Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Data Descriptor
- Open access
- Published: 14 April 2023

A comprehensive dataset of annotated brain metastasis MR images with clinical and radiomic data
- Beatriz Ocaña-Tienda ORCID: orcid.org/0000-0001-8931-3836 1 ,
- Julián Pérez-Beteta ORCID: orcid.org/0000-0003-0317-6215 1 ,
- José D. Villanueva-García 1 ,
- José A. Romero-Rosales ORCID: orcid.org/0000-0001-5154-3740 1 ,
- David Molina-García ORCID: orcid.org/0000-0002-6104-3894 1 ,
- Yannick Suter ORCID: orcid.org/0000-0003-1822-948X 2 ,
- Beatriz Asenjo 3 ,
- David Albillo ORCID: orcid.org/0000-0002-4496-6849 4 ,
- Ana Ortiz de Mendivil 5 ,
- Luis A. Pérez-Romasanta 6 ,
- Elisabet González-Del Portillo 6 ,
- Manuel Llorente 4 ,
- Natalia Carballo 4 ,
- Fátima Nagib-Raya 3 ,
- Maria Vidal-Denis 3 ,
- Belén Luque 1 ,
- Mauricio Reyes ORCID: orcid.org/0000-0002-2434-9990 2 ,
- Estanislao Arana 7 &
- Víctor M. Pérez-García ORCID: orcid.org/0000-0002-6575-495X 1
Scientific Data volume 10 , Article number: 208 ( 2023 ) Cite this article
6673 Accesses
8 Citations
20 Altmetric
Metrics details
- Applied mathematics
- Translational research
Brain metastasis (BM) is one of the main complications of many cancers, and the most frequent malignancy of the central nervous system. Imaging studies of BMs are routinely used for diagnosis of disease, treatment planning and follow-up. Artificial Intelligence (AI) has great potential to provide automated tools to assist in the management of disease. However, AI methods require large datasets for training and validation, and to date there have been just one publicly available imaging dataset of 156 BMs. This paper publishes 637 high-resolution imaging studies of 75 patients harboring 260 BM lesions, and their respective clinical data. It also includes semi-automatic segmentations of 593 BMs, including pre- and post-treatment T1-weighted cases, and a set of morphological and radiomic features for the cases segmented. This data-sharing initiative is expected to enable research into and performance evaluation of automatic BM detection, lesion segmentation, disease status evaluation and treatment planning methods for BMs, as well as the development and validation of predictive and prognostic tools with clinical applicability.
Similar content being viewed by others
A large open access dataset of brain metastasis 3D segmentations on MRI with clinical and imaging information
Divya Ramakrishnan, Leon Jekel, … Mariam S. Aboian
Enhancing the REMBRANDT MRI collection with expert segmentation labels and quantitative radiomic features
Anousheh Sayah, Camelia Bencheqroun, … Yuriy Gusev
Raidionics: an open software for pre- and postoperative central nervous system tumor segmentation and standardized reporting
David Bouget, Demah Alsinan, … Ingerid Reinertsen
Background & Summary
Brain metastases (BMs) represent the most common intracranial neoplasm in adults. They affect around 20% of all cancer patients 1 , 2 , 3 , 4 , 5 , 6 , and are among the main complications of lung, breast and colorectal cancers, melanoma or renal cell carcinomas 1 , 2 , 3 , 4 . The increasing availability of systemic treatments has improved the prognosis of patients with primary tumors, leading to an increase in the probability of developing BMs 2 , 3 , 6 , 7 .
BMs often appear as multiple lesions, with only around 25% of patients harboring a single BM 2 , 8 . On magnetic resonance imaging (MRI) studies, they are found to present contrast-enhancing features. Contrast-enhanced T1-weighted (CE-T1-W) MRI is the gold standard imaging sequence for BMs, providing information about lesion size, morphology and surrounding healthy structures 7 , 9 . T2-weighted imaging and fluid attenuation inversion recovery (FLAIR) MRI sequences are also used to help in identifying BMs, due to the surrounding edema found in many BM lesions 1 , 5 , 7 .
Treatment of BMs typically includes a combination of radiotherapy, chemotherapy, immunotherapy, targeted therapies, and/or surgery 1 , 2 , 3 . Radiotherapy schemes include whole brain radiation therapy and stereotactic radiosurgery (SRS). SRS is considered the standard of care in patients with limited metastatic burden 6 , 7 , 9 , 10 , 11 .
The clinical management of BMs undergoing radiotherapy requires time-consuming processes such as lesion identification and segmentation 2 , 3 , 12 . Time spent on those tasks could be reduced with the aid of semi-automatic or automatic computer-guided algorithms. Machine learning (ML) and deep learning (DL) techniques are being developed for different problems related to BMs, such as: automatic BM detection 5 , 6 , 7 , 12 , 13 , 14 , segmentation 11 , 13 , 14 , 15 and differential diagnosis of BMs from other brain tumors 7 , 12 , 16 . AI algorithms may also reduce human errors in all of those jobs that result from heavy workloads, allowing for increased reproducibility 6 , 12 .
Another problem in which AI can be helpful is the differentiation between post-treatment BM progression and radiation necrosis, a transient inflammatory effect after SRS. These two situations have overlapping features on MRI sequences, which makes it challenging to distinguish them visually 7 , 9 , 10 . Incorrect classification leads to unnecessary treatments and substantial patient harm. For this reason, AI methods have have been developed to automatically distinguish them 7 , 9 .
Finally, the development of prognostic and predictive metrics using the information contained in medical images is of the utmost importance because of the clinical implications. For BMs, the Graded Prognostic Assessment (GPA) index is the most popular clinically-validated prognostic scale 1 , 3 . However, it does not use any imaging information, but only clinical variables. In this sense, the field of Radiomics has the potential to improve the prognostic and predictive value of GPA and set the ground for novel indexes 17 , 18 . Radiomic-based research in brain tumors has been huge, and a variety of parameters have been studied 4 , 7 , 16 , 19 , 20 , 21 , 22 . Additionally, while morphological features obtained from MRI have proven effective in the setting of other brain tumors, little research has been done on their utility for BMs. 23 , 24 , 25 , 26 , 27 , 28 , 29 . The calculation of those biomarkers relies on brain tumor segmentations. Several approaches constructed using ML and DL algorithms have been proposed in the literature to automate this procedure 11 , 12 , 30 , 31 , 32 , 33 , 34 . However, due to the lack of large BM public datasets, there is no common ground on which they can be properly compared.
Publicly available datasets of BMs are limited. The most popular repository of images for cancer research is The Cancer Imaging Archive (TCIA) 35 , including more than 140 imaging repositories of different human cancers. However, in the case of BMs, only one database including 156 whole brain MRI studies have been found available 14 . This leads to the fact that while there is a good amount of public data for the much less frequent primary brain tumors such as glioblastoma, available datasets for BMs are scarce.
This study tries to solve that problem by contributing longitudinal magnetic resonance imaging studies of 75 BM patients, harboring 260 BM lesions, for a total of 637 imaging studies. Imaging studies include pretreatment post-contrast T1-w sequences, and most of them include other sequences such as T1, T2, FLAIR, DWI, etc. Semi-automatic segmentations of 154 different BMs for a total of 593 post-contrast T1-W segmentations are also provided with the dataset. These data are accompanied by an extensive database including clinical data and a set of morphological and radiomic-based features obtained from the segmentations.
MRI studies in our dataset have four times the number of segmentations than those currently publicly available 14 . Additionally, we make public three excel files, one of which contains clinical data, including patient information, details about the primary tumor, details about treatments, and the date of the patient’s death, as opposed to the already published one, which only contains information about the histology of the primary tumor.
Subject characteristics
Data collected include the follow-up imaging studies and clinical data of 75 BM patients from 5 different medical institutions. Inclusion criteria was defined as: deceased adult patients with pathologically confirmed diagnosis of BM between January 1, 2005 and December 31, 2021, availability of imaging studies with at least the post-contrast T1-w high-resolution sequence (pixel spacing ≤2 mm., slice thickness ≤2 mm., no gap between slices), no noise or artifacts in the images, and availability of basic clinical data (age at diagnosis, sex, treatment schemes followed, survival, etc.). Primary tumors were: Non-small cell lung cancer (NSCLC) (n = 38), small cell lung cancer (SCLC) (n = 5), breast cancer (n = 22), melanoma (n = 6), ovarian cancer (n = 2), kidney cancer (n = 1) and uterine cancer (n = 1).
The 75 patients included had a total of 260 BMs with a total of 637 imaging studies. Of those, 593 studies were semi-automatically segmented as described below.
Image acquisition
All post-contrast T1-W sequences were obtained after intravenous administration of a single dose of contrast. The 593 imaging sequences segmented were acquired with a 1-T (n = 8), 1.5-T (n = 550) or 3.0-T (n = 35) MR imaging scanners. Regarding the MR imaging vendors, General Electric (n = 225), Philips (n = 197), and Siemens (n = 171) medical systems were used. Other image parameters are described in Table 1 .
Segmentation procedure
Segmentation was performed using an in-house semi-automatic segmentation procedure 26 , 28 . Tumors were automatically delineated by using a gray-level threshold chosen to identify the largest contrast-enhancing tumoral volume. Then, a biomedical engineer/applied mathematician (B. O.-T.) carefully corrected each segmentation, slice by slice, using a brushing/pixel-removing tool. The segmentation process is summarized in Fig. 1 . The outcome was cross-checked by three researchers with more than seven years of expertise on MRI (D. M.-G., J. P.-B., V. M. P.-G.) and then corrected by one of the radiologists participating in the study (B.A, A.O.M, D.A, L.A.P.-R., E.A.). The raw medical images in DICOM format were used in this procedure, so they were not modified to perform the tumor segmentations.
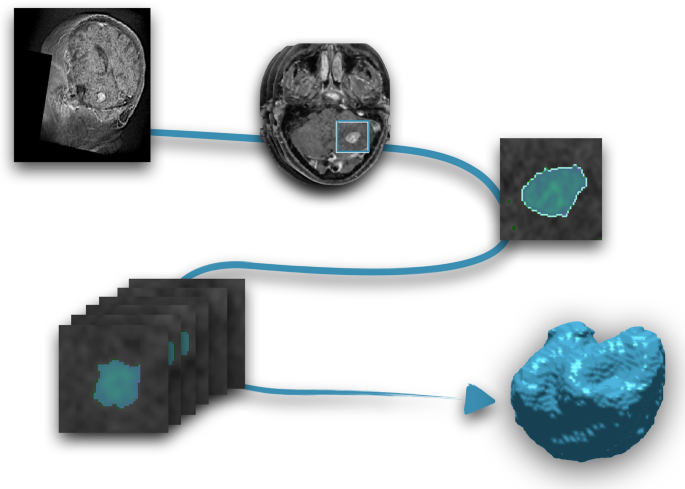
Image segmentation procedure. From the MR images (T1-W with contrast), each slice was semi-automatically segmented and manually corrected. Once every slice was segmented, the last step was the three-dimensional reconstruction of the tumor.
Clinical data and anonymization
Clinical data were collected for the 75 patients. For each patient, age at diagnosis and sex, primary tumor type and subtype, molecular markers (e.g. EGFR, ALK and ROS1 for lung cancer) and tumor stage were taken. Also, the GPA index 1 , 3 , was included for a subset of institutions. Regarding each BM, the ID (a number to differentiate it from other BMs in the same patient), location in the brain (frontal, temporal, parietal and occipital, right and left side), date of appearance on MRI, and treatments received were recorded. For each treatment, the type of treatment, doses, fractions, date of start and date of end were recorded. The dates of follow-up MRI studies available were also included. Radionecrosis was confirmed for 39 lesions.
The first step of the data anonymization was performed at the institutions of origin of the data. Such a step included patient and center data anonymization. An additional more profound anonymization was performed using the clinical trials processor from the medical imaging resource center 36 . Within that step, all private DICOM tags and all tags containing sensitive or identifying information as well as all dates were modified such that for every subject, the imaging study where the first BM was initially identified corresponds to January 1st, 1900. The anonymized times were computed taking as reference that time point, in days, which means that negative numbers identified treatments prior to the diagnosis of the BM. The relative differences in times for the different events for each patient were preserved. The last anonymization step was a defacing process that made impossible the facial reconstruction. After this whole process, patient records were finally reviewed independently by three of the authors (B. O.-T., J. P.-B., and J. A. R.-R.).
Morphological parameters
Different morphological parameters were computed from the segmentations and gathered in the database, including the following:
For each focus, three different types of volumes were computed: the contrast-enhancing ( V CE ), necrotic (or non-enhancing) ( V N ) and total volume ( V = V CE + V N ).
Contrast-enhancing spherical rim width (CE rim width)
Obtained for each focus from the CE and necrotic volumes as
By assuming that the areas of necrotic tissue and the entire tumor are spherical, this feature calculates the average width of the CE areas. Additional information and illustrations of tumors with high and low CE rim widths, can be found in 29 .
Obtained by reconstructing the tumor surface using the Matlab “isosurface” command from the discrete sets of voxels characterizing the tumor.
Surface regularity
It is a dimensionless ratio between the volume of the segmented tumor divided by the volume of a spherical tumor with the same surface. For each focus, it was calculated as
The range for this parameter is 0 (for tumors with highly uneven surfaces) and 1 (for spherical tumors). Additional information and illustrations of tumors with high and low CE rim widths, can be found in 17 .
Maximum diameter
It provides the largest longitudinal measure of the tumor and is computed for each focus as the maximum distance between two points located on the surface of the CE tumor.
Radiomic-based features
A total of 110 different features were extracted with the open-source Python package PyRadiomics version 2.2.0 37 . This feature dataset includes 16 shape descriptors and different measures of the intensity distribution and texture within the segmentation labels. The intensity features include simple first-order statistics (19 features), those derived from the gray-level co-occurrence matrix (GLCM, 24 features), gray-level run-length matrix (GLRLM, 16 features), gray-level size-zone matrix (GLSZM, 16 features), neighboring gray-tone difference matrix (NGTDM, 5 features), and gray-level dependence matrix (14 features). The features were extracted from the original image sequence after z-score normalization, intensity scaling by a factor of 100 and subsequently shifting by 300 (i. e. three standard deviations) to ensure most intensity values are positive for the first-order features and geometry tolerance 0.04. Other specific tasks may require different feature extraction procedures 18 .
No voxel resampling prior to feature extraction was used to maintain the information as unaltered as possible. Since the algorithm to extract image features is shared, any user can redo the extraction by applying any resampling.
Atlas location features
Affine registration was used to align all subjects to MNI atlas space 38 using the mri_robust_register 39 . The centroid of each separate metastasis lesion was listed and may be used to efficiently identify the location and affected brain region.
Ethical approval
We have complied with all relevant ethical regulation and all subjects included in the study are deceased. Human data were obtained in the framework of the study OpenBTAI (Open database of Brain Tumors for studies in Artificial Intelligence), a retrospective, multicenter, nonrandomized study approved by the corresponding institutional review boards: Fundación Instituto Valenciano de Oncología (2021-05), Hospital Universitario HM Sanchinarro (21.06.1858-GHM), Hospital Universitario 12 de Octubre (21/711), Hospital General Universitario de Ciudad Real (12/2021), Hospital Regional Universitario de Málaga (24/06/2021), Hospital Universitario y Politécnico La Fe (2021-504-1), MD Anderson Cancer Center (01/06/2021), Hospital Universitario de Salamanca (2021 10 879), Complejo Hospitalario Universitario de Toledo (29/9/2021-770) and Hospital Universitario Marqués de Valdecilla (14/2021 – 10/09/2021).
Data Records
All data records collected for this manuscript are available at the Figshare Repository 40 and on the webpage https://molab.es where the number of cases will be expanded.
Raw medical images for each follow-up study have been stored using the Digital Imaging and Communications in Medicine image file format (DICOM, ISO 12052). Tumor segmentations and the corresponding images have been stored in The Neuroimaging Informatics Technology Initiative (NIfTI) format, maintaining raw medical image coordinates, since no preprocessing was used to perform the manual segmentations. We have uploaded six zip files with the DICOMS images, one containing all the segmentations (files ended _msk.nii) and one containing the corresponding images (files ended _img.nii) to each of the segmentations available. Also, three excel files containing: (1) all the clinical data, (2) morphological parameters measured directly from the segmentations, and (3) radiomic-based features computed for each follow-up study segmented are included together with the imaging data.
Technical Validation
Data collection.
The collaborating expert board-certified neuroradiologists identified and collected the 637 follow-up studies of the 75 BM patients included in the study. Only confirmed BM patients were included in the study, and primary tumors for each patient were pathologically confirmed and verified prior to inclusion in the study.
Data curation and testing of the inclusion criteria was performed by a biomedical engineer/applied mathematician with more than seven years experience in management of medical images (B. O.-T., D. M.-G., J. P.-B. and V. M. P.-G.) and then cross-checked by a different expert.
Segmentation method
All semi-automatic segmentations performed in this study were carefully validated by an expert radiologist after have been performed by experienced experts in the management of medical images and cross-checked by a different expert. A reproducibility study for the methodology was performed in 26 , showing its reliability.
Each segmentation mask contains two labels for each BM: labels ending in 1 correspond to contrast-enhancing (CE) parts of the tumor; labels ending in 2 represent the non-enhancing or necrotic area of the tumor. Features were extracted for CE and necrotic zones and also were computed for the combination of both.
Comparison between measurements obtained and radiomic features
Two excel files are provided with features from the segmented images. One of them contains some morphological variables computed directly from the manual segmentation while the other is a radiomic-based set of features.
Usage Notes
The whole dataset can be downloaded from the figshare repository 40 . To process the provided images and segmentations, it is highly recommended that medical imaging tools be used, which handle consistently the physical space and orientation of the images. We verified that all the Nifti files (segmentations and images) can be loaded correctly with FSLeyes v1.3.0 ( https://www.fsl.fmrib.ox.ac.uk ) (FMRIB Centre, Oxford, UK) and DICOM files could be easily loaded using Horos v3.3.6 ( https://www.horosproject.org ).
Code availability
We provide the code used to extract the features with PyRadiomics at https://github.com/ysuter/OpenBTAI-radiomics . For reproducibility and convenience in case any user wants to customize the extraction, all the.py files needed and a “readme” file are available.
Achrol, A. S. et al . Brain metastases. Nature Rev Dis Primers. 5 (5), 1–26 (2019).
Google Scholar
Nayak, L., Lee, E. Q. & Wen, P. Y. Epidemiology of brain metastases. Curr Oncol Rep. 14 , 48–54 (2012).
Article PubMed Google Scholar
Lignelli, A. & Khandji, A. G. Review of imaging techniques in the diagnosis and management of brain metastases. Neurosurg Clin N Am. 22 , 15–25 (2011).
Kniep, H. C. et al . Radiomics of brain MRI: utility in prediction of metastatic tumor type. Radiology. 290 , 479–487 (2019).
Dikici, E. et al . Automated brain metastases detection framework for T1-weighted contrast-enhanced 3D MRI. IEEE J Biomed Health Inform. 24 (10), 2883–2893 (2020).
Cho, S. J. et al . Brain metastasis detection using machine learning: a systematic review and meta-analysis. Neuro Oncol. 23 (2), 214–225 (2021).
Article MathSciNet PubMed Google Scholar
Tong, E., McCullagh, K. L. & lv, M. Advanced imaging of brain metastases: from augmenting visualization and improving diagnosis to evaluating treatment response. Front Neurol. 11 , 1–14 (2020).
Article Google Scholar
Wolpert, F. et al . Risk factors for the development of epilepsy in patients with brain metastases. Neuro Oncol. 22 (55), 718–728 (2020).
Kim, H. Y. et al . Classification of true progression after radiotherapy of brain metastasis on MRI using artificial intelligence: a systematic review and meta-analysis. Neuro Oncol Adv. 3 (1), 1–12 (2021).
Gagliardi, F. et al . Role of stereotactic radiosurgery for the treatment of brain metastasis in the era of immunotherapy: A systematic review on current evidences and predicting factors. Critical Reviews in Oncology Hematology. 165 , 103431 (2021).
Bousabarah, K. et al . Deep convolutional neural networks for automated segmentation of brain metastases trained on clinical data. Radiat Oncol. 15 , 87 (2020).
Article PubMed PubMed Central Google Scholar
Xue, J. et al . Deep learning-based detection and segmentation-assisted management of brain metastases. Neuro Oncol. 22 (4), 505–514 (2020).
Charron, O. et al . Automatic detection and segmentation of brain metastases on multimodal MR images with a deep convolutional neural network. Comput Biol Med. 95 , 43–54 (2018).
Grøvik, E. et al . Deep learning enables automatic detection and segmentation of brain metastases on multi-sequence MRI. J Magnet Reson Imag. 51 (1), 175–182 (2019).
Liu, Y. et al . A deep convolutional neural network-based automatic delineation strategy for multiple brain metastases stereotactic radiosurgery. Plos One. 12 (10), e0185844 (2017).
Bae, S. et al . Robust performance of deep learning for distinguishing glioblastoma from single brain metastasis using radiomic features: model development and validation. Sci Rep. 10 (1), 12110 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Gillies, R. J., Kinahan, P. E. & Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 278 (2), 563–577 (2016).
Mouraviev, A. et al . Use of radiomics for the prediction of local control of brain metastases after stereotactic radiosurgery. Neuro-Oncology. 22 (6), 797–805 (2020).
Molina, D. et al . Tumour heterogeneity in glioblastoma assessed by MRI texture analysis: a potential marker of survival. Br J Radiol. 89 (1064), 20160242 (2016).
Suter, Y. et al . Radiomics for glioblastoma survival analysis in pre-operative MRI: exploring feature robustness, class boundaries, and machine learning techniques. Cancer Imaging 20 , 55 (2020).
Baid, U. et al . Overall survival prediction in glioblastoma with radiomic features using machine learning. Front Comput Neurosci. 14 , 61 (2020).
Narang, S., Lehrer, M., Yang, D., Lee, J. & Rao, A. Radiomics in glioblastoma: current status, challenges and opportunities. Trasl Cancer Res. 5 (4), 383–397 (2016).
Article CAS Google Scholar
Pérez-Beteta, J. et al . Glioblastoma: does the pre-treatment geometry matter? A postcontrast T1 MRI-based study. Eur Radiol. 27 (3), 1096–1104 (2017).
Wangaryattawanich, P. et al . Multicenter imaging outcomes study of The Cancer Genome Atlas glioblastoma patient cohort: imaging predictors of overall and progression-free survival. Neuro Oncol. 17 (11), 1525–1537 (2015).
Grabowski, M. M. et al . Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 121 (5), 1115–1123 (2014).
Pérez-Beteta, J. et al . Tumor surface regularity at MR imaging predicts survival and response to surgery in patients with glioblastoma. Radiology. 288 (1), 218–225 (2018).
Ellingson, B. M., Bendszus, M., Sorensen, A. G. & Pope, W. B. Emerging techniques and technologies in brain tumor imaging. Neuro Oncol. 16 (7), 12–23 (2014).
Pérez-Beteta, J. et al . Morphological MRI-based features provide pretreatment survival prediction in glioblastoma. Eur Radiol. 29 (4), 1968–1977 (2019).
Cui, Y. et al . Prognostic imaging biomarkers in glioblastoma: Development and independent validation on the basis of multiregion and quantitative analysis of MR images. Radiology. 278 (2), 546–553 (2016).
Menze, B. H. et al . The multimodal brain tumor image segmentation benchmark (BRATS). IEEE T Med Imaging. 34 (10), 1993–2024 (2015).
Ermiş, E. et al . Fully automated brain resection cavity delineation for radiation target volume definition in glioblastoma patients using deep learning. Radiat Oncol. 15 , 100 (2020).
Porz, N. et al . Multi-modal glioblastoma segmentation: man versus machine. Plos one. 9 (5), e96873 (2014).
Article ADS PubMed PubMed Central Google Scholar
Kamnitsas, K. et al . Efficient multi-scale 3D CNN with fully connected CRF for accurate brain lesion segmentation. Med Image Anal. 36 , 61–78 (2017).
Meier, R. et al . Clinical evaluation of a fully-automatic segmentation method for longitudinal brain tumor volumetry. Sci Rep. 6 , 23376 (2016).
Clark, K. et al . The Cancer Imaging Archive (TCIA): Maintaining and operating a public information repository. Journal of Digital Imaging. 26 (6), 1045–1057 (2013).
Aryanto, K. Y. E., Oudkerk, M. & van Ooijen, P. M. A. Free DICOM de-identification tools in clinical research: functioning and safety of patient privacy. European radiology 25 (12), 3685–3695 (2015).
Article CAS PubMed PubMed Central Google Scholar
van Griethuysen, J. J. et al . Computational radiomics system to decode the radiographic phenotype. Cancer Res. 77 (21), e104–e107 (2017).
Mazziotta, J. et al . A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM). Philos. Trans. Roy. Soc. Lond. Ser. B: Biol. Sci. 356 (1412), 1293–1322 (2001).
Reuter, M., Rosas, H. D. & Fischl, B. Highly accurate inverse consistent registration: a robust approach. NeuroImage 53 (4), 1181–1196 (2010).
Ocaña-Tienda, B. et al . Brain Metastasis MR images with segmentations, clinical data, morphological measurements and radiomic features, Figshare , https://doi.org/10.6084/m9.figshare.c.6194104.v1 (2023).
Download references
Acknowledgements
This research has been supported by grants awarded to V.M. P.-G. by the James S. Mc. Donnell Foundation, United States of America, 21st Century Science Initiative in Mathematical and Complex Systems Approaches for Brain Cancer (collaborative award 220020560, https://doi.org/10.37717/220020560 ), Ministerio de Ciencia e Innovación, Spain (grant numbers PID2019-110895RB-I00 and PDC2022-133520-I00) and Junta de Comunidades de Castilla-La Mancha (SBPLY/21/180501/000145). BOT is supported by the Spanish Ministerio de Ciencia e Innovación (grant PRE2020-092178).
Author information
Authors and affiliations.
