
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.


In Spring 2021, the National Library of Medicine (NLM) PubMed® Special Query on this page will no longer be curated by NLM. If you have questions, please contact NLM Customer Support at https://support.nlm.nih.gov/
This chart lists the major biomedical research reporting guidelines that provide advice for reporting research methods and findings. They usually "specify a minimum set of items required for a clear and transparent account of what was done and what was found in a research study, reflecting, in particular, issues that might introduce bias into the research" (Adapted from the EQUATOR Network Resource Centre ). The chart also includes editorial style guides for writing research reports or other publications.
See the details of the search strategy. More research reporting guidelines are at the EQUATOR Network Resource Centre .
Last Reviewed: April 14, 2023
When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections
- Collections Home
- About Collections
- Browse Collections
- Calls for Papers
Reporting Guidelines
Reporting guidelines are statements intended to advise authors reporting research methods and findings. They can be presented as a checklist, flow diagram or text, and describe what is required to give a clear and transparent account of a study's research and results. These guidelines are prepared through consideration of specific issues that may introduce bias, and are supported by the latest evidence in the field. The Reporting Guidelines Collection highlights articles published across PLOS journals and includes guidelines and guidance, commentary, and related research on guidelines. This collection features some of the many resources available to facilitate the rigorous reporting of scientific studies, and to improve the presentation and evaluation of published studies.
Image Credit: CCAC North Library, Flickr
- From the Blogs
- Observational & Epidemiological Research
- Randomized Controlled Trials
- Systematic Reviews & Meta-Analyses
- Diagnostic & Prognostic Research
- Animal & Cell Models
- General Guidance
- Image credit CCAC North Library, Flickr.com Speaking of Medicine Maximizing the Impact of Research: New Reporting Guidelines Collection from PLOS – Speaking of Medicine September 3, 2013 Amy Ross, Laureen Connell
- Image credit 10.1371/journal.pmed.1001885 PLOS Medicine The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement October 6, 2015 Eric I. Benchimol, Liam Smeeth, Astrid Guttmann, Katie Harron, David Moher, Irene Petersen, Henrik T. Sørensen, Erik von Elm, Sinéad M. Langan, RECORD Working Committee
- Image credit 10.1371/journal.pmed.0040297 PLOS Medicine Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration October 16, 2007 Jan P Vandenbroucke, Erik von Elm, Douglas G Altman, Peter C Gøtzsche, Cynthia D Mulrow, Stuart J Pocock, Charles Poole, James J Schlesselman, Matthias Egger, for the STROBE Initiative
- Image credit 10.1371/journal.pmed.0040296 PLOS Medicine The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies October 16, 2007 Erik von Elm, Douglas G Altman, Matthias Egger, Stuart J Pocock, Peter C Gøtzsche, Jan P Vandenbroucke, for the STROBE Initiative
- Image credit PLOS PLOS Medicine Reporting Guidelines for Survey Research: An Analysis of Published Guidance and Reporting Practices August 2, 2011 Carol Bennett, Sara Khangura, Jamie Brehaut, Ian Graham, David Moher, Beth Potter, Jeremy Grimshaw
- Image credit 10.1371/journal.pone.0064733 PLOS ONE Impact of STROBE Statement Publication on Quality of Observational Study Reporting: Interrupted Time Series versus Before-After Analysis August 26, 2013 Sylvie Bastuji-Garin, Emilie Sbidian, Caroline Gaudy-Marqueste, Emilie Ferrat, Jean-Claude Roujeau, Marie-Aleth Richard, Florence Canoui-Poitrine
- Image credit 10.1371/journal.pone.0094412 PLOS ONE The Reporting of Observational Clinical Functional Magnetic Resonance Imaging Studies: A Systematic Review April 22, 2014 Qing Guo, Melissa Parlar, Wanda Truong, Geoffrey Hall, Lehana Thabane, Margaret McKinnon, Ron Goeree, Eleanor Pullenayegum
- Image credit 10.1371/journal.pone.0101176 PLOS ONE A Review of Published Analyses of Case-Cohort Studies and Recommendations for Future Reporting June 27, 2014 Stephen Sharp, Manon Poulaliou, Simon Thompson, Ian White, Angela Wood
- Image credit 10.1371/journal.pone.0103360 PLOS ONE Outlier Removal and the Relation with Reporting Errors and Quality of Psychological Research July 29, 2014 Marjan Bakker, Jelte Wicherts
- Image credit 10.1371/journal.pmed.1000022 PLOS Medicine STrengthening the REporting of Genetic Association Studies (STREGA)— An Extension of the STROBE Statement February 3, 2009 Julian Little, Julian P.T Higgins, John P.A Ioannidis, David Moher, France Gagnon, Erik von Elm, Muin J Khoury, Barbara Cohen, George Davey-Smith, Jeremy Grimshaw, Paul Scheet, Marta Gwinn, Robin E Williamson, Guang Yong Zou, Kim Hutchings, Candice Y Johnson, Valerie Tait, Miriam Wiens, Jean Golding, Cornelia van Duijn, John McLaughlin, Andrew Paterson, George Wells, Isabel Fortier, Matthew Freedman, Maja Zecevic, Richard King, Claire Infante-Rivard, Alex Stewart, Nick Birkett
- Image credit 10.1371/journal.pmed.1001117 PLOS Medicine STrengthening the Reporting of OBservational studies in Epidemiology – Molecular Epidemiology (STROBE-ME): An Extension of the STROBE Statement October 25, 2011 Valentina Gallo, Matthias Egger, Valerie McCormack, Peter Farmer, John Ioannidis, Micheline Kirsch-Volders, Giuseppe Matullo, David Phillips, Bernadette Schoket, Ulf Strömberg, Roel Vermeulen, Christopher Wild, Miquel Porta, Paolo Vineis
- Image credit PLOS PLOS Medicine Observational Studies: Getting Clear about Transparency August 26, 2014 The PLoS Medicine Editors
- Image credit 10.1371/journal.pmed.0050020 PLOS Medicine CONSORT for Reporting Randomized Controlled Trials in Journal and Conference Abstracts: Explanation and Elaboration January 22, 2008 Sally Hopewell, Mike Clarke, David Moher, Elizabeth Wager, Philippa Middleton, Douglas G Altman, Kenneth F Schulz, and the CONSORT Group
- Image credit PLOS PLOS ONE Endorsement of the CONSORT Statement by High-Impact Medical Journals in China: A Survey of Instructions for Authors and Published Papers February 13, 2012 Xiao-qian Li, Kun-ming Tao, Qinghui Zhou, David Moher, Hong-yun Chen, Fu-zhe Wang, Chang-quan Ling
- Image credit PLOS PLOS ONE Assessing the Quality of Reports about Randomized Controlled Trials of Acupuncture Treatment on Diabetic Peripheral Neuropathy July 2, 2012 Chen Bo, Zhao Xue, Guo Yi, Chen Zelin, Bai Yang, Wang Zixu, Wang Yajun
- Image credit 10.1371/journal.pone.0065442 PLOS ONE Reporting Quality of Social and Psychological Intervention Trials: A Systematic Review of Reporting Guidelines and Trial Publications May 29, 2013 Sean Grant, Evan Mayo-Wilson, G. J. Melendez-Torres, Paul Montgomery
- Image credit 10.1371/journal.pone.0084779 PLOS ONE Are Reports of Randomized Controlled Trials Improving over Time? A Systematic Review of 284 Articles Published in High-Impact General and Specialized Medical Journals December 31, 2013 Matthew To, Jennifer Jones, Mohamed Emara, Alejandro Jadad
- Image credit 10.1371/journal.pone.0086360 PLOS ONE Assessment of the Reporting Quality of Randomized Controlled Trials on Treatment of Coronary Heart Disease with Traditional Chinese Medicine from the Chinese Journal of Integrated Traditional and Western Medicine: A Systematic Review January 28, 2014 Fan Fang, Xu Qin, Sun Qi, Zhao Jun, Wang Ping, Guo Rui
- Image credit 10.1371/journal.pmed.1001666 PLOS Medicine Evidence for the Selective Reporting of Analyses and Discrepancies in Clinical Trials: A Systematic Review of Cohort Studies of Clinical Trials June 24, 2014 Kerry Dwan, Douglas Altman, Mike Clarke, Carrol Gamble, Julian Higgins, Jonathan Sterne, Paula Williamson, Jamie Kirkham
- Image credit 10.1371/journal.pone.0110229 PLOS ONE Systematic Evaluation of the Patient-Reported Outcome (PRO) Content of Clinical Trial Protocols October 15, 2014 Derek Kyte, Helen Duffy, Benjamin Fletcher, Adrian Gheorghe, Rebecca Mercieca-Bebber, Madeleine King, Heather Draper, Jonathan Ives, Michael Brundage, Jane Blazeby, Melanie Calvert
- Image credit 10.1371/journal.pone.0110216 PLOS ONE Patient-Reported Outcome (PRO) Assessment in Clinical Trials: A Systematic Review of Guidance for Trial Protocol Writers October 15, 2014 Melanie Calvert, Derek Kyte, Helen Duffy, Adrian Gheorghe, Rebecca Mercieca-Bebber, Jonathan Ives, Heather Draper, Michael Brundage, Jane Blazeby, Madeleine King
- Image credit 10.1371/journal.pmed.1000261 PLOS Medicine Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT Statement June 8, 2010 Hugh MacPherson, Douglas G. Altman, Richard Hammerschlag, Li Youping, Wu Taixiang, Adrian White, David Moher, on behalf of the STRICTA Revision Group
- Image credit PLOS PLOS Medicine Comparative Effectiveness Research: Challenges for Medical Journals April 27, 2010 Harold C. Sox, Mark Helfand, Jeremy Grimshaw, Kay Dickersin, the PLoS Medicine Editors , David Tovey, J. André Knottnerus, Peter Tugwell
- Image credit PLOS PLOS Medicine Reporting of Systematic Reviews: The Challenge of Genetic Association Studies June 26, 2007 Muin J Khoury, Julian Little, Julian Higgins, John P. A Ioannidis, Marta Gwinn
- Image credit 10.1371/journal.pmed.0040078 PLOS Medicine Epidemiology and Reporting Characteristics of Systematic Reviews March 27, 2007 David Moher, Jennifer Tetzlaff, Andrea C Tricco, Margaret Sampson, Douglas G Altman
- Image credit 10.1371/journal.pone.0027611 PLOS ONE From QUOROM to PRISMA: A Survey of High-Impact Medical Journals’ Instructions to Authors and a Review of Systematic Reviews in Anesthesia Literature November 16, 2011 Kun-ming Tao, Xiao-qian Li, Qing-hui Zhou, David Moher, Chang-quan Ling, weifeng yu
- Image credit 10.1371/journal.pone.0075122 PLOS ONE Testing the PRISMA-Equity 2012 Reporting Guideline: the Perspectives of Systematic Review Authors October 10, 2013 Belinda Burford, Vivian Welch, Elizabeth Waters, Peter Tugwell, David Moher, Jennifer O'Neill, Tracey Koehlmoos, Mark Petticrew
- Image credit 10.1371/journal.pone.0092508 PLOS ONE The Quality of Reporting Methods and Results in Network Meta-Analyses: An Overview of Reviews and Suggestions for Improvement March 26, 2014 Brian Hutton, Georgia Salanti, Anna Chaimani, Deborah Caldwell, Chris Schmid, Kristian Thorlund, Edward Mills, Lucy Turner, Ferran Catala-Lopez, Doug Altman, David Moher
- Image credit 10.1371/journal.pone.0096407 PLOS ONE Blinded by PRISMA: Are Systematic Reviewers Focusing on PRISMA and Ignoring Other Guidelines? May 1, 2014 Padhraig Fleming, Despina Koletsi, Nikolaos Pandis
- Image credit 10.1371/journal.pmed.1000097 PLOS Medicine Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement July 21, 2009 David Moher, Alessandro Liberati, Jennifer Tetzlaff, Douglas G. Altman, The PRISMA Group
- Image credit 10.1371/journal.pmed.1001333 PLOS Medicine PRISMA-Equity 2012 Extension: Reporting Guidelines for Systematic Reviews with a Focus on Health Equity October 30, 2012 Vivian Welch, Mark Petticrew, Peter Tugwell, David Moher, Jennifer O'Neill, Elizabeth Waters, Howard White
- Image credit 10.1371/journal.pmed.1001419 PLOS Medicine PRISMA for Abstracts: Reporting Systematic Reviews in Journal and Conference Abstracts April 9, 2013 Elaine Beller, Paul Glasziou, Douglas Altman, Sally Hopewell, Hilda Bastian, Iain Chalmers, Peter Gotzsche, Toby Lasserson, David Tovey
- Image credit PLOS PLOS Medicine Many Reviews Are Systematic but Some Are More Transparent and Completely Reported than Others March 27, 2007 The PLoS Medicine Editors
- Image credit 10.1371/journal.pmed.1000100 PLOS Medicine The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration July 21, 2009 Alessandro Liberati, Douglas G. Altman, Jennifer Tetzlaff, Cynthia Mulrow, Peter C. Gøtzsche, John P. A. Ioannidis, Mike Clarke, P. J. Devereaux, Jos Kleijnen, David Moher
- Image credit 10.1371/journal.pone.0007753 PLOS ONE Quality and Reporting of Diagnostic Accuracy Studies in TB, HIV and Malaria: Evaluation Using QUADAS and STARD Standards November 13, 2009 Patricia Scolari Fontela, Nitika Pant Pai, Ian Schiller, Nandini Dendukuri, Andrew Ramsay, Madhukar Pai
- Image credit 10.1371/journal.pmed.1001531 PLOS Medicine Use of Expert Panels to Define the Reference Standard in Diagnostic Research: A Systematic Review of Published Methods and Reporting October 15, 2013 Loes Bertens, Berna Broekhuizen, Christiana Naaktgeboren, Frans Rutten, Arno Hoes, Yvonne van Mourik, Karel Moons, Johannes Reitsma
- Image credit 10.1371/journal.pone.0085908 PLOS ONE The Assessment of the Quality of Reporting of Systematic Reviews/Meta-Analyses in Diagnostic Tests Published by Authors in China January 21, 2014 Long Ge, Jian-cheng Wang, Jin-long Li, Li Liang, Ni An, Xin-tong Shi, Yin-chun Liu, JH Tian
- Image credit 10.1371/journal.pmed.1000420 PLOS Medicine Strengthening the Reporting of Genetic Risk Prediction Studies: The GRIPS Statement March 15, 2011 A. Cecile J. W. Janssens, John P. A. Ioannidis, Cornelia M. van Duijn, Julian Little, Muin J. Khoury, for the GRIPS Group
- Image credit 10.1371/journal.pmed.1001216 PLOS Medicine Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration May 29, 2012 Doug Altman, Lisa McShane, Willi Sauerbrei, Sheila Taube
- Image credit 10.1371/journal.pmed.1001381 PLOS Medicine Prognosis Research Strategy (PROGRESS) 3: Prognostic Model Research February 5, 2013 Ewout Steyerberg, Karel Moons, Danielle van der Windt, Jill Hayden, Pablo Perel, Sara Schroter, Richard Riley, Harry Hemingway, Douglas Altman
- Image credit 10.1371/journal.pmed.1001380 PLOS Medicine Prognosis Research Strategy (PROGRESS) 2: Prognostic Factor Research February 5, 2013 Richard Riley, Jill Hayden, Ewout Steyerberg, Karel Moons, Keith Abrams, Panayiotis Kyzas, Nuria Malats, Andrew Briggs, Sara Schroter, Douglas Altman, Harry Hemingway
- Image credit 10.1371/journal.pmed.1001671 PLOS Medicine Improving the Transparency of Prognosis Research: The Role of Reporting, Data Sharing, Registration, and Protocols July 8, 2014 George Peat, Richard Riley, Peter Croft, Katherine Morley, Panayiotis Kyzas, Karel Moons, Pablo Perel, Ewout Steyerberg, Sara Schroter, Douglas Altman, Harry Hemingway
- Image credit 10.1371/journal.pone.0007824 PLOS ONE Survey of the Quality of Experimental Design, Statistical Analysis and Reporting of Research Using Animals November 30, 2009 Carol Kilkenny, Nick Parsons, Ed Kadyszewski, Michael F. W. Festing, Innes C. Cuthill, Derek Fry, Jane Hutton, Douglas G. Altman
- Image credit 10.1371/journal.pmed.1001489 PLOS Medicine Threats to Validity in the Design and Conduct of Preclinical Efficacy Studies: A Systematic Review of Guidelines for In Vivo Animal Experiments July 23, 2013 Valerie Henderson, Jonathan Kimmelman, Dean Fergusson, Jeremy Grimshaw, Dan Hackam
- Image credit 10.1371/journal.pone.0088266 PLOS ONE Five Years MIQE Guidelines: The Case of the Arabian Countries February 4, 2014 Afif Abdel Nour, Esam Azhar, Ghazi Damanhouri, Stephen Bustin
- Image credit 10.1371/journal.pone.0101131 PLOS ONE The Quality of Methods Reporting in Parasitology Experiments July 30, 2014 Oscar Flórez-Vargas, Michael Bramhall, Harry Noyes, Sheena Cruickshank, Robert Stevens, Andy Brass
- Image credit 10.1371/journal.pbio.1000412 PLOS Biology Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research June 29, 2010 Carol Kilkenny, William J. Browne, Innes C. Cuthill, Michael Emerson, Douglas G. Altman
- Image credit 10.1371/journal.pbio.1001481 PLOS Biology Whole Animal Experiments Should Be More Like Human Randomized Controlled Trials February 12, 2013 Beverly Muhlhausler, Frank Bloomfield, Matthew Gillman
- Image credit 10.1371/journal.pbio.1001756 PLOS Biology Two Years Later: Journals Are Not Yet Enforcing the ARRIVE Guidelines on Reporting Standards for Pre-Clinical Animal Studies January 7, 2014 David Baker, Katie Lidster, Ana Sottomayor, Sandra Amor
- Image credit PLOS PLOS Biology Reporting Animal Studies: Good Science and a Duty of Care June 29, 2010 Catriona J. MacCallum
- Image credit PLOS PLOS Biology Open Science and Reporting Animal Studies: Who’s Accountable? January 7, 2014 Catriona MacCallum, Jonathan Eisen, Emma Ganley
- Image credit PLOS PLOS Computational Biology Minimum Information About a Simulation Experiment (MIASE) April 28, 2011 Dagmar Waltemath, Richard Adams, Daniel A. Beard, Frank T. Bergmann, Upinder S. Bhalla, Randall Britten, Vijayalakshmi Chelliah, Michael T. Cooling, Jonathan Cooper, Edmund J. Crampin, Alan Garny, Stefan Hoops, Michael Hucka, Peter Hunter, Edda Klipp, Camille Laibe, Andrew K. Miller, Ion Moraru, David Nickerson, Poul Nielsen, Macha Nikolski, Sven Sahle, Herbert M. Sauro, Henning Schmidt, Jacky L. Snoep, Dominic Tolle, Olaf Wolkenhauer, Nicolas Le Novère
- Image credit 10.1371/journal.pmed.0050139 PLOS Medicine Guidelines for Reporting Health Research: The EQUATOR Network’s Survey of Guideline Authors June 24, 2008 Iveta Simera, Douglas G Altman, David Moher, Kenneth F Schulz, John Hoey
- Image credit 10.1371/journal.pmed.1000217 PLOS Medicine Guidance for Developers of Health Research Reporting Guidelines February 16, 2010 David Moher, Kenneth F. Schulz, Iveta Simera, Douglas G. Altman
- Image credit 10.1371/journal.pone.0035621 PLOS ONE Are Peer Reviewers Encouraged to Use Reporting Guidelines? A Survey of 116 Health Research Journals April 27, 2012 Allison Hirst, Douglas Altman
- Image credit PLOS PLOS Neglected Tropical Diseases Research Ethics and Reporting Standards at PLoS Neglected Tropical Diseases October 31, 2007 Gavin Yamey
- Image credit PLOS PLOS Medicine Better Reporting, Better Research: Guidelines and Guidance in PLoS Medicine April 29, 2008 The PLoS Medicine Editors
- Image credit CCAC North Library, Flickr.com PLOS Medicine Better Reporting of Scientific Studies: Why It Matters August 27, 2013 The PLoS Medicine Editors
- Image credit 10.1371/journal.pone.0097492 PLOS ONE Do Editorial Policies Support Ethical Research? A Thematic Text Analysis of Author Instructions in Psychiatry Journals June 5, 2014 Daniel Strech, Courtney Metz, Hannes Knüppel
Reporting Guidelines
It is important that your manuscript gives a clear and complete account of the research that you have done. Well reported research is more useful and complete reporting allows editors, peer reviewers and readers to understand what you did and how.
Poorly reported research can distort the literature, and leads to research that cannot be replicated or used in future meta-analyses or systematic reviews.
You should make sure that you manuscript is written in a way that the reader knows exactly what you did and could repeat your study if they wanted to with no additional information. It is particularly important that you give enough information in the methods section of your manuscript.
To help with reporting your research, there are reporting guidelines available for many different study designs. These contain a checklist of minimum points that you should cover in your manuscript. You should use these guidelines when you are preparing and writing your manuscript, and you may be required to provide a completed version of the checklist when you submit your manuscript.
The EQUATOR (Enhancing the Quality and Transparency Of health Research) Network is an international initiative that aims to improve the quality of research publications. It provides a comprehensive list of reporting guidelines and other material to help improve reporting.
A list full of all of the reporting guidelines endorsed by the EQUATOR Network can be found here . Some of the reporting guidelines for common study designs are:
- Randomized controlled trials – CONSORT
- Systematic reviews – PRISMA
- Observational studies – STROBE
- Case reports – CARE
- Qualitative research – COREQ
- Pre-clinical animal studies – ARRIVE
Peer reviewers may be asked to use these checklists when assessing your manuscript. If you follow these guidelines, editors and peer reviewers will be able to assess your manuscript better as they will more easily understand what you did. It may also mean that they ask you for fewer revisions.
Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals
- 1 Executive Managing Editor, JAMA and the JAMA Network, Chicago, Illinois
- 2 Deputy Managing Editor, JAMA Network, Chicago, Illinois
- 3 Managing Editor, JAMA , Chicago, Illinois
- Editorial The Reporting of Race and Ethnicity in Medical and Science Journals Annette Flanagin, RN, MA; Tracy Frey, BA; Stacy L. Christiansen, MA; Howard Bauchner, MD JAMA
- Editorial Guidance on Race, Ethnicity, and Origin as Proxies for Genetic Ancestry in Biomedical Publication W. Gregory Feero, MD, PhD; Robert D. Steiner, MD; Anne Slavotinek, MBBS, PhD; Tiago Faial, PhD; Michael J. Bamshad, MD; Jehannine Austin, PhD, CGC; Bruce R. Korf, MD, PhD; Annette Flanagin, RN, MA; Kirsten Bibbins-Domingo, PhD, MD, MAS JAMA
The goal of this guidance is to provide recommendations and suggestions that encourage fairness, equity, consistency, and clarity in use and reporting of race and ethnicity in medical and science journals. As previously summarized, “terminology, usage, and word choice are critically important, especially when describing people and when discussing race and ethnicity. Inclusive language supports diversity and conveys respect. Language that imparts bias toward or against persons or groups based on characteristics or demographics must be avoided.” 1
With the publication of an earlier version of this guidance, 1 comments were invited, and helpful assessments and comments were received from numerous reviewers, scholars, and researchers, who provided valuable feedback and represented diverse expertise and opinions. After thorough review of these comments (some of which did not agree with others) and additional research and discussion, the guidance was revised and updated, and additional formal review was obtained. In this Editorial, we present the updated guidance, and we sincerely thank the many reviewers for their contributions, each of whom are listed in the Acknowledgment at the end of this article.
This guidance continues to acknowledge that race and ethnicity are social constructs as well as the important sensitivities and controversies related to use of these terms and associated nomenclature in medical and health research, education, and practice. Thus, for content published in medical journals, language and terminology must be accurate, clear, and precise, and must reflect fairness, equity, and consistency in use and reporting of race and ethnicity. The guidance also acknowledges that the reporting of race and ethnicity should not be considered in isolation and should be accompanied by reporting of other sociodemographic factors and social determinants, including concerns about racism, disparities, and inequities, and the intersectionality of race and ethnicity with these other factors.
The guidance defines commonly used terms associated with race and ethnicity and acknowledges that these terms and definitions have changed, that some are out of date, and that the nomenclature will continue to evolve. Other topics addressed include relevant concerns and controversies in health care and research, including the intersectionality of ancestry and heritage, social determinants of health, and other socioeconomic, structural, institutional, cultural, and demographic factors; reporting of race and ethnicity in research articles; use of racial and ethnic collective or umbrella terms, capitalization, and abbreviations; listing racial and ethnic categories in alphabetical order vs order by majority; adjectival vs noun usage for categories of race and ethnicity; geographic origin and regionalization considerations; and brief guidance for journals and publishers that collect demographic data on editors, authors, and peer reviewers. Examples are provided to help guide authors and editors.
This updated guidance on reporting race and ethnicity is being added to the Inclusive Language section of the AMA Manual of Style: A Guide for Authors and Editors as subsection 11.2.3, Race and Ethnicity (see below). 2 This section of the Correct and Preferred Usage chapter of the style manual also includes subsections that address reporting concerns and preferred nomenclature for sex and gender, sexual orientation, age, socioeconomic status, and persons with diseases, disorders, or disabilities. Each of these subsections also will be reviewed and updated.
With this publication, this guidance will be freely available on the JAMA website and in the AMA Manual of Style , 2 and linked from the JAMA Network journals’ Instructions for Authors. Others are welcome to use and cite this guidance.
This guidance is not intended to be final but is presented with the understanding that monitoring will continue, and further updates will be provided as needed. Continual review of the terms and language used in the reporting of race and ethnicity is critically important as societal norms continue to evolve.
Section 11.12.3. Race and Ethnicity
Race and ethnicity are social constructs, without scientific or biological meaning. The indistinct construct of racial and ethnic categories has been increasingly acknowledged, and concerns about use of these terms in medical and health research, education, and practice have been progressively recognized. Accordingly, for content published in medical and science journals, language and terminology must be accurate, clear, and precise and must reflect fairness, equity, and consistency in use and reporting of race and ethnicity. (Note: historically, although inappropriately, race may have been considered a biological construct; thus, older content may characterize race as having biological significance.)
One of the goals of this guidance is to encourage the use of language to reduce unintentional bias in medical and science literature. The reporting of race and ethnicity should not be considered in isolation but should be accompanied by reporting of other sociodemographic factors and social determinants, including concerns about racism, disparities, and inequities, and the intersectionality of race and ethnicity with these other factors.
