- Skip to main menu
- Skip to user menu

10 Clinical Research Career Paths
- Industry Features
- General Careers Advice
In 2020, the global Clinical Trials market was estimated at $44.3 billion, and this is expected to grow at an annual rate of 5.7% between 2021 and 2028. The National Institute for Health Research (NIHR) also recorded that between April 2020 and March 2021, 1,390,483 participants took part in Clinical Research across England, which is almost double the numbers from the previous year.
In this article, we look at 10 different career paths within Clinical Research, with an outline of some of the most common responsibilities for each role…
Clinical Trials Manager / Administrator
Clinical Trials Managers / Administrators are responsible for the administrative aspects of clinical trials. Their duties often include:
- Preparing essential documents and ensuring documentation is kept private and confidential.
- Attending safety and study start-up meetings and coordinating investigator meetings.
- Managing clinical trial supplies.
- Reviewing trial protocols and identifying any protocol issues.
- Processing and tracking payments to investigator sites.
More information on the role of a Clinical Trials Manager can be found here.
Clinical Research Associate (CRA)
CRAs are responsible for organising and administering clinical trials and are typically involved in all stages of a trial, from identifying investigator sites to closing down the trial. The responsibilities of a CRA can include:
- Identifying suitable facilities to be used as trial sites and selecting an investigator to be responsible for the site.
- Briefing trial investigators and instructing clinicians on how the trial should be conducted.
- Writing up clinical trial methodologies and designing trial materials.
- Monitoring the progress of clinical trials and preparing final reports.
- Designing and authenticating data collection forms and managing regulatory applications/approvals.
More information on the role of a Clinical Research Associate can be found here.
Clinical Project Manager
Clinical Project Managers are responsible for managing the workers involved in clinical research projects, ensuring protocol compliance whilst coordinating projects to meet clinical objectives. The main responsibilities of a Clinical Project Manager may include:
- Overseeing the enrolment of subjects into clinical trials by assessing the eligibility of potential subjects and tracking the enrolment status of suitable participants.
- Ensuring compliance with protocols and informing investigators of any protocol issues.
- Monitoring study activities to ensure the study remains on schedule and is kept within allocated budgets.
- Maintaining records of study activity, including records of side effect data.
More information on the role of a Clinical Project Manager can be found here.
Pharmacovigilance / Drug Safety Officer
Pharmacovigilance Officers, also known as Drug Safety Officers, are responsible for ensuring that new and existing drugs on the market are safe for patients, and for identifying any issues with these drugs. They may be responsible for:
- Monitoring the effectiveness of new drugs and pharmaceutical products already on the market.
- Monitoring adverse effects to new or existing drugs and flag any early warning signs of these to minimise risk.
- Conducting interviews with patients and healthcare professionals.
- Completing safety update reports and conducting safety audits.
Study Start Up Associate
Study Start Up Associates are integral in making sure that clinical research sites are well prepared to begin a new trial. They can be involved in the following:
- Executing start-up activities before site activation including preparing consent forms, identifying new investigator sites, allocating study budgets, and supporting patient recruitment and retention.
- Ensuring physicians working at research sites are prepared to begin trials.
- Obtaining appropriate ethics and regulatory approvals and ensuring research operations comply with protocols.
- Analysing study start-up metrics to ensure efficiency and identifying areas for development, including in terms of start-up timelines.
More information on the role of a Study Start Up Associate can be found here.
Clinical Research Nurse
Clinical Research Nurses help to improve patient care by supporting patients through their treatment, ensuring they are both safe and fully informed of the study activities. Some of their main responsibilities could include:
- Helping to develop new treatments and care pathways for patients.
- Aiding data collection activities.
- Ensuring patients give full consent prior to being enrolled in clinical trials and making sure patients fully understand all aspects of the study before doing so.
- Assisting the principal investigator with pre-study preparation and study start-up activities, including preparing protocols for regulatory and ethical approval, and attending investigator meetings.
- Arranging appointments for potential and enrolled trial participants.
More information on the role of a Clinical Research Nurse can be found here.
Clinical Research Scientist
Clinical Research Scientists are responsible for undertaking medical research in research labs to find more effective ways of diagnosing and curing a variety of illnesses. They may also be responsible for:
- Interacting with patients taking experimental treatments to understand the effectiveness of these treatments and to investigate new ways of improving their wellbeing.
- Working with other medical staff to advise on how to use products and equipment already on or coming to the market.
- Analysing data to further develop treatments and test any new methods of diagnosis and treatment.
Clinical Investigator
Clinical Investigators ensure that the investigation is meeting research expectations and is conducted in line with the investigator statement, investigational plan, and all necessary regulations. By doing so, they protect the welfare of clinical trial participants as well as the integrity of the resulting data. Their responsibilities can include:
- Meeting specific guidelines and/or requirements set by applicable regulatory and ethical bodies.
- Conducting or supervising research to ensure the investigational plan and corresponding study protocols are being followed.
- Notifying relevant bodies of any changes in research activity, including any unanticipated obstacles that may introduce risk to study participants.
- Ensuring informed consent has been obtained from all participants.
- Maintaining records of the clinical studies and preparing reports to be sent to investigation sponsors and other relevant bodies.
Patient Recruitment Specialist
Patient Recruitment Specialists are responsible for recruitment-related activities. Their main responsibilities include:
- Recruiting participants in line with protocol-specific inclusion and exclusion criteria.
- Tracking recruitment progress and developing new and existing recruitment strategies.
- Contacting potential participants to assess eligibility and to schedule site visits.
- Ensure patient information is accurately collected and entered into the relevant database and is protected.
Biostatistician
Biostatisticians provide statistical support to clinical studies and work across all study phases. Typically, their work can include:
- Obtaining clinical data from the Clinical Data Manager to undertake necessary statistical analyses. Interpreting the meanings of statistical outputs resulting from different analyses.
- Assisting the Clinical Trial Manager in writing up the final technical paper for the study, sharing findings from statistical analyses.
- Analysing safety and efficacy data and applying statistical methods to develop the science of data analysis.
More information on the role of a Biostatistician can be found here.
Current Opportunities in Clinical Research…
Take a look at current opportunities in Clinical Research here and set up job alerts to be notified of the latest opportunities in the industry.
* Article updated March 2024
Related links
- Jobs in Clinical Research
- More Careers Advice
Share this article
Related articles