Mathematical Oncology Laboratory (MOLAB), University of Castilla-La Mancha, Ciudad Real, Spain
Beatriz Ocaña-Tienda, Julián Pérez-Beteta, José D. Villanueva-García, José A. Romero-Rosales, David Molina-García, Belén Luque & Víctor M. Pérez-García
Medical Image Analysis Group, ARTORG Research Center, Bern, Switzerland
Yannick Suter & Mauricio Reyes
Radiology Department, Hospital Regional Universitario de Málaga, Málaga, Spain
Beatriz Asenjo, Fátima Nagib-Raya & Maria Vidal-Denis
Radiology Department, MD Anderson Cancer Center, Madrid, Spain
David Albillo, Manuel Llorente & Natalia Carballo
Radiology Department, Sanchinarro University Hospital, HM Hospitales, Madrid, Spain
Ana Ortiz de Mendivil
Radiation Oncology Department, Hospital Universitario de Salamanca, Salamanca, Spain
Luis A. Pérez-Romasanta & Elisabet González-Del Portillo
Radiology Department, Fundación Instituto Valenciano de Oncología, Valencia, Spain
Estanislao Arana
You can also search for this author in PubMed Google Scholar
Contributions
B.O.-T., J.P.-B., D.M.-G., M. R., E.A. and V. M.P.-G. designed research; B.O.-T. performed the segmentations; Y.S. performed full data anonymization; B.A., D.A., A.O.M., L.A.P.-R., E.G.P., M.L., N.C., F.N.-R., M.V.-D., B.L. and E.A. collected data; B.O.-T., D.M.-G., J.P.-B., J.A.R.-R. and V.M.P.-G. analyzed data; D.M.-G. and V.M.P.-G. wrote the paper; All authors revised and corrected the manuscript. B.O.-T. and J.P.-B. contributed equally to the paper and V.M.P.-G and E. A. are both joint senior authors of this manuscript.
Corresponding authors
Correspondence to Beatriz Ocaña-Tienda or Estanislao Arana .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ocaña-Tienda, B., Pérez-Beteta, J., Villanueva-García, J.D. et al. A comprehensive dataset of annotated brain metastasis MR images with clinical and radiomic data. Sci Data 10 , 208 (2023). https://doi.org/10.1038/s41597-023-02123-0
Download citation
Received : 14 September 2022
Accepted : 30 March 2023
Published : 14 April 2023
DOI : https://doi.org/10.1038/s41597-023-02123-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Divya Ramakrishnan
- Mariam S. Aboian
Scientific Data (2024)
Developing a Radiomics Atlas Dataset of normal Abdominal and Pelvic computed Tomography (RADAPT)
- Elisavet Kapetanou
- Stylianos Malamas
- Michail E. Klontzas
Journal of Imaging Informatics in Medicine (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Advertisement
Systematic review of the management of brain metastases from hormone receptor positive breast cancer
- Open access
- Published: 08 March 2023
- Volume 162 , pages 45–57, ( 2023 )
Cite this article
You have full access to this open access article
- Shirley Jusino ORCID: orcid.org/0000-0001-7960-9334 1 ,
- Camilo E. Fadul ORCID: orcid.org/0000-0001-7459-7661 2 &
- Patrick Dillon ORCID: orcid.org/0000-0003-0622-725X 3
2723 Accesses
6 Citations
Explore all metrics
Introduction
Brain metastases are a common cause of morbidity and mortality in patients with breast cancer. Local central nervous system (CNS) directed therapies are usually the first line treatment for breast cancer brain metastases (BCBM), but those must be followed by systemic therapies to achieve long-term benefit. Systemic therapy for hormone receptor (HR + ) breast cancer has evolved in the last 10 years, but their role when brain metastases occur is uncertain.
We performed a systematic review of the literature focused on management of HR + BCBM by searching Medline/PubMed, EBSCO, and Cochrane databases. The PRISMA guidelines were used for systematic review.
Out of 807 articles identified, 98 fulfilled the inclusion criteria in their relevance to the management of HR + BCBM.
Conclusions
Similar to brain metastases from other neoplasms, local CNS directed therapies are the first line treatment for HR + BCBM. Although the quality of evidence is low, after local therapies, our review supports the combination of targeted and endocrine therapies for both CNS and systemic management. Upon exhaustion of targeted/endocrine therapies, case series and retrospective reports suggest that certain chemotherapy agents are active against HR + BCBM. Early phase clinical trials for HR + BCBM are ongoing, but there is a need for prospective randomized trials to guide management and improve patients’ outcome.
Similar content being viewed by others

Reirradiation versus systemic therapy versus combination therapy for recurrent high-grade glioma: a systematic review and meta-analysis of survival and toxicity
Ravi Marwah, Daniel Xing, … Sweet Ping Ng

SEOM-GEICAM-SOLTI clinical guidelines for early-stage breast cancer (2022)
Francisco Ayala de la Peña, Silvia Antolín Novoa, … Eva Ciruelos

First-line therapy with palbociclib in patients with advanced HR+/HER2− breast cancer: The real-life study PALBOSPAIN
N. Martínez-Jañez, M. Bellet Ezquerra, … F. Moreno Antón
Avoid common mistakes on your manuscript.
Breast cancer is the most commonly diagnosed cancer in women worldwide, with brain metastases being a major cause of morbidity and mortality [ 1 ]. It is estimated that 10–24% of metastatic breast cancers (MBC) seed the brain (30% per autopsy series) [ 2 , 3 , 4 ], and, in the United States, it is the second most frequent malignancy to cause brain metastases [ 5 ]. Approximately 7% of patients with MBC will have brain metastases at diagnosis (synchronous) while 17% will appear later on the course of the disease (metachronous) [ 6 , 7 ]. Young age, lymph node positivity, and tumor characteristics (stage, grade, size, and Ki-67 index) correlate with higher incidence of breast cancer brain metastases (BCBM) [ 7 , 8 , 9 ]. In a recent meta-analysis, BCBM were found in 15% of patients with hormone receptor positive (HR + ) and about 50% of HER2 + breast cancers [ 10 ].
Several prospective trials provide evidence to support management guidelines of HER2 + BCBM [ 11 ], but for patients with HR + /HER2 − , the subtype with the highest absolute incidence of brain metastases, the evidence is scant and retrospective [ 12 ]. We performed a systematic review of the published data on approved and emerging systemic treatment options that could support their use for patients with HR + BCBM.
Literature search
We conducted a systematic literature review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [ 13 ]. We queried MEDLINE/PubMed and Cochrane Library for articles published between January 1964 and June 2022 using key terms to access clinical trials and original articles on current treatment options for HR + BCBM. The search included combinations of the following keywords “HR + breast cancer”, “ER + breast cancer” “brain metastases”, “surgical resection”, “radiation therapy”, “systemic therapy”, “immunotherapy”, “chemotherapy”, and “targeted therapy”. The MEDLINE/PubMed, Cochrane Library, and EBSCO Essentials databases were searched on June 25, 2022. Abstracts and presentations from national meetings from 2019 to 2022 were also searched.
Study inclusion and analysis
One author (SJ) screened all article abstracts and selected potential papers for inclusion. Another author (PD) determined if the selected papers met the inclusion/exclusion criteria. Studies were included if a primary or secondary analysis examined treatment safety or efficacy in HR + BCBM. We excluded studies if they were not in English, were not peer reviewed, or were a letter or commentary article. Additionally, studies focused on leptomeningeal metastases were excluded. We included case reports, meta-analyses, reviews, and relevant retrospective and prospective studies that enrolled any BCBM participant with or without a pre-planned analysis of BCBM outcomes.
The search in MEDLINE/PubMed and Cochrane Library yielded 748 articles that we screened for eligibility by title and abstract (Fig. 1 ). Additionally, we included 59 articles that we identified in the references. Of the 820 articles, we excluded 722 that did not meet the inclusion criteria, leaving 98 included in this systematic review.
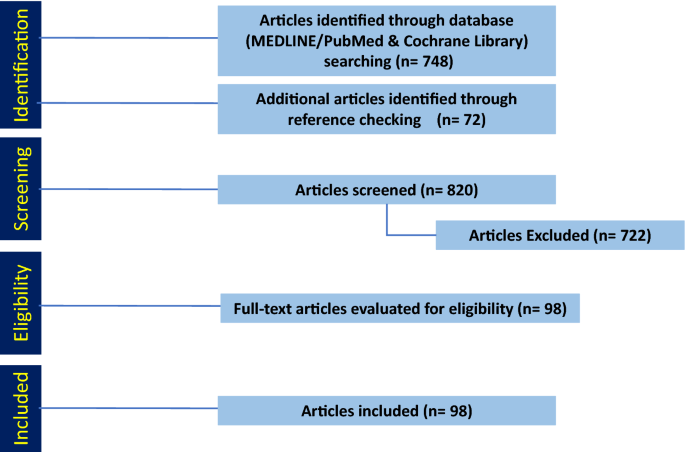
PRISMA diagram detailing the database search outcomes. A total of 748 articles were identified through database search. Another 72 additional articles were identified through references and added. Based on our inclusion and exclusion criteria 123 articles were included
Local therapy for HR + BCBM
The recommendations for local therapy (surgery and radiation) for HR + BCBM are similar to those for brain metastases from other types of cancer and previously reviewed [ 14 ]. The use of local brain directed therapy depends on the patient’s functional status, the extent of systemic extra-neural disease, the number of metastases, the neurologic symptoms, and other comorbidities. Although there are no prospective randomized studies comparing surgery and stereotactic radiosurgery (SRS) for a single brain metastasis, surgical resection is considered when complete resection with low morbidity is feasible and when there is diagnostic uncertainty, bulky disease, high symptom burden, or when a very favorable extracranial disease profile exists. Resection followed by whole brain radiotherapy (WBRT) improved survival when compared to no adjuvant post-operative radiotherapy [ 15 , 16 ]. A concern associated with WBRT is the long-term effect on neurocognitive function; thus, strategies to reduce the incidence include WBRT with hippocampal avoidance (HA) [ 17 , 18 ] and memantine treatment [ 19 ].
Meanwhile, SRS is often the preferred approach to treat limited volume brain metastases. Metastatic volumes greater than 10 cm 3 and progressive extra-cranial disease at the time of SRS were associated with worse survival for patients with BCBM [ 20 ]. Although the indication for SRS had previously been the presence of four brain metastases or less, recent guidelines from national societies suggest that some patients with more than four brain metastases may benefit from SRS [ 14 , 21 , 22 , 23 ]. Moreover, SRS has the potential to reduce the risk of long-term radiation-induced neurocognitive impairment, while improving the quality of life [ 24 ]. In most instances, a case-by-case assessment by a multidisciplinary group with consideration of risk factors is the preferred approach.
Systemic therapy
After local therapies, patients with BCBM may benefit from systemic treatment due to the high frequency of additional recurrences both in the CNS and extra-neural. For HR + BCBM, targeted therapy is preferred for first- and second-line systemic treatments, while cytotoxic chemotherapy is reserved for later lines of treatment or cases with refractory disease (Fig. 2 ).
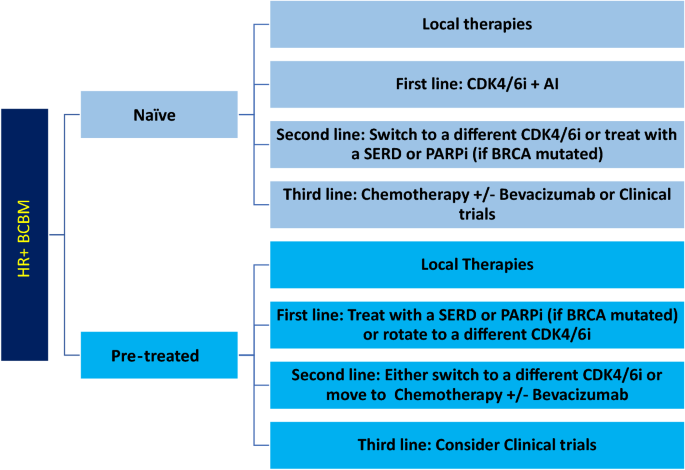
Suggested Line of Treatment for HR + BCBM. Local therapies (i.e., surgical resection and radiation) should be attempted first in naïve or pre-treated patients. Then, first, second-, and third-line systemic approach should be followed. HR + hormone receptor positive, BCBM breast cancer brain metastases, CDK4/6i cyclin dependent kinase 4/6 inhibitors, AI aromatase inhibitors, SERD selective estrogen receptor degraders, PARPi poly adenosine diphosphate-ribose polymerase inhibitors
Targeted therapy: CDK 4/6 inhibitors: palbociclib, ribociclib, and abemaciclib
Although the three FDA-approved CDK4/6i cross the blood–brain barrier (BBB), their clinical CNS efficacy is unproven. Palbociclib and abemaciclib are substrates of efflux transporters P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), while ribociclib is a substrate for P-gp [ 25 ]. Despite the limitations of CNS drug exposure from a pharmacologic standpoint, there are reports of clinical activity against HR + BCBM.
Palbociclib was the first CDK4/6i approved for the treatment of HR + /HER2 − MBC with or without visceral metastasis based on two randomized clinical trials [ 26 ]. Both studies allowed patients with brain metastases, but only two and five patients were accrued, respectively (no data on CNS outcome is available). The information on treatment arm for patients who developed new brain metastases while on study was not disclosed.
Ribociclib was FDA-approved based on the results from the MONALEESA-2 [ 27 , 28 ] and MONALEESA-3 [ 29 ] studies. The MONALEESA-2 study excluded patients with brain metastases [ 28 ]. In the MONALEESA-3 trial, eight of the 726 patients randomized (2:1) to receive ribociclib plus fulvestrant or placebo plus fulvestrant [ 30 ] had stable brain metastases, but no specific CNS outcome data is available.
Abemaciclib was FDA-approved following the results of a phase II single arm and two randomized clinical trials [ 31 , 32 , 33 ]. However, all three studies excluded patients with brain metastases. On the other hand, a single-arm phase II study evaluated the intracranial overall response rate (ORR) in HR + BCBM brain or leptomeningeal metastases treated with abemaciclib [ 34 ]. The patients, grouped by tumor subtype, were treated with either abemaciclib or the standard of care therapy. Despite achieving excellent CSF drug concentration with an intracranial ORR of 5.2% and an intracranial clinical benefit rate (complete responses + partial responses + stable disease) of 24% in HR + /HER2 − patients, the study did not meet its primary endpoint of an intracranial ORR ≥ of 15%.
All three CDK4/6i have published case reports [ 35 , 36 ] suggesting clinical activity, but there are no large controlled clinical trials demonstrating improved outcomes with these drugs for patients with HR + BCBM. Furthermore, there is scant information about appearance of new CNS metastases to draw conclusions about their ability to prevent development of BCBM. Based on their potential clinical activity and acceptable toxicity profile, an expert opinion suggested the use of CDK4/6i for patients with HR + BCBM [ 37 ].
Data on re-treatment with CDK4/6i will be available as per the MAINTAIN clinical trial (clinicaltrials.gov NCT02632045), although this study excludes patients with active CNS metastases. Clinical trials with new CDK4/6i [dalpiciclib (NCT05586841)] and CDK2i [fadraciclib (NCT02552953)] are ongoing, but these studies exclude patients with active CNS metastases.
Endocrine therapy: tamoxifen, anastrozole, letrozole, and exemestane
Aromatase inhibitors (AI) are potentially active for the treatment of BCBM as they lower both serum and CSF concentrations of estradiol [ 38 ]. However, the only publications suggesting AI (or tamoxifen) have activity for HR + BCBM are case series and reports [ 39 , 40 , 41 , 42 , 43 ]. Their potential efficacy is in the setting of BCBM naïve to endocrine therapies, but limited in tumors harboring ESR1 mutations or other endocrine resistance mechanisms [ 44 ]. A retrospective study of 198 patients with HR + BCBM found that the median OS was significantly longer in patients who received endocrine therapy after a diagnosis of BCBM compared with patients who did not receive it (15 versus 4 months) [ 45 ]. Thus, for patients with newly diagnosed HR + BCBM, it is reasonable to continue or start endocrine therapies in the setting of brain metastases, but combination therapy with a targeted agent is generally preferred.
Endocrine therapy: fulvestrant
Fulvestrant is the only FDA-approved selective estrogen receptor degrader (SERD) for breast cancer although several novel oral SERDs are in late-stage of development. Fulvestrant did not readily cross the intact BBB in animal studies [ 46 ] but two case series have suggested activity in patients with BCBM [ 47 , 48 ]. The largest monotherapy fulvestrant study included patients with stable brain metastases, but outcomes for this specific group were not reported [ 49 ]. A phase II study [ 50 ] compared fulvestrant alone or in combination with capivasertib, an AKT inhibitor, in postmenopausal women with aromatase inhibitor-resistant HR + /HER2 − MBC, showing a significantly longer PFS of the combination over monotherapy (10.3 versus 4.8 months, n = 71). Although patients with BCBM were included, their outcomes were not reported. There are several ongoing trials using fulvestrant alone or in combination with novel agents, which allow inclusion of patients with BCBM (Table 1 ).
Targeted therapy: PI3K/mTOR inhibitors
Everolimus is an mTOR inhibitor approved for late-stage HR + MBC based on a randomized phase III trial (n = 724) [ 51 ] that suggested that the combination with exemestane offers a PFS benefit versus exemestane alone. While this study excluded BCBM, another phase II trial for BCBM, tested the CNS response rate to everolimus, trastuzumab, and vinorelbine [ 52 ] in HER2 + BCBM. The CNS response rate was 4%, the median intracranial time to progression was 3.9 months, and the median OS was 12.2 months, but the study did not meet its primary endpoint. A retrospective study of everolimus in patients with MBC and prior treatment observed a PFS of 6.8 months [ 53 ]. Nine patients with BCBM achieved a PFS of 6 months.
Alternatively, alpelisib may be an option in selected patients with PIK3CA mutations and brain metastases. Case reports (n = 4) [ 54 ] and a real world dataset with four additional cases (PFS of 43 days) [ 55 ] suggest that alpelisib may have CNS activity. Ongoing studies are examining either alpelisib or next-generation PI3K inhibitors in MBC and BCBM (NCT05230810).
Targeted therapy: PARP inhibitors
Olaparib, a PARP inhibitor with CNS penetration [ 56 ], has FDA approval in patients with MBC and a germline mutation in BRCA1 or BRCA2 genes. In an open-label phase III trial [ 57 ], monotherapy olaparib was compared with standard therapy in patients with a germline BRCA mutation and HER2 − MBC. The median PFS was significantly longer in the olaparib (7.0 months) than in the standard therapy group (4.2 months), but there were no significant differences in OS [ 58 ]. This study did not report on brain metastases. Another phase II study demonstrated that olaparib is an effective and tolerable treatment in patients with MBC (brain metastases allowed) and germline PALB2 or somatic BRCA1 and BRCA2 mutations [ 59 ]; there was no report of BCBM efficacy.
Targeted therapy: bevacizumab
Bevacizumab is a vascular endothelial growth factor (VEGF) inhibitor that improved PFS in patients with MBC treated in either the first-line or the second-line setting when combined with chemotherapy [ 60 , 61 , 62 , 63 , 64 ]. However, bevacizumab ultimately had no effect on OS and the FDA indication in breast cancer was rescinded in 2011. However, phase II clinical trials [ 65 , 66 ] have shown that bevacizumab may be a reasonable option as an adjuvant to cytotoxic chemotherapy in BCBM.
Chemotherapy
Existing practice guidelines for treatment of MBC recommend sequential endocrine/targeted therapy until available agents have been exhausted before deploying systemic cytotoxic chemotherapy [ 67 ]. It is unclear if this recommendation applies to BCBM. Although cytotoxic agents may be faster acting against BCBM than certain targeted/endocrine therapies, it may be at the cost of greater toxicity. Several studies that report activity for cytotoxic agents against BCBM fail to describe cohort characteristics including receptor status, undermining the establishment of their efficacy among the distinct breast cancer subtypes [ 68 , 69 , 70 , 71 , 72 ].
Capecitabine is often the first chemotherapy attempted for HR + BCBM [ 10 , 73 ], because it is thought to penetrate the BBB [ 74 ]. A retrospective study [ 75 ] and a phase I trial [ 69 ] reported responses in the brain. Likewise, methotrexate penetrates the BBB and exhibited PR (28%) responses in a retrospective study [ 71 ]. A non-randomized prospective study reported that treatment with the CMF (cyclophosphamide, methotrexate, and fluorouracil) or FAC (5-fluorouracil, doxorubicin, and cyclophosphamide) regimens led to a 59% CNS response [ 76 ]. Furthermore, a prospective study ( n = 56) revealed that cisplatin and etoposide resulted in CNS response, including seven CR, 14 PR, and 12 SD [ 77 ]. Other drugs that cross the BBB and have reported clinical data include temozolomide [ 78 ], doxil [ 79 ], eribulin [ 80 ] and irinotecan [ 81 ].
Combination local and systemic therapy
The combination of chemotherapy and radiation may have synergistic effect against brain metastases. A prospective study compared the efficacy and impact on the quality of life of WBRT and chemotherapy in patients with BCBM [ 81 ]. This study randomized 58 patients stratified according to breast cancer subtypes to receive WBRT alone or WBRT plus carboplatin. The ORR was 34.4% for WBRT alone and 79.3% when combined with cisplatin. The OS (15.9 versus 11.3 months) and the PFS (10.2 versus 6.8 months) were significantly longer in the WBRT plus chemotherapy group when compared to the WBRT cohort. Karnofsky Performance Status scores significantly improved after WBRT plus chemotherapy compared to WBRT alone, while the combination had similar adverse reactions.
A phase I trial showed that bevacizumab combined with WBRT was safe and generated response in patients with brain metastases from solid tumors (n = 19), including breast cancer (n = 13) [ 82 ]. There was an 87.5% response rate at the highest dosing level (WBRT 30 Gy in 10 fractions and bevacizumab 15 mg/kg on days 1, 15, and 29).
Specifically, for patients with HR + BCBM, a retrospective study of concurrent radiotherapy with CDK4/6i, palbociclib (n = 34) or abemaciclib (n = 2), resulted in brain metastases local control at 12 weeks of 91.7% [ 83 ]. This outcome is provocative but there is need for prospective controlled studies to support any recommendation on the combination of radiation and CDK4/6i for patients with HR + BCBM.
Emerging therapies
Immunotherapy/antibody–drug conjugates.
Immunotherapy is not approved for metastatic HR + breast cancer (aside from rare patients with high tumor mutational burden or mismatch repair deficient cancers). A phase II (NCT02886585) study is evaluating the safety and efficacy of pembrolizumab, a checkpoint inhibitor (PD-1), in CNS metastases (brain and leptomeningeal) from multiple tumors (including breast cancer). Preliminary results from this study suggest efficacy of pembrolizumab in the treatment of leptomeningeal disease from solid tumor malignancies (n = 20, including 7 HR + /HER2- and 3 HR + /HER2 + ) [ 84 ], but results pertaining to brain metastases have yet to be published.
Recent phase I and II studies have shown positive results with trastuzumab deruxtecan, an antibody–drug conjugate linked to a topoisomerase I inhibitor in patients with HER2 low MBC [ 85 , 86 ]. A phase III trial [ 87 ] evaluated the efficacy and safety of trastuzumab deruxtecan (n = 373, HR + = 331) in HER2 low MBC patients compared to physician’s choice of chemotherapy (eribulin, capecitabine, paclitaxel, or gemcitabine) (n = 184, HR + = 163). Trastuzumab deruxtecan significantly prolonged median PFS (10.1 versus 5.4 months) and OS (23.9 versus 17.5 months) when compared to the control arm. In the trastuzumab deruxtecan and the chemotherapy cohorts, 5.4% and 4.3% of patients had brain metastases. The brain metastases ORR was 67.4% [ 88 ] suggesting that trastuzumab deruxtecan has activity in patients with HR + , HER2 low CNS metastases.
New compounds
Sacituzmab govitecan and Elacestrant received indications in HR + breast cancer in 2023 and will be studied for activity in HR + BCBM (no CNS efficacy data available to date). Multiple drugs with potential efficacy in HR + BCBM are being studied in preclinical and clinical studies. A highlight is ANG1005, which consist of three paclitaxel molecules covalently linked to Angiopep-2 and crosses the BBB via the LRP1 (low-density lipoprotein receptor-related protein 1) transport system [ 89 ]. An open-label phase II study in BCBM (n = 72, 39 HR + ) revealed an 8% intracranial ORR, better for patients with HER2 + (14%) than those with HER2 − (3%).
Another phase I study [ 90 ] evaluated the optimal dose for an AKT inhibitor (MK-2206) administered in combination with anastrozole, fulvestrant, or both in postmenopausal women with HR + /HER2 − MBC (n = 30). Nineteen patients had visceral involvement (including brain metastases). Preliminary results showed PR in 7.7% of the patients and a CBR of 36.7% and ORR rate of 15.4%. The most common adverse events were rash (33.3%), hyperglycemia (20%), hypophosphatemia (16.7%), and fatigue (10%).
Recommendations
There is no level 1 evidence based on prospective randomized clinical trials to provide guidance on systemic therapies for HR + BCBM. The current potentially effective first-line systemic therapies for HR + BCBM, (Fig. 2 ) include CDK4/6i (palbociclib, ribociclib, or abemaciclib) in combination with aromatase inhibitors, or SERDs. Potential options for second-line systemic treatments include trastuzumab deruxtecan if HER2 low , CDK4/6i rotation, a mTORC1 inhibitor, a PARP inhibitor if BRCA mutated, or other molecularly targeted inhibitors such as alpelisib (usually given with an endocrine agent). Pre-treated patients may have endocrine resistance (i.e., ESR1 mutation), thus, a personalized approach based on molecular testing may be of benefit. Upon exhaustion of targeted/endocrine therapies, chemotherapy agents such as capecitabine, trastuzumab deruxtecan, eribulin or others (with or without bevacizumab) could be an option (Table 2 ).
Expert opinions/recommendation in the area of HR + BCBM are limited since many published studies fail to disclose the receptor status or to make direct correlations between receptor status, brain metastases, and treatment response. Furthermore, at least 20% of BCBM have receptors that differ from the primary cancer [ 91 , 92 , 93 , 94 , 95 , 96 , 97 ].
Despite the advances in systemic therapies for HR + breast cancer, the treatment of brain metastases remains a major therapeutic challenge that requires a multidisciplinary approach. The contemporary recommendations for the treatment of HR + BCBM involve local therapies; maximal local control with surgery, SRS and WBRT with the option of repeated local therapy for recurrence whenever feasible [ 14 ].
Clinical trials are increasingly available for patients with BCBM (Table 1 ), but the field needs randomized clinical trials of new drug candidates that include patients with BCBM and report separately on their outcomes. Research into distinct biomarkers BCBM that could aid in early detection and improve personalized targeted therapy is needed. Screening for brain metastases in patients with MBC is not generally recommended; however, approximately 20% [ 98 ] of patients with BCBM are asymptomatic. Asymptomatic patients have less CNS metastatic burden and better outcomes than patients who are symptomatic [ 99 ]. Noninvasive techniques such as liquid biopsy presents an emerging aspect of breast cancer care that may help improve future CNS surveillance.