When reporting the results of research that includes racial and ethnic disparities and inequities, authors are encouraged to provide a balanced, evidence-based discussion of the implications of the findings for addressing institutional racism and structural racism as these affect the study population, disease or disorder studied, and the relevant health care systems. For example, Introduction and Discussion sections of manuscripts could include implications of historical injustices when describing the differences observed by race and ethnicity. Such discussion of implications can use specific words, such as racism , structural racism , racial equity , or racial inequity , when appropriate.
Definitions
The definitions provided herein focus on reporting race and ethnicity. Definitions of broader terms (eg, disparity, inequity, intersectionality, and others) will be included in the overarching Inclusive Language section that contains this subsection.
“Race and ethnicity are dynamic, shaped by geographic, cultural, and sociopolitical forces.” 3 Race and ethnicity are social constructs and with limited utility in understanding medical research, practice, and policy. However, the terms may be useful as a lens through which to study and view racism and disparities and inequities in health, health care, and medical practice, education, and research. 3 - 5 Terms and categories used to define and describe race and ethnicity have changed with time based on sociocultural shifts and greater awareness of the role of racism in society. This guidance is presented with that understanding, and updates have been and will continue to be provided as needed.
The terms race (first usage dating back to the 1500s) and ethnicity (first usage dating back to the late 1700s) 6 have changed and continue to evolve semantically. The Oxford English Dictionary currently defines race as “a group of people connected by common descent or origin” or “any of the (putative) major groupings of mankind, usually defined in terms of distinct physical features or shared ethnicity” and ethnicity as “membership of a group regarded as ultimately of common descent, or having a common national or cultural tradition.” 7 For example, in the US, ethnicity has referred to Hispanic or Latino, Latina, or Latinx people. Outside of the US, other terms of ethnicity may apply within specific nations or ancestry groups. As noted in a lexicographer’s post on the Conscious Style Guide , race and ethnicity are difficult to untangle. 6 In general, ethnicity has historically referred to a person’s cultural identity (eg, language, customs, religion) and race to broad categories of people that are divided arbitrarily but based on ancestral origin and physical characteristics. 6 Definitions that rely on external determinations of physical characteristics are problematic and may perpetuate racism. In addition, there is concern about whether these and other definitions are appropriate or out-of-date 8 and whether separation of subcategories of race from subcategories of ethnicity could be discriminatory, especially when used by governmental agencies and institutions to guide policy, funding allocations, budgets, and data-driven business and research decisions. 9 Thus, proposals have been made that these terms be unified into an aggregate, mutually exclusive set of categories as in “race and ethnicity.” 10 (See Additional Guidance for Use of Racial and Ethnic Collective Terms.)
The term ancestry refers to a person’s country or region of origin or an individual’s lineage of descent. Another characteristic of many populations is genetic admixture , which refers to genetic exchange among people from different ancestries and may correlate with an individual’s risk for certain genetic diseases. 3 Ancestry and genetic admixture may provide more useful information about health, population health, and genetic variants and risk for disease or disorders than do racial and ethnic categories. 3
Although race and ethnicity have no biological meaning, the terms have important, albeit contested, social meanings. Neglecting to report race and ethnicity in health and medical research disregards the reality of social stratification, injustices, and inequities and implications for population health, 3 , 4 and removing race and ethnicity from research may conceal health disparities. Thus, inclusion of race and ethnicity in reports of medical research to address and further elucidate health disparities and inequities remains important at this time.
According to the “Health Equity Style Guide for the COVID-19 Response: Principles and Preferred Terms for Non-Stigmatizing, Bias-Free Language” of the Centers for Disease Control and Prevention (CDC), racism is defined as a “system of structuring opportunity and assigning value based on the social interpretation of how one looks...(“race”), that unfairly disadvantages some individuals and communities, unfairly advantages other individuals and communities, and undermines realization of the full potential of our whole society through the waste of human resources.” 11 Note that racism and prejudice can occur without phenotypic discrimination.
Jones 12 and the CDC style guide 11 have defined 3 levels of racism.
Systemic, institutionalized, and structural racism : “Structures, policies, practices, and norms resulting in differential access to the goods, services, and opportunities of society by ‘race’ (eg, how major systems—the economy, politics, education, criminal justice, health, etc—perpetuate unfair advantage).” 11 The Associated Press (AP) Stylebook advises to not shorten these terms to “racism,” to avoid confusion with the other definitions. 13
Interpersonal and personally mediated racism : “Prejudice and discrimination, where prejudice is differential assumptions about the abilities, motives, and intents of others by ‘race,’ and discrimination is differential actions towards others by ‘race.’ These can be either intentional or unintentional.” 11
Internalized racism : “Acceptance by members of the stigmatized ‘races’ of negative messages about their own abilities and intrinsic worth.” 11
Concerns, Sensitivities, and Controversies in Health Care and Research
There are many examples of reported associations between race and ethnicity and health outcomes, but these outcomes may also be intertwined with ancestry and heritage, social determinants of health, as well as socioeconomic, structural, institutional, cultural, demographic, or other factors. 3 , 4 , 14 Thus, discerning the roles of these factors is difficult. For example, a person’s ancestral heritage may convey certain health-related predispositions (eg, cystic fibrosis in persons of Northern European descent and sickle cell disease reported among people whose ancestors were from sub-Saharan Africa, India, Saudi Arabia, and Mediterranean countries); however, such perceptions have resulted in underdiagnosis of these conditions in other populations. 15
Also, certain groups may bear a disproportionate burden of disease compared with other groups, but this may reflect individual and systemic disparities and inequities in health care and social determinants of health. For example, according to the US National Cancer Institute, the rates of cervical cancer are higher among Hispanic/Latina women and Black/African American women than among women of other racial or ethnic groups, with Black/African American women having the highest rates of death from the disease, but social determinants of health and inequities are also associated with a high prevalence of cervical cancer among these women. 16 The American Heart Association summarizes similar disparities in cardiovascular disease among Black individuals in the US compared with those from other racial and ethnic groups. 17
Identifying the race or ethnicity of a person or group of participants, along with other sociodemographic variables, may provide information about participants included in a study and the potential generalizability of the results of a study and may identify important disparities and inequities. Researchers should aim for inclusivity by providing comprehensive categories and subcategories where applicable. Many people may identify with more than 1 race and ethnicity; therefore, categories should not be considered absolute or viewed in isolation.
However, there is concern about the use of race in clinical algorithms and some health-based risk scores and databases because of inapplicability to some groups and the potential for discrimination and inappropriate clinical decisions. For example, the use of race to estimate glomerular filtration rates among Black adults has become controversial for several reasons. 18 - 21 Oversimplification of racial dichotomies can be harmful, such as in calculating kidney function, especially with racial inequities in kidney care. In this context, health inequities among populations should be addressed rather than focusing solely on differences in racial categories (eg, Black vs White adults with kidney disease). 21 Another example is the Framingham Risk Score, which was originally developed from a cohort of White, middle-class participants in the US included in the Framingham Heart Study and may not accurately estimate risk in other racial and ethnic populations. Similar concerns have been raised about genetic risk studies based on specific populations or that do not include participants from other groups (eg, a genome-wide association study that reports a genetic association with a specific disease or disorder based solely on a population of European descent). 22 Use caution in interpreting or generalizing findings from studies of risk based on populations of individuals representing specific or limited racial and ethnic categories.
Guidance for Reporting Race and Ethnicity in Research Articles
The JAMA Network journals include the following guidance for reporting race and ethnicity and other demographic information in research articles in the Instructions for Authors. 23
Demographic Information
Aggregate, deidentified demographic information (eg, age, sex, race and ethnicity, and socioeconomic indicators) should be reported for research reports along with all prespecified outcomes. Demographic variables collected for a specific study should be indicated in the Methods section. Demographic information assessed should be reported in the Results section, either in the main article or in an online supplement or both. If any demographic characteristics that were collected are not reported, the reason should be stated. Summary demographic information (eg, baseline characteristics of study participants) should be reported in the first line of the Results section of the Abstract.
With regard to the collection and reporting of demographic data on race and ethnicity:
The Methods section should include an explanation of who identified participant race and ethnicity and the source of the classifications used (eg, self-report or selection, investigator observed, database, electronic health record, survey instrument).
If race and ethnicity categories were collected for a study, the reasons that these were assessed also should be described in the Methods section. If collection of data on race and ethnicity was required by the funding agency, that should be noted.
Specific racial and ethnic categories are preferred over collective terms, when possible. Authors should report the specific categories used in their studies and recognize that these categories will differ based on the databases or surveys used, the requirements of funders, and the geographic location of data collection or study participants. Categories included in groups labeled as “other” should be defined.
Categories should be listed in alphabetical order in text and tables.
Race and ethnicity categories of the study population should be reported in the Results section.
Reporting race and ethnicity in this study was mandated by the US National Institutes of Health (NIH), consistent with the Inclusion of Women, Minorities, and Children policy. Individuals participating in the poststudy survey were categorized as American Indian or Alaska Native, Asian, Black or African American, Hispanic or Latino, Native Hawaiian or Other Pacific Islander, or White based on the NIH Policy on Reporting Race and Ethnicity Data. Children’s race and ethnicity were based on the parents’ report. Race was self-reported by study participants, and race categories (Black and White) were defined by investigators based on the US Office of Management and Budget’s Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. Given that racial residential segregation is distinctively experienced by Black individuals in the US, the analytical sample was restricted to participants who self-identified as Black. In this genome-wide association study, participants were from 8 African countries (ie, Kenya, Mozambique, Namibia, Nigeria, South Africa, Sudan, Uganda, and Zambia). Any Black African group from any of the 8 African countries (mostly of Bantu descent) was included in the Black African cohort. The South African group composed primarily of multiple racial categories, comprising any admixture combination of individuals of European, Southeast Asian, South Asian, Bantu-speaking African, and/or indigenous Southern African hunter-gatherer ancestries (Khoikhoi, San, or Bushmen), was renamed admixed African individuals. The race and ethnicity of an individual was self-reported. Data for this study included US adults who self-reported as non-Hispanic Black (hereafter, Black), Hispanic or Latino, and non-Hispanic White (hereafter, White) individuals. We excluded individuals who self-reported being Asian or of other race and ethnicity (which included those who were American Indian or Alaska Native and Native Hawaiian or Other Pacific Islander) because of small sample sizes.
Additional Guidance for Use of Racial and Ethnic Collective Terms
Specific racial and ethnic terms are preferred over collective terms, when possible. Authors should report the specific categories used in their studies and recognize that these categories will differ based on the databases or surveys used, the requirements of funders, and the geographic location of data collection or study participants.
When collective terms are used, merging of race and ethnicity with a virgule as “race/ethnicity” is no longer recommended. Instead, “race and ethnicity” is preferred, with the understanding that there are numerous subcategories within race and ethnicity. Given that a virgule often means “and/or,” which can be confusing, do not use the virgule construction in this context (see also 8.4, Forward Slash [Virgule, Solidus]).
The general term minorities should not be used when describing groups or populations because it is overly vague and implies a hierarchy among groups. Instead, include a modifier when using the word “minority” and do not use the term as a stand-alone noun, for example, racial and ethnic minority groups and racial and ethnic minority individuals . 11 , 24 However, even this umbrella term may not be appropriate in some settings. Other terms such as underserved populations (eg, when referring to health disparities among groups) or underrepresented populations (eg, when referring to a disproportionately low number of individuals in a workforce or educational program) may be used provided the categories of individuals included are defined at first mention. 25 The term minoritized may be acceptable as an adjective provided that the noun(s) that it is modifying is included (eg, “racial and ethnic minoritized group”). Groups that have been historically marginalized could be suitable in certain contexts if the rationale for this designation is provided and the categories of those included are defined or described at first mention. 11
The nonspecific group label “other” for categorizing race and ethnicity is uninformative and may be considered pejorative. However, the term is sometimes used for comparison in data analysis when the numbers of those in some subgroups are too small for meaningful analyses. The term should not be used as a “convenience” grouping or label unless it was a prespecified formal category in a database or research instrument. In such cases, the categories included in “other” groups should be defined and reported. Authors are advised to be as specific as possible when reporting on racial and ethnic categories (even if these categories contain small numbers). If the numbers in some categories are so small as to potentially identify study participants, the specific numbers and percentages do not need to be reported provided this is noted. For cases in which the group “other” is used but not defined, the author should be queried for further explanation.
The terms multiracial and multiethnic are acceptable in reports of studies if the specific categories these terms comprise are defined or if the terms were predefined in a study or database to which participants self-selected. If the criteria for data quality and confidentiality are met, at a minimum, the number of individuals identifying with more than 1 race should be reported. Authors are encouraged to provide greater detail about the distribution of multiple racial and ethnic categories if known. In general, the term mixed race may carry negative connotations 13 and should be avoided, unless it was specifically used in data collection; in this case, the term should be defined, if possible. To the extent possible, the specific type of multiracial and multiethnic groups should be delineated.
In this study, 140 participants (25%) self-reported as multiracial, which included 100 (18%) identifying as Asian and White and 40 (7%) as Black and White.
Other terms may enter the lexicon as descriptors or modifiers for racial and ethnic categories of people. For example, the term people of color was introduced to mean all racial and ethnic groups that are not considered White or of European ancestry and as an indication of antiracist, multiracial solidarity. However, there is concern that the term may be “too inclusive,” to the point that it erases differences among specific groups. 13 , 26 - 28 There are similar concerns about use of the collective and abbreviated terms for Black, Indigenous, and people of color ( BIPOC ) and Black, Asian, and minority ethnic ( BAME ) (commonly used in the UK). Criticism of these terms has noted that they disregard individuals’ identities, do not include all underrepresented groups, eliminate differences among groups, and imply a hierarchy among them. 13 , 26 - 28 Although these terms may be used colloquially (eg, within an opinion article), preference is to describe or define the specific racial or ethnic categories included or intended to be addressed. These terms should not be used in reports of research, unless the terms are included in a database on which a study is based or specified in a research data collection instrument (eg, survey questionnaire).
In agreement with other guides, 13 , 24 other terms related to colors, such as brown and yellow , should not be used to describe individuals or groups. These terms may be less inclusive than intended or considered pejorative or a racial slur.
In addition, avoid collective reference to racial and ethnic minority groups as “non-White.” If comparing racial and ethnic groups, indicate the specific groups. Researchers should avoid study designs and statistical comparisons of White groups vs “non-White” groups and should specify racial and ethnic groups included and conduct analyses comparing the specific groups. If such a comparison is justified, authors should explain the rationale and specify what categories are included in the “non-White” group.
Capitalization
The names of races, ethnicities, and tribes should be capitalized, such as African American, Alaska Native, American Indian, Asian, Black, Cherokee Nation, Hispanic, Kamba, Kikuyu, Latino, and White. There may be sociopolitical instances in which context may merit exception to this guidance, for example, in an opinion piece for which capitalization could be perceived as inflammatory or inappropriate (eg, “white supremacy”).
Adjectival Usage for Specific Categories
Racial and ethnic terms should not be used in noun form (eg, avoid Asians , Blacks , Hispanics , or Whites ); the adjectival form is preferred (eg, Asian women, Black patients , Hispanic children , or White participants ) because this follows AMA style regarding person-first language. The adjectival form may be used as a predicate adjective to modify the subject of a phrase (eg, “the patients self-identified as Asian, Black, Hispanic, or White”). 11
Most combinations of proper adjectives derived from geographic entities are not hyphenated when used as racial or ethnic descriptors. Therefore, do not hyphenate terms such as Asian American , African American , Mexican American, and similar combinations, and in compound modifiers (eg, African American patient ).
Geographic Origin and Regionalization Considerations
Awareness of the relevance of geographic origin and regionalization associated with racial and ethnic designations is important. In addition, preferred usage may change about the most appropriate designation. For example, the term Caucasian had historically been used to indicate the term White , but it is technically specific to people from the Caucasus region in Eurasia and thus should not be used except when referring to people from this region.
The terms African American or Black may be used to describe participants in studies involving populations in the US, following how such information was recorded or collected for the study. However, the 2 terms should not be used interchangeably in reports of research unless both terms were formally used in the study, and the terms should be used consistently within a specific article. For example, among Black people residing in the US, those from the Caribbean may identify as Black but not as African American, whereas Black people whose families have been in the US for several generations may identify as Black and African American. When a study includes individuals of African ancestry in the diaspora, the term Black may be appropriate because it does not obscure cultural and linguistic nuances and national origins, such as Dominican, Haitian, and those of African sovereign states (eg, Kenyan, Nigerian, Sudanese), provided that the term was used in the study.
The term Asian is a broad category that can include numerous countries of origin (eg, Cambodia, China, India, Japan, Korea, Malaysia, Pakistan, the Philippine Islands, Thailand, Vietnam, and others) and regions (eg, East Asia, South Asia, Southeast Asia). 29 The term may be combined with those from the Pacific Islands as in Asian or Pacific Islander . The term Asian American is acceptable when describing those who identify with Asian descent among the US population. However, reporting of individuals’ self-identified countries of origin is preferred when known. As with other categories, the formal terms used in research collection should be used in reports of studies.
In reference to persons indigenous to North America (and their descendants), American Indian or Alaska Native is generally preferred to the broader term Native American . However, the term Indigenous is also acceptable. There are also other specific designations for people from other locations, such as Native Hawaiian and Pacific Islander . 29 , 30 If appropriate, specify the nation or peoples (eg, Inuit , Iroquois , Mayan, Navajo , Nez Perce , Samoan ). Many countries have specific categories for Indigenous peoples (eg, First Nations in Canada and Aboriginal in Australia). Capitalize the first word and use lowercase for people when describing persons who are Indigenous or Aboriginal (eg, Indigenous people, Indigenous peoples of Canada , Aboriginal people ). Lowercase indigenous when referring to objects, such as indigenous plants .
Hispanic , Latino or Latina , Latinx , and Latine are terms that have been used for people living in the US of Spanish-speaking or Latin American descent or heritage, but as with other terms, they can include people from other geographic locations. 29 , 30 Hispanic historically has been associated with people from Spain or other Spanish-speaking countries in the Western hemisphere (eg, Cuba, Central and South America, Mexico, Puerto Rico); however, individuals and some government agencies may prefer to specify country of origin. 29 - 31 Latino or Latina are broad terms that have been used for people of origin or descent from Cuba, Mexico, Puerto Rico, and some countries in Central America, South America, and the Caribbean, but again, individuals may prefer to specify their country of origin. 29 - 31 When possible, a more specific term (eg, Cuban , Cuban American , Guatemalan, Latin American , Mexican , Mexican American , Puerto Rican ) should be used. However, as with other categories, the formal terms used in research collection should be used for reports of studies. For example, some US agencies also include Spanish origin when listing Hispanic and Latino. The terms Latinx and Latine are acceptable as gender-inclusive or nonbinary terms for people of Latin American cultural or ethnic identity in the US. However, editors should avoid reflexively changing Latino and Latina to Latinx or vice versa and should follow author preference. Authors of research reports, in turn, should use the terms that were prespecified in their study (eg, via participant self-report or selection, investigator observed, database, electronic health record, survey instrument).
Description of people as being of a regional descent (eg, of African, Asian, European, or Middle Eastern or North African descent) is acceptable if those terms were used in formal research. However, it is preferable to identify a specific country or region of origin when known and pertinent to the study.
For the genome-wide association study discovery stage, study participants of African ancestry were recruited from Ghana, Nigeria, South Africa, and the US, where the same phenotype definition was applied to diagnose primary open-angle glaucoma. The second validation meta-analysis included individuals with primary open-angle glaucoma and matched control individuals from Mali, Cameroon, Nigeria (Lagos, Kaduna, and Enugu), Brazil, Saudi Arabia, the Democratic Republic of the Congo, Morocco, and Peru.
