- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
February 2, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked

New therapeutic strategy for metastatic prostate cancer patients resistant to standard treatment
by Germans Trias i Pujol Research Institute

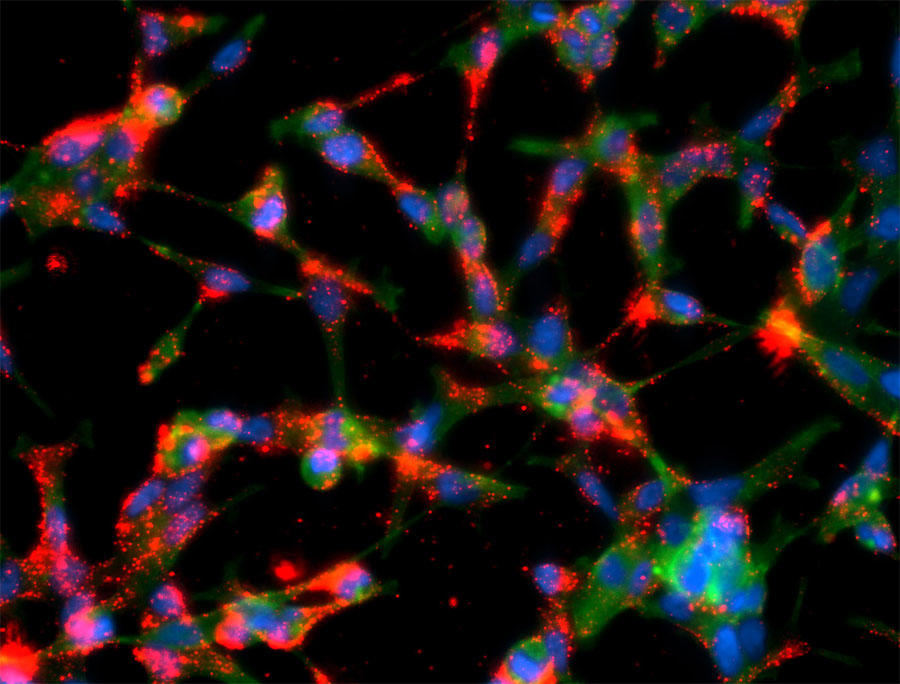
A team of researchers from the Badalona Applied Research Group in Oncology (B·ARGO) and the Urologic Tumors Unit of the Institut Català d'Oncologia (ICO) and the Germans Trias i Pujol Research Institute (IGTP) have found a new therapeutic strategy for patients with a specific subtype of metastatic prostate cancer resistant to standard chemotherapy treatment with docetaxel.
In this study, published in the journal Frontiers in Pharmacology , they propose a new treatment based on a combination of kinase inhibitors in patients who inevitably stop responding to docetaxel .
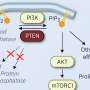
The team found that resistance to this drug is associated with the hyperactivation of the cellular pathways PI3K/AKT and MEK/ERK and have explored the possibility of inhibiting these pathways as a new therapeutic strategy in patients who maintain the function of PTEN, a negative regulatory protein of the PI3K/AKT pathway.
The results of the study have been satisfactory and, for this reason, the team wants to conduct a clinical trial to assess the safety and efficacy of this combination in patients with prostate cancer resistant to docetaxel.
Vicenç Ruiz de Porras and Adrià Bernat-Peguera, ICO-IGTP researchers and co-first authors of the study, state that the results of this study "open the door to a new therapeutic strategy for those patients with PTEN wild-type tumors, who have progressed to docetaxel and in whom, unlike PTEN null patients , the efficacy of AKT inhibitors in monotherapy has not been demonstrated."
Explore further
Feedback to editors

Scientists identify rare gene variants which confer up to 6-fold increase in risk of obesity

Diabetes drug shows promise against Parkinson's in clinical study

Study: Life expectancy increased as world addressed major killers, though poor pandemic management slowed progress
11 hours ago

Researchers map how the brain regulates emotions
13 hours ago

Paper: Policy reforms urgently needed to mitigate racial disparities in perinatal mental health conditions
14 hours ago

Earlier menopause plus high cardiovascular risk may lead to cognitive problems later
Users actively seek and share child sexual abuse material on Tor, but help is available to those willing to stop
Hepatitis C cases dropped in the US. Health officials aren't sure if it's a blip or a trend

Team moves forward in developing a vaccine for the 'zombie drug' xylazine
15 hours ago
Immunotherapy for Alzheimer's disease shows promise in mouse study
Related stories.

Protein discovery could help solve prostate cancer drug resistance
Jan 22, 2024

Discoveries suggest new breast cancer treatment
Oct 15, 2020

Newly identified personalized immunotherapy combination treats an aggressive form of advanced prostate cancer
Mar 3, 2023
Researchers characterize the tumor suppressor activity of the PTEN protein in melanoma
Jan 9, 2024

Cancer-causing mutations rewire growth signaling in prostate cancer model
Sep 11, 2023

Experimental drug shows promise
Jun 14, 2017
Recommended for you
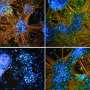
Researchers make mice a more powerful tool to study a wide range of human diseases
16 hours ago

A molecular route to decoding synaptic specificity and nerve cell communication
19 hours ago

New study targets major risk factor for gastric cancer

Study suggests lung cancer does not decrease in line with reduced smoking
Apr 2, 2024
AI's ability to detect tumor cells could be key to more accurate bone cancer prognoses
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Eur Urol Open Sci
- v.36; 2022 Feb
A Systematic Review of Patients’ Values, Preferences, and Expectations for the Treatment of Metastatic Prostate Cancer
Martin j. connor.
a Imperial Prostate, Division of Surgery, Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, London, UK
b Imperial Urology, Charing Cross Hospital, Imperial College Healthcare NHS Trust, London, UK
Mesfin G. Genie
c Health Economic Research Unit (HERU), Institute of Applied Health Sciences, University of Aberdeen, Aberdeen, UK
David Burns
Edward j. bass, michael gonzalez.
d Department of Oncology, Charing Cross Hospital, Imperial College Healthcare NHS Trust, London, UK
Naveed Sarwar
Alison falconer, stephen mangar, tim dudderidge.
e Department of Urology, University Hospital Southampton NHS Foundation Trust, Southampton, UK
Vincent Khoo
f Department of Clinical Oncology, The Royal Marsden Hospital & Institute of Cancer Research, London, UK
Mathias Winkler
Hashim u. ahmed, verity watson, associated data.
Advances in systemic agents have increased overall survival for men diagnosed with metastatic prostate cancer. Additional cytoreductive prostate treatments and metastasis-directed therapies are under evaluation. These confer toxicity but may offer incremental survival benefits. Thus, an understanding of patients’ values and treatment preferences is important for counselling, decision-making, and guideline development.
To perform a systematic review of patients’ values, preferences, and expectations regarding treatment of metastatic prostate cancer.
Evidence acquisition
The MEDLINE, Embase, and CINAHL databases were systematically searched for qualitative and preference elucidation studies reporting on patients’ preferences for treatment of metastatic prostate cancer. Certainty of evidence was assessed using Grading of Recommendation, Assessment, Development and Evaluation (GRADE) or GRADE Confidence in the Evidence from Reviews of Qualitative Research (CERQual). The protocol was registered on PROSPERO as CRD42020201420.
Evidence synthesis
A total of 1491 participants from 15 studies met the prespecified eligibility for inclusion. The study designs included were discrete choice experiments ( n = 5), mixed methods ( n = 3), and qualitative methods ( n = 7). Disease states reported per study were: metastatic castration-resistant prostate cancer in nine studies (60.0%), metastatic hormone-sensitive prostate cancer in two studies (13.3%), and a mixed cohort in four studies (26.6%). In quantitative preference elicitation studies, patients consistently valued treatment effectiveness and delay in time to symptoms as the two top-ranked treatment attributes (low or very low certainty). Patients were willing to trade off treatment-related toxicity for potential oncological benefits (low certainty). In qualitative studies, thematic analysis revealed cancer progression and/or survival, pain, and fatigue as key components in treatment decisions (low or very low certainty). Patients continue to value oncological benefits in making decisions on treatments under qualitative assessment.
Conclusions
There is limited understanding of how patients make treatment and trade-off decisions following a diagnosis of metastatic prostate cancer. For appropriate investment in emerging cytoreductive local tumour and metastasis-directed therapies, we should seek to better understand how this cohort weighs the oncological benefits against the risks.
Patient summary
We looked at how men with advanced (metastatic) prostate cancer make treatment decisions. We found that little is known about patients’ preferences for current and proposed new treatments. Further studies are required to understand how patients make decisions to help guide the integration of new treatments into the standard of care.
Take Home Message
A systematic review of patients’ values, preferences, and expectations for treatment of metastatic prostate cancer revealed that treatment effectiveness and delay in time to symptoms were consistently valued as the two most important attributes in quantitative studies (low or very low certainty). In qualitative studies, patients identified cancer progression and/or survival, pain, and fatigue as key (low or very low certainty). Greater understanding of how patients make trade-off decisions is needed for appropriate investment in emerging cytoreductive local tumour and metastasis-directed therapies.
1. Introduction
In contrast to localised prostate cancer, patients with metastatic prostate cancer have distant spread of disease that is not curable [1] . This disease state has primarily been managed using androgen deprivation therapy (ADT) via medical or surgical castration [1] . In isolation, this intervention can lead to disease progression from metastatic hormone-sensitive prostate cancer (mHSPC) to the androgen-independent state of metastatic castration-resistant prostate cancer (mCRPC) within 11–18 mo, limiting overall survival (OS) [2] , [3] .
Recent advances in systemic therapy (eg, docetaxel, abiraterone acetate, enzalutamide, and apalutamide) have resulted in a dramatic improvement in median OS for patients with mHSPC at 4.8 yr [4] , [5] , [6] , [7] . To gain a further oncological benefit, there has been a move to explore local cytoreductive treatments of the primary prostate tumour and its metastases in both mHSPC and mCRPC [1] , [8] , [9] . Research is particularly focused on patients with a limited number of metastases, or oligometastatic disease [10] . Local prostate interventions include cytoreductive external beam radiotherapy, cytoreductive radical prostatectomy, and cytoreductive minimally invasive ablative therapies [1] , [11] , [12] , [13] , [14] , [15] . In addition, metastasis-directed interventions include stereotactic ablative radiation therapy (SABR), lutetium-177 prostate-specific membrane antigen ligands, radium-223, and metastasectomy [8] , [16] , [17] .
These novel interventions offer significant oncological promise for patients with metastatic prostate cancer [1] . Furthermore, secondary benefits may also arise from the avoidance or delay of second- and third-line systemic agents and their associated toxicity [16] , [18] . However, each specific treatment is not without its own treatment-related risk (eg, death) and significant side effects may occur (eg, urinary incontinence, fatigue) [18] . Thus, an understanding of patients’ values and preferences for management is important for patient counselling, decision-making, and guideline development.
This systematic review synthesises the evidence from quantitative preference elicitation studies and qualitative studies reporting on patients’ values, preferences, and expectations in the treatment of metastatic prostate cancer.
2. Evidence acquisition
This prospectively registered (PROSPERO, CRD42020201420) systematic review was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines [19] , [20] .
2.1. Search strategy
A systematic search of the MEDLINE, CINAHL, and Embase databases was carried out, with searches of reference lists of eligible studies to capture additional relevant articles that met our inclusion and exclusion criteria. In brief, the search key terms included “prostate neoplasm/adenocarcinoma” and “metastasis/oligmetastasis/advanced/stage IV/metastatic” and “preference elicitation/discrete choice experiment/stated preference/part-worth utility/functional measurement/paired comparison/pairwise choice/conjoint analysis/conjoint measurement/best-worst scale/contingent valuation/standard gable/time-tradeoff/willingness-to-pay/willingness-to-accept”. The detailed search strategy is provided in the Supplementary material . Search results were limited to the English language and from database inception until November 1, 2020. Review articles, letters, and conference abstracts were excluded at this stage.
The titles and abstracts were reviewed independently by three authors (D.B., M.G.G., V.W.) and adjudicated by a fourth author (M.J.C.). The eligibility criteria were then applied. Any disparities that arose were discussed with the co-authors until agreement was reached. Agreement was verified by a fifth author (H.U.A.) where required. The full text of the remaining articles was reviewed independently by four authors (D.B., M.G.G., V.W., M.J.C.).
2.2. Inclusion and exclusion criteria
We included quantitative preference elicitation studies (ie, discrete choice experiments [DCEs], time trade-off [TTO], and standard gamble) and qualitative studies (ie, interviews, focus groups) reporting on patient preferences for the treatment of metastatic prostate cancer. Studies were excluded if they involved (1) nonmetastatic disease or (2) a mixed cohort of disease states (ie, localised and metastatic) or mixed primary cancers if study outcomes were not presented separately by disease state.
2.3. Data extraction
The following data were extracted from all the studies included: reference, authors, publishing journal, year of publication, disease state of the patient population, study size, age, study design or methodology, treatment evaluated, main topic in relation to the study purpose, primary results, and conclusions.
2.4. Assessment of methodological quality
For quantitative studies, methodological quality was assessed using the Purpose, Respondents, Explanation, Findings, and Significance (PREFS) quality assessment checklist, which was developed to assess the quality of studies in systematic reviews of patient preference literature ( Supplementary Table 1 ) [21] . For qualitative studies, methodological quality was assessed using the Standards for Reporting Qualitative Research (SRQR) criteria ( Supplementary Table 4 ) [22] .
2.5. Risk of bias
For quantitative studies, risk of bias (RoB) was assessed using an RoB tool covering (1) sample selection, (2) response (or attrition) rate, (3) choice and administration of the methodology, (4) outcome (or health state) presentation, and (5) respondent understanding and data analysis ( Supplementary Table 2 ). In accordance with previous systematic reviews, high RoB was assigned when the measurement instrument was not valid. If the measurement instrument was valid, RoB was designated as low if there were no individual items marked as high RoB and as moderate if not more than two items had moderate RoB [23] . For qualitative studies, RoB was assessed using the SRQR criteria ( Supplementary Table 4 ) [22] . Studies with a total score of less than 20 were deemed to have high methodological limitation (RoB).
2.6. Assessment of certainty of evidence
The certainty of evidence presented was assessed using Grading of Recommendation, Assessment, Development and Evaluation (GRADE) and GRADE Confidence in the Evidence from Reviews of Qualitative Research (CERQual) for quantitative and qualitative studies, respectively [24] , [25] .
2.7. Data analysis
A narrative synthesis (quantitative studies) and a thematic analysis (qualitative studies) of the collected data were undertaken with presentation of an interpretation of major findings in the context of the current field [26] . All discrete data points were analysed using SPSS version 27.0 (IBM Corp., Armonk, NY, USA). A meta-analysis of quantitative studies was not performed given the heterogeneous pool of study populations, designs, and outcomes reported.
3. Evidence synthesis
3.1. quantity of evidence and characteristics of the studies included.
Of the 573 articles identified, 15 studies with a total of 1491 participants met the prespecified eligibility for inclusion in this systematic review, as outlined in the PRISMA-P flow diagram ( Fig. 1 ) [27] , [28] , [29] , [30] , [31] , [32] , [33] , [34] , [35] , [36] , [37] , [38] , [39] , [40] , [41] . The mean number of participants per study was 99 (standard deviation [SD] 117.14). The study designs reported for the articles included were DCEs ( n = 5), mixed methods ( n = 3), and qualitative methods ( n = 7; Table 1 ). The mean age reported for participants ranged from 69.1 to 75.4 yr. The disease state of the participants was mCRPC in nine studies (60.0%), mHSPC in two studies (13.3%), and a mixed mCRPC/mHSPC cohort in four studies (26.6%).

Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) flowchart.
Characteristics and design of the studies included
NR = not reported; SD = standard deviation; A = academic; C = commercial; M – Mixed; TTO = time trade-off; DCE = discrete choice experiment; MMS = mixed-methods study; SSI = semi-structured interview; SI = structured interview; mHSPC = metastatic hormone-sensitive prostate cancer; mCRPC = metastatic castration-resistant prostate cancer; ONJ = osteonecrosis of the jaw; CTx = chemotherapy; AA = abiraterone acetate; VA = Veterans Affairs; NCI = National Cancer Institute; SRQR = Standards for Reporting Qualitative Research; PREFS = Purpose, Respondents, Explanation, Findings, and Significance for preference and qualitative studies.
Treatments evaluated in the studies included chemotherapy ( n = 3; 20.0%), abiraterone acetate ( n = 4; 26.7%), enzalutamide ( n = 2; 13.3%), radium-223 ( n = 1; 6.7%), radiotherapy ( n = 1; 6.7%), any systemic therapy ( n = 1; 6.7%), any hormonal therapy ( n = 4; 26.7%), and any bone-targeted agent ( n = 3; 20.0%). In total, eight out of 15 studies (53.3%) were commercially funded ( Table 1 ). Author groups from the UK accounted for the largest number of studies (37%; Supplementary Fig. 1 ).
3.2. Methodological quality and RoB
Methodological validity assessments for each study are listed in Supplementary Tables 1–4 . Of the five quantitative studies, four (80%) reported a high response rate, one (20%) tested participant understanding, and all studies analysed the data correctly ( Supplementary Table 2 ). For the quantitative studies, the mean PREFS quality score was 4 (SD 0). In terms of validity assessment, none of the studies justified their forced-choice study design; three studies (60%) did not report details of their experimental design. All studies piloted the data collection tool with the target population before implementing the main survey. Two (40%) met most of the analysis criteria. The mean SRQR quality score for the qualitative studies was 18.4 (SD 3.4; Supplementary Table 4 ). A single quantitative preference study was deemed to have high RoB ( n = 1; 20%). Five qualitative studies were deemed to have high RoB ( n = 5; 50%).
3.3. Results
3.3.1. quantitative treatment preference studies.
A summary of the demographics and study design of the quantitative preference studies ( n = 5) is presented in Table 1 [27] , [28] , [29] , [30] , [31] . In all studies, participants were asked to choose between two treatment alternatives and were not given the option to report that they would not be treated. None of the studies justified this study design. The number of treatment attributes evaluated ranged from two to seven (mean 5, SD 2). Overall, treatment effectiveness, delay in time to symptoms, and fatigue emerged as the predominant treatment-related preferences that patients valued ( Table 2 ).
Summary of major of findings for patient preferences and values in quantitative studies
RAI = relative attribute importance; RI = relative importance; OR = odds ratio.
3.3.1.1. mHSPC
de Freitas and colleagues [27] explored how 152 patients with mHSPC perceived the risks and benefits of hypothetical abiraterone acetate and docetaxel treatment in three European countries. The study included six treatment attributes: mode of administration, tiredness and fatigue, treatment effectiveness, bone pain, nausea and vomiting, and risk of infection. The authors reported that treatment effectiveness was the main objective for patients, and that patients wanted to avoid uncontrolled pain. In terms of relative attribute importance (RAI), the treatment attribute ranking was treatment effectiveness (RAI 7.25) followed by pain (RAI 6.26), risk of nausea (RAI 4.12), vomiting (RAI 3.17), risk of fatigue (RAI 2.24), and mode of administration (RAI 2.09) [27] .
3.3.1.2. mCRPC
In the mCRPC setting, Eliasson and colleagues [28] explored hypothetical treatment options for 285 patients across the UK and Europe. The study included seven treatment attributes: effectiveness (delay in months before chemotherapy), steroid use, possible drug interactions (additional hospital visits for monitoring), cognitive impairment described as “fogginess” (effects on cognition and memory), fatigue, food restrictions, and bone pain. The findings were presented in terms of odds ratios (ORs) and the results suggest that patients prefer treatments that fully control bone pain (OR 12.06, 95% confidence interval [CI] 10.55–13.80) and those that delay chemotherapy (OR 1.72, 95% CI 1.54–1.92). In addition, patients seem to prefer treatments with a lower risk of “fogginess” (OR 2.11, 95% CI 1.84–2.42), a lower risk of fatigue (OR 1.36, 95% CI 1.21–1.52), and fewer additional hospital visits (OR 1.24, 95% CI 1.11–1.39) [28] .
The concordance of treatment preferences between patients and physicians in mCRPC was explored in a study of 103 patients in Japan [29] . The study included four attributes: quality of life, effectiveness, side effects, and accessibility. In terms of the relative importance (RI) of attributes, the preference ranking among patients was effectiveness (RI 32%) followed by accessibility of treatment (RI 26%), quality of life (RI 23%), and side effects (RI 19%).
With regard to bone-targeted and systemic agents in the mCRPC setting, Uemura et al [30] explored preferences associated with various treatments (radium-223, abiraterone acetate, and docetaxel) for 133 patients in Japan. The study included six attributes: OS length, time to a symptomatic skeletal event (SSE), administration method, reduction in the risk of bone pain, treatment-associated risk of fatigue, and lost workdays. Patients ranked their preferences as fatigue (RI 24.9%) followed by reduction in the risk of bone pain (RI 23.2%) and OS length (RI 19.2%). The authors compared preferences across symptomatic and asymptomatic patients and found that symptomatic patients placed significantly more importance on delaying an SSE. The authors concluded that patients with CRPC were more concerned about reduced quality of life from side effects of treatment than extension of survival.
Hauber et al [31] explored preferences for bone-targeted agents among 401 patients with mixed disease states in the UK and Sweden. The study used two TTO questions to assess patients’ trade-offs between avoiding metastasis-induced bone complications and longer survival. The results showed that patients were willing to trade up to 5 mo of survival to prevent bone complications.
3.3.2. Qualitative studies
A summary of the demographics and study design of the qualitative studies is presented in Table 1 [32] , [33] , [34] , [35] , [36] , [37] , [38] , [39] , [40] , [41] . A complete list of all findings by study is available in Supplementary Table 7 . Thematic analysis revealed the following key themes: cancer progression and/or survival; pain; fatigue; and other symptoms (sexual dysfunction, bothersome lower urinary tract symptoms [LUTS]; Table 3 ) [26] .
Summary of major findings for patient preferences and values in qualitative studies
LUTS = lower urinary tract symptoms; QoL = quality of life.
Cancer progression and/or OS benefits related to treatment were a key theme extracted from five of the studies [35] , [36] , [37] , [38] , [39] . Dearden et al [37] undertook semistructured interviews with 38 patients with mCRPC who were receiving a novel antiandrogen therapy (abiraterone acetate or enzalutamide). Patients were satisfied with these therapies, specifically with reductions in prostate-specific antigen levels and the extended survival quality.
Burbridge et al [35] carried out semistructured interviews with 25 patients diagnosed with mCRPC. Of these patients, 83.3% said they would have taken a medication to delay (metastasis) progression if one had been available, irrespective of side effects. Ito et al [34] conducted semistructured interviews with 31 patients with mHSPC across Europe and the UK who were receiving docetaxel. They found that at the beginning of therapy, men were willing to take docetaxel to prolong their life, despite being fearful of the potential side effects and impact on their daily lives.
Fatigue was a key theme related to treatments identified in five of the studies [32] , [34] , [35] , [36] , [38] . Catt et al [36] undertook structured interviews with 37 patients with mCRPC, exploring experiences of treatment decisions, perceived benefits and harms of treatment, and the effects on patients’ lives. At 3 mo after starting a systemic therapy, 42% of patients said that fatigue was the worst treatment‐related side effect. Burbridge et al [35] also found that more than 75% of men with mCRPC reported fatigue or extreme tiredness (“ Whatever [I do] is exhausting ”). In the study by Ito et al [34] , fatigue was a significant treatment-related side-effect reported by up to 60.9% of the patients interviewed.
Pain was identified as a theme in two studies [35] , [36] . Burbridge et al [35] found that pain was one of the most frequent symptoms reported by more than 75% of patients (“I had a lot of pain”; “The pain comes and goes and I usually feel it somewhere in my back”) . Catt et al [36] also found that pain was the worst symptom reported by most patients (46%), although nearly one-fifth (19%) made comments attributing the pain to causes other than prostate cancer (“ I think the pain in my hip could be rheumatic”; “My pain in the lower back and shoulder are due to degeneration ”).
Other symptoms related to treatments and local disease were sexual dysfunction and bothersome LUTS, reported in four studies [32] , [35] , [38] , [41] . It is known from earlier work in the era before docetaxel that andropause symptoms (including sexual dysfunction) related to ADT administration were a significant consideration for patients in deciding on whether to commence treatment and a source of treatment regret [32] , [33] , [38] . Burbridge et al [35] found that bothersome LUTS were reported by more than 75% of men. Patients were willing to consider supportive treatment to alleviate these symptoms, probably caused by progression of an untreated local tumour.
Grunfeld et al [38] found that most patients reported hot flashes and night sweats, gynaecomastia, cognitive decline, and changes in sexual dysfunction (“ That the erection is rather painful is somewhat of a disincentive to trying it too often ”) as the most frequent adverse effects, affecting everyday functioning. Some patients felt that there was no need for treatment as they were older and single, whereas other reported a belief that the negative aspects outweighed the benefits.
3.4. Discussion
3.4.1. principal findings.
This systematic review addresses the evidence from both quantitative and qualitative studies reporting on patients’ values, preferences, and expectations in relation to their treatment for metastatic prostate cancer. In quantitative preference elicitation studies, patients consistently valued treatment effectiveness and delay in time to symptoms as the two most highly ranked treatment attributes (low to very low certainty; Table 2 ). Patients were willing to trade treatment-related toxicity for potential oncological benefits (low certainty). With rapidly emerging local tumour treatments and metastasis-directed therapies now available to patients, these findings are an important consideration for patients and their clinicians.
Qualitative thematic analysis revealed cancer progression or survival, pain, and fatigue as key to treatment decisions (low to very low certainty; Table 3 ). Patients continue to value oncological benefits in making decisions regarding treatments. However, in the subgroup of symptomatic patients, treatments that could alleviate pain were highly valued even at the expense of survival benefits (very low certainty).
Furthermore, treatment inducing fatigue had a significant negative impact on remaining quality of life (very low certainty). The fact that ionising radiation directed to metastases may secondarily exacerbate or induce fatigue highlights just one example of the difficult decision-making balance that patients and clinicians face [42] .
3.4.2. Comparison with prior reviews and guidelines
To the best of our knowledge, this is the first systematic review to evaluate patients’ preference and values for treatments following a diagnosis of metastatic prostate cancer. Prior systematic reviews of patients’ preferences involved patients with localised prostate cancer, in which the marginal gains in absolute survival advantage (up to 5% over 10–15 yr) and side effects associated with radical prostate treatment remain the predominant issues [43] , [44] . In the noncurative setting, it can be assumed that patients’ treatment preferences are entirely different.
The landmark STAMPEDE (arm H) study of 2061 men with newly diagnosed metastatic prostate cancer receiving additional local prostate radiotherapy compared to those receiving systemic therapy alone demonstrated a significant OS advantage for patients with low-volume disease in the radiotherapy arm (3-yr OS: 81% vs 73%; hazard ratio 0.68, 95% CI 0.52–0.90; p = 0.007) [11] .
Against this background, international prostate cancer guidelines have incorporated radiotherapy into the standard of care [45] , [46] . However, some guidelines recommend dose and fractionation schedules (eg, 36 Gy in 6 fraction) that specifically reduce hospital attendances on the basis that patients would value such an approach in the decision-making process [45] . However, there is no robust evidence detailing how patients balance the risks against the benefits of new treatments applied to this setting to support such a recommendation [18] .
3.4.3. Strength and limitations
This is the first study to use an expert panel of urologists, oncologists, and health economists to develop a priori criteria for conducting a systematic review on this topic. This methodological rigour enabled us to summarise the key findings and rate the certainty of the evidence presented using the GRADE and GRADE CERQual criteria, respectively.
Unfortunately, the varied study designs and outcomes reported in the quantitative preference studies precluded a meta-analysis. Furthermore, the diverse qualitative studies reported are likely to reflect the heterogeneous pool of patients included in interviews (varied disease states, metastatic burden, asymptomatic vs symptomatic disease).
Finally, although bone-targeted agents were evaluated in this systematic review, the majority of studies focused on existing systemic therapies. No studies specifically reported on cytoreductive radical prostatectomy, minimally invasive ablative therapies, metastasectomy, or SABR. We are thus unable to report on patient preferences and values with regard to these treatments.
3.4.4. Unanswered questions and future research
This systematic review has predominantly highlighted patient preferences in the context of being offered established systemic therapy options and a limited number of bone-targeted agents. Therefore, our overall understanding of how novel surgical and radiotherapy treatment options are valued by patients remains limited.
However, results support a number of these treatment options continue to be published following robust trial evaluation [8] , [16] , [17] . We therefore propose that a reappraisal of patient preferences is now required to permit integration of new treatments into existing standard-of-care pathways. This could take the form of a prospective stand-alone study or indeed could be integrated into ongoing studies during longitudinal follow-up (eg, {"type":"clinical-trial","attrs":{"text":"NCT01751438","term_id":"NCT01751438"}} NCT01751438 , {"type":"clinical-trial","attrs":{"text":"NCT03456843","term_id":"NCT03456843"}} NCT03456843 , {"type":"clinical-trial","attrs":{"text":"NCT02454543","term_id":"NCT02454543"}} NCT02454543 , {"type":"clinical-trial","attrs":{"text":"NCT03988686","term_id":"NCT03988686"}} NCT03988686 , {"type":"clinical-trial","attrs":{"text":"NCT02742675","term_id":"NCT02742675"}} NCT02742675 , ISRCTN15704862, {"type":"clinical-trial","attrs":{"text":"NCT03655886","term_id":"NCT03655886"}} NCT03655886 , {"type":"clinical-trial","attrs":{"text":"NCT03678025","term_id":"NCT03678025"}} NCT03678025 , and {"type":"clinical-trial","attrs":{"text":"NCT03763253","term_id":"NCT03763253"}} NCT03763253 ).
The IP5-MATTER study ( {"type":"clinical-trial","attrs":{"text":"NCT04590976","term_id":"NCT04590976"}} NCT04590976 ) is a multicentre discrete-choice experiment, currently in its accrual phase, evaluating 300 patients with de novo synchronous mHSPC [47] . This trial is designed to evaluate novel treatments (cytoreductive radical prostatectomy, external beam radiotherapy, minimally invasive ablative therapy, and SABR) in addition to systemic therapy for the first time. The study is collecting data on patient characteristics (eg, age, comorbidities) and will offer an insight into whether these also have an impact on patient preferences [48] .
It can be hypothesised that the results from studies can then be combined with the effect sizes from future reported interventional randomised trials to determine if, on average, patients are willing to accept the potential effect sizes that are reported in these studies [12] , [14] .
Finally, research on patients’ values, preferences, and expectations for treatment should be cognisant of an emerging theme of treatment regret that has been reported for localised disease [49] . One option for mitigating such levels of treatment regret is to assist in the informed decision-making process. It is possible that once novel treatment pathways are established for metastatic prostate cancer, the findings from patient preference elucidation studies (such as DCEs) may be integrated into further work towards the creation of decision treatment aids (DTAs). While residual uncertainty regarding the role of DCEs in the development of such DTAs remains, the methodology is currently being validated in localised prostate cancer and other studies on benign surgical strategies. If proven, this approach may offer utility in the development of any future DTAs for this specific cohort of patients [50] , [51] , [52] .
4. Conclusions
There is currently limited understanding of patients’ preferences for treatment, and thus trade-off decisions, following a new diagnosis of metastatic prostate cancer. For appropriate investment in emerging cytoreductive prostate and metastasis-directed treatment options that are most acceptable to patients, attempts to formalise our understanding of the trade-offs between oncological benefits and risks in this cohort should be performed.
Author contributions: Martin J. Connor had full access to all the data in the study and takes responsibility for the data integrity and the accuracy of the data analysis.
Study concept and design: Connor, Genie, Burns.
Acquisition of data : Connor, Genie.
Analysis and interpretation of data : Connor, Genie.
Drafting of the manuscript: Connor, Genie.
Critical revision of the manuscript for important intellectual content: Connor, Burns, Genie, Bass, Winkler, Khoo, Ahmed, Watson, Dudderidge, Sarwar, Gonzalez, Mangar, Falconer.
Statistical analysis: Connor, Genie.
Obtaining funding : None.
Administrative, technical, or material support : None.
Supervision: Ahmed, Winkler, Khoo, Watson.
Other: None.
Financial disclosures: Martin J. Connor certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Martin J. Connor receives grant funding from the Wellcome Trust and University College London Hospitals Charity. Vincent Khoo is supported by personal fees and nonfinancial support from Accuray, Astellas, Bayer, Janssen, and Boston Scientific. Hashim U. Ahmed is supported by core funding from the UK National Institute of Health Research (NIHR) Imperial Biomedical Research Centre and funding from the Wellcome Trust, Medical Research Council (UK), Prostate Cancer UK, Cancer Research UK, The BMA Foundation, The Urology Foundation, The Imperial Health Charity, Sonacare, Trod Medical, and Sophiris Biocorp for trials and studies in prostate cancer; is a paid medical consultant for Sophiris Biocorp, Sonacare, and BTG/Galil; and is a paid proctor for high-intensity focused ultrasound, cryotherapy, and Rezūm water vapour therapy. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: None.
Associate Editor: Guillaume Ploussard
Appendix A Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2021.10.003 .

