An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Turk J Pharm Sci
- v.18(3); 2021 Jun


Development and Validation of an HPLC Method Using an Experimental Design for Analysis of Amlodipine Besylate and Enalapril Maleate in a Fixed-dose Combination
Diren sarisaltik yasin.
1 Dicle University Faculty of Pharmacy, Department of Pharmaceutical Technology, Diyarbakır, Turkey
Alev ARSLANTÜRK BİNGÜL
2 Dicle University Faculty of Science, Department of Chemistry, Diyarbakır, Turkey
Alptuğ KARAKÜÇÜK
3 Gazi University Faculty of Pharmacy, Department of Pharmaceutical Technology, Ankara, Turkey
4 Ankara Medipol University Faculty of Pharmacy, Department of Pharmaceutical Technology, Ankara, Turkey
Zeynep Şafak TEKSİN
Objectives:.
The aim of this study was to develop and optimize a simple, cost-effective, and robust high-performance liquid chromatography (HPLC) method by taking an experimental design approach to the assay and dissolution analysis of amlodipine besylate and enalapril maleate from a fixed-dose combination tablet.
Materials and Methods:
The chromatographic analysis was performed on a C18 column (4.6x250 mm id., particle size of 5 μm). The injection volume was 5 μL, and the detection wavelength was 215 nm. A Box-Behnken design was used to test the robustness of the method. The flow rate (1, 1.2, and 1.4 mL/min), column temperature (25°C, 30°C, and 35°C), methanol ratio of the mobile phase (5, 10, and 15%), and pH of the mobile phase (2.8, 3, and 3.2) were selected as independent variables. The method was validated according to International Conference on Harmonization guidelines. Dissolution of the tablets was performed by using USP apparatus 2 and analyzed using the optimized HPLC method. Multivariate linear regression analysis and ANOVA were used in the statistical evaluation.
Linear models were fitted for all variables. The flow rate was the most significant factor affecting the APIs’ concentrations. The optimized method included the following parameters: Column temperature of 25°C, 10% methanol as the mobile phase, pH of 2.95, and flow rate of 1.205 mL/min. Retention times were 3.8 min and 7.9 min for enalapril and amlodipine, respectively. The method was found to be linear in the range of 0.8-24 μg/mL (R 2 >0.999) and 1.6-48 μg/mL (R 2 >0.999) for amlodipine and enalapril, respectively. Both APIs were dissolved more than 85% within 10 min.
Conclusion:
The experimental design was proved as a useful tool for the determination and separation of enalapril maleate and amlodipine besylate in dosage forms. The optimized method can be used for in vitro performance and quality control tests of fixed-dose tablet combinations containing enalapril maleate and amlodipine besylate.
INTRODUCTION
At the early stages of the treatment of hypertension, it can be useful to choose monotherapy to observe the effect and the side effects of the drug. However, monotherapy can be insufficient to reach the target blood pressure in a majority of patients. 1 , 2 , 3 A greater therapeutic benefit can be achieved with two or even more antihypertensive drugs. 4 Therefore, fixed-dose combinations (FDCs) are frequently used in cardiovascular diseases such as hypertension. In order to develop an FDC product including two drugs, certain conditions must be met. For instance, a synergistic effect can be observed using two drugs together, or a side effect related to a drug may be eliminated using the other drug concurrently. 5 In the treatment of hypertension, there is a synergistic effect between calcium channel blockers (CCBs) and angiotensin-converting enzyme inhibitors (ACEIs). In addition, ACEIs such as enalapril prevent peripheral edema caused by CCBs such as amlodipine. 6
Amlodipine is a long-acting CCB that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. It is indicated for the treatment of hypertension and coronary artery disease when used alone or in combination with another antihypertensive agent. 7 Amlodipine is given orally as besylate in general, but doses are calculated in terms of amlodipine base. A dose of 6.94 mg of amlodipine besylate is equivalent to 5 mg of amlodipine base. The recommended dose of amlodipine is 5-10 mg once daily. 8 Since amlodipine is a weak base, it exhibits high solubility in physiological pH values. Although the bioavailability of amlodipine is approximately 60%-65%, it is defined as a highly permeable drug because of the 90%-95% excretion rate as an inactive metabolite in the urine Shohin et al. 9 Amlodipine is a class 1 drug according to the Biopharmaceutics Classification System (BCS). 9 , 10 , 11
Enalapril is the ethyl ester of enalaprilat, an ACEI indicated for the treatment of hypertension and heart failure. Enalapril is available as maleate salt in the drug market. Enalapril maleate is a white crystalline powder sparingly soluble in water. Although the solubility is 25 mg/mL at pH 3.5, it increases to 200 mg/mL at pH 7.0. It is defined as BCS class 3 with high solubility but low permeability properties. 12
There are high-performance liquid chromatography (HPLC) methods recommended in United States Pharmacopeia (USP42) for analysis of amlodipine besylate 13 and enalapril maleate, 14 separately and a few liquid chromatography methods are available in the literature for analyses of amlodipine, 15 and enalapril, 16 , 17 individually or in combination with other drugs. 18 , 19 , 20 , 21 , 22 , 23 However, these methods are not suitable for the separation of amlodipine and enalapril in the same dosage unit. Nevertheless, there are three published articles for HPLC analysis of amlodipine besylate and enalapril maleate together in dosage forms. 24 , 25 , 26 However these methods contain a high ratio of organic solvents in the mobile phase, which is environmentally inappropriate according to the green chemistry approach. An important principle of green chemistry is to reduce toxic organic solvents and to consume safer chemicals. 27 , 28 Relating to the green analytical chemistry approach, Korany et al. 27 recommended reducing the acetonitrile amount in the methods and using multiparameter methods such as design of experiment (DOE) instead of the one factor at a time (OFAT) approach. 28 In the method developed by Chaudhari 24 , the mobile phase contains 50% acetonitrile and 40% methanol and a higher injection volume (20 µL), which increases the consumption of mobile phase and the linearity range was comparatively narrow (0.5-6 µg/mL and 0.5-8 µg/mL for enalapril and amlodipine, respectively). In another method, the mobile phase includes 60% acetonitrile, the injection volume was 20 µL, and the linearity range was not suitable for lower concentrations (20-100 µg/mL), which might be essential for the initial points of the dissolution tests. 25 In the method developed by Masih et al. 26 , 50% 1N HCl and 50% methanol were included in the mobile phase, and the injection volume was 10 µL. Additionally, none of the studies include the application of DOE in robustness testing in validation for amlodipine besylate and enalapril maleate. Furthermore, there is no dissolution analysis of enalapril and amlodipine in the combined dosage form in the literature.
DOE is a well-defined mathematical methodology to demonstrate how to obtain maximum reliable and valuable scientific information by performing minimal experiments. 29 In this technique, the effects of multiple variations on one or more responses can be investigated at the same time, instead of changing OFAT. Although conventional developmental approaches are mainly empirical and are often conducted using the changing OFAT method, DOE provides the facility of performing systematic and multivariate experiments in order to entirely understand the process and to assess the statistical significance of the variables. 30 , 31 By creating experimental matrix, DOE allows faster visualization and determination of more factors at a time. 32 Besides, in OFAT approach factors are evaluated independently, so it is assumed that the factors do not influence each other. However, the potential interactions between the factors can be identified using the appropriate DOE model. 33 , 34 In the pharmaceutical field, DOE helps to understand the effects of the critical formulation and process variables on the final product. 35 , 36 DOE can be used for factor screening and characterization of a new system or optimization of a characterized system. Factors are independent variables that might affect the results of critical responses. For instance, in an analytical method development process, the flow rate can be an independent factor that has potential effects on the peak area of the analyte. In a screening design it is aimed to investigate numerous factors that might affect the response and to discover the factor which has the most significant influence on the responses. 37 On the other hand, in an optimization process, the main objective of which is to define the optimal conditions and settings for the factors. 38 In case more than one factor must be examined, the multivariate optimization designs can be reasonable in order to evaluate different factors at the same time and to determine if interactions exist between factors. 37 , 38
In analytical chemistry, DOE can be used for chromatographic analytical method development to optimize the sampling preparational, column, detector, instrumental, or environmental factors. 31 , 39 Similarly, analytical method validation parameters such as accuracy, linearity, precision, or robustness can be performed by experimental design approaches. 29 , 40 , 41 , 42 , 43 , 44 , 45 , 46 Using DOE in validation studies is recommended in the International Conference on Harmonization (ICH) guidelines. 27 , 47 There have been many studies in which DOE was applied to robustness. 31 , 32 , 43 , 48 , 49 Experimental design targeting robustness is a good approach to fully understand the factors with effects on the responses and provide maximum information about the method in a short time. Robustness should be built into methods in the pre-validation stages; otherwise, a robustness test performed too late has a risk of obtaining inappropriate results which can cause redevelopment and revalidation. 50 Therefore, a robustness test in the earlier stage of the method development process leads to a saving of effort, time, and money. Experimental data obtained from early stages can aid in performance method evaluation and can be used to guide further method development. 51
Optimization can be performed by using response surface methodology (RSM) designs such as the Box-Behnken design (BBD) and the central composite design (CCD). 49 , 52 The BBD is a second-order design that allows investigation of numerous factors with three levels. It is preferable to the CCD because it prevents an unrealistic extreme scenario by creating the experimental matrix without containing extreme points in the same experiment. 33 , 52 BBD is used in analytical method optimization in many studies. 6 , 48 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65
In this study, a simple, rapid and robust HPLC method with photodiode array (PDA) detection at 215 nm was developed for the determination and separation of amlodipine besylate and enalapril maleate in FDC tablets. This method, which is available for assay and dissolution studies, was fast, environmentally friendly, and more cost-effective than the earlier published methods. 24 , 25 , 26 In this study, DOE was adapted to the robustness parameter of the analytical method for determining amlodipine and enalapril together. DOE principles were used in the method development of amlodipine and enalapril for the first time. The validation of the method was performed according to the ICH Q2 (R1) guideline. 47 The BBD was used for the optimization of the method. The optimized HPLC method was applied to dissolution and assay analysis of an in-house FDC tablet including amlodipine and enalapril.
MATERIALS AND METHODS
Materials and reagents.
HPLC-grade methanol, o-phosphoric acid and hydrochloric acid 37% were obtained from Merck, Germany. Amlodipine besylate (Hetero Drugs, India) and enalapril maleate (Zheijiang Huahai, China) were kindly gifted by Nobel Pharma, Turkey.
The FDC tablet contains 6.94 mg of amlodipine besylate and 10 mg of enalapril maleate as APIs.
The HPLC system was a Shimadzu chromatographic system (Japan) with LC-20AD pump, SPD-M20A PDA detector at a wavelength of 215 nm, a reversed phase C18 column (4.6x250 mm id., particle size of 5 µm) from Waters ® (USA). The HPLC system was controlled by LC Solution Software. Design Expert ® Version 9 (Stat-Ease Inc, USA) was used for the experimental design and statistical analysis of data. A pH meter (PASS1 P11-BNC-Bante, England) was used to control the aqueous buffer. Dissolution test was performed with Pharmatest ® Dissolution System (Germany).
Chromatographic conditions
The mobile phase was a mixture of methanol and water (pH adjusted to 3.0 with o-phosphoric acid) in the proportion of 10:90 (v:v). The injection volume of the samples was 5 µL. The flow rate was 1.2 mL/min. The detector wavelength was 215 nm and the column temperature was 30°C.
Preparation of standard solutions
The standard solution was prepared according to the following process: 6.94 mg of amlodipine besylate (equivalent to 5 mg amlodipine base) and 10 mg of enalapril maleate were weighed and transferred to a 50 mL volumetric flask and diluted to the appropriate volume with 0.1N HCl. This solution included 0.1 mg/mL of amlodipine base and 0.2 mg/mL of enalapril maleate. The calculations were performed considering amlodipine base and enalapril as maleate salts because of the dose proportionality in market products .
Calibration procedure
Calibration series were prepared in volumetric flasks by the appropriate dilution of standard solution with 0.1N HCl. The calibration curve was plotted with eight concentrations in the range of 0.8-24 µg/mL for amlodipine and 1.6-48 µg/mL for enalapril (as maleate). The experiments were performed in three replicates for each level. The linearity of the calibration curve was evaluated by the linear regression statistics of concentrations against peak area.
Statistical analysis
Experimental design.
Experimental plan, data analysis and optimization process were executed in Design Expert ® Version 9 by using the BBD. The BBD is a three-level and multi-factor design which is a combination of 2K factorial and balanced incomplete block designs. In this study, four factors with three levels for each were determined as given in Table 1 .

