- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- Resources for Hematology Fellows
- American Society of Hematology
- Hematopoiesis Case Studies

Case Study: 44-Year-Old Man with Fever, Abdominal Pain, and Pancytopenia
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
A 44-year-old man presents with fever, abdominal pain, and fatigue. His physical examination shows splenomegaly. His laboratory results are as follows:
The patient is transfused several units of packed red blood cells without significant correction of his anemia, and instead, his pancytopenia worsens. Peripheral smear shows pancytopenia without blasts, tear drop cells, or dysplasia. A bone marrow biopsy demonstrates the following:
- Aplastic anemia
- Acute promyelocytic leukemia
- Myelofibrosis
- Hemophagocytic lymphohistiocytosis
- Myelodysplastic syndrome
Explanation
The most likely diagnosis is hemophagocytic lymphohistiocytosis (HLH). The patient fulfills at least five of the main nine diagnostic criteria of HLH including fever, splenomegaly, cytopenia, elevated ferritin, low fibrinogen, and evidence of hemophagcytosis on bone marrow, as demonstrated in the pictures that a histiocytes engulfing a nucleated red cell (Figure 1) and a neutrophil (Figure 2).
Myelofibrosis can be associated with splenomegaly, but is less likely here since no marrow fibrosis or tear drop cells reported. Myelodysplastic syndrome is a possible cause of pancytopenia, but no dysplasia was noted on peripheral smear or in the bone marrow. Acute promyelocytic leukemia can be associated with DIC and low fibrinogen on presentation, but should have a hypercellular bone marrow with predominance of promyelocytes. Patients with aplastic anemia are found to have profound hypocellular bone marrow, but no hemophagocytes should be found.
Case study submitted by Tzu-Fei Wang, MD, The Ohio State University, Columbus, OH
American Society of Hematology. (1). Case Study: 44-Year-Old Man with Fever, Abdominal Pain, and Pancytopenia. Retrieved from https://www.hematology.org/education/trainees/fellows/case-studies/male-fever-abdominal-pain-pancytopenia .
American Society of Hematology. "Case Study: 44-Year-Old Man with Fever, Abdominal Pain, and Pancytopenia." Hematology.org. https://www.hematology.org/education/trainees/fellows/case-studies/male-fever-abdominal-pain-pancytopenia (label-accessed April 02, 2024).
"American Society of Hematology." Case Study: 44-Year-Old Man with Fever, Abdominal Pain, and Pancytopenia, 02 Apr. 2024 , https://www.hematology.org/education/trainees/fellows/case-studies/male-fever-abdominal-pain-pancytopenia .
Citation Manager Formats
- Case report
- Open access
- Published: 03 November 2022
Aplastic anemia: a new complication in the recent mysterious hepatitis outbreak among children worldwide: two case reports
- Ali Ghanei-Shahmirzadi 1 ,
- Hamid Reihani 1 ,
- Ali Abbasi-Kashkooli 1 ,
- Fereshteh Karbasian 2 ,
- Seyyed Bozorgmehr Hedayati 3 ,
- Mohammadreza Bordbar 3 ,
- Maryam Ataollahi 4 ,
- Seyed Mohsen Dehghani 4 &
- Bita Geramizadeh 5 , 6
Journal of Medical Case Reports volume 16 , Article number: 422 ( 2022 ) Cite this article
3164 Accesses
1 Citations
Metrics details
Recently, an unknown hepatitis outbreak among children has concerned many individuals worldwide. These cases are frequently reported, mainly from Europe and other countries. In this study, we present two similar patients, who, to the best of our knowledge, are the first cases reported in the Middle East (Shiraz, Fars Province, Iran). Unlike in similar cases reported up until 30 April 2022, our patients’ hepatitis eventually resulted in aplastic anemia.
Case presentation
In this study, we present cases of two Iranian boys aged 13 and 8 years with hepatitis of unknown origin who developed aplastic anemia in the course of hospitalization.
Conclusions
Hepatitis-associated aplastic anemia is a well-known immune-mediated form of aplastic anemia that we detected in our patients and treated with immunosuppressive therapy. One patient established a satisfactory response to the treatment, but unfortunately, the other was declared brain dead.
Peer Review reports
Introduction
Since November 2021, there have been reports of an unexplained hepatitis outbreak in several countries. Hepatitis can occur from various causes, including viral infections, autoimmune disorders, toxins, and medications [ 1 ]. In April 2022, 145 children with unknown severe hepatitis were reported in the UK, and nine other cases were reported in Alabama [ 2 , 3 ]. Furthermore, there have been similar reports from Denmark, Spain, Ireland, and Netherlands [ 3 ]. At the same time, two children were referred to our center in Shiraz, Iran with hepatitis manifestations, where further laboratory evaluations ruled out all regular causalities. Besides their hepatitis, we noticed pancytopenia in their complete blood count (CBC) test, which raised suspicion for probable bone marrow disorder. Therefore, a bone marrow biopsy was done, and the results were in favor of aplastic anemia.
Aplastic anemia is a rare but life-threatening condition in which bone marrow failure and hypocellularity result in progressive pancytopenia [ 4 , 5 ]. Aplastic anemia has been categorized into acquired and inherited types [ 6 ]. Acquired aplastic anemia is due to an abnormal immune response triggered by different environmental exposures, including drugs, toxins, and viral infections [ 6 ]. It appears that cytotoxic lymphocytes and type I cytokines have a role in autoimmune aplastic anemia, and evidence of low quantity and/or function of T-regulatory cells has been found [ 7 , 8 ]. Since our patients did not have any history of inherited aplastic anemia or any other risk factor for acquired aplastic anemia, we considered hepatitis to be the underlying cause of their aplastic anemia. Therefore, our diagnosis became hepatitis-associated aplastic anemia (HAAA). HAAA is a well-known immune-mediated form of acquired aplastic anemia in which an acute hepatitis episode results in acute or chronic bone marrow failure accompanied by pancytopenia [ 9 , 10 ]. HAAA was first mentioned in two cases in 1955 [ 11 , 12 ], but the number of reports reached more than 200 cases by 1975 [ 13 ]. Later on, it was documented in up to 2–5% of aplastic anemia cases in the West [ 14 , 15 ] and 4–10% of the cases in the Far East [ 16 ]. Consequently, owing to the extent of this new hepatitis outbreak among children and considering the rareness of having two patients in such a short period with this infrequent diagnosis in our center, we informed the healthcare providers of other aspects of this new outbreak in the hopes of achieving a faster diagnosis and, therefore, better outcome and prognosis for the patients. We present two cases of HAAA who were referred to our center.
A 13-year-old Iranian boy came to our pediatric emergency department, a referral center affiliated with Shiraz University of Medical Sciences, with the chief complaint of yellowish skin and two red spots on his right leg. Furthermore, his mother mentioned that he had nosebleeds for a week prior to the admission. He developed icterus 2 months before the admission, and after preliminary laboratory evaluations, which revealed elevated liver enzymes, he was diagnosed with hepatitis. One day before admission, his mother suddenly saw some petechiae-like lesions on his right leg, so she brought the boy to our center. We decided to check CBC and performed liver function tests (LFTs). His preadmission medications included folic acid 5 mg once a day and ursodeoxycholic acid 300 mg twice a day. Physicians had prescribed these drugs because of his previous hepatitis diagnosis. On our primary physical examination, his sclera appeared icteric. He had an ulcer on his lower lip. His lungs were clear, and his heart sounds were normal. On abdominal examination, his liver seemed to be slightly enlarged. He also had evidence of ecchymosis on his right leg.
Laboratory investigations revealed pancytopenia on the first CBC [white blood cell count (WBC) 900/µl, hemoglobin (HB) 7.8 g/dl, platelet count (PLT) 4000/µl]. His liver enzymes were elevated [aspartate transferase (AST) 68 U/L, alanine transaminase (ALT) 174 U/L, total bilirubin 1 mg/dL, direct bilirubin 0.29 mg/dL, gamma-glutamyl transpeptidase 26 U/L] as they had been over the past 2 months. Intending to find the cause of his hepatitis, we checked for common viral hepatitis causes, including hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), cytomegalovirus (CMV), and Epstein–Barr virus (EBV), which were all negative. We checked anti-LKM antibody (Ab), anti-dsDNA Ab, cANCA, and pANCA to rule out the possibility of autoimmune disorders, though we did not find any of them to be positive. We also checked ceruloplasmin and serum copper levels to rule out Wilson’s disease, and neither was in favor of Wilson. We checked coronavirus disease 2019 (COVID-19) immunoglobulin (Ig)G and IgM, which were both negative. We also asked about his past drug history (including herbal drugs) and any potential toxin exposure, but we did not find that could have caused hepatitis. Moreover, we performed chromosome breakage test, which was negative, ruling out Fanconi anemia.
In terms of his pancytopenia, we performed bone marrow aspiration and biopsy. The results showed severe hypocellularity (approximately 15%), which was low for his age and suggestive of aplastic anemia. Pictures of bone marrow biopsy and aspiration are shown in Fig. 1 . Immunophenotyping by flow cytometry was done on his bone marrow sample, and there was no evidence of leukemia or lymphoma. Our treatment plan was immunosuppressive therapy (IST). The patient was started on equine antithymocyte globulin (ATG) (40 mg/kg/day for 4 days), prednisolone (0.5 mg/kg/day), and cyclosporine (10 mg/kg/day). After receiving treatment, his CBC stabilized (enough to discharge the patient from hospital with further follow-up scheduled) 10 days after IST initiation, and eventually, he was discharged in good condition.
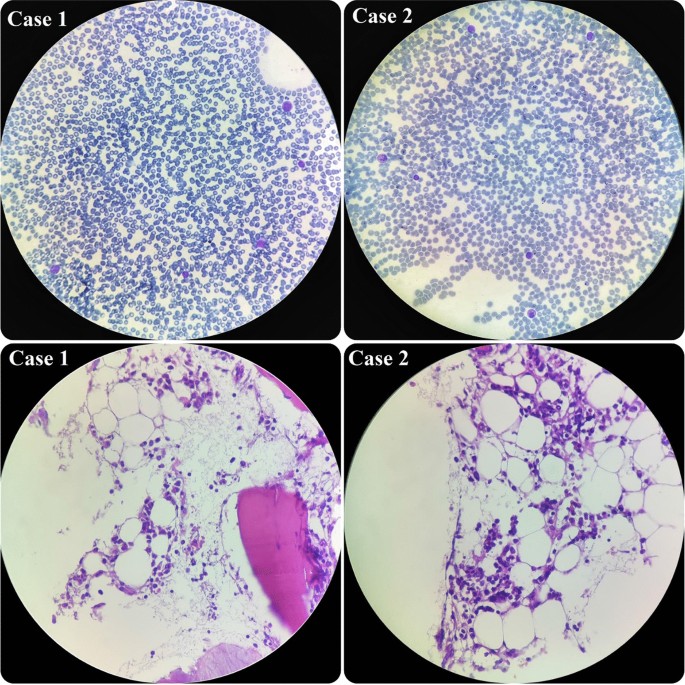
Hypocellular smear from bone marrow aspiration revealing a few scattered myeloid cells and lymphocytes. On bone marrow biopsy, which revealed hypocellularity for his age, intertrabecular marrow spaces were shown to have been replaced by fatty cells beside the presence of some nucleated cells, including lymphocytes, plasma cells, and erythroid cells
An 8-year-old Iranian boy was brought to our emergency department with the chief complaint of yellowish skin and abdominal distension. He was relatively well until 3 weeks prior to admission. His initial symptoms were anorexia, weakness, and fever, which led him to a medical center. Primary laboratory evaluations were done there, and he was diagnosed with fulminant hepatitis on the basis of elevated international normalized ratio (INR). He also had extremely high liver enzymes. At that medical center, he had received supportive care. Then, since our hospital is the referral liver transplant center in Iran, his parents brought him to our emergency department. On initial presentation, he was severely icteric. His abdomen was distended, and ascites and hepatomegaly were observed.
