Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Literature Review | Guide, Examples, & Templates

How to Write a Literature Review | Guide, Examples, & Templates
Published on January 2, 2023 by Shona McCombes . Revised on September 11, 2023.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research that you can later apply to your paper, thesis, or dissertation topic .
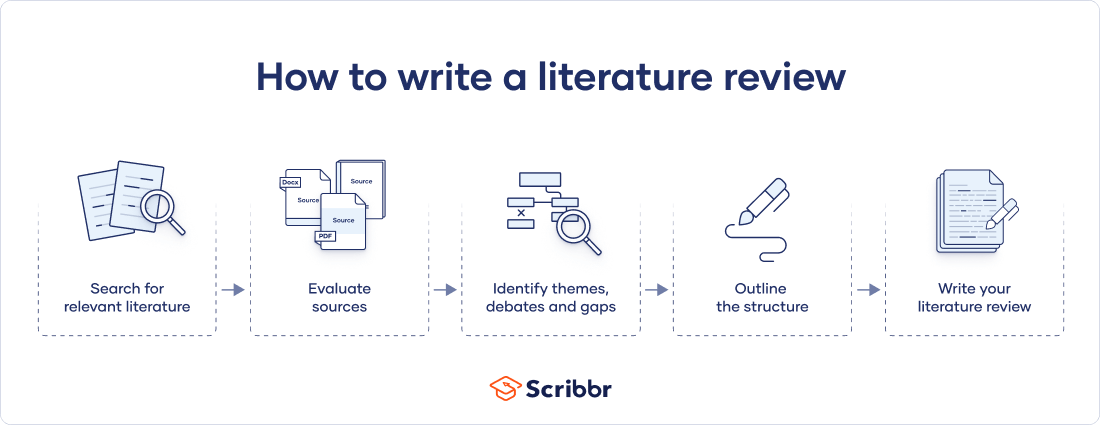
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates, and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarize sources—it analyzes, synthesizes , and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
What is the purpose of a literature review, examples of literature reviews, step 1 – search for relevant literature, step 2 – evaluate and select sources, step 3 – identify themes, debates, and gaps, step 4 – outline your literature review’s structure, step 5 – write your literature review, free lecture slides, other interesting articles, frequently asked questions, introduction.
- Quick Run-through
- Step 1 & 2
When you write a thesis , dissertation , or research paper , you will likely have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and its scholarly context
- Develop a theoretical framework and methodology for your research
- Position your work in relation to other researchers and theorists
- Show how your research addresses a gap or contributes to a debate
- Evaluate the current state of research and demonstrate your knowledge of the scholarly debates around your topic.
Writing literature reviews is a particularly important skill if you want to apply for graduate school or pursue a career in research. We’ve written a step-by-step guide that you can follow below.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research problem and questions .
Make a list of keywords
Start by creating a list of keywords related to your research question. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list as you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some useful databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can also use boolean operators to help narrow down your search.
Make sure to read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
You likely won’t be able to read absolutely everything that has been written on your topic, so it will be necessary to evaluate which sources are most relevant to your research question.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models, and methods?
- Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible , and make sure you read any landmark studies and major theories in your field of research.
You can use our template to summarize and evaluate sources you’re thinking about using. Click on either button below to download.
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It is important to keep track of your sources with citations to avoid plagiarism . It can be helpful to make an annotated bibliography , where you compile full citation information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
The only proofreading tool specialized in correcting academic writing - try for free!
The academic proofreading tool has been trained on 1000s of academic texts and by native English editors. Making it the most accurate and reliable proofreading tool for students.
Try for free
To begin organizing your literature review’s argument and structure, be sure you understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly visual platforms like Instagram and Snapchat—this is a gap that you could address in your own research.
There are various approaches to organizing the body of a literature review. Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarizing sources in order.
Try to analyze patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organize your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text , your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, you can follow these tips:
- Summarize and synthesize: give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: don’t just paraphrase other researchers — add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically evaluate: mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: use transition words and topic sentences to draw connections, comparisons and contrasts
In the conclusion, you should summarize the key findings you have taken from the literature and emphasize their significance.
When you’ve finished writing and revising your literature review, don’t forget to proofread thoroughly before submitting. Not a language expert? Check out Scribbr’s professional proofreading services !
This article has been adapted into lecture slides that you can use to teach your students about writing a literature review.
Scribbr slides are free to use, customize, and distribute for educational purposes.
Open Google Slides Download PowerPoint
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarize yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your thesis or dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, September 11). How to Write a Literature Review | Guide, Examples, & Templates. Scribbr. Retrieved April 9, 2024, from https://www.scribbr.com/dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, what is a theoretical framework | guide to organizing, what is a research methodology | steps & tips, how to write a research proposal | examples & templates, unlimited academic ai-proofreading.
✔ Document error-free in 5minutes ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
- Search Menu
- Advance articles
- Editor's Choice
- Supplements
- French Abstracts
- Portuguese Abstracts
- Spanish Abstracts
- Author Guidelines
- Submission Site
- Open Access
- About International Journal for Quality in Health Care
- About the International Society for Quality in Health Care
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact ISQua
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, results of data synthesis, conclusions, acknowledgements.
- < Previous
How are medication errors defined? A systematic literature review of definitions and characteristics
- Article contents
- Figures & tables
- Supplementary Data
M. Lisby, L.P. Nielsen, B. Brock, J. Mainz, How are medication errors defined? A systematic literature review of definitions and characteristics, International Journal for Quality in Health Care , Volume 22, Issue 6, December 2010, Pages 507–518, https://doi.org/10.1093/intqhc/mzq059
- Permissions Icon Permissions
Multiplicity in terminology has been suggested as a possible explanation for the variation in the prevalence of medication errors. So far, few empirical studies have challenged this assertion. The objective of this review was, therefore, to describe the extent and characteristics of medication error definitions in hospitals and to consider the consequences for measuring the prevalence of medication errors.
Studies were searched for in PubMed, PsychINFO, Embase and CINAHL employing primary search terms such as ‘medication errors’ and ‘adverse drug events’. Peer-reviewed articles containing these terms as primary end-points were included. Study country, year, aim, design, data-collection methods, sample-size, interventions and main results were extracted.
Forty-five of 203 relevant studies provided a generic definition of medication errors including 26 different forms of wordings. The studies conducted in nine countries represented a variety of clinical settings and the approach was mainly descriptive. Of utmost importance is the documented prevalence of medication errors, which ranged from 2 to 75% with no associations found between definitions and prevalence.
Inconsistency in defining medication errors has been confirmed. It appears that definitions and methods of detection rather than being reproducible and reliable methods are subject to the individual researcher's preferences. Thus, application of a clear-cut definition, standardized terminology and reliable methods has the potential to greatly improve the quality and consistency of medication error reporting. Efforts to achieve a common accepted definition that defines the scope and content are therefore needed.
In the Harvard Medical Practice studies of adverse events in hospitals, medication errors were found to be the main contributor constituting around one in five of the events, which were subsequently confirmed in comparable studies and studies of adverse drug events (ADEs) [ 1–4 ]. This has led to an increased focus on epidemiology and prevention of medication error in hospital settings around the world prompting numerous studies [ 5–13 ]. However, this contribution has not provided clarity or consistent findings with respect to medication errors. Quite the contrary, there appears to be a multiplicity of terms involved in defining the clinical range of medication errors and classifying consequences e.g. error, failure, near miss, rule violation, deviation, preventable ADE and potential ADE [ 14–18 ]. Moreover, it has been suggested that this inconsistency has contributed to the substantial variation in the reported occurrences of medication errors [ 19–21 ]. Thus, compared with other epidemiological fields in health care, no single definition is currently being used to determine medication errors although attempts to develop an international definition have been made e.g. National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) [ 22 ], which is clearly reflected in the referred literature. As an important consequence, this lack of clarity hinders reliable comparison of findings across studies, clinical settings and countries.
Another obstacle to achieving consensus of a common definition is the different approaches towards interpreting and detecting medication errors such as the system-oriented approach using mandatory or voluntary-based reporting systems in contrast to the epidemiological approach using specific research methods. Choice of reliable methods, including denominators, data completeness and systematic data collection, is essential in the epidemiological approach. However, these considerations are likely to be secondary in a system approach where causation is paramount. Unfortunately, identifying error causes without consistent, reliable measures is unlikely to lead to well-targeted prevention strategies. So far, the literature has mainly emphasized the problem of inconsistent use of definitions and data collection methods, whereas few studies have explored medical error subsets, and these have often been in specific clinical settings or particular to specific patient safety organizations [ 5 , 19–21 , 23–25 ]. Most importantly, no studies have to our knowledge systematically provided an overview of the extent of existing definitions and their possible impact on the occurrence of medication errors. Hence, the objective of this study was to investigate definitions of medication errors and, furthermore, to describe characteristics as well as to assess whether or not there were any associations between definitions and observed prevalence in hospitals.
Data sources
A systematic search of studies related to medication errors was performed in the databases on 18 December 2006 in PubMed (1951), Embase (1948), CINAHL (1981) and PsycINFO (1806) using the following key terms: ‘medication errors’, ‘adverse drug events’, ‘adverse drug events and errors’ and ‘medication errors and adverse drug events’ (Fig. 1 ). To capture all possible studies of medication errors in hospitals, the search was not restricted to MeSH terms in PubMed. However, a comparable search using the MeSH term ‘medication errors’ was performed in which all studies from the key term search could be retrieved.
Summary of search and review process.
Study selection
Only studies performed in hospital settings having medication errors and/or ADEs as the main objective were included in the present review, excluding studies performed solely in primary health care and literature reviews of medication errors and ADE (Fig. 1 ). Although there is no reason to believe that the occurrence of medication errors would be significantly different from hospitals, primary health care was excluded in this review due to assumed differences in medication handling and to the limited amount of completed medication error research in primary health care when the literature search was conducted. Finally, the search was limited to peer-reviewed studies with abstracts and studies presented in English.
First, titles and abstracts were examined in accordance with inclusion and exclusion criteria. Secondly, papers that met the inclusion criteria or papers in which inclusion could not be determined directly e.g. whether a setting was representing primary care were obtained. Thirdly, all duplicates between databases, papers that did not meet the inclusion criteria or papers that could not be obtained were excluded.
Data extraction
Definitions of medication errors and ADEs were registered along with included error types and whether the paper focused on ordering, dispensing, administering and monitoring. Moreover, general information regarding journal, author, year, title, aim, setting, participants, design, methods, intervention, results and evidence level were registered in an Access database.
Determination of evidence level was based on modified Oxford Criteria (Table 1 ). Studies in which evidence level could not be determined on behalf of available information, were discussed with a clinical pharmacologist and a professor in Public Health.
Levels of evidence, Oxford Centre for Evidence-based Medicine (2001) and pharmaco-epidemiological study design
In Table 1 , study-designs, in respectively, Oxford Centre for Evidence-based Medicine (therapy/prevention/aetiology/harm) and in the Pharmaco-epidemiological literature are provided along with the matching evidence levels (right column). In the present review, the evidence levels of the included studies were classified in accordance to these study-designs, as appropriate.
Due to the obvious lack of standard methodology and outcome measures, data could not be statistically summarized. However, prevalences of medication errors were reported for studies in which denominators were accessible. In pre–post studies and controlled studies, only prevalences of medication errors at baseline or from a control group were presented, whereas no prevalences could be calculated in studies using data from reporting systems [ 26 ]. Definitions were analysed with regard to similarities in content leading to the following five categories: (i) studies using the term error; (ii) studies using the NCC MERP definition; (iii) studies using failure; (iv) studies using deviation; and, finally (v) other terms. In each category, definitions from included studies were presented along with study characteristics. Finally, possible tendencies towards associations between definitions and prevalences were examined.
The literature search revealed 203 eligible papers (Fig. 1 ) of which 45 (23%) included a generic definition of medication errors. An additional 30 studies included a stage-specific definition; 22 prescribing, 3 in dispensing, 5 in administering and, finally, 4 studies contained a definition of intravenous errors. However, in 124 studies, no definitions were provided.
Overall characteristics
The 45 included studies were published in 26 different peer-reviewed journals in the period from 1984 to 2006 with half of them in the period 2005–06. The majority of studies were conducted in North America, representing 36 studies; 2 were done in Australia, 6 in Western Europe and, finally, 1 in Asia. The studies were conducted in a variety of clinical settings with almost 50% assessing more than one type of setting e.g. medical and surgical departments. Moreover, 20 studies included only adults, 9 studies only children, 9 studies both adults and children and, finally, 8 studies included other types of participants e.g. nurses and pharmacists. In 13 studies an intervention was addressed of which 9 were technologies in the medication process (e.g. computerized order entry (CPOE) either alone or combined with clinical decision support (CDS) systems, dose dispensing systems and infusion pumps with CDS). Descriptive designs were employed in 37 studies, whereas 2 studies were conducted as randomized clinical controlled studies, 1 as a case–control study and 1 as a prospective cohort study, and, finally, 4 studies were conducted using other designs e.g. case reports. Nine out of 10 studies were classified as evidence level IV or V, and, finally, chart review and reporting systems were the most frequently used methods to detect medication errors.
Prevalence of medication errors
In 21 of 45 studies, it was not possible to determine a prevalence of medication errors due to lack of valid denominators. These were in particular studies using reporting systems, interview and questionnaires as data collection method. Overall, a prevalence of 75% was found, with the majority being below <10% (Tables 2–4 ).
Studies using errors in definition of medication errors
a Oxford Centre for Evidence-based Medicine Levels of Evidence. Abbreviations: P: prescription; T: transcription; D: dispensing; A: administration; CPOE: computerized order entry; CDS: clinical decision support; OE: opportunities for errors; MEOS: medication error outcome scale. b Pre-intervention. c Preventable ADE + potential ADE.
Studies using the NCC MERP definition of medication errors
NCC MERP definition: ‘Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in control of the health-care professional, patient or consumer. Such events may be related to professional practice, health-care products, procedures and systems, including prescribing; order communication; product labelling, packaging and nomenclature; compounding; dispensing; distribution; administration; education; monitoring; and use.’ a Oxford Centre for Evidence-based Medicine Levels of Evidence. Abbreviations: P: prescription; T: transcription; D: dispensing; A: administration; CPOE: computerized order entry; CDS: clinical decision support; N/A: not applicable.
Studies using ‘failure, deviation or other’ terms in definition of medication errors
a Oxford Centre for Evidence-based Medicine Levels of Evidence. Abbreviations: AUS: Australia; UK: United Kingdom; P: prescription; T: transcription; D: dispensing; A: administration; CPOE: computerized order entry; CDS: clinical decision support; OE: opportunities for errors; N/A: not applicable; MEOS: medication error outcome scale. b Prevalence from a control group or pre-intervention.
An average of nine error types (min/max: 1/38) were identified in 38 of the 45 studies. In seven studies no error types were included due to study design. The study having one error type, namely, overdose (gentamicin) accounted for the highest prevalence in the review. Unfortunately, it was not possible to retrieve prevalence in the study using the highest number of error types, as data were collected from voluntary reporting. Dosing errors were the most frequent single error type, and in studies including all stages in the medication process, prescribing errors accounted for the highest percentage.
Definitions
Of the 45 definitions, 26 differed in wording and/or content. One definition used harm or potential for harm as a criterion for medication error, whereas one explicitly included intercepted medication errors [ 27–29 ]. Finally, five definitions were limited to deviations between ordered and administered drugs and doses [ 30–34 ]. In all other definitions no restrictions were specified.
A crude categorization of the revealed definitions was performed based on similarities in wording and/or content. Tables 2–4 provide an overview of definitions and characteristics of each study. Table 2 shows 15 definitions using the word ‘error/s’ followed by information about included stages in the medication process. In seven definitions, information regarding injury or intercepted errors is stated. Table 3 reveals characteristics of 17 studies using the definition from NCC MERP [ 22 ]. Finally, Table 4 presents five definitions using failure instead of error; five focusing on deviations between ordered and administered drugs/doses and three using other definitions.
Trends towards association between definition and prevalence
In the first category (Table 2 ), it was possible to ascertain prevalence in all studies ranging from 2 to 75% with the two European studies as the main contributors. In the second category (Table 3 ), which included studies using the definition from NCC MERP, it was possible to retrieve prevalence in 1 out of 17 studies, due to the use of reporting systems. This study revealed a prevalence of 8%. In the third category (Table 4 ) consisting of five studies using the term ‘failure’, it was not possible to provide information about prevalence due to study design and data collection methods. Finally, in the fourth category (Table 4 ), a prevalence of 3–16% was observed in studies focusing on deviations between ordered and administered drugs/doses.
To our knowledge, this is the first study to systematically explore the extent and impact of generic definitions of medication errors in hospital settings. The literature review confirmed an inconsistent use of definitions. However, other aspects have to be considered in order to explain the variation in prevalence of medication errors, as interpretation of the included definitions did not suggest any tendencies.
It is of particular relevance that fewer than half of the studies explicitly defined medication errors either as an overall definition (generic) or a stage/route-specific term. Furthermore, fewer than a quarter presented a generic definition despite that being the main objective of the studies. Thus, the inconsistency in terminology only represents the tip of the iceberg. Additionally, the present review has confirmed that the overall poor understanding of the epidemiology of medication errors can, at least, partly be explained by choice of design, data collection methods and study population, including denominators [ 19–21 , 23 ]. Based on these shortcomings, we have revealed a prevalence of 2–75% in studies that included a generic definition of medication error.
The second important problem is the choice of denominator or study population. It has previously been suggested that to use opportunities for errors rather than number of patients as denominator reduces the risk of case-mix bias [ 26 ]. Here we demonstrated a variety of denominators including drug order, doses, opportunities for errors, patients, nurses, reports and triggers. In addition, the frequent use of a reporting system excluded calculation of valid prevalence in almost half of all the studies thereby increasing the lack of clarity.
Thirdly, the impact of error types should be considered. It could be assumed that increasing the number of error types being measured, would automatically result in higher occurrences of medication errors due to an increased probability of detecting more errors. However, the study with the highest prevalence of errors (75%) in the present review included only one error type, namely, dosing errors, which conflicts with this assumption [ 35 ]. On the other hand, not all error types are mutually exclusive e.g. dosing errors, which inevitably includes all errors resulting in wrong or omitted dose (under-dose, overdose, omission of dose). Thus, the number of error types has to be weighed against type of error and the sensitivity of error detection methods. Unfortunately, the present review did not provide sufficient information on the impact of error types with regard to prevalence.
Finally, choice of data collection method should be considered important. Previously, chart review has been considered as the most appropriate method to detect prescribing errors and direct observation the most sensitive method to detect dispensing and administration errors, as opposed to voluntary reporting, which was found to be the least sensitive method [ 32 , 36 ]. In recent years the availability of computer-generated signals in error detection has increased, which allow an objective detection of all incidents that have been defined as an error in the computer. Thus, it can be assumed that such systems will increase the detection of systematic documented electronic data such as dosing of gentamicin [ 35 ]. In the present review the most frequently applied error detection method was chart review, which might have contributed to an underestimation of the occurrence of medication errors when applied to detection of errors other than prescribing.
Definition and prevalence
Interestingly, definitions, which at first glance appeared to be similar (Table 2 ), turned out to have the widest range in prevalence of medication errors. A closer scrutiny revealed that 10 of 15 studies in this category were affiliated with the same institutions in Boston, USA [ 7 , 15 , 28 , 37–43 ]. In addition, these studies demonstrated the lowest occurrence of medication errors ranging from 2 to 8% regardless of whether intercepted errors were included or not, suggesting consistency in error detection methods. However, prevalence in the two studies from Europe exceeded the American studies by as much as eight times, despite use of virtually identical definitions [ 10 , 35 ]. No obvious circumstances can explain these extreme differences, apart from use of data collection methods, as the study with the highest prevalence used computer-generated signals to detect dosing errors [ 35 ].
The majority of studies used the definition by NCC MERP. Unfortunately, it was only possible to retrieve one valid prevalence of medication errors [ 44 ]. This definition was initially developed for medication error reporting and, therefore, was an obvious choice for studies using reporting systems, which was the case for almost all the studies in this category [ 22 ]. An important drawback to reporting systems is an increased risk of underestimating the occurrence of medication errors due to the reporter's awareness of errors, attitudes towards reporting errors and fear of sanctions [ 45 ]. In addition, reporting systems are by nature denominator free as they do not provide information on the whole population; on the contrary, retrospective fitted denominators, such as time period or admissions, are frequently employed to demonstrate error rates [ 26 ]. Thus, reports of incidence or prevalence in studies using reporting systems should be avoided or interpreted with caution. Unfortunately, this expelled a unique opportunity to compare prevalence in studies using identical definitions.
Surprisingly, only 1 of the 45 definitions restricted medication errors to failures that either result in harm or have the potential to lead to harm [ 27 ]. Contrary to other definitions of medication errors in the present review, this approach relates process and outcome factors within the same definition, which previously has been suggested as minimizing the risk of accepting associations between errors and processes as synonyms for causation [ 46 ]. Moreover, this definition has been tested in an Australian study, in which it proved to be the most robust among other definitions, when evaluated in comparison with different medication error scenarios [ 25 ]. However, due to the design of this study it was not possible to elucidate that a restricted definition would lead to lower occurrences of medication errors compared with more profound definitions [ 15 ].
Finally, definitions that considered a medication error as a deviation between an ordered and administered drug and dose seemed to be more homogeneous with regard to prevalence despite representing different countries and employing different study designs [ 30 , 32–34 , 47 ]. However, these studies predominantly used the same types of denominator (opportunities for errors; doses and orders) as well as the most sensitive and appropriate data collection methods, e.g. direct observation in studies of dispensing and administration errors. A possible explanation for this consistency is the clear-cut limitation to deviations, which might appear simpler and be a less subjective approach in determination of medication errors. However, this approach excludes prescribing errors, as prescriptions serve as the gold standard in these definitions.
Limitations
The aim of this review was to investigate the multiplicity of definitions used in studies having medication errors and/or ADEs as the main objective. Hence, the characteristics and prevalence reported here might not reflect the overall occurrence of medication errors. However, it could be assumed that prevalence ranging from 2 to 75% represents the vast majority of studies in medication errors. Secondly, the literature search was limited to four major databases and restricted to papers in the English language. It is, therefore, possible that studies that would have met the inclusion criteria, were not indexed by these databases or were published in other languages than English. Nevertheless, due to experience from the current literature search, in which studies from a time span of >20 years were included, we assume that studies that might have been unintentionally disregarded in the search strategy would rather have added to the current inconsistency than contributed to clarification of the terminology. Third, the groupings we selected were somewhat arbitrary and this might have affected our chances of seeing an effect.
In the present systematic literature review of 45 studies we have confirmed inconsistency in defining medication errors as well as lack of definitions. Most of the definitions were profound, including minor deviations as well as fatal errors, whereas a single definition was restricted to harmful or potentially harmful errors.
Most importantly, it appears that definitions of medication errors and methods of detection, rather than being reproducible and reliable methods, are subject to individual researcher's preferences. Thus, it is obvious that application of a clear-cut definition, standardized terminology and reliable methods will greatly improve the quality and consistency of medication error findings. Efforts to achieve a commonly accepted definition that defines the scope and content is required in future studies.
We would like to thank, Prof. D.W. Bates, Harvard Medical School and Harvard School of Public Health, Boston, MA, for commenting on the present article.
Google Scholar
- medication errors
Supplementary data
Email alerts, citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1464-3677
- Print ISSN 1353-4505
- Copyright © 2024 International Society for Quality in Health Care and Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Libraries | Research Guides
Literature reviews, what is a literature review, learning more about how to do a literature review.
- Planning the Review
- The Research Question
- Choosing Where to Search
- Organizing the Review
- Writing the Review
A literature review is a review and synthesis of existing research on a topic or research question. A literature review is meant to analyze the scholarly literature, make connections across writings and identify strengths, weaknesses, trends, and missing conversations. A literature review should address different aspects of a topic as it relates to your research question. A literature review goes beyond a description or summary of the literature you have read.
- Sage Research Methods Core Collection This link opens in a new window SAGE Research Methods supports research at all levels by providing material to guide users through every step of the research process. SAGE Research Methods is the ultimate methods library with more than 1000 books, reference works, journal articles, and instructional videos by world-leading academics from across the social sciences, including the largest collection of qualitative methods books available online from any scholarly publisher. – Publisher
- Next: Planning the Review >>
- Last Updated: Jan 17, 2024 10:05 AM
- URL: https://libguides.northwestern.edu/literaturereviews