The significant factors in the model were determined by multivariate linear regression analysis and ANOVA F-test and its lack of fit with a confidence interval of 95% for each response. Significant factors were determined by the probability level that the p value is less than 0.05 and one-factor graphs.
Assay in FDC tablets
The FDC tablet containing amlodipine besylate and enalapril maleate was prepared by using direct compression method. For assay of the tablets, 10 tablets for each product were selected at random and weighed. Then these tablets were powdered, and a quantity of the powder (equivalent to 5 mg of amlodipine and 10 mg of enalapril maleate) was accurately weighed and transferred to a 50 mL volumetric flask. A 30 mL volume of diluent solution (0.1N HCl) was added and mixed for 15 min in magnetic stirrer. Then, it was diluted with the same solution to the volume and mixed in an ultrasonic bath for 10 min. A 4 mL volume of this solution was transferred to a 25 mL volumetric flask and diluted to the volume using the same solvent and was held in an ultrasonic bath for 5 min. The samples were filtered through a syringe tip filter of 0.45-µm pore size and then analyzed using HPLC.
Dissolution studies
Dissolution studies were performed using USP apparatus II (paddle method) in 0.1N HCl (pH 1.2). The dissolution volume was 900 mL, and the temperature was 37°C±0.5°C. The paddle rotational speed was 75 rpm. Samples (2 mL) were withdrawn at 10, 20, 30, 45, and 60 min, and the same amount of fresh media was replaced. The samples were filtered through 0.45-µm membrane filters to vials and analyzed by the optimized HPLC method. The dissolution profiles were evaluated as the cumulative drug dissolved (%) over time. All experiments were performed in n=3 and the cumulative amounts were evaluated as the mean ± standard deviation (SD).
RESULTS AND DISCUSSION
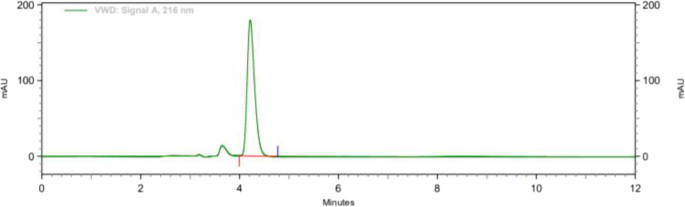
The chromatograms of diluent (blank) and those obtained from the standard solutions of amlodipine and enalapril are given in Figure 1 , ,2 2 respectively. The initial method provided good separation in a short time of 3.8 min for enalapril and 7.9 min for amlodipine. This level of separation is acceptable in a conventional method development process. A robustness study with DOE was also performed.

Chromatogram of the placebo (blank medium) for specificity testing
PDA: Photodiode array

