An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci


Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives
Stroke is the second leading cause of death and a major contributor to disability worldwide. The prevalence of stroke is highest in developing countries, with ischemic stroke being the most common type. Considerable progress has been made in our understanding of the pathophysiology of stroke and the underlying mechanisms leading to ischemic insult. Stroke therapy primarily focuses on restoring blood flow to the brain and treating stroke-induced neurological damage. Lack of success in recent clinical trials has led to significant refinement of animal models, focus-driven study design and use of new technologies in stroke research. Simultaneously, despite progress in stroke management, post-stroke care exerts a substantial impact on families, the healthcare system and the economy. Improvements in pre-clinical and clinical care are likely to underpin successful stroke treatment, recovery, rehabilitation and prevention. In this review, we focus on the pathophysiology of stroke, major advances in the identification of therapeutic targets and recent trends in stroke research.
1. Introduction
Stroke is a neurological disorder characterized by blockage of blood vessels. Clots form in the brain and interrupt blood flow, clogging arteries and causing blood vessels to break, leading to bleeding. Rupture of the arteries leading to the brain during stroke results in the sudden death of brain cells owing to a lack of oxygen. Stroke can also lead to depression and dementia.
Until the International Classification of Disease 11 (ICD-11) was released in 2018, stroke was classified as a disease of the blood vessels. Under the previous ICD coding rationale, clinical data generated from stroke patients were included as part of the cardiovascular diseases chapter, greatly misrepresenting the severity and specific disease burden of stroke. Due to this misclassification within the ICD, stroke patients and researchers did not benefit from government support or grant funding directed towards neurological disease. After prolonged advocacy from a group of clinicians, the true nature and significance of stroke was acknowledged in the ICD-11; stroke was re-categorized into the neurological chapter [ 1 ]. The reclassification of stroke as a neurological disorder has led to more accurate documentation of data and statistical analysis, supporting improvements in acute healthcare and acquisition of research funding for stroke.
2. Epidemiology of Stroke
Stroke is the second leading cause of death globally. It affects roughly 13.7 million people and kills around 5.5 million annually. Approximately 87% of strokes are ischemic infarctions, a prevalence which increased substantially between 1990 and 2016, attributed to decreased mortality and improved clinical interventions. Primary (first-time) hemorrhages comprise the majority of strokes, with secondary (second-time) hemorrhages constituting an estimated 10–25% [ 2 , 3 ]. The incidence of stroke doubled in low-and-middle income countries over 1990–2016 but declined by 42% in high-income countries over the same period. According to the Global Burden of Disease Study (GBD), although the prevalence of stroke has decreased, the age of those affected, their sex and their geographic location mean that the socio-economic burden of stroke has increased over time [ 3 ].
Age-specific stroke : The incidence of stroke increases with age, doubling after the age of 55 years. However, in an alarming trend, strokes in people aged 20–54 years increased from 12.9% to 18.6% of all cases globally between 1990 and 2016. Nevertheless, age-standardized attributable death rates decreased by 36.2% over the same period [ 3 , 4 , 5 ]. The highest reported stroke incidence is in China, where it affects an estimated 331–378 individuals per 100,000 life years. The second-highest rate is in eastern Europe (181–218 per 100,000 life years) and the lowest in Latin America (85–100 per 100,000 life years) [ 3 ].
Gender-specific stroke : The occurrence of stroke in men and women also depends on age. It is higher at younger ages in women, whereas incidence increases slightly with older age in men. The higher risk for stroke in women is due to factors related to pregnancy, such as preeclampsia, contraceptive use and hormonal therapy, as well as migraine with aura. Atrial fibrillation increases stroke risk in women over 75 years by 20%. Based on the National Institutes of Health Stroke Scale (0 = no stroke, 1–4 = minor stroke, 5–15 = moderate stroke, 15–20 = moderate/severe stroke, 21–42 = severe stroke), mean stroke severity was estimated at 10 for women and 8.2 for men. Both brain infarction and intracerebral hemorrhage (ICH) are common in men, but cardioembolic stroke, a more severe form of stroke, is more prevalent among women. The fatality rate for stroke is also higher among women [ 5 , 6 , 7 ]. Women live longer than men, which is one reason for their higher incidence of stroke; another important concern is women’s delay in accepting help for ongoing symptoms [ 8 ]. For men, the most common causes of stroke are tobacco smoking, excessive alcohol consumption, myocardial infarction and arterial disorders [ 9 ].
Geographic and racial variation : As noted earlier, stroke incidence varies considerably across the globe. A global population-based study of the prevalence of stroke and related risks examined demography, behavior, physical characteristics, medical history and laboratory reports, and revealed the contribution of exposure to air pollution and particulate matter to stroke mortality [ 10 ]. Another population-based study, conducted in north-eastern China, is thought to be broadly representative of the disease situation in developing countries. It found hypertension to be a statistically significant risk for stroke, specifically ischemic stroke [ 11 ]. A study conducted in the United States (US) also identified hypertension as a major cause of stroke and described geographical variation in symptomatic intensity in stroke sufferers. Insufficient physical activity, poor food habits and nicotine and alcohol consumption were considered added risks [ 12 ]. Differences in exposure to environmental pollutants, such as lead and cadmium, also influenced stroke incidences across regions. This study also revealed differences in stroke incidence between non-Hispanic white and black populations aged 40–50 years [ 13 ].
Socioeconomic variation : There is a strong inverse relationship between stroke and socioeconomic status, attributable to inadequate hospital facilities and post-stroke care among low-income populations [ 14 ]. A case study conducted in the US showed that people with high financial status had better stroke treatment options than deprived individuals [ 15 ]. A study in China linked low income and lack of health insurance to prevention of secondary stroke attack [ 16 ]. Research conducted in Austria associated level of education with take-up of treatments such as echocardiography and speech therapy; however, there was no difference in administration of thrombolysis, occupational therapy, physiotherapy or stroke care for secondary attack by socioeconomic status [ 17 ]. Similarly, in the Scottish healthcare system, basic treatments like thrombolysis were provided irrespective of the economic status of patients [ 18 ].
3. Pathophysiology of Stroke
Stroke is defined as an abrupt neurological outburst caused by impaired perfusion through the blood vessels to the brain. It is important to understand the neurovascular anatomy to study the clinical manifestation of the stroke. The blood flow to the brain is managed by two internal carotids anteriorly and two vertebral arteries posteriorly (the circle of Willis). Ischemic stroke is caused by deficient blood and oxygen supply to the brain; hemorrhagic stroke is caused by bleeding or leaky blood vessels.
Ischemic occlusions contribute to around 85% of casualties in stroke patients, with the remainder due to intracerebral bleeding. Ischemic occlusion generates thrombotic and embolic conditions in the brain [ 19 ]. In thrombosis, the blood flow is affected by narrowing of vessels due to atherosclerosis. The build-up of plaque will eventually constrict the vascular chamber and form clots, causing thrombotic stroke. In an embolic stroke, decreased blood flow to the brain region causes an embolism; the blood flow to the brain reduces, causing severe stress and untimely cell death (necrosis). Necrosis is followed by disruption of the plasma membrane, organelle swelling and leaking of cellular contents into extracellular space [ 20 ], and loss of neuronal function. Other key events contributing to stroke pathology are inflammation, energy failure, loss of homeostasis, acidosis, increased intracellular calcium levels, excitotoxicity, free radical-mediated toxicity, cytokine-mediated cytotoxicity, complement activation, impairment of the blood–brain barrier, activation of glial cells, oxidative stress and infiltration of leukocytes [ 21 , 22 , 23 , 24 , 25 ].
Hemorrhagic stroke accounts for approximately 10–15% of all strokes and has a high mortality rate. In this condition, stress in the brain tissue and internal injury cause blood vessels to rupture. It produces toxic effects in the vascular system, resulting in infarction [ 26 ]. It is classified into intracerebral and subarachnoid hemorrhage. In ICH, blood vessels rupture and cause abnormal accumulation of blood within the brain. The main reasons for ICH are hypertension, disrupted vasculature, excessive use of anticoagulants and thrombolytic agents. In subarachnoid hemorrhage, blood accumulates in the subarachnoid space of the brain due to a head injury or cerebral aneurysm ( Figure 1 ) [ 27 , 28 ].

Molecular mechanism of stroke.
4. Risk Factors for Stroke
As noted earlier, the risk of stroke increases with age and doubles over the age of 55 years in both men and women. Risk is increased further when an individual has an existing medical condition like hypertension, coronary artery disease or hyperlipidemia. Nearly 60% of strokes are in patients with a history of transient ischemic attack (TIA). Some of the risk factors for stroke are modifiable, and some are non-modifiable ( Figure 2 ).

Risk factors associated with stroke.
4.1. Non-Modifiable Risk Factors
These include age, sex, ethnicity, TIA and hereditary characteristics. In the US in 2005, the average age of incidence of stroke was 69.2 years [ 2 , 29 , 30 ]. Recent research has indicated that people aged 20–54 years are at increasing risk of stroke, probably due to pre-existing secondary factors [ 31 ]. Women are at equal or greater risk of stroke than men, irrespective of age [ 32 ]. US research shows that Hispanic and black populations are at higher risk of stroke than white populations; notably, the incidence of hemorrhagic stroke is significantly higher in black people than in age-matched white populations [ 33 , 34 , 35 ].
Transient ischemic attack is classified as a mini stroke; the underlying mechanism is the same as for full-blown stroke. In TIA, the blood supply to part of the brain is blocked temporarily. It acts as a warning sign before the actual event, providing an opportunity to change lifestyle and commence medications to reduce the chance of stroke [ 36 , 37 ].
Genetics contribute to both modifiable and non-modifiable risk factors for stroke. Genetic risk is proportional to the age, sex and race of the individual [ 38 , 39 ], but a multitude of genetic mechanisms can increase the risk of stroke. Firstly, a parental or family history of stroke increases the chance of an individual developing this neurological disorder. Secondly, a rare single gene mutation can contribute to pathophysiology in which stroke is the primary clinical manifestation, such as in cerebral autosomal dominant arteriopathy. Thirdly, stroke can be one of many after-effects of multiple syndromes caused by genetic mutation, such as sickle cell anemia. Fourthly, some common genetic variants are associated with increased stroke risk, such as genetic polymorphism in 9p21 [ 40 ]. A genome-wide association study of stroke showed high heritability (around 40%) for large blood vessel disease, and low heritability (16.7%) for small vessel disorders. Recent evidence suggests that studying heritability will improve the understanding of stroke sub-types, improve patient management and enable earlier and more efficient prognosis [ 5 , 41 ].
4.2. Modifiable Risk Factors
These are of paramount importance, because timely and appropriate medical intervention can reduce the risk of stroke in susceptible individuals. The major modifiable risk factors for stroke are hypertension, diabetes, lack of physical exercise, alcohol and drug abuse, cholesterol, diet management and genetics.
Hypertension : It is one of the predominant risk factors for stroke. In one study, a blood pressure (BP) of at least 160/90 mmHg and a history of hypertension were considered equally important predispositions for stroke, with 54% of the stroke-affected population having these characteristics [ 42 , 43 ]. BP and prevalence of stroke are correlated in both hypertensive and normal individuals. A study reported that a 5–6 mm Hg reduction in BP lowered the relative risk of stroke by 42% [ 44 ]. Randomized trials of interventions to reduce hypertension in people aged 60+ have shown similar results, lowering the incidences of symptoms of stroke by 36% and 42%, respectively [ 45 , 46 ].
Diabetes : It doubles the risk of ischemic stroke and confers an approximately 20% higher mortality rate. Moreover, the prognosis for diabetic individuals after a stroke is worse than for non-diabetic patients, including higher rates of severe disability and slower recovery [ 47 , 48 ]. Tight regulation of glycemic levels alone is ineffective; medical intervention plus behavioral modifications could help decrease the severity of stroke for diabetic individuals [ 49 ].
Atrial fibrillation (AF) : AF is an important risk factor for stroke, increasing risk two- to five-fold depending upon the age of the individual concerned [ 50 ]. It contributes to 15% of all strokes and produces more severe disability and higher mortality than non-AF-related strokes [ 51 ]. Research has shown that in AF, decreased blood flow in the left atrium causes thrombolysis and embolism in the brain. However, recent studies have contradicted this finding, citing poor evidence of sequential timing of incidence of AF and stroke, and noting that in some patients the occurrence of AF is recorded only after a stroke. In other instances, individuals harboring genetic mutations specific to AF can be affected by stroke long before the onset of AF [ 52 , 53 ]. Therefore, we need better methods of monitoring the heart rhythms that are associated with the vascular risk factors of AF and thromboembolism.
Hyperlipidemia : It is a major contributor to coronary heart disease, but its relationship to stroke is complicated. Total cholesterol is associated with risk of stroke, whereas high-density lipoprotein (HDL) decreases stroke incidence [ 54 , 55 , 56 ]. Therefore, evaluation of lipid profile enables estimation of the risk of stroke. In one study, low levels of HDL (<0.90 mmol/L), high levels of total triglyceride (>2.30 mmol/L) and hypertension were associated with a two-fold increase in the risk of stroke-related death in the population [ 55 ].