Our initial laboratory investigations showed severe elevation of his liver enzymes (AST 1615 U/L, ALT 1880 U/L, total bilirubin 47 mg/dL, direct bilirubin 22.8 mg/dL). At that time, he also had coagulation disorder [prothrombin time (PT) 21.1 seconds and INR 2.5]. Moreover, we detected anemia and mild leukopenia in his CBC, but his platelet count was normal (WBC 2900/µl, HB 8.3 g/dl, PLT 190,000/µl). We started searching for the cause of his hepatitis. After checking viral and immunological markers, the only noticeable item we found was a positive COVID-19 IgG Ab. Like the previous patient, he did not report any history of exposure to drugs (including herbal drugs) or hepatotoxic toxins. Moreover, Fanconi anemia was ruled out for the patient by negative chromosome breakage test.
During the admission course, his situation improved and his liver enzymes began to decrease, but he suddenly developed petechiae on his left hand. So, we immediately checked the CBC, which revealed severe pancytopenia (WBC 500/µl, HB 7.1g/dl, PLT 20,000/µl). His platelet count dropped drastically. Therefore, we planned for bone marrow aspiration and biopsy, and the result showed severe hypocellularity, approximately 20%, indicating aplastic anemia. Pictures of bone marrow aspiration and biopsy are shown in Fig. 1 .
Since his WBC count was very low, we could not consider a bone marrow transplant, and we decided to try immunosuppressive therapy for him, as the other patient had responded well to it. Unfortunately, after receiving the third dose of ATG, he had an episode of generalized tonic–clonic seizure, and he did not show any response to the treatment until that time. Hence, we stopped the chemotherapy and transferred him to the pediatric intensive care unit (ICU) ward. Unfortunately, he was declared brain dead, due to the low platelet number and coagulopathy. Our patients’ characteristics are presented in Table 1 .
Since January 2022, the world has faced an unknown hepatitis outbreak primarily reported in Europe and the USA, mainly in children under 10 years of age [ 2 , 17 ]. As we have been struggling with COVID 19 during the past 2 years, it is essential to clarify different aspects of this new challenge as soon as possible. Undoubtedly, the most crucial element is to determine the cause, as well as finding proper treatment and identifying the short- and long-term complications of this new hepatitis outbreak. Right now, the leading hypothesis about the source of this outbreak is an adenovirus [ 2 ]. However, it is not yet determined whether we are dealing with a new variant or whether the social distancing in these 2 years resulted in fewer exposures to the virus, making children’s naive immune system more susceptible to the previously existing types. It is noteworthy that adenovirus has been known previously to cause acute hepatitis in immunosuppressive children [ 18 ].
As of 29 April 2022, according to the World Health Organization (WHO) and UK Health Security Agency (UKHSA), there are at least 200 cases of acute hepatitis of unknown origin that have been reported from 11 countries [ 2 , 3 ]. Interestingly, 40 out of 53 patients in the UK and 9 out of 9 cases in Alabama that were tested for adenovirus had a positive result [ 2 , 17 ]. Moreover, the WHO stated that adenovirus had been detected in at least 74 cases [ 3 ]. Unfortunately, owing to the lack of laboratory equipment, we could not confirm adenovirus infection in our patients. However, our second case had a positive COVID-19 IgG test. It should be mentioned that, in the majority of confirmed cases, patients were not infected by the COVID-19 virus or vaccinated with COVID-19 vaccines at the time of developing hepatitis. Therefore, we can argue that there is no relation between this outbreak and COVID-19 infection. However, we should consider the high rate of COVID-19 over recent months, especially in children and particularly in England, which has more cases than other countries. This may result in the presentation of a new hepatitis type or mislead us because of its constant presence during this time. Therefore, it is better to perform further experiments to find more substantial evidence.
Severe aplastic anemia usually develops 2–3 months after acute hepatitis attack in patients with HAAA [ 9 ]. In our patients, the delay between hepatitis attack and aplastic anemia was close to 2 months as well. In both cases, we started immunosuppressive therapy as soon as the diagnosis of aplastic anemia was confirmed, which consisted of a combination of cyclosporine, ATG, and steroids. Previous studies have shown a 30–70% response to immunosuppressive therapy treatment in children with acquired aplastic anemia [ 19 , 20 , 21 ]. We tried the same regimen on our patients, and one of them benefited from this treatment. However, the other patient did not respond well owing to his poor condition, and eventually, he was declared brain dead. Although we used this particular IST regimen in our patients, we recommend to test other accepted regimens as well and compare and analyze the results to identify the best treatment.
Hepatitis-associated aplastic anemia (HAAA) is a well-known immune-mediated form of aplastic anemia that we detected in our patients. To the best of our knowledge, our study is the first to report the co-occurrence of aplastic anemia with the recent unknown outbreak of hepatitis among children. Therefore, we recommend being alert to hepatitis cases that develop signs and symptoms of pancytopenia, and performing further follow-ups for early diagnosis of potential aplastic anemia.
Availability of data and materials
Data of the patient can be requested from the authors. Do not hesitate to get in touch with the corresponding author if you are interested in these data.
Abbreviations
- Hepatitis-associated aplastic anemia
Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394(10201):869–81.
Article CAS PubMed Google Scholar
Increase in hepatitis (liver inflammation) cases in children under investigation https://www.gov.uk/government/news/increase-in-hepatitis-liver-inflammation-cases-in-children-under-investigation#full-publication-update-history : UK Health Security Agency; 2022.
Multi-Country—Acute, severe hepatitis of unknown origin in children https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON376 : WHO; 2022.
Miano M, Dufour C. The diagnosis and treatment of aplastic anemia: a review. Int J Hematol. 2015;101(6):527–35.
Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–19.
Article CAS PubMed PubMed Central Google Scholar
Altay D, Yılmaz E, Özcan A, Karakükçü M, Ünal E, Arslan D. Hepatitis-associated aplastic anemia in pediatric patients: single center experience. Transfus Apheres Sci. 2020;59(6): 102900.
Article Google Scholar
Solomou EE, Rezvani K, Mielke S, Malide D, Keyvanfar K, Visconte V, et al . Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007;110(5):1603–6.
Shi J, Ge M, Lu S, Li X, Shao Y, Huang J, et al . Intrinsic impairment of CD4+ CD25+ regulatory T cells in acquired aplastic anemia. Blood. 2012;120(8):1624–32.
Gonzalez-Casas R, Garcia-Buey L, Jones E, Gisbert J, Moreno-Otero R. Systematic review: hepatitis-associated aplastic anaemia—a syndrome associated with abnormal immunological function. Aliment Pharmacol Ther. 2009;30(5):436–43.
Rauff B, Idrees M, Shah SAR, Butt S, Butt AM, Ali L, et al . Hepatitis associated aplastic anemia: a review. Virol J. 2011;8(1):1–6.
Fomina L. K voprosu obizmenenii krovetvoreniia pri zabolevaniiakh pecheni. Sov Med. 1955;19(6):28–31.
CAS PubMed Google Scholar
Lorenz E, Quaiser K. Panmyelopathy following epidemic hepatitis. Wien Med Wochenschr. 1955;105(1):19–22.
Hagler L, Pastore RA, Bergin JJ, Wrensch MR. Aplastic anemia following viral hepatitis: report of two fatal cases and literature review. Medicine. 1975;54(2):139–64.
Böttiger L, Westerholm B. Aplastic anaemia: aplastic anaemia and infectious hepatitis. Acta Med Scand. 1972;192(1–6):323–6.
PubMed Google Scholar
Mary J, Baumelou E, Guiguet M. Epidemiology of aplastic anemia in France: a prospective multicentric study. Blood. 1990;75(8):1646–53.
Young NS, Issaragrasil S, Chieh CEW, Takaku F. Aplastic anaemia in the Orient. Br J Haematol. 1986;62(1):1–6.
Stubblefield W. Investigations of nine young children with adenovirus are underway www.alabamapublichealth.gov2022 .
Matoq A, Salahuddin A. Acute hepatitis and pancytopenia in healthy infant with adenovirus. Case Rep Pediatr. 2016;2016.
Locasciulli A, Oneto R, Bacigalupo A, Socié G, Korthof E, Bekassy A, et al . Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92(1):11–8.
Osugi Y, Yagasaki H, Sako M, Kosaka Y, Taga T, Ito T, et al . Antithymocyte globulin and cyclosporine for treatment of 44 children with hepatitis associated aplastic anemia. Haematologica. 2007;92(12):1687–90.
Jeong DC, Chung NG, Cho B, Zou Y, Ruan M, Takahashi Y, et al . Long-term outcome after immunosuppressive therapy with horse or rabbit antithymocyte globulin and cyclosporine for severe aplastic anemia in children. Haematologica. 2014;99(4):664.
Download references
Acknowledgements
The authors are grateful to the patients and their parents for their participation in the current study.
Author information
Authors and affiliations.
Student Research Committee, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
Ali Ghanei-Shahmirzadi, Hamid Reihani & Ali Abbasi-Kashkooli
Department of Pediatric Gastroenterology, Shiraz University of Medical Sciences, Shiraz, Iran
Fereshteh Karbasian
Hematology research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Seyyed Bozorgmehr Hedayati & Mohammadreza Bordbar
Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Maryam Ataollahi & Seyed Mohsen Dehghani
Shiraz Transplant Research Center (STRC), Shiraz University of Medical Sciences, Shiraz, Iran
Bita Geramizadeh
Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran
You can also search for this author in PubMed Google Scholar
Contributions
SD, FK, MA, BG, and MB designed the study and revised the manuscript. HR, AG, SH, and AA were in charge of collecting data and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Fereshteh Karbasian .
Ethics declarations
Ethics approval and consent to participate.
Our study has been reviewed and approved by the Medical Ethics Committee of Shiraz University of Medical Sciences.
Consent for publication
Written informed consent was obtained from the patients’ legal guardians for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ghanei-Shahmirzadi, A., Reihani, H., Abbasi-Kashkooli, A. et al. Aplastic anemia: a new complication in the recent mysterious hepatitis outbreak among children worldwide: two case reports. J Med Case Reports 16 , 422 (2022). https://doi.org/10.1186/s13256-022-03542-0
Download citation
Received : 08 June 2022
Accepted : 25 July 2022
Published : 03 November 2022
DOI : https://doi.org/10.1186/s13256-022-03542-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aplastic anemia
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Aplastic anemia
On this page, coping and support, preparing for your appointment.
