Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions

Sickle Cell Disease : A Review
- 1 Division of General Pediatrics, Boston University School of Medicine, Boston Medical Center, Boston, Massachusetts
- 2 Division of Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston
- 3 School of Medicine, Division of Hematology and Oncology, University of California Davis, Sacramento
- Comment & Response A Review of Sickle Cell Disease—Reply Patricia L. Kavanagh, MD; Titilope Fasipe, MD, PhD; Ted Wun, MD JAMA
- Comment & Response A Review of Sickle Cell Disease Nikolaos Vlachadis, MD, DMD, MPH, MSc, DSc; Nikolaos Vrachnis, MD, PhD JAMA
- JAMA Clinical Guidelines Synopsis Diagnosis and Management of Priapism Richard J. Fantus, MD; Robert E. Brannigan, MD; Andrew M. Davis, MD, MPH JAMA
Importance Sickle cell disease (SCD) is an inherited disorder of hemoglobin, characterized by formation of long chains of hemoglobin when deoxygenated within capillary beds, resulting in sickle-shaped red blood cells, progressive multiorgan damage, and increased mortality. An estimated 300 000 infants are born annually worldwide with SCD. Most individuals with SCD live in sub-Saharan Africa, India, the Mediterranean, and Middle East; approximately 100 000 individuals with SCD live in the US.
Observations SCD is diagnosed through newborn screening programs, where available, or when patients present with unexplained severe atraumatic pain or normocytic anemia. In SCD, sickling and hemolysis of red blood cells result in vaso-occlusion with associated ischemia. SCD is characterized by repeated episodes of severe acute pain and acute chest syndrome, and by other complications including stroke, chronic pain, nephropathy, retinopathy, avascular necrosis, priapism, and leg ulcers. In the US, nearly all children with SCD survive to adulthood, but average life expectancy remains 20 years less than the general population, with higher mortality as individuals transition from pediatric to adult-focused health care systems. Until 2017, hydroxyurea, which increases fetal hemoglobin and reduces red blood cell sickling, was the only disease-modifying therapy available for SCD and remains first-line therapy for most individuals with SCD. Three additional therapies, L-glutamine, crizanlizumab, and voxelotor, have been approved as adjunctive or second-line agents. In clinical trials, L-glutamine reduced hospitalization rates by 33% and mean length of stay from 11 to 7 days compared with placebo. Crizanlizumab reduced pain crises from 2.98 to 1.63 per year compared with placebo. Voxelotor increased hemoglobin by at least 1 g/dL, significantly more than placebo (51% vs 7%). Hematopoietic stem cell transplant is the only curative therapy, but it is limited by donor availability, with best results seen in children with a matched sibling donor. While SCD is characterized by acute and chronic pain, patients are not more likely to develop addiction to pain medications than the general population.
Conclusions and Relevance In the US, approximately 100 000 people have SCD, which is characterized by hemolytic anemia, acute and chronic pain, acute chest syndrome; increased incidence of stroke, nephropathy, and retinopathy; and a life span that is 20 years shorter than the general population. While hydroxyurea is first-line therapy for SCD, L-glutamine, crizanlizumab, and voxelotor have been approved in the US since 2017 as adjunctive or second-line treatments, and hematopoietic stem cell transplant with a matched sibling donor is now standard care for severe disease.
Read More About
Kavanagh PL , Fasipe TA , Wun T. Sickle Cell Disease : A Review . JAMA. 2022;328(1):57–68. doi:10.1001/jama.2022.10233
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 April 2024
Multi-center study on mortality in children, and adults with sickle cell anemia-risk factors and causes of death
- Salam Alkindi 1 ,
- Salma Al-Jadidi 1 ,
- Safa Al-Adawi 1 ,
- Refaat Abdullah Elsadek 2 ,
- Ali Al Madhani 3 ,
- Maryam Al-Nabhani 4 &
- Anil V. Pathare 1
Scientific Reports volume 14 , Article number: 8584 ( 2024 ) Cite this article
235 Accesses
7 Altmetric
Metrics details
- Health care
- Medical research
- Risk factors
Sickle cell disease (SCD) is a major public health burden worldwide with increasing morbidity and mortality. The study evaluates the risk factors associated with mortality in SCD patients, between the years 2006 and 2020 at three hospitals in Oman. The analysis includes clinical manifestations, haematological, biochemical, and radiological parameters, use of antibiotics, and blood and exchange transfusions. Our cohort included 123 patients (82 males, 41 females), with a median age of 27 (Interquartile Range 21–35 years). SCD related complications included acute chest syndrome (ACS) in 52.8%, splenic sequestration in 21.1%, right upper quadrant syndrome in 19.5%, more than > 6 VOC/year in 17.9%, and stroke in 13.8%. At the terminal admission, patients had cough, reduced O 2 saturation, crepitation and fever in 24.4%, 49.6%, 53.6% and 68.3% respectively. Abnormal chest X-ray and chest CT scan were seen in 57.7%, and 76.4% respectively. Laboratory parameters showed a significant drop in hemoglobin (Hb) and platelet counts from baseline, with a significant rise in WBC, LDH and CRP from baseline ( p < 0.05, Wilcoxon Signed Ranks test). All patients received antibiotics, whereas, 95.9% and 93.5% received simple blood transfusions, and exchange transfusions respectively, and 66.6% required non-invasive ventilation. Among the causes of death, ACS is seen in 32 (26%), sepsis in 49 (40%), and miscellaneous in 42 (34%). Sudden death was seen in 32 (26%) of patients. Male gender, with low HbF, rapid drop in Hb and platelet, and increased in WBC, LDH, ferritin, and CRP, correlated significantly with mortality in this cohort.
Similar content being viewed by others

Acute kidney injury
John A. Kellum, Paola Romagnani, … Hans-Joachim Anders

Diagnosis and management of Guillain–Barré syndrome in ten steps
Sonja E. Leonhard, Melissa R. Mandarakas, … Bart C. Jacobs

Antoni Torres, Catia Cilloniz, … Tom van der Poll
Introduction
Sickle cell disease (SCD) is one of the commonest monogenic diseases with highest prevalence in sun-saharan Africa (500–2000/100.000) 1 . It is caused by a single amino acid mutation, where glutamic acid is replaced by valine in the beta chain of hemoglobin molecule 2 , 3 . The hemoglobin-S polymerizes in post-capillary venules, causing the red blood cells to assume a distorted, sickle-like shape.
Sickle cell disease is a major public health problem in Oman with a marked increase in morbidity and mortality. The estimated sickle cell gene frequency is between 5.8%, and 5.1% with the prevalence of sickle cell trait and SCD is 4.8% and 0.3% respectively 4 , 5 .
Sickle cell disease is characterized by repetitive episodes of vaso-occlusion causing painful episodes, hemolytic anemia, and increased risk of infections impacting severely on survival. Survival estimates however, have continued to improve, with over 95% of SCD patients reaching adulthood, at least in the developed world 6 . In 1994, Platt reported the median survival for patients with HbSS/Sβ 0 thalassemia to be 42–48 years for males and females respectively 7 . This has increased to 53 and 58 years in Jamaica by 2001 8 , and 58 years in the United States in 2014 9 . In recent years, patients are reported to live into their eighth decade 10 . This improvement is attributed to the widespread use of newborn screening programs, penicillin prophylaxis, the universal vaccination program, and the establishment of a comprehensive clinical care program (CCCP) with Hydroxyurea, and blood transfusions 11 .
Although survival rate for children and adults with SCD has improved, however there are still patients who are lost prematurely 11 , 12 , 13 , 14 , 15 . There are many causes of death including sepsis, painful episodes precipitating acute chest syndrome (ACS), and stroke. Previous studies have attempted at identifying risk factors for death including the development of dactylitis, co-inheritance of alpha thalassemia, high Hb F, low baseline Hb, and high WBC count as predictors of severe adverse events 13 , 14 . Some of these were substantiated in subsequent studies, and others were not. The data on specific causes and risk factors for mortality in our country are not available. In a study by Tawfic et al. 16 , ACS was the main cause of ICU admission in patients with SCD. Further, the use of inotropic support and/or mechanical ventilation was an indicator of a high mortality rate among these patients. In another study in children with SCD, Jaiyesimi and Kasem 17 showed that ACS was common irrespective of SCD severity, and all patients appeared to be at risk, but it was increased by Vaso-occlusive crisis (VOC).
Although ACS has remained a leading cause of death, there are no established risk factors for death in patients with SCD in Oman. We are presenting death from three different hospitals in Oman, to identify the main causes of death and its associated risk factors.
Materials and methods
In this retrospective study, 123 SCD patients, who died between 2006 and 2020 at three hospitals in Oman were enrolled, after written approval from the Medical Research Ethics Committee (MREC # 1322), College of Medicine & Health Sciences. All methods were carried out in accordance with relevant guidelines and regulations of the Institution where this research was carried out. Due to the retrospective nature of the study, the need for informed consent was waived by the Medical Research Ethics Committee (MREC #1322). The inclusion criteria included SCD patients of any age, who died between 2006 and 2020. The data was obtained from the electronic patient record system and included demography, the season during death, previous SCD manifestations such as the frequency of painful crisis, ACS, splenic and hepatic sequestration, dactylitis, and stroke. We also analyzed the hematological, biochemical, and radiological parameters at baseline (stable during Outpatient visit), and in the terminal event, as well as the use of antibiotics, ventilatory support, and use of blood /exchange transfusions.
The laboratory results were obtained at baseline and at the terminal event. Hemoglobin and platelet counts were obtained at baseline and nadir, whereas, reticulocyte counts, and white blood cell count (WBC) were recorded at baseline and their maximum. Biochemical parameters included C-reactive protein (CRP), Liver function tests, and Serum LDH and were collected on arrival and at maximum during the terminal event. Additionally, data on basal serum ferritin and HbF were also collected.
Definitions of parameters and SCD complications
To classify the time of death, the year was divided into two seasons based on a mean temperature of 33 °C, between April and August, and a mean temperature of 25 °C between September and March. Stroke was defined as acute neurologic syndrome due to vascular occlusion or hemorrhage in which neurologic symptoms or signs lasted more than 24 h. ACS was defined as the presence of new pulmonary infiltrates on a chest X-ray film, CT scan of the chest, or both, in association with acute respiratory tract symptoms. Sudden death was defined as death from any cause happening within 24 h after hospital admission. Acute splenic sequestration was defined as a decrease from baseline in the hemoglobin level or hematocrit of at least 20 percent, plus a simultaneous increase in the size of the spleen to at least 2 cm below the left costal margin. Right upper quadrant syndrome was defined as SCD painful crisis with signs and symptoms affecting the liver or gallbladder.
Clinical symptoms recorded included temperature, respiratory symptoms, and O 2 saturation by pulse oximetry. Radiology studies including chest X-ray, CT scan and ultra sound of abdomen. Further, SCD therapeutic management protocols like non-invasive ventilatory support, antibiotics, blood transfusion and blood exchange were also recorded. Terminal events were defined as the last hospital admission leading to death of the patient. Causes of death were classified to ACS (clinical & radiological signs), sepsis (clinical, and laboratory evidence with positive microbial culture) and miscellaneous (all others). Miscellaneous causes include cardiac events, pulmonary embolism, stroke, RTA and malignancy.