For example, it is generally preferable to describe persons of Asian ancestry according to their country or regional area of origin (eg, Cambodian , Chinese , Indian , Japanese , Korean , Sri Lankan , East Asian , Southeast Asian ). Similarly, study participants from the Middle Eastern and North African region should be described using their nation of origin (eg, Egyptian , Iranian , Iraqi , Israeli , Lebanese ) when possible. Individuals of Middle Eastern and North African descent who identify with Arab ancestry and reside in the US may be referred to as Arab American . In such cases, researchers should report how categories were determined (eg, self-reported or selected by study participants or from demographic data in databases or other sources).
Note that Arab and Arab American, Asian and Asian American, Chinese and Chinese American , Mexican and Mexican American , and so on are not equivalent or interchangeable.
For studies that use national databases or include participants in a single country, a term for country of origin can be included if the term was provided at data collection (eg, Chinese American and Korean American for a study performed in the US, or Han Chinese and Zhuang Chinese for a study conducted in China). Again, how these designations were determined (eg, self-reported or selected or by other means) should be reported.
Abbreviations
Generally, abbreviations of categories for race and ethnicity should be avoided unless necessary because of space constraints (eg, in tables and figures) or to avoid long, repetitive strings of descriptors. If used, any abbreviations should be clearly explained parenthetically in text or in table and figure footnotes or legends.
Guidance for Journals and Publishers That Collect Data on Editors, Authors, and Peer Reviewers
Journals and publishers that collect race and ethnicity data on editors, editorial board members, authors, and peer reviewers should follow principles of confidentiality, privacy, and inclusivity and should permit individuals to self-identify or opt out of such identification. The Joint Commitment on Action for Inclusion and Diversity in Publishing is developing an international list of terms for journals and publishers that collect information on race and ethnicity. 32
Journals that collect information on race and ethnicity should not permit editorial decisions to be influenced by the demographic characteristics of authors, peer reviewers, editorial board members, or editors. In addition, the collection and use of such data should respect privacy regulations and be secured to prevent disclosure of personally identifiable information. Individual personally identifiable information of authors and peer reviewers should not be accessible to anyone involved in editorial decisions. Such data may be used in aggregate to benchmark and monitor strategies to promote and improve the diversity of journals.
Corresponding Author: Annette Flanagin, RN, MA, JAMA and the JAMA Network, 330 N Wabash Ave, Chicago, IL 60611 ( [email protected] ).
Conflict of Interest Disclosures: Ms Christiansen is chair and Mss Flanagin and Frey are members of the AMA Manual of Style committee.
Members of the AMA Manual of Style Committee : Stacy L. Christiansen, MA, managing editor, JAMA ; Miriam Y. Cintron, BA, assistant managing editor, JAMA ; Angel Desai, MD, MPH, associate editor, JAMA Network Open , and assistant professor, University of California, Davis; Annette Flanagin, RN, MA, executive managing editor, JAMA and JAMA Network; Phil B. Fontanarosa, MD, MBA, interim editor in chief, JAMA and JAMA Network; Tracy Frey, BA, deputy managing editor, JAMA Network; Iris Y. Lo, BA, assistant deputy managing editor, JAMA Network; Connie Manno, ELS, freelance manuscript editing director, JAMA Network; and Christopher C. Muth, MD, senior editor, JAMA , and assistant professor, Rush University Medical Center.
Additional Contributions: We thank the following individuals for their thoughtful review and comments that helped shape this guidance: Nadia N. Abuelezam, ScD, Boston College William F. Connell School of Nursing; Adewole S. Adamson, MD, MPP, Associate Editor, JAMA Dermatology , University of Texas at Austin, Internal Medicine, Division of Dermatology; Dewesh Agrawal, MD, Children’s National Hospital, and Departments of Pediatrics and Emergency Medicine, George Washington University School of Medicine and Health Sciences; Kristine J. Ajrouch, PhD, Department of Sociology, Eastern Michigan University; Germine H. Awad, PhD, University of Texas at Austin; Maria Baquero, PhD, MPH, senior social epidemiologist, Research and Evaluation Unit, Bureau of Epidemiology Services, Division of Epidemiology, New York City Department of Health and Mental Hygiene; Aisha Barber, MD, MEd, Children’s National Hospital, and Department of Pediatrics, George Washington University School of Medicine and Health Sciences; Ashley Bennett, MD, Children's Hospital Los Angeles; Howard Bauchner, MD, former editor in chief, JAMA and JAMA Network; Khadijah Breathett, MD, MS, Department of Medicine, University of Arizona; Thuy D. Bui, MD, Department of Medicine, Global Health/Underserved Populations Track, University of Pittsburgh School of Medicine; Kim Campbell, BS, senior manuscript editor, JAMA ; Jessica Castner, PhD, RN-BC, editor in chief, Journal of Emergency Nursing ; Linh Hồng Chương, MPH, Department of Health Policy & Management, UCLA Fielding School of Public Health; Francisco Cigarroa, MD, Department of Surgery, Director, Transplant Center, UT Health, San Antonio, and JAMA editorial board member; Desiree M. de la Torre, MPH, MBA, Community Affairs & Population Health Improvement, Children’s National Hospital; Christopher P. Duggan, MD, MPH, Center for Nutrition at Boston Children's Hospital and Harvard Medical School; Torian Easterling, MD, New York City Department of Health and Mental Hygiene; Enrique Escalante, MD, MSHS, director of Diversity Recruitment and Inclusion, Children’s National Hospital, and Department of Pediatrics, George Washington University School of Medicine and Health Sciences; Lisa Evans, JD, Scientific Workforce Diversity Officer, National Institutes of Health; Olanrewaju O. Falusi, MD, Children’s National Hospital; Stephanie E. Farquhar, PhD, MHS, New York City Department of Health and Mental Hygiene; Glenn Flores, MD, Department of Pediatrics, University of Miami Miller School of Medicine, Holtz Children’s Hospital, Jackson Health System; Barbara Gastel, MD, MPH, Texas A&M College of Medicine, Humanities in Medicine; L. Hannah Gould, PhD, New York City Department of Health and Mental Hygiene; Carmen E. Guerra, MD, MSCE, vice chair of Diversity and Inclusion, Department of Medicine, Perelman School of Medicine, University of Pennsylvania; P. Michael Ho, MD, PhD, University of Colorado, Aurora; Said Ibrahim, MD, MPH, MBA, Division of Healthcare Delivery Science & Innovation, Weill Cornell Medicine; Omer Ilahi, MD, Southwest Orthopedic Group; Alireza Hamidian Jahromi, MD, Rush University Medical Center; Quita Highsmith, MBA, chief diversity officer, Genentech; Renee M. Johnson, PhD, MPH, Department of Mental Health, Johns Hopkins Bloomberg School of Public Health; Kamlesh Khunti, PhD, MD, University of Leicester; Trevor Lane, MA, DPhil, AsiaEdit; Cara Lichtenstein, MD, MPH, Children’s National Hospital; Francie Likis, DrPh, editor in chief, Journal of Midwifery & Women's Health ; Preeti N. Malani, MD, MSJ, associate editor, JAMA , and Department of Medicine, Division of Infectious Disease, University of Michigan; Jacqueline McGrath, PhD, RN, School of Nursing, UT Health San Antonio; Michelle Morse, MD, New York City Department of Health and Mental Hygiene; Brahmajee K. Nallamothu, MD, MPH, University of Michigan, Ann Arbor; Peter Olson, MA, manuscript editing coordinator, JAMA Network; Manish Pareek, MD, University of Leicester; Monica Peek, MD, MPH, Department of Medicine, Chicago Center for Diabetes Translational Research, University of Chicago; Jose S. Pulido, MD, MS, MPH, MBA, Wills Eye Hospital, and Departments of Ophthalmology and Molecular Medicine, Mayo Clinic; Zudin Puthucheary, MBBS, PhD, BMedSci, William Harvey Research Institute, Barts and The London School of Medicine & Dentistry, Queen Mary University of London, Royal London Hospital, Barts Health NHS Trust; Sanjay Ramakrishnan, MBBS, Nuffield Department of Medicine, University of Oxford; Jeanne M. Regnante, chief health equity and diversity officer, LUNGevity Foundation; Allie Reynolds, Princeton University; Ash Routen, PhD, University of Leicester; James B. Ruben, MD, Folsom, California (retired); Randall L. Rutta, National Health Council; Deborah Salerno, PhD, senior medical writer, Salerno Scientific; Kanade Shinkai, MD, editor, JAMA Dermatology , and Department of Dermatology, University of California San Francisco; Erica S. Spatz, MD, MHS, Yale School of Medicine; Jenna Rose Stoehr, BA, Northwestern University Feinberg School of Medicine; L. Tantay, MA, New York City Department of Health and Mental Hygiene; Ayanna Vasquez, MD, MS, New York City Department of Health and Mental Hygiene; and Jason H. Wasfy, MD, MPhil, Massachusetts General Hospital.
See More About
Flanagin A , Frey T , Christiansen SL , AMA Manual of Style Committee. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA. 2021;326(7):621–627. doi:10.1001/jama.2021.13304
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Guidelines for Reporting Your Research
Reporting standards, bias and language.
- Language Services and Resources for Non-Native English Speakers
ASHA encourages the use of relevant reporting guidelines to help promote the transparency and reproducibility of scientific research. Although the submission of completed checklists for the relevant guidelines (and flow diagram, if applicable) alongside your manuscript is not required, we do strongly encourage that clinical studies appearing in ASHA journals meet recognized standards for designing and implementing their studies and reporting the findings:
- Articles reporting randomized clinical trials should follow the Consolidated Standards of Reporting Trials ( CONSORT ).
- Nonrandomized clinical evaluations should follow the Transparent Reporting of Evaluations with Nonrandomized Designs ( TREND ) statement.
- Studies of diagnostic accuracy should meet the Standards for Reporting of Diagnostic Accuracy ( STARD ).
Additional standards and checklists may be relevant depending on the type of study conducted. Therefore, authors are encouraged to review the Enhancing the QUAlity and Transparency of health Research ( EQUATOR ) information in the Reporting Standards section
EQUATOR Network
Enhancing the QUAlity and Transparency of health Research, better known as EQUATOR, is an international initiative with aims very much in alignment the goals of the ASHA Journals Program . The EQUATOR Network mission is to achieve accurate, complete, and transparent reporting of all health research studies to support research reproducibility and usefulness. To support EQUATOR’s goals, ASHA encourages the use of a relevant reporting guideline when writing any communication sciences and disorders research manuscript. It is hoped that by utilizing the appropriate reporting guidelines, the quality of research reports will be improved, enabling easier evaluation and better clinical applicability.
We invite you to submit completed checklists for the relevant guidelines (and flow diagram if applicable) alongside your manuscript, indicating the manuscript page on which each checklist item is found. Editable checklists for reporting guidelines can be found on the EQUATOR Network site ( www.equator-network.org ), which also gives general information on how to choose the correct guideline and why guidelines are important. Using a checklist helps to ensure you have used a guideline correctly.
At minimum, your article should report the content addressed by each item of the identified checklist or state that the item was not considered in the study and, if relevant, the reason why not (for example, if you did not use anonymizing, your article should explain this). Meeting these basic reporting requirements will greatly improve the value of your manuscript, may facilitate/enhance the peer review process, and may enhance its chances for eventual publication.
While checklists are not required, we encourage you to complete a checklist because this helps you to check that you have included all of the important information in your article, and because our editors and reviewers will also be asked to make use of reporting guidelines when peer reviewing submitted manuscripts. If the checklist indicates an item that you have not addressed in your manuscript, please either explain in the manuscript text why this information is not relevant to your study or add the relevant information.
Common Study Types and Appropriate Guidelines
Some common study types and the appropriate guidelines are listed below. If you cannot find an appropriate guideline here, search the full EQUATOR database . You may need to use more than one guideline, depending on your research. For example, if you randomly assigned human participants to one of two interventions, then conducted unstructured interviews with each participant, you will need to use CONSORT , COREQ , and TIDIER together. To make sure you collect all of the relevant guidelines, check each major heading, even if you have already found a relevant guideline under a previous major heading.
If you are reporting a protocol
Use the SPIRIT guideline for the protocol of a clinical trial. Use the PRISMA-P guideline for the protocol of a systematic review.
If you are reporting a review of a section of the existing literature
Use the ENTREQ guideline for a review of studies that use descriptive data, such as unstructured interviews (qualitative data). Use the MOOSE guideline for a review of observational studies. The PRISMA guideline should be used only for systematic reviews.
If you are reporting on animal research
Use the ARRIVE (or the UK version ) guideline for research on animals in a lab.
If you are reporting descriptive data (either alone or alongside quantitative analysis)
Use the COREQ guideline for reporting unstructured interviews and focus groups. Use the CARE guideline for reporting one case study or a series of case studies. Use the SRQR guideline for any other descriptive data (qualitative research).
If you are reporting research into diagnosis
Use the STARD guideline if you compared the accuracy of a diagnostic test with an established reference standard test. Use the REMARK guideline if you evaluated the prognostic value of a biomarker. Use the TRIPOD guideline if you developed, validated, or updated a prognostic or diagnostic prediction modelling tool.
If you are reporting research into an intervention or treatment on people
Use the TIDIER guideline to fully describe your intervention. Use the CHEERS guideline for an economic evaluation of the interventions.
If you are reporting research into an intervention, treatment, exposure, or protective factor on people
Use the CARE guideline for reporting one case study or a series of case studies. If you selected your participants before they received the intervention/exposure/etc. under study, AND You controlled which intervention/exposure/etc. they each received, AND You used a random allocation method to decide which intervention/exposure/etc. they each received (i.e., a randomised controlled trial) THEN , use the CONSORT guideline or one of its extensions. If you selected your participants after they received the intervention/exposure/etc. under study, OR You selected your participants before they received the intervention/exposure/etc. under study AND you did not control which intervention/exposure/etc. they received (they decided/their doctor decided/life just happened) (i.e., an observational study), THEN , use the STROBE guideline or one of its extensions. If you selected your participants before they received the intervention/exposure/etc. under study, AND If CARE, CONSORT, and STROBE are not applicable to your research AND You used a non-random way to decide which intervention/exposure/etc. your participants received, such as which hospital they went to or what their clinical symptoms were. (i.e., a non-randomised trial) THEN , use the TREND guideline.
The ASHA Journals Program follows the Publication Manual of the American Psychological Association (7th ed.) style , which reminds authors to be mindful of using language that is free of bias—or the suggestion thereof. ASHA’s official statement on language and terminology related to disability and differing abilities is as follows:
ASHA adheres to the style guide of the American Psychological Association (APA) in using person-first or identity-first language to describe attributes and diagnoses of individuals or groups of people. When there is a preference, ASHA honors that preference. For more information, see APA’s style guidelines on bias-free language .
Per APA style, “It is unacceptable to use constructions that might imply prejudicial beliefs or perpetuate biased assumptions against persons on the basis of age, disability, gender, participation in research, racial or ethnic identity, sexual orientation, socioeconomic status, or some combination of these or other personal factors (e.g., marital status, immigration status, religion)” (p. 131).
However, when an author expresses a preference for identity-first language, ASHA honors that preference. Per APA style, “As always, good judgment is required—these are not rigid rules. If your writing reflects respect for your participants and your readers, and if you write with appropriate specificity and precision, you contribute to the goal of accurate, unbiased communication” (p. 131).
For many more details and examples on APA style recommendations for ensuring bias-free language—including entire sections on age, disability, gender, participation in research, racial and ethnic identity, sexual orientation and preference, socioeconomic status, and intersectionality—see APA’s online guide to bias-free language .
Bias Examples
Using the term "normal".
The use of “normal” to describe human beings is avoided because it suggests, incorrectly, that the other groups are somehow “abnormal.” Thus, ASHA rewords a phrase such as “normal participants” to “typical participants” to avoid describing a person as “normal.” In a similar way, we rephrase “normal-hearing participants” to “participants with normal hearing” to adhere to person-first language (which, in this instance, would be the most acceptable style).
APA discusses this in its section on false hierarchies in the chapter on bias-free language: “Compare groups with care. Bias occurs when authors use one group (often their own group) as the standard against which others are judged (e.g., using citizens of the United States as the standard without specifying why that group was chosen). For example, usage of “normal” may prompt readers to make the comparison with “abnormal,” thus stigmatizing individuals with differences” (p. 134).
What is normal, anyway?
The University of Kansas University Research & Training Center on Independent Living has this to say on the portrayal of disabled people and the question of what normal really is: “About one in five Americans has a disability. . . . People with disabilities, like all people, have strengths and weaknesses, successes and failures, and hopes and dreams. People with disabilities are the largest minority group in America, and the only group that any person can join at any time—through disease, traumatic event, or other reason. Like other minority groups, there is much diversity within the disability community, and people with disabilities don’t want to be stereotyped when their stories are told. . . . By following these guidelines, you can contribute to this momentum, portraying people with disabilities in an objective, authentic way” (from Guidelines: How to Write About People With Disabilities , 9 th ed.) .
For more information, see APA’s online General Principles for Reducing Bias .
Gender Breakdown of Participants
In listing the gender breakdown of a group of participants, authors should list not only the number of male participants but also the number of female participants, even if the number of female participants can be safely assumed. For example, “30 participants (10 men)” would be expanded to “30 participants (10 men, 20 women).”
Gender Identity and Sexual Orientation
To ensure that your language is free of bias related to gender identity and sexual orientation, we recommend that you adhere to APA style principles that address this topic. As stated in the APA Publication Manual, “Gender offers an added layer of specificity when interpreting patterns or phenomena of human behavior. However, the terms related to gender and sex are often conflated, making precision essential to writing about gender and/or sex without bias. The language related to gender identity and sexual orientation has also evolved rapidly, and it is important to use the terms people use to describe themselves” (p. 138, Section 5.5, para 1).
For more information—including comprehensive subsections on gender versus sex, gender identity, the reporting of gender, transgender and gender-nonconforming people, sex assignment, gender and noun/pronoun usage, terms that imply binaries, and specific examples of bias-free language—see https://apastyle.apa.org/style-grammar-guidelines/bias-free-language/gender
"Deaf" as an Adjective
ASHA has been informed by members of the Deaf community that using “deaf” as an adjective to describe human participants (e.g., “deaf participants”) is acceptable and is not seen as biased by the Deaf community. Therefore, no changes would be made in this example.
APA style uses “Deaf” as one very specific example in its overall discussion on eliminating bias related to disabilities: “The members of some groups of people with disabilities—effectively subcultures within the larger culture of disability—have particular ways of referring to themselves that they would prefer others to adopt. When you use the disability language choices made by groups of disabled individuals, you honor their preferences. For example, some Deaf individuals culturally prefer to be called “Deaf” (capitalized) rather than “people with hearing loss” or “people who are deaf” (Dunn & Andrews, 2015). Likewise, use the term “hard of hearing” rather than “hearing-impaired.” Honoring the preference of the group is not only a sign of professional awareness and respect for any disability group but also a way to offer solidarity” (p. 136).
The University of Kansas Research & Training Center on Independent Living, which publishes Guidelines: How to Write About People With Disabilities (a highly respected publication that is now in its 9th edition), says this:
Deaf refers to a degree of hearing loss that is significant enough to prevent understanding speech through the ear. Deaf people usually identify as the Deaf or Deaf community (with a capital D) rather than people who are deaf. Deaf people are part of a specific community made up of those who share a common culture and sign language. Hearing impairment, hearing loss, deafness, or hearing difficulties are generic terms used by some individuals to indicate any degree of hearing loss, from mild to profound, although some perceive the term hearing impaired as negative. Members of the Deaf community do not use any of these terms as they are medically defined. Hard of hearing refers to those with a mild to moderate hearing loss who may use technology for amplification. A person who has hearing difficulties may have speech difficulties, too, but deafness does not affect mental abilities. Say deaf woman or hard of hearing boy. People who have some degree of hearing and vision loss prefer the term Deaf-Blind. Never use deaf and dumb or deaf mute.
For more information on writing about individuals in the Deaf community, see APA’s online guide to disability language .
Disability Language: General Overview
Per APA style, “The members of some groups of people with disabilities—effectively subcultures within the larger culture of disability—have particular ways of referring to themselves that they would prefer others to adopt. When you use the disability language choices made by groups of disabled individuals, you honor their preferences.”
But, as we all know, language and terminology are constantly changing. And it’s up to us, as authors and publishers, to stay in tune with the preferred language and terminology of the times. “The language to use where disability is concerned is evolving,” APA style explains. “The overall principle for using disability language is to maintain the integrity (worth and dignity) of all individuals as human beings. Authors who write about disability are encouraged to use terms and descriptions that both honor and explain person-first and identity-first perspectives. Language should be selected with the understanding that the expressed preference of people with disabilities regarding identification supersedes matters of style” ( APA Publication Manual, 2020, p. 136).
For more information, see APA’s online guide to disability language .
Language Services
Before submitting a manuscript to an ASHA journal, authors whose first language is not English may choose to have their manuscript professionally edited. Such editing will check for grammar, spelling, and punctuation; improve clarity and word choice; and ensure that the tone and style is appropriate for the journal. Below is a list of organizations we partner with for language services.
ASHA author services, powered by Editage, are designed to support authors with not only end-to-end pre-submission manuscript preparation (including language services for non-native English speakers), but also post-publication research communication. All the author support services are available at a subsidized cost to ASHA authors. Authors submitting their manuscripts to journals published by ASHA will also have free access to a wealth of educational resources powered by Editage Insights, the author education arm of Editage.
Learn more by selecting the Editage logo above to go to the ASHA Author Services page , powered by Editage.
Author Resource Center
Related content, aja special issue: internet and audiology, select papers from the 45th clinical aphasiology conference, improved review process with new editorial board structure, now in effect, quick resources.
- EQUATOR Network library of reporting guidelines
- APA Style Blog posts on bias-free language
- ASHA Author Services, powered by Editage
- AuthorAid support for researchers in developing countries.
- EQUATOR Network resources in Portuguese and Spanish
- Hinari Access to Research for Health Programme