Survival from HR + breast cancer is improving as drugs that are more effective become available, but as patients with MBC live longer, the likelihood of CNS relapse increases. The recommendations for local therapies are robust, but systemic therapy recommendation are limited by the quality of evidence. There is urgency to study new and potentially more effective therapies in well-designed, clinical trials to improve outcomes of the growing population with breast cancer and brain metastases.
Data Availability
Not applicable
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Article PubMed Google Scholar
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872. https://doi.org/10.1200/JCO.2004.12.149
Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, Debled M, Mouret-Reynier MA, Goncalves A, Dalenc F, Delaloge S, Campone M, Augereau P, Ferrero JM, Levy C, Fumet JD, Lecouillard I, Cottu P, Petit T, Uwer L, Jouannaud C, Leheurteur M, Dieras V, Robain M, Chevrot M, Pasquier D, Bachelot T (2019) Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer 121:991–1000. https://doi.org/10.1038/s41416-019-0619-y
Article CAS PubMed PubMed Central Google Scholar
Tsukada Y, Fouad A, Pickren JW, Lane WW (1983) Central nervous system metastasis from breast carcinoma. Autopsy study Cancer 52:2349–2354. https://doi.org/10.1002/1097-0142(19831215)52:12%3c2349::aid-cncr2820521231%3e3.0.co;2-b
Article CAS PubMed Google Scholar
Khan M, Arooj S, Li R, Tian Y, Zhang J, Lin J, Liang Y, Xu A, Zheng R, Liu M, Yuan Y (2020) Tumor primary site and histology subtypes role in radiotherapeutic management of brain metastases. Front Oncol 10:781. https://doi.org/10.3389/fonc.2020.00781
Article PubMed PubMed Central Google Scholar
Van Mechelen M, Van Herck A, Punie K, Nevelsteen I, Smeets A, Neven P, Weltens C, Han S, Vanderstichele A, Floris G, Lobelle JP, Wildiers H (2020) Behavior of metastatic breast cancer according to subtype. Breast Cancer Res Treat 181:115–125. https://doi.org/10.1007/s10549-020-05597-3
Koniali L, Hadjisavvas A, Constantinidou A, Christodoulou K, Christou Y, Demetriou C, Panayides AS, Pitris C, Pattichis CS, Zamba-Papanicolaou E, Kyriacou K (2020) Risk factors for breast cancer brain metastases: a systematic review. Oncotarget 11:650–669. https://doi.org/10.18632/oncotarget.27453
Graesslin O, Abdulkarim BS, Coutant C, Huguet F, Gabos Z, Hsu L, Marpeau O, Uzan S, Pusztai L, Strom EA, Hortobagyi GN, Rouzier R, Ibrahim NK (2010) Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol 28:2032–2037. https://doi.org/10.1200/JCO.2009.24.6314
Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S, Valabrega G, Aglietta M, Montemurro F (2014) Metastatic breast cancer subtypes and central nervous system metastases. Breast 23:623–628. https://doi.org/10.1016/j.breast.2014.06.009
Kuksis M, Gao Y, Tran W, Hoey C, Kiss A, Komorowski AS, Dhaliwal AJ, Sahgal A, Das S, Chan KK, Jerzak KJ (2021) The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol 23:894–904. https://doi.org/10.1093/neuonc/noaa285
Ramakrishna N, Anders CK, Lin NU, Morikawa A, Temin S, Chandarlapaty S, Crews JR, Davidson NE, Franzoi MAB, Kirshner JJ, Krop IE, Patt DA, Perlmutter J, Giordano SH (2022) Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol 40:2636–2655. https://doi.org/10.1200/JCO.22.00520
Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, Claus EB, Lee EQ, Wen PY, Haas-Kogan DA, Alexander BM, Lin NU, Aizer AA (2017) Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol 3:1069–1077. https://doi.org/10.1001/jamaoncol.2017.0001
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson NTN, Gondi V, Jordan JT, Lassman AB, Maues J, Mohile N, Redjal N, Stevens G, Sulman E, van den Bent M, Wallace HJ, Weinberg JS, Zadeh G, Schiff D (2022) Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol 40:492–516. https://doi.org/10.1200/JCO.21.02314
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500. https://doi.org/10.1056/NEJM199002223220802
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR et al (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33:583–590. https://doi.org/10.1002/ana.410330605
Han YM, Cai G, Chai WM, Xu C, Cao L, Ou D, Chen JY, Kirova YM (2017) Radiological distribution of brain metastases and its implication for the hippocampus avoidance in whole brain radiotherapy approach. Br J Radiol 90:20170099. https://doi.org/10.1259/bjr.20170099
Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP (2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 32:3810–3816. https://doi.org/10.1200/JCO.2014.57.2909
Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, Choucair A, Fox S, Suh JH, Roberge D, Kavadi V, Bentzen SM, Mehta MP, Watkins-Bruner D, Radiation Therapy Oncology G (2013) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 15:1429–1437. https://doi.org/10.1093/neuonc/not114
Wilson TG, Robinson T, MacFarlane C, Spencer T, Herbert C, Wade L, Reed H, Braybrooke JP (2020) Treating brain metastases from breast cancer: outcomes after stereotactic radiosurgery. Clin Oncol (R Coll Radiol) 32:390–396. https://doi.org/10.1016/j.clon.2020.02.007
Ramakrishna N, Temin S, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, Giordano SH, Gonzalez-Angulo AM, Kirshner JJ, Krop I, Levinson J, Modi S, Patt DA, Perez EA, Perlmutter J, Winer EP, Lin NU (2014) Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:2100–2108. https://doi.org/10.1200/JCO.2013.54.0955
Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ, Gillin MT, Mohideen N, Hahn CA, Chang EL (2012) Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2:210–225. https://doi.org/10.1016/j.prro.2011.12.004
Grandhi R, Kondziolka D, Panczykowski D, Monaco EA 3rd, Kano H, Niranjan A, Flickinger JC, Lunsford LD (2012) Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg 117:237–245. https://doi.org/10.3171/2012.4.JNS11870
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044. https://doi.org/10.1016/S1470-2045(09)70263-3
Groenland SL, Martinez-Chavez A, van Dongen MGJ, Beijnen JH, Schinkel AH, Huitema ADR, Steeghs N (2020) Clinical Pharmacokinetics and Pharmacodynamics of the Cyclin-Dependent Kinase 4 and 6 Inhibitors Palbociclib, Ribociclib, and Abemaciclib. Clin Pharmacokinet 59:1501–1520. https://doi.org/10.1007/s40262-020-00930-x
Turner NC, Finn RS, Martin M, Im SA, DeMichele A, Ettl J, Dieras V, Moulder S, Lipatov O, Colleoni M, Cristofanilli M, Lu DR, Mori A, Giorgetti C, Iyer S, Bartlett CH, Gelmon KA (2018) Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol 29:669–680. https://doi.org/10.1093/annonc/mdx797
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, Andre F, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Petrakova K, Hart LL, Villanueva C, Chan A, Jakobsen E, Nusch A, Burdaeva O, Grischke EM, Alba E, Wist E, Marschner N, Favret AM, Yardley D, Bachelot T, Tseng LM, Blau S, Xuan F, Souami F, Miller M, Germa C, Hirawat S, O’Shaughnessy J (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738–1748. https://doi.org/10.1056/NEJMoa1609709
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Petrakova K, Blackwell KL, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Mondal S, Su F, Miller M, Elmeliegy M, Germa C, O’Shaughnessy J (2018) Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 29:1541–1547. https://doi.org/10.1093/annonc/mdy155
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martin M, Nusch A, Sonke GS, De la Cruz-Merino L, Beck JT, Pivot X, Vidam G, Wang Y, Rodriguez Lorenc K, Miller M, Taran T, Jerusalem G (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36:2465–2472. https://doi.org/10.1200/JCO.2018.78.9909
Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S, Petrakova K, Valeria Bianchi G, Martin M, Nusch A, Sonke GS, De la Cruz-Merino L, Beck JT, Ji Y, Wang C, Deore U, Chakravartty A, Zarate JP, Taran T, Fasching PA (2021) Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol 32:1015–1024. https://doi.org/10.1016/j.annonc.2021.05.353
Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V, Patt D, Wildiers H, Hudis CA, O’Shaughnessy J, Zamora E, Yardley DA, Frenzel M, Koustenis A, Baselga J (2017) MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(−) metastatic breast cancer. Clin Cancer Res 23:5218–5224. https://doi.org/10.1158/1078-0432.CCR-17-0754
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Conte P, Lu Y, Barriga S, Hurt K, Frenzel M, Johnston S, Llombart-Cussac A (2020) The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol 6:116–124. https://doi.org/10.1001/jamaoncol.2019.4782
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Tredan O, Chen SC, Manso L, Freedman OC, Garnica Jaliffe G, Forrester T, Frenzel M, Barriga S, Smith IC, Bourayou N, Di Leo A (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638–3646. https://doi.org/10.1200/JCO.2017.75.6155
Tolaney SM, Sahebjam S, Le Rhun E, Bachelot T, Kabos P, Awada A, Yardley D, Chan A, Conte P, Dieras V, Lin NU, Bear M, Chapman SC, Yang Z, Chen Y, Anders CK (2020) A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin Cancer Res 26:5310–5319. https://doi.org/10.1158/1078-0432.CCR-20-1764
Troussier I, Canova C, Klausner G (2020) Complete response of leptomeningeal carcinomatosis secondary to breast cancer. Breast 54:328–330. https://doi.org/10.1016/j.breast.2020.11.019
Radke I, von Wahlde MK, Schulke C, Tio J (2020) Ribociclib in breast cancer brain metastases: a case report. Breast Care (Basel) 15:543–547. https://doi.org/10.1159/000504405
Schlam I, Tolaney SM (2021) Is there a role for CDK 4/6 inhibitors in breast cancer brain metastases? Oncotarget 12:873–875. https://doi.org/10.18632/oncotarget.27904
Azcoitia I, Mendez P, Garcia-Segura LM (2021) Aromatase in the human brain. Androg Clin Res Ther 2:189–202. https://doi.org/10.1089/andro.2021.0007
Madhup R, Kirti S, Bhatt ML, Srivastava PK, Srivastava M, Kumar S (2006) Letrozole for brain and scalp metastases from breast cancer—a case report. Breast 15:440–442. https://doi.org/10.1016/j.breast.2005.07.006
Goyal S, Puri T, Julka PK, Rath GK (2008) Excellent response to letrozole in brain metastases from breast cancer. Acta Neurochir (Wien) 150:613–614. https://doi.org/10.1007/s00701-008-1576-z . ( discussion 614-615 )
Ito K, Ito T, Okada T, Watanabe T, Gomi K, Kanai T, Mochizuki Y, Amano J (2009) A case of brain metastases from breast cancer that responded to anastrozole monotherapy. Breast J 15:435–437. https://doi.org/10.1111/j.1524-4741.2009.00756.x
Almajed MM, Esfahani K, Pelmus M, Panasci L (2016) Complete response and long-term survival of leptomeningeal carcinomatosis from breast cancer with maintenance endocrine therapy. BMJ Case Rep. https://doi.org/10.1136/bcr-2016-215525
Saha P, Amico AL, Olopade OI (2016) Long-term disease-free survival in a young patient with hormone receptor-positive breast cancer and oligometastatic disease in the brain. Clin Breast Cancer 16:e61-63. https://doi.org/10.1016/j.clbc.2016.02.013
Turner NC, Swift C, Kilburn L, Fribbens C, Beaney M, Garcia-Murillas I, Budzar AU, Robertson JFR, Gradishar W, Piccart M, Schiavon G, Bliss JM, Dowsett M, Johnston SRD, Chia SK (2020) ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: a combined analysis of the phase III SoFEA and EFECT trials. Clin Cancer Res 26:5172–5177. https://doi.org/10.1158/1078-0432.CCR-20-0224
Bergen ES, Berghoff AS, Medjedovic M, Rudas M, Fitzal F, Bago-Horvath Z, Dieckmann K, Mader RM, Exner R, Gnant M, Zielinski CC, Steger GG, Preusser M, Bartsch R (2019) Continued endocrine therapy is associated with improved survival in patients with breast cancer brain metastases. Clin Cancer Res 25:2737–2744. https://doi.org/10.1158/1078-0432.CCR-18-1968
Howell A, Osborne CK, Morris C, Wakeling AE (2000) ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer 89:817–825. https://doi.org/10.1002/1097-0142(20000815)89:4%3c817::aid-cncr14%3e3.0.co;2-6
Wang Q, Sun B, Liu C, Shi S, Ding L, Liu J, Wu S (2019) Brain metastases from breast cancer may respond to endocrine therapy: report of two cases. Onco Targets Ther 12:1389–1393. https://doi.org/10.2147/OTT.S188143
Rusz O, Koszo R, Dobi A, Csenki M, Valicsek E, Nikolenyi A, Uhercsak G, Cserhati A, Kahan Z (2018) Clinical benefit of fulvestrant monotherapy in the multimodal treatment of hormone receptor and HER2 positive advanced breast cancer: a case series. Onco Targets Ther 11:5459–5463. https://doi.org/10.2147/OTT.S170736
Robertson JFR, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, Shparyk Y, Cardona-Huerta S, Cheung KL, Philco-Salas MJ, Ruiz-Borrego M, Shao Z, Noguchi S, Rowbottom J, Stuart M, Grinsted LM, Fazal M, Ellis MJ (2016) Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 388:2997–3005. https://doi.org/10.1016/S0140-6736(16)32389-3
Jones RH, Casbard A, Carucci M, Cox C, Butler R, Alchami F, Madden TA, Bale C, Bezecny P, Joffe J, Moon S, Twelves C, Venkitaraman R, Waters S, Foxley A, Howell SJ (2020) Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 21:345–357. https://doi.org/10.1016/S1470-2045(19)30817-4
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529. https://doi.org/10.1056/NEJMoa1109653
Van Swearingen AED, Siegel MB, Deal AM, Sambade MJ, Hoyle A, Hayes DN, Jo H, Little P, Dees EC, Muss H, Jolly T, Zagar TM, Patel N, Miller CR, Parker JS, Smith JK, Fisher J, Shah N, Nabell L, Nanda R, Dillon P, Abramson V, Carey LA, Anders CK (2018) LCCC 1025: a phase II study of everolimus, trastuzumab, and vinorelbine to treat progressive HER2-positive breast cancer brain metastases. Breast Cancer Res Treat 171:637–648. https://doi.org/10.1007/s10549-018-4852-5
Shen XB, Li GL, Zheng YB, Chen ZH, Cao WM, Wang XJ, Shao XY (2021) Combined everolimus and endocrine therapy in advanced HR-positive, HER2-negative Chinese breast cancer patients: a retrospective study. Ann Transl Med 9:1334. https://doi.org/10.21037/atm-21-3840
Batalini F, Moulder SL, Winer EP, Rugo HS, Lin NU, Wulf GM (2020) Response of brain metastases from PIK3CA-mutant breast cancer to alpelisib. JCO Precis Oncol. https://doi.org/10.1200/PO.19.00403
Miller J, Armgardt E, Svoboda A (2022) The efficacy and safety of alpelisib in breast cancer: a real-world analysis. J Oncol Pharmacy Pract. https://doi.org/10.1177/10781552221096413
Article Google Scholar
Song YK, Kim MJ, Kim MS, Lee JH, Chung SJ, Song JS, Chae YJ, Lee KR (2022) Role of the efflux transporters Abcb1 and Abcg2 in the brain distribution of olaparib in mice. Eur J Pharm Sci 173:106177. https://doi.org/10.1016/j.ejps.2022.106177
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377:523–533. https://doi.org/10.1056/NEJMoa1706450
Robson M, Ruddy KJ, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Li W, Tung N, Armstrong A, Delaloge S, Bannister W, Goessl C, Degboe A, Hettle R, Conte P (2019) Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Cancer 120:20–30. https://doi.org/10.1016/j.ejca.2019.06.023
Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, Shah PD, Ballinger TJ, Yang ES, Vinayak S, Melisko M, Brufsky A, DeMeo M, Jenkins C, Domchek S, D’Andrea A, Lin NU, Hughes ME, Carey LA, Wagle N, Wulf GM, Krop IE, Wolff AC, Winer EP, Garber JE (2020) TBCRC 048: Phase II study of Olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 38:4274–4282. https://doi.org/10.1200/JCO.20.02151
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676. https://doi.org/10.1056/NEJMoa072113
Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G (2010) Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28:3239–3247. https://doi.org/10.1200/JCO.2008.21.6457
Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O’Shaughnessy J (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29:1252–1260. https://doi.org/10.1200/JCO.2010.28.0982
Miles D, Cameron D, Bondarenko I, Manzyuk L, Alcedo JC, Lopez RI, Im SA, Canon JL, Shparyk Y, Yardley DA, Masuda N, Ro J, Denduluri N, Hubeaux S, Quah C, Bais C, O’Shaughnessy J (2017) Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): a double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer 70:146–155. https://doi.org/10.1016/j.ejca.2016.09.024
Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS (2011) RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29:4286–4293. https://doi.org/10.1200/JCO.2010.34.1255
Leone JP, Emblem KE, Weitz M, Gelman RS, Schneider BP, Freedman RA, Younger J, Pinho MC, Sorensen AG, Gerstner ER, Harris G, Krop IE, Morganstern D, Sohl J, Hu J, Kasparian E, Winer EP, Lin NU (2020) Phase II trial of carboplatin and bevacizumab in patients with breast cancer brain metastases. Breast Cancer Res 22:131. https://doi.org/10.1186/s13058-020-01372-w
Lu YS, Chen TW, Lin CH, Yeh DC, Tseng LM, Wu PF, Rau KM, Chen BB, Chao TC, Huang SM, Huang CS, Shih TT, Cheng AL, Taiwan Breast Cancer C (2015) Bevacizumab preconditioning followed by Etoposide and Cisplatin is highly effective in treating brain metastases of breast cancer progressing from whole-brain radiotherapy. Clin Cancer Res 21:1851–1858. https://doi.org/10.1158/1078-0432.CCR-14-2075
Lin NU, Gaspar LE, Soffietti R (2017) Breast cancer in the central nervous system: multidisciplinary considerations and management. Am Soc Clin Oncol Educ Book 37:45–56. https://doi.org/10.14694/EDBK_175338 . ( 10.1200/EDBK_175338 )
Rosner D, Nemoto T, Lane WW (1986) Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer 58:832–839. https://doi.org/10.1002/1097-0142(19860815)58:4%3c832::aid-cncr2820580404%3e3.0.co;2-w
Rivera E, Meyers C, Groves M, Valero V, Francis D, Arun B, Broglio K, Yin G, Hortobagyi GN, Buchholz T (2006) Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer 107:1348–1354. https://doi.org/10.1002/cncr.22127
Wang ML, Yung WK, Royce ME, Schomer DF, Theriault RL (2001) Capecitabine for 5-fluorouracil-resistant brain metastases from breast cancer. Am J Clin Oncol 24:421–424. https://doi.org/10.1097/00000421-200108000-00026
Lassman AB, Abrey LE, Shah GD, Panageas KS, Begemann M, Malkin MG, Raizer JJ (2006) Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol 78:255–260. https://doi.org/10.1007/s11060-005-9044-6
Vinolas N, Graus F, Mellado B, Caralt L, Estape J (1997) Phase II trial of cisplatinum and etoposide in brain metastases of solid tumors. J Neurooncol 35:145–148. https://doi.org/10.1023/a:1005835430489
Bailleux C, Eberst L, Bachelot T (2021) Treatment strategies for breast cancer brain metastases. Br J Cancer 124:142–155. https://doi.org/10.1038/s41416-020-01175-y
Mata JF, Garcia-Manteiga JM, Lostao MP, Fernandez-Veledo S, Guillen-Gomez E, Larrayoz IM, Lloberas J, Casado FJ, Pastor-Anglada M (2001) Role of the human concentrative nucleoside transporter (hCNT1) in the cytotoxic action of 5[Prime]-deoxy-5-fluorouridine, an active intermediate metabolite of capecitabine, a novel oral anticancer drug. Mol Pharmacol 59:1542–1548. https://doi.org/10.1124/mol.59.6.1542
Ekenel M, Hormigo AM, Peak S, Deangelis LM, Abrey LE (2007) Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol 85:223–227. https://doi.org/10.1007/s11060-007-9409-0
Boogerd W, Dalesio O, Bais EM, van der Sande JJ (1992) Response of brain metastases from breast cancer to systemic chemotherapy. Cancer 69:972–980. https://doi.org/10.1002/1097-0142(19920215)69:4%3c972::aid-cncr2820690423%3e3.0.co;2-p
Franciosi V, Cocconi G, Michiara M, Di Costanzo F, Fosser V, Tonato M, Carlini P, Boni C, Di Sarra S (1999) Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer 85:1599–1605
Trudeau ME, Crump M, Charpentier D, Yelle L, Bordeleau L, Matthews S, Eisenhauer E (2006) Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada—Clinical Trials Group (NCIC-CTG). Ann Oncol 17:952–956. https://doi.org/10.1093/annonc/mdl056
Linot B, Campone M, Augereau P, Delva R, Abadie-Lacourtoisie S, Nebout-Mesgouez N, Capitain O (2014) Use of liposomal doxorubicin-cyclophosphamide combination in breast cancer patients with brain metastases: a monocentric retrospective study. J Neurooncol 117:253–259. https://doi.org/10.1007/s11060-014-1378-5
Fabi A, Terrenato I, Vidiri A, Villani V, Tanzilli A, Airoldi M, Pedani F, Magri V, Palleschi M, Donadio M, Catania G, Nistico C, Carapella C, Ruda R, Pace A, Maschio M, Telera S, Cognetti F, Aino (2021) Eribulin in brain metastases of breast cancer: outcomes of the EBRAIM prospective observational trial. Future Oncol 17:3445–3456. https://doi.org/10.2217/fon-2021-0300
Kai Xu YH, Yao J, Zhong Xu, He X (2021) Whole-brain radiotherapy and chemotherapy in the treatment of patients with breast cancer and brain metastases. Int J Clin Exp Med 14:1250–1257
Google Scholar
Levy C, Allouache D, Lacroix J, Dugue AE, Supiot S, Campone M, Mahe M, Kichou S, Leheurteur M, Hanzen C, Dieras V, Kirova Y, Campana F, Le Rhun E, Gras L, Bachelot T, Sunyach MP, Hrab I, Geffrelot J, Gunzer K, Constans JM, Grellard JM, Clarisse B, Paoletti X (2014) REBECA: a phase I study of bevacizumab and whole-brain radiation therapy for the treatment of brain metastasis from solid tumours. Ann Oncol 25:2351–2356. https://doi.org/10.1093/annonc/mdu465
Kim KN, Shah P, Clark A, Freedman GM, Dastgheyb S, Barsky AR, Dreyfuss AD, Taunk NK (2021) Safety of cyclin-dependent kinase4/6 inhibitor combined with palliative radiotherapy in patients with metastatic breast cancer. Breast 60:163–167. https://doi.org/10.1016/j.breast.2021.10.001
Brastianos PK, Lee EQ, Cohen JV, Tolaney SM, Lin NU, Wang N, Chukwueke U, White MD, Nayyar N, Kim A, Alvarez-Breckenridge C, Krop I, Mahar MK, Bertalan MS, Shaw B, Mora JL, Goss N, Subramanian M, Nayak L, Dietrich J, Forst DA, Nahed BV, Batchelor TT, Shih HA, Gerstner ER, Moy B, Lawrence D, Giobbie-Hurder A, Carter SL, Oh K, Cahill DP, Sullivan RJ (2020) Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med 26:1280–1284. https://doi.org/10.1038/s41591-020-0918-0
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, Hirai T, Atsumi R, Nakada T, Hayakawa I, Abe Y, Agatsuma T (2016) DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 22:5097–5108. https://doi.org/10.1158/1078-0432.CCR-15-2822
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T (2016) Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 107:1039–1046. https://doi.org/10.1111/cas.12966
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, Lee KS, Niikura N, Park YH, Xu B, Wang X, Gil-Gil M, Li W, Pierga JY, Im SA, Moore HCF, Rugo HS, Yerushalmi R, Zagouri F, Gombos A, Kim SB, Liu Q, Luo T, Saura C, Schmid P, Sun T, Gambhire D, Yung L, Wang Y, Singh J, Vitazka P, Meinhardt G, Harbeck N, Cameron DA, Investigators DE-BT (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9–20. https://doi.org/10.1056/NEJMoa2203690
Hurvitz S ea (2021) Trastuzumab deruxtecan (T-DXd; DS-8201a) vs trastuzumab emtansine (T-DM1) in patients with HER2+ metastatic breast cancer., 2021 San Antonio Breast Cancer Symposium
Kumthekar P, Tang SC, Brenner AJ, Kesari S, Piccioni DE, Anders C, Carrillo J, Chalasani P, Kabos P, Puhalla S, Tkaczuk K, Garcia AA, Ahluwalia MS, Wefel JS, Lakhani N, Ibrahim N (2020) ANG1005, a brain-penetrating peptide-drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clin Cancer Res 26:2789–2799. https://doi.org/10.1158/1078-0432.CCR-19-3258
Ma CX, Sanchez C, Gao F, Crowder R, Naughton M, Pluard T, Creekmore A, Guo Z, Hoog J, Lockhart AC, Doyle A, Erlichman C, Ellis MJ (2016) A phase I study of the AKT inhibitor MK-2206 in combination with hormonal therapy in postmenopausal women with estrogen receptor-positive metastatic breast cancer. Clin Cancer Res 22:2650–2658. https://doi.org/10.1158/1078-0432.CCR-15-2160
Bachmann C, Grischke EM, Fehm T, Staebler A, Schittenhelm J, Wallwiener D (2013) CNS metastases of breast cancer show discordant immunohistochemical phenotype compared to primary. J Cancer Res Clin Oncol 139:551–556. https://doi.org/10.1007/s00432-012-1358-0
Bachmann C, Grischke EM, Staebler A, Schittenhelm J, Wallwiener D (2013) Receptor change-clinicopathologic analysis of matched pairs of primary and cerebral metastatic breast cancer. J Cancer Res Clin Oncol 139:1909–1916. https://doi.org/10.1007/s00432-013-1511-4
Kaidar-Person O, Meattini I, Jain P, Bult P, Simone N, Kindts I, Steffens R, Weltens C, Navarria P, Belkacemi Y, Lopez-Guerra J, Livi L, Baumert BG, Vieites B, Limon D, Kurman N, Ko K, Yu JB, Chiang V, Poortmans P, Zagar T (2018) Discrepancies between biomarkers of primary breast cancer and subsequent brain metastases: an international multicenter study. Breast Cancer Res Treat 167:479–483. https://doi.org/10.1007/s10549-017-4526-8
Sava A, Costea CF, Vatavu R, Grigore M, Turliuc MD, Dumitrescu GF, Eva L, Motoc AGM, Stan CI, Gavril LC, Scripcariu SI (2021) Brain metastases originating in breast cancer: clinical-pathological analysis and immunohistochemical profile. Rom J Morphol Embryol 62:435–444. https://doi.org/10.47162/RJME.62.2.09
Timmer M, Werner JM, Rohn G, Ortmann M, Blau T, Cramer C, Stavrinou P, Krischek B, Mallman P, Goldbrunner R (2017) Discordance and conversion rates of progesterone-, estrogen-, and HER2/neu-receptor status in primary breast cancer and brain metastasis mainly triggered by hormone therapy. Anticancer Res 37:4859–4865. https://doi.org/10.21873/anticanres.11894
Kotecha R, Tonse R, Rubens M, McDermott MW, Odia Y, Appel H, Mehta MP (2021) Systematic review and meta-analysis of breast cancer brain metastasis and primary tumor receptor expression discordance. Neurooncol Adv 3:vdab010. https://doi.org/10.1093/noajnl/vdab010
Priedigkeit N, Hartmaier RJ, Chen Y, Vareslija D, Basudan A, Watters RJ, Thomas R, Leone JP, Lucas PC, Bhargava R, Hamilton RL, Chmielecki J, Puhalla SL, Davidson NE, Oesterreich S, Brufsky AM, Young L, Lee AV (2017) Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol 3:666–671. https://doi.org/10.1001/jamaoncol.2016.5630
Niikura N, Hayashi N, Masuda N, Takashima S, Nakamura R, Watanabe K, Kanbayashi C, Ishida M, Hozumi Y, Tsuneizumi M, Kondo N, Naito Y, Honda Y, Matsui A, Fujisawa T, Oshitanai R, Yasojima H, Tokuda Y, Saji S, Iwata H (2014) Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat 147:103–112. https://doi.org/10.1007/s10549-014-3090-8
Laakmann E, Witzel I, Neunhoffer T, Weide R, Schmidt M, Park-Simon TW, Mobus V, Mundhenke C, Polasik A, Lubbe K, Hesse T, Riecke K, Thill M, Fasching PA, Denkert C, Fehm T, Nekljudova V, Rey J, Loibl S, Muller V (2020) Characteristics and clinical outcome of breast cancer patients with asymptomatic brain metastases. Cancers (Basel). https://doi.org/10.3390/cancers12102787
Download references
Acknowledgements
The authors thank the University of Virginia Summer Medical Research Internship Program for support.