Statistical analysis
The statistical package for social science (IBM SPSS, USA ver.23, Armonk, NY) was used to analyze the collected data. Normally distributed data were characterized as mean with standard deviation, whereas, data that was not normally distributed was characterized as median with interquartile range (IQR) for continuous variables and percentage and frequency for categorical variables. Wilcoxon Signed Ranks test was used to test the significance of the association between subgroups for various clinical and laboratory parameters. An alpha of < 0.05 was considered to be the statistically significant p value.
123 patients (82 males, 41 females) who were enrolled in the study had a median age (IQR) of 27 (21–35) years, with a range between 1.5 to 79 years. Painful VOC episodes > 6/year were seen in almost one-fifth of this cohort (17.9%), while most patients had a significant past history of SCD-related complications including ACS (52.8%), splenic sequestration (21.1%), right upper quadrant syndrome (19.5%) and stroke (13.8%) (Table 1 ). At the terminal event, fever, cough, abnormal findings in chest examination, and reduced O 2 saturation (pulse oximetry) were seen in 68.3%, 24.4%, 53.6%, and 49.6% respectively.
Amongst the haematology parameters, there was a significant drop in the median hemoglobin and platelet counts from baseline, with a significant rise in the WBC counts ( p < 0.05, Wilcoxon signed ranks test). The median level of baseline hemoglobin (g/dl) dropped significantly from 9.5 to 6.8 g/dl. Reticulocytes were elevated at 5.75%. The median level of white blood cell count (× 10 9 /L) increased significantly to 21 from a baseline level of 11 ( p < 0.05). The median platelet count (× 10 9 /L) dropped significantly to 80 from a baseline level of 319 ( p < 0.05). Amongst the biochemical parameters, the median CRP levels (mg/L) significantly increased from 43 at arrival to the hospital, to the median CRP max value of 171 ( p < 0.05). Similarly, the median serum bilirubin levels (mg/dl) rose significantly from 34 at admission to 105 ( p < 0.05). Further the median serum LDH levels (U/L) also significantly increased from 652 at admission to 1616 at the time of the terminal event ( p < 0.05).
Chest X-ray was abnormal in 71 (57.7%) patients, whereas, the ultrasound showed abnormalities among 54 patients (43.9%). Although a CT scan was done only in 94 patients, it was abnormal in 84 (89.3%) patients. All these patients received antibiotics, whereas, simple and exchange blood transfusions were given in 118 (95.9%) and 115 (93.5%) of patients respectively. Further, 82 patients (66.6%) required ventilatory support.
At the terminal event, 32 (26%) patients died within 24 h of admission to the hospital. 49 (40%) died with sepsis, 32 (26%) died due to ACS associated with multi-organ failure, while the remaining 42 (34%) died due to miscellaneous causes including cardiac events, pulmonary embolism, stroke, RTA, and some died at home. (Table 2 ) Mortality was significantly correlated with a cutoff of basal Hb, basal WBC count, and HbF% of 7 gm/dl, 15 × 10 9 /L, and 8.5% respectively. Leukocytosis was seen in a small number of patients at baseline, but it was significantly high at the time of death. In addition, HbF was a strong predictor of death in all groups. The majority of patients 89 (72.4%) who died had a HbF < 8.5%. About 30% of all the patients, had baseline thrombocytosis, however many of the patients had thrombocytopenia at the time of death. Splenectomy (surgical or auto-splenectomy) was seen in about 50% of all the groups, but no significant differences between all causes of death.
In addition, death due to ACS was significantly seen in the colder months of the year, ( p = 0.04), and sudden death was more likely to happen in ACS, and sepsis group. When we looked at miscellaneous causes of death, cardiac events were most frequent, followed by pulmonary embolism, and stroke. There were a few cases, who died at home, RTA, or cancer (Fig. 1 ).
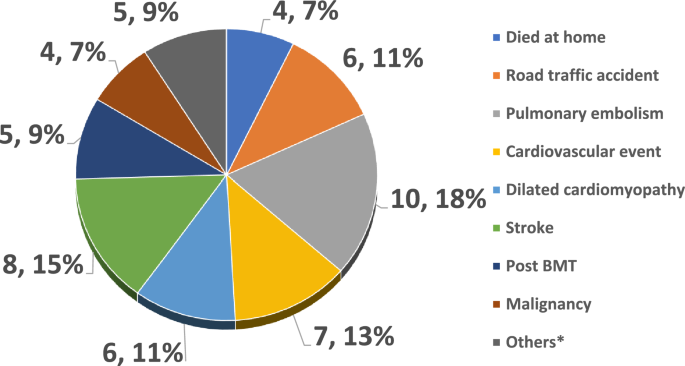
Details of miscellaneous causes (n, %).
Table 3 shows a comparison of the various risk factors affecting mortality from a literature review. It shows that although survival has improved, patients still die prematurely, especially males; and in this study, the overall proportion of death among males was double that seen in females (66% vs 33%) although the gender ratio in the general SCD Omani patients is 0.51 19 .
Amongst 67 cases with sepsis, 53 (79.1%) were culture-proven, with 11 (20.7%) patients showing multiple microorganisms, while bacterial, fungal, and viral microorganisms were isolated in 32 (60.4%), 6 (11.3%), and 4 (7.5%) respectively (Table 4 ). Among the positive cultures, gram-negative organisms were the commonest, and the highest was seen in the sepsis group, with an increasing number of fungal and viral illnesses. Also, it’s important to note that 16.5% of the cohort had serological evidence of viral hepatitis B, C, and E.
Although survival has improved in SCD, patients still die prematurely, especially males; and in this retrospective study, the overall proportion of death among males was double that seen in females. Similarly, the median age in males was lower (26.5 v/s 28 years), as compared to the females, but this difference was not statistically significant. Comparable findings were also reported in other studies from the region and beyond 14 , 15 , 18 , 20 , 21 .
Karacaoglu et al. 20 , from Turkey, demonstrated that there were more deaths among males, than females, and the mean age of death in their female population was 40.1 years. Although patients are still dying young, data from a French SCD cohort in children under the age of 5 years showed that there is an improvement in survival attributed to the establishment of what is known as complete comprehensive patient care for SCD 21 . Al-Suliman et al. 22 in a study on adults from the eastern province in Saudi Arabia reported a higher prevalence of males 55.8% versus females 44.2% female, with the mean age of the male patients being 30 ± 14 years (range, 16–67 years) and of the female patients 27 ± 13 years (range, 14–67 years). The first Brazilian single institutional study in 2017 showed that the mortality rate was 18.87% among adult SCD patients 23 . Further, combined data between 1997 and 2017, from Brazil showed that mortality was more in the males (50.4%), and patients aged between 25 and 34 years had a higher incidence of deaths 24 . In another study from Brazil between 2000 and 2018, during the entire period the mean ages at death is significantly lower for males, than for females, being 29.4 (± 19.6) versus 33.3 (± 20.3) years respectively 25 . In a study from the UK, the CCU mortality for SCD patients was 19.6% between 2000 and 2007, with the mean ages for males and females respectively being 32.6 and 34.25 years 26 . Further, in a study from the USA with data spanning over 27 years between 1979 and 2005, the mean age at death was significantly different for males (33.4 years) than for females (36.9 years) 27 . Nonetheless, in recent years, patients are reported to live into their eighth decade as reported by Ballas 10 and indeed in this study cohort, we had a SCD patient who died at the age of 79.
Significantly VOC (> 6 episodes/year) were seen in about 20% of cases, reflecting that recurrent VOC remains a marker of severe disease, and these patients accumulate disease-related end-organ damage that leads to their mortality 28 . However, about two-thirds of patients in this cohort (63.4%) had relatively mild disease, indicating the need for intense vigilance in picking up alarm signs for early mortality in some of these relatively mild disease cases.
Many of our patients had a history of ACS, stroke, hepatic & splenic sequestration as well as dactylitis, which were shown to be independent predictors of early childhood mortality in SCD patients 7 , 8 , 9 , 20 . Sixty-five (52.8%) patients had a prior history of ACS, one of the major complications of SCD, and contributed to the terminal event in 25(20.3%) of this cohort. Furthermore, 84 (68.3%) patients presented with fever, cough (24.4%), and 61(49.6%) had reduced O 2 saturation, while 67 (54.5%) had abnormal findings in the chest examination in the terminal episode, with 82 (66.6%) needing ventilatory support. Similarly, 71 (57.7%) patients in this cohort had an abnormal chest X-ray, which was one of the defining features of ACS, and 77.8% had an abnormal CT scan of the chest. Importantly, Knight-Madden et al. 29 , and Tawfiq et al. 16 have shown that mechanical ventilation was found to predict mortality and increased utilization of hospital resources. In this cohort 66.6% needed ventilator assistance, the majority of these patients received simple blood transfusions [118 (95.9%)] and many needed exchange transfusions [115 (93.5%)] as well.
Sepsis seems to be a major contributor to the terminal event as seen in 49 (40%) of cases in this cohort. These patients were characterized by a significantly high CRP, and LDH along with a significant rise in the WBC counts from the baseline, and a significant drop in platelets 30 . Among patients with sepsis, almost 80% of these patients had positive microbial cultures confirming sepsis. These patients received multiple broad-spectrum antibiotics during their terminal event. In the cooperative study of SCD from the USA, only a few bacteria were detected in lung samples at bronchoscopy, possibly since bronchoscopy was performed after empiric antibiotic administration, which is a universal practice 31 . Furthermore, even among SCD patients who had an autopsy done on them, sepsis was identified as the leading cause of death in a postmortem study 32 . More recent paper by Ballas indicated that sepsis contributed to death in a significant number of patients with SCD 33 .
Among the miscellaneous causes, multiorgan failure with sudden death including cardiac events with dilated cardiomayopathy seem to be a leading cause, although pulmonary embolism, stroke and RTA were also other significant causes of mortality in this cohort. Sudden death with bone marrow fat emboli seems to be associated with HbSC and HbSβ + Thalassaemia or the milder SCD phenotypes 34 . However, in this SCD patient cohort HbSβ + Thalassaemia was seen in 22.5% with no HbSC patient and 75.5% patients having HbSS genotype. This is similar to findings from other studies 15 , 18 . Similarly pulmonary embolism (PE), stroke, and RTA were also significant causes of mortality in this cohort. PE is the leading presentation of thromboembolic complications in SCD 35 , 36 , and stroke prevalence was relatively low in our population (probably due to the high prevalence of alpha thalassemia, Alkindi et al. 5 ); however it remains as an important cause of morality. RTA contributed to about 5% of overall total deaths, although no data is available if it was linked to opioid use while driving, on not 33 . Among the miscellaneous also few patients had Bone marrow transplant with GVHD and died with sepsis, and also few patients had malignancy including acute myeloid leukemia, multiple myeloma, sarcoma and myelodysplasia.
Laboratory data showed that there was a statistically significant drop in levels of hemoglobin and platelets count at the terminal event in this study cohort (Table 1 ). A low hemoglobin level has been previously shown to correlate with an increased risk of death with stroke 18 . Although the majority of patients had a baseline Hb > 7 g/dl, the median Hb fell from 9.5 to 6.8, during the terminal event, in all causes of death (Table 2 ). On the contrary, literature reports show that a higher hemoglobin (Hb) level correlated with an increased risk of acute chest syndrome and painful crisis 18 . This was also seen in this study, using the cutoff basal Hb > 7 g/dl and basal WBC > 15 × 10 9 /L (Table 2 ). Consistent with this, many patients in this cohort needed either simple transfusion (95.9%) or exchange transfusions (93.5%).
