CASE REPORT article
Case report: successful treatment of human diabetic foot ulcer using low-intensity diagnostic ultrasound combined with microbubbles: two cases.

- 1 Department of Ultrasound, the General Hospital of Western Theater Command, Chengdu, China
- 2 Department of Endocrinology, the General Hospital of Western Theater Command, Chengdu, China
Background: Diabetic foot ulcer (DFU) is one of the serious complications of diabetes, which has high disability rate and mortality. Low-intensity ultrasound combined with microbubbles in blood circulation can enhance the blood perfusion effect of local soft tissue, which has the potential to promote the healing of diabetic ulcer. Here, we report how this method was used to help the healing of two patients with chronic refractory DFUs.
Case Presentation: In case 1, a 56-year-old man with 3-years history of type 2 diabetes had a 3.0×2.0 cm ulcer which infected with staphylococcus aureus on his right calf for more than half a month. In case 2, a 70-year-old man with 10-years history of type 2 diabetes presented with an 8-month right heel ulcer that developed to 7.5×4.6 cm. And he also had hyperlipidemia, hypertension, and renal impairment. Both patients were enrolled in our study to receive treatment of low-intensity diagnostic ultrasound (LIDUS) combined with microbubbles. They were discharged after a 20-minute daily standard treatment for 7 consecutive days. The ulcers in both cases completely healed in 60 days and 150 days, respectively, and haven’t recurred for more than one year of follow-up.
Conclusion: It is feasible, safe, and effective to use commercial LIDUS combined with commercial microbubbles in the treatment of diabetic lower extremity ulcers. This study may provide an innovative and non-invasive method for the treatment of DFUs.

Introduction
Diabetic foot ulcer, as one of the serious complications of diabetes, has brought heavy economic and public health burden to the society due to its high incidence (15-25%) ( 1 ), high disability rate and high mortality ( 2 ) in diabetic patients.
Microcirculatory dysfunction is an important cause of DFU. On one side, hyperglycemia and hyperinsulinemia promote characteristic extensive endothelial hyperplasia, basement membrane thickening, and even calcification in arterioles, leading to ischemia-hypoxia and poor perfusion in foot soft tissue ( 3 ). On the other, hyperglycemia and oxidative stress lead to endothelial dysfunction, characterized by impaired auto-regulation of micro vessels and a blunted response to vasodilatory stimuli, thereby exacerbating functional perfusion defects in the limbs ( 4 ).
Endovascular shear force is an important means to regulate endothelium-derived vasoactive substances and control micro vasodilation ( 5 ). According to this, a series of related drugs and modified endogenous active substances have been developed to treat tissue ischemia through improving microcirculation perfusion.
Low-intensity pulse ultrasound, is a kind of ultrasonic energy mainly with mechanical effect, but not thermal effect. The shear force, micro jet and shock wave generated by ultrasonic pulse produce a series of physical and biological effects, which are widely used in the therapeutic field. Perfusion effect is one of these effects, which is to enhance local blood perfusion in tissues by setting appropriate acoustic parameters ( 6 ). The microbubbles in the circulation, as cavitation nuclei, could make the ultrasound produce very high shear force and multiply the effect of local blood flow enhancement. Therefore, when low-intensity ultrasound is combined with microbubbles, it has a very good potential for the treatment of tissue ischemic diseases.
In this report, we presented two complete healing cases of refractory DFU treated by commercial LIDUS combined with commercial microbubbles for the first time.
Materials and methods
A GE LOGIQ 9 ultrasound scanner (GE Healthcare, Waukesha, WI) equipped with a 9L Linear array probe (GE Healthcare) was used for both conventional ultrasonography and Contrast-Enhanced Ultrasonography (CEUS). In conventional ultrasonography, thyroid imaging mode was used with a frequency of 9MHz and an imaging depth of 4cm. In CEUS, “Contrast” key was clicked.
An Acuson S2000 ultrasound scanner (SIMENS Healthcare, Erlangen, Germany) equipped with a 9L4 Linear array probe was used for all treatments. Contrast pulse sequencing (CPS) mode were used to monitor microbubble perfusion and an intermittent flash of high MI impulses. The frequency of flash was set at 4 MHz, Imaging depth at 4 cm, with a frame rate of 50 frames per second and an MI of 0.86 (79% acoustic output power).
Same CEUS imaging sections of the ulcerative and surrounding soft tissue before and after treatment were used for perfusion evaluation, chartered with adjacent vessels or bony structures. All parameters of ultrasound were consistent in both patients during diagnosis and treatment.
The microbubbles used for ultrasonic diagnosis and treatment were SonoVue (Bracco Imaging Scandinavia AB, Oslo, Norway), a commercial ultrasound contrast agent. The microbubble suspension with a concentration of 11.8 mg/mL were prepared according to the manufacturer’s instructions, with 59 mg sulfur hexafluoride lyophilized powder mixed with 5 mL of normal saline. A 140 ×110 ×7 mm acoustic coupling pad (Foshan SiEn Technology Co., LTD., Guangzhou, China) was used during imaging and treatment procedures for better coupling.
After routine clinical debridement of the wound, the acoustic coupling pad was placed on the ulcerated skin area. CEUS was first performed on local tissue, and 2.4 ml microbubble suspension was injected rapidly through the cubital vein, followed by flushing with 5 ml normal saline. CPS angiography combined with microbubble Flash mode was used for treatment: “Microbubble Flash → Microbubble Contrast → Microbubble Flash → Microbubble Contrast”. In the first 5 minutes, the remaining circulating microbubbles from previous CEUS were used to mediate ultrasound therapy. Then another 5 ml of the prepared microbubble suspension was taken and injected slowly and continuously through the vein for 10 min. Finally, the remaining circulating microbubbles were used again to mediate ultrasound treatment for 5 min, and the total time of ultrasound treatment was 20 min. The ultrasound treatment cycle was 7 days, once a day, and the treatment process was the same for each time. Follow-up observation was conducted for 6 months.
Case presentation
A 56-year-old man with 3-years history of type 2 diabetes fell to the ground while cycling two weeks ago, resulting in a skin ulceration on his right calf. He received basic debridement and daily dressing change at local hospital but the ulcer did not heal. Subsequently, the patient was admitted to the department of Endocrinology in our hospital. The patient was not taking any medication at admission.
Physical examination showed an ulcerated surface on the patient’s medial right calf, about 3.0 ×2.0 cm in size, with a depth of about 6 mm ( Figure 1A ). The granulation tissue was relatively fresh, with a little dark red bloody exudate on the surface, and the surrounding soft tissues were red, swollen and slightly tender. Laboratory examination showed increased fasting blood glucose (16.2 mmol/L), increased HbA1c (7.10%), normal liver and renal function indexes. Secretion culture from the ulcer indicated an infection of Staphylococcus epidermidis ( Table 1 ). Magnetic resonance imaging (MRI) examination showed that the ulcer did not involve bone tissue ( Figure 1B ). Color Doppler Flow Imaging (CDFI) showed no significant abnormalities in peripheral arteries ( Figure 1C ). Ankle-brachial index (ABI) was normal (ABI=1.20), Current perception threshold (CPT) was 0.00, and there was no abnormal sensation ( Table 1 ). Based on these evidence, the patient preliminary diagnosed as diabetic foot ulcer (Grade 3 of Wagner classification).
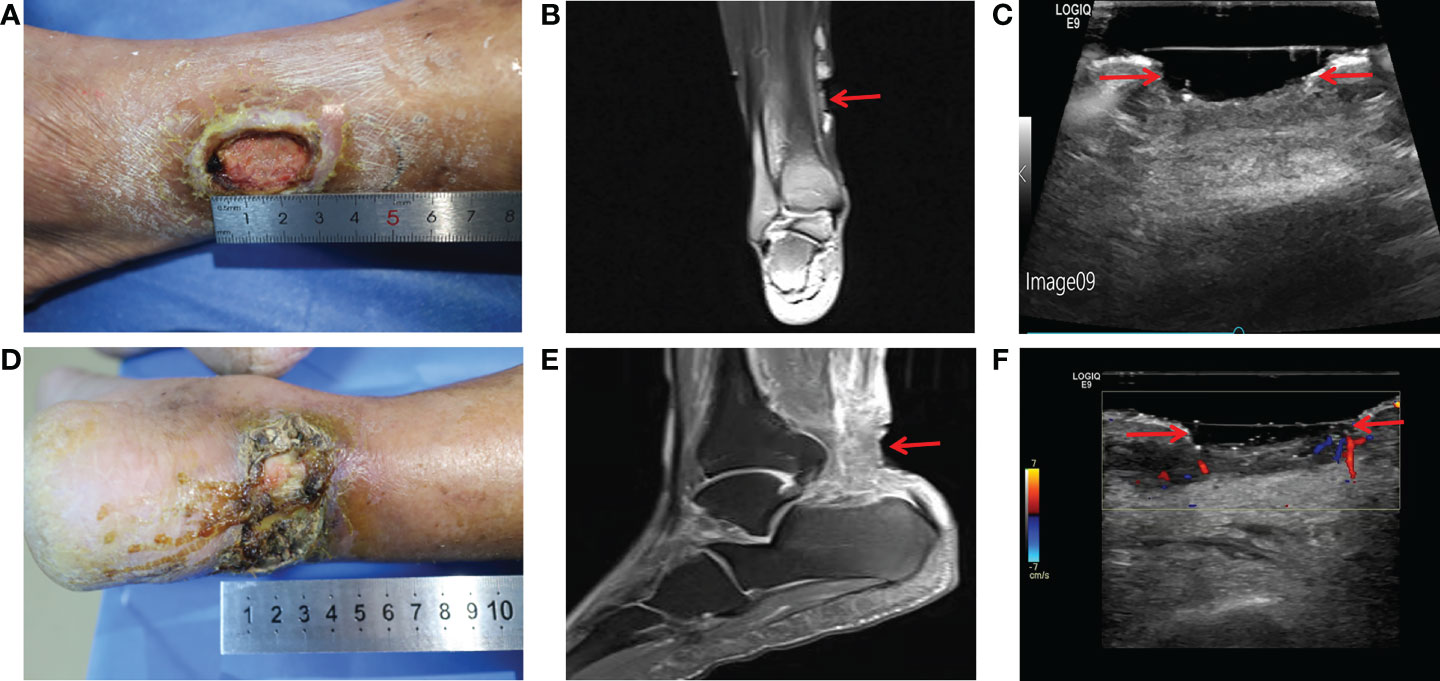
Figure 1 Imaging diagnosis of diabetic lower extremity ulcers before treatment for case 1 (A-C) and case 2 (D-F) . (A) Picture showed an ulcerated surface on the inner skin of the right calf, about 3.0×2.0 cm in size and 6 mm in depth; (B) MRI showed a local subcutaneous soft tissue defect at the medial margin of the right calf, with swelling in the margin and adjacent soft tissue space; (C) Gray-scale sonography showed a heterogeneous low echo area in the subcutaneous soft tissue of the medial side of the right calf; (D) Picture showed an ulcerated surface in the skin of the right heel, about 7.5 × 4.6 cm in size and 4 mm in depth, with necrosis and exudation; (E) MRI showed extensive swelling of the soft tissue and fascia in the lower part of the right calf with unclear and disordered layers; (F) CDFI showed local skin defects and discontinuity in the skin of the right heel. The blood flow signal in the low echo surface was not obvious, and a little blood flow signal could be seen in the periphery. The red arrows indicate the ulcer defects.
