Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Biofuels articles from across Nature Portfolio
Biofuels are fuels produced from hydrocarbon-rich living organisms (biomass) — such as plants or microalgae — by thermal, chemical or biochemical conversion processes. As with fuels, biofuels such as biodiesel, biogas and syngas are combusted to generate energy.
Related Subjects
- Bioalcohols
- Biomethanol
- Green diesel
- Solid biofuels
Latest Research and Reviews
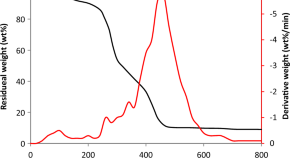
Experimental investigation on utilization of Sesbania grandiflora residues through thermochemical conversion process for the production of value added chemicals and biofuels
- Kedri Janardhana
- C. Sowmya Dhanalakshmi
- Melvin Victor De Poures
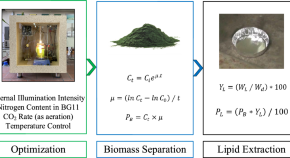
Lipids productivity of cyanobacterium Anabaena vaginicola in an internally illuminated photobioreactor using LED bar lights
- Hootan Goldoost
- Farzaneh Vahabzadeh
- Narges Fallah

Hydrogen storage and geo-methanation in a depleted underground hydrocarbon reservoir
Geologic formations could be used for hydrogen storage and conversion to methane, yet technical feasibility is unclear as field-scale data are lacking. Here the authors perform field tests demonstrating that hydrogen can be stored and microbially converted to methane in a depleted underground hydrocarbon reservoir.
- Cathrine Hellerschmied
- Johanna Schritter
- Andreas P. Loibner

Exergy-energy, sustainability, and emissions assessment of Guizotia abyssinica (L.) fuel blends with metallic nano additives
- M. S. Abishek
- Sabindra Kachhap
- Ali ELrashidi

Van Krevelen diagrams based on machine learning visualize feedstock-product relationships in thermal conversion processes
Feedstock properties play a crucial role in thermal conversion processes for power generation, fuel production, or chemical synthesis, but understanding the influence of these many properties on treatment performance is a complex task. Here, a series of van Krevelen diagrams were generated to illustrate the impact of H/C and O/C ratios of feedstock on the products obtained from six commonly used thermochemical processes.
- Yiying Wang
- Chi-Hwa Wang
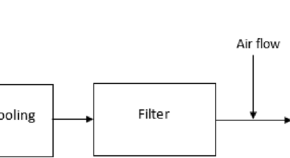
Startup process, safety and risk assessment of biomass gasification for off-grid rural electrification
- Md Mashiur Rahman
- Ulrik Birk Henriksen
- Daniel Ciolkosz
News and Comment
Impact of soil carbon changes.
- James Gallagher

Réunion’s search for energy self-sufficiency
Whether the French island succeeds in producing all of its electricity depends not only on technology, but also on social and political will.
- Rachel Nuwer

A pathway to bio-based aromatics
Aromatic compounds have broad applications in our daily life, but their production currently relies heavily on fossil resources. Now, a strategy enables synthesis of benzenoid aromatics from bioderived feedstock, paving the way to the more sustainable production of aromatics.
- Shuizhong Wang
- Guoyong Song

How to rescue biofuels from a sustainable dead end
An environmentally friendly path forwards for liquid fuel derived from plants will depend on smarter agriculture and smarter regulation.
- Peter Fairley
A fit transportation
Recent policies are promoting the conditions for a transformation of the transportation sector worldwide. Here, we look at the example from the European Union and reflect on the opportunities that initiatives such as Fit for 55 represent for catalysis science.

Bugging out for jet fuel
As an alternative to fossil fuels for shipping, aviation and rocketry, high-energy polycyclopropanated fuels can be generated using a bacterial host.
- Stacey-Lynn Paiva
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Forum article
- Open access
- Published: 18 May 2017
Biofuel research: perceptions of power and transition
- Lena Partzsch 1
Energy, Sustainability and Society volume 7 , Article number: 14 ( 2017 ) Cite this article
13k Accesses
8 Citations
1 Altmetric
Metrics details
Whether biofuels represent a sustainable innovation, a creative alternative, or a gold rush, very much depends on our perception of power and change with regard to sustainability. This article provides an overview of existing understandings of power in the research on biofuels, including positive perceptions that often lead to more optimistic evaluations of biofuels. It exposes the diversity with which one can understand power through three ideal type concepts: “power with,” “power to,” and “power over”. Integrating these concepts in one power framework allows for examining how the three dimensions interrelate with each other and developing the contours of a power lens on biofuel governance and research. With the 2007–2008 food price crisis, critics re-politicized the governance of biofuels. Several farmer associations have completely turned against biofuels. The article argues that this rejection of biofuels is due to a limited perception of power as a coercion and manipulation (power over). While the current governance of biofuels basically reproduces systems and positions, we should start to more seriously and intensively ask questions of where, when, and how the governance of biofuels may also allow for “green” resistance (power to) and collective empowerment (power with).
Introduction
Whether biofuels represent a sustainable innovation, a creative alternative or a gold rush [ 1 ], very much depends on our perception of power and change with regard to sustainability. This leads to the challenge of how to conceptualize these understandings. I gather diverse perceptions of power and illustrate them for biofuel research. The aim is to initiate a broader, more comprehensive debate across ontological and epistemological differences in this field of research. To begin the discussion, I introduce key components of the debate by identifying different perceptions of power that are common to research on biofuels along three ideal type conceptions:
Power with means collective empowerment through convincing and learning with and from each other. It refers to processes of developing shared values, finding common ground, and generating collective strengths [ 2 ]. Based on this understanding of power, biofuels can potentially be a sustainable innovation that serves the common good (climate protection, energy security, regional development, etc.) (e.g., [ 3 , 4 ]).
Power to corresponds to the ability of agents “to get things done” [ 5 ]. While Pitkin [ 6 ] defines power to as non-relational, Barnett and Duvall [ 7 ] define power to as tied to social relations of constitution that define who the actors are, along with their capacities and practices. Footnote 1 Scholars, who take a perspective of power to, may highlight the agency of producing biofuels as a creative alternative in hitherto fossil fuel-dependent societies (e.g., [ 8 , 9 ]).
Power over describes the direct and indirect ability of powerful actors, structures, and discourses to influence the actions and even the thoughts of others. It is based on power concepts by Dahl [ 10 ], Bachrach and Baratz [ 11 ], and Lukes [ 12 ], among others. I also discuss concepts of discursive power under this category (e.g., [ 13 , 14 ]), while I am aware that these concepts partly fall under the category of power to [ 7 ]. From a perspective of power over, biofuels can be seen as a gold rush: While everybody expected sudden wealth in this new field, there are very few winners and many losers (e.g. [ 15 , 16 ]).
I chose this tripartite approach as a framework for my article, because it is most comprehensive and makes an extension of the power discussion on biofuels possible. At the same time, the framework allows for the discussion of the well-known grouping of the four “faces of power” under the category of power over [ 17 , 18 ]. I will argue that in the research on biofuels, the understandings of power as power with and power to tend to prevail, even when they are not made explicit. This means that scholars have overemphasized the potential of biofuels as a creative alternative to fossil fuels and sustainable innovation for rural development. Concepts of power over have only more recently been applied, specifically since research has started to explicitly issue power. This has, in particular, been used to explain why any process of governing biofuels (biofuel governance) did not lead to urgent sustainability transitions, and why the biofuel boom should rather be seen as a gold rush. Scholars have demonstrated that the development of biofuels markets benefitted large companies and conglomerates [ 19 ]. Critical and post-structuralist perspectives have helped to understand this development by exploring structures and discourses favoring them [ 20 ]. Scholars have used Foucault’s concepts to outline how scientific knowledge practices render the very essence of problems (and solutions) raised on the biofuel agenda [ 21 , 22 ].
This article involves first of all implicit and explicit understandings of power (how do biofuel researchers think and talk about power?). These understandings are expressed in empirical research, as I will demonstrate below, and they hence also allow for an illustration of the practice of biofuel governance (how is power exercised in and through biofuel governance?). This makes the article also relevant for political practice. We should understand, not only in theoretical but also in practical terms, how we effectuate or prevent changes towards a more sustainable supply of energy and transport fuel. As in analytical heuristics, it is not possible to offhand separate power with , power to , and power over in empirical research. These categories shine multiple lights on different aspects of the same empirical phenomena. In practice, these forms of power exercise are mostly interrelated. My less concern is to weigh and compare the pros and cons of each perspective, but rather to outline an agenda for a multidimensional analysis of all three mechanisms of power and their interrelations.
In order to get the full picture of how change happens, we should understand how different perspectives add on to each other (besides overlaps and contradictions). To do this, I will begin by describing each perspective in itself. Based on a survey on biofuel research, I will give references for each perspective. These references are only illustrative. Then, I will exemplify the interrelations between each of these perspectives with respect to biofuel research. I explain how power imbalances can affect processes of power with and power to . Again, scholars have demonstrated how large conglomerates have manipulated biofuel governance in their favor, and why therefore the biofuels boom should be considered as a gold rush. However, I argue that interrelations may also work the other way around, and this is particularly relevant to the main argument of this article. Biofuels as a creative alternative and a sustainable innovation may also provoke changes in existing relations of power over and contribute to address asymmetries and inequalities in agrifood and transport systems. We need a multidimensional power approach to explore these interrelations.
Biofuel: sustainable innovation (power with)
Research on biofuel governance and other studies in the field of sustainability are most often based on a positive perception of power in the sense of power with . Power with is a term that refers to processes of developing shared values, finding common ground, and generating collective strengths [ 2 ]. This conception does not necessarily refer to the diffusion of already existing (predefined) norms. Rather, power with implies learning processes that allow actors to question self-perceptions and to actively build up a new awareness of individuals or groups [ 23 , 24 ]. In this vein, with regard to biofuels, scholars have assumed that collective empowerment and solidarity are possible and that biofuel technologies as a “sustainable innovation” can pave the way to post-carbon societies [ 25 , 26 ].
Power with is often linked to Arendt’s definition of power [ 27 ]. Footnote 2 According to Arendt, power always refers to a group or to a collective of individuals:
Power corresponds to the human ability not just to act but to act in concert. Power is never the property of an individual; it belongs to a group and remains in existence only so long as the group keeps together. When we say of somebody that he is ‘in power’ we actually refer to his being empowered by a certain number of people to act in their name ([ 28 ]: 44). Footnote 3
Research on environmental leadership (e.g., [ 29 ]) in pioneer countries, such as Germany and France in the biofuel sector [ 3 , 30 ], most obviously reflects such an understanding of power. Leaders or pioneers are empowered to act in the name of others from this perspective (while they dominate others from a perspective of power over , see below). In this sense, (Young [ 31 ]: 285) defines leadership in the interest of common welfare:
Leadership (…) refers to the actions of individuals who endeavor to solve or circumvent the collective action problems that plague the efforts of parties seeking to reap joint gains in processes of institutional bargaining.
Leaders and pioneers do not enforce their own interests against or over others; rather they seek “to reap joint gains” of environmentalism. Environmental leadership studies, based on such an understanding of power, usually follow the discourse of Ecological Modernization that highlights flexible and cost-efficient problem solving. Ecological modernization outlines a win-win storyline of environmental protection that benefits green (biofuel) business as much as the environment [ 32 , 33 ]. From this perspective, those who are neither leaders nor pioneers are considered free-riders or laggards , rather than subordinates. Non-leaders also benefit, at least in the long run, from power (with), since biofuels are expected to tackle common problems, such as climate change, enhance energy security, and to contribute to regional development [ 3 , 34 ]. Policies promoting biofuels are hence per se seen to be desirable since, from this perspective, they serve everybody’s interest.
Scholars have extensively analyzed the emergence, diffusion, efficiency, and effectiveness of policies promoting biofuels, with the (at least implicit) aim to foster their adoption and implementation [ 30 , 35 ]. In this context, policy learning and experiments have been gaining momentum [ 9 , 26 ]. Deliberative processes, including third-party certification schemes, were initiated and observed with the aim to introduce sustainable biofuel production schemes that would integrate those formerly excluded stakeholders with new technology; in everyday practice, every actor in the field would then become a winner [ 4 , 36 ].
Scholars who share this perspective of power as power with do not think in dichotomies such as winners - losers or good-bad . Instead, they understand power (or similar concepts, such as leadership) as serving the common good (climate protection, energy security, and sustainability). As there are no subordinates from this power perspective, no imperative follows to empower or to resist. The empowerment of non-leaders is not an issue because scholars assume that, in principle, they are also interested in developing sustainable innovations and that they likewise benefit from respective leadership efforts.
Biofuel: creative alternative and “green” resistance (power to)
While power with pertains to collective empowerment and solidarity, power to refers to single actors and separate groups, such as farmers, co-operatives, and individual processors who were initially key players in pioneering biofuel regions [ 19 ]. Accordingly, biofuels are often seen as an opportunity to empower green ideas and values. Pitkin [ 6 ] emphasizes how power can be non-relational, since an actor may have the power to accomplish something all by him- or herself. This understanding of power is related to the development of an individual identity; self-confidence and consciousness raising [ 23 ]. It is here where Nussbaum’s and Sen’s [ 37 ] capability approach comes in, which defines power as “a capability to act upon one’s environment” [ 38 ]. For example, an individual farmer can simply start to produce and use biomass-based fuels without any permission or interference from another actor, such as the petrol industry. However, constructivist research has demonstrated how every actor or group is defined through socially constituted relations that, at least indirectly, shape the actions of individuals [ 7 ]: only a farmer who receives knowledge about alternative technologies may effectively implement them.
Power to can be linked to Parsons’ definition of power as the ability “to get things done” [ 5 ]. It highlights a productive agency, especially in the cases where actors’ goals are opposed or resisted. Biofuel research by small farmers and rural communities is often based on this perception of power [ 9 , 39 ]. Scholars highlight the potential of biofuels for rural development by providing new markets for agricultural production. They assume that through the introduction of radically new technologies in niches, farmers are able to empower themselves in an attempt of an “agro-ecological revolution” [ 8 ]. They highlight the self-empowering agency of hitherto marginalized people to become “energy sheiks” [ 40 ], based on biomass production.
Scholars, who take a perspective of power to , focus on the productive agency of the biofuel sector. They are interested in the empowerment of alternative ideas and values which, in the case of biofuels, allow for transforming fossil fuel-dependent societies. These alternative agents criticize the practices or the authority of the dominant, carbon-intense system and refuse to reproduce their own positions in this system. Their non-conformism is perceived to serve the common good as they develop alternative technologies required by everyone in a world beyond petrol. From a perspective of power to and in difference to a perspective of power with , there are only a limited number of transformational agents: not everybody in the field is assumed to be a “winner” in the first place; there are only a few “energy sheiks”. However, scholars see an imperative to act based on normatively prior “green” values, for example, climate protection and sustainability (and everybody benefits from the realization of these values).
Biofuel: gold rush (power over)
Scholars who explicitly issue power in the context of biofuels usually perceive power as asymmetric. Biofuel governance is seen as a zero-sum game which produces winners and losers. From this perspective, powerful actors, structures, and discourses in the field of biofuel governance influence the actions and even the thoughts of others. In the following, I will illustrate this perspective, further differentiating the “four faces” of power over (see Table 1 ): visible , hidden , invisible , and unconscious power [ 2 , 41 ]. (the fourth dimension does not understand power as a zero-sum game and can also be added to power to , see the first footnote.)
In the first dimension, agents exercise visible power when they directly influence political decision-makers based on their material and ideational resources [ 42 ]. What is visible is not the power as such, but rather its physical means such as lobbying activities, party financing, and armed force. (Dahl [ 10 ]: 201) defines: “A has power over B to the extent that he can get B to do something that B would not otherwise do” (emphasis added). Any kind of state force implementing objectives of sustainability by top downregulation means exercising direct power. Non-state actors may also play a role in this game. Coase [ 43 ] explains this for business firms. Also when Pilgrim and Harvey [ 44 ] demonstrate how NGO lobbying significantly affected biofuel policy changes and sustainability regulation in the UK and in Europe, they assume that NGOs enforce their ideas against others in an arena of obviously competing demands.
The second dimension of hidden power refers to power not obviously opposed by anyone. Bachrach and Baratz [ 11 ] speak of “two faces of power” emphasizing that some issues never even make it onto the political agenda and are dismissed before observable negotiations start. For a long time, the EU issued biofuels only in the context of climate change, completely neglecting aspects of competing food demands and land use change in the Global South [ 45 , 46 ]. Scholars demonstrating such hidden aspects apply this second dimension of power over to analyze biofuel governance.
The traditional conception of structural (hidden) power in international relations aims to address the coercion resulting from the capital mobility of transnational corporations. Threats to shift investments abroad do not even need to be voiced in order to influence policies in their favor [ 42 , 47 ]. More recent studies point to the fact that businesses also exercise structural power by self-regulation and public-private partnerships; these types of governance allow business actors to actively set rules, for example, for the “sustainable” production of biofuels at the expense of state actors [ 42 , 48 ]. In addition, as public authorities have faced challenges in facilitating the implementation of their sustainability criteria outside their jurisdictions, the EU has started to use these private schemes to verify compliance with sustainability criteria in biofuel production outside its own territory [ 49 , 50 ]. As a result, following this perspective, power in the global political economy has been diffused, leaving biofuel conglomerates with considerable power over others [ 51 ].
Further, scholars are increasingly focusing on power relations linked to latent conflicts of interest. In the third dimension, invisible power comes to play as a result of norms and ideas [ 41 ]. Research analyzes discourses, communication practices, cultural values and institutions, which all work to shape relevant thoughts and actions [ 12 ]. With regard to biofuels, Munro [ 22 ] has shown how, in the United States, a powerful coalition of agricultural interests manipulated the governance of biofuels by linking it to public concerns about climate change and energy security. In consequence, corn biofuel received political support, tax reductions, and subsidies. Likewise, Puttkammer and Grethe [ 52 ] have found a coalition of biofuel advocates to dominate the public discourse in Germany, while scientists who doubted the efficiency of biofuels could not make their voice heard. The discourse only shifted with the 2007–2008 food price crisis when scholars demystified the “ethanol bubble” [ 53 ] and outlined potentially devastating implications for global poverty and food security. Experts, NGOs, and business actors who have challenged the sustainability of biofuels on many fronts began to be heard [ 20 , 22 ].
For the most part, these discourse scholars blame other scholars who apply a perspective of power with for neglecting and postponing important questions of social justice linked to biofuel production [ 21 , 54 ]. Win-win rhetoric is demonstrated to manifest global power asymmetries rather than to contribute to more ecology and fairness [ 22 , 53 ]. From this perspective, pioneers and leaders, whose role Young [ 31 ] and Bernard and Prieur [ 30 ], among others, consider to be positive, only serve dominant interests and prevent a more fundamental social transformation to sustainability. With reference to the International Political Economy, most scholars deny a simple confrontation of biofuel proponents (or pioneers) and opponents (or laggards). In this vein, Levidow [ 55 ] outlines how the EU can continue “its global plunder of resources” because it pursues global leadership for sustainable biofuels. Silva-Castaneda [ 56 ] demonstrates how, in Indonesia, some NGOs decided to participate in the Roundtable on Sustainable Palm Oil (RSPO), a certification process initiated by the WWF, among others. The local NGOs managed to include important clauses regarding indigenous and land rights in the RSPO standard. In practice, however, auditors rarely recognize as valid evidence the forms of proof put forward by local communities, and global conglomerates could even use the standards to increase their primacy vis-à-vis local farmers [ 56 ]. These examples reveal power over within multi-stakeholder processes.
Studies demonstrate that the expansion of biofuels in countries of the Global South was only possible through the partial neglect (simplification) of their cultural and ecological diversity [ 57 ]. Nygren [ 58 ] illustrates how leading retailers, in negotiation with environmental organizations, have guided consumers’ expectations of certified Southern forest products by building images of Southern community forest producers as authentic and exotic others . She concludes that certification as a market-based form of governance has only had a limited impact on altering the unequal relationship characteristic of global networks of production and consumption.
With reference to Foucault [ 13 ] and Bourdieu [ 59 ], we can capture links between knowledge, power, and politics in a fourth dimension of power over [ 17 ]. Critical and (post-) structuralist approaches understand power in a way that everything is socially constructed. Scholars analyze the normative impact on (supposed) losers, such as farmers in the Global South, as well as on (supposed) winners, such as major agribusiness actors. All actors work to mainly reproduce systems and positions [ 60 ]. With regard to biofuels, several studies have highlighted the central role of knowledge and framing [ 15 , 16 , 21 ]. Drawing on Foucault, Kuchler and Linnér [ 21 ] have analyzed the discursive practices of the three major international organizations focused on food and agriculture, energy, and climate with regard to biofuels over the last 20 years: the UN Food and Agriculture Organization (FAO), the International Energy Agency (IEA), and the Intergovernmental Panel on Climate Change (IPCC). They found that, in contrast to pro and contra accounts, the arguments of all three organizations reflected a policy consensus based on the mainstream notion of industrial agricultural production, promoting the intensification and expansion of rural production. The biofuel discourse has further constituted a concatenation of the three issues of agricultural production, energy security, and climate change mitigation. When the discourse shifted with the 2007–2008 food price crisis, all the three major organizations adapted to this shift [ 21 ]. Instead of exercising power over by manipulating discourses on biofuels according to specific pro or contra interests, the organizations were found to rather reproduce hegemonic discourses and their own positions.
The gold rush metaphor is used a lot to describe the situation of biofuels from a power over perspective [ 1 ]. Biofuel production, like gold mining, is unprofitable for most farmers, just like it was for diggers and mine owners. Both biofuel production and gold mining can in addition have very negative environmental effects. While, however, people are made to believe that everyone can become abundantly wealthy (“energy sheiks”), only some few investors make large fortunes. Applying discursive approaches of power over , we can argue that even such investors and major businesses are subject to and not only conscious manipulators of discourses of agricultural intensification and economic growth. The analysis of power over helps to understand why change to more sustainable transport and agricultural systems does not happen. However, as I argue in this article, it falls short on explaining when and why there also sometimes is disruptive change and empowerment.
Power to change: interrelations between power with, power to, and power over
While the perspectives of power with and power to (over-) emphasize the potential for change with regard to biofuels, scholars with understandings of power over often exaggerate their negative impacts. The tripartite framework allows for the combining of different analytical perspectives and to examine their interrelations. While the three categories are first of all analytical heuristics, they also stand for different mechanisms of the exercise of power (see Fig. 1 ). Power over affects what is considered a “sustainable innovation” and “creative alternative”. Research has demonstrated this. However, I argue that it is also possible the other way round: there are situations in which power with and power to can address power imbalances and prevent a situation in which there are only a few winners and many losers as a result of biofuel governance.
Agent-based power
As shown in Fig. 1 , besides considering material and ideational sources of power, we also need to consider different mechanisms of power (over/to/with), since they lead to different results of power (leading to a new distribution of sources in a circular process, see the arrow at the bottom of Fig. 1 ). Biofuels per se are neither a sustainable innovation, a creative alternative nor a gold rush. The three metaphors exemplify three different results of power: the exercise of power over leads to a gold rush situation. So, if scholars only ask for power over , they will always find winners and losers. By contrast, if we ask for the exercise of power to , we may find that biofuels are creative alternative. Finally, the exercise of power with can be exemplified by a case of finding an agreement on sustainability criteria of biofuel production. To demonstrate overlaps, especially, in terms of the results of power, I used dashed lines in Fig. 1 .
When, in the field of biofuels, scholars explicitly issue power, they generally use concepts of power over to explain why governance and research in this field have a blind spot for power asymmetries [ 49 , 53 ]. Biofuel opponents may have accomplished a shift in the biofuel discourse after the 2007–2008 food price crisis [ 20 , 22 ]. However, overriding power asymmetries have prevented a structural change in both the energy/transport and the agricultural sectors. The trend is now definitely towards large companies and conglomerates [ 49 , 50 ].
However, the fact that biofuels have caused no structural change and have disadvantaged rather than empowered small farmers in the Global South, does not mean that a structural change is impossible. What I want to argue in this article is that exercising consensual forms of power (power with) as well as self-empowerment and resistance (power to) can also eclipse and overcome power asymmetries (power over). Empirical research on deliberative processes suggests that communication and common action never happen among equals and that they are never free from any form of power over [ 36 , 61 ]. Hence, we need to understand power with as a form of exercising power, which is strategic (bargaining) as well as communicative (arguing). A crucial part of this process is the orientation of agents involved in processes of biofuel governance. If actors are open to changing their positions and developing shared understandings, transitions to sustainability can follow from dialogues [ 61 , 62 ].
Following this perspective, even if small farmers in the Global South have fewer capabilities compared to conglomerates from the EU and the United States, this does not mean that they have no possibility to act independently from them. For example, sugar is costly to establish, and thus is economically most efficient at large plantation scales. However, Jatropha can more readily be produced through outgrower schemes as it is less capital intensive [ 9 , 49 ]. While currently almost all bio-ethanol is produced from grain or sugarcane and therefore competes with food purposes, other efficient and economically viable technologies for ethanol production are available [ 63 ]. The production of perennial energy crops, such as grasses and trees, and crop residues, such as straw, are seen to require fewer inputs and less prime land [ 64 ].
Under specific conditions, empowerment is possible; processes of power with and power to can have a (positive) impact on unwanted relations of power over . For example, processes of stakeholder dialogue and certification demonstrate that an agreement beyond the lowest common denominator is possible. In addition, they can weaken the perceived legitimacy of powerful actors that are producing biofuels unsustainably. The critical discourse on biomass certification has issued consumers’ accountability for harmful social and environmental effects in countries of production [ 55 , 65 ]. When the legitimacy of unconditional import as well as of private certification schemes was put into question [ 50 ], transnational conglomerates lost ideational and material resources on which their power over others was based. In the agrifood sector, we can clearly see that certification has become a new normative obligation [ 66 ].
We can observe various kinds of empowerment and resistance related to biofuels. While Nygren [ 58 ] argues that certification schemes reproduce (inferior) positions of southern producers as authentic and exotic others, she does not completely deny that certification had a positive impact on altering asymmetries in global networks of production and consumption. Silva-Castaneda’s [ 56 ] study discloses new ways in which local communities can legally prove their land rights, for instance, by video documentation to replace missing formal documents or destructed land marks.
Scholars have described movements, such as Via Campesina, in terms of exercising power over and opposing transnational agriculture corporations [ 67 ]. In terms of reducing and overcoming power asymmetries, however, what is most striking is the fact that small farmers within this movement exercise power to by doing healthy and sustainable agriculture independently of the major agribusinesses to which, from a power over perspective, they would only be subordinated. At the same time, when producing organically, small farmers do not reproduce the system of industrial agricultural production (and their inferior positions within that system). So, their way of farming can be considered as a creative alternative and as a way of resistance. Moreover, within this movement of Via Campesina, despite widely different internal cultures, farmers also exercise power with by (re-) constituting a new shared peasant identity. From a perspective of power with, we can argue that, in the long run, everybody, even from outside this movement, may benefit and share norms and values developed here such as sustainability in farming. The movement delegitimizes the acquisition of land by established conglomerates (“land grabbing”), whose ideational sources of power shrink in consequence. The visible result is a new, more equal, and just distribution of (power) resources through land reforms.
Conclusions
This article should not only encourage a debate on power issues with regard to biofuels, but moreover, develop the debate more comprehensively. When political power has been analyzed in the context of biofuels, this has happened so far through using confrontational or structuralist and discursive approaches that are based on an understanding of power over . Respective scholars have accused other researchers of neglecting “real power concentrations” in the biofuels industries. Often quite rightly: biofuel research has neglected the limits of win-win for a very long time. Scholars have taken sides and normatively inflated their own pro biofuel position, while they have dispatched their adversaries as laggards with regard to the future of transport and agriculture. Of course, not every (supposedly) sustainable innovation is necessarily good in the sense that it is completely uncontroversial (even if there is no visible opposition as in the case of biofuels for a long time). In this context, the question of power essentially addresses the re-politicization of decisions perceived to be urgent and without alternative. With the 2007–2008’s shift in discourse, critics re-politicized the governance of biofuels. Several farmer associations have completely turned against biofuels. I argue that this rejection of biofuels is due to a limited perception of power as power over .
Why does it make sense to complement such a perception of power over ? Why does a multidimensional power framework make more sense? Naming different perspectives, as done here, with one and the same term—“power”—means, first, to put them on one normative level. Gold rush (power over) is a term with strongly negative connotations, on the one hand, and leads to normatively inflating sustainable innovations (power with) and creative resistance (power to), on the other. This is often unjustified because the exercise of power with and power to are not per se more legitimate forms of achieving social change. For example, preventing greenhouse gas emissions “from above” can be quite legitimate.
Secondly, as illustrated in this article, all three conceptions of power are already used in research on biofuels (although sometimes only implicitly; this should change). My hope is that this article addresses diverse communities and overcomes boundaries between them with this multidimensional power approach (in particular, between those who still celebrate biofuels as a “sustainable innovation” and those scholars who completely condemn them because of related power asymmetries). Especially those whose research is (implicitly) based on understandings of power as power with and power to could take stronger reference to researchers taking a critical viewpoint on their studies (power over)—in particular, through showing how consensual forms of power exercise (power with) and resistance and empowerment (power to) not only reproduce power asymmetries but also help overcome them. If we look at the gold rush metaphor from a perspective of power to , we may see that there is a lot of entrepreneurship involved in the discovery of gold deposits. From the perspective of power with , we may also see that people in the field of gold mining as well as of biofuel production find common ground among diverse interests and organize with each other.
Third, convincing and learning (power with) as well as creative ability (power to) and coercion and manipulation (power over) do not completely capture concrete change processes. The analytical categories applied in this paper help to cluster the various understandings of power in biofuel research, but they also reflect different mechanisms of power in reality. Power with perspectives focus on the benefits of biofuels (sustainable innovation); power to focuses on how new actors develop alternatives to fossil (and nuclear)-based economies; power over points to the limits of change because of the dominance of specific actors, structures, and discourses. The common terminology allows that the three perspectives on power are not considered as mutually exclusive (different interpretations of the same phenomenon), but as supplementary (different aspects of a change process). It becomes possible to examine their interrelations and their supplementary potential. With this article, I hope to have given an impetus for further research in this direction. A comprehensive analysis of power in diverse parts of biofuel research and governance is definitely a prerequisite for more seriously and intensively exploring questions of where, when, and how the governance of biofuels may also allow for “green” resistance and collective empowerment.
If actors create (reproduce) discourses and structures, I call this power to . Most constructivist studies however deal with identifying dominant (hegemonic) structures and discourses over others that are unconsciously reproduced, i.e., power over .
Power with is not identical to Arendt’s understanding of power or its empirical operationalization hardly accomplishes Arendt’s demands. So deliberative theories of democracy build upon her understanding of power without finding it comprehensively implemented in reality [ 61 , 68 , 69 ]. In difference to deliberative processes, power with encompasses communicative as well as common action.
An example, to which Arendt refers in a footnote to her definition of power, is the student protests at Berkeley and elsewhere at the end of the 1960s. She contrasts the power of the students—“obviously the strongest power on every campus simply because of the students’ superior number” ([ 28 ]: 44)—to the violence of the university authorities. An individual student leader ‘in power’ would speak on behalf of the movement.
Balkema A, Pols AJ (2015) Biofuels: sustainable innovation or gold rush? Identifying responsibilities for biofuel innovations. In: Koops B-J, Oosterlaken I, Romijn H et al (eds) Responsible innovation 2: concepts, approaches, and applications. Springer, New York, pp 283–303
Google Scholar
Partzsch L (2015) Kein Wandel ohne Macht–Nachhaltigkeitsforschung braucht ein mehrdimensionales Machtverständnis. GAIA 24(1):48–56
Article Google Scholar
Sordaa G, Banseb M, Kemfert C (2010) An overview of biofuel policies across the world. Energy Policy 38(11):6977–6988
Vermeulen S, Dufey A, Vorley B (2008) Biofuels: making tough choices. The International Institute for Environment and Development (IIED) (2)., pp 1–2
Parsons T (1963) On the concept of political power. Proc Am Philos Soc 107(3):232–262
Pitkin HF (1985) Wittgenstein and justice: on the significance of Ludwig Wittgenstein for social and political thought. University of California Press, Berkeley
Barnett M, Duvall R (2005) Power in global governance. In: Barnett MN, Duvall R (eds) Power in global governance. Cambridge University Press, Cambridge, pp 1–32
Altieri MA, Toledo VM (2011) The agroecological revolution in Latin America: rescuing nature, ensuring food sovereignty and empowering peasants. J Peasant Stud 38(3):587–612
van Eijcka J, Romijn H (2008) Prospects for Jatropha biofuels in Tanzania: an analysis with strategic niche management. Energy Policy 36(1):311–325
Dahl RA (1957) The concept of power. Behav Sci 2(3):201–215
Bachrach P, Baratz MS (1962) Two faces of power. Am Polit Sci Rev 4(56):947–952
Lukes S (1974) Power: a radical view. Macmillan, London
Book Google Scholar
Foucault M (1982) The subject and power. Crit Inq 8(4):777–795
Laclau E, Mouffe C (1985) Hegemony and socialist strategy: towards a radical democatic politics. Verso, London
Baka J (2014) What wastelands? A critique of biofuel policy discourse in South India. Geoforum 54(6):315–323
Hunsberger C (2010) The politics of Jatropha-based biofuels in Kenya: convergence and divergence among NGOs, donors, government officials and farmers. J Peasant Stud 37(4):939–962
Digeser P (1992) The fourth face of power. J Polit 54(4):977–1007
Haugaard M (2012) Rethinking the four dimensions of power: domination and empowerment. J Polit Power 5(1):33–54
Hunt SC, Sawin JL, Stair P (2006) Cultivating renewable alternatives to oil. In: Worldwatch Institute (ed) State of the world 2006: Special focus: India and China. Earthscan, London, pp 61–77
Sengers F, Raven R, van Venrooij A (2010) From riches to rags: biofuels, media discourses, and resistance to sustainable energy technologies. Energy Policy 38(9):5013–5027
Kuchler M, Linnér B-O (2012) Challenging the food vs. fuel dilemma: genealogical analysis of the biofuel discourse pursued by international organizations. Food Policy 37(5):581–588
Munro B (2015) The lost innocenece of ethanol: Power, knowledge, discourse, and the U.S. biofuel policy: Doctoral dissertation. Kansas State University, Kansas
Eyben R, Harris C, Pettit J (2006) Introduction: exploring power for change. IDS Bull 37(6):1–10
Gaard G (2010) Women, water, energy: an ecofeminist approach. In: Brown PG, Schmidt JJ (eds) Water ethics: foundational readings for students and professionals. Island Press, Washington, DC, pp 59–75
Balat M, Balat H (2009) Recent trends in global production and utilization of bio-ethanol fuel. Appl Energy 86(11):2273–2282
Nill J, Kemp R (2009) Evolutionary approaches for sustainable innovation policies: from niche to paradigm? Res Policy 38(4):668–680
Allen A (1998) Rethinking power. Hypatia 13(1):21–40
Arendt H (1970) On violence. Harcourt, Brace & World, New York
Skodvin T, Andresen S (2006) Leadership revisited. Glob Environ Polit 6(3):13–27
Bernard F, Prieur A (2007) Biofuel market and carbon modeling to analyse French biofuel policy. Energy Policy 35:5991–6002
Young OR (1991) Political leadership and regime formation: on the development of institutions in international society. Int Organ 45(3):281–308
Huber J (2000) Towards industrial ecology: sustainable development as a concept of ecological modernization. J Environ Policy Plan 2(4):269–285
Jänicke M (2008) Ecological modernisation: new perspectives. J Clean Prod 16(5):557–565
Bomb C, Deurwaarder E, Kaberger T (2007) Biofuels for transport in Europe: lessons from Germany and the UK. Energy Policy 35(4):2256–2267
Foxona T, Pearson P (2008) Overcoming barriers to innovation and diffusion of cleaner technologies: some features of a sustainable innovation policy regime. J Clean Prod 16(1):S148–S161
Longstaff H, Secko DM (2014) Assessing the quality of a deliberative democracy mini-public event about advanced biofuel production and development in Canada. Public Understanding of Science (online)., pp 1–10
Nussbaum M, Sen AK (1987) Internal criticism and Indian rationalist traditions. World Institute for Development Economics Research of the United Nations University, Helsinki
Scholtes F (2009) Analysing and explaining power a capability perspective, Bonn
Malik US, Ahmed M, Sombilla MA et al (2009) Biofuels production for smallholder producers in the Greater Mekong Sub-region. Appl Energy 86(S1):S58–S68
Rathke G-W, Diepenbrock W (2006) Mit Biomasse zum ‘Energie-Scheich’?: Stand, Ertragspotenziale und Perspektiven. Chancen für Landwirtschaft und angrenzende Industriezweige. In: DLG (ed) (Deutsche Landwirtschafts-Gesellschaft) Zukunftsstandort Deutschland: Strategien Für Die Landwirtschaft. DLG-Verlag, Frankfurt a.M
Gaventa J (2006) Finding the spaces for change: a power analysis. IDS Bull 37(6):23–33
Fuchs D (2005) Understanding business power in global governance. Nomos, Baden-Baden
Coase RH (1988) The firm, the market, and the law. University of Chicago Press, Chicago
Pilgrim S, Harvey M (2010) Battles over biofuels in Europe: NGOs and the politics of markets. Sociol Res Online 13(3):4
Stattman SL, Gupta A (2015) Negotiating authority in the global biofuel governance: Brazil and the EU in the WTO. Glob Environ Polit 15(1):41–59
Afionis S, Stringer LC (2012) European Union leadership in biofuels regulation: Europe as a normative power? J Clean Prod 32(1):114–123
Altvater E, Mahnkopf B (1999) Grenzen der Globalisierung: Ökonomie, Ökologie und Politik in der Weltgesellschaft, 4th edn. Westfälisches Dampfboot, Münster
Levin K, Cashore B, Koppell J (2009) Can non-state certification systems bolster state-centered efforts to promote sustainable development through the clean development mechanism? Wake Forest Law Review 44(1):777–798
Dauvergne P, Neville KJ (2010) Forests, food, and fuel in the tropics: the uneven social and ecological consequences of the emerging political economy of biofuels. J Peasant Stud 37(4):631–660
Ponte S, Daugbjerg C (2015) Biofuel sustainability and the formation of transnational hybrid governance. Environ Polit 24(1):96–114
Henriksen LF (2015) The global network of biofuel sustainability standards-setters. Environ Polit 24(1):115–137
Puttkammer J, Grethe H (2015) The public debate on biofuels in Germany: who drives the discourse? Ger J Agric Econ 64(4):263–273
Runge CF, Senauer B (2007) How biofuels could starve the poor. Foreign Aff 86(3):41–53
Horlings LG, Marsden TK (2011) Towards the real green revolution? Exploring the conceptual dimensions of a new ecological modernisation of agriculture that could ‘feed the world’. Glob Environ Chang 21(2):441–452
Levidow L (2013) EU criteria for sustainable biofuels: accounting for carbon, depoliticizing plunder. Geoforum 44(1):211–223
Silva-Castaneda L (2012) A forest of evidence: third-party certification and multiple forms of proof—a case study of oil palm plantations in Indonesia. Agric Hum Values 29(3):361–370
Selfa T, Bain C, Moreno R et al (2015) Interrogating social sustainability in the biofuels sector in Latin America: tensions between global standards and local experiences in Mexico, Brazil, and Colombia. Environmental Management
Nygren A (2015) Governance and images: representations of certified southern producers in high-quality design markets. Environ Values 24(3):391–412
Bourdieu P (1987) Sozialer Sinn: Kritik der theoretischen Vernunft. Suhrkamp, Frankfurt a.M
Guzzini S (1993) Structural power: the limits of neorealist power analysis. Int Organ 47(3):443–478
Dryzek JS (2000) Deliberative democracy and beyond: liberals, critics, contestations. Oxford University Press, New York
Prittwitz V (ed) (1996) Verhandeln und Argumentieren. Leske + Budrich, Opladen
Mussatto SI, Dragone G, Guimarães PM et al (2010) Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv 28(6):817–830
Shortall O (2014) Rethinking bioenergy from an agricultural perspective: ethical issues raised by perennial energy crop and crop residue production for energy in the UK and Denmark: PhD thesis. University of Nottingham, Nottigham
Partzsch L (2011) The legitimacy of biofuel certification. Agric Hum Values 28(3):413–425
Kalfagianni A (2015) ‘Just food’: the normative obligations of private agrifood governance. Glob Environ Chang 31(1):174–186
Martínez-Torres ME, Rosset PM (2010) La Vía Campesina: the birth and evolution of a transnational social movement. J Peasant Stud 37(1):149–175
Habermas J (1998) Between facts and norms: contributions to a discourse theory of law and democracy. MIT Press, Cambridge
Young IM (2001) Activist challenges to deliberative democracy. Polit Theo 29(5):670–690
Partzsch L, Fuchs DA (2012) Philanthropy: power with in international relations. J Polit Power 5(3):359–376
Guzzini S (2007) The concept of power: a constructivist analysis. In: Berenskoetter F, Williams MJ (eds) Power in world politics. Routledge, New York, pp 23–42
Download references
Acknowledgements
The research received no funding.
Competing interests
The author declares that she has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and affiliations.
Sustainability Governance, Institute of Environmental Social Sciences and Geography, University of Freiburg, Tennenbacher Strasse 4, D-79106, Freiburg, Germany
Lena Partzsch
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Lena Partzsch .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Cite this article.
Partzsch, L. Biofuel research: perceptions of power and transition. Energ Sustain Soc 7 , 14 (2017). https://doi.org/10.1186/s13705-017-0116-1
Download citation
Received : 01 February 2017
Accepted : 04 May 2017
Published : 18 May 2017
DOI : https://doi.org/10.1186/s13705-017-0116-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Energy, Sustainability and Society
ISSN: 2192-0567
- General enquiries: [email protected]
Advertisement
A Comprehensive Review of Feedstocks as Sustainable Substrates for Next-Generation Biofuels
- Published: 07 April 2022
- Volume 16 , pages 105–122, ( 2023 )
Cite this article
- Aditi Singh 1 , 2 ,
- Priya Prajapati 1 , 3 ,
- Shaili Vyas 1 , 3 ,
- Vivek Kumar Gaur 4 , 5 ,
- Raveendran Sindhu 6 ,
- Parameswaran Binod 7 ,
- Vinod Kumar 8 ,
- Reeta Rani Singhania 9 ,
- Mukesh Kumar Awasthi 10 ,
- Zengqiang Zhang 10 &
- Sunita Varjani ORCID: orcid.org/0000-0001-6966-7768 1
1957 Accesses
14 Citations
1 Altmetric
Explore all metrics
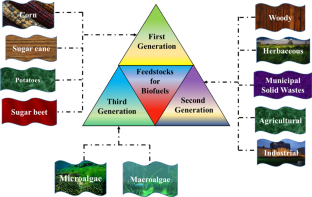
With the rise in global population, industrialization, and economic expansion, the persistent overconsumption of conventional fossil fuels has resulted in the depletion of fossil fuel reserves. This has fuelled the need to investigate and boost scientific research efforts on sustainable and renewable bioenergy feedstock. A substitute that can minimize reliance on nonrenewable energy resources while also lowering greenhouse gas emissions. In this context, biofuels have received a great deal of interest in recent years as a prospective substitute for conventional fossil fuels. The prime reason behind this is the feedstock utilized in their synthesis. The feedstocks employed here are environmentally safe, nontoxic, and emit little to no pollution. These feedstocks are classified into four generations: first, second, third, and fourth. Food crops and lignocellulosic biomass and waste constitute first- and second-generation feedstocks. The third- and fourth-generation feedstock is microalgae. This paper provides a comprehensive overview of feedstocks utilized to produce biofuels, including the various pre-treatment methods, strategies, and techno-economic analysis in order to pave the way for next-generation biofuels. It also covers the advantages, drawbacks, challenges, and current developments.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price excludes VAT (USA) Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Biofuels Production from Diverse Bioresources: Global Scenario and Future Challenges

