- You are here:
- American Chemical Society
- Discover Chemistry

Recent advancements in water treatment
For immediate release, acs news service weekly presspac: january 19, 2022.
Generating clean, safe water is becoming increasingly difficult. Water sources themselves can be contaminated, but in addition, some purification methods can cause unintended harmful byproducts to form. And not all treatment processes are created equal with regard to their ability to remove impurities or pollutants. Below are some recent papers published in ACS journals that report insights into how well water treatment methods work and the quality of the resulting water. Reporters can request free access to these papers by emailing newsroom@acs.org .
“Drivers of Disinfection Byproduct Cytotoxicity in U.S. Drinking Water: Should Other DBPs Be Considered for Regulation?” Environmental Science & Technology Dec.15, 2021
In this paper, researchers surveyed both conventional and advanced disinfection processes in the U.S., testing the quality of their drinking waters. Treatment plants with advanced removal technologies, such as activated carbon, formed fewer types and lower levels of harmful disinfection byproducts (known as DBPs) in their water. Based on the prevalence and cytotoxicity of haloacetonitriles and iodoacetic acids within some of the treated waters, the researchers recommend that these two groups be considered when forming future water quality regulations.
“Complete System to Generate Clean Water from a Contaminated Water Body by a Handmade Flower-like Light Absorber” ACS Omega Dec. 9, 2021 As a step toward a low-cost water purification technology, researchers crocheted a coated black yarn into a flower-like pattern. When the flower was placed in dirty or salty water, the water wicked up the yarn. Sunlight caused the water to evaporate, leaving the contaminants in the yarn, and a clean vapor condensed and was collected. People in rural locations could easily make this material for desalination or cleaning polluted water, the researchers say.
“Data Analytics Determines Co-occurrence of Odorants in Raw Water and Evaluates Drinking Water Treatment Removal Strategies” Environmental Science & Technology Dec. 2, 2021
Sometimes drinking water smells foul or “off,” even after treatment. In this first-of-its-kind study, researchers identified the major odorants in raw water. They also report that treatment plants using a combination of ozonation and activated carbon remove more of the odor compounds responsible for the stink compared to a conventional process. However, both methods generated some odorants not originally present in the water.
“Self-Powered Water Flow-Triggered Piezocatalytic Generation of Reactive Oxygen Species for Water Purification in Simulated Water Drainage” ACS ES&T Engineering Nov. 23, 2021
Here, researchers harvested energy from the movement of water to break down chemical contaminants. As microscopic sheets of molybdenum disulfide (MoS2) swirled inside a spiral tube filled with dirty water, the MoS2 particles generated electric charges. The charges reacted with water and created reactive oxygen species, which decomposed pollutant compounds, including benzotriazole and antibiotics. The researchers say these self-powered catalysts are a “green” energy resource for water purification.
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News . ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org .
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Media Contact
ACS Newsroom newsroom@acs.org

Discover Chemistry —Menu
- News Releases
- ACS in the News
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Research on drinking water purification technologies for household use by reducing total dissolved solids (TDS)
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Redlands East Valley High School, Redlands, California, United States of America
- Bill B. Wang

- Published: September 28, 2021
- https://doi.org/10.1371/journal.pone.0257865
- Reader Comments
This study, based in San Bernardino County, Southern California, collected and examined tap water samples within the area to explore the feasibility of adopting non-industrial equipment and methods to reduce water hardness and total dissolved solids(TDS). We investigated how water quality could be improved by utilizing water boiling, activated carbon and sodium bicarbonate additives, as well as electrolysis methods. The results show that heating is effective at lower temperatures rather than long boils, as none of the boiling tests were lower than the original value. Activated carbon is unable to lower TDS, because it is unable to bind to any impurities present in the water. This resulted in an overall TDS increase of 3.5%. However, adding small amounts of sodium bicarbonate(NaHCO 3 ) will further eliminate water hardness by reacting with magnesium ions and improve taste, while increasing the pH. When added to room temperature tap water, there is a continuous increase in TDS of 24.8% at the 30 mg/L mark. The new findings presented in this study showed that electrolysis was the most successful method in eliminating TDS, showing an inverse proportion where an increasing electrical current and duration of electrical lowers more amounts of solids. This method created a maximum decrease in TDS by a maximum of 22.7%, with 3 tests resulting in 15.3–16.6% decreases. Furthermore, when water is heated to a temperature around 50°C (122°F), a decrease in TDS of around 16% was also shown. The reduction of these solids will help lower water hardness and improve the taste of tap water. These results will help direct residents to drink more tap water rather than bottled water with similar taste and health benefits for a cheaper price as well as a reduction on plastic usage.
Citation: Wang BB (2021) Research on drinking water purification technologies for household use by reducing total dissolved solids (TDS). PLoS ONE 16(9): e0257865. https://doi.org/10.1371/journal.pone.0257865
Editor: Mahendra Singh Dhaka, Mohanlal Sukhadia University, INDIA
Received: June 22, 2021; Accepted: September 14, 2021; Published: September 28, 2021
Copyright: © 2021 Bill B. Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: The author received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The concentration of total dissolved solids(TDS) present in water is one of the most significant factors in giving water taste and also provides important ions such as calcium, magnesium, potassium, and sodium [ 1 – 3 ]. However, water with high TDS measurements usually indicates contamination by human activities, such as soil and agricultural runoff caused by irrigation, unregulated animal grazing and wildlife impacts, environmentally damaging farming methods such as slash and burn agriculture, and the overuse of nitrate-based fertilizer [ 4 , 5 ], etc. Around tourist areas as well as state parks, these factors will slowly add up over time and influence the water sources nearby [ 5 ]. Water that flows through natural springs and waterways with high concentrations of organic salts within minerals and rocks, or groundwater that originates from wells with high salt concentration will also result in higher particle measurements [ 6 ].
Water sources can be contaminated by substances and ions such as nitrate, lead, arsenic, and copper [ 7 , 8 ] and may cause many health problems related to heavy metal consumption and poisoning. Water reservoirs and treatments plants that do not consider water contamination by motor vehicles, as well as locations that struggle to provide the necessary components required for water treatment will be more prone to indirect contamination [ 9 – 11 ]. Many plants are effective in ensuring the quality and reduction of these contaminants, but often leave out the secondary considerations, The United States Environmental Protection Agency(US EPA)’s secondary regulations recommend that TDS should be below 500 mg/L [ 2 ], which is also supported by the World Health Organization(WHO) recommendation of below 600 mg/L and an absolute maximum of less than 1,000 mg/L [ 3 ]. These substances also form calcium or magnesium scales within water boilers, heaters, and pipes, causing excess buildup and drain problems, and nitrate ions may pose a risk to human health by risking the formation of N -nitroso compounds(NOC) and less public knowledge about such substances [ 12 – 15 ]. Nitrates can pose a non-carcinogenic threat to different communities, but continue to slip past water treatment standards [ 15 ]. Furthermore, most people do not tolerate or prefer water with high hardness or chlorine additives [ 16 ], as the taste changes tremendously and becomes unpreferable. Even so, TDS levels are not accounted for in mandatory water regulations, because the essential removal of harmful toxins and heavy metals is what matters the most in water safety. Some companies indicate risks in certain ions and alkali metals, showing how water hardness is mostly disregarded and is not as well treated as commercial water bottling companies [ 17 , 18 ].
