WHAT ARE YOU LOOKING FOR?
Key searches, sweeping review reveals latest evidence on the diagnosis, treatment, and monitoring of adhd.
A comprehensive literature review from USC researchers summarizes the most robust findings on addressing ADHD in children and adolescents, which will inform updated clinical practice guidelines from the American Academy of Pediatrics.
Photo/iStock
Hundreds of studies are published each year on attention deficit hyperactivity disorder (ADHD), but more work is needed to ensure those findings improve lives.
With input from expert stakeholders across the field, researchers at the Southern California Evidence Review Center , part of the Keck School of Medicine of USC , have synthesized the latest insights so that they can ultimately inform clinical practice. Broadly, they found that both medications and psychosocial treatments work for treating ADHD and that children with the condition can and do get better.
“We have more research than ever on ADHD, but we need to summarize it in a reliable and valid way,” said Susanne Hempel, PhD , a professor of clinical population and public health sciences at the Keck School of Medicine and director of the Southern California Evidence Review Center, who oversaw the work.
The team, which included researchers from the Southern California Evidence-based Practice Center, the Keck School of Medicine’s division of child psychiatry and the Children’s Hospital Los Angeles Behavioral Health Institute, reviewed more than 23,000 publications on ADHD. Their work was commissioned by the Agency for Healthcare Research and Quality and funded by the Patient-Centered Outcomes Research Institute.
The results, just published in two companion papers in the journal Pediatrics , answer big questions about what works to effectively diagnose and treat ADHD, and point to ongoing gaps in the research, including how best to monitor the condition’s progression over time. Clinicians selected by the American Academy of Pediatrics (AAP) will now use the evidence review to create updated clinical guidelines that inform best practices in ADHD care across the nation.
“Parents, teachers and providers need evidence-based information about ADHD,” Hempel said. “We included only the most robust studies in our review, which enables us to make strong evidence statements.”
New findings on diagnosis and treatment
Before beginning the literature review, the research team developed their questions and protocols in collaboration with ADHD experts across the field to ensure they were asking and answering questions that could directly benefit patients, families and providers. During the process, the researchers also posted their preliminary findings and welcomed feedback during a 45-day public comment period.
The team conducted an extensive search that was not restricted to diagnostic tools or treatment approaches already known to be effective. From more than 23,000 publications, the researchers selected 550 studies for the final analysis. Studies were selected if they met the team’s inclusion criteria, which prioritized rigorous study designs such as randomized controlled trials.
For diagnosis of ADHD, many tools are available, including parent and teacher rating scales, patient self-reports, neuropsychological tests, EEG approaches, imaging, biomarkers, activity monitoring and observation. For several approaches, the researchers found a substantial variation in results, with some studies indicating a given method was highly effective and others indicating that it performed poorly. “We’re getting better at diagnosing ADHD, but research is still characterized by a lot of variation,” Hempel said.
Many treatments for ADHD have been rigorously tested, building a strong evidence base for medications (including both stimulants and non-stimulants), as well as psychosocial approaches, such as behavior modification. Other non-drug treatments the team analyzed include cognitive training, neurofeedback, physical exercise, nutrition and supplements, parent support, and school interventions.
“Medications have the strongest evidence for improving not only ADHD symptoms, but also other problems that often accompany ADHD, such as oppositional and disruptive behaviors,” said Bradley Peterson, MD, director of the Institute for the Developing Mind at Children’s Hospital Los Angeles (CHLA) and the lead author of the review.
Monitoring ADHD over time
In addition to reviewing the evidence on diagnosis and treatment, the researchers explored what is known about ongoing monitoring of ADHD: How can providers assess whether a child or adolescent needs to continue treatment for the condition? Experts across the field agreed that the question is a critical one, but few studies have explored the question. The evidence review team concluded that more research is needed on monitoring ADHD over time.
The publications will now be used to support an update of the AAP’s clinical practice guidelines for ADHD , providing up-to-date advice for how best to diagnose, evaluate and treat the condition.
“The overarching takeaway: ADHD is treatable. There are lots of studies that can show us that children absolutely can get better,” Hempel said.
About this research
In addition to Drs. Peterson and Hempel, the study’s other authors are Joey Trampush, Morah Brown, Margaret Maglione, Maria Bolshakova, Mary Rozelle, Jeremy Miles, Sheila Pakdaman and Aneesa Motala among others from the Southern California Evidence Review Center, Keck School of Medicine, University of Southern California.
This work is supported by the Agency for Healthcare Research and Quality [Contract No. 75Q80120D00009] and the Patient-Centered Outcomes Research Institute [Publication No. 2023-SR-03].
- Press Release
- Population and Public Health Sciences

The Palgrave Handbook of Male Psychology and Mental Health pp 291–307 Cite as

Attention Deficit Hyperactivity Disorder (ADHD): A Case Study and Exploration of Causes and Interventions
- Bijal Chheda-Varma 5
- First Online: 02 March 2019
3206 Accesses
The male to female ratio of ADHD is 4:1. This chapter on ADHD provides a wide perspective on understanding, diagnosis and treatment for ADHD. It relies on a neurodevelopmental perspective of ADHD. Signs and symptoms of ADHD are described through the DSM-V criteria. A case example (K, a patient of mine) is illustrated throughout the chapter to provide context and illustrations, and demonstrates the relative merits of “doing” (i.e. behavioural interventions) compared to cognitive insight, or medication alone. Finally, a discussion of the Cognitive Behavioral Modification Model (CBM) for the treatment of ADHD provides a snapshot of interventions used by clinicians providing psychological help.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Alderson, R. M., Hudec, K. L., Patros, C. H. G., & Kasper, L. J. (2013). Working memory deficits in adults with attention-deficit/hyperactivity disorder (ADHD): An examination of central executive and storage/rehearsal processes. Journal of Abnormal Psychology, 122 (2), 532–541. http://dx.doi.org/10.1037/a0031742 .
Arcia, E., & Conners, C. K. (1998). Gender differences in ADHD? Journal of Developmental and Behavioral Pediatrics, 19 (2), 77–83. http://dx.doi.org/10.1097/00004703-199804000-00002 .
Barkley, R. A. (1990). Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment . New York: Guildford.
Google Scholar
Barkley, R. A. (1997). ADHD and the nature of self-control . New York: Guilford Press.
Barkley, R. A. (2000). Commentary on the multimodal treatment study of children with ADHD. Journal of Abnormal Child Psychology, 28 (6), 595–599. https://doi.org/10.1023/A:1005139300209 .
Article Google Scholar
Barkley, R., Knouse, L., & Murphy, K. Correction to Barkley et al. (2011). Psychological Assessment [serial online]. June 2011; 23 (2), 446. Available from: PsycINFO, Ipswich, MA. Accessed December 11, 2014.
Beck, A. T. (1976). Cognitive therapy and the emotional disorders . New York, NY: International Universities Press.
Brown, T. E. (2005). Attention deficit disorder: The unfocused mind in children and adults . New Haven, CT: Yale University Press.
Brown, T. (2013). A new understanding of ADHD in children and adults . New York: Routledge.
Chacko, A., Kofler, M., & Jarrett, M. (2014). Improving outcomes for youth with ADHD: A conceptual framework for combined neurocognitive and skill-based treatment approaches. Clinical Child and Family Psychology Review . https://doi.org/10.1007/s10567-014-0171-5 .
Chronis, A., Jones, H. A., Raggi, V. L. (2006, August). Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. Clinical Psychology Review, 26 (4), 486–502. ISSN 0272-7358. http://dx.doi.org/10.1016/j.cpr.2006.01.002 .
Curatolo, P., D’Agati, E., & Moavero, R. (2010). The neurobiological basis of ADHD. Italian Journal of Pediatrics, 36 , 79. http://doi.org/10.1186/1824-7288-36-79 . http://www.sciencedirect.com/science/article/pii/S0272735806000031 .
Curtis, D. (2010). ADHD symptom severity following participation in a pilot, 10-week, manualized, family-based behavioral intervention. Child & Family Behavior Therapy, 32 , 231–241. https://doi.org/10.1080/07317107.2010.500526 .
De Young, R. (2014). Using the Stroop effect to test our capacity to direct attention: A tool for navigating urgent transitions. http://www.snre.umich.edu/eplab/demos/st0/stroopdesc.html .
Depue, B. E., Orr, J. M., Smolker, H. R., Naaz, F., & Banich, M. T. (2015). The organization of right prefrontal networks reveals common mechanisms of inhibitory regulation across cognitive, emotional, and motor processes. Cerebral Cortex (New York, NY: 1991), 26 (4), 1634–1646.
D’Onofrio, B. M., Van Hulle, C. A., Waldman, I. D., Rodgers, J. L., Rathouz, P. J., & Lahey, B. B. (2007). Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Archives of General Psychiatry, 64, 1296–1304 [PubMed].
DSM-V. (2013). Diagnostic and statistical manual of mental disorders . American Psychological Association.
Eisenberg, D., & Campbell, B. (2009). Social context matters. The evolution of ADHD . http://evolution.binghamton.edu/evos/wp-content/uploads/2012/02/eisenberg-and-campbell-2011-the-evolution-of-ADHD-artice-in-SF-Medicine.pdf .
Gizer, I. R., Ficks, C., & Waldman, I. D. (2009). Hum Genet, 126 , 51. https://doi.org/10.1007/s00439-009-0694-x .
Hinshaw, S. P., & Scheffler, R. M. (2014). The ADHD explosion: Myths, medication, money, and today’s push for performance . New York: Oxford University Press.
Kapalka, G. M. (2008). Efficacy of behavioral contracting with students with ADHD . Boston: American Psychological Association.
Kapalka, G. (2010). Counselling boys and men with ADHD . New York: Routledge, Taylor & Francis Group.
Book Google Scholar
Knouse, L. E., et al. (2008, October). Recent developments in psychosocial treatments for adult ADHD. National Institute of Health, 8 (10), 1537–1548. https://doi.org/10.1586/14737175.8.10.1537 .
Laufer, M., Denhoff, E., & Solomons, G. (1957). Hyperkinetic impulse disorder in children’s behaviour problem. Psychosomatic Medicine, 19, 38–49.
Raggi, V. L., & Chronis, A. M. (2006). Interventions to address the academic impairment of children and adolescents with ADHD. Clinical Child and Family Psychology Review, 9 (2), 85–111. https://doi.org/10.1007/s10567-006-0006-0 .
Ramsay, J. R. (2011). Cognitive behavioural therapy for adult ADHD. Journal of Clinical Outcomes Management, 18 (11), 526–536.
Retz, W., & Retz-Junginger, P. (2014). Prediction of methylphenidate treatment outcome in adults with attention deficit/hyperactivity disorder (ADHD). European Archives of Psychiatry and Clinical Neuroscience . https://doi.org/10.1007/s00406-014-0542-4 .
Safren, S. A., Otto, M. W., Sprich, S., Winett, C. L., Wilens, T. E., & Biederman, J. (2005, July). Cognitive-behavioral therapy for ADHD in medication-treated adults with continued symptoms. Behaviour Research and Therapy, 43 (7), 831–842. ISSN 0005-7967. http://dx.doi.org/10.1016/j.brat.2004.07.001 . http://www.sciencedirect.com/science/article/pii/S0005796704001366 .
Sibley, M. H., Kuriyan, A. B., Evans, S. W., Waxmonsky, J. G., & Smith, B. H. (2014). Pharmacological and psychosocial treatments for adolescents with ADHD: An updated systematic review of the literature. Clinical Psychology Review, 34 (3), 218–232. https://doi.org/10.1016/j.cpr.2014.02.001 .
Simchon, Y., Weizman, A., & Rehavi, M. (2010). The effect of chronic methylphenidate administration on presynaptic dopaminergic parameters in a rat model for ADHD. European Neuropsychopharmacology, 20 (10), 714–720. ISSN 0924-977X. https://doi.org/10.1016/j.euroneuro.2010.04.007 . http://www.sciencedirect.com/science/article/pii/S0924977X10000891 .
Swanson, J. M., & Castellanos, F. X. (2002). Biological bases of ADHD: Neuroanatomy, genetics, and pathophysiology. In P. S. Jensen & J. R. Cooper (Eds.), Attention deficit hyperactivity disorder: State if the science, best practices (pp. 7-1–7-20). Kingston, NJ: Civic Research Institute.
Toplak, M. E., Connors, L., Shuster, J., Knezevic, B., & Parks, S. (2008, June). Review of cognitive, cognitive-behavioral, and neural-based interventions for attention-deficit/hyperactivity disorder (ADHD). Clinical Psychology Review, 28 (5), 801–823. ISSN 0272-7358. http://dx.doi.org/10.1016/j.cpr.2007.10.008 . http://www.sciencedirect.com/science/article/pii/S0272735807001870 .
Wu, J., Xiao, H., Sun, H., Zou, L., & Zhu, L.-Q. (2012). Role of dopamine receptors in ADHD: A systematic meta-analysis. Molecular Neurobiology, 45 , 605–620. https://doi.org/10.1007/s12035-012-8278-5 .
Download references
Author information
Authors and affiliations.
Foundation for Clinical Interventions, London, UK
Bijal Chheda-Varma
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
UCL, London, UK
John A. Barry
Norfolk and Suffolk NHS Foundation Trust, Wymondham, UK
Roger Kingerlee
Change, Grow, Live, Dagenham/Southend, Essex, UK
Martin Seager
Community Interest Company, Men’s Minds Matter, London, UK
Luke Sullivan
Rights and permissions
Reprints and permissions
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter.
Chheda-Varma, B. (2019). Attention Deficit Hyperactivity Disorder (ADHD): A Case Study and Exploration of Causes and Interventions. In: Barry, J.A., Kingerlee, R., Seager, M., Sullivan, L. (eds) The Palgrave Handbook of Male Psychology and Mental Health. Palgrave Macmillan, Cham. https://doi.org/10.1007/978-3-030-04384-1_15
Download citation
DOI : https://doi.org/10.1007/978-3-030-04384-1_15
Published : 02 March 2019
Publisher Name : Palgrave Macmillan, Cham
Print ISBN : 978-3-030-04383-4
Online ISBN : 978-3-030-04384-1
eBook Packages : Behavioral Science and Psychology Behavioral Science and Psychology (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
Participants
Interventions, conclusions, acknowledgments, improving care management in attention-deficit/hyperactivity disorder: an rct.
POTENTIAL CONFLICT OF INTEREST: Drs Fiks and Grundmeier are the inventors of the Care Assistant, which was used as the patient portal for patients with attention-deficit/hyperactivity disorder in this study; the other authors have indicated they have no potential conflicts of interest to disclose.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
James P. Guevara , Thomas J. Power , Katherine Bevans , Lisa Snitzer , Siobhan Leavy , Denise Stewart , Caroline Broomfield , Salima Shah , Robert Grundmeier , Jeremy J. Michel , Steven Berkowitz , Nathan J. Blum , Matthew Bryan , Heather Griffis , Alexander G. Fiks; Improving Care Management in Attention-Deficit/Hyperactivity Disorder: An RCT. Pediatrics August 2021; 148 (2): e2020031518. 10.1542/peds.2020-031518
Download citation file:
- Ris (Zotero)
- Reference Manager
Video Abstract
To compare the effectiveness of care management combined with a patient portal versus a portal alone for communication among children with attention-deficit/hyperactivity disorder (ADHD).
Randomized controlled trial conducted at 11 primary care practices. Children aged 5 to 12 years old with ADHD were randomly assigned to care management + portal or portal alone. The portal included parent-reported treatment preferences and goals, medication side effects, and parent- and teacher-reported ADHD symptom scales. Care managers provided education to families; communicated quarterly with parents, teachers, and clinicians; and coordinated care. The main outcome, changes in the Vanderbilt Parent Rating Scale (VPRS) score as a measure of ADHD symptoms, was assessed using intention-to-treat analysis.
A total of 303 eligible children (69% male; 46% Black) were randomly assigned, and 273 (90%) completed the study. During the 9-month study, parents in the care management + portal arm communicated inconsistently with care managers (mean 2.2; range 0–6) but similarly used the portal (mean 2.3 vs 2.2) as parents in the portal alone arm. In multivariate models, VPRS scores decreased over time (Adjusted β = −.015; 95% confidence interval −0.023 to −0.07) in both groups, but there were no intervention-by-time effects (Adjusted β = .000; 95% confidence interval −0.011 to 0.012) between groups. Children who received ≥2 care management sessions had greater reductions in VPRS scores than those with fewer sessions.
Results did not provide evidence that care management combined with a patient portal was different from portal use alone among children with ADHD. Both groups demonstrated similar reductions in ADHD symptoms. Those families with greater care management engagement demonstrated greater reductions than those with less engagement.
Electronic patient portals and care managers have been used to facilitate communication among clinicians and families of children with mental health disorders. The relative effectiveness of patient portals vis-à-vis care managers on outcomes in attention-deficit/hyperactivity disorder is not known.
In this randomized comparative effectiveness trial that included 303 children with attention-deficit/hyperactivity disorder, there were no significant intervention-by-time differences in parent-reported outcomes between groups. Care management did not enhance communication and improve outcomes beyond a patient portal alone.
Attention-deficit/hyperactivity disorder (ADHD), characterized by inattention, impulsivity, and hyperactivity, is the most common chronic neurobehavioral disorder in children. 1 , 2 Prevalence among children in the United States has risen to 12%. 3 Effectiveness of treatment of ADHD, supported by clinical trials, consists of psychotropic medications, such as methylphenidate, and behavior therapy, alone or in combination. 2 , 4 Unfortunately, adherence to ADHD treatment is poor, limiting treatment effectiveness. 5 , 6
Shared decision-making (SDM) may be helpful for conditions like ADHD that have evidence-based options and variation in how families weigh options. 7 SDM is a process in which families and clinicians jointly engage in decision-making, exchange information and treatment preferences, and work to decide on a treatment plan. 8 Because children with ADHD use services across multiple systems, poor communication between families and health and education systems can limit SDM and lead to poor adherence to treatment. 9
Two strategies have been proposed to enhance communication and promote greater SDM in ADHD. One is the use of electronic patient portals, online health care applications that allow patients to interact and communicate with providers and manage their health. 10 – 12 Portals designed for ADHD have been found to increase exchange of information between clinicians, parents, and teachers. 13 Another is the use of care managers, who function to promote patient engagement and coordinate care across care systems. In studies of adolescents and adults with depression, care managers have demonstrated favorable findings regarding depressive symptoms and functioning. 14 – 16 Data are limited regarding the use of care managers with ADHD. 17 Given the growing access to patient portals with ADHD-specific components, we sought to determine the comparative effectiveness of patient portals combined with care managers versus patient portals alone on ADHD symptoms, goal attainment, and patient-reported outcome (PRO) measures. We postulated that the combination of patient portals and care managers would be associated with greater reductions in ADHD symptoms over time than portal use alone and that effects would vary by race and/or ethnicity and income.