Voydeya approved in the EU as add-on treatment to ravulizumab or eculizumab for adults with the rare disease PNH who have residual haemolytic anaemia

Vertex and TreeFrog Therapeutics Announce Licensing Agreement and Collaboration to Optimize Production of Vertex’s Cell Therapies for Type 1 Diabetes

European Commission Approves Pfizer’s EMBLAVEO® for Patients with Multidrug-Resistant Infections and Limited Treatment Options
Latest articles, about vectura.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, what is clinical research.
Clinical research occurs in many formats and can involve anyone. Learn how you can participate and contribute to medical advances.
This page last reviewed on September 29, 2016
Connect with Us
- More Social Media from NIH

News & information
Keep up with the latest from r&d partners, 10 clinical research career paths and progression opportunities, felicia rodriguez.
- October 30, 2023
Clinical research careers contribute to the development of safe and effective treatments and therapies for patients. The responsibilities may vary based on the organization, therapeutic area, and specific study requirements, but they all share the common goal of advancing medical science and improving healthcare outcomes.
Progressing in clinical research jobs involves a combination of experience, education, certifications, and networking. All of these are fairly essential for career growth, although the specific path and opportunities may vary depending on your interests, the organization you work for, and the area you specialize in.
In this guide, we explore some of the many careers in clinical research, from entering the profession to potential progression opportunities.
Clinical research career paths
Clinical research careers can follow a range of routes. Here are ten clinical research jobs you can go into, along with their responsibilities.
1. Clinical research coordinator (CRC) An entry-level role, CRCs assist with patient recruitment, obtaining informed consent, data collection, and ensuring protocol adherence. They coordinate study visits, maintain documentation, and communicate with investigators.
2. Clinical research associate (CRA) Entry-level clinical research associate jobs involve monitoring clinical trial sites, verifying data, ensuring regulatory compliance and study protocols, and assessing the safety and wellbeing of study subjects.
3. Clinical trial manager Clinical trial managers oversee all operations of a clinical trial, from study initiation to close-out. They manage budgets, timelines, and teams of CRAs, ensuring that trials are executed successfully.
4. Clinical project manager Clinical project managers manage and oversee multiple trials within a program. They collaborate with cross-functional teams, manage resources, and ensure that each phase of a project aligns with organizational goals.
5. Regulatory affairs specialist Regulatory affairs specialists are largely responsible for the administrative side of compliance. They compile, submit, and maintain regulatory documents for approval. You’ll have to stay informed on changing regulations and liaise with regulatory agencies, ensuring that all policies are adhered to.
6. Data manager Data managers manage clinical trial data, overseeing data collection, cleaning, and database management. They ensure data quality and see to it that all standards are followed, working closely with biostatisticians.
7. Clinical research scientist Clinical research scientists design study protocols, collect and analyze data, and interpret results. They also write study reports and publish findings in scientific journals.
8. Medical monitor Medical monitors oversee patient and subject safety during the trial, review adverse events, and make recommendations for study adjustments or halts based on medical knowledge.
9. Clinical quality assurance auditor Auditors conduct regular inspections and audits to adhere to regulations and quality standards. They identify non-compliance issues and recommend corrective actions.
10. Clinical research consultant Consultants provide expert guidance on various aspects of clinical research, including study design, regulatory strategies, and data analysis. They work independently or with organizations to solve complex problems.
Clinical research progression opportunities
To advance in clinical research careers, you can further your education by pursuing advanced degrees. These might include a Master’s in Clinical Research or an MBA. You could also obtain certifications relevant to your role, like the Clinical Research Professional (CCRP) or Project Management Professional (PMP).
Networking can also help you to get ahead by building rewarding relationships, connecting with peers and mentors in the industry and learning from more senior professionals. This could involve attending conferences, joining professional organizations, and asking to shadow leaders.
Here’s more detail on clinical research progression, and areas the above ten roles can move into.
1. Senior CRC or Clinical Research Associate (CRA) Clinical research coordinators (CRCs) can progress to a senior CRC or clinical research associate (CRA). Responsibilities include more independent study management, training junior coordinators, and handling complex trials.
2. Senior CRA or clinical trial manager Clinical research associates (CRAs) can progress to a senior CRA or clinical trial manager position. The duties of senior clinical research associate jobs are focused on starting to mentor junior CRAs. Clinical trial managers take on even more responsibility by overseeing an entire team of CRAs.
3. Clinical project manager or senior clinical trial manager Clinical trial managers can progress to clinical project manager roles, which involve managing the entire project portfolio. Senior clinical trial managers then demonstrate their capabilities by handling significantly more complex trials.
4. Director of clinical operations Clinical project managers can look to become directors of clinical operations. Directors play a pivotal role in overseeing the management and execution of clinical research programs within an organization, including teams of project managers and CRAs.
5. Regulatory affairs manager or director Over time, regulatory affairs specialists might be able to take up a manager position, which would involve handling larger portfolios of products. Eventually, you could become a director, overseeing an entire regulatory department.
6. Senior data manager or clinical data scientist Data managers often move into senior roles, which means managing larger datasets. You could then set your sights on becoming a data scientist, specializing in data analysis.
7. Senior research scientist or director of clinical research With experience, clinical research scientists can aim to become senior scientists. You would take on more significant research projects, possibly with the goal of becoming a director. This would mean running an entire research department.
8. Chief medical officer (CMO) Medical monitors can progress to chief medical officers (CMOs), who are responsible for all medical aspects of clinical research within an organization.
9. Senior auditor or quality assurance manager Later in their careers, clinical quality assurance auditors might become senior auditors, overseeing an audit team. You could then take up a quality assurance manager position, managing the entire quality assurance program.
10. Clinical research consultant As a consultant, your options for progression are slightly different. Rather than looking to move into a different role, the goal is usually to build a larger client base and gain expertise in specific therapeutic areas. Your earnings and reputation can then grow as you become an expert in the field.
Progressing your career with R&D Partners clinical research staffing agency
R&D Partners are dedicated to helping you excel in the rewarding field of clinical research. As experienced clinical research job recruiters, we understand that a rewarding career in this industry can shape the future of healthcare, making a positive impact on people’s lives.
Whether you’re looking for entry-level clinical research associate jobs or senior leadership roles, our goal is to provide you with insights, strategies, and guidance to chart your path to success in this ever-evolving industry. As partners to many leading life science organizations on the east and west coast, we can bring you exclusive career opportunities not available anywhere else.
Contact our friendly team to discuss your career options, or browse our current opportunities in clinical research careers.
Our global FSP & strategic staffing firm specializes in Scientific, Clinical Research, and Engineering.
Explore our top job opportunities.
We offer life sciences niche functional service provider & strategic staffing solutions to help you reach your goals.