In Southern California, water quality is not as well maintained than the northern counties as most treatment plants in violation of a regulation or standard are located in Central-Southern California [ 19 ], with southern counties having the largest number of people affected [ 20 ]. This study is focused on the Redlands area, which has had no state code violations within the last decade [ 21 ]. A previous study has analyzed TDS concentrations throughout the Santa Ana Basin, and found concentrations ranging from 190–600 ppm as treated wastewater and samples obtained from mountain sites, taking into account the urban runoff and untreated groundwater as reasons for elevated levels of TDS but providing no solution in helping reduce TDS [ 22 ]. Also, samples have not been taken directly through home water supplies, where the consumer is most affected. Other water quality studies in this region have been focused on the elimination of perchlorates in soil and groundwater and distribution of nitrates, but such research on chemicals have ceased for the last decade, demonstrated by safe levels of perchlorates and nitrates in water reports [ 23 , 24 ]. In addition to these studies, despite the improving quality of the local water treatment process, people prefer bottled water instead of tap water because of the taste and hardness of tap water [ 25 ]. Although water quality tests are taken and documented regularly, the taste of the water is not a factor to be accounted for in city water supplies, and neither is the residue left behind after boiling water. The residue can build up over time and cause appliance damage or clogs in drainage pipes.
This study will build upon previous analyses of TDS studies and attempt to raise new solutions to help develop a more efficient method in reducing local TDS levels, as well as compare current measurements to previous analyses to determine the magnitude to which local treatment plants have improved and regulated its treatment processes.
Several methods that lower TDS are reviewed: boiling and heating tap water with and without NaHCO₃, absorption by food-grade activated carbon [ 26 , 27 ], and battery-powered electrolysis [ 28 – 30 ]. By obtaining water samples and determining the difference in TDS before and after the listed experiments, we can determine the effectiveness of lowering TDS. The results of this study will provide options for residents and water treatment plants to find ways to maintain the general taste of the tap water, but also preserve the lifespan of accessories and pipelines. By determining a better way to lower TDS and treat water hardness, water standards can be updated to include TDS levels as a mandatory measurement.
Materials and methods
All experiments utilized tap water sourced from Redlands homes. This water is partially supplied from the Mill Creek (Henry Tate) and Santa Ana (Hinckley) Water Sheds/Treatment Plants, as well as local groundwater pumps. Water sampling and sourcing were done at relatively stable temperatures of 26.9°C (80.42°F) through tap water supplies. The average TDS was measured at 159 ppm, which is slightly lower than the reported 175 ppm by the City of Redlands. Permission is obtained by the author from the San Bernardino Municipal Water Department website to permit the testing procedures and the usage of private water treatment devices for the purpose of lowering water hardness and improving taste and odor. The turbidity was reported as 0.03 Nephelometric Turbidity Units (NTU) post-treatment. Residual nitrate measured at 2.3mg/L in groundwater before treatment and 0.2 mg/L after treatment and perchlorate measured at 0.9 μg/L before treatment, barely staying below the standard of 1 μg/L; it was not detected within post-treatment water. Lead content was not detected at all, while copper was detected at 0.15 mg/L.
For each test, all procedures were done indoors under controlled temperatures, and 20 L of fresh water was retrieved before each test. Water samples were taken before each experimental set and measured for TDS and temperature, and all equipment were cleaned thoroughly with purified water before and after each measurement. TDS consists of inorganic salts and organic material present in solution, and consists mostly of calcium, magnesium, sodium, potassium, carbonate, chloride, nitrate, and sulfate ions. These ions can be drawn out by leaving the water to settle, or binding to added ions and purified by directly separating the water and ions. Equipment include a 50 L container, 1 L beakers for water, a graduated cylinder, a stir rod, a measuring spoon, tweezers, a scale, purified water, and a TDS meter. A standard TDS meter is used, operated by measuring the conductivity of the total amount of ionized solids in the water, and is also cleaned in the same manner as aforementioned equipment. The instrument is also calibrated by 3 pH solutions prior to testing.All results were recorded for and then compiled for graphing and analysis.
Heating/Boiling water for various lengths of time
The heating method was selected because heat is able to break down calcium bicarbonate into calcium carbonate ions that are able to settle to the bottom of the sample. Four flasks of 1 L of tap water were each heated to 40°C, 50°C, 60°C, and 80°C (104–176°F) and observed using a laser thermometer. The heated water was then left to cool and measurements were made using a TDS meter at the 5, 10, 20, 30, and 60-minute marks.
For the boiling experiments, five flasks of 1 L of tap water were heated to boil at 100°C (212°F). Each flask, which was labeled corresponding to its boiling duration, was marked with 2, 4, 6, 10, and 20 minutes. Each flask was boiled for its designated time, left to cool under open air, and measurements were made using a TDS meter at the 5, 10, 20, 30, 60, and 120-minute marks. The reason that the boiling experiment was extended to 120 minutes was to allow the water to cool down to room temperature.
Activated carbon as a water purification additive
This test was performed to see if food-grade, powdered activated carbon had any possibility of binding with and settling out residual particles. Activated carbon was measured using a milligram scale and separated into batches of 1, 2, 4, 5, 10, 30, and 50 mg. Each batch of the activated carbon were added to a separate flask of water and stirred for five minutes, and finally left to settle for another five minutes. TDS measurements were recorded after the water settled.
Baking soda as a water purification additive
To lower scale error and increase experimental accuracy, a concentration of 200 mg/L NaHCO₃ solution was made with purified water and pure NaHCO₃. For each part, an initial TDS measurement was taken before each experiment.
In separate flasks of 1 L tap water, each labeled 1, 2, 4, 5, 10, and 30 mg of NaHCO 3 , a batch was added to each flask appropriately and stirred for 5 minutes to ensure that everything dissolved. Measurements were taken after the water was left to settle for another 5 minutes for any TDS to settle.
Next, 6 flasks of 1 L tap water were labeled, with 5 mg (25 mL solution) of NaHCO₃ added to three flasks and 10 mg (50 mL solution) of NaHCO₃ added to the remaining three. One flask from each concentration of NaHCO₃ was boiled for 2 mins., 4 mins., or 6 mins., and then left to cool. A TDS measurement was taken at the 5, 10, 20, 30, 60, and 120-minute marks after removal from heat.
Electrolysis under low voltages
This test was performed because the ionization of the TDS could be manipulated with electricity to isolate an area of water with lower TDS. For this test, two 10cm long graphite pieces were connected via copper wiring to a group of batteries, with each end of the graphite pieces submerged in a beaker of tap water, ~3 cm apart.
Using groups of 1.5 V double-A batteries, 4 beakers with 40mL of tap water were each treated with either 7.5, 9.0, 10.5, and 12.0 V of current. Electrolysis was observed to be present by the bubbling of the water each test, and measurements were taken at the 3, 5, 7, and 10 minute marks.
Results/Discussion
Heating water to various temperatures until the boiling point.
The goal for this test was to use heat to reduce the amount of dissolved oxygen and carbon dioxide within the water, as shown by this chemical equation: Heat: Ca(HCO 3 ) 2 → CaCO 3 ↓ + H 2 O + CO 2 ↑.
This would decompose ions of calcium bicarbonate down into calcium carbonate and water and carbon dioxide byproducts.
Patterns and trends in decreasing temperatures.
The following trend lines are based on a dataset of changes in temperature obtained from the test results and graphed as Fig 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0257865.g001
To predict the precise temperature measurements of the tap water at 26.9°C, calculations were made based on Fig 1 . The fitting equations are in the format, y = a.e bx . The values for the fitting coefficients a and b, and correlation coefficient R 2 are listed in Table 1 as column a, b and R 2 . The calculated values and the target temperature are listed in Table 1 .
https://doi.org/10.1371/journal.pone.0257865.t001
Fig 2 was obtained by compiling TDS results with different temperatures and times.
https://doi.org/10.1371/journal.pone.0257865.g002
The fitting equations for Fig 2 are also in the format, y = a.e bx . The fitting coefficients a and b, and correlation coefficient R 2 values are listed in Table 2 . Based on the fitting curves in Fig 2 and the duration to the target temperature in Table 1 , We calculated the TDS at 26.9°C as listed in column calculated TDS in Table 2 based on the values we reported on Fig 2 .
https://doi.org/10.1371/journal.pone.0257865.t002
Based on the heating temperature and the calculated TDS with the same target water temperature, we obtained the following heating temperature vs TDS removal trend line and its corresponding fitting curve in Table 2 .
