- Open access
- Published: 09 May 2019

Alzheimer’s disease: risk factors and potentially protective measures
- Marcos Vinícius Ferreira Silva 1 ,
- Cristina de Mello Gomide Loures 1 ,
- Luan Carlos Vieira Alves 1 ,
- Leonardo Cruz de Souza 2 ,
- Karina Braga Gomes Borges 1 &
- Maria das Graças Carvalho 1
Journal of Biomedical Science volume 26 , Article number: 33 ( 2019 ) Cite this article
86k Accesses
374 Citations
542 Altmetric
Metrics details
Alzheimer’s disease (AD) is the most common type of dementia and typically manifests through a progressive loss of episodic memory and cognitive function, subsequently causing language and visuospatial skills deficiencies, which are often accompanied by behavioral disorders such as apathy, aggressiveness and depression. The presence of extracellular plaques of insoluble β-amyloid peptide (Aβ) and neurofibrillary tangles (NFT) containing hyperphosphorylated tau protein (P-tau) in the neuronal cytoplasm is a remarkable pathophysiological cause in patients’ brains. Approximately 70% of the risk of developing AD can be attributed to genetics. However, acquired factors such as cerebrovascular diseases, diabetes, hypertension, obesity and dyslipidemia increase the risk of AD development. The aim of the present minireview was to summarize the pathophysiological mechanism and the main risk factors for AD. As a complement, some protective factors associated with a lower risk of disease incidence, such as cognitive reserve, physical activity and diet will also be addressed.
Introduction
Alzheimer’s disease (AD) is the most common type of dementia [ 1 ], affecting at least 27 million people and corresponding from 60 to 70% of all dementias cases [ 2 ]. The occurrence of this disease also has a huge impact on life of patient’s family, in addition to a high financial cost to society [ 3 ]. From an anatomopathological point of view, AD is characterized by two prototypical lesions: 1) senile plaques, composed of a nucleus of β-amyloid protein accumulation (Aβ42), as extra-cellular lesions and 2) neurofibrillary tangles composed of phosphorylated tau protein (P-tau) and which are intraneuronal findings [ 4 ]. Deposition of β-amyloid protein can also occur in capillaries walls, arteries and arterioles causing amyloid cerebral angiopathy leading to degeneration of vascular wall componentes and worsening of blood flow, besides predisposing to intraparenchymal hemorrhages [ 5 ].
AD typically manifests through a progressive loss of episodic memory and cognitive function, with later deficiency of language and visuospatial abilities. Such changes are often accompanied by behavioral disorders such as apathy, aggressiveness and depression [ 6 ]. It should be noted that there is an important subgroup of AD patients who do not present a typically amnestic picture, manifesting non-amnestic deficits from the onset of symptoms [ 7 ]. Structural neuroimaging, with a pattern of hippocampal and parietal atrophy in typical cases reinforces the diagnosis [ 8 ]. Patients who meet typical disease characteristics, excluding other causes such as vascular and fronto-temporal dementias, have a probable diagnosis of AD [ 6 ]. Definitive diagnosis of the disease is usually carried out only through postmortem examination, whose purpose is to demonstrate histologically the neurofibrillary tangles and the senile plaques [ 9 ].
Pathophysiology of Alzheimer’s disease
The presence of extracellular plaques of insoluble β-amyloid peptide (Aβ) and neurofibrillary tangles (NFT) of P-tau in neuronal cytoplasm is the hallmark of AD [ 10 ]. Although the mechanisms by which these changes lead to cognitive decline are still debated, these deposits are believed to lead to atrophy and death of neurons resulting from excitotoxicity processes [excessive stimulation of neurotransmitter receptors in neuronal membranes], collapse in calcium homeostasis, inflammation and depletion of energy and neuronal factors. As a result of this process, damage to neurons and synapses involved in memory processes, learning and other cognitive functions lead to the aforementioned cognitive decline [ 11 ].
According to amyloid cascade theory (one of the most accepted theories about AD pathogenesis, although still debated), the cerebral accumulation of Aβ peptide, resulting from the imbalance between production and clearance of this protein, is the main event causing the disease, being other events observed (including the formation of NFT) resulting from this process [ 12 ].
The Aβ peptide, which has 36 to 43 aminoacids, is derived from amyloid precursor protein (APP) enzymatic proteolysis, a physiologically produced protein that plays important roles in brain homeostasis [ 13 , 14 ]. The APP gene is located on chromosome 21, which explains the higher incidence of early-onset AD in individuals with 21 trisomy (Down Syndrome) and in individuals with APP gene locus duplication [a rare form of early onset of familial origin]. It is believed that overexpression of APP results in an increase of cerebral Aβ peptide, and consequently, in its deposition [ 15 ].
Two main pathways for APP processing are now recognized: a non-amyloidogenic α-secretase-mediated pathway and an amyloidogenic β-and γ-secretase-mediated pathway. Cleavage of APP by α-secretase results in a soluble molecule, sAPPα, which has probable neuroprotective function, playing important roles in the plasticity and survival of neurons and protection against excitotoxicity [ 16 , 17 ]. The Aβ peptide is produced by APP cleavage by a β-secretase (mainly BACE1 enzyme). In this pathway, APP is cleaved by β-secretase to give a APP soluble fragment (sAPPβ, a mediator related to neuronal death), and a carboxy-terminal complex linked to cell membrane. The latter is cleaved by a γ-secretase complex composed by 4 proteins: presenilin 1 or 2, nicastrin, APH-1 (formerly pharynx-defective-1) and and PEN-2 (presenilin enhancer-2), to give rise to the Aβ peptide. Aβ peptides ranging in size from 38 to 43 aminoacids are generated with predominance of the 40 aminoacid form (Aβ 40), followed by 42 (Aβ 42) [ 17 , 18 ]. In physiological conditions, the amyloidogenic and non-amyloidogenic pathways coexist in equilibrium, the latter being favored preferentially [ 19 ].
The Aβ42 peptide is more prone to aggregation than Aβ40. Immunohistochemical analyses indicate that Aβ42 is initially deposited and found at higher concentrations in the amyloid plaques observed in AD patients [ 20 ]. Several studies showed that CSF Aβ42 levels are surrogate markers of underlying brain amyloidosis [ 21 , 22 ]. On the contrary, the correlation between serum Aβ42 levels and cerebral amyloidosis is not yet demonstrated. A decrease in Aβ42 levels is observed in cerebrospinal fluid of AD subjects, which can be explained in part by higher deposition of β-amyloid plaques [ 23 ]. As additional evidence of Aβ42 peptide and the AD pathophysiology, it is further noted that mutations in APP and presenilin genes, which give rise to early-onset familial AD forms, lead to a relative increase in Aβ42 levels [ 20 ].
Aβ peptides, under physiological conditions, are produced primarily in monomeric forms with synapses protective function. However, the accumulation of this protein leads to formation of fibrils that accumulate in senile plaques. High levels of Aβ may lead to oligomeric products formation (dimers, trimers, tetramers) leading to neuronal toxicity and degeneration (both by interaction with cell membranes and their receptors, and by direct interference in intracellular processes), interfering with the function and survival of cholinergic, serotonergic, noradrenergic and dopaminergic neurons, reducing their control over the amyloidogenic pathway and favoring the accumulation of insoluble Aβ peptide [ 19 , 24 ].
The exact mechanism by which deposition of Aβ peptide promotes NFT formation of hyperphosphorylated tau protein is not known. Blurton-Jones & Laferla (2006) [ 25 ] suggest four basic mechanisms:
The Aβ peptide promotes the activation of specific kinases (GSK3β, e.g.) that catalyze the hyperphosphorylation of tau protein, leading to its conformation change and formation of NFT;
Neuroinflammation promoted by the deposition of Aβ peptide leads to the production of proinflammatory cytokines that stimulate the phosphorylation of tau protein;
Reduced capacity of degradation of tau protein by the proteasome, in a process induced by Aβ peptide;
Defects in axonal transport promoted by Aβ peptide lead to inadequate localization of tau protein and its messenger RNA, which can lead to hyperphosphorylation and aggregation in NFT.
Tau protein is a microtubule-associated protein, produced by alternative splicing of the MAPT gene, located on chromosome 17 (17q21). Six isoforms of tau protein are produced by this process [ 26 ]. The main known physiological functions of this protein are the stimulation of tubulin polymerization, microtubules stabilization and intracellular organelles transport by microtubules. Once hyperphosphorylated, the protein loses its functions in the synthesis and stabilization of microtubules, leading to neuronal damage and promoting cytotoxicity [ 27 ]. Histological analyses demonstrate that both the load and the distribution of NFT in brain tissue correlate better with the severity of cognitive deficit than the Aβ peptide deposits [ 28 ].
- Genetic risk factors
AD can be classified by the age of onset of the first symptoms. Early-onset AD affects individuals under 65 years of age, accounting for about 4–6% of cases of AD, while the late form AD affects individuals aged 65 years or older. Besides the age of onset of symptoms, the early and late forms of AD differ in other clinical, neuropsychological, neuropathological and neuroimaging variables [ 29 ].
According to Ballard et al. (2011) [ 1 ] about 70% of the risk of developing AD can be attributed to genetics. Early AD usually occurs due to mutations in genes APP, PSEN1 and PSEN2 (genes of amyloid precursor protein, presenilin 1 and presenilin 2, respectively), whereas late-form AD is mainly associated with a polymorphism in APOE gene (apolipoprotein E gene), especially the presence of ε4 allele [ 30 , 31 ].
More than 30 dominant mutations have already been found in APP gene (located in chromosome 21q21) and are associated with about 15% of cases of early-onset autosomal dominant AD. Mutations in PSEN1 gene (located at 14q24.3) are associated with 80% of cases of early-onset AD, whereas 5% of cases are associated with PSEN2 mutations (located at 1q31-q42) [ 32 ]. Most of APP gene mutations, as well as PSEN1 mutations, lead to an increase in Aβ42: Aβ40 ratio, either by Aβ42 increased expression, reduction of Aβ40, or both. This deregulation favors early Aβ deposition in brain tissue favoring the amyloidogenic cascade [ 33 ]. It is believed that there are other genes besides APP, PSEN1 and PSEN2 involved in the pathogenesis of early-onset AD, as demonstrated by Campion et al. (1999) [ 34 ].
Apolipoprotein E (ApoE) is a protein involved in lipid metabolism encoded by APOE gene, located on chromosome 19. There are three APOE alleles described (ε2, ε3 and ε4, giving rise to apoE2, apoE3 and apoE4 isoforms), present in population at different frequencies (ε2: 5–10%, ε3: 65–70% and ε4: 15–20%). A study by Corbo and Scacchi (1999) [ 35 ] showed that there is a great variability in the APOE allele distribution among the different populations, with ε2 frequencies varying from 0.0 in some Native American populations up to 0.145 in Papuans. The ε 4 frequencies obtained by the authors range from 0.052 (Sardinians) to 0.407 (Pygmies). The ε4 allele is the main risk factor for late-onset AD. The presence of ε4 in heterozygosity increases 3-fold the risk of AD developing, whereas in homozygosis, the risk is increased 12-fold. Conversely, the presence of ε2 allele reduces the risk of AD developing [ 36 , 37 ].
The causes of the association between apoE are not yet fully understood, although some mechanisms have been proposed, and presented consistent results in clinical and in vitro studies. Among these studies, some show that apoE is able to bind to Aβ peptide. While the apoE4 isoform binds to Aβ peptide promoting its polymerization in fibrils and its deposition, apoE2 and apoE3 forms are more efficient in promoting the clearance of this peptide, reducing its deposition in brain tissue [ 38 ]. ApoE has neuroprotective effects and is able to act on neurons development, with apoE2 and apoE3 performing better than apoE4. Additionally, it is observed that protease-generated apoE fragments have toxic effects, which may lead to neuronal injury and favor Aβ peptide deposition [ 38 , 39 ].
More recently it was observed that rare alterations in the triggering receptor expressed on myeloid cells 2 ( TREM2 ) gene elevated the risk ratio by 2.9% for AD development [ 40 , 41 ]. The pathophysiological mechanism by which the deficiency in the gene increases the risk ratio for AD still needs to be better clarified. The gene is located on chromosome 6p21 [ 42 ] and the TREM2 protein is a highly expressed receptor on the surface of microglia, phagocytic cells of central nervous system, and has the function of modulating phagocytic and inflammatory responses in central nervous system [ 43 ]. Activation of microglia through the interaction of TREM2 and DAP12 stimulates the production of CCL19 and CCL21 chemokines and phagocytosis [ 44 ]. In knockout models for the TREM2 receptor it was observed that phagocytic capacity of apoptotic neuronal cell bodies was deficient [ 44 ]. Thus the accumulation of these cellular debris would promote a proinflammatory microenvironment [ 44 ]. Xiang et al. (2016) [ 45 ] observed that the removal capacity of Aβ peptide deposits is impaired in TREM2 receptor deficiency and would favor amyloid plaques accumulation.
- Acquired risk factors
A number of acquired factors increase the risk of developing AD. Among those factors are cerebrovascular diseases (most commonly reported risk factor), diabetes, hypertension, obesity and dyslipidemia [ 46 ]. The association of these risk factors to AD development will be described in the following subsections, as well as some protective factors associated with a lower risk of disease incidence, such as cognitive reserve, physical activity and diet as reported by Mayeux & Stern (2012) [ 46 ].
Cerebrovascular diseases
Cerebrovascular diseases and AD share many risk factors, which often overlap. Cerebrovascular changes such as hemorrhagic infarcts, small and large ischemic cortical infarcts, vasculopathies, and changes in cerebral white matter are known to increase the risk of dementia. Postmortem analyses of the brains of patients with AD indicate the presence of parenchymal vascular disease (amyloid angiopathy by Aβ peptide and small vessels arteriolosclerotic disease), with hemorrhagic outbreaks and infarcts being found in more than 50% of them [ 47 , 48 ]. According to Liu et al., (2015) [ 49 ], neuropathological findings indicate that between 6 and 47% of individuals with dementia have a simultaneous occurrence of cerebrovascular disease. These observations point to the potential role of homeostatic mechanisms in AD and lead to question whether the dementias in which vascular processes are involved are fundamentally different from those related to accumulation of Aβ42 and tau proteins or if both pathological processes produce synergistic effects on cognitive function [ 9 ].
According to the “double-stroke” theory of AD, vascular risk factors (“first stroke”) lead to dysfunction in blood-brain barrier and reduction in cerebral blood flow, with decreased blood supply to the region (oligoemia). This event leads to neuronal damage by non-amyloidogenic and amyloidogenic pathways. Firstly, the dysfunction of blood-brain barrier leads to oligoemia and the accumulation of neurotoxic molecules, events associated with the occurrence of multiple focal ischemic infarcts and micro-injuries resulting from hypoxia, causing neuronal damage. In the amyloidogenic pathway, vascular injury leads to increased expression and processing of APP, resulting in an increase in Aβ peptide. In addition, damage to blood-brain barrier leads to decreased clearance of Aβ peptide. The accumulation of amyloid in brain (“second stroke”) amplifies neuronal dysfunction and speeds up neurodegeneration process. Both Aβ peptide accumulation and hypoperfusion lead to hyperphosphorylation of tau protein, promoting the formation of NFT [ 50 ].
Hypertension
A longitudinal study carried out by Skoog et al. (1996) demonstrated that hypertension is capable of leading to increased risk of developing AD [ 51 ]. Other studies have confirmed this association, indicating that hypertension, especially when present in middle age, negatively affects cognitive performance at more advanced ages, and this association becomes weaker with age [ 52 ]. Hypertension is capable of causing changes in the vascular walls which can lead to hypoperfusion, ischemia and cerebral hypoxia, contributing to trigger the development of AD. Studies demonstrate that cerebral ischemia is capable of leading to the accumulation of APP and Aβ, in addition to stimulating the expression of presenilin, involved in Aβ synthesis. Hypertension may also lead to dysfunction in the blood-brain barrier, an event associated with the genesis of AD by previously discussed mechanisms [ 53 ].
Type 2 diabetes
Epidemiological studies indicate a clear association between type 2 diabetes mellitus and the increased risk of developing AD. Several mechanisms for this association are suggested, including insulin resistance and insulin deficiency, impaired insulin receptor, toxicity of hyperglycemia, adverse effects due to advanced glycation end products, cerebrovascular damage, vascular inflammation and others [ 54 ].
The use of animal models was able to demonstrate that deficiency or resistance to insulin are able to stimulate the action of β and γ-secretases, besides promoting reduction of Aβ clearance, leading to its accumulation in brain tissue. Insulin resistance or deficiency are still capable of inducing hyperphosphorylation of tau protein, leading to NFT formation. Insulin and insulin-like growth factor bind to insulin receptor, leading to its autophosphorylation and activation. Activation of this receptor leads to phosphorylation of phosphoinositide 3-kinase (PI3K) enzyme, which in turn phosphorylates and inhibits glycogen synthase kinase 3β (GSK3β) enzyme, which is important for tau protein phosphorylation. Thus, insulin deficiency / resistance leads to GSK3β abnormal activation, and consequently, to an increase of p-tau formation [ 55 ].
In addition to the mechanisms discussed earlier, studies have reported that advanced glycation end products (AGEs) induce neuronal death through activation of cell death pathways, in addition to stimulating APP processing through increased expression of complexes β and γ-secretases (BACE and PSEN1), in a process involving reactive oxygen species generation [ 56 ]. In addition, Aβ peptide may undergo non-enzymatic glycation, making it an AGE more neurotoxic than its non-glycated form [ 57 ].
The role of obesity as a risk factor for AD development is still uncertain, with studies presenting rather heterogeneous results. According to a meta-analysis developed by Profenno, Porsteinsson, & Faraone (2010) [ 58 ], obesity (Body Mass Index - BMI ≥30 kg / m 2 ) is significantly and independently associated with AD developing risk. On the other hand, a meta-analysis conducted by Fitzpatrick et al. (2009) [ 59 ] indicated that obesity in middle age is a risk factor for dementia development (hazard ratio - HR: 1.39; 95% CI: 1.03–1.87), while in later stages of life, obesity is inversely correlated with the risk of dementia (HR: 0.63; 95% CI: 0.44–0.91). The same authors have also reported that below-ideal weight (BMI < 20 kg / m 2 ) is also associated with an increased risk of dementia (HR: 1.62, 95% CI: 1.02–2.64). Weight loss at more advanced ages occurs in concomitance to other comorbidities and is often indicative of poor health, and may even precede dementia onset within 10 years. Another meta-analysis conducted by Anstey et al. (2011) [ 60 ] indicated that both low weight and overweight as well as obesity in middle age are associated with a higher risk of developing AD in late life.
Dyslipidemia
Elevated cholesterol levels have been proposed as risk factors for the development of AD. Studies have already demonstrated 10% higher cholesterol levels in patients with AD, compared to healthy individuals [ 61 ]. Hypercholesterolemia is a risk factor both for atherosclerosis development and AD development as well as other neurodegenerative diseases [ 62 ].
Hypercholesterolemia increases AD risk primarily because of its effects on the blood-brain barrier. Studies have shown that elevated circulating cholesterol levels are capable of compromising integrity in blood-brain barrier [ 62 ], resulting in mechanisms previously discussed. In addition, experimental studies using animal models demonstrate that hypercholesterolemia is associated with increased Aβ peptide deposition, in addition to increased NFT formation, cognitive decline, neuroinflammation, dysfunction of cholinergic neurons and the presence of cerebral microhemorrhages compatible with AD [ 63 , 64 ].
In observational studies a beneficial effect was observed in the users of statins as the reduction in AD incidence or improvement in the disease progression [ 65 , 66 , 67 ]. However, clinical studies to date have not demonstrated benefit of statins treatment and protection against cognitive decline in AD patients at various stages of disease [ 68 , 69 , 70 , 71 , 72 ]. Contrary to meta-analysis findings conducted by Song et al. (2013) [ 73 ] who observed a lower risk of developing AD in statins users, a Cochrane meta-analysis [ 74 ] did not observe difference in disease outcome as well as alteration in mini-mental status examination (MMSE) in patients using or not statins. However, some important questions regarding the clinical studies are pointed out, i.e., whether treatment initiated in middle age prior disease onset would also have a beneficial effect in elderly, or whether in people with AD family history the treatment would be effective in comparison to those without this background.
Marital status, stress, depression and inadequate sleep
Widowhood status has been reported as an important risk factor AD. A cohort study by Håkansson et al. (2009) [ 75 ] shows that widowed individuals have an increased risk of developing AD compared to married or cohabiting individuals and that this effect is more pronounced in carriers of the APOE ε4 allele. Other studies, such as that by Fan et al. (2015) [ 76 ] demonstrated an association between the risk of all-cause dementia and widow status. A meta-analysis by Sommerlad et al. (2018) [ 77 ] reported an association between widowhood and all-cause dementia, but the same association was not found between widowhood and AD or vascular dementia.
Studies in animal models of AD have shown that stress, characterized as hyperactivation of the hypothalamic, pituitary and adrenal axis (HPA) leading to an increase in cortisol production, causes an increase in Aβ peptide deposition in regions of the brain such as hypothalamus and prefrontal cortex [ 78 , 79 , 80 ]. Carroll et al. (2011) [ 81 ] have observed that the prolonged stress caused by this hyperactivation also causes an increase in the accumulation of hyperphosphorylated tau and neurodegeneration in mice. In humans, increased levels of cortisol were observed in patients with AD compared to the control group [ 82 , 83 , 84 ]. Huang et al. (2009) [ 85 ] observed in a 2-year follow-up of patients with AD that the higher cortisol levels correlated with the faster progression of the disease, worsened in the MMSE and smaller volume of the hippocampus region when observed by resonance. The authors of this study argue that hippocampal atrophy causes a disinhibition effect on the HPA axis, which would cause elevation in cortisol levels as a consequence of the pathophysiological process of AD. Toledo et al. (2012) [ 86 ], observed in a sample of 26 patients with AD that the increase in cortisol levels is correlated with the deposition of the Aβ peptide observed by means of pittsburgh compound b-positron emission tomography (PiB-PET). Ennis et al. (2017) [ 87 ], in a 10-year longitudinal study with 1025 participants observed an increased risk of 1.31 for the development of AD and elevation in cortisol levels that were dosed in 24-h urine samples. However, this result contrasts with that observed in the Rotterdan study [ 88 ] in blood samples collected in the morning when there was no correlation between cortisol levels and AD or dementia in general.
Early adult depression is a risk factor for the development of dementia at more advanced age including AD [ 89 , 90 , 91 ]. Zverova et al. (2013) [ 83 ] observed a greater odds ratio for cognitive decline in the presence of cortisol levels and patients with AD and symptoms of depression. Wu et al. (2018) [ 92 ] observed in some patients with major depression in middle age hippocampal atrophy and Aβ peptide deposition observed by PET indicating that the protein metabolism may be altered in patients with depression.
According to a study published by Proserpio et al. (2018) [ 93 ], sleep disorders have a bidirectional relationship with AD: sleep disorders arise during the early stages of dementia and tend to worsen with the onset of dementia. Similarly, sleep disorders can lead to an increased risk of dementia. A meta-analysis by Shi et al. (2018) [ 94 ] demonstrated that individuals with sleep disorders have an increased risk of developing dementia. More specifically, individuals with insomnia are at high risk for developing AD but not for vascular dementia or other causes. Similarly, individuals with sleep disordered breathing had an increased risk of developing all-cause dementia, AD, and vascular dementia.
Smoking may affect the risk of developing AD by various mechanisms. It is known that it is able to raise the generation of free radicals, increasing oxidative stress, and to promote pro-inflammatory action in the immune system, leading to the activation of phagocytes and consequently, additional oxidative damage. In addition, smoking may lead to cerebrovascular diseases, which increase the risk of AD [ 95 , 96 ]. In a meta-analysis performed by Cataldo et al. (2010), an analysis of 8 case-control studies with affiliations with the tobacco industry suggested a protective effect of smoking in relation to AD (odds ratio (OR): 0.91, 95% CI 0.75–1, 10). In contrast, 14 cohort studies with no association with the tobacco industry demonstrated an increased relative risk for smokers (Relative Risk (RR): 1.45; 95% CI, 1.16–1.80) [ 97 ]. According to Durazzo et al. (2014), the sum of the evidence presented today in the literature is enough for the cessation of smoking to be recommended in order to reduce the incidence of dementia [ 96 ].
Protective factors
Cognitive reserve.
It has been observed in many cases a discrepancy between the degree of brain damage found in histopathological analyses and the severity of cognitive decline. To explain these findings, the theory of cognitive reserve was proposed, which postulates that the gap between brain injury and clinical manifestations is attributable to cognitive reserve capacity. This can be subdivided into two models: brain reserve model or threshold, and cognitive reserve model and / or compensation. The first is based on the amount of available neural substrate (eg, brain size, synapses density or dendritic branching), while the latter focuses on the more efficient ability to use the preexisting brain network in healthy individuals and on the recruitment of more resources to support normal functioning in presence of brain damage [ 98 ].
Several elements are associated with a greater cognitive reserve, such as educational level, occupational activities, leisure activities, physical activities and the integrity of relationships network [ 98 , 99 ]. A study conducted by Stern et al. (1994) [ 100 ] indicated that individuals with low level of schooling and low level of professional achievement had an approximately two-fold increased risk of developing dementia. Similarly, another study indicated that individuals with a higher level of leisure activities performance had a lower risk of developing dementia [ 101 ].
Physical activity
A meta-analysis developed by Hamer & Chida (2009) [ 102 ] indicated that physical activity practice is able to reduce AD risk by 45%. This protective effect is related to several mechanisms, such as reduction of blood pressure, obesity and proinflammatory activity besides the improvement in lipid profile and endothelial function. In addition, adaptations that occur in response to exercise can lead to a better cerebral blood flow and, consequently, better oxygenation of important areas for cognitive function [ 102 ]. It is also believed that physical activity is able to prevent AD by increasing neurotrophic factors such as BDNF (Brain Derived Neurotrophic Factor), IGF-1 (Insulin-Like Growth Factor), VEGF (Vascular Endothelial Growth Factor), stimulating neurogenesis and synaptic plasticity; and by the reduction of free radicals in the hippocampus, as well as increase of superoxide dismutase and eNOS (endothelial nitric oxide synthase) [ 103 ]. Studies have shown that the practice of physical activities is capable of promoting an increase in hippocampal volume, in addition to increasing plasma BDNF concentrations in healthy elderly, indicating a possible neuroprotective effect. It was also reported that in the AD elderly, practice of physical activities correlates positively with the levels of BDNF [ 104 ], which is a growth factor associated with the development and survival of neurons and synapses [ 105 ].