About the ASHA Journals
ASHA publishes four peer-reviewed scholarly journals and one peer-reviewed scholarly review journal pertaining to the general field of communication sciences and disorders (CSD) and to the professions of audiology and speech-language pathology. These journals are the American Journal of Audiology ; American Journal of Speech-Language Pathology ; Journal of Speech, Language, and Hearing Research ; Language, Speech, and Hearing Services in Schools ; and Perspectives of the ASHA Special Interest Groups . These journals have the collective mission of disseminating research findings, theoretical advances, and clinical knowledge in CSD.
Connect with the ASHA Journals
Subscribe to the asha journals, additional author services.
ASHA Author Services Portal

© 1997-2024 American Speech-Language-Hearing Association Privacy Notice Terms of Use

Strengthening the reporting of observational studies in epidemiology
What is STROBE?
STROBE stands for an international, collaborative initiative of epidemiologists, methodologists, statisticians, researchers and journal editors involved in the conduct and dissemination of observational studies, with the common aim of STrengthening the Reporting of OBservational studies in Epidemiology .
For STROBE-related entries in PubMed click here .
Aims and use of STROBE
Incomplete and inadequate reporting of research hampers the assessment of the strengths and weaknesses of the studies reported in the medical literature. Readers need to know what was planned (and what was not), what was done, what was found, and what the results mean. Recommendations on the reporting of studies that are endorsed by leading medical journals can improve the quality of reporting.
Observational research comprises several study designs and many topic areas. We aimed to establish a checklist of items that should be included in articles reporting such research – the STROBE Statement. We considered it reasonable to initially restrict the recommendations to the three main analytical designs that are used in observational research: cohort, case-control, and cross-sectional studies. We want to provide guidance on how to report observational research well. Our recommendations are not prescriptions for designing or conducting studies. Also, the checklist is not an instrument to evaluate the quality of observational research.
Further use
The STROBE initiative should be seen as an ongoing process, with future revisions of the recommendations based on comments, critique and new evidence. We welcome translations into other languages and extensions to other observational study designs, for example nested case-control studies, and specific topic areas, for example, genetic and molecular epidemiology.
Note : We ask anyone intending to use the STROBE Statement for further extensions, translations or other STROBE-related work to contact the coordinating group through this website first. This will allow to coordinate efforts and to avoid duplication. The authors of the original STROBE articles hold the copyright. Please, contact us if you wish to re-publish STROBE material in additional journals, books or other media.
All documents and publications produced by the STROBE Initiative are open-access and available for download on this website.

Enhancing the QUAlity and Transparency Of health Research
- Courses & events
- Librarian Network
- Search for reporting guidelines
Use your browser's Back button to return to your search results
Standards for reporting qualitative research: a synthesis of recommendations
Reporting guidelines for main study types, translations.
Some reporting guidelines are also available in languages other than English. Find out more in our Translations section .
- About the Library
For information about Library scope and content, identification of reporting guidelines and inclusion/exclusion criteria please visit About the Library .
Visit our Help page for information about searching for reporting guidelines and for general information about using our website.
Library index
- What is a reporting guideline?
- Browse reporting guidelines by specialty
- Reporting guidelines under development
- Translations of reporting guidelines
- EQUATOR Network reporting guideline manual
- Reporting guidelines for animal research
- Guidance on scientific writing
- Guidance developed by editorial groups
- Research funders’ guidance on reporting requirements
- Professional medical writing support
- Research ethics, publication ethics and good practice guidelines
- Links to other resources
- Correspondence
- Open access
- Published: 07 November 2012
Reporting guidelines for modelling studies
- Carol Bennett 1 , 2 &
- Douglas G Manuel 1 , 2 , 3 , 4 , 5 , 6
BMC Medical Research Methodology volume 12 , Article number: 168 ( 2012 ) Cite this article
11k Accesses
33 Citations
7 Altmetric
Metrics details
Modelling studies are used widely to help inform decisions about health care and policy and their use is increasing. However, in order for modelling to gain strength as a tool for health policy, it is critical that key model factors are transparent so that users of models can have a clear understanding of the model and its limitations.Reporting guidelines are evidence-based tools that specify minimum criteria for authors to report their research such that readers can both critically appraise and interpret study findings. This study was conducted to determine whether there is an unmet need for population modelling reporting guidelines.
We conducted a review of the literature to identify: 1) guidance for reporting population modelling studies; and, 2) evidence on the quality of reporting of population modelling studies. Guidance for reporting was analysed using a thematic approach and the data was summarised as frequencies. Evidence on the quality of reporting was reviewed and summarized descriptively.
There were no guidelines that specifically addressed the reporting of population modelling studies. We identified a number of reporting guidelines for economic evaluation studies, some of which had sections that were relevant population modelling studies. Amongst seven relevant records, we identified 69 quality criteria that have distinct reporting characteristics. We identified two papers that addressed reporting practices of modelling studies. Overall, with the exception of describing the data used for calibration, there was little consistency in reporting.
Conclusions
While numerous guidelines exist for developing and evaluating health technology assessment and economic evaluation models, which by extension could be applicable to population modelling studies, there is variation in their comprehensiveness and in the consistency of reporting these methods. Population modelling studies may be an area which would benefit from the development of a reporting guideline.
Peer Review reports
Introduction
Modelling studies are used widely to help inform decisions about health care and policy and their use is increasing [ 1 , 2 ]. A model is “an analytical methodology that accounts for events over time and across populations, that is based on data drawn from primary or secondary sources…” and in the context of health care-evaluation “…whose purpose is to estimate the effects of an intervention on valued health consequences and costs” [ 3 ]. Its value lies not only in its results, but also in its ability to reveal the connections between its data and assumptions and model outputs [ 3 ]. But, as pointed out by Garrison, models don’t have to be mathematically sophisticated to be hard to follow [ 4 ]. For these reasons, a model should not be a “black box” for the end-user but be as transparent as possible [ 3 ].
To address the problem of poorly reported research, multiple reporting guidelines have been developed and validated for use with a number of study designs. Reporting guidelines are evidence-based tools that employ expert consensus to specify minimum criteria for authors to report their research such that readers can both critically appraise and interpret study findings [ 5 , 6 ]. The EQUATOR Network, an international initiative whose aim is to improve the reliability of medical research by promoting transparent and accurate reporting of research studies, indexes more than 100 reporting guidelines on their Web site ( http://www.equator-network.org ).
The growth in the number and range of reporting guidelines has prompted guidance on how to develop one using a well-structured development process [ 6 ]. This study addresses the needs assessment-that is, to determine whether there is a need for population modelling reporting guidelines. More specifically, the objectives of our study were: to locate and assess any existing reporting guidelines for population modelling studies; to identify key quality criteria for the reporting of population modelling studies; and to determine if and how these criteria are being reported in the literature.
We began this process with a search of the MEDLINE electronic database (MEDLINE (1950 – February 2011) via Ovid. Our electronic search strategy (see appendix), developed in consultation with a library scientist, was pragmatically designed to avoid being overwhelmed with irrelevant records. We hand-searched the reference lists and used the related articles feature in PubMED for all papers meeting our eligibility criteria. In addition, we reviewed relevant textbooks and Web sites. One reviewer screened the titles and abstracts of all unique citations to identify papers that met our inclusion criteria—that is, English language papers that provided explicit guidance on the reporting of population modelling studies or provided evidence on the quality of reporting of population modelling studies in the health science literature. The full-text report of each record passing title/abstract screening was retrieved and reviewed by the research team and its inclusion/exclusion status was established.
For records that provided explicit guidance on reporting of population modelling studies, the list of criteria identified was analysed using a thematic approach and the data was summarised as frequencies. For those papers that presented evidence on the quality of reporting of population modelling studies, we identified the aspects of reporting that were assessed and summarised the results descriptively.
Results and discussion
We identified 806 unique records through our search strategy, 30 full-text articles were reviewed to determine eligibility (Figure 1 ).
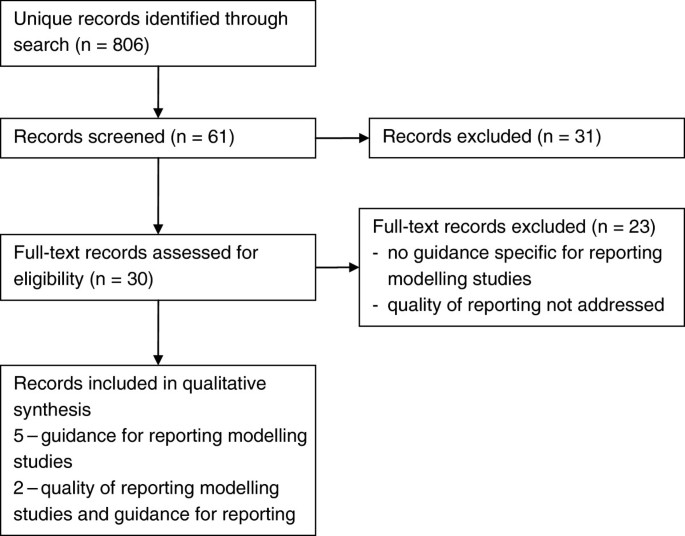
Flow diagram of records – guidelines for reporting modelling studies and evidence on the quality of reporting of modelling studies.
Existence of guidelines for modelling studies
There were no guidelines that specifically addressed the reporting of population modelling studies. However, there were a number of reporting guidelines for economic evaluation studies: one of which was related to modelling [ 7 ] and one included a section which focused on the generalisability of modelling studies [ 8 ]. Additionally, we identified one paper that provided reporting guidance for a specific aspect of simulation modelling methodology – calibration [ 9 ].
Numerous guidelines have been published defining good practice for the conduct of economic evaluations in general and model-based evaluations in particular. We identified two papers that provided guidance for assessing the quality of decision-analytic modelling studies [ 3 , 10 ] and one paper that provided guidance for assessing validation of population-based disease simulation models [ 2 ].
Identification of key reporting items
Amongst the relevant records that were analysed, we identified 69 quality criteria that have distinct reporting characteristics (Table 1 ).
We identified 22 items relating to the structure of the model and broadly classified them into 10 domains: 1) statement of decision problem/objective; 2) statement of scope/perspective; 3) rationale for structure; 4) structural assumptions; 5) strategies/comparators; 6) model type; 7) time horizon; 8) disease states/pathways; 9) cycle length; and, 10) parsimony.
We identified 28 items related to data issues and broadly classified them into 11 domains: 1) data identification; 2) data modelling; 3) baseline data; 4) treatment effects; 5) risk factors; 6) data incorporation; 7) assessment of uncertainty; 8) methodological; 9) structural; 10) heterogeneity; and, 11) parameter.
We identified 14 items related to consistency (internal and external) and validity (output plausibility and predictive validity). The final five items fell under computer implementation, transparency or funding.
The items are not mutually exclusive, and there is overlap if one takes into account implicit and explicit considerations. Even considering this, the records differed in terms of their comprehensiveness and the areas of model quality they considered. No item was identified by all of the resources, one item appeared in five lists, four items appeared in four lists, three items appeared in 17 lists and the remainder of the items appeared in only one or two lists (Table 1 ).
Quality of reporting
We identified two papers that addressed reporting practices of modelling studies, the first of which was a systematic review of coronary heart disease policy models [ 11 ].
The authors evaluated 75 papers on the basis of whether a sensitivity analysis was carried out, the validity was checked, data quality was reported, illustrative examples were provided, if the model was potentially available to the reader (transparency), and if potential limitations were specified or discussed. This evaluation was based on authors reporting on the specific item in the articles.
Relatively few papers included in the review reported on quality issues: sensitivity analysis and assessment of validity were reported in very few models, 33% provided illustrative examples, working versions of the model were available in 10%, and 19% reported on limitations of their methodology,
The second paper examining the reporting practices of modelling studies looked more specifically at the reporting of calibration methods in 154 cancer simulation models [ 9 ]. Data elements abstracted included whether model validation was mentioned (52%) and if a description of the calibration protocol was provided (66%). The authors further characterized calibration protocols by five components. A description of the data used as calibration targets was reported by 95% of the studies and goodness-of-fit metrics were reported in 54% of the studies. However, the search algorithm used for selected alternative parameter values, the criteria for identifying parameter sets that provide an acceptable model fit, and the stopping criteria were not well reported (quantitative values not provided).
Few studies were identified that addressed the quality of reporting of population modelling studies. Overall, with the exception of describing the data used for calibration, there is little consistency in the reporting of items that have been identified as key quality items.
Population modelling studies can fill an important role for policy makers. Their ability to synthesize data from multiple sources and estimate the effects of interventions can be invaluable, especially in areas where primary data collection may be infeasible. However, in order for modelling to gain strength as a tool for health policy, it is critical that key model factors are made transparent so that users of models have a clear understanding of the model and its limitations.
While numerous guidelines exist for developing and evaluating health technology assessment and economic evaluation models, which by extension can be applicable to population modelling studies, there is variation in their comprehensiveness and in the consistency of reporting these methods. There is evidence to suggest that key items are under-reported.
In other areas where reporting guidelines have been developed, there has been a favourable impact on the transparency and accuracy of reporting [ 12 – 15 ]. Population modelling studies may be another area which would benefit from the development of a reporting guideline. Moher and colleagues have outlined the importance of a structured approach to the development of reporting guidelines [ 6 ]. This paper provides results from initial steps in this structure approach. Future work should focus on identifying key information related to potential sources of bias in population modelling studies and identifying a multidisciplinary expert panel to steer the guideline development process.
Search strategy
"Reproducibility of Results"
Quality control/
((valid$ or reliab$ or quality or accura$) adj2 (result$ or report$ or data)).tw.
(good adj1 practice$).tw.
Guidelines as Topic/
(guideline$ or checklist$).tw.
(model$ adj3 (stud$ or method$ or process$ or simulation)).tw.
(modelling or modeling).tw.
Research design/
Decision Support Techniques/
published literature.tw.
16 7 and 10 and 15
Weinstein MC, Toy EL, Sandberg EA, Neumann PJ, Evans JS, Kuntz KM, Graham JD, Hammitt JK: Modeling for health care and other policy decisions: uses, roles, and validity. Value Health. 2001, 4: 348-361. 10.1046/j.1524-4733.2001.45061.x.
Article CAS PubMed Google Scholar
Kopec JA, Fines P, Manuel DG, Buckeridge DL, Flanagan WM, Oderkirk J, Abrahamowicz M, Harper S, Sharif B, Okhmatovskaia A, Sayre EC, Rahman MM, Wolfson MC: Validation of population-based disease simulation models: a review of concepts and methods. [Review]. BMC Publ Health. 2010, 10: 710-10.1186/1471-2458-10-710.
Article Google Scholar
Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR, ISPOR Task Force on Good Research Practices: Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health. 2003, 6: 9-17. 10.1046/j.1524-4733.2003.00234.x.
Article PubMed Google Scholar
Garrison LP: The ISPOR Good Practice Modeling Principles–a sensible approach: be transparent, be reasonable. Value Health. 2003, 6: 6-8. 10.1046/j.1524-4733.2003.00003.x.
Enhancing the Quality and Transparency of Health Research (Equator Network) An introduction to reporting guidelines. http://www.equator-network.org Accessed 2012-03-01.
Moher D, Schulz KF, Simera I, Altman DG: Guidance for developers of health research reporting guidelines. PLoS Medicine / Public Library of Science. 2010, 7: e1000217-
Google Scholar
Nuijten MJ, Pronk MH, Brorens MJ, Hekster YA, Lockefeer JH, de Smet PA, Bonsel G, van der Kuy A: Reporting format for economic evaluation. Part II: Focus on modelling studies. Pharmacoeconomics. 1998, 14: 259-268. 10.2165/00019053-199814030-00003.
Drummond M, Manca A, Sculpher M: Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int J Technol Assess Health Care. 2005, 21: 165-171.
PubMed Google Scholar
Stout NK, Knudsen AB, Kong CY, McMahon PM, Gazelle GS: Calibration methods used in cancer simulation models and suggested reporting guidelines. [Review] [180 refs]. PharmacoEconomics. 2009, 27: 533-545. 10.2165/11314830-000000000-00000.
Article PubMed PubMed Central Google Scholar
Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, Woolacoot N, Glanville J: Review of guidelines for good practice in decision-analytic modelling in health technology assessment. [Review] [62 refs]. 2001, Winchester, England: Health Technology Assessment, 8: iii-iiv.
Unal B, Capewell S, Critchley JA: Coronary heart disease policy models: a systematic review. [Review] [51 refs]. BMC Publ Health. 2006, 6: 213-10.1186/1471-2458-6-213.
Smidt N, Rutjes AW, van der Windt DA, Ostelo RW, Bossuyt PM, Reitsma JB, Bouter LM, de Vet HC: The quality of diagnostic accuracy studies since the STARD statement: has it improved?. Neurology. 2006, 67: 792-797. 10.1212/01.wnl.0000238386.41398.30.
Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, Gaboury I: Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006, 185: 263-267.
Smith BA, Lee HJ, Lee JH, Choi M, Jones DE, Bausell RB, Broome ME: Quality of reporting randomized controlled trials (RCTs) in the nursing literature: application of the consolidated standards of reporting trials (CONSORT). Nurs Outlook. 2008, 56: 31-37. 10.1016/j.outlook.2007.09.002.
Prady SL, Richmond SJ, Morton VM, Macpherson H: A systematic evaluation of the impact of STRICTA and CONSORT recommendations on quality of reporting for acupuncture trials. PLoS One. 2008, 3: e1577-10.1371/journal.pone.0001577. Electronic Resource.
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/12/168/prepub
Download references
Acknowledgments
We thank Sascha Davis, MLIS (Librarian, The Ottawa Hospital) for her assistance with designing the electronic search strategy used in this study.
Funding support: Canadian Institutes of Health Research STAR Emerging Team Grant.
Author information
Authors and affiliations.
Clinical Epidemiology Program, Ottawa Hospital Research Institute, 1053 Carling Avenue, Ottawa, K1Y 4E9, Canada
Carol Bennett & Douglas G Manuel
ICES@uOttawa, Institute for Clinical Evaluative Sciences, 1053 Carling Avenue, Ottawa, K1Y 4E9, Canada
Department of Family Medicine, University of Ottawa, Ottawa, K1H 8M5, Canada
Douglas G Manuel
CT Lamont Primary Health Care Research Centre, University of Ottawa, Ottawa, ON, Canada
Bruyere Research Institute, University of Ottawa, Ottawa, ON, Canada
Department of Epidemiology and Community Medicine, The University of Ottawa, Ottawa, ON, Canada
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Carol Bennett .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
Concept and design (CB, DM); acquisition of data (CB); analysis and interpretation of data (CB, DM); drafting of the manuscript (CB); critical revision of the manuscript for important intellectual content (CB, DM); and final approval of the version to be published (CB, DM). Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Bennett, C., Manuel, D.G. Reporting guidelines for modelling studies. BMC Med Res Methodol 12 , 168 (2012). https://doi.org/10.1186/1471-2288-12-168
Download citation
Received : 11 June 2012
Accepted : 26 October 2012
Published : 07 November 2012
DOI : https://doi.org/10.1186/1471-2288-12-168
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Reporting Guideline
- Economic Evaluation Study
- Guideline Development Process
- Explicit Guidance
- Calibration Protocol
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
- Privacy Policy
Buy Me a Coffee

