An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Orthop Case Rep
- v.6(3); Jul-Aug 2016

How much Literature Review is enough for a Case Report?
Ashok shyam.
1 Indian Orthopaedic Research Group, Thane, India
Literature review is an essential part of any scientific document. It’s the foundation on which the premise of scientific research is built. Every scientific study has to be seen in context of the existing knowledge and literature. However literature is growing at exponential rate today and especially with so many online journals today it’s really difficult to review all literature. This poses unique challenge to authors who wish to submit a case report for Journal of Orthopaedic Case Reports.
First issue is how much important is literature review for a case report. From early days of case reports, literature review is been the most important cornerstone of a case report. Especially since earlier only rare cases were considered as case reports [ 1 ]. So to establish the rarity of the case it was important to thoroughly review the literature. For us at Journal of Orthopaedic Case Report, literature review is one of the most significant part of the case report. Most of the times it is the section which decides on acceptance or rejection of borderline cases. For cases that are rare we recommend our authors to do a thorough review of literature and also prepare a table of literature review which should be added to the manuscript. For cases where is the focus is on a specific clinical learning point, the literature review needs to be more exhaustive. When a clinical point has to be made, the literature review should cover all the alternative plans that are already described in literature or at least similar cases that have used a different plan. Many cases that are published in Journal of Orthopaedic Case Reports are more on clinical strategies that are improvised and applied to clinical cases. In these scenario we wish our authors to provide rationale for their choices with back up from literature. Also a thorough review of all exiting strategies should be added to the review. The intention is to make the reader aware of all existing management options and then understand why particular option was chosen in the particular patient. This will also help effective communication between the authors and reviewers and ultimately the readers.
The second issue is how much literature to review. With so much literature available at times literature review may miss important article which is relevant to the case. At times it happens that too much literature is added to the article making it bulky and in need for repeated editing. To resolve these issues we would recommend authors to do a thorough PubMed and google Scholar review which will include most important of the articles. If the number of articles relevant to your search are exceeding thirty you may apply filters like selecting articles published in last 5 years or selecting articles only from PubMed. Selecting article only on PubMed will help get the most relevant articles in your review but an additional scrutiny of google result will help you not miss out on important articles. Google will most of the times provide a very comprehensive list of articles and it may need some effort on part of authors to find the relevant articles. But combining both search strategies will definitely make the review more complete and relevant. The number of references should be limited to maximum of 30 for journal of orthopaedic case reports. This will again require authors to review select the most relevant articles from the literature search. Try and include the most recent articles and also from the most relevant authors and medical centres. In case a particular authors has written multiple articles on similar topic, one of his most relevant can be included while others can be left. Articles that are most close to your clinical scenario should be preferred over other articles. Also articles that have chosen strategies similar to yours should be preferred. If the number of articles is still much more, try to limit the references in terms of geography and include articles that are relevant in your country or geographical location to make is more comparable. Also patient profiles can be matched in terms of age and gender to provide for a better comparison between your case and the case from literature. As far as possible provide a literature review table with list of articles, authors names, number of patients, interventions, results and complications to provide a complete perspective to reader. Review of complications needs a special mention and it should be a part of every surgical case that is reported.
In the end try and weave a continuous narrative including parts from your case and reference to context from literature and make an interesting story for readers and editors. We all love a good story which is told in a well woven text and has relevant learning points underlined.
Editor-Journal of Orthopaedic Case Reports
Conflict of Interest: Nil
Source of Support: None
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Literature Review | Guide, Examples, & Templates
How to Write a Literature Review | Guide, Examples, & Templates
Published on January 2, 2023 by Shona McCombes . Revised on September 11, 2023.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research that you can later apply to your paper, thesis, or dissertation topic .
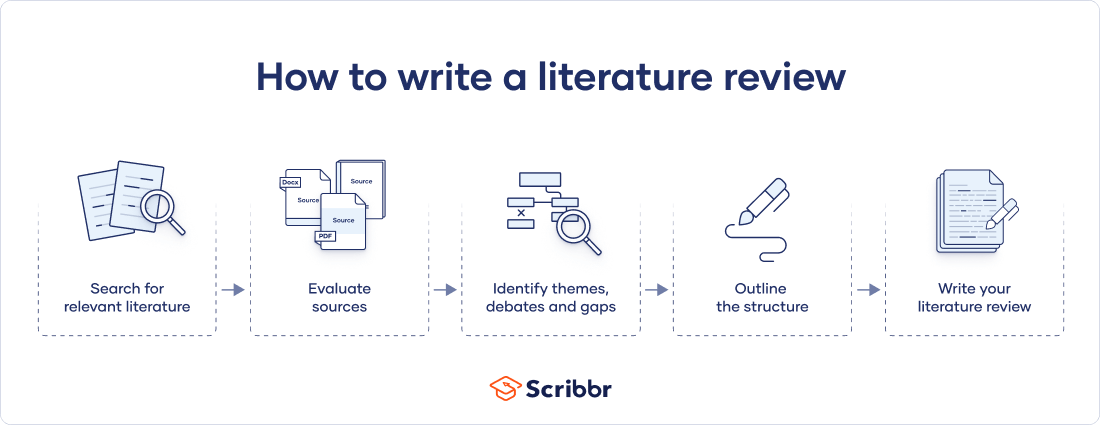
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates, and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarize sources—it analyzes, synthesizes , and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
What is the purpose of a literature review, examples of literature reviews, step 1 – search for relevant literature, step 2 – evaluate and select sources, step 3 – identify themes, debates, and gaps, step 4 – outline your literature review’s structure, step 5 – write your literature review, free lecture slides, other interesting articles, frequently asked questions, introduction.
- Quick Run-through
- Step 1 & 2
When you write a thesis , dissertation , or research paper , you will likely have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and its scholarly context
- Develop a theoretical framework and methodology for your research
- Position your work in relation to other researchers and theorists
- Show how your research addresses a gap or contributes to a debate
- Evaluate the current state of research and demonstrate your knowledge of the scholarly debates around your topic.
Writing literature reviews is a particularly important skill if you want to apply for graduate school or pursue a career in research. We’ve written a step-by-step guide that you can follow below.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research problem and questions .
Make a list of keywords
Start by creating a list of keywords related to your research question. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list as you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some useful databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can also use boolean operators to help narrow down your search.
Make sure to read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
You likely won’t be able to read absolutely everything that has been written on your topic, so it will be necessary to evaluate which sources are most relevant to your research question.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models, and methods?
- Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible , and make sure you read any landmark studies and major theories in your field of research.
You can use our template to summarize and evaluate sources you’re thinking about using. Click on either button below to download.
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It is important to keep track of your sources with citations to avoid plagiarism . It can be helpful to make an annotated bibliography , where you compile full citation information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
The only proofreading tool specialized in correcting academic writing - try for free!
The academic proofreading tool has been trained on 1000s of academic texts and by native English editors. Making it the most accurate and reliable proofreading tool for students.
Try for free
To begin organizing your literature review’s argument and structure, be sure you understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly visual platforms like Instagram and Snapchat—this is a gap that you could address in your own research.
There are various approaches to organizing the body of a literature review. Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarizing sources in order.
Try to analyze patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organize your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text , your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, you can follow these tips:
- Summarize and synthesize: give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: don’t just paraphrase other researchers — add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically evaluate: mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: use transition words and topic sentences to draw connections, comparisons and contrasts
In the conclusion, you should summarize the key findings you have taken from the literature and emphasize their significance.
When you’ve finished writing and revising your literature review, don’t forget to proofread thoroughly before submitting. Not a language expert? Check out Scribbr’s professional proofreading services !
This article has been adapted into lecture slides that you can use to teach your students about writing a literature review.
Scribbr slides are free to use, customize, and distribute for educational purposes.
Open Google Slides Download PowerPoint
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarize yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your thesis or dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, September 11). How to Write a Literature Review | Guide, Examples, & Templates. Scribbr. Retrieved April 1, 2024, from https://www.scribbr.com/dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, what is a theoretical framework | guide to organizing, what is a research methodology | steps & tips, how to write a research proposal | examples & templates, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 January 2023
A student guide to writing a case report
- Maeve McAllister 1
BDJ Student volume 30 , pages 12–13 ( 2023 ) Cite this article
21 Accesses
Metrics details
As a student, it can be hard to know where to start when reading or writing a clinical case report either for university or out of special interest in a Journal. I have collated five top tips for writing an insightful and relevant case report.
A case report is a structured report of the clinical process of a patient's diagnostic pathway, including symptoms, signs, diagnosis, treatment planning (short and long term), clinical outcomes and follow-up. 1 Some of these case reports can sometimes have simple titles, to the more unusual, for example, 'Oral Tuberculosis', 'The escapee wisdom tooth', 'A difficult diagnosis'. They normally begin with the word 'Sir' and follow an introduction from this.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
Purchase on Springer Link
Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Guidelines To Writing a Clinical Case Report. Heart Views 2017; 18 , 104-105.
British Dental Journal. Case reports. Available online at: www.nature.com/bdj/articles?searchType=journalSearch&sort=PubDate&type=case-report&page=2 (accessed August 17, 2022).
Chate R, Chate C. Achenbach's syndrome. Br Dent J 2021; 231: 147.
Abdulgani A, Muhamad, A-H and Watted N. Dental case report for publication; step by step. J Dent Med Sci 2014; 3 : 94-100.
Download references
Author information
Authors and affiliations.
Queen´s University Belfast, Belfast, United Kingdom
Maeve McAllister
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Maeve McAllister .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
McAllister, M. A student guide to writing a case report. BDJ Student 30 , 12–13 (2023). https://doi.org/10.1038/s41406-023-0925-y
Download citation
Published : 30 January 2023
Issue Date : 30 January 2023
DOI : https://doi.org/10.1038/s41406-023-0925-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Writing a Case Report: Literature Review
- Introduction
- Literature Review
- Additional Help
Common Questions about Literature Reviews
- What a Literature Review is NOT
- What is a Literature Review?
- How is a Literature Review different from an academic research paper?
- Why do we write Literature Reviews?
- Who writes Literature Reviews?
OK. You’ve got to write a literature review. You dust off a novel and a book of poetry, settle down in your chair, and get ready to issue a “thumbs up” or “thumbs down” as you leaf through the pages. “Literature review” done. Right?
Wrong! The “literature” of a literature review refers to any collection of materials on a topic, not necessarily the great literary texts of the world. “Literature” could be anything from a set of government pamphlets on British colonial methods in Africa to scholarly articles on the treatment of a torn ACL. And a review does not necessarily mean that your reader wants you to give your personal opinion on whether or not you liked these sources.
A literature review discusses published information in a particular subject area, and sometimes information in a particular subject area within a certain time period.
A literature review can be just a simple summary of the sources, but it usually has an organizational pattern and combines both summary and synthesis. A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information. It might give a new interpretation of old material or combine new with old interpretations. Or it might trace the intellectual progression of the field, including major debates. And depending on the situation, the literature review may evaluate the sources and advise the reader on the most pertinent or relevant.
The main focus of an academic research paper is to develop a new argument, and a research paper is likely to contain a literature review as one of its parts. In a research paper, you use the literature as a foundation and as support for a new insight that you contribute. The focus of a literature review, however, is to summarize and synthesize the arguments and ideas of others without adding new contributions.
Literature reviews provide you with a handy guide to a particular topic. If you have limited time to conduct research, literature reviews can give you an overview or act as a stepping stone. For professionals, they are useful reports that keep them up to date with what is current in the field. For scholars, the depth and breadth of the literature review emphasizes the credibility of the writer in his or her field. Literature reviews also provide a solid background for a research paper’s investigation. Comprehensive knowledge of the literature of the field is essential to most research papers.
Literature reviews are written occasionally in the humanities, but mostly in the sciences and social sciences; in experiment and lab reports, they constitute a section of the paper. Sometimes a literature review is written as a paper in itself.
Things to consider before starting your Literature Review
- Find models
- Narrow your topic
- Consider whether your sources are current
If your assignment is not very specific, seek clarification from your instructor:
- Roughly how many sources should you include?
- What types of sources (books, journal articles, websites)?
- Should you summarize, synthesize, or critique your sources by discussing a common theme or issue?
- Should you evaluate your sources?
- Should you provide subheadings and other background information, such as definitions and/or a history?
Look for other literature reviews in your area of interest or in the discipline and read them to get a sense of the types of themes you might want to look for in your own research or ways to organize your final review. You can simply put the word “review” in your search engine along with your other topic terms to find articles of this type on the Internet or in an electronic database. The bibliography or reference section of sources you’ve already read are also excellent entry points into your own research.
There are hundreds or even thousands of articles and books on most areas of study. The narrower your topic, the easier it will be to limit the number of sources you need to read in order to get a good survey of the material. Your instructor will probably not expect you to read everything that’s out there on the topic, but you’ll make your job easier if you first limit your scope.
And don’t forget to tap into your professor’s (or other professors’) knowledge in the field. Ask your professor questions such as: “If you had to read only one book from the 90’s on topic X, what would it be?” Questions such as this help you to find and determine quickly the most seminal pieces in the field.
Some disciplines require that you use information that is as current as possible. In the sciences, for instance, treatments for medical problems are constantly changing according to the latest studies. Information even two years old could be obsolete. However, if you are writing a review in the humanities, history, or social sciences, a survey of the history of the literature may be what is needed, because what is important is how perspectives have changed through the years or within a certain time period. Try sorting through some other current bibliographies or literature reviews in the field to get a sense of what your discipline expects. You can also use this method to consider what is currently of interest to scholars in this field and what is not.
Strategies for writing a Literature Review
- Finding a focus
- Convey it to your reader
- Consider organization
A literature review, like a term paper, is usually organized around ideas, not the sources themselves as an annotated bibliography would be organized. This means that you will not just simply list your sources and go into detail about each one of them, one at a time. No. As you read widely but selectively in your topic area, consider instead what themes or issues connect your sources together. Do they present one or different solutions? Is there an aspect of the field that is missing? How well do they present the material and do they portray it according to an appropriate theory? Do they reveal a trend in the field? A raging debate? Pick one of these themes to focus the organization of your review.
A literature review may not have a traditional thesis statement (one that makes an argument), but you do need to tell readers what to expect. Try writing a simple statement that lets the reader know what is your main organizing principle. Here are a couple of examples:
- The current trend in treatment for congestive heart failure combines surgery and medicine.
- More and more cultural studies scholars are accepting popular media as a subject worthy of academic consideration.
You’ve got a focus, and you’ve stated it clearly and directly. Now what is the most effective way of presenting the information? What are the most important topics, subtopics, etc., that your review needs to include? And in what order should you present them? Develop an organization for your review at both a global and local level:
First, cover the basic categories
Just like most academic papers, literature reviews also must contain at least three basic elements: an introduction or background information section; the body of the review containing the discussion of sources; and, finally, a conclusion and/or recommendations section to end the paper. The following provides a brief description of the content of each:
- Introduction: Gives a quick idea of the topic of the literature review, such as the central theme or organizational pattern.
- Body: Contains your discussion of sources and is organized either chronologically, thematically, or methodologically (see below for more information on each).
- Conclusions/Recommendations: Discuss what you have drawn from reviewing literature so far. Where might the discussion proceed?
Begin Writing
- Use evidence
- Be selective
- Use quotes sparingly
- Summarize and synthesize
- Keep your own voice
- Use caution when paraphrasing
Once you’ve settled on a general pattern of organization, you’re ready to write each section. There are a few guidelines you should follow during the writing stage as well. Here is a sample paragraph from a literature review about sexism and language to illuminate the following discussion:
However, other studies have shown that even gender-neutral antecedents are more likely to produce masculine images than feminine ones (Gastil, 1990). Hamilton (1988) asked students to complete sentences that required them to fill in pronouns that agreed with gender-neutral antecedents such as “writer,” “pedestrian,” and “persons.” The students were asked to describe any image they had when writing the sentence. Hamilton found that people imagined 3.3 men to each woman in the masculine “generic” condition and 1.5 men per woman in the unbiased condition. Thus, while ambient sexism accounted for some of the masculine bias, sexist language amplified the effect. (Source: Erika Falk and Jordan Mills, “Why Sexist Language Affects Persuasion: The Role of Homophily, Intended Audience, and Offense,” Women and Language19:2).
In the example, the writers refer to several other sources when making their point. A literature review in this sense is just like any other academic research paper. Your interpretation of the available sources must be backed up with evidence to show that what you are saying is valid.
Select only the most important points in each source to highlight in the review. The type of information you choose to mention should relate directly to the review’s focus, whether it is thematic, methodological, or chronological.
Falk and Mills do not use any direct quotes. That is because the survey nature of the literature review does not allow for in-depth discussion or detailed quotes from the text. Some short quotes here and there are okay, though, if you want to emphasize a point, or if what the author said just cannot be rewritten in your own words. Notice that Falk and Mills do quote certain terms that were coined by the author, not common knowledge, or taken directly from the study. But if you find yourself wanting to put in more quotes, check with your instructor.
Remember to summarize and synthesize your sources within each paragraph as well as throughout the review. The authors here recapitulate important features of Hamilton’s study, but then synthesize it by rephrasing the study’s significance and relating it to their own work.
While the literature review presents others’ ideas, your voice (the writer’s) should remain front and center. Notice that Falk and Mills weave references to other sources into their own text, but they still maintain their own voice by starting and ending the paragraph with their own ideas and their own words. The sources support what Falk and Mills are saying.
When paraphrasing a source that is not your own, be sure to represent the author’s information or opinions accurately and in your own words. In the preceding example, Falk and Mills either directly refer in the text to the author of their source, such as Hamilton, or they provide ample notation in the text when the ideas they are mentioning are not their own, for example, Gastil’s. For more information, please see our student handbook .
Revise, revise, revise
Draft in hand? Now you’re ready to revise. Spending a lot of time revising is a wise idea, because your main objective is to present the material, not the argument. So check over your review again to make sure it follows the assignment and/or your outline. Then, just as you would for most other academic forms of writing, rewrite or rework the language of your review so that you’ve presented your information in the most concise manner possible. Be sure to use terminology familiar to your audience; get rid of unnecessary jargon or slang. Finally, double check that you’ve documented your sources and formatted the review appropriately for your discipline.
Writing a Literature Review
How to Organize
- Chronological
- By publication
- Methodological
- Other Sections
Once you have the basic categories in place, then you must consider how you will present the sources themselves within the body of your paper. Create an organizational method to focus this section even further.
To help you come up with an overall organizational framework for your review, consider the following scenario:
You’ve decided to focus your literature review on materials dealing with sperm whales. This is because you’ve just finished reading Moby Dick, and you wonder if that whale’s portrayal is really real. You start with some articles about the physiology of sperm whales in biology journals written in the 1980’s. But these articles refer to some British biological studies performed on whales in the early 18th century. So you check those out. Then you look up a book written in 1968 with information on how sperm whales have been portrayed in other forms of art, such as in Alaskan poetry, in French painting, or on whale bone, as the whale hunters in the late 19th century used to do. This makes you wonder about American whaling methods during the time portrayed in Moby Dick, so you find some academic articles published in the last five years on how accurately Herman Melville portrayed the whaling scene in his novel.
Now consider some typical ways of organizing the sources into a review:
If your review follows the chronological method, you could write about the materials above according to when they were published. For instance, first you would talk about the British biological studies of the 18th century, then about Moby Dick, published in 1851, then the book on sperm whales in other art (1968), and finally the biology articles (1980s) and the recent articles on American whaling of the 19th century. But there is relatively no continuity among subjects here. And notice that even though the sources on sperm whales in other art and on American whaling are written recently, they are about other subjects/objects that were created much earlier. Thus, the review loses its chronological focus.
Order your sources by publication chronology, then, only if the order demonstrates a more important trend. For instance, you could order a review of literature on biological studies of sperm whales if the progression revealed a change in dissection practices of the researchers who wrote and/or conducted the studies.
A better way to organize the above sources chronologically is to examine the sources under another trend, such as the history of whaling. Then your review would have subsections according to eras within this period. For instance, the review might examine whaling from pre-1600-1699, 1700-1799, and 1800-1899. Under this method, you would combine the recent studies on American whaling in the 19th century with Moby Dick itself in the 1800-1899 category, even though the authors wrote a century apart.
Thematic reviews of literature are organized around a topic or issue, rather than the progression of time. However, progression of time may still be an important factor in a thematic review. For instance, the sperm whale review could focus on the development of the harpoon for whale hunting. While the study focuses on one topic, harpoon technology, it will still be organized chronologically. The only difference here between a “chronological” and a “thematic” approach is what is emphasized the most: the development of the harpoon or the harpoon technology. But more authentic thematic reviews tend to break away from chronological order. For instance, a thematic review of material on sperm whales might examine how they are portrayed as “evil” in cultural documents. The subsections might include how they are personified, how their proportions are exaggerated, and their behaviors misunderstood. A review organized in this manner would shift between time periods within each section according to the point made.
A methodological approach differs from the two above in that the focusing factor usually does not have to do with the content of the material. Instead, it focuses on the “methods” of the researcher or writer. For the sperm whale project, one methodological approach would be to look at cultural differences between the portrayal of whales in American, British, and French art work. Or the review might focus on the economic impact of whaling on a community. A methodological scope will influence either the types of documents in the review or the way in which these documents are discussed.
Once you’ve decided on the organizational method for the body of the review, the sections you need to include in the paper should be easy to figure out. They should arise out of your organizational strategy. In other words, a chronological review would have subsections for each vital time period. A thematic review would have subtopics based upon factors that relate to the theme or issue.
Sometimes, though, you might need to add additional sections that are necessary for your study, but do not fit in the organizational strategy of the body. What other sections you include in the body is up to you. Put in only what is necessary. Here are a few other sections you might want to consider
- Current Situation: Information necessary to understand the topic or focus of the literature review.