An official website of the Department of Health & Human Services
- Search All AHRQ Sites
- Email Updates

1. Use quotes to search for an exact match of a phrase.
2. Put a minus sign just before words you don't want.
3. Enter any important keywords in any order to find entries where all these terms appear.
- The PSNet Collection
- All Content
- Perspectives
- Current Weekly Issue
- Past Weekly Issues
- Curated Libraries
- Clinical Areas
- Patient Safety 101
- The Fundamentals
- Training and Education
- Continuing Education
- WebM&M: Case Studies
- Training Catalog
- Submit a Case
- Improvement Resources
- Innovations
- Submit an Innovation
- About PSNet
- Editorial Team
- Technical Expert Panel
How are medication errors defined? A systematic literature review of definitions and characteristics.
Lisby M, Nielsen LP, Brock B, et al. How are medication errors defined? A systematic literature review of definitions and characteristics. International Journal for Quality in Health Care. 2010;22(6). doi:10.1093/intqhc/mzq059.
This systematic review found wide variation in how medication errors are defined between studies. This variation has significant implications for determining the prevalence of medication errors. Prior commentaries have noted the need for standardized, universally applicable definitions of adverse drug events.
How should medication errors be defined? Development and test of a definition. May 30, 2012
Errors in the medication process: frequency, type, and potential clinical consequences. March 6, 2005
Identifying high-risk medication: a systematic literature review. August 13, 2014
Case study: getting boards on board at Allen Memorial Hospital, Iowa Health System. April 2, 2008
Association of hospital participation in a regional trauma quality improvement collaborative with patient outcomes. June 20, 2018
Selection of indicators for continuous monitoring of patient safety: recommendations of the project 'safety improvement for patients in Europe.' June 10, 2009
Novel analysis of clinically relevant diagnostic errors in point-of-care devices. October 19, 2011
Measuring patient safety climate: a review of surveys. October 12, 2005
National Patient Safety Foundation agenda for research and development in patient safety. March 27, 2005
Physician assistants and the disclosure of medical error. June 18, 2014
Insulin dosing error in a patient with severe hyperkalemia. January 17, 2018
Effective interventions and implementation strategies to reduce adverse drug events in the Veterans Affairs (VA) system. February 20, 2008
Implementing an error disclosure coaching model: a multicenter case study. February 22, 2017
Building safer systems by ecological design: using restoration science to develop a medication safety intervention. April 12, 2006
Disclosing adverse events in clinical practice: the delicate act of being open. February 2, 2022
Use of a safety climate questionnaire in UK health care: factor structure, reliability and usability. November 22, 2006
Using standardised patients in an objective structured clinical examination as a patient safety tool. March 6, 2005
Time of day effects on the incidence of anesthetic adverse events. August 23, 2006
Discrimination, abuse, harassment, and burnout in surgical residency training. November 20, 2019
Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. March 27, 2005
Relationship between tort claims and patient incident reports in the Veterans Health Administration. April 21, 2005
An intervention to decrease patient identification band errors in a children's hospital. May 12, 2010
"Every error counts": a web-based incident reporting and learning system for general practice. August 20, 2008
Effects of skilled nursing facility structure and process factors on medication errors during nursing home admission. November 5, 2014
Frequency and type of situational awareness errors contributing to death and brain damage: a closed claims analysis. May 24, 2017
A simulation design for research evaluating safety innovations in anaesthesia. January 28, 2009
Teaching teamwork during the Neonatal Resuscitation Program: a randomized trial. June 20, 2007
Implementation of bar-code medication administration to reduce patient harm. February 20, 2019
Design and implementation of a point-of-care computerized system for drug therapy in Stockholm metropolitan health region--bridging the gap between knowledge and practice. August 29, 2007
Analysis of suicides reported as adverse events in psychiatry resulted in nine quality improvement initiatives. July 21, 2021
Nil per os orders for imaging: a teachable moment. September 27, 2017
An observational study of direct oral anticoagulant awareness indicating inadequate recognition with potential for patient harm. April 13, 2016
Clinical pharmacists and inpatient medical care: a systematic review. May 17, 2006
Safety learning among young newly employed workers in three sectors: a challenge to the assumed order of things. November 17, 2021
Competencies for patient safety and quality improvement: a synthesis of recommendations in influential position papers. April 6, 2016
Patterns of potential opioid misuse and subsequent adverse outcomes in Medicare, 2008 to 2012. June 6, 2018
Video capture of clinical care to enhance patient safety. March 1, 2006
Do faculty and resident physicians discuss their medical errors? October 15, 2008
Getting teams to talk: development and pilot implementation of a checklist to promote interprofessional communication in the OR. October 19, 2005
Why psychiatry is different--challenges and difficulties in managing a nosocomial outbreak of coronavirus disease (COVID-19) in hospital care. January 20, 2021
The "Seven Pillars" response to patient safety incidents: effects on medical liability processes and outcomes. September 7, 2016
Regional surveillance of emergency-department visits for outpatient adverse drug events. April 22, 2009
Errors with concentrated epinephrine in otolaryngology. September 3, 2008
Doctors debate safety of their white coats. December 2, 2015
Multicentre study to develop a medication safety package for decreasing inpatient harm from omission of time-critical medications. March 4, 2015
Strengthening leadership as a catalyst for enhanced patient safety culture: a repeated cross-sectional experimental study. June 22, 2016
Errors during the preparation of drug infusions: a randomized controlled trial. August 22, 2012
The impact of a tele-ICU on provider attitudes about teamwork and safety climate. May 26, 2010
Non-intercepted dose errors in prescribing antineoplastic treatment: a prospective, comparative cohort study. March 11, 2015
Polypharmacy in hospitalized older adult cancer patients: experience from a prospective, observational study of an oncology-acute care for elders unit. August 5, 2009
Anaesthetists' management of oxygen pipeline failure: room for improvement. January 31, 2007
Attitudes and barriers to incident reporting: a collaborative hospital study. February 22, 2006
Using simulation to identify sources of medical diagnostic error in child physical abuse. April 27, 2016
An alternative strategy for studying adverse events in medical care. March 27, 2005
A prospective study of patient safety in the operating room. February 22, 2006
Wrong-site sinus surgery in otolaryngology. August 11, 2010
Reducing serious safety events and priority hospital-acquired conditions in a pediatric hospital with the implementation of a patient safety program. June 6, 2018
The SAGES Fundamental Use of Surgical Energy program (FUSE): history, development, and purpose. February 14, 2018
Does a suggested diagnosis in a general practitioners' referral question impact diagnostic reasoning: an experimental study. April 27, 2022
Adverse-event-reporting practices by US hospitals: results of a national survey. January 7, 2009
Involvement of parents in critical incidents in a neonatal-paediatric intensive care unit. December 16, 2009
Using computerized virtual cases to explore diagnostic error in practicing physicians. February 13, 2019
The role of housestaff in implementing medication reconciliation on admission at an academic medical center. June 16, 2010
The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. April 19, 2006
National Partnership for Maternal Safety: Consensus Bundle on Venous Thromboembolism. December 7, 2016
Enhancing psychological safety in mental health services. June 9, 2021
Teamwork behaviours and errors during neonatal resuscitation. March 24, 2010
The use of medical emergency teams in medical and surgical patients: impact of patient, nurse and organisational characteristics. October 29, 2008
Relationship between complaints and quality of care in New Zealand: a descriptive analysis of complainants and non-complainants following adverse events. February 15, 2006
Augmenting health care failure modes and effects analysis with simulation. March 5, 2014
Medication safety program reduces adverse drug events in a community hospital. June 22, 2005
Nurses' perspective on a serious adverse drug event. March 6, 2005
Disclosing large scale adverse events in the US Veterans Health Administration: lessons from media responses. April 13, 2016
Beyond "see one, do one, teach one": toward a different training paradigm. February 25, 2009
The experiences of risk managers in providing emotional support for health care workers after adverse events. May 11, 2016
Risk managers' descriptions of programs to support second victims after adverse events. May 13, 2015
Interprofessional education in team communication: working together to improve patient safety. March 27, 2013
Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. February 7, 2018
Preventable errors in organ transplantation: an emerging patient safety issue? July 11, 2012
Levels of agreement on the grading, analysis and reporting of significant events by general practitioners: a cross-sectional study. October 29, 2008
The impact of trained assistance on error rates in anaesthesia: a simulation-based randomised controlled trial. February 25, 2009
The effect of executive walk rounds on nurse safety climate attitudes: a randomized trial of clinical units. April 27, 2005
Association of surgical resident wellness with medical errors and patient outcomes. May 6, 2020
Relationship between patient complaints and surgical complications. February 15, 2006
Error rating tool to identify and analyse technical errors and events in laparoscopic surgery. September 11, 2013
The influence of standardisation and task load on team coordination patterns during anaesthesia inductions. April 29, 2009
Incident reporting system does not detect adverse drug events: a problem for quality improvement. March 27, 2005
A systematic review of clinical decision support systems for clinical oncology practice. May 15, 2019
Development and validation of a taxonomy of adverse handover events in hospital settings. February 18, 2015
Evaluation of reasons why surgical residents exceeded 2011 duty hour requirements when offered flexibility. June 20, 2018
Error, stress, and teamwork in medicine and aviation: cross sectional surveys. December 21, 2005
Readiness for organisational change among general practice staff. April 28, 2010
Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. April 2, 2008
The costs of adverse drug events in hospitalized patients. March 27, 2005
Medication administration discrepancies persist despite electronic ordering. November 28, 2007
A family-centered rounds checklist, family engagement, and patient safety: a randomized trial. May 31, 2017
Improving team information sharing with a structured call-out in anaesthetic emergencies: a randomized controlled trial. March 12, 2014
Communication failures contributing to patient injury in anaesthesia malpractice claims. September 1, 2021
Are informed policies in place to promote safe and usable EHRs? A cross-industry comparison. March 8, 2017
Evaluation of adverse drug events and medication discrepancies in transitions of care between hospital discharge and primary care follow-up. October 29, 2014
Thematic reviews of patient safety incidents as a tool for systems thinking: a quality improvement report. May 17, 2023
Driving Learning and Improvement After RCA2 Event Reviews. January 26, 2023 - January 26, 2023
HSIB Education. October 19, 2022
Interventions to reduce medication dispensing, administration, and monitoring errors in pediatric professional healthcare settings: a systematic review. September 29, 2021
Critical incidents involving the medical emergency team: a 5-year retrospective assessment for healthcare improvement. April 28, 2021
Suicide as an incident of severe patient harm: a retrospective cohort study of investigations after suicide in Swedish healthcare in a 13-year perspective. March 31, 2021
Learning from incident reporting? Analysis of incidents resulting in patient injuries in a web-based system in Swedish health care. December 9, 2020
How incident reporting systems can stimulate social and participative learning: a mixed-methods study. September 2, 2020
Register-based research of adverse events revealing incomplete records threatening patient safety. August 19, 2020
Identifying no-harm incidents in home healthcare: a cohort study using trigger tool methodology. August 5, 2020
Apparent cause analysis: a safety tool. May 20, 2020
Medical teamwork and the evolution of safety science: a critical review. March 11, 2020
The Field Guide to Human Error Investigations, Third Edition. August 24, 2017
Monitoring the anaesthetist in the operating theatre—professional competence and patient safety. March 1, 2017
Measurement of patient safety: a systematic review of the reliability and validity of adverse event detection with record review. September 28, 2016
Is there evidence for a better health care for cancer patients after a second opinion? A systematic review. September 28, 2016
Healthcare staff wellbeing, burnout, and patient safety: a systematic review. August 24, 2016
Performance of the Global Assessment of Pediatric Patient Safety (GAPPS) tool. June 15, 2016
Vaccination errors in general practice: creation of a preventive checklist based on a multimodal analysis of declared errors. June 15, 2016
Medical error—the third leading cause of death in the US. May 11, 2016
Patients' views of adverse events in primary and ambulatory care: a systematic review to assess methods and the content of what patients consider to be adverse events. February 17, 2016
Aviation and healthcare: a comparative review with implications for patient safety. February 3, 2016
Interorganizational complexity and organizational accident risk: a literature review. November 25, 2015
Interventions to reduce nurses' medication administration errors in inpatient settings: a systematic review and meta-analysis. September 30, 2015
The influence of context on the effectiveness of hospital quality improvement strategies: a review of systematic reviews. September 16, 2015
Ethical issues in patient safety research: a systematic review of the literature. September 9, 2015
"First, know thyself": cognition and error in medicine. June 3, 2015
Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. May 6, 2015
Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. March 11, 2015
Peer review of medical practices: missed opportunities to learn. January 28, 2015

Connect With Us
Sign up for Email Updates
To sign up for updates or to access your subscriber preferences, please enter your email address below.
Agency for Healthcare Research and Quality
5600 Fishers Lane Rockville, MD 20857 Telephone: (301) 427-1364
- Accessibility
- Disclaimers
- Electronic Policies
- HHS Digital Strategy
- HHS Nondiscrimination Notice
- Inspector General
- Plain Writing Act
- Privacy Policy
- Viewers & Players
- U.S. Department of Health & Human Services
- The White House
- Don't have an account? Sign up to PSNet
Submit Your Innovations
Please select your preferred way to submit an innovation.
Continue as a Guest
Track and save your innovation
in My Innovations
Edit your innovation as a draft
Continue Logged In
Please select your preferred way to submit an innovation. Note that even if you have an account, you can still choose to submit an innovation as a guest.
Continue logged in
New users to the psnet site.
Access to quizzes and start earning
CME, CEU, or Trainee Certification.
Get email alerts when new content
matching your topics of interest
in My Innovations.