The relationship between the effects of diet and the risk of developing AD was based on certain patterns that were associated with lower or higher risk of developing AD [ 106 ]. As an example, Mediterranean diet is rich in unsaturated fats and antioxidants which confers a protection factor, as diets rich in saturated and trans fats and low levels of anti-oxidants are associated with higher risk of developing AD [ 106 , 107 ]. Some dietary components are essential for neurocognition protection such as dietary fatty acids, including fish oil; antioxidants, such as vitamins E and C; fruits and vegetables; vitamins B6, B12 (cobalamine) and folate, in addition to caloric restriction [ 108 ]. Antioxidants are able to prevent damage caused by reactive oxygen species in addition to stabilizing the membranes; docosahexaenoic acid (DHA) helps clear the Aβ peptide and, together with choline and uridine, aid in the synthesis of the neuronal membrane [ 106 ]. Phospholipid composition is essential in neuronal membrane function. Thus, adequate intake of DHA, eicosapentaenoic acid (EPA), uridine monophosphate, choline, folate, vitamins B6, B12, C, and E, and selenium contributes to a better synthesis of phospholipids and, consequently, to synaptic function preservation and against neurodegeneration [ 106 ]. Nerve synapses consist mainly of neuronal membranes, and neuronal and synaptic losses observed in AD have been related to degeneration and alteration in the composition of these membranes [ 109 ]. Brain aging associated with changes in lipid composition is well studied for treatment and prevention purposes with phospholipids such as phosphatidylcholine and phosphatidylserine that could favor cognitive improvement [ 110 ]. The OmegAD study (a set of double-blind, placebo-controlled clinical trials involving AD patients which evaluated the effects of omega-3 fatty acids (n − 3 FAs) daily supplementation in patients with mild to moderate AD) showed that after six months, DHA (1.7 g) and EPA (0.6 g) supplementation demonstrated benefits such as preservation of cognitive performance, increase in plasma and CSF (Cerebrospinal fluid) levels of n − 3 FAs, DHA and EPA (and negative correlation between DHA and total / phosphorylated tau levels in CSF), reduction in cytokine release pro-inflammatory by blood peripheral mononuclear cells (PBMC), modulation in the expression of genes involved in the regulation of inflammation in PBMC, elevation in transthyretin plasma levels (a protein that binds to AB and which may influence its deposition in the brain), and increase in body weight and BMI. However, the literature data do not support the benefits of 3-FA supplementation in preventing cognitive decline in elderly subjects [ 111 ].
Epidemiological studies have observed a relationship between serum levels of vitamin D reduction, especially 25-hydroxyvitamin D, and AD development [ 112 , 113 , 114 ]. Vitamin D is an important steroid hormone that acts on calcium metabolism and bone regulation, and has some functions in central nervous system, such as regulation of neurotrophic factors, calcium homeostasis, acts on oxidative stress mechanisms, immune system modulation and inflammation [ 115 ]. In the case of inflammation, vitamin D deficiency causes an increase in the amyloidogenic pathway due to elevation of BACE1 and APP cleavage and decrease of Aβ degradation [ 116 ]. Briones & Darwish (2012) [ 117 ] reported a BACE1 and Aβ peptide reduction after vitamin D supplementation in elderly rats. It has also been observed that vitamin D acts on macrophages in order to promote clearence of Aβ peptide [ 118 , 119 ]. In AD patients mutations were also observed in vitamin D receptor (VDR) gene, which would favor the onset of the disease [ 120 ]. To date, no large randomized clinical trial has been conducted on the effect of vitamin D supplementation on the cognition of AD patients. However, in smaller or cohort studies, the results of using high doses of vitamin D and cognitive improvement are divergent [ 121 , 122 , 123 , 124 ]. Vitamin D deficiency should be screened and supplemented in the elderly population due to its high prevalence, but this treatment is not specific for cognitive improvement.
Estrogen (hormone replacement therapy)
Estrogen roles in sex organs are well understood, but it has recently been observed that local production of estrogen plays specific roles in tissues in which it is produced, with or without dependence on circulating estrogen [ 125 ]. Estrogen, especially estradiol, is able to prevent mitochondrial dysfunction in nerve cells, neuroinflammation and assist in DNA repair mechanisms [ 126 ], thus presenting neuroprotective effect [ 126 , 127 ]. The results observed in epidemiological studies are inconsistent [ 128 , 129 ]. Some studies have not observed a beneficial effect of hormone replacement therapy, estrogen or combination therapy on the risk of developing AD [ 130 , 131 ]. Other studies reported a beneficial effect on cognition protection in women receiving hormone replacement therapy at different ages after the onset of menopause [ 132 , 133 , 134 , 135 ]. Inconsistent epidemiological findings, in addition to other factors such as increased risk of deep venous thrombosis, hormone replacement therapy is not recommended in order to prevent cognitive decline and AD development [ 136 ].
Other relevant factors and conclusion
The main pathophysiological mechanisms of AD are amyloidosis and tau-related neurodegeneration, and have specific topographical and chronological pathways. For instance, brain amyloidosis starts in neocortical regions and then affects subcortical structures [ 137 ]. On the other hand, neurodegeneration first appear on locus coeruleus and then spreads through transentorrinal area and neocortical regions [ 137 ]. Cognitive and behavioral features of AD are significantly correlated to the topographical distribution of neurofibrillary tangles.
There is great variability in topographical patterns of pathological findings in AD, causing great phenotypical variability [ 7 ], with atypical presentations of the disease [ 138 ]. It is not clear how risk and beneficial factors may modulate the topographical progression of amyloidosis and neurodegeneration.
The effects of modifiable risk factors on cross-sectional cognition have been the target of multiple WRAP (The Wisconsin Registry for Alzheimer’s Prevention) investigations. This study has investigated risk factors for AD in middle age, since this phase of life is less studied in relation to the later stages of aging. However, this is a critical time because it is when the Alzheimer’s pathology begins and thus, when its trajectory can be modified through pharmacological approaches and / or lifestyle changes. Within this context, the WRAP study, reported by Johnson et al. (2018), suggest that social engagement, physical and cognitive activities, glucose regulation, stress and sleep, in addition to cardiovascular and metabolic risks are interventional parameters that may improve brain health and reduce the likelihood and severity of AD pathology. These authors conclude that a good health and a salutary lifestyle are factors associated not only with better cognition and brain structure but also the lower AD pathophysiologic burden [ 139 ].
The studies of genetic risk factors are important to better elucidate the pathophysiological processes in the development of AD. However, such factors are not passible to any intervention until now. Faced to this scenario, modifiable risk factors such as diabetes, hypertension and dyslipidemia and others previously mentioned should be closely monitored to prevent complications favoring cognitive decline or even to improve the quality of life of patients with AD. In this context, it should also be emphasized that factors considered protective, such as physical exercise, diet and cognitive stimuli should be strongly and widely encouraged, so that such theoretically preventive measures can be adopted by the population contributing to reduce risk of this disease. Since no current drug intervention can modify the pathophysiological mechanisms related to the development of this devastating disease, adoption of these measures constitutes an important strategy for clinical management in order to prevent or postpone cognitive decline.
Abbreviations
- Alzheimer’s disease
Advanced glycation end products
Apolipoprotein E (ApoE)
Amyloid precursor protein
β-amyloid peptide
Brain Derived Neurotrophic Factor
Body Mass Index
Cerebrospinal fluid
Docosahexaenoic acid
Endothelial nitric oxide synthase
Eicosapentaenoic acid
Hypothalamic, pituitary and adrenal axis
Hazard Ratio
Insulin-Like Growth Factor
Omega-3 fatty acids
Neurofibrillary tangles
Peripheral blood mononuclear cells
Phosphorylated tau protein
Vitamin D receptor
Vascular Endothelial Growth Factor
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377(9770):1019–31.
Article PubMed Google Scholar
Ferreira D, Perestelo-Perez L, Westman E, Wahlund LO, Sarria A, Serrano-Aguilar P. Meta-review of CSF Core biomarkers in Alzheimer's disease: the state-of-the-art after the new revised diagnostic criteria. Front Aging Neurosci. 2014;6:47.
PubMed PubMed Central Google Scholar
IDd A, FHdEdM G, Forlenza OV, UdS P, HLd B, et al. Alzheimer disease: correlation between memory and autonomy. Rev psiquiatr clín. 2005;32(3):131–6.
Article Google Scholar
Shinohara M, Sato N, Shimamura M, Kurinami H, Hamasaki T, Chatterjee A, et al. Possible modification of Alzheimer's disease by statins in midlife: interactions with genetic and non-genetic risk factors. Front Aging Neurosci. 2014;6:71.
Article PubMed PubMed Central Google Scholar
Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, et al. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease. Neuron. 2010;66(5):695–709.
Article CAS PubMed PubMed Central Google Scholar
Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer's Disease. N Engl J Med. 2012;367:795–804.
Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10(9):785–96.
de Souza LC, Sarazin M, Teixeira-Junior AL, Caramelli P, Santos AE, Dubois B. Biological markers of Alzheimer's disease. Arq Neuropsiquiatr. 2014;72(3):227–31.
Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE. Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimers Dement. 2013;9(1):76–92.
Kang S, Lee YH, Lee JE. Metabolism-centric overview of the pathogenesis of Alzheimer's disease. Yonsei Med J. 2017;58(3):479–88.
Mucke L. Neuroscience: Alzheimer's disease. Nature. 2009;461:895–7.
Article CAS PubMed Google Scholar
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–6.
Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329–44.
Muller UC, Deller T, Korte M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat Rev Neurosci. 2017;18(5):281–98.
Doran E, Keator D, Head E, Phelan MJ, Kim R, Totoiu M, et al. Down syndrome, partial trisomy 21, and absence of Alzheimer's disease: the role of APP. J Alzheimers Dis. 2017;56(2):459–70.
Gupta A, Goyal R. Amyloid beta plaque: a culprit for neurodegeneration. Acta Neurol Belg. 2016;116(4):445–50.
Zhang YW, Thompson R, Zhang H, Xu H. APP processing in Alzheimer's disease. Mol Brain. 2011;4:3.
Zhang H, Ma Q, Zhang YW, Xu H. Proteolytic processing of Alzheimer's beta-amyloid precursor protein. J Neurochem. 2012;120(Suppl 1):9–21.
Martorana A, Di Lorenzo F, Belli L, Sancesario G, Toniolo S, Sallustio F, et al. Cerebrospinal fluid Abeta42 levels: when physiological become pathological state. CNS Neurosci Ther. 2015;21(12):921–5.
Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. Jama. 2000;283(12):1571–7.
Grimmer T, Riemenschneider M, Forstl H, Henriksen G, Klunk WE, Mathis CA, et al. Beta amyloid in Alzheimer's disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65(11):927–34.
Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66(3):382–9.
Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003;2(10):605–13.
Lee SJ, Nam E, Lee HJ, Savelieff MG, Lim MH. Towards an understanding of amyloid-beta oligomers: characterization, toxicity mechanisms, and inhibitors. Chem Soc Rev. 2017;46(2):310–23.
Blurton-Jones M, Laferla FM. Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res. 2006;3(5):437–48.
Kovacs GG. Invited review: neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol. 2015;41(1):3–23.
Khan SS, Bloom GS. Tau: the Center of a Signaling Nexus in Alzheimer's disease. Front Neurosci. 2016;10:31.
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–81.
Mendez MF. Early-onset Alzheimer disease. Neurol Clin. 2017;35(2):263–81.
Giri M, Zhang M, Lu Y. Genes associated with Alzheimer's disease: an overview and current status. Clin Interv Aging. 2016;11:665–81.
Cacace R, Sleegers K, Van Broeckhoven C. Molecular genetics of early-onset Alzheimer's disease revisited. Alzheimers Dement. 2016;12(6):733–48.
Calero M, Gómez-Ramos A, Calero O, Soriano E, Avila J, Medina M. Additional mechanisms conferring genetic susceptibility to Alzheimer’s disease. Front Cell Neurosci. 2015;9:138.
Bekris LM, Yu CE, Bird TD, Tsuang DW. Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol. 2010;23(4):213–27.
Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999;65:664–70.
Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann Hum Genet. 1999;63(Pt 4):301–10.
Karch CM, Goate AM. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77(1):43–51.
Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl). 2016;94:739–46.
Article CAS Google Scholar
Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63(3):287–303.
Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: from lipid transport to neurobiology. Prog Lipid Res. 2011;50(1):62–74.
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368(2):107–16.
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368(2):117–27.
Allcock RJ, Barrow AD, Forbes S, Beck S, Trowsdale J. The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol. 2003;33(2):567–77.
Jiang T, Zhang YD, Gao Q, Ou Z, Gong PY, Shi JQ, et al. TREM2 ameliorates neuronal tau pathology through suppression of microglial inflammatory response. Inflammation. 2018;41(3):811–23.
Mecca C, Giambanco I, Donato R, Arcuri C. Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int J Mol Sci. 2018;19(1):318.
Article CAS PubMed Central Google Scholar
Xiang X, Werner G, Bohrmann B, Liesz A, Mazaheri F, Capell A, et al. TREM2 deficiency reduces the efficacy of immunotherapeutic amyloid clearance. EMBO Mol Med. 2016;8:992–1004.
Mayeux R, Stern Y. Epidemiology of Alzheimer Disease. Cold Spring Harb Perspect Med. 2012;2:a006239.
Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–52.
Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol. 2016;131(5):645–58.
Liu W, Wong A, Law AC, Mok VC. Cerebrovascular disease, amyloid plaques, and dementia. Stroke. 2015;46(5):1402–7.
Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–38.
Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141–5.
Staessen JA, Richart T, Birkenhager WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49(3):389–400.
Skoog I, Gustafson D. Update on hypertension and Alzheimer's disease. Neurol Res. 2006;28(6):605–11.
Li X, Song D, Leng SX. Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clin Interv Aging. 2015;10:549–60.
Kimura N. Diabetes mellitus induces Alzheimer's disease pathology: histopathological evidence from animal models. Int J Mol Sci. 2016;17(4):503.
Ko SY, Ko HA, Chu KH, Shieh TM, Chi TC, Chen HI, et al. The Possible Mechanism of Advanced Glycation End Products (AGEs) for Alzheimer’s Disease. PLoS One. 2015;10.
Li XH, Du LL, Cheng XS, Jiang X, Zhang Y, Lv BL, et al. Glycation exacerbates the neuronal toxicity of beta-amyloid. Cell Death Dis. 2013;4:e673.
Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67(6):505–12.
Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT Jr, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336–42.
Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12(5):e426–37.
Popp J, Meichsner S, Kolsch H, Lewczuk P, Maier W, Kornhuber J, et al. Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer's disease. Biochem Pharmacol. 2013;86(1):37–42.
Xue-shan Z, Juan P, Qi W, Zhong R, Li-hong P, Zhi-han T, et al. Imbalanced cholesterol metabolism in Alzheimer's disease. Clin Chim Acta. 2016;456:107–14.
Ricciarelli R, Canepa E, Marengo B, Marinari UM, Poli G, Pronzato MA, et al. Cholesterol and Alzheimer's disease: a still poorly understood correlation. IUBMB Life. 2012;64(12):931–5.
Ullrich C, Pirchl M, Humpel C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol Cell Neurosci. 2010;45:408–17.
Hendrie HC, Hake A, Lane K, Purnell C, Unverzagt F, Smith-Gamble V, et al. Statin use, incident dementia and Alzheimer disease in elderly African Americans. Ethn Dis. 2015;25(3):345–54.
Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam study. J Neurol Neurosurg Psychiatry. 2009;80(1):13–7.
Lin FC, Chuang YS, Hsieh HM, Lee TC, Chiu KF, Liu CK, et al. Early statin use and the progression of Alzheimer disease: a Total population-based case-control study. Medicine (Baltimore). 2015;94(47):e2143.
Simons M, Schwarzler F, Lutjohann D, von Bergmann K, Beyreuther K, Dichgans J, et al. Treatment with simvastatin in normocholesterolemic patients with Alzheimer's disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol. 2002;52(3):346–50.
Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77(6):556–63.
Feldman HH, Doody RS, Kivipelto M, Sparks DL, Waters DD, Jones RW, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74(12):956–64.
Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–30.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22.
Song Y, Nie H, Xu Y, Zhang L, Wu Y. Association of statin use with risk of dementia: a meta-analysis of prospective cohort studies. Geriatr Gerontol Int. 2013;13(4):817–24.
McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;(1):Cd003160. https://doi.org/10.1002/14651858.CD003160.pub3 .
Håkansson K, Rovio S, Helkala EL, Vilska AR, Winblad B, Soininen H, et al. Association between mid-life marital status and cognitive function in later life: population based cohort study. BMJ. 2009;339:b2462
Fan LY, Sun Y, Lee HJ, Yang SC, Chen TF, Lin KN, et al. Marital status, lifestyle and dementia: a Nationwide survey in Taiwan. PLoS One. 2015;10(9):e0139154.
Sommerlad A, Ruegger J, Singh-Manoux A, Lewis G, Livingston G. Marriage and risk of dementia: systematic review and meta-analysis of observational studies. J Neurol Neurosurg Psychiatry. 2018;89(3):231–8.
Ray B, Gaskins DL, Sajdyk TJ, Spence JP, Fitz SD, Shekhar A, et al. Restraint stress and repeated corticotrophin-releasing factor receptor activation in the amygdala both increase amyloid-beta precursor protein and amyloid-beta peptide but have divergent effects on brain-derived neurotrophic factor and pre-synaptic proteins in the prefrontal cortex of rats. Neuroscience. 2011;184:139–50.
Lesuis SL, Maurin H, Borghgraef P, Lucassen PJ, Van Leuven F, Krugers HJ. Positive and negative early life experiences differentially modulate long term survival and amyloid protein levels in a mouse model of Alzheimer's disease. Oncotarget. 2016;7(26):39118–35.
Justice NJ, Huang L, Tian JB, Cole A, Pruski M, Hunt AJ Jr, et al. Posttraumatic stress disorder-like induction elevates beta-amyloid levels, which directly activates corticotropin-releasing factor neurons to exacerbate stress responses. J Neurosci. 2015;35(6):2612–23.
Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, et al. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J Neurosci. 2011;31(40):14436–49.
Lara VP, Caramelli P, Teixeira AL, Barbosa MT, Carmona KC, Carvalho MG, et al. High cortisol levels are associated with cognitive impairment no-dementia (CIND) and dementia. Clin Chim Acta. 2013;423:18–22.
Zverova M, Fisar Z, Jirak R, Kitzlerova E, Hroudova J, Raboch J. Plasma cortisol in Alzheimer's disease with or without depressive symptoms. Med Sci Monit. 2013;19:681–9.
Wang LY, Raskind MA, Wilkinson CW, Shofer JB, Sikkema C, Szot P, et al. Associations between CSF cortisol and CSF norepinephrine in cognitively normal controls and patients with amnestic MCI and AD dementia. Int J Geriatr Psychiatry. 2018;33(5):763–8.
Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer's disease. J Clin Neurosci. 2009;16(10):1283–6.
Toledo JB, Toledo E, Weiner MW, Jack CR, Jagust W, Lee VMY, et al. Cardiovascular risk factors, cortisol, and amyloid-β deposition in Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2012;8(6):483–9.
Ennis GE, An Y, Resnick SM, Ferrucci L, O'Brien RJ, Moffat SD. Long-term cortisol measures predict Alzheimer disease risk. Neurology. 2017;88:371–8.
Schrijvers EM, Direk N, Koudstaal PJ, Kirschbaum C, Hofman A, Tiemeier H, et al. Associations of serum cortisol with cognitive function and dementia: the Rotterdam study. J Alzheimers Dis. 2011;25(4):671–7.
Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–31.
Ricci S, Fuso A, Ippoliti F, Businaro R. Stress-induced cytokines and neuronal dysfunction in Alzheimer's disease. J Alzheimers Dis. 2012;28(1):11–24.
Vilalta-Franch J, Lopez-Pousa S, Llinas-Regla J, Calvo-Perxas L, Merino-Aguado J, Garre-Olmo J. Depression subtypes and 5-year risk of dementia and Alzheimer disease in patients aged 70 years. Int J Geriatr Psychiatry. 2013;28(4):341–50.
Wu KY, Lin KJ, Chen CH, Chen CS, Liu CY, Huang SY, et al. Diversity of neurodegenerative pathophysiology in nondemented patients with major depressive disorder: Evidence of cerebral amyloidosis and hippocampal atrophy. Brain Behav. 2018;8(7):e01016.
Proserpio P, Arnaldi D, Nobili F, Nobili L. Integrating sleep and Alzheimer's disease pathophysiology: hints for sleep disorders management. J Alzheimers Dis. 2018;63(3):871–86.
Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16.
Traber MG, van der Vliet A, Reznick AZ, Cross CE. Tobacco-related diseases. Is there a role for antioxidant micronutrient supplementation? Clin Chest Med. 2000;21(1):173–87 x.
Durazzo TC, Mattsson N, Weiner MW. Smoking and increased Alzheimer's disease risk: a review of potential mechanisms. Alzheimers Dement. 2014;10(3 Suppl):S122–45.
Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer's disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis. 2010;19(2):465–80.
Xu W, Yu JT, Tan MS, Tan L. Cognitive reserve and Alzheimer's disease. Mol Neurobiol. 2015;51(1):187–208.
Tucker AM, Stern Y. Cognitive reserve in aging. Curr Alzheimer Res. 2011;8(4):354–60.
Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. Jama. 1994;271(13):1004–10.
Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57(12):2236–42.
Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39(1):3–11.
Mendiola-Precoma J, Berumen LC, Padilla K, Garcia-Alcocer G. Therapies for prevention and treatment of Alzheimer's disease. Biomed Res Int. 2016;2016:2589276.
Paillard T, Rolland Y, de Souto Barreto P. Protective effects of physical exercise in Alzheimer's disease and Parkinson's disease: a narrative review. J Clin Neurol. 2015;11(3):212–9.
Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function*. Annu Rev Neurosci. 2001;24:677–736.
de Wilde MC, Vellas B, Girault E, Yavuz AC, Sijben JW. Lower brain and blood nutrient status in Alzheimer's disease: Results from meta-analyses. Alzheimers Dement (N Y). 2017;3:416–31.
Google Scholar
Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–25.
Smith PJ, Blumenthal JA. Diet and Neurocognition: review of evidence and methodological considerations. Curr Aging Sci. 2010;3(1):57–66.
van Wijk N, Broersen LM, de Wilde MC, Hageman RJ, Groenendijk M, Sijben JW, et al. Targeting synaptic dysfunction in Alzheimer's disease by administering a specific nutrient combination. J Alzheimers Dis. 2014;38(3):459–79.
Olivera-Pueyo J, Pelegrin-Valero C. Dietary supplements for cognitive impairment. Actas Esp Psiquiatr. 2017;45(Supplement):37–47.
PubMed Google Scholar
Fraga VG, Carvalho MDG, Caramelli P, de Sousa LP, Gomes KB. Resolution of inflammation, n-3 fatty acid supplementation and Alzheimer disease: a narrative review. J Neuroimmunol. 2017;310:111–9.
Knekt P, Saaksjarvi K, Jarvinen R, Marniemi J, Mannisto S, Kanerva N, et al. Serum 25-hydroxyvitamin d concentration and risk of dementia. Epidemiology. 2014;25(6):799–804.
Shen L, Ji HF. Vitamin D deficiency is associated with increased risk of Alzheimer's disease and dementia: evidence from meta-analysis. Nutr J. 2015;14:76.
Licher S, de Bruijn R, Wolters FJ, Zillikens MC, Ikram MA, Ikram MK. Vitamin D and the risk of dementia: the Rotterdam study. J Alzheimers Dis. 2017;60(3):989–97.
Landel V, Annweiler C, Millet P, Morello M, Féron F. Vitamin D, Cognition and Alzheimer’s Disease: The Therapeutic Benefit is in the D-Tails. J Alzheimers Dis. 2016;53:419–44.
Grimm MOW, Thiel A, Lauer AA, Winkler J, Lehmann J, Regner L, et al. Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation. Int J Mol Sci. 2017;18(12):2764.
Briones TL, Darwish H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J Neuroinflammation. 2012;9:244.
Mizwicki MT, Menegaz D, Zhang J, Barrientos-Duran A, Tse S, Cashman JR, et al. Genomic and nongenomic signaling induced by 1alpha,25(OH)2-vitamin D3 promotes the recovery of amyloid-beta phagocytosis by Alzheimer's disease macrophages. J Alzheimers Dis. 2012;29(1):51–62.
Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, Abel K, et al. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer's disease patients. J Alzheimers Dis. 2009;17(3):703–17.
Gezen-Ak D, Dursun E, Bilgic B, Hanagasi H, Ertan T, Gurvit H, et al. Vitamin D receptor gene haplotype is associated with late-onset Alzheimer's disease. Tohoku J Exp Med. 2012;228(3):189–96.
Annweiler C, Herrmann FR, Fantino B, Brugg B, Beauchet O. Effectiveness of the combination of memantine plus vitamin D on cognition in patients with Alzheimer disease: a pre-post pilot study. Cogn Behav Neurol. 2012;25(3):121–7.
Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Herrmann FR, et al. Higher vitamin D dietary intake is associated with lower risk of alzheimer's disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205–11.
Stein MS, Scherer SC, Ladd KS, Harrison LC. A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer's disease. J Alzheimers Dis. 2011;26(3):477–84.
Miller BJ, Whisner CM, Johnston CS. Vitamin D supplementation appears to increase plasma Abeta40 in vitamin D insufficient older adults: a pilot randomized controlled trial. J Alzheimers Dis. 2016;52(3):843–7.
Li R, Cui J, Shen Y. Brain sex matters: estrogen in cognition and Alzheimer’s disease. Mol Cell Endocrinol. 2014;389(0):13–21.
Zárate S, Stevnsner T, Gredilla R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front Aging Neurosci. 2017;9:430.
Depypere H, Vierin A, Weyers S, Sieben A. Alzheimer's disease, apolipoprotein E and hormone replacement therapy. Maturitas. 2016;94:98–105.
Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74.
Dye RV, Miller KJ, Singer EJ, Levine AJ. Hormone replacement therapy and risk for neurodegenerative diseases. Int J Alzheimers Dis. 2012;2012:258454.
Imtiaz B, Taipale H, Tanskanen A, Tiihonen M, Kivipelto M, Heikkinen AM, et al. Risk of Alzheimer's disease among users of postmenopausal hormone therapy: a nationwide case-control study. Maturitas. 2017;98:7–13.
Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative memory study: a randomized controlled trial. Jama. 2003;289(20):2651–62.
Wharton W, Baker LD, Gleason CE, Dowling M, Barnet JH, Johnson S, et al. Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer's disease: results of a randomized controlled trial. J Alzheimers Dis. 2011;26(3):495–505.
Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76(1):103–5.
Espeland MA, Rapp SR, Manson JE, Goveas JS, Shumaker SA, Hayden KM, et al. Long-term effects on cognitive trajectories of postmenopausal hormone therapy in two age groups. J Gerontol A Biol Sci Med Sci. 2017;72(6):838–45.
Fox M, Berzuini C, Knapp LA. Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer's risk in a cohort of British women. Psychoneuroendocrinology. 2013;38(12):2973–82.
Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:Cd004143.
Mattei TA. Is it all about contact? Neurodegeneration as a “protein freeze tag game” inside the central nervous system. Front Neurol. 2013;4:75.
Warren JD, Fletcher PD, Golden HL. The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol. 2012;8(8):451–64.
Johnson SC, Koscik RL, Jonaitis EM, Clark LR, Mueller KD, Berman SE, et al. The Wisconsin registry for Alzheimer's prevention: a review of findings and current directions. Alzheimers Dement (Amst). 2018;10:130–42.
Download references
Acknowledgements
Not applicable
CNPq, CAPES and Fapemig
Availability of data and materials
Author information, authors and affiliations.
Faculdade de Farmácia, Universidade Federal de Minas Gerais, Avenida Presidente Antônio Carlos, 6627 – Pampulha, Belo Horizonte, Minas Gerais, 31270-901, Brazil
Marcos Vinícius Ferreira Silva, Cristina de Mello Gomide Loures, Luan Carlos Vieira Alves, Karina Braga Gomes Borges & Maria das Graças Carvalho
Faculdade de Medicina, Universidade Federal de Minas Gerais, Av. Prof. Alfredo Balena, 190 - Santa Efigênia, Belo Horizonte, Minas Gerais, 30130-100, Brazil
Leonardo Cruz de Souza
You can also search for this author in PubMed Google Scholar
Contributions
MVFS, CGML and LCVA drafted the manuscript. LCS, KBGB and MGC reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Marcos Vinícius Ferreira Silva .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Silva, M.V.F., Loures, C.d.M.G., Alves, L.C.V. et al. Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci 26 , 33 (2019). https://doi.org/10.1186/s12929-019-0524-y
Download citation
Received : 19 January 2019
Accepted : 18 April 2019
Published : 09 May 2019
DOI : https://doi.org/10.1186/s12929-019-0524-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Journal of Biomedical Science
ISSN: 1423-0127
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