The authors declare that a support was received from the University of Virginia Summer Medical Research Internship Program. No other funds, grants or other support were received during the preparation of the manuscript. This research was supported by Office of Extramural Research, National Institutes of Health (Grant 2P30CA044579-26, 2P30CA044579-26).
Author information
Authors and affiliations.
Ponce Health Sciences University, Ponce, PR, 00716, USA
Shirley Jusino
Division of Neuro-Oncology, Department of Neurology, University of Virginia, Charlottesville, VA, 22908, USA
Camilo E. Fadul
Division of Hematology/Oncology, University of Virginia, Charlottesville, VA, 22908, USA
Patrick Dillon
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by SJ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Patrick Dillon .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 31 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Jusino, S., Fadul, C.E. & Dillon, P. Systematic review of the management of brain metastases from hormone receptor positive breast cancer. J Neurooncol 162 , 45–57 (2023). https://doi.org/10.1007/s11060-023-04276-9
Download citation
Received : 25 January 2023
Accepted : 23 February 2023
Published : 08 March 2023
Issue Date : March 2023
DOI : https://doi.org/10.1007/s11060-023-04276-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Brain metastases
- Find a journal
- Publish with us
- Track your research

BrainMetShare
Dataset description.

A brain MRI dataset to develop and test improved methods for detection and segmentation of brain metastases. The dataset includes 156 whole brain MRI studies, including high-resolution, multi-modal pre- and post-contrast sequences in patients with at least 1 brain metastasis accompanied by ground-truth segmentations by radiologists.
Canonical URL
https://stanfordaimi.azurewebsites.net/datasets/ae0182f1-d5b6-451a-8177-d1f39f01…
Full Description
About 2% of all patients with a primary neoplasm will be diagnosed with brain metastases at the time of their initial diagnosis. As we are getting better at controlling primary cancers, even more patients eventually present with such lesions. Given that brain metastases are often quite treatable with surgery or stereotactic radiosurgery, accurate segmentation of brain metastases is a common job for radiologists. Having algorithms to help detect and localize brain metastasis could relieve radiologists from this tedious but crucial task. Given the success of recent AI techniques on other segmentation tasks, we have put together this gold-standard, labeled MRI dataset to allow for the development and testing of new techniques in these patients with the hopes of spurring research in this area.
Dataset Details
This is a dataset of 156 pre- and post-contrast whole brain MRI studies in patients with at least 1 cerebral metastasis. Mean patient age was 63±12 years (range: 29–92 years). Primary malignancies included lung (n = 99), breast (n = 33), melanoma (n = 7), genitourinary (n = 7), gastrointestinal (n = 5), and miscellaneous cancers (n = 5). The specific primary malignancies for each case are included in an excel sheet that can be downloaded with the data. 64 (41%) had 1–3 metastases, 47 (30%) had 4–10 metastases, and 45 (29%) had >10 metastases. Lesion sizes varied from 2 mm to over 4 cm and were scattered in every region of the brain parenchyma, i.e., the supratentorial and infratentorial regions, as well as the cortical and subcortical structures. It includes 4 different 3D sequences (T1 spin-echo pre-contrast, T1 spin-echo post-contrast, T1 gradient-echo post (using an IR-prepped FSPGR sequence), T2 FLAIR post) in the axial plane, co-registered to each other, resampled to 256 x 256 pixels. The nominal in-plane resolution is 0.94 mm and the through-plane resolution is 1.0 mm. Standard dose (0.1 mmol/kg) gadolinium contrast agents were used for all cases. All the images have been skull-stripped by using the Brain Extraction Tool (BET) (Smith SM. Fast robust automated brain extraction. Hum Brain Map. 2002;17:143–155). The brain masks were generated from the precontrast T1-weighted 3D CUBE imaging series using the nordicICE software package (NordicNeuroLab, Bergen, Norway) and propagated to the other sequences.
Assignment of Labels
For 105 cases, we include radiologist-drawn segmentations of the metastatic lesions, stored in folder ‘mets_stanford_release_train’. The segmentations were based on the T1 gradient-echo post-contrast images. The remaining 51 cases are unlabeled and stored in ‘mets_stanford_release_test’. There are 5 folders for each subject in the training group – folder ‘0’ contains T1 gradient-echo post images; folder ‘1’ contains T1 spin-echo pre images; folder ‘2’ contains T1 spin-echo post images; folder ‘3’ contains T2 FLAIR post images; folder ‘seg’ contains a binary mask of the segmented metastases (0, 255). There are 4 folders for each subject in the testing group, which are labelled identically, except for the absence of folder ‘seg’.
Additional Information
More detailed information on this dataset and the Stanford group’s initial performance on this data set can be found in Grøvik et al., Deep Learning Enables Automatic Detection and Segmentation of Brain Metastases on Multisequence MRI, JMRI 2019; 51(1):175-182.
We would like to thank the team involved with labeling and preparing the data and for checking it for potential PHI: Darvin Yi, Endre Grovik, Elizabeth Tong, Michael Iv, Daniel Rubin, Greg Zaharchuk, and Ghiam Yamin, and the Division of Neuroimaging at Stanford for supporting this project.
Grøvik et al., Deep Learning Enables Automatic Detection and Segmentation of Brain Metastases on Multisequence MRI, JMRI 2019; 51(1):175-182 also available on ArXiv ( https://arxiv.org/abs/1903.07988 ).

Terms & Conditions
Stanford university school of medicine brain mets dataset research use agreement.
By registering for downloads from Brain Mets Dataset, you are agreeing to this Research Use Agreement, as well as to the Terms of Use of the Stanford University School of Medicine website as posted and updated periodically at http://www.stanford.edu/site/terms/ .
1. Permission is granted to view and use Brain Mets Dataset without charge for personal, non-commercial research purposes only. Any commercial use, sale, or other monetization is prohibited.
2. Other than the rights granted herein, the Stanford University School of Medicine (“School of Medicine”) retains all rights, title, and interest in the Brain Mets Dataset.
3. You may make a verbatim copy of the Brain Mets Dataset for personal, non-commercial research use as permitted in this Research Use Agreement. If another user within your organization wishes to use the Brain Mets Dataset, they must register as an individual user and comply with all the terms of this Research Use Agreement.
4. YOU MAY NOT DISTRIBUTE, PUBLISH, OR REPRODUCE A COPY of any portion or all of the Brain Mets Dataset to others without specific prior written permission from the School of Medicine.
5. YOU MAY NOT SHARE THE DOWNLOAD LINK to the Brain Mets dataset to others. If another user within your organization wishes to use the Brain Mets Dataset, they must register as an individual user and comply with all the terms of this Research Use Agreement.
6. You must not modify, reverse engineer, decompile, or create derivative works from the Brain Mets Dataset. You must not remove or alter any copyright or other proprietary notices in the Brain Mets Dataset.
7. The Brain Mets Dataset has not been reviewed or approved by the Food and Drug Administration, and is for non-clinical, Research Use Only. In no event shall data or images generated through the use of the Brain Mets Dataset be used or relied upon in the diagnosis or provision of patient care.
8. THE Brain Mets DATASET IS PROVIDED "AS IS," AND STANFORD UNIVERSITY AND ITS COLLABORATORS DO NOT MAKE ANY WARRANTY, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE, NOR DO THEY ASSUME ANY LIABILITY OR RESPONSIBILITY FOR THE USE OF THIS Brain Mets DATASET.
9. You will not make any attempt to re-identify any of the individual data subjects. Re-identification of individuals is strictly prohibited. Any re-identification of any individual data subject shall be immediately reported to the School of Medicine.
10. Any violation of this Research Use Agreement or other impermissible use shall be grounds for immediate termination of use of this Brain Mets Dataset. In the event that the School of Medicine determines that the recipient has violated this Research Use Agreement or other impermissible use has been made, the School of Medicine may direct that the undersigned data recipient immediately return all copies of the Brain Mets Dataset and retain no copies thereof even if you did not cause the violation or impermissible use.
In consideration for your agreement to the terms and conditions contained here, Stanford grants you permission to view and use the Brain Mets Dataset for personal, non-commercial research. You may not otherwise copy, reproduce, retransmit, distribute, publish, commercially exploit or otherwise transfer any material.
Limitation of Use
You may use the Brain Mets Dataset for legal purposes only.
You agree to indemnify and hold Stanford harmless from any claims, losses or damages, including legal fees, arising out of or resulting from your use of the Brain Mets Dataset or your violation or role in violation of these Terms. You agree to fully cooperate in Stanford’s defense against any such claims. These Terms shall be governed by and interpreted in accordance with the laws of California.
DOWNLOAD DATASET
- Open access
- Published: 03 April 2024
Mesothelin promotes brain metastasis of non-small cell lung cancer by activating MET
- Shengkai Xia 1 na1 ,
- Wenzhe Duan 1 na1 ,
- Mingxin Xu 1 na1 ,
- Mengqi Li 1 ,
- Mengyi Tang 1 ,
- Song Wei 2 ,
- Manqing Lin 1 ,
- Encheng Li 1 ,
- Wenwen Liu 1 , 3 &
- Qi Wang 1 , 3
Journal of Experimental & Clinical Cancer Research volume 43 , Article number: 103 ( 2024 ) Cite this article
434 Accesses
1 Altmetric
Metrics details
Brain metastasis (BM) is common among cases of advanced non-small cell lung cancer (NSCLC) and is the leading cause of death for these patients. Mesothelin (MSLN), a tumor-associated antigen expressed in many solid tumors, has been reported to be involved in the progression of multiple tumors. However, its potential involvement in BM of NSCLC and the underlying mechanism remain unknown.
The expression of MSLN was validated in clinical tissue and serum samples using immunohistochemistry and enzyme-linked immunosorbent assay. The ability of NSCLC cells to penetrate the blood-brain barrier (BBB) was examined using an in vitro Transwell model and an ex vivo multi-organ microfluidic bionic chip. Immunofluorescence staining and western blotting were used to detect the disruption of tight junctions. In vivo BBB leakiness assay was performed to assess the barrier integrity. MET expression and activation was detected by western blotting. The therapeutic efficacy of drugs targeting MSLN (anetumab) and MET (crizotinib/capmatinib) on BM was evaluated in animal studies.
MSLN expression was significantly elevated in both serum and tumor tissue samples from NSCLC patients with BM and correlated with a poor clinical prognosis. MSLN significantly enhanced the brain metastatic abilities of NSCLC cells, especially BBB extravasation. Mechanistically, MSLN facilitated the expression and activation of MET through the c-Jun N-terminal kinase (JNK) signaling pathway, which allowed tumor cells to disrupt tight junctions and the integrity of the BBB and thereby penetrate the barrier. Drugs targeting MSLN (anetumab) and MET (crizotinib/capmatinib) effectively blocked the development of BM and prolonged the survival of mice.
Conclusions
Our results demonstrate that MSLN plays a critical role in BM of NSCLC by modulating the JNK/MET signaling network and thus, provides a potential novel therapeutic target for preventing BM in NSCLC patients.
Introduction
Lung cancer is one of the most lethal and aggressive malignancies. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of the total incidence of lung cancer and adenocarcinoma is the most common pathological type of NSCLC [ 1 ]. Approximately 50% of NSCLC patients eventually develop brain metastasis (BM), and the median survival of lung cancer patients with brain metastasis is only 4-6 months [ 2 , 3 ]. The identification of oncogenic driver gene alterations has refined the staging of NSCLC, and targeted drugs against these oncogenes have dramatically improved the treatment outcomes. MET is one of the most common oncogenes in NSCLC. It encodes MET, also known as hepatocyte growth factor receptor (HGFR), which is characterized as a high affinity transmembrane receptor tyrosine kinase (RTK). Its activation in a phosphorylated state (p-MET) has been shown to play a critical role in NSCLC tumor progression and invasion [ 4 ]. Inhibition of p-MET significantly reduces the cellular activity and aggressiveness of NSCLC cells, thereby significantly reducing the incidence of BM [ 5 ]. Capmatinib, a selective MET inhibitor which was approved by the Food and Drug Administration (FDA) in 2020, has shown activity in malignancies with MET activation including the advanced NSCLC [ 6 , 7 ]. It is worth noting that crizotinib, which was approved by the FDA in 2016, although primarily indicated for the anaplastic lymphoma kinase (ALK)-rearranged lung cancer in clinical application [ 8 ], also has an inhibitory effect on MET. However, their specific therapeutic effects on NSCLC BM need to be further clarified.
The blood-brain barrier (BBB), which is composed of a network of closely opposed endothelial cells in the cerebral capillaries and characterized by the presence of continuous tight junctions (TJs), serves as a barrier between the peripheral circulatory system and neural tissue [ 9 ]. The “opening” of TJs by tumor cells in order to penetrate the BBB is the decisive rate-limiting step in the development of BM. However, the underlying mechanisms remain largely unknown.
Mesothelin (MSLN) is a tumor differentiation antigen that has recently been found to be overexpressed in many types of solid tumors, including lung cancer [ 10 , 11 , 12 ]. Abnormal MSLN expression promotes tumor development by inducing tumor cell proliferation, anti-apoptosis and metastasis [ 13 , 14 , 15 ]. Targeted therapies against MSLN have showed antitumor activaties in MSLN-positive tumors [ 16 , 17 ]. In lung cancer, MSLN promotes epithelial-mesenchymal transition (EMT) and stemness of tumor cells, tumorwhich may facilitate the occurrence of BM [ 18 ]. Recently, MSLN-specific cellular immune responses were identified in the blood of patients with BM and regarded as a predictor for survival, which indicates the involvement of MSLN in BM [ 19 ].
In the present study, we determined the high expression of MSLN and its promotive role in BM of NSCLC, and revealed the underlying mechanism that MSLN promotes tumor cell extravasation across the BBB by facilitating the expression and activation of MET through the c-Jun N-terminal kinase (JNK) signalling pathway. Our results also showed that targeting MSLN with anetumab or MET with crizotinib or capmatinib effectively prevents the development of BM in vivo .
Cell culture and drugs
Human bronchial epithelial cells (16HBE cells), human lung fibroblasts (HFL1 cells), human monocyte cells (THP-1 cells), normal bronchial epithelial cells (BEAS-2B cells) and lung cancer cell lines (H1299, H2030, PC9, H1975, H460, and H226 cells) were purchased from the Chinese Academy of Medical Sciences (Beijing, China). The brain metastatic lung cancer cell line H2030-BrM and PC9-BrM was generated by injecting H2030 cells and PC9 cells into the left ventricle of immunodeficient mice and isolating the metastatic cells from harvested areas of brain metastases. Human lung microvascular endothelial cells (hPMECs), human brain microvascular endothelial cells (hBMVECs) and human astrocytes (HA-1800) were purchased from Sciencell (Sciencell, USA) and cultured in the appropriate medium recommended by the manufacturer. The above cell types were cultured at 37°C in humidified air with 5% CO 2 . The different cell types were authenticated by short tandem repeat profiling and tested for mycoplasma contamination.
JNK-IN-8 (HY-13319), Crizotinib (HY-50878), Anetumab (HY-P99352) and Capmatinib (HY-13404) were purchased from MedChemexpress (USA).
SDS-PAGE and western blot analysis
Cells were lysed in a mixture of radioimmunoprecipitation assay (RIPA) protein lysis buffer (Thermo Scientific) containing the protease inhibitor phenylmethylsulfonyl fluoride (PMSF, Beyotime) and a phosphatase inhibitor cocktail (Sigma-Aldrich) at 4°C for 45 min. The BCA Protein Quantification Kit (Thermo Fisher Scientific) was used to measure protein concentrations. Proteins (30 μg/lane) were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels and transferred to nitrocellulose membranes (Millipore, USA). Each membrane was blocked with protein-free rapid blocking buffer (EpiZyme, Shanghai) for 15 min and then incubated overnight at 4°C with the following antibodies: anti-E-cadherin (1:500, 13-1700, Invitrogen), anti-N-cadherin (1:200, sc-393933, Santa Cruz Biotechnology), anti-Slug (1:500, ab27568, Abcam), anti-matrix metalloproteinase 7 (MMP7, 1:1000, ab205525, Abcam), anti-MSLN (1:3000, ab133489, Abcam), anti-glyceraldehyde phosphate dehydrogenase (GAPDH, 1:5000, 10494-1-AP, Proteintech), anti-cleaved poly(ADP-ribose) polymerase (PARP, 1:1000, #5625, Cell Signaling Technology), anti-cleaved caspase-3 (1:1000, #9661, Cell Signaling Technology), anti-vascular endothelial (VE)-cadherin (1:1000, ab205336, Abcam), anti-junctional adhesion molecule (JAM)-A (1:1000, ab269948, Abcam), anti-claudin 5 (1:1000, ab131259, Abcam), anti-phosphorylated (p)-MET (1:5000, ab68141, Abcam), anti-MET (ab51067, 1:5000, Abcam), anti-p-JNK (1:1000, #4668, Cell Signaling Technology), and anti-t-JNK (1:1000, #9252, Cell Signaling Technology). The next day, the membranes were washed with Tris-buffered saline with Tween 20 (TBST) for 8 min at room temperature twice and then incubated with secondary antibodies (1:5000, SA00001-2, Proteintech; 1:5000, SA00001-1, Proteintech) for 1 h at room temperature. The membranes were then washed three times with TBST for 8 min each, followed by scanning and visualization of the immunoreactivity by enhanced chemiluminescence (ECL, Advansta). Protein expression in three independent experiments was quantified using ImageJ software (National Institutes of Health, USA).
Clinical samples
Tissue samples from 70 NSCLC patients and serum samples from 154 participants (untreated patients with NSCLC or primary brain tumor and healthy volunteers) were collected from the Second Affiliated Hospital of Dalian Medical University, Dalian, China. The diagnosis of NSCLC and primary brain tumor was confirmed by pathology (surgical resection and/or biopsy). All patients with advanced NSCLC and primary brain tumors completed baseline brain MRI examinations at the time of initial diagnosis and before receiving anti-tumor therapy. Written informed consent was obtained from all participants. This study was approved by the Ethics Review Committee of the Second Hospital of Dalian Medical University (2020-020). In addition, the information of 478 lung cancer patients used by the GEPIA database ( http://gepia.cancer-pku.cn/ ) was obtained from the TCGA database (Table S 1 ) [ 20 ].
Enzyme-linked immunosorbent assay (ELISA)
Target protein concentrations were measured using a Human MMP7 ELISA Kit (Elabscience) and a Human MSLN ELISA Kit (Omnimabs). We followed the manufacturer’s instructions and measured the optical density (OD) of the solution in each well at 450 nm. Finally, the protein concentration in each sample was obtained by comparison with the standard curve.
Immunohistochemistry (IHC) staining and scoring
Surgical specimens were embedded in paraffin and sectioned for IHC analysis. The tissue sections were dewaxed, hydrated, and rinsed in running water for 10 min. The sections were then soaked in boiling sodium citrate antigen repair solution for 20 min before addition of endogenous peroxidase blocker followed by dropwise addition of normal goat serum working solution for blocking. Solutions of anti-MSLN antibody (1:100, Proteintech, 66404-1-Ig) and anti-MET antibody (1:150, Proteintech, 25869-1-AP) were added for incubation overnight at 4°C. The following day, biotin-labeled secondary antibody was added dropwise followed by dropwise addition of horse radish peroxidase(HRP)-labeled streptavidin working solution. The tissue sections were then stained with diaminobenzidine(DAB), rinsed with tap water, stained with hematoxylin for 20s, and rinsed again before being dehydrated, cleared, and sealed. IHC images were quantitatively assessed and automatically scored using the IHC Profiler open source plugin[ 21 ].
Establishment of stable overexpression and knockdown cell lines
PC9 and PC9-BrM cells were transfected with viral vectors for MSLN overexpression and knockdown, respectively, according to the manual for lentivirus use (Shanghai Genechem Co., Ltd.), and the overexpressed or knockdown cells were screened with puromycin (1 µg/ml) for 1 week. The target sequences of MSLN shRNA1 and shRNA2 were 5'- GGAUGAGCUCUACCCACAATT-3' and 5'-CUUGCUUUCCAGAACAUGATT-3', respectively. PC9-BrM cells were transfected with MET-specific small interfering RNAs (siRNAs) (siRNA-1: 5'- GCCUGAAUGAUGAUGACAUUCUTT-3' and siRNA-2:5'- GCUGGUGGCACUUUACUUATT -3') using Lipofectamine 2000 or control siRNA (GenePharma) for 48 h. MSLN knockdown in PC9-BrM cells (PC9-BrM-SH2) was achieved by transfection of the cells with MET plasmid or negative control plasmid using Lipofectamine 2000 for 48 h, after which the cells were screened with G418 for 1 week. The efficiency of overexpression or knockdown was assessed by western blotting.
Wound healing assays
Cells were inoculated in 6-well plates, and once they reached confluency, wounds were created using a sterile 100-μl pipette tip. The cells were washed in suspension with phosphate-buffered saline (PBS) and imaged. After 24 h of incubation in serum-free medium, the healing process of cells migrating to cover the wound area was observed microscopically and imaged. Cellular wound healing rates were analyzed using ImageJ software.
Transwell migration and invasion assays
Cell suspensions with a cell density of 2×10 6 cells/ml in 200 µl of serum-free medium were added to Transwell chambers. For invasion experiments, the Transwell membrane was wrapped in advance with a matrix gel (BD, USA). The lower chamber was then spiked with medium containing 20% fetal bovine serum and incubated for 24 h. The cells were then fixed in 4% paraformaldehyde for 20 min and stained with crystal violet for 20 min. The stromal gel and cells were removed from the Transwell chamber layer with a cotton swab and photographed under a microscope to observe and count the cells. The data were analyzed using ImageJ software.
Trans-endothelial assays
hBMVECs (1×10 5 ) and HA1800 cells (1×10 5 ) were inoculated in the upper and bottom wells of a Transwell chamber and cultured until complete monolayers had formed. Brain metastatic cells (4×10 5 ) in medium containing 1% serum were inoculated in the top inserts, and 500 µl of medium with 20% serum was added to the bottom chamber. After 24 h, the green fluorescent protein (GFP)-labeled BM cells that had invaded via the membrane were photographed on a fluorescent microscope for counting.
Trans-BBB assays on a microfluidic chip
A bionic multi-organ microfluidic chip that allows real-time visual monitoring of the entire BM process was fabricated as previously described [ 22 ]. Briefly, tumor cells were edited to stably express GFP, while hBMVECs were labeled red with the Cell Tracker TM CM-Dil dye (Invitrogen, USA) according to the manufacturer’s instructions. After the biomimetic “lung” organ and “brain” organ were constructed, tumor cells were introduced to the upstream “lung” organ to allow the occurrence of BM. The trans-BBB events were then observed using on inverted fluorescent microscope. The observation starting time was designated as the time when the first cell reached the downstream vascular channel along with the fluid, and the images were captured after 36 h.
Generation of conditioned medium
Tumor cells were incubated in serum-free medium on 6-well plates for 24 h. After 24 h, tumor cell supernatant samples were centrifuged and filtered to remove cellular debris. The collected conditioned medium was then stored at -80℃ until further use.
Immunofluorescence (IF) staining
For immunofluorescent staining, hBMVECs were washed three times with PBS, fixed in 4% paraformaldehyde, and permeabilized in 0.1% Triton X-100 solution (Sigma, USA) for 10 min. For blocking, 3% Bovine serum albumin (BSA) solution was added for 30 min, and then cells were incubated with primary antibody (anti-JMA-A, Abcam; anti-VE-cadherin and anti-claudin 5, Invitrogen; 1:50 dilution) overnight at 4°C. After three washes with PBS, the cells were incubated in solution of fluorescein isothiocyanate (FITC)-labeled secondary antibody (1:100 dilution; Proteintech, USA) at room temperature. Cell nuclei were stained with 1 µg/ml 4',6-diamidino-2-phenylindole (DAPI) (1:1000 dilution; Sigma, USA) for 10 min at room temperature. Images were obtained using a confocal microscope (Leica TCS SP5II, Germany).
