Our latest white paper on Literature Monitoring Challenges and Opportunities is out. Get it here!

- Oct 11, 2022

Pharmacovigilance Case Studies from the Scientific Literature
Updated: Nov 8, 2023
At this year's World Drug Safety Conferences in Amsterdam and Boston, biologit presented two case studies detecting relevant safety events from the scientific literature using biologit MLM-AI's unique models fine-tuned for pharmacovigilance.
In this article, we share the case study results and the full presentations in case you missed this year's event.
The Value of Open Access for Safety Data - A COVID-19 Case Study
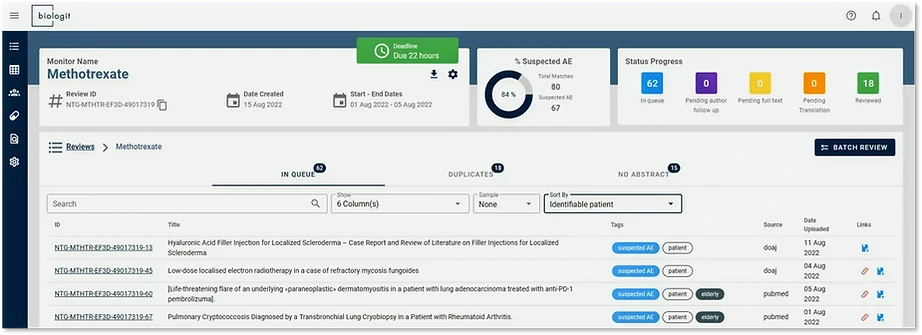
Biologit MLM-AI includes high quality scientific databases out of the box , including PubMed and two large open access databases: Crossref and DOAJ .
We conducted a search for Covid-19 vaccines from June 2021 to April 2022. To reduce screening volume, the study took advantage of MLM-AI's automatic article de-duplication and AI-based screening capabilities .
The goal was to quickly narrow down to potential ICSR articles. Out of 9948 obtained hits, a total of 1946 unique articles ( 19% ) were found from open access literature. From those 359 (18% ) were flagged by MLM-AI as containing a suspected safety event, and finally 24 valid ICSRs were identified coming exclusively from open access sources (Crossref or DOAJ):

Below are two examples of ICSR articles found in the open access literature as part of this study:

"COVID vaccine related lower limb gangrene: the first case report"
International Surgery Journal (India), Oct 2021
"Bilateral adrenal haemorrhage with renal infarction after ChAdOx1 nCoV-19 AstraZeneca vaccination"
BIR Case Reports (UK), January 2022
👉 Get the complete presentation from World Drug Safety Conference Europe (Amsterdam)
Case Study: CAR-T Cells
The second case study presented at World Drug Safety Congress US (Boston) involved an exploratory analysis of CAR-T Cell therapies. The study involved searching the literature from January 2022 to August 2022 using biologit MLM-AI, and taking advantage of the tool's AI capabilities to zoom in on the most relevant articles:

Once results were filtered for articles with relevant safety data, the team was able to conduct an analysis of events reported in each study (full details in the presentation below).
👉 Get the complete presentation from World Drug Safety Conference Americas (Boston)
More Case Studies
To learn more, check out these other case studies produced by the biologit team:
The power of open access adverse events searches - a case study with Crossref and DOAJ by Naveen Basar .
The use of AI in Biomedical Literature as a Trigger to Identify Antimicrobial Resistance by Natasha Troy
About biologit MLM-AI
Biologit MLM-AI is a complete literature screening platform built for pharmacovigilance teams. Its flexible workflow, unified scientific database, and unique AI productivity features deliver fast, inexpensive, and fully traceable results for any screening needs.
- Biologit: Case studies
Recent Posts
The power of open access adverse events searches - a case study with Crossref and DOAJ
The use of AI in Biomedical Literature to Identify Antimicrobial Resistance
Comentarios

