- Open access
- Published: 02 December 2021

Dopamine, behavior, and addiction
- Roy A. Wise ORCID: orcid.org/0000-0002-9115-6654 1 , 2 &
- Chloe J. Jordan 3
Journal of Biomedical Science volume 28 , Article number: 83 ( 2021 ) Cite this article
25k Accesses
23 Citations
3 Altmetric
Metrics details
Addictive drugs are habit-forming. Addiction is a learned behavior; repeated exposure to addictive drugs can stamp in learning. Dopamine-depleted or dopamine-deleted animals have only unlearned reflexes; they lack learned seeking and learned avoidance. Burst-firing of dopamine neurons enables learning—long-term potentiation (LTP)—of search and avoidance responses. It sets the stage for learning that occurs between glutamatergic sensory inputs and GABAergic motor-related outputs of the striatum; this learning establishes the ability to search and avoid. Independent of burst-firing, the rate of single-spiking—or “pacemaker firing”—of dopaminergic neurons mediates motivational arousal. Motivational arousal increases during need states and its level determines the responsiveness of the animal to established predictive stimuli. Addictive drugs, while usually not serving as an external stimulus, have varying abilities to activate the dopamine system; the comparative abilities of different addictive drugs to facilitate LTP is something that might be studied in the future.
Addictive drugs are habit-forming. Here we use the phase “habit-forming broadly [ 1 ] to refer to the entire progression toward the stimulus–response end habits [ 2 ] discussed in the specialist literature.Rewards are habit-forming because predictive stimuli—reward-predictors as well as punishment-predictors—come to cause dopaminergic burst-firing, and burst-firing enhances or enables the separate development of long-term potentiation (LTP) and long-term depression (LTD) of learned connections between other systems: glutamatergic input pathways and GABAergic output pathways [ 3 ]. The primary source of these is in the striatum; the striatum receives sensory inputs from the cortex and sends motor-related outputs that are essential for food-searching and punishment-avoidance. The primary evidence for dopaminergic involvement in reward-driven learning comes from studies of genetically altered mice that cannot synthesize dopamine in the brain. These mice appear normal when born, but they fail to learn food-seeking and, after weaning, die of starvation unless they are force-fed [ 4 ]. Such animals have only unconditioned reflexes—“consummatory” reflexes [ 5 ]—and, having not learned to feed,—an “appetitive” response [ 5 ]—also fail to learn to seek or avoid other rewards. That dopamine is critical for such learning is evident from the dopaminergic recordings of Schultz and colleagues [ 6 , 7 ] and from recent optogenetic studies that confirm dopaminergic activation as rewarding [ 8 , 9 , 10 , 11 ].
Dopamine-deficient animals
Dopamine-deficient animals are born with minimal—learned in utero—knowledge of the environment. These animals have normal reflexes: they can swallow food if it is placed in their mouth [ 12 ] and they escape from aversive stimuli [ 13 , 14 , 15 ]. They do not, however, learn to search for rewards or avoid aversive stimuli [ 4 , 16 ]. They must learn to approach the environmental cues that guide behavior, leading to food and other rewards [ 17 , 18 ], just as they must learn to avoid predictive stimuli that warn of punishers. Learned approach to rewards is an appetitive behavior and is essential for addiction as well as for feeding; indeed, the strongest evidence of drug self-administration involved responses to predictors because the animals, in these cases, rarely have sensory contact with the drug itself [ 19 ].
Dopaminergic burst-firing enables environmental learning
Genetically modified neonates that cannot synthesize dopamine in the brain [ 4 ], like adult animals with their dopamine systems lesioned [ 16 ] or blocked pharmacologically [ 20 , 21 , 22 ]—dopamine challenged animals—fail to find and eat external foods [ 20 , 22 ] and fail to seek and consume addictive drugs [ 3 , 23 , 24 , 25 ] or other rewards [ 21 , 26 , 27 ].
The dopamine system is activated by three kinds of external stimuli: rewarding stimuli, punishing stimuli, and novel stimuli. When activated by rewards or punishers, portions of the dopamine system discharge in bursts [ 6 , 28 , 29 , 30 , 31 , 32 ], whereas other portions are inhibited [ 33 , 34 , 35 ]. Dopaminergic burst discharges involve two or more linked spikes with progressively decreasing amplitude and short inter-discharge intervals (about 60 ms between the first and second discharge and about 120 ms between subsequent discharges) [ 36 ]. These discharges cause very local accumulations of dopamine in the striatum, at local peaks of 100 nM or greater concentration, as measured by fast-scan cyclic voltammetry (FSCV) [ 37 ].
The primary sensory inputs to the dopamine system—driving this release—are glutamatergic and perhaps cholinergic terminals from cell bodies of the latero-dorsal and pedunculopontine tegmental nuclei [ 38 , 39 ]; similar burst firing can be activated as well by direct glutamatergic stimulation of dopaminergic neurons in isolated brain slices [ 40 ]. In dopamine-intact animals, dopaminergic neurons burst-fire in response not just to rewards or punishers but also to stimuli that reliably precede—and thus predict—rewards and punishers [ 6 , 7 , 41 ].
The burst-firing in response to predictors of rewards or punishers develops with age, as the animal learns about the environment. The burst-responses should not really be seen as travelling from the unconditioned rewards and punishers to their predictors; rather, the process of burst-firing develops anew in response to predictors that involve a Hebbian mechanism [ 42 , 43 ]. Hebb has postulated a mechanism by which repeated synaptic input from a (predictor) cell that reliably precedes another (reward) neuron becomes linked to its target. As responses to predictors develop, the burst-responding in response to the actual rewards or punishers is temporarily lost; responsiveness, however—in this case inhibition of firing—appears when the reward or punisher fails to appear at the expected time [ 44 ]. When burst-firing develops in response to reward-predictors it enables cellular learning in surrounding synapses; these are glutamate-GABA synapses localized within microns of the sites of dopamine release.
Burst-firing of the dopamine system is only a first step in the learning; the formation of the synapses for searching develops in other cellular elements. Dopamine bursting enables development of long-term potentiation (LTP) and long-term depression (LTD), and, in the striatum, this occurs between glutamatergic sensory inputs and GABAergic motor-related outputs [ 45 , 46 ]. Dopamine in the striatum reaches and binds to high-affinity D2 dopamine receptors and low-affinity D1 receptors [ 48 , 49 ]. At high affinity D2 receptors significant binding occurs, making D2 receptors particularly sensitive to phasic decreases in dopamine release. At low affinity D1 receptors less dopamine should be bound, making D1 receptors particularly sensitive to phasic increases in dopamine release. Movements result when D1 receptors are activated and inhibition of movement results when D2 receptors are activated [ 9 ]. In behaving animals, activation of D1 and D2 are momentary complements; their activations occur concurrently [ 50 ]. Concurrent activation presumably involves activating one subset of muscles (D1) to do something while inhibiting (D2) other sets of muscles, antagonistic muscles, that would normally interfere with the elicited action. The reward-predicting stimuli that lead an animal to anticipate rewards—both natural rewards and drug rewards—are established by this kind of learning [ 3 , 25 ].
Dopaminergic pacemaker-firing modulates motivation
Whereas burst responding of the dopamine system is elicited by external stimuli, dopaminergic single discharges also spontaneously occur; these discharges are identified as pacemaker firing because they result from a depolarizing current within the dopaminergic cells themselves [ 51 ]. Such discharges can be seen in brain slice preparations even when, in vitro, excitatory inputs have been eliminated [ 40 ]. Pacemaker firing is slower than burst firing; it occurs at about half the frequency of burst-firing [ 51 ]. The rate of pacemaker firing is modulated by two sources: by increases or decreases of inhibitory inputs from GABA-containing cells [ 52 ] and by hormones and peptides that act at receptors on dopaminergic neurons themselves [ 53 , 54 , 55 ] or that act through their inputs [ 55 , 56 , 57 ]. The tonic modulation of the dopamine system—pacemaker firing, supplemented by episodes of burst-firing—is a correlate of, presumably a cause of, motivational arousal.
Motivational arousal is a state variable; it regulates readiness to respond to external stimuli. While rewards and punishers elicit responses regardless of emotional state, it is predictors of rewards or punishers that depend on motivational arousal. In resting animals, it is pacemaker firing that varies as a function of internal state and determines when, and to what degree, the animal responds to reward-predictors. Burst-firing can also influence motivational arousal; consider the behavior of an animal when a pheromone-emitting conspecific passes nearby. Motivational arousal varies over time and, in resting animals, determines when a previously sated animal starts to become hungry and interested in seeking food.
In a resting animal, the release of dopamine is detected historically by microdialysis [ 58 ]. Baseline levels of dopamine are estimated to be around 5 nM [ 59 , 60 ]; microdialysis can measure dopamine levels this low and much lower; microdialysis—in tetrodotoxin-treated animals—can measure dopamine at 1% of baseline levels [ 61 ]. However, microdialysis is an insensitive measure, averaging rather than giving data from single cells; it involves the sampling of extracellular fluids through large (~ 250 microns) push–pull cannulae in the brain, in contact with many dopamine terminals, and it usually gives averages taken over minutes or tens of minutes. One possibility is that basal dopamine levels are near 5 nM at all points throughout the striatum; alternatively, it is possible that microdialysis simply reflects the average of large fluctuations around some unknown actual baseline level. In contrast, however, the alternative—FSCV, for example—measures individual elevations and does not have the sensitivity to detect the low levels of dopamine in resting animals; it is insensitive to dopamine at concentrations below 20 nM [ 37 ] and uses “background subtraction” to isolate dopamine fluctuations from noise [ 62 ]. FSCV measures peak concentrations that are isolated both in localization and in time. Because the degree of temporal and spatial heterogeneity is not known, it is not clear the degree to which these isolated dopamine peaks contribute to the motivational arousal in active animals. More recently developed techniques involving optical technology, calcium imaging, and genetically-encoded fluorescent protein sensors [ 63 ] will give us better methods for assessing pacemaker dopamine discharge.
The evidence implicating a causal role of dopamine in motivational function comes from experiments where the dopamine system has been experimentally manipulated [ 25 ]. These include the following: Animals with partial dopamine depletion show reduced energy in learned tasks [ 64 ]. Parkinsonian patients with decreased dopamine levels have deficits in speed of hand movements [ 65 ] and in willingness to squeeze a dynamometer [ 66 ]; when dopamine is replaced by L-DOPA administration, these symptoms decrease [ 66 ]. Amphetamine, which augments dopamine release, causes humans to increase effort for monetary rewards [ 67 ]. A dopamine uptake inhibitor that doubles baseline dopamine levels increases willingness to work for high-carbohydrate pellets [ 68 ]. Restoring dopamine by re-establishing synthesis in dopaminergic neurons restores locomotion and food-seeking in dopamine-deficient mice [ 69 , 70 ].
The fact that dopamine-depleted animals already have responses to rewards and punishers allows a stronger definition of motivation than has been offered in the past; the level of motivation varies with responsiveness to predictive stimuli in the environment. The distinction here is between predictive stimuli that lead toward or away from rewards or punishers and rewarding and punishing stimuli themselves, to which dopamine-depleted animals continue to respond.
Motivation is not a linear function of dopamine levels and may vary with noradrenergic as well as dopaminergic motivation. Motivation is low when dopamine levels are low, and it increases as dopamine levels start to increase. However, when dopamine levels are doubled or tripled—such levels as are induced by self-administered doses of amphetamine [ 71 ], cocaine [ 72 ], or opiates [ 73 ]—motivation is lost [ 74 ]. Thus the relation that links dopamine level with motivation appears to be a classic “U”-shaped function; such functions have traditionally been associated with arousal and motivation [ 75 , 76 ].
Predictive cues can become aversive
Wheeler and colleagues have suggested conditions in which cocaine-predictive cues can become associated with negative affect [ 77 , 78 , 79 ]. The first of these papers discussed “cocaine predictive” cues, but the second paper more correctly characterized them as cues of “delayed cocaine delivery.” The parameters of establishing the association of a sweet-tasting substance with aversive conditioning are of particular interest, in part because people who use addictive drugs sometimes appear to do so in anticipation of, or in fear of, expected aversive symptoms.
In the Wheeler studies, animals were given series of 30 or 45 min-long, intra-oral taste stimuli that preceded 2 h sessions of intravenous saline or cocaine self-administration. After several days of training the facial expression elicited by the taste cues [ 77 ] and the effect of these cues on dopamine release [ 78 ] were determined. A taste cue that preceded subsequent saline self-administration caused licking and lateral tongue movements—these are responses driven by sweet solutions—whereas cues that predicted delayed cocaine self-administration had come to cause gagging and gaping—the responses to aversive quinine solutions—[ 77 ]. Moreover, the cue predicting saline self-administration increased dopamine release, whereas the cue predicting cocaine self-administration inhibited dopamine release [ 77 ]. The critical factor here is that it was the predictor of delayed cocaine availability that became aversive. Delayed cocaine availability is not well associated with dopamine release; dopamine release is directly controlled by what happens in seconds after the prediction [ 44 ]. The immediate consequence—for the dopamine system—of the cue that predicted delayed cocaine was the absence of dopamine it caused after training with series of 29 or 44 one-minute cue exposures.
Dopamine and addictive drugs
Roles for dopamine in reward theory [ 80 , 81 , 82 ] and a role of reward in addiction [ 83 ] were established shortly after dopamine was established as a neurotransmitter. Dopamine was first identified with reward function from anatomical [ 84 ] and pharmacological evidence [ 85 , 86 , 87 , 88 , 89 ]; it was subsequently implicated as well in motivational function [ 90 , 91 , 92 ]. Dopamine has broadly been associated with the rewarding effects of addictive drugs, particularly in the process of establishing habitual drug intake [ 24 , 93 , 94 ]. However, dopamine plays strongly established roles in the addictive properties of some drugs but is implicated by minimal evidence in others.
Amphetamine and cocaine The role of dopamine in the rewarding effects of the psychomotor stimulants—amphetamine and cocaine—are strongly established. Self-administered doses of amphetamine [ 71 ] or cocaine [ 72 ] elevate dopamine levels over four-fold. Dopamine antagonists at high doses block amphetamine and cocaine self-administration [ 86 , 95 , 96 ]. At low doses the antagonists cause compensatory increases in responding, suggesting that the rewarding effects of the stimulants has been attenuated [ 86 , 95 , 96 ]. Dopamine-selective lesions cause immediate loss of cocaine self-administration when the lesions are complete [ 97 ] and temporary loss when they are incomplete [ 98 ]. These lesioned animals continue to lever-press for the direct dopamine agonist, apomorphine, following these lesions, confirming that the lesioned animals remember their training history and have normal motor capacity [ 97 , 98 ]. Finally, cocaine and amphetamine induces long-term synaptic changes in glutamate-GABA synapses in the striatum [ 99 , 100 , 101 ].
Opiates Heroin self-administration is also clearly dopamine-dependent. It more than triples resting dopamine levels [ 73 ], and while the role of dopamine in opiate addiction has been questioned [ 102 ], evidence from intravenous heroin self-administration studies makes it clear that animals usually request additional heroin each time their dopamine levels fall below about twice-normal levels [ 103 ]. An important possibility in experiments blocking opiate self-administration with dopamine antagonists is that the antagonists act not only at post-synaptic receptors but also at dopamine autoreceptors [ 104 ] where they increase dopamine firing and dopamine release. By increasing dopamine release—as heroin alone does not—dopamine antagonists elevate extracellular dopamine at the nerve terminal, desensitizing the system to the antagonist and, in this case, requiring more heroin to be effective. In any case, dopamine antagonists do block opiate self-administration [ 102 ]; the lack of compensatory increases in responding for heroin following low doses of dopamine antagonists [ 102 ] does not [ 105 ] rule out a role for dopamine in opiate reward. Studies of opiate-conditioned place preferences adds to the evidence that opiates are habit-forming—place-preferences address the first element of search-habits, the locomotion to the place where drugs are available—and that their habit-forming effects are blocked by dopamine antagonists [ 106 , 107 ].
Nicotine Self-administration of nicotine also appears to be dopamine-dependent. Nicotine self-administration causes burst-firing of dopaminergic neurons [ 108 , 109 ] and elevates dopamine levels to 150–200% of baseline [ 110 ]. It is disrupted by selective dopaminergic antagonists [ 111 ] and selective neurochemical lesions [ 112 ]. Nicotine acts at sites and on receptors expressed by dopamine neurons and inhibitory controllers of dopamine neurons, such as local GABAergic cells within the ventral tegmental area (VTA). Deletion of nicotinic receptor subunits, such as β2, abolishes nicotine-induced dopamine release and attenuates nicotine self-administration, and re-expression of β2 restores nicotine’s rewarding effects [ 113 , 114 , 115 ]. Nicotine causes conditioned place preferences; this is blocked with dopamine antagonists [ 116 ]. Nicotine enables LTP in glutamatergic inputs to the dopamine system and primes the ability of cocaine to induce LTP in the amygdala [ 117 , 118 ], a structure anatomically related to the striatum [ 119 ].
Ethanol The evidence that dopamine is important for the rewarding effects of ethanol is also substantial but weaker than that supporting dopamine involvement in stimulant or opiate reward. Part of the problem is that we still have no animal model of self-administration that is sufficient to maintain intoxication [ 120 ]; rats can be induced to drink alcohol [ 121 , 122 , 123 , 124 ], and animals can be made physically dependent on alcohol [ 125 , 126 ], however, even in already dependent rats, voluntary self-administration that maintains dependence is not seen. Ethanol (and ethanol withdrawal) increases burst-firing in dopaminergic animals [ 127 , 128 ]; ethanol also increases pacemaker dopaminergic firing [ 129 ]. Ethanol can increase dopamine levels to 150–200% of baseline [ 94 ], and increases dopamine cell burst-firing as well as pacemaker-like firing in the VTA; note, however, that a subset of VTA dopamine neurons are instead inhibited by ethanol [ 128 ] and this might also be important.
Dopamine antagonists decrease lever-pressing for ethanol in a sucrose-fading procedure [ 130 , 131 ]; this is done in animals that were experienced with ethanol and during intervals of alcohol deprivation. However, the case of alcohol is unusual. In a conditioned place preference study, alcohol is reported to be dopamine-dependent in alcohol-naive animals but not in withdrawn, experienced, animals [ 132 ]. One possibility is that a dopamine-independent pathway is also involved in ethanol reinforcement [ 132 , 133 ]. Ethanol enhances synaptic plasticity in the striatum [ 101 ].
Cannabis There are many cannabinoids, some of which have psychoactive effects and remain to be studied. The primary psychoactive ingredient in marijuana is ∆9 -tetrahydrocannabinol (THC). While some studies have reported that this agent is self-administered intravenously by rodents [ 134 ] and non-human primates [ 135 ] and increases striatal dopamine levels [ 136 , 137 , 138 , 139 ], other studies suggest that THC is not very rewarding in other animals, such that THC self-administration is modest and difficult to sustain [ 140 , 141 ]. Newer rodent models of edible or vaporized THC self-administration hold promise [ 142 , 143 ]. However, species differences in cannabinoid receptor expression and distribution, particularly in the VTA, may underlie differences in the rewarding effects of THC between humans, non-human primates and rodents [ 144 ].
THC is an unusual agent; two of its endogenous analogues—anandamide, 2-arachidonylglycerol—are expressed by dopaminergic (and other) neurons and are released when dopaminergic neurons fire; they influence dopamine turnover through actions on inputs to the dopamine system [ 145 , 146 ]. THC is self-administered into two dopamine-rich regions, the posterior VTA where mesolimbic dopamine cell bodies are found and the posterior ventral striatum, where terminals of that system terminate [ 147 ]; these sites of action implicate THC’s actions on the dopamine system in reward function and the use of central drug self-administration suggests that site-specificity is important here.
Barbiturates and benzodiazepines Much less is known about self-administered doses of barbiturates or benzodiazepines. Barbiturates [ 148 , 149 ] and benzodiazepines [ 150 , 151 ] are self-administered both intravenously and intracranially into the VTA [ 152 , 153 ] by animals. Benzodiazepines increase VTA dopamine neuron firing and induce LTP in glutamatergic inputs to VTA dopamine neurons through positive modulation of local GABA A receptors [ 154 , 155 , 156 , 157 ]. At experimenter-selected doses they elevate dopamine levels [ 158 , 159 , 160 , 161 ] and it has been suggested that they are addictive for this reason [ 24 ].
Caffeine Caffeine is self-administered by animals [ 148 , 162 , 163 ] and produces conditioned flavor preferences (low doses) or conditioned place aversions (high doses) in rats when injected intraperitoneally or directly into the VTA [ 164 ]. A dopamine antagonist injected into the shell of the ventral striatum blocks these place preferences, whereas the antagonist injected into the core of the ventral striatum blocks the conditioned aversive effects [ 165 ]. Volatized, inhaled caffeine increases extracellular dopamine levels in the nucleus accumbens shell [ 166 ]. The mechanism by which caffeine does so is not clear. The main actions of caffeine are mediated through actions at adenosine receptors that form heteromers with dopamine receptors. However, in human Positron Emission Tomography (PET) studies, caffeine increases D2/D3 receptor availability in the ventral striatum, suggesting caffeine alone does not directly increase dopamine levels in this region [ 167 ]. Other studies suggest that caffeine enhances the rewarding effects of other manipulations, such as exercise [ 168 ] or ethanol consumption [ 65 , 169 ].
Conclusions
Learned behavior—perhaps all or almost-all learned behavior—depends on dopamine function; dopamine deficient animals fail to learn to search for food or other rewards and fail to learn to avoid predictable punishers. Dopamine neurons discharge in bursts when triggered by external stimuli, and this burst-firing enables formation of potentiated glutamate-GABA signaling that is critical for learned searching. Dopamine neurons also discharge in slower single-impulse pacemaker firing and the rate of this firing appears to determine motivation in resting (inanimate) animals. The ability of addictive drugs to cause burst-like discharges in the dopamine system is the broadly assumed correlate of addiction, but the direct evidence for this assumption is linked strongly to amphetamine, cocaine, and opiates; the evidence is weaker for nicotine and alcohol, cannabis, barbiturates, benzodiazepines, and caffeine. The abilities of different addictive drugs to enable long-term potentiation and facilitate habit formation via dopaminergic mechanisms should be compared in future studies.
Availability of data and materials
Not applicable.
Singer BF, Fadanelli M, Kawa AB, Robinson TE. Are cocaine-seeking “habits” necessary for the development of addiction-like behavior in rats? J Neurosci. 2018;38(1):60–73.
Article CAS PubMed PubMed Central Google Scholar
Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4–5):407–19.
Article CAS PubMed Google Scholar
Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94.
Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann NY Acad Sci. 2008;1129:35–46.
Craig W. Appetites and aversions as constituents of instincts. Biol Bull. 1918;34:91–107.
Article Google Scholar
Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–63.
Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–13.
Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–33.
Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15(6):816–8.
Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, Ikemoto S. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J Neurosci. 2014;34(3):817–22.
Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K, Janak PH. Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLoS ONE. 2014;9(4):e94771.
Article PubMed PubMed Central Google Scholar
Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83(7):1197–209.
Cooper BR, Breese GR, Grant LD, Howard JL. Effects of 6-hydroxydopamine treatments on active avoidance responding: evidence for involvement of brain dopamine. J Pharmacol Exp Ther. 1973;185(2):358–70.
CAS PubMed Google Scholar
Fibiger HC, Phillips AG, Zis AP. Deficits in instrumental responding after 6-hydroxydopamine lesions of the nigro-neostriatal dopaminergic projection. Pharmacol Biochem Behav. 1974;2(1):87–96.
Barbano MF, Wang HL, Zhang S, Miranda-Barrientos J, Estrin DJ, Figueroa-González A, et al. VTA glutamatergic neurons mediate innate defensive behaviors. Neuron. 2020;107(2):368-82.e8.
Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand. 1971;367:95–122.
Article CAS Google Scholar
Bindra D. Neuropsychological interpretation of the effects of drive and incentive-motivation on general activity and instrumental behavior. Psychol Rev. 1968;75:1–22.
Bolles RC. Reinforcement, expectancy, and learning. Psychol Rev. 1972;79:394–409.
Wise RA. Brain reward circuitry: Insights from unsensed incentives. Neuron. 2002;36:229–40.
Wise RA, Spindler J, deWit H, Gerber GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science. 1978;201(4352):262–4.
Gerber GJ, Sing J, Wise RA. Pimozide attenuates lever pressing for water reinforcement in rats. Pharmacol Biochem Behav. 1981;14(2):201–5.
Wise RA, Schwartz HV. Pimozide attenuates acquisition of lever pressing for food in rats. Pharmacol Biochem Behav. 1981;15:655–6.
Wise RA. Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Sci. 1982;5:39–87.
Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–92.
Wise RA, Robble MA. Dopamine and addiction. Ann Rev Psychol. 2020;71:79–106.
Robinson DL, Phillips PE, Budygin EA, Trafton BJ, Garris PA, Wightman RM. Sub-second changes in accumbal dopamine during sexual behavior in male rats. NeuroReport. 2001;12(11):2549–52.
El-Guebaly N, Mudry T, Zohar J, Tavares H, Potenza MN. Compulsive features in behavioural addictions: the case of pathological gambling. Addiction (Abingdon, England). 2012;107(10):1726–34.
Strecker RE, Jacobs BL. Substantia nigra dopaminergic unit activity in behaving cats: effect of arousal on spontaneous discharge and sensory evoked activity. Brain Res. 1985;361(1–2):339–50.
Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J Neurophysiol. 1986;56:1439–61.
Horvitz JC, Stewart T, Jacobs BL. Burst activity of ventral tegmental dopamine neurons is elicited by sensory stimuli in the awake cat. Brain Res. 1997;759(2):251–8.
Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. PNAS. 2009;106(12):4894–9.
Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459(7248):837–41.
Matsumoto H, Tian J, Uchida N, Watabe-Uchida M. Midbrain dopamine neurons signal aversion in a reward-context-dependent manner. eLife. 2016;5.
Juarez B, Han MH. Diversity of dopaminergic neural circuits in response to drug exposure. Neuropsychopharmacology. 2016;41(10):2424–46.
Verharen JPH, Luijendijk MCM, Vanderschuren L, Adan RAH. Dopaminergic contributions to behavioral control under threat of punishment in rats. Psychopharmacology. 2020;237(6):1769–82.
Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–90.
Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, et al. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem. 2012;121(2):252–62.
Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–73.
Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. PNAS. 2006;103(13):5167–72.
Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9(10):3463–81.
Kim KM, Baratta MV, Yang A, Lee D, Boyden ES, Fiorillo CD. Optogenetic mimicry of the transient activation of dopamine neurons by natural reward is sufficient for operant reinforcement. PLoS ONE. 2012;7(4):e33612.
Hebb DO. The organization of behavior. New York: Wiley; 1949.
Google Scholar
Hebb DO. Essay on mind. New York: Erlbaum; 1980.
Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9.
Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13:1071–7.
Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30(5):211–9.
Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30(5):228–35.
Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30(3):767–77.
Neve KA, Neve RL. Molcular biology of dopamine receptors. In: Neve KA, Neve RL, editors. The dopamine receptors. Totowa: Humana Press; 1997. p. 27–76.
Chapter Google Scholar
Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494(7436):238–42.
Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4(11):2866–76.
Grace AA. The regulation of dopamine neuron activity as determine by in vivo and in vitro intracellular recordings. In: Chiodo LA, Freeman A, editors. Neurophysiology of dopaminergic systems: current status and Clinical perspectives. Detroit: Lakeshor Publishing; 1987. p. 1–66.
Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964(1):107–15.
Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51(6):811–22.
Jerlhag E, Egecioglu E, Dickson SL, Engel JA. Glutamatergic regulation of ghrelin-induced activation of the mesolimbic dopamine system. Addiction Biol. 2011;16(1):82–91.
Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10(2):89–98.
Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Amer J Physiol Endocrinol Metab. 2013;305(11):E1367–74.
Tossman U, Ungerstedt U. Microdialysis in the study of extracellular levels of amino acids in the rat brain. Acta Physiol Scand. 1986;128(1):9–14.
Parsons LH, Justice JB. Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. J Neurochem. 1992;58:212–8.
Sam PM, Justice JB Jr. Effect of general microdialysis-induced depletion on extracellular dopamine. Anal Chem. 1996;68(5):724–8.
Westerink BHC, Tuntler J, Damsma G, Rollema H, De Vries JB. The use of tetrodotoxin for the characterization of drug-enhanced dopamine release in conscious rats studied by brain dialysis. Naunyn-Schmiedeberg’s Arch Pharmacol. 1987;336:502–7.
Howell JO, Kuhr WG, Ensman RE, Wightman RM. Background subtraction for rapid scan voltammetry. J Electroanalytical Chem Interfacial Electrochem. 1986;209:77–90.
Yocky AG, Covey DP. Evolution of in vivo dopamine monitoring techniques. Pharmacol Biochem Behav. 2021;200:173078.
Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191(3):461–82.
Mazzoni P, Hristova A, Krakauer JW. Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J Neurosci. 2007;27(27):7105–16.
Chong TT, Bonnelle V, Manohar S, Veromann KR, Muhammed K, Tofaris GK, et al. Dopamine enhances willingness to exert effort for reward in Parkinson’s disease. Cortex. 2015;69:40–6.
Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci. 2011;31(46):16597–602.
Yohn SE, Errante EE, Rosenbloom-Snow A, Somerville M, Rowland M, Tokarski K, et al. Blockade of uptake for dopamine, but not norepinephrine or 5-HT, increases selection of high effort instrumental activity: implications for treatment of effort-related motivational symptoms in psychopathology. Neuropharmacol. 2016;109:270–80.
Szczypka MS, Mandel RJ, Donahue BA, Snyder RO, Leff SE, Palmiter RD. Viral gene delivery selectively restores feeding and prevents lethality of dopamine-deficient mice. Neuron. 1999;22(1):167–78.
Heusner CL, Hnasko TS, Szczypka MS, Liu Y, During MJ, Palmiter RD. Viral restoration of dopamine to the nucleus accumbens is sufficient to induce a locomotor response to amphetamine. Brain Res. 2003;980(2):266–74.
Ranaldi R, Pocock D, Zereik R, Wise RA. Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous D-amphetamine self- administration. J Neurosci. 1999;19(10):4102–9.
Wise RA, Newton P, Leeb K, Burnette B, Pocock P, Justice JB. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology. 1995;120:10–20.
Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995;21(2):140–8.
Wise RA. Cognitive factors in addiction and nucleus accumbens function: some hints from rodent models. Psychobiology. 1999;27:300–10.
Hebb DO. Drives and the C.N.S. (conceptual nervous system). Psychol Rev. 1955;62:243–54.
Malmo RB. Activation: a neuropsychological dimension. Psychol Rev. 1959;66:367–86.
Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57(5):774–85.
Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, et al. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69(11):1067–74.
Wheeler DS, Robble MA, Hebron EM, Dupont MJ, Ebben AL, Wheeler RA. Drug predictive cues activate aversion-sensitive striatal neurons that encode drug seeking. J Neurosci. 2015;35(18):7215–25.
Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152(2):215–47.
Fibiger HC. Drugs and reinforcement mechanisms: a critical review of the catecholamine theory. Ann Rev Pharmacol Toxicol. 1978;18:37–56.
Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–83.
Pickens R, Harris WC. Self-administration of d-amphetamine by rats. Psychopharmacologia. 1968;12:158–63.
Crow TJ. A map of the rat mesencephalon for electrical self-stimulation. Brain Res. 1972;36:265–73.
Lippa AS, Antelman SM, Fisher AE, Canfield DR. Neurochemical mediation of reward: a significant role for dopamine. Pharmacol Biochem Behav. 1973;1:23–8.
Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–9.
Fouriezos G, Wise RA. Pimozide-induced extinction of intracranial self-stimulation: response patterns rule out motor or performance deficits. Brain Res. 1976;103(2):377–80.
Wasserman EM, Gomita Y, Gallistel CR. Pimozide blocks reinforcement but not priming from MFB stimulation in the rat. Pharmacol Biochem Behav. 1982;17:783–7.
Ettenberg A, Camp CH. Haloperidol induces a partial reinforcement extinction effect in rats: implications for a dopamine involvement in food reward. Pharmacol Biochem Behav. 1986;25:813–21.
Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–9.
Ikemoto S, Panksepp J. Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci. 1996;110(2):331–45.
Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–30.
Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. J Abnorm Psychol. 1988;97(2):118–32.
Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. PNAS. 1988;85:5274–8.
Yokel RA, Wise RA. Attenuation of intravenous amphetamine reinforcement by central dopamine blockade in rats. Psychopharmacology. 1976;48:311–8.
de Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol. 1977;31(4):195–203.
Article PubMed Google Scholar
Roberts DCS, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-OHDA lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–7.
Roberts DCS, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–20.
Kombian SB, Malenka RC. Simultaneous LTP of non-NMDA- and LTD of NMDA-receptor-mediated responses in the nucleus accumbens. Nature. 1994;368:242–6.
Nishioku T, Shimazoe T, Yamamoto Y, Nakanishi H, Watanabe S. Expression of long-term potentiation of the striatum in methamphetamine-sensitized rats. Neurosci Lett. 1999;268(2):81–4.
Avchalumov Y, Trenet W, Piña-Crespo J, Mandyam C. SCH23390 reduces methamphetamine self-administration and prevents methamphetamine-induced striatal LTD. Int J Mol Sci. 2020;21(18):6491.
Article CAS PubMed Central Google Scholar
Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–9.
Pocock D, Leeb K, Wise RA. Elevations and phasic fluctuations in nucleus accumbens dopamine during IV heroin self-administration. Soc Neurosci Abstr. 1994;20:1234.
Aghajanian GK, Bunney BS. Dopamine “autoreceptors”: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn-Schmiedeberg’s Arch Pharmacol. 1977;297(1):1–7.
Wise RA. Intravenous drug self-administration: a special case of positive reinforcement. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. p. 117–41.
Bozarth MA, Wise RA. Heroin reward is dependent on a dopaminergic substrate. Life Sci. 1981;29(18):1881–6.
Bechara A, Harrington F, Nader K, van der Kooy D. Neurobiology of motivation: Double dissociation of two motivational mechanisms mediating opiate reward in drug-naive versus drug-dependent rats. Behav Neurosci. 1992;106:798–807.
Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiologica Scandanavica. 1986;128:351–8.
Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem Pharmacol. 2013;86(8):1181–93.
Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314.
Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology. 1991;104:171–6.
Corrigall WA, Franklin KBJ, Coen KM, Clarke P. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–9.
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–7.
Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436(7047):103–7.
Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S, et al. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol Psychiat. 2013;18(3):382–93.
Acquas E, Carboni E, Leone P, Di Chiara G. SCH 23390 blocks drug-conditioned place-preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade? Psychopharmacology. 1989;99(2):151–5.
Huang YY, Kandel DB, Kandel ER, Levine A. Nicotine primes the effect of cocaine on the induction of LTP in the amygdala. Neuropharmacology. 2013;74:126–34.
Chen YH, Hsieh TH, Kuo TT, Kao JH, Ma KH, Huang EY, et al. Release parameters during progressive degeneration of dopamine neurons in a mouse model reveal earlier impairment of spontaneous than forced behaviors. J Neurochem. 2019;150(1):56–73.
Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21(8):323–31.
Wise RA. Maximization of ethanol intake in the rat. Advances in Exper Med Biol. 1975;59:279–94.
Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29(3):203–10.
Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exper Res. 1986;10:436–42.
Sinclair JD, Hyytiä P, Nurmi M. The limited access paradigm: description of one method. Alcohol (Fayetteville, NY). 1992;9(5):441–4.
Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exper Res. 2008;32(10):1816–23.
Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–90.
Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol (Fayetteville, NY). 1996;13(2):163–70.
Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98(4):2297–310.
Doyon WM, Ostroumov A, Ontiveros T, Gonzales RA, Dani JA. Ethanol produces multiple electrophysiological effects on ventral tegmental area neurons in freely moving rats. Addiction Biol. 2021;26(2):e12899.
Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508(1):65–9.
Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology. 1992;109:92–8.
Samson HH, Hodge CW, Tolliver GA, Haraguchi M. Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull. 1993;30:133–41.
Ting AKR, Dockstader C, Heinmiller A, Grieder T, van der Kooy D. GABA(A) receptors mediate the opposing roles of dopamine and the tegmental pedunculopontine nucleus in the motivational effects of ethanol. Eur J Neurosci. 2009;29(6):1235–44.
Laviolette SR, van der Kooy D. GABA(A) receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. Eur J Neurosci. 2001;13(5):1009–15.
Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activataion of mesolimbic dopamine ransmission by a common µ 1 opioid receptor mechanism. Science. 1997;276:2048–50.
Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology. 2003;169(2):135–40.
Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990;102(2):156–62.
Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48(8):1105–16.
Oleson EB, Cachope R, Fitoussi A, Tsutsui K, Wu S, Gallegos JA, et al. Cannabinoid receptor activation shifts temporally engendered patterns of dopamine release. Neuropsychopharmacology. 2014;39(6):1441–52.
Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34(3):759–66.
Mansbach RS, Nicholson KL, Martin BR, Balster RL. Failure of Delta(9)-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behav Pharmacol. 1994;5(2):219–25.
Wakeford AGP, Wetzell BB, Pomfrey RL, Clasen MM, Taylor WW, Hempel BJ, et al. The effects of cannabidiol (CBD) on ∆ 9 -tetrahydrocannabinol (THC) self-administration in male and female Long-Evans rats. Exper Clin Psychopharmacol. 2017;25(4):242–8.
Freels TG, Baxter-Potter LN, Lugo JM, Glodosky NC, Wright HR, Baglot SL, et al. Vaporized cannabis extracts have reinforcing properties and support conditioned drug-seeking behavior in rats. J Neurosci. 2020;40(9):1897–908.
Smoker MP, Mackie K, Lapish CC, Boehm SL 2nd. Self-administration of edible Δ(9)-tetrahydrocannabinol and associated behavioral effects in mice. Drug Alcohol Depend. 2019;199:106–15.
Jordan CJ, Xi ZX. Progress in brain cannabinoid CB(2) receptor research: from genes to behavior. Neurosci Biobehav Rev. 2019;98:208–20.
Mateo Y, Johnson KA, Covey DP, Atwood BK, Wang HL, Zhang S, et al. Endocannabinoid actions on cortical terminals orchestrate local modulation of dopamine release in the nucleus accumbens. Neuron. 2017;96(5):1112-26.e5.
Davis MI, Crittenden JR, Feng AY, Kupferschmidt DA, Naydenov A, Stella N, et al. The cannabinoid-1 receptor is abundantly expressed in striatal striosomes and striosome-dendron bouquets of the substantia nigra. PLoS ONE. 2018;13(2):e0191436.
Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26(18):4901–7.
Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969;16:30–48.
Pickens R, Muchow D, DeNoble V. Methohexital-reinforced responding in rats: effects of fixed ratio size and injection dose. J Pharmacol Exp Ther. 1981;216(2):205–9.
Griffiths RR, Lukas SE, Bradford LE, Brady JV, Snell JD. Self-injection of barbiturates and benzodiazepines in baboons. Psychopharmacology. 1981;75:101–9.
Szostak C, Finlay JM, Fibiger HC. Intravenous self-administration of the short-acting benzodiazepine midazolam in the rat. Neuropharmacology. 1987;26:1673–6.
Ikemoto S, Kohl RR, McBride WJ. GABA(A) receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem. 1997;69:137–43.
Ikemoto S, Murphy JM, McBride WJ. Regional differences within the rat ventral tegmental area for muscimol self-infusions. Pharmacol Biochem Behav. 1998;61:87–92.
Heikkinen AE, Möykkynen TP, Korpi ER. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology. 2009;34(2):290–8.
Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463(7282):769–74.
Soden ME, Chung AS, Cuevas B, Resnick JM, Awatramani R, Zweifel LS. Anatomic resolution of neurotransmitter-specific projections to the VTA reveals diversity of GABAergic inputs. Nat Neurosci. 2020;23:968.
Vashchinkina E, Panhelainen A, Aitta-Aho T, Korpi ER. GABAA receptor drugs and neuronal plasticity in reward and aversion: focus on the ventral tegmental area. Front Pharmacol. 2014;5:256.
Di Chiara G, Imperato A. Preferential stimulation of dopamine release in the nucleus accumbens by opiates, alcohol, and barbiturates: studies with transcerebral dialysis in freely moving rats. Ann NY Acad Sci. 1986;473:367–81.
O’Brien DP, White FJ. Inhibition of non-dopamine cells in the ventral tegmental area by benzodiazepines: relationship to A10 dopamine cell activity. Eur J Pharmacol. 1987;142:343–54.
Schelp SA, Brodnik ZD, Rakowski DR, Pultorak KJ, Sambells AT, Espana RA, et al. Diazepam concurrently increases the frequency and decreases the amplitude of transient dopamine release events in the nucleus accumbens. J Pharmacol Exp Ther. 2018;364(1):145–55.
van der Kooij MA, Hollis F, Lozano L, Zalachoras I, Abad S, Zanoletti O, et al. Diazepam actions in the VTA enhance social dominance and mitochondrial function in the nucleus accumbens by activation of dopamine D1 receptors. Mol Psychiatry. 2018;23(3):569–78.
Horger BA, Wellman PJ, Morien A, Davies BT, Schenk S. Caffeine exposure sensitizes rats to the reinforcing effects of cocaine. NeuroReport. 1991;2:53–6.
Bradley CA, Palmatier MI. Intravenous and oral caffeine self-administration in rats. Drug Alcohol Depend. 2019;203:72–82.
Myers KP, Izbicki EV. Reinforcing and aversive effects of caffeine measured by flavor preference conditioning in caffeine-naive and caffeine-acclimated rats. Physiol Behav. 2006;88(4–5):585–96.
Yee M, Maal-Bared G, Ting AKR, Chwalek M, Mackay-Clackett I, Bergamini M, et al. Segregation of caffeine reward and aversion in the rat nucleus accumbens shell versus core. Eur J Neurosci. 2020;52(3):3074–86.
Galvalisi M, Prieto JP, Martinez M, Abin-Carriquiry JA, Scorza C. Caffeine induces a stimulant effect and increases dopamine release in the nucleus accumbens shell through the pulmonary inhalation route of administration in rats. Neurotox Res. 2017;31(1):90–8.
Volkow ND, Wang GJ, Logan J, Alexoff D, Fowler JS, Thanos PK, et al. Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Trans Psychiat. 2015;5:e549.
Zheng X, Takatsu S, Wang H, Hasegawa H. Acute intraperitoneal injection of caffeine improves endurance exercise performance in association with increasing brain dopamine release during exercise. Pharmacol Biochem Behav. 2014;122:136–43.
Holstein SE, Barkell GA, Young MR. Caffeine increases alcohol self-administration, an effect that is independent of dopamine D(2) receptor function. Alcohol (Fayetteville, NY). 2021;91:61–73.
Download references
Acknowledgements
Author information, authors and affiliations.
Intramural Research Program, National Institute on Drug Abuse, 250 Mason Lord Drive, Baltimore, MD, USA
Roy A. Wise
Behavior Genetics Laboratory, McLean Hospital, Harvard Medical School, 115 Mill Street, Belmont, MA, 02478, USA
Division of Alcohol, Drugs and Addiction, Department of Psychiatry, Harvard Medical School, McLean Hospital, 115 Mill Street, Belmont, MA, 02478, USA
Chloe J. Jordan
You can also search for this author in PubMed Google Scholar
Contributions
RAW and CJJ each read the cited articles, planned the review, wrote the review, responded to reviewer comments, and modified the references for citation style. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Roy A. Wise .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wise, R.A., Jordan, C.J. Dopamine, behavior, and addiction. J Biomed Sci 28 , 83 (2021). https://doi.org/10.1186/s12929-021-00779-7
Download citation
Received : 11 May 2021
Accepted : 19 November 2021
Published : 02 December 2021
DOI : https://doi.org/10.1186/s12929-021-00779-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Journal of Biomedical Science
ISSN: 1423-0127
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
HYPOTHESIS AND THEORY article
The dopamine hypothesis of drug addiction and its potential therapeutic value.