Clinical Research
Careers at iqvia.
Drive the evolution of clinical development.
Not ready to apply? Join our Global Talent Network .
Make an impact on patient health
We are taking clinical research to the next level. Leverage data, in-house technologies and analytics to enable evidenced-based solutions that will help reimagine clinical development and improve patient outcomes.
Joining IQVIA means unlocking access to supportive leadership, a wide variety of career opportunities and technology-enabled resources that make doing your job more efficient. Here, you'll gain a sense of pride that comes with being part of something bigger than you ever imagined.
Featured Clinical Research Areas
Monitoring careers.
Our clinical research associates play a vital role in driving the evolution of clinical development. They bring passion, ambition and a deep level of expertise that’s used to help solve complex clinical issues while ensuring adherence to regulations and sponsor requirements.

Clinical Operations Careers
From strategy development to trial execution to regulatory management, embark on a fulfilling career on IQVIA’s Clinical Operations team. Pair your drug development process expertise and our in-house technologies to deliver best-in-class customer and site experiences, and more notably, make a meaningful impact on improving patient lives.

Clinical Project Management & Leadership Careers
Play a pivotal role in driving clinical trial delivery through right first-time behavior and risk-based project management with a career in Clinical Project Management / Leadership at IQVIA. With a steadfast focus on quality, meticulous financial control and strong communication skills, you’ll serve as a trusted liaison between IQVIA and our customers. Spearhead medical breakthroughs that will leave a lasting impact on patients around the globe.

Clinical Data Management Careers
Transform clinical trial efficiency and deliver medical breakthroughs faster. Clinical Data Management oversees the acquisition, validation and analysis of complex clinical trial data, ensuring its accuracy and compliance with regulatory guidelines. Put your passions to work with a career contributing to life-changing research that positively impacts patient outcomes and shapes the future of healthcare.
Statistical Services Careers
A Statistical Services career means harnessing the power of diverse datasets and strategic methodologies to unlock new possibilities for clinical trials. Working cross-functionally with teams around the world, you’ll develop innovative statistical strategies and analytical solutions to support evidence-based decision-making and help customers navigate the complexities of drug development and regulatory submissions.

At IQVIA, I always value our ability to positively impact patient lives, the supportive leadership and the abundance of growth opportunities available. I know my goal of becoming a Regional Head or Alliance Lead is achievable here.

Christy Willetts
Associate Director, Clinical Operations
Not only does IQVIA provide us excellent with standard training, but we have resources to help us gain knowledge and soft skills as well as supportive colleagues to help problem solve and navigate the changing day-to-day responsibilities of a CRA. I also love our passion for continuous growth and the ample development opportunities that will enable me a successful future here.

Senior Clinical Research Associate
IQVIA invests in me, awakens my talent, and helps me develop skills for new opportunities. As a Project Leader, I’m building teams and processes to support all stages of the project lifecycle. What makes it all worth it? Everything I do contributes to the improvement of patients’ health and well-being.

Nadezhda Simeonova
Senior Clinical Project Manager
When I started my career at IQVIA, I was impressed by the in-house technology. Ten years later, I’m even more impressed. But for me, it’s the people. I’ve had the opportunity to mentor project leaders as well as be a mentee. Growing with my colleagues makes me feel closer and that we’re all empowering each other to be our best.

Julie Lightfoot
Clinical Project Management Director
What you can expect
Working in Clinical Research at IQVIA, regardless of your role, you will thrive within our dynamic culture and experience:
Professional Development
Work-life balance, supportive leadership, best-in-class training, collaboration, explore clinical research jobs.
- R1412234 Clinical Vendor Program Manager - Novartis Dedicated Learn more Multiple Locations
- R1392915 Clinical Trial Manager (Local) Learn more Multiple Locations
- R1412882 Clinical Project Management Director, CNS, Global Project Leadership Learn more Multiple Locations
Join our Global Talent Network
Let’s stay connected. Sign up to receive alerts when new opportunities become available that match your career ambitions.
Job Category Select a Job Category Administrative Support Advanced Analytics Business Systems Analysis Client Services Clinical Data Management Clinical Operations Clinical Project Management/Leadership Clinical Trial Supply & Logistics Connected Devices Consulting Contract Management Database Management Systems Epidemiology Finance Human Resources Information Security Internships IT Design & Development IT Infrastructure IT Support Lab Science Lab Science Advisors Lab Services Laboratory Laboratory Projects Legal and Regulatory Lifecycle Safety Marketing Medical Medical Affairs Medical Communications Medical Sales & Services Monitoring Patient Centric Services (PCS) Phase 1/Clinic Operations Product Support (Tier 3) Production Project and Program Management QA & Testing Quality Assurance Sales Sales Support Software Development Engineering Statistician Strategic Supplier Services Strategy and Corporate Development Technical Writing
Remote Select... Yes
Areas of Interest
Confirm Email
Search for a location and select one from the list of suggestions. Select a job category from the list of options. Finally, click “Add” to create your job alert. Multiple job alerts may be created by repeating these steps.
Explore life at IQVIA

- Clinical Research Job Opportunities
The road to your future starts here!
To post a job, please click here .
Careers & Services
- Clinical Research Services


