
- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search

IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)

UCL Research Ethics
- Advice on writing an information sheet and consent form

Writing a Participant Information Sheet and Consent Form
Recruitment documents help people make informed choices about whether to participate in a research study. Find out how to write a Participant Information Sheet, example forms and further guidance.
Writing a Participant Information Sheet
Participant Information Sheets must be designed to assist participants to make informed choices. Potential recruits must be given sufficient information to allow them to decide whether or not they want to take part. The process of obtaining consent and the accompanying documentation must be approved by a research ethics committee and, where only verbal consent to research is contemplated include consideration of an appropriate process for witnessing the consent.
Researchers must take the steps necessary to ensure that all participants in the research understand the process in which they are to be engaged, including why their participation is necessary, how it will be used, and how and to whom it will be reported so that the prospective participant can make an informed decision about whether they really do want to take part.
It is highly recommended that the information provided is presented on headed paper and is accurate, clear and simple so that someone with a reading age of 8 would understand the contents (use short words, sentences and paragraphs). The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon and abbreviations, bias, coercion or any inappropriate inducements.
What should the Participant Information Sheet include:
- A friendly invitation to participate.
- A brief and simple explanation of the purposes of the research and a statement explaining how the participant was chosen and how many other participants will be involved in the study.
- A statement that participation is voluntary; refusal to participate will involve no penalty or loss of benefits to which the participant is otherwise entitled; and the participant may discontinue participation at any time without penalty or loss of benefits.
- A thorough explanation of the expected duration of participation in the research and the procedures to be followed.
- A description of any reasonably foreseeable risks or discomforts and any benefits to the participant. For research involving more than minimal risk, an explanation as to whether any compensation or any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.
- A statement describing the extent, if any, to which confidentiality of records identifying the participant will be maintained.
- It is considered good practice for researchers to debrief participants at the conclusion of the research and to provide them with copies of any reports or other publications arising from their participation.
- If appropriate, a statement indicating that the data might be used for additional or subsequent research.
- An explanation of who to contact for answers to pertinent questions about the research and the rights of the participant and who to contact in the event of a research-related injury to the participant.
- If applicable, a statement declaring that each researcher who may have access to children (aged under 18) or vulnerable adults has undergone a satisfactory criminal records check.
- Remember to thank your participant for considering taking part in the study and include a statement indicating that the research study has been approved by the UCL Research Ethics Committee.
Language and layout
It is highly recommended that the information provided is presented on headed paper and is accurate, clear, and simple. The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon, and abbreviations, bias, coercion, or any inappropriate inducements.
The following points should be considered when writing an information sheet:
- Use clear, non-technical language. We recommend that you refer to the Plain English Campaign
- Use appropriate language for the target audience. For example, consider the different ways needed to communicate with primary school children as opposed to their teachers, or people with expertise in the area of study as opposed to people with no such knowledge
- Divide the text into paragraphs for ease of reading
- Consider using sub-headings for clarity, such as questions and answers
- Make sure the font and font size are legible.
Ask someone else to review your information sheet before it is circulated.
- Template Participant Information Sheet (Word)
- Template Consent Form (Word)
- Guidance on obtaining consent from research participants online (for online and in-person study designs)
Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL
- Recording & Obtaining Consent
UCL Research Ethics Committee Guidance Note 2: Extract from Nuffield Council on Bioethics website
Page last updated: April 2023

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )
- Privacy Policy
Buy Me a Coffee

Home » Informed Consent in Research – Types, Templates and Examples
Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
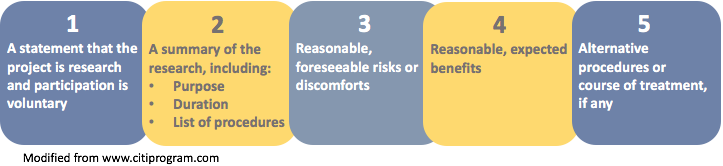
Note: Element number 5 (alternative procedures) applies primarily to clinical research.

General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
- Brief protocol for exempt research including data management and security questionnaire
Informed Consent Guidance
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Informed Consent Templates (2018 Common Rule)
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 05/8/2023.
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 05/08/2023
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools.
Other Templates
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects.
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
Child Assent and Parental Permission
- Child assent ages 3-6
- Child assent ages 7-11
- Child assent ages 12-14
- Parent permission
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections

Informed Consent and Consent Forms for Research Participants
Informed consent is a communication process by which researchers reach agreement with people about whether they wish to participate in research. Confusing informed consent with a signed consent form may violate the ethical intent of informed consent, which is to communicate clearly and respectfully, to foster trust, comprehension, and good decision making, and to ensure that participation is voluntary.
Consent forms are often written in “legalese” and are long, complex, and often inappropriate to the culture or language of the potential subject, insulting, and virtually impossible for most people to comprehend. They convey to some the impression that signing such a formal-looking document commits them to participation. Among subjects who willingly sign documents, most sign the consent form without reading it.
How has this come to pass? Early concern with ethics of human research was about biomedical research and focused on the necessity of obtaining informed consent. Over the decades, the elements of informed consent have grown in number, as has the idea that informed consent is a form that is to be signed by the subject. According to the Federal Regulation of Human Research 46.117(a):
Except as provided in paragraph (c) of this section, informed consent shall be documented by the use of a written consent form approved by the IRB and signed by the subject or the subject’s legally authorized representative. A copy shall be given to the person signing the form.
However, many researchers and the Institutional Review Boards that govern their research fail to recognize that 46.117(c) provides for a waiver of signed consent forms:
(c) An IRB may waive the requirement for the investigator to obtain a signed consent form for some or all subjects if it finds either: (1) That the only record linking the subject and the research would be the consent document and the principal risk would be potential harm resulting from a breach of confidentiality. Each subject will be asked whether the subject wants documentation linking the subject with the research, and the subject’s wishes will govern, or (2) That the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context.
The reason for obtaining a signed consent form has always been much more to protect the researcher and the institution than to serve the interests of the research subject. In case the subject claims later that consent was inadequate or omitted, the researcher can counter by showing the form. Recently, the Office of Human Research Protection has imposed highly publicized and costly sanctions against a few research institutions. Understandably, IRBs and research administrators consider it in their self-interest to make highly conservative decisions. Since IRBs must take steps to justify waiving documentation of informed consent by deeming the research to be minimal risk, many consider it safer not to do so, fearing that such an action might leave them open to questions by the OHRP. Thus, the reason for obtaining a signed consent form is typically to protect the institution, not the subject. Researchers, science, institutions, subjects, and IRBs would all be better off if they made intelligent interpretations of the requirements of the Common Rule.
The Social and Behavioral Sciences Working Group has made various recommendations based on the Common Rule, designed to guide social and behavioral researchers and IRBs out of such conundrums. The authors, both members of the Working Group, developed recommendations concerning informed consent, some of which are summarized here:
1. Informed consent should take the form of an open, easily understood communication process. Typically, this means a friendly verbal exchange between researcher and subject, with a written summary of the information for the subject to keep, as appropriate. (The copy for the subject to keep would be inappropriate if the written record of the subject’s participation could be damaging to the subject, as when the research is about domestic violence, or illegal behavior). Both the verbal and written discussion should be brief, and simply phrased at such a level that all of the subjects can understand it.
2. Subjects must receive enough easily understood, accurate information to judge whether the risk or inconvenience involved is at a level they can accept. The responsibility rests with the investigator to describe any risks accurately and understandably. There are many kinds of minor or everyday risks or inconveniences that most persons would gladly undertake if it were their choice to do so, but which they would not wish to have imposed upon them unilaterally. However, some may make a rational decision that the experience would be too stressful, risky, or unpleasant for some idiosyncratic reason that applies to them and not to other subjects.
3. Especially when the research procedure is long and complex, the researcher must make it quite clear that the subject is free to ask questions at any time. Informed consent, as a conversation (not a form), needs to be available throughout the research, as subjects do not necessarily develop questions or concerns about their participation until they are well into the research experience. For example, a discussion of confidentiality may not capture subjects’ attention or comprehension until they are asked some quite personal questions in the ensuing research experience.
4. When subjects can readily refuse to participate by hanging up the phone or tossing out a mailed survey, the informed consent can be extremely brief (a sentence or two). Courtesy and professionalism require that the identity of the researcher and research institution be mentioned, along with the nature and purpose of the research. However, if there are no risks, benefits, or confidentiality issues involved, these topics and the right to refuse to participate need not be mentioned, as such details would be gratuitous and might decrease participation by implying greater risk that actually exists. If the researcher has any connection with the institution at which the subjects receive health care or other essential services, it is necessary to mention the right of the research subject to refuse or withdraw without prejudice. Such rights may be honored implicitly by making it clear that you are asking their permission to involve them as research subjects.
5. Verbal informed consent need not be detailed and written consent is not appropriate when the research is not concerned with sensitive personal information and when subjects are peers or superiors of the researcher.
6. The cultural norms and life-styles of subjects should be considered when deciding how to approach informed consent. For example, research on homeless injection drug users should probably be preceded by a several week-long process of “hanging out” and talking with them. The resulting informal communication will raise issues they wish to discuss with the researcher. The conditions under which the research is conducted can then be negotiated orally between the researcher and the community members, as appropriate. Written documents and signed forms would expose subjects to risk of arrest and serve no redeeming purpose.
7. A wide range of media are appropriate for administering informed consent. Video tapes, brochures, group discussions, web sites, community newsletters, and the “grape vine” can be more appropriate ways of communicating with potential subjects than the potentially confusing formal consent forms that often are used.
8. When written or signed consent places subjects at risk, it must be waived. There are times when the written record is the only evidence that the subject has participated in a study in which there is acknowledgement or appearance of situations that would place the subject at risk.
9. When it is important to have some record of the informed consent but when written or signed consent would place the subject at risk or be difficult for the subject to read, one useful procedure is to have a trusted colleague witness the verbal consent.
10. Community consultation, or meeting with community leaders of the potential subjects, is a useful way to plan research that is likely to raise sensitive questions among those to be studied and members of their community. This is not a substitute for individual informed consent, but often clears the way for potential subjects to be ready to decide whether to participate.
11. In certain circumstances, persons are not in a position to decide whether to consent until immediately after their participation, e.g., in brief sidewalk interviews, which persons are likely to welcome.
12. Some research cannot validly be conducted if all details are disclosed at the outset. The alternatives to outright deception of subjects are to a) obtain permission to provide only a description of what the subject will experience, with an agreement that the full details of the study will be disclosed afterward; b) obtain permission to engage in concealment or deception with the understanding that pilot research has shown that peers of the subject do not find such concealment or deception objectionable and that a full explanation will follow their participation, c) explain that the subject might be enrolled in one of several possible conditions and to gain permission to disclose in which of these the subject was actually enrolled after his or her participation is completed.
Author’s Note: The Social and Behavioral Sciences Working Group (formerly a part of the National Human Research Protections Advisory Council but now an independent body) chaired by Felice Levine helped to develop these ideas.
Reference Melton, G., Levine, R. J., Koocher, G., Rosethal, R., & Thompson, W. (1988). Community Consultation in Socially Sensitive Research: Lessons from Clinical Trials on Treatments for AIDS. American Psychologist , 43, 573-581.
APS regularly opens certain online articles for discussion on our website. Effective February 2021, you must be a logged-in APS member to post comments. By posting a comment, you agree to our Community Guidelines and the display of your profile information, including your name and affiliation. Any opinions, findings, conclusions, or recommendations present in article comments are those of the writers and do not necessarily reflect the views of APS or the article’s author. For more information, please see our Community Guidelines .
Please login with your APS account to comment.
About the Authors
Joan Sieber is professor of psychology at California State University, Hayward. She received her bachelor's, master's, and doctorate from the University of Delaware. Robert J. Levine is professor of medicine and co-chair of the interdisciplinary bioethics project at Yale University. He is also the founding editor of IRB: A Review of Human Subjects Research.