The Quintessence of Basic and Clinical Research and Scientific Publishing pp 645–656 Cite as
Literature Reviews: An Overview of Systematic, Integrated, and Scoping Reviews
- John R. Turner 4
- First Online: 01 October 2023
698 Accesses
Literature reviews are a main part of the research process. Literature Reviews can be stand-alone research projects, or they can be part of a larger research study. In both cases, literature reviews must follow specific guidelines so they can meet the rigorous requirements for being classified as a scientific contribution. More importantly, these reviews must be transparent so that they can be replicated or reproduced if desired. The rigorous requirements set out by the National Science Foundation (NSF) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) aim to support researchers in conducting literature reviews as well as address the replication crisis that has challenged scientific disciplines over the past decade. The current chapter identifies some of the requirements along with highlighting different types of reviews and recommendations for conducting a rigorous review.
- Literature review
- Integrated review
- Systematic review
- Scoping review
- Cooper’s taxonomy
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Begley GC, Ioannidis JPA (2015) Reproducibility in science: improving the standard for basic and preclinical research. Circ Res 116:116–126. https://doi.org/10.1161/CIRCRESAHA.114.303819
Article CAS PubMed Google Scholar
Romero F (2019) Philosophy of science and the replicability crisis. Philos Compass 14:e12633. https://doi.org/10.1111/phc3.12633
Article Google Scholar
Van Bavel JJ, Mende-Siedlecki P, Brady WJ, Reinero DA (2016) Contextual sensitivity in scientific reproducibility. Proc Natl Acad Sci U S A 113:6454–6459. https://doi.org/10.1073/pnas.1521897113
Article CAS PubMed PubMed Central Google Scholar
Ioannidis JPA (2012) Why science is not necessarily self-correcting. Perspect Psychol Sci 7:645–654. https://doi.org/10.1177/1745691612464056
Article PubMed Google Scholar
Bollen K, Cacioppo JT, Kaplan RM, Krosnick JA, Olds JL (2015) Social, behavioral, and economic sciences perspectives on robust and reliable science. https://www.nsf.gov/sbe/AC_Materials/SBE_Robust_and_Reliable_Research_Report.pdf . Accessed 15 Sept 2022
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372. https://doi.org/10.1136/bmj.n71
Imel S (2011) Writing a literature review. In: Tonette RS, Hatcher T (eds) The handbook of scholarly writing and publishing. Jossey-Bass, San Francisco, CA, pp 145–160
Google Scholar
Boote DN, Beile P (2005) Scholars before researchers: on the centrality of the dissertation literature review in research preparation. Educ Res 34:3–15. https://doi.org/10.3102/0013189X034006003
Bryman A (2008) Social research methods. Oxford University Press, New York, NY
Taylor D (n.d.) The literature review: a few tips on conducting it. https://advice.writing.utoronto.ca/types-of-writing/literature-review/ . Accessed 1 Oct 2022
Torraco RJ (2005) Writing integrative literature reviews: guidelines and examples. Hum Resour Dev Rev 4(3):356–367. https://doi.org/10.1177/1534484305278283
Cooper HM (1988) Organizing knowledge syntheses: a taxonomy of literature reviews. Knowl Soc 1:104–126. https://doi.org/10.1007/BF03177550
Cooper H (2010) Research synthesis and meta-analysis. 4th ed. Applied social research methods series, vol 2. Sage, Los Angelas, CA
Cooper H (2003) Psychological bulletin: editorial. Psychol Bull 129:3–9. https://doi.org/10.1037/0033-2909.129.1.3
Hart C (2018) Doing a literature review: releasing the research imagination, 2nd edn. Sage, Los Angelas, CA
Tricco AC, Lillie E, Zarin W, O’Brien K, Colquhoun H, Kastner M et al (2016) A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol 16:15. https://doi.org/10.1186/s12874-016-0116-4
Article PubMed PubMed Central Google Scholar
Torraco RJ (2016) Writing integrative literature reviews: using the past and present to explore the future. Hum Resour Dev Rev 15:404–428. https://doi.org/10.1177/1534484316671606
Doty DH, Glick WH (1994) Typologies as a unique form of theory building: toward improved understanding and modeling. Acad Manag Rev 19:230–251. https://doi.org/10.5465/AMR.1994.9410210748
Leedy PD, Ormrod JE (2005) Practical research: planning and design, 8th edn. Pearson, Upper Saddle River, NJ
Ragins BR (2012) Reflections on the craft of clear writing. Acad Manag Rev 37:493–501. https://doi.org/10.5465/amr.2012.0165
King S (2000) On writing: a memoir of the craft. Scribner, New York, NY
Torraco RJ (2016) Research methods for theory building in applied disciplines: a comparative analysis. Adv Dev Hum Resour 4:355–376. https://doi.org/10.1177/1523422302043008
Pendleton-Jullian AM, Brown JS (2018) Design unbound: designing for emergence in a white water world. MIT Press, Cambridge, MA
Book Google Scholar
Simon HA (2019) The sciences of the artificial [reissue of 3rd ed.]. MIT Press, Cambridge, MA
Klir GJ (2009) W. Ross Ashby: a pioneer of systems science. Int J Gen Syst 38:175–188. https://doi.org/10.1080/03081070802601434
Reen J (2020) The evolution of knowledge: rethinking science for the anthropocene. Princeton University Press, Princeton, NJ
Download references
Conflict of Interest
No conflicts of interest.
Author information
Authors and affiliations.
University of North Texas, Denton, TX, USA
John R. Turner
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to John R. Turner .
Editor information
Editors and affiliations.
Retired Senior Expert Pharmacologist at the Office of Cardiology, Hematology, Endocrinology, and Nephrology, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, MD, USA
Gowraganahalli Jagadeesh
Professor & Director, Research Training and Publications, The Office of Research and Development, Periyar Maniammai Institute of Science & Technology (Deemed to be University), Vallam, Tamil Nadu, India
Pitchai Balakumar
Division Cardiology & Nephrology, Office of Cardiology, Hematology, Endocrinology and Nephrology, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, MD, USA
Fortunato Senatore
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter.
Turner, J.R. (2023). Literature Reviews: An Overview of Systematic, Integrated, and Scoping Reviews. In: Jagadeesh, G., Balakumar, P., Senatore, F. (eds) The Quintessence of Basic and Clinical Research and Scientific Publishing. Springer, Singapore. https://doi.org/10.1007/978-981-99-1284-1_38
Download citation
DOI : https://doi.org/10.1007/978-981-99-1284-1_38
Published : 01 October 2023
Publisher Name : Springer, Singapore
Print ISBN : 978-981-99-1283-4
Online ISBN : 978-981-99-1284-1
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Subject List
- Take a Tour
- For Authors
- Subscriber Services
- Publications
- African American Studies
- African Studies
- American Literature
- Anthropology
- Architecture Planning and Preservation
- Art History
- Atlantic History
- Biblical Studies
- British and Irish Literature
- Childhood Studies
- Chinese Studies
- Cinema and Media Studies
- Communication
- Criminology
- Environmental Science
- Evolutionary Biology
- International Law
- International Relations
- Islamic Studies
- Jewish Studies
- Latin American Studies
- Latino Studies
- Linguistics
- Literary and Critical Theory
- Medieval Studies
- Military History
- Political Science
- Public Health
- Renaissance and Reformation
- Social Work
- Urban Studies
- Victorian Literature
- Browse All Subjects
How to Subscribe
- Free Trials
In This Article Expand or collapse the "in this article" section Literature Reviews
Introduction, what is a literature review.
- Literature Reviews for Thesis or Dissertation
- Stand-alone and Systemic Reviews
- Purposes of a Literature Review
- Texts on Conducting a Literature Review
- Identifying the Research Topic
- The Persuasive Argument
- Searching the Literature
- Creating a Synthesis
- Critiquing the Literature
- Building the Case for the Literature Review Document
- Presenting the Literature Review
Related Articles Expand or collapse the "related articles" section about
About related articles close popup.
Lorem Ipsum Sit Dolor Amet
Vestibulum ante ipsum primis in faucibus orci luctus et ultrices posuere cubilia Curae; Aliquam ligula odio, euismod ut aliquam et, vestibulum nec risus. Nulla viverra, arcu et iaculis consequat, justo diam ornare tellus, semper ultrices tellus nunc eu tellus.
- Higher Education Research
- Meta-Analysis and Research Synthesis in Education
- Methodologies for Conducting Education Research
- Mixed Methods Research
- Philosophy of Education
- Politics of Education
- Qualitative Data Analysis Techniques
Other Subject Areas
Forthcoming articles expand or collapse the "forthcoming articles" section.
- Gender, Power, and Politics in the Academy
- Girls' Education in the Developing World
- Non-Formal & Informal Environmental Education
- Find more forthcoming articles...
- Export Citations
- Share This Facebook LinkedIn Twitter
Literature Reviews by Lawrence A. Machi , Brenda T. McEvoy LAST REVIEWED: 21 April 2021 LAST MODIFIED: 27 October 2016 DOI: 10.1093/obo/9780199756810-0169
Literature reviews play a foundational role in the development and execution of a research project. They provide access to the academic conversation surrounding the topic of the proposed study. By engaging in this scholarly exercise, the researcher is able to learn and to share knowledge about the topic. The literature review acts as the springboard for new research, in that it lays out a logically argued case, founded on a comprehensive understanding of the current state of knowledge about the topic. The case produced provides the justification for the research question or problem of a proposed study, and the methodological scheme best suited to conduct the research. It can also be a research project in itself, arguing policy or practice implementation, based on a comprehensive analysis of the research in a field. The term literature review can refer to the output or the product of a review. It can also refer to the process of Conducting a Literature Review . Novice researchers, when attempting their first research projects, tend to ask two questions: What is a Literature Review? How do you do one? While this annotated bibliography is neither definitive nor exhaustive in its treatment of the subject, it is designed to provide a beginning researcher, who is pursuing an academic degree, an entry point for answering the two previous questions. The article is divided into two parts. The first four sections of the article provide a general overview of the topic. They address definitions, types, purposes, and processes for doing a literature review. The second part presents the process and procedures for doing a literature review. Arranged in a sequential fashion, the remaining eight sections provide references addressing each step of the literature review process. References included in this article were selected based on their ability to assist the beginning researcher. Additionally, the authors attempted to include texts from various disciplines in social science to present various points of view on the subject.
Novice researchers often have a misguided perception of how to do a literature review and what the document should contain. Literature reviews are not narrative annotated bibliographies nor book reports (see Bruce 1994 ). Their form, function, and outcomes vary, due to how they depend on the research question, the standards and criteria of the academic discipline, and the orthodoxies of the research community charged with the research. The term literature review can refer to the process of doing a review as well as the product resulting from conducting a review. The product resulting from reviewing the literature is the concern of this section. Literature reviews for research studies at the master’s and doctoral levels have various definitions. Machi and McEvoy 2016 presents a general definition of a literature review. Lambert 2012 defines a literature review as a critical analysis of what is known about the study topic, the themes related to it, and the various perspectives expressed regarding the topic. Fink 2010 defines a literature review as a systematic review of existing body of data that identifies, evaluates, and synthesizes for explicit presentation. Jesson, et al. 2011 defines the literature review as a critical description and appraisal of a topic. Hart 1998 sees the literature review as producing two products: the presentation of information, ideas, data, and evidence to express viewpoints on the nature of the topic, as well as how it is to be investigated. When considering literature reviews beyond the novice level, Ridley 2012 defines and differentiates the systematic review from literature reviews associated with primary research conducted in academic degree programs of study, including stand-alone literature reviews. Cooper 1998 states the product of literature review is dependent on the research study’s goal and focus, and defines synthesis reviews as literature reviews that seek to summarize and draw conclusions from past empirical research to determine what issues have yet to be resolved. Theoretical reviews compare and contrast the predictive ability of theories that explain the phenomenon, arguing which theory holds the most validity in describing the nature of that phenomenon. Grant and Booth 2009 identified fourteen types of reviews used in both degree granting and advanced research projects, describing their attributes and methodologies.
Bruce, Christine Susan. 1994. Research students’ early experiences of the dissertation literature review. Studies in Higher Education 19.2: 217–229.
DOI: 10.1080/03075079412331382057
A phenomenological analysis was conducted with forty-one neophyte research scholars. The responses to the questions, “What do you mean when you use the words literature review?” and “What is the meaning of a literature review for your research?” identified six concepts. The results conclude that doing a literature review is a problem area for students.
Cooper, Harris. 1998. Synthesizing research . Vol. 2. 3d ed. Thousand Oaks, CA: SAGE.
The introductory chapter of this text provides a cogent explanation of Cooper’s understanding of literature reviews. Chapter 4 presents a comprehensive discussion of the synthesis review. Chapter 5 discusses meta-analysis and depth.
Fink, Arlene. 2010. Conducting research literature reviews: From the Internet to paper . 3d ed. Los Angeles: SAGE.
The first chapter of this text (pp. 1–16) provides a short but clear discussion of what a literature review is in reference to its application to a broad range of social sciences disciplines and their related professions.
Grant, Maria J., and Andrew Booth. 2009. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Information & Libraries Journal 26.2: 91–108. Print.
DOI: 10.1111/j.1471-1842.2009.00848.x
This article reports a scoping review that was conducted using the “Search, Appraisal, Synthesis, and Analysis” (SALSA) framework. Fourteen literature review types and associated methodology make up the resulting typology. Each type is described by its key characteristics and analyzed for its strengths and weaknesses.
Hart, Chris. 1998. Doing a literature review: Releasing the social science research imagination . London: SAGE.
Chapter 1 of this text explains Hart’s definition of a literature review. Additionally, it describes the roles of the literature review, the skills of a literature reviewer, and the research context for a literature review. Of note is Hart’s discussion of the literature review requirements for master’s degree and doctoral degree work.
Jesson, Jill, Lydia Matheson, and Fiona M. Lacey. 2011. Doing your literature review: Traditional and systematic techniques . Los Angeles: SAGE.
Chapter 1: “Preliminaries” provides definitions of traditional and systematic reviews. It discusses the differences between them. Chapter 5 is dedicated to explaining the traditional review, while Chapter 7 explains the systematic review. Chapter 8 provides a detailed description of meta-analysis.
Lambert, Mike. 2012. A beginner’s guide to doing your education research project . Los Angeles: SAGE.
Chapter 6 (pp. 79–100) presents a thumbnail sketch for doing a literature review.
Machi, Lawrence A., and Brenda T. McEvoy. 2016. The literature review: Six steps to success . 3d ed. Thousand Oaks, CA: Corwin.
The introduction of this text differentiates between a simple and an advanced review and concisely defines a literature review.
Ridley, Diana. 2012. The literature review: A step-by-step guide for students . 2d ed. Sage Study Skills. London: SAGE.
In the introductory chapter, Ridley reviews many definitions of the literature review, literature reviews at the master’s and doctoral level, and placement of literature reviews within the thesis or dissertation document. She also defines and differentiates literature reviews produced for degree-affiliated research from the more advanced systematic review projects.
back to top
Users without a subscription are not able to see the full content on this page. Please subscribe or login .
Oxford Bibliographies Online is available by subscription and perpetual access to institutions. For more information or to contact an Oxford Sales Representative click here .
- About Education »
- Meet the Editorial Board »
- Academic Achievement
- Academic Audit for Universities
- Academic Freedom and Tenure in the United States
- Action Research in Education
- Adjuncts in Higher Education in the United States
- Administrator Preparation
- Adolescence
- Advanced Placement and International Baccalaureate Courses
- Advocacy and Activism in Early Childhood
- African American Racial Identity and Learning
- Alaska Native Education
- Alternative Certification Programs for Educators
- Alternative Schools
- American Indian Education
- Animals in Environmental Education
- Art Education
- Artificial Intelligence and Learning
- Assessing School Leader Effectiveness
- Assessment, Behavioral
- Assessment, Educational
- Assessment in Early Childhood Education
- Assistive Technology
- Augmented Reality in Education
- Beginning-Teacher Induction
- Bilingual Education and Bilingualism
- Black Undergraduate Women: Critical Race and Gender Perspe...
- Blended Learning
- Case Study in Education Research
- Changing Professional and Academic Identities
- Character Education
- Children’s and Young Adult Literature
- Children's Beliefs about Intelligence
- Children's Rights in Early Childhood Education
- Citizenship Education
- Civic and Social Engagement of Higher Education
- Classroom Learning Environments: Assessing and Investigati...
- Classroom Management
- Coherent Instructional Systems at the School and School Sy...
- College Admissions in the United States
- College Athletics in the United States
- Community Relations
- Comparative Education
- Computer-Assisted Language Learning
- Computer-Based Testing
- Conceptualizing, Measuring, and Evaluating Improvement Net...
- Continuous Improvement and "High Leverage" Educational Pro...
- Counseling in Schools
- Critical Approaches to Gender in Higher Education
- Critical Perspectives on Educational Innovation and Improv...
- Critical Race Theory
- Crossborder and Transnational Higher Education
- Cross-National Research on Continuous Improvement
- Cross-Sector Research on Continuous Learning and Improveme...
- Cultural Diversity in Early Childhood Education
- Culturally Responsive Leadership
- Culturally Responsive Pedagogies
- Culturally Responsive Teacher Education in the United Stat...
- Curriculum Design
- Data Collection in Educational Research
- Data-driven Decision Making in the United States
- Deaf Education
- Desegregation and Integration
- Design Thinking and the Learning Sciences: Theoretical, Pr...
- Development, Moral
- Dialogic Pedagogy
- Digital Age Teacher, The
- Digital Citizenship
- Digital Divides
- Disabilities
- Distance Learning
- Distributed Leadership
- Doctoral Education and Training
- Early Childhood Education and Care (ECEC) in Denmark
- Early Childhood Education and Development in Mexico
- Early Childhood Education in Aotearoa New Zealand
- Early Childhood Education in Australia
- Early Childhood Education in China
- Early Childhood Education in Europe
- Early Childhood Education in Sub-Saharan Africa
- Early Childhood Education in Sweden
- Early Childhood Education Pedagogy
- Early Childhood Education Policy
- Early Childhood Education, The Arts in
- Early Childhood Mathematics
- Early Childhood Science
- Early Childhood Teacher Education
- Early Childhood Teachers in Aotearoa New Zealand
- Early Years Professionalism and Professionalization Polici...
- Economics of Education
- Education For Children with Autism
- Education for Sustainable Development
- Education Leadership, Empirical Perspectives in
- Education of Native Hawaiian Students
- Education Reform and School Change
- Educational Statistics for Longitudinal Research
- Educator Partnerships with Parents and Families with a Foc...
- Emotional and Affective Issues in Environmental and Sustai...
- Emotional and Behavioral Disorders
- Environmental and Science Education: Overlaps and Issues
- Environmental Education
- Environmental Education in Brazil
- Epistemic Beliefs
- Equity and Improvement: Engaging Communities in Educationa...
- Equity, Ethnicity, Diversity, and Excellence in Education
- Ethical Research with Young Children
- Ethics and Education
- Ethics of Teaching
- Ethnic Studies
- Evidence-Based Communication Assessment and Intervention
- Family and Community Partnerships in Education
- Family Day Care
- Federal Government Programs and Issues
- Feminization of Labor in Academia
- Finance, Education
- Financial Aid
- Formative Assessment
- Future-Focused Education
- Gender and Achievement
- Gender and Alternative Education
- Gender-Based Violence on University Campuses
- Gifted Education
- Global Mindedness and Global Citizenship Education
- Global University Rankings
- Governance, Education
- Grounded Theory
- Growth of Effective Mental Health Services in Schools in t...
- Higher Education and Globalization
- Higher Education and the Developing World
- Higher Education Faculty Characteristics and Trends in the...
- Higher Education Finance
- Higher Education Governance
- Higher Education Graduate Outcomes and Destinations
- Higher Education in Africa
- Higher Education in China
- Higher Education in Latin America
- Higher Education in the United States, Historical Evolutio...
- Higher Education, International Issues in
- Higher Education Management
- Higher Education Policy
- Higher Education Student Assessment
- High-stakes Testing
- History of Early Childhood Education in the United States
- History of Education in the United States
- History of Technology Integration in Education
- Homeschooling
- Inclusion in Early Childhood: Difference, Disability, and ...
- Inclusive Education
- Indigenous Education in a Global Context
- Indigenous Learning Environments
- Indigenous Students in Higher Education in the United Stat...
- Infant and Toddler Pedagogy
- Inservice Teacher Education
- Integrating Art across the Curriculum
- Intelligence
- Intensive Interventions for Children and Adolescents with ...
- International Perspectives on Academic Freedom
- Intersectionality and Education
- Knowledge Development in Early Childhood
- Leadership Development, Coaching and Feedback for
- Leadership in Early Childhood Education
- Leadership Training with an Emphasis on the United States
- Learning Analytics in Higher Education
- Learning Difficulties
- Learning, Lifelong
- Learning, Multimedia
- Learning Strategies
- Legal Matters and Education Law
- LGBT Youth in Schools
- Linguistic Diversity
- Linguistically Inclusive Pedagogy
- Literacy Development and Language Acquisition
- Literature Reviews
- Mathematics Identity
- Mathematics Instruction and Interventions for Students wit...
- Mathematics Teacher Education
- Measurement for Improvement in Education
- Measurement in Education in the United States
- Methodological Approaches for Impact Evaluation in Educati...
- Mindfulness, Learning, and Education
- Motherscholars
- Multiliteracies in Early Childhood Education
- Multiple Documents Literacy: Theory, Research, and Applica...
- Multivariate Research Methodology
- Museums, Education, and Curriculum
- Music Education
- Narrative Research in Education
- Native American Studies
- Note-Taking
- Numeracy Education
- One-to-One Technology in the K-12 Classroom
- Online Education
- Open Education
- Organizing for Continuous Improvement in Education
- Organizing Schools for the Inclusion of Students with Disa...
- Outdoor Play and Learning
- Outdoor Play and Learning in Early Childhood Education
- Pedagogical Leadership
- Pedagogy of Teacher Education, A
- Performance Objectives and Measurement
- Performance-based Research Assessment in Higher Education
- Performance-based Research Funding
- Phenomenology in Educational Research
- Physical Education
- Podcasts in Education
- Policy Context of United States Educational Innovation and...
- Portable Technology Use in Special Education Programs and ...
- Post-humanism and Environmental Education
- Pre-Service Teacher Education
- Problem Solving
- Productivity and Higher Education
- Professional Development
- Professional Learning Communities
- Program Evaluation
- Programs and Services for Students with Emotional or Behav...
- Psychology Learning and Teaching
- Psychometric Issues in the Assessment of English Language ...
- Qualitative, Quantitative, and Mixed Methods Research Samp...
- Qualitative Research Design
- Quantitative Research Designs in Educational Research
- Queering the English Language Arts (ELA) Writing Classroom
- Race and Affirmative Action in Higher Education
- Reading Education
- Refugee and New Immigrant Learners
- Relational and Developmental Trauma and Schools
- Relational Pedagogies in Early Childhood Education
- Reliability in Educational Assessments
- Religion in Elementary and Secondary Education in the Unit...
- Researcher Development and Skills Training within the Cont...
- Research-Practice Partnerships in Education within the Uni...
- Response to Intervention
- Restorative Practices
- Risky Play in Early Childhood Education
- Scale and Sustainability of Education Innovation and Impro...
- Scaling Up Research-based Educational Practices
- School Accreditation
- School Choice
- School Culture
- School District Budgeting and Financial Management in the ...
- School Improvement through Inclusive Education
- School Reform
- Schools, Private and Independent
- School-Wide Positive Behavior Support
- Science Education
- Secondary to Postsecondary Transition Issues
- Self-Regulated Learning
- Self-Study of Teacher Education Practices
- Service-Learning
- Severe Disabilities
- Single Salary Schedule
- Single-sex Education
- Single-Subject Research Design
- Social Context of Education
- Social Justice
- Social Network Analysis
- Social Pedagogy
- Social Science and Education Research
- Social Studies Education
- Sociology of Education
- Standards-Based Education
- Statistical Assumptions
- Student Access, Equity, and Diversity in Higher Education
- Student Assignment Policy
- Student Engagement in Tertiary Education
- Student Learning, Development, Engagement, and Motivation ...
- Student Participation
- Student Voice in Teacher Development
- Sustainability Education in Early Childhood Education
- Sustainability in Early Childhood Education
- Sustainability in Higher Education
- Teacher Beliefs and Epistemologies
- Teacher Collaboration in School Improvement
- Teacher Evaluation and Teacher Effectiveness
- Teacher Preparation
- Teacher Training and Development
- Teacher Unions and Associations
- Teacher-Student Relationships
- Teaching Critical Thinking
- Technologies, Teaching, and Learning in Higher Education
- Technology Education in Early Childhood
- Technology, Educational
- Technology-based Assessment
- The Bologna Process
- The Regulation of Standards in Higher Education
- Theories of Educational Leadership
- Three Conceptions of Literacy: Media, Narrative, and Gamin...
- Tracking and Detracking
- Traditions of Quality Improvement in Education
- Transformative Learning
- Transitions in Early Childhood Education
- Tribally Controlled Colleges and Universities in the Unite...
- Understanding the Psycho-Social Dimensions of Schools and ...
- University Faculty Roles and Responsibilities in the Unite...
- Using Ethnography in Educational Research
- Value of Higher Education for Students and Other Stakehold...
- Virtual Learning Environments
- Vocational and Technical Education
- Wellness and Well-Being in Education
- Women's and Gender Studies
- Young Children and Spirituality
- Young Children's Learning Dispositions
- Young Children's Working Theories
- Privacy Policy
- Cookie Policy
- Legal Notice
- Accessibility
Powered by:
- [66.249.64.20|193.7.198.129]
- 193.7.198.129