In a previous prospective study by Plat et al. 7 , the most straightforward laboratory risk factor related to the cause of death was fetal hemoglobin level. Patients with high levels had an improved life expectancy, while adults who had low levels of fetal hemoglobin as children were likely to die earlier than those who had high levels 7 . Thus, when using a cutoff of HbF of 8.5, there was a clear protective effect of high HbF against all types of death, with a significant proportion of these deaths occurring in the low HbF group, ( p < 0.05, Wilcoxon signed ranks test, Table 1 ). This may reflect the heterogeneity of HbS-associated haplotypes in Oman. Although the Arab-Indian haplotype is common in the Eastern provinces in Saudi Arabia 22 in Oman, it only constitutes 25% in the SCD patient population, while Benin and Bantu haplotypes form 50% and 25% respectively 37 . Another, confounding factor related to HbF is hydroxyurea use, which is seen in 36% of this current SCD cohort.
An elevated WBC count was an independent predictor of disease severity as seen in our study. Quinn and Miller 38 in a prospective study reported that leukocytes are known to be involved in the process of vaso-occlusion, and leukocytosis in adults is associated with an increased frequency of ACS and death 39 . Our patients also showed a significant rise in the WBC counts in the terminal event and using a cutoff of 15 × 10 9 /L, it was shown that there was a statistically significant rise in the WBC counts with a median WBC count of 24 and IQR between 20 and 33 (Table 1 ) and this correlated significantly with sepsis during the terminal event.
High serum ferritin was thought to be one of the associated features of mortality among patients with SCD, reflecting both iron overload, as well as inflammatory status. Increased gastrointestinal absorption of iron has been reported in sickle cell disease, because of the associated chronic hemolysis, and anemia. In our study cohort, the median serum ferritin (ng/L) was 1064 with IQR between 323 and 2987. Interestingly, Akinbami et al. 40 , recently reported that 90% of subjects with sickle cell disease had normal iron stores.
Elevated CRP can occur in SCD, during steady state, and in crisis, as reported by us and Okocha et al. 41 , 42 . It represents an underlying inflammatory/ infective, process or tissue necrosis and showed a statistically significant rise, in our cohort, especially among patients who died of sepsis/ multi-organ failure (Table 1 ). In our cohort, the basal median CRP was high (43 mg/L) and showed a statistically significant rise in the terminal episode with a median of 171 ( p < 0.05). Among 67 cases with sepsis, 54 (79.1%) were culture-proven, with 11 (20.7%) patients, showing multiple microorganisms, while bacterial, fungal, and viral microorganisms were isolated in 32 (60.4%), 6 (11.3%), and in 4 (7.5%) respectively. The most frequent organisms were gram-negative organisms, similar to the recent report of the rising gram-negative organisms, reflecting an increasing use of external lines and acquisition of hospital acquired infections 43 .
Our study demonstrated a high serum LDH, high serum bilirubin, and high CRP that further increased significantly toward the terminal event. Several studies have shown these observations and they indicate the degree of inflammation, tissue necrosis, infarction, and haemolysis that is accentuated during the terminal phase 29 , 44 , 45 . Our study demonstrated a statistically significant rise in these parameters in the terminal episode substantiating the above (Table 1 ). These factors with the associated leukocytosis, thrombocytopenia, and drop in hemoglobin heralded the onset of multiorgan failure in this cohort.
The cause of death was established by the course of illness during hospitalization, supportive laboratory evidence and telephonic enquiries with relatives in case of out of hospital deaths of these patients. Although ACS has remained a leading cause of death, there are no established risk factors for death in patients with SCD in Oman. In a study from Oman by Tawfic et al. 16 ACS was the main cause of ICU admission in patients with SCD. Further, the use of inotropic support and/or mechanical ventilation was an indicator of high mortality rate among these patients. Jaiyesimi and Kasem 17 showed that ACS was common irrespective of SCD severity in children from Oman, and all patients appeared to be at risk, but it was increased in patients with vaso-occlusive crisis.
When we looked at the time of death, we observed increased mortality due to ACS and sepsis during the colder months of the year (September to March). This is not unusual as viral infections such as respiratory syncytial virus and seasonal influenza predominate during that period and it is known to be one of the leading viral causes of ACS. Our own data and others showed similar patterns 46 , 47 . We also observed that over 30% of deaths occurred within 24 h of hospital admission. This data is also similar to the previous studies and the highest numbers were reported in the ACS and sepsis groups 48 . In the absence of post-mortem studies, (which are rarely accepted by the patient’s relatives), the exact cause will remain elusive or putative, although the recent literature is suggestive of increased cardiovascular events in this situation.
SCD is a complex condition, with life-threatening complications that interfere with the patient’s normal life owing to repeated painful episodes, and the associated multi-system involvement. Although this is a retrospective study, however, it identified that ACS and sepsis were the most important preventable risk factor associated with mortality in this SCD patient cohort. A high index of suspicion and vigilance is required for patients presenting with fever, tachycardia, and signs of ACS, associated with a drop in Hb, with raised WBC, CRP, LDH, and ferritin, to identify these patients early and initiate appropriate rapid management protocols.
In summary, the ability to identify the risk factors that are associated with increased mortality among SCD patients permits accurate prognostication and provides effective prophylactic management strategies. This study shows that male gender, low HbF, substantial drop in hemoglobin and platelet, as well as increased in WBC counts, serum LDH, ferritin and CRP, correlated significantly with mortality risk during the terminal event in patients with SCD.
Data availability
Data is available on request from the authors. The corresponding author can be contacted for details of the study.
Colombatti, R., Birkegard, C. & Medici, M. PB2215—Global epidemiology of sickle cell disease: A systematic review. HemaSphere 6 , 2085–2086. https://doi.org/10.1097/01.HS9.0000851688.00394.f4 (2022).
Article PubMed Central Google Scholar
Ware, R. E., de Montalembert, M., Tshilolo, L. & Abboud, M. R. Sickle cell disease. Lancet 390 (10091), 311–323. https://doi.org/10.1016/S01406736(17)30193-9 (2017).
Article PubMed Google Scholar
Bunn, H. F. Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 337 (11), 762–769. https://doi.org/10.1056/NEJM199709113371107 (1997).
Article CAS PubMed Google Scholar
Al-Riyami, A. A., Suleiman, A. J., Afifi, M., Al-Lamki, Z. M. & Daar, S. A community-based study of common hereditary blood disorders in Oman. East Mediterr. Health J. 7 (6), 1004–1011 (2001).
Alkindi, S. et al. Forecasting hemoglobinopathy burden through neonatal screening in Omani neonates. Hemoglobin. 34 (2), 135–144. https://doi.org/10.3109/03630261003677213 (2010).
Telfer, P. et al. Clinical outcomes in children with sickle cell disease living in England: A neonatal cohort in East London. Haematologica 92 (7), 905–912. https://doi.org/10.3324/haematol.10937 (2007).
Platt, O. S. et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 330 (23), 1639–1644. https://doi.org/10.1056/NEJM199406093302303 (1994).
Wierenga, K. J., Hambleton, I. R. & Lewis, N. A. Survival estimates for patients with homozygous sickle-cell disease in Jamaica: A clinic-based population study. Lancet. 357 (9257), 680–683. https://doi.org/10.1016/s0140-6736(00)04132-5 (2001).
Elmariah, H. et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am. J. Hematol. 89 (5), 530–535. https://doi.org/10.1002/ajh.23683 (2014).
Article CAS PubMed PubMed Central Google Scholar
Ballas, S. K., Pulte, E. D., Lobo, C. & Riddick-Burden, G. Case series of octogenarians with sickle cell disease. Blood. 128 (19), 2367–2369. https://doi.org/10.1182/blood-2016-05-715946 (2016).
Quinn, C. T., Rogers, Z. R., McCavit, T. L. & Buchanan, G. R. Improved survival of children and adolescents with sickle cell disease. Blood 115 (17), 3447–3452. https://doi.org/10.1182/blood-2009-07-233700 (2010).
Vichinsky, E., Hurst, D., Earles, A., Kleman, K. & Lubin, B. Newborn screening for sickle cell disease: Effect on mortality. Pediatrics. 81 (6), 749–755 (1988).
Yanni, E., Grosse, S. D., Yang, Q. & Olney, R. S. Trends in pediatric sickle cell disease-related mortality in the United States, 1983–2002. J. Pediatr. 154 (4), 541–545. https://doi.org/10.1016/j.jpeds.2008.09.052 (2009).
Vichinsky, E. P. et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N. Eng. J. Med. 342 (25), 1855–1865. https://doi.org/10.1056/NEJM200006223422502 (2000).
Article CAS Google Scholar
Leikin, S. L. et al. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatrics 84 (3), 500–508 (1989).
Tawfic, Q. A. et al. Adult sickle cell disease: A five-year experience of intensive care management in a university hospital in Oman. Sultan Qaboos Univ. Med. J. 12 (2), 177–183. https://doi.org/10.12816/0003110 (2012).
Article PubMed PubMed Central Google Scholar
Jaiyesimi, O. & Kasem, M. Acute chest syndrome in Omani children with sickle cell disease: Epidemiology and clinical profile. Ann. Trop. Paediatr. 27 (3), 193–199. https://doi.org/10.1179/146532807x220307 (2007).
Miller, S. T. et al. Prediction of adverse outcomes in children with sickle cell disease. N. Engl. J. Med. 342 (2), 83–89. https://doi.org/10.1056/NEJM200001133420203 (2000).
https://data.gov.om/OMPOP2016/population?region=1000010&indicator=1000250&nationality=1000000&sex=1000000&age-group=1000000&frequency=A .
Karacaoglu, P. K. et al. East Mediterranean region sickle cell disease mortality trial: Retrospective multicenter cohort analysis of 735 patients. Ann. Hematol. 95 (6), 993–1000. https://doi.org/10.1007/s00277-016-2655-5 (2016).
Desselas, E. et al. Mortality in children with sickle cell disease in mainland France from 2000 to 2015. Haematologica 105 (9), e440-443. https://doi.org/10.3324/haematol.2019.237602 (2020).
Al-Suliman, A. et al. Patterns of mortality in adult sickle cell disease in the Al-Hasa region of Saudi Arabia. Ann. Saudi Med. 26 (6), 487–488. https://doi.org/10.5144/0256-4947.2006.487 (2006).
de Castro Lobo, C. L. et al. Mortality in children, adolescents and adults with sickle cell anemia in Rio de Janerio, Brazil. Hematol. Transfus. Cell Ther. 40 (1), 37–42. https://doi.org/10.1016/j.bjhh.2017.09.006 (2018).
Article Google Scholar
Mota, F. M. et al. Analysis of the temporal trend of mortality from sickle cell anemia in Brazil. Rev. Bras. Enferm. 75 (4), e20210640. https://doi.org/10.1590/0034-7167-2021-0640 (2022).
Santo, A. H. Sickle cell disease related mortality in Brazil, 2000–2018. Hematol. Transfus. Cell Ther. 44 (2), 177–185. https://doi.org/10.1016/j.htct.2020.09.154 (2022).