- History: The chronological progression of the field, the literature, or an idea that is necessary to understand the literature review, if the body of the literature review is not already a chronology.
- Methods and/or Standards: The criteria you used to select the sources in your literature review or the way in which you present your information. For instance, you might explain that your review includes only peer-reviewed articles and journals.
- Questions for Further Research : What questions about the field has the review sparked? How will you further your research as a result of the review?
- Works Consulted
We consulted these works while writing this handout. This is not a comprehensive list of resources on the handout’s topic, and we encourage you to do your own research to find additional publications. Please do not use this list as a model for the format of your own reference list, as it may not match the citation style you are using. For guidance on formatting citations, please see the OCOM Library AMA Guide . We revise these tips periodically and welcome feedback.
Anson, Chris M. and Robert A. Schwegler. The Longman Handbook for Writers and Readers. 6th edition. New York: Longman, 2010.
Jones, Robert, Patrick Bizzaro, and Cynthia Selfe. The Harcourt Brace Guide to Writing in the Disciplines. New York: Harcourt Brace, 1997.
Lamb, Sandra E. How to Write It: A Complete Guide to Everything You’ll Ever Write. Berkeley, Calif.: Ten Speed Press, 1998.
Rosen, Leonard J. and Laurence Behrens. The Allyn and Bacon Handbook. 4th edition. Boston: Allyn and Bacon, 2000.
Troyka, Lynn Quitman. Simon and Schuster Handbook for Writers. Upper Saddle River, N.J.: Prentice Hall, 2002.

- << Previous: Introduction
- Next: Examples >>
- Last Updated: Nov 2, 2023 2:12 PM
- URL: https://ocomlibrary.libguides.com/casereport
OCOM Library
Email us at [email protected]
Phone us at 503-253-3443x132
Visit us at 75 NW Couch St, Portland, OR 97209
- Case Reports
- Open access
- Published: 25 February 2021
Renal leiomyoma: a case report and literature review
- Sajal Gerdharee 1 ,
- Abrie van Wyk 2 ,
- Heidi van Deventer ORCID: orcid.org/0000-0001-7472-1773 1 &
- Andre van der Merwe 1
African Journal of Urology volume 27 , Article number: 39 ( 2021 ) Cite this article
2657 Accesses
1 Citations
Metrics details
Renal leiomyomas are exceptionally rare, benign, mesenchymal tumours originating from smooth muscle in the kidney. Historically, because of their small size, most renal leiomyoma cases were discovered incidentally based on autopsy findings. However, since the advent and improved access to imaging modalities such as ultrasound and computed tomography (CT), renal leiomyomas are being discovered more frequently. Although usually incidental discoveries, clinical presenting signs and symptoms comprise abdominal or flank pain, a palpable flank mass, and haematuria in 20% of those with symptoms.
Case presentation
We study the case of an incidentally found, asymptomatic, left kidney mass that presented in a 60-year-old female. Initial suspicions on CT imaging of either renal cell carcinoma or oncocytoma resulted in a radical nephrectomy of the left kidney. Postoperative pathological examination of the mass revealed a renal leiomyoma; a rare, benign tumour that is mostly indistinguishable from malignant tumours on imaging.
Conclusions
With the current availability of ultrasonography and CT, they are often discovered incidentally, and the radiological differential diagnoses are often inadequate or challenging in such cases. The gold standard management of these suspicious cancer cases is still a radical nephrectomy with postoperative pathological and immunohistochemical analysis. Due to its benign nature, patients enjoy excellent prognoses without recurrence. We discuss and briefly review the relevant literature of the clinical, imaging and pathological features of renal leiomyomas and those of the differential diagnoses.
1 Background
Renal leiomyomas are exceptionally rare, benign, mesenchymal tumours originating from smooth muscle in the kidney. Renal smooth muscle can be found within the renal capsule, calyces, pelvis, and blood vessels; hence, any of these areas can be a site of origin for leiomyomas.
Steiner and colleagues found that of symptomatic leiomyomas, 53% of tumours were localised subcapsular, 37% capsular, and 10% within the renal pelvis [ 1 ].
Historically, because of their small size, most renal leiomyoma cases were discovered incidentally based on autopsy findings [ 2 ]. However, since the advent and improved access to imaging modalities such as ultrasound and CT, renal leiomyomas are being discovered more frequently.
Although usually incidental discoveries, clinical presenting signs and symptoms comprise abdominal or flank pain, a palpable flank mass, and haematuria in 20% of those with symptoms. Cases suggest a higher prevalence in adult females, with an average age of 42 at presentation [ 1 , 3 ]. Leiomyomas of the kidney are even rarer in the paediatric population, and there have been very few reported cases of these tumours in the literature. Some cases of interest included a six-year-old boy with an initially suspected Wilms tumour; a leiomyoma occurring in a transplanted kidney in association with the Epstein–Barr virus (EBV), five years post renal transplant; and a vascular renal leiomyoma in an 11-year-old with bilateral RCC [ 4 ].
Over ten years, the Brady Urological Institute reviewed several consecutive nephrectomies as a result of renal tumours. Of a total of 1030 nephrectomies, renal leiomyomas comprised 0.3% of the total, and 1.5% of all benign tumours removed [ 5 ].
In terms of etiological factors leading to the development of a renal leiomyoma, there are various theories and suggested causative factors. Krishnan and colleagues showed a possible causal relationship between Epstein-Barr virus and renal leiomyoma in immunocompromised patients, while Tsujimura and colleagues suggested a possible link with tuberous sclerosis; a condition more commonly associated with renal angiomyolipoma (AML) [ 6 , 7 ].
When considering the relevant differential diagnosis concerning clinical presentation and imaging studies, three renal tumours that are more commonly found are AML, oncocytoma, and the diagnostic importance that lies in distinguishing them from the malignant RCC [ 8 ]. Renal cell carcinomas make up the majority of contrast-enhancing small renal masses, the most prevalent histological type being the clear cell RCC. Frank and colleagues demonstrated a clear cell RCC incidence of 55% after 2770 surgeries (radical nephrectomies or nephron-sparing) for solid, unilateral, non-metastatic tumours [ 9 ].
AMLs are benign tumours in the perivascular epithelioid cell tumour family, comprising variable mixtures of thick-walled blood vessels, mature fat, and smooth muscle. It may sometimes be represented by dominance of the smooth muscle component, hence its likeness to a leiomyoma. Furthermore, they are characterised by smooth muscle and melanocytic marker (HMB-45) co-expression [ 10 ]. In contrast to this, renal leiomyoma tumour cells typically test negative for HMB-45, but express smooth muscle actin and desmin. Angiomyolipoma is also relatively well-distinguished radiologically as an enhancing mass with macroscopic fat, devoid of calcification. Small, fat-poor tumours can be more challenging to distinguish from RCC [ 11 ].
Oncocytoma often displays the appearance of a well-circumscribed, homogeneous, and solid lesion, and may often demonstrate central scarring—features that are not mutually exclusive with the presentation of RCC [ 12 ]. Postoperatively the pathologist also needs to differentiate leiomyoma from leiomyosarcoma [ 10 ]. Histological features associated with leiomyosarcoma are tumour necrosis, nuclear pleomorphism, and increased mitotic activity [ 13 ].
2 Case presentation
2.1 clinical history.
The patient under study is a 60-year-old female, previously diagnosed with hypertension, diabetes mellitus (type II) and congestive cardiac failure. After a routine chest radiograph displayed suspicious nodularity in both lung apices, a CT was performed, and the nodules were queried to be post-infective changes or suspicious of metastases. An abdominal CT was then performed in search of a primary neoplastic lesion and a mixed, solid-cystic mass in the lower pole of the left kidney was discovered. The mass was asymptomatic, and she had no associated pain, discomfort or haematuria.
2.2 Investigations
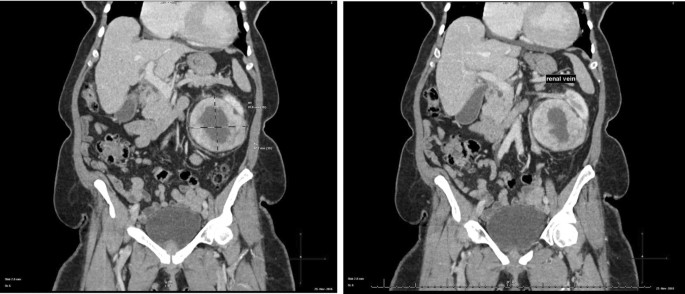
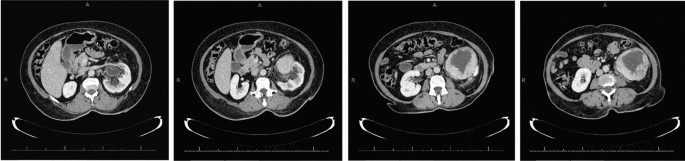
The abdominal CT showed a large solid/cystic tumour in the lower pole of the left kidney. The dimensions of the renal mass was 9 cm × 8 cm, demonstrating peripheral soft tissue enhancement with a central, cystic component measuring 12 HU (Hounsfield Units). It was highly suspicious of an RCC with central necrosis. Two months later, a repeat contrasted CT was done to evaluate for the progression of the suspicious lung lesions, which may have indicated metastases, and for the appearance of new intra-abdominal metastases. CT findings were unchanged from before, as seen in Figs. 1 and 2 .
Abdominal CT demonstrating renal mass
There was displacement of the lower pole of the kidney with marked compression of the proximal ureter, which was splayed around the mass—resulting in mild hydronephrosis. The mass demonstrated fat stranding and bordered the retroperitoneal fascia but did not bridge or enter the peritoneal cavity. Similarly, renal vessels were splayed but not infiltrated, with no signs of neovascularisation. There was no clear claw sign demonstrated to suggest renal origin, and no associated lymphadenopathy or distant metastases. These features were not in keeping with an aggressive RCC, and a possible diagnosis of a renal oncocytoma was considered. However, it is noted that the absence of a renal claw sign and clear origin from the renal cortex remains atypical for either of the diagnoses mentioned.
The patient was prepared for surgery and underwent a radical nephrectomy. The postoperative course was uneventful, and the patient was discharged.
Histological sections of the tumour displayed a well-circumscribed, unencapsulated mass comprising spindled cells with a fascicular growth pattern (Fig. 3 ). There was central cystic degeneration, associated with degenerative atypia but without any mitotic figures noted. There was no evidence of vascular invasion or necrosis. Immunohistochemically, the lesion was strongly positive for desmin and smooth muscle actin and negative for HMB-45 and melan A. Chromogenic in situ hybridisation for Epstein-Barr encoded RNA was negative, excluding EBV associated smooth muscle neoplasm, which may have similar morphology. These results, in keeping with clinically absent features of tuberous sclerosis, favoured a diagnosis of a renal leiomyoma. There was unfortunately no mention made by the histological report of whether the tumour originated from capsular or subcapsular renal tissue.
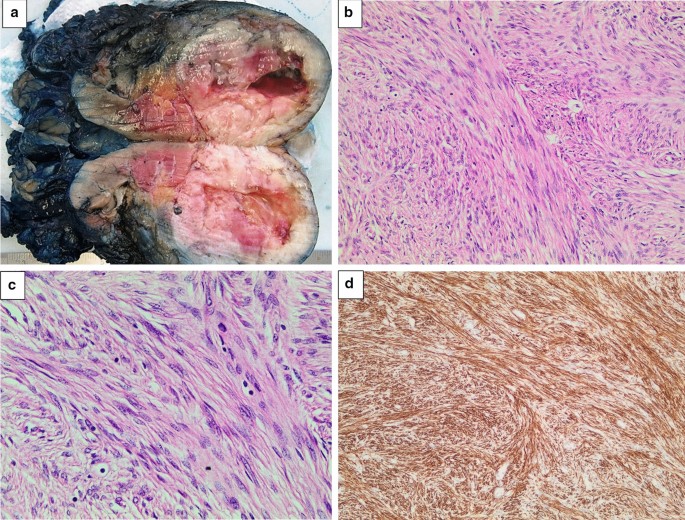
a Left kidney bisected. A round, well-circumscribed tumour with central cystic degeneration is present in the lower pole of the kidney. b The tumour is composed of spindle-shaped cells with eosinophilic cytoplasm growing in intersecting fascicles. Haematoxylin and eosin, original magnification × 200. c The nuclei of the tumour cells often have rounded ends (“cigar-shaped” nuclei). Scattered lymphocytes are noted in the background. Significant pleomorphism, tumour necrosis and increased mitotic activity are absent. Haematoxylin and eosin, original magnification × 400. d An immunohistochemical stain for desmin shows strong and diffuse cytoplasmic staining. Original magnification × 200
3 Discussion
The literature reviewed comprised entirely of case studies that included a diagnosis of renal leiomyoma, within no specific time period. Provided that the diagnosis of a renal leiomyoma was made, there were no other exclusion criteria other than the patient’s age being older than 16 years—of which no such cases were eliminated thereby. Cases were reviewed and added on a case-to-case basis as they were found. Search engines utilised in English, comprised primarily of PubMed, and the university library e-databases.
We reviewed 20 cases of renal leiomyomas in adults (19 from the literature and one of our own), summarised in Table 1 . The mean age was 49, with a 70% predominance of female patients. Sixty-eight per cent of these cases were symptomatic before the discovery of their renal leiomyomas. Of those cases reporting signs, 53% had abdominal pain, 32% had a flank mass, while only 26% were haematuric. Only one of nineteen presented with all three clinical features simultaneously, while 32% had at least two of the features present. In cases where tumour origin was mentioned, there was a 50% predominance of capsular leiomyomas, and only two cases were located in the subcapsular area. Of the cases that reported immunohistochemical testing, 100% of tumours tested negative for HMB-45. All the tumours that were tested for smooth muscle actin and/or desmin were positive for these markers. Tumour sizes ranged from 1.3 to 32 cm, with an average diameter of 9.6 cm.
Some of these results differ slightly from the literature represented earlier concerning presentation, which is likely due to the sample size and lack of reporting on the various clinical and pathological characteristics in many of these cases.
3.1 Pre-operative diagnostics
On ultra-sonographic imaging, leiomyomas typically appear hypoechoic with either a solid or a cystic appearance [ 10 ]. Some helpful CT features were pointed out by Derchi and colleagues to help with the differential diagnosis. Firstly, the density before contrast of all leiomyomas displayed a likeness to that of muscle and was hyper-dense. After the addition of contrast, the masses had lower enhancement in comparison with the renal parenchyma. Secondly, the lesions display no infiltration of neighbouring tissue and are usually well-circumscribed and located peripherally. These features see the addition of a renal leiomyoma to the differential; however, malignant disease still cannot be excluded. The presence of infiltration virtually excludes a benign renal leiomyoma [ 1 , 14 ].
The size and enhancement of incidentally discovered, small, asymptomatic renal masses may be of value in determining the likely nature of such lesions.
A study of 2770 renal tumours found that almost half the number of non-metastatic tumours smaller than 1 cm were benign, while tumours of 7 cm or larger comprised only 6.3% of benign tumours [ 9 ]. O’Connor and colleagues discovered incidental renal lesions greater than 1 cm in 433 of 3001 (14%) in asymptomatic adults after an abdominopelvic CT for screening colonography. Of these, 87% were radiologically characterised as benign and a further 13% of “indeterminate significance” [ 15 ]. Radiologically, the enhancement of a renal lesion is considered a valuable characteristic in determining the nature of the lesion. While there is some consensus that enhancement of 20 HU or more is significant in identifying malignant lesions, some authors suggest that lesions displaying 10 HU or more should still be considered significantly suspicious of malignancy. Therefore, lesions displaying attenuation from 10 HU to 19 HU are considered “indeterminate”.
This determination has some implications when considering the possibility of pre-operative diagnosis in the form of CT-guided core biopsies. Although it remains controversial, Eshed and colleagues believe that due to the low complication rate and value in implementing disease-tailored management, indication for core biopsies should include radiographically indeterminate lesions and small renal masses. In agreement, Romero and colleagues suggest that there is a place for biopsy and conservative management in patients with radiographic and clinical evidence of a diagnosis other than RCC. These lesions with benign biopsy results can then undergo resection, re-evaluation or close clinical or imaging surveillance [ 5 , 16 ]. Where availability and resources allow for such, the use of Magnetic Resonance Imaging (MRI) in the diagnosis of renal masses is another area of diagnostics which may benefit pre-operative diagnostics. However, in a resource-poor setting, this modality is a less feasible option than CT due to limited availability in the public sector, and cost. It is thus not further discussed in this paper.
Currently, management of larger lesions still holds surgical resection as its gold standard, with radical nephrectomy being the usual approach to suspicious lesions. Lesions equal to, or smaller than, 4 cm allow consideration for nephron-sparing resections, and smaller renal masses can be biopsied—although still considered a somewhat controversial practice [ 1 ]. In the ideal setting, nephron-sparing resection could also be a consideration, given the availability of extemporaneous examination during the surgical procedure. Likewise, the same remains true with regard to performing an initial renal biopsy for immunohistological diagnosis. The indications for either of which would also need to be correlated with pre-operative imaging findings.
4 Conclusion
Accurate detection of renal leiomyomas may be challenging in the pre-operative setting, given the variety of findings on imaging. There are very few characteristics that may help rule in or rule out the leiomyoma amongst its differential, especially when suspicious or malignant lesions have to be considered. Pre-operative, image-guided core biopsy would undoubtedly aid in distinguishing the leiomyoma. However, its use is dependent on multiple factors ranging from clinical presentation to size and contrast enhancement of the lesion, and availability of resources. In addition to being viewed as a controversial practice, the place for biopsy in the resource-poor setting is yet to be determined. Until more rigid indications are established surrounding conservative treatment, definitive management will invariably be surgical, as these patients enjoy an excellent prognosis without recurrence.
Availability of data and materials
Data sharing does not apply to this article as no datasets were generated or analysed during the current study.
Abbreviations
Angiomyolipoma
Computed tomography
Epstein–Barr virus
Hounsfield units
Renal cell carcinoma
Steiner M, Quinlan D, Goldman S, Millmond S, Hallowell M, Stutzman R et al (1990) Leiomyoma of the kidney: presentation of 4 new cases and the role of computerized tomography. J Urol 143(5):994–998
Article CAS Google Scholar
Belis J, Post G, Rochman S, Franklin MD (1979) Genitourinary leiomyomas. Urology 13(4):424–429
Andreoiu M, Drachenberg D, Macmahon R (2009) Giant renal leiomyoma: a case report and brief review of the literature. Can UrolAssoc J 3(5):58–60
Google Scholar
Nakib G, Mahgoub N, Calcaterra V, Pelizzo G (2017) Renal leiomyoma in pediatric age: a rare case report with review of the literature. J PediatrSurg Case Rep 27:43–46
Romero F, Kohanim S, Lima G, Permpongkosol S, Fine S, Kavoussi L (2005) Leiomyomas of the kidney: emphasis on conservative diagnosis and treatment. Urology 66(6):1319.e1–3
Article Google Scholar
Krishnan R, Freeman J, Creager A (1999) Epstein–Barr virus induced renal leiomyoma. J Urol 161(1):212
Tsujimura A, Miki T, Sugao H, Takaha M, Takeda M, Kurata A (1996) Renal leiomyoma associated with tuberous sclerosis. UrolInt 57(3):192–193
CAS Google Scholar
Nagar A, Raut A, Narlawar R, Bhatgadde V, Rege S, Thapar V (2004) Giant renal capsular leiomyoma: study of two cases. Br J Radiol 77(923):957–958
Frank I, Blute M, Cheville J, Lohse C, Weaver A, Zincke H (2003) Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 170(6 Pt 1):2217–2220
Brunocilla E, Pultrone C, Schiavina R, Vagnoni V, Caprara G, Martorana G (2012) Renal leiomyoma: case report and literature review. Can UrolAssoc J 6(2):E87-90
Kim J, Park S, Shon J, Cho K (2004) Angiomyolipoma with minimal fat: differentiation from renal cell carcinoma at biphasic helical CT. Radiology 230(3):677–684
Perez-Ordonez B, Hamed G, Campbell S, Erlandson RA, Russo P, Gaudin PB et al (1997) Renal oncocytoma: a clinicopathologic study of 70 cases. Am J SurgPathol 21(8):871–883
Hogan A, Smyth G, D’Arcy C, O’Brien A, Quinlan D (2008) Renal capsular leiomyoma. Urology 71(6):1226.e1–3
Derchi L, Grenier N, Heinz-Peer G, Dogra V, Franco F, Rollandi G et al (2008) Imaging of renal leiomyomas. ActaRadiol 49(7):833–838
O’Connor S, Pickhardt P, Kim D, Oliva M, Silverman S (2011) Incidental Finding of renal masses at unenhanced CT: prevalence and analysis of features for guiding management. Am J Roentgenol 197(1):139–145
Eshed I, Elias S, Sidi A (2004) Diagnostic value of CT-guided biopsy of indeterminate renal masses. ClinRadiol 59(3):262–267
Mitra B, Debnath S, Pal M, Paul B, Saha T, Maiti A (2012) Leiomyoma of kidney: an Indian experience with literature review. Int J Surg Case Rep 3(11):569–573
Larbcharoensub N, Limprasert V, Pangpunyakulchai D, Sanpaphant S, Wiratkapun C, Kijvikai K (2017) Renal leiomyoma: a case report and review of the literature. Urol Case Rep 7(13):3–5
Akbulut F, Şahan M, Üçpınar B, Savun M, Özgör F, Arslan B et al (2016) A rare case of renal leiomyoma treated with laparoscopic partial nephrectomy. Med Bull Haseki 54(1):41–43
Kocak C, Kabay S, Isler B (2015) Leiomyoma of the renal vein: a rare tumor presenting as a renal mass. Case Rep Urol 5(2015):1–3
Lal A, Galwa R, Chandrasekar P, Sachdeva M, Vashisht R, Khandelwal N (2009) A huge renal capsular leiomyoma mimicking retroperitoneal sarcoma. Saudi J Kidney Dis Transpl 20(6):1069–1071
PubMed Google Scholar
Takezaki T, Nakama M, Ogawa A (1985) Renal leiomyoma with extensive cystic degeneration. Urology 25(4):401–403
Fu L, Humphrey P, Adeniran A (2015) Renal leiomyoma. J Urol 193(3):997–998
Patil P, McKenney J, Trpkov K, Hes O, Montironi R, Scarpelli M et al (2015) Renal leiomyoma: a contemporary multi-institution study of an infrequent and frequently misclassified neoplasm. Am J SurgPathol 39(3):349–356
Download references
Acknowledgements
No funding was received for this study.