Clinical Research Coordinator Associate
🔍 school of medicine, stanford, california, united states.
Clinical Research Coordinator for Cognitive Aging, Dementia, and Parkinson’s disease
Do you want to help Stanford University develop world-leading research to better diagnose and treat people suffering from Parkinson’s disease, Dementia with Lewy Bodies and Alzheimer’s disease?
If you…
- Enjoy working directly with patients and their families.
- Are exceptionally organized and can balance multiple projects at one time.
- Love learning new skills, such as how to administer neurological and memory tests.
- Are a proven quick learner who doesn’t need handholding to get started.
Then we offer…
- The opportunity to contribute to understanding Alzheimer’s disease and Parkinson’s disease.
- Challenging but positive environment where you will always learn new things.
- Teamwork and camaraderie.
- Competitive full-time salary.
- Excellent Stanford benefits.
The Poston Lab recruits and follows older normal adults; older adults with mild cognitive impairment; and patients with Parkinson’s disease, Dementia with Lewy Bodies, Alzheimer’s disease, and related disorders. These studies collect information on memory and other mental abilities, which we link with brain imaging, biochemical and genetic markers, and autopsy results. Most research participants will have Parkinson’s disease, Dementia with Lewy Bodies, Alzheimer's disease, or another neurodegenerative disorder; some participants will be not have any impairment.
The Poston Lab seeks a full-time Clinical Research Coordinator Associate. The desired candidate is self-motivated, detail-oriented, relatively independent, patient, punctual, and conscientious, with excellent interpersonal skills and excellent communication skills in English. Preference will be given to qualified candidates, who are also native/fluent in Spanish (written and verbal). Under the supervision of Dr. Poston and other investigators at the Stanford Alzheimer’s Disease Research Center (ADRC), main duties include in-person and telephone recruitment of research participants; obtaining informed consent; scheduling and coordination of research participant visits; maintaining longitudinal contact with participants by phone, email and other means; data collection (including psychometric data), scoring, and data entry. Training will be provided to administer research questionnaires and administer psychometric tests.
We like working with other smart, motivated, fun people looking to better the lives of our patients through scientific discovery. In addition to submitting your on-line resume, please include a cover letter describing your prior clinical research experience and the top five attributes or experiences that make this the right job for you. For more information about our lab and research, please see http://neurology.stanford.edu/labs/postonlab/ .
Duties include*:
- Serve as primary contact with research participants, sponsors, and regulatory agencies. Coordinate studies from startup through close-out.
- Determine eligibility of and gather consent from study participants according to protocol. Assist in developing recruitment strategies.
- Coordinate collection of study specimens and processing.
- Collect and manage patient and laboratory data for clinical research projects. Manage research project databases, develop flow sheets and other study related documents, and complete study documents/case report forms.
- Ensure compliance with research protocols, and review and audit case report forms for completion and accuracy with source documents. Prepare regulatory submissions, and ensure institutional Review Board renewals are completed.
- Assemble study kits for study visits, monitor scheduling of procedures and charges, coordinate documents, and attend monitoring meetings with sponsors, acting as primary contact.
- Monitor expenditures and adherence to study budgets and resolve billing issues in collaboration with finance and/or management staff.
- Interact with the principal investigator regularly, ensuring patient safety and adherence to proper study conduct.
- Ensure essential documentation and recording of patient and research data in appropriate files per institutional and regulatory requirements.
- Participate in monitor visits and regulatory audits.
*- Other duties may also be assigned.
DESIRED QUALIFICATIONS:
- Excellent verbal and written communication skills in English required.
- Excellent verbal and written communication skills in Spanish desired.
- Prior experience with patients or research subjects is desired. Preference given to applicants with previous experience, particularly clinical research experience, with older adults who may have neurological impairment (e.g., Parkinson’s disease) or cognitive impairment (e.g., Alzheimer’s disease).
- Ability to communicate clearly and empathetically with research participants and their families.
- Strong interpersonal skills, including the ability to work easily with research participants and research team members.
- Strong general computer skills and ability to quickly learn and master computer programs.
- Strong analytical skills and experience with computer spreadsheets and database software.
- Proficiency with Microsoft Office and Excel.
- Ability to work under deadlines with general guidance.
EDUCATION & EXPERIENCE (REQUIRED):
Two year college degree and two years related work experience or a Bachelor’s degree in a related field or an equivalent combination of related education and relevant experience.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
- Strong interpersonal skills.
- Proficiency with Microsoft Office.
- Knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
Society of Clinical Research Associates or Association of Clinical Research Professionals certification is preferred.
PHYSICAL REQUIREMENTS*:
- Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
- Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
- Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
*- Consistent with its obligations under the law, the University will provide reasonable accommodation to any employee with a disability who requires accommodation to perform the essential functions of his or her job.
WORKING CONDITIONS:
Occasional evening and weekend hours.
WORK STANDARDS:
- Interpersonal Skills: Demonstrates the ability to work well with Stanford colleagues and clients and with external organizations.
- Promote Culture of Safety: Demonstrates commitment to personal responsibility and value for safety; communicates safety concerns; uses and promotes safe behaviors based on training and lessons learned.
- Subject to and expected to comply with all applicable University policies and procedures, including but not limited to the personnel policies and other policies found in the University's Administrative Guide, http://adminguide.stanford.edu .
The expected pay range for this position is $31.73 to $36.54 per hour.
Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs.
At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website ( https://cardinalatwork.stanford.edu/benefits-rewards ) provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process.
Consistent with its obligations under the law, the University will provide reasonable accommodations to applicants and employees with disabilities. Applicants requiring a reasonable accommodation for any part of the application or hiring process should contact Stanford University Human Resources by submitting a contact form .
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned.
- Schedule: Full-time
- Job Code: 1013
- Employee Status: Regular
- Requisition ID: 102979
- Work Arrangement : On Site
My Submissions
Track your opportunities.
Similar Listings
School of Medicine, Stanford, California, United States
📁 Research
Post Date: Jan 29, 2024
Post Date: Feb 15, 2023
Post Date: Aug 05, 2022
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs
Clinical Research Coordinator Technician / Assistant
How to apply.
A cover letter is required for consideration for this position and should be attached as the first page of your resume. The cover letter should address your specific interest in the position and outline skills and experience that directly relate to this position.
This position may independently provide study coordination for simple and moderately complex clinical research studies. As a member of a coordination team, this position may help support a portfolio of projects with varying levels of complexity. Mastery of all job duties from the CRC-Assistant position on the Michigan Medicine CRC Career Ladder is required.
Mission Statement
Michigan Medicine improves the health of patients, populations and communities through excellence in education, patient care, community service, research and technology development, and through leadership activities in Michigan, nationally and internationally. Our mission is guided by our Strategic Principles and has three critical components; patient care, education and research that together enhance our contribution to society.
Responsibilities*
Experience as part of a team with all 8 competency domains is expected:
- Scientific Concepts and Research Design
- Ethical Participant Safety Considerations
- Investigational Products Development and Regulation
- Clinical Study Operations (GCPs)
- Study and Site Management
- Data Management and Informatics
- Leadership and Professionalism
- Communication and Teamwork
Supervision Received:
This position receives direct supervision and reports directly to the unit Administrator, CRC-Lead, or CRC-Project Manager.
Supervision Exercised:
Required Qualifications*
Clinical Research Technician
- Associate's Degree in Health Science or an equivalent combination of related education and experience is necessary.
- Minimum 1 year of directly related experience in clinical research and clinical trials is necessary. (Please review SoCRA's Definition of a Clinical Research Professional for qualifying experience prior to applying.) or An advanced degree in a health-related areas such as: Health Sciences, Behavioral Sciences, Public Health, Health Care Administration, Clinical Research Administration, Social Work, Psychology, Epidemiology, Foreign MD. or Minimum 3 years of human subject experience (clinical, lab or health regulations) such as related patient care, related community health and wellness, related clinical information, and research.
Clinical Research Assistant
- High School Diploma or GED
Desired Qualifications*
Bachelor's degree in Health Science or an equivalent combination of related education and experience is desirable. An understanding of medical terminology, experience in a large complex health care setting, ability to effectively communicate with staff and faculty of all levels, and knowledge of university policies and procedures is desirable.