The following tests can help diagnose aplastic anemia:
- Blood tests. Normally, red blood cell, white blood cell and platelet levels stay within certain ranges. In aplastic anemia all three of these blood cell levels are low.
- Bone marrow biopsy. A doctor uses a needle to remove a small sample of bone marrow from a large bone in your body, such as your hipbone. The sample is examined under a microscope to rule out other blood-related diseases. In aplastic anemia, bone marrow contains fewer blood cells than normal. Confirming a diagnosis of aplastic anemia requires a bone marrow biopsy.

Bone marrow exam
In a bone marrow aspiration, a health care provider uses a thin needle to remove a small amount of liquid bone marrow, usually from a spot in the back of your hipbone (pelvis). A bone marrow biopsy is often done at the same time. This second procedure removes a small piece of bone tissue and the enclosed marrow.
Once you've received a diagnosis of aplastic anemia, you might need other tests to determine the cause.
More Information
- Bone marrow biopsy
Treatments for aplastic anemia, which will depend on the severity of your condition and your age, might include observation, blood transfusions, medications, or bone marrow transplantation. Severe aplastic anemia, in which your blood cell counts are extremely low, is life-threatening and requires immediate hospitalization.
Blood transfusions
Although not a cure for aplastic anemia, blood transfusions can control bleeding and relieve symptoms by providing blood cells your bone marrow isn't producing. You might receive:
- Red blood cells. These raise red blood cell counts and help relieve anemia and fatigue.
- Platelets. These help prevent excessive bleeding.
While there's generally no limit to the number of blood transfusions you can have, complications can sometimes arise with multiple transfusions. Transfused red blood cells contain iron that can accumulate in your body and can damage vital organs if an iron overload isn't treated. Medications can help rid your body of excess iron.
Over time your body can develop antibodies to transfused blood cells, making them less effective at relieving symptoms. The use of immunosuppressant medication makes this complication less likely.
Stem cell transplant
A stem cell transplant to rebuild the bone marrow with stem cells from a donor might be the only successful treatment option for people with severe aplastic anemia. A stem cell transplant, also called a bone marrow transplant, is generally the treatment of choice for people who are younger and have a matching donor — most often a sibling.
If a donor is found, your diseased bone marrow is first depleted with radiation or chemotherapy. Healthy stem cells from the donor are filtered from the blood. The healthy stem cells are injected intravenously into your bloodstream, where they migrate to the bone marrow cavities and begin creating new blood cells.
The procedure requires a lengthy hospital stay. After the transplant, you'll receive drugs to help prevent rejection of the donated stem cells.
A stem cell transplant carries risks. Your body may reject the transplant, leading to life-threatening complications. In addition, not everyone is a candidate for transplantation or can find a suitable donor.
Immunosuppressants
For people who can't undergo a bone marrow transplant or for those whose aplastic anemia is due to an autoimmune disorder, treatment can involve drugs that alter or suppress the immune system (immunosuppressants).
Drugs such as cyclosporine (Gengraf, Neoral, Sandimmune) and anti-thymocyte globulin suppress the activity of immune cells that are damaging your bone marrow. This helps your bone marrow recover and generate new blood cells. Cyclosporine and anti-thymocyte globulin are often used together.
Corticosteroids, such as methylprednisolone (Medrol, Solu-Medrol), are often used with these drugs.
Although effective, these drugs further weaken your immune system. It's also possible for anemia to return after you stop these drugs.
Bone marrow stimulants
Certain drugs — including colony-stimulating factors, such as sargramostim (Leukine), filgrastim (Neupogen) and pegfilgrastim (Neulasta), epoetin alfa (Epogen/Procrit), and eltrombopag (Promacta) — help stimulate the bone marrow to produce new blood cells. Growth factors are often used with immune-suppressing drugs.
Antibiotics, antivirals
Having aplastic anemia weakens your immune system, which leaves you more prone to infections.
If you have aplastic anemia, see your doctor at the first sign of infection, such as a fever. You don't want the infection to get worse, because it could prove life-threatening. If you have severe aplastic anemia, your doctor might prescribe antibiotics or antiviral medications to help prevent infections.
Other treatments
Aplastic anemia caused by radiation and chemotherapy treatments for cancer usually improves after those treatments stop. The same is true for most other drugs that induce aplastic anemia.
Pregnant women with aplastic anemia are treated with blood transfusions. For many women, pregnancy-related aplastic anemia improves once the pregnancy ends. If that doesn't happen, treatment is still necessary.
- Blood transfusion
- Bone marrow transplant
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
If you have aplastic anemia, take care of yourself by:
- Resting when you need to. Anemia can cause fatigue and shortness of breath with even mild exertion. Take a break and rest when you need to.
- Avoiding contact sports. Because of the risk of bleeding associated with a low platelet count, avoid activities that can cause a cut or fall.
- Protecting yourself from germs. Wash your hands frequently and avoid sick people. If you develop a fever or other indicators of an infection, see your doctor for treatment.
Tips to help you and your family better cope with your illness include:
- Research your disease. The more you know, the better prepared you'll be to make treatment decisions.
- Ask questions. Be sure to ask your doctor about anything related to your disease or treatment that you don't understand. It might help you to record or write down what your doctor tells you.
- Be vocal. Don't be afraid to express your concerns to your doctor or other health care professionals treating you.
- Seek support. Ask family and friends for emotional support. Ask them to consider becoming blood donors or bone marrow donors. It might help to talk to others coping with the disease. Ask your doctor if he or she knows of local support groups, or contact the Aplastic Anemia and MDS International Foundation. It offers a peer support network and can be reached at 800-747-2820.
- Take care of yourself. Proper nutrition and sleep are important to optimize blood production.
Start by making an appointment with your primary care doctor. He or she might then refer you to a doctor who specializes in treating blood disorders (hematologist). If aplastic anemia comes on suddenly, your treatment might begin in the emergency room.
Here's some information to help you get ready for your appointment.
What you can do
Make a list of:
- Your symptoms and when they began
- Key personal information, including any recent life changes, such as a new job, particularly one that exposes you to chemicals
- Medications, vitamins and other supplements you take, including doses
- Questions to ask your doctor
Take a family member or a friend with you to your doctor, if possible, to help you remember the information you're given.
For aplastic anemia, questions to ask your doctor include:
- What's the most likely cause of my symptoms?
- Are there other possible causes for my symptoms?
- What's my prognosis?
- What treatments are available, and which do you recommend?
- Are there alternatives to the primary approach that you're suggesting?
- I have another health condition. How can I best manage them together?
- Do you have brochures or other printed material I can have? What websites do you recommend?

What to expect from your doctor
Your doctor is likely to ask you questions, such as:
- Have you had recent infections?
- Have you bled unexpectedly?
- Are you more tired than usual?
- Does anything seem to improve your symptoms?
- Does anything appear to worsen your symptoms?
Feb 11, 2022
- Ferri FF. Anemia, aplastic. In: Ferri's Clinical Advisor 2020. Elsevier; 2020. https://www.clinicalkey.com. Accessed Nov. 21, 2019.
- AskMayoExpert. Aplastic anemia (adult). Mayo Clinic; 2019.
- Peslak SA, et al. Diagnosis and treatment of aplastic anemia. Current Treatment Options in Oncology. 2018; doi:10.1007/s11864-017-0511-z.
- Olson TS. Aplastic anemia: Pathogenesis; clinical manifestations; and diagnosis. https://www.uptodate.com/contents/search. Accessed Nov. 16, 2019.
- Aplastic anemia. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/aplastic-anemia. Accessed Nov. 16, 2019.
- Olson TS. Treatment of aplastic anemia in adults. https://www.uptodate.com/contents/search. Accessed Nov. 16, 2019.
- Aplastic anemia. Aplastic Anemia and MDS International Foundation. https://www.aamds.org/diseases/aplastic-anemia. Accessed Nov. 16, 2019.
- DeZern AE, et al. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematology/Oncology Clinics of North America. 2018; doi:10.1016/j.hoc.2018.04.001.
- Symptoms & causes
- Doctors & departments
- Diseases & Conditions
- Aplastic anemia diagnosis & treatment
- Bone marrow
Associated Procedures
Products & services.
- A Book: Living Medicine
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
CON-XXXXXXXX
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Enter search terms to find related medical topics, multimedia and more.
Advanced Search:
- Use “ “ for exact phrases.
- For example: “pediatric abdominal pain”
- Use – to remove results with certain keywords.
- For example: abdominal pain -pediatric
- Use OR to account for alternate keywords.
- For example: teenager OR adolescent
- IN THIS TOPIC
Patient-specific Issues in Evaluation and Management
General trends in evaluation and management, aplastic anemia: management of pediatric patients.
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
- Open the PDF for in another window
Eva C. Guinan; Aplastic Anemia: Management of Pediatric Patients. Hematology Am Soc Hematol Educ Program 2005; 2005 (1): 104–109. doi: https://doi.org/10.1182/asheducation-2005.1.104
Download citation file:
- Ris (Zotero)
- Reference Manager
Aplastic anemia is a term describing the common findings of pancytopenia and marrow hypoplasia arising from a variety of disease states, including acquired aplastic anemia and a variety of congenital marrow failure states. The management of children with these disorders has been confounded by difficulties of diagnosis. The availability of molecular testing has assisted in partial resolution of this problem but has raised new issues, such as the potential of genetic predisposition and the management of asymptomatic individuals with molecular markers. Longitudinal data from large cohort studies and disease registries are providing a rational basis for making more informed treatment decisions for children with these disorders. In particular, the ability to subset patients more accurately has improved triage of treatments. Approaches to hematopoietic stem cell transplantation (SCT), using both conventional and alternative donors, are changing rapidly, and the long-term sequelae of newer approaches are not entirely clear. Improved diagnosis and longer survival have fostered an understanding of the multidisciplinary approach necessary to manage both the underlying problems and the significant sequelae of treatment in both acquired and congenital disease.
Bone marrow failure syndromes encompass a number of moderately well described entities, defined largely by clinical presentation rather than results of specific testing, that share the common findings of peripheral blood cytopenia in the setting of marrow hypoplasia. While an increasing number of specific genetic abnormalities have been associated with different congenital marrow failure syndromes over the past few years, only a proportion of patients within each congenital disease category have the mutations described. This suggests both that many other mutations remain to be identified and that many combinations of events, genetic and environmental, can combine to yield similar clinical syndromes. Nonetheless, the availability of such “genetic testing” has revealed increasing numbers of individuals who by clinical criteria appear to have idiopathic aplastic anemia (AA) and appear phenotypically normal yet have molecular hallmarks of congenital marrow failure syndromes. 1 , 2 The issues of misdiagnosis and therefore mismanagement have thus become more prominent. Additionally, management of the asymptomatic individual now presents itself as a clinical problem—one that is largely unexplored—and the question of whether mutations confer predisposition versus establish diagnosis, as well as the role of somatic mutation, will need to be considered in devising management strategies. The following sections present some implications of our altered knowledge for management of children with marrow failure. This discussion is not meant to be inclusive. The first section emphasizes examples bearing on how the rapidly evolving array of diagnostic tests and epidemiologic information might best be incorporated into caring for individual patients while the second provides a brief summary of general trends in treatment.