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from different groups of cells and reverse transcribed to cDNA using the One-Step gDNA Removal and cDNA Synthesis SuperMix (AT311, Transgen Biotech, Beijing, China) according to the manufacturer’s protocol. The relative levels of MSLN mRNA transcripts, MET mRNA transcripts, and GAPDH transcripts were quantified by qRT-PCR using Top Green qPCR SuperMix (AQ131, Transgen Biotech) and the following specific primers. The primer sequences were: h-MSLN-F 5'-CTGGAAGCCTGCGTGGAT-3′ and h-MSLN-R 5'-CCAGGTGCTGGATCACAGACT-3′; h-MET-F 5'- TCCAGGCAGTGCAGCATGTA-3′ and h-MET-R 5'-TCAAGGATTTCACAGCACAGTGA-3′; h-MMP7-F 5'- AGAGATCCCCCTGCATTTCA-3′ and h-MMP7-R 5'- GCCCATCAAATGGGTAGGAGT-3′;
h-GAPDH-F 5'-CATGAGAAGTATGACAACAGCCT-3′ and h GAPDH-R 5'-AGTCCTTCCACGATACCAAAGT-3′. All data were analyzed by the 2-ΔΔCt method.
Animal study
Four-week-old female BALB-c-nu mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (China). The PC9 cell line stably expressing GFP-luciferase fusion protein was constructed [ 22 ], cultured and collected in a cell suspension of 1×10 7 cells/ml. After the mice were anesthetized, 100 µl of cell suspension was injected into the left ventricle of each mouse. After retro-orbital injection of D-Luciferin (150 mg/kg body weight; Promega, USA) at the indicated time points, images of mouse tumor metastases were acquired using the IVIS Spectrum Xenogen instrument (PerkinElmer, USA). In vivo imaging software (version 2.50) was used to analyze the bioluminescence images. All animal experiments were performed in accordance with a protocol approved by the Animal Protection and Use Committee of Dalian Medical University.
BBB leakiness assay
Mice were injected in the tail vein with 100mg/kg Texas Red dextran (70,000 MW, Thermo Fisher Scientific, D1864). After 3 hours, mice were injected in the tail vein with 10 mg/kg DyLight 488-Lycopersicon Esculentum Lectin (LEL) (Thermo Fisher Scientific, L32470). After 10 minetes, each mouse was anaesthetised and perfused with ice PBS until there was no blood, followed by 4% paraformaldehyde for 3-5 min. Brain tissue was extracted and immersed in 30% sucrose overnight. Tissue cryosections with 6 µm thick were stained with DAPI (Solarbio) and images were obtained with an Leica TCS SP5II confocal microscopy (Leica). Three random areas of each section were collected and three sections of each brain were examined.
Statistical analysis
The data are expressed as the mean ± standard deviation (SD). The data were plotted using GraphPad Prism 8.0 software and then statistically analyzed using Statistical Package for the Social Sciences (SPSS) 19.0 software. To identify statistical differences between groups, the data were compared among experimental groups using analysis of variance (ANOVA), t-test or chi-square test. Statistical significance was defined by P <0.05.
Increased MSLN expression correlates with BM of NSCLC
To identify proteins potentially involved in the BM of NSCLC, we employed two NSCLC cell lines, PC9 (EGFR Dexon19 mutation) and H2030 (K-ras G12C mutation) cells, to develop a high-brain metastatic subpopulation (PC9-BrM and H2030-BrM, Fig. 1 A, Fig. S 1 A) and performed further proteomics analysis in PC9 and PC9-BrM cells to characterize the protein expression profile found in BM in our previous work[ 22 , 23 ]. Our results showed that the expression of MSLN was significantly up-regulated in the protein profiling (Fig. 1 B), and the increased MSLN expression was verified by western blotting in both PC9-BrM and H2030-BrM cells compared to the respective parental cells (Fig. 1 C). We also measured MSLN expression in a normal human bronchial epithelial cell line (BEAS2B) and in five NSCLC cell lines and found that MSLN was not detected in non-cancerous BEAS2B cells but was clearly expressed in the NSCLC cell lines characterized by preferential BM capacity, such as in PC9, H460 and H226 cells (Fig. S 1 B) [ 24 , 25 ].
MSLN expression is increased in NSCLC patients with BM. A Schematic illustration of the selection process of brain metastasis(BM) derivatives in mice. Parent cells PC9 and H2030 were inoculated into the left ventricle of nude mice to isolate and collect tumor cells with BM. The selection process was carried out twice, and the high-brain metastatic subpopulation (PC9-BrM and H2030-BrM cell lines) were collected. B Differential protein volcano map between PC9-BrM cells and PC9 cells. C Western blot analysis showed that PC9-BrM and H2030-BrM cells with high metastatic activity had higher MSLN protein levels. D Representative images and quantification analysis of MSLN staining in primary lung tumor (LT, n =36) and NSCLC-derived brain metastases (BM, n =34) surgical specimens. (scale bar, 200 μm) . E ELISA detection of MSLN expression in serum of all patients and control groups. HC, healthy controls ( n =24). ELC, early-stage NSCLC ( n =22). BoM, lung cancer with bone metastasis ( n =23). LM, lung cancer with live metastasis ( n =20). LCBM, lung cancer with brain metastasis ( n =42). PBT, primary brain tumor ( n =23). F Kaplan-Meier analysis of the overall survival of 478 lung cancer patients in the GEPIA database. (Data are presented as mean ± SD)
We next examined the expression of MSLN in clinical samples. 34 brain metastatic tumors were obtained from NSCLC BM patients while 36 primary lung tumors were obtained from patients with early stage NSCLC. We performed IHC for MSLN and found that MSLN expression was higher in the BM tissues than in the neoplastic tissues in situ (Fig. 1 D, Table S 2 ). Recent studies have indicated that the presence of soluble MSLN in serum samples may also be a potential serum biomarker for malignancies [ 26 ]. Hence, we also detected the levels of MSLN in serum samples. Serum samples were collected from an untreated patients cohort ( n =154) including 107 patients with NSCLC, 23 with primary brain tumors (PBT) and 24 healthy controls (HC). The 107 NSCLC patients included 22 cases of early lung cancer (ELC), 23 cases with bone metastasis (BoM), 20 cases with liver metastasis (LM) and 42 cases with lung cancer brain metastasis (LCBM). The clinicopathological imaging characteristics of the cohort are shown in Table S 3 . We found that the average MSLN level of the ELC group was higher than that of the HC group. Further comparison between the LCBM group and the ELC group showed that the MSLN level in the LCBM group was higher than that in the ELC group (Fig. 1 E). In the analysis of the correlation between serum MSLN expression and clinicopathological imaging characteristics of lung cancer patients with BM, it was found that the serum level of MSLN was significantly correlated with smoking history, BM maximum diameter, meningeal metastasis, number of primary lung lesions, pleural effusion, and epidermal growth factor receptor (EGFR) mutation status (Table S 4 ). Moreover, analysis of the GEPIA public database showed that high MSLN expression in NSCLC patients was significantly associated with low survival ( n =478, Fig. 1 F). Together these findings indicate that MSLN is involved in malignant progression of lung cancer and may play an important role in promoting BM in NSCLC.
MSLN promotes the migration and invasion of NSCLC brain metastatic cells in vitro
It is well known that tumor cells undergo EMT to become aggressive and migratory, and this is an important event in tumor metastasis and tumor progression [ 27 ]. Our previous study found that the brain metastatic cell line PC9-BrM exhibits a mesenchymal-like phenotype and strong ability to migrate and invade [ 22 ], as do H2030-BrM cells as demonstrated in this study (Fig. S 2 ). Some studies have concluded that MSLN plays an important role in tumor migration and invasion [ 28 , 29 ]. Hence, we hypothesized that MSLN plays a role in the process of BM of NSCLC. In our experiments, knockdown of MSLN significantly reduced the migratory and invasive capacity of brain metastatic NSCLC cells (PC9-BrM and H2030-BrM), whereas overexpression of MSLN increased the migratory and invasive capacity of the parental cells (PC9 and H2030, respectively), as evidenced by the wound healing and Transwell assays (Fig. 2 A-C, Fig. S 3 ). These results suggest that MSLN significantly promotes the migration and invasion of NSCLC brain metastatic cells. In addition, we examined the expression of EMT markers by western blotting and found that knockdown of MSLN reversed the mesenchymal phenotype of PC9-BrM cells based on increased E-cadherin expression and decreased N-cadherin and Slug expression (Fig. 2 D), while overexpression of MSLN facilitated EMT of PC9 cells (Fig. 2 E).
MSLN promotes the migration and invasion of NSCLC cells in vitro . A Representative images and quantitative results of western blotting showing MSLN expression after transfection of PC9-BrM and PC9 cells with lentivirus. B The effect of MSLN expression on the migration capacity in PC9-BrM and PC9 cells assessed in a wound-healing assay (scale bar, 100 µm). C Transwell migration and invasion assays to determine the effect of altered MSLN expression on the migration and invasion of NSCLC cells (scale bar, 200 µm). D, E Western blot analysis of E-cadherin, N-cadherin, Slug and MMP7 expression in PC9-BrM cells and PC9 cells after alteration of MSLN expression. F-G The expression of MMP7 by PC9 cells and PC9-BrM cells was detected by qRT-PCR (F) and ELISA (G). (PC9-NC, PC9 cells transfected with negative control plasmid. PC9-OE, PC9 cells transfected with MSLN plasmid. PC9-BrM-NC, PC9-BrM cells transfected with negative control shRNA. PC9-BrM-SH1, PC9-BrM cells transfected with MSLN-targeted shRNA1. PC9-BrM-SH2, PC9-BrM cells transfected with MSLN-targeted shRNA2. Data are presented as mean ± SD, ns, no significance)
Because the expression of matrix metalloproteinase family members (MMPs) is essential for tumor progression and metastasis [ 28 , 29 , 30 ], we further analyzed whether MMPs are involved in the MSLN-induced enhanced aggression of brain metastatic cells. We found that MMP7, rather than the more widely studied MMP2/9, was regulated by MSLN. The level of cellular and secretory MMP7, as determined by Western blotting, qRT-PCR and ELISA, was significantly decreased by MSLN deletion in PC9-BrM cells and was increased by MSLN overexpression in PC9 cells (Fig. 2 D-G). In summary, our data indicate that MSLN plays a promotive role in the enhanced migration and invasion of NSCLC brain metastatic cells.
MSLN helps brain metastatic cells penetrate the BBB by degrading inter-endothelial TJs
The BBB can restrict the invasion of many pathogens, and therefore, the crossing of the BBB by tumor cells is a critical step in BM [ 31 ]. We found that the highly brain metastatic cells were more capable of penetrating an endothelial cell layer than were the parental cells (Fig. S 4 A) [ 22 ]. To further investigate the role of MSLN in BM, we examined the trans-endothelial cell migration ability of brain metastatic cells with or without MSLN interference, using an in vitro BBB model and an ex vivo bionic BBB microfluidic chip model established previously [ 22 ]. The in vitro BBB model was established using Transwell chambers coated with human brain microvascular endothelial cells (hBMVECs) and primary human astrocytes (HA-1800), while the ex vivo bionic BBB model was established on a well-designed microfluidic chip where HA-1800 cells were introduced into the brain parenchyma chamber and hBMVECs were introduced into the vascular channels for co-culture with HA-1800 cells under the dynamic flow shear force (Fig. 3 A). The results showed that silencing MSLN resulted in a significant decrease in the ability of brain metastatic NSCLC cells to penetrate the endothelium (Fig. 3 B, Fig. S 4 B). Consistently, trans-endothelial migration was significantly increased among parental cells overexpressing MSLN (Fig. 3 C, Fig. S 4 C). These results suggest that MSLN helps metastatic NSCLC cells to cross the BBB. TJs, the key structures that maintain the barrier function of the BBB, mainly consist of ocludin, junctional adhesion molecules(JAMs), claudins, zonula occludens (ZO), and calmodulin (VE-cadherin), which form a junctional complex to maintain the stability of the barrier. Disruption of the junctional complex leads to the loss of cell-cell contacts and the formation of cellular gaps, which creates the opportunity for tumor cells to cross the BBB [ 31 ].
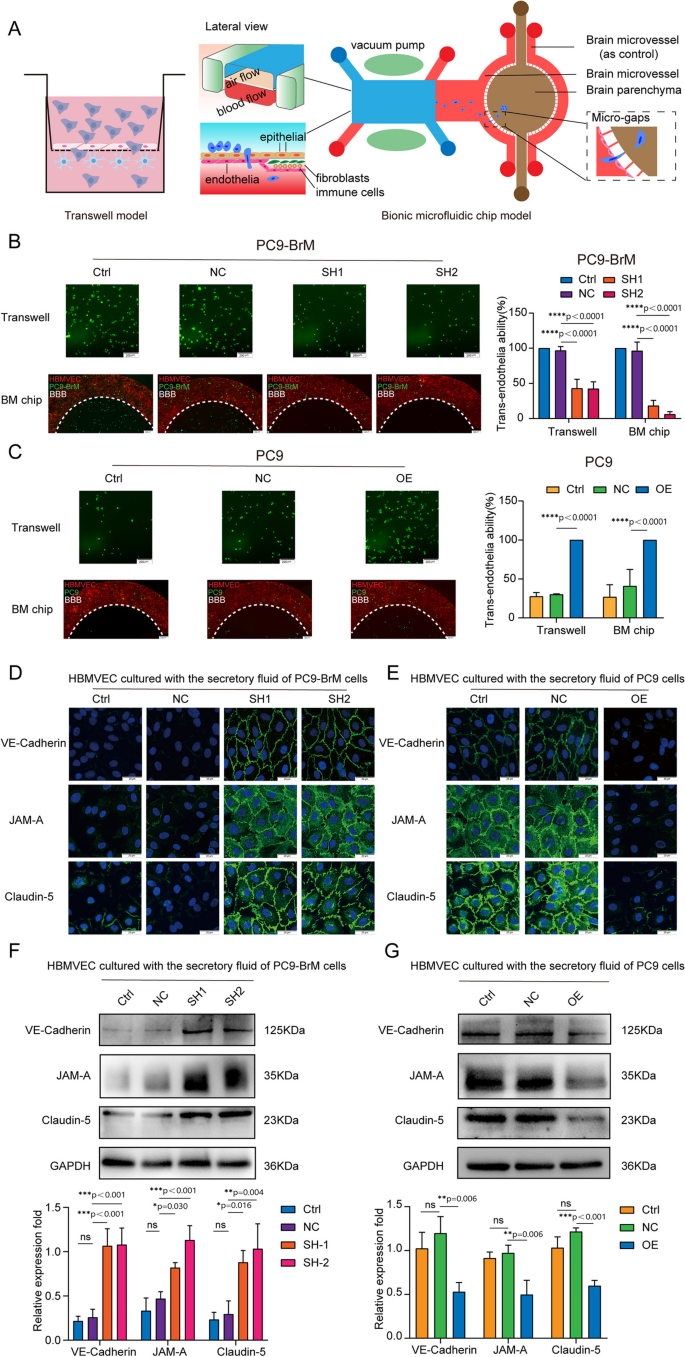
MSLN promotes NSCLC cells penetration of endothelium by promoting cleavage of endothelial TJ proteins. A Schematic diagrams of a classic in vitro blood-brain barrier (BBB) model and the multi-organ microfluidic chip. B , C Representative images of the ability of tumor cells to penetrate the BBB in the Transwell assay and the chip (scale bar, 200 µm). D , E Representative confocal microscopy images showing the distribution of VE-cadherin, JAM-A and claudin-5 in a hBMVEC monolayer (scale bar, 20 µm). F, G Western blot analysis of VE-cadherin, JAM-A and claudin-5 expression in hBMVECs after treatment with conditioned medium from the indicated tumor cells. (TJ, tight junction. PC9-NC, PC9 cells transfected with negative control plasmid. PC9-OE, PC9 cells transfected with MSLN plasmid. PC9-BrM-NC, PC9-BrM cells transfected with negative control shRNA. PC9-BrM-SH1, PC9-BrM cells transfected with MSLN-targeted shRNA1. PC9-BrM-SH2, PC9-BrM cells transfected with MSLN-targeted shRNA2. Data are presented as mean ± SD, ns, no significance)
We next investigated whether MSLN in NSCLC cells affected the expression of these junctional complex proteins by hBMVECs. We treated hBMVEC monolayers for 24 h with conditioned medium obtained over a 24 h period from the indicated tumor cells and then observed the distribution of VE-cadherin, JAM-A and claudin-5 expression by immunofluorescence imaging. The image results showed that the distributions of VE-cadherin, JAM-A and claudin-5 became discontinuous and vastly diminished in hBMVEC monolayers treated with conditioned medium derived from tumor cells with relatively high expression of MSLN (Fig. 3 D, E). We further quantified the expression of TJ complex proteins by western blotting. Consistent with the immunofluorescence observations, we found that conditioned medium from tumor cells with MSLN knockdown did not induce as much degradation of the cadherin, JAM-A and claudin-5 expression patterns among hBMVEC monolayers (Fig. 3 F). Conversely, the degradation of these endothelial junction proteins was significantly increased after treatment of hBMVECs with conditioned medium from MSLN-overexpressing parental cells (Fig. 3 G). These results indicate that MSLN promotes the ability of brain metastatic cells to penetrate the BBB by destroying TJ complexes.
Disruptive effect of MSLN on TJs is dependent on MET expression
Studies have shown that MET plays a biological role in the BM of many tumor cells [ 5 , 32 ], including the ability of the cells to cross the BBB [ 33 ]. We assessed the co-expression of MSLN and MET in surgical specimens of primary lung tumors (LT, n =25) and brain metastases of lung cancer (BM, n =25), and found that both MSLN and MET were expressed at elevated levels in surgical specimens of brain metastases of lung cancer, and showed a close correlation (Fig. 4 A-C). In NSCLC lines, western blot analysis further confirmed that the expression levels of MET and p-MET decreased with knockdown of MSLN, whereas overexpression of MSLN promoted the cellular expression levels of MET and p-MET (Fig. 4 D-E, Fig. S 5 A). To determine whether MET is involved in MSLN-regulated tumor cell penetration of the BBB, we knocked down MET in PC9-BrM cells with siRNA (Fig. 4 F) and then overexpressed MSLN in PC9-BrM cells in which we had previously knocked down MSLN (PC9-BrM-SH2) (Fig. 4 G). In vitro and ex vivo trans-BBB assays showed that MET knockdown inhibited the ability of PC9-BrM cells to penetrate the BBB, whereas MET overexpression relieved the suppression of the trans-BBB ability of PC9-BrM-SH2 cells (Fig. 4 H, Fig. S 5 B). We further evaluated expression of the junctional proteins of the BBB after treatment of hBMVECs with conditioned medium from the indicated metastatic cells. The results showed that MET knockdown inhibited the ability of brain metastatic cells to degrade TJ complexes, whereas overexpression of MET significantly enhanced the TJ complex degrading capacity of PC9-BrM-SH2 cells (Fig. 4 I, J). Taken together, these data suggest that the effect of MSLN on tumor cell penetration of the BBB is dependent on MET.
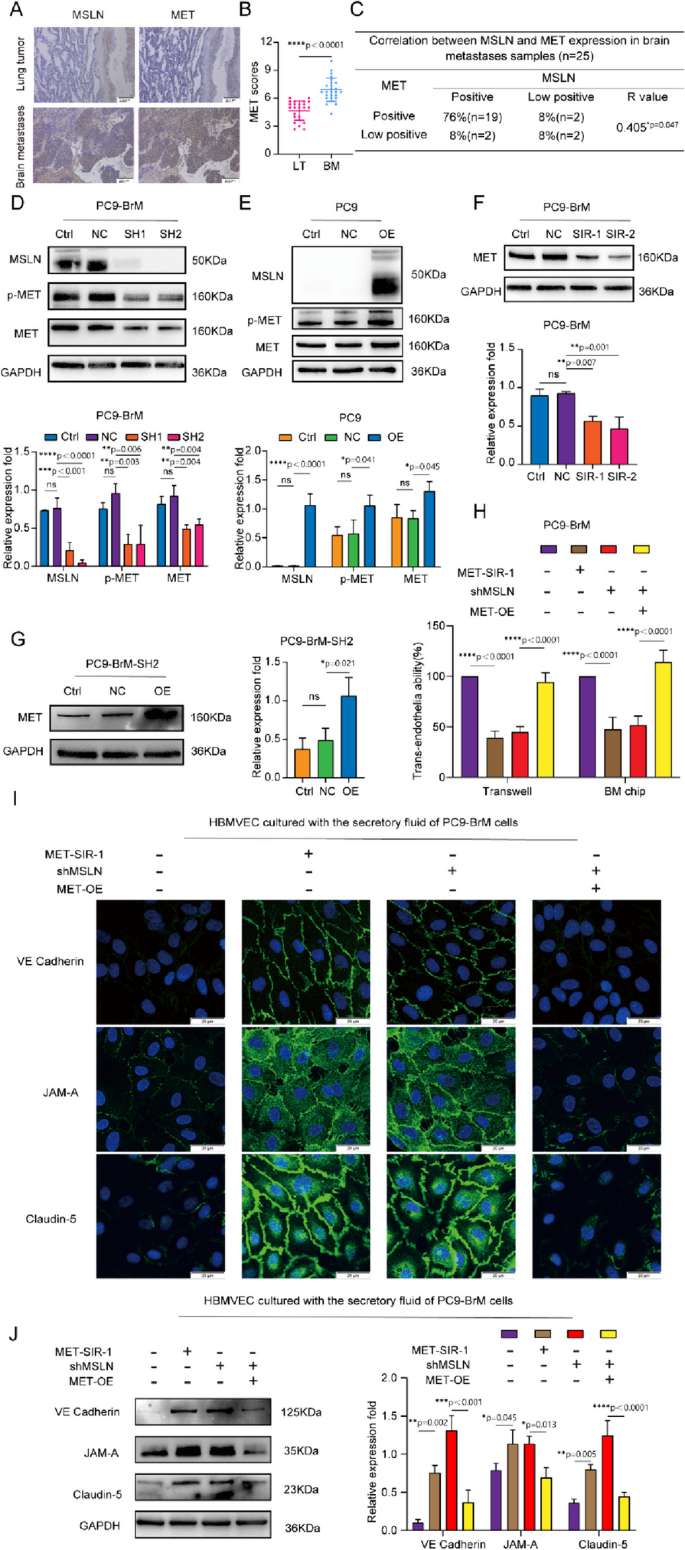
The effect of MSLN on NSCLC penetration of the BBB is dependent on MET. A,B Representative images and quantification analysis of MSLN and MET staining in primary lung tumor (LT, n =25) and lung cancer-derived brain metastases (BM, n =25) surgical specimens (scale bar, 200 μm). C Correlation of MSLN and MET protein expression in BM surgical specimens. D, E Representative western blot images showing the expression of MSLN, p-MET and MET in the indicated cells. F, G Representative images and quantitative results of western blotting for MET expression in tumor cells after transfection. H Effect of MET on the ability of NSCLC cells to penetrate the endothelium. I, J After treatment of hBMVECs with conditioned medium from the designated tumor cells for 24 h, western blot analysis and immunofluorescence detection showed the distribution of VE-cadherin, JAM-A and claudin-5 expression in hBMVEC monolayers (scale bar, 20 μm). (PC9-NC, PC9 cells transfected with negative control plasmid. PC9-OE, PC9 cells transfected with MSLN plasmid. PC9-BrM-NC, PC9-BrM cells transfected with negative control shRNA. PC9-BrM-SH1, PC9-BrM cells transfected with MSLN-targeted shRNA1. PC9-BrM-SH2, PC9-BrM cells transfected with MSLN-targeted shRNA2. PC9-BrM-SIR-1, PC9-BrM cells transfected with MET-targeted siRNA-1. PC9-BrM-SIR-2, PC9-BrM cells transfected with MET-targeted siRNA-2. PC9-BrM-SH2-NC, PC9-BrM-SH2 cells transfected with negative control plasmid. PC9-BrM-SH2-OE, PC9-BrM-SH2 cells transfected with MET plasmid. MET-SIR-1, PC9-BrM cells transfected with MET-targeted siRNA-1. shMSLN, PC9-BrM cells transfected with MSLN-targeted shRNA1. MET-OE, PC9-BrM cells transfected with MET plasmid.Data are presented as mean ± SD, ns, no significance)
MSLN regulates the expression and phosphorylation of MET through the JNK signaling pathway in brain metastatic cells
Previous studies reported that activation of JNK promotes NSCLC metastasis by activating MMPs [ 34 ]. In addition, enhancement of JNK signaling promotes activation of MMP9, which further promotes the degradation of TJ proteins and leakage of the BBB [ 35 ]. To further determine the potential mechanisms underlying the effect of MSLN on MET expression, we detected JNK activity and MET expression in PC9-BrM cells transfected with MSLN-targeted shRNA or treated with a JNK inhibitor, JNK-IN-8, or a MET inhibitor, crizotinib. Western blot analysis demonstrated that both knockdown of MSLN and JNK-IN-8 treatment led to inhibited expression and phosphorylation of MET along with suppression of JNK activity in brain metastatic cells, whereas overexpression of MSLN significantly enhanced JNK activity in these cells (Fig. 5 A-B, F, Fig. S 5 A, C). Notably, JNK-IN-8 treatment did not affect the MSLN expression but did efficiently attenuate MET activation in NSCLC cells overexpressing MSLN, whereas crizotinib had no effect on MSLN expression and JNK signaling (Fig. 5 C-D, E-G). Taken together, these results suggest that MSLN regulates the expression and phosphorylation of MET through the JNK signaling pathway in brain metastatic cells.