Home » Research Report – Example, Writing Guide and Types
Research Report – Example, Writing Guide and Types
Table of Contents

Research Report
Definition:
Research Report is a written document that presents the results of a research project or study, including the research question, methodology, results, and conclusions, in a clear and objective manner.
The purpose of a research report is to communicate the findings of the research to the intended audience, which could be other researchers, stakeholders, or the general public.
Components of Research Report
Components of Research Report are as follows:
Introduction
The introduction sets the stage for the research report and provides a brief overview of the research question or problem being investigated. It should include a clear statement of the purpose of the study and its significance or relevance to the field of research. It may also provide background information or a literature review to help contextualize the research.
Literature Review
The literature review provides a critical analysis and synthesis of the existing research and scholarship relevant to the research question or problem. It should identify the gaps, inconsistencies, and contradictions in the literature and show how the current study addresses these issues. The literature review also establishes the theoretical framework or conceptual model that guides the research.
Methodology
The methodology section describes the research design, methods, and procedures used to collect and analyze data. It should include information on the sample or participants, data collection instruments, data collection procedures, and data analysis techniques. The methodology should be clear and detailed enough to allow other researchers to replicate the study.
The results section presents the findings of the study in a clear and objective manner. It should provide a detailed description of the data and statistics used to answer the research question or test the hypothesis. Tables, graphs, and figures may be included to help visualize the data and illustrate the key findings.
The discussion section interprets the results of the study and explains their significance or relevance to the research question or problem. It should also compare the current findings with those of previous studies and identify the implications for future research or practice. The discussion should be based on the results presented in the previous section and should avoid speculation or unfounded conclusions.
The conclusion summarizes the key findings of the study and restates the main argument or thesis presented in the introduction. It should also provide a brief overview of the contributions of the study to the field of research and the implications for practice or policy.
The references section lists all the sources cited in the research report, following a specific citation style, such as APA or MLA.
The appendices section includes any additional material, such as data tables, figures, or instruments used in the study, that could not be included in the main text due to space limitations.
Types of Research Report
Types of Research Report are as follows:
Thesis is a type of research report. A thesis is a long-form research document that presents the findings and conclusions of an original research study conducted by a student as part of a graduate or postgraduate program. It is typically written by a student pursuing a higher degree, such as a Master’s or Doctoral degree, although it can also be written by researchers or scholars in other fields.
Research Paper
Research paper is a type of research report. A research paper is a document that presents the results of a research study or investigation. Research papers can be written in a variety of fields, including science, social science, humanities, and business. They typically follow a standard format that includes an introduction, literature review, methodology, results, discussion, and conclusion sections.
Technical Report
A technical report is a detailed report that provides information about a specific technical or scientific problem or project. Technical reports are often used in engineering, science, and other technical fields to document research and development work.
Progress Report
A progress report provides an update on the progress of a research project or program over a specific period of time. Progress reports are typically used to communicate the status of a project to stakeholders, funders, or project managers.
Feasibility Report
A feasibility report assesses the feasibility of a proposed project or plan, providing an analysis of the potential risks, benefits, and costs associated with the project. Feasibility reports are often used in business, engineering, and other fields to determine the viability of a project before it is undertaken.
Field Report
A field report documents observations and findings from fieldwork, which is research conducted in the natural environment or setting. Field reports are often used in anthropology, ecology, and other social and natural sciences.
Experimental Report
An experimental report documents the results of a scientific experiment, including the hypothesis, methods, results, and conclusions. Experimental reports are often used in biology, chemistry, and other sciences to communicate the results of laboratory experiments.
Case Study Report
A case study report provides an in-depth analysis of a specific case or situation, often used in psychology, social work, and other fields to document and understand complex cases or phenomena.
Literature Review Report
A literature review report synthesizes and summarizes existing research on a specific topic, providing an overview of the current state of knowledge on the subject. Literature review reports are often used in social sciences, education, and other fields to identify gaps in the literature and guide future research.

Research Report Example
Following is a Research Report Example sample for Students:
Title: The Impact of Social Media on Academic Performance among High School Students
This study aims to investigate the relationship between social media use and academic performance among high school students. The study utilized a quantitative research design, which involved a survey questionnaire administered to a sample of 200 high school students. The findings indicate that there is a negative correlation between social media use and academic performance, suggesting that excessive social media use can lead to poor academic performance among high school students. The results of this study have important implications for educators, parents, and policymakers, as they highlight the need for strategies that can help students balance their social media use and academic responsibilities.
Introduction:
Social media has become an integral part of the lives of high school students. With the widespread use of social media platforms such as Facebook, Twitter, Instagram, and Snapchat, students can connect with friends, share photos and videos, and engage in discussions on a range of topics. While social media offers many benefits, concerns have been raised about its impact on academic performance. Many studies have found a negative correlation between social media use and academic performance among high school students (Kirschner & Karpinski, 2010; Paul, Baker, & Cochran, 2012).
Given the growing importance of social media in the lives of high school students, it is important to investigate its impact on academic performance. This study aims to address this gap by examining the relationship between social media use and academic performance among high school students.
Methodology:
The study utilized a quantitative research design, which involved a survey questionnaire administered to a sample of 200 high school students. The questionnaire was developed based on previous studies and was designed to measure the frequency and duration of social media use, as well as academic performance.
The participants were selected using a convenience sampling technique, and the survey questionnaire was distributed in the classroom during regular school hours. The data collected were analyzed using descriptive statistics and correlation analysis.
The findings indicate that the majority of high school students use social media platforms on a daily basis, with Facebook being the most popular platform. The results also show a negative correlation between social media use and academic performance, suggesting that excessive social media use can lead to poor academic performance among high school students.
Discussion:
The results of this study have important implications for educators, parents, and policymakers. The negative correlation between social media use and academic performance suggests that strategies should be put in place to help students balance their social media use and academic responsibilities. For example, educators could incorporate social media into their teaching strategies to engage students and enhance learning. Parents could limit their children’s social media use and encourage them to prioritize their academic responsibilities. Policymakers could develop guidelines and policies to regulate social media use among high school students.
Conclusion:
In conclusion, this study provides evidence of the negative impact of social media on academic performance among high school students. The findings highlight the need for strategies that can help students balance their social media use and academic responsibilities. Further research is needed to explore the specific mechanisms by which social media use affects academic performance and to develop effective strategies for addressing this issue.
Limitations:
One limitation of this study is the use of convenience sampling, which limits the generalizability of the findings to other populations. Future studies should use random sampling techniques to increase the representativeness of the sample. Another limitation is the use of self-reported measures, which may be subject to social desirability bias. Future studies could use objective measures of social media use and academic performance, such as tracking software and school records.
Implications:
The findings of this study have important implications for educators, parents, and policymakers. Educators could incorporate social media into their teaching strategies to engage students and enhance learning. For example, teachers could use social media platforms to share relevant educational resources and facilitate online discussions. Parents could limit their children’s social media use and encourage them to prioritize their academic responsibilities. They could also engage in open communication with their children to understand their social media use and its impact on their academic performance. Policymakers could develop guidelines and policies to regulate social media use among high school students. For example, schools could implement social media policies that restrict access during class time and encourage responsible use.
References:
- Kirschner, P. A., & Karpinski, A. C. (2010). Facebook® and academic performance. Computers in Human Behavior, 26(6), 1237-1245.
- Paul, J. A., Baker, H. M., & Cochran, J. D. (2012). Effect of online social networking on student academic performance. Journal of the Research Center for Educational Technology, 8(1), 1-19.
- Pantic, I. (2014). Online social networking and mental health. Cyberpsychology, Behavior, and Social Networking, 17(10), 652-657.
- Rosen, L. D., Carrier, L. M., & Cheever, N. A. (2013). Facebook and texting made me do it: Media-induced task-switching while studying. Computers in Human Behavior, 29(3), 948-958.
Note*: Above mention, Example is just a sample for the students’ guide. Do not directly copy and paste as your College or University assignment. Kindly do some research and Write your own.
Applications of Research Report
Research reports have many applications, including:
- Communicating research findings: The primary application of a research report is to communicate the results of a study to other researchers, stakeholders, or the general public. The report serves as a way to share new knowledge, insights, and discoveries with others in the field.
- Informing policy and practice : Research reports can inform policy and practice by providing evidence-based recommendations for decision-makers. For example, a research report on the effectiveness of a new drug could inform regulatory agencies in their decision-making process.
- Supporting further research: Research reports can provide a foundation for further research in a particular area. Other researchers may use the findings and methodology of a report to develop new research questions or to build on existing research.
- Evaluating programs and interventions : Research reports can be used to evaluate the effectiveness of programs and interventions in achieving their intended outcomes. For example, a research report on a new educational program could provide evidence of its impact on student performance.
- Demonstrating impact : Research reports can be used to demonstrate the impact of research funding or to evaluate the success of research projects. By presenting the findings and outcomes of a study, research reports can show the value of research to funders and stakeholders.
- Enhancing professional development : Research reports can be used to enhance professional development by providing a source of information and learning for researchers and practitioners in a particular field. For example, a research report on a new teaching methodology could provide insights and ideas for educators to incorporate into their own practice.
How to write Research Report
Here are some steps you can follow to write a research report:
- Identify the research question: The first step in writing a research report is to identify your research question. This will help you focus your research and organize your findings.
- Conduct research : Once you have identified your research question, you will need to conduct research to gather relevant data and information. This can involve conducting experiments, reviewing literature, or analyzing data.
- Organize your findings: Once you have gathered all of your data, you will need to organize your findings in a way that is clear and understandable. This can involve creating tables, graphs, or charts to illustrate your results.
- Write the report: Once you have organized your findings, you can begin writing the report. Start with an introduction that provides background information and explains the purpose of your research. Next, provide a detailed description of your research methods and findings. Finally, summarize your results and draw conclusions based on your findings.
- Proofread and edit: After you have written your report, be sure to proofread and edit it carefully. Check for grammar and spelling errors, and make sure that your report is well-organized and easy to read.
- Include a reference list: Be sure to include a list of references that you used in your research. This will give credit to your sources and allow readers to further explore the topic if they choose.
- Format your report: Finally, format your report according to the guidelines provided by your instructor or organization. This may include formatting requirements for headings, margins, fonts, and spacing.
Purpose of Research Report
The purpose of a research report is to communicate the results of a research study to a specific audience, such as peers in the same field, stakeholders, or the general public. The report provides a detailed description of the research methods, findings, and conclusions.
Some common purposes of a research report include:
- Sharing knowledge: A research report allows researchers to share their findings and knowledge with others in their field. This helps to advance the field and improve the understanding of a particular topic.
- Identifying trends: A research report can identify trends and patterns in data, which can help guide future research and inform decision-making.
- Addressing problems: A research report can provide insights into problems or issues and suggest solutions or recommendations for addressing them.
- Evaluating programs or interventions : A research report can evaluate the effectiveness of programs or interventions, which can inform decision-making about whether to continue, modify, or discontinue them.
- Meeting regulatory requirements: In some fields, research reports are required to meet regulatory requirements, such as in the case of drug trials or environmental impact studies.
When to Write Research Report
A research report should be written after completing the research study. This includes collecting data, analyzing the results, and drawing conclusions based on the findings. Once the research is complete, the report should be written in a timely manner while the information is still fresh in the researcher’s mind.
In academic settings, research reports are often required as part of coursework or as part of a thesis or dissertation. In this case, the report should be written according to the guidelines provided by the instructor or institution.
In other settings, such as in industry or government, research reports may be required to inform decision-making or to comply with regulatory requirements. In these cases, the report should be written as soon as possible after the research is completed in order to inform decision-making in a timely manner.
Overall, the timing of when to write a research report depends on the purpose of the research, the expectations of the audience, and any regulatory requirements that need to be met. However, it is important to complete the report in a timely manner while the information is still fresh in the researcher’s mind.
Characteristics of Research Report
There are several characteristics of a research report that distinguish it from other types of writing. These characteristics include:
- Objective: A research report should be written in an objective and unbiased manner. It should present the facts and findings of the research study without any personal opinions or biases.
- Systematic: A research report should be written in a systematic manner. It should follow a clear and logical structure, and the information should be presented in a way that is easy to understand and follow.
- Detailed: A research report should be detailed and comprehensive. It should provide a thorough description of the research methods, results, and conclusions.
- Accurate : A research report should be accurate and based on sound research methods. The findings and conclusions should be supported by data and evidence.
- Organized: A research report should be well-organized. It should include headings and subheadings to help the reader navigate the report and understand the main points.
- Clear and concise: A research report should be written in clear and concise language. The information should be presented in a way that is easy to understand, and unnecessary jargon should be avoided.
- Citations and references: A research report should include citations and references to support the findings and conclusions. This helps to give credit to other researchers and to provide readers with the opportunity to further explore the topic.
Advantages of Research Report
Research reports have several advantages, including:
- Communicating research findings: Research reports allow researchers to communicate their findings to a wider audience, including other researchers, stakeholders, and the general public. This helps to disseminate knowledge and advance the understanding of a particular topic.
- Providing evidence for decision-making : Research reports can provide evidence to inform decision-making, such as in the case of policy-making, program planning, or product development. The findings and conclusions can help guide decisions and improve outcomes.
- Supporting further research: Research reports can provide a foundation for further research on a particular topic. Other researchers can build on the findings and conclusions of the report, which can lead to further discoveries and advancements in the field.
- Demonstrating expertise: Research reports can demonstrate the expertise of the researchers and their ability to conduct rigorous and high-quality research. This can be important for securing funding, promotions, and other professional opportunities.
- Meeting regulatory requirements: In some fields, research reports are required to meet regulatory requirements, such as in the case of drug trials or environmental impact studies. Producing a high-quality research report can help ensure compliance with these requirements.
Limitations of Research Report
Despite their advantages, research reports also have some limitations, including:
- Time-consuming: Conducting research and writing a report can be a time-consuming process, particularly for large-scale studies. This can limit the frequency and speed of producing research reports.
- Expensive: Conducting research and producing a report can be expensive, particularly for studies that require specialized equipment, personnel, or data. This can limit the scope and feasibility of some research studies.
- Limited generalizability: Research studies often focus on a specific population or context, which can limit the generalizability of the findings to other populations or contexts.
- Potential bias : Researchers may have biases or conflicts of interest that can influence the findings and conclusions of the research study. Additionally, participants may also have biases or may not be representative of the larger population, which can limit the validity and reliability of the findings.
- Accessibility: Research reports may be written in technical or academic language, which can limit their accessibility to a wider audience. Additionally, some research may be behind paywalls or require specialized access, which can limit the ability of others to read and use the findings.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Research Paper Conclusion – Writing Guide and...

Appendices – Writing Guide, Types and Examples

Scope of the Research – Writing Guide and...