In Fig 1 , a trend in the rate of cooling is seen, where a higher heating temperature creates a steeper curve. During the first five minutes of cooling, the water cools quicker as the absorbed heat is quickly released into the surrounding environment. By the 10-minute mark, the water begins to cool in a linear rate of change. One detail to note is that the 100°C water cools quicker than the 80°C and eventually cools even faster than the 60°C graph. Table 1 supports this observation as the duration to target temperature begins to decrease from a maximum point of 94.8 mins to 80.95 mins after the 80°C mark.
As shown in Fig 2 , all TDS values decrease as the temperature starts to cool to room temperature, demonstrating a proportional relationship where a lower temperature shows lower TDS. This can partially be explained by the ions settling in the flasks. Visible particles can also be observed during experimentation as small white masses on the bottom, as well as a thin ring that forms where the edge of the water contacts the flask. When the water is heated to 40°C and cooled, a 3.8% decrease in TDS is observed. When 50°C is reached, the TDS drops at its fastest rate from an initial value of 202 ppm to 160 ppm after 60 minutes of settling and cooling. The TDS measurements in these experiements reach a maximum of 204 ppm at the 60°C mark. However, an interesting phenomenon to point out is that the water does not hit a new maximum at 100°C. meaning that TDS reaches a plateau at 60°C. Also, the rate of decrease begins to slow down after 20 minutes, showing that an unknown factor is affecting the rate of decrease. It is also hypothesized that the slight increase in TDS between the 5–20 minute range is caused by a disturbance in the settling of the water, where the temperature starts to decrease at a more gradual and constant rate. The unstable and easy formation of CaCO 3 scaling has also been the subject of a study of antiscaling methods, which also supports the result that temperature is a significant influence for scale formation [ 12 ].
In Table 2 , calculations for TDS and the time it takes for each test to cool were made. Using the data, it is determined that the test with 50°C water decreased the most by 16% from the initial measurement of 159ppm. This means that it is most effective when water is heated between temperatures of 40–60°C when it comes to lowering TDS, with a difference of ~7–16%. When water is heated to temperatures greater than 80°C, the water begins to evaporate, increasing the concentration of the ions, causing the TDS to increase substantially when cooled to room temperature.
Finally, in Fig 3 , a line of best fit of function f(x) = -0.0007x 3 + 0.1641x 2 –10.962x + 369.36 is used with R 2 = 0.9341. Using this function, the local minimum of the graph would be reached at 48.4°C.
https://doi.org/10.1371/journal.pone.0257865.g003
This data shows that heating water at low temperatures (i.e. 40–50°C) may be more beneficial than heating water to higher temperatures. This study segment has not been presented in any section within the United States EPA Report on water management for different residual particles/substances. However, warmer water temperatures are more prone to microorganism growth and algal blooms, requiring more intensive treatment in other areas such as chlorine, ozone, and ultraviolet disinfection.
Using the specific heat capacity equation, we can also determine the amount of energy and voltage needed to heat 1 L of water up to 50°C: Q = mcΔT, where c, the specific heat capacity of water, is 4.186 J/g°C, ΔT, the change in temperature from the experimental maximum to room temperature, is 30°C, and m, the mass of the water, is 1000 g. This means that the amount of energy required will be 125580 J, which is 0.035 kWh or 2.1 kW.
After taking all of the different measurements obtained during TDS testing, and compiling the data onto this plot, Fig 4 is created with a corresponding line of best fit:
https://doi.org/10.1371/journal.pone.0257865.g004
In Fig 4 , it can be observed that the relationship between the temperature of the water and its relative TDS value is a downwards facing parabolic graph. As the temperature increases, the TDS begins to decrease after the steep incline at 50–60°C. The line of best fit is represented by the function f(x) = -0.0142x 2 + 2.258x + 105.84. R 2 = 0.6781. Because the R 2 value is less than expected, factors such as the time spent settling and the reaction rate of the ions should be considered. To determine the specifics within this experiment, deeper research and prolonged studies with more highly accurate analyses must be utilized to solve this problem.
Boiling water for various amounts of time
Trend of boiling duration and rate of cooling..
Using the same methods to create the figures and tables for the previous section, Fig 5 depicts how the duration of time spent boiling water affects how fast the water cools.
https://doi.org/10.1371/journal.pone.0257865.g005
As seen in Fig 5 , within the first 10 minutes of the cooling time, the five different graphs are entwined with each other, with all lines following a similar pattern. However, the graph showing 20 minutes of boiling is much steeper than the other graphs, showing a faster rate of cooling. This data continues to support a previous claim in Fig 2 , as this is most likely represented by a relationship a longer the boil creates a faster cooling curve. This also shows that the first 5 minutes of cooling have the largest deviance compared to any other time frame.
The cooling pattern is hypothesized by possible changes in the orderly structure of the hydrogen bonds in the water molecules, or the decreased heat capacity of water due to the increasing concentration of TDS.
Effect on TDS as boiling duration increases.
In Fig 6 , all lines except for the 20-minute line are clustered in the bottom area of the graph. By excluding the last measurement temporarily due to it being an outlier, we have observed that the difference between the initial and final TDS value of each test decreases.
https://doi.org/10.1371/journal.pone.0257865.g006
Despite following a similar trend of an increase in TDS at the start of the tests and a slow decrease overtime, this experiment had an interesting result, with the final test measuring nearly twice the amount of particles compared to any previous tests at 310 ppm, as shown in Fig 6 . It is confirmed that the long boiling time caused a significant amount of water to evaporate, causing the minerals to be more concentrated, thus resulting in a 300 ppm reading. Fig 6 follows the same trend as Fig 2 , except the TDS reading veers away when the boiling duration reaches 20 minutes. Also, with the long duration of heating, the water has developed an unfavorable taste from intense concentrations of CaCO₃. This also causes a buildup of a thin crust of CaCO₃ and other impurities around the container that is difficult to remove entirely. This finding is in accordance with the introductory statement of hot boiling water causing mineral buildups within pipes and appliances [ 9 ]. A TDS reading of 300ppm is still well below federal secondary standards of TDS, and can still even be compared to bottled water, in which companies may fluctuate and contain 335ppm within their water [ 1 , 2 ].
This experiment continues to stupport that the cooling rate of the water increases as the time spent boiling increases. Based on this test, a prediction can be made in which an increased concentration of dissolved solids lowers the total specific heat capacity of the sample, as the total volume of water decreases. This means that a method can be derived to measure TDS using the heat capacity of a tap water mixture and volume, in addition to current methods of using the electrical conductivity of aqueous ions.
Adding food-grade activated carbon to untreated tap water
Fig 7 presents a line graph with little to no change in TDS, with an initial spike from 157 to 163 ppm. The insoluble carbon remains in the water and shows no benefit.
https://doi.org/10.1371/journal.pone.0257865.g007
The food-grade activated carbon proved no benefit to removing TDS from tap water, and instead added around 5–7 ppm extra, which settled down to around +4 ppm at 120 minutes. The carbon, which is not 100% pure from inorganic compounds and materials present in the carbon, can dissolve into the water, adding to the existing concentration of TDS. Furthermore, household tap water has already been treated in processing facilities using a variety of filters, including carbon, so household charcoal filters are not effective in further reducing dissolved solids [ 18 ].