Chromatogram of enalapril (8 μg/mL, as maleate) and amlodipine (4 μg/mL) in the initial method
Robustness with DOE principles
According to the ICH Q2 (R1), in a robust method, small variations in certain method parameters do not affect the reliability and results of the method. 47 These small variations are important for the pharmaceutical industry in terms of the transfer of the analytical method from research and development to the quality control laboratory or from one company to another. In other words, it is the indication of the strength of the method. 51 In order to assess the concurrent influences of the changes in factors on the defined responses, a multivariate analysis by DOE is recommended in robustness studies. 43 DOE is used in analytical method development for two main purposes: To determine the most significant factor influencing the response of the study and to discover the optimized value of the factors for best results for the response. 37
The DOE plan in a robustness test includes the following stages: 31
Selection of factors and their levels
Robustness studies are an excellent opportunity to apply statistical experimental design to provide data-based control of the method. 51 Since there are many factors that might affect the method, it is vital to choose the right factors. In robustness studies of liquid chromatography, the most frequently preferred factors are the pH of the mobile phase, analysis time, flow rate, column type, temperature, composition of the mobile phase, detection wavelength, chosen filters, or the variations in sample preparation such as dilution, shaking time, or heating temperature. 39 , 51 It should be noted that there are no absolute truths in selecting factors in a DOE process; the chosen factors should comply with the purpose. According to ICH Q2 (R1), the following variations were recommended for the robustness test of HPLC methods: 1) pH of the mobile phase, 2) composition of the mobile phase, 3) column type, 4) temperature, and 5) flow rate. Except for the column type, all recommended factors (mobile phase ratio, pH, flow rate, and column temperature) were investigated in this study. The chosen factors and their pre-defined levels have the potential to affect the method depending on the analyst, laboratory or equipment, and environmental conditions. 47
After selecting the factors, it is necessary to define their levels. In a two-level model such as Plackett-Burman Design (PBD) or two-level factorial designs, a maximum and a minimum limit are required for the factor values. In three-level designs, additional middle values, which generally represent the target or the expected value, are added to the design. Defining the levels is a critical step in experimental design. Particularly in two-level designs in which inappropriate levels were used, inaccurate and low-quality results can be obtained. 33 In order to avoid this problem, a three-level BBD design is preferred. The levels of the factors are usually defined symmetrically around the nominal level, which is the middle level in a three-level design. The interval chosen between the levels is generally decided according to the operator’s personal experiences or anticipated changes from one laboratory to another. For example, if the developed method will be transferred to another laboratory, the pH can be measured using a pH meter with a small deviation, so pH should be considered as critical. The pH of a solution varies with a deviation of 0.02 with a confidence limit of 95%. 50 Therefore, this limit is acceptable for the pH in a robustness test. The interval of pH was ±0.02 in this study. The levels of column temperature were decided ±5°C as recommended in the article by Vander-Heyden et al. 50 , which was aimed to guide a robustness parameter in method development. The levels of other factors, selected as 5% for mobile phase composition and 0.2 mL/min for flow rate, were in agreement with previous similar studies. 32 , 43 , 65
Defining responses to be investigated
In the HPLC studies where robustness was investigated by DOE, various responses such as peak area, peak height, determined concentration, retention time, tailing factor, theoretical plate number, and resolution were used. The most important selection criterion for a response to use in factor evaluation is ease of measurement. 39 Additionally, using a large number of responses can lead to confusion when interpreting the results. Therefore, API concentrations calculated from the peak areas were selected as responses in this study.
Choosing an experimental design
A suitable experimental design should be selected based on the aim of the study. In case a large number of factors might affect the method, the aim can be to discard some factors that have no significant effect on the response. For this purpose, a screening design such as PBD can be used. On the other hand, if the main objective is to investigate the effects of the relatively lower number of factors deeply, or optimize the most effective factors, optimization designs should be preferred. 31 Generally, optimization is carried out following determination of the most significant factors by screening design. In case there is a factor known to be highly effective in the separation (such a flow rate or temperature), optimization designs can be preferred directly. 37 In this study, factors that may affect the results, such as the column temperature, flow rate, and composition of the mobile phase, were chosen with the purpose of performing an optimization. Another reason for choosing an RSM design is to observe any interaction between the factors.
The most used RSM designs are CCD and BBD. BBD requires the fewest experiments among the RSM designs because it does not contain values that are maximum or minimum values in the experimental matrix. 33 Since BBD requires fewer experiments, and the experimental matrix does not contain the highest or lowest level in the combination, this experimental design prevents an unrealistic extreme scenario. Therefore, the experiment number, time, and cost are reduced. BBD can evaluate the linear and non-linear effects of factors. 34 , 66 Thus, BBD was selected for the experimental plan, data analysis and optimization process using the Design Expert ® Version 9 software.
Execution of experiments
Experimental executions were computed by Design Expert Software. Robustness was assessed by using BBD with 29 runs. Experimental design and calculated concentrations of enalapril (as maleate) and amlodipine and the corresponding responses are given in Table 2 .

Statistical evaluation of responses and their interpretations
The best fit model was linear for all factors and their responses. In the literature, linear analysis is frequently indicated and recommended in robustness tests. 29 , 30 Therefore, our results were as expected. Linear models are used to show the main effects of factors.
The equation model for Y 1 (enalapril concentration) and Y 2 (amlodipine concentration) was as follows:
Y 1 =32.32+0.079X 1 -5.32X 2 +0.11X 3 +0.51X 4 (Equation 1)
Y 2 =16.19+0.12X 1 -2.72X 2 +0.020X 3 +0.021X 4 (Equation 2)
Where, X 1 is column temperature, X 2 is flow rate, X 3 is the methanol ratio in the mobile phase, and X 4 is the pH of the mobile phase.
The ANOVA results are given in Table 3 . The significant effects showed a p value less than 0.05, a low SD (CV %), and a high adjusted R-square (adj R 2 ) value indicating a good relationship between the experimental data and those of the fitted model. The predicted R-square (pred R 2 ) value was in agreement with the adj R 2 for all responses.

The one-factor graphs ( Figure 3 , ,4) 4 ) demonstrated that the flow rate was the most significant factor on the responses; inverse proportionality was found (p<0.05). It was revealed that the most critical factor in robustness is the flow rate. The methanol ratio in mobile phase, temperature, and pH had no significant effect on the calculated concentrations of amlodipine and enalapril in defined levels. Kovacs et al. 30 have evaluated the same factors in their robustness test with different responses such as peak asymmetry and retention time. They found that the proportion of methanol in the mobile phase had a significant effect on the retention time of strontium ranelate. Similarly, Dhumal et al. 32 found that the proportion of methanol in the mobile phase and the flow rate had a negative effect, while the pH had a positive effect on the peak area and the determined tapentadol concentration. In another study, in which the same factors and different responses (tailing factor, retention time and theoretical plate) were used, the most effective factors were found to be the methanol composition and pH. 45 However, the significance of factors depends on the APIs and chromatographic conditions. If we had defined our levels more broadly for other factors (methanol ratio, temperature, and pH) or if we had assessed more responses such as tailing factor or resolution we might have observed a meaningful effect with other factors. However, this was not considered to be an error in the design because the DOE is specific to the purpose. In this study, we would like to see how possible rational changes would affect the analytical results, rather than creating a design space based on the extreme values of factors.

A-D) One-factor graphs of the main effects of the factors on amlodipine concentration

A-D) One-factor graphs of the main effects of the factors on enalapril concentration
Two-way interactions between independent variables were found to be insignificant (p>0.05). Therefore, a simple screening design, such as a PBD, which is the most popular design in robustness evaluation, might be used in this study. 37 However, since PBD is a two-level design, it can cause inaccurate statistical evaluations when unsuitable factor levels are selected or when there might be an interaction between the factors. If an experimental model is needed to determine tolerable variations, an optimization design is recommended by Sahu et al. 31 For this reason, as discussed before, we preferred a BBD that contained a third level (target middle level) and provided more information about the method. There have been similar studies with other drugs in which calculated drug concentrations were the only response and flow rate was the only significant factor in the response. 43 , 46
Optimization
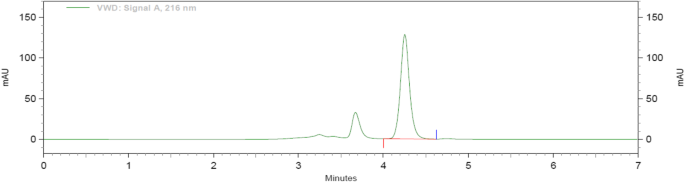
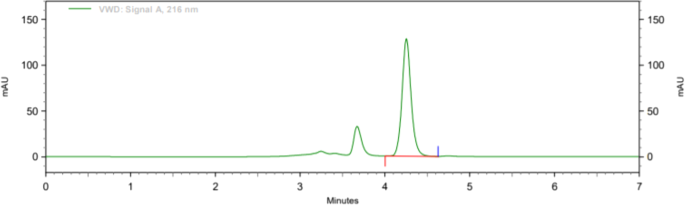
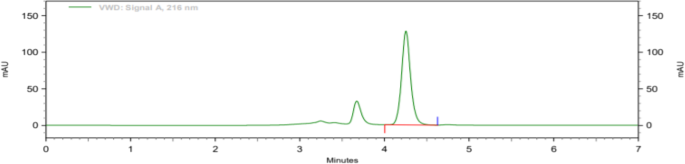
Following linear model fitting, an optimization run was performed, and factor settings were defined using the prediction spreadsheet of the software ( Figure 5 ). The final optimized parameters were a flow rate of 1.205 mL/min, pH of 2.95, and column temperature of 25°C. The factors described in the optimization were very close to the nominal levels in the BBD design. Non-etheless, these minor changes caused a better peak shape for amlodipine and a lower tailing factor (from 1.417 to 1.164, p<0.05) ( Figure 6 ). Retention times were not changed in the method with 3.8 min and 7.9 min for enalapril and amlodipine, respectively.