Work Schedule
This position will primarily support work M-F during normal business hours.
Work Locations
Underfill statement.
This position may be underfilled at the CRC-Assistant title based on selected candidates’ qualifications.
Additional Information
Michigan Medicine is firmly committed to advancing inclusion, diversity, equity, accessibility, and belonging, which are core to the culture and values of the Medical School Office of Research. Our community supports recruiting and cultivating a diverse workforce as a reflection of our commitment to serve the diverse people of Michigan and the world. We strive to create a work culture where each team member feels respected, valued, and safe.
Background Screening
Michigan Medicine conducts background screening and pre-employment drug testing on job candidates upon acceptance of a contingent job offer and may use a third party administrator to conduct background screenings. Background screenings are performed in compliance with the Fair Credit Report Act. Pre-employment drug testing applies to all selected candidates, including new or additional faculty and staff appointments, as well as transfers from other U-M campuses.
Application Deadline
Job openings are posted for a minimum of seven calendar days. The review and selection process may begin as early as the eighth day after posting. This opening may be removed from posting boards and filled anytime after the minimum posting period has ended.
U-M EEO/AA Statement
The University of Michigan is an equal opportunity/affirmative action employer.
- About AstraZeneca
Life at AstraZeneca
- Our Locations
- Information Technology
- BioPharmaceuticals
- BioPharmaceuticals R&D
- International
- Enabling functions
- Oncology R&D
- Early Talent
Inclusion & Diversity
- Application Hints & Tips
AstraZeneca in Russia
In Russia, career growth is an important part of your journey with us. Teamwork, determination and continuous innovation are ingrained in our culture, and as you develop in these areas within our dynamic environment, you’ll discover much more about what you can do.
Keyword Search
City, State, or ZIP
At AstraZeneca Russia, we put science at the centre of everything we do. Having a clear focus on patients and an unquestionable passion for technology gives us the grounding we need to set new standards for medicine across Russia and Eurasia. Our 2000 talented colleagues work across 72 cities, including our head office in Moscow and our production site in the Kaluga region. Here, we push boundaries in Oncology, Rare Diseases, and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Join us to be at the forefront of medical science and help patients all across the country.

Did you know? In 2019-2022, AstraZeneca was recognized as the best employer in Russia according to the Top Employers Institute for the highest standards in HR management.
Moscow
With 280 hard-working people in our Moscow head office, situated in the main business district, Moscow City, we deliver results across all our functions – from Commercial and Clinical Research to HR and Business Development. Our modern office environment fosters effective cross-functional collaboration, communication, and exchange of ideas.
With social zones, amphitheater space (which is also used for town hall meetings), coffee points, the city’s biggest Encore Fitness Centre, and even a ballroom, there are many places to bring out the best in one another and spark great ideas. Plus, with our offices situated on the 29 th and 30 th floor of the 49-storey OKO Tower, the views alone are enough to inspire your creative thinking.

Our Kaluga production facility, based in the Vorsino Industrial Park, is a centre of excellence for quality and manufacturing. Here, we produce medicines for the whole of Russia, ensuring that we meet the needs of patients across the region.
We have a strong and respected reputation, with our site obtaining the ‘Certificate of Trust’ from Kaluga Region State Labour Inspection in 2017. This is proven in the way that our teams work together and share their achievements. It’s also proven in the way that we look after our people, offering them a place where they can feel at home. From relaxing lounge zones and a small gym to our canteen, this is an office where people can get to know each other, while enjoying thrilling work every day.

At AstraZeneca we believe in the potential of our people and we’ll develop you beyond what you thought possible. Wherever you’re based, you can expect to develop your career in a vibrant culture that sparks innovation and collaboration.
– Alina Mantseva, Area HR Director Russia & Eurasia
To learn more about our corporate culture and career opportunities, please visit our website .
- Google+
What we offer
To share our appreciation of the value you bring and the ideas you provide, we offer both company-wide and Russia-specific benefits. Globally, we offer internal learning opportunities, structured training and flexible benefits chosen by you. And in Russia, we take the standard work-life balance options to a whole new level. With 33 days annual leave, maternity leave and child care of up to three years and five extra days of overtime leave. And that’s not all:
Award-winning
As well as being a 2019-2022 Top Employer for our ‘people-first’ approach, we’re recognised as a leading company by several other awards bodies for our methods in business and beyond. For 11 years (2011-2021) AstraZeneca was a “Dream Employer” according to Medpred.ru and a TOP-2 among pharmaceutical companies in the HeadHunter employers ranking in 2021 and 2022. AstraZeneca won a number of business awards, including Randstad Award Russia and WOW!HR Award.
Collaborative
Here, collaboration is a big part of our culture, both professionally and socially. We hold annual events, weekly activities, and celebrations. We even encourage colleagues to enjoy flexible hours, with the working day starting between 8am and 10am and finishing between 5pm and 7pm.
As a global company whose purpose is to help people, it’s in our nature to find ways of doing good for the world. As a part of this, we do a lot for charity. Since 2014, AstraZeneca has been implementing a Young Health Program which is aimed at promoting healthy lifestyle among teenagers from orphanages. Since 2021 AstraZeneca has been supporting “Children at Home” Info Centre, set up by “Volunteers to Help Orphans” Fund to provide foster children and their parents with counseling to help them cope with the negative effects of the orphanage experience, improve their mental health and raise chances for a successful future and healthy life.
Featured roles
- Оператор-наладчик Russia
- Менеджер по ИТ поддержке Moscow, Russia
- Территориальный Менеджер по работе с ключевыми клиентами / Красноярск Moscow, Russia
- Старший менеджер по продукту Moscow, Russia
Kaluzhskaya oblast', Russia
1-й Восточный пассаж, 8, Dobrino, Kaluzhskaya oblast', Russia, 249022
Krasnogvardeyskiy lane, Moscow
1st Krasnogvardeyskiy lane 21, bld.1, floor 30, Moscow, Russia, 123112