In an era of molecular diagnostics and computer assistance for evidence-based practice, the roles of history and physical exam are at risk as the basis for clinical guidance. While determining the onset, duration, and severity of signs and symptoms related to poor marrow function may be of diminished value in discriminating diagnoses, a sophisticated history at diagnosis and thereafter remains critical to guiding management. For example, issues related to pubertal progression appear nowhere on the list of differential diagnoses for marrow failure, are unlikely to appear as part of a “clinical practice guideline,” and may not appear to be salient when faced with new onset marrow failure in a child. However, it is exactly this information that will assist in forming a prospective plan for management of menstruation, either suppression of menses in anemic and thrombocytopenic post-menarchal females or prospective counseling for pre-menarchal but clearly pubertal female patients and their families. To extend this example, the same information about pubertal status will guide thinking regarding potential sperm banking for pubertal male patients who may go on to receive chemotherapy such as cyclophosphamide or undergo hematopoietic stem cell transplantation (SCT) conditioning. As the care plan for aplastic patients with matched siblings may evolve rapidly, prospective recognition of these details and their management are critical. As the patient population is sufficiently rare and each patient sufficiently unique, it is my experience that the responsibility for thinking early and iteratively about the complex subspecialty issues surrounding the marrow failure patient will rest solely on the shoulders of the hematologist. The above is only one of many examples.
Whereas histories attempting to establish drug/toxin exposure may guide management and counseling, time spent attempting to establish an infectious history is unlikely to be of much use in establishing a specific diagnosis or management plan. In contrast, a history and a physical exam guided by the epidemiologic information from increasingly sizable and well-organized databases having entered patients with various congenital marrow failure syndromes 3 , 4 are likely to have management significance above and beyond diagnosis. While a description of similar hematologic or physical findings in family members may help to substantiate or debunk a hypothetical inherited disorder, the now aggregated patient data demonstrate that the concordance of clinical phenotype and disease severity within families, including those with the same genetic mutation, may be quite limited. 5 , – 7 The extended family may contain individuals with characteristic physical findings but no known hematologic disease and vice versa. Indeed, the effects of variable penetrance as well as the influence of other non-disease specific genes on the hematologic and nonhematologic expression of these syndromes appear to be significant.
Given the still incomplete tool kit of diagnostics to determine with certainty the patient with idiopathic versus congenital marrow failure as well as the divergence in treatment options, tolerability of treatment, and the open-ended questions relating to management of previously undiagnosed family members, all the potential avenues of ascertainment should be explored. The family history for leukemia should be extended to include a family history of osteogenic sarcoma, squamous cell carcinoma of head and neck and/or genitourinary tract, and breast cancer, excess risks that marrow failure disease-specific registries have helped to define. 5 , 8 , – 10 In addition to the alteration in breadth of the family history, however, the most critical difference over time has been how it will inform management of the entire kindred, ranging from providing appropriate reassurance for those in whom no “genetic” disorder can currently be established to genetic testing and cancer surveillance for those with congenital marrow failure.
This broad view of the family as a diagnostic and management “continuum” is one salient difference between management of adult and pediatric marrow failure patients. In part this is driven by the prominence of SCT in the management of children with idiopathic marrow failure 11 as well as its role in the management of those with congenital disease. 12 , 13 An understanding of hematopoietic stem cell donor options is necessary to effectively triage potential therapies for the patient, and thus consideration of the state of health of siblings and other potential family donors should be undertaken earlier than might be the case with adult patients. Early involvement of genetic counseling may be appropriate, particularly because families of pediatric patients are often still establishing their families and there may be ongoing or planned pregnancies that become medically relevant. In an era of pre-implantation genetic diagnosis, this becomes especially important. Parenthetically, the importance of carrier status in donors is incompletely understood for any of the marrow failure states but will doubtless become better appreciated. A recent case report suggests that a carrier for c-MPL mutation in congenital amegakaryocytic thrombocytopenia (CAMT) can successfully serve as a bone marrow donor. 14
While the importance of meticulously examining the patient for stigmata of a congenital syndrome is obvious, the registry and case compilation data have put the problem of unequivocally identifying and initiating treatment for the patient with idiopathic AA in sharp relief. A significant proportion of patients with the inherited syndromes have normal physical exams. 1 , – 3 Therefore, absence of characteristic findings in the patient or family is insufficient to eliminate consideration of congenital syndromes as a cause of marrow failure and should be viewed with caution as sufficient criteria to initiate therapy for acquired AA. In contrast, the presence, type and severity of somatic abnormalities can provide very useful information for management above and beyond diagnosis. For example, a severe somatic phenotype has been associated with shorter time to onset of hematologic abnormalities and subsequent risk for leukemia in patients with Fanconi anemia (FA). 15 These data are likely to become increasingly robust and useful over time and should be used to assist in decision making about treatment initiation, frequency of surveillance and whether and when to consider SCT or participation in relevant phase I studies.
Blood counts at diagnosis and thereafter significantly impact management, acutely and long term. Since the literature of response and prognosis in AA rests on published diagnostic peripheral blood and bone marrow criteria, strict adherence to these standards is important. Although we tend to think of AA as pancytopenia, it is important to recognize that bilineage failure fulfills these criteria and should be evaluated and managed accordingly. In addition to definition, an appreciation of the importance of severity has evolved over time. Severe AA (SAA) is generally defined by the presence of neutrophil counts less than 500/μL and what is often referred to as very severe AA (VSAA) as the presence of neutrophil counts less than 200/μL. 16 Moderate or non-severe refers to marrow failure with less dramatic findings than those noted above, These values have some prognostic value and have been used to analyze results of various treatment strategies. 16 , – 19 Of course, counts vary over time, sometimes rather broadly, and the implications of these swings on expectations of efficacy for potential therapy remain somewhat indeterminate. While immediate SCT from a matched family donor is the treatment of choice for most children with SAA, 11 these counts may guide the triage of alternative therapies for those for whom such SCT is not an option. 20 , 21 For example, although good outcomes reported for patients with moderate AA may encourage a minimalist approach to therapy, the combination of antithymocyte globulin (ATG) and cyclosporine has been demonstrated to be better than that of cyclosporine alone, suggesting that less aggressive approaches may not be prudent. 22 Patients with SAA fare better with combination immunosuppression than with single agents, but the addition of granulocyte colony-stimulating factor (G-CSF) has not further improved overall response or survival in this group. 17 , 18 , 23 The outcome of treatment of young patients with VSAA has variably been worse, equivalent or better than that of children with SAA, 17 , 19 , 24 but certainly results with the use of multi-agent immunosuppression appear favorable enough to encourage use of immunosuppression versus alternative donor SCT.
Use of blood counts in establishing transfusion threshold varies by practitioner. Leukodepletion techniques have decreased rates of allosensitization overall with some consistent findings in AA. 25 However, transfusion-related problems of sensitization, iron overload and infection persist and confound the supportive care of pancytopenia. Prediction of significant hemorrhage is imprecise, and general guidelines for platelet support, in particular, should be constantly reviewed in view of individual history, exam and infectious status. Iron status should be followed and where iron accumulation appears particularly rapid, evaluation for genetic predisposition to iron retention may be helpful. Chelation should be initiated at a trigger ferritin level that is determined relative to the ability of the patient and family to support this treatment. The efficacy and toxicity of newer oral agents should be revisited as more data become available. Persistent neutropenia poses a risk of bacterial and fungal infection. It is not currently standard practice to use prophylactic antibiotics although individual history of specific infection may support subsequent prophylaxis. There is little evidence to guide these decisions. The frequency of Pneumocystis carinii pneumonia (PCP) and significant viral infections in these populations is quite limited. While it seems reasonable to provide PCP prophylaxis for patients receiving multi-agent immunosuppression, neither optimal duration nor efficacy of such therapy is prospectively established.
The bone marrow aspirate provides another readily available guide to management. Subtleties of the various marrow failure states aside, the most important role is in dismissing the presence of malignancy and in establishing the index of suspicion for myelodysplasia (MDS). There is frequently some dyserythropoiesis in patients with marrow failure, regardless of cause, making the always thorny issue of MDS difficult to address. Pediatricians may be less adept than their adult peers in evaluating MDS morphologically because it occurs so infrequently. Even so, patients with clinical idiopathic AA and classic “empty” marrows may have clonal chromosomal findings common to MDS. It is not infrequent for the pancytopenic marrow to yield poor specimens; it is incumbent upon the practitioner to establish whether the laboratory obtained sufficient cells and, within reason, to pursue attempts to get an adequate cytogenetic assessment. The cultural issues of parents and pediatricians around procedures in children are many, but in this case the importance of the information should be the primary driver of practice. Abnormal cytogenetic results will impact on diagnosis and management. SCT is a widely reported therapy for children with MDS; the intensity of conditioning differs for SAA and MDS, depending upon donor selection, with significant consequences regarding potential relapse of MDS. Although some patients with MDS may have hematologic improvement with ATG, 26 , 27 I am unaware of an informative experience in children and this would not be a current standard approach to care. The activity of thalidomide in MDS, and the dramatic effect of treatment with the thalidomide derivative lenalidomide in adults with 5q– MDS, suggest that therapeutic choices for MDS patients will increase. 28 , 29 The tolerability and suitability of newer agents for children, let alone their efficacy in contrast with chemotherapy or SCT, will be important to establish and then incorporate into optimal treatment strategies.
Chromosomal findings also influence the management of patients with congenital diseases. For example, development of genetic and partial trisomies and tetrasomies of chromosome 3q in patients with FA have been associated with particularly rapid, fatal complications. 30 Conversely, the presence of isochromosome 7q in patients with Shwachman-Diamond syndrome (SDS) has been associated with failure to progress to hematologic malignancy. 31 , 32 Patients with SAA and 13q– have been reported to have a high response rate to immunosuppression and low risk of progression to MDS, although the youngest patient reported was 19. 33 While the identification of such specific findings in patients mandates careful reassessment of therapeutic strategies, the implications of other cytogenetic abnormalities are subject to some debate. The observation of “transient” abnormalities has further clouded this area in both acquired and congenital marrow failure management. 32 , 34 However, incorporation of more frequent and more effectively analyzed cytogenetic sampling of hematopoietic cells into routine care is wise, and more standard responses to cytogenetic information in longitudinally followed patients will evolve shortly.
Associations of specific mutations with natural history of patients with congenital disease are also impacting management strategies. After cloning of the first FA gene, FANCC , the International Fanconi Anemia Registry (IFAR) was used to determine that IVS4 or exon 14 mutations defined a particularly high risk group for early onset disease and bad outcome among FANCC patients. 35 The European FA Research Group later studied 245 patients from 179 families in 2000, at a time when only 4 FA genes ( FANCA, FANCC, FANCF and FANCG ) had been cloned. 15 Even so, specific (null) mutations were readily shown to be associated with earlier onset of hematologic abnormalities, higher incidences of leukemia and/or higher incidences of somatic abnormalities. Patients with the biallelic FANCD1 mutations (i.e., BRCA2 ) have particularly early onset of leukemia. 36 Similarly, recent reports have provided helpful data on the incidence, prevalence, time to occurrence and outcome of a variety of malignancies in patients with marrow failure states. 3 , 9 , 10 In some cases, the incidence of solid malignancies, such as squamous cell carcinomas in FA patients, is sufficiently high that routine surveillance seems clearly indicated. 8 , 37 This cancer risk may be modified by treatment choices or sequelae of treatment. For example, secondary malignancies in FA patients and in AA patients appear increased in those having experienced graft-versus-host disease (GVHD). 8 , 12 , 38 It is likely that this sort of data will increasingly drive clinical decision-making.