Optimization conditions of independent variables according to the Design Expert ® Software

Chromatograms of enalapril (8 μg/mL, as maleate) and amlodipine (4 μg/mL) in the optimized method
The optimized method was validated based on international guidelines.
The linearity of the peak area versus concentration was shown in the range of 0.8-24 µg/mL for amlodipine and 1.6-48 µg/mL for enalapril (as maleate). Linearity results were given in Table 4 . The linearity range was kept wider than the previously published methods. 24 , 25 , 26 The lower concentrations are considered for the first minutes of the dissolution study, and higher values are for the assay.

Accuracy was demonstrated using six different solutions, containing 1.39, 2.78, 5.56, 12, 16, and 19.2 µg/mL of amlodipine and 2.78, 5.56, 11.12, 24, 32, and 38.4 µg/mL of enalapril maleate. Recovery values were obtained within the range of 98.6%-101.6%. The low value of relative standard deviation (RSD) less than 1% indicates that the proposed method is accurate. Results are presented in Table 5 .

Repeatability
Repeatability is also termed intraday precision and provides information about the precision under the same operating conditions in a short time interval. 47 Repeatability was assessed using 10 determinations of the solutions including 16 µg/mL of amlodipine and 32 µg/mL of enalapril maleate. The recovery values were 99.9±0.31% and 100±0.07% for amlodipine and enalapril maleate, respectively.
The RSDs were 0.307% and 0.0711% for amlodipine and enalapril maleate, respectively.
Intermediate precision
Intermediate precision was assessed using the interday variations. Two different concentrations (4 and 16 µg/mL for amlodipine and 8 and 32 µg/mL for enalapril maleate) were analyzed on three consecutive days. The RSD values of interday precision were less than 1%, confirming the method precision. The results are given in Table 6 .

The low RSD value for intermediate precision and repeatability of the method as well as within-day and day-to-day variation suggested that the method was precise within the range of measurement.
Limit of detection (LOD) and limit of quantification (LOQ)
LOD and LOQ were calculated based on the SD of the response and the slope by using the equations below:
L O D = 3 . 3 × σ S ( Equation 3 )
L O Q = 10 × σ S ( Equation 4 )
where s is the SD of the response, and S is the slope of the calibration curve. According to the equations, LOD values were 0.0631 µg/mL and 0.0424 µg/mL and LOQ were 0.19 µg/mL and 0.129 µg/mL for amlodipine and enalapril maleate, respectively.
The LOD and LOQ results suggested that the method was highly sensitive.
The drugs dissolved in 0.1N HCl were stable when stored at 25°C for 72 hours. After 72 hours, drug recovery values were 99.7% for amlodipine and 99.4% for enalapril maleate.
Assay in tablets
The optimized method was used for the assay of amlodipine and enalapril in FDC tablets. An additional peak from excipients was not observed. The results were in the range of the labeled amount ±5% for both drugs ( Table 7 ).

Dissolution
Dissolution was performed with the in-house FDC tablet by using USP apparatus II in 0.1N HCl. 0.1N HCl was selected as the model dissolution medium. The proposed HPLC method was available for dissolution of FDC tablets. Both amlodipine and enalapril were dissolved more than 85% within 10 min. Dissolution profiles of amlodipine and enalapril were given in Figure 7 . The dissolution media of 0.1N HCl replaces the artificial stomach medium that is frequently used with the purpose of formulation development and quality control. For using this analytical method for other dissolution media such as pH 4.5 or pH 6.8 there might be small modifications in chromatographic conditions.

Dissolution results of amlodipine and enalapril in an in-house FDC product (n=3)
FDC: Fixed-dose combination
In conclusion, an accurate, precise, specific, and environmentally appropriate HPLC method was developed and validated for amlodipine besylate and enalapril maleate in the typical dosage unit. The BBD, an optimization design, was used to evaluate the operational factors in a robustness test, and validation was performed according to international guidelines. The developed method was more economic and suitable for green chemistry with less solvent consumption, which improved column performance. The method was applied to assay and dissolution studies and was found suitable for quality control tests and in vitro performance of pharmaceutical dosage forms for a fixed-dose tablet combination containing amlodipine besylate and enalapril maleate for the treatment of hypertension.
Acknowledgments
The authors would like to thank Nobel Pharma (Turkey) for providing amlodipine besylate and enalapril maleate as gift samples.
Conflicts of interest: No conflict of interest was declared by the authors. The authors alone are responsible for the content and writing of the paper.
- Open access
- Published: 19 July 2021
Development and validation of RP-HPLC method for estimation of brexpiprazole in its bulk and tablet dosage form using Quality by Design approach
- Amol S. Jagdale 1 ,
- Nilesh S. Pendbhaje 2 ,
- Rupali V. Nirmal 1 ,
- Poonam M. Bachhav 1 &
- Dayandeo B. Sumbre 1
Future Journal of Pharmaceutical Sciences volume 7 , Article number: 142 ( 2021 ) Cite this article
7000 Accesses
3 Citations
Metrics details
A new, sensitive, suitable, clear, accurate, and robust reversed-phase high-performance liquid chromatography (RP-HPLC) method for the determination of brexpiprazole in bulk drug and tablet formulation was developed and validated in this research. Surface methodology was used to optimize the data, with a three-level Box-Behnken design. Methanol concentration in the mobile phase, flow rate, and pH were chosen as the three variables. The separation was performed using an HPLC method with a UV detector and Openlab EZchrom program, as well as a Water spherisorb C 18 column (100 mm × 4.6; 5m). Acetonitrile was pumped at a flow rate of 1.0 mL/min with a 10 mM phosphate buffer balanced to a pH of 2.50.05 by diluted OPA (65:35% v/v) and detected at 216 nm.
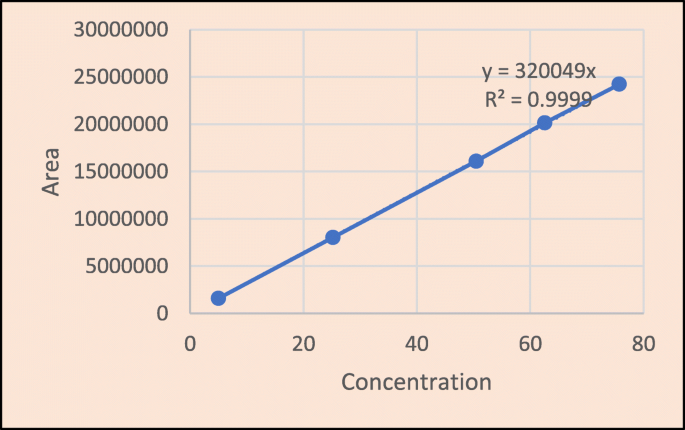
The developed RP-HPLC method yielded a suitable retention time for brexpiprazole of 4.22 min, which was optimized using the Design Expert-12 software. The linearity of the established method was verified with a correlation coefficient (r 2 ) of 0.999 over the concentration range of 5.05–75.75 g/mL. For API and formulation, the percent assay was 99.46% and 100.91%, respectively. The percentage RSD for the method’s precision was found to be less than 2.0%. The percentage recoveries were discovered to be between 99.38 and 101.07%. 0.64 μg/mL and 1.95 μg/mL were found to be the LOD and LOQ, respectively.
The developed and validated RP-HPLC system takes less time and can be used in the industry for routine quality control/analysis of bulk drug and marketed brexpiprazole products.
Graphical abstract
Brexpiprazole is 7-[4-[4-(1-benzothiophen-4-yl) piperazin-1-yl] piperazin-1-yl] piperazin-1-yl] piperazin-1-yl] piperazin-1-yl] piperazin-1-yl] piperazin-1 butoxy] However, The USFDA approved quinolin-2 (1H)-one in 2015, and it is marketed as Rexulti, a generic name coined by Otsuka in Japan and marketed by Lundbeck in the USA for the treatment of schizophrenia as a monotherapy and as an adjunctive treatment to antidepressants in the treatment of major depressive disorder [ 1 , 2 , 3 , 4 , 5 ]. Early treatment with aripiprazole can result in problematic akathisia. Brexpiprazole may be less likely than aripiprazole to induce akathisia. This will be a big benefit, but there is not much experience with the drug yet. Brexpiprazole, like aripiprazole, is a partial agonist of the dopamine D2 receptor and has mild effects on QTc. Brexpiprazole is likely to have a wide dose range in clinical practice due to the function of CYP2D6 in its metabolism [ 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 ].
According to a literature review, there are few publications on UV-visible spectroscopy and HPLC, but no one has used Quality by Design. To ensure process consistency throughout the product lifecycle, simple validated RP-HPLC methods for the determination of brexpiprazole in pharmaceutical dosage forms must be established using the Quality by Design (QbD) approach as per ICH Q8 (R2) guidelines [ 8 , 15 , 16 , 17 , 18 , 19 ].
Materials and reagents
Alkem Laboratories Limited, Mumbai, donated brexpiprazole (see Fig. 1 ). Merck provided HPLC grade methanol, acetonitrile, orthophosphoric acid (OPA), and analytical grade ethanol, DMF, DMSO, and HCl. Siddhi Lab provided the HPLC grade water.
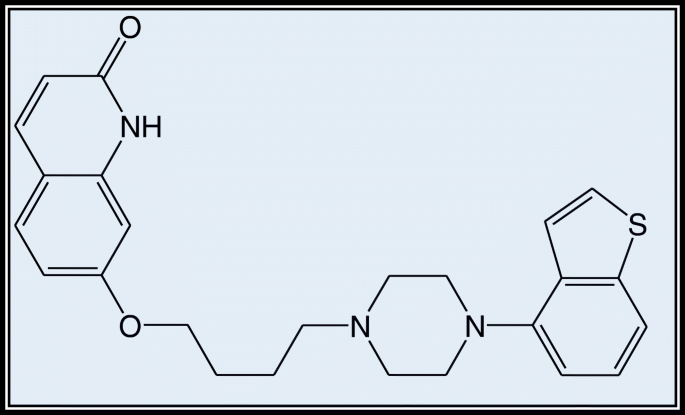
Molecular structure of brexpiprazole
Instrumentation and software
An Agilent HPLC system with DEAX02386 pump and autosampler with UV-visible detector served as the chromatographic system (DEACX16446). For data collection and processing, the chromatograms were registered using Openlab EZChrom on a Windows-based computer system. Brexpiprazole concentrations were determined using a HyPURITY C 18 (100mm × 4.6mm ID, particle size 5μ) column.
QbD software
Design Expert® software (Design Expert trial version 12.0.10.0; State-Ease Inc., Minneapolis, MN, USA)
Preparations of solutions
Preparation of standard stock solution.
The standard solution was made by dissolving 10 mg of brexpiprazole in a 100-mL clean and dry volumetric flask, then adding approximately 70 mL of methanol to fully dissolve it and fill the flask to the mark with methanol (100 μg/mL).
Sample preparation
Ten milligrams brexpiprazole was correctly weighed and transferred to a 100-mL volumetric flask. Fifty milliliters diluent was added and sonicated to fully remove it. Using diluent, dilute the mixture by another 10 to 20 mL.