We conducted a prospective randomized comparative effectiveness trial involving 11 primary care pediatric practices affiliated with a large children’s hospital from March 10, 2016, to April 12, 2019. The practices included 5 urban practices and 6 suburban practices that used a common electronic health record (EHR) and an ADHD-specific patient portal known as the ADHD Care Assistant. 18 , 19 Five of the practices had colocated behavioral health services. We recruited the practices using letters of invitation and in-person presentations. The study was approved by the Institutional Review Board at the Children’s Hospital of Philadelphia and was registered at Clinicaltrials.gov (NCT02716324) before recruitment of participants.
Children were eligible for the study if they received care at a participating practice, had an ADHD diagnosis code ( International Classification of Diseases, Ninth Revision [ICD-9] code 314) recorded at an ambulatory visit in the past year, and were aged 5 through 12 years old. Children were excluded if they had a diagnosis of autism spectrum disorder (ICD-9 code 299), conduct disorder (ICD-9 code 312), psychosis (ICD-9 code 298), bipolar disorder (ICD-9 code 296) or suicidal ideation or intent (ICD-9 codes E950.0–E958.9) in the past 12 months. Lists of potentially eligible children were identified from EHR records and were verified by primary care clinicians at the practices. A random sample of at least 300 eligible children stratified by practice, sex, and age group (5–7 vs 8–12 years old) were selected to achieve a representative sample. Parents of selected children were mailed a recruitment letter and a stamped self-addressed postcard to opt out. Families who did not opt out within 2 weeks were called to screen for eligibility, provide study information, and schedule an enrollment visit. We randomly selected additional children from the same strata as those who declined participation, were ineligible, or were unable to be contacted. Children were consented and randomly assigned 1:1 within strata to the 2 groups by the study biostatistician using a random number generator.
We sought to compare the effectiveness of an ADHD portal embedded in the EHR (portal alone) with the portal combined with an ADHD care manager (care management + portal). The ADHD portal, known as the ADHD Care Assistant, was designed to (1) collect and share patient and family treatment preferences and goals with a clinician; (2) trend ADHD symptoms, performance impairment ratings, medication side effects, treatment receipt, and medication side effects by using electronically submitted parent and teacher reports; (3) provide a repository of ADHD educational materials; and (4) support information sharing between parents and teachers ( Fig 1 ). The ADHD Care Assistant enabled clinicians to send survey-driven ADHD symptom rating scales to a child’s parent and teacher via e-mail. Data collected through this system were displayed directly to the clinician within the EHR. Parents were provided an opportunity to view teacher reports, and teachers could view parent-submitted reports with parental consent. 20 The frequency of e-mails from the portal varied from biweekly to every 3 months at the discretion of the clinician in consultation with the family. Parent and teacher usage of the ADHD Care Assistant during study participation was extracted from the EHR.

Design for the ADHD Care Assistant portal. The figure shows the design for the ADHD SDM portal. The portal is able to (1) capture and share patient and family treatment preferences and goals; (2) monitor symptoms, treatment receipt, and side effects as well as goal attainment; and (3) facilitate communication between parent, teachers, and primary care clinicians. Data on behavior therapy and school-based intervention receipt are not available in the ADHD Care Assistant.
ADHD care managers were bachelor’s-trained individuals who were responsible for communicating information and facilitating coordination of care. The care managers confirmed family treatment preferences and goals, provided education on the treatment of ADHD and associated conditions, monitored attainment of parent-directed goals and emerging issues, and provided resources and assistance with concerns for patients with ADHD and families. The care managers sought to contact families, teachers, and clinicians by phone, text message, or e-mail every 3 months or sooner if problems arose. The care managers completed a fidelity checklist after each encounter to assess self-reported task completion (0, not completed; 1, partially completed; 2, fully completed) and summarized the sessions as a telephone encounter in the EHR.
We collected demographic information (child age, child sex, child race and/or ethnicity, child Supplemental Security Income [SSI] status, parental education level, urban or suburban residence, free or reduced lunch status, and school type [public, charter, or private]) at baseline. In addition, we geocoded participant home addresses and used American Community Survey tract-level data to obtain median family neighborhood income levels. ADHD medication (stimulants, α-agonists, and atomoxetine) fills and dates for each child during the study period were determined by abstraction from the EHR.
Change in ADHD symptoms was the primary outcome. We used the ADHD symptom subscale of the Vanderbilt Parent Rating Scale (VPRS) to assess ADHD symptoms. 21 The VPRS has been shown to have excellent internal consistency (α = .90–.94) and concurrent validity ( r = 0.79) in relation to diagnostic interviews. The VPRS includes 18 items that correspond to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition symptom criteria and has a 4-point Likert response category (never [0] to very often [3]) for each item. We summed items on the VPRS to obtain an overall symptom score ranging from 0 to 54, with lower scores indicating fewer ADHD symptoms.
Changes in goal attainment and PROs were secondary outcomes. We identified family treatment goals through use of the ADHD Preference and Goals Instrument, a parent-reported 46-item tool that queries parents on their treatment preferences (medications and/or behavioral therapy) and goals for treatment. 22 A primary goal for each family was identified and categorized as academic, behavioral, or relational by consensus of the study team. Goal attainment scaling (GAS) was used to allow parents to rate the degree to which their goal was met over time and was scaled from 0 (no change) to 6 (goal completely met). 23 – 25 PRO measures were completed by a parent proxy and by children aged ≥8 for school performance, student engagement, peer relationships, family belonging, and teacher connectedness. These PRO measures consisted of Patient-Reported Outcomes Measurement Information System short forms and Healthy Pathway scales, which are brief, reliable, and precise measures of patient-reported health status. 26 – 29 Higher GAS and PRO scores indicate better outcomes.
Outcome measures were collected at baseline and at 3-month intervals for 9 to 12 months after enrollment by using Research Electronic Data Capture e-mail surveys. Research staff, who were blinded to participants’ study arms, contacted nonresponders at each study visit by phone to complete the surveys. In addition, we obtained VPRS scores from the ADHD portal, if available within 30 days of the corresponding Research Electronic Data Capture survey, for nonresponders.
We employed imputation methods for missing items for 2 of the outcomes (VPRS and PRO measures). If a participant had 1 or 2 items missing within 1 of the 2 domains on the VPRS or any of the PRO measures, the average of the nonmissing items for the domain or PRO measure was assigned to obtain a score. No between–study arm differences between pre- and postimputation VPRS and PRO mean scores were observed for cases and controls. VPRS subdomains and PRO measures with >2 missing items were not imputed. We elected not to impute missing GAS scores.
To determine differences in outcomes between groups, we followed the standard of an intention-to-treat repeated-measures longitudinal analysis. To check on the adequacy of randomization, patient characteristics were compared between groups. To assess bivariate associations between study arm and outcome at each of the study visits, t tests were used for ADHD symptoms, parent and child PRO measures, and goal attainment. To assess differences in outcomes, we used linear mixed-effects models to account for missing outcome values under a missing-at-random assumption. We examined results from analogous generalized estimating equations models to examine the robustness of our conclusions to model selection. 30 , 31 Intervention-by-time interactions for each outcome were used to represent the adjusted difference in outcome measures between the 2 groups over time. Models were adjusted for seasonality (fall, winter, spring, and summer), child age (5–7 and 8–12 years), child sex, child race and/or ethnicity (white, Black, Hispanic, or other), free or reduced school lunch, metropolitan status (urban or suburban), child SSI status, parent education level (up to high school, some college, or college degree), school type (public, private, or charter), median neighborhood income, and ADHD medication status. To account for clustering, clinic site was included in each model as a random effect. With a sample of 300, we had power of 0.87 to detect a difference of 2.5 points on the VPRS scores between groups, assuming α = .05, 80% follow-up, and moderate correlation ( r = 0.6) over 9 months. The difference of 2.5 points represented a small clinically meaningful effect size from our pilot study. We conducted a sensitivity analyses to examine the impact of intervention dosage (ie, number of care management sessions) on ADHD symptoms. All analyses were conducted by using Stata 15 statistical software (Stata Corp, College Station, TX).
We identified 3118 potential participants aged 5 to 12 years old with ADHD from the 11 practices ( Fig 2 ). Of these, we randomly selected and stratified 960 children. We sent 875 recruitment letters, but 572 were not enrolled for the following reasons: 112 declined participation, 174 were ineligible, and 286 were unable to be reached. Thus, we enrolled and randomly assigned 303 eligible children: 154 to care management + portal and 149 to portal alone. Of these, 273 (90.1%) completed the study, as defined by completing the final VPRS: 143 (93%) in care management + portal and 130 (87%) in portal alone.

Participant flow through the study.
After randomization, children in both study arms had similar sociodemographic characteristics ( Table 1 ). The average age was 8.5 years old, with most (91%) ≥8 years of age. Two-thirds were male. Children were racially and socioeconomically diverse, with 51% residing in urban locations. More than half qualified for free or reduced school lunch (54%), and more than half attended public schools (60%). As expected, a slight majority (53%) of children reported receiving an ADHD medication at baseline.
Demographic Characteristics of Study Participants at Baseline
Participants were children aged 5–12 y old with ADHD who were recruited from 11 primary care pediatric practices. Numbers may not add to column totals because of missing data.
Over the course of the study period, 206 (68%) study participants used the ADHD portal to complete a parent-reported VPRS ( Table 2 ). The average number of VPRSs completed was similar in both arms: 2.3 in care management + portal and 2.2 in portal alone. Nearly one-third of participants (30%) had a teacher use the patient portal to complete a Vanderbilt Teacher Rating Scale. In care management + portal, most parents (96%) had at least 1 care management session. One-third of children (34%) had a teacher engage with the care managers. When care manager fidelity checklists were examined, 66% of parent and 63% of teacher sessions were rated as fully completed on all relevant items (data not shown) and the rest as partially completed or not relevant.
Portal and Care Manager Sessions Completed, by Study Arm
Participants were children aged 5–12 y old with ADHD who were recruited from 11 primary care pediatric practices. Numbers may not add to column totals because of missing data. Families of all participants were contacted at least every 3 mo to complete an online ADHD portal survey of ADHD symptoms. Only families of participants in the care manager + portal group were contacted at least every 3 mo to participate in a care management session. n/a, not applicable.
Completion of a survey through the ADHD portal by either a parent or a teacher constituted a portal session.
Table 3 shows the primary outcome, mean VPRS scores, by study visit and group. All 303 participants completed study visit 1, 258 (85%) completed study visit 2, 236 (78%) completed study visit 3, and 273 (90%) completed study visit 4. Mean VPRS scores decreased over time, indicating clinical improvement in ADHD symptoms, but there were no statistically significant differences in mean scores between groups at any study visit. GAS, parent proxy–reported PRO, and child-reported PRO scores did not change over time and did not differ between groups at any study visit (Supplemental Tables 5 through 7).
VPRS Scores by Study Visit Between Groups
Participants were children aged 5–12 y old with ADHD who were recruited from 11 primary care pediatric practices. Differences in mean VPRS scores at study visits were assessed by using the t test.
To assess changes between groups in VPRS scores, results from the previously described model are presented in Table 4 . The intervention-by-time interaction in the full model was not significant (β = .00; 95% confidence interval [CI] −.01 to .01), indicating no difference between groups in changes in VPRS scores over time. In models without the interaction term, time (days) was significant, suggesting that VPRS scores decreased an average of 0.015 points per day or roughly 4 points over the course of the 9-month intervention period for both groups, a clinically meaningful improvement. Urban and medication status were also both significant, indicating that children residing in urban compared with suburban residences had VPRS scores that were greater and children on ADHD medications, as opposed to no medications, had VPRS scores that were lower. There were no adverse effects from either intervention identified, and interactions of intervention by race or income were not significant, suggesting no heterogeneity of treatment effects. Results from the generalized estimating equations model were consistent with results from the linear mixed-effects model.
Adjusted VPRS Scores
Participants were children aged 5–12 y old with ADHD who were recruited from 11 primary care pediatric practices. After imputation, there were 1034 individual person–time points. Linear mixed-effects models regressed VPRS scores on intervention status, time (days), intervention by time, race, urbanicity, education, medication status, median household income, and season and a random effect for clinic site.
Medication status reported by parent at time of survey; if medication status was missing on the self-reported survey, the status was included from the EHR at a clinic visit within 1 mo of the corresponding survey date.
We conducted a sensitivity analysis to determine if there was a dose-response in which greater engagement among care management + portal participants resulted in greater declines in VPRS scores. After adjustment for seasonality, we found that those participants who received ≥2 care management sessions experienced statistically significantly greater decreases in VPRS scores (−4.7; 95% CI −8.0 to −1.4) than those with 1 or 0 sessions. There were no demographic differences by the number of care management sessions attended. This nonrandomized result suggests that greater engagement of participants within the care management intervention resulted in greater symptom improvement.
In this comparative effectiveness study of 2 communication strategies, an electronic patient portal combined with a care manager versus a portal alone, we found no difference over time in primary or secondary outcomes between the 2 groups. We observed an overall improvement in ADHD symptoms over time in both groups but little to no change in goal attainment or PROs at any time point. This suggests that care management did not improve ADHD symptoms over and above that of the patient portal alone.
Our finding that children in both groups showed improvement in ADHD symptoms over time is consistent with previous studies employing patient portals for ADHD. Epstein et al 13 found that community pediatricians using an ADHD portal were significantly more likely to collect information from parents and teachers than those using usual care. Nagykaldi et al 32 found that the use of a portal focused on preventive care resulted in increased patient activation and greater patient-centered care, and users were more likely to receive needed preventive care.
There was variable engagement by parents with the care manager. It is not entirely clear why parents did not consistently engage to a greater extent with the care manager than with the portal as we had postulated. Our results revealed modest engagement by parents and teachers with care managers. Care managers were instructed to engage with parents and teachers virtually at least every 3 months; however, care managers found it challenging to contact parents and teachers to schedule sessions. In some instances, care managers were unable to identify contact information for teachers despite calls to schools. Given the virtual nature of the care management intervention in this study, face-to-face contact was lacking and may have contributed to the inconsistent engagement. In a study of community health workers, those workers who provided face-to-face communication saw more beneficial effects on the quality of care and a reduction in hospital days. 33 In addition, systematic reviews of collaborative care trials among adults with depression have consistently demonstrated that care management involving face-to-face contact is associated with modest but sustained improvement in depression outcomes (standardized mean difference 0.25; 95% CI 0.18 to 0.32) compared with usual care. 34 , 35 Our preplanned sensitivity analysis revealed that those with a greater number of care management sessions had greater reductions in ADHD symptoms than those with fewer sessions, suggesting the importance of family engagement for this intervention to be effective.
There were several limitations to our study findings. First, our study was conducted in a single geographic area within an integrated pediatric health care system. Results may not be generalizable to other geographic areas or other health care systems. Second, our care managers primarily employed electronic means of communication, which may have limited engagement, as discussed above. Third, we did not enroll teachers in our study because of challenges in connecting with and obtaining formal approval from schools. This limitation did not permit us to interview teachers to discern their perceptions of the interventions. Finally, we lacked a no intervention control group, because use of the electronic portal was considered standard of care at our institution during the study period. This limited our ability to formally test benefits of the portal over no portal care.
In this comparative effectiveness study, we found no evidence that care management combined with a patient portal produced patient outcomes different from those of a patient portal alone among school-aged children with ADHD. Both groups demonstrated similar reductions in ADHD symptoms over time. Overall, there was variable engagement by parents with care managers, which likely limited its impact. Those families with greater care management engagement, as demonstrated by the number of sessions attended, showed greater reductions in ADHD symptoms over time than those with less. There was no heterogeneity of treatment effects as a function of race and/or ethnicity or household income. In future studies, researchers investigating the effects of care management should consider methods (eg, face-to-face meetings) to better engage families and teachers.
We thank the Pediatric Research Consortium (PeRC) at the Children’s Hospital of Philadelphia and all participating practices and clinicians for their support of this study. We also thank Stephanie Liu, MS, for her assistance with data collection.
Dr Guevara conceptualized and designed the study, oversaw the data collection and analysis, drafted the initial manuscript, and revised the manuscript; Drs Power and Bevans, Ms Snitzer, Dr Leavy, Ms Stewart, and Drs Grundmeier, Berkowitz, Blum, Bryan, and Fiks helped conceptualize and design the study, interpreted the data analysis, and critically reviewed the manuscript for intellectual content; Ms Broomfield and Ms Shah conducted the data collection, assisted with the data analysis, and critically reviewed the manuscript for intellectual content; Drs Michel and Griffis conducted the data analysis and critically reviewed the manuscript for intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Drs Guevara and Griffis had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This work was presented in part at the annual meeting of the Pediatric Academic Societies; April 27–30, 2019; Baltimore, MD; and at the International Forum on Quality and Safety in Healthcare; September 18–20, 2019; Taipei, Taiwan.
A complete, cleaned, and deidentified data set (including a data dictionary) will be made available to other investigators after all analyses have been conducted and after publication of this article. To obtain this data set, investigators may contact the study principal investigator, who will provide a data sharing agreement. The data sharing agreement will permit the data set to be shared once an institutional review board protocol has been approved at the investigators’ home institution and the investigators have signed a pledge to not attempt to identify individual study subjects. The data set will be made available on a CD-ROM or through a secure FTP site.
This trial has been registered at www.clinicaltrials.gov (identifier NCT02716324).
Dr Berkowitz’s current affiliation is Department of Psychiatry, University of Colorado, Denver, Colorado.
FUNDING: Funding was provided by the Patient Centered Outcomes Research Institute (PCORI), award (CDR-1408-20669). The statements in this article are solely the responsibility of the authors and do not necessarily reflect the views of the Patient-Centered Outcomes Research Institute, its Board of Governors, or its Methodology Committee.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2021-050766 .
attention-deficit/hyperactivity disorder
confidence interval
electronic health record
goal attainment scaling
International Classification of Diseases, Ninth Revision
patient-reported outcome
shared decision-making
Supplemental Security Income
Vanderbilt Parent Rating Scale
Competing Interests
Supplementary data.
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account

Patient Case Study
Case: johnny (8-year old male), diagnosis: adhd.
Johnny was an eight year-old boy with a diagnosis of ADHD when he and his mother came to our practice seeking help for his symptoms. For years, Johnny had struggled with aggressive, impulsive and oppositional tendencies. While he was highly intelligent, his school performance suffered due to struggles with emotional management.
As an infant, Johnny was colicky and was prescribed multiple rounds of antibiotics due to recurrent ear infections, ultimately needing tubes to help drain the middle ear. He also struggled with encopresis.
Upon a physical exam, it was noted that Johnny had an obvious and inflamed “lip-licking ring” around his mouth along with nasal congestion. A comprehensive battery of testing only found a few abnormalities. Johnny was heterozygous for a single nucleotide polymorphism of the methylenetetrahydrofolate reductase (MTHFR) enzyme at position 677 with a single C to T substitution. Trace metal hair and organic acid testing were all within normal limits. Based on the symptoms and a history of ear infections, dairy was removed from the diet. Lithium orotate, at one mg twice daily, was prescribed to help reduce his aggressive, impulsive and oppositional tendencies.