A Broad Introduction to First-, Second-, and Third-Generation Biofuels

Biofuels: The Environment-Friendly Energy Carriers
Data availability.
All data and material used for preparing the manuscript appear in the submitted article.
Fang Y, Paul MC, Varjani S et al (2021) Concentrated solar thermochemical gasification of biomass: principles, applications, and development. Renew Sustain Energy Rev 150:111484. https://doi.org/10.1016/j.rser.2021.111484
Article CAS Google Scholar
Ramos JL, García-Lorente F, Valdivia M, Duque E (2017) Green biofuels and bioproducts: bases for sustainability analysis. Microb Biotechnol 10:1111–1113. https://doi.org/10.1111/1751-7915.12768
Article PubMed PubMed Central Google Scholar
Papong S, Rewlay-ngoen C, Itsubo N, Malakul P (2017) Environmental life cycle assessment and social impacts of bioethanol production in Thailand. J Clean Prod 157:254–266. https://doi.org/10.1016/j.jclepro.2017.04.122
Article Google Scholar
Pisarello ML, Maquirriain M, Sacripanti Olalla P et al (2018) Biodiesel production by transesterification in two steps: Kinetic effect or shift in the equilibrium conversion? Fuel Process Technol 181:244–251. https://doi.org/10.1016/j.fuproc.2018.09.028
Hirani AH, Javed N, Asif M et al (2018) A review on first-and second-generation biofuel productions. In: Biofuels: Greenhouse Gas Mitigation and Global Warming. Springer, New Delhi, pp 141–154. https://doi.org/10.1007/978-81-322-3763-1_8
Kumar P, Varkolu M, Mailaram S et al (2019) Biorefinery Polyutilization Systems : Production of Green Transportation Fuels From Biomass. Polygeneration with Polystorage for Chemical and Energy Hubs. pp 373–407. https://doi.org/10.1016/B978-0-12-813306-4.00012-4
Hassan SS, Williams GA, Jaiswal AK (2019) Moving towards the second generation of lignocellulosic biorefineries in the EU: drivers, challenges, and opportunities. Renew Sustain Energy Rev 101:590–599. https://doi.org/10.1016/j.rser.2018.11.041
Fiorentino G, Zucaro A, Ulgiati S (2019) Towards an energy efficient chemistry. Switching from fossil to bio-based products in a life cycle perspective. Energy 170:720–729. https://doi.org/10.1016/j.energy.2018.12.206
Leong WH, Lim JW, Lam MK et al (2018) Third generation biofuels: a nutritional perspective in enhancing microbial lipid production. Renew Sustain Energy Rev 91:950–961. https://doi.org/10.1016/j.rser.2018.04.066
Loredo-trevi A, Pinales-m D, Aguilar N et al (2021) Bioresource technology high-pressure technology for Sargassum spp biomass pretreatment and fractionation in the third generation of bioethanol production. Bioresour Technol 329:1–10. https://doi.org/10.1016/j.biortech.2021.124935
Abdullah B, Anuar S, Muhammad S et al (2019) Fourth generation biofuel: a review on risks and mitigation strategies. Renew Sustain Energy Rev 107:37–50. https://doi.org/10.1016/j.rser.2019.02.018
Atelge MR, Atabani AE, Banu JR et al (2020) A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 270:117494. https://doi.org/10.1016/j.fuel.2020.117494
Liu X, Lee C, Kim JY (2020) Thermal hydrolysis pre-treatment combined with anaerobic digestion for energy recovery from organic wastes. J Mater Cycles Waste Manag 22:1370–1381. https://doi.org/10.1007/s10163-020-01025-2
Hosseini Koupaie E, Dahadha S, Bazyar Lakeh AA et al (2019) Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-a review. J Environ Manage 233:774–784. https://doi.org/10.1016/j.jenvman.2018.09.106
Article CAS PubMed Google Scholar
Solarte-Toro JC, Romero-García JM, Martínez-Patiño JC et al (2019) Acid pretreatment of lignocellulosic biomass for energy vectors production: a review focused on operational conditions and techno-economic assessment for bioethanol production. Renew Sustain Energy Rev 107:587–601. https://doi.org/10.1016/j.rser.2019.02.024
Tsegaye B, Balomajumder C, Roy P (2019) Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: an overview and future prospect. Bull Natl Res Cent 43:51. https://doi.org/10.1186/s42269-019-0094-x
Rajendran K, Drielak E, Sudarshan Varma V et al (2018) Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production–a review. Biomass Convers Biorefinery 8:471–483. https://doi.org/10.1007/s13399-017-0269-3
Lee M, Lin YL, Chiueh P Te, Den W (2020) Environmental and energy assessment of biomass residues to biochar as fuel: a brief review with recommendations for future bioenergy systems. J Clean Prod 251:119714. https://doi.org/10.1016/j.jclepro.2019.119714
Pramanik SK, Suja FB, Zain SM, Pramanik BK (2019) The anaerobic digestion process of biogas production from food waste: prospects and constraints. Bioresour Technol Reports 8:100310. https://doi.org/10.1016/j.biteb.2019.100310
Jang Y, Malaviya A, Lee J et al (2013) Metabolic engineering of Clostridium acetobutylicum for the enhanced production of isopropanol-butanol-ethanol fuel mixture. Biotechnol Prog 29:1083–1088
Rezania S, Oryani B, Cho J et al (2020) Different pretreatment technologies of lignocellulosic biomass for bioethanol production: an overview. Energy 199:117457. https://doi.org/10.1016/j.energy.2020.117457
Su T, Zhao D, Khodadadi M, Len C (2020) Lignocellulosic biomass for bioethanol: recent advances, technology trends and barriers to industrial development. Curr Opin Green Sustain Chem. https://doi.org/10.1016/j.cogsc.2020.04.005
Manmai N, Unpaprom Y, Ramaraj R (2021) Bioethanol production from sunflower stalk: application of chemical and biological pretreatments by response surface methodology (RSM). Biomass Conv Bioref 11:1759–1773. https://doi.org/10.1007/s13399-020-00602-7
Yaashikaa PR, Kumar PS, Saravanan A et al (2020) Bioconversion of municipal solid waste into bio-based products: a review on valorisation and sustainable approach for circular bioeconomy. Sci Total Environ 748:141312
Susmozas A, Martín-Sampedro R, Ibarra D et al (2020) Process Strategies for the Transition of 1G to Advanced Bioethanol Production Processes. 8(10):1310. https://doi.org/10.3390/pr8101310
Costa D, Jesus J, Virgínio e Silva J et al (2018) Life cycle assessment of bioethanol production from sweet potato (Ipomoea batatas L.) in an experimental plant. Bioenerg Res 11:715–725. https://doi.org/10.1007/s12155-018-9932-1
Khan MT, Khan IA, Yasmeen S, Nizamani GS, Afghan S (2019) Sugarcane biofuels and bioenergy production in pakistan: current scenario, potential, and future avenues. Sugarcane Biofuels, 175-202. https://doi.org/10.1007/978-3-030-18597-8_9
Alavijeh RS, Karimi K, Berg C Van Den (2020) An integrated and optimized process for cleaner production of ethanol and biodiesel from corn stover by Mucor indicus. J Clean Prod 249:119321. https://doi.org/10.1016/j.jclepro.2019.119321
Sivamani S, Pandian A, Muthusamy C et al (2018) Evaluation of the potential of cassava-based residues for biofuels production. Rev Environ Sci Bio/Technology. https://doi.org/10.1007/s11157-018-9475-0
Kumar J, Reetu S (2014) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. Biotech 5:337–353. https://doi.org/10.1007/s13205-014-0246-5
Manochio C, Andrade BR, Rodriguez RP, Moraes BS (2017) Ethanol from biomass: a comparative overview. Renew Sustain Energy Rev 80:743–755. https://doi.org/10.1016/j.rser.2017.05.063
Su T, Zhao D, Khodadadi M, Len C (2020) Lignocellulosic biomass for bioethanol: recent advances, technology trends, and barriers to industrial development. Curr Opin Green Sustain Chem 24:56–60. https://doi.org/10.1016/j.cogsc.2020.04.005
Chen L, Gu W, Gui X et al (2018) Complete genome sequence of Bacillus velezensis 157 isolated from Eucommia ulmoides with pathogenic bacteria inhibiting and lignocellulolytic enzymes production by SSF. 3 Biotech 8:114. https://doi.org/10.1007/s13205-018-1125-2
Prasad RK, Chatterjee S, Mazumder PB et al (2019) Bioethanol production from waste lignocelluloses: a review on microbial degradation potential. ECSN. https://doi.org/10.1016/j.chemosphere.2019.05.142
Dutta K, Daverey A, Lin J (2014) Evolution retrospective for alternative fuels: first to fourth generation. Renew Energy 69:114–122. https://doi.org/10.1016/j.renene.2014.02.044
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status , challenges , and optimization of a sustainable and renewable industry for biofuels , feed , and other products. Microb Cell Fact 17:36. https://doi.org/10.1186/s12934-018-0879-x
Jhang S, Lin Y, Chen K et al (2020) Evaluation of fuel consumption, pollutant emissions and well-to-wheel GHGs assessment from a vehicle operation fueled with bioethanol, gasoline and hydrogen. Energy 209:118436. https://doi.org/10.1016/j.energy.2020.118436
Riccio A, Chianese E, Tirimberio G, Prati MV (2017) Emission factors of inorganic ions from road traffic: a case study from the city of Naples ( Italy ). Transp Res Part D 54:239–249. https://doi.org/10.1016/j.trd.2017.05.008
Di P, Finore I, Poli A et al (2019) The production of second generation bioethanol: the biotechnology potential of thermophilic bacteria. J Clean Prod 233:1410–1417. https://doi.org/10.1016/j.jclepro.2019.06.152
Koehler N, Mccaherty J, Wilson C et al (2019) 2019 ETHANOL INDUSTRY OUTLOOK [Ebook] (1st ed.). Renewable Fuels Association. Retrieved 19 December 2021, from http://www.cornlp.com/Adobe/outlook2019.pdf
Niphadkar S, Bagade P, Ahmed S (2017) Bioethanol production: insight into past, present and future perspectives. Biofuels 0:1–10. https://doi.org/10.1080/17597269.2017.1334338
Pandiyan K, Singh A, Singh S et al (2019) Technological interventions for utilization of crop residues and weedy biomass for second generation bio-ethanol production. Renew Energy 132:723–741. https://doi.org/10.1016/j.renene.2018.08.049
Satari B, Karimi K, Kumar R (2019) Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: a review. Royal Society of Chemistry
Book Google Scholar
Ayodele BV, Alsaffar MA, Mustapa SI (2019) An overview of integration opportunities for sustainable bioethanol production from first- and second-generation sugar-based feedstocks. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.118857
Rastogi M, Shrivastava S (2017) Recent advances in second generation bioethanol production: an insight to pretreatment, sacchari fi cation and fermentation processes. Renew Sustain Energy Rev 80:330–340. https://doi.org/10.1016/j.rser.2017.05.225
Nair RB, Lennartsson PR, Taherzadeh MJ (2017) Bioethanol production from agricultural and municipal wastes. Current Developments in Biotechnology and Bioengineering 157–190. https://doi.org/10.1016/B978-0-444-63664-5.00008-3
Chen J, Zhang B, Luo L et al (2021) A review on recycling techniques for bioethanol production from lignocellulosic biomass. Renew Sustain Energy Rev 149:111370. https://doi.org/10.1016/j.rser.2021.111370
Sharma B, Larroche C, Dussap C (2020) Comprehensive assessment of 2G bioethanol production. Bioresour Technol 313:123630. https://doi.org/10.1016/j.biortech.2020.123630
Singh D, Sharma D, Soni SL et al (2020) A comprehensive review on 1st-generation biodiesel feedstock palm oil: production , engine performance , and exhaust emissions. Bioenerg Res 14:1–22. https://doi.org/10.1007/s12155-020-10171-2
Singh D, Sharma D, Soni SL et al (2019) Review article A review on feedstocks , production processes, and yield for different generations of biodiesel. Fuel 262:116553. https://doi.org/10.1016/j.fuel.2019.116553
Sawasdee V, Haosagul S, Pisutpaisal N (2019) ScienceDirect Co-digestion of waste glycerol and glucose to enhance biogas production. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2019.03.144
Fonseca JM, Teleken JG, Almeida VDC, Silva C (2019) Biodiesel from waste frying oils : Methods of production and puri fi cation. Energy Convers Manag 184:205–218. https://doi.org/10.1016/j.enconman.2019.01.061
Ambat I, Srivastava V, Sillanpää M (2018) Recent advancement in biodiesel production methodologies using various feedstock: a review. Renew Sustain Energy Rev 90:356–369. https://doi.org/10.1016/j.rser.2018.03.069
Mohanty SS, Koul Y, Varjani S et al (2021) A critical review on various feedstocks as sustainable substrates for biosurfactants production: a way towards cleaner production. Microb Cell Fact 20:1–13
Hosseinzadeh-bandbafha H, Tabatabaei M, Aghbashlo M, Khanali M (2018) A comprehensive review on the environmental impacts of diesel / biodiesel additives. Energy Convers Manag 174:579–614. https://doi.org/10.1016/j.enconman.2018.08.050
Hazrat MA, Rasul MG, Khan MMK et al (2019) ScienceDirect ScienceDirect ScienceDirect Emission characteristics of waste tallow and waste cooking oil Emission characteristics of waste on tallow waste cooking oil based ternary biodiesel fuels based ternary biodiesel fuels Assessing of using the heat. Energy Procedia 160:842–847. https://doi.org/10.1016/j.egypro.2019.02.149
Rene ER, Ge J, Kumar G et al (2020) Resource recovery from wastewater, solid waste, and waste gas: engineering and management aspects. Environ Sci Pollut Res 27:17435–17437. https://doi.org/10.1007/s11356-020-08802-4
Sharma PK, Sharma D, Soni SL et al (2019) Characterization of the non-road modified diesel engine using a novel entropy-vikor approach: experimental investigation and numerical simulation. J Energy Resour Technol 141(8):082208. https://doi.org/10.1115/1.4042717
Das M, Sarkar M, Datta A, Santra AK (2018) Study on viscosity and surface tension properties of biodiesel-diesel blends and their effects on spray parameters for CI engines. Fuel 220:769–779. https://doi.org/10.1016/j.fuel.2018.02.021
Azhagurajan KVA, Vedaraman SCVN (2017) Rice husk as nanoadditive in diesel – biodiesel fuel blends used in diesel engine. J Therm Anal Calorim. https://doi.org/10.1007/s10973-017-6692-7
Srivastava RK, Shetti NP, Raghava K, Tejraj R (2020) Biofuels, biodiesel and biohydrogen production using bioprocesses A review United States of America. Environ Chem Lett. https://doi.org/10.1007/s10311-020-00999-7
Khan MA, Ngo HH, Guo W et al (2017) Biohydrogen production from anaerobic digestion and its potential as renewable energy. Renew Energy. https://doi.org/10.1016/j.renene.2017.04.029
Usman TMM, Banu R, Gunasekaran M, Kumar G (2019) Corresponding author Details. Bioresour Technol Rep 7:100287. https://doi.org/10.1016/j.biteb.2019.100287
Xd TX, Xd DX, Xd DX (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86. https://doi.org/10.1016/j.pecs.2017.05.004
Kannah RY, Kavitha S, Sivashanmugham P et al (2018) ScienceDirect Biohydrogen production from rice straw: effect of combinative pretreatment, modelling assessment and energy balance consideration. Int J Hydrogen Energy 44(4):2203-2215. https://doi.org/10.1016/j.ijhydene.2018.07.201
Argun H, Dao S (2016) ScienceDirect Bio-hydrogen production from waste peach pulp by dark fermentation: effect of inoculum addition. Int J Hydrogen Energy 42:2569–2574. https://doi.org/10.1016/j.ijhydene.2016.06.225
Kant S, Sadashiv S, Ashok A et al (2021) Science of the total environment renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci Total Environ 765:144429. https://doi.org/10.1016/j.scitotenv.2020.144429
Haron R, Mat R, Amran T et al (2017) Overview on utilization of biodiesel by-product for biohydrogen production. J Clean Prod. https://doi.org/10.1016/j.jclepro.2017.10.160
Shanmugam S, Hari A, Pandey A, Mathimani T (2020) Comprehensive review on the application of inorganic and organic nanoparticles for enhancing biohydrogen production. Fuel 270:117453. https://doi.org/10.1016/j.fuel.2020.117453
Sarangi PK, Nanda S (2020) Biohydrogen production through dark fermentation. Chem Eng Technol 43(4): 601–612. https://doi.org/10.1002/ceat.201900452
Jarunglumlert T, Prommuak C, Putmai N (2017) Scaling-up bio-hydrogen production from food waste : Feasibilities and challenges. Int J Hydrogen Energy 43(2):634–648. https://doi.org/10.1016/j.ijhydene.2017.10.013
Mirmohamadsadeghi S, Karimi K, Tabatabaei M, Aghbashlo M (2019) Biogas production from food wastes: A review on recent developments and future perspectives. Bioresour Technol Rep 7:100202. https://doi.org/10.1016/j.biteb.2019.100202
Felipe I, Duarte N, Vieira B et al (2018) Resources, conservation & recycling assessment of potential biogas production from multiple organic wastes in Brazil : Impact on energy generation, use, and emissions abatement. Resour Conserv Recycl 131:54–63. https://doi.org/10.1016/j.resconrec.2017.12.012
Wesley O, Yaqian A, Ange Z et al (2017) A review of biogas utilisation, purification and upgrading technologies. Waste and Biomass Valorization. https://doi.org/10.1007/s12649-016-9826-4
Zhu T, Curtis J, Clancy M (2019) Promoting agricultural biogas and biomethane production: lessons from cross-country studies. Renew Sustain Energy Rev 114:109332. https://doi.org/10.1016/j.rser.2019.109332
Lyng K, Elstad A, Jørgen O (2018) Relation between greenhouse gas emissions and economic pro fi t for different con figurations of biogas value chains: a case study on different levels of sector integration. J Clean Prod 182:737–745. https://doi.org/10.1016/j.jclepro.2018.02.126
Kapoor R, Ghosh P, Kumar M et al (2020) Valorization of agricultural waste for biogas based circular economy in India: a research outlook. Bioresour Technol 304:123036. https://doi.org/10.1016/j.biortech.2020.123036
Zareei S (2017) Evaluation of biogas potential from livestock manures and rural wastes using GIS in Iran. Renew Energy. https://doi.org/10.1016/j.renene.2017.11.026
Paudel SR, Banjara SP, Choi OK et al (2017) Pretreatment of agricultural biomass for anaerobic digestion: current state and challenges. Bioresour Technol 245:1194–1205. https://doi.org/10.1016/j.biortech.2017.08.182
Arun N, Dalai AK (2019) Environmental and socioeconomic impact assessment of biofuels from lignocellulosic biomass. Lignocellulosic Biomass to Liquid Biofuels, 283–299. https://doi.org/10.1016/B978-0-12-815936-1.00009-5
Nicoletti J, Ning C, You F (2019) Incorporating agricultural waste-to-energy pathways into biomass product and process network through data-driven nonlinear adaptive robust optimization. Energy 180:556–571. https://doi.org/10.1016/j.energy.2019.05.096
Hafid HS, Rahman NAA, Shah UKM et al (2017) Feasibility of using kitchen waste as future substrate for bioethanol production: a review. Renew Sustain Energy Rev 74:671–686. https://doi.org/10.1016/j.rser.2017.02.071
Rizwan M, Saif Y, Almansoori A, Elkamel A (2018) Optimal processing route for the utilization and conversion of municipal solid waste into energy and valuable products. J Clean Prod 174:857–867. https://doi.org/10.1016/j.jclepro.2017.10.335
Zahedi S, Sales D, García-Morales JL, Solera R (2018) Obtaining green energy from dry-thermophilic anaerobic co-digestion of municipal solid waste and biodiesel waste. Biosyst Eng 170:108–116
Moreno-Garcia L, Adjallé K, Barnabé S, Raghavan GSV (2017) Microalgae biomass production for a biorefinery system: Recent advances and the way towards sustainability. Renew Sustain Energy Rev 76:493–506. https://doi.org/10.1016/j.rser.2017.03.024
Mathur R, Srivastava VK (2019) Crop Residue Burning: Effects on Environment. Greenhouse Gas Emissions, 127–140. https://doi.org/10.1007/978-981-13-3272-2_9
Bundhoo ZMA (2018) Solid waste management in least developed countries: current status and challenges faced. J Mater Cycles Waste Manag 20:1867–1877. https://doi.org/10.1007/s10163-018-0728-3
Sharma BK, Chandel MK (2017) Life cycle assessment of potential municipal solid waste management strategies for Mumbai, India. Waste Manag Res 35:79–91. https://doi.org/10.1177/0734242X16675683
Article PubMed Google Scholar
Mishra B, Varjani S, Agrawal DC et al (2020) Engineering biocatalytic material for the remediation of pollutants: a comprehensive review. Environ Technol Innov 20:101063. https://doi.org/10.1016/j.eti.2020.101063
Rajmohan KS, Chandrasekaran R, Varjani S (2020) A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J Microbiol 60:125–138. https://doi.org/10.1007/s12088-019-00841-x
Article CAS PubMed PubMed Central Google Scholar
Saritha M, Prasad Tollamadugu NVKV (2018) The status of research and application of biofertilizers and biopesticides: Global scenario. Recent Developments in Applied Microbiology and Biochemistry, 195–207. https://doi.org/10.1016/B978-0-12-816328-3.00015-5
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sustain Energy Rev 90:877–891. https://doi.org/10.1016/j.rser.2018.03.111
García-Condado S, López-Lozano R, Panarello L et al (2019) Assessing lignocellulosic biomass production from crop residues in the European Union: modelling, analysis of the current scenario and drivers of interannual variability. GCB Bioenergy 11:809–831. https://doi.org/10.1111/gcbb.12604
Ingle AP, Rathod J, Pandit R et al (2017) Comparative evaluation of free and immobilized cellulase for enzymatic hydrolysis of lignocellulosic biomass for sustainable bioethanol production. Cellulose 24:5529–5540. https://doi.org/10.1007/s10570-017-1517-1
Singhvi MS, Gokhale DV (2019) Lignocellulosic biomass: hurdles and challenges in its valorization. Appl Microbiol Biotechnol 103:9305–9320. https://doi.org/10.1007/s00253-019-10212-7
Shrestha S, Fonoll X, Khanal SK, Raskin L (2017) Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: current status and future perspectives. Bioresour Technol 245:1245–1257. https://doi.org/10.1016/j.biortech.2017.08.089
Liang G, Li Y, Yang C et al (2020) Ash properties correlated with diverse types of biomass derived from power plants: an overview. Energy Sources, Part A Recover Util Environ Eff 00:1–12. https://doi.org/10.1080/15567036.2020.1804012
Masto RE, Pandit A, Kumar S et al (2020) Comparative evaluation of aquatic biomass feedstocks for energy application and potential for extraction of plant nutrients from their ash. Biomass Bioenerg 142:105783. https://doi.org/10.1016/j.biombioe.2020.105783
Abraham A, Mathew AK, Park H et al (2020) Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour Technol 301:122725. https://doi.org/10.1016/j.biortech.2019.122725
Varjani S, Shah AV, Vyas S, Srivastava VK (2021) Processes and prospects on valorizing solid waste for the production of valuable products employing bio-routes: a systematic review. Chemosphere 282:130954. https://doi.org/10.1016/j.chemosphere.2021.130954
Trinh LTP, Lee YJ, Park CS, Bae HJ (2019) Aqueous acidified ionic liquid pretreatment for bioethanol production and concentration of produced ethanol by pervaporation. J Ind Eng Chem 69:57–65. https://doi.org/10.1016/j.jiec.2018.09.008
Qu J, Sun Y, Awasthi MK et al (2021) Effect of different aerobic hydrolysis time on the anaerobic digestion characteristics and energy consumption analysis. Bioresour Technol 320:124332. https://doi.org/10.1016/j.biortech.2020.124332
Montoya-Rosales JJ, Peces M, González-Rodríguez LM et al (2020) A broad overview comparing a fungal, thermal and acid pre-treatment of bean straw in terms of substrate and anaerobic digestion effect. Biomass Bioenerg 142:1–8. https://doi.org/10.1016/j.biombioe.2020.105775
Chowdhury S, Kim GH, Bolan N, Longhurst P (2019) A critical review on risk evaluation and hazardous management in carcass burial. Process Saf Environ Prot 123:272–288. https://doi.org/10.1016/j.psep.2019.01.019
Biruntha M, Mariappan P, Karunai Selvi B et al (2020) Vermiremediation of urban and agricultural biomass residues for nutrient recovery and vermifertilizer production. Waste and Biomass Valorization 11:6483–6497. https://doi.org/10.1007/s12649-019-00899-0
Moustakas K, Parmaxidou P, Vakalis S (2020) Anaerobic digestion for energy production from agricultural biomass waste in Greece: capacity assessment for the region of Thessaly. Energy 191:116556. https://doi.org/10.1016/j.energy.2019.116556
Ren HY, Kong F, Zhao L et al (2019) Enhanced co-production of biohydrogen and algal lipids from agricultural biomass residues in long-term operation. Bioresour Technol 289:121774. https://doi.org/10.1016/j.biortech.2019.121774
Khattab SMR, Watanabe T (2019) Bioethanol From sugarcane bagasse: status and perspectives. Bioethanol Production from Food Crops, 187–212. https://doi.org/10.1016/B978-0-12-813766-6.00010-2
Bozkurt YC, Apul OG (2020) Critical review for microwave pretreatment of waste-activated sludge prior to anaerobic digestion. Curr Opin Environ Sci Heal 14:1–9. https://doi.org/10.1016/j.coesh.2019.10.003
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energy Combust Sci 38:449–467. https://doi.org/10.1016/j.pecs.2012.03.002
Shah AV, Srivastava VK, Mohanty SS, Varjani S (2021) Municipal solid waste as a sustainable resource for energy production: state-of-the-art review. J Environ Chem Eng 9:105717. https://doi.org/10.1016/j.jece.2021.105717