Preparation of diluted OPA
Pipette 5 mL of OPA into a 50-mL volumetric flask and top up with water to reach the desired amount. Sonicate for 5 min after thoroughly mixing.
Preparation of 1.0% OPA in water
One milliliter OPA was blown out of the solution and moved to a 100-mL volumetric flask, which was filled to the mark with water. Sonicate for 5 min after thoroughly mixing.
Preparation of 10.00 mM phosphate buffer in water
Weigh 1.36 g of OPA and dissolve it in 1000 mL of water, adjusting the pH to 2.0 (±) 0.05 with the diluted OPA solution.
Determination of detection wavelength
Between 200 and 400 nm, the standard solution was scanned. As shown in Fig. 2 , the wavelength of maximum absorption for drug was determined to be 216 nm.
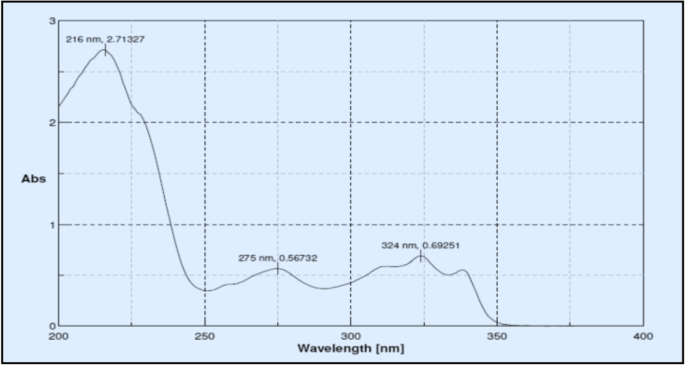
UV spectrum of brexpiprazole in methanol
Method development by QbD approach
Application of design of experiments for method optimization.
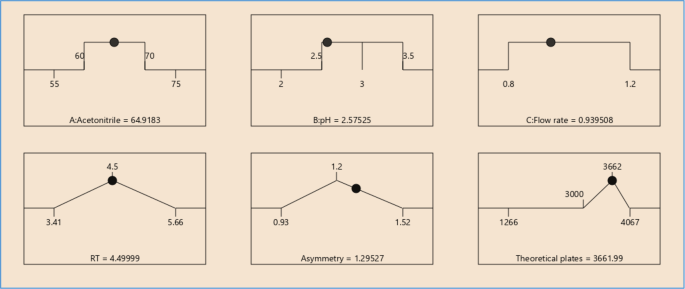
To investigate the effect of three factors on the two primary response variables, 33 randomized response surface designs with a Box-Behnken design were used with 17 trial runs. Three variables were analyzed at three levels in this design, and experimental trials were conducted in all three possible combinations. Flow rates (X1), pH (X2), and mobile phase composition (X3) were designated as independent variables, while retention time (RT), asymmetry, and theoretical plates were designated as dependent variables. The data was then entered into the Design Expert 12.0.10.0 software and evaluated using the ANOVA test. To assess the effect of flow rate, pH, and mobile phase composition on dependent variables, the results were subjected to the 3-dimensional response surface methodology. Table 1 shows the likely trial runs using 3 3 Box-Behnken designs. Table 2 shows the experimental results and selected method conditions.
Analysis of the sample
Brexpiprazole drug (api).
The drug sample solution was prepared by liquifying 10 mg of brexpiprazole API into a 100-mL volumetric flask, adding 70 mL of methanol to fully melt it by sonication, and then adjusting the volume with solvents (100g/mL). Filtered through a suitable filter, and a sufficient amount of the sample solution was discarded. Using methanol (50g/mL), dilute 5 mL of the filtrate solution to 10 mL.
Tablet formulation
Keep in mind, in the market, there is a tablet called Rexulti. However, it is not available in India. There are three doses available: 1, 2, and 4 mg. The sample preparation will be seen on a dosage of 4 mg, which is a higher dose. For preparing lab-level tablet mixture, approximately 150 mg average weight is taken into account. The formula for one tablet is shown in Table 3 .
Weigh 10 mg of brexpiprazole powder into a 100-mL flask, add about 70 mL of methanol to completely solubilize it by sonication, and complete volume up to spot with the methanol (100 g/mL). Filtered through a suitable filter, and a sufficient amount of the sample solution was discarded. Five milliliters filtrate solution was diluted to 10 mL with methanol (50 g/mL).
Control strategy
Filtration study.
Filtration experiment using centrifuged (unfiltered) sample and filtered test solution. During the filtration process, 5 mL of the aliquot sample was discarded and 0.45 m PVDF 0.45 and 0.45 m Nylon syringe filters were used.
Stability of analytical solution
A stability analysis will be carried out on both the normal and test solutions. A test sample of Rexulti tablet will be used to determine the stability of the test solution. The stability test will be carried out in a standard laboratory environment.
The solution will be held in a brightly lit laboratory for 12 to 24 h before being analyzed. The discrepancy between the test solution’s results at each stability time point and the original will be calculated for the test solution stability analysis. The discrepancy between the effects of the stability time point and the original will be calculated in a standard solution stability analysis.
Method validation
The developed method for estimating brexpiprazole was validated for the following parameters using ICH Q2 (R1) guidelines [ 20 , 21 , 22 , 23 , 24 , 25 ].
Specificity
To demonstrate the method’s precision, the following solutions will be prepared and injected (double-checked the peak purity).
Blank (methanol as a diluent)
Brexpiprazole standard solution
Brexpiprazole sample solution
Placebo treatments
Linearity and range
The statistical treatment of test results obtained by examination of samples with analyte concentrations around the claimed spectrum determines the analytical method’s linearity. As a function of analyte concentration, the region is graphically plotted. Curve fitting percentages are measured.
Accuracy (%recovery)
The accuracy will be tested in the range of 50 to 150% of the working concentration of 4 mg strength. Every occurs solution will be prepared in triplicate. A placebo will be included in the experiment. For each study, the percent recovery was determined.
There are two levels of precision: repeatability and intermediate precision. It is carried out on a sample API.
Repeatability (intraday precision)
Intermediate precision (interday precision)
The API test sample was created from scratch. As shown below, these samples were injected under various chromatographic conditions.
Flow rate changes (20% of the total)
A change in wavelength (3 nm)
± 2°C increase in column oven temperature
The limit of detection (LOD) and limit of quantification (LOQ) were calculated separately using the following equations based on the standard deviation of the y-intercept and the slope of the calibration curve, respectively.
Optimization of mobile phase
Methanol: water (70:30), acetonitrile: water (70:30), acetonitrile: 1% OPA in water (80:20), and acetonitrile: 10 mM phosphate buffer were among the mobile phases that were optimised, shown in Fig. 3 . Acetonitrile: 10 mM phosphate buffer adjusted pH 2.5 by OPA (80:20), acetonitrile: 10 mM phosphate buffer adjusted pH 2.5 by OPA (65:35), acetonitrile: 10 mM phosphate buffer adjusted pH 2.5 by OPA (65:35). As a result, chromatographic conditions in trial were used for process validation, as shown in Fig. 4 : Acetonitrile: 10 mM phosphate buffer modified pH 2.5 by OPA (65:35) provides a better peak, lower retention time, and tailing factor. Typical chromatogram of optimized run is shown in Fig. 5 . Analytical data for typical chromatogram of optimized run are shown in Table 4 .
Solutions for optimized run
Typical chromatogram obtained from optimized mobile phase
Typical chromatogram of optimized run
Optimization of various parameters for analysis of brexpiprazole using HPLC (by using central composite design)
Design summary for optimization is given in Table 5 . Obtained solution for optimized formulation is given in Table 6 .
System suitability test (SST)
It was observed from the data tabulated that the method complies with system suitability parameters. Hence, it can be concluded that the system suitability parameter meets the requirement of method validation. Typical chromatogram of SST for brexpiprazole is shown in Fig. 6 . Analytical data of system suitability test are given in Table 7 .
Typical chromatogram of SST for brexpiprazole
Filter test
Both filters PVDF and Nylon pass the criteria for filter study; hence, both filters can be used because %absolute difference is NMT 2.0, and it follows acceptance criteria. Analytical data of filter test are given in tabular form in Table 8 . Typical chromatogram of unfiltered sample, sample filtered through 0.45 μ PVDF filter, and sample filtered through 0.45 μ Nylon filter is shown in Figs. 7 , 8 , and 9 respectively.
Typical chromatogram of unfiltered sample
Typical chromatogram of sample filtered through 0.45μ PVDF filter
Typical chromatogram of sample filtered through 0.45μ Nylon filter
Solution stability
Both standard solution and sample solution were found stable for 24 h; hence, prepared solution can be used up to 24 h. (User can check solution stability even after 24 h if he/she wants to inject solution after 24 h.) Analytical data are given in Table 9 .
Blank and placebo solution are not having interference at R.T. of brexpiprazole. Peak purity for both standard as well as sample was within limits. Sample solution exhibits the same R.T. as that of standard solution. Hence, developed chromatographic method passed the criteria for specificity. Result of specificity is given in Table 10 .
%Recovery was found well within acceptance range (98.00 to 102.0%) at all three levels. Result and statistical data of accuracy are given in Table 11 .
%RSD for 12 samples (precision and intermediate precision samples) NMT 2.0%. The %RSD of method precision is 0.53 and 0.495. Therefore, the HPLC method for the determination of brexpiprazole is precise. Analytical data of both precision of brexpiprazole is given in Table 12 .
From the calibration curve, we had to conclude that brexpiprazole shows linear response in the range of 5.05–75.75 μg/mL. The regression value was found well within the limit. Result and statistical data of linearity of brexpiprazole are given in Table 13 . Linearity graph of brexpiprazole is shown in Fig. 10 .
Linearity graph of brexpiprazole