Adding sodium bicarbonate solution to boiled tap water
As seen in Fig 8 , after adding 1 mg of NaHCO 3 in, the TDS rises to 161 ppm, showing a minuscule increase. When 4 mg was added, the TDS drops down to 158 ppm. Then, when 5 mg was added, a sudden spike to 172 ppm was observed. This means that NaHCO 3 is able to ionize some Ca 2+ and Mg 2+ ions, but also adds Na + back into the water. This also means that adding NaHCO 3 has little to no effect on TDS, with 4mg being the upper limit of effectiveness.
https://doi.org/10.1371/journal.pone.0257865.g008
To examine whether or not the temperature plays a role in the effectiveness in adding NaHCO 3 , a boiling experiment was performed, and the data is graphed in Fig 9 .
https://doi.org/10.1371/journal.pone.0257865.g009
Fig 9 presents the relationship between the amount of common baking soda(NaHCO₃) added, the boiling time involved, and the resulting TDS measurements. After boiling each flask for designated amounts of time, the results showed a downward trend line from a spike but does not reach a TDS value significantly lower than the initial sample. It is apparent that the NaHCO₃ has not lowered the TDS of the boiling water, but instead adds smaller quantities of ions, raising the final value. This additive does not contribute to the lowering of the hardness of the tap water. However, tests boiled with 5 mg/L of baking soda maintained a downward pattern as the water was boiled for an increasing amount of time, compared to the seemingly random graphs of boiling with 10 mg/L.
In some households, however, people often add NaHCO₃ to increase the pH for taste and health benefits. However, as shown in the test results, it is not an effective way of reducing TDS levels in the water [ 10 , 16 ], but instead raises the pH, determined by the concentration added. Even under boiling conditions, the water continues to follow the trend of high growth in TDS, of +25–43 ppm right after boiling and the slow drop in TDS (but maintaining a high concentration) as the particles settle to the bottom.
Utilizing the experimental results, we can summarize that after adding small batches of NaHCO3 and waiting up to 5 minutes will reduce water hardness making it less prone to crystallizing within household appliances such as water brewers. Also, this process raises the pH, which is used more within commercial water companies. However, the cost comes at increasing TDS.
Using electrolysis to treat TDS in tap water
Different voltages were passed through the water to observe the change in TDS overtime, with the data being compiled as Fig 10 .
https://doi.org/10.1371/journal.pone.0257865.g010
The process of electrolysis in this experiment was not to and directly remove the existing TDS, but to separate the water sample into three different areas: the anode, cathode, and an area of clean water between the two nodes [ 19 ]. The anions in the water such as OH - , SO 4 2- , HCO 3 - move to the anode, while the cations such as H + , Ca 2+ 、Mg 2+ 、Na + move to the cathode. The middle area would then be left as an area that is more deprived of such ions, with Fig 10 proving this.
As shown in Fig 10 , electrolysis is effective in lower the TDS within tap water. Despite the lines being extremely tangled and unpredictable, the general trend was a larger decrease with a longer duration of time. At 10 minutes, all lines except 10.5 V are approaching the same value, meaning that the deviation was most likely caused by disturbances to the water during measurement from the low volume of water. With each different voltage test, a decrease of 12.7% for 6.0 V, 14.9% for 9.0 V, 22.7% for 10.5 V. and 19.5% for 12.0 V respectfully were observed. In the treatment of wastewate leachate, a study has shown that with 90 minutes of electrical treatment, 34.58% of TDS content were removed, supporting the effectiveness of electricity and its usage in wastewater treatment [ 29 ].
This experiment concludes that electrolysis is effective in lowering TDS, with the possibility to improve this process by further experimentation, development of a water cleaning system utilizing this cathode-anode setup to process water. This system would be a more specific and limited version of a reverse osmosis system by taking away ions through attraction, rather than a filter.
The Southern Californian tap water supply maintains TDS values below the federal regulations. However, crystalline scale buildup in household appliances is a major issue as it is hard to clean and eliminate. To easily improve the taste and quality of tap water at home as well as eliminating the formation of scales, the following methods were demonstrated as viable:
- By heating water to around 50°C (122°F), TDS and water hardness will decrease the most. Also, the boiling process is effective in killing microorganisms and removing contaminants. This process cannot surpass 10 minutes, as the concentration of the ions in the water is too high, which poses human health risks if consumed. These, along with activated carbon and NaHCO₃ additives, are inefficient methods that have minimal effects for lowering TDS.
- Electrolysis is one of the most effective methods of eliminating TDS. Experiments have proven that increased current and duration of time helps lower TDS. However, this method has yet to be implemented into conventional commercial water filtration systems.
Also, some observations made in these experiments could not be explained, and require further research and experimentation to resolve these problems. The first observation is that TDS and increasing water temperature maintain a parabolic relationship, with a maximum being reached at 80°C, followed by a gradual decrease. The second observation is that when water is boiled for an increased duration of time, the rate of cooling also increases.
This experiment utilized non-professional scientific equipment which are prone to mistakes and less precise. These results may deviate from professionally derived data, and will require further study using more advanced equipment to support these findings.
Acknowledgments
The author thanks Tsinghua University Professor and PLOS ONE editor Dr. Huan Li for assisting in experimental setups as well as data processing and treatment. The author also thanks Redlands East Valley High School’s Dr. Melissa Cartagena for her experimental guidance, and Tsinghua University Professor Dr. Cheng Yang for proofreading the manuscript.
- View Article
- Google Scholar
- 2. United States Environmental Protection Agency. 2018 Edition of the Drinking Water Standards and Health Advisories Tables (EPA 822-F-18-001). US EPA, Washington D.C., USA, 2018; pp. 9–19.
- 3. World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum. WHO, Geneva, Switzerland, 2017; pp. 7, 219–230, 423.
- PubMed/NCBI
- 6. Chloride, Salinity, and Dissolved Solids. 2019 Mar 1, [cited on 20 September 2020] Available from: https://www.usgs.gov/mission-areas/water-resources/science/chloride-salinity-and-dissolved-solids?qt-science_center_objects=0#qt-science_center_objects .
- 13. Shoukat, Ammara, Hussain, M., Shoukat, Asra. Effects of Temperature on Total dissolved Solid in water. Water Quality Study Conference, Mehran University Sindh, Pakistan, February 2020.
- 17. United States Environmental Protection Agency. Drinking Water Treatment Plant Residuals Management—Technical Report: Summary of Residuals Generation, Treatment, and Disposal at Large Community Water Systems. US EPA, Washington D.C., USA, 2011; pp. 177–182.
- 19. Exceedance and Compliance Status of Public Water Systems. 2012 Feb 1, [cited 22 September 2020] Available from: https://www.arcgis.com/apps/MapJournal/index.html?appid=143794cd74e344a29eb8b96190f4658b# .
- 20. 2019 Water Quality Status Report California. California Water Boards, 2019 July 1, [cited 22 September 2020] Available from: https://gispublic.waterboards.ca.gov/portal/apps/MapJournal/index.html?appid=6cde29ac0afc4d55b0fdaaae6bfc1aa4 .
- 21. City of Redlands—Water Quality Consumer Confidence Reports. City of Redlands, 2010–2020, [cited 23 September 2020] Available from: https://www.cityofredlands.org/post/water-quality .
- 25. Consumers’ Preference For Bottled Water Is Growing And They Want It Available Wherever Drinks Are Sold. 2019 Jan 8, [cited 23 September 2020] Available from: https://www.bottledwater.org/consumers’-preference-bottled-water-growing-and-they-want-it-available-wherever-drinks-are-sold .
Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.
Purpose-led Publishing is a coalition of three not-for-profit publishers in the field of physical sciences: AIP Publishing, the American Physical Society and IOP Publishing.
Together, as publishers that will always put purpose above profit, we have defined a set of industry standards that underpin high-quality, ethical scholarly communications.