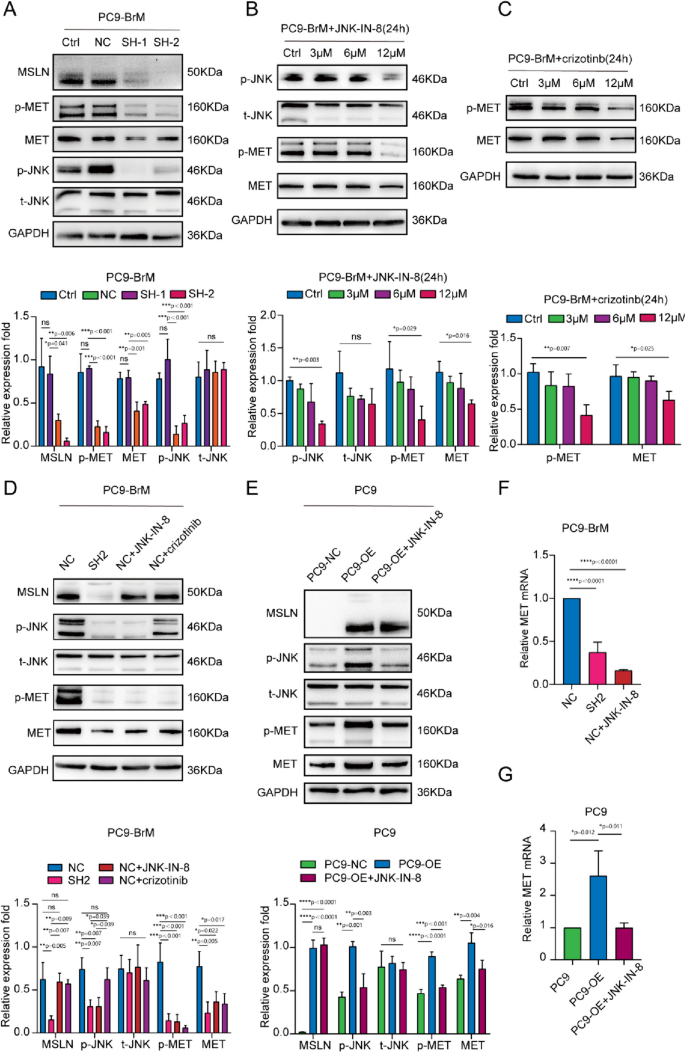
MSLN regulates MET phosphorylation as well as protein and mRNA expression in NSCLC cells through the JNK signaling pathway. A Representative western blot images showing MSLN, p-JNK, t-JNK, p-MET and MET expression levels after MSLN knockdown in PC9-BrM cells. B, C PC9-BrM cells were treated with JNK-IN-8 and crizotinib at different concentrations of 0, 3, 6, and 12 µM for 24 h, and the expression of the indicated molecules was detected by western blotting. D-G PC9-BrM-NC cells, PC9-BrM-SH2 cells, PC9-NC cells and PC9-OE cells were incubated in serum-free medium for 24 h, and then PC9-BrM-NC cells and PC9-OE cells were treated with the designated inhibitors at 12 µM for 24 h. The expression of the indicated molecules was then detected by western blotting and qRT-PCR. (PC9-NC, PC9 cells transfected with negative control plasmid. PC9-OE, PC9 cells transfected with MSLN plasmid. PC9-BrM-NC, PC9-BrM cells transfected with negative control shRNA. PC9-BrM-SH1, PC9-BrM cells transfected with MSLN-targeted shRNA1. PC9-BrM-SH2, PC9-BrM cells transfected with MSLN-targeted shRNA2. Data are presented as mean ± SD, ns, no significance)
Targeting MSLN and MET therapeutically inhibits BM in vivo
A previous study demonstrated that anetumab can specifically target MSLN-positive tumors and inhibit tumor growth in subcutaneous and orthotopic xenograft models [ 36 ], and another study reported that anetumab has preliminary anti-tumor activity in patients with MSLN-positive solid tumors in a phase I study [ 37 ]. The non-selective tyrosine kinase inhibitor (TKI) for MET crizotinib has been widely used in the clinical treatment of lung cancer patients including those with BM [ 38 ], and the selevtive MET-TKI capmatinib, which also inhibits the phosphorylation of MET in PC9-BrM cells (Fig. S 6 ), has been recently approved and applied for NSCLC treatment [ 39 ]. We further evaluated the therapeutic efficacies of MSLN- or MET-targeting therapies in an in vivo preclinical BM model. For establishment of the mouse models, PC9-BrM cells (control) or PC9-BrM cells with MSLN knockdown (shMSLN) were introduced into nude mice by intracardiac injection. Anetumab, crizotinib and capmatinib were administered separately or in combination to mice inoculated with PC9-BrM cells. An in vivo BBB leakiness assay was performed by intravenous injection of Texas Red-Dextran (70,000 MW), DyLight 488-Lycopersicon Esculentum Lectin (LEL) on the 10th day. Dextran was used as an indicator of BBB leakiness while LEL was used to label the BBB. The diffused dextran indicated the impared BBB in mice injected by PC9-BrM cells (Control) while the dextran diffusion was significantly suppressed once MSLN and MET are targeted separately or jointly (Fig. 6 A). As evidenced by the regular weekly bioluminescence images, it was found that anetumab, crizotinib and genetic silencing of MSLN all significantly inhibited the occurrence of BM in vivo and prolonged the survival of mice (Fig. 6 B-D). Capmatinib also showed significantly inhibitory effect on the development of BM, and the improvement in the survival of mice was observed in the combined treatment group with capmatinib and anetumab (Fig. 6 E-G). However, combined treatment with anetumab and crizotinib did not result in prolonged survival of the mice, with most mice dying within 20 days without any secondary metastases. Overall, our in vivo results suggest that therapies targeting MSLN and MET exhibited remarkable therapeutic efficacy for inhibiting the BM.
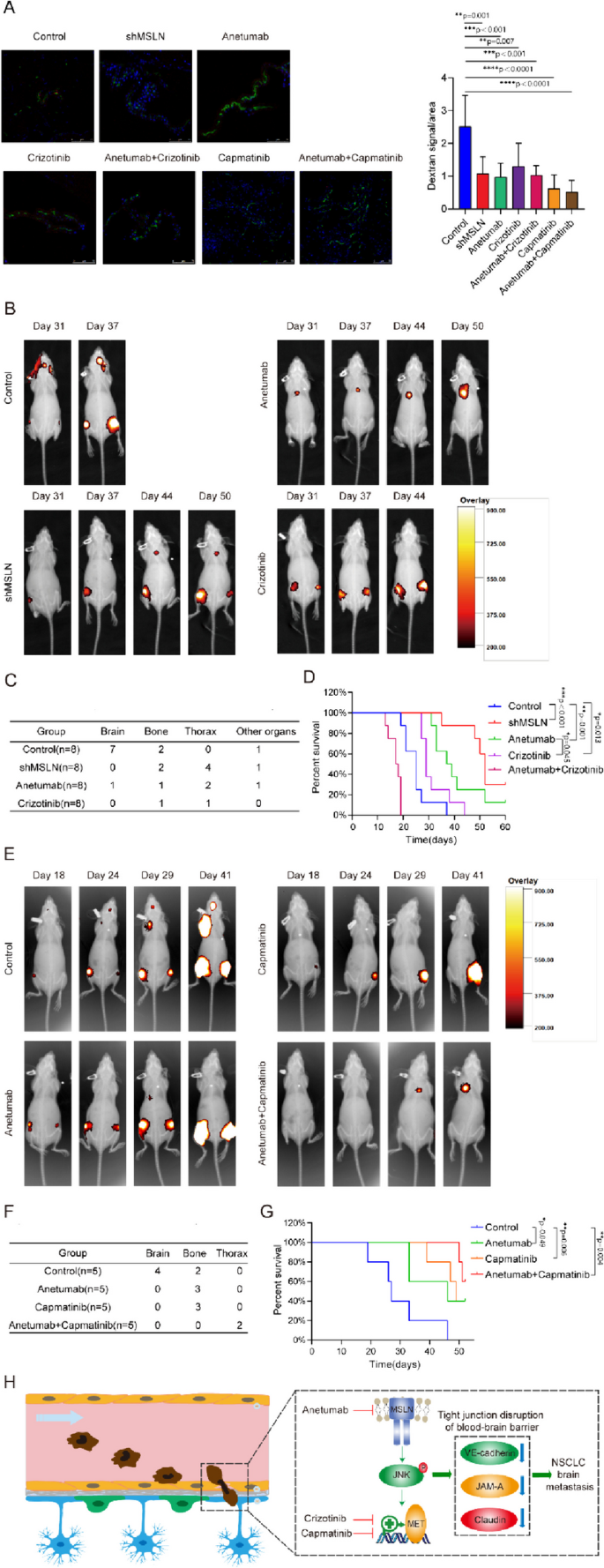
Targeting MSLN and MET therapeutically inhibits lung cancer BM in vivo . PC9-BrM cells and PC9-BrM cells with MSLN knockdown (shMSLN) were injected into nude mice via the left ventricle. Nude mice injected with PC9-BrM cells were randomly assigned to the following administration regimen groups and continued to be administered for 5 weeks from the day 3 post-injection: Control, placebo administration; anetumab, anetumab (0.2 mg/kg) intravenously weekly; crizotinib, crizotinib (5 mg/kg) intraperitoneally every 2 days; anetumab+crizotinib, anetumab (0.2 mg/kg) intravenously weekly and crizotinib (5 mg/kg) intraperitoneally every 2 days; capmatinib, oral capmatinib (10mg/kg) daily; anetumab+capmatinib, anetumab (0.2 mg/kg) intravenously weekly and oral capmatinib (10mg/kg) daily. A Fluorescent images showing BBB permeability of the mouse brains after intravenous injection of Texas Red-Dextran (70,000 MW), DyLight 488-Lycopersicon Esculentum Lectin (LEL) ( n =3 in each group). Cell nuclei are stained with DAPI (blue). Scale bar, 75 μm. B Representative biofluorescence images of each group at the indicated time. C Results for distant metastasis in each group. D Survival curves for the different groups ( n =8 in each group). E Representative biofluorescence images of each group at the indicated times. F Results for distant metastasis in each group. G Survival curves for the different groups ( n =5 in each group). H Schematic description of the role of MSLN in promoting lung cancer BM by disrupting the BBB. (Data are presented as mean ± SD)
MSLN, a cell surface glycoprotein, is highly expressed in various tumor tissues [ 18 , 28 , 29 , 40 , 41 ], while it is found at very low levels in normal human tissues [ 10 , 42 ]. A study showed that knockdown of MSLN significantly inhibits in vitro cell adhesion, migration, and invasion (critical steps necessary for metastasis), and also reverses EMT and attenuates stem cell properties in lung cancer cells [ 18 ]. In this study, we found that MSLN is not only a specific expression of the tumor antigen in situ in lung cancer compared to paracancer, but its expression is further elevated in brain metastases. This means that MSLN may play an important role in the pathological process of BM. Since soluble MSLN in serum samples may also be a potential serum biomarker for malignancies [ 26 ], we also found that the MSLN level of the ELC group was higher than that of the HC group, and the MSLN level in the LCBM group was even higher than that of the ELC group, indicating the important significance of MSLN in NSCLC that different threshold expression levels can help to diagnose primary lung cancer and secondary BM and predict the risk of lung cancer BM. In addition, serum levels of MSLN were found to be significantly higher in the LCBM group than in the PBT group. Since there is no evidence that MSLN is a tumor antigen in PBT, this finding suggests that serum MSLN may be useful in identifying primary and secondary intracranial tumors. Therefore, MSLN is a good indicator of NSCLC progression, especially for BM.
Tumor cell penetration of the BBB is the rate-limiting step in BM [ 43 , 44 ]. To study the pathology of BM, we used both a conventional Transwell model and our constructed multi-organ microfluidic chip to study tumor cell extravasation across the BBB. The 'BBB' constructed on the chip mimics the physiological microenvironment in terms of structural integrity and barrier function, and allows real-time visualisation of the entire tumor BM process, which is not possible with the Transwell system or animal models [ 22 ]. Our experiments with both models have shown that MSLN promotes the crossing of the BBB by NSCLC cells. BBB disruption is necessary for tumor cell migration across the endothelium and is achieved by degradation of brain endothelial cell junction proteins. We treated brain endothelial cells with conditioned medium from brain metastatic cells and found that knockdown of MSLN inhibited the degradation of VE-cadherin, JAM-A and claudin-5 on brain endothelial cells, significantly reducing the number of tumor cells migrating across the endothelial layer.
With the development of prevention and evidence-based medicine, it has been considered more important to prevent metastasis than to treat it. Based on our findings that MSLN promotes BM by encouraging NSCLC cells to cross the BBB, a key rate-limiting link, targeting MSLN is expected to be a therapeutic strategy for preventing BM in NSCLC. As MSLN expression is rather low in most normal tissues, but highly elevated in tumors, the current main strategies for targeting MSLN include tumor vaccines, antibody-based therapies and chimeric antigen receptor T-cell (CAR-T) therapies. The combination of the bacterial vaccine CRS-207, an attenuated form of a Listeria monocytogenes vector overexpressing human MSLN, with pemetrexed/cisplatin chemotherapy provided objective disease control in unresectable malignant pleural mesothelioma and induced significant clinical responses, suggesting that tumor vaccines may be potential candidates for cancer therapy [ 45 ]. Anetumab ravtansine (ARav) is a novel antibody-drug conjugate currently in clinical trials for several malignancies that express MSLN. The antibody binds MSLN with high affinity and induces internalisation of DM4 (the conjugate combines with ravtansine). Once inside the cell, the SPDB (N-succinimidyl 4-(2-pyridyldithio)butnoate) junction is cleaved [ 46 ] and DM4 binds to microtubule proteins, disrupting microtubule dynamics and thereby inhibiting cell division and proliferation. In vivo , ARav showed potent anti-tumor activity against MSLN-expressing mesothelioma, pancreatic and ovarian xenografts from cancer patients [ 36 ]. Among immunotherapies, CAR-T therapy is considered one of the most promising new approaches for cancer treatment. CAR-T cells are engineered T cells that produce an artificial T receptor targeting a specific protein. To date, fourth-generation CARs favor the secretion of cytokines (including IL-12 and IL-15) and thus strongly influence the immune components of the tumor microenvironment [ 47 ]. Preclinical studies in a mouse model of metastatic pancreatic adenocarcinoma demonstrated that CAR-T cells targeting MSLN can induce tumor cytotoxicity and eradicate lung metastases [ 48 , 49 ]. In an in situ mouse model of mesothelioma, local intrapleural injection of CAR-T cells targeting MSLN produced potent anti-tumor activity that correlated with their proliferation and persistence after 200 days [ 50 ]. Similar results were recently reported in a preclinical model of gastric cancer following peritumor injection of CAR-T cells targeting MSLN [ 51 ]. In the present study, we found that anetumab reduced the incidence of lung cancer BM and effectively prolonged the survival of mice. These results provide support for the further investigation of MSLN-targeted therapy in patients with NSCLC BM.
In this study, we further elucidated the mechanism by which MSLN promotes NSCLC cell trans-BBB and found that MSLN-mediated BBB disruption by NSCLC cells is dependent on MET expression and activation. In NSCLC, the three main mechanisms of MET dysregulation include protein overexpression, MET exon 14 jump mutation (METex14) or gene amplification. The MET protein encoded by the MET gene is a tyrosine kinase receptor. Upon activation, MET dimerisation and tyrosine phosphorylation occur, which activates downstream signalling pathways such as PI3K/AKT, RAS/MAPK, STAT and Wnt/β-catenin, etc., which promote the survival, proliferation, invasion and drug resistance in lung cancer [ 52 ]. MET knockdown was found to significantly reduce the incidence of BM from NSCLC cells in vitro [ 5 ]. Increased plasma soluble Met (sMet) levels are associated with lower overall survival in NSCLC patients [ 53 ], supporting the results of other studies which showed that MET overexpression and amplification are associated with poor prognosis in NSCLC patients [ 54 , 55 , 56 , 57 ]. As a result, capmatinib, a selective MET inhibitor, was approved by the FDA recently. In a clinical trial, the combination of capmatinib with EGFR-TKIs is determined as a promising treatment option for patients with EGFR-mutated, MET-dysregulated NSCLC and particularly for patients with MET-amplified tumors [ 58 ]. Capmatinib showed a clinically meaningful rate of anti-tumor activity and an acceptable safety profile in pretreated advanced NSCLC patients with either MET gene copy number (GCN) ≥6 and/or METex14 mutation [ 59 ]. Crizotinib, an FDA-approved small molecule inhibitor of the ALK, MET and ROS1 tyrosine kinases for advanced NSCLC [ 60 , 61 , 62 , 63 ], has shown satisfactory antitumor activity [ 64 ]. A recent study has reported the sensitivity to crizotinib-targeted therapy in patients with BM from NSCLC with concomitant activation of MET receptors and ALK fusion genes [ 65 ]. In the present study, we found that crizotinib and capmatinib significantly inhibited the occurrence of BM in vivo and prolonged the survival of the mice. Noteworthy, animal studies indicated that combination of crizotinib and anetumab lead to shorter survival while combination of capmatinib and anetumab showed a better efficiency. We hypothesized that crizotinib may cause more toxic side effects since it does not only target the MET. Moreover, in vivo tolerance to the combination of crizotinib and anetumab needs to be further explored. Taken together, these data support the potential targeted use of the MET selective TKI capmatinib or the MET non-selective TKI crizotinib according to the driver gene characteristics of patients with advanced NSCLC to provide preventive strategies for BM.
Our study provides evidence that MSLN promotes MET expression and activation via the JNK signalling pathway, which helps tumor cells degrade TJs of the BBB, thereby promoting the development of BM. MSLN can be used not as a biomarker for the diagnosis and prognosis of NSCLC, but also as an effective target for the therapies for patients with BM. Application of MSLN-targeted inhibitor (anetumab) or MET-targeted inhibitors (crizotinib/capmatinib) provides new preventive strategies for NSCLC BM (Fig. 6 H).
There remain some possible limitations in this study. First, as our clinical samples are from a single center, the sample size is limited. Future multicenter and large-scale studies are warranted to further verify the conclusions. Secondly, our in vivo and in vitro studies were mainly conducted by using lung adenocarcinoma cell lines. Squamous and large cell lung cancer cell lines with brain metastasis characteristics need to be established in the future. Finally, our in vivo experiments were conducted in nude mice which excluded the regulation of MSLN on immunity and its effect on BM outcomes. It is also unknown whether the drugs will induce immune-related responses in vivo which may thus affect the therapeutic efficiency.
Availability of data and materials
All data are available from the Prof. Qi Wang upon reasonable request.
Abbreviations
Brain metastasis
Non-small cell lung cancer
Blood-brain barrier
c-Jun N-terminal kinase
Hepatocyte growth factor receptor
Transmembrane receptor tyrosine kinase
Phosphorylated MET
Food and Drug Administration
Anaplastic lymphoma kinase
Tight junctions
Epithelial-mesenchymal transition
Human bronchial epithelial cells
Human lung microvascular endothelial cells
Human lung fibroblasts
Human monocyte cells
Normal bronchial epithelial cells
Human brain microvascular endothelial cells
Human astrocytes
Radioimmunoprecipitation assay
Sodium dodecyl sulfate
Polyacrylamide gel electrophoresis
Matrix metalloproteinase 7
Glyceraldehyde phosphate dehydrogenase
Vascular endothelial
Tris-buffered saline with Tween
Enhanced chemiluminescence
Enzyme-linked immunosorbent assay
Optical density
Horse radish peroxidase
Diaminobenzidine
Phosphate-buffered saline
Green fluorescent protein
Immunofluorescence
Bovine serum albumin
Fluorescein isothiocyanate
4',6-diamidino-2-phenylindole
Quantitative reverse transcription-polymerase chain reaction
Standard deviation
Statistical Package for the Social Sciences
Analysis of variance
Primary brain tumors
Healthy controls
Early lung cancer
Bone metastasis
Liver metastasis
Lung cancer brain metastasis
Epidermal growth factor receptor
Matrix metalloproteinase family members
Junctional adhesion molecules
Tyrosine kinase inhibitor
Lycopersicon Esculentum Lectin
Chimeric antigen receptor T-cell
Anetumab ravtansine
The conjugate combines with ravtansine
N-succinimidyl 4-(2-pyridyldithio)butnoate
Soluble Met
Gene copy number
ROS proto-oncogene 1, receptor tyrosine kinase
Phosphoinositide 3-kinase
Mitogen-activated protein kinase
Signal transducer and activator of transcription
MET exon 14 jump mutation
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94.
Article PubMed Google Scholar
Sorensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6(9):1474–80.
Article CAS PubMed Google Scholar
Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19(1):e43–55.
Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65(4):1479–88.
Breindel JL, Haskins JW, Cowell EP, Zhao M, Nguyen DX, Stern DF. EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res. 2013;73(16):5053–65.
Article CAS PubMed PubMed Central Google Scholar
Tan AC, Tan DSW. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J Clin Oncol. 2022;40(6):611–25.
Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(10):944–57.
Camidge D, Otterson G, Clark J, Ignatius Ou S, Weiss J, Ades S, et al. Crizotinib in Patients With MET-Amplified NSCLC. 2021;16(6):1017-29.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25.
Morello A, Sadelain M, Adusumilli PS. Mesothelin-Targeted CARs: driving T cells to solid tumors. Cancer Discov. 2016;6(2):133–46.
Cristaudo A, Foddis R, Vivaldi A, Guglielmi G, Dipalma N, Filiberti R, et al. Clinical significance of serum mesothelin in patients with mesothelioma and lung cancer. Clin Cancer Res. 2007;13(17):5076–81.
Ho M, Bera TK, Willingham MC, Onda M, Hassan R, FitzGerald D, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13(5):1571–5.
Servais EL, Colovos C, Rodriguez L, Bograd AJ, Nitadori J, Sima C, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res. 2012;18(9):2478–89.
Kachala SS, Bograd AJ, Villena-Vargas J, Suzuki K, Servais EL, Kadota K, et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin Cancer Res. 2014;20(4):1020–8.
Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis. 2011;32(7):1013–24.
Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5(208):208ra147.
Article PubMed PubMed Central Google Scholar
Wittwer NL, Staudacher AH, Liapis V, Cardarelli P, Warren H, Brown MP. An anti-mesothelin targeting antibody drug conjugate induces pyroptosis and ignites antitumor immunity in mouse models of cancer. J Immunother Cancer. 2023;11(3):e006274.
He X, Wang L, Riedel H, Wang K, Yang Y, Dinu CZ, et al. Mesothelin promotes epithelial-to-mesenchymal transition and tumorigenicity of human lung cancer and mesothelioma cells. Mol Cancer. 2017;16(1):63.
Zhenjiang L, Rao M, Luo X, Sandberg E, Bartek J Jr, Schoutrop E, et al. Mesothelin-specific immune responses predict survival of patients with brain metastasis. EBioMedicine. 2017;23:20–4.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–102.
Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9(5): e96801.
Liu W, Song J, Du X, Zhou Y, Li Y, Li R, et al. AKR1B10 (Aldo-keto reductase family 1 B10) promotes brain metastasis of lung cancer cells in a multi-organ microfluidic chip model. Acta Biomater. 2019;91:195–208.
Liu W, Zhou Y, Duan W, Song J, Wei S, Xia S, et al. Glutathione peroxidase 4-dependent glutathione high-consumption drives acquired platinum chemoresistance in lung cancer-derived brain metastasis. Clin Transl Med. 2021;11(9):e517.
Navab R, Liu J, Seiden-Long I, Shih W, Li M, Bandarchi B, et al. Co-overexpression of Met and hepatocyte growth factor promotes systemic metastasis in NCI-H460 non-small cell lung carcinoma cells. Neoplasia. 2009;11(12):1292–300.
Zhang H, Wang Y, Chen Y, Sun S, Li N, Lv D, et al. Identification and validation of S100A7 associated with lung squamous cell carcinoma metastasis to brain. Lung Cancer. 2007;57(1):37–45.
Burt BM, Lee HS, Lenge De Rosen V, Hamaji M, Groth SS, Wheeler TM, et al. Soluble Mesothelin-Related Peptides to Monitor Recurrence After Resection of Pleural Mesothelioma. Ann Thorac Surg. 2017;104(5):1679-87.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73.
Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep. 2013;3:1870.
Chang MC, Chen CA, Chen PJ, Chiang YC, Chen YL, Mao TL, et al. Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. Biochem J. 2012;442(2):293–302.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67.
Custodio-Santos T, Videira M, Brito MA. Brain metastasization of breast cancer. Biochim Biophys Acta Rev Cancer. 2017;1868(1):132–47.
Xing F, Liu Y, Sharma S, Wu K, Chan MD, Lo HW, et al. Activation of the c-met pathway mobilizes an inflammatory network in the brain microenvironment to promote brain metastasis of breast cancer. Cancer Res. 2016;76(17):4970–80.
Choi YP, Lee JH, Gao MQ, Kim BG, Kang S, Kim SH, et al. Cancer-associated fibroblast promote transmigration through endothelial brain cells in three-dimensional in vitro models. Int J Cancer. 2014;135(9):2024–33.
Ochieng JK, Kundu ST, Bajaj R, Leticia Rodriguez B, Fradette JJ, Gibbons DL. MBIP (MAP3K12 binding inhibitory protein) drives NSCLC metastasis by JNK-dependent activation of MMPs. Oncogene. 2020;39(43):6719–32.
Liao B, Geng L, Zhang F, Shu L, Wei L, Yeung PKK, et al. Adipocyte fatty acid-binding protein exacerbates cerebral ischaemia injury by disrupting the blood-brain barrier. Eur Heart J. 2020;41(33):3169–80.
Golfier S, Kopitz C, Kahnert A, Heisler I, Schatz CA, Stelte-Ludwig B, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther. 2014;13(6):1537–48.
Hassan R, Blumenschein GR Jr, Moore KN, Santin AD, Kindler HL, Nemunaitis JJ, et al. First-in-human, multicenter, phase I dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J Clin Oncol. 2020;38(16):1824–35.
Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–8.
Mathieu L, Larkins E, Akinboro O, Roy P, Amatya A, Fiero M, et al. FDA Approval Summary: capmatinib and tepotinib for the treatment of metastatic NSCLC harboring MET exon 14 skipping mutations or alterations. Clin Cancer Res. 2022;28(2):249–54.
Cheng WF, Huang CY, Chang MC, Hu YH, Chiang YC, Chen YL, et al. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br J Cancer. 2009;100(7):1144–53.
Okła K, Surówka J, Frąszczak K, Czerwonka A, Kaławaj K, Wawruszak A, et al. Assessment of the clinicopathological relevance of mesothelin level in plasma, peritoneal fluid, and tumor tissue of epithelial ovarian cancer patients. Tumour Biol. 2018;40(10):1010428318804937.
Chang K, Pai LH, Batra JK, Pastan I, Willingham MC. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res. 1992;52(1):181–6.