Encyclopedia of Evidence in Pharmaceutical Public Health and Health Services Research in Pharmacy pp 1–12 Cite as
Pharmacovigilance to Inform Drug Safety: Challenges and Opportunities
- Satabdi Chatterjee 2 &
- Rajender R. Aparasu 3
- Living reference work entry
- First Online: 14 January 2023
45 Accesses
Pharmacovigilance is vital to ensure the short-term and long-term safety of medications and other medicinal substances. Both active and passive pharmacovigilance surveillance systems have an important role to play in pharmacovigilance. Therefore, the scope of pharmacovigilance has also increased across the world due to changes in regulatory and nonregulatory processes. This chapter describes the activities and role of pharmacovigilance to inform drug safety and discusses the challenges and opportunities in this field. The challenges include changing regulatory process, lack of standardization of data systems for evaluation, the safety of herbal and combination medications, and medications for rare diseases. With the advent of improved methods to evaluate drug safety, availability of real-world data, advanced methods such as machine learning approaches, and other technical tools, the opportunities in pharmacovigilance have increased. Overall, there exists a strong future for pharmacovigilance to improve the safety of medications.
- Pharmacovigilance
- Drug safety
- Opportunities
This is a preview of subscription content, log in via an institution .
Ahmad A, Patel I, Sanyal S, Balkrishnan R, Mohanta GP. A study on drug safety monitoring program in India. Indian J Pharm Sci. 2014;76(5):379–86.
Google Scholar
Aparasu RR, Chatterjee S, Mehta S, Chen H. Risk of death in dual-eligible nursing home residents using typical or atypical antipsychotic agents. Med Care. 2012;50(11):961–9. https://doi.org/10.1097/MLR.0b013e31826ec185 .
Article Google Scholar
Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317–8. https://doi.org/10.1001/jama.2017.18391 .
Beninger P. Pharmacovigilance: an overview. Clin Ther. 2018;40(12):1991–2004. https://doi.org/10.1016/j.clinthera.2018.07.012 . Epub 2018 Aug 17
Article CAS Google Scholar
Chatterjee S, Bali V, Carnahan RM, Johnson ML, Chen H, Aparasu RR. Anticholinergic medication use and risk of dementia among elderly nursing home residents with depression. Am J Geriatr Psychiatry. 2016;24(6):485–95.
Chen JH, Asch SM. Machine learning and prediction in medicine – beyond the peak of inflated expectations. N Engl J Med. 2017;376(26):2507–9.
European Medicines Agency (EMA). Eudravigilance 2020. Available at: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance . Accessed Aug 2021
Fischbach LA, Martin BC. Cohort and case-control studies. In: Aparasu RR, Bentley JP, editors. Principles of research design and drug literature evaluation. 2e ed. McGraw Hill; 2020.
Future Market Insights (FMI) Outlook. Pharmacovigilance Market: Global Industry Analysis and Opportunity Assessment, 2015–2020. Available at: https://www.futuremarketinsights.com/reports/pharmacovigilance-market . Accessed Aug 2021.
Gliklich RE, Dreyer NA, Leavy MB, ed. Registries for Evaluating Patient Outcomes: A User’s Guide [Internet]. 3rd ed. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. Report No.: 13(14)-EHC111.
Health Canada. Canada vigilance adverse reaction online database. 2013. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-database/instructions-canada-vigilance-adverse-reaction-online-database.html#a1 . Accessed Aug 2021.
Kim JH, Scialli AR. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci. 2011;122:1–6.
Malikova MA. Practical applications of regulatory requirements for signal detection and communications in pharmacovigilance. Ther Adv Drug Saf. 2020;
Martin LG, Hanssens Y, Paudyal V. Overview of this issue: pharmacovigilance, what is new? Int J Clin Pharm. 2018;40(4):737–9. https://doi.org/10.1007/s11096-018-0719-4 .
Mort JR, Shiyanbola OO. Case reports and case series. In: Aparasu RR, Bentley JP, editors. Principles of research design and drug literature evaluation. 2e ed. McGraw Hill; 2020.
Murali K, Kaur S, Prakash A, Medhi B. Artificial intelligence in pharmacovigilance: practical utility. Indian J Pharmacol. 2019;51(6):373–6.
Murray MD. Use of data from electronic health records for pharmacoepidemiology. Curr Epidemiol Rep. 2014;1:186–93.
National Institutes of Health (NIH). List of Registries. 2021. Available at: https://www.nih.gov/health-information/nih-clinical-research-trials-you/list-registries . Accessed Aug 2021.
Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a Clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140:795–801.
Platt R, Brown JS, Robb M, McClellan M, Ball R, Nguyen MD, Sherman RE. The FDA sentinel initiative – an evolving National Resource. N Engl J Med. 2018;379(22):2091–3.
Safety Guidelines – International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). Available at: https://www.ich.org/page/safety-guidelines . Accessed Aug 2021.
Sardella M, Belcher G. Pharmacovigilance of medicines for rare and ultrarare diseases. Ther Adv Drug Saf. 2018;9(11):631–8.
Shaw D, Graeme L, Pierre ED, Kelvin C. Pharmacovigilance of herbal medicine. J Ethnopharmacol. 2012;140:513–8.
Strom B. Overview of automated databases in pharmacoepidemiology. In: Brian L, Strom SEK, Hennessy S, editors. Pharmacoepidemiology. 5th ed. Philadelphia: Wiley; 2012.
Chapter Google Scholar
Talbot J, Nilsson B. Pharmacovigilance in pharmaceutical industry. Br J Clin Pharmacol. 1998;45:427–31.
The Framingham Heart Study. Available at: https://framinghamheartstudy.org/files/2021/07/FHS-Laying-the-Foundation-from-NIH.pdf . Accessed Aug 2021
The Observational Health Data Sciences and Informatics (OHDSI). 2021. Available at: https://ohdsi.org/ . Accessed Aug 2021.
US Food and Drug Administration. FDA adverse event reporting system latest quarterly data files. 2014. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm . Accessed Jul 2021.
US Food and Drug Administration. Providing Postmarket periodic safety reports in the ICH E2C(R2) format (periodic benefit-risk evaluation report) – Food and Drug Administration 2016. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/providing-postmarket-periodic-safety-reports-ich-e2cr2-format-periodic-benefit-risk-evaluation . Accessed Aug 2021.
Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, eds. Developing a protocol for observational comparative effectiveness research: a User’s guide. AHRQ Publication No. 12(13)-EHC099. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Available at: www.effectivehealthcare.ahrq.gov/Methods-OCER.cfm . Accessed Aug 2021.
WHO: International Drug Monitoring: The Role of the Hospital. Technical Report Series No. 425. Geneva: WHO, 1969.
WHO. The importance of pharmacovigilance – safety monitoring of medicinal products. Geneva: World Health Organization; 2002. Available at: http://apps.who.int/medicinedocs/en/d/Js4893e . Accessed Aug 2021.
WHO. A practical handbook on the pharmacovigilance of antiretroviral medicines. Geneva: World Health Organization; 2009. Available at: http://apps.who.int/iris/bitstream/handle/10665/44236/9789241547949_eng.pdf?sequence=1 . Accessed Aug 2021.
WHO Toolkit. Module 10. Pharmacovigilance. Available at: https://www.who.int/hiv/pub/10.pdf . Accessed Jul 2021.
WHO VigiBase. Available at: https://www.who-umc.org/vigibase/vigibase/ . Accessed Jul 2021.
Download references
Author information
Authors and affiliations.
Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA
Satabdi Chatterjee
Department of Pharmaceutical Health Outcomes and Policy, College of Pharmacy, University of Houston, Houston, TX, USA
Rajender R. Aparasu
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Rajender R. Aparasu .
Section Editor information
Pharmacy and Pharmacology, University of Bath, Bath, United Kingdom
Prasad Nishtala
Rights and permissions
Reprints and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this entry
Cite this entry.