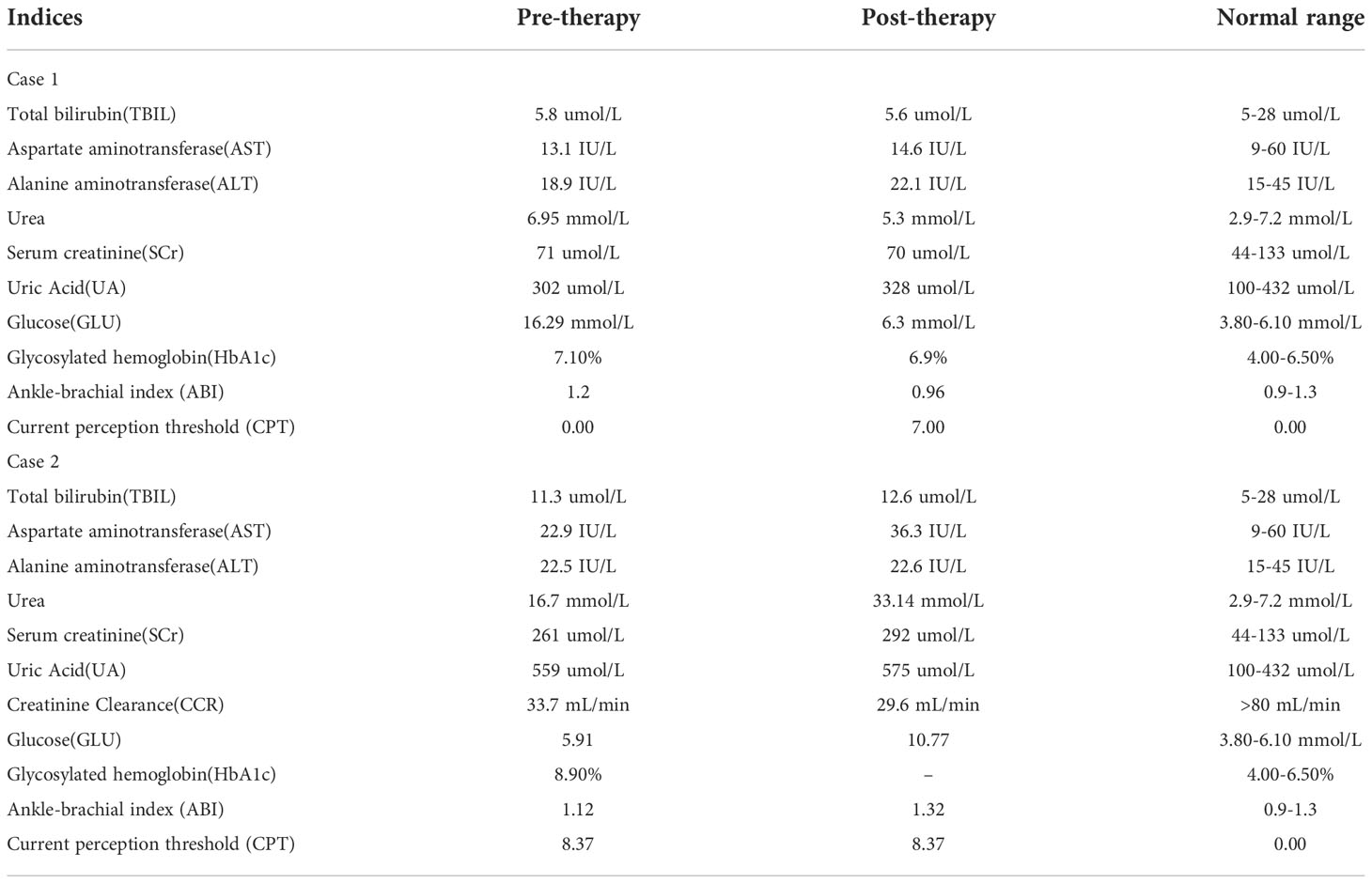
Table 1 Details of blood routine, blood glucose level and other testing.
After admission, the patient received antibiotic therapy for 8 days (intravenous cefotiam hydrochloride, 1g/8h, once a day). The ulcer wound dressing were changed once a day. For blood glucose control, the patient also received subcutaneous injection of recombinant human insulin (4 IU, three times a day), before each meal, subcutaneous injection of protamine human insulin (10 IU, once a day), at 22:00 every day, oral metformin hydrochloride sustained-release tablet (0.5 g, twice a day), oral sitagliptin phosphate tablet (100 mg, once a day). LIDUS combined with microbubbles therapy was performed once a day from the 5th day of admission ( Figure 2A ). On the 8th day of anti-infection, there was no purulent secretion in the wound, and the redness and swelling of the surrounding block were alleviated. On the 12th day of blood glucose control, the blood glucose level reduced to 6.3 mmol/L ( Table 1 ). On the 12th day after enrolling in the LIDUS therapy, the ulcer area also decreased from 3.0 ×2.0 cm to 2.8 × 1.4 cm and its depth decreased from 6 mm to 4 mm, filled with granulation tissue, no purulent exudation was present. The patient was then discharged and continued to receive standardized blood sugar control treatment. Follow-up found the ulcer skin recovered 60 days after enrolling in LIDUS therapy ( Figures 2B–F ). Liver and kidney function were reexamined after ultrasound combined with microbubble treatment, and no abnormalities were found. Ankle brachial index was 0.96, slightly decreased. The CPT grade was 7.0, indicating mild hypoesthesia ( Table 1 ). After discharge on the 12th day, the following medications were given to control glycemic for the next 15 days, subcutaneous injections of recombinant insulin lyprol (10 IU-8 IU, twice a day), before breakfast and dinner, oral metformin hydrochloride sustained-release tablet (0.5 g, twice a day), oral sitagliptin phosphate tablet(100 mg, once a day). Subsequently, the patient stopped glucose-controlling drugs by himself, and wound dressing was changed daily. To be clear, the timeline of entire treatment process of this case was presented in Figure 3 .
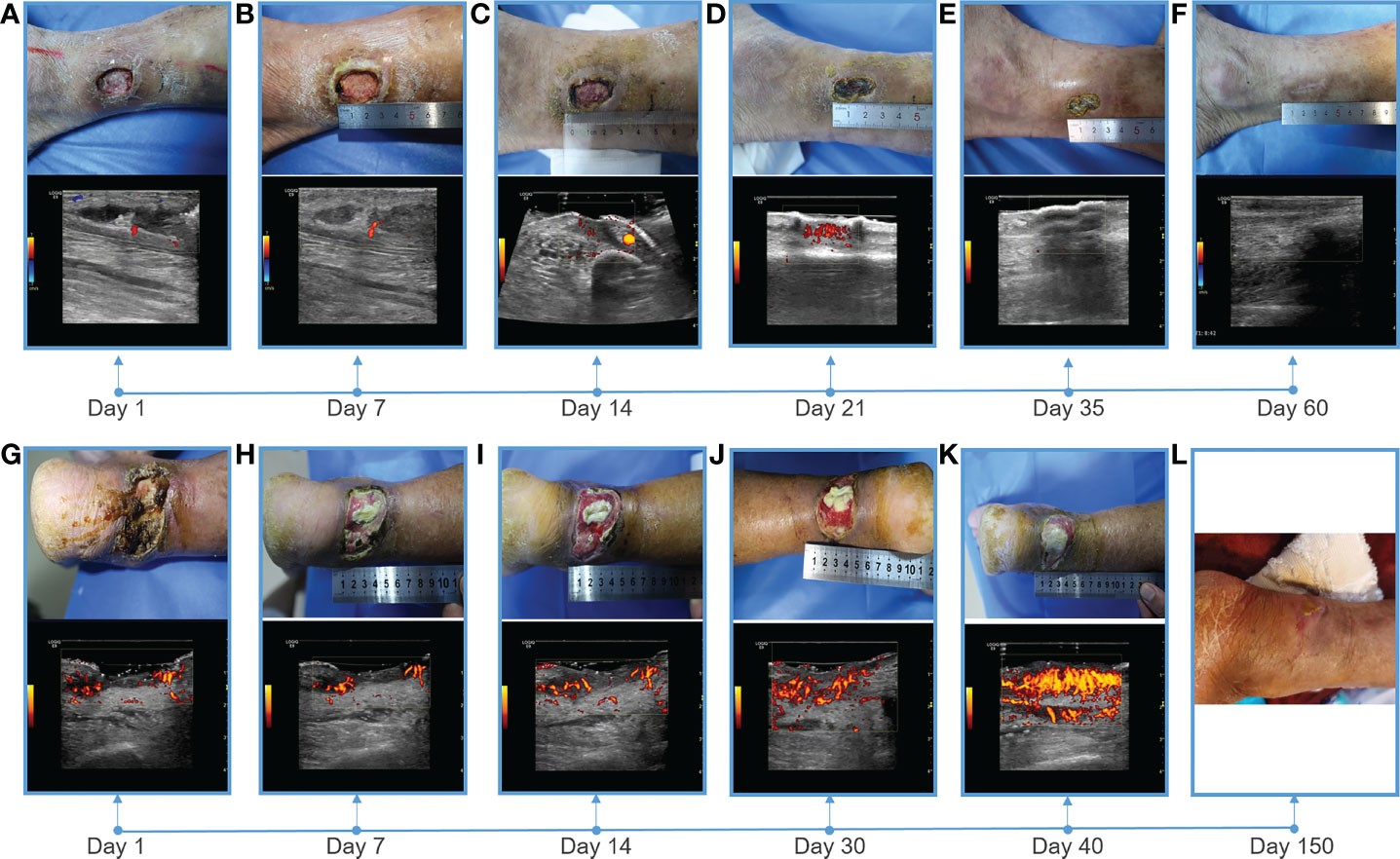
Figure 2 Showing of skin wounds and CDFI or PDI ((Power Doppler Imaging)) ultrasound imaging for progress in the treatment of diabetic lower extremity ulcers in two cases. Wound conditions at the (A) 1th, (B) 7th, (C) 14th, (D) 21th, (E) 35th, and (F) 60th day of post therapy showed the gradual healing of ulceration for case 1; Wound conditions at the (G) 1th, (H) 7th, (I) 14th, (J) 30th, (K) 40th, and (L) 150th day of post therapy showed the gradual healing of ulceration for case 2. PDI showed that the blood flow of the soft tissue around the ulcer gradually increased, and the blood flow was very abundant before healing.
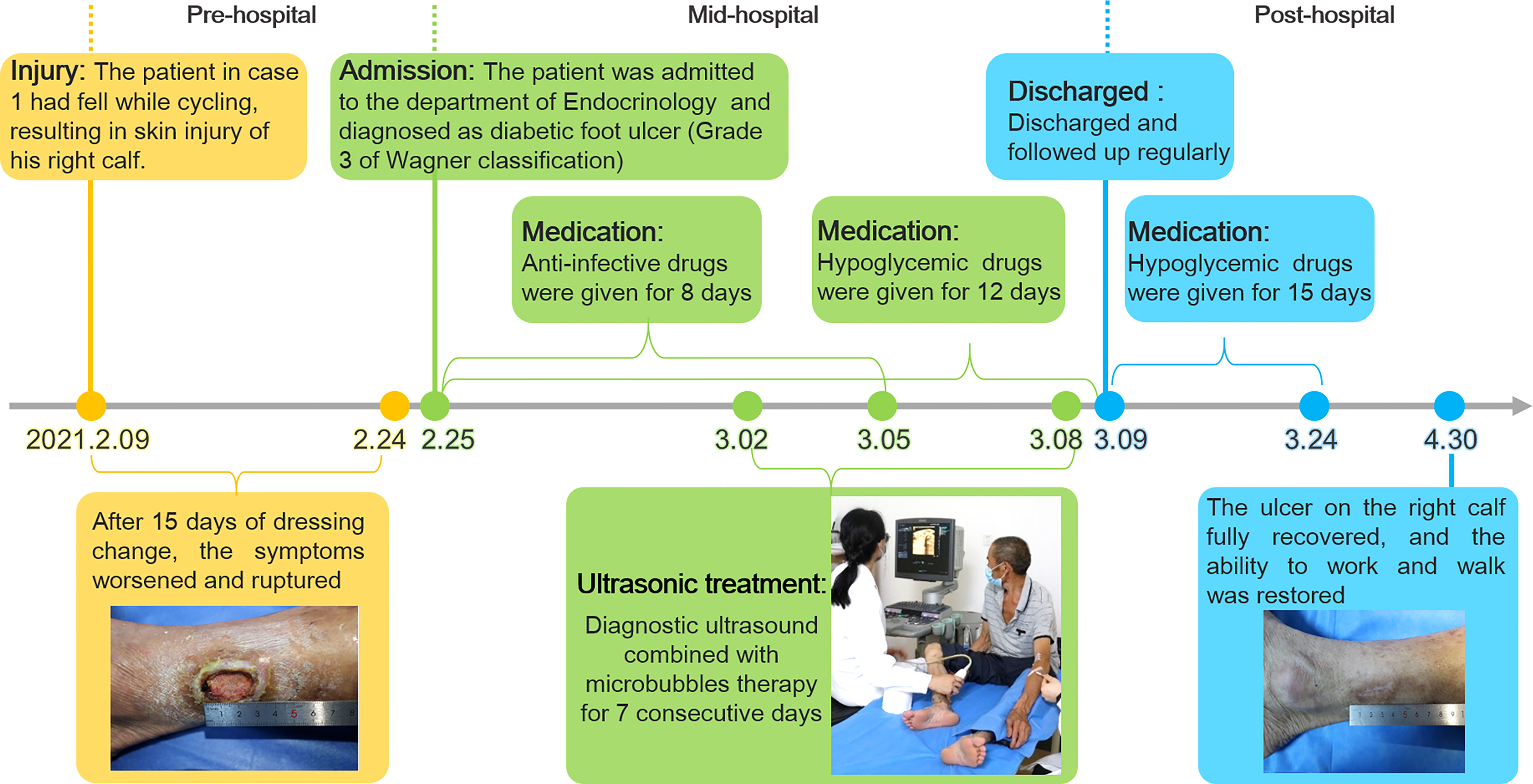
Figure 3 The timeline of the treatment process of case 1 from the day of injury to the day of healing.