Ruan D, Zhou Z, Pang H et al (2019) Enhancing methane production of anaerobic sludge digestion by microaeration: enzyme activity stimulation, semi-continuous reactor validation and microbial community analysis. Bioresour Technol 289:121643. https://doi.org/10.1016/j.biortech.2019.121643
Wu Y, Ge S, Xia C et al (2021) Application of intermittent ball milling to enzymatic hydrolysis for efficient conversion of lignocellulosic biomass into glucose. Renew Sustain Energy Rev 136:110442. https://doi.org/10.1016/j.rser.2020.110442
Sekar M, Mathimani T, Alagumalai A et al (2021) A review on the pyrolysis of algal biomass for biochar and bio-oil – bottlenecks and scope. Fuel 283:119190. https://doi.org/10.1016/j.fuel.2020.119190
Vaish B, Sharma B, Srivastava V et al (2019) Energy recovery potential and environmental impact of gasification for municipal solid waste. Biofuels 10:87–100. https://doi.org/10.1080/17597269.2017.1368061
Nile A, Nile SH, Kim DH et al (2018) Valorization of onion solid waste and their flavonols for assessment of cytotoxicity, enzyme inhibitory and antioxidant activities. Food Chem Toxicol 119:281–289. https://doi.org/10.1016/j.fct.2018.02.056
Rosen Y, Mamane H, Gerchman Y (2019) Short ozonation of lignocellulosic waste as energetically favorable pretreatment. Bioenergy Res 12:292–301. https://doi.org/10.1007/s12155-019-9962-3
Saggi SK, Gupta G, Dey P (2017) Biological pretreatment of lignocellulosic biomaterials. Adv Biofeedstocks Biofuels 1:97–119. https://doi.org/10.1002/9781119117322.ch5
Tsapekos P, Kougias PG, Angelidaki I (2018) Mechanical pretreatment for increased biogas production from lignocellulosic biomass; predicting the methane yield from structural plant components. Waste Manag 78:903–910. https://doi.org/10.1016/j.wasman.2018.07.017
Wu D, Wei Z, Qu F et al (2020) Effect of Fenton pretreatment combined with bacteria inoculation on humic substances formation during lignocellulosic biomass composting derived from rice straw. Bioresour Technol 303:122849. https://doi.org/10.1016/j.biortech.2020.122849
Vinayak V, Khan MJ, Varjani S et al (2021) Microbial fuel cells for remediation of environmental pollutants and value addition: special focus on coupling diatom microbial fuel cells with photocatalytic and photoelectric fuel cells. J Biotechnol 338:5–19. https://doi.org/10.1016/j.jbiotec.2021.07.003
Nagarajan D, Varjani S, Lee DJ, Chang JS (2021) Sustainable aquaculture and animal feed from microalgae – nutritive value and techno-functional components. Renew Sustain Energy Rev 150:111549. https://doi.org/10.1016/j.rser.2021.111549
Czatzkowska M, Harnisz M, Korzeniewska E, Koniuszewska I (2020) Inhibitors of the methane fermentation process with particular emphasis on the microbiological aspect: A review. Energy Sci Eng 8:1880–1897. https://doi.org/10.1002/ese3.609
Kushkevych I, Kobzová E, Vítězová M et al (2019) Acetogenic microorganisms in operating biogas plants depending on substrate combinations. Biologia (Bratisl) 74:1229–1236. https://doi.org/10.2478/s11756-019-00283-2
Sudhakar MP, Kumar BR, Mathimani T, Arunkumar K (2019) A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J Clean Prod 228:1320–1333. https://doi.org/10.1016/j.jclepro.2019.04.287
Gan YY, Chen WH, Ong HC et al (2020) Effects of dry and wet torrefaction pretreatment on microalgae pyrolysis analyzed by TG-FTIR and double-shot Py-GC/MS. Energy 210:118579. https://doi.org/10.1016/j.energy.2020.118579
Isa MH, Wong LP, Bashir MJK et al (2020) Improved anaerobic digestion of palm oil mill effluent and biogas production by ultrasonication pretreatment. Sci Total Environ 722:137833. https://doi.org/10.1016/j.scitotenv.2020.137833
Whiting K, Carmona LG, Sousa T (2017) A review of the use of exergy to evaluate the sustainability of fossil fuels and non-fuel mineral depletion. Renew Sustain Energy Rev 76:202–211. https://doi.org/10.1016/j.rser.2017.03.059
Azizi K, Keshavarz Moraveji M, Abedini Najafabadi H (2018) A review on bio-fuel production from microalgal biomass by using pyrolysis method. Renew Sustain Energy Rev 82:3046–3059. https://doi.org/10.1016/j.rser.2017.10.033
Zabed HM, Akter S, Yun J et al (2019) Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew Sustain Energy Rev 105:105–128. https://doi.org/10.1016/j.rser.2019.01.048
Fasciotti M (2017) Perspectives for the use of biotechnology in green chemistry applied to biopolymers, fuels and organic synthesis: from concepts to a critical point of view. Sustain Chem Pharm 6:82–89. https://doi.org/10.1016/j.scp.2017.09.002
Bharagava RN, Chowdhary P (2018) Emerging and eco-friendly approaches for waste management. https://doi.org/10.1007/978-981-10-8669-4
Mohd Azhar SH, Abdulla R, Jambo SA et al (2017) Yeasts in sustainable bioethanol production: A review. Biochem Biophys Reports 10:52–61. https://doi.org/10.1016/j.bbrep.2017.03.003
Bolatkhan K, Kossalbayev BD, Zayadan BK et al (2019) Hydrogen production from phototrophic microorganisms: reality and perspectives. Int J Hydrogen Energy 44:5799–5811. https://doi.org/10.1016/j.ijhydene.2019.01.092
Zhang J, Zhang X, Yang M et al (2021) Transforming lignocellulosic biomass into biofuels enabled by ionic liquid pretreatment. Bioresour Technol 322:124522. https://doi.org/10.1016/j.biortech.2020.124522
Vasudev V, Ku X, Lin J (2019) Kinetic study and pyrolysis characteristics of algal and lignocellulosic biomasses. Bioresour Technol 288:121496. https://doi.org/10.1016/j.biortech.2019.121496
Obileke KC, Nwokolo N, Makaka G et al (2021) Anaerobic digestion: technology for biogas production as a source of renewable energy—a review. Energy Environ 32:191–225. https://doi.org/10.1177/0958305X20923117
Kumar A, Samadder SR (2017) A review on technological options of waste to energy for effective management of municipal solid waste. Waste Manag 69:407–422. https://doi.org/10.1016/j.wasman.2017.08.046
Schmieder H, Abeln J, Boukis N et al (2000) Hydrothermal gasification of biomass and organic wastes. J Supercrit Fluids 17:145–153
Sharma HK, Xu C, Qin W (2019) Biological Pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste and Biomass Valorization 10:235–251. https://doi.org/10.1007/s12649-017-0059-y
Abuabdou SMA, Ahmad W, Aun NC, Bashir MJK (2020) A review of anaerobic membrane bioreactors (AnMBR) for the treatment of highly contaminated landfill leachate and biogas production: effectiveness, limitations and future perspectives. J Clean Prod 255:120215. https://doi.org/10.1016/j.jclepro.2020.120215
Srivastava SK (2020) Advancement in biogas production from the solid waste by optimizing the anaerobic digestion. Waste Dispos Sustain Energy 2:85–103. https://doi.org/10.1007/s42768-020-00036-x
Chen YC, Tsai PY (2017) Evaluating the operational risks of biomedical waste using failure mode and effects analysis. Waste Manag Res 35:593–601. https://doi.org/10.1177/0734242X17700717
Mengistu T, Gebrekidan H, Kibret K et al (2018) Comparative effectiveness of different composting methods on the stabilization, maturation and sanitization of municipal organic solid wastes and dried faecal sludge mixtures. Environ Syst Res 6:5. https://doi.org/10.1186/s40068-017-0079-4
Moset V, de Almeida Neves Xavier C, Feng L et al (2018) Combined low thermal alkali addition and mechanical pre-treatment to improve biogas yield from wheat straw. J Clean Prod 172:1391–1398. https://doi.org/10.1016/j.jclepro.2017.10.173
Feng RZ, Zaidi AA, Zhang K, Shi Y (2019) Optimisation of microwave pretreatment for biogas enhancement through anaerobic digestion of microalgal biomass. Period Polytech Chem Eng 63:65–72. https://doi.org/10.3311/PPch.12334
Cho D-W, Tsang DCW, Kim S et al (2018) Thermochemical conversion of cobalt-loaded spent coffee grounds for production of energy resource and environmental catalyst. Bioresour Technol 270:346–351
Han Y, Zhuo Y, Peng D et al (2017) Influence of thermal hydrolysis pretreatment on organic transformation characteristics of high solid anaerobic digestion. Bioresour Technol 244:836–843. https://doi.org/10.1016/j.biortech.2017.07.166
Bahreini G, Nazari L, Ho D et al (2020) Enzymatic pre-treatment for enhancement of primary sludge fermentation. Bioresour Technol 305:123071. https://doi.org/10.1016/j.biortech.2020.123071
Kumar S, Smith SR, Fowler G et al (2017) Challenges and opportunities associated with waste management in India. R Soc Open Sci 4(3):160764. https://doi.org/10.1098/rsos.160764
Scown CD, Baral NR, Yang M et al (2021) Technoeconomic analysis for biofuels and bioproducts. Curr Opin Biotechnol 67:58–64
Balasubramani R, Muthunarayanan V, Periakaruppan R et al (2021) Treatment of Waste. Waste Valorisation, 33–49. https://doi.org/10.1002/9781119502753.ch3
Zhu Q, Li G, Jiang Z et al (2020) Investigating the variation of dissolved organic matters and the evolution of autotrophic microbial community in composting with organic and inorganic carbon sources. Bioresour Technol 304:123013. https://doi.org/10.1016/j.biortech.2020.123013
Coarita Fernandez H, Teixeira Franco R, Bayard R, Buffiere P (2020) Mechanical pre-treatments evaluation of cattle manure before anaerobic digestion. Waste and Biomass Valorization 11:5175–5184. https://doi.org/10.1007/s12649-020-01022-4
Khanh Nguyen V, Kumar Chaudhary D, Hari Dahal R et al (2021) Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 285:119105. https://doi.org/10.1016/j.fuel.2020.119105
Ma X, Yu M, Song N et al (2020) Effect of ethanol pre-fermentation on organic load rate and stability of semi-continuous anaerobic digestion of food waste. Bioresour Technol 299:122587. https://doi.org/10.1016/j.biortech.2019.122587
Moya D, Aldás C, López G, Kaparaju P (2017) Municipal solid waste as a valuable renewable energy resource: a worldwide opportunity of energy recovery by using Waste-To-Energy Technologies. Energy Procedia 134:286–295. https://doi.org/10.1016/j.egypro.2017.09.618
Pandey BK, Vyas S, Pandey M, Gaur A (2016) Municipal solid waste to energy conversion methodology as physical, thermal, and biological methods. Curr Sci Perspect 2:39–44
Google Scholar
Nath BK, Chaliha C, Bhuyan B et al (2018) GIS mapping-based impact assessment of groundwater contamination by arsenic and other heavy metal contaminants in the Brahmaputra River valley: a water quality assessment study. J Clean Prod 201:1001–1011. https://doi.org/10.1016/j.jclepro.2018.08.084
Qian C, Li Q, Zhang Z et al (2020) Prediction of higher heating values of biochar from proximate and ultimate analysis. Fuel 265:116925. https://doi.org/10.1016/j.fuel.2019.116925
Gaur VK, Sharma P, Sirohi R et al (2020) Assessing the impact of industrial waste on environment and mitigation strategies: a comprehensive review. J Hazard Mater 398:123019. https://doi.org/10.1016/j.jhazmat.2020.123019
Sharma P, Gaur VK, Kim S, Pandey A (2019) Microbial strategies for bio-transforming food waste into resources. Bioresour Technol 299:122580. https://doi.org/10.1016/j.biortech.2019.122580
Dornau A, Robson JF, Thomas GH, McQueen-Mason SJ (2020) Robust microorganisms for biofuel and chemical production from municipal solid waste. Microb Cell Fact 19:1–18
Noh HJ, Woo JE, Lee SY, Jang Y-S (2018) Metabolic engineering of Clostridium acetobutylicum for the production of butyl butyrate. Appl Microbiol Biotechnol 102:8319–8327
Thapa B, Patidar SK, Khatiwada NR et al (2019) Production of ethanol from municipal solid waste of India and Nepal. Waste Valorisation and Recycling, 47–58. https://doi.org/10.1007/978-981-13-2784-1_5
Solera R (2018) Science Direct Obtaining green energy from dry-thermophilic anaerobic co-digestion of municipal solid waste and biodiesel waste. 170:108–116. https://doi.org/10.1016/j.biosystemseng.2018.04.005
Zahedi S (2018) Energy efficiency: importance of indigenous microorganisms contained in the municipal solid wastes. Waste Manag 78:763–769
Byadgi SA, Kalburgi PB (2016) Production of bioethanol from waste newspaper. Procedia Environ Sci 35:555–562
Prasoulas G, Gentikis A, Konti A et al (2020) Bioethanol production from food waste applying the multienzyme system produced on-site by Fusarium oxysporum F3 and mixed microbial cultures. Fermentation 6:39
Matsakas L, Gao Q, Jansson S et al (2017) Green conversion of municipal solid wastes into fuels and chemicals. Electron J Biotechnol 26:69–83
Chen G, Ali I, Raza S et al (2020) ScienceDirect Hydrogen-rich syngas production from municipal solid waste gasification through the application of central composite design : An optimization study. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2020.09.118
Experimental I, Gandidi IM, Susila MD (2017) Author ’ s accepted manuscript production of valuable pyrolytic oils from mixed municipal solid waste ( msw ) in Indonesia using Reference: To appear in: Case Studies in thermal engineering waste (MSW) in indonesia using non-isothermal and isothermal. Case Stud Therm Eng. https://doi.org/10.1016/j.csite.2017.08.003
Verma M, Godbout S, Brar SK et al (2012) Biofuels production from biomass by thermochemical conversion technologies. Int J Chem Eng 1-18. https://doi.org/10.1155/2012/542426
Mahesh D, Ahmad S, Kumar R et al (2021) Hydrothermal liquefaction of municipal solid wastes for high quality bio-crude production using glycerol as co-solvent. Bioresour Technol 339:125537
Shah AV, Singh A, Sabyasachi S, Kumar V (2022) Bioresource technology organic solid waste: biorefinery approach as a sustainable strategy in circular bioeconomy. Bioresour Technol 349:126835. https://doi.org/10.1016/j.biortech.2022.126835
Soleymani M, Rosentrater KA (2017) Techno-economic analysis of biofuel production from macroalgae (seaweed). Bioengineering 4:92
Vasco-Correa J, Khanal S, Manandhar A, Shah A (2018) Anaerobic digestion for bioenergy production: global status, environmental and techno-economic implications, and government policies. Bioresour Technol 247:1015–1026
Hannula I (2016) Hydrogen enhancement potential of synthetic biofuels manufacture in the European context: a techno-economic assessment. Energy 104:199–212
Phillips SD, Tarud JK, Biddy MJ, Dutta A (2011) Gasoline from woody biomass via thermochemical gasification, methanol synthesis, and methanol-to-gasoline technologies: a technoeconomic analysis. Ind Eng Chem Res 50:11734–11745
Sindhu R, Binod P, Pandey A et al (2019) Biofuel Production from biomass: toward sustainable development. Current Developments In Biotechnology and Bioengineering 79–92. https://doi.org/10.1016/B978-0-444-64083-3.00005-1
Torres M, Janaína N, Luana M, Basso G (2020) Biofuels and their connections with the sustainable development goals : a bibliometric and systematic review. Environ Dev Sustain. https://doi.org/10.1007/s10668-020-01110-4
Adegboye MF, Ojuederie OB, Talia PM, Babalola OO (2021) Bioprospecting of microbial strains for biofuel production: metabolic engineering, applications, and challenges. Biotechnol Biofuels 14:1–21
Chen G, Jamro IA, Samo SR et al (2020) Hydrogen-rich syngas production from municipal solid waste gasification through the application of central composite design: an optimization study. Int J Hydrogen Energy 45:33260–33273
Gandidi IM, Susila MD, Pambudi NA (2017) Production of valuable pyrolytic oils from mixed municipal solid waste (MSW) in Indonesia using non-isothermal and isothermal experimental. Case Stud Therm Eng 10:357–361
Boscagli C, Morgano MT, Raffelt K et al (2018) Influence of feedstock, catalyst, pyrolysis and hydrotreatment temperature on the composition of upgraded oils from intermediate pyrolysis. Biomass Bioenerg 116:236–248
Download references
Acknowledgements
Aditi Singh, Priya Prajapati, and Shaili Vyas are grateful to authorities of GPCB for allowing them to undergo an internship with “A scheme on Project, Thesis or Internship at GPCB.” The authors would like to thank Gujarat Pollution Control Board for encouragement and support during manuscript preparation.
Author information
Authors and affiliations.
Gujarat Pollution Control Board, Gandhinagar, 382 010, Gujarat, India
Aditi Singh, Priya Prajapati, Shaili Vyas & Sunita Varjani
Central University of Gujarat, Gandhinagar, 382030, Gujarat, India
Aditi Singh
Kadi Sarva Vishwavidyalaya, Gandhinagar, Gujarat, 382015, India
Priya Prajapati & Shaili Vyas
Amity Institute of Biotechnology, Amity University Uttar Pradesh, Lucknow Campus, Lucknow, India
Vivek Kumar Gaur
Centre for Energy and Environmental Sustainability, Lucknow, India
Department of Food Technology, T K M Institute of Technology, Kollam, 691 505, Kerala, India
Raveendran Sindhu
CSIR-National Institute for Interdisciplinary Science and Technology (NIIST), Trivandrum, 695 019, Kerala, India
Parameswaran Binod
CSIR-Indian Institute of Integrative Medicine, Jammu, 180001, India
Vinod Kumar
Department of Marine Environmental Engineering, National Kaohsiung University of Science and Technology, Kaohsiung City, 81157, Taiwan
Reeta Rani Singhania
College of Natural Resources and Environment, Northwest A& F University, Yangling, Shaanxi Province, 712100, People’s Republic of China
Mukesh Kumar Awasthi & Zengqiang Zhang
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sunita Varjani .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Singh, A., Prajapati, P., Vyas, S. et al. A Comprehensive Review of Feedstocks as Sustainable Substrates for Next-Generation Biofuels. Bioenerg. Res. 16 , 105–122 (2023). https://doi.org/10.1007/s12155-022-10440-2
Download citation
Received : 06 January 2022
Accepted : 18 March 2022
Published : 07 April 2022
Issue Date : March 2023
DOI : https://doi.org/10.1007/s12155-022-10440-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lignocellulose
- Pre-treatment
- Value creation
- Find a journal
- Publish with us
- Track your research
- Articles in Press
- Current Issue
Journal Archive
Join our Community on:
Biofuel Research Journal`s Official Channel on WeChat (in Mandarin Chinese)

Latest News
Biofuel Research Journal
has been accepted to be indexed by Inspec!

"Inspec, produced by The
Institution of Engineering
and Technology , has been
the definitive engineering
and physics research database
for over 50 years"
Read more about Inspec on Clarivate TM
has been accepted to be indexed by Ei Compendex (Engineering Village)!
Read more here !
Read the Editor`s Pick: Biogas Collection here !
is now indexed by ProQuest!

We are delighted to announce .... Read more here !
Celebrating a year of growth
for Biofuel Research Journal !

This year marks a year of growth .... Read more here !

Biofuel Research Journal (BRJ) is a leading, peer-reviewed academic journal dedicated to publishing high-quality research on biofuels, bioproducts, and related biomass-derived materials and technologies. BRJ is an open-access online journal and completely free-of-charge, aiming to advance knowledge and understanding of sustainable energy solutions, environmental protection, and the circular economy through cutting-edge research and innovative applications. The journal welcomes original articles, review papers, case studies, short communications, and hypotheses in the following areas:
- Biofuels and Bioproducts: Exploring the production, characterization, and application of various biofuels, such as biodiesel, bioalcohols (bioethanol, biobutanol), biogas (biomethane, biohydrogen), algal biofuels, and other emerging biofuel sources. Additionally, BRJ encourages research on bioproducts, including biocomposites, bio-based smart materials, and innovative applications in the fields of food, pharmaceuticals, and beyond.
- Biomass Valorization: Investigating efficient and sustainable methods for biomass conversion, including biorefineries, bioprocesses, and advanced biomass utilization techniques to maximize energy and value-added product yields.
- Biomass-Derived Materials for Energy and Storage Systems: Advancing research on biomass-derived materials with potential applications in energy conversion and storage systems, including fuel cells, batteries, supercapacitors, and photovoltaic modules.
- Biomass-Derived Materials for Environmental Sustainability: Exploring using biomass-derived catalysts and materials for carbon dioxide capture and conversion to mitigate greenhouse gas emissions. Additionally, researching biomass-derived catalysts for air, soil, and water pollution mitigation contributes to improved environmental quality.
- Biomass-Derived Materials for Sustainable Applications: Investigating the use of biomass-derived materials in food applications, such as sustainable packaging materials or functional food ingredients, as well as their potential in biomedical applications, drug delivery systems, tissue engineering, and regenerative medicine.
- Biomass-Derived Materials for Catalytic Applications: Advancing the understanding and development of biomass-derived materials with catalytic properties, enabling green and sustainable manufacturing processes and chemical transformations.
- Techno-Economic and Environmental Assessments: Analyzing the techno-economic feasibility and environmental sustainability (life cycle assessment, exergy, emergy, risk assessment) of biofuel, bioproduct, and biomass-derived materials production and application pathways, ensuring their compatibility with international standards (e.g., PAS 2050:2011 and ISO 14040:2006).
- Climate Change and Sustainability: Investigating the impacts of biofuel and bioproduct production and consumption on climate change and promoting innovative, low-carbon pathways for their production and use. Articles focusing on strategies for limiting greenhouse gas emissions and promoting circular economy principles in the bioenergy sector are encouraged.
- Novel Processing and Integrated Systems: Highlighting innovative approaches and integrated systems for biofuel and bioproduct processing, as well as energy audits for production plants to enhance efficiency and sustainability.
- Artificial Photosynthesis for Biofuels Production: Exploring cutting-edge research on artificial photosynthesis as a potential green route for biofuels production.
- Promotion of Biofuels and Bioproducts in Developing Economies: Encouraging research on the promotion and adoption of biofuels and bioproducts in developing economies to foster indigenous development and sustainable energy solutions.
- Biofuels and Bioproducts in Circular Economy: Addressing the role of biofuels and bioproducts in the circular economy framework, focusing on resource efficiency, waste valorization, and sustainability.
- Biofuels and Bioproducts Finance: Exploring financial aspects related to biofuels and bioproducts, including investment strategies, funding mechanisms, and economic considerations for the development and commercialization of sustainable bioenergy solutions.
BRJ aims to foster interdisciplinary collaborations among researchers, engineers, scientists, policymakers, and industry experts to accelerate the adoption of sustainable energy solutions and foster a greener future. The journal is committed to maintaining the highest standards of peer review and editorial integrity, ensuring that only high-quality and impactful research is published.
Biofuel Research Journal is indexed in Scopus and Web of Science .
BRJ currently has no fees. Learn More .
Editor-in-Chief: Vijai Kumar Gupta
> View Full Editorial Board
Editorial Board
10.18331/BRJ2024.11.1.1
- PDF 190.06 K
Research Paper
Critical impacts of energy targeting on the sustainability of advanced biobutanol separation.
Pages 1999-2012
10.18331/BRJ2024.11.1.2
Keikhosro Karimi; Benyamin Khoshnevisan; Joeri F.M. Denayer
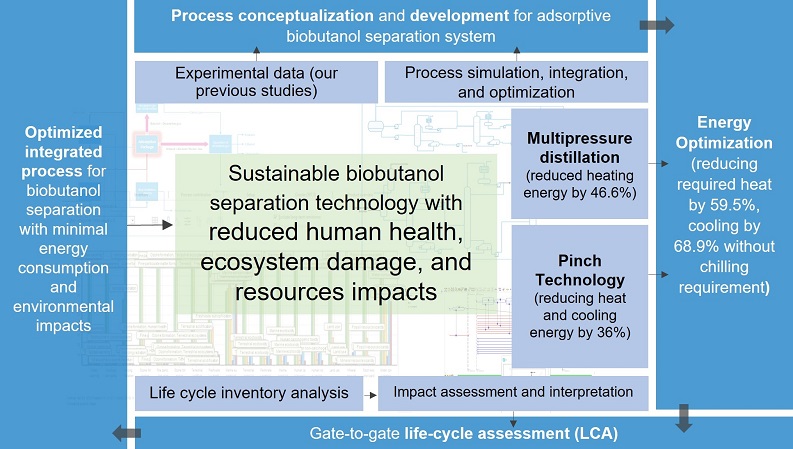
- View Article
Artificial humic substances as sustainable carriers for manganese: Development of a novel bio-based microfertilizer
Pages 2013-2024
10.18331/BRJ2024.11.1.3
Alexander Volikov; Helen Schneider; Nadezda V. Tarakina; Nader Marzban; Markus Antonietti; Svitlana Filonenko
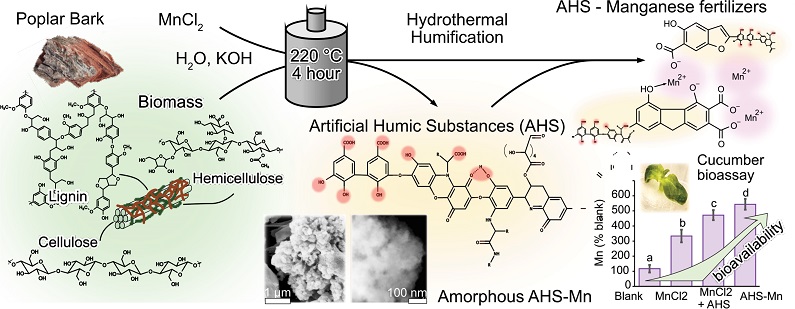

Lime-assisted hydrothermal humification and carbonization of sugar beet pulp: Unveiling the yield, quality, and phytotoxicity of products
Pages 2025-2039
10.18331/BRJ2024.11.1.4
Mona Ghaslani; Reza Rezaee; Omid Aboubakri; Ehsan Sarlaki; Thomas Hoffmann; Afshin Maleki; Nader Marzban
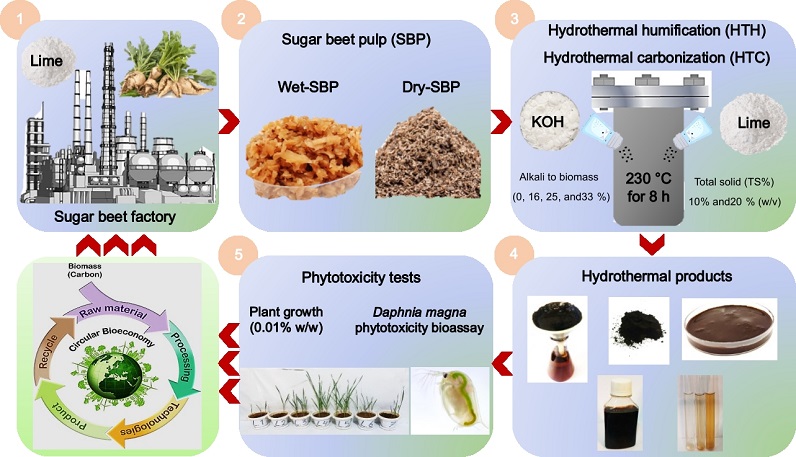
Review Paper
New trends in microbial lipid-based biorefinery for fermentative bioenergy production from lignocellulosic biomass.
Pages 2040-2064
10.18331/BRJ2024.11.1.5
Salauddin Al Azad; Meysam Madadi; Guojie Song; Chihe Sun; Fubao Sun
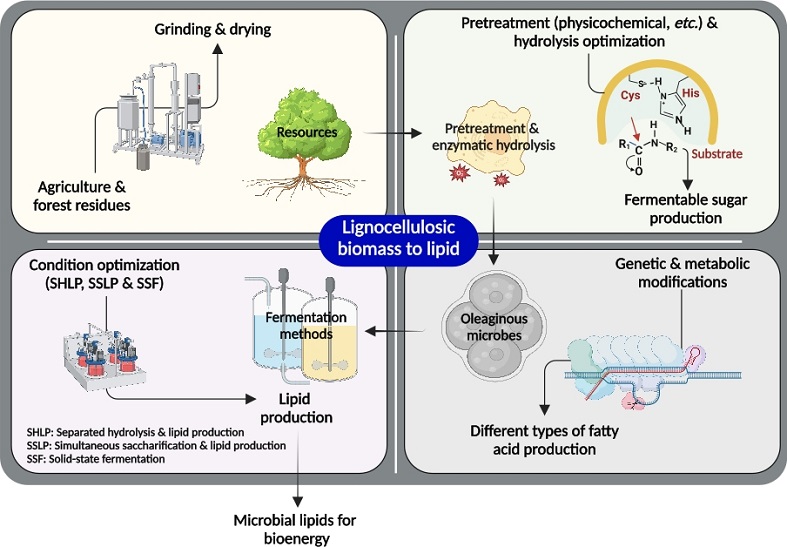
Publication Information
Indexing and abstracting.