Chatterjee, S., Aparasu, R.R. (2022). Pharmacovigilance to Inform Drug Safety: Challenges and Opportunities. In: Encyclopedia of Evidence in Pharmaceutical Public Health and Health Services Research in Pharmacy. Springer, Cham. https://doi.org/10.1007/978-3-030-50247-8_33-1
Download citation
DOI : https://doi.org/10.1007/978-3-030-50247-8_33-1
Received : 06 June 2022
Accepted : 11 June 2022
Published : 14 January 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-50247-8
Online ISBN : 978-3-030-50247-8
eBook Packages : Springer Reference Biomedicine and Life Sciences Reference Module Biomedical and Life Sciences
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
Pharmacovigilance Analytics
Your best resource for PV analytics news, content and innovation!
Individual Case Safety Reports (ICSR) in Pharmacovigilance: Essential Tools for Drug Safety Monitoring
December 25, 2023 by Jose Rossello 2 Comments
Pharmacovigilance plays a critical role in ensuring the safety and efficacy of drugs, as well as the ongoing monitoring of their potential adverse effects. One integral component of pharmacovigilance is the Individual Case Safety Report (ICSR), which consists of detailed information on adverse drug reactions (ADRs) or other drug-related problems reported by patients, healthcare professionals, or pharmaceutical companies. These reports serve as valuable sources of data for regulatory authorities, enabling them to continually assess and manage potential risks and benefits associated with medicinal products.
The management and processing of ICSRs are critical aspects of pharmacovigilance operations, from collection and data entry to evaluation and clinical review . Ensuring the quality and accuracy of ICSRs is essential for identifying new safety signals and making informed decisions about drug safety . With advanced technologies , such as machine learning and artificial intelligence , the processing of ICSRs has become more efficient and streamlined, facilitating the timely detection of emerging safety concerns and aiding pharmacovigilance professionals in their work.
Key Takeaways
- ICSRs play a crucial role in pharmacovigilance, providing essential data on adverse drug reactions and safety concerns.
- Proper management and processing of ICSRs contribute significantly to the identification of new safety signals and informed decision-making.
- Advancements in technology have streamlined the handling of ICSRs, improving efficiency and facilitating timely detection of safety issues.
Basics of ICSR
Definition and purpose.
Individual Case Safety Reports (ICSR) are crucial components of pharmacovigilance , the science of monitoring and analyzing the safety of medicines . The primary objective of ICSRs is to detect, assess, and prevent adverse drug reactions (ADRs) that may occur during the use of a medicinal product. By gathering data on patients’ adverse events , ICSRs play a vital role in identifying new safety signals and enhancing the knowledge of already-known risks associated with specific drugs.
Types of ICSRs
There are two main types of ICSRs: expedited and non-expedited. Expedited ICSRs refer to reports of serious or unexpected ADRs that require prompt attention and action from regulatory authorities. Non-expedited ICSRs, on the other hand, collect information on non-serious or expected adverse events and are submitted according to a predefined schedule.
ICSRs can further be classified based on their source, such as spontaneous reports (submitted voluntarily by patients, healthcare professionals, or manufacturers) and solicited reports (collected through clinical trials, post-authorization safety studies, or patient registries).
Components of an ICSR
An ICSR must contain certain essential components for it to be valid and useful for pharmacovigilance purposes. These components are:
- Identified Patient : The patient who experienced the adverse event must be clearly identified, either by initials, demographics, or a unique code.
- Medicinal Product : The drug or medicinal product associated with the adverse event must be specified, including its brand name and active ingredients.
- Adverse Event(s) : A description of the adverse events experienced by the patient, including the nature, severity, and duration of the reaction.
- Source : The source of the report, such as spontaneous or solicited, and the reporter’s contact information (e.g., healthcare professional, patient, or regulatory authorities).
A well-constructed ICSR should also include a report title , which summarizes the main aspects of the case, and a case narrative , providing a detailed account of the patient’s experience, including the sequence of events, medical history, and any relevant laboratory results or diagnostic procedures.
ICSRs are submitted to national and regional pharmacovigilance centers and serve as an essential tool for ensuring patient safety and the effective monitoring of drug safety profiles.
ICSR Management Process
Case intake and triage.
The first step in managing Individual Case Safety Reports (ICSRs) is case intake and triage. During this stage, pharmacovigilance professionals receive ICSRs from various sources, including healthcare professionals and consumers. After receiving the reports, they perform an initial assessment to classify them based on factors such as seriousness, listedness, and causality. The classification helps prioritize which cases require immediate attention and further investigation.
Data Entry and Coding
Once the ICSRs are classified, pharmacovigilance professionals proceed to data entry and coding. They input the relevant data elements from the reports into a database, ensuring the information is accurately captured. During this process, standardized coding terminologies, such as MedDRA, are utilized to translate medical and drug-related information into coded data.
- Data elements : demographic information, reporter details, adverse event description, drug information, medical history, and other relevant details.
- Coded data : ensures consistency, enables better data analysis, and facilitates communication between various regulatory authorities and organizations.
Medical Review
After data entry and coding, a medical reviewer, typically a healthcare professional with specialized training in pharmacovigilance, conducts a medical review of the ICSRs. This review process involves:
- Verification of the coded data and case narratives.
- Evaluation of the seriousness assessment and re-evaluating, if necessary.
- Assessing the listedness of the adverse event, comparing it to the approved product label and established safety profiles.
- Establishing causality between the drug and the reported adverse event, which may involve reviewing medical history and concomitant medications.
The medical review helps ensure the accuracy and completeness of the data, as well as identify any potential new safety signals.
Quality Control
The final stage in the ICSR management process is quality control (QC). During this phase, another pharmacovigilance professional conducts an independent review of the case to verify the accuracy, consistency, and completeness of the information. They check the following components:
- Data quality: ensuring all the necessary data elements are captured, and the coded data is accurate.
- Documentation grading: evaluating whether the case documentation is complete and properly graded based on source (e.g., healthcare professional, consumer) and nature of the event (e.g., serious, non-serious).
- Quality check: identifying and addressing any discrepancies or missing information before the ICSR is finalized and submitted to the relevant regulatory authorities.
Implementing a robust ICSR management process helps ensure the integrity of the data used in pharmacovigilance activities, leading to better identification of safety signals and more effective action by regulatory authorities.
ICSR Data Standards
International standards and guidelines.