Upon follow-up, Johnny’s demeanor had dramatically improved. He was cooperative and emotionally engaging. The lip-licking ring had resolved. Both Johnny’s mother and Johnny were excited about the improvements and ready to continue integrative treatment.
Case Summary
Chronic ear infections are often a symptom of food allergies. While the research is not exhaustive, studies have found consistent correlations between cow’s milk allergy and middle ear infections in children ( Bhombal 2006 , Juntti 1999 ). Removing dairy products often results in a reduction or elimination of ear infections.
And while lithium is a well-known treatment for bipolar, it also has well-documented anti-aggressive effects ( Müller-Oerlinghausen 2010 ). In children struggling with oppositional and aggressive tendencies, low-dose lithium can be a straightforward treatment to improve symptoms.
Want to learn nutritional and functional medicine strategies like these to help your patients? Enroll in our comprehensive Fellowship for mental health providers! Book a private phone call with Dr. James Greenblatt to learn more today.
Bhombal S, Bothwell MR, Bauer SM. Prevalence of elevated total IgE and food allergies in a consecutive series of ENT pediatric patients. Otolaryngol Head Neck Surg . 2006;134(4):578-580. doi:10.1016/j.otohns.2005.11.041
Juntti H, Tikkanen S, Kokkonen J, Alho OP, Niinimäki A. Cow’s milk allergy is associated with recurrent otitis media during childhood. Acta Otolaryngol . 1999;119(8):867-873. doi:10.1080/00016489950180199

Home About Faculty Affiliate Program Contact
Courses Conferences Webinars Integrative Psychiatry Fellowship
Articles Books Low-Dose Lithium Provider Directory
The information on this site is not intended or implied to be a substitute for professional medical advice, diagnosis or treatment. Always seek the guidance of your doctor or other qualified health professional with any questions you may have regarding your health or a medical condition. The views and opinions expressed by those on this website are entirely their own. James Greenblatt, MD, assumes no responsibility whatsoever for the content of any such views or opinions, or for any information provided by any such individual.
By accessing or utilizing www.PsychiatryRedefined.org in any way, any user thereof releases James Greenblatt, MD (including Comprehensive Psychiatric Resources, Inc. d/b/a Psychiatry Redefined™, his principals, employees, agents and assigns) from and against any and all liability of any kind whatsoever, and indemnifies James Greenblatt, MD, against any and all claims of any kind.
Privacy Policy & Course Policies | Terms & Conditions
© 2024 Psychiatry Redefined. Psychiatry Redefined™. All Rights Reserved.
- What is Functional Psychiatry?
- New? Start Here
- Affiliate Program
- Schedule & Curriculum
- Certification
- Discovery Calls
- Testimonials
- Scholarships
- Discovery Calls & Webinars
- Child & Adolescent Mental Health Training
- Depression Intensive
- Depression & Mood Disorders Training
- View All Courses
- CME Courses
- Course Subscriptions
- Dr. Carson Course Bundle
- New! Photobiomodulation (PBM)
- Anorexia Nervosa
- Anxiety & OCD
- Endocannabinoids, CBD & Women’s Health
- Medication Side Effects
- Suicide Prevention
- Upcoming Webinars
- Webinar Library
- Psychiatry Reimagined 2024 Conference
- Lithium Orotate
- Nutritional Psychiatry for Anorexia
- Nutritional Psychiatry for BPD
- Supplement Protocols for Clinicians
- FMMH 2023 Annual Conference
- Low-Dose Lithium
- Binge-Eating & Food Addiction
- Child & Adolescent Psychiatry
- Functional Medicine for Psychiatry
- Ketogenic Diets
- Genetic Testing
- NE Conference on ADHD
- Well Woman Conference
- Enews Issues
- FinallyFocused.org
- Supplements
- Provider Directory
- Enewsletter
- JamesGreenblattMD.com
- Course Log-In
Privacy Overview
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Thieme Open Access

ADHD: Current Concepts and Treatments in Children and Adolescents
Renate drechsler.
1 Department of Child and Adolescent Psychiatry and Psychotherapy, University Hospital of Psychiatry, University of Zurich, Zurich, Switzerland
Silvia Brem
2 Neuroscience Center Zurich, Swiss Federal Institute of Technology and University of Zurich, Zurich, Switzerland
Daniel Brandeis
3 Department of Child and Adolescent Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim/Heidelberg University, Mannheim, Germany
4 Zurich Center for Integrative Human Physiology, University of Zurich, Zurich, Switzerland
Edna Grünblatt
Gregor berger, susanne walitza.
Attention deficit hyperactivity disorder (ADHD) is among the most frequent disorders within child and adolescent psychiatry, with a prevalence of over 5%. Nosological systems, such as the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) and the International Classification of Diseases, editions 10 and 11 (ICD-10/11) continue to define ADHD according to behavioral criteria, based on observation and on informant reports. Despite an overwhelming body of research on ADHD over the last 10 to 20 years, valid neurobiological markers or other objective criteria that may lead to unequivocal diagnostic classification are still lacking. On the contrary, the concept of ADHD seems to have become broader and more heterogeneous. Thus, the diagnosis and treatment of ADHD are still challenging for clinicians, necessitating increased reliance on their expertise and experience. The first part of this review presents an overview of the current definitions of the disorder (DSM-5, ICD-10/11). Furthermore, it discusses more controversial aspects of the construct of ADHD, including the dimensional versus categorical approach, alternative ADHD constructs, and aspects pertaining to epidemiology and prevalence. The second part focuses on comorbidities, on the difficulty of distinguishing between “primary” and “secondary” ADHD for purposes of differential diagnosis, and on clinical diagnostic procedures. In the third and most prominent part, an overview of current neurobiological concepts of ADHD is given, including neuropsychological and neurophysiological researches and summaries of current neuroimaging and genetic studies. Finally, treatment options are reviewed, including a discussion of multimodal, pharmacological, and nonpharmacological interventions and their evidence base.
Introduction
With a prevalence of over 5%, attention deficit hyperactivity disorder (ADHD) is one of the most frequent disorders within child and adolescent psychiatry. Despite an overwhelming body of research, approximately 20,000 publications have been referenced in PubMed during the past 10 years, assessment and treatment continue to present a challenge for clinicians. ADHD is characterized by the heterogeneity of presentations, which may take opposite forms, by frequent and variable comorbidities and an overlap with other disorders, and by the context-dependency of symptoms, which may or may not become apparent during clinical examination. While the neurobiological and genetic underpinnings of the disorder are beyond dispute, biomarkers or other objective criteria, which could lead to an automatic algorithm for the reliable identification of ADHD in an individual within clinical practice, are still lacking. In contrast to what one might expect after years of intense research, ADHD criteria defined by nosological systems, such as the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) and the International Classification of Diseases, editions 10 and 11 (ICD-10/11) have not become narrower and more specific. Rather, they have become broader, for example, encompassing wider age ranges, thus placing more emphasis on the specialist's expertise and experience. 1 2 3
Definitions and Phenomenology
Adhd according to the dsm-5 and icd-10/11.
ADHD is defined as a neurodevelopmental disorder. Its diagnostic classification is based on the observation of behavioral symptoms. ADHD according to the DSM-5 continues to be a diagnosis of exclusion and should not be diagnosed if the behavioral symptoms can be better explained by other mental disorders (e.g., psychotic disorder, mood or anxiety disorder, personality disorder, substance intoxication, or withdrawal). 1 However, comorbidity with other mental disorders is common.
In the DSM-5, the defining symptoms of ADHD are divided into symptoms of inattention (11 symptoms) and hyperactivity/impulsivity (9 symptoms). 1 The former differentiation between subtypes in the DSM-IV proved to be unstable and to depend on the situational context, on informants, or on maturation, and was therefore replaced by “presentations.” 4 Thus, the DSM-5 distinguishes between different presentations of ADHD: predominantly inattentive (6 or more out of 11 symptoms present), predominantly hyperactive/impulsive (6 or more out of 9 symptoms present), and combined presentation (both criteria fulfilled), as well as a partial remission category. Symptoms have to be present in two or more settings before the age of 12 years for at least 6 months and have to reduce or impair social, academic, or occupational functioning. In adolescents over 17 years and in adults, five symptoms per dimension need to be present for diagnosis. 1 In adults, the use of validated instruments like the Wender Utah rating scale is recommended. 5
In contrast, the ICD-10 classification distinguishes between hyperkinetic disorder of childhood (with at least six symptoms of inattention and six symptoms of hyperactivity/impulsivity, present before the age of 6 years) and hyperkinetic conduct disorder, a combination of ADHD symptoms and symptoms of oppositional defiant and conduct disorders (CD). 3 In the ICD-11 (online release from June 2018, printed release expected 2022), the latter category has been dropped, as has the precise age limit (“onset during the developmental period, typically early to mid-childhood”). Moreover, the ICD-11 distinguishes five ADHD subcategories, which match those of the DSM-5: ADHD combined presentation, ADHD predominantly inattentive presentation, ADHD predominantly hyperactive/impulsive presentation and two residual categories, ADHD other specified and ADHD nonspecified presentation. For diagnosis, behavioral symptoms need to be outside the limits of normal variation expected for the individual's age and level of intellectual functioning. 2
Overlapping Constructs: Sluggish Cognitive Tempo and Emotional Dysregulation
Sluggish cognitive tempo (SCT) is a clinical construct characterized by low energy, sleepiness, and absent-mindedness, and is estimated to occur in 39 to 59% of (adult) individuals with ADHD. 6 7 The question of whether SCT might constitute a feature of ADHD or a separate construct that overlaps with ADHD inattention symptoms is unresolved. 8 While current studies indicate that SCT might be distinct and independent from hyperactivity/impulsivity, as well as from inattention dimensions, it remains uncertain whether it should be considered as a separate disorder. 8 9 Twin studies have revealed a certain overlap between SCT and ADHD, especially with regard to inattention symptoms, but SCT seems to be more strongly related to nonshared environmental factors. 10
Emotion dysregulation is another associated feature that has been discussed as a possible core component of childhood ADHD, although it is not included in the DSM-5 criteria. Deficient emotion regulation is more typically part of the symptom definition of other psychopathological disorders, such as oppositional defiant disorder (ODD), CD, or disruptive mood dysregulation disorder (DSM-5; for children up to 8 years). 11 However, an estimated 50 to 75% of children with ADHD also present symptoms of emotion dysregulation, for example, anger, irritability, low tolerance for frustration, and outbursts, or sometimes express inappropriate positive emotions. The presence of these symptoms increases the risk for further comorbidities, such as ODD and also for anxiety disorders. 12 13 For adult ADHD, emotional irritability is a defining symptom according to the Wender Utah criteria, and has been confirmed as a primary ADHD symptom by several studies (e.g., Hirsch et al). 5 14 15
Whether emotion dysregulation is inherent to ADHD, applies to a subgroup with combined symptoms and a singular neurobiological pathway, or is comorbid with but independent of ADHD, is still a matter of debate (for a description of these three models; Shaw et al 13 ). Faraone et al 12 distinguished three ADHD prototypes with regard to deficient emotion regulation: ADHD prototype 1 with high-emotional impulsivity and deficient self-regulation, prototype 2 with low-emotional impulsivity and deficient self-regulation, and prototype 3 with high-emotional impulsivity and effective self-regulation. All three prototypes are characterized by an inappropriate intensity of emotional response. While prototypes 1 and 3 build up their responses very quickly, prototype 2 is slower to respond but experiences higher subjective emotional upheaval than is overtly shown in the behavior. Prototypes 1 and 2 both need more time to calm down compared with prototype 3 in which emotional self-regulation capacities are intact.
Dimensional versus Categorical Nature of ADHD
Recent research on subthreshold ADHD argues in favor of a dimensional rather than categorical understanding of the ADHD construct, as its core symptoms and comorbid features are dimensionally distributed in the population. 16 17 18 Subthreshold ADHD is common in the population, with an estimated prevalence of approximately 10%. 19 According to Biederman and colleagues, clinically referred children with subthreshold ADHD symptoms show a similar amount of functional deficits and comorbid symptoms to those with full ADHD, but tend to come from higher social-class families with fewer family conflicts, to have fewer perinatal complications, and to be older and female (for the latter two, a confound with DSM-IV criteria cannot be excluded). 20
Temperament and Personality Approaches to ADHD
Another approach which is in accordance with a dimensional concept is to analyze ADHD and categorize subtypes according to temperament/personality traits (for a review and the different concepts of temperament see Gomez and Corr 21 ). Temperament/personality traits are usually defined as neurobiologically based constitutional tendencies, which determine how the individual searches for or reacts to external stimulation and regulates emotion and activity. While temperament traits per se are not pathological, extreme variations or specific combinations of traits may lead to pathological behavior. This approach has been investigated in several studies by Martel and colleagues and Nigg, 22 23 24 who employed a temperament model comprising three empirically derived domains 25 26 : (1) negative affect, such as tendencies to react with anger, frustration, or fear; (2) positive affect or surgency which includes overall activity, expression of happiness, and interest in novelty; and (3) effortful control which is related to self-regulation and the control of action. The latter domain shows a strong overlap with the concept of executive function. 27 In a community sample, early temperamental traits, especially effortful control and activity level, were found to potentially predict later ADHD. 28 Karalunas et al 29 30 distinguished three temperament profiles in a sample of children with ADHD: one with normal emotional functioning; one with high surgency, characterized by high levels of positive approach-motivated behaviors and a high–activity level; and one with high negative (“irritable”) affect, with the latter showing the strongest, albeit only moderate stability over 2 years. Irritability was not reducible to comorbidity with ODD or CD and was interpreted as an ADHD subgroup characteristic with predictive validity for an unfavorable outcome. These ADHD temperament types were distinguished by resting-state and peripheral physiological characteristics as measured by functional magnetic resonance imaging (fMRI). 29
Epidemiology and Prevalence
While ADHD seems to be a phenomenon that is encountered worldwide, 31 prevalence rates and reported changes in prevalence are highly variable, depending on country and regions, method, and sample. 32 A meta-analysis by Polanczyk et al 32 yielded a worldwide prevalence rate of 5.8% in children and adolescents. 33 In an update published 6 years later, the authors did not find evidence for an increase in prevalence over a time span of 30 years. Other meta-analyses reported slightly higher (e.g., 7.2%) 34 or lower prevalence rates, which seems to be attributable to the different criteria adopted for defining ADHD. Prevalence rates in children and adolescents represent averaged values across the full age range, but peak prevalence may be much higher in certain age groups, for example, 13% in 9-year-old boys. 35 Universal ADHD prevalence in adults is estimated to lie at 2.8%, with higher rates in high-income (3.6%) than in low-income (1.4%) countries. 36 True prevalence rates (also called community prevalence, e.g., Sayal et al 37 ) should be based on population-based representative health surveys, that is, the actual base rate of ADHD in the population, in contrast to the administrative base rate, which is related to clinical data collection (Taylor 38 ). Recent reports on the increase in ADHD rates usually refer to administrative rates, drawn from health insurance companies, from the number of clinical referrals for ADHD, 39 clinical case identification estimates, or from the percentage of children taking stimulant medication (prescription data). Changes in these rates may be influenced by increased awareness, destigmatization, modifications in the defining criteria of ADHD, or altered medical practice. According to a recent U.S. health survey on children and adolescents (4–17 years), in which parents had to indicate whether their child had ever been diagnosed with ADHD, the percentage of diagnoses increased from 6.1% in 1997 to 10.2% in 2016. 40 A representative Danish survey based on health registry, data collected from 1995 to 2010 reported that ADHD incidence rates increased by a factor of approximately 12 (for individuals aged 4–65 years) during this period. Moreover, the gender ratio decreased from 7.5:1 to 3:1 at early school age and from 8.1:1 to 1.6:1 in adolescents in the same time frame, 41 42 probably indicating an improved awareness of ADHD symptoms in girls. In other countries, it is assumed that girls are still underdiagnosed. 38
Population register data show that the use of stimulants for ADHD has increased considerably worldwide. 43 In most countries, an increase in stimulant medication use has been observed in children since the 1990s (e.g., United Kingdom from 0.15% in 1992 to 5.1% in 2012/2013), 44 45 but in some European countries, stimulant prescription rates for children and adolescents have remained stable or decreased over the last 5 to 10 years (e.g., Germany). 35 In the United States, the prescription of methylphenidate peaked in 2012 and has since been slightly decreasing, while the use of amphetamines continues to rise. 46
Comorbidity, Differential Diagnosis, and Clinical Assessment
Comorbidity.
ADHD is characterized by frequent comorbidity and overlap with other neurodevelopmental and mental disorders of childhood and adolescence. The most frequent comorbidities are learning disorders (reading disorders: 15–50%, 4 dyscalculia: 5–30%, 47 autism spectrum disorder, which since the DSM-5 is no longer viewed as an exclusion criterion for ADHD diagnosis: 70–85%, 48 49 tic/Tourette's disorder and obsessive compulsive disorder: 20%, and 5%, 50 developmental coordination disorder: 30–50%, 51 depression and anxiety disorders: 0–45%, 52 53 and ODD and CD: 27–55% 54 ). ADHD increases the risk of substance misuse disorders 1.5-fold (2.4-fold for smoking) and problematic media use 9.3-fold in adolescence 55 56 and increases the risk of becoming obese 1.23-fold for adolescent girls. 57 58 59 It is also associated with different forms of dysregulated eating in children and adolescents. Enuresis occurs in approximately 17% of children with ADHD, 60 and sleep disorders in 25 to 70%. 61 Frequent neurological comorbidities of ADHD include migraine (about thrice more frequent in ADHD than in typically developing [TD] children) 62 63 64 and epilepsy (2.3 to thrice more frequent in ADHD than in TD children). 65 66 The risk of coexisting ADHD being seen as a comorbid condition and not the primary diagnosis is considerably enhanced in many childhood disorders of different origins. For example, the rate of comorbid ADHD is estimated at 15 to 40% 67 68 in children with reading disorders and at 26 to 41% 69 70 in children with mild intellectual dysfunction. While comorbidity in neurodevelopmental disorders may arise from a certain genetic overlap (see details under genetic associations), ADHD symptoms are also present in several disorders with well-known and circumscribed genetic defects, normally not related to ADHD (e.g., neurofibromatosis, Turner's syndrome, and Noonan's syndrome) 71 or disorders with nongenetic causes, such as traumatic brain injuries, pre-, peri- or postnatal stroke, or syndromes due to toxic agents, such as fetal alcohol syndrome. Comorbid ADHD is estimated in 20 to 50% of children with epilepsy, 72 73 in 43% of children with fetal alcohol syndrome, 74 and in 40% of children with neurofibromatosis I. 75 ADHD is three times more frequent in preterm-born children than in children born at term and four times more frequent in extremely preterm-born children. 76
Differential Diagnosis, Primary and Secondary ADHD
A range of medical and psychiatric conditions show symptoms that are also present in primary ADHD. The most important medical conditions which are known to “mimic” ADHD and need to be excluded during the diagnostic process are epilepsy (especially absence epilepsy and rolandic epilepsy), thyroid disorders, sleep disorder, drug interaction, anemia, and leukodystrophy. 77 78 The most important psychiatric conditions to be excluded are learning disorder, anxiety disorders, and affective disorders, while an adverse home environment also needs to be excluded.