- Reference Manager
- Simple TEXT file
People also looked at
Review article, scope of algae as third generation biofuels.

- Biochemical Conversion Division, Sardar Swaran Singh National Institute of Renewable Energy, Kapurthala, Punjab, India
An initiative has been taken to develop different solid, liquid, and gaseous biofuels as the alternative energy resources. The current research and technology based on the third generation biofuels derived from algal biomass have been considered as the best alternative bioresource that avoids the disadvantages of first and second generation biofuels. Algal biomass has been investigated for the implementation of economic conversion processes producing different biofuels such as biodiesel, bioethanol, biogas, biohydrogen, and other valuable co-products. In the present review, the recent findings and advance developments in algal biomass for improved biofuel production have been explored. This review discusses about the importance of the algal cell contents, various strategies for product formation through various conversion technologies, and its future scope as an energy security.
Introduction
The requirement of energy for the mankind is increasing day by day. The major source of energy is based on fossil fuels only. Thus, the scarcity of fossil fuels, rising price of petroleum based fuels, energy protection, and increased global warming resulted in focusing on renewable energy sources such as solar, wind, hydro, tidal, and biomass worldwide ( Goldemberg and Guardabassi, 2009 ; Dragone et al., 2010 ; Rajkumar et al., 2014 ).
Different biomass from various sources like agricultural, forestry, and aquatic have been taken into consideration as the feedstocks for the production of several biofuels such as biodiesel ( Boyce et al., 2008 ; Yanqun et al., 2008 ), bioethanol ( Behera et al., 2014 ), biohydrogen ( Marques et al., 2011 ), bio-oil ( Shuping et al., 2010 ), and biogas ( Hughes et al., 2012 ; Singh et al., 2014 ). However, the environmental impact raised from burning of fuels has a great impact on carbon cycle (carbon balance), which is related to the combustion of fossil fuels. Besides, exhaustion of different existing biomass without appropriate compensation resulted in huge biomass scarcity, emerging environmental problems such as deforestation and loss of biodiversity ( Goldemberg, 2007 ; Li et al., 2008 ; Saqib et al., 2013 ).
Recently, researchers and entrepreneurs have focused their interest, especially on the algal biomass as the alternative feedstock for the production of biofuels. Moreover, algal biomass has no competition with agricultural food and feed production ( Demirbas, 2007 ). The photosynthetic microorganisms like microalgae require mainly light, carbon dioxide, and some nutrients (nitrogen, phosphorus, and potassium) for its growth, and to produce large amount of lipids and carbohydrates, which can be further processed into different biofuels and other valuable co-products ( Brennan and Owende, 2010 ; Nigam and Singh, 2011 ). Interestingly, the low content of hemicelluloses and about zero content of lignin in algal biomass results in an increased hydrolysis and/or fermentation efficiency ( Saqib et al., 2013 ). Other than biofuels, algae have applications in human nutrition, animal feed, pollution control, biofertilizer, and waste water treatment ( Thomas, 2002 ; Tamer et al., 2006 ; Crutzen et al., 2007 ; Hsueh et al., 2007 ; Choi et al., 2012 ). Therefore, the aim of the current review is to explore the scope of algae for the production of different biofuels and evaluation of its potential as an alternative feedstock.
Algae: Source of Biofuels
Generally, algae are a diverse group of prokaryotic and eukaryotic organisms ranging from unicellular genera such as Chlorella and diatoms to multicellular forms such as the giant kelp, a large brown alga that may grow up to 50 m in length ( Li et al., 2008 ). Algae can either be autotrophic or heterotrophic. The autotrophic algae require only inorganic compounds such as CO 2 , salts, and a light energy source for their growth, while the heterotrophs are non-photosynthetic, which require an external source of organic compounds as well as nutrients as energy sources ( Brennan and Owende, 2010 ). Microalgae are very small in sizes usually measured in micrometers, which normally grow in water bodies or ponds. Microalgae contain more lipids than macroalgae and have the faster growth in nature ( Lee et al., 2014a ). There are about more than 50,000 microalgal species out of which only about 30,000 species have been taken for the research study ( Surendhiran and Vijay, 2012 ; Richmond and Qiang, 2013 ; Rajkumar et al., 2014 ). The short harvesting cycle of algae is the key advantage for its importance, which is better than other conventional crops having harvesting cycle of once or twice in a year ( Chisti, 2007 ; Schenk et al., 2008 ). Therefore, the main focus has been carried out on algal biomass for its application in biofuel area.
There are several advantages of algal biomass for biofuels production: (a) ability to grow throughout the year, therefore, algal oil productivity is higher in comparison to the conventional oil seed crops; (b) higher tolerance to high carbon dioxide content; (c) the consumption rate of water is very less in algae cultivation; (d) no requirement of herbicides or pesticides in algal cultivation; (e) the growth potential of algal species is very high in comparison to others; (f) different sources of wastewater containing nutrients like nitrogen and phosphorus can be utilized for algal cultivation apart from providing any additional nutrient; and (g) the ability to grow under harsh conditions like saline, brackish water, coastal seawater, which does not affect any conventional agriculture ( Spolaore et al., 2006 ; Dismukes et al., 2008 ; Dragone et al., 2010 ). However, there are several disadvantages of algal biomass as feedstock such as the higher cultivation cost as compared to conventional crops. Similarly, harvesting of algae require high energy input, which is approximately about 20–30% of the total cost of production. Several techniques such as centrifugation, flocculation, floatation, sedimentation, and filtration are usually used for harvesting and concentrating the algal biomass ( Demirbas, 2010 ; Ho et al., 2011 ).
The algae can be converted into various types of renewable biofuels including bioethanol, biodiesel, biogas, photobiologically produced biohydrogen, and further processing for bio-oil and syngas production through liquefaction and gasification, respectively ( Kraan, 2013 ). The conversion technologies for utilizing algal biomass to energy sources can be categorized into three different ways, i.e., biochemical, chemical, and thermochemical conversion and make an algal biorefinery, which has been depicted in Figure 1 . The biofuel products derived from algal biomass using these conversion routes have been explored in detail in the subsequent sections.
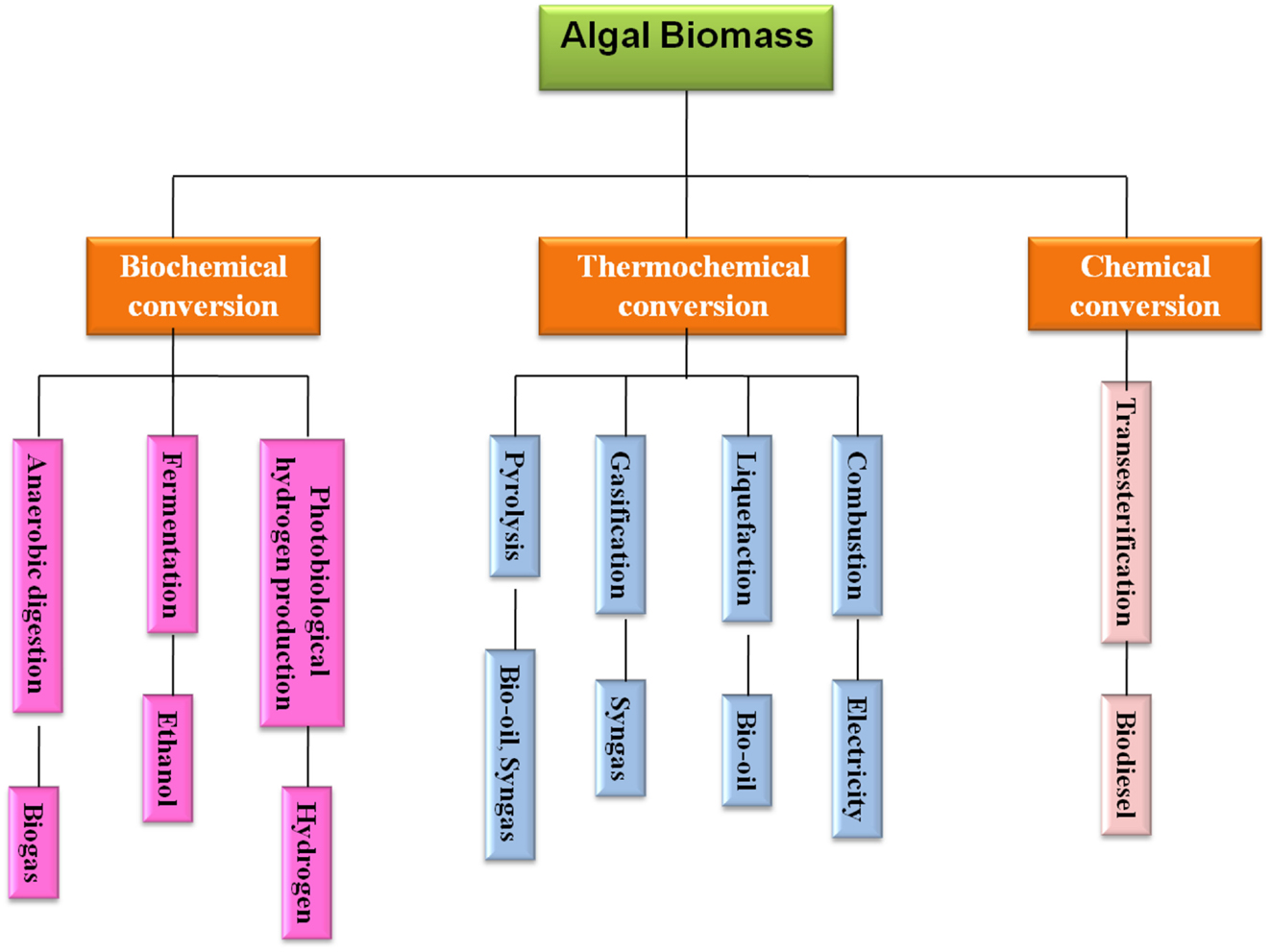
Figure 1. Algal biomass conversion process for biofuel production .
Biodiesel Production
Biodiesel is a mixture of monoalkyl esters of long chain fatty acids [fatty acid methyl esters (FAME)], which can be obtained from different renewable lipid feedstocks and biomass. It can be directly used in different diesel engines ( Clark and Deswarte, 2008 ; Demirbas, 2009 ). Studies to explore the microalgae as feedstock for the production of liquid fuels had been started for the mid-1980s. In order to solve the energy crisis, the extraction of lipids from diatoms was attempted by some German scientists during the period of World War-II ( Cohen et al., 1995 ). The higher oil yield in algal biomass as compared to oil seed crops makes the possibility to convert into the biodiesel economically using different technologies. A comparative study between algal biomass and terrestrial plants for the production of biodiesel has been depicted in Table 1 . The oil productivity (mass of oil produced per unit volume of the microalgal broth per day) depends on the algal growth rate and the biomass content of the species. The species of microalgae such as Kirchneriella lunaris , Ankistrodesmus fusiformis , Chlamydocapsa bacillus , and Ankistrodesmus falcatus with high levels of polyunsaturated FAME are generally preferred for the production of biodiesel ( Nascimento et al., 2013 ). They commonly multiply their biomass with doubling time of 24 h during exponential growth. Oil content of microalgae is generally found to be very high, which exceed up to 80% by weight of its dry biomass. About 5,000–15,000 gal of biodiesel can be produced from algal biomass per acre per year, which reflects its potentiality ( Spolaore et al., 2006 ; Chisti, 2007 ).
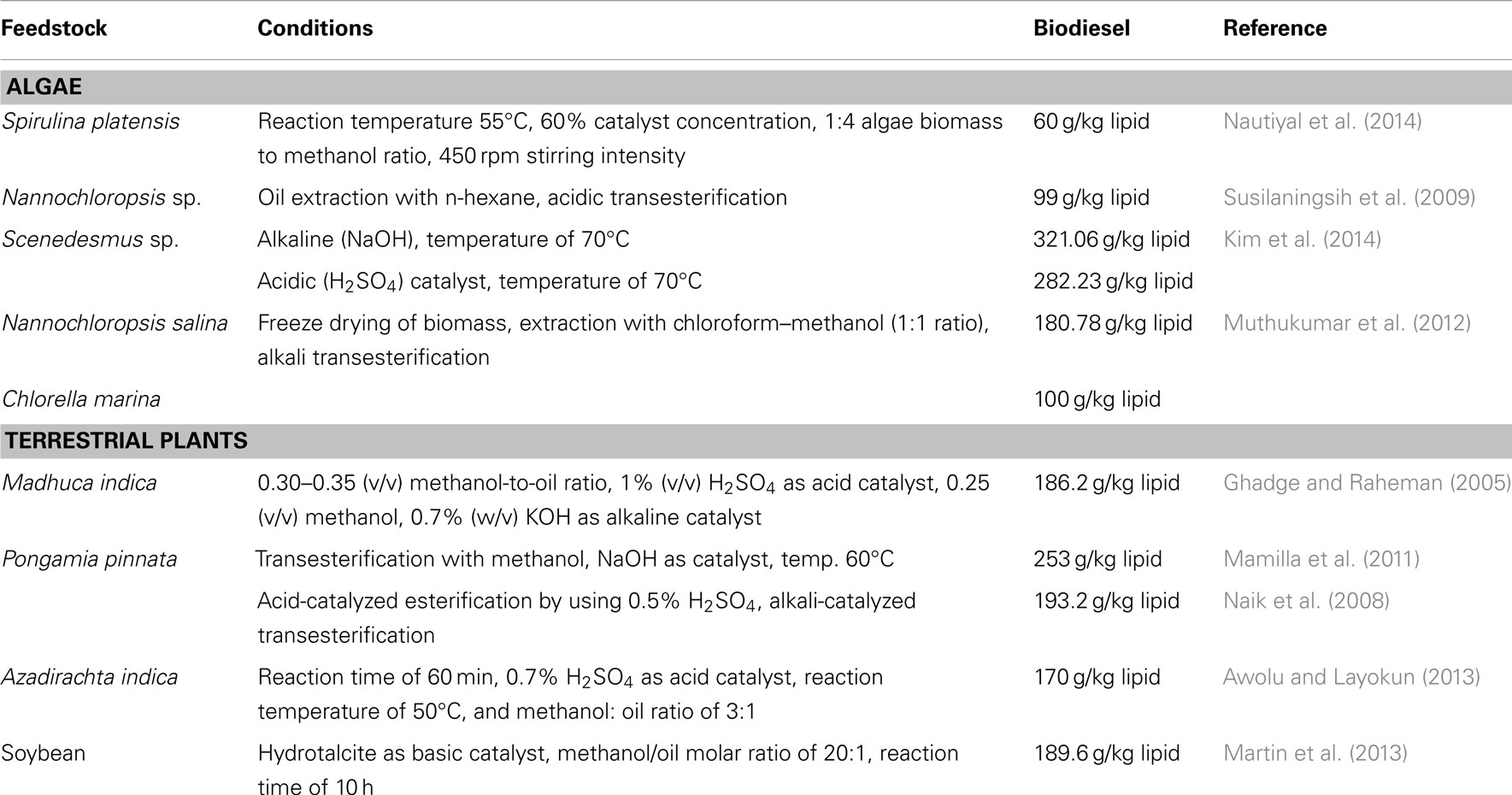
Table 1 . Comparative study between algal biomass and terrestrial plants for biodiesel production .
However, there are some standards such as International Biodiesel Standard for Vehicles (EN14214) and American Society for Testing and Materials (ASTM), which are required to comply with the algal based biodiesel on the physical and chemical properties for its acceptance as substitute to fossil fuels ( Brennan and Owende, 2010 ). The higher degree of polyunsaturated fatty acids of algal oils as compared to vegetable oils make susceptible for oxidation in the storage and further limits its utilization ( Chisti, 2007 ). Some researchers have reported the different advantages of the algal biomass for the biodiesel production due to its high biomass growth and oil productivity in comparison to best oil crops ( Chisti, 2007 ; Hossain et al., 2008 ; Hu et al., 2008 ; Rosenberg et al., 2008 ; Schenk et al., 2008 ; Rodolfi et al., 2009 ; Mutanda et al., 2011 ).
Algal biodiesel production involves biomass harvesting, drying, oil extraction, and further transesterification of oil, which have been described as below.
Harvesting and Drying of Algal Biomass
Unicellular microalgae produce a cell wall containing lipids and fatty acids, which differ them from higher animals and plants. Harvesting of algal biomass and further drying is important prior to mechanical and solvent extraction for the recovery of oil. Macroalgae can be harvested using nets, which require less energy while microalgae can be harvested by some conventional processes, which include filtration ( Rossignol et al., 1999 ) flocculation ( Liu et al., 2013 ; Prochazkova et al., 2013 ), centrifugation ( Heasman et al., 2008 ), foam fractionation ( Csordas and Wang, 2004 ), sedimentation, froth floatation, and ultrasonic separation ( Bosma et al., 2003 ). Selection of harvesting method depends on the type of algal species.
Drying is an important method to extend shelf-life of algal biomass before storage, which avoids post-harvest spoilage ( Munir et al., 2013 ). Most of the efficient drying methods like spray-drying, drum-drying, freeze drying or lyophilization, and sun-drying have been applied on microalgal biomass ( Leach et al., 1998 ; Richmond, 2004 ; Williams and Laurens, 2010 ). Sun-drying is not considered as a very effective method due to presence of high water content in the biomass ( Mata et al., 2010 ). However, Prakash et al. (2007) used simple solar drying device and succeed in drying Spirulina and Scenedesmus having 90% of moisture content. Widjaja et al. (2009) showed the effectiveness of drying temperature during lipid extraction of algal biomass, which affects both concentration of triglycerides and lipid yield. Further, all these processes possess safety and health issues ( Singh and Gu, 2010 ).
Extraction of Oil from Algal Biomass
Unicellular microalgae produce a cell wall containing lipids and fatty acids, which differ them from higher animals and plants. In the literature, there are different methods of oil extraction from algae, such as mechanical and solvent extraction ( Li et al., 2014 ). However, the extraction of lipids from microalgae is costly and energy intensive process.
Mechanical oil extraction
The oil from nuts and seeds is extracted mechanically using presses or expellers, which can also be used for microalgae. The algal biomass should be dried prior to this process. The cells are just broken down with a press to leach out the oil. About 75% of oil can be recovered through this method and no special skill is required ( Munir et al., 2013 ). Topare et al. (2011) extracted oil through screw expeller by mechanical pressing (by piston) and osmotic shock method and recovered about 75% of oil from the algae. However, more extraction time is required as compared to other methods, which make the process unfavorable and less effective ( Popoola and Yangomodou, 2006 ).
Solvent based oil extraction
Oil extraction using solvent usually recovers almost all the oil leaving only 0.5–0.7% residual oil in the biomass. Therefore, the solvent extraction method has been found to be suitable method rather than the mechanical extraction of oil and fats ( Topare et al., 2011 ). Solvent extraction is another method of lipid extraction from microalgae, which involves two stage solvent extraction systems. The amount of lipid extracted from microalgal biomass and further yield of highest biodiesel depends mainly on the solvent used. Several organic solvents such as chloroform, hexane, cyclo-hexane, acetone, and benzene are used either solely or in mixed form ( Afify et al., 2010 ). The solvent reacts on algal cells releasing oil, which is recovered from the aqueous medium. This occurs due to the nature of higher solubility of oil in organic solvents rather than water. Further, the oil can be separated from the solvent extract. The solvent can be recycled for next extraction. Out of different organic solvents, hexane is found to be most effective due to its low toxicity and cost ( Rajvanshi and Sharma, 2012 ; Ryckebosch et al., 2012 ).
In case of using mixed solvents for oil extraction, a known quantity of the solvent mixture is used, for example, chloroform/methanol in the ratio 2:1 (v/v) for 20 min using a shaker and followed by the addition of mixture, i.e., chloroform/water in the ratio of 1:1 (v/v) for 10 min ( Shalaby, 2011 ). Similarly, Pratoomyot et al. (2005) extracted oil from different algal species using the solvent system chloroform/methanol in the ratio of 2:1 (v/v) and found different fatty acid content. Ryckebosch et al. (2012) optimized an analytical procedure and found chloroform/methanol in the ratio 1:1 as the best solvent mixture for the extraction of total lipids. Similarly, Lee et al. (1998) extracted lipid from the green alga Botryococcus braunii using different solvent system and obtained the maximum lipid content with chloroform/methanol in the ratio of 2:1. Hossain et al., 2008 used hexane/ether in the ratio 1:1 (v/v) for oil extraction and allowed to settle for 24 h. Using a two-step process, Fajardo et al. (2007) reported about 80% of lipid recovery using ethanol and hexane in the two steps for the extraction and purification of lipids. Therefore, a selection of a most suitable solvent system is required for the maximum extraction of oil for an economically viable process.
Lee et al. (2009) compared the performance of various disruption methods, including autoclaving, bead-beating, microwaves, sonication, and using 10% NaCl solution in the extraction of Botryococcus sp., Chlorella vulgaris , and Scenedesmus sp, using a mixture of chloroform and methanol (1:1).
Transesterification
This is a process to convert algal oil to biodiesel, which involves multiple steps of reactions between triglycerides or fatty acids and alcohol. Different alcohols such as ethanol, butanol, methanol, propanol, and amyl alcohol can be used for this reaction. However, ethanol and methanol are used frequently for the commercial development due to its low cost and its physical and chemical advantages ( Bisen et al., 2010 ; Surendhiran and Vijay, 2012 ). The reaction can be performed in the presence of an inorganic catalyst (acids and alkalies) or lipase enzyme. In this method, about 3 mol of alcohol are required for each mole of triglyceride to produce 3 mol of methyl esters (biodiesel) and 1 mol of glycerol (by-product) ( Meher et al., 2006 ; Chisti, 2007 ; Sharma and Singh, 2009 ; Surendhiran and Vijay, 2012 ; Stergiou et al., 2013 ) (Figure 2 ). Glycerol is denser than biodiesel and can be periodically or continuously removed from the reactor in order to drive the equilibrium reaction. The presence of methanol, the co-solvent that keeps glycerol and soap suspended in the oil, is known to cause engine failure ( Munir et al., 2013 ). Thus, the biodiesel is recovered by repeated washing with water to remove glycerol and methanol ( Chisti, 2007 ).
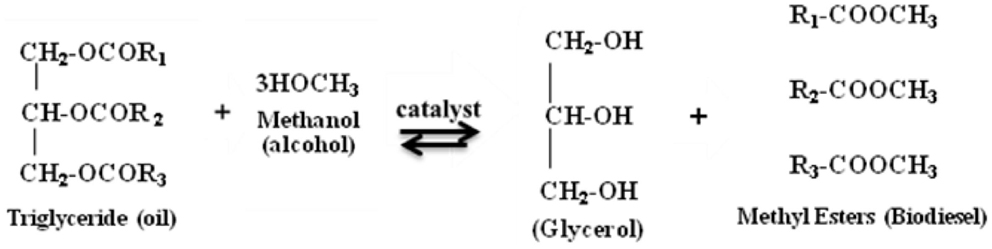
Figure 2. Transesterification of oil to biodiesel . R 1–3 are hydrocarbon groups.
The reaction rate is very slow by using the acid catalysts for the conversion of triglycerides to methyl esters, whereas the alkali-catalyzed transesterification reaction has been reported to be 4000 times faster than the acid-catalyzed reaction ( Mazubert et al., 2013 ). Sodium and potassium hydroxides are the two commercial alkali catalysts used at a concentration of about 1% of oil. However, sodium methoxide has become the better catalyst rather than sodium hydroxide ( Singh et al., 2006 ).
Kim et al. (2014) used Scenedesmus sp. for the biodiesel production through acid and alkali transesterification process. They reported 55.07 ± 2.18%, based on lipid by wt of biodiesel conversion using NaOH as an alkaline catalyst than using H 2 SO 4 as 48.41 ± 0.21% of biodiesel production. In comparison to acid and alkalies, lipases as biocatalyst have different advantages as the catalysts due to its versatility, substrate selectivity, regioselectivity, enantioselectivity, and high catalytic activity at ambient temperature and pressure ( Knezevic et al., 2004 ). It is not possible by some lipases to hydrolyze ester bonds at secondary positions, while some other group of enzymes hydrolyzes both primary and secondary esters. Another group of lipases exhibits fatty acids selectivity, and allow to cleave ester bonds at particular type of fatty acids. Luo et al. (2006) cloned the lipase gene lipB68 and expressed in Escherichia coli BL21 and further used it as a catalyst for biodiesel production. LipB68 could catalyze the transesterification reaction and produce biodiesel with a yield of 92% after 12 h, at a temperature of 20°C. The activity of the lipase enzyme with such a low temperature could provide substantial savings in energy consumption. However, it is rarely used due to its high cost ( Sharma et al., 2001 ).
Extractive transesterification
It involves several steps to produce biodiesel such as drying, cell disruption, oils extraction, transesterification, and biodiesel refining ( Hidalgo et al., 2013 ). The main problems are related with the high water content of the biomass (over 80%), which overall increases the cost of whole process.
In situ transesterification
This method skips the oil extraction step. The alcohol acts as an extraction solvent and an esterification reagent as well, which enhances the porosity of the cell membrane. Yields found are higher than via the conventional route, and waste is also reduced. Industrial biodiesel production involves release of extraction solvent, which contributes to the production of atmospheric smog and to global warming. Thus, simplification of the esterification processes can reduce the disadvantages of this attractive bio-based fuel. The single-step methods can be attractive solutions to reduce chemical and energy consumption in the overall biodiesel production process ( Patil et al., 2012 ). A comparison of direct and extractive transesterification is given in Table 2 .
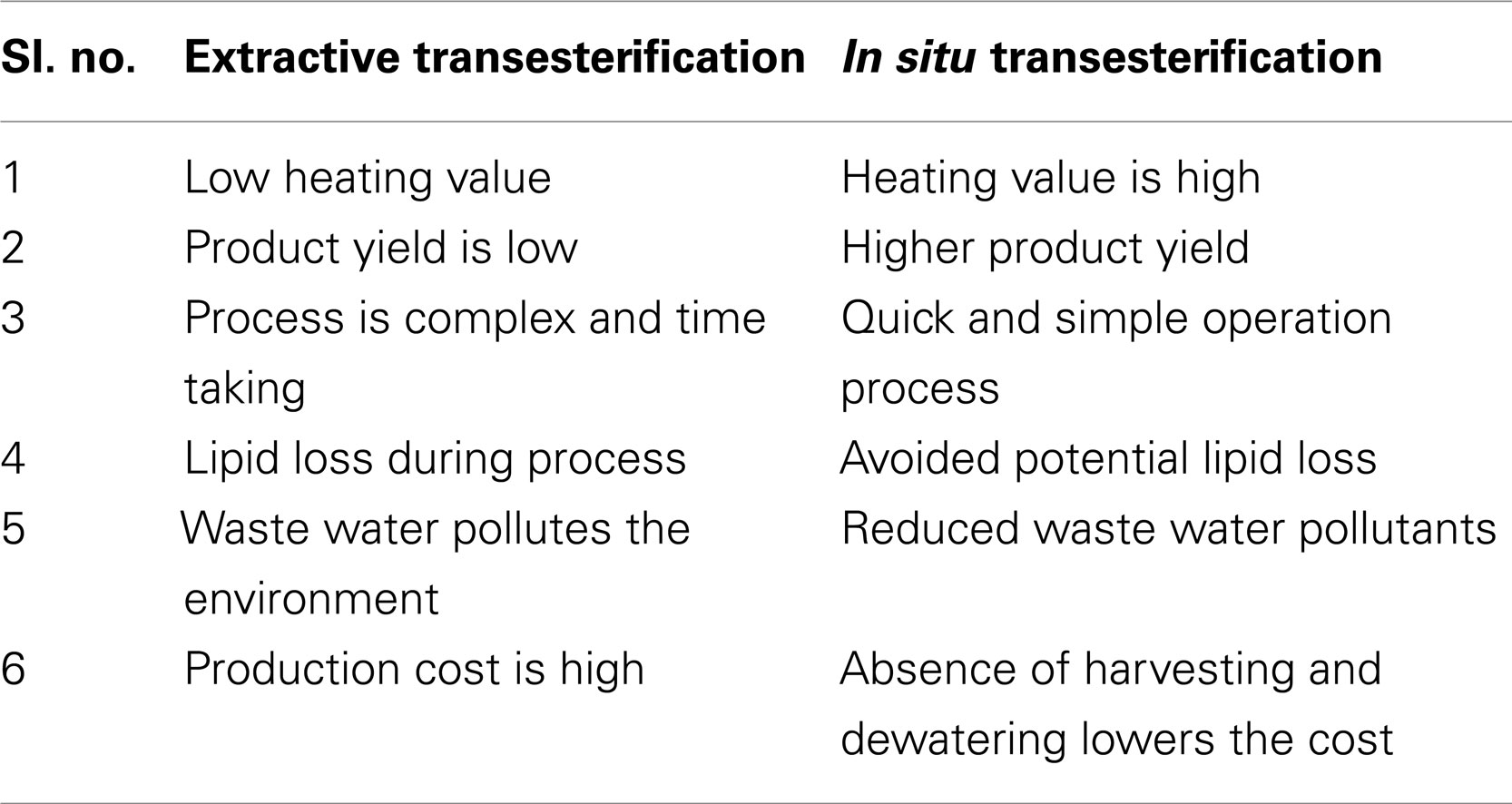
Table 2 . Comparison of extractive transesterification and in situ methods ( Haas and Wagner, 2011 ) .
Bioethanol Production
Several researchers have been reported bioethanol production from certain species of algae, which produce high levels of carbohydrates as reserve polymers. Owing to the presence of low lignin and hemicelluloses content in algae in comparison to lignocellulosic biomass, the algal biomass have been considered more suitable for the bioethanol production ( Chen et al., 2013 ). Recently, attempts have been made (for the bioethanol production) through the fermentation process using algae as the feedstocks to make it as an alternative to conventional crops such as corn and soyabean ( Singh et al., 2011 ; Nguyen and Vu, 2012 ; Chaudhary et al., 2014 ). A comparative study of algal biomass and terrestrial plants for the production of bioethanol has been given in Table 3 . There are different micro and macroalgae such as Chlorococcum sp., Prymnesium parvum , Gelidium amansii , Gracilaria sp., Laminaria sp., Sargassum sp., and Spirogyra sp., which have been used for the bioethanol production ( Eshaq et al., 2011 ; Rajkumar et al., 2014 ). These algae usually require light, nutrients, and carbon dioxide, to produce high levels of polysaccharides such as starch and cellulose. These polysaccharides can be extracted to fermentable sugars through hydrolysis and further fermentation to bioethanol and separated through distillation as shown in Figure 3 .
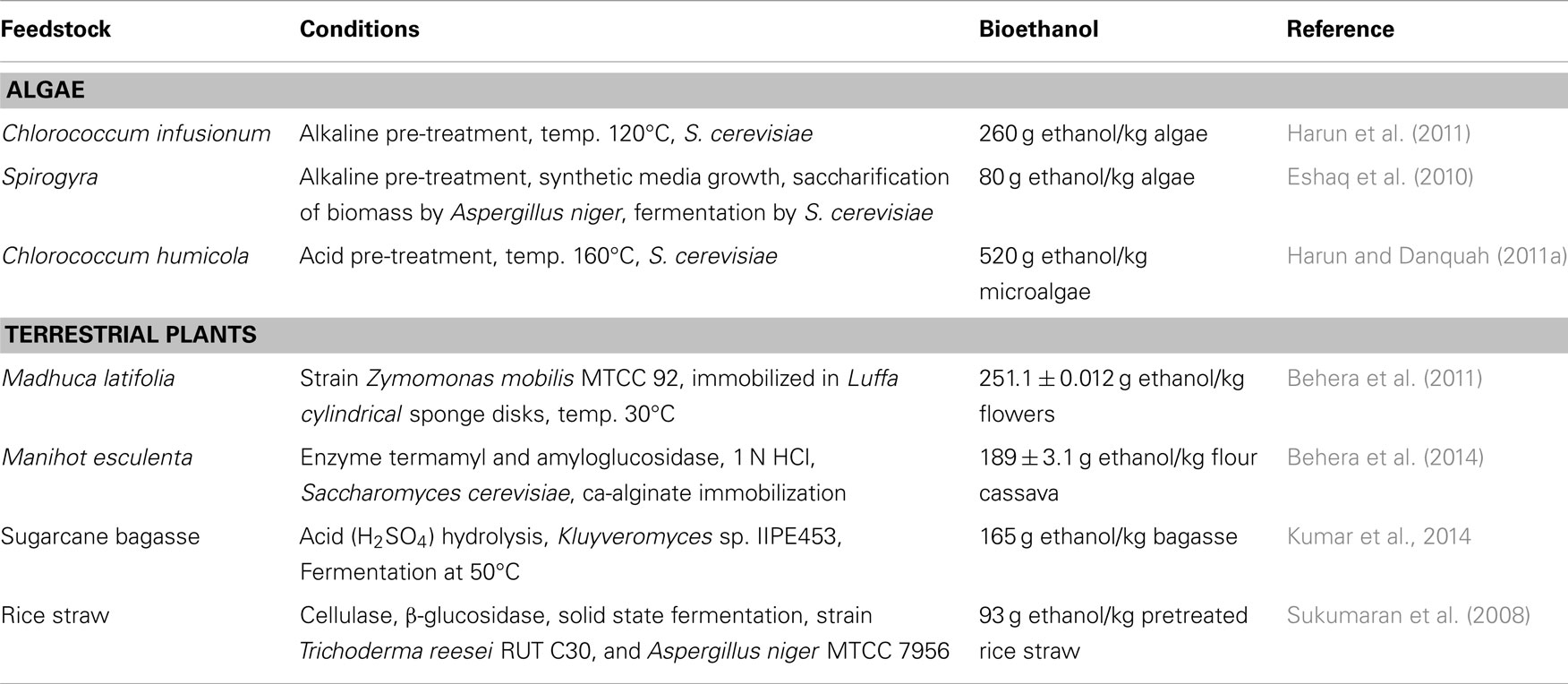
Table 3 . Comparative study between algal biomass and terrestrial plants for bioethanol production .
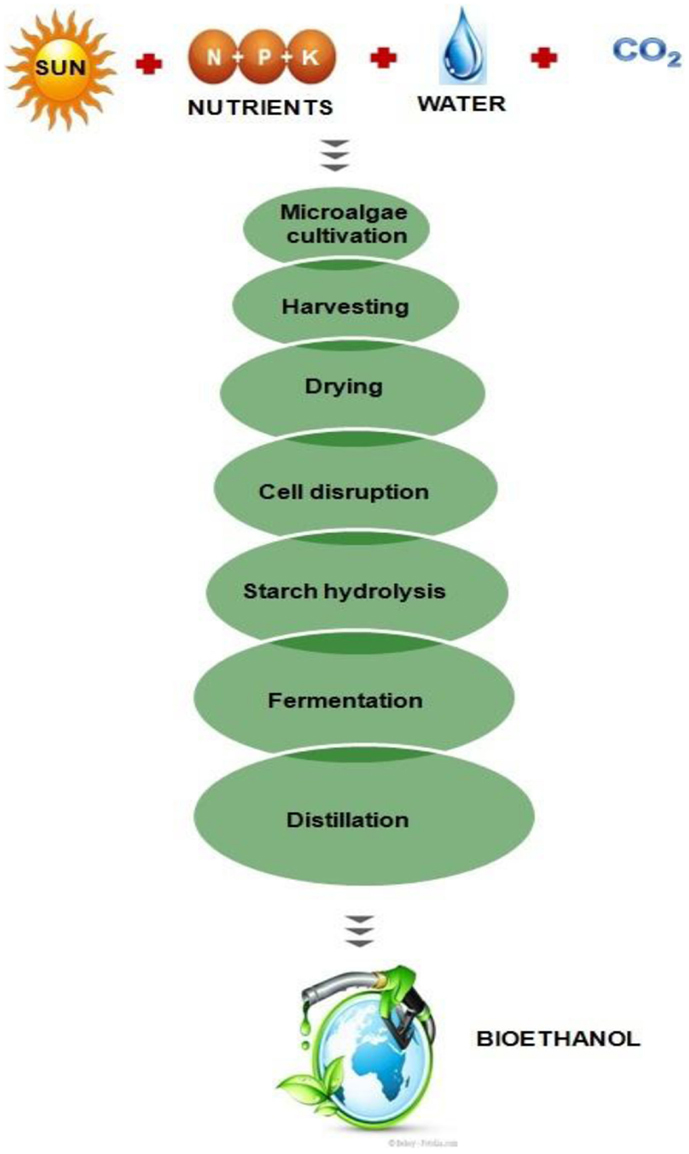
Figure 3. Process for bioethanol production from microalgae .
Pre-Treatment and Saccharification
It has been reported that, the cell wall of some species of green algae like Spirogyra and Chlorococcum contain high level of polysaccharides. Microalgae such as C. vulgaris contains about 37% of starch on dry weight basis, which is the best source for bioethanol with 65% conversion efficiency ( Eshaq et al., 2010 ; Lam and Lee, 2012 ). Such polysaccharide based biomass requires additional processing like pre-treatment and saccharification before fermentation ( Harun et al., 2010 ). Saccharification and fermentation can also be carried out simultaneously using an amylase enzyme producing strain for the production of ethanol in a single step. Bioethanol from microalgae can be produced through the process, which is similar to the first generation technologies involving corn based feedstocks. However, there is limited literature available on the fermentation process of microalgae biomass for the production of bioethanol ( Schenk et al., 2008 ; John et al., 2011 ).
The pre-treatment is an important process, which facilitates accessibility of biomass to enzymes to release the monosaccharides. Acid pre-treatment is widely used for the conversion of polymers present in the cell wall to simple forms. The energy consumption in the pre-treatment is very low and also it is an efficient process ( Harun and Danquah, 2011a , b ). Yazdani et al. (2011) found 7% (w/w) H 2 SO 4 as the promising concentration for the pre-treatment of the brown macroalgae Nizimuddinia zanardini to obtain high yield of sugars without formation of any inhibitors. Candra and Sarinah (2011) studied the bioethanol production using red seaweed Eucheuma cottonii through acid hydrolysis. In this study, 5% H 2 SO 4 concentration was used for 2 h at 100°C, which yielded 15.8 g/L of sugars. However, there are other alternatives to chemical hydrolysis such as enzymatic digestion and gamma radiation to make it more sustainable ( Chen et al., 2012 ; Yoon et al., 2012 ; Schneider et al., 2013 ).
Similar to starch, there are certain polymers such as alginate, mannitol, and fucoidan present in the cell wall of various algae, which requires additional processing like pre-treatment and saccharification before fermentation. Another form of storage carbohydrate found in various brown seaweeds and microalgae is laminarin, which can be hydrolyzed by β-1,3-glucanases or laminarinases ( Kumagai and Ojima, 2010 ). Laminarinases can be categorized into two groups such as exo- and endo-glucanases based on the mode of hydrolysis, which usually produces glucose and smaller oligosaccharides as the end product. Both the enzymes are necessary for the complete digestion of laminarin polymer ( Lee et al., 2014b ).