Gardner, K. et al. Outcome of adults with sickle cell disease admitted to critical care—Experience of a single institution in the UK. Br. J. Hematol. 150 , 610–613. https://doi.org/10.1111/j.1365-2141.2010.08271.x (2010).
Lanzkron, S., Carroll, C. P. & Haywood, C. Mortality rates and age at death from sickle cell disease: US, 1979–2005. Public Health Rep. 128 , 110–116. https://doi.org/10.1177/003335491312800206 (2013).
Darbari, D. S. et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLoS One. 8 (11), e79923. https://doi.org/10.1371/journal.pone.0079923 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar
Knight-Madden, J. M., Barton-Gooden, A., Weaver, S. R., Reid, M. & Greenough, A. Mortality, asthma, smoking and acute chest syndrome in young adults with sickle cell disease. Lung. 191 (1), 95–100. https://doi.org/10.1007/s00408-012-9435-3 (2013).
Shome, D. K. et al. The platelet count and its implications in sickle cell disease patients admitted for intensive care. Indian J. Crit. Care Med. 22 (8), 585–590. https://doi.org/10.4103/ijccm.IJCCM_49_18 (2018).
Castro, O. et al. The acute chest syndrome in sickle cell disease: Incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 84 (2), 643–649 (1994).
Manci, E. A. et al. Causes of death in sickle cell disease: An autopsy study. Br. J. Haematol. 123 (2), 359–365. https://doi.org/10.1046/j.1365-2141.2003.04594.x (2003).
Ballas, S. K. Opioids are not a major cause of death of patients with sickle cell disease. Ann. Hematol. 100 (5), 1133–1138. https://doi.org/10.1007/s00277-021-04502-2 (2021).
Tsitsikas, D. A. et al. Revisiting fat embolism in sickle syndromes: Diagnostic and emergency therapeutic measures. Br. J. Haematol. 186 , e112–e115. https://doi.org/10.1111/bjh.15941 (2019).
Naik, R. P., Streiff, M. B., Haywood, C., Segal, J. B. & Lanzkron, S. Venous thromboembolism incidence in the cooperative study of sickle cell disease. J. Thromb. Haemost. 12 (12), 2010–2016. https://doi.org/10.1111/jth.12744 (2014).
Alkindi, S. et al. Predicting risk factors for thromboembolic complications in patients with sickle cell anaemia—Lessons learned for prophylaxis. J. Int. Med. Res. 49 (12), 3000605211055385. https://doi.org/10.1177/03000605211055385 (2021).
Daar, S., Hussain, H. M., Gravell, D., Nagel, R. L. & Krishnamoorthy, R. Genetic epidemiology of HbS in Oman: Multicentric origin for the βS gene. Am J Hematol 64 (1), 39–46. https://doi.org/10.1002/(sici)1096-8652(200005)64:1%3c39::aid-ajh7%3e3.0.co;2-# (2000).
Quinn, C. T. & Miller, S. T. Risk factors and prediction of outcomes in children and adolescents who have sickle cell anemia. Hematol. Oncol. Clin. N. Am. 18 (6), 1339–1354. https://doi.org/10.1016/j.hoc.2004.07.004 (2004).
Article MathSciNet Google Scholar
Alkindi, S. et al. Predictors of impending acute chest syndrome in patients with sickle cell anaemia. Sci. Rep. 10 (1), 2470. https://doi.org/10.1038/s41598-020-59258-y (2020).
Akinbami, A. A. et al. Serum ferritin levels in adults with sickle cell disease in Lagos, Nigeria. J. Blood Med. 4 , 59–63. https://doi.org/10.2147/JBM.S42212 (2013).
Pathare, A. et al. Cytokine profile of sickle cell disease in Oman. Am. J. Hematol. 77 (4), 323–328. https://doi.org/10.1002/ajh.20196 (2004).
Okocha, C. et al. C-reactive protein and disease outcome in Nigerian sickle cell disease patients. Ann. Med. Health Sci. Res. 4 (5), 701–705. https://doi.org/10.4103/2141-9248.141523 (2014).
Al-Tawfiq, J. A., Rabaan, A. A. & AlEdreesi, M. H. Frequency of bacteremia in patients with sickle cell disease: A longitudinal study. Ann. Hematol. 100 (6), 1411–1416. https://doi.org/10.1007/s00277-021-04523-x (2021).
Maitre, B. et al. Acute chest syndrome in adults with sickle cell disease. Chest. 117 (5), 1386–1392. https://doi.org/10.1378/chest.117.5.1386 (2000).
Allareddy, V. et al. Outcomes of acute chest syndrome in adult patients with sickle cell disease: Predictors of mortality. PLoS One 9 (4), e94387. https://doi.org/10.1371/journal.pone.0094387 (2014).
Sadreameli, S. C., Reller, M. E., Bundy, D. G., Casella, J. F. & Strouse, J. J. Respiratory syncytial virus and seasonal influenza cause similar illnesses in children with sickle cell disease. Pediatr. Blood Cancer. 61 (5), 875–878. https://doi.org/10.1002/pbc.24887 (2014).
Alkindi, S., Al-Yahyai, T., Raniga, S., Boulassel, M. R. & Pathare, A. Respiratory viral infections in sickle cell anemia: Special emphasis on H1N1 co-infection. Oman Med. J. 35 (6), e197. https://doi.org/10.5001/omj.2020.89 (2020).
Nze, C. et al. Sudden death in sickle cell disease: Current experience. Br. J. Haematol. 188 (4), e43–e45. https://doi.org/10.1111/bjh.16314 (2020).
Download references
Acknowledgements
We wish to thank Professor Samir K. Ballas for his constructive comments on the manuscript, and the hospital administration for allowing the use of hospital material in this study.
Author information
Authors and affiliations.
Department of Haematology, College of Medicine and Health Sciences, Sultan Qaboos University, P. O. Box 35, 123, Muscat, Oman
Salam Alkindi, Salma Al-Jadidi, Safa Al-Adawi & Anil V. Pathare
Department of Medicine, Nizwa Hospital, Nizwa, Oman
Refaat Abdullah Elsadek
Department of Medicine, Sohar Hospital, Sohar, Oman
Ali Al Madhani
Emergency Department, Royal Hospital, Muscat, Oman
Maryam Al-Nabhani
You can also search for this author in PubMed Google Scholar
Contributions
All authors have made substantial contributions and have seen and approved the final version of manuscript. (1) S.A.K., S.A.J., S.A.A., R.A.E. and A.A.M. were fully involved in the conception and design of the study, acquisition, analysis and interpretation of data, (2) S.A.K., and A.V.P. were instrumental in the drafting the article and critical appraisal before submission.
Corresponding author
Correspondence to Salam Alkindi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Alkindi, S., Al-Jadidi, S., Al-Adawi, S. et al. Multi-center study on mortality in children, and adults with sickle cell anemia-risk factors and causes of death. Sci Rep 14 , 8584 (2024). https://doi.org/10.1038/s41598-024-58328-9
Download citation
Received : 20 June 2023
Accepted : 27 March 2024
Published : 13 April 2024
DOI : https://doi.org/10.1038/s41598-024-58328-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sickle cell disease
- Predictors of mortality
- Acute chest syndrome
- Sudden death
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Micromachines (Basel)

Techniques for the Detection of Sickle Cell Disease: A Review
Wjdan a. arishi.
1 Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, King Abdulaziz University, P.O. Box 80402, Jeddah 21589, Saudi Arabia; as.ude.uak.uts@1000ihsiraw
Hani A. Alhadrami
2 Molecular Diagnostic laboratory, King Abdulaziz University Hospital, King Abdulaziz University, P.O. BOX 80402, Jeddah 21589, Saudi Arabia
Mohammed Zourob
3 Department of Chemistry, Alfaisal University, Al Zahrawi Street, Al Maather, AlTakhassusi Rd, Riyadh 11533, Saudi Arabia
Sickle cell disease (SCD) is a widespread disease caused by a mutation in the beta-globin gene that leads to the production of abnormal hemoglobin called hemoglobin S. The inheritance of the mutation could be homozygous or heterozygous combined with another hemoglobin mutation. SCD can be characterized by the presence of dense, sickled cells that causes hemolysis of blood cells, anemia, painful episodes, organ damage, and in some cases death. Early detection of SCD can help to reduce the mortality and manage the disease effectively. Therefore, different techniques have been developed to detect the sickle cell disease and the carrier states with high sensitivity and specificity. These techniques can be screening tests such as complete blood count, peripheral blood smears, and sickling test; confirmatory tests such as hemoglobin separation techniques; and genetic tests, which are more expensive and need to be done in centralized labs by highly skilled personnel. However, advanced portable point of care techniques have been developed to provide a low-cost, simple, and user-friendly device for detecting SCD, for instance coupling solubility tests with portable devices, using smartphone microscopic classifications, image processing techniques, rapid immunoassays, and sensor-based platforms. This review provides an overview of the current and emerging techniques for sickle cell disease detection and highlights the different potential methods that could be applied to help the early diagnosis of SCD.

1. Introduction
Sickle-cell disease (SCD) is a multisystem disorder related to acute illness, painful episodes, and gradual organ damage [ 1 ]. Sickle cell anemia is caused by point mutations in the HBB gene, which codes for β-subunit, where adenine is substituted by thymine (GAG > GTG) at codon 6 of the HBB gene. As a result of nucleotide substitution, the amino acid is altered, and glutamic acid is replaced by valine resulting in hemoglobin S (HbS) formation. Hb S polymerizes in a deoxygenated state and forms rigid, less soluble sickle-shaped cells [ 1 , 2 ]. SCD arises when inheriting two mutated alleles βS/βS (homozygous) or in the case of inheriting different types of mixed heterozygous alleles such as sickle-β-thalassemia HbSβ-thalassemia, sickle-hemoglobin C disease (HbSC), and other combinations. When the sickle cell trait (SCT)is heterozygous βA/βS, it means only one allele is affected and produces insoluble hemoglobin, and the other gene is wildtype and produces normal hemoglobin [ 3 ]. The pathogenesis mechanism of SCD depends on the polymerization of hemoglobin S, which is triggered by the lower oxygen affinity [ 4 ]. Polymerization alters the physical properties of red blood cells, such as shape and cell membrane, leading to dehydration of cells and increased polymerization. Repeated polymerization and sickling of cells lead to the formation of irreversibly sickle cell [ 4 ]. This accelerates cell destruction and reduces cells’ lifespan by ≥75%, resulting in hemolytic anemia [ 5 ]. In addition, the polymerized cell cannot move easily in the small blood vessels, resulting in the blockage of the vessel, i.e. vaso-occlusion [ 5 , 6 ]. The most common acute complication of SCD is acute vaso-occlusive crises (VOC) that cause pain crisis and acute chest syndrome, which is considered the major cause of hospitalization and death among SCD patients [ 4 , 7 ]. Chronic complications of SCD start to appear with age as organ failure due to the progressive ischemia leads to earlier death, cerebrovascular disease, pulmonary hypertension, retinopathy, and priapism. In addition, complications during pregnancy include preeclampsia and preterm delivery [ 8 , 9 ]. Children with SCD who live in Sub-Saharan Africa have a high mortality rate estimated at 50–80% by five years old. The most common cause of death in children is infection, including invasive pneumococcal disease and malaria [ 1 , 9 ]. In developed countries, the life expectancy of SCD patients has been improved by early diagnosis, comprehensive treatment, and general medical care. Therefore, early detection supports the effective management of the disease [ 10 ].