Alcohol and drug abuse : The relationship between stroke risk and alcohol intake follows a curvilinear pattern, with the risk related to the amount of alcohol consumed daily. Low to moderate consumption of alcohol (≤2 standard drinks daily for men and ≤1 for women) reduces stroke risk, whereas high intake increases it. In contrast, even low consumption of alcohol escalates the risk of hemorrhagic stroke [ 57 , 58 , 59 ]. Regular use of illegitimate substances such as cocaine, heroin, phencyclidine (PCP), lysergic acid diethylamide (LSD), cannabis/marijuana or amphetamines is related to increased risk of all subtypes of strokes [ 60 ]. Illicit drug use is a common predisposing factor for stroke among individuals aged below 35 years. US research showed that the proportion of illicit drug users among stroke patients aged 15–44 years was six times higher than among age-matched patients admitted with other serious conditions [ 61 ]. However, there is no strong evidence to confirm these findings, and the relationship between these drugs and stroke is anecdotal [ 62 ].
Smoking : Tobacco smoking is directly linked to increased risk of stroke. An average smoker has twice the chance of suffering from a stroke of a non-smoker. Smoking contributes to 15% of stroke-related mortality. Research suggests that an individual who stops smoking reduces the relative risk of stroke, while prolonged second-hand smoking confers a 30% elevation in the risk of stroke [ 63 , 64 , 65 ].
Insufficient physical inactivity and poor diet are associated with increased risk for stroke. Lack of exercise increases the chances of stroke attack in an individual. Insufficient physical activity is also linked to other health issues like high BP, obesity and diabetes, all conditions related to high stroke incidence [ 66 , 67 ]. Poor diet influences the risk of stroke, contributing to hypertension, hyperlipidemia, obesity and diabetes. Certain dietary components are well known to heighten risk; for example, excessive salt intake is linked to high hypertension and stroke. Conversely, a diet high in fruit and vegetables (notably, the Mediterranean diet) has been shown to decrease the risk of stroke [ 68 , 69 , 70 , 71 , 72 ].
5. Animal Models of Stroke
Animal models usually used for research include induced, spontaneous, negative and orphan models. In the induced model, a disease condition is induced in the animal with a view to studying the effects, whereas in the spontaneous model, an animal is selected with a similar disease state naturally present in the model. Negative animal models are used to study the resistance mechanisms underlying a particular disease condition. Orphan models are deployed to understand the pathology of a newly characterized disease in human subjects [ 73 , 74 ].
Many animal models have been developed to study the pathophysiology associated with stroke; they offer several advantages over studying stroke in humans or in vitro. The nature of stroke in humans is unpredictable, with diverse clinical manifestation and localization, whereas animal models are highly predictable and reproducible. Pathophysiological investigation often requires direct access to brain tissue, which is possible with animal models but not in humans. Moreover, current imaging techniques are unable to characterize events occurring within the first few minutes of a stroke. Finally, some aspects of stroke, such as vasculature and perfusion, cannot be studied in in vitro models [ 75 ]. Different stroke models used in animals are described in the session below ( Table 1 ).
Advantages and disadvantages of the stroke models.
The intraluminal suture MCAo model : The middle cerebral artery (MCA) is vulnerable to ischemic insult and occlusion in humans, accounting for 70% of stroke-related disability. This disease model has been widely studied in rat and mouse models, with more than 2600 experiments conducted [ 76 , 77 ]. The MCAo procedure is minimally invasive; it involves occlusion of the carotid artery by insertion of a suture until it interrupts blood flow to the MCA. This procedure is applied for time periods such as 60 or 90 min or permanently, to induce infarction, and has a success rate of 88–100% in rats and mice [ 78 ]. The most commonly used animal for studying pre-clinical stroke is the Sprague–Dawley rat, which has a small infarct volume [ 79 ]. In mice, C57BL/6 and SV129 are commonly used to introduce MCA infarction. The reproducibility of the technique depends on a multitude of factors, such as the animal strain, suture diameter, body weight and age. The advantage of this model is that it mimics the human ischemic stroke and displays similar penumbra [ 80 ]. The MCAo model is appropriate for reproducing ischemic stroke and associated clinical manifestations such as neuronal cell death, cerebral inflammation and blood–brain barrier damage [ 75 ].
Craniectomy model : This model uses a surgical procedure for inducing occlusion in the artery. In this technique, a neurological deficit can be induced in mice by electrocoagulation causing permanent insult or a microaneurysm until blood flow is interrupted. Alternatively, three-vessel occlusion is used, reducing the blood flow and resulting in damaged tissue. The infarct volume differs depending on whether the occlusion is permanent or transient [ 81 , 82 , 83 ]. A study conducted in neonatal P14–P18 rats mimicked pediatric stroke in a younger human population; a 3-h occlusion was performed to induce lesions affecting 40–50% of the brain [ 84 ]. Similarly, in P7 rats, oedema formation was observed in the MCA, followed by microglial infiltration. The P12 CB-17 is another animal model used for stroke research, mainly due to low variability in occlusion insult to the brain [ 85 ]. The other advantages of this model include reproducible infarct size and neurofunctional deficits, reduced mortality and visual ratification. The CB-17 model was successfully used to reproduce cerebral infarction and long-term survival rate, and to study ischemic reperfusion. Researchers showed that reperfusion supports neuron survival, rescues vascular phenotypes and is associated with functional recovery after stroke [ 86 ].
The Levine–Rice model : It involves histological examination and behavioral tests in rat pups, and it is used to study neonatal hypoxic-ischemic stroke [ 87 ]. In this model, a unilateral ligation is followed by reperfusion and recovery. Later, the animal is placed in a hypoxic chamber to understand neonatal stroke pathophysiology as well as regenerative and rehabilitative therapeutic possibilities. P7 rat animal models are commonly used to study the clinical manifestations of hypoxic-ischemic injury [ 88 , 89 , 90 ].
Photo-thrombosis model : This model is based on photo-oxidation of the vasculature leading to lesion formation in the cortex and striatum. In this method, the skull is irradiated with a photoactive dye that causes endothelial damage, intraparenchymal vessel aggregation and platelet stimulation in the affected area. It is injected intraperitoneally in mice and intravenously in rats [ 91 ]. This model is highly reproducible, with a low mortality rate and no surgery. The pathophysiology of this method is slightly different to that seen in human stroke due to little collateral blood flow or formation of ischemic penumbra. However, recent researchers modified the photothrombotic ischemia model to include hypoperfusion in an attempt to mimic penumbra. It has also been deployed in freely moving mice to evaluate the development of motor cortex ischemia and motor deficits. This model permits assessment of the ongoing infarction and improves our understanding of the neuronal insult and repair process [ 92 , 93 ].
Endothelin-1 model: Endothelin-1 (ET-1) : ET-1 is a small peptide molecule produced by smooth muscle cells and the endothelium. It is a paracrine factor that restricts the vascular system through cell-specific receptors. Ischemic lesion is induced by stereotaxic injection of ET-1 directly into the exposed MCA in the intracerebral or cortex region [ 94 ]. ET-1 administration was observed to cause 70–90% reduction in cerebral blood flow, followed by reperfusion [ 95 ]. This technique is minimally invasive, has a low death rate and can be applied to deep and superficial brain regions. It is appropriate for long-term lesion studies, and the lesion size can be controlled by regulating ET-1 concentration, which is critical for reproducibility [ 95 ]. ET-1 is expressed by both neurons and astrocytes, which may decrease the stringency of interpretation of neuronal dysfunction in stroke [ 96 ]. A study in juvenile P21 rats used ET-1 to induce focal lesion in the striatum [ 97 ]. Similarly, aged P12 and P25 rats showed neuronal damage and lesion formation after injection of ET-1 into the hippocampus [ 98 ].
The embolic stroke model : It includes microsphere, macrosphere and thromboembolic models. The microsphere model involves introduction of spheres of diameter 20–50 μm into the circulatory system using a microcatheter to form multifocal infarcts [ 99 ]. Macrospheres are 100–400 μm in diameter and introduced into the intracerebral artery (ICA) to produce reproducible lesions in the MCA [ 100 ]. In the thromboembolic model, thrombin is directly injected to form clots in the ICA or MCA. The volume of the infarct depends upon the size of the clot formed [ 101 ]. This model closely resembles the type of stroke seen in humans. Prior study of clots induced by this model in mice have showed that they are mainly comprised of polymerized fibrin with few cells and platelets present, and 75% of clots exhibit platelet/fibrin build-up and deposition of neutrophils, monocytes and erythrocytes [ 102 ].
Neurorehabilitation in animal models : Various rehabilitative devices and forced training strategies have been deployed in stroke-affected animals to study neurological behavior. Robotic and electric devices have also been developed for training purposes in animal models to evaluate the functionality and effectiveness of the rehabilitation process. Similarly, forced exercise regimes, such as running on a treadmill or task-oriented motor training, are used to study rehabilitation scope in humans. Housing environments that provide social, motor and sensory stimuli and support cell engraftment, creating a more realistic approximation of human treatment, can be tested using animal models [ 103 , 104 , 105 ].
Animal models in biomaterial testing : Animal models have been well characterized for the study of brain tissues via brain atlases ( http://www.med.harvard.edu/AANLIB/ , https://portal.brain-map.org ) for the required species. Stereotaxic techniques are utilized to introduce biomaterials or cells into particular coordinates of the target tissue. Microlesions can be studied precisely, and targeted localization can be confirmed using magnetic resonance imaging (MRI)-based lesion cartography [ 106 , 107 , 108 ].
6. Prevention and Treatment Strategies for Stroke
Stroke prevention involves modifying risk factors within a population or individuals, while stroke management depends on treating its pathophysiology. Despite an enormous amount of research into stroke over the last two decades, no simple means of treating or preventing all the clinical causes of stroke has been established. The overall direction of current stroke research is to generate novel therapies that modulate factors leading to primary and secondary stroke. Recent and current strategies for stroke prevention and treatment are discussed below ( Figure 3 ).

Stroke therapy. This represents the overall process to manage the incidence of stroke.
Excitotoxicity : Neuronal death is a key manifestation of stroke. A key reason for this phenomenon is neuronal depolarization and inability to maintain membrane potential within the cell. This process is mediated by glutamate receptors N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), which were among the first neuroprotective agents tested in stroke prevention. However, the untimely release of glutamate overpowers the system that removes glutamate from the cell and causes abnormal release of NMDA and AMPA molecules, leading to uninhibited calcium influx and protein damage. As a result, these agents have not been shown to reduce neuronal death in human subjects. Targeting the molecular pathways downstream of excitotoxicity signaling, rather than directly targeting glutamatergic signaling, might reduce the side effects of the process [ 109 , 110 ].
Gamma aminobutyric acid (GABA) agonists : Clomethiazole is a GABA agonist that has been tested for its ability to improve stroke symptoms in patients, but failed to reduce the toxicity induced by the glutamate receptor [ 111 ].
Sodium (Na + ) channel blockers : Na + channel blockers have been used as neuroprotective agents in various animal models of stroke. They prevent neuronal death and reduce white matter damage. Many voltage-gated Na + channel blockers have been tested in clinical trials, but most have proved to be ineffective [ 112 ]. Mexiletine is a neuroprotectant and Na + channel blocker that proved effective in grey and white matter ischemic stroke, though further evaluation is required to confirm its role [ 113 ]. Lubeluzole was shown to reduce mortality in stroke in initial clinical trials, but successive trials failed to reproduce similar outcomes. Similarly, sipatrigine is a Na + and Ca 2+ channel blocker which failed in a Phase II clinical trial in stroke patients. Amiodarone was shown to aggravate brain injury due to defective transportation and accumulation of Na + ions in the brain after stroke [ 114 ].
Calcium (Ca 2+ ) channel blockers : Voltage-dependent Ca 2+ ion channel blockers have been shown to decrease the ischemic insult in animal models of brain injury. The Ca 2+ ion chelator DP-b99 proved efficient and safe in Phase I and II clinical trials when administered to stroke patients. Similarly, Phase II trials significantly improved clinical symptoms in stroke patients treated within 12 h of onset [ 115 ]. In another study, Ca 2+ channel blockers reduced the risk of stroke by 13.5% in comparison to diuretics and β-blockers [ 116 ].