We’ll keep you up-to-date
Sign up to be the first to receive job updates.
Email Address
Confirm Email
The success of AstraZeneca is founded on innovation, creativity and diversity. Find out more.
We are AstraZeneca, one of the world’s most forward-thinking and connected Biopharmaceutical companies. Find out more.
At AstraZeneca, our purpose is to help patients all over the world by delivering life-changing medicines as one collaborative team. Find out more.

Great culture, great work assignments, supportive management. Rotation opportunity within the company. They value inclusion and diversity.
Skip to Content
Goodbye to noncompete agreements? What the FTC ruling could mean for workers and businesses
- Share via Twitter
- Share via Facebook
- Share via LinkedIn
- Share via E-mail
In a decision that could free millions of workers to quit jobs and join competitors or form their own companies, the Federal Trade Commission (FTC) voted Tuesday to ban noncompete agreements. These contracts, which are widespread throughout industries, prevent employees from leaving a company to join a competitor or from forming a competing firm of their own.

About one in five American workers, from minimum wage employees to CEOs, are bound by noncompetes, according to the FTC. The new regulations, set to take effect in August, would lead to wage increases totaling $300 billion per year, the agency said.
In its ruling , the FTC cited research co-authored by Tony Tong , professor of strategy and entrepreneurship in the Leeds School of Business, which found that not only do noncompete agreements limit worker mobility, they can lead to economic concentration and reduced competition. On the flipside, banning noncompetes should increase innovation and entrepreneurship, since workers and ideas can more easily move among companies.
CU Boulder Today sat down with Tong, also the chair of Leeds’ Strategy, Entrepreneurship and Operations Division, to discuss the impact of the FTC’s decision on employees and businesses.
What does this ruling mean for workers?
It’s a big win, especially for workers who earn a lower salary but have signed noncompete agreements, because their mobility was unnecessarily restrained by such agreements. It’s important to bear in mind that noncompetes are not just used for the highest-paid executives; they’re used very broadly, even in Colorado, which has a relatively relaxed stance.
Unlike highly paid executives who have bargaining power when it comes to negotiating with employers, rank-and-file workers don’t have a lot of choices and often have to sign the agreement to maintain employment, especially after they've turned down other offers. A lot of employees don’t know that they need to sign one until after they’ve accepted a job offer and walked into the workplace the first day. And if you’re an average worker, you can’t walk away at that point.
There’s a small downside, though. When employers are able to enforce noncompete agreements, they should be more willing to invest in training and give employees the knowledge related to the company and the industry. Now they may be more reluctant to do that for new employees, for whom that kind of training is very important. The burden of receiving that training might be shifted a bit more to the employees themselves.
What will the impacts be for businesses?
Certain industries like finance and biotech will be especially unhappy because proprietary information or knowledge is key to their success. Larger firms also don't like it; they want their employees to be with them for a long time. Their argument is that noncompetes protect their proprietary information, and with enforceable noncompetes, they’re more willing to invest in employees.
The FTC’s counterargument is that larger firms could use other means to protect proprietary information like nondisclosure agreements, and every state has a trade secret law. You don’t need to go so far as limiting someone’s mobility to protect your proprietary information.
In general, though, banning noncompete agreements is good news for small businesses. It's much easier for them to hire new employees, and it’s good for entrepreneurship. Venture capital firms seem to be reacting positively because they invest in startups.
Your research explores how noncompete agreements affect the broader economy. What were your findings?
The research, which was led by a then-doctoral student at Leeds, Kenneth Younge (who was a faculty member at Purdue University’s Krannert School of Management when the paper was published), showed that when Michigan accidentally changed its law to enforce noncompetes, the rate of mergers and acquisitions increased.
Acquirers saw companies based in Michigan as more valuable in the short run, because these companies could “trap” their employees for some time. Mergers and acquisitions can lead to market concentration. However, related research also shows Michigan’s noncompete enforcement led many knowledge workers to move to other states later.
Looking ahead, what other outcomes are possible from this ruling?
A lot of organizations are suing the FTC, like the U.S. Chamber of Commerce, but these suits could take a long time to get through the system.
When the ruling goes into effect, I anticipate seeing more employees switching jobs, though not a big surge. They will be less restrained for sure, but people move jobs for many reasons other than noncompetes. Companies, too, will be digesting and taking other measures to retain employees. They can do a lot of other things to protect proprietary information. And even if one or two employees leave, it’s not going to cause a lot of damage to the company unless the whole team is poached.
The FTC’s rule makes sense in my opinion. Noncompetes have often been used by companies strategically for reasons other than protecting proprietary information. And some companies are found to be abusing the use of noncompetes, for example by asking new recruits to sign these agreements only after they have accepted the job and turned down other offers; by using vague language in the agreements to cover unnecessarily broad terms; or by threatening to sue employees even if they leave for good reasons.
Also, at the end of the day, I don't think in our free economy you can limit people's movement. Employees have the right to seek a higher wage and move to the company they want to work for.
Overall, this is good news for seniors graduating from universities. Especially in a place like Colorado—we have a dynamic economy, and startups are emerging every day, creating a diverse set of opportunities young employees should take advantage of to get the experiences that will benefit their careers.
- Business & Entrepreneurship
News Headlines
CU Boulder Today regularly publishes Q&As with our faculty members weighing in on news topics through the lens of their scholarly expertise and research/creative work. The responses here reflect the knowledge and interpretations of the expert and should not be considered the university position on the issue. All publication content is subject to edits for clarity, brevity and university style guidelines .
Related Articles