- University of Texas Libraries

Literature Reviews
- What is a literature review?
- Steps in the Literature Review Process
- Define your research question
- Determine inclusion and exclusion criteria
- Choose databases and search
- Review Results
- Synthesize Results
- Analyze Results
- Librarian Support
What is a Literature Review?
A literature or narrative review is a comprehensive review and analysis of the published literature on a specific topic or research question. The literature that is reviewed contains: books, articles, academic articles, conference proceedings, association papers, and dissertations. It contains the most pertinent studies and points to important past and current research and practices. It provides background and context, and shows how your research will contribute to the field.
A literature review should:
- Provide a comprehensive and updated review of the literature;
- Explain why this review has taken place;
- Articulate a position or hypothesis;
- Acknowledge and account for conflicting and corroborating points of view
From S age Research Methods
Purpose of a Literature Review
A literature review can be written as an introduction to a study to:
- Demonstrate how a study fills a gap in research
- Compare a study with other research that's been done
Or it can be a separate work (a research article on its own) which:
- Organizes or describes a topic
- Describes variables within a particular issue/problem
Limitations of a Literature Review
Some of the limitations of a literature review are:
- It's a snapshot in time. Unlike other reviews, this one has beginning, a middle and an end. There may be future developments that could make your work less relevant.
- It may be too focused. Some niche studies may miss the bigger picture.
- It can be difficult to be comprehensive. There is no way to make sure all the literature on a topic was considered.
- It is easy to be biased if you stick to top tier journals. There may be other places where people are publishing exemplary research. Look to open access publications and conferences to reflect a more inclusive collection. Also, make sure to include opposing views (and not just supporting evidence).
Source: Grant, Maria J., and Andrew Booth. “A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies.” Health Information & Libraries Journal, vol. 26, no. 2, June 2009, pp. 91–108. Wiley Online Library, doi:10.1111/j.1471-1842.2009.00848.x.
Meryl Brodsky : Communication and Information Studies
Hannah Chapman Tripp : Biology, Neuroscience
Carolyn Cunningham : Human Development & Family Sciences, Psychology, Sociology
Larayne Dallas : Engineering
Janelle Hedstrom : Special Education, Curriculum & Instruction, Ed Leadership & Policy
Susan Macicak : Linguistics
Imelda Vetter : Dell Medical School
For help in other subject areas, please see the guide to library specialists by subject .
Periodically, UT Libraries runs a workshop covering the basics and library support for literature reviews. While we try to offer these once per academic year, we find providing the recording to be helpful to community members who have missed the session. Following is the most recent recording of the workshop, Conducting a Literature Review. To view the recording, a UT login is required.
- October 26, 2022 recording
- Last Updated: Oct 26, 2022 2:49 PM
- URL: https://guides.lib.utexas.edu/literaturereviews

Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

How are medication errors defined? A systematic literature review of definitions and characteristics

2010, International Journal for Quality in Health Care
Related Papers
Scandinavian Journal of Public Health
Birgitte Brock
British Journal of Clinical Pharmacology
Jeffrey Aronson
Systematic Reviews in Pharmacy
Ratanto Ratanto
The medication process is carried out by a professional team, namely pharmacists, doctors and nurses. Aim: To identify and analyse the type and factors that influence medication error in Hospital and also the responsibility of each profession in ensuring drug safety. Method: The scoping review is carried out through the study method, with keywords; error medication, safety medication, medication and nurse responsibility, medication and doctor's responsibility, drug and pharmacist responsibility, determinants of error medication, how to reduce error medication. The manuscript database is accessed from Google Scholar, with an English and Indonesian language and published in 2021. Result: A total of twenty-one (21) studies from fourteen countries matched with the inclusion criteria. Scoping area described error medication types. determinant of MEs, roles of the health provider and effort to reduce MEs incident. Conclusion: Medication errors are dangerous incidents so that each heal...
PsycEXTRA Dataset
European Journal of Hospital Pharmacy
Pietro Minuz
International Journal of Clinical Practice
Christine Lu
Rabiu Abubakar , Nordin Bin Simbak
The primary goal of medicine is to achieve positive therapeutic outcome while carefully minimizing patient risk. However, with the advancement made in the technology of drug discovery and formulation new medicines are flooding to the drug-market. Although newly launched medicines are opening lot more avenues and opportunities for patient care but also harboring new hazards. Medication errors (MEs) are common in health care system all over world. These errors are more dangerous especially in developing countries were patients’ right is not well protected. It contributes significantly to drug-related complications which range from mild damage to more severe event leading to hospitalization. Various health care professionals’ attitudes as well as system failure contribute to MEs. It has become necessary for every health care professional to understand the nature and sources of MEs and try to find solution. Sources of MEs are multi-factorials and multi-disciplinary that require careful detection, assessment and intervention. Several MEs preventive strategies were identified which if properly implemented will significantly improve health care delivery services. The purpose of this work is to highlight the role of health care professionals in MEs; identify the common sources of MEs and discuss the proper MEs preventive strategies.
South Asian Research Journal of Pharmaceutical Sciences
Daniel Singh
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
What is a literature review?

A literature review is a critical analysis of the literature related to your research topic. It evaluates and critiques the literature to establish a theoretical framework for your research topic and/or identify a gap in the existing research that your research will address.
A literature review is not a summary of the literature. You need to engage deeply and critically with the literature. Your literature review should show your understanding of the literature related to your research topic and lead to presenting a rationale for your research.
A literature review focuses on:
- the context of the topic
- key concepts, ideas, theories and methodologies
- key researchers, texts and seminal works
- major issues and debates
- identifying conflicting evidence
- the main questions that have been asked around the topic
- the organisation of knowledge on the topic
- definitions, particularly those that are contested
- showing how your research will advance scholarly knowledge (generally referred to as identifying the ‘gap’).
This module will guide you through the functions of a literature review; the typical process of conducting a literature review (including searching for literature and taking notes); structuring your literature review within your thesis and organising its internal ideas; and styling the language of your literature review.
The purposes of a literature review
A literature review serves two main purposes:
1) To show awareness of the present state of knowledge in a particular field, including:
- seminal authors
- the main empirical research
- theoretical positions
- controversies
- breakthroughs as well as links to other related areas of knowledge.
2) To provide a foundation for the author’s research. To do that, the literature review needs to:
- help the researcher define a hypothesis or a research question, and how answering the question will contribute to the body of knowledge;
- provide a rationale for investigating the problem and the selected methodology;
- provide a particular theoretical lens, support the argument, or identify gaps.
Before you engage further with this module, try the quiz below to see how much you already know about literature reviews.
Research and Writing Skills for Academic and Graduate Researchers Copyright © 2022 by RMIT University is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lau F, Kuziemsky C, editors. Handbook of eHealth Evaluation: An Evidence-based Approach [Internet]. Victoria (BC): University of Victoria; 2017 Feb 27.