- © 2023
Alzheimer’s Disease Research
What Has Guided Research So Far and Why It Is High Time for a Paradigm Shift
- Christian Behl 0
Institute of Pathobiochemistry, University Medical Center of the Johannes Gutenberg University, Mainz, Germany
You can also search for this author in PubMed Google Scholar
- Aims to answer why—after more than 100 years of Alzheimer's research—there is still no convincing therapy available
- Informs on leading perspectives and key developments of Alzheimer's research from its beginnings up until today
- Promotes a paradigm shift in Alzheimer's Disease research and a greater openness towards new disease hypotheses
9608 Accesses
4 Citations
36 Altmetric
- Table of contents
About this book
Authors and affiliations, about the author, bibliographic information.
- Publish with us
Buying options
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
This is a preview of subscription content, log in via an institution to check for access.
Table of contents (21 chapters)
Front matter, introduction.
Christian Behl
The Psychiatrist and Pathologist Aloysius Alzheimer and His Seminal Findings
Alzheimer’s disease research after 1945: the recommencement, alzheimer’s research goes deeper: ultrastructural electron microscopy studies, focus on neurochemistry led to the cholinergic hypothesis of alzheimer’s disease, the glutamatergic hypothesis of alzheimer’s disease, biochemistry and genetics point out a prime suspect for causing alzheimer’s disease, getting to the bottom of it: amyloid beta peptide is derived from a larger precursor, step by step toward an amyloid beta peptide-based hypothesis of alzheimer’s disease, concerns about the amyloid cascade hypothesis and reappraisals, ignorance or conspiracy or just an amyloid firewall that blocks alternative ideas, in the slip stream of amyloid: the tau and tangle hypothesis, focus on tauopathies and beyond, alzheimer’s research gains momentum and spreads out, the amyloid cascade hypothesis has to deliver, finally, beyond app, psen1, psen2, and apoe: what else does the genome tell us, alternative hypotheses and observations that were somehow lost on the way, is the persistence of the amyloid cascade hypothesis a result of constant confirmation bias, driving forces of alzheimer’s research directions.
This book highlights the key phases and central findings of Alzheimer’s Disease research since the introduction of the label ‘Alzheimer’s Disease’ in 1910. The author, Christian Behl, puts dementia research in the context of the respective zeitgeist and summarizes the paths that have led to the currently available Alzheimer’s drugs. As the reader is taken through the major developments in Alzheimer's Disease research, particularly over the past thirty years, Behl poses critical questions: Why are the exact causes of Alzheimer's Disease still in the dark, despite all the immense, worldwide research efforts in academia as well as in the pharmaceutical industry? Why has the majority of an entire research field kept focusing on a single hypothesis that establishes the deposition of the amyloid beta peptide in the brain as the key trigger of Alzheimer's pathology, even though this concept has still not been convincingly proven in the clinics? Are there other hypotheses that might explainthe pathogenesis of this complex brain disease, and if so, why were these perspectives not adequately followed?
In this book, Behl tries to answer these questions. Starting with the historical background, the author illustrates the long and arduous research journey, its numerous setbacks, and the many alternative explanations for the disease, which have started gaining increasing attention and acceptance in the Alzheimer’s research community only more recently.
With his deep dive into the history and progression of this research, including the most recent developments, Behl explains why he believes that it is high time to promote a paradigm shift in Alzheimer’s Disease research.
- Alzheimer Clinics
- Alzheimer Therapy
- Alzheimer's Disease
- Amyloid Plaques
- Amyloid-Cascade-Hypothesis
- Agenda Setting
- Aternative Hypotheses
- Risk Factors
Christian Behl is Professor of Pathobiochemistry and Director of the Institute of Pathobiochemistry at the University Medical Center of the Johannes Gutenberg University Mainz, Germany. He has been closely following Alzheimer’s Disease research since the early 1990’s, when he first got involved into the field himself during his time at the Salk Institute for Biological Studies, La Jolla, USA. He stayed active in the field all through his research station at the Max Planck Institute of Psychiatry, Munich, Germany, and later in Mainz. There his current research (in Mainz) focuses on the cellular degradation mechanism autophagy in the context of neurodegeneration and aging. For quite some time Behl has been an active advocate for widening the focus of Alzheimer’s Disease research to improve the understanding of this complex, age-related brain disorder. Behl is member of several scientific boards, including the German Alzheimer Foundation.
Book Title : Alzheimer’s Disease Research
Book Subtitle : What Has Guided Research So Far and Why It Is High Time for a Paradigm Shift
Authors : Christian Behl
DOI : https://doi.org/10.1007/978-3-031-31570-1
Publisher : Springer Cham
eBook Packages : Biomedical and Life Sciences , Biomedical and Life Sciences (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2023
Hardcover ISBN : 978-3-031-31569-5 Published: 14 July 2023
Softcover ISBN : 978-3-031-31572-5 Due: 14 August 2023
eBook ISBN : 978-3-031-31570-1 Published: 13 July 2023
Edition Number : 1
Number of Pages : XXV, 652
Number of Illustrations : 9 b/w illustrations, 107 illustrations in colour
Topics : Neurosciences , Neurology , Physiology , Cognitive Psychology , Neurosciences , Neurosciences
Policies and ethics
- Find a journal
- Track your research
A cultural approach to dementia prevention
- An Introduction to Alzheimer’s Disease: What is it?

By: Adrianna Fusco
Introduction: Alzheimer’s disease, something we hear about online, in commercials, on news stations, and in many other parts of life. However, we are never told much about Alzheimer’s disease other than the devastating impacts it has. What is Alzheimer’s disease? What are the symptoms or signs to look out for? How does it progress? What causes it? How can it be prevented?
What is it? Alzheimer’s disease is a form of dementia, which is just an umbrella term used to describe loss of memory, language, problem solving, and other thinking abilities. More specifically, Alzheimer’s diseaseis a progressive, neurodegenerative disease that is categorized by a loss of memory, along with basic life skills like eating, bathing, talking, etc.
Symptoms: Common symptoms include: memory loss, paranoia, depression, anger, aggression, anxiety, apathy, loneliness, and psychosis. These symptoms vary from person to person.
Progress: As mentioned above, Alzheimer’s disease is a progressive disease. This means that it develops and gets worse over time. In the first stages of Alzheimer’s disease, there is usually very mild memory loss or problems with thinking abilities. The person may have a hard time remembering where they placed something or have a hard time recalling the right word to say. However, they still are independent, meaning they can still take care of themselves and do things like driving.
During the middle stages of Alzheimer’s disease, the cognitive processes get worse. Now the person may not be able to remember their personal history, like their address or phone number. They also may have a hard time recalling memories or remembering something from their past. The person is no longer able to take care of themselves because in this stage, they tend to forget where they are and often have a hard time using the bathroom or getting dressed appropriately for the day. An example of this is the person wearing shorts in the winter. Along with the cognitive changes, the person may begin to feel sad, lonely, anxious, and paranoid. The symptoms vary from person to person.
When the person hits stage 2, they will need a caregiver to assist them with their tasks and the caregiving will increase as the disease progresses. However, it’s important to help them without trying to do everything for them. They are still adults and they want to be treated as such, so it’s important to still let them have at least some control over their life. Whether that’s letting them do simply chores, like folding clothes, or doing activities, like arts and crafts. This will help provide a sense of normalcy.
The final stage of Alzheimer’s disease is when people begin to lose sense and control of the environment around them. By this point, the cognitive abilities of the individual have tremendously decreased. They can no longer speak in long formulated sentences, instead they speak in short fragments or words. They have trouble completing everyday tasks like walking, sitting, eating, and drinking. This means that they require around the clock assistance to make sure that they are remembering to eat and to help them eat. In general, the assistance is meant to make sure the person is safe and is living to their best ability. At this point, the individuals are very susceptible to infections. When the symptoms and daily conditions get really bad, usually, families turn to hospice care, so that the patient is comfortable at the end of their life. Hospice care also provides emotional support to loved ones, which is vital. Losing a loved one can cause serious emotional and mental strain, so that support is important.
The cause of Alzheimer’s disease is still being researched, but researchers have identified what they believe to be the main culprits of the disease: plaques and tangles.
Plaques are deposits of amyloid beta that forms between nerve cells that blocks the signals and stops the right materials from being sent to the nerve for survival. In a healthy brain, amyloid beta is used to help support neural repair and growth. However, in Alzheimer’s disease, there is an overproduction of this amyloid beta protein that disturbs these cells and eventually causes the death of the cells. The death of the old cells causes the loss of old memories and information. The blocking of nerve cells can stop the production of new connections, which means short term memories are not being accurately encoded in the brain to become long term memories.
Tangles are made up of twisted tau that builds up between cells. In a healthy brain, tau is used to help support neural strength and is important in keeping stability in the cells. However, a build up leads to the cells not being able to receive signals and the supplies it needs to function (i.e. energy). These lead to death of the cells, leading to loss of information and life skills.
There is also a biomarker known as APOE-4, that is thought to predispose people to Alzheimer’s disease. This gene along with some environmental stressors could affect whether someone gets the disease and the progression of it. However, a lot of research is still being conducted on this topic and we are constantly rerouting what we know, as new information is found.
Alzheimer’s disease is a terrible disease that claims the lives of a lot of people every year. It’s important to know the signs and to check up with your doctor when anything seems unusual. Alzheimer’s disease and dementia are not a normal part of aging, so see your doctor if you notice any issues with your memory. The earlier the disease is detected, the better it can be treated.
Stay tuned for more blog posts about Alzheimer’s disease, including a look into the mental health of caregivers, prevention, treatment, and more! We also will be writing posts about interviews with doctors, as well as posts about brain health!
Thank you for reading!
References:
“Alzheimer’s Caregivers: 8 Tips for People Caring for a Loved One With Alzheimer’s Disease or Dementia: Caregivers.” 30Seconds Health ,
30seconds.com/health/tip/14389/Alzheimers-Caregivers-8-Tips-for-People-Caring-for-a-Loved-One-With -Alzheimers-Disease-or-Dementia.
Mayeux, Richard, et al. “Treatment of Alzheimer’s Disease: NEJM.” Edited by Alastair J.J. Wood, New England Journal of Medicine , 16 Mar. 2000, www.nejm.org/doi/pdf/10.1056/NEJM199911253412207.
NHS Choices, NHS, 10 May 2018,
www.nhs.uk/conditions/alzheimers-disease/causes/#:~:text=Alzheimer’s%20disease%20is%20thought%2 0to,form%20tangles%20within%20brain%20cells.
Porsteinsson, Anton P., et al. “Neuropsychiatric Symptoms in Dementia: A Cause or Consequence?” American Journal of Psychiatry , American Psychiatric Association Publishing, 30 Apr. 2015, ajp.psychiatryonline.org/doi/10.1176/appi.ajp.2015.15030277#:~:text=The%20term%20neuropsychiatric %20symptoms%20describes%20heterogeneous%20behavioral%20or,agitation%2C%20anxiety%2C%20 apathy%2C%20depression%2C%20psychosis%2C%20and%20sleep%20disturbance.
“Stages of Alzheimer’s.” Alzheimer’s Disease and Dementia , www.alz.org/alzheimers-dementia/stages.
“What Is Alzheimer’s?” Alzheimer’s Disease and Dementia ,
www.alz.org/alzheimers-dementia/what-is-alzheimers.
129 Alzheimer’s Disease Essay Topics & Examples
If you’re writing about patients with memory loss or dementia care and treatment, this article will be of use. Our team has prepared Alzheimer’s disease essay examples and topics below.
🏆 Best Alzheimer’s Disease Essay Examples & Topics
💡 most interesting alzheimer’s disease topics to write about, 📌 simple & easy alzheimer’s disease research topics, 👍 good research topics about alzheimer’s disease, ❓ research questions about alzheimer’s disease.
- The Case Study of Patient With Late-Stage Alzheimer’s Disease In the majority of cases of Alzheimer’s, it has been shown that patients are unable to make decisions on their own and are also unable to communicate their assent verbally.
- Therapeutic Dogs, Dementia, Alzheimer’s and Fluid Intelligence It is worth noting that with dementia, the patient has a speech disorder and a personality change in the early stages of the pathology.
- The Alzheimer’s Association Dementia Care Practice Therefore, achieving the philosophy and recommendations of the association is a shared responsibility between doctors, patients, and caregivers. Ultimately, CAPD tests the functionalities of the patient ranging from the psychomotor activities, perceptions, awareness, and orientations, […]
- Dementia, Alzheimer, and Delirium in an Elderly Woman Additionally, she struggles with identifying the appropriate words to use in dialogue and changes the topic. Timing: While in the middle of conversations and public places like supermarkets.
- Alzheimer’s Disease Diagnosis and Intervention The accumulation of plaques and tangles in the brain is a hallmark of the disease, resulting in the death of neurons and a decline in mental capacity.
- Alzheimer’s Disease: Assessment and Intervention The caregiver is recommended to install safety locks and alarms on all doors and windows to prevent the patient from leaving the apartment without supervision.
- Diagnosis of Alzheimer’s or Mild Cognitive Impairment Additionally, it could be mild cognitive impairment as the state shares symptoms with early-onset Alzheimer’s, and if there would be a decline of the signs in the future.
- Management of a Patient With Alzheimer’s: Case Study The correlation between this issue and the probability of the emergence of AD in elderly citizens is proved by the scholars who examined the impact of the quality of air on a person’s health.
- Bilinguals’ Cognitive-Linguistic Abilities and Alzheimer’s Disease This irregularity is reflected in the preserved linguistic abilities, including code-switching and semantic fluency, and the declined functions in translation, picture naming, and phonemic fluency, calling for improved therapy and testing practices.
- Managing Dementia and Alzheimer’s Disease The PICOT question is “In the care of Alzheimer’s and dementia patients, does integrated community-based care as compared to being in a long-term care facility improve outcome throughout the remainder of their lives”.
- Pathophysiology of Alzheimer’s Disease The study will discuss the pathophysiology of Alzheimer’s disease, such as risk factors, cellular involvement, genetic influences, and the interventions of the available therapy’s pharmacological Interventions.
- Alzheimer’s Disease: Definition, Stages, Diagnosis Alzheimer’s disease is the most common type of dementia, and it is a condition in which the brain stops appropriately performing its functions.
- Fall Risk Assessment of Alzheimer’s Patient The nurse answers questions about the old lady helps fill the Stay Independent brochure and assists the observing physician in carrying the various clinical tests on the patient.
- Alzheimer’s Disease in an Iranian Patient The patient in the company of his son returns to the clinic after four weeks. Since the patient shows no side effects of the disease and an increase in Exelon to 6 mg orally BID […]
- Mr. Akkad and Alzheimer’s Disease: Case Study The onset of the symptoms is reported to have been within the past two years, but the situation has begun to deteriorate, prompting Mr.
- Alzheimer’s Disease: History, Mechanisms and Treatment Nevertheless, researchers state that the development of Alzheimer’s is impacted by the formation of protein plaques and tangles in the brain.
- Alzheimer’s Disease: Causes and Treatment AD is associated with different changes, both cognitive and behavioral. A patient can observe some or all of them depending on the development of the disease.
- Frontotemporal Dementia vs. Alzheimer’s Disease in a Patient Moreover, Alzheimer’s disease affects hypertrophies in the hippocampus as the initial part is involved in the brain’s memory areas and spatial orientation.
- Alzheimer’s Disease: Diagnostic and Treatment Alzheimer’s disease is a progressive degenerative disorder that causes a deterioration of mental and cognitive abilities.
- The Effect of Music on People With Alzheimer’s Disease The evidence suggests that one of the most prominent effects of music on patients with Alzheimer’s disease is autobiographical memory preservation alongside the stimulation of both sympathetic and parasympathetic nervous systems.
- Community Health: Alzheimer’s Disease The community nurse’s role is to develop and participate in primary, secondary, and tertiary preventive strategies and to provide a wide range of nursing care services while maintaining the health and wellbeing of individuals with […]
- Challenges of Living With Alzheimer Disease The medications make the condition of the patient better during the first stages of the disease. During the middle stage of the disease, the symptoms worsen.
- The Burden of Alzheimer’s Disease Assessing the appropriateness and effectiveness of reducing the cost of providing care for patients with Alzheimer remains a major issue that needs to be addressed.
- Chronic Care For Alzheimer’s Disease The application of the Chronic Care Model, in its turn, will serve as the foundation for building the patient’s awareness about their condition, thus, improving the patient’s quality of life and creating the environment, in […]
- Synopsis of Research Studies of Individuals Afflicted by Mild Alzheimer’s Disease The research questions in the articles were tailored along the various physical activities that can assist patients affected by Alzheimer Disease.
- Alzheimer’s Disease and Naturopathic Medicine The main feature of AD is the aggregation of -amyloid. However, application of natural therapies to prohibit the process of the pathways can slow the progress of AD.
- Brain Reduction and Presence of Alzheimer’s Disease The purpose of the study was to examine the correlation between brain reduction and the presence of Alzheimer’s disease. The researchers wanted to examine the nature of such changes in elderly individuals at low risk […]
- Alzheimer Related Morbidity and Death Among New Yorkers Generally, Alzheimer disease is a form of dementia, which inflicts a loss of memory, thinking and behavior. The proportion of ethnic and racial diversity in the US is increasing.
- Human Disorders: Alzheimer’s Disease and Dementia The brain shows notable changes in Alzheimer’s disease notably, development of tangles in deep areas of the brain and also formation of plagues in other areas.
- Environmental Interview on a Patient With Alzheimer Disease In the 1980s, delusions and hallucinations were added as signs of the disease. Researches in the 1960’s show a link between cognitive reduction and the number of ailments in the brain.
- Alzheimer’s Disease Article and Clinical Trial This study shows that environmental hazards, in this case lead, increase the risk of developing Alzheimer’s disease and that the development period is crucial for determining future vulnerability to neurodegeneration and Alzheimer’s disease.
- Alzheimer’s Disease: Regarding Physiology However, one clear aspect of the development of this disease arises from a very complex chain of activities taking place in the brain over a long period of time.
- Mapping the Neurofibrillary Degeneration From Alzheimer’s Disease Patient This is an analytic review of the studies elaborating on the relationship of hyperphosphorylated tau proteins to the development of Alzheimer’s disease and focusing on the antigen capture ELISA specific for p-tau proteins.
- Alzheimer’s Disease: Key Aspects This event constitutes part of a broader campaign, which includes fundraising, information support, and promotion of specialized care for everybody suffering from the disease.
- Role of Alzheimer’s Disease Advanced in Our Understanding of the Aging Process Aging on the hand can be defined as the accumulation of different harmful changes in the tissues and cells that raises the possibility of disease and death.
- Depression and Alzheimer’s Disease Moretti et al have studied the relationship between depression and Alzheimer’s disease and explored whether depression is a symptom of AD or comorbidity.
- Alzheimer’s Disease: Medical Analysis Such gene-associated markers have been characterized, in particular the apolipoprotein E gene, which was linked to chromosome# 19, and was responsible for accumulation of A by way of binding to this protein.
- Diabetic Teaching Plan for Alzheimer’s Patient He knows the purposes and some of the steps and needs to be taught again to regain his independence in monitoring his blood glucose level.
- Comparing Alzheimer’s Disease and Parkinson’s Disease There are many superficial similarities between Alzheimer’s disease and Parkinson’s disease primarily in some symptoms and age-group of persons afflicted by these two diseases.
- Alzheimer’s Disease and Long Term Care Alzheimer’s disease is a progressive disease in which memory impairment and disturbances in reasoning and perception are the primary symptoms. Also, well-known skills and recognition of objects and person is diminished in this stage of […]
- The Effects of Alzheimer’s Disease on Family Members The disease develops gradually and is said to be a disease of the old because it relates to the inability to remember.
- Alzheimer’s Disease in Science Daily News Article The news article accurately reports the focus of the study in the diagnosis of AD. Hence, the news article accurately presents that the diagnostic method is important in the diagnosis and prognosis of AD among […]
- Dancing and Risk of Alzheimer’s Disease Despite the fact that there is no effective treatment for Alzheimer’s disease, scientists discovered that dancing could help reduce the severity of the disorder as this activity involves simultaneous brain functioning, which helps to affect […]
- Alzheimer’s Disease Prevalence and Prevention The estimated global prevalence of Alzheimer’s disease is 50 million and is projected to triple by 2050 due to growth in the older generation. According to Alzheimer’s Association, AD is the fifth-ranking killer of persons […]
- Alzheimer’s Disease: Managing Cognitive Dysfunction In the majority of cases, Alzheimer’s disease turns out to be the cause of this problem. Alzheimer’s disease can be caused by different risk factors, but in the majority of cases, it is associated with […]
- Alzheimer’s Disease in Newspaper Articles The number of patients diagnosed with Alzheimer’s and diabetes in the United States, and indeed globally, has increased significantly in the last few years. This means that the main interest of such collaboration is to […]
- Alzheimer’s and Cardiovascular Diseases Progress While the design of the study involves a review of the existing papers and a compilation of their key results, the information provided by the authors is nonetheless crucial to the understanding of the issue.
- Heart Disease and Alzheimer’s in Adult Women Education and Employment History: The patient reported she is a college graduate and has a master’s degree in Victorian Literature. The patient is currently working full-time as a Literature professor at UC Berkeley, in a […]
- The Alzheimer’s Disease Concept In simple words, it is the condition caused by the negative changes in the human brain that, as the end result, leads to memory loss and some behavioral issues that worsen the quality of patient’s […]
- Alzheimer’s Disease, Its Nature and Diagnostics According to the Alzheimer’s Association, this condition is the sixth leading cause of lethal outcomes in the United States. The most frequent symptoms of Alzheimer’s disease include problems with memory, reasoning, thinking processes, perception, and […]
- Alzheimer’s Disease in Medical Research The existing data proposes that if the illness is distinguished before the commencement of evident warning signs, it is probable that the treatments founded on the facts of fundamental pathogenesis will be of assistance in […]
- Alzheimer’s Disease and Antisocial Personality Disorder Since there is currently no cure for Alzheimer’s disease, the future of the nursing care for the people that have the identified disorder concerns mostly maintaining the patient’s quality of life.
- Plasma Amyloid-Beta and Alzheimer’s Disease The impact of AD on public health includes increased rates of informal care and the direct charges of communal care. The aim of this study is to find the precise relationship between plasma amyloid beta […]
- Age Ailment: Dementia and Alzheimer’s Disease It is a time for one to clean the mind and take time to do what matters most in life. With an increased level of technological advancements, a digital sabbatical is mandatory to lower the […]
- Psychology Issues: Alzheimer’s Disease Alzheimer’s disease is a psychological disorder that involves the progressive destruction of brain cells and reduction in the proper functioning of the brain.
- Importance of Drug Therapy in Management of Alzheimer’s Disease The effects of Alzheimer’s disease can be controlled by early detection. Most studies are based on the effects of drug therapy mild Alzheimer’s patients.
- The Development of Alzheimer’s Disease and It’s Effect on the Brain Research studies have revealed that prevalence of the Alzheimer’s disease is increasing exponentially due to change in lifestyles and the incurable nature of the disease.
- Treatment of Alzheimer’s Disease According to documented research, Alzheimer’s disease is the primary cause of dementia affecting close to half a million people in the United Kingdom and five million in the United States.
- Health Care for Elderly People With Alzheimer’s Disease C’s condition is not likely to affect the relationship between her and her relatives if they are sensible toward her. C is to take her to a nursing home for the elderly.
- Diagnosis of Alzheimer’s Disease The most remarkable feature of the disease is the loss of ability to remember events in an individual’s life. According to the latter hypothetical medical study, it has been exemplified that the presence of deposits […]
- Concept and Treatment of the Alzheimer Disorder This implies that cognitive and natural therapies are highly perceived to be effective as opposed to pharmacological treatments. One cannot ignore the fact that both cognitive and natural therapies have become widely accepted in treating […]
- Understanding Alzheimer’s Disease Among Older Population After the 65 years, it has been found that the probability of developing Alzheimer’s disease doubles after every 5 years and as a result, by the age of 85 years, the risk of acquiring the […]
- Concepts of Alzheimer’s Disease The brain changes are the same in both men and women suffering from Alzheimer’s disease. There is also a significant increase in the death of the neurons leading to the shrinking of the affected regions.
- Alzheimer’s Association Of Neurological Disorders And Stroke
- The Potential Treatment of Alzheimer’s Disease: Through CRISPR-Cas9 Genome Editing
- Alzheimer’s Condition as an Enemy of Mental Health
- Vitamin A as a Potential Therapy to Prevent Alzheimer’s Disease
- The Relationship Between Gender And Alzheimer’s Disease
- The Stages and Treatments of Alzheimer’s Disease
- The Clinical Description of the Causes, Symptoms and Treatment of Alzheimer’s Disease
- The Description of Alzheimer’s Disease and Its Statistics in America
- The Psychological Symptoms Of Alzheimer’s The Cognitive Symptoms
- Varying Aspects of Alzheimer’s Disease and Implementations
- The Effects Of Alzheimer’s And Dementia Among Elderly
- The Early Symptoms and Progression of Alzheimer’s Disease
- Watching a Loved One Slip Away from Alzheimer’s Disease
- The Differences Between Dementia And Alzheimer’s Dementia
- A History of Alzheimer’s Disease and Why it is Still One of the Most Researched Diseases Today
- A Healthy Lifestyle Might Help Combat Parkinson’s Disease And Alzheimer’s Disease
- The Studies Of Music And How It May Not Help The Alzheimer’s Disease
- The Trials of Caring For A Loved One With Alzheimer’s Disease
- Alzheimer’s Disease A Progressive And Fatal Disease Of The Brain
- The Effects of Dementia and Alzheimer’s Disease on Caregivers and the Care Needed for Suffering Patients
- The Psychologist’s Role in Addressing Family and Community Problems for Families with Alzheimer’s Disease
- Alzheimer’s Disease and Its Effect on the Patient and Care Giver
- The Statistics of Prevalence of Alzheimer’s Disease in the 21st Century
- The Link Between Down Syndrome and Alzheimer’s Disease
- The Pathophysiology Of Alzheimer’s Disease
- The Causes, Symptoms and Treatment of Alzheimer’s Disease
- The Focus on Alzheimer’s Disease in the Documentary Black Daises for the Bride
- The Physiology and Genetics Behind Alzheimer’s Disease
- The Early Manifestations of Alzheimer’s Disease
- The Role Of Gamma Secretase In Alzheimer’s Disease
- The Lack Of Early Detection Of Alzheimer’s Disease
- The Representation of Alzheimer’s Disease and Its Impact in the Film Still Alice
- The Possible Link of the Human Immune System to Alzheimer’s Disease
- The Study of Alzheimer’s Disease and Its Affect on the Elderly
- The Characteristics, History, Symptoms, Statistics, and Treatment of Alzheimer’s Disease, a Degenerative Brain Disease
- The Triggers, Progression, and Treatment of Alzheimer’s Disease
- Traumatic Brain Injury and Alzheimer’s Disease
- The Positive Impact of Exercise in Protecting the Brain from Alzheimer’s Disease
- Three Primary Types of Dementia: Alzheimer’s Disease, Vascular Dementia
- The Causes, Risks, Factors, and Stages of Alzheimer’s Disease
- The Contingent Valuation Method in Health Care: An Economic Evaluation of Alzheimer’s Disease
- What Is the Difference Between Dementia and Alzheimer’s Disease?
- What Is the Main Cause of Alzheimer’s Disease?
- How Do You Prevent Alzheimer’s Disease?
- Who Is at High Risk for Alzheimer’s Disease?
- What Foods Cause Alzheimer’s Disease?
- Do Alzheimer’s Disease Patients Sleep a Lot?
- Do Alzheimer’s Disease Patients Know They Have It?
- Do Alzheimer’s Disease Patients Feel Pain?
- What Is the Best Treatment for Alzheimer’s Disease?
- How Long Do Alzheimer’s Disease Patients Live?
- What Do Alzheimer’s Disease Patients Think?
- Do People with Alzheimer’s Disease Have Trouble Walking?
- Is End Stage Alzheimer’s Disease Painful?
- What Are the Final Stages of Alzheimer’s Disease Before Death?
- Does Alzheimer’s Disease Run in Families?
- Should You Tell Alzheimer’s Disease Patients the Truth?
- Why Do Alzheimer’s Disease Patients Stop Talking?
- How Do You Know When an Alzheimer’s Disease Patient Is Dying?
- Which Is Worse: Dementia or Alzheimer’s Disease?
- What to Say to Someone Who Has Alzheimer’s Disease?
- How Does Alzheimer’s Disease Affect Eyes?
- Are Alzheimer’s Disease Patients Happy?
- What Are the Warning Signs of Alzheimer’s Disease?
- What Is the Best Way to Help Someone with Alzheimer’s Disease?
- What Are Good Activities for Alzheimer’s Disease Patients?
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 21). 129 Alzheimer’s Disease Essay Topics & Examples. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/
"129 Alzheimer’s Disease Essay Topics & Examples." IvyPanda , 21 Feb. 2024, ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
IvyPanda . (2024) '129 Alzheimer’s Disease Essay Topics & Examples'. 21 February.
IvyPanda . 2024. "129 Alzheimer’s Disease Essay Topics & Examples." February 21, 2024. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
1. IvyPanda . "129 Alzheimer’s Disease Essay Topics & Examples." February 21, 2024. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
Bibliography
IvyPanda . "129 Alzheimer’s Disease Essay Topics & Examples." February 21, 2024. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
- Brain Titles
- Diabetes Questions
- Dementia Research Ideas
- Cardiovascular Diseases Titles
- Dyslexia Topics
- OCD Essay Titles
- Depression Essay Topics
- Disease Questions
- Disorders Ideas
- Nervous System Research Topics
- Pathogenesis Research Ideas
- Caregiver Topics
- Health Promotion Research Topics
- Neuropsychology Topics
- Therapeutics Research Ideas
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NATURE INDEX
- 13 March 2024
Researchers call for a major rethink of how Alzheimer’s treatments are evaluated
- Esther Landhuis 0
Esther Landhuis is a science journalist in the San Francisco Bay Area, California.
You can also search for this author in PubMed Google Scholar