The Individual Case Safety Reports (ICSR) in pharmacovigilance play a vital role in monitoring and evaluating the safety of medicinal products. To ensure consistency, reliability, and efficient communication, international standards and guidelines have been established. The International Conference on Harmonisation (ICH) sets protocols for the content and structure of ICSRs, providing unified data elements and terminologies.
Electronic Transmission Specifications
In order to streamline the reporting process and facilitate the exchange of ICSR data between different pharmacovigilance agencies, electronic transmission specifications have been developed. The electronic format for ICSR data communication is governed by the ISO/HL7 27953 standard. This international standard provides a comprehensive framework for the exchange of safety information in a secure and efficient manner.
The use of electronic submissions enables regulatory authorities and pharmaceutical companies to improve their pharmacovigilance activities. It helps reduce manual intervention, error rates, and processing times, leading to more efficient safety reporting.
Data Quality and Completeness
Ensuring high-quality data is essential for maintaining the integrity of ICSRs and deriving meaningful insights from them. To promote the accuracy and consistency of ICSR data, the completeness score is measured. A completeness score is an indicator that assesses the quality and coverage of the data elements present in an ICSR.
Several data quality issues can arise during the collection, processing, and analysis of ICSRs. Identifying and mitigating these systematic data quality issues is crucial for improving the overall quality of ICSR data. Some factors that contribute to data quality include:
- Timeliness of submissions
- Consistency in data capture methods
- Appropriate coding of adverse events
- Accurate documentation of relevant medical history
Implementing standard practices, training programs, and periodic data quality assessments can help address these issues, thereby ensuring the reliability and effectiveness of ICSRs in pharmacovigilance.
ICSR in Regulatory Context
Individual Case Safety Reports (ICSRs) play a crucial role in the regulatory context of pharmacovigilance, ensuring the safety and efficacy of medicinal products. ICSRs are submitted to regulatory authorities for both pre-market review and approval, as well as post-market safety surveillance. In this section, we will examine the importance of ICSRs in these different stages and look into the most relevant pharmacovigilance legislation.
Post-Market Safety Surveillance
Post-market safety surveillance entails monitoring the adverse drug reactions (ADRs) and other safety-related issues in medicinal products once they are approved for use. ICSRs become an indispensable tool for health authorities, medical professionals, and pharmaceutical companies to detect and evaluate possible safety signals in the post-market phase.
One example of a post-market safety surveillance system is the FDA Adverse Event Reporting System (FAERS), which collects and analyzes ICSRs from healthcare professionals, patients, and manufacturers. Here, the ICSRs are thoroughly assessed by the regulators to determine if a safety signal warrants further actions such as product labeling updates, additional post-market studies, or even a product recall in severe cases.
Pre-Market Review and Approval
Before a medicinal product can be released in the market, it undergoes rigorous pre-market review and approval processes by regulatory bodies such as the FDA. During this stage, ICSRs serve as crucial data sources for safety assessments, helping the authorities decide whether to approve, modify, or reject a specific drug.
Pharmaceutical companies submit ICSRs as part of their regulatory submissions package, which also includes preclinical and clinical trial data, to showcase the drug’s safety profile. Regulators meticulously scrutinize the submitted ICSR data to evaluate the benefit-risk balance and make informed decisions on the suitability of a product for market release.
Pharmacovigilance Legislation
To ensure compliance and maintain a high level of safety in drug development and distribution, several pharmacovigilance legislations have been established globally. These laws and regulations provide a framework for reporting requirements, safety assessments, and post-market actions. They mandate the timely submission of ICSRs by the concerned parties, such as pharmaceutical companies and health professionals, ensuring that potential safety issues are dealt with promptly and effectively.
As an example, the FDA has established a Safety Reporting Portal where ICSRs can be submitted electronically, streamlining the reporting process and making it more efficient. Appropriate adherence to these legislations not only guarantees the safety and efficacy of approved products but also contributes to fostering a strong culture of pharmacovigilance and vigilance in the medical community.
In conclusion, ICSRs hold a pivotal position in the regulatory context of pharmacovigilance. They are crucial in both post-market safety surveillance and pre-market review and approval, ensuring that medicinal products uphold high safety standards. Strict adherence to pharmacovigilance legislation and timely submission of ICSRs play a vital role in safeguarding public health and minimizing the potential risks associated with medicinal products.
Signal Detection and Management
Identification of safety signals.
In pharmacovigilance, the continuous monitoring of Individual Case Safety Reports (ICSRs) is essential for detecting potential safety signals. A safety signal refers to new information about a drug that suggests there may be an association between the drug and an adverse event. Signal detection relies on ICSRs and safety databases to identify new safety signals and assess their impact on public health. Advancements in technology, such as VigiFlow and VigiLyze, have improved the ways these signals are managed and assessed for potential risks.
Safety signals can be detected through various methods, including the review of ICSRs, statistical analysis, and the use of machine learning algorithms . These methods allow pharmacovigilance experts to identify and track new safety signals and proactively address any potential risks.
Assessment and Prioritization
Once a safety signal is identified, it’s essential to assess and prioritize it to determine its clinical significance. Assessment involves evaluating the strength of the signal and the quality of available data to establish whether a causal relationship exists between the drug and the adverse event. Various tools and approaches are available for signal assessment, such as the clinical utility score for prioritization and the VigiGrade tool, which evaluates the quality of ICSRs.
In addition, the severity of the potential adverse event and the number of affected patients are considered when prioritizing signals. High-priority safety signals typically warrant further investigation and possible regulatory action to protect public health.
Regulatory Action and Communication
Following the assessment and prioritization of a safety signal, appropriate regulatory actions may be taken to mitigate the associated risks. Actions can range from updating product labels and issuing drug safety alerts to requesting additional post-marketing studies or, in severe cases, withdrawing the drug from the market.
Communication plays a vital role in managing safety signals effectively. Regulatory agencies, such as the World Health Organization, often collaborate with national pharmacovigilance centers to monitor and share safety information. The communication of emerging safety signals between organizations and healthcare professionals is crucial to ensure timely action to protect public health.
In conclusion, ICSRs are an essential component of pharmacovigilance and play a vital role in signal detection and management. The constant monitoring and evaluation of safety signals help ensure the safety of drugs and contribute to maintaining public trust in healthcare systems.