Detection of hemoglobin S and diagnosis of sickle cell disease depend mainly on the clinical laboratory, where a combination of biochemical and molecular tests is used in the detection and confirmation of the diagnosis [ 11 ]. The most popular methods for detecting these diseases are the full count of blood cells, Hb electrophoresis, and high-performance liquid chromatography (HPLC). These methods are considered the gold standard in the diagnosis of SCD [ 12 ].
2. Clinical Picture of the Inherited Hemoglobin Disorders
Sickle cell disease causes a variety of different illnesses; the most common disorder is sickle cell anemia HbSS with the genotype βs/βs. Other forms of the SCD are formed with a combination of βS mutation with other HBB mutations, such as sickle-hemoglobin C disease (HbSC) and sickle-β-thalassemia (either HbSβ+ or HbSβ0). β0 means there is no β-globin synthesis, while β+ means reduced production of β-globin [ 13 ]. The most severe forms of SCD are HbSS and HbSβ0, and they show same clinical picture. HbSC and HbSβ+ are considered the less severe forms of SCD [ 14 ]. The clinical picture of the sickle-β-thalassemia ranges from asymptomatic to severe state similar to HbSS sickle cell anemia [ 15 ], while, in some HbSC cases, severe and life-threatening complications will appear [ 16 ]. Some genetic factors can modify the sickle cell’s clinical expression when co-inherited with the βS gene, such as α-globin gene mutations, either one-gene deletion or two-gene deletion [ 17 ].
3. Techniques and Assays to Diagnose and Monitor SCD
Several techniques and assays are used for the detection and monitoring of the sickle disease. These techniques can be divided into two main categories: (1) currently used methods in the diagnosis of SCD; and (2) innovative techniques which are mostly still in the research stage. Several reviews have been published related to the development of point of care (POC) SCD detection [ 18 , 19 , 20 ]. Table 1 lists the different technologies developed for the diagnosis and monitoring of the SCD.
Technologies for sickle-cell disease (SCD) diagnosis and monitoring.
4. Current Techniques to Diagnose and Monitor SCD
4.1. complete blood cell count.
The complete blood count (CBC) is a primary test to characterize the different types of anemia. However, the hemoglobin mutation will affect the hematological parameters, showing a variable change [ 20 ]. Patients with homozygous SS and heterozygous S/β° mutations usually present with hemolytic anemia where the red blood cells (RBCs), hemoglobin and hematocrit are low. In contrast, the counts of white blood cells (WBC) and platelet are elevated, and they can fluctuate. However, reticulocyte counts are variable and depend on different factors such as the degree of anemia caused by the cells hemolysis, sequestration, and bone marrow response to anemia [ 18 , 20 ]. Mean corpuscular volume (MCV) is usually elevated in SCD patients receiving hydroxyurea. Moreover, elevated red cell distribution width (RDW) is seen in SCD patients because of RBCs’ different subpopulations. Although CBC is widely used to describe the hematological parameter as valuable information, it is insufficient to give a complete picture of patients’ diagnoses [ 18 , 20 ].
4.2. Peripheral Blood Smear
The peripheral blood smear (PBF) is usually done after spotting abnormality in the automation counts and is considered a landmark of any hematological evaluation. PBF examines the morphology of the blood cell and evaluates any microscopic changes, which can provide valuable information that helps in the diagnoses of the different types of anemia [ 21 ]. In sickle cell anemia, moderate to severe anisopoikilocyte is seen with a variable number of elongated sickle cells, which is best observed when the red blood cells are deprived of oxygen [ 22 ]. The preparation of these blood smear slides is relatively simple, rapid, and inexpensive. Although peripheral blood smear is an informative hematological test, it relies on the pathologist’s skills, and the availability of trained pathologists is limited. Furthermore, the blood film analysis is too complicated due to the changes in the cell’s edge, location, shape, and size. As a result, a computerized system has been developed to provide a more accessible way to recognize the type of anemia [ 23 ].
4.3. Solubility Sickling Test
Sickling tests are mainly based on the polymerization of HbS in the deoxygenated state. The solubility test is the most widely used nowadays; its principle is based on the insolubility of Hb-S in the presence of concentrated phosphate buffer, a hemolyzing agent, and sodium dithionate. These agents crystalize the HbS and precipitate the cells, which refract the light and cause solution turbidity. The result is compared with negative and positive controls [ 24 ].
This test is easy to perform and inexpensive. It suffers from a false-negative result when utilized for newborns, due to the presence of a high amount of hemoglobin F and when the HbS is less than 10% of the total hemoglobin [ 25 ]. Furthermore, false-negative results are observed in patients with coinheritance of α-thalassemia trait and severe anemia. In contrast, false positives are observed in patients with high serum viscosity, erythrocytosis, highly marked leukocytosis and in some cases of anemia. Moreover, the sickle solubility tests cannot differentiate between sickle cells trait (SCT) and SCD, and they are insensitive to the detection of hemoglobin AS (HbAS)[ 25 , 26 ].These disadvantages make them difficult to use in screening programs [ 27 ].
4.4. Hemoglobin Electrophoresis
Electrophoresis is a type of chromatography techniques, and it is considered as one of the important tests used to detect Hb variants [ 11 ]. In this test, an electrical field is applied to facilitate the migration of electrically charged molecules. The first described hemoglobin variant Hb-S by using electrophoresis was in 1949. To identify hemoglobin variants, different pH and mediums are used, either cellulose acetate electrophoreses at alkaline pH or citrate agar at acidic pH [ 28 ].
Alkaline electrophoresis is a diagnostic tool that has been used to detect thalassemia and sickle cell anemia at pH 8.4. First, a hemolysate is prepared from the red blood cells; then, it is added to a cellulose strip and run-in buffer at a constant voltage in an electrophoresis chamber [ 29 ]. As a result, the different hemoglobin types with different net charges are separated into various bands depending on their mobility. Hemoglobin electrophoresis can differentiate between HbS and HbC, which are the most clinically significant variants. However, electrophoresis does not distinguish between hemoglobin variants with the same electrical charges and gives the same migration patterns, such as HbD and HbG, which comigrate with HbS; HbE and Hb0-Arab have similar migration to the HbC molecules [ 30 ]. Furthermore, alkaline electrophoresis can be affected by the presence of large amounts of hemoglobin F in newborns, which can dominate the smaller electrophoresis band. Therefore, extra care should be taken to reliably detect the HbS. In addition, smaller bands such as HbA2, HbH, and Hb Bart’s may be missed. Therefore, a more efficient test should be used as a diagnostic test to overcome these limitations [ 31 ].
Citrate agar electrophoresis is performed in acidic pH 6.0–6.2, and it depends on the interaction of the agaropectin in the gel mixture with the structural changes of the Hb [ 28 ]. Most hemoglobin variants that comigrate at alkaline pH can be separated effectively using citrate agar electrophoresis [ 32 , 33 ]. Citrate agar electrophoresis is not affected by the high amount of hemoglobin F in newborns; thus, it can be used as a diagnostic test for sickle cell disease at birth. However, it is laborious and challenging to perform in limited resources areas [ 28 , 34 ].
Capillary electrophoresis has been documented to separate Hb fractions and diagnose sickle cell disease and thalassemia. The capillary electrophoresis separates the protein in an untreated fused-silica column reliably [ 35 ]. Fully automated methods such as CAPILLARYS 2 system has been available in the market since the early 2000s. This method has eight parallel fused silica columns where multiple samples can be analyzed, and each column can be used for at least 3000 runs. The hemolysates are prepared automatically from red cell pellets [ 11 ]. The reference ranges for HbA2 are adapted to be 2.1–3.2% and <0.8% for HbF. However, in the presence of different Hb variants, Capillary Zone Electrophoresis (CZE) is better than HPLC for quantifying HbA2 except in the presence of HbC [ 36 ]. Moreover, a fully automated Neonat Fast Hb device with CAPILLARYS cord blood mode can analyze dried blood spots on filter paper and liquid cord blood. Thus, it can be used in the neonate screening test. These advantages make the CAPILLARYS instrument the first-line test for screening hemoglobinopathies in newborn and adult patients [ 36 ].
4.5. Isoelectric Focusing
Isoelectric focusing (IEF) is a high-resolution method for separating proteins depends on their isoelectric points (pI). The Hb molecules travel across a pH gradient until they reach their isoelectric points where the net charge is zero. The Hb molecules precipitate and appear as a sharp band [ 37 ]. This technique can detect HbS and HbA easily in a high concentration of HbF. Moreover, it separates Hb D-Punjab from HbS. Generally, it can provide the result within 45 min [ 37 ]. Although IEF is relatively expensive and requires highly trained personnel to interpret the results due to the larger number of bands, it is still considered the standard test for newborn screening, as it needs a very small volume of sample and can be used with a dried blood spot [ 38 , 39 ].
4.6. High Performance Liquid Chromatography
HPLC is documented to separate the hemoglobin fractions as they have different interaction with the stationary phase [ 40 ]. HPLC detects different types of hemoglobin based on the retention time and shape of the peak [ 41 ]. Each hemoglobin has a specific retention time and can be compared with the retention time of the known hemoglobin fractions [ 11 ]. HPLC is used to detect and quantify HbF, Hb A2, HbS, HbC, Hb Barts, and other Hb variants [ 11 ]. Developing a fully automated HPLC would be useful in testing a large number of samples accurately. HPLC shows better sensitivity in separation of hemoglobin variants than electrophoresis [ 42 ].
HPLC is much less labor-intensive and more reliable for monitoring patients under blood transfusion or hydroxyurea [ 43 ]. However, HPLC is an expensive machine and cannot differentiate among all variants with the same retention time. For example, all Hb variants with a similar retention time to HbS are eluted out with the HbS peak. Therefore, it can misdiagnose new variants that mimic HbS. Thus, HPLC cannot stand alone as a diagnostic test and should be done along with a confirmatory test such as DNA analysis before giving a final diagnosis [ 44 ].
4.7. Genetic Test
The genetic study is important for the precise detection of the various types of sickle cell disease, based on the detection of β-globin mutations that lead to sickle cell disease development [ 45 ].
4.7.1. Polymerase Chain Reaction (PCR)-Based Techniques
Polymerase chain reaction is one of the most powerful diagnostic techniques, where special enzymes are used to amplify specific parts of the genetic materials to millions of copies, using specific primers. PCR can detect well known single genes or several genes in a single tube [ 46 ]. The PCR program involves denaturation, annealing, and elongation, which is repeated for 20–40 thermal cycles. Then, the result can be detected by gel electrophoresis, sequencing, melting curve analysis, or monitoring the change in the fluorescence. PCR sensitivity and specificity have revolutionized the prenatal and neonatal diagnostic field. Several PCR-based techniques are documented to detect βs mutations, such as high-resolution melting (HRM) analysis, which is simple, sensitive, and cost-effective for use in mass screening of SCD genotypes [ 47 ]. Another simple, low-cost PCR-based technique has been developed using bi-directional allele-specific amplification (ASA) and a hot star system to provide more specific single-tube genotyping, where the point mutation of sickle cell anemia is used as the SNP model. In addition, discriminatory conditions have enabled the determination of homozygous and heterozygous states based on the different band sizes on the agarose gel electrophoresis [ 46 , 48 ]. The amplification-refractory mutation system (ARMS) is a simple technique for detecting point mutation or small deletion. The ARMS principle is to use primers with specific sequences to allow the amplification of DNA in the presence of the target allele. Therefore, the detection of the target allele is based on the presence of the PCR product. The alleles can then be differentiated on agarose gel with different band sizes [ 49 ]. ARMS has been mostly used in prenatal diagnosis by detection of sickle cell mutation in the fetal sample. The ARMS’s sensitivity has been measured by comparing the result to identify the presence of hemoglobin variants by HPLC [ 50 ]. Wu et al. demonstrated an allele-specific oligonucleotide (ASO) hybridization to detect sickle cell mutation using two PCR primers. One primer was used for the normal allele and the other one for the mutated allele. The primer is joined to the complementary sequence and amplified, which in turn releases the fluorescent label that determines the amount of the target. This method can differentiate between the allelic variation [ 51 ].
