Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character

Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- March 01, 2024 | VOL. 181, NO. 3 CURRENT ISSUE pp.171-254
- February 01, 2024 | VOL. 181, NO. 2 pp.83-170
- January 01, 2024 | VOL. 181, NO. 1 pp.1-82
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Substance Use Disorders and Addiction: Mechanisms, Trends, and Treatment Implications
- Ned H. Kalin , M.D.
Search for more papers by this author
The numbers for substance use disorders are large, and we need to pay attention to them. Data from the 2018 National Survey on Drug Use and Health ( 1 ) suggest that, over the preceding year, 20.3 million people age 12 or older had substance use disorders, and 14.8 million of these cases were attributed to alcohol. When considering other substances, the report estimated that 4.4 million individuals had a marijuana use disorder and that 2 million people suffered from an opiate use disorder. It is well known that stress is associated with an increase in the use of alcohol and other substances, and this is particularly relevant today in relation to the chronic uncertainty and distress associated with the COVID-19 pandemic along with the traumatic effects of racism and social injustice. In part related to stress, substance use disorders are highly comorbid with other psychiatric illnesses: 9.2 million adults were estimated to have a 1-year prevalence of both a mental illness and at least one substance use disorder. Although they may not necessarily meet criteria for a substance use disorder, it is well known that psychiatric patients have increased usage of alcohol, cigarettes, and other illicit substances. As an example, the survey estimated that over the preceding month, 37.2% of individuals with serious mental illnesses were cigarette smokers, compared with 16.3% of individuals without mental illnesses. Substance use frequently accompanies suicide and suicide attempts, and substance use disorders are associated with a long-term increased risk of suicide.
Addiction is the key process that underlies substance use disorders, and research using animal models and humans has revealed important insights into the neural circuits and molecules that mediate addiction. More specifically, research has shed light onto mechanisms underlying the critical components of addiction and relapse: reinforcement and reward, tolerance, withdrawal, negative affect, craving, and stress sensitization. In addition, clinical research has been instrumental in developing an evidence base for the use of pharmacological agents in the treatment of substance use disorders, which, in combination with psychosocial approaches, can provide effective treatments. However, despite the existence of therapeutic tools, relapse is common, and substance use disorders remain grossly undertreated. For example, whether at an inpatient hospital treatment facility or at a drug or alcohol rehabilitation program, it was estimated that only 11% of individuals needing treatment for substance use received appropriate care in 2018. Additionally, it is worth emphasizing that current practice frequently does not effectively integrate dual diagnosis treatment approaches, which is important because psychiatric and substance use disorders are highly comorbid. The barriers to receiving treatment are numerous and directly interact with existing health care inequities. It is imperative that as a field we overcome the obstacles to treatment, including the lack of resources at the individual level, a dearth of trained providers and appropriate treatment facilities, racial biases, and the marked stigmatization that is focused on individuals with addictions.
This issue of the Journal is focused on understanding factors contributing to substance use disorders and their comorbidity with psychiatric disorders, the effects of prenatal alcohol use on preadolescents, and brain mechanisms that are associated with addiction and relapse. An important theme that emerges from this issue is the necessity for understanding maladaptive substance use and its treatment in relation to health care inequities. This highlights the imperative to focus resources and treatment efforts on underprivileged and marginalized populations. The centerpiece of this issue is an overview on addiction written by Dr. George Koob, the director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and coauthors Drs. Patricia Powell (NIAAA deputy director) and Aaron White ( 2 ). This outstanding article will serve as a foundational knowledge base for those interested in understanding the complex factors that mediate drug addiction. Of particular interest to the practice of psychiatry is the emphasis on the negative affect state “hyperkatifeia” as a major driver of addictive behavior and relapse. This places the dysphoria and psychological distress that are associated with prolonged withdrawal at the heart of treatment and underscores the importance of treating not only maladaptive drug-related behaviors but also the prolonged dysphoria and negative affect associated with addiction. It also speaks to why it is crucial to concurrently treat psychiatric comorbidities that commonly accompany substance use disorders.
Insights Into Mechanisms Related to Cocaine Addiction Using a Novel Imaging Method for Dopamine Neurons
Cassidy et al. ( 3 ) introduce a relatively new imaging technique that allows for an estimation of dopamine integrity and function in the substantia nigra, the site of origin of dopamine neurons that project to the striatum. Capitalizing on the high levels of neuromelanin that are found in substantia nigra dopamine neurons and the interaction between neuromelanin and intracellular iron, this MRI technique, termed neuromelanin-sensitive MRI (NM-MRI), shows promise in studying the involvement of substantia nigra dopamine neurons in neurodegenerative diseases and psychiatric illnesses. The authors used this technique to assess dopamine function in active cocaine users with the aim of exploring the hypothesis that cocaine use disorder is associated with blunted presynaptic striatal dopamine function that would be reflected in decreased “integrity” of the substantia nigra dopamine system. Surprisingly, NM-MRI revealed evidence for increased dopamine in the substantia nigra of individuals using cocaine. The authors suggest that this finding, in conjunction with prior work suggesting a blunted dopamine response, points to the possibility that cocaine use is associated with an altered intracellular distribution of dopamine. Specifically, the idea is that dopamine is shifted from being concentrated in releasable, functional vesicles at the synapse to a nonreleasable cytosolic pool. In addition to providing an intriguing alternative hypothesis underlying the cocaine-related alterations observed in substantia nigra dopamine function, this article highlights an innovative imaging method that can be used in further investigations involving the role of substantia nigra dopamine systems in neuropsychiatric disorders. Dr. Charles Bradberry, chief of the Preclinical Pharmacology Section at the National Institute on Drug Abuse, contributes an editorial that further explains the use of NM-MRI and discusses the theoretical implications of these unexpected findings in relation to cocaine use ( 4 ).