The above section is intended to convey the message that decision-making for pediatric patients with marrow failure is highly individualized. However, there are some general management precepts related simply to diagnosis per se. Children with SAA or VSAA, like younger adults, appear best served by matched family SCT, which results in 70%–90% chance of long-term survival. 11 , 20 , 21 No alternative treatment comes close to achieving these results. However, the issues of substituting one chronic disease (GVHD) for another (aplasia) remain appreciable; while rates have decreased, acute GVHD grade II–IV and chronic GVHD of any severity still occur in approximately 20%–25% of patients. In general these percentages are lower, but not zero, for younger children. The long-term sequelae include uncertain effects on fertility and some increased risk of second malignancies such as skin and thyroid cancer, although radiation-free regimens commonly used in this setting are associated with high likelihood of preserved fertility 39 and little second malignancy. 38 For the child lacking such a donor, the data cited above support use of multiagent immunosuppression containing at least ATG, steroids and cyclosporine. 19 , – 21 If patients respond, slow weaning of cyclosporine and careful follow-up is advisable as the recurrence rate is substantial. 18 For patients who do not have a clinically satisfactory response, another trial of ATG may be attempted or SCT from a suitably matched unrelated donor (URD) may be considered. Alternative SCT results have improved in recent years. 40 , 41 In my own experience, the likelihood of response to retreatment is less than that which is published and my experience with URD SCT significantly better, but each practitioner will need to weigh the literature against local results and opinions of other consultants in coming to closure with patients and families. While the rate of response to immunosuppression has remained relatively constant since the introduction of multi-agent approaches, there has been significant improvement in alternative SCT outcomes and the most contemporary data should be reviewed. Use of cyclophosphamide absent stem cell support is controversial, 42 , 43 but until fully resolved, this and other possible novel studies should also be discussed with the family. The decision of whether and when to proceed to the curative potential of SCT with its significant and unpredictable side effect profile is complex and depends upon an appropriate review of results and complications by the physician or consultants and subsequent interpretation of that information by patient/family groups with diverse beliefs and risk-taking profiles. In any case, non-transplanted patients require close assessment not only for hematologic status but also for evolution of MDS and other secondary malignancies. 8 , 11 , 38
The generic triage of therapy for patients with marrow failure states is even less orderly. The FA Research Foundation, for example, has sponsored two international consensus conferences to bring multidisciplinary subspecialists together with a view toward promulgating helpful clinical management guidelines (available at www.fanconi.org ). In brief, these guidelines suggest critical hematologic values or findings at which therapy should be initiated and suggest an approach to the triage of therapies. In FA and Diamond-Blackfan anemia (DBA), the excellent results of matched sibling SCT 12 , 13 in alleviating marrow failure mandate early consideration of this treatment where available. However, the sequelae of GVHD and conditioning toxicity in terms of quality of life and second malignancy remain considerable even in this group. 12 , 13 Significant improvements in alternative donor SCT in FA have been reported, but these results are based on relatively few patients with still limited follow-up. 44 Alternative donor SCT results in DBA appear unsatisfactory, but little experience with current regimens is reported. 13 Both related and URD SCT for dyskeratosis congenita (DC) are associated with significant acute and chronic toxicity; similar to the situation with FA, SCT does not abrogate and may even exacerbate the other systemic complications of DC. 45 , 46 Patients with SDS have also had excessive toxicity with SCT, although the outcome may be better for those with AA than those with hematologic malignancy. 31
The role of non-SCT supportive therapies is similar to that in SAA, requiring careful attention to blood products, iron and infectious propensity. This is similarly true for patients with DBA. The consequences of transfusion- and steroid-related toxicity have been clarified and need to be integrated into decision-making and subsequent management. 3 For example, prospective counseling around high-risk activities (e.g., use of sunscreen, sexual transmission of papilloma virus) and the need for cancer screening are an extremely important component of care for patients with FA.
The non-hematological management of congenital marrow failure patients is also being informed by the improved patient recognition and characterization discussed above. Awareness of these non-hematologic issues, many of which are critical for maximal functioning and quality of life, falls squarely upon the hematologist as few other specialists have sufficient exposure to patients with these diagnoses to spontaneously initiate appropriate evaluations or follow-up. Liaisons with other subspecialists, including adult head and neck specialists in the case of DC and FA, is critical in effecting the most appropriate management of these complex patients.
Thus, we are now in an era in which there is a richness of available detail regarding the natural history of patients with increasingly well understood, or at least classifiable, disorders. The availability of longitudinal clinical data for both acquired and congenital disease patients in concert with molecular and cytogenetic information provides an enormous resource that should rationalize the evaluation and management and assist hematologists in advocacy for this very complex patient group.
- Previous Article
- Next Article
Email alerts
Affiliations, american society of hematology.
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
A case-control study of aplastic anemia
Affiliation.
- 1 Department of Epidemiology, Johns Hopkins School of Hygiene and Public Health, Baltimore, MD.
- PMID: 2915573
- DOI: 10.1016/0145-2126(89)90025-8
A case-control interview study of aplastic anemia was conducted to evaluate suspected risk factors. Cases (N = 59) newly diagnosed during 1975-82 at 25 Baltimore area hospitals were compared with 59 individually matched (on age, sex and race) controls selected by random digit dialing. The average educational level was less for cases than controls. The major job-related findings were a significant excess for occupational exposure to paint (OR = 6.1; 95% C.I. = 1.2-29.7), further substantiated by a positive dose-response relationship, although painters were not at excess risk. An increased risk of occupational exposure to viruses (OR = 9.0; 95% C.I. = 0.8-105.6) was noted. Additional evidence implicating viral factors included a significant association with prior history of hepatitis (OR = 9.0; 95% C.I. = 1.0, 84.2) and an elevated risk for pre-diagnostic receipt of blood transfusions (OR = 7.1; 95% C.I. = 0.7-68.4). Risks were not increased for other occupational, residential, personal, or medical treatment exposures or for other viral infections, medical conditions, smoking or alcohol consumption prior to diagnosis. Because of the small number of subjects studied and the multiple comparisons examined, these findings should be interpreted cautiously and confirmation should be undertaken in larger, population-based studies.
Publication types
- Research Support, U.S. Gov't, P.H.S.
- Anemia, Aplastic / epidemiology
- Anemia, Aplastic / etiology*
- Child, Preschool
- Hepatitis / complications
- Middle Aged
- Occupational Diseases / complications
- Retrospective Studies
- Risk Factors
- Transfusion Reaction
Grants and funding
- R01 CA 24757/CA/NCI NIH HHS/United States
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Diagnosis and Treatment of Aplastic Anemia
Scott a. peslak.
1 Division of Hematology and Oncology, Department of Medicine, Hospital of the University of Pennsylvania, Perelman Center for Advanced Medicine, 3400 Civic Center Blvd, Philadelphia, PA 19406, USA
Timothy Olson
2 Comprehensive Bone Marrow Failure Center, Children’s Hospital of Philadelphia, 3615 Civic Center Boulevard, Philadelphia, PA 19104
3 Division of Oncology, Department of Pediatrics, Children’s Hospital of Philadelphia, 3615 Civic Center Boulevard, Philadelphia, PA 19104
Daria V. Babushok
Opinion statement.
Acquired aplastic anemia (AA) is a rare, life-threatening bone marrow failure (BMF) disorder that affects patients of all ages and is caused by lymphocyte destruction of early hematopoietic cells. Diagnosis of AA requires a comprehensive approach with prompt evaluation for inherited and secondary causes of bone marrow aplasia, while providing aggressive supportive care. The choice of frontline therapy is determined by a number of factors including AA severity, age of the patient, donor availability, and access to optimal therapies. For newly diagnosed severe aplastic anemia, bone marrow transplant should be pursued in all pediatric patients and in younger adult patients when a matched sibling donor is available. Frontline therapy in older adult patients and in all patients lacking a matched sibling donor involves immunosuppressive therapy (IST) with horse antithymocyte globulin and cyclosporine A. Recent improvements in upfront therapy include encouraging results with upfront closely matched unrelated donor transplants in younger patients and the emerging benefits of eltrombopag combined with initial IST, with randomized studies underway. In the refractory setting, several therapeutic options exist, with improving outcomes of matched unrelated donor and haploidentical bone marrow transplantation as well as the addition of eltrombopag to the non-transplant AA armamentarium. With the recent appreciation of frequent clonal hematopoiesis in AA patients and with the growing use of next-generation sequencing in the clinic, utmost caution should be exercised in interpreting the significance of somatic mutations in AA. Future longitudinal studies of large numbers of patients are needed to determine the prognostic significance of somatic mutations and to guide optimal surveillance and treatment approaches to prevent long term clonal complications.
Introduction
Aplastic anemia (AA) is a rare, immune-mediated hematopoietic disorder associated with significant morbidity and mortality. In patients with suspected AA, rapid and accurate diagnosis and concomitant supportive care are critical. Historically, immunosuppressive therapy (IST) and bone marrow transplantation (BMT) in eligible patients have been the mainstay of AA treatment [ 1 ]. However, new frontline and salvage therapies are fundamentally changing how we approach therapy of AA, particularly in adult patients [ 2 – 4 ]. In pediatric patients, new transplant strategies and improvements in supportive care have led to greatly improved outcomes and increasing use of BMT in both upfront and refractory settings [ 5 ]. Furthermore, recent recognition of frequent clonal hematopoiesis in AA has changed our understanding of this immune-mediated blood disorder, reframing how we view somatic changes and a diagnosis of myelodysplastic syndrome (MDS) in patients with AA [ 6 , 7 ]. Here, we present a comprehensive review of the diagnosis and treatment of AA, focusing on recent studies.
Clinical presentation and epidemiology
AA should be suspected in patients presenting with pancytopenia and a hypocellular bone marrow. Typical symptoms include fatigue and easy bruising or bleeding; infections may be present, but generally there is no long-standing illness. There is a well-recognized bimodal age distribution with one peak in mid to late childhood and another in the elderly [ 8 ]. The estimated annual incidence of AA is ~2 cases per million in Europe and North America, with a 2–3 fold higher incidence in East Asia [ 8 ]. In ~10% of patients, a history of non-viral hepatitis can precede the onset of AA [ 9 ]; an uncommon association with eosinophilic fasciitis has also been reported [ 10 ]. With rare exceptions, such as chloramphenicol, antiepileptics, and the emerging link to immunotherapies [ 8 , 11 ], a causal relationship to medications or toxins can be difficult to establish.
Diagnosis and severity stratification
When AA is suspected, a comprehensive evaluation should be performed rapidly to exclude other mimicking conditions ( Figure 1 , Table 1 ). A baseline evaluation requires a full history and physical exam, a complete blood count with differential, a blood smear, a reticulocyte count, and a bone marrow aspirate with a core biopsy, with ancillary studies including cytogenetics and fluorescence in situ hybridization (FISH).