Author information
Authors and affiliations.
Division of Urology, Faculty of Medicine and Health Sciences, Tygerberg Hospital, Stellenbosch University, P.O. Box 19063, Tygerberg, 7505, South Africa
Sajal Gerdharee, Heidi van Deventer & Andre van der Merwe
Division of Anatomical Pathology, NHLS Tygerberg and University of Stellenbosch, Cape Town, South Africa
Abrie van Wyk
You can also search for this author in PubMed Google Scholar
Contributions
SG did the write up of the case report and literature review as part of his undergraduate studies. HVD and AVDM contributed equally to the writing of the manuscript. AVW reviewed the pathology section of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Heidi van Deventer .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval was obtained from the Health Research Ethics Committee of Stellenbosch University with the reference number of UC19/10/048. Consent to participate is not applicable.
Consent for publication
Written informed consent for publication of the patient’s clinical details and clinical images was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Gerdharee, S., van Wyk, A., van Deventer, H. et al. Renal leiomyoma: a case report and literature review. Afr J Urol 27 , 39 (2021). https://doi.org/10.1186/s12301-021-00143-z
Download citation
Received : 27 November 2019
Accepted : 12 February 2021
Published : 25 February 2021
DOI : https://doi.org/10.1186/s12301-021-00143-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Renal leiomyoma
- Case report
- Renal leiomyoma diagnosis
- Literature review
- Renal leiomyoma imaging

CC0006 Basics of Report Writing
Structure of a report (case study, literature review or survey).
- Structure of report (Site visit)
- Citing Sources
- Tips and Resources
The information in the report has to be organised in the best possible way for the reader to understand the issue being investigated, analysis of the findings and recommendations or implications that relate directly to the findings. Given below are the main sections of a standard report. Click on each section heading to learn more about it.
- Tells the reader what the report is about
- Informative, short, catchy
Example - Sea level rise in Singapore : Causes, Impact and Solution
The title page must also include group name, group members and their matriculation numbers.
Content s Page
- Has headings and subheadings that show the reader where the various sections of the report are located
- Written on a separate page
- Includes the page numbers of each section
- Briefly summarises the report, the process of research and final conclusions
- Provides a quick overview of the report and describes the main highlights
- Short, usually not more than 150 words in length
- Mention briefly why you choose this project, what are the implications and what kind of problems it will solve
Usually, the abstract is written last, ie. after writing the other sections and you know the key points to draw out from these sections. Abstracts allow readers who may be interested in the report to decide whether it is relevant to their purposes.
Introduction
- Discusses the background and sets the context
- Introduces the topic, significance of the problem, and the purpose of research
- Gives the scope ie shows what it includes and excludes
In the introduction, write about what motivates your project, what makes it interesting, what questions do you aim to answer by doing your project. The introduction lays the foundation for understanding the research problem and should be written in a way that leads the reader from the general subject area of the topic to the particular topic of research.
Literature Review
- Helps to gain an understanding of the existing research in that topic
- To develop on your own ideas and build your ideas based on the existing knowledge
- Prevents duplication of the research done by others
Search the existing literature for information. Identify the data pertinent to your topic. Review, extract the relevant information for eg how the study was conducted and the findings. Summarise the information. Write what is already known about the topic and what do the sources that you have reviewed say. Identify conflicts in previous studies, open questions, or gaps that may exist. If you are doing
- Case study - look for background information and if any similar case studies have been done before.
- Literature review - find out from literature, what is the background to the questions that you are looking into
- Site visit - use the literature review to read up and prepare good questions before hand.
- Survey - find out if similar surveys have been done before and what did they find?
Keep a record of the source details of any information you want to use in your report so that you can reference them accurately.
Methodology
Methodology is the approach that you take to gather data and arrive at the recommendation(s). Choose a method that is appropriate for the research topic and explain it in detail.
In this section, address the following: a) How the data was collected b) How it was analysed and c) Explain or justify why a particular method was chosen.
Usually, the methodology is written in the past tense and can be in the passive voice. Some examples of the different methods that you can use to gather data are given below. The data collected provides evidence to build your arguments. Collect data, integrate the findings and perspectives from different studies and add your own analysis of its feasibility.
- Explore the literature/news/internet sources to know the topic in depth
- Give a description of how you selected the literature for your project
- Compare the studies, and highlight the findings, gaps or limitations.
- An in-depth, detailed examination of specific cases within a real-world context.
- Enables you to examine the data within a specific context.
- Examine a well defined case to identify the essential factors, process and relationship.
- Write the case description, the context and the process involved.
- Make sense of the evidence in the case(s) to answer the research question
- Gather data from a predefined group of respondents by asking relevant questions
- Can be conducted in person or online
- Why you chose this method (questionnaires, focus group, experimental procedure, etc)
- How you carried out the survey. Include techniques and any equipment you used
- If there were participants in your research, who were they? How did you select them and how may were there?
- How the survey questions address the different aspects of the research question
- Analyse the technology / policy approaches by visiting the required sites
- Make a detailed report on its features and your understanding of it
Results and Analysis
- Present the results of the study. You may consider visualising the results in tables and graphs, graphics etc.
- Analyse the results to obtain answer to the research question.
- Provide an analysis of the technical and financial feasibility, social acceptability etc
Discussion, Limitation(s) and Implication(s)
- Discuss your interpretations of the analysis and the significance of your findings
- Explain any new understanding or insights that emerged as a result of your research
- Consider the different perspectives (social, economic and environmental)in the discussion
- Explain the limitation(s)
- Explain how could what you found be used to make a difference for sustainability
Conclusion and Recommendations
- Summarise the significance and outcome of the study highlighting the key points.
- Come up with alternatives and propose specific actions based on the alternatives
- Describe the result or improvement it would achieve
- Explain how it will be implemented
Recommendations should have an innovative approach and should be feasible. It should make a significant difference in solving the issue under discussion.
- List the sources you have referred to in your writing
- Use the recommended citation style consistently in your report
Appendix (if necessary/any)
Include any material relating to the report and research that does not fit in the body of the report, in the appendix. For example, you may include survey questionnaire and results in the appendix.
- << Previous: Structure of a report
- Next: Structure of report (Site visit) >>
- Last Updated: Jan 12, 2024 11:52 AM
- URL: https://libguides.ntu.edu.sg/report-writing
You are expected to comply with University policies and guidelines namely, Appropriate Use of Information Resources Policy , IT Usage Policy and Social Media Policy . Users will be personally liable for any infringement of Copyright and Licensing laws. Unless otherwise stated, all guide content is licensed by CC BY-NC 4.0 .
CASE REPORT article
Case report: a case report and literature review on severe bullous skin reaction induced by anti-pd-1 immunotherapy in a cervical cancer patient.

- 1 The Fifth Department of Oncology, Jinshazhou Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2 The Second Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou, China
- 3 Pediatric Ward, Henan Province Hospital of TCM, Zhengzhou, China
- 4 Department of Oncology, Fuda Cancer Hospital Guangzhou, Guangzhou, China
Background: Anti-programmed cell death protein 1 (PD-1) has been successfully used in carcinomas treatment. However, it causes significant adverse effects (AEs), including cutaneous reactions, particularly the life-threatening severe bullous skin reactions (SBSR) and toxic epidermal necrolysis (TEN).
Case summary: Herein, we described for the first time a case report of SBSR induced by anti-PD-1 therapy in a cervical cancer patient. In addition, we revised existing literature on anti-PD-1 induced cutaneous reactions. We reported a cervical cancer patient who was treated with four successive cycles of Sintilimab and Toripalimab injections and developed systemic rashes, bullae, and epidermal desquamation, which worsened and led to infection, eventually causing death after being unresponsive to aggressive treatments.
Conclusion: Anti-PD-1 antibodies commonly cause skin toxicity effects, some of which may be deadly. Therefore, healthcare providers should observe early symptoms and administer proper treatment to prevent aggravation of symptoms.
Introduction
Cervical cancer is the fourth most common cancer in women ( Colombo et al., 2012 ). Its main treatment consists of platinum-based chemotherapy, with limited therapeutic outcomes and severe side effects ( Greer et al., 2010 ; Pfaendler and Tewari, 2016 ). Since the introduction of programmed death 1 (PD-1) protein monoclonal antibodies, they have shown outstanding clinical efficacy in multiple cancer types, including advanced cervical cancer ( Martínez and Del Campo, 2017 ; Wang and Li, 2019 ). In this regard, Pembrolizumab was used for advanced cervical cancer in the KEYNOTE-028 clinical study, demonstrating the efficacy of PD-1/PD-L1 inhibitors in advanced cervical cancer ( Frenel et al., 2017 ). PD-1 monoclonal antibodies have been shown to potentiate T lymphocytes cytotoxic activity against tumor cells and control tumor growth ( Pedoeem et al., 2014 ; Tumeh et al., 2014 ). Most patients tolerated anti-PD-1 therapy, whereas some presented toxic and side effects ( Champiat et al., 2016 ). Major anti-PD-1 associated adverse effects (AEs) included skin toxicity, endocrine reaction, gastrointestinal reaction, hepatitis, and renal dysfunction ( Hofmann et al., 2016 ; Ansell, 2017 ; Nishijima et al., 2017 ; O’Kane et al., 2017 ; Tie et al., 2017 ; Gubens et al., 2019 ). The most common AEs involved skin reactions such as lichenoid reaction, eczema, vitiligo, and pruritus ( Joseph et al., 2015 ; Robert et al., 2015a ; Weber et al., 2015 ; Ciccarese et al., 2016 ; Hwang et al., 2016 ; Yang et al., 2019 ). However, the most severe skin reaction observed was toxic epidermal necrolysis (TEN) in three cases of malignant melanoma ( Nayar et al., 2016 ; Vivar et al., 2017 ; Logan et al., 2020 ).
Case Presentations
The patient was a 38-year-old Asian female. In June 2019, cervical tumor with invasion of the uterine wall, bladder and rectum walls, and anterior sacral and bilateral inguinal lymphadenopathies was detected by magnetic resonance imaging, which was prescribed because she presented vaginal bleeding. Biopsy pathological results suggested cervical squamous cell carcinoma, FIGO stage IVA. On August 18, 2019, she was intravenously (i. v.) administered 240 mg paclitaxel +90 mg cisplatin chemotherapy, along with 200 mg Sintilimab at 21-days cycle intervals. On September 9, 2019, the patient received a second cycle of the same dose of Sintilimab and chemotherapy. Sintilimab is an innovative monoclonal antibody targeting PD-1, jointly developed by Innovent and Lilly in China, which has been granted marketing approval by the China Food and Drug Administration. The drug was granted orphan drug status by the FDA in April 2020 for the treatment of esophageal cancer. Because of financial issues, Sintilimab was switched to 240 mg Toripalimab in the third cycle on October 1, 2019, for two consecutive weeks per cycle, whereas the chemotherapy regimen remained unaltered. Toripalimab is also an anti-PD-1 monoclonal antibody produced in China. In March 2020, Toripalimab was granted orphan drug status by the US FDA in combination with acytinib for the treatment of mucosal melanoma. A follow-up exam after the third cycle showed progressive disease. In the fourth cycle on October 21, 2019, we modified chemotherapy to 240 mg paclitaxel and 135 mg nedaplatin, combined with 200 mg Sintilimab. Six days after the fourth cycle of treatment, she presented with rashes. Large erythema was observed in many parts of the body, along with some prominent skin areas and pigmentation ( Figure 1 ) and she was given the antihistamine diphenhydramine. The patient further developed shortness of breath and edema of both lower limbs, which was considered a heart failure condition. Cardiotonic, diuretic, and vasodilator agents were then provided. In addition, red blood cell transfusion was given, because her hemoglobin was 61 g/L.
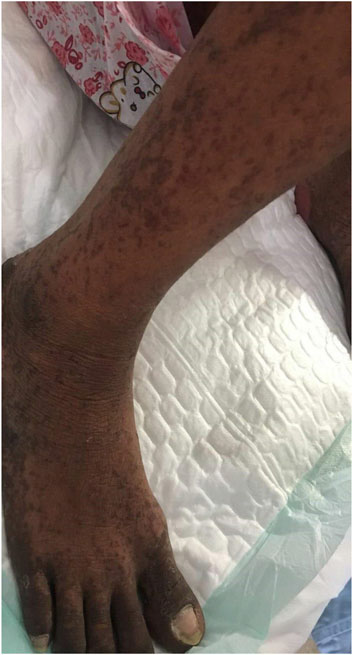
FIGURE 1 . Maculopapular skin rash on legs and feet.
After antihistamine treatment, she presented with worsening symptoms, with increased erythema, local transparent blisters, and tender epidermal loss of less than 10% of the body surface area (BSA). Furthermore, she developed mild swelling of oropharyngeal mucosa with repeated bleeding and scabbing, resulting in mouth opening and swallowing difficulties ( Figure 2 ), but refused to undergo skin biopsy. Stevens-Johnson syndrome (SJS) was diagnosed by a professor of dermatology, and we started i. v. administration of 80 mg/d methylprednisolone, combined with 0.4 g/kg/d immunoglobulins injection every day. We also improved the skin, mouth, and perineal mucous membranes care. Six days after medication, the epidermal peeling aggravated. Clear blisters locally appeared, presenting apparent perineal skin damage, with skin loss over 30% of BSA ( Figure 3 ) . The patient also presented fever, with respective C-reactive protein and neutrophil values of 117.33 mg/L and 89.20%. The skin lesion developed to SBSR, accompanied by infection, which was treated with antibiotics. The epidermal exfoliation and infection continued to worsen, and a disturbance of consciousness was observed. The patient died 33 days after the fourth cycle of medication, probably due to sepsis.
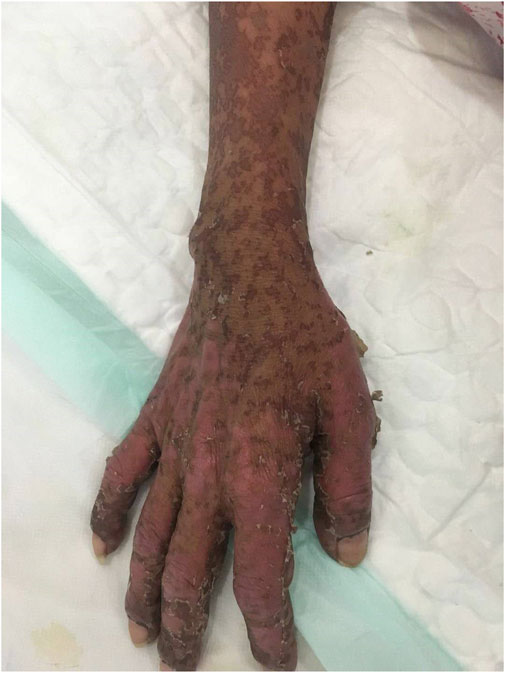
FIGURE 2 . Epidermal exfoliation of upper limbs (BSA less than 10%).
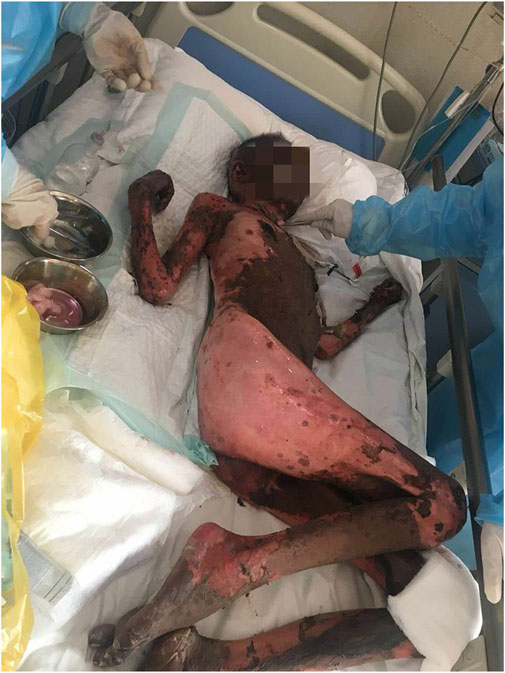
FIGURE 3 . A large exfoliated area of the body (BSA more than 30%).
Cutaneous Adverse Effects of Anti-Programmed Death-1 Therapy
Although docetaxel and paclitaxel share the same pharmaceutical composition, cases of SBSR -causing paclitaxel have not been reported to date ( Ohlmann et al., 2007 ; Kattan et al., 2008 ; Cohen et al., 2019 ). The toxicity spectrum of anti-PD-1 is prone to skin-related reactions, excluding the possibility that other drugs used by the patient may cause SBSR, which we believed it was related to anti-PD-1 treatment.
PD-1/PD-L1 pathway inhibits T cell activation, inducing lymphocyte apoptosis, and maintains autoimmune tolerance. In the tumor microenvironment, tumor cells bind to PD-1 on the lymphocyte surface through PD-L1 overexpression to inhibit lymphocytes function, thus escaping the immune surveillance and destruction ( Dong and Chen, 2006 ). PD-1 is an important target in anti-tumor therapy because the aforementioned inhibitory signaling pathways can be blocked, enhancing T cells immune response ( Topalian et al., 2012 ).
In major lung cancer related studies, CheckMate 017 and CheckMate 057 ( Borghaei et al., 2015 ; Brahmer et al., 2015 ) revealed that anti-PD-1 AEs in lung cancer patients were mainly grade 1–2, in KEYNOTE-010 ( Herbst et al., 2016 ), two cases (less than 1%) were reported as grade 3–4 rash, with a median time of AEs of 5–7 weeks.
In the study of malignant melanoma, CheckMate 066 ( Robert et al., 2015a ) reported one case of grade 3–4 rash and one case of grade 3–4 pruritus. The incidence of grade 3–4 cutaneous AEs was 0.5%. CheckMate 037 and KEYNOTE-002 did not report grade 3–4 cutaneous AEs ( Ribas et al., 2015 ; Weber et al., 2015 ). The incidence of Nivolumab and Pembrolizumab associated rashes was 14.3 and 16.7% respectively, and the incidence of rash over grade 3 was 1.2 and 1.9% respectively, according to the meta-analysis of V. R. Belum ( Belum et al., 2016 ). Skin reactions generally occur two to 3 weeks after the administration of the immune inhibitor ( Kumar et al., 2017 ). Studies have shown that progression‐free survival and overall survival in cancer patients with skin reactions are longer than those without skin reactions, after immunotherapy ( Sanlorenzo et al., 2015 ; Hasan Ali et al., 2016 ; Imafuku et al., 2017 ; Min Lee et al., 2018 ; Quach et al., 2019 ). A similar conclusion was reported in a study of immunotherapy combined with radiotherapy ( Haratani et al., 2018 ). Quach HT ( Quach et al., 2019 ) retrospective analysis of 318 patients treated with anti-PD-1 monoclonal showed that patients who experienced skin toxicity 3 months after the application of the immune inhibitors, had better therapeutic outcomes than those who did not show skin issues, and that a higher response rate was observed in patients with vitiligo and pruritus than patients with only vitiligo.
Clinical manifestations of severe bullous skin reaction caused by PD-1 monoclonal antibody are very similar to those observed in SJS/TEN. However, the former characterizes by the duration of drug application, the involvement of the mucous membrane, and the degree of skin peeling ( Lipowicz et al., 2013 ; Zhao et al., 2018 ). Research by Robin Reschke et al. (2019) showed that among all the current reports of serious skin reactions caused by checkpoint inhibitor therapy, only a small number are typical SJS/TEN. Therefore, clinicians, especially oncologists, should differentiate them by considering clinical evidence and if necessary, request a dermatologist assistance in diagnosis and treatment.
Case Reports on Toxic Epidermal Necrolysis Induced by Immunotherapy
Although mild dermatotoxicity indicates a better clinical outcome, severe dermatotoxicity may result in treatment interruption ( Macdonald et al., 2015 ), and sometimes it may become a life-threatening condition ( Nayar et al., 2016 ; Vivar et al., 2017 ; Logan et al., 2020 ). TEN is the most serious type of drug eruption, characterized by extensive epidermal exfoliation and blistering ( Roujeau and Stern, 1994 ), with a mortality of 25–50% ( Mockenhaupt et al., 2008 ; Kinoshita and Saeki, 2017 ).
Three cases of TEN induced by anti-PD-1 in melanoma have been reported to date ( Nayar et al., 2016 ; Vivar et al., 2017 ; Logan et al., 2020 ), all of which were treated with Nivolumab, and a previous history of Ipilimumab or were treated with these drugs in combination. TEN developed after the second or third cycle of Nivolumab. In all cases, TEN was treated with immunoglobulins (IVIg), two patients used cyclosporines, and other two used glucocorticoids. All patients finally died, including those reported in this article ( Table 1 ).