Treatment Implications of Understanding Brain Function During Early Abstinence in Patients With Alcohol Use Disorder
Developing a better understanding of the neural processes that are associated with substance use disorders is critical for conceptualizing improved treatment approaches. Blaine et al. ( 5 ) present neuroimaging data collected during early abstinence in patients with alcohol use disorder and link these data to relapses occurring during treatment. Of note, the findings from this study dovetail with the neural circuit schema Koob et al. provide in this issue’s overview on addiction ( 2 ). The first study in the Blaine et al. article uses 44 patients and 43 control subjects to demonstrate that patients with alcohol use disorder have a blunted neural response to the presentation of stress- and alcohol-related cues. This blunting was observed mainly in the ventromedial prefrontal cortex, a key prefrontal regulatory region, as well as in subcortical regions associated with reward processing, specifically the ventral striatum. Importantly, this finding was replicated in a second study in which 69 patients were studied in relation to their length of abstinence prior to treatment and treatment outcomes. The results demonstrated that individuals with the shortest abstinence times had greater alterations in neural responses to stress and alcohol cues. The authors also found that an individual’s length of abstinence prior to treatment, independent of the number of days of abstinence, was a predictor of relapse and that the magnitude of an individual’s neural alterations predicted the amount of heavy drinking occurring early in treatment. Although relapse is an all too common outcome in patients with substance use disorders, this study highlights an approach that has the potential to refine and develop new treatments that are based on addiction- and abstinence-related brain changes. In her thoughtful editorial, Dr. Edith Sullivan from Stanford University comments on the details of the study, the value of studying patients during early abstinence, and the implications of these findings for new treatment development ( 6 ).
Relatively Low Amounts of Alcohol Intake During Pregnancy Are Associated With Subtle Neurodevelopmental Effects in Preadolescent Offspring
Excessive substance use not only affects the user and their immediate family but also has transgenerational effects that can be mediated in utero. Lees et al. ( 7 ) present data suggesting that even the consumption of relatively low amounts of alcohol by expectant mothers can affect brain development, cognition, and emotion in their offspring. The researchers used data from the Adolescent Brain Cognitive Development Study, a large national community-based study, which allowed them to assess brain structure and function as well as behavioral, cognitive, and psychological outcomes in 9,719 preadolescents. The mothers of 2,518 of the subjects in this study reported some alcohol use during pregnancy, albeit at relatively low levels (0 to 80 drinks throughout pregnancy). Interestingly, and opposite of that expected in relation to data from individuals with fetal alcohol spectrum disorders, increases in brain volume and surface area were found in offspring of mothers who consumed the relatively low amounts of alcohol. Notably, any prenatal alcohol exposure was associated with small but significant increases in psychological problems that included increases in separation anxiety disorder and oppositional defiant disorder. Additionally, a dose-response effect was found for internalizing psychopathology, somatic complaints, and attentional deficits. While subtle, these findings point to neurodevelopmental alterations that may be mediated by even small amounts of prenatal alcohol consumption. Drs. Clare McCormack and Catherine Monk from Columbia University contribute an editorial that provides an in-depth assessment of these findings in relation to other studies, including those assessing severe deficits in individuals with fetal alcohol syndrome ( 8 ). McCormack and Monk emphasize that the behavioral and psychological effects reported in the Lees et al. article would not be clinically meaningful. However, it is feasible that the influences of these low amounts of alcohol could interact with other predisposing factors that might lead to more substantial negative outcomes.