4.7.2. Restriction Fragment Length Polymorphism
Restriction fragment length polymorphism (RFLP) is used to detect sickle cell disease based on restriction enzymes, which remove the recognition site at the βs mutated gene [ 52 ]. For example, MstII is one of the first described restriction enzymes; it cuts the DNA in the sequence CCTNAGG (where N represents any nucleotide). Therefore, when thymine replaces the adenine, it removes the recognition site for MstII restrictase, as shown in Figure 1 . After separation, the number of bands resulting from the enzyme cutting indicates the number of mutations. In a healthy individual with (βA βA), the gene is cut by the MstII restrictase and yields two bands, as shown in Figure 1 a. In homozygotes, the restrictase cuts both genes, and two short bands appear. In the sickle cell trait (βAβS), no cut is made in the βS, so a single band appears; however, the βA gene is cleaved, and two bands appear, as shown in Figure 1 b. In sickle cell anemia homozygous (βSβS), there is no enzyme cutting due to the mutation in both genes, so a single wide band appears, as shown in Figure 1 c [ 52 ]. Another restriction enzyme has been used in sickle cell detection is Ddel I. The mutation caused sickle cells Anemia(SCA) removes the restriction site of Ddel I, 5′-GTNAG-3′. As a result, bands with different lengths appear depending on the presence of sickle cell anemia mutation [ 53 , 54 ].

Restriction fragment length polymorphism (RFLP) for Sickle cell anemia: ( a ) normal gene(βAβA); ( b ) sickle cell trait (βAβS); and ( c ) sickle cell anemia (βSβS).
4.7.3. DNA Microarrays and Sequencing Techniques
DNA microarrays consist of a large number of immobilized DNA oligonucleotide spots on the array surface, where hybridization events occur with complementary sequences, which in turn indicate the concentrations of the nucleic acids [ 55 ]. Microarrays have been used in genome-wide association studies (GWASs) to identify the presence of single nucleotide polymorphisms (SNPs) in a single run, as well as the copy number of variants [ 55 , 56 ]. Hamda et al. developed a novel database that combines the gene expression with genome-wide association study (GWAS), using homozygous SS microarray datasets to determine SCD transcriptomic profile [ 56 , 57 ].
Next-generation sequencing (NGS), which is deep DNA sequencing, has been used to identify different types of mutation. NGS can be run for the whole-exome sequencing (WES) or whole-genome sequencing (WGS). These techniques have been used widely for genetic analyses to predict sickle cell disease’s severity and progression, which can help make a treatment decision, discover new therapies, and develop novel diagnostic assays [ 58 ]. WES is performed to determine single-nucleotide variants (SNVs) in sickle cell mutation by sequencing the coding region of the β-globin gene. This procedure gives a full description of the β-globin gene accurately [ 59 ]. Few studies have utilized this approach to identify genetic modifications in the SCD severity. One study reported an increase in the number of strokes in African Americans due to mutation in GOLGB1 and ENPP1 [ 60 ]. Another study pointed out that mutation in SALL2 is associated with a high level of HbF in response to hydroxyurea [ 61 ]. Variants in MBL2 and KLRC3 were observed more frequently among adult SCD with hyperhemolysis syndrome than controls [ 62 ]. Whole-genome sequencing helps analyze the entire genome, identify the genomic modification of SCD, and create the Sickle Genome Project (SGP). It helped develop a robust pipeline for the correct identification of SNPs [ 63 ]. Furthermore, it confirmed the association of the SCD phenotypes with common genetic modifiers, including fetal hemoglobin BCL11A, HBB, UGT1A1, and APOL1. This technique will help in precision medicine to make better treatment decisions and discover new treatments [ 59 ].
5. Innovative Techniques for the Diagnosis and Monitoring of SCD
5.1. image processing techniques.
Image processing techniques play an essential role in the analysis of red blood cells. Blood cell disorders can be classified based on different features: the cell shape, central pallor diameter, target flag, etc. [ 64 ]. The cells can also be classified based on the image features by using segmentation and artificial neural network [ 65 ].
Chy and Rahaman developed an automated method to detect sickle cell anemia (SCA) using an image processing technique. An algorithm is used to automate the detection of sickle cells found in thin blood smears. The first step in this technique is to take blood images using a camera connected to a light microscope. Then, a pre-processing step converts the images into grayscale, enhances the image, and passes it through the median filter to reduce the noise. After that, the RBCs are segmented through a segmentation threshold, followed by a morphological operation for the image to remove the unwanted objects. The features of the images are created based on color, texture, and the cells’ geometry. As a final step, the computer classifier is trained to assess the picture. In total, 120 photos were used to assess this technique: 80 for training and 40 images for testing. The authors reported 95% accuracy and 96.55% sensitivity [ 66 ].
Alzubaidi et al. employed deep learning models to detect SCA and classify the red blood cells based on the microscopic images. The models were able to extract and implement the classification functions automatically in one shot. Moreover, they developed three deep learning models to determine and categorize the red blood cells based on the shapes: round shape indicating normal cells, elongated shape indicating sickle cells, and other blood shapes. The researcher focused on resolving the lack of training data, where they used the transfer learning technique. The study employed 626 images; 202 were classified as circular; 211 images were identified as elongated; and 213 as other cell shapes. The model achieved 99.54% accuracy and 99.98% when they used the same model plus a multi-class support vector machine [ 67 ].
De Haan et al. combined a smartphone microchip, a microscope, and machine learning algorithms to develop an affordable, portable, and rapid screening test for sickle cell anemia. This module uses two deep neural networks: The first one enhances the picture taken by the smartphone microscope. The second one complements the first neural network by enhancing the picture and performing semantic segmentation between the normal RBCs and sickle RBCs within the blood film. Finally, these segmented images are used to help the diagnoses of the sickle cell disease patients. This method achieved around 98% accuracy using 96 samples; 32 were SCD thin blood smears and 64 normal thin blood smears [ 22 ]. A smartphone-based image acquisition process has been developed for imaging the RBCs from the SCD patients under oxygen control. This method can automatically distinguish the normal RBCs from the sickled RBCs based on the morphology change, using image processing (MATLAB R2019a) to analyze the image and quantify the sickled cells. This advanced technique is cheap and easy to use [ 68 ].
The image processing methods provide automated interpreting of the blood cell images, minimizing errors, which can effectively monitor the SCD patient’s status [ 66 , 67 ]. However, the image processing techniques have some drawbacks: they cannot distinguish between the different types of the SCD; a high concentration of HbF can affect the polymerization of HbS, which can exclude the application of these tests to newborn screening [ 9 ]; they cannot classify RBCs accurately because they rely on binary classification, which ignores other blood cells shapes; and they are time consuming and require special equipment such as digital camera or smartphones [ 67 ].
5.2. Emerging Flow Cytometry
Conventional flow cytometry techniques have been used to detect sickle cells based on fluorescent markers or cellular morphology [ 69 ]. Advanced flow cytometry based on imaging techniques has been demonstrated to enhance the sensitivity by combining cell population analysis and morphological data. Beers et al. developed an imaging flow cytometry assay (SIFCA) and software algorithm to distinguish between sickle RBCs and normal RBCs based on their morphology. SIFCA is performed by diluting the peripheral blood sample, deoxygenating the cells by reducing the oxygen to 2% for 2 h, and then analyze it using imaging flow cytometry. Finally, the cells are classified based on the morphology into sickled and normal cells by using algorithm software. The authors analyzed 100 images of normal cells and 100 images of sickle cell, and they reported 100% sensitivity and 99.1% specificity. The study proved that SIFCA can assess sickling tendency in SCA patients to identify the severity of the disease and drug monitoring [ 70 , 71 ].
Cai et al. developed in-vitro photoacoustic flow cytometry (PAFC) for morphological detection of sickle cells containing hemoglobin S. They employed photothermal and photoacoustic spectra for determination of hemoglobin heterogeneity and accumulation of the HbS in sickle RBCs. The sickled RBCs showed 2–4-fold lower linear mode than normal RBCs. This method is useful in monitoring the sickling states [ 72 ]. Liua et al. developed microfluidic flow cytometry based on the electrical impedance spectroscopy. This technique detects the changes in the electrical impedance resulted from the change in the cells’ shape from the round soluble cells to sickle rigid cells under hypoxic condition. In this study, the cells were obtained from a healthy donor and three sickle cell patients, and the difference in the electrical impedance was measured to show the difference between normal cells and sickled cells. They showed that the electrical impedance signal can be used as an indicator of the cell sickling events. However, it is still unclear if these novel flow cytometry techniques can be used to monitor disease severity or if they can distinguish between sickle cell trait and sickle cell disease [ 19 , 73 ].
5.3. Mechanical Differentiation of Sickle Cells
The deformability of red blood cells is a crucial determinant of blood flow in circulation. In sickle cell disease, RBCs are mechanically fragile and poorly deformable, resulting in impaired blood flow. This feature of the sickle cell can be used to monitor the disease severity and the sickling event [ 74 ].
Brandao et al. developed an optical tweezer to capture red blood cells (RBC) by dragging them through a viscous fluid (human AB plasma) to measure the elasticity of the cells. In this study, the RBC deformability was measured in 10 homozygous patients (HbSS), 5 patients taking hydroxyurea (HU) for six months (HbSS/HU), 10 patients with sickle cell trait (HbAS), and 35 normal controls. The RBCs deformability was lower in the patients with HbSS and HbAS; however, in patients taking hydroxyurea HbSS/HU, the cells’ deformability was found to be similar to the normal control cells. These results show that optical tweezers have the potential to be used to monitor hydroxyurea response in sickle cell disease, but they cannot be used to distinguish between different types of hemoglobin diseases [ 74 ].
Qiang et al. demonstrated an amplitude-modulated electrodeformation in microfluidic for identifying the mechanical fatigue in single cells. This method depends on the cell’s mechanical fatigue, which leads to deterioration of the physical properties by subjecting the cells to static loads. In this method, a constant amplitude fatigue load is applied to deform the RBCs, and, with more fatigue cycles, the cells progressively lose their ability to stretch. Moreover, cyclic deformation was shown to be a fast method to deform RBCs under static deformation at the same maximum load. This testing platform can provide the possibility of flexible detection of sickle cells, but it is not yet validated in sickle cell detection [ 75 ].