Initial screening evaluation of a patient with aplastic anemia is required to document pancytopenia with a hypocellular marrow, followed by testing to exclude alternative diagnoses. Aplastic anemia severity and outcomes of a transplant evaluation factor into determining an optimal treatment strategy. Patients with Severe or Very Severe Aplastic Anemia (SAA/VSAA) 40 years of age or younger with an HLA-matched sibling donor should undergo an evaluation for an allogeneic bone marrow transplant; older patients or patients without an HLA-identical sibling donor should be evaluated for frontline immunosuppressive therapy (IST) with horse ATG and CsA. **Based on recent data showing superior hematologic outcomes with the addition of eltrombopag [ 4 ], addition of 6 months of eltrombopag to standard IST in patients without pre-existing cytogenetic abnormalities can be considered. Cyclosporine A should be continued for ~12 months of therapy, followed by a slow taper to reduce relapse rates. Salvage therapies include alternative transplant modalities and a variety of nontransplant options. AA, aplastic anemia; PNH, paroxysmal nocturnal hemoglobinuria; 6p CN-LOH, copy number-neutral loss of heterozygosity of chromosome arm 6p; alloSCT, allogeneic stem cell transplant; NSAA, nonsevere aplastic anemia; SAA, severe aplastic anemia; VSAA, very severe aplastic anemia. *Cellularity criteria are determined on adequate bone marrow biopsy, and hypoplastic marrow can either be diagnosed on total cellularity or on bone marrow biopsy with <50 percent normal cellularity in which < 30 percent of the cells are hematopoietic. HLA, human leukocyte antigen; alloBMT, allogeneic bone marrow transplant; IST, immunosuppressive therapy; hATG, horse antithymocyte globulin; CsA, cyclosporine A; CR, complete response; PR, partial response; Cy, cyclophosphamide; MRD, matched related donor; MUD, matched unrelated donor ; haplo BMT, haploidentical bone marrow transplant.
A variety of testing modalities in addition to a detailed personal/family history and exposure history are required both in the initial screening evaluation as well as the subsequent exclusion of alternative diagnoses.
CBC, complete blood count; LDH, lactate dehydrogenase; MPN myeloproliferative neoplasm; FISH , fluorescence in-situ hybridization; MDS, myelodysplastic syndrome; HIV, human immunodeficiency virus; EBV, Epstein-Barr virus; CMV, cytomegalovirus; DEB, diepoxybutane; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; IBMF , inherited bone marrow failure; HLH , hemophagocytic lymphohistiocytosis; LGL , large granular lymphocyte.
The search for alternative etiologies ( Figure 1 , Table 1 ) should focus on ascertainment of drug and toxin exposures, signs and symptoms suggestive of autoimmune or rheumatologic diseases, family and/or personal history suggestive of an inherited BMF disorder, infections, and nutritional deficiencies. Exclusion of inherited BMF is particularly relevant in children and younger adults, where, at a minimum, an evaluation should include a detailed family history looking for lifelong cytopenias, congenital anomalies, cancers, and lung and liver pathology; in addition, patients should be screened for Fanconi anemia by testing the patient’s lymphocytes for sensitivity to crosslinking agents and for Dyskeratosis congenita by measuring lymphocyte telomere lengths [ 12 ]. Lymphocyte telomere lengths may also be low in AA, particularly hepatitis-associated AA, requiring careful interpretation [ 13 ]. Additional causes of acquired BMF include autoimmune marrow aplasia due to a clonal T- or NK- large granular lymphocyte (LGL) expansion [ 14 ], which can be evaluated by T cell receptor rearrangement studies paired with lymphocyte flow cytometry. Morphologic and cytogenetic analyses are used to evaluate for hypoplastic MDS [ 15 ], although limited cellularity frequently precludes informative morphology assessment. Because of their association with acquired AA, detection of a paroxysmal nocturnal hemoglobinuria (PNH) clone (seen in up to 50% of AA patients) or copy number-neutral loss of heterozygosity of chromosome arm 6p (6p CN-LOH, seen in about 12% of AA patients) can be helpful in supporting the diagnosis of AA.
Once the diagnostic evaluation is complete, treatment is guided by the AA severity, established by the Camitta criteria ( Figure 1 ) [ 16 , 17 ]. For younger patients with severe aplastic anemia (SAA) or very severe aplastic anemia (VSAA), a transplant evaluation should be rapidly initiated. A referral to a tertiary center that specializes in the care of AA patients should be strongly considered.
Supportive Care
Throughout the diagnostic and treatment process, patients must be provided aggressive supportive care. Generally, restrictive transfusion targets (hemoglobin > 7 g/dL, platelets > 10,000 cells/μL) are preferred, especially in potential transplant candidates, given the risk of alloimmunization and transfusional iron overload [ 18 ]. Irradiated blood products should be used to prevent transfusion-associated graft-versus-host disease (GVHD). Because of the high mortality due to invasive mold infections, particularly Aspergillus species, antifungal prophylaxis with voriconazole or posaconazole should be used in patients with severe neutropenia (absolute neutrophil count < 500 cells/μL) [ 18 ]. Pneumocystis jirovecii pneumonia (PJP) prophylaxis should be used during the period of lymphopenia following ATG therapy, ideally selecting an alternative to trimethoprim-sulfamethoxazole because of its myelosuppressive effects. Antimicrobial prophylaxis with quinolone antibiotics in patients with VSAA can reduce the risk of gram-negative sepsis, but routine use of prophylactic antibiotics in patients with higher neutrophil counts is not advised in order to limit antibiotic resistance. Because granulocyte-colony-stimulating factor (G-CSF) does not improve overall survival when added to IST [ 19 , 20 ], routine G-CSF use outside of episodes of febrile neutropenia remains controversial [ 21 ]. The benefits and risks of vaccines in AA also remain controversial due to the risk of immune activation, with some AA guidelines recommending against vaccinations outside of the post-transplant setting [ 21 ].
Transplant-based therapies for SAA/VSAA
Patient selection.
In patients with SAA and VSAA eligible for transplant-based therapy, age remains the major factor predicting survival after matched sibling donor (MSD) allogeneic transplantation. A retrospective analysis from the Center for International Blood and Marrow Transplant Research (CIBMTR) of over 1,300 patients receiving MSD-BMT showed the adjusted 5-year overall survival (OS) of 53% in patients over the age of 40 years, as compared to 82% for patients under 20 years and 72% for patients aged 20–40 years [ 22 ]. The differences were primarily due to increased GVHD, infections, and delayed platelet recovery in the older cohort. In addition, these patients were more likely to have received prior IST, and/or to have additional comorbidities with poorer performance status and a longer interval between diagnosis and BMT [ 22 ]. Although outcomes in older patients transplanted with fludarabine-containing regimens have been more encouraging, these data are limited to retrospective analyses [ 23 ]. Thus, the current standard of care for patients older than 40 years is frontline IST, while BMT is the treatment of choice for children and young adults with SAA who have a MSD ( Figure 1 ).
Donor choice
Historically, frontline transplantation for SAA in patients under 40 years of age has been largely limited to MSD transplants [ 24 , 25 ]. However, a recent retrospective analysis of approximately 1,450 patients with AA transplanted between 2005 and 2009 showed no significant difference in OS between MSD and matched unrelated donor (MUD) transplant, although rates of acute and chronic GVHD were higher with MUD-BMT [ 26 ]. An analysis of 29 pediatric patients treated with Fludarabine/Cyclophosphamide/ATG (FCC) conditioning followed by unrelated donor transplantation showed similar overall and progression-free survival as compared to historical MSD-BMT controls, and superior outcomes compared to IST [ 5 ], suggesting that front-line therapy with MUD-BMT may be considered upfront in selected patients under age 20. Randomized trials are underway to compare outcomes of upfront MUD-BMT versus IST in pediatric patients without a matched sibling donor (Pediatric Blood and Marrow Transplant Consortium and the North American Pediatric Aplastic Anemia Consortium); studies exploring frontline MUD-BMT in adults under 40 are also ongoing (Blood and Marrow Transplant Clinical Trials Network). Pending these prospective studies, IST remains the standard upfront AA therapy in patients without MSD [ 26 , 5 ].
The outcomes of mismatched or haploidentical donor transplantation in AA have also improved. In a prospective multicenter study of 101 AA patients receiving haploidentical transplants in China, 94% of patients achieved successful engraftment with 3-year overall and failure free survival of 89% and 86%, respectively [ 27 ]. A registry-based comparison of upfront haploidentical and MSD transplantation in 158 consecutive SAA patients in China have shown similar high rates of engraftment and OS, but significantly higher rates of grade III–IV acute GVHD (10% versus 1.5%) and chronic GVHD (31% versus 4.4%) for haploidentical transplants [ 28 ]. A more recent study of 16 patients receiving haploidentical or unrelated donor transplants with post-transplant cyclophosphamide showed encouraging results with 100% engraftment and no instances of grade 3 or higher GVHD [ 29 ]. Novel approaches including co-infusion of mesenchymal stem cells [ 30 ] and selective T cell Receptor αβ depletion [ 31 ] are being explored.
Graft source
Bone marrow grafts have been shown to produce superior OS compared to peripheral blood stem cell (PBSC) grafts in both pediatric [ 32 ] and adult [ 33 ] AA patients, due to lower rates of GVHD. More recent efforts to improve outcomes with PBSC showed encouraging results with partial T cell depletion [ 34 ]; larger randomized prospective studies are needed to confirm the efficacy and safety of this approach.
Conditioning Regimens
The standard conditioning regimen for MSD-BMT in younger patients is 200 mg/kg cyclophosphamide with antithymocyte globulin (ATG), with three-year survival rates of 92% [ 35 ]. However, subsequent studies in older transplant recipients (age > 30) did not show a survival benefit when compared to IST [ 36 ]. To reduce toxicity in older patients, newer regimens have incorporated fludarabine with lower-dose cyclophosphamide and with ATG (FCA) or alemtuzumab (FCC), with improved OS [ 37 – 39 ]. A CIBMTR analysis of 833 AA bone marrow transplants evaluated the role of ATG source on transplant outcomes, and demonstrated that rabbit ATG (Thymoglobulin, Sanofi, France) results in lower rates of acute and chronic GVHD for MSD transplants, improves survival, and lowers rates of acute GVHD for MUD transplants [ 40 ]. Conditioning for MUD and haploidentical transplants also includes 200 cGy total body irradiation [ 41 ].
Non-transplant therapy of AA
Immunosuppression.
For patients older than 40 years with newly-diagnosed SAA/VSAA or younger patients without an MSD, immunosuppression with ATG and cyclosporine A (CsA) continues to be the recommended frontline therapy ( Figure 1 ) [ 42 ], offering outcomes comparable to allogeneic BMT with reduced morbidity in older patients [ 43 , 44 ]. Horse ATG is the recommended ATG source, based on a randomized-controlled trial of 120 patients showing a superior overall response (68% compared to 37%) and OS (96% compared to 76%) for horse ATG-based IST compared to rabbit ATG-based IST [ 45 ].