A 70-year-old male patient diagnosed with type 2 diabetes for more than 10 years had poor glycemic control due to irregular medication. The patient found an ulcer on his right heel without any known injuries eight months ago. After removing the black scab on the surface of the ulcer by himself, the ulcer was getting worse and the patient was subsequently admitted to the Endocrinology Department of our hospital. Since the onset of the ulcer, the patient had complained about a progressively deterioration in mental, physical, appetite and sleep. The patient also had a history of alcohol abuse for more than 30 years (about 500mL strong wine a day).
Physical examination revealed a skin defect about 7.5 × 4.6 cm in size and 4 mm in depth, with black crusts and yellowish exudate, surrounding skin redness and swelling, and pain when walking and pressing the wound ( Figure 1D ). Laboratory examination showed normal fasting blood glucose (PP 5.91 mmol/L), increased HbA1c (8.90%), normal liver function, and Renal insufficiency (blood urea (16.76 mmol/L, serum creatinine 261 umol/L, and serum uric acid 559 umol/L) ( Table 1 ).Blood pressure test showed hypertension (160/110 mmHg). MRI examination showed that the ulcer did not involve bone tissue ( Figure 1E ). CDFI showed mild atherosclerosis of lower extremity arteries ( Figure 1F ). Ankle-brachial index was in the normal range, and the CPT grade was 8.37, suggesting moderate hypoesthesia. Based on these evidence, the patient preliminary diagnosed as type 2 diabetes, diabetic foot, hyperlipidemia, hypertension and renal impairment in the outpatient department of endocrinology of our hospital.
Heel ulcer debridement was performed on the patient first ( Figure 2G ). After debridement, the ulcer surface showed no obvious granulation tissue and light red color, and the Achilles tendon was partially necrotic and pale color with partial yellowness. After 7 consecutive days of ultrasound combined with microbubble therapy, the ulcer area did not change significantly ( Figure 2H ). On the 7th day, the granulation tissue grew obviously, and the tendon tissue grew slightly ( Figure 2H ). On the 14th day, the wound contracted slightly and granulation tissue grew with bright red color ( Figure 2I ). On the 30th day, the granulation tissue tended to fill the wound, and a little epidermal tissue grew around it ( Figure 2J ). On the 40th day, the wound contracted and became slightly smaller, granulation tissue protruded from the skin surface, and the tendon tissue was completely repaired with normal color ( Figure 2K ). During the LIDUS therapy, the patient’s local pain and itching gradually increased. Subsequently, the patient was to be treated by skin grafting in the burn department. However, due to the long-term high blood glucose (about 10 mmol/L), the surgeon suggested controlling blood glucose before operating. With no other treatment, after 150 days, the wound was covered with epidermis and the ulcer was basically healed ( Figure 2L ). There was no significant change in liver and kidney function before and after ultrasound treatment, ankle-brachial index increased (ABI=1.32), indicating arterial stiffness, and the CPT grade was 8.37, indicating moderate hypoesthesia ( Table 1 ). The patient was treated only in the outpatient department, and glycemic control was simply by oral metformin hydrochloride sustained-release tablets (0.5g, twice a day), which was not effective.
The therapeutic effect in 2 patients was positive and encouraging. The size and depth of the ulcer determined the time to cure, and the ulcer completely healed in 60 to 150 days, and the patients were able to live independently. The safety of the treatment process was also verified. There was no significant difference in liver and kidney function in two patients before and after LIDUS therapy. In addition, there was no ecchymosis on the local body surface, and no thrombosis and other adverse events occurred in local veins and arteries. What is noteworthy is patient 2, whose wound was large and blood sugar fluctuated for a long time and remained high. After careful surgical evaluation, skin grafting was finally abandoned to close the wound. Unexpectedly, after 150 days of slow growth, the skin healed on its own. Both patients were followed up for more than one year and had no recurrence of ulcers. At a recent follow-up visit, Patient 1 said that compared with other methods he had known, receiving our treatment was like undergoing ultrasound examination, which was painless, non-invasive, easy to adhere to, and had definite efficacy, and he was happy to share this treatment with other patients.
To our knowledge, this was the first human trail that used LIDUS combined with microbubbles to treat diabetic foot ulcer. Almost all previous studies on ultrasonic treatment of diabetic foot ulcer were in vitro or preclinical ( 7 – 9 ). Until now, the study of low-intensity ultrasonic cavitation in the treatment of human diabetic foot ulcer has not been reported. The only known clinical application of LIDUS plus microbubbles is in the field of tumor therapy. Kotopoulis ( 10 ) and Liuzheng ( 11 ) have respectively used this method to enhance microcirculation blood supply to pancreatic and breast cancer tumors, and improve the efficacy of chemotherapy.
Blood flow enhancement by LIDUS is the premise of this clinical experiment, which was found in our previous animal studies. When MI was set to 0.3, 5 minutes after ultrasound combined with microbubbles irradiation for VX2 tumor, the tumor blood supply was significantly increased by contrast enhanced ultrasound by a direct visualization method and TIC curve quantitative analysis ( 12 ). Similarly, in this study, after the soft tissue around DFU was treated for 20 minutes, its blood perfusion was observed increased by direct visualization, and reached a higher peak intensity (-46 to -42dB) by quantitative analysis in a shorter time and decreased more slowly after treatment. The results showed that vascular resistance of muscle tissue decreased and blood perfusion increased after treatment. In vivo studies have reported that low-intensity ultrasound combined with microbubbles irradiating muscle tissue for more than 10 minutes can reverse ischemia up to 24 hours ( 6 ).
As a chronic refractory wound, diabetic ulcer healing also involves cell proliferation, angiogenesis and other processes. Several studies have explored the molecular mechanism of low-intensity ultrasound combined with microbubble therapy, namely, the enhanced effect of local blood flow is related to the increased synthesis of local vasodilators Nitric oxide (NO) and prostaglandin ( 13 ), while the production of ATPase makes cell proliferation and metabolism more active ( 6 ). At the same time, this method can increase vascular endothelial growth factor (VEGF) and other growth factors and promote angiogenesis ( 14 ). Hypoxia-inducible factor-α (HIF-α) and the activation of immune pathway are also involved in this process ( 15 , 16 ). In this case, the wounds of the 2 patients were gradually and slowly healed after short treatment. The timing and molecular mechanism of initiating active wound repair and continuing to heal need to be further studied.
Diagnostic low-intensity ultrasound was selected in this study primarily for the safety of human trials. Due to the strict FDA restrictions ( 17 ), the acoustic intensity of diagnostic ultrasound is constrained within an admissible range (0.05-0.5 W/cm 2 ) in the form of low energy. As the cavitation nuclei, the microbubbles can reduce the cavitation threshold and enhance the cavitation effect ( 18 , 19 ). Moreover, the visualization advantage of diagnostic low-intensity ultrasound enables it to observe the whole process of microbubble perfusion and rupture. In the experiment, it was observed that the muscular tissue perfusion microbubbles disappeared after a flash operation. From this, it can be speculated that the existing parameter Settings caused Sonoporation in the treatment, and the physical effects such as shear waves and micro jets may promote the occurrence of healing events. High parameters of MI (0.89 and 0.86), sound power (79%) and Frame (50) were set to improve the duty cycle of treatment pulses, and Flash mode was selected to promote the occurrence of cavitation through breaking microbubbles. The possibility of cavitation induced by commercial equipment was also confirmed by Lindar (6) and Kitoplis ( 10 ).
The importance of this study may be reflected in the following aspects: firstly, it was a real world clinical study in humans, and secondly, the therapeutic equipments and microbubbles used are commercial products, which could make this technology promising for clinical promotion and could provide a new non-invasive method and idea for the treatment of diabetic ulcers. The limitations of this study mainly focus on the small sample size, the interpretation of the results needs to be cautious, and more samples need to be accumulated to further verify the treatment effect.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of The General Hospital of Western Theater Command of PLA. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JT, RW and ZC: conception and design of the work. XZ and LP: data collection. XZ, YC and RW: Image analysis and interpretation, manuscript writing, and critical revision of the article. ZC: approval of the final version of the article.
This work received grants from Key Research and Development Program of Science and Technology Department of Sichuan Province (2020YFS0122), and Project of Hospital management of General Hospital of Western Theater Command of PLA (2019ZY11).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med (2017) 376:2367–75. doi: 10.1056/NEJMra1615439
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Costa RHR, Cardoso NA, Procopio RJ, Navarro TP, Dardik A, de Loiola Cisneros L. Diabetic foot ulcer carries high amputation and mortality rates, particularly in the presence of advanced age, peripheral artery disease and anemia. Diabetes Metab Synd (2017) 11 Suppl 2:S583–S7. doi: 10.1016/j.dsx.2017.04.008
CrossRef Full Text | Google Scholar
3. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol (2020) 18:117–24. doi: 10.2174/1570161117666190502103733
4. Chapouly C, Yao Q, Vandierdonck S, Larrieu-Lahargue F, Mariani JN, Gadeau A-P, et al. Impaired hedgehog signalling-induced endothelial dysfunction is sufficient to induce neuropathy: implication in diabetes. Cardiovasc Res (2016) 109:217–27. doi: 10.1093/cvr/cvv263
5. Hogan B, Shen Z, Zhang H, Misbah C, Barakat AI. Shear stress in the microvasculature: influence of red blood cell morphology and endothelial wall undulation. Biomech Model Mechan (2019) 18:1095–109. doi: 10.1007/s10237-019-01130-8
6. Belcik JT, Davidson BP, Xie A, Wu MD, Yadava M, Qi Y, et al. Augmentation of muscle blood flow by ultrasound cavitation is mediated by ATP and purinergic signaling. Circulation (2017) 135:1240–52. doi: 10.1161/CIRCULATIONAHA.116.024826
7. Chen L, Zheng Q, Chen X, Wang J, Wang L. Low-frequency ultrasound enhances vascular endothelial growth factor expression, thereby promoting the wound healing in diabetic rats. Exp Ther Med (2019) 18:4040–8. doi: 10.3892/etm.2019.8051
8. Vander Horst MA, Raeman CH, Dalecki D, Hocking DC. Time- and dose-dependent effects of pulsed ultrasound on dermal repair in diabetic mice. Ultrasound Med Biol (2021) 47:1054–66. doi: 10.1016/j.ultrasmedbio.2020.12.024
9. Wakabayashi N, Sakai A, Takada H, Hoshi T, Sano H, Ichinose S, et al. Noncontact phased-array ultrasound facilitates acute wound healing in mice. Plast Reconstr Surg (2020) 145:348E–59E. doi: 10.1097/PRS.0000000000006481
10. Kotopoulis S, Dimcevski G, Gilja OH, Hoem D, Postema M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: A clinical case study. Med Phys (2013) 40(7):072902. doi: 10.1118/1.4808149
11. Chen X, Qiao X, Cuo YI, Liu Y, Zhu Q, Rong Y, et al. Microbubble mediated diagnostic ultrasound enhance blood perfusion of breast cancer. J Clin Ultrasound Med (2018) 020:82–5. doi: 10.16245/j.cnki.issn1008-6978.2018.02.004
12. Qiao X, Chen Z, Yi C, Gao W, Gao S, Liu Z. Vascular effect of rabbit VX2 tumor induced by diagnostic ultrasound with microbubbles. J Clin Ultrasound Med (2017) 019:217–21. doi: 10.16245/j.cnki.issn1008-6978.2017.04.001
13. Suchkova VN, Baggs RB, Sahni SK, Francis CW. Ultrasound improves tissue perfusion in ischemic tissue through a nitric oxide dependent mechanism. Thromb Haemostasis (2002) 88:865–70. doi: 10.1055/s-0037-1613315
14. Deng L-D, Qi L, Suo Q, Wu S-J, Mamtilahun M, Shi R-B, et al. Transcranial focused ultrasound stimulation reduces vasogenic edema after middle cerebral artery occlusion in mice. Neural Regener Res (2022) 17:2058–63. doi: 10.4103/1673-5374.335158
15. Maan ZN, Januszyk M, Rennert RC, Duscher D, Rodrigues M, Fujiwara T, et al. Noncontact, low-frequency ultrasound therapy enhances neovascularization and wound healing in diabetic mice. Plast Reconstr Surg (2014) 134:402E–11E. doi: 10.1097/PRS.0000000000000467
16. Zhang Z-C, Yang Y-L, Li B, Hu X-C, Xu S, Wang F, et al. Low-intensity pulsed ultrasound promotes spinal fusion by regulating macrophage polarization. BioMed Pharmacother (2019) 120:109499. doi: 10.1016/j.biopha.2019.109499
17. U.S. Department of Health and Human Services, Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. Food and Drug Administration (2008).