Research Contribution – Thesis Guide

Research Problem – Examples, Types and Guide

Institutional Review Board – Application Sample...
The RePORT Expenditures and Results (RePORTER) module allows users to search a repository of NIH-funded research projects and access publications and patents resulting from NIH funding. Enter just about anything in the RePORTER Quick Search box above (text, PI names, project numbers, fiscal year, agency) or launch the Advanced Search to precisely configure searches using separate search fields.
RePORTER Home Advanced Search
Enter abstracts or other scientific text and Matchmaker will return lists of similar projects from RePORTER or program officials associated with those projects. These matches are based on the terms and concepts used in the submitted text. Matchmaker summarizes the projects by the program official, institute or center, review panel, and activity code.
Find a Match
Awards by Location
NIH Awards by Location - Explore year-by-year NIH funding by institution, state, congressional district, and more!
Categorical Spending
Categorical Spending displays the annual support level for various research, condition, and disease categories based on grants, contracts, and other funding mechanisms used across the National Institutes of Health (NIH), as well as disease burden data. The NIH does not expressly budget by category. The annual estimates reflect amounts that change as a result of science, actual research projects funded, and the NIH budget.
See Details
NIH Data Book
The NIH Data Book (NDB) provides basic summary statistics on extramural grants and contract awards, grant applications, the organizations that NIH supports, the trainees and fellows supported through NIH programs, and the national biomedical workforce.
Launch Data Book
Research Portfolio Online Reporting Tools (RePORT)
In addition to carrying out its scientific mission, the NIH exemplifies and promotes the highest level of public accountability. To that end, the Research Portfolio Online Reporting Tools provides access to reports, data, and analyses of NIH research activities, including information on NIH expenditures and the results of NIH supported research.
NIH COVID-19 Research Explore NIH’s comprehensive summary of ongoing COVID-19 research, including funding, clinical trials, and more. Preview a modernized RePORTER , which offers faster searches and mobile-friendly interface. Subscribe to the NIH RePORT ListServ to stay up to date on changes to these sites.
RePORT Statistics
Projects by institute/center, projects by state.

Trends in Major Fields of Study of NIH-Supported Ph.D. Recipients

Research News
News highlights.
- Webinar – NIH Simplified Review Framework for Research Project Grants (RPGs): Implementation and Impact on Funding Opportunities - Mar 2024
- New Location for NIH Public Access Policy Content & Resources - Mar 2024
- How Implementing a 2022 Law is Helping Us Ensure Safe and Respectful Workplaces - Mar 2024
- End of an Era: CD/DVD Format No Longer Allowed for Videos Submitted as Application Materials - Mar 2024
- How Many Researchers: The FY 2023 Cumulative Investigator Rate - Mar 2024
NIH… Turning Discovery Into Health ®
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Research articles
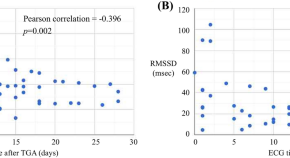
Changes in heart rate variability over time from symptom onset of transient global amnesia
- Sue Hyun Lee
- Kyung Min Kim
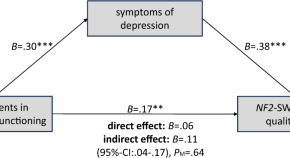
The impact of mental health on health-related quality of life in patients with NF2-related Schwannomatosis
- Anna Freier
- Anna C. Lawson McLean
- Johannes Kruse

Association of the fat mass index with hepatic steatosis and fibrosis: evidence from NHANES 2017–2018
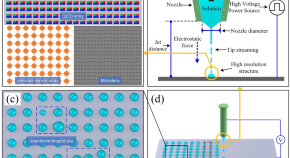
Preparation of high-resolution micro/nano dot array by electrohydrodynamic jet printing with enhanced uniformity
- Zhouping Yin
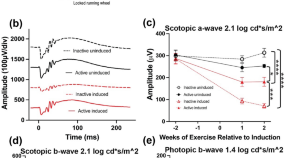
Voluntary exercise preserves visual function and reduces inflammatory response in an adult mouse model of autosomal dominant retinitis pigmentosa
- Katie L. Bales
- Austin M. Karesh
- Machelle T. Pardue
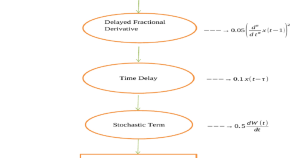
Numerical simulation of a fractional stochastic delay differential equations using spectral scheme: a comprehensive stability analysis
- Sami Ullah Khan
- Atif M. Alamri
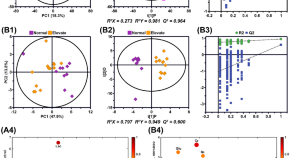
Metabolic responses to the occurrence and chemotherapy of pancreatic cancer: biomarker identification and prognosis prediction
- Tianhong Teng
- Heguang Huang
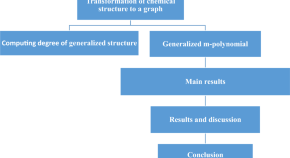
On topological analysis of two-dimensional covalent organic frameworks via M-polynomial
- Muhammad Farhan Hanif
- Samuel Asefa Fufa
Unbiased serum metabolomic analysis in cats with naturally occurring chronic enteropathies before and after medical intervention
- Maria Questa
- Bart C. Weimer
- Sina Marsilio
The clinical evaluation of a widefield lens to expand the field of view in optical coherence tomography (OCT-A)
- Fritz Soecknick
- Katharina Breher
- Focke Ziemssen
Modeling liquid rate through wellhead chokes using machine learning techniques
- Mohammad-Saber Dabiri
- Fahimeh Hadavimoghaddam
- Abdolhossein Hemmati-Sarapardeh
Levenberg–Marquardt deep neural watermarking for 3D mesh using nearest centroid salient point learning
- Modigari Narendra
- M. L. Valarmathi
- Amir H. Gandomi
Enhancement of magnetization and optical properties of CuFe 2 O 4 /ZnFe 2 O 4 core/shell nanostructure
- A. M. Faramawy
- H. M. El-Sayed
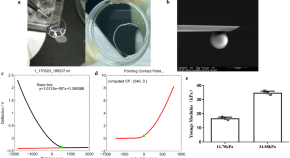
Balanced activation of Nrf-2/ARE mediates the protective effect of sulforaphane on keratoconus in the cell mechanical microenvironment
- Ruixing Liu
- Xiaoming Yan
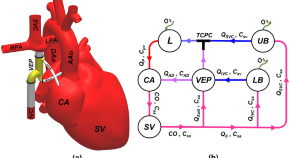
In vitro hemodynamic performance of a blood pump for self-powered venous assist in univentricular hearts
- Reza Rasooli
- Henrik Holmstrom
- Aksel Hiorth
Community detection in hypergraphs via mutual information maximization
- Jürgen Kritschgau
- Daniel Kaiser
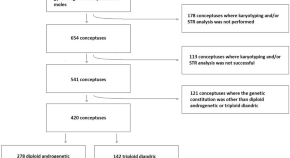
Aneuploidy is frequent in heterozygous diploid and triploid hydatidiform moles
- L. Andreasen
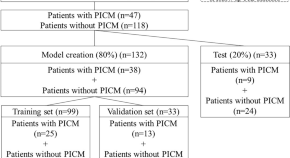
Prediction of pacemaker-induced cardiomyopathy using a convolutional neural network based on clinical findings prior to pacemaker implantation
- Mitsunori Oida
- Takuya Mizutani
- Issei Komuro
Numerical simulation of shoegear-rail coupling vibration under different initial contact forces
- Peihuo Peng
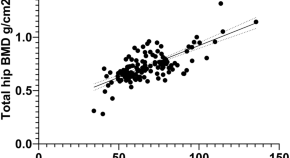
New point-of-care calcaneal ultrasound densitometer (Osteosys BeeTLE) compared to standard dual-energy X-ray absorptiometry (DXA)
- Giovanni Adami
- Maurizio Rossini
- Roberto Lovato
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
House Republican budget calls for raising the retirement age for Social Security

WASHINGTON — A new budget by a large and influential group of House Republicans calls for raising the Social Security retirement age for future retirees and restructuring Medicare.
The proposals, which are unlikely to become law this year, reflect how many Republicans will seek to govern if they win the 2024 elections. And they play into a fight President Joe Biden is seeking to have with former President Donald Trump and the Republican Party as he runs for re-election.
The budget was released Wednesday by the Republican Study Committee , a group of more than 170 House GOP lawmakers, including many allies of Republican presidential nominee Donald Trump. Apart from fiscal policy, the budget endorses a series of bills “designed to advance the cause of life,” including the Life at Conception Act, which would aggressively restrict abortion and potentially threaten in vitro fertilization , or IVF, by establishing legal protections for human beings at “the moment of fertilization.” It has recently caused consternation within the GOP following backlash to an Alabama Supreme Court ruling that threatened IVF.
The RSC, which is chaired by Rep. Kevin Hern, R-Okla., counts among its members Speaker Mike Johnson, R-La., and his top three deputies in leadership. Johnson chaired the RSC from 2019 to 2021; his office did not immediately respond when asked about the new budget.
For Social Security, the budget endorses "modest adjustments to the retirement age for future retirees to account for increases in life expectancy." It calls for lowering benefits for the highest-earning beneficiaries. And it emphasizes that those ideas are not designed to take effect immediately: "The RSC Budget does not cut or delay retirement benefits for any senior in or near retirement."
The new budget also calls for converting Medicare to a "premium support model," echoing a proposal that Republican former Speaker Paul Ryan had rallied support for. Under the new RSC plan, traditional Medicare would compete with private plans and beneficiaries would be given subsidies to shop for the policies of their choice. The size of the subsidies could be pegged to the "average premium" or "second lowest price" in a particular market, the budget says.
The plan became a flashpoint in the 2012 election, when Ryan was GOP presidential nominee Mitt Romney's running mate, and President Barack Obama charged that it would "end Medicare as we know it." Ryan defended it as a way to put Medicare on better financial footing, and most of his party stood by him.
Medicare is projected to become insolvent in 2028, and Social Security will follow in 2033. After that, benefits will be forcibly cut unless more revenues are added.
Biden has blasted Republican proposals for the retirement programs, promising that he will not cut benefits and instead proposing in his recent White House budget to cover the future shortfall by raising taxes on upper earners.
The RSC budget also presents a conundrum for Trump, who has offered shifting rhetoric on Social Security and Medicare without proposing a clear vision for the future of the programs.
Notably, the RSC budget presents three possible options to address the projected insolvency of the retirement programs: raise taxes, transfer money from the general fund or reduce spending to cover the shortfall.
It rejects the first two options.
"Raising taxes on people will further punish them and burden the broader economy–something that the spend and print regime has proven to be disastrous and regressive," the budget says, adding that the committee also opposes "a multi-trillion-dollar general fund transfer that worsens our fiscal situation."
That leaves spending cuts.
The RSC budget launches blistering criticism at "Obamacare," or the Affordable Care Act, and calls for rolling back its subsidies and regulations that were aimed at extending insurance coverage.
Sahil Kapur is a senior national political reporter for NBC News.
- Share full article
Advertisement
Supported by
Led by Its Youth, U.S. Sinks in World Happiness Report
For the first time since the first World Happiness Report was issued in 2012, the United States was not ranked among the world’s Top 20 happiest countries. The drop was driven by people under 30.