However, the picture is complex, as many differential diagnoses may also occur as comorbidities. For instance, bipolar disorder, which is frequently diagnosed in children and adolescents in the United States but not in Europe, is considered as a differential diagnosis to ADHD, but ADHD has also been found to be a comorbidity of bipolar disorder in 21 to 98% of cases. 79 Similarly, absence epilepsy is a differential diagnosis of ADHD but is also considered to be a frequent comorbidity, occurring in 30 to 60% of children with absence epilepsy. 80 The prevalence of the ADHD phenotype in benign childhood epilepsy with centrotemporal spikes (rolandic epilepsy) lies at 64 to 65%, 81 and is possibly related to the occurrence of febrile convulsions. 82 The literature often does not draw a clear distinction between an ADHD phenotype, which includes all types of etiologies and causes, and a yet to be specified developmental ADHD “genotype.” Some authors use terms, such as “idiopathic” ADHD, 83 “primary,” or “genotypic” ADHD, 84 in contrast to ADHD of circumscribed origin other than developmental, the latter being referred to as ADHD “phenotype,” or “phenocopy,” 85 or “ADHD-like.” 86 “Secondary ADHD” usually refers to newly acquired ADHD symptoms arising after a known event or incident, for example, a head trauma or stroke. After early childhood stroke, the ADHD phenotype occurs in 13 to 20% of cases, and after pediatric traumatic brain injury, ADHD symptoms are observed in 15 to 20% of children. 87 Having ADHD considerably increases the risk of suffering a traumatic brain injury, 88 89 90 and most studies on secondary ADHD after traumatic brain injury control for or compare with premorbid ADHD (e.g., Ornstein et al 91 ). Whether and to what extent “phenotypic” and “genotypic” ADHD need to be distinguished on a phenomenological level is not clear. It is possible that shared neurobiological mechanisms will prevail and that genetic vulnerability and epigenetic factors may play a role in both types. For example, James et al 86 compared neurophysiological markers in two groups of adolescents with ADHD, one born very preterm and the other born at term. While the authors found very similar ADHD-specific markers in the two groups, some additional deficits only emerged in the preterm group, indicating more severe impairment. Other examples are rare genetic diseases with known genetic defects, which are often comorbid with ADHD. One may ask whether, for example, ADHD in Turner's syndrome should be considered as a rare genetic ADHD variant and count as genotypic ADHD, or whether it results from a different genetic etiology, with the status of an ADHD phenotype.
Clinical Diagnostic Procedure
Clinical assessment in children should mainly be based on a clinical interview with parents, including an exploration of the problems, the detailed developmental history of the child including medical or psychiatric antecedents, information on family functioning, peer relationships, and school history. According to the guidelines of the National Institute for Health and Care Excellence (NICE) in the United Kingdom, this may also include information on the mental health of the parents and the family's economic situation. The child's mental state should be assessed, possibly using a standardized semistructured clinical interview containing ADHD assessments (e.g., Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime version, DSM-5) 92 93 and by observer reports. The exploration should cover behavioral difficulties and strengths in several life contexts, for example, school, peer relationships, and leisure time. The use of informant rating scales, such as Conners' Rating Scales, 3rd edition, 94 or the Strengths and Difficulties Questionnaire 95 may be useful, but diagnosis should not be solely based on rating scales (NICE, AWFM ADHD). 96 97 A further interview should be conducted with the child or adolescent to gain a picture of the patient's perspective on current problems, needs, and goals, even though self-reports are considered less reliable for diagnosis. Information should also be obtained from the school, for example, by face-to-face or telephone contact with the teacher and, if possible, by direct school-based observation. A medical examination should be performed to exclude somatic causes for the behavioral symptoms and to gain an impression of the general physical condition of the patient. Current guidelines do not recommend including objective test procedures (intelligence and neuropsychological tests), neuroimaging, or neurophysiological measures in routine ADHD assessment but do suggest their use as additional tools when questions about cognitive functions, academic problems, coexisting abnormalities in electroencephalography (EEG), or unrecognized neurological conditions arise. After completion of the information gathering, the NICE guidelines recommend a period of “watchful waiting” for up to 10 weeks before delivering a formal diagnosis of ADHD. A younger age of the diagnosed child relative to his/her classmates has to be mentioned as one of the many pitfalls in the assessment of ADHD. It has been shown that the youngest children in a class have the highest probability of being diagnosed with ADHD and of being medicated with stimulants. 98
There is consensus that the diagnosis of ADHD requires a specialist, that is, a child psychiatrist, a pediatrician, or other appropriately qualified health care professionals with training and expertise in diagnosing ADHD. 97
Current Neurobiological and Neuropsychological Concepts
Neuropsychology, neuropsychological pathways and subgroups.
ADHD is related to multiple underlying neurobiological pathways and heterogeneous neuropsychological (NP) profiles. Twenty-five years ago, ADHD was characterized as a disorder of inhibitory self-control, 54 and an early dual pathway model distinguished between an inhibitory/executive function pathway and a motivational/delay aversion pathway (also called “cool” and “hot” executive function pathways in later publications), which are related to distinct neurobiological networks. 99 100 101 Still, the two systems may also interact. 102
Since then, other pathways have been added, such as time processing, 103 but a definitive number of possible pathways is difficult to define. For example, Coghill and colleagues 104 differentiated six cognitive factors in children with ADHD (working memory, inhibition, delay aversion, decision-making, timing, and response time variability) derived from seven subtests of the Cambridge neuropsychological test automated battery. Attempts to empirically classify patients into subgroups with selective performance profiles departing from comprehensive NP data collection were inconclusive. For example, using delay aversion, working memory, and response-time tasks, Lambek and colleagues 105 expected to differentiate corresponding performance profile subgroups in children with ADHD. However, their analysis resulted in subgroups differentiated by the severity of impairments, and not by selective profiles. Other empirical studies using latent profile or cluster analysis of NP tasks in large ADHD samples have differentiated three 106 107 or four 108 NP profile groups, which all included children with ADHD, as well as TD children, differing in severity but not in the type of profile. This might indicate that the identified NP deficit profiles were not ADHD-specific, but rather reflected characteristic distributions of NP performances, which are also present in the general population, with extreme values in children with ADHD. Some other empirical studies in the search for subgroups, however, identified ADHD-specific performance profiles (“poor cognitive control,” 109 “with attentional lapses and fast processing speed” 110 ), among other profiles being shared with TD controls. Obviously, divergent results regarding subgrouping may also be related to differing compilations of tested domains, consequently leading to a limited comparability of these studies.
Which Neuropsychological Functions are Impaired in ADHD and When?
A meta-analysis conducted in 2005 identified consistent executive function deficits with moderate effect sizes in children with ADHD in terms of response inhibition, vigilance, working memory, and planning. 67 Since then, a vast number of studies on NP deficits in children with ADHD compared with TD controls have been published. A recent meta-analysis included 34 meta-analyses on neurocognitive profiles in ADHD (all ages) published until 2016, referring to 12 neurocognitive domains. 111 The authors found that 96% of all standardized mean differences were positive in favor of the control group. Unweighted effect sizes ranged from 0.35 (set shifting) to 0.66 (reaction time variability). Weighted mean effect sizes above 0.50 were found for working memory (0.54), reaction time variability (0.53), response inhibition (0.52), intelligence/achievement (0.51), and planning/organization (0.51). Effects were larger in children and adolescents than in adults. The other domains comprised vigilance, set shifting, selective attention, reaction time, fluency, decision making, and memory.
Nearly every neuropsychological domain has been found to be significantly impaired in ADHD compared with TD controls, though effect sizes are often small. This includes, for example, altered perception (e.g., increased odor sensitivity 112 ; altered sensory profile 113 ; impaired yellow/blue color perception, e.g., Banaschewski et al, 114 for review, see Fuermaier et al 115 ), emotional tasks (e.g., facial affect discrimination), 116 social tasks (e.g., Marton et al 117 ), communication, 118 and memory. 119 Several of the described impairments may be related to deficient top-down cognitive control and strategic deficits, 120 121 122 but there is also evidence for basic processing deficits. 123
Neuropsychological Deficits as Mediators of Gene-Behavior Relations
A vast amount of research has been devoted to the search for neuropsychological endophenotypes (or intermediate phenotypes) for ADHD, that is, neurobiologically based impairments of NP performance characteristic of the disorder that may also be found in nonaffected close relatives. ADHD neuropsychological endophenotypes are assumed to mediate genetic risk from common genetic variants. 124 So far, deficits in working memory, reaction-time variability, inhibition, time processing, response preparation, arousal regulation, and others have been identified as probable endophenotypes for ADHD. 124 125 126 127 Genetic studies indicate an association of an ADHD-specific polygenetic general risk score (i.e., the total number of genetic variants that may be associated with ADHD, mostly related to dopaminergic transmission) with working memory deficits and arousal/alertness, 124 or with a lower intelligence quotient (IQ) and working memory deficits, 128 respectively. More specifically, a link of ADHD-specific variants of DAT1 genes with inattention and hyperactivity symptoms seems to be mediated by inhibitory control deficits. 129
Individual Cognitive Profiles and the Relevance of Cognitive Testing for the Clinical Assessment
Heterogeneity is found with regard to profiles, as well as with regard to the severity of cognitive impairment in individuals with ADHD, as measured by standardized tests. ADHD does not necessarily come with impaired neuropsychological test performance: about one-third of children with ADHD will not present any clinically relevant impairment, while another one-third shows unstable or partial clinical impairment, and about another one-third performs below average in NP tests. The classic concept of NP impairment, which assumes relative stability over time, possibly does not apply to NP deficits observed in ADHD, or only to a lesser extent. For the larger part, the manifestation of performance deficits may depend on contextual factors, 130 such as reward, or specifically its timing, amount, and nature, or on energetic factors, 131 for example, the rate of stimulus presentation or the activation provided by the task.
Many studies have shown that behavioral ratings of ADHD symptoms or questionnaires on executive function deficits are not, or at best weakly, correlated with NP test performance, even when both target the same NP domain. 132 133 In consequence, questionnaires on executive functioning are not an appropriate replacement for neuropsychological testing. Likewise, ADHD symptom rating scales do not predict results of objective attention or executive function tests and vice versa. Although mild intellectual disability and low IQ are more typically associated with the disorder, ADHD can be encountered across the entire IQ spectrum, including highly gifted children. 134 Therefore, an intelligence test should be part of the diagnostic procedure, but is not mandatory according to ADHD guidelines. In some children, intellectual difficulties and not ADHD may be the underlying cause for ADHD-like behaviors, while in other children with ADHD, academic underachievement despite a high IQ may be present.
It has been argued that symptoms defining ADHD may be understood as dimensional markers of several disorders belonging to an ADHD spectrum and, in consequence, the diagnosis of these behavioral symptoms should be the starting point for a more in-depth diagnosis rather than the endpoint. 135 This should include the cognitive performance profile. The ADHD behavioral phenotype predicts neither NP impairment nor intellectual achievement in the individual case, and objective testing is the only way to obtain an accurate picture of the child's cognitive performance under standardized conditions. Its goal is not ADHD classification, but rather to obtain the best possible understanding of the relation between cognitive functioning and behavioral symptoms for a given patient, to establish an individually tailored treatment plan.
Neurophysiology
Neurophysiological methods like EEG, magnetoencephalography, and event-related potentials (ERPs) as task-locked EEG averages capture brain functions in ADHD at high (ms) temporal resolution. The approach covers both fast and slow neural processes and oscillations, and clarifies the type and timing of brain activity altered in ADHD at rest and in tasks. It reveals neural precursors, as well as correlates, and consequences of ADHD behavior. 136 Neurophysiological and particularly EEG measures also have a long and controversial history as potential biomarkers of ADHD. Current evidence clarifies how multiple pathways and deficits are involved in ADHD at the group level, but recent attempts toward individual clinical translation have also revealed considerable heterogeneity, which does not yet support a clinical application for diagnostic uses or treatment personalization, as explained below.
Resting Electroencephalography
The EEG is dominated by oscillations in frequency bands ranging from slow δ (<4 Hz) and θ (4–7 Hz) via α (8–12 Hz) to faster β (13–30 Hz) and γ (30–100 Hz) band activity. The spectral profile reflects maturation and arousal, with slow frequencies dominating during early childhood and slow-wave sleep. Source models can link scalp topography to brain sources and distributed networks.
Initial studies suggested a robust link between ADHD diagnosis and resting EEG markers of reduced attention, hypoarousal, or immaturity, such as increased θ and an increased θ/β ratio (TBR). However, more recent studies, 137 138 some with large samples, 139 140 failed to replicate a consistent TBR increase in ADHD. Instead, the results indicated heterogeneous θ and β power deviations in ADHD not explained by ADHD subtype and psychiatric comorbidity. 141 A cluster analysis of EEG in children with ADHD also revealed considerable heterogeneity regarding θ excess and β attenuation in ADHD. While several clusters with EEG patterns linked to underarousal and immaturity could be identified, only three of the five EEG clusters (60% of the cases with ADHD) had increased θ. 139 Several recent θ and TBR studies that no longer found TBR association with ADHD diagnosis still replicated the reliable age effects, 137 138 142 confirming the high quality of these studies. Increasing sleepiness in adolescents, 143 or shorter EEG recordings, may have reduced the sensitivity to time effects and state regulation deficits in ADHD, 136 144 potentially contributing to these replication failures. Also, conceptualizing TBR as a marker of inattention or maturational lag may be too simple, since θ activity can also reflect concentration, cognitive effort, and activation. 145 146
During sleep, stage profiles reveal no consistent deviations in ADHD, but the slow-wave sleep topography is altered. In particular, frontal slow waves are reduced, leading to a more posterior topography as observed also in younger children. 147 This delayed frontalization can be interpreted as a maturational delay in ADHD, in line with a cluster of resting EEG, changes in task related ERPs during response inhibition, 148 and structural magnetic resonance imaging (MRI) findings. 149
Task Related Event-Related Potentials
Task-related processing measures, particularly ERPs, have critically advanced our understanding of ADHD through their high-time resolution, which can separate intact and compromised brain functions. ERPs have revealed impairments during preparation, attention, inhibition, action control, as well as error, and reward processing, with partly distinct networks but often present during different phases of the same task. In youth and adults with ADHD, the attentional and inhibitory P3 components and the preparatory contingent negative variation (CNV) component are most consistently affected, but state regulation and error or reward processing are also compromised. 136 150 Activity during preparation, attention, or inhibition is typically weaker and more variable but not delayed. This often occurs in task phases without visible behavior and precedes the compromised performance. Familial and genetic factors also modulate these markers of attention and control. Some impairment is also observed in nonaffected siblings or in parents without ADHD, 151 152 and genetic correlates often implicate the dopamine system. 125 Some ERP changes, like the attenuated CNV during preparation, remain stable throughout maturation, and are also markers of persistent ADHD, while other markers, such as the inhibition related P3, remain attenuated despite clinical remission. 148 153
Overall, the ERP results confirm attentional, cognitive, and motivational, rather than sensory or motor impairments in ADHD, in line with current psychological and neurobiological models. However, different ERP studies hardly used the same tests and measures, so valid statements regarding classification accuracy and effect size are particularly difficult, 154 and there is an urgent need for meta-analyses regarding the different ERPs.
Clinical Translation
Despite published failures to replicate robust TBR based classification of ADHD, a TBR-based EEG test was recently approved by the U.S. Food and Drug Administration to assist ADHD diagnosis. 155 Although not promoted as a stand-alone test, children with suspected ADHD, and increased TBR were claimed to likely meet full diagnostic criteria for ADHD; while children with suspected ADHD but no TBR increase should undergo further testing, as they were likely to have other disorders better explaining ADHD symptoms (see also DSM-5 exclusionary criterion E).
This multistage diagnostic approach could possibly identify a homogeneous neurophysiological subgroup, but it omits critical elements of careful, guideline-based ADHD diagnostics. Reliability and predictive value of the TBR remain untested, and the increasing evidence for poor validity of TBR renders it unsuitable for stand-alone ADHD diagnosis. Accordingly, the use of TBR as a diagnostic aid was broadly criticized. 156 157
In sum, the recent literature suggests that neither TBR nor other single EEG or ERP markers are sufficient to diagnose ADHD and are not recommended for clinical routine use, in line with the increasing evidence for heterogeneity in ADHD.
Combining measures across time, frequency, and tasks or states into multivariate patterns may better characterize ADHD. The potential of such approaches is evident in improved classification using machine-learning algorithms based on combinations of EEG measures 142 or EEG and ERP measures. 138 158 However, claims of high-classification accuracies up to 95% (e.g., Mueller et al 158 ) require further independent replication and validation with larger samples, and plausible mapping to neural systems and mechanisms. Modern pattern classification is particularly sensitive to uncontrolled sample characteristics and needs validation through independent large samples. 159
Focusing on EEG-based prediction rather than diagnosis may hold more promise for clinical translation, and may utilize the EEG heterogeneity in clinical ADHD samples. For example, early studies on predicting stimulant response suggested that children with altered wave activity, in particular increased TBR, θ or α slowing, respond well to stimulant medication. However, in recent prospective work with a large sample, TBR was not predictive, and α slowing allowed only limited prediction in a male adolescent subgroup. 160
Predicting response to intense nonpharmacological treatment is of particular interest given the high costs and time requirements. Promising findings have been reported for one neurofeedback study, where α EEG activity and stronger CNV activity together predicted nearly 30% of the treatment response. 161 Still, the lack of independent validation currently allows no clinical application.
In conclusion, neurophysiological measures have clarified a rich set of distinct impairments but also preserved functions which can also serve as markers of persistence or risk. These markers may also contribute in the classification of psychiatric disorders based on neuromarkers (research domain criteria approach). As potential predictors of treatment outcome they may support precision medicine, and proof-of-concept studies also highlight the potential of multivariate profiling. The findings also demonstrate the challenge with this approach, including notable replication failures, and generalizability of most findings remains to be tested. Neurophysiological markers are not ready to serve as tools or aids to reliably diagnose ADHD, or to personalize ADHD treatment in individual patients.
Neuroimaging
Modern brain imaging techniques have critically contributed to elucidating the etiology of ADHD. While MRI provides detailed insights into the brain microstructure, such as for example gray matter volume, density, cortical thickness, or white matter integrity, fMRI allows insights into brain functions through activation and connectivity measures with high–spatial resolution.