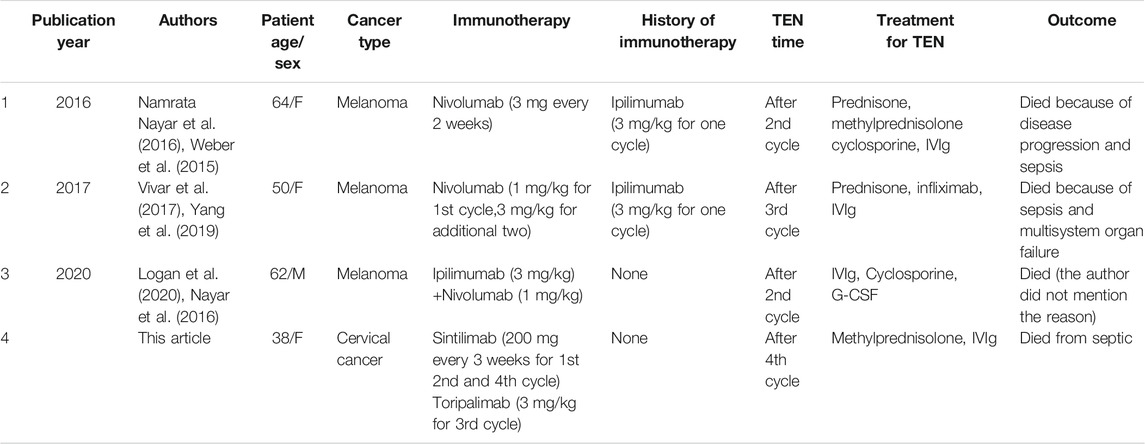
TABLE 1 . Reported cases of TEN caused by anti-PD-1 monoclonal antibodies therapy.
The incidence of CTLA-4 adverse reactions is as high as 60%, and skin and gastrointestinal tract is the most easily affected organs ( Hodi et al., 2010 ). The incidence of grade 3–4 adverse events was 20% ( Schachter et al., 2017 ). At present, it is believed that the cause of CTLA-4 adverse reactions was the excessive immune response to normal organs after activation of T cells ( Blansfield et al., 2005 ). However, the mechanism that causes TEN is still unclear.
Relationship Between Anti-Programmed Death-1 Monoclonal Antibodies and Cutaneous Reactions
Immune-related AEs may damage any organ in the body ( Michot et al., 2016 ), mostly skin ( Minkis et al., 2013 ; Abdel-Rahman et al., 2015 ; Naidoo et al., 2015 ). However, the mechanism of anti-PD-1-induced skin toxicity has not yet been elucidated ( Maloney et al., 2020 ). PD-1/PD-L1 pathway plays an important role in autoimmunity, preventing T cells from responding to autoantigens ( Francisco et al., 2010 ). After anti-PD-1 monoclonal antibody administration, this balance may be broken, causing T cells to attack normal and tumor cells, leading to toxic and side effects ( Berman et al., 2015 ). In the PD-1 knockout mouse model, Okazaki et al. (2003) demonstrated that PD-1 blockade not only affected Treg function, but also participated in the production of autoantibodies. Similar results were observed in melanoma patients treated with Nivolumab ( Kanameishi et al., 2016 ). The three reported cases of TEN induced by anti-PD-1 had a medical history of anti-CTLA-4. Gu et al. (2019) meta-analysis showed that anti-PD-1/PD-L1 combined with anti-CTLA-4 caused a higher incidence of adverse reactions and was prone to treatment discontinuation, as shown in Check-Mate 067 study ( Larkin et al., 2015 ). However, results evidenced higher remission rates when two immune inhibitors were used in combination ( Robert et al., 2015b ). Choi et al. (2019) reported a case of liver cancer patient who successfully switched to pembrolizumab due to nivolumab allergy. Therefore, different anti-PD-1 monoclonal antibodies share the same mechanism, but may cause different reactions. Our patients presented with TEN after using two different anti-PD-1 monoclonal antibodies. However, interaction between these two drugs may not be discarded.
Treatment of Skin Reactions Caused by Immunotherapy
Early treatment of rash caused by immune agents is particularly important ( O’Kane et al., 2017 ). In general, patients can be topically treated with glucocorticoid ointment and orally with antipruritics (mainly antihistamines) if the patient presents itching. For grade 3–4 toxic side effects caused by anti-PD-1 monoclonal antibodies, glucocorticoids should be orally administered and anti-PD-1 should be discontinued until glucocorticoid dose reduction therapy is completed ( Eigentler et al., 2016 ; Weber et al., 2017 ). The drug should be immediately discontinued and the patient should be hospitalized if TEN develops. SJS/TEN treatment guidelines from the United Kingdom ( Creamer et al., 2016 ) and the literature review of Friedman et al. (2016) indicate that glucocorticoids, immunoglobulins, and cyclosporine may be used in TEN therapy but such treatment has not been clearly demonstrated over supportive therapy alone. Previous studies ( de Sica-Chapman et al., 2010 ) have shown that G-CSF has immunomodulatory effects and promotes epithelial regeneration. SJS/TEN treatment by plasmapheresis has also been reported ( Narita et al., 2011 ). In this regard, Koštál used plasmapheresis therapy in four patients presenting TEN who did not respond to glucocorticoids and immunoglobulin. Their symptoms improved and necrotic epithelium began to repair ( Kostal et al., 2012 ). Skin care played an important role in the recovery from TEN ( Cooper, 2012 ).
Although most skin reactions caused by anti-PD-1 monoclonal antibodies were mild, they may still cause fatal skin toxicity. Therefore, early recognition, intervention, and treatment are essential to avoid further development of skin reactions. In this regard, early application of glucocorticoids may slow the response.

Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Jinshazhou Hospital of Guangzhou University of Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
The original manuscript was written by XL, L-XQ, and Y-MR, reviewed and edited by CH. All authors read and approved the final manuscript. XL, L-XQ and Y-MR contribute equally to this work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to express their gratitude to EditSprings ( https://www.editsprings.com/ ) for the expert linguistic services provided.
Abbreviations
AEs, adverse effects; BSA, body surface area; PD-1, Anti-programmed cell death protein 1; TEN, toxic epidermal necrolysis.
Abdel-Rahman, O., ElHalawani, H., and Fouad, M. (2015). Risk of Cutaneous Toxicities in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors: a Meta-Analysis. Future Oncol. 11 (17), 2471–2484. doi:10.2217/fon.15.118
PubMed Abstract | CrossRef Full Text | Google Scholar
Ansell, S. M. (2017). Nivolumab in the Treatment of Hodgkin Lymphoma. Clin. Cancer Res. 23 (7), 1623–1626. doi:10.1158/1078-0432.CCR-16-1387
Belum, V. R., Benhuri, B., Postow, M. A., Hellmann, M. D., Lesokhin, A. M., Segal, N. H., et al. (2016). Characterisation and Management of Dermatologic Adverse Events to Agents Targeting the PD-1 Receptor. Oxford, England : 1990. Eur. J. Cancer. 60, 12–25. doi:10.1016/j.ejca.2016.02.010
Berman, D., Korman, A., Peck, R., Feltquate, D., Lonberg, N., and Canetta, R. (2015). The Development of Immunomodulatory Monoclonal Antibodies as a New Therapeutic Modality for Cancer: the Bristol-Myers Squibb Experience. Pharmacol. Ther. 148, 132–153. doi:10.1016/j.pharmthera.2014.11.017
Blansfield, J. A., Beck, K. E., Tran, K., Yang, J. C., Hughes, M. S., Kammula, U. S., et al. (2005). Cytotoxic T-Lymphocyte-Associated Antigen-4 Blockage Can Induce Autoimmune Hypophysitis in Patients with Metastatic Melanoma and Renal Cancer. J. Immunother. 28 (6), 593–598. doi:10.1097/01.cji.0000178913.41256.06
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus Docetaxel in Advanced Nonsquamous Non-small-cell Lung Cancer. N. Engl. J. Med. 373 (17), 1627–1639. doi:10.1056/NEJMoa1507643
Brahmer, J., Reckamp, K. L., Baas, P., Crinò, L., Eberhardt, W. E., Poddubskaya, E., et al. (2015). Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-small-cell Lung Cancer. N. Engl. J. Med. 373 (2), 123–135. doi:10.1056/NEJMoa1504627
Champiat, S., Lambotte, O., Barreau, E., Belkhir, R., Berdelou, A., Carbonnel, F., et al. (2016). Management of Immune Checkpoint Blockade Dysimmune Toxicities: a Collaborative Position Paper. Ann. Oncol. 27 (4), 559–574. doi:10.1093/annonc/mdv623
Choi, B., McBride, A., and Scott, A. J. (2019). Treatment with Pembrolizumab after Hypersensitivity Reaction to Nivolumab in a Patient with Hepatocellular Carcinoma. Am. J. Health Syst. Pharm. 76 (21), 1749–1752. doi:10.1093/ajhp/zxz189
Ciccarese, C., Alfieri, S., Santoni, M., Santini, D., Brunelli, M., Bergamini, C., et al. (2016). New Toxicity Profile for Novel Immunotherapy Agents: Focus on Immune-Checkpoint Inhibitors. Expert Opin. Drug Metab. Toxicol. 12 (1), 57–75. doi:10.1517/17425255.2016.1120287
Cohen, E. E. W., Soulières, D., Le Tourneau, C., Dinis, J., Licitra, L., Ahn, M. J., et al. (2019). Pembrolizumab versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-And-Neck Squamous Cell Carcinoma (KEYNOTE-040): a Randomised, Open-Label, Phase 3 Study. Lancet 393 (10167), 156–167. doi:10.1016/S0140-6736(18)31999-8
Colombo, N., Carinelli, S., Colombo, A., Marini, C., Rollo, D., and Sessa, C. (2012). Cervical Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 23 (Suppl. 7), vii27–32. doi:10.1093/annonc/mds268
Cooper, K. L. (2012). Drug Reaction, Skin Care, Skin Loss. Crit. Care Nurse. 32 (4), 52–59. doi:10.4037/ccn2012340
CrossRef Full Text | Google Scholar
Creamer, D., Walsh, S. A., Dziewulski, P., Exton, L. S., Lee, H. Y., Dart, J. K. G., et al. (2016). UK Guidelines for the Management of Stevens-Johnson Syndrome/toxic Epidermal Necrolysis in Adults 2016. J. Plast. Reconstr. Aesthet. Surg. 69 (6), e119–e153. doi:10.1016/j.bjps.2016.01.034
de Sica-Chapman, A., Williams, G., Soni, N., and Bunker, C. B. (2010). Granulocyte colony-stimulating Factor in Toxic Epidermal Necrolysis (TEN) and Chelsea & Westminster TEN Management Protocol [corrected]. Br. J. Dermatol. 162 (4), 860–865. doi:10.1111/j.1365-2133.2009.09585.x
Dong, H., and Chen, X. (2006). Immunoregulatory Role of B7-H1 in Chronicity of Inflammatory Responses. Cell Mol Immunol. 3 (3), 179–187.
PubMed Abstract | Google Scholar
Eigentler, T. K., Hassel, J. C., Berking, C., Aberle, J., Bachmann, O., Grünwald, V., et al. (2016). Diagnosis, Monitoring and Management of Immune-Related Adverse Drug Reactions of Anti-PD-1 Antibody Therapy. Cancer Treat. Rev. 45, 7–18. doi:10.1016/j.ctrv.2016.02.003
Francisco, L. M., Sage, P. T., and Sharpe, A. H. (2010). The PD-1 Pathway in Tolerance and Autoimmunity. Immunol. Rev. 236, 219–242. doi:10.1111/j.1600-065X.2010.00923.x
Frenel, J. S., Le Tourneau, C., O'Neil, B., Ott, P. A., Piha-Paul, S. A., Gomez-Roca, C., et al. (2017). Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results from the Phase Ib KEYNOTE-028 Trial. J. Clin. Oncol. 35 (36), 4035–4041. doi:10.1200/JCO.2017.74.5471
Friedman, C. F., Proverbs-Singh, T. A., and Postow, M. A. (2016). Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2 (10), 1346–1353. doi:10.1001/jamaoncol.2016.1051
Greer, B. E., Koh, W. J., Abu-Rustum, N. R., Apte, S. M., Campos, S. M., Chan, J., et al. (2010). Cervical Cancer. J. Natl. Compr. Canc Netw. 8 (12), 1388–1416. doi:10.6004/jnccn.2010.0104
Gu, L., Khadaroo, P. A., Su, H., Kong, L., Chen, L., Wang, X., et al. (2019). The Safety and Tolerability of Combined Immune Checkpoint Inhibitors (Anti-PD-1/pd-L1 Plus Anti-CTLA-4): a Systematic Review and Meta-Analysis. BMC cancer. 19 (1), 559. doi:10.1186/s12885-019-5785-z
Gubens, M. A., Sequist, L. V., Stevenson, J. P., Powell, S. F., Villaruz, L. C., Gadgeel, S. M., et al. (2019). Pembrolizumab in Combination with Ipilimumab as Second-Line or Later Therapy for Advanced Non-small-cell Lung Cancer: KEYNOTE-021 Cohorts D and H. Lung Cancer. 130, 59–66. doi:10.1016/j.lungcan.2018.12.015
Haratani, K., Hayashi, H., Chiba, Y., Kudo, K., Yonesaka, K., Kato, R., et al. (2018). Association of Immune-Related Adverse Events with Nivolumab Efficacy in Non-small-cell Lung Cancer. JAMA Oncol. 4 (3), 374–378. doi:10.1001/jamaoncol.2017.2925
Hasan Ali, O., Diem, S., Markert, E., Jochum, W., Kerl, K., French, L. E., et al. (2016). Characterization of Nivolumab-Associated Skin Reactions in Patients with Metastatic Non-small Cell Lung Cancer. Oncoimmunology 5 (11), e1231292. doi:10.1080/2162402X.2016.1231292
Herbst, R. S., Baas, P., Kim, D. W., Felip, E., Pérez-Gracia, J. L., Han, J. Y., et al. (2016). Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-small-cell Lung Cancer (KEYNOTE-010): a Randomised Controlled Trial. Lancet 387 (10027), 1540–1550. doi:10.1016/S0140-6736(15)01281-7
Hodi, F. S., O'Day, S. J., McDermott, D. F., Weber, R. W., Sosman, J. A., Haanen, J. B., et al. (2010). Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 363 (8), 711–723. doi:10.1056/NEJMoa1003466
Hofmann, L., Forschner, A., Loquai, C., Goldinger, S. M., Zimmer, L., Ugurel, S., et al. (2016). Cutaneous, Gastrointestinal, Hepatic, Endocrine, and Renal Side-Effects of Anti-PD-1 Therapy. (Oxford, England : 1990). Eur. J. Cancer. 60, 190–209. doi:10.1016/j.ejca.2016.02.025
Hwang, S. J., Carlos, G., Wakade, D., Byth, K., Kong, B. Y., Chou, S., et al. (2016). Cutaneous Adverse Events (AEs) of Anti-programmed Cell Death (PD)-1 Therapy in Patients with Metastatic Melanoma: A Single-Institution Cohort. J. Am. Acad. Dermatol. 74 (3), 455–e1.e451. doi:10.1016/j.jaad.2015.10.029
Imafuku, K., Yoshino, K., Ymaguchi, K., Tsuboi, S., Ohara, K., and Hata, H. (2017). Nivolumab Therapy before Vemurafenib Administration Induces a Severe Skin Rash. J. Eur. Acad. Dermatol. Venereol. 31 (3), e169–e171. doi:10.1111/jdv.13892
Joseph, R. W., Cappel, M., Goedjen, B., Gordon, M., Kirsch, B., Gilstrap, C., et al. (2015). Lichenoid Dermatitis in Three Patients with Metastatic Melanoma Treated with Anti-PD-1 Therapy. Cancer Immunol. Res. 3 (1), 18–22. doi:10.1158/2326-6066.CIR-14-0134
Kanameishi, S., Otsuka, A., Nonomura, Y., Fujisawa, A., Endo, Y., and Kabashima, K. (2016). Idiopathic Thrombocytopenic Purpura Induced by Nivolumab in a Metastatic Melanoma Patient with Elevated PD-1 Expression on B Cells. Ann. Oncol. 27 (3), 546–547. doi:10.1093/annonc/mdv580
Kattan, J. G., Farhat, F. S., Chahine, G. Y., Nasr, F. L., Moukadem, W. T., Younes, F. C., et al. (2008). Weekly Docetaxel, Zoledronic Acid and Estramustine in Hormone-Refractory Prostate Cancer (HRPC). Invest. New Drugs. 26 (1), 75–79. doi:10.1007/s10637-007-9074-3
Kinoshita, Y., and Saeki, H. (2017). A Review of Toxic Epidermal Necrolysis Management in Japan. Allergol. Int. 66 (1), 36–41. doi:10.1016/j.alit.2016.06.001
Kostal, M., Blaha, M., Lanska, M., Koštálová, M., Bláha, V., Štepánová, E., Malý, J., et al. (2012). Beneficial Effect of Plasma Exchange in the Treatment of Toxic Epidermal Necrolysis: a Series of Four Cases. J. Clin. Apher. 27 (4), 215–220. doi:10.1002/jca.21213
Kumar, V., Chaudhary, N., Garg, M., Floudas, C. S., Soni, P., and Chandra, A. B. (2017). Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 8, 49. doi:10.3389/fphar.2017.00049
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Cowey, C. L., Lao, C. D., et al. (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 373 (1), 23–34. doi:10.1056/NEJMoa1504030
Lipowicz, S., Sekula, P., Ingen-Housz-Oro, S., Liss, Y., Sassolas, B., Dunant, A., et al. (2013). Prognosis of Generalized Bullous Fixed Drug Eruption: Comparison with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Br. J. Dermatol. 168 (4), 726–732. doi:10.1111/bjd.12133
Logan, I. T., Zaman, S., Hussein, L., and Perrett, C. M. (2020). Combination Therapy of Ipilimumab and Nivolumab-Associated Toxic Epidermal Necrolysis (TEN) in a Patient with Metastatic Melanoma: A Case Report and Literature Review. J. Immunother. 43 (3), 89–92. doi:10.1097/CJI.0000000000000302
Macdonald, J. B., Macdonald, B., Golitz, L. E., LoRusso, P., and Sekulic, A. (2015). Cutaneous Adverse Effects of Targeted Therapies: Part II: Inhibitors of Intracellular Molecular Signaling Pathways. J. Am. Acad. Dermatol. 72 (2), 221–228. quiz 237-228. doi:10.1016/j.jaad.2014.07.033
Maloney, N. J., Ravi, V., Cheng, K., Bach, D. Q., and Worswick, S. (2020). Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis-like Reactions to Checkpoint Inhibitors: a Systematic Review. Int. J. Dermatol. 59 (6), e183–e188. doi:10.1111/ijd.14811
Martínez, P., and Del Campo, J. M. (2017). Pembrolizumab in Recurrent Advanced Cervical Squamous Carcinoma. Immunotherapy 9 (6), 467–470. doi:10.2217/imt-2016-0119
Michot, J. M., Bigenwald, C., Champiat, S., Collins, M., Carbonnel, F., Postel-Vinay, S., et al. (2016). Immune-related Adverse Events with Immune Checkpoint Blockade: a Comprehensive Review. Eur. J. Cancer . Oxford, England : 1990 54, 139–148. doi:10.1016/j.ejca.2015.11.016
Min Lee, C. K., Li, S., Tran, D. C., Zhu, G. A., Kim, J., Kwong, B. Y., et al. (2018). Characterization of Dermatitis after PD-1/pd-L1 Inhibitor Therapy and Association with Multiple Oncologic Outcomes: A Retrospective Case-Control Study. J. Am. Acad. Dermatol. 79 (6), 1047–1052. doi:10.1016/j.jaad.2018.05.035
Minkis, K., Garden, B. C., Wu, S., Pulitzer, M. P., and Lacouture, M. E. (2013). The Risk of Rash Associated with Ipilimumab in Patients with Cancer: a Systematic Review of the Literature and Meta-Analysis. J. Am. Acad. Dermatol. 69 (3), e121–8. doi:10.1016/j.jaad.2012.12.963
Mockenhaupt, M., Viboud, C., Dunant, A., Naldi, L., Halevy, S., Bouwes Bavinck, J. N., et al. (2008). Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study. J. Invest. Dermatol. 128 (1), 35–44. doi:10.1038/sj.jid.5701033
Naidoo, J., Page, D. B., Li, B. T., Connell, L. C., Schindler, K., Lacouture, M. E., et al. (2015). Toxicities of the Anti-PD-1 and Anti-PD-L1 Immune Checkpoint Antibodies. Ann. Oncol. 26 (12), 2375–2391. doi:10.1093/annonc/mdv383
Narita, Y. M., Hirahara, K., Mizukawa, Y., Kano, Y., and Shiohara, T. (2011). Efficacy of Plasmapheresis for the Treatment of Severe Toxic Epidermal Necrolysis: Is Cytokine Expression Analysis Useful in Predicting its Therapeutic Efficacy? J. Dermatol. 38 (3), 236–245. doi:10.1111/j.1346-8138.2010.01154.x
Nayar, N., Briscoe, K., and Fernandez Penas, P. (2016). Toxic Epidermal Necrolysis-like Reaction with Severe Satellite Cell Necrosis Associated with Nivolumab in a Patient with Ipilimumab Refractory Metastatic Melanoma. J. Immunother. 39 (3), 149–152. doi:10.1097/CJI.0000000000000112
Nishijima, T. F., Shachar, S. S., Nyrop, K. A., and Muss, H. B. (2017). Safety and Tolerability of PD-1/pd-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist 22 (4), 470–479. doi:10.1634/theoncologist.2016-0419
O'Kane, G. M., Labbé, C., Doherty, M. K., Young, K., Albaba, H., and Leighl, N. B. (2017). Monitoring and Management of Immune-Related Adverse Events Associated with Programmed Cell Death Protein-1 Axis Inhibitors in Lung Cancer. Oncologist 22 (1), 70–80. doi:10.1634/theoncologist.2016-0164
Ohlmann, C. H., Kohlmorgen, S., Sahi, D., Engelmann, U., and Heidenreich, A. (2007). Lethal Course after Chemotherapy with Docetaxel. Acute Liver Failure with Accompanying Erythema Multiforme Major. Urologe A. 46 (10), 1425–1427. doi:10.1007/s00120-007-1367-9
Okazaki, T., Tanaka, Y., Nishio, R., Mitsuiye, T., Mizoguchi, A., Wang, J., et al. (2003). Autoantibodies against Cardiac Troponin I Are Responsible for Dilated Cardiomyopathy in PD-1-Deficient Mice. Nat. Med. 9 (12), 1477–1483. doi:10.1038/nm955
Pedoeem, A., Azoulay-Alfaguter, I., Strazza, M., Silverman, G. J., and Mor, A. (2014). Programmed Death-1 Pathway in Cancer and Autoimmunity. Clin. Immunol. 153 (1), 145–152. doi:10.1016/j.clim.2014.04.010
Pfaendler, K. S., and Tewari, K. S. (2016). Changing Paradigms in the Systemic Treatment of Advanced Cervical Cancer. Am. J. Obstet. Gynecol. 214 (1), 22–30. doi:10.1016/j.ajog.2015.07.022
Quach, H. T., Dewan, A. K., Davis, E. J., Ancell, K. K., Fan, R., Ye, F., et al. (2019). Association of Anti-programmed Cell Death 1 Cutaneous Toxic Effects with Outcomes in Patients with Advanced Melanoma. JAMA Oncol. 5 (6), 906–908. doi:10.1001/jamaoncol.2019.0046
Reschke, R., Mockenhaupt, M., Simon, J. C., and Ziemer, M. (2019). Severe Bullous Skin Eruptions on Checkpoint Inhibitor Therapy - in Most Cases Severe Bullous Lichenoid Drug Eruptions. J. Dtsch Dermatol. Ges. 17 (9), 942–948. doi:10.1111/ddg.13876
Ribas, A., Puzanov, I., Dummer, R., Schadendorf, D., Hamid, O., Robert, C., et al. (2015). Pembrolizumab versus Investigator-Choice Chemotherapy for Ipilimumab-Refractory Melanoma (KEYNOTE-002): a Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 16 (8), 908–918. doi:10.1016/S1470-2045(15)00083-2
Robert, C., Long, G. V., Brady, B., Dutriaux, C., Maio, M., Mortier, L., et al. (2015). Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 372 (4), 320–330. doi:10.1056/NEJMoa1412082
Robert, C., Schachter, J., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2015). Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 372 (26), 2521–2532. doi:10.1056/NEJMoa1503093
Roujeau, J. C., and Stern, R. S. (1994). Severe Adverse Cutaneous Reactions to Drugs. N. Engl. J. Med. 331 (19), 1272–1285. doi:10.1056/NEJM199411103311906
Sanlorenzo, M., Vujic, I., Daud, A., Algazi, A., Gubens, M., Luna, S. A., et al. (2015). Pembrolizumab Cutaneous Adverse Events and Their Association with Disease Progression. JAMA Dermatol. 151 (11), 1206–1212. doi:10.1001/jamadermatol.2015.1916
Schachter, J., Ribas, A., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2017). Pembrolizumab versus Ipilimumab for Advanced Melanoma: Final Overall Survival Results of a Multicentre, Randomised, Open-Label Phase 3 Study (KEYNOTE-006). Lancet 390 (10105), 1853–1862. doi:10.1016/S0140-6736(17)31601-X
Tie, Y., Ma, X., Zhu, C., Mao, Y., Shen, K., Wei, X., et al. (2017). Safety and Efficacy of Nivolumab in the Treatment of Cancers: A Meta-Analysis of 27 Prospective Clinical Trials. Int. J. Cancer. 140 (4), 948–958. doi:10.1002/ijc.30501
Topalian, S. L., Hodi, F. S., Brahmer, J. R., Gettinger, S. N., Smith, D. C., McDermott, D. F., et al. (2012). Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 366 (26), 2443–2454. doi:10.1056/NEJMoa1200690
Tumeh, P. C., Harview, C. L., Yearley, J. H., Shintaku, I. P., Taylor, E. J., Robert, L., et al. (2014). PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 515 (7528), 568–571. doi:10.1038/nature13954
Vivar, K. L., Deschaine, M., Messina, J., Divine, J. M., Rabionet, A., Patel, N., et al. (2017). Epidermal Programmed Cell Death-Ligand 1 Expression in TEN Associated with Nivolumab Therapy. J. Cutan. Pathol. 44 (4), 381–384. doi:10.1111/cup.12876
Wang, Y., and Li, G. (2019). PD-1/PD-L1 Blockade in Cervical Cancer: Current Studies and Perspectives. Front. Med. 13 (4), 438–450. doi:10.1007/s11684-018-0674-4
Weber, J. S., D'Angelo, S. P., Minor, D., Hodi, F. S., Gutzmer, R., Neyns, B., et al. (2015). Nivolumab versus Chemotherapy in Patients with Advanced Melanoma Who Progressed after Anti-CTLA-4 Treatment (CheckMate 037): a Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 16 (4), 375–384. doi:10.1016/S1470-2045(15)70076-8
Weber, J. S., Hodi, F. S., Wolchok, J. D., Topalian, S. L., Schadendorf, D., Larkin, J., et al. (2017). Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 35 (7), 785–792. doi:10.1200/JCO.2015.66.1389
Yang, W., Li, S., and Yang, Q. (2019). Risk of Dermatologic and Mucosal Adverse Events Associated with PD-1/pd-L1 Inhibitors in Cancer Patients: A Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore) 98 (20), e15731. doi:10.1097/MD.0000000000015731
Zhao, C. Y., Hwang, S. J. E., Consuegra, G., Chou, S., and Fernandez-Peñas, P. (2018). Anti-programmed Cell Death-1 Therapy-Associated Bullous Disorders: a Systematic Review of the Literature. Melanoma Res. 28 (6), 491–501. doi:10.1097/CMR.0000000000000500
Keywords: severe bullous skin reactions, literature review, case report, toxic epidermal necrolysis, cervical cancer, PD-1
Citation: Li X, Qu L-X, Ren Y-M and Hu C (2021) Case Report: A Case Report and Literature Review on Severe Bullous Skin Reaction Induced by anti-PD-1 Immunotherapy in a Cervical Cancer Patient. Front. Pharmacol. 12:707967. doi: 10.3389/fphar.2021.707967
Received: 11 May 2021; Accepted: 12 August 2021; Published: 24 August 2021.