Increased Comorbidity Between Substance Use and Psychiatric Disorders in Sexual Identity Minorities
There is no question that victims of societal marginalization experience disproportionate adversity and stress. Evans-Polce et al. ( 9 ) focus on this concern in relation to individuals who identify as sexual minorities by comparing their incidence of comorbid substance use and psychiatric disorders with that of individuals who identify as heterosexual. By using 2012−2013 data from 36,309 participants in the National Epidemiologic Study on Alcohol and Related Conditions–III, the authors examine the incidence of comorbid alcohol and tobacco use disorders with anxiety, mood disorders, and posttraumatic stress disorder (PTSD). The findings demonstrate increased incidences of substance use and psychiatric disorders in individuals who identified as bisexual or as gay or lesbian compared with those who identified as heterosexual. For example, a fourfold increase in the prevalence of PTSD was found in bisexual individuals compared with heterosexual individuals. In addition, the authors found an increased prevalence of substance use and psychiatric comorbidities in individuals who identified as bisexual and as gay or lesbian compared with individuals who identified as heterosexual. This was most prominent in women who identified as bisexual. For example, of the bisexual women who had an alcohol use disorder, 60.5% also had a psychiatric comorbidity, compared with 44.6% of heterosexual women. Additionally, the amount of reported sexual orientation discrimination and number of lifetime stressful events were associated with a greater likelihood of having comorbid substance use and psychiatric disorders. These findings are important but not surprising, as sexual minority individuals have a history of increased early-life trauma and throughout their lives may experience the painful and unwarranted consequences of bias and denigration. Nonetheless, these findings underscore the strong negative societal impacts experienced by minority groups and should sensitize providers to the additional needs of these individuals.
Trends in Nicotine Use and Dependence From 2001–2002 to 2012–2013
Although considerable efforts over earlier years have curbed the use of tobacco and nicotine, the use of these substances continues to be a significant public health problem. As noted above, individuals with psychiatric disorders are particularly vulnerable. Grant et al. ( 10 ) use data from the National Epidemiologic Survey on Alcohol and Related Conditions collected from a very large cohort to characterize trends in nicotine use and dependence over time. Results from their analysis support the so-called hardening hypothesis, which posits that although intervention-related reductions in nicotine use may have occurred over time, the impact of these interventions is less potent in individuals with more severe addictive behavior (i.e., nicotine dependence). When adjusted for sociodemographic factors, the results demonstrated a small but significant increase in nicotine use from 2001–2002 to 2012–2013. However, a much greater increase in nicotine dependence (46.1% to 52%) was observed over this time frame in individuals who had used nicotine during the preceding 12 months. The increases in nicotine use and dependence were associated with factors related to socioeconomic status, such as lower income and lower educational attainment. The authors interpret these findings as evidence for the hardening hypothesis, suggesting that despite the impression that nicotine use has plateaued, there is a growing number of highly dependent nicotine users who would benefit from nicotine dependence intervention programs. Dr. Kathleen Brady, from the Medical University of South Carolina, provides an editorial ( 11 ) that reviews the consequences of tobacco use and the history of the public measures that were initially taken to combat its use. Importantly, her editorial emphasizes the need to address health care inequity issues that affect individuals of lower socioeconomic status by devoting resources to develop and deploy effective smoking cessation interventions for at-risk and underresourced populations.
Conclusions
Maladaptive substance use and substance use disorders are highly prevalent and are among the most significant public health problems. Substance use is commonly comorbid with psychiatric disorders, and treatment efforts need to concurrently address both. The papers in this issue highlight new findings that are directly relevant to understanding, treating, and developing policies to better serve those afflicted with addictions. While treatments exist, the need for more effective treatments is clear, especially those focused on decreasing relapse rates. The negative affective state, hyperkatifeia, that accompanies longer-term abstinence is an important treatment target that should be emphasized in current practice as well as in new treatment development. In addition to developing a better understanding of the neurobiology of addictions and abstinence, it is necessary to ensure that there is equitable access to currently available treatments and treatment programs. Additional resources must be allocated to this cause. This depends on the recognition that health care inequities and societal barriers are major contributors to the continued high prevalence of substance use disorders, the individual suffering they inflict, and the huge toll that they incur at a societal level.
Disclosures of Editors’ financial relationships appear in the April 2020 issue of the Journal .