Delayed Maturation and Persistent Alterations in the Brain Microstructure in ADHD
The brain undergoes pronounced developmental alterations in childhood and adolescence. Gray matter volume and cortical thickness show nonlinear inverted U -shaped trajectories of maturation with a prepubertal increase followed by a subsequent decrease until adulthood while white matter volume progressively increases throughout adolescence and early adulthood in a rather linear way. 162 163 164 165 Large variations of the maturational curves in different brain regions and subregions suggest that phylogenetically older cortical areas mature earlier than the newer cortical regions. Moreover, brain areas associated with more basic motor or sensory functions mature earlier than areas associated with more complex functions including cognitive control or attention. 163 164 Altered maturation of the cortex for ADHD has been reported for multiple areas and cortical dimensions, 166 167 mainly in the form of delayed developmental trajectories in ADHD but recently also as persistent reductions, particularly in the frontal cortex. 168 Such findings speak for delayed maturation in specific areas rather than a global developmental delay of cortical maturation in ADHD. Microstructural alterations in ADHD have been associated with a decreased intracranial volume 169 and total brain size reduction of around 3 to 5%. 100 168 170 In accordance, increasing ADHD symptoms in the general population correlated negatively with the total brain size. 171 A meta-analysis (Frodl et al) and a recent cross-sectional mega- and meta-analysis (Hoogman et al) indicate that such reductions in brain volume may be due to decreased gray matter volumes in several subcortical structures, such as the accumbens, amygdala, caudate, hippocampus, and putamen but also cortical areas (prefrontal, the parietotemporal cortex) and the cerebellum. 170 172 173 174 175 176 177 Effects sizes of subcortical alterations were highest in children with ADHD and the subcortical structures showed a delayed maturation. 169 Moreover, higher levels of hyperactivity/impulsivity in children were associated with a slower rate of cortical thinning in prefrontal and cingulate regions. 167 178 Differences in brain microstructure have also been reported in a meta-analysis for white matter integrity as measured with diffusion tensor imaging in tracts subserving the frontostriatal-cerebellar circuits. 179 To summarize, diverse neuroanatomical alterations in total brain volume and multiple cortical and subcortical dimensions characterize ADHD. These alterations are most pronounced in childhood and suggest a delayed maturation of specific cortical and subcortical areas along with some persistent reductions in frontal areas in a subgroup of ADHD patients with enduring symptoms into adulthood.
Alterations in the Brain Function of Specific Networks in ADHD
Specific functional networks, mainly those involved in inhibition, attention processes, cognitive control, reward processing, working memory, or during rest have been intensively studied in ADHD using fMRI in the past. Alterations have been reported in the corresponding brain networks and the main findings are summarized below.
Atypical Resting State Connectivity in Children with ADHD
Resting state examines spontaneous, low frequency fluctuations in the fMRI signal during rest, that is , in absence of any explicit task. 180 Resting state networks describe multiple brain regions for which the fMRI signal is correlated (functionally connected) at rest, but the same networks may coactivate also during task-based fMRI. 181 One important resting state network, the so-called default mode network (DMN), comprises brain areas that show higher activation during wakeful rest and deactivations with increasing attentional demands. 182 183 While the DMN usually shows decreasing activation with increasing attentional demands, the cognitive control network shows an opposite pattern and increases its activation. This inverse correlation of DMN and the cognitive control networks is diminished or absent in children and adults with ADHD and may explain impaired sustained attention through attentional lapses that are mediated by the DMN. 181 184 185 186 In addition, a more diffuse pattern of resting state networks connectivity and a delayed functional network development in children with ADHD have been reported. 187 Finally, atypical connectivity in cognitive and limbic cortico-striato-thalamo-cortical loops of patients with ADHD suggest that the neural substrates may either reside in impaired cognitive network and/or affective, motivational systems. 181
Altered Processing of Attention and Inhibition in Fronto-basal Ganglia Circuits in ADHD
Meta-analyses summarizing the findings of functional activation studies report most consistent alterations in brain activation patterns as hypoactivation of the frontoparietal network for executive functions and the ventral attention system for attentional processes in children with ADHD. 188 189 190 More specifically, motor or interference inhibition tasks yielded consistent decreases in a (right lateralized) fronto-basal ganglia network comprising supplementary motor area, anterior cingulate gyrus, left putamen, and right caudate in children with ADHD. 189 190 For tasks targeting attentional processes, decreased activation in a mainly right lateralized dorsolateral fronto-basal ganglia-thalamoparietal network characterized children with ADHD. Depending on the task, hyperactivation can cooccur in partly or distinct cerebellar, cortical, and subcortical regions. 188 189 190
Altered Reward Processing and Motivation
Emotion regulation and motivation is mediated by extended orbitomedial and ventromedial frontolimbic networks in the brain. 191 Abnormal sensitivity to reward seems to be an important factor in the etiology of ADHD as suggested by several models of ADHD, 192 193 194 mainly due to a hypofunctioning dopaminergic system. 195 In accordance, impairments in specific signals that indicate violations of expectations, the so called reward prediction errors (RPE), were shown in the medial prefrontal cortex of adolescents with ADHD during a learning task. 196 RPE signals are known to be encoded by the dopaminergic system of the brain, and deficient learning and decision making in ADHD may thus be a consequence of impaired RPE processing. 196 Abnormal activation has also been reported for the ventral striatum during reward anticipation and in other cortical and subcortical structures of the reward circuitry. 197
Normalization of Atypical Activation and Brain Structural Measures after Treatment
Stimulant medication and neurofeedback studies have pointed to a certain normalization of dysfunctional activation patterns in critical dorsolateral frontostriatal and orbitofrontostriatal regions along with improvements in ADHD symptoms. 198 199 200 201 Also, brain microstructure, especially the right caudate, has shown some gradual normalization with long-term stimulant treatment. 176 190
To conclude, a wide range of neuroimaging studies reveal relatively consistent functional deficits in ADHD during executive functions, including inhibitory control, working memory, reward processes, and attention regulation but also during rest. Some of these alterations are more persistent, others are specific to children and may thus represent a developmental delay. Specific treatments showed trends toward a normalization of alterations in brain microstructure and functional networks.
Genetic Associations with ADHD and ADHD Related Traits
From family studies, as well as twin studies, the heritability for ADHD has been estimated to be between 75 upto 90%. 202 Moreover, the heritability was found to be similar in males and females and for inattentive and hyperactive-impulsive components of ADHD. 202 Interestingly, a strong genetic component was also found when the extreme and subthreshold continuous ADHD trait symptoms were assessed in the Swedish twins. 19 Even over the lifespan, adult ADHD was found to demonstrate high heritability that was not affected by shared environmental effects. 203 Recently, structural and functional brain connectivity assessed in families affected by ADHD has been shown to have heritable components associated with ADHD. 204 Similarly, the heritability of ERPs elicited in a Go/No-Go-task measuring response inhibition known to be altered in ADHD, was found to be significantly heritable. 205
In several studies, ADHD-related traits have also shown significant heritability. For example, in two independent, population based studies, significant single nucleotide polymorphism heritability estimates were found for attention-deficit hyperactivity symptoms, externalizing problems, and total problems. 206 In another study, investigating the two opposite ends of ADHD symptoms, low-extreme ADHD traits were significantly associated with shared environmental factors without significant heritability. 207 While on the other hand, high-extreme ADHD traits showed significant heritability without shared environmental influences. 207 A crossdisorder study including 25 brain disorders from genome wide association studies (GWAS) of 265,218 patients and 784,643 controls, including their relationship to 17 phenotypes from 1,191,588 individuals, could demonstrate significant shared heritability. 208 In particular, ADHD shared common risk variants with bipolar disorder, major depressive disorder, schizophrenia, and with migraine. 208 Indeed, in general, population-based twin studies suggest that genetic factors are associated with related-population traits for several psychiatric disorders including ADHD. 209 This suggests that many psychiatric disorders are likely to be a continuous rather than a categorical phenotype.
Though ADHD was found to be highly heritable, the underlying genetic risk factors are still not fully revealed. The current consensus suggests, as in many other psychiatric disorders, a multifactorial polygenic nature of the common disorder. Both common genetic variants studied by hypothesis-driven candidate gene association or by the hypothesis-free GWAS could only reveal the tip of the iceberg. Through the candidate gene approach, only very few findings could show replicable significant association with ADHD, as reported by meta-analysis studies for the dopaminergic, noradrenergic, and serotonergic genes. 210 211 Several GWAS have been conducted followed by meta-analysis, which again failed reaching genome-wide significant results. 212 213 214 215 216 217 218 219 220 221 222 223 224 However, recently, the first genome-wide significance has been reached in a GWAS meta-analysis consisting of over 20,000 ADHD patients and 35,000 controls. 225 Twelve independent loci were found to significantly associate with ADHD, including genes involved in neurodevelopmental processes, such as FOX2 and DUSP6 . 225 But even in these findings the effect sizes are rather small to be used for diagnostic tools. Therefore, polygenic risk score approaches have emerged as a possible tool to predict ADHD. 202 Yet this approach needs further investigation now that genome-wide significance has been reached by Demontis et al. 225 However, at this point, it is not yet possible to exclude that rare SNPs of strong effect may also be responsible (similar to breast cancer) for a small proportion of ADHD cases due to the heterogeneity of symptomatology, illness course, as well as biological marker distribution, as outlined above.
Multimodal Treatment of ADHD
A variety of national and international guidelines on the assessment and management of ADHD have been published over the last 10 years, not only for clinicians but also for patients and caregivers. 96 97 226 227 228 All guidelines recommend a multimodal treatment approach in which psychoeducation forms a cornerstone of the treatment and should be offered to all of those receiving an ADHD diagnosis, as well as to their families and caregivers.
According to the NICE Guidelines, the first step is always a planning process for the multimodal treatment with respect to the psychological, behavioral, and occupational or educational needs of the child and his/her family. 97 This planning phase could be organized as a “round table” with the child, parents, and other caregivers. The following aspects should be taken into account: the severity of ADHD symptoms and impairment, the relative impact of other neurodevelopmental or mental health conditions and how these affect or may affect everyday life (including sleep). In addition, resilience and protective factors, as well as the goals of the child and family, should be considered in the intervention process. The participation of child and parents in the planning and treatment process is more centrally outlined in recent guidelines and is emphasized in detail for the different treatment steps (e.g., NICE and S3 Guidelines). 96 97 The participation process is not just a one-time dialogue but should rather continue throughout all steps of the treatment process. Benefits and harms of nonpharmacological and pharmacological treatments should be discussed carefully and on the basis of the latest evidence. Preferences and concerns, and the importance of adherence to treatment, should be discussed and taken into account within the treatment process. Patients and their families or caregivers should be reassured, as appropriate that they can revisit decisions about treatments.
Multimodal treatment approaches also advocate a systematic adaptive procedure that combines different treatment modules according to the needs and situation of the patient and family. This may, for instance, include a first stage in which parent counseling is initiated, a second-stage encompassing, for example, individual behavioral therapy for the child, while the parents participate in a parent training program in parallel, followed by a third stage in which stimulant medication is started, etc. 229 230 Environment-centered interventions aim at the counseling or training of parents or the instruction of teachers at school or preschool. Parent training programs may be administered individually or in groups and have shown positive effects on parenting skills, ADHD behavior, and comorbid conduct problems. 231 232 233 Family therapy for ADHD focuses on the ADHD family, with the ADHD patient being a part of the family system with dysfunctional interactional patterns. 234 School-based interventions may target (1) the conditions in the classroom, for example, by minimizing distractions; (2) the instruction of the teacher, for example, by suggesting more appropriate teaching methods or by promoting peer tutoring; or (3) the student, for example, by improving self-management and social skills, or by helping to cope with stigma. 235 236 237
Pharmacological Approaches
Starting medication.
All medication for ADHD should only be initiated by a health care professional with training and expertise in diagnosing and managing ADHD. The expert should be familiar with the pharmacokinetic profiles and bioavailability of all the short- and long-acting preparations available for ADHD. The following parameters should be considered before first medication: medical history of the child but possibly also of the parents, current medication, height and weight, baseline pulse and blood pressure, a cardiovascular assessment, and an electrocardiogram if the treatment may affect the QT interval. A cardiology expert opinion should be sought before starting medication for ADHD if there is a history of congenital heart disease, previous cardiac surgery, or a history of sudden death in a first-degree relative under the age of 40 years, or if the blood pressure is consistently above the 95th centile for age and height for children and young people.

Age-Specific Needs
Treatment recommendations are often based on the specific needs of children, youth, or adults. 97 226 According to the NICE guidelines 97 and also pharmacological recommendations (e.g., Walitza and colleagues 238 239 ), a distinction should also be made between children under 5 years of age or preschool children, and school children. For the younger children (under 5 years of age), parent or career training programs and parent group training programs are always first-line treatments. Medication for children under 5 years with ADHD should only be given following a second specialist opinion from an ADHD service with expertise in managing ADHD in young children (ideally from a tertiary service). For children over 5 years of age, education and information about the causes and impact of ADHD and advice on parenting strategies should be offered, as well as liaison with school, college, or university if consent to do so is provided. 97 Children aged 5 years and over and young people should only receive medication if the ADHD symptoms are still causing a persistent significant impairment in at least one life domain after environmental modifications have been implemented and evaluated.
Selection of Pharmacotherapy
In Europe, methylphenidate either as short- or long-acting preparation is the first-line medication for ADHD across the life span. Second-line medications are lisdexamfetamine, atomoxetine, and guanfacine. A switch to lisdexamfetamine is only recommended if children have first undergone at least a 6-week trial of methylphenidate at an adequate dose and have not derived sufficient benefit in terms of reduced ADHD symptoms and associated impairment, or if patients experience adverse side effects. 238 The Canadian Guidelines (2018) recommend an individual treatment approach, which can start with different options, and if medication is to be used, long-acting formulations of psychostimulants or atomoxetine are always the first choice. 226 Comorbid disorders may necessitate adjustments to the treatment plan or alternative treatments.
According to the NICE guidelines, atomoxetine and guanfacine should only be offered if patients cannot tolerate methylphenidate or lisdexamfetamine or if their symptoms have not responded to separate 6-week trials of methylphenidate and lisdexamfetamine, having considered alternative preparations and adequate doses. 97
Evidence for ADHD Medications
In the first “gold standard” study comparing the different treatment approaches for ADHD alone and in combination (National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD [MTA study]), the effects of both pharmacological therapy (methylphenidate and intensive counseling) and of multimodal therapy (methylphenidate and intensive behavioral therapy) were significantly more effective after 14 months than behavioral therapy alone or than the “standard” therapy (treatment as usual in the community) of the control group. The multimodal therapy was not significantly superior to pharmacological therapy alone, but did result in significant improvements in ADHD symptoms at a lower dosage of methylphenidate. 240 241 242 Since the MTA study, numerous studies have investigated methylphenidate, amphetamine, and nonstimulants like atomoxetine or α 2 -adrenoceptor agonists, such as clonidine and guanfacine, regarding different aspects of effectiveness and tolerability.
The psychostimulants methylphenidate and amphetamine are the most effective agents for the treatment of core ADHD symptoms, with a favorable efficacy and adverse event profile. 243 244 245 Compared with methylphenidate and amphetamine, which both show immediate symptom reduction, the full effects of atomoxetine and guanfacine on reducing ADHD symptoms usually only unfold after some weeks of administration. Atomoxetine and guanfacine are not controlled substances, and are licensed in various European countries and in the United States for treatment of ADHD in children above the age of 6 years. Both have been shown to be effective in decreasing ADHD core symptoms with an effect size of around 0.7, which is somewhat lower than the effect size for methylphenidate, depending on the underlying studies (e.g., Sallee et al 246 ).
Management Strategies and Duration of Pharmacological Treatment
Following an adequate dosage of medication ( Table 1 ) and treatment response, medication for ADHD should be titrated to an optimized dosage with regard to the clinical efficacy, safety, and side effects, which should be continued for as long as it remains clinically necessary and effective. This should be reviewed at least annually, also with a planned “medication break” to decide whether there is a continuing need for care. 238 239 However, there is little available empirical evidence to guide clinicians on questions, such as the optimum duration of treatment and when it is appropriate to consider drug discontinuation. As ADHD can persist into adulthood, decisions on treatment discontinuation need to be taken on a case-by-case basis. 226
Abbreviations: ADHD, attention deficit hyperactivity disorder; max. maximum.
Adapted from (1) Walitza S, Romanos M, Greenhill LL, Banaschewski T. Attention-Deficit/Hyperactivity Disorders. In: Gerlach M, Warnke A, Greenhill LL, eds. Psychiatric Drugs in Children and Adolescents. Wien: Springer; 2014:369–381 238 and (2) Walitza S, Gerlach M, Romanos M, Renner T. Psychostimulanzien und andere Arzneistoffe, die zur Behandlung der Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung (ADHS) angewendet werden. In: Gerlach M, Mehler-Wex C, Walitza S, Warnke A, Wewetzer C, eds. Neuro-/Psychopharmaka im Kindes- und Jugendalter: Grundlagen und Therapie. Berlin, Heidelberg: Springer Berlin Heidelberg; 2016:289–331. 239
Among the most frequent side effects of psychostimulant therapy ( Table 2 ) are reduced appetite and sleep disturbances. 247 Appetite reduction following treatment initiation with an ADHD drug often attenuates with time. Reduced appetite at mealtimes can be avoided by taking the medication after meals rather than before. Should a clinically significant lack of appetite persist, dosage reduction (by one-fourth or half tablet of methylphenidate), discontinuation (rarely necessary), or switching to a different formulation or medication should be considered.
Adapted from (1) Walitza S, Romanos M, Greenhill LL, Banaschewski T. Attention-Deficit/Hyperactivity Disorders. In: Gerlach M, Warnke A, Greenhill LL, eds. Psychiatric Drugs in Children and Adolescents. Wien: Springer; 2014:369–381 238 ; (2) Walitza S, Gerlach M, Romanos M, Renner T. Psychostimulanzien und andere Arzneistoffe, die zur Behandlung der Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung (ADHS) angewendet werden. In: Gerlach M, Mehler-Wex C, Walitza S, Warnke A, Wewetzer C, eds. Neuro-/Psychopharmaka im Kindes- und Jugendalter: Grundlagen und Therapie. Berlin, Heidelberg: Springer Berlin Heidelberg; 2016:289–331 239 ; (3) Huang YS, Tsai MH. Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge. CNS Drugs 2011;25:539–554; (4) Storebo OJ, Pedersen N, Ramstad E et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents - assessment of adverse events in non-randomized studies. Cochrane Database Syst Rev 2018;5:CD012069284; and (5) Wigal T, Greenhill L, Chuang S et al. Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry 2006;45:1294–1303.
Nonpharmacological Treatments
Cognitive behavioral therapy.