Venture Partners report highlights growing innovation pipeline, national recognition

US labor market can affect ‘people who are not even here’

Working with Colorado, for Colorado—and its future
- Arts & Humanities
- Climate & Environment
- Education & Outreach
- Health & Society
- Law & Politics
- Science & Technology
Campus Community
- Administration
- Announcements & Deadlines
- Career Development
- Getting Involved
- Mind & Body
Events & Exhibits
- Arts & Culture
- Conferences
- Lectures & Presentations
- Performances & Concerts
- Sports & Recreation
- Workshops & Seminars
Subscribe to CUBT
Sign up for Alerts
Administrative eMemos
Buff Bulletin Board
Events Calendar

An official website of the United States government
Here’s how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Take action
- Report an antitrust violation
- File adjudicative documents
- Find banned debt collectors
- View competition guidance
- Competition Matters Blog
New HSR thresholds and filing fees for 2024
View all Competition Matters Blog posts
We work to advance government policies that protect consumers and promote competition.
View Policy
Search or browse the Legal Library
Find legal resources and guidance to understand your business responsibilities and comply with the law.
Browse legal resources
- Find policy statements
- Submit a public comment

Vision and Priorities
Memo from Chair Lina M. Khan to commission staff and commissioners regarding the vision and priorities for the FTC.
Technology Blog
Consumer facing applications: a quote book from the tech summit on ai.
View all Technology Blog posts
Advice and Guidance
Learn more about your rights as a consumer and how to spot and avoid scams. Find the resources you need to understand how consumer protection law impacts your business.
- Report fraud
- Report identity theft
- Register for Do Not Call
- Sign up for consumer alerts
- Get Business Blog updates
- Get your free credit report
- Find refund cases
- Order bulk publications
- Consumer Advice
- Shopping and Donating
- Credit, Loans, and Debt
- Jobs and Making Money
- Unwanted Calls, Emails, and Texts
- Identity Theft and Online Security
- Business Guidance
- Advertising and Marketing
- Credit and Finance
- Privacy and Security
- By Industry
- For Small Businesses
- Browse Business Guidance Resources
- Business Blog
Servicemembers: Your tool for financial readiness
Visit militaryconsumer.gov
Get consumer protection basics, plain and simple
Visit consumer.gov
Learn how the FTC protects free enterprise and consumers
Visit Competition Counts
Looking for competition guidance?
- Competition Guidance
News and Events
Latest news, williams-sonoma will pay record $3.17 million civil penalty for violating ftc made in usa order.
View News and Events
Upcoming Event
Commissioner bedoya speaks at loyola university school of law’s 24th annual loyola antitrust colloquium.
View more Events
Sign up for the latest news
Follow us on social media
--> --> --> --> -->

Playing it Safe: Explore the FTC's Top Video Game Cases
Learn about the FTC's notable video game cases and what our agency is doing to keep the public safe.
Latest Data Visualization
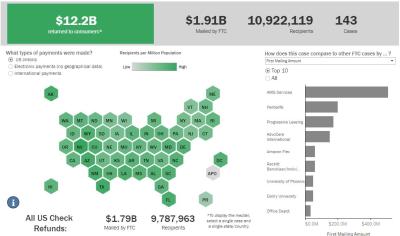
FTC Refunds to Consumers
Explore refund statistics including where refunds were sent and the dollar amounts refunded with this visualization.
About the FTC
Our mission is protecting the public from deceptive or unfair business practices and from unfair methods of competition through law enforcement, advocacy, research, and education.
Learn more about the FTC

Meet the Chair
Lina M. Khan was sworn in as Chair of the Federal Trade Commission on June 15, 2021.
Chair Lina M. Khan
Looking for legal documents or records? Search the Legal Library instead.
- Cases and Proceedings
- Premerger Notification Program
- Merger Review
- Anticompetitive Practices
- Competition and Consumer Protection Guidance Documents
- Warning Letters
- Consumer Sentinel Network
- Criminal Liaison Unit
- FTC Refund Programs
- Notices of Penalty Offenses
- Advocacy and Research
- Advisory Opinions
- Cooperation Agreements
- Federal Register Notices
- Public Comments
- Policy Statements
- International
- Office of Technology Blog
- Military Consumer
- Consumer.gov
- Bulk Publications
- Data and Visualizations
- Stay Connected
- Commissioners and Staff
- Bureaus and Offices
- Budget and Strategy
- Office of Inspector General
- Careers at the FTC
Fact Sheet on FTC’s Proposed Final Noncompete Rule
- Competition
- Office of Policy Planning
- Bureau of Competition
The following outline provides a high-level overview of the FTC’s proposed final rule :
- Specifically, the final rule provides that it is an unfair method of competition—and therefore a violation of Section 5 of the FTC Act—for employers to enter into noncompetes with workers after the effective date.
- Fewer than 1% of workers are estimated to be senior executives under the final rule.
- Specifically, the final rule defines the term “senior executive” to refer to workers earning more than $151,164 annually who are in a “policy-making position.”
- Reduced health care costs: $74-$194 billion in reduced spending on physician services over the next decade.
- New business formation: 2.7% increase in the rate of new firm formation, resulting in over 8,500 additional new businesses created each year.
- This reflects an estimated increase of about 3,000 to 5,000 new patents in the first year noncompetes are banned, rising to about 30,000-53,000 in the tenth year.
- This represents an estimated increase of 11-19% annually over a ten-year period.
- The average worker’s earnings will rise an estimated extra $524 per year.
The Federal Trade Commission develops policy initiatives on issues that affect competition, consumers, and the U.S. economy. The FTC will never demand money, make threats, tell you to transfer money, or promise you a prize. Follow the FTC on social media , read consumer alerts and the business blog , and sign up to get the latest FTC news and alerts .
Press Release Reference
Contact information, media contact.
Victoria Graham Office of Public Affairs 415-848-5121