1 US Department of Health and Human Services: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality: National Survey on Drug Use and Health 2018. Rockville, Md, SAMHSA, 2019 ( https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2018-NSDUH ) Google Scholar
2 Koob GF, Powell P, White A : Addiction as a coping response: hyperkatifeia, deaths of despair, and COVID-19 . Am J Psychiatry 2020 ; 177:1031–1037 Link , Google Scholar
3 Cassidy CM, Carpenter KM, Konova AB, et al. : Evidence for dopamine abnormalities in the substantia nigra in cocaine addiction revealed by neuromelanin-sensitive MRI . Am J Psychiatry 2020 ; 177:1038–1047 Link , Google Scholar
4 Bradberry CW : Neuromelanin MRI: dark substance shines a light on dopamine dysfunction and cocaine use (editorial). Am J Psychiatry 2020 ; 177:1019–1021 Abstract , Google Scholar
5 Blaine SK, Wemm S, Fogelman N, et al. : Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation . Am J Psychiatry 2020 ; 177:1048–1059 Abstract , Google Scholar
6 Sullivan EV : Why timing matters in alcohol use disorder recovery (editorial). Am J Psychiatry 2020 ; 177:1022–1024 Abstract , Google Scholar
7 Lees B, Mewton L, Jacobus J, et al. : Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the Adolescent Brain Cognitive Development Study . Am J Psychiatry 2020 ; 177:1060–1072 Link , Google Scholar
8 McCormack C, Monk C : Considering prenatal alcohol exposure in a developmental origins of health and disease framework (editorial). Am J Psychiatry 2020 ; 177:1025–1028 Abstract , Google Scholar
9 Evans-Polce RJ, Kcomt L, Veliz PT, et al. : Alcohol, tobacco, and comorbid psychiatric disorders and associations with sexual identity and stress-related correlates . Am J Psychiatry 2020 ; 177:1073–1081 Abstract , Google Scholar
10 Grant BF, Shmulewitz D, Compton WM : Nicotine use and DSM-IV nicotine dependence in the United States, 2001–2002 and 2012–2013 . Am J Psychiatry 2020 ; 177:1082–1090 Link , Google Scholar
11 Brady KT : Social determinants of health and smoking cessation: a challenge (editorial). Am J Psychiatry 2020 ; 177:1029–1030 Abstract , Google Scholar
- Cited by None

- Substance-Related and Addictive Disorders
- Addiction Psychiatry
- Transgender (LGBT) Issues
- Open access
- Published: 21 June 2021
A review of research-supported group treatments for drug use disorders
- Gabriela López 1 ,
- Lindsay M. Orchowski ORCID: orcid.org/0000-0001-9048-3576 2 ,
- Madhavi K. Reddy 3 ,
- Jessica Nargiso 4 &
- Jennifer E. Johnson 5
Substance Abuse Treatment, Prevention, and Policy volume 16 , Article number: 51 ( 2021 ) Cite this article
26k Accesses
7 Citations
3 Altmetric
Metrics details
This paper reviews methodologically rigorous studies examining group treatments for interview-diagnosed drug use disorders. A total of 50 studies reporting on the efficacy of group drug use disorder treatments for adults met inclusion criteria. Studies examining group treatment for cocaine, methamphetamine, marijuana, opioid, mixed substance, and substance use disorder with co-occurring psychiatric conditions are discussed. The current review showed that cognitive behavioral therapy (CBT) group therapy and contingency management (CM) groups appear to be more effective at reducing cocaine use than treatment as usual (TAU) groups. CM also appeared to be effective at reducing methamphetamine use relative to standard group treatment. Relapse prevention support groups, motivational interviewing, and social support groups were all effective at reducing marijuana use relative to a delayed treatment control. Group therapy or group CBT plus pharmacotherapy are more effective at decreasing opioid use than pharmacotherapy alone. An HIV harm reduction program has also been shown to be effective for reducing illicit opioid use. Effective treatments for mixed substance use disorder include group CBT, CM, and women’s recovery group. Behavioral skills group, group behavioral therapy plus CM, Seeking Safety, Dialectical behavior therapy groups, and CM were more effective at decreasing substance use and psychiatric symptoms relative to TAU, but group psychoeducation and group CBT were not. Given how often group formats are utilized to treat drug use disorders, the present review underscores the need to understand the extent to which evidence-based group therapies for drug use disorders are applied in treatment settings.
Drug use disorders are a significant public health concern in the United States. According to the National Epidemiologic Survey of Alcohol and Related Conditions-III, the lifetime prevalence rate of DSM-5 drug use disorders is 9.9%, which includes amphetamine, cannabis, club drug, cocaine, hallucinogen, heroin, opioid, sedative/tranquilizer, and solvent/inhalant use disorders [ 1 ]. Drug use disorders are defined in terms of eleven criteria including physiological, behavioral and cognitive symptoms, as well as consequences of criteria, any two of which qualify for a diagnosis [ 2 , 3 ]. The individual and community costs of drug use are estimated at over $193 billion [ 4 , 5 ] and approximately $78.5 billion [ 6 ] for opioids alone. Consequences include overdose [ 7 ], mental health problems [ 8 ], and a range of medical consequences such as human immunodeficiency virus [ 9 , 10 ], hepatitis C virus [ 9 ], and other viral and bacterial infections [ 11 ].
Evidence-based practice was formally defined by Sackett et al. [ 12 ] in 1996 to refer to the “conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients” (p. 71). In 2006, the American Psychological Association [ 13 ] developed a policy on evidence-based practice (EBP) of psychotherapy, which emphasized the integration of best research evidence (i.e., data from meta-analyses, randomized controlled trials, effectiveness trials, and other forms of systematic case studies and reviews) with clinical expertise and judgment to deliver treatment in the context of a patient’s individual needs, preferences and culture. The shift towards EBP for substance use disorders has multiple benefits for practitioners and patients, including an increased focus on the implementation of treatments that are safe and cost-effective [ 14 ]. A recent survey of clinicians’ practices with substance use treatment found that clinicians often conducted therapy in groups [ 15 ]. While most clinicians who completed the survey reported use of evidence-based treatment practices (EBT) some also reported the use of non-EBT practices [ 15 ]. Ensuring that clinicians can readily access information regarding the current state of evidence regarding group-based therapies for substance use disorders is critical for fostering increased use of EBTs.