- ‘G. Minardi’ Cognitive Neuroscience Laboratory, Department of Drug Sciences, University of Sassari, Sassari, Italy
Dopamine (DA) transmission is deeply affected by drugs of abuse, and alterations in DA function are involved in the various phases of drug addiction and potentially exploitable therapeutically. In particular, basic studies have documented a reduction in the electrophysiological activity of DA neurons in alcohol, opiate, cannabinoid, and other drug-dependent rats. Further, DA release in the Nucleus accumbens (Nacc) is decreased in virtually all drug-dependent rodents. In parallel, these studies are supported by increments in intracranial self stimulation (ICSS) thresholds during withdrawal from alcohol, nicotine, opiates, and other drugs of abuse, thereby suggesting a hypofunction of the neural substrate of ICSS. Accordingly, morphological evaluations fed into realistic computational analysis of the medium spiny neuron of the Nacc, post-synaptic counterpart of DA terminals, show profound changes in structure and function of the entire mesolimbic system. In line with these findings, human imaging studies have shown a reduction of dopamine receptors accompanied by a lesser release of endogenous DA in the ventral striatum of cocaine, heroin, and alcohol-dependent subjects, thereby offering visual proof of the “ dopamine-impoverished” addicted human brain. The lasting reduction in physiological activity of the DA system leads to the idea that an increment in its activity, to restore pre-drug levels, may yield significant clinical improvements (reduction of craving, relapse, and drug-seeking/taking). In theory, it may be achieved pharmacologically and/or with novel interventions such as transcranial magnetic stimulation (TMS). Its anatomo-physiological rationale as a possible therapeutic aid in alcoholics and other addicts will be described and proposed as a theoretical framework to be subjected to experimental testing in human addicts.
Drug addiction is a brain disease that produces profound modifications in human behavior ( Hyman, 2007 ; Koob and Volkow, 2010 ), with important negative consequences at various levels, including personal health, employment, family interactions, and society in general ( Chandler et al., 2009 ). Therapeutic possibilities for this devastating illness are, with some rare exceptions, limited to pharmacologic treatments that are largely unsatisfactory ( Koob et al., 2009 ; Leggio et al., 2010 ; Swift, 2010 ). From here the necessity to develop new therapeutic hypothesis/interventions independent from those commonly employed.
Transcranial magnetic stimulation (TMS), through generation of an electromagnetic field capable of crossing painlessly through the skull and influencing the underlying brain matter, appears to be a promising candidate for treating addictive behaviors ( Barr et al., 2008 ; Feil and Zangen, 2010 ) and other brain diseases ( Kobayashi and Pascual-Leone, 2003 ). In brief, this relatively new method allows modulation of discrete brain areas of the awake and conscious subject under study. The pulsatile electromagnetic field generated around the coil crosses the skull and is capable of directly exciting/inhibiting neurons in the underlying cortices ( Padberg and George, 2009 ). Commonly employed as a research tool, TMS is recently affirming its role as a potential therapeutic means approved by the Food and Drug Administration for brain pathologies such as drug-resistant major depression, bipolar syndrome, and negative symptoms of schizophrenia. In the drug addiction field, the therapeutic potential of TMS has been tested in nicotine-dependent subjects ( Lang et al., 2008 ; Amiaz et al., 2009 ), cocaine addicts ( Boutros et al., 2001 , 2005 ; Sundaresan et al., 2007 ; Politi et al., 2008 ), and alcoholics ( Conte et al., 2008 ; Mishra et al., 2010 ). Although the results are certainly encouraging, the disparity of clinical outcomes evaluated in different studies and diversity of pattern/site/methodology of stimulation precludes direct comparisons and hampers firm conclusions. However, in those studies in which craving was measured ( Politi et al., 2008 ; Amiaz et al., 2009 ; Mishra et al., 2010 ) significant reductions have been found, thus encouraging further experimental scrutiny. At present, we are evaluating anti-craving and alcohol-intake efficacy of TMS in alcoholics (Addolorato et al., in preparation), short and long-term cocaine intake in treatment-seeking cocaine addicts (Pedetti et al., in preparation), and money/cocaine choice in a lab study of cocaine addicts non-seeking treatment (Martinez et al., in preparation). Nevertheless, the brain site(s) to be stimulated/inhibited and the stimulation parameters (i.e., frequency of stimulation, number of session etc.,) are matters of intense debate and an appropriate rationale is needed.
Dopamine as a Possible Therapeutic Target
The role of central DA systems in the acute effects of drugs of abuse was recognized long ago ( Wise, 1980 , 1987 ; Di Chiara and Imperato, 1988 ). Even before ( Ahlenius et al., 1973 ), attempts were made to prevent human alcohol-induced euphoria through administration of the DA synthesis inhibitor alpha methyl-para-tyrosine. Although theoretically ineccepibile, this approach (reduction of drug-induced DA increments to prevent abuse) is unlikely to have a practical validity as any compound with DA antagonistic (i.e., neuroleptics) properties is known to be aversive in humans. On the other hand, widely documented experimental evidence suggests that the mesolimbic dopamine system is “hypofunctional” in the addicted brain ( Melis et al., 2005 ). In brief, the hypothesis contends that decreased DA function in addicted subjects results in a decreased interest to non-drug-related stimuli and increased sensitivity to the drug of choice ( Melis et al., 2005 ), leading to propose that restoring DA function might be therapeutically advantageous.
Alcohol-dependent (in the present context the term “dependent,” when referred to a non-human experimental subject, indicates a condition in which the subject has shown unequivocally a proof of dependency, i.e., somatic signs of withdrawal) rats show a profound reduction of spontaneous firing rate and burst firing of antidromically identified Nucleus accumbens (Nacc)-projecting ventral tegmental area (VTA) DA-containing neurons in rats ( Diana et al., 1993 ) and mice ( Bailey et al., 2001 ) resulting in a concomitant reduction of microdialysate DA in the Nacc ( Rossetti et al., 1992 ; Diana et al., 1993 ; Barak et al., 2011 ). Further, the reduced dopaminergic activity outlasts somatic signs of alcohol-withdrawal ( Diana et al., 1996 , 2003 ) thereby suggesting a role for DA in the lasting consequences of alcohol dependence while excluding the possibility of a DA role in somatic aspects of withdrawal. Further, original (pre-dependence) DA levels in the Nacc are restored when ethanol is self ( Weiss et al., 1996 ) and/or passively administered ( Diana et al., 1993 , 1996 ). These observations are paralleled by intracranial self stimulation (ICSS) studies showing that ethanol-withdrawn rats are capable of maintaining the ICSS behavior provided that the stimulus current intensity is increased ( Schulteis et al., 1995 ). This important observation strongly indicates that the neural substrate responsible for maintaining the ICSS behavior is hyperpolarized, or more refractory, in the alcohol-dependent subject as compared with its control. Since the neural substrate of ICSS involves DA axons ( Yeomans, 1989 ; Yeomans et al., 1993 ) near the stimulating electrode, the results are complementary to those reported above and well support a deficitary function of DA neurons. In addition, the perseverance of the reduction in DA activity (beyond resolution of somatic signs of withdrawal) has also been documented in morphine-dependent rats ( Diana et al., 1999 ), while a dichotomy between DA function and somatic withdrawal has been observed in cannabinoid–withdrawn rats ( Diana et al., 1998 ). Similarly, conditioned heroin withdrawal decreases reward sensitivity ( Kenny et al., 2006 ) which persists well beyond the initial phase of withdrawal. These findings, observed across different addicting compounds and experimental conditions, suggest that DA hypofunction persists over time, although reverting to “normality” ( Diana et al., 1999 , 2006 ), eventually with species-specific time course.
In addition to basic literature, reports in humans are also supportive of a compromised role of DA transmission in alcoholics. While alcohol increases DA release in healthy subjects ( Boileau et al., 2003 ) with some gender differences ( Urban et al., 2010 ), a reduced number of DA receptors has been observed ( Volkow et al., 1996 ; Martinez et al., 2005 ) in alcoholics that appears to be accompanied by a blunted DA release ( Martinez et al., 2005 , 2007 ; Volkow et al., 2007 ). While the reduced number of DA receptors could be, at first sight, be viewed as suggesting an increased DA release, it should be noted that by administering the DA inhibitor alpha methyl-para-tyrosine, Martinez et al. (2009) were able to exclude this possibility. Indeed, while healthy controls do show an increased raclopride binding after acute alpha methyl-para-tyrosine administration, cocaine-dependent subjects do not (or to a significantly lesser extent; Martinez et al., 2009 ). Similar results were obtained with the dopamine releasing agent methylphenidate ( Volkow et al., 2007 ) and amphetamine ( Martinez et al., 2005 ) in alcoholics. Notably, artificially increasing the brain levels of DAD2 receptors, using a replication-deficient adenoviral vector containing the rat cDNA insert for DAD2 into the Nacc, reduces alcohol intake in spontaneously drinking rats, thereby offering the counterproof that a potentiation of DA transmission may have beneficial effects on alcohol-seeking and alcohol-taking, in experimental models ( Thanos et al., 2001 , 2004 ). In line with this conclusion, a spontaneous high number of DA D2 receptors has been shown to have a protective role in non-alcoholic members of alcoholic families ( Volkow et al., 2006 ). These findings further support the notion that the number of DA receptors (and consequently DA transmission) inversely correlates with alcohol drinking.
These observations may suggest that “ boosting” DA neurons to produce more available DA in the synaptic cleft could alleviate some of the symptoms of addiction and alcoholism, thereby acquiring a therapeutic character. In theory, this could be achieved by two different strategies: (1) DA-potentiating drugs and (2) TMS. Both possibilities are discussed below.
Dopamine-Potentiating Drugs
Although medications that increase DA activity could be effective in treating alcohol abuse disorders, conflicting results have been produced ( Swift, 2010 ). For example, it was suggested that the DA agonist bromocriptine reduced drinking in alcoholics ( Lawford et al., 1995 ), but a randomized, double-blind, placebo-controlled study using a long-acting injectable bromocriptine preparation in 366 alcoholic-dependent individuals did not find difference in alcohol relapse between medication and placebo ( Naranjo et al., 1997 ). Another example is the stimulant medication modafinil (DA indirect agonist), found to improve cognition in 40 alcoholics with organic brain syndrome, but effects on drinking could not be measured ( Saletu et al., 1990 ). However, modafinil reduced cocaine use in a placebo-controlled study with 62 cocaine-dependent individuals ( Dackis and O’Brien, 2005 ), while another trial did not find differences between modafinil and placebo tested for methamphetamine users ( Shearer et al., 2010 ). While evidence for the use of DA agonists as a treatment for alcohol and/or substance use disorders is inconclusive ( Swift, 2010 ), there has been a revived interest for these drugs, possibly because adequate neurobiological rationale ( Melis et al., 2005 ) is now available. For example, aripiprazole ( Semba et al., 1995 ; Burris et al., 2002 ; Shapiro et al., 2003 ) a partial DA agonist which in principle should antagonize DA when tone is high, whereas should increase DA transmission when basic tone is low, represents a proposed treatment for alcohol abuse disorders ( Kenna et al., 2009 ). Human laboratory alcohol studies have shown that aripiprazole reduces drinking ( Kranzler et al., 2008 ), especially in the more impulsive alcoholic ( Voronin et al., 2008 ). An fMRI study demonstrated that aripiprazole significantly attenuates neural activity in the ventral striatum in response to alcohol cues ( Myrick et al., 2010 ) thereby suggesting a therapeutic potential for cue-induced relapse. Further, a 12-week, double-blind, placebo-controlled treatment study with 295 alcohol-dependent individuals found that aripiprazole initially decreased heavy drinking days compared to placebo, but this significant effect was not present when the target dose of 30 mg was reached ( Anton et al., 2008 ). This trial also showed greater side-effects and greater study discontinuation in the aripiprazole arm, as compared to placebo ( Anton et al., 2008 ). Interestingly, an open-label study of aripiprazole ( Martinotti et al., 2009 ) and a recent human laboratory study ( Kenna et al., 2009 ) suggests that lower doses of aripiprazole (5–15 mg per day) may be better tolerated and still reduce drinking with effects on relapse comparable to those obtained with the opiate antagonist naltrexone ( Martinotti et al., 2009 ).
In summary, dopamine plays a key role in the addiction process, but significant side-effects have limited the use of medications that work directly on the dopaminergic system. The use of DA partial agonists with lower side effect profiles, and appropriate dosing represent important directions for future research in this area.
Transcranial Magnetic Stimulation
Increasing DA tone with appropriate pharmacological tools, is only one of the possible strategies. Endogenous activity of DA-containing neurons can be augmented with non-pharmacological tools such as TMS ( Strafella et al., 2001 ) thereby providing, in principle, an adjunct to the “therapeutic arsenal” against addiction, endowed with lesser systemic side-effects and limited contraindications. However, while the rationale is “neurochemical” for pharmacological agents (neurotransmitter receptors, brain area etc.,) , it must be anatomically based for TMS. Being that DA-containing neurons are located deeply in the brainstem (thereby making the neurons inaccessible to direct TMS stimuli) it becomes unavoidable to reach them indirectly through neurons located elsewhere in the brain. The dorsolateral prefrontal cortex (DLPfcx) by projecting monosynaptically to the rat ( Carr and Sesack, 2000 ) and primate ( Frankle et al., 2006 ) VTA may serve this function. These studies show a projection from the PFC to midbrain DA neurons, terminating both within the SN proper as well as in the VTA. They arise from a broad region of the PFC, including the DLPfcx, cingulate, and orbital cortices. Indeed, these pyramidal neurons (Figure 1 ) could be exploited as the primary target of the TMS stimulus and their increased activity to produce, ultimately, an enhancement in DA availability in the synaptic cleft in the Nacc. Schematically, the hypothesized circuit (Figure 2 ) would be the following: TMS → DLPfcx → VTA → DA increase in forebrain projection site (i.e., Nacc). In this context, it is imperative to employ stimulation parameters consonant with the physiological activity of the system under study to restore pre-drug DA levels. For instance, it has been shown that DLPfcx stimulation produces bursts in rat DA neurons ( Gariano and Groves, 1988 ; Murase et al., 1993 ), highlighting the importance of stimulation parameters. Indeed, burst firing is more efficacious than single spiking (of identical frequency but evenly spaced action potentials) in inducing DA release in terminal areas ( Gonon, 1988 ; Manley et al., 1992 ). Consistently, the role of DLPfcx in regulating basal DA activity through the VTA has been reported ( Taber et al., 1995 ; Karreman and Moghaddam, 1996 ).
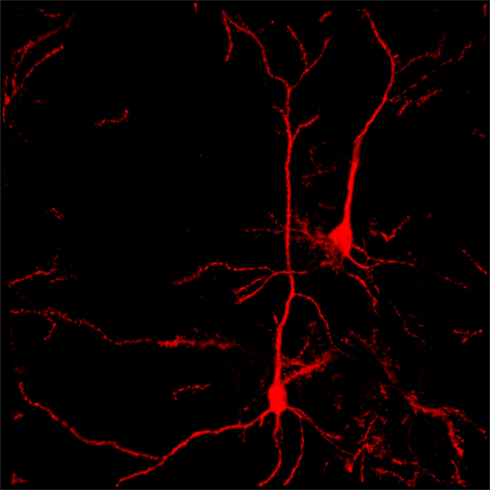
Figure 1. Confocal reconstruction of Golgi-stained pyramidal neurons from DLPfcx obtained by a projection of 55 scans for a depth of 27.5 μm in the z -axis . DLPfxc may represent a useful target for rTMS stimulation.
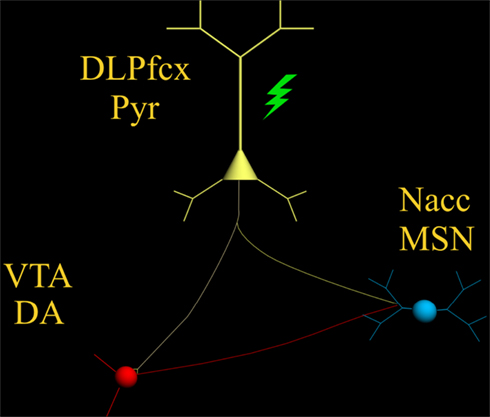
Figure 2. The scheme illustrates the proposed circuit to be activated by the TMS stimulus (green) which, by activating the pyramidal neuron (yellow) with its neurotransmitter glutamate, would excite: (1) DA-containing neurons of the VTA (red) and (2) MSN of the Nacc (blue) .
Among the various factors that are likely to influence its efficacy, the importance of the baseline cortical activation state on the impact of TMS is fundamental ( Silvanto and Pascual-Leone, 2008 ). This state-dependency is key as the neural impact of any external stimulus represents an interaction with the ongoing brain activity at the time of stimulation. The effects of any external stimulus are therefore not only determined by the properties of that stimulus but also by the activation state of the brain. Accordingly, it has been shown that baseline cortical activity determines whether TMS hampers or hastens behavior ( Silvanto et al., 2008 ). The state-dependency principle described above would also apply to the state of the DA system. The hypodopaminergic state ( Melis et al., 2005 ) should then “amplify” the effect of TMS as compared with that expected in a normo-functioning DA system.
The responsivity of the neuron(s) to electrical and synaptic stimuli is strictly dependent on its morphological features, which in turn, are deeply modified by drugs of abuse ( Robinson and Kolb, 2004 ) and withdrawal from chronic treatment with opiates ( Sklair-Tavron et al., 1996 ; Spiga et al., 2003 , 2005 ), cannabis derivatives/analogs ( Spiga et al., 2010 ), and psychostimulants ( Robinson and Kolb, 1997 ) have been shown to produce reductions in DA cells size ( Sklair-Tavron et al., 1996 ; Spiga et al., 2003 ), paralleled by persistently ( Diana et al., 2006 ) altered patterns of synaptic connectivity, and spines density in the Nacc and Pfcx ( Robinson and Kolb, 1997 ). These architectural changes would be expected to modify intrinsic spontaneous action potential generating capacity and responsiveness of the system to the TMS stimuli. Accordingly, realistic computational analysis ( Spiga et al., 2010 ) of cannabis-dependent rats, generated by input of experimentally verified morphometrical and electrophysiological properties, predicts a lower action potential generation of Nacc medium spiny neuron (MSN). These results suggest that MSN, of cannabis-dependent rats are likewise hypofunctional. Considering that the main drive of these neurons is cortical glutamate (Glu; see discussion in Spiga et al., 2010 , and references therein; Kalivas and Hu, 2006 ) it raises the possibility of a reduction of Glu as a causal factor. This finding, thus offers the additional possibility that stimulation of these units through TMS may be advantageous in restoring pre-drug physiological activity. Indeed, TMS cortical application should increase the activity of glutamate-containing cortico-fugal fibers monosynaptically impinging upon the spine’s heads of Nacc MSN ( Groenewegen et al., 1991 ). Considering the fundamental role Glu plays in synaptic plasticity ( Russo et al., 2010 ), its role could also be exploited in LTP-like stimulation parameters, ultimately aimed at producing lasting and enduring restoration of original physiological activity. These characteristics must be considered and coherently inserted into a framework to obtain optimal stimulation parameters. In vivo recordings of VTA-projecting DLPfcx neurons do fire spontaneously around 4–6 Hz ( Pistis et al., 2001 ) and a TMS stimulus frequency of 10 Hz could be a reasonable frequency to obtain a significant increase in VTA-projecting neurons aimed at stimulating the “ deficient” dopamine system and its post-synaptic counterpart (i.e., MSN of the Nacc).
Another factor to be considered is that all previous studies (see above) applied the TMS stimulus monolaterally, yet obtaining a reduction of alcohol craving ( Mishra et al., 2010 ). While alcohol intake was not measured, and contralateral effects cannot be excluded a priori , it is possible that application of TMS bilaterally, as in the case of the H-coil ( Feil and Zangen, 2010 ), would yield stronger cortical activation (larger number of fibers activated) with an increased probability of a significant increment of bilateral DA release. It should be noted that unilateral TMS application has already been reported to increase DA release ( Strafella et al., 2001 ) omolaterally in the human striatum, as well as in rodents ( Keck et al., 2002 ; Zangen and Hyodo, 2002 ), and even in morphine-withdrawn rats ( Erhardt et al., 2004 ), thereby supporting the rationale outlined above. Although Strafella et al. (2001) proposed activation of (Glu-containing) cortico-fugal fibers making synaptic contact with DA-containing terminals in the ventral striatum, to explain their results, it should be noted that the existence of axo-axonic contacts has always being questioned based on the lack of appropriate anatomical observations ( Groenewegen et al., 1991 ; Meredith et al., 2008 ).
While many technical details for optimal stimulation parameters need further investigation and optimization, the TMS appears to deserve careful experimental scrutiny as a potential therapeutic tool in alcoholics and other addicts. Indeed, with its nearly absent systemic effects, minimal side-effects, and a low degree of invasiveness, TMS may offer the first opportunity for an efficacious, non-pharmacological, therapeutic tool in alcoholism and other chemical dependencies. If appropriately combined with a solid neurobiological rationale (DA system), it may offer a unique opportunity for developing further the first “ electrophysiological” approach in studying and eventually treating the devastating and widespread brain disease of addiction.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported, in part, by grants from MIUR (PRIN. N°2004052392) and Dipartimento Politiche Antidroga. The author wish to thank S. Spiga for elaborating iconographic material presented.
Ahlenius, S., Carlsson, A., Engel, J., Svensson, T., and Södersten, P. (1973). Antagonism by alpha methyltyrosine of the ethanol-induced stimulation and euphoria in man. Clin. Pharmacol. Ther. 14, 586–591.
Pubmed Abstract | Pubmed Full Text
Amiaz, R., Levy, D., Vainiger, D., Grunhaus, L., and Zangen, A. (2009). Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 104, 653–660.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Anton, R. F., Kranzler, H., Breder, C., Marcus, R. N., Carson, W. H., and Han, J. (2008). A randomized, multicenter, double-blind, placebo-controlled study of the efficacy and safety of aripiprazole for the treatment of alcohol dependence. J. Clin. Psychopharmacol. 28, 5–12.
Bailey, C. P., O’Callaghan, M. J., Croft, A. P., Manley, S. J., and Little, H. J. (2001). Alterations in mesolimbic dopamine function during the abstinence period following chronic ethanol consumption. Neuropharmacology 41, 989–999.
Barak, S., Carnicella, S., Yowell, Q. W., and Ron, D. (2011). Glial cell line-derived neurotrophic factor reverses alcoho-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J. Neurosci. 31, 9885–9894.
Barr, M. S., Fitzgerald, P. B., Farzan, F., George, T. P., and Daskalakis, Z. J. (2008). Transcranial magnetic stimulation to understand the pathophysiology and treatment of substance use disorders. Curr. Drug Abuse Rev. 1, 328–339.
Boileau, I., Assaad, J. M., Pihl, R. O., Benkelfat, C., Leyton, M., Diksic, M., Tremblay, R. E., and Dagher, A. (2003). Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 15, 226–231.
CrossRef Full Text
Boutros, N. N., Lisanby, S. H., McClain-Furmanski, D., Oliwa, G., Gooding, D., and Kosten, T. R. (2005). Cortical excitability in cocaine-dependent patients: a replication and extension of TMS findings. J. Psychiatr. Res. 39, 295–302.
Boutros, N. N., Lisanby, S. H., Tokuno, H., Torello, M. W., Campbell, D., Berman, R., Malison, R., Krystal, J. H., and Kosten, T. (2001). Elevated motor threshold in drug-free, cocaine-dependent patients assessed with transcranial magnetic stimulation. Biol. Psychiatry 49, 369–373.
Burris, K. D., Molski, T. F., Xu, C., Ryan, E., Tottori, K., Kikuchi, T., Yocca, F. D., and Molinoff, P. B. (2002). Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J. Pharmacol. Exp. Ther. 302, 381–389.
Carr, D. B., and Sesack, S. R. (2000). Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J. Neurosci. 20, 3864–3873.
Chandler, R. K., Fletcher, B. W., and Volkow, N. D. (2009). Treating drug abuse and addiction in the criminal justice system: improving public health and safety. JAMA 301, 183–190.
Conte, A., Attilia, M. L., Gilio, F., Iacovelli, E., Frasca, V., Bettolo, C. M., Gabriele, M., Giacomelli, E., Prencipe, M., Berardelli, A., Ceccanti, M., and Inghilleri, M. (2008). Acute and chronic effects of ethanol on cortical excitability. Clin. Neurophysiol. 119, 667–674.
Dackis, C., and O’Brien, C. (2005). Neurobiology of addiction: treatment and public policy ramifications. Nat. Neurosci. 8, 1431–1436.
Di Chiara, G., and Imperato, A. (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U.S.A. 85, 5274–5278.
Diana, M., Brodie, M., Muntoni, A., Puddu, M. C., Pillolla, G., Steffensen, S., Spiga, S., and Little, H. J. (2003). Enduring effects of chronic ethanol in the CNS: basis for alcoholism. Alcohol. Clin. Exp. Res. 27, 354–361.
Diana, M., Melis, M., Muntoni, A. L., and Gessa, G. L. (1998). Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc. Natl. Acad. Sci. U.S.A. 18, 10269–10273.
Diana, M., Muntoni, A. L., Pistis, M., Melis, M., and Gessa, G. L. (1999). Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur. J. Neurosci. 11, 1037–1041.
Diana, M., Pistis, M., Carboni, S., Gessa, G. L., and Rossetti, Z. L. (1993). Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc. Natl. Acad. Sci. U.S.A. 90, 7966–7969.
Diana, M., Pistis, M., Muntoni, A., and Gessa, G. L. (1996). Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: Evidence of protracted abstinence. Neuroscience 71, 411–415.
Diana, M., Spiga, S., and Acquas, E. (2006). Persistent and reversible morphine withdrawal-induced morphological changes in the nucleus accumbens. Ann. N. Y. Acad. Sci. 1074, 446–457.
Erhardt, A., Sillaber, I., Welt, T., Müller, M. B., Singewald, N., and Keck, M. E. (2004). Repetitive transcranial magnetic stimulation increases the release of dopamine in the nucleus accumbens shell of morphine-sensitized rats during abstinence. Neuropsychopharmacology 29, 2074–2080.
Feil, J., and Zangen, A. (2010). Brain stimulation in the study and treatment of addiction. Neurosci. Biobehav. Rev. 34, 559–574.
Frankle, W. G., Laruelle, M., and Haber, S. N. (2006). Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection. Neuropsychopharmacology 31, 1627–1636.
Gariano, R. F., and Groves, P. M. (1988). Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res. 462, 194–198.
Gonon, F. (1988). Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience 24, 19–28.
Groenewegen, H. J., Berendse, H. W., Meredith, G. E., Haber, S. N., Voorn, P., Wolters, J. G., and Lohman, A. H. M. (1991). “Functional anatomy of the ventral, limbic system-innervated striatum,” in The Mesolimbic Dopamine System: From Motivation to Action , eds P. Willner and J. Scheel-Krüger (New York: Wiley), 19–59.
Hyman, S. E. (2007). The neurobiology of addiction: implications for voluntary control of behavior. Am. J. Bioeth. 7, 8–11.
Kalivas, P. W., and Hu, X. T. (2006). Exciting inhibition in psychostimulation addiction. Trends Neurosci. 29, 610–616.
Karreman, M., and Moghaddam, B. (1996). The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J. Neurochem. 66, 589–598.
Keck, M. E., Welt, T., Müller, M. B., Erhardt, A., Ohl, F., Toschi, N., Holsboer, F., and Sillaber, I. (2002). Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology 43, 101–109.
Kenna, G. A., Leggio, L., and Swift, R. M. (2009). A safety and tolerability laboratory study of the combination of aripiprazole and topiramate in volunteers who drink alcohol. Hum. Psychopharmacol. 24, 465–472.
Kenny, P. J., Chen, S. A., Kitamura, O., Markou, A., and Koob, G. F. (2006). Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J. Neurosci. 26, 5894–5900.
Kobayashi, M., and Pascual-Leone, A. (2003). Transcranial magnetic stimulation in neurology. Lancet Neurol. 2, 145–156.
Koob, G. F., Kenneth Lloyd, G., and Mason, B. J. (2009). Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat. Rev. Drug Discov. 8, 500–515.
Koob, G. F., and Volkow, N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238.
Kranzler, H. R., Covault, J., Pierucci-Lagha, A., Chan, G., Douglas, K., Arias, A. J., and Oncken, C. (2008). Effects of aripiprazole on subjective and physiological responses to alcohol. Alcohol. Clin. Exp. Res. 32, 573–579.
Lang, N., Hasan, A., Sueske, E., Paulus, W., and Nitsche, M. A. (2008). Cortical hypoexcitability in chronic smokers? A transcranial magnetic stimulation study. Neuropsychopharmacology 33, 2517–2523.
Lawford, B. R., Young, R. M., Rowell, J. A., Qualichefski, J., Fletcher, B. H., Syndulko, K., Ritchie, T., and Noble, E. P. (1995). Bromocriptine in the treatment of alcoholics with the D2 dopamine receptor A1 allele. Nat. Med. 1, 337–341.
Leggio, L., Cardone, S., Ferrulli, A., Kenna, G. A., Diana, M., Swift, R. M., and Addolorato, G. (2010). Turning the clock ahead: potential preclinical and clinical neuropharmacological targets for alcohol dependence. Curr. Pharm. Des. 16, 2159–2181.
Manley, L. D., Kuczenski, R., Segal, D. S., Young, S. J., and Groves, P. M. (1992). Effects of frequency and pattern of medial forebrain bundle stimulation on caudate dialysate dopamine and serotonin. J. Neurochem. 58, 1491–1498.
Martinez, D., Gil, R., Slifstein, M., Hwang, D. R., Huang, Y., Perez, A., Kegeles, L., Talbot, P., Evans, S., Krystal, J., Laruelle, M., and Abi-Dargham, A. (2005). Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol. Psychiatry 58, 779–786.
Martinez, D., Greene, K., Broft, A., Kumar, D., Liu, F., Narendran, R., Slifstein, M., Van Heertum, R., and Kleber, H. D. (2009). Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am. J. Psychiatry 166, 1170–1177.
Martinez, D., Kim, J. H., Krystal, J., and Abi-Dargham, A. (2007). Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin. N. Am. 17, 539–555.
Martinotti, G., Di Nicola, M., Di Giannantonio, M., and Janiri, L. (2009). Aripiprazole in the treatment of patients with alcohol dependence: a double-blind, comparison trial vs. naltrexone. J. Psychopharmacol. 23, 123–129.
Melis, M., Spiga, S., and Diana, M. (2005). The dopamine hypothesis of drug addiction: hypodopaminergic state. Int. Rev. Neurobiol. 63, 101–154.
Meredith, G. E., Baldo, B. A., Andrezjewsky, M. E., and Kelley, A. E. (2008). The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct. Funct. 213, 17–27.
Mishra, B. R., Nizamie, S. H., Das, B., and Praharaj, S. K. (2010). Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 105, 49–55.
Murase, S., Grenhoff, J., Chouvet, G., Gonon, F. G., and Svensson, T. H. (1993). Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci. Lett. 157, 53–56.
Myrick, H., Li, X., Randall, P. K., Henderson, S., Voronin, K., and Anton, R. F. (2010). The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J. Clin. Psychopharmacol. 30, 365–372.
Naranjo, C. A., Dongier, M., and Bremner, K. E. (1997). Long-acting injectable bromocriptine does not reduce relapse in alcoholics. Addiction 92, 969–978.
Padberg, F., and George, M. S. (2009). Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp. Neurol. 219, 2–13.
Pistis, M., Porcu, G., Melis, M., Diana, M., and Gessa, G. L. (2001). Effects of cannabinoids on prefrontal neuronal responses to ventral tegmental area stimulation. Eur. J. Neurosci. 14, 96–102.
Politi, E., Fauci, E., Santoro, A., and Smeraldi, E. (2008). Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am. J. Addict. 17, 345–346.
Robinson, T. E., and Kolb, B. (1997). Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 17, 8491–8497.
Robinson, T. E., and Kolb, B. (2004). Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47(Suppl. 1), 33–46.
Rossetti, Z. L., Melis, F., Carboni, S., Diana, M., and Gessa, G. L. (1992). Alcohol withdrawal in rats is associated with a marked fall in extraneuronal dopamine. Alcohol. Clin. Exp. Res. 16, 529–532.
Russo, S. J., Dietz, D. M., Dumitriu, D., Morrison, J. H., Malenka, R. C., and Nestler, E. J. (2010). The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 33, 267–276.
Saletu, B., Saletu, M., Grünberger, J., Frey, R., Zatschek, I., and Mader, R. (1990). On the treatment of the alcoholic organic brain syndrome with an alpha-adrenergic agonist modafinil: double-blind, placebo-controlled clinical, psychometric and neurophysiological studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 14, 195–214.
Schulteis, G., Markou, A., Cole, M., and Koob, G. F. (1995). Decreased brain reward produced by ethanol withdrawal. Proc. Natl. Acad. Sci. U.S.A. 20, 5880–5884.
Semba, J., Watanabe, A., Kito, S., and Toru, M. (1995). Behavioural and neurochemical effects of OPC-14597, a novel antipsychotic drug, on dopaminergic mechanisms in rat brain. Neuropharmacology 34, 785–791.
Shapiro, D. A., Renock, S., Arrington, E., Chiodo, L. A., Liu, L. X., Sibley, D. R., Roth, B. L., and Mailman, R. (2003). Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 28, 1400–1411.
Shearer, J., Shanahan, M., Darke, S., Rodgers, C., van Beek, I., McKetin, R., and Mattick, R. P. (2010). A cost-effectiveness analysis of modafinil therapy for psychostimulant dependence. Drug Alcohol Rev. 29, 235–242.
Silvanto, J., Cattaneo, Z., Battelli, L., and Pascual-Leone, A. (2008). Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. J. Neurophysiol. 99, 2725–2730.
Silvanto, J., and Pascual-Leone, A. (2008). State-dependency of transcranial magnetic stimulation. Brain Topogr. 21, 1–10.
Sklair-Tavron, L., Shi, W. X., Lane, S. B., Harris, H. W., Bunney, B. S., and Nestler, E. J. (1996). Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc. Natl. Acad. Sci. U.S.A. 1, 11202–11207.
Spiga, S., Lintas, A., Migliore, M., and Diana, M. (2010). Altered architecture and functional consequences of the mesolimbic dopamine system in cannabis dependence. Addict Biol. 15, 266–276.
Spiga, S., Puddu, M. C., Pisano, M., and Diana, M. (2005). Morphine withdrawal-induced morphological changes in the nucleus accumbens. Eur. J. Neurosci. 22, 2332–2340.
Spiga, S., Serra, G. P., Puddu, M. C., Foddai, M., and Diana, M. (2003). Morphine withdrawal-induced abnormalities in the VTA: confocal laser scanning microscopy. Eur. J. Neurosci. 17, 605–612.
Strafella, A. P., Paus, T., Barrett, J., and Dagher, A. (2001). Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 21, RC157.
Sundaresan, K., Ziemann, U., Stanley, J., and Boutros, N. (2007). Cortical inhibition and excitation in abstinent cocaine-dependent patients: a transcranial magnetic stimulation study. Neuroreport 18, 289–292.
Swift, R. M. (2010). Medications acting on the dopaminergic system in the treatment of alcoholic patients. Curr. Pharm. Des. 16, 2136–2140.
Taber, M. T., Das, S., and Fibiger, H. C. (1995). Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J. Neurochem. 65, 1407–1410.
Thanos, P. K., Taintor, N. B., Rivera, S. N., Umegaki, H., Ikari, H., Roth, G., Ingram, D. K., Hitzemann, R., Fowler, J. S., Gatley, S. J., Wang, G. J., and Volkow, N. D. (2004). DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol. Clin. Exp. Res. 28, 720–728.
Thanos, P. K., Volkow, N. D., Freimuth, P., Umegaki, H., Ikari, H., Roth, G., Ingram, D. K., and Hitzemann, R. (2001). Overexpression of dopamine D2 receptors reduces alcohol self-administration. J. Neurochem. 78, 1094–1103.
Urban, N. B., Kegeles, L. S., Slifstein, M., Xu, X., Martinez, D., Sakr, E., Castillo, F., Moadel, T., O’Malley, S. S., Krystal, J. H., and Abi-Dargham, A. (2010). Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [11C] raclopride. Biol. Psychiatry 68, 689–696.
Volkow, N. D., Wang, G. J., Begleiter, H., Porjesz, B., Fowler, J. S., Telang, F., Wong, C., Ma, Y., Logan, J., Goldstein, R., Alexoff, D., and Thanos, P. K. (2006). High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch. Gen. Psychiatry 63, 999–1008.
Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Hitzemann, R., Ding, Y. S., Pappas, N., Shea, C., and Piscani, K. (1996). Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol. Clin. Exp. Res. 20, 1594–1598.
Volkow, N. D., Wang, G. J., Telang, F., Fowler, J. S., Logan, J., Jayne, M., Ma, Y., Pradhan, K., and Wong, C. (2007). Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J. Neurosci. 27, 12700–12706.
Voronin, K., Randall, P., Myrick, H., and Anton, R. (2008). Aripiprazole effects on alcohol consumption and subjective reports in a clinical laboratory paradigm-possible influence of self-control. Alcohol. Clin. Exp. Res. 32, 1954–1961.
Weiss, F., Parsons, L. H., Schulteis, G., Hyytia, P., Lorang, M. T., Bloom, F. E., and Koob, G. F. (1996). Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J. Neurosci. 16, 3474–3485.
Wise, R. A. (1980). Action of drugs of abuse on brain reward systems. Pharmacol. Biochem. Behav. 13(Suppl. 1), 213–223.
Wise, R. A. (1987). The role of reward pathways in the development of drug dependence. Pharmacol. Ther. 35, 227–263.
Yeomans, J. S. (1989). Two substrates for medial forebrain bundle self-stimulation: myelinated axons and dopamine axons. Neurosci. Biobehav. Rev. 13, 91–98.
Yeomans, J. S., Mathur, A., and Tampakeras, M. (1993). Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopamine neurons. Behav. Neurosci. 107, 1077–1087.
Zangen, A., and Hyodo, K. (2002). Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport 13, 2401–2405.
Keywords: addiction, dopamine, rTMS, dopamine agents, VTA, prefrontal cortex
Citation: Diana M (2011) The dopamine hypothesis of drug addiction and its potential therapeutic value. Front. Psychiatry 2 :64. doi: 10.3389/fpsyt.2011.00064
Received: 14 September 2011; Paper pending published: 07 October 2011; Accepted: 02 November 2011; Published online: 29 November 2011.
Reviewed by:
Copyright: © 2011 Diana. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Marco Diana, ‘G. Minardi’ Cognitive Neuroscience Laboratory, Department of Drug Sciences, University of Sassari, Via Muroni n. 23, Sassari, Italy. e-mail: dsfdiana@uniss.it
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Evidence Backs Gateway Hypothesis in Drug Addiction
- Aaron Levin
Search for more papers by this author
A study in mice adds insights to epidemiological research on ways in which users arrive at their drugs of addiction.
Chickens or eggs?
It’s an old argument, but addiction specialists are still scrapping over which drug of abuse users adopt first.