Markou et al. (2013) saccharified the biomass of Spirulina ( Arthrospira platensis ), fermented the hydrolyzate and obtained the maximum ethanol yield of 16.32 and 16.27% (g ethanol /g biomass ) produced after pre-treatment with 0.5 N HNO 3 and H 2 SO 4 , respectively. Yanagisawa et al. (2011) investigated the content of polysaccharide materials present in three types of seaweeds such as sea lettuce ( Ulva pertusa ), chigaiso ( Alaria crassifolia ), and agar weed ( Gelidium elegans ). These seaweeds contain no lignin, which is a positive signal for the hydrolysis of polysaccharides without any pre-treatment. Singh and Trivedi (2013) used Spirogyra biomass for the production of bioethanol using Saccharomyces cerevisiae and Zymomonas mobilis . In a method, they followed acid pre-treatment of algal biomass and further saccharified using α-amylase producing Aspergillus niger . In another method, they directly saccharified the biomass without any pre-treatment. The direct saccharification process resulted in 2% (w/w) more alcohol in comparison to pretreated and saccharified algal biomass. This study revealed that the pre-treatment with different chemicals are not required in case of Spyrogyra , which reflects its economic importance for the production of ethanol. Also, cellulase enzyme has been used for the saccharification of algal biomass containing cellulose. However, this enzyme system is more expensive than amylases and glucoamylases, and doses required for effective cellulose saccharification are usually very high. Trivedi et al. (2013) applied different cellulases on green alga Ulva for saccharification and found highest conversion efficiency of biomass into reducing sugars by using cellulase 22119 rather than viscozyme L, cellulase 22086 and 22128. In this experiment, they found a maximum yield of sugar 206.82 ± 14.96 mg/g with 2% (v/v) enzyme loading for 36 h at a temperature of 45°C.
Fermentation
There are different groups of microorganisms like yeast, bacteria, and fungi, which can be used for the fermentation of the pretreated and saccharified algal biomass under anaerobic process for the production of bioethanol ( Nguyen and Vu, 2012 ). Nowadays, S. cerevisiae and Z. mobilis have been considered as the bioethanol fermenting microorganisms. However, fermentation of mannitol, a polymer present in certain algae is not possible in anaerobic condition using these well known microorganisms and requires supply of oxygen during fermentation, which is possible only by Zymobacter palmae ( Horn et al., 2000 ).
Certain marine red algae contain agar, a polymer of galactose and galactopyranose, which can be used for the production of bioethanol ( Yoon et al., 2010 ). The biomass of red algae can be depolymerized into different monomeric sugars like glucose and galactose. In addition to mannitol and glucose, brown seaweeds contain about 14% of extra carbohydrates in the form of alginate ( Wargacki et al., 2012 ). Horn et al. (2000) reported the presence of alginate, laminaran, mannitol, fucoidan, and cellulose in some brown seaweeds, which are good source of sugars. They fermented brown seaweed extract having mannitol using bacteria Z. palmae and obtained an ethanol yield of about 0.38 g ethanol/g mannitol.
In the literature, there are many advantages supporting microalgae as the promising substrate for bioethanol production. Hon-Nami (2006) used Chlamydomonas perigranulata algal culture and obtained different by-products such as ethanol and butanediol. Similarly, Yanagisawa et al. (2011) obtained glucose and galactose through the saccharification of agar weed (red seaweed) containing glucan and galactan and obtained 5.5% of ethanol concentration through fermentation using S. cerevisiae IAM 4178. Harun et al. (2010) obtained 60% more ethanol in case of lipid extracted microalgal biomass rather than intact algal biomass of Chlorococcum sp. This shows the importance of algal biomass for the production of both biodiesel and bioethanol.
Biogas Production
Recently, biogas production from algae through anaerobic digestion has received a remarkable attention due to the presence of high polysaccharides (agar, alginate, carrageenan, laminaran, and mannitol) with zero lignin and low cellulose content. Mostly, seaweeds are considered as the excellent feedstock for the production of biogas. Several workers have demonstrated the fermentation of various species of algae like Scenedesmus , Spirulina , Euglena , and Ulva for biogas production ( Samson and Leduy, 1986 ; Yen and Brune, 2007 ; Ras et al., 2011 ; Zhong et al., 2012 ; Saqib et al., 2013 ). The production of biogas using algal biomass in comparison to some terrestrial plants is shown in Table 4 .
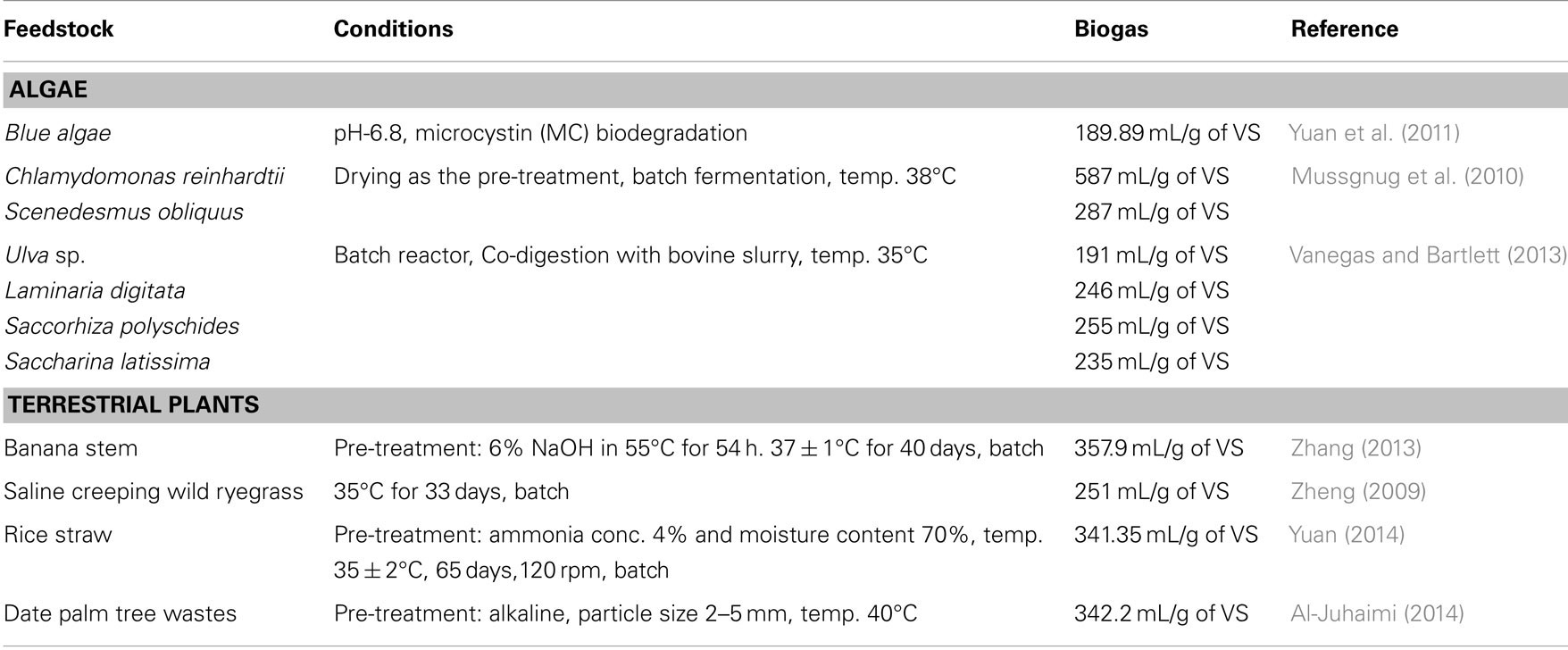
Table 4 . Comparative study between algal biomass and terrestrial plants for biogas production .
Biogas is produced through the anaerobic transformation of organic matter present in the biodegradable feedstock such as marine algae, which releases certain gases like methane, carbon dioxide, and traces of hydrogen sulfide. The anaerobic conversion process involves basically four main steps. In the first step, the insoluble organic material and higher molecular mass compounds such as lipids, carbohydrates, and proteins are hydrolyzed into soluble organic material with the help of enzyme released by some obligate anaerobes such as Clostridia and Streptococci . The second step is called as acidogenesis, which releases volatile fatty acids (VFAs) and alcohols through the conversion of soluble organics with the involvement of enzymes secreted by the acidogenic bacteria. Further, these VFAs and alcohols are converted into acetic acid and hydrogen using acetogenic bacteria through the process of acetogenesis, which finally metabolize to methane and carbon dioxide by the methanogens ( Cantrell et al., 2008 ; Vergara-Fernandez et al., 2008 ; Brennan and Owende, 2010 ; Romagnoli et al., 2011 ).
Sangeetha et al. (2011) reported the anaerobic digestion of green alga Chaetomorpha litorea with generation of 80.5 L of biogas/kg of dry biomass under 299 psi pressure. Vergara-Fernandez et al. (2008) evaluated digestion of the marine algae Macrocystis pyrifera and Durvillaea antarctica marine algae in a two-phase anaerobic digestion system and reported similar biogas productions of 180.4 (±1.5) mL/g dry algae/day with a methane concentration around 65%. However, in case of algae blend, same methane content was observed with low biogas yield. Mussgnug et al. (2010) reported biogas production from some selected green algal species like Chlamydomonas reinhardtii and Scenedesmus obliquus and obtained 587 and 287 mL biogas/g of volatile solids, respectively. Further, there are few studies, which have been conducted with microalgae showing the effect of different pre-treatment like thermal, ultrasound, and microwave for the high production of biogas ( Gonzalez-Fernandez et al., 2012a , b ; Passos et al., 2013 ).
However, there are different factors, which limit the biogas production such as requirement of larger land area, infrastructure, and heat for the digesters ( Collet et al., 2011 ; Jones and Mayfield, 2012 ). The proteins present in algal cells increases the ammonium production resulting in low carbon to nitrogen ratio, which affects biogas production through the inhibition of growth of anaerobic microorganisms. Also, anaerobic microorganisms are inhibited by the sodium ions. Therefore, it is recommended to use the salt tolerating microorganisms for the anaerobic digestion of algal biomass ( Yen and Brune, 2007 ; Brennan and Owende, 2010 ; Jones and Mayfield, 2012 ).
Biohydrogen Production
Recently, algal biohydrogen production has been considered to be a common commodity to be used as the gaseous fuels or electricity generation. Biohydrogen can be produced through different processes like biophotolysis and photo fermentation ( Shaishav et al., 2013 ). Biohydrogen production using algal biomass is comparative to that of terrestrial plants (Table 5 ). Park et al. (2011) found Gelidium amansii (red alga) as the potential source of biomass for the production of biohydrogen through anaerobic fermentation. Nevertheless, they found 53.5 mL of H 2 from 1 g of dry algae with a hydrogen production rate of 0.518 L H 2 /g VSS/day. The authors found an inhibitor, namely, 5-hydroxymethylfurfural (HMF) produced through the acid hydrolysis of G. amansii that decreases about 50% of hydrogen production due to the inhibition. Thus, optimization of the pre-treatment method is an important step to maximize biohydrogen production, which will be useful for the future direction ( Park et al., 2011 ; Shi et al., 2011 ). Saleem et al. (2012) reduced the lag time for hydrogen production using microalgae Chlamydomonas reinhardtii by the use of optical fiber as an internal light source. In this study, the maximum rate of hydrogen production in the presence of exogenic glucose and optical fiber was reported to be 6 mL/L culture/h, which is higher than other reported values.
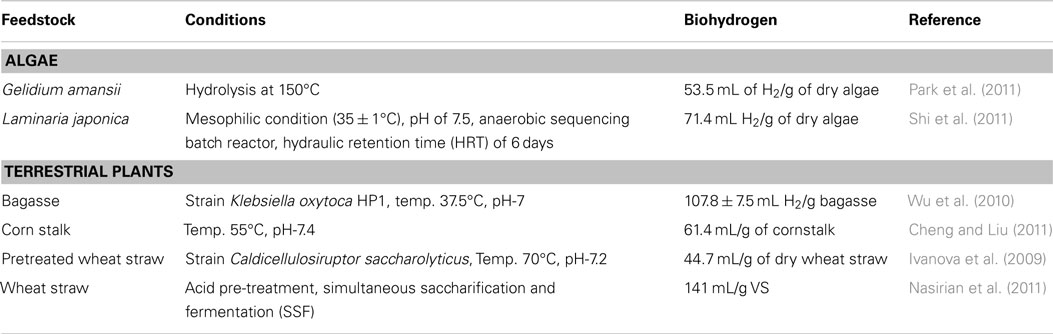
Table 5 . Comparative study between algal biomass and terrestrial plants for biohydrogen production .
Some of microalgae like blue green algae have glycogen instead of starch in their cells. This is an exception, which involves oxidation of ferrodoxin by the hydrogenase enzyme activity for the production of hydrogen in anaerobic condition. However, another function of this enzyme is to be involved in the detachment of electrons. Therefore, different researchers have focused for the identification of these enzyme activities having interactions with ferrodoxin and the other metabolic functions for microalgal photobiohydrogen production. They are also involved with the change of these interactions genetically to enhance the biohydrogen production ( Gavrilescu and Chisti, 2005 ; Hankamer et al., 2007 ; Wecker et al., 2011 ; Yacoby et al., 2011 ; Rajkumar et al., 2014 ).
Bio-Oil and Syngas Production
Bio-oil is formed in the liquid phase from algal biomass in anaerobic condition at high temperature. The composition of bio-oil varies according to different feedstocks and processing conditions, which is called as pyrolysis ( Iliopoulou et al., 2007 ; Yanqun et al., 2008 ). There are several parameters such as water, ash content, biomass composition, pyrolysis temperature, and vapor residence time, which affect the bio-oil productivity ( Fahmi et al., 2008 ). However, due to the presence of water, oxygen content, unsaturated and phenolic moieties, crude bio-oil cannot be used as fuel. Therefore, certain treatments are required to improve its quality ( Bae et al., 2011 ). Bio-oils can be processed for power generation with the help of external combustion through steam and organic rankine cycles, and stirling engines. However, power can also be generated through internal combustion using diesel and gas-turbine engines ( Chiaramonti et al., 2007 ). In literature, there are limited studies on algae pyrolysis compared to lignocellulosic biomass. Although, high yields of bio-oil occur through fluidized-bed fast pyrolysis processes, there are several other pyrolysis modes, which have been introduced to overcome their inherent disadvantages of a high level of carrier gas flow and excessive energy inputs ( Oyedun et al., 2012 ). Demirbas (2006) investigated suitability of the microalgal biomass for bio-oil production and found the superior quality than the wood. Porphy and Farid (2012) produced bio-oil from pyrolysis of algae ( Nannochloropsis sp.) at 300°C after lipid extraction, which composed of 50 wt% acetone, 30 wt% methyl ethyl ketone, and 19 wt% aromatics such as pyrazine and pyrrole. Similarly, Choi et al. (2014) carried out pyrolysis study on a species of brown algae Saccharina japonica at a temperature of 450°C and obtained about 47% of bio-oil yield.
Gasification is usually performed at high temperatures (800–1000°C), which converts biomass into the combustible gas mixture through partial oxidation process, called syngas or producer gas. Syngas is a mixture of different gases like CO, CO 2 , CH 4 , H 2 , and N 2 , which can also be produced through normal gasification of woody biomass. In this process, biomass reacts with oxygen and water (steam) to generate syngas. It is a low calorific gas, which can be utilized in the gas turbines or used directly as fuel. Different variety of biomass feedstocks can be utilized for the production of energy through the gasification process, which is an added advantage ( Carvalho et al., 2006 ; Prins et al., 2006 ; Lv et al., 2007 ).
Conclusion and Future Perspectives
Recently, it is a challenge for finding different alternative resources, which can replace fossil fuels. Due to presence of several advantages in algal biofuels like low land requirement for biomass production and high oil content with high productivity, it has been considered as the best resource, which can replace the liquid petroleum fuel. However, one of its bottlenecks is the low biomass production, which is a barrier for industrial production. Also, another disadvantage includes harvesting of biomass, which possesses high energy inputs. For an economic process development in comparison to others, a cost-effective and energy efficient harvesting methods are required with low energy input. Producing low-cost microalgal biofuels requires better biomass harvesting methods, high biomass production with high oil productivity through genetic modification, which will be the future of algal biology. Therefore, use of the standard algal harvesting technique, biorefinery concept, advances in photobioreactor design and other downstream technologies will further reduce the cost of algal biofuel production, which will be a competitive resource in the near future.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to Prof. Y. K. Yadav, Director, NIRE, Kapurthala for his consistent support to write this review paper. The authors greatly acknowledge the Ministry of New and Renewable Energy, New Delhi, Govt. of India, for providing funds to carry out research work.
Afify, A. M. M., Shanab, S. M., and Shalaby, E. A. (2010). Enhancement of biodiesel production from different species of algae. Grasas y Aceites 61, 416–422. doi: 10.3989/gya.021610
CrossRef Full Text | Google Scholar
Al-Juhaimi, F. Y. (2014). Biogas production through the anaerobic digestion of date palm tree wastes – process optimization. Bioresources 9, 3323–3333. doi:10.15376/biores.9.2
Awolu, O. O., and Layokun, S. K. (2013). Optimization of two-step transesterification production of biodiesel from neem ( Azadirachta indica ) oil. Int. J. Energy Environ. 4, 39. doi:10.1186/2251-6832-4-39
Bae, Y. J., Ryu, C., Jeon, J. K., Park, J., Suh, D. J., Suh, Y. W., et al. (2011). The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour. Technol. 102, 3512–3520. doi:10.1016/j.biortech.2010.11.023
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Behera, S., Mohanty, R. C., and Ray, R. C. (2011). Ethanol production from mahula ( Madhuca latifolia L.) flowers using free and immobilized (in Luffa cylindrical L. sponge discs) cells of Zymomonas mobilis MTCC 92. Ann. Microbiol. 61, 469–474. doi:10.1007/s13213-010-0160-y
Behera, S., Mohanty, R. C., and Ray, R. C. (2014). Batch ethanol production from cassava ( Manihot esculenta Crantz.) flour using Saccharomyces cerevisiae cells immobilized in calcium alginate. Ann. Microbiol. doi:10.1007/s13213-014-0918-8
Bisen, P. S., Sanodiya, B. S., Thakur, G. S., Baghel, R. K., and Prasad, G. B. K. S. (2010). Biodiesel production with special emphasis on lipase-catalyzed transesterification. Biotechnol. Lett. 32, 1019–1030. doi:10.1007/s10529-010-0275-z
Bosma, R., Van Spronsen, W. A., Tramper, J., and Wijffels, R. H. (2003). Ultrasound a new s separation technique to harvest microalgae. J. Appl. Phycol. 15, 143–153. doi:10.1023/A:1023807011027
Boyce, A. N., Chowd-Hury, P., and Naqiuddin, M. (2008). Biodiesel fuel production from algae as renewable energy. Am. J. Biochem. Biotechnol. 4, 250–254. doi:10.3844/ajbbsp.2008.250.254
Brennan, L., and Owende, P. (2010). Biofuels from microalgae-a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energ. Rev. 14, 557–577. doi:10.1016/j.rser.2009.10.009
Candra, K. P., and Sarinah, S. (2011). Study on bioethanol production using red seaweed Eucheuma cottonii from Bontang sea water. J. Coastal Dev. 15, 45–50. doi:10.4172/1410-5217.10000328
Cantrell, K. B., Ducey, T., Ro, K. S., and Hunt, P. G. (2008). Livestock waste-to-bioenergy generation opportunities. Bioresour. Technol. 99, 7941–7953. doi:10.1016/j.biortech.2008.02.061
Carvalho, A. P., Meireles, L. A., and Malcata, F. X. (2006). Microalgal reactors: a review of enclosed system designs and performances. Biotechnol. Prog. 22, 1490–1506. doi:10.1002/bp060065r
Chaudhary, L., Pradhan, P., Soni, N., Singh, P., and Tiwari, A. (2014). Algae as a feedstock for bioethanol production: new entrance in biofuel world. Int. J. Chem. Technol. Res. 6, 1381–1389.
Google Scholar
Chen, C. Y., Zhao, X. Q., Yen, H. W., Ho, S. H., Cheng, C. L., Bai, F., et al. (2013). Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 78, 1–10. doi:10.1016/j.bej.2013.03.006
Chen, R., Yue, Z., Deitz, L., Liu, Y., and Liao, W. (2012). Use of an algal hydrolysate to improve enzymatic hydrolysis of lignocellulose. Bioresour. Technol. 108, 149–154. doi:10.1016/j.biortech.2011.12.143
Cheng, X. Y., and Liu, C. Z. (2011). Hydrogen production via thermophilic fermentation of cornstalk by Clostridium thermocellum . Energy Fuel 25, 1714–1720. doi:10.1021/ef2000344
Chiaramonti, D., Oasmaa, A., and Solantausta, Y. (2007). Power generation using fast pyrolysis liquids from biomass. Renew. Sustain. Energ. Rev. 11, 1056–1086. doi:10.1016/j.rser.2005.07.008
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306. doi:10.1016/j.biotechadv.2007.02.001
Choi, J. H., Woo, H. C., and Suh, D. J. (2014). Pyrolysis of seaweeds for bio-oil and bio-char production. Chem. Eng. Trans. 37, 121–126. doi:10.1016/j.biortech.2014.09.068
Choi, W., Han, J., Lee, C., Song, C., Kim, J., Seo, Y., et al. (2012). Bioethanol production from Ulva pertusa kjellman by high-temperature liquefaction. Chem. Biochem. Eng. 26, 15–21.
Clark, J., and Deswarte, F. (2008). Introduction to Chemicals from Biomass . Wiley series in renewable resources. Hoboken: John Wiley & Sons.
Cohen, Z., Norman, H. A., and Heimer, Y. M. (1995). Microalgae as a source of x-3 fatty acids. World Rev. Nutr. Diet. 77, 1–31.
Collet, P., Helias, A., Lardon, L., Ras, M., Goy, R. A., and Steyer, J. P. (2011). Life-cycle assessment of microalgae culture coupled to biogas production. Bioresour. Technol. 102, 207–214. doi:10.1016/j.biortech.2010.06.154
Crutzen, P. J., Mosier, A. R., Smith, K. A., and Winiwarter, W. (2007). N 2 O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Atmos. Chem. Phys. 7, 11191–11205. doi:10.5194/acpd-7-11191-2007
Csordas, A., and Wang, J. (2004). An integrated photobioreactor and foam fractionation unit for growth and harvest of Chaetoceros sp in open systems. Aquac. Eng. 30, 15–30. doi:10.1016/j.aquaeng.2003.07.001
Demirbas, A. (2006). Oily products from mosses and algae via pyrolysis. Energy Source 28, 933–940. doi:10.1080/009083190910389
Demirbas, A. (2007). Progress and recent trends in biofuels. Prog. Energy Combust. Sci. 33, 1–18. doi:10.1016/j.pecs.2006.06.001
Demirbas, A. (2009). Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 50, 14–34. doi:10.1016/j.enconman.2008.09.001
Demirbas, A. (2010). Use of algae as biofuel sources. Energy Convers. Manag. 51, 2738–2749. doi:10.1016/j.enconman.2010.06.010
Dismukes, G. C., Carrieri, D., Bennette, N., Ananyev, G. M., and Posewitz, M. C. (2008). Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 19, 235–240. doi:10.1016/j.copbio.2008.05.007
Dragone, G., Fernandes, B., Vicente, A. A., and Teixeira, J. A. (2010). “Third generation biofuels from microalgae,” in Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology , ed. A. Mendez-Vilas (Madrid: Formatex), 1315–1366
Eshaq, F. S., Ali, M. N., and Mohd, M. K. (2010). Spirogyra biomass a renewable source for biofuel (bioethanol) production. Int. J. Eng. Sci. Technol. 2, 7045–7054.
Eshaq, F. S., Ali, M. N., and Mohd, M. K. (2011). Production of bioethanol from next generation feed-stock alga Spirogyra species. Int. J. Eng. Sci. Technol. 3, 1749–1755.
Fahmi, R., Bridgwater, A. V., Donnison, I., and Yates, N. (2008). The effect of lignin and inorganic species in biomass on pyrolysis oil yield, quality and stability. Fuel 87, 1230–1240. doi:10.1016/j.fuel.2007.07.026
Fajardo, A. R., Cerdan, L. E., Medina, A. R., Fernandez, F. G. A., Moreno, P. A. G., and Grima, E. M. (2007). Lipid extraction from the microalga Phaeodactylum tricornutum . Eur. J. Lipid Sci. Technol. 109, 120–126. doi:10.1002/ejlt.200600216
Gavrilescu, M., and Chisti, Y. (2005). Biotechnology – a sustainable alternative for chemical industry. Biotechnol. Adv. 23, 471–499. doi:10.1016/j.biotechadv.2005.03.004
Ghadge, S. V., and Raheman, H. (2005). Biodiesel production from mahua ( Madhuca indica ) oil having high free fatty acids. Biomass Bioenergy 28, 601–605. doi:10.1016/j.biombioe.2004.11.009
Goldemberg, J. (2007). Ethanol for a sustainable energy future. Science 315, 808–810. doi:10.1126/science.1137013
Goldemberg, J., and Guardabassi, P. (2009). Are biofuels a feasible option? Energy Policy 37, 10–14. doi:10.1016/j.enpol.2008.08.031
Gonzalez-Fernandez, C., Sialve, B., Bernet, N., and Steyer, J. P. (2012a). Comparison of ultrasound and thermal pretreatment of Scenedesmus biomass on methane production. Bioresour. Technol. 110, 610–616. doi:10.1016/j.biortech.2012.01.043
Gonzalez-Fernandez, C., Sialve, B., Bernet, N., and Steyer, J. P. (2012b). Thermal pretreatment to improve methane production of Scenedesmus biomass. Biomass Energy 40, 105–111. doi:10.1016/j.biombioe.2012.02.008
Haas, M. I., and Wagner, K. (2011). Simplifying biodiesel production: the direct or in situ transesterification of algal biomass. Eur. J. Lipid Sci. Technol. 113, 1219–1229. doi:10.1002/ejlt.201100106
Hankamer, B., Lehr, F., Rupprecht, J., Mussgnug, J. H., Posten, C., and Kruse, O. (2007). Photosynthetic biomass and H 2 production by green algae: from bioengineering to bioreactor scale up. Physiol. Plant. 131, 10–21. doi:10.1111/j.1399-3054.2007.00924.x
Harun, R., and Danquah, M. K. (2011a). Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem . 46, 304–309. doi:10.1016/j.procbio.2010.08.027
Harun, R., and Danquah, M. K. (2011b). Enzymatic hydrolysis of microalgae biomass for bioethanol production. Chem. Eng. J. 168, 1079–1084. doi:10.1016/j.cej.2011.01.088
Harun, R., Danquah, M. K., and Forde-Gareth, M. (2010). Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 85, 199–203. doi:10.1002/jctb.2287
Harun, R., Jason, W. S. Y., Cherrington, T., and Danquah, M. K. (2011). Exploring alkaline pretreatment of microalgal biomass for bioethanol production. Appl. Energy 88, 3464–3467. doi:10.1016/j.apenergy.2010.10.048
Heasman, M., Diemar, J. O., Connor, W., Shushames, T., Foulkes, L., and Nell, J. (2008). Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve molluscs – a summary. Aquac. Res. 31, 637–659. doi:10.1046/j.1365-2109.2000.318492.x
Hidalgo, P., Toro, C., Ciudad, G., and Navia, R. (2013). Advances in direct transesterification microalgal biomass for biodiesel production. Rev. Environ. Sci. Biotechnol. 12, 179–199. doi:10.1007/s11157-013-9308-0
Ho, S. H., Chen, C. Y., Lee, D. J., and Chang, J. S. (2011). Perspectives on microalgal Co 2 -emission mitigation systems-a review. Biotechnol. Adv. 29, 189–198. doi:10.1016/j.biotechadv.2010.11.001
Hon-Nami, K. (2006). A unique feature of hydrogen recovery in endogenous starch to alcohol fermentation of the marine microalga Chlamydomonas perigranulata . Appl. Biochem. Biotechnol. 131, 808–828. doi:10.1385/ABAB:131:1:808
Horn, H. J., Aasen, I. M., and Østgaard, M. (2000). Production of ethanol from mannitol by Zymobacter palmae . J. Ind. Microbiol. Biotechnol. 24, 51–57. doi:10.1038/sj.jim.2900771
Hossain, A. B. M. S., Salleh, A., Boyce, A. N., Chowd-Hury, P., and Naqiuddin, M. (2008). Biodiesel fuel production from algae as renewable energy. Am. J. Biochem. Biotechnol. 4, 250–254. doi:10.3844/ajbbsp.2008.250.254
Hsueh, H. T., Chu, H., and Yu, S. T. (2007). A batch study on the bio-fixation of carbon dioxide in the absorbed solution from a chemical wet scrubber by hot spring and marine algae. Chemosphere 66, 878–886. doi:10.1016/j.chemosphere.2006.06.022
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M., et al. (2008). Microalgal triacylglycerols as feedstocks for biofuels production: perspectives and advances. Plant J. 54, 621–639. doi:10.1111/j.1365-313X.2008.03492.x
Hughes, A. D., Kelly, M. S., Black, K. D., and Stanley, M. S. (2012). Biogas from macroalgae: is it time to revisit the idea? Biotechnol. Biofuels 5, 1–7. doi:10.1186/1754-6834-5-86
Iliopoulou, E. F., Antonakou, E. V., Karakoulia, S. A., Vasalos, I. A., Lappas, A. A., and Triantafyllidis, K. S. (2007). Catalytic conversion of biomass pyrolysis products by mesoporous materials: effect of steam stability and acidity of Al-MCM-41 catalysts. Chem. Eng. J. 134, 51–57. doi:10.1016/j.cej.2007.03.066
Ivanova, G., Rakhely, G., and Kovacs, K. (2009). Thermophilic biohydrogen production from energy plants by Caldicellulosiruptor saccharolyticus and comparison with related studies. Int. J. Hydrogen Energy 34, 3659–3670. doi:10.1016/j.ijhydene.2009.02.082
John, R. P., Anisha, G. S., Nampoothiri, K. M., and Pandey, A. (2011). Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour. Technol. 102, 186–193. doi:10.1016/j.biortech.2010.06.139
Jones, C. S., and Mayfield, S. P. (2012). Algae biofuels: versatility for the future of bioenergy. Curr. Opin. Biotechnol. 23, 346–351. doi:10.1016/j.copbio.2011.10.013