Handbook of eHealth Evaluation: An Evidence-based Approach [Internet].
Chapter 9 methods for literature reviews.
Guy Paré and Spyros Kitsiou .
9.1. Introduction
Literature reviews play a critical role in scholarship because science remains, first and foremost, a cumulative endeavour ( vom Brocke et al., 2009 ). As in any academic discipline, rigorous knowledge syntheses are becoming indispensable in keeping up with an exponentially growing eHealth literature, assisting practitioners, academics, and graduate students in finding, evaluating, and synthesizing the contents of many empirical and conceptual papers. Among other methods, literature reviews are essential for: (a) identifying what has been written on a subject or topic; (b) determining the extent to which a specific research area reveals any interpretable trends or patterns; (c) aggregating empirical findings related to a narrow research question to support evidence-based practice; (d) generating new frameworks and theories; and (e) identifying topics or questions requiring more investigation ( Paré, Trudel, Jaana, & Kitsiou, 2015 ).
Literature reviews can take two major forms. The most prevalent one is the “literature review” or “background” section within a journal paper or a chapter in a graduate thesis. This section synthesizes the extant literature and usually identifies the gaps in knowledge that the empirical study addresses ( Sylvester, Tate, & Johnstone, 2013 ). It may also provide a theoretical foundation for the proposed study, substantiate the presence of the research problem, justify the research as one that contributes something new to the cumulated knowledge, or validate the methods and approaches for the proposed study ( Hart, 1998 ; Levy & Ellis, 2006 ).
The second form of literature review, which is the focus of this chapter, constitutes an original and valuable work of research in and of itself ( Paré et al., 2015 ). Rather than providing a base for a researcher’s own work, it creates a solid starting point for all members of the community interested in a particular area or topic ( Mulrow, 1987 ). The so-called “review article” is a journal-length paper which has an overarching purpose to synthesize the literature in a field, without collecting or analyzing any primary data ( Green, Johnson, & Adams, 2006 ).
When appropriately conducted, review articles represent powerful information sources for practitioners looking for state-of-the art evidence to guide their decision-making and work practices ( Paré et al., 2015 ). Further, high-quality reviews become frequently cited pieces of work which researchers seek out as a first clear outline of the literature when undertaking empirical studies ( Cooper, 1988 ; Rowe, 2014 ). Scholars who track and gauge the impact of articles have found that review papers are cited and downloaded more often than any other type of published article ( Cronin, Ryan, & Coughlan, 2008 ; Montori, Wilczynski, Morgan, Haynes, & Hedges, 2003 ; Patsopoulos, Analatos, & Ioannidis, 2005 ). The reason for their popularity may be the fact that reading the review enables one to have an overview, if not a detailed knowledge of the area in question, as well as references to the most useful primary sources ( Cronin et al., 2008 ). Although they are not easy to conduct, the commitment to complete a review article provides a tremendous service to one’s academic community ( Paré et al., 2015 ; Petticrew & Roberts, 2006 ). Most, if not all, peer-reviewed journals in the fields of medical informatics publish review articles of some type.
The main objectives of this chapter are fourfold: (a) to provide an overview of the major steps and activities involved in conducting a stand-alone literature review; (b) to describe and contrast the different types of review articles that can contribute to the eHealth knowledge base; (c) to illustrate each review type with one or two examples from the eHealth literature; and (d) to provide a series of recommendations for prospective authors of review articles in this domain.
9.2. Overview of the Literature Review Process and Steps
As explained in Templier and Paré (2015) , there are six generic steps involved in conducting a review article:
- formulating the research question(s) and objective(s),
- searching the extant literature,
- screening for inclusion,
- assessing the quality of primary studies,
- extracting data, and
- analyzing data.
Although these steps are presented here in sequential order, one must keep in mind that the review process can be iterative and that many activities can be initiated during the planning stage and later refined during subsequent phases ( Finfgeld-Connett & Johnson, 2013 ; Kitchenham & Charters, 2007 ).
Formulating the research question(s) and objective(s): As a first step, members of the review team must appropriately justify the need for the review itself ( Petticrew & Roberts, 2006 ), identify the review’s main objective(s) ( Okoli & Schabram, 2010 ), and define the concepts or variables at the heart of their synthesis ( Cooper & Hedges, 2009 ; Webster & Watson, 2002 ). Importantly, they also need to articulate the research question(s) they propose to investigate ( Kitchenham & Charters, 2007 ). In this regard, we concur with Jesson, Matheson, and Lacey (2011) that clearly articulated research questions are key ingredients that guide the entire review methodology; they underscore the type of information that is needed, inform the search for and selection of relevant literature, and guide or orient the subsequent analysis. Searching the extant literature: The next step consists of searching the literature and making decisions about the suitability of material to be considered in the review ( Cooper, 1988 ). There exist three main coverage strategies. First, exhaustive coverage means an effort is made to be as comprehensive as possible in order to ensure that all relevant studies, published and unpublished, are included in the review and, thus, conclusions are based on this all-inclusive knowledge base. The second type of coverage consists of presenting materials that are representative of most other works in a given field or area. Often authors who adopt this strategy will search for relevant articles in a small number of top-tier journals in a field ( Paré et al., 2015 ). In the third strategy, the review team concentrates on prior works that have been central or pivotal to a particular topic. This may include empirical studies or conceptual papers that initiated a line of investigation, changed how problems or questions were framed, introduced new methods or concepts, or engendered important debate ( Cooper, 1988 ). Screening for inclusion: The following step consists of evaluating the applicability of the material identified in the preceding step ( Levy & Ellis, 2006 ; vom Brocke et al., 2009 ). Once a group of potential studies has been identified, members of the review team must screen them to determine their relevance ( Petticrew & Roberts, 2006 ). A set of predetermined rules provides a basis for including or excluding certain studies. This exercise requires a significant investment on the part of researchers, who must ensure enhanced objectivity and avoid biases or mistakes. As discussed later in this chapter, for certain types of reviews there must be at least two independent reviewers involved in the screening process and a procedure to resolve disagreements must also be in place ( Liberati et al., 2009 ; Shea et al., 2009 ). Assessing the quality of primary studies: In addition to screening material for inclusion, members of the review team may need to assess the scientific quality of the selected studies, that is, appraise the rigour of the research design and methods. Such formal assessment, which is usually conducted independently by at least two coders, helps members of the review team refine which studies to include in the final sample, determine whether or not the differences in quality may affect their conclusions, or guide how they analyze the data and interpret the findings ( Petticrew & Roberts, 2006 ). Ascribing quality scores to each primary study or considering through domain-based evaluations which study components have or have not been designed and executed appropriately makes it possible to reflect on the extent to which the selected study addresses possible biases and maximizes validity ( Shea et al., 2009 ). Extracting data: The following step involves gathering or extracting applicable information from each primary study included in the sample and deciding what is relevant to the problem of interest ( Cooper & Hedges, 2009 ). Indeed, the type of data that should be recorded mainly depends on the initial research questions ( Okoli & Schabram, 2010 ). However, important information may also be gathered about how, when, where and by whom the primary study was conducted, the research design and methods, or qualitative/quantitative results ( Cooper & Hedges, 2009 ). Analyzing and synthesizing data : As a final step, members of the review team must collate, summarize, aggregate, organize, and compare the evidence extracted from the included studies. The extracted data must be presented in a meaningful way that suggests a new contribution to the extant literature ( Jesson et al., 2011 ). Webster and Watson (2002) warn researchers that literature reviews should be much more than lists of papers and should provide a coherent lens to make sense of extant knowledge on a given topic. There exist several methods and techniques for synthesizing quantitative (e.g., frequency analysis, meta-analysis) and qualitative (e.g., grounded theory, narrative analysis, meta-ethnography) evidence ( Dixon-Woods, Agarwal, Jones, Young, & Sutton, 2005 ; Thomas & Harden, 2008 ).
9.3. Types of Review Articles and Brief Illustrations
EHealth researchers have at their disposal a number of approaches and methods for making sense out of existing literature, all with the purpose of casting current research findings into historical contexts or explaining contradictions that might exist among a set of primary research studies conducted on a particular topic. Our classification scheme is largely inspired from Paré and colleagues’ (2015) typology. Below we present and illustrate those review types that we feel are central to the growth and development of the eHealth domain.
9.3.1. Narrative Reviews
The narrative review is the “traditional” way of reviewing the extant literature and is skewed towards a qualitative interpretation of prior knowledge ( Sylvester et al., 2013 ). Put simply, a narrative review attempts to summarize or synthesize what has been written on a particular topic but does not seek generalization or cumulative knowledge from what is reviewed ( Davies, 2000 ; Green et al., 2006 ). Instead, the review team often undertakes the task of accumulating and synthesizing the literature to demonstrate the value of a particular point of view ( Baumeister & Leary, 1997 ). As such, reviewers may selectively ignore or limit the attention paid to certain studies in order to make a point. In this rather unsystematic approach, the selection of information from primary articles is subjective, lacks explicit criteria for inclusion and can lead to biased interpretations or inferences ( Green et al., 2006 ). There are several narrative reviews in the particular eHealth domain, as in all fields, which follow such an unstructured approach ( Silva et al., 2015 ; Paul et al., 2015 ).
Despite these criticisms, this type of review can be very useful in gathering together a volume of literature in a specific subject area and synthesizing it. As mentioned above, its primary purpose is to provide the reader with a comprehensive background for understanding current knowledge and highlighting the significance of new research ( Cronin et al., 2008 ). Faculty like to use narrative reviews in the classroom because they are often more up to date than textbooks, provide a single source for students to reference, and expose students to peer-reviewed literature ( Green et al., 2006 ). For researchers, narrative reviews can inspire research ideas by identifying gaps or inconsistencies in a body of knowledge, thus helping researchers to determine research questions or formulate hypotheses. Importantly, narrative reviews can also be used as educational articles to bring practitioners up to date with certain topics of issues ( Green et al., 2006 ).
Recently, there have been several efforts to introduce more rigour in narrative reviews that will elucidate common pitfalls and bring changes into their publication standards. Information systems researchers, among others, have contributed to advancing knowledge on how to structure a “traditional” review. For instance, Levy and Ellis (2006) proposed a generic framework for conducting such reviews. Their model follows the systematic data processing approach comprised of three steps, namely: (a) literature search and screening; (b) data extraction and analysis; and (c) writing the literature review. They provide detailed and very helpful instructions on how to conduct each step of the review process. As another methodological contribution, vom Brocke et al. (2009) offered a series of guidelines for conducting literature reviews, with a particular focus on how to search and extract the relevant body of knowledge. Last, Bandara, Miskon, and Fielt (2011) proposed a structured, predefined and tool-supported method to identify primary studies within a feasible scope, extract relevant content from identified articles, synthesize and analyze the findings, and effectively write and present the results of the literature review. We highly recommend that prospective authors of narrative reviews consult these useful sources before embarking on their work.
Darlow and Wen (2015) provide a good example of a highly structured narrative review in the eHealth field. These authors synthesized published articles that describe the development process of mobile health ( m-health ) interventions for patients’ cancer care self-management. As in most narrative reviews, the scope of the research questions being investigated is broad: (a) how development of these systems are carried out; (b) which methods are used to investigate these systems; and (c) what conclusions can be drawn as a result of the development of these systems. To provide clear answers to these questions, a literature search was conducted on six electronic databases and Google Scholar . The search was performed using several terms and free text words, combining them in an appropriate manner. Four inclusion and three exclusion criteria were utilized during the screening process. Both authors independently reviewed each of the identified articles to determine eligibility and extract study information. A flow diagram shows the number of studies identified, screened, and included or excluded at each stage of study selection. In terms of contributions, this review provides a series of practical recommendations for m-health intervention development.
9.3.2. Descriptive or Mapping Reviews
The primary goal of a descriptive review is to determine the extent to which a body of knowledge in a particular research topic reveals any interpretable pattern or trend with respect to pre-existing propositions, theories, methodologies or findings ( King & He, 2005 ; Paré et al., 2015 ). In contrast with narrative reviews, descriptive reviews follow a systematic and transparent procedure, including searching, screening and classifying studies ( Petersen, Vakkalanka, & Kuzniarz, 2015 ). Indeed, structured search methods are used to form a representative sample of a larger group of published works ( Paré et al., 2015 ). Further, authors of descriptive reviews extract from each study certain characteristics of interest, such as publication year, research methods, data collection techniques, and direction or strength of research outcomes (e.g., positive, negative, or non-significant) in the form of frequency analysis to produce quantitative results ( Sylvester et al., 2013 ). In essence, each study included in a descriptive review is treated as the unit of analysis and the published literature as a whole provides a database from which the authors attempt to identify any interpretable trends or draw overall conclusions about the merits of existing conceptualizations, propositions, methods or findings ( Paré et al., 2015 ). In doing so, a descriptive review may claim that its findings represent the state of the art in a particular domain ( King & He, 2005 ).
In the fields of health sciences and medical informatics, reviews that focus on examining the range, nature and evolution of a topic area are described by Anderson, Allen, Peckham, and Goodwin (2008) as mapping reviews . Like descriptive reviews, the research questions are generic and usually relate to publication patterns and trends. There is no preconceived plan to systematically review all of the literature although this can be done. Instead, researchers often present studies that are representative of most works published in a particular area and they consider a specific time frame to be mapped.
An example of this approach in the eHealth domain is offered by DeShazo, Lavallie, and Wolf (2009). The purpose of this descriptive or mapping review was to characterize publication trends in the medical informatics literature over a 20-year period (1987 to 2006). To achieve this ambitious objective, the authors performed a bibliometric analysis of medical informatics citations indexed in medline using publication trends, journal frequencies, impact factors, Medical Subject Headings (MeSH) term frequencies, and characteristics of citations. Findings revealed that there were over 77,000 medical informatics articles published during the covered period in numerous journals and that the average annual growth rate was 12%. The MeSH term analysis also suggested a strong interdisciplinary trend. Finally, average impact scores increased over time with two notable growth periods. Overall, patterns in research outputs that seem to characterize the historic trends and current components of the field of medical informatics suggest it may be a maturing discipline (DeShazo et al., 2009).
9.3.3. Scoping Reviews
Scoping reviews attempt to provide an initial indication of the potential size and nature of the extant literature on an emergent topic (Arksey & O’Malley, 2005; Daudt, van Mossel, & Scott, 2013 ; Levac, Colquhoun, & O’Brien, 2010). A scoping review may be conducted to examine the extent, range and nature of research activities in a particular area, determine the value of undertaking a full systematic review (discussed next), or identify research gaps in the extant literature ( Paré et al., 2015 ). In line with their main objective, scoping reviews usually conclude with the presentation of a detailed research agenda for future works along with potential implications for both practice and research.
Unlike narrative and descriptive reviews, the whole point of scoping the field is to be as comprehensive as possible, including grey literature (Arksey & O’Malley, 2005). Inclusion and exclusion criteria must be established to help researchers eliminate studies that are not aligned with the research questions. It is also recommended that at least two independent coders review abstracts yielded from the search strategy and then the full articles for study selection ( Daudt et al., 2013 ). The synthesized evidence from content or thematic analysis is relatively easy to present in tabular form (Arksey & O’Malley, 2005; Thomas & Harden, 2008 ).
One of the most highly cited scoping reviews in the eHealth domain was published by Archer, Fevrier-Thomas, Lokker, McKibbon, and Straus (2011) . These authors reviewed the existing literature on personal health record ( phr ) systems including design, functionality, implementation, applications, outcomes, and benefits. Seven databases were searched from 1985 to March 2010. Several search terms relating to phr s were used during this process. Two authors independently screened titles and abstracts to determine inclusion status. A second screen of full-text articles, again by two independent members of the research team, ensured that the studies described phr s. All in all, 130 articles met the criteria and their data were extracted manually into a database. The authors concluded that although there is a large amount of survey, observational, cohort/panel, and anecdotal evidence of phr benefits and satisfaction for patients, more research is needed to evaluate the results of phr implementations. Their in-depth analysis of the literature signalled that there is little solid evidence from randomized controlled trials or other studies through the use of phr s. Hence, they suggested that more research is needed that addresses the current lack of understanding of optimal functionality and usability of these systems, and how they can play a beneficial role in supporting patient self-management ( Archer et al., 2011 ).
9.3.4. Forms of Aggregative Reviews
Healthcare providers, practitioners, and policy-makers are nowadays overwhelmed with large volumes of information, including research-based evidence from numerous clinical trials and evaluation studies, assessing the effectiveness of health information technologies and interventions ( Ammenwerth & de Keizer, 2004 ; Deshazo et al., 2009 ). It is unrealistic to expect that all these disparate actors will have the time, skills, and necessary resources to identify the available evidence in the area of their expertise and consider it when making decisions. Systematic reviews that involve the rigorous application of scientific strategies aimed at limiting subjectivity and bias (i.e., systematic and random errors) can respond to this challenge.
Systematic reviews attempt to aggregate, appraise, and synthesize in a single source all empirical evidence that meet a set of previously specified eligibility criteria in order to answer a clearly formulated and often narrow research question on a particular topic of interest to support evidence-based practice ( Liberati et al., 2009 ). They adhere closely to explicit scientific principles ( Liberati et al., 2009 ) and rigorous methodological guidelines (Higgins & Green, 2008) aimed at reducing random and systematic errors that can lead to deviations from the truth in results or inferences. The use of explicit methods allows systematic reviews to aggregate a large body of research evidence, assess whether effects or relationships are in the same direction and of the same general magnitude, explain possible inconsistencies between study results, and determine the strength of the overall evidence for every outcome of interest based on the quality of included studies and the general consistency among them ( Cook, Mulrow, & Haynes, 1997 ). The main procedures of a systematic review involve:
- Formulating a review question and developing a search strategy based on explicit inclusion criteria for the identification of eligible studies (usually described in the context of a detailed review protocol).
- Searching for eligible studies using multiple databases and information sources, including grey literature sources, without any language restrictions.
- Selecting studies, extracting data, and assessing risk of bias in a duplicate manner using two independent reviewers to avoid random or systematic errors in the process.
- Analyzing data using quantitative or qualitative methods.
- Presenting results in summary of findings tables.
- Interpreting results and drawing conclusions.
Many systematic reviews, but not all, use statistical methods to combine the results of independent studies into a single quantitative estimate or summary effect size. Known as meta-analyses , these reviews use specific data extraction and statistical techniques (e.g., network, frequentist, or Bayesian meta-analyses) to calculate from each study by outcome of interest an effect size along with a confidence interval that reflects the degree of uncertainty behind the point estimate of effect ( Borenstein, Hedges, Higgins, & Rothstein, 2009 ; Deeks, Higgins, & Altman, 2008 ). Subsequently, they use fixed or random-effects analysis models to combine the results of the included studies, assess statistical heterogeneity, and calculate a weighted average of the effect estimates from the different studies, taking into account their sample sizes. The summary effect size is a value that reflects the average magnitude of the intervention effect for a particular outcome of interest or, more generally, the strength of a relationship between two variables across all studies included in the systematic review. By statistically combining data from multiple studies, meta-analyses can create more precise and reliable estimates of intervention effects than those derived from individual studies alone, when these are examined independently as discrete sources of information.
The review by Gurol-Urganci, de Jongh, Vodopivec-Jamsek, Atun, and Car (2013) on the effects of mobile phone messaging reminders for attendance at healthcare appointments is an illustrative example of a high-quality systematic review with meta-analysis. Missed appointments are a major cause of inefficiency in healthcare delivery with substantial monetary costs to health systems. These authors sought to assess whether mobile phone-based appointment reminders delivered through Short Message Service ( sms ) or Multimedia Messaging Service ( mms ) are effective in improving rates of patient attendance and reducing overall costs. To this end, they conducted a comprehensive search on multiple databases using highly sensitive search strategies without language or publication-type restrictions to identify all rct s that are eligible for inclusion. In order to minimize the risk of omitting eligible studies not captured by the original search, they supplemented all electronic searches with manual screening of trial registers and references contained in the included studies. Study selection, data extraction, and risk of bias assessments were performed independently by two coders using standardized methods to ensure consistency and to eliminate potential errors. Findings from eight rct s involving 6,615 participants were pooled into meta-analyses to calculate the magnitude of effects that mobile text message reminders have on the rate of attendance at healthcare appointments compared to no reminders and phone call reminders.
Meta-analyses are regarded as powerful tools for deriving meaningful conclusions. However, there are situations in which it is neither reasonable nor appropriate to pool studies together using meta-analytic methods simply because there is extensive clinical heterogeneity between the included studies or variation in measurement tools, comparisons, or outcomes of interest. In these cases, systematic reviews can use qualitative synthesis methods such as vote counting, content analysis, classification schemes and tabulations, as an alternative approach to narratively synthesize the results of the independent studies included in the review. This form of review is known as qualitative systematic review.