Reviewed by:
Copyright © 2021 Li, Qu, Ren and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Li, [email protected]
† These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Search Menu
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- Author Guidelines
- Open Access
- Why Publish
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Acknowledgments.
- < Previous
A Case Report and Literature Review of Portal Vein Thrombosis Associated with Cytomegalovirus Infection in Immunocompetent Patients
- Article contents
- Figures & tables
- Supplementary Data
A. Squizzato, W. Ageno, A. Cattaneo, N. Brumana, A Case Report and Literature Review of Portal Vein Thrombosis Associated with Cytomegalovirus Infection in Immunocompetent Patients, Clinical Infectious Diseases , Volume 44, Issue 2, 15 January 2007, Pages e13–e16, https://doi.org/10.1086/509641
- Permissions Icon Permissions
We describe a young man with acute portal vein thrombosis (PVT) and cytomegalovirus (CMV) infection, and we review the literature regarding the association between PVT and CMV in immunocompetent patients. Published data suggest that CMV hepatitis and, possibly, other types of acute viral hepatitis could be a local risk factor for acute PVT.
Several local and systemic factors are involved in the pathogenesis of acute portal vein thrombosis (PVT). In particular, intra-abdominal inflammatory processes, such as acute and chronic pancreatitis, cholecystitis, and appendicitis, are well recognized risk factors of PVT [ 1 ]. Neither acute hepatitis nor acute cytomegalovirus (CMV) infection—which is both a hypothesized systemic procoagulant risk factor and a certain cause of viral hepatitis—is usually reported as a potential risk factor for PVT [ 1–3 ]. We describe a young immunocompetent man who experienced prompt resolution of an asymptomatic acute PVT in a highly likely case of CMV infection and review the available evidence of the natural history of acute CMV-mediated PVT.
Case report . A 34-year-old white man with an echographic diagnosis of PVT was admitted to a tertiary care hospital (Ospedale di Circolo, Varese, Italy) on 26 March 2005. His past medical, family, and social history was unremarkable, apart from a spontaneous pneumothorax at the age of 13 years. In the 2 weeks prior to his admission to the hospital, he was unsuccessfully treated with antibiotic therapy (ampicillin and erythromycin) by a general practitioner for a persistent fever associated with cough. He was initially referred to the emergency department of a primary care hospital, where abdominal color Doppler ultrasonography was performed after the detection of elevated transaminase, lactate dehydrogenase, and D-dimer levels. The ultrasound revealed a partial thrombosis of the left branch of the portal vein and a moderately enlarged liver and spleen. For this reason, he was transferred to the Department of Clinical Medicine at Ospedale di Circolo.
At admission, the patient was asymptomatic; in particular, he did not report any abdominal symptoms. He was still receiving antibiotic therapy and was not receiving any additional medication. On physical examination, his blood pressure was 140/90 mm Hg, he had a regular pulse rate of 96 beats/min, his body temperature was 38.3°C, and his blood oxygen saturation level on breathing air was 97%. Abdominal palpation revealed a mild enlargment of the liver and of the spleen during deep inspiration. Serum testing revealed a mild elevation of aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels (57 U/L, 94 U/L, and 866 U/L, respectively) and a marked elevation of the D-dimer level (1010 µg/L; cutoff value, <200 µg/L). Other laboratory test results were normal, except for a relative lymphocytosis (61.3%). Both electrocardiogram and chest radiograph findings were normal. Urine and blood culture results were negative for bacterial growth.
An urgent computed tomographic scan of the abdomen using intravenous iodine contrast medium confirmed a nonenhancing filling defect within the lumen of the left branch of the portal vein. Organ masses, which are possible local causes of portal vein thrombosis, were excluded, apart from 1-cm-diameter lymphonodes located at the hepatic ileum. The patient was given a full therapeutic dose of low molecular weight heparin, enoxaparin (8000 IU twice daily), and, few days later, 5 mg of warfarin. He continued to receive warfarin, with a target international normalized ratio of 2.5, for the following 6 months.
During the patient's hospitalization, an extensive serological screening was performed with the aim to identify any possible viral cause of hepatitis and persistent fever; test results for hepatitis B surface antigen, antihepatitis B core antigen IgG, hepatitis C virus IgG, hepatitis A virus total immunoglobulin and IgM, Toxoplasma gondii IgM, Epstein-Barr anti-viral capsid antigen IgM, HIV-1 and -2 total IgG, and parvovirus B19 antiantigen recombinant VP2 IgM antibodies were all negative. Rubella IgM and herpes simplex 1 and 2 IgM index test results were not conclusive. The CMV IgM index value was 2.5 (cutoff value, >1.1), the IgG antibody level was 1.3 UI/mL (cutoff value, >1.0 UI/mL), and IgG avidity was 10% (low avidity, <35%); 8 months later, at a follow-up visit, IgM levels were normal, and the IgG level was 3.8 UI/mL.
On 5 April 2005, an abdominal CT using iodine contrast medium revealed a complete resolution of the PVT. At discharge from the hospital, on 7 April 2005, the patient was asymptomatic and afebrile, but alanine aminotransferase and aspartate aminotransferase levels remained increased (164 and 479 IU/dL, respectively). The conclusive diagnosis was acute PVT associated with a probable acute CMV infection, because no other obvious causes could explain clinical and laboratory data.
Extensive screening for thrombophilia was performed after discontinuation of oral anticoagulation therapy—that is, 8 months after the diagnosis of thrombosis. The results of this screening were negative. Wild-type factor II and V genes were present, protein C resistance was absent, a screening test for protein C and S had negative results, antithrombin activity was 102%, factor VIII activity was 150% (reference range, 50%–200%), homocysteine level was 9.7 µmol/L, and normal levels of IgM and IgG anticardiolipin antibodies, normal levels of IgM and IgG anti-β glycoprotein 1 antibodies, as well as negative lupus anticoagulant (activated thromboplastin time and dilute Russell's viper venom time), were present. At that time, aspartate aminotransferase and alanine aminotransferase levels were within the reference range (20–29 IU/L). The results of an HIV test were negative.
Discussion . Venous thromboembolism is a disease with multiple causes, often involving acquired or environmental risk factors, as well as a genetic predisposition [ 4 ]. As with PVT, single or multiple prothrombotic disorders are frequently associated with local precipitating factors, such as cirrhosis, cancer, or local inflammation [ 2 ]. In our patient, it is likely that both a systemic procoagulant state and local inflammation coexisted.
Acute infections are associated with a transient increased risk of venous thromboembolic events [ 5 ]. In particular, some viral infections almost invariably lead to hemostatic abnormalities that range from insignificant laboratory changes to severe disseminated intravascular coagulation [ 6 ]. A direct infection of endothelial cells and systemic inflammation lead to an activation of coagulation due to tissue factor-mediated thrombin generation, down-regulation of physiological anticoagulant mechanism, and inhibition of fibrinolysis [ 3 ]. In vitro studies indicate that CMV has a procoagulant effect: endothelial cells turn in a procoagulant state as CMV directly infects endothelium and causes membrane perturbation. Intrinsic CMV procoagulant properties start and/or amplify the hemostatic imbalance [ 3 ].
Furthermore, as in other acute viral infections, systemic inflammatory response syndrome and inflammatory changes in the surrounding tissues could be associated with viral acute hepatitis; propagation of acute liver inflammation to the endothelium of the portal vein system by contiguity could activate the coagulation system and increase the risk of PVT. To further support our hypothesis that acute viral hepatitis is a relevant pathogenetic factor in developing acute PVT and, in particular, in developing acute CMV-mediated PVT, we performed an extensive literature search of the Embase and Medline databases for articles published up to May 2006, to correctly describe the natural history of acute CMV-mediated PVT. No language restrictions were applied, and reference lists of all included studies were manually searched for other potential eligible studies. Our search results revealed that no case-control or cohort studies of acute CMV-mediated PVT have been published. We identified only 10 published case reports, all of which involved immunocompetent patients, 1 of whom was an infant [ 7–15 ] ( table 1 ). Including our case, adult patients had a mean age of 34 years, and 6 patients were male (60%). Three patients (30%) did not complain of abdominal pain. Known venous thromboembolic risk factors were absent in 4 patients (40%). Eight patients (80%) experienced a complete recanalization of the portal vein that was evident at the radiologic follow-up, which was performed a median of 3 months after the PVT. Two patients (20%) experienced a complete resolution of PVT in <12 days. All 11 patients (100%) had elevated aspartate aminotransferase and/or alanine aminotransferase serum levels.
Published cases of acute portal vein thrombosis (PVT) associated with cytomegalovirus (CMV) infection.
Why is viral hepatitis not usually reported as a cause of acute PVT? Published data suggest that a rapid resolution of the thrombus can occur, even without anticoagulation therapy [ 8 ]. This does not occur in more usual sites of venous thrombosis: 38.8% of patients with a deep venous thrombosis of the lower limbs experienced resolution of the thrombus after 6 months of anticoagulation therapy [ 16 ]. Moreover, mild symptomatic or asymptomatic clinical presentation can occur: 30% of acute PVT cases were detected accidentally, because patients did not complain of any abdominal pain. In our patient, PVT was completely asymptomatic; it was discovered inadvertently by ultrasonography, and it resolved completely after 10 days of antithrombotic therapy.
In conclusion, our case report reinforces the available evidence that suggests that, at the very least, acute CMV hepatitis—and, possibly, acute viral hepatitis—should be added to the list of risk factors of acute PVT. As well, these conditions should be routinely investigated in etiological clinical studies to definitively assess their causal role in acute PVT in immunocompetent patients.
We thank Professors D. Valla and N. Casadevall for providing a full paper of a published case report.
Potential conflicts of interest . All authors: no conflicts.
Google Scholar
- hepatitis, viral, acute
- immunocompetence
- cytomegalovirus infections
- cytomegalovirus
- portal vein thrombosis
Email alerts
More on this topic, related articles in pubmed, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Advertisement
Post-COVID-19 arthritis: a case report and literature review
- Case Based Review
- Open access
- Published: 15 February 2021
- Volume 40 , pages 3357–3362, ( 2021 )
Cite this article
You have full access to this open access article
- M. Gasparotto 1 na1 ,
- V. Framba 2 na1 ,
- C. Piovella 2 ,
- A. Doria 1 &
- Luca Iaccarino 1 , 3
9338 Accesses
69 Citations
18 Altmetric
Explore all metrics
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) is the novel pathogen responsible for the coronavirus disease 19 (COVID-19) outbreak. Researchers and clinicians are exploring the pathogenetic mechanisms of the viral-induced damage and growing interest is focusing on the short-term and long-term immune-mediated consequences triggered by the infection. We will focus on post-SARS-CoV2 infection arthritis which may arise as a new pathological condition associated with COVID-19. In this article, we describe a case of acute oligoarthritis occurring 13 days after a SARS-CoV2 severe pneumonia in a middle-aged Caucasian man and we go over a brief review of the current available literature. We hypothesize that molecular mimicry might be the basic immunological mechanism responsible for the onset of COVID-19-related arthritis based on the current knowledge of SARS-CoV2 and on the known pathogenetic mechanism of viral-induced arthritis.
Similar content being viewed by others
Reactive arthritis after COVID-19: a case-based review
Burhan Fatih Kocyigit & Ahmet Akyol
Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment
Sanket Shah, Debashish Danda, … Vir Singh Negi
Post SARS-CoV-2 infection reactive arthritis: a brief report of two pediatric cases
Reza Sinaei, Sara Pezeshki, … Nava Gharaei
Avoid common mistakes on your manuscript.
Introduction
In the midst of COVID-19 outbreak, researchers from all over the world are studying the pathogenetic mechanisms of SARS-CoV2 respiratory infection but growing interest is also focusing on the immune-mediated consequences that could be secondarily triggered by the virus. The most severe cases are characterized by a marked pro-coagulant state [ 1 , 2 ] and by an inflammatory cytokine storm similar to that found in macrophage activation syndrome [ 3 , 4 , 5 ]. A dysregulated hyperimmune response definitely contributes to the severity of damage and may elicit autoimmune processes in predisposed individuals. Viral infections are supposed to be involved in the pathogenesis of many rheumatological conditions and several cases of autoimmune-induced diseases after SARS-CoV2 infection are reported in the literature [ 6 , 7 , 8 , 9 ].
Case report description
We describe a case of acute arthritis after a SARS-CoV2 infection in a 60-year-old Caucasian man without relevant comorbidities (Table 1 ). In April 2020, he was hospitalized for hyperpyrexia, headache, asthenia, and a worsening dyspnea. At the emergency room (ER), the thoracic ultrasound and chest X-ray revealed an interstitial pneumonia; a nasopharyngeal swab was positive for SARS-CoV2 and his blood test revealed a marked inflammatory state characterized by CRP (C-reactive protein) 240 mg/L, fibrinogen 9.83 g/L, interleukin-6 162 ng/L, ferritin 944 μg/L, and D-dimer 993 μg/L. He was admitted to the Internal Medicine department and treated with azithromycin, ceftriaxone, hydroxychloroquine (HCQ) (400 mg/die), anticoagulation for thromboembolism prophylaxis, and low-flow oxygen. For progressive respiratory failure, he was referred to the intensive care unit where he underwent nasotracheal intubation and received broad-spectrum antibiotics (meropenem, linezolid), antimycotic prophylaxis, continuous diuretic infusion, noradrenalin for hemodynamic support, and therapeutic dose of anticoagulants for elevation in D-dimer values. Due to a progressive improvement of respiratory gas exchange and chest X-ray, he was extubated after 10 days. He was discharged in good general conditions and low-grade inflammation on blood tests after overall 19 days of hospitalization. The weekly surveillance nasopharyngeal swabs for SARS-CoV2 persisted negative. Nevertheless, 13 days after discharge, he complained tenderness of the right ankle, knee, and hip in association with low-grade fever. He presented to the ER with oligoarthritis of the right lower limb and high CRP level (237 mg/L) on blood tests. Physical and ultrasound examination confirmed slight right ankle inflammation and clear right knee arthritis. Arthrocentesis led to the evacuation of 20 cc of a cloudy, yellow, and highly inflammatory synovial fluid (SF) (Fig. 1 ) whose analysis revealed 20.000/mm 3 white blood cells of which 90% polymorphonucleates and 10% monocytes; no crystals were detected. Synovial RT-PCR (real-time polymerase chain reaction) for SARS-CoV2, as well as SF culture for bacterial agents, was negative (Table 2 ). He denied any infectious symptom, recent history of physical trauma, dyspnea, previous episode of arthritis, dactylitis, conjunctivitis or uveitis nor inflammatory diarrhea, and personal or familial history of psoriasis. To proceed with further investigations, the patient was hospitalized again. A new nasopharyngeal swab and a negative research of SARS-CoV2 nucleic acid on sputum excluded a recurrence of the systemic infection while serology showed indisputable seroconversion (a-SARS-CoV2 IgG 37.120 KUA/L and a-SARS-CoV2 IgM 9.163 KUA/L). Urine and blood cultures were negative and procalcitonin within the normal range; urethral swab and stool culture did not show evidence of bacterial infection. The in-depth examination for systemic rheumatic causes of arthritis were negative including antinuclear antibodies (ANA), extractable antinuclear antibodies, rheumatoid factor (RF), anti-citrullinated peptide, and HLA-B27 typing (Table 3 ). The knees, ankles, and hip X-ray did not show erosions or intra-articular calcifications (Fig. 2 ). Even though the patient’s SF was markedly inflammatory, which is an infrequent finding in infectious-related arthritis, the temporal relation with SARS-CoV2 infection made the hypothesis of post-viral acute arthritis the most probable. A nonsteroidal anti-inflammatory (NSAID) therapy with ibuprofen 600-mg; bid was started with clinical benefit and decrease of CRP. The patient was discharged after 9 days and continued the NSAIDs for other 3 weeks. Up to 6 months after therapy discontinuation, he presented no signs of arthritis recurrence.