We are proudly declaring that science is our only shareholder.
Nanotechnology: an approach for water purification-review
Rama Sharma 1
Published under licence by IOP Publishing Ltd IOP Conference Series: Materials Science and Engineering , Volume 1116 , International Conference on Futuristic and Sustainable Aspects in Engineering and Technology (FSAET 2020) 18th-19th December 2020, Mathura, India Citation Rama Sharma 2021 IOP Conf. Ser.: Mater. Sci. Eng. 1116 012007 DOI 10.1088/1757-899X/1116/1/012007
Article metrics
1231 Total downloads

Share this article
Author e-mails.
Author affiliations
1 Department of Biotechnology, GLA University, Mathura
Buy this article in print
Clean water is the global need and need of life for all the human kinds. But the clean water resources are being contaminated in present time. Nanotechnology is an easy and practical approach to clean waste water by using different methods. Different types of bacteria, toxic chemicals like arsenic, mercury etc., and sediments can be removed by using nanotechnology. Nanomaterial based devices are being used for water purification. Nano filtration method has advantages over other conventional method as low pressure is required to pass the water through filters and these filters can be cleaned easily by back flushing. Smooth interior of carbon nanotubes make them convenient for the removal of almost all types of water contaminants. Because of larger surface area nanostructured materials have advantages over conventional micro structured materials.
Export citation and abstract BibTeX RIS
Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence . Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 20 March 2008
Science and technology for water purification in the coming decades
- Mark A. Shannon 1 , 4 ,
- Paul W. Bohn 1 , 2 ,
- Menachem Elimelech 1 , 3 ,
- John G. Georgiadis 1 , 4 ,
- Benito J. Mariñas 1 , 5 &
- Anne M. Mayes 1 , 6
Nature volume 452 , pages 301–310 ( 2008 ) Cite this article
69k Accesses
6522 Citations
244 Altmetric
Metrics details
One of the most pervasive problems afflicting people throughout the world is inadequate access to clean water and sanitation. Problems with water are expected to grow worse in the coming decades, with water scarcity occurring globally, even in regions currently considered water-rich. Addressing these problems calls out for a tremendous amount of research to be conducted to identify robust new methods of purifying water at lower cost and with less energy, while at the same time minimizing the use of chemicals and impact on the environment. Here we highlight some of the science and technology being developed to improve the disinfection and decontamination of water, as well as efforts to increase water supplies through the safe re-use of wastewater and efficient desalination of sea and brackish water.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
A critical review of point-of-use drinking water treatment in the United States
Jishan Wu, Miao Cao, … Eric M. V. Hoek
Recent developments in hazardous pollutants removal from wastewater and water reuse within a circular economy
Menatalla Ahmed, Musthafa O. Mavukkandy, … Shadi W. Hasan
Electrolysis of low-grade and saline surface water
Wenming Tong, Mark Forster, … Pau Farràs
Montgomery, M. A. & Elimelech, M. Water and sanitation in developing countries: including health in the equation. Environ. Sci. Technol. 41 , 17–24 (2007)
Article ADS PubMed Google Scholar
Lima, A. A. M. et al. Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern brazil. J. Infect. Dis. 181 , 1643–1651 (2000)
Article CAS PubMed Google Scholar
Behrman, J. R., Alderman, H. & Hoddinott, J. Hunger and malnutrition. in Copenhagen Consensus—Challenges and Opportunities (London, 2004) OCLC 57489365 (London School of Hygiene and Tropical Medicine, 2004); 〈 http://www.copenhagenconsensus.com/Files/Filer/CC/Papers/ Hunger%5Fand%5FMalnutrition%5F070504.pdf 〉
Google Scholar
Singh, P. & Bengtson, L. The impact of warmer climate on melt and evaporation for the rainfed, snowfed and glacierfed basins in the Himalayan region. J. Hydrol. 300 , 140–154 (2005)
Article ADS Google Scholar
Shiyin, L., Wenxin, S., Shen, Y. & Li, G. Glacier changes since the Little Ice Age maximum in the western Qilian Shan, northwest China, and consequences of glacier runoff for water supply. J. Glaciol. 49 , 117–124 (2003)
Barnett, T. P., Adam, J. C. & Lettenmaier, D. P. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 438 , 303–309 (2005)
Article ADS CAS PubMed Google Scholar
Bradley, R. S., Vuille, M., Diaz, H. F. & Vergara, W. Threats to water supplies in the tropical Andes. Science 312 , 1755–1756 (2006)
van der Kooij, D. in Heterotrophic Plate Counts and Drinking-water Safety: The Significance of HPCs for Water Quality and Human Health (eds Bartram, J., Cotruvo, J., Exner, M., Fricker, C. & Glasmacher, A.) 199–232 (IWA Publishing, World Health Organization, Geneva, 2003)
Pitman, G. K. Bridging Troubled Waters—Assessing The World Bank Water Resources Strategy (World Bank Publications, Washington DC, 2002)
World Health Organization. Emerging Issues in Water and Infectious Disease 1–22 (World Health Organization, Geneva, 2003)
United States Environmental Protection Agency. 40 CFR parts 9, 141 & 142 National Primary Drinking Water Regulations: Long term 2 enhanced surface water treatment rule; final rule. Federal Register 71 , 653–702 (2006)
United States Environmental Protection Agency. 40 CFR parts 9, 141, & 142 National Primary Drinking Water Regulations: Stage 2 disinfectants and disinfection byproducts rule; final rule. Federal Register 71 , 388–493 (2006)
Krasner, S. W. et al. Occurrence of a new generation of disinfection byproducts. Environ. Sci. Technol. 40 , 7175–7185 (2006)
Muellner, M. G. et al. Haloacetonitriles vs. regulated haloacetic acids: are nitrogen-containing DBPs more toxic? Environ. Sci. Technol. 41 , 645–651 (2007)
Centers for Disease Control and Prevention. Safe Water Systems for the Developing World: A Handbook for Implementing Household-Based Water Treatment and Safe Storage Projects (CDC, Atlanta, 2000)
Simonet, J. & Gantzer, C. Inactivation of poliovirus 1 and f-specific RNA phages and degradation of their genomes by UV irradiation at 254 nanometers. Appl. Environ. Microbiol. 72 , 7671–7677 (2006)
Article CAS PubMed PubMed Central Google Scholar
Nuanualsuwan, S. & Cliver, D. O. Capsid functions of inactivated human picornaviruses and feline calicivirus. Appl. Environ. Microbiol. 69 , 350–357 (2003)
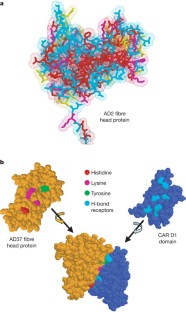
Coyne, C. B. & Bergelson, J. M. CAR: A virus receptor within the tight junction. Adv. Drug Deliv. Rev. 57 , 869–882 (2005)