“Common factors will explain the use of drugs in general, and specific factors will explain why young people use specific drugs and do so in a particular sequence,” according to Nobel Prize–winning psychiatrist Eric Kandel, M.D., and his wife and collaborator, sociologist Denise Kandel, Ph.D., about their research on how tobacco use can serve as a “gateway” to cocaine addiction.
Do people with addiction just latch onto the first drug that comes their way, or is there a predictable escalation in their choice of substance?
“The debate is still going on,” said Denise Kandel, Ph.D., a professor of sociomedical sciences in psychiatry at Columbia University and chief of the Department of the Epidemiology of Substance Abuse at the New York State Psychiatric Institute.
Kandel is a longtime proponent of the “gateway hypothesis” of drug use: “a well-defined developmental sequence of drug use occurs that starts with a legal drug and proceeds to illegal drugs.”
Her epidemiological studies have shown that 87.9 percent of 18- to-34 year-old cocaine users had smoked cigarettes before using cocaine, but only 3.5 percent used cocaine before smoking cigarettes.
A second model of addiction posits a “common liability” to drug use—that is, an underlying general vulnerability for drug use.
Now, a combination of epidemiological and molecular research demonstrates a priming effect of nicotine on the brain that enhances the physiological response to cocaine, supporting the gateway model, according to Kandel and her husband, psychiatrist Eric Kandel, M.D., the Nobel Prize–winning professor of neuroscience and psychiatry at Columbia University and a senior investigator at the Howard Hughes Medical Institute, writing together in the September 4 New England Journal of Medicine .
“If you give an animal nicotine before you give it cocaine, it dramatically enhances the effects of cocaine, while cocaine has no effects on nicotine,” said Eric Kandel in an interview with Psychiatric News . “We showed this at the levels of gene expression and chromatin structure.”
“This is a very provocative study that asks questions with important public-health implications,” said Joni Rutter, Ph.D., director of the Division of Basic Neuroscience and Behavioral Research at the National Institute on Drug Abuse.
“In this specific drug pair, this may be a model that works,” Rutter told Psychiatric News . “It may not in other drugs of abuse, but the questions certainly could be asked. The molecular mechanisms they studied are intriguing and are certainly testable.”
Eric Kandel, his longtime collaborator Amir Levine, Ph.D., and their colleagues set out to examine several parameters of drug-use sequence.
One behavioral test, locomotor sensitization, showed that mice given nicotine in their drinking water for seven days, followed by co-administration of nicotine and cocaine for four days, displayed increased activity compared with both controls and mice getting cocaine only.
A second test, conditioned place preference, also showed that mice thus primed preferred sites associated with cocaine, compared with mice getting cocaine only.
An electrophysiologial test in the nuclear accumbens supplied more evidence.
“We found that just one injection of cocaine in a mouse given nicotine for seven days led to a marked reduction in long-term potentiation that started immediately after stimulation and persisted for up to 180 minutes,” wrote the Kandels. “Nicotine alone, cocaine alone for seven days, or seven days of cocaine followed by 24 hours of nicotine did not alter long-term potentiation.”
Finally, gene expression studies found that nicotine reduced histone deacetylase activity, thus increasing acetylation of the histones H3 and H4 at the FosB promoter in the striatum, and creating an environment conducive to FosB expression, which contributes to addictive behavior in mice.
Nicotine’s priming effect occurred only if the cocaine was first administered at the same time as nicotine, indicating that the latter may enhance the physiological effects of cocaine.
“For all the measures we studied—locomotor sensitization, conditioned place preference, long-term potentiation, and expression—reversing the order of nicotine and cocaine exposure was ineffective: cocaine did not enhance the effect of nicotine,” noted the Kandels.
“This shows that smoking is not only dangerous in its own right, but it’s capable of potentiating at least one more dangerous drug,” said Eric Kandel. “We’re now looking at whether alcohol has a similar effect or whether nicotine has similar effects on other drugs.”
The Kandels suggested that perhaps the two hypotheses about the route to addiction can be reconciled. “[W]e believe that the gateway hypothesis and the common liability model are complementary,” they concluded. “Common factors will explain the use of drugs in general, and specific factors will explain why young people use specific drugs and do so in a particular sequence.”
Their research must be replicated by independent labs but, if validated, might change the way cocaine abuse is treated, said Rutter.
“Providers treating cocaine addiction would also address nicotine use,” she said. “Nicotine replacement therapy thus might not be a good choice, and behavioral approaches might work better. The Kandels have done a nice job of setting the table for asking those kinds of questions.”
Those questions might involve gender or age, since the Kandels’ lab work was carried out only in adult male mice, she said.
Rodents starting nicotine in adolescence consume more than those who start as adults, so a study of nicotine priming should also extend to adolescents, said both Rutter and the Kandels.
“And if nicotine primes the brain for cocaine, and if that holds up in real-world settings, does it also prime for other risky, impulsive behaviors or addictions, like obesity?” she asked, referring to the fact that many women gain weight when they quit smoking.
The Kandels’ cross-disciplinary work adds new insights into the process of addiction and, if one is needed, yet another reason to keep young people from starting to smoke. ■
“A Molecular Basis for Nicotine as a Gateway Drug” can be accessed here .

Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- April 01, 2024 | VOL. 181, NO. 4 CURRENT ISSUE pp.255-346
- March 01, 2024 | VOL. 181, NO. 3 pp.171-254
- February 01, 2024 | VOL. 181, NO. 2 pp.83-170
- January 01, 2024 | VOL. 181, NO. 1 pp.1-82
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Substance Use Disorders and Addiction: Mechanisms, Trends, and Treatment Implications
- Ned H. Kalin , M.D.
Search for more papers by this author
The numbers for substance use disorders are large, and we need to pay attention to them. Data from the 2018 National Survey on Drug Use and Health ( 1 ) suggest that, over the preceding year, 20.3 million people age 12 or older had substance use disorders, and 14.8 million of these cases were attributed to alcohol. When considering other substances, the report estimated that 4.4 million individuals had a marijuana use disorder and that 2 million people suffered from an opiate use disorder. It is well known that stress is associated with an increase in the use of alcohol and other substances, and this is particularly relevant today in relation to the chronic uncertainty and distress associated with the COVID-19 pandemic along with the traumatic effects of racism and social injustice. In part related to stress, substance use disorders are highly comorbid with other psychiatric illnesses: 9.2 million adults were estimated to have a 1-year prevalence of both a mental illness and at least one substance use disorder. Although they may not necessarily meet criteria for a substance use disorder, it is well known that psychiatric patients have increased usage of alcohol, cigarettes, and other illicit substances. As an example, the survey estimated that over the preceding month, 37.2% of individuals with serious mental illnesses were cigarette smokers, compared with 16.3% of individuals without mental illnesses. Substance use frequently accompanies suicide and suicide attempts, and substance use disorders are associated with a long-term increased risk of suicide.
Addiction is the key process that underlies substance use disorders, and research using animal models and humans has revealed important insights into the neural circuits and molecules that mediate addiction. More specifically, research has shed light onto mechanisms underlying the critical components of addiction and relapse: reinforcement and reward, tolerance, withdrawal, negative affect, craving, and stress sensitization. In addition, clinical research has been instrumental in developing an evidence base for the use of pharmacological agents in the treatment of substance use disorders, which, in combination with psychosocial approaches, can provide effective treatments. However, despite the existence of therapeutic tools, relapse is common, and substance use disorders remain grossly undertreated. For example, whether at an inpatient hospital treatment facility or at a drug or alcohol rehabilitation program, it was estimated that only 11% of individuals needing treatment for substance use received appropriate care in 2018. Additionally, it is worth emphasizing that current practice frequently does not effectively integrate dual diagnosis treatment approaches, which is important because psychiatric and substance use disorders are highly comorbid. The barriers to receiving treatment are numerous and directly interact with existing health care inequities. It is imperative that as a field we overcome the obstacles to treatment, including the lack of resources at the individual level, a dearth of trained providers and appropriate treatment facilities, racial biases, and the marked stigmatization that is focused on individuals with addictions.
This issue of the Journal is focused on understanding factors contributing to substance use disorders and their comorbidity with psychiatric disorders, the effects of prenatal alcohol use on preadolescents, and brain mechanisms that are associated with addiction and relapse. An important theme that emerges from this issue is the necessity for understanding maladaptive substance use and its treatment in relation to health care inequities. This highlights the imperative to focus resources and treatment efforts on underprivileged and marginalized populations. The centerpiece of this issue is an overview on addiction written by Dr. George Koob, the director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and coauthors Drs. Patricia Powell (NIAAA deputy director) and Aaron White ( 2 ). This outstanding article will serve as a foundational knowledge base for those interested in understanding the complex factors that mediate drug addiction. Of particular interest to the practice of psychiatry is the emphasis on the negative affect state “hyperkatifeia” as a major driver of addictive behavior and relapse. This places the dysphoria and psychological distress that are associated with prolonged withdrawal at the heart of treatment and underscores the importance of treating not only maladaptive drug-related behaviors but also the prolonged dysphoria and negative affect associated with addiction. It also speaks to why it is crucial to concurrently treat psychiatric comorbidities that commonly accompany substance use disorders.
Insights Into Mechanisms Related to Cocaine Addiction Using a Novel Imaging Method for Dopamine Neurons
Cassidy et al. ( 3 ) introduce a relatively new imaging technique that allows for an estimation of dopamine integrity and function in the substantia nigra, the site of origin of dopamine neurons that project to the striatum. Capitalizing on the high levels of neuromelanin that are found in substantia nigra dopamine neurons and the interaction between neuromelanin and intracellular iron, this MRI technique, termed neuromelanin-sensitive MRI (NM-MRI), shows promise in studying the involvement of substantia nigra dopamine neurons in neurodegenerative diseases and psychiatric illnesses. The authors used this technique to assess dopamine function in active cocaine users with the aim of exploring the hypothesis that cocaine use disorder is associated with blunted presynaptic striatal dopamine function that would be reflected in decreased “integrity” of the substantia nigra dopamine system. Surprisingly, NM-MRI revealed evidence for increased dopamine in the substantia nigra of individuals using cocaine. The authors suggest that this finding, in conjunction with prior work suggesting a blunted dopamine response, points to the possibility that cocaine use is associated with an altered intracellular distribution of dopamine. Specifically, the idea is that dopamine is shifted from being concentrated in releasable, functional vesicles at the synapse to a nonreleasable cytosolic pool. In addition to providing an intriguing alternative hypothesis underlying the cocaine-related alterations observed in substantia nigra dopamine function, this article highlights an innovative imaging method that can be used in further investigations involving the role of substantia nigra dopamine systems in neuropsychiatric disorders. Dr. Charles Bradberry, chief of the Preclinical Pharmacology Section at the National Institute on Drug Abuse, contributes an editorial that further explains the use of NM-MRI and discusses the theoretical implications of these unexpected findings in relation to cocaine use ( 4 ).
Treatment Implications of Understanding Brain Function During Early Abstinence in Patients With Alcohol Use Disorder
Developing a better understanding of the neural processes that are associated with substance use disorders is critical for conceptualizing improved treatment approaches. Blaine et al. ( 5 ) present neuroimaging data collected during early abstinence in patients with alcohol use disorder and link these data to relapses occurring during treatment. Of note, the findings from this study dovetail with the neural circuit schema Koob et al. provide in this issue’s overview on addiction ( 2 ). The first study in the Blaine et al. article uses 44 patients and 43 control subjects to demonstrate that patients with alcohol use disorder have a blunted neural response to the presentation of stress- and alcohol-related cues. This blunting was observed mainly in the ventromedial prefrontal cortex, a key prefrontal regulatory region, as well as in subcortical regions associated with reward processing, specifically the ventral striatum. Importantly, this finding was replicated in a second study in which 69 patients were studied in relation to their length of abstinence prior to treatment and treatment outcomes. The results demonstrated that individuals with the shortest abstinence times had greater alterations in neural responses to stress and alcohol cues. The authors also found that an individual’s length of abstinence prior to treatment, independent of the number of days of abstinence, was a predictor of relapse and that the magnitude of an individual’s neural alterations predicted the amount of heavy drinking occurring early in treatment. Although relapse is an all too common outcome in patients with substance use disorders, this study highlights an approach that has the potential to refine and develop new treatments that are based on addiction- and abstinence-related brain changes. In her thoughtful editorial, Dr. Edith Sullivan from Stanford University comments on the details of the study, the value of studying patients during early abstinence, and the implications of these findings for new treatment development ( 6 ).
Relatively Low Amounts of Alcohol Intake During Pregnancy Are Associated With Subtle Neurodevelopmental Effects in Preadolescent Offspring
Excessive substance use not only affects the user and their immediate family but also has transgenerational effects that can be mediated in utero. Lees et al. ( 7 ) present data suggesting that even the consumption of relatively low amounts of alcohol by expectant mothers can affect brain development, cognition, and emotion in their offspring. The researchers used data from the Adolescent Brain Cognitive Development Study, a large national community-based study, which allowed them to assess brain structure and function as well as behavioral, cognitive, and psychological outcomes in 9,719 preadolescents. The mothers of 2,518 of the subjects in this study reported some alcohol use during pregnancy, albeit at relatively low levels (0 to 80 drinks throughout pregnancy). Interestingly, and opposite of that expected in relation to data from individuals with fetal alcohol spectrum disorders, increases in brain volume and surface area were found in offspring of mothers who consumed the relatively low amounts of alcohol. Notably, any prenatal alcohol exposure was associated with small but significant increases in psychological problems that included increases in separation anxiety disorder and oppositional defiant disorder. Additionally, a dose-response effect was found for internalizing psychopathology, somatic complaints, and attentional deficits. While subtle, these findings point to neurodevelopmental alterations that may be mediated by even small amounts of prenatal alcohol consumption. Drs. Clare McCormack and Catherine Monk from Columbia University contribute an editorial that provides an in-depth assessment of these findings in relation to other studies, including those assessing severe deficits in individuals with fetal alcohol syndrome ( 8 ). McCormack and Monk emphasize that the behavioral and psychological effects reported in the Lees et al. article would not be clinically meaningful. However, it is feasible that the influences of these low amounts of alcohol could interact with other predisposing factors that might lead to more substantial negative outcomes.
Increased Comorbidity Between Substance Use and Psychiatric Disorders in Sexual Identity Minorities
There is no question that victims of societal marginalization experience disproportionate adversity and stress. Evans-Polce et al. ( 9 ) focus on this concern in relation to individuals who identify as sexual minorities by comparing their incidence of comorbid substance use and psychiatric disorders with that of individuals who identify as heterosexual. By using 2012−2013 data from 36,309 participants in the National Epidemiologic Study on Alcohol and Related Conditions–III, the authors examine the incidence of comorbid alcohol and tobacco use disorders with anxiety, mood disorders, and posttraumatic stress disorder (PTSD). The findings demonstrate increased incidences of substance use and psychiatric disorders in individuals who identified as bisexual or as gay or lesbian compared with those who identified as heterosexual. For example, a fourfold increase in the prevalence of PTSD was found in bisexual individuals compared with heterosexual individuals. In addition, the authors found an increased prevalence of substance use and psychiatric comorbidities in individuals who identified as bisexual and as gay or lesbian compared with individuals who identified as heterosexual. This was most prominent in women who identified as bisexual. For example, of the bisexual women who had an alcohol use disorder, 60.5% also had a psychiatric comorbidity, compared with 44.6% of heterosexual women. Additionally, the amount of reported sexual orientation discrimination and number of lifetime stressful events were associated with a greater likelihood of having comorbid substance use and psychiatric disorders. These findings are important but not surprising, as sexual minority individuals have a history of increased early-life trauma and throughout their lives may experience the painful and unwarranted consequences of bias and denigration. Nonetheless, these findings underscore the strong negative societal impacts experienced by minority groups and should sensitize providers to the additional needs of these individuals.
Trends in Nicotine Use and Dependence From 2001–2002 to 2012–2013
Although considerable efforts over earlier years have curbed the use of tobacco and nicotine, the use of these substances continues to be a significant public health problem. As noted above, individuals with psychiatric disorders are particularly vulnerable. Grant et al. ( 10 ) use data from the National Epidemiologic Survey on Alcohol and Related Conditions collected from a very large cohort to characterize trends in nicotine use and dependence over time. Results from their analysis support the so-called hardening hypothesis, which posits that although intervention-related reductions in nicotine use may have occurred over time, the impact of these interventions is less potent in individuals with more severe addictive behavior (i.e., nicotine dependence). When adjusted for sociodemographic factors, the results demonstrated a small but significant increase in nicotine use from 2001–2002 to 2012–2013. However, a much greater increase in nicotine dependence (46.1% to 52%) was observed over this time frame in individuals who had used nicotine during the preceding 12 months. The increases in nicotine use and dependence were associated with factors related to socioeconomic status, such as lower income and lower educational attainment. The authors interpret these findings as evidence for the hardening hypothesis, suggesting that despite the impression that nicotine use has plateaued, there is a growing number of highly dependent nicotine users who would benefit from nicotine dependence intervention programs. Dr. Kathleen Brady, from the Medical University of South Carolina, provides an editorial ( 11 ) that reviews the consequences of tobacco use and the history of the public measures that were initially taken to combat its use. Importantly, her editorial emphasizes the need to address health care inequity issues that affect individuals of lower socioeconomic status by devoting resources to develop and deploy effective smoking cessation interventions for at-risk and underresourced populations.
Conclusions
Maladaptive substance use and substance use disorders are highly prevalent and are among the most significant public health problems. Substance use is commonly comorbid with psychiatric disorders, and treatment efforts need to concurrently address both. The papers in this issue highlight new findings that are directly relevant to understanding, treating, and developing policies to better serve those afflicted with addictions. While treatments exist, the need for more effective treatments is clear, especially those focused on decreasing relapse rates. The negative affective state, hyperkatifeia, that accompanies longer-term abstinence is an important treatment target that should be emphasized in current practice as well as in new treatment development. In addition to developing a better understanding of the neurobiology of addictions and abstinence, it is necessary to ensure that there is equitable access to currently available treatments and treatment programs. Additional resources must be allocated to this cause. This depends on the recognition that health care inequities and societal barriers are major contributors to the continued high prevalence of substance use disorders, the individual suffering they inflict, and the huge toll that they incur at a societal level.
Disclosures of Editors’ financial relationships appear in the April 2020 issue of the Journal .
1 US Department of Health and Human Services: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality: National Survey on Drug Use and Health 2018. Rockville, Md, SAMHSA, 2019 ( https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2018-NSDUH ) Google Scholar
2 Koob GF, Powell P, White A : Addiction as a coping response: hyperkatifeia, deaths of despair, and COVID-19 . Am J Psychiatry 2020 ; 177:1031–1037 Link , Google Scholar
3 Cassidy CM, Carpenter KM, Konova AB, et al. : Evidence for dopamine abnormalities in the substantia nigra in cocaine addiction revealed by neuromelanin-sensitive MRI . Am J Psychiatry 2020 ; 177:1038–1047 Link , Google Scholar
4 Bradberry CW : Neuromelanin MRI: dark substance shines a light on dopamine dysfunction and cocaine use (editorial). Am J Psychiatry 2020 ; 177:1019–1021 Abstract , Google Scholar
5 Blaine SK, Wemm S, Fogelman N, et al. : Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation . Am J Psychiatry 2020 ; 177:1048–1059 Abstract , Google Scholar
6 Sullivan EV : Why timing matters in alcohol use disorder recovery (editorial). Am J Psychiatry 2020 ; 177:1022–1024 Abstract , Google Scholar
7 Lees B, Mewton L, Jacobus J, et al. : Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the Adolescent Brain Cognitive Development Study . Am J Psychiatry 2020 ; 177:1060–1072 Link , Google Scholar
8 McCormack C, Monk C : Considering prenatal alcohol exposure in a developmental origins of health and disease framework (editorial). Am J Psychiatry 2020 ; 177:1025–1028 Abstract , Google Scholar
9 Evans-Polce RJ, Kcomt L, Veliz PT, et al. : Alcohol, tobacco, and comorbid psychiatric disorders and associations with sexual identity and stress-related correlates . Am J Psychiatry 2020 ; 177:1073–1081 Abstract , Google Scholar
10 Grant BF, Shmulewitz D, Compton WM : Nicotine use and DSM-IV nicotine dependence in the United States, 2001–2002 and 2012–2013 . Am J Psychiatry 2020 ; 177:1082–1090 Link , Google Scholar
11 Brady KT : Social determinants of health and smoking cessation: a challenge (editorial). Am J Psychiatry 2020 ; 177:1029–1030 Abstract , Google Scholar
- Cited by None