Role of Eltrombopag in Frontline Therapy
One of the most promising recent treatments for AA is the oral thrombopoietin (TPO) receptor agonist eltrombopag, previously approved for treatment of chronic idiopathic thrombocytopenic purpura [ 46 ]. Studies in mouse models showed that signaling via the TPO receptor c-mpl is necessary for hematopoietic stem cell maintenance and downstream expansion and differentiation [ 47 , 48 ]. Interestingly, patients with congenital amegakaryocytic thrombocytopenia who have mutations in c-mpl can progress to aplastic anemia, suggesting that a deficiency in TPO signaling may play a role in AA pathogenesis [ 49 ]. Based on these studies, eltrombopag was initially tested as monotherapy in a Phase 2 study of 43 patients with relapsed/refractory SAA with persistent thrombocytopenia following IST [ 2 , 3 ]. The overall response rate after 3–4 months of therapy was 40% (17 of 43). Remarkably, one patient achieved a trilineage response, and 8 patients had neutrophil responses including 4 with severe neutropenia. With long-term treatment, 7 patients achieved trilineage responses. An early signal of increased clonal evolution in this high-risk population was also noted with 19% (8 of 43) patients developing cytogenetic abnormalities during follow-up [ 2 , 3 ].
Based on these encouraging data, a phase 1/2 study evaluated whether the addition of eltrombopag to frontline IST can further improve patient outcomes [ 4 ]. 92 patients were assigned to receive daily eltrombopag in addition to horse ATG and CsA in three separate cohorts starting eltrombopag at either day 0 or day 14 and continuing for 3 or 6 months. Because of the concern for karyotypic evolution [ 2 , 3 ], patients with cytogenetic abnormalities were excluded. The overall response rate at 6 months in the three cohorts ranged between 80–94%, as compared to 66% in the composite historical cohort of 102 patients [ 50 , 45 ]. There was also an encouraging improvement in the rate of complete response, at 36% with eltrombopag as compared to a historical estimate of 10% with standard IST. During an initial phase of the study CsA was discontinued at 6 months, with a relapse rate of 32%–54%, leading to a protocol amendment to extend CsA duration to 2 years [ 4 ]. Encouragingly, the rates of chromosomal aberrations were similar to those in historical controls, ~8% at 2 years of follow-up [ 4 ]. Several prospective randomized trials combining eltrombopag therapy with IST are underway to confirm these findings and to better assess long-term efficacy and safety, particularly clonal evolution ( Table 2 ). However, given these encouraging early results and low observed toxicity, we believe that in selected newly diagnosed SAA/VSAA patients without pre-existing karyotypic abnormalities, addition of six months of eltrombopag to upfront standard IST should be considered.
Eltrombopag in the treatment of aplastic anemia.
SAA, severe aplastic anemia; VSAA, very severe aplastic anemia; NSAA, nonsevere aplastic anemia; PLT, platelet; ORR, overall response rate; CR, complete response; FA, Fanconi anemia; DC, Dyskeratosis congenita , hATG , horse antithymocyte globulin; CsA , cyclosporine A; IST, immunosuppressive therapy; EMBT-SAA, European Society for Blood and Marrow Transplantation Severe Aplastic Anemia; RACE , Randomized multicenter study comparing horse Antithymocyte globuline (hATG) + Cyclosporine A (CsA) with or without Eltrombopag; EMAA, Efficacy and Safety of Eltrombopag + CsA in Patients with Moderate Aplastic Anemia; SOAR, Eltrombopag Combined With Cyclosporine as First Line Therapy in Patients With Severe Acquired Aplastic Anemia.
Cyclosporine Maintenance and Taper
Although there are no definitive data on the optimal duration of CsA, it is clear that early discontinuation leads to a high rate of early relapse. An 11-year follow-up of a randomized trial of horse ATG with or without CsA showed that CsA maintenance delays relapses. 26% of patients required CsA for greater than 6 months due to recurrent cytopenias on discontinuation [ 43 ]. Follow-up of two National Institutes of Health cohorts totaling 102 patients treated with standard IST for whom CsA taper was started at 6 months showed a cumulative relapse rate of 33% at 5 years of follow-up, with a median time to relapse of 2 years [ 51 ]. Compared to historical cohorts that discontinued CsA at 6 months, a tapering regimen delayed relapse by approximately 1 year [ 51 ]. The high relapse rates of 32%–54% in the recent study of eltrombopag added to upfront IST were also attributed to early CsA discontinuation, and were improved with extending CsA to a 2 year maintenance [ 4 ]. There are limited data on the rate of CsA taper, although an analysis of 33 pediatric AA patients suggested that a slower taper of <0.3mg/kg/month may lead to fewer relapses [ 52 ]. Putting these data together, our practice ( Figure 1 ) is to continue full dose CsA, targeting therapeutic CsA trough of 200–300 mcg/L, for approximately 12 months after horse ATG therapy and until achievement of stable and maximally-improved blood counts, at which time we initiate a slow taper with no more than 10% dose reduction at a time over the course of approximately one year.
Treatment of nonsevere aplastic anemia (NSAA)
Unlike the fairly defined guidelines for frontline treatment of SAA/VSAA ( Figure 1 ), the approach to nonsevere aplastic anemia (NSAA) is more nuanced. The natural history of patients with NSAA has been evaluated in several retrospective analyses, showing progression of cytopenias in ~20–67% of NSAA patients [ 53 – 56 ]. Interestingly, NSAA patients managed in more recent years (1997–2002) appear to have worse outcomes with a 56% 10-year survival as compared to 70% in NSAA patients treated between 1991–1996; this was associated with a significantly longer interval from diagnosis to treatment in the more recent cohort (52 versus 102 days) [ 57 ]. Expert AA guidelines recommend treating NSAA patients if they have transfusion dependence or neutropenia [ 21 ]. A prospective randomized study compared horse ATG and CsA to CsA monotherapy in 114 NSAA patients, showing significantly higher overall response (74% versus 46%) in the ATG and CsA arm [ 58 ]. Transfusion independence was achieved in 90% of ATG/CsA-treated patients compared to only 67% of patients receiving CsA alone; 5-year OS was equivalent in both groups [ 58 ]. The outcomes of a recent cohort of 95 Japanese NSAA patients treated with horse ATG and CsA were less encouraging, showing a lower 6-month response rate of 55% and a 10-year failure free survival of 44%, with the majority of patients needing second- and third-line therapies. The median time to initial treatment was 47 days [ 59 ].
More recently, eltrombopag has been proposed as a potential option for NSAA patients, with a number of ongoing studies studying the safety and efficacy of eltrombopag in combination with CsA in NSAA ( Table 2 ) [ 60 ]. Given the excellent tolerability and efficacy of eltrombopag in the relapsed/refractory and first-line SAA/VSAA settings ( Table 2 ) [ 2 – 4 ], we anticipate that eltrombopag-containing regimens would be similarly beneficial in NSAA and may allow for improved outcomes with lower toxicities in this population.
Several other treatments have been investigated in NSAA; however, to date, none have been demonstrated to be superior to standard IST. In 45 patients with NSAA treated with a recombinant humanized anti-IL2 receptor antibody daclizumab, 42% achieved a hematologic response at 3 months [ 61 ], although only 25% achieved transfusion independence at ~5 years of follow-up [ 62 ]. An antihelminthic agent levamisole, associated with immunomodulatory activity, was tested in combination with CsA in 118 Chinese patients with NSAA; the study found a nearly 100% overall response rate in 42 patients with newly-diagnosed NSAA and 87% overall response rate in chronic NSAA [ 63 ], suggesting that CsA combined with levamisole may be a promising therapy to be evaluated in future randomized studies.
Clonal evolution
The improvement in long-term survival of AA patients led to an increased appreciation of the long-term clonal sequelae of AA. ~15% of AA patients treated with IST go on to develop the late complications of MDS and acute myeloid leukemia (AML) [ 64 , 65 , 43 ]. Approximately 10% of AA patients (range 3–26%) develop cytogenetic changes during the course of their disease (reviewed in [ 7 ]), most commonly monosomy 7/del (7q) and trisomy 8, as well as del (13q), and trisomies of chromosomes 6, 15 and 21. In the context of AA, monosomy 7 has been found to correlate with a poor prognosis, including a worse response to IST and increased progression to MDS, while del (13q) and trisomy 8 are associated with an improved response to IST and a better prognosis (reviewed in [ 6 , 7 ]).
Newer techniques combining single nucleotide polymorphism arrays (SNP-A) and next-generation sequencing (NGS) have allowed to more precisely evaluate clonal hematopoiesis in AA. The majority of AA patients, including over 60% of children with AA, develop clonal genetic changes [ 66 , 67 ]. Several recent reviews comprehensively addressed this topic [ 6 , 7 , 68 ]. The most common clonal abnormality in AA is the development of PNH clones, which can be detected by flow cytometry as cells lacking glycophophatidyl-inositol-linked proteins due to a somatic mutation in the PIGA gene [ 69 , 70 ], found in up to 50% of AA patients. The second most common is somatic loss of human leukocyte antigen (HLA) loci, that are detected as either regions of acquired 6p CN-LOH or as inactivating mutations in HLA class I genes in approximately 17% of AA patients [ 71 – 74 ]. Both PNH clones and HLA loss are hypothesized to occur due to immune escape, and, if present, can be helpful in corroborating a diagnosis of AA. The presence of even a subclinical PNH clone has been found to correlate with an improved response to IST [ 75 – 79 , 70 ]. In contrast to PNH, the prognostic impact of somatic HLA loss is less clear [ 71 , 72 ], with emerging data suggesting that HLA loss may be best viewed as a marker of a higher immune pathogenicity of a patient’s inherited HLA alleles [ 74 , 73 ].
Using targeted NGS of genes recurrently mutated in hematologic malignancies, several groups identified somatic mutations in MDS-associated genes in AA, detected in up to a third of adult AA patients [ 80 – 83 , 66 ]. The most commonly mutated malignancy-associated genes in AA are ASXL1 , BCOR / BCORL1 , and DNMT3A [ 66 , 81 , 82 ]. The prognostic implications of somatic mutations in MDS-associated genes are not clear, due to the lack of prospective, long-term studies of large numbers of patients carrying these mutations. Nevertheless, available data suggest that mutations in BCOR and BCORL1 may be predictive of an improved response to IST [ 66 ], and together with mutations in PIGA , comprise a group with “favorable” prognosis [ 66 ]. In the comprehensive study of clonal hematopoiesis in AA by Yoshizato and colleagues, no other genes were identified to have prognostic significance individually, perhaps due to the limited statistical power, although several genes ( DNMT3A, ASXL1, TP53, RUNX1 , and CSMD1 ) were associated with worse OS when analyzed in aggregate [ 66 ]. Putting together the available data, it is clear that the majority of pediatric and adult AA patients develop clonal hematopoiesis, with a large fraction of adult AA patients having detectable somatic mutations in genes that are frequently altered in aging and MDS. However, only ~15% of AA patients progress to the late complications of MDS and leukemia after 10 years of follow-up [ 64 ]. Thus, in the absence of prospective longitudinal studies evaluating the prognosis of AA patients carrying specific MDS-associated somatic mutations, we advise caution when factoring the presence of somatic mutations into therapeutic decisions in this patient population.