Matching Psychology Training to Job Market Realities
APS President Wendy Wood discusses how graduate programs can change the habit of focusing on academic-career preparation.

Scientists Propose Upgrades to Research-Methods Education for Psychology Students
Many undergraduate psychology courses fail to ensure students fully understand research design and analysis. An international team of psychological scientists have recommended some systemic steps to remedy that shortcoming.

How Many Peer-Reviewed Articles Do You Need to Earn Tenure?
Developmental psychologist James Byrnes explores updated norms, historical trends, and strategies of successful candidates for publishing peer-reviewed articles.
Privacy Overview
- IRB-SBS Home
- Contact IRB-SBS
- IRB SBS Staff Directory
- IRB SBS Board Members
- About the IRB-SBS
- CITI Training
- Education Events
- Virginia IRB Consortium
- IRB-SBS Learning Shots
- HRPP Education & Training
- Student Support
- Access iProtocol
- Getting Started
- iProtocol Question Guide
- iProtocol Management
- Protocol Review Process
- Certificate of Confidentiality
- Deception and/or Withholding Information from a Participant
- Ethnographic Research
- IRB-SBS 101
- IRB-SBS Glossary
- Participant Pools
- Paying Participants
- Research in an Educational Setting
- Research in an International Setting and/or Location
- Risk-Sensitive Populations
- Student Researchers and Faculty Sponsors
- Study Funding and the IRB
- Understanding Risk in Research
- Vulnerable Participants
- IRB-SBS PAM & Ed
- Federal Regulations
- Ethical Principals
- Partner Offices
- Determining Human Subjects Research
- Determining HSR or SBS