Antioxidants : Reactive oxygen species produced in the normal brain are balanced by antioxidants generated in a responsive mechanism. However, in the ischemic stroke model, excess production of free radicals and inactivation of detoxifying agents cause redox disequilibrium. This phenomenon leads to oxidative stress, followed by neuronal injury. Therefore, antioxidants are employed in treatment of acute stroke to inhibit or scavenge free radical production and degrade free radicals in the system. In one study, antioxidant AEOL 10,150 (manganese (III) meso-tetrakis (di-N-ethylimidazole) porphyrin) effectively regulated the gene expression profiles specific to inflammation and stress response to decrease the ischemic damage and reperfusion in stroke patients [ 117 ]. In another, deferoxamine was shown to regulate the expression of hypoxia-inducible factor-1, a transcriptional factor regulated by oxygen levels, which in turn switched on other genes like vascular endothelial growth factor and erythropoietin. This mechanism, studied in an animal stroke model, proved beneficial in reducing lesion size and improving sensorimotor capabilities [ 118 , 119 ]. Similarly, NXY-059 compound acts as a scavenger to eliminate free radicals and decrease neurological deficits. The Stroke-Acute-Ischemic-NXY-Treatment-I (SAINT) clinical trial showed the efficacy and safety of NXY-059, but SAINT II failed to reproduce the positive effect of this drug in stroke patients [ 120 , 121 ]. In another study, researchers employed intravenous injection of antioxidants directly into mice brains to understand the benefits of route of administration. This method reduced neurological defects, but had minimal influence on brain damage [ 122 ].
6.1. Reperfusion
The intravenous thrombolytics (IVT) : The IVT treatment paradigm was originally developed to treat coronary thrombolysis but was found to be effective in treating stroke patients. The efficiency of thrombolytic drugs depends on factors including the age of the clot, the specificity of the thrombolytic agent for fibrin and the presence and half-life of neutralizing antibodies [ 123 ]. The drugs used in IVT treatment aim to promote fibrinolysin formation, which catalyzes the dissolution of the clot blocking the cerebral vessel. The most effective IVT drug, recombinant tissue plasminogen activator (rt-PA, or alteplase), was developed from research conducted by the US National Institute of Neurological Disorders and Stroke (NINDS) [ 124 ]. However, European Cooperative Acute Stroke Study (ECASS and ECASS II) researchers were unable to reproduce NINDS’ results. Later, it was found that this drug was effective in reducing clot diameter in stroke patients within three hours of incidence. The Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS-MOST) confirmed the efficacy and safety of alteplase within the designated time frame [ 125 ]. Another category of thrombolytics, consisting of fibrin and non-fibrin drugs, is used for treatment of stroke symptoms. Fibrin activators like alteplase, reteplase and tenecteplase convert plasminogen to plasmin directly, whereas non-fibrin activators like the drugs streptokinase and staphylokinase do so indirectly [ 123 ].
Intra-arterial thrombolysis (IAT) : IAT is another approach designed to combat acute stroke. This treatment is most effective in the first six hours of onset of MCA occlusion, and requires experienced clinicians and angiographic techniques [ 115 ]. Prolyse in Acute Cerebral Thromboembolism II (PROACT II) and Middle Cerebral Artery Embolism Local Fibrinolytic Intervention (MELT) were randomized clinical trials (RCTs) undertaken to test the efficacy and safety of a recombinant pro-urokinase drug [ 126 , 127 ], but did not produce any data useful for stroke treatment. Thrombolytics and glycoprotein IIb/IIIa antagonists were combined in two small clinical trials; this approach was helpful in treating atherosclerotic occlusions but less effective for cardioembolism [ 128 , 129 ]. The Interventional Management of Stroke (IMS) III trial tested IVT and IAT together to assess the benefits of combining rapid administration of therapy (IVT) and a superior recanalization methodology for faster relief (IAT) [ 130 ]. The IMS III trial was fruitful with bridging therapy (combination of IVT and IAT) as compared to IVT alone. There was an increase of 69.6% in the recanalization rate using bridging therapy in stroke patients [ 131 , 132 ].
Fibrinogen-depleting agents : Research has found a strong correlation between high fibrinogen levels in stroke patients and poor diagnosis for clinical outcomes. Fibrinogen-depleting agents decrease blood plasma levels of fibrinogen, hence reduce blood thickness and increase blood flow. They also remove the blood clot in the artery and restore blood flow in the affected regions of the brain. However, although some RCTs of defibrinogen therapy identified beneficial effects of fibrinogen-depleting agents in stroke patients, others failed to show positive effects on clinical outcomes after stroke [ 133 ]. Moreover, some studies reported bleeding after treatment with defibrinogen agents. Ancrod is a defibrinogenating agent derived from snake venom that has been studied for its ability to treat ischemic stroke within three hours of onset [ 134 ]. The European Stroke Treatment with Ancrod Trial (ESTAT) concluded that controlled administration of ancrod at 70 mg/dL fibrinogen was efficacious and safe, and achieved lower prevalence of ICH than observed at lower fibrinogen levels [ 135 ].
6.2. Others
Antihypertensive therapy : Hypertension is a risk factor for stroke. There are many reasons for high BP in stroke, including a history of hypertension, acute neuroendocrine stimulation, increased intracranial pressure, stress linked to hospital admission and intermittent painful spells [ 136 ]. Correct treatment of high BP during stroke is uncertain due to contradictory outcomes of clinical studies. Some research shows positive correlations between high BP and stroke-related mortality, hematoma expansion or intracerebral damage, suggesting that high BP should be treated. In other studies, low BP levels led to tissue perfusion and increased lesion size, thereby worsening the clinical outcome [ 137 , 138 ]. The multi-center Acute Candesartan Cilexetil Therapy in Stroke Survivors (ACCESS) Phase II study proved that taking medication (candesartan) for BP during stroke was safe, with no orchestrated cerebrovascular events reported due to hypotension. Similar research has been performed with antihypertensive drugs, such as the Continue Or Stop post Stroke Antihypertensives Collaborative Study (COSSACS) to study the efficacy of antihypertensive therapy in stroke; the Control of Hypertension and Hypotension Immediately Post Stroke (CHHIPS) study, designed to determine the cut-off value for BP during an attack; and the Scandinavian Candesartan Acute Stroke Trial (SCAST), which aimed to measure the effectiveness of the drug candesartan on stroke and cardiovascular disease [ 115 , 139 ]. In the COSSACS study, continuing antihypertensive drugs for a two-week period produced no extra harm as compared to stopping it and might be associated with reduced two-week mortality in patients with ischemic stroke [ 140 ]. The CHHIPS study demonstrated that a relatively moderate reduction in blood pressure lowered the mortality rate [ 141 ], whereas the SCAST study suggested that a careful BP-lowering treatment was associated with a higher risk of poor clinical outcome [ 142 ].
Glucose management : Hyperglycemia (elevated blood glucose) is common in stroke patients, so targeting blood glucose levels is an efficient stroke management strategy. Hyperglycemia > 6.0 mmol/L (108 mg/dL) is observed in most stroke patients; it initiates lipid peroxidation and cell lysis in compromised tissue, leading to stroke complications. An experimental study conducted in a rat model of collagenase-induced ICH found that hyperglycemia worsens edema formation and increases cell death, accelerating the course of ischemic injury. Increased blood glucose level is also associated with progression of infarction, reduced recanalization and poor clinical outcome [ 143 ]. Continuous glucose monitoring systems have been deployed to reduce stroke-related risks in both diabetic and non-diabetic stroke patients [ 144 ].
Antiplatelet therapy : This therapy is used for acute ischemic stroke management and for prevention of stroke incidence. It is also vital in controlling non-cardioembolic ischemic stroke and TIA. Antiplatelet agents like aspirin, clopidogrel and ticagrelor are the most widely used drugs administered to stroke sufferers within the first few days of attack [ 145 ]. Dual antiplatelet therapy, which involves a combination of clopidogrel, prasugrel or ticagrelor with aspirin, has become popular; many studies have tested the efficacy and safety of this dual therapy. It has been claimed that clopidogrel and aspirin combination therapy is most beneficial if introduced within 24 h of stroke and continued for 4–12 weeks [ 146 ].
Stem cell therapy : It offers promising therapeutic opportunities, safety and efficacy to stroke patients. Research on embryonic stem cells, mesenchymal cells and induced pluripotent stem cells has assessed their potential for tissue regeneration, maintenance, migration and proliferation, rewiring of neural circuitry and physical and behavioral rejuvenation [ 147 ]. Recently, a new type of mesenchymal stem cells (MSCs), called multilineage differentiating stress-enduring (Muse) cells, has been found in connective tissue. These cells offer great regenerative capacity and have been tested as a stroke treatment. After intravenous transplantation of Muse cells in a mouse model, they were found to engraft into the damaged host tissue and differentiate to provide functional recovery in the host [ 148 ]. Neovascularization is another mode of action of cell therapies in stroke; studies conducted in vitro and in vivo have shown that transplanted cells promote angiogenesis [ 149 , 150 ]. Furthermore, multiple stroke studies have reported that MSCs stimulate neurogenesis; this was confirmed in human embryonic neural stem cells using BrdU-labelling [ 151 , 152 ]. Stem cell therapy enhances the proliferation of neural stem cells and neuritogenesis [ 153 ]. Careful experimental design and clinical trials of stem cell therapies are likely to usher in a new era of treatment for stroke by promoting neurogenesis, rebuilding neural networks and boosting axonal growth and synaptogenesis.
Neural repair : This is an alternative therapy to neuroprotection. It is used to rejuvenate the tissue when the damage is already done and is therefore not time-bound but is most effective when administered 24 h after stroke attack. Many animal models have been used in attempts to stimulate neurogenesis and initiate the neuronal repair process [ 154 ]. Neural repair utilizes stem cell therapy to initiate repair mechanisms through cell integration into the wound or use of neurotrophic factors to block neuronal growth inhibitors. These cells may be channeled to any injured region to facilitate greater synaptic connectivity. Clinical trials using neural stem cells have proven beneficial in stroke patients. However, trials of myelin-associated glycoprotein, neurite outgrowth inhibitor (NOGO) proteins and chondroitin sulphate proteoglycans have shown these agents to be insufficiently effective; more clinical trials are required to increase treatment efficacy [ 155 ]. Biological intrusions may foster regeneration of newer cells, improve axonal guidance and enhance neural circuitry. Pharmacological and immunological interventions may target receptors to provide signaling cues for regeneration or block inhibitory factors in stroke-affected regions of the brain [ 156 ].
Rehabilitation : Stroke can leave individuals with short- and long-term disabilities. Daily activities like walking and toileting are often affected, and sensorimotor and visual impairment are common. Rehabilitation aims to reinforce the functional independence of people affected by stroke [ 157 ]. It includes working with patients and families to provide supportive services and post-stroke guidance after 48 h of stroke attack in stable patients. Stroke rehabilitation may involve physical, occupational, speech and/or cognitive therapy. It is designed to assist patients to recover problem-solving skills, access social and psychological support, improve their mobility and achieve independent living. Rehabilitation may also include neurobiological tasks designed to lessen the impact of cognitive dysfunction and induce synaptic plasticity, as well as long-term potentiation [ 158 , 159 ]. Neuromodulators play a vital role in triggering expression of specific genes that promote axon regeneration, dendritic spine development, synapse formation and cell replacement therapy. Task-oriented approaches, like arm training and walking, help stroke patients to manage their physical disability, and visual computer-assisted gaming activities have been used to enhance visuomotor neuronal plasticity [ 160 ].
7. Trends in Stroke Research
The incidence of stroke-related emergencies has decreased substantially over recent years due to improved understanding of the pathophysiology of stroke and identification of new drugs designed to treat the multitude of possible targets. Technological advancements like telestroke [ 161 ] and mobile stroke [ 162 ] units have reduced mortality and morbidity. Therefore, stroke management systems should include post-stroke care facilities on top of existing primary care and access to occupational, speech or any physical therapy following hospital discharge. Hospitals should develop standardized policies to handle emergencies in a timely fashion to avoid casualties and prevent secondary stroke [ 163 ]. Recently, the role of physiotherapists has emerged as an important aspect of post-stroke care management. Physiotherapists have initiated clinical trials of stroke recovery processes and rehabilitation therapy sessions. One ongoing study includes a strategy to manage disability by improving mobility using treadmill exercise, electromechanical device therapy and circuit class therapy [ 164 , 165 ]. Stroke Recovery and Rehabilitation Roundtables bring physiotherapists and other experts together to recommend research directions and produce guidance for the post-stroke healthcare system. Optimized delivery of stroke care systems and access to rehabilitation services are the future of healthcare for stroke [ 166 ].
Animal models used in stroke research reflect only a portion of the consequences of the condition in human subjects. Moreover, experiments conducted within a single laboratory are often constrained in terms of their research output. In vivo animal models of stroke should include aged populations to maximize their relevance, but most recent studies involve young and adult animals. Stroke studies should be conducted in both male and female subjects to exclude gender bias, and should take account of other confounders like hypertension, diabetes and obesity. All these issues make stroke research complex and expensive, and imply that it should be carried out collaboratively, across multiple labs. Ideally, an international multicenter platform for clinical trials would be established to increase the validity of research outcomes with respect to efficacy, safety, translational value, dose–response relationships and proof-of-principle. This strategy will help to overcome the current hurdles in transforming laboratory data into therapeutics for stroke.