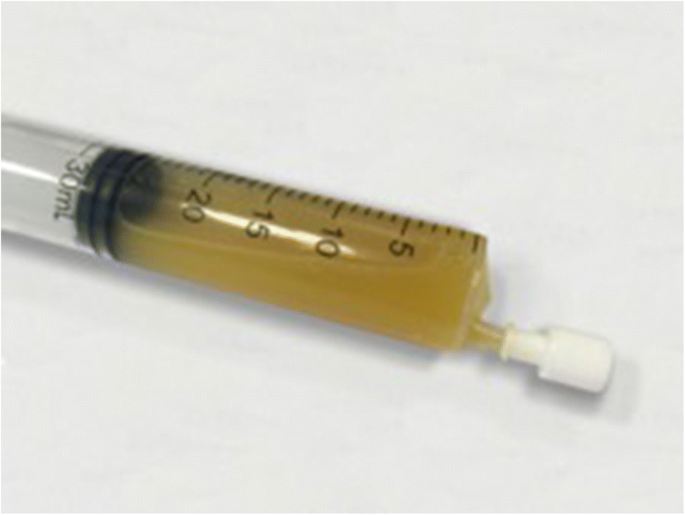
Patient’s synovial fluid
Knees X-ray
Discussion and review of the literature
The pathogenesis of viral associated arthritis is only partially understood but one of the mechanisms supposed to mediate the activation of the inflammatory process is molecular mimicry [ 16 ], well known to be responsible for eliciting autoimmune responses in predisposed individuals [ 17 , 18 ]. Examples of molecular mimicry concerning SARS-CoV2 are reported [ 18 ] and this mechanism is hypothetically involved in the pathogenesis of both the acute systemic infection and the post-infective viral-related immunological consequences [ 19 , 20 ]. Previous and actual studies demonstrate that coronaviruses share molecular epitopes with human proteins (e.g., spike glycoprotein S) that play a key role to host cell invasion and escape immune response attacks, giving to the infectious agent an immune-evasive capacity [ 21 , 22 ]. SASR-CoV2 shares three sequences of six amino acids with as many brainstem human proteins and the cross-reaction between human and viral epitopes may lead to brainstem damage and respiratory failure [ 23 ]. Other suggestive examples of molecular mimicry-driven diseases after COVID-19 come from recent publications reporting cases of Guillain-Barre and Miller-Fisher syndrome [ 24 , 25 ]. Mimicking epitopes may also be present in synovial membrane and cause, with similar mechanism, an acute local inflammation.
To study in depth the relationship between SARS-CoV2 infection and post-COVID-19 arthritis, we performed a comprehensive search on PubMed of all the reported cases of acute arthritis in patients with SARS-CoV2 infection from January 2020 to October 2020 combining the following keywords: acute arthritis, reactive arthritis, viral arthritis, COVID-19, coronavirus, and SARS-CoV2. We considered only English-written case reports of adult patients. Thirteen articles met our searching inclusion criteria; four were excluded because they are not pertinent with the purpose of our review, one because it was a correspondence letter to an already considered article with no case report included in the text, and two because of describing cases of drug-induced gouty arthritis or patients with previous history of gout and therefore not strictly categorizable in among the group of post-COVID-19 arthritis. The six articles which satisfied inclusion and exclusion criteria refer to 6 case reports [ 10 , 11 , 12 , 13 , 14 , 15 ] which are included in Table 3 .
The low prevalence of this clinical condition we found in patients with COVID-19 could be due to the use of HCQ and corticosteroids for the treatment of the viral infection which may prevent or weaken the inflammatory joint manifestations. Despite HCQ has not demonstrated to be effective in the treatment of COVID-19 [ 26 ], it has proven efficacy in the management of systemic rheumatological diseases, especially with inflammatory joint involvement. HCQ is the anchor drug in systemic lupus erythematosus, acting as immunomodulator, and prevents or mitigates lupus clinical manifestation in autoantibody positive asymptomatic subjects [ 27 ]; it is also part of the treatment for the milder forms of rheumatoid arthritis and Sjogren syndrome with frequent episodes of joint pain [ 28 ].
Analyzing more specifically the six cases of suspected COVID-19-related arthritis, it is apparent how the SF analysis was not performed in three cases [ 13 , 14 , 15 ]; therefore a microcrystalline etiology cannot be certainly ruled out. In the remaining three cases [ 10 , 11 , 12 ], monosodium urate and calcium pyrophosphate crystals were not detected at polarized light microscope examination, thus configuring an essential step forward in the exclusion diagnostic process. No other chemical-physical characteristics, including the number of white blood cells and their differential count, have been reported. As shown in Table 3 , the lag time between SARS-CoV2 infection and onset of arthritis is variable but joint symptoms generally present days after the acute viral infection and usually during the healing period. Clinical and epidemiological data show a prominent involvement of lower limb joints with mono- or oligoarticular symptoms and a predilection for male sex. The clinical presentation that emerges from these case reports may deviate from the classic picture of a viral-related arthritis where joint involvement usually occurs during the viremia period and presents with a polyarticular pattern sometimes resembling rheumatoid arthritis [ 29 ].
Viral-related arthritis remains, in the most cases, a diagnosis of exclusion and this underlies the importance to exhaustively perform all the tests to rule out other possible diagnosis. Unfortunately, the diagnostic workup carried out in the reported cases is often partial and incomplete. In particular, a broad microbiological investigation comprehensive of blood, urine and stool cultures, urethral swab, and serological tests for bacteria responsible for reactive arthritis is in some cases lacking. By contrast, in our case report, the chemical-physical characteristics and microbiologic cultures of SF excluded septic and microcrystalline arthritis; broad-spectrum microbiological and serological tests for the common agent of bacterial reactive arthritis did not show any evidence of active or recent infections; the autoantibody immune profile resulted negative too. Finally, RT-PCR for the detection of SARS-CoV2 nucleic acids did not show the presence of the virus in the SF and this validates the hypothesis of an immune-mediated process.
Interestingly, all these suspected post-COVID-19 arthritis cases share a complete and prompt response to NSAID and/or glucocorticoids that, together with the onset timing and joint localization of arthritis, play in favor of a strict relation with SARS-CoV2 infection.
If we suppose a molecular mimicry-based pathogenesis, where the antibody response to the virus is crucial to induce joint inflammation, the rapid lowering of post-infection immunity along weeks could in turn have contributed to the fading of arthritic manifestations. Finally, this pathogenetic hypothesis, based on immune system hyperactivation, could also explain why arthritis has been reported only in patients with a severe infection; in milder forms of COVID-19, joint involvement may have a subclinical course and therefore less frequently come to medical attention.
Conclusions
Before COVID-19 outbreak, no cases of coronavirus-related arthritis have been reported in literature but SARS-CoV2 represents a new devastating entity still under study on worldwide scale. Many steps forward in the comprehension of the viral pathogenicity have been made since the start of the pandemic, but much of the infection-related consequences remain to be discovered. A growing number of cases of COVID-19-related arthritis are being reported in literature, configuring this condition worthy of further study. Complete clinical and laboratory data, SF analysis, and a strict follow-up of the patient are of paramount importance to perform a careful differential diagnosis and to better define the characteristic of inflammatory joint involvement related to SARS-CoV2 infection.
Data availability
All data relevant to the clinical case are included in the article.
Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, Navalesi P, Simioni P (2020) COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost 120:998–1000. https://doi.org/10.1055/s-0040-1710018
Article PubMed PubMed Central Google Scholar
Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S (2020) Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 173:268–277. https://doi.org/10.7326/M20-2003
Article PubMed Google Scholar
McGonagle D, Sharif K, O’Regan A et al (2020) The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 19:102537. https://doi.org/10.1016/j.autrev.2020.102537
Article CAS PubMed PubMed Central Google Scholar
Bindoli S, Felicetti M, Sfriso P, Doria A (2020) The amount of cytokine-release defines different shades of Sars-Cov2 infection. Exp Biol Med 245:1–7. https://doi.org/10.1177/1535370220928964
Article CAS Google Scholar
Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF, Caricchio R, Mahmud S, Hazen MM, Halyabar O, Hoyt KJ, Han J, Grom AA, Gattorno M, Ravelli A, Benedetti F, Behrens EM, Cron RQ, Nigrovic PA (2020) On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheum 72:1059–1063. https://doi.org/10.1002/art.41285
Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, Nguyen EL, Barsh GR, Maskatia S, Mathew R (2020) COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr 10:537–540. https://doi.org/10.1542/hpeds.2020-0123
Viner RM, Whittaker E (2020) Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet 395:1741–1743. https://doi.org/10.1016/S0140-6736(20)31129-6
Harzallah I, Debliquis A, Drénou B (2020) Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost 18:2064–2065. https://doi.org/10.1111/jth.14867
Article CAS PubMed Google Scholar
Andina D, Noguera-Morel L, Bascuas-Arribas M, Gaitero-Tristán J, Alonso-Cadenas JA, Escalada-Pellitero S, Hernández-Martín Á, Torre-Espi M, Colmenero I, Torrelo A (2020) Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol 37:406–411. https://doi.org/10.1111/pde.14215
Yokogawa N, Minematsu N, Katano H, Suzuki T (2020) Case of acute arthritis following SARS-CoV2 infection. Ann Rheum Dis 0:1. https://doi.org/10.1136/annrheumdis-2020-218281
Liew IY, Mak TM, Cui L, Vasoo S, Lim XR (2020) A case of reactive arthritis secondary to coronavirus disease 2019 infection. J Clin Rheumatol 26:233. https://doi.org/10.1097/RHU.0000000000001560
Ono K, Kishimoto M, Shimasaki T, Uchida H, Kurai D, Deshpande GA, Komagata Y, Kaname S (2020) Reactive arthritis after COVID-19 infection. RMD Open 6:e001350. https://doi.org/10.1136/rmdopen-2020-001350
Saricaoglu EM, Hasanoglu I, Guner R (2020) The first reactive arthritis case associated with COVID-19 [Online ahead of print]. J Med Virol. https://doi.org/10.1002/jmv.26296
Danssaert Z, Raum G, Hemtasilpa S (2020) Reactive arthritis in a 37-year-old female with SARS-CoV2 infection. Cureus 12:e9698. https://doi.org/10.7759/cureus.9698
Parisi S, Borrelli R, Bianchi S, Fusaro E (2020) Viral arthritis and COVID-19. Lancet Rheumatol 2:e655–e657. https://doi.org/10.1016/S2665-9913(20)30348-9
Fujinami RS, von Herrath MG, Christen U, Whitton JL (2006) Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev 19:80–94. https://doi.org/10.1128/CMR.19.1.80-94.2006
Cusick MF, Libbey JE, Fujinami RS (2012) Molecular mimicry as a mechanism of autoimmune disease. Clin Rev Allergy Immunol 42:102–111. https://doi.org/10.1007/s12016-011-8294-7
Cappello F (2020) Is COVID-19 a proteiform disease inducing also molecular mimicry phenomena? Cell Stress Chaperones 25:381–382. https://doi.org/10.1007/s12192-020-01112-1
Cappello F, Marino Gammazza A, Dieli F, Conway de Macario E, Macario AJL (2020) Does SARS-CoV-2 trigger stress-induced autoimmunity by molecular mimicry? A hypothesis. J Clin Med 9:2038. https://doi.org/10.3390/jcm9072038
Article CAS PubMed Central Google Scholar
Angileri F, Legare S, Marino Gammazza A, Conway de Macario E, JL Macario A, Cappello F (2020) Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun Rev 19:102591. https://doi.org/10.1016/j.autrev.2020.102591
Hwa KY, Lin WM, Hou YI, Yeh TM (2008) Peptide mimicrying between SARS coronavirus spike protein and human proteins reacts with SARS patient serum. J Biomed Biotechnol 2008:326464–326468. https://doi.org/10.1155/2008/326464
Tim Chew F, Ong SY, Hew CL (2003) Severe acute respiratory syndrome coronavirus and viral mimicry. Lancet 361:2081. https://doi.org/10.1016/S0140-6736(03)13652-5
Article PubMed Central Google Scholar
Lucchese G, Flöel A (2020) Molecular mimicry between SARS-CoV-2 and respiratory pacemaker neurons. Autoimmun Rev 19:102556. https://doi.org/10.1016/j.autrev.2020.102556
Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, Baldanti F, Daturi R, Postorino P, Cavallini A, Micieli G (2020) Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med 382:2574–2576. https://doi.org/10.1056/NEJMc2009191
Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S et al (2020) Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 95:e601–e605. https://doi.org/10.1212/WNL.0000000000009619
Ibáñez S, Martínez O, Valenzuela F, Silva F, Valenzuela O (2020) Hydroxychloroquine and chloroquine in COVID-19: should they be used as standard therapy? Clin Rheumatol 39(8):2461–2465. https://doi.org/10.1007/s10067-020-05202-4
James JA, Kim-Howard XR, Bruner BF, Jonsson MK, McClain M, Arbuckle MR, Walker C, Dennis GJ, Merrill JT, Harley JB (2007) Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus 16(6):401–409. https://doi.org/10.1177/0961203307078579
Schrezenmeier E, Dörner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 16:155–166. https://doi.org/10.1038/s41584-020-0372-x
Marks M, Marks JL (2016) Viral arthritis. Clin Med (Lond) 16:129–134. https://doi.org/10.7861/clinmedicine.16-2-129
Article Google Scholar
Download references
Open Access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement.
Author information
M. Gasparotto and V. Framba contributed equally to this work.
Authors and Affiliations
Rheumatology Unit, Department of Medicine-DIMED, University of Padua, Padua, Italy
M. Gasparotto, A. Doria & Luca Iaccarino
Internal Medicine Unit and Regional Liver Disease Reference Centre, Department of Medicine – DIMED, University of Padua, Padua, Italy
V. Framba & C. Piovella
Division of Rheumatology, University of Padova, Via Giustiniani, 2, 35128, Padova, Italy
Luca Iaccarino
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Luca Iaccarino .
Ethics declarations
Disclosures, ethical approval.
Not applicable; ethical approval not required.
Patient consent for publication
Consent is obtained directly from patient, signed on July 15, 2020.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Gasparotto, M., Framba, V., Piovella, C. et al. Post-COVID-19 arthritis: a case report and literature review. Clin Rheumatol 40 , 3357–3362 (2021). https://doi.org/10.1007/s10067-020-05550-1
Download citation
Received : 10 November 2020
Revised : 09 December 2020
Accepted : 13 December 2020
Published : 15 February 2021
Issue Date : August 2021
DOI : https://doi.org/10.1007/s10067-020-05550-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Molecular mimicry
- Reactive arthritis
- Find a journal
- Publish with us
- Track your research
- Case Report
- Open access
- Published: 02 April 2024
Infection may play an important role in the pathogenesis of alveolar osteonecrosis following facial herpes zoster: a case report and literature review
- Kaikai Huang 1 ,
- Youyuan Wang 2 ,
- Yuhua Huang 1 ,
- Shanshan Han 1 ,
- Yu Yang 1 ,
- Pinghua Qu 1 ,
- Baoying Liang 1 ,
- Qingyu Zhen 1 ,
- Wenting Chen 3 &
- Ying Lin 1
BMC Oral Health volume 24 , Article number: 409 ( 2024 ) Cite this article
Metrics details
Herpes zoster (HZ) is one of the most common skin diseases caused by viruses. Facial HZ develops when the varicella-zoster virus affects the trigeminal nerve, and alveolar osteonecrosis is a rare complication. However, the exact pathogenesis of postherpetic alveolar osteonecrosis remains unclear.
Case description
We encountered a patient who presented to the dermatology clinic with facial HZ and tooth exfoliation in the upper right jaw, and panoramic radiography revealed decreased bone density and poor alveolar socket healing in his right maxilla. Biopsy of the alveolar process revealed fragments of nonvital lamellar bone, which were devoid of osteoblasts and osteocytes and were surrounded by numerous neutrophils and bacterial aggregates. Thus, the diagnosis of alveolar osteonecrosis following facial HZ was confirmed. He then underwent resection of the osteonecrotic tissue. The pathological findings of postoperative tissue were similar to those of previous biopsies. Varicella-zoster virus and multiple types of bacteria were detected through next-generation sequencing, and the species of bacteria were consistent with the results of bacterial culture. Antibiotics and valaciclovir were administered during the perioperative period. The patient showed good recovery at the 9-month follow-up.
Conclusions
The coexistence of bacterial and viral infection may play an important role in the pathogenesis of alveolar osteonecrosis following HZ. To our knowledge, we are the first to directly explore microbial pathogens in a case of postherpetic alveolar osteonecrosis through next-generation sequencing and bacterial culture. We recommend that oral examinations be carefully conducted for patients who are diagnosed with facial HZ, even if their facial rashes have faded away. We suggest that a prolonged and full-dose antiviral therapy course may be beneficial for the treatment of facial HZ with intraoral lesions. The implementation of dental preventive measures should be considered for patients with facial HZ. The application of antibiotics and excision of necrotic bone may reduce the abundance of bacteria in lesions and improve wound healing.
Peer Review reports
Herpes zoster (HZ) is one of the most common skin diseases caused by viruses, and up to one-third of humans may be affected during their lives [ 1 ]. Facial HZ develops when varicella-zoster virus (VZV) affects the trigeminal nerve [ 2 ]. Herpetic neuralgia and Ramsay Hunt syndrome are well-known complications of facial HZ [ 2 , 3 ]. Another rare, severe complication is alveolar osteonecrosis, which can be easily overlooked, as it may occur long after the onset of HZ [ 4 ]. Only 46 such cases had been reported as of 2014 [ 4 ].
Alveolar osteonecrosis is a severe bone disease (osteonecrosis) that affects the jaws (the maxilla and the mandible). The definitive diagnosis of alveolar osteonecrosis depends on the pathological characteristics of osteonecrosis. Alveolar osteonecrosis is usually considered related to certain kinds of drugs (medication-related osteonecrosis of the jaws (MRONJ) due to antiangiogenic agents or antiresorptive drugs such as bisphosphonates and denosumab), radiotherapy (osteoradionecrosis), bacterial infection (osteomyelitis) and metastatic jaw disease [ 5 , 6 , 7 ]. However, the exact pathogenesis of postherpetic alveolar osteonecrosis remains unclear.
Herein, we report the case of a patient with HZ and ipsilateral tooth exfoliation who was later diagnosed with alveolar osteonecrosis. We demonstrate the important role of infection in the pathogenesis of alveolar osteonecrosis through pathological characteristics, next-generation sequencing (NGS) and bacterial culture.
Case presentation
A 67-year-old man presented to the dermatology clinic of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine with a 5-week history of erythema and clustered blisters accompanied by great pain in the right face. He had a severe toothache in the upper right jaw, visited the stomatologist in a local hospital 4 weeks prior and was diagnosed with acute periodontitis and HZ. He was prescribed intravenous ceftizoxime 1 g/d and metronidazole 0.5 g/d, as well as oral acetaminophen for 2 weeks. However, the rashes on his face worsened, and he was subsequently referred to the dermatology clinic in the local hospital. A 10-day regimen of oral valacyclovir 1 g twice a day was initiated. However, the patient’s intense pain was not relieved and four teeth of his upper right jaw exfoliated in succession 10 days before he visited our clinic.
The patient had a 30-year on-and-off history of toothache. He saw the stomatologist and took painkillers at the very beginning. Then, he took metamizole sodium and phenylbutazone tablets every time the toothache attacked, and he hardly went to the hospital to receive standardized treatment, even when he lost several molar teeth many years earlier. The patient also had a history of hypertension and infection with hepatitis B virus (HBV) for years. He had no previous history of tumors, local radiotherapy or other therapy with antiangiogenic agents or antiresorptive drugs such as bisphosphonates and denosumab.
Extraoral examination revealed pigmentation and scars on the right half of the face (Fig. 1 a). On intraoral examination, it was found that there was a complete loss of crowns from teeth 11 to 17 and 35; there was also some tooth decay, gingival recession, and exposure of the alveolar process in the first quadrant of the maxillary arch extending from teeth 11 to 14 (Fig. 1 b). Residual roots of teeth 14, 36 and 48 were also observed.
Clinical findings. ( a ) Pigmentation and scars on the right face after herpes zoster. ( b ) Tooth exfoliation, gingival recession, and exposure of the alveolar process in the first quadrant of the maxillary arch extending from tooth 11 to 14. ( c ) Extensive osteonecrosis was excised during debridement. ( d ) Granulation tissue formation was observed three weeks after debridement
Laboratory tests yielded the following results: normal coagulation function, blood glucose, routine urine tests, routine stool tests, electrocardiography and chest radiography. The screening result for human immunodeficiency virus (HIV) antibody was negative. Quantitative analysis of HBV DNA yielded a value of 4.18 × 10 5 IU/mL. Routine blood examination revealed an elevated white blood cell count of 10.84 × 10 9 /L. CRP was 20.8 mg/L. ALT and serum creatinine were slightly elevated at 54 U/L and 138 µmol/L, respectively. Color ultrasonography showed multiple hepatic cysts and renal cysts. Panoramic radiography was conducted 10 days after tooth exfoliation and revealed decreased bone density and poor alveolar socket healing in his right maxilla. Decayed teeth, residual roots of teeth and periapical cysts were found (Fig. 2 a). Computed tomography examination revealed empty tooth sockets on the right side of the maxilla (Fig. 2 b).