- Substance-Related and Addictive Disorders
- Addiction Psychiatry
- Transgender (LGBT) Issues
The dopamine hypothesis of drug addiction: hypodopaminergic state
Affiliation.
- 1 B.B. Brodie Department of Neuroscience, University of Cagliari, 09042 Monserrato, Italy.
- PMID: 15797467
- DOI: 10.1016/S0074-7742(05)63005-X
Publication types
- Research Support, Non-U.S. Gov't
- Brain / metabolism*
- Brain / pathology
- Dopamine / metabolism*
- Substance-Related Disorders / etiology
- Substance-Related Disorders / pathology
- Substance-Related Disorders / physiopathology*
- Share full article
Advertisement
Supported by
A Conversation With …
Teen Drug Use Habits Are Changing, For the Good. With Caveats.
Dr. Nora Volkow, who leads the National Institutes of Drug Abuse, would like the public to know things are getting better. Mostly.

By Matt Richtel
Historically speaking, it’s not a bad time to be the liver of a teenager. Or the lungs.
Regular use of alcohol, tobacco and drugs among high school students has been on a long downward trend.
In 2023, 46 percent of seniors said that they’d had a drink in the year before being interviewed; that is a precipitous drop from 88 percent in 1979, when the behavior peaked, according to the annual Monitoring the Future survey, a closely watched national poll of youth substance use. A similar downward trend was observed among eighth and 10th graders, and for those three age groups when it came to cigarette smoking. In 2023, just 15 percent of seniors said that they had smoked a cigarette in their life, down from a peak of 76 percent in 1977 .
Illicit drug use among teens has remained low and fairly steady for the past three decades, with some notable declines during the Covid-19 pandemic.
In 2023, 29 percent of high school seniors reported using marijuana in the previous year — down from 37 percent in 2017, and from a peak of 51 percent in 1979.
There are some sobering caveats to the good news. One is that teen overdose deaths have sharply risen, with fentanyl-involved deaths among adolescents doubling from 2019 to 2020 and remaining at that level in the subsequent years.
Dr. Nora Volkow has devoted her career to studying use of drugs and alcohol. She has been the director of the National Institute on Drug Abuse since 2003. She sat down with The New York Times to discuss changing patterns and the reasons behind shifting drug-use trends.
What’s the big picture on teens and drug use?
People don’t really realize that among young people, particularly teenagers, the rate of drug use is at the lowest risk that we have seen in decades. And that’s worth saying, too, for legal alcohol and tobacco.
What do you credit for the change?
One major factor is education and prevention campaigns. Certainly, the prevention campaign for cigarette smoking has been one of the most effective we’ve ever seen.
Some of the policies that were implemented also significantly helped, not just making the legal age for alcohol and tobacco 21 years, but enforcing those laws. Then you stop the progression from drugs that are more accessible, like tobacco and alcohol, to the illicit ones. And teenagers don’t get exposed to advertisements of legal drugs like they did in the past. All of these policies and interventions have had a downstream impact on the use of illicit drugs.
Does social media use among teens play a role?
Absolutely. Social media has shifted the opportunity of being in the physical space with other teenagers. That reduces the likelihood that they will take drugs. And this became dramatically evident when they closed schools because of Covid-19. You saw a big jump downward in the prevalence of use of many substances during the pandemic. That might be because teenagers could not be with one another.
The issue that’s interesting is that despite the fact schools are back, the prevalence of substance use has not gone up to the prepandemic period. It has remained stable or continued to go down. It was a big jump downward, a shift, and some drug use trends continue to slowly go down.
Is there any thought that the stimulation that comes from using a digital device may satisfy some of the same neurochemical experiences of drugs, or provide some of the escapism?
Yes, that’s possible. There has been a shift in the types of reinforcers available to teenagers. It’s not just social media, it’s video gaming, for example. Video gaming can be very reinforcing, and you can produce patterns of compulsive use. So, you are shifting one reinforcer, one way of escaping, with another one. That may be another factor.
Is it too simplistic to see the decline in drug use as a good news story?
If you look at it in an objective way, yes, it’s very good news. Why? Because we know that the earlier you are using these drugs, the greater the risk of becoming addicted to them. It lowers the risk these drugs will interfere with your mental health, your general health, your ability to complete an education and your future job opportunities. That is absolutely good news.
But we don’t want to become complacent.
The supply of drugs is more dangerous, leading to an increase in overdose deaths. We’re not exaggerating. I mean, taking one of these drugs can kill you.
What about vaping? It has been falling, but use is still considerably higher than for cigarettes: In 2021, about a quarter of high school seniors said that they had vaped nicotine in the preceding year . Why would teens resist cigarettes and flock to vaping?
Most of the toxicity associated with tobacco has been ascribed to the burning of the leaf. The burning of that tobacco was responsible for cancer and for most of the other adverse effects, even though nicotine is the addictive element.
What we’ve come to understand is that nicotine vaping has harms of its own, but this has not been as well understood as was the case with tobacco. The other aspect that made vaping so appealing to teenagers was that it was associated with all sorts of flavors — candy flavors. It was not until the F.D.A. made those flavors illegal that vaping became less accessible.
My argument would be there’s no reason we should be exposing teenagers to nicotine. Because nicotine is very, very addictive.
Anything else you want to add?
We also have all of this interest in cannabis and psychedelic drugs. And there’s a lot of interest in the idea that psychedelic drugs may have therapeutic benefits. To prevent these new trends in drug use among teens requires different strategies than those we’ve used for alcohol or nicotine.
For example, we can say that if you take drugs like alcohol or nicotine, that can lead to addiction. That’s supported by extensive research. But warning about addiction for drugs like cannabis and psychedelics may not be as effective.
While cannabis can also be addictive, it’s perhaps less so than nicotine or alcohol, and more research is needed in this area, especially on newer, higher-potency products. Psychedelics don’t usually lead to addiction, but they can produce adverse mental experiences that can put you at risk of psychosis.
Matt Richtel is a health and science reporter for The Times, based in Boulder, Colo. More about Matt Richtel

Are There Racial Differences in Opioid Abuse Among Seniors?
New research finds older americans, black or white, abuse opioids equally..
Posted April 12, 2024 | Reviewed by Tyler Woods
- What Is Addiction?
- Find a therapist to overcome addiction

Prescription opioid misuse is a very serious problem in the United States. The misuse of prescription opioids is typically defined as: (a) use of a prescription opioid without a prescription of one’s own; (b) use in greater amounts, more often, or longer than one was told to take them; or (c) use in any other way not authorized by a physician. Prescription opioids used for pain relief are generally safe when taken for a short time and as prescribed, but they are prone to misuse because they can cause euphoria, pain relief, and extreme relaxation. About 9 million Americans report misusing a prescription opioid at least once in the past year. This is concerning because misuse of prescription opioids carries a significant risk of adverse health outcomes.
Misuse of prescription opioids has been linked to accidental overdose, hospitalizations, criminal justice involvement, and mental health issues, among other problems. Prescription opioid misuse among older adults, however, may be an area of particular concern. For example, one study found that prescription opioid misuse more than tripled (.3-1 percent) between 1992-2002 among Americans age 55+. More recent data show that rates continued to rise among that same age group, increasing from 1-1.7 percent between 2003-2013. Another concerning trend is that poison control center call data show an increase (from 2006 to 2013) among older adults in opioid-related deaths and opioid use with suicidal intent. Collectively, these studies show a clear upward trend.
These increases are of particular concern because older adults are more vulnerable to the adverse effects of prescription opioids than younger age groups. Not only do older adults experience a variety of age-related pharmacokinetic changes and a greater tendency toward poly -pharmacy (i.e., the simultaneous use of multiple prescription medications), they are at elevated risk due to social problems (e.g., financial difficulties and social isolation ), psychological issues ( depression and loss of memory ), and physical ailments (e.g., lack of mobility and general ill health). Older adults also tend to have access to large amounts of prescription opioids as they are one of the largest demographics of Americans prescribed opioids for pain management . These unique risk factors make older adults vulnerable to prescription opioid misuse and the associated consequences of such use.
What is unclear, however, is whether rates are higher among White older adults than Black older adults. Generally speaking, rates have historically been higher among White adults than Black adults, but it’s not clear whether these differences persist into older adulthood. Also, research on older adult prescription opioid misuse is rare, and there hasn’t been a study on this topic in several years.
Fortunately, a recent study that used nationally representative data sought to compare prevalence rates among White and Black older adults. This study found that rates of prescription opioid misuse were actually equal among Black (2.5 percent) and White (2.5 percent) older adults. This result is somewhat surprising, given that numerous other studies have found prescription opioid misuse to be generally higher among White people than Black people. Equal prevalence challenges the notion of prescription opioid misuse as solely a ‘White problem.’
Since the start of the opioid crisis in the late 1990s, the problem has been framed as an issue that Black Americans have been largely insulated from. However, this result provides evidence to the contrary and suggests that a demographic shift in the U.S. opioid crisis has taken place. Behavioral health providers should be aware of this demographic shift and be mindful that White and Black older adults are now equally at risk of prescription opioid misuse. This means prevention and treatment efforts need to be targeted equitably at both Black and White older adults.

Khary Rigg, Ph.D., is an Associate Professor in the Department of Mental Health Law & Policy at the University of South Florida. His research focuses on substance use disorders.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Teletherapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Coronavirus Disease 2019
- Affective Forecasting
- Neuroscience
University of Utah Hospital
General questions.
- Billing & Insurance
- Health Care Home
- Press Releases
University of Utah announces major funding for new addiction treatment research
Media contact:.
Patricia Brandt Manager, Public Relations and Communications, Huntsman Mental Health Institute University of Utah Health Email: Patricia.Brandt @hsc.utah.edu
Salt Lake City (April 10, 2024) - Worldwide, someone dies from drug or alcohol addiction every four minutes. Now, researchers at Huntsman Mental Health Institute at the University of Utah have been selected by Wellcome Leap to research a new treatment for substance use disorder as part of a $50 million commitment to develop innovative treatments.

Brian J. Mickey, MD, PhD, professor of psychiatry at Huntsman Mental Health Institute (pictured top left), will lead the team of investigators with expertise in psychiatry, biomedical engineering, neuroscience, radiology, and social work to research a new, noninvasive treatment for addiction. Co-principal investigators include Jan Kubanek, PhD , (pictured top center), and Taylor Webb, PhD (pictured top right); co-investigators include (from left to right) Eric Garland, PhD, LCSW ; Rana Jawish, MD ; Vincent Koppelmans, PhD ; and Tom Riis, PhD.
The research will be funded by the Untangling Addiction program, which is a $50 million program founded by Wellcome Leap , to develop scalable measures to assess addiction susceptibility, quantify the risks stemming from addiction, and develop innovative treatments.
“Substance use disorder is a significant global health problem, and yet the treatment options are limited,” Mickey said. “We’re developing a non-invasive intervention for preventing and treating addiction, chronic pain, and depression. This funding will help us validate and generate the data to support the next critical step: an efficacy trial to determine the effectiveness of the intervention.”
Mickey’s team will use a novel ultrasound-based device to modulate deep brain regions and behaviors associated with opioid addiction. The goal will be to ultimately develop this approach into an individually targeted therapeutic intervention for a range of addictions. “Addictions are brain illnesses that have enormous negative impact on individuals, families, and society,” Mickey said. “A major reason that addictions have been difficult to prevent—and treat—is that they are driven by dysfunction of deep brain regions that are challenging to access. Many psychiatric problems such as depression, anxiety, and addiction are caused by malfunction of brain circuits. This project is an example of our mission to understand how these neural circuits are dysregulated and to develop novel, circuit-targeted interventions that return the brain to a healthy state.”
"We are proud to bring Wellcome Leap's innovative problem-solving and funding approach to our research enterprise at the University of Utah," said Taylor Randall, President , University of Utah. "To have our mental health researchers contributing to pioneering work on addiction treatment reaffirms our commitment to improving lives through discovery."
“What makes research like this so impactful is that it brings together a variety of disciplines to help solve complex problems in mental health,” said Mark Hyman Rapaport, MD , CEO of Huntsman Mental Health Institute. “This is particularly timely news given the groundbreaking of a new translational research building on campus focused on mental health and the brain. Our nation is in a mental health crisis, but there is hope if we can think differently and work together to change this trajectory.”
About Huntsman Mental Health Institute
Huntsman Mental Health Institute at University of Utah Health brings together 75 years of patient care, research, and education into one of the nation's leading academic medical centers focused on mental health. Nestled in the campus of University of Utah, Huntsman Mental Health Institute serves the community with 1,600 faculty and staff in 20 locations providing inpatient and outpatient services for youth, teens, and adults as well as a comprehensive crisis care model which includes the nationally recognized SafeUT app and the 988 Crisis hotline for Utah. Our mission is to advance mental health knowledge, hope, and healing for all. Learn more at: HMHI.utah.edu and join the conversation on Instagram , Facebook , TikTok , X and LinkedIn .
- huntsman mental health institute
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Neuropsychopharmacology
- v.42(5); 2017 Apr
Testing the Gateway Hypothesis
Michael l miller.
1 Departments of Psychiatry and Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Yasmin L Hurd
The gateway drug hypothesis refers to the pattern of substance use during adolescence whereby legal substances, such as nicotine and alcohol, precede the progressive use of illicit substances like cocaine and heroin. This concept of a gateway progression related to addiction vulnerability has had important implications with respect to biology, society, and public policy. The gateway drug hypothesis was initially formulated by epidemiological studies which, aiming to characterize the natural history of substance use, were not designed to address the issue of ‘causality’ and therefore garnered substantial controversy. Specifically, opponents argued that since the initial studies could not distinguish between a drug’s direct effect and other confounding variables (eg, environment), the term gateway drug was misleading. To address such criticisms, controlled animal experiments over the past few decades have helped to provide causal neurobiological insights relevant to the gateway hypothesis and drug addiction vulnerability.
Fredriksson et al (2016) , being a recent example, tested the gateway hypothesis by assessing the effect of alcohol exposure on future cocaine use disorder in self-administering rats. Based on a comprehensive set of experiments, the researchers ultimately found that exposure to moderate or high amounts of alcohol beginning in late adolescence/early adulthood (and extending for 7 weeks) does not impact subsequent cocaine self-administration when examined later in adulthood. This study importantly builds on previous animal studies that, as opposed to assessing self-administration, had measured behavioral proxies of addiction such as novelty-seeking and conditioned place preference. The current findings nicely contribute to the field’s growing knowledge base, but the multifaceted complexity of substance use disorder requires several factors to be kept in mind that, if incorporated into future experiments, may address remaining questions that thoroughly evaluate the gateway drug hypothesis.
Given that the brain’s vulnerability to a drug is dynamic, and affected by age, one of the most important gateway considerations is the developmental period of drug exposure. Fredriksson et al (2016) started alcohol administration on either postnatal day 44 or 64, and these ages—roughly corresponding to late adolescence and early adulthood, respectively, in humans—were selected because they matched the onset of most binge drinking behavior. Although binge drinking does occur late, the predisposing pattern of substance use described by the gateway drug hypothesis refers to the initial and continued casual use of a substance, which occur during early to mid-adolescence and therefore precedes binge drinking behavior. The importance of age of onset to potential gateway sensitivity is illustrated by studies showing that drug exposure during adulthood does not alter the subsequent sensitivity to a particular substance as it does if administered during the adolescent period ( Spear, 2016 ).
Another interesting factor is the interaction between the specific exposure type and the drug-class whose vulnerability is altered. Although evidence supporting a gateway relationship between alcohol and psychostimulants remains mixed and controversial, the literature supports a relatively consistent association between early alcohol exposure (either adolescent or gestational) and future alcohol consumption ( Spear, 2016 ). The notion of drug–drug specificity applies to other drug classes as well, with studies consistently suggesting that adolescent nicotine exposure sensitizes an individual to psychostimulants, but not alcohol use, while adolescent cannabinoid exposure sensitizes an individual to opioid but not psychostimulant use. These drug–drug interactions are likely driven by the drug’s direct neurobiological effect on neurodevelopmental processes, in addition to interactions with other factors, such as sex, genetic background, and other environmental influences that complicate these associations. Indeed, there is a complex interplay between genetic and environmental influences such that studies of discordant mono and dizygotic twins report that early initiation of alcohol use (and other commonly available drugs) is largely driven by environmental influences in early adolescence with a shift of genetics assuming increased importance across adolescent development ( Dick et al , 2016 ). Recapitulating this complex interaction in animal models is challenging but will provide important neurobiological insights.
Although not addressed in the initial gateway hypothesis landmark study, an increasing effort in the field has been focused on understanding the relevance of sex in addiction vulnerability. In general, human investigations indicate that males are more at risk for substance use disorders even though females are more sensitive to the reinforcing properties of drugs. For instance, it has been reported that adolescent alcohol exposure has a long-term effect into adulthood particularly for females with increased acquisition of cocaine self-administration behavior, but not in males ( Mateos-Garcia et al , 2015 ). The data remains limited to make definitive conclusions regarding gateway-like effects in males and females to help discern sex-dependent relationships in relation to the progression of addiction.
Finally, an intriguing consideration that has come to attention in recent years is the aspect of transgenerational transmission. The possibility that early drug use might alter addiction risk in humans has inspired recent interest about the potential long-lasting impact not only within their lifetime but even across generations. One of the first studies in this regard related to paternal alcohol exposure in which it was demonstrated to influence the adult offspring in regard to their sensitivity (locomotor response) to amphetamine ( Abel, 1993 ). In addition, our lab, which has focused on Δ 9 -tetrahydrocannabinol (the psychoactive component of cannabis), identified significant cross-generational effects insomuch that parental exposure during adolescence increases the subsequent adult offspring’s heroin self-administration behavior that coincided with disrupted striatal neuroplasticity and epigenetic reprogramming ( Szutorisz and Hurd, 2016 ). Thus, cross-generational gateway-like effects may also be of relevant to understanding the vulnerability of drug addiction seen in adults.
Overall, the concept of the gateway hypothesis has inspired a large body of research, but there remain significant gaps of knowledge before we are able to fully accept or refute the hypothesis. Despite the growing number of published papers relevant to the gateway drug hypothesis, many complex factors still have not been thoroughly addressed to determine causality in animal models even excluding human-specific confounds that impact interpretation such as social, psychological, and legal considerations. As the legality regarding substances of abuse evolve, becoming more relaxed in many jurisdictions, understanding the effect of drug exposure during critical periods of neurodevelopment, particularly adolescence, is essential.
Funding and disclosure
The authors declare no conflict of interest.
Acknowledgments
YLH supported by NIH/NIDA grants DA030359 and DA033660 and MLM supported by NIH/NIDA Predoctoral Training grant F30-DA038954. Invited commentary on: ‘Prior Exposure to Alcohol has no Effect on Cocaine Self-Administration and Relapse in Rats: Evidence from a Rat Model that does not Support the Gateway Hypothesis’ by Fredriksson et al (2016) .
- Abel EL (1993). Paternal alcohol exposure and hyperactivity in rat offspring: effects of amphetamine . Neurotoxicol Teratol 15 : 445–449. [ PubMed ] [ Google Scholar ]
- Dick DM, Adkins AE, Kuo SI (2016). Genetic influences on adolescent behavior . Neurosci Biobehav Rev 70 : 198–205. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fredriksson I, Adhikary S, Steensland P, Vendruscolo LF, Bonci A, Shaham Y et al (2016). Prior exposure to alcohol has no effect on cocaine self-administration and relapse in rats: evidence from a rat model that does not support the gateway hypothesis . Neuropsychopharmacology (e-pub ahead of print). [ PMC free article ] [ PubMed ]
- Mateos-Garcia A, Manzanedo C, Rodriguez-Arias M, Aguilar MA, Reig-Sanchis E, Navarro-Frances CI et al (2015). Sex differences in the long-lasting consequences of adolescent ethanol exposure for the rewarding effects of cocaine in mice . Psychopharmacology 232 : 2995–3007. [ PubMed ] [ Google Scholar ]
- Spear LP (2016). Consequences of adolescent use of alcohol and other drugs: Studies using rodent models . Neurosci Biobehav Rev 70 : 228–243. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Szutorisz H, Hurd YL (2016). Epigenetic effects of cannabis exposure . Biol Psychiatry 79 : 586–594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 11, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Q&A: Can weight loss drugs help in addiction treatment?
by Christine Yu, Pennsylvania State University