Special Considerations
Vaccines and pharmacovigilance.
Vaccines play a critical role in public health, making their safety monitoring a top priority. Within the field of pharmacovigilance, specific attention is given to vaccine safety through systems like the Vaccine Adverse Event Reporting System (VAERS) . VAERS serves as a database for collecting reports of adverse events associated with vaccines. The submission of individual case safety reports (ICSRs) in the context of vaccines helps to identify potential safety issues and enables health authorities to take timely and appropriate actions.
Unique Identification Challenges
One challenge in the ICSR process is the identification of patients and reporters. Ensuring that the identifiable patient and identifiable reporter information is accurate and complete is crucial for the analysis of adverse events. Confidentiality concerns might lead to incomplete patient profiles or missing reporter details, hindering the correct assessment of the event. Careful handling of these sensitive data points while retaining the essential information for analysis is a delicate balance that must be achieved in pharmacovigilance.
An example of the information to be collected and verified includes:
- Patient demographics (age, gender)
- Reporter’s contact information
- Drug or vaccine details
- Adverse events
Event-Based Reporting Nuances
Pharmacovigilance reporting often deals with specific events such as an overdose or an unexpected adverse event. In these cases, ICSRs provide insight into the occurrence of such incidents and contribute to the overall understanding of potential risks.
In the case of an overdose or unexpected events, the following information should be included in the ICSR:
- Description of the event
- Dose and frequency of drug or vaccine administration
- Medical history and concomitant medications
- Clinical course and patient outcome
By considering these special aspects when managing ICSRs, the overall process of pharmacovigilance becomes more efficient and effective in ensuring the safety and well-being of patients using various medical products.
ICSR Systems and Operations
Pharmacovigilance databases.
Individual Case Safety Reports (ICSR) are essential components of a robust pharmacovigilance program. These reports aid in identifying and evaluating potential safety signals in medicines and healthcare products. Pharmaceutical companies and regulatory authorities rely on comprehensive safety databases to collect, manage, and analyze ICSRs. Safety databases serve as the main repositories for the vast amount of data that arise from adverse event reporting.
Some of the key features of safety databases include:
- Data standardization : Ensuring data consistency with globally accepted formats, like electronic submissions.
- Data integrity : Protecting data from unauthorized access, and maintaining accuracy and reliability during data management processes.
- Efficient data retrieval : Providing advanced search capabilities to allow users to access and extract data easily.
Automation and Technological Advances
As the volume of ICSRs increases, pharmacovigilance operations need to embrace automation and technological advancements to enhance efficiency and manage the workload. One notable development in this space involves integrating deep learning approaches and artificial intelligence ( AI ) to automate ICSR processing 1 . This not only accelerates the process but also improves detection accuracy and operational efficiency.
Alongside AI, technological advancements such as natural language processing (NLP), predictive analytics , and advanced algorithms enhance the quality of data analysis, reduce manual intervention, and optimize the pharmacovigilance process . Many pharmaceutical firms and regulators have started exploring and adopting these innovations.
Global and Regional Practices
Pharmacovigilance practices vary across different countries due to diverse regulatory requirements and local practices. For instance, the Pharmacovigilance Programme of India (PvPI) operates under the Indian Pharmacopoeia Commission and the Ministry of Health and Family Welfare, Government of India 2 . It maintains a centralized database for managing ICSRs submitted by various organizations, focusing on promoting patient safety and ensuring efficient reporting processes nationwide. The success of regional pharmacovigilance operations, such as PvPI, depends on capabilities to adapt global best practices while addressing local needs and requirements.
In conclusion, the ICSR systems and operations play a crucial role in the overall pharmacovigilance landscape. With modern technologies and efficient practices, these systems ensure patient safety and build the foundation for a safer healthcare environment.
- [HTML] Training augmented intelligent capabilities for pharmacovigilance : applying deep-learning approaches to individual case safety report processing ↩
- [PDF] A REVIEW ON INTRODUCTION TO PHARMACOVIGILANCE AND CASE STUDIES OF INDIVIDUAL CASE SAFETY REPORTS FROM DIFFERENT SOURCE ↩
Frequently Asked Questions
What constitutes a valid individual case safety report for submission.
A valid Individual Case Safety Report (ICSR) for submission in pharmacovigilance consists of specific and detailed information. This generally includes an identifiable reporter, a description of the adverse event, a clear link to the medicinal product, and relevant patient data. The report should be well-documented, following a standardized format to ensure high-quality data for further analysis.
Which data sources are typically utilized for generating Individual Case Safety Reports?
Various data sources contribute to generating ICSRs. Some common sources include spontaneous reports from healthcare professionals or patients, case reports from clinical trials, literature reviews, and post-marketing surveillance studies. These sources provide valuable insights and help in identifying new safety signals in pharmacovigilance.
What are the key benefits of submitting and analyzing Individual Case Safety Reports?
Submitting and analyzing ICSRs plays a crucial role in pharmacovigilance. It aids in identifying new safety signals and strengthening the existing safety profile of medicinal products. Analyzing ICSR data helps in detecting potential adverse drug reactions, thereby protecting public health and improving patient safety. Furthermore, it informs regulatory actions and contributes to the development of risk mitigation strategies.
How are Individual Case Safety Reports integrated into the broader scope of pharmacovigilance activities?
ICSR management is a critical component of pharmacovigilance activities. Integration of ICSR data, when combined with other sources like drug utilization studies and epidemiological research, helps in developing a comprehensive understanding of drug safety profiles. This integrated approach enables better decision-making for regulatory actions, risk evaluation, and effective communication of safety information to healthcare professionals and patients.
What are the mandatory reporting timeframes for ICSRs in post-marketing surveillance and clinical trials?
For ICSRs in post-marketing surveillance, the mandatory reporting timeframe might vary depending on jurisdiction. However, the general guideline requires that serious adverse events be reported within 15 days and non-serious adverse events within 30 to 60 days. Regarding clinical trials, ICSRs should be reported within 7 days for fatal or life-threatening events and within 15 days for other serious events.
What career opportunities exist within the field of ICSR management and analysis?
Careers within the field of ICSR management and analysis include roles such as pharmacovigilance associates, drug safety officers, and safety data analysts. These professionals are responsible for handling ICSR-related tasks like data processing, quality control, signal detection, and reporting, as well as collaborating with regulatory authorities and other healthcare stakeholders. Opportunities exist across pharmaceutical companies, contract research organizations, and regulatory bodies.
- Term: Clinical Review
- Term: Drug Safety
- Term: Solicited Reports
- Term: Clinical Trial
- Term: Signal Detection
- Term: Individual Case Safety Report (ICSR)
- Term: Signal
Reader Interactions
[…] last subsection of the PADER includes Individual Case Safety Reports (ICSRs), which represent detailed descriptions of individual adverse drug experiences. These […]
[…] inclusive of all new or updated safety information. It should detail exposure data, summarize individual case safety reports (ICSRs), describe study results, and outline any changes in the benefit-risk […]
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