By Sopan Deb
Each year, it’s no surprise that Finland tops the annual World Happiness Report. And this year was no different, marking the country’s seventh consecutive year doing so — though some Finns have bristled at the title .
But the 2024 report, released on Wednesday , had a note of alarm that was less about who was at the top of the rankings and more about who wasn’t: Americans — particularly those under 30 — have become drastically less happy in recent years.
The report, compiled annually by a consortium of groups including the United Nations and Gallup, was the latest data point in what some researchers have described as a crisis among America’s youth.
For the first time since the first World Happiness Report was published in 2012, the United States fell out of the Top 20 and dropped to 23rd, pushed down by cratering attitudes of Americans under 30.
Americans have long been an unhappy bunch. They have never ranked in the Top 10 of the World Happiness Report, which is based on how respondents in different countries rate their own happiness.
But this was the first time that the consortium separated results by age, finding disparities in the views of younger and older Americans. Among the 143 countries surveyed , the United States ranked 10th for people 60 and older, but 62nd for people under 30. The happiest young people are in Lithuania, while the unhappiest are in Afghanistan.
“I have never seen such an extreme change,” John Helliwell, an economist and a co-author of the report, said in an interview, referring to the drop in happiness among younger people. “This has all happened in the last 10 years, and it’s mainly in the English-language countries. There isn’t this drop in the world as a whole.”
To collect the data, Dr. Helliwell and his collaborators interviewed about 1,000 people in each of the more than 130 countries surveyed annually from 2021 to 2023. Respondents were asked — among other prompts — to think of their life as a ladder and to rate it on a scale of one to 10, with 10 being the best possible life.
Dr. Lorenzo Norris, an associate professor of psychiatry at George Washington University, who was not part of the World Happiness study, cited the disruptions to life brought about by the coronavirus pandemic as a chief cause of mental health challenges among younger Americans.
“The literature is clear in practice — the effect that this had on socialization, pro-social behavior, if you will, and the ability for people to feel connected and have a community,” Dr. Norris said of the pandemic. “Many of the things that would have normally taken place for people, particularly high school young adults, did not take place,” he added. “And that is still occurring.”
Jade Song, a 27-year-old novelist , counted herself among those who had become increasingly unhappy in recent years.
“It’s mostly because as an adult you suddenly become aware of all the world news and you pay attention more to what you can control, and you realize that there is so little you can control,” Ms. Song, who was not part of the study, said in an interview. “Even if you’re going to protests or paying your rent and bills all on time, it’s so difficult, especially now, to break free from how you’re living your life when you realize how little impact your actions actually have on a broader level.”
In 2022, a Harvard University study showed that well-being among young adults in the United States had declined in the previous 20 years. Young people — those between the ages of 18 and 25 — reported the lowest levels of happiness compared with other age groups, as well as the poorest mental and physical health, sense of purpose, character, virtue, close social relationships and financial stability. Similar findings have emerged in Britain and Canada.
“One factor, which we’re all thinking about, is social media,” said Dr. Robert Waldinger, the director of the Harvard Study of Adult Development. “Because there’s been some research that shows that depending on how we use social media, it lowers well-being, it increases rates of depression and anxiety, particularly among young girls and women, teenage girls.”
In addition, Dr. Waldinger said, the negative feedback loop from news consumption has become a contributing factor.
“There’s also a lot of anxiety about the state of the world,” he said. “About climate change. About all of the polarization that we’re seeing.”
Of course, the United States is not the only country dealing with the pandemic, social media and climate change. But in some other countries, such as Croatia, Switzerland and Austria, the World Happiness Report shows that young people are becoming happier.
Happiness has long been an object of fascination in the United States. The right to the “pursuit of happiness,” of course, appears early in the Declaration of Independence as a self-evident truth. Exploring it as a concept has been a mainstay of American pop culture. Think of the earworm hits “Happy” by Pharrell Williams or “Don’t Worry Be Happy” by Bobby McFerrin. In the television show “Mad Men,” Don Draper laments: “What is happiness? It’s a moment before you need more happiness .”
“ Part of the problem is that we have this huge expectation of happiness in America,” said Eric Weiner, the author of “The Geography of Bliss,” and so we suffer partly from the unhappiness of not being happy and the expectation that we should be happy. And not every country in the world has that.”
For that book, Mr. Weiner, a former foreign correspondent for National Public Radio, traveled to several countries ranked among the world’s happiest places.
“There’s an assumption that if you’re American, you’re wealthy and you’re high tech and you’re successful; you should be happy,” he said. “There’s a lot of data that shows that the greater your expectations, the less you’re happy.”
The expectations for young people like Ms. Song, the novelist, said have shifted.
“We have less to look forward to,” she said. “Because in the future, there’s going to be climate change that will affect the way we live. I think there’s less of a clear-cut trajectory for our life paths, because for so long, it was so easy just to know that you could go get married and have your 2.5 kids, and then pay for your house. But now that path is a lot more closed.”
There is a silver lining, though, for the report released on Wednesday, Dr. Helliwell said.
“ A, this angst is very local and, B, it’s very recent, which means, C, it’s not fundamental and going to last forever,” he said. “If it has been created that quickly, it could be removed that quickly.”
Sopan Deb is a Times reporter covering breaking news and culture. More about Sopan Deb
Managing Anxiety and Stress
Stay balanced in the face of stress and anxiety with our collection of tools and advice..
How are you, really? This self-guided check-in will help you take stock of your emotional well-being — and learn how to make changes .
These simple and proven strategies will help you manage stress , support your mental health and find meaning in the new year.
First, bring calm and clarity into your life with these 10 tips . Next, identify what you are dealing with: Is it worry, anxiety or stress ?
Persistent depressive disorder is underdiagnosed, and many who suffer from it have never heard of it. Here is what to know .
If you notice drastic shifts in your mood during certain times of the year, you could have seasonal affective disorder. Here are answers to your top questions about the condition .
How much anxiety is too much? Here is how to establish whether you should see a professional about it .
An avocado a day may improve overall diet quality, researchers report
Eating one avocado per day may improve overall diet quality, according to a team led by researchers in Penn State's Department of Nutritional Sciences. Poor diet quality is a risk factor for many diseases, including heart disease, and many American adults have poor diet quality and do not meet key dietary recommendations provided by the Dietary Guidelines for Americans.
This study was led by Kristina Petersen, associate professor of nutritional sciences, and Penny Kris-Etherton, retired Evan Pugh University Professor of Nutritional Sciences, and recently published in the journal Current Developments in Nutrition . The researchers examined how a food-based intervention -- one avocado per day -- impacts overall diet quality.
"Avocados are a nutrient-dense food, containing a lot of fiber and other important nutrients. We wanted to see if regular intake of this food would lead to an increase in diet quality," Petersen said. "Previous observational research suggests avocado consumers have higher diet quality than non-consumers. So, we developed this study to determine if there is a causational link between avocado consumption and overall diet quality."
Petersen stated that because only 2% of American adults are regular avocado consumers, the researchers wanted to determine if including avocados in an individual's daily diet could significantly increase their diet quality.
Researchers conducted phone interviews with participants before the study began and at a few points throughout to determine what their dietary intake was like in the previous 24 hours and evaluated their diets using the Healthy Eating Index to determine how well they adhered to the Dietary Guidelines for Americans. Adherence to the guidelines was used as a measure of overall diet quality.
The study consisted of 1,008 participants who were split into two groups. One group continued their usual diet and limited their avocado intake during the 26-week study, while the other group incorporated one avocado per day into their diet.
"We found that the participants who had an avocado per day significantly increased their adherence to dietary guidelines," Petersen said. "This suggests that strategies, like eating one avocado per day, can help people follow dietary guidelines and improve the quality of their diets."
Although researchers said they were not surprised to see that eating avocados daily improved diet quality, they had not predicted how participants were able to achieve it.
"We determined that participants were using avocados as a substitute for some foods higher in refined grains and sodium," Petersen said. "In our study, we classified avocados as a vegetable and did see an increase in vegetable consumption attributed to the avocado intake, but also participants used the avocados to replace some unhealthier options."
According to Petersen, having poor diet quality substantially increases the risk for conditions like heart disease, type 2 diabetes, kidney disease and many other preventable diseases.
"By improving people's adherence to dietary guidelines, we can help to reduce their risk of developing these chronic conditions and prolong healthy life expectancy," Petersen said.
Petersen has also conducted similar studies investigating the impact of food-based interventions, including the relationship between pistachios and diet quality, but said that more research is needed to determine what other food-based strategies can be used to improve people's adherence to dietary guidelines.
"In studies like this one, we are able to determine food-based ways to improve diet quality, but behavioral strategies are also needed to help people adhere to dietary guidelines and reduce their risk of chronic disease," Petersen said.
Other contributors to the study include Sydney Smith and David M. Reboussin, Wake Forest University School of Medicine; Alice H. Lichtenstein and Nirupa R. Matthan, Tufts University; Zhaoping Li, David Geffen School of Medicine at the University of California, Los Angeles; and Joan Sabate, Sujatha Rajaram and Gina Segovia-Siapco, Loma Linda University.
The Avocado Nutrition Center supported this study. The funder did not influence the data analysis, data interpretation or writing of the published study.
- Diet and Weight Loss
- Cholesterol
- Food and Agriculture
- Agriculture and Food
- Veterinary Medicine
- Healthy diet
- Atkins Diet
- Micronutrient
- Mediterranean diet
- Diabetic diet
Story Source:
Materials provided by Penn State . Original written by Brooke Pier. Note: Content may be edited for style and length.
Journal Reference :
- Kristina S Petersen, Sydney Smith, Alice H Lichtenstein, Nirupa R Matthan, Zhaoping Li, Joan Sabate, Sujatha Rajaram, Gina Segovia-Siapco, David M Reboussin, Penny M Kris-Etherton. One Avocado per Day as Part of Usual Intake Improves Diet Quality: Exploratory Results from a Randomized Controlled Trial . Current Developments in Nutrition , 2024; 8 (2): 102079 DOI: 10.1016/j.cdnut.2024.102079
Cite This Page :
Explore More
- Next-Gen Solar Cells: Perovskite Semiconductors
- When Faces Appear Distorted: Rare Condition
- End of Planet Formation
- Enormous Ice Loss from Greenland Glacier
- Signs of Life Detectable in Single Ice Grain
- Tudor Era Horse Cemetery
- When Do Babies Become Conscious?
- Secrets of the Van Allen Belt Revealed
- Robotic Prostheses, Exoskeletons
- Bronze Age Families: Clothing, Recipes, Pets
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Multidiscip Healthc
Information bias in health research: definition, pitfalls, and adjustment methods
Alaa althubaiti.
Department of Basic Medical Sciences, College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
As with other fields, medical sciences are subject to different sources of bias. While understanding sources of bias is a key element for drawing valid conclusions, bias in health research continues to be a very sensitive issue that can affect the focus and outcome of investigations. Information bias, otherwise known as misclassification, is one of the most common sources of bias that affects the validity of health research. It originates from the approach that is utilized to obtain or confirm study measurements. This paper seeks to raise awareness of information bias in observational and experimental research study designs as well as to enrich discussions concerning bias problems. Specifying the types of bias can be essential to limit its effects and, the use of adjustment methods might serve to improve clinical evaluation and health care practice.
Introduction
Bias can be defined as any systematic error in the design, conduct, or analysis of a study. In health studies, bias can arise from two different sources; the approach adopted for selecting subjects for a study or the approach adopted for collecting or measuring data from a study. These are, respectively, termed as selection bias and information bias. 1 Bias can have different effects on the validity of medical research findings. In epidemiological studies, bias can lead to inaccurate estimates of association, or over- or underestimation of risk parameters. Allocating the sources of bias and their impacts on final results are key elements for making valid conclusions. Information bias, otherwise known as misclassification, is one of the most common sources of bias that affects the validity of health research. It originates from the approach that is utilized to obtain or confirm study measurements. These measurements can be obtained by experimentation (eg, bioassays) or observation (eg, questionnaires or surveys).
Medical practitioners are conscious of the fact that the results of their investigation can be deemed invalid if they do not account for major sources of bias. While a number of studies have discussed different types of bias, 2 – 4 the problem of bias is still frequently ignored in practice. Often bias is unintentionally introduced into a study by researchers, making it difficult to recognize, but it can also be introduced intentionally. Thus, bias remains a very sensitive issue to address and discuss openly. The aim of this paper is to raise the awareness of three specific forms of information bias in observational and experimental medical research study designs. These are self-reporting bias, and the often-marginalized measurement error bias, and confirmation bias. We present clear and simple strategies to improve the decision-making process. As will be seen, specifying the type of bias can be essential for limiting its implications. The “Self-reporting bias” section discusses the problem of bias in self-reporting data and presents two examples of self-reporting bias, social desirability bias and recall bias. The “Measurement error bias” section describes the problem of measurement error bias, while the “Confirmation bias” section discusses the problem of confirmation bias.
Self-reporting bias
Self-reporting is a common approach for gathering data in epidemiologic and medical research. This method requires participants to respond to the researcher’s questions without his/her interference. Examples of self-reporting include questionnaires, surveys, or interviews. However, relative to other sources of information, such as medical records or laboratory measurements, self-reported data are often argued to be unreliable and threatened by self-reporting bias.
The issue of self-reporting bias represents a key problem in the assessment of most observational (such as cross-sectional or comparative, eg, case–control or cohort) research study designs, although it can still affect experimental studies. Nevertheless, when self-reporting data are correctly utilized, they can help to provide a wider range of responses than many other data collection instruments. 5 For example, self-reporting data can be valuable in obtaining subjects’ perspectives, views, and opinions.
There are a number of aspects of bias that accompany self-reported data and these should be taken into account during the early stages of the study, particularly when designing the self-reporting instrument. Bias can arise from social desirability, recall period, sampling approach, or selective recall. Here, two examples of self-reporting bias are discussed: social desirability and recall bias.
Social desirability bias
When researchers use a survey, questionnaire, or interview to collect data, in practice, the questions asked may concern private or sensitive topics, such as self-report of dietary intake, drug use, income, and violence. Thus, self-reporting data can be affected by an external bias caused by social desirability or approval, especially in cases where anonymity and confidentiality cannot be guaranteed at the time of data collection. For instance, when determining drug usage among a sample of individuals, the results could underestimate the exact usage. The bias in this case can be referred to as social desirability bias.
Overcoming social desirability bias
The main strategy to prevent social desirability bias is to validate the self-reporting instrument before implementing it for data collection. 6 – 11 Such validation can be either internal or external. In internal validation, the responses collected from the self-reporting instrument are compared with other data collection methods, such as laboratory measurements. For example, urine, blood, and hair analysis are some of the most commonly used validation approaches for drug testing. 12 – 14 However, when laboratory measurements are not available or it is not possible to analyze samples in a laboratory for reasons such as cost and time, external validation is often used. There are different methods, including medical record checks or reports from family or friends to examine externally the validity of the self-reporting instrument. 12 , 15
Note that several factors must be accounted for in the design and planning of the validation studies, and in some cases, this can be very challenging. For example, the characteristics of the sample enrolled in the validation study should be carefully investigated. It is important to have a random selection of individuals so that results from the validation can be generalized to any group of participants. When the sampling approach is not random and subjective, the results from the validation study can only apply to the same group of individuals, and the differences between the results from validation studies and self-reporting instruments cannot be used to adjust for differences in any group of individuals. 12 , 16 Hence, when choosing a predesigned and validated self-reporting instrument, information on the group of participants enrolled in the validation process should be obtained. This information should be provided as part of the research paper and if not, further communication is needed with the authors of the work in order to obtain them. For example, if the target of the study is to examine drug use among the general population with no specific background, then a self-reporting instrument that has been validated on a sample of the population having general characteristics should be used. In addition, combining more than one validation technique or the use of multiple data sources may increase the validity of the results.
Moreover, the possible effects of social desirability on study outcomes should be identified during the design phase of the data collection method. As such, measurement scales such as Marlowe–Crowne Social Desirability Scale 17 or Martin–Larsen Approval Motivation score 18 would be useful to identify and measure the social desirability aspect of the self-reported information.
Recall bias
Occasionally, study participants can erroneously provide responses that depend on his/her ability to recall past event. The bias in this case can be referred to as recall bias, as it is a result of recall error. This type of bias often occurs in case–control or retrospective cohort study designs, where participants are required to evaluate exposure variables retrospectively using a self-reporting method, such as self-administered questionnaires. 19 – 21
While the problems posed by recall bias are no less than those caused by social desirability, recall bias is more common in epidemiologic and medical research. The effect of recall bias has been investigated extensively in the literature, with particular focus on survey methods for measuring dietary or food intake. 22 – 25 If not given proper consideration, it can either underestimate or overestimate the true effect or association. For example, a recall error in a dietary survey may result in underestimates of the association between dietary intake and disease risk. 24
Overcoming recall bias
To overcome recall bias, it is important to recognize cases where recall errors are more likely to occur. Recall bias was found to be related to a number of factors, including length of the recall period (ie, short or long times of clinical assessment), characteristics of the disease under investigation (eg, acute, chronic), patient/sample characteristics (eg, age, accessibility), and study design (eg, duration of study). 26 – 30 For example, in a case–control study, cases are often more likely to recall exposure to risk factors than healthy controls. As such, true exposure might be underreported in healthy controls and overreported in the cases. The size of the difference between the observed rates of exposure to risk factors in cases and controls will consequently be inflated, and, in turn, the observed odds ratio would also increase.
Many solutions have proven to be useful for minimizing and, in some cases, eliminating recall bias. For example, to select the appropriate recall period, all the above-mentioned factors should be considered in relation to recall bias. Previous literature showed that a short recall period is preferable to a long one, particularly when asking participants about routine or frequent events. In addition, the recall period can be stratified according to participant demographics and the frequency of events they experienced. For example, when participants are expected to have a number of events to recall, they can be asked to describe a shorter period than those who would have fewer events to recall. Other methods to facilitate participant’s recall include the use of memory aids, diaries, and interviewing of participants prior to initiating the study. 31
However, when it is not possible to eliminate recall errors, it is important to obtain information on the error characteristics and distribution. Such information can be obtained from previous or pilot studies and is useful when adjusting the subsequent analyses and choosing a suitable statistical approach for data analysis. It must be borne in mind that there are fundamental differences between statistical approaches to make adjustments that address different assumptions about the errors. 22 , 32 – 36 When conducting a pilot study to examine error properties, a high level of accuracy and careful planning are needed, as validation largely depends on biological testing or laboratory measurements, which, besides being costly to conduct, are often subject to measurement errors. For example, in a validation study to estimate sodium intake using a 24-hour urinary excretion method, the estimated sodium intake tended to be lower than the true amount. 25 Despite these potential shortcomings, the use of biological testing or laboratory measurements is one of the most credible approaches to validate self-reported data. More information on measurement errors is provided in the next section.
It is important to point out that overcoming recall bias can be difficult in practice. In particular, bias often accompanies results from case–control studies. Hence, case–control studies can be conducted in order to generate a research hypothesis, but not to evaluate prognoses or treatment effects. Finally, more research is needed to assess the impact of recall bias. Studies to evaluate the agreements between responses from self-reporting instruments and gold-standard data sources should be conducted. Such studies can provide medical researchers with information concerning the validity of the self-reporting instrument before utilizing it in a study or for a disease under investigation. Other demographic factors associated with recall bias can also be identified. For instance, a high agreement was found between self-reported questionnaires and medical record diagnoses of diseases such as diabetes, hypertension, myocardial infarction, and stroke but not for heart failure. 37
Measurement error bias
Device inaccuracy, environmental conditions in the laboratory, or self-reported measurements are all sources of errors. If these errors occur, observed measurements will differ from the actual values, and this is often referred to as measurement error, instrumental error, measurement imprecision, or measurement bias. These errors are encountered in both observational (such as cohort studies) and experimental (such as laboratory tests) study designs. For example, in an observational study of cardiovascular disease, measurements of blood cholesterol levels (as a risk factor) often included errors.
An analysis that ignores the effect of measurement error on the results can be referred to as a naïve analysis. 22 Results obtained from using naïve analysis can be potentially biased and misleading. Such results can include inconsistent (or biased) and/or inefficient estimators of regression parameters, which may yield poor inferences about confidence intervals and the hypothesis testing of parameters. 22 , 34
However, random sampling should not be confused with measurement error variability. Commonly used statistical methods can address the sampling variability during data analysis, but they do not account for uncertainty due to measurement error.
Measurement error bias has rarely been discussed or adjusted for in the medical research literature, except in the field of forensic medicine, where forensic toxicologists have undoubtedly the most theoretical understanding of measurement bias as it is particularly relevant for their type of research. 38 Known examples of measurement error bias have also been reported for blood alcohol content analyses. 38 , 39
Systematic and random error
Errors could occur in a random or systematic manner. When errors are systematic, the observed measurements deviate from true values in a consistent manner, that is, they are either consistently higher or lower than the true values. For example, a device could be calibrated improperly and subtract a certain amount from each measurement. By not accounting for this deviation in the measurement, the results will contain systematic errors and in this case, true measurements would be underestimated.
For random errors, the deviation of the observed from true values is not consistent, causing errors to occur in an unpredictable manner. Such errors will follow a distribution, in the simplest case a gaussian (also called normal or bell-shaped) distribution, and will have a mean and standard deviation. When the mean is zero, the measured value should be reported within an interval around zero and an estimated amount of deviation from the actual value. When the target value is reported to fall within a range or interval of minimum and maximum levels, the size of the interval depends mainly on the size of measurement errors, that is, the larger the errors, the larger the uncertainty and hence the wider the intervals, which could affect the precision level.
Random errors could also be proportional to the measured amount. In this case, errors can be referred to as multiplicative or non-gaussian errors. 36 These random errors occur due to uncontrollable and possibly unknown experimental factors, such as laboratory environment conditions that affect concentrations in biological experiments. Examples of non-gaussian errors can be found in breath alcohol measurements, in which the variability around the measurement increases with increasing alcohol concentrations. 40 – 42
Adjusting for measurement error bias
The type and distribution of measurement errors determines the type of adjusting method. 34 When errors are systematic, calibration methods can be used to reduce their effects on the results. These methods are based on a reference measurement that can be obtained from a previous or pilot study, and used as the correct quantity to calibrate the study measurements. As such, simple mathematical tools can be used if the errors are estimated. The adjustment methods for systematic errors are simpler to apply than those for random errors.
Significant efforts have been made to develop sophisticated statistical approaches that adjust for the effect of random measurement errors. 34 Commonly available and popular statistical software packages, such as R Software Package ( http://www.r-project.org ) and the Stata (Stata Corporation, College Station, TX, USA) include features that allow adjustments to be made for random measurement errors. Some of the bias adjustment methods include simulation–extrapolation, regression calibration, and the instrumental variable approach. 34 In order to select the best adjustment approach, knowledge of the error properties is essential. For example, the amount of standard deviation and the shape of error distribution should be identified through a previous or pilot study. Therefore, evaluation of the measuring technique is recommended to identify the error properties before starting the actual measuring procedure. Error properties should also be identified for survey measurement errors, in which methods for examining the reliability and validity of the survey can be used such as test–retest and record checks.
A simpler approach used by practitioners to minimize errors in epidemiologic studies is replication; in this method, replicates of the risk factor (eg, long-term average nutrients) are available and the mean of these values is calculated and used to present an approximate value relative to the actual value. 43 These replicates can also be used to estimate the measurement error variance and apply an adjusted statistical approach.
Confirmation bias
Placing emphasis on one hypothesis because it does not contradict investigator beliefs is called confirmation bias, otherwise known as confirmatory, ascertainment, or observer bias. Confirmation bias is a type of psychological bias in which a decision is made according to the subject’s preconceptions, beliefs, or preferences. Such bias results from human errors, including imprecision and misconception. Confirmation bias can also emerge owing to overconfidence, which results in contradictory evidence being ignored or overlooked. 44 In medicine, confirmation bias is one of the main reasons for diagnostic errors and may cause inaccurate diagnosis and improper treatment management. 45 – 47
An understanding of how the results of a medical investigation are affected by confirmation bias is important. Many studies have demonstrated that any aspect of investigation that requires human judgment is subject to confirmation bias, 48 – 50 which was also found to influence the inclusion and exclusion criteria of randomized controlled trial study designs. 51 There are many examples of confirmation bias in the medical literature, some of which are even illustrated in DNA matching. 16
Overcoming confirmation bias
Researchers have shown that not accounting for confirmation bias could affect the reliability of the investigation. Several studies in the literature also suggest a number of approaches for dealing with this type of bias. An approach that is often used is to conduct multiple and independent checks on study subjects across different laboratories or through consultation with other researchers who may have differing opinions. Through this approach, scientists can seek independent feedback and confirmation. 52 The use of blinding or masking procedures, whether single- or double-blinded, is important for enhancing the reliability of scientific investigations. These approaches have proven to be very useful in clinical trials, as they protect final conclusions from confirmation bias. The blinding may involve participant, treating clinician, recruiter, and/or assessor.
In addition, researchers should be encouraged to evaluate evidence objectively, taking into account contradictory evidence, and alter perspectives through specific education and training programs, 53 , 54 with no overcorrection or change in the researcher’s decision making. 55
However, the problem with the above suggestions is that they become ineffective if specific factors of bias are not accounted for. For example, researchers could reach conclusions in haste due to external pressure to obtain results, which can be particularly true in highly sensitive clinical trials. Bias in such cases is a very sensitive issue, as it might affect the validity of the investigation. We can, however, avoid the possibility of such bias by developing and following well-designed study protocols.
Finally, in order to overcome confirmation bias and enhance the reliability of investigations, it is important to accept that bias is a part of investigations. Quantifying this inevitable bias and its potential sources must be part of well-developed conclusions.
Bias in epidemiologic and medical research is a major problem. Understanding the possible types of bias and how they affect research conclusions is important to ensure the validity of findings. This work discussed some of the most common types of information bias, namely self-reporting bias, measurement error bias, and confirmation bias. Approaches for overcoming bias through the use of adjustment methods were also presented. A summary of study types with common data collection methods, type of information bias and adjusting or preventing strategies is presented in Table 1 . The framework described in this work provides epidemiologists and medical researchers with useful tools to manage information bias in their scientific investigations. The consequences of ignoring this bias on the validity of the results were also described.
Type of study designs, common data collection methods, type of bias, and adjusting strategies
Bias is often not accounted for in practice. Even though a number of adjustment and prevention methods to mitigate bias are available, applying them can be rather challenging due to limited time and resources. For example, measurement error bias properties might be difficult to detect, particularly if there is a lack of information about the measuring instrument. Such information can be tedious to obtain as it requires the use of validation studies and, as mentioned before, these studies can be expensive and require careful planning and management. Although conducting the usual analysis and ignoring measurement error bias may be tempting, researchers should always follow the practice of reporting any evidence of bias in their results.
In order to minimize or eliminate bias, careful planning is needed in each step of the research design. For example, several rules and procedures should be followed when designing self-reporting instruments. Training of interviewers is important in minimizing such type of bias. On the other hand, the effect of measurement error can be difficult to eliminate since measuring devices and algorithms are often imperfect. A general rule is to revise the level of accuracy of the measuring instrument before utilizing it for data collection. Such adjustments should greatly reduce any possible defects. Finally, confirmation bias can be eliminated from the results if investigators take into account different factors that can affect human judgment.
Researchers should be familiar with sources of bias in their results, and additional effort is needed to minimize the possibility and effects of bias. Increasing the awareness of the possible shortcomings and pitfalls of decision making that can result in bias should begin at the medical undergraduate level and students should be provided with examples to demonstrate how bias can occur. Moreover, adjusting for bias or any deficiency in the analysis is necessary when bias cannot be avoided. Finally, when presenting the results of a medical research study, it is important to recognize and acknowledge any possible source of bias.
The author reports no conflicts of interest in this work.
Read our research on: TikTok | Podcasts | Election 2024
Regions & Countries
How hispanic americans get their news, u.s.-born latinos overwhelmingly prefer to get their news in english; about half of immigrant latinos prefer it in spanish.