Appendix A. Supplementary data
The following are the Supplementary data to this article:
- Introduction
- Conclusions
- Article Information
Blue lines represent use of upfront docetaxel (data available from March 2017). Orange lines represent use of an androgen receptor pathway inhibitor starting within 6 months (solid line) or between 6 and 24 months after diagnosis (dashed line).
Overall survival was estimated using a flexible parametric model standardized for baseline clinical characteristics according to last calendar period (2017-2020). Five-year restricted mean survival (RMS) was used to describe the increase in survival time. Survival estimates and 95% CI are also reported in eTable 1 in Supplement 1 . Numbers at risk were extracted from the unadjusted analysis.
Ten-year overall survival was estimated by use of a flexible parametric model standardized to baseline characteristics of men diagnosed in 2020. The 10-year restricted mean survival (RMS) was used to report the increase in survival time. Dashed lines represent lower and upper limits of the 95% CIs.
eFigure 1. Median and lower quartile level of PSA for men with de novo mCSPC in 2008-2020
eFigure 2. Unadjusted five-year overall survival for men with de novo mCSPC diagnosed in 2008-2020
eTable 1. Use of imaging techniques in men with de novo mCSPC in 2013-2020 in the National Prostate Cancer Register (NPCR) of Sweden
eTable 2. Five-year crude survival in Kaplan-Meier analysis and standardized survival in parametric models
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Corsini C , Garmo H , Orrason AW , Gedeborg R , Stattin P , Westerberg M. Survival Trend in Individuals With De Novo Metastatic Prostate Cancer After the Introduction of Doublet Therapy. JAMA Netw Open. 2023;6(10):e2336604. doi:10.1001/jamanetworkopen.2023.36604
Manage citations:
© 2024
- Permissions
Survival Trend in Individuals With De Novo Metastatic Prostate Cancer After the Introduction of Doublet Therapy
- 1 Department of Surgical Sciences, Uppsala University, Uppsala, Sweden
- 2 Division of Experimental Oncology/Unit of Urology, URI Institution, IRCCS San Raffaele Hospital, Milan, Italy
- 3 Medical Products Agency, Stockholm, Sweden
Question Has the introduction of doublet therapy in individuals with de novo metastatic castration-sensitive prostate cancer been associated with changes in survival on a population basis in Sweden?
Findings In this nationwide cohort study, upfront treatment with doublet therapy among 11 382 individuals between 2008 and 2020 with de novo metastatic castration-sensitive prostate cancer increased from 1% in 2016 to 44% in 2020. Mean survival in individuals with de novo metastatic castration-sensitive prostate cancer increased 6 months during the first 5 years of follow-up.
Meaning In parallel with improvements in treatment of advanced prostate cancer, a clinically meaningful increase in mean survival was observed in this study.
Importance Recently, life-prolonging treatments for patients with advanced prostate cancer have been introduced in clinical practice.
Objective To investigate if the introduction of doublet therapy is associated with changes in survival on a population-basis.
Design, Setting, and Participants This nationwide population-based cohort study used data from the Prostate Cancer data Base Sweden from 2008 to 2020. Men registered with de novo metastatic castration-sensitive prostate cancer (mCSPC) were included.
Exposure The proportion of men with mCSPC who received doublet therapy, ie, androgen deprivation therapy plus androgen receptor pathway inhibitor drugs or chemotherapy was assessed.
Main Outcomes and Measures Standardized overall survival, taking age, comorbidity, and cancer characteristics into consideration, was estimated by use of a parametric survival model.
Results A total of 11 382 men were included in this study (median [IQR] age, 74.0 [68-81] years). There was a shift toward less advanced prostate cancer during the study period with a decrease in median (IQR) prostate-specific antigen at diagnosis in men with mCSPC from 145 (39-571) ng/mL to 107 (27-426) ng/mL. Upfront treatment with doublet therapy in these men simultaneously increased from 1% (7 of 991) in 2016 to 44% (402 of 922) in 2020. The adjusted 5-year overall survival increased from 26% (95% CI, 25%-28%) from 2008 to 2012 to 35% (95% CI, 31%-40%) from 2017 to 2020. During the first 5 years after diagnosis, there was an increase in mean survival of 6 months, from 2.7 (95% CI, 2.6-2.8) years from 2008 to 2012 to 3.2 (95% CI, 3.1-3.1) years from 2017 to 2020.
Conclusions and Relevance In parallel with improvements in treatment of advanced prostate cancer, a clinically meaningful increase in mean survival was observed in men with de novo mCSPC in Sweden between 2008 and 2020 in this study.
In randomized clinical trials (RCT), the addition of docetaxel or an androgen receptor pathway inhibitor (ARPi) drugs to standard androgen deprivation therapy (ADT), ie, doublet therapy, increased survival in individuals with de novo metastatic castration-sensitive prostate cancer (mCSPC), ie, metastatic disease at diagnosis. 1 - 9 The improvement was most pronounced in patients with high-volume disease. 1 , 10 In the STAMPEDE trial, abiraterone acetate with prednisone improved 5-year overall survival to 60% compared with 41% in the group treated with ADT alone. 6 Similarly, in the LATITUDE trial, median overall survival was 53.3 months in men with de novo metastatic high-risk cancer treated with doublet therapy with abiraterone acetate, compared with 36.5 months in the placebo group. 7 Novel ARPis such as enzalutamide and apalutamide, when combined with ADT, have also been demonstrated to significantly increase progression-free survival 11 and overall survival. 8 , 9
Consequently, guidelines from the European Association of Urology and the National Swedish Guidelines now recommend doublet therapy for men with mCSPC. 12 , 13 As a result, use of doublet therapy has increased substantially in Sweden, and in 2021 approximately half of all individuals with de novo mCSPC received doublet therapy. At the same time, earlier detection of metastatic disease has led to lower tumor burden in patients with de novo mCSPC, as mirrored by lower prostate-specific antigen (PSA) levels at diagnosis. 14 The aim of this study was to investigate if the increased use of doublet therapy in men with de novo mCSPC in Sweden has been accompanied by improvements in survival, taking other temporal changes into consideration.
The National Prostate Cancer Register (NPCR) of Sweden captures 98% of all incident prostate cancer cases compared with the Swedish Cancer Registry to which reporting is mandated by law. 15 In the Prostate Cancer data Base Sweden (PCBaSe), NPCR has been enriched with data from other registers including the Patient Registry, the Cause of Death Registry, and the Prescribed Drug Registry, by use of the Swedish person identity number as previously described in detail. 16 The Swedish Research Ethics Authority approved the study. The requirement for informed consent was waived by this authority. The study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
The study population consisted of men registered from 2008 to 2020 in NPCR with de novo mCSPC defined by the presence of skeletal or visceral metastases on radionuclide bone scan, computed tomography, positron emission tomography (PET)/computed tomography, magnetic resonance imaging, or radiograph imaging.
Data on use of the ARPi drugs abiraterone (Anatomical Therapeutic Chemical code L02BX03), enzalutamide (L02BB04), and apalutamide (L02BB05) were based on filled prescriptions for these drugs in the Prescribed Drug Registry. Data on treatment with docetaxel were available in NPCR from March 2017. Upfront treatment was defined as treatment given within 6 months from diagnosis. The use of ARPis was further assessed at 6 to 24 months from date of diagnosis.
Data were extracted from NPCR on PSA, mode of cancer detection (nonorganized screening or workup of men with low urinary tract symptoms, bone pain, or hematuria), Gleason score, clinical stage according to TNM (tumor, node, metastasis) classification, and primary treatment.
We estimated life expectancy at the time of diagnosis by use of age and 2 measures of comorbidity, a drug comorbidity index (DCI) and a newly created multidimensional comorbidity index (MDCI). 17 MDCI was based on hospital discharge diagnoses registered in the National Patient Registry during 10 years prior to date of prostate cancer diagnosis, while the DCI was based on filled prescriptions in the Prescribed Drug Registry during the year prior to diagnosis. We optimized a previously described method to calculate the life expectancy by using the MDCI instead of Charlson Comorbidity Index in the model. 18
Outcomes were overall survival and cause-specific survival according to date and cause of death as registered in the Cause of Death Registry. Follow-up started at date of diagnosis and ended on December 31, 2022, or at date of death, whichever event came first.
Survival was estimated annually and in 3 calendar periods corresponding to the gradual uptake of doublet therapy, ie, 2008 to 2012, 2013 to 2016, and 2017 to 2020. All analyses were stratified according to age at diagnosis (<60, 60-69, 70-79, and ≥80 years). Crude survival was described with Kaplan-Meier curves. Differences in survival due to heterogeneous treatment intensity over calendar periods were expected to become evident after 6 months from start of treatment so hazards were not expected to be proportional. 19 We estimated standardized survival curves using a parametric gamma survival model allowing for nonproportional hazards, comparing the 3 study periods. 20 We standardized according to the case mix of men diagnosed from 2017 to 2020 by adjusting for age, PSA, Gleason score, clinical T stage, mode of cancer detection, primary treatment, and comorbidity by use of DCI and MDCI. Adjusted annual survival was estimated similarly, standardized according to the case mix of men diagnosed in 2020, and the 10-year survival trend was estimated after 2022 for men diagnosed between 2013 and 2020. To estimate the magnitude of difference in survival between the 3 calendar periods and annually, we calculated the restricted mean survival at 5 and 10 years. 21 , 22 Model fit was assessed by comparing the parametric survival curves with the corresponding Kaplan-Meier curves.
Missing data for PSA, T stage, Gleason score, primary treatment, and mode of detection was imputed (5 times) using multiple imputation. 23 Confidence intervals were computed by use of bootstrapping (500 resamplings) followed by multiple imputation with the boot multiple imputation percentile method. 24 Statistical analyses were performed with R version 3.5.3 (R Foundation).
Age at diagnosis and burden of comorbidities of the 11 382 men diagnosed with de novo mCSPC remained essentially stable during the study from 2008 to 2020 (median [IQR] age, 74.0 [68-81] years) ( Table ). However, there was a shift toward less advanced prostate cancer, eg, the proportion of men with T4 tumors decreased slightly from 20% (694 of 3465) from 2008 to 2012 to 16% (344 of 3973) from 2017 to 2020, and the proportion of men with symptoms at diagnosis decreased from 83% (2870 of 3465) to 68% (2700 of 3973). The median (IQR) PSA at diagnosis decreased from 145 (39-571) ng/mL (to convert to micrograms per liter, multiply by 1) from 2008 to 2012 to 107 (27-426) ng/mL from 2017 to 2020. In men aged 75 to 79 years, median PSA decreased from 125 ng/mL to 58 ng/mL, and for men 80 years or older from 200 ng/mL to 116 ng/mL, whereas virtually no change was observed for men 74 years or younger (eFigure 1 in Supplement 1 ). Among men diagnosed with de novo metastatic disease, there were only minor changes in use of imaging techniques during the study period (eTable 1 in Supplement 1 ).
Upfront treatment with doublet therapy for de novo mCSPC increased from 1% (7 of 991) in 2016 to 44% (402 of 922) in 2020 ( Figure 1 ). Upfront use of docetaxel increased from 3% (26 of 980) in 2017 to 20% (183 of 922) in 2020 and upfront use of ARPis increased from 1% (7 of 991) in 2016 to 27% (245 of 922) in 2020. Treatment with docetaxel and ARPis was more common in younger compared with older men. In 2017, 7% (8 of 107) of men younger than 65 years received docetaxel, while in 2020, the percentage was 42% (53 of 126). No man older than 80 years was treated with docetaxel in 2017 and 1% (3 of 258) was treated with docetaxel in 2020. Similarly, there was a stronger increase in use of ARPis among men younger than 65 years from 2% (2 of 132) in 2016 to 31% (39 of 126) in 2020 than for men older than 80 years, with corresponding percentages of 1% (3 of 296) and 17% (44 of 258). The use of ARPis 6 to 24 months after diagnosis was negligible prior to 2012 and increased to 19% (174 of 922) in 2020.
Standardized 5-year overall survival increased from 26% (95% CI, 25%-28%) from 2008 to 2012 to 35% (95% CI, 31%-40%) from 2017 to 2020. This corresponded to an increase of 6 months in mean survival 5 years after diagnosis, from 2.7 years (95% CI, 2.6-2.8 years) to 3.2 years (95% CI, 3.1-3.2 years) ( Figure 2 ; eTable 2 in Supplement 1 ). In men older than 80 years, the increase was less pronounced, with an increase of 3.6 months in mean survival.
The parametric survival models fitted the observed survival data described by the Kaplan-Meier curves well (eFigure 2 and eTable 2 in Supplement 1 ). Cause-specific survival mirrored the overall survival, and the temporal trends were similar.
The 10-year overall survival increased from 9% (95% CI, 8%-10%) for men diagnosed in 2008 to 11% (95% CI, 11%-13%) for men diagnosed in 2012, with a further estimated increase to 18% (95% CI, 16%-20%) in 2020 ( Figure 3 ). For the whole study group, an increase was estimated in mean survival at 10 years of follow-up from 3.3 (95% CI, 3.2-3.5) years for men diagnosed in 2008 to 4.6 (95% CI, 4.5-4.8) years for men diagnosed in 2020. The estimated survival increase was lower in men older than 80 years.
In this nationwide population-based study in Sweden, the addition of ARPi or docetaxel to standard ADT increased substantially between 2017 and 2020. In 2020, approximately 50% of patients with de novo mCSPC received doublet therapy. Between 2008 and 2020, mean survival increased with 6 months after 5 years of follow-up in all individuals with de novo mCSPC, taking changes in age, comorbidity, and cancer characteristics into account, supporting that doublet therapy is effective in clinical practice on a population basis.
Flexible parametric survival models were used to analyze survival, standardizing for changes in baseline characteristics during the study period and estimating long-term survival after 2022. The parametric gamma survival model allowed for nonproportional hazards that were expected since heterogeneous treatment intensity over calendar periods would affect survival only after around 6 months from start of treatment. 20 Restricted mean survival is a useful measure to summarize changes in survival since it accounts for shape of the entire survival curve during follow-up in contrast to median survival that represents a snapshot of the survival at the time when 50% of men have died. The increase in restricted mean survival is likely a conservative estimate of the improvement in long-term survival since we expect the time period–specific survival curves to remain separated during most of the remaining follow-up. Nevertheless, our estimation of survival beyond the observed follow-up should be interpreted with caution particularly since it does not account for future changes in treatment that are likely to increase survival even further in individuals with mCSPC.
In parallel to the introduction of doublet therapy, there continued to be a decrease in metastatic burden, mirrored by lower levels of PSA in individuals with mCSPC. This may partly explain the survival improvement in the last study period; however, the improvement remained after standardization for changes in cancer characteristics, including PSA levels. Furthermore, the biggest decrease in median PSA was observed among the oldest men for whom survival increased less than for the youngest men who received doublet therapy more often but whose median PSA did not decrease. There were minimal changes in the use of imaging during the study period, although there was a rise in prostate-specific membrane antigen PET use in the final year. Further studies are needed to determine the impact on survival of more sensitive imaging modalities, such as magnetic resonance imaging and prostate-specific membrane antigen PET. 25 , 26
Unlike RCTs, observational studies may be biased from confounding, with fitter and younger men being more likely to receive more active treatment. 27 - 30 To avoid this selection bias, we assessed survival in all men with de novo mCSPC, ie, including men who did not receive doublet therapy. In several RCTs, a substantially better survival has been observed for patients treated with ARPis or docetaxel compared with individuals receiving standard of care with ADT only, with the strongest effect observed when doublet therapy was used upfront. 2 , 7 - 9 , 31 In the STAMPEDE trial, the 5-year overall survival was 60% in individuals undergoing ADT plus ARPi compared with 41% in individuals undergoing ADT only, 32 whereas in our study, 5-year overall survival was 50% in men younger than 74 years in the latest calendar period, which seems reasonable given that about half of these men received doublet therapy. Men in our study population had comparable median PSA as men in the STAMPEDE trial (103 ng/mL vs 97 ng/mL), suggesting that the disease burden was quite similar. 5 , 33
This study has several strengths. The PCBaSe captures virtually all individuals diagnosed with prostate cancer in Sweden with registration of comprehensive data of cancer characteristics at diagnosis as well as primary treatment, complete capture of filled prescriptions, and complete follow-up of mortality. Comorbidity was assessed by use of 2 new indices based on comprehensive data in the Patient Registry and the Prescribed Drug Registry. The recency of the data is another strength of our study, with a last date of follow-up in December 2022.
There are also some limitations to our study. Although there were no substantial changes in the diagnostic workup, eg, imaging, unmeasured and unknown changes over calendar time may have affected survival. For example, information on the extent of bone metastases was only available from 2018, precluding us to analyze high- vs low-volume disease. Furthermore, there is no information on PSA levels during follow-up in NPCR or in any other nationwide register, so we could not assess progression-free survival. Upfront treatment with docetaxel has been captured in NPCR since March 2017; however, it was used before that date mostly among individuals with mCRPC. Use of docetaxel in patients with mCSPC was correctly registered in NPCR in 84% in an audit of 500 health care records. We did not have information on the use of docetaxel in individuals with mCRPC. However, the survival benefit of docetaxel in individuals with mCRPC is limited compared with upfront use in men with de novo mCSPC. 5 , 34 Overall, 2% of patients were treated with both docetaxel and ARPi within the first 6 months. We do not know why both drugs were used nor do we know the date for start of docetaxel treatment. We speculate that intolerance to docetaxel made the clinician switch to an ARPi.
A clinically meaningful increase in long-term survival was observed in men diagnosed with de novo mCSPC between 2008 and 2020 in Sweden. We argue that the main reason for this improvement was the increased upfront use of doublet therapy, combining ADT with docetaxel or an ARPi. Continued increase in use of doublet therapy and the introduction of triplet therapy (ADT plus docetaxel plus ARPis) 31 will likely increase survival further in men with metastatic prostate cancer.
Accepted for Publication: August 24, 2023.
Published: October 2, 2023. doi:10.1001/jamanetworkopen.2023.36604
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Corsini C et al. JAMA Network Open .
Corresponding Author: Marcus Westerberg, PhD, Regional Cancer Center Midsweden, Uppsala University Hospital, 75237 Uppsala, Sweden ( [email protected] ).
Author Contributions: Drs Corsini and Westerberg had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Corsini, Garmo, Gedeborg, Stattin, Westerberg.
Acquisition, analysis, or interpretation of data: Corsini, Garmo, Wilberg Orrason, Stattin, Westerberg.
Drafting of the manuscript: Corsini, Gedeborg, Westerberg.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Corsini, Garmo, Westerberg.
Obtained funding: Stattin.
Administrative, technical, or material support: Stattin.
Supervision: Garmo, Stattin, Westerberg.
Conflict of Interest Disclosures: None reported.