Imaging manifestations. ( a ) Panoramic radiography showed decreased bone density and poor alveolar socket healing in the right maxilla. Decayed teeth (red arrowhead), residual roots of teeth (yellow arrowhead) and periapical cysts (green arrowhead) were found. ( b ) Computed tomography examination revealed empty tooth sockets (white arrowhead) on the right side of the maxilla
Biopsy was conducted from a piece of alveolar process and adjacent mucous membrane. Hyperplasia of the squamous epithelium with no atypia was observed in the oral mucosa. Fibrinoid necrosis of some vascular walls, lumen occlusion, and infiltration of histiocytes and neutrophils were found beneath the mucosa (Fig. 3 a). There were also some fragments of nonvital lamellar bone, which were devoid of osteoblasts and osteocytes and were surrounded by numerous neutrophils and bacterial aggregates (Fig. 3 b). Thus, the diagnosis of alveolar osteonecrosis following facial HZ was confirmed.
The patient was then transferred to the Department of Oral and Maxillofacial Surgery in Sun Yat-sen Memorial Hospital for resection of the osteonecrotic tissue. Extensive malodorous osteonecrosis was observed in the right maxilla during debridement (Fig. 1 c). The pathological findings of postoperative tissue were similar to those of previous biopsies (Fig. 3 c). Bacterial aggregates could be seen inside the marrow cavity by Periodic Acid-Schiff staining (Fig. 3 d).
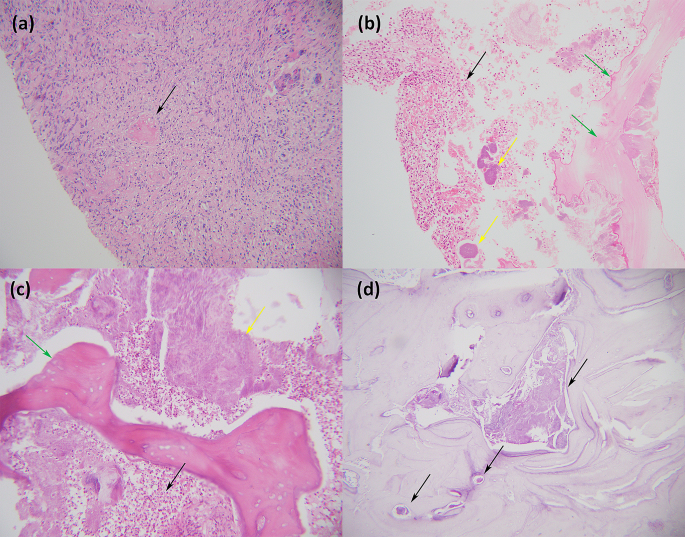
Histological findings. ( a ) The biopsy of the mucous membrane adjacent to the alveolar process revealed infiltration of histiocytes and neutrophils beneath the mucosa. The arrowhead indicates lumen occlusion (Haematoxylin and Eosin ×200). ( b ) The biopsy of the alveolar process showed fragments of nonvital lamellar bone (green arrowhead), which were devoid of osteoblasts and osteocytes and were surrounded by numerous neutrophils (black arrowhead) and bacterial aggregates (yellow arrowhead) (Haematoxylin and Eosin ×200). ( c ) The pathological findings of postoperative tissue were similar to those of previous biopsies, and osteonecrosis (green arrowhead), numerous neutrophils (black arrowhead) and bacterial aggregates (yellow arrowhead) were observed (Haematoxylin and Eosin ×200). ( d ) Arrowheads indicate bacterial aggregates inside the marrow cavity (Periodic Acid-Schiff staining ×100)
A necrotic bone tissue sample was taken for bacterial culture under aerobic and anaerobic conditions. Prevotella denticola , Streptococcus intermedius , Actinomycetes oris , and Actinomyces viscosus were then confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Moreover, NGS was conducted from a piece of alveolar process and adjacent mucous membrane. Following DNA extraction, DNA libraries were constructed and sequenced by the MGISEQ-2000 platform [ 8 ]. High-quality sequencing data were generated by removing low-quality reads, followed by computational subtraction of human host sequences mapped to the human reference genome (hg19) using Burrows‒Wheeler Alignment [ 9 ]. The remaining data obtained by removal of low-complexity reads were classified by simultaneous alignment to the Pathogens Metagenomics Database (PMDB), consisting of bacteria, fungi, viruses and parasites. Finally, Prevotella, Streptococcus, Lactobacillus, Veillonella, Actinomyces, Candida , VZV, HBV, human gammaherpesvirus 4 and Torque teno virus (TTV) were detected by NGS. Thus, the coexistence of bacterial and viral infection was confirmed.
During the perioperative period, a 7-day regimen of antiviral treatment (oral valacyclovir 1 g twice a day) and antibiotic therapy (intravenous cefathiamidine 2 g twice a day for 3 days and oral cefuroxime 0.25 g twice a day for 4 days successively) were administered. Granulation tissue formation was observed on the surface of the alveolar wound three weeks after debridement (Fig. 1 d). At a follow-up 9 months later, no further tooth exfoliation was found.
Discussion and conclusions
Although there was no apparent osteonecrosis of the jaw according to panoramic radiographs or CT scans, biopsy of the alveolar process revealed the typical pathological characteristics of osteonecrosis. Thus, the diagnosis of alveolar osteonecrosis following facial HZ can be confirmed before surgery. Alveolar osteonecrosis may appear 9 to 150 days after the onset of facial HZ [ 10 ], and tooth exfoliation is one of its most important clinical manifestations [ 11 , 12 ]. This phenomenon could hardly be explained by coincidence, as reported cases of tooth exfoliation have always occurred on the same side as facial HZ [ 4 ]. There must be some underlying factors associated with facial HZ with alveolar osteonecrosis.
MRONJ and osteoradionecrosis could be ruled out in the present case, as the patient did not have a related history. Histological findings did not support metastatic jaw diseases. Some scholars believe that local vasculitis caused by viruses [ 13 ], vasoconstriction through sympathetic innervation [ 14 ], mechanical compression of the alveolar artery by the swollen alveolar nerve [ 10 ], or a hypercoagulable state may be involved in the pathogenesis of postherpetic alveolar osteonecrosis. In our case, the pathological findings of fibrinoid necrosis on the vascular wall and lumen occlusion may support the hypothesis that vascular factors also partially contributed to postherpetic alveolar osteonecrosis.
Notably, the patient had a long-term history of toothache but did not receive standard treatment. Decayed teeth, residual roots of teeth and periapical cysts indicated poor oral hygiene and chronic oral diseases with possible bacterial colonization of the patient. Four teeth of his upper right jaw exfoliated successively after the onset of ipsilateral facial HZ, and interestingly, the adjacent teeth of the upper left jaw seemed not to be affected. An immunosuppressive state, absence of early standardized antiviral treatment, underlying diseases such as tumors, tuberculosis, HIV or HBV infection, and advanced age are considered risk factors for alveolar osteonecrosis in patients with facial HZ involving the maxillary and/or mandibular branch of the trigeminal nerve [ 11 , 12 , 14 , 15 ]. Ipsilateral lesions on the buccal mucosa, labial mucosa, tongue, alveolar ridge and soft palate can be affected in facial HZ cases [ 16 ]. A subsequent serious bacterial infection, such as septicemia, may occur following HZ [ 17 ]. It has been reported that existing periodontitis or pulpitis may lead to more severe alveolar osteonecrosis [ 9 ]. Thus, it is reasonable to infer that VZV infection may lead to severe damage to the oral mucosa, which aggravates chronic oral diseases and facilitates bacterial infection.
In view of the lack of in-depth discussion about infection in the previous literature, we attempted to apply comprehensive techniques to analyze the pathogens of postherpetic alveolar osteonecrosis, including bacterial culture, NGS sequencing and histopathological examination. To our knowledge, we are the first to directly explore microbial pathogens in cases of postherpetic alveolar osteonecrosis through NGS sequencing and bacterial culture. The poor oral hygiene, bacterial aggregates observed in the bone marrow cavity of the necrotic bone upon histopathological examination, VZV and multiple types of bacteria detected through NGS sequencing which were consistent with the results of bacterial culture, strongly indicated that the coexistence of bacterial and viral infection may play an important role in the pathogenesis of alveolar osteonecrosis following HZ.
On the other hand, chronic oral diseases may lead to localized immunosuppression, which possibly increases the risk of VZV reactivation. The role of local factors in the outbreak of HZ has been discussed in some studies. It has been reported that HZ can occur in affected sites after local radiotherapy, intra-articular corticosteroid injection and surgical operations [ 18 , 19 , 20 , 21 ]. The risk of developing HZ in breast cancer patients who have received postoperative radiotherapy may be 3- to 5-fold higher than the incidence in the general population [ 21 ]. Obviously, the patient in this case had chronic oral diseases before HZ onset. However, whether preexisting chronic oral diseases may increase the risk of developing HZ remains to be verified by studies on a large sample of patients.
Besides VZV, we should notice that some other viruses such as HBV, human gammaherpesvirus 4 and TTV were also detected by NGS. High levels of HBV have been confirmed in the blood by quantitative analysis as the case description above. HBV can cause hepatitis, fibrosis, cirrhosis, hepatocellular carcinoma and liver failure [ 22 ]. Human gammaherpesvirus 4 , also known as Epstein-Barr virus (EBV), infects more than 95% of the world’s population and is associated with some kinds of lymphoma, nasopharyngeal carcinoma and infectious mononucleosis [ 23 ]. EBV establishes a life-long persistence in the human host by infecting B cells, and the cycling of latency and reactivation is ongoing in all infected individuals [ 23 ]. TTV DNAemia is universal among the global population and there is now a widespread consensus that TTV should be considered a commensal because no evidence supports a causal association with any human disease [ 24 ]. It was unavoidable that the local tissue taken for NGS examination in our case would contain a small amount of blood. Thus, it was reasonable to infer that the HBV, EBV and TTV we detected by NGS originated from the blood. To our knowledge, there are currently no studies reporting the pathogenesis of HBV, EBV and TTV in alveolar osteonecrosis. On the other hand, VZV establishes latency in the cell bodies of axons after primary infection [ 25 ]. When reactivated, VZV travels within the axon in anterograde manner to reach the innervated skin and mucous membrane where it causes HZ, characterized by a localized painful vesicular rash [ 25 ]. Thus, only when VZV is reactivated can it be detected in local tissue. In addition, postherpetic alveolar osteonecrosis always occurs at the same innervated part of HZ [ 4 ]. Therefore, it is reasonable to infer that the VZV we detected in local tissue by NGS is involved in the pathogenesis of postherpetic alveolar osteonecrosis.
Based on the pathogenesis of the disease we discussed above, we recommend that oral examinations be carefully conducted for patients who are diagnosed with facial HZ, even if their facial rashes have faded away. In particular, patients may be first seen in the dermatology department, and they should be referred to the stomatology department for consultation and evaluation.
Early antiviral treatment is important for HZ. The course of antiviral treatment is usually 7 days [ 26 ]. In patients who continue to develop new vesicles or who have cutaneous, ocular, neurologic, or motor complications after 7 days of antiviral therapy, extending the duration of antiviral therapy for more than 7 days is recommended [ 26 ]. However, there is not yet a guideline regarding the recommended antiviral therapy course for the treatment of facial HZ with intraoral lesions. The NGS results of the abovementioned patient demonstrated the existence of VZV in oral lesions at 5 weeks after the onset of HZ. Thus, we suggest that a prolonged and full-dose antiviral therapy course may be beneficial for the treatment of facial HZ with intraoral lesions, especially when the intraoral mucosa has not recovered after a conventional 7-day therapy course.
In considering prevention, we can refer to MRONJ, as bacterial infection (mainly with actinomycetes) is believed to play an important role in MRONJ [ 12 , 27 ]. The implementation of dental preventive measures in solid tumor patients with bone metastases treated with bisphosphonates may help to decrease the occurrence of MRONJ from 3.2 to 1.3% [ 28 ]. We should also strengthen oral health education and nursing practices regarding facial HZ. The application of early antiviral treatment and antibiotics and the excision of necrotic bone would help to improve wound healing to the greatest extent [ 29 ]. The bacteria most frequently associated with MRONJ are Streptococcus species (spp.), Prevotella spp., Actinomyces spp., Veillonella spp., and Parvimonas micra [ 30 ]. The bacteria are most susceptible to the cephalosporins cefotaxime, cefuroxime and β-lactam antibiotics with β-lactamase inhibitors [ 30 ]. The pathogenic agents we detected in this case of postherpetic alveolar osteonecrosis were in accordance with MRONJ, indicating that it was reasonable to choose antibiotics for postherpetic alveolar osteonecrosis according to MRONJ.
The removal of necrotic bone may reduce the abundance of bacteria in lesions, especially in deep tissue [ 30 ]. In some previous cases of postherpetic alveolar osteonecrosis, the patient underwent more than one operation [ 2 , 31 ]. Clinicians should attach importance to timely debridement; this was also a key experience in our successful treatment of this patient.
In conclusion, alveolar osteonecrosis is a rare, severe complication of HZ and may occur long after the onset of HZ. Tooth exfoliation is a sign of alveolar osteonecrosis. We have been the first to directly explore the microbial pathogens in a case of postherpetic alveolar osteonecrosis through NGS sequencing and bacterial culture. We suggest that the coexistence of bacterial and viral infection may play an important role in the pathogenesis of alveolar osteonecrosis following HZ.
Data availability
All data underlying the findings and outcome are presented as part of the article and no supplementary source data are required.
Abbreviations
- Herpes zoster
Next-generation sequencing
Hepatitis B virus
Human immunodeficiency virus
Varicella-zoster virus
Epstein-Barr virus
Torque teno virus
Medication-related osteonecrosis of the jaw
Sato K, Adachi K, Nakamura H, Asano K, Watanabe A, Adachi R, et al. Burden of herpes zoster and postherpetic neuralgia in Japanese adults 60 years of age or older: results from an observational, prospective, physician practice-based cohort study. J Dermatol. 2017;44(4):414–22.
Article PubMed Google Scholar
Rudd T, Chai BY, Gurunluoglu R, Glasgow M. Mandibular osteonecrosis and Ramsay Hunt syndrome following a case of herpes zoster. J Oral Maxillofac Surg. 2014;72(10):e19741–6.
Article Google Scholar
Mahajan VK, Ranjan N, Sharma S, Sharma NL. Spontaneous tooth exfoliation after trigeminal herpes zoster: a case series of an uncommon complication. Indian J Dermatol. 2013;58(3):244.
Article PubMed PubMed Central Google Scholar
Cloarec N, Zaegel-Faucher O, Bregigeon S, Cano CE, Chossegros C, Wajszczak B, et al. Mandibular osteonecrosis and dental exfoliation after trigeminal zoster in an HIV-infected patient: case report and review of the literature. AIDS. 2014;28(3):448–50.
Güll FD, Deppe H, Kesting M, Schwarzer C. Periodontal disease-like bone loss after adjuvant radiotherapy in the head and neck region: a case report and review of the literature. Quintessence Int. 2017;48(6):451–57.
PubMed Google Scholar
Fliefel R, Tröltzsch M, Kühnisch J, Ehrenfeld M, Otto S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int J Oral Maxillofac Surg. 2015;44(5):568–85.
Article CAS PubMed Google Scholar
Otto S, Aljohani S, Fliefel R, Ecke S, Ristow O, Burian E, et al. Infection as an important factor in medication-related osteonecrosis of the jaw (MRONJ). Med (Kaunas). 2021;57(5):463.
Google Scholar
Jeon YJ, Zhou Y, Li Y, Guo Q, Chen J, Quan S, et al. The feasibility study of non-invasive fetal trisomy 18 and 21 detection with semiconductor sequencing platform. PLoS ONE. 2014;9(10):e110240.
Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60.
Article CAS PubMed PubMed Central Google Scholar
Arikawa J, Mizushima J, Higaki Y, Hoshino J, Kawashima M. Mandibular alveolar bone necrosis after trigeminal herpes zoster. Int J Dermatol. 2004;43(2):136–7.
Siwamogstham P, Kuansuwan C, Reichart PA. Herpes zoster in HIV infection with osteonecrosis of the jaw and tooth exfoliation. Oral Dis. 2006;12(5):500–5.
Mendieta C, Miranda J, Brunet LI, Gargallo J, Berini L. Alveolar bone necrosis and tooth exfoliation following herpes zoster infection: a review of the literature and case report. J Periodontol. 2005;76(1):148–53.
Melanson M, Chalk C, Georgevich L, Fett K, Lapierre Y, Duong H, et al. Varicella-Zoster virus DNA in CSF and arteries in delayed contralateral hemiplegia: evidence for viral invasion of cerebral arteries. Neurology. 1996;47(2):569–70.
van Heerden WF, McEachen SE, Boy SC. Alveolar bone necrosis and tooth exfoliation secondary to herpes zoster in the setting of HIV/AIDS. AIDS. 2005;19(18):2183–4.
Gupta S, Sreenivasan V, Patil PB. Dental complications of herpes zoster: two case reports and review of literature. Indian J Dent Res. 2015;26(2):214–9.
Preeti N, Harshkant G, Pooja S, Palak JC. Herpes zoster on the face in the elderly. BMJ Case Rep. 2014; 2014: bcr2013200101.
Woznowski M, Quack I, Bölke E, Peiper M, Matuschek C, Gatermann SG, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 2010;15(9):410–4.
Fernandes NF, Malliah R, Stitik TP, Rozdeba P, Lambert WC, Schwartz RA. Herpes zoster following intra-articular corticosteroid injection. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18(1):28–30.
Chaitanya EV, Shruthi TV, Shah NA. Late onset DCR surgical site herpes zoster in an immunocompetent patient: a rare presentation. Indian J Ophthalmol. 2020;68(1):202–3.
Choi HJ, Kim JH, Lee YM. Herpes zoster developing within recent subciliary incision scar. J Craniofac Surg. 2012;23(3):930–1.
Dunst J, Steil B, Furch S, Fach A, Bormann G, Marsch W. Herpes zoster in breast cancer patients after radiotherapy. Strahlenther Onkol. 2000;176(11):513–6.
Shih C, Yang CC, Choijilsuren G, Chang CH, Liou AT. Hepatitis B virus. Trends Microbiol. 2018;26(4):386–7.
Damania B, Kenney SC, Raab-Traub N. Epstein-Barr virus : biology and clinical disease. Cell. 2022;185(20):3652–70.
Redondo N, Navarro D, Aguado JM, Fernández-Ruiz M. Viruses, friends, and foes: the case of Torque Teno virus and the net state of immunosuppression. Transpl Infect Dis. 2022;24(2):e13778.
Tommasi C, Breuer J. The biology of Varicella-Zoster virus replication in the skin. Viruses. 2022;14(5):982.
Robert HD, Robert WJ, Judith B, John WG, Myron JL, Miroslav B, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–26.
Naik NH, Russo TA. Bisphosphonate-related osteonecrosis of the jaw: the role of actinomyces. Clin Infect Dis. 2009;49(11):1729–32.
Ripamonti CI, Maniezzo M, Campa T, Fagnoni E, Brunelli C, Saibene G, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20(1):137–45.
Lambade P, Lambade D, Saha TK, Dolas RS, Pandilwar PK. Maxillary osteonecrosis and spontaneous teeth exfoliation following herpes zoster. Oral Maxillofac Surg. 2012;16(4):369–72.
Zirk M, Wenzel C, Buller J, Zöller JE, Zinser M, Peters F. Microbial diversity in infections of patients with medication-related osteonecrosis of the jaw. Clin Oral Investig. 2019;23(5):2143–51.
Yin M, Huang P, Yang S, Wang W. Ramsay Hunt syndrome and mandibular alveolar bone necrosis following herpes zoster: a case report and literature review. Front Neurol. 2022;13:1073607.
Download references
Acknowledgements
We thank Dr. Zhexun Huang from Department of implantation, Stomatological Hospital, School of Stomatology, Southern Medical University for professional advice on discussion in this case.
This work was supported by National Natural Science Foundation of China (No.31972856), Top Talents Project of Guangdong Provincial Hospital of Chinese Medicine (No.BJ2022YL08) and Chinese Medicine Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (No.YN2022QN25).
Author information
Authors and affiliations.
The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, 510000, China
Kaikai Huang, Yuhua Huang, Shanshan Han, Yu Yang, Pinghua Qu, Baoying Liang, Qingyu Zhen & Ying Lin
Department of Oral and Maxillofacial Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 510000, China
Youyuan Wang
The Second Clinical School of Guangzhou University of Chinese Medicine, Guangzhou, 510000, China
Wenting Chen
You can also search for this author in PubMed Google Scholar
Contributions
KH and YL were responsible for writing and revision of the manuscript. KH, YW and YH contributed to the oral examination and treatment of the patient. SH, BL, YY and PQ were responsible for collecting clinical and laboratory data. WC and QZ were responsible for literature review. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Ying Lin .
Ethics declarations
Ethics approval and consent to participate.
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (G2023-06). The patient signed a written consent stating the approval for participation in this report.
Consent for publication
Written informed consent has been obtained from the patient to publish this paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Huang, K., Wang, Y., Huang, Y. et al. Infection may play an important role in the pathogenesis of alveolar osteonecrosis following facial herpes zoster: a case report and literature review. BMC Oral Health 24 , 409 (2024). https://doi.org/10.1186/s12903-024-04202-z
Download citation
Received : 25 January 2024
Accepted : 28 March 2024
Published : 02 April 2024
DOI : https://doi.org/10.1186/s12903-024-04202-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Tooth exfoliation
- Alveolar osteonecrosis
- Complication
BMC Oral Health
ISSN: 1472-6831
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Case report
- Open access
- Published: 31 March 2024
Atypical lipoma of the right piriformis muscle: a case report and review of the literature
- Xiao Qiu 1 ,
- Xiaoyong Luo 1 &
- Renmei Wu 1
Journal of Medical Case Reports volume 18 , Article number: 189 ( 2024 ) Cite this article
32 Accesses
1 Altmetric
Metrics details
Piriformis muscle mass is rare, which is particular for intrapiriformis lipoma. Thus far, only 11 cases of piriformis muscle mass have been reported in the English literature. Herein, we encountered one patient with intrapiriformis lipoma who was initially misdiagnosed.