Although any effort to summarize a literature as large and complex as the psychological treatment literature is useful, there are several limitations. With few exceptions, research-supported treatment lists categorize treatments by formal change theory (e.g., cognitive-behavioral, interpersonal) and describe little about the context, format, or setting in which treatments were conducted and tested [ 16 ]. As a result, it is often difficult to ascertain from existing resources whether research supported treatments were conducted in group or individual format. A group format is often used in substance use treatment [ 17 ] and aftercare programs [ 18 , 19 , 20 , 21 , 22 ] . The discrepancy between the wide-spread use of group therapy in clinical practice and the relative paucity of research on the efficacy of group treatments has been noted by treatment researchers [ 23 ] and clinicians [ 24 ]. According to Lundahl’s [ 25 ] 2010 meta-analysis of studies evaluating the efficacy of motivational interviewing (MI), a commonly used treatment for substance use disorders, examination of the 119 studies concluded that studies of MI in a group format were too rare to draw solid conclusions about the efficacy of group MI. Also, it is possible that efficacy of treatments developed for individual delivery will be altered when delivered in a group format and vice versa. Given the limited empirical inquiry on group treatments for substance use, a framework organizing the literature on the efficacy of group therapy to treat substance use disorders would be useful. There is also a need for a more recent rigorous review of the empirical evidence to support group-based treatments for substance use disorders. Over 15 years ago, Weiss and colleagues conducted a review of 24 treatment outcome studies within the substance use disorder intervention literature comparing group therapy to other treatments conditions (i.e., no group therapy, individual therapy, group therapy plus individual therapy), and found no differences between group and individual therapy [ 26 ].
Given the importance of understanding the current evidence base for group-delivered treatments for substance use disorders, the present review sought to provide a summary of the literature on the benefits of group treatments for drug use disorders. Group treatments are potentially cost-effective, widely disseminable, and adaptable to a variety of populations but are lagging individual treatments in terms of research attention. Thus, highlighting characteristics of group treatments that are potentially efficacious is of import to stimulate further empirical inquiry. The review is organized by drug type (cocaine, methamphetamine, marijuana, opiate, mixed substance use disorders; SUD) and co-occurring SUD and psychiatric problems. We excluded studies focused on alcohol use disorder alone as this literature is summarized elsewhere (see Orchowski & Johnson, 2012). Given research suggesting that several factors impact outcomes of group treatments, including formal change theory driving the treatment approach (i.e., cognitive-behavioral, motivational interviewing), as well as patient factors [ 27 ], the review begins by first reviewing each theory of change (i.e., type of treatment), and then concludes by summarizing the research examining the extent to which patient factors influence the efficacy of group treatments for SUD.
To locate studies that evaluated a group treatment for SUD that met review inclusion criteria, the authors conducted a comprehensive literature search of PsycINFO and MedLine through 2020. Three individuals then examined abstracts of the articles for relevance. In addition, the authors utilized the reference lists of review studies and meta-analyses of SUD- treatments to locate additional studies that might meet the review inclusion criteria. The authors and a research assistant then reviewed full articles with relevance to the current study and excluded any studies that did not meet the review inclusion criteria (see Fig. 1 ).
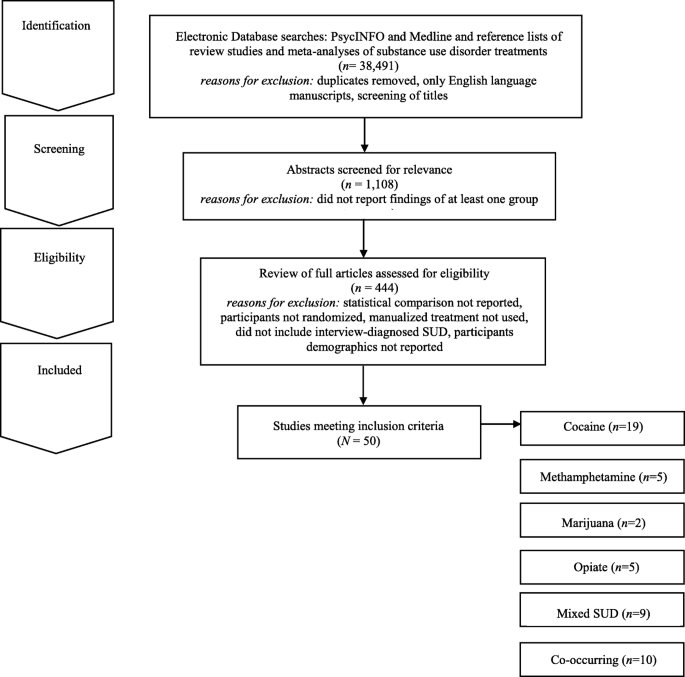
Electronic Search Strategy Flowchart
For inclusion in the review, studies needed to meet the following criteria: 1) report the findings of at least one group treatment; 2) provide at least one statistical comparison between the group treatment and a control condition; 3) randomize participants between the group treatment and control condition; 4) utilize a manualized treatment; 5) include patients with an interview-diagnosed SUD; and 6) provide information regarding the demographic characteristics of the participants in the study. Studies’ methods and results were used for data extraction. Studies which maintained a primary focus on the treatment of SUD, but also included treatment of a co-occurring psychiatric condition, were included in the review. Studies which included alcohol use as a comorbid diagnosis along another substance use were included. Studies examining the efficacy of group treatment for only alcohol use were excluded. The final set of articles included were 50 research studies that utilized a group treatment modality for the treatment of SUD, including separately examining cocaine, methamphetamine, marijuana, opioid, mixed substance, or SUD with comorbid psychiatric problems in adults.