Credit: Taj Francis
In January 2023, the US Food and Drug Administration (FDA) approved lecanemab — an antibody medication that decreases β-amyloid protein build-up in the brain — as a treatment for Alzheimer’s disease. Pivotal evidence came from a large, randomized trial of people with early-stage Alzheimer’s , which afflicts around 32 million people worldwide. By the end of that 18-month study 1 , patients in the placebo group scored on average 1.66 points worse than their performance at baseline on a standard dementia test, which assesses cognitive and functional changes over time through interviews with a patient and their caregiver. The mean score of treated participants, by comparison, worsened by 1.21 points — a 27% slowing of cognitive decline.
But is this improvement meaningful for patients and their families?

Nature Index 2024 Health sciences
There are two major categories of drugs used to treat Alzheimer’s disease and other progressive conditions: symptomatic drugs, which treat the symptoms, and disease-modifying drugs, which target the root cause. Donepezil and rivastigmine, for example, are symptomatic drugs that boost the activity of chemicals in the brain to compensate for declines in cognitive and memory function caused by Alzheimer’s disease, but they cannot stop its progression . Lecanemab, developed jointly by Japanese pharmaceutical company Eisai and American biotechnology firm Biogen, targets the underlying issue of amyloid build-up in the brain, and in doing so, could fundamentally change the course of the disease.
An important feature of disease-modifying drugs is that their benefits are cumulative. Studies of patients with multiple sclerosis , for example, have shown the benefits of starting disease-modifying drugs earlier in the course of the disease compared with later, including improved mortality rates and reduced disability in the long term. Being able to quantify how long a disease-modifying drug can delay or halt the progression of Alzheimer’s disease could change how researchers understand — and communicate — its benefits.
In studies of potential disease-modifying drugs for Alzheimer’s disease, there has always been a tension between being able to produce a treatment effect and being able to measure it, says Suzanne Hendrix, statistician and founder of the clinical trials consulting firm Pentara in Salt Lake City, Utah. Clinical trials generally enrol early-stage patients — those with mild cognitive impairment and evidence of brain amyloid — because amyloid-targeting therapies have the best chance of working if given well before the disease takes hold. But in the early stages, patients deteriorate so gradually that it can be difficult to perceive the impact of a disease-modifying drug using standardized tests.
At a scientific meeting in 2009, Hendrix recalls being pulled aside by an executive at Eisai, who told her: “Nobody’s measuring this disease right. Until we measure the most progressive aspects of disease, we’re not going to be able to see treatment effects.”
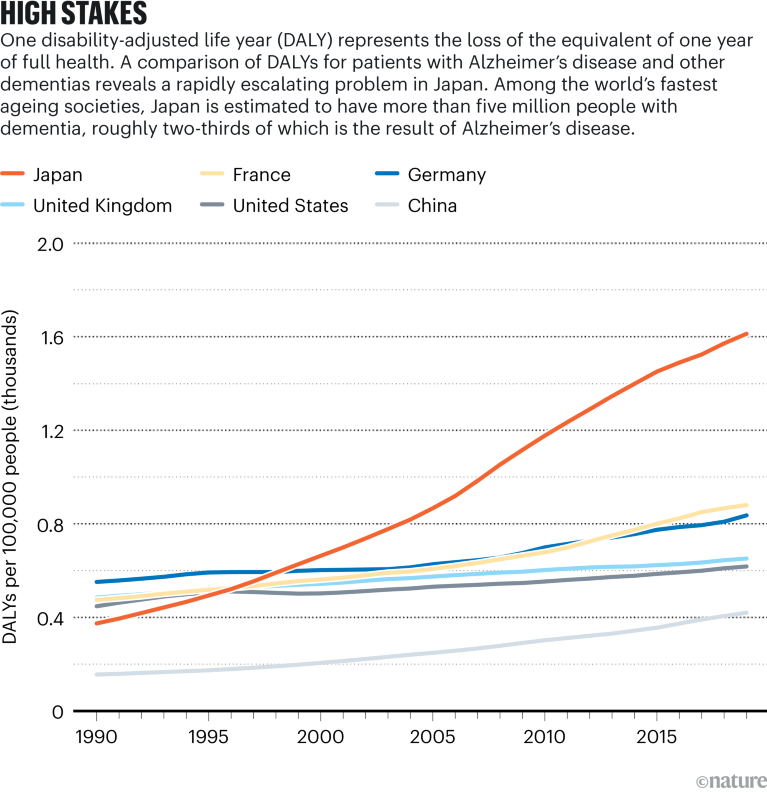
Source: Institute for Health Metrics and Evaluation; Cummings, J. L., Goldman, D. P., Simmons-Stern, N. R., Ponton, E. Alzheimers Dement. 18 , 469–477 (2022)
Hendrix and other researchers are exploring time-based metrics as a new approach. Savings of time, measured as prolonged quality of life after 18 months of treatment, for example, is “much easier to talk about” than point differences on cognitive and functional scales, says Lars Rau Raket, a statistician at the Copenhagen, Denmark, branch of US pharmaceutical company Eli Lilly. For early-stage Alzheimer’s patients , says Raket, “it’s about how much you can extend the time in the ‘good parts’ — in the milder stages of disease”.
Straight line to time
To come up with a time-based approach, Hendrix and her colleagues pooled parts of several rating scales from standard dementia tests to develop a new tool called that picks up on subtle changes that occur in early Alzheimer’s. By zeroing in on where changes are more pronounced in these early stages, such as a diminished ability to juggle tasks or to recall past events, the team could track the progression of several key features of the disease.
To measure the effectiveness of disease-modifying treatments on these key features as units of time, the researchers used clinical outcomes from placebo and treated participants in a phase II trial of another amyloid-lowering therapy, donanemab . They calculated that over the 76-week duration of the trial, overall disease progression was delayed by 5.2 months.
In a paper published last year 2 , when he was working for Danish firm Novo Nordisk, in a lab just outside Copenhagen, Raket took a similar approach to calculating treatment effects in terms of time. But their methods differed in some ways. Whereas Hendrix’s work focused on calculating time savings across multiple outcomes, Raket used multiple models to calculate time savings for each outcome measure.
The idea of time-based models seems to be gaining traction. They were used as exploratory measures in a phase III trial of donanemab, conducted by Eli Lilly and Company, and published in JAMA last year 3 . Eisai also showed a time-based analysis in a 2022 presentation of its phase III lecanemab data at the Clinical Trials on Alzheimer’s Disease meeting in San Francisco. In those analyses, participants treated with lecanemab took 25.5 months to reach the same degree of worsening on a common dementia test as the placebo group did at 18 months — a time saving of 7.5 months.
Raket says he has been approached by several people in the pharmaceutical industry and academia, and some are working with him to apply the concept to their research. At the 2023 Alzheimer’s Association International Conference in Amsterdam, Raket and his collaborators in the United States, Canada and Europe compared time-based models with conventional statistical approaches for progressive diseases, and analysed how delays in disease progression calculated with time-based methods translate to treatment differences on standard cognitive tests. “I haven’t experienced this kind of interest in my work before,” he says. Raket predicts that an increasing number of trials in the neurodegeneration space will be reporting time-savings estimates in the years to come.
Broad impacts
Beyond Alzheimer’s disease, time-saved models could be applied to other progressive conditions, including Parkinson’s disease and amyotrophic lateral sclerosis (ALS). Cancer and cardiovascular disease studies, which tend to focus on events — delaying relapse or death, or cutting the risk of heart attacks, for instance — are less suited to models that track progression. If, however, heart disease were conceptualized as a gradual worsening of blood pressure or cholesterol over time, and treatment could be shown to slow the rate of deterioration, the time-saved approach could be used to measure the treatment benefit, says Hendrix.
One benefit of time-based methods is that they could help make clinical trials less prone to being skewed by outliers , says Geert Molenberghs, a biostatistician at KU Leuven and Hasselt University, both in Belgium, who collaborates with Hendrix. For example, a small subset of people with early Alzheimer’s disease deteriorate unusually quickly. If these rapid decliners are in the treated group, they could potentially mask a drug benefit, says Molenberghs. The details become “very technical”, he says, but with time-based approaches, these rare individuals “are less influential. They have less capacity to overturn the statistics.”
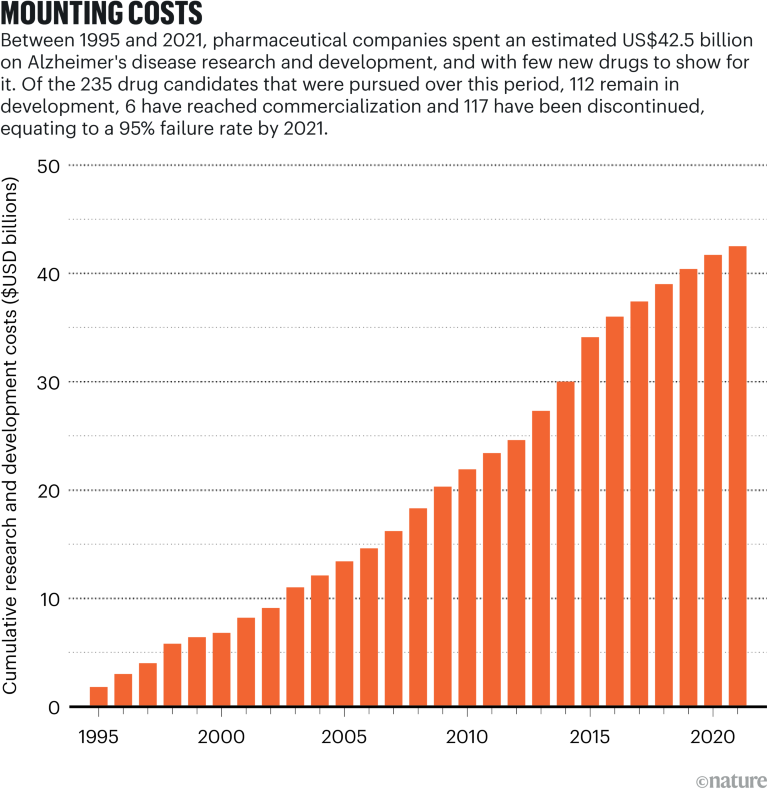
Time-based metrics could impact broader conversations with health economists and policymakers. “The idea that you could take somebody who’s already in their senior years and keep them functional and not needing 24/7 care — that’s incredibly valuable information for making estimates about the true burden or cost of the disease to caregivers and society,” says John Harrison, chief science officer at Scottish Brain Sciences, a research institute in Edinburgh, Scotland. “It’s a very neat communications tool which feeds into estimates of progression, cost, strategy and, one hopes, legislation and planning.”
There are open questions that might need to be addressed before time-saved models are more widely applied in clinical trials. One is that, although time progresses linearly, not all points on that line are equally meaningful. For example, the anti-amyloid mechanism might only be beneficial in the early stages of Alzheimer’s disease, says Ron Petersen, a neurologist at Mayo Clinic in Rochester, Minnesota. “By the time the person progresses to, say, moderate dementia, modifying amyloid probably isn’t going to make any difference.”
Hendrix is hopeful that the time-saved idea can be further developed and applied to clinical trials in the future, because it could make a big difference in tracking not only how effective new disease-modifying drugs are, but also in helping Alzheimer’s patients and their families to better understand the progression of the disease and how they can plan for it.
Ultimately, as more studies “start focusing on how much time we’ve saved people, all of the effects that we see will be more relevant” to people’s daily lives, Hendrix says.
Nature 627 , S18-S20 (2024)
doi: https://doi.org/10.1038/d41586-024-00756-8
This article is part of Nature Index 2024 Health sciences , an editorially independent supplement. Advertisers have no influence over the content.
Van Dyck, C. H. et al. N. Engl. J. Med. 388 , 9–21 (2023).
Article PubMed Google Scholar
Raket, L. L. Stat. Med. 41 , 5537–5557 (2022).
Sims, J. R. et al. JAMA 330 , 512–527 (2023).
Download references
Related Articles

Partner content: A holistic approach to health in Japan
Partner content: Can we increase the safety of carbon nanotubes?
Partner content: Building for a digitized, personalized future
Partner content: Translating basic research into medical advances
Partner content: Meds, meals and microbes: gut health on the cancer front line
Partner content: A hotspot for research and development of medical AI
Partner content: What are we learning from the world’s myopia capital?
Partner content: Systems biology: a network view of disease could unearth hidden targets
Partner content: Learning from the pupil: the power of pupillometry
Partner content: Does green tea offer cognitive and sleep benefits?
Partner content: A team effort delivers a new tool for fighting neuroblastoma
Partner content: Shaping the future of medical science - A multidisciplinary paradigm
Partner content: Harnessing AI to see a patient’s unique patterns
Partner content: Improving the outlook for chronic kidney disease
Partner content: From medical robots to a mouse metaverse
Partner content: What comes after clinical trials?
Partner content: Making vitamin D detection much more accessible
Partner content: A new injectable gel could seal wounds after cancer surgery
Partner content: The surprising interplay between metabolism and mind
Partner content: Detecting atrial fibrillation while there’s still time to act
- Alzheimer's disease
- Medical research
- Health care
How to make an old immune system young again
News 27 MAR 24

Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity
Article 27 MAR 24

Pregnancy advances your ‘biological’ age — but giving birth turns it back
News 22 MAR 24
Anti-ageing antibodies revive the immune system
News & Views 27 MAR 24

Abortion-pill challenge provokes doubt from US Supreme Court
News 26 MAR 24

The future of at-home molecular testing
Outlook 21 MAR 24
Postdoctoral Fellowships-Metabolic control of cell growth and senescence
Postdoctoral positions in the team Cell growth control by nutrients at Inst. Necker, Université Paris Cité, Inserm, Paris, France.
Paris, Ile-de-France (FR)
Inserm DR IDF Paris Centre Nord
Zhejiang Provincial Hospital of Chinese Medicine on Open Recruitment of Medical Talents and Postdocs
Director of Clinical Department, Professor, Researcher, Post-doctor
Hangzhou, Zhejiang, China
The First Affiliated Hospital of Zhejiang Chinese Medical University
Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Warmly Welcomes Talents Abroad
“Qiushi” Distinguished Scholar, Zhejiang University, including Professor and Physician
No. 3, Qingchun East Road, Hangzhou, Zhejiang (CN)
Sir Run Run Shaw Hospital Affiliated with Zhejiang University School of Medicine
Global Faculty Recruitment of School of Life Sciences, Tsinghua University
The School of Life Sciences at Tsinghua University invites applications for tenure-track or tenured faculty positions at all ranks (Assistant/Ass...
Beijing, China
Tsinghua University (The School of Life Sciences)
Faculty Positions at Great Bay University, China
We are now seeking outstanding candidates in Physics, Chemistry and Physical Sciences.
Dongguan, Guangdong, China
Great Bay University, China (GBU)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Ask a doc: 'How can I reduce the risk of Alzheimer’s disease?' Here are 3 tips
W hile there currently is no cure for Alzheimer’s disease — the most common type of dementia — there are healthy steps a person can take to reduce their risk or possibly prevent it.
Jessica Caldwell, PhD, a neuropsychologist with Cleveland Clinic, shared her top tips for keeping Alzheimer’s at bay.
She has three.
Caldwell first recommends incorporating exercise into one's daily routine.
THESE ADULT VACCINES COULD REDUCE SENIORS’ RISK OF ALZHEIMER’S, STUDY FINDS: ‘HEIGHTENED IMMUNE RESPONSE’
"The reason exercise is so important is that it multitasks," Caldwell said in commentary provided by Cleveland Clinic.
READ ON THE FOX NEWS APP
"First and foremost, when you exercise, a chemical is released in your brain immediately and over the long term that supports your memory system in the brain."
Exercise can also help sharpen the ability to grow new neural pathways and learn new things, the doctor added.
Studies have shown that resistance training and physical exercise can decrease the formation of beta-amyloid plaques, which are proteins that build up in the brain and lead to the development of Alzheimer's disease.
LOSS OF SMELL COULD BE WARNING SIGN FOR FUTURE ALZHEIMER’S DISEASE, RESEARCHERS SAY
"In addition, exercise can aid in reducing stress hormones and inflammation in the body – both of which, if chronic, can cause problems for your memory system and your Alzheimer’s disease risk," Caldwell said.
Any kind of moderate-intensity exercise, like a brisk walk, will provide benefits, Caldwell said. Strive for a goal of 150 minutes per week, she recommends.
The doctor’s second tip for preventing Alzheimer’s disease is to get enough sleep — ideally between seven and eight continuous hours per night.
If you don’t get proper sleep , it could impact your memory the next day, Caldwell warned.
DEMENTIA-DEPRESSION CONNECTION: EARLY SADNESS CAN LEAD TO LATER COGNITIVE ISSUES, STUDY FINDS
"When we sleep, during certain stages of our sleep and not others, our brain actually clears debris," she said.
"One of the types of debris our brain clears is amyloid, the protein that builds up in unhelpful and pathological ways when it comes to Alzheimer’s disease."
Finally, Caldwell recommends adopting a Mediterranean diet, which focuses on eating healthy fats, whole foods, leafy greens, whole grains, fruits, nuts, seeds, and herbs and spices.
"Research has shown this kind of diet is good for your brain and heart health," she said.
In March, researchers from the Rush University Medical Center in Chicago, Illinois, analyzed the autopsy results of 581 participants of the Rush Memory and Aging Project.
The participants had provided their complete dietary information at the start of the study.
As Fox News Digital reported in March, those who followed a Mediterranean diet — particularly eating green, leafy vegetables — showed fewer signs of Alzheimer’s in their brain tissue.
There are currently more than six million Americans living with Alzheimer's in the U.S., according to the Alzheimer’s Association.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
That number is expected to grow to nearly 13 million by 2050.
Original article source: Ask a doc: 'How can I reduce the risk of Alzheimer’s disease?' Here are 3 tips

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Dementia prevention, intervention, and care: 2020 report of the Lancet Commission
Gill livingston.
a Division of Psychiatry, University College London, London, UK
d Camden and Islington NHS Foundation Trust, London, UK
Jonathan Huntley
Andrew sommerlad.
f National Ageing Research Institute and Academic Unit for Psychiatry of Old Age, University of Melbourne, Royal Melbourne Hospital, Parkville, VIC, Australia
Clive Ballard
g University of Exeter, Exeter, UK
Sube Banerjee
h Faculty of Health: Medicine, Dentistry and Human Sciences, University of Plymouth, Plymouth, UK
Carol Brayne
i Cambridge Institute of Public Health, University of Cambridge, Cambridge, UK
Alistair Burns
j Department of Old Age Psychiatry, University of Manchester, Manchester, UK
Jiska Cohen-Mansfield
k Department of Health Promotion, School of Public Health, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
l Heczeg Institute on Aging, Tel Aviv University, Tel Aviv, Israel
m Minerva Center for Interdisciplinary Study of End of Life, Tel Aviv University, Tel Aviv, Israel
Claudia Cooper
Sergi g costafreda.
n Department of Preventive and Social Medicine, Goa Medical College, Goa, India
b Dementia Research Centre, UK Dementia Research Institute, University College London, London, UK
o Institute of Neurology, National Hospital for Neurology and Neurosurgery, University College London Hospitals NHS Foundation Trust, London, UK
Laura N Gitlin
p Center for Innovative Care in Aging, Johns Hopkins University, Baltimore, MA, USA
Robert Howard
Helen c kales.
r Department of Psychiatry and Behavioral Sciences, UC Davis School of Medicine, University of California, Sacramento, CA, USA
Mika Kivimäki
c Department of Epidemiology and Public Health, University College London, London, UK
Eric B Larson
s Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA
Adesola Ogunniyi
t University College Hospital, Ibadan, Nigeria
Vasiliki Orgeta
Karen ritchie.
u Inserm, Unit 1061, Neuropsychiatry: Epidemiological and Clinical Research, La Colombière Hospital, University of Montpellier, Montpellier, France
v Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, UK
Kenneth Rockwood
w Centre for the Health Care of Elderly People, Geriatric Medicine Dalhousie University, Halifax, NS, Canada