Case presentation
The patient is a 50-year-old Chinese man. He complained of osphyalgia, right buttock pain, and radiating pain from the right buttock to the back of the right leg. Both ultrasound and magnetic resonance imaging demonstrated a cyst-like mass in the right piriformis muscle. Ultrasonography-guided aspiration was performed on this patient first, but failed. He was then recommended to undergo mass resection and neurolysis of sciatic nerve. Surprisingly, final histology revealed the diagnosis of intrapiriformis lipoma. The patient exhibited significant relief of symptoms 3 days post-surgery.
Diagnosis and differential diagnosis of radicular pain are potentially challenging but necessary. Atypical lipoma is prone to be misdiagnosed, especially in rare sites. It is notable for clinicians to be aware of the presence of intrapiriformis lipoma to avoid misdiagnosis and inappropriate treatment.
Peer Review reports
Piriformis syndrome (PS), also known as sciatic nerve outlet syndrome, caused by compression of the sciatic nerve by the piriformis muscle, is characterized by occasional sciatic-type pain, tingling, and numbness in the buttock along the sciatic nerve pathway down to the lower thigh and the calf [ 1 ]. The causes of PS are diversified, including inflammatory, traumatic, tumoral, and malformative factors [ 2 , 3 ]. PS triggered by space-occupying lesions of the piriformis muscle is very rare. Up to date, only 11 cases of piriformis muscle mass have been reported in the English literature [ 4 , 5 , 6 , 7 , 8 , 9 , 10 ].
Lipomas are one of the most common mesenchymal neoplasms and can occur in any region of the body that contains fat component, including the subcutaneous soft tissues, mediastinum, retroperitoneum, bones, or along the gastrointestinal tract [ 11 ]. Intrapiriformis lipoma is rare and the diagnosis might be intractable when presenting atypical. In addition, misdiagnosis can lead to inappropriate treatment, which causes unsatisfactory outcomes. Here, we present a case of a large intrapiriformis lipoma that was initially misdiagnosed, highlighting that clinicians should be aware that intrapiriformis lipoma might harbor atypical manifestations upon examination.
A 50-year-old Chinese man presented to the orthopedics department with chief complaints of osphyalgia, right buttock pain, and radiating pain from the right buttock to the back of the right leg. The right buttock pain was the most prominent symptom. The pain was accelerated by movement and relieved by lying supine, which induced abnormal walk in the patient. No previous relevant treatment or surgery was reported. Additionally, there was no significant relevant family or social history.
Lasegue’s sign and its strengthening test were positive. Physical examination also demonstrated the positive findings of right femoral nerve traction test and Faber test, and the limitation of right hip abduction was observed. Neurological examination of the lower limb did not demonstrate any loss of sensation or reduced muscle power in any of the nerve root distributions. Non-remarkable finding was revealed after the abdominal examination.
As no apparent abnormalities were indicated upon the plain radiograph imaging of his lumbar spine, magnetic resonance imaging (MRI) scan of the lumbar/sacral area of the spine was then suggested, showing lumbar disc herniation (LDH), which did not account for the patient’s predominant right buttock pain. Thus, the musculoskeletal ultrasound (MSK-US) for the sciatic nerve scanning was performed, implying that the right sciatic nerve was pushed by an anechoic mass within the right piriformis muscle measuring 6 cm mediolateral, 2.3 cm anteroposterior, and 2.6 cm craniocaudal. The lobulated mass was cystic-like with regular margins and no posterior wall enhancement (Fig. 1 ). Subsequently, further MRI of the pelvis and ipsilateral hip indicated a cystic-like lesion in piriformis muscle region with low T1 signal and high T2 signal, and the maximum measurement was about 3.1 cm mediolateral and 2.2 cm anteroposterior (Fig. 2 ). Considering these results, a piriformis ganglion was suspected, and the differential diagnoses included hematoma, metastatic tumor, and so forth.
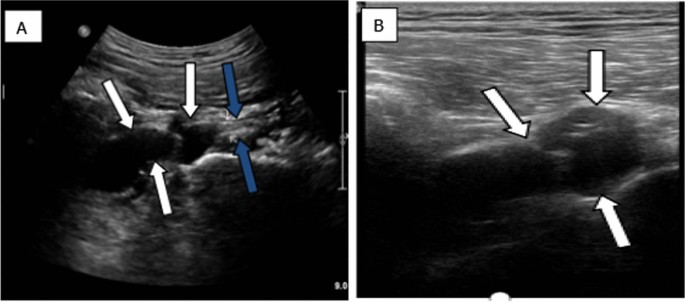
Sonographic examination showing a separate anechoic mass (white arrows) above the outlet of the right piriformis muscle pushing the right sciatic nerve (blue arrows). A Sagittal view(low- frequency probe); B Transverse view (high-frequency probe)
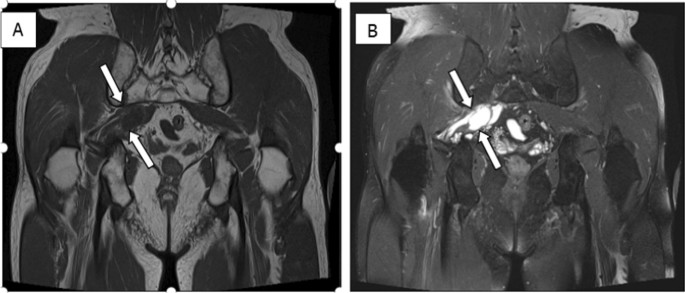
MRI demonstrating multiple cysts (arrows) in piriformis muscle region with long T1 ( A ) and long T2 ( B ) signals
Aiming to achieve the final diagnosis, the ultrasonography-guided aspiration was conducted, but failed due to unextracted cystic fluid. In addition, significant resistance was encountered when injecting with physiological saline. As for the undefined nature of the mass and the associated serious symptoms, malignancy could not be excluded; the patient was suggested to undergo piriformis muscle mass resection and neurolysis of sciatic nerve. Operative finding showed the compression of right sciatic nerve by a fat-like mass at the lower margin of piriformis muscle measuring 5 cm mediolateral, 2 cm anteroposterior, and 2 cm craniocaudal. Final histology revealed that the lesion was fibrous adipose tissue, which was consistent with diagnosis of lipoma (Fig. 3 ). The patient exhibited significant relief of symptoms 3 days post-surgery. No recurrence of relevant symptoms was observed during 24-month follow-up period.
Under microscope inspection, removed specimen revealed fibrous adipose tissue within the lesion, consistent with lipoma
Lower back pain can present with radicular pain caused by lumbosacral nerve root pathology. As a major cause of lower back pain, sciatica, and radicular leg pain, LDH is usually the first considered diagnosis. Similarly, in our case LDH was initially considered according to the MRI of the lumbar/sacral spine. However, the primary pain in the right buttock of this patient was unexplained on the diagnosis of LDH.
PS, also known as sciatic nerve outlet syndrome, is a type of sciatic neuralgia caused by compression of the piriformis muscle on the sciatic nerve. Typical manifestations include buttock pain and radiating pain in the innervated area of the sciatic nerve. In general, etiologies are composed of traumatic bleeding, adhesions, scars, anatomical variations, and so forth [ 2 ]. Of note, intrapiriformis lesion enlarging the muscle may be the common cause of sciatic nerve compression-induced secondary PS, whereas PS triggered by space-occupying lesions of the piriformis muscle is very rare. To the best of our knowledge, only 11 cases have been reported in the literature thus far; these patients and our present case are summarized in Table 1 [ 4 , 5 , 6 , 7 , 8 , 9 , 10 ], among which only 1 case of intramuscular lipomas occurring within the piriformis muscle leading to secondary PS have been previously reported in the literature [ 6 ].
Lipomas can be classified into superficial and deep lesions according to the location. Deep-seated lipomas are less common than superficial lipomas, which may be located under the muscle (submuscular), within the muscle (intramuscular), between the muscles (intermuscular), or on top of the muscle (supramuscular) [ 11 ]. Clinically, lipomas often present as asymptomatic slow-growing mass or swelling with no palpable mass. The application of ultrasound (US) in the examination of lipomas is very frequent. Usually, superficial lipomas might manifest as a hyperechoic solid mass without posterior acoustic enhancement or show as a isoechoic mass on gray-scale US. Compared with superficial lipomas, the deep-seated type can present as various US characteristics. In addition, few reports in the literature show the hypoechoic, isoechoic, or anechoic properties of deep ones [ 12 , 13 , 14 ]. However, the intrapiriformis lipoma in our case was featured as an anechoic lesion, usually indicated as cystic lesions. The MRI of the pelvis and ipsilateral hip showed the same signal characteristics as those of water on all sequences. Therefore, the lesion within piriformis muscle region was then misdiagnosed as ganglion and distinguished from neuschwannoma, liposarcoma, hematoma, lymphoma, metastatic tumor, and so on. Therefore, ultrasonography-guided aspiration was performed while noncystic fluid was extracted.
The echogenicity of lipomas may range from hyperechoic to anechoic, depending on the component percentage of connective tissue and other reflective interfaces presented within a lipoma [ 15 ]. It has been postulated that US and MRI appearance of lipomas are largely dependent on the internal cellularity, specifically on the proportion of fat and water within the lesion [ 16 ]. When the proportion of water in the lipoma is high, it may present the same imaging characteristics as this case.
Generally, intrapiriformis lipoma does not require treatment in the absence of symptoms, while for our case, considering the serious symptoms of this patient and undefined nature, even including malignancy, after series of examinations, surgical treatment was recommended. Fortunately, the patient showed significant relief of symptoms 3 days after surgery. No recurrence of associated symptoms was observed during 24-month follow-up period.
Despite the potentially significant challenges for the diagnosis and differential diagnosis of radicular pain, it is highly necessary and essential. It is notable that medical practitioners should be aware of this condition and exclude space-occupying lesions of piriformis muscles when encountering patients presenting with radicular pain. Our case highlighted the atypical manifestations of lipomas in rare areas such as piriformis muscles, for which condition-adequate examinations should be performed and surgery might be finally suggested to reach the final diagnosis, thus avoiding misdiagnosis and inappropriate treatment and increasing the life quality of these patients.
Availability of data and materials
The authors of this manuscript are willing to provide additional information regarding the case report.
Abbreviations
Musculoskeletal ultrasound
Magnetic resonance imaging
Piriformis syndrome
Lumbar disc herniation
Hopayian K, Song F, Riera R, Sambandan S. The clinical features of the piriformis syndrome: a systematic review. Eur Spine J. 2010;19:2095–109.
Article PubMed PubMed Central Google Scholar
Kirschner JS, Foye PM, Cole JL. Piriformis syndrome, diagnosis and treatment. Muscle Nerve. 2009;40(1):10–8.
Article PubMed Google Scholar
Byrd JWT. Piriformis syndrome. Oper Tech Sports Med. 2005;13(1):71–9.
Article Google Scholar
Salar O, Flockton H, Singh R, Reynolds J. Piriformis muscle metastasis from a rectal polyp. Case Rep. 2012. https://doi.org/10.1136/bcr-2012-007208 .
Domínguez-Páez M, De Miguel-Pueyo LS, Medina-Imbroda JM, González-García L, Moreno-Ramírez V, Martín-Gallego A, et al . Sciatica secondary to extrapelvic endometriosis affecting the piriformis muscle. Case report. Neurocirugia (Astur). 2012;23(4):170–4.
Drampalos E, Sadiq M, Thompson T, Lomax A, Paul A. Intrapiriformis lipoma: an unusual cause of piriformis syndrome. Eur Spine J. 2015;24:551–4.
Park JH, Jeong HJ, Shin HK, Park SJ, Lee JH, Kim E. Piriformis ganglion: an uncommon cause of sciatica. Orthop Traumatol Surg Res. 2016;102(2):257–60.
Article CAS PubMed Google Scholar
Lodin J, Brušáková Š, Kachlík D, Sameš M, Humhej I. Acute piriformis syndrome mimicking cauda equina syndrome: illustrative case. J Neurosurg Case Lessons. 2021;2(17): CASE21252.
Sanuki N, Kodama S, Seta H, Sakai M, Watanabe H. Radiation therapy for malignant lumbosacral plexopathy: a case series. Cureus. 2022;14(1): e20939.
PubMed PubMed Central Google Scholar
Ward TRW, Garala K, Dos Remedios I, Lim J. Piriformis syndrome as a result of intramuscular haematoma mimicking cauda equina effectively treated with piriformis tendon release. BMJ Case Reports CP. 2022;15(3): e247988.
Paunipagar BK, Griffith J, Rasalkar DD, Chow LTC, Kumta SM, Ahuja A. Ultrasound features of deep-seated lipomas. Insights Imaging. 2010;1(3):149–53.
Goldberg BB. Ultrasonic evaluation of superficial masses. J Clin Ultrasound. 1975;3(2):91–4.
Rahmani G, McCarthy P, Bergin D. The diagnostic accuracy of ultrasonography for soft tissue lipomas: a systematic review. Acta radiologica open. 2017;6(6):2058460117716704.
Fujioka K. A comparison between superficial and deep-seated lipomas on high-resolution ultrasonography: with RTE and MRI appearances. Biomed J Sci Tech Res. 2019;19:14220–4.
Google Scholar
Behan M, Kazam E. The echographic characteristics of fatty tissues and tumors. Radiology. 1978;129(1):143–51.
Inampudi P, Jacobson JA, Fessell DP, Carlos RC, Patel SV, Delaney-Sathy LO, et al . Soft-tissue lipomas: accuracy of sonography in diagnosis with pathologic correlation. Radiology. 2004;233(3):763–7.
Download references
Acknowledgements
The author(s) gratefully acknowledge the useful suggestions given by Dr Ji-Bin Liu of Thomas Jefferson University, and the author(s) thank Xiaobo Luo for providing language assistance during article writing.
Not applicable.
Author information
Authors and affiliations.
Department of Ultrasound, Suining Central Hospital, 127 Desheng West Road, Suining, Sichuan, China
Xiao Qiu, Xiaoyong Luo & Renmei Wu
You can also search for this author in PubMed Google Scholar
Contributions
Renmei Wu contributed to the collection of the medical history data. Xiao Qiu contributed to the manuscript preparation of this case report. Xiaoyong Luo supervised the case report.
Corresponding author
Correspondence to Renmei Wu .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was approved by the ethics review board of Suining Central Hospital (no. LLSLH20220011).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The author(s) declared no potential conflicts of interest regarding the publication of this manuscript.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Qiu, X., Luo, X. & Wu, R. Atypical lipoma of the right piriformis muscle: a case report and review of the literature. J Med Case Reports 18 , 189 (2024). https://doi.org/10.1186/s13256-024-04507-1
Download citation
Received : 04 April 2022
Accepted : 13 March 2024
Published : 31 March 2024
DOI : https://doi.org/10.1186/s13256-024-04507-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Piriformis muscle
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Airway Management for Emergency Spinal Epidural Hematoma Evacuation With Awake Spine Surgery: Case Report and Literature Review.
BACKGROUND: Postoperative spinal epidural hematomas (pSEHs) are a rare complication of microdiscectomy surgery. The hematoma may be unnoticed intraoperatively, but timely treatment may prevent permanent neurologic impairment. Airway management in patients with a full stomach is generally performed with rapid sequence intubation and general anesthesia. Awake spine surgery without intravenous analgesia or sedation may be beneficial in patients with a full stomach who are at higher risk for pulmonary aspiration with general anesthesia due to a loss of non-per-oral (NPO) status. The authors propose that it can also be performed in cases of urgent/emergent postsurgical epidural hematoma evacuation. METHODS: We present the airway management of a 41-year-old man who underwent a minimally invasive microdiscectomy with normal strength immediately after surgery but developed progressive weakness with right foot dorsiflexion, right extensor hallucis longus muscle weakness, and progressive right lower extremity ascending numbness over the course of the first 2 hours after surgery due to an epidural hematoma. RESULTS: The patient underwent urgent awake epidural hematoma evacuation with a spinal anesthetic. Afterward, the patient recovered neurological function and was discharged the following morning. CLINICAL RELEVANCE: pSEHs are a rare complication of microdiscectomy surgery. The purpose of this article is to describe the novel use of awake spine surgery in emergent epidural hematoma evacuation and demonstrate its feasibility. CONCLUSIONS: In emergencies, when a patient is not NPO, awake spine surgery can safely be performed with no sedation, ensuring the patient can protect their airway and avoid the risk of aspiration.
Duke Scholars
Published In
Publication date, start / end page, related subject headings.
- 3202 Clinical sciences
- 1109 Neurosciences

IMAGES
VIDEO
COMMENTS
From early days of case reports, literature review is been the most important cornerstone of a case report. Especially since earlier only rare cases were considered as case reports . So to establish the rarity of the case it was important to thoroughly review the literature. For us at Journal of Orthopaedic Case Report, literature review is one ...
The definition of a case report or a case series is not well defined in the literature and has been defined variously by different journals and authors. However, the basic definition of a case report is the detailed report of an individual including aspects like exposure, symptoms, signs, intervention, and outcome.
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
When writing a title, it may be best to avoid terms such as "case report," "review of literature," "unique," "rare," "first-report"; these do not add value to the presentation. Introduction. This must introduce the condition and clearly state why the case report is worth reading. It may also contain a brief mention of the ...
Sometimes case reports include a short literature review, if you think it is worthwhile, include it. 2. Describe your patient and follow ... case report for publication; step by step. J Dent Med ...
Sometimes case reports include a short literature review, if you think it is worthwhile, include it. 2. Describe your patient and follow the diagnostic pathway. For example - Patient X is a 10 ...
A literature review can be just a simple summary of the sources, but it usually has an organizational pattern and combines both summary and synthesis. A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information.
Andreoiu M, Drachenberg D, Macmahon R (2009) Giant renal leiomyoma: a case report and brief review of the literature. Can UrolAssoc J 3(5):58-60. Google Scholar Nakib G, Mahgoub N, Calcaterra V, Pelizzo G (2017) Renal leiomyoma in pediatric age: a rare case report with review of the literature. J PediatrSurg Case Rep 27:43-46
Literature review Explore the literature/news/internet sources to know the topic in depth; Give a description of how you selected the literature for your project; Compare the studies, and highlight the findings, gaps or limitations. Case study An in-depth, detailed examination of specific cases within a real-world context.
Table 1 Levels of evidence The definition of a case report or a case series is not well defined in the literature and has been defined variously by different journals and authors. However, the ...
Keywords: severe bullous skin reactions, literature review, case report, toxic epidermal necrolysis, cervical cancer, PD-1. Citation: Li X, Qu L-X, Ren Y-M and Hu C (2021) Case Report: A Case Report and Literature Review on Severe Bullous Skin Reaction Induced by anti-PD-1 Immunotherapy in a Cervical Cancer Patient. Front.
We describe a young immunocompetent man who experienced prompt resolution of an asymptomatic acute PVT in a highly likely case of CMV infection and review the available evidence of the natural history of acute CMV-mediated PVT. Case report. A 34-year-old white man with an echographic diagnosis of PVT was admitted to a tertiary care hospital ...
We will focus on post-SARS-CoV2 infection arthritis which may arise as a new pathological condition associated with COVID-19. In this article, we describe a case of acute oligoarthritis occurring 13 days after a SARS-CoV2 severe pneumonia in a middle-aged Caucasian man and we go over a brief review of the current available literature. We ...
an abstract, an introduction with a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, and a brief summary of the case and a conclusion.21,22 Tables, figures, graphs, and illustrations comprise the supplementary parts and will enhance the case report's flow and ...
Case Report; Open access; Published: 02 April 2024; Infection may play an important role in the pathogenesis of alveolar osteonecrosis following facial herpes zoster: a case report and literature review. Kaikai Huang 1, Youyuan Wang 2, Yuhua Huang 1, Shanshan Han 1, Yu Yang 1, Pinghua Qu 1, Baoying Liang 1, Qingyu Zhen 1, Wenting Chen 3 ...
Background Piriformis muscle mass is rare, which is particular for intrapiriformis lipoma. Thus far, only 11 cases of piriformis muscle mass have been reported in the English literature. Herein, we encountered one patient with intrapiriformis lipoma who was initially misdiagnosed. Case presentation The patient is a 50-year-old Chinese man. He complained of osphyalgia, right buttock pain, and ...
We present a case of cortical laminar necrosis after severe hydrocephalus to highlight considerations for multimodal cerebral autoregulation monitoring to determine mean arterial pressure (MAP) thresholds during neurological emergencies, as well as postoperative head imaging for patients with ventriculoperitoneal shunts (VPS).
DOI: 10.1016/j.heliyon.2024.e28511 Corpus ID: 268745905; Sebaceous hyperplasia of the eyelid: A comprehensive case report and literature review @article{Ma2024SebaceousHO, title={Sebaceous hyperplasia of the eyelid: A comprehensive case report and literature review}, author={Mingshen Ma and Rui Liu and Jing Li and Hang Yang and Runzi Yang and Jian-Min Ma}, journal={Heliyon}, year={2024}, url ...
We report a novel variant in exon 5 (186 nucleotides) of the ASNS gene. Previous studies reported three mutations in exon 5; however, these variants were compound heterozygous (Chen et al., 2019; Schleinitz et al., 2018; Staklinski et al., 2023), and our case is the first homozygous pathogenic variant in exon 5. One reason that might explain ...
Airway Management for Emergency Spinal Epidural Hematoma Evacuation With Awake Spine Surgery: Case Report and Literature Review. Int J Spine Surg. 2024 Mar 4;18(1):69-72. Published In. Int J Spine Surg. DOI. 10.14444/8569. ISSN. 2211-4599. Publication Date. March 4, 2024. Volume. 18. Issue. 1. Start / End Page. 69 / 72.