A rigorous example of one such review in the eHealth domain is presented by Mickan, Atherton, Roberts, Heneghan, and Tilson (2014) on the use of handheld computers by healthcare professionals and their impact on access to information and clinical decision-making. In line with the methodological guidelines for systematic reviews, these authors: (a) developed and registered with prospero ( www.crd.york.ac.uk/ prospero / ) an a priori review protocol; (b) conducted comprehensive searches for eligible studies using multiple databases and other supplementary strategies (e.g., forward searches); and (c) subsequently carried out study selection, data extraction, and risk of bias assessments in a duplicate manner to eliminate potential errors in the review process. Heterogeneity between the included studies in terms of reported outcomes and measures precluded the use of meta-analytic methods. To this end, the authors resorted to using narrative analysis and synthesis to describe the effectiveness of handheld computers on accessing information for clinical knowledge, adherence to safety and clinical quality guidelines, and diagnostic decision-making.
In recent years, the number of systematic reviews in the field of health informatics has increased considerably. Systematic reviews with discordant findings can cause great confusion and make it difficult for decision-makers to interpret the review-level evidence ( Moher, 2013 ). Therefore, there is a growing need for appraisal and synthesis of prior systematic reviews to ensure that decision-making is constantly informed by the best available accumulated evidence. Umbrella reviews , also known as overviews of systematic reviews, are tertiary types of evidence synthesis that aim to accomplish this; that is, they aim to compare and contrast findings from multiple systematic reviews and meta-analyses ( Becker & Oxman, 2008 ). Umbrella reviews generally adhere to the same principles and rigorous methodological guidelines used in systematic reviews. However, the unit of analysis in umbrella reviews is the systematic review rather than the primary study ( Becker & Oxman, 2008 ). Unlike systematic reviews that have a narrow focus of inquiry, umbrella reviews focus on broader research topics for which there are several potential interventions ( Smith, Devane, Begley, & Clarke, 2011 ). A recent umbrella review on the effects of home telemonitoring interventions for patients with heart failure critically appraised, compared, and synthesized evidence from 15 systematic reviews to investigate which types of home telemonitoring technologies and forms of interventions are more effective in reducing mortality and hospital admissions ( Kitsiou, Paré, & Jaana, 2015 ).
9.3.5. Realist Reviews
Realist reviews are theory-driven interpretative reviews developed to inform, enhance, or supplement conventional systematic reviews by making sense of heterogeneous evidence about complex interventions applied in diverse contexts in a way that informs policy decision-making ( Greenhalgh, Wong, Westhorp, & Pawson, 2011 ). They originated from criticisms of positivist systematic reviews which centre on their “simplistic” underlying assumptions ( Oates, 2011 ). As explained above, systematic reviews seek to identify causation. Such logic is appropriate for fields like medicine and education where findings of randomized controlled trials can be aggregated to see whether a new treatment or intervention does improve outcomes. However, many argue that it is not possible to establish such direct causal links between interventions and outcomes in fields such as social policy, management, and information systems where for any intervention there is unlikely to be a regular or consistent outcome ( Oates, 2011 ; Pawson, 2006 ; Rousseau, Manning, & Denyer, 2008 ).
To circumvent these limitations, Pawson, Greenhalgh, Harvey, and Walshe (2005) have proposed a new approach for synthesizing knowledge that seeks to unpack the mechanism of how “complex interventions” work in particular contexts. The basic research question — what works? — which is usually associated with systematic reviews changes to: what is it about this intervention that works, for whom, in what circumstances, in what respects and why? Realist reviews have no particular preference for either quantitative or qualitative evidence. As a theory-building approach, a realist review usually starts by articulating likely underlying mechanisms and then scrutinizes available evidence to find out whether and where these mechanisms are applicable ( Shepperd et al., 2009 ). Primary studies found in the extant literature are viewed as case studies which can test and modify the initial theories ( Rousseau et al., 2008 ).
The main objective pursued in the realist review conducted by Otte-Trojel, de Bont, Rundall, and van de Klundert (2014) was to examine how patient portals contribute to health service delivery and patient outcomes. The specific goals were to investigate how outcomes are produced and, most importantly, how variations in outcomes can be explained. The research team started with an exploratory review of background documents and research studies to identify ways in which patient portals may contribute to health service delivery and patient outcomes. The authors identified six main ways which represent “educated guesses” to be tested against the data in the evaluation studies. These studies were identified through a formal and systematic search in four databases between 2003 and 2013. Two members of the research team selected the articles using a pre-established list of inclusion and exclusion criteria and following a two-step procedure. The authors then extracted data from the selected articles and created several tables, one for each outcome category. They organized information to bring forward those mechanisms where patient portals contribute to outcomes and the variation in outcomes across different contexts.
9.3.6. Critical Reviews
Lastly, critical reviews aim to provide a critical evaluation and interpretive analysis of existing literature on a particular topic of interest to reveal strengths, weaknesses, contradictions, controversies, inconsistencies, and/or other important issues with respect to theories, hypotheses, research methods or results ( Baumeister & Leary, 1997 ; Kirkevold, 1997 ). Unlike other review types, critical reviews attempt to take a reflective account of the research that has been done in a particular area of interest, and assess its credibility by using appraisal instruments or critical interpretive methods. In this way, critical reviews attempt to constructively inform other scholars about the weaknesses of prior research and strengthen knowledge development by giving focus and direction to studies for further improvement ( Kirkevold, 1997 ).
Kitsiou, Paré, and Jaana (2013) provide an example of a critical review that assessed the methodological quality of prior systematic reviews of home telemonitoring studies for chronic patients. The authors conducted a comprehensive search on multiple databases to identify eligible reviews and subsequently used a validated instrument to conduct an in-depth quality appraisal. Results indicate that the majority of systematic reviews in this particular area suffer from important methodological flaws and biases that impair their internal validity and limit their usefulness for clinical and decision-making purposes. To this end, they provide a number of recommendations to strengthen knowledge development towards improving the design and execution of future reviews on home telemonitoring.
9.4. Summary
Table 9.1 outlines the main types of literature reviews that were described in the previous sub-sections and summarizes the main characteristics that distinguish one review type from another. It also includes key references to methodological guidelines and useful sources that can be used by eHealth scholars and researchers for planning and developing reviews.
Typology of Literature Reviews (adapted from Paré et al., 2015).
As shown in Table 9.1 , each review type addresses different kinds of research questions or objectives, which subsequently define and dictate the methods and approaches that need to be used to achieve the overarching goal(s) of the review. For example, in the case of narrative reviews, there is greater flexibility in searching and synthesizing articles ( Green et al., 2006 ). Researchers are often relatively free to use a diversity of approaches to search, identify, and select relevant scientific articles, describe their operational characteristics, present how the individual studies fit together, and formulate conclusions. On the other hand, systematic reviews are characterized by their high level of systematicity, rigour, and use of explicit methods, based on an “a priori” review plan that aims to minimize bias in the analysis and synthesis process (Higgins & Green, 2008). Some reviews are exploratory in nature (e.g., scoping/mapping reviews), whereas others may be conducted to discover patterns (e.g., descriptive reviews) or involve a synthesis approach that may include the critical analysis of prior research ( Paré et al., 2015 ). Hence, in order to select the most appropriate type of review, it is critical to know before embarking on a review project, why the research synthesis is conducted and what type of methods are best aligned with the pursued goals.
9.5. Concluding Remarks
In light of the increased use of evidence-based practice and research generating stronger evidence ( Grady et al., 2011 ; Lyden et al., 2013 ), review articles have become essential tools for summarizing, synthesizing, integrating or critically appraising prior knowledge in the eHealth field. As mentioned earlier, when rigorously conducted review articles represent powerful information sources for eHealth scholars and practitioners looking for state-of-the-art evidence. The typology of literature reviews we used herein will allow eHealth researchers, graduate students and practitioners to gain a better understanding of the similarities and differences between review types.
We must stress that this classification scheme does not privilege any specific type of review as being of higher quality than another ( Paré et al., 2015 ). As explained above, each type of review has its own strengths and limitations. Having said that, we realize that the methodological rigour of any review — be it qualitative, quantitative or mixed — is a critical aspect that should be considered seriously by prospective authors. In the present context, the notion of rigour refers to the reliability and validity of the review process described in section 9.2. For one thing, reliability is related to the reproducibility of the review process and steps, which is facilitated by a comprehensive documentation of the literature search process, extraction, coding and analysis performed in the review. Whether the search is comprehensive or not, whether it involves a methodical approach for data extraction and synthesis or not, it is important that the review documents in an explicit and transparent manner the steps and approach that were used in the process of its development. Next, validity characterizes the degree to which the review process was conducted appropriately. It goes beyond documentation and reflects decisions related to the selection of the sources, the search terms used, the period of time covered, the articles selected in the search, and the application of backward and forward searches ( vom Brocke et al., 2009 ). In short, the rigour of any review article is reflected by the explicitness of its methods (i.e., transparency) and the soundness of the approach used. We refer those interested in the concepts of rigour and quality to the work of Templier and Paré (2015) which offers a detailed set of methodological guidelines for conducting and evaluating various types of review articles.
To conclude, our main objective in this chapter was to demystify the various types of literature reviews that are central to the continuous development of the eHealth field. It is our hope that our descriptive account will serve as a valuable source for those conducting, evaluating or using reviews in this important and growing domain.
- Ammenwerth E., de Keizer N. An inventory of evaluation studies of information technology in health care. Trends in evaluation research, 1982-2002. International Journal of Medical Informatics. 2004; 44 (1):44–56. [ PubMed : 15778794 ]
- Anderson S., Allen P., Peckham S., Goodwin N. Asking the right questions: scoping studies in the commissioning of research on the organisation and delivery of health services. Health Research Policy and Systems. 2008; 6 (7):1–12. [ PMC free article : PMC2500008 ] [ PubMed : 18613961 ] [ CrossRef ]
- Archer N., Fevrier-Thomas U., Lokker C., McKibbon K. A., Straus S.E. Personal health records: a scoping review. Journal of American Medical Informatics Association. 2011; 18 (4):515–522. [ PMC free article : PMC3128401 ] [ PubMed : 21672914 ]
- Arksey H., O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005; 8 (1):19–32.
- A systematic, tool-supported method for conducting literature reviews in information systems. Paper presented at the Proceedings of the 19th European Conference on Information Systems ( ecis 2011); June 9 to 11; Helsinki, Finland. 2011.
- Baumeister R. F., Leary M.R. Writing narrative literature reviews. Review of General Psychology. 1997; 1 (3):311–320.
- Becker L. A., Oxman A.D. In: Cochrane handbook for systematic reviews of interventions. Higgins J. P. T., Green S., editors. Hoboken, nj : John Wiley & Sons, Ltd; 2008. Overviews of reviews; pp. 607–631.
- Borenstein M., Hedges L., Higgins J., Rothstein H. Introduction to meta-analysis. Hoboken, nj : John Wiley & Sons Inc; 2009.
- Cook D. J., Mulrow C. D., Haynes B. Systematic reviews: Synthesis of best evidence for clinical decisions. Annals of Internal Medicine. 1997; 126 (5):376–380. [ PubMed : 9054282 ]
- Cooper H., Hedges L.V. In: The handbook of research synthesis and meta-analysis. 2nd ed. Cooper H., Hedges L. V., Valentine J. C., editors. New York: Russell Sage Foundation; 2009. Research synthesis as a scientific process; pp. 3–17.
- Cooper H. M. Organizing knowledge syntheses: A taxonomy of literature reviews. Knowledge in Society. 1988; 1 (1):104–126.
- Cronin P., Ryan F., Coughlan M. Undertaking a literature review: a step-by-step approach. British Journal of Nursing. 2008; 17 (1):38–43. [ PubMed : 18399395 ]
- Darlow S., Wen K.Y. Development testing of mobile health interventions for cancer patient self-management: A review. Health Informatics Journal. 2015 (online before print). [ PubMed : 25916831 ] [ CrossRef ]
- Daudt H. M., van Mossel C., Scott S.J. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. bmc Medical Research Methodology. 2013; 13 :48. [ PMC free article : PMC3614526 ] [ PubMed : 23522333 ] [ CrossRef ]
- Davies P. The relevance of systematic reviews to educational policy and practice. Oxford Review of Education. 2000; 26 (3-4):365–378.
- Deeks J. J., Higgins J. P. T., Altman D.G. In: Cochrane handbook for systematic reviews of interventions. Higgins J. P. T., Green S., editors. Hoboken, nj : John Wiley & Sons, Ltd; 2008. Analysing data and undertaking meta-analyses; pp. 243–296.
- Deshazo J. P., Lavallie D. L., Wolf F.M. Publication trends in the medical informatics literature: 20 years of “Medical Informatics” in mesh . bmc Medical Informatics and Decision Making. 2009; 9 :7. [ PMC free article : PMC2652453 ] [ PubMed : 19159472 ] [ CrossRef ]
- Dixon-Woods M., Agarwal S., Jones D., Young B., Sutton A. Synthesising qualitative and quantitative evidence: a review of possible methods. Journal of Health Services Research and Policy. 2005; 10 (1):45–53. [ PubMed : 15667704 ]
- Finfgeld-Connett D., Johnson E.D. Literature search strategies for conducting knowledge-building and theory-generating qualitative systematic reviews. Journal of Advanced Nursing. 2013; 69 (1):194–204. [ PMC free article : PMC3424349 ] [ PubMed : 22591030 ]
- Grady B., Myers K. M., Nelson E. L., Belz N., Bennett L., Carnahan L. … Guidelines Working Group. Evidence-based practice for telemental health. Telemedicine Journal and E Health. 2011; 17 (2):131–148. [ PubMed : 21385026 ]
- Green B. N., Johnson C. D., Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. Journal of Chiropractic Medicine. 2006; 5 (3):101–117. [ PMC free article : PMC2647067 ] [ PubMed : 19674681 ]
- Greenhalgh T., Wong G., Westhorp G., Pawson R. Protocol–realist and meta-narrative evidence synthesis: evolving standards ( rameses ). bmc Medical Research Methodology. 2011; 11 :115. [ PMC free article : PMC3173389 ] [ PubMed : 21843376 ]
- Gurol-Urganci I., de Jongh T., Vodopivec-Jamsek V., Atun R., Car J. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database System Review. 2013; 12 cd 007458. [ PMC free article : PMC6485985 ] [ PubMed : 24310741 ] [ CrossRef ]
- Hart C. Doing a literature review: Releasing the social science research imagination. London: SAGE Publications; 1998.
- Higgins J. P. T., Green S., editors. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Hoboken, nj : Wiley-Blackwell; 2008.
- Jesson J., Matheson L., Lacey F.M. Doing your literature review: traditional and systematic techniques. Los Angeles & London: SAGE Publications; 2011.
- King W. R., He J. Understanding the role and methods of meta-analysis in IS research. Communications of the Association for Information Systems. 2005; 16 :1.
- Kirkevold M. Integrative nursing research — an important strategy to further the development of nursing science and nursing practice. Journal of Advanced Nursing. 1997; 25 (5):977–984. [ PubMed : 9147203 ]
- Kitchenham B., Charters S. ebse Technical Report Version 2.3. Keele & Durham. uk : Keele University & University of Durham; 2007. Guidelines for performing systematic literature reviews in software engineering.
- Kitsiou S., Paré G., Jaana M. Systematic reviews and meta-analyses of home telemonitoring interventions for patients with chronic diseases: a critical assessment of their methodological quality. Journal of Medical Internet Research. 2013; 15 (7):e150. [ PMC free article : PMC3785977 ] [ PubMed : 23880072 ]
- Kitsiou S., Paré G., Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. Journal of Medical Internet Research. 2015; 17 (3):e63. [ PMC free article : PMC4376138 ] [ PubMed : 25768664 ]
- Levac D., Colquhoun H., O’Brien K. K. Scoping studies: advancing the methodology. Implementation Science. 2010; 5 (1):69. [ PMC free article : PMC2954944 ] [ PubMed : 20854677 ]
- Levy Y., Ellis T.J. A systems approach to conduct an effective literature review in support of information systems research. Informing Science. 2006; 9 :181–211.
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P. A. et al. Moher D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine. 2009; 151 (4):W-65. [ PubMed : 19622512 ]
- Lyden J. R., Zickmund S. L., Bhargava T. D., Bryce C. L., Conroy M. B., Fischer G. S. et al. McTigue K. M. Implementing health information technology in a patient-centered manner: Patient experiences with an online evidence-based lifestyle intervention. Journal for Healthcare Quality. 2013; 35 (5):47–57. [ PubMed : 24004039 ]
- Mickan S., Atherton H., Roberts N. W., Heneghan C., Tilson J.K. Use of handheld computers in clinical practice: a systematic review. bmc Medical Informatics and Decision Making. 2014; 14 :56. [ PMC free article : PMC4099138 ] [ PubMed : 24998515 ]
- Moher D. The problem of duplicate systematic reviews. British Medical Journal. 2013; 347 (5040) [ PubMed : 23945367 ] [ CrossRef ]
- Montori V. M., Wilczynski N. L., Morgan D., Haynes R. B., Hedges T. Systematic reviews: a cross-sectional study of location and citation counts. bmc Medicine. 2003; 1 :2. [ PMC free article : PMC281591 ] [ PubMed : 14633274 ]
- Mulrow C. D. The medical review article: state of the science. Annals of Internal Medicine. 1987; 106 (3):485–488. [ PubMed : 3813259 ] [ CrossRef ]
- Evidence-based information systems: A decade later. Proceedings of the European Conference on Information Systems ; 2011. Retrieved from http://aisel .aisnet.org/cgi/viewcontent .cgi?article =1221&context =ecis2011 .
- Okoli C., Schabram K. A guide to conducting a systematic literature review of information systems research. ssrn Electronic Journal. 2010
- Otte-Trojel T., de Bont A., Rundall T. G., van de Klundert J. How outcomes are achieved through patient portals: a realist review. Journal of American Medical Informatics Association. 2014; 21 (4):751–757. [ PMC free article : PMC4078283 ] [ PubMed : 24503882 ]
- Paré G., Trudel M.-C., Jaana M., Kitsiou S. Synthesizing information systems knowledge: A typology of literature reviews. Information & Management. 2015; 52 (2):183–199.
- Patsopoulos N. A., Analatos A. A., Ioannidis J.P. A. Relative citation impact of various study designs in the health sciences. Journal of the American Medical Association. 2005; 293 (19):2362–2366. [ PubMed : 15900006 ]
- Paul M. M., Greene C. M., Newton-Dame R., Thorpe L. E., Perlman S. E., McVeigh K. H., Gourevitch M.N. The state of population health surveillance using electronic health records: A narrative review. Population Health Management. 2015; 18 (3):209–216. [ PubMed : 25608033 ]
- Pawson R. Evidence-based policy: a realist perspective. London: SAGE Publications; 2006.
- Pawson R., Greenhalgh T., Harvey G., Walshe K. Realist review—a new method of systematic review designed for complex policy interventions. Journal of Health Services Research & Policy. 2005; 10 (Suppl 1):21–34. [ PubMed : 16053581 ]
- Petersen K., Vakkalanka S., Kuzniarz L. Guidelines for conducting systematic mapping studies in software engineering: An update. Information and Software Technology. 2015; 64 :1–18.
- Petticrew M., Roberts H. Systematic reviews in the social sciences: A practical guide. Malden, ma : Blackwell Publishing Co; 2006.
- Rousseau D. M., Manning J., Denyer D. Evidence in management and organizational science: Assembling the field’s full weight of scientific knowledge through syntheses. The Academy of Management Annals. 2008; 2 (1):475–515.
- Rowe F. What literature review is not: diversity, boundaries and recommendations. European Journal of Information Systems. 2014; 23 (3):241–255.
- Shea B. J., Hamel C., Wells G. A., Bouter L. M., Kristjansson E., Grimshaw J. et al. Boers M. amstar is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. Journal of Clinical Epidemiology. 2009; 62 (10):1013–1020. [ PubMed : 19230606 ]
- Shepperd S., Lewin S., Straus S., Clarke M., Eccles M. P., Fitzpatrick R. et al. Sheikh A. Can we systematically review studies that evaluate complex interventions? PLoS Medicine. 2009; 6 (8):e1000086. [ PMC free article : PMC2717209 ] [ PubMed : 19668360 ]
- Silva B. M., Rodrigues J. J., de la Torre Díez I., López-Coronado M., Saleem K. Mobile-health: A review of current state in 2015. Journal of Biomedical Informatics. 2015; 56 :265–272. [ PubMed : 26071682 ]
- Smith V., Devane D., Begley C., Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. bmc Medical Research Methodology. 2011; 11 (1):15. [ PMC free article : PMC3039637 ] [ PubMed : 21291558 ]
- Sylvester A., Tate M., Johnstone D. Beyond synthesis: re-presenting heterogeneous research literature. Behaviour & Information Technology. 2013; 32 (12):1199–1215.
- Templier M., Paré G. A framework for guiding and evaluating literature reviews. Communications of the Association for Information Systems. 2015; 37 (6):112–137.
- Thomas J., Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. bmc Medical Research Methodology. 2008; 8 (1):45. [ PMC free article : PMC2478656 ] [ PubMed : 18616818 ]
- Reconstructing the giant: on the importance of rigour in documenting the literature search process. Paper presented at the Proceedings of the 17th European Conference on Information Systems ( ecis 2009); Verona, Italy. 2009.
- Webster J., Watson R.T. Analyzing the past to prepare for the future: Writing a literature review. Management Information Systems Quarterly. 2002; 26 (2):11.
- Whitlock E. P., Lin J. S., Chou R., Shekelle P., Robinson K.A. Using existing systematic reviews in complex systematic reviews. Annals of Internal Medicine. 2008; 148 (10):776–782. [ PubMed : 18490690 ]
This publication is licensed under a Creative Commons License, Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0): see https://creativecommons.org/licenses/by-nc/4.0/
- Cite this Page Paré G, Kitsiou S. Chapter 9 Methods for Literature Reviews. In: Lau F, Kuziemsky C, editors. Handbook of eHealth Evaluation: An Evidence-based Approach [Internet]. Victoria (BC): University of Victoria; 2017 Feb 27.
- PDF version of this title (4.5M)
- Disable Glossary Links
In this Page
- Introduction
- Overview of the Literature Review Process and Steps
- Types of Review Articles and Brief Illustrations
- Concluding Remarks
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Chapter 9 Methods for Literature Reviews - Handbook of eHealth Evaluation: An Ev... Chapter 9 Methods for Literature Reviews - Handbook of eHealth Evaluation: An Evidence-based Approach
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Impulsive suicide attempts: a systematic literature review of definitions, characteristics and risk factors
Affiliations.
- 1 Australian Institute for Suicide Research and Prevention, National Centre of Excellence in Suicide Prevention, World Health Organisation Collaborating Centre for Research and Training in Suicide Prevention, Griffith University, Australia. Electronic address: [email protected].
- 2 Australian Institute for Suicide Research and Prevention, National Centre of Excellence in Suicide Prevention, World Health Organisation Collaborating Centre for Research and Training in Suicide Prevention, Griffith University, Australia; Griffith Health Institute, Australia.
- 3 Australian Institute for Suicide Research and Prevention, National Centre of Excellence in Suicide Prevention, World Health Organisation Collaborating Centre for Research and Training in Suicide Prevention, Griffith University, Australia.
- PMID: 25299440
- DOI: 10.1016/j.jad.2014.08.044
Background: Extensive research on impulsive suicide attempts, but lack of agreement on the use of this term indicates the need for a systematic literature review of the area. The aim of this review was to examine definitions and likely correlates of impulsive attempts.
Methods: A search of Medline, Psychinfo, Scopus, Proquest and Web of Knowledge databases was conducted. Additional articles were identified using the cross-referencing function of Google Scholar.
Results: 179 relevant papers were identified. Four different groups of research criteria used to assess suicide attempt impulsivity emerged: (a) time-related criteria, (b) absence of proximal planning/preparations, (c) presence of suicide plan in lifetime/previous year, and (d) other. Subsequent analysis used these criteria to compare results from different studies on 20 most researched hypotheses. Conclusions regarding the characteristics of impulsive attempts are more consistent than those on the risk factors specific to such attempts. No risk factors were identified that uniformly related to suicide attempt impulsivity across all criteria groups, but relationships emerged between separate criteria and specific characteristics of suicide attempters.
Limitations: Only published articles were included. Large inconsistencies in methods of the studies included in this review prevented comparison of effect sizes.
Conclusions: The vast disparities in findings on risk factors for impulsive suicide attempts among different criteria groups suggest the need to address the methodological issues in defining suicide attempt impulsivity before further research into correlates of such attempts can effectively progress. Specific recommendations are offered for necessary research.
Keywords: Impulsive suicide; Impulsivity; Planned suicide; Planning; Suicide attempts.
Copyright © 2014 Elsevier B.V. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Systematic Review
- Impulsive Behavior*
- Risk Factors
- Suicide, Attempted / psychology*
- Suicide, Attempted / statistics & numerical data*