In recent years, the popularity of drugs like Ozempic and Wegovy has skyrocketed. While this new class of drugs, called GLP-1 receptor agonist drugs, are approved for use in diabetes and for weight loss, researchers have found that they might help with other conditions too, like cardiovascular disease and addiction. They've made such a splash that the journal Science named GLP-1 drugs the 2023 Breakthrough of the Year .
Among those investigating the potential of GLP-1 drugs for the treatment of addiction are Patricia "Sue" Grigson, professor and chair of the department of neural and behavioral sciences at Penn State College of Medicine, and Scott Bunce, associate professor in the department of psychiatry and behavioral health at Penn State College of Medicine.
In the United States, one person dies from an overdose every five minutes, according to the White House Office of National Drug Control Policy. Grigson and Bunce are among the first to investigate whether GLP-1 drugs could play a role in the treatment of opioid use disorder. In February, Grigson presented early results from a small clinical trial at the American Association for the Advancement of Science conference in Denver.
And the results, she says, look promising. Later this year, Grigson and Bunce plan to begin a larger clinical trial of a GLP-1 drug to treat opioid addiction in the outpatient setting.
Penn State News caught up with Grigson and Bunce to discuss their work.
There's a lot of buzz about drugs like Ozempic and how they may be helpful for more than just weight loss. When did you start to think that they might have a role in addiction medicine?
Grigson: For decades people have thought about addiction as hijacking the brain's reward pathway. We started thinking about people's behavior and the lengths they will go to satisfy their need for their substance of choice. If it's a physiological need, we wondered if a drug that elicits satiety or fullness could be helpful. That led us to GLP-1 receptor agonists.
In our lab, we mostly look at opioid use disorder. We completed our first preclinical study in 2017. Since then, we've found that GLP-1 agonists work very nicely in preclinical models. We've found that they reduce relapse to heroin and fentanyl seeking whether elicited by cues, stress, or the drug itself and reduce heroin and fentanyl-induced seeking behavior in both male and female rats.
But we wanted to translate our data and study this in human participants . Scott and I joined forces and were awarded a grant from the NIH Heal Initiative. We started a small clinical trial in 2019.
You recently presented early findings from a study with participants in a residential treatment facility for opioid use disorder. Can you tell me about the study?
Grigson: This was a fully randomized, double blind, placebo-controlled trial with 20 participants. It was conducted at the Caron Treatment Centers, a residential treatment facility in Wernersville [Pennsylvania]. Half of the participants were given the GLP-1 drug liraglutide, and the other half received placebo. All participants were given their choice of taking an approved medication for opioid use disorder, in this case, buprenorphine.
Bunce: Safety was an important consideration when we designed the study. It was important that we design it around a clinical setting with a medical center on-site.
What did you measure?
Bunce: Our hypothesis is that these drugs can reduce craving in individuals with an opioid use disorder, which will help them refrain from misusing opioids. Other investigators, like Lorenzo Leggio at the National Institute on Alcohol Abuse and Alcoholism (NIAAA), have been looking at the potential to use a GLP-1 drugs to reduce the misuse of other addictive substances, such as alcohol, for a number of years. But this is the first study to address this issue in opioids.
Measuring craving, however, can be a bit of a moving target, and difficult to capture. Using a methodology known as ecological momentary assessment, or EMA, we asked participants to use a smartphone app to gather in-the-moment data four times a day. In those real-time surveys, participants reported not only on craving, but also on their moods, stress, nausea, sleep, fatigue and pain in that specific moment in time.
What did you find?
Grigson: We saw a 40% reduction in opioid craving among participants who were taking the GLP-1 drugs compared to those who received the placebo. It was a significant reduction, equivalent to the percent reduction in craving that Scott and his team have previously seen following two weeks of intensive residential treatment at Caron.
The GLP-1 drugs reduced craving beginning with the lowest dose of liraglutide, even when patients were reporting high levels of stress. Those on placebo usually experienced an increase in craving in the afternoon or evening. Our data showed that craving among those who were on liraglutide stayed flat.
Bunce: Patients have told me that it slows down their need for immediate gratification of their craving, allowing them to make better—and healthier—decisions. It's like craving food. Most of us have had days when we craved pizza or chocolate. One way this medication appears to work is to minimize that drive, allowing you to slow down and make a healthy choice.
But there is still a lot that we do not know. In no way are we saying, "take this medicine and you will not need a medication for opioid use disorder, such as buprenorphine or methadone." It is possible, but there is not enough evidence to support that approach at this time.
What's next for your work?
Grigson: We're really encouraged. The data is promising but we have to see it in a larger clinical trial.
We're starting a larger outpatient study this summer or fall where we will recruit 200 people across three sites—Penn State's Pennsylvania Psychiatric Institute, New York University and the University of Maryland. Timothy Brick, associate professor in Penn State's College of Health and Human Development, and Jennifer Nyland, assistant professor in the department of neural and behavioral sciences at Penn State College of Medicine, will join the team as principal investigators.
This will be a randomized, placebo-controlled clinical trial, so we will evaluate participants taking the GLP-1 receptor agonist semaglutide, the medication that is in Ozempic and Wegovy, compared to those who will be on placebo. Because our preliminary data suggested that patients did better in the study if they were on both the GLP-1 drug and medication for opioid use disorder, in this study, half of the participants will be on methadone and half will be on buprenorphine for opioid use disorder treatment.
Each participant will be evaluated for three months. It will take approximately two years to collect data on 200 participants across the three sites.
There are some pluses with using semaglutide. First, it is a once-a-week injection, whereas liraglutide is once daily, so this may be more tolerable and less time-consuming for participants. Previous studies also have found that semaglutide has fewer gastrointestinal side effects.
Is the hope that the U.S. Food and Drug Administration (FDA) might approve these drugs for the treatment of opioid use disorder? If GLP-1 agonist drugs are already approved for human use, does that fast-track things?
Bunce: Yes. If we demonstrate that these medications are efficacious in reducing craving and the return to opioid use, it is a high priority for the National Institute on Drug Abuse (NIDA) to have the FDA approve these medications as a treatment for opioid use disorder. And certainly, a medication that has already been approved for use in humans is a huge time saver and one of the reasons we looked at these existing medications. Further, inclusion of the safety measures in the first study, even though they were burdensome, helped validate the safety of these medications in individuals with an opioid use disorder.
Grigson: If our data show that it is safe and is saving lives, it might be possible to move it quickly through the FDA, but we will have to wait to see what will happen. We are hopeful.
Explore further
Feedback to editors


Researchers demonstrate miniature brain stimulator in humans
17 minutes ago
Study reveals potential to reverse lung fibrosis using the body's own healing technique
17 hours ago
Researchers discover cell 'crosstalk' that triggers cancer cachexia

Study improves understanding of effects of household air pollution during pregnancy

Wearable sensors for Parkinson's can improve with machine learning, data from healthy adults
18 hours ago

New insights on B cells: Researchers explore building better antibodies and curbing autoimmune diseases

Grieving pet owners comforted by 'supernatural' interactions

Study shows AI improves accuracy of skin cancer diagnoses

Cell's 'garbage disposal' may have another role: Helping neurons near skin sense the environment

Chlamydia vaccine shows promise in early trial
Related stories.

Rural Americans are going without meds to fight opioid, alcohol addictions
Mar 26, 2024

Suvorexant may ease symptoms during opioid withdrawal therapy
Jul 12, 2022
Mindfulness could help women with opioid use disorder better control drug urges
Dec 5, 2023

Rapid high-dose buprenorphine treatment strategy found to reduce opioid withdrawal in individuals using fentanyl
Nov 8, 2023
Clinical trial looks at tramadol for opioid withdrawal
Jul 12, 2017

Risk of overdose higher when opioid agonists are prescribed with other medicines
Aug 3, 2023
Recommended for you

Pandemic drinking hit middle-aged women hardest, study finds
19 hours ago

Researchers shed light on the molecular causes of different functions of opioid receptors

Asia-Pacific gets new weapon in fight against drug-resistant TB
Apr 12, 2024

Scientists say outdated diabetes drug still has something to offer
Apr 11, 2024

In the drive to deprescribe, heartburn drug study teaches key lessons

Study supports use of cystic fibrosis drug in infants from four weeks of age
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 09 November 2020
Habit, choice, and addiction
- Y. Vandaele ORCID: orcid.org/0000-0002-8389-8850 1 &
- S. H. Ahmed 2 , 3
Neuropsychopharmacology volume 46 , pages 689–698 ( 2021 ) Cite this article
8044 Accesses
31 Citations
47 Altmetric
Metrics details
Addiction was suggested to emerge from the progressive dominance of habits over goal-directed behaviors. However, it is generally assumed that habits do not persist in choice settings. Therefore, it is unclear how drug habits may persist in real-world scenarios where this factor predominates. Here, we discuss the poor translational validity of the habit construct, which impedes our ability to determine its role in addiction. New evidence of habitual behavior in a drug choice setting are then described and discussed. Interestingly, habitual preference did not promote drug choice but instead favored abstinence. Here, we propose several clues to reconcile these unexpected results with the habit theory of addiction, and we highlight the need in experimental research to face the complexity of drug addicts’ decision-making environments by investigating drug habits in the context of choice and in the presence of cues. On a theoretical level, we need to consider more complex frameworks, taking into account continuous interactions between goal-directed and habitual systems, and alternative decision-making models more representative of real-world conditions.
You have full access to this article via your institution.
Similar content being viewed by others

A systematic review and multivariate meta-analysis of the physical and mental health benefits of touch interventions
Julian Packheiser, Helena Hartmann, … Frédéric Michon

Microdosing with psilocybin mushrooms: a double-blind placebo-controlled study
Federico Cavanna, Stephanie Muller, … Enzo Tagliazucchi

Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers
Joseph M. Rootman, Pamela Kryskow, … Zach Walsh
Introduction
Tobacco, alcohol, and substance use disorders, which will be referred to as addiction in the present review, are all driven by a transition toward compulsive drug use characterized by a loss of control over drug intake, persistent drug use despite dreadful consequences, and frequent episodes of relapse. Among recreational users, only a subset ultimately lose control over drug use and develop an addiction. To explain this transition, several, often overlapping, theories have been proposed [ 1 ]. Among them, the influential but controversial habit theory of addiction posits that the transition to addiction emerges from the progressive development and dominance of drug habits over goal-directed control [ 2 , 3 ]. Although drug habits appear omnipresent in any form of addiction, whether formation or expression of drug habits contribute to the transition to addiction remains a matter of debate.
The involvement of automatic processes in addiction was suggested 30 years ago in the seminal work of Tiffany [ 4 ]. Several diagnostic criteria for SUD are consistent with the concept of drug habit; notably, the persistence of drug use when it is no longer pleasurable and despite negative consequences, the high reactivity to drug-associated cues and context, and the fact that addictive behaviors appear out of voluntary control [ 1 , 5 , 6 ]. Habits are defined as automatic responses elicited by antecedent stimuli without deliberation or representation of the consequences of one’s action. Because habits do not depend on the response–outcome association underlying goal-directed behavior, they are generally operationalized as an absence of goal-directed behavior; that is, actions not affected by a reduction of the outcome value and/or by a degradation of the response–outcome contingency are under habitual control (Box 1 ) [ 7 , 8 ]. Although these tests typically answer a yes-or-no question, habit and goal-directed systems likely control behavior along a continuum, and the balance between these two systems would be shifted toward habit in SUD.
However, the relation between drug use and habit remains controversial in humans, with mixed results and significant discrepancies [ 9 , 10 ]. Furthermore, although the literature in rodents converges to show that drug exposure promotes habit, how drug habits favor further drug use and, ultimately, the transition to addiction remains unclear. In this review, we try to address this question by reviewing behavioral evidence supporting the habit theory of addiction in rodents and discussing important limitations, notably the absence of habit in choice settings. We then present new evidence of habitual behavior in a drug choice setting and propose several clues to explain our unexpected results in the light of the habit theory of addiction. We propose new perspectives on this theory that embrace the complexity of the decision-making environment of drug addicts and of interactions between decision-making processes.
Box 1 Experimental tests of habitual control
In contrast to goal-directed behavior, habit does not depend on the current motivational value of the outcome and on the knowledge of a causal relationship between the response and the outcome. Thus, reducing the value of the outcome and/or the contingency between the response and the outcome does not affect habitual behavior but reduces responding under goal-directed control (Balleine and Dickinson [ 8 ]; Dickinson [ 41 ]; Dickinson and Balleine [ 7 ]).
Outcome devaluation : the value of a reward is typically reduced by sensory-specific satiety or by pairing the consumption of the reward with an injection of lithium chloride to induce conditioned taste aversion (CTA) (Adams and Dickinson 1981 [ 129 ]; Balleine and Dickinson [ 8 ]; Colwill 1993 [ 126 ]; Dickinson and Balleine [ 7 ]; Rescorla 1987 [ 128 ]). Responding for the devalued outcome is then tested under extinction and compared to a control condition in which the outcome is not devalued.
Contingency degradation : the contingency between the response and the outcome can be degraded by providing noncontingent delivery of one outcome, while maintaining another response–outcome association intact. For instance, one action (R1) is performed to obtain a reward (O1), while another action (R2) gives access to another reward (O2). During the test, one of the outcomes (i.e., O1) is delivered non contingently such that its delivery is equally probable following a response or not (that is, p (O1/R1) = p (O1/~R1) = 0.5). The contingency of this R1–O1 association is thus degraded. The alternative R2–O2 contingency remains intact. Goal-directed performance of the degraded response should be reduced compared to the non-degraded alternative (Colwill 1993 [ 126 ]; Dickinson and Mulatero 1989 [ 127 ]). Conversely, insensitivity to this procedure indicates that performance is under habitual control.
Drugs promote habit
A large number of studies in rodents show that drugs of abuse promote habit. Following drug self-administration training, drugs can be devalued using either sensory-specific satiety or CTA before responding for the drug is tested under extinction (Box 1 ). Using this procedure, it was shown that responding for ethanol [ 11 , 12 , 13 , 14 , 15 , 16 , 17 ], cocaine [ 18 , 19 ], and nicotine [ 20 , 21 ] becomes habitual after various length of training. In some studies, the transition to habit was faster for the drug compared to a nondrug reward suggesting stronger facilitation of habit formation for drug seeking [ 11 , 13 , 15 , 18 , 21 ]. Interestingly, studies in which rats are trained to self-administer cocaine or heroin in a seeking-taking schedule (e.g., heterogeneous chains; seeking RI30—taking FR1 on separate levers) reveal that rats correctly encode the contingency between the seeking response, the taking response and the outcome, indicating that their behavior is under goal-directed control [ 22 , 23 ]. However, it was also shown that the cocaine-seeking response becomes insensitive to extinction of the cocaine taking response following extended self-administration training, suggesting a shift to habitual control [ 24 ].
Numerous studies show that passive drug exposure is sufficient to promote habitual responding for nondrug rewards. For instance, while lever pressing for a solution of 20% sucrose remains under goal-directed control after 8 weeks of training, home-cage access to ethanol during instrumental training renders the behavior habitual [ 11 ]. Ethanol-induced facilitation of habitual responding for food was also found following chronic intermittent exposure to ethanol vapor [ 25 ]. Passive cocaine [ 26 , 27 ] or amphetamine [ 28 , 29 , 30 ] exposure also rendered responding for a nondrug reward insensitive to devaluation by specific satiety or CTA. Interestingly, even limited post-training exposure to cocaine was sufficient to observe habitual responding for food rewards [ 31 ], a results not replicated with amphetamine [ 32 ]. Drug-induced facilitation of habit was also demonstrated in studies showing insensitivity to degradation of instrumental contingency (Box 1 ) following ethanol exposure [ 16 ] or repeated injections of cocaine [ 33 ]. However, two studies have found that exposure to cocaine increased rather than decreased sensitivity to contingency degradation [ 34 , 35 ]. Overall, besides few exceptions [ 32 , 35 , 36 ], the literature in rodents converges to show that various drugs of abuse shift the balance toward habit.
Limitations to the habit theory of addiction
Although drugs of abuse generally promote habit, a very specific set of conditions is typically required to observe habit in rodents. First, the schedule of reinforcement (i.e., random interval) can bias action control toward habit by reducing the contingency and contiguity between response and reinforcement [ 37 , 38 , 39 ]. Second, extended operant training can also be required to induce an observable shift toward habit [ 40 , 41 , 42 ]. For instance, drug seeking is goal-directed after limited training in the seeking-taking schedule [ 22 , 23 , 24 ] but becomes habitual after extended training [ 24 ]. Long training is also required to observe the development of alcohol and nicotine habits [ 11 , 20 ]. Lack of choice seems to be a prerequisite for observing habits during testing. When animals have concurrent access to at least two rewarded responses, their behavior remains sensitive to outcome devaluation, even after extended training [ 42 , 43 , 44 ] or cocaine exposure [ 34 ]. Furthermore, the degree of reward predictability seems to play a significant role in habit expression [ 45 , 46 , 47 ]. When uncertainty about task contingencies is introduced before testing, this can be sufficient to render habitual behavior, goal-directed again [ 45 , 46 ]. Finally, expression of habit is typically observed under conditions of extinction. Indeed, when the devalued reinforcer is delivered during reacquisition tests, instrumental responding for drug or nondrug rewards generally becomes sensitive to outcome devaluation [ 15 , 18 , 21 , 28 , 30 , 40 , 41 ].
If we consider that behavior remains goal-directed when there is a simple choice between two options, the hypothesis that drug habits contribute to compulsive drug use and ultimately addiction is difficult to reconcile with real-world scenarios, in which drug addicts typically face a multitude of drug and nondrug alternatives [ 10 ]. The apparent incompatibility between choice and habit raises another paradox that extends beyond the question of addiction: if this incompatibility were genuine, then how habitual behaviors could be so ubiquitous in everyday life with its rich array of choices and options? In real-world scenarios, habits must somehow be compatible with choice, if only to minimize the costs associated with computationally demanding goal-directed decision-making processes [ 48 , 49 ]. Another factor limiting the ecological relevance of animal research on habits is that habits have only been observed under extinction conditions, mainly to avoid incentive learning and reengagement of goal-directed control [ 15 , 18 , 21 , 40 , 41 ]. However, extinction conditions rarely occur in real-world drug use scenarios, in which drug seeking is typically reinforced [ 10 ]. Although current animal models appear to fail to demonstrate habit in conditions of higher face validity, the difficulty of observing habit in drug users could also indicate that habit is not an underlying process driving addiction. One way to address this issue is to improve the validity of the habit construct, mainly impeded by the apparent impossibility of observing habit under conditions of choice and reinforcement. However, two recent studies provide new evidence of habit in a drug choice setting and under conditions of reinforcement.
New evidence of habitual responding for nondrug reinforcers in a drug choice setting
We have recently found that in rats given a choice between a noncaloric solution of saccharin and an intravenous dose of cocaine, responding for saccharin is habitual [ 50 ]. Indeed, preference for saccharin was maintained following saccharin devaluation by sensory-specific satiety, in a test conducted under extinction (Fig. 1A, B ). In fact, we observed an effect of reward directly reflecting rats’ preference for saccharin, but no effect of devaluation on saccharin- and cocaine-seeking behavior (Fig. 1A, B ). This insensitivity of saccharin preference to devaluation was replicated using CTA (Fig. 1D, E ). Importantly, devaluation of saccharin was verified by showing a reduction of saccharin consumption in the devalued group compared to the non-devalued group for both devaluation methods (Fig. 1C, F ).
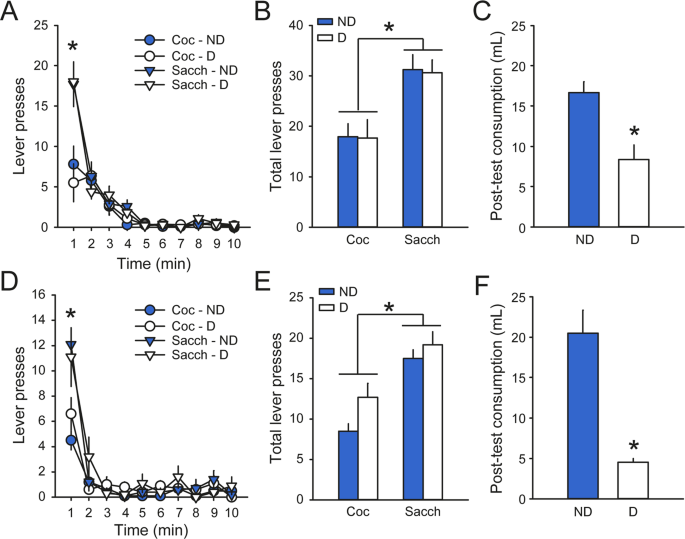
A – C Responding for saccharin is not reduced following saccharin devaluation by specific satiety. A Rats’ performance on the cocaine and saccharin levers did not differ between the devalued group (D; white) and the non-devalued group (ND; blue) across 1 min time bins in the extinction test. * p < 0.05 Coc vs. Sacch. B The total number of lever presses was higher on the saccharin lever compared to the cocaine lever but was not affected by devaluation. * p < 0.05 Coc vs. Sacch. C Saccharin was correctly devalued as measured by a reduction in posttest consumption of saccharin in the D group compared to the ND group. D – F Preference for saccharin is also insensitive to saccharin devaluation by CTA. D , E Rats responded more on the saccharin lever compared to the cocaine lever but did not differ as a function of devaluation. * p < 0.05 Coc vs. Sacch. F Devaluation of saccharin was confirmed during the test of consumption immediately after the extinction session. Adapted from [ 50 ].
Another study from our laboratory tested the sensitivity of the rats’ preference to changes in the current value of the nondrug option, in conditions of choice and reinforcement [ 51 ]. Specifically, water-restricted rats were trained to choose between water and cocaine. Preference was assessed across repeated cycles of water restriction and satiation (Fig. 2A ). 1 h or 2 h presession access to water (1h-Ø and 2h-Ø sessions) had no effect on preference and only moderately suppressed water consumption during water trials (Fig. 2A, B ). Thus, water was also made available during every intertrial intervals (ITI) of the session (Free-Water condition, FW sessions). This resulted in a drastic suppression of water consumption during water trials, indicating successful devaluation (Fig. 2B ). However, rats kept preferentially selecting the water option, even though they consumed little of it. Importantly, experiencing the devalued outcome during ITI and water trials did not reverse preference toward the still valued drug option by reengaging goal-directed control, indicating that preference for water was habitual and inflexible.
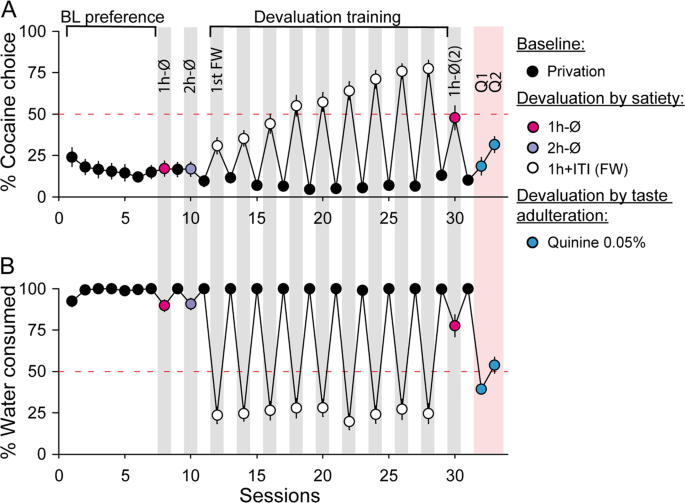
Water-restricted rats offered a choice between water and cocaine expressed a robust preference for water (black; baseline preference under water deprivation). Water was then partially devalued with 1 h (1h-Ø, pink) and 2 h free-water access (2h-Ø, purple) before the choice session. Water preference was not affected ( A ) but there was moderate suppression of water consumption. B Thus, free-water access was also introduced during each intertrial interval (ITI) of choice sessions in addition to the hour of water presession access (white; 1 h + ITI, Free-Water FW). Although this condition drastically suppressed water consumption from the first FW session ( B ), nine sessions were needed to observe a complete reversal of preference ( A ). Following this devaluation training, 1 h water access was sufficient to raise cocaine preference to 50% in a second 1h-Ø choice session (pink). Finally, devaluation of water by taste adulteration with quinine (blue) only moderately affected preference ( A ) despite a strong suppression of water consumption ( B ). Adapted from [ 51 ].
A progressive reversal of preference toward the drug was observed across nine cycles of water restriction and satiation, indicating that preference can only change after repeated training with the novel water value. These results could be well explained in the context of model-based (MB) and model-free (MF) control, used as proxies for goal-directed and habitual control, respectively (Box 2 ) [ 48 , 52 , 53 , 54 ]. The slow reversal of preference observed in our study is what would be expected under MF control, which depends on iterative and retrospective learning of an action’s values in a given “state”. Thus, rats may have learned to compute the actions’ value from the start of the session, based on their motivational state. In other words, rats learn to select water when thirsty, and cocaine when sated, without relying on the expected current value of these two rewards. To test this hypothesis, rats were tested again with 1 h water access before the session but not during ITI (1h-Ø; Fig. 2A ). Although this condition moderately decreased consumption during water trials, the preference for cocaine increased to 50% and was significantly higher than cocaine preference before devaluation training under the same conditions. These results suggest that during devaluation training, rats learn to use their motivational state as a discriminative cue to predict the most valuable option, under MF control. Alternatively, since rats became sensitive to the altered outcome value in the presence of an altered interoceptive state (water satiation), it could be argued that rats progressively learned to reengage MB goal-directed control. Yet, rats maintained their preference for water following quinine-induced devaluation, despite a significant suppression of water consumption (Fig. 2A, B ), indicating that rats cannot flexibly adjust their preference in response to outcome devaluation using another modality (e.g., taste instead of motivational state). A more parsimonious hypothesis is that rats learned instead to select options according to their motivational state under MF control (i.e., select water when thirsty), without relying on the outcome value per se.
Box 2 Model-based and model-free control
Algorithms in reinforcement learning, namely MB and MF learning, have been developed to account for the trade-off between decision speed and accuracy. MB and MF learning formalize the well-documented distinction between goal-directed and habitual behavior, respectively. MB algorithms prospectively learn an internal model of the world, and store a representation of the environment structure (i.e., a cognitive map) in order to compute the expected value of all available courses of actions by iteratively estimating their consequences. MB learning is therefore accurate, but laborious. On the other hand, MF algorithms store and retrieve options “cached values”: the long-run expected value of each action, acquired by iteratively updating actions value through repeated experience of the outcome. This simplified learning model is fast and efficient at the cost of inflexibility: the stored values may be invalid and produce suboptimal choices following changes in task contingencies.
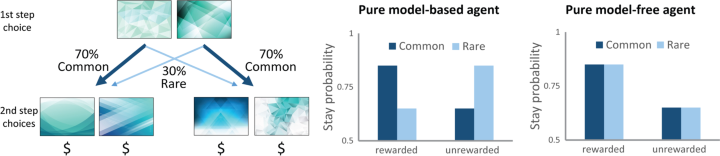
Possible explanations
The results described above are surprising since responding for the nondrug reward was habitual despite choice and reinforcement. In the following subsection, we will discuss possible explanations for these unexpected results.
Both experiments included prior training in the discrete-trial choice schedule to assess preference under baseline conditions. In this procedure, the lever insertion and retraction at each trial constitute salient cues predicting reward availability and delivery, respectively. By reducing uncertainty about reward delivery and alleviating the need for attentional monitoring, these cues can promote the rapid development of habit [ 47 , 55 , 56 ]. Indeed, arbitration between MF and MB control has been suggested to rely on the relative uncertainty of predictions from each system [ 52 , 57 ]. In procedures involving discrete trials, the low uncertainty about MF predictions derived from the lever cues through reinforcement learning is hypothesized to favor habit. This could explain why habitual responding for sucrose is observed after only five sessions whereas 8 weeks of training are not sufficient to observe habit when these cues are not available [ 11 , 55 ]. Therefore, habitual preference in the two studies described above may be promoted by the structure of the discrete-trial choice procedure. It is noteworthy that studies showing goal-directed choice between two nondrug rewards use self-paced random-ratio or -interval schedules, in absence of reward-predictive cues and thus, under conditions of higher reward uncertainty [ 34 , 42 , 44 , 58 ].
The strong initial preference for the alternative nondrug reward in our studies indicates large difference in outcome values [ 50 , 51 ]. In contrast, studies showing goal-directed choice between two response–outcome associations typically use equally valuable rewards [ 42 , 43 , 44 , 59 , 60 , 61 , 62 ]. In this condition, the brain chooses advantageously by assigning and comparing options value and selecting the response associated with the highest value [ 63 , 64 , 65 , 66 ]. Consequently, decision-making remains under goal-directed control—driven by a representation of the options’ value—when choice outcomes are difficult to distinguish [ 67 ]. However, when there is a clear difference in outcome values, choice may not require effortful outcome representation but could instead rely on MF stimulus–response policy, slowly updated based on prior reward history [ 48 ]. This is indeed what we observed when assessing rats’ preference across repeated cycles of water restriction and satiation [ 51 ]. The facilitation of MF control in our experimental choice setting is also in accordance with the arbitration model of Daw et al. based on the relative uncertainty of MB vs. MF predictions [ 52 , 57 ]. While an increase in task complexity is predicted to favor MB control, the strong difference between value of drug and nondrug rewards combined with the high predictability of reward delivery provided by lever cues should favor MF control.
Reframing the habit theory of addiction
In the two studies described above, habitual responding did not promote drug choice but instead favored abstinence. How can we reconcile these results with the habit theory of addiction? In the following section, we will discuss new avenues to reframe the habit theory of addiction by embracing the complexity of (1) drug addicts’ decision-making environment and (2) interactions between decision-making processes.
Facing the complexity of drug addicts’ environment
The discrete-trial choice procedure developed in our laboratory has been used as a rodent model of addiction to isolate a minority of vulnerable rats that prefer the drug, when the large majority prefers the alternative nondrug reward [ 68 , 69 , 70 , 71 ]. It is perhaps not surprising that population-wide behavior in rats does not reflect the behavior of the subgroup of individuals losing control over drug use and developing SUD. Future research will assess possible development of habitual cocaine preference in the subset of cocaine-preferring rats.
Although our research departs from the mainstream in showing habitual preference for a nondrug reward in a drug choice setting, there are commonalities with the literature on the role of reward-predictive cues in biasing behavior toward habit. In rodents, it was shown that providing reward-predictive cues—the insertion and retraction of the lever—reduces uncertainty about reward delivery and favors habit [ 55 , 56 ]. In this context, the lever cue could act as a noncontingent discriminative stimulus signaling the contingency between the response and the reward [ 72 ]. Discriminative cues predictive of drug availability have been shown to produce drug seeking in animal models of relapse [ 72 , 73 , 74 , 75 , 76 ]. Interestingly, when smokers are required to choose between cigarette and food rewards, the presentation of discriminative cigarette cues (cigarette pictures) biased preference toward cigarettes, an effect that was not reduced by tobacco devaluation using health warning or satiety [ 77 , 78 ]. This result suggests that habitual behavior is more strongly bounded by discriminative environmental stimuli and less controlled by the primary drug reinforcement itself.
Noncontingent Pavlovian cues can also directly interact with instrumental reward-seeking behavior, a phenomenon known as “Pavlovian to instrumental transfer” (PIT). Pavlovian cues can elicit a representation of the outcome identity and enhance instrumental responding for that same outcome specifically, independently of the current outcome value (specific-PIT) [ 42 , 79 , 80 ]. Specific-PIT can therefore counteract goal-directed responding by enhancing responding for an outcome predicted by a cue, despite devaluation of this outcome by satiety [ 81 , 82 ]. However, the role of PIT in addiction remains unclear [ 83 ] and this process is presumably rare in human drug-seeking behavior, which is generally reinforced by contingent drug exposure. Instead, Pavlovian cues are more likely to influence drug-seeking behavior when they are contingent with drug delivery and come to function as conditioned reinforcers (CR), by acquiring motivational salience through repeated pairing with the drug [ 72 , 84 ]. Although numerous studies demonstrate the fundamental role of CR in producing and maintaining drug-seeking behaviors [ 72 , 75 , 85 ], how resistant habitual behaviors are to changes in CR remains relatively unexplored. More generally, the fundamental role of Pavlovian cues in the control of reward-seeking behaviors remains largely overlooked in tasks employing self-paced free-operant schedules in absence of conditioned and discriminative stimuli.
Because of the multiple interactions between cues, actions and outcomes, task structure plays a fundamental role in the orchestration of associative control during choice behavior. Moving forward, it is fundamental to face the associative complexity underlying drug choice in addiction to understand how interactions between stimuli, actions, and outcomes shape individuals’ choices between drug and nondrug rewards.
Facing the complexity of interactions between decision-making processes
The habit theory of addiction is limited by the difficulty of observing habits in real-world settings and evidence that drug-seeking behaviors are primarily goal-directed [ 5 , 10 ]. It could be argued that behavioral persistence toward a devalued goal results from an excessively strong motivation for the goal rather than from an action executed “out of habit”. Indeed, it was recently suggested that excessive goal-directed control would drive the transition to addiction [ 10 ]. Interestingly, evidence suggests that rats showing compulsive-like methamphetamine self-administration (i.e., resistance to footshock punishment) exhibited hyperactivity in the orbitofrontal cortex (OFC) to dorsomedial striatum (DMS) pathways, and lower engagement of the medial prefrontal cortex (mPFC)—ventrolateral striatum circuitry [ 86 ]. Furthermore, in a model of optogenetic dopamine neurons self-stimulation [ 87 ], it was shown that potentiation of the OFC to dorsal striatum synaptic pathway drives compulsive-like reinforcement [ 88 ]. Given the established role of OFC in encoding of value during goal-directed behavior, these results suggest that compulsive-like drug use may be driven by an overestimation of drug value relative to punishment [ 89 ]. Furthermore, impairment of executive functioning resulting from drug-induced dysfunctions in PFC activity can disrupt inhibitory control, resulting in an inability to suppress strong motivation after a change in contingencies [ 89 , 90 , 91 ]. Together, these studies suggest that compulsive-like drug use is driven by excessive goal-directed motivation for the drug.
Evidence of a shift from ventromedial to dorsolateral striatum in striato-nigro-striatal dopaminergic pathways, which is proposed to underlie the transition from goal-directed to habitual control over drug seeking remains limited. Indeed, studies demonstrating this shift during cocaine self-administration under a second-order schedule of reinforcement did not assess whether behavior was habitual [ 92 ]. Although a shift from ventromedial to dorsolateral striatal (DLS) dopamine release has been observed during cocaine self-administration, this shift was suggested to promote refinement of instrumental learning rather than escalated and compulsive-like cocaine seeking [ 93 ]. Numerous studies suggest that DMS and DLS are sequentially involved during early and late instrumental training, when behavior is goal-directed or habitual, respectively [ 94 , 95 , 96 , 97 ]. This dissociation between DMS and DLS has also been reported following ethanol and cocaine self-administration [ 11 , 24 ]. Furthermore, dopamine transmission in the DMS and DLS is required for early and late performance of cue-mediated cocaine seeking, respectively [ 98 ]. However, the hypothesis of sequential involvement of DMS and DLS across habitual learning has been recently challenged [ 56 ] and whether this serial recruitment in dorsostriatal activity is accelerated by drug exposure remains unknown. Clearly, more research is needed to demonstrate a shift in meso-nigro-striatal dopaminergic signaling and dorsostriatal activity in the context of habitual drug-seeking behavior.
Although some neurobiological evidence suggests that addiction is associated with excessive goal-directed drug seeking while other studies seem to indicate a shift toward DLS-dependent drug-seeking habits, drug-related behaviors may not be exclusively habitual or goal directed. There are instances of both goal-directed and habitual behavior in drug addiction. Some strategies developed by drug addicts to acquire money, procure the drug and consume it are undoubtedly goal-directed in that they are highly flexible, driven by expectation of drug effects, and involve careful assessment of risks and benefits [ 5 , 99 ]. On the other hand, some drug-related behaviors can also be conceived as habitual, for instance, the first cigarette smoked in the morning. Therefore, instead of asking whether drug-seeking behavior is goal-directed or habitual, it may be more relevant to consider exercise of goal-directed control as a gradient and to determine how tilted the balance on that gradient is. However, tasks assessing individual sensitivity to outcome devaluation typically answer a yes-or-no question [ 100 ]. In humans, the 2-step task (Box 2 ) was developed to estimate individual reliance on MB and MF control [ 48 , 52 , 53 , 54 ] and is more suitable to measure the relative strength of both systems (but see [ 101 ]). Using this procedure, several studies have shown correlation between drug use and the strength of MB control [ 102 , 103 ]. Recent adaptation of this task in rodents [ 104 , 105 , 106 ] will provide further information about the relative contribution of MB and MF systems in animal models of addiction [ 107 ].
Studies using the 2-step task converge to suggest that goal-directed and habitual control are engaged in parallel and that subjects rely on both systems to make decisions [ 53 , 108 ]. Several neurocomputational models suggest that habitual and goal-directed processes are intermingled under a hierarchical decision-making structure. Keramati et al. proposed an integrative “plan-until-habit” model in which MF cached values are directly integrated into MB prospective planning [ 49 ]. Along the same line, Dezfouli and colleagues proposed that goal-directed choices can be executed under habitual control [ 109 , 110 , 111 , 112 ]. Alternatively, another model suggests that habitual control can be exerted over goal selection. Selected goals are then reached with deliberation and planning [ 113 ]. Although these models propose opposite relationships between goal-directed and habitual systems, all share the assumption that humans constantly and flexibly engage habitual and goal-directed control under hierarchical levels in the decision-making structure. Further blurring the frontier between goal-directed and habitual behaviors, several researchers suggest that habits are by essence goal driven [ 114 , 115 ].
One key problem of goal-directed, MB strategy is the high computational demand for implementation. In theory, to make decisions under MB control, agents build a decision tree of all possible states and actions and navigate in this “cognitive map” to estimate the long-run worth of each available outcome [ 48 ]. In the forest of decision-tree possibilities in real-world settings, considering all the available options is not possible; relevant paths must be somehow preselected [ 116 ]. For instance, possible outcomes in a choice situation may be irrelevant and not considered in the first place. We have recently shown in rats that options can be available but not considered in the associative structure of the task, despite the engagement of goal-directed control [ 117 ]. In this task, we allowed rats to exert goal-directed control over the occurrence of choice trials by requiring them to nosepoke in a hole for the presentation of cocaine and saccharin levers (Fig. 3A ). As expected, we found that rats preferred saccharin over cocaine but intriguingly, this preference was exclusive in the majority of rats (Fig. 3B ). When the interest for saccharin was temporarily lost due to repeated choice (i.e., specific satiety), rats preferred to pause for long periods before reinitiating a choice trial for saccharin, instead of switching to cocaine (Fig. 3C ). To explain this suboptimal behavior, we suggested that rats are preferentially associating the initiation of behavioral sequences with saccharin, thereby ignoring the drug reward. These results show that in some situations, choice outcomes can be available but ignored, even when responding is under goal-directed control [ 117 ].
A Rats are required to nosepoke in a hole under a fixed ratio 10 to trigger the presentation of two levers. Two consecutive presses on the left or right lever result in the delivery of saccharin or an intravenous infusion of cocaine, respectively. B In this procedure, rats expressed a strong preference for saccharin. Interestingly, this preference was exclusive for a majority of rats (right panel). C Analysis of choice patterns reveals that rats choosing saccharin exclusively did so in bouts of varying lengths separated by pauses, during which they did not self-initiated any trial for cocaine, despite transient saccharin devaluation by sensory-specific satiety. This behavior represents an opportunity cost because the duration of pauses is sufficient to earn several cocaine injections (right panel). Adapted from [ 117 ].
These results raise an intriguing question; is it possible to select an option among several choice outcomes without actually choosing between them? Instead of comparing and choosing between options, subjects may only consider the relevant options successively and decide whether to accept or reject them. This is the principle of sequential choice models, which assume that in nature, simultaneous encounters are rare and that mechanisms of choice may be evolutionarily adapted to sequential encounters [ 118 , 119 , 120 , 121 , 122 , 123 , 124 ]. Applying this model to the discrete-trial choice procedure, choice between drug and nondrug rewards may not involve simultaneous choice with comparison of options value. Instead, only the relevant preferred option would be considered. Since choices are exclusive in this procedure, habitual selection of the nondrug reward with a short latency automatically foregoes the opportunity to select cocaine. Likewise, drug addicts are unlikely to simultaneously choose between drug and nondrug rewards by comparing options values; they may instead decide whether to carry out their drug-seeking sequence. Therefore, experimental settings involving simultaneous choice between options comparable in value in both human and rodent studies may preclude the observation of habit by requiring assessment and comparison of options’ value, thereby reengaging goal-directed control. Yet, this “artificial” choice setting may not represent the true decision-making structure faced by drug users in real-world environment. Although more research is needed to assess the validity of these sequential choice models, this new framework could resolve the challenge of the exponential computational cost of MB strategies in real-world environment and the expression of habit despite choice in our experiments [ 50 , 51 ], and in the broader context of drug-seeking in addiction.
We hope it is clear from this review that habits alone cannot account for the development of compulsive drug use and that drug habits are not necessary [ 125 ], nor sufficient [ 89 ] to explain the transition to addiction. However, this does not preclude a role for habits in addiction. Then, to what extent are drug habits actually involved? To answer this question, we suffer from several limitations. The structure of our procedures generally favors reengagement of goal-directed control precluding correct assessment of habit. Experiments in animals suffer from a paucity of reward-predictive cues, which does not reflect the sensorial and associative richness of drug addicts’ environment and does not facilitate the development of habit by reducing reinforcement uncertainty. Finally, investigations are limited by too narrow views that drug-seeking behavior should be either habitual or goal-directed. Moving forward, we propose to better design instrumental tasks, in the presence of choice and reward-predictive cues, and under conditions of high reinforcement predictability to favor implementation of simple stimulus–response MF policies. Alternative task structures involving sequential rather than simultaneous choice should also be considered. On a theoretical level, we may need to consider a more complex framework taking into account (1) the continuous arbitration between goal-directed and habitual systems, (2) the hierarchical decision-making architectures combining these two systems and (3) alternative sequential decision-making models suggesting that individuals may consider one option at a time when making decisions. Although much remains to be done, our hope is that this review opens up new perspectives to determine the role of habit and choice in addiction.
Funding and disclosure
This work was supported by the French Research Council (CNRS), the Université de Bordeaux, the French National Agency (ANR-2010-BLAN-1404-01), the Ministère de l’Enseignement Supérieur et de la Recherche (MESR), the Fondation pour la Recherche Médicale (FRM DPA20140629788), and the Peter und Traudl Engelhorn foundation. The authors declare no competing interests.
Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci. 2008;31:415–87.
PubMed PubMed Central Google Scholar
Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23–50.
PubMed Google Scholar
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9.
CAS PubMed Google Scholar
Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–68.
Heather N. Is the concept of compulsion useful in the explanation or description of addictive behaviour and experience? Addict Behav Rep. 2017;6:15–38.
Ostlund SB, Balleine BW. On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov Today Dis Model 2008;5:235–45.
Google Scholar
Dickinson A, Balleine B. Motivational control of instrumental action. Anim Learn Behav. 1994;22:1–18.
Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–19.
Hogarth L, Lam-Cassettari C, Pacitti H, Currah T, Mahlberg J, Hartley L, et al. Intact goal-directed control in treatment-seeking drug users indexed by outcome-devaluation and Pavlovian to instrumental transfer: critique of habit theory. Eur J Neurosci. 2018;50:2513–2525.
Hogarth L. Addiction is driven by excessive goal-directed drug choice under negative affect: translational critique of habit and compulsion theory. Neuropsychopharmacology. 2020;45:720–735. https://doi.org/10.1038/s41386-020-0600-8 .
Corbit L, Nie H, Janak P. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry. 2012;72:389–95.
Barker JM, Zhang H, Villafane JJ, Wang TL, Torregrossa MM, Taylor JR. Epigenetic and pharmacological regulation of 5HT3 receptors controls compulsive ethanol seeking in mice. Eur J Neurosci. 2014;39:999–1008.
Dickinson A, Wood N, Smith JW. Alcohol seeking by rats: action or habit? Q J Exp Psychol B 2002;55:331–48.
Lopez MF, Becker HC, Chandler LJ. Repeated episodes of chronic intermittent ethanol promote insensitivity to devaluation of the reinforcing effect of ethanol. Alcohol. 2014;48:639–45.
CAS PubMed PubMed Central Google Scholar
Mangieri RA, Cofresí RU, Gonzales RA. Ethanol seeking by long evans rats is not always a goal-directed behavior. PLoS ONE. 2012;7:1–13.
Mangieri RA, Cofresí RU, Gonzales RA. Ethanol exposure interacts with training conditions to influence behavioral adaptation to a negative instrumental contingency. Front Behav Neurosci. 2014;8:220.
Corbit LH, Nie H, Janak PH. Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Front Behav Neurosci. 2014;8:1–9.
Miles FJ, Everitt BJ, Dickinson A. Oral cocaine seeking by rats: action or habit? Behav Neurosci. 2003;117:927–38.
Leong KC, Berini CR, Ghee SM, Reichel CM. Extended cocaine-seeking produces a shift from goal-directed to habitual responding in rats. Physiol Behav. 2016;164:330–5.
Clemens KJ, Lay BP, Holmes NM. Extended nicotine self-administration increases sensitivity to nicotine, motivation to seek nicotine and the reinforcing properties of nicotine-paired cues. Addict Biol. 2015;22:400–410.
Loughlin A, Funk D, Coen K, Lê AD. Habitual nicotine-seeking in rats following limited training. Psychopharmacology. 2017;234:2619–2629.
Olmstead MC, Lafonda MV, Everittb BJ, Dickinsonb A. Cocaine seeking by rats is a goal-directed action. Behav Neurosci. 2001;115:394–402.
Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4:943–7.
Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–63.
Renteria R, Baltz ET, Gremel CM. Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat Commun. 2018;9:1–11.
CAS Google Scholar
Corbit LH, Chieng BC, Balleine BW. Effects of repeated cocaine exposure on habit learning and reversal by N-acetylcysteine. Neuropsychopharmacology. 2014;39:1893–901.
LeBlanc KH, Maidment NT, Ostlund SB. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS ONE. 2013;8:e61355:1-10.
Nelson A, Killcross S. Amphetamine exposure enhances habit formation. J Neurosci. 2006;26:3805–12.
Nordquist RE, Voorn P, de Mooij-van Malsen JG, Joosten RNJMA, Pennartz CMA, Vanderschuren LJMJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. Eur Neuropsychopharmacol. 2007;17:532–40.
Nelson AJD, Killcross S, Leblanc KH. Accelerated habit formation following amphetamine exposure is reversed by D 1, but enhanced by D 2, receptor antagonists. Front Neurosci. 2013;7:1–13.
Schmitzer-Torbert N, Apostolidis S, Amoa R, O’Rear C, Kaster M, Stowers J, et al. Post-training cocaine administration facilitates habit learning and requires the infralimbic cortex and dorsolateral striatum. Neurobiol Learn Mem. 2015;118:105–12.
Shiflett MW. The effects of amphetamine exposure on outcome-selective Pavlovian-instrumental transfer in rats. Psychopharmacology. 2012;223:361–70.
Gourley SL, Olevska A, Gordon J, Taylor JR. Cytoskeletal determinants of stimulus-response habits. J Neurosci. 2013;33:11811–6.
Halbout B, Liu AT, Ostlund SB. A closer look at the effects of repeated cocaine exposure on adaptive decision-making under conditions that promote goal-directed control. Front Psychiatry. 2016;7:1–12.
Phillips GD, Vugler A. Effects of sensitization on the detection of an instrumental contingency. Pharmacol Biochem Behav. 2011;100:48–58.
Son JH, Latimer C, Keefe KA. Impaired formation of stimulus-response, but not action-outcome, associations in rats with methamphetamine-induced neurotoxicity. Neuropsychopharmacology. 2011;36:2441–51.
Dickinson A, Nicholas DJ, Adams CD. The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. Q J Exp Psychol Sect B. 1983;35:35–51.
Derusso AL, Fan D, Gupta J, Shelest O, Costa RM, Yin HH. Instrumental uncertainty as a determinant of behavior under interval schedules of reinforcement. Front Integr Neurosci. 2010;4:1–8.
Urcelay GP, Jonkman S. Delayed rewards facilitate habit formation delayed rewards facilitate habit formation. J Exp Psychol Anim Learn Cogn. 2019;45:413–421.
Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol Sect B. 1982;34:77–98.
Dickinson A. Actions and habits: the development of behavioural autonomy. Philos Trans R Soc B Biol Sci. 1985;308:67–78.
Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. J Exp Psychol Anim Behav Process. 2004;30:104–17.
Colwill RM, Triola SM. Instrumental responding remains under the control of the consequent outcome after extended training. Behav Process. 2002;57:51–64.
Kosaki Y, Dickinson A. Choice and contingency in the development of behavioral autonomy during instrumental conditioning. J Exp Psychol Anim Behav Process. 2010;36:334–42.
Trask S, Shipman ML, Green JT, Bouton ME. Some factors that restore goal-direction to a habitual behavior. Neurobiol Learn Mem. 2020;169:107161.
Bouton ME, Broomer MC, Rey CN, Thrailkill EA. Unexpected food outcomes can return a habit to goal-directed action. Neurobiol Learn Mem. 2020;169:1–9.
Thrailkill EA, Trask S, Vidal P, Alcalá JA, Bouton ME. Stimulus control of actions and habits: a role for reinforcer predictability and attention in the development of habitual behavior. J Exp Psychol Anim Learn Cogn. 2018;44:370–84.
Dolan RJ, Dayan P. Goals and habits in the brain. Neuron. 2013;80:312–25.
Keramati M, Smittenaar P, Dolan RJ, Dayan P. Adaptive integration of habits into depth-limited planning defines a habitual-goal-directed spectrum. Proc Natl Acad Sci USA. 2016;113:12868–73.
Vandaele Y, Guillem K, Ahmed SH, Ahmed SH. Habitual preference for the nondrug reward in a drug choice setting. Front Behav Neurosci. 2020;14:1–9.
Vandaele Y, Vouillac-Mendoza C, Ahmed SH. Inflexible habitual decision-making during choice between cocaine and a nondrug alternative. Transl Psychiatry. 2019;9:109.
Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8:1704–11.
Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69:1204–15.
Doya K, Samejima K, Katagiri K, Kawato M. Multiple model-based reinforcement learning. Neural Comput. 2002;14:1347–69.
Vandaele Y, Pribut HJ, Janak PH. Lever insertion as a salient stimulus promoting insensitivity to outcome devaluation. Front Integr Neurosci. 2017;11:1–13.
Vandaele Y, Mahajan NR, Ottenheimer DJ, Richard JM, Mysore SP, Janak PH. Distinct recruitment of dorsomedial and dorsolateral striatum erodes with extended training. Elife. 2019;8:1–29.
Lee SW, Shimojo S, O’Doherty JP. Neural computations underlying arbitration between model-based and model-free learning. Neuron. 2014;81:687–99.
Colwill RM, Rescorla RA. Instrumental responding remains sensitive to reinforcer devaluation after extensive training. J Exp Psychol Anim Behav Process. 1985;11:520–36.
Parkes SL, Balleine BW. Incentive memory: evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. J Neurosci. 2013;33:8753–63.
Parkes SL, Bradfield LA, Balleine BW. Interaction of insular cortex and ventral striatum mediates the effect of incentive memory on choice between goal-directed actions. J Neurosci. 2015;35:6464–71.
Balleine BW, Killcross AS, Dickinson A. The effect oflesions ofthe basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23:666–75.
Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146:145–57.
Glimcher PW, Rustichini A. Neuroeconomics: the consilience of brain and decision. Science. 2004;306:447–52.
Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–56.
Rushworth MF, Mars RB, Summerfield C. General mechanisms for making decisions? Curr Opin Neurobiol. 2009;19:75–83.
Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol. 2010;20:262–70.
Keramati M, Dezfouli A, Piray P. Speed/accuracy trade-off between the habitual and the goal-directed processes. PLoS Comput Biol. 2011;7:e1002055.
Lenoir M, Augier E, Vouillac C, Ahmed SH. A choice-based screening method for compulsive drug users in rats. Curr Protoc Neurosci. 2013;1:1–17.
Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, et al. Cocaine is low on the value ladder of rats: Possible evidence for resilience to addiction. PLoS ONE. 2010;5:e11592:1–14.
Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS ONE. 2007;2:e698:1–10.
Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–25.
Namba MD, Tomek SE, Olive MF, Beckmann JS, Gipson CD. The winding road to relapse: forging a new understanding of cue-induced reinstatement models and their associated neural mechanisms. Front Behav Neurosci. 2018;12:1–22.
Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: Effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA. 2000;97:4321–6.
Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–72.
Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20.
Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc B Biol Sci. 2008;363:3233–43.
Hogarth L, Chase HW. Parallel goal-directed and habitual control of human drug-seeking: implications for dependence vulnerability. J Exp Psychol Anim Behav Process. 2011;37:261–76.
Hogarth L. Goal-directed and transfer-cue-elicited drug-seeking are dissociated by pharmacotherapy: evidence for independent additive controllers. J Exp Psychol Anim Behav Process. 2012;38:266–78.
Corbit LH, Janak PH, Balleine BW. General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci. 2007;26:3141–9.
Rescorla RA. Transfer of instrumental control mediated by a devalued outcome. Anim Learn Behav. 1994;22:27–33.
Watson P, Wiers RW, Hommel B, de Wit S. Working for food you don’t desire. Cues interfere with goal-directed food-seeking. Appetite 2014;79:139–48.
Van Steenbergen H, Watson P, Wiers RW, Hommel B, de Wit S. Dissociable corticostriatal circuits underlie goal-directed vs. cue-elicited habitual food seeking after satiation: evidence from a multimodal MRI study. Eur J Neurosci. 2017;46:1815–1827.
Lamb RJ, Schindler W, Pinkston JW. Conditioned stimuli’s role in relapse: pre-clinical research on pavlovian instrumental transfer. Psychopharmacology. 2016;233:1933–44.
Kelleher RT, Gollub LR. A review of positive conditioned reinforcement. J Exp Anal Behav. 1962;5:543–97.
Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–76.
Hu Y, Salmeron BJ, Krasnova IN, Gu H, Lu H, Bonci A, et al. Compulsive drug use is associated with imbalance of orbitofrontal- And prelimbic-striatal circuits in punishment-resistant individuals. Proc Natl Acad Sci USA. 2019;116:9066–71.
Pascoli V, Terrier J, Hiver A, Lüscher C. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron. 2015;88:1054–66.
Pascoli V, Hiver A, Van Zessen R, Loureiro M, Achargui R, Harada M, et al. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature. 2018;564:366–71.
Lüscher C, Robbins TW, Everitt BJ. The transition to compulsion in addiction. Nat Rev Neurosci. 2020;21:247–63.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2012;12:652–69.
Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–66.
Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–41.
Willuhn I, Burgeno LM, Everitt BJ, Phillips PEM. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci USA. 2012;109:20703–8.
Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–23.
Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–9.
Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–76.
Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52.
Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37:2456–66.
Preble E. Taking care of business—the heroin user’ s life on the street. Int J Addict. 1969;4:1–24.
Schreiner DC, Renteria R, Gremel CM. Fractionating the all-or-nothing definition of goal-directed and habitual decision-making. J Neurosci Res. 2019;98:998–1006.
Feher da Silva C, Hare TA. Humans primarily use model-based inference in the two-stage task. Nat Hum Behav. 2020. https://doi.org/10.1038/s41562-020-0905-y .
Article PubMed Google Scholar
Byrne KA, Otto AR, Pang B, Patrick CJ, Worthy DA. Substance use is associated with reduced devaluation sensitivity. Cogn Affect Behav Neurosci. 2019;19:40–55.
Gillan CM, Kosinski M, Whelan R, Phelps EA, Daw ND. Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. Elife. 2016;5:e11305:1–24.
Miller KJ, Botvinick MM, Brody CD. Dorsal hippocampus contributes to model-based planning. Nat Neurosci. 2017;20:1269–76.
Groman SM, Massi B, Mathias SR, Lee D, Taylor JR. Model-free and model-based influences in addiction-related behaviors. Biol Psychiatry. 2019;85:936–45.
Groman SM, Massi B, Mathias SR, Curry DW, Lee D, Taylor JR. Neurochemical and behavioral dissections of decision-making in a rodent multistage task. J Neurosci. 2019;39:295–306.
Fraser KM, Janak PH. How does drug use shift the balance between model-based and model-free control of decision making? Biol Psychiatry. 2019;85:886–8.
Otto AR, Gershman SJ, Markman AB, Daw ND. The curse of planning. Psychol Sci. 2013;24:751–61.
Dezfouli A, Balleine BW. Actions, action sequences and habits: evidence that goal-directed and habitual action control are hierarchically organized. PLoS Comput Biol. 2013;9:e1003364.
Dezfouli A, Balleine BW. Habits, action sequences and reinforcement learning. Eur J Neurosci. 2012;35:1036–51. https://doi.org/10.1038/s41386-020-0600-8 .
Dezfouli A, Lingawi NW, Balleine BW. Habits as action sequences: hierarchical action control and changes in outcome value. Philos Trans R Soc L B Biol Sci. 2014;369:20130482.
Balleine BW, Dezfouli A. Hierarchical action control: adaptive collaboration between actions and habits. Front Psychol. 2019;10:1–13.
Cushman F, Morris A. Habitual control of goal selection in humans. Proc Natl Acad Sci USA. 2015;112:13817–22.
Wood W, Neal DT. A new look at habits and the habit—goal interface. Psychol Rev. 2007;114:843–63.
Kruglanski AW, Szumowska E. Habitual behavior is goal-driven. Perspect Psychol Sci. 2020. https://doi.org/10.1177/1745691620917676 .
Huys QJM, Eshel N, O’Nions E, Sheridan L, Dayan P, Roiser JP. Bonsai trees in your head: how the pavlovian system sculpts goal-directed choices by pruning decision trees. PLoS Comput Biol. 2012;8:1–13.
Vandaele Y, Vouillac-Mendoza C, Ahmed SH. Cocaine falls into oblivion during volitional initiation of choice trials. Addict Biol. 2020. In press.
Shapiro MS, Siller S, Kacelnik A. Simultaneous and sequential choice as a function of reward delay and magnitude: normative, descriptive and process-based models tested in the European Starling (Sturnus vulgaris). J Exp Psychol Anim Behav Process. 2008;34:75–93.
Freidin E, Aw J, Kacelnik A. Sequential and simultaneous choices: testing the diet selection and sequential choice models. Behav Process. 2009;80:218–23.
Freidin E, Kacelnik A. Rational choice, context dependence, and the value of information in European starlings (Sturnus vulgaris). Science. 2011;334:1000–2.
Vasconcelos M, Monteiro T, Aw J, Kacelnik A. Choice in multi-alternative environments: a trial-by-trial implementation of the sequential choice model. Behav Process. 2010;84:435–9.
Vasconcelos M, Monteiro T, Kacelnik A. Context-dependent preferences in starlings: linking ecology, foraging and choice. PLoS ONE. 2013;8:1–8.
Mobbs D, Trimmer PC, Blumstein DT, Dayan P. Foraging for foundations in decision neuroscience: insights from ethology. Nat Rev Neurosci. 2018;19:419–27.
Grace RC. Acquisition of choice in concurrent chains: assessing the cumulative decision model. Behav Process. 2016;126:82–93.
Singer BF, Fadanelli M, Kawa AB, Robinson TE. Are cocaine-seeking “ habits” necessary for the development of addiction-like behavior in rats? J Neurosci. 2017;38:60–73.
Colwill RM. An associative analysis of instrumental learning. Curr Dir Psychol Sci. 1993;2:111–6.
Dickinson A, Mulatero CW. Reinforcer specificity of the suppression of instrumental performance on a non-contingent schedule. Behav Processes. 1989;19:167–80.
Rescorla RA. A Pavlovian analysis of goal-directed behavior. American Psychologist 1987;42:119–29.
Adams CD, Dickinson A. Instrumental responding following reinforcer devaluation. Q J Exp Psychol Sect B Comp Physiol Psychol. 1981;33:109–121.
Gläscher J, Daw N, Dayan P, O'Doherty JP. States versus Rewards: Dissociable Neural Prediction Error Signals Underlying Model-Based and Model-Free Reinforcement Learning. Neuron 2010;66:585–95.
Download references
Acknowledgements
We thank Christophe Bernard, Mathieu Louvet, and Eric Wattelet for administrative assistance. We also thank Dr. Patricia Janak for her helpful comments on a previous version of the review, and Emma Chaloux-Pinette for proofreading the paper.
Author information
Authors and affiliations.
Department of Psychiatry, Lausanne University Hospital, Lausanne, Switzerland
Y. Vandaele
Institut des Maladies Neurodégénératives, Université de Bordeaux, Bordeaux, France
S. H. Ahmed
Institut des Maladies Neurodégénératives, CNRS, Bordeaux, France
You can also search for this author in PubMed Google Scholar
Contributions
YV drafted the first version of the paper; SHA and YV revised and edited the paper; YV and SHA approved the final version of the paper.
Corresponding author
Correspondence to Y. Vandaele .
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Vandaele, Y., Ahmed, S.H. Habit, choice, and addiction. Neuropsychopharmacol. 46 , 689–698 (2021). https://doi.org/10.1038/s41386-020-00899-y
Download citation
Received : 24 August 2020
Revised : 07 October 2020
Accepted : 19 October 2020
Published : 09 November 2020
Issue Date : March 2021
DOI : https://doi.org/10.1038/s41386-020-00899-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Glun2b inhibition confers resilience against long-term cocaine-induced neurocognitive sequelae.
- Elizabeth G. Pitts
- Shannon L. Gourley
Neuropsychopharmacology (2023)
Task parameters influence operant response variability in mice
- Emma G. Follman
- Maxime Chevée
- Erin S. Calipari
Psychopharmacology (2023)
Sorry—Bad Habit! Validation of the German Self-Report Habit Index with a Test for Its Relation to Potentially Addictive Forms of Health-Risk Behaviors
- Mareile Opwis
- Eva Catrin Bartel
- Jennifer Schmidt
International Journal of Mental Health and Addiction (2023)
Selling visibility-boosts on dating apps: a problematic practice?
- Bouke de Vries
Ethics and Information Technology (2023)
A neuroeconomic signature of opioid craving: How fluctuations in craving bias drug-related and nondrug-related value
- Kathryn Biernacki
- Silvia Lopez-Guzman
- Anna B. Konova
Neuropsychopharmacology (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Humphrey Fellows and NIDA INVEST Postdoctoral Fellows Visit NIDA