Elizabeth L Sampson
e Barnet, Enfield, and Haringey Mental Health Trust, London, UK
Quincy Samus
q Department of Psychiatry and Behavioral Sciences, Johns Hopkins University, Baltimore, MA, USA
Lon S Schneider
x Department of Psychiatry and the Behavioural Sciences and Department of Neurology, Keck School of Medicine, Leonard Davis School of Gerontology of the University of Southern California, Los Angeles, CA, USA
Geir Selbæk
y Norwegian National Advisory Unit on Ageing and Health, Vestfold Hospital Trust, Tønsberg, Norway
z Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway
aa Geriatric Department, Oslo University Hospital, Oslo, Norway
ab Department Psychosocial and Community Health, School of Nursing, University of Washington, Seattle, WA, USA
Naaheed Mukadam
Associated data, executive summary.
The number of older people, including those living with dementia, is rising, as younger age mortality declines. However, the age-specific incidence of dementia has fallen in many countries, probably because of improvements in education, nutrition, health care, and lifestyle changes. Overall, a growing body of evidence supports the nine potentially modifiable risk factors for dementia modelled by the 2017 Lancet Commission on dementia prevention, intervention, and care: less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, and low social contact. We now add three more risk factors for dementia with newer, convincing evidence. These factors are excessive alcohol consumption, traumatic brain injury, and air pollution. We have completed new reviews and meta-analyses and incorporated these into an updated 12 risk factor life-course model of dementia prevention. Together the 12 modifiable risk factors account for around 40% of worldwide dementias, which consequently could theoretically be prevented or delayed. The potential for prevention is high and might be higher in low-income and middle-income countries (LMIC) where more dementias occur.
Our new life-course model and evidence synthesis has paramount worldwide policy implications. It is never too early and never too late in the life course for dementia prevention. Early-life (younger than 45 years) risks, such as less education, affect cognitive reserve; midlife (45–65 years), and later-life (older than 65 years) risk factors influence reserve and triggering of neuropathological developments. Culture, poverty, and inequality are key drivers of the need for change. Individuals who are most deprived need these changes the most and will derive the highest benefit.
Policy should prioritise childhood education for all. Public health initiatives minimising head injury and decreasing harmful alcohol drinking could potentially reduce young-onset and later-life dementia. Midlife systolic blood pressure control should aim for 130 mm Hg or lower to delay or prevent dementia. Stopping smoking, even in later life, ameliorates this risk. Passive smoking is a less considered modifiable risk factor for dementia. Many countries have restricted this exposure. Policy makers should expedite improvements in air quality, particularly in areas with high air pollution.
We recommend keeping cognitively, physically, and socially active in midlife and later life although little evidence exists for any single specific activity protecting against dementia. Using hearing aids appears to reduce the excess risk from hearing loss. Sustained exercise in midlife, and possibly later life, protects from dementia, perhaps through decreasing obesity, diabetes, and cardiovascular risk. Depression might be a risk for dementia, but in later life dementia might cause depression. Although behaviour change is difficult and some associations might not be purely causal, individuals have a huge potential to reduce their dementia risk.
In LMIC, not everyone has access to secondary education; high rates of hypertension, obesity, and hearing loss exist, and the prevalence of diabetes and smoking are growing, thus an even greater proportion of dementia is potentially preventable.
Amyloid-β and tau biomarkers indicate risk of progression to Alzheimer's dementia but most people with normal cognition with only these biomarkers never develop the disease. Although accurate diagnosis is important for patients who have impairments and functional concerns and their families, no evidence exists to support pre-symptomatic diagnosis in everyday practice.
Our understanding of dementia aetiology is shifting, with latest description of new pathological causes. In the oldest adults (older than 90 years), in particular, mixed dementia is more common. Blood biomarkers might hold promise for future diagnostic approaches and are more scalable than CSF and brain imaging markers.
Wellbeing is the goal of much of dementia care. People with dementia have complex problems and symptoms in many domains. Interventions should be individualised and consider the person as a whole, as well as their family carers. Evidence is accumulating for the effectiveness, at least in the short term, of psychosocial interventions tailored to the patient's needs, to manage neuropsychiatric symptoms. Evidence-based interventions for carers can reduce depressive and anxiety symptoms over years and be cost-effective.
Keeping people with dementia physically healthy is important for their cognition. People with dementia have more physical health problems than others of the same age but often receive less community health care and find it particularly difficult to access and organise care. People with dementia have more hospital admissions than other older people, including for illnesses that are potentially manageable at home. They have died disproportionately in the COVID-19 epidemic. Hospitalisations are distressing and are associated with poor outcomes and high costs. Health-care professionals should consider dementia in older people without known dementia who have frequent admissions or who develop delirium. Delirium is common in people with dementia and contributes to cognitive decline. In hospital, care including appropriate sensory stimulation, ensuring fluid intake, and avoiding infections might reduce delirium incidence.
Key messages
- • New evidence supports adding three modifiable risk factors—excessive alcohol consumption, head injury, and air pollution—to our 2017 Lancet Commission on dementia prevention, intervention, and care life-course model of nine factors (less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, and infrequent social contact).
- • Modifying 12 risk factors might prevent or delay up to 40% of dementias.
- • Prevention is about policy and individuals. Contributions to the risk and mitigation of dementia begin early and continue throughout life, so it is never too early or too late. These actions require both public health programmes and individually tailored interventions. In addition to population strategies, policy should address high-risk groups to increase social, cognitive, and physical activity; and vascular health.
- • Aim to maintain systolic BP of 130 mm Hg or less in midlife from around age 40 years (antihypertensive treatment for hypertension is the only known effective preventive medication for dementia).
- • Encourage use of hearing aids for hearing loss and reduce hearing loss by protection of ears from excessive noise exposure.
- • Reduce exposure to air pollution and second-hand tobacco smoke.
- • Prevent head injury.
- • Limit alcohol use, as alcohol misuse and drinking more than 21 units weekly increase the risk of dementia.
- • Avoid smoking uptake and support smoking cessation to stop smoking, as this reduces the risk of dementia even in later life.
- • Provide all children with primary and secondary education.
- • Reduce obesity and the linked condition of diabetes. Sustain midlife, and possibly later life physical activity.
- • Addressing other putative risk factors for dementia, like sleep, through lifestyle interventions, will improve general health.
- • Many risk factors cluster around inequalities, which occur particularly in Black, Asian, and minority ethnic groups and in vulnerable populations. Tackling these factors will involve not only health promotion but also societal action to improve the circumstances in which people live their lives. Examples include creating environments that have physical activity as a norm, reducing the population profile of blood pressure rising with age through better patterns of nutrition, and reducing potential excessive noise exposure.
- • Dementia is rising more in low-income and middle-income countries (LMIC) than in high-income countries, because of population ageing and higher frequency of potentially modifiable risk factors. Preventative interventions might yield the largest dementia reductions in LMIC.
For those with dementia, recommendations are:
- • Post-diagnostic care for people with dementia should address physical and mental health, social care, and support. Most people with dementia have other illnesses and might struggle to look after their health and this might result in potentially preventable hospitalisations.
- • Specific multicomponent interventions decrease neuropsychiatric symptoms in people with dementia and are the treatments of choice. Psychotropic drugs are often ineffective and might have severe adverse effects.
- • Specific interventions for family carers have long-lasting effects on depression and anxiety symptoms, increase quality of life, are cost-effective and might save money.
Acting now on dementia prevention, intervention, and care will vastly improve living and dying for individuals with dementia and their families, and thus society.
Introduction
Worldwide around 50 million people live with dementia, and this number is projected to increase to 152 million by 2050, 1 rising particularly in low-income and middle-income countries (LMIC) where around two-thirds of people with dementia live. 1 Dementia affects individuals, their families, and the economy, with global costs estimated at about US$1 trillion annually. 1
We reconvened the 2017 Lancet Commission on dementia prevention, intervention, and care 2 to identify the evidence for advances likely to have the greatest impact since our 2017 paper and build on its work. Our interdisciplinary, international group of experts presented, debated, and agreed on the best available evidence. We adopted a triangulation framework evaluating the consistency of evidence from different lines of research and used that as the basis to evaluate evidence. We have summarised best evidence using, where possible, good- quality systematic reviews, meta-analyses, or individual studies, where these add important knowledge to the field. We performed systematic literature reviews and meta-analyses where needed to generate new evidence for our analysis of potentially modifiable risk factors for dementia. Within this framework, we present a narrative synthesis of evidence including systematic reviews and meta-analyses and explain its balance, strengths, and limitations. We evaluated new evidence on dementia risk in LMIC; risks and protective factors for dementia; detection of Alzheimer's disease; multimorbidity in dementia; and interventions for people affected by dementia.
Nearly all the evidence is from studies in high-income countries (HIC), so risks might differ in other countries and interventions might require modification for different cultures and environments. This notion also underpins the critical need to understand the dementias related to life-course disadvantage—whether in HICs or LMICs.
Our understanding of dementia aetiology is shifting. A consensus group, for example, has described hippocampal sclerosis associated with TDP-43 proteinopathy, as limbic-predominant age-related TDP-43 encephalopathy (LATE) dementia, usually found in people older than 80 years, progressing more slowly than Alzheimer's disease, detectable at post-mortem, often mimicking or comorbid with Alzheimer's disease. 3 This situation reflects increasing attention as to how clinical syndromes are and are not related to particular underlying pathologies and how this might change across age. More work is needed, however, before LATE can be used as a valid clinical diagnosis.
The fastest growing demographic group in HIC is the oldest adults, those aged over 90 years. Thus a unique opportunity exists to focus on both human biology, in this previously rare population, as well as on meeting their needs and promoting their wellbeing.
Prevention of dementia
The number of people with dementia is rising. Predictions about future trends in dementia prevalence vary depending on the underlying assumptions and geographical region, but generally suggest substantial increases in overall prevalence related to an ageing population. For example, according to the Global Burden of Diseases, Injuries, and Risk Factors Study, the global age-standardised prevalence of dementia between 1990 and 2016 was relatively stable, but with an ageing and bigger population the number of people with dementia has more than doubled since 1990. 4
However, in many HIC such as the USA, the UK, and France, age-specific incidence rates are lower in more recent cohorts compared with cohorts from previous decades collected using similar methods and target populations 5 ( figure 1 ) and the age-specific incidence of dementia appears to decrease. 6 All-cause dementia incidence is lower in people born more recently, 7 probably due to educational, socio-economic, health care, and lifestyle changes. 2 , 5 However, in these countries increasing obesity and diabetes and declining physical activity might reverse this trajectory. 8 , 9 In contrast, age-specific dementia prevalence in Japan, South Korea, Hong Kong, and Taiwan looks as if it is increasing, as is Alzheimer's in LMIC, although whether diagnostic methods are always the same in comparison studies is unclear. 5 , 6 , 7

Incidence rate ratio comparing new cohorts to old cohorts from five studies of dementia incidence 5
IIDP Project in USA and Nigeria, Bordeaux study in France, and Rotterdam study in the Netherlands adjusted for age. Framingham Heart Study, USA, adjusted for age and sex. CFAS in the UK adjusted for age, sex, area, and deprivation. However, age-specific dementia prevalence is increasing in some other countries. IID=Indianapolis–Ibadan Dementia. CFAS=Cognitive Function and Ageing Study. Adapted from Wu et al, 5 by permission of Springer Nature.
Modelling of the UK change suggests a 57% increase in the number of people with dementia from 2016 to 2040, 70% of that expected if age-specific incidence rates remained steady, 10 such that by 2040 there will be 1·2 million UK people with dementia. Models also suggest that there will be future increases both in the number of individuals who are independent and those with complex care needs. 6
In our first report, the 2017 Commission described a life-course model for potentially modifiable risks for dementia. 2 Life course is important when considering risk, for example, obesity and hypertension in midlife predict future dementia, but both weight and blood pressure usually fall in later life in those with or developing dementia, 9 so lower weight and blood pressure in later life might signify illness, not an absence of risk. 11 , 12 , 13 , 14 We consider evidence on other potential risk factors and incorporate those with good quality evidence in our model.
Figure 2 summarises possible mechanisms of protection from dementia, some of which involve increasing or maintaining cognitive reserve despite pathology and neuropathological damage. There are different terms describing the observed differential susceptibility to age-related and disease-related changes and these are not used consistently. 15 , 16 A consensus paper defines reserve as a concept accounting for the difference between an individual's clinical picture and their neuropathology. It, divides the concept further into neurobiological brain reserve (eg, numbers of neurones and synapses at a given timepoint), brain maintenance (as neurobiological capital at any timepoint, based on genetics or lifestyle reducing brain changes and pathology development over time) and cognitive reserve as adaptability enabling preservation of cognition or everyday functioning in spite of brain pathology. 15 Cognitive reserve is changeable and quantifying it uses proxy measures such as education, occupational complexity, leisure activity, residual approaches (the variance of cognition not explained by demographic variables and brain measures), or identification of functional networks that might underlie such reserve. 15 , 16 , 17 , 18 , 19 , 20

Possible brain mechanisms for enhancing or maintaining cognitive reserve and risk reduction of potentially modifiable risk factors in dementia
Early-life factors, such as less education, affect the resulting cognitive reserve. Midlife and old-age risk factors influence age-related cognitive decline and triggering of neuropathological developments. Consistent with the hypothesis of cognitive reserve is that older women are more likely to develop dementia than men of the same age, probably partly because on average older women have had less education than older men. Cognitive reserve mechanisms might include preserved metabolism or increased connectivity in temporal and frontal brain areas. 17 , 18 , 19 , 20 , 21 People in otherwise good physical health can sustain a higher burden of neuropathology without cognitive impairment. 22 Culture, poverty, and inequality are important obstacles to, and drivers of, the need for change to cognitive reserve. Those who are most deprived need these changes the most and will derive the highest benefit from them.
Smoking increases air particulate matter, and has vascular and toxic effects. 23 Similarly air pollution might act via vascular mechanisms. 24 Exercise might reduce weight and diabetes risk, improve cardiovascular function, decrease glutamine, or enhance hippocampal neurogenesis. 25 Higher HDL cholesterol might protect against vascular risk and inflammation accompanying amyloid-β (Aβ) pathology in mild cognitive impairment. 26
Dementia in LMIC
Numbers of people with dementia in LMIC are rising faster than in HIC because of increases in life expectancy and greater risk factor burden. We previously calculated that nine potentially modifiable risk factors together are associated with 35% of the population attributable fraction (PAFs) of dementia worldwide: less education, high blood pressure, obesity, hearing loss, depression, diabetes, physical inactivity, smoking, and social isolation, assuming causation. 2 Most research data for this calculation came from HIC and there is a relative absence of specific evidence of the impact of risk factors on dementia risk in LMIC, particularly from Africa and Latin America. 27
Calculations considering country-specific prevalence of the nine potentially modifiable risk factors indicate PAF of 40% in China, 41% in India and 56% in Latin America with the potential for these numbers to be even higher depending on which estimates of risk factor frequency are used. 28 , 29 Therefore a higher potential for dementia prevention exists in these countries than in global estimates that use data predominantly from HIC. If not currently in place, national policies addressing access to education, causes and management of high blood pressure, causes and treatment of hearing loss, socio-economic and commercial drivers of obesity, could be implemented to reduce risk in many countries. The higher social contact observed in the three LMIC regions provides potential insights for HIC on how to influence this risk factor for dementia. 30 We could not consider other risk factors such as poor health in pregnancy of malnourished mothers, difficult births, early life malnutrition, survival with heavy infection burdens alongside malaria and HIV, all of which might add to the risks in LMIC.
Diabetes is very common and cigarette smoking is rising in China while falling in most HIC. 31 A meta-analysis found variation of the rates of dementia within China, with a higher prevalence in the north and lower prevalence in central China, estimating 9·5 million people are living with dementia, whereas a slightly later synthesis estimated a higher prevalence of around 11 million. 30 , 32 These data highlight the need for more focused work in LMIC for more accurate estimates of risk and interventions tailored to each setting.
Specific potentially modifiable risk factors for dementia
Risk factors in early life (education), midlife (hypertension, obesity, hearing loss, traumatic brain injury, and alcohol misuse) and later life (smoking, depression, physical inactivity, social isolation, diabetes, and air pollution) can contribute to increased dementia risk ( table 1 ). Good evidence exists for all these risk factors although some late-life factors, such as depression, possibly have a bidirectional impact and are also part of the dementia prodrome. 33 , 34
PAF for 12 dementia risk factors
Data are relative risk (95% CI) or %. Overall weighted PAF=39·7%. PAF=population attributable fraction.
In the next section, we briefly describe relevant newly published and illustrative research studies that add to the 2017 Commission's evidence base, including risks and, for some, mitigation. We have chosen studies that are large and representative of the populations, or smaller studies in areas where very little evidence exists. We discuss them in life-course order and within the life course in the order of magnitude of population attributable factor.
Education and midlife and late-life cognitive stimulation
Education level reached.
Higher childhood education levels and lifelong higher educational attainment reduce dementia risk. 2 , 35 , 36 , 37 New work suggests overall cognitive ability increases, with education, before reaching a plateau in late adolescence, when brain reaches greatest plasticity; with relatively few further gains with education after age 20 years. 38 This suggests cognitive stimulation is more important in early life; much of the apparent later effect might be due to people of higher cognitive function seeking out cognitively stimulating activities and education. 38 It is difficult to separate out the specific impact of education from the effect of overall cognitive ability, 38 , 39 and the specific impact of later-life cognitive activity from lifelong cognitive function and activity. 39 , 40
Cognitive maintenance
One large study in China tried to separate cognitive activity in adulthood from activities for those with more education, by considering activities judged to appeal to people of different levels of education. 40 It found people older than 65 years who read, played games, or bet more frequently had reduced risk of dementia (n=15 882, odds ratio [OR]=0·7, 95% CI 0·6–0·8). The study excluded people developing dementia less than 3 years after baseline to reduce reverse causation.
This finding is consistent with small studies of midlife activities which find them associated with better late-life cognition; so for example, in 205 people aged 30–64 years, followed up until 66–88 years, travel, social outings, playing music, art, physical activity, reading, and speaking a second language, were associated with maintaining cognition, independent of education, occupation, late-life activities, and current structural brain health. 41 Similarly, engaging in intellectual activity as adults, particularly problem solving, for 498 people born in 1936, was associated with cognitive ability acquisition, although not the speed of decline. 42
Cognitive decline
The use it or lose it hypothesis suggests that mental activity, in general, might improve cognitive function. People in more cognitively demanding jobs tend to show less cognitive deterioration before, and sometimes after retirement than those in less demanding jobs. 43 , 44 One systematic review of retirement and cognitive decline found conflicting evidence. 45 Subsequently, a 12-year study of 1658 people found older retirement age but not number of years working, was associated with lower dementia risk. 46 Those retiring because of ill health had lower verbal memory and fluency scores than those retiring for other reasons. 47 Another study found a two-fold increase in episodic memory loss attributable to retirement (n=18 575, mean age 66 years), compared to non-retirees, adjusting for health, age, sex, and wealth. 48 Similarly, in a cohort of 3433 people retiring at a mean age of 61 years, verbal memory declined 38% (95% CI 22–60) faster than before retirement. 44 In countries with younger compared to higher retirement ages, average cognitive performance drops more. 49
Cognitive interventions in normal cognition and mild cognitive impairment
A cognitive intervention or cognition-orientated treatment comprises strategies or skills to improve general or specific areas of cognition. 50 Computerised cognitive training programmes have increasingly replaced tasks that were originally paper-and-pencil format with computer-based tasks for practice and training. 51
Three systematic reviews in the general population found no evidence of generalised cognition improvement from specific cognitive interventions, including computerised cognitive training, although the domain trained might improve. 52 , 53 , 54
A meta-analysis of 17 controlled trials of at least 4 hours of computerised cognitive training, (n=351, control n=335) for mild cognitive impairment, found a moderate effect on general cognition post-training (Hedges' g=0·4, 0·2–0·5); 55 however few high quality studies and no long-term high quality evidence about prevention of dementia currently exists. A meta-analysis of 30 trials of computerised, therapy-based and multimodal interventions for mild cognitive impairment found an effect on activities of daily living (d=0·23) and metacognitive outcomes (d=0·30) compared to control. 56 A third systematic review identified five high quality studies, four group-delivered and one by computer, and concluded the evidence for the effects of cognitive training in mild cognitive impairment was insufficient to draw conclusions. 53 A comprehensive, high quality, systematic overview of meta-analyses of cognitive training in healthy older people, those with mild cognitive impairment and those with dementia, found that most were of low standard, were positive and most reached statistical significance but it was unclear whether results were of clinical value because of the poor standard of the studies and heterogeneity of results ( figure 3 ). 51

Pooled results of meta-analyses investigating objective cognitive outcomes of cognition-oriented treatment in older adults with and without cognitive impairment
K represents the number of primary trials included in the analysis. If a review reported several effect sizes within each outcome domain, a composite was created and k denotes the range of the number of primary trials that contributed to the effect estimate. AMSTAR=A MeaSurement Tool to Assess systematic Reviews (max score 16). Adapted from Gavelin et al, 51 by permission of Springer Nature.
In the only randomised controlled trial (RCT) of behavioural activation (221 people) for cognition in amnestic mild cognitive impairment, behavioural activation versus supportive therapy was associated with a decreased 2-year incidence of memory decline (relative risk [RR] 0·12, 0·02–0·74). 57
Hearing impairment
Hearing loss had the highest PAF for dementia in our first report, using a meta-analysis of studies of people with normal baseline cognition and hearing loss present at a threshold of 25 dB, which is the WHO threshold for hearing loss. In the 2017 Commission, we found an RR of 1·9 for dementia in populations followed up over 9–17 years, with the long follow-up times making reverse causation bias unlikely. 2 A subsequent meta-analysis using the same three prospective studies measuring hearing using audiometry at baseline, found an increased risk of dementia (OR 1·3, 95% CI 1·0–1·6) per 10 dB of worsening of hearing loss. 58 A cross-sectional study of 6451 individuals designed to be representative of the US population, with a mean age of 59·4 years, found a decrease in cognition with every 10 dB reduction in hearing, which continued to below the clinical threshold so that subclinical levels of hearing impairment (below 25 dB) were significantly related to lower cognition. 59
Although the aetiology still needs further clarification, a small US prospective cohort study of 194 adults without baseline cognitive impairment, (baseline mean age 54·5 years), and at least two brain MRIs, with a mean of 19 years follow-up, found that midlife hearing impairment measured by audiometry, is associated with steeper temporal lobe volume loss, including in the hippocampus and entorhinal cortex. 60
Hearing aids
A 25-year prospective study of 3777 people aged 65 years or older found increased dementia incidence in those with self-reported hearing problems except in those using hearing aids. 61 Similarly, a cross–sectional study found hearing loss was only associated with worse cognition in those not using hearing aids. 62 A US nationally representative survey of 2040 people older than 50 years, tested every two years for 18 years, found immediate and delayed recall deteriorated less after initiation of hearing aid use, adjusting for other risk factors. 63 Hearing aid use was the largest factor protecting from decline (regression coefficient β for higher episodic memory 1·53; p<0·001) adjusting for protective and harmful factors. The long follow-up times in these prospective studies suggest hearing aid use is protective, rather than the possibility that those developing dementia are less likely to use hearing aids. Hearing loss might result in cognitive decline through reduced cognitive stimulation.
Traumatic brain injury (TBI)
The International Classification of Disease (ICD) defines mild TBI as concussion and severe TBI as skull fracture, oedema, brain injury or bleed. Single, severe TBI is associated in humans, and mouse models, with widespread hyperphosphorylated tau pathology, and mice with APOE ε4 compared to APOE ε3 allele have more hippocampal hyper-phosphorylated tau after TBI. 64 , 65 TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. 66 A nationwide Danish cohort study of nearly 3 million people aged 50 years or older, followed for a mean of 10 years, found an increased dementia (HR 1·2, 95% CI 1·2–1·3) and Alzheimer's disease risk (1·2, 1·1–1·2). 67 Dementia risk was highest in the 6 months after TBI (4·1, 3·8–4·3) and increased with number of injuries in people with TBI (one TBI 1·2, 1·2–1·3; ≥5 TBIs 2·8, 2·1–3·8). Risk was higher for TBI than fractures in other body areas (1·3, 1·3–1·3) and remained elevated after excluding those who developed dementia within 2 years after TBI, to reduce reverse causation bias. 67
Similarly, a Swedish cohort of over 3 million people aged 50 years or older, found TBI increased 1-year dementia risk (OR 3·5, 95% CI 3·2–3·8); and risk remained elevated, albeit attenuated over 30 years (1·3, 1·1–1·4). 68 ICD defined single mild TBI increased the risk of dementia less than severe TBI and multiple TBIs increased the risk further (OR 1·6, 95% CI 1·6–1·7 for single TBI; 2·1, 2·0–2·2 for more severe TBI; and 2·8, 2·5–3·2 for multiple TBI). A nested case control study of early onset clinically diagnosed Alzheimer's disease within an established cohort also found TBI was a risk factor, increasing with number and severity. 69 A stronger risk of dementia was found nearer the time of the TBI, leading to some people with early-onset Alzheimer's disease.
Military veterans have a high risk of occupational TBI, and formal record keeping allows long-term follow-up. A study of 178 779 veterans with TBI with propensity-matched veterans without TBI found dementia risk was associated with TBI severity (HR 2·4, 95% CI 2·1–2·7 for mild TBI without loss of consciousness; 2·5, 2·3–2·8 for mild TBI with loss of consciousness; and 3·8, 3·6–3·9 for moderate to severe TBI). 70 Similarly women veterans with TBI had increased risk of dementia compared to those without TBI (1·5, 1·0–2·2). 71
A cohort study of 28 815 older adults with concussion, found the risk of dementia doubled, with 1 in 6 developing dementia over a mean follow-up of 3·9 years, although those taking statins had a 13% reduced risk of dementia compared to those who were statin-free. They suggest future RCTs as statins might mitigate injury-related brain oedema, oxidative stress, amyloid protein aggregation, and neuroinflammation. 72
The term chronic traumatic encephalopathy describes sports head injury, which is not yet fully characterised and covers a broad range of neuropathologies and outcomes, with current views largely conjecture. 73 The evidence has subsequently been strengthened by a study on Scottish former soccer players reporting that they are more likely than controls to have Alzheimer's disease specified on their death certificates (HR 5·1, 95% CI 2·9–8·8) and to have been prescribed any dementia-related medications (OR 4·9, 95% CI 3·8–6·3) but not on medical records. 74 The study controlled for socio-economic class based on residential address, which in footballers might be less linked to level of education.
Hypertension
Persistent midlife hypertension is associated with increased risk of a late life dementia. In the Framingham Offspring cohort comprising 1440 people, elevated systolic blood pressure (≥140 mm Hg in midlife; mean age 55 years) was associated with an increased risk of developing dementia (HR 1·6, 95% CI 1·1–2·4) over an 18 year follow-up period. 12 In this study risk increased further if hypertension persisted into later life (mean age 69 years; HR 2·0, 95% CI 1·3–3·1). In the same cohort, people in late midlife (mean age 62 years) with ideal cardiovascular parameters (current non-smoker, body mass index [BMI] 18·5–25 kg/m 2 , regular physical activity, healthy diet, optimum blood pressure <120/<80 mm Hg, cholesterol, and normal fasting blood glucose) were compared to people with at least one of these risks. 75 Those with ideal cardiovascular parameters had a lower 10-year risk of all-cause dementia (HR 0·8, 95% CI 0·1–1·0), vascular dementia (0·5, 0·3–0·8) and clinically diagnosed Alzheimer's disease (0·8, 0·6–1·0). In a UK cohort study of 8639 civil servants, a single measure of systolic blood pressure of 130 mm Hg or higher at age 50 years but not at age 60 or 70 years was associated with increased risk of dementia (1·4, 1·1–1·7). 13 In those with persistent systolic blood pressure of 130 mm Hg or higher, from mean age 45 to 61 years, dementia risk is increased even if free of cardiovascular disease relative to those without hypertension (1·3, 1·0–1·7).
A further cohort study has provided potential insights into mechanisms, reporting that midlife hypertension, defined as from age 40 years, was associated with reduced brain volumes and increased white matter hyperintensity volume but not amyloid deposition. 76 Of note, blood pressure declines in later life and this decline is associated with and, potentially caused by, dementia development (HR 2·4, 95% CI 1·4–4·2). 12 , 13 , 77
Antihypertensive drugs, aspirin, and statins
The US and Puerto Rico Systolic Blood Pressure Intervention Trial (SPRINT) in 9361 hypertensive adults aged 50 years and older, was stopped early because of significantly fewer cardiovascular events and deaths occurring in the intensive treatment arm (aiming for systolic <120 mm Hg, n=4678) in comparison with standard treatment (systolic <140 mm Hg, n=4683). 78 Cognitive assessment continued after stopping the trial intervention in SPRINT MIND. 79 In the intensive compared with the standard treatment group, there were 7·2 dementia cases as opposed to 8·6 cases/1000 person-years (HR 0·8; 95% CI 0·7–1·0) within on average 2 years from the end of the intervention period and 5 years after baseline. Pre-specified secondary outcomes were also reduced in the intensive arm for mild cognitive impairment (14·6 vs 18·3 cases/1000 person-years; HR 0·8, 95% CI 0·7–1·0), combined mild cognitive impairment or dementia (20·2 vs 24·1 cases/1000 person-years; HR 0·9, 95% CI 0·7–1·0) 79 making this the first trial to suggest reduction of risk for mild cognitive impairment. Those who were lost to follow-up were at greater risk of dementia than those who continued but follow-up rates did not differ according to intervention group. 80
Four meta-analyses of blood pressure medications to lower high blood pressure with six studies overlap have provided combined estimates of effects. All meta-analyses suggest reduced dementia in those in the interventions arms for outcomes of any dementia as well as clinically diagnosed Alzheimer's disease. The first included randomised controlled trials (RCTs) of any drug to lower blood pressure and reported a reduction in risk of around 10% at marginal significance (RR 0·9, 95% CI 0·9–1·0). 81 Meta-regression showed risk lowered more if the achieved systolic pressure differential was larger between the intervention and control group. The second included 15 trials and observational studies of diuretics involving 52 599 people (median age 76 years) with 6·1 years median follow-up (dementia HR 0·8, 95% CI 0·8–0·9 and Alzheimer's disease 0·8, 0·7–0·9). 82 The third included used individual participant data from six observational studies; (dementia 0·9, 0·8–1·0 and Alzheimer's disease 0·8, 0·7–1·0; figure 4 ). 83 The fourth focused on people prescribed calcium channel blocker only, included 10 RCTs and observational studies comprising 75 239 hypertensive older adults (median age 72 years, median follow-up 8·2 years) found lowered dementia risk (RR 0·7, 95% CI 0·6–0·9). 84 A 2019 meta-analysis addressing which class of anti-hypertensive drug to use to lower risk of either incident dementia or cognitive decline, found over 50 000 participants in 27 studies and reported no consistent difference in effect according to which class of drug was used. 85