Advancements in stem cell technologies and genomics have led to regenerative therapy to rebuild neural networks and repair damaged neurons due to ischemic insult [ 167 , 168 ]. The WIP1 gene is a regulator of Wnt signaling and a promising target for drug development. Studies in mice models showed that knockdown of WIP1 downregulates the stroke functional recovery process after injury, and that the presence of this gene regulates neurogenesis through activation of β-Catenin/Wnt signaling [ 169 ]. Similarly, NB-3 (contactin-6) plays a vital role in neuroprotection, as shown by knockdown of NB-3 in mice after stroke attack. NB-3-deficient mice had increased brain damage after MCAo, which also affected neurite outgrowth and neuronal survival rate. NB-3 is believed to have therapeutic benefits for ischemic insult [ 170 ]. Therefore, WIP1 and NB-3 are promising candidates for future drug trials. This is a vast field, and more research must be conducted in the coming years to enable the development of therapeutic drugs.
Numerous natural compounds have proven to be beneficial for stroke prevention and treatment. They can be synthesized at a lower cost than synthetic compounds and offer competitive efficacy and safety. Honokiol is a natural product that showed neuroprotective effects in animal models, and appears to have a role in reducing oxidative stress and inhibiting inflammatory responses [ 171 ]. Gastrodin, a compound extracted from Gastrodia elata , is a promising candidate in stroke treatment. In a mouse model, it improved neurogenesis and activated β-Catenin-dependent Wnt signaling to provide neuroprotection after ischemic insult. It also has antioxidative effects which protects the neural progenitor cells from neuron functional impairment. Gastrodin’s safety has been proved in clinical trials, hence it is an option for stroke management in the coming years [ 172 ].
The Utstein methodology is a process of standardizing and reporting research on out-of-hospital stroke and defining the essential elements of management tools. Its growing popularity led to the establishment of the Global Resuscitation Alliance (GRA), an organization that governs best practices. The primary aim of GRA is to facilitate stroke care from pre-hospital admission to rehabilitation and recovery. It has developed 10 guidelines to ensure smooth transitioning of services during and after attack. It has implemented a stroke registry, public awareness and educational programs, promoted techniques for early stroke recognition by first responders, sought to optimize prehospital and in-hospital stroke care, advocated the use of advanced neuroimaging techniques and promoted a culture of excellence. The Utstein community has developed comprehensive plans to improve early diagnosis and treatment of stroke patients globally [ 173 ].
Future clinical trials should aim not only to determine the efficacy and safety of drugs but to characterize recovery and clinical outcomes. Clinical trials of pharmacological therapies for post-stroke recovery should adhere to the following guidelines [ 174 ]. Patients should be enrolled within two weeks of stroke whenever possible. Studies should include sampling from a multicenter platform and include global scale criteria for data analysis. The underlying mechanism of action of the tested drugs on target molecules should be thoroughly understood. Secondary measurements like day-to-day progress of recovery, length of rehabilitation, treatment endpoint analysis and any other compounding factors should also be recorded. Overall, research on stoke management has advanced rapidly in recent years and is certain to make additional valuable discoveries through the application of new technologies in hypothesis-driven clinical trials.
8. Translational Challenges for the Current Stroke Therapeutic Strategies
Stroke research has seen fundamental advancements over recent years. The improvements in the selection of animal models, imaging techniques and methodological progress have led to immense drug targets and therapeutic interventions. In spite of this, the subsequent clinical trials failed to prove pre-clinical outcomes. Recanalization therapy showed some promising results in the clinical trials but only a small section of stroke patients benefited from this treatment [ 175 ]. Hence, the translational potential of stroke research is still under-investigated.
The key challenges that hinder the smooth transition of pre-clinical research into successful drugs include relevant endpoint selection, confounding diseases models like hypertension and diabetes, modelling age and gender effects in stroke patients, development of medical devices, investigating medical conditions that co-exist during stroke incidence, reproducibility of pre-clinical stroke research data and modelling functional and behavioral outcome [ 176 , 177 , 178 ]. Multiple causality of the stroke occurrence is another problem that is often over-looked. Homogeneity in stroke models to exhibit the broad spectrum of stroke pathophysiology associated with ischemic lesions or cortical or intracerebral damage is critical. Therefore, stroke animal models that target specific causes of stroke should be included. Latent interaction between comorbidities and stroke treatment should be identified to increase the safety and efficacy of the clinical outcome [ 179 ]. Short-term experimental trials often result in failed therapeutic development due to false-negative outcomes in the clinical settings [ 180 ]. Understanding the functional and behavioral output which might mislead true recovery is problematic in clinical trials wherein animal models have greater ability to mask the functional benefits [ 181 ]. This affects the affecting translational capability of the research. Adapting a combined approach to model recovery and rehabilitation is also important for successful transition.
One of the other problems with the clinical trials for stroke is the lack of efficient data management. The impact of large data generated from numerous clinical experiments is over-whelming and there should be a standardized system to manage such data. Moreover, these data should be deposited into a public data repository for easy access.
Industry and academic corroborations in stroke research are critical to improve the translational value [ 182 ]. A consensus between industry and academic interests is vital for successful transition. The industry collaborations are mostly monetary driven and have time constraints which might compromise the pre-clinical study protocol design, appropriate sample sizes and overestimation of treatment effects. IP protection and publication of research data may discord between these groups. A multicenter approach, long-term collaborations, effective project management, use of advanced methodologies and establishment of functional endpoints will probably advance the translational roadblocks in stroke research [ 183 ].
9. Conclusions
Stroke is the second leading cause of death and contributor to disability worldwide and has significant economic costs. Thus, more effective therapeutic interventions and improved post-stroke management are global health priorities. The last 25 years of stroke research has brought considerable progress with respect to animal experimental models, therapeutic drugs, clinical trials and post-stroke rehabilitation studies, but large gaps of knowledge about stroke treatment remain. Despite our increased understanding of stroke pathophysiology and the large number of studies targeting multiple pathways causing stroke, the inability to translate research into clinical settings has significantly hampered advances in stroke research. Most research has focused on restoring blood flow to the brain and minimizing neuronal deficits after ischemic insult. The major challenges for stroke investigators are to characterize the key mechanisms underlying therapies, generate reproducible data, perform multicenter pre-clinical trials and increase the translational value of their data before proceeding to clinical studies.
Author Contributions
Conceptualization, D.K.; writing—original draft preparation, D.K.; writing—review and editing, Z.X.; funding acquisition, Z.X. All authors have read and agreed to the published version of the manuscript.
This research and The APC was funded by Apex Biotech Research.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Open access
- Published: 23 November 2020
Commercial head-mounted display virtual reality for upper extremity rehabilitation in chronic stroke: a single-case design study
- Mattias Erhardsson 1 , 2 ,
- Margit Alt Murphy ORCID: orcid.org/0000-0002-3192-7787 1 &
- Katharina S. Sunnerhagen 1
Journal of NeuroEngineering and Rehabilitation volume 17 , Article number: 154 ( 2020 ) Cite this article
5721 Accesses
28 Citations
3 Altmetric
Metrics details
Rehabilitation is crucial for maximizing recovery after stroke. Rehabilitation activities that are fun and rewarding by themselves can be more effective than those who are not. Gamification with virtual reality (VR) exploits this principle. This single-case design study probes the potential for using commercial off-the-shelf, room-scale head-mounted virtual reality for upper extremity rehabilitation in individuals with chronic stroke, the insights of which can inform further research.
A heterogeneous volunteer sample of seven participants living with stroke were recruited through advertisement. A single-case design was employed with a 5-week baseline (A), followed by a 10-week intervention (B) and a 6-month follow-up. Upper extremity motor function was assessed with validated kinematic analysis of drinking task. Activity capacity was assessed with Action Research Arm Test, Box and Block Test and ABILHAND questionnaire. Assessments were done weekly and at follow-up. Playing games on a VR-system with head-mounted display (HTC Vive) was used as rehabilitation intervention. Approximately 300 games were screened and 6 tested. Visual analysis and Tau-U statistics were used to interpret the results.
Visual analysis of trend, level shift and overlap as well as Tau-U statistics indicated improvement of Action Research Arm Test in six participants. Four of these had at least a moderate Tau-U score (0.50–0.92), in at least half of the assessed outcomes. These four participants trained a total of 361 to 935 min. Two out of four participants who were able to perform the drinking task, had the highest training dose (> 900 min) and showed also improvements in kinematics. The predominant game played was Beat Saber. No serious adverse effects related to the study were observed, one participant interrupted the intervention phase due to a fall at home.
Conclusions
This first study of combining commercial games, a commercial head-mounted VR, and commercial haptic hand controls, showed promising results for upper extremity rehabilitation in individuals with chronic stroke. By being affordable yet having high production values, as well as being an easily accessible off-the-shelf product, this variant of VR technology might facilitate widespread adaption. Insights garnered in this study can facilitate the execution of future studies.
Trial registration The study was registered at researchweb.org (project number 262331, registered 2019-01-30, https://www.researchweb.org/is/vgr/project/262331 ) prior to participant enrolment.
Post-stroke sequelae can encompass any number of domains associated with cerebral function, including motor, sensory, language and cognitive functions. Upper extremity motor function is affected in approximately 50% of patients early after stroke [ 1 ]. About 1/3 of those with early upper extremity impairment will achieve full dexterity in the chronic stage of recovery [ 2 ] Rehabilitation is crucial for maximizing recovery from neurological conditions, including stroke. Most of the rehabilitation interventions are concentrated to the first 3 to 6 months after stroke, although the need remains for years to come [ 3 ]. Rehabilitation activities that are more engaging, e.g. virtual reality (VR), can be more effective compared to conventional rehabilitation [ 4 , 5 ]. VR has been shown to improve upper extremity functioning when used in addition to conventional rehabilitation [ 6 , 7 ]. A rehabilitation activity that is enjoyable can also enhance adherence and long-term use. Gaming augmented with visual and audio feedback exploits neurophysiological reward mechanisms e.g. by engaging dopaminergic reward systems, which can enhance brain plasticity [ 8 , 9 ].
The VR research field is heterogeneous and has been likened to the “Wild West” [ 10 ]. VR systems within the rehabilitation context can be grouped into systems that are customized for rehabilitation [ 11 ], and those who are off-the-shelf systems for a broader entertainment market [ 12 ]. The advantage of customized systems developed for rehabilitation purposes is that they follow rehabilitation principles and can thus be intrinsically useful for rehabilitation. Commercial off-the-shelf systems on the other hand can be both more economical, entertaining as well as have a higher product quality, but in turn do require adaptation to find its place as a rehabilitation tool.
Although the layman might term only head-mounted displays (HMD) as VR, console games [ 13 , 14 , 15 , 16 ], 3D-monitors [ 17 ], and HMD [ 11 , 18 , 19 , 20 ] are all denoted as VR within academic literature [ 10 , 21 ]. On this spectrum, HMD represents the most immersive VR technology. Literature pertaining to VR, based on HMD for upper extremity stroke rehabilitation, is limited [ 21 ]. Rehabilitation approaches tested with HMD VR include both custom hardware and software [ 11 ], as well as off-the-shelf hardware with custom software [ 19 , 20 ]. However, combining both off-the-shelf hardware and software seems to be unexplored ground in the field of stroke rehabilitation.
Upper extremity rehabilitation can benefit from technology that stimulate involved neurological pathways [ 22 ]. These pathways can be stimulated in room-scale HMD VR systems with haptic hand controls, where sensors track hand and head movement in 6 degrees of freedom. The market for commercial off-the-shelf room-scale VR was dominated by Oculus Rift, HTC Vive and PlayStation VR at the start of this study (early spring 2019). Among these, HTC Vive was brought to bear for this study as it was simpler to set up and use than Oculus Rift and had a far greater repertoire of games available than PlayStation VR. A monitor displays approximately what the user sees in the HMD, facilitating demonstrations by, and support from accompanying personnel.
The overall aim of this study was to explore what potential commercial off-the-shelf, head-mounted display, room-scale virtual reality has for chronic stroke rehabilitation with focus on upper extremity functioning. The results can help lay the foundations for future larger-scale studies. The study aimed also to provide further insights on which HMD-VR games can be suitable for people with chronic stroke, who might benefit most, and which outcome measures might be most suitable for evaluation.
The SCRIBE reporting guideline checklist was utilized for this article [ 23 ]. The study was registered at researchweb.org (project number 262331) prior to participant enrolment [ 24 ].
Study design
Initial forays into novel interventions require development and piloting before larger randomization trials [ 25 , 26 ]. Thus, a multiple-participant, single-case design was chosen since it is sensitive to individual improvement and is of appropriate scope for a small-scale rehabilitation study [ 27 , 28 ]. The single-case design employed in this study consisted of a baseline phase (phase A), an intervention phase (phase B), as well as a 6-month follow up. Baseline included 5 assessments performed once a week. During the 10 weeks intervention phase the assessments were performed once a week. While this non-randomized, non-blinded study design is unable to determine causal relationships, it can demonstrate temporal correlations.