Cognitive behavioral therapy (CBT) is a form of behavioral intervention which aims at reducing ADHD behaviors or associated problems by enhancing positive behaviors and creating situations in which desired behaviors may occur. In the case of preschool and young school children, CBT focuses on parents and educators, who are instructed and trained to act according to CBT principles, while older children and adolescents may be trained directly to use more appropriate behavioral strategies. 248 CBT and its more specific forms (e.g., social skills training, training of planning and organizational skills, and self-management techniques) have positive effects on behavior, parenting skills, child–parent relationships, and certain daily living skills, 232 249 although effects on ADHD core symptoms are inconsistent and relatively low when only blinded assessments are considered. 250 A recent meta-analysis suggested that the combined treatment of medication with CBT is more efficacious than stimulant medication alone (with an estimated standardized mean difference of 0.5). 251
Neuropsychological Treatments
In cognitive training interventions, either PC-supported or in a manualized format, cognitive exercises that tap into cognitive domains, such as working memory or inhibitory control, are performed in a repetitive manner and with increasing difficulty. The evidence base for this type of intervention is poor according to recent studies (e.g., Bikic et al 252 ) and metastudies (e.g., Cortese et al 253 ). While some “near-transfer” improvements in neuropsychological tests tapping into the trained domain are probable, the evidence for “far transfer” to academic achievements or to the ADHD symptom level is weak. Most studies, however, used the same kind of cognitive training with all participants, irrespective of their actual individual cognitive difficulties. Moreover, they did not adhere to theoretically based training principles, which recommend domain-specific training for the functional improvement of a selective neuropsychological deficit. Possibly, future approaches that combine repetitive exercise and top-down strategy application may provide larger benefits for children with ADHD.
In neurofeedback training (NF), EEG activity measured by one or more electrodes applied to the head is transformed into a visual or acoustic signal and fed back online, for example, by a stimulus moving up and down. By steering the stimulus on the screen, the participant may gain control over his/her EEG activity. Many different training protocols have been applied to ADHD. Those which have received the best evaluation are the NF training of the θ/β frequency bands ratio (the goal is generally to decrease θ and to increase β frequencies) and the training of slow cortical potentials (learning to intentionally increase and decrease cortical excitability over short periods of time). However, “normalizing” an ADHD-specific deviant EEG pattern can no longer qualify as a meaningful goal, as no characteristic ADHD pattern seems to exist (Loo et al, 254 see neurophysiology section), although gaining control over one's brain activity and over attentional states continues to be a valid treatment goal. According to parent ratings, clinical improvements after NF are stronger and longer-lasting compared with other behavioral treatment methods, but teacher ratings usually fail to yield significant effects. 255 Recent research has focused on the specificity of treatment effects, defined as the association between the learned regulation of EEG activity and the behavioral outcome. 256 To date, there is no convincing evidence that the learned control over brain activity is responsible for the observed behavioral improvements. Instead, nonspecific treatment effects, such as improved self-efficacy, positive reinforcement, and learning to sit still, seem to contribute in large part to the positive clinical outcome.
Methodologically more sophisticated NF approaches, such as tomographic NF, 257 fMRI-NF, 258 or near-infrared spectroscopy feedback (feedback of hemoglobin oxygenation) 259 are still in the experimental stage.
Noninvasive Brain Stimulation
Repetitive transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) represent other potential means to modulate cortical activity. Therefore, these approaches may also be promising in terms of improving clinical and cognitive ADHD symptoms such as inattention and impulsiveness. 258 260 261 262 Based on a meta-analysis, Westwood et al 263 suggested that left and/or right prefrontal stimulation may improve performance in attention, inhibition and/or working memory tasks. However, these approaches are not yet recommended by therapy guidelines.
Alternative Nonpharmacological Treatment Methods
Mindfulness training, physical activity, and yoga seem to have positive effects on ADHD behavior, but for the time being, the scientific evidence is weak and these treatments are seen at best as complementary to other interventions. 264 265 266 267 268 Digital home treatment programs or support apps are currently being developed for ADHD patients or their parents 269 270 ; their usefulness or clinical validity still needs to be tested. Children and adolescents with ADHD often show a great affinity with digital media, which may improve compliance, but one has to take into account that the rate of problematic internet use and gaming is enhanced in youth with ADHD (estimated at 37% in ADHD vs. 12% in TD). 271 Free fatty acid supplementation has been described to bring about small but significant reductions in ADHD symptoms even with probably blinded assessments (standardized mean difference = 0.16). 250
Long-Term Outcome
Follow-up studies have reported divergent results, with some reporting high rates of persistence until adulthood (up to 79%), 153 and others showing much higher rates of remission from childhood to adolescence (e.g., 45–55% of syndromal remissions). 272 273 274 Recent population-based studies from Brazil, the United Kingdom, and New Zealand have claimed that a large portion of de novo ADHD cases emerge at adult age, 275 276 277 but these results can probably be explained by methodological artifacts and missed subthreshold cases. 76 278 279 However, meta-analytic findings by Bonvicini et al 280 indicate that in part, different genes and polymorphisms seem to contribute to childhood ADHD and adulthood ADHD, lending some genetic plausibility to findings of a late manifestation of the disorder. According to the MTA study, the contribution of interventions administered during childhood to outcome in adulthood is negligible, but controlled intervention was limited to a relatively short period of time (14 months). 281 Neurobiologically, the course of ADHD may be explained by different models. 274 According to the first model, remission at adult age may be reduced to the normalization of brain functions through maturation. A second model explains remission through the recruitment of compensatory brain functions. The third model claims that brain function anomalies show life-long persistence, even though behavioral dysfunction may have remitted. 274 Possibly, all of these models, and probably additional ones too (see e.g., Doehnert et al 148 ), apply to different subgroups of patients or functions and may account for the divergent results in the literature.
Conflict of Interest D.B. reports having served as an unpaid scientific advisor for an EU-funded neurofeedback trial unrelated to the present work.
S.W. reports grants from Gertrud Thalmann Fonds of the UPK Basel, Collaborative Project, grants from Ebnet Foundation, grants from Mensia Technologies SA & EU H2020 SME Instrument, grants from University Medical Center Utrecht & Stanley Medical Research Institute, Collaborative Project, grants from Swiss National Foundation, Investigator Initiated Clinical Trial, other from Thieme Neuropychopharmakologie des Kindes und Jugendalters, outside the submitted work; and S.W. has received in the last 5 years royalities from Thieme Hogrefe, Kohlhammer, Springer, Beltz. S.W. has received lecture honoraria from Opopharma in the last 5 years. Her work was supported in the last 5 years by the Swiss National Science Foundation (SNF), diff. EU FP7s, HSM Hochspezialisierte Medizin of the Kanton Zurich, Switzerland, Bfarm Germany, ZInEP, Hartmann Müller Stiftung, Olga Mayenfisch, Gertrud Thalmann Fonds. Outside professional activities and interests are declared under the link of the University of Zurich www.uzh.ch/prof/ssl-dir/interessenbindungen/client/web/ .
Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions
Acetaminophen Use During Pregnancy and Children’s Risk of Autism, ADHD, and Intellectual Disability
- 1 Department of Global Public Health, Karolinska Institutet, Stockholm, Sweden
- 2 Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden
- 3 Clinical Epidemiology Division, Department of Medicine, Solna, Karolinska Institutet, Stockholm, Sweden
- 4 Department of Women’s Health, Division of Obstetrics, Karolinska University Hospital, Stockholm, Sweden
- 5 Department of Neonatology, Sachs’ Children and Youth Hospital, Stockholm, Sweden
- 6 Centre for Epidemiology and Community Medicine, Region Stockholm, Stockholm, Sweden
- 7 Department of Epidemiology and Biostatistics, Drexel University Dornsife School of Public Health, Philadelphia, Pennsylvania
- 8 A.J. Drexel Autism Institute, Drexel University, Philadelphia, Pennsylvania
Question Does acetaminophen use during pregnancy increase children’s risk of neurodevelopmental disorders?
Findings In this population-based study, models without sibling controls identified marginally increased risks of autism and attention-deficit/hyperactivity disorder (ADHD) associated with acetaminophen use during pregnancy. However, analyses of matched full sibling pairs found no evidence of increased risk of autism (hazard ratio, 0.98), ADHD (hazard ratio, 0.98), or intellectual disability (hazard ratio, 1.01) associated with acetaminophen use.
Meaning Acetaminophen use during pregnancy was not associated with children’s risk of autism, ADHD, or intellectual disability in sibling control analyses. This suggests that associations observed in other models may have been attributable to confounding.
Importance Several studies suggest that acetaminophen (paracetamol) use during pregnancy may increase risk of neurodevelopmental disorders in children. If true, this would have substantial implications for management of pain and fever during pregnancy.
Objective To examine the associations of acetaminophen use during pregnancy with children’s risk of autism, attention-deficit/hyperactivity disorder (ADHD), and intellectual disability.
Design, Setting, and Participants This nationwide cohort study with sibling control analysis included a population-based sample of 2 480 797 children born in 1995 to 2019 in Sweden, with follow-up through December 31, 2021.
Exposure Use of acetaminophen during pregnancy prospectively recorded from antenatal and prescription records.
Main Outcomes and Measures Autism, ADHD, and intellectual disability based on International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision codes in health registers.
Results In total, 185 909 children (7.49%) were exposed to acetaminophen during pregnancy. Crude absolute risks at 10 years of age for those not exposed vs those exposed to acetaminophen were 1.33% vs 1.53% for autism, 2.46% vs 2.87% for ADHD, and 0.70% vs 0.82% for intellectual disability. In models without sibling control, ever-use vs no use of acetaminophen during pregnancy was associated with marginally increased risk of autism (hazard ratio [HR], 1.05 [95% CI, 1.02-1.08]; risk difference [RD] at 10 years of age, 0.09% [95% CI, −0.01% to 0.20%]), ADHD (HR, 1.07 [95% CI, 1.05-1.10]; RD, 0.21% [95% CI, 0.08%-0.34%]), and intellectual disability (HR, 1.05 [95% CI, 1.00-1.10]; RD, 0.04% [95% CI, −0.04% to 0.12%]). To address unobserved confounding, matched full sibling pairs were also analyzed. Sibling control analyses found no evidence that acetaminophen use during pregnancy was associated with autism (HR, 0.98 [95% CI, 0.93-1.04]; RD, 0.02% [95% CI, −0.14% to 0.18%]), ADHD (HR, 0.98 [95% CI, 0.94-1.02]; RD, −0.02% [95% CI, −0.21% to 0.15%]), or intellectual disability (HR, 1.01 [95% CI, 0.92-1.10]; RD, 0% [95% CI, −0.10% to 0.13%]). Similarly, there was no evidence of a dose-response pattern in sibling control analyses. For example, for autism, compared with no use of acetaminophen, persons with low (<25th percentile), medium (25th-75th percentile), and high (>75th percentile) mean daily acetaminophen use had HRs of 0.85, 0.96, and 0.88, respectively.
Conclusions and Relevance Acetaminophen use during pregnancy was not associated with children’s risk of autism, ADHD, or intellectual disability in sibling control analysis. This suggests that associations observed in other models may have been attributable to familial confounding.
Read More About
Ahlqvist VH , Sjöqvist H , Dalman C, et al. Acetaminophen Use During Pregnancy and Children’s Risk of Autism, ADHD, and Intellectual Disability. JAMA. 2024;331(14):1205–1214. doi:10.1001/jama.2024.3172
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Copyright © 2024 OccupationalTherapy.com - All Rights Reserved
Pediatric Case Study: Child with ADHD
This course focuses on a case study for a 9-year-old male with ADHD experiencing occupational challenges in education, ADL, IADL, and social participation.
Course created on January 30, 2020
Course Type : Video, Text
CEUs/Hours Offered: AOTA/0.1 Intermediate, OT Service Delivery; CE Broker/1.0 Home Study, General (FL), Patient Related (AL), General Continuing Education (GA), Direct Client/patient Services In Occupational Therapy (SC), Related To OT (AZ), Related To OT (LA), Directly Related To OT (MS), Directly Related To OT (TN), CE Broker #20-766056; IACET/0.1; IBCCES/1.0; NBCOT PDUs/1.25 Intermediate, Pediatrics
Learning Outcomes
No more reviews to load.
Presented By

Nicole Quint Dr.OT, OTR/L
Nicole Quint has been an occupational therapist for over 15 years, currently serving as an Associate Professor in the Occupational Therapy Department at Nova Southeastern University, teaching in both the Masters and Doctoral programs. She provides outpatient pediatric OT services, specializing in children and adolescents with Sensory Processing Disorder and concomitant disorders. She also provides consultation services for schools, professional development, and special education services. She provides continuing education on topics related to SPD, pediatric considerations on the occupation of sleep, occupational therapy and vision, reflective therapist, executive functions, leadership in occupational therapy and social emotional learning.
Course participation information
To ensure you are ready to participate, please complete our short Test Drive to prepare your computer to view the course.
View Course Help
Full attendance is required, and the times you log in and out will be recorded and documented. If you log in to a live webinar late or if you log out early, you may not be able to earn CEU.
Passing an online exam and completing a course evaluation will be required to earn continuing education credit.
Live Webinars allow presenter and participant interaction. The exam and course evaluation for these courses must be completed within 7 days of the event.
On-demand courses include texts, video and audio recordings of live webinars, and multimedia formats. The exam and course evaluation for on-demand courses must be completed within 30 days of course registration.
To participate in the course, complete the exam and course evaluation, and earn continuing education credit, you must be a OccupationalTherapy.com member. Participants must complete the entire course; partial credit is not allowed.
OccupationalTherapy.com is committed to ensuring accessibility to the widest possible audience. We are continually improving the user experience for everyone. If you have questions, requests, or would like to report an accessibility-related issue, please email [email protected] . We will review your request and respond in a timely manner.
Visit our Contact us page or give us a call if you have questions.
American Occupational Therapy Association
OccupationalTherapy.com is an AOTA Approved Provider of professional development, #7659. This distance learning - independent course is offered at 0.1 CEUs/contact hours (Intermediate level, OT Service Delivery Area). The assignment of AOTA CEUs does not imply endorsement of specific course content, products, or clinical procedures by AOTA.
OccupationalTherapy.com is an approved provider for CE Broker, provider #50-14558. This course is offered for 1.0 hours. If you are an OT/OTA in Alabama, Arizona, Florida, Georgia, Kansas, Louisiana, Michigan, Mississippi, Oklahoma, South Carolina or Tennessee, CE Broker may be of interest to you.
International Association for Continuing Education and Training
continu ed , LLC, DBA OccupationalTherapy.com, is accredited by the International Association for Continuing Education and Training (IACET). continu ed complies with the ANSI/IACET Standard, which is recognized internationally as a standard of excellence in instructional practices. As a result of this accreditation, continu ed is authorized to issue the IACET CEU. continu ed , LLC, is authorized by IACET to offer 0.1 CEUs for this program.
International Board of Credentialing and Continuing Education Standards
The continu ed family of websites, including OccupationalTherapy.com, is a Certified Training Partner of IBCCES. This course is offered for a maximum of 1.0 CE hours for the autism certifications issued by IBCCES.
National Board for Certification in Occupational Therapy
OccupationalTherapy.com is a NBCOT® Professional Development Provider. This course is offered for 1.25 NBCOT PDUs (Intermediate level, Pediatrics Area). NBCOT® is a registered trademark of The National Board for Certification in Occupational Therapy, Inc.
Our site uses cookies to improve your experience. By using our site, you agree to our Privacy Policy .
- Open access
- Published: 23 November 2023
Risk factors associated with newly diagnosed attention-deficit/hyperactivity disorder in adults: a retrospective case-control study
- Jeff Schein 1 ,
- Martin Cloutier 2 ,
- Marjolaine Gauthier-Loiselle 2 ,
- Rebecca Bungay 2 ,
- Emmanuelle Arpin 2 ,
- Annie Guerin 2 &
- Ann Childress 3
BMC Psychiatry volume 23 , Article number: 870 ( 2023 ) Cite this article
1955 Accesses
18 Altmetric
Metrics details
Knowledge of risk factors for attention-deficit/hyperactivity disorder (ADHD) may facilitate early diagnosis; however, studies examining a broad range of potential risk factors for ADHD in adults are limited. This study aimed to identify risk factors associated with newly diagnosed ADHD among adults in the United States (US).
Eligible adults from the IQVIA PharMetrics® Plus database (10/01/2015-09/30/2021) were classified into the ADHD cohort if they had ≥ 2 ADHD diagnoses (index date: first ADHD diagnosis) and into the non-ADHD cohort if they had no observed ADHD diagnosis (index date: random date) with a 1:3 case-to-control ratio. Risk factors for newly diagnosed ADHD were assessed during the 12-month baseline period; logistic regression with stepwise variable selection was used to assess statistically significant association. The combined impact of selected risk factors was explored using common patient profiles.
A total of 337,034 patients were included in the ADHD cohort (mean age 35.2 years; 54.5% female) and 1,011,102 in the non-ADHD cohort (mean age 44.0 years; 52.4% female). During the baseline period, the most frequent mental health comorbidities in the ADHD and non-ADHD cohorts were anxiety disorders (34.4% and 11.1%) and depressive disorders (27.9% and 7.8%). Accordingly, a higher proportion of patients in the ADHD cohort received antianxiety agents (20.6% and 8.3%) and antidepressants (40.9% and 15.8%). Key risk factors associated with a significantly increased probability of ADHD included the number of mental health comorbidities (odds ratio [OR] for 1 comorbidity: 1.41; ≥2 comorbidities: 1.45), along with certain mental health comorbidities (e.g., feeding and eating disorders [OR: 1.88], bipolar disorders [OR: 1.50], depressive disorders [OR: 1.37], trauma- and stressor-related disorders [OR: 1.27], anxiety disorders [OR: 1.24]), use of antidepressants (OR: 1.87) and antianxiety agents (OR: 1.40), and having ≥ 1 psychotherapy visit (OR: 1.70), ≥ 1 specialist visit (OR: 1.30), and ≥ 10 outpatient visits (OR: 1.51) (all p < 0.05). The predicted risk of ADHD for patients with treated anxiety and depressive disorders was 81.9%.
Conclusions
Mental health comorbidities and related treatments are significantly associated with newly diagnosed ADHD in US adults. Screening for patients with risk factors for ADHD may allow early diagnosis and appropriate management.
Peer Review reports
Attention-deficit/hyperactivity disorder (ADHD) is a debilitating neurodevelopmental condition with an estimated prevalence of 4.4% among adults in the United States (US) [ 1 ]. ADHD is traditionally perceived as a childhood disorder [ 2 ]; hence, underdiagnosis, delayed diagnosis, and undertreatment of ADHD are believed to be common among adults [ 3 , 4 ].