PHARMACOVIGILANCE-AN EMERGENCE A Case Study

Pharmacovigilance (PV), defined by the world health organization (WHO) as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. Emergence of PV activities and the awareness would play a vital role in ensuring that doctors, healthcare professional, together with the patient, have enough information to make an educated decision when it comes to choosing a drug for treatment and eventually achieving the patient safety in large.
Related Papers
International Journal of Pharmacovigilance
Vrish Ashwlayan
International Journal of Advanced Research
Vikram Musale
Priti Vohra
IJPSM Journal , HM MAHAJAN
The Pharmacovigilance Programme of India (PvPI) was launched by Central Drug Standard Control Organization under the tutelage of Ministry of Health and Family Welfare, Government of India in July 2010. The main obstacle is under reporting which curbs the functioning of PvPI. AIMS AND OBJECTIVES: To identify practical approaches towards increasing effectiveness of PvPI in order to formulate a practicable rather than ideal on-paper plan. MATERIALS AND METHODS: After discussing various issues in regular clinical meets at our institute (NKPSIMS, Nagpur, M.S.) realistically implementable recommendations were taken into considerations without being overenthusiastic to contribute towards PvPI. Some of them were tested at our institute like compulsion for each undergraduate student to report at least two Adverse Drug Reactions (ADRs), lectures, Continued Medical Education (CME) and workshops on Pharmacovigilance. RESULTS: The results of these interventions were more than encouraging since we saw increased reporting, although results of some interventions were not quantitatively measurable. CONCLUSION: The present study has looked into several major aspects of the issue of underreporting of ADRs, and we suggest credible and practically executionable recommendations at each level of healthcare in order to increase reporting and help PvPI serve its purpose- to increase patient safety in terms of drug related problems. The present paper is a clamor for action by the medical fraternity and the regulatory establishments.
THE PROFESSIONAL MEDICAL JOURNAL
Saba Zubair
Israel Journal of Health Policy Research
Irene Fermont
International Journal of Advanced Research in Science, Communication and Technology
Sanjay Bais
In order for clinical practise, public health efforts, and effective drug regulatory systems to function effectively, pharmacovigilance—the term used to describe the processes for recording and analysing adverse drug reactions—must be implemented. A high level of skill is required to grasp pharmacovigilance in order to swiftly identify pharmacological dangers and to defend the product against an unjustified withdrawal. The volume of data handled has increased as a result of the reporting of number of the adverse drug reactions (ADRs). The present global network of pharmacovigilance centres, which is supervised by the Uppsala Monitoring Center, would be strengthened by an independent review procedure. This would consider disputed and important pharmaceutical safety problems that might have a detrimental effect on public health across international borders. Recently, the main goal of pharmacovigilance has been to identify previously unrecognised or poorly understood adverse drug react...
Pharmacovigilance [Working Title]
Rajvi Patel
Siniša Franjić
Pharmacovigilance is a set of activities related to the detection, assessment, understanding, prevention and treatment of drug side effects as well as new knowledge about the harmful effects of drug use. A side effect is any harmful and unwanted reaction to a drug. This includes side effects that occur as a result of overdose, misuse, abuse, medical errors, as well as side effects that result from occupational exposure. An unexpected adverse reaction is any adverse reaction whose nature, severity or outcome is not in accordance with that stated in the approved summary of product characteristics, or in the drug examiner instructions for medical products which are in the phase of clinical trials.
sarfaraz Kazi Masoodali
Pharmacovigilance is the science & activities involved in identifying recognizing, assessing, checking, & preventing adverse effect of pharmaceutical products. Pharmacovigilance is the science & activities of pharmacists, crucial in maintaining safe &reasonable medicine use. This study examines the perspective of drug store students on pharmacovigilance & ADR Announcement in under graduate drug store education. Clinical Research encompasses the entire process from lab to consumer market, identifying promising drug. Pre-clinical studies or animal studies examine safety, toxicity, & efficacy. Pharmacovigilance is crucial in healthcare, assessing drug interactions & their effect on human. Pharmacogenetics & pharmacogenomics are essential in Clinical Research, as they determine human genome variation & disease susceptibility, aiding early drug discovery. Pharmacovigilance is crucial tool used to monitor the side effects & related problems of medicines & vaccines, ensuring their safety & effectiveness in the market. The relevant details are presented in this Chapter. Key words: Pharmacovigilance, Clinical Trials, ADRs, Pharmaceutical Product, pharmacy, Safety, Toxicity, Efficacy, Marketing
RELATED PAPERS
Produsen Paving Block Termurah Di Pasirkuda Bogor
Produsen Paving Block Termurah
Rebecca Schild
ADLFI. Archéologie de la France - Informations
Denis Maréchal
Lourdes Angulo Salazar
Proceedings of the National Academy of Sciences
Merran Matthews
Anadolu Üniversitesi Sosyal Bilimler Dergisi
Sebnem Ozdemir
Surgery for Obesity and Related Diseases
Ashley Bohon
Harri Kiiskinen
Transgenic Research
Zeitschrift für Kristallographie Supplements
Ana Labrador
International Journal of New Education
Tania Katherine Sanchez Hurtado
Enfermedades Infecciosas y Microbiología Clínica
José Luis Pérez Arellano
Journal of Mechanical Design
G.Giridhar Reddy
CERN European Organization for Nuclear Research - Zenodo
Luis Eduardo Andrade Alcívar
Zbornik radova Uciteljskog fakulteta Prizren-Leposavic
Ljiljana Paunović
CADERNOS DE FINANÇAS PÚBLICAS
Paulo de Melo Jorge
Journal of Archaeological Method and Theory
Kalle Lindholm
sewa villa lembang bandung
Turkish Journal of Medical Sciences
Mehmet Akif Buyukbese
Francisco Maciá Pérez
Oral Presentations
Andreina Poggi
Orvosi Hetilap
Karoly Szuhai
Call Girls in Karkardooma
Call Girls in Delhi 9 5 6 0 2 6 6 9 1 4 Escorts Service
Francisco Javier Trigueros Cano
See More Documents Like This
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Welcome to Contract Pharma
Login join welcome to -->, subscribe free magazine enewsletter magazine enewsletter --> checkout.

The Future of Pharmacovigilance is Proactive
Data and AI can unlock the path to proactivity.

- Clinical Trials
- Drug Development
- Information Technology
- Prozomix and Ginkgo Bioworks Partner to Build Out Production of Next-Gen Enzyme Plates
- CytomX Achieves $5M Astellas Milestone
- Genmab to Acquire ProfoundBio for $1.8B
- Fanapt Approved by FDA for Treatment of Bipolar I Disorder
- Autolomous and BioCentriq Partner to Transform Operations
- Implementing an Automated DOE in Twin Screw Wet Granulation
- Sustainable Pharmaceutical Packaging
- Pre-filled Syringe Packaging Trends
- Liquid Pharmaceutical Filling: Small, Smaller, Micro Batches
- Newsmakers Q&A: BioVectra
- Multi-particulate Formulations – Tablets or Capsules?
- Manufacturing Equipment Trends
- OSD Outsourcing: Savvy CMOs Hone Skills as Complex APIs Fuel Demand
- Catalent Acquisition by Novo Holdings: Evolution of the Industry(?)
- The Road Ahead for CDMOs in 2024
Cookies help us to provide you with an excellent service. By using our website, you declare yourself in agreement with our use of cookies. You can obtain detailed information about the use of cookies on our website by clicking on "More information”. Got It
- Privacy Policy
- Terms And Conditions

Latest Breaking News From Nutraceuticals World

Latest Breaking News From Coatings World

Latest Breaking News From Medical Product Outsourcing

Latest Breaking News From Contract Pharma

Latest Breaking News From Beauty Packaging

Latest Breaking News From Happi

Latest Breaking News From Ink World

Latest Breaking News From Label & Narrow Web

Latest Breaking News From Nonwovens Industry

Latest Breaking News From Orthopedic Design & Technology

Latest Breaking News From Printed Electronics Now
Copyright © 2024 Rodman Media. All rights reserved. Use of this constitutes acceptance of our privacy policy The material on this site may not be reproduced, distributed, transmitted, or otherwise used, except with the prior written permission of Rodman Media.
AD BLOCKER DETECTED Our website is made possible by displaying online advertisements to our visitors. Please consider supporting us by disabling your ad blocker.