CAS PubMed Google Scholar
Jia W, Martin TA, Zhang G, Jiang WG. Junctional adhesion molecules in cerebral endothelial tight junction and brain metastasis. Anticancer Res. 2013;33(6):2353–9.
PubMed Google Scholar
Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11(5):352–63.
Hassan R, Alley E, Kindler H, Antonia S, Jahan T, Honarmand S, et al. Clinical Response of Live-Attenuated, Listeria monocytogenes Expressing Mesothelin (CRS-207) with Chemotherapy in Patients with Malignant Pleural Mesothelioma. Clin Cancer Res. 2019;25(19):5787–98.
Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117(12):1736–42.
Qin L, Zhao R, Li P. Incorporation of functional elements enhances the antitumor capacity of CAR T cells. Exp Hematol Oncol. 2017;6:28.
He J, Zhang Z, Lv S, Liu X, Cui L, Jiang D, et al. Engineered CAR T cells targeting mesothelin by piggyBac transposon system for the treatment of pancreatic cancer. Cell Immunol. 2018;329:31–40.
Sun Q, Zhou S, Zhao J, Deng C, Teng R, Zhao Y, et al. Engineered T lymphocytes eliminate lung metastases in models of pancreatic cancer. Oncotarget. 2018;9(17):13694–705.
Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151.
Lv J, Zhao R, Wu D, Zheng D, Wu Z, Shi J, et al. Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer. J Hematol Oncol. 2019;12(1):18.
Remon J, Hendriks LEL, Mountzios G, Garcia-Campelo R, Saw SPL, Uprety D, et al. MET alterations in NSCLC-current perspectives and future challenges. J Thorac Oncol. 2023;18(4):419–35.
Gao H-F, Li A-N, Yang J-J, Chen Z-H, Xie Z, Zhang X-C, et al. Soluble c-met levels correlated with tissue c-met protein expression in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2017;18(1):85–91.
Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol. 2012;9(6):314–26.
Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, et al. MET Exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent met genomic amplification and c-met overexpression. J Clin Oncol. 2016;34(7):721–30.
Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14(10):2895–9.
Turke AB, Zejnullahu K, Wu Y-L, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17(1):77–88.
Wu YL, Zhang L, Kim DW, Liu X, Lee DH, Yang JC, et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib After Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients With EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36(31):3101–9.
Schuler M, Berardi R, Lim WT, de Jonge M, Bauer TM, Azaro A, et al. Molecular correlates of response to capmatinib in advanced non-small-cell lung cancer: clinical and biomarker results from a phase I trial. Ann Oncol. 2020;31(6):789–97.
Shaw AT, Kim D-W, Nakagawa K, Seto T, Crinó L, Ahn M-J, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94.
Kwak EL, Bang Y-J, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703.
Ou SHI, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6(5):942–6.
Bergethon K, Shaw AT, Ou S-HI, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863-70.
Camidge DR, Otterson GA, Clark JW, Ignatius Ou S-H, Weiss J, Ades S, et al. Crizotinib in Patients With MET-Amplified NSCLC. J Thorac Oncol. 2021;16(6):1017–29.
Wang P, Xiao P, Ye Y, Liu P, Han L, Dong L, et al. Rapid response of brain metastasis to crizotinib in a patient with KLC1-ALK fusion and MET gene amplification positive non-small cell lung cancer: a case report. Cancer Biol Med. 2017;14(2):183–6.
Download references
Acknowledgements
We appreciate the support of the Clinical Medical Research Center of Liaoning Province and the Dalian Respiratory Protection Engineering Center Laboratory.
This work was supported by grant from the National Natural Science Foundation of China (No. 82027805, 82103054 and 81972916), Liaoning Revitalization Talents Program (XLYC2002013), and the Science and Technology Innovation Foundation of Dalian (2020JJ25CY018, 2021JJ12SN42), Liaoning Province Science and Technology Programme(2023-BSBA-091), Dalian Science and Technology Talent Innovation Support Plan (2022RQ037), Beijing Natural Science Foundation (7232020).
Author information
Shengkai Xia, Wenzhe Duan and Mingxin Xu contributed equally to this work.
Authors and Affiliations
Department of Respiratory Medicine, The Second Hospital, Dalian Medical University, Dalian, China
Shengkai Xia, Wenzhe Duan, Mingxin Xu, Mengqi Li, Mengyi Tang, Manqing Lin, Encheng Li, Wenwen Liu & Qi Wang
Department of Oncology, Beijing Chest Hospital, Capital Medical University, Beijing, China
Department of Scientific Research Center, The Second Hospital, Dalian Medical University, Dalian, China
Wenwen Liu & Qi Wang
You can also search for this author in PubMed Google Scholar
Contributions
S.X, W.D and W.L designed and analyzed all experiments. S.X, W.D and W.L performed cell assays, animal experiments, IHC staining assays and write the manuscript. M.X performed the microfluidic chip assays. M.L collected the clinical samples. M.T and S.W. provided protocols and technical input. M.L. helps the tissue staining. Q.W, W.L and E.L conceived and supervised the project, and revised the manuscript. All authors read and approved the submitted manuscript.
Corresponding authors
Correspondence to Encheng Li , Wenwen Liu or Qi Wang .
Ethics declarations
Ethics approval and consent to partcipate.
Animal study was approved by the Animal Ethics Review Committee of Dalian Medical University. Clinical study was approved by the Ethics Review Committee of the Second Hospital of Dalian Medical University.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., supplementary material 3., supplementary material 4., supplementary material 5., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Xia, S., Duan, W., Xu, M. et al. Mesothelin promotes brain metastasis of non-small cell lung cancer by activating MET. J Exp Clin Cancer Res 43 , 103 (2024). https://doi.org/10.1186/s13046-024-03015-w
Download citation
Received : 23 November 2023
Accepted : 18 March 2024
Published : 03 April 2024
DOI : https://doi.org/10.1186/s13046-024-03015-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Brain metastasis (BM)
- Blood-brain barrier (BBB)
Journal of Experimental & Clinical Cancer Research
ISSN: 1756-9966
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Search form
New state of mind: rethinking how researchers understand brain activity.

(© stock.adobe.com)
Understanding the link between brain activity and behavior is among the core interests of neuroscience. Having a better grasp of this relationship will both help scientists understand how the brain works on a basic level and uncover what specifically goes awry in cases of neurological and psychological disease.
One way that researchers study this connection is through what are known as “brain states,” patterns of neural activity or connectivity that emerge during specific cognitive tasks and are common enough in all individuals that they become predictable. Another, newer, approach is the study of brain waves, rhythmic, repetitive patterns of brain cell activity caused by synchronization across cells.
In a new paper, two Yale researchers propose that these two ways of thinking about brain activity may not represent separate events but two aspects of the same occurrence. Essentially, they suggest that though brain states are traditionally thought of as a snapshot of brain activity while waves are more like a movie, they’re capturing parts of the same story.
Reconsidering these two approaches in this context, the researchers say, could help both fields benefit from the methods and knowledge of the other and advance our understanding of the brain.
Inspired by ecological, conservation, and Indigenous philosophies, Maya Foster, a third-year Ph.D. student in the Department of Biomedical Engineering, began pursuing this idea once she joined the lab of Dustin Scheinost , an associate professor in the Department of Radiology and Biomedical Imaging at Yale School of Medicine.
They are co-authors of the new paper , published April 5 in the journal Trends in Cognitive Sciences.
“ We’re arguing that rather than a brain state being one single thing, it’s a collection of things, a collection of discrete patterns that emerge in time in a predictable way,” she said.
In an interview with Yale News, Foster and Scheinost describe their proposal, and discuss how they might help researchers better understand the mysteries of the brain. This interview has been edited and condensed.
When did you start to consider these might be two aspects of the same occurrence?
Maya Foster: This has been on my mind even before I came to this lab. I was reading a book — “Erosion: Essays of Undoing” by Terry Tempest Williams — and she talks about how human-made machinery like helicopters cause vibrations that interrupt the natural pulse of things and cause things like rock formations to fall apart. Relatedly, there are a lot of Indigenous populations that believe everything has a pulse. And that got me thinking of the brain and whether we have some type of resonance or vibration that can be disrupted.
Then I joined this lab and Dustin let me experiment with a lot of different things. During one of those experiments, I input some data into a particular analysis and the outputs looked wave-like, and patterns emerged and then repeated. That took me down a whole rabbit hole of research literature and there was a lot of evidence for this idea of wave-like patterns in brain states.
What are the benefits of considering brain states as wave-like?
Foster: I think it creates a synergy where both sides — the brain state folks and the brain wave folks — benefit by learning from each other. And maybe the gaps in knowledge we have now when it comes to how brain activity relates to behavior might be filled by both groups working together.
Dustin Scheinost: Brain waves are newer in this field and they’re complex. And any time you can take something new and relate it to something old — brain states in this case — it gives you a natural jumping off point. You can bring along everything you’ve learned so far. It’s kind of like not throwing the baby out with the bath water. We don’t need to drop brain states. They’ve informed us, but we can go in a different direction with them too.
How are you proposing researchers consider brain states and brain waves now?
Foster: Borrowing from physics, when you analyze light, it can be a discrete point — a photon — or it can be wave-like. And that’s one way we’re thinking about this. Similarly, depending on how you analyze brain states you can get static patterns, much like a photon, or you if you look at activity more dynamically, certain patterns start to occur more than once over time, kind of like a wave.
So we’re arguing that rather than a brain state being one single thing, it’s a collection of things, a collection of discrete patterns that emerge in time in a predictable way.
For example, if we measured four distinct patterns in brain activity as someone completed a cognitive task, a brain state could be that pattern one emerges, then pattern three, then two, then four, and that series might repeat over time. And when that repetition stops, that would be the end of that particular brain state.
You also draw comparisons to the musical technique known as “fugue.” How does that fit with how you’re visualizing these phenomena?
Foster: I’m a music person, so that’s where this came from. In a fugue, you have a basic melody and then that melody emerges later in the music in different forms and formats. For instance, the melody will play, then some other music comes in, then the melody returns with the same rhythm and time sequence but maybe it’s in a different key.
Fugues are cyclical and wave-like, they have distinct groups of notes, and there’s a systematic repetition and sometimes layering of the main melody. We’re arguing that brain states are also wave-like, have distinct patterns of brain activity, and display systematic repetition and layering of sequential patterns.
How are you hoping other researchers respond to your argument?
Foster: I would love feedback, honestly. There is evidence for what we’re proposing but when it comes to implementing these ideas going forward, it would be helpful to have a conversation about how that might work. There are a lot of different strategies and I’m interested in a broader conversation about how we as researchers might go about studying this.
What’s it like as someone who has been in this field for a while to have a student come in with a new idea like this?
Scheinost: You can get set in your ways as a researcher and you need new ideas, new creativity. Sometimes they may sound outlandish when you first hear them. But then you ruminate, and they start to take form. And it’s fun. That’s really where the fun of this job is, to hear new ideas and see how people discuss and debate them.
Health & Medicine
Media Contact
Fred Mamoun: [email protected] , 203-436-2643

In Memoriam
Bartlett, among the first U.S. scholars to study in the Qing archives

How to prevent pickleball injuries

From Yale to the White House: Making a career in policy and public service

A whole-person approach to mental health
- Show More Articles
Help | Advanced Search
Computer Science > Computer Vision and Pattern Recognition
Title: umbrae: unified multimodal decoding of brain signals.
Abstract: We address prevailing challenges of the brain-powered research, departing from the observation that the literature hardly recover accurate spatial information and require subject-specific models. To address these challenges, we propose UMBRAE, a unified multimodal decoding of brain signals. First, to extract instance-level conceptual and spatial details from neural signals, we introduce an efficient universal brain encoder for multimodal-brain alignment and recover object descriptions at multiple levels of granularity from subsequent multimodal large language model (MLLM). Second, we introduce a cross-subject training strategy mapping subject-specific features to a common feature space. This allows a model to be trained on multiple subjects without extra resources, even yielding superior results compared to subject-specific models. Further, we demonstrate this supports weakly-supervised adaptation to new subjects, with only a fraction of the total training data. Experiments demonstrate that UMBRAE not only achieves superior results in the newly introduced tasks but also outperforms methods in well established tasks. To assess our method, we construct and share with the community a comprehensive brain understanding benchmark BrainHub. Our code and benchmark are available at this https URL .
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Neurooncol Adv
- v.2(1); Jan-Dec 2020

Medical management of brain metastases
Burkhardt Brain Tumor and Neuro-Oncology Center, Cleveland Clinic, Cleveland, Ohio, USA
Yasmeen Rauf
Manmeet s ahluwalia.
The development of brain metastases occurs in 10–20% of all patients with cancer. Brain metastases portend poor survival and contribute to increased cancer mortality and morbidity. Despite multimodal treatment options, which include surgery, radiotherapy, and chemotherapy, 5-year survival remains low. Besides, our current treatment modalities can have significant neurological comorbidities, which result in neurocognitive decline and a decrease in a patient’s quality of life. However, innovations in technology, improved understanding of tumor biology, and new therapeutic options have led to improved patient care. Novel approaches in radiotherapy are minimizing the neurocognitive decline while providing the same therapeutic benefit. In addition, advances in targeted therapies and immune checkpoint inhibitors are redefining the management of lung and melanoma brain metastases. Similar approaches to brain metastases from other primary tumors promise to lead to new and effective therapies. We are beginning to understand the appropriate combination of these novel approaches with our traditional treatment options. As advances in basic and translational science and innovative technologies enter clinical practice, the prognosis of patients with brain metastases will continue to improve.
An estimated 10–20% of patients with cancer will be diagnosed with brain metastases over their disease course. 1 , 2 However, the true incidence is likely higher as autopsy studies have reported metastases in 30–40% of patients with cancer. 3 , 4 As advances in therapy lead to prolonged survival after the initial cancer diagnosis, clinical trial enrollment increases, increasing the frequency of staging MRIs, reported incidence of brain metastasis will likely continue to increase. 5 Brain metastases generally portend a poor prognosis and even those with the most favorable prognostic factors have an overall 2-year and 5-year survival of 8.1% and 2.4% across all primary tumors. 2 While traditional treatment options including surgery and radiotherapy remain standard approaches for treating brain metastases, advances in targeted therapeutics and immunotherapies and providing exciting new treatment options for these patients.
Epidemiology
The three most common primary tumors associated with brain metastases, and the primary focus of this review, are lung (20–56%), breast (5–20%), and melanoma (7–16%) accounting for 67–80% of all brain metastases. 6–8 Within each primary tumor, the molecular subtype and previous treatments also play a role in the incidence of brain metastases. For example, in non-small-cell lung cancer (NSCLC), about 25–40% of patients will develop brain metastases, but in patients with anaplastic lymphoma kinase (ALK) rearrangements that have failed first-line ALK inhibitors, the incidence of brain metastases is between 45% and 70%. 9 , 10 In addition, in breast cancer, women with human epidermal growth factor receptor 2 ( ERBB2 or HER2 ) amplification or triple-negative hormone receptor status are at a higher risk of developing brain metastases compared to women with ER-positive or PR-positive cancers. 11
The risk of developing brain metastases also increases with more advanced primary disease. 12 In HER2 -positive breast cancer, the incidence of brain metastases increases from 1.1% to 11.5% in patients with distant metastases compared to those without. 11 The risk of brain metastases also varies by age and is dependent on the primary tumor location. For breast cancer the risk is highest in younger patients between 20 and 39, in lung cancer the highest risk is between 40 and 49, whereas in melanoma, renal cell carcinoma (RCC), and colorectal cancer the highest risk is between 50 and 59. 13 Together this epidemiological data highlight the different trends in brain metastases across primary tumor types as well as the unique characteristics of each.
Patients with brain metastases have a dismal prognosis with 2-year and 5-year overall survival of 8.1% and 2.4% across all primary tumors. Various prognostic scores have been developed to classify the disease severity and guide the aggressiveness of therapy, including inclusion in clinical trials. In 2008, a prognostic score was developed that analyzed 1960 patients and took into account additional clinical variables. In the graded prognostic assessment (GPA), patients are given a score based on age, KPS, number of central nervous system (CNS) metastases, and the presence or absence of extracranial metastases. The GPA splits patients into 4 different groups, those with the best score having a median survival of 11 months compared to those with the worst score have a prognosis of 2.6 months. 14 This score remained the standard until the advent of targeted therapeutics shifted the treatment of lung cancer brain metastases and the GPA no longer predicted survival in these patients. Therefore, a lung-specific GPA that took into account the molecular profile of the tumors (Lung-molGPA) was developed. 15 Additional prognostic scores have also been developed and the constant in all of these is the inclusion of KPS. 16 , 17 Similar GPA scores exist for melanoma, RCC, and breast cancer brain metastases. 18–20 Finally, a nomogram for predicting individual survival probabilities has been developed utilizing the Radiation Therapy Oncology Group (RTOG) database. 21
Overview of Treatment Options
Surgery and radiotherapy have long been the cornerstone for the management of brain metastases. Until recently, systemic drug therapies have shown limited efficacy in the management of brain metastases. Lack of permeability of drugs through the blood–brain barrier (BBB) is often thought to be one reason for this low efficacy.
Even temozolomide, which is standard of care for patients with glioblastoma, has shown marginal benefit in the treatment of brain metastases. As a single agent, the overall response rate intracranially of temozolomide for brain metastases was less than 10% across multiple primary tumor types. 22 Additionally, temozolomide has minimal efficacy on the primary tumor, with extracerebral response rates ranging from 3% to 43% depending on the primary tumor. 22
Similarly, chemotherapies given for the primary tumor demonstrate very little intracranial efficacy. However, as discussed below, advances in immunotherapy and targeted therapies are beginning to demonstrate intracranial efficacy ( Table 1 ).
Significant Trials in Radiotherapy and Radiosurgery for Brain Metastases
Whole-Brain Radiation Therapy
Historically, whole-brain radiation therapy (WBRT) was the standard treatment for most patients with brain metastases. Two trials in the early 1990s demonstrated that surgery in addition to radiation provided survival benefits and improved local control. 23 , 24 WBRT has remained the most commonly used treatment for brain metastases due to its accessibility, quick initiation, the ability to control visible and occult lesions, as well as symptom improvement. However, in the last decade, the use of WBRT has been decreasing. 25 This is in part due to the decline in neurocognitive function seen in patients treated with WBRT. Fatigue, somnolence, learning, and memory impairments, which often occur with WBRT, are less frequent with the use of stereotactic radiosurgery (SRS). 26 To study the treatment effects of WBRT and SRS on neurocognitive function, validated, objective psychometric tests are often used and include Hopkins Verbal Learning Test, Controlled Oral Word Association, Grooved Pegboard Test, and Trail Making A and B tests. These are often performed at baseline and followed over time. In a study that randomized 213 patients to either WBRT plus SRS or SRS alone found at 3 months greater cognitive deterioration and decreased quality of life in patients treated with the WBRT plus SRS. For long-term survivors, the difference in cognitive deterioration was also seen at 12 months. 26
One method of minimizing the neurotoxicity of WBRT is the concurrent treatment with N- methyl- d -aspartate glutamine receptor blocker memantine. Radiation to the brain is known to cause overexcitation of the brain, altering the NMDA to GABA receptor ratio, at times resulting in neuronal cell death. 27 Memantine was shown to decrease time to cognitive decline and increase executive function, processing speed and delayed recognition. 28 This has led the congress of neurological surgeons to recommend memantine for 6 months after WBRT. 29 Another method currently being investigated to minimize the neurotoxicity has been hippocampus-sparing WBRT. Data from a phase III trial comparing hippocampal-avoidance WBRT plus memantine to WBRT plus memantine alone were recently published. The authors found that even with memantine, hippocampal avoidance added a significant ability to preserve neurocognitive function at both 4 and 6 months. 30 , 31
WBRT has traditionally played a significant role in the management of small-cell lung cancer (SCLC). Earlier studies demonstrated an overall survival benefit from prophylactic cranial irradiation (PCI) in patients with limited but stable extracranial disease. 32 , 33 In a meta-analysis of 7 trials of 987 patients published in 1999 comparing PCI versus observation with a positive response to initial treatment, those receiving PCI had an improvement in survival at 3 years from 15.3% to 20.7% ( P = .01). 32 However, in a recent phase III randomized trial in Japan, the median survival for patients receiving PCI was worse than those with observational MRIs. The median survivals were 11.6 months and 13.7 months, respectively, and this trended toward significance (hazard ratio [HR] 1.27, P = .094). 34 This new data has brought into question the efficacy of PCI for patients with SCLC.
Stereotactic Radiosurgery
SRS, in contrast to WBRT, involves the precise focusing of radiation from multiple angles to provide a confined area of high-dose radiation. This decreases the dose of radiation reaching healthy tissue and allows avoidance of radiation-sensitive tissue like the optic nerve. SRS plus WBRT was initially shown to improve intracranial control rates as well as improve overall survival. 35 However, multiple follow-up studies failed to replicate the overall survival advantage. 26 , 35–38 Based on this data, the US and European guidelines recommend against the addition of WBRT to SRS for patients with less than 4 brain metastases. 39 , 40
Advances in radiosurgery technology have made it possible to treat tens of brain metastases if desired. In a Japanese prospective observational study following almost 1200 patients treated with SRS alone, they found no difference in overall survival between patients with 2–4 versus 5–10 brain metastases (HR 0.97, P = .78; P non-inferiority <.0001). Two phase III prospective clinical trials are attempting to provide level 1 evidence for the efficacy of SRS versus WBRT for patients with 4 or more brain metastases ( {"type":"clinical-trial","attrs":{"text":"NCT01592968","term_id":"NCT01592968"}} NCT01592968 and {"type":"clinical-trial","attrs":{"text":"NCT02353000","term_id":"NCT02353000"}} NCT02353000 ).
Postoperative WBRT has been considered standard of care after resection of a single metastasis. 23 , 41 However, with the increased concern of WBRT-associated neurocognitive decline, the role of SRS in these patients was investigated. In a phase III trial comparing SRS to WBRT in the postoperative setting, the cognitive-deterioration-free survival was longer in patients assigned to the SRS group (HR 0.47, P < .0001). The cognitive deterioration at 6 months was less frequent in the SRS group (52% vs 85%, P < .00031). There was no statistical difference in overall survival. 42
In order to determine if SRS was necessary in the postoperative setting, a study was done to compare SRS to the resection cavity and observation with SRS performed only to remaining intact brain metastases. The authors found that the 1-year local control rate was 43% in the observation group and 72% in the SRS group ( P = .015). 42
Additionally, postoperative SRS is associated with increased rates of leptomeningeal disease, especially in the posterior fossa and in breast cancer, compared to postoperative WBRT. 43–45 Due to these risks some are investigating the use of preoperative SRS, which has shown to have similar rates of development of leptomeningeal disease compared to WBRT. 46 , 47 However, the data for its efficacy in this setting are limited to retrospective reports. 44 Combined, these results establish SRS as an effective adjuvant therapy to surgical resection.
Another advance in the area of radiotherapy is hypofractionated SRS, which typically includes 3–5 treatments at a decreased dose. This decreases toxicity around important structures like the brainstem and optic nerve. This strategy also led to low levels of radiation necrosis and improves local control after fractionated stereotactic radiation therapy for brain metastases. 48–52 Also, because SRS alone does not treat microscopic disease, while WBRT is thought to, patients have higher rates of both local and distant recurrence of brain metastases when compared to WBRT plus SRS. 53 A meta-analysis including tumors from multiple primaries with 1–4 intracranial lesions calculated an HR for local control of 2.61 ( P < .0001) and 2.15 ( P < .0001) for distant brain control favoring WBRT and SRS. However, no difference in overall survival was observed (HR 0.98, P = .88). 53 It has been shown that distant failure after upfront SRS is correlated with an increasing number of brain metastases, lowest SRS dose, and melanoma histology. 54 Repeat courses of SRS in these patients can allow patients to maintain neurocognitive function and their quality of life. 55 , 56 Finally, there is great interest in the coordination of radiation therapy and immunotherapy and preliminary evidence suggests concurrent therapy may increase the intracranial efficacy. 57
Neurosurgical resection can be useful in a selected patient population; however, due to the potential comorbidities, surgery is not recommended for everyone. Surgery can be helpful for tissue diagnosis, cerebral decompression, reducing mass effect, and vasogenic edema. With the advent of stereotactic neurosurgical techniques, minimally invasive surgical resection is now possible. From a therapeutic perspective, adjuvant radiotherapy is always necessary to provide any survival benefit. Currently, the European Association of Neuro-oncology (EANO) guidelines recommend surgical resection when the systemic disease is absent or controlled and the KPS is 60 or more. Additionally, surgical resection should be considered for lesions at least 3 cm in diameter, lesions with necrotic appearance and edema/mass effect, posterior fossa lesions associated with hydrocephalus, and lesions located in symptomatic eloquent areas. 39
In addition to direct therapeutic advantages, histopathological analysis of tissue may be necessary for diagnosis and molecular profiling of the tumor. With the development of genetic sequencing, the long hypothesized difference between the primary tumor and the brain metastases has been confirmed. A recent study performed whole-exome sequencing on 86 matched brain metastases, primary tumors, and normal tissue. 58 The authors found that while tumors shared a common ancestor, they continued to evolve independently. In 53% of cases, the authors found clinically informative alterations in the brain metastases not detected in the primary tumor. Besides, spatially and temporally separated brain metastasis were similar but highly divergent from distal extracranial metastases. 58 This knowledge suggests that molecular profiling of surgical biopsies may provide clinical benefit, especially with the further development of immunotherapies and targeted therapies.
Role of Steroids and Anti-Epileptic Drugs
Approximately 20–40% of patients with brain tumors have experienced a seizure episode before or at the time of diagnosis. Another 20–45% will develop seizures at some point during their treatment. 59 These statistics make prophylactic anti-epileptic drugs (AEDs) an attractive treatment option. However, the side effects of AEDs include myelosuppression, cognitive impairment, immunosuppression, and liver dysfunction. Despite numerous studies, there is no evidence for prophylactic AEDs use in seizure-naive patients. This led the Congress of Neurological Surgeons to conclude that prophylactic AEDs are not indicated in seizure-naive patients with metastatic brain tumors preoperatively, intraoperatively, or postoperatively. 60 It is important to note that AEDs are recommended in all patients who have experienced a seizure.
Corticosteroids are prescribed for brain metastases to control mass effect and minimize neurological symptoms. Recent guidelines from the Congress of Neurological Surgeons outline the current recommendations for the appropriate setting and the choice of steroid. 61 Dexamethasone is the drug of choice and should always be tapered as quickly as clinically tolerated. In patients with mild symptoms, temporary steroids are recommended for symptomatic relief related to intracranial pressure and edema with a dose starting at 4–8 mg/day of dexamethasone. In patients with moderate to severe symptoms, doses as high as 16 mg/day can be considered.