The diagnostic challenges of ADHD are partially attributable to the frequent comorbid mental disorders [ 5 , 6 ]. Certain mental health comorbidities, such as anxiety and depressive disorders, share overlapping symptoms with ADHD [ 7 , 8 ], potentially leading to misdiagnosis or delayed diagnosis. Studies have suggested that about one-fifth of adults seeking psychiatric services and reporting for other mental health conditions were later found to have ADHD [ 9 , 10 , 11 ]. The World Health Organization Mental Health Survey has also reported that among US adults with ADHD identified through diagnostic interviews, approximately half had received some form of treatment for their emotional or behavioral problems in the past year, but only 13.2% were treated specifically for ADHD [ 12 ]. Clinicians’ lack of awareness or training on adult ADHD may also hinder ADHD diagnosis [ 4 ]. A US medical record-based study found that 56% of adults with ADHD had not received a prior diagnosis of the condition despite complaining about ADHD symptoms to other healthcare professionals in the past [ 13 ]. Other reasons adding to the diagnostic challenge of ADHD in adults may include patient’s fear of stigma and masking behaviors developed over the years [ 4 , 14 ].
ADHD is associated with a wide range of psychosocial, functional, and occupational problems in adults [ 15 ]. A delay in diagnosis, or undiagnosed and ultimately untreated ADHD, may lead to poor clinical and functional outcomes even if comorbidities are treated [ 16 ]. Conversely, early identification of ADHD may allow better symptom management and improve patient functioning and quality of life. To facilitate diagnosis, risk factors are commonly used to predict disease development and aid clinicians to identify at-risk patients [ 17 ]. However, there is a paucity of large studies examining a broad range of potential risk factors for an ADHD diagnosis in adults. Prior studies have reported certain patient characteristics, such as presence of anxiety disorders, depressive disorders, sleep impairments, eating disorders, and childhood illnesses or health events (e.g., obesity, head injuries, infections) that may be associated with ADHD [ 18 , 19 , 20 , 21 , 22 , 23 ]. Yet, most of these studies have examined a single or a few factors, and many were conducted in pediatric ADHD populations primarily outside of the US.
Knowledge on patient characteristics associated with a higher risk of ADHD in adults and the patient journey prior to a clinical ADHD diagnosis may facilitate early diagnosis and the provision of appropriate management. The current study was conducted to identify risk factors for newly diagnosed ADHD in adult patients using a large claims database in the US. The potential utility of the results was also demonstrated through exploring the combined impact of selected risk factors on ADHD risk prediction using fictitious common patient profiles.
Data source
Data from the IQVIA PharMetrics® Plus (IQVIA) database covering the period of October 1, 2015, to September 30, 2021, were used. The IQVIA database contains integrated claims data of over 190 million beneficiaries across the US and includes information on inpatient and outpatient diagnoses and procedures, prescription fills, patients’ pharmacy and medical benefits, inpatient stays, and provider details. Additional data elements encompass dates of service, demographic variables, plan type, payer type, and start and stop dates of health plan enrollment. Data are de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA); therefore, no review by an institutional review board nor informed consent was required per Title 45 of CFR, Part 46.101(b)(4) [ 24 ].
Study design and patient populations
A retrospective case-control study design was used. Eligible adults were classified into two cohorts based on the presence of ADHD diagnoses (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] F90.x): the ADHD cohort comprised patients with ≥ 2 ADHD diagnoses recorded on a medical claim on distinct dates at any time during their continuous health plan enrollment; and the non-ADHD cohort comprised patients without any ADHD diagnoses recorded on a medical claim at any time during their continuous health plan enrollment. To account for large differences in sample size and to retain statistical power, a 1:3 case-to-control ratio was used. Specifically, eligible patients were randomly selected into the non-ADHD cohort such that the total number of patients in the non-ADHD cohort was three times that of the ADHD cohort.
The index date was defined as the first observed ADHD diagnosis among the ADHD cohort and a randomly selected date among the non-ADHD cohort. To allow sufficient time to capture potential risk factors for ADHD, patients were required to have ≥ 12 months of continuous health plan enrollment prior to the index date. The baseline period was defined as the 12 months pre-index.
Study measures and outcomes
Patient characteristics and potential risk factors for newly diagnosed ADHD were assessed during the baseline period for each cohort, separately. Potential risk factors considered in this study were identified through a targeted literature review and observable variables in the data and included demographic characteristics (i.e., age, sex, regions of residence, calendar year of index date), clinical characteristics (i.e., physical and mental health comorbidities), pharmacological treatments (i.e., medications for common ADHD comorbidities), healthcare resource utilization (i.e., number of psychotherapy, inpatient, emergency room, outpatient, and specialist [psychiatrist, neurologist] visits). Risk factors for ADHD in this study were identified from potential risk factors that had statistically significant association with newly diagnosed ADHD, as described in the next section.
Statistical analyses
Descriptive statistics were used to summarize baseline patient characteristics and potential risk factors for newly diagnosed ADHD. Means, medians, and standard deviations (SDs) were reported for continuous variables; frequency counts and percentages were reported for categorical variables.
Univariate statistics were used to compare potential risk factors between the ADHD and non-ADHD cohorts. The magnitude of the difference between cohorts was assessed by calculating the standardized differences (std. diff.) for both continuous and categorical variables.
Logistic regression model with stepwise variable selection was used to assess statistically significant association between potential risk factors and ADHD diagnosis. Potential risk factors were eligible for inclusion in the logistic regression based on their univariate association with ADHD diagnosis (i.e., std. diff. >0.10). Potential risk factors presented in < 0.5% of the sample were discarded. Variables included in the last iteration of the stepwise selection process were considered as risk factors of the study outcome. The association between risk factors and ADHD diagnosis were reported as odds ratios (ORs) along with their 95% confidence intervals (CIs) and p-values.
To facilitate the interpretation of the regression analyses, the predicted risk of ADHD based on regression coefficient estimates was evaluated for six fictitious common patient profiles corresponding to patients who harbor selected combinations of ADHD risk factors. This exploratory analysis allowed for the estimation of how the risk of having ADHD would vary had the same person had additional risk factors but otherwise the same characteristics.
Patient characteristics and potential risk factors
The total sample comprised 1,348,136 patients, including 337,034 in the ADHD cohort and 1,011,102 in the non-ADHD cohort (Fig. 1 ). Table 1 presents the patient characteristics and potential risk factors (i.e., characteristics with a std. diff. >0.10) by cohort.
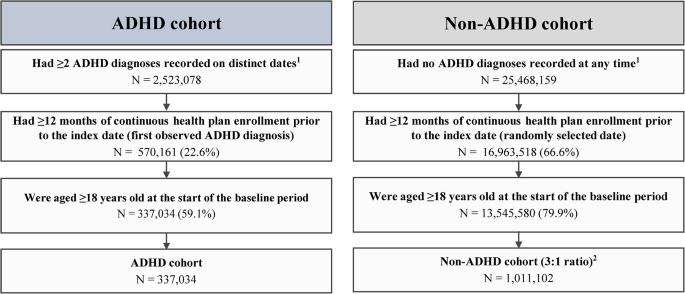
Sample selection flowchart. ADHD, attention-deficit/hyperactivity disorder; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification
1 ADHD was defined as ICD-10-CM codes: F90.x
2 Eligible patients were randomly selected into the non-ADHD cohort such that the total number of patients in the non-ADHD cohort is 3 times that of the ADHD cohort to account for large differences in sample size
Demographic characteristics
As of index date, the ADHD cohort was younger than the non-ADHD cohort (mean age: 35.2 and 44.0 years; std. diff. = 0.68). In both cohorts, slightly over half of the patients were female (54.5% and 52.4%; std. diff. = 0.04), and the South was the most represented region (48.7% and 42.5%; std. diff. = 0.13).
Clinical characteristics
During the baseline period, the most frequent physical comorbidities in the ADHD and non-ADHD cohorts were hypertension (12.4% and 21.3%; std. diff. = 0.24), obesity (10.0% and 9.4%; std. diff. = 0.02), and chronic pulmonary disease (9.0% and 7.2%; std. diff. = 0.07).
A lower proportion of patients had no mental health comorbidities in the ADHD cohort than the non-ADHD cohort (42.0% and 70.8%; std. diff. = 0.61). The mean ± SD number of mental health comorbidities was 1.2 ± 1.4 in the ADHD cohort and 0.5 ± 0.9 in the non-ADHD cohort (std. diff. = 0.65). The most frequent mental health comorbidities in the ADHD and non-ADHD cohorts were anxiety disorders (34.4% and 11.1%; std. diff. = 0.58), depressive disorders (27.9% and 7.8%; std. diff. = 0.54), sleep-wake disorders (13.2% and 7.7%; std. diff. = 0.18), trauma- and stressor-related disorders (12.4% and 3.4%; std. diff. = 0.34), and substance-related and addictive disorders (9.4% and 5.0%; std. diff. = 0.17).
Pharmacological treatments
A higher proportion of patients in the ADHD than the non-ADHD cohort received antidepressants (40.9% and 15.8%; std. diff. = 0.58), antianxiety agents (20.6% and 8.3%; std. diff. = 0.36), anticonvulsants (16.1% and 6.8%; std. diff. = 0.29), and antipsychotics (7.2% and 1.5%; std. diff. = 0.28).
Healthcare resource utilization
The ADHD cohort, relative to the non-ADHD cohort, had generally higher mean ± SD rates of healthcare resource utilization, including more psychotherapy visits (2.9 ± 8.8 and 0.6 ± 4.0; std. diff. = 0.34), emergency room visits (0.6 ± 1.7 and 0.4 ± 1.2; std. diff. = 0.14), outpatient visits (12.7 ± 16.5 and 8.3 ± 12.4; std. diff. = 0.30), and specialist visits (1.0 ± 4.0 and 0.2 ± 1.8; std. diff. = 0.24); the number of inpatient visits were similar between cohorts (0.1 ± 0.4 and 0.1 ± 0.3; std. diff. = 0.04).
Association between risk factors and ADHD diagnosis
The risk factors with a significant association with an ADHD diagnosis are presented in Fig. 2 . Demographically, being younger and living in the South were risk factors for having an ADHD diagnosis (OR for age: 0.95; OR for region of residence using South as a reference: Midwest, 0.79; West, 0.70; Northwest, 0.67; all p < 0.05).
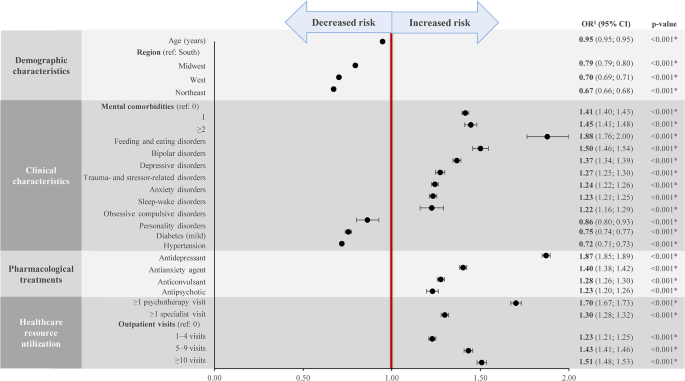
ADHD, attention-deficit/hyperactivity disorder; CI, confidence interval; OR, odds ratio
*Statistically significant at the 5% level
1 Estimated from logistic regression analyses
Other key risk factors associated with a significantly increased probability of having an ADHD diagnosis included the number of mental health comorbidities (OR for 1 comorbidity: 1.41; ≥2 comorbidities: 1.45); certain mental health comorbidities, including feeding and eating disorders (OR: 1.88), bipolar disorders (OR: 1.50), depressive disorders (OR: 1.37), trauma- and stressor-related disorders (OR: 1.27), anxiety disorders (OR: 1.24), sleep-wake disorders (OR: 1.23), and obsessive compulsive disorders (OR: 1.22); use of antidepressants (OR: 1.87) and antianxiety agents (OR: 1.40); and having ≥ 1 psychotherapy visit (OR: 1.70), ≥ 1 specialist visit (OR: 1.30), and ≥ 10 outpatient visits (OR: 1.51) (all p < 0.05).
Predicted risk of ADHD for patient profiles with selected risk factors
Selected risk factors identified from the logistic regression analyses were used to create fictitious common patient profiles to demonstrate their combined impact on the predicted risk of having an ADHD diagnosis (Fig. 3 ). Five of the six profiles correspond to patients with the same demographic characteristics (i.e., aged 35 years and living in the South) but vary in terms of the number (i.e., 1 or ≥ 2) and types of mental health comorbidities (i.e., anxiety disorder and/or depressive disorder), the pharmacological treatment received (i.e., antianxiety and/or antidepressant agent, or no treatment), and the level of healthcare resource utilization (i.e., number of psychotherapy, specialist, and outpatient visits). The remaining profile corresponds to low-risk patients with no relevant risk factors for ADHD.
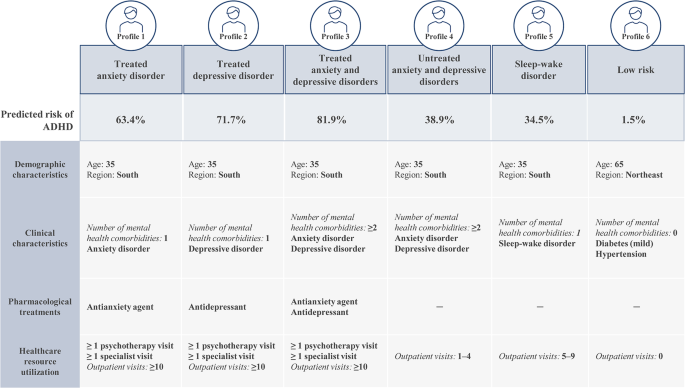
Predicted risk of ADHD for selected patient profiles
ADHD, attention-deficit/hyperactivity disorder
Based on these patient profiles, the predicted risk of ADHD was the highest among patients with treated anxiety and depressive disorders (profile 3). More specifically, a patient presenting with the characteristics described in this profile would have an 81.9% likelihood of being diagnosed with ADHD in the coming year. The profile with the next highest predicted risk of ADHD was patients with treated depressive disorder (profile 2; 71.7%), followed by patients with treated anxiety disorder (profile 1; 63.4%). Profiles corresponding to a moderate predicted risk of ADHD included patients with untreated anxiety and depressive disorders (profile 4; 38.9%) and patients with sleep-wake disorder (profile 5; 34.5%). The predicted risk for ADHD among low-risk patients (profile 6) was 1.5%.
This large retrospective case-control study has identified a broad range of risk factors associated with ADHD in adults and quantified the added likelihood of an ADHD diagnosis contributed by each factor. Certain mental health comorbidities and their associated treatments and care were found to be significantly associated with newly diagnosed ADHD in adults. Specifically, the presence of common mental health comorbidities of ADHD such as anxiety and depressive disorders was associated with 24% and 37% increased risk of having an ADHD diagnosis, respectively. The use of pharmacological treatments for these conditions such as antianxiety agents and antidepressants was associated with an increased risk of having an ADHD diagnosis of 40% and 87%, respectively; having at least one prior psychotherapy visit was also associated with a 70% increased risk. Demographically, being younger and living in the South were found to be risk factors for having an ADHD diagnosis. The combined impact of selected risk factors on the predicted ADHD risk was explored through specific patient profiles, which demonstrated how the findings may be interpreted in clinical settings. The presence of a combination of risk factors may suggest that a patient is at a high risk of having undiagnosed ADHD and signify the need for further assessments. Collectively, findings of this study have extended our understanding on the patient path to ADHD diagnosis as well as the characteristics and clinical events that could suggest undiagnosed ADHD in adults.
Most prior studies examining characteristics associated with ADHD have focused on a single or a few factors, and many were conducted in pediatric populations [ 18 , 19 , 20 , 21 , 22 , 23 ]. Nonetheless, the risk factors for ADHD identified in the current study are largely aligned with the literature. For instance, among prior research in adults, a multicenter patient register study found that at the time of first ADHD diagnosis, mental health comorbidities were present in two-thirds of the patients; patients on average presented with 2.4 comorbidities, with the most common comorbidities being substance use disorders, anxiety disorders, mood disorders, and personality disorders [ 6 ]. Another study among adult members of two large managed healthcare plans found that compared with individuals without ADHD, those screened positive for ADHD through a telephone survey but had no documented ADHD diagnosis (i.e., the undiagnosed group) had significantly higher rates of mental health comorbidities (e.g., anxiety, depression, bipolar disorder) and were more likely to receive medications for a mental health condition [ 25 ]. In line with these findings, the current exploratory patient profile analyses also suggest that patients with more mental health comorbidities and have received the associated pharmacological treatments and care are at a higher risk of having undiagnosed ADHD than those with fewer or untreated mental health comorbidities.
The current study also found that an overall higher healthcare resource utilization was a characteristic associated with newly diagnosed ADHD among adult patients. A potential interpretation of this finding is that an individual who experienced ADHD-related symptoms might visit a psychologist or physician frequently to seek help for the symptoms; thus, a high level of prior healthcare resource utilization may be a sign that an individual could have undiagnosed ADHD. Clinical judgement should be applied to determine whether further evaluation for ADHD is needed on a case-by-case basis considering the presence of other high-risk characteristics.
The diagnosis of ADHD can be challenging, particularly among adults [ 2 , 3 ]. The current study suggests that information on patient characteristics, such as the presence of mental health comorbidities and healthcare resource utilization history, may be used to aid clinicians identify adult patients at risk of ADHD and minimize missed opportunity to provide a timely diagnosis of ADHD and the proper care. Notably, underdiagnosis or a delayed diagnosis of ADHD leads to undertreatment and can adversely affect patients’ occupational achievements, diminish self-esteem, and hamper interpersonal relationships, considerably reducing the quality of life [ 8 ]. ADHD in adults has also been shown to be associated with approximately $123 billion total societal excess costs in the US [ 26 ]. Consequently, early detection and treatment of ADHD may have the potential to alleviate the large patient and societal burden associated with the condition.
It is worth mentioning that causes for ADHD is multifactorial, and multiple risk factors may contribute to the risk of having ADHD [ 15 ]. Some risk factors in the literature (e.g., genetics and environmental factors [ 27 , 28 ]) are not available in claims data, and these factors are important to consider when establishing an ADHD diagnosis. Nonetheless, the risk factors identified in this study were generated based on a large sample size (over 1.3 million adults), and as exemplified by the exploratory patient profiles, the presence of multiple risk factors was associated with an overall higher risk of having undiagnosed ADHD. Together, these findings would help inform clinicians on the types of high-risk patient profiles that should raise a red flag for potential ADHD and prompt further clinical assessments, such as family psychiatric history and diagnostic interviews. As such, findings of this study may facilitate early diagnosis and appropriate management of ADHD among adults, which may in turn improve patient outcomes.