Participants
Recruitment was conducted through advertisement at patient organizations and support groups, local health-care providers, as well as informing participants from other studies. This resulted in a volunteer sample of 7 individuals with chronic stroke (Fig. 1 ). Individuals who indicated interest were interviewed to determine if it was plausible they would fulfil inclusion/exclusion criteria. If the person didn’t think it was possible to somehow hold an object like a remote control and press any button with the affected hand, then this was interpreted as likely having too low upper extremity function. If not excluded by the interview, a physical visit was booked to confirm the inclusion according to defined criteria.
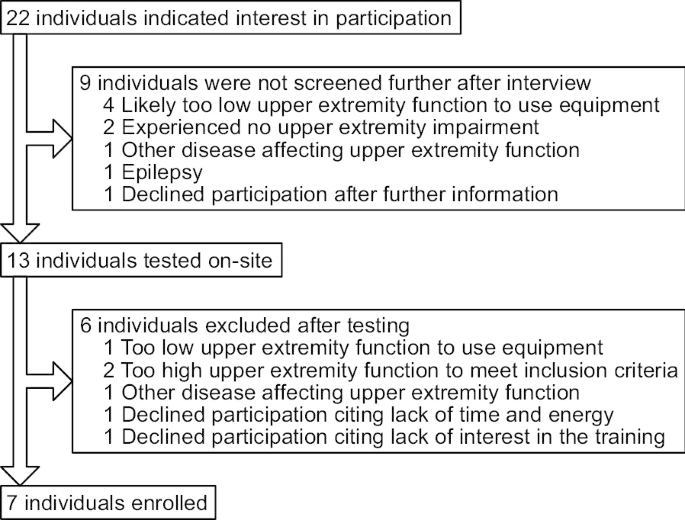
Participant recruitment flowchart
The inclusion criteria were: stroke at least 6 months prior and a residual upper extremity deficit identified by at least one of the following criteria: Fugl-Meyer Assessment of Upper Extremity motor score ≤ 60 [ 29 ], Action Research Arm Test (ARAT) score ≤ 51 [ 30 ], Box and Blocks Test (BBT) at least 6 blocks less compared to normative value and the non-affected hand [ 31 , 32 ]. Diagnosed with, or under investigation for, any other condition than stroke that affects the upper extremity function, not being able to adhere to protocol or use the equipment, e.g. due to having too low upper extremity motor function or being unable to visit the site at least once a week for 15 weeks entailed study exclusion. Finally, to err on the side of caution of the HTC Vive safety instructions, having a history of photosensitive seizures or a pacemaker also excluded participation.
Outcome measures
A battery of recommended clinical and kinematic assessments with strong psychometric properties covering body function and activity domains according to International Classification of Functioning and Health (ICF) were used to assess upper extremity functioning [ 33 , 34 , 35 ]. Clinical background data were collected through interviews and medical records. All assessments were administered by a trained medical researcher in a random order within the assessment session. During the intervention phase, the assessments took place prior to the training session when they occurred on the same day. Weekly time spent on assessments varied within the approximate range of 15–60 min between participants. Assessments took place in a research facility close to the University hospital.
Activity domain outcome measures
Action Research Arm Test (ARAT) evaluates the upper extremity activity capacity and includes 19 items divided into four subtests (grasp, grip, pinch, gross movement) [ 36 , 37 ]. A maximum score of 57 in ARAT indicates full capacity, and the minimally clinically important difference (MCID) is 5.7 [ 30 ]. The Box and Blocks Test (BBT) assesses gross motor ability to grasp and move the largest number of small wooden cubes (2.5 cm) from one box to another in 1 min [ 32 ], and the minimally detectable change (MDC) is 5.5 [ 31 ]. The ABILHAND questionnaire evaluates the person’s perceived difficulty of performing bimanual daily activities [ 38 ]. The score is expressed as logits after a Rasch based conversion and the values range from approximately -6 to 6. A score of 0 means that most of the activities are difficult to perform, and the MCID is 0.26–0.35 [ 39 ].
Body function domain outcome measures—kinematics
Validated and recommended kinematic measures of movement time, smoothness and compensatory trunk displacement, calculated from a 3D movement analysis of the drinking task, were used to evaluate changes in movement performance and quality [ 35 , 40 ]. These 3 measures are valid, reliable and sensitive to change in stroke population and cover the key elements of motor deficits in stroke [ 41 , 42 , 43 , 44 ]. Kinematic data was acquired with a 5-camera high speed (240 Hz) motion capture system (Qualisys AB, Gothenburg, Sweden). The cameras emit infra-red light that is reflected by the circular markers placed on the anatomical landmarks on the body. The 8 markers were placed on the tested hand (III metacarpophalangeal joint), wrist (styloid process of ulna), elbow (lateral epicondyle), on both shoulders (acromion), trunk (sternum), forehead and the drinking cup [ 40 ]. Kinematic data was analyzed and filtered (6-Hz Butterworth filter) in the Matlab software (R2019B, The Mathworks Inc). The drinking task included reaching and grasping the glass, lifting it to the mouth and drinking a sip of water, placing the glass back on the table and returning the hand back on the edge of the table. The starting position was standardized to the body size and the glass was positioned at 30 cm distance from the table edge, which was within the reach of the hand when the back was against the backrest of the chair. The trunk was not restrained, and the participants were instructed to perform the task with the affected arm in a self-paced speed as naturally as possible. After few familiarization trials the task was repeated 10 times with approximately 5 s rest between each trial. The mean of 10 trials was used as test result.
Movement time was defined as time required to complete the entire drinking task. The start and end of the movement was identified from the point where the hand marker surpassed the 2% of the maximum velocity of reaching or returning phase, respectively [ 40 , 41 ]. The reference value for healthy controls for movement time of the drinking task is 6.28 s (SD 0.98) as reported previously [ 35 ]. Movement smoothness was defined as the number of movement units (NMU) identified from the tangential velocity profile of the hand marker. A movement unit was defined as the difference between a local minimum and the next maximum velocity value that exceeded the amplitude limit of 20 mm/s, where the time between two subsequent peaks had to be at least 150 ms. The minimum possible value for NMU was 4 including one predominant peak for each movement phase (reach, transport forward, transport back and return of the hand). The reference value for healthy controls for movement smoothness is 6.0 units (SD 1.0) [ 35 ]. Trunk displacement was defined as maximal forward displacement of the sternal marker in the sagittal plane from the initial position during the entire task. The reference value for healthy controls for trunk displacement is 3.3 cm (SD 1.6) [ 35 ].
Additional clinical assessments
In order to keep the assessment time short compared to training time the additional assessments of sensorimotor function were only performed before and after intervention as well as at 6 months follow-up. The Fugl-Meyer Assessment-Upper Extremity (FMA-UE) including assessment of sensation, range of motion and pain was used [ 45 ]. Upper extremity spasticity at the elbow and wrist joint was assessed by the modified Ashworth Scale [ 46 ]. Physical activity level was assessed by Saltin-Grimby Physical Activity Level Scale (SGPALS) at baseline and at 6-moths follow-up [ 47 ].
Intervention
The intervention consisted of playing VR games on the HTC Vive (HTC Corporation, New Taipei City, Republic of China). The intervention took place in a dedicated VR-room at the research facility. The participants themselves booked access to the system. They were encouraged to play as much as possible. A researcher was present at every training session. During each session the researcher noted what application(s) the participant used and how long these were used. Other observations, participants’ thoughts and experiences and possible adverse effects were recorded as field notes. Participants scored their perceived physical exertion after every training session on Borg Rating of Perceived Exertion, of which a median value for all sessions was calculated (Table 1 ) [ 48 ].
Approximately 300 games out of the 3000 available for HTC Vive were screened for its potential to be used in the study. Screening was done by the first author (ME), who has extensive gaming experience, by systematically browsing the Steam store page. VR games were sorted by falling popularity and about 300 of the most popular games were assessed for their suitability. If a game was not disregarded outright the first author looked at gameplay clips, read reviews, and if available played demo versions. Six games were promising enough to be downloaded and tested. Five were made available to the participants, as one game was deemed to present an unacceptable risk of falling. Participants decided themselves, but with guidance, what and how to play. Beat Saber was the overwhelmingly most common game to see play, NVIDIA VR funhouse saw some play, while the other games saw little to no play. Beat Saber is a rhythm-based game while NVIDA VR funhouse consists of several carnival-style mini-games. See Additional file 1 for further details about the games and the screening process.
In Beat Saber, the user has one lightsaber in each hand, which is used to cut blocks to the sound of music. The gameplay forces the player to use both hands. The difficulty can be adjusted in a massive range, which stretches from beyond the best of human capacity to a negligible difficulty level. There is a risk of sensory overload even at the lowest difficulties, although lowering the volume and enabling options reducing special effects can somewhat mitigate this. The gameplay itself do not require the user to press buttons, allowing individuals with severe upper extremity impairment to play it e.g. through attachment of the hand controller to the hand with Velcro straps. It was designed to be played standing but can be played sitting. See Fig. 2 for a visualization of a participant playing Beat Saber.

Screenshot of Mixed Reality footage of a participant playing Beat Saber. Mixed Reality was provided by the 3rd party software LIV, and was captured with OBS Studio
Statistical and visual analysis
Visual analysis is the accepted norm within single-case design studies, but other than that there’s no consensus as to what other of the multitude of available data analysis methods that should be used [ 27 , 28 , 49 , 50 , 51 , 52 ]. Visual analysis was conducted as follows: (i) determine stable baseline trend; (ii) to compare trends, levels and variability within and between phases; and (iii) to assess overlap and consistency of patterns [ 49 ]. Immediacy of effect [ 49 ] was not assessed since rehabilitation interventions are not expected to yield immediate effects after initiation of the intervention. The visual analysis was first conducted independently by two authors (ME, MAM) and then jointly to reach a consensus.
Of available analytical statistics techniques for single-case design data [ 53 ], the post-hoc statistical analysis of Tau-U was deemed the best fit for this study’s data [ 54 ]. Tau-U can adjust for the baseline trends evident in our data, and unlike the majority of other tests it is also applicable to ordinal data. The Tau-U summary index Tau-U A vs. B − trend A can be understood as an effect size coefficient, showing the proportion of the data that improves from baseline to intervention after adjusting for the baseline trend [ 54 , 55 ]. Follow-up phase data was not included in the Tau-U calculation. The magnitude of Tau-U statistics were interpreted similarly to correlation and effect size statistics: 0.00–0.25 (very low), 0.26–0.49 (low), 0.50– 0.69 (moderate), 0.70–0.89 (high) and 0.90–1.00 (very high) [ 56 ]. Tau-U A vs B − trend A summary indices were calculated with RStudio version 1.2.5042 [ 57 ] running R 4.0.0 with Rtools40 installed [ 58 ], using the packages SingleCaseES version 0.4.3 [ 59 ] and readxl version 1.3.1 [ 60 ]. See Additional file 2 for the R script and Additional file 3 for the excel file which was loaded into R.
The demographic and clinical characteristics of the participants along with number of assessments, training sessions and training time are shown in Table 1 . The recruited participants hailed from different ethnicities and had an educational background spanning less than 9 years education to completed third cycle education, with the most common background being Swedish ethnicity and more than 12 years of education. According to the medical records, one participant had some residual perceptual and cognitive deficits, although these deficits were relatively mild and did not interfere with co-operation during testing or intervention. None of the participants had documented neglect, and two participants had some residual communication difficulties (slower in speaking). All participants completed the baseline assessments and were followed-up 6 months post intervention.
Training time was mostly limited by the participants’ time and energy, and not by limitations in system accessibility or capacity. Three participants (P1, P3, P5) trained approximately 3 times per week and therefore reached the highest training dose of 739–915 min in total. See Additional file 4 for further details on the training sessions. Three participants (P2, P4, P6) attended fewer sessions due to practical reasons such difficulties arranging transport or having a busy schedule. One participant (P7) was interrupted from training after 4 training sessions due to a hip fracture after a fall at home. This was also the reason for missing data points on assessments during the intervention phase in P7. For this reason, the P7 was excluded from the visual analysis.
No serious adverse effects were observed during or after training. One participant felt slightly unsteady for a few hours post-training after the first training sessions. Another participant perceived standing on a virtual platform in the Beat Saber game as somewhat intimidating. The median physical exertion after a training was graded by participants between 12 and 18 (Table 1 ).
Participants were overwhelmingly positive when asked about their thoughts on the training session; all participants expressed that training was “good” and/or “fun” during the intervention phase. They often commented the improvement they saw in their in-game performance. Participants described a positive feeling of being in another world in which they could move more easily. Other improvements that they mentioned were for upper extremity functioning (P4), pain (P4), spasticity (P3), neglect (P7) and walking (P6). P1 explicitly said he did not perceive any improvement outside of the game. The attending researcher noted that two participants (P1 and P3) became independent in using the VR system.