Google Scholar
18. Khanna A, Nelmes RT, Gougoulias N, Maffulli N, Gray J. The effects of LIPUS on soft-tissue healing: a review of literature. Bbrit Med Bull (2009) 89:169–82. doi: 10.1093/bmb/ldn040
19. Guo X, Li Q, Zhang Z, Zhang D, Tu J. Investigation on the inertial cavitation threshold and shell properties of commercialized ultrasound contrast agent microbubbles. J Acoust Soc Am (2013) 134:1622–31. doi: 10.1121/1.4812887
Keywords: diabetic foot ulcer, diagnostic ultrasound, microbubble, ultrasound treatment, case report
Citation: Zhang X, Cheng Y, Pei L, Tao J, Wang R and Chen Z (2022) Case report: Successful treatment of human diabetic foot ulcer using low-intensity diagnostic ultrasound combined with microbubbles: Two cases. Front. Endocrinol. 13:1046896. doi: 10.3389/fendo.2022.1046896
Received: 17 September 2022; Accepted: 09 November 2022; Published: 25 November 2022.
Reviewed by:
Copyright © 2022 Zhang, Cheng, Pei, Tao, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Chen, [email protected] ; Rui Wang, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Diabetic Foot Ulcers: Case Presentations
- First Online: 20 June 2020
Cite this chapter

- Animesh Hazari 3 &
- G. Arun Maiya 4
420 Accesses
In the previous chapter, we have learnt about various foot complications in diabetes mellitus which is collectively known as the diabetic foot syndrome. Diabetic foot ulcers (DFU) are the most devastating foot complications that affect an individual in all aspects of life. In this chapter, we shall focus on few case histories of patients with diabetic foot ulceration along with their clinical presentation. This would be very useful for readers to understand the clinical presentation on DFU, its associated problems, overall ill effects of diabetes mellitus for DFS which could also affect other domains of life significantly. We shall only present the case history of few patients here and deal with possible non-surgical management techniques in the upcoming chapters. The patients were recruited for a research study at Centre for Diabetic Foot Care, Kasturba Hospital, Manipal, Karnataka, India.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Author information
Authors and affiliations.
LFAMS, Lovely Professional University, Jalandhar, Punjab, India
Animesh Hazari
Centre for Diabetic Foot Care and Research, MAHE, Manipal, Karnataka, India
G. Arun Maiya
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Animesh Hazari .
Rights and permissions
Reprints and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Hazari, A., Maiya, G.A. (2020). Diabetic Foot Ulcers: Case Presentations. In: Clinical Biomechanics and its Implications on Diabetic Foot. Springer, Singapore. https://doi.org/10.1007/978-981-15-3681-6_7
Download citation
DOI : https://doi.org/10.1007/978-981-15-3681-6_7
Published : 20 June 2020
Publisher Name : Springer, Singapore
Print ISBN : 978-981-15-3680-9
Online ISBN : 978-981-15-3681-6
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
Diabetic foot
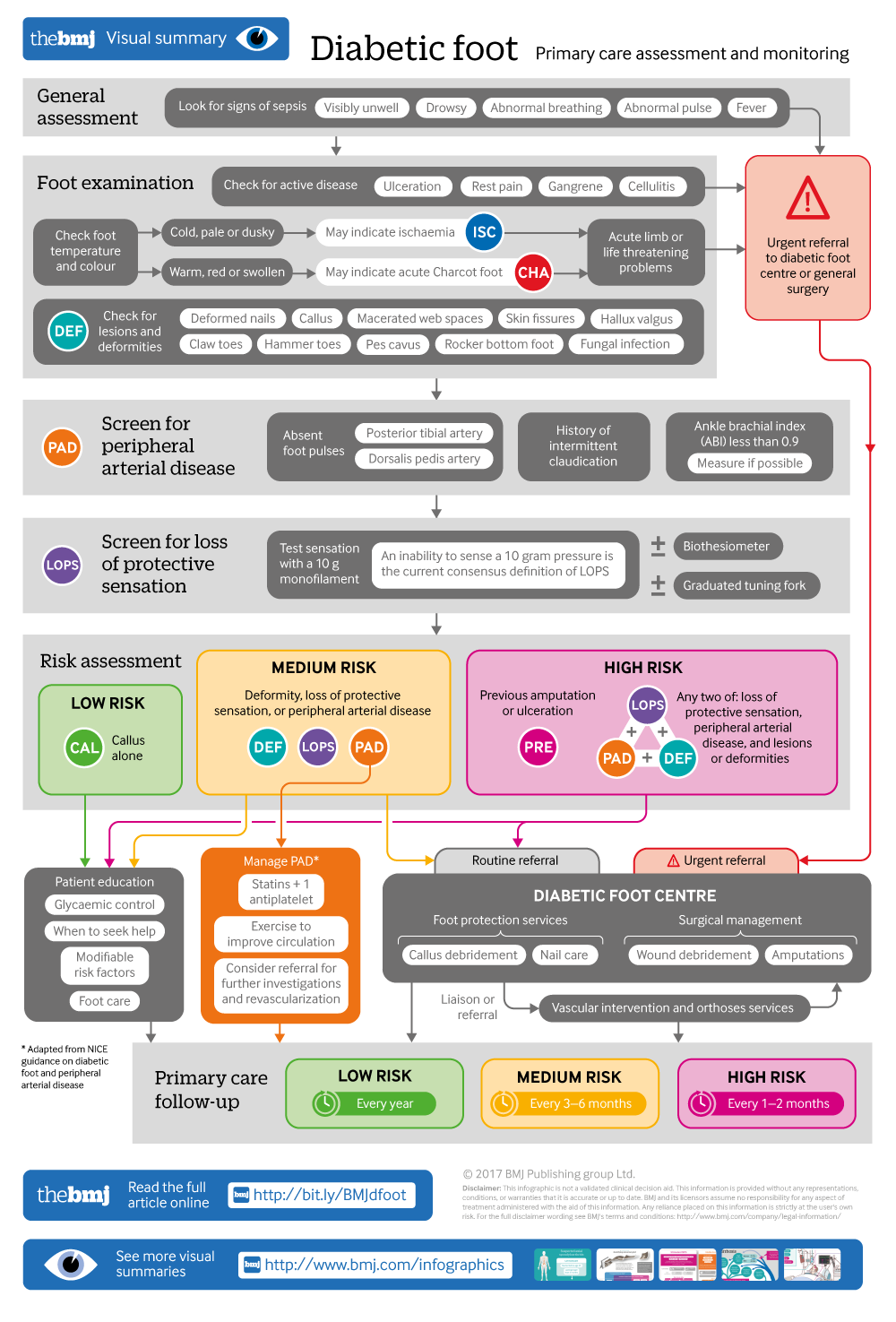
Infographic available
Click here for a visual overview of the primary care assessment and monitoring of a diabetic foot
- Related content
- Peer review
- Satish Chandra Mishra , consultant surgeon and scientist 1 ,
- Kunal C Chhatbar , consultant surgeon 2 ,
- Aditi Kashikar , research assistant 3 ,
- Abha Mehndiratta , technical adviser 4
- 1 Department of Surgery, Bhabha Atomic Research Centre Hospital, Mumbai, India
- 2 KHM Hospital, Mumbai, India
- 3 Seth Gordhandas Sunderdas Medical College and King Edward Memorial Hospital, Mumbai, India
- 4 Global Health and Development Group, Imperial College London, St Mary’s Hospital, London, UK
- Correspondence to: A Mehndiratta abha{at}mail.harvard.edu
What you need to know
Diabetic foot can be prevented with good glycaemic control, regular foot assessment, appropriate footwear, patient education, and early referral for pre-ulcerative lesions
Examine the feet of people with diabetes for any lesions and screen for peripheral neuropathy and peripheral arterial disease, which can lead to injuries or ulceration
Refer patients with foot ulceration and signs of infection, sepsis, or ischaemia immediately to a specialised diabetic foot centre for surgical care, revascularisation, and rehabilitation
Foot disease affects nearly 6% of people with diabetes 1 and includes infection, ulceration, or destruction of tissues of the foot. 2 It can impair patients’ quality of life and affect social participation and livelihood. 3 Between 0.03% and 1.5% of patients with diabetic foot require an amputation. 4 Most amputations start with ulcers and can be prevented with good foot care and screening to assess the risk for foot complications. 5 We provide an update on the prevention and initial management of diabetic foot in primary care.
Sources and selection criteria
This clinical update is based on recommendations in the standard treatment guideline, The diabetic foot: prevention and management in India 2016, published by the Indian Ministry of Health and Family Welfare. 33 A multidisciplinary guideline development group consisting of surgeons, primary care practitioners, and a patient representative developed these guidelines, with inputs from experts in diabetes, diabetic foot rehabilitation, and vascular surgery. The group included representation from rural and urban India, and public and private sectors.
The guideline development group selected recommendations from the National Institute for Health and Care Excellence clinical guideline 19. Diabetic foot problems: prevention and management. Updated 2016, International Working Group on the Diabetic Foot guidance on the prevention of foot ulcers in at-risk patients with diabetes 2015, National Institute for Health and Care Excellence. Peripheral arterial disease: diagnosis and management. Guideline 147, 2012, and Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections, 2012. 9 10 21 32 Some recommendations were adopted unchanged, whereas others were adapted taking into account the challenges of a low resource setting, such as availability of public and private health infrastructure, equipment, staffing, and current capacity at different levels of care.
What causes diabetic foot?
Uncontrolled diabetes contributes to the development of neuropathy and peripheral arterial disease by complex metabolic pathways. 6 Loss of sensation caused by peripheral neuropathy, ischaemia due to peripheral arterial disease, or a combination of these may lead to foot ulcers. A systematic review (78 studies from 84 cohorts) reports a prevalence of 0.003-2.8% for diabetes related peripheral neuropathy and 0.01-0.4% for diabetes related peripheral arterial disease. 4 Figure 1 ⇓ depicts factors that contribute to foot complications.
Fig 1 Risk factors and mechanism for foot ulcer and amputation
- Download figure
- Open in new tab
- Download powerpoint
Diabetes is also implicated in Charcot arthropathy, which involves progressive destruction of the bones, joints, and soft tissues, most commonly in the ankle and foot. Diabetes related Charcot’s arthropathy has a reported prevalence between 0.08% and 13%, but there are no high quality epidemiological studies on Charcot’s foot. 7 8 A combination of neuropathy, abnormal loading of foot, repeated micro trauma, and metabolic abnormalities of bone leads to inflammation, causing osteolysis, fractures, dislocation, and deformities.
In low and middle income countries barefoot walking, lack of awareness, delay in seeking care, and shortage of trained healthcare providers and foot care services are common factors that add to the burden of foot disease.
How is it diagnosed?
A thorough foot examination is important to detect the disease early. Screening for peripheral neuropathy and peripheral arterial disease can help identify patients at risk of foot ulcers. A history of ulcers or amputations and poor glycaemic control increase the risk.
Assess the patient’s general condition for signs of toxicity or sepsis such as feeling unwell, looking sick, showing abnormal behaviour, circulation, or respiration, with or without fever. Examine the feet at each follow-up visit for active disease such as ulceration or gangrene (fig 2 ⇓ ). Look for lesions such as fungal infection, cracks and skin fissures, deformed nails, macerated web spaces, calluses, and deformities such as hammer toes, claw toes, and pes cavus, which increase the risk of ulceration (fig 3 ⇓ ). Feel the temperature of the feet with the dorsum of your hand. A cold foot might suggest ischaemia, and increased warmth with redness and swelling might suggest inflammation such as acute Charcot foot or cellulitis.
Fig 2 Gangrene and ulcer in foot at high risk (previous toe amputation)
Fig 3 Hammer toe deformity with callus and ulcer. Hammer toe is caused by weakened muscles in the foot. The joint connecting the foot with the toe bends upwards (metatarsophalangeal extension) and the joint in middle of the toe bends downwards towards the floor (proximal interphalangeal flexion). This results in the toe curling under the foot and being subjected to excessive ground reaction forces during walking.