Consent Templates
The templates below were created to help you create the documents you will need to communicate to participants what they will do in the study. The documents you provide participants will range from recruitment materials to post-debrief consent forms, and you need to submit everything that you provide to a participant to our Board for review. For more information about the consent process see Consent .
- General Consent Template : This form covers all of the basic elements that are required for a consent document. Even if you don't plan to use this exact document, refer to it to ensure that you have all of the appropriate elements in place in your consent procedure.
- Electronic Consent Template : This form is modeled after the General Consent Template with some modifications that make it more appropriate for an online format. For more information about this template, see Electronic Consent .
- Parent Consent : If you are including minors in your study, you will need to provide a consent form for parents and an age appropriate assent form for minors. This form is a guide for creating a parent informed consent document. This form can also be used as a guide for surrogate consent procedures.
- Minor Assent (for ages 13-17) : This template provides the basic elements required for older minors to provide assent and could also be used as a model for higher functioning individuals with diminished mental capacity.
- Minor Assent (for ages 7-12) : This template provides the basic elements required for younger minors to provide assent and could also be used as a model for higher functioning individuals with diminished mental capacity. For children younger than 7, assent forms are not required but include information in the consent section regarding what you will say to them about the study (where appropriate).
- Capacity to Consent Template : For some participant populations, it may be necessary to determine if a participant is able to provide consent; if not, a surrogate can be used (you will also need a surrogate consent form and participant assent form, similar to the parent/child consent/assent forms).
- GDPR Informed Consent Addendum : If you are collecting data from citizens of the European Union or the United Kingdom, you will need to provide additional information to your participants, per the GDPR. For more information, see the Research in an International Setting and/or Location and International Research Data Source .
- Study Information Sheet : While many studies do not require researchers to collected signed consent forms, we generally require that participants receive a Study Information Sheet to provide them with information about the study. This information can be provided as a paper document at the beginning of a survey.
- Electronic Study Information Page : This template is similar to the Study Information Sheet with modification for an electronic delivery. For more information about this template, see Electronic Consent .
- Parent Notification Template : Typically used for studies in an educational setting (particularly where the study is exempt but parent notification is still required), this template is a guide for creating a notification letter to send home to parents.
- Oral Consent Card : Typically used in anthropology studies where the participant may be uncomfortable with a form and/or unable to use it, the Oral Consent Card provides all of the elements required for consent in a bullet format so that the researcher can refer to each point as he or she is obtaining consent from the participant.
- Oral Consent Template : This form is also used in situations where the participant is uncomfortable with a form and/or unable to use it. It is more suited to non-anthropology research (though anthropologists are welcome to refer to it as well).
- Sample Debriefing Form : A debriefing form is a summary of the study given to a participant in a deception study and/or a study that includes students from a participant pool. The purpose is to educate participants about the study and to provide them with resources, particularly if the study is upsetting.
- Advertising Flyer Template : Recruitment materials are part of the consent process and it is important that participants are accurately informed about the study throughout the process. You are not required to use this flyer template (it is a model appropriate for a flyer posted around campus), but it is important that you follow the guide provided in Recruitment .
- ResearchMatch Advertising Template : The NIH funds a free and secure recruitment tool called ResearchMatch that helps to connect researchers with volunteers that are interested in participating in studies. If you are interested in using ResearchMatch to help advertise for your study, complete this ResearchMatch Advertising Template and upload.
- Materials Release Form : The data you collect from your participants may be useful in other spheres, such as an educational tool and/or library archive. Using data in this manner is beyond the scope of the study and you should seek additional permission to use the participant's data in this way. This form allows a participant to declare how they would like their materials to be used by the researcher if the researcher wants to use the materials in situations beyond the study.
- Data Release Form : This form is similar to the Post-Debrief Consent Form; it is used when a participant has been recorded or photographed without their knowledge.
- Post-Debrief Consent Form : This form is used in a deception study after the deception is revealed to the participant. The participant is given an opportunity to decide if they still want to participate after the true purpose of the study is revealed.
- The title of protocol must match the title on all consent forms. The title must be relevant, appropriate, and easy to understand. Include the project title on all pages of the consent form.
- List the page numbers on all pages of the consent form in the standard format: Page 1.
- Delete all colored text from the final copy of your form. The colored text is for explanation purposes only.
- Make sure that the form matches the descriptions in the protocol and vice versa.
- Include all relevant information in the consent form rather than referring to previous verbal explanations. The consent form should provide a complete explanation of what the participant is agreeing to do in the study.
- Be aware of the needs of the participant. Avoid using jargon and acronyms that the participant may not understand; make sure the reading comprehension level is appropriate.
- Do not use statements that make implicit demands on participants to participate , e.g., "You will enjoy and benefit from participating in this study."
- Prepare the consent forms in the standard format provided in the template, with all headings addressed. Use the standard language provided on the template where appropriate.
- Please proofread the consent forms for grammar and spelling errors.
- Do not use language that revokes a participant’s legal rights . A consent form is not a legal document.
- Do not require the participants to sign consent to long statements written in first person , e.g., “I agree to participate in this research study. I understand that the risks are minimal and that I will receive no benefits. I know how to withdraw from this study. I will receive $X in payment for participating. I understand that if I withdraw from the study before my participation is complete, I will receive prorated payment according to the following schedule . . . I agree not to hold the researchers liable for any injuries resulting from participation in this study. . .” DO ask participants to sign consent to a simple agreement statement at the end of the consent form: “I agree to participate in the research study described above.”
Participant information sheets & informed consent forms
How to provide information and seek consent from research participants.
Potential participants need information on which to base their choice to take part in clinical research. It is important that information given to participants before obtaining their written informed consent is clear and concise and fully explains all aspects of the research.
The HRA (Health Research Authority) provides detailed guidelines to help avoid delay at sponsor and ethics review. Make sure that you use the latest version.
Please also refer to:
Guidance for researchers seeking sponsorship
Template consent form
Participant information sheets.
Providing an information sheet is just one part of seeking the consent of participants. The REC will consider the whole process.
- The level of detail should be appropriate to the complexity of the study.
- Write in simple, non-technical terms that a lay person will understand.
Discussion is the most effective way to ensure consent is 'informed'. It is important that the person seeking consent spends time going through written information and does not simply give it to the participant to read on their own. This should be outlined at the top of the information sheet, perhaps suggesting how long it may take.
Depending on your research goal, you may need to consider a number of information sheets as follows:
- participant information sheet for adults
- information sheet for parents/guardians (if involving children/young people)
- information sheet(s) for children/young people
- consultee information sheet (for consultees of adults lacking capacity to consent)
Information sheets for minors
Where the Clinical Trial Regulations apply (ie for CTIMPs), a minor is defined as someone under the age of 16. Where common law applies (all research not covered by the regulations), the law states that the age of majority is 18.
Be clear in all documentation about whether you are seeking consent or assent (seeking the child’s agreement) and, if in doubt, contact the Research Governance, Ethics & Assurance Team .
Consent requires a full explanation of the study. Assent requires a clear explanation (comprehensible rather than comprehensive) as consent will be sought from the parent.
Information sheets should be designed for each appropriate age range to reflect comprehension and development, for example:
- children or young people 11–15 years
- children 6–10 years
- children 5 years and under: predominantly pictorial, with very simple sentences to be shown/read to the child. Parents need to provide consent since assent is not sought from children under 5.
Ideally such material should be shorter than that designed for adults, whilst retaining all relevant information.
Informed consent forms
The consent form is to be designed so that the participant is consenting to everything described in the text of the information sheet. The purpose of the form is to record the participant’s decision to take part.
For some studies, specific text may be needed to cover important issues, especially if additional elements are optional for the participant. These may include:
- additional invasive tests or samples required for study purposes only (ie additional to normal care)
- consent to use of audio/video-taping, with possible use of verbatim quotation or use of photographs
- transfer of data/samples to countries with less data protection
- agreement to receive individual feedback from testing
- requirement to contact their GP
For University-sponsored studies where samples are collected, specific wording is required. The first statement is essential; others may be used (and adapted) where required for a particular study.
- I consider these samples a gift to the University of Oxford and I understand I will not gain any direct personal benefit from this.
- I understand that data obtained from my samples may be used for commercial purposes and that I will not gain any financial compensation should this occur.
- I give permission for genetic testing to be carried out on these samples.
You should consider whether leftover samples taken for research could be a valuable resource to other researchers in the future. If so, then retention should be covered in the protocol and information sheet, and the following statement included on the consent form:
- I give consent for samples to be retained and used in future research studies.
Depending on your research goal, you may need to consider a number of forms as follows:
- consent form for adults
- consent form for parents/guardians (if involving young people)
- assent form (for young people who are agreeing to take part)
- consultee declaration form (for adults lacking capacity to consent)
RGEA TEAM
Related links
- Study preparation
- Research governance & classification
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Breathe (Sheff)
- v.14(2); 2018 Jun