Salvage therapy for relapsed and refractory aplastic anemia
Despite overall improvement in AA outcomes, ~33–35% of patients who initially respond to IST will relapse during or after CsA taper, while another 35% will be refractory to frontline IST [ 45 , 1 ]. Most patients (~60–68%) who relapse following an initial response to IST can be salvaged with full-dose CsA monotherapy and/or a second course of IST with rabbit ATG and CsA [ 84 , 52 , 85 , 42 , 86 ] or transplant. Alternatives to ATG and CsA in relapsed disease include alemtuzumab, which, in a single-arm prospective study of 25 patients with relapsed AA, was shown to produce a hematologic response in 56% of patients, with 86% 3-year survival [ 87 ]. Because most patients will respond to a second round of IST [ 84 ], in adults, transplant therapies are usually reserved for relapsed patients who failed an attempt of salvage with a second course of immunosuppression, while excellent outcomes in children with salvage BMT after IST failure make MUD-BMT a reasonable second-line option [ 88 ].
Compared to relapsed AA patients who previously responded to IST, patients with primary refractory AA have worse outcomes, and only ~30% of refractory AA patients can be salvaged with rabbit ATG and CsA [ 84 ]. The failure-free survival in refractory pediatric patients treated with second-line IST can be as poor as 9.5%, as compared to >80% for salvage SCT [ 89 ]. Thus, refractory AA patients should be evaluated for salvage allogeneic transplant options, which may include HLA identical sibling, matched unrelated, or haploidentical bone marrow transplantation, depending on donor availability. Among non-transplant therapies, eltrombopag has hematologic response rates of ~40% in refractory AA, including some trilineage responses, and represents an important option, particularly for older adults or patients who are poor transplant candidates [ 2 , 3 ].
There are several additional second- and third-line treatment options for refractory AA, of which danazol and alemtuzumab are more commonly used. In 48 patients with refractory AA randomized to receive alemtuzumab versus rabbit ATG with CsA, alemtuzumab was comparable to rabbit ATG arm, with a hematologic response of 37% and a 3-year survival of 83% [ 87 ]. Although androgen therapy has not been found to improve survival in combination with first-line IST [ 90 , 91 ], a study of 16 refractory AA patients suggests that androgens may be helpful, particularly in female patients [ 92 ]. Cyclophosphamide in moderate to high doses also has efficacy in refractory AA [ 93 – 95 ], but significant toxicity with prolonged neutropenia and high rates of infectious have largely limited its use [ 96 , 97 ].
AA is a rare, life-threatening, BMF syndrome that requires a systematic and timely approach to diagnosis and treatment. For a younger patient with a MSD, allogeneic BMT remains the standard frontline therapy, while other patients should receive frontline immunosuppression with horse ATG and CsA. Emerging data suggest that addition of eltrombopag to frontline IST can further improve outcomes and that outcomes following upfront MUD BMT may now be equivalent to MSD-BMT, at least in pediatric patients. Long-term prospective studies are underway to confirm the safety and efficacy of these approaches. As the outcomes of MUD and haploidentical transplantation improve and with emergence of eltrombopag as an effective agent in refractory AA, we expect that outcomes of patients with refractory AA will improve. Finally, with the recent findings of frequent clonal hematopoiesis in the majority of AA patients, utmost caution should be exercised in the interpretation of molecular changes which are common in this patient population and, in the absence of long-term prospective studies, do not have well-defined prognostic implications.
Acknowledgments
This work was supported by NHLBI T32 HL007439-38 grant to S.A.P, NHLBI K08 HL122306 to T.O., and NHLBI K08 HL132101-01 and AA & MDS International Foundation Research Grant to D.V.B, and and NIH/NIDDK R24DK103001 for Penn/CHOP AA studies.
Compliance with Ethics Guidelines
Conflict of Interest
The authors have no conflicts of interests to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance

IMAGES
VIDEO
COMMENTS
Patients with aplastic anemia are found to have profound hypocellular bone marrow, but no hemophagocytes should be found. Case study submitted by Tzu-Fei Wang, MD, The Ohio State University, Columbus, OH. Citations. Citations APA. American Society of Hematology. (1). Case Study: 44-Year-Old Man with Fever, Abdominal Pain, and Pancytopenia. ...
References. Aplastic anemia is a disease with a long history. The first case description was published by Paul Ehrlich in 1888, the term "anemia aplastique" originated with Louis Henri Vaquez ...
Introduction. Aplastic anemia (AA) is a rare condition characterized by the combination of hypoplasia or aplasia of the bone marrow and pancytopenia in at least two of the three main lines of cells: red blood cells (RBCs), white blood cells (WBCs), and platelets [].An estimated incidence of this disease is 0.6 to 6.1/million per year with a sex ratio of about 1:1 [].
In a case-based format, this review emphasizes the newer data on molecular (somatic and germline) findings in AA and how they are (or are not) helpful during diagnosis. ... Epidemiology of aplastic anemia: a study of 1324 cases. Hematology. 2020; 25 (1):48-54. [Google Scholar] 10. Mukherjee S, Sekeres MA..
Fanconi anemia is the most common hereditary cause. It presents in the late first decade with pancytopenia, organ hypoplasia, and bone defects including abnormal radii, absent thumbs, and short stature. Seronegative hepatitis is responsible for 5% to 10% of total cases. Telomerase defects are found in 5% to 10% of adult-onset aplastic anemia.
In this study, we present cases of two Iranian boys aged 13 and 8 years with hepatitis of unknown origin who developed aplastic anemia in the course of hospitalization. Hepatitis-associated aplastic anemia is a well-known immune-mediated form of aplastic anemia that we detected in our patients and treated with immunosuppressive therapy.
n engl j med 385;16 nejm.org October 14, 2021 1513 Case Records of the Massachusetts General Hospital lege campus at the start of the coronavirus dis-
Aplastic anaemia is a rare, previously fatal condition with a significantly improved survival rate owing to advances in understanding of the pathophysiology and improved treatment strategies including haematopoietic stem cell transplantation. Although a rare condition, aplastic anaemia continues to present a high burden for affected patients ...
Aplastic Anemia. A plastic anemia is a disease with a long history. The first case description was published by Paul Ehrlich in 1888, the term "anemia aplas-tique" originated with Louis Henri ...
A Young Adult with Aplastic Anemia and Gray Hair. Eva C. Guinan1,2,3,4* and Suneet Agarwal2,3,4,5. 1 Departments of Radiation Oncology and 2 Pediatric Oncology, Dana-Farber Cancer Institute, Boston, MA; 3 Division of Hematology/Oncology, Boston Children's Hospital, Boston, MA; 4 Harvard Medical School, Boston, MA; 5 Harvard Stem Cell ...
Acquired severe aplastic anemia (SAA) is a rare hematologic disease associated with significant morbidity and mortality. Immune destruction of hemopoietic stem cells plays an important role in pathogenesis, as shown by successful treatment with immunosuppressive agents, leading to transfusion independence or complete recovery of peripheral blood counts in a proportion of patients.
Despite the precision of its diagnostic criteria, aplastic anemia has always been a diagnosis of exclusion. No single test allows us to reliably diagnose idiopathic aplastic anemia, but the field has advanced considerably in terms of awareness of and diagnosis of other disorders resulting in a similar or indistinguishable hematologic phenotype. 1-4 Consequently, the diagnostic evaluation has ...
Aplastic anemia has also been associated with pregnancy, which interestingly enough was also the purported cause for the development of AA of the case first described by Dr. Paul Ehrlich. 3, 16, 17 Infection with human immunodeficiency virus (HIV) is associated with the development of AA, although this association is limited to case reports and ...
Treatment. Treatments for aplastic anemia, which will depend on the severity of your condition and your age, might include observation, blood transfusions, medications, or bone marrow transplantation. Severe aplastic anemia, in which your blood cell counts are extremely low, is life-threatening and requires immediate hospitalization.
Aplastic anemia is an historic disease. The first patient was described by the young Paul Ehrlich in 1885, "anemia aplastique" originated with Vaquez in 1904, and its clinical features were described by Cabot and other pathologists in the early 20 th century. In the modern era, an almost uniformly fatal prognosis, mainly for young persons ...
ABSTRACT. Objective: Prevalence of aplastic anemia (AA) is high in the Asian population. This study was done to explore the etiology and association of AA with various socio-economic and environmental factors. Study design and setting: Study included 1324 consecutive AA cases registered at Armed Forces Bone Marrow Transplant Centre Rawalpindi, Pakistan, from March 2001 to August 2016.
January 25, 2023. Researchers reported on a patient with untreated aplastic anemia who presented with classical paroxysmal nocturnal hemoglobinuria (PNH). The case study was published in Cureus. A 29-year-old man presented with a weeklong history of generalized weakness and intermittent nausea. He was previously diagnosed with aplastic anemia ...
The bone marrow failure (BMF) state of aplastic anemia (AA) is marked by cytopenias and ineffective hematopoiesis. 1 AA confers a significant risk for morbidity and death as a result of its progressivenatural history and/or complications related to suboptimal therapy. 2, 3 Without definitive treatment, mortalityfrom severe AA (SAA) approaches 70% at 2 years. 4 Establishing an accurate etiology ...
Case Studies /. Anemia in a 42-year-old woman. Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada) — dedicated to using leading-edge science to save and improve lives around the world. Learn more about the MSD Manuals and our commitment to Global Medical Knowledge.
Aplastic anemia is a term describing the common findings of pancytopenia and marrow hypoplasia arising from a variety of disease states, includin ... In any case, non-transplanted ... an international Fanconi anemia registry study. Blood. 1997; 90: 105 -110. 36. Wagner JE, Tolar J, Levran O, et al. Germline mutations in BRCA2: shared genetic ...
A case-control interview study of aplastic anemia was conducted to evaluate suspected risk factors. Cases (N = 59) newly diagnosed during 1975-82 at 25 Baltimore area hospitals were compared with 59 individually matched (on age, sex and race) controls selected by random digit dialing. The average educational level was less for cases than controls.
1. Introduction. Aplastic anemia (AA) is a syndrome of bone marrow failure, characterized by peripheral blood cytopenias and decreased bone marrow hematopoietic function [Citation 1].The annual incidence of AA is approximately 2-2.3 per 1,000,000 in Europe and North America, while the incidence in Asia is 7.4 per 1,000,000 [Citation 2].In almost all population-based studies the sex ratio is ...
Acquired aplastic anemia (AA) is a rare, life-threatening bone marrow failure (BMF) disorder that affects patients of all ages and is caused by lymphocyte destruction of early hematopoietic cells. Diagnosis of AA requires a comprehensive approach with prompt evaluation for inherited and secondary causes of bone marrow aplasia, while providing ...
Our case study emphasizes the significance of early diagnosis, appropriate genetic testing, and cautious use of chemotherapeutic agents. ... Ashkenazi Jews have a disease carrier frequency of one in 89. 2 It is the most common inherited form of aplastic anemia.
BloodandMarrow TransplantClinica l TrialsNetwor k Re-Admission/Ho nFor WebVersion:1.0; 4.07;05-24 Segment(PROTSEG): DateofAdmission 1-Co 1-Co 1-Co 1-Co 1-Co 1-Co 1-Co 1-Co 1-Co 1-