Peripheral neuropathy
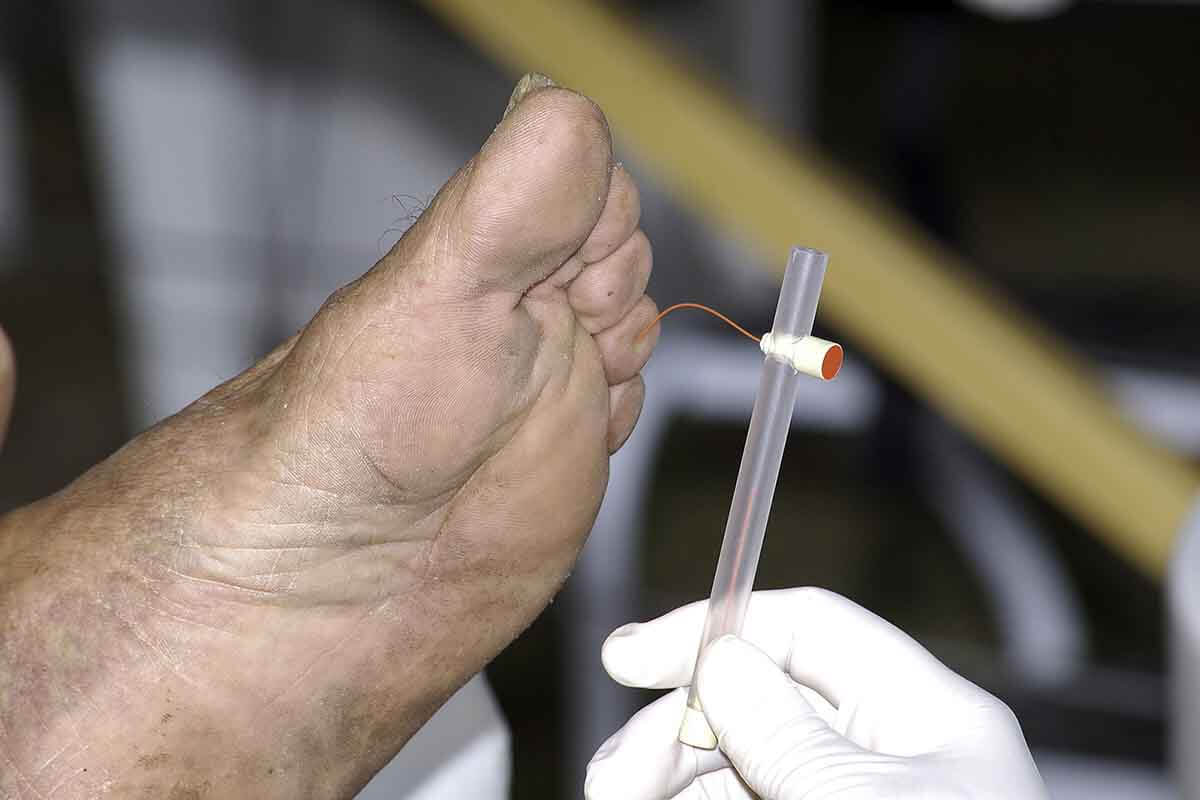
The aim of screening is to identify patients with loss of protective sensation in the feet. Most guidelines recommend the 10 g monofilament for neuropathy assessment (fig 4 ⇓ ) in people with diabetes. 9 10 This monofilament exerts a 10 g buckling force when it bends. An inability to sense a 10 g pressure is the current consensus definition of loss of protective sensation. The test is portable, cheap, and easy to perform (box 1). 12 15 Despite the widespread use of the monofilament test, its accuracy in diagnosing neuropathy is variable. 16 The test may be combined with another test to screen for neuropathy, such as a biothesiometer or a graduated tuning fork (Rydel Seiffer) to assess vibration perception threshold. 17 18
Fig 4 Monofilament test: testing sites and application. The nine plantar sites are the distal great toe; third toe; fifth toe; first, third, and fifth metatarsal heads; medial foot, lateral foot, and heel; and one dorsal site
Box 1: Monofilament test (fig 4 ⇑ )
Procedure —Ask the patient to sit or lie down with both legs stretched out and soles exposed. Explain the procedure and make him or her familiar with the sensation by applying the monofilament on a sensitive area such as the palm. Ask the patient to close his or her eyes and to say “yes” every time touch is felt on the soles, no matter how lightly it is perceived. Place the monofilament at 90° to the skin and press it till it buckles to 1 cm, then hold there for 1-2 seconds and remove. 11 Test different sites in a random sequence with a pause (sham application) to prevent the patient from guessing the next application. If the patient fails to respond at a site, revisit the same site two more times in a random sequence during the assessment. If the patient does not perceive the sensation all the three times, then record the result as loss of protective sensation. 11 Loss of protective sensation even at a single site puts the patient at risk for foot complications.
Test sites and threshold —Most studies recommend testing at 10 sites.
Inability to perceive a 10 g monofilament three times at even a single site means the patient has loss of protective sensation. 11 12
Inter-observer variability —This is reported to be more on the heels, with a higher chance of a false positive result. 13 Exercise caution before labelling a heel as insensate, especially if screening a population where barefoot walking is common.
Durability of monofilaments —Monofilaments tend to fatigue with repeated use, and a 24 hour recovery period is recommended after 100 compression cycles. 14 Replace a monofilament after three months of regular use.
Peripheral arterial disease
Ask for a history of intermittent claudication and rest pain, which suggest peripheral arterial disease. 19 Palpate the posterior tibial artery and dorsalis pedis artery in both feet and record pulsations as absent or present. 20
The ankle brachial index is an adjunct measure to diagnose peripheral arterial disease. 19 21 It is the ratio of the highest systolic blood pressure at the ankle (dorsalis pedis artery or posterior tibial artery) to the systolic blood pressure at the arm, and is measured using a Doppler device. 10 See box 2 on grading the severity of obstruction. Measurement of the ankle brachial index is user dependent. People with diabetes can often have falsely raised ankle brachial index levels as a result of poor compressibility from calcified arteries. 21 Furthermore, availability of equipment, time constraints, and lack of training are reported as major barriers to ankle brachial index testing in primary care. 23 24 25
Box 2: Ankle brachial index
The severity of peripheral arterial disease is interpreted 22 :
0.91-1.3—Normal
0.70-0.90—Mild obstruction
0.40-0.69—Moderate obstruction
<0.40—Severe obstruction
>1.3—Poorly compressible vessel
On the basis of this initial assessment, patients can be categorised as having a low, moderate, or high risk of diabetic foot (see infographic). 9
How can it be prevented?
Regular foot examination.
The suggested frequency for follow-up is based on expert consensus (see infographic). For people at low risk, continue annual foot assessments as they could progress to moderate or high risk. Emphasise the importance of foot care and monitoring glycaemic control.
More frequent follow-up is advised in patients at moderate or high risk, such as those with a foot deformity or with a diagnosis of peripheral neuropathy or peripheral arterial disease at initial assessment. Repeat testing for neuropathy is not necessary if diagnosed previously. Neuropathy reversal is not established in studies. A quick inspection for a breach in skin integrity or ulceration should suffice. Patients with asymptomatic peripheral arterial disease may be followed up in primary care and managed as in guidelines for peripheral arterial disease. 21
Refer patients with calluses and deformed toe nails to preventive podiatry services for basic nail and skin care, including debridement of calluses. Timely referral to foot protection services for control of risk factors in patients with diabetes prevents infection, gangrene, amputation, or death, and reduces hospital admissions and costs. 9
Glycaemic control
Early and good glycaemic control is effective in preventing neuropathy but there is a lack of studies to show that glycaemic control reverses neuropathy. 26 Discuss optimal blood sugar and glycated haemoglobin (HbA 1c ) targets with patients and monitor these as per standard guidelines for diabetes care to prevent or slow the progression of peripheral neuropathy. 27 28
Patient education
Offer people with diabetes or their caregivers, or both, oral and written information on:
The importance of blood glucose control and modifiable cardiovascular risk factors such as diet, exercise, body weight, and cessation of smoking.
The importance of foot care and advice on basic foot care (see box 3). While offering advice consider the patient’s cultural practices and religious beliefs as well as social and family support.
The person’s current risk of developing a foot problem.
When to seek professional help and who to contact in foot emergencies.
Box 3: Tips on foot care for people with diabetes 19
Inspect both feet daily, including the area between the toes. Ask a caregiver to do this if you are unable to.
Wash the feet daily with water at room temperature, with careful drying, especially between the toes.
Use lubricating oils or creams for dry skin, but not between the toes.
Cut nails straight across.
Do not remove corns and calluses using a chemical agent or plaster. They should not be excised at home and must be managed by trained staff.
Always wear socks with shoes and check inside shoes for foreign objects before wearing them.
Avoid walking barefoot at all times.
Ensure a qualified healthcare provider examines your feet regularly.
Notify the healthcare provider at once if a blister, cut, scratch, or sore develops.
Evidence for the effectiveness of patient education on foot care is lacking. A Cochrane review of 11 randomised controlled trials concluded that brief foot care education alone does positively influence patient knowledge and behaviour in the short term, but it is ineffective in preventing diabetic foot ulcers. Education in a structured, organised, and repetitive manner, combined with preventive interventions may, however, prevent foot problems. 29 Although the International Working Group on the Diabetic Foot acknowledges the limited evidence on long term efficacy of patient education, it recommends some form of patient education to improve their foot care knowledge and behaviour. 10
Occlusive footwear causes sweating and can predispose to fungal infection, 30 31 particularly in tropical countries. Ideally, footwear for people with diabetes should have a wide toe box, soft cushioned soles, extra depth to accommodate orthoses if required, and laces or Velcro for fitting and adjustments. A new pair of shoes can be worn for a short while daily until comfortable. Patient compliance to prescribed footwear is usually poor, particularly at home where they are more active. 29 Patients with plantar ulcers at forefoot or heel may be offered offloading footwear (fig 5 ⇓ ) to allow ulcer healing and prevent recurrence.
Fig 5 Offloading footwear reduces pressure on a specific part of the foot to allow an ulcer on that part to heal or to prevent new ulcers. The top figure shows footwear that reduced pressure on the forefoot and the footwear shown underneath allows pressure on the heel to be offloaded
When to refer?
Refer immediately patients with a life threatening or limb threatening problem such as foot ulceration with fever or any signs of sepsis; ulceration with limb ischaemia; gangrene, or a suspected deep seated soft tissue or bone infection usually indicated by either a grossly swollen foot with shiny skin and patches of discoloration or a gritty feel to the bone during a probe to bone test in an open wound. 9 Refer to a specialised diabetic foot centre or to general surgery for wound care, offloading, revascularisation if needed, and rehabilitation.
Explain to patients the need to seek specialist care to limit complications. Provide detailed and clear communication before patients are referred so that multidisciplinary care can be facilitated at the earliest opportunity.
Before referral, wash the ulcer with clean water or saline and apply a sterile inert dressing such as a saline soaked gauze to control exudates and maintain a warm, moist environment for healing. Avoid microbicidal agents such as hydrogen peroxide, povidone iodine, or chlorhexidine to clean or dress the ulcer as these are cytotoxic. Costly antimicrobial dressings are not recommended. 9 Adjust dressings, footwear, and ambulation to avoid weight bearing on an ulcerated foot. 32 Early and aggressive treatment to control infection is important, especially in the presence of an ulcer. Start antibiotic treatment according to antibiotic policy based on local resistance patterns. Before starting antibiotics, take a piece of soft tissue from the base of the ulcer for culture and sensitivity, or take a deep swab for culture. 9 Refer urgently, within one or two days, patients with a history of rest pain, uncomplicated ulcer, or acute Charcot foot. 9 For patients with rest pain or intermittent claudication, offer referral to vascular intervention services for further investigations such as Duplex ultrasonography, and consideration for revascularisation. 21
The management and referral pathways between primary care, specialty diabetic foot centres, and multidisciplinary foot care services need to be integrated (see infographic).
How can diabetic foot care services be organised in India?
Nearly 415 million people globally have diabetes, with 75% living in low and middle income countries. In India about 70 million people have diabetes, and the number is projected to rise to 125 million by 2040. 34
The National Institute for Health and Care Excellence guideline on diabetic foot recommends a three tier system for foot care: primary healthcare for preventive services and appropriate referral of diabetic foot; foot protection services at community level for podiatric care and management of simple foot problems; and multidisciplinary foot care services at tertiary level to handle complex foot problems. 9
In low and middle income countries, primary care doctors are not trained in diabetic foot care, podiatry as a discipline is emerging, and multidisciplinary foot care services are available at few tertiary care centres.