It should be noted that several studies that met inclusion criteria were not reported in the present review because they did not report the use of a specific screening instrument for SUD as a part of the study inclusion/exclusion criteria. These studies are as follows and include these comparisons: group-based relational therapy [ 28 ] two studies by Guydish et al. [ 29 , 30 ] comparing a day treatment program to residential treatment (RT) program, a day treatment program to a coping skills group [ 31 ], standard care to a harm reduction group [ 32 ], 12 step group to a CBT group [ 33 ], medical management treatment (MMT) with CBT group to an MMT plus treatment reinforcement plan [ 34 ], treatment as usual to contingency management (CM) [ 35 ], professionally led recovery training group to treatment as usual (TAU) [ 36 ], two 4 month residential treatment programs [ 37 ], varying lengths of therapeutic community program (TPC) with and without relapse prevention [ 38 ], and Information and Referral plus peer advocacy to a Motivational group with CBT group [ 39 ].
Review of evidence-based theories of change
The 50 research studies meeting inclusion criteria tested the following group treatment modalities: contingency management (CM), motivational interviewing (MI), relapse prevention (RP), social support (SS), cognitive-behavioral (CBT), coping skills (CS), harm reduction (HR), cognitive therapy (CT), drug counseling (DC), recovery training (RT), standard group therapy (SGT), family therapy (FT), intensive group therapy (IGT), 12 step facilitation group therapy (12SG), relational psychotherapy mothers’ group (RPMG), psychoeducational therapy group (PET), behavioral skills (BS), and seeking safety (SS). Below, we briefly review the theory of change that drives each of these treatments.
Several treatment approaches are grounded in behavioral therapies and/or cognitive therapies. Broadly, cognitive therapy is an approach that focuses exclusively on targeting thoughts that are identified as part of a diagnosis or behavioral problem [ 28 ]. Cognitive-behavioral (CBT) therapy is an approach that targets specific symptoms, thoughts, and behaviors that are identified as part of a diagnosis or presenting problem [ 28 ]. Under the umbrella of CBT several other treatment modalities exist. For example, relapse prevention is a CBT treatment that hypothesizes that there are cognitive, behavioral, and affective mechanism that underlie the process of relapse [ 40 ]. Recovery training is a more specific form of relapse prevention, including education on addiction and recovery and reinforcing relapse prevention skills (e.g., understanding triggers, coping with cravings etc.) [ 41 , 42 ]. Other treatments focus on coping skills more broadly. For example, coping skills treatments include a focus on components of adaptability in interpersonal relationships, thinking and feeling, as well as approaches to self and life [ 28 ]. Some treatment approaches also recognize that individuals may not be ready to change their substance use. For example, motivational interviewing is often described as a therapy guiding technique in which the therapist is a helper in the behavior change process and expressed acceptance of the patient [ 43 ]. Standard group therapy includes 90 min sessions approximately twice a week in a group setting, [ 44 ] whereas intensive group therapy is a heavier dose of standard group therapy that includes 120-min sessions up to five times a week [ 44 ]. Psychoeducational therapy group focused on providing information on the immediate and delayed problems of substance use disorders to patients [ 45 ]. Lastly, dialectical behavior therapy (DBT) is a type of CBT therapy that focuses on helping regulate intense emotional states and provides skills to reduce arousal levels, and increase mindfulness, emotional regulation, and interpersonal skills [ 46 ].
Grounded within behavioral therapies, are behavioral skills training which focused on developing behaviors that are adaptive [ 28 ]. Contingency management is a type of behavioral therapy in which patients are reinforced or rewarded for positive behavioral change [ 47 ]. Harm reduction is a term for interventions aiming to reduce the problematic effects of behaviors [ 48 ]. Several treatment approaches also focus on interpersonal networks and building interpersonal skills. For example, social support is any psychological resources provided by a social network to help patients cope with stress [ 49 ]. Twelve-step facilitation group therapy is a more specific form of social support, which focuses on introducing patients to the 12 steps of alcoholics anonymous or related groups (i.e., cocaine or narcotics anonymous) to encourage 12-step meeting attendance in their community [ 33 , 50 ]. Seeking Safety is a present-focused and empowerment-based intervention focused on coping skills that emphasizes the importance of safety within interpersonal relationships [ 51 ]. Drug counseling describes treatment that aims to facilitate abstinence, encourage mutual support, and provide coping skills [ 52 ]. Finally, family therapy is a family-based intervention that aims to change, parenting behaviors and family interactions [ 53 ]. Overall, there are many overlapping components and skill sets in the models discussed above (See Table 1 ).