Associations of antihypertensive medication use with incident dementia in those with high blood pressure
Adapted from Ding et al, 83 by permission of Elsevier.
A Cochrane review reported good evidence that statins given to older people at risk of vascular disease do not prevent cognitive decline or dementia. 86 One RCT found 100 mg aspirin versus placebo in 19 114 healthy adults older than 65 years did not reduce dementia (HR 1·0, 95% CI 0·8–1·2), death, physical disability, or cardiovascular disease over a period of 4·7 years. 87
Physical inactivity, exercise, and fitness
Studies of physical activity are complex. Patterns of physical activity change with age, generation, and morbidity and are different across sex, social class, and cultures. The studies suggest a complicated relationship with the potential for both risk reduction and reverse causation.
Meta-analyses of longitudinal observational studies of 1–21 years duration showed exercise to be associated with reduced risk of dementia. 2 A further overview of systematic reviews concluded that there is convincing evidence for physical activity protecting against clinically diagnosed Alzheimer's disease. 88
Since the 2017 Commission, the HUNT study of 28 916 participants aged 30–60 years has been published, reinforcing the previous literature in this area. At least weekly midlife moderate-to-vigorous physical activity (breaking into a sweat) was associated with reduced dementia risk over a 25-year period of follow-up (HR 0·8, 95% CI 0·6–1·1) but the confidence intervals were wide. 89 In contrast the Whitehall Study reporting on the 28-year follow-up of 10 308 people, found that more than 2·5 hours of self-reported moderate-to-vigorous physical activity per week, lowered dementia risk over 10, but not 28 years. 33 Very long-term studies are unusual; however, one 44-year study recruited 191 women (mean age 50) purposively to be representative of the Swedish population and reported that 32% of the participants with low baseline peak fitness, 25% with medium, and 5% with high fitness developed dementia (high vs medium HR 0·1, 95% CI 0·03–0·5, low vs medium 1·4, 0·7–2·8). 90
An individual-level meta-analysis of 19 observational studies of relatively younger adults included 404 840 participants' data (mean baseline age 45·5 years; mean follow-up duration 14·9 years), reporting an increased incidence of all-cause dementia (HR 1·4, 95% CI 1·2–1·7) and clinically diagnosed Alzheimer's disease (1·4, 1·1–1·7) in those who were physically inactive in the 10-year period before diagnosis. 91 Notably, however, no difference in dementia risk measured 10–15 years before time of dementia incidence was found except in those with comorbid cardio-metabolic disease (RR 1·3, 95% CI 0·8–2·1).
People might stop exercising due to prodromal dementia so inactivity might be either a consequence or a cause or both in dementia and might be more of a risk in those with cardiovascular morbidity. As with other outcomes, exercise might be required to be sustained and continue nearer the time of risk. 92
Trials of exercise
Since the 2017 Commission several meta-analyses and systematic reviews have been published with three high quality meta-analyses which we include. The first included 39 RCTs with an unclear total number of participants examining moderate or vigorous exercise of any frequency lasting 45–60 min per session in cognitively normal adults aged older than 50 years. This analysis reported global cognitive improvements (standard mean difference [SMD]=0·3, 95% CI 0·2–0·4) for moderate or vigorous resistance (13 studies) or aerobic exercise (18 studies) lasting 45–60 min per session with no difference between them but no effect found for yoga. 93 A second meta-analysis of RCTs in people with mild cognitive impairment found global cognition improved in the intervention group (0·3, 0·1–0·5) with aerobic exercise having a higher effect (0·6, 0·5–0·6). 94 This study did not have dementia as an outcome measure. A third meta-analysis of RCTs of longer term exercise found five studies (four lasting 12 months and one 24 months) with 2878 participants with normal baseline cognition. 95 The incidence of dementia was 3·7% (n=949) for exercisers and 6·1% (n=1017) for controls (random effect RR 0·6, 95% CI 0·3–1·1; fixed effect as no evidence of heterogeneity 0·7, 0·4–1·0). The authors concluded that the study showed no significant effect of exercise for reducing dementia, mild cognitive impairment, or clinically significant cognitive decline but was underpowered. WHO guidelines have been published since the 2017 Commission, suggesting specific activity levels drawing on these, and one further systematic review which considered sex differences on the effect of exercise. 96 , 97 It concluded the evidence points towards physical activity having a small, beneficial effect on normal cognition, with a possible effect in mild cognitive impairment, mostly due to aerobic exercise. 97 Evidence about the effect of specific types of exercise, such as progressive muscle resistance training, on dementia risk is scarce.
In the 2017 Commission we reported on diabetes as a risk factor for dementia. Distinguishing between treated and untreated diabetes as a risk factor for dementia is challenging in observational studies. In a pooled meta-analysis from over 2·3 million individuals with type 2 diabetes across 14 cohort studies, including 102 174 with dementia, diabetes was associated with an increased risk of any dementia (RR 1·6, 95% CI 1·5–1·8 for women and 1·6, 1·4–1·8 for men). 98 The risk of dementia increased with the duration and severity of diabetes. The effect of different diabetic medications on cognition or dementia outcomes remains unclear as few studies have investigated this area. 99 However, one meta-analysis of cohort studies of diabetes reported that, cross sectionally, people with diabetes taking metformin had lower prevalence of cognitive impairment (three studies OR 0·6, 95% CI 0·4–0·8) and, longitudinally, reduced dementia incidence (six studies HR 0·8, 95% CI 0·4–0·9) compared with those taking other medications or no medication. 100 However another analysis did not find a protective effect of metformin for incident dementia (three studies, RR 1·1, 95% CI 0·5–2·4) with possible harm with insulin therapy (1·2, 1·1–1·4); but this did not account for severity of diabetes of those with type 2 diabetes on insulin. 99 A Cochrane review reported intensive compared to standard diabetes control trials with 5 year follow up (n=11 140), showing no impact on cognitive decline (1·0, 95% CI 0·9–1·1) or dementia (1·3, 0·9–1·9). 101
Overall type 2 diabetes is a clear risk factor for development of future dementia; however, whether any particular medication ameliorates this risk is unclear. Intensive diabetic control does not decrease the risk of dementia.
Combined cardiovascular risk factors
Studies of individual cardiovascular risk factors usually control for other cardiovascular risks, which cluster in individual people. This does not take into account the combinations and contexts in which risk occurs. A UK study of 7899 people aged 50 years followed up for 25 years, calculated a cardiovascular health score based on four behaviour-related (smoking, diet, physical activity, BMI) and three biological (fasting glucose, blood cholesterol, blood pressure) metrics each coded on a three-point scale (0, 1, 2). 100 A better score was associated with a lower risk of dementia (HR 0·9, 95% CI 0·9–1·0 per 1 point scale increment), for both behaviour-related (HR/1 point increment in subscales 0·9, 95% CI 0·8–0·9) and biological subscales (0·9, 0·8–1·0), maintained in people free of cardiovascular disease over the follow-up (0·9, 95% CI 0·8–1·0). These authors also reported an association of the score on the scale with hippocampal atrophy and total brain volume but not white matter hyperintensities. This finding underlines the importance of clustering of cardiovascular risk factors in midlife, as studies of individual risk factors in this sample had not shown a significant association, when controlling for other individual risks. 33
Excessive alcohol consumption
Heavy drinking is associated with brain changes, cognitive impairment, and dementia, a risk known for centuries. 102 An increasing body of evidence is emerging on alcohol's complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record based studies. Alcohol is strongly associated with cultural patterns and other sociocultural and health-related factors, making it particularly challenging to understand the evidence base.
A French 5-year longitudinal study of over 31 million people admitted to hospital, found alcohol use disorders (harmful use or dependence as defined in ICD) were associated with increased dementia risk, calculated separately for men and women (women HR 3·3, 95% CI 3·3–3·4, men 3·4, 3·3–3·4). 103 The relationship of dementia with alcohol use disorders was particularly clear in the earlier onset dementias (age less than 65 years) in which 56·6% had an alcohol use disorder noted in their records (n=57 353; 5·2% all dementias).
A systematic review incorporating 45 studies of light to moderate drinking using a variety of definitions reported a reduced risk of dementia compared with not drinking (RR 0·7; 95% CI 0·6–0·91). 104 Risk was not reported separately for men and women. Drinking less than 21 units of alcohol per week (1 unit of alcohol=10 mL or 8 g pure alcohol) might be associated with a lower risk of dementia. 105 , 106 A 5-year follow-up study of 13 342 men and women volunteers from UK biobank aged 40–73 years who drank, included few heavy drinkers and did not analyse abstainers. 106 The study reported that those who drank more than 12 units per week declined slightly more in reaction time in a perceptual matching task than those who drank less (β2=−0·07, 95% CI −0·09 to −0·04). 106 The UK Whitehall study with 23 years follow-up, included 9087 participants aged 35–55 years at baseline. 107 Drinking more than 21 units per week and long-term abstinence were both associated with a 17% (95% CI 4–32 and 13–23 respectively) increase in dementia compared to drinking less than 14 units. Drinking more than 14 units was also associated with right sided hippocampal atrophy on MRI. 108
Weight control and obesity
Overweight is an emerging concern, given the changing BMI across the world's ageing population. New evidence supports the relationship between increased BMI and dementia from a review of 19 longitudinal studies including 589 649 people aged 35 to 65 years, followed up for up to 42 years. It reported obesity (BMI ≥30; RR 1·3, 95% CI 1·1–1·6) but not being overweight (BMI 25–30; 1·1, 1·0–1·2) was associated with late-life dementia. 109 In a further meta-analysis of individual level data from 1·3 million adults (aged ≥18 years), which included two studies from the meta-analysis cited above, 109 higher body mass measured before probable preclinical and prodromal dementia was associated with increased dementia risk (RR 1·3, 1·1–1·7/5-unit increase in BMI). 11
Weight loss in midlife and dementia risk
A meta-analysis of seven RCTs (468 participants) and 13 longitudinal studies (551 participants) of overweight and obese adults without dementia, mean age 50 years, found weight loss of 2 kg or more in people with BMI greater than 25 was associated with a significant improvement in attention and memory. All but one of the studies included participants aged younger than 65 years. The RCTs reported memory improvement over 8–48 weeks (SMD=0·4, 95% CI 0·2–0·6) and short-term longitudinal studies found improvement over a median of 24 weeks (SMD=0·7, 95% CI 0·5–0·8); however, data about the long-term effects or the effect of weight loss in preventing dementia are absent. 110
Smokers are at higher risk of dementia than non-smokers, 2 and at a higher risk of premature death before the age at which they might have developed dementia, introducing some bias and uncertainty in the association between smoking and risk of dementia. 111 , 112 Stopping smoking, even when older, reduces this risk. Among 50 000 men aged older than 60 years, stopping smoking for more than 4 years, compared to continuing, substantially reduced dementia risk over the subsequent 8 years (HR 0·9; 95% CI 0·7–1·0). 113 Worldwide, 35% of non-smoking adults and 40% of children are estimated to be exposed to second-hand smoke; 114 although literature on the impact of this exposure and dementia risk is scarce. One study indicated that in women aged 55–64 years, second-hand smoke exposure was associated with more memory deterioration and the risk increased with exposure duration even after controlling for other confounding factors. 115
Depression is associated with dementia incidence, with a variety of possible psychological or physiological mechanisms. It is also part of the prodrome and early stages of dementia. Reverse causation is possible whereby depressive symptoms result from dementia neuropathology that occurs years before clinical dementia onset. These explanations are not mutually exclusive. As in diabetes, few studies considering depression as a risk factor for dementia have distinguished between treated and untreated depression. In a meta-analysis of 32 studies, with 62 598 participants, with follow-up from 2 to 17 years, a depressive episode was a risk factor for dementia (pooled effect size 2·0, 95% CI 1·7–2·3). 116 Meta-regression analysis revealed a non-significant trend for the association between depression and incident dementia to be weaker when the length of follow-up was longer. The Norwegian HUNT study, suggested that symptoms of psychological distress predicted dementia 25 years later however with wide bounds of uncertainty (HR 1·3, 95% CI 1·0–1·7). 89 Two further studies differentiate between late-life and earlier life depressive symptoms. The UK Whitehall study, in a follow-up of 10 189 people, reports that in late life these symptoms increase dementia risk but not at younger ages (follow-up 11 years HR 1·7; 95% CI 1·2–2·4; follow-up 22 years 1·0, 0·7–1·4). 34 , 117 A 14-year longitudinal study of 4922 initially cognitively healthy men, aged 71–89 years, found depression was associated with 1·5 (95% CI 1·2- 2·0) times the incidence of dementia but this association was accounted for by people developing dementia within 5 years of depression. 118 The use of antidepressants did not decrease this risk.
A study of 755 people with mild cognitive impairment and with a history of depression from the Australian longitudinal Alzheimer's Disease Neuroimaging Initiative, considered the effect of selective serotonin-reuptake inhibitor (SSRI) treatment, such as citalopram, known to reduce amyloid plaque generation and plaque formation in animal models. 119 The study found that more than 4 years of such treatment was associated with delayed progression to clinically diagnosed Alzheimer's disease. People treated with antidepressants seem likely to differ from those who are not treated. Thus, the question of whether antidepressant treatment mitigates dementia risk remains open.
Social contact
Social contact, now an accepted protective factor, enhances cognitive reserve or encourages beneficial behaviours, although isolation might also occur as part of the dementia prodrome. Several studies suggest that less social contact increases the risk of dementia. Although most people in mid and later life are married, by the time they reach older age, disproportionate numbers of women are widowed as they outlive their husbands, thus reducing their social contact. In these generations, marital status is therefore an important contributor to social engagement. Additionally, most marriages are in the relatively young, and married people usually have more interpersonal contact than do single people—this gives a long-term estimate of the effect of social contact. A systematic review and meta-analysis including 812 047 people worldwide found dementia risk to be elevated in lifelong single (RR 1·4, 95% CI 1·1–1·9) and widowed people (1·2, 1·0–1·4), compared with married people and the association was consistent in different sociocultural settings. 120 Studies adjusted for sex and we do not know if a differential risk between men and women exists. Differences persisted in studies that adjusted for education and physical health so might be attributable to married people having more social contact, rather than solely because they tend to have better physical health and more education, although residual confounding is possible. A systematic review and meta-analysis of 51 longitudinal cohort studies of social isolation and cognition included 102 035 participants aged 50 or more years at baseline, with follow-up of 2–21 years. 121 High social contact (measured through either or both of social activity and social network) was associated with better late-life cognitive function (r=0·05, 95% CI: 0·04–0·065) and no differences according to sex or length of time followed up.
A new meta-analysis found that in long-term studies (≥10 years), good social engagement was modestly protective (n=8876, RR=0·9, 95% CI 0·8–1·0); but loneliness was not associated with dementia risk. 122 No long term (>10 years) studies of loneliness and dementia outcomes have been done.
A UK 28-year follow-up study of 10 308 people found that more frequent social contact at age 60 years was associated with lower dementia risk over 15 years of follow-up (HR for one standard deviation social contact frequency 0·9, 95% CI 0·8–1·0). This finding suggests more frequent social contact during late middle age is associated with a modest reduction in dementia risk, independent of socio-economic and other lifestyle factors. 123 A Japanese longitudinal cohort study of 13 984 adults aged older than 65 years with a mean of 10 years follow-up calculated a five-point social contact scale based on: marital status; exchanging support with family members; having contact with friends; participating in community groups; and engaging in paid work. It found the score to be linearly associated with reduced dementia risk; those who scored highest on the five-point scale were 46% less likely to develop incident dementia compared with those in the lowest category. 124
Despite clear cultural variation in the meaning and perception of social isolation, findings of protective effect of more social contact are largely consistent in different settings and for either sex across the studies and meta-analyses. 118 , 120 , 121
Social interventions
Little evidence of the effects of social interventions on dementia exists but a systematic review of low quality RCTs of 576 adults aged 60 or more years with normal cognition found facilitated meeting and discussion groups were associated with improved global cognition and increased brain volume at follow-up. 118
Air pollutants
Air pollution and particulate pollutants are associated with poor health outcomes, including those related to non-communicable diseases. Attention has turned to their potential effect on the brain. Animal models suggest airborne particulate pollutants accelerate neurodegenerative processes through cerebrovascular and cardiovascular disease, Aβ deposition, and amyloid precursor protein processing. 125 , 126 Although the higher levels of dementia from air pollutants are still subject to the potential for residual confounding, the effects on animal models are evidence of physiological effects over and above those driven by life-course deprivation.
High nitrogen dioxide (NO 2 ) concentration (>41·5 μg/m 3 ; adjusted HR 1·2, 95% CI 1·0–1·3), fine ambient particulate matter (PM) 2·5 from traffic exhaust (1·1, 1·0–1·2) 127 , 128 , 129 and PM 2·5 from residential wood burning (HR=1·6, 95% CI 1·0–2·4 for a 1 μg/m 3 increase) are associated with increased dementia incidence. Traffic often produces NO 2 and PM 2·5 and it is hard to separate their effects, although evidence for additive effects of different pollutants exists. 127 , 128 , 129 A systematic review of studies until 2018 including 13 longitudinal studies with 1–15 years follow-up of air pollutants exposure and incident dementia, found exposure to PM 2·5, NO 2 , and carbon monoxide were all associated with increased dementia risk. 24 The attributable burden of dementia and excess death from PM 2·5 in one large 10-year US study was particularly high in Black or African American individuals and socio-economically disadvantaged communities and related to particulate PM 2·5 concentrations above the US guidelines. 130
Mechanisms by which sleep might affect dementia remain unclear, but sleep disturbance has been linked with β-amyloid (Aβ) deposition, 131 , 132 reduced glymphatic clearance pathways activation, 133 low grade inflammation, increased Tau, hypoxia 132 , 134 and cardiovascular disease. 135 Sleep disturbance is hypothesised to increase inflammation which raises Aβ burden, leading to Alzheimer's disease and further sleep disturbance. 136
Two meta-analyses showed similar findings. The first was a synthesis of longitudinal studies with an average of 9·5 years follow-up and the second reported cross-sectional and prospective cohort studies of mixed quality with different methods of measuring sleep. Sleep disturbances were defined broadly, often self-reported and including short and long sleep duration, poor sleep quality, circadian rhythm abnormality, insomnia, and obstructive sleep apnoea. All these disturbances were associated with a higher risk of all-cause dementia (RR 1·2; 95% CI 1·1–1·3) 137 and clinically diagnosed Alzheimer's disease (1·6, 1·3–1·9) compared with no sleep disturbance, although not all cohort studies excluded those with cognitive impairment or dementia at baseline from their analyses. 138 A U-shaped association has been reported between sleep duration and risk of mild cognitive impairment or dementia with higher risks of dementia with less than 5 hours (HR=2·6; 95% CI 1·4–5·1) compared with more than 5 and less than 7 and more than 10 hours sleep (2·2, 1·4–3·5) and risks for all-cause dementia and clinically diagnosed Alzheimer's disease being similar. 135 , 139 , 140 , 141
The postulated mechanisms of reduced sleep leading to accumulation of Alzheimer's type pathology is inconsistent with the evidence that both more sleep and less sleep are associated with increased risk of dementia. New onset late-life sleep disturbance, a few years before clinical dementia, might be part of the natural history of the dementia syndrome, appearing to be a risk factor, or reflect other disorders, for example, mood disturbances or cardiovascular disease. 135 , 142 Hypnotic use might increase risks although this is unclear and a 2018 study 139 suggests that findings of a connection were related to reverse causality and confounders. 143 When benzodiazepine use was considered, in one study, sleep length was no longer significant 139 but not in all studies. 135 Those taking hypnotics were at greater risk of dementia than those who did not regardless of sleep duration. 139 Medication for sleep disturbance might be harmful and benzodiazepines are associated with falls, hospital admissions, and possibly dementia. 139 , 144
Nutrition and dietary components are challenging to research with controversies still raging around the role of many micronutrients and health outcomes in dementia. Observational studies have focused on individual components ranging from folate and B vitamins, Vitamin C, D, E, and selenium amongst others as potential protective factors. 88 There has been a move towards considering the evidence base for whole diets in the last 5 years, particularly high plant intake such as in the Mediterranean diet (high intake of vegetables, legumes, fruits, nuts, cereals, and olive oil; low intake of saturated lipids and meat) or the similar Nordic diet, rather than individual nutrients, which might reduce cognitive decline and dementia. 145 One example is a longitudinal cohort study of 960 participants, ages 58–99 years, in which those reporting the highest intake of green leafy vegetables, equivalent to 1·3 servings per day, had less cognitive decline over 4·7 years than those reporting the lowest intake (β=0·05 standardised units 95% CI 0·02–0·07). 146 The authors report this difference as being equivalent to being 11 years younger. A further prospective cohort study with three midlife dietary assessments in 8255 people, followed up for a mean of nearly 25 years, found neither healthy dietary pattern nor Mediterranean diet protected from dementia, except in those with cardiovascular disease, suggesting that diet might influence dementia risk by protecting from the excess risk of cardiovascular risk factors. 147
Dietary interventions
As well as whole diets, there has been some interest in multi-nutrient interventions. A systematic review and a Cochrane review including RCTs of supplements (A, B, C, D, and E; calcium, zinc, copper, and multivitamins trials, n-3 fatty acids, antioxidant vitamins, and herbs) found a lack of evidence for supplement use to preserve cognitive function or prevent dementia in middle-aged (45–64 years) or older people (aged 65 years and older). 148 , 149 Cochrane reviews found no evidence for beneficial effects on cognition of those with mild cognitive impairment of supplementation with B vitamins for 6 to 24 months 150 or with vitamin E in preventing progression from mild cognitive impairment to dementia. 151 A 24-month RCT of 311 people of a multi-nutrient drink containing docosahexaenoic acid, vitamins B12, B6, folic acid, and other nutrients; found no significant effect on preventing cognitive deterioration in prodromal Alzheimer's disease. 152 The authors comment that the control group's cognitive decline was much lower than expected, leading to an inadequately powered trial.
Meta-analysis of two RCTs with 471 participants with normal cognition found the Mediterranean diet improved global cognition compared to controls (SMD 0·2, 95% CI 0·0–0·4). 153 A further meta-analysis identified five RCTs (n=1888) with a weak effect on global cognition (SMD 0·2, 95% Cl 0·0–0·5) 154 but no benefit of Mediterranean diet for incident cognitive impairment or dementia.
The WHO guidelines recommend a Mediterranean diet to reduce the risk of cognitive decline or dementia, as it might help and does not harm, but conclude Vitamins B and E, polyunsaturated fatty acid, and multicomplex supplementation should not be recommended. 97
Trials of combination strategies to prevent dementia
The FINGER RCT was a 2-year multidomain intervention to prevent cognitive decline and dementia in 1260 people with cardiovascular risk factors aged 60–77 years, recruited from a Finnish national survey. Similar multidomain studies were discussed in the 2017 Commission. 2 FINGER found a small group reduction in cognitive decline in the intervention group compared with control (comprehensive neuropsychological test battery Z score 0·02, 95% Cl 0·00–0·04) regardless of baseline sociodemographic, socio-economic, cognitive, or cardiovascular status. 155 However, in a subgroup analysis, greater beneficial effects were observed on processing speed in individuals with higher baseline cortical thickness in Alzheimer's disease areas. 156
The Healthy Ageing Through Internet Counselling in the Elderly (HATICE) study recruited 2724 older people (≥65 years) in the Netherlands, Finland, and France with two or more cardiovascular risk factors. 157 , 158 It compared an interactive internet platform plus remote support by a coach, aiming to improve self-management of vascular risk factors, with a non-interactive control platform with basic health information. A small improvement in the cardiovascular risk composite primary outcome was observed in the intervention group compared with the control group at 18 months, mainly through weight loss, and the dementia risk score was slightly lower in those who received the intervention (mean difference −0·15, 95% CI −0·3 to −0·0). A larger effect was observed in the younger age group (65–70 years) and those with the lowest level of education, who had a higher baseline risk, suggesting that targeting high-risk populations might be more effective. Several multidomain preventive trials are ongoing—for example, World Wide FINGERS .
Total PAF calculation
We incorporated excessive alcohol consumption, TBI, and air pollution into our life-course model of dementia, as well as the original nine risk factors, because of the updated evidence. To calculate new RRs for excessive alcohol consumption, TBI and air pollution, we systematically reviewed the literature and did new meta-analyses for excessive alcohol consumption and TBI. For the other nine factors, we used values for RR and risk factors prevalence from our previous analysis and calculated communality using the same method as in the 2017 Commission. 2
PAF calculation
We used a representative sample of over 10 000 UK community-dwelling adults, to calculate communality (clustering of risk factors) of 11 risk factors for which data existed, 159 to allow calculation of each factor's unique risk. As we could find no datasets measuring TBI, with the other 11 risk factors of interest, we could not calculate its communality. We therefore used the mean of the other 11 communalities to calculate a weighted PAF, so we could include TBI. We used cohabitation as a proxy measure for social contact, and urbanicity for air pollution exposure. Our analysis found four principal components, explaining 55% of the total variance between the eleven risk factors, suggesting substantial overlap. The appendix (p 2) shows the PAF formula and the steps in calculating communality and we detail our new meta-analyses next, which we used to update the figure and perform our new calculations.
Incorporation of the new chosen risks in new systematic reviews
We searched, from inception to Oct 29, 2019, Embase, Allied, and Complementary Medicine, MEDLINE, and PsycINFO terms “dementia” OR “dement*” OR “AD” OR “VaD”, “Alzheimer*” AND “alcohol” OR “ethanol” OR “alcohol*” OR “drink*” OR “drunk*” to update an earlier review. 160 We used inclusion criteria: original population-based cohort studies measuring drinking during midlife, as alcohol intake tends to fall with age; 161 alcohol consumption quantified at baseline by units or number of drinks (one drink, 1·5 units) per week; and all-cause dementia ascertained at follow-up using validated clinical measures. We contacted authors for additional data. 162 Three studies met our inclusion criteria. 107 , 162 , 163 We converted HRs to RRs 164 and used raw data 162 to calculate RR, 165 for our random effects meta-analysis using Generic Inverse Variance Methods. The RR associated with drinking—more than 21 units (168 g) of alcohol weekly—compared with lighter drinking was 1·18 (95% Cl 1·06–1·31; figure 5 ). We used Health Survey England figures for heavier drinking prevalence to calculate PAF as we could not find a worldwide estimate. The weighted PAF was 0·8.