We recommend training primary care doctors in diabetic foot care, particularly in countries with a high burden of diabetes. Referral hospitals should develop diabetic foot centres under the specialty of general surgery. These centres would provide foot protection services such as callus debridement and nail care, and surgeries such as wound debridement and minor or major amputations. Multidisciplinary foot care services should be provided at all tertiary level hospitals with facilities for vascular intervention and orthoses.
Education into practice
In your practice, what proportion of people with diabetes have had a foot evaluation in the past 12 months?
Describe how you would screen patients with diabetes for peripheral neuropathy and peripheral arterial disease.
How would you advise a patient with diabetes about foot care?
How patients were involved in the creation of this article
No patients were involved in the creation of this article.
Additional resources
For healthcare providers.
Indian Ministry of Health and Family Welfare. Standard treatment guidelines: The diabetic foot: prevention and management in India, 2016. http://clinicalestablishments.nic.in/En/1068-standard-treatment-guidelines.aspx http://clinicalestablishments.nic.in/WriteReadData/5381.pdf
International Working Group on the Diabetic Foot. Guidance on footwear and offloading interventions to prevent and heal foot ulcers in people with diabetes. www.iwgdf.org/files/2015/website_footwearoffloading.pdf
National Institute for Health and Care Excellence clinical guideline on diabetic foot problems: prevention and management, 2015. www.nice.org.uk/guidance/ng19/chapter/1-recommendations
National Institute for Health and Care Excellence clinical guideline on peripheral arterial disease: diagnosis and management 2012, updated 2017. www.nice.org.uk/guidance/cg147 .
Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections, 2012. https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/cis346
For patients*
NHS Choices. Diabetes. www.nhs.uk/Conditions/Diabetes/Pages/Diabetes.aspx
NHS Choices. How to look after your feet if you have diabetes. www.nhs.uk/Livewell/foothealth/Pages/Diabetesandfeet.aspx
NHS Choices. Why feet sensations are lost and how to take care of them. www.nhs.uk/Conditions/Peripheral-neuropathy/Pages/Complications.aspx
NHS Choices. What does a podiatrist do and how can a podiatrist help you? www.nhs.uk/livewell/foothealth/pages/foot-problems-podiatrist.aspx
NHS Choices. How do common foot problems look? www.nhs.uk/Tools/Pages/Foot-problems-a-visual-guide.aspx
*All these web links are freely available on the internet.
Suggestions for future research
Does grading the severity of peripheral arterial disease using the ankle brachial index help guide interventions to prevent foot ulcers in people with diabetes?
What is the sensitivity of the monofilament test to diagnose peripheral neuropathy, and the interobserver variation among trained providers?
What model of patient education is effective in preventing diabetic foot complications?
Contributors: SM, KC, and AM conceived and designed the review. SM and KC created the first draft. AM and AK revised the content and approved the final version to be published. All authors act as guarantors.
Funding: The Indian Ministry of Health and Family Welfare funded the process for development of the standard treatment guideline on Diabetic Foot. The Department for International Development funded the technical assistance provided by Global Health and Development Group (formerly NICE International) to the Guideline Development Group on diabetic foot.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following: SM, KC, and AK were members of the guideline development group for the standard treatment guideline on the diabetic foot: prevention and management in India, 2016 published by the Ministry of Health and Family Welfare, government of India. AM provided technical input on methodology to this guideline development group.
Provenance and peer review: This article is one of a series commissioned by the BMJ from the Global Health and Development Group at Imperial College London (formerly NICE International) as part of the International Decision Support Initiative ( www.idsihealth.org ). The BMJ retained full editorial control over external peer review, editing, and publication. Open access fees are funded by the Bill and Melinda Gates Foundation.
Patient consent: All photographs have been included after taking patient consent.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/ .
- ↵ Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (†). Ann Med 2017 ; 359 : 106 - 16 . doi:10.1080/07853890.2016.1231932 pmid:27585063 . OpenUrl
- ↵ Schaper NC, Apelqvist J, Bakker K. The international consensus and practical guidelines on the management and prevention of the diabetic foot. Curr Diab Rep 2003 ; 359 : 475 - 9 . doi:10.1007/s11892-003-0010-4 pmid:14611743 . OpenUrl
- ↵ Jeffcoate W, Bakker K. World Diabetes Day: footing the bill. Lancet 2005 ; 359 : 1527 . doi:10.1016/S0140-6736(05)66437-9 pmid:15866295 . OpenUrl
- ↵ Lazzarini PA, Hurn SE, Fernando ME, et al. Prevalence of foot disease and risk factors in general inpatient populations: a systematic review and meta-analysis. BMJ Open 2015 ; 359 : e008544 . doi:10.1136/bmjopen-2015-008544 pmid:26597864 . OpenUrl
- ↵ Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005 ; 359 : 217 - 28 . doi:10.1001/jama.293.2.217 pmid:15644549 . OpenUrl
- ↵ Bhat S, Mary S, Giri AP, Kulkarni MJ. Advanced glycation end products in diabetic complications. In: Mechanisms of vascular defects in diabetes mellitus . Springer International, 2017:423-49.
- ↵ Frykberg RG, Belczyk R. Epidemiology of the Charcot foot. Clin Podiatr Med Surg 2008 ; 359 : 17 - 28, v . doi:10.1016/j.cpm.2007.10.001 pmid:18165108 . OpenUrl
- ↵ Rogers LC, Frykberg RG, Sanders LJ. The diabetic Charcot foot: recognition, evaluation and management. In: Armstrong DG, Lavery LA, eds. Clinical care of the diabetic foot . 3rd ed. 2016: 99.
- ↵ International Guidelines Team. National Institute for Health and Care Excellence clinical guideline 19. Diabetic foot problems: prevention and management. Updated 2016. www.nice.org.uk/guidance/ng19 .
- ↵ Bus SA, van Netten JJ, Lavery LA, et al. International Working Group on the Diabetic Foot guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab Res Rev 2016 ; 359 : 16 - 24 . doi:10.1002/dmrr.2696 pmid:26334001 . OpenUrl
- ↵ British Columbia Provincial Nursing Skin and Wound Committee. Procedure: Monofilament testing for loss of protective sensation of diabetic/neuropathic feet for adults and children. 2014:1-3. www.clwk.ca/buddydrive/file/procedure-monofilament-testing/?download=106%253Aprocedure-monofilament-testing-for-lops .
- ↵ Smieja M, Hunt DL, Edelman D, Etchells E, Cornuz J, Simel DL. International Cooperative Group for Clinical Examination Research. Clinical examination for the detection of protective sensation in the feet of diabetic patients. J Gen Intern Med 1999 ; 359 : 418 - 24 . doi:10.1046/j.1525-1497.1999.05208.x pmid:10417599 . OpenUrl
- ↵ Mythili A, Kumar KD, Subrahmanyam KA, Venkateswarlu K, Butchi RG. A comparative study of examination scores and quantitative sensory testing in diagnosis of diabetic polyneuropathy. Int J Diabetes Dev Ctries 2010 ; 359 : 43 - 8 . doi:10.4103/0973-3930.60007 pmid:20431806 . OpenUrl
- ↵ Booth J, Young MJ. Differences in the performance of commercially available 10-g monofilaments. Diabetes Care 2000 ; 359 : 984 - 8 . doi:10.2337/diacare.23.7.984 pmid:10895851 . OpenUrl
- ↵ Mayfield JA, Sugarman JR. The use of the Semmes-Weinstein monofilament and other threshold tests for preventing foot ulceration and amputation in persons with diabetes. J Fam Pract 2000 ; 359 ( Suppl ): S17 - 29 . pmid:11093555 . OpenUrl
- ↵ Dros J, Wewerinke A, Bindels PJ, van Weert HC. Accuracy of monofilament testing to diagnose peripheral neuropathy: a systematic review. Ann Fam Med 2009 ; 359 : 555 - 8 . doi:10.1370/afm.1016 pmid:19901316 . OpenUrl
- ↵ Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 2000 ; 359 : 606 - 11 . doi:10.2337/diacare.23.5.606 pmid:10834417 . OpenUrl
- ↵ Vijay V, Snehalatha C, Seena R, Ramachandran A. The Rydel Seiffer tuning fork: An inexpensive device for screening diabetic patients with high-risk foot. Pract Diabetes Int 2001 ; 359 : 155 - 6 doi:10.1002/pdi.170 . OpenUrl
- ↵ Hinchliffe R, Brownrigg J, Apelqvist J, et al. International Working Group on the Diabetic Foot guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev 2016 ; 359 : 37 - 44 . doi:10.1002/dmrr.2698 pmid:26332424 . OpenUrl
- ↵ Damir A. Clinical assessment of diabetic foot patient. J Int Med Sci Acad 2011 ; 359 : 199 - 203 . OpenUrl
- ↵ National Institute for Health and Care Excellence. Peripheral arterial disease: diagnosis and management. Guideline 147, 2012. www.nice.org.uk/guidance/cg147 .
- ↵ American Diabetes Association. Epidemiology and impact of peripheral arterial disease in people with diabetes. Diabetes 2003 ; 359 : 3333 - 41 . OpenUrl
- ↵ Mohler ER 3rd, , Treat-Jacobson D, Reilly MP, et al. Utility and barriers to performance of the ankle-brachial index in primary care practice. Vasc Med 2004 ; 359 : 253 - 60 . doi:10.1191/1358863x04vm559oa pmid:15678616 . OpenUrl
- ↵ Haigh KJ, Bingley J, Golledge J, Walker PJ. Barriers to screening and diagnosis of peripheral artery disease by general practitioners. Vasc Med 2013 ; 359 : 325 - 30 . doi:10.1177/1358863X13505673 pmid:24105616 . OpenUrl
- ↵ Chaudru S, de Müllenheim P-Y, Le Faucheur A, Kaladji A, Jaquinandi V, Mahé G. Training to perform ankle-brachial index: systematic review and perspectives to improve teaching and Learning. Eur J Vasc Endovasc Surg 2016 ; 359 : 240 - 7 . doi:10.1016/j.ejvs.2015.09.005 pmid:26602321 . OpenUrl
- ↵ Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep 2014 ; 359 : 528 . doi:10.1007/s11892-014-0528-7 pmid:25139473 . OpenUrl
- ↵ Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: A position statement by the American diabetes association. Diabetes Care 2017 ; 359 : 136 - 54 . doi:10.2337/dc16-2042 pmid:27999003 . OpenUrl
- ↵ American Diabetes Association. Standards of medical care in diabetes—2017. Abridged for primary care providers. Clin Diabetes 2017 ; 359 : 5 - 26 . pmid:28144042 . OpenUrl
- ↵ Dorresteijn JAN, Valk GD. Patient education for preventing diabetic foot ulceration. Diabetes Metab Res Rev 2012 ; 359 ( Suppl 1 ): 101 - 6 . doi:10.1002/dmrr.2237 pmid:22271733 . OpenUrl
- ↵ Thomas J, Jacobson GA, Narkowicz CK, Peterson GM, Burnet H, Sharpe C. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther 2010 ; 359 : 497 - 519 . doi:10.1111/j.1365-2710.2009.01107.x pmid:20831675 . OpenUrl
- ↵ Ameen M. Epidemiology of superficial fungal infections. Clin Dermatol 2010 ; 359 : 197 - 201 . doi:10.1016/j.clindermatol.2009.12.005 pmid:20347663 . OpenUrl
- ↵ Lipsky BA, Berendt AR, Cornia PB, et al. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012 ; 359 : 132 - 73 doi:10.1093/cid/cis346 . OpenUrl
- ↵ Ministry Health and Family Welfare of India. Standard treatment guidelines: The diabetic foot: prevention and management in India, 2016. Ministry Health and Family Welfare, India. 2016. www.nhm.gov.in/nrhm-instate/520-standard-treatment-guidelines.html
- ↵ International Diabetes Federation. IDF diabetes atlas—2015, 7th ed. IDF, 2015. www.diabetesatlas.org/resources/2015-atlas.html .
- Diabetes & Primary Care
- Vol:24 | No:05
Interactive case study: Problems with the diabetic foot
- 25 Oct 2022
Share this article + Add to reading list – Remove from reading list ↓ Download pdf
Diabetes & Primary Care ’s series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes.
Around one in three people with diabetes will develop a foot ulcer within their lifetime. Primary care plays a critical role in identifying problems with the diabetic foot, and in responding rapidly and appropriately to them. The three mini-case studies developed for this issue of the journal take us through the basic considerations of identifying and managing problems with the diabetic foot.
The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Working through the case studies will improve our knowledge and problem-solving skills in diabetes care by encouraging us to make evidence-based decisions in the context of individual cases.