The findings of the current study should be considered in light of certain limitations inherent to retrospective databases using claims data, including the risk of data omissions, coding errors, and the presence of rule-out diagnosis. Nonetheless, while few studies specifically assessed the validity of ICD-10-CM codes for ADHD diagnoses in claims data, literature evidence has suggested high accuracy of ICD-9-CM codes in identifying neurodevelopmental disorders, including ADHD, and a good correspondence between the ICD-9 and − 10 codes is expected [ 29 , 30 ]. Furthermore, ICD codes have been widely used in the literature to identify ADHD diagnoses in claims-based analyses [ 31 , 32 , 33 ]. Meanwhile, as the study included commercially insured patients, the sample may not be representative of the entire ADHD population in the US. Furthermore, potential risk factors were limited to information available in health insurance claims data only, which may lack relevant information related to ADHD, such as presence of childhood ADHD, family history, or environmental factors. In addition, some characteristics may interact with multiple variables such that their association with an ADHD diagnosis may already be captured by other variables; as such, a characteristic with an OR of less than 1 should not be interpreted as having a protective effect against an ADHD diagnosis but rather that the characteristic alone may be insufficient to prompt screening for ADHD. Lastly, findings from this retrospective observational analysis should be interpreted as measures of association; no causal inference can be drawn.
This large retrospective case-control study found that mental health comorbidities and related treatments and care are significantly associated with newly diagnosed ADHD in US adults. The presence of a combination of risk factors may suggest that a patient is at a high risk of having undiagnosed ADHD. The results of this study provide insights on the path to ADHD diagnosis and may aid clinicians identify at-risk patients for screening, which may facilitate early diagnosis and appropriate management of ADHD.
Data Availability
The data that support the findings of this study are available from IQVIA but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding author (email: [email protected]) upon reasonable request and with permission of IQVIA.
Abbreviations
- Attention-deficit/hyperactivity disorder
Confidence intervals
Health Insurance Portability and Accountability Act
International Classification of Diseases, Tenth Revision, Clinical Modification
Standard deviation
Standardized difference
United States
Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–23.
Article PubMed PubMed Central Google Scholar
Weibel S, Menard O, Ionita A, Boumendjel M, Cabelguen C, Kraemer C, et al. Practical considerations for the evaluation and management of attention deficit hyperactivity disorder (ADHD) in adults. Encephale. 2020;46(1):30–40.
Article CAS PubMed Google Scholar
Ginsberg Y, Quintero J, Anand E, Casillas M, Upadhyaya HP. Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: a review of the literature. Prim Care Companion CNS Disord. 2014;16(3).
Prakash J, Chatterjee K, Guha S, Srivastava K, Chauhan VS. Adult attention-deficit hyperactivity disorder: from clinical reality toward conceptual clarity. Ind Psychiatry J. 2021;30(1):23–8.
Kooij JJ, Huss M, Asherson P, Akehurst R, Beusterien K, French A, et al. Distinguishing comorbidity and successful management of adult ADHD. J Atten Disord. 2012;16(5 Suppl):3S–19S.
Article PubMed Google Scholar
Pineiro-Dieguez B, Balanza-Martinez V, Garcia-Garcia P, Soler-Lopez B, The CAT Study Group. Psychiatric comorbidity at the time of diagnosis in adults with ADHD: the CAT study. J Atten Disord. 2016;20(12):1066–75.
D’Agati E, Curatolo P, Mazzone L. Comorbidity between ADHD and anxiety disorders across the lifespan. Int J Psychiatry Clin Pract. 2019;23(4):238–44.
Goodman DW, Thase ME. Recognizing ADHD in adults with comorbid mood disorders: implications for identification and management. Postgrad Med. 2009;121(5):20–30.
Almeida MLG, Hernandez GAO, Ricardo-Garcell J. ADHD prevalence in adult outpatients with nonpsychotic psychiatric illnesses. J Atten Disord. 2007;11(2):150–6.
Article Google Scholar
Nylander L, Holmqvist M, Gustafson L, Gillberg C. ADHD in adult psychiatry. Minimum rates and clinical presentation in general psychiatry outpatients. Nord J Psychiatry. 2009;63(1):64–71.
Deberdt W, Thome J, Lebrec J, Kraemer S, Fregenal I, Ramos-Quiroga JA, et al. Prevalence of ADHD in nonpsychotic adult psychiatric care (ADPSYC): a multinational cross-sectional study in Europe. BMC Psychiatry. 2015;15:242.
Fayyad J, De GR, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402–9.
Faraone SV, Spencer TJ, Montano CB, Biederman J. Attention-deficit/hyperactivity disorder in adults: a survey of current practice in psychiatry and primary care. Arch Intern Med. 2004;164(11):1221–6.
Lebowitz MS. Stigmatization of ADHD: a developmental review. J Atten Disord. 2016;20(3):199–205.
Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020.
Pawaskar M, Fridman M, Grebla R, Madhoo M. Comparison of quality of life, productivity, functioning and self-esteem in adults diagnosed with ADHD and with symptomatic ADHD. J Atten Disord. 2020;24(1):136–44.
Offord DR, Kraemer HC. Risk factors and prevention. Evid Based Ment Health. 2000;3(3):70–1.
Carpena MX, Munhoz TN, Xavier MO, Rohde LA, Santos IS, Del-Ponte B, et al. The role of sleep duration and sleep problems during childhood in the development of ADHD in adolescence: findings from a population-based birth cohort. J Atten Disord. 2020;24(4):590–600.
Liu CY, Asherson P, Viding E, Greven CU, Pingault JB. Early predictors of de novo and subthreshold late-onset ADHD in a child and adolescent cohort. J Atten Disord. 2021;25(9):1240–50.
Meinzer MC, Pettit JW, Viswesvaran C. The co-occurrence of attention-deficit/hyperactivity disorder and unipolar depression in children and adolescents: a meta-analytic review. Clin Psychol Rev. 2014;34(8):595–607.
Mowlem FD, Rosenqvist MA, Martin J, Lichtenstein P, Asherson P, Larsson H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur Child Adolesc Psychiatry. 2019;28(4):481–9.
So M, Dziuban EJ, Pedati CS, Holbrook JR, Claussen AH, O’Masta B et al. Childhood physical health and attention deficit/hyperactivity disorder: a systematic review and meta-analysis of modifiable factors. Prev Sci. 2022.
Levy LD, Fleming JP, Klar D. Treatment of refractory obesity in severely obese adults following management of newly diagnosed attention deficit hyperactivity disorder. Int J Obes (Lond). 2009;33(3):326–34.
U.S. Department of Health and Human Services. 45 CFR 46: pre-2018 requirements [Available from: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html#46.101 .
Able SL, Johnston JA, Adler LA, Swindle RW. Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychol Med. 2007;37(1):97–107.
Schein J, Adler LA, Childress A, Gagnon-Sanschagrin P, Davidson M, Kinkead F, et al. Economic burden of attention-deficit/hyperactivity disorder among adults in the United States: a societal perspective. J Manag Care Spec Pharm. 2022;28(2):168–79.
PubMed Google Scholar
Palladino VS, McNeill R, Reif A, Kittel-Schneider S. Genetic risk factors and gene-environment interactions in adult and childhood attention-deficit/hyperactivity disorder. Psychiatr Genet. 2019;29(3):63–78.
Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013;54(1):3–16.
Straub L, Bateman BT, Hernandez-Diaz S, York C, Zhu Y, Suarez EA, et al. Validity of claims-based algorithms to identify neurodevelopmental disorders in children. Pharmacoepidemiol Drug Saf. 2021;30(12):1635–42.
Gruschow SM, Yerys BE, Power TJ, Durbin DR, Curry AE. Validation of the use of electronic health records for classification of ADHD status. J Atten Disord. 2019;23(13):1647–55.
Classi PM, Le TK, Ward S, Johnston J. Patient characteristics, comorbidities, and medication use for children with ADHD with and without a co-occurring reading disorder: a retrospective cohort study. Child Adolesc Psychiatry Ment Health. 2011;5:38.
Shi Y, Hunter Guevara LR, Dykhoff HJ, Sangaralingham LR, Phelan S, Zaccariello MJ, et al. Racial disparities in diagnosis of attention-deficit/hyperactivity disorder in a US national birth cohort. JAMA Netw Open. 2021;4(3):e210321.
Schein J, Childress A, Adams J, Gagnon-Sanschagrin P, Maitland J, Qu W, et al. Treatment patterns among children and adolescents with attention-deficit/hyperactivity disorder in the United States - A retrospective claims analysis. BMC Psychiatry. 2022;22(1):555.
Article CAS PubMed PubMed Central Google Scholar
Download references
Acknowledgements
Medical writing assistance was provided by Flora Chik, PhD, MWC, an employee of Analysis Group, Inc., and funded by Otsuka Pharmaceutical Development & Commercialization, Inc.
Financial support for this research was provided by Otsuka Pharmaceutical Development & Commercialization, Inc. The study sponsor was involved in several aspects of the research, including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Author information
Authors and affiliations.
Otsuka Pharmaceutical Development & Commercialization, Inc, 508 Carnegie Center, Princeton, NJ, 08540, USA
Jeff Schein
Analysis Group, Inc, 1190 avenue des Canadiens-de-Montréal, Tour Deloitte, Suite 1500, Montréal, QC, H3B 0G7, Canada
Martin Cloutier, Marjolaine Gauthier-Loiselle, Rebecca Bungay, Emmanuelle Arpin & Annie Guerin
Center for Psychiatry and Behavioral Medicine, 7351 Prairie Falcon Rd STE 160, Las Vegas, NV, 89128, USA
Ann Childress
You can also search for this author in PubMed Google Scholar
Contributions
MC, MGL, RB, EA, and AG contributed to study conception and design, collection and assembly of data, and data analysis and interpretation. JS and AC contributed to study conception and design, data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Corresponding author
Correspondence to Rebecca Bungay .
Ethics declarations
Ethics approval and consent to participate.
The research was conducted according to the principles of the Declaration of Helsinki. Data analyzed in this study are de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA); therefore, no review by an institutional review board nor informed consent was required per Title 45 of CFR, Part 46.101(b)(4) [ 24 ].
Consent for publication
Not applicable.
Competing interests
JS is an employee of Otsuka Pharmaceutical Development & Commercialization, Inc. AC received research support from Allergan, Takeda/Shire, Emalex, Akili, Ironshore, Arbor, Aevi Genomic Medicine, Neos Therapeutics, Otsuka, Pfizer, Purdue, Rhodes, Sunovion, Tris, KemPharm, Supernus, and the U.S. Food and Drug Administration; was on the advisory board of Takeda/Shire, Akili, Arbor, Cingulate, Ironshore, Neos Therapeutics, Otsuka, Pfizer, Purdue, Adlon, Rhodes, Sunovion, Tris, Supernus, and Corium; received consulting fees from Arbor, Ironshore, Neos Therapeutics, Purdue, Rhodes, Sunovion, Tris, KemPharm, Supernus, Corium, Jazz, Tulex Pharma, and Lumos Pharma; received speaker fees from Takeda/Shire, Arbor, Ironshore, Neos Therapeutics, Pfizer, Tris, and Supernus; and received writing support from Takeda /Shire, Arbor, Ironshore, Neos Therapeutics, Pfizer, Purdue, Rhodes, Sunovion, and Tris. MC, MGL, RB, EA, and AG are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Otsuka Pharmaceutical Development & Commercialization, Inc.
Previous presentation
Part of the material in this manuscript was presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 2023 conference held on May 7–10, 2023, in Boston, MA, as a poster presentation.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Schein, J., Cloutier, M., Gauthier-Loiselle, M. et al. Risk factors associated with newly diagnosed attention-deficit/hyperactivity disorder in adults: a retrospective case-control study. BMC Psychiatry 23 , 870 (2023). https://doi.org/10.1186/s12888-023-05359-7
Download citation
Received : 05 May 2023
Accepted : 07 November 2023
Published : 23 November 2023
DOI : https://doi.org/10.1186/s12888-023-05359-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anxiety disorder
- Depressive disorder
- Comorbidity
- Risk factor
BMC Psychiatry
ISSN: 1471-244X
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
The present study analyzes a specific case of ADHD with predominantly inattentive presentation, covering monopolar electroencephalogram recording (brain mapping called MiniQ) and intervention via neurofeedback. ... Based on the information obtained over the evaluation of the case, and considering the prior diagnosis from her pediatric ...
We identified diagnostic accuracy studies in 12 databases published from 1980 through June 2023.STUDY SELECTION. Any ADHD tool evaluation for the diagnosis of ADHD, requiring a reference standard of a clinical diagnosis by a mental health specialist.DATA EXTRACTION. ... Joanna King, and Robyn Wheatley from the American Academy of Pediatrics ...
Only a very small number of studies (33 of 312) reported on outcomes at or beyond 12 months of follow-up (see Online Appendix). Many did not report on key outcomes of this review. Studies evaluating combined psychosocial and medication interventions, such as the multimodal treatment of ADHD study, 28 did not find sustained effects beyond 12 ...
up the case study, names those activities in brief without the full details and explicit information each client-family receives in why and how to implement the program. Go to www.handle.org for more information. The HANDLE® Institute 7 Mt. Lassen Drive, Suite B110 San Rafael, CA 94903 415-479-1800
Introduction. Attention deficit hyperactivity disorder (ADHD) is a frequent mental disorder with childhood onset and a worldwide prevalence of at least 2.8% ().It is characterized by the three core symptoms of attention deficit, hyperactivity, and impulsivity manifesting since childhood ().Adult ADHD is also commonly associated with different comorbidities (3, 4), particularly obsessive ...
Children with ADHD are 50% less likely to participate in sports than children with asthma (Tanden et al., 2019). I find that amazing. Kids with ADHD also have a higher incidence of screen time usage, and we know that that is always a challenge (Tanden et al., 2019). Childhood ADHD is also associated with obesity.
ETIOLOGY. ADHD is a disorder with multiple etiologies. Combinations of genetic, neurological, and environmental factors contribute to pathogenesis and its heterogeneous phenotype ().Evidence from family, twin, and adoption studies has suggested strongly that ADHD is a highly hereditary, polygenic disorder ().Gene variants predicting risk for ADHD are important for brain development, cell ...
Despite increased awareness, Attention-deficit hyperactivity disorder (ADHD) is a chronic condition that affects 8% to 12% of school-aged children and contributes significantly to academic and social impairment. There is currently broad agreement on evidence-based best practices of ADHD identification and diagnosis, therapeutic approach, and ...
A third author was consulted in case of disagreement. To extract the data from each study, a data collection table was created. ... it was found evidence of comparative effectiveness of NF and CogT for children with ADHD. As other studies point out, ... Pediatrics. 2018; 141:e20180094. doi: 10.1542/peds.2018-0094. [Google Scholar] 22. Razoki B ...
Clinicians selected by the American Academy of Pediatrics (AAP) will now use the evidence review to create updated clinical guidelines that inform best practices in ADHD care across the nation. "Parents, teachers and providers need evidence-based information about ADHD," Hempel said. "We included only the most robust studies in our review ...
Barkley in 2000 presented a commentary on the concerns with the multimodal treatment study of ADHD (Multimodal Treatment of ADHD). The MTA study was known as a landmark in the history of treatment research for ADHD. It evaluated the combined treatment approach for ADHD that included medication and behavioural approaches.
This paper presents a case study using monopolar electroencephalogram recording (brain mapping known as MiniQ) for subsequent use in an intervention with neurofeedback for a 10-year-old girl presenting predominantly inattentive ADHD. ... and considering the prior diagnosis from her pediatric neurologist, the subject presented ADHD with ...
Case Study 1 - Jack Jack is a 7 year old male Grade 1 student who lives in Toronto with his parents. He is the only child to two parents, both of whom have completed post-graduate education. There is an extended family history of Attention Deficit/Hyperactivity Disorder (ADHD), mental health concerns as well as academic excellence.
A total of 303 eligible children (69% male; 46% Black) were randomly assigned, and 273 (90%) completed the study. During the 9-month study, parents in the care management + portal arm communicated inconsistently with care managers (mean 2.2; range 0-6) but similarly used the portal (mean 2.3 vs 2.2) as parents in the portal alone arm.
This case study illustrates a behavioral treatment of "Peter," a 4-year-old male with attention deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder. Multiple evidence-based treatment procedures were implemented, affording the opportunity to explore issues common to the clinical application of empirically supported ...
Case Summary. Chronic ear infections are often a symptom of food allergies. While the research is not exhaustive, studies have found consistent correlations between cow's milk allergy and middle ear infections in children (Bhombal 2006, Juntti 1999). Removing dairy products often results in a reduction or elimination of ear infections.
ADHD increases the risk of substance misuse disorders 1.5-fold (2.4-fold for smoking) and problematic media use 9.3-fold in adolescence 55 56 and increases the risk of becoming obese 1.23-fold for adolescent girls. 57 58 59 It is also associated with different forms of dysregulated eating in children and adolescents.
Despite increased awareness, Attention-deficit hyperactivity disorder (ADHD) is a chronic condition that affects 8% to 12% of school-aged children and contributes significantly to academic and social impairment. There is currently broad agreement on evidence-based best practices of ADHD identification and diagnosis, therapeutic approach, and ...
Williams J., Sharp GB, Lange B., et al. Differentiating between seizures and attention deficit hyperactivity disorder (ADHD) in a pediatric population. Clin Pediatr. 2002;41:565-568. Google Scholar
Pediatric patients presenting with Attention-Deficit Hyperactivity Disorder (ADHD or ADD) are a common occurrence in naturopathic practice. While the state of the research is evolving, there is ...
The ADHD case patient group included 18,756 participants aged 5 to 18 years (mean = 8.3 years, SD = 2.6 years), and the control group included 37,512 in the matching of 1:2. ... The present study showed that pediatric ADHD is associated with significantly higher rates of all types of pediatric infectious diseases, use of all kinds of anti ...
The Avon Longitudinal Study of Parents and Children found that birthing parent's ADHD polygenic risk was associated with acetaminophen use in pregnancy. 23 The Norwegian Mother, Father, and Child Cohort Study (MoBa) found that birthing parent's ADHD polygenic risk was associated with pregnancy pain, migraine, and use of pain medications ...
The ADHD case patient group included 18,756 participants aged 5 to 18 years (mean = 8.3 years, SD = 2.6 years), and the control group included 37,512 in the matching of 1:2. ... The present study showed that pediatric ADHD is associated with significantly higher rates of all types of pediatric infectious diseases, use of all kinds of anti ...
Pediatric Case Study: Child with ADHD. Course: #4577 Level: Intermediate 1 Hour 4060 Reviews. This course focuses on a case study for a 9-year-old male with ADHD experiencing occupational challenges in education, ADL, IADL, and social participation. Course created on January 30, 2020. Pediatrics Early Intervention and School-Based. Preview Exam.
Yet, most of these studies have examined a single or a few factors, and many were conducted in pediatric ADHD populations primarily outside of the US. ... This large retrospective case-control study found that mental health comorbidities and related treatments and care are significantly associated with newly diagnosed ADHD in US adults. The ...
Case Study Corner: Follow These Guidelines for Successful ADHD Reporting Outcomes. Published on Wed Oct 25, 2017. Pay attention to payer rules for testing, treatment options. It's a common problem that seems to be growing. The Centers for Disease Control (CDC) estimates that it affects 10 percent of children between the ages of 4 and17.