On November 29 and 30, 2023, the NIDA International Program invited 11 2023-2024 Hubert H. Humphrey Drug Use and Addiction Research fellows at Virginia Commonwealth University as well as three NIDA INVEST Postdoctoral fellows to an orientation meeting at NIDA, to visit the NIDA facilities and learn more about NIDA’s programs.
The fellows heard from NIDA Deputy Director Dr. Wilson Compton and Dr. Elizabeth Barfield from the Office of Science Policy and Communications (OSPC). They also attended presentations about various NIDA divisions, programs, and initiatives. Dr. Keisher Highsmith from the Division of Epidemiology, Services and Prevention Research (DESPR) provided an overview of NIDA’s Services Research Branch, and Dr. Ivan Montoya described the activities and priorities of the Division of Therapeutics and Medical Consequences. Dr. Tisha Wiley and Dr. Julia Zur, also both from DESPR, highlighted two of NIDA’s large initiatives, the Justice Community Opioid Innovation Network (JCOIN) and the Harm Reduction Research Network. Dr. Flora Katz from the NIH Fogarty International Center provided information on opportunities offered by the center, and Health Resources and Services Administration representatives, Ms. Alexa Ofori and Ms. Megan Meacham, gave an overview of the Federal Office of Rural Health Policy. The fellows also learned from OSPC’s Mr. Brian Marquis about National Drug and Alcohol Facts Week®, a health observance to inspire dialogue about the science of drug use and addiction among youth, educators, health care providers, community partners, and scientists.
The participating fellows greatly appreciated the chance to learn more about NIDA and its programs and to form connections with both other fellows and NIDA staff that may shape not only their fellowship but also their future work after they return to their home countries. According to INVEST Fellow Ilia Nadareishvili (Georgia), “The NIDA Fellows’ Orientation was the best opportunity to see and learn about NIDA International’s work and meet the officials in both formal and informal environments. Activities like these shape our careers and give motivation to double efforts in the work we do. The visit gave me a chance to meet amazing people from across the world. I realized how different we, the NIDA INVEST fellows, are in our backgrounds and work, but how this different work eventually contributes to the common vision and goal.” He particularly enjoyed the opportunity to also meet the Humphrey fellows. “It was even more exciting to learn experiences and stories of Humphrey fellows. We are considering doing work together, and to discuss this and other topics, I did a follow-up visit to Virginia Commonwealth University in Richmond. There, I was kindly invited to join some of the Humphrey fellows’ activities, contributing to my awareness on substance use prevention and recovery initiatives and further strengthening the connection with the fellows.”
Humphrey Fellow of Public Health Policy Ahmed Nawaz (Pakistan) noted the benefits of the meeting to his work, saying, “The NIDA Fellows Orientation Meeting was an invaluable experience that provided me with deep insights about the U.S. health systems, expanded my professional network, and equipped me with essential tools to advance skills to help devise more effective strategies for drug-demand reduction in Pakistan.”
Humphrey Fellow Thinzar Tun (Myanmar) particularly enjoyed learning more about the institute whose resources she has already been using frequently in her work. “During my 20 years of work in the drug addiction field in Myanmar, the first scientific resource that I use whenever I prepare substance use disorder trainings or advocacy for the community is NIDA, since our country has limited resources on substance use. I am honored and humbled to meet with the NIDA and NIH team in person and learn more from you all during the orientation session of my Humphrey fellowship journey. I never thought that I will have that chance in my life—but it did happen!” she said.

IMAGES
VIDEO
COMMENTS
Drug addiction is a brain disease that produces profound modifications in human behavior (Hyman, 2007; Koob and Volkow, 2010), with important negative consequences at various levels, including personal health, employment, family interactions, and society in general (Chandler et al., 2009).Therapeutic possibilities for this devastating illness are, with some rare exceptions, limited to ...
Drug consumption is driven by a drug's pharmacological effects, which are experienced as rewarding, and is influenced by genetic, developmental, and psychosocial factors that mediate drug accessibility, norms, and social support systems or lack thereof. The reinforcing effects of drugs mostly depend on dopamine signaling in the nucleus accumbens, and chronic drug exposure triggers ...
The dopamine theory of reward and addiction, which states that dopamine release mediates reward and thus leads to addiction, has had huge traction. However, it became accepted as a 'universal ...
The characterisation of the role of glia and the extracellular matrix (ECM) in drug-induced synaptic plasticity is an exciting emerging field of drug addiction research as it comes with promising ...
Addictive drugs are habit-forming. Addiction is a learned behavior; repeated exposure to addictive drugs can stamp in learning. Dopamine-depleted or dopamine-deleted animals have only unlearned reflexes; they lack learned seeking and learned avoidance. Burst-firing of dopamine neurons enables learning—long-term potentiation (LTP)—of search and avoidance responses. It sets the stage for ...
The Dopamine Hypothesis of Drug Addiction and Its Potential Therapeutic Value. Dopamine (DA) transmission is deeply affected by drugs of abuse, and alterations in DA function are involved in the various phases of drug addiction and potentially exploitable therapeutically. In particular, basic studies have documented a reduction in the ...
1. Introduction. The concept of "gateway hypothesis" has been studied since the 1970s (Kandel, 1975, Kandel and Faust, 1975) as the theory suggests that an adolescent's early experimentation with alcohol or tobacco or cannabis escalates to more addictive illicit drugs later in adulthood (Lynskey et al., 2003).Most commonly used illicit substances include heroin/opioids, cocaine and or ...
Abstract and Figures. Dopamine (DA) transmission is deeply affected by drugs of abuse, and alterations in DA function are involved in the various phases of drug addiction and potentially ...
The gateway drug hypothesis refers to the pattern of substance use during adolescence whereby legal substances, such as nicotine and alcohol, precede the progressive use of illicit substances like ...
In addition, we show that some (universal) recovery processes transcend the context of addiction. Research on stigma shows that focusing on deficits, while neglecting resilience, capacity, and humanity, reinforces the devaluation of people with drug addiction (del Vecchio, 2006). Thus, recognizing the commonness of some recovery experiences ...
A leading hypothesis guiding current molecular and cellular research into drug addiction conceptualizes key aspects of addiction as a form of memory in which common neuroplasticity mechanisms that mediate normal learning and memory processes are 'hijacked' by exposure to drugs of abuse to produce pathologic addiction-related memories.
Abstract. This chapter presents the dopamine (DA) hypothesis of drug addiction. Drug addiction is a brain disorder caused by the repetitive use of various chemicals that alter the normal ...
A second model of addiction posits a "common liability" to drug use—that is, an underlying general vulnerability for drug use. Now, a combination of epidemiological and molecular research demonstrates a priming effect of nicotine on the brain that enhances the physiological response to cocaine, supporting the gateway model, according to ...
In Addiction: A disorder of choice, Gene Heyman surveys a broad array of evidence—historical, anthropological, survey, clinical, and laboratory-based to build an argument about the role of basic choice processes in the phenomena that comprise drug addiction. He makes a compelling, multifaceted argument that conceptualizing drug addiction as a chronic disease (like schizophrenia or diabetes ...
Introduction. Addiction is a psychiatric disorder characterized by a pathological and compulsive pattern of drug-seeking and drug-taking behaviors that occupy an extraordinary amount of an individual's time and efforts, leading to significant functional impairments to meet the responsibilities of work, school, or home ().Data from the 2013 National Survey on Drug Use and Health suggested ...
The numbers for substance use disorders are large, and we need to pay attention to them. Data from the 2018 National Survey on Drug Use and Health suggest that, over the preceding year, 20.3 million people age 12 or older had substance use disorders, and 14.8 million of these cases were attributed to alcohol.When considering other substances, the report estimated that 4.4 million individuals ...
The dopamine hypothesis of drug addiction: hypodopaminergic state. The dopamine hypothesis of drug addiction: hypodopaminergic state Int Rev Neurobiol. 2005:63:101-54. doi: 10.1016/S0074-7742(05)63005-X. ... Research Support, Non-U.S. Gov't Review MeSH terms Animals ...
Neuropsychopharmacology 42 , 985-986 ( 2017) Cite this article. The gateway drug hypothesis refers to the pattern of substance use during adolescence whereby legal substances, such as nicotine ...
Ketamine is a chemical compound used as an anesthetic in humans and animals. It was developed decades ago as a less toxic alternative to the drug phencyclidine (PCP), which was also developed as an anesthetic. A ketamine derivative, esketamine (under the brand name Spravato ®), is approved by the U.S. Food and Drug Administration (FDA) for treatment-resistant depression in adults.
Addiction is an SSA journal publishing peer-reviewed research reports on pharmalogical and behavioural addictions spanning many different disciplines. Abstract Background and aims Xylazine is a non-opioid sedative which has spread rapidly throughout the US illicit drug supply. This study aimed to describe the spread of xylazine throughout the ...
Dr. Nora Volkow, who leads the National Institutes of Drug Abuse, would like the public to know things are getting better. Mostly. By Matt Richtel Historically speaking, it's not a bad time to ...
Misuse of prescription opioids has been linked to accidental overdose, hospitalizations, criminal justice involvement, and mental health issues, among other problems. Prescription opioid misuse ...
According to James, the orexin 1 receptor is important for stress, panic, motivation, and craving, including for drugs of abuse and food, while orexin 2 receptor is more important for wakefulness and arousal. ... "His research into addiction disorders could become the key in addressing an issue that impacts so many people and families around ...
Worldwide, someone dies from drug or alcohol addiction every four minutes. Now, researchers at Huntsman Mental Health Institute at University of Utah have been selected by Wellcome Leap to research a new treatment for substance use disorder as part of a $50 million commitment to develop innovative treatments. Brian J. Mickey, MD, PhD, Professor of Psychiatry at Huntsman Mental Health Institute ...
Testing the Gateway Hypothesis. The gateway drug hypothesis refers to the pattern of substance use during adolescence whereby legal substances, such as nicotine and alcohol, precede the progressive use of illicit substances like cocaine and heroin. This concept of a gateway progression related to addiction vulnerability has had important ...
In recent years, the popularity of drugs like Ozempic and Wegovy has skyrocketed. While this new class of drugs, called GLP-1 receptor agonist drugs, are approved for use in diabetes and for ...
"Insights from nicotine help to explain psychiatric and addictive drugs, and nicotine addiction remains one of the best-studied psychiatric disorders." Lester's Langley Award lecture, presented at the society's annual meeting in Edinburgh, Scotland, is titled, "Toward a Wearable Continuous Monitor for the Personal Pharmacokinetics of Nicotine."
Here, we propose several clues to reconcile these unexpected results with the habit theory of addiction, and we highlight the need in experimental research to face the complexity of drug addicts ...
On November 29 and 30, 2023, the NIDA International Program invited 11 2023-2024 Hubert H. Humphrey Drug Use and Addiction Research fellows at Virginia Commonwealth University as well as three NIDA INVEST Postdoctoral fellows to an orientation meeting at NIDA, to visit the NIDA facilities and learn more about NIDA's programs.