Pew Research Center conducted this study to understand Hispanic Americans’ habits around news and information, including the languages in which they consume news and their engagement with Hispanic media outlets.
Most of the questions in this report are from Pew Research Center’s 2023 National Survey of Latinos, a survey of 5,078 U.S. Hispanic adults conducted Nov. 6-19, 2023. This includes 1,524 Hispanic adults on the Center’s American Trends Panel (ATP) and 3,554 Hispanic adults on Ipsos’ KnowledgePanel . Respondents on both panels are recruited through national, random sampling of residential addresses. Recruiting panelists by phone or mail ensures that nearly all U.S. adults have a chance of selection. This gives us confidence that any sample can represent the whole population, or in this case the whole U.S. Hispanic population. (For more information, watch our Methods 101 explainer on random sampling.)
To further ensure the survey reflects a balanced cross-section of the nation’s Hispanic adults, the data is weighted to match the U.S. Hispanic adult population by age, gender, education, nativity, Hispanic origin group and other categories. Read more about the ATP’s methodology . Refer to the topline for the questions used for our National Survey of Latinos , along with responses, and to methodology for more details.
The questions about how often people get news from various platforms, which platforms they prefer for getting news, and which social media sites people get news from are from an ATP survey of 8,842 U.S. adults, including 1,193 Hispanic adults, conducted Sept. 25-Oct. 1, 2023. Refer to the topline for t he questions used for this survey , along with responses, and to the methodology for more details.
Pew Research Center is a subsidiary of The Pew Charitable Trusts, its primary funder. This is the latest report in Pew Research Center’s ongoing investigation of the state of news, information and journalism in the digital age, a research program funded by The Pew Charitable Trusts, with generous support from the John S. and James L. Knight Foundation.
The terms Hispanic and Latino are used interchangeably in this report.
Hispanic/Latino Americans, Hispanic/Latino adults , and Hispanics/Latinos are used interchangeably in this report to refer to survey respondents who self-identify as Hispanic or Latino in the United States. They include those who say their race is White, Black, Asian or some other race and those who identify as multiracial. Hispanic/Latino Americans live in the U.S. but are not necessarily U.S. citizens.
U.S. born refers to people born in the 50 states or the District of Columbia.
Immigrant refers to people born outside the 50 states or D.C. For the purposes of this report, immigrants include those born in Puerto Rico or another U.S. territory. Although individuals born in Puerto Rico are U.S. citizens by birth, they are grouped with immigrant respondents because they were born into a Spanish-dominant culture and because on many points their attitudes, views and beliefs more closely resemble those of Hispanics born outside the U.S. than Hispanics born in the 50 states or D.C., and even U.S.-born Hispanics who identify as being of Puerto Rican origin.
Second generation refers to people born in the 50 states or D.C. who have at least one parent born in a different country, Puerto Rico or another U.S. territory.
Third generation or higher refers to people born in the 50 states or D.C. who have two parents born in the 50 states or D.C.
Language dominance is a composite measure based on self-described assessments of speaking and reading abilities. Spanish-dominant people are more proficient in Spanish than in English (i.e., they speak and read Spanish “very well” or “pretty well” but rate their English ability lower). Bilingual refers to people who are proficient in both English and Spanish. English-dominant people are more proficient in English than in Spanish.
“Middle income” is defined here as two-thirds to double the median annual family income for panelists on the American Trends Panel. “Lower income” falls below that range; “upper income” falls above it. Refer to the methodology for more details.
Hispanic news outlets are those outlets that focus on providing news and information specifically to Hispanic audiences. These can include newspapers, radio or TV stations, podcasts, or social media accounts created for and by Hispanic people. Their content could be in Spanish, English, both languages or another language.
Country of origin refers to the country that survey respondents, their parents or their Hispanic ancestors came from.
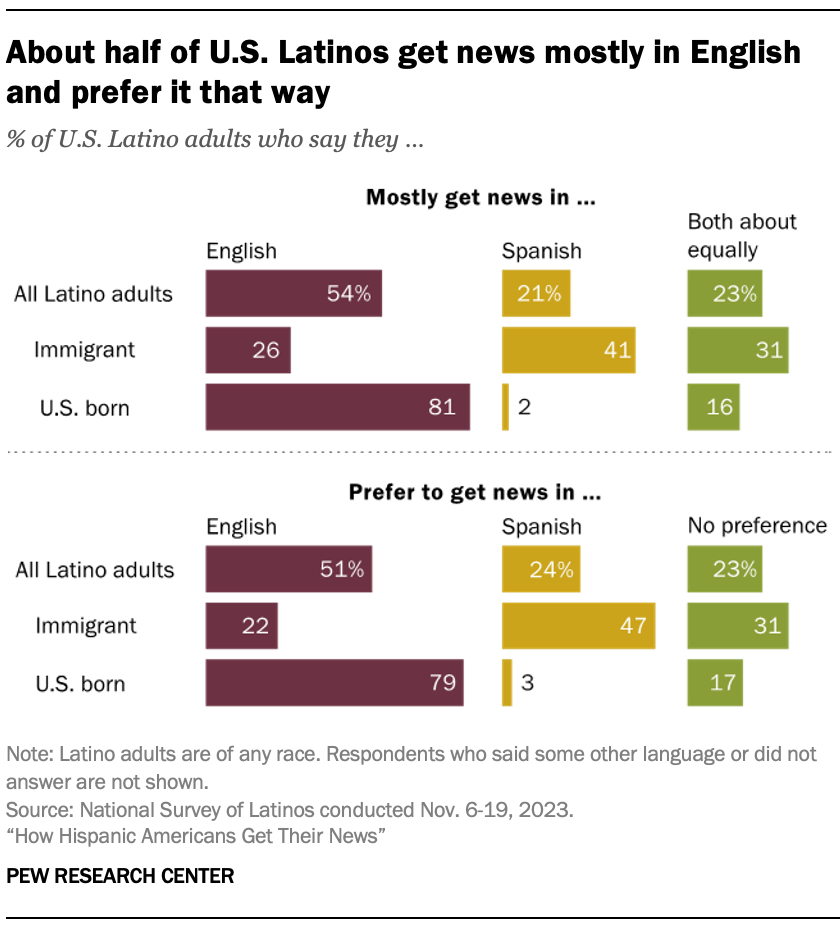
Just over half of U.S. Hispanic adults (54%) get their news mostly in English – far higher than the share who get their news mostly in Spanish (21%). About a quarter of Hispanic Americans (23%) say they consume news in both languages about equally.
There is an almost identical pattern on the question of preferred language for news: 51% prefer to get their news in English, 24% prefer Spanish and 23% say they do not have a preference.
But a new Pew Research Center survey of adults who identify as Hispanic or Latino finds major differences in news consumption habits between U.S.-born Hispanics and those who immigrated from other countries .
While U.S.-born Latinos overwhelmingly get their news in English, and prefer it in English, those born outside the United States have much more varied habits: 41% get their news mostly in Spanish, 26% get it primarily in English and 31% do both about equally. Similarly, 47% of Latino immigrants prefer to get their news in Spanish, while 22% prefer English and 31% do not express a preference.
Among Latino immigrants, those who have spent more years in the U.S. are less inclined than more recent arrivals to get news in Spanish, and more inclined to get it in English. There is little difference in the shares who get news in both languages about equally.
Jump to more information on the languages in which U.S. Latinos consume news.
We asked these questions to better understand how a group that makes up nearly one-in-five Americans stays informed, especially as its demographics and use of Spanish continue to change. Immigrants are declining as a share of all U.S. Hispanics , and the share of Hispanics who speak Spanish at home has also dropped – even though the number of Hispanics who speak Spanish at home has increased due to overall growth in the Hispanic population.
Other key findings about Hispanics’ news consumption include:
Most Latino adults prefer digital devices for news
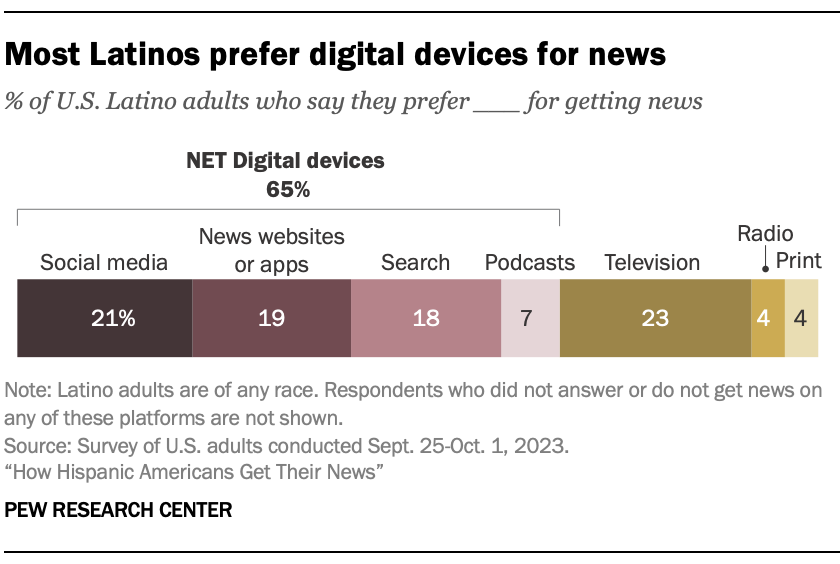
Latinos get their news from a variety of sources, but most say they prefer to use digital devices over other platforms. Nearly nine-in-ten (87%) say they get news from digital devices at least sometimes, and 65% say they prefer this form of news over TV, radio or print. Digital devices have become an increasingly common source for news among Latinos – and among Americans overall – in recent decades, a shift driven by the rise of the internet .
Latinos are more likely than White Americans (55%) and Black Americans (50%) to prefer getting news from digital devices. Latinos also are more likely than White and Black adults to get news from social media, at least in part because Latino adults tend to be younger than other groups, and young adults are more inclined to use social media for news.
Nearly three-quarters of Latino adults under 50 (73%) prefer to get their news on digital devices, including 27% who prefer social media specifically.
Jump to more information on the platforms where U.S. Latinos get news.
Attention to news is declining among U.S. Latinos
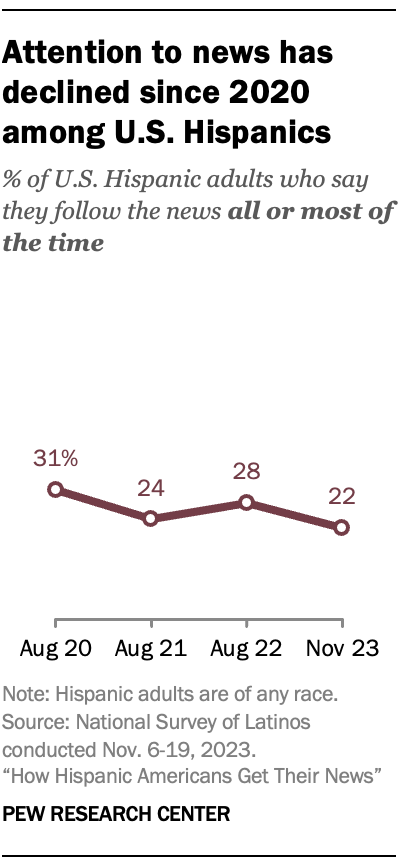
About one-in-five Latino adults (22%) say they follow the news all or most of the time, while an additional 36% follow the news some of the time. The share of Latinos who follow the news all or most of the time has fluctuated in recent years but has dropped by 9 percentage points between 2020 (31%) and 2023 (22%), similar to a pattern seen across the general U.S. public .
In recent years, Hispanic Americans have followed the news less closely than Black and White Americans. Again, the high share of young adults within the Hispanic population plays a role, because young people are less likely to follow the news closely. Among Hispanic adults ages 18 to 29, just 10% say they follow the news all or most of the time – far below the share of Hispanics ages 65 and older who do so (44%).
Jump to more information on U.S. Hispanics’ news consumption habits.
Half of Hispanic adults get news from Hispanic news outlets
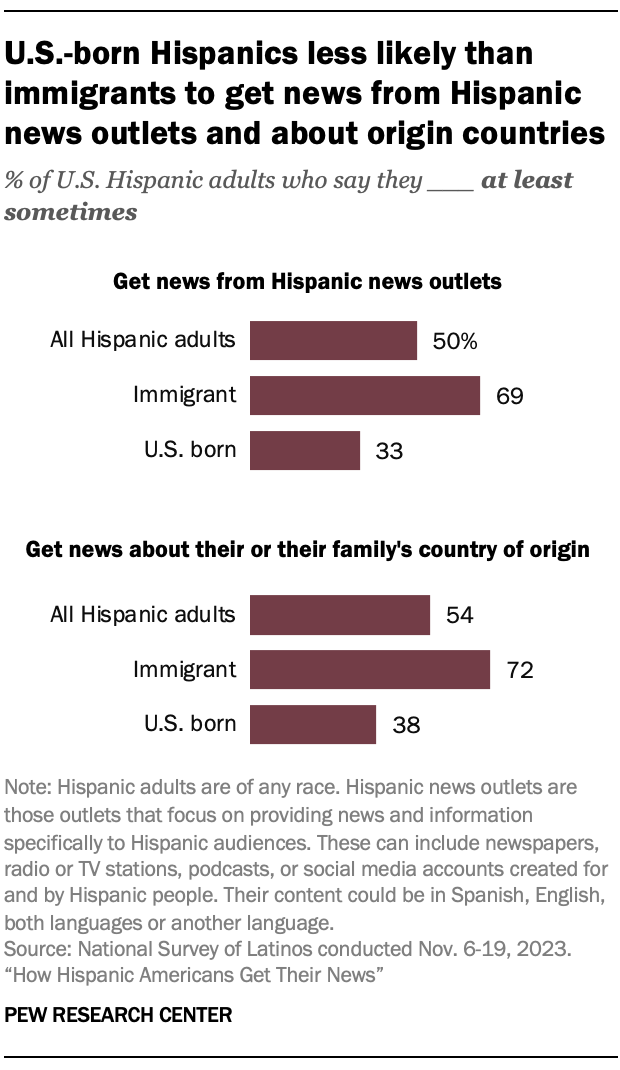
Half of U.S. Hispanic adults say they at least sometimes get news from Hispanic news outlets – those that specifically cater to Hispanic audiences. This includes 21% who say they do this extremely or very often. Just over half of Hispanics (54%) get news about their or their family’s country of origin at least sometimes, including 24% who do this often.
Hispanic immigrants are much more likely than U.S.-born Hispanics to get news from Hispanic outlets and about their origin country. In both cases, about seven-in-ten immigrants say they at least sometimes get these types of news: 69% get news from Hispanic outlets and 72% get news about their country of origin. Among Hispanic adults who were born in the U.S., 33% at least sometimes get news from Hispanic outlets, and 38% get news about their family’s country of origin.
There are further differences among U.S.-born Hispanics: Those whose parents were also born in the U.S. are even less likely than those with one or more immigrant parent to get these types of news.
Jump to more information on Hispanic news outlets and news about Hispanic Americans’ origin countries.
Sign up for The Briefing
Weekly updates on the world of news & information
Report Materials
Table of contents, latinos’ views on the migrant situation at the u.s.-mexico border, 8 facts about black americans and the news, news platform fact sheet, latinos’ views of and experiences with the spanish language, hispanic and black news media fact sheet, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .

IMAGES
VIDEO
COMMENTS
11. Preliminary guideline for reporting bibliometric reviews of the biomedical literature (BIBLIO) : a minimum requirements. 12. Appropriate design and reporting of superiority, equivalence and non-inferiority clinical trials incorporating a benefit-risk assessment: the BRAINS study including expert workshop. 13.
For reporting animal research and peer-reviewers of animal research studies. Scientists developed the guidelines, originally published in PLOS Biology, in consultation with the scientific community as part of a National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3R).
Reporting guidelines have been developed to help improve the reporting of specific study designs. If followed by authors this should enable users to understand the design, conduct and analysis of the research, to critically appraise and review the findings and interpret the conclusions appropriately [ 4 ]. A guideline is a checklist, diagram or ...
Reporting guidelines are statements intended to advise authors reporting research methods and findings. They can be presented as a checklist, flow diagram or text, and describe what is required to give a clear and transparent account of a study's research and results. These guidelines are prepared through consideration of specific issues that may introduce bias, and are supported by the latest ...
Some of the reporting guidelines for common study designs are: Randomized controlled trials - CONSORT. Systematic reviews - PRISMA. Observational studies - STROBE. Case reports - CARE. Qualitative research - COREQ. Pre-clinical animal studies - ARRIVE. Peer reviewers may be asked to use these checklists when assessing your manuscript.
Reporting guidelines list and describe the key information that authors should include in a manuscript that describes a research study or literature review, to ensure that readers can understand, evaluate, and/or replicate the study or review. 1 Reporting guidelines complement a journal's instructions for authors, which tend to focus on ...
Checklist for qualitative studies. Compose a title that includes the term "qualitative", the population, condition, place and time. Use a structured abstract format with the following section headings: Background, Objective, Methods, Findings and Conclusion. Identify the topic of the study and why it is important.
As a response to a low quality of reporting of medical research, guidelines for several different types of study design have been developed to secure accurate reporting and transparency for reviewers and readers from the scientific community. Herein, we review and discuss the six most widely accepted and used guidelines: PRISMA, CONSORT, STROBE ...
Appropriate criteria for evaluating rigor in qualitative research depend on the specifics of the approach and methods used, but inadequate reporting hinders the ability of readers to interpret and assess the strength of any qualitative study. 1-5 The Standards for Reporting Qualitative Research (SRQR) 5 and the Consolidated Criteria for Reporting Qualitative Research (COREQ) 4 are 2 attempts ...
The reporting of diagnostic test accuracy studies, screening studies, therapeutic studies, systematic reviews and meta-analyses, cost-effectiveness studies, recommendations and/or guidelines, and medical education studies is discussed in this article. The available guidelines, which can be found at the EQUATOR network, are summarized in Table 5.
This article is the first in a series of two articles that will review how to report and how to critically appraise research in health care. In this article, the reporting of diagnostic test accuracy and screening studies, therapeutic studies, systematic reviews and meta-analyses, cost-effectiveness studies, recommendations and/or guidelines, and medical education studies is discussed.
When reporting the results of research that includes racial and ethnic disparities and inequities, authors are encouraged to provide a balanced, evidence-based discussion of the implications of the findings for addressing institutional racism and structural racism as these affect the study population, disease or disorder studied, and the ...
Studies of diagnostic accuracy should meet the Standards for Reporting of Diagnostic Accuracy . Additional standards and checklists may be relevant depending on the type of study conducted. Therefore, authors are encouraged to review the Enhancing the QUAlity and Transparency of health Research ( EQUATOR ) information in the Reporting Standards ...
Table 1: Standards for Reporting Qualitative Research (SRQR) a. To explicate how the final set of standards reflect the material in the original sources, two of us (B.O., D.A.C.) selected by consensus the 25 most complete sources of recommendations and identified which standards reflected the concepts found in each original source (see Table 2 ).
The NIH IRP has revised its policies on reporting research events and non-compliance in human subjects research. Investigators are expected to be familiar with and comply fully with the reporting requirements as specified in . Policy 801 Reporting Research Events. and with the requirements specified . Policy 802 Non-compliance in Human Subjects ...
Recommendations on the reporting of studies that are endorsed by leading medical journals can improve the quality of reporting. Observational research comprises several study designs and many topic areas. We aimed to establish a checklist of items that should be included in articles reporting such research - the STROBE Statement.
Reporting of qualitative research studies. Full bibliographic reference: O'Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89(9):1245-1251. Language: English: PubMed ID: 24979285: Relevant URLs
Methods. We conducted a review of the literature to identify: 1) guidance for reporting population modelling studies; and, 2) evidence on the quality of reporting of population modelling studies. Guidance for reporting was analysed using a thematic approach and the data was summarised as frequencies. Evidence on the quality of reporting was ...
Thesis is a type of research report. A thesis is a long-form research document that presents the findings and conclusions of an original research study conducted by a student as part of a graduate or postgraduate program. It is typically written by a student pursuing a higher degree, such as a Master's or Doctoral degree, although it can also ...
Research Portfolio Online Reporting Tools(RePORT) In addition to carrying out its scientific mission, the NIH exemplifies and promotes the highest level of public accountability. To that end, the Research Portfolio Online Reporting Tools provides access to reports, data, and analyses of NIH research activities, including information on NIH ...
Registered Report (18) Year. All. All; 2024 (6655) 2023 (22198) 2022 (21835) 2021 (23320) 2020 (21191) ... A radiographic and histological study to compare red (650 nm) versus near infrared (810 ...
The report, once completed, will help guide innovation and investments in menopause-related research and care across the federal government and research community.
Many studies are rejected for publication because of criticism that a study is underpowered, though many more studies are published despite this. 74 Reporting how a sample size was predetermined based on power analyses conducted during the experimental design stage is a good way to avoid this criticism.
A new study projects that global fertility rates, which have been declining in all countries since 1950, will continue to plummet through the end of the century, resulting in a profound ...
The budget was released Wednesday by the Republican Study Committee, a group of more than 170 House GOP lawmakers, including many allies of Republican presidential nominee Donald Trump.Apart from ...
The report, compiled annually by a consortium of groups including the United Nations and Gallup, was the latest data point in what some researchers have described as a crisis among America's youth.
Petersen has also conducted similar studies investigating the impact of food-based interventions, including the relationship between pistachios and diet quality, but said that more research is ...
A study of over 20,000 adults found that those who followed an 8-hour time-restricted eating schedule, a type of intermittent fasting, had a 91% higher risk of death from cardiovascular disease. ... Statements and conclusions of studies that are presented at the American Heart Association's scientific meetings are solely those of the study ...
The issue of self-reporting bias represents a key problem in the assessment of most observational (such as cross-sectional or comparative, eg, case-control or cohort) research study designs, although it can still affect experimental studies. Nevertheless, when self-reporting data are correctly utilized, they can help to provide a wider range ...
Pew Research Center conducted this study to understand Hispanic Americans' habits around news and information, including the languages in which they consume news and their engagement with Hispanic media outlets. ... Most of the questions in this report are from Pew Research Center's 2023 National Survey of Latinos, a survey of 5,078 U.S ...