How to obtain informed consent for research
1 University of Messina, “G. Martino” Hospital, Messina, Italy
Amelia Licari
2 University of Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
Current biomedical research on human subjects requires clinical trial, which is defined as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions [ i.e. drugs, cells or other biological products, surgical procedures, devices] to evaluate the effects on health outcomes” [1]. In our modern ethical conception, all research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent, which is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate”, as stated in the International Council for Harmonisation Good Clinical Practice guidelines [2]. Informed consent is documented by means of a written, signed and dated informed consent form. This form is required in the following cases: 1) when the research involves patients, children, incompetent/incapacitated persons, healthy volunteers, immigrants or others ( e.g. prisoners); 2) when the research uses/collects human genetic material, biological samples or personal data [3].
Short abstract
The process of obtaining informed consent for clinical trials is tightly regulated; complications arise in circumstances when consent may be waived, or when needed from vulnerable populations http://ow.ly/rEMe30j5MVq
Current biomedical research on human subjects requires clinical trial, which is defined as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions [ i.e. drugs, cells or other biological products, surgical procedures, devices] to evaluate the effects on health outcomes” [ 1 ]. In our modern ethical conception, all research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent, which is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate”, as stated in the International Council for Harmonisation Good Clinical Practice guidelines [ 2 ]. Informed consent is documented by means of a written, signed and dated informed consent form. This form is required in the following cases: 1) when the research involves patients, children, incompetent/incapacitated persons, healthy volunteers, immigrants or others ( e.g. prisoners); 2) when the research uses/collects human genetic material, biological samples or personal data [ 3 ].
The informed consent form must be written in language easily understood by the subjects, it must minimise the possibility of coercion or undue influence, and the subject must be given sufficient time to consider participation. However, informed consent is not merely a form that is signed, but is a process in which the subject has an understanding of the research and its risks, and it is tightly described in ethical codes and regulations for human subject research [ 2 ].
Educational aims
- To provide a comprehensive overview of issues in obtaining informed consent in clinical research.
- To describe the process of obtaining informed consent in clinical trials.
- To highlight the circumstances under which informed consent can be waived.
- To review the setting of obtaining informed consent from “vulnerable populations”.
The informed consent process
The voluntary expression of the consent by a competent subject and the adequate information disclosure about the research are critical and essential elements of the informed consent process [ 4 ]. Competent subjects able to comprehend the research-related information should personally decide and provide the consent on research participation. Conditions posing practical challenges in obtaining informed consent from the real subject may include situations of medical emergency or obtaining consent from “vulnerable” subjects and/or children [ 5 ].
Research-related information must be presented to enable people to voluntarily decide whether or not to participate as a research subject. For an ethically valid consent, information provided to a research subject should include, but not be limited to: information about the health condition for which the research is proposed; details of the nature and purpose of the research; the expected duration of the subject’s participation; a detailed description of study treatment or intervention and of any experimental procedures (including, in the case of randomised clinical trials (RCTs), also blinding and randomisation); a statement that participation in research is voluntary; probable risks and benefits associated with research participation; details of the nature of the illness and possible outcome if the condition is left untreated; availability, risks and benefits of alternative treatments; information about procedures adopted for ensuring data protection/confidentiality/privacy, including duration of storage of personal data; details about the handling of any incidental findings of the research; description of any planned genetic tests; details of insurance coverage in case of injury; reference contacts for any further answers to pertinent questions about the research and the subject’s rights and in case of any research-related injury to the subject; and any other information that seems necessary for an informed decision to be taken by the subject. Of particular importance, a statement offering the subject the opportunity to withdraw at any time from the research without consequences must be provided during the information disclosure [ 2 ]. Specific information should be provided in case of research projects involving children, incapacitated adults not able to give informed consent, illiterate populations, etc. (as will be described later in this article).
The information about the research should be given by a physician or by other individuals ( i.e. researchers) with appropriate scientific training and qualifications [ 6 ]. Furthermore, the location where the informed consent is being discussed, and the subject’s physical, emotional and psychological capability, must be taken into consideration when taking consent from a human subject.
Informed consent: when is it not necessary?
After institutional review board (IRB) or independent ethics committee approval is achieved, obtaining informed consent from each human subject prior to his/her participation in clinical trial is mandatory [ 5 ]. However, when specific circumstances occur, the informed consent can be waived, and “research without consent” is possible, which allows enrolment of patients without their consent, under strict regulation [ 7 ]. In order that research without consent is considered justifiable, the following three conditions have to be met: 1) it is impracticable to obtain consent, 2) the research does not infringe the principle of self-determination, and 3) the research provides significant clinical relevance [ 8 ].
The first condition, of “impracticability”, occurs when obtaining informed consent is burdened by high impact in terms of time and economic resources or could compromise the study’s validity [ 8 ]. The second condition means that, although physicians are requested to ensure that the patient has understood the aim of the research and the risks and/or benefits associated with study participation, the researchers are also advised to respect the patient’s decision-making capacity, not interfering with his/her decisions and acting always in the patient’s best interest [ 9 ]. The third condition leads to justification of waiving consent when the clinical relevance and public health importance are potentially high [ 8 ].
The formal literature identifies different types of RCTs and classifies them into three macro-areas: 1) RCTs based on infeasibility of informed consent; 2) RCTs that omit informed consent only for control groups; and 3) RCTs that omit informed consent entirely.
RCTs based on infeasibility of informed consent
Emergency clinical studies, involving critically ill subjects, represent an exception to the requirement of informed consent. The investigated life-saving therapy and the medical intervention may be required immediately, not permitting the researchers to wait and respect all procedures of obtaining informed consent. Within this context, the researchers will be able to proceed with patient recruitment, also without the subject’s consent to treatment, when, prior to the study, the IRB has ascertained the presence of mandatory conditions ( table 1 ) [ 10 ].
Table 1
Conditions to be met in emergency clinical study
Cluster randomised studies include cluster-cluster and individual-cluster research [ 11 ]. In cluster-cluster designs ( e.g. studies on infectious disease prevention), the intervention involves the entire target community, so that single subjects cannot refuse it [ 12 ]. Conversely, in individual-cluster designs ( e.g. studies on primary care), although the intervention involves all the selected community, the right to refuse treatment is allowed. Under this circumstance, the omission of informed consent is justified only when the treatment refusal undermines the validity of the research study and/or procedures [ 13 ].
RCTs that omit informed consent only for control groups
In Zelen’s single-consent model ( e.g. RCTs in infectious or oncological diseases), randomisation occurs prior to any consent, and informed consent is sought only from individuals assigned to experimental treatment [ 14 ]. In the control group, the physicians do not make substantial changes in routine patient care, so informed consent is not required for patient enrolment [ 8 ].
In order to improve study recruitment, Zelen developed the double-consent design. Specifically, informed consent is requested for subjects to be involved in the study but not for the randomisation, preventing psychological distress [ 14 ].
In follow-up studies, the nested consent model ( e.g. for single cohort studies) or cohort multiple RCTs model ( e.g. for multiple cohort studies) is applied. In these variants, patients give their consent for prospective follow-up; however, they remain blinded to any randomised experimental interventions [ 15 ].
In trials using the model of “consent to postponed information”, the informed consent process is carried out after the study is completed [ 16 ].
All these RCT types aim to avoid unnecessary stress in patients who will not receive the new promising experimental treatment. Moreover, these clinical study designs do not affect the standard therapeutic approach or infringe the rights of the patients in the control group; therefore, the clinical trial can proceed without obtaining informed consent [ 8 ].
RCTs that omit informed consent entirely
Based on the fact that patients are assigned to standard care interventions, no informed consent is sought either in low-risk pragmatic RCTs [ 17 ] or in prompted optional randomisation trials [ 18 , 19 ]. However, in a low-risk pragmatic RCT, patients do not have the possibility to choose one of the two standard treatments, whereas in a prompted optional randomisation trial, both the researchers and the enrolled patients can choose one type of treatment over another, despite the randomisation results [ 6 ].
Special needs: vulnerable patients