Based on the calibration curve, we can deduce that brexpiprazole has a linear response in the 5.05–75.75 g/mL range. The regression value was discovered to be well within the acceptable range. Data for calibration curve of brexpiprazole is shown in Table 14 .
It may be calculated based on the standard deviation (SD) of the response and slope of the curve (S). Result of detection limit is given in Table 15 . Calibration curve of brexpiprazole for LOD and LOQ is given in Fig. 11 .
Calibration curve of brexpiprazole for LOD and LOQ
The robustness of an analytical method is determined by analysis of aliquots from homogenous lots by differing physical parameters that may differ but are still within the specified parameters of the assay. Analytical interpretation is given in Table 16 .
The aim of this project was to create a simple, reliable, precise, and appropriate RP-HPLC system using the Quality by Design (QbD) approach. DOE results, including ANOVA, diagnostic graphs, and model graphs, were examined for each factor. The effect of each factor on the response result was investigated in this result.
In terms of analytical method creation and validation, the results of all system suitability parameters were appropriate within the limits specified by applying ICH (Q2 R1) guidelines, indicating that the system is functioning properly and can provide accurate and precise results. The established method’s analysis results were validated in terms of linearity, accuracy, precision, and robustness, as well as the detection and quantification limits.
The developed method has many advantages, according to Mondal et al., including reproducibility of findings, rapid interpretation, easy sample preparation, and improved selectivity and sensitivity. The developed method can be used for routine research in the pharmaceutical industry for the bulk drug brexpiprazole as well as the pharmaceutical dosage type since it is stable and reproducible and takes less time [ 10 ].
According to the above experimental results, this newly developed method for estimating brexpiprazole was found to be simple, precise, and accurate, with a shorter retention time that makes it more acceptable and cost effective, and it can be effectively applied for routine analysis in research institutions, quality control departments in industries, and approved testing laboratories.
Availability of data and materials
Data and material are available upon request.
• https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/205422s003lbl.pdf
• https://www.clinicaltrialsarena.com/projects/rexulti-brexpiprazole-treatment-major-depressive-disorder-schizophrenia/
• www.drugbank.com
• https://www.drugs.com/monograph/brexpiprazole.html
Abbreviations
Reversed-phase high-performance liquid chromatography
Percentage recovery
Limit of detection
Limit of quantification
Quality by Design
United States Food and Drug Administration
International Council for Harmonization of Technical Requirement for Pharmaceutical for Human Use
Orthophosphoric acid
Dichloromethane
Dimethyl sulfoxide
Hydrochloric acid
Polyvinylidene fluoride
Analysis of variance
Active pharmaceutical ingredient
Greig SL (2015) Brexpiprazole: first global approval. Drugs 75(14):1687–1697. https://doi.org/10.1007/s40265-015-0462-2
Article CAS PubMed Google Scholar
Rexulti® (brexpiprazole) [full prescribing information]. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/205422s003lbl.pdf Accessed 18 Jan 2016.
Stahl SM (2016) Mechanism of action of brexpiprazole: comparison with aripiprazole. CNS Spectr 21(1):1–6. https://doi.org/10.1017/S1092852915000954
Article PubMed Google Scholar
https://www.clinicaltrialsarena.com/projects/rexulti-brexpiprazole-treatment-major-depressive-disorder-schizophrenia/
Maeda et al (2014) Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulators. J Pharmacol Exp Ther 350(3):589–604. https://doi.org/10.1124/jpet.114.213793
Hope J, Castle D, Keks NA (2018) Brexpiprazole: a new leaf on the partial dopamine agonist branch. Australas Psychiatry 26(1):92–94. https://doi.org/10.1177/1039856217732473
Sravani A, Naga Durga CH, Divya U, Suneetha CH, Suresh P, Tirumaleswara Rao B (2017) Method development and validation for the estimation of brexpiprazole in drug substance by RP-HPLC method. Indo Am J Pharm Res 7(05):8560–8564
CAS Google Scholar
Bhawar HS, Thete S, Shinde GS (2019) Development and validation of stability indicating RP-HPLC method for estimation of brexpiprazole from bulk and tablet form. J Drug Deliv Therapeut 9(4):141–145
Jaiswal CC, Patel HU (2020) Development and validation of stability indicating RP-HPLC method for estimation of brexpiprazole in tablet. World J Pharmacy Pharm Sci 9(6):1568–1582
Mondal S, Kumar VG, Mondal P (2018) New spectrophotometric techniques for the estimation of brexpiprazole in tablet dosage form. Int J Pharm Sci Res 10(5):2151–2455
Google Scholar
Patel P, Mashru R (2020) Design, optimization, and validation of chemometrics assisted spectrophotometric methods for simultaneous determination of brexpiprazole and aripiprazole
Pulusu VS, Routhu KC, Chikkaswamy SB (2019) Quantitative determination of brexpiprazole by RP-HPLC method. Pharmaceutica Analytica Acta 10(2):610 1-5
Article Google Scholar
www.drugbank.com
https://www.drugs.com/monograph/brexpiprazole.html
ICH Harmonized Tripartite Guideline. (2009). Pharmaceutical development Q8(R2) International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. http://www.ich.org/fileadmin/public_web_site/ich_products/guidelines/quality/Q8_R1/step4/Q8_R2_guideline.pdf
ICH Harmonized Tripartite Guideline. Quality risk management Q9 (2005) International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf
ICH Harmonized Tripartite Guideline. Pharmaceutical quality systems Q10 (2008) International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use. http://www.ich.org/fileadmin/public_web_site/ich_products/guidelines/quality/Q10/step4/Q10_guideline.pdf
Gundala A, Bharathi K, Prasad KVSRG (2018) Analytical quality by design approach in RP-HPLC method development for the assay of pitavastatin in tablet dosage form. Int J Pharm Sci Res 9(11):4992–5001
Bhatt DA, Rane SI (2011) QbD approach to analytical RPHPLC method development and its validation. Int J Pharm Pharm Sci 3(1):179–187
International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use ICH harmonized tripartite guideline validation of analytical procedures: text and methodology Q2(R1) Validation. https://www.ich.org/page/quality-guidelines\
Vidushi Y, Meenakshi B (2017) A review on HPLC method development and validation. Res J Life Sci, Bioinform, Pharm Chem Sci 2(6):178
Pendhbaje NS, Nirmal RV, Jamdhade AA, Pathan SM (2021) Method development and validation by HPLC: a brief review. Res Rev: J Pharm Sci 12(1):27–39p
Bose A (2014) HPLC calibration process parameters in terms of system suitability test. Austin Chromatogr 1(2):1–4
Sabir AM (2013) HPLC method development and validation -a review. In Res J Pharm 4(4):39–46
Pendbhaje NS, Jamdhade AA, Pathan SM, Nirmal RV (2021) A review on quantification of brexpiprazole in its bulk and pharmaceutical dosage form by various analytical methods. Int J Pharm Res Appl 6(1):1118–1132
Download references
Acknowledgements
Authors express their sincere gratitude to N.D.M.V.P. College of Pharmacy, Nashik, and Sanjivani College of Pharmaceutical Education and Research, Kopargaon, for continuous motivation, support, and guidance for research activity and for providing all required facilities to accomplish the entitled work.
Not applicable
Author information
Authors and affiliations.
MVPs College of Pharmacy, Nashik-422 002, Department of Pharmaceutical Chemistry, Savitribai Phule Pune University, Pune, MS, India
Amol S. Jagdale, Rupali V. Nirmal, Poonam M. Bachhav & Dayandeo B. Sumbre
SRE’S, Sanjivani College of Pharmaceutical Education and Research, Kopargaon, India
Nilesh S. Pendbhaje
You can also search for this author in PubMed Google Scholar
Contributions
R.N. contributed to literature survey and performed practical work and thesis typing, N.P. supported in performing experimental work, A.S. had contributed to guide the whole work, and P.B. and D.S. contributed to editing in thesis typing. The authors read and approved the final manuscript and approve the submission.
Corresponding author
Correspondence to Rupali V. Nirmal .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Jagdale, A.S., Pendbhaje, N.S., Nirmal, R.V. et al. Development and validation of RP-HPLC method for estimation of brexpiprazole in its bulk and tablet dosage form using Quality by Design approach. Futur J Pharm Sci 7 , 142 (2021). https://doi.org/10.1186/s43094-021-00293-5
Download citation
Received : 24 February 2021
Accepted : 25 June 2021
Published : 19 July 2021
DOI : https://doi.org/10.1186/s43094-021-00293-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Brexpiprazole
- Acetonitrile
- Development
- My Shodhganga
- Receive email updates
- Edit Profile
Shodhganga : a reservoir of Indian theses @ INFLIBNET
- Shodhganga@INFLIBNET
- Koneru Lakshmaiah Education Foundation
- Department of Chemistry
Items in Shodhganga are licensed under Creative Commons Licence Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0).