Seiradake, E., Lortat-Jacob, H., Billet, O., Kremer, E. J. & Cusack, S. Structural and mutational analysis of human Ad37 and canine adenovirus 2 fiber heads in complex with the D1 domain of coxsackie and adenovirus receptor. J. Biol. Chem. 281 , 33704–33716 (2006)
Hawkins, C. L., Pattison, D. I. & Davies, M. J. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 25 , 259–274 (2003)
Nightingdale, Z. D. et al. Relative reactivity of lysine and other peptides-bound amino acids to oxidation by hypochlorite. Free Radic. Biol. Med. 29 , 425–433 (2000)
Article Google Scholar
Bergt, C., Fu, X., Huq, N. P., Kao, J. & Heinecke, J. W. Lysine residues direct the chlorination of tyrosines in Y XX K motifs of apolipoprotein A-I when hypochlorous acid oxidizes high density lipoprotein. J. Biol. Chem. 279 , 7856–7866 (2004)
Pattison, D. I. & Davies, M. J. Kinetic analysis of the role of histidine chloramines in hypochlorous acid mediated protein oxidation. Biochemistry 44 , 7378–7387 (2005)
Medina-Kauwe, L. K. Endocytosis of adenovirus and adenovirus capsid proteins. Adv. Drug Deliv. Rev. 55 , 1485–1496 (2003)
Yates, M. V., Malley, J., Rochelle, P. & Hoffman, R. Effect of adenovirus resistance on UV disinfection requirements: A report on the state of adenovirus science. J. Am. Water Works Assoc. 98 , 93–106 (2006)
Article CAS Google Scholar
Li, Q., Liang, W. & Shang, J. K. Enhanced visible-light absorption from PdO nanoparticles in nitrogen-doped titanium oxide thin films. Appl. Phys. Lett. 90 , 063109 (2007)
Article ADS CAS Google Scholar
Fu, P., Luan, Y. & Dai, X. Preparation of activated carbon fibers supported TiO2 photocatalyst and evaluation of its photocatalytic reactivity. J. Mol. Catal. Chem. 221 , 81–88 (2004)
Medina-Valtierra, J., Garcia-Servin, J., Frausto-Reyes, C. & Calixto, S. The photocatalytic application and regeneration of anatase thin films with embedded commercial TiO2 particles deposited on glass microrods. Appl. Surf. Sci. 252 , 3600–3608 (2006)
Changrani, R. G. & Raupp, G. B. Two-dimensional heterogeneous model for a reticulated-foam photocatalytic reactor. Am. Inst. Chem. Eng. J. 46 , 829–842 (2000)
Molinari, R., Palmisano, L., Drioli, E. & Schiavello, M. Studies on various reactor configurations for coupling photocatalysis and membrane processes in water purification. J. Membr. Sci. 206 , 399–415 (2002)
Lin, H. & Valsaraj, K. T. Development of an optical fiber monolith reactor for photocatalytic wastewater treatment. J. Appl. Electrochem. 35 , 699–708 (2005)
Blanco-Galvez, J., Fernandez-Ibanez, P. & Malato-Rodriguez, S. Solar photocatalytic detoxification and disinfection of water: Recent overview. J. Solar Energy Eng. 129 , 4–15 (2007)
Gill, L. W. & McLoughlin, O. A. Solar disinfection kinetic design parameters for continuous flow reactors. J. Solar Energy Eng. 129 , 111–118 (2007)
Schwarzenbach, R. P. et al. The challenge of micropollutants in aquatic systems. Science 313 , 1072–1077 (2006)
Sarkar, S. et al. Well-head arsenic removal units in remote villages of Indian subcontinent: Field results and performance evaluation. Water Res. 39 , 2196–2206 (2005)
Khan, A. H. et al. Appraisal of a simple arsenic removal method for groundwater of Bangladesh. J. Environ. Sci. Health Part A 35 , 1021–1041 (2000)
Silliman, S. E., Boukari, M., Crane, P., Azonsi, F. & Neal, C. R. Observations on elemental concentrations of groundwater in central Benin. J. Hydrol. 335 , 374–388 (2007)
Rasul, S. B. et al. Electrochemical measurement and speciation of inorganic arsenic in groundwater of Bangladesh. Talanta 58 , 33–43 (2002)
Chen, Z. L., Akter, K. F., Rahman, M. M. & Naidu, R. Speciation of arsenic by ion chromatography inductively coupled plasma mass spectrometry using ammonium eluents. J. Sep. Sci. 29 , 2671–2676 (2006)
Sultan, J. & Gabryelski, W. Structural identification of highly polar nontarget contaminants in drinking water by ESI-FAIMS-Q-TOF-MS. Anal. Chem. 78 , 2905–2917 (2006)
Kuo, T.-C. et al. Gateable nanofluidic interconnects for multilayered microfluidic separational systems. Anal. Chem. 75 , 1861–1867 (2003)
Liu, J. W. et al. A catalytic beacon sensor for uranium with parts-per-trillion sensitivity and millionfold selectivity. Proc. Natl Acad. Sci. USA 104 , 2056–2061 (2007)
Article ADS CAS PubMed PubMed Central Google Scholar
Chang, I. H. et al. Miniaturized lead sensor based on lead-specific DNAzyme in a nanocapillary interconnected microfluidic device. Environ. Sci. Technol. 39 , 3756–3761 (2005)
Zhu, P. X. et al. Detection of water-borne E. coli O157 using the integrating waveguide biosensor. Biosens. Bioelectron. 21 , 678–683 (2005)
Snyder, S. A. et al. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 202 , 156–181 (2007)
Davis, A. P., Sheppard, D. N. & Smith, B. D. Development of synthetic membrane transporters for anions. Chem. Soc. Rev. 36 , 348–357 (2007)
Snyder, S. A., Vanderford, B. J. & Rexing, D. J. Trace analysis of bromate, chlorate, iodate, and perchlorate in natural and bottled waters. Environ. Sci. Technol. 39 , 4586–4593 (2005)
Garelick, H., Dybowska, A., Valsami-Jones, E. & Priest, N. D. Remediation technologies for arsenic contaminated drinking waters. J. Soils Sediments 5 , 182–190 (2005)
Schideman, L. C., Marinas, B. J., Snoeyink, V. L. & Campos, C. Three-component competitive adsorption model for fixed-bed and moving-bed granular activated carbon adsorbers. Part I. Model development. Environ. Sci. Technol. 40 , 6805–6811 (2006)
Magnuson, M. L. & Speth, T. F. Quantitative structure—Property relationships for enhancing predictions of synthetic organic chemical removal from drinking water by granular activated carbon. Environ. Sci. Technol. 39 , 7706–7711 (2005)
Yavuz, C. T. et al. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 314 , 964–967 (2006)
Article PubMed Google Scholar
Fournier, D., Hawari, J., Streger, S. H., McClay, K. & Hatzinger, P. B. Biotransformation of N-nitrosodimethylamine by Pseudomonas mendocina KR1. Appl. Environ. Microbiol. 72 , 6693–6698 (2006)
Kraemer, S. M., Xu, J. D., Raymond, K. N. & Sposito, G. Adsorption of Pb(II) and Eu(III) by oxide minerals in the presence of natural and synthetic hydroxamate siderophores. Environ. Sci. Technol. 36 , 1287–1291 (2002)
Chaplin, B. P., Roundy, E., Guy, K. A., Shapley, J. R. & Werth, C. J. Effects of natural water ions and humic acid on catalytic nitrate reduction kinetics using an alumina supported Pd-Cu catalyst. Environ. Sci. Technol. 40 , 3075–3081 (2006)
Daiger, G. T., Rittmann, B. E., Adham, S. & Andreottola, G. Are membrane bioreactors ready for widespread application? Environ. Sci. Technol. 39 , 399A–406A (2005)
Yang, W. B., Cicek, N. & Ilg, J. State-of-the-art of membrane bioreactors: worldwide research and commercial applications in North America. J. Membr. Sci. 270 , 201–211 (2006)
Bixio, D. et al. Wastewater reuse in Europe. Desalination 189 , 89–101 (2006)