21. Narrative writing
Narrative writing is an important part of Pharmacovigilance and in patient safety as well.
A narrative is a brief summary of specific events experienced by patients, during the course of a clinical trial/treatment. Narrative writing involves multiple activities such as generation of patient profiles, review of data sources, and identification of events for which narratives are required.
To provide a concise summary of identified/specific adverse events (AEs) occurring in a patient to conclude causal relationship between the drug and event.
The objective of the narrative is to summarize all relevant clinical and related information, including patient characteristics, therapy details, prior medical history, clinical course of the event(s), laboratory evidence and any other information that supports or refuses a diagnosis for an ADR. The information should be presented in a logical time sequence.
Regulatory Perspectives:
The ICH guideline (E2B) on data elements and specifications for electronic reporting of individual ADR cases states that company narratives are required for all serious reactions.
Narratives are expected to be submitted for all cases reported expeditiously to any regulatory authority, but are useful and should be made available when needed for other types of reports and purposes.
As per International Conference on Harmonisation (ICH) E3, a patient narrative should describe :
- The nature, intensity and outcome of the event
- Clinical course leading to the event
- Timing of study drug administration
- Relevant laboratory measures
- Counter measures
- Action taken with the study drug in relation to the event
- Post mortem findings (if applicable)
- Investigator’s and sponsor’s opinion on causality
- Additionally, patient identifier, age, gender, clinical condition, disease being treated, relevant medical history, concomitant and prior medications should be included.
- All this information is extracted from the source files (e.g. Council for International Organisations of Medical Sciences [CIOMS] form.
Flow of Narrative:
- Report type and reporter information
- Patient demographics
- Patient medical history and concomitant medication information
- Suspect product information timing and conditions surrounding the onset of the reaction(s)
- Clinical course of the events, with an indication of timing of event corresponding to drug administration
- Nature, intensity/severity, and outcome of the event
- Relevant laboratory findings
- Treatment administered for the event
- Action taken with respect to the drug
- Dechallenge and rechallenge information, if applicable
- Postmortem findings (if applicable)
- Outcome of event.
- Clinical relation of event with suspect drug
- zthe original reporter’s clinical assessment
- The narrative preparer’s (sponsor’s) medical evaluation and comment.
Standard narrative template:
This initial [serious/non-serious] [spontaneous/literature] report, which originated from [country] was received by [Marketing Partner’s Name] on [date DD MMM YYYY].
Information has been received from a [reporter] concerning a [age] year old [male/female].
The patient’s medical history of [history, including the duration of concurrent illness, age at diagnosis, date of diagnosis or onset date not reported].
Concomitant therapy included [generic name of relevant concomitant drugs with/without indication if appropriate. Or provide a general statement such as subject was also receiving multiple concomitant medications, with or without indications (for example, he/she was taking multiple medications for pain, hypertension and depression). Note that concomitant medications include those medications taken within a reasonable time frame (30 days) prior to the onset of an adverse event and medications taken by the subject at the time of the reported adverse event.]
The patient commenced treatment with [SUSPECT PRODUCT], dose, frequency, on date for indication.
On [date MM DDD YYYY], the subject presented with event [detailed event description including signs, symptoms and details about hospitalisation including prolongation of existing hospitalisation]. which required hospitalisation.
The patient’s laboratory results on [DD MMM YYYY] were as follows: [list relevant physical findings, exams and laboratory results].
Corrective treatment for the event included [describe medications, procedures, tests, investigations etc.].
Action taken with suspect drug included (discontinued/temporarily stopped/dose decreased/dose increased/no change/unknown).
State outcome of event following treatment (recovered, unresolved,recovered with sequelae (describe sequelae), or death). Note if event resolved spontaneously].
Fatal patient outcome details: include date and cause of death, the timing of death in relation to the event onset and suspect drug therapy duration.
The reporter assessed the events as being serious/non serious [if serious, state reported seriousness criteria] physicians assessment of intensity of event (e.g. mild, moderate, severe) and relatedness of event to products include reported rationale for causality assessment.]
[Company’s medical assessment and comment: Include the facts that the company believe are relevant to the case].
For clinical trial cases narrative should start with:
- Protocol/Study ID: XXXX
- Study Title/Study description: Post-Marketing Surveillance of DRUG mg (ingredient) to Evaluate Its Safety and Efficacy.
- Screening number/ Randomization No: XXX-XX-XXX
- Patient ID/Subject ID: XXXX-XXXX
Follow-up Information:
When relevant new information becomes available, a follow-up narrative may need to be written depending on the amount and importance of the information. There are three options for incorporating the new information:
- Prepare an entirely new narrative
- Add new information in a separate additional paragraph
- Highlight in some way (e.g., bold or underline) the newly added follow-up material interspersed within the original narrative.
The Working Group’s preference is as follows:
Every effort should be made to blend the follow-up details into the original narrative, as usual in chronological order, to avoid repetition and contradictions.
Follow-up information received on [DD-MMM-YYYY] from [source] (or Source Data Verification received on…., or Data Correction Made on…., etc): (Briefly describe additions, corrections)
Important Points to Remember
- Narrative should be precise and concise.
- Double check spell mistakes, spaces, format, flow of narrative in
- agreed chronology
- Do not repeat the information.
- Avoid using short forms in narrative.
- Do not change the meaning of narrative by adding own supportive words/conclusion. The verbatim should be written as it is presented in source document. Whenever possible, the reporter’s exact (verbatim) words for the suspected adverse reaction(s) should be used. Use quotation marks to present strange verbatim terms.
- Use paragraphs to present narrative in style and logical format.
- Narratives should be written in the third person using the past tense.
- In general, abbreviations and acronyms should not be used. If used abbreviations should be expanded once. Relevant laboratory results are an exception but it is important that values be quoted in SI units, with an option to include additional units as well.
- Time to onset of an event from the start of treatment should generally be given in the most appropriate time units (e.g., days or hours or weeks), but actual dates can also be included if considered helpful to the reader.
- If detailed supplementary records are important to a case (e.g., an autopsy report), their availability should be mentioned in the narrative.
- Information may be provided by more than one person (e.g., original reporter plus supplementary information from a specialist); all source(s) of additional material should be specified.
- When there is conflicting information provided from different sources, this should be mentioned and the sources identified.
- If it is suspected that an adverse reaction resulted from misprescribing (e.g., wrong drug or wrong dose) or other medication error, judgmental comments should not be included in the narrative due to the legal implications. However, it is important to state the facts (e.g., ‘‘four times the normal dose had been administered,’’ ‘‘prescription was misread and a contraindicated drug for this patient was given,’’ etc.).
Example of a Standard Narrative Template:
Case reference number 12345678 is a spontaneous case report sent by a hospital pharmacist.
This report refers to an 84-year-old Caucasian male patient who experienced myocardial infarction while on qweasytrol.
The patient’s past medical history included gastric ulcer, asthma, and hypertension. At the time of the event the patient had Lyme Disease and severe headache. The patient previously took steroids (1990), cimetidine (1996), and tetracycline (09-Sep-1999). The patient had a history of allergy to penicillin and gin.
The patient started taking qweasytrol from 01-Jan-2000 at 1:00 PM, at an unspecified dose for vomiting. Some 12 hours later, and 10 minutes following the latest dose, the patient developed rash, dyspnea and queasiness. Over the period of the next two days, the patient also developed chest pain and later unconsciousness.
Relevant laboratory test results include elevated CK-MB and relevant physical signs were hypertension, fourth heart sound and bradycardia. The patient was hospitalized. Qweasytrol was discontinued on 08-Jan-2000. The eventual diagnosis made on the 10-Jan-2000 was myocardial infarction. The patient was treated for the event with a beta-blocker.
The patient died on 12-Jan-2000 from myocardial infarction; no autopsy was done. Death occurred 12 days after the treatment with qweasytrol began and 4 days after it was discontinued.
The cardiologist cited in the pharmacist’s report considered the myocardial infarction possibly related to qweasytrol. In his opinion, other possible etiological factors include hypertension and the patient’s age.
The company believes the following facts were also relevant in this case: as a highly selective epsilon G2 receptor antagonist, there was no known plausible mechanism by which the drug would cause a myocardial infarction.
Examples of Acceptable Company Clinical Evaluation Comments in narrative:
- The available pre-clinical data did not suggest a possibility that the subject drug would induce.
- As only limited information has been obtained so far, it is difficult to assess a cause and effect relationship.
- The temporal relationship (6 weeks) between the onset of the event and administration of drug x, which has a one-hour half-life, makes any causal relationship unlikely.
- The reported event is a well-known class effect (specify drug class here). However, this is the first reported case with.
- There is no plausible mechanism to implicate the subject drug.
- It is of interest to note that the patient was subsequently rechallenged at the same dose without recurrence of the adverse effect.
- The skin test with drug x, performed immediately after the event, was negative.
- The co-medications y and z should also be considered causative; the reported event is labeled for both drugs.
- The medication was not administered according to the dosage recommendation for the drug.
- The investigator on follow-up has changed his assessment from ‘‘probably’’ to ‘‘probably not’’ for the following reasons —.
- The benefit-risk relationship of drug x is not affected by this report.
Examples of Unacceptable Company Clinical Evaluation Comments in narrative:
- The investigator changed his assessment from ‘‘probably’’ to ‘‘probably not’’ on follow-up. [Without a reason, such a statement should not be made.]
- The company view is that the event is not due to the subject drug. [Inadequate without a reason given].
- No comment. [Under some regulatory requirements, such as in Germany, Austria and Japan, some company opinion is expected]
10 Comments
Dear Ramya, Well said about narration,👍
Hai Ramya I am really appreciate you🙏 easy to understand,
Precise and clear information on narrative writing…very informative and helpful…..Thank you….
very helpful, thank you
very precise infromation on narrative writing thank you
Afetr reading this i got a clarity on narration. Thankful to you
Very informative and precise, much helpful…thanks
Thank you, ma’am, for your valuable guidance.
Pingback: Mixing OTC and Prescription Drugs in Heart Disease: A Comprehensive Guide – World Of Medic
Hi i am venkatapanchumarthi. Awesome.I read the beautiful article i recent days it gives very informartion its helped me a lot.Thanks for sharing your kowledge.
Leave a Reply Cancel reply