Meta-analysis of relative risk of dementia associated with drinking more than 21 units of alcohol per week in midlife compared to lighter consumption of alcohol
To estimate the RR of TBI of all severities for all cause dementia, we searched Embase, Medline, and PsycINFO from Jan 1, 2016, to Oct 21, 2019, updating an earlier search, 166 using terms (“traumatic brain injury” or “head injury” or “brain injury” or TBI) AND (neurodegeneration or “cognitive dysfunction” or dementia or “Alzheimer's disease” or “Parkinson's disease” or “frontotemporal dementia”). We converted HR figures to RR. 164 , 167 We used inclusion criteria: original population-based cohort studies, baseline TBI of all severities reported, and all-cause dementia ascertained at follow-up using validated clinical measures. We combined four new studies meeting inclusion criteria 67 , 68 , 71 , 168 with the four studies meeting criteria from the original review in a random effects meta-analysis. 166 The pooled RR was 1·84 (95% CI 1·54–2·20) for all cause dementia from all severities of TBI ( figure 6 ) although there was heterogeneity in study-specific estimates, possibly because of different populations. We used the TBI adult population prevalence of 12·1% from a meta-analysis to calculate PAF. 173 The weighted PAF was 3·4.

Meta-analysis of relative risk of all-cause dementia associated with all severity midlife traumatic brain injury
A 2019 systematic review synthesised observational studies, finding consistently increased risk of dementia from air pollution, but heterogeneous comparator groups precluded meta-analysis. 24 We updated the search, using the same search terms and searching MEDLINE, Embase, and PsycINFO from Sept 20, 2018, (the end date of the last search) to Oct 22, 2019. We included longitudinal studies with assessment of all cause air pollution exposure; use of formal assessment of cognitive function at baseline; report of incident all-cause dementia, data from adults (age ≥18 years); and a minimum follow-up of 6 months. As meta-analysis was not possible, we used data from the only study of all-cause air pollution with the outcome of all-cause dementia, with low-moderate risk of bias. This population-based, observational cohort was from Canada, where pollutant concentrations are among the lowest in the world and examined 2 066 639 people, with a mean baseline age of 67 years. 174 We calculated the RR of dementia for those in the three highest quartiles compared to the lowest was 1·09 (1·07–1·11). The attributable fraction for exposure to the highest three quartiles versus the lowest quartile of PM 2·5 and NO 2 was 6·1% (4·8–7·5). The weighted PAF was 2·3.
Table 1 displays the prevalence, communality, relative risk, unweighted and weighted PAFs adjusted for communality. Figure 7 shows the updated life-course model of potentially modifiable risk factors for dementia, including the three new risk factors.

Population attributable fraction of potentially modifiable risk factors for dementia
Strengths and limitations
This Commission is the most comprehensive analysis to date and updates the 2017 Commission with emerging risk factor evidence convincing enough to calculate PAF for potentially reversible risk factors. We reviewed the literature systematically for the chosen risk factors and provided illustrative new literature to update our synthesis and identify data to calculate communality. We find a hopeful picture with an estimate of around 40% of all cases of dementia being associated with 12 potentially modifiable risk factors.
We have made assumptions to calculate this new model. We used global figures for dementia risk although we know the risk factors prevalence varies between countries and most global research is from HIC, so LMIC are under-represented because of lack of data. We have assumed a causal relationship between risk factors and dementia, although we have been cautious and not included risk factors with less good evidence. No single database exists with all 12 risk factors together, but we found 11 of the factors in a UK database and used the mean figure for communality calculations for TBI. We calculated communality for the other 11. We do not know how far findings of communality in other geographical populations might differ, or in those with a differing distribution of age groups or sex. We found that social isolation was not explicitly measured and had to use proxies, such as cohabitation when considering prevalence, which are approximate.
Specifically, evidence for the association of alcohol misuse with dementia comes from HIC and future studies from LMIC are needed to complete the picture. Exposure to air pollution changes over a lifetime and is inextricably linked to poverty and deprivation. However, the effects on animal models suggests specific physiological effects over and above those driven by life-course deprivation. We also considered the overlap with education for this and other risk factors and the correction for education, strongly inversely linked to deprivation, will address at least some of the confounding. However, the results in one study which reported the effect of air pollution on incident dementia showed very little difference in estimates before and after adjustment for education and other risk factors, suggesting little residual confounding exists. 174 We were also unable to meta-analyse data on pollution and thus unlike the other relative risks, the figure comes from only one study, from an area of low pollution so is likely to be an underestimate.
The longitudinal evidence linking potentially modifiable risk factors to dementia generally fulfils causality criteria in observational data (strength, consistency, biological plausibility, temporality, dose–response, coherence, and quasi-experimental studies, for example, more education or using hearing aids). When measuring a risk nearer to the age of dementia onset, then it is more likely that prodromal change affects, or even causes it. Alternatively, a risk factor might act on preclinical pathology or even cause dementia near the time of exposure. Thus, excessive alcohol, and TBI are particularly important in young-onset dementia, although many early onset dementias relate to genetic risks. Risk factors might also matter more at a time of higher biological vulnerability, which the studies we have drawn on cannot establish. The length of exposure required for risk or protection effect, and their inter-relationships as they change across life is unclear—it seems probable that longer or more intense exposure has stronger effects. Additionally, as our communality figures show, risk factors overlap. We cannot establish from these data if having multiple risk factors has an additive or synergistic effect. Association does not prove causation, however, as already noted, the reductions in prevalence and incidence in several HIC suggests that at least some of the risk factors estimated here do have a causal relationship with the clinical expression of dementia.
Key points and recommendations
We judge that sufficient new evidence supports adding three additional modifiable risk factors for dementia to our 2017 Commission model (excessive alcohol, traumatic brain injury, and air pollution). We have been able to add updated evidence on the nine risk factors implicated in the 2017 Commission (education, hypertension, hearing impairment, smoking, obesity, depression, inactivity, diabetes, and social contact). Reduction of these risk factors might be protective for people with or without a genetic risk, although study findings have not been entirely consistent. 175 , 176 , 177 , 178 As we noted in the 2017 Commission, others have previously calculated an estimate of the risk associated with APOε4 at 7% taking into account some other risk factors and this estimate highlights how relatively important potentially modifiable risk factors are in dementia. 2 , 179
For some risk factors, the pattern of risk and the individual's other health, both physical and mental, might be especially important. Currently, the evidence suggests a Mediterranean or Scandinavian diet might have value in preventing cognitive decline in people with intact cognition, particularly as one component of a healthy lifestyle, although how long the exposure has to be or during which ages is unclear. We do not recommend taking additional vitamins, oils, or mixed dietary supplements as a means of preventing dementia as extensive testing in trials has not led to signals of beneficial effects.
Data from RCTs on interventions to prevent cognitive decline, all-cause dementia, or Alzheimer's disease are few. For some key life influences, only observational data, particularly related to natural experiments such as changing the statutory education age, are possible. These influences should be investigated systematically wherever possible. Others can theoretically be investigated but the long follow-up required for midlife risk and protective factors and non-random attrition in longer studies are challenging. Using intermediate endpoints, such as cognition, and dementia onset in research remains uncertain because no intermediate markers with such a close relationship to dementia outcomes exist that it would be possible to predict with certainty for any given individual, age, and sex. Overall, the evidence for treating hypertension is strongest and high blood pressure throughout midlife increases the risk of dementia even without stroke.
Although a need for more evidence is apparent, recommendations should not wait, as clear indications of ways to reduce the chances of developing dementia without causing harm will also lead to other health and wellbeing benefits.
Our recommended strategies for dementia risk reduction include both population-wide and targeted interventions ( panel ). It is important to remember that more socially disadvantaged groups, including Black, Asian, and minority ethnic groups, are particularly at risk.
Recommended strategies for dementia risk reduction
Risks are particularly high in more socially disadvantaged populations including in Black, Asian, and minority ethnic groups.
Population-wide
- • Prioritise childhood education for all, worldwide
- • Implement social public health policies that reduce hypertension risk in the entire population
- • Develop policies that encourage social, cognitive, and physical activity across the life course for all (with no evidence for any specific activities being more protective)
- • Scrutinise the risks for hearing loss throughout the life course, to reduce the risk of exposure to this risk factor
- • Reduce the risk of serious brain trauma in relevant settings, including occupational and transport
- • National and international policies to reduce population exposure to air pollution
- • Continue to strengthen national and international efforts to reduce exposure to smoking, both for children and adults, and to reduce uptake and encourage cessation
Targeted on individuals
- • Treat hypertension and aim for SBP <130 mm Hg in midlife
- • Use hearing aids for hearing loss; we need to help people wear hearing aids as many find them unacceptable, too difficult to use, or ineffective
- • Avoid or discourage drinking 21 or more units of alcohol per week
- • Prevent head trauma where an individual is at high risk
- • Stopping smoking is beneficial regardless of age
- • Reduce obesity and the linked condition of diabetes by healthy food availability and an environment to increase movement
- • Sustain midlife, and possibly late-life physical activity
Although we have more to learn about effectiveness, avoiding or delaying even a proportion of potentially modifiable dementias should be a national priority for all.
Interventions and care in dementia
Not all dementia will be preventable and we present the latest evidence on intervention and care for dementia. To date the emphasis has been on specific subtypes of dementia, most notably on Alzheimer's disease, which has been conceptualised over the years in a variety of changing diagnostic criteria—eg, DSM IV and DSM V. 180 , 181 Intense efforts have been put into biomarkers for early preclinical detection of the disease process before it becomes dementia. Biomarkers need to show reliability and validity, and for dementias they also need to be very closely and clearly related to clinical syndrome outcomes in the way that, for example, human papillomavirus is for cervical cancer, and hypertension has been for stroke.
Biomarkers and detection of Alzheimer's disease
Markers of neurodegeneration linked to clinical dementia include brain volume loss—ie, hippocampal volume loss and entorhinal cortex and medial temporal cortical thinning—seen in structural imaging. The most studied molecular markers are in Alzheimer's disease and are amyloid and tau, which PET and CSF detect clinically. The prevalence of particular pathologies at different ages is important in interpretation of such studies. So, for example, population derived studies show increases in plaques in the population from less than 3% at age 50–59 years to around 40% at age 80–89 years. 182
Amyloid imaging
Amyloid imaging detects amyloid in the brain with high sensitivity and specificity in both cognitively normal and people with Alzheimer's disease when the gold-standard comparison is either neuropathology or clinical diagnosis, distinguishing Alzheimer's disease from other neurodegenerative conditions. 183 Amyloid imaging is not a diagnostic test for dementia. A US study of randomly selected older people from the community recruited 1671 people (mean age of 71 years). 182 The prevalence of PET detected amyloid positivity increased from 2·7% (95% CI 0·5–4·9) of people without cognitive impairment aged 50–59 years to 41·3% (95% CI 33·4–49·2%) aged 80–89 years. 182 In 10-year follow-up PET positivity was associated with a higher probability of developing Alzheimer's disease compared with those who were amyloid negative (HR 2·6, 95% CI 1·4–4·9). In participants with mild cognitive impairment who were amyloid positive the probability (HR 1·9, 95% CI 0·9–3·9) was not very different to those who were amyloid negative (1·6, 0·8–3·4).
Similarly, an 8-year follow-up study of 599 volunteers (average age 70 years) in Australia found that cognitively normal PET amyloid-positive people had an elevated risk of developing Alzheimer's disease compared with amyloid negative (17·7% vs 8·1%; OR 2·4, 95% CI 1·5–4·0). 184 Over 80% of the 266 people who were PET amyloid-positive did not go onto develop a cognitive impairment within 8 years, showing positive status does not predict impairment for most people in a timeframe that might be a useful prognostic window. Follow-up at 5 years of amyloid-positive participants with normal cognition or mild cognitive impairment versus amyloid negative people found the same pattern of increased risk (2·6, 1·4–4·9). Risk also increases per 1 year of age (HR 1·05, 95% CI 0·55–2·0/year), and APOEε4 status (2·6, 1·4–5·0). 184
Most people who are amyloid positive with no other markers have not developed Alzheimer's disease dementia during their lifetime. A model of lifetime risks of people who are amyloid positive without any other biomarkers finds it to be 8·4% for a 90-year-old woman who is cognitively normal at baseline, 23·5% for a 75-year-old woman and 29·3% for a 65-year-old woman. 185 The 10-year risk is considerably less, so a 65-year-old woman with only amyloid biomarkers but who is cognitively normal and has no neurodegeneration has a 10-year Alzheimer's disease risk of 2·5% and a man 2·3%, but the risk is higher with accompanying neurodegeneration ( table 2 ). 185
Ten-year risks by age of developing Alzheimer's disease for women based on amyloidosis alone and in the presence of neurodegeneration and mild cognitive impairment
Data are relative risk (95% CI) or %. Reproduced from Brookmeyer and Abdalla 185 by permission of Elsevier.
Overall, the knowledge of PET-measured amyloid and tau status and MRI-derived cortical thickness in a general population derived sample, only adds a small improvement, which might not be clinically important for predicting memory decline over a model with clinical and genetic variables. 186
Using amyloid PET in patients with cognitive impairment of uncertain causes, results in changes to the clinical diagnosis of Alzheimer's disease 187 and sometimes to medication prescription. We do not know whether PET use improves patient care or decreases care costs. Many people have a mixed cause of dementia and a positive result does not indicate only Alzheimer's disease.
Fluid biomarkers
PET imaging is very costly (US$3000 in the USA) and although used in some clinical settings remains the topic of research to understand its usefulness in broader populations. Fluid biomarkers—ie, blood and cerebrospinal fluid tests—have become a more practical focus of interest since it has become possible to measure specific proteins linked to the proteins associated with the neuropathologies of Alzheimer's disease. 188 A composite blood biomarker for amyloid tested in a discovery dataset and then a validation cohort of participants aged 60–90 years who were already taking part in studies in Japan or Australia had areas under the receiver operating characteristic curves of 96·7% for discovery and 94·1% for validation. The blood biomarker had sensitivity and specificity above 80% against amyloid PET measurement 188 and correlated with CSF concentrations of Aβ1–42. These results are similar to other amyloid blood biomarkers 189 , 190 and harmonisation to a common reference standard is now vital. Although CSF Aβ1–42/1–40 ratio and amyloid PET are now considered interchangeable, 191 CSF tau biomarkers have only correlated weakly with brain tau as currently measured by radioligands. 192 Neurofilament light protein is measured in many cohorts; however, it is non-specific. People with Huntington's disease, multiple sclerosis, mild cognitive impairment, and Alzheimer's disease might have raised blood neurofilament light concentrations, which are a marker of neurodegeneration. 193 , 194 , 195
Key points and conclusions
To be useful in clinical practice biomarkers must be well understood in the populations to which they are going to be applied, including the effects of age and sex on results. There is now reasonable evidence that amyloid and tau measured by PET or in fluid indicate increased risk for development of cognitive impairment in older adults but at the individual level prognostication is not possible as most cognitively normal people with these markers do not develop dementia within a clinically relevant timeframe. Negative amyloid results can be useful for ruling out current Alzheimer's pathology in people with cognitive impairment when the cause is uncertain and show an individual is unlikely to develop Alzheimer's disease during the next few years. High neurofilament light concentrations indicate a neurodegenerative process but not its cause. The value of biomarkers, in terms of diagnostic value, has not been addressed in different representative populations and particularly not in those from LMIC. The potential advantages of blood biomarkers are their low cost and their wider acceptability and applicability in many settings. In many areas of medicine more reliable diagnostic tests have improved research, including epidemiological and public health research and trials, to help distinguish cause from symptom (tuberculosis from a fever) or assess risk factor and disease (hypercholesterolaemia and ischaemic heart disease). Those biomarkers developed for the underlying biology of the dementia syndrome are subject to the same assessment of value.
Principles of intervention in people with dementia
In the 2017 Commission, we discussed that when concerns are raised by patients or family, an accurate diagnosis is helpful. Such a diagnosis provides a gateway to intervention and services where available, for planning for possible futures, and support for family, as well as to research. Unfortunately, these services are not always available. National plans for dementia support timely diagnosis and offer help to individuals and their families.
We did not address screening of those not presenting with concerns but rigorous systematic reviews by the US Task Force on Prevention have found an absence of evidence of benefit and harm. 196 The first trial of population screening took place in the USA, screening 4005 primary care patients aged 65 years or older. No clear benefit or harm in terms of quality of life, mood, or increasing diagnostic rates was found. 197 Other strategies might become more valuable in time such as sensitive awareness of risk factors, when routine records suggest an individual might be deteriorating cognitively. 198
People with dementia have complex problems with symptoms in many domains. Those providing support and any interventions must consider the person as a whole, as well as their context and their close carers, whether family or friends. Individuals' medical, cognitive, psychological, environmental, cultural, and social needs must be given consideration. 2 In the context of under provision of services, this notion is and will continue to be a challenge. Dementia, as an illness which affects cognition by definition, affects the ability to organise activities and people with dementia often need help to do what they enjoy—for example, listen to music, or go to gardens and parks. Wellbeing is one of the goals of dementia care.
Interventions once a diagnosis has been made
Cholinesterase inhibitors have a useful, modest role in improving cognition and activities of daily living in patients with mild-to-moderate Alzheimer's disease and memantine can be prescribed in combination or each drug used separately for moderate and severe Alzheimer's disease. 2 , 199 , 200 However, although available in most countries these drugs are no longer remunerated in France because it is felt that they offer only a small benefit while shifting clinician's attention from other interventions. Whether non-prescribing of this drug will help patients by removing an intervention with known benefit or be detrimental to them is unknown. 201 No advances have been reported in Aβ therapeutics, with negative results from phase 3 trials of monoclonal antibodies (eg, solanezumab, crenezumab) and inhibitors of β-secretase, a protease involved in the production of Aβ peptides. 202 Aducanumab previously abandoned as futile now has further unpublished results. Three 5HT6 antagonists and the calcium channel blocker nilvadipine 203 , 204 have also been ineffective. These drugs also show substantial impact during treatments at so-called therapeutic concentrations on the leakiness of blood vessels. The long-term impact of such side-effects is unknown. Anti-tau, anti-amyloid, and anti-inflammatory drugs continue to be in focus and some argue that pre-symptomatic interventions are necessary, especially if targeting Aβ production, but no evidence of efficacy 205 and some evidence of worsening target symptoms currently exists. 206
Cognitive training in people with dementia
A meta-analysis of 12 controlled trials of 389 people with mild dementia, completing 4 or more hours of group-based computerised cognitive training (mean age 66–81 years, 63·5% female participants), found a small, statistically significant beneficial effect on overall cognition, driven by two trials of virtual reality or Video games (SMD=0·3, 95% CI 0·0–0·5), one with a low and one with a high risk of bias. 55
A Cochrane review 207 found 33 trials of cognitive training, only one of which overlapped with the study above, with around 2000 participants with mild-to-moderate dementia, most with a high or uncertain risk of bias. 207 People completing cognitive training, compared with usual treatment or non-specific activities, had small-to-moderate effects on overall cognition (SMD 0·4, 95% CI 0·2–0·6) and specific cognitive abilities such as verbal fluency and improvements lasted for a few months to 1 year. No direct evidence was observed to suggest that cognitive training was better than cognitive stimulation therapy.
Exercise and physical activity
The Dementia and Physical Activity RCT 208 found moderate-to-high intensity aerobic and strength exercise training did not slow cognitive impairment in people with mild-to-moderate dementia but improved physical fitness. The US Reducing Disability in Dementia study 209 implemented an at-home multicomponent intervention including exercise education, training to increase pleasant events, and activator-behaviour-consequence problem-solving approach over 6 weeks by case managers in 255 community dwelling people with dementia older than 60 years and their family carer and were able to follow up 140 (54·9%). The study found increased physical activity; days of taking 30 or more minutes of exercise (effect size 0·6, 95% CI 0·4–0·8 after the treatment and 0·3, 0·1–0·5 at 13 months) in a before and after intervention comparison.
Interventions for neuropsychiatric symptoms of dementia
Neuropsychiatric symptoms are common and often clustered in people with dementia. These symptoms might precede dementia and are associated with tau and amyloid neuropathology. 210 This suggests that underlying neurobiological mechanisms might underpin neuropsychiatric symptoms. However, other drivers relating to the personal history and the environment of the person with dementia are also likely to exist. Neurodegeneration could lead to increased vulnerability to stressors or triggers. Genetics, cognitive reserve, resilience, medical comorbidities, and environment including responses of carers might modify these relationships. Needs and responses will also be individual and relate to a person's own social, cultural, and historical context. First-line assessment and management of neuropsychiatric symptoms should focus on basic health: describe and diagnose symptoms; look for causes such as pain (using validated pain assessments might help), illness, discomfort, hunger, loneliness, boredom, lack of intimacy and worry that could cause the behaviours and alleviate these while considering risks of harm. 2
No new evidence of medication effectiveness for these symptoms exists; risperidone in low doses (0·5 mg daily) and some other antipsychotics are sometimes effective but often ineffective and have adverse effects. 2 Specific initiatives have led to a decrease in antipsychotic prescriptions for people with dementia, although often replaced with other psychotropics ( figure 8 ), such as benzodiazepines, antidepressants, and mood stabilisers. 211 These psychotropics lack evidence of efficacy for neuropsychiatric symptoms but show clear evidence of possible harm; for example, trazodone and benzodiazepines increase fall-related injuries. 144 Major policy changes should be assessed carefully, within and across countries for unintended consequences (and perhaps unexpected benefits) and their costs.