Readers are invited to respond to the questions by typing in your answers. In this way, we are actively involved in the learning process, which is hopefully a much more effective way to learn.
By actively engaging with these case histories, I hope you will feel more confident and empowered to manage such presentations effectively in the future.
Glenda is 62 years old and has had type 2 diabetes for 5 years. She reports an uncomfortable tingling sensation in both her feet that is most troublesome at night, and the feeling of walking on pebbles.
How would you interpret Glenda’s symptoms?
56-year-old Sam has established type 2 diabetes and peripheral neuropathy. An area under his left foot has been weeping, but is not painful. Examination reveals an area of callus and frank ulceration under the first metatarsal head. There is a little bleeding, but no pus discharge or indication of cellulitis. The foot is not deformed, and feels warm with good pulses. A dense sensory peripheral neuropathy is confirmed.
What is your assessment of Sam’s problem?
Proma is a 57-year-old Asian woman with type 2 diabetes. Two years ago she suffered a myocardial infarction. She has a small new ulcer on her right foot, without cellulitis or discharge. The posterior tibial pulse is absent and the dorsalis pedis pulse is weak. The foot is cool, with a dusky tinge to the toes and dystrophic toenails. Peripheral sensation is intact. There is excessive wear on the inside of the lateral aspect of her shoe at the site corresponding to her ulcer.
What is the likely underlying cause of Proma’s foot ulcer?
By working through these interactive case studies, we will consider the following issues and more:
- Causes of peripheral neuropathy.
- Pharmacological management of peripheral neuropathic pain in diabetes.
- Prevention of diabetic foot problems in primary care.
- Neuropathic and ischaemic origins of foot ulcers.
Click here to see the case study.
Interactive case study: Non-diabetic hyperglycaemia – Prediabetes
Diabetes distilled: smoking cessation cuts excess mortality rates after as little as 3 years, impact of freestyle libre 2 on diabetes distress and glycaemic control in people on twice-daily pre-mixed insulin, updated guidance from the pcds and abcd: managing the national glp-1 ra shortage, diabetes distilled: fib-4 – a diagnostic and prognostic marker for liver and cardiovascular events and mortality, at a glance factsheet: tirzepatide for management of type 2 diabetes, editorial: lipid management, tirzepatide and hybrid closed-loop: what does new nice guidance recommend.

Diagnosing and managing non-diabetic hyperglycaemia.
17 Apr 2024

The mortality benefits of smoking cessation may be greater and accrue more rapidly than previously understood.

Expanding CGM eligibility criteria to include this patient group may be beneficial.
27 Mar 2024

Advice on selecting alternative glucose-lowering therapies when GLP-1 RAs used in the management of type 2 diabetes in adults are unavailable.
22 Mar 2024
Sign up to all DiabetesontheNet journals
- CPD Learning
- Journal of Diabetes Nursing
- Diabetes Care for Children & Young People
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Yeungnam Med Sci
- v.40(4); 2023 Oct
- PMC10626291
The pathophysiology of diabetic foot: a narrative review
Department of Orthopaedic Surgery, Kosin University College of Medicine, Busan, Korea
An aging population and changes in dietary habits have increased the incidence of diabetes, resulting in complications such as diabetic foot ulcers (DFUs). DFUs can lead to serious disabilities, substantial reductions in patient quality of life, and high financial costs for society. By understanding the etiology and pathophysiology of DFUs, their occurrence can be prevented and managed more effectively. The pathophysiology of DFUs involves metabolic dysfunction, diabetic immunopathy, diabetic neuropathy, and angiopathy. The processes by which hyperglycemia causes peripheral nerve damage are related to adenosine triphosphate deficiency, the polyol pathway, oxidative stress, protein kinase C activity, and proinflammatory processes. In the context of hyperglycemia, the suppression of endothelial nitric oxide production leads to microcirculation atherosclerosis, heightened inflammation, and abnormal intimal growth. Diabetic neuropathy involves sensory, motor, and autonomic neuropathies. The interaction between these neuropathies forms a callus that leads to subcutaneous hemorrhage and skin ulcers. Hyperglycemia causes peripheral vascular changes that result in endothelial cell dysfunction and decreased vasodilator secretion, leading to ischemia. The interplay among these four preceding pathophysiological factors fosters the development and progression of infections in individuals with diabetes. Charcot neuroarthropathy is a chronic and progressive degenerative arthropathy characterized by heightened blood flow, increased calcium dissolution, and repeated minor trauma to insensate joints. Directly and comprehensively addressing the pathogenesis of DFUs could pave the way for the development of innovative treatment approaches with the potential to avoid the most serious complications, including major amputations.
Introduction
Diabetic foot ulcers (DFUs) are ulcers that arise on the feet of individuals with diabetes and are a major concern. These ulcers stem from the deterioration of the skin or mucosal tissue on the feet and are particularly susceptible to exacerbation by conditions such as diabetic neuropathy and peripheral vascular disease. Upon occurrence, DFUs can culminate in foot amputation, inflicting a substantial psychological burden on patients. This unfortunate outcome can lead to a reduction in one's daily activities, culminating in a decline in both physical capabilities and social engagement, leading to a serious reduction in the patient's quality of life and high financial costs for society [ 1 ].
As the average age of the population increases worldwide, the number of patients with diabetes, and accordingly, the number of patients with DFUs, is also increasing. The lifetime prevalence of DFUs in the population with diabetes is 15% to 25%, and the recurrence rate of DFUs within 5 years is high, ranging from 50% to 70% [ 2 - 4 ].
Understanding the pathophysiology of DFUs is crucial for effective management and prevention, facilitating early diagnosis, informed decision-making, personalized treatment, improved wound healing, fewer complications, preventive actions, and research advancements.
Pathophysiology of diabetic foot
The pathophysiology of diabetic ulcers involves metabolic causes, neuropathy, angiopathy, and changes in the immune system. The interaction between metabolic dysfunction, diabetic immunopathy, diabetic neuropathy, and diabetic angiopathy promotes the development and progression of diabetic foot infections (DFIs) and may lead to diabetic neuroarthropathy ( Figs. 1 , ,2 2 ).

Diagram of pathophysiological factors in diabetic foot. The interaction of metabolic dysfunction, diabetic immunopathy, diabetic neuropathy, and diabetic angiopathy promotes the development and progression of diabetic foot infections and may lead to diabetic neuroarthropathy.

Pathological pathways in the progression of diabetic foot. A schematic diagram representing the pathophysiology of DFUs via different pathological pathways. Endothelial dysfunction, ischemia, neuroarthropathy, and impaired immunology contribute to the pathophysiology of DFUs. ATP, adenosine triphosphate; PKC, protein kinase C; ROS, reactive oxygen species; DFU, diabetic foot ulcer.
1. Metabolic dysfunction
Diabetes affects epineural microvessels and reduces blood supply to the nerves of patients with diabetes [ 5 ]. Apart from the vascular supply, the peculiar anatomy of the peripheral nervous system may explain the predisposition of its most distal parts to diabetes. The axon is covered with Schwann cells, but the distal axon is too weak because the neuronal cell body is relatively small compared to the very long axon neurite. Therefore, distal axons are vulnerable when diabetes affects nerves [ 6 ].
The processes by which hyperglycemia causes peripheral nerve damage are related to adenosine triphosphate (ATP) deficiency, the polyol pathway, oxidative stress, protein kinase C (PKC) activity, and proinflammatory processes.
An insufficient ATP supply hampers axonal transport, particularly in mitochondria-rich axons that provide nerve energy, thus promoting axonal injury and diabetic neuropathy. The inability to counter excessive oxidative stress due to inadequate ATP levels damages the axons during hyperglycemia, causing axonal degeneration or apoptosis [ 7 ]. Oxidative stress negatively affects multiple biochemical pathways.
The polyol pathway involves the conversion of glucose to sorbitol by aldose reductase (AR) and the subsequent conversion of sorbitol to fructose by sorbitol dehydrogenase. In diabetes, elevated glucose levels boost the affinity of AR for glucose, leading to increased sorbitol production. Accumulated sorbitol reduces Na + K + -ATPase activity, thereby diminishing nerve cell reserves and conduction velocities. The hyperglycemia-induced polyol pathway also increases oxidative stress due to nicotinamide adenine dinucleotide phosphate (NADPH) depletion via the pentose phosphate pathway, which is essential for glutathione generation [ 8 ]. Excess fructose accelerates glycation and NADPH consumption and exacerbates intracellular oxidative stress [ 9 ]. This disturbance results in decreased antioxidant levels and increased production of reactive oxygen species (ROS), which play pivotal roles in diabetes-related complications. The activation of AR increases polyol flux and causes neuropathy; however, neuropathic changes have also been observed in AR-deficient diabetic mice [ 10 ]. Further research is required to establish a direct link between AR and diabetic neuropathy.
PKC is a member of the serine/threonine protein kinase family [ 11 ] and is involved in various cellular responses associated with diabetes [ 12 ]. Hyperglycemia triggers glycolysis and activates PKC [ 13 ]. Vascular dysfunction caused by PKC activation promotes diabetic microvascular complications, which primarily alter blood flow [ 14 ], extracellular matrix synthesis, basement membrane thickening [ 15 ], vascular permeability [ 16 ], and angiogenesis [ 17 ].
Low-grade intraneural inflammation is an aspect of diabetic neuropathy. Systemic proinflammatory activity in human sensorimotor diabetic neuropathy has recently been reported [ 18 ]. This mild inflammatory process is a common terminal pathway in diabetic neuropathy and is associated with the degeneration of intraepidermal nerve fibers.
2. Diabetic immunopathy
In the context of hyperglycemia, the suppression of endothelial nitric oxide (NO) production inhibits NO synthase, leading to an elevated level of ROS, notably superoxide radicals. This heightened ROS level subsequently triggers an increase in hydrogen peroxide concentrations. Consequently, highly reactive hydroxyl radicals can be formed that cause cellular damage. The combined action of NO and superoxide results in the production of peroxynitrite, which, in turn, affects endothelial vasodilation and mediates lipid peroxidation. This process sets the stage for heightened concentrations of low-density lipoproteins, followed by the development of microcirculation atherosclerosis, elevated inflammation, abnormal intimal growth, platelet aggregation, and thrombosis [ 19 ].
Hyperglycemia contributes to excess hydrogen peroxide, intensifying oxidative stress and related products [ 20 ]. These products stimulate the generation of advanced glycation end-products (AGEs) [ 21 ]. Decoupling of NO synthase reduces NO production, resulting in impaired wound healing [ 22 ]. In the context of wound healing, the controlled generation and elimination of ROS are vital. However, diabetic wounds exhibit elevated ROS levels, which further impede the healing process. The heightened presence of ROS not only slows wound healing but also leads to excessive oxidative stress.
3. Diabetic neuropathy
Peripheral neuropathy is the most common intractable complication of diabetes [ 23 , 24 ]. More than 60% of DFUs result from an underlying neuropathy [ 25 ]. The duration of diabetes and glycated hemoglobin levels are strongly associated with the prevalence of neuropathy [ 26 , 27 ].
Blood supply to the peripheral nerves is insufficient, blood flow is easily damaged, and automatic regulation of blood flow is impaired. These anatomical features enable us to understand why peripheral nerve neuropathy differs from other complications [ 28 ]. These features make peripheral nerves vulnerable to ischemia.
In diabetic neuropathy, damaged nerve endings lead to pain perception due to disrupted action potentials, inducing hyperexcitability [ 29 ]. Upregulated voltage-gated sodium channels (Nav), including Nav1.3 and Nav1.7, in diabetic animals influence diabetic neuropathy progression, while reduced Nav1.8 and Nav1.6, along with abnormal phosphorylation of Nav1.6, Nav1.7, and Nav1.8, contribute to nociceptive nerve fiber irregularities [ 30 , 31 ]. Patients with diabetic neuropathy display increased nodal Na + currents that intensify peripheral nerve hyperexcitability.
Altered K + voltage-dependent channels (Kv) affect resting membrane potential and increase neuronal excitability [ 32 ]. Reduced Kv currents in diabetic rats elevate excitability and peptide release, and these factors could contribute to peripheral nerve hyperexcitability in diabetes.
The development of neuropathy in affected patients results from hyperglycemia-induced metabolic abnormalities [ 33 , 34 ]. Diabetic neuropathy affects the sensory, motor, and autonomic nervous systems.