Kim, G. V., Choi, W. Y., Kang, D. H., Lee, S. Y., and Lee, H. Y. (2014). Enhancement of biodiesel production from marine alga, Scenedesmus sp. through in situ transesterification process associated with acidic catalyst. BioMed. Res. Int. 2014:391542 doi:10.1155/2014/391542
Knezevic, Z. D., Siler-Marinkovic, S. S., and Mojovic, L. V. (2004). Immobilized lipases as practical catalysts. Acta Period. Technol. 35, 151–164. doi:10.2298/APT0435151K
Kraan, S. (2013). Mass cultivation of carbohydrate rich microalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Change . 18, 27–46. doi:10.1007/s11027-010-9275-5
Kumagai, Y., and Ojima, T. (2010). Isolation and characterization of two types of β-1,3-glucanases from the common sea hare Aplysia kurodai . Comp. Biochem. Physiol. Biochem. Mol. Biol. 155, 138–144. doi:10.1016/j.cbpb.2009.10.013
Kumar, S., Dheeran, P., Singh, S. P., Mishra, I. M., and Adhikari, D. K. (2014). Bioprocessing for bagasse hydrolysate for ethanol and xylitol production using thermotolerant yeast. Biprocess Biosyst. Eng. 38, 39–47. doi:10.1007/s00449-014-1241-2
Lam, M. K., and Lee, K. T. (2012). Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol. Adv. 30, 630–690. doi:10.1016/j.biotechadv.2011.11.008
Leach, G., Oliveira, G., and Morais, R. (1998). Spray-drying of Dunaliella salina to produce a b-carotene rich powder. J. Ind. Microbiol. Biotechnol. 20, 82–85. doi:10.1038/sj.jim.2900485
Lee, A., Lewis, D., and Ashman, P. (2009). Microbial flocculation, a potentially low-cost harvesting technique for marine microalgae for the production of biodiesel. J. Appl. Phycol . 21, 559–567. doi:10.1007/s10811-008-9391-8
Lee, K., Eisterhold, M. L., Rindi, F., Palanisami, S., and Nam, P. K. (2014a). Isolation and screening of microalgae from natural habitats in the Midwestern United States of America for biomass and biodiesel sources. J. Nat. Sci. Biol. Med. 5, 333–339. doi:10.4103/0976-9668.136178
Lee, K. C., Takamitsu, A., Ibrahim, D., Kosugi, A., Prawitwong, P., Lan, D., et al. (2014b). Purification and characterization of a thermostable laminarinase from Penicillium rolfsii c3-2(1) IBRL. Bioresource 9, 1072–1084. doi:10.15376/biores.9.1
Lee, S. J., Yoon, B. D., and Oh, H. M. (1998). Rapid method for the determination of lipid from the green alga Botryococcus braunii . Biotechnol. Tech. 12, 553–556. doi:10.1023/A:1008811716448
Li, Y., Horsman, M., Wu, N., Lan, C. Q., and Dubois-Calero, N. (2008). Biofuels from microalgae. Biotechnol. Progr. 24, 815–820. doi:10.1021/bp070371k
Li, Y., Naghdi, F. G., Garg, G., Adarme-Vega, T. C., Thurecht, K. J., Ghafor, W. A., et al. (2014). A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microb. Cell Fact. 13, 1–9. doi:10.1186/1475-2859-13-14
Liu, J., Zhu, Y., Tao, Y., Zhang, Y., Li, A., Li, T., et al. (2013). Freshwater microalgae harvested via flocculation induced by pH decrease. Biotechnol. Biofuels 6, 98. doi:10.1186/1754-6834-6-98
Luo, Y., Zheng, Y., Jiang, Z., Ma, Y., and Wei, D. (2006). A novel psychrophilic lipase from Pseudomonas fluorescens with unique property in chiral resolution and biodiesel production via transesterification. Appl. Microbiol. Biotechnol. 73, 349–355. doi:10.1007/s00253-006-0478-3
Lv, P., Yuan, Z., Wu, C., Ma, L., Chen, Y., and Tsubaki, N. (2007). Biosyngas production from biomass catalytic gasification. Energy. Convers. Manag. 48, 1132–1139. doi:10.1016/j.enconman.2006.10.014
Mamilla, V. R., Mallikarjun, M. V., and Rao, G. L. N. (2011). Preparation of biodiesel from Karanja oil. Int. J. Energy Eng. 1, 94–100. doi:10.5963/IJEE0102008
Markou, G., Angelidaki, I., Nerantzis, E., and Georgakakis, D. (2013). Bioethanol production by carbohydrate-enriched biomass of Arthrospira ( Spirulina ) platensis . Energies 6, 3937–3950. doi:10.3390/en6083937
Marques, A. E., Barbosa, A. T., Jotta, J., Coelho, M. C., Tamagnini, P., and Gouveia, L. (2011). Biohydrogen production by Anabaena sp. PCC 7120 wild-type and mutants under different conditions: light, nickel, propane, carbon dioxide and nitrogen. Biomass Bioenergy 35, 4426–4434. doi:10.1016/j.biombioe.2011.08.014
Martin, M., Pires, R. F., Alves, M. J., Hori, C. E., Reis, M. H. M., and Cardoso, V. L. (2013). Transesterification of soybean oil for biodiesel production using hydrotalcite as basic catalyst. Chem. Eng. Trans. 32, 817–822. doi:10.3303/CET1332137
Mata, T. M., Martins, A., and Caetano, N. S. (2010). Microalgae for biodiesel production and other applications: a review. Ren. Sust. Energy. Rev . 14, 217–232. doi:10.1016/j.rser.2009.07.020
Mazubert, A., Poux, M., and Aubin, J. (2013). Intensified processes for FAME production from waste cooking oil: a technological review. Chem. Eng. J. 233, 201–223. doi:10.1016/j.cej.2013.07.063
Meher, L. C., Vidya, S. D., and Naik, S. N. (2006). Technical aspects of biodiesel production by transesterification – a review. Renew. Sustain. Energ. Rev. 10, 248–268. doi:10.1016/j.biotechadv.2014.10.003
Munir, N., Sharif, N., Naz, S., Saleem, F., and Manzoor, F. (2013). Harvesting and processing of microalgae biomass fractions for biodiesel production (a review). Sci. Tech. Dev. 32, 235–243.
Mussgnug, J. H., Klassen, V., Schluter, A., and Kruse, O. (2010). Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 150, 51–56. doi:10.1016/j.jbiotec.2010.07.030
Mutanda, T., Ramesh, D., Karthikeyan, S., Kumari, S., Anandraj, A., and Bux, F. (2011). Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol. 102, 57–70. doi:10.1016/j.biortech.2010.06.077
Muthukumar, A., Elayaraja, S., Ajithkumar, T. T., Kumaresan, S., and Balasubramanian, T. (2012). Biodiesel production from marine microalgae Chlorella marina and Nannochloropsis salina . J. Petrol. Technol Altern. Fuels 3, 58–62. doi:10.5897/JPTAF12.010
Naik, M., Meher, L. C., Waik, S. N., and Das, L. M. (2008). Production of biodiesel from high free fatty acid Karanja ( Pongamia pinnata ) oil. Biomass Bioenergy 32, 354–357. doi:10.1016/j.biombioe.2007.10.006
Nascimento, I. A., Marques, S. S. I., Cabanelas, I. T. D., Pereira, S. A., Druzian, J. I., de Souza, C. O., et al. (2013). Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenerg. Res. 6, 1–13. doi:10.1007/s12155-012-9222-2
Nasirian, N., Almassi, M., Minaei, S., and Widmann, R. (2011). Development of a method for biohydrogen production from wheat straw by dark fermentation. Int. J. Hydrogen Energy 36, 411–420. doi:10.1016/j.ijhydene.2010.09.073
Nautiyal, P., Subramanian, K. A., and Dastidar, M. G. (2014). Kinetic and thermodynamic studies on biodiesel production from Spirulina platensis algae biomass using single stage extraction-transesterification process. Fuel 135, 228–234. doi:10.1016/j.fuel.2014.06.063
Nguyen, T. H. M., and Vu, V. H. (2012). Bioethanol production from marine algae biomass: prospect and troubles. J. Viet. Env. 3, 25–29. doi:10.13141/jve.vol3.no1
Nigam, P. S., and Singh, A. (2011). Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 37, 52–68. doi:10.1016/j.pecs.2010.01.003
Oyedun, A. O., Lam, K. L., Gebreegziabher, T., Lee, H. K. M., and Hui, C. W. (2012). Optimisation of operating parameters in multi-stage pyrolysis. Chem. Engg. Trans. 29, 655–660. doi:10.3303/CET1229110
Park, J. H., Yoon, J. J., Park, H. D., Kim, Y. J., Lim, D. J., and Kim, S. H. (2011). Feasibility of biohydrogen production from Gelidium amansii . Int. J. Hydrogen Energy 36, 13997–14003. doi:10.1016/j.ijhydene.2011.04.003
Passos, F., Sole, M., Garcia, J., and Ferrer, I. (2013). Biogas production from microalgae grown in wastewater: effect of microwave pretreatment. Appl. Energy 108, 168–175. doi:10.1016/j.watres.2013.10.013
Patil, P. D., Gude, V. G., Mannarswamy, A., Cooke, P., Nirmalakhandan, N., Lammers, P., et al. (2012). Comparison of direct transesterification of algal biomass under supercritical methanol and microwave irradiation conditions. Fuel 97, 822–831. doi:10.1016/j.fuel.2012.02.037
Popoola, T. O. S., and Yangomodou, O. D. (2006). Extraction, properties and utilization potentials of cassava seed oil. Biotechnology 5, 38–41. doi:10.3923/biotech.2006.38.41
Porphy, S. J., and Farid, M. M. (2012). Feasibility study for production of biofuel and chemicals from marine microalgae Nannochloropsis sp. based on basic mass and energy analysis. ISRN Renew. Energ. 2012:156824. doi:10.5402/2012/156824
Prakash, J., Pushparaj, B., Carlozzi, P., Torzillo, G., Montaini, E., and Materassi, R. (2007). Microalgal biomass drying by a simple solar device. Int. J. Solar Energy 18, 303–311. doi:10.1080/01425919708914325
Pratoomyot, J., Srivilas, P., and Noiraksar, T. (2005). Fatty acids composition of 10 microalgal species. J. Sci. Technol. 27, 1179–1187.
Prins, M. J., Ptasinski, K. J., and Janssen, F. J. J. G. (2006). More efficient biomass gasification via torrefaction. Energy 31, 3458–3470. doi:10.1016/j.energy.2006.03.008
Prochazkova, G., Safarik, I., and Branyik, T. (2013). Harvesting microalgae with microwave synthesized magnetic microparticles. Bioresour. Technol. 130, 472–477. doi:10.1016/j.biortech.2012.12.060
Rajkumar, R., Yaakob, Z., and Takriff, M. S. (2014). Potential of the micro and macro algae for biofuel production: a brief review. Bioresour. 9, 1606–1633. doi:10.15376/biores.9.1
Rajvanshi, S., and Sharma, M. P. (2012). Microalgae: a potential source of biodiesel. J. Sustain. Bioenergy Syst. 2, 49–59. doi:10.4236/jsbs.2012.23008
Ras, M., Lardon, L., Bruno, S., Bernet, N., and Steyer, J. P. (2011). Experimental study on a coupled process of production and anaerobic digestion of Chlorella vulgaris . Bioresour. Technol. 102, 200–206. doi:10.1016/j.biortech.2010.06.146
Richmond, A. (2004). Handbook of Microalgal Culture: Biotechnology and Applied Phycology . Osney Mead, Oxford: Blackwell Science Ltd.
Richmond, A., and Qiang, H. (2013). Handbook of Microalgal Culture: Applied Phycology and Biotechnology , Second Edn. Hoboken, NJ: Wiley-Blackwell.
Rodolfi, L., Zittelli, G. C., Bassi, N., Padovani, G., Biondi, N., Bonini, G., et al. (2009). Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102, 100–112. doi:10.1002/bit.22033
Romagnoli, F., Blumberga, D., and Gigli, E. (2011). Biogas from marine macroalgae: a new environmental technology-life cycle inventory for a further LCA. Environ. Climate Technol. 4, 97–108. doi:10.2478/v10145-010-0024-5
Rosenberg, J. N., Oyler, G. A., Wilkinson, L., and Betenbaugh, M. J. (2008). A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 19, 430–436. doi:10.1016/j.copbio.2008.07.008
Rossignol, N., Vandanjon, L., Jaoue, P., and Quemeneur, F. (1999). Membrane technology for the continuous separation of microalgae/culture medium: compared performances of cross-flow microfiltration and ultrafiltration. Aquac. Eng. 20, 191–208. doi:10.1016/S0144-8609(99)00018-7
Ryckebosch, E., Muylaert, K., and Foubert, I. (2012). Optimization of an analytical procedure for extraction of lipids from microalgae. J. Am. Oil Chem. Soc. 89, 189–198. doi:10.1007/s11746-011-1903-z
Saleem, M., Chakrabarti, M. H., Raman, A. A. A., Hasan, D. B., Daud, W. M. A. W., and Mustafa, A. (2012). Hydrogen production by Chlamydomonas reinhardtii in a two-stage process with and without illumination at alkaline pH. Int. J. Hydrogen Energy 37, 4930–4934. doi:10.1016/j.ijhydene.2011.12.115
Samson, R., and Leduy, A. (1986). Detailed study of anaerobic digestion of Spirulina maxima algal biomass. Biotechnol. Bioeng. 28, 1014–1023. doi:10.1002/bit.260280712
Sangeetha, P., Babu, S., and Rangasamy, R. (2011). Potential of green alga Chaetomorpha litorea (Harvey) for biogas production. Int. J. Curr. Sci. 1, 24–29.
Saqib, A., Tabbssum, M. R., Rashid, U., Ibrahim, M., Gill, S. S., and Mehmood, M. A. (2013). Marine macroalgae Ulva : a potential feed-stock for bioethanol and biogas production. Asian J. Agri. Biol. 1, 155–163.
Schenk, P., Thomas-Hall, S., Stephens, E., Marx, U., Mussgnug, J., Posten, C., et al. (2008). Second generation biofuels: high efficiency microalgae for biodiesel production. Bioenergy Res . 1, 20–43. doi:10.1007/s12155-008-9008-8
Schneider, R. C. S., Bjerk, T. R., Gressler, P. D., Souza, M. P., Corbelline, V. A., and Lobo, E. A. (2013). “Potential production of biofuel from microalgae biomass produced in wastewater,” in Biodiesel – Feedstocks, Production and Applications , ed. Z. Fang (Rijeka: InTech). doi:10.5772/52439
Shaishav, S., Singh, R. N., and Satyendra, T. (2013). Biohydrogen from algae: fuel of the future. Int. Res. J. Env. Sci. 2, 44–47.
Shalaby, E. A. (2011). “Algal biomass and biodiesel production,” in Biodiesel – Feedstocks and Processing Technologies , ed. S. Margarita (Rijeka: InTech), 111–132. ISBN: 978-953-307-713-0.
Sharma, R., Chisti, Y., and Banerjee, U. C. (2001). Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 19, 627–662. doi:10.1016/S0734-9750(01)00086-6
Sharma, Y. C., and Singh, B. (2009). Development of biodiesel: current scenario. Renew. Sustain. Energ. Rev. 13, 1646–1651. doi:10.1016/j.biortech.2010.06.057
Shi, X., Jung, K. W., Kim, H., Ahn, Y. T., and Shin, H. S. (2011). Direct fermentation of Laminaria japonica for biohydrogen production by anaerobic mixed cultures. Int. J. Hydrogen Energy 36, 5857–5864. doi:10.1016/j.ijhydene.2011.01.125
Shuping, Z., Yulong, W., Mingde, Y., Kaleem, I., Chun, L., and Tong, J. (2010). Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy 35, 5406–5411. doi:10.1016/j.energy.2010.07.013
Singh, A., He, B., Thompson, J., and Gerpen, J. V. (2006). Process optimization of biodiesel production using alkaline catalysts. Appl. Eng. Agric. 22, 597–600. doi:10.13031/2013.21213
Singh, A., Nigam, P. S., and Murphy, J. D. (2011). Mechanism and challenges in commercialisation of algal biofuels. Bioresour. Technol. 102, 26–34. doi:10.1016/j.biortech.2010.06.057
Singh, D. P., and Trivedi, R. K. (2013). Production of biofuel from algae: an economic and eco-friendly resource. Int. J. Sci. Res. 2, 352–357.
Singh, J., and Gu, S. (2010). Commercialization potential of microalgae for biofuels production. Renew. Sustain. Energ. Rev. 14, 2596–2610. doi:10.1016/j.rser.2010.06.014
Singh, R., Behera, S., Yadav, Y. K., and Kumar, S. (2014). “Potential of wheat straw for biogas production using thermophiles,” in Recent Advances in Bio-Energy Research , eds S. Kumar, A. K. Sarma, S. K. Tyagi, and Y. K. Yadav (Kapurthala: SSS-National Institute of Renewable Energy), 242–249.
Spolaore, P., Joannis-Cassan, C., Duran, E., and Isambert, A. (2006). Commercial applications of microalgae. J. Biosci. Bioeng. 101, 87–96. doi:10.1263/jbb.101.87
Stergiou, P. Y., Foukis, A., Filippou, M., Koukouritaki, M., Parapouli, M., Theodorou, L. G., et al. (2013). Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 31, 1846–1859. doi:10.1016/j.biotechadv.2013.08.006
Sukumaran, R. K., Singhania, R., Mathew, G. M., and Pandey, A. (2008). Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew. Energ. 34, 421–424. doi:10.1016/j.renene.2008.05.008
Surendhiran, D., and Vijay, M. (2012). Microalgal biodiesel-acomprehensive review on the potential and alternative biofuel. Res. J. Chem. Sci. 2, 71–82.
Susilaningsih, D., Djohan, A. C., Widyaningrum, D. N., and Anam, K. (2009). Biodiesel from indigenous Indonesian marine microalgae Nanochloropsis sp. J. Biotechnol. Res. Trop. Reg. 2, 1–4.
Tamer, E., Amin, M. A., Ossama, E. T., Bo, M., and Benoit, G. (2006). Biological treatment of industrial wastes in a photobioreactor. Water Sci. Technol. 53, 117–125. doi:10.2166/wst.2006.344
Thomas, D. N. (2002). Seaweeds . Washington, DC: Smithsonian Books, Natural History Museum.
Topare, N., Rout, S. J., Renge, V. C., Khedkar, S. V., Chavan, Y. P., and Bhagat, S. L. (2011). Extraction of oil from algae by solvent extraction and oil expeller method. Int. J. Chem. 9, 1746–1750.
Trivedi, N., Gupta, V., Reddy, C. R., and Jha, B. (2013). Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga Ulva fasciata Delile. Bioresour. Technol. 150, 106–112. doi:10.1016/j.biortech.2013.09.103
Vanegas, C. H., and Bartlett, J. (2013). Green energy from marine algae: biogas production and composition from the anaerobic digestion of Irish seaweed species. Environ. Technol. 34, 2277–2283. doi:10.1080/09593330.2013.765922
Vergara-Fernandez, A., Vargas, G., Alarcon, N., and Velasco, A. (2008). Evaluation of marine algae as a source of biogas in a two-stage anaerobic reactor system. Biomass Bioenergy 32, 338–344. doi:10.1016/j.biombioe.2007.10.005
Wargacki, A. J., Leonard, E., Win, M. N., Regitsky, D. D., Santos, C. N., Kim, P. B., et al. (2012). An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335, 308–313. doi:10.1126/science.1214547
Wecker, M. S. A., Meuser, J. E., Posewitz, M. C., and Ghirardi, M. L. (2011). Design of a new biosensor for algal H 2 production based on the H 2 -sensing system of Rhodobacter capsulatus . Int. J. Hydrogen Energy 36, 11229–11237. doi:10.1016/j.ijhydene.2011.05.121
Widjaja, A., Chien, C. C., and Ju, Y. H. (2009). Study of increasing lipid production from fresh water microalgae Chlorella vulgaris . J. Taiwan Inst. Chem. Eng. 40, 13–20. doi:10.1016/j.jtice.2008.07.007
Williams, P. J. L. B., and Laurens, L. M. L. (2010). Microalgae as biodiesel and biomass feedstocks: review and analysis of the biochemistry, energetics and economics. Energy Environ. Sci 3, 554–590. doi:10.1039/b924978h
Wu, X., Li, Q., Dieudonne, M., Cong, Y., Zhou, J., and Long, M. (2010). Enhanced H 2 gas production from bagasse using adhE inactivated Klebsiella oxytoca HP1 by sequential dark-photo fermentations. Bioresour. Technol. 101, 9605–9611. doi:10.1016/j.biortech.2010.07.095
Yacoby, I., Pochekailov, S., Topotrik, H., Ghirardi, M. L., King, P. W., and Zhang, S. (2011). Photosynthetic electron partitioning between (FeFe)-hydrogenase and ferrodoxin: NADP+-oxidoreductase (FNR) enzymes in vitro. Proc. Natl. Acad. Sci. U.S.A. 108, 9396–9401. doi:10.1073/pnas.1103659108
Yanagisawa, M., Nakamura, K., Ariga, O., and Nakasaki, K. (2011). Production of high concentrations of bioethanol from seaweeds that contain easily hydrolysable polysaccharides. Process Biochem. 46, 2111–2116. doi:10.4161/bioe.23396
Yanqun, L., Mark, H., Nan, W., Christopher, Q. L., and Nathalie, D. C. (2008). Biofuels from microalgae. Biotechnol. Progress. 24, 815–820.
Yazdani, P., Karimi, K., and Taherzadeh, M. J. (2011). “Improvement of enzymatic hydrolysis of a marine macro-alga by dilute acid hydrolysis pretreatment,” in World Renewable Energy Congress (Bioenergy Technology), 8-13 May , ed. B. Moshfegh (Linkoping, Sweden), 186–191.
Yen, H. W., and Brune, D. E. (2007). Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol. 98, 130–134. doi:10.1016/j.biortech.2005.11.010
Yoon, J. J., Kim, Y. J., Kim, S. H., Ryu, H. J., Choi, J. Y., Kim, G. S., et al. (2010). Production of polysaccharides and corresponding sugars from red seaweed. Adv. Mater. Res. 9, 463–466. doi:10.1016/j.biortech.2008.11.053
Yoon, M., Choi, J. I., Lee, J. W., and Park, D. H. (2012). Improvement of saccharification process for bioethanol production from Undaria sp. by gamma irradiation. Radiat. Phys. Chem. 81, 999–1002. doi:10.1016/j.radphyschem.2011.11.035
Yuan, H. (2014). Effect of ammoniation pretreatment at low moisture content on anaerobic digestion performance of rice straw. Bioresources 9, 6707–6718. doi:10.15376/biores.9.4
Yuan, X., Shi, X., Zhang, D., Qiu, Y., Guo, R., and Wang, L. (2011). Biogas production and microcystin biodegradation in anaerobic digestion of blue algae. Energy Environ. Sci. 4, 1511–1515. doi:10.1039/c0ee00452a
Zhang, C. (2013). Alkaline pretreatment for enhancement of biogas production. Bioresour. Technol. 149, 353–358. doi:10.1016/j.biortech.2013.09.070
Zheng, Y. (2009). Anaerobic digestion of saline creeping wild ryegrass for biogas production and pretreatment of particleboard material. Bioresour. Technol. 100, 1582–1588. doi:10.1016/j.biortech.2008.08.048
Zhong, W., Zhang, Z., Luo, Y., Qiao, W., Xiao, M., and Zhang, M. (2012). Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour. Technol. 114, 181–186. doi:10.1016/j.biortech.2012.02.111
Keywords: algae, microalgae, biofuels, bioethanol, biogas, biodiesel, biohydrogen
Citation: Behera S, Singh R, Arora R, Sharma NK, Shukla M and Kumar S (2015) Scope of algae as third generation biofuels. Front. Bioeng. Biotechnol. 2 :90. doi: 10.3389/fbioe.2014.00090
Received: 31 July 2014; Accepted: 29 December 2014; Published online: 11 February 2015.
Reviewed by:
Copyright: © 2015 Behera, Singh, Arora, Sharma, Shukla and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sachin Kumar, Biochemical Conversion Division, Sardar Swaran Singh National Institute of Renewable Energy, Jalandhar-Kapurthala Road, Wadala Kalan, Kapurthala 144601, Punjab, India e-mail: sachin.biotech@gmail.com
This article is part of the Research Topic
Marine biomolecules
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Biofuels from algae: challenges and potential
Michael hannon.
1 San Diego Center for Algal Biotechnology, University of California San Diego, Division of Biology, La Jolla, CA, USA
Javier Gimpel
Miller tran, beth rasala, stephen mayfield.
Algae biofuels may provide a viable alternative to fossil fuels; however, this technology must overcome a number of hurdles before it can compete in the fuel market and be broadly deployed. These challenges include strain identification and improvement, both in terms of oil productivity and crop protection, nutrient and resource allocation and use, and the production of co-products to improve the economics of the entire system. Although there is much excitement about the potential of algae biofuels, much work is still required in the field. In this article, we attempt to elucidate the major challenges to economic algal biofuels at scale, and improve the focus of the scientific community to address these challenges and move algal biofuels from promise to reality.
Importance & challenges of algal biofuels
The global economy requires fossil hydrocarbons to function, from producing plastics and fertilizers to providing the energy required for lighting, heating and transportation. With our increasing population and expanding economy, there will be increased fossil fuel use. As countries improve their gross domestic product per capita, data suggest that their fossil fuel use will increase, and competition for these limited resources will increase. In addition, there comes increasing atmospheric CO 2 concentration, and the potential for significant greenhouse gas-mediated climate change [ 1 ], which now seems likely to affect all parts of the world. Finally, petroleum, which is partially derived from ancient algae deposits, is a limited resource that will eventually run out or become too expensive to recover [ 2 – 4 ]. These factors are driving the development of renewable energy sources that can supplant fossil fuels, and allow greater access to fuel resources for all nations, while greatly reducing carbon emissions into the atmosphere. A number of technologies have been examined as renewable energy sources and, although no single strategy is likely to provide a total solution, it seems possible that a combination of strategies can be employed that will substantially decrease our dependence on fossil fuels [ 4 ]. The challenge that remains is to develop renewable energy industries that operate sustainably and can be cost competitive with existing energy options.
Fossil fuels are used for the generation of electrical power, as well as liquid fuels. There are a variety of renewable or low atmospheric pollution technologies that can generate electrical power, including solar, wind, hydroelectric, geothermal and nuclear. However, renewable technologies to supplement or replace liquid fossil fuels are still in their early developmental stages. The International Energy Agency expects that biofuels will contribute 6% of total fuel use by 2030, but could expand significantly if undeveloped petroleum fields are not accessed or if substantial new fields are not identified ( Figure 1 ). The most promising sustainable alternatives are almost exclusively categorized under the moniker ‘biofuels’. This term describes a diverse range of technologies that generate fuel with at least one component based on a biological system. The major technologies presently employed for biofuels begin with terrestrial plants and culminate with ethanol, whether this is corn starch to sugar to ethanol, or sugarcane sugars to ethanol. The regional success of some of these strategies is well noted; in particular, the sugarcane-to-ethanol production in Brazil [ 5 ]. To a lesser degree, oils from terrestrial plants – for example, soy and palm – are used to produce biodiesel. These strategies are functional at the small scale; however, as their use has increased, it is evident that they are not sustainable, owing to the enormous amount of agricultural land that would be required to supplant a significant fraction of petroleum using this strategy [ 6 , 7 ]. A number of hybrid strategies have been discussed or are currently being deployed. Examples of such strategies include conversion of cellulose to sugars for fermentation into fuel, and gasification of residual biomass into syngas that can then be used to produce liquid fuels [ 8 ]. Although each of these strategies is being used to produce fuels, they are insufficient to accommodate the global demand for liquid fuels.