Two participants had undergone a 3-weeks intensive rehabilitation between the last intervention and 6-monts follow-up assessment (P2 and P6), and one (P2) had participated in another 6-weeks interventional study targeting upper extremity function. Other participants did not report any changes in their rehabilitation. None of the participants had changed their overall physical activity level as measured with SGPALS.
Outcomes were plotted on an outcome basis, with activity domain outcomes in Fig. 3 and body function domain outcomes in Fig. 4 . Outcomes plotted on an individual basis with additional annotation is available in Additional file 5 .
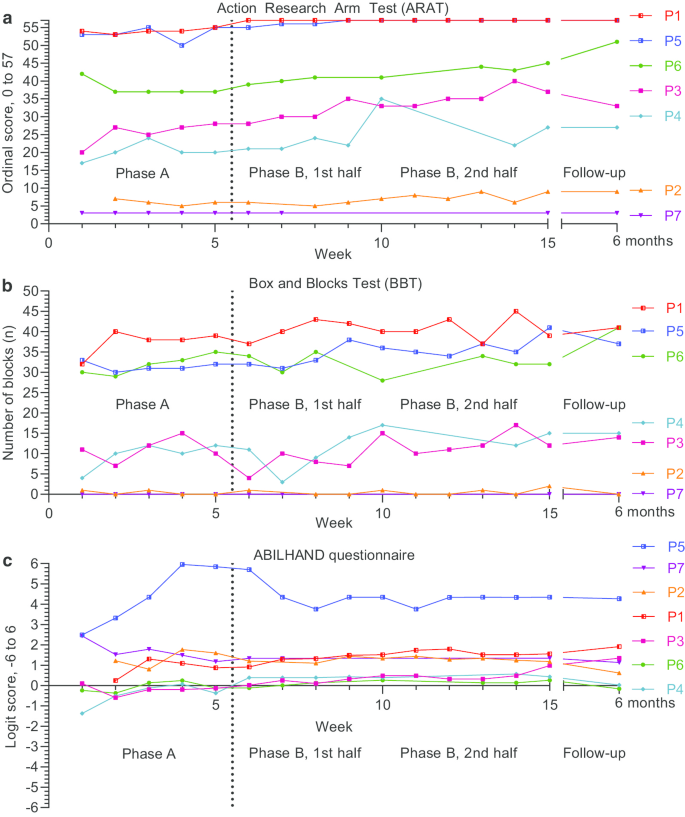
Activity domain outcome measures: Action Research Arm Test ( a ), Box and Blocks Test ( b ) and the ABILHAND ( c ) questionnaire. Outcomes were assessed on a weekly basis during baseline (Phase A) and intervention (Phase B), and at 6-month follow-up
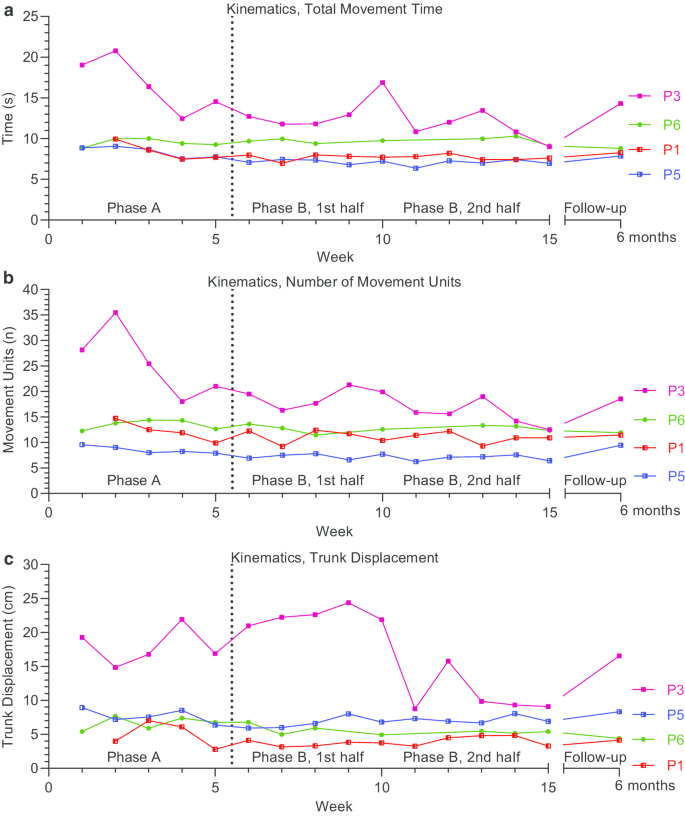
Kinematic body function outcome measures: Total Movement Time ( a ), Number of Movement Units ( b ) and Trunk Displacement ( c ). Outcomes were assessed on a weekly basis during baseline (Phase A) and intervention (Phase B), and at 6-month follow-up
Action Research Arm Test
Regarding baseline stability and trend, visual analysis indicated a relatively stable baseline for all six participants (Fig. 3 a), although in two participants (P3 and P4) a positive trend which plateaus in the end of the baseline could be observed. In phase B, trends remain positive for all participants except P1 and P5 who reached the ceiling effect early the intervention phase. All six showed increased levels during the phase B. This level shift was in particular visible in the second half of the phase B. Three participants (P1, P3, P5) had little to no overlap between phase A and B, while P2, P4, P6 had some overlap.
The Tau-U scores for ARAT were compatible with the improved levels, as well as the relatively unchanged trends from baseline to intervention found in the visual analysis (Table 2 ). Improvements detected during intervention phase compared to baseline after adjusting for the baseline trend was large or very large (Tau-U > 0.70) in four participants, and moderate (Tau-U > 0.50) in two participants. The first high score in P6, resulted in an increased overlap between phases in the visual analysis, but due to lower following scores at baseline, the Tau-U still showed large effect in this participant. In three participants (P3, P4, and P6) the ARAT scores were large than the MCID of 5.7 points in the end of the intervention phase when compared to the last baseline score. In two participants (P1 and P5) a ceiling effect was present in ARAT, and improvement beyond the MCID could not be detected.
At 6 months follow-up the ARAT scores showed further improvement in one participant (P6), while in all others the ARAT remained slightly higher than the scores at baseline (Fig. 3 a).

Box and Blocks Test
Visual analysis showed fair baseline stability. Baseline trends were either neutral (P2, P3) or plateaued (P1, P5, P4), although P6 had a continuously positive baseline trend (Fig. 3 b). P5 showed a clear positive trend during the intervention. While overall variability was high for most participants in both phases, the greatest variability could be seen for P3 in the baseline, which then decreased throughout the intervention. Levels increased between baseline and the second half of the intervention for 3 participants (P1, P4 and P5).
This level shift with low overlap was supported by the moderate to large Tau-U scores for P1 and P5, respectively (Table 2 ). The very low Tau-U for P4 could be explained by the plateauing baseline trend, as well as the low scores in the first 3 weeks of the intervention. Only one participant (P5) showed a change large than the MDC of 5.5 cubes in the end of the intervention phase when compared to the last baseline score.
Follow-up scores were analogous to the latter half of the intervention, except for P6 who scored substantially higher. P6 had limited training during the intervention (198 min, Table 1 ) yet participated in 3 weeks intensive rehabilitation prior to the follow-up assessment.
ABILHAND questionnaire
Visual analysis exhibited plateauing baselines around 0 logits (P3, P4, P6), 1.25 logits (P1, P2), or close to the ceiling effect of 6 logits (P5) (Fig. 3 c). Interventional trends were on a whole neutral, although P3 and P1 presented a positive trend in the first and in the second half of the intervention phase, respectively. There were little to no interphase overlap in phase B for P1, P3 and P4. Notably, interindividual comparative scores for the self-perceived manual ability measured by ABILHAND differed from all other performance-based outcomes, which shared more consistent patterns. For example, P2, who showed the lowest motor function in performance tests scored on par with or higher than most other participants in ABILHAND.
The low overlap with increased levels as manifested with the visual analysis for 3 participants were supported by the large (P1 and P4) and very large (P3) Tau-U scores (Table 2 ). In four participants (P1, P3, P4, and P6) the ABILHAND logits were large than the MCID of 0.35 logits in the end of the intervention phase when compared to the last baseline score.
The 6-month follow-up revealed decreased ABILHAND logits compared to the interventional phase scores for all participants except P1 and P3.
Body functions domain outcome measures—kinematics
Four participants (P1, P3, P5, P6) with sufficient motor function were able to perform the kinematic drinking task with the more-affected arm (Fig. 4 ). A clear negative and thus improving trend in movement time and movement units was seen with visual analysis during the baseline for P3, while this trend was less pronounced in P1 and P5. During the intervention phase, the levels improved, i.e. were lower, and there was a little to no interphase overlap in P3 and P5 concerning movement time (Fig. 4 a) and movement units (Fig. 4 b). The visual analysis showed a high degree of variability in P3 throughout both phases for all 3 kinematic variables, while it was more modest for the other participants. P3 also showed improved levels in all three kinematic measures, mainly in the second half of the intervention phase. These observed improvements in P3 were beyond the established clinically important difference of 2.4 s, 3.3 movement units and 2.0 cm for the movement time, number of movement units and trunk displacement, respectively [ 43 ]. A small improvement in trunk displacement was noted for the P6 in the intervention phase (Fig. 4 C).
The improved levels seen in the visual analysis were supported by Tau-U in all cases, showing moderate to large effect (Tau-U 0.66 to 0.88), except for the late improvement observed in P3 in trunk displacement that showed very low effect (Tau-U 0.08) (Table 2 ).
In summary, two out of four participants (P3 and P5) tested with kinematics improved in terms of movement time and movement units, as substantiated with both visual and statistical analysis. Two out of four (P3 and P6) improved in trunk displacement, in which the late improvements in trunk displacement in P3 were not supported by Tau-U analysis.
After 6 months, the observed improvements showed, however, some reversion in most of the participants. The 6 months follow-up measures were akin to their respective final two baseline measurements for all participants except P6, who achieved personal bests.
Scores from the additional clinical assessments of sensorimotor function assessed pre- and post-intervention as well as at 6 months follow-up show relatively stable values over the course of the study (Table 3 ). The change in FMA-UE scores between pre- and post-intervention varied between 0–5 points.
This study evaluated the potential effects of commercial head-mounted virtual reality on upper extremity functioning in individuals with chronic stroke. Acceptable adherence to the training protocol was reached in 6 out of 7 participants and no serious adverse effects were observed during or after the intervention. The most played VR game was a rhythm-based game that did not require any manipulation of the buttons by the player and allowed a variety of adjustments in terms of speed, gaming difficulty and other personal preferences. Thus, most of the training was focused on the arm and hand movements, and less on the finger movements. A positive trend of improvement, at least in one outcome measure, was observed in all 6 participants adhering to the training protocol. Independent of the impairment level, all 6 participants showed improvements in upper extremity activity capacity, assessed with ARAT. The 3 participants who had the highest training dose (739–915 active training minutes) showed improvements in 3 to 5 outcome measures out of total 6. These findings indicate that commercial HMD VR might be a useful tool for people with chronic stroke to improve their upper extremity functioning.
Who might benefit most from VR training?
Results indicated noticeable improvements in five participants (P1, P3, P4, P5, P6). One participant (P2) with poor initial motor function did reach the cut-off value indicating a moderate effect in statistical analysis for ARAT, although this finding was less pronounced in the visual analysis. One participant (P7), who needed to interrupt the intervention premature due to a fall at home did not show any change. Interestingly, the group who responded better to the intervention had baseline ARAT and FMA-UE scores above 15 and 30 points, respectively, in contrast to P2 who had ARAT below 10 and FMA-UE 20 points. Thus possibly, the VR intervention, as used in this study, might have the best benefit for those with moderate to mild upper extremity impairment. Similar to our results, the non-immersive VR based gaming demonstrated positive effects primary in those with moderate to mild upper extremity impairment [ 6 , 16 ]. Thus, possible effects of VR gaming, immersive or not, in people with poor motor function after stroke remain unknown.
As for the dose, three participants (P1, P3 and P5) with the highest training dose showed improvements in several outcomes. Nevertheless, even participants with lower dose showed improvements in some outcome measures. The only exception was the participant with severe motor impairment (P2) and the participant who interrupted the study. A training time around 500 min, as used in a large randomized control trial using video-based non-immersive VR gaming (Nintendo Wii) as add-on therapy, showed significant positive effects on activity performance time in subacute stroke [ 16 ]. An appropriate dose for HMD VR is largely unknown, although based on our data, a time between 200 and 900 min had some effect for upper extremity functioning in people with chronic stroke. However, training levels of 900 min, which means at least 30 min of VR training 3 times per week for 10 weeks, might have a higher potential effect on upper extremity functioning.
What outcomes should be used?