Group-based cocaine use treatments for adults
Nineteen studies were identified that targeted cocaine use and utilized some form of group therapy, the most of any drug in this review (see Table 2 ). Overall, the studies showed that all of the group therapy modalities included in this review generally reduced cocaine use when compared to treatment as usual (TAU), including day hospital groups [ 54 ]. Two studies, Magura et al. (1994) and Magura et al. (2002) did not find group differences between 8 months CBT and 8 months of TAU that consisted of methadone maintenance therapy among 141 patients with cocaine disorder [ 60 , 69 ]. When compared directly, individuals in CBT groups achieved longer abstinence than individuals in 12 step facilitation groups [ 33 ] or low intensity groups [ 64 , 65 ]. However, in another study, individuals with cocaine dependence receiving 12-step based Group Drug Counseling (GDC; similar to 12-step facilitation) had similar cocaine abstinence outcomes with or without additional individual CBT [ 41 ]. This may suggest that group 12-step facilitation is an effective intervention for cocaine dependence. Two studies demonstrated the superiority of CM groups for reducing cocaine use as compared to CBT [ 62 ] or TAU groups [ 61 , 62 ] at 12 weeks [ 54 ], 17 weeks [ 53 ], 26 weeks [ 53 ] and 52 weeks follow up [ 51 ]. Therefore, CBT group therapy and contingency management groups appear to be more effective at reducing cocaine use than TAU groups.
Group-based methamphetamine use treatments for adults
Only five treatment studies were identified that examined group treatments for methamphetamine use (see Table 3 ). Three studies found longer periods of abstinence for the group treatment (CM or drug+CM) than for TAU or non-CM conditions. The first study conducted by Rawson and colleagues compared matrix model (MM) with TAU in eight community outpatient settings [ 71 ]. The MM consisted of CBT groups, family education groups, social support groups, and individual counseling sessions along with weekly urine screens for 16 weeks. Participants in the MM condition attended more sessions, stayed in treatment longer, had more than twice as many contacts, evidence longer abstinence and greater self-reported psychosocial functioning relative to the TAU group. However, these significant differences did not persist 6 months later at follow-up.
Shoptaw et al. (2006) [ 73 ] compared four groups for treating methamphetamine dependence sertraline + CM, sertraline only, placebo + CM, and placebo [ 73 ]. Additionally, all participants attended a relapse prevention group conducted three times a week over a 14-week period. Findings provided support for the efficacy of CM for amphetamine use disorders. Group treatment (CM or drug + CM) was more effective for sustaining longer periods of abstinence relative to TAU or non-CM conditions. Roll et al. [ 72 ] found that effects of CM relative to TAU became larger as the duration of CM increased. Jaffe et al. [ 70 ] evaluated a culturally tailored intervention for 145 methamphetamine dependent gay and bisexual males. Participants in the Gay Specific CBT condition reported the most rapid decline in levels of methamphetamine use relative to standard CBT, CBT + CM, suggesting benefits for culturally appropriate group methamphetamine interventions.
Group-based marijuana use treatments for adults
Two studies examining group treatments for adults with marijuana use disorders were identified (see Table 4 ). Both studies were conducted by the same research group, utilizing the same inclusion criteria for marijuana use (50 times in 90 days). The studies examined group relapse prevention (RP) [ 76 ], specifically designed for adult marijuana users. The first trial [ 75 ] ( n = 212) comparing relapse prevention to a social support group found participants in both group treatment conditions did well overall, with two-thirds (65%) reporting abstinence of marijuana use for 2 weeks after session 4 or the quit date and 63% reporting abstinence during the last 2 weeks of treatment. Gender differences emerged; no differences between group treatments were found for women, but men in the relapse prevention group reported reduced marijuana use at the 3-month follow-up compared to men in the social support group.
A second trial [ 74 ] randomized participants to 14 sessions of group RP enhanced with cognitive behavioral skills training, two sessions of motivational interviewing (MI) with feedback and advice on cognitive behavioral skills (modeled after the Drinkers Check-up) [ 77 ], or a 4-month delayed treatment control (DTC) group which consisted of the RP group or individual MI treatment of the participants choosing. Compared to individuals randomly assigned to the DTC condition, participants in the group RP and individual MI conditions evidenced a significantly greater reduction in marijuana use and related problems over 16-month follow-up. However, examination of participants’ reactions to DTC assignment indicated that participants who felt that changing their marijuana use was their own responsibility were more likely than those who did not to change their use patterns without treatment engagement.