Literature Reviews
- Types of reviews
- Getting started
Types of reviews and examples
Choosing a review type.
- 1. Define your research question
- 2. Plan your search
- 3. Search the literature
- 4. Organize your results
- 5. Synthesize your findings
- 6. Write the review
- Artificial intelligence (AI) tools
- Thompson Writing Studio This link opens in a new window
- Need to write a systematic review? This link opens in a new window

Contact a Librarian
Ask a Librarian
- Meta-analysis
- Systematized
Definition:
"A term used to describe a conventional overview of the literature, particularly when contrasted with a systematic review (Booth et al., 2012, p. 265).
Characteristics:
- Provides examination of recent or current literature on a wide range of subjects
- Varying levels of completeness / comprehensiveness, non-standardized methodology
- May or may not include comprehensive searching, quality assessment or critical appraisal
Mitchell, L. E., & Zajchowski, C. A. (2022). The history of air quality in Utah: A narrative review. Sustainability , 14 (15), 9653. doi.org/10.3390/su14159653
Booth, A., Papaioannou, D., & Sutton, A. (2012). Systematic approaches to a successful literature review. London: SAGE Publications Ltd.
"An assessment of what is already known about a policy or practice issue...using systematic review methods to search and critically appraise existing research" (Grant & Booth, 2009, p. 100).
- Assessment of what is already known about an issue
- Similar to a systematic review but within a time-constrained setting
- Typically employs methodological shortcuts, increasing risk of introducing bias, includes basic level of quality assessment
- Best suited for issues needing quick decisions and solutions (i.e., policy recommendations)
Learn more about the method:
Khangura, S., Konnyu, K., Cushman, R., Grimshaw, J., & Moher, D. (2012). Evidence summaries: the evolution of a rapid review approach. Systematic reviews, 1 (1), 1-9. https://doi.org/10.1186/2046-4053-1-10
Virginia Commonwealth University Libraries. (2021). Rapid Review Protocol .
Quarmby, S., Santos, G., & Mathias, M. (2019). Air quality strategies and technologies: A rapid review of the international evidence. Sustainability, 11 (10), 2757. https://doi.org/10.3390/su11102757
Grant, M.J. & Booth, A. (2009). A typology of reviews: an analysis of the 14 review types and associated methodologies. Health Information & Libraries Journal , 26(2), 91-108. https://www.doi.org/10.1111/j.1471-1842.2009.00848.x
Developed and refined by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), this review "map[s] out and categorize[s] existing literature on a particular topic, identifying gaps in research literature from which to commission further reviews and/or primary research" (Grant & Booth, 2009, p. 97).
Although mapping reviews are sometimes called scoping reviews, the key difference is that mapping reviews focus on a review question, rather than a topic
Mapping reviews are "best used where a clear target for a more focused evidence product has not yet been identified" (Booth, 2016, p. 14)
Mapping review searches are often quick and are intended to provide a broad overview
Mapping reviews can take different approaches in what types of literature is focused on in the search
Cooper I. D. (2016). What is a "mapping study?". Journal of the Medical Library Association: JMLA , 104 (1), 76–78. https://doi.org/10.3163/1536-5050.104.1.013
Miake-Lye, I. M., Hempel, S., Shanman, R., & Shekelle, P. G. (2016). What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Systematic reviews, 5 (1), 1-21. https://doi.org/10.1186/s13643-016-0204-x
Tainio, M., Andersen, Z. J., Nieuwenhuijsen, M. J., Hu, L., De Nazelle, A., An, R., ... & de Sá, T. H. (2021). Air pollution, physical activity and health: A mapping review of the evidence. Environment international , 147 , 105954. https://doi.org/10.1016/j.envint.2020.105954
Booth, A. (2016). EVIDENT Guidance for Reviewing the Evidence: a compendium of methodological literature and websites . ResearchGate. https://doi.org/10.13140/RG.2.1.1562.9842 .
Grant, M.J. & Booth, A. (2009). A typology of reviews: an analysis of the 14 review types and associated methodologies. Health Information & Libraries Journal , 26(2), 91-108. https://www.doi.org/10.1111/j.1471-1842.2009.00848.x
"A type of review that has as its primary objective the identification of the size and quality of research in a topic area in order to inform subsequent review" (Booth et al., 2012, p. 269).
- Main purpose is to map out and categorize existing literature, identify gaps in literature—great for informing policy-making
- Search comprehensiveness determined by time/scope constraints, could take longer than a systematic review
- No formal quality assessment or critical appraisal
Learn more about the methods :
Arksey, H., & O'Malley, L. (2005) Scoping studies: towards a methodological framework. International Journal of Social Research Methodology , 8 (1), 19-32. https://doi.org/10.1080/1364557032000119616
Levac, D., Colquhoun, H., & O’Brien, K. K. (2010). Scoping studies: Advancing the methodology. Implementation Science: IS, 5, 69. https://doi.org/10.1186/1748-5908-5-69
Example :
Rahman, A., Sarkar, A., Yadav, O. P., Achari, G., & Slobodnik, J. (2021). Potential human health risks due to environmental exposure to nano-and microplastics and knowledge gaps: A scoping review. Science of the Total Environment, 757 , 143872. https://doi.org/10.1016/j.scitotenv.2020.143872
A review that "[compiles] evidence from multiple...reviews into one accessible and usable document" (Grant & Booth, 2009, p. 103). While originally intended to be a compilation of Cochrane reviews, it now generally refers to any kind of evidence synthesis.
- Compiles evidence from multiple reviews into one document
- Often defines a broader question than is typical of a traditional systematic review
Choi, G. J., & Kang, H. (2022). The umbrella review: a useful strategy in the rain of evidence. The Korean Journal of Pain , 35 (2), 127–128. https://doi.org/10.3344/kjp.2022.35.2.127
Aromataris, E., Fernandez, R., Godfrey, C. M., Holly, C., Khalil, H., & Tungpunkom, P. (2015). Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. International Journal of Evidence-Based Healthcare , 13(3), 132–140. https://doi.org/10.1097/XEB.0000000000000055
Rojas-Rueda, D., Morales-Zamora, E., Alsufyani, W. A., Herbst, C. H., Al Balawi, S. M., Alsukait, R., & Alomran, M. (2021). Environmental risk factors and health: An umbrella review of meta-analyses. International Journal of Environmental Research and Public Dealth , 18 (2), 704. https://doi.org/10.3390/ijerph18020704
A meta-analysis is a "technique that statistically combines the results of quantitative studies to provide a more precise effect of the result" (Grant & Booth, 2009, p. 98).
- Statistical technique for combining results of quantitative studies to provide more precise effect of results
- Aims for exhaustive, comprehensive searching
- Quality assessment may determine inclusion/exclusion criteria
- May be conducted independently or as part of a systematic review
Berman, N. G., & Parker, R. A. (2002). Meta-analysis: Neither quick nor easy. BMC Medical Research Methodology , 2(1), 10. https://doi.org/10.1186/1471-2288-2-10
Hites R. A. (2004). Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environmental Science & Technology , 38 (4), 945–956. https://doi.org/10.1021/es035082g
A systematic review "seeks to systematically search for, appraise, and [synthesize] research evidence, often adhering to the guidelines on the conduct of a review" provided by discipline-specific organizations, such as the Cochrane Collaboration (Grant & Booth, 2009, p. 102).
- Aims to compile and synthesize all known knowledge on a given topic
- Adheres to strict guidelines, protocols, and frameworks
- Time-intensive and often takes months to a year or more to complete
- The most commonly referred to type of evidence synthesis. Sometimes confused as a blanket term for other types of reviews
Gascon, M., Triguero-Mas, M., Martínez, D., Dadvand, P., Forns, J., Plasència, A., & Nieuwenhuijsen, M. J. (2015). Mental health benefits of long-term exposure to residential green and blue spaces: a systematic review. International Journal of Environmental Research and Public Health , 12 (4), 4354–4379. https://doi.org/10.3390/ijerph120404354
"Systematized reviews attempt to include one or more elements of the systematic review process while stopping short of claiming that the resultant output is a systematic review" (Grant & Booth, 2009, p. 102). When a systematic review approach is adapted to produce a more manageable scope, while still retaining the rigor of a systematic review such as risk of bias assessment and the use of a protocol, this is often referred to as a structured review (Huelin et al., 2015).
- Typically conducted by postgraduate or graduate students
- Often assigned by instructors to students who don't have the resources to conduct a full systematic review
Salvo, G., Lashewicz, B. M., Doyle-Baker, P. K., & McCormack, G. R. (2018). Neighbourhood built environment influences on physical activity among adults: A systematized review of qualitative evidence. International Journal of Environmental Research and Public Health , 15 (5), 897. https://doi.org/10.3390/ijerph15050897
Huelin, R., Iheanacho, I., Payne, K., & Sandman, K. (2015). What’s in a name? Systematic and non-systematic literature reviews, and why the distinction matters. https://www.evidera.com/resource/whats-in-a-name-systematic-and-non-systematic-literature-reviews-and-why-the-distinction-matters/

- Review Decision Tree - Cornell University For more information, check out Cornell's review methodology decision tree.
- LitR-Ex.com - Eight literature review methodologies Learn more about 8 different review types (incl. Systematic Reviews and Scoping Reviews) with practical tips about strengths and weaknesses of different methods.
- << Previous: Getting started
- Next: 1. Define your research question >>
- Last Updated: Apr 3, 2024 12:40 PM
- URL: https://guides.library.duke.edu/lit-reviews

Services for...
- Faculty & Instructors
- Graduate Students
- Undergraduate Students
- International Students
- Patrons with Disabilities
- Harmful Language Statement
- Re-use & Attribution / Privacy
- Support the Libraries