The VR training, as used in this study, was primarily expected to target the activity domain of functioning. The gaming is a task- and performance-oriented exercise, which do not emphasize the quality of movement nor whether the task is accomplished by using a certain muscle group or movement. Accordingly, in the current study, the training effect was most evident in the activity domain as assessed by the ARAT. Thereby, ARAT, as an activity level assessment could be recommended as a first-choice outcome for similar interventional studies. Activity level outcome, the Wolf Motor Function Test, was used as primary endpoint in a large randomized control trial using video-based non-immersive VR gaming [ 16 ]. The task execution time was significantly improved after VR intervention, although this result was not superior to the same dose recreational activity intervention [ 16 ].
In the current study, we also included kinematic movement analysis of the drinking task to objectively evaluate the changes in movement performance and quality. Since this task necessitates an ability to grasp the glass and drink a sip water with the more-affected arm, only 4 out of 7 participants were able to perform the task. After VR training, two participants showed consistently shorter movement times accompanied by improved movement smoothness; and in two, the compensatory forward movement of trunk during task execution was decreased. However, improvements larger than the established clinically important difference were only present for all three kinematics (movement time, smoothness and trunk displacement) in one participant (P3) [ 43 ]. Kinematics have previously only sparsely used to evaluate the effects of VR gaming and might therefore provide new insights on possible effects on motor performance and quality [ 61 , 62 ]. While a shorter movement time reported in this study echoed results from previous research [ 16 ] which showed improvements in task execution time after video-based VR intervention, improvements in movement smoothness are novel. These results are promising and show that some improvements might as well be expected in the domain of motor function when measured objectively with kinematic analysis.
As a fast, mobile and straight-forward test yielding data on a discrete integer scale as opposed to an ordinal scale, BBT is appropriate as a composite measure for speed and precision of finger, hand and arm function [ 32 ]. Limitations of this outcome became, however, visible in the present study. A relatively large variability coupled with the seemingly low responsiveness seen in the data may have compromised the statistical power. A higher floor effect of BBT than ARAT was also observed for P2.
While there seemed to be a high degree of agreement of inter-individual relative scores between outcomes, the self-perceived manual ability in daily activities as assessed by ABILHAND, displayed incongruent patterns. Discrepancies between observed and self-reported measures are not uncommon and can partly be explained by inter-personal differences in perception of problems and how these affect the daily life activities. In chronic stroke, one in five showed discrepancies between self-reported and observed upper extremity outcomes [ 63 ]. With this background, the self-reported outcome measures are often advocated to be included in clinical studies in order to cover and better understand patient perspectives [ 63 , 64 , 65 ].
How did the VR gaming work?
Players with severe upper extremity impairment could play Beat Saber on HTC Vive, albeit by using Velcro straps to retain the grasp of the hand control. To overcome this limitation, recently available lighter and more ergonomic hand controls with built-in straps, such as Valve Index, could be considered. Finger tracking independent of hand controls, such as Leap Motion [ 66 ], represent another opportunity for participant interaction when the technology has been developed further. Meanwhile, if Beat Saber would be the sole available game appropriate for the intervention then the stand-alone and thus much more affordable Oculus Quest could be used instead.
The median physical exertion after training, as reported by the participants, ranged between 12 and 18. This corresponds to somewhat hard to very hard perceived exertion, on the Borg RPE scale. Similar exertion rates have been reported previously in intervention studies using video-based non-immersive VR gaming in stroke [ 16 , 67 ]. Even when the focus of this study was not on cardiovascular health, previous research using VR-based gaming have shown positive effects to fitness and physical activity [ 68 ]. Taken the low levels of physical activity often described in people with stroke, playing engaging VR games might be beneficial from an exercise point of view [ 69 ].
Screening 300 of the approximately 5000 titles available for HTC Vive primarily yielded 1 game. This indicate that while the assortment of commercial HMD VR games is staggering, the amount of games appropriate for upper extremity rehabilitation post-stroke is miniscule. Other HMD VR rehabilitation studies using commercial hardware use custom rehabilitation software, often developed by the researchers themselves [ 19 , 20 , 70 , 71 ]. It seems, however, that the custom hardware has limited production values and weren’t developed with the help of commercial game developers in order to maximize intrinsic gameplay rewards, i.e. making the game itself as fun as possible in order to boost adherence and the exploitation of reward mechanisms. Reaching out to successful game developers in order to modify existing commercial games or to develop novel ones with rehabilitation in mind seem to be untested.
Strength and limitations
With 7 participants and 6 outcome measures, this represented a large single case design study. Furthermore, the recruited participants were a heterogeneous group, which is a strength for single case design. The results from the current study can guide researchers to select suitable study design, what outcome(s) to assess, what participants to recruit and how many are needed when designing larger studies. An appropriate next step could be a phase 2 VR study with focus on feasibility, acceptability and initial clinical efficacy [ 10 ]. The open commercial nature of both the hardware and software used in this study facilitate adoption in wider research community, both in terms of ease of access and cost.
Like a few previous rehabilitation studies [ 64 , 65 ], the customary visual analysis was complemented with Tau-U [ 53 ], which strengthens the findings. Baseline Corrected Tau (BC-Tau) was another possibility [ 63 ], however the baseline correction of BC-Tau was strict and depended on only adjusting for statistically significant baseline trends, which were an issue since Monte Carlo simulations for BC-Tau indicated exceptionally low power for baselines consisting of 5 measurements [ 63 ]. A strength BC-Tau would have over Tau-U if it could appropriately adjust for baseline trend in our data, is that unlike Tau-U, BC-Tau yields further statistics such as p-values.
A downside of the intervention, at least in the context of this study, was the requirement of having a researcher, experienced with both rehabilitation and VR gaming, on site for all training sessions. Furthermore, the single case design was not experimental and thus did not permit causal inference. A common motif in baseline measurements was the initial improvement that plateaued towards end of the phase. While the relative stability of plateaued values was acceptable for comparing level changes between phases in our single case design, similar baseline changes would make it difficult to establish causality [ 27 , 49 ]. Considering these baseline changes as well as the slow improvement that one would expect from a successful chronic stroke rehabilitation intervention, an experimental single case design might not be appropriate for a next phase study which attempt to determine the interventions effectiveness. The baseline improvements seen in this study might partially have caused by familiarization and training effects in some of the participants due to the repetitive scheme of the assessments. In some participants, the baseline assessments might as well be acting as somewhat of a rehabilitation activity by themselves. The heterogeneity of the participants extended to upper extremity function, so much so that floor and ceiling effects of several outcomes came into play, masking potential changes. Due to the study design, the results cannot be generalized to the stroke population at large and need to be interpreted individually.
The results generated in this study indicate that off-the-shelf, room-scale head-mounted-display VR has potential for upper extremity rehabilitation in individuals with chronic stroke. The selection of available games appropriate for stroke population is, however, limited. Taken the promising results along with experiences and lessons learned, phase 2 studies evaluating feasibility and initial efficacy are warranted. Future studies may want to aim for 200–900 min total training time, perhaps toward the upper portion of the range. In the long run, these future studies may enable the addition of another fun and cost-effective tool in the arsenal of rehabilitation health-care professionals.
Availability of data and materials
All data generated or analyzed during this study is included in this published article and its additional files (Additional file 4 ).
Abbreviations
- Virtual reality
- Head-mounted display
Box and blocks test
Fugl-Meyer Assessment of Upper Extremity
Modified Ashworth Scale
Saltin-Grimby Physical Activity Level Scale
International Classification of Functioning, Disability and Health
Minimally clinically important difference
Minimally detectable change
Persson HC, Parziali M, Danielsson A, Sunnerhagen KS. Outcome and upper extremity function within 72 hours after first occasion of stroke in an unselected population at a stroke unit. A part of the SALGOT study. BMC Neurol. 2012;12:162.
Article PubMed PubMed Central Google Scholar
Nijland RH, van Wegen EE, van der Harmeling Wel BC, Kwakkel G. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke. 2010;41(4):745–50.
Article PubMed Google Scholar
Tistad M, von Koch L, Sjostrand C, Tham K, Ytterberg C. What aspects of rehabilitation provision contribute to self-reported met needs for rehabilitation one year after stroke-amount, place, operator or timing? Health Expect. 2013;16(3):e24-35.
Lohse KR, Hilderman CG, Cheung KL, Tatla S, Van der Loos HF. Virtual reality therapy for adults post-stroke: a systematic review and meta-analysis exploring virtual environments and commercial games in therapy. PLoS ONE. 2014;9(3):e93318.
Article PubMed PubMed Central CAS Google Scholar
Howard MC. A meta-analysis and systematic literature review of virtual reality rehabilitation programs. Comput Hum Behav. 2017;70:317–27.
Article Google Scholar
Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2017;11:CD008349.
PubMed Google Scholar
Aminov A, Rogers JM, Middleton S, Caeyenberghs K, Wilson PH. What do randomized controlled trials say about virtual rehabilitation in stroke? A systematic literature review and meta-analysis of upper-limb and cognitive outcomes. J Neuroeng Rehabil. 2018;15(1):29.
Marsh R, Hao X, Xu D, Wang Z, Duan Y, Liu J, et al. A virtual reality-based FMRI study of reward-based spatial learning. Neuropsychologia. 2010;48(10):2912–21.
Tran DA, Pajaro-Blazquez M, Daneault JF, Gallegos JG, Pons J, Fregni F, et al. Combining dopaminergic facilitation with robot-assisted upper limb therapy in stroke survivors: a focused review. Am J Phys Med Rehabil. 2016;95(6):459–74.
Birckhead B, Khalil C, Liu X, Conovitz S, Rizzo A, Danovitch I, et al. Recommendations for methodology of virtual reality clinical trials in health care by an international working group: iterative study. JMIR Ment Health. 2019;6(1):e11973.
Crosbie JH, Lennon S, McGoldrick MC, McNeill MD, McDonough SM. Virtual reality in the rehabilitation of the arm after hemiplegic stroke: a randomized controlled pilot study. Clin Rehabil. 2012;26(9):798–806.
Article CAS PubMed Google Scholar
McNulty PA, Thompson-Butel AG, Faux SG, Lin G, Katrak PH, Harris LR, et al. The efficacy of Wii-based movement therapy for upper limb rehabilitation in the chronic poststroke period: a randomized controlled trial. Int J Stroke. 2015;10(8):1253–60.
Nguyen AV, Ong YA, Luo CX, Thuraisingam T, Rubino M, Levin MF, et al. Virtual reality exergaming as adjunctive therapy in a sub-acute stroke rehabilitation setting: facilitators and barriers. Disabil Rehabil Assist Technol. 2019;14(4):317–24.
Demers M, Chan Chun Kong D, Levin MF. Feasibility of incorporating functionally relevant virtual rehabilitation in sub-acute stroke care: perception of patients and clinicians. Disabil Rehabil Assist Technol. 2019;14(4):361–7.
Warland A, Paraskevopoulos I, Tsekleves E, Ryan J, Nowicky A, Griscti J, et al. The feasibility, acceptability and preliminary efficacy of a low-cost, virtual-reality based, upper-limb stroke rehabilitation device: a mixed methods study. Disabil Rehabil. 2019;41(18):2119–34.
Saposnik G, Cohen LG, Mamdani M, Pooyania S, Ploughman M, Cheung D, et al. Efficacy and safety of non-immersive virtual reality exercising in stroke rehabilitation (EVREST): a randomised, multicentre, single-blind, controlled trial. Lancet Neurol. 2016;15(10):1019–27.
Broeren J, Claesson L, Goude D, Rydmark M, Sunnerhagen KS. Virtual rehabilitation in an activity centre for community-dwelling persons with stroke. The possibilities of 3-dimensional computer games. Cerebrovas Dis. 2008;26(3):289–96.
Borrego A, Latorre J, Alcaniz M, Llorens R. Comparison of oculus rift and HTC Vive: feasibility for virtual reality-based exploration, navigation, exergaming, and rehabilitation. Games Health J. 2018;7(3):151–6.
Lee SH, Jung HY, Yun SJ, Oh BM, Seo HG. Upper Extremity rehabilitation using fully immersive virtual reality games with a head mount display: a feasibility study. PMR J Injury Funct Rehabil. 2020;12(3):257–62.
Weber LM, Nilsen DM, Gillen G, Yoon J, Stein J. Immersive virtual reality mirror therapy for upper limb recovery after stroke: a pilot study. Am J Phys Med Rehabil. 2019;98(9):783–8.
Tieri G, Morone G, Paolucci S, Iosa M. Virtual reality in cognitive and motor rehabilitation: facts, fiction and fallacies. Expert Rev Med Devices. 2018;15(2):107–17.
Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, Dispa D, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci. 2016;10:442.
Article CAS PubMed PubMed Central Google Scholar
Tate RL, Perdices M, Rosenkoetter U, McDonald S, Togher L, Shadish W, et al. The Single-Case Reporting Guideline In BEhavioural Interventions (SCRIBE) 2016: explanation and elaboration. Archiv Sci Psychol. 2016;4(1):10–31.
Google Scholar
Sunnerhagen KS. Implementation of off-the-shelf room-scale VR for upper extremity rehabilitation in chronic stroke patients: a single subject design pilot study 2019. https://www.researchweb.org/is/vgr/project/262331 . Accessed 16 July 2019
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud. 2013;50(5):587–92.