Lung Cancer
Lung cancer accounts for the greatest proportion of brain metastases and portends a dismal prognosis. 62 Brain metastases can arise from both NSCLC and SCLC. Until recently, surgery and WBRT or SRS were used to treat NSCLC brain metastases. Due to the advances in the understanding of the biology of NSCLC brain metastasis, there is an increasing role of targeted drugs and immunotherapy in the treatment of these ( Table 2 ).
Significant Trials in Lung Cancer Brain Metastases
The identification of targetable genetic alterations has led to exciting new therapies for NSCLC. The Lung Cancer Mutation Consortium found that targetable oncogenic drivers have been identified in 64% of patients with NSCLC adenocarcinoma. 63 In addition, oncogenic driver mutations can be identified in up to 80% of squamous NSCLC. However, most of these mutations do not have currently approved therapy. 64 , 65 The most recent NSCLC guidelines published by the National Comprehensive Cancer Network (NCCN), 2019 version 4, recommend that the 9 genes related to targeted therapy that should be tested include EGFR, KRAS , HER2 , ALK , ROS1 , MET , BRAF , RET , and NTRK . 66 As mentioned above, recent studies have highlighted genetic evolution from the primary tumor to the brain metastases, suggesting that additional and/or alternative mutation may be driving intracranial progression. 58 Currently, however, retesting the genetic profile of brain metastases is not standard of care.
Epidermal Growth Factor Receptor
Due to the identification of epidermal growth factor receptor (EGFR) overexpression in NSCLC, there was great excitement around EGFR inhibitors in the early 2000s. However, early unselected clinical trials demonstrated limited clinical efficacy. 67–70 It was not until 3 papers published in 2004 demonstrated that activating mutations in the EGFR gene were required for sensitivity to gefitinib and erlotinib, first-generation EGFR tyrosine kinase inhibitors (TKIs). 71–73 EGFR mutations were found to occur at higher rates in never-smokers, and the rate of EGFR mutation patients is highest in the Asian population. 74 The initial retrospective data on the intracranial efficacy in patients harboring EGFR mutations reported intracranial response rates ranging from 42% to 82%. 75–77
The first prospective data on intracranial efficacy compared responses to EGFR TKIs in patients with or without EGFR mutations. A phase II study in China found that in patients with asymptomatic brain metastases, EGFR mutations led to a significantly increased overall survival compared to wild-type patients (37.5 months vs 18.4 months, P = .02). 78 Another phase II trial of the first-generation TKI gefitinib, where all patients had an EGFR mutation and untreated brain metastases, the response rate was 87.8% with an overall survival of 21.9 months.
Unfortunately, the response duration of first-generation EGFR TKIs is often limited due to secondary mutations, primarily threonine–methionine substitution on codon 790 (T790M). 79 , 80
The second-generation EGFR TKI afatinib has also shown intracranial activity. In the LUX-Lung 3 trial, the median time to CNS progression was longer in the afatinib group compared to the chemotherapy group (15.2 months [95% CI 7.7–29.0] vs 5.7 months [95% CI 2.6–8.2]). 81 Additionally, the LUX-Lung 6 trial also demonstrated increased time to CNS progression group (15.2 months [95% CI 3.8–23.7] vs 7.3 months [95% CI 3.7–10.9]). 81 A combined analysis demonstrated a significantly prolonged progression-free survival (PFS) (8.2 vs 5.4 months; HR 0.50, P = .0297).
The third-generation EGFR TKI osimertinib was developed to be effective against the T790M mutation, which is frequently identified after treatment with first-generation TKIs. In a trial for first-line treatment of EGFR-mutated NSCLC comparing osimertinib to first-generation EGFR TKIs, 6% of patients had CNS progression in the osimertinib group compared to 15% in the standard EGFR-TKI group. In addition, osimertinib significantly increased PFS compared to first-generation TKIs (HR 0.46, 95% CI 0.37–0.57). 82 In a subgroup analysis of a trial comparing osimertinib to pemetrexed plus carboplatin or cisplatin in patients who fail first-generation EGFR TKIs, among the 144 patients with brain metastases, the median PFS was longer in the osimertinib group (8.5 months vs 4.2 months; HR 0.32, 95% CI 0.21–0.49). 83 Together, this data consistently have shown better intracranial activity of osimertinib compared to first-generation EGFR TKIs and cytotoxic chemotherapies and is currently considered as first-line treatment for patients with NSCLC. A recent phase III study compared icotinib alone versus WBRT. This study found a significantly improved intracranial PFS in the icotinib alone group (HR 0.56, P < .014). There was no survival benefit in the icotinib alone arm. 84 This drug is only currently approved in China, but highlights the possibility of improved intracranial control with systemic targeted therapies over traditional local therapies.
Anaplastic Lymphoma Kinase
In 2007, the gene anaplastic lymphoma kinase ( ALK ) was found fused with echinoderm microtubule-associated protein-like 4 (EMLA4) gene in patients with NSCLC. 85 Three to seven percent of patients with NSCLC have ALK translocations, and when treated with platinum-based chemotherapy, there is no difference in overall survival. Patients with ALK translocations also have a higher risk of developing brain metastases. 86 The advent of ALK inhibitors has rapidly improved the prognosis of these patients.
ALK inhibitor trials included prospective tumor genotyping, which lead to more rapid and widespread use of these drugs. In the randomized controlled clinical trial for the first-generation ALK inhibitor, crizotinib, 79 patients with stable brain metastases were enrolled. Those patients treated with crizotinib had significantly higher intracranial disease control at 12 and 24 weeks (12 weeks: 85% vs 45%, P < .001; 24 weeks: 56% vs 25%, P = .006). 87 However, resistance to these ALK inhibitors was common and eventually, intracranial progression was seen in most patients.
Second-generation ALK inhibitors, ceritinib, alectinib, and brigatinib, were the next class of ALK inhibitors that were developed. 88–90 In a phase II trial of ceritinib, of the 100 patients who had baseline brain metastases, there was a 45% intracranial response rate (95% CI 23.1–68.5%) with an 80% intracranial disease control rate. 91 In phase I/II study, patients with crizotinib-resistant ALK -rearranged NSCLC were treated with alectinib. Of the 21 patients with baseline brain metastases, 11 had an objective response, 6 of which were complete responses. 92 This led to a phase III trial comparing alectinib versus crizotinib in ALK inhibitor naive patients who found a significant improvement in PFS (HR 0.08, 95% CI 0.01–0.61). Within this study, the HR for intracranial PFS was 0.51 (95% CI 0.16–1.64). 93 , 94 In a second phase III trial comparing alectinib to crizotinib, patients treated with alectinib, only 12% had CNS progression compared to 45% of patients treated with crizotinib (HR 0.16, 95% CI 0.10–0.28, P < .001). In addition, CNS complete response was significantly more likely in the alectinib group compared to the crizotinib group (45% vs 9%, P -value <.001). 95 The combination of these trials demonstrates the intracranial efficacy of second-generation ALK inhibitors. The first FDA-approved third-generation ALK inhibitor was lorlatinib. Lorlatinib was designed to penetrate the BBB and has broad ALK mutational coverage. In phase II clinical trial, in patients with at least one prior ALK inhibitor, 51 of 81 patients had an intracranial response leading to a 63% response rate (95% CI 51.5–73.4%). 96 This data led to the accelerated approval of lorlatinib for patients who have progressed on crizotinib and at least one other ALK inhibitor for metastatic disease; or whose disease has progressed on alectinib or ceritinib as the first ALK inhibitor therapy for metastatic disease. Currently, ALK -positive patients and patients with EGFR-mutated lung cancer who have asymptomatic brain metastases may be treated with only targeted therapy and have local therapy omitted until progression.
Immune Checkpoint Inhibitors
In addition to targeted therapies, immunotherapies are also rapidly altering the treatment of NSCLC. In particular, the anti-PD-1 antibodies pembrolizumab and nivolumab and the PD-L1 antibody atezolizumab have all shown efficacy in NSCLC. 97–99 PD-L1 expression within the lung tumor is indicative of survival; however, oftentimes PD-L1 expression in an intracranial lesion is unknown. A study of 73 lung cancer patients with paired samples of the primary tumor and brain metastases evaluated the tumor PD-L1 expression and tumor microenvironment PD-L1 expression. 100 The authors found that in 14% of cases, there was a disagreement between the primary site and the brain metastases in tumor cell PD-L1 expression. Additionally, the authors found disagreement in tumor-infiltrating lymphocytes in 26% of cases. Another study found that 7 of 32 patients with NSCLC had PDL1 expression more than 5% in their tumor. 101 This suggested different expression in the brain metastases, and the primary tumor is possible. However, routine testing of PD-L1 in brain metastases is currently not the standard of care.
Several retrospective studies have investigated the intracranial efficacy of immunotherapy for NSCLC brain metastases. In an Italian series of 409 patients with asymptomatic brain metastases, the disease control rate was 40%. 102 In a French study of 130 patients with brain metastases, 37% had either stable disease or partial response with an overall survival of 6.6 months. 103 In a phase II study of pembrolizumab in patients with NSCLC brain metastases, 33% of patients had an intracranial response. 104 , 105 In a follow-up abstract investigating the durability of the response, the authors reported a CNS PFS of 10.7 months with 31% of patients surviving at least 2 years. 105 In the phase III KEYNOTE 189 trial of pembrolizumab plus chemotherapy versus chemotherapy alone the HR for patients with stable brain metastases was 0.36 (95% CI 0.20–0.62), supporting the efficacy of pembrolizumab in patients with brain metastases. 98
Breast Cancer
Breast cancer is the second most common cancer leading to brain metastases. 5 Triple-negative breast cancer patients are most at risk for the development of brain metastases, with a median overall survival of fewer than 6 months. 106 , 107 Unfortunately, targeted therapies for brain metastases in this population are lacking, and these patients are primarily treated with chemotherapy. 108 Recently, the FDA approved atezolizumab (a PD-L1 inhibitor), a class of drugs that have shown some efficacy in brain metastases from melanoma and NSCLC. However, the phase III clinical trial that led to its approval only included patients with asymptomatic treated CNS metastases. While the number of patients in this subgroup was small, there was no statistical difference in PFS between the atezolizumab plus Nab-Paclitaxel versus the placebo plus Nab-Paclitaxel (HR 0.86, 95% CI 0.50–1.49) ( Table 3 ). 109
Significant Trials in Breast Cancer Brain Metastases
Human Epidermal Growth Factor Receptor 2
In 20–30% of breast cancers, the human EGFR 2 (HER2) is over-expressed. HER2 -directed drugs include trastuzumab, pertuzumab, ado-trastuzumab emtansine, neratinib, tucatinib, and lapatinib. 110 A study investigating 377 women with CNS metastasis from HER2 -positive breast cancer found that those with brain metastases were younger and more likely to have a higher disease burden. 111 The median time to CNS progression was 13.3 months and those treated with trastuzumab had a significant improvement in median overall survival (17.5 months vs 3.8 months) and was significant on the multivariable analysis (HR 0.33, P < .001). Two other studies have also demonstrated improved overall survival of trastuzumab in patients with brain metastases. 112 , 113
In a phase II trial investigating the small molecule inhibitor lapatinib with capecitabine in patients with untreated brain metastases, 29 of 45 patients had objective CNS response. 114 A study was done to investigate the ability of lapatinib to prevent brain metastases. In the study, HER2 -positive metastatic breast cancer patients were treated with either lapatinib or trastuzumab in combination with capecitabine. This trial was closed early due to poor accrual, but the authors ultimately found that the incidence of CNS metastases as the first site of relapse was 3% for the lapatinib group versus 5% for the trastuzumab group ( P = .36). 115
Neratinib is a small molecule irreversible TKI of EGFR, HER2 , and HER4 that was hypothesized to have efficacy against brain metastases. As a monotherapy, the intracranial response rate was only 8%; however, in combination with capecitabine, the response rate was 49%. 116 , 117 As a result, the NCCN guidelines include neratinib with capecitabine as an option for the management of HER2 -positive breast cancer brain metastases. 108 Additionally, the combination of HER2 -directed therapy with SRS has been shown to increase local tumor control. 118 , 119
Tucatinib is another small, selective HER2 TKI that results in less diarrhea and skin toxicities. 120 , 121 A phase I study which combined tucatinib with trastuzumab reported that the combination led to an intracranial objective response rate of 12%. 122 When tucatinib and trastuzumab were combined with capecitabine, 42% of patients had an intracranial objective response. 121 A phase II trial that includes patients with progressive brain metastases (NCT02614794n) is currently investigating this combination.
Hormone Receptor-Positive Disease
The current guidelines for patients with hormone receptor-positive disease recommend endocrine therapy as first-line treatment. 123 Interestingly, the concentration of tamoxifen and its metabolites can be up to 46-fold higher in the brain tissue compared with serum. 124 Additionally, because aromatase inhibitors work by inhibiting the generation of estrogens in the ovaries (premenopausal women) and peripheral tissue (postmenopausal women), this class of drugs does not require brain penetration in order to reduce the levels of estrogen in the brain. However, the survival data supporting endocrine therapy for the treatment of brain metastases are relatively weak and limited. 125 , 126 Whole-exome sequencing of 21 patients with breast cancer found frequent alterations of the CDK and PI3K pathways and that these changes were often unique to brain metastases. 58 As a result, the oral CDK inhibitor abemaciclib was studied in the phase III MONARCH trial and showed significantly prolonged PFS (HR 0.54, P = .000021), but the trial excluded patients with brain metastases. 127 An ongoing clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT02308020","term_id":"NCT02308020"}} NCT02308020 ) is testing the intracranial efficacy of abemaciclib. Early data from this trial demonstrated an intracranial response in 2 of 23 patients. 128
Melanoma is the third most frequent of the solid tumors that metastasizes to the brain. 7 Estimates predict that up to 75% of patients with metastatic melanoma will have evidence of CNS involvement at the time of autopsy. 129 The key driver mutations in melanoma involve CDKN2A , BRAF , NRAS , and KIT . 130 Of these, mutations to v-RAF murine sarcoma viral oncogene homology B ( BRAF ) is present in up to 50% of advanced melanoma patients, the majority resulting from a substitution of valine to glutamate at codon 600 (V600E) or valine to lysine at the same codon (V600K) ( Table 4 ). 131 , 132
Significant Trials in Melanoma Brain Metastases
BRAF Inhibitors
While patients with brain metastases were excluded from the majority of the initial phase III trial for the approval of BRAF inhibitors, the phase II trial BREAK-MB was the first to specifically investigate the intracranial efficacy. In this study, 172 melanoma patients were treated with oral dabrafenib and the authors found a 39% response rate in patients who had not previously received local treatment and 31% in those who had. 132 In a phase II study of Vemurafenib in 146 patients, the authors found that 18% of patients with previously untreated brain metastases had intracranial response. 133 Unfortunately, the response to BRAF inhibitors is limited to a few months and most patients will have disease recurrence within 12 months. 134
The tumors often become resistant to the BRAF inhibition through the mutations resulting in the reactivation of the MAPK pathway. In order to counter this, MEK inhibitors are often combined with BRAF inhibitors. In a phase II trial with dabrafenib plus trametinib intracranial response was seen between 44% and 59% of patients depending on previous therapies, suggesting the efficacy of the combination. However, the duration of the intracranial response was relatively short, ranging from 4.5 to 8.3 months. 135 Additional phase II trials are currently underway investigating the efficacy of the combination of these drugs (Vemurafenib plus combimetinib {"type":"clinical-trial","attrs":{"text":"NCT02537600","term_id":"NCT02537600"}} NCT02537600 and {"type":"clinical-trial","attrs":{"text":"NCT03430947","term_id":"NCT03430947"}} NCT03430947 , and Dabrafenib plus trametinib {"type":"clinical-trial","attrs":{"text":"NCT02974803","term_id":"NCT02974803"}} NCT02974803 ) with radiosurgery.
The most promising shift in melanoma brain metastasis care has been the development of immune checkpoint inhibitors. Immune checkpoint inhibitors demonstrate a more durable response compared to BRAF inhibitors. The anti-CTLA4 monoclonal antibody ipilimumab was the first to demonstrate intracranial efficacy. In a phase II trial, patients were separated into 2 groups, those who were not receiving corticosteroids (cohort A) and those who required corticosteroids for symptomatic control (cohort B). The intracranial disease control rate was 24% in cohort A and 10% in cohort B. More striking was the difference in overall survival between the 2 groups 7 months versus 3.7 months. 136 In another phase II trial of patients with untreated brain metastases treated with pembrolizumab, 26% of patients had an intracranial response, with 48% of patients alive at 24 months. 137
Even more impressive has been the results of CheckMate-204, a phase II clinical trial that enrolled 90 patients with asymptomatic brain metastases and treated with a combination of nivolumab and ipilimumab. Among the 94 patients treated, the rate of intracranial clinical benefit was 57% with a complete intracranial response of 26%. 138 In a similar phase II trial comparing the combination of ipilimumab and nivolumab versus nivolumab alone. In the combination arm, the intracranial response rate was 46% versus 20% in the nivolumab alone arm. However, overall survival was similar between the groups. Of note, the third arm with symptomatic metastases or leptomeningeal disease had significantly worse outcomes. 139 In patients with symptomatic brain metastases who received at least one dose of both ipilimumab and nivolumab had an intracranial response rate of 16.7%. 140
While these results strongly suggest the durable intracranial efficacy of combination immunotherapy, they were not powered to determine the difference in overall survival. A phase III trial currently recruiting patients is powered to investigate differences in survival in melanoma brain metastases ( {"type":"clinical-trial","attrs":{"text":"NCT02460068","term_id":"NCT02460068"}} NCT02460068 ). Another phase II trial is comparing the efficacy of ipilimumab plus nivolumab plus SRS compared to ipilimumab plus nivolumab alone and is designed to determine differences in neurologic specific survival at 12 months ( {"type":"clinical-trial","attrs":{"text":"NCT03340129","term_id":"NCT03340129"}} NCT03340129 ). 141 Finally, the efficacy of pembrolizumab in patients with brain metastases is also under investigation ( {"type":"clinical-trial","attrs":{"text":"NCT02886585","term_id":"NCT02886585"}} NCT02886585 ). In the melanoma arm of this trial between cycles 1 and 2 of pembrolizumab, SRS will be administered.
Future Directions and Conclusion
Advances in our ability to identify actionable mutations in patients with brain metastases have enabled the development of more advanced trial designs. The Alliance A071701 trial will build off these advances in patients with brain metastases, primarily from lung and breast primary tumors. In this trial, patients with progressive brain metastases who have tissue (brain or extracranial) available for sequencing will be assigned into 1 of 3 cohorts based on genetic alterations. Actionable alterations in the CDK pathway will be treated with abemaciclib as above. Mutations in the PI3K/AKT/mTOR pathways will be treated with the PI3K inhibitor entrectinib. Finally, patients with ALK/NTRK/ROS1 translocations will be treated with an inhibitor of this pathway, GDC-0084. The primary endpoint in this trial will be the CNS response rate.
In the last decade advancements in our understanding of brain metastases and the development of new therapies have provided a new outlook on brain metastases. Developments in radiation therapies with the increased use of SRS and hippocampal sparing WBRT may limit the neurocognitive decline that has been a staple of radiation treatment for many years. In addition, the presence of the BBB led to the historical viewpoint that systemic therapies played little role in the management of brain metastases. Neurocognitive decline and the patient’s quality of life must always be at the forefront of any therapeutic advancement. The presence of BBB led to the historical viewpoint that systemic therapies played little role in the management of brain metastasis. However, advances in targeted therapies and immune checkpoint inhibitors are providing novel medical therapeutics. Moving forward, the appropriate combination of these novel approaches with focused forms of radiation will be an active form of clinical investigation. The new age of precision medicine will enable clinicians to better estimate a patient’s prognosis and help identify appropriate management options promising future improvement in the management of brain metastases and better prognosis for patients. 142
Funding and Conflict of Interest Statement
Conflict of Interest for Manmeet Ahluwalia includes receipt of grants/research supports: Astrazeneca, Abbvie, BMS, Bayer, Incyte, Pharmacyclics, Novocure, Merck. Receipt of honoraria or consultation fees: Elsevier, Wiley, Astrazeneca, Abbvie, VBI Vaccines, Flatiron, Varian Medical Systems, Prime Education, Bayer, Karyopharm, Tocagen, Forma therapeutics. Stock shareholder: Doctible, Mimivax. Other authors have no conflict to report.

IMAGES
VIDEO
COMMENTS
Introduction. Brain metastases (BM) are the most common intracranial neoplasms in adults and are the primary cause of neurologic complications resulting from systemic cancers [].Left untreated, afflicted patients suffer a dismal 3-month median survival, however, the rapid advancement in imaging and therapeutics, including systemic treatments in the form of immunotherapy in combination with ...
Introduction. Brain metastasis continues to represent a significant source of morbidity and mortality in patients with different types of systemic cancer, as first recognized by Bucholz in 1898. 1 The true incidence of brain metastasis is difficult to determine accurately. Most previous estimates emerged from historical neurosurgical series, and because neurosurgeons were reluctant to operate ...
Brain metastases (BM) affect up to one-third of adults with solid tumor malignancies and are associated with significant cancer patient morbidity, anxiety, and mortality. Approximately 70,000-400,000 patients will develop BM each year in the USA [ 1, 2, 3 ]. Consequentially, BM represent an important public health care burden that is also ten ...
1. Epidemiology of Brain Metastases. Cancer is the second most prevalent cause of death worldwide [], with lung, breast, colorectal, and prostate being the most frequently affected organs [].Tumor cell seeding into the central nervous system [], mostly localized in the brain parenchyma, the cerebrospinal fluid (CSF), the dura, and the bone structures of the skull, is a frequent complication of ...
Abstract. Brain metastasis, which commonly arises in patients with lung cancer, breast cancer and melanoma, is associated with poor survival outcomes and poses distinct clinical challenges. The ...
Brain metastases (BMs) represent the most common intra-cranialmalignanciesinadults,accountingforupto50%ofall brain tumors.1 The incidence of BM has been increasing in recent years, likely due to the availability of effective sys-temic therapies for primary cancers, the immunological nature of the brain as a sanctuary site, along with advances
Nature Reviews Cancer 20 , 1 ( 2020) Cite this article. This Focus issue highlights current research into the unique biology of brain tumours and brain metastasis and how this research might ...
Brain metastases (BMs) represent the most common intracranial neoplasm in adults. They affect around 20% of all cancer patients 1,2,3,4,5,6, and are among the main complications of lung, breast ...
Abstract. Brain metastases are intracranial recurrence of extracranial malignant tumors with a high incidence and poor prognosis. Owing to the particularity of intracranial localization, clinical diagnosis (neuroimaging and biopsy) of brain metastases is associated with shortcomings such as delayed diagnosis and biopsy invasiveness.
In the United States, it is estimated that between 8% and 10% of patients with cancer will develop brain metastases representing approximately 200,000 new patients with brain metastases every year. 1 The point prevalence of brain metastases on initial diagnosis varies widely between different cancer histologies. For example, the incidence proportion of patients with metastatic cancer who have ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Brain metastases are a common ...
Brain metastases are an increasing global public health concern, even as survival rates improve for patients with metastatic disease. Both metastases and the sequelae of their treatment are key determinants of the inter-related priorities of patient survival, function, and quality of life, mandating a multidimensional approach to clinical care and research. At a virtual National Cancer ...
Abstract: Cerebral metastases or brain metastases (brain mets) is the most common malignant intracranial cancer among all. brain tumors. Brain mets are the secondary tumors migrated from the ...
This review focuses on the management of brain metastases. The four main modes of therapy are discussed: whole brain radiation therapy (WBRT), surgery, radiosurgery, and chemotherapy. Young patients with limited extracranial disease may benefit from surgical resection of a single brain metastasis, and from radiosurgery (or stereotactic radiotherapy) if two to four brain metastases are present ...
Introduction Brain metastases are a common cause of morbidity and mortality in patients with breast cancer. Local central nervous system (CNS) directed therapies are usually the first line treatment for breast cancer brain metastases (BCBM), but those must be followed by systemic therapies to achieve long-term benefit. Systemic therapy for hormone receptor (HR+) breast cancer has evolved in ...
Within 2 years of diagnosis, almost 80% of SCLC patients experience brain metastases (BMs). 2 The median survival for patients with untreated SCLC-BMs is approximately 3 months. 3 Most BMs from SCLC are multifocal, making whole brain radiotherapy (WBRT) the preferred treatment option for patients with SCLC BMs. 4 Previous researches have ...
This is a dataset of 156 pre- and post-contrast whole brain MRI studies in patients with at least 1 cerebral metastasis. Mean patient age was 63±12 years (range: 29-92 years). Primary malignancies included lung (n = 99), breast (n = 33), melanoma (n = 7), genitourinary (n = 7), gastrointestinal (n = 5), and miscellaneous cancers (n = 5).
Background Brain metastasis (BM) is common among cases of advanced non-small cell lung cancer (NSCLC) and is the leading cause of death for these patients. Mesothelin (MSLN), a tumor-associated antigen expressed in many solid tumors, has been reported to be involved in the progression of multiple tumors. However, its potential involvement in BM of NSCLC and the underlying mechanism remain ...
Previous research has shown that the incidence of BM is ten times higher than all primary brain tumors combined. 20 Most BM originates from lung and breast cancer, and results in high mortality and poor prognosis. 21 Brain metastases occur in 10%-30% of patients with metastatic breast cancer, 22 and the median time from diagnosis of breast ...
Another, newer, approach is the study of brain waves, rhythmic, repetitive patterns of brain cell activity caused by synchronization across cells. In a new paper, two Yale researchers propose that these two ways of thinking about brain activity may not represent separate events but two aspects of the same occurrence.
We address prevailing challenges of the brain-powered research, departing from the observation that the literature hardly recover accurate spatial information and require subject-specific models. To address these challenges, we propose UMBRAE, a unified multimodal decoding of brain signals. First, to extract instance-level conceptual and spatial details from neural signals, we introduce an ...
The development of brain metastases occurs in 10-20% of all patients with cancer. Brain metastases portend poor survival and contribute to increased cancer mortality and morbidity. Despite multimodal treatment options, which include surgery, radiotherapy, and chemotherapy, 5-year survival remains low. Besides, our current treatment modalities ...