A “vulnerable population” is defined as a disadvantaged community subgroup unable to make informed choices, protect themselves from inherent or intended risks, or keep their own interests safeguarded [ 20 ]. In the health domain, “vulnerable populations” refers to physical vulnerability ( e.g. pregnant women, fetuses, children, orphans, students, employees, prisoners, the military, and those who are chronically or terminally ill), psychological vulnerability (cognitively and intellectually impaired individuals) and social vulnerability (those who are homeless, from ethnic minorities, are immigrants or refugees) [ 20 ].
Due to a compromised free will and inability to make conscious decisions, several ethical dilemmas (related to communications, privacy and treatment) often arise when research involves these populations. Guaranteeing protection of rights, safety, data privacy and confidentiality of vulnerable subjects are prerogatives of good clinical practice, and law dispositions are regulated and strictly monitored by the applicable authorities [ 21 ].
Physical vulnerability
For a long time, pregnant women were excluded from clinical research because of their “vulnerability”. Although pregnant women are able to make informed and conscious choices, they have been considered “vulnerable” due to the potential risks to the fetus, who is also considered as a “patient” [ 22 ]. More recently, with the consideration of pregnant women as “scientifically complex” rather than “vulnerable” subjects, it has been permitted to involve this category in research trials [ 23 ]. The “scientific complexity” reflects both ethical and physiological complexity. The ethical aspects are secondary to the need to find a balance between interests of the fetus and the mother. The physiological aspects are strictly related to the pregnancy status [ 24 ].
Research studies involving pregnant women and fetuses have to satisfy specific federal regulations ( table 2 ). The following appropriate precautions should be taken in research studies involving pregnant women: no pregnant woman may be involved as a subject in a human clinical research project unless the purpose of the research is to meet the health needs of the mother and the fetus will be placed at risk only to the minimum extent necessary to meet such needs, or the risk to the fetus is minimal [ 25 ].
Table 2
Conditions to be met in research studies involving pregnant women and fetuses
Researchers can enrol pregnant women only when the mother and/or the father are legally competent. In fact, the consent to participate in research may be either self-directed (only the mother’s consent is required) or made with the guidance of the woman’s partner. However, the father’s consent need not be obtained when: 1) the research activity is directed to the health needs of the mother; 2) the father’s identity is doubtful; 3) the father is absent; or 4) a pregnancy from rape has occurred [ 26 ]. The consent signature requirements from the mother and father are summarised in table 3 . Once the informed consent is obtained, the pregnant women will be included into any phase of the study unless the research project will be compromised or the patient’s health (mother and/or fetus) will be in danger.
Table 3
Consent signature requirements for pregnant women and children
# : consent requirements are the same whether the risk is “no more than minimal” or “more than minimal”.
Medical students and employees, who take part in numerous aspects of patient care in primary, secondary and tertiary care settings, are often invited to participate in human studies as volunteers. Frequently, the requesting researcher is their supervisor or instructor, who may push them to participate in the study, which can negatively influence their decision and also violate the consent legitimacy. Therefore, in order to protect these subjects against “coercion” or “undue influence”, when an investigator wishes to recruit medical students or employees, they must first obtain IRB approval for inclusion in the study of these vulnerable subgroups [ 27 ].
Prisoners, defined as any individual involuntarily confined or detained in a penal institution, are considered as “vulnerable” because they may be coerced into study participation, and also, due to both cognitive and psychiatric disorders, they can show an impaired ability to provide voluntary informed consent [ 28 ]. To protect this population, the Office for Human Research Protections has stipulated federal regulations according to which the only studies that may involve prisoners are those with independent and valid reasons for involving them ( table 4 ) [ 25 ].
Table 4
Studies that may involve prisoners
Due to the context of war in which they work, as well as the critical care setting in which they are treated, military subjects often receive medical care and/or participate in biomedical research under an “implied consent” condition. Moreover, the superior–subordinate relationship contributes to favour coercion or undue influence, making this population vulnerable [ 29 ]. To curb this phenomenon and to ensure that participation is truly voluntary, the US Dept of Defense agencies have adopted requirements similar to those that govern medical research that applies to the civilian population. Accordingly, the medical research recruitment session happens in the absence of superiors, and the informed consent is obtained prior to participating in a medical research study. The presence of an ombudsman guarantees and verifies that the participation is voluntary and that the information provided during recruitment is complete, accurate and clear. A payment as an incentive is acceptable but it must not be used to legitimise a coercive interference. Additional protection is provided to students at service academies, especially those aged <18 years. However, when emergency research is conducted or the research study advances the development of a medical product needed by the armed forces, informed consent will not be required [ 29 ].
Psychological vulnerability
Mental disability may compromise the self-determination and decision-making capacities [ 30 ]. Researchers interested in enrolling individuals with cognitive disorders are invited to apply different strategies to promote a better understanding of information-gathering processes. Simplifying the questions and content, adopting supportive technologies, using a more simple language, and spending more time for the information process have been suggested as useful and valid measures. When all these strategies prove to be insufficient, the investigators are required to obtain consent from a legally authorised representative [ 30 ].
Social vulnerability
Similarly to other vulnerable populations, research involving the homeless, ethnic minorities, immigrants and refugees is regulated by laws and specific procedures. Cultural and language differences, “undocumented” migrant status, and the precarious legal positions of these subjects raise several ethical issues, such as whether the participation is truly voluntary, or there are unrealistic expectations, or any benefits for their “status”.
Obtaining informed consent in these groups is extremely complex. A friendly procedure has been identified as the best way to adequately involve these vulnerable groups. A health centre or community building could represent an accessible location. The reimbursement of travel expenses for applicants can be a valid solution to obtain a representative sample for the clinical research. Clear and simple language, emphasising confidentiality, with the help of professional interpreters, can tempt migrants to sign the consent form. Lastly, the possibility of receiving something back in return for their contribution may enable successful enrolment of migrants in research [ 31 ].
Special needs: children
Because of their young age as well as their limited emotional and intellectual abilities, children are considered to be legally incompetent to give valid informed consent; thus, to enrol a child in a research study, the permission by at least one parent or legal representative is mandatory ( table 3 ). For subjects aged <18 years, biological or adoptive parents or legal guardians (persons having both legal capacity and responsibility) can give consent on behalf of their child, exercising free power of choice without any form of coercion. While married mothers and fathers both have parental responsibility, unmarried parents can exert parental responsibility only if they are named individually on the child’s birth certificate. Also, divorced parents maintain parental responsibility, but it is necessary to know to whom the child’s custody has been assigned [ 32 ]. However, on this matter, the European laws and regulations are not harmonised and several discrepancies are present in each country [ 33 ].
Despite potential benefits for the research subjects, the failure of parents to give consent (or their refusal to give consent) is not a rare circumstance [ 34 ]. It can be the case that researchers are dealing with underage parents, so that, although underage parents are responsible for representing their children, as minors themselves they are not considered to be sufficiently mature; therefore, they will be not able to give valid consent. Literacy and socioeconomic levels have been identified as the most common reasons for parental non-response [ 34 ]. Clarity and adequate explanation of research information materials should be part of effective planning to overcome language and social barriers.
In clinical studies in which the adopted methodology constitutes “less than minimal risks” for children, passive parental consent represents a possible way to more easily obtain informed parental consent [ 34 ]. Furthermore, parents can be informed with regard to a possible study involving their children, and, at the time of data collection, only the child’s assent is required. In fact, although the child’s decision-making capacity and understanding of the research project in which he/she will be involved may be limited, the Medical Research Council have shown that, when study details are provided and communicated in a clear and adequate manner, the child can be able to reach a decision and participate consciously in the research [ 35 ]. “Assent” is the term coined to express the child’s willingness to participate in clinical trials despite their young age. The “assent” should include and respect the following key points: 1) helping the child to acquire disease awareness; 2) explaining the potential impact of the experimental treatment; 3) evaluating the child’s ability to understand and adapt to new situations or challenges; and 4) positively influencing the patient’s willingness to participate in clinical trials [ 36 ]. Although the “assent” is not mandatory for research offering a direct benefit for the child, it arises from the need to respect paediatric research subjects [ 37 ]. The evaluation of the capacity to provide the “assent” is based on developmental stage, intellectual abilities and life or disease experience. Usually, the cut-off age of 7 years is used for the beginning of logical thought processes and rational decision making [ 38 ]. However, “assent” for children aged <7 years can be also required once the ability to read and write has been verified [ 32 ]. Figures 1 and and2 2 summarise the parental and assent permission requirements, respectively.