(A) Global liquid fuel use in 2006 was predominantly (96.3%) conventional petroleum, with slightly less than 1% being biofuels. (B) In 2030, the International Energy Agency estimates that 29% of liquid fuels will originate from current conventional oil sources, 57% will be from undeveloped or unidentified conventional oil sources and 6% will be biofuels [ 4 ]. The large gray area of undeveloped or unidentified sources provides ample and possibly necessary expansion for nonconventional sources.
In this article, we discuss the potential of a burgeoning alternative strategy: microalgae-produced liquid fuels. The high lipid content, high growth rate and ability to rapidly improve strains and produce co-products, without competing for arable land, make algae an exciting addition to the sustainable fuel portfolio. This article will focus on the requirements for establishing microalgae as an environmentally and economically viable platform, with an emphasis on combining fuel production with production of co-products, which we view as an essential strategy for the economic viability and, hence, broad adoption, of this potential fuel source ( Figure 2 ).

These improvements will require years of research and cover (A) bioprospecting for high-oil-producing, low-input-requiring species; (B) engineering to improve growth, harvesting and nutrient recycling; (C ) further strain improvement through breeding, selection and random mutagenesis; and (D) bioengineering to improve fuel traits, produce co-products and crop protection. Estimates given in this figure are for illustration purposes based on our best guesses. We believe that bioprospecting has high potential to identify a solid biofuel species in the next few years but subsequent improvement of that species, as well as solving engineering challenges to improve cost efficiency, will not occur as rapidly.
Present economic reality of liquid fuels
There are considerable challenges to making biofuels capable of competing with petroleum. Certainly, a premium price is warranted for clean fuels (fuels that have a 50% lower CO 2 cradle-to-grave footprint than petroleum); however, estimated costs of a barrel of algae-based fuel using current technology is US$300–2600, compared with $40–80 (2009) for petroleum [ 9 – 12 ]. Although some estimates for a barrel of algae oil in specific regions reach as low as $84 [ 13 ]. The higher dollar estimates are more common and similar to our own estimates, and exclude algal oil from the current liquid fuel market. The challenges and strategies to tackle this economic discrepancy are discussed in this article.
Benefits of microalgal biofuels
Microalgae are a diverse group of single-celled organisms that have the potential to offer a variety of solutions for our liquid transportation fuel requirements through a number of avenues. Algal species grow in a wide range of aquatic environments, from freshwater through saturated saline. Algae efficiently use CO 2 , and are responsible for more than 40% of the global carbon fixation, with the majority of this productivity coming from marine microalgae [ 14 , 15 ]. Algae can produce biomass very rapidly, with some species doubling in as few as 6 h, and many exhibiting two doublings per day [ 16 , 17 ]. All algae have the capacity to produce energy-rich oils, and a number of microalgal species have been found to naturally accumulate high oil levels in total dry biomass [ 18 ]. For example, some Botryococcus spp. have been identified that have up to 50% of their dry mass stored as long-chain hydrocarbons [ 19 ]. With potentially millions of species, algal diversity gives researchers many options for identifying production strains and also provides sources for genetic information that can be used to improve these production strains. The microalgal species being investigated as potential biofuel crops originate from groups whose ancestral relationships are significantly broader than the most diverse land plants, providing a wealth of genetic diversity [ 20 , 21 ]. The groups most often considered when discussing microalgae are diatoms, green algae, golden brown, prymnesiophytes, eustigmatophytes and cyanobacteria [ 16 ], and members from all of these groups have been examined as potential fuel production strains. However, it should be noted that cyanobacteria are not algae but a class of photosynthetic bacteria.
Microalgae have additional advantages over terrestrial plants. Since they are single-celled organisms that duplicate by division, high-throughput technologies can be used to rapidly evolve strains. This can reduce processes that take years in crop plants, down to a few months in algae. Algae have a reduced impact on the environment compared with terrestrial sources of biomass used for biofuels [ 9 ]. They can be grown on land that would not be used for traditional agricultural, and are very efficient at removing nutrients from water. Thus, not only would production of algae biofuels minimize land use compared with biofuels produced from terrestrial plants but, in the process of culturing these microalgae, waste streams can be remediated. Potential waste streams include municipal wastewater to remove nitrates and phosphates before discharge, and flue gas of coal or other combustible-based power plants to capture sulfates and CO 2 [ 22 – 24 ]. Algae production strains also have the potential to be bioengineered, allowing improvement of specific traits [ 25 , 26 ] and production of valuable co-products, which may allow algal biofuels to compete economically with petroleum. These characteristics make algae a platform with a high potential to produce cost-competitive biofuels.
Challenges for algal fuel commercialization
The high growth rates, reasonable growth densities and high oil contents have all been cited as reasons to invest significant capital to turn algae into biofuels. However, for algae to mature as an economically viable platform to offset petroleum and, consequently, mitigate CO 2 release, there are a number of hurdles to overcome ranging from how and where to grow these algae, to improving oil extraction and fuel processing. The algal biofuels production chain is outlined in Figure 3 and shows that the major challenges include strain isolation, nutrient sourcing and utilization, production management, harvesting, coproduct development, fuel extraction, refining and residual biomass utilization.

Improved strains, as well as downstream efficiency, are integral aspects of the algae biofuel production strategy.
Making algal growth & harvesting more efficient
Improved engineering will make a significant impact on algae biofuel production. These improvements include efficient strategies for nutrient circulation and light exposure, and have been reviewed elsewhere [ 27 , 28 ]. In brief, there are significant challenges for engineers to either design photobioreactors (PBRs) that are cheap enough for large-scale deployment, or for engineers and biologists to combine forces to develop species that grow efficiently in low-cost open systems [ 29 ]. PBRs have advantages over open systems in that they can more easily maintain axenic cultures, and can maintain more controlled growth environments, which may lead to increases in productivity; however, contained systems are challenged by efficiencies in gas exchange and a requirement for supplemental cooling [ 28 ]. Despite the advantages of decreased contamination and increased productivity, it is unclear whether PBRs will ever become cost competitive with open pond systems. Regardless of the growth strategy employed, substantial improvements over current technologies for the growth, harvesting and extracting oil from algae need to be made, and coordinated efforts will be needed to couple engineering advances with improved production strains.
Improving oil extraction & downstream processing
Oil extraction is another challenge that is most easily addressed from the engineering side. There are three major strategies for extracting oil from algae: oil press/expeller, hexane extraction, and supercritical CO 2 fluid extraction [ 30 ]. These technologies have all been successfully demonstrated but are relatively expensive, either in terms of equipment needed or energy required to extract the oil. Fortunately, all are amenable to engineering improvements. Once extracted, because crude algae oil is chemically similar to crude fossil fuel oil, the engineering challenges associated with algae oil conversion to usable liquid fuels are similar to those already well managed by petroleum companies, although improved catalysts will be required to improve gasoline production from bio-oil [ 31 ]. Because of these similarities, it seems reasonable to assume that collaborations between algae production companies and major oil companies are likely, since these companies have extensive experience maximizing downstream processing efficiencies.
Regardless of the growth strategy employed and efficiency of oil extraction, the scale of implementation that is required to replace a meaningful amount of fossil fuel is significant. In 2008, the USA alone required 19,497,950 barrels of oil per day [ 32 ]. For algae, or any other biofuel feedstock, to impact this number, significant acreage must be dedicated to production facilities, with estimates suggesting that 30 million acres will be required to meet US oil demand. Different models have been presented for how large-scale aquaculture can be achieved. Although both terrestrial strategies and marine strategies may be required, in this article we focus on the terrestrial aquaculture, since marine strategies are completely unknown at present and may require engineering significantly different from what is practiced today. The terrestrial models use land that is not presently used for food agriculture, and has minimal known environmental or other significant economic utility.
Water is potentially a major limiting factor in algal growth. Expansion of algal growth into nonarable land will require water; fortunately, many of these regions have substantial alkaline or saline water reservoirs beneath them, providing a significant source of nonpotable water that is suitable for growth of many algal species. Perhaps surprisingly, algae grown in open ponds have water requirements per unit area similar to that of cotton or wheat, but less than that of corn, to replenish the water lost in evaporation (for an overview of water requirements of terrestrial plants used in biofuel production see [ 33 ]). It is imperative when considering broad deployment of algae, to consider water use to avoid a future ‘water versus fuel’ debate. Although substantial alkaline reserves are available, water will remain a central issue for algae biofuels production and will need to be considered carefully as the industry expands.
Nutrient challenge
Algae require nutrients, light, water and a carbon source, most often CO 2 , for efficient growth. The major nutrients required by most algae include phosphorous, nitrogen, iron and sulfur. Often, the nutrient requirement necessary for algal growth is ignored, since algae are very efficient at sequestering these nutrients when present in their environment [ 34 , 35 ]. Changes in nutrient load and algal growth have been studies extensively in terms of eutrophication of lakes and coastal regions, but not as heavily in terms of productivity in large-scale aquaculture [ 36 , 37 ]. If terrestrial agriculture is a model for some of the challenges for algal aquaculture, then providing sufficient nutrients for large-scale algal growth is a significant challenge. Micro- and macro-nutrient supplements, or fertilizer, account for significant costs in the current terrestrial agriculture industry [ 38 ], and biofuels are not expected to be an exception. The use of fertilizers has been increasing globally. Unfortunately, many fertilizer components are generated from fossil fuels or mined and, as such, they are not renewable [ 39 – 42 ]. Algae, similar to plants, require sources of phosphorus, nitrogen and potassium, which are the major components of agricultural fertilizers, and large-scale aquaculture will impact these already limited supplies. In addition, optimal growth of many algal species requires chelated iron and sulfur.
Phosphorous makes up slightly less then 1% of total algal biomass and is required at approximately 0.03–0.06% in the medium to sustain algal growth. Fertilizers in the USA used for agriculture currently contain a less than optimal concentration of phosphate owing to limited supplies. Presently, less than 40 million tons of phosphate is mined from the USA annually, and the maximum phosphate production from this mining peaked in the late 1980s. If algal biofuels are to completely replace petroleum in the USA, an additional 53 million tons of phosphate must be acquired annually. This is a significant challenge, given that the total amount of phosphate in the USA is estimated to be approximately 2.8 billion tons. This leaves few options other then efficient recycling the phosphate back into the algae ponds or significantly increasing mining output, a prospect that would seem to provide a temporary solution at best.
Nitrogen, unlike phosphorous, is not limited in supply but is often a limiting macronutrient when it comes to plant and algae growth. Algae require nitrogen to be fixed into ammonia, nitrates and similar molecules, in order to be used as a nutrient source [ 43 ]. Some bacteria, such as rhizobia, have the ability to fix their own nitrogen and some form symbiotic relationships with terrestrial plants, providing the plants with this crucial nutrient to sustain protein and nucleic acid synthesis [ 42 , 44 ]. Some cyanobacteria also have the ability to fix nitrogen, while almost all algal species identified to date require an exogenous source of fixed nitrogen, and most prefer ammonia, as it is less energetically demanding than nitrate or nitrite [ 45 ]. Providing a cheap source of fixed nitrogen will be important for algae biofuel production, and the possibility of using nitrogen-fixing cyanobacteria to supply this nitrogen may help minimize these costs [ 46 ].
In the open oceans, iron is a major limiting nutrient for algal growth, as demonstrated by the induction of algal blooms by the addition of exogenous iron to open oceans [ 47 ]. Interestingly, the addition of iron to induce an algal bloom has been considered and tested as a strategy to sequester CO 2 [ 47 – 49 ]. Biologically, iron is required for electron transport in all known photosynthetic organisms, including Chlamydomonas reinhardtii , and is typically found in iron-sulfur clusters in a variety of photosynthetic proteins [ 50 ]. Iron in its oxidized form is not optimal for uptake, and most algae prefer chelated iron. Fortunately, iron can be easily acquired and is more available than many of the other required nutrients.
Sulfur, in addition to its key role in the electron transport chain, is also required for protein synthesis and lipid metabolism. Sulfur deficiency has been shown to limit algal density and stunt growth [ 51 ]. Thus, it seems likely that sulfur will be important for optimal algal growth, and cost/benefit analysis will need to be considered to determine the optimal amount of sulfur to add to the media for the best economic return.
The acquisition of the aforementioned nutrients, as well as potassium and at least nine other micro- and macro-nutrients, should not be overlooked when considering the implications of scaling algal biofuel production to meaningful levels [ 52 ]. Many of the nutrients may be supplemented by combining nutrient-rich waste water or agricultural runoff with algal growth facilities, streamlining water remediation and optimizing economic fuel production. These strategies appear to be viable at some scale; however, alternative possibilities must also be developed. Ultimately, a combination of methods may be required, and perhaps a recycling of micro- and macro-nutrients will have to be developed for algae-based biofuels to reach a capacity that impacts present fossil fuel use. One of the most promising techniques for recycling nutrients in algal ponds is to use anaerobic digestion [ 53 ]. This bacterial process produces methane gas, while keeping the majority of the nutrients in a bacterial slurry that can be killed and the mix used for algal fertilizer. Methane gas is not currently a high-value commodity , but can help provide energy to operate algae farms, and cheap anaerobic digestion will preclude producing some types of higher value proteins in the algae. Therefore, a balance should be reached between efficient anaerobic digestion and high-value co-products, as shown in Figure 4 .

This is a model of how we expect nutrient utilization to occur as the field matures. Algae will be harvested and the oil will be extracted, the remaining biomass (carbohydrates/proteins) will either be recycled for nutrients through anaerobic digestion or similar means, producing methane gas and a nutrient-rich slurry, which can then be fed back into the algal pond, rather than exogenously produced fertilizers, or used to for high-value co-products, ranging from industrial enzymes, nutraceuticals or animal feed stocks. Some of these nutrients can be recycled through waste water, while others will be lost due to runoff.
Crop protection: minimizing algae death from biotic & abiotic factors
Much like terrestrial monocultures, large algal monocultures will be invaded by pests and pathogens, and therefore, crop protection is a major challenge to algal pond sustainability . Identifying strains resistant to pathogens, along with many other strategies, will need to be employed. These strategies, discussed later, may include engineering specific pest resistance into production species that have robust growth characteristics and significant lipid composition. Other approaches may include using multiple species, which may be sufficient to the slow spread of specific pests and minimize crop loss in large algal facilities.
Microalgal growth facilities can be an excellent habitat for a wide variety of undesirable guests. In most cases, these will be detrimental for algal growth by acting as competitors (other algae with low oil production or bacteria), parasites (virus, fungus or protozoans) or predators (protozoans, fungus or aquatic invertebrates [ 54 – 57 ]. Algae biofuel projects are considering both open and closed systems. These options have significantly different challenges. Closed systems, such as PBRs, have the potential to minimize contamination, but this comes at a high capital expense. Outdoor pond systems have lower initial capital costs, but historically these open pond systems have relied mainly on outcompeting contaminating organisms by using densely grown axenic (or nearly axenic) starter cultures [ 58 , 59 ]. This high-density inoculation allows algae populations to expand rapidly, minimizing end-product loss due to contamination. Unfortunately, this strategy might not be feasible for the extremely large culture volumes required for biofuel production, especially if continuous harvesting strategies are employed. Another solution to minimize contamination is to use microalgae that can grow under extreme conditions, which are not suitable for most of the potential contaminants. This would be the case with Dunaliella salina and Arthrospira , which can withstand up to 35% salinity and pH 10, respectively [ 59 – 61 ]. Unfortunately, not all production strains can survive in extreme conditions, and there is still the possibility for an extremophile contamination to arise [ 62 ].
Microalgae have developed morphological, behavioral and chemical mechanisms for defending themselves from pathogens and predators. Chemical defense is widely present in the ‘algae group’ against bacteria, fungus, protozoans, aquatic invertebrates, other algae and even viruses [ 63 – 65 ]. The majority of antibiotic extracts studied so far have been from marine macro- and micro-algae [ 65 – 67 ]; however, they are also present in many freshwater species [ 67 – 69 ]. Most antibiotics from microalgae have come from cyanobacteria, haptophytes, chrysophytes, diatoms, dinoflagellates and chlorophytes. The chemical nature of these substances is very diverse, including fatty acids, bromophenols, tanins, polysaccharides, alcohols, halogenated compounds, peptides, lipopeptides, alkaloids, amides, tertiary sulfoniums, and many other unique substances [ 65 , 66 ]. Some of these chemicals accumulate within cells so they only act after the algae is damaged or ingested. In other species, toxins are secreted into the media by the algae to avoid negative interactions [ 70 ]. The mode of action of toxins against predators can be further classified into acute toxicity, reduced fitness (growth reduction and/or reduced progeny), feeding inhibition and avoidance. Morphological and behavioral defense mechanisms also complement the chemical repertoire against algae grazers [ 57 , 70 , 71 ].
Given these natural defense mechanisms, it seems wise to take advantage of them, along with other strategies adapted from agriculture, to secure ‘crop’ protection for biofuel production. The simplest solution would be to pick a production strain with extremophile characteristics and a broad repertoire of antibiotic properties. However, this might not occur naturally in a single species, so the next simplest solution would be to coculture a set of microalgae that synergistically contribute to protect the entire crop. Additionally, a single species could be engineered to produce one or more of these algal antibiotics or other natural products [ 63 , 72 , 73 ]. However, these are mostly secondary metabolites that require several enzymes to be synthesized. As an alternative, antimicrobial peptides (AMPs) could be expressed from a single heterologous gene, as has been shown in the nucleus and chloroplast of plants [ 74 , 75 ]. Some of these molecules have been shown to be broad-spectrum antibacterial, antifungal or antiprotozoal agents for which pathogens have a limited capability to develop resistance [ 76 , 77 ]. Moreover, they can also be specifically designed and screened for a specific crop protection function [ 78 ].
Alternative bactericidal proteins (non-AMPs) have been expressed in algae. The Chlorella ellipsoidea nuclear genome was engineered to produce rabbit neutrophil peptide-1, which proved to be effective agent against human pathogens in vitro [ 79 ]. Transformed Chlamydomonas reindhartii chloroplast accumulated 5% total soluble protein (TSP) of the mammary-associated serum amyloid A3 peptide, but its antimicrobial activity was not tested [ 80 , 81 ]. Recently, expression of bovine lactoferricin was achieved in nuclear transformants of Nannochloropsis oculata . Algal extracts from strains expressing this protein were effective against Escherichia coli and Vibrio parahemolyticus [ 82 ].
The aforementioned proteins might be effective against microorganisms but aquatic invertebrates could still feed freely on these algae. A future solution might come from the expression of ‘insecticidal’ proteins, such as those with similar function to the ones from Bacillus thuringensis and Bacillus sphaericus . These proteins have already been expressed in plants and cyanobacteria, and shown to be detrimental towards aquatic insects, aquatic larvae and daphnids [ 73 , 83 – 85 ]. On the other hand, some aquatic invertebrates might be beneficial for algae, and could be used as a biological control strategy. Certain species have strict preference for prey other than algae, such as the heterotrophic protists [ 57 , 70 ]. For example, copepods have been shown to directly contribute to the blooming of the alga Phaeocystis (Haptophyceae) by selectively eating its protozoan predators [ 86 ].
Fundamentally, the challenges described can be overcome in algae, as they have in terrestrial crops, but this may require many years of basic research to understand algal/pathogen interactions that impact crop production. In addition, we will need to balance the cost of solutions relative to increase productivity and, hence. return on investment, and this analysis may prove difficult in the short term, as there are little fundamental data to base this analysis on.
Competition with petroleum: getting the price right
With current estimates of algal-based biofuels ranging from US$300–2600 per barrel based on current technology, technical hurdles need to be overcome to improve this price. Some of these improvements can come from improving growth strategies and engineering, as discussed previously, but improvements can also come from optimizing the use of the entire organism. Although the final price of a barrel of algae oil when production goes to large scale is difficult to extrapolate from the present small production facilities, system improvements will certainly bring costs down. Figure 5 illustrates our estimates of the relative impacts of technological improvements on the economic viability of algae biofuels. Most analysts do not predict full parity with petroleum in the near future. More likely, the initial selling point of algal fuels will be approximately twofold higher than petroleum, but the environmental costs will be substantially lower than our current strategy of depending on fossil fuels.