O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open. 2019;9(8):e029954.
Zhan S, Ottenbacher KJ. Single subject research designs for disability research. Disabil Rehabil. 2001;23(1):1–8.
Lobo MA, Moeyaert M, Baraldi Cunha A, Babik I. Single-case design, analysis, and quality assessment for intervention research. J Neurol Phys Ther. 2017;41(3):187–97.
Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791–8.
Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82(1):14–9.
Chen HM, Chen CC, Hsueh IP, Huang SL, Hsieh CL. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair. 2009;23(5):435–40.
Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39(6):386–91.
Alt Murphy M, Resteghini C, Feys P, Lamers I. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. 2015;15:29.
Kwakkel G, Lannin NA, Borschmann K, English C, Ali M, Churilov L, et al. Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2017;12(5):451–61.
Kwakkel G, van Wegen EEH, Burridge JH, Winstein CJ, van Dokkum LEH, Alt Murphy M, et al. Standardized measurement of quality of upper limb movement after stroke: consensus-based core recommendations from the second stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. 2019;33(11):951–8.
Nordin A, Alt Murphy M, Danielsson A. Intra-rater and inter-rater reliability at the item level of the Action Research Arm Test for patients with stroke. J Rehabil Med. 2014;46(8):738–45.
Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the Action Research Arm test. Neurorehabil Neural Repair. 2008;22(1):78–90.
Penta M, Tesio L, Arnould C, Zancan A, Thonnard JL. The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: Rasch-based validation and relationship to upper limb impairment. Stroke. 2001;32(7):1627–34.
Wang TN, Lin KC, Wu CY, Chung CY, Pei YC, Teng YK. Validity, responsiveness, and clinically important difference of the ABILHAND questionnaire in patients with stroke. Arch Phys Med Rehabil. 2011;92(7):1086–91.
Alt Murphy M, Murphy S, Persson HC, Bergstrom UB, Sunnerhagen KS. Kinematic analysis using 3D motion capture of drinking task in people with and without upper-extremity impairments. J Vis Exp. 2018;133:e57228.
Alt Murphy M, Willen C, Sunnerhagen KS. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabil Neural Repair. 2011;25(1):71–80.
Alt Murphy M, Willen C, Sunnerhagen KS. Movement kinematics during a drinking task are associated with the activity capacity level after stroke. Neurorehabil Neural Repair. 2012;26(9):1106–15.
Alt Murphy M, Willen C, Sunnerhagen KS. Responsiveness of upper extremity kinematic measures and clinical improvement during the first three months after stroke. Neurorehabil Neural Repair. 2013;27(9):844–53.
Thrane G, Alt Murphy M, Sunnerhagen KS. Recovery of kinematic arm function in well-performing people with subacute stroke: a longitudinal cohort study. J Neuroeng Rehabil. 2018;15(1):67.
Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31.
CAS PubMed Google Scholar
Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–7.
Grimby G, Borjesson M, Jonsdottir IH, Schnohr P, Thelle DS, Saltin B. The, “Saltin-Grimby Physical Activity Level Scale” and its application to health research. Scand J Med Sci Sports. 2015;25(Suppl 4):119–25.
Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81.
U.S. Department of Education IoES, National Center for Education Evaluation and Regional Assistance. What Works Clearinghouse, Standards Handbook, Version 4.0. 2017.
Tate RL, Perdices M, Rosenkoetter U, Wakim D, Godbee K, Togher L, et al. Revision of a method quality rating scale for single-case experimental designs and n-of-1 trials: the 15-item Risk of Bias in N-of-1 Trials (RoBiNT) Scale. Neuropsychol Rehabil. 2013;23(5):619–38.
Chen M, Hyppa-Martin JK, Reichle JE, Symons FJ. Comparing single case design overlap-based effect size metrics from studies examining speech generating device interventions. Am J Intellect Dev Disabil. 2016;121(3):169–93.
Manolov R, Losada JL, Chacon-Moscoso S, Sanduvete-Chaves S. Analyzing two-phase single-case data with non-overlap and mean difference indices: illustration, software tools, and alternatives. Front Psychol. 2016;7(32):32.
PubMed PubMed Central Google Scholar
Manolov R, Moeyaert M. Recommendations for choosing single-case data analytical techniques. Behav Ther. 2017;48(1):97–114.
Parker RI, Vannest KJ, Davis JL, Sauber SB. Combining nonoverlap and trend for single-case research: Tau-U. Behav Ther. 2011;42(2):284–99.
Brossart DF, Laird VC, Armstrong TW, Walla P. Interpreting Kendall’s Tau and Tau-U for single-case experimental designs. Cogent Psychol. 2018. https://doi.org/10.1080/23311908.2018.1518687 .
Daniel WW. Biostatistics: Basic Concepts and Methodology for the Health Sciences. Hoboken: Wiley; 2010.
RStudio Team. RStudio: Integrated Development Environment for R. Boston: RStudio, Inc.; 2020.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020.
Pustejovsky JE, Swan DM. SingleCaseES: A calculator for single-case effect sizes. 2019.
Wickham H, Bryan J. readxl: Read Excel Files. 2019.
Hussain N, Sunnerhagen KS, Alt Murphy M. End-point kinematics using virtual reality explaining upper limb impairment and activity capacity in stroke. J Neuroeng Rehabil. 2019;16(1):82.
Spitzley KA, Karduna AR. Feasibility of using a fully immersive virtual reality system for kinematic data collection. J Biomech. 2019;87:172–6.
Essers B, Meyer S, De Bruyn N, Van Gils A, Boccuni L, Tedesco Triccas L, et al. Mismatch between observed and perceived upper limb function: an eye-catching phenomenon after stroke. Disabil Rehabil. 2019;41(13):1545–51.
Hussain N, Alt Murphy M, Lundgren-Nilsson A, Sunnerhagen KS. Relationship between self-reported and objectively measured manual ability varies during the first year post-stroke. Sci Rep. 2020;10(1):5093.
van Lieshout EC, Visser-Meily JMA, Nijland RH, Dijkhuizen RM, Kwakkel G. Comparison of self-reported vs observational clinical measures of improvement in upper limb capacity in patients after stroke. Journal of rehabilitation medicine. 2020;52(4):jrm00051.
Wang ZR, Wang P, Xing L, Mei LP, Zhao J, Zhang T. Leap Motion-based virtual reality training for improving motor functional recovery of upper limbs and neural reorganization in subacute stroke patients. Neur Regener Res. 2017;12(11):1823–31.
Turkbey TA, Kutlay S, Gok H. Clinical feasibility of Xbox KinectTM training for stroke rehabilitation: a single-blind randomized controlled pilot study. J Rehabil Med. 2017;49(1):22–9.
Staiano AE, Beyl RA, Guan W, Hendrick CA, Hsia DS, Newton RL Jr. Home-based exergaming among children with overweight and obesity: a randomized clinical trial. Pediatr Obesity. 2018;13(11):724–33.
Article CAS Google Scholar
Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–53.
Micarelli A, Viziano A, Micarelli B, Augimeri I, Alessandrini M. Vestibular rehabilitation in older adults with and without mild cognitive impairment: effects of virtual reality using a head-mounted display. Arch Gerontol Geriatr. 2019;83:246–56.
Huang X, Naghdy F, Du H, Naghdy G, Murray G. Design of adaptive control and virtual reality-based fine hand motion rehabilitation system and its effects in subacute stroke patients. Comput Methods Biomech Biomed Eng Imaging Vis. 2017;6(6):1–9.
Download references
Acknowledgements
First and foremost, we thank the participants for their time and energy. We would also like to thank the local patient organizations and support groups (Strokeföreningen Göteborg med omnejd, Stroke Mitt I Livet-SMIL and Hjärnpunkten) for help with recruitment, and the local health-care providers (Stroke Forum, Senioren, Nötkärnan Bergsjön, Närhälsan Sävedalen) spreading information about the study.
Open Access funding provided by Gothenburg University Library. This project was funded by the Swedish Research Council (VR 2017-00946), the Swedish Heart-Lung Foundation, the Swedish Brain Foundation, Promobilia and the Norrbacka Eugenia Foundation, the Swedish Society for Medical Research (S19-0074), the Swedish state under an agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG-718711, ALFGBG-775561, ALFGBG-826331).
Author information
Authors and affiliations.
Institute of Neuroscience and Physiology, Clinical Neuroscience, Rehabilitation Medicine, Sahlgrenska Academy, University of Gothenburg, Per Dubbsgatan 14, 3rd Floor, 41345, Gothenburg, Sweden
Mattias Erhardsson, Margit Alt Murphy & Katharina S. Sunnerhagen
Institute of Biomedicine, Medical Biochemistry and Cell Biology, Sahlgrenska Academy, University of Gothenburg, Medicinaregatan 9 A, 413 90, Gothenburg, Sweden
Mattias Erhardsson
You can also search for this author in PubMed Google Scholar
Contributions
ME initiated and designed the study, collected and analyzed the data, and drafted the manuscript. MAM designed the study, analyzed the data and contributed substantially to the writing. KSS initiated and designed the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Margit Alt Murphy .
Ethics declarations
Ethics approval and consent to participate.
Ethical application was approved by the Swedish Ethical Review Authority 2019-02-19 (DNR 2019-00467 / 1075-18). All participants gave their written and oral informed consent to participate in the study.
Consent for publication
Written, informed consent to publish anonymized individual data was obtained from all participants. Furthermore, a separate written informed consent was obtained for publication of anonymized images and videos from all individuals where this was recorded.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Describes the screening and testing process for the games.
Additional file 2.
The R script which was used to calculate the Tau-U A vs. B − trend A summary index for the primary outcomes.
Additional file 3.
The excel file with primary outcomes which was imported to R for the Tau-U analysis.
Additional file 4.
This excel file contains all data which was generated with or analyzed in this study. It is divided into 3 tabs: Primary outcomes, Additional assessments, and Training sessions.
Additional file 5.
Outcomes plotted on an individual level. The dotted lines are linear models fit to the corresponding phase. The area shaded grey around the fit line is the 95% confidence interval for the model. The horizontal black lines are the medians for Phase A, 1st, and 2nd half of phase B respectively. The vertical bar between phase A and B is the MCD (BBT) or MCID (all other outcomes) for the outcome, anchored at the phase A median.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Erhardsson, M., Alt Murphy, M. & Sunnerhagen, K.S. Commercial head-mounted display virtual reality for upper extremity rehabilitation in chronic stroke: a single-case design study. J NeuroEngineering Rehabil 17 , 154 (2020). https://doi.org/10.1186/s12984-020-00788-x
Download citation
Received : 03 June 2020
Accepted : 13 November 2020
Published : 23 November 2020
DOI : https://doi.org/10.1186/s12984-020-00788-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Upper extremity
- Rehabilitation
- Video games
Journal of NeuroEngineering and Rehabilitation
ISSN: 1743-0003
- Submission enquiries: [email protected]
LSVT BIG in late stroke rehabilitation: A single-case experimental design study
- PMID: 30862183
- DOI: 10.1177/0008417419832951
Background.: Late stroke rehabilitation interventions often target impairment with limited carryover to daily occupation.
Purpose.: This study explored whether the LSVT BIG program could lead to improved performance in client-identified occupations and decreased impairment late poststroke.
Method.: A single-case experimental design with one repetition was completed. Participants were two adults who had experienced a stroke 3 and 12 years previously. Each participant selected up to six occupational goals, and the intervention was applied to half. Repeated measures were taken using the Canadian Occupational Performance Measure and the Rating of Everyday Arm-Use in the Community and Home. Additional measures of performance and impairment were applied pre- and postintervention.
Findings.: Performance improved on either self-assessment or blinded-rater assessment for all but one activity (trained or untrained).
Implications.: LSVT BIG is a promising intervention to improve occupational performance. Further research is required to clarify elements of the program essential to improving occupational performance.
Keywords: Détermination des objectifs; Ergothérapie; Goal setting; Habiletés motrices; Motor skills; Occupational performance; Occupational therapy; Rendement occupationnel.
- Activities of Daily Living
- Disability Evaluation*
- Exercise Test
- Middle Aged
- Occupational Therapy*
- Recovery of Function
- Single-Case Studies as Topic
- Stroke Rehabilitation*
- Surveys and Questionnaires

IMAGES
VIDEO
COMMENTS
In the US in 2005, the average age of incidence of stroke was 69.2 years [ 2, 29, 30 ]. Recent research has indicated that people aged 20–54 years are at increasing risk of stroke, probably due to pre-existing secondary factors [ 31 ]. Women are at equal or greater risk of stroke than men, irrespective of age [ 32 ].
Purpose.: This study explored whether the LSVT BIG program could lead to improved performance in client-identified occupations and decreased impairment late poststroke. Method.: A single-case experimental design with one repetition was completed. Participants were two adults who had experienced a stroke 3 and 12 years previously.