IMAGES
VIDEO
COMMENTS
Here are four steps you can take to become a researcher: 1. Take relevant classes. Clinical researchers typically pursue an undergraduate degree in biology, chemistry, medicine, psychology or a related field. Many also earn a master's, especially if they hope to work at a university or pharmaceutical company.
Here's a list of steps on how to find clinical research jobs: 1. Obtain qualifications. When looking for a clinical research job, you may have a better chance of receiving an employment offer if you possess sufficient education and certification requirements. Most clinical researchers have a bachelor's degree in life science or a health discipline.
A clinical researcher, also known as a principal investigator (PI), is a medical scientist who evaluates the safety and effectiveness of medications, diagnostic tests, therapeutic treatments and medical devices. The clinical research specialist is the professional who assists the clinical researcher or PI in the day-to-day operations of ...
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
New Jersey. $110,000 - $145,000 a year. Contract. 8 hour shift. Easily apply. Stay current with best practices and research in speech-language pathology. Contracts start in July and Aug and run through the 2024-2025 school year. Today. View similar jobs with this employer.
Clinical Research Nurse. Clinical Research Nurses help to improve patient care by supporting patients through their treatment, ensuring they are both safe and fully informed of the study activities. Some of their main responsibilities could include: Helping to develop new treatments and care pathways for patients. Aiding data collection activities.
To earn this certification, you must have one of the following: At least two years of clinical research experience or 3,500 hours of part-time experience in the past five years. A degree in clinical research and at least one year of full-time experience. A certificate in clinical research, a bachelor's or associate degree in health science ...
From a US national research authority. Clinical research occurs in many formats and can involve anyone. Learn how you can participate and contribute to medical advances. This page last reviewed on September 29, 2016.
Clinical research is a branch of medical research that involves people and aims to determine the effectiveness ( efficacy) and safety of medications, devices, diagnostic products, and treatment regimens intended for improving human health. [1] [2] These research procedures are designed for the prevention, treatment, diagnosis or understanding ...
A clinical research associate (CRA) is a health care or life sciences professional who oversees clinical trials on behalf of pharmaceutical companies, medical research institutes and government agencies.. What You Need to Know to Become a Clinical Research Associate (CRA) CRAs are sometimes called clinical monitors or trial monitors. A key part of the job is to monitor Good Clinical Practice ...
Clinical Research Coordinator. Nellis AFB, NV. $33.00 - $38.00 Per Hour (Employer est.) Easy Apply. 2 years healthcare related field experience in human research, development, test, and evaluation within the last five years). Job Types: Full-time, Contract.….
Clinical Research Associate (CRA) or Study Monitor. Drug Safety Specialist. Biostatistician. Study Manager/Project Manager. Data Scientist, Clinical Data Coordinator, Analyst, or Manager. Quality Assurance Specialist, Auditor. Clinical Business Analyst. Medical Writer. *Requires a medical degree.
Clinical research career paths. Clinical research careers can follow a range of routes. Here are ten clinical research jobs you can go into, along with their responsibilities. 1. Clinical research coordinator (CRC) An entry-level role, CRCs assist with patient recruitment, obtaining informed consent, data collection, and ensuring protocol ...
Washington, DC. Actively Hiring. 2 days ago. Today's top 357,000+ Clinical Research jobs in United States. Leverage your professional network, and get hired. New Clinical Research jobs added daily.
Explore Clinical Research Jobs. R1412057 Vendor Program Manager Learn more Multiple Locations. R1397954 Study Project Lead, Associate Director, Single Sponsor Dedicated Learn more Multiple Locations. R1403611 Senior Study Lead Learn more Multiple Locations. See All Clinical Research Jobs.
Job Title Company / Organization Location More Information; Other: Clinical Research Specialist - Investigator Initiated: Arthrex, Inc. Naples, FL : Details : Other: Clinical Research Technical Writer (Remote) Arthrex, Inc. Naples, FL: Details : Clinical Research Coordinator: Divisional Manager of Clinical Research Operations, Psychiatry Department
Clinical Research Coordinator for Cognitive Aging, Dementia, and Parkinson's disease ... email and other means; data collection (including psychometric data), scoring, and data entry. ... The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a ...
142,262 "clinical Research" jobs available on Indeed.com. Apply to Clinical Research Associate, Clinical Research Coordinator, Site Director and more!
Mastery of all job duties from the CRC-Assistant position on the Michigan Medicine CRC Career Ladder is required. Mission Statement. ... (Please review SoCRA's Definition of a Clinical Research Professional for qualifying experience prior to applying.) or An advanced degree in a health-related areas such as: Health Sciences, Behavioral Sciences ...
Gilead Sciences | 613,369 followers on LinkedIn. Creating Possible | At Gilead, we set - and achieve - bold ambitions to create a healthier world for all people. From our pioneering virology ...
For 11 years (2011-2021) AstraZeneca was a "Dream Employer" according to Medpred.ru and a TOP-2 among pharmaceutical companies in the HeadHunter employers ranking in 2021 and 2022. AstraZeneca won a number of business awards, including Randstad Award Russia and WOW!HR Award.
Apply for Sr CRA/Principal CRA - Southwest job with Thermo Fisher Scientific in Remote, California, United States of America. Clinical Research jobs at Thermo Fisher Scientific
Search jobs in Moscow, ID. Get the right job in Moscow with company ratings & salaries. 3,127 open jobs in Moscow. Get hired!
A competent Clinical Research Associate should be able to perform various duties and responsibilities. Proper fulfillment of a Clinical Research Associate's duties and responsibilities brings success to your company. Clinical Research Associates should assist in organizing and monitoring the different stages of clinical trials.
The Federal Trade Commission decision means employees would have more freedom to job hop while companies may invest less in training, ... The research, which was led by a then-doctoral student at Leeds, Kenneth Younge (who was a faculty member at Purdue University's Krannert School of Management when the paper was published), showed that when ...
The following outline provides a high-level overview of the FTC's proposed final rule:. The final rule bans new noncompetes with all workers, including senior executives after the effective date.
The construction of one of the largest projects of Moscow healthcare - the new medical and diagnostic complex of the MCC named after A.S. Loginov has been completed. Today the center was visited by the Deputy Prime Minister Tatyana Golikova and the Mayor of MoscowSergei Sobyanin. For 3 years, a state-of-the-art clinic was erected on the site of ...
Adolescent Health Provider (NP or PA) Children's Clinic 4.2. Billings, MT 59102. $90,000 - $125,000 a year. Full-time + 1. 24 to 40 hours per week. Monday to Friday + 3. Easily apply. Minimum of 2 years of clinical experience working as a provider in a clinical setting required, preferably in adolescent health or a related field.