In our model, our starting strain, with characteristics in brackets, has a break-even price of US$21.33. Significant improvements in all factors (blue) or exceptional improvements in all factors (pink) have break-even prices of US$4.23 or US$1.58 before co-products are considered. Our model consists of seven major factors: 1) annual maintenance costs: this includes personnel, land taxes, fertilizer costs, upkeep, water and power; 2) harvesting costs: this cost is incurred every time algae reaches harvesting stage which is a function of growth rate, and maximum growth density (it includes costs for water extraction, oil extraction and oil transport from harvest site to sales destination); 3) pond depth: this is how deep the ponds can be made while the algae still maintain optimal growth rates. The remaining four are characteristics of the algae; 4) lipid content; 5) growth rate; 6) maximum growth density; 7) marketable co-products produced by the algae. Based on our analysis, improvements in all seven factors are required for algae to come close to competitive with petroleum. Maximizing the last four characteristics require good crop protection, stressing the importance of developments in this field before the large capital investment required to build full scale algae biofuel farms. It is important to note this economic model does not include the initial capital costs to build the initial farms.
From the bench to the pond: strategies to make algae biofuels viable
Taking algae from a potential biofuel producer to large-scale production will be dictated by a combination of factors that include economic viability, and the perceived value of CO 2 mitigation by this technology. In addition, early successes in both business and academic environments will promote funding of the research necessary to optimize and validate this technology. To get a sense of the potential progress required, we generated a simplified economic analysis using a Scenedesmus spp., which produced 0.21 g/l/day of biomass, with a lipid content of 21% [ 18 ]. In our model, petroleum-based oil would have to cost approximately $710 per barrel for this organism to be economically viable at these growth and lipid accumulation rates using existing production technologies. One surprising result from this model is the importance of growth density in comparison to growth rate. Although growth rate is important for overall productivity, our model predicts that higher growth densities improve economic viability more rapidly than a proportional increase in growth rate, since the expense of harvesting and fuel extraction outweighs the capital expense of building a larger facility to get the same overall total production. If we assume that, with thorough characterization of extant species, we can identify a species that has a growth rate of 0.3 g/l/day and 40% oil content, then the resulting price of algae oil would be approximately $310 per barrel. This number could potentially be improved with breeding and selection or molecular genetics to further optimize the production strain, but there is no guarantee that a strain with these characteristics will be identified owing to the complex interaction between growth rates and oil accumulation. Similarly, there is no way to validate that our model is correct, since a number of costs implicit in the model are estimates based on previous work of others [ 9 – 12 ], and extrapolated from traditional agriculture data [ 39 ]. In the following sections, we discuss the major strategies to overcome this price gap.
Bioprospecting: utilizing natural diversity to increase productivity
Algae are an extremely diverse group that contains many thousands of known species, and potentially hundreds of thousands. The great diversity of algal species provides a wide range of starting strains for fuel production. This presents an incredible opportunity, but also a significant challenge. Characterizing species for application in industrial processes requires substantial effort. To move a species into an applied pipeline after initial species identification, significant physiological, biochemical and genetic characterization must occur. This characterization includes establishing optimal growth conditions (i.e., temperature, nutrient levels, salinity and pH), growth characterization (i.e., rate of growth and final culture density), and analysis of metabolite accumulation (i.e., lipid composition and accumulation). In parallel, functional genomics (genomics, proteomics and metabolomics) can provide insight into metabolic pathways present in these species and provide a foundation for future metabolic engineering. The US government-sponsored Aquatic Species Program (ASP) provided initial characterization of a few hundred species, from the 1970s through to the mid-1990s [ 16 ]. This work has continued in a number of laboratories around the world, but remains an area of biology that is largely unexplored. General descriptions of the few algal classes in which one or more species has been characterized now follow.
This diverse group, with more than 100,000 estimated species, is currently one of the most prolific primary marine producers. Furthermore, it is believed that some oil deposits originated from diatom biomass [ 87 ]. Diatoms are distinguished by their ornate bipartite shells, which are composed primarily of polymerized silicates. In silica-limited environments, diatoms have been found to accumulate lipids; however, growth is reduced [ 88 ]. Although this class represents a relatively untapped pool of biodiversity for biofuels, only a limited amount of research has been done to understand how these algae accumulate lipids, while the majority of the work has focused on understanding how diatoms generate their shells. In addition, diatom species have been identified that generate a number of interesting biomolecules that have human health benefits or commercial application, such as omega-3 fatty acids [ 89 , 90 ]. Breeding approaches, as well as using forward genetics to better understand their basic biology, has been hampered by the fact that they are diploid during vegetative growth, unlike many other algal groups. Although other diploid species have become foundational model organisms (e.g. Arabidopsis , Drosophila and mice), the diatom sexual lifecycle is poorly understood, making them recalcitrant to traditional forward genetic exploration. Whole-genome sequences have been released for two species, Thalassiosira pseudonana and Phaeodactylum tricornutum , and recent work has shown stable nuclear transformation of these two species and a few other diatoms [ 91 – 94 ]. The sequenced genomes, in conjunction with nuclear transformation, may allow reverse genetic approaches, such as siRNAs, to dissect the molecular pathways regulating diatom metabolism, allowing us to improve their potential as biofuel-generating organisms and our understanding of their basic biology [ 95 ].
Chlorophyceae & trebouxiophyceae
These green algae are the most heavily studied algal groups, primarily owing to the establishment of the chlorophyceae, C. reinhardtii , as an algal model organism. One reason for this focus on green algae is their shared common ancestry with vascular plants. The extant green algae consist of approximately 8000 species, and member species can be found throughout freshwater and marine habitats, and a few species (e.g., D. salina ) have been shown to survive in high/saturated saline solutions. Green algae have been used extensively in aquaculture, mainly for the production of secondary metabolites, such as β-carotene and astaxanthin. The genomes of a number of green algae have been sequenced, and molecular tools are in place to transform both the chloroplast and nuclear genome of C. reinhardtii [ 96 – 98 ]. Current work is underway to transform plastids of additional species in our laboratory, as well as others, while nuclear transformation has been achieved in a number of other green algae species, such as members of the Chlorella and volvox genuses [ 99 , 100 ]. In terms of oil production, of the published algal species, members of the Scenedesmus genus have been identified as potential oil-producing species, with both rapid growth, as well as relatively high lipid content [ 18 , 101 , 102 ]. In addition, a number of species have been identified that produce interesting metabolites, such as Botryococcus spp. that produce the triterpenoid botryoccenes, a potential fuel molecule that requires minimal refining [ 103 ].
Cyanobacteria
Cyanobacteria were initially examined for biofuel production and found to accumulate relatively low levels of lipids [ 16 ]; however, recently, there has been renewed interest in cyanobacteria as a potential biofuel source because of the ability of some species to grow in extreme environments, and the potential to rapidly engineer these species. Although lipid production in cyanobacteria may never reach that of the eukaryotic algae, the ability to easily manipulate the cyanobacterial genome may allow them to be engineered to produce other biofuel precusor molecules. Cyanobacteria may also be able to be manipulated to secrete biofuel molecules. Another potential advantage of some cyanobacteria is their ability to fix nitrogen. Although growing two or more species as a consortia has not been carefully modeled to date, it is possible that a nitrogen-fixing cyanobacteria, cocultured with a lipid-rich algae, may form a synergistic relationship, allowing the total biomass density to expand above current estimates, while minimizing the cost of adding nitrates to the system. In fact, recently, a diatom species has been characterized, which has endosymbiosed a nitrogen-fixing cyanobacterium, suggesting that such relationships are indeed beneficial to at least one of the two species [ 104 , 105 ].
Through further study of these diverse classes, we may be able to identify new species with improved growth characteristics, improved fuel production or natural co-products, which can improve the viability of algal biofuels. In addition, genomic data from these species can be used as sources of new genes and, combined with screening for natural products, may illuminate new metabolic pathways, which may be useful for engineering other species.
Growth of algal strains
A key characteristic for determining the viability of newly identified algal species as potential biofuel strains is their growth characteristics. Growth rates and maximum growth densities must be characterized in terms of real-world growth conditions rather than laboratory conditions. These conditions can be simulated to some degree in the laboratory by using appropriate media, mixing condition and light regimes; however, production estimates to date, which are primarily based on laboratory conditions in enclosed photobioreactors, will need to be recalculated for industrially scalable systems before they can be considered meaningful for biofuel production, whether they are open ponds or advanced photobioreactors. While useful knowledge can be gained from these types of investigations, in order for true economic viability to be assessed, and for algae to move forward from the bench to the pond, all aspects of algal physiology must be assessed under real-world conditions, rather than the theoretically achievable results under idealized conditions.
Optimizing the growth of algae in open ponds is a key component of reaching economic viability, and remains a major challenge for the industry. Identifying species that grow well under these conditions is a focal point of research in many laboratories. Algae can grow in a wide variety of temperatures, with growth being limited primarily by nutrient availability and light. Light provides the energy for carbon fixation, and is converted to chemical energy through photosynthesis, providing the building blocks for biofuel production. Algae growth rates are often limited by light penetration into the ponds from both self-shading and light absorption by the water, and these constraints are major determining factors of pond depth. The identification of production species that have adapted to harvest shorter wavelengths of light, which have greater water depth penetration, may allow algal pond depth to be increased beyond the current 15–30 cm [ 106 ]. This will decrease the total land required for algal biofuel production.
To date, algal growth in open pond systems have been able to produce 0.06–0.231 g/l/day of biomass [ 16 , 106 , 107 ] and nearly 3 g/l/day in bioreactors [ 108 ]. These numbers may improve through genetics and breeding, as has been accomplished in crop species, as demonstrated by Montsanto and Pioneer's successes in improving the yields of maize or improvements in engineering for photobioreactors. Direct molecular genetic modification may also aid in improving growth characteristics. The failure to change metabolism owing to environmentally adverse conditions may be disadvantageous for survival in a competitive environment; however, impeding these pathways may allow for more consistent yields in partially controlled environments [ 109 ], such as those being considered for algal biofuel production. In addition, a number of engineering strategies have been examined to increase both light and nutrient utilization, which is discussed elsewhere [ 110 ]. At least one algal company has explored eliminating the light component entirely, growing algae nonphotosynthetically, using a reduced carbon source (Solazyme). In these conditions, algal biomass can accumulate more rapidly and reach higher densities, since they avoid issues with shading and have a readily available carbon source. However, since the vast majority of reduced carbon sources are derived from photosynthesis, this strategy simply displaces the need for photosynthesis to another plant, while adding the burden of shipping the reduced carbon source to the site of algal fermentation. In addition, adding a reduced carbon source to algae cultures will increase the presence of unwanted pests, potentially making the scale-up to the volumes required for significant biofuel production problematic.
Breeding & classical genetics to improve & identify traits
Artificial selection of desired traits in agricultural plants has probably been occurring since the dawn of agriculture. The two traditional strategies have been identification of traits of interest from the naturally occurring diversity of the crop species, and selectively combining these traits through interbreeding. A clear success of this strategy is the diversity of Brassica oleracea cultivars from broccoli to cabbage. Directed and successful breeding of some species of microalgae is possible, but little is known about the sexual lifecycle of the vast majority of species. This is partly owing to the enormous diversity in the algae groups, from the diploid diatoms to the haploid chlorophyceae, such that processes occurring in one algae species are not necessarily applicable to other species. However, perhaps more limiting is the general lack of information on the entire lifecycle of many algae, mainly owing to their small size and aqueous lifecycle. There is potential to improve algae for biofuels by developing the tools for selective breeding and using these to move traits that have an impact on biofuels between closely related species, or to improve specific strains of one species. These breeding strategies have an advantage, in that polygenic traits can be moved between strains; however, currently mutagenesis and molecular genetics are at the forefront of algal strain improvement.
'Omics of algal biofuels
In the past 10 years, biological sciences have seen an explosion of strategies that examine entire classes of molecules from a whole organism or cell type, collectively describe as 'omes, including genomes, proteomes, transcriptomes, lipidomes and others. Technologies used to study 'omes strive to analyze entire molecule classes, rather than by piecemeal. The relative merits and weaknesses of these strategies can be debated; however, these 'omic fields will play a vital role when characterizing new species, as researchers expand beyond classical model organisms. These technologies have been especially valuable in unicellular organisms. Broad application in the natural product field is already occurring to identify operons that may encode enzymes to produce new valuable products through comparative genomics. As organism complexity increases, the challenges of 'omic strategies increase. Fortunately, microalgae are amenable to many of the 'omic techniques developed for bacteria and single-celled eukaryotes, such as S. cerevisae. The technologies to apply 'omics to micro-algae are constantly increasing, and we now describe some of the broad applicability of these technologies to improve algal biofuels.
Sorting through the broad range of species being considered for algal biofuels requires a concerted effort to evaluate a number of traits. The primary traits of interest are growth rates, growth density, lipid accumulation, resistance to predators and harvestability. Many of these are probably multigene traits, and species with sequenced genomes will offer significant advantages to those without. We can use comparative genomics to understand key aspects of the basic biology of algae, and whole-genome sequences significantly improve the ease of bioengineering, from the traditional breeding and mapping of genes that control a trait, to knock-out or -down genes that affect pathways of interest. Genomes, when well annotated, not only provide knowledge of metabolic pathways and enzymes that are present in the system, but also help in identification of elements that will be required for regulating gene expression, including promoters and regulatory elements, both of which aid in improved metabolic engineering. Although genome sequencing was once a rate-limiting factor, advances in sequencing technologies have significantly reduced the time and cost of sequencing entire genomes, making high-throughput genome sequencing feasible. To date, a number of algal genomes have been sequenced, or are in process of being sequenced and these numbers are expected to expand dramatically as potential biofuel strains are identified from environmental isolates.
As mentioned previously, the possibility of finding biofuel production strains that combine rapid growth rates, high lipid yields and an ability to grow to relatively high densities while ideally having good crop protection and harvestability characteristics is low. However, upon identifying strains that have one or more of these characteristics, advanced genomic approaches should help elucidate the pathways that allow these traits to persist. By gaining an increased understanding of how extant species maintain these distinct traits, researchers will more rapidly discern whether a new species has potential for development as a biofuel production strain. Although groups are assembling significant genomic data because of the high efficiency and relatively ‘low’ cost of full-genome sequencing, there is a disconnection between sequence generation and production of useful genetic information, including correlation of biological function with a specific gene or cluster of genes. Fortunately, high-throughput sequencing strategies can be adapted to generate transcriptome data, either by aligning cDNA sequence data to an assembled genome or through de novo transcriptome assembly [ 111 , 112 ]. This is a good first step in understanding gene expression in production species; however, an emphasis must be placed on correlating gene expression with phenotype in these species.
Certainly, approaches can be used to rapidly identify genes that correlate with high lipid content, but these are only possible with high-throughput sequencing. In order for these approaches to yield results, basic characterization of species must also occur. For biofuels, the basic traits mentioned should be considered, and independent groups from the academic and the commercial sector will need to continue to define the most desirable characteristics. As these traits are identified, there is potential to use modified quantitative trait loci (QTL) strategies, and apply these across species to identify orthologs that regulate similar traits in other species [ 113 ]. These strategies, and other technologies, may expedite the identification of genes that have the potential to improve biofuel viability and the engineering of production strains.
Bioengineering: improving traits through synthetic biology
Identification of an ideal, unmodified biofuel organism that fits into the established infrastructure for harvesting, extraction and purification, and is economically viable, is a possibility; however, a much more likely scenario is the identification of a variety of species that each have one or a few of these desirable traits. These traits, when engineered into a single strain, may be sufficient to result in an economically viable production strain. In addition to strain improvements in fuel production, using genes identified from other algae species may allow for improved expression of heterologous proteins, which either have high value as a protein coproduct or enzymatically produce a high-value coproduct. Both of these strategies are being investigated to improve the economics of algal biofuels.
Transformation technologies
To date, only a few algal species have been successfully genetically manipulated. These modifications have come in the form of induced random mutagenesis to identify new genes that play important roles in processes of interest. Additional mutagenic strategies include insertional mutagenesis of the nuclear genome, allowing the isolation of tagged mutant genes, although there is some debate on whether this strategy has strong preference for specific genomic regions [ 114 ]. Heterologous gene expression has also been used as a means to modify biological function, and the nuclear genomes of a number of algae have been transformed, with a variety of reporter genes, as well as drug-resistance genes; however, extensive analysis of transgene expression has only been performed in Chlamydomonas . In Chlamydomonas , researchers have encountered problems with transgene silencing [ 115 ]. Strategies to mitigate nuclear silencing have been attempted, including identifying strains that have mutations in the silencing pathways [ 115 , 116 ]. In addition to the nuclear genome, the plastid genome of Chlamydomonas has been successfully transformed and has become a consistent method for heterologous protein expression [ 117 , 118 ]. Although C. reinhardtii may not be a good biofuel production species, the technologies established in this species have potential for application in other algal species. We will discuss some of the successes and failures in algal transformation and their implications in biofuels.
The development of heterologous gene expression tools in algae have followed a path similar that that used in other research organisms. A common strategy to verify that a phenotype of interest is caused by a mutation in a specific gene is to insert a second, wild-type copy of the gene into the host genome to test whether this transgene can complement the mutant phenotype. This strategy is especially valuable in organisms that are haploid during the majority of their lifecycle, such as the chlorophyte alga, because it allows researchers to determine whether loss-of-function or gain-of-function mutations cause the observed phenotype. Early successes in nuclear transformation of C. reinhardtii started in the late 1980s [ 119 , 120 ], with high-efficiency transformation of the nuclear genome first described in 1990 [ 121 , 122 ]. These strategies were quickly adopted for gene identification of loss-of-function mutations by transforming large cosmid, yeast or bacterial artificial chromosomes (YACs or BACs) containing pieces of the C. reinhardtii genome into mutant C. reinhardtii backgrounds [ 123 – 125 ], allowing researchers to clone genes based on transcomplementation. Nuclear gene expression for transcomplementation has been used extensively in C. reinhardtii for gene identification with relatively good success; however, algal nuclear transformation has also been used for heterologous gene expression.
The goal of heterologous gene expression was originally to select transformants that had taken up foreign DNA and separate them from those that did not. Initially, the potential for this approach was determined by complementing a photosynthetically deficient mutant with the endogenous OEE-1 gene [ 122 ], since heterologous marker genes had shown little success. As genome information became more available, it became clear that the C. reinhardtii nuclear genome contained significant GC codon bias, suggesting failure of nuclear heterologous gene expression maybe owing to incorrect codon usage of the inserted gene, although this bias does not preclude heterologous gene expression [ 126 ]. However, improving codon optimization did improve selectable marker expression and, thus, improve their use [ 127 ]. However, despite the improvement in codon optimization, it was found that C. reinhardtii can and often does efficiently silence or downregulate nonrequired heterologous genes when expressed at high levels, perhaps as a defense against viral infection.
Overcoming this nuclear gene silencing in C. reinhardtii has been an ongoing challenge. A number of mutants have been identified that have decreased gene silencing capabilities, often through the disruption of the small RNA pathways [ 115 ]. In addition, strategies that couple heterologous gene expression to expression of genes that provide a significant advantage to the algae have also found significant success. Interestingly, not all algae appear to show this aggressive silencing of heterologous genes. The diatoms Phaeodactylum tricornutum and Thalassiosira pseudonana have both shown stable integration and expression of marker genes, including genes that do not necessarily impart a significant advantage (e.g., GFP ); however, extensive data on the expression of heterologous genes in most algae, including these diatoms, is still being generated. For a list of microalgal species that have been transformed, please see Table 1 . Improving our ability to manipulate the nuclear genome of many of these species in a stable and reproducible fashion will greatly facilitate our ability to produce biofuels and bioproducts.
Transformed microalgae species.
Stable chloroplast transformation was first accomplished in the green algae C. reinhartdii in 1989 when Boynton and co-workers restored the photosynthetic capacity of a chloroplast mutant by cell bombardment with high-velocity microprojectiles coated with the wild-type gene [ 129 ]. In contrast to nuclear transformation, chloroplast transformation occurs through homologous recombination rather than random integration and does not show gene silencing. As shown in Table 1 , more then 20 different algae have now been transformed in either the nucleus or plastid, with all major classes having at least one report of foreign gene expression.
The first attempts at expression of recombinant proteins in the chloroplast of C. reinhardtii involved the bacterial neomycin phosphotransferase [ 148 ] and β-glucuronidase genes [ 149 ], both driven by C. reinhardtii chloroplast promoters. These studies showed stable accumulation of recombinant mRNAs, but no protein accumulation could be detected. Using chloroplast promoters and 5′ untranslated regions (UTRs) to drive the expression of the bacterial aadA gene, encoding aminoglycoside adenine transferase (AAD), stable accumulation of a foreign protein in transgenic chloroplasts was first reported in 1991 as the ability of the transformed cells to resist spectinomycin treatment and by an enzymatic assay of AAD activity [ 150 ]. The first identification of foreign protein expression using western blot analysis was shown in 1999 for the bacterial B(β)-glucuronidase (GUS) reporter protein, driven by either the rbcL , psbA or atpA promoters and 5′ UTRs [ 151 ]. Similarly, expression of Renilla luciferase protein was also shown by western blot analysis in 1999 [ 152 ]. Although protein accumulation in each of these initial studies appeared to be very low, probably less than 0.01% of soluble protein, the recovery of enzymatic activity of the recombinant proteins in all cases indicated that properly folded foreign proteins could be expressed in chloroplasts of C. reinhardtii . More recently, as our understanding of chloroplast gene regulation has improved, reports have shown recombinant proteins accumulating as high as 10–20% of total soluble protein [ 80 , 153 ]. However, there is significantly more to be learned, since different lines that have identical plastid insertions can have quite disparate protein accumulation. This suggests that second site mutations (probably nuclear) may be induced during the chloroplast transformation event.
Although protein expression in algae has been improved over the last 20 years, application of these technologies outside of the laboratory has been limited. The growing interest in biofuels, has renewed interest in improving heterologous protein expression for real-world applications, with a goal for heterologous protein expression of increased lipid production. This was attempted by simply overexpressing the acetyl-CoA carboxylase (ACC) in the diatom Cyclotella cryptica to improve lipid content by increasing the first enzyme in the lipid biogenesis pathway. Unfortunately, this initial experiment did not yield increased lipid production [ 16 ]. As the complexities of lipid regulation have been further elucidated, the possibilities for modifying lipid metabolism have increased [ 154 ]. These modifications may increase lipid content, as well as generate easily processed lipids for fuels. Another major recent focus in biofuels is lipid secretion. Cyanobacteria have been engineered to secrete as much as 133 mg/l/day [ 155 ]. The ability to secrete lipids may improve efficiency in harvesting and nutrient utilization, as well as provide some protection from contamination. Similar strategies have been proposed for diatoms and other eukaryotic algae but have yet to be implemented to our knowledge. Although lipid quantity and secretion have seen limited success, improvements in lipid composition and content in higher plants have been attempted. For the most part, these improvements have been undertaken to modify the carbon chain length and degrees of saturation to produce neutraceuticals, such as omega-3 and omega-6 fatty acids [ 89 , 156 , 157 ]. These successful experiments suggest that improving lipid production in algae is a tenable option to improve biofuel charactieristics.
Improving fuel molecules
Algae can be harnessed to produce a number of molecules that can be used for fuels. The current trend is to use the fatty acids that are naturally produced in algae, since many species produce these lipids at a substantial percentage of their total mass. In-depth discussions of algal lipid production have been reviewed elsewhere [ 158 – 160 ]. In brief, for fatty acid production, acetyl CoA is converted to malonyl CoA through the activity of acetyl CoA carboxylase (ACCase). Fatty acid synthase (FAS) then transfers the malonyl moiety from the malonyl CoA to an acyl carrier protein (ACP), generating malonyl-ACP. Subsequent condensation steps catalyzed by FAS proteins elongate the growing acyl chain, while elongases and desaturases further modify the fatty acids. Most algae species accumulate fatty acids with a range in length of 16–20 carbons, with no to as many as five sites of unsaturation. Although the fatty acid composition of different algae species is distinct, once extracted, its chemical profile is not expected to be substantially different from the chemical mixes currently found in sweet light crude oil. The enzymes involved in oil production may be able to be manipulated, thus increasing the oil production of these species, and the resulting oil can be used after being transesterified as diesel or undergo carbon cracking to produce biogasoline or biojet fuels [ 161 ]. To maximize fuel production and minimize costs, knowing the exact lipid content in terms of carbon chain length is important, so that chemical modification can be optimized. Lipid composition can be determined through lipid extraction, fractionation and subsequent mass spectrometry analysis. Different methods of lipid extraction from hexane extraction to pressing strategies extract lipids with different efficiencies and can yield different lipid profiles and returns. It is important that the extraction strategies be optimized depending on downstream industrial applications.
Although harvesting endogenous lipids, or even improved lipids through bioengineering is the most likely strategy for biofuel production, algae can produce a number of other potential fuel molecules. Much discussion has surrounded the potential of developing a hydrogen economy. A number of algal species can be induced to produce hydrogen gas using the reducing potential of photosynthesis in a reaction catalyzed by a hydrogenase [ 162 ]; however, both the movement towards a hydrogen economy and the ability to engineer algal species to produce substantial amounts of hydrogen in a regulated manner have moved forward at a slower rate than once predicted. Hydrogen production in algae has been reviewed elsewhere [ 162 ]. In addition to hydrogen, algae produce hexose sugars, which can be fermented to produce ethanol, butanol and potentially longer chain hydrocarbons. Although this strategy has not been extensively studied, the high photosynthetic efficiency of algae coupled with high biomass per area and the low cellulose content of many species makes algae a candidate that could be considered for subsequent fermentation.
Although both hydrogen- and sugar-based biofuel production could potentially impact the use of petroleum fuels, algae have the potential to produce a number of secondary metabolites that have characteristics much closer to existing petroleum fuels. The most promising of these are the terpenes, which offer a potential new fuel source outside of fatty acids that is compatible with our existing fuel framework. Terpenes are polymers of isoprene C 5 H 8 . If produced in high quantities, hemiterpenes (single isoprene units) and monoterpenes (C 10 H 16 ) could function in current gasoline engines with only minor engine modifications [ 163 ]. Longer isoprene chains could be used for biodiesel or cracked into biogasoline or jet fuels. To date, terpene accumulation for fuels has not been a large focus of algal biology, since many terpenes and terpene derivatives have properties that make them more valuable for other uses, such as flavor, fragrances and antibiotics [ 164 , 165 ]. However, if algae can be engineered to produce higher levels of certain terpenes, then these molecules present an alternative to fatty acids as an algal biofuel.
Identifying natural traits to improve the economics of production strains
In addition to improved lipid production, other strain-specific improvements are being considered, including crop protection, salt tolerance, growth at high pH, improved nutrient utilization and traits that lead to more efficient harvesting, such as flocculation. The economic impact from these improvements will allow for decrease operational costs. For example, flocculation will allow improved harvesting by making it easier to concentrate algae, decreasing the cost of water extraction. A variety of algae have been characterized for their fouling of marine equipment. Some of the qualities that cause fouling include rapid growth on specific substrates. Further studies of these attributes may lead to improved flocculation and extraction, by understanding how and why these species grow and adhere to these specific substrates. These data may allow these traits to be exploited, so that algae will aggregate after a specific induction event or to a specific surface.
In general, the literature on algal strain improvements is minimal; however, extrapolation from successes in other system may provide a blueprint for some of these improvements. One such improvement is better salt tolerance conferred through expression of glycinebetaine and polyamines in terrestrial plants [ 166 , 167 ]. Despite the potential value of these improvements, their real value must be determined experimentally in each species.
Improved economics through the production of natural co-products
The extraction and sale of natural or engineered co-products along with algae oil could positively impact the economics of algae-based biofuels. If the infrastructure for algae fuel production is in place, expansion of these facilities to include protein or other coproduct purification can be added at a fraction of total cost. The postprocessing residue from algae oil extraction consists primarily of proteins and carbohydrates. Conventional use of these by-products might include anaerobic digestion to generate methane gas [ 163 ], combustion for energy production or, perhaps, use as animal feed, although algae are not presently sold as animal feed outside of the aquaculture industry. The high protein content of most microalgae and their amino acid composition makes them suitable for human and animal nutrition. The cyanobacteria Arthrospira (i.e., Spirulina) has a 60–71% dry-weight protein content, and is widely used as a food supplement for humans, cattle, poultry, aquarium fish, ornamental birds and horses [ 168 ]. Algae biomass is also an essential source of nutrients for fish, mollusk and shrimp in the aquaculture industry. The most popular algae genera are Tetraselmis , Nannochloropsis , Isochrysis , Pavlova , Navicula , Nitzschia , Chaetoceros , Skeletonema , Phaeodactylum and Thalassiosira [ 169 , 170 ]. Chlorella is also regarded as an excellent nutrient source for humans but it also produces a high valuable molecule, β-1,3-glucan. This polysaccharide is a recognized immunostimulator, a free radical scavenger and a reducer of blood lipids [ 171 ].
Given their diverse nature, microalgae can produce a wide variety of nutrients and secondary metabolites that are beneficial for human or animals. Valuable current or potential co-products include carotenoids, and long-chain polyunsaturated fatty acids (LCPUFAs). Microalgae can also produce a wide variety of useful carotenoids, such as lutein, zeaxanthin, lycopene, bixin, β-carotene and astaxanthin. However, commercial production is mainly confined to the latter two [ 172 – 174 ]. β-carotene is produced by the marine algae D. salina , which can accumulate up to 14% of its dry weight as this pigment under stress conditions [ 175 ]. This carotenoid is an orange pigment, widely used as a natural food colorant. It is also a strong antioxidant and a precursor of vitamin A [ 176 , 177 ]. The main producer of astaxanthin is the freshwater algae, Haematococcus pluvialis , which can accumulate up to 4% of its dry weight as this pigment [ 178 ]. Astaxanthin is a red pigment, mainly used as a feed additive for coloring salmon, carp, red seabream, shrimp and chickens. It is also used as a food supplement for humans, given that it is an extraordinary antioxidant [ 179 , 180 ].
Microalgae can also synthesize LCPUFAs, including omega-3 and omega-6. These are essential for humans and marine animals but they are only available in a very limited selection of foods [ 181 , 182 ]. Docosahexaenoic acid (DHA) is an omega-3 fatty acid commercially produced by Crypthecodinium and Schizochytrium for infant formulas and aquaculture feeds. Algae can also efficiently produce other important LCPUFAs but are currently not the main commercial source of these fatty acids. These LCPUFAs include eicosapentanoic acid (produced by Nannochloropsis , Phaeodactylum and Nitzschia ), arachidonic acid (produced by Porphyridium ) and γ-linoleic acid (produced by Arthrospira ) [ 183 ].
Additional minor commercial products from microalgae are phycobiliproteins, used as food and research dyes ( Arthrospira and Porphyridium ) [ 184 , 185 ], extracts for cosmetics ( Nannochloropsis and Dunaliella ) [ 186 ], and stable isotope biomolecules used for research ( Phaeodactylum and Arthrospira ) [ 187 ].
Microalgae can synthesize many other unique molecules with commercial potential, such as toxins, vitamins, antibiotics, sterols, lectins, mycosporine-like amino acids, halogenated compounds and polyketides. In some instances, the expression of molecules that improve crop protection may also have pharmaceutical value. For further reading see [ 183 , 188 – 192 ].
These natural co-products have potential to provide a bridge while the economics of algal biofuels improve. Early on, owing to the large market for fuels and companies establishing niches, it is most likely that diverse coproduct-producing strains will be used, rather than an optimal single strain. In addition, many of these co-products will be coextracted with the lipids using current strategies, decreasing their value as a coproduct. Improving extraction techniques or dedicating a percentage of the algal crop to these higher value products depending on demand (e.g., with terrestrial agriculture) may further close the economic gap between petroleum and algae biofuels ( Table 2 ).
Potential algae co-products.
Byproducts economics
Co-products derived from the protein fraction of algae.
An alternative coproduct strategy is to genetically engineer algae to produce higher value proteins, specifically industrial enzymes and animal feed supplements. These co-products would enhance the value of the protein residual from biofuel production strains. The current market values for recombinant proteins range between US$5 and $10,000 per kg. Although the higher value end of this spectrum consists of small niche markets, there are many industrial enzymes that can be produced, with values ranging between $25 and $50 per kg, and for which large markets currently exist [ 25 ]. With sufficient expression and economic purification of these protein co-products, the price gap between fossil fuels and algae-based biofuels can be minimized.
Future perspective
We have discussed strategies to make algae-based fuels costs competitive with petroleum. Bioprospecting is of importance to identify algal species that have desired traits (e.g. high lipid content, growth rates, growth densities and/or the presence of valuable co-products), while growing on low-cost media. Despite the potential of this strategy, the most likely scenario is that bioprospecting will not identify species that are cost competitive with petroleum, and subsequent genetic engineering and breeding will be required to bring these strains to economic viability. The range of potential for engineering algae is just beginning to be realized, from improving lipid biogenesis and improving crop protection, to producing valuable enzyme or protein co-products. No sustainable technology is without its challenges but blind promotion of those technologies without honest consideration of the long-term implications may lead to the acceptance of strategies whose long-term consequences outweigh their short-term benefits. We have presented what we view as the most important current and upcoming challenges of algae biofuels but, as with any new industry, the more we learn the more we realize that challenges exist that we had not foreseen. Even given these uncertainties, we believe that fuel production from algae can be cost competitive and widely scalable and deployable in the next 7–10 years, but only if we continue to expand our understanding of these amazing organisms as we expand our ability to engineer them for the specific task of developing a new energy industry.
Acknowledgments
Stephen Mayfield is a founder of and has a financial interest in Sapphire Energy an algal biofuel company, but this work should not be considered to reflect the views of Sapphire Energy. This report was supported by a grant from the US Air Force #FA9550-09-1-0336. Special thanks to Michael Burkart and Evan Stephens for their comments.
Financial & competing interests disclosure : The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪ ▪ of considerable
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Save to Library
- Last »
- Biomass Processing Follow Following
- Bioprocessing Follow Following
- Bioproducts Follow Following
- Biochemical Engineering Follow Following
- Bioenergy Follow Following
- Water Treatment Follow Following
- Wastewater Treatment Follow Following
- Water quality Follow Following
- Environmental Engineering Follow Following
- Chemical Engineering Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Publishing
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

IMAGES
VIDEO
COMMENTS
However, scientific research has shown that various biofuels differ massively in the greenhouse gas balance when compared with petrol despite the potential advantages. Based on the techniques used for processing the fuel and production of the feedstock, certain crops may also emit more greenhouse gases than fossil fuels do [52], [53]. 3.2.
This paper reviews and compares different strategies for biofuel production and concludes about their reliability against conventional fuels. It considers the feasibility and effectiveness behind using first, second and third-generation biofuels and draws a comparison between top countries their energy consumption rate and methods for reducing it.
Second- and third-generation biofuels are often referred to as 'advanced biofuels' as their production techniques or pathways are still in the research and development, pilot or demonstration phase. In this paper, the terminology 'first, second and third generation' has been selected and followed throughout.
Biofuels are fuels produced from hydrocarbon-rich living organisms (biomass) — such as plants or microalgae — by thermal, chemical or biochemical conversion processes. As with fuels, biofuels ...
Predominantly, biofuels are produced from photosynthetic organisms. such as photosynthetic bacteria, micro- and macro-algae and vascular land plants. The. primary products of biofuel may be in a ...
Biofuel technology has evolved through several generations of significant advancements. The predominant problem with first-generation biofuels is that they are derived from food crops (e.g., corn and sugar cane), which require fertilization, water, and soil, and thus directly compete with food production. ... This is a novel research area that ...
Biofuels are a source of cleaner energy to promote a cleaner environment. India initiated bio-fuel production nearly a decade ago to reduce its dependence on imported oil and thus improve energy ...
As biofuel research continues at an unprecedented rate, the development of new feedstocks and improvements in bioenergy production processes provide the key to the transformation of biomass into a global energy resource. ... Once your paper has been assessed for suitability by the editor, it will then be double anonymized peer-reviewed by ...
Research on biofuel governance and other studies in the field of sustainability are most often based on a positive perception of power in the sense of power with.Power with is a term that refers to processes of developing shared values, finding common ground, and generating collective strengths [].This conception does not necessarily refer to the diffusion of already existing (predefined) norms.
This paper provides a comprehensive overview of feedstocks utilized to produce biofuels, including the various pre-treatment methods, strategies, and techno-economic analysis in order to pave the way for next-generation biofuels. ... Third-generation biofuels are now in the research and development stage. The financial viability of these cycles ...
Abstract. Bioethanol, a renewable and sustainable b iofuel, has eme rged as a promising. solution to address environmental and energy challenges. This comprehensive. review explores the historical ...
Cover art by BiofuelResJ. ©2024. Biofuel Research Journal (BRJ) is a leading, peer-reviewed academic journal dedicated to publishing high-quality research on biofuels, bioproducts, and related biomass-derived materials and technologies. BRJ is an open-access online journal and completely free-of-charge, aiming to advance knowledge and ...
Research on the production of biofuels and bioenergy from the organic fraction of municipal solid waste and refuse-derived fuel was presented by Verhe et al. (2020). The updated research ... The paper and cardboard wastes were first pulped to a dry matter consistency of 10% w/w and heated to 50°C to increase slurry homogeneity and specific ...
Biofuels are fossil fuel alternatives produced from agricultural biomass or other organic matter; considered sustainable, eco-friendly, and bioeconomic biofuels have come up as a topic of discussion for over a decade. Their practical use depends on the production methods, low cost-technology implementation, and substrate used.
The current research and technology based on the third generation biofuels derived from algal biomass have been considered as the best alternative bioresource that avoids the disadvantages of first and second generation biofuels. ... H. W., and Brune, D. E. (2007). Anaerobic co-digestion of algal sludge and waste paper to produce methane ...
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on BIOFUEL PRODUCTION. Find methods information, sources, references or conduct a literature review on ...
Previous and predicted global petroleum sources (A) Global liquid fuel use in 2006 was predominantly (96.3%) conventional petroleum, with slightly less than 1% being biofuels.(B) In 2030, the International Energy Agency estimates that 29% of liquid fuels will originate from current conventional oil sources, 57% will be from undeveloped or unidentified conventional oil sources and 6% will be ...
Abstract. This critical review summarizes the utilization of algae as the resilient source for biofuel. The paper validates the different stages in generation of biofuels and provides a clarity on III generation biofuels. The microalgae is focused as an incredible source and a detailed discussion has been carried out from the cultivation ...
An Overview of Biofuel. Muhammad Arshad, Muhammad Anjum Zia, Farman Ali Shah. and Mushtaq Ahmad. Abstract Fossil fuels applications are linked with current widely held environ-. mental issues. The ...
This paper aims to provide a bibliometric analysis of publication trends on the themes of biomass and bioenergy worldwide. A wide range of studies have been performed in the field of the usage of biomass for energy production, in order to... more. Download. by Dr. Abdul-Sattar Nizami.
Microalgae or seaweed (Macroalgae) are considered superior compared with terrestrial plants - in terms of solar energy storage, nutrient assimilation and potential for biofuel production - due to ...
Biodiesel production is a promising and important field of research because the relevance it gains from the rising petroleum price and its environmental advantages. This paper reviews the history and recent developments of Biodiesel, including the different types of biodiesel, the characteristics, processing and economics of Biodiesel industry.