The evidence of sensory neuropathy includes hyperalgesia, paresthesia, and allodynia. Sensory neuropathy causes loss of protective sensations. As a result, the risk for trauma is significantly elevated [ 35 , 36 ] and injuries often go unnoticed for weeks. Motor neuropathy can manifest as atrophy of the small foot muscles, resulting in malpositioning of the toes (claw toe) [ 37 ]. These muscle changes can cause foot deformities leading to biomechanical abnormalities. Glycosylation of tendons induces stiffness and shortening, potentially giving rise to foot deformities, such as claw toes and hammer toes, along with Achilles tendon stiffening, which elevates the pressure on the forefoot [ 38 ]. The combination of sensory and motor peripheral neuropathy results in an unequal foot load with an unsteady gait. Over time, hyperkeratosis develops because of neuropathy and an elevated plantar pressure load. Autonomic neuropathy reduces the function of sweat and sebaceous glands of the feet, resulting in dry skin and fissures. Furthermore, it diminishes the neuroinflammatory responses to noxious stimuli [ 39 ]. Consequently, the natural ability of the foot to moisturize is lost, and the skin becomes more susceptible to injury and infection. The interaction between sensory, motor, and autonomic neuropathy can form a callus in the foot. After repeated exposure to external or minor trauma, skin ulcers form through subcutaneous hemorrhage ( Fig. 3 ) [ 40 ].

The process from callus formation to ulceration formation in patients with diabetes. The interaction of sensory, motor, and autonomic neuropathy can form a callus in the foot. After repeated exposure to external or minor trauma, skin ulcers form through subcutaneous hemorrhages. Adapted from Armstrong et al. [ 40 ] with permission of the New England Journal of Medicine .
4. Diabetic angiopathy
Hyperglycemia causes endothelial damage, dyslipidemia, and increased platelet viscosity and activity, leading to atherosclerosis. Hyperglycemia also causes peripheral vascular changes, resulting in endothelial cell dysfunction and decreased vasodilator secretion [ 25 , 41 ]. Peripheral vascular constriction and hypercoagulation lead to ischemia, which increases the risk of skin ulcers. Peripheral arterial disease is an important cause of foot ulcers in approximately 50% of cases [ 42 ]. An insufficient peripheral blood supply delays wound healing and exacerbates infections [ 43 ].
In diabetic neuropathy, the perception of pain arises from impaired nerve endings that disrupt the normal progression of action potentials. Atherosclerosis is the primary contributor to vascular mortality in type 2 diabetes. This process is driven by endothelial cell dysfunction, a consequence of factors like hypertension, insulin resistance, and hyperglycemia. The resulting dysfunction not only hampers the production of NO by endothelial cells but also triggers signals that encourage the proliferation of vascular smooth muscle cells through the mitogen-activated protein kinase pathway. Elevated glucose levels counteract the vasodilatory effects of NO and increase the levels of antifibrinolytic and prothrombotic molecules [ 4 ].
The intricate relationship among insulin resistance, hyperglycemia, and dyslipidemia underscores the nature of diabetes. Through extensive research, four interconnected pathways have been revealed, shedding light on the connection between hyperglycemia and endothelial cell damage, resulting in shared biochemical abnormalities. Elevated glucose exposure spurs the production of mitochondrial superoxide, thereby influencing downstream pathways including the hexosamine and PKC pathways. Hyperglycemia-induced mitochondrial generation of ROS affects both endothelial cells and platelets, inciting platelet aggregation and cytokine release [ 4 , 44 ].
In the context of diabetes, targeting the excessive production of mitochondrial ROS is promising for addressing vascular dysfunction. Gliclazide, a sulfonylurea, possesses antioxidant and anti-aggregation properties that can potentially enhance vascular regulation and mitigate oxidative stress. It effectively interferes with AGE-induced vascular endothelial growth factor expression while modulating signaling pathways intricately linked to diabetic angiopathy [ 45 ].
5. Diabetic foot infections
The interplay of metabolic factors, immunopathy, diabetic neuropathy, and diabetic angiopathy promotes the development and progression of infections, ischemic ulcers, and gangrene in diabetic individuals, potentially culminating in amputation ( Fig. 4 ) [ 46 , 47 ].

Development of diabetic foot ulcers due to diabetic neuropathy. The interplay of metabolic factors, immunopathy, diabetic neuropathy, and diabetic angiopathy fosters the development and progression of infections, ischemic ulcers, or gangrene in individuals with diabetes. Adapted from Schaper et al. [ 47 ] with permission from John Wiley & Sons, Ltd.
A range of microorganisms can trigger DFIs ( Fig. 3 ) [ 40 ], with Staphylococcus aureus being the most prevalent. Among DFIs, methicillin-resistant S. aureus (MRSA) is found in 16.78% to 30% of cases [ 48 ]. Although MRSA infections do not appear to affect mortality, they are associated with increased hospitalization rates and increased risks of limb amputation [ 49 ]. Amputation, as a preventive measure against the spread of infection, was shown to extend life expectancy by 2 years in half of the studied subjects with diabetes [ 50 , 51 ]. Nevertheless, the survival rate of patients with diabetes and ulcerative infections remains at only 56% 5 years after the initial onset of ulcers [ 51 ]. Collectively, these findings underscore the urgency of enhanced ulcer prevention and prompt diagnosis of DFIs.
6. Neuroarthropathy
Charcot neuroarthropathy, commonly referred to as Charcot foot, is a chronic and progressive degenerative arthropathy that arises from disrupted sensory innervation of the affected joint. This insidious and destructive condition primarily affects the foot bones, leading to deformities that can trigger ulcer formation and subsequent disabilities. Charcot foot development is characterized by joint subluxation and dislocation, bone osteolysis, fragmentation, and soft-tissue edema [ 47 ].
Deterioration of the autonomic nervous system in individuals with diabetes mellitus leads to increased local blood supply, resulting in elevated resting blood flow compared to that in individuals who are non-diabetic. This heightened blood flow combined with increased calcium dissolution promotes osteoclastic bone activity and damage. Another theory suggests that repeated minor trauma to the insensate joints contributes to fracture and disintegration.
In patients with Charcot foot, proinflammatory proteins exhibit distinct expression patterns under modulated stimulation. The production of proinflammatory cytokines, such as tumor necrosis factor-α and interleukin (IL)-1β, triggers uncontrolled osteolysis. These cytokines upregulate the expression of receptor activator of nuclear factor (NF)-κB ligand (RANKL), which in turn promotes osteoclast maturation through NF-κB activation, while the anti-inflammatory peptides IL-4 and IL-10 are downregulated.
A hallmark deformity associated with Charcot foot is midfoot collapse, often termed the “rocker-bottom” foot deformity. Hallux valgus and loose bodies in the joint cavity may also be present. These deformities increase susceptibility to recurrent ulcerations [ 52 ].
Hyperglycemia induces diabetic ulcers through several processes. The interactions among metabolic dysfunction, diabetic immunopathy, diabetic neuropathy, and diabetic angiopathy promote the development and progression of DFIs and may lead to diabetic neuroarthropathy. A direct and detailed approach to understanding the pathogenesis of DFUs will enable the development of new treatment methods and prevent the worst outcomes of diabetic ulcers such as major amputation.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.

IMAGES
VIDEO
COMMENTS
Diabetic foot case presentation. This document presents a case study of a 56-year-old man with type 2 diabetes presenting with a non-healing ulcer on his right foot following toe amputation. It provides details of his medical history, examination findings, lab investigations, and discusses diabetic foot ulcers and complications.
Approximately between 15 and 25% of diabetic patients will present with foot ulcers throughout their life, this being the main cause of non-traumatic amputation worldwide. The overall prevalence rate of this complication is between 1.3 and 4.8%. These are developed by the convergence of several predisposing, triggering and aggravating factors.
Diabetic foot ulcer (DFU) is one of the serious complications of diabetes, which has high disability rate and mortality. ... Case Presentation. In case 1, a 56-year-old man with 3-years history of type 2 diabetes had a 3.0×2.0 cm ulcer which infected with staphylococcus aureus on his right calf for more than half a month. In case 2, a 70-year ...
Case Presentation: In case 1, a 56-year-old man with 3-years history of type 2 diabetes had a 3.0×2.0 cm ulcer which infected with staphylococcus aureus on his right calf for more than half a month. In case 2, a 70-year-old man with 10-years history of type 2 diabetes presented with an 8-month right heel ulcer that developed to 7.5×4.6 cm.
Grade 4 diabetic foot with necrotizing fasciitis is a severe infection that can cause septicemia, amputation and even death. ... Case presentation Patient history. A 40-year-old male patient with DM was admitted to our department with a complaint of progressive ulceration of the right foot for 4 days duration. Four days before, the patient ...
Non-enzymatic glycation contractures. increased plantar pressures ‒ Skin integrety ‒ Nail Care. Hospitalized diabetic foot ulcer patients can expect a 59% longer length of stay. Patient with diabetes are 15 times more likely to require a major amputation ‒ 14% to 24% DM ulcers will result in an amputation.
Diabetes mellitus effects around 10% of the whole population and unhealed diabetic foot ulcer is one of the most debilitating and economically burdensome conditions. ... Lavery L A, et al. The costs of diabetic foot: the economic case for the limb salvage team. J Vas Surg 2010; 52(3): 17S-22S. Crossref. PubMed. Google Scholar. 6. Strauss MB ...
intervals with measurements of diabetic foot wounds to monitor reduction of wound size and healing progress (Grade 1C) Recommendation 1.1: We recommend evaluation for infection on initial presentation of all diabetic foot wounds, with initial sharp débridement of all infected diabetic ulcers, and urgent surgical intervention for foot infections
Currently the patient is on insulin with antibiotic, antiplatelet, and calcium channel blockers. Summary: The common case presentation on diabetic foot ulcers with their associated problems has been discussed in this chapter. We find that among Indian population, the most common sites of ulcer is metatarsal heads and heel.
Diabetic foot ulcers (DFU) are the most devastating foot complications that affect an individual in all aspects of life. In this chapter, we shall focus on few case histories of patients with diabetic foot ulceration along with their clinical presentation. This would be very useful for readers to understand the clinical presentation on DFU, its ...
Case presentation1.1.1. ... The acutely inflamed foot is one of the most fatal presentations of diabetic foot complications. These complications may present initially as minor trauma, which can rapidly progress to a limb-threatening infection, a sudden deterioration of a known underlying neuropathic diabetic foot ulcer, or limb ischemia. ...
In the diagnosis of diabetic foot ulcers or pre-ulcerative lesions, the following should be taken into account: History of trauma. History of puncture wound (with or without shoe gear) History of change in shoe gear. History of deformity, either acquired or congenital. History of callus or blister. History of wound care management.
#### What you need to know Foot disease affects nearly 6% of people with diabetes1 and includes infection, ulceration, or destruction of tissues of the foot.2 It can impair patients' quality of life and affect social participation and livelihood.3 Between 0.03% and 1.5% of patients with diabetic foot require an amputation.4 Most amputations start with ulcers and can be prevented with good ...
Diabetes & Primary Care's series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes.. Around one in three people with diabetes will develop a foot ulcer within their lifetime. Primary care plays a critical role in identifying problems with the diabetic foot, and in responding rapidly and ...
DIABETIC FOOT SOAP CASE PRESENTATION.pdf. Education. 1 of 33. Download now. Download to read offline. CASE PRESENTATION ON DIABETIC FOOT PRESENTED. INTRODUCTION- DEFINITION Diabetic foot is. Wound classification system- Stages. RISK FACTORS • Age.
Proper diabetic foot care is a crucial aspect of diabetes treatment for limb preservation. One of the leading causes of hospitalization and amputation in patients with diabetes is foot ulcerations. The majority of diabetic foot complications result from ischemia, neuropathy, and/or infection. Patients with diabetes and neuropathy have a 7 to 10 ...
Case Study: Treating A Patient With A Chronic Diabetic Foot Ulcer. This author navigates the complex issues in treating an elderly patient with a heavily exudative diabetic foot ulcer, which has recurred over an 11-year period. This case is compelling for several reasons. It illustrates the importance of the team approach to limb salvage in a ...
Diabetic Foot - Case Presentation - Free download as Powerpoint Presentation (.ppt), PDF File (.pdf), Text File (.txt) or view presentation slides online. diabetic foot
Diabetic foot ulcers (DFUs) are ulcers that arise on the feet of individuals with diabetes and are a major concern. These ulcers stem from the deterioration of the skin or mucosal tissue on the feet and are particularly susceptible to exacerbation by conditions such as diabetic neuropathy and peripheral vascular disease.
Case study presentation on diabetic foot ulcer.pptx - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. Scribd is the world's largest social reading and publishing site. ...
CASE PRESENT A TION: A 71 years old lady, known case of diabetes insipidus type2 poorly controlled, hypertension, Afib, peripheral vascular disease, history of right foot amputations, left femoral ...