Kimura, K., Yamato, N., Yamamura, H. & Watanabe, Y. Membrane fouling in pilot-scale membrane bioreactors (MBRs) treating municipal wastewater. Environ. Sci. Technol. 39 , 6293–6299 (2005)
Ulbricht, M. & Belfort, G. Surface modification of ultrafiltration membranes by low temperature plasma.2. Graft polymerization onto polyacrylonitrile and polysulfone. J. Membr. Sci. 111 , 193–215 (1996)
Carroll, T., Booker, N. A. & Meier-Haack, J. Polyelectrolyte-grafted microfiltration membranes to control fouling by natural organic matter in drinking water. J. Membr. Sci. 203 , 3–13 (2002)
Deratani, A., Li, C. L., Wang, D. M. & Lai, J. Y. New trends in the preparation of polymeric membranes for liquid filtration. Ann. Chim.-Sci. Mater. 32 , 107–118 (2007)
Hester, J. F., Banerjee, P. & Mayes, A. M. Preparation of protein-resistant surfaces on poly(vinylidene fluoride) membranes via surface segregation. Macromolecules 32 , 1643–1650 (1999)
Hester, J. F. & Mayes, A. M. Design and performance of foul-resistant poly(vinylidene fluoride) membranes prepared in a single step by surface segregation. J. Membr. Sci. 202 , 119–135 (2002)
Wang, Y. Q. et al. Remarkable reduction of irreversible fouling and improvement of the permeation properties of poly(ether sulfone) ultrafiltration membranes by blending with pluronic F127. Langmuir 21 , 11856–11862 (2005)
Asatekin, A., Kang, S., Elimelech, M. & Mayes, A. M. Anti-fouling ultrafiltration membranes containing polyacrylonitrile- graft -poly(ethylene oxide) comb copolymer additives. J. Membr. Sci. 298 , 136–146 (2007)
Kang, S., Asatekin, A., Mayes, A. M. & Elimelech, M. Protein antifouling mechanisms of PAN UF membranes incorporating PAN-g-PEO additive. J. Membr. Sci. 298 , 42–50 (2007)
Ulbricht, M. Advanced functional polymer membranes. Polymer 47 , 2217–2262 (2006)
Akthakul, A., Salinaro, R. F. & Mayes, A. M. Antifouling polymer membranes with sub-nanometer size selectivity. Macromolecules 37 , 7663–7668 (2004)
Zhou, M., Kidd, T. J., Noble, R. D. & Gin, D. L. Supported lyotropic liquid crystal polymer membranes: promising materials for molecular-size-selective aqueous nanofiltration. Adv. Mater. 17 , 1850–1853 (2005)
Asatekin, A. et al. Antifouling nanofiltration membranes for membrane bioreactors from self-assembling graft copolymers. J. Membr. Sci. 285 , 81–89 (2006)
Revanur, R., McCloskey, B., Breitenkamp, K., Freeman, B. D. & Emrick, T. Reactive amphiphilic graft copolymer coatings applied to polyvinylidene fluoride ultrafiltration membranes. Macromolecules 40 , 3624–3630 (2007)
Yang, S. Y. et al. Nanoporous membranes with ultrahigh selectivity and flux for the filtration of viruses. Adv. Mater. 18 , 709–712 (2006)
Phillip, W. A., Rzayev, J., Hillmyer, M. A. & Cussler, E. L. Gas and water liquid transport through nanoporous block copolymer membranes. J. Membr. Sci. 286 , 144–152 (2006)
Nunes, S. P., Sforca, M. L. & Peinemann, K.-V. Dense hydrophilic composite membranes for ultrafiltration. J. Membr. Sci. 106 , 49–56 (1995)
Yoon, K. et al. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polym. 47 , 2434–2441 (2006)
Lu, Y., Suzuki, T. & Zhang, W. Moore, J. S. &Mariñas, B. J. Nanofiltration membranes based on rigid star amphiphiles. Chem. Mater. 19 , 3194–3204 (2007)
Zhou, Y. & Tol, R. S. J. Evaluating the costs of desalination and water transport. Wat. Resour. Res. 41 , W03003,–1–10 (2005)
Veerapaneni, S., Long, B., Freeman, S. & Bond, R. Reducing energy consumption for seawater desalination. J. Am. Water Works Assoc. 99 , 95–106 (2007)
Morgan, L. A. et al. Solar distillation: a promising alternative for water provision with free energy, simple technology and a clean environment. Desalination 116 , 45–56 (1998)
Bourounia, K., Chaibib, M. T. & Tadrist, L. Water desalination by humidification and dehumidification of air: state of the art. Desalination 137 , 167–176 (2001)
McCutcheon, J. R., McGinnis, R. L. & Elimelech, M. A novel ammonia-carbon dioxide forward (direct) osmosis desalination process. Desalination 174 , 1–11 (2005)
Mathioulakis, E., Belessiotis, V. & Delyannis, E. Desalination by using alternative energy: review and state-of-the-art. Desalination 203 , 346–365 (2007)
Alonitis, S. A., Kouroumbas, K. & Vlachakis, N. Energy consumption and membrane replacement cost for seawater RO desalination plants. Desalination 157 , 151–158 (2003)
Seacord, T. F., Coker, S. D. & MacHarg, J. Affordable desalination collaboration 2005 results. In International Desalination And Water Reuse Quarterly (Green Global Publications, Anaheim, California, 2006)
Spiegler, K. S. & El-Sayed, Y. M. The energetics of desalination processes. Desalination 134 , 109–128 (2001)
Hummer, G., Rasaiah, J. C. & Nowotyta, J. P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414 , 188–190 (2001)
Kalra, A., Garde, S. & Hummer, G. Osmotic water transport through carbon nanotube membranes. Proc. Natl Acad. Sci. USA 100 , 10175–10180 (2003)
Hinds, B. J. et al. Aligned multiwalled carbon nanotube membranes. Science 303 , 62–65 (2003)
Article ADS PubMed CAS Google Scholar
Holt, J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312 , 1034–1037 (2006)
Fornasiero, F. et al. Ion exclusion by sub 2-nm carbon nanotube pores. Proc. Natl. Acad. Sci. USA (in the press)
Walz, T., Smith, B. L., Zeidel, M. L., Engel, A. & Agre, P. Biologically-active 2-dimensional crystals of aquaporin chip. J. Biol. Chem. 269 , 1583–1586 (1994)
Qiao, R., Georgiadis, J. G. & Aluru, N. R. Differential ion transport induced electroosmosis and internal recirculation in heterogeneous osmosis membranes. Nano Lett. 6 , 995–999 (2006)
Ishida, H., Donowaki, K., Inoue, Y., Qi, Z. & Sokabe, M. Synthesis and ion channel formation of novel cyclic peptides containing a non-natural amino acid. Chem. Lett. Jpn 26 , 935–954 (1997)
Nednoor, P., Gavalas, V. G., Chopra, N., Hinds, B. J. & Bachas, L. G. Carbon nanotube based biomimetic membranes: mimicking protein channels regulated by phosphorylation. J. Mater. Chem. 17 , 1755–1757 (2007)
Parsegian, A. Energy of an ion crossing a low dielectric membrane: solutions to four relevant electrostatic problems. Nature 221 , 844–846 (1969)
Facciotti, M. T., Rouhani-Manshadi, S. & Glaeser, R. M. Energy transduction in transmembrane ion pumps. Trends Biochem. Sci. 29 , 445–451 (2004)
Martz, E. Protein explorer: easy yet powerful macromolecular visualization. Trends Biochem. Sci. 27 , 107–109 (2002)
van Raaij, M. J., Louis, N., Chroboczek, J. & Cusack, S. Structure of the human adenovirus serotype 2 fiber head domain at 1.5 Å resolution. Virology 262 , 333–343 (1999)
Download references
Acknowledgements
We acknowledge the US National Science Foundation Science and Technology Center, WaterCAMPWS , Center for Advanced Materials for the Purification of Water with Systems.
Author information
Authors and affiliations.