IMAGES
VIDEO
COMMENTS
This case report is an example of post-marketing drug surveillance and pharmacovigilance of a widely used antibiotic through which the drug-safety database can be updated to educate and alert subsequent prescribers as to the potential effect. Patient histories can be viewed as vital statistics and, when incorporated into the electronic health ...
Pharmacovigilance (PV) has grown significantly in India in the last couple of decades. ... for example., case receipt team, triage team, data entry team, and quality control team. Below is the process flowchart [Figure 1] of the steps required in case processing. ... even within the same study. In fatal cases, the case processor will have to ...
A Practical Guide on Pharmacovigilance for Beginners. PREFACE. The Authors are pleased to present the First edition of "A Practical Guide on Pharmacovigilance for Beginners". This book is sketched to provide a concise introduction along with practical applications of pharmacovigilance that medical students, post graduates in pharmacology ...
After nearly 12 decades of pharmaceutical catastrophes and the associated groundbreaking regulatory innovations, pharmacovigilance has come down to us in the present day as 3 interlocking core disciplines: case management, signal management, and benefit-risk management. This review provides a state-of-the-art introduction to the great variety of sources of safety information, both dependent ...
case studies on how different countries from every region in the world have implemented processes to address pharmacovigilance (PV) for biotherapeutic medicines. More specifically, the project had three objectives: • Improve understanding of the different approaches adopted globally in the six World Health Organization regions;
In line with this, [16] study revealed. poor budgeting for pharmacovigilance in Nigeria. This scenario. has hindered mostly the monitory, assessment and detection of. drugs adverse effect on ...
Case reports may act as early-warning signals, which can subsequently be more thoroughly interrogated by analytical epidemiological studies. Even if many of these case reports do not lead to changes in the drug reference information, 6, 7 such reports (both published and unpublished) have guided numerous pharmacovigilance decisions. 8-10 They ...
The second case study presented at World Drug Safety Congress US (Boston) involved an exploratory analysis of CAR-T Cell therapies. The study involved searching the literature from January 2022 to August 2022 using biologit MLM-AI, and taking advantage of the tool's AI capabilities to zoom in on the most relevant articles:
"Input" constitutes the case management pharmacovigilance activity and refers to the source of information that is added to the database in a systematic, consistent, and timely manner, following globally recognized coding format and guidelines. ... and others. The Framingham Heart Study is an example of a prospective cohort study designed ...
Pharmacovigilance (PV) is a relatively new discipline in the pharmaceutical industry. Having undergone rapid growth over the past 2 decades, PV now touches many other disciplines in the research and development enterprise. With its growth has come a heightened awareness and interest in the medical community about the roles that PV plays. This article provides insights into the background and ...
Pharmacovigilance plays a critical role in ensuring the safety and efficacy of drugs, as well as the ongoing monitoring of their potential adverse effects. One integral component of pharmacovigilance is the Individual Case Safety Report (ICSR), which consists of detailed information on adverse drug reactions (ADRs) or other drug-related problems reported by patients, healthcare professionals ...
South American Journal of Medicine, Volume-2, Issue-2, 2014 PHARMACOVIGILANCE-AN EMERGENCE A Case Study By Dr Deven V Parmar1 and Dr. Dharani Munirathinam2, USA (1Faculty at Fellow American College Of Clinical Pharmacology 2 Pharmacovigilance consultant, ACCP Member) Email:- [email protected] INTRODUCTION Pharmacovigilance (PV), defined by the world health organization (WHO) as the ...
Pharmacovigilance case processing helps to get clear information regarding the drug and outcomes by using various software technologies. Arugs database, cemflow, vigibase and ICSR are few software ...
Expert's Opinion. The Future of Pharmacovigilance is Proactive. Data and AI can unlock the path to proactivity. Bruce Palsulich, Vice President of Safety, Oracle Life Sciences04.03.24. The pace of drug therapy approvals has reached astounding highs. In 2023 alone, the FDA approved 55 new drugs. However, each treatment comes with the potential ...
After nearly 12 decades of pharmaceutical catastrophes and the associated groundbreaking regulatory innovations, pharmacovigilance has come down to us in the present day as 3 interlocking core disciplines: case management, signal management, and benefit-risk management. This review provides a state-of-the-art introduction to the great variety ...
Narrative writing is an important part of Pharmacovigilance and in patient safety as well. A narrative is a brief summary of specific events experienced by patients, during the course of a clinical trial/treatment. Narrative writing involves multiple activities such as generation of patient profiles, review of data sources, and identification ...