Proportion of patients with a diagnosis of dementia prescribed an antipsychotic drug (A) and those prescribed an anxiolytic, hypnotic, or antidepressant (B)
CPRD=Clinical Practice Research Datalink. Reproduced from Donegan et al, 211 by permission of Elsevier.
Evidence is slowly accumulating for the effectiveness, at least in the short term, of person-centred evidence-based psychosocial interventions. In Germany, a 6-month cluster RCT of nurse-delivered, supervised dementia care management used a computer-assisted nurse assessment to determine personalised intervention modules, then a multi-disciplinary team discussion and agreement with the physician for 634 people (mean age 80 years) with dementia living at home with a primary carer or alone. 212 The mean mini mental state examination (MMSE) was 23, only 38% had a formal diagnosis of dementia; the majority of participants (51%) had mild dementia but some had moderate and some severe dementia. The intervention consisted of psychosocial management of treatment and care, medication management and carer support, and education and discussion with a psychiatrist or neurologist. The intervention, compared with care as usual, was associated with better outcomes for neuropsychiatric symptoms (Neuropsychiatric Inventory [NPI] score −7·5, 95% CI −11·1 to −3·8), however this effect could be because of deterioration in care as usual (in the care as usual group NPI increased from 7·2 to 15·2; in the intervention group NPI increased from 7·6 to 8·2). This between-group reduction in neuropsychiatric symptoms was greater than that expected, extrapolating from other study results, with antipsychotic medication. Effects on quality of life were only apparent for those people living with a carer.
An eight-session home-based tailored activity programme RCT, tailored both to the person with dementia living at home and to a family member compared with eight telephone-based education sessions, recruited 160 participants with 64% follow-up, imputing values for the rest. 213 The study reported a large reduction in overall neuropsychiatric symptoms immediately after the intervention, which were better in the group receiving home-based tailored activity programme on the neuropsychiatric inventory (mean difference in score 24·3, 95% CI 3·1–45·6), and on functional dependence and pain but this was not sustained 4 months later. Non-completers had more severe neuropsychiatric symptoms.
Since the 2017 Commission two new systematic reviews of antidepressants to treat depression in dementia reported moderate quality evidence that antidepressant treatment for people with dementia does not lead to better control of symptomatology compared with placebo. 214 , 215
Agitation is distressing for people with dementia and those around them, and contributes substantially to the overall costs as the level of agitation increases. 216 The body of evidence on this key behaviour is growing, mostly focused on care-home settings. These findings are valuable as these populations are most affected; however, because many people with dementia reside at home a major gap in knowledge remains.
Care home residents with agitation often find sitting still difficult and therefore might not be included in activities. 217 , 218 Two new cluster RCTs of professionals delivering multicomponent, interdisciplinary, interventions in care homes successfully reduced agitation. The WHELD study 219 included participants with or without neuropsychiatric symptoms and provided person-centred care, aiming to improve communication with people with dementia. It implemented social, sensory experiences or other activities; educated about antipsychotic review; and addressed physical problems, finding lower Cohen Mansfield Agitation Inventory (CMAI) at 9 months (MD −4·3 points, 95% CI −7·3 to −1·2). 219 The TIME study 220 for people with moderate-to-high levels of agitation consisted of a manual-based comprehensive assessment of the resident and structured case conference for the staff and doctor, to create a tailored plan, and then implement it. This intervention led to reduced agitation at 8 weeks (NPI −1·1 points, 95% CI −0·1 to −2·1; CMAI −4·7 points, −0·6 to −8·8) and 12 weeks (NPI −1·6, −0·6 to −2·7; CMAI −5·9, −1·7 to −10·1). 220 These effect sizes are similar to those seen for medications, but without harmful side-effects. 2 , 221 A further RCT studied a six-session intervention with staff in groups, teaching staff to understand agitation as related to medical, psychological, or social unmet needs and to implement strategies to meet these needs, using the describe, investigate, create, and evaluate approach. 222 The intervention did not reduce agitation symptoms, although it was cost-effective, improving quality of life. 223 Overall, the current evidence for agitation in care homes favours multi-component interventions by clinical staff, including considering if drugs might harm, and not drug interventions. Thus a major gap remains in knowledge about people living at home who comprise the majority of those with dementia.
Psychotic symptoms in dementia
People with dementia might be wrongly thought to have delusions when they misremember, and new psychotic symptoms are often due to delirium, thus thorough assessment of symptoms is essential. 2 Management of psychosis in dementia should start with non-pharmacological interventions; however, evidence for effectiveness of these interventions for psychosis in dementia is weaker than for agitation. 224 Antipsychotics for psychosis in dementia should be prescribed in as low a dose and for the shortest duration possible. 2 However, a Cochrane review of antipsychotics withdrawal found two trials with participants with dementia who had responded to antipsychotic treatment. These reported that stopping antipsychotics was associated with symptomatic relapse 225 suggesting the need for caution in any medication withdrawal in this group. There was low-quality evidence that, in general, discontinuation might make little or no difference to overall neuropsychiatric symptoms, adverse events, quality of life or cognitive function. 226
Apathy might be conceptualised as the opposite of engagement, comprising reduced interest, initiative, and activity. Like people without dementia, those with dementia engage more in preferred activities, but require additional support to do so. 227 A study in care homes observed engagement increased during activities in those who attended the groups. 228 A Cochrane review of the few people who had been in drug RCTs of methylphenidate versus placebo for apathy in dementia found small improvements on the apathy evaluation scale (MD −5·0, 95% CI −9·6 to−0·4, n=145, three studies, low-quality evidence) but not on the NPI apathy subscale (MD −0·1, 95% CI −3·9 to 3·7, n=85, two studies). 229
There is no evidence that medication for sleep in dementia is effective 230 and considerable evidence for harm—ie, earlier death, increased hospitalisation, and falls—exists. 139 , 144 Testing of non-pharmacological interventions is ongoing. 231
Carer distress related to neuropsychiatric symptoms rather than the dementia symptoms was associated in one study with increased use and costs of health services, 232 highlighting the need for effectively identifying, educating, and supporting distressed carers. An RCT 233 reporting 6-year follow-up after the eight session STrAtegies for RelaTives intervention—manual-based coping intervention delivered by supervised psychology graduates—found continuing effectiveness for depressive symptoms in carers (adjusted MD −2·00; 95% CI −3·4 to −0·6) and risk of case-level depression, with patient-related cost being approximately 3 times lower than those who did not receive the intervention (median £5759 vs £16 964 in the final year; p=0·07). 233 Another US study 234 followed up 663 people, mean age 77 years, 55% women. Caregiver depression rather than symptoms of people with dementia predicted emergency department use for people with dementia, with a 73% (RR 1·73, 95% CI 1·3–2·3) increase. 234
Functioning
A UK RCT of 14 sessions of cognitive rehabilitation focused on individual goal attainment with therapy delivered at home by an occupational therapist or nurse to 475 participants with mild-to-moderate dementia (MMSE ≥18 for inclusion; mean 24) and a family carer. 235 Individuals had two or three goals; the most common was engaging in activities (21% of goals). The intervention group reported increased goal attainment over 3 and 9 months compared with usual treatment (effect size 0·8, 95% CI 0·6–1·0 at both 3 and 9 months). 235 The treatment did not improve participants' quality of life, mood, self-efficacy, cognition, carer stress, or health status and was not cost-effective. A systematic review 236 of RCTs without meta-analysis for overall effect size, concluded that all interventions which had improved functioning in people living with dementia in the community have been individual rather than group interventions. These were: in-home physiotherapist delivered aerobic exercise (two studies, larger one positive, 140 people with Alzheimer's disease; smaller study negative, 30 people with Alzheimer's disease), individualised cognitive rehabilitation (mild or moderate dementia; two studies; 257 cognitive reserve intervention groups and 255 controls), and in-home activities-focused occupational therapy (people with mild to moderate dementia, three studies, 201 intervention, 191 controls) reduced functional decline compared to controls but group-exercise and reminiscence therapies were ineffective. 236
People with dementia have other illnesses
Multimorbidity is a huge challenge in dementia, not only because people with dementia have increased rates of other illnesses, but also because they often find it particularly difficult to organise care. People with dementia might forget to tell their family or health professionals of symptoms, struggle to understand or follow agreed plans, and are more likely to forget to drink and eat, increasing falling and infection rates. 237 People with dementia consult primary care less often 238 and have fewer dental visits 239 than those without dementia and their family members, if involved, often feel they lack knowledge to assist. 240 Health-care professionals need education to be more comfortable, understanding, and positive in communicating with people with dementia. 241
Around 70–80% of people diagnosed with dementia in primary care have at least two other chronic illnesses. 242 , 243 People who are physically more frail are more likely to have dementia, but the relationship between pathology and symptoms in these people is comparatively weak suggesting that dementia might be from other causes. 22 Compared to the general older population, people with dementia have increased rates of cerebrovascular disease, 243 , 244 , 245 , 246 stroke, 247 Parkinson's disease, 243 , 245 diabetes, 245 , 247 skin ulcers, anxiety and depression, 243 , 245 pneumonia, incontinence, and electrolyte disturbance. 245 Multimorbidity in people with dementia is associated with faster functional decline 248 and worse quality of life for people with dementia and their family carers. 249
Dementia and COVID-19
Severe acute respiratory syndrome coronavirus 2, was first identified in patients with viral pneumonia in Hubei province, China. 250 Severity and mortality of the associated disease (COVID-19) worsen with increasing age 251 and with pre-existing illnesses such as hypertension and diabetes, 252 and thus many people with dementia are at particular risk. Death certificates from the UK indicate that dementia and Alzheimer's disease were the most common underlying conditions, specified in 11 950 deaths (25·6% of all deaths involving COVID-19) in March to May, 2020. 253 Many charities, practitioners, and academics supporting people with dementia have issued guidance based on current evidence and best practice, including advance consideration of whether people would wish to be hospitalised if they develop severe COVID-19. Concern has been expressed that the illness and consequent distancing might increase family carer stress, loneliness, neuropsychiatric symptoms and use of psychotropic medication, and lead to complications, including future dementia. Interventions delivered remotely through technology have also been implemented in some places. 254 , 255 , 256 , 257
People with dementia might struggle to adhere to measures to reduce virus transmission, as they might not understand or remember about required changes to behaviour, such as physical distancing and hygiene, leading to increased risk to themselves and their carers. 258 They might additionally be vulnerable if they depend on others for daily activities or personal care, as this necessitates close personal contact.
This situation is particularly concerning in those care homes, where many residents have dementia and where many COVID-19 deaths have occurred in many countries 259 , 260 , 261 with reports of more than half of residents being admitted to hospital. In US nursing homes, among 10 576 people with confirmed COVID-19, residents living with dementia made up 52% of COVID-19 cases; yet, accounted for 72% of all deaths (an increased risk of 1·7). 262 The number of people living together in care homes means that the infection of an individual, either staff or resident, could endanger more people than in traditional or family households. Although evidence exists that if staff are sufficiently and rigorously protected they are unlikely to develop COVID-19, many staff have become unwell and some have died. 263 , 264 Illness means that there are fewer people to care for residents at a time when they need particularly high levels of care. This situation is particularly relevant in the care of residents with dementia, if they are expected to remain in their own rooms, rather than eating and participating in activities with others. Staff or residents might also be moved between care homes and increase risk in other homes. 261 Restrictions on visitors to private homes, care homes, and hospitals might cause greater distress for people with dementia and they might not understand why people are wearing masks, recognise who is behind it, or understand speech when lips are covered. Lack of restrictions means that the visitors might also be at elevated risk. 261
The impacts of COVID-19 on people with dementia might be particularly severe in LMICs, due to smaller health budgets for testing and protective equipment, capacity of health-care systems, quality of care home provision and patterns of workforce mobility. 264
Thus, people with dementia are particularly vulnerable to COVID-19 because of their age, multimorbidity, and difficulties in maintaining physical distancing. 250 , 251 , 252
We recommend rigorous public health measures of protective equipment and hygiene, including not moving staff or residents between care homes or admitting new residents when their COVID-19 status is unknown, should mitigate impacts on people with dementia. It is also imperative that there is frequent and regular testing of staff in care homes for infection, ensuring staff have sick pay so that they do not come in when symptomatic and interim care is being set up for people discharged from hospital so that only those who are COVID-19 free come to live in care homes. Resident testing should encompass asymptomatic as well as symptomatic people, when there is exposure within the home to COVID-19. In the future, many homes might be able to start to provide oxygen therapy so that those who do not want to be admitted to hospital are still able to access oxygen therapy. In addition, it is also important to reduce isolation by providing the necessary equipment and a brief training to relatives on how to protect themselves and others from COVID-19; so that they can visit their relatives with dementia in nursing homes safely when it is allowed. Further evidence is needed to inform responses to this and future public health emergencies.
Hospital admissions
Hospitalisation in people with dementia is associated with adverse, unintended consequences, including distress, functional and cognitive decline, and high economic costs. 265 , 266 , 267 People with dementia have 1·4 to 4 times more hospital admissions than others with similar illnesses. 266 , 268 , 269 , 270
A systematic review and meta-analysis including 34 studies of 277 432 people with dementia found that in the six studies which compared the two groups, people with dementia had increased hospital admissions compared with those without dementia, after adjusting for age, sex, and physical comorbidity (RR 1·4, 95% CI 1·2–1·7; figure 9 ). 271 Hospitalisation rates in people with dementia ranged from 0·37 to 1·26 per person-year in high-quality studies. Admissions are often for conditions that might be manageable in the community (potentially preventable hospitalisations). 268 People with dementia experience longer and more frequent admissions and readmissions; health-care expenditure for people with moderate-severe dementia is around double that of people without dementia. 269 , 272 , 273 Early detection and management of physical ill-health in people with dementia, particularly of pain, falls, diabetes, incontinence, and sensory impairment, is important. 199 , 274 , 275 However, no intervention has successfully reduced number of hospital admissions of community-dwelling people with dementia, 276 although education, exercise, rehabilitation, and telemedicine have reduced admissions for older people without dementia. 277

Systematic review and meta-analysis of hospitalisation rates of people with dementia compared to those without dementia controlled for age and sex
Reproduced from Shepherd et al, 271 by permission of Springer Nature.
High-quality care for people with dementia takes longer than caring for others with the same condition. 278 Recognition of dementia in hospital inpatients is necessary for optimum care, 279 but dementia is often undetected or unrecorded. 280 In the UK however, detection rates have increased over the past 10 years. 281
Physical illness, delirium, and dementia
Dementia and delirium frequently occur together. In one hospital inpatients' survey nearly 35% of those older than 80 years experienced delirium; those with prior cognitive impairment had 15 times the risk of developing delirium than those without (OR 15·3, 95% CI 5·2–45·4). 282 People with delirium without known dementia are more likely to be diagnosed with dementia in the future than others, either because of pre-existing undiagnosed dementia or cognitive impairment, present in 20·7% (95% CI 11·9–29·5) and 37·8% (27·3–88·3) respectively of one cohort, or because delirium has neurotoxic effects and so precipitates dementia. 283 People with similar neuropathology show faster cognitive decline if they develop delirium than if they do not. 284 Additionally, older people without dementia declined cognitively more than twice as fast after an emergency hospital admission for any cause, compared with those not admitted, suggesting any severe illness is associated with cognitive decline. 285 Risk factors for delirium in dementia include sensory impairment, pain, polypharmacy, dehydration, intercurrent illnesses, such as urinary tract infections or faecal impaction, and an unfamiliar or changing environment. 286 Delirium in older people should prompt consideration of underlying dementia.
Most research on delirium prevention has been in people without dementia. It suggests targeting hydration, stopping medication predisposing to delirium, monitoring the depth of anaesthesia, and sleep promotion. However, no evidence for medication efficacy, including cholinesterase inhibitors, antipsychotic medication, or melatonin exists. 287 , 288 , 289 The Hospital Elder Life Program 290 —an intervention to prevent delirium in those admitted to hospital—reduces delirium incidence and includes people who are cognitively impaired. This multidisciplinary treatment consists of daily visits, orientation, therapeutic activities, sleep enhancement, early mobilisation, vision and hearing adaptation, fluid repletion, infection prevention and management of constipation, pain, and hypoxia, and feeding assistance. 290
A network meta-analysis of drugs for prevention and treatment of delirium did not include studies of people with dementia, thus we cannot use this to recommend drugs for people with dementia and delirium as this research might be inapplicable to them. 291
Little high-quality research exists on managing delirium in dementia. One RCT compared care at a specialist medical and mental health unit to usual care for 600 confused people older than 65 years, acutely admitted to hospital and found no difference in the primary outcome of days spent at home or in hospital, but increased family satisfaction. 292 A further RCT of cognitively stimulating activities for people with delirium in dementia did not improve the delirium. 293 No definitive evidence that any medication improves delirium in people with dementia exists: cholinesterase inhibitors, antipsychotics, and sedating benzodiazepines are ineffective and antipsychotics and benzodiazepines are associated with mortality and morbidity. 265 , 288 , 294 , 295 , 296 , 297 Given the risk of dementia in people who develop delirium, its prevention, and possibly advances in its management, might offer a means for dementia prevention. 298
Link between very old age, frailty, and dementia
The fastest growing demographic group in most advanced countries are people aged 90 years and older. One well characterised post-mortem cohort of the oldest old (n=1079; mean age 90 years) dying with dementia, found that neuropathological features of Alzheimer's disease account for about half of the cognitive decline seen as people diagnosed with Alzheimer's disease had mixed causes of dementia. 299 Although Alzheimer's disease neuropathology was the commonest cause of dementia, Alzheimer's disease changes rarely occurred on their own, so only 9% of people with dementia had pure Alzheimer's disease pathology. 300 People who have Alzheimer's disease pathology without developing dementia tend to have fewer age-related health deficits than those who develop it with even low concentrations of plaques and tangles. 301 A moderation analysis showed that the relationship between Alzheimer's disease pathology and dementia status differed according to level of frailty (adjusted for age, sex, and education) with increasing frailty weakening the relationship between Alzheimer's disease pathology and dementia ( figure 10 ). 22 As with delirium, some of this additional health risk might be modifiable. This approach suggests a new type of therapy focus on specific age-related processes that underpin many diseases of late life might reduce the incidence or severity of dementia.

Moderation analyses of the relationship between Alzheimer's disease pathology and clinical diagnosis of Alzheimer's dementia (adjusted for age, sex, and education)
As frailty increased, the odds of a neuropathological diagnosis of Alzheimer disease corresponding to a clinical diagnosis decreased. Reproduced from Wallece et al, 22 by permission of Elsevier.
End-of-life care in dementia
The numbers of people dying with dementia are increasing but the evidence for the best end-of-life care is scarce. Trends in age-standardised death rates (3·6%) for dementia increased slightly between 1990–2016, with pronounced increases in the USA and Japan and decreases in western Europe and central Latin America. 4 Dementia is more readily being included on death certificates, which accounts for some of the rise. The increase might be related to dementia manifesting at later ages, with higher physical frailty 22 leading to a faster decline.
Most people with dementia might die while still in the mild-to-moderate stages whereas only about a quarter of those dying with dementia have severe dementia. 302 , 303 The trajectory of dementia is often unpredictable 304 and palliative care initiation should reflect need not prognosis.
Decision making about end of life is complex and simple rules of thumb, co-designed with staff and carers, provided clarity in some small studies. 304 One RCT testing decision-aids about families' and doctors' goals of care for people with advanced dementia led to increased palliative care content in care plans. 305 , 306 In a 9-month UK prospective study, 85 care home residents with advanced dementia from 14 homes were likely to be living with distressing symptoms, specifically agitation (54%) or pain (61% on movement). 304
Capacity to make abstract decisions, including about the future, might be lost early in dementia. 307 Therefore, advance care planning, designed to empower people with dementia and improve quality of dying, might theoretically be something everyone should do before developing dementia. 308 However, people might not be able to predict their future wishes. This might explain why family carer proxies show only low-to-moderate agreement with stated end-of-life treatment preferences of people with dementia. 309 Advance care planning might, however, reduce carers' uncertainty in decision making and improve perceptions of quality of care. 310
Partners of people dying with dementia experience poorer mental health than those facing bereavement from other causes 311 possibly because of long and difficult caring responsibilities. This might be ameliorated through sensitive and timely information, particularly regarding the progression of dementia, 312 individually or through family and staff case-conferencing. 313 , 314
Conclusions
Knowledge about risk factors and potential prevention, detection, and diagnosis of dementia is improving although significant gaps remain. 315 In this Commission report, we have specified policy and individual changes to delay the onset of cognitive impairment and dementia and better ways to support and treat people with dementia and their families and to improve their quality of life.
Interventions, including organisation of the complex physical illness and social needs, to support people affected by dementia can have a huge effect when taken as a whole. Our ambition is for worldwide provision of resources for an adequate level of wellbeing to people with dementia and their carers with a better evidence base to guide individual care and policy making alike. With good quality care, people can live well with dementia and families can feel supported.
Acknowledgments
We are partnered by University College London (UCL), the Alzheimer's Society, UK, the Economic and Social Research Council, and Alzheimer's Research UK, and would like to thank them for financial help. These organisations funded the fares, accommodation, and food for the Commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication. We would like to thank Bernadette Courtney, Jacques Gianino, and Nuj Monowari, from UCL, London, UK, for their administrative help, including managing finances, booking rooms and food, and setting up a website supported by the University College London Hospitals National Institute for Health Research Biomedical Research Centre. We would like thank Henrik Zetterberg for advice on biomarkers and dementia.
Contributors
GL, JH, AS, and NM contributed to literature searches and quality assessments for systematic reviews. JH and NM performed meta-analyses. GL, JH, AS, and NM conceived the new PAF calculation and NM led the statistical analysis. GL, JH, AS, NM, DA, CLB, SB, AB, JC-M, CC, SGC, NF, RH, HCK, EBL, VO, KRi, KRo, ELS, QS, LSS, and GS attended the conference to discuss the content. GL, JH, EBL, AS, DA, and ELS wrote first drafts of sections of the paper. GL wrote the first draft of the whole paper and revisions of drafts. CBa reviewed and contributed to revision of the final drafts. All authors contributed to sections of the reports and all revised the paper for important intellectual content.
Declaration of interests
AS reports grants from Wellcome Trust (200163/Z/15/Z), outside the submitted work. DA reports grants from Eli Lilly, during the conduct of the study. CBa reports grants and personal fees from Aca-dia and Lundbeck; and personal fees from Roche, Otsuka, Biogen, Eli Lilly, and Pfizer, outside the sub-mitted work. SB reports grants and personal fees from AbbVie, personal fees and non-financial sup-port from Eli Lilly, and personal fees from Eleusis, Daval International, Boehringer Ingelheim, Axovant Sciences, Lundbeck, and Nutricia, outside the submitted work; and he has been employed by the Department of Health for England. NF reports non-financial support from Eli Lilly, outside the submitted work. LNG and her institutions (Johns Hopkins University, Baltimore, MD, USA, Drexel University, Philadelphia, PA, USA, and Thomas Jefferson University, Philadelphia, PA, USA) are entitled to receive royalties from fees associated with online training for the tailored activity program, which is an evidence-based program referenced in the Review. RH reports grants from Department of Health, NIHR HTA Programme, outside the submitted work; and he is a Scientific Trustee of the charity Alzheimer's Research UK. MK reports grants from the UK Medical Research Council (S011676, R024227), NordForsk (the Nordic Programme on Health and Welfare, 75021) and the Academy of Finland (311492), outside the submitted work. EBL reports other (royalties) from UpToDate, outside the submitted work. KRo reports personal fees from Clinical Cardio Day-Cape Breton University, Sydney, NS, Canada, CRUIGM-Montreal, Jackson Laboratory, Bar Harbor, MA, USA (speaker fees), MouseAge, Rome, Italy (speaker fees), Lundbeck, Frontemporal Dementia Study-Group, SunLife Insurance, Japan, outside the submitted work. He is a President and Chief Science Officer of DGI Clinical, which in the last 5 years has contracts with pharma and device manufacturers (Baxter, Baxalta, Shire, Hollister, Nutricia, Roche, Otsuka) on individualised outcome measurement. In 2017, he attended an advisory board meeting with Lundbeck. He is also Associate Director of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes of Health Research, and with additional funding from the Alzheimer Society of Canada and several other charities, as well as, in its first phase (2013-2018), from Pfizer Canada and Sanofi Canada. He receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research, and research support from the Canadian Institutes of Health Research, the QEII Health Science Centre Foundation, the Capital Health Research Fund and the Fountain Family Innovation Fund of the QEII Health Science Centre Foundation. LSS reports grants and personal fees from Eli Lilly, Merck, and Roche/Genentech; personal fees from Avraham, Boehringer Ingelheim, Neurim, Neuronix, Cognition, Eisai, Takeda, vTv, and Abbott; and grants from Biogen, Novartis, Biohaven, and Washington University DIAN-TU, outside the submitted work. The remaining authors declare no conflict of interests.
Supplementary Material
Uncited references.

IMAGES
COMMENTS
1. Introduction. Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles (Figure 1) as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain, the medial temporal ...
Introduction. Alzheimer disease (AD) is one of the greatest medical care challenges of our century and is the main cause of dementia. In total, 40 million people are estimated to suffer from dementia throughout the world, and this number is supposed to become twice as much every 20 years, until approximately 2050. 1 Because dementia occurs mostly in people older than 60 years, the growing ...
Rate of decline of memory (M) over time (t, months to years). Memory declines slowly in normal aging (1). Alzheimer's disease is marked by more rapid cognitive decline, often starting earlier in life (2). Current therapies enhance cognition without changing the rate of decline in AD (3). The anticipated effect of novel therapies is reduction ...
Alzheimer's disease (AD) is the most common type of dementia [], affecting at least 27 million people and corresponding from 60 to 70% of all dementias cases [].The occurrence of this disease also has a huge impact on life of patient's family, in addition to a high financial cost to society [].From an anatomopathological point of view, AD is characterized by two prototypical lesions: 1 ...
Over the past two decades, the landscape of dementia research has changed drastically due to advances in knowledge at the molecular, cellular, animal, and human levels. Advances have not been limited to the Alzheimer's disease spectrum but include improved understanding of other disorders that can also lead to dementia. In this Anniversary Round-up, I discuss what I consider to be the most ...
Alzheimer disease is a neurodegenerative disorder that causes cognitive impairment. This Primer by Knopman et al. reviews the epidemiology of cognitive manifestations and risk factors, summarizes ...
This book highlights the key phases and central findings of Alzheimer's Disease research since the introduction of the label 'Alzheimer's Disease' in 1910. The author, Christian Behl, puts dementia research in the context of the respective zeitgeist and summarizes the paths that have led to the currently available Alzheimer's drugs.
A brain scan reveals the extent of damage caused by Alzheimer's disease. Credit: Zephyr/Science Photo Library ... After spending more than 30 frustrating years in Alzheimer's research, she ...
A range of blood-based biomarkers have shown high specificity for Alzheimer's disease (AD) pathophysiology with phosphorylated-tau (p-tau) being the most promising test. Here, the authors show ...
The federal government's Alzheimer's and related dementias research strategy focuses on engaging a cross-disciplinary team of geneticists, epidemiologists, gerontologists, behavioral scientists, disease and structural biologists, pharmacologists, clinical researchers, and others to bring the greatest and most diverse expertise to the field.
Alzheimer's disease (AD) is the most common cause of dementia and is clinically characterized b y a progression from episodic memory problems to a slow general decline of cognitive function. [1]
Suddenly, AD dementia went from a relatively rare condition to a major public health issue. This led to greater attention to the disease by the public and at the National Institutes of Health, which established the National Alzheimer's Disease Research Center program to study the cause, neuropathology, and clinical characteristics of AD.
Seven recent papers amplify advances in Alzheimer's research. New findings from big-data and open-science research are revealing clues about the molecular mechanisms of Alzheimer's disease and new ways to discover potential therapeutic targets and biomarkers. These new discoveries were made by six research teams participating in the ...
Progress with Treatments for Alzheimer's Disease. Allan I. Levey, M.D., Ph.D. An estimated 50 million people worldwide have dementia, mostly due to Alzheimer's disease. The inexorable ...
The Nature paper has been cited in about 2300 scholarly articles—more than all but four other Alzheimer's basic research reports published since 2006, according to the Web of Science database. Since then, annual NIH support for studies labeled "amyloid, oligomer, and Alzheimer's" has risen from near zero to $287 million in 2021.
Similarly, in Western Europe, dementia affects ~2.5% of people aged 65-69 years, escalating to about 40% of those aged 90-94 years , and by 2050, there will likely be up to 18.9 million ...
Current Alzheimer Research. Articles & Issues. Menu. Articles & Issues. Latest issue; All issues ... Towards the Integrative Theory of Alzheimer's Disease: Linking Molecular Mechanisms of Neurotoxicity, Beta-amyloid Biomarkers, and the Diagnosis ... More opportunities to publish your research: Browse open Calls for Papers beta. ISSN: 1567 ...
To advance research into Alzheimer's disease and Alzheimer's disease-related dementias (AD/ADRD), NIA funds centers and programs that gather, coordinate, and share data. These resources are available to investigators who are researching the many facets of AD/ADRD in order to better understand the disease and develop effective treatments and ...
1. Introduction. Alzheimer's disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ shutdown [1,2,3,4,5].
In this Double Take video from the New England Journal of Medicine, Drs. Nathaniel Chin and Stephen Salloway define the stages of dementia and the effect of each stage on the activities of daily ...
More specifically, Alzheimer's diseaseis a progressive, neurodegenerative disease that is categorized by a loss of memory, along with basic life skills like eating, bathing, talking, etc. Common symptoms include: memory loss, paranoia, depression, anger, aggression, anxiety, apathy, loneliness, and psychosis. These symptoms vary from person ...
Alzheimer's Disease Prevalence and Prevention. The estimated global prevalence of Alzheimer's disease is 50 million and is projected to triple by 2050 due to growth in the older generation. According to Alzheimer's Association, AD is the fifth-ranking killer of persons […] Dancing and Risk of Alzheimer's Disease.
In a paper published last year 2, when he was working for Danish firm Novo Nordisk, in a lab just outside Copenhagen, Raket took a similar approach to calculating treatment effects in terms of ...
Dementia is an acquired loss of cognition in multiple cognitive domains sufficiently severe to affect social or occupational function. In the US, Alzheimer's disease (AD) affects 5.8 million people. However, dementia is commonly associated with more than one neuropathology, usually AD with cerebrovascular pathology.
Research Paper Alzheimers Disease - Free download as PDF File (.pdf), Text File (.txt) or read online for free. research paper alzheimers disease
A doctor points out evidence of Alzheimer's disease on PET scans at the Center for Alzheimer Research and Treatment at Brigham And Women's Hospital in Boston, Massachusetts.
All these disturbances were associated with a higher risk of all-cause dementia (RR 1·2; 95% CI 1·1-1·3) 137 and clinically diagnosed Alzheimer's disease (1·6, 1·3-1·9) compared with no sleep disturbance, although not all cohort studies excluded those with cognitive impairment or dementia at baseline from their analyses. 138 A U ...