Du et al. developed a microfluidic-based method to detect and quantify sickle cells by modulating the disease’s pathophysiology. In this study, the kinetics of cell sickling, unsickling, and cell rheology were investigated by exposing the cells to different hypoxic conditions to mimic microvasculature scenarios, sickling events, and hydroxyurea therapy. The microfluidic chip is a double-layer device consisting of a cell channel, polydimethylsiloxane film in the middle, and a gas channel where the O 2 concentration is controlled. In the deoxygenated state, when the oxygen is less than 5%, the shape of the RBCs that contain HbS change, and form sickled cells within 12 s. This method has been used to monitor sickling events and hydroxyurea therapy [ 76 ].
Javidi et al. developed a spatiotemporal analysis of cell membrane fluctuations for the diagnosis of SCD. The test is based on using a hologram video containing either normal RBCs or sickled RBCs. The video was recorded using a low-cost, compact, 3D-printed shearing interferometer. Each hologram film was reconstructed and formed a spatiotemporal data cube. These data were extracted by calculating the standard deviations and the mean of the cell membrane fluctuations at every location over time. This resulted in a two-dimensional map of the standard deviation and mean. This method can be considered as low-cost, fast, and it does not need trained personnel to run. The accuracy of the results could be enhanced by combining it with the machine learning approach [ 77 ].
Techniques that depend on the detection of sickle cells’ mechanical deformability can only be used as a monitoring test in SCA, as they cannot indicate the disease’s severity in heterozygous states.
5.4. Lateral Flow Immunoassay
Lateral flow assays (LFAs) are widely used as portable platforms in biomedical detection. Kanter et al. demonstrated a sensing platform called Sickle SCAN. It is used to detect normal hemoglobin HbAA, sickle cell trait HbAS, hemoglobin C trait HbAC, sickle-hemoglobin C disease HbSC, and hemoglobin C disease HbCC, as shown in Figure 2 . The test used polyclonal antibodies on lateral flow chromaographic immunoassay against hemoglobin S, hemoglobin C, and hemoglobin A to detect the different types of SCD qualitatively. The polyclonal antibodies are attached on the test strip; the sample migrates in the absorbent pads, where the antibody conjugated to the nanoparticles binds to the hemoglobin; and then both migrate to the test strip. The hemoglobin binds with the corresponding antibody and produces blue lines, as shown in Figure 2 [ 78 ]. The Sickle SCAN cartridge contains four detection bands: the control band, normal HbA, HbS band, and HbC band. The test can be performed within minutes and costs a few dollars per test [ 78 ]. Mcgann et al. tested the validation of the Sickle SCAN assay to detect different hemoglobin. The test was performed using 139 whole blood samples (venous samples, dried blood spots, and spiked blood samples), and the results were compared to capillary electrophoresis (CZE) [ 79 ] The test’s accumulative sensitivity and specificity for HbSS were 98.4% and 98.6%, respectively. The cumulative sensitivity and specificity for the diagnosis of HbSC disease were 100%. A neonate sample with a high amount of HbF was tested and demonstrated that the detection of HbS or HbC was not affected by the high concentration of HbF. Furthermore, they examined the test’s storage condition and documented that the device can be stored at 37 °C for 30 days. However, the test suffers from some limitations such as misinterpretation of the result due to visual reading, cross-reactivity of the polyclonal antibody, and false-positive results in detecting the HbA heterozygous with HbS. The authors recommended another validation for the sickle SCAN in primary healthcare centers [ 79 ].

The Sickle SCAN based on lateral flow immunoassay to detect sickle-cell disease (SCD): normal hemoglobin HbAA ( a ); sickle cell trait HbAS ( b ); sickle cell anemia HbSS ( c ); hemoglobin C trait ( d ); and sickle cell-hemoglobin C disease HbSC ( e ). Created with BioRender.com.
Another new device, called HemoTypeSC assay, has been developed based on competitive lateral flow immunochromatographic assay (LFA), which uses specific antibodies against hemoglobin A, S, and C ( Figure 3 ). The test strip consists of laminated fiberglass, sample pads, nitrocellulose impregnated with the antibody for different Hb, and a cellulose wicking pad. The dried blood sample is diluted in detergent and non-specific blocking buffer. The test strip is placed in the test vial to migrate across the strip. As a result of this, a red line appears, as shown in Figure 3 . It was reported that the result could be obtained in 20 min with 100% sensitivity [ 80 ]. The advantage of HemoTypeSC is that it can differentiate the normal Hemoglobin HbAA (three bands appear control S and C; the A band is absent) ( Figure 3 a), sickle cell trait HbAS (two bands appear; control, and C; the A, and S bands are absent) ( Figure 3 b), sickle cell anemia HbSS (three bands appear: control, A and C; the S band is absent) ( Figure 3 c) hemoglobin C trait HbAC (two bands will appear; control, and S; the C and A band are absent) ( Figure 3 d), and hemoglobin SC disease HbSC (two bands will appear; control and A; the S and C bands are absent) ( Figure 3 e). The advantages of the HemoTypeSC assay are that it is rapid, portable, cost-effective, and has promising characteristics to improve the diagnosis of SCD. However, it cannot detect HbF and HbA2 [ 80 , 81 ]. Nnodu et al. performed a validation study for HemoTypeSC by screening 1121 samples from babies, and they compared the results with HPLC. The discrepancies were confirmed by molecular diagnosis. The test showed 93.4% sensitivity and 99.9% specificity. The accuracy of HemoTypeSC was 99.1% [ 81 ]. Another validation study was performed in Uganda, where 1000 sickle cell disease phenotypes were tested and validated with CZE. The study reported ≥99.5% sensitivity and ≥99.9% specificity. Two samples with HbSS phenotype were missed and identified as HbAS. It was found that both patients had blood transfusions recently [ 82 ]. HemoTypeSC was tested in Côte D’Ivoire by analyzing 336 children: 100 children already diagnosed with hemoglobin disease and 236 as participants. The sensitivity and specificity of detection hemoglobin A, S, and C were 98.2% and 99.7%, respectively [ 83 ]. Unfortunately, the test was not able to differentiate between HbSS and sickle-β0-thalassemia, as well as showed misinterpretation of the result in patients who received blood transfusions recently [ 81 ].

HemoTypeSC based on lateral flow immunoassay to detect SCD: normal hemoglobin HbAA ( a ); sickle cell trait HbAS ( b ); sickle cell anemia HbSS ( c ); hemoglobin C trait HbAC ( d ); and hemoglobin SC disease HbSC ( e ). Created with BioRender.com.
5.5. Density-Based Separation
Density-based separation detects sickled RBCs using aqueous multi-phase systems based on cell density measurements (AMPS). The dense sickle cells can be distinguished from normal cells by the two-phase AMPS system ( Figure 4 a, HbAA and HbAS (1), HbSS and HbSC (2)) with 90% sensitivity and 97% specificity. In contrast, the three-phase systems ( Figure 4 b) reported 91% sensitivity and 88% specificity [ 84 ]. The test only requires around 5 µL of the blood sample, which is mixed in capillary tubes with aqueous polymeric solutions. Upon 10 min centrifugation, the dense RBC precipitates and forms a layer in the bottom of the tube, which indicates the SCD [ 84 ]. The three-phase systems in Figure 4 b were integrated with an optical reader to enable the distinction between HbSS and HbSC ( Figure 4 b, HbAA and HbAS (1), HbSS (2), HbSC (3)). The test is rapid and straightforward; however, this technique cannot distinguish between HbAA and HbAS ( Figure 4 b(1)). In addition, the accuracy of the test was compromised in cases with elevated HbF levels, such as in newborns and the Arab-Indian haplotype, due to the absence of dense RBCs. Furthermore, many health conditions, treatments, and medications can affect the RBC density and the test’s validity [ 18 ]. Kumar et al. validated the AMPS test, where they tested 505 samples. They reported 86% sensitivity and 60% specificity for SCD-AMPS-2. However, the sensitivity was dropped in children from 6 months to 1 year of age to 84%. Furthermore, the study documented 75% sensitivity and 60% specificity for SCD-AMPS-3, while the diagnostic accuracy was 69% for SCD-AMPS-3 and 77% for SCD-AMPS-2 [ 85 ].

Multi-phase systems by cell density measurements (AMPS) to detects sickled RBCs: ( a ) two-phase AMPS HbAA, with HbAS (1) and HbSS and HbSC (2); and ( b ) three-phase AMPS, with HbAA and HbAS (1), HbSS (2), and HbSC (3). Created with BioRender.com.
A Portable smartphone platform has been developed to detect sickle cell using less than 1 μL of blood sample, based on the high density of sickle cells under hypoxic conditions. The blood sample is mixed with sodium metabisulfite to induce dehydration and cell sickling. The sample is illuminated with light-emitting diode (LED). Then, the image is magnified by an optical lens. Permanent magnets have been used for the magnetic levitation of the RBCs to observe the sickle cells image. The advantage of employing magnetic levitation is that it eliminates the need for the microscope or centrifugation [ 86 ].
5.6. Paper-Based Hemoglobin Solubility Test
It is a technique that depends on the filtration properties of the paper substrate and on the insolubility of the HbS, where it can be visually interpreted to detect the presence of hemoglobin S. In this test, one drop of the blood sample is mixed with a hemoglobin solubility agent with a ratio of 1:10. Then, the mixture is placed onto chromatography paper followed by staining. A different stained blood pattern forms based on the hemoglobin, as shown in Figure 5 . These stains are used to determine HbSS ( Figure 5 a), the carrier HbAS ( Figure 5 b), and normal hemoglobin HbAA ( Figure 5 c). The test shows 94.2% sensitivity and 97.7% specificity for the visual detection of the HbS. Furthermore, the test can be performed within 20 min [ 87 ]. Moreover, the test’s sensitivity is increased when combined with an image analysis algorithm, which quantifies the Hb in the sample based on the intensity of the color in the center spot. This test has several advantages: simple fabrication, easy to use as it needs single step, affordable, and low-cost (costs less than a dollar per test) [ 87 , 88 ]. However, this test is affected by the blood clotting, preventing the blood from wicking through the paper substrate. Furthermore, the test is unable to differentiate between HbSC and HbAS. Finally, this test is not reliable for newborns due to a high level of HbF, which prevents the polymerization and precipitation of hemoglobin S [ 87 , 88 ]. Validation of the paper-based screening test was performed by Piety et al., who tested 226 samples and compared the results with IEF electrophoresis to distinguish HbAS and HbSS from HbAA patients. The study reported 94.2% sensitivity, 97.7% specificity, and 96.9% accuracy [ 89 ]. The authors employed automated image analysis C-index to distinguish between HbSC and HbAS. They demonstrated 100% sensitivity and 59% specificity. The high sensitivity but relatively low specificity indicated that the C-index could be used to exclude patients without the disease. Furthermore, this test cannot detect sickle cell disease in neonates due to the high level of HbF [ 89 ].

Paper-based hemoglobin solubility test. Created with BioRender.com.