- Open access
- Published: 09 April 2024
Patient characteristics of, and remedial interventions for, complaints and medico-legal claims against doctors: a rapid review of the literature
- Timothy J. Schultz ORCID: orcid.org/0000-0003-1419-3328 1 ,
- Michael Zhou 2 ,
- Jodi Gray 1 ,
- Jackie Roseleur 1 ,
- Richard Clark 1 , 3 ,
- Dylan A. Mordaunt 1 , 4 ,
- Peter D. Hibbert 5 , 6 ,
- Georgie Haysom 7 &
- Michael Wright 7 , 8
Systematic Reviews volume 13 , Article number: 104 ( 2024 ) Cite this article
Metrics details
It is uncertain if patient’s characteristics are associated with complaints and claims against doctors. Additionally, evidence for the effectiveness of remedial interventions on rates of complaints and claims against doctors has not been synthesised.
We conducted a rapid review of recent literature to answer: Question 1 “What are the common characteristics and circumstances of patients who are most likely to complain or bring a claim about the care they have received from a doctor?” and Question 2 “What initiatives or interventions have been shown to be effective at reducing complaints and claims about the care patients have received from a doctor?”. We used a systematic search (most recently in July 2023) of PubMed, Scopus, Web of Science and grey literature. Studies were screened against inclusion criteria and critically appraised in duplicate using standard tools. Results were summarised using narrative synthesis.
From 8079 search results, we reviewed the full text of 250 studies. We included 25 studies: seven for Question 1 (6 comparative studies with controls and one systematic review) and 18 studies for Question 2 (14 uncontrolled pre-post studies, 2 comparative studies with controls and 2 systematic reviews). Most studies were set in hospitals across a mix of medical specialties.
Other than for patients with mental health conditions (two studies), no other patient characteristics demonstrated either a strong or consistent effect on the rate of complaints or claims against their treating doctors.
Risk management programs (6 studies), and communication and resolution programs (5 studies) were the most studied of 6 intervention types. Evidence for reducing complaints and medico-legal claims, costs or premiums and more timely management was apparent for both types of programs. Only 1 to 3 studies were included for peer programs, medical remediation, shared decision-making, simulation training and continuing professional development, with few generalisable results.
Few patient characteristics can be reliably related to the likelihood of medico-legal complaints or claims. There is some evidence that interventions can reduce the number and costs of claims, the number of complaints, and the timeliness of claims. However, across both questions, the strength of the evidence is very weak and is based on only a few studies or study designs that are highly prone to bias.
Peer Review reports
Up to 10% of hospital patients experience an adverse event [ 1 ]. Medical negligence or the failure to meet the standard of care reasonably expected of an ‘average’ doctor is a contributing factor to a small proportion of adverse events [ 1 , 2 ]. Medico-legal claims seeking compensation for medical negligence may be filed against doctors by patients through civil litigation. For less serious events or to express dissatisfaction with care, patients may also make a formal complaint, either directly to their care provider or the provider’s employer or to medical and other regulators and health complaints entities [ 3 ].
Doctors’ demographic (e.g. gender, age, years spent in practice) and workplace-related factors (e.g. greater number of patient lists) are associated with the risk of complaints and malpractice claims [ 4 , 5 ]. It is less clear what, if any, patient characteristics are associated with complaints and claims, and anecdotal evidence suggests that the rate of complaints and claims is rising [ 6 ]. Though females may be more likely to complain, and complaints and claims are often raised by patients’ living or bereaved relatives [ 7 , 8 ], there are no relevant systematic reviews on this topic. This led to the following review question (Question 1) “What are the common characteristics and circumstances of patients who are most likely to complain or bring a claim about the care they have received from a doctor?”.
In addition to the impact on patient wellbeing, doctors involved in adverse events experience serious emotional and psychological impacts [ 9 ]. Additionally, the financial cost to health systems from medico-legal claims is significant, potentially jeopardising the long-term financial sustainability of some public health systems [ 10 ]. Doctors, hospitals, health services, health regulators, representative medical organisations and medical insurers are therefore all highly motivated to provide safe, high-quality care that minimises complaints and claims against them, their staff, stakeholders and members. For example, medical colleges, practitioner regulation boards and medical indemnity insurers maintain professional standards of their members and conduct activities such as continuing professional development (CPD) [ 11 ], remediation programs [ 12 ] and communication and resolution programs (CRPs) [ 13 ]. Despite a recent scoping review describing how remediation programs are delivered to regulated health professionals [ 14 ], there is no substantive review of the literature across the wide range of stakeholders and potential interventions applicable to reduce complaints and claims against doctors. We therefore posed the following additional review question (Question 2): “What initiatives or interventions have been shown to be effective at reducing complaints and claims about the care patients have received from a doctor?” [ 6 ].
Review objective and research questions
The purpose of this review was to provide an evidence-based foundation to understand which patient factors influence complaints or claims and what interventions can support a reduction in complaints or claims [ 6 ]. This information could be used by clinicians, hospital administrators, healthcare regulators and medical indemnity insurers to inform their practice and policy. For the purposes of this study, a “claim” was defined as an assertion of wrongdoing that forms the basis for a request for compensation [ 15 ]; an “unwarranted” claim occurred when the care provided had not been below the expected standard and the complaint was not otherwise warranted [ 6 ].
A protocol defining the scope of the review (PEO/PICO, inclusion and exclusion criteria, search strategy and limits) was developed according to Sax Institute guidelines [ 16 ] but was not prospectively registered. The review was conducted according to guidance provided by the Cochrane Rapid Review method [ 17 ] and the SelecTing Approaches for Rapid Reviews (STARR) approach [ 18 ]. The updated Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist was used to report review findings [ 19 ].
Scope of the review
The review focussed on health systems of high-income Commonwealth countries including Australia, New Zealand, Canada and the United Kingdom (UK). Additionally, studies from the United States of Amercia (USA), Ireland and Western Europe were included to inform the review. The review focussed on the peer-reviewed literature although grey literature of similar quality was also searched. The review was conducted over an 8-week period from September to October 2022. The search was repeated in September 2023.
Inclusion and exclusion criteria
The inclusion and exclusion criteria for Question 1 and Question 2 are included in Table 1 . The settings were hospitals (excluding the emergency department), primary care and secondary care. Regulatory complaints, complaints to practices or hospitals and claims for compensation were included, while complaints on social media were excluded. For Question 1, the review focussed on correlations between the ‘exposure’ (e.g. patient characteristics) and the number, type or nature of complaints/claims. For Question 2, the review included interventions implemented primarily to reduce the number of complaints/claims against doctors, although other secondary outcomes included the costs of claims or insurance premiums, the duration of the claims management process, doctor risk profile or performance, doctor confidence/knowledge/satisfaction, workplace culture, and patient outcomes (e.g. morbidity) or patient satisfaction.
Only English language studies using quantitative study designs included in the National Health and Medical Research Council (NHRMC) guidelines [ 20 ] were included (e.g. ranging from level I systematic review, level II randomised controlled trial, level III pseudorandomised trial/comparative study with or without concurrent controls, and level IV case series with either post-test or pre-test/post-test outcomes). Cross-sectional studies were excluded.
Search strategy and selection criteria
Given the aetiological nature of studies relevant to Question 1 in particular, we used a PEO approach (Participant, Exposure, Outcome) [ 21 ] to frame the search strategy (see Supplementary Table S 1 , S 2 , S 3 ). Terms relating to ‘participants’ included doctors and health services. Terms relating to ‘exposure’ included patient characteristics (such as demographics, socio-economic status, and health literacy) for Question 1, and patient safety interventions (such as checklists, care bundles and teamwork) or clinical risk management programs (such as medical education, risk mitigation, peer program and communication and resolution) for Question 2. Terms relating to ‘outcomes’ included malpractice, negligence, complaint, claim management and medico-legal.
We searched three bibliographic databases (PubMed, Scopus and Web of Science) and grey literature sources (Google, Proquest Theses, GreyLit.org and Mednar) for relevant studies. The reference lists and citation searching of included studies were included as other search methods. To ensure applicability to a modern healthcare system only studies published since 2011 were included. The search was conducted first in September 2022 and then repeated in July 2023.
Screening based on title and abstract was conducted independently in pairs by four members of the research team (TS, MZ, JG, JR) following training on two sets of 100 studies.
Quality appraisal
The quality of included studies was appraised independently in pairs by four members of the research team (TS, JG, JR, PH) using AMSTAR 2 for systematic reviews [ 22 ] and National Institute of Health tools for case–control studies and uncontrolled pre-post studies [ 23 ]. These tools include 16 items (systematic reviews) or 12 items (case–control studies and uncontrolled pre-post), which were scored as ‘Yes’, ‘No’, ‘Not applicable’ or ‘Cannot determine’ [ 23 ], AMSTAR 2 also uses ‘Probably yes’.
Data collection
Data was extracted from each paper into a Microsoft Excel spreadsheet that had been pilot tested by three reviewers. Extraction was conducted by a single reviewer (TS or MZ) and then checked by a second reviewer (JG, JR).
A narrative synthesis was used to describe the key findings for both review questions. For review Question 1, results are presented separately for each patient characteristic, grouped according to patient demographics (e.g. age, sex, complainant), patient risk factors (e.g. American Society of Anaesthesiologists’ (ASA) score, the existence of a mental disorder, re-operation) and the therapeutic context (e.g. aspects of treatment, diagnosis, setting and/or phase of care including length of stay (LOS) and complications). For review Question 2, results are presented for seven different types of programs implemented to reduce the number of complaints and/or claims against doctors. The consistency, clinical impact, generalisability, and applicability of study findings were appraised using the NHRMC matrix which ranks each component’s strength using a four-point scale (excellent, good, satisfactory and poor) [ 20 ].
Literature search
Nearly 8900 studies were identified across the search strategy, of which 255 full texts were reviewed (Fig. 1 ). Of these, 230 were excluded as not relevant or due to an ineligible study design. A total of seven studies were included for Question 1, and 18 studies were included for Question 2 (Supplementary Table S 4 ).
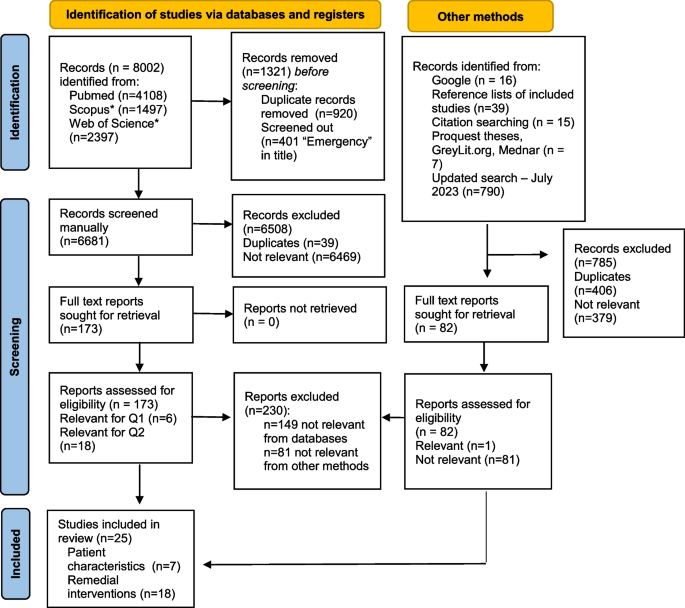
PRISMA study flow diagram [ 19 ]. * filters applied to these search results (Australia, New Zealand, Canada, UK)
The characteristics of the studies included for Question 1 are presented in Table 2 . There were six comparative studies with concurrent controls (three from the USA [ 24 , 25 , 26 ], two from the UK [ 27 , 28 ]) and one from Italy [ 29 ] and one systematic reviews of non-randomised control trials [ 3 ]. The in-patient hospital setting was most common ( n = 5) across a range of specialties and conditions, most commonly surgery. In total, there were 27 variables reported across the seven studies, 17 of these were included in multiple studies. Sex ( n = 6) and age ( n = 5) were the most frequently recorded patient demographics. For patient risk factors, ASA score, mental disorders, tobacco use and body mass index (BMI) > 30 were measured in two studies. For therapeutic context, LOS, setting, complications and treatment were measured in two studies.
Quality assessment is summarised in Table 2 , Supplementary Table S 5 (comparative studies) and Supplementary Table S 6 (systematic reviews). For the 6 comparative studies, 6 to 10 (mean 8.3, SD = 1.4) of 12 criteria were met; for the systematic review, 4 of the 16 criteria were met (or probably met).
In general, there was very limited evidence for the existence of significant relationships between patient characteristics and the rate of complaints or claims (Table 3 ). For demographics, one study identified that a 10-year increase in the age of paediatric surgery patients led to a near 50% greater odds (OR = 1.47, CI 1.04–2.08) of a complaint and that male gender reduced odds of a complaint in adults by 34% (OR = 0.66, CI 0.47–0.92) [ 25 ]. However, sex and age were not significant predictors in five and four other studies, respectively. A systematic review of 36 studies (comprising 44,211 complaints) estimated that 64% of complainants were patients and 26% were family members; the remaining 10% was not specified [ 3 ]. Of patient risk factors, patients with mental, behavioural, or neurodevelopmental disorders were significantly more likely to complain following hand and upper extremity surgery [ 24 ] and spine surgery [ 26 ] (Table 3 ).
In terms of therapeutic context, there were lower odds of a complaint for two procedural features: (i) use of a general anaesthetic in both paediatric and adult populations provided odds ratios, respectively, of 0.22 (CI 0.07–0.62) and 0.67 (CI 0.47–0.95) compared to no general anaesthetic, and (ii) a 1-h delay in actual start time led to slightly higher odds of a complaint, more notably in paediatrics (OR = 1.27, CI 1.10–1.47) than in adults (OR = 1.05, CI 0.95–1.16) [ 25 ]. The odds of a complaint were seven times greater for patients undergoing surgery (CI 5.2–9.6) [ 26 ]. The overuse of non-beneficial interventions and underuse of treatment escalation plans predicted complaints from the next-of-kin of patients who died in hospital [ 28 ]. For example, treatment escalation limitation plans were used significantly less frequently in complaints (23.8% versus 47.2%, P = 0.013) [ 28 ]. Other components of therapeutic context, including LOS, setting, and experiencing complications and harms, were not significant predictors of complaints (Table 3 ).
Uncontrolled pre-post studies ( n = 14) were the most common study design included for Question 2, followed by comparative studies with concurrent controls ( n = 2) and systematic reviews ( n = 2) (Table 4 ). Studies were set in the USA ( n = 12) [ 13 , 15 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 ], Canada ( n = 2) [ 40 , 41 ], the UK [ 12 ], Ireland [ 42 ] and New Zealand [ 43 ] ( n = 1, each). The studies addressed malpractice claims ( n = 9), complaints ( n = 5), and regulatory notifications ( n = 2) and a mix of outcomes ( n = 1). In-patient hospital ( n = 11) was the most common setting, followed by mixed ( n = 4), primary care and secondary care ( n = 1, each). There were seven types of interventions for Question 2 studies: risk management ( n = 6), CRPs ( n = 5) (note one study [ 31 ] assessed both), medical remediation ( n = 3), peer program ( n = 2) and, CPD, simulation training and shared decision-making ( n = 1, each). Quality assessment is summarised in Table 3 , Supplementary Table S 5 (comparative studies), Table S 7 (uncontrolled pre-post studies) and Supplementary Table S 6 (systematic reviews). Eight of the 12 criteria were met for the one comparative study; 3 to 11 of the 12 criteria were met for the 14 uncontrolled pre-post studies (mean 7.6, SD = 2.6); and 8 and 11 of the 16 criteria were met for the two systematic reviews.
Findings and definitions for Question 2 across the seven types of interventions and eight included outcomes are presented in Table 5 . No studies examined doctor satisfaction or patient outcomes (such as mortality or morbidity).
The six studies of risk management programs [ 31 , 32 , 34 , 38 , 40 , 42 ], also called risk reduction programs, were heterogeneous in nature, and included enhanced evaluation of, and response to, complaints [ 42 ], active engagement of physicians in risk assessment [ 32 ], lectures followed by a mock lawsuit [ 34 ], and education [ 38 , 40 ]. Evidence from these studies of risk management programs supported reductions in claims, complaints and claims costs (Table 5 ). Other benefits included more timely complaints management, improved patient safety culture and staff confidence.
Evidence for communication and resolution programs (CRPs, five studies [ 13 , 15 , 31 , 33 , 35 ]) was consistent across four studies. There were lower rates of claims and complaints, lower claim amounts, and faster resolution of claims following the implementation of CRPs (Table 5 ) [ 15 , 31 , 33 , 35 ]. However, results were less supportive in a study using an interrupted time series (ITS) design [ 13 ]. One study demonstrated improved patient satisfaction [ 33 ].
Three studies of medical remediation showed either a reduction in claims rates [ 12 ] or an improved doctor risk profile [ 29 , 43 ].
Two studies of peer review, or the use of peer messengers, demonstrated a reduction in either complaint rates [ 36 ] or improved doctor risk profile [ 37 ] (Table 5 ).
A systematic review of five studies concluded that there was insufficient evidence to determine whether or not shared decision-making reduces claims [ 44 ]. A retrospective pre-post program evaluation of simulation training on malpractice claims among obstetrician-gynaecologists reported that the rate of claims after simulation training was halved to 5.7 claims per 100 physician years of coverage. Attending more sessions was associated with a greater reduction in claims, although there was no difference in the total costs of paid claims before and after the training [ 39 ].
In one included study of CPD, doctors who reported participation in CPD activities were significantly less likely (OR 0.60; CI 0.39 to 0.95) to receive quality of care-related complaints than those who did not report participating in CPD [ 41 ]. Participants in group-based CPD were less likely (OR 0.68; CI 0.47 to 0.98) to receive quality of care-related complaints than individual or assessment-based CPD [ 41 ].
Summary of the evidence
A summary of the included studies’ evidence base, consistency, clinical impact, generalisability and applicability is included in Table 6 . The evidence base was rated as poor for both Question 1 and 2 (Table 6 ). Consistency and clinical impact were slightly higher for Question 2 than Question 1, whereas generalisability and applicability were satisfactory for both Question 1 and Question 2.
This review has identified a clear lack of recent high-quality studies to inform an in-depth understanding of either review Question 1 or Question 2. For Question 1, seven patient characteristics were associated with patients’ likelihood to complain or make a medico-legal claim against a doctor; however, only one of these findings (presence of a mental disorder) was replicated. This may be related to the paucity of studies, for example, only half of the patient characteristics were evaluated in more than one study. While more studies were included for Question 2, the low quality of the predominant study design (case series) severely limits the strength of the review’s findings.
The main finding for Question 1 of a relationship between a patient’s mental health status and complaint behaviour may reflect non-modifiable associations between underlying mental health conditions, poorer outcomes and reduced satisfaction after surgery [ 24 , 26 ]. Alternatively, the finding may reflect the impact of stigma experienced by these patients in healthcare settings. Mental illness-related stigma is prevalent in healthcare [ 51 ]. Stigma creates barriers to accessing healthcare, such as delays in help-seeking, treatment discontinuation, suboptimal therapeutic relationships, patient safety concerns and poorer quality care [ 52 ]. The presence of these barriers may be associated with a complaint about a healthcare provider.
Findings for Question 2 offer some evidence to support most of the included interventions, particularly risk management programs and CRPs. Some of the commonly occurring attributes of risk management programs were the evaluation and analysis of complaints and claims, targeted medico-legal education, and implementation of patient safety measures. The majority of the risk management programs were developed and delivered internally, either at the level of hospital department [ 38 ], hospital-wide [ 32 , 34 ] or general practice-level [ 42 ]. Local contextualisation, incorporating the site-specific nature of malpractice claims and legislation, and delivery of risk management programs apparently enhance the acceptability of risk management programs for surgeons, in particular [ 53 , 54 , 55 ]. Nevertheless, in one study, the Society of Obstetricians and Gynaecologists of Canada partnered with a healthcare insurance representative body to support the international expansion of a risk management program [ 40 ].
Studies of CRPs were generally consistent in showing lower rates of claims and complaints, lower claim amounts, and faster resolution of claims following the implementation of CRPs. However, limited adherence to the key components of CRP, including a proliferation of partial apology laws, may detract from the effectiveness of CRP in meeting the needs of injured patients [ 56 , 57 , 58 ]. Patients involved in CRP have expressed a greater desire for information provision from hospitals about efforts to prevent recurrences of the event [ 59 ].
Interventions such as caps on compensation, attorney fees, and alternative payment systems and liabilities [ 31 ] were excluded from the review as they are not doctor-directed interventions. The impacts of these medical malpractice reforms have been recently summarised [ 60 , 61 ].
The small number of included studies (Question 1) and the low quality of included studies (Question 2) represent major gaps in the evidence. For Question 1, there were a large number of excluded studies that were uncontrolled or unadjusted cross-sectional studies of complaints or claims that simply report the underlying characteristics of a claims database. Due to the lack of a control group, these studies do not provide particularly useful insights into the relationship between patient characteristics and the rate of complaints or claims. While more studies were included for Question 2, the predominant study design (i.e. uncontrolled pre-post) is weak as it does not permit adjustments for other secular trends in claims or confounders, or include control sites. Therefore, very little strength could be offered for recommendations emanating from either Question 1 or Question 2.
For Question 1, only one study specified whether a complaint was warranted or unwarranted [ 41 ]. No study included both types of complaints to determine predictors of successful interventions targeting unwarranted claims/complaints. The finding that a substantial subset of complaints originate from non-patient sources is likely to reduce the predictive value of patient characteristics for claims and complaints in this analysis. For Question 2, no studies assessed staff satisfaction or patient outcomes, such as mortality or morbidity. Additionally, there is rarely any evidence provided about generalisability or the potential for implementation and sustainability of the intervention, and most studies are limited to a single hospital/health service. Only one included study reported on the impact on organisational culture [ 40 ] or patient satisfaction [ 33 ].
All stages of the rapid review were conducted independently in duplicate to minimise the risk of errors. However, we only included studies published since 2011. This may have excluded relevant, older literature, which may be a limitation to this rapid review. Additionally, we filtered search results from the Scopus and Web of Science databases to countries with similar health systems (Australia, New Zealand, Canada and the UK) and screened out studies with ‘emergency’ in the title.
Conclusions
Despite substantial efforts made to collect information about patient complaints and claims, research has generally failed to robustly determine patient characteristics associated with complaints and claims. There is a small amount of evidence that patients with mental health conditions are more likely to complain.
The evidence for the effectiveness of interventions to reduce the likelihood of a doctor receiving a complaint or claim is also weak, as it is dominated by low-quality, uncontrolled pre-post studies. Only one or two studies were included for five types of programs (peer programs, medical remediation, shared decision-making, simulation training and CPD). More evidence, however, offers support for the effectiveness of risk management programs and CRPs in reducing complaints and claims.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Runciman W, Merry A, Walton M. Safety and Ethics in Healthcare: A Guide to Getting it Right. Aldershot: Ashgate; 2007.
Google Scholar
Grober ED, Bohnen JMA. Defining medical error. Can J Surg. 2005;48(1):39–44.
PubMed PubMed Central Google Scholar
Reader TW, Gillespie A, Roberts J. Patient complaints in healthcare systems: a systematic review and coding taxonomy. BMJ Qual Saf. 2014;23(8):678–89.
Article PubMed PubMed Central Google Scholar
Austin EE, Do V, Nullwala R, Fajardo Pulido D, Hibbert PD, Braithwaite J, et al. Systematic review of the factors and the key indicators that identify doctors at risk of complaints, malpractice claims or impaired performance. BMJ Open. 2021;11(8):e050377.
Unwin E, Woolf K, Wadlow C, Potts HWW, Dacre J. Sex differences in medico-legal action against doctors: a systematic review and meta-analysis. BMC Med. 2015;13(1):172.
Institute S. Evidence Check: Doctors challenges and Patient complaints. Sydney: Sax Insititute; 2022.
Daniel AE, Burn RJ, Horarlk S. Patients’ complaints about medical practice. MJA. 1999;170(12):598–602.
CAS PubMed Google Scholar
Vincent C, Young M, Phillips A. Why do people sue doctors? A study of patients and relatives taking legal action. Lancet. 1994;343(8913):1609–13.
Article CAS PubMed Google Scholar
Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726.
Article CAS PubMed PubMed Central Google Scholar
Forrest C, O'Donoghue K, Collins DC, O'Reilly S. Current Irish medicolegal landscape: an unsustainable trajectory. BMJ Open Qual. 2023;12(3).
Forsetlund L, O'Brien MA, Forsén L, Mwai L, Reinar LM, Okwen MP, et al. Continuing education meetings and workshops: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews. 2021(9).
O’Brien M, Dinwoodie M, Hartwig B, Blaney D. The Clinical Communication Program: an effective intervention for reducing future risk for high-risk physicians. Asia Pacific J Health Manage. 2014;9(1):8–13.
Kachalia A, Sands K, Niel MV, Dodson S, Roche S, Novack V, et al. Effects of a communication-and-resolution program on hospitals’ malpractice claims and costs. Health Aff. 2018;37(11):1836–44.
Article Google Scholar
Kennedy G, Jacobs N, Freemark L, Madan S, Chan N, Tran Y, et al. Remediation programs for regulated health care professionals: a scoping review. J Contin Educ Health Prof. 2022;42(1):36–46.
Article PubMed Google Scholar
Adams MA, Elmunzer BJ, Scheiman JM. Effect of a health system’s medical error disclosure program on gastroenterology-related claims rates and costs. Am J Gastroenterol. 2014;109(4):460–4.
Sax Institute. Evidence Check: a fast and accurate summary of the latest health evidence. Sydney: Sax Institute; 2022. [Available from: https://www.saxinstitute.org.au/wp-content/uploads/2022-Evidence-Check-Flyer.pdf .
Garritty C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C, et al. Cochrane rapid reviews methods group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2021;130:13–22.
Pandor A, Kaltenthaler E, Martyn-St James M, Wong R, Cooper K, Dimairo M, et al. Delphi consensus reached to produce a decision tool for SelecTing Approaches for Rapid Reviews (STARR). J Clin Epidemiol. 2019;114:22–9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
National Health and Medical Research Council. NHMRC levels of evidence and grades for recommendations for guideline developers. Canberra: NHMRC; 2009.
Moola S, Munn Z, Sears K, Sfetcu R, Currie M, Lisy K, et al. Conducting systematic reviews of association (etiology): the Joanna Briggs Institute’s approach. Int J Evid Based Healthc. 2015;13(3):163–9.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
NIH. Study Quality Assessment Tools 2021. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools .
Grandizio LC, Barreto Rocha DF, Piper JP, Hayes DS, Klena JC. An analysis of formal patient complaints and malpractice events involving hand and upper extremity surgeons. J Am Acad Orthop Surg. 2021;29(15):659–65.
Kynes JM, Schildcrout JS, Hickson GB, Pichert JW, Han X, Ehrenfeld JM, et al. An analysis of risk factors for patient complaints about ambulatory anesthesiology care. Anesth Analg. 2013;116(6):1325–32.
Rae M, Barreto Rocha DF, Hayes DS, Haak M, Maniar H, Grandizio LC. Formal Patient Complaints and Malpractice Events Involving Orthopedic Spine Surgeons: A Ten-Year Analysis. Spine (Phila Pa 1976). 2022;47(14):E521-e6.
Jones K, Davies B, Stubbs DJ, Komashie A, Burnstein RM, Hutchinson P, et al. Can compliment and complaint data inform the care of individuals with chronic subdural haematoma (cSDH)? BMJ Open Qual. 2021;10(3).
Robin Taylor D, Bouttell J, Campbell JN, Lightbody CJ. A case-controlled study of relatives’ complaints concerning patients who died in hospital: the role of treatment escalation/limitation planning. Int J Qual Health C. 2020;32(3):212–8.
Facchin F, Pagani A, Perozzo FAG, Scarpa C, Bassetto F, Vindigni V. Litigation Cases After Post-Bariatric Surgery: Lesson from the Past. Aesthetic Plast Surg. 2023.
Cosman BC, Alverson AD, Boal PA, Owens EL, Norcross WA. Assessment and remedial clinical education of surgeons in California. Arch Surg. 2011;146(12):1411–5.
Cardoso R, Zarin W, Nincic V, Barber SL, Gulmezoglu AM, Wilson C, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6(1):181.
Diraviam SP, Sullivan PG, Sestito JA, Nepps ME, Clapp JT, Fleisher LA. Physician engagement in malpractice risk reduction: A UPHS case study. Jt Comm J Qual Patient Saf. 2018;44(10):605–12.
PubMed Google Scholar
Fustino NJ, Moore P, Viers S, Cheyne K. Improving patient experience of care providers in a multispecialty ambulatory pediatrics practice. Clin Pediatr (Phila). 2019;58(1):50–9.
Juo YY, Lewis C, Hanna C, Reber HA, Tillou A. An innovative approach for familiarizing surgeons with malpractice litigation. J Surg Educ. 2019;76(1):127–33.
LeCraw FR, Montanera D, Jackson JP, Keys JC, Hetzler DC, Mroz TA. Changes in liability claims, costs, and resolution times following the introduction of a communication-and-resolution program in Tennessee. J Patient Saf Risk Manage. 2018;23(1):13–8.
Nassiri AM, Pichert JW, Domenico HJ, Galloway MB, Cooper WO, Bennett ML. Unsolicited patient complaints among otolaryngologists. Otolaryngol Head Neck Surg. 2019;160(5):810–7.
Pichert JW, Moore IN, Karrass J, Jay JS, Westlake MW, Catron TF, et al. An intervention model that promotes accountability: peer messengers and patient/family complaints. Jt Comm J Qual Patient Saf. 2013;39(10):435–46.
Raper SE, Rose D, Nepps ME, Drebin JA. Taking the initiative: risk-reduction strategies and decreased malpractice costs. J Am Coll Surg. 2017;225(5):612–21.
Schaffer AC, Babayan A, Einbinder JS, Sato L, Gardner R. Association of simulation training with rates of medical malpractice claims among obstetrician-gynecologists. Obstet Gynecol. 2021;138(2):246–52.
Milne JK, Walker DE, Vlahaki D. Reflections on the Canadian MORE(OB) obstetrical risk management programme. Best Pract Res Clin Obstet Gynaecol. 2013;27(4):563–9.
Wenghofer EF, Campbell C, Marlow B, Kam SM, Carter L, McCauley W. The effect of continuing professional development on public complaints: a case-control study. Med Educ. 2015;49(3):264–75.
Barragry RA, Varadkar LE, Hanlon DK, Bailey KF, O’Dowd TC, O’Shea BJ. An analytic observational study on complaints management in the general practice out of hours care setting: who complains, why, and what can we do about it? BMC Fam Pract. 2016;17(1):87.
Lillis S, Takai N, Francis S. Long-term outcomes of a remedial education program for doctors with clinical performance deficits. J Contin Educ Heal Prof. 2014;34(2):96–101.
Durand MA, Moulton B, Cockle E, Mann M, Elwyn G. Can shared decision-making reduce medical malpractice litigation? A systematic review. BMC Health Serv Res. 2015;15.
Price T, Archer J. UK policy on doctor remediation: trajectories and challenges. J Contin Educ Health Prof. 2017;37(3):207–11.
Price T, Brennan N, Wong G, Withers L, Cleland J, Wanner A, et al. Remediation programmes for practising doctors to restore patient safety: the RESTORE realist review. Health Serv Deliv Res. 2021;9(11):1–116.
Rout A, Roberts P. Peer review in nursing and midwifery: a literature review. J Clin Nurs. 2008;17(4):427–42.
Travaglia J, Debono D. Peer review in medicine: a comprehensive review of the literature. Sydney: Centre for Clinical Governance Research in Health; 2009.
Lateef F. Simulation-based learning: Just like the real thing. J Emerg Trauma Shock. 2010;3(4):348–52.
Royal Australasian College of Physicians. Continuing Professional Development (CPD) Participation Policy. Sydney: RACP; 2020.
Corrigan PW, Druss BG, Perlick DA. The impact of mental illness stigma on seeking and participating in mental health care. Psychol Sci Public Interest. 2014;15(2):37–70.
Knaak S, Mantler E, Szeto A. Mental illness-related stigma in healthcare: barriers to access and care and evidence-based solutions. Healthc Manage Forum. 2017;30(2):111–6.
Raper SE, Gupta M, Okusanya O, Morris JB. Improving communication skills: a course for academic medical center surgery residents and faculty. J Surg Educ. 2015;72(6):e202–11.
Raper SE, Joseph J, Seymour WG, Sullivan PG. Tipping the scales: educating surgeons about medical malpractice. J Surg Res. 2016;206(1):206–13.
Raper SE, Joseph J. Informed consent for academic surgeons: a curriculum-based update. MedEdPORTAL. 2020;16:10985.
Ross NE, Newman WJ. The role of apology laws in medical malpractice. J Am Acad Psychiatry Law. 2021;49(3):406–14.
Fields AC, Mello MM, Kachalia A. Apology laws and malpractice liability: what have we learned? BMJ Qual Saf. 2021;30(1):64–7.
Gallagher TH, Mello MM, Sage WM, Bell SK, McDonald TB, Thomas EJ. Can communication-and-resolution programs achieve their potential? Five key questions Health Affairs. 2018;37(11):1845–52.
Moore J, Bismark M, Mello MM. Patients’ experiences with communication-and-resolution programs after medical injury. JAMA Intern Med. 2017;177(11):1595–603.
Liu J, Hyman DA. The impact of medical malpractice reforms. Annual Rev Law Soc Sci. 2020. p. 405–19.
Agarwal R, Gupta A, Gupta S. The impact of tort reform on defensive medicine, quality of care, and physician supply: A systematic review. Health Services Research. 2019 2019/08//:851+.
Download references
Acknowledgements
We acknowledge the support of Eileen Goldberg and Richa Jaswal who brokered the review through the Sax Institute.
The project was funded by Avant Insurance Limited, Australia, which advised on the study protocol and approved publication. The authors alone are responsible for the views expressed in this review, and they do not necessarily represent the decisions, policies, or views of Avant Insurance Limited, Australia.
Author information
Authors and affiliations.
College of Medicine and Public Health, Flinders Health and Medical Research Institute, Flinders University, Adelaide, Australia
Timothy J. Schultz, Jodi Gray, Jackie Roseleur, Richard Clark & Dylan A. Mordaunt
College of Nursing and Health Sciences, Flinders University, Adelaide, Australia
Michael Zhou
HealthFX, Melbourne, Australia
Richard Clark
Southern Adelaide Local Health Network, Adelaide, Australia
Dylan A. Mordaunt
Australian Institute of Health Innovation, Macquarie University, Sydney, Australia
Peter D. Hibbert
IIMPACT in Health, Allied Health and Human Performance, University of South Australia, Adelaide, Australia
Avant Mutual, Sydney, Australia
Georgie Haysom & Michael Wright
Centre for Health Economics Research and Evaluation, University of Technology Sydney, Sydney, Australia
Michael Wright
You can also search for this author in PubMed Google Scholar
Contributions
TJS obtained funding, developed review methods, conducted the search, screened, critically appraised, extracted data, interpreted results, and wrote the manuscript. MZ screened, extracted data, interpreted results, and wrote the manuscript. JG and JR screened, critically appraised, extracted data; RC and DAM interpreted results. PDH critically appraised, interpreted results. GH and MW developed the protocol and interpreted results. All authors reviewed the manuscript and approved the submitted version. All authors are personally accountable for their own contributions.
Corresponding author
Correspondence to Timothy J. Schultz .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
Authors TJS, MZ, JG, JR, RC, DAM, and PDH declare no competing interests.
Authors GH and MW are employees of Avant Insurance Limited, Australia, a provider of medico-legal insurance.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
Pubmed search - 8 September 2022. Table S2. Scopus search - 8 September 2022. Table S3. Web of Science - 8 September 2022. Table S4. Summary of study design for included studies for Question 1 and 2 using NHMRC levels of evidence [20]. Table S5. Summary of quality appraisal for eight comparative studies with concurrent controls, six for Question 1 (Q1) and two for Question 2 (Q2). Table S6. Summary of quality appraisal for three systematic reviews (one for Question 1 (Q1) and two for Question 2 (Q2)). Table S7. Summary of quality appraisal for 14 uncontrolled pre-post studies for Question 2 (Q2).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Schultz, T.J., Zhou, M., Gray, J. et al. Patient characteristics of, and remedial interventions for, complaints and medico-legal claims against doctors: a rapid review of the literature. Syst Rev 13 , 104 (2024). https://doi.org/10.1186/s13643-024-02501-8
Download citation
Received : 14 December 2023
Accepted : 20 February 2024
Published : 09 April 2024
DOI : https://doi.org/10.1186/s13643-024-02501-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medico-legal claims
- Communication and resolution program
- Risk management program
- Patient characteristics
- Patient safety
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
In the present systematic literature review of 45 studies we have confirmed inconsistency in defining medication errors as well as lack of definitions. Most of the definitions were profound, including minor deviations as well as fatal errors, whereas a single definition was restricted to harmful or potentially harmful errors.
Inconsistency in defining medication errors has been confirmed. It appears that definitions and methods of detection rather than being reproducible and reliable methods are subject to the individual researcher's preferences. Thus, application of a clear-cut definition, standardized terminology and reliable methods has the potential to greatly ...
A literature review is a review and synthesis of existing research on a topic or research question. A literature review is meant to analyze the scholarly literature, make connections across writings and identify strengths, weaknesses, trends, and missing conversations. A literature review should address different aspects of a topic as it ...
This literature review shows there is no widely accepted definition nor a preferred classification system used for innovations in radiotherapy. Overall, two major approaches were found to categorize innovations, suggesting that key characteristics of radiotherapy interventions exist and can be used to categorise innovations.
A formal literature review is an evidence-based, in-depth analysis of a subject. There are many reasons for writing one and these will influence the length and style of your review, but in essence a literature review is a critical appraisal of the current collective knowledge on a subject. Rather than just being an exhaustive list of all that ...
The reviewed definitions have been developed using a variety of methods including theoretical approaches involving review, synthesis, and/or expansion of existing theories of wisdom or related constructs (7, 12, 24, 25); prototypical studies involving methods requiring participants to provide or rate wisdom-related characteristics followed by ...
This systematic review found wide variation in how medication errors are defined between studies. This variation has significant implications for determining the prevalence of medication errors. Prior commentaries have noted the need for standardized, universally applicable definitions of adverse drug events.
A literature review is probably the most common academic writing activity that is performed by scholars and graduate students. Imel [] identified a literature review as being either part of a larger study or as a research effort on its own.As a part of a larger study, Imel [] identified the literature is "the foundation for the study."It has been suggested that the literature review for a ...
A systematic literature review of definitions and characteristics.}, author={Marianne Lisby and Lars Peter Nielsen and Lars Peter Nielsen and Birgitte Brock and Birgitte Brock and Jan Mainz and Jan Mainz}, journal={International journal for quality in health care : journal of the International Society for Quality in Health Care}, year={2010 ...
The term literature review can refer to the process of doing a review as well as the product resulting from conducting a review. The product resulting from reviewing the literature is the concern of this section. Literature reviews for research studies at the master's and doctoral levels have various definitions.
A literature or narrative review is a comprehensive review and analysis of the published literature on a specific topic or research question. The literature that is reviewed contains: books, articles, academic articles, conference proceedings, association papers, and dissertations. It contains the most pertinent studies and points to important ...
A systematic literature review of definitions and characteristics . × Close Log In. Log in with Facebook Log in ... Lisby M, Nielsen LP, Brock B and Mainz J. How are medication errors defined: a systematic review of definitions and characteristics. Int J Qual Health Care 2010;22(6):507−18. [19] Miller MR, Clark JS and Lehman CU. Computer ...
A literature review serves two main purposes: 1) To show awareness of the present state of knowledge in a particular field, including: seminal authors. the main empirical research. theoretical positions. controversies. breakthroughs as well as links to other related areas of knowledge. 2) To provide a foundation for the author's research.
9.3. Types of Review Articles and Brief Illustrations. EHealth researchers have at their disposal a number of approaches and methods for making sense out of existing literature, all with the purpose of casting current research findings into historical contexts or explaining contradictions that might exist among a set of primary research studies conducted on a particular topic.
Background: Extensive research on impulsive suicide attempts, but lack of agreement on the use of this term indicates the need for a systematic literature review of the area. The aim of this review was to examine definitions and likely correlates of impulsive attempts. Methods: A search of Medline, Psychinfo, Scopus, Proquest and Web of Knowledge databases was conducted.
A review of the available literature indicated that medication discrepancies and errors are characterised in several ways [25]. In the absence of a gold-standard definition, and for the purpose of ...
To date, there has been no systematic review of the literature on impulsive suicide attempts. The present paper sought to address this gap. Its purpose was to examine how impulsive suicide attempts have been defined by researchers and to establish, as far as possible, the likely correlates of those attempts described as impulsive. 2. Method
Prominent definitions The most referenced PSS definition in the literature review was the definition by Mont from 2002, which was referred to 18 times in the primary literature: “A system of products, services, supporting networks and infrastructure that is designed to be: competitive, satisfy customer needs and have a lower environmental ...
This article examines the literature discussing open peer review, identifies common open peer review definitions, and describes eight common characteristics of open peer review: signed review, disclosed review, editor-mediated review, transparent review, crowd-sourced review, pre-publication review, synchronous review, and post-publication review.
Types of reviews and examples. Definition: "A term used to describe a conventional overview of the literature, particularly when contrasted with a systematic review (Booth et al., 2012, p. 265). Characteristics: Example: Mitchell, L. E., & Zajchowski, C. A. (2022). The history of air quality in Utah: A narrative review.
This article attempts to sort out the definitions cited so far by important organizations and key academics. The review was based on deep literature study has attempted to be inclusive and consistent.
The literature review for BIM definitions included the most important, well-known literature—books by well-known BIM practitioners and scientific articles by prominent BIM researchers. ... " BIM is a digital representation of the physical and functional characteristics of an object. As such, it serves as a common knowledge resource for ...
Background It is uncertain if patient's characteristics are associated with complaints and claims against doctors. Additionally, evidence for the effectiveness of remedial interventions on rates of complaints and claims against doctors has not been synthesised. Methods We conducted a rapid review of recent literature to answer: Question 1 "What are the common characteristics and ...
As a major solution to climate change, the low-carbon transition of energy systems has received growing attention in the past decade. This paper presents a bibliometric review of the literature on the low-carbon transition of energy systems from an engineering management perspective. First, the definition and boundaries of the energy system transition are clarified, covering transformation of ...