Group-based opiate use treatments for adults
Five group treatment studies for opioid use were identified (see Table 5 ). Two studies compared the effectiveness of pharmacotherapy plus group therapies [ 79 , 80 , 81 ] to pharmacotherapy alone in samples of opioid dependent persons, and both found that adding group treatment improved outcomes. The first study compared Naltrexone with monthly medical monitoring visits to an enhanced group condition (EN) consisting of Naltrexone plus a Matrix Method (MM) [ 79 ]. MM consisted of hourly individual sessions, 90-min CBT group, and 60 min of cue-exposure weekly for weeks 1–12; hourly individual sessions and CBT group sessions for weeks 13–26; and 90-min social support group sessions for weeks 27–52. Results found that EN participants took more study medication, were retained in treatment longer, used less opioids while in treatment, and showed greater improvement on psychological and affective dimensions than Naltrexone only participants. No difference by treatment condition was found at 6- and 12-month follow-ups. Similarly, Scherbaum et al. [ 80 ] compared routine Methadone Maintenance Therapy (MMT) with routine MMT plus group CBT psychotherapy (20 90-min sessions for 20 weeks). MMT plus group CBT participants showed less drug use than participants in the MMT group (i.e., control group). In contrast, a higher dose of group therapy provided without methadone maintenance was less effective for heroin use than was a lower dose of group therapy with methadone maintenance (Sees et al. [ 81 ]. This suggests that the combination of pharmacotherapy and group therapy for opioid use is optimal.
Shaffer et al. [ 22 ] compared psychodynamic group therapy with a hatha yoga group. All participants received methadone maintenance and individual therapy. No differences between two treatment conditions were found. For all participants, longer participation in treatment was associated with reduction in drug use and criminal activity. Lastly, Des Jarlais et al. [ 78 ] compared a group social learning AIDS/drug injection treatment program (4 sessions, 60–90 min, over 2 weeks) to a control condition. All participants received information about AIDS and HIV antibody test counseling. Compared to control participants, intervention participants reported lower rates of drug injection over time.
Group treatments for mixed SUD for adults
Nine treatment studies were identified that targeted mixed substance use with group treatments (see Table 6 ). Three involved CBT. Downey et al. [ 82 ] compared group CBT plus individual CBT to group CBT plus vouchers in a sample of 14 polysubstance users (cocaine and heroin) maintained on buprenorphine. The study was significantly underpowered and they found no significant differences on treatment outcomes. Marques and Formiogioni [ 84 ] compared individual CBT to group CBT in a sample of 155 participants with alcohol and/or drug dependence. They found that both formats resulted in similar outcomes, with higher compliance in the group CBT participants (66.7% compliance with treatment). Rawson et al. [ 87 ] compared three 16-week treatments: CM, group CBT, and CM plus group CBT, among 171 participants with cocaine disorder or methamphetamine abuse. They found that CM produced better retention and lower rates of stimulant use than CBT during treatment, but CBT produced comparable longer-term outcomes.
Two studies involved Group Drug Counseling (GDC). Greenfield et al. [ 52 ] compared a group drug counseling (GDC) (mixed gender) to a women’s recovery group (WRG) that both met weekly, for 12 weeks, for 90-min sessions among 44 participants that had a substance use disorder other than nicotine. WRG evidenced significantly greater reductions in drug and alcohol use over the follow up compared with GDC. Schottenfeld et al. [ 88 ] compared GDC (weekly, 1-h group sessions) to a community reinforcement approach (CRA; twice weekly sessions for the first 12 weeks and then weekly the following 12 weeks) among 117 patients with an opioid and cocaine use disorder. There were no differences in retention or drug use.
Remaining studies examined other interventions. Margolin et al. [ 83 ] compared an HIV Harm reduction program (HHRP) that met twice weekly for 2 h to an active control group that met six times in a sample of 90 HIV-seropositive methadone-maintained injection drug users with opioid dependence, and abuse or dependence on cocaine. At follow up, they had lower addiction severity scores and were less likely to have engaged in high risk behaviors compared to control. McKay et al. [ 85 ] compared weekly phone monitoring and counseling plus a support group in the first 4 weeks (TEL), twice-weekly individualized relapse prevention, and twice-weekly standard group counseling (STND) among 259 referred participants with alcohol use disorder or cocaine disorder. STND resulted in more days abstinent than TEL. Nemes et al. [ 86 ] compared a 12-month group program (10 months inpatient and 2 months outpatient) to an abbreviated group program (6 months inpatient, 6 months outpatient) among 412 patients with multiple drug/alcohol use disorders. Results indicated that both groups had reduction in arrests and drug use. There were no significant difference between groups. Lastly, Smith et al. [ 89 ] compared a standard treatment program (STP, daily group counseling, family outreach, 12-step program introduction, four 2 h sessions for family) to an enhanced treatment program (ETP; twice weekly group on relapse prevention and interpersonal violence in additional to all STP components) among 383 inpatient veterans meeting for an alcohol, cocaine, or amphetamine use disorder. Results indicated that ETP had enhanced abstinence rates at 3-month and 12-month follow up compared to