NSF STC WaterCAMPWS, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801, USA,
Mark A. Shannon, Paul W. Bohn, Menachem Elimelech, John G. Georgiadis, Benito J. Mariñas & Anne M. Mayes
Department of Chemical and Biomolecular Engineering and Department of Chemistry, University of Notre Dame, Notre Dame, Indiana 46556, USA,
Paul W. Bohn
Department of Environmental and Chemical Engineering, Yale University, New Haven, Connecticut 06520, USA,
Menachem Elimelech
Department of Mechanical Science and Engineering, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801, USA,
Mark A. Shannon & John G. Georgiadis
Department of Civil and Environmental Engineering, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801, USA,
Benito J. Mariñas
Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA,
Anne M. Mayes
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mark A. Shannon .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Shannon, M., Bohn, P., Elimelech, M. et al. Science and technology for water purification in the coming decades. Nature 452 , 301–310 (2008). https://doi.org/10.1038/nature06599
Download citation
Received : 14 July 2007
Accepted : 14 December 2007
Issue Date : 20 March 2008
DOI : https://doi.org/10.1038/nature06599
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Bio-inspired design of next-generation ultrapermeable membrane systems.
- Mingheng Li
npj Clean Water (2024)
Wood-inspired metamaterial catalyst for robust and high-throughput water purification
Nature Communications (2024)
Air-gap-assisted solvothermal process to synthesize unprecedented graphene-like two-dimensional TiO2 nanosheets for Na+ electrosorption/desalination
- Billel Salhi
- Nadeem Baig
- Ismail Abdulazeez
A bio-based nanofibre hydrogel filter for sustainable water purification
- Meihui Jiang
- Chuyan Jing
Nature Sustainability (2024)
Solvent effects in anion recognition
- Sophie C. Patrick
- Paul D. Beer
- Jason J. Davis
Nature Reviews Chemistry (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Closing the water cycle by either desalination or wastewater purification promises to provide virtually unlimited volumes of freshwater: in principle, it would enable an increase in water ...
Dec.15, 2021. In this paper, researchers surveyed both conventional and advanced disinfection processes in the U.S., testing the quality of their drinking waters. Treatment plants with advanced removal technologies, such as activated carbon, formed fewer types and lower levels of harmful disinfection byproducts (known as DBPs) in their water.
In 2019, about 6% of public water utilities in the U.S. had a health-based violation. Due to the high risk of exposure to various contaminants in drinking water, point-of-use (POU) drinking water ...
something new, like "to write new versus with a false despair". This does not take the practicability of the research into account; it also does not stimulate applications in large-scale water purification.In addition, in the information age, data management has flownfrom cutting-edge fieldsto every aspect of our daily life. Therefore, in ...
Robots for water remediation. Micro- and nanorobots aim to accelerate and improve the water decontamination process. The synergistic action between their active motion and their material ...
Over the years, few review articles have focused on the importance and applicability of AI in water treatment processes. For instance, Li et al. reviewed the most recent developments and applications of AI technologies in drinking water treatment plants [17].Likewise, innovations in the desalination process by AI were comprehended [19].Generally, previous studies have considered a single AI ...
Agricultural development, extensive industrialization, and rapid growth of the global population have inadvertently been accompanied by environmental pollution. Water pollution is exacerbated by the decreasing ability of traditional treatment methods to comply with tightening environmental standards. This review provides a comprehensive description of the principles and applications of ...
1. Introduction. Giving people easy access to inexpensive, clean water that meets their expanding demand is a major challenge facing the world. The most significant issues that make it harder for the water supply system to function are population expansion, global climate change, and water pollution [1].By 2025, half of the world's population will live in areas with water scarcity, according ...
In recent years, many specialists have looked at the use of carbon nanotubes in the purification of water. According to research, microwave-heated MWCNTs have been shown to remove Zn(II) from an aqueous solution with a clearance rate greater than 99%. ... Water purification with TiO 2 -polymer composites have been intensively researched ...
Despite the most innovative processes used to remove these compounds, their persistence in drinking water is still a reality. Several research works have reported on the development of new targeted strategies for the removal of these compounds from water by membrane filtration [31,32,33].
Filtration is a water treatment system that involves the separation of solids from liquids using a porous medium to remove suspended solids, colloids, and other substances (Charles 2009;Zeki 2009
The development of technological advancement may help to overcome these effects. Particularly nanotechnology plays a pivotal role to overcome these concerns. The enormous potential of nanotechnology was proved on pursuing research and became a water treatment tool of the 21st century (Khan et al., 2019). Nanotechnology was put forward to ...
This review paper focuses on the various types of Filtration techniques. available and whic h tec hnique is most suitable for which t ype of region. W e. will study the t ypes of natural ...
This study, based in San Bernardino County, Southern California, collected and examined tap water samples within the area to explore the feasibility of adopting non-industrial equipment and methods to reduce water hardness and total dissolved solids(TDS). We investigated how water quality could be improved by utilizing water boiling, activated carbon and sodium bicarbonate additives, as well ...
Furthermore, considering the importance of drinking water at the regional and global level, there is a need for an updated study to identify new trends in water treatment research based on information provided by Scopus and WoS, two of the main multidisciplinary academic databases worldwide (Visser et al., Citation 2021>).
Sustainable and affordable supply of clean, safe, and adequate water is one of the most challenging issues facing the world. Membrane separation technology is one of the most cost-effective and widely applied technologies for water purification. Polymeric membranes such as cellulose-based (CA) membranes and thin-film composite (TFC) membranes have dominated the industry since 1980.
Fundamentals and applications in water treatment. Nature Water 2 , 101 ( 2024) Cite this article. 1370 Accesses. 2 Altmetric. Metrics. For papers regarding water and wastewater treatment, we are ...
hydrogel-based solar water purification have appeared recently. Although the development of hydrogel-materials for solar water purification is still in its early stages, many research papers have been published in this field. A timely, thorough review of the recent achievements will highlight key research
Rapid progress developing novel materials has propelled photocatalysis to the forefront of sustainable wastewater treatments. This review presents the latest progress on applications of photocatalytic wastewater treatment. Our focus is on strategies for improving performance. Challenges and outlooks in this promising field are also discussed.
Nanotechnology is an easy and practical approach to clean waste water by using different methods. Different types of bacteria, toxic chemicals like arsenic, mercury etc., and sediments can be removed by using nanotechnology. Nanomaterial based devices are being used for water purification. Nano filtration method has advantages over other ...
Water purification systems for domestic use have drawn much of attention over the past few years. This can be related to improvement of public health and concerns for water contamination. ... Brunelle JA, Carlos JP. Recent trends in dental caries in U.S. children and the effect of water fluoridation. J Dent Res. 1990; 69: 723-727. [Google ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... New methods of water treatment ...
As of today, the latest water purification technologies are nanotechnology, acoustic nanotube, photocatalytic water purification, aquaporin inside TM, and automatic variable filtration. The use of technologies in water treatment is mainly due to three main reasons: a new standard for water quality, an increase in water contamination, and cost.
Recent research on the transport of water through hydrophobic double-walled carbon nanotubes is promising, demonstrating water fluxes that are over three orders of magnitude higher than those ...
With ongoing climate change, new threats appear to the operation of water supply systems (WSSs), which are related to the amount of available drinking water resources, its quality, the operation of existing water supply infrastructure and changes in consumer behavior. The paper presents a bibliometric analysis of the state of knowledge on the impact of climate change on the failure of water ...
The area of water purification has been one of the most dynamic research fields in recent years with strong public policy implications with over 39,000 papers. Similarly, the areas of nanomaterials and nanoprocesses have been one of the most dynamic research fields in recent years with significant public policy implications with over 1,000,000 ...
It has become necessary to develop effective strategies to prevent and reduce water pollution as a result of the increase in dangerous pollutants in water reservoirs. Consequently, there is a need to design new catalyst materials to promote the efficiency of advanced oxidation processes (AOPs) in the field of wastewater treatment plant to ensure the mineralization of trace organic contaminants.