A microchip electrophoresis paper-based has been developed to identify and quantify hemoglobin types, including HbA, A2, F, S, and C [ 90 ]. This microchip is named HemeChip platform, and it consists of a cartridge where the hemoglobin variants are isolated, pictured, and tracked during electrophoresis. HemeChip is based on separating the hemoglobin using cellulose acetate electrophoresis [ 18 , 90 ]. The HemeChip test is performed by mixing the blood sample with deionized water, and then the microchip is placed inside the HemeChip reader and an electrical field is applied, which results in separating the Hb. As a result, each Hb moves a unique distance across the paper strip. Real-time images are taken during the test and analyzed automatically using custom built-in software [ 90 ]. This test has a number of advantages such as low-cost, disposable, portable, high accuracy, easy to perform, and can be completed in less than 10 min [ 18 ].
5.7. Sensors Based Techniques
5.7.1. fluorescence based optofluidic resonator.
An optofluidic resonator based on the waveguide has been used to identify the interaction of Fe 2+ and Fe 3+ within protoporphyrin IX in phosphate buffer saline. The metal clad waveguide optofluidic resonator is used for the real-time detection using a small sample volume. The Fe 2+ molecule shows a fluorescent peak which corresponds to the normal hemoglobin, while the Fe 3+ fluorescent peaks are correlated with the S hemoglobin of sickle-cell disease patients [ 91 ].
5.7.2. Sensors Based on Electrical Impedance Signal
The electrical impedance signal is a sensitive indicator of the disease severity and red blood cell sickling events. Combining the microfluidic chip with the electrical impedance provides a promising method for identifying sickling and unsickling processes in SCD patients [ 92 ]. Liu et al. developed a microfluidics-based electrochemical impedance sensor to determine the SCD red blood cells’ electrical properties under oxygen control conditions. The electrical impedance of sickle cells shows a significant difference between normoxic and hypoxic conditions [ 93 ]. These methods have been developed to monitor the cell sickling, and they do not accurately describe the severity of heterozygosis SCD.
5.7.3. Quartz Crystal Microbalance (QCM)
The quartz crystal microbalance (QCM) sensor mainly depends on the change in the frequency of a quartz crystal resonator due to the mass variation at the crystal surface [ 94 ]. Quartz crystal microbalance has been used to determine the morphological change of RBCs. Combining quartz crystal microbalance and a novel mathematical model provides complete information about the changes in the RBC’s elasticity. This sensor can differentiate between normal biconcave discoid RBCs and sickled cells and can be used as monitoring test [ 95 ].
5.7.4. Genosensors
Brazaca et al. demonstrated an electrochemical nanosensor to detect SCA trait based on the immobilization of mutated single-strand DNA on a gold electrode. The hybridization between the probe and target DNA is measured using electrochemical impedance spectroscopy (EIS). The sensor allows the detection of the SCA trait individuals and provides genetic consulting [ 96 ]. An oligonucleotide sensor has been developed for the detection of β-globin point mutation caused by sickle cell anemia. The sensor employs luminescence resonance energy transfer between photon upconverting nanoparticles (donor) and conventional fluorophore (acceptor). The sensor can determine the matched target in random oligonucleotides sequences with high sensitivity and specificity [ 97 ]. SPR biosensor has been documented to detect point mutations in the HBB gene. The technology depends on the immobilization of wild-type and βS mutated probes. The probes can differentiate between wild-type alleles and mutated ones. This technique is affordable and allows real-time detection of single-point mutations, responsible for sickle cell disease, making it suitable for effective prenatal diagnosis. This sensor needs a PCR product, which limits the ability to use it at POC [ 98 ]. The specificity of the genosensors depends on the probe design and the immobilization methods. These sensors can be improved to detect different types of SCD accurately and not be affected by the presence of different hemoglobin fractions.
5.8. The Pyrosequencing Technique
The pyrosequencing technique (PyS) has been used to identify different types of homozygous or heterozygous SCD. This test aims to sequence hemoglobin of a small number of patients accurately to differentiate between βSβS and Sβ0 thalassemia and detect HbC mutations. The difference in the mutations is only in a single nucleotide, which is hard to detect with conventional tests. PyS was compared with sanger sequencing, and it was found to be able to correctly identify βSβS with 98.7% accuracy, sickle cell-hemoglobin C disease with 98.7%, and the heterozygous with 92.2%. It was also proven to be satisfactory for β+ and β0 mutations found in SCD patients. However, several samples were wrongly classified by PyS, due to the low level of the pyrogram peaks [ 99 ]. This sequencing technique has significant potential to provide an accurate diagnosis of SCD disease and differentiate between the types of the disease, and it can be used in neonatal screening.
6. Conclusions
Sickle cell disease diagnosis has been part of the clinical laboratory for almost a century. In the last few decades, studies have enhanced the knowledge about the physiology and biochemistry of these genetic disorders to improve the detection and help in developing advanced strategies to combine molecular techniques and classical biochemical methods. Regardless of the methods implemented, the outcomes must be correlated with the clinical picture. To conclude, most hemoglobin variants can be identified and controlled by RBC indices, HPLC results, and family studies. However, the drawbacks associated with the diagnostic approaches should be known to prevent a false-negative diagnosis. Genetic tests are recommended to validate borderline cases and detect unusual and novel variants. Recently, different portable and rapid devices have been described to diagnose SCD, including platforms based on immune assay, density-based separation, and sensor-based technologies.
Author Contributions
W.A.A. did the original draft preparation. M.Z. supervised it, review and edited it to improve it. M.Z. also secured the funding. H.A.A. participated in writing some parts and the proof reading. All authors have read and agreed to the published version of the manuscript.
The authors extend their appreciation to the Deputyship for Research& Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number 492.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

VIDEO
COMMENTS
Sickle cell disease (SCD) refers to a group of hemoglobinopathies that include mutations in the gene encoding the beta subunit of hemoglobin. The first description of SCA 'like' disorder was provided by Dr. Africanus Horton in his book The Disease of Tropical Climates and their treatment (1872). However, it was not until 1910 when Dr. James B Herrick and Dr. Ernest Irons reported noticing ...
Sickle cell disease (SCD), a group of inherited hemoglobinopathies characterized by mutations that affect the β-globin chain of hemoglobin, affects approximately 100,000 people in the USA and more than 3 million people worldwide [1, 2].SCD is characterized by chronic hemolytic anemia, severe acute and chronic pain as well as end-organ damage that occurs across the lifespan.
Sickle-cell anaemia (also known as sickle-cell disorder or sickle-cell disease) is a common genetic condition due to a haemoglobin disorder - inheritance of mutant haemoglobin genes from both parents. Such haemoglobinopathies, mainly thalassaemias and sickle-cell anaemia, are globally widespread. About 5% of the world's population carries ...
Sickle cell disease (SCD) is a monogenetic disorder due to a single base-pair point mutation in the β-globin gene resulting in the substitution of the amino acid valine for glutamic acid in the β-globin chain. Phenotypic variation in the clinical presentation and disease outcome is a characteristic feature of the disorder.
1564 n engl j med 376;16 nejm.org April 20, 2017 The new england journal of medicine Figure 2. Common Clinical Complications of Sickle Cell Disease. Data are from Rees et al.3 and Serjeant.22 ...
Sickle cell disease (SCD), which aects approximately 100,000 individuals in the USA and more than 3 million world-wide, is caused by mutations in the βb globin gene that result in sickle hemoglobin production. Sickle hemoglobin polymerization leads to red blood cell sickling, chronic hemolysis and vaso-occlusion. Acute and chronic pain as well
Newby et al. have now demonstrated durable editing of the sickle-cell disease allele in the β-globin gene into a non-pathogenic variant in human-patient hematopoietic stem and progenitor cells ...
Abstract: This paper reviews Sickle cell anaemia.Sickle cell anaemia is a homozygous form of HbS (HbSS).This result. from sin gle point replacement of glut amine by valine at position 6 of β ...
Sickle cell disease and its variants constitute the most common inherited blood disorders affecting millions of individuals worldwide. Significant information regarding the nature of the genetic mutations and modifier genes that result in increased or decreased severity of the disease are available. In recent years, detailed data regarding molecular genetics, pathophysiology, mechanisms for ...
Sickle cell disease (SCD) is a collection of inherited blood disorders that affect a substantial number of people in the U.S., particularly African Americans. People with SCD have an abnormal type of hemoglobin, Hb S, which polymerizes when deoxygenated, causing the red blood cells to become misshapen and rigid. Individuals with SCD are at higher risk of morbidity and mortality from infections ...
Comprehensive Sickle Cell Center 1900 University Boulevard 513 Tinsley Harrison Birmingham, AL 35294-0006 Tel: (205) 975-2281 Fax: (205) 975-5264 E-mail: [email protected] Clinton Joiner, M.D., Ph.D. Associate Professor of Pediatrics Director, Comprehensive Sickle Cell Center Children's Hospital Medical Center 3333 Burnett Avenue, OSB 4
Introduction. Sickle cell disease (SCD) is an inherited blood disorder that first appeared in the Western literature in 1910 when Dr. James Herrick described a case of severe malaise and anemia in a 20-year-old dental student from Grenada (Herrick, 1910).On examining his blood smear, he noticed many bizarrely shaped red blood cells, leading him to surmise that "…the cause of the disease ...
Abstract. ImportanceSickle cell disease (SCD) is an inherited disorder of hemoglobin, characterized by formation of long chains of hemoglobin when deoxygenated within capillary beds, resulting in sickle-shaped red blood cells, progressive multiorgan damage, and increased mortality. An estimated 300 000 infants are born annually worldwide with SCD.
Sickle cell anemia, which is also called sickle cell disease (SCD), is a hematological disorder that causes occlusion in blood vessels, leading to hurtful episodes and even death. The key function ...
Sickle cell disease (SCD) is one of the commonest monogenic diseases with highest prevalence in sun-saharan Africa (500-2000/100.000) 1. It is caused by a single amino acid mutation, where ...
Abstract. Sickle cell anemia is an inherited disorder of the globin chains that causes hemolysis and chronic organ damage. This activity reviews the pathophysiology, presentation, complications ...
Impact of SARS-CoV-2 infection on pain crisis and acute chest syndrome in patients with sickle cell anemia: A retrospective multi-cohort study based on US national data from 2020 to 2022 Juan Alvarado , Keval Yerigeri , Anita Boakye , Christina Randolph , Aparna Roy , Grace Onimoe ,
Semantic Scholar extracted view of "Sickle cell anemia: hepatic macrophages to the rescue." ... Showing 1 through 3 of 0 Related Papers. 9 References; Related Papers ... Semantic Scholar is a free, AI-powered research tool for scientific literature, based at the Allen Institute for AI. Learn More. About About Us Meet the Team Publishers Blog ...
Introduction. Sickle cell disease (SCD), a group of inherited hemoglobinopathies characterized by mutations that affect the β-globin chain of hemoglobin, affects approximately 100,000 people in the USA and more than 3 million people worldwide [1, 2].SCD is characterized by chronic hemolytic anemia, severe acute and chronic pain as well as end-organ damage that occurs across the lifespan.
This review provides an overview of the current and emerging techniques for sickle cell disease detection and highlights the different potential methods that could be applied to help the early diagnosis of SCD. Keywords: sickle cell anemia, hemoglobinopathies, detection, diagnosis, point of care. 1.
sickle cell disease, low oxygen tension promotes red blood cell sickling and repeated damage of cell membrane and decrease the cell's elasticity. 2-The rigid blood cells are unable to deform as they pass through narrow capillaries, leading to vessel. 3-The actual anemia of the illness is caused by rapid hemolysis because short RBC life, the