- Conference Proceedings - APTICON
- Editorial Board
- Journal Metrics
- Past Editors
- Press Release
- Manuscript Submissions
- Instruction to Authors
- Article Processing Charges
- Plagiarism Policy
- For Librarians
- Search Articles
Stability Indicating Assay for the Determination of Bilastine in Bulk Drug and Method Development Validation by RP-HPLC Using Analytical Quality by Design Approaches
Authors and affiliation (s):.
Anjali Nayak 1,* , R Maria Danish Alwin 2 , Sangeetha G 2 , Sowmya C Y 2 , Yashwanth V Reddy 2 , Raman Dang 3
1 Department of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, Bangalore, Karnataka, INDIA.
2 Department of Quality Assurance, Krupanidhi College of Pharmacy, Bangalore, Karnataka, INDIA.
3 Principal, Krupanidhi College of Pharmacy, Bangalore, Karnataka, INDIA
Background: Regulatory organizations have acknowledged the need for systematic rules for understanding development as a result of the large increase in concerns and criticism regarding the quality and pharmaceutical products. Bilastine is a second generation antihistamine medication. Generally, it is used for treatment of allergic rhino conjunctivitis and urticaria (hives). Objectives: The current study outlines the methodical design and validation of a reversed-phase high-performance liquid chromatographic method for the estimation of Bilastine in bulk drugs using AQbD approach. Materials and Methods: Using Box Behnken design, the critical method parameters were methodically optimized. Risk estimation matrix was performed and Critical Analytical Attributes, Critical Method Attributes were correlated to identify risk factors of method development. A reverse phase column in isocratic elution mode with mobile phase NaH 2 PO 4 buffer and methanol of different ratio and flow rate 1 mL/min was set for RP-HPLC method development. Results: Chromatographic separation was accomplished on INTERSIL C8 column. The optimized and predicted data from JMP PRO 14 software consist of mobile phase 0.1N NaH 2 PO 4 (60%): Methanol (40%), pumped at a flow rate of 1 mL/min gave the higher desirability function of 77%. LOD and LOQ are, respectively, 0.005 mcg/mL and 0.016 mcg/ mL. The Rt of Bilastine was discovered to be 1.894 min. The created method was approved and validated in accordance with ICH Q2 (R1) recommendations. Conclusion: The chosen models were determined to be significant with p <0.05. The validation parameter findings were within the permitted range. Forcefully testing the drug's stability under various stress situations revealed considerable degradation in the presence of heat.
Keywords: AQbD, Bilastine, Analytical target profile, Critical method attributes, Design of experiments, RP-HPLC, Validation.
- PDF (PDF, 423.6 KB)
Browse Issues
- Latest Issue
- Past Issues
Impact Factor
IJPER - An Official Publication of Association of Pharmaceutical Teachers of India is pleased to announce continued growth in the Latest Release of Journal Citation Reports (source: Web of Science Data).
Impact Factor® as reported in the 2023 Journal Citation Reports® (Clarivate Analytics, 2023): 0.8
Recent Publications
- Scientific Validation of Traditional Detoxification Process and Evaluation of its Impact on Anti-Microbial Potency, Phytochemical and Heavy Metals in Nigella sativa
- Systemic Effect of Human Follicular Fluid from Endometriotic and Healthy Subjects on Female Mice
- Development and Validation of a Stability Indicating HPTLC Method for Simultaneous Estimation of Telmisartan and Gallic Acid as per ICH Q1A (R2)
- Rapid, Simple and Low-Cost Reverse-Phase High-Performance Liquid Chromatography Tool for Estimation of Methotrexate: Testing Application in Standard Laboratory Bulk, Marketed Dosage Form, and Release kinetics in Nanoformulation
The Official Journal of Association of Pharmaceutical Teachers of India (APTI) (Registered under Registration of Societies Act XXI of 1860 No. 122 of 1966-1967, Lucknow)
Indian Journal of Pharmaceutical Education and Research (IJPER) [ ISSN-0019-5464] is the official journal of Association of Pharmaceutical Teachers of India (APTI) and is being published since 1967.
DOI HISTORY
IJPER uses reference linking service using Digital Object Identifiers ( DOI ) by Crossref. Articles from the year 2013 are being assigned DOIs for its permanent URLs

IMAGES
VIDEO
COMMENTS
Results: Linear models were fitted for all variables. The flow rate was the most significant factor affecting the APIs' concentrations. The optimized method included the following parameters: Column temperature of 25°C, 10% methanol as the mobile phase, pH of 2.95, and flow rate of 1.205 mL/min. Retention times were 3.8 min and 7.9 min for enalapril and amlodipine, respectively.
Validation is the process of establishing the performance characteristics and limitations of a method and identification of the influences which may change these characteristics and to what extent ...
know the analytical method validation of HPLC as per USP and ICH guidelines. Keywords Validation, HPLC, USP, ICH, Regulatory, QC Lab Introduction Method validation is defined as the process which proves that an implied analytical method is acceptable for its intended purpose, determined by means of well- documented experimental studies.
The present study describes the risk based HPLC method development and validation of ceftriaxone sodium in pharmaceutical dosage form. An efficient experimental design based on central composite design of two key components of the RP-HPLC method (mobile phase and pH) is presented. The chromatographic conditions were optimized with the Design ...
II. Guidelines for analytical method development and validation of biotechnological synthesis of drugs. Production of a chiral steroid as model. Johan Lindholm, Monika Johansson and Torgny Fornstedt. Journal of Chromatography B, 791 (2003), 323-336. III. Investigation of the adsorption behaviour of a chiral model com-pound on Kromasil CHI-TBB.
This review gives information regarding various stages involved in development and validation of HPLC method. Validation of HPLC method as per ICH Guidelines covers all the performance characteristics of validation, like Accuracy, precision, specificity, linearity, range and limit of detection, limit of quantification, robustness and system ...
Received 04 Novem ber 2015; accep ted 20 November 2015. Abstract. HPLC is the dominant separ ation tech nique in modern pharmaceutical and bio medical anal ysis because it r esults in hi ghly ...
method development, optimization, and validation processes. Because of its advantages such as rapidity, specificity, accuracy, precision, and ease of automation, the HPLC method can be used to analyze the majority of drugs in multicomponent dosage forms. HPLC method development and validation are critical in new drug
The issues pertinent to designing HPLC method development and validation and identification of the influences which may change these characteristics and to what extent are discussed. High performance liquid chromatography (HPLC) is an essential analytical tool in assessing drug product. HPLC methods should be able to separate, detect, and quantify the various drugs and drug related degradants ...
This thesis concerns the development and validation of high performance liquid chromatography (HPLC) methods aimed for two industrially important areas: (i) analysis of biotechnological synthesis a ...
The Shodhganga@INFLIBNET Centre provides a platform for research students to deposit their Ph.D. theses and make it available to the entire scholarly community in open access. Shodhganga@INFLIBNET. Jawaharlal Nehru Technological University, Anantapuram. Department of Pharmaceutical Sciences.
HPLC Method Development: Analytical method development and validation are critical steps in the discovery, development, and manufacturing of pharmaceuticals. These techniques are used to ensure the identity, purity, potency, and performance of pharmaceutical products. When developing methods, there are numerous factors to consider.
Validation is the process of establishing the performance and limitations of any technique and identification of various products which may change their characteristics. This article discusses the strategies and the issues pertinent to modelling of HPLC method development and validation. Key words - HPLC, Impurities, Method development, Validation
HPLC methods development and validation play important roles in new discovery, development, manufacture of pharmaceutical drugs and various other studies related to humans and animals. An analytical procedure is developed to test a defined characteristic of the drug substance or drug product against established acceptance criteria for ...
A new, sensitive, suitable, clear, accurate, and robust reversed-phase high-performance liquid chromatography (RP-HPLC) method for the determination of brexpiprazole in bulk drug and tablet formulation was developed and validated in this research. Surface methodology was used to optimize the data, with a three-level Box-Behnken design. Methanol concentration in the mobile phase, flow rate, and ...
For HPLC Method Development Agilent make of HPLC is used using Phosphate Buffer, Acetonitrile, and Methanol in different Proportions as per the drug under Study. newlineFor application of QBD Approach, Design Expert®13 software was used. newlineAfter Method Development as per ICH guidelines Validation has been performed .In Linearity ...
The steps involved in developing a stability-indicating HPLC method influences the analysis of degradation products/impurities in stability study and its validation demonstrate the suitability for its intended purpose. HPLC method development and validation play important role in the discovery, development and manufacture of pharmaceutical products. This article mainly focuses on the ...
the validation work proceeds. Quite often method validation evolves from method development and so the two activities are often closely tied, with the validation study employing the techniques and steps in the anal ysis as defined by the method development. Analytical methods need to be validated or revalidated
Validation is an analytical method that provides information about various parameters such as accuracy, precision, linearity, Limit if Detection, Limit of Quantification, specificity, robustness, range. As per ICH guidelines, the validation should be done. This article was prepared with the aim to review the method development and validation of ...
and quality reliably, in a cost-effective manner. Development of a method is essential for discovery, development, and evaluation of medicines in the pharmaceutical formulation. The main aim of this review article was to check the development and validation of the procedure employed for the
The analytical quantification of the different dose-banded (DB) of the infusion bags prepared in advance were achieved using this new reversed HPLC-DAD/ELSD analytical method in less than 4 min. For a same batch of mAbs, DAD generated nearly uniform response and showed a slightly superior accuracy than did ELSD.
Amazon Web Services
Development and Validation of Stability Indicating RP HPLC Method for the Simultaneous Determination of Selective Pharmaceutical Drugs: Researcher: Nekkala Kalpana: Guide(s): J V Shanmukha Kumar: Keywords: Chemistry Chemistry Analytical Physical Sciences: University: Koneru Lakshmaiah Education Foundation: Completed Date: 2020: Abstract ...
Risk estimation matrix was performed and Critical Analytical Attributes, Critical Method Attributes were correlated to identify risk factors of method development. A reverse phase column in isocratic elution mode with mobile phase NaH 2 PO 4 buffer and methanol of different ratio and flow rate 1 mL/min was set for RP-HPLC method development.
Validation studies demonstrated that the RP-HPLC method of finerenone in both bulk drug & pharmaceutical fixed dosage forms is simple, specific, rapid, reliable & reproducible. I have done research and achieved the method development & validation of highly accurate, sensitive, precise, rapid gradient system RP-HPLC method of finerenone in both bulk drug & pharmaceutical fixed dosage forms. The ...
The results indicate that the method was sensitive and could detect and quantify lower levels of cyclophosphamide and its related substances, and thus can be used for the stability-indicating analysis of cyclophosphamide and its related substances in pharmaceutical formulations. Objective: The current study aimed to develop a simple, sensitive, and precise high-performance liquid ...