Flow chart of parental permission requirements.

Flow chart of child assent requirements.
When conducting clinical research, the obtaining of informed consent is required. Informed consent is a procedure through which a competent subject, after having received and understood all the research-related information, can voluntarily provide his or her willingness to participate in a clinical trial. However, when it is impracticable to obtain consent, and the research does not infringe the principle of self-determination and also provides significant clinical relevance, the researcher is legally authorised to proceed without informed consent. Furthermore, in order to preserve the self-determination and decision-making rights, specific law dispositions are applied when vulnerable populations are enrolled in clinical trials.
Self-evaluation questions
- a) Diagnosis
- b) Risks and benefits of treatment
- c) Alternatives to treatment
- d) Family’s wishes
- a) When a minor is considered as emancipated
- b) When a patient is found to be incompetent
- c) When immediate treatment is necessary to prevent death or permanent impairment
- d) When the subject is aged >18 years
- a) Minor is married or divorced
- b) Minor on active duty in the US armed forces
- c) Minor is considered self-sufficient by a court
- d) Minor having a son
Suggested answers
- All research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent.
- Voluntary expression of consent and adequate information disclosure about the research are critical and essential elements of the informed consent process.
- When specific circumstances occur, informed consent can be waived: if it is impracticable to obtain consent, if the research does not infringe the principle of self-determination, and if the research provides significant clinical relevance.
- Participation of vulnerable patients in clinical trials is regulated by specific law dispositions.
Conflict of interest: None declared.