Funding/Support: Funding was received from Swedish Cancer Society (grant number 2022-2051) and Region Uppsala. The public health care administration for Region Uppsala in Sweden has, on behalf of the National Prostate Cancer Register (NPCR, agreements on subscriptions for quarterly reports from Patient-Overview Prostate Cancer within NPCR with Astellas, Janssen, and Bayer, as well as research projects with Astellas, Bayer, and Janssen.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The Medical Products Agency is a Swedish government agency. The views expressed in this article may not represent the views of the Medical Products Agency.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: This project was made possible by the continuous work of the National Prostate Cancer Register (NPCR) of Sweden steering group: Ingela Franck Lissbrant, David Robinson, Johan Styrke, Johan Stranne, Jon Kindblom, Camilla Thellenberg, Andreas Josefsson, Ingrida Verbiené, Hampus Nugin, Stefan Carlsson, Anna Kristiansen, Mats Andén, Thomas Jiborn, Olof Ståhl, Olof Akre, Per Fransson, Eva Johansson, Magnus Törnblom, Fredrik Jäderling, Marie Hjälm Eriksson, Lotta Renström, Jonas Hugosson, Ola Bratt, Maria Nyberg, Fredrik Sandin, Fredrik Sandin, Maria Brus, Mats Lambe, Anna Hedström, Nina Hageman, Christofer Lagerros, Hans Joelsson, and Gert Malmberg. They were not compensated. This manuscript was finalized at The Bergman Estate in Fårö, Sweden, and we thank the Bergman Foundation for their hospitality.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Advances in Prostate Cancer Research
Nanoparticles are tested as a means to deliver drugs to prostate cancer cells.
NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat prostate cancer. Most men diagnosed with prostate cancer will live a long time, but challenges remain in choosing the best treatments for individuals at all stages of the disease.
This page highlights some of the latest research in prostate cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.
Studying Early Detection for Men at High Risk
Men with certain inherited genetic traits are at increased risk for developing prostate cancer. Examples of such traits include inherited BRCA gene mutations and Lynch syndrome . No clear guidelines exist for when or how—or if—to screen men at high genetic risk for prostate cancer.
NCI researchers are using magnetic resonance imaging (MRI) of the prostate in men at high risk to learn more about how often and how early these cancers occur. They’re also testing whether regular scans in such men can detect cancers early, before they spread elsewhere in the body ( metastasize ).
Diagnosing Prostate Cancer
Improving biopsies for prostate cancer.
Traditionally, prostate cancer has been diagnosed using needles inserted into the prostate gland in several places under the guidance of transrectal ultrasound (TRUS) imaging to collect samples of tissue. This approach is called systematic biopsy .
However, ultrasound does not generally show the location of cancer within the prostate. It is mainly used to make sure the biopsy needles go into the gland safely. Therefore, biopsy samples using ultrasound guidance can miss cancer altogether. Or they may identify low-grade cancer while missing areas of high-grade , potentially more aggressive cancer.
Some doctors, concerned that a systematic biopsy showing only low-grade cancer could have missed a high-grade cancer, may suggest surgery or radiation. However, in some cases these treatments will be for a cancer that may have never caused a problem, which is considered overtreatment .
Using MRI and ultrasound . Scientists at NCI have developed a procedure that combines magnetic resonance imaging (MRI) with TRUS for more accurate prostate biopsies. MRI can locate potential areas of cancer within the gland but is not practical for real-time imaging to guide a prostate biopsy. The procedure, known as MRI-targeted biopsy, uses computers to fuse an MRI image with an ultrasound image. This lets doctors use ultrasound guidance to take biopsy samples of areas of possible cancer seen on MRI.
NCI researchers have found that combining MRI-targeted biopsy with systematic biopsy can increase the detection of high-grade prostate cancers while decreasing detection of low-grade cancers that are unlikely to progress.
Testing machine learning . Researchers are testing the use of machine learning , also called artificial intelligence (AI), to better recognize suspicious areas in a prostate MRI that should be biopsied. AI is also being developed to help pathologist s who aren't prostate cancer experts accurately assess prostate cancer grade . Cancer grade is the most important factor in determining the need for treatment versus active surveillance .
Finding small amounts of prostate cancer using imaging and PSMA
NCI-supported researchers are developing new imaging techniques to improve the diagnosis of recurrent prostate cancer. A protein called prostate-specific membrane antigen (PSMA) is found in large amounts—and almost exclusively—on prostate cells. By fusing a molecule that binds to PSMA to a compound used in PET imaging, scientists have been able to see tiny deposits of prostate cancer that are too small to be detected by regular imaging.
The Food and Drug Administration (FDA) has approved two such compounds for use in PET imaging of men with prostate cancer. These approvals are for men whose cancer may have spread to other parts of the body but is still considered curable, either with surgery or other treatments.
The ability to detect very small amounts of metastatic prostate cancer could help doctors and patients make better-informed treatment decisions. For example, if metastatic cancer is found when a man is first diagnosed, he may choose an alternative to surgery because the cancer has already spread. Or doctors may be able to treat cancer recurrence—either in the prostate or metastatic disease—earlier. which may lead to better survival. Studies are being done to determine if such early detection can improve outcomes.
As part of the Cancer Moonshot℠ , NCI researchers are testing whether PSMA-PET imaging can also identify men who are at high risk of their cancer recurring. Such imaging may eventually be able to help predict who needs more aggressive treatment—such as radiation therapy in addition to surgery—after diagnosis.
Research teams are also looking at:
- whether certain patterns seen on PSMA tests taken over time may indicate an increased risk of recurrence after initial treatment.
- how small metastases discovered with PSMA change over time , with or without treatment.
New Prostate Cancer Treatments
Standard treatments for prostate cancer that has not spread elsewhere in the body are surgery or radiation therapy (RT), with or without hormone therapy .
Active surveillance is also an option for men who have a low risk of their cancer spreading. This means monitoring the cancer with regular biopsies and holding off on treatment unless there is evidence of progression. Rates of active surveillance more than doubled between 2014 and 2021 , to almost 60% of US men diagnosed with low-risk prostate cancer.
Hormone therapy for prostate cancer
Over the last decade, several new approaches to hormone therapy for advanced or metastatic prostate cancer have been approved for clinical use.
Many prostate cancers that originally respond to treatment with standard hormone therapy become resistant over time, resulting in castrate-resistant prostate cancer (CRPC). Four newer drugs have been shown to extend survival in some groups of men with CRPC. All inhibit the action of hormones that drive CRPC:
- enzalutamide (Xtandi)
- abiraterone (Zytiga)
- darolutamide (Nubeqa)
- apalutamide (Erleada)
These drugs are now also used in some people whose prostate cancer still responds to standard hormone therapies but has spread elsewhere in the body (metastasized).
Scientists are continuing to study novel treatments and drugs, along with new combinations of existing treatments, in men with metastatic and castration-resistant prostate cancer.
PARP inhibitors for prostate cancer
A PARP inhibitor is a substance that blocks an enzyme in cells called PARP. PARP helps repair DNA when it becomes damaged. Some prostate tumors have genetic defects that limit their ability to repair DNA damage. Such tumors may be sensitive to PARP inhibitors.
Two PARP inhibitors, olaparib (Lynparza) and rucaparib (Rubraca) , have been approved for some men whose prostate cancer has such genetic defects and has metastasized , and whose disease has stopped responding to standard hormone treatments. Ongoing studies are looking at combing PARP inhibitors with hormone therapies.
Immunotherapy: vaccines for prostate cancer
Immunotherapies are treatments that harness the power of the immune system to fight cancer. These treatments can either help the immune system attack the cancer directly or stimulate the immune system in a more general way.
Vaccines and checkpoint inhibitors are two types of immunotherapy being tested in prostate cancer. Treatment vaccines are injections that stimulate the immune system to recognize and attack a tumor.
One type of treatment vaccine called sipuleucel-T (Provenge) is approved for men with few or no symptoms from metastatic CRPC.
Immunotherapy: checkpoint inhibitors for prostate cancer
An immune checkpoint inhibitor is a type of drug that blocks proteins on immune cells, making the immune system more effective at killing cancer cells.
Two checkpoint inhibitors, pembrolizumab (Keytruda) and dostarlimab (Jemperli) have been approved for the treatment of tumors, including prostate cancers, that have specific genetic features . Pembrolizumab has also been approved for any tumor that has metastasized and has a high number of genetic mutations .
But relatively few prostate cancers have these features, and prostate cancer in general has largely been resistant to treatment with checkpoint inhibitors and other immunotherapies, such as CAR T-cell therapy .
Research is ongoing to find ways to help the immune system recognize prostate tumors and help immune cells penetrate prostate tumor tissue. Studies are looking at whether combinations of immunotherapy drugs, or immunotherapy drugs given with other types of treatment, may be more effective in treating prostate cancer than single immunotherapies alone.
Targeted radiation therapy and PSMA
Scientists have developed targeted therapies based on PSMA, the same protein that is being tested for imaging prostate cancer. For treatment, the molecule that targets PSMA is chemically linked to a radioactive compound . This new compound can potentially find, bind to, and kill prostate cancer cells throughout the body.
In a recent clinical trial, men with a type of advanced prostate cancer who received a PSMA-targeting drug lived longer than those who received standard therapies . This trial led to FDA approval of the drug, Lu177-PSMA-617 (Pluvicto) , to treat some people with metastatic prostate cancer. Ongoing and planned clinical trials are testing PSMA-targeting drugs in patients with earlier stages of prostate cancer, and in combination with other treatments, including targeted therapies like PARP inhibitors and immunotherapy.
Personalized clinical trials for prostate cancer
Research is uncovering more information about the genetic changes that happen as prostate cancers develop and progress. Although early-stage prostate cancer has relatively few genetic changes compared with other types of cancer, researchers have learned that metastatic prostate cancers usually accumulate more mutations as they spread through the body.
These mutations may make men with metastatic prostate cancers candidates for what are called “basket” clinical trials of new drugs. Such trials enroll participants based on the mutations found in their cancer, not where in the body the cancer arose. In the NCI-MATCH trial , a high percentage of enrolled men with advanced prostate cancer had mutations that could potentially be targeted with investigational drugs.
NCI-Supported Research Programs
Many NCI-funded researchers working at the National Institutes of Health campus, as well as across the United States and world, are seeking ways to address prostate cancer more effectively. Some of this research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some is more clinical, seeking to translate basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in prostate cancer.
- The Cancer Biomarkers Research Group promotes research on cancer biomarkers and manages the Early Detection Research Network (EDRN) . EDRN is a network of NCI-funded institutions that are collaborating to discover and validate early detection biomarkers.
- Within the Center for Cancer Research , the Prostate Cancer Multidisciplinary Clinic (PCMC) provides comprehensive consultations on diagnosis and treatment options to people with newly-diagnosed prostate cancer.
- The Prostate Specialized Programs of Research Excellence (Prostate SPOREs) are designed to quickly move basic scientific findings into clinical settings. The Prostate SPOREs support the development of new therapies and technologies and studies to better understand how to prevent, monitor, and treat prostate cancer.
- The NCI Cancer Intervention and Surveillance Modeling Network (CISNET) focuses on using modeling to improve our understanding of which men are most likely to benefit from PSA-based screening. CISNET also studies treatment strategies for prostate cancer and approaches for reducing prostate cancer disparities.
- The NCI Genitourinary Malignancies Center of Excellence (GUM-COE) brings together scientists studying genitourinary cancers (GU) from across NCI’s Center for Cancer Research and the Division of Cancer Epidemiology and Genetics, as well as investigators who study GU malignancies in other institutes of NIH. The goal is to provide a centralized resource and infrastructure to accelerate the discovery, development, and delivery of interventions for the prevention, diagnosis, and treatment of these cancers.
- The Research on Prostate Cancer in Men with African Ancestry (RESPOND) study is the largest-ever coordinated research effort to study biological and non-biological factors associated with aggressive prostate cancer in African American men. The study , launched by NCI and the National Institute on Minority Health and Health Disparities in partnership with the Prostate Cancer Foundation, is looking at the environmental and genetic factors related to the aggressiveness of prostate cancer in African American men to better understand why they disproportionally experience aggressive disease.
Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for prostate cancer prevention , screening , and treatment .
Prostate Cancer Research Results
The following are some of our latest news articles on prostate cancer research:
- Enzalutamide Gets Added Approval for Prostate Cancer That Hasn’t Spread
- FDA Approves New Initial Treatment Option for Some Metastatic Prostate Cancers
- Is a Genomic Test Better at Finding Aggressive Prostate Cancer?
- Active Surveillance for Low-Risk Prostate Cancer Continues to Rise
- Darolutamide Extends Survival for Some People with Metastatic Prostate Cancer
- Shorter, More Intensive Radiation Safe after Surgery for Prostate Cancer
View the full list of Prostate Cancer Research Results and Study Updates .
Prostate cancer: Newly-developed inhibitor shows massive potential
More than 65,000 men fall ill with prostate cancer each year in Germany. Twelve thousand of them develop a treatment-resistant form which eventually ends in death. Now, a team of researchers from the Medical Faculty at the University of Freiburg has developed an active substance that might in future represent a new treatment option. This substance, known as KMI169, targets an enzyme that plays an important role in the development of prostate cancer. The inhibitor displayed massive potential in among others cancer cells that were resistant to conventional treatments.
Researchers from the Department of Urology at the Freiburg University Medical Center as well as the Institut für Pharmazeutische Wissenschaften at the University of Freiburg published their study in Nature Communications on 2 January 2024 .
"We've had our eye on the enzyme KMT9 as a possible target in prostate cancer for a long time. The development of this specific inhibitor is now a decisive step in combating prostate cancer far more effectively," explains study head Professor Roland Schüle, Academic Director of the Department of Urology at the Freiburg University Medical Center and Dr. Eric Metzger, group leader in Schüle's department.The substance's potential use against treatment-resistant forms of cancer makes it especially valuable. "This treatment-resistance means that the classic antihormonal treatment often fails within a few months and the disease then progresses rapidly. The inhibitor we've developed offers us a highly innovative therapeutic approach here," says Schüle.
New approach also relevant to bladder cancer
Using cell cultures, the groups headed by Schüle and co-author Professor Manfred Jung, head of the Chemical Epigeneticsgroup of the Institut für Pharmazeutische Wissenschaften, have shown that the enzyme KMT9, known as a methyltransferase, is a critical factor in the development and progress of certain types of cancer such as prostate or bladder cancer. "The inhibitor fits snugly like a key in its lock and blocks the functioning of KMT9 and therefore also the growth of both prostate and bladder cancer cells," says Jung. The development of KMI169 was guided by crystal structure analysis of KMT9 and numerous other studies. "We modified the compound many times to increase its potency, selectivity and medicinal properties."
- Men's Health
- Prostate Cancer
- Breast Cancer
- Diseases and Conditions
- Prostate Health
- Lung Cancer
- Prostate cancer
- Colorectal cancer
- Breast cancer
- Malignant melanoma
- Ovarian cancer
- Stomach cancer
- Cervical cancer
- Hip dysplasia
Story Source:
Materials provided by University of Freiburg . Note: Content may be edited for style and length.
Journal Reference :
- Sheng Wang, Sebastian O. Klein, Sylvia Urban, Maximilian Staudt, Nicolas P. F. Barthes, Dominica Willmann, Johannes Bacher, Manuela Sum, Helena Bauer, Ling Peng, Georg A. Rennar, Christian Gratzke, Katrin M. Schüle, Lin Zhang, Oliver Einsle, Holger Greschik, Calum MacLeod, Christopher G. Thomson, Manfred Jung, Eric Metzger, Roland Schüle. Structure-guided design of a selective inhibitor of the methyltransferase KMT9 with cellular activity . Nature Communications , 2024; 15 (1) DOI: 10.1038/s41467-023-44243-6
Cite This Page :
Explore More
- 100 Kilometers of Quantum-Encrypted Transfer
- Intelligent Liquid
- New Approach to Searching for Dark Matter
- We've Had Bird Evolution All Wrong
- Physical Activity Protects Against Chronic Pain
- Australia On Track for Decades-Long Megadroughts
- Speed of Visual Perception Ranges Widely
- 3D Printed Replica of an Adult Human Ear
- Extremely Fast Wound Healing: New Treatment
- Micro-Lisa! Novel Nano-Scale Laser Writing
Trending Topics
Strange & offbeat.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Clinical Research
- Open access
- Published: 27 March 2024
Real-world overall survival with abiraterone acetate versus enzalutamide in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer
- Daniel J. George ORCID: orcid.org/0000-0002-0836-8542 1 ,
- Krishnan Ramaswamy ORCID: orcid.org/0000-0003-0890-0268 2 ,
- Hongbo Yang 3 ,
- Qing Liu 3 ,
- Adina Zhang 3 ,
- Alexandra Greatsinger 3 ,
- Jasmina Ivanova ORCID: orcid.org/0000-0002-4028-9676 2 ,
- Betty Thompson 2 ,
- Birol Emir 2 ,
- Agnes Hong 2 , 4 &
- Stephen J. Freedland ORCID: orcid.org/0000-0002-8104-6419 5 , 6
Prostate Cancer and Prostatic Diseases ( 2024 ) Cite this article
437 Accesses
2 Altmetric
Metrics details
- Cancer therapy
- Prostate cancer
There are no large head-to-head phase 3 clinical trials comparing overall survival (OS) for abiraterone and enzalutamide. This study used Medicare claims data to compare OS in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC) who initiated abiraterone or enzalutamide.
This retrospective analysis of the Medicare database (2009–2020) included adult men with ≥1 claim for prostate cancer, metastatic diagnosis, and no prior chemotherapy or novel hormone therapy who initiated first-line (1L) abiraterone or enzalutamide in the index period (September 10, 2014 to May 31, 2017). Cox proportional-hazards models with inverse probability treatment-weighting (IPTW) were used to compare OS between abiraterone- and enzalutamide-treated patients, adjusting for baseline characteristics. Subgroup analyses by baseline characteristics were also conducted.
Overall, 5506 patients who received 1L abiraterone ( n = 2911) or enzalutamide ( n = 2595) were included. Median follow-up was comparable in both cohorts (abiraterone, 19.1 months; enzalutamide, 20.3 months). IPTW-adjusted median OS (95% CI) was 20.6 months (19.7‒21.4) for abiraterone and 22.5 months (21.2‒23.8) for enzalutamide, with an IPTW-adjusted hazard ratio (95% CI) of 1.10 (1.04–1.16). Median OS was significantly shorter for abiraterone versus enzalutamide in patients ≥75 years old; White patients; patients with baseline diabetes, cardiovascular disease, both diabetes and cardiovascular disease, and renal disease; and across all socioeconomic strata.
Conclusions
In the Medicare chemotherapy-naïve mCRPC population, 1L abiraterone was associated with worse OS versus enzalutamide in the overall population and among subgroups with older age and comorbidities, supporting findings from previous real-world studies and demonstrating a disparity in outcomes.
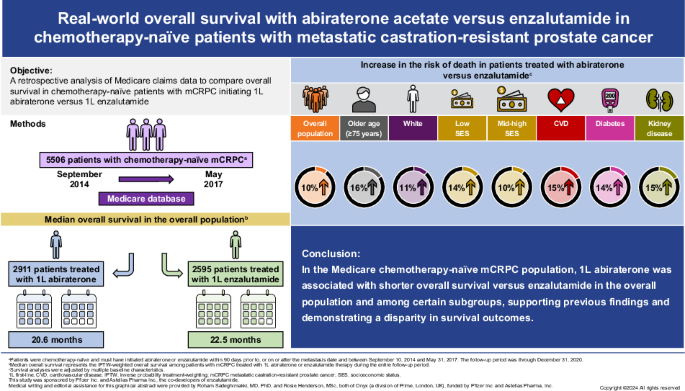
Similar content being viewed by others

Survival outcomes in patients with chemotherapy-naive metastatic castration-resistant prostate cancer treated with enzalutamide or abiraterone acetate
Scott T. Tagawa, Krishnan Ramaswamy, … Daniel J. George

Overall survival and adverse events after treatment with darolutamide vs. apalutamide vs. enzalutamide for high-risk non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis
Mike Wenzel, Luigi Nocera, … Pierre I. Karakiewicz
Survival of veterans treated with enzalutamide and abiraterone for metastatic castrate resistant prostate cancer based on comorbid diseases
Martin W. Schoen, Kenneth R. Carson, … Kristen M. Sanfilippo
Introduction
Prostate cancer (PC) is the most common cancer, excluding non-melanoma skin cancer, and the second leading cause of cancer death among men in the United States (US). In the COU-AA-302 (NCT00887198) and PREVAIL (NCT01212991) clinical trials, abiraterone and enzalutamide, respectively, showed clinically meaningful improvements in survival compared with placebo as first-line (1L) treatments in patients with metastatic castration-resistant PC (mCRPC) [ 1 , 2 ]. As such, US guidelines recommend continued androgen deprivation therapy (ADT) in combination with either abiraterone plus prednisone or enzalutamide as the preferred novel hormone therapies (NHTs) for the treatment of NHT-naïve mCRPC [ 3 ]. Abiraterone is an androgen biosynthesis inhibitor, that inhibits 17α-hydroxylase/C17,20-lyase (CYP17), while enzalutamide is an androgen receptor inhibitor. There are no large head-to-head phase 3 clinical trials comparing the efficacy of abiraterone to enzalutamide as 1L treatment for mCRPC, with overall survival (OS) as the primary endpoint.
Several meta-analyses of clinical trials have demonstrated worse radiographic progression-free survival in patients with mCRPC following 1L treatment with abiraterone versus enzalutamide [ 4 , 5 , 6 ]. However, clear evidence of OS differences is not available from these meta-analyses and the reported findings are limited by the considerable heterogeneity in clinical trial populations and designs, and the reliance on immature OS data at the time of primary endpoint readout in some studies.
Multiple real-world studies have compared the OS associated with 1L abiraterone versus enzalutamide in patients with mCRPC. Six large studies (each >1000 patients), using data from the US Veteran’s Health Administration (VHA) [ 7 , 8 ], US Flatiron electronic medical record (EMR) [ 9 ], French National Health System [ 10 ], and Taiwan National Health Insurance databases [ 11 , 12 ], demonstrated statistically significant detriment in OS for abiraterone compared with enzalutamide in this population. Among studies with smaller sample sizes (100‒1000 patients), one reported a significantly reduced OS with abiraterone versus enzalutamide [ 13 ] and eight found comparable OS for 1L abiraterone and enzalutamide [ 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 ]. Of note, a non-significant trend for worse survival with abiraterone versus enzalutamide was observed in five of these studies [ 14 , 17 , 18 , 20 , 21 ]. Importantly, no study to date has shown improved OS for abiraterone versus enzalutamide.
Given the observed OS differences for 1L abiraterone versus enzalutamide in mCRPC studies using French and Taiwanese national datasets, it is valuable to examine these survival differences in a US national dataset, such as Medicare, which is more broadly representative of the population than the US VHA and US Flatiron EMR datasets. Medicare is the primary insurer for men aged ≥65 years in the US and, as the majority (~88%) of the deaths from PC occur in this age group, with a median age at death of 79 years, it is important to assess survival differences within this population [ 22 , 23 ].
This study aimed to compare OS in chemotherapy-naïve patients with mCRPC initiating 1L abiraterone versus enzalutamide in the US Medicare population. Based on multiple prior real-world studies, we hypothesized that OS would be worse in abiraterone-treated patients relative to enzalutamide-treated patients.
Data source
This was a retrospective, observational study of administrative claims data from the Centers for Medicare and Medicaid Services 100% Medicare fee-for-service database from January 1, 2009 to December 31, 2020. Medicare is a US national program that provides access to health insurance for Americans aged ≥65 years, certain disabled patients aged <65 years, and patients with end-stage renal disease [ 24 ]. This study was determined to be exempt from review by the New England Institutional Review Board.
Patient identification
Patients who were ≥18 years of age with ≥1 medical claim with a PC diagnosis code (ICD-9-CM: 185; ICD-10-CM: C61); metastatic disease; evidence of surgical castration any time before the index date or medical castration lasting ≥8 weeks within 1 year before index date; and a post-castration prescription claim for abiraterone or enzalutamide were included in the study (Fig. S1 ).
Patients were chemotherapy-naïve and must have initiated abiraterone or enzalutamide within 90 days prior to the metastasis date, or on or after the metastasis date and between September 10, 2014 and May 31, 2017 to ensure that both therapies were approved for chemotherapy-naïve mCRPC and prior to disclosure of clinical trial data for abiraterone use in metastatic castration-sensitive PC (mCSPC). The index date was defined as the initiation date of abiraterone or enzalutamide. The start of the index period was based on the date of the US Food and Drug Administration (FDA) approval of enzalutamide for chemotherapy-naïve mCRPC. Abiraterone was approved for use in chemotherapy-naïve mCRPC in 2012. Enzalutamide was approved for use in chemotherapy-naïve mCRPC in September 2014. The end of the index period was shortly before the public disclosure of the clinical trial data on abiraterone efficacy in mCSPC [ 25 ], and was selected to ensure patients with mCSPC were excluded.
Patients were included in two distinct cohorts of abiraterone- and enzalutamide-treated patients based on their index prescription, using an intention-to-treat study design.
Patient characteristics
Demographic characteristics (e.g., age, race, region, and socioeconomic status [SES]), clinical characteristics, comorbidities during the baseline period, prior treatments, and healthcare resource use were assessed on or within the 365 days prior to the index date.
Definitions for baseline characteristics and administrative codes for defining comorbidities are presented in the Supplementary Information and Table S1 .
Outcome measures
Length of follow-up, treatment patterns and duration, and OS were assessed from the index date to the earliest of death, disenrollment from Medicare, or the end of data availability.
OS was defined as the time from the index date to death from any cause. Treatment duration of the index prescription was defined as the time from the index date to the discontinuation date. Discontinuation was defined as the earliest of: (1) death, (2) last observed administration plus day of supply associated with last administration, or (3) day before the start of next line of therapy (LOT). Time to subsequent therapy was defined as the time from the index date to the start of next LOT.
Statistical analysis
Means and standard deviations were estimated for continuous baseline variables. Counts and percentages were estimated for categorical baseline variables. Standardized mean difference (SMD) was calculated for each baseline variable. Treatment sequences of up to three treatment regimens were reported in Sankey diagrams. Kaplan–Meier analyses were conducted to describe time-to-event outcomes (i.e., OS, treatment duration, and time to subsequent treatment). Unadjusted and inverse probability treatment-weighting (IPTW)-adjusted Cox proportional-hazards models adjusting for baseline characteristics were fitted to compare time-to-event outcomes in the overall study population as well as in subgroups ( Supplementary Information ). Hazard ratios (HRs) with their 95% confidence intervals (CIs) and p -values were estimated from the Cox models using enzalutamide as the reference cohort.
Sensitivity analysis
A sensitivity analysis was conducted to test the robustness of the primary analysis by adjusting the logistic regression model for additional covariates ( Supplementary Information ).
Subgroup analysis by baseline characteristics
Subgroup analyses were conducted to compare OS between abiraterone and enzalutamide, defined by baseline characteristics: age (≥75 years, <75 years), race (White, Black), SES (low, middle/high), presence of comorbidities (cardiovascular disease [CVD], diabetes, liver disease, and renal disease).
Subgroup analysis by subsequent treatment
Subgroup analysis was conducted to compare median OS between abiraterone and enzalutamide in patients who received 1L treatment with abiraterone or enzalutamide without any subsequent treatment. Additional exploratory analyses were conducted in the following subgroups: (1) patients who switched from abiraterone to enzalutamide and vice versa; (2) patients who switched from abiraterone or enzalutamide to chemotherapy; (3) patients who switched from abiraterone or enzalutamide to another LOT, i.e., non-NHT and non-chemotherapy second-line (2L) regimens, or received >2 LOTs.
Patient population
Overall, 5506 patients with chemotherapy-naïve mCRPC who initiated 1L abiraterone or enzalutamide were identified: 2911 in the abiraterone cohort and 2595 in the enzalutamide cohort (Fig. S1 ).
Baseline demographic and clinical characteristics were generally similar between the cohorts (Table 1 ; Table S2 ). Patients in the abiraterone cohort had a higher use of long-term corticosteroids during the baseline period than patients in the enzalutamide cohort (14.7% vs. 7.9%, SMD = 21.5%). Individual relevant comorbidities were largely similar; however, baseline diabetes was less common in the abiraterone cohort than in the enzalutamide cohort (31.5% vs. 36.8%, SMD = −11.1%).
Treatment duration
Median (95% CI) treatment duration was numerically shorter for the abiraterone cohort (6.7 [6.3‒7.0] months) compared with the enzalutamide cohort (7.4 [7.0‒7.9] months), but the HR was not statistically significant (IPTW-adjusted HR 1.04 [95% CI 0.99–1.10]). In contrast, the median (95% CI) time to subsequent treatment was significantly shorter for the abiraterone cohort (14.5 [13.4‒15.4] months) compared with the enzalutamide cohort (16.7 [15.9‒17.8] months; IPTW-adjusted HR 1.14 [95% CI 1.06–1.22]; p < 0.001).
Overall survival in the overall population
Median follow-up was similar for both cohorts (abiraterone: 19.1 months; enzalutamide: 20.3 months). The IPTW-adjusted median OS was 20.6 months for the abiraterone cohort and 22.5 months for the enzalutamide cohort. Patients who received abiraterone at index had an increased risk of death compared with patients who received enzalutamide (IPTW-adjusted HR 1.10; 95% CI: 1.04–1.16; p < 0.001) (Fig. 1 ).
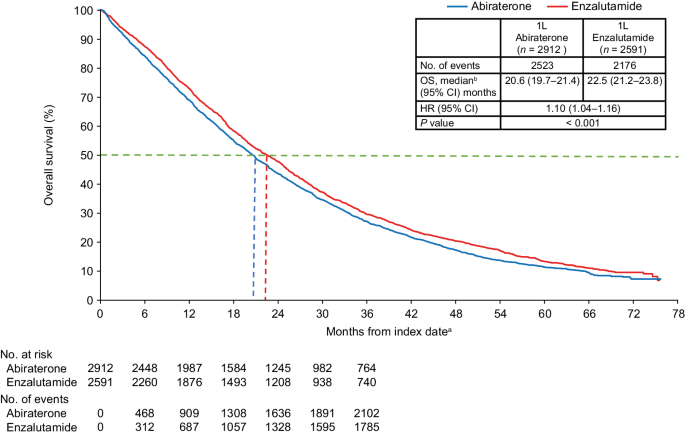
1L first-line, ADT androgen deprivation therapy, CCI Charlson Comorbidity Index, CI confidence interval, HR hazard ratio, IPTW inverse probability treatment-weighting, mCRPC metastatic castration-resistant prostate cancer, NHT novel hormone therapy, OS overall survival, PC prostate cancer, SES socioeconomic status. a The index date was defined as the first initiation of abiraterone or enzalutamide within 90 days prior to or any time after a metastatic disease diagnosis following PC diagnosis, and during the index period of September 10, 2014 through May 31, 2017. b Median OS represents the IPTW-weighted OS among patients with mCRPC treated with 1L abiraterone or enzalutamide therapy during the entire follow-up period. Propensity scores for IPTW were generated by adjusting for baseline characteristics including age, race, geographic regions, SES, site of metastasis, liver metastasis, time from diagnosis to metastasis, time from metastasis to index date, time from ADT start to index date, radical prostatectomy, prior first-generation antiandrogens, prior chronic corticosteroid use, opioid analgesic use, comorbidities during baseline (CCI components, type I and type II diabetes, cardiovascular disease, and anemia), PC-related hospitalization, PC-related emergency room visit, all-cause hospitalization, and all-cause emergency room visits.
In the sensitivity analysis using additional covariate adjustments, the results were identical to the main analysis (Fig. S2 ).
Overall survival in subgroups defined by baseline characteristics
Shorter survival for abiraterone-treated patients versus enzalutamide-treated patients was found for multiple subgroups defined by baseline characteristics (Fig. 2 ). Worse survival for abiraterone versus enzalutamide was observed in patients ≥75 years old (HR 1.16, p < 0.001), White patients (HR 1.11, p = 0.002), patients with low and middle/high SES (HR 1.14, p = 0.040; and HR 1.10, p = 0.007, respectively), patients with baseline CVD (HR 1.15, p < 0.001), diabetes (HR 1.14, p = 0.009), both CVD and diabetes (HR 1.15, p = 0.008), and renal disease (HR 1.15, p = 0.018). There was no difference in OS between abiraterone and enzalutamide for Black patients and patients aged <75 years.
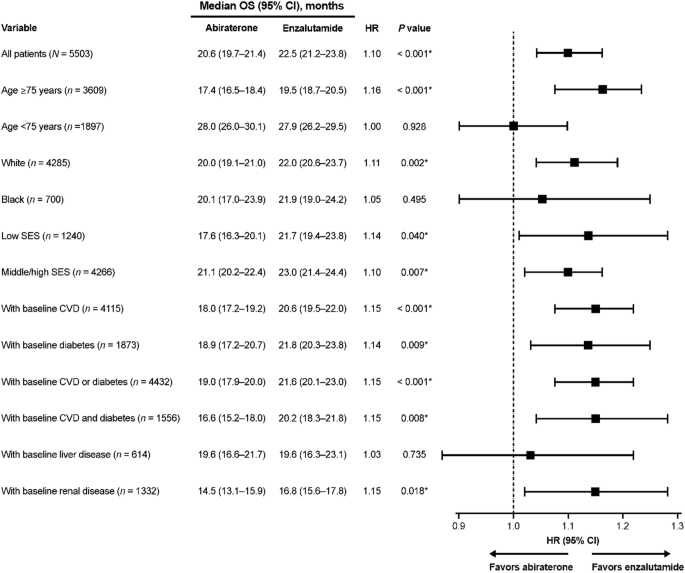
*Indicates a statistically significant association. CI confidence interval, CVD cardiovascular disease, HR hazard ratio, IPTW inverse probability treatment-weighting, mCRPC metastatic castration-resistant prostate cancer, OS overall survival, SES socioeconomic status.
Overall survival in subgroups defined by subsequent treatments
IPTW-adjusted median OS and IPTW-adjusted HRs for the subgroups defined by subsequent treatments are reported in Table 2 . While OS was generally shorter for abiraterone compared with enzalutamide when stratified by subsequent treatment, this did not always reach statistical significance.
Treatment patterns
Comparable proportions of patients in the abiraterone ( n = 1758, 60.4%) and enzalutamide ( n = 1510, 58.2%) cohorts received at least one new subsequent FDA-approved therapy in addition to ADT or older first-generation antiandrogens following their index treatment (Fig. 3 ). Among the 2238 patients who did not receive a subsequent therapy, 13.0% continued on 1L treatment (abiraterone or enzalutamide), 66.4% discontinued 1L treatment, and 20.6% died on 1L treatment.
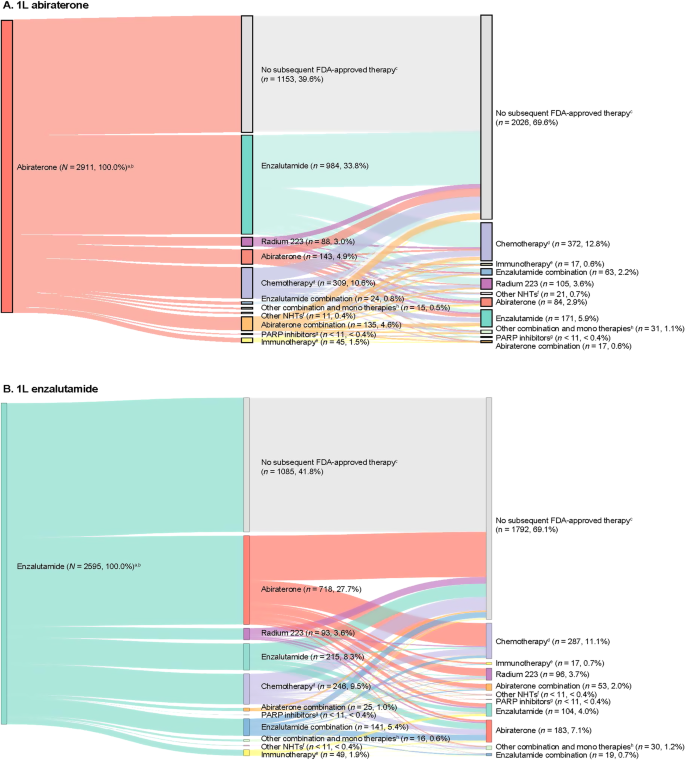
A Patients who initiated abiraterone as 1L treatment and B patients who initiated enzalutamide as 1L treatment. 1L first-line, 2L second-line, 3L third-line, ADT androgen deprivation therapy, FDA US Food and Drug Administration, LOT line of therapy, mCRPC metastatic castration-resistant prostate cancer, NHT novel hormonal therapy, PARP poly (adenosine diphosphate ribose) polymerase. a Percentages are reported out of the total number of patients. b 2L and 3L indicate treatment regimens after abiraterone ( A ) or enzalutamide ( B ), which were assessed during the follow-up period. c No new subsequent FDA-approved therapy was defined as patients who did not receive NHTs, chemotherapy, immunotherapy, radium-223, ketoconazole, or PARP inhibitors as a subsequent line additional to ADT or older first-generation antiandrogens following their index treatment. d Chemotherapy includes cabazitaxel, docetaxel, carboplatin, cisplatin, oxaliplatin, and mitoxantrone. e Immunotherapy includes sipuleucel-T and pembrolizumab. f Other NHTs include apalutamide and darolutamide. g PARP inhibitors include olaparib and rucaparib. h Other combinations and monotherapies include cabazitaxel + carboplatin, carboplatin + docetaxel, docetaxel + cisplatin, cabazitaxel + docetaxel, carboplatin + olaparib, cisplatin + docetaxel, docetaxel + radium-223, ketoconazole + docetaxel, ketoconazole + sipuleucel-T, mitoxantrone + carboplatin, sipuleucel-T + radium-223, ketoconazole, etc.
The most common 2L therapy for patients who received abiraterone at index was enzalutamide ( n = 984, 33.8%), and abiraterone for patients who received enzalutamide at index ( n = 718, 27.7%; Fig. 3 ). Among patients who received abiraterone or enzalutamide at index and received 2L therapy, 50.3% ( n = 885) and 53.2% ( n = 803) received a third line (3L) of therapy, respectively. Chemotherapy was the most common 3L therapy.
To our knowledge, this is the first retrospective real-world analysis assessing OS for abiraterone versus enzalutamide in mCRPC using the Medicare database. This study demonstrated worse survival associated with abiraterone compared with enzalutamide in an older population of patients with chemotherapy-naïve mCRPC (mean age of 78 years), which is largely representative of the US population at risk. Medicare represents a broad-based elderly US population for which survival differences may reflect both the efficacy and the tolerability of treatment. The 10% decrement in survival associated with abiraterone relative to enzalutamide reported here, in this frail population, is clinically significant and may have important implications for clinicians deciding between these treatment options that have identical indications for patients with mCRPC. Indeed, our subgroup analyses suggest that in patients with cardiovascular and metabolic comorbidities, treatment with abiraterone is associated with an even shorter proportional survival than treatment with enzalutamide.
The results of this analysis suggesting worse OS associated with abiraterone versus enzalutamide in the mCRPC population (HR 1.10, 95% CI: 1.04–1.16) are in line with previous analyses of large real-world studies using data from administrative claims and EMR [ 7 , 8 , 9 , 10 , 11 , 12 ]. In a study using the French National Health Data System ( N = 10308; 2014‒2018), abiraterone was associated with shorter median OS compared with enzalutamide (31.7 vs. 34.2 months) [ 10 ]. Likewise, two retrospective analyses of the Taiwan National Health Insurance data (each >1000 patients) found significantly lower propensity-score-adjusted OS rates for 1L abiraterone versus 1L enzalutamide in the overall mCRPC population (49.51% vs. 57.6%, p = 0.003; and 46.3% vs. 59.4%, p < 0.001, respectively) [ 11 , 12 ].
Real-world studies in selected US populations have reported similar OS differences. In a study using the US VHA database (2014‒2018) in chemotherapy-naïve patients with mCRPC ( N = 3174), median OS was shorter with 1L abiraterone versus 1L enzalutamide (25.9 vs. 29.6 months, p = 0.001) [ 7 ]. Another VHA-based real-world study ( N = 5822; 2014‒2017) also found significantly shorter median OS for abiraterone-treated patients compared with enzalutamide-treated patients (22.1 vs. 24.2 months, p = 0.001) [ 8 ]. Furthermore, a retrospective cohort study of mCRPC patients who received 1L systemic therapy using the US Flatiron EMR database (2012–2018) found shorter median OS for abiraterone versus enzalutamide among 2615 non-Hispanic White men (17 vs. 20 months; adjusted HR 1.21, 95% CI: 1.06‒1.38) [ 9 ]. Together, these results present robust evidence to support a reduced OS associated with 1L abiraterone compared with 1L enzalutamide in chemotherapy-naïve mCRPC patients.
In contrast to these large real-world studies, institutional cohort and population-based studies have reported comparable OS among mCRPC patients receiving 1L abiraterone or enzalutamide, although there was a non-significant trend favoring enzalutamide in several studies [ 14 , 15 , 17 , 18 , 20 , 21 , 26 , 27 ]. However, these studies were smaller in size and underpowered to detect modest differences. Ultimately, Medicare represents the broadest real-world population database of patients with advanced PC in the US. Thus, it is critically important to determine if outcomes differ between these two NHTs as the most common standard-of-care options for patients with mCRPC.
Importantly, the threshold for tolerance of inferior outcomes in subgroups of patients undergoing therapeutic interventions in advanced cancers has come under increasing scrutiny [ 28 ]. In this study, significant abiraterone-associated detriments in OS persisted in several subgroups defined by baseline characteristics, including patients aged ≥75 years and those with CVD, diabetes, or both. This is supported by Schoen et al. who reported significantly shorter OS in patients aged ≥75 years and patients with CVD or diabetes who received abiraterone versus enzalutamide in VHA medical facilities [ 8 ]. The finding of poorer OS in older patients or patients with CVD treated with abiraterone versus enzalutamide, if causal, may stem from the greater cardiovascular toxicity associated with abiraterone plus prednisone relative to enzalutamide [ 29 , 30 , 31 , 32 ]. This may also explain the finding of poorer OS with abiraterone versus enzalutamide in the overall study population, as the majority of patients in this study were older and had baseline CVD. Importantly, we found no subgroups in which abiraterone was associated with an improvement in OS compared with enzalutamide.
Consistent with the findings of the Flatiron study by Marar et al. [ 9 ], our analysis by race demonstrated reduced OS with 1L abiraterone versus 1L enzalutamide in White patients, but no difference in Black patients. A limitation of both the current study and the Flatiron study [ 9 ], is that the proportion of Black patients included was low (13%). Other studies suggest that there may be a race-treatment effect, with a lower risk of death for Black patients versus White patients with mCRPC receiving 1L NHT [ 33 , 34 ], but small sample sizes of Black patients limit the power for definitive conclusions at this time. Of note, unlike the current study, where we found no differences in OS for Black patients receiving abiraterone or enzalutamide, a recent analysis of the VHA dataset (2011–2017) that included a larger proportion of Black patients with mCRPC (23%) found significantly shorter OS associated with 1L abiraterone versus enzalutamide among Black patients (21.3 vs. 24.5 months, p < 0.001) [ 35 ]. Thus, further work is needed to understand the effects of these treatments in other datasets that may have a larger proportion of Black patients.
When assessing OS in subgroups defined by subsequent treatments, the largest subgroup of patients (~41%) received 1L NHT only. Notably, abiraterone was associated with a significantly shorter median OS versus enzalutamide in this subgroup (10.6 vs. 13.6 months, p = 0.001). This is consistent with the VHA and Flatiron studies showing that approximately 50% of patients with mCRPC received only one line of NHT; and abiraterone was associated with significantly reduced OS compared with enzalutamide [ 7 , 8 , 9 ]. While these are considered post-baseline subgroups and no adjustment for time-varying covariates was performed, the consistency of these results across datasets suggests that a significant number of patients with mCRPC will only receive one NHT and the choice of 1L NHT may have significant survival implications. The observed treatment patterns in this study were consistent with previously published real-world studies in the US [ 7 , 8 , 9 , 36 ].
This study has some limitations. As this was a retrospective cohort study of the Medicare population, findings may not be applicable to the general population. Given the lack of specific diagnosis codes for mCRPC, assumptions were made in selecting patients with mCRPC based on clinical input and initiation of treatment with abiraterone and enzalutamide, during a period when both were approved for mCRPC only and before public disclosure of the abiraterone clinical trial findings in mCSPC. Furthermore, this study may be limited by residual confounding as we were unable to adjust survival outcomes by several potentially confounding clinical factors (e.g., performance status, laboratory measurements, tumor grade, and metastatic disease burden) due to their unavailability in the Medicare data. Our analysis utilized Medicare claim data which are inherently subject to inaccuracies in the coding of diagnoses and therapies. In addition, claims data do not provide information about the causes of change in treatment. However, as inaccuracies in data tend to bias the results to the null, it is possible our study misestimated the survival detriment associated with abiraterone. Variables such as time from first PC diagnosis to metastatic diagnosis and time from ADT to index date may be truncated because claims data were available after patients became eligible and were enrolled in Medicare. Finally, a filled pharmacy claim does not guarantee that the patient used the prescribed treatment.
In conclusion, in the Medicare chemotherapy-naïve mCRPC population, patients initiating 1L abiraterone had significantly shorter survival and increased risk of death compared with patients initiating 1L enzalutamide. Abiraterone-associated survival detriments were observed in patients with older age, White patients, low and middle/high SES, and certain comorbidities. These findings support previous real-world studies of large databases reporting worse OS associated with 1L abiraterone versus enzalutamide in this patient population. The reproducibility of these results across varied populations represents mounting evidence of a significant difference in comparative effectiveness among the two NHTs. Given the significant proportion of patients who ultimately receive only one line of therapy for mCRPC and the lack of any subgroups demonstrating improved survival with abiraterone, these data should support greater use of enzalutamide in this patient population. Future studies utilizing different data sources to fully determine the impact of 1L abiraterone compared with enzalutamide on patient survival in mCRPC should ensure an appropriate balance between statistical power and the ability to detect clinically meaningful differences, as null findings seem to be exclusively found in smaller studies that were likely underpowered.
Data availability
The data that support the findings of this study are available from Medicare, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Code availability
The code cannot be made publicly available due to confidentiality and proprietary rights. The macros, methods, and model specifics can be shared upon request.
Armstrong AJ, Lin P, Tombal B, Saad F, Higano CS, Joshua AM, et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer from the PREVAIL trial. Eur Urol. 2020;78:347–57.
Article CAS PubMed Google Scholar
Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60.
Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline PART II. J Urol. 2021;205:22–9.
Article PubMed Google Scholar
McCool R, Fleetwood K, Glanville J, Arber M, Goodall H, Naidoo S. Systematic review and network meta-analysis of treatments for chemotherapy-naive patients with asymptomatic/mildly symptomatic metastatic castration-resistant prostate cancer. Value Health. 2018;21:1259–68.
Fang M, Nakazawa M, Antonarakis ES, Li C. Efficacy of abiraterone and enzalutamide in pre-and postdocetaxel castration-resistant prostate cancer: a trial-level meta-analysis. Prostate Cancer. 2017;2017:8560827.
Article PubMed PubMed Central Google Scholar
Chopra A, Georgieva M, Lopes G, Yeo CM, Haaland B. Abiraterone or enzalutamide in advanced castration‐resistant prostate cancer: an indirect comparison. Prostate. 2017;77:639–46.
Tagawa ST, Ramaswamy K, Huang A, Mardekian J, Schultz NM, Wang L, et al. Survival outcomes in patients with chemotherapy-naive metastatic castration-resistant prostate cancer treated with enzalutamide or abiraterone acetate. Prostate Cancer Prostatic Dis. 2021;24:1032–40.
Article CAS PubMed PubMed Central Google Scholar
Schoen MW, Carson KR, Eisen SA, Bennett CL, Luo S, Reimers MA, et al. Survival of veterans treated with enzalutamide and abiraterone for metastatic castrate resistant prostate cancer based on comorbid diseases. Prostate Cancer Prostatic Dis. 2023;26:743–50.
Marar M, Long Q, Mamtani R, Narayan V, Vapiwala N, Parikh RB. Outcomes among African American and Non-Hispanic White men with metastatic castration-resistant prostate cancer with first-line Abiraterone. JAMA Netw Open. 2022;5:e2142093.
Scailteux L-M, Campillo-Gimenez B, Kerbrat S, Despas F, Mathieu R, Vincendeau S, et al. Overall survival among chemotherapy-naive patients with castration-resistant prostate cancer under abiraterone versus enzalutamide: a direct comparison based on a 2014–2018 French population study (the SPEAR Cohort). Am J Epidemiol. 2021;190:413–22.
Lin Y-T, Huang Y-C, Liu C-K, Lee T-S, Chen M, Chien Y-N. Treatment-emergent co-morbidities and survival in patients with metastatic castration-resistant prostate cancer receiving abiraterone or enzalutamide. Front Pharm. 2021;12:669236.
Article CAS Google Scholar
Li P-Y, Lu Y-H, Chen C-Y. Comparative effectiveness of abiraterone and enzalutamide in patients with metastatic castration-resistant prostate cancer in Taiwan. Front Oncol. 2022;12:822375.
Demirci A, Bilir C, Gülbağcı B, Hacıbekiroğlu İ, Bayoğlu İV, Bilgetekin İ, et al. Comparison of real-life data of abiraterone acetate and enzalutamide in metastatic castration-resistant prostate cancer. Sci Rep. 2021;11:14131.
Chen HK, Su PJ, Wang YL, Chang KC, Su YL, Chang PH, et al. Long-term use and risk of major adverse cardiac events: Comparing enzalutamide and abiraterone in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer. Int J Cancer. 2023;152:1191–201.
Chowdhury S, Bjartell A, Lumen N, Maroto P, Paiss T, Gomez-Veiga F, et al. Real-world outcomes in first-line treatment of metastatic castration-resistant prostate cancer: the Prostate Cancer Registry. Target Oncol. 2020;15:301–15.
Anton A, Pillai S, Semira MC, Wong S, Shapiro J, Weickhardt A, et al. Real-world first-line systemic therapy patterns in metastatic castration-resistant prostate cancer. BJUI Compass. 2022;3:205–13.
Al-Ali BM, Eredics K, Madersbacher S, Schauer I. Abiraterone acetate, enzalutamide and their sequence for castration-resistant prostate cancer. Wien Klin Wochenschr. 2018;130:659–64.
Soleimani M, Zou K, Sunderland K, Struss W, Eigl BJ, Nappi L, et al. Effectiveness of first-line abiraterone versus enzalutamide among patients ≥80 years of age with metastatic castration-resistant prostate cancer: a retrospective propensity score–weighted comparative cohort study. Eur J Cancer. 2021;152:215–22.
Baillie K, Mueller T, Pan J, Laskey J, Bennie M, Crearie C, et al. Use of record linkage to evaluate treatment outcomes and trial eligibility in a real-world metastatic prostate cancer population in Scotland. Pharmacoepidemiol Drug Saf. 2020;29:653–63.
Das P, Taylor S, Price J, Jones M, Martin-Fernandez C, Ali A, et al. Abiraterone acetate plus Prednisone/Prednisolone compared with Enzalutamide in metastatic castration resistant prostate cancer before or after chemotherapy: a retrospective study of real-world data (ACES). BJUI Compass. 2020;1:21–31.
Alkan A, Güç ZG, Gürbüz M, Özgün G, Değirmencioğlu S, Doğan M, et al. Enzalutamide versus abiraterone acetate as first-line treatment of castration resistant metastatic prostate cancer in geriatric (≥75) patients. J Mens Health 2021;17:128–34.
Google Scholar
National Cancer Institute. SEER Cancer Stat Facts: Prostate Cancer. 2023. https://seer.cancer.gov/statfacts/html/prost.html .
Clark R, Vesprini D, Narod SA. The effect of age on prostate cancer survival. Cancers (Basel). 2022;14:4149.
Centers for Medicare & Medicaid Services. Medicare and You Handbook. Baltimore, MD: U.S. Department of Health & Human Services; 2023.
Fizazi K, Tran N, Fein LE, Matsubara N, Rodríguez Antolín A, Alekseev BY, et al. LATITUDE: a phase III, double-blind, randomized trial of androgen deprivation therapy with abiraterone acetate plus prednisone or placebos in newly diagnosed high-risk metastatic hormone-naive prostate cancer. Abstracts of the 2017 ASCO Annual Meeting II. J Clin Oncol. 2017;35:LBA3–LBA3.
Article Google Scholar
Banna GL, Urzia V, Benanti C, Pitrè A, Lipari H, Di Quattro R, et al. Adherence to abiraterone or enzalutamide in elderly metastatic castration-resistant prostate cancer. Support Care Cancer. 2020;28:4687–95.
Briones Carvajal JR, Naimi MF, Zhang L, Emmenegger U. Real-world comparison of abiraterone (A) versus enzalutamide (E) for first-line therapy of metastatic castration-resistant prostate cancer (mCRPC). Abstracts of the American Society of Clinical Oncology 2021 Genitourinary Cancers Symposium. J Clin Oncol. 2021;39:133.
Cuzick J, Sasieni P. Interpreting the results of noninferiority trials—a review. Br J Cancer. 2022;127:1755–9.
Hu J, Aprikian AG, Vanhuyse M, Dragomir A. Comparative cardiovascular safety of novel hormonal agents in metastatic castration-resistant prostate cancer using real-world data. Clin Genitourin Cancer. 2022;20:17–24.
Lu-Yao G, Nikita N, Keith SW, Nightingale G, Gandhi K, Hegarty SE, et al. Mortality and hospitalization risk following oral androgen signaling inhibitors among men with advanced prostate cancer by pre-existing cardiovascular comorbidities. Eur Urol. 2020;77:158–66.
Shore ND, Saltzstein D, Sieber P, Mehlhaff B, Gervasi L, Phillips J, et al. Results of a real-world study of enzalutamide and abiraterone acetate with prednisone tolerability (REAAcT). Clin Genitourin Cancer. 2019;17:457–63.e456.
Riekhof F, Yan Y, Bennett CL, Sanfilippo KM, Carson KR, Chang S-H, et al. Hospitalizations among veterans treated for metastatic prostate cancer with abiraterone or enzalutamide. Clin Genitourin Cancer. 2023;S1558-7673:00173–8.
George DJ, Ramaswamy K, Huang A, Russell D, Mardekian J, Schultz NM, et al. Survival by race in men with chemotherapy-naive enzalutamide- or abiraterone-treated metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2021;25:524–30.
George DJ, Halabi S, Fleming MT, Heath EI, Tutrone RF, Sutton L, et al. A prospective trial of apalutamide and abiraterone acetate plus prednisone in Black and White men with metastatic castrate-resistant prostate cancer. Abstracts of the 2023 American Society of Clinical Oncology Annual Meeting. J Clin Oncol. 2023;41:5015.
Cheranda N, Luo S, Sanfilippo KM, Eisen SA, Schoen MW. Survival in Black and non-Black patients with metastatic prostate cancer. Abstracts of the 2023 American Society of Clinical Oncology Genitourinary Cancers Symposium. J Clin Oncol. 2023;41:101.
George DJ, Sartor O, Miller K, Saad F, Tombal B, Kalinovský J, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18:284–94.
Download references
Acknowledgements
Medical writing and editorial support were provided by Tom Fresen, MSc, Roham Sadeghimakki, MD, PhD, Megan Christian, MBiolSci, and Rosie Henderson, MSc, of Onyx, (a division of Prime, London, UK), and funded by Pfizer Inc. and Astellas Pharma, Inc., the co-developers of enzalutamide. The authors were involved in the collection and interpretation of information provided in the manuscript, and ultimate responsibility for opinions and conclusions lies with the authors.
This study was sponsored by Pfizer Inc. and Astellas Pharma Inc., the co-developers of enzalutamide.
Author information
Authors and affiliations.
Duke Cancer Institute, Durham, NC, USA
Daniel J. George
Pfizer Inc., New York, NY, USA
Krishnan Ramaswamy, Jasmina Ivanova, Betty Thompson, Birol Emir & Agnes Hong
Analysis Group, Inc., Boston, MA, USA
Hongbo Yang, Qing Liu, Adina Zhang & Alexandra Greatsinger
Formerly of Astellas Pharma, Northbrook, IL, USA
Cedars-Sinai Medical Center, Los Angeles, CA, USA
Stephen J. Freedland
Durham VA Medical Center, Durham, NC, USA
You can also search for this author in PubMed Google Scholar
Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: KR, HY, QL, JI, BT, BE, AH, and SJF. Acquisition of data: HY, QL, AZ, and AG. Analysis and interpretation of data: DJG, KR, HY, QL, AZ, AG, JI, BT, BE, AH, and SJF. Drafting of manuscript: HY, QL, AZ, AG, and BE. Reading and interpretation of literature: DJG, KR, HY, QL, AZ, AG, JI, BT, AH, and SJF. Critical revision of manuscript for important intellectual content: DJG, KR, HY, QL, AZ, AG, JI, BT, BE, AH, and SJF. Statistical analysis: AZ and AG. Obtaining funding: None. Administrative, technical, or material support: None. Supervision: SJF. Other: None.
Corresponding author
Correspondence to Daniel J. George .
Ethics declarations
Competing interests.
DJG reports being a consultant for Bayer, Exelixis, Pfizer Inc., Sanofi, Astellas Pharma Inc., Innocrin Pharma, Bristol Meyers Squibb, Genentech, Janssen, Merck Sharp & Dohme, Sumitomo Pharma America, Inc. (formerly Myovant Sciences), AstraZeneca, Michael J. Hennessy Associates, Constellation Pharmaceuticals, Physicians’ Education Resource, Propella Therapeutics, RevHealth, Xcures, Novartis, Dendreon, Acerta, and Calithera Biosciences. KR, AH, JI, BT, and EB are employees of Pfizer Inc. HY, QL, AZ, and AG are employees of Analysis Group, which was a paid consultant to Pfizer in connection with the development of this manuscript. SJF reports being a consultant for Astellas Pharma Inc., AstraZeneca, Bayer, Clovis Oncology, Exact Sciences Corporation, Janssen Biotech, Merck, Pfizer Inc., Sanofi, Sumitomo Pharma America, Inc. (formerly Myovant Sciences), and Tempus.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
George, D.J., Ramaswamy, K., Yang, H. et al. Real-world overall survival with abiraterone acetate versus enzalutamide in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00816-0
Download citation
Received : 12 September 2023
Revised : 20 February 2024
Accepted : 26 February 2024
Published : 27 March 2024
DOI : https://doi.org/10.1038/s41391-024-00816-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Company Profile
- Company Fundamentals
- Company Financials
- Fibrogen Inc 's ROI
- Fibrogen Inc 's Revenue per Employee
- Fibrogen Inc 's Business Description
- Fibrogen Inc 's Officers & Directors
- Charts & Quotes
- Fibrogen Inc 's News
- Company Suppliers
- Company Competitors
- Company Markets & Customers
- Economic Indicators
- At a Glance
- Performance by Industry
- Growth Rates by Industry
- Profitability by Industry
- Valuation by Industry
- Financial Strength by Industry
- Advance Monthly Sales
- Consumer Price Index CPI
- Producer Price Index PPI
- Retail Inventories
- Personal Income
- Gross Domestic Product GDP
- Money Supply
- Industrial Production
- Productivity
- Employment Situation
- U.S. International Trade
- Factory orders
- Durable Goods
- Construction Spending
- Housing Starts
- Vehicle Unit Sales
- Cryptocurrencies
- Sectors & Industries
- Commodities
- Foreign Exchange
- Stock Performance Screening
- Growth Rates Screening
- Profitability Screening
- Valuation Screening
- Dividend Screening
- Financial Strength Screening
- Efficiency Screening
- Largest Companies
- Mgmt. Effectiveness Screening
- News at a Glance
- Stock News
- Economy News
- Financial Terms
- Technical Analysis
- Fundamental Analysis
- Energy Terms
- Manufacturing Terms
- Transportation Terms
- Health Care
- Insurance Terms
- Economy Terms
- Hotel & Leisure Terms
- Dividend News
- News on Company's Stock Splits
Promising Results of FG-3246 in Phase 1 Monotherapy Study for Metastatic Castration-Resistant Prostate Cancer

Previous News
Niu Technologies Reports Strong Sales Volume for Q1 2024 Amidst Growing Demand for Urban Mobility Solutions
Grifols Procleix ArboPlex Assay: Pioneering Arbovirus Screening with CE Mark for the First 4-in-1 NAT
Michelin Star-led Food Company and EnWave Collaborate: An Evaluation of Revolutionary Food Dehydration Technology
Richmond American's Volusia County Spectacle: An Inside Look at Booming Growth, Deteriorating Returns, and Building Steadies Amid Economic Turbulence
Volaris Soars with Impressive March 2024 Traffic Results, Exemplifying Stability and Growth in the Airline Industry
FMC Corporation's Strategic Appointments Steer the Company Towards Future Growth
Ashland Announces Executive Organization Changes to Drive Growth in Personal Care and Specialty Additives
Ethan Allen Reveals Fiscal 2024 Q3 Results, Investors Await Insights into Post-Pandemic Recovery
Brigadier General (Retired) Bobbi Doorenbos Joins Kratos Board of Directors to Strengthen Leadership and Drive Growth
XPO's Journey: Navigating Through Turbulent Waters and Paving the Future of Corporate Logistics.
- FibroGen-stock
- News for FibroGen
FibroGen Announces Topline Results from Phase 1 Monotherapy Study of FG-3246 in Patients with Metastatic Castration-Resistant Prostate Cancer
- FG-3246 demonstrated efficacy in adenocarcinoma selected cohorts receiving biologically active doses of FG-3246 at ≥ 1.2 mg/kg in heavily pre-treated, biomarker unselected patients:
- Median radiographic progression free survival of 8.7 months
- PSA50 response in 36% of patients
- Confirmed radiographic objective response rate of 20%, with a median duration of response of 7.5 months
- FG-3246 demonstrated an acceptable safety profile; adverse events consistent with those observed in other antibody drug conjugate therapies with a MMAE payload
- Company plans to meet with the U.S. Food and Drug Administration (FDA) to discuss development pathway; Phase 2 initiation is anticipated in 2H 2024
SAN FRANCISCO, April 02, 2024 (GLOBE NEWSWIRE) -- FibroGen, Inc. (NASDAQ: FGEN) today announced topline data from the Fortis Therapeutics-sponsored Phase 1 study of FG-3246 (also known as FOR46), a potential first-in-class anti-CD46 antibody drug conjugate (ADC) with an MMAE-containing payload, in a dose-escalation and dose-expansion trial enrolling patients with metastatic castration-resistant prostate cancer (mCRPC) whose tumors have progressed on at least one androgen receptor-signaling inhibitor (ARSI).
“We are delighted to showcase the latest encouraging clinical data from the FOR46-001 Phase 1 ADC trial,” said Deyaa Adib, M.D., Chief Medical Officer of FibroGen. “We observed a median radiographic progression free survival of 8.7 months in heavily pre-treated patients, who received biologically active doses of FG-3246 in the second line or later setting prior to chemotherapy. These Phase 1 data provide evidence of a favorable safety profile and promising clinical activity as further evidenced by prostate-specific antigen reduction of ≥ 50% and shrinking of measurable disease. We look forward to publishing the totality of the Phase 1 data as we advance the program further in the clinic.”
In the Phase 1 dose-escalation portion of the study, ascending dose levels of FG-3246 were administered every 3 weeks. In the dose-expansion arm of the trial, patients were treated at the 2.7 mg/kg adjusted body weight dosing (AjBW) until disease progression. The endpoints were safety, tolerability, and anti-tumor activity as measured by the decline of prostate-specific antigen (PSA) from baseline, objective tumor response rate in patients with measurable disease, and radiographic progression free survival (rPFS).
The completed Phase 1 trial includes a total of 56 patients from the dose-escalation and dose-expansion cohorts. The efficacy analysis includes patients who received a starting dose of FG-3246 of ≥ 1.2 mg/kg in the dose-escalation cohort, and patients who received 2.7 mg/kg AjBW with a histologic diagnosis of adenocarcinoma in the dose-expansion cohort. Patients were heavily pre-treated, having received a median of 5 lines of therapy prior to receiving FG-3246.
In the efficacy analysis, PSA reductions of ≥ 50% were observed in 36% of PSA evaluable patients. For RECIST evaluable patients, 20% met the criteria of a partial response, or tumor reduction in size of ≥ 30%, with a median duration of response of 7.5 months. The median rPFS in this heavily pre-treated patient population was 8.7 months.
The most frequent adverse events were consistent with other MMAE-based ADCs and included infusion related reactions, fatigue, weight loss, neutropenia, and peripheral neuropathy.
“The results from the FOR46-001 Phase 1 study are promising, demonstrating a manageable safety profile and continued robust signals of clinical activity,” stated Dr. Rahul Aggarwal, Professor of Medicine at University of California San Francisco and Lead Investigator of the study. “The observed median radiographic progression free survival of 8.7 months in patients treated with a starting FG-3246 dose of 1.2 mg/kg and higher is quite favorable and highlights the therapeutic potential of FG-3246 as a new ADC aimed at a novel target. These findings warrant further investigation and hold promise for addressing the therapeutic needs of patients with CD46 positive prostate cancer. We are also excited about potential combinations with FG-3246 and will be presenting investigator sponsored trial data of FG-3246 in combination with enzalutamide at the upcoming ASCO 2024 annual meeting.”
Earlier data from the FOR46-001 trial had been presented at the American Society for Clinical Oncology (ASCO) 2022 annual meeting 1 , and complete results from the study are being submitted to a medical journal for publication in 2024.
About the Phase 1 Study FOR46-001 ( NCT03575819 ) is a Phase 1, dose-escalation study to evaluate multiple doses of IV-administered FG-3246 (also known as FOR46) in patients with mCRPC who have progressive disease on at least one ARSI, followed by a dose-expansion cohort, to evaluate the safety, tolerability, PK, biological activity, and preliminary evidence of anti-tumor activity of FG-3246 in this patient population.
Thirty-three (33) patients were enrolled in the dose-escalation phase of the study at doses between 0.1 mg/kg and 3.0 mg/kg every three weeks (Q3W), with adjusted body weight dosing (AjBW) used at most dose levels above 2.1 mg/kg. Safety and tolerability of FG-3246 were evaluated in the dose-escalation period of the study.
Twenty-three (23) patients were enrolled in the dose-expansion period of the study; 18 patients with adenocarcinoma mCRPC (Cohort 1) and five patients with neuroendocrine prostate cancer (Cohort 2). All patients in the expansion cohorts were treated at 2.7 mg/kg AjBW to a maximum of 270 mg every three weeks.
The safety profile of FG-3246 was characterized, and anti-tumor activity of FG-3246 in adenocarcinoma patients dosed at ≥ 1.2 mg/kg was evaluated.
About Metastatic Castration-Resistant Prostate Cancer Prostate cancer develops when malignant cells form and grow in the prostate gland. Prostate cancer is the most common cancer in men in the United States, who currently have a 1 in 8 lifetime risk of developing the disease. 2 Approximately 290,000 new diagnoses of prostate cancer and nearly 35,000 deaths were estimated in the U.S. in 2023. 2 Metastatic castration-resistant prostate cancer (mCRPC) is a form of advanced prostate cancer that shows signs of growth, even with low levels of testosterone. 2 With mCRPC, the cancer stops responding to hormone therapies and can be life-threatening if it spreads to other parts of the body such as nearby lymph nodes, bones, the bladder, rectum, liver, lungs, and the brain. Death from prostate cancer is typically the result of mCRPC, with a 5-year survival rate of 34% 3 , and the unfortunate reality remains that mCRPC is an incurable disease. 4
About FG-3246 FG-3246 (also known as FOR46) is a potential first-in-class fully human antibody-drug conjugate (ADC), exclusively in-licensed from Fortis Therapeutics, and is being developed by FibroGen for metastatic castration-resistant prostate cancer and other tumor types. FG-3246 binds to an epitope of CD46, a cell receptor target, that induces internalization upon antibody binding, is present at high levels in prostate cancer and other tumor types, and demonstrates very limited expression in most normal tissues. FG-3246 is comprised of an anti-CD46 antibody, YS5, linked to the anti-mitotic agent, MMAE, which is a clinically and commercially validated ADC payload. FG-3246 has demonstrated anti-tumor activity in both preclinical and clinical studies. FG-3246 is currently in an ongoing Phase 1/2 study being conducted at UCSF to evaluate it in combination with enzalutamide with initial data expected in mid-2024, and a biomarker trial using a PET biomarker for CD46 using the same antibody backbone. We anticipate the initiation of the Phase 2 trial in metastatic castration-resistant prostate cancer in the second half of 2024. FG-3246 is an investigational drug and not approved for marketing by any regulatory authority.
About FibroGen FibroGen, Inc. is a biopharmaceutical company focused on accelerating the development of novel therapies at the frontiers of cancer biology. Pamrevlumab, a fully human anti-CTGF monoclonal antibody, is in clinical development for the treatment of metastatic pancreatic cancer and locally advanced unresectable pancreatic cancer (LAPC). Roxadustat (爱瑞卓®, EVRENZO TM ) is currently approved in China, Europe, Japan, and numerous other countries for the treatment of anemia in chronic kidney disease (CKD) patients on dialysis and not on dialysis. Roxadustat is in clinical development for chemotherapy-induced anemia (CIA) and a Supplemental New Drug Application (sNDA) has been accepted for review by the China Health Authority. FG-3246 (also known as FOR46), a first-in-class antibody-drug conjugate (ADC) targeting CD46 is in development for the treatment of metastatic castration-resistant prostate cancer. This program also includes the development of an associated CD46-targeted PET biomarker. In addition, FibroGen has expanded its research and development portfolio to include two immuno-oncology product candidates for the treatment of solid tumors. For more information, please visit www.fibrogen.com.
Forward-Looking Statements This release contains forward-looking statements regarding FibroGen’s strategy, future plans and prospects, including statements regarding its clinical programs and those of its collaboration partner Fortis, and UCSF. These forward-looking statements include, but are not limited to, statements regarding the efficacy, safety, and potential success of FibroGen product candidates, and statements about FibroGen’s plans and objectives and typically are identified by use of terms such as “may,” “will,” “should,” “on track,” “could,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue” and similar words, although some forward-looking statements are expressed differently. FibroGen’s actual results may differ materially from those indicated in these forward-looking statements due to risks and uncertainties related to the continued progress and timing of its various programs, including the enrollment and results from ongoing and potential future clinical trials, and other matters that are described in FibroGen’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, as filed with the Securities and Exchange Commission (SEC), including the risk factors set forth therein. Investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this release, and FibroGen undertakes no obligation to update any forward-looking statement in this press release, except as required by law.
References: 1. Aggarwal, R. et al. Journal of Clinical Oncology 2022 40:16_suppl, 3001-3001 2. Schaeffer, E. et al. https://jnccn.org/view/journals/jnccn/21/10/article-p1067.xml 3. Seer.cancer.gov https://seer.cancer.gov/statfacts/html/prost.html 4. Dong, L. et al. Asian Journal of Urology (2019) 6, 26e41
FibroGen, Inc.
Investors: David DeLucia, CFA Vice President of Corporate FP&A / Investor Relations [email protected]
Media: Meichiel Keenan Director, Investor Relations and Corporate Communications [email protected]

FibroGen News MORE
Related stocks.

IMAGES
VIDEO
COMMENTS
In 2021, it is estimated that 26% of new noncutaneous cancer cases will be because of prostate cancer resulting in 11% of cancer-related deaths in the United States, making it the most common malignancy in men and the second leading cause of cancer mortality. 1 Following onset of metastatic disease, the disease is invariably fatal with a 5-year survival rate of only 30%.
Additionally, in patients with metastatic castration-resistant prostate cancer, there are many new therapeutic approaches. Chemotherapy alone has been the standard of care in this situation. In the last years, some new therapeutic options have been developed, which led to an improved survival after progression under chemotherapy.
In June, the FDA approved a new treatment for the most advanced type of prostate cancer. Patients who have this condition, which is called metastatic castration-resistant prostate cancer (mCRPC), have few therapeutic options, so the approval helps to fill an urgent need. mCRPC sets in when the front-line hormonal therapies that doctors use ...
For men with an initial diagnosis of metastatic prostate cancer, continuous androgen-deprivation therapy represented the standard of care from 1941 until 2015, when two trials (Androgen Ablation ...
Few areas in oncology have seen as much progress in recent years as metastatic prostate cancer. In a masterful review of the current landscape of management of metastatic prostate cancer, Sayegh et al 1 incorporate recent advances to provide a thorough overview of evolving treatment paradigms in this field. When I discuss the management of prostate cancer with patients—and when I describe ...
With more than 1.4 million new cases in 2020 and an increasing incidence trend, prostate cancer maintains its position as the second most diagnosed cancer among men worldwide. 1 De novo metastatic prostate cancer accounts for around 15% of all prostate cancer cases in western countries, but it is responsible for the majority of prostate cancer-related deaths and imposes a significant socio ...
With over 288,000 new cases of prostate cancer diagnosed in the United States in 2023, prostate cancer remains the most common solid malignancy in men [1]. ... Hui Ting Ruan c Translational Hematology and Oncology Research, Taussig Cancer Institute, Cleveland Clinic, Case Comprehensive Cancer Center, ... Patients with metastatic prostate cancer ...
Metastatic castration-resistant prostate cancer is a heterogeneous disease with poor outcomes. 1-6 Tumors in up to 30% of patients harbor deleterious aberrations in genes involved in repairing DNA ...
About 10 million men are presently living with a diagnosis of prostate cancer, and approximately 700 000 of these are living with metastatic disease. 1. , 2. Metastatic prostate cancer accounts for more than 400 000 deaths annually, and this mortality is expected to more than double by 2040.
The results were impressive. The five-year survival rate for newly diagnosed metastatic prostate cancer is only around 30% (see 'Surviving metastases'). But after more than four years, all the ...
New therapeutic strategy for metastatic prostate cancer patients resistant to standard treatment. A team of researchers from the Badalona Applied Research Group in Oncology (B·ARGO) and the ...
A new trial is being planned to confirm these results. ... in a small study including 13 patients with metastatic prostate cancer, ... a trial of the ECOG-ACRIN cancer research group (E1809). ...
Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial
Prostate cancer: highlights from research Download PDF. OUTLOOK; 14 September 2022 ... Latest on: Cancer. Video: Cancer-busting vaccines ... Metastatic prostate cancer: seeking a fresh chance of ...
Bone metastases are devastating developments of cancers that originate in other organs. In the case of metastatic prostate cancer, bone metastases represent an incurable, painful, and often deadly development, killing more than 30,000 American men every year. Unlike many other tumor types, metastatic prostate cancer responds poorly to immune-based therapies, but a new study published in Cancer ...
In 2020, there were an estimated 1,414,259 new cases of prostate cancer globally with an age standardized incidence rate (ASIR) of 30.7 per 100,000 males. There were an estimated 375,304 prostate cancer-related deaths with an age standardized mortality rate (ASMR) of 7.7 per 100,000 males [ 3 ]. In the United States, there will be an estimated ...
Evidence synthesis. A total of 1491 participants from 15 studies met the prespecified eligibility for inclusion. The study designs included were discrete choice experiments (n = 5), mixed methods (n = 3), and qualitative methods (n = 7).Disease states reported per study were: metastatic castration-resistant prostate cancer in nine studies (60.0%), metastatic hormone-sensitive prostate cancer ...
Causes of Death During Each Latency Period After Diagnosis of Metastatic Prostate Cancer. View Large Download. ... for SMRs—linked to county attributes—total U.S., 1969-2017 counties, National Cancer Institute, DCCPS, Surveillance Research Program. Released April 2019, based on the November 2018 submission. ... Get the latest research based ...
Key Points. Question Has the introduction of doublet therapy in individuals with de novo metastatic castration-sensitive prostate cancer been associated with changes in survival on a population basis in Sweden?. Findings In this nationwide cohort study, upfront treatment with doublet therapy among 11 382 individuals between 2008 and 2020 with de novo metastatic castration-sensitive prostate ...
This page highlights some of the latest research in prostate cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies. ... Over the last decade, several new approaches to hormone therapy for advanced or metastatic prostate cancer ...
Dec 12 2023 Columbia University Irving Medical Center. Immunotherapy has been disappointing as a prostate cancer treatment, but a new Columbia study suggests that the powerful treatments have ...
New, advanced treatments for prostate cancer provide patients with a multitude of options. ... Radium-223 is given to patients with metastatic prostate cancer that has spread to the bones and has shown resistance to hormonal therapy. ... what we strive to bring as Johns Hopkins faculty is the clinical and research expertise to look at the whole ...
Jan. 5, 2021 — New research has identified a new mechanism in which prostate cancer cells can 'switch' character and become resistant to therapy. These findings are an important development in ...
Metastatic castration-resistant prostate cancer. Patients in this situation who have progressed on taxane-based chemotherapy as well as a next-generation hormonal agent have the option to enroll ...
Full Title A Phase 3, Open-label, Randomized, Prospective Study of Apalutamide with Continued Versus Intermittent Androgen-Deprivation Therapy (ADT) following PSA Response in Participants with Metastatic Castration-Sensitive Prostate Cancer (mCSPC) Purpose Prostate cancers initially need the male hormone testosterone for growth. Hormone therapies that lower the level of testosterone are among ...
There is increasing demand worldwide to develop diagnostic and therapeutic (theranostic) markers for prostate cancer. One target of interest is prostate-specific membrane antigen (PSMA), a protein which is overexpressed in prostate cancer cells. Over the past decade, a growing body of literature has demonstrated that radiolabeled ligands that target PSMA show favorable clinical response and ...
This retrospective analysis of the Medicare database (2009-2020) included adult men with ≥1 claim for prostate cancer, metastatic diagnosis, and no prior chemotherapy or novel hormone therapy ...
This article provides an overview of the topline results from the Phase 1 study conducted by FibroGen, Inc. and sponsored by Fortis Therapeutics. The study evaluated the potential of FG-3246, an anti-CD46 antibody drug conjugate (ADC) with an MMAE-containing payload, as a treatment option for patients with metastatic castration-resistant prostate cancer (mCRPC) who have previously progressed ...
Apr 03, 2024. Men with metastatic hormone-sensitive prostate cancer who experience baseline bone pain have worse survival outcomes despite treatment intensification. Research findings presented at the 2024 ASCO Genitourinary Symposium revealed the significance of baseline bone pain as a prognostic marker for overall survival (OS) in men with ...
Prostate cancer is the most common cancer in men in the United States, who currently have a 1 in 8 lifetime risk of developing the disease. 2 Approximately 290,000 new diagnoses of prostate cancer ...