The Sheridan Libraries
- Write a Literature Review
- Sheridan Libraries
- Find This link opens in a new window
- Evaluate This link opens in a new window

Not every source you found should be included in your annotated bibliography or lit review. Only include the most relevant and most important sources.

Get Organized
- Lit Review Prep Use this template to help you evaluate your sources, create article summaries for an annotated bibliography, and a synthesis matrix for your lit review outline.
Summarize your Sources
Summarize each source: Determine the most important and relevant information from each source, such as the findings, methodology, theories, etc. Consider using an article summary, or study summary to help you organize and summarize your sources.
Paraphrasing
- Use your own words, and do not copy and paste the abstract
- The library's tutorials about plagiarism are excellent, and will help you with paraphasing correctly
Annotated Bibliographies
Annotated bibliographies can help you clearly see and understand the research before diving into organizing and writing your literature review. Although typically part of the "summarize" step of the literature review, annotations should not merely be summaries of each article - instead, they should be critical evaluations of the source, and help determine a source's usefulness for your lit review.
Definition:
A list of citations on a particular topic followed by an evaluation of the source’s argument and other relevant material including its intended audience, sources of evidence, and methodology
- Explore your topic.
- Appraise issues or factors associated with your professional practice and research topic.
- Help you get started with the literature review.
- Think critically about your topic, and the literature.
Steps to Creating an Annotated Bibliography:
- Find Your Sources
- Read Your Sources
- Identify the Most Relevant Sources
- Cite your Sources
- Write Annotations
Annotated Bibliography Resources
- Purdue Owl Guide
- Cornell Annotated Bibliography Guide
- << Previous: Evaluate
- Next: Synthesize >>
- Last Updated: Sep 26, 2023 10:25 AM
- URL: https://guides.library.jhu.edu/lit-review
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- QuestionPro

- Solutions Industries Gaming Automotive Sports and events Education Government Travel & Hospitality Financial Services Healthcare Cannabis Technology Use Case NPS+ Communities Audience Contactless surveys Mobile LivePolls Member Experience GDPR Positive People Science 360 Feedback Surveys
- Resources Blog eBooks Survey Templates Case Studies Training Help center
Home Surveys Academic Research
Research Summary: What is it & how to write one

The Research Summary is used to report facts about a study clearly. You will almost certainly be required to prepare a research summary during your academic research or while on a research project for your organization.
If it is the first time you have to write one, the writing requirements may confuse you. The instructors generally assign someone to write a summary of the research work. Research summaries require the writer to have a thorough understanding of the issue.
This article will discuss the definition of a research summary and how to write one.
What is a research summary?
A research summary is a piece of writing that summarizes your research on a specific topic. Its primary goal is to offer the reader a detailed overview of the study with the key findings. A research summary generally contains the article’s structure in which it is written.
You must know the goal of your analysis before you launch a project. A research overview summarizes the detailed response and highlights particular issues raised in it. Writing it might be somewhat troublesome. To write a good overview, you want to start with a structure in mind. Read on for our guide.
Why is an analysis recap so important?
Your summary or analysis is going to tell readers everything about your research project. This is the critical piece that your stakeholders will read to identify your findings and valuable insights. Having a good and concise research summary that presents facts and comes with no research biases is the critical deliverable of any research project.
We’ve put together a cheat sheet to help you write a good research summary below.
Research Summary Guide
- Why was this research done? – You want to give a clear description of why this research study was done. What hypothesis was being tested?
- Who was surveyed? – The what and why or your research decides who you’re going to interview/survey. Your research summary has a detailed note on who participated in the study and why they were selected.
- What was the methodology? – Talk about the methodology. Did you do face-to-face interviews? Was it a short or long survey or a focus group setting? Your research methodology is key to the results you’re going to get.
- What were the key findings? – This can be the most critical part of the process. What did we find out after testing the hypothesis? This section, like all others, should be just facts, facts facts. You’re not sharing how you feel about the findings. Keep it bias-free.
- Conclusion – What are the conclusions that were drawn from the findings. A good example of a conclusion. Surprisingly, most people interviewed did not watch the lunar eclipse in 2022, which is unexpected given that 100% of those interviewed knew about it before it happened.
- Takeaways and action points – This is where you bring in your suggestion. Given the data you now have from the research, what are the takeaways and action points? If you’re a researcher running this research project for your company, you’ll use this part to shed light on your recommended action plans for the business.
LEARN ABOUT: Action Research
If you’re doing any research, you will write a summary, which will be the most viewed and more important part of the project. So keep a guideline in mind before you start. Focus on the content first and then worry about the length. Use the cheat sheet/checklist in this article to organize your summary, and that’s all you need to write a great research summary!
But once your summary is ready, where is it stored? Most teams have multiple documents in their google drives, and it’s a nightmare to find projects that were done in the past. Your research data should be democratized and easy to use.
We at QuestionPro launched a research repository for research teams, and our clients love it. All your data is in one place, and everything is searchable, including your research summaries!
Authors: Prachi, Anas
MORE LIKE THIS

Raked Weighting: A Key Tool for Accurate Survey Results
May 31, 2024

Top 8 Data Trends to Understand the Future of Data
May 30, 2024

Top 12 Interactive Presentation Software to Engage Your User
May 29, 2024

Trend Report: Guide for Market Dynamics & Strategic Analysis
Other categories.
- Academic Research
- Artificial Intelligence
- Assessments
- Brand Awareness
- Case Studies
- Communities
- Consumer Insights
- Customer effort score
- Customer Engagement
- Customer Experience
- Customer Loyalty
- Customer Research
- Customer Satisfaction
- Employee Benefits
- Employee Engagement
- Employee Retention
- Friday Five
- General Data Protection Regulation
- Insights Hub
- Life@QuestionPro
- Market Research
- Mobile diaries
- Mobile Surveys
- New Features
- Online Communities
- Question Types
- Questionnaire
- QuestionPro Products
- Release Notes
- Research Tools and Apps
- Revenue at Risk
- Survey Templates
- Training Tips
- Uncategorized
- Video Learning Series
- What’s Coming Up
- Workforce Intelligence
- USC Libraries
- Research Guides
Organizing Your Social Sciences Research Paper
- 7. The Results
- Purpose of Guide
- Design Flaws to Avoid
- Independent and Dependent Variables
- Glossary of Research Terms
- Reading Research Effectively
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Applying Critical Thinking
- Choosing a Title
- Making an Outline
- Paragraph Development
- Research Process Video Series
- Executive Summary
- The C.A.R.S. Model
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Secondary Sources
- Tiertiary Sources
- Scholarly vs. Popular Publications
- Qualitative Methods
- Quantitative Methods
- Insiderness
- Using Non-Textual Elements
- Limitations of the Study
- Common Grammar Mistakes
- Writing Concisely
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Generative AI and Writing
- USC Libraries Tutorials and Other Guides
- Bibliography
The results section is where you report the findings of your study based upon the methodology [or methodologies] you applied to gather information. The results section should state the findings of the research arranged in a logical sequence without bias or interpretation. A section describing results should be particularly detailed if your paper includes data generated from your own research.
Annesley, Thomas M. "Show Your Cards: The Results Section and the Poker Game." Clinical Chemistry 56 (July 2010): 1066-1070.
Importance of a Good Results Section
When formulating the results section, it's important to remember that the results of a study do not prove anything . Findings can only confirm or reject the hypothesis underpinning your study. However, the act of articulating the results helps you to understand the problem from within, to break it into pieces, and to view the research problem from various perspectives.
The page length of this section is set by the amount and types of data to be reported . Be concise. Use non-textual elements appropriately, such as figures and tables, to present findings more effectively. In deciding what data to describe in your results section, you must clearly distinguish information that would normally be included in a research paper from any raw data or other content that could be included as an appendix. In general, raw data that has not been summarized should not be included in the main text of your paper unless requested to do so by your professor.
Avoid providing data that is not critical to answering the research question . The background information you described in the introduction section should provide the reader with any additional context or explanation needed to understand the results. A good strategy is to always re-read the background section of your paper after you have written up your results to ensure that the reader has enough context to understand the results [and, later, how you interpreted the results in the discussion section of your paper that follows].
Bavdekar, Sandeep B. and Sneha Chandak. "Results: Unraveling the Findings." Journal of the Association of Physicians of India 63 (September 2015): 44-46; Brett, Paul. "A Genre Analysis of the Results Section of Sociology Articles." English for Specific Speakers 13 (1994): 47-59; Go to English for Specific Purposes on ScienceDirect;Burton, Neil et al. Doing Your Education Research Project . Los Angeles, CA: SAGE, 2008; Results. The Structure, Format, Content, and Style of a Journal-Style Scientific Paper. Department of Biology. Bates College; Kretchmer, Paul. Twelve Steps to Writing an Effective Results Section. San Francisco Edit; "Reporting Findings." In Making Sense of Social Research Malcolm Williams, editor. (London;: SAGE Publications, 2003) pp. 188-207.
Structure and Writing Style
I. Organization and Approach
For most research papers in the social and behavioral sciences, there are two possible ways of organizing the results . Both approaches are appropriate in how you report your findings, but use only one approach.
- Present a synopsis of the results followed by an explanation of key findings . This approach can be used to highlight important findings. For example, you may have noticed an unusual correlation between two variables during the analysis of your findings. It is appropriate to highlight this finding in the results section. However, speculating as to why this correlation exists and offering a hypothesis about what may be happening belongs in the discussion section of your paper.
- Present a result and then explain it, before presenting the next result then explaining it, and so on, then end with an overall synopsis . This is the preferred approach if you have multiple results of equal significance. It is more common in longer papers because it helps the reader to better understand each finding. In this model, it is helpful to provide a brief conclusion that ties each of the findings together and provides a narrative bridge to the discussion section of the your paper.
NOTE: Just as the literature review should be arranged under conceptual categories rather than systematically describing each source, you should also organize your findings under key themes related to addressing the research problem. This can be done under either format noted above [i.e., a thorough explanation of the key results or a sequential, thematic description and explanation of each finding].
II. Content
In general, the content of your results section should include the following:
- Introductory context for understanding the results by restating the research problem underpinning your study . This is useful in re-orientating the reader's focus back to the research problem after having read a review of the literature and your explanation of the methods used for gathering and analyzing information.
- Inclusion of non-textual elements, such as, figures, charts, photos, maps, tables, etc. to further illustrate key findings, if appropriate . Rather than relying entirely on descriptive text, consider how your findings can be presented visually. This is a helpful way of condensing a lot of data into one place that can then be referred to in the text. Consider referring to appendices if there is a lot of non-textual elements.
- A systematic description of your results, highlighting for the reader observations that are most relevant to the topic under investigation . Not all results that emerge from the methodology used to gather information may be related to answering the " So What? " question. Do not confuse observations with interpretations; observations in this context refers to highlighting important findings you discovered through a process of reviewing prior literature and gathering data.
- The page length of your results section is guided by the amount and types of data to be reported . However, focus on findings that are important and related to addressing the research problem. It is not uncommon to have unanticipated results that are not relevant to answering the research question. This is not to say that you don't acknowledge tangential findings and, in fact, can be referred to as areas for further research in the conclusion of your paper. However, spending time in the results section describing tangential findings clutters your overall results section and distracts the reader.
- A short paragraph that concludes the results section by synthesizing the key findings of the study . Highlight the most important findings you want readers to remember as they transition into the discussion section. This is particularly important if, for example, there are many results to report, the findings are complicated or unanticipated, or they are impactful or actionable in some way [i.e., able to be pursued in a feasible way applied to practice].
NOTE: Always use the past tense when referring to your study's findings. Reference to findings should always be described as having already happened because the method used to gather the information has been completed.
III. Problems to Avoid
When writing the results section, avoid doing the following :
- Discussing or interpreting your results . Save this for the discussion section of your paper, although where appropriate, you should compare or contrast specific results to those found in other studies [e.g., "Similar to the work of Smith [1990], one of the findings of this study is the strong correlation between motivation and academic achievement...."].
- Reporting background information or attempting to explain your findings. This should have been done in your introduction section, but don't panic! Often the results of a study point to the need for additional background information or to explain the topic further, so don't think you did something wrong. Writing up research is rarely a linear process. Always revise your introduction as needed.
- Ignoring negative results . A negative result generally refers to a finding that does not support the underlying assumptions of your study. Do not ignore them. Document these findings and then state in your discussion section why you believe a negative result emerged from your study. Note that negative results, and how you handle them, can give you an opportunity to write a more engaging discussion section, therefore, don't be hesitant to highlight them.
- Including raw data or intermediate calculations . Ask your professor if you need to include any raw data generated by your study, such as transcripts from interviews or data files. If raw data is to be included, place it in an appendix or set of appendices that are referred to in the text.
- Be as factual and concise as possible in reporting your findings . Do not use phrases that are vague or non-specific, such as, "appeared to be greater than other variables..." or "demonstrates promising trends that...." Subjective modifiers should be explained in the discussion section of the paper [i.e., why did one variable appear greater? Or, how does the finding demonstrate a promising trend?].
- Presenting the same data or repeating the same information more than once . If you want to highlight a particular finding, it is appropriate to do so in the results section. However, you should emphasize its significance in relation to addressing the research problem in the discussion section. Do not repeat it in your results section because you can do that in the conclusion of your paper.
- Confusing figures with tables . Be sure to properly label any non-textual elements in your paper. Don't call a chart an illustration or a figure a table. If you are not sure, go here .
Annesley, Thomas M. "Show Your Cards: The Results Section and the Poker Game." Clinical Chemistry 56 (July 2010): 1066-1070; Bavdekar, Sandeep B. and Sneha Chandak. "Results: Unraveling the Findings." Journal of the Association of Physicians of India 63 (September 2015): 44-46; Burton, Neil et al. Doing Your Education Research Project . Los Angeles, CA: SAGE, 2008; Caprette, David R. Writing Research Papers. Experimental Biosciences Resources. Rice University; Hancock, Dawson R. and Bob Algozzine. Doing Case Study Research: A Practical Guide for Beginning Researchers . 2nd ed. New York: Teachers College Press, 2011; Introduction to Nursing Research: Reporting Research Findings. Nursing Research: Open Access Nursing Research and Review Articles. (January 4, 2012); Kretchmer, Paul. Twelve Steps to Writing an Effective Results Section. San Francisco Edit ; Ng, K. H. and W. C. Peh. "Writing the Results." Singapore Medical Journal 49 (2008): 967-968; Reporting Research Findings. Wilder Research, in partnership with the Minnesota Department of Human Services. (February 2009); Results. The Structure, Format, Content, and Style of a Journal-Style Scientific Paper. Department of Biology. Bates College; Schafer, Mickey S. Writing the Results. Thesis Writing in the Sciences. Course Syllabus. University of Florida.
Writing Tip
Why Don't I Just Combine the Results Section with the Discussion Section?
It's not unusual to find articles in scholarly social science journals where the author(s) have combined a description of the findings with a discussion about their significance and implications. You could do this. However, if you are inexperienced writing research papers, consider creating two distinct sections for each section in your paper as a way to better organize your thoughts and, by extension, your paper. Think of the results section as the place where you report what your study found; think of the discussion section as the place where you interpret the information and answer the "So What?" question. As you become more skilled writing research papers, you can consider melding the results of your study with a discussion of its implications.
Driscoll, Dana Lynn and Aleksandra Kasztalska. Writing the Experimental Report: Methods, Results, and Discussion. The Writing Lab and The OWL. Purdue University.
- << Previous: Insiderness
- Next: Using Non-Textual Elements >>
- Last Updated: May 30, 2024 9:38 AM
- URL: https://libguides.usc.edu/writingguide
Bulk Content Generator
Brand Voice
AI Text Editor
Research Summary Generator
Craft a detailed research synopsis utilizing the given data for an all-encompassing understanding.
Try Research Summary for free →
Research Summary
Learn how to provide the key points, main findings, and any other relevant information for the research you want to summarize
1 variation
What is a Research Summary?
Have you ever found yourself drowning in a sea of research articles, struggling to make sense of it all? Well, fear not! A research summary is here to save the day. But what exactly is a research summary, and how can it help you navigate the vast ocean of information?
A research summary is a concise and informative overview of a research article, report, or thesis. It aims to provide the reader with a clear understanding of the study's purpose, methods, results, and conclusions without having to read the entire document. Think of it as a mini-version of the original work that highlights its most important aspects.
Now that we know what a research summary is let's dive into why they're so beneficial.
The Benefits of Research Summaries
Research summaries offer several advantages for both readers and writers:
Time-saving : Reading a well-written research summary can save you hours of sifting through dense academic papers. It allows you to quickly grasp the key points and decide if you want to explore the full document further.
Improved comprehension : By breaking down complex ideas into digestible chunks, research summaries make it easier for readers to understand the material. This is particularly helpful for those who are new to a topic or have limited knowledge in the field.
Enhanced communication : Research summaries enable researchers to share their findings with a wider audience, including non-experts and industry professionals. This can lead to increased collaboration and knowledge exchange across disciplines.
Better decision-making : For professionals who rely on evidence-based practices, research summaries provide an accessible way to stay informed about the latest developments in their field. This enables them to make better decisions based on up-to-date information.
With these benefits in mind, let's explore some tips for writing an effective research summary.
Tips for Writing a Great Research Summary
Creating an engaging and informative research summary doesn't have to be a daunting task. Here are some tips to help you craft the perfect summary:
Know your audience : Consider who will be reading your summary and tailor your language and content accordingly. If you're writing for a general audience, avoid jargon and technical terms. If your readers are experts in the field, focus on the most relevant and novel aspects of the research.
Be concise : A research summary should be brief yet informative. Aim to capture the essence of the study without getting bogged down in unnecessary details.
Use clear language : Write in simple, straightforward sentences that are easy to understand. Avoid flowery language or complex sentence structures that may confuse readers.
Highlight key points : Focus on the main elements of the study, such as its purpose, methods, results, and conclusions. Make sure these points stand out by using headings, bullet points, or bold text.
Stay objective : Present the information in a neutral tone and avoid expressing personal opinions or biases. Stick to the facts and let your readers draw their own conclusions.
Proofread : Before submitting your research summary, take the time to proofread it carefully for grammar, spelling, and punctuation errors. A polished summary will make a better impression on your readers.
Generate the Perfect Research Summary with Our Research Summary Generator
Now that we've covered what a research summary is, its benefits, and tips for writing one – wouldn't it be great if there was a tool that could generate a perfect research summary every single time? Well, guess what? There is!
With our Research Summary Generator, you can create an engaging and informative summary in just a few clicks. Say goodbye to hours spent poring over dense academic papers and hello to quick, easy-to-understand summaries tailored to your needs.
Give it a try today and see how our Research Summary Generator can revolutionize your research process!
Example outputs
This Research Summary Generator saves you time and effort by summarizing your research findings in a clear and concise manner, allowing you to easily communicate your results to others.
The Effects of Exercise on Mental Health
A study was conducted to investigate the effects of exercise on mental health. Participants were randomly assigned to either an exercise group or a control group. The exercise group engaged in moderate-intensity aerobic exercise for 30 minutes, three times per week for eight weeks. The results showed that the exercise group had significantly lower levels of depression and anxiety compared to the control group.
Keywords: exercise, mental health, depression, anxiety
The impact of social media on body image.
This study aimed to examine the impact of social media on body image. A sample of young adults completed surveys assessing their use of social media and their perceptions of their own body image. Results indicated that individuals who spent more time on social media reported greater dissatisfaction with their bodies. Additionally, exposure to images of thin and fit individuals on social media was associated with increased body dissatisfaction.
Keywords: social media, body image, young adults, body dissatisfaction
The benefits of meditation for stress reduction.
This meta-analysis aimed to evaluate the effectiveness of meditation for stress reduction. A total of 18 randomized controlled trials were included in the analysis. Results showed that meditation was effective in reducing perceived stress, with larger effect sizes observed for mindfulness-based interventions. Furthermore, the benefits of meditation appeared to be maintained over time.
Keywords: meditation, stress reduction, mindfulness, meta-analysis
What other amazing things can this template help you create.
✔ Meta Title
✔ Meta Description
✔ Extract keywords
✔ Feature Image
✔ Soon Internal Linking
Who needs Research Summary Generator?
Researchers
Graduate students
Business professionals
Frequently asked questions
- How does the Research Summary Generator work? Simply input your research findings into the generator, and it will automatically summarize them in a clear and concise manner.
- Can I customize the generated summary? Yes, you can edit the summary as needed to ensure it accurately reflects your research findings.
- Is the Research Summary Generator free to use? Yes, the generator is completely free to use with no limitations.
Jump to navigation

Cochrane Training
Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence.
Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Key Points:
- A ‘Summary of findings’ table for a given comparison of interventions provides key information concerning the magnitudes of relative and absolute effects of the interventions examined, the amount of available evidence and the certainty (or quality) of available evidence.
- ‘Summary of findings’ tables include a row for each important outcome (up to a maximum of seven). Accepted formats of ‘Summary of findings’ tables and interactive ‘Summary of findings’ tables can be produced using GRADE’s software GRADEpro GDT.
- Cochrane has adopted the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) for assessing certainty (or quality) of a body of evidence.
- The GRADE approach specifies four levels of the certainty for a body of evidence for a given outcome: high, moderate, low and very low.
- GRADE assessments of certainty are determined through consideration of five domains: risk of bias, inconsistency, indirectness, imprecision and publication bias. For evidence from non-randomized studies and rarely randomized studies, assessments can then be upgraded through consideration of three further domains.
Cite this chapter as: Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
14.1 ‘Summary of findings’ tables
14.1.1 introduction to ‘summary of findings’ tables.
‘Summary of findings’ tables present the main findings of a review in a transparent, structured and simple tabular format. In particular, they provide key information concerning the certainty or quality of evidence (i.e. the confidence or certainty in the range of an effect estimate or an association), the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes. Cochrane Reviews should incorporate ‘Summary of findings’ tables during planning and publication, and should have at least one key ‘Summary of findings’ table representing the most important comparisons. Some reviews may include more than one ‘Summary of findings’ table, for example if the review addresses more than one major comparison, or includes substantially different populations that require separate tables (e.g. because the effects differ or it is important to show results separately). In the Cochrane Database of Systematic Reviews (CDSR), all ‘Summary of findings’ tables for a review appear at the beginning, before the Background section.
14.1.2 Selecting outcomes for ‘Summary of findings’ tables
Planning for the ‘Summary of findings’ table starts early in the systematic review, with the selection of the outcomes to be included in: (i) the review; and (ii) the ‘Summary of findings’ table. This is a crucial step, and one that review authors need to address carefully.
To ensure production of optimally useful information, Cochrane Reviews begin by developing a review question and by listing all main outcomes that are important to patients and other decision makers (see Chapter 2 and Chapter 3 ). The GRADE approach to assessing the certainty of the evidence (see Section 14.2 ) defines and operationalizes a rating process that helps separate outcomes into those that are critical, important or not important for decision making. Consultation and feedback on the review protocol, including from consumers and other decision makers, can enhance this process.
Critical outcomes are likely to include clearly important endpoints; typical examples include mortality and major morbidity (such as strokes and myocardial infarction). However, they may also represent frequent minor and rare major side effects, symptoms, quality of life, burdens associated with treatment, and resource issues (costs). Burdens represent the impact of healthcare workload on patient function and well-being, and include the demands of adhering to an intervention that patients or caregivers (e.g. family) may dislike, such as having to undergo more frequent tests, or the restrictions on lifestyle that certain interventions require (Spencer-Bonilla et al 2017).
Frequently, when formulating questions that include all patient-important outcomes for decision making, review authors will confront reports of studies that have not included all these outcomes. This is particularly true for adverse outcomes. For instance, randomized trials might contribute evidence on intended effects, and on frequent, relatively minor side effects, but not report on rare adverse outcomes such as suicide attempts. Chapter 19 discusses strategies for addressing adverse effects. To obtain data for all important outcomes it may be necessary to examine the results of non-randomized studies (see Chapter 24 ). Cochrane, in collaboration with others, has developed guidance for review authors to support their decision about when to look for and include non-randomized studies (Schünemann et al 2013).
If a review includes only randomized trials, these trials may not address all important outcomes and it may therefore not be possible to address these outcomes within the constraints of the review. Review authors should acknowledge these limitations and make them transparent to readers. Review authors are encouraged to include non-randomized studies to examine rare or long-term adverse effects that may not adequately be studied in randomized trials. This raises the possibility that harm outcomes may come from studies in which participants differ from those in studies used in the analysis of benefit. Review authors will then need to consider how much such differences are likely to impact on the findings, and this will influence the certainty of evidence because of concerns about indirectness related to the population (see Section 14.2.2 ).
Non-randomized studies can provide important information not only when randomized trials do not report on an outcome or randomized trials suffer from indirectness, but also when the evidence from randomized trials is rated as very low and non-randomized studies provide evidence of higher certainty. Further discussion of these issues appears also in Chapter 24 .
14.1.3 General template for ‘Summary of findings’ tables
Several alternative standard versions of ‘Summary of findings’ tables have been developed to ensure consistency and ease of use across reviews, inclusion of the most important information needed by decision makers, and optimal presentation (see examples at Figures 14.1.a and 14.1.b ). These formats are supported by research that focused on improved understanding of the information they intend to convey (Carrasco-Labra et al 2016, Langendam et al 2016, Santesso et al 2016). They are available through GRADE’s official software package developed to support the GRADE approach: GRADEpro GDT (www.gradepro.org).
Standard Cochrane ‘Summary of findings’ tables include the following elements using one of the accepted formats. Further guidance on each of these is provided in Section 14.1.6 .
- A brief description of the population and setting addressed by the available evidence (which may be slightly different to or narrower than those defined by the review question).
- A brief description of the comparison addressed in the ‘Summary of findings’ table, including both the experimental and comparison interventions.
- A list of the most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes.
- A measure of the typical burden of each outcomes (e.g. illustrative risk, or illustrative mean, on comparator intervention).
- The absolute and relative magnitude of effect measured for each (if both are appropriate).
- The numbers of participants and studies contributing to the analysis of each outcomes.
- A GRADE assessment of the overall certainty of the body of evidence for each outcome (which may vary by outcome).
- Space for comments.
- Explanations (formerly known as footnotes).
Ideally, ‘Summary of findings’ tables are supported by more detailed tables (known as ‘evidence profiles’) to which the review may be linked, which provide more detailed explanations. Evidence profiles include the same important health outcomes, and provide greater detail than ‘Summary of findings’ tables of both of the individual considerations feeding into the grading of certainty and of the results of the studies (Guyatt et al 2011a). They ensure that a structured approach is used to rating the certainty of evidence. Although they are rarely published in Cochrane Reviews, evidence profiles are often used, for example, by guideline developers in considering the certainty of the evidence to support guideline recommendations. Review authors will find it easier to develop the ‘Summary of findings’ table by completing the rating of the certainty of evidence in the evidence profile first in GRADEpro GDT. They can then automatically convert this to one of the ‘Summary of findings’ formats in GRADEpro GDT, including an interactive ‘Summary of findings’ for publication.
As a measure of the magnitude of effect for dichotomous outcomes, the ‘Summary of findings’ table should provide a relative measure of effect (e.g. risk ratio, odds ratio, hazard) and measures of absolute risk. For other types of data, an absolute measure alone (such as a difference in means for continuous data) might be sufficient. It is important that the magnitude of effect is presented in a meaningful way, which may require some transformation of the result of a meta-analysis (see also Chapter 15, Section 15.4 and Section 15.5 ). Reviews with more than one main comparison should include a separate ‘Summary of findings’ table for each comparison.
Figure 14.1.a provides an example of a ‘Summary of findings’ table. Figure 15.1.b provides an alternative format that may further facilitate users’ understanding and interpretation of the review’s findings. Evidence evaluating different formats suggests that the ‘Summary of findings’ table should include a risk difference as a measure of the absolute effect and authors should preferably use a format that includes a risk difference .
A detailed description of the contents of a ‘Summary of findings’ table appears in Section 14.1.6 .
Figure 14.1.a Example of a ‘Summary of findings’ table
Summary of findings (for interactive version click here )
a All the stockings in the nine studies included in this review were below-knee compression stockings. In four studies the compression strength was 20 mmHg to 30 mmHg at the ankle. It was 10 mmHg to 20 mmHg in the other four studies. Stockings come in different sizes. If a stocking is too tight around the knee it can prevent essential venous return causing the blood to pool around the knee. Compression stockings should be fitted properly. A stocking that is too tight could cut into the skin on a long flight and potentially cause ulceration and increased risk of DVT. Some stockings can be slightly thicker than normal leg covering and can be potentially restrictive with tight foot wear. It is a good idea to wear stockings around the house prior to travel to ensure a good, comfortable fit. Participants put their stockings on two to three hours before the flight in most of the studies. The availability and cost of stockings can vary.
b Two studies recruited high risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous two years, large varicose veins or, in one of the studies, participants taller than 190 cm and heavier than 90 kg. The incidence for the seven studies that excluded high risk participants was 1.45% and the incidence for the two studies that recruited high-risk participants (with at least one risk factor) was 2.43%. We have used 10 and 30 per 1000 to express different risk strata, respectively.
c The confidence interval crosses no difference and does not rule out a small increase.
d The measurement of oedema was not validated (indirectness of the outcome) or blinded to the intervention (risk of bias).
e If there are very few or no events and the number of participants is large, judgement about the certainty of evidence (particularly judgements about imprecision) may be based on the absolute effect. Here the certainty rating may be considered ‘high’ if the outcome was appropriately assessed and the event, in fact, did not occur in 2821 studied participants.
f None of the other studies reported adverse effects, apart from four cases of superficial vein thrombosis in varicose veins in the knee region that were compressed by the upper edge of the stocking in one study.
Figure 14.1.b Example of alternative ‘Summary of findings’ table
14.1.4 Producing ‘Summary of findings’ tables
The GRADE Working Group’s software, GRADEpro GDT ( www.gradepro.org ), including GRADE’s interactive handbook, is available to assist review authors in the preparation of ‘Summary of findings’ tables. GRADEpro can use data on the comparator group risk and the effect estimate (entered by the review authors or imported from files generated in RevMan) to produce the relative effects and absolute risks associated with experimental interventions. In addition, it leads the user through the process of a GRADE assessment, and produces a table that can be used as a standalone table in a review (including by direct import into software such as RevMan or integration with RevMan Web), or an interactive ‘Summary of findings’ table (see help resources in GRADEpro).
14.1.5 Statistical considerations in ‘Summary of findings’ tables
14.1.5.1 dichotomous outcomes.
‘Summary of findings’ tables should include both absolute and relative measures of effect for dichotomous outcomes. Risk ratios, odds ratios and risk differences are different ways of comparing two groups with dichotomous outcome data (see Chapter 6, Section 6.4.1 ). Furthermore, there are two distinct risk ratios, depending on which event (e.g. ‘yes’ or ‘no’) is the focus of the analysis (see Chapter 6, Section 6.4.1.5 ). In the presence of a non-zero intervention effect, any variation across studies in the comparator group risks (i.e. variation in the risk of the event occurring without the intervention of interest, for example in different populations) makes it impossible for more than one of these measures to be truly the same in every study.
It has long been assumed in epidemiology that relative measures of effect are more consistent than absolute measures of effect from one scenario to another. There is empirical evidence to support this assumption (Engels et al 2000, Deeks and Altman 2001, Furukawa et al 2002). For this reason, meta-analyses should generally use either a risk ratio or an odds ratio as a measure of effect (see Chapter 10, Section 10.4.3 ). Correspondingly, a single estimate of relative effect is likely to be a more appropriate summary than a single estimate of absolute effect. If a relative effect is indeed consistent across studies, then different comparator group risks will have different implications for absolute benefit. For instance, if the risk ratio is consistently 0.75, then the experimental intervention would reduce a comparator group risk of 80% to 60% in the intervention group (an absolute risk reduction of 20 percentage points), but would also reduce a comparator group risk of 20% to 15% in the intervention group (an absolute risk reduction of 5 percentage points).
‘Summary of findings’ tables are built around the assumption of a consistent relative effect. It is therefore important to consider the implications of this effect for different comparator group risks (these can be derived or estimated from a number of sources, see Section 14.1.6.3 ), which may require an assessment of the certainty of evidence for prognostic evidence (Spencer et al 2012, Iorio et al 2015). For any comparator group risk, it is possible to estimate a corresponding intervention group risk (i.e. the absolute risk with the intervention) from the meta-analytic risk ratio or odds ratio. Note that the numbers provided in the ‘Corresponding risk’ column are specific to the ‘risks’ in the adjacent column.
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding intervention risk is obtained as:
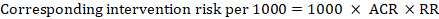
As an example, in Figure 14.1.a , the meta-analytic risk ratio for symptomless deep vein thrombosis (DVT) is RR = 0.10 (95% CI 0.04 to 0.26). Assuming a comparator risk of ACR = 10 per 1000 = 0.01, we obtain:
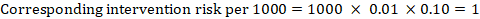
For the meta-analytic odds ratio (OR) and assumed comparator risk, ACR, the corresponding intervention risk is obtained as:
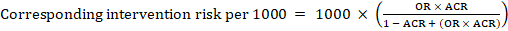
Upper and lower confidence limits for the corresponding intervention risk are obtained by replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.10 with 0.04, then with 0.26, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
When dealing with risk ratios, it is critical that the same definition of ‘event’ is used as was used for the meta-analysis. For example, if the meta-analysis focused on ‘death’ (as opposed to survival) as the event, then corresponding risks in the ‘Summary of findings’ table must also refer to ‘death’.
In (rare) circumstances in which there is clear rationale to assume a consistent risk difference in the meta-analysis, in principle it is possible to present this for relevant ‘assumed risks’ and their corresponding risks, and to present the corresponding (different) relative effects for each assumed risk.
The risk difference expresses the difference between the ACR and the corresponding intervention risk (or the difference between the experimental and the comparator intervention).
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding risk difference is obtained as (note that risks can also be expressed using percentage or percentage points):
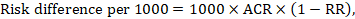
As an example, in Figure 14.1.b the meta-analytic risk ratio is 0.41 (95% CI 0.29 to 0.55) for diarrhoea in children less than 5 years of age. Assuming a comparator group risk of 22.3% we obtain:
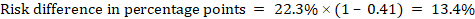
For the meta-analytic odds ratio (OR) and assumed comparator risk (ACR) the absolute risk difference is obtained as (percentage points):
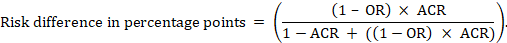
Upper and lower confidence limits for the absolute risk difference are obtained by re-running the calculation above while replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.41 with 0.28, then with 0.55, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
14.1.5.2 Time-to-event outcomes
Time-to-event outcomes measure whether and when a particular event (e.g. death) occurs (van Dalen et al 2007). The impact of the experimental intervention relative to the comparison group on time-to-event outcomes is usually measured using a hazard ratio (HR) (see Chapter 6, Section 6.8.1 ).
A hazard ratio expresses a relative effect estimate. It may be used in various ways to obtain absolute risks and other interpretable quantities for a specific population. Here we describe how to re-express hazard ratios in terms of: (i) absolute risk of event-free survival within a particular period of time; (ii) absolute risk of an event within a particular period of time; and (iii) median time to the event. All methods are built on an assumption of consistent relative effects (i.e. that the hazard ratio does not vary over time).
(i) Absolute risk of event-free survival within a particular period of time Event-free survival (e.g. overall survival) is commonly reported by individual studies. To obtain absolute effects for time-to-event outcomes measured as event-free survival, the summary HR can be used in conjunction with an assumed proportion of patients who are event-free in the comparator group (Tierney et al 2007). This proportion of patients will be specific to a period of time of observation. However, it is not strictly necessary to specify this period of time. For instance, a proportion of 50% of event-free patients might apply to patients with a high event rate observed over 1 year, or to patients with a low event rate observed over 2 years.
As an example, suppose the meta-analytic hazard ratio is 0.42 (95% CI 0.25 to 0.72). Assuming a comparator group risk of event-free survival (e.g. for overall survival people being alive) at 2 years of ACR = 900 per 1000 = 0.9 we obtain:
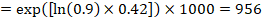
so that that 956 per 1000 people will be alive with the experimental intervention at 2 years. The derivation of the risk should be explained in a comment or footnote.
(ii) Absolute risk of an event within a particular period of time To obtain this absolute effect, again the summary HR can be used (Tierney et al 2007):
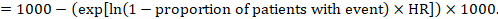
In the example, suppose we assume a comparator group risk of events (e.g. for mortality, people being dead) at 2 years of ACR = 100 per 1000 = 0.1. We obtain:
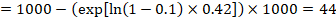
so that that 44 per 1000 people will be dead with the experimental intervention at 2 years.
(iii) Median time to the event Instead of absolute numbers, the time to the event in the intervention and comparison groups can be expressed as median survival time in months or years. To obtain median survival time the pooled HR can be applied to an assumed median survival time in the comparator group (Tierney et al 2007):
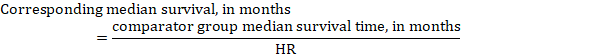
In the example, assuming a comparator group median survival time of 80 months, we obtain:
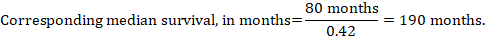
For all three of these options for re-expressing results of time-to-event analyses, upper and lower confidence limits for the corresponding intervention risk are obtained by replacing HR by its upper and lower confidence limits, respectively (e.g. replacing 0.42 with 0.25, then with 0.72, in the example). Again, as for dichotomous outcomes, such confidence intervals do not incorporate uncertainty in the assumed comparator group risks. This is of special concern for long-term survival with a low or moderate mortality rate and a corresponding high number of censored patients (i.e. a low number of patients under risk and a high censoring rate).
14.1.6 Detailed contents of a ‘Summary of findings’ table
14.1.6.1 table title and header.
The title of each ‘Summary of findings’ table should specify the healthcare question, framed in terms of the population and making it clear exactly what comparison of interventions are made. In Figure 14.1.a , the population is people taking long aeroplane flights, the intervention is compression stockings, and the control is no compression stockings.
The first rows of each ‘Summary of findings’ table should provide the following ‘header’ information:
Patients or population This further clarifies the population (and possibly the subpopulations) of interest and ideally the magnitude of risk of the most crucial adverse outcome at which an intervention is directed. For instance, people on a long-haul flight may be at different risks for DVT; those using selective serotonin reuptake inhibitors (SSRIs) might be at different risk for side effects; while those with atrial fibrillation may be at low (< 1%), moderate (1% to 4%) or high (> 4%) yearly risk of stroke.
Setting This should state any specific characteristics of the settings of the healthcare question that might limit the applicability of the summary of findings to other settings (e.g. primary care in Europe and North America).
Intervention The experimental intervention.
Comparison The comparator intervention (including no specific intervention).
14.1.6.2 Outcomes
The rows of a ‘Summary of findings’ table should include all desirable and undesirable health outcomes (listed in order of importance) that are essential for decision making, up to a maximum of seven outcomes. If there are more outcomes in the review, review authors will need to omit the less important outcomes from the table, and the decision selecting which outcomes are critical or important to the review should be made during protocol development (see Chapter 3 ). Review authors should provide time frames for the measurement of the outcomes (e.g. 90 days or 12 months) and the type of instrument scores (e.g. ranging from 0 to 100).
Note that review authors should include the pre-specified critical and important outcomes in the table whether data are available or not. However, they should be alert to the possibility that the importance of an outcome (e.g. a serious adverse effect) may only become known after the protocol was written or the analysis was carried out, and should take appropriate actions to include these in the ‘Summary of findings’ table.
The ‘Summary of findings’ table can include effects in subgroups of the population for different comparator risks and effect sizes separately. For instance, in Figure 14.1.b effects are presented for children younger and older than 5 years separately. Review authors may also opt to produce separate ‘Summary of findings’ tables for different populations.
Review authors should include serious adverse events, but it might be possible to combine minor adverse events as a single outcome, and describe this in an explanatory footnote (note that it is not appropriate to add events together unless they are independent, that is, a participant who has experienced one adverse event has an unaffected chance of experiencing the other adverse event).
Outcomes measured at multiple time points represent a particular problem. In general, to keep the table simple, review authors should present multiple time points only for outcomes critical to decision making, where either the result or the decision made are likely to vary over time. The remainder should be presented at a common time point where possible.
Review authors can present continuous outcome measures in the ‘Summary of findings’ table and should endeavour to make these interpretable to the target audience. This requires that the units are clear and readily interpretable, for example, days of pain, or frequency of headache, and the name and scale of any measurement tools used should be stated (e.g. a Visual Analogue Scale, ranging from 0 to 100). However, many measurement instruments are not readily interpretable by non-specialist clinicians or patients, for example, points on a Beck Depression Inventory or quality of life score. For these, a more interpretable presentation might involve converting a continuous to a dichotomous outcome, such as >50% improvement (see Chapter 15, Section 15.5 ).
14.1.6.3 Best estimate of risk with comparator intervention
Review authors should provide up to three typical risks for participants receiving the comparator intervention. For dichotomous outcomes, we recommend that these be presented in the form of the number of people experiencing the event per 100 or 1000 people (natural frequency) depending on the frequency of the outcome. For continuous outcomes, this would be stated as a mean or median value of the outcome measured.
Estimated or assumed comparator intervention risks could be based on assessments of typical risks in different patient groups derived from the review itself, individual representative studies in the review, or risks derived from a systematic review of prognosis studies or other sources of evidence which may in turn require an assessment of the certainty for the prognostic evidence (Spencer et al 2012, Iorio et al 2015). Ideally, risks would reflect groups that clinicians can easily identify on the basis of their presenting features.
An explanatory footnote should specify the source or rationale for each comparator group risk, including the time period to which it corresponds where appropriate. In Figure 14.1.a , clinicians can easily differentiate individuals with risk factors for deep venous thrombosis from those without. If there is known to be little variation in baseline risk then review authors may use the median comparator group risk across studies. If typical risks are not known, an option is to choose the risk from the included studies, providing the second highest for a high and the second lowest for a low risk population.
14.1.6.4 Risk with intervention
For dichotomous outcomes, review authors should provide a corresponding absolute risk for each comparator group risk, along with a confidence interval. This absolute risk with the (experimental) intervention will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the absolute effect in the same format as the risks with comparator intervention (see Section 14.1.6.3 ), for example as the number of people experiencing the event per 1000 people.
For continuous outcomes, a difference in means or standardized difference in means should be presented with its confidence interval. These will typically be obtained directly from a meta-analysis. Explanatory text should be used to clarify the meaning, as in Figures 14.1.a and 14.1.b .
14.1.6.5 Risk difference
For dichotomous outcomes, the risk difference can be provided using one of the ‘Summary of findings’ table formats as an additional option (see Figure 14.1.b ). This risk difference expresses the difference between the experimental and comparator intervention and will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the risk difference in the same format as assumed and corresponding risks with comparator intervention (see Section 14.1.6.3 ); for example, as the number of people experiencing the event per 1000 people or as percentage points if the assumed and corresponding risks are expressed in percentage.
For continuous outcomes, if the ‘Summary of findings’ table includes this option, the mean difference can be presented here and the ‘corresponding risk’ column left blank (see Figure 14.1.b ).
14.1.6.6 Relative effect (95% CI)
The relative effect will typically be a risk ratio or odds ratio (or occasionally a hazard ratio) with its accompanying 95% confidence interval, obtained from a meta-analysis performed on the basis of the same effect measure. Risk ratios and odds ratios are similar when the comparator intervention risks are low and effects are small, but may differ considerably when comparator group risks increase. The meta-analysis may involve an assumption of either fixed or random effects, depending on what the review authors consider appropriate, and implying that the relative effect is either an estimate of the effect of the intervention, or an estimate of the average effect of the intervention across studies, respectively.
14.1.6.7 Number of participants (studies)
This column should include the number of participants assessed in the included studies for each outcome and the corresponding number of studies that contributed these participants.
14.1.6.8 Certainty of the evidence (GRADE)
Review authors should comment on the certainty of the evidence (also known as quality of the body of evidence or confidence in the effect estimates). Review authors should use the specific evidence grading system developed by the GRADE Working Group (Atkins et al 2004, Guyatt et al 2008, Guyatt et al 2011a), which is described in detail in Section 14.2 . The GRADE approach categorizes the certainty in a body of evidence as ‘high’, ‘moderate’, ‘low’ or ‘very low’ by outcome. This is a result of judgement, but the judgement process operates within a transparent structure. As an example, the certainty would be ‘high’ if the summary were of several randomized trials with low risk of bias, but the rating of certainty becomes lower if there are concerns about risk of bias, inconsistency, indirectness, imprecision or publication bias. Judgements other than of ‘high’ certainty should be made transparent using explanatory footnotes or the ‘Comments’ column in the ‘Summary of findings’ table (see Section 14.1.6.10 ).
14.1.6.9 Comments
The aim of the ‘Comments’ field is to help interpret the information or data identified in the row. For example, this may be on the validity of the outcome measure or the presence of variables that are associated with the magnitude of effect. Important caveats about the results should be flagged here. Not all rows will need comments, and it is best to leave a blank if there is nothing warranting a comment.
14.1.6.10 Explanations
Detailed explanations should be included as footnotes to support the judgements in the ‘Summary of findings’ table, such as the overall GRADE assessment. The explanations should describe the rationale for important aspects of the content. Table 14.1.a lists guidance for useful explanations. Explanations should be concise, informative, relevant, easy to understand and accurate. If explanations cannot be sufficiently described in footnotes, review authors should provide further details of the issues in the Results and Discussion sections of the review.
Table 14.1.a Guidance for providing useful explanations in ‘Summary of findings’ (SoF) tables. Adapted from Santesso et al (2016)
14.2 Assessing the certainty or quality of a body of evidence
14.2.1 the grade approach.
The Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) has developed a system for grading the certainty of evidence (Schünemann et al 2003, Atkins et al 2004, Schünemann et al 2006, Guyatt et al 2008, Guyatt et al 2011a). Over 100 organizations including the World Health Organization (WHO), the American College of Physicians, the American Society of Hematology (ASH), the Canadian Agency for Drugs and Technology in Health (CADTH) and the National Institutes of Health and Clinical Excellence (NICE) in the UK have adopted the GRADE system ( www.gradeworkinggroup.org ).
Cochrane has also formally adopted this approach, and all Cochrane Reviews should use GRADE to evaluate the certainty of evidence for important outcomes (see MECIR Box 14.2.a ).
MECIR Box 14.2.a Relevant expectations for conduct of intervention reviews
For systematic reviews, the GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. Assessing the certainty of a body of evidence involves consideration of within- and across-study risk of bias (limitations in study design and execution or methodological quality), inconsistency (or heterogeneity), indirectness of evidence, imprecision of the effect estimates and risk of publication bias (see Section 14.2.2 ), as well as domains that may increase our confidence in the effect estimate (as described in Section 14.2.3 ). The GRADE system entails an assessment of the certainty of a body of evidence for each individual outcome. Judgements about the domains that determine the certainty of evidence should be described in the results or discussion section and as part of the ‘Summary of findings’ table.
The GRADE approach specifies four levels of certainty ( Figure 14.2.a ). For interventions, including diagnostic and other tests that are evaluated as interventions (Schünemann et al 2008b, Schünemann et al 2008a, Balshem et al 2011, Schünemann et al 2012), the starting point for rating the certainty of evidence is categorized into two types:
- randomized trials; and
- non-randomized studies of interventions (NRSI), including observational studies (including but not limited to cohort studies, and case-control studies, cross-sectional studies, case series and case reports, although not all of these designs are usually included in Cochrane Reviews).
There are many instances in which review authors rely on information from NRSI, in particular to evaluate potential harms (see Chapter 24 ). In addition, review authors can obtain relevant data from both randomized trials and NRSI, with each type of evidence complementing the other (Schünemann et al 2013).
In GRADE, a body of evidence from randomized trials begins with a high-certainty rating while a body of evidence from NRSI begins with a low-certainty rating. The lower rating with NRSI is the result of the potential bias induced by the lack of randomization (i.e. confounding and selection bias).
However, when using the new Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool (Sterne et al 2016), an assessment tool that covers the risk of bias due to lack of randomization, all studies may start as high certainty of the evidence (Schünemann et al 2018). The approach of starting all study designs (including NRSI) as high certainty does not conflict with the initial GRADE approach of starting the rating of NRSI as low certainty evidence. This is because a body of evidence from NRSI should generally be downgraded by two levels due to the inherent risk of bias associated with the lack of randomization, namely confounding and selection bias. Not downgrading NRSI from high to low certainty needs transparent and detailed justification for what mitigates concerns about confounding and selection bias (Schünemann et al 2018). Very few examples of where not rating down by two levels is appropriate currently exist.
The highest certainty rating is a body of evidence when there are no concerns in any of the GRADE factors listed in Figure 14.2.a . Review authors often downgrade evidence to moderate, low or even very low certainty evidence, depending on the presence of the five factors in Figure 14.2.a . Usually, certainty rating will fall by one level for each factor, up to a maximum of three levels for all factors. If there are very severe problems for any one domain (e.g. when assessing risk of bias, all studies were unconcealed, unblinded and lost over 50% of their patients to follow-up), evidence may fall by two levels due to that factor alone. It is not possible to rate lower than ‘very low certainty’ evidence.
Review authors will generally grade evidence from sound non-randomized studies as low certainty, even if ROBINS-I is used. If, however, such studies yield large effects and there is no obvious bias explaining those effects, review authors may rate the evidence as moderate or – if the effect is large enough – even as high certainty ( Figure 14.2.a ). The very low certainty level is appropriate for, but is not limited to, studies with critical problems and unsystematic clinical observations (e.g. case series or case reports).
Figure 14.2.a Levels of the certainty of a body of evidence in the GRADE approach. *Upgrading criteria are usually applicable to non-randomized studies only (but exceptions exist).
14.2.2 Domains that can lead to decreasing the certainty level of a body of evidence
We now describe in more detail the five reasons (or domains) for downgrading the certainty of a body of evidence for a specific outcome. In each case, if no reason is found for downgrading the evidence, it should be classified as 'no limitation or not serious' (not important enough to warrant downgrading). If a reason is found for downgrading the evidence, it should be classified as 'serious' (downgrading the certainty rating by one level) or 'very serious' (downgrading the certainty grade by two levels). For non-randomized studies assessed with ROBINS-I, rating down by three levels should be classified as 'extremely' serious.
(1) Risk of bias or limitations in the detailed design and implementation
Our confidence in an estimate of effect decreases if studies suffer from major limitations that are likely to result in a biased assessment of the intervention effect. For randomized trials, these methodological limitations include failure to generate a random sequence, lack of allocation sequence concealment, lack of blinding (particularly with subjective outcomes that are highly susceptible to biased assessment), a large loss to follow-up or selective reporting of outcomes. Chapter 8 provides a discussion of study-level assessments of risk of bias in the context of a Cochrane Review, and proposes an approach to assessing the risk of bias for an outcome across studies as ‘Low’ risk of bias, ‘Some concerns’ and ‘High’ risk of bias for randomized trials. Levels of ‘Low’. ‘Moderate’, ‘Serious’ and ‘Critical’ risk of bias arise for non-randomized studies assessed with ROBINS-I ( Chapter 25 ). These assessments should feed directly into this GRADE domain. In particular, ‘Low’ risk of bias would indicate ‘no limitation’; ‘Some concerns’ would indicate either ‘no limitation’ or ‘serious limitation’; and ‘High’ risk of bias would indicate either ‘serious limitation’ or ‘very serious limitation’. ‘Critical’ risk of bias on ROBINS-I would indicate extremely serious limitations in GRADE. Review authors should use their judgement to decide between alternative categories, depending on the likely magnitude of the potential biases.
Every study addressing a particular outcome will differ, to some degree, in the risk of bias. Review authors should make an overall judgement on whether the certainty of evidence for an outcome warrants downgrading on the basis of study limitations. The assessment of study limitations should apply to the studies contributing to the results in the ‘Summary of findings’ table, rather than to all studies that could potentially be included in the analysis. We have argued in Chapter 7, Section 7.6.2 , that the primary analysis should be restricted to studies at low (or low and unclear) risk of bias where possible.
Table 14.2.a presents the judgements that must be made in going from assessments of the risk of bias to judgements about study limitations for each outcome included in a ‘Summary of findings’ table. A rating of high certainty evidence can be achieved only when most evidence comes from studies that met the criteria for low risk of bias. For example, of the 22 studies addressing the impact of beta-blockers on mortality in patients with heart failure, most probably or certainly used concealed allocation of the sequence, all blinded at least some key groups and follow-up of randomized patients was almost complete (Brophy et al 2001). The certainty of evidence might be downgraded by one level when most of the evidence comes from individual studies either with a crucial limitation for one item, or with some limitations for multiple items. An example of very serious limitations, warranting downgrading by two levels, is provided by evidence on surgery versus conservative treatment in the management of patients with lumbar disc prolapse (Gibson and Waddell 2007). We are uncertain of the benefit of surgery in reducing symptoms after one year or longer, because the one study included in the analysis had inadequate concealment of the allocation sequence and the outcome was assessed using a crude rating by the surgeon without blinding.
(2) Unexplained heterogeneity or inconsistency of results
When studies yield widely differing estimates of effect (heterogeneity or variability in results), investigators should look for robust explanations for that heterogeneity. For instance, drugs may have larger relative effects in sicker populations or when given in larger doses. A detailed discussion of heterogeneity and its investigation is provided in Chapter 10, Section 10.10 and Section 10.11 . If an important modifier exists, with good evidence that important outcomes are different in different subgroups (which would ideally be pre-specified), then a separate ‘Summary of findings’ table may be considered for a separate population. For instance, a separate ‘Summary of findings’ table would be used for carotid endarterectomy in symptomatic patients with high grade stenosis (70% to 99%) in which the intervention is, in the hands of the right surgeons, beneficial, and another (if review authors considered it relevant) for asymptomatic patients with low grade stenosis (less than 30%) in which surgery appears harmful (Orrapin and Rerkasem 2017). When heterogeneity exists and affects the interpretation of results, but review authors are unable to identify a plausible explanation with the data available, the certainty of the evidence decreases.
(3) Indirectness of evidence
Two types of indirectness are relevant. First, a review comparing the effectiveness of alternative interventions (say A and B) may find that randomized trials are available, but they have compared A with placebo and B with placebo. Thus, the evidence is restricted to indirect comparisons between A and B. Where indirect comparisons are undertaken within a network meta-analysis context, GRADE for network meta-analysis should be used (see Chapter 11, Section 11.5 ).
Second, a review may find randomized trials that meet eligibility criteria but address a restricted version of the main review question in terms of population, intervention, comparator or outcomes. For example, suppose that in a review addressing an intervention for secondary prevention of coronary heart disease, most identified studies happened to be in people who also had diabetes. Then the evidence may be regarded as indirect in relation to the broader question of interest because the population is primarily related to people with diabetes. The opposite scenario can equally apply: a review addressing the effect of a preventive strategy for coronary heart disease in people with diabetes may consider studies in people without diabetes to provide relevant, albeit indirect, evidence. This would be particularly likely if investigators had conducted few if any randomized trials in the target population (e.g. people with diabetes). Other sources of indirectness may arise from interventions studied (e.g. if in all included studies a technical intervention was implemented by expert, highly trained specialists in specialist centres, then evidence on the effects of the intervention outside these centres may be indirect), comparators used (e.g. if the comparator groups received an intervention that is less effective than standard treatment in most settings) and outcomes assessed (e.g. indirectness due to surrogate outcomes when data on patient-important outcomes are not available, or when investigators seek data on quality of life but only symptoms are reported). Review authors should make judgements transparent when they believe downgrading is justified, based on differences in anticipated effects in the group of primary interest. Review authors may be aided and increase transparency of their judgements about indirectness if they use Table 14.2.b available in the GRADEpro GDT software (Schünemann et al 2013).
(4) Imprecision of results
When studies include few participants or few events, and thus have wide confidence intervals, review authors can lower their rating of the certainty of the evidence. The confidence intervals included in the ‘Summary of findings’ table will provide readers with information that allows them to make, to some extent, their own rating of precision. Review authors can use a calculation of the optimal information size (OIS) or review information size (RIS), similar to sample size calculations, to make judgements about imprecision (Guyatt et al 2011b, Schünemann 2016). The OIS or RIS is calculated on the basis of the number of participants required for an adequately powered individual study. If the 95% confidence interval excludes a risk ratio (RR) of 1.0, and the total number of events or patients exceeds the OIS criterion, precision is adequate. If the 95% CI includes appreciable benefit or harm (an RR of under 0.75 or over 1.25 is often suggested as a very rough guide) downgrading for imprecision may be appropriate even if OIS criteria are met (Guyatt et al 2011b, Schünemann 2016).
(5) High probability of publication bias
The certainty of evidence level may be downgraded if investigators fail to report studies on the basis of results (typically those that show no effect: publication bias) or outcomes (typically those that may be harmful or for which no effect was observed: selective outcome non-reporting bias). Selective reporting of outcomes from among multiple outcomes measured is assessed at the study level as part of the assessment of risk of bias (see Chapter 8, Section 8.7 ), so for the studies contributing to the outcome in the ‘Summary of findings’ table this is addressed by domain 1 above (limitations in the design and implementation). If a large number of studies included in the review do not contribute to an outcome, or if there is evidence of publication bias, the certainty of the evidence may be downgraded. Chapter 13 provides a detailed discussion of reporting biases, including publication bias, and how it may be tackled in a Cochrane Review. A prototypical situation that may elicit suspicion of publication bias is when published evidence includes a number of small studies, all of which are industry-funded (Bhandari et al 2004). For example, 14 studies of flavanoids in patients with haemorrhoids have shown apparent large benefits, but enrolled a total of only 1432 patients (i.e. each study enrolled relatively few patients) (Alonso-Coello et al 2006). The heavy involvement of sponsors in most of these studies raises questions of whether unpublished studies that suggest no benefit exist (publication bias).
A particular body of evidence can suffer from problems associated with more than one of the five factors listed here, and the greater the problems, the lower the certainty of evidence rating that should result. One could imagine a situation in which randomized trials were available, but all or virtually all of these limitations would be present, and in serious form. A very low certainty of evidence rating would result.
Table 14.2.a Further guidelines for domain 1 (of 5) in a GRADE assessment: going from assessments of risk of bias in studies to judgements about study limitations for main outcomes across studies
Table 14.2.b Judgements about indirectness by outcome (available in GRADEpro GDT)
Intervention:
Comparator:
Direct comparison:
Final judgement about indirectness across domains:
14.2.3 Domains that may lead to increasing the certainty level of a body of evidence
Although NRSI and downgraded randomized trials will generally yield a low rating for certainty of evidence, there will be unusual circumstances in which review authors could ‘upgrade’ such evidence to moderate or even high certainty ( Table 14.3.a ).
- Large effects On rare occasions when methodologically well-done observational studies yield large, consistent and precise estimates of the magnitude of an intervention effect, one may be particularly confident in the results. A large estimated effect (e.g. RR >2 or RR <0.5) in the absence of plausible confounders, or a very large effect (e.g. RR >5 or RR <0.2) in studies with no major threats to validity, might qualify for this. In these situations, while the NRSI may possibly have provided an over-estimate of the true effect, the weak study design may not explain all of the apparent observed benefit. Thus, despite reservations based on the observational study design, review authors are confident that the effect exists. The magnitude of the effect in these studies may move the assigned certainty of evidence from low to moderate (if the effect is large in the absence of other methodological limitations). For example, a meta-analysis of observational studies showed that bicycle helmets reduce the risk of head injuries in cyclists by a large margin (odds ratio (OR) 0.31, 95% CI 0.26 to 0.37) (Thompson et al 2000). This large effect, in the absence of obvious bias that could create the association, suggests a rating of moderate-certainty evidence. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. However, if the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0, then some hesitation would be appropriate in the decision to rate up for a large effect. Another situation allows inference of a strong association without a formal comparative study. Consider the question of the impact of routine colonoscopy versus no screening for colon cancer on the rate of perforation associated with colonoscopy. Here, a large series of representative patients undergoing colonoscopy may provide high certainty evidence about the risk of perforation associated with colonoscopy. When the risk of the event among patients receiving the relevant comparator is known to be near 0 (i.e. we are certain that the incidence of spontaneous colon perforation in patients not undergoing colonoscopy is extremely low), case series or cohort studies of representative patients can provide high certainty evidence of adverse effects associated with an intervention, thereby allowing us to infer a strong association from even a limited number of events.
- Dose-response The presence of a dose-response gradient may increase our confidence in the findings of observational studies and thereby enhance the assigned certainty of evidence. For example, our confidence in the result of observational studies that show an increased risk of bleeding in patients who have supratherapeutic anticoagulation levels is increased by the observation that there is a dose-response gradient between the length of time needed for blood to clot (as measured by the international normalized ratio (INR)) and an increased risk of bleeding (Levine et al 2004). A systematic review of NRSI investigating the effect of cyclooxygenase-2 inhibitors on cardiovascular events found that the summary estimate (RR) with rofecoxib was 1.33 (95% CI 1.00 to 1.79) with doses less than 25mg/d, and 2.19 (95% CI 1.64 to 2.91) with doses more than 25mg/d. Although residual confounding is likely to exist in the NRSI that address this issue, the existence of a dose-response gradient and the large apparent effect of higher doses of rofecoxib markedly increase our strength of inference that the association cannot be explained by residual confounding, and is therefore likely to be both causal and, at high levels of exposure, substantial. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. Here, the fact that the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0 might make some hesitate in the decision to rate up for a large effect
- Plausible confounding On occasion, all plausible biases from randomized or non-randomized studies may be working to under-estimate an apparent intervention effect. For example, if only sicker patients receive an experimental intervention or exposure, yet they still fare better, it is likely that the actual intervention or exposure effect is larger than the data suggest. For instance, a rigorous systematic review of observational studies including a total of 38 million patients demonstrated higher death rates in private for-profit versus private not-for-profit hospitals (Devereaux et al 2002). One possible bias relates to different disease severity in patients in the two hospital types. It is likely, however, that patients in the not-for-profit hospitals were sicker than those in the for-profit hospitals. Thus, to the extent that residual confounding existed, it would bias results against the not-for-profit hospitals. The second likely bias was the possibility that higher numbers of patients with excellent private insurance coverage could lead to a hospital having more resources and a spill-over effect that would benefit those without such coverage. Since for-profit hospitals are likely to admit a larger proportion of such well-insured patients than not-for-profit hospitals, the bias is once again against the not-for-profit hospitals. Since the plausible biases would all diminish the demonstrated intervention effect, one might consider the evidence from these observational studies as moderate rather than low certainty. A parallel situation exists when observational studies have failed to demonstrate an association, but all plausible biases would have increased an intervention effect. This situation will usually arise in the exploration of apparent harmful effects. For example, because the hypoglycaemic drug phenformin causes lactic acidosis, the related agent metformin was under suspicion for the same toxicity. Nevertheless, very large observational studies have failed to demonstrate an association (Salpeter et al 2007). Given the likelihood that clinicians would be more alert to lactic acidosis in the presence of the agent and over-report its occurrence, one might consider this moderate, or even high certainty, evidence refuting a causal relationship between typical therapeutic doses of metformin and lactic acidosis.
14.3 Describing the assessment of the certainty of a body of evidence using the GRADE framework
Review authors should report the grading of the certainty of evidence in the Results section for each outcome for which this has been performed, providing the rationale for downgrading or upgrading the evidence, and referring to the ‘Summary of findings’ table where applicable.
Table 14.3.a provides a framework and examples for how review authors can justify their judgements about the certainty of evidence in each domain. These justifications should also be included in explanatory notes to the ‘Summary of Findings’ table (see Section 14.1.6.10 ).
Chapter 15, Section 15.6 , describes in more detail how the overall GRADE assessment across all domains can be used to draw conclusions about the effects of the intervention, as well as providing implications for future research.
Table 14.3.a Framework for describing the certainty of evidence and justifying downgrading or upgrading
14.4 Chapter information
Authors: Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Acknowledgements: Andrew D Oxman contributed to earlier versions. Professor Penny Hawe contributed to the text on adverse effects in earlier versions. Jon Deeks provided helpful contributions on an earlier version of this chapter. For details of previous authors and editors of the Handbook , please refer to the Preface.
Funding: This work was in part supported by funding from the Michael G DeGroote Cochrane Canada Centre and the Ontario Ministry of Health.
14.5 References
Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, Mills E, Heels-Ansdell D, Johanson JF, Guyatt G. Meta-analysis of flavonoids for the treatment of haemorrhoids. British Journal of Surgery 2006; 93 : 909-920.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr., Zaza S. Grading quality of evidence and strength of recommendations. BMJ 2004; 328 : 1490.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011; 64 : 401-406.
Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, Mears D, Schemitsch EH, Heels-Ansdell D, Devereaux PJ. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004; 170 : 477-480.
Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Annals of Internal Medicine 2001; 134 : 550-560.
Carrasco-Labra A, Brignardello-Petersen R, Santesso N, Neumann I, Mustafa RA, Mbuagbaw L, Etxeandia Ikobaltzeta I, De Stio C, McCullagh LJ, Alonso-Coello P, Meerpohl JJ, Vandvik PO, Brozek JL, Akl EA, Bossuyt P, Churchill R, Glenton C, Rosenbaum S, Tugwell P, Welch V, Garner P, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 1: a randomized trial shows improved understanding of content in summary of findings tables with a new format. Journal of Clinical Epidemiology 2016; 74 : 7-18.
Deeks JJ, Altman DG. Effect measures for meta-analysis of trials with binary outcomes. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in Context . 2nd ed. London (UK): BMJ Publication Group; 2001. p. 313-335.
Devereaux PJ, Choi PT, Lacchetti C, Weaver B, Schünemann HJ, Haines T, Lavis JN, Grant BJ, Haslam DR, Bhandari M, Sullivan T, Cook DJ, Walter SD, Meade M, Khan H, Bhatnagar N, Guyatt GH. A systematic review and meta-analysis of studies comparing mortality rates of private for-profit and private not-for-profit hospitals. Canadian Medical Association Journal 2002; 166 : 1399-1406.
Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Statistics in Medicine 2000; 19 : 1707-1728.
Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology 2002; 31 : 72-76.
Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane Review. Spine 2007; 32 : 1735-1747.
Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann H. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336 : 3.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011a; 64 : 383-394.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW, Jr., Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schünemann HJ. GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011b; 64 : 1283-1293.
Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schünemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015; 350 : h870.
Langendam M, Carrasco-Labra A, Santesso N, Mustafa RA, Brignardello-Petersen R, Ventresca M, Heus P, Lasserson T, Moustgaard R, Brozek J, Schünemann HJ. Improving GRADE evidence tables part 2: a systematic survey of explanatory notes shows more guidance is needed. Journal of Clinical Epidemiology 2016; 74 : 19-27.
Levine MN, Raskob G, Landefeld S, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 : 287S-310S.
Orrapin S, Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database of Systematic Reviews 2017; 6 : CD001081.
Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2007; 4 : CD002967.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, Lasserson T, Opiyo N, Kunnamo I, Sinclair D, Garner P, Treweek S, Tovey D, Akl EA, Tugwell P, Brozek JL, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. Journal of Clinical Epidemiology 2016; 74 : 28-39.
Schünemann HJ, Best D, Vist G, Oxman AD, Group GW. Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. Canadian Medical Association Journal 2003; 169 : 677-680.
Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, Fahy BF, Gould MK, Horan KL, Krishnan JA, Manthous CA, Maurer JR, McNicholas WT, Oxman AD, Rubenfeld G, Turino GM, Guyatt G. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006; 174 : 605-614.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW, Jr., Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008a; 336 : 1106-1110.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, Muti P, Jaeschke R, Guyatt GH. GRADE: assessing the quality of evidence for diagnostic recommendations. ACP Journal Club 2008b; 149 : 2.
Schünemann HJ, Mustafa R, Brozek J. [Diagnostic accuracy and linked evidence--testing the chain]. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2012; 106 : 153-160.
Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, Shea B, Wells G, Helfand M. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013; 4 : 49-62.
Schünemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? Journal of Clinical Epidemiology 2016; 75 : 6-15.
Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, Morgan RL, Gartlehner G, Kunz R, Katikireddi SV, Sterne J, Higgins JPT, Guyatt G, Group GW. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2018.
Spencer-Bonilla G, Quinones AR, Montori VM, International Minimally Disruptive Medicine W. Assessing the Burden of Treatment. Journal of General Internal Medicine 2017; 32 : 1141-1145.
Spencer FA, Iorio A, You J, Murad MH, Schünemann HJ, Vandvik PO, Crowther MA, Pottie K, Lang ES, Meerpohl JJ, Falck-Ytter Y, Alonso-Coello P, Guyatt GH. Uncertainties in baseline risk estimates and confidence in treatment effects. BMJ 2012; 345 : e7401.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JPT. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355 : i4919.
Thompson DC, Rivara FP, Thompson R. Helmets for preventing head and facial injuries in bicyclists. Cochrane Database of Systematic Reviews 2000; 2 : CD001855.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8 .
van Dalen EC, Tierney JF, Kremer LCM. Tips and tricks for understanding and using SR results. No. 7: time‐to‐event data. Evidence-Based Child Health 2007; 2 : 1089-1090.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.
- Privacy Policy

Home » Research Findings – Types Examples and Writing Guide
Research Findings – Types Examples and Writing Guide
Table of Contents

Research Findings
Definition:
Research findings refer to the results obtained from a study or investigation conducted through a systematic and scientific approach. These findings are the outcomes of the data analysis, interpretation, and evaluation carried out during the research process.
Types of Research Findings
There are two main types of research findings:
Qualitative Findings
Qualitative research is an exploratory research method used to understand the complexities of human behavior and experiences. Qualitative findings are non-numerical and descriptive data that describe the meaning and interpretation of the data collected. Examples of qualitative findings include quotes from participants, themes that emerge from the data, and descriptions of experiences and phenomena.
Quantitative Findings
Quantitative research is a research method that uses numerical data and statistical analysis to measure and quantify a phenomenon or behavior. Quantitative findings include numerical data such as mean, median, and mode, as well as statistical analyses such as t-tests, ANOVA, and regression analysis. These findings are often presented in tables, graphs, or charts.
Both qualitative and quantitative findings are important in research and can provide different insights into a research question or problem. Combining both types of findings can provide a more comprehensive understanding of a phenomenon and improve the validity and reliability of research results.
Parts of Research Findings
Research findings typically consist of several parts, including:
- Introduction: This section provides an overview of the research topic and the purpose of the study.
- Literature Review: This section summarizes previous research studies and findings that are relevant to the current study.
- Methodology : This section describes the research design, methods, and procedures used in the study, including details on the sample, data collection, and data analysis.
- Results : This section presents the findings of the study, including statistical analyses and data visualizations.
- Discussion : This section interprets the results and explains what they mean in relation to the research question(s) and hypotheses. It may also compare and contrast the current findings with previous research studies and explore any implications or limitations of the study.
- Conclusion : This section provides a summary of the key findings and the main conclusions of the study.
- Recommendations: This section suggests areas for further research and potential applications or implications of the study’s findings.
How to Write Research Findings
Writing research findings requires careful planning and attention to detail. Here are some general steps to follow when writing research findings:
- Organize your findings: Before you begin writing, it’s essential to organize your findings logically. Consider creating an outline or a flowchart that outlines the main points you want to make and how they relate to one another.
- Use clear and concise language : When presenting your findings, be sure to use clear and concise language that is easy to understand. Avoid using jargon or technical terms unless they are necessary to convey your meaning.
- Use visual aids : Visual aids such as tables, charts, and graphs can be helpful in presenting your findings. Be sure to label and title your visual aids clearly, and make sure they are easy to read.
- Use headings and subheadings: Using headings and subheadings can help organize your findings and make them easier to read. Make sure your headings and subheadings are clear and descriptive.
- Interpret your findings : When presenting your findings, it’s important to provide some interpretation of what the results mean. This can include discussing how your findings relate to the existing literature, identifying any limitations of your study, and suggesting areas for future research.
- Be precise and accurate : When presenting your findings, be sure to use precise and accurate language. Avoid making generalizations or overstatements and be careful not to misrepresent your data.
- Edit and revise: Once you have written your research findings, be sure to edit and revise them carefully. Check for grammar and spelling errors, make sure your formatting is consistent, and ensure that your writing is clear and concise.
Research Findings Example
Following is a Research Findings Example sample for students:
Title: The Effects of Exercise on Mental Health
Sample : 500 participants, both men and women, between the ages of 18-45.
Methodology : Participants were divided into two groups. The first group engaged in 30 minutes of moderate intensity exercise five times a week for eight weeks. The second group did not exercise during the study period. Participants in both groups completed a questionnaire that assessed their mental health before and after the study period.
Findings : The group that engaged in regular exercise reported a significant improvement in mental health compared to the control group. Specifically, they reported lower levels of anxiety and depression, improved mood, and increased self-esteem.
Conclusion : Regular exercise can have a positive impact on mental health and may be an effective intervention for individuals experiencing symptoms of anxiety or depression.
Applications of Research Findings
Research findings can be applied in various fields to improve processes, products, services, and outcomes. Here are some examples:
- Healthcare : Research findings in medicine and healthcare can be applied to improve patient outcomes, reduce morbidity and mortality rates, and develop new treatments for various diseases.
- Education : Research findings in education can be used to develop effective teaching methods, improve learning outcomes, and design new educational programs.
- Technology : Research findings in technology can be applied to develop new products, improve existing products, and enhance user experiences.
- Business : Research findings in business can be applied to develop new strategies, improve operations, and increase profitability.
- Public Policy: Research findings can be used to inform public policy decisions on issues such as environmental protection, social welfare, and economic development.
- Social Sciences: Research findings in social sciences can be used to improve understanding of human behavior and social phenomena, inform public policy decisions, and develop interventions to address social issues.
- Agriculture: Research findings in agriculture can be applied to improve crop yields, develop new farming techniques, and enhance food security.
- Sports : Research findings in sports can be applied to improve athlete performance, reduce injuries, and develop new training programs.
When to use Research Findings
Research findings can be used in a variety of situations, depending on the context and the purpose. Here are some examples of when research findings may be useful:
- Decision-making : Research findings can be used to inform decisions in various fields, such as business, education, healthcare, and public policy. For example, a business may use market research findings to make decisions about new product development or marketing strategies.
- Problem-solving : Research findings can be used to solve problems or challenges in various fields, such as healthcare, engineering, and social sciences. For example, medical researchers may use findings from clinical trials to develop new treatments for diseases.
- Policy development : Research findings can be used to inform the development of policies in various fields, such as environmental protection, social welfare, and economic development. For example, policymakers may use research findings to develop policies aimed at reducing greenhouse gas emissions.
- Program evaluation: Research findings can be used to evaluate the effectiveness of programs or interventions in various fields, such as education, healthcare, and social services. For example, educational researchers may use findings from evaluations of educational programs to improve teaching and learning outcomes.
- Innovation: Research findings can be used to inspire or guide innovation in various fields, such as technology and engineering. For example, engineers may use research findings on materials science to develop new and innovative products.
Purpose of Research Findings
The purpose of research findings is to contribute to the knowledge and understanding of a particular topic or issue. Research findings are the result of a systematic and rigorous investigation of a research question or hypothesis, using appropriate research methods and techniques.
The main purposes of research findings are:
- To generate new knowledge : Research findings contribute to the body of knowledge on a particular topic, by adding new information, insights, and understanding to the existing knowledge base.
- To test hypotheses or theories : Research findings can be used to test hypotheses or theories that have been proposed in a particular field or discipline. This helps to determine the validity and reliability of the hypotheses or theories, and to refine or develop new ones.
- To inform practice: Research findings can be used to inform practice in various fields, such as healthcare, education, and business. By identifying best practices and evidence-based interventions, research findings can help practitioners to make informed decisions and improve outcomes.
- To identify gaps in knowledge: Research findings can help to identify gaps in knowledge and understanding of a particular topic, which can then be addressed by further research.
- To contribute to policy development: Research findings can be used to inform policy development in various fields, such as environmental protection, social welfare, and economic development. By providing evidence-based recommendations, research findings can help policymakers to develop effective policies that address societal challenges.
Characteristics of Research Findings
Research findings have several key characteristics that distinguish them from other types of information or knowledge. Here are some of the main characteristics of research findings:
- Objective : Research findings are based on a systematic and rigorous investigation of a research question or hypothesis, using appropriate research methods and techniques. As such, they are generally considered to be more objective and reliable than other types of information.
- Empirical : Research findings are based on empirical evidence, which means that they are derived from observations or measurements of the real world. This gives them a high degree of credibility and validity.
- Generalizable : Research findings are often intended to be generalizable to a larger population or context beyond the specific study. This means that the findings can be applied to other situations or populations with similar characteristics.
- Transparent : Research findings are typically reported in a transparent manner, with a clear description of the research methods and data analysis techniques used. This allows others to assess the credibility and reliability of the findings.
- Peer-reviewed: Research findings are often subject to a rigorous peer-review process, in which experts in the field review the research methods, data analysis, and conclusions of the study. This helps to ensure the validity and reliability of the findings.
- Reproducible : Research findings are often designed to be reproducible, meaning that other researchers can replicate the study using the same methods and obtain similar results. This helps to ensure the validity and reliability of the findings.
Advantages of Research Findings
Research findings have many advantages, which make them valuable sources of knowledge and information. Here are some of the main advantages of research findings:
- Evidence-based: Research findings are based on empirical evidence, which means that they are grounded in data and observations from the real world. This makes them a reliable and credible source of information.
- Inform decision-making: Research findings can be used to inform decision-making in various fields, such as healthcare, education, and business. By identifying best practices and evidence-based interventions, research findings can help practitioners and policymakers to make informed decisions and improve outcomes.
- Identify gaps in knowledge: Research findings can help to identify gaps in knowledge and understanding of a particular topic, which can then be addressed by further research. This contributes to the ongoing development of knowledge in various fields.
- Improve outcomes : Research findings can be used to develop and implement evidence-based practices and interventions, which have been shown to improve outcomes in various fields, such as healthcare, education, and social services.
- Foster innovation: Research findings can inspire or guide innovation in various fields, such as technology and engineering. By providing new information and understanding of a particular topic, research findings can stimulate new ideas and approaches to problem-solving.
- Enhance credibility: Research findings are generally considered to be more credible and reliable than other types of information, as they are based on rigorous research methods and are subject to peer-review processes.
Limitations of Research Findings
While research findings have many advantages, they also have some limitations. Here are some of the main limitations of research findings:
- Limited scope: Research findings are typically based on a particular study or set of studies, which may have a limited scope or focus. This means that they may not be applicable to other contexts or populations.
- Potential for bias : Research findings can be influenced by various sources of bias, such as researcher bias, selection bias, or measurement bias. This can affect the validity and reliability of the findings.
- Ethical considerations: Research findings can raise ethical considerations, particularly in studies involving human subjects. Researchers must ensure that their studies are conducted in an ethical and responsible manner, with appropriate measures to protect the welfare and privacy of participants.
- Time and resource constraints : Research studies can be time-consuming and require significant resources, which can limit the number and scope of studies that are conducted. This can lead to gaps in knowledge or a lack of research on certain topics.
- Complexity: Some research findings can be complex and difficult to interpret, particularly in fields such as science or medicine. This can make it challenging for practitioners and policymakers to apply the findings to their work.
- Lack of generalizability : While research findings are intended to be generalizable to larger populations or contexts, there may be factors that limit their generalizability. For example, cultural or environmental factors may influence how a particular intervention or treatment works in different populations or contexts.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples

Institutional Review Board – Application Sample...

Evaluating Research – Process, Examples and...
- Affiliate Program

- UNITED STATES
- 台灣 (TAIWAN)
- TÜRKIYE (TURKEY)
- Academic Editing Services
- - Research Paper
- - Journal Manuscript
- - Dissertation
- - College & University Assignments
- Admissions Editing Services
- - Application Essay
- - Personal Statement
- - Recommendation Letter
- - Cover Letter
- - CV/Resume
- Business Editing Services
- - Business Documents
- - Report & Brochure
- - Website & Blog
- Writer Editing Services
- - Script & Screenplay
- Our Editors
- Client Reviews
- Editing & Proofreading Prices
- Wordvice Points
- Partner Discount
- Plagiarism Checker
- APA Citation Generator
- MLA Citation Generator
- Chicago Citation Generator
- Vancouver Citation Generator
- - APA Style
- - MLA Style
- - Chicago Style
- - Vancouver Style
- Writing & Editing Guide
- Academic Resources
- Admissions Resources
How to Write the Results/Findings Section in Research
What is the research paper Results section and what does it do?
The Results section of a scientific research paper represents the core findings of a study derived from the methods applied to gather and analyze information. It presents these findings in a logical sequence without bias or interpretation from the author, setting up the reader for later interpretation and evaluation in the Discussion section. A major purpose of the Results section is to break down the data into sentences that show its significance to the research question(s).
The Results section appears third in the section sequence in most scientific papers. It follows the presentation of the Methods and Materials and is presented before the Discussion section —although the Results and Discussion are presented together in many journals. This section answers the basic question “What did you find in your research?”
What is included in the Results section?
The Results section should include the findings of your study and ONLY the findings of your study. The findings include:
- Data presented in tables, charts, graphs, and other figures (may be placed into the text or on separate pages at the end of the manuscript)
- A contextual analysis of this data explaining its meaning in sentence form
- All data that corresponds to the central research question(s)
- All secondary findings (secondary outcomes, subgroup analyses, etc.)
If the scope of the study is broad, or if you studied a variety of variables, or if the methodology used yields a wide range of different results, the author should present only those results that are most relevant to the research question stated in the Introduction section .
As a general rule, any information that does not present the direct findings or outcome of the study should be left out of this section. Unless the journal requests that authors combine the Results and Discussion sections, explanations and interpretations should be omitted from the Results.
How are the results organized?
The best way to organize your Results section is “logically.” One logical and clear method of organizing research results is to provide them alongside the research questions—within each research question, present the type of data that addresses that research question.
Let’s look at an example. Your research question is based on a survey among patients who were treated at a hospital and received postoperative care. Let’s say your first research question is:
“What do hospital patients over age 55 think about postoperative care?”
This can actually be represented as a heading within your Results section, though it might be presented as a statement rather than a question:
Attitudes towards postoperative care in patients over the age of 55
Now present the results that address this specific research question first. In this case, perhaps a table illustrating data from a survey. Likert items can be included in this example. Tables can also present standard deviations, probabilities, correlation matrices, etc.
Following this, present a content analysis, in words, of one end of the spectrum of the survey or data table. In our example case, start with the POSITIVE survey responses regarding postoperative care, using descriptive phrases. For example:
“Sixty-five percent of patients over 55 responded positively to the question “ Are you satisfied with your hospital’s postoperative care ?” (Fig. 2)
Include other results such as subcategory analyses. The amount of textual description used will depend on how much interpretation of tables and figures is necessary and how many examples the reader needs in order to understand the significance of your research findings.
Next, present a content analysis of another part of the spectrum of the same research question, perhaps the NEGATIVE or NEUTRAL responses to the survey. For instance:
“As Figure 1 shows, 15 out of 60 patients in Group A responded negatively to Question 2.”
After you have assessed the data in one figure and explained it sufficiently, move on to your next research question. For example:
“How does patient satisfaction correspond to in-hospital improvements made to postoperative care?”
This kind of data may be presented through a figure or set of figures (for instance, a paired T-test table).
Explain the data you present, here in a table, with a concise content analysis:
“The p-value for the comparison between the before and after groups of patients was .03% (Fig. 2), indicating that the greater the dissatisfaction among patients, the more frequent the improvements that were made to postoperative care.”
Let’s examine another example of a Results section from a study on plant tolerance to heavy metal stress . In the Introduction section, the aims of the study are presented as “determining the physiological and morphological responses of Allium cepa L. towards increased cadmium toxicity” and “evaluating its potential to accumulate the metal and its associated environmental consequences.” The Results section presents data showing how these aims are achieved in tables alongside a content analysis, beginning with an overview of the findings:
“Cadmium caused inhibition of root and leave elongation, with increasing effects at higher exposure doses (Fig. 1a-c).”
The figure containing this data is cited in parentheses. Note that this author has combined three graphs into one single figure. Separating the data into separate graphs focusing on specific aspects makes it easier for the reader to assess the findings, and consolidating this information into one figure saves space and makes it easy to locate the most relevant results.
Following this overall summary, the relevant data in the tables is broken down into greater detail in text form in the Results section.
- “Results on the bio-accumulation of cadmium were found to be the highest (17.5 mg kgG1) in the bulb, when the concentration of cadmium in the solution was 1×10G2 M and lowest (0.11 mg kgG1) in the leaves when the concentration was 1×10G3 M.”
Captioning and Referencing Tables and Figures
Tables and figures are central components of your Results section and you need to carefully think about the most effective way to use graphs and tables to present your findings . Therefore, it is crucial to know how to write strong figure captions and to refer to them within the text of the Results section.
The most important advice one can give here as well as throughout the paper is to check the requirements and standards of the journal to which you are submitting your work. Every journal has its own design and layout standards, which you can find in the author instructions on the target journal’s website. Perusing a journal’s published articles will also give you an idea of the proper number, size, and complexity of your figures.
Regardless of which format you use, the figures should be placed in the order they are referenced in the Results section and be as clear and easy to understand as possible. If there are multiple variables being considered (within one or more research questions), it can be a good idea to split these up into separate figures. Subsequently, these can be referenced and analyzed under separate headings and paragraphs in the text.
To create a caption, consider the research question being asked and change it into a phrase. For instance, if one question is “Which color did participants choose?”, the caption might be “Color choice by participant group.” Or in our last research paper example, where the question was “What is the concentration of cadmium in different parts of the onion after 14 days?” the caption reads:
“Fig. 1(a-c): Mean concentration of Cd determined in (a) bulbs, (b) leaves, and (c) roots of onions after a 14-day period.”
Steps for Composing the Results Section
Because each study is unique, there is no one-size-fits-all approach when it comes to designing a strategy for structuring and writing the section of a research paper where findings are presented. The content and layout of this section will be determined by the specific area of research, the design of the study and its particular methodologies, and the guidelines of the target journal and its editors. However, the following steps can be used to compose the results of most scientific research studies and are essential for researchers who are new to preparing a manuscript for publication or who need a reminder of how to construct the Results section.
Step 1 : Consult the guidelines or instructions that the target journal or publisher provides authors and read research papers it has published, especially those with similar topics, methods, or results to your study.
- The guidelines will generally outline specific requirements for the results or findings section, and the published articles will provide sound examples of successful approaches.
- Note length limitations on restrictions on content. For instance, while many journals require the Results and Discussion sections to be separate, others do not—qualitative research papers often include results and interpretations in the same section (“Results and Discussion”).
- Reading the aims and scope in the journal’s “ guide for authors ” section and understanding the interests of its readers will be invaluable in preparing to write the Results section.
Step 2 : Consider your research results in relation to the journal’s requirements and catalogue your results.
- Focus on experimental results and other findings that are especially relevant to your research questions and objectives and include them even if they are unexpected or do not support your ideas and hypotheses.
- Catalogue your findings—use subheadings to streamline and clarify your report. This will help you avoid excessive and peripheral details as you write and also help your reader understand and remember your findings. Create appendices that might interest specialists but prove too long or distracting for other readers.
- Decide how you will structure of your results. You might match the order of the research questions and hypotheses to your results, or you could arrange them according to the order presented in the Methods section. A chronological order or even a hierarchy of importance or meaningful grouping of main themes or categories might prove effective. Consider your audience, evidence, and most importantly, the objectives of your research when choosing a structure for presenting your findings.
Step 3 : Design figures and tables to present and illustrate your data.
- Tables and figures should be numbered according to the order in which they are mentioned in the main text of the paper.
- Information in figures should be relatively self-explanatory (with the aid of captions), and their design should include all definitions and other information necessary for readers to understand the findings without reading all of the text.
- Use tables and figures as a focal point to tell a clear and informative story about your research and avoid repeating information. But remember that while figures clarify and enhance the text, they cannot replace it.

Step 4 : Draft your Results section using the findings and figures you have organized.
- The goal is to communicate this complex information as clearly and precisely as possible; precise and compact phrases and sentences are most effective.
- In the opening paragraph of this section, restate your research questions or aims to focus the reader’s attention to what the results are trying to show. It is also a good idea to summarize key findings at the end of this section to create a logical transition to the interpretation and discussion that follows.
- Try to write in the past tense and the active voice to relay the findings since the research has already been done and the agent is usually clear. This will ensure that your explanations are also clear and logical.
- Make sure that any specialized terminology or abbreviation you have used here has been defined and clarified in the Introduction section .
Step 5 : Review your draft; edit and revise until it reports results exactly as you would like to have them reported to your readers.
- Double-check the accuracy and consistency of all the data, as well as all of the visual elements included.
- Read your draft aloud to catch language errors (grammar, spelling, and mechanics), awkward phrases, and missing transitions.
- Ensure that your results are presented in the best order to focus on objectives and prepare readers for interpretations, valuations, and recommendations in the Discussion section . Look back over the paper’s Introduction and background while anticipating the Discussion and Conclusion sections to ensure that the presentation of your results is consistent and effective.
- Consider seeking additional guidance on your paper. Find additional readers to look over your Results section and see if it can be improved in any way. Peers, professors, or qualified experts can provide valuable insights.
One excellent option is to use a professional English proofreading and editing service such as Wordvice, including our paper editing service . With hundreds of qualified editors from dozens of scientific fields, Wordvice has helped thousands of authors revise their manuscripts and get accepted into their target journals. Read more about the proofreading and editing process before proceeding with getting academic editing services and manuscript editing services for your manuscript.
As the representation of your study’s data output, the Results section presents the core information in your research paper. By writing with clarity and conciseness and by highlighting and explaining the crucial findings of their study, authors increase the impact and effectiveness of their research manuscripts.
For more articles and videos on writing your research manuscript, visit Wordvice’s Resources page.
Wordvice Resources
- How to Write a Research Paper Introduction
- Which Verb Tenses to Use in a Research Paper
- How to Write an Abstract for a Research Paper
- How to Write a Research Paper Title
- Useful Phrases for Academic Writing
- Common Transition Terms in Academic Papers
- Active and Passive Voice in Research Papers
- 100+ Verbs That Will Make Your Research Writing Amazing
- Tips for Paraphrasing in Research Papers
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Malays Fam Physician
- v.2(1); 2007
Summarizing Research Findings: Systematic Review and Meta-Analysis
MSc, PhD, Lecturer, Health Research Development Unit (HeRDU), Faculty of Medicine, University of Malaya
The explosion of biomedical publishing makes keeping up with the primary studies an impossible task. The often disparate, confusing and contradicting findings of individual studies makes healthcare professionals turn to review articles where knowledge has been collated and published in summaries. Narrative reviews lack rigorous, systematic and reproducible synthesis. In contrast, systematic reviews are conducted using systematic and explicit methods to identify, select and critically appraise relevant research, and to collect and analyse data from the studies that are included in the review. The final pathway for systematic review is a statistical summary of the results of primary studies, or meta-analysis. This article provides some guidelines to health care providers in understanding the key aspects of systematic review and meta-analysis. Steps involved in systematic review are discussed. The potential pitfall of meta-analysis was also explored.
THE NEED FOR REVIEWS
The huge amount of medical information available and its exponential growth have become common problem in the literature of biomedical information. Health care practitioners face the explosion in biomedical knowledge which makes keeping up with the primary research an impossible feat. There are approximately 17,000 new biomedical books published every year along with 30,000 biomedical journals, with an annual increase of 7%. For instance, MEDLINE alone contains more than eleven million citations, and more than 400,000 articles are added to the file each year. 1 Majority health care providers noted that the current volume of scientific literature is unmanageable 2 and often do not have sufficient time for reading medical journals as the information explosion continues. 3 Further, some of these studies could be unclear, confusing or may also have contradicting results.
THE NARRATIVE REVIEWS
To make this task easier and manageable for health care providers as well as decision makers, reviews are often among their information resources. Reviews have always been a part of the medical literature. Traditionally, medical research has been integrated in the narrative or nonsystematic form. An expert in a particular field will review studies, decided on the relevance, and highlight the findings, both in terms of results and, to a lesser degree the methodology. 4 Such narrative reviews tend to be unsystematic and susceptible to many biases. Firstly, no systematic approach is prescribed to obtain the primary data and to integrate the data. Often, subjective judgment of the reviewer was used. There were often no explicit standards exist to assess the quality of review. Moreover, narrative reviewer also does not synthesize data quantitatively.
A CALL FOR SYSTEMATIC REVIEWS
A systematic review is defined by the Cochrane Handbook as ‘A review of a clearly formulated question that uses systematic and explicit methods to identify, select and critically appraise relevant research, and to collect and analyse data from the studies that are included in the review’. In contrast to narrative review, systematic review allows readers to appraise how the review was conducted and synthesized. It is of particular value in bringing together a number of separately conducted studies, sometimes with conflicting findings, and synthesizing their results. Systematic reviews have been proven to be able to yield valid, precise, and widely applicable answers to clinical questions. 5 In short, systematic reviews summarise large amounts of information and are more likely than individual trials to describe the true clinical effect of an intervention. Thus, systematic reviews have come to play a central role in informing clinical decisions and guidelines. A systematic review is also often called an ‘overview’.
A meta-analysis takes a systematic review one step further by statistically pooling the results of combinable studies. Since its introduction, meta-analysis has established itself as an influential branch of clinical epidemiology and health services research, with hundreds of meta-analyses published in the medical literature each year. 6
THE PROCESS OF SYSTEMATIC REVIEW
Systematic review should be carefully planned with a detailed written protocol prepared in advance as any other search project. Systematic review involves several discrete steps and the steps are summarised below.
Step 1: Formulate review question . This requires the formulation of a clear statement of relevant patient groups, intervention of interest, as well as outcomes. The details are used to select studies for inclusion in the review.
Step 2: Locate studies . Systematic review must be undertaken in accordance with a predefined search strategy that would allow the completeness of the search to be assessed. Search strategies should consider the following sources: The Cochrane Controlled Trials Register (CCTR), other electronic databases and trials registered not covered by CCTR, checking reference lists, hand searching of key journals and personal communication with experts in the fields. The selection of primary studies is governed by inclusion and exclusion criteria that are initially specified when the protocol is defined.
Step 3: Appraising the quality of studies . After an exhaustive search, all possible primary studies that have been identified need to be assessed for eligibility for inclusion. Application of stringent inclusion/exclusion criteria should be addressed for example types of participants, interventions, outcomes, study designs and methodological quality. Independent assessment by more than one observer is desirable.
Step 4: Combining the results. The findings from combinable individual primary studies are then pooled to produce an ‘overall estimate’ on the clinical effectiveness of the intervention. The aggregation can be qualitative, or more appropriate, by statistically combining the data produced by individual studies into a single summary estimate. The statistical pooling of data is termed meta-analysis ( Figure 1 ). In meta-analysis, results from studies are combined using ‘inverse variance method’, whereby larger studies and studies with less random variation are given greater weight than smaller studies.

Meta-analysis: Data from several different studies are combined and produce a single estimate
Meta-analysis can only be undertaken when studies address the same question, administer the intervention in a similar manner or measure the same outcomes. When studies differ in one or more of these components, meta-analysis is not appropriate. Therefore, systematic review may or may not include meta-analysis.
In meta-analysis, for outcomes measured on a continuous scale, the weighted mean difference is commonly used. For outcomes measured on a dichotomous scale, common approaches include the use of odds ratio or relative risk. There are two approaches for combining the data: fixed-effects model assume that an intervention has a single true effect whereas random-effects models assume that an effect may vary across studies. 7 The results of meta-analysis can be displayed graphically (Forest plot) to allow a visual comparison of findings of individual studies ( Figure 2 ).

Forest plot of meta-analysis
Systematic review should continue with an investigation of the reasons for heterogeneity. Subgroup analysis, sensitivity analysis and meta-regression are frequently used to investigate heterogeneity of individual studies in meta-analysis. One of the major drawbacks to using meta-analysis is the possibility of publication bias. One way to investigate whether a review is subject to publication bias is to prepare a ‘funnel plot’ ( Figure 3 ) and examine this for signs of asymmetry. 8

Funnel plot showing evidence of publication bias
Step 5: Interpret results. The findings from systematic review and statistical pooling of the studies then need to be interpreted, discussed and set out the implications for practice or further research. Issues such as the quality and heterogeneity of the included studies plus the possible impact of bias need to be discussed.
PITFALLS AND PROBLEMS
Meta-analyses have received mixed receptions. Some see meta-analysis as an exercise of ‘mega-silliness’, 9 ‘a tool has become a weapon’ 10 and a number of statisticians think that meta-analysis represents the unacceptable face of statisticism. 11 There are also those that still prefer the conventional narrative review article. 12 The mixed receptions were due to opposite conclusions observed in some systematic reviews that address the same issue. 13 , 14 Also, meta-analyses of small trials were discovered to contradict by a single large randomized trial. 15
Publication bias could be a serious problem for meta-analyses, secondly, studies may be of varying quality. Clearly the quality of trials included in a systematic review and meta-analysis is of crucial importance and should be of high methodological quality as well as free from biases. Meta-analysis should therefore be considered only within the framework of systematic review that has been prepared using a systematic approach to mitigate all kinds of biases and explicitly address the issue of the completeness of the evidence identified, the quality of component studies and the combinability of studies. 15 The Cochrane Collaboration has been established to overcome this problem by providing high quality and authoritative systematic reviews and meta-analyses. 16 , 17 The collaboration not only ensures that high-quality reviews are conducted but also update reviews when new evidence becomes available.
Systematic review is an invaluable resource for both clinicians and researchers. However, not all reviews are systematic and even those that are described as systematic may be methodologically flawed. Nonetheless, a high quality systematic review provides the best available evidence. The usefulness of a systematic review can further be enhanced by statistical summary of the results by meta-analytical technique. Pooling individual studies may reduce the risk of random error, increase statistical power and allow for a more accurate estimate of effect size.
SOME TERMINOLOGIES
Bias (synonym: systematic error): the distortion of the outcome, as a result of a known or unknown variable other than intervention (i.e. the tendency to produce results that depart from the “true” result).
Cochrane Collaboration : The Cochrane Collaboration is an international organization that aims to help people make well-informed decisions about healthcare by preparing, maintaining & promoting the accessibility of systematic reviews of the effects of healthcare interventions.
Cochrane Controlled Trials Register (CCTR): CCTR is a database of references to controlled trials in health care.
Critical appraisal : systematically finding, appraising and interpreting evidence of effectiveness. It is aimed to examine research evidence to assess its validity, results and relevance before using it to inform a decision.
Cumulative meta-analysis : the repeated performance of meta-analysis whenever a new trial becomes available for inclusion. In cumulative meta-analysis studies are added one at a time in a specified order.
Effect size : refers to the size of a relationship between an expose and an outcome. The term is applied to measurement of the differences in the outcome between the study groups. Relative risk, odds ratio, and risk differences can be defined as effect sizes for dichotomous scale. Effect size of continuous variable is the standardized mean differences.
Fixed-effect model : a mathematical model that combines the results of studies that assume the effect of the intervention is constant in all subject population studied. Only within study variation is included when assessing the uncertainty of results.
Forest plot : a forest plot presents the means and variance for the difference for each pooled primary study. The line represents the standard error of the difference, the box represents the mean difference and its size proportional to the number of subjects in the study. The bottom entry in a forest plot is the summary estimate of the treatment difference and confidence interval for the summary difference ( Figure 2 ).
Funnel plot : a graphical method of assessing bias; the effect size of each study is plotted against some measure of study information. If the shape of the plot resembles an inverted funnel, it can be stated that there is no evidence of publication bias within the systematic review ( Figure 3 ).
Heterogeneity : the variability between studies in terms of key characteristics (i.e. ecological variables) quality (i.e. methodology) or effect (i.e. results). Statistical tests of heterogeneity may be used to assess whether the observed variability in effect size (i.e. study results) is greater than that expected to occur purely by chance.
Meta-regression : a multivariable model investigating effect size from individual studies, generally weighted by sample size, as a function of various study characteristics (i.e. to investigate whether study characteristics are influencing effect size).
Outlier : an outlier study in meta-analysis is study that results very different from the rest of the studies. Outlier could alter the conclusions of a meta-analysis.
Overall estimate : is the pooled estimate from a meta-analysis. The overall estimate from a meta-analysis is always displayed with its confidence interval.
Primary studies : Individual studies contributing to a systematic review are called primary studies whereas a systematic review is a form of a secondary study.
Publication bias : publication bias refers to the problem that positive results are more likely to be published than negative results and this may therefore give a misleading assessment of the impact of an intervention. Publication bias can be examined via a funnel plot.
Random-effects model : a mathematical model for combining the results of studies that allow for variation in the effect of the intervention amongst the subject populations studied. Both within-study variation and between-study variation is included when assessing the uncertainty of results.
Review : article that summarizes a number of primary studies and discusses the effectiveness of a particular intervention. It may not be a systematic review.
Search strategy : a description of the methodology used to locate and identify research articles pertinent to a systematic review, as specified within the relevant protocol. It includes a list of search terms, based on the subject, intervention and outcome of the review, to be used when searching electronic databases, websites, reference lists and when engaging with personal contacts. If required, the strategy may be modified once the search has commenced.
Sensitivity analysis : repetition of the analysis using different sets of assumptions in order to determine the impact of variation arising from these assumptions, or uncertain decisions, on the results of a systematic review.
Subgroup analysis : used to determine if the effects of an intervention vary between subgroups in the systematic review.
Weighted mean difference : a method used to combine measures on continuous scales (where the mean, standard deviation and sample size in each group are known) and the weight given to each study is determined by the precision of its estimate of effect.
Cochrane Systematic Review and SEA-ORCHID Project
Cochrane systematic reviews combine the results of the best medical research using rigorous methods, and are regarded as the gold standard of reference for health care professionals. Malaysia has relatively minor involvement in Cochrane Collaboration despite its economic growth and the fast improving standard of medical care. It is likely that clinical questions with high relevance to Malaysia are therefore not being addressed in Cochrane reviews.
The SEA-ORCHID project, which stands for South East Asia Optimising Reproductive and Child Health Outcomes in Developing Countries Project, is a five-year project (2003 to 2008) aiming to promote the synthesis and application of high level clinical evidence on issues relevant to this region, focusing on maternal and child health but also involving other related disciplines. Jointly funded by the Wellcome Trust and the Australian National Health and Medical Research Council and supported by the Cochrane Australasian Centre, the project activities include regular Cochrane Systematic Review Workshop and work-in sessions throughout the country. This is a good opportunity for the pool of clinical and research talents in our country to contribute in synthesizing the best clinical evidence and making a significant impact on evidence-based health care.
If you are interested in authoring or co-authoring a Cochrane review, you will be guided at every step by experienced reviewers leading to its publication in the Cochrane Library. In this workshop, you will also hear the experiences of people who are in the process of developing a protocol or review.
For further information please contact:
- Professor Jackie Ho [email protected]
- Dr Lai Nai Ming [email protected]

The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.
- Scoping Review
- Open access
- Published: 24 May 2024
Impact of climate change on the global circulation of West Nile virus and adaptation responses: a scoping review
- Hao-Ran Wang 1 , 2 ,
- Tao Liu 1 , 2 ,
- Xiang Gao 1 , 2 ,
- Hong-Bin Wang 1 , 2 &
- Jian-Hua Xiao ORCID: orcid.org/0000-0003-1109-9133 1 , 2
Infectious Diseases of Poverty volume 13 , Article number: 38 ( 2024 ) Cite this article
327 Accesses
1 Altmetric
Metrics details
West Nile virus (WNV), the most widely distributed flavivirus causing encephalitis globally, is a vector-borne pathogen of global importance. The changing climate is poised to reshape the landscape of various infectious diseases, particularly vector-borne ones like WNV. Understanding the anticipated geographical and range shifts in disease transmission due to climate change, alongside effective adaptation strategies, is critical for mitigating future public health impacts. This scoping review aims to consolidate evidence on the impact of climate change on WNV and to identify a spectrum of applicable adaptation strategies.
We systematically analyzed research articles from PubMed, Web of Science, Scopus, and EBSCOhost. Our criteria included English-language research articles published between 2007 and 2023, focusing on the impacts of climate change on WNV and related adaptation strategies. We extracted data concerning study objectives, populations, geographical focus, and specific findings. Literature was categorized into two primary themes: 1) climate-WNV associations, and 2) climate change impacts on WNV transmission, providing a clear understanding. Out of 2168 articles reviewed, 120 met our criteria. Most evidence originated from North America (59.2%) and Europe (28.3%), with a primary focus on human cases (31.7%). Studies on climate-WNV correlations ( n = 83) highlighted temperature (67.5%) as a pivotal climate factor. In the analysis of climate change impacts on WNV ( n = 37), most evidence suggested that climate change may affect the transmission and distribution of WNV, with the extent of the impact depending on local and regional conditions. Although few studies directly addressed the implementation of adaptation strategies for climate-induced disease transmission, the proposed strategies ( n = 49) fell into six categories: 1) surveillance and monitoring (38.8%), 2) predictive modeling (18.4%), 3) cross-disciplinary collaboration (16.3%), 4) environmental management (12.2%), 5) public education (8.2%), and 6) health system readiness (6.1%). Additionally, we developed an accessible online platform to summarize the evidence on climate change impacts on WNV transmission ( https://2xzl2o-neaop.shinyapps.io/WNVScopingReview/ ).
Conclusions
This review reveals that climate change may affect the transmission and distribution of WNV, but the literature reflects only a small share of the global WNV dynamics. There is an urgent need for adaptive responses to anticipate and respond to the climate-driven spread of WNV. Nevertheless, studies focusing on these adaptation responses are sparse compared to those examining the impacts of climate change. Further research on the impacts of climate change and adaptation strategies for vector-borne diseases, along with more comprehensive evidence synthesis, is needed to inform effective policy responses tailored to local contexts.
West Nile virus (WNV), the most widely distributed flavivirus globally, is a significant mosquito-borne virus [ 1 ]. It was first isolated in 1937 from the blood of a febrile woman in the West Nile region of Uganda. The earliest reported outbreaks occurred in the 1950s near Haifa, Israel [ 2 ]. Since the 1950s, WNV outbreaks have primarily occurred in Israel and various African countries [ 3 , 4 ]. However, the epidemiology of WNV appears to have shifted since the 1990s due to the globalization of human trade and travel [ 1 ]. WNV was first detected in New York City in 1999 and subsequently spread rapidly throughout the entire Western Hemisphere, including the United States (US), Canada, and Argentina [ 5 , 6 , 7 ]. Concurrently, epidemic activity increased in Europe, the Middle East, and Russia [ 3 , 4 , 8 ]. In 2018, Europe experienced an unprecedented WNV epidemic, with human cases exceeding 1900, seven times higher than in previous seasons [ 9 ]. In 2020, locally transmitted human cases of WNV were reported for the first time in the Netherlands and Germany [ 10 , 11 ]. Evidence suggests interactive WNV cycles on all continents except Antarctica [ 1 ].
The establishment of ongoing WNV transmission relies on the interactions among the virus, vectors, hosts, and environmental factors [ 12 ]. WNV can infect a wide range of vertebrate species, including most mammals, birds, and some reptiles and amphibians [ 13 , 14 ]. Birds, serving as the primary amplifying hosts, play a crucial role in WNV proliferation. While humans and horses are susceptible to WNV, they are considered dead-end hosts [ 15 ]. In humans, WNV often results in asymptomatic or mild illness, but approximately 1 in 150 cases progress to neuroinvasive disease, potentially leading to encephalitis or death [ 16 ]. The primary vectors for WNV transmission are mosquitoes, particularly those belonging to the Culex genus. Mosquito bites are responsible for the vast majority of human WNV infections, although the virus can also spread through blood transfusions, organ transplantations, and potentially breastfeeding [ 17 ]. Given that WNV is transmitted by mosquitoes, its distribution depends on environmental conditions and is susceptible to the impacts of climate change [ 18 ]. For example, higher temperatures can accelerate viral replication, shorten the extrinsic incubation period in mosquitoes, promote vector abundance, enhance transmission efficiency, expand the suitability of vector habitats, and increase the probability of avian migration across regions [ 19 , 20 ]. Additionally, precipitation patterns have a significant impact on mosquito breeding and abundance, thus affecting the spread and geographical distribution of WNV [ 18 ].
The current body of evidence strongly indicates that climate change directly impacts the spread and proliferation of vector-borne illnesses, including WNV [ 21 ]. Numerous studies have demonstrated that areas vulnerable to WNV transmission could expand or shift due to climate elements. This encompasses projecting future global climate change scenarios, examining how vector species respond to environmental shifts in laboratory settings, and conducting field research in regions where outbreaks occur. There is some evidence of WNV emerging or re-emerging in high-latitude regions and at the edges of current endemic zones [ 22 , 23 , 24 , 25 , 26 ]. For example, in North America, the suitable range for WNV is projected to extend northward and to higher altitudes by 2050 and 2080, potentially leading to new infections in both native and non-native species [ 22 ]. In Europe, increased WNV cases and new outbreak locations are predicted under future climate scenarios, especially at the margin of current transmission areas [ 23 ]. In South America, high risk areas for WNV might shift between 2046–2065 and 2081–2100, with more pronounced changes under high greenhouse gas emission scenarios, potentially altering the current WNV distributions in some countries (e.g., parts of Bolivia, Paraguay, and Brazil) [ 24 ]. Moreover, existing surveillance data support the overall trend of heightened WNV risk due to climate change. For instance, in the Powder River Basin of Montana and Wyoming, US, the WNV mortality rate in the wild bird population was significantly higher in 2003 (the sixth most sweltering summer historically) than in 2004 and 2005 [ 25 ]. In Germany, the extreme heat in the summer of 2018 (the second most sweltering and desiccated summer historically) theoretically played a pivotal role in reducing the average extrinsic incubation period in mosquitoes, resulting in rapid viral amplification and increased transmission risks to vertebrate hosts [ 26 ]. However, the impact of climate change on WNV distribution may vary geographically, and some areas may see a decrease in cases. For example, while Keyel et al. predicted a general increase in WNV cases in 2021, a subsequent study indicated that future cases may decrease in areas outside the boundaries of the original study area in New York [ 27 , 28 ].
While efforts to mitigate climate change are essential to reduce CO 2 emissions and lessen potential future impacts, there is an increasing need to focus on adaptation strategies as well. These include various short-term measures at different levels to address the immediate effects of climate change [ 29 ]. Adaptation approaches aim to enhance resilience in health systems, preparing them to manage and minimize the health consequences of climate change [ 29 ]. Given the commitments countries have made to the Paris Agreement and Sustainable Development Goals, along with the growing global evidence base for climate change's impact on disease spread, nations have begun developing and implementing policy responses as components of national climate adaptation plans [ 30 ]. Insights into the expected magnitude of climate change impacts on WNV and associated adaptive responses can help inform best practices to mitigate public health impacts from the climate-induced spread of disease.
Contemporary prioritization in Canada of investigative pursuits on emerging human and animal diseases under climate change scenarios indicated that WNV is a disease requiring primary attention [ 31 , 32 ]. Since the Intergovernmental Panel on Climate Change (IPCC) Fourth Assessment Report in 2007, the health impacts of climate change have garnered significant research focus [ 33 ]. This attention has increased further following the Fifth Assessment Report in 2014 and the 2015 Lancet Commission on Climate Change and Health, leading to a growth in the number of related publications [ 34 , 35 ]. In addition to highlighting the impacts of climate change, these articles also emphasize to some degree specific interventions or policy responses within defined countries and regions. To our knowledge, a comprehensive review of the global impacts and adaptation responses related to climate change and WNV has not been conducted. Such a review is necessary to consolidate existing evidence, explore how climate change influences the spread of WNV, and identify the most effective strategies for developing adaptation policies.
In summary, this scoping review aims to address two core questions:
What types of evidence exist regarding the impact of climate change on the global transmission of WNV?
What adaptation measures have been proposed or implemented in response to climate change?
Our primary focus is to elucidate the climatic drivers of WNV to better inform these strategies. This approach is intended to serve as a foundation for future research that may delve into comprehensive public health policies and adaptation measures.
Protocol and registration
We used a scoping review methodology to select studies for inclusion in this synthesis. Our review followed an established protocol, guided by the PRISMA Scoping Review Extension (PRISMA-SCR) and published scoping review methodology [ 36 , 37 , 38 ]. It was registered with the OSF Registries ( https://osf.io/9j2as ) on December 25, 2023, to ensure transparency [ 39 , 40 ].
Search strategy
We conducted systematic searches across four databases—PubMed (MEDLINE), Web of Science, Scopus, and EBSCOhost—to identify relevant peer-reviewed publications on climate change and WNV between January 2007 and December 2023 without imposing language restrictions. Our literature searches employed terminology related to climate change and the diseases of interest. Terms for climate change were taken from the search strategy used in Sweileh’s (2020) bibliometric analysis of climate change and health publications: “climat* Change” OR “global warming” OR “changing climate” OR “climate variability” OR “greenhouse gas” OR “rising temperature” OR “extreme weather” OR “greenhouse effect” [ 41 ]. Disease-specific terms included were: “West Nile virus” OR “WNV host” OR “WNV vector”. Full search strategies for each database are provided in supplementary materials (Additional file 1 ).
This search strategy was designed to comprehensively capture all original studies examining the associations between meteorological, climatological, ecological, or environmental change factors and the transmission dynamics, outbreaks, risks, or adaptations of WNV. By conducting systematic searches across key databases, supplemented by targeted topic strings, our strategy ensures reproducibility and effectively summarizes contemporary evidence illuminating the connections between WNV and climate amidst escalating changes.
Eligibility criteria
The criteria for including and excluding articles in our analysis are outlined in Table 1 . We examined literature since 2007 to capture research conducted after the IPCC Fourth Assessment Report’s release, representing a milestone driving expanded climate-health investigations [ 33 , 41 ]. Focusing on this period enhances relevance and rigor by concentrating on studies consciously examining climate-related impacts during intensifying change. Further augmenting stringency, we concentrated solely on original quantitative and qualitative investigations published in English-language peer-reviewed academic journals. Together these boundaries help systematically extract recent high-quality evidence elucidating shifting WNV transmission dynamics amidst climate change while delineating adaptations instituted since an authoritative global assessment.
Screening and study selection
We used the systematic review software NoteExpress 3.8.0.9455 (Beijing Aegean Sea Software Company, Beijing, China) to implement standardized screening and selection procedures. Two independent reviewers carried out an initial screening of titles and abstracts to filter articles that met basic eligibility criteria, with a third reviewer resolving any discrepancies. Subsequently, these two reviewers conducted full-text evaluations of the retained articles to ensure compliance with all inclusion criteria as outlined in the predefined protocol. Any disputes again triggered third-reviewer arbitration to achieve consensus.
Data extraction
We used a predefined covidence data extraction framework to systematically characterize key article features including 1) identifiers like title, author(s), and year; 2) specific objectives, study populations, WNV research priority (primary/secondary), and geographic focus; and 3) findings of the paper, such as nature of the evidence for climate change impacts on disease emergence, transmission or spread and/or policy responses, interventions or adaptations [ 42 , 43 ].
We categorized the geographic focus of articles into six regions: North America, South America, Europe, Africa, Asia, and Oceania, with multi-regional studies classified as global. The study populations analyzed included humans, mosquitoes, birds, and horses. Investigations encompassing more than one species were labeled as ‘multiple species’, and studies that did not specify their focus were marked as ‘unspecified’. The central disease under investigation in all articles was WNV. Articles primarily focused on WNV dynamics were categorized under ‘primary’ interest level, while those analyzing WNV in conjunction with other vector-borne diseases were deemed of ‘secondary’ interest.
The findings of the paper regarding evidence or arguments presented on the impacts of climate change (including extreme weather, rising temperatures, and/or climate variability) on WNV emergence, transmission, or spread were recorded. To clearly understand the impacts of climate change on WNV, articles were grouped into two main categories: 1) climate-WNV associations, and 2) climate change impacts on WNV, as categorized by Kulkarni et al. in their study of the impact of climate change on global malaria and dengue fever [ 38 ]. The articles defined as climate-WNV associations mainly refer to the impacts of climatic and seasonal factors (e.g. temperature, precipitation, and seasonal variations) on WNV transmission and spread within a certain time frame. Articles defined as climate change impacts on WNV are further categorized into two types: those with clear evidence of climate change or climate anomalies during the study period affecting WNV transmission and spread, and those with projections of future WNV transmission and spread under climate change scenarios.
The findings of the paper pertaining to evidence for policy responses, interventions, or adaptive measures addressing the impacts of climate change on disease emergence, transmission, or spread were documented. Specifically, the nature of the evidence or arguments presented regarding policy measures, interventions, and/or adaptations to mitigate the effects of climate change on the emergence, propagation, or spread of WNV were recorded. The United Nations Environmental Program (UNEP) handbook on methodologies for assessing climate change impacts and adaptation strategies outlines a typology of adaptation measures to safeguard human health from climate change [ 44 ]. These encompassed five categories of measures: (1) surveillance and monitoring, (2) infrastructure development, (3) public education, (4) technology or engineering strategies, and (5) medical interventions. The content of the article on adaptation strategies is categorized according to the UNEP manual and in the context of the WNV case.
Quality assessment of included literature
The quality of the included articles was assessed using the Joanna Briggs Institute Prevalence Critical Appraisal Tool [ 45 ]. All selected studies were scored using the 10 quality control items suggested by the tool. A score of one was awarded for each item fulfilled while a zero score was awarded for each unmet item. Score aggregates were generated and studies were classified as either low (0–3), moderate (4–6), or high (7–10) quality.
Web development
Most reviews traditionally present evidence in a tabular format, which consumes a considerable portion of the article’s space and often hinders easy navigation through the key information [ 36 , 38 ]. In this study, we used the R Shiny interactive web application framework to develop an online-accessible website that presents evidence on the impact of climate change on WNV transmission and dissemination [ 46 ]. This website allows visitors to query and download information on the effects of climate change on WNV transmission and spread at any time and from any location. This method provides a novel way to access and understand the synthesized evidence in a clearer and more convenient manner.
Characteristics of included studies
Initially, 2168 articles were retrieved from four databases: Web of Science, PubMed, Scopus, and EBSCOhost. After removing 896 duplicates, 1272 articles remained (Fig. 1 ). Following title and abstract screening, 1068 articles were excluded as irrelevant, leaving 204 for full-text review. This resulted in 105 articles meeting inclusion criteria, focusing on the association between climate/weather and WNV or its transmission due to climate changes.
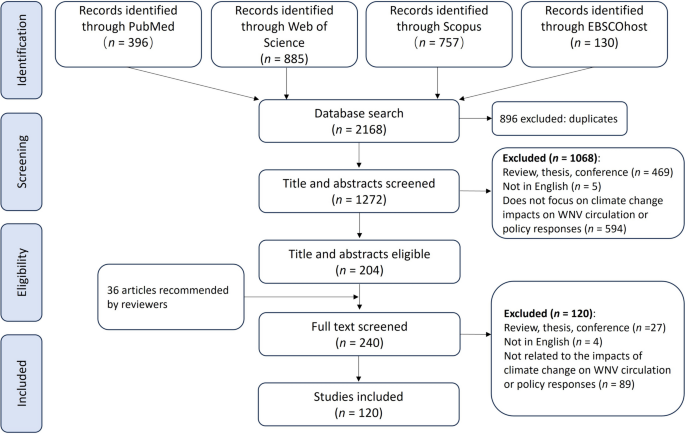
Flowchart diagram illustrating the article search and selection process
To comprehensively cover literature on the impact of climate change on WNV, we used specific search terms based on key themes from prior studies [ 37 , 38 ]. Although these terms helped in retrieving targeted and relevant literature, their specificity might have restricted the scope, possibly excluding significant studies that broader terms could have included. Hence, the reviewers recommended 36 relevant articles, which we screened and retained 15 articles according to the inclusion criteria.
The comprehensive review included 120 studies divided into two categories: 83 studies focused on the associations between climate/weather and WNV, and 37 studies examined the impacts of climate change on WNV transmission. All the reviewed evidence and related adaptation responses are available for exploration and download through a dedicated Shiny web application ( https://2xzl2o-neaop.shinyapps.io/WNVScopingReview/ ).
Publication year
The number of published studies on climate change and WNV has increased over time, with a sharp rise observed after 2013 (Fig. 2 ). Regarding the temporal distribution of relevant literature, two key observations can be made.
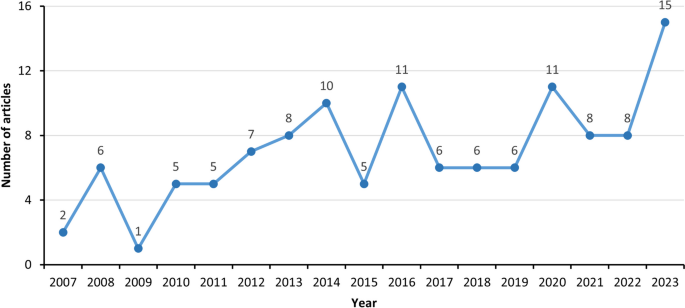
Distribution of the publication years in all articles included from 2007 to 2023
First, only 26 articles were published between 2007 and 2012, of which 21 articles focused on the associations between climate/weather [ 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 ] and 5 articles examined the impacts of climate change on WNV [ 25 , 68 , 69 , 70 , 71 ]. The earliest study on climate/weather factors and WNV, published in 2007, analyzed the association between precipitation and human WNV incidence in the US during 2002–2004. The first article on the impacts of climate change on WNV, published in 2007, investigated WNV prevalence in wild Greater Sage-Grouse populations across Montana and Wyoming during 2003–2005. The relatively small number of studies before 2013 indicates that relevant research was still in its infancy stage.
Second, most studies on this topic ( n = 94) emerged after 2013, corresponding to the release of the IPCC Fifth Assessment Report in 2014 and the Lancet Commission on Climate and Health in 2015 [ 34 , 35 ]. As authoritative reviews synthesizing the state-of-the-art science on anthropogenic climate change and its health consequences, these landmark reports have stimulated new research assessing climate impacts on infectious diseases like WNV.
Study location
The geographical distribution of study locations examined in the articles is shown in Fig. 3 . The most frequently studied region was North America, representing 59.2% of articles ( n = 71). Within North America, 53 articles focused on the US [ 25 , 27 , 28 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 68 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 ], 17 on Canada [ 31 , 32 , 63 , 64 , 69 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 ], and 1 covered the entire continent [ 22 ]. Europe was the second most studied region, accounting for 28.3% of articles ( n = 34) [ 23 , 26 , 65 , 70 , 71 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 ]. The other world regions assessed were Asia ( n = 4; 3.3%) [ 66 , 146 , 147 , 148 ], Africa ( n = 4; 3.3%) [ 67 , 149 , 150 , 151 ], South America ( n = 2; 1.7%) [ 24 , 152 ], and Oceania ( n = 1; 0.8%) [ 153 ]. Only 4 articles (3.3%) [ 51 , 154 , 155 , 156 ] included multiple global regions and were classified as the “global” studies.
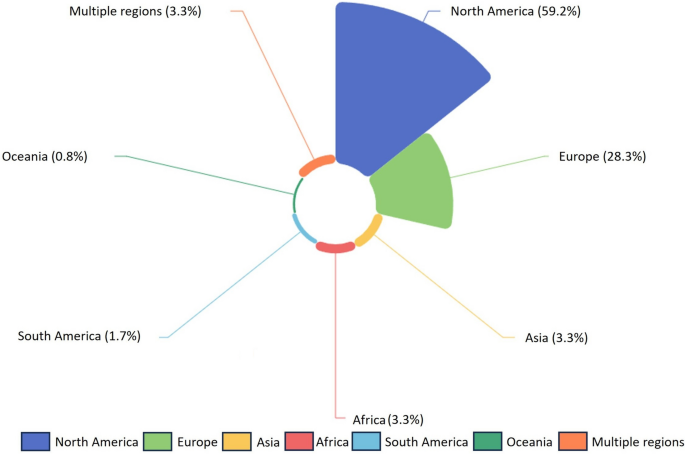
Geographical distribution of the study areas in all articles included from 2007 to 2023
Research on WNV has focused on two regions, North America and Europe, which corresponds to the high incidence and disease burden from epidemics reported in these two regions over the past two decades. In the US, between 2007 and 2022, there were 32,600 confirmed or suspected human WNV cases reported to the Centers for Disease Control and Prevention, particularly concentrated in California, Colorado, and Texas [ 157 ]. WNV remains the leading cause of mosquito-borne disease in the US, accounting for 83.0% of the reported cases in 2020 [ 158 ]. In Canada, since the virus's emergence in 2001, there have been over 5000 lab-confirmed human cases, with around 20.0% of patients experiencing neurological complications [ 159 , 160 ]. Additionally, it is estimated that up to 27,000 cases may have gone unreported, given the largely asymptomatic nature of WNV infection [ 160 ]. Similarly severe WNV outbreaks have hit Europe in recent years — its 2018 epidemic exceeded 1900 confirmed human cases, surpassing all previous years in scale and distribution [ 161 ]. The heavy health and economic toll has reasonably triggered intensive research interests in examining environmental risk factors such as climate change. Study interests and public health priorities understandably tend to align with acute epidemic events and tangible disease burden.
Research on WNV in regions like Asia, Africa, South America, and Oceania has been comparatively sparse. This imbalance may stem from various factors, such as a lower prioritization due to limited epidemiological data and clinical cases, often attributed to suboptimal surveillance systems. Additionally, the allocation of public health resources in these regions might be challenged by competing health issues, alongside barriers to conducting coordinated multi-national research. For example, in South America, inconsistencies between actual and reported WNV cases arise from symptomatic similarity with other arboviruses and limitations in differential laboratory diagnostics [ 24 ]. Moreover, mild and self-resolving cases may remain undocumented. Meanwhile, more severe cases can also be under-diagnosed, owing to a lack of accessible healthcare facilities and logistical constraints on sample transportation and testing [ 12 ].
Regional differences in climate, vector ecology, and host community characteristics contribute to variations in WNV transmission patterns and health impacts. For example, the primary vectors of WNV display distinct seasonality under varying climatic conditions [ 68 ]. Furthermore, viral strains may evolve different levels of pathogenicity in diverse host species and environmental settings [ 84 ]. Consequently, collaborative multi-regional research is essential to formulate prevention policies that are specifically tailored to different regions. Additionally, integrating knowledge and assessment tools is crucial to further understand the environmental and social factors driving WNV transmission.
Study population
The majority of research articles ( n = 103; 85.8%) focused exclusively on WNV, its vectors, or hosts. The remaining 17 articles (14.2%) examined WNV in conjunction with other mosquito-borne diseases, such as dengue fever and Rift Valley fever. The most studied subject was human WNV cases (Fig. 4 ), examined in 31.7% of articles ( n = 38) [ 23 , 27 , 47 , 48 , 52 , 53 , 54 , 56 , 58 , 59 , 62 , 66 , 71 , 73 , 78 , 82 , 83 , 90 , 96 , 98 , 102 , 109 , 118 , 122 , 124 , 125 , 126 , 128 , 130 , 131 , 132 , 133 , 134 , 135 , 140 , 143 , 145 , 148 ]. Mosquito vectors[ 49 , 55 , 57 , 61 , 65 , 68 , 69 , 72 , 74 , 80 , 84 , 87 , 88 , 91 , 92 , 93 , 95 , 97 , 101 , 103 , 105 , 107 , 108 , 110 , 111 , 116 , 121 , 127 , 129 , 136 , 139 , 144 , 150 , 152 , 153 , 155 ] and multi-species [ 22 , 26 , 28 , 51 , 60 , 64 , 67 , 70 , 75 , 76 , 77 , 79 , 81 , 86 , 94 , 99 , 100 , 106 , 117 , 119 , 123 , 141 , 142 , 146 ] were investigated in 36 (30.0%) and 24 (20.0%) studies, respectively. A smaller percentage of articles ( n = 10, 8.3%) failed to specify the study population [ 24 , 31 , 32 , 51 , 112 , 113 , 120 , 151 , 154 , 156 ]. Limited studies focused solely on bird hosts ( n = 7, 5.8%) [ 25 , 50 , 89 , 104 , 114 , 115 , 138 ] or equine hosts ( n = 5, 4.2%) [ 63 , 85 , 137 , 147 , 149 ].
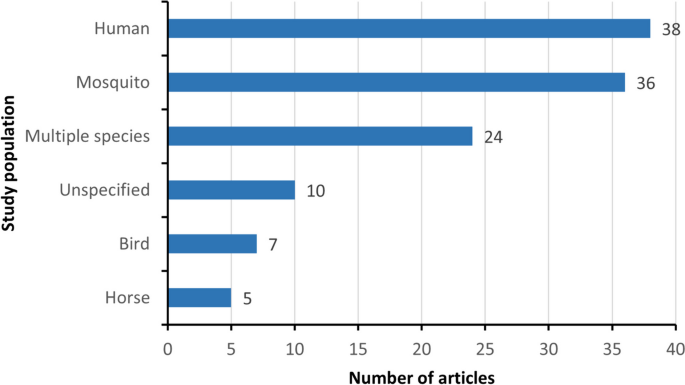
Distribution of the study populations in all articles included from 2007 to 2023
The majority of WNV research has focused on human infection. It's estimated that about 1 in 150 infected individuals develop a severe, long-lasting illness [ 162 ]. High incidence rates in humans have been linked to environmental factors such as extensive irrigated croplands and rural settings [ 54 ]. Mosquito vectors, particularly Culex species, play a crucial role in WNV transmission cycles, with their abundance influenced by factors like the urban heat island effect, the presence of water bodies, and the extent of irrigated farmland [ 54 , 129 ].
However, there is a significant gap in the number of animal-focused studies compared to human studies. In North America, over 28,000 equine cases of WNV have been reported since 1999 [ 163 ]. Additionally, in the US alone, the virus has impacted over 300 bird species, with estimated deaths in the millions [ 164 ]. Juvenile dispersing birds have been demonstrated to play a vital role in the long-distance dispersal and rapid spatial spread of introduced WNV strains across North America [ 165 ]. Given the importance of the role of animals in the transmission and evolution of WNV, there is a need to strengthen research on the impacts of climate change on the transmission and spread of WNV in animals.
Climate-WNV associations
Among the 83 articles examining climate/weather associations with WNV, temperature was the most studied factor ( n = 56, 67.5%) [ 28 , 47 , 49 , 51 , 52 , 54 , 55 , 58 , 59 , 60 , 61 , 63 , 64 , 66 , 72 , 75 , 76 , 79 , 80 , 81 , 82 , 84 , 86 , 87 , 88 , 89 , 92 , 93 , 94 , 98 , 105 , 107 , 108 , 109 , 110 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 132 , 134 , 137 , 138 , 139 , 147 , 150 ]. All these studies showed increased WNV transmission probabilities or cases within certain temperature ranges. Precipitation was assessed in 34 studies (41.0%), with 13 showing a positive correlation, 13 indicating a negative correlation, 7 revealing mixed positive/negative correlations, and 1 indicating no correlation with WNV risk [ 28 , 47 , 48 , 52 , 53 , 54 , 55 , 58 , 63 , 67 , 72 , 77 , 79 , 81 , 82 , 86 , 87 , 88 , 92 , 93 , 98 , 105 , 109 , 110 , 125 , 126 , 128 , 131 , 136 , 137 , 139 , 146 , 149 , 150 ]. Drought events and warmer winters were investigated less frequently, in 8 (9.7%) [ 28 , 56 , 72 , 74 , 85 , 119 , 126 , 146 ] and 5 (6.0%) [ 50 , 65 , 83 , 92 , 106 ] studies, respectively. Four articles (4.8%) showed a correlation between humidity and WNV risk, with 3 [ 47 , 80 , 91 ] showing a positive correlation and 1 [ 119 ] showing a negative correlation. Nine studies (10.8%) found links between WNV activity and climate-driven seasonal shifts [ 57 , 61 , 62 , 73 , 78 , 90 , 95 , 96 , 133 ], while 2 (2.4%) reported increased transmission associated with flooding events [ 135 , 153 ]. Three studies (3.6%) reported the correlation between WNV risk and winds/hurricanes [ 91 , 97 , 119 ].
Temperature and WNV
Ambient temperature is a critical driver influencing WNV transmission through direct and indirect impacts on vectors and hosts [ 49 , 71 ]. Specifically, higher temperatures accelerate viral replication and shorten the incubation period in mosquitoes, fuel vector population growth, increase transmission efficiency, and expand vector habitat suitability [ 49 ]. In Israel, positive temperature anomalies were linked to greater mosquito abundance and ensuing human cases [ 66 ]. Similarly in Canada, higher mean temperatures are associated with increased Culex populations and elevated WNV infections [ 129 ]. Moreover, there is a trend towards increased risk around large metropolitan areas characterized by urban heat islands, for example in the United Kingdom [ 129 ]. Phenomena such as warm winters and hot summers due to increased temperatures have also contributed to the rise in WNV infection rates [ 50 , 65 , 83 , 106 ]. The mean temperature is a strong predictor of the presence of WNV in Culex mosquitoes, and this relationship is unimodal [ 76 ]. The optimal temperature range for WNV transmission is identified as 22.7–30.2 °C [ 75 ]. Outside this range, particularly at temperatures below 17.0 °C, vector competence significantly declines, reaching a relative risk near zero [ 76 ]. It is important to note that this lower temperature threshold can vary among different vector species. Moreover, extreme heat events may further amplify outbreak magnitude [ 65 , 66 ]. However, it is important to note that an increase in temperature does not necessarily mean an increase in disease incidence altogether. For example, temperatures above 30 °C reduced survival of Culex tarsalis and slowed the growth of WNV in Culex mosquito [ 166 ].
In addition, ambient temperature rise under climate change may indirectly alter WNV transmission by shifting bird host ecology and associated vector exposures. Models project warming could expand bird infection prevalence to higher latitudes as longer activity seasons enable more transmission events [ 114 ]. While temperature alone allows increased vector habitat suitability and viral replication at mid-range optima, cascading impacts on avian immunity, migration timing, vector-host overlaps, and habitat ranges could potentially override direct effects. For instance, warmer climates have prompted earlier nesting in British birds, potentially leading to offspring hatching during peak mosquito seasons, increasing young birds’ exposure to vectors [ 167 ]. This phenomenon is exemplified in Caillouët et al.’s study, which demonstrates how the end of the nesting season aligns with higher mosquito populations, potentially escalating WNV transmission risks during these periods [ 168 ].
Precipitation and WNV
The positive correlation between elevated rainfall pre-outbreak and intensified WNV vector abundance/infection has been well documented [ 47 , 125 , 126 ]. For example, a 10 cm rise in summer precipitation was associated with 0.39 more WNV-positive Culex mosquitoes per 1000 tested in South Africa [ 67 ]. In the US, every 1 cm precipitation increase was linked to a 15% greater WNV incidence [ 86 ]. In Australia and Srpska, flooding due to extreme precipitation events creates favorable conditions for WNV transmission, as waterlogged environments can support larger populations of waterbirds and mosquitoes, increasing the likelihood of virus spread [ 135 , 153 ]. However, precipitation effects on WNV vary across regions and timescales, likely due to place-based differences in viral strain, vector, and host ecology. For example, a negative correlation between total monthly precipitation and the number of WNV cases was observed in Europe [ 128 ]. Similarly, in years of increased human WNV incidence in Israel, there was a significant decrease in spring precipitation [ 146 ]. In America, extreme drought caused by extremely low precipitation is a potential amplifier of WNV virus transmission and can further increase the risk of WNV transmission [ 56 , 85 ]. The cause of this phenomenon may be related to the fact that below-average precipitation creates limited water resources for mosquitoes, thereby increasing close contact between hosts and infected mosquitoes at remaining water sources [ 169 ]. In addition, both positive and negative correlations of precipitation on WNV incidence have been observed in the eastern and western parts of the US at different time scales [ 53 , 82 ].
Humidity, wind speed and WNV
Humidity and wind speed play important and complex roles in WNV transmission dynamics, but the impacts vary widely across ecosystems. For instance, higher humidity increased the probability of human infection with WNV in the US [ 47 ], and positive correlations were found between soil moisture and vector indices [ 80 ]. However, a Greek study conversely found negative relative humidity-WNV case correlations [ 119 ]. A study in New York and Connecticut showed an inverse U-shaped relationship between soil moisture and WNV-infected mosquitoes, with high infection associated with drought, but also an increase associated with wetter conditions—both patterns can be present at the same time [ 27 ]. Meanwhile, wind may impact disease transmission by influencing mosquito movement. For example, low wind speeds were found to be associated with the capture of WNV-infected mosquitoes during the same week that human cases of WNV emerged in Greece [ 119 ]. This may be related to the fact that high wind speeds reduce the chances of a mosquito blood meal, thus reducing the chances of human WNV cases [ 119 ]. Additional hypotheses, including storm roles in bird migration contributing to WNV transmission [ 170 ], require further investigation.
Climate-driven seasonal shifts and WNV
Climate-driven seasonal shifts are also important factors influencing WNV spread and outbreak magnitudes. For example, Texan counties experience major spikes following wet springs and hot, dry summers [ 73 ]. In Suffolk County, warm and dry conditions in early spring have been shown to increase WNV infection in Culex mosquitoes [ 74 ]. Patterns of dry, hot temperatures following wet years also increase WNV infections [ 78 ]. Broader European analyses suggest that anomalous seasonal temperatures and dry winters exacerbate seasonal amplification and drive WNV outbreaks [ 133 ]. These climate-mediated seasonal effects likely arise through multiple mechanisms affecting vector reproduction, host immunity, viral replication rates, and transmission efficiency at different phases [ 170 ]. As climate change intensifies precipitation variability and seasonal temperature extremes, such seasonal shift tipping points may become more frequent. Therefore, improved surveillance programs that are responsive to emerging seasonal shifts remain essential for predicting and mitigating transmission at fine geographic and temporal scales.
While climatic factors have a significant impact on the spread and transmission of WNV, many other factors also influence the complexity of the transmission dynamics. Land use, global trade, bird migration patterns, landscape features, and socioeconomics also partially determine the geographic distribution of infections [ 50 , 80 , 86 ]. For example, areas with older infrastructure, lower incomes, high percentages of cropland, and large rural populations have more landscape features and environmental conditions favorable to vector habitat, which increases local WNV risk [ 54 , 80 ]. Therefore, operationalizing the “One Health” paradigm through collaborative surveillance, modeling, and mitigation across veterinary, human, wildlife and environmental health remains imperative for fully anticipating and responding to shifting WNV.
Climate change impacts on WNV
Among the 37 articles examining climate change impacts on WNV, the majority ( n = 28; 75.7%) predicted the impact of climate change on WNV [ 22 , 23 , 24 , 27 , 31 , 32 , 69 , 70 , 99 , 100 , 101 , 102 , 103 , 104 , 111 , 112 , 113 , 114 , 115 , 116 , 141 , 142 , 143 , 145 , 151 , 152 , 155 , 156 ]. Specifically, high latitude regions, areas with immunocompromised populations, locations prone to extreme weather events, and marginalized communities were expected to be more affected [ 22 , 24 , 103 ]. Additionally, 8 articles (21.6%) provided substantial evidence that climatic variability phenomena have already affected the transmission and distribution of WNV during recent outbreaks [ 25 , 26 , 68 , 71 , 140 , 144 , 148 , 154 ]. Only 1 article (2.7%) focused on developing a national indicator framework for monitoring climate change impacts on infectious diseases [ 51 ].
Evidence of future climate change impacts on WNV
In the review, most evidence predicts that future climate change may affect the spread and distribution of WNV [ 22 , 23 , 24 , 111 , 114 , 151 , 155 , 156 ]. In North America, the projected climatic suitability range for WNV in 2050 and 2080 is expected to expand northward and into high-altitude areas, potentially leading to infections in novel and native hosts [ 22 ]. In Europe, studies project heightened WNV infection rates and new endemic areas under future climate scenarios, particularly at the margin of current transmission zones (e.g., eastern Croatia, northeastern and northwestern Turkey) [ 23 ]. Notably, recent evidence also confirms local transmissions as far north as Germany and the Netherlands, indicating an expansion of risk areas beyond those previously identified [ 10 , 11 ]. In South America, high-risk areas for WNV may shift between 2046–2065 and 2081–2100, becoming more pronounced under high greenhouse gas emission scenarios, potentially altering the current WNV distribution in some countries (e.g. parts of Bolivia, Paraguay, and Brazil) [ 24 ]. In Morocco, the suitable habitat range for Cx. pipiens is projected to expand into new central and southeastern areas by 2050, increasing the risk of WNV transmission [ 151 ].
Current evidence of climate change impacts on WNV
In addition to predictive studies on the future, existing evidence also demonstrates that climatic variability phenomena have already affected the transmission and distribution of WNV in some regions [ 25 , 26 , 144 , 148 ]. In the Powder River Basin of Montana and Wyoming in the US, WNV-related mortality rates in bird populations were significantly higher in 2003, the sixth warmest summer on record, than in 2004 and 2005, the 86th and 41st warmest, respectively [ 25 ]. Although this increase in mortality coincided with higher temperatures, it is crucial to consider that 2003 also marked a period of the virus’s initial introduction into the region. This introduction likely contributed significantly to the observed mortality rates, as populations are often most vulnerable when a pathogen first emerges. In Germany, the extreme heat of the summer of 2018 (the second hottest and most arid summer on record locally) was speculated to be an important reason for the decreased mean extrinsic incubation period values in mosquitoes, leading to rapid viral amplification and increased risk of transmission to vertebrate hosts [ 26 ]. Additionally, the detection of WNV-infected Uranotaenia unguiculata in northern Germany in 2016 presents another case of climate change driving the northward spread of mosquito species and WNV [ 144 ]. In Israel, an intense heat wave and a spike in summer temperatures were observed during WNV outbreaks [ 148 ].
The extent of climate change impacts on WNV transmission depends on local regional conditions, including population immunity levels and vector abundance [ 99 , 101 , 103 ]. In areas where comprehensive vaccination programs for animals susceptible to WNV, such as horses, are in place, alongside robust public health infrastructure and strong vector monitoring and control systems, the impact of WNV may be significantly mitigated or even negligible [ 103 ]. For example, predictions for the island scrub-jay in California showed that vaccinating ≥ 60 individuals during WNV outbreaks could decrease the risk from ≥ 22% to ≤ 5% [ 104 ]. Undoubtedly, strengthening broad-spectrum socioecological resilience through surveillance, preparedness, vector management, and medical capacity building remains paramount for sustainable health amidst climate and global change [ 101 ]. However, these anthropogenic measures require considerable regional coordination and resource mobilization, frequently lacking in disproportionately impacted communities. Therefore, actualizing equitable and adaptive WNV resilience necessitates comprehensively integrating climatological, environmental, veterinary, wildlife, genetic, immunological, and public health data into prediction frameworks and response protocols prioritizing vulnerable populations. International organizations must lead in facilitating such collaborative resilience measures globally.
Adaptation strategies to address climate-driven WNV transmission and spread
Among all 120 reviewed articles, 49 proposed or discussed adaptive strategies against WNV risks in response to climate change. These measures were categorized into six groups based on UNEP criteria and the case of WNV (Fig. 5 ) [ 44 ]: surveillance and monitoring ( n = 19; 38.8%) [ 22 , 23 , 56 , 65 , 69 , 75 , 85 , 92 , 99 , 100 , 101 , 106 , 117 , 120 , 123 , 135 , 148 , 151 , 152 ]; predictive models ( n = 9; 18.4%) [ 49 , 70 , 74 , 81 , 84 , 98 , 103 , 105 , 111 ]; cross-disciplinary/border cooperation ( n = 8; 16.3%) [ 24 , 51 , 80 , 126 , 131 , 133 , 141 , 156 ]; environmental management ( n = 6; 12.2%) [ 25 , 87 , 95 , 104 , 142 , 145 ]; health system preparation ( n = 4; 8.2%) [ 27 , 57 , 102 , 121 ]; and public education ( n = 3; 6.1%) [ 86 , 113 , 118 ]. A brief overview table of identified adaptation strategies is provided (Additional file 2 ), with details accessible on the project website under “Detailed adaptation strategies”.
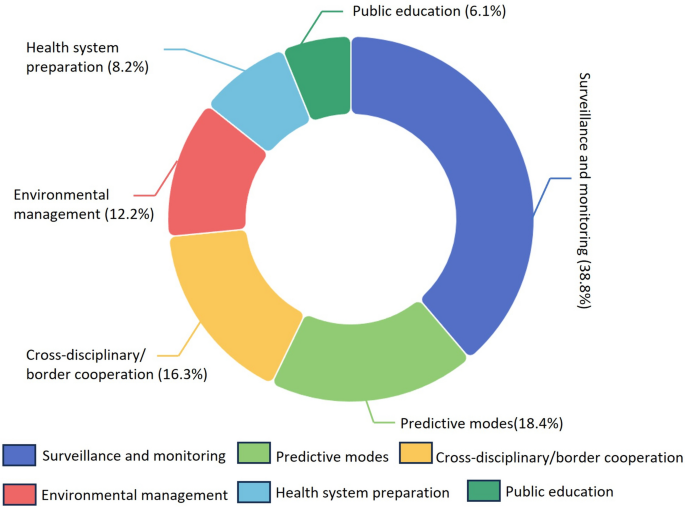
Classification of 49 articles that proposed or discussed adaptive strategies against West Nile virus risks in response to climate change, divided into six categories based on United Nations Environmental Program criteria
Monitoring and surveillance
Most studies reviewed highlight that monitoring and surveillance are the most critical means of preventing and controlling the spread of WNV under climate change scenarios. Specifically, surveillance should concentrate on high-risk populations, vector populations, wildlife and domestic animals, migrating birds, and neglected areas. As Skaff et al. noted, identifying consistencies between highly susceptible communities and local climates approaching critical thermal thresholds can enhance infectious disease prevention efficacy amidst climate change [ 75 ]. Additionally, Semenza et al. recommended fortifying epidemiological monitoring for neuroinvasive diseases potentially indicative of WNV to expand healthcare provider awareness of clinical manifestations and strengthen diagnostic testing capabilities [ 23 ]. They also advised augmenting blood donation screening and transportation safeguards while at the same time accounting for climate change in formulating robust WNV contamination prevention protocols [ 23 ]. Moreover, numerous studies have suggested that a more granular analysis of meteorological and entomological factors could improve comprehension of intricate WNV transmission dynamics [ 31 , 56 , 65 , 101 , 106 , 151 , 152 ]. Concurrently, research and control programs must localize to maximize relevance for regional climate change impacts [ 101 ]. Furthermore, public health agencies and vector control teams should amplify efforts to continuously track distributions to minimize human infection risks [ 151 , 152 ]. Meanwhile, WNV surveillance systems should be strengthened with host monitoring and regular risk assessment, especially for rural livestock, long-distance migratory birds, and wildlife with high mobility [ 117 , 123 ]. Domestic livestock, particularly horses in high-risk areas, should be vaccinated to enhance their immunity and prevent mortality and morbidity [ 85 ]. Routine surveillance should also be conducted in neglected areas (e.g., areas thought not to be transmitted zones and poor areas) [ 22 ]. Based on the results of data analysis from surveillance and monitoring, preventive and control strategies need to be adjusted accordingly to cope with changing infectious diseases.
Predictive models
Beyond intensifying surveillance, advancing predictive models and early warning systems remain vital for honing outbreak preparedness and rapid response. Sophisticated predictive tools enabling localized risk projections and efficient resource allocation can dramatically amplify intervention impact [ 38 ]. Ideally, such systems would synthesize meteorological, biological, genetic, ecological, entomological, and epidemiological data for accurate emergence prediction across scales [ 49 , 70 , 74 , 81 , 84 , 103 , 105 , 111 ]. Developing predictive models by linking laboratory-observed environmental transmission patterns to actual transmission patterns is crucial to accurately predicting the impact of climate change on WNV and other vector-borne pathogens [ 49 ]. Most importantly, next-generation frameworks must address substantial knowledge gaps around viral evolution, vector-host mutations, species migration and adaptation capacity, infection-recovery dynamics, and anthropogenic environmental change impacts on virus shifting dynamics [ 70 , 103 , 111 ]. Advancing models encompassing this intrinsic biocomplexity and policy-environment feedback remains essential to preempt unprecedented post-climate change outbreaks through context-specific preparation and response. International alliances should prioritize pioneering these innovations in prediction science alongside flexible surveillance strengthening for integrated epidemic resilience. Beyond informing ongoing emergence, these efforts will uncover complex ecological interconnectivity in the face of convergence across climate and global changes.
Cross-disciplinary/border cooperation
As climate change accelerates, advanced WNV prevention and control requires integrating “One Health” approaches across human, veterinary, wildlife, and environmental health sectors. Multidisciplinary collaboration enables the holistic elucidation of shifting transmission dynamics for accurate risk prediction, alert activation, and adaptive response [ 126 , 133 ]. Specifically, increased data sharing between public health, vector control, and meteorological agencies, coupled with artificial intelligence integration, can exponentially improve monitoring sensitivity, early warning trigger development, and outbreak interception agility [ 24 , 80 ]. Additionally, transregional information exchange and coordination remain imperative for refining control strategies and resource allocation amidst climate and global change [ 156 ]. The 2018 European WNV emergency exemplified the superiority of integrated “One Health” surveillance, ensuring targeted data-driven countermeasures, bridging counties halted uncontrolled cross-border transmission [ 141 ]. Given the existential threat of vector-borne diseases necessitates all governmental and international institutions prioritizing and operationalizing such interdisciplinary preparedness and response architectures. This obligation will grow increasingly urgent as environments continue transforming unprecedentedly.
Other adaptive strategies
Of all the studies reviewed, there are fewer strategies related to public education, environmental management, and health system preparedness. However, adapting to the growing threat of WNV under climate change will require multifaceted strategies across environmental management, public awareness-raising, and health system preparedness. Effective environmental management to suppress vector populations, including the elimination of mosquito breeding grounds and the establishment of secondary conserved populations for possible vaccination, forms a crucial first line of defense against WNV [ 25 , 104 , 142 ]. However, this must be coupled with sustained public education campaigns to promote protective behaviors among individuals and vigilant surveillance efforts to enable early response [ 86 , 113 , 118 ]. Finally, health systems must enhance their capacity for detecting WNV outbreaks in vectors and hosts, allowing timely intervention measures, as well as boosting clinical diagnosis and treatment capacity [ 102 , 121 ].
Future work
While this review concentrated on the climatic aspects of WNV transmission, it sets the stage for subsequent in-depth analyses of adaptation strategies within the public health domain. Future studies could adopt a One Health approach or leverage the UNEP framework to explore diverse responses to WNV, thereby enriching the dialogue between climate science and public health policy.
Climate change may affect the transmission and distribution of WNV, with the extent of the impact depending on local and regional conditions. Surveillance and monitoring stand out as the most recommended adaptation tactics to address the spread of WNV under climate change scenarios. However, far fewer studies have explicitly focused on adaptation strategies than have investigated the impacts of climate change. Further research on the impacts of climate change and adaptation strategies for vector-borne diseases, as well as more comprehensive evidence synthesis, are needed to inform effective policy responses tailored to local contexts.
Our findings highlight the significant role of climate factors in the transmission dynamics of WNV. However, acknowledging the limitations of our focus, we propose future research to extensively explore adaptation strategies that address these climatic challenges. Such efforts would provide comprehensive insights that are crucial for the development of robust public health policies.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- West Nile virus
Intergovernmental Panel on Climate Change
United Nations Environmental Program
Kramer LD, Ciota AT, Kilpatrick AM. Introduction, spread, and establishment of West Nile virus in the Americas. J Med Entomol. 2019;56(6):1448–55.
Article PubMed PubMed Central Google Scholar
Bernkopf H, Levine S, Nerson R. Isolation of West Nile virus in Israel. Infect Dis. 1953;93:207–18.
Article CAS Google Scholar
Murgue B, Zeller H, Deubel V. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. Curr Top Microbiol Immunol. 2002;267:195–221.
CAS PubMed Google Scholar
Johnson N, de FernándezMarco M, Giovannini A, et al. Emerging mosquito-borne threats and the response from European and Eastern Mediterranean countries. Int J Environ Res Public Health. 2018;15(12):2775.
Nash D, Mostashari F, Fine A, Miller J, O’leary D, Murray K, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–14.
Article CAS PubMed Google Scholar
Petersen LR, Hayes EB. West Nile virus in the Americas. Med Clin North Am. 2008;92:1307–22.
Article PubMed Google Scholar
Lindsey NP, Staples JE, Lehman JA, Fischer M. Surveillance for human West Nile virus disease-United States, 1999–2008. MMWR Surveill Summ. 2010;59:1–17.
PubMed Google Scholar
Haussig JM, Young JJ, Gossner CM, Mezei E, Bella A, Sirbu A, et al. Early start of the West Nile fever transmission season 2018 in Europe. Euro Surveill. 2018;23(32):1800428.
Camp JV, Nowotny N. The knowns and unknowns of West Nile virus in Europe: what did we learn from the 2018 outbreak? Expert Rev Anti-Infect Ther. 2020;18(2):145–54.
Pietsch C, Michalski D, Münch J, Petros S, Bergs S, Trawinski H, et al. Autochthonous West Nile virus infection outbreak in humans, Leipzig, Germany, August to September 2020. Euro Surveill. 2020;25(46):2001786.
Article CAS PubMed PubMed Central Google Scholar
Vlaskamp DRM, Thijsen SFT, Reimerink J, Hilkens P, Bouvy WH, Bantjes SE, et al. First autochthonous human West Nile virus infections in the Netherlands, July to August 2020. Euro Surveill. 2020;25(46):2001904.
Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81.
Kilpatrick AM, Ladeau SL, Marra PP. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk. 2007;124(4):1121–36.
Article Google Scholar
Gómez A, Kilpatrick AM, Kramer LD, Dupuis AP, Maffei JG, Goetz SJ, et al. Land use and West Nile virus seroprevalence in wild mammals. Emerg Infect Dis. 2008;14(6):962.
David S, Abraham AM. Epidemiological and clinical aspects on West Nile virus, a globally emerging pathogen. Infect Dis. 2016;48:571–86.
Centers for Disease Control. West Nile virus - statistics & maps in 2018. https://www.cdc.gov/westnile/statsmaps/index.html . Accessed 13 June 2023.
Ciota AT. West Nile virus and its vectors. Curr Opin Insect Sci. 2017;22:28–36.
Kilpatrick AM. Globalization, land use, and the invasion of West Nile virus. Science. 2011;334(6054):323–7.
Jia Y, Moudy RM, Dupuis AP II, Ngo KA, Maffei JG, Jerzak GV, et al. Characterization of a small plaque variant of West Nile virus isolated in New York in 2000. Virology. 2007;367:339–47.
Kunkel KE, Novak RJ, Lampman RL, Gu W. Modeling the impact of variable climatic factors on the crossover of Culex restauns and Culex pipiens (Diptera: Culicidae), vectors of West Nile virus in Illinois. Am J Trop Med Hyg. 2006;74:16–173.
Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Beagley J, Belesova K, et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. Lancet. 2021;397(10269):129–70.
Harrigan RJ, Thomassen HA, Buermann W, Smith T. A continental risk assessment of West Nile virus under climate change. Global Change Biol. 2014;20(8):2417–25.
Semenza JC, Tran A, Espinosa L, Sudre B, Domanovic D, Paz S. Climate change projections of West Nile virus infections in Europe: implications for blood safety practices. Environ Health. 2016;15(1):125–36.
Google Scholar
Lorenz C, de Azevedo TS, Chiaravalloti-Neto F. Impact of climate change on West Nile virus distribution in South America. Trans R Soc Trop Med Hyg. 2022;116(11):1043–53.
Walker BL, Naugle DE, Doherty KE, Cornish TE. West Nile virus and greater sage-grouse: estimating infection rate in a wild bird population. Avian Dis. 2007;51(3):691–6.
Ziegler U, Lühken R, Keller M, Cadar D, van der Grinten E, Michel F, et al. West Nile virus epizootic in Germany, 2018. Antivir Res. 2019;162:39–43.
Keyel AC. Patterns of West Nile virus in the Northeastern United States using negative binomial and mechanistic trait-based models. GeoHealth. 2023;7(4):e2022GH000747.
Keyel AC, Elison Timm O, Backenson PB, Prussing C, Quinones S, McDonough KA, et al. Seasonal temperatures and hydrological conditions improve the prediction of West Nile virus infection rates in Culex mosquitoes and human case counts in New York and Connecticut. Plos One. 2019;14(6): e0217854.
Chersich MF, Wright CY. Climate change adaptation in South Africa: a case study on the role of the health sector. Global Health. 2019;15:1–16.
Bardosh KL, Ryan S, Ebi K, Welburn S, Singer B. Addressing vulnerability, building resilience: community-based adaptation to vector-borne diseases in the context of global change. Infect Dis Pover. 2017;6(1):166.
Cox R, Sanchez J, Revie CW. Multi-criteria decision analysis tools for prioritising emerging or re-emerging infectious diseases associated with climate change in Canada. Plos One. 2013;8(8): e68338.
Hongoh V, Michel P, Gosselin P, Samoura K, Ravel A, Campagna C, et al. Multi-stakeholder decision aid for improved prioritization of the public health impact of climate sensitive infectious diseases. Int J Environ Res Public Health. 2016;13(4):419.
Pachauri RK, Reisinger A. Climate Change 2007: synthesis Report. Contribution of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. Geneva, Switzerland: IPCC; 2007. p. 104.
Pachauri RK, Allen MR, Barros VR, Broome J, Cramer W, Christ R, Climate change, et al. Synthesis Report, Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC. 2014;2014:151.
Watts N, Adger WN, Agnolucci P. Health and climate change: policy responses to protect public health. Lancet. 2015;386(10006):1861–914.
Eder M, Cortes F, de SiqueiraFilhaTeixeira N, de Franca Araújo GV, Degroote S, Braga C, et al. Scoping review on vector-borne diseases in urban areas: transmission dynamics, vectorial capacity and co-infection. Infect Dis Poverty. 2018;7(1):1–24.
Orr M, Inoue Y, Seymour R, Dingle G. Impacts of climate change on organized sport: a scoping review. WIREs Clim Change. 2022;13(3): e760.
Kulkarni MA, Duguay C, Ost K. Charting the evidence for climate change impacts on the global spread of malaria and dengue and adaptive responses: a scoping review of reviews. Global Health. 2022;18(1):1–18.
Schultz A, Goertzen L, Rothney J, Wener P, Enns J, Halas G, et al. A scoping approach to systematically review published reviews: adaptations and recommendations. Res Synth Methods. 2018;9(1):116–23.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73.
Sweileh WM. Bibliometric analysis of peer-reviewed literature on climate change and human health with an emphasis on infectious diseases. Glob Health. 2020;16(1):44.
Masson-Delmotte VP, Zhai P, Pirani SL, Connors C, Péan S, Berger N, et al. IPCC. Summary for Policymakers. In: Climate change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2021.
IPCC. An IPCC Special Report on the impacts of global warming of 1.5°C above preindustrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. https://pure.iiasa.ac.at/id/eprint/15716/1/SR15_TS_High_Res.pdf . Accessed 29 Jan 2019.
Feenstra JF, Burton I, Smith JB, Tol RS. Handbook on Methods for Climate Change Impact Assessment and Adaptation Strategies. Amsterdam: Vrije University; 1998.
Isaiah PM, Sólveig Palmeirim M, Steinmann P. Epidemiology of pediatric schistosomiasis in hard-to-reach areas and populations: a scoping review. Infect Dis Poverty. 2023;12(1):37.
Wang H, Guo T, Wang Z, Xiao J, Gao L, Gao X, et al. PreCowKetosis: A Shiny web application for predicting the risk of ketosis in dairy cows using prenatal indicators. Comput Electron Agr. 2023;206: 107697.
Soverow JE, Wellenius GA, Fisman DN, Mittleman MA. Infectious disease in a warming world: how weather influenced West Nile virus in the United States (2001–2005). Environ Health Persp. 2009;117(7):1049–52.
Wang G, Minnis RB, Belant JL, Wax CL. Dry weather induces outbreaks of human West Nile virus infections. BMC Infect Dis. 2010;10:38–38.
Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. Plos Pathog. 2008;4(6):e1000092.
LaDeau SL, Calder CA, Doran PJ, Marra PP. West Nile virus impacts in American crow populations are associated with human land use and climate. Ecol Res. 2011;26:909–16.
Liu H, Weng Q. Environmental factors and risk areas of West Nile Virus in southern California, 2007–2009. Environ Model Assess. 2012;17:441–52.
Walsh MG. The role of hydrogeography and climate in the landscape epidemiology of West Nile virus in New York State from 2000 to 2010. Plos One. 2012;7(2): e30620.
Landesman WJ, Allan BF, Langerhans RB, Knight TM, Chase JM. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector-Borne Zoonot. 2007;7(3):337–43.
Wimberly MC, Hildreth MB, Boyte SP, Lindquist E, Kightlinger L. Ecological niche of the 2003 West Nile virus epidemic in the northern Great Plains of the United States. Plos One. 2008;3(12): e3744.
Deichmeister JM, Telang A. Abundance of West Nile virus mosquito vectors in relation to climate and landscape variables. J Vector Ecol. 2011;36(1):75–85.
DeGroote JP, Sugumaran R, Brend SM, Tucker BJ, Bartholomay LC. Landscape, demographic, entomological, and climatic associations with human disease incidence of West Nile virus in the state of Iowa, USA. Int J Health Geogr. 2008;7(1):1–16.
Shaman J, Harding K, Campbell SR. Meteorological and hydrological influences on the spatial and temporal prevalence of West Nile virus in Culex mosquitoes, Suffolk County. New York J Med Entomol. 2011;48(4):867–75.
Winters AM, Eisen RJ, Lozano-Fuentes S, Moore CG, Pape WJ, Eisen L. Predictive spatial models for risk of West Nile virus exposure in eastern and western Colorado. Am J Trop Med Hyg. 2008;79(4):581.
Chuang TW, Wimberly MC. Remote sensing of climatic anomalies and West Nile virus incidence in the northern Great Plains of the United States. Plos One. 2012;7(10): e46882.
Hartley DM, Barker CM, Le Menach A, Niu T, Gaff HD, Reisen WK. Effects of temperature on emergence and seasonality of West Nile virus in California. Am J Trop Med Hyg. 2012;86(5):884.
Ruiz MO, Chaves LF, Hamer GL, Sun T, Brown WM, Walker ED, et al. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasites Vector. 2010;3(1):1–16.
Shaman J, Day JF, Komar N. Hydrologic conditions describe West Nile virus risk in Colorado. Int J Environ Res Public He. 2010;7(2):494–508.
Epp TY, Waldner C, Berke O. Predictive risk mapping of West Nile virus (WNV) infection in Saskatchewan horses. Can J Vet Res. 2011;75(3):161–70.
PubMed PubMed Central Google Scholar
Roth D, Henry B, Mak S, Fraser M, Taylor M, Li M, et al. West Nile virus range expansion into British Columbia. Emerg Infect Dis. 2010;16(8):1251.
Platonov AE, Tolpin VA, Gridneva KA, Titkov AV, Platonova OV, Kolyasnikova NM, et al. The incidence of West Nile disease in Russia in relation to climatic and environmental factors. Int J Environ Res Public Health. 2014;11(2):1211–32.
Paz S, Albersheim I. Influence of warming tendency on Culex pipiens population abundance and on the probability of West Nile fever outbreaks (Israeli case study:2001–2005). EcoHealth. 2008;5:40–8.
Uejio CK, Kemp A, Comrie AC. Climatic controls on West Nile virus and Sindbis virus transmission and outbreaks in South Africa. Vector-Borne Zoonot. 2012;12(2):117–25.
Chaves LF, Hamer GL, Walker ED, Brown WM, Ruiz MO, Kitron UD. Climatic variability and landscape heterogeneity impact urban mosquito diversity and vector abundance and infection. Ecosphere. 2011;2(6):1–21.
Hongoh V, Berrang-Ford L, Scott ME, Lindsay LR. Expanding geographical distribution of the mosquito, Culex pipiens , in Canada under climate change. Appl Geogr. 2012;33:53–62.
Gale P, Brouwer A, Ramnial V, Kelly L, Kosmider R, Fooks AR, et al. Assessing the impact of climate change on vector-borne viruses in the EU through the elicitation of expert opinion. Epidemiol Infect. 2010;138(2):214–25.
Paz S, Malkinson D, Green MS, Tsioni G, Papa A, Danis K, et al. Permissive summer temperatures of the 2010 European West Nile fever upsurge. Plos One. 2013;8(2): e56398.
Johnson BJ, Sukhdeo MVK. Drought-induced amplification of local and regional West Nile virus infection rates in New Jersey. J Med Entomol. 2013;50(1):195–204.
Ukawuba I, Shaman J. Association of spring-summer hydrology and meteorology with human West Nile virus infection in West Texas, USA, 2002–2016. Parasites Vector. 2018;11:1–15.
Little E, Campbell SR, Shaman J. Development and validation of a climate-based ensemble prediction model for West Nile virus infection rates in Culex mosquitoes, Suffolk County New York. Parasites Vector. 2016;9(1):1–13.
Skaff NK, Cheng Q, Clemesha RES, Collender PA, Gershunov A, Head JR, et al. Thermal thresholds heighten sensitivity of West Nile virus transmission to changing temperatures in coastal California. Proc Biol Sci. 2020;287(1932):20201065.
Shocket MS, Verwillow AB, Numazu MG, Slamani H, Cohen JM, El Moustaid F, et al. Transmission of West Nile and five other temperate mosquito-borne viruses peaks at temperatures between 23 C and 26 C. Elife. 2020;9: e58511.
Crowder DW, Dykstra EA, Brauner JM, Duffy A, Reed C, Martin E, et al. West Nile virus prevalence across landscapes is mediated by local effects of agriculture on vector and host communities. Plos One. 2013;8(1): e55006.
Smith KH, Tyre AJ, Hamik J, Hayes MJ, Zhou Y, Dai L. Using climate to explain and predict West Nile Virus risk in Nebraska. GeoHealth. 2020;4(9):e2020GH000244.
Tokarz RE, Smith RC. Crossover dynamics of Culex (Diptera: Culicidae) vector populations determine WNV transmission intensity. J Med Entomol. 2020;57(1):289–96.
Lockaby G, Noori N, Morse W, Zipperer W, Kalin L, Governo R, et al. Climatic, ecological, and socioeconomic factors associated with West Nile virus incidence in Atlanta, Georgia, USA. J Vector Ecol. 2016;41(2):232–43.
Uelmen JA, Brokopp C, Patz J. A 15 year evaluation of West Nile Virus in Wisconsin: effects on wildlife and human health. Int J Environ Res Public Health. 2020;17(5):1767.
Hahn MB, Monaghan AJ, Hayden MH, Eisen RJ, Delorey MJ, Lindsey NP, et al. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am J Trop Med Hyg. 2015;92(5):1013.
Wimberly MC, Lamsal A, Giacomo P, Chuang TW. Regional variation of climatic influences on West Nile virus outbreaks in the United States. Am J Trop Med Hyg. 2014;91(4):677.
Fay RL, Ngo KA, Kuo L, Willsey GG, Kramer LD, Ciota AT. Experimental evolution of West Nile virus at higher temperatures facilitates broad adaptation and increased genetic diversity. Viruses. 2021;13(10):1889.
Humphreys JM, Pelzel-McCluskey AM, Cohnstaedt LW, McGregor BL, Hanley KA, Hudson AR, et al. Integrating spatiotemporal epidemiology, eco-phylogenetics, and distributional ecology to assess West Nile disease risk in horses. Viruses. 2021;13(9):1811.
Hernandez E, Torres R, Joyce AL. Environmental and sociological factors associated with the incidence of West Nile virus cases in the Northern San Joaquin Valley of California, 2011–2015. Vector-Borne Zoonot. 2019;19(11):851–8.
Myer MH, Campbell SR, Johnston JM. Spatiotemporal modeling of ecological and sociological predictors of West Nile virus in Suffolk County, NY, mosquitoes. Ecosphere. 2017;8(6): e01854.
Myer MH, Johnston JM. Spatiotemporal Bayesian modeling of West Nile virus: Identifying risk of infection in mosquitoes with local-scale predictors. Sci Total Environ. 2019;650:2818–29.
Kala AK, Tiwari C, Mikler AR, Atkinson SF. A comparison of least squares regression and geographically weighted regression modeling of West Nile virus risk based on environmental parameters. Peer J. 2017;5: e3070.
Day JF, Shaman J. Using hydrologic conditions to forecast the risk of focal and epidemic arboviral transmission in peninsular Florida. J Med Entomol. 2014;45(3):458–65.
Peper ST, Dawson DE, Dacko N, Athanasiou K, Hunter J, Loko F, et al. Predictive modeling for West Nile virus and mosquito surveillance in Lubbock Texas. J Am Mosquito Contr. 2018;34(1):18–24.
Poh KC, Chaves LF, Reyna-Nava M, Roberts CM, Fredregill C, Bueno R Jr, et al. The influence of weather and weather variability on mosquito abundance and infection with West Nile virus in Harris County, Texas, USA. Sci Total Environ. 2019;675:260–72.
Shand L, Brown WM, Chaves LF, Goldberg TL, Hamer GL, Haramis L, et al. Predicting West Nile virus infection risk from the synergistic effects of rainfall and temperature. J Med Entomol. 2016;53(4):935–44.
Mori H, Wu J, Ibaraki M, Schwartz FW. Key factors influencing the incidence of West Nile virus in Burleigh County, North Dakota. Int J Environ Res Public Health. 2018;15(9):1928.
Ward MJ, Sorek-Hamer M, Henke JA, Little E, Patel A, Shaman J, et al. A spatially resolved and environmentally informed forecast model of West Nile virus in Coachella Valley, California. GeoHealth. 2023;7(12):e2023GH000855.
Gorris ME, Randerson JT, Coffield SR, Treseder KK, Zender CS, Xu C, Manore CA. Assessing the influence of climate on the spatial pattern of West Nile virus incidence in the United States. Environ Health Perspect. 2023;131(4):047016.
Huang X, Athrey GN, Kaufman PE, Fredregill C, Slotman MA. Effective population size of Culex quinquefasciatus under insecticide-based vector management and following Hurricane Harvey in Harris County Texas. Front Genet. 2023;14:1297271.
Holcomb KM, Mathis S, Staples JE, Fischer M, Barker CM, Beard CB, et al. Evaluation of an open forecasting challenge to assess skill of West Nile virus neuroinvasive disease prediction. Parasite Vector. 2023;16(1):11.
Paull SH, Horton DE, Ashfaq M, Rastogi D, Kramer LD, Diffenbaugh NS, et al. Drought and immunity determine the intensity of West Nile virus epidemics and climate change impacts. P Roy Soc B-Biol Sci. 1848;2017(284):20162078.
Keyel AC, Raghavendra A, Ciota AT, Elison TO. West Nile virus is predicted to be more geographically widespread in New York State and Connecticut under future climate change. Global Change Biol. 2021;27(21):5430–45.
Morin CW, Comrie AC. Regional and seasonal response of a West Nile virus vector to climate change. Proc Natl Acad Sci U S A. 2013;110(39):15620–5.
Filippelli GM, Freeman JL, Gibson J, Jay S, Moreno-Madriñán MJ, Ogashawara I, et al. Climate change impacts on human health at an actionable scale: a state-level assessment of Indiana, USA. Clim Change. 2020;163(4):1985–2004.
Brown HE, Young A, Lega J, Andreadis TG, Schurich J, Comrie A. Projection of climate change influences on US West Nile virus vectors. Earth Interact. 2015;19(18):1–18.
Bakker VJ, Sillett TS, Boyce WM, Doak DF, Vickers TW, Reisen WK, et al. Translocation with targeted vaccination is the most effective strategy to protect an island endemic bird threatened by West Nile virus. Divers Distrib. 2020;26(9):1104–15.
Chen CC, Epp T, Jenkins E, Waldner C, Curry PS, Soos C, et al. Modeling monthly variation of Culex tarsalis (Diptera: Culicidae) abundance and West Nile Virus infection rate in the Canadian Prairies. Int J Environ Res Public Health. 2013;10(7):3033–51.
Mallya S, Sander B, Roy-Gagnon MH, Taljaard M, Jolly A, Kulkarn MA. Factors associated with human West Nile virus infection in Ontario: a generalized linear mixed modelling approach. BMC Infect Dis. 2018;18(1):1–9.
Temple SD, Manore CA, Kaufeld KA. Bayesian time-varying occupancy model for West Nile virus in Ontario Canada. Stoch Environ Res Risk Assess. 2022;36(8):2337–52.
Talbot B, Kulkarni MA, Rioux-Rousseau M, Siebels K, Kotchi SO, Ogden NH, et al. Ecological niche and positive clusters of two West Nile virus vector in Ontario Canada. EcoHealth. 2023;20(3):249–62.
Albrecht L, Kaufeld KA. Investigating the impact of environmental factors on West Nile virus human case prediction in Ontario Canada. Front Public Health. 2023;11:1100543.
Baril C, Pilling BG, Mikkelsen MJ, Sparrow JM, Duncan CAM, Koloski CW, et al. The influence of weather on the population dynamics of common mosquito vector species in the Canadian Prairies. Parasite Vector. 2023;16(1):153.
Chen CC, Jenkins E, Epp T, Waldner C, Curry PS, Soos C. Climate change and West Nile virus in a highly endemic region of North America. Int J Environ Res Public Health. 2013;10(7):3052–71.
Otten A, Fazil A, Chemeris A, Breadner P, Ng V. Prioritization of vector-borne diseases in Canada under current climate and projected climate change. Microbial Risk Anal. 2020;14:100089.
Hongoh V, Campagna C, Panic M, Samuel O, Gosselin P, Waaub JP, et al. Assessing interventions to manage West Nile virus using multi-criteria decision analysis with risk scenarios. Plos One. 2016;11(8): e0160651.
Tam BY, Tsuji LJS. West Nile virus in American crows ( Corvus brachyrhynchos ) in Canada: projecting the influence of climate change. GeoJournal. 2016;81:89–101.
Tam BY, Martin I, Tsuji LJS. Geospatial analysis between the environment and past incidences of West Nile virus in bird specimens in Ontario Canada. GeoJournal. 2014;79:805–17.
Rakotoarinia MR, Seidou O, Lapen DR, Leighton PA, Ogden NH, Ludwig A. Future land-use change predictions using Dyna-Clue to support mosquito-borne disease risk assessment. Environ Monit Assess. 2023;195(7):815.
Di Pol G, Crotta M, Taylor RA. Modelling the temperature suitability for the risk of West Nile Virus establishment in European Culex pipiens populations. Transbound Emerg Dis. 2022;69(5):1787–99.
Coroian M, Petrić M, Pistol A, Sirbu A, Domșa C, Mihalca AD. Human West Nile Meningo-Encephalitis in a highly endemic country: a complex epidemiological analysis on biotic and abiotic risk factors. Int J Environ Res Public Health. 2020;17(21):8250.
Stilianakis NI, Syrris V, Petroliagkis T, Pärt P, Gewehr S, Kalaitzopoulou S, et al. Identification of climatic factors affecting the epidemiology of human West Nile virus infections in northern Greece. Plos One. 2016;11(9): e0161510.
Vogels CB, Hartemink N, Koenraadt CJM. Modelling West Nile virus transmission risk in Europe: effect of temperature and mosquito biotypes on the basic reproduction number. Sci Rep. 2017;7(1):5022.
Fros JJ, Geertsema C, Vogels CB, Roosjen PP, Failloux AB, Vlak JM, et al. West Nile virus: high transmission rate in north-western European mosquitoes indicates its epidemic potential and warrants increased surveillance. Plos Neglect Trop Dis. 2015;9(7):e0003956.
Tran A, Sudre B, Paz S, Rossi M, Desbrosse A, Chevalier V, et al. Environmental predictors of West Nile fever risk in Europe. Int J Health Geogr. 2014;13:1–11.
Radojicic S, Zivulj A, Petrovic T, Nisavic J, Milicevic V, Sipetic-Grujicic S, et al. Spatiotemporal analysis of West Nile virus epidemic in South Banat District, Serbia, 2017–2019. Animals. 2021;11(10):2951.
Platonov AE, Fedorova MV, Karan LS, Shopenskaya TA, Platonova OV, Zhuravlev VI. Epidemiology of West Nile infection in Volgograd, Russia, in relation to climate change and mosquito (Diptera: Culicidae) bionomics. Parasitol Res. 2008;1(103):45–53.
Moirano G, Gasparrini A, Acquaotta F, Fratianni S, Merletti F, Maule M, et al. West Nile virus infection in Northern Italy: Case-crossover study on the short-term effect of climatic parameters. Environ Res. 2018;167:544–9.
Marcantonio M, Rizzoli A, Metz M, Rosà R, Marini G, Chadwick E, et al. Identifying the environmental conditions favouring West Nile virus outbreaks in Europe. Plos One. 2015;10(3): e0121158.
Mihailović DT, Petrić D, Petrović T, Hrnjaković-Cvjetković I, Djurdjevic V, Nikolić-Đorić E, et al. Assessment of climate change impact on the malaria vector Anopheles hyrcanus, West Nile disease, and incidence of melanoma in the Vojvodina Province (Serbia) using data from a regional climate model. Plos One. 2020;15(1): e0227679.
Trájer AJ, Bede-Fazekas Á, Bobvos J, Páldy A. Seasonality and geographical occurrence of West Nile fever and distribution of Asian tiger mosquito. Q J Hung Meteorol Se. 2014;118(1):19–40.
Townroe S, Callaghan A. British container breeding mosquitoes: the impact of urbanisation and climate change on community composition and phenology. Plos One. 2014;9(4): e95325.
Paz S. West Nile Virus Eruptions in Summer 2010–What Is the Possible Linkage with Climate Change? Netherlands: National Security and Human Health Implications of Climate Change. Springer; 2012. p. 253–60.
Mavrakis A, Papavasileiou C, Alexakis D, Papakitsos EC, Salvati L. Meteorological patterns and the evolution of West Nile virus in an environmentally stressed Mediterranean area. Environ Monit Assess. 2021;193:1–11.
Vlasova NV, Masyagutova LM, Abdrakhmanova ER, Rafikova LA, Chudnovets GM. A conceptual scheme of a predictive-analytical model for describing incidence of west nile fever based on weather and climate estimation (exemplified by the Volgograd region). Health Risk Anal. 2022;4:124.
Farooq Z, Rocklöv J, Wallin J, Abiri N, Sewe MO, Sjödin H, et al. Artificial intelligence to predict West Nile virus outbreaks with ecoclimatic drivers. Lancet Reg Health Eu. 2022;17:100370.
Marini G, Pugliese A, Wint W, Alexander NS, Rizzoli A, Rosà R. Modelling the West Nile virus force of infection in the European human population. One Health. 2022;15: 100462.
Vukmir NR, Bojanić J, Mijović B, Roganović T, Aćimović J. Did intensive floods influence higher incidence rate of the West Nile virus in the population exposed to flooding in the Republic of Srpska in 2014. Arch Vet Med. 2019;12(1):21–32.
Krol L, Blom R, Dellar M, van der Beek JG, Stroo ACJ, van Bodegom PM, et al. Interactive effects of climate, land use and soil type on Culex pipiens/torrentium abundance. One Health. 2023;17: 100589.
Magallanes S, Llorente F, Ruiz-López MJ, de la PuenteMartinez - J, Soriguer R, Calderon J, et al. Long-term serological surveillance for West Nile and Usutu virus in horses in south-West Spain. One Health. 2023;17:100578.
Niczyporuk JS, Kozdrun W, Czujkowska A, Blanchard Y, Helle M, Dheilly NM, et al. West Nile virus lineage 2 in free-living Corvus cornix birds in Poland. Trop Med Infect Dis. 2023;8(8):417.
Angelou A, Gewehr S, Mourelatos S, Kioutsioukis I. Early warning impact of temperature and rainfall anomalies onto West Nile virus human cases. Environ Sci Proc. 2023;26(1):93.
Watts MJ, iMonteys VS, Mortyn PG, Kotsila P. The rise of West Nile virus in Southern and Southeastern Europe: A spatial–temporal analysis investigating the combined effects of climate, land use and economic changes. One Health. 2021;13:100315.
Lourenço J, Barros SC, Zé-Zé L, Damineli DSC, Giovanetti M, Osório HC, et al. West Nile virus transmission potential in Portugal. Comms Biol. 2022;5(1):6.
Ewing DA, Purse BV, Cobbold CA, White SM. A novel approach for predicting risk of vector-borne disease establishment in marginal temperate environments under climate change: West Nile virus in the UK. J R Soc Interface. 2021;18(178):20210049.
Trájer AJ. Meteorological conditions associated with West Nile fever incidences in mediterranean and continental climates in Europe. Idojaras. 2017;121:303–28.
Tippelt L, Walther D, Kampen H. The thermophilic mosquito species Uranotaenia unguiculata Edwards, 1913 (Diptera: Culicidae) moves north in Germany. Parasitol Res. 2017;116:3437–40.
Farooq Z, Sjödin H, Semenza JC, Tozan Y, Sewe MO, Wallin J, Rocklöv J. European projections of West Nile virus transmission under climate change scenarios. One Health. 2023;16: 100509.
Aharonson-Raz K, Lichter-Peled A, Tal S, Gelman B, Cohen D, Klement E, et al. Spatial and temporal distribution of West Nile virus in horses in Israel (1997–2013)-From endemic to epidemics. Plos One. 2014;9(11):e113149.
Ahmadnejad F, Otarod V, Fathnia A, Ahmadabadi A, Fallah MH, Zavareh A, et al. Impact of climate and environmental factors on West Nile virus circulation in Iran. J Arthropod Borne Dis. 2016;10(3):315.
Salama M, Amitai Z, Lustig Y, Mor Z, Weiberger M, Chowers M, et al. Outbreak of West Nile virus disease in Israel (2015): A retrospective analysis of notified cases. Travel Med Infect Dis. 2018;28:41–5.
Calistri P, Ippoliti C, Candeloro L, Benjelloun A, El Harrak M, Bouchra B, et al. Analysis of climatic and environmental variables associated with the occurrence of West Nile virus in Morocco. Prev Vet Med. 2013;110(3–4):549–53.
Velu RM, Kwenda G, Bosomprah S, Chisola MN, Simunyandi M, Chisenga CC, et al. Ecological niche modeling of Aedes and Culex mosquitoes: a risk map for Chikungunya and West Nile viruses in Zambia. Viruses. 2023;15(9):1900.
Outammassine A, Zouhair S, Loqman S. Rift Valley fever and West Nile virus vectors in Morocco: Current situation and future anticipated scenarios. Transbound Emerg Dis. 2022;69(3):1466–78.
Figueroa DP, Scott S, González CR, Bizama G, Flores Mara R, Bustamante R, et al. Estimating the climate change consequences on the potential distribution of Culex pipiens L. 1758, to assess the risk of West Nile virus establishment in Chile. Gatana. 2020;84(1):46–53.
Huang B, Prow NA, van den Hurk AF, Allcock RJ, Moore PR, Doggett SL, et al. Archival isolates confirm a single topotype of West Nile virus in Australia. Plos Negl Trop Dis. 2016;10(12):e0005159.
Anyamba A, Small JL, Britch SC, Tucker CJ, Pak EW, Reynolds CA, et al. Recent weather extremes and impacts on agricultural production and vector-borne disease outbreak patterns. Plos One. 2014;9(3): e92538.
Samy AM, Elaagip AH, Kenawy MA, Ayres CF, Peterson AT, Soliman DE. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus , vector of West Nile virus and lymphatic filariasis. Plos One. 2016;11(10): e0163863.
Negev M, Paz S, Clermont A, Pri-Or NG, Shalom U, Yeger T, et al. Impacts of climate change on vector borne diseases in the Mediterranean Basin—implications for preparedness and adaptation policy. Int J Environ Res Public Health. 2015;12(6):6745–70.
CDC. Historic Data for WNV, 1999–2022. https://www.cdc.gov/westnile/statsmaps/historic-data.html . Accessed 11 Oct 2023.
Soto RA, Hughes ML, Staples JE, Lindsey NP. West Nile virus and other domestic nationally notifiable arboviral diseases-United States, 2020. MMWR Morb Mortal Wkly Rep. 2022;71(18):628.
Zheng H, Drebot MA, Coulthart MB. West Nile virus in Canada: ever-changing, but here to stay Canada. Commun Dis Rep. 2014;40(10):173–7.
Public Health Agency of Canada. West Nile virus and other mosquito-borne disease national surveillance report. https://www.canada.ca/en/public-health/services/diseases/west-nile-virus/surveillance-west-nile-virus.html . Accessed 11 March 2024.
Young JJ, Haussig JM, Aberle SW, Pervanidou D, Riccardo F, Sekulić N, et al. Epidemiology of human West Nile virus infections in the European Union and European Union enlargement countries, 2010 to 2018. Eurosurveillance. 2021;26(19):2001095.
CDC. FAQ: general questions about West Nile virus. 2015. http://www.cdc.gov/westnile/faq/genQuestions.html . Accessed 13 Jun 2023.
Komar N. West Nile virus: epidemiology and ecology in North America. Adv Virus Res. 2003;61:185–234.
George TL, Harrigan RJ, LaManna JA, DeSante DF, Saracco JF, Smith TB. Persistent impacts of West Nile virus on North American bird populations. Proc Natl Acad Sci U S A. 2015;112(46):14290–4.
Hamer GL, Walker ED, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, et al. Rapid amplification of West Nile virus: the role of hatch-year birds. Vector Borne Zoonotic Dis. 2008;8(1):57–68.
Reisen WK. Effect of temperature on Culex tarsalis (Diptera: Culicidae) from the Coachella and San Joaquin valleys of California. J Med Entomol. 1995;32:636–45.
Cotton PA. Avian migration phenology and global climate change. Proc Natl Acad Sci U S A. 2003;100:12219–22.
Caillouët KA, Riggan AE, Bulluck LP, Carlson JC, Sabo RT. Nesting bird “host funnel” increases mosquito-bird contact rate. J Med Entomol. 2013;50(2):462–6.
Shaman J, Day JF, Stieglitz M. Drought induced amplification and epidemic transmission of West Nile virus in southern Florida. J Med Entomol. 2005;42:134–41.
Paz S. Climate change impacts on West Nile virus transmission in a global context. Philos Trans R Soc Lond B Biol Sci. 2015;370(1665):20130561.
Download references
Acknowledgements
Not applicable.
This work was supported by the National Natural Science Foundation of China (No.31802217).
Author information
Authors and affiliations.
Department of Veterinary Surgery, Northeast Agricultural University, Harbin, 150030, Heilongjiang, People’s Republic of China
Hao-Ran Wang, Tao Liu, Xiang Gao, Hong-Bin Wang & Jian-Hua Xiao
Heilongjiang Key Laboratory for Laboratory Animals and Comparative Medicine, College of Veterinary Medicine, Northeast Agricultural University, Harbin, 150030, Heilongjiang, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
HRW participated in the design, study selection, data extraction and analysis, and write-up of this study. TL, XG, and HBW participated in the design of this study and revised the manuscript. JHX participated in the revision of the manuscript. All authors approved the final version.
Corresponding author
Correspondence to Jian-Hua Xiao .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wang, HR., Liu, T., Gao, X. et al. Impact of climate change on the global circulation of West Nile virus and adaptation responses: a scoping review. Infect Dis Poverty 13 , 38 (2024). https://doi.org/10.1186/s40249-024-01207-2
Download citation
Received : 03 January 2024
Accepted : 17 May 2024
Published : 24 May 2024
DOI : https://doi.org/10.1186/s40249-024-01207-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Climate change
- Vector-borne pathogens
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Gene variants foretell the biology of future breast cancers in Stanford Medicine study
In a finding that vastly expands the understanding of tumor evolution, researchers discover genetic biomarkers that can predict the breast cancer subtype a patient is likely to develop.
May 30, 2024 - By Krista Conger

Stanford Medicine researchers found that inherited gene sequences can predict what type of breast cancer a patient is likely to develop, along with how aggressive that cancer may be. Emily Moskal
A Stanford Medicine study of thousands of breast cancers has found that the gene sequences we inherit at conception are powerful predictors of the breast cancer type we might develop decades later and how deadly it might be.
The study challenges the dogma that most cancers arise as the result of random mutations that accumulate during our lifetimes. Instead, it points to the active involvement of gene sequences we inherit from our parents — what’s known as your germline genome — in determining whether cells bearing potential cancer-causing mutations are recognized and eliminated by the immune system or skitter under the radar to become nascent cancers.
“Apart from a few highly penetrant genes that confer significant cancer risk, the role of hereditary factors remains poorly understood, and most malignancies are assumed to result from random errors during cell division or bad luck,” said Christina Curtis , PhD, the RZ Cao Professor of Medicine and a professor of genetics and of biomedical data science. “This would imply that tumor initiation is random, but that is not what we observe. Rather, we find that the path to tumor development is constrained by hereditary factors and immunity. This new result unearths a new class of biomarkers to forecast tumor progression and an entirely new way of understanding breast cancer origins.”
Curtis is the senior author of the study, which will be published May 31 in Science . Postdoctoral scholar Kathleen Houlahan , PhD, is the lead author of the research.
“Back in 2015, we had posited that some tumors are ‘born to be bad’ — meaning that their malignant and even metastatic potential is determined early in the disease course,” Curtis said. “We and others have since corroborated this finding across multiple tumors, but these findings cast a whole new light on just how early this happens.”
A new take on cancer’s origin
The study, which gives a nuanced and powerful new understanding of the interplay between newly arisen cancer cells and the immune system, is likely to help researchers and clinicians better predict and combat breast tumors.
Currently, only a few high-profile cancer-associated mutations in genes are regularly used to predict cancers, but these account for a small minority of cases. Those include BRCA1 and BRCA2, which occur in about one of every 500 women and confer an increased risk of breast or ovarian cancer, and rarer mutations in a gene called TP53 that causes a disease called Li Fraumeni syndrome, which predisposes to childhood and adult-onset tumors.

Christina Curtis
The findings suggest there are tens or hundreds of additional gene variants — identifiable in healthy people — that through interactions with the immune system pull the strings that determine why some people remain cancer-free throughout their lives.
“Our findings not only explain which subtype of breast cancer an individual is likely to develop,” Houlahan said, “but they also hint at how aggressive and prone to metastasizing that subtype will be. Beyond that, we speculate that these inherited variants may influence a person’s risk of developing breast cancer. However, future studies will be needed to examine this.”
The genes we inherit from our parents are known as our germline genome. They’re mirrors of our parents’ genetic makeup, and they can vary among people in small ways that give some of us blue eyes, brown hair or type O blood. Some inherited genes include mutations that confer increased cancer risk from the get-go, such as BRCA1, BRCA2 and TP53.
In contrast, most cancer-associated genes are part of what’s known as our somatic genome. As we live our lives, our cells divide and die in the tens of millions. Each time the DNA in a cell is copied, mistakes happen and mutations can accumulate. DNA in tumors is often compared with the germline genomes in blood or normal tissues in an individual to pinpoint which changes likely led to the cell’s cancerous transformation.
Classifying breast cancers
In 2012, Curtis began a deep dive — assisted by machine learning — into the types of somatic mutations that occur in thousands of breast cancers. She was eventually able to categorize the disease into 11 subtypes with varying prognoses and risk of recurrence, finding that four of the 11 groups were significantly more likely to recur even 10 or 20 years after diagnosis — critical information for clinicians making treatment decisions and discussing long-term prognoses with their patients.
Prior studies had shown that people with inherited BRCA1 mutations tend to develop a subtype of breast cancer known as triple negative breast cancer. This correlation implies some behind-the-scenes shenanigans by the germline genome that affects what subtype of breast cancer someone might develop.
“We wanted to understand how inherited DNA might sculpt how a tumor evolves,” Houlahan said. To do so, they took a close look at the immune system.
It’s a quirk of biology that even healthy cells routinely decorate their outer membranes with small chunks of the proteins they have bobbing in their cytoplasm — an outward display that reflects their inner style.

Kathleen Houlahan
The foundations for this display are what’s known as HLA proteins, and they are highly variable among individuals. Like fashion police, immune cells called T cells prowl the body looking for any suspicious or overly flashy bling (called epitopes) that might signal something is amiss inside the cell. A cell infected with a virus will display bits of viral proteins; a sick or cancerous cell will adorn itself with abnormal proteins. These faux pas trigger the T cells to destroy the offenders.
Houlahan and Curtis decided to focus on oncogenes, normal genes that, when mutated, can free a cell from regulatory pathways meant to keep it on the straight and narrow. Often, these mutations take the form of multiple copies of the normal gene, arranged nose to tail along the DNA — the result of a kind of genomic stutter called amplification. Amplifications in specific oncogenes drive different cancer pathways and were used to differentiate one breast cancer subtype from another in Curtis’ original studies.
The importance of bling
The researchers wondered whether highly recognizable epitopes would be more likely to attract T cells’ attention than other, more modest displays (think golf-ball-sized, dangly turquoise earrings versus a simple silver stud). If so, a cell that had inherited a flashy version of an oncogene might be less able to pull off its amplification without alerting the immune system than a cell with a more modest version of the same gene. (One pair of overly gaudy turquoise earrings can be excused; five pairs might cause a patrolling fashionista T cell to switch from tutting to terminating.)
The researchers studied nearly 6,000 breast tumors spanning various stages of disease to learn whether the subtype of each tumor correlated with the patients’ germline oncogene sequences. They found that people who had inherited an oncogene with a high germline epitope burden (read: lots of bling) — and an HLA type that can display that epitope prominently — were significantly less likely to develop breast cancer subtypes in which that oncogene is amplified.
There was a surprise, though. The researchers found that cancers with a large germline epitope burden that manage to escape the roving immune cells early in their development tended to be more aggressive and have a poorer prognosis than their more subdued peers.
“At the early, pre-invasive stage, a high germline epitope burden is protective against cancer,” Houlahan said. “But once it’s been forced to wrestle with the immune system and come up with mechanisms to overcome it, tumors with high germline epitope burden are more aggressive and prone to metastasis. The pattern flips during tumor progression.”
“Basically, there is a tug of war between tumor and immune cells,” Curtis said. “In the preinvasive setting, the nascent tumor may initially be more susceptible to immune surveillance and destruction. Indeed, many tumors are likely eliminated in this manner and go unnoticed. However, the immune system does not always win. Some tumor cells may not be eliminated and those that persist develop ways to evade immune recognition and destruction. Our findings shed light on this opaque process and may inform the optimal timing of therapeutic intervention, as well as how to make an immunologically cold tumor become hot, rendering it more sensitive to therapy.”
The researchers envision a future when the germline genome is used to further stratify the 11 breast cancer subtypes identified by Curtis to guide treatment decisions and improve prognoses and monitoring for recurrence. The study’s findings may also give additional clues in the hunt for personalized cancer immunotherapies and may enable clinicians to one day predict a healthy person’s risk of developing an invasive breast cancer from a simple blood sample.
“We started with a bold hypothesis,” Curtis said. “The field had not thought about tumor origins and evolution in this way. We’re examining other cancers through this new lens of hereditary and acquired factors and tumor-immune co-evolution.”
The study was funded by the National Institutes of Health (grants DP1-CA238296 and U54CA261719), the Canadian Institutes of Health Research and the Chan Zuckerberg Biohub.

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers

Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Scientists identify mechanism behind drug resistance in malaria parasite
Press contact :.

Previous image Next image
Share this news article on:
Related links.
- Peter Dedon
- Antimicrobial Resistance Interdisciplinary Research Group
- Singapore-MIT Alliance for Research and Technology (SMART)
- Department of Biological Engineering
Related Topics
- Drug resistance
- Antibiotics
- Biological engineering
- Drug development
- International initiatives
- Collaboration
Related Articles

Satellite-based method measures carbon in peat bogs

Newly discovered bacterial communication system aids antimicrobial resistance

SMART launches research group to advance AI, automation, and the future of work

A novel combination therapy counters antibiotic-resistant Mycobacterium abscessus infections
Previous item Next item
More MIT News

MIT Corporation elects 10 term members, two life members
Read full story →

Diane Hoskins ’79: How going off-track can lead new SA+P graduates to become integrators of ideas

Chancellor Melissa Nobles’ address to MIT’s undergraduate Class of 2024

Noubar Afeyan PhD ’87 gives new MIT graduates a special assignment

Commencement address by Noubar Afeyan PhD ’87

President Sally Kornbluth’s charge to the Class of 2024
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
America’s best decade, according to data
One simple variable, more than anything, determines when you think the nation peaked.

How do you define the good old days?
Department of Data

The plucky poll slingers at YouGov, who are consistently willing to use their elite-tier survey skills in service of measuring the unmeasurable, asked 2,000 adults which decade had the best and worst music, movies, economy and so forth, across 20 measures . But when we charted them, no consistent pattern emerged.
We did spot some peaks: When asked which decade had the most moral society, the happiest families or the closest-knit communities, White people and Republicans were about twice as likely as Black people and Democrats to point to the 1950s. The difference probably depends on whether you remember that particular decade for “Leave it to Beaver,” drive-in theaters and “12 Angry Men” — or the Red Scare, the murder of Emmett Till and massive resistance to school integration.
“This was a time when Repubs were pretty much running the show and had reason to be happy,” pioneering nostalgia researcher Morris Holbrook told us via email. “Apparently, you could argue that nostalgia is colored by political preferences. Surprise, surprise.”
And he’s right! But any political, racial or gender divides were dwarfed by what happened when we charted the data by generation. Age, more than anything, determines when you think America peaked.
So, we looked at the data another way, measuring the gap between each person’s birth year and their ideal decade. The consistency of the resulting pattern delighted us: It shows that Americans feel nostalgia not for a specific era, but for a specific age.
The good old days when America was “great” aren’t the 1950s. They’re whatever decade you were 11, your parents knew the correct answer to any question, and you’d never heard of war crimes tribunals, microplastics or improvised explosive devices. Or when you were 15 and athletes and musicians still played hard and hadn’t sold out.
Not every flavor of nostalgia peaks as sharply as music does. But by distilling them to the most popular age for each question, we can chart a simple life cycle of nostalgia.
The closest-knit communities were those in our childhood, ages 4 to 7. The happiest families, most moral society and most reliable news reporting came in our early formative years — ages 8 through 11. The best economy, as well as the best radio, television and movies, happened in our early teens — ages 12 through 15.
GET CAUGHT UP Summarized stories to quickly stay informed

Chief Justice Roberts declines to meet with Democrats on court ethics

Will Trump go to jail? Can he be president? What’s next after guilty verdict?

Bruhat Soma wins Scripps National Spelling Bee in second-ever spell-off

How a fed up carpenter found his stolen power tools — and 15,000 others

Here’s how long it takes workers to become 401(k) millionaires
Slightly spendier activities such as fashion, music and sporting events peaked in our late teens — ages 16 through 19 — matching research from the University of South Australia’s Ehrenberg-Bass Institute, which shows music nostalgia centers on age 17 .
YouGov didn’t just ask about the best music and the best economy. The pollsters also asked about the worst music and the worst economy. But almost without exception, if you ask an American when times were worst, the most common response will be “right now!”
This holds true even when “now” is clearly not the right answer. For example, when we ask which decade had the worst economy, the most common answer is today. The Great Depression — when, for much of a decade, unemployment exceeded the what we saw in the worst month of pandemic shutdowns — comes in a grudging second.
To be sure, other forces seem to be at work. Democrats actually thought the current economy wasn’t as bad as the Great Depression. Republicans disagreed. In fact, measure after measure, Republicans were more negative about the current decade than any other group — even low-income folks in objectively difficult situations.
So, we called the brilliant Joanne Hsu, director of the University of Michigan’s Surveys of Consumers who regularly wrestles with partisan bias in polling.
Hsu said that yes, she sees a huge partisan split in the economy, and yes, Republicans are far more negative than Democrats. But it hasn’t always been that way.
“People whose party is in the White House always have more favorable sentiment than people who don’t,” she told us. “And this has widened over time.”
In a recent analysis , Hsu — who previously worked on some of our favorite surveys at the Federal Reserve — found that while partisanship drove wider gaps in economic expectations than did income, age or education even in the George W. Bush and Barack Obama years, they more than doubled under Donald Trump as Republicans’ optimism soared and Democrats’ hopes fell.
Our attitudes reversed almost the instant President Biden took office, but the gap remains nearly as wide. That is to say, if we’d asked the same questions about the worst decades during the Trump administration, Hsu’s work suggests the partisan gap could have shriveled or even flipped eyeglasses over teakettle.
To understand the swings, Hsu and her friends spent the first part of 2024 asking 2,400 Americans where they get their information about the economy. In a new analysis , she found Republicans who listen to partisan outlets are more likely to be negative, and Democrats who listen to their own version of such news are more positive — and that Republicans are a bit more likely to follow partisan news.
But while Fox and friends drive some negativity, only a fifth of Republicans get their economic news from partisan outlets. And Democrats and independents give a thumbs down to the current decade, too, albeit at much lower rates.
There’s clearly something more fundamental at work. As YouGov’s Carl Bialik points out, when Americans were asked last year which decade they’d most want to live in, the most common answer was now. At some level then, it seems unlikely that we truly believe this decade stinks by almost every measure.
A deeper explanation didn’t land in our laps until halfway through a Zoom call with four well-caffeinated Australian marketing and consumer-behavior researchers: the Ehrenberg-Bass folks behind the music study we cited above. (Their antipodean academic institute has attracted massive sponsorships by replacing typical corporate marketing fluffery with actual evidence.)
Their analysis began when Callum Davies needed to better understand the demographics of American music tastes to interpret streaming data for his impending dissertation. Since they were already asking folks about music, Davies and his colleagues decided they might as well seize the opportunity to update landmark research from Holbrook and Robert Schindler about music nostalgia.
Building on the American scholars’ methods, they asked respondents to listen to a few seconds each of 34 songs , including Justin Timberlake’s “Sexy Back” and Johnny Preston’s “ Running Bear .” Then respondents were asked to rate each song on a zero-to-10 scale. (In the latter case, we can’t imagine the high end of the scale got much use, especially if the excerpt included that song’s faux-tribal “hooga-hooga” chant and/or its climactic teen drownings.)
Together, the songs represented top-10 selections from every even-numbered year from 1950 (Bing and Gary Crosby’s “Play a Simple Melody”) to 2016 (Rihanna’s “Work”), allowing researchers to gather our preferences for music released throughout our lives.
Like us, they found that you’ll forever prefer the music of your late teens. But their results show one big difference: There’s no sudden surge of negative ratings for the most recent music.
Marketing researcher Bill Page said that by broadly asking when music, sports or crime were worst, instead of getting ratings for specific years or items, YouGov got answers to a question they didn’t ask.
“When you ask about ‘worst,’ you’re not asking for an actual opinion,” Page said. “You’re asking, ‘Are you predisposed to think things get worse?’”
“There’s plenty of times surveys unintentionally don’t measure what they claim to,” his colleague Zac Anesbury added.
YouGov actually measured what academics call “declinism,” his bigwig colleague Carl Driesener explained. He looked a tiny bit offended when we asked if that was a real term or slang they’d coined on the spot. But in our defense, only a few minutes had passed since they had claimed “cozzie livs” was Australian for “the cost of living crisis.”
Declinists believe the world keeps getting worse. It’s often the natural result of rosy retrospection, or the idea that everything — with the possible exception of “Running Bear” — looks better in memory than it did at the time. This may happen in part because remembering the good bits of the past can help us through difficult times, Page said.
It’s a well-established phenomenon in psychology, articulated by Leigh Thompson, Terence Mitchell and their collaborators in a set of analyses . They found that when asked to rate a trip mid-vacation, we often sound disappointed. But after we get home — when the lost luggage has been found and the biting-fly welts have stopped itching — we’re as positive about the trip as we were in the early planning stage. Sometimes even more so.
So saying the 2020s are the worst decade ever is akin to sobbing about “the worst goldang trip ever” at 3 a.m . in a sketchy flophouse full of Russian-speaking truckers after you’ve run out of cash and spent three days racing around Urumqi looking for the one bank in Western China that takes international cards.
A few decades from now, our memories shaped by grainy photos of auroras and astrolabes, we’ll recall only the bread straight from streetside tandoor-style ovens and the locals who went out of their way to bail out a couple of distraught foreigners.
In other words, the 2020s will be the good old days.
Greetings! The Department of Data curates queries. What are you curious about: How many islands have been completely de-ratted? Where is America’s disc-golf heartland? Who goes to summer camp? Just ask!
If your question inspires a column, we’ll send you an official Department of Data button and ID card. This week’s buttons go to YouGov’s Taylor Orth, who correctly deduced we’d be fascinated by decade-related polls, and Stephanie Killian in Kennesaw, Ga., who also got a button for our music column , with her questions about how many people cling to the music of their youth.


Scientist III
- Madison, Wisconsin
- SCHOOL OF MEDICINE AND PUBLIC HEALTH/NEUROSCIENCE
- Staff-Full Time
- Opening at: May 31 2024 at 12:20 CDT
- Closing at: Jun 14 2024 at 23:55 CDT
Job Summary:
We seek an expert in multi-photon microscopy to direct and contribute to the integration of intravital microscopy into the research programs of multiple laboratories in the School of Medicine and Public Health. The position offers an exciting opportunity for a highly motivated scientist to collaborate and contribute to groundbreaking research in neuroscience and other disciplines. The Scientist will be responsible for liaising with multiple research groups, and customizing the design and execution of experiments utilizing live fluorescence imaging. The scientist will also be a vital contributor to data analysis, publication of research findings, and grant proposal preparation. Located on an isthmus between lakes Mendota and Monona, the city of Madison, WI is consistently ranked one of the best places to live is the US. With an extensive network of parks and bike trails, a thriving arts and cultural scene and a resilient economy, Madison is one of the fastest growing cities in the Midwest.
Responsibilities:
- 30% Identifies research problems and develops highly complex research methodologies and procedures. Publishes and presents results to help advance research
- 30% Collects and analyzes highly complex research data, conducts experiments and interviews, and documents results according to established policies and procedures
- 5% Conducts literature reviews, prepares reports and materials and, disseminates information to appropriate entities
- 5% Attends and assists with the facilitation of scholarly events and presentations in support of continued professional development and the dissemination of research information
- 10% Identifies, writes, or assists in developing grant opportunities, grant applications, and proposals to secure research funding
- 10% May supervise the day-to-day activities of a research unit and staff and resolve routine personnel issues
- 5% Serves as an institutional subject matter expert and liaison with key internal and external stakeholders providing expert level information and representing the interests of a specialized research area
- 5% Monitors program budget and approves unit expenditures
Institutional Statement on Diversity:
Diversity is a source of strength, creativity, and innovation for UW-Madison. We value the contributions of each person and respect the profound ways their identity, culture, background, experience, status, abilities, and opinion enrich the university community. We commit ourselves to the pursuit of excellence in teaching, research, outreach, and diversity as inextricably linked goals. The University of Wisconsin-Madison fulfills its public mission by creating a welcoming and inclusive community for people from every background - people who as students, faculty, and staff serve Wisconsin and the world. For more information on diversity and inclusion on campus, please visit: Diversity and Inclusion
Required PhD in a life science discipline
Qualifications:
Required: - At least 3 years of experience with intravital imaging - Record of accomplishment in experimental design and execution Preferred: - Experience with dynamic signal in vivo imaging (i.e. GECIs or other) - Ability to work independently and collaboratively with students postdocs and faculty - Excellent communication and interpersonal skills - Experience with Bruker or Prairie multi-photon microscope
Full Time: 100% It is anticipated this position requires work be performed in-person, onsite, at a designated campus work location.
Appointment Type, Duration:
Ongoing/Renewable
Minimum $80,000 ANNUAL (12 months) Depending on Qualifications Employees in this position can expect to receive benefits such as generous vacation, holidays, and sick leave; competitive insurances and savings accounts; retirement benefits. Benefits information can be found at ( https://hr.wisc.edu/benefits/ ). This position is eligible for a hiring bonus of 10% of the starting salary.
Additional Information:
University sponsorship is not available for this position. The selected applicant will be responsible for ensuring their continuous eligibility to work in the United States (i.e. a citizen or national of the United States, a lawful permanent resident, a foreign national authorized to work in the United States without the need of an employer sponsorship) on or before the effective date of appointment. This position is an ongoing position that will require continuous work eligibility. UW-Madison is not an E-Verify employer, and therefore, is not eligible to employ F1-OPT STEM Extension participants. If you are selected for this position you must provide proof of work authorization and eligibility to work.
How to Apply:
Your application must be received through the Jobs at UW portal to be considered as a candidate. Applications submitted outside of this system will not be considered. To apply for this position, please click on the "Apply Now" button. You will be asked to upload a current resume/CV and a cover letter briefly describing your qualifications and experience. You will also be asked to provide contact information for three (3) references, including your current/most recent supervisor during the application process. References will not be contacted without prior notice.
Betsy Quinlan [email protected] 608-439-9026 Relay Access (WTRS): 7-1-1. See RELAY_SERVICE for further information.
Official Title:
Scientist III(RE045)
Department(s):
A53-MEDICAL SCHOOL/NEUROSCIENCE
Employment Class:
Academic Staff-Renewable
Job Number:
The university of wisconsin-madison is an equal opportunity and affirmative action employer..
You will be redirected to the application to launch your career momentarily. Thank you!
Frequently Asked Questions
Applicant Tutorial
Disability Accommodations
Pay Transparency Policy Statement
Refer a Friend
You've sent this job to a friend!
Website feedback, questions or accessibility issues: [email protected] .
Learn more about accessibility at UW–Madison .
© 2016–2024 Board of Regents of the University of Wisconsin System • Privacy Statement

IMAGES
VIDEO
COMMENTS
A research summary is a brief yet concise version of the research paper for a targeted audience. Read more to find out about structure of a research summary, tips to write a good research summary, and common mistakes to write a research summary. ... Additionally, there needs to be a short but thorough explanation of how the findings of the ...
Research Summary. Definition: A research summary is a brief and concise overview of a research project or study that highlights its key findings, main points, and conclusions. It typically includes a description of the research problem, the research methods used, the results obtained, and the implications or significance of the findings.
A research summary is a crucial piece of writing that serves as an insightful overview of your research on a specific topic. As a researcher, it is essential to provide your reader with a detailed summary of the key findings from your study. When crafting a research summary, it is important to consider the structure of the article and the goal ...
A research article usually has seven major sections: Title, Abstract, Introduction, Method, Results, Discussion, and References. The first thing you should do is to decide why you need to summarize the article. If the purpose of the summary is to take notes to later remind yourself about the article you may want to write a longer summary ...
Reporting Research Results in APA Style | Tips & Examples. Published on December 21, 2020 by Pritha Bhandari.Revised on January 17, 2024. The results section of a quantitative research paper is where you summarize your data and report the findings of any relevant statistical analyses.. The APA manual provides rigorous guidelines for what to report in quantitative research papers in the fields ...
A summary should be written objectively and in a way that covers the article in sufficient detail—accurately yet briefly—to allow a reader to quickly absorb its significance. 3.1 Do some groundwork. Skim the article to get a rough idea of each section and the significance of the content. Read the paper in more depth.
Table of contents. When to write a summary. Step 1: Read the text. Step 2: Break the text down into sections. Step 3: Identify the key points in each section. Step 4: Write the summary. Step 5: Check the summary against the article. Other interesting articles. Frequently asked questions about summarizing.
Summarize each source: Determine the most important and relevant information from each source, such as the findings, methodology, theories, etc. Consider using an article summary, or study summary to help you organize and summarize your sources. ... Appraise issues or factors associated with your professional practice and research topic. Help ...
Empirical paper: Summarize your findings. In an empirical paper, this is the time to summarize your key findings. Don't go into great detail here (you will have presented your in-depth results and discussion already), but do clearly express the answers to the research questions you investigated.
Recent research suggests that study participants desire to have research findings returned to them, and that doing so can help build trust and encourage future research participation. A Narrative Summary is a written summary of your study's research findings. It is a useful way to succinctly summarize the purpose, main findings, and impact of ...
A research summary is a piece of writing that summarizes your research on a specific topic. Its primary goal is to offer the reader a detailed overview of the study with the key findings. A research summary generally contains the article's structure in which it is written. You must know the goal of your analysis before you launch a project.
Draft Introduction for Summary of Findings: In the introduction for the Summary of Findings, assert that you have answered your research questions. At a minimum you would tell the reader how many findings emerged and describe them in a sentence each. Most important is the findings you present in chapter 5 reflect and match what is significant ...
Scholarcy's AI summarization tool is designed to generate accurate, reliable article summaries. Our summarizer tool is trained to identify key terms, claims, and findings in academic papers. These insights are turned into digestible Summary Flashcards. Scroll in the box below to see the magic ⤸. The knowledge extraction and summarization ...
For most research papers in the social and behavioral sciences, there are two possible ways of organizing the results. Both approaches are appropriate in how you report your findings, but use only one approach. Present a synopsis of the results followed by an explanation of key findings. This approach can be used to highlight important findings.
A research summary is a concise and informative overview of a research article, report, or thesis. It aims to provide the reader with a clear understanding of the study's purpose, methods, results, and conclusions without having to read the entire document. Think of it as a mini-version of the original work that highlights its most important ...
These formats are supported by research that focused on improved understanding of the information they intend to convey (Carrasco-Labra et al 2016, Langendam et al 2016, Santesso et al 2016). ... Summary of findings (for interactive version click here) Compression stockings compared with no compression stockings for people taking long flights.
Qualitative Findings. Qualitative research is an exploratory research method used to understand the complexities of human behavior and experiences. Qualitative findings are non-numerical and descriptive data that describe the meaning and interpretation of the data collected. Examples of qualitative findings include quotes from participants ...
Step 1: Consult the guidelines or instructions that the target journal or publisher provides authors and read research papers it has published, especially those with similar topics, methods, or results to your study. The guidelines will generally outline specific requirements for the results or findings section, and the published articles will ...
The final pathway for systematic review is a statistical summary of the results of primary studies, or meta-analysis. This article provides some guidelines to health care providers in understanding the key aspects of systematic review and meta-analysis. Steps involved in systematic review are discussed. The potential pitfall of meta-analysis ...
Bass EB, Shekelle P, Treadwell J, Rosen M, Mull NK, Stewart CM, Motala A, Zhang A, Sharma, R. Making Healthcare Safer IV—Summary of Findings on Year 1 Topics. (Prepared by the Johns Hopkins, ECRI-Penn, and Southern California Evidence-based Practice Centers under Contract No. 75Q80120D00003). AHRQ Publication No. 23(24)-EHC019-15.
Like an abstract in a published research article, the purpose of an article summary is to give the reader a brief, structured overview of the study. To write a good summary, identify what information is important and condense that information for your reader. The better you understand a subject, the easier it is to explain it thoroughly and ...
In summary, HPAI H5-positive milk poses a risk when consumed untreated, but heat inactivation under the laboratory conditions used here reduces HPAI H5 virus titers by more than 4.5 log units.
Checklist: Research results 0 / 7. I have completed my data collection and analyzed the results. I have included all results that are relevant to my research questions. I have concisely and objectively reported each result, including relevant descriptive statistics and inferential statistics. I have stated whether each hypothesis was supported ...
SUMMARY: Findings of research misconduct have been made against Darrion Nguyen (Respondent), who was formerly a Laboratory Technician, Division of Pediatric Neurology and Developmental Neuroscience, Baylor College of Medicine (BCM). Respondent engaged in research misconduct in research supported by U.S. Public Health Service (PHS) funds ...
Researchers from Cleveland Clinic and IBM recently published findings in the Journal of Chemical Theory and Computation that could lay the groundwork for applying quantum computing methods to protein structure prediction. This publication is the first peer-reviewed quantum computing paper from the Cleveland Clinic-IBM Discovery Accelerator ...
Background West Nile virus (WNV), the most widely distributed flavivirus causing encephalitis globally, is a vector-borne pathogen of global importance. The changing climate is poised to reshape the landscape of various infectious diseases, particularly vector-borne ones like WNV. Understanding the anticipated geographical and range shifts in disease transmission due to climate change ...
In a finding that vastly expands the understanding of tumor evolution, researchers discover genetic biomarkers that can predict the breast cancer subtype a patient is likely to develop. ... the Canadian Institutes of Health Research and the Chan Zuckerberg Biohub. Krista Conger Krista Conger is a senior science writer in the Office of ...
The research sets the foundation for the development of better tools to study RNA modifications and their role in resistance while simultaneously opening new avenues for drug development. RNA-modifying enzymes, especially those linked to resistance, are currently understudied, and they are attractive targets for the development of new and more ...
America's best decade, according to data. One simple variable, more than anything, determines when you think the nation peaked. Mike Lee and his daughter, Zoey, play at Alethia Tanner Park in D ...
Job Summary: We seek an expert in multi-photon microscopy to direct and contribute to the integration of intravital microscopy into the research programs of multiple laboratories in the School of Medicine and Public Health. The position offers an exciting opportunity for a highly motivated scientist to collaborate and contribute to groundbreaking research in neuroscience and other disciplines.