COMMENTS
Oral Consent Template. Guidance for Protocols Involving Oral Consent. Debriefing Template. Guidance and Template for Debriefing Participants. Studies Involving Children (Assent/Permission Forms) Parent-Guardian Permission for Studies Involving Children. Sample Parental Notification Form. Sample Child Assent Form. Performance Release for Minors ...
Standard Informed Consent Template for Research . Use this template if your research is . NOT Federally-sponsored. AND. participants are . adults. Black "we" and "our" (for researcher); not third person (e.g., "we will ask participants"). • Avoid Common Problems with Consent Forms. Read these tips! 1.
Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
If you like, a summary of the results of the study will be sent to you. If you have any other concerns about your rights as a research participant that have not been answered by the investigators, you may contact the Smith College Institutional Review Board at [email protected] or (413) 585-3562.
'Annotated' Template Example Consent Form. Template Consent Form (Word) Further Guidance. Guidance on obtaining consent from research participants online (for online and in-person study designs) Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL. Recording & Obtaining Consent
The forms should be provided to participants in addition to the main study consent form. The language in these forms can also be adapted and added to consent forms for studies in which COVID-19 screening and testing procedures are being done for study purposes, i.e., the results of the screening and/or testing will be used as study data. To do ...
Participant Consent Form This template is designed primarily for those doing qualitative interviews with adults from non-vulnerable populations and dealing with non-sensitive topics. The form would be different in the case of focus groups or quantitative research. If conducting research with vulnerable populations and / or sensitive topics please
This template is a guide to help researchers design study information sheets and consent forms. It has been designed with reference to HRA Participant Information Sheet Preparation Guidance. Sample text is italicised. Alter or delete as required as you produce the draft. Standard required text is underlined.
this section, and all sections of the consent form, use simple, plain English. This section is required.) Procedures to be followed: (Here describe the research procedures to be followed, including duration of the subject's participation. Experimental procedures must be identified. This section is required.) Risks to study participants:
2023-04-10. Assent Form Ages 7-14. 2023-06-27. Consent Addendum for Unencrypted Communication. 2020-10-26. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information.
Questions from potential participants about the research study are directed to the principal investigator (and/or primary contact). The consent form must include their name, title(s), phone number and/or email. Question about participant's rights or research-related injury should be directed to the Institutional Review Board (IRB): Crown ...
Whenever you are proposing research with human participants you must provide a form, known as an Informed Consent Form (ICF), with each proposal to indicate that the research participant has decided to ... storage and future use of samples, this separate consent form should provide the reasons for requesting storage of the samples (or data ...
Consent Template Exempt Research This consent form is an example, ... This is an addendum to be used in conjunction with the consent form to allow participants to choose which genetic research results they would like to have returned to them. RoR Addendum: 6-13-2019:
Consent Form *. Study Title. We are asking you to be in a research study. You do not have to be in the study. If you say yes, you can quit the study at any time. Please take as much time as you need to make your choice. Your medical care will not change in any way if you say no.
Informed Consent in Research. Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of ...
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent ...
Updated June 23, 2023. A research informed consent form is used for the purpose of freeing students/faculty of any liability while performing a research study with human participants. Not only does the consent form liberate the researchers of accountability, it briefs the participants of how the research will be conducted, presented and reported.
Sample Informed Consent Form - ©NCPI. The following is a sample consent form for a research project. It is a research project on faculty life on campus, carried out by the principle investigator (PI) of this project from the fake-named Century University. The interviewer (the investigator) should have the interviewee read this form carefully ...
Confusing informed consent with a signed consent form may violate the ethical intent of informed consent, which is to communicate clearly and respectfully, to foster trust, comprehension, and good decision making, and to ensure that participation is voluntary.
Consent Templates. The templates below were created to help you create the documents you will need to communicate to participants what they will do in the study. The documents you provide participants will range from recruitment materials to post-debrief consent forms, and you need to submit everything that you provide to a participant to our ...
Here's a research consent form template created by our VP of User Research (and User Research Yearbook Class of '22 member), Roberta Dombrowski. This template was created specifically for moderated research studies, but can easily be adapted for unmoderated studies as well. *Note: You probably know this but….
It is important that information given to participants before obtaining their written informed consent is clear and concise and fully explains all aspects of the research. The HRA (Health Research Authority) provides detailed guidelines to help avoid delay at sponsor and ethics review. Make sure that you use the latest version. Please also ...
The consent signature requirements from the mother and father are summarised in table 3. Once the informed consent is obtained, the pregnant women will be included into any phase of the study unless the research project will be compromised or the patient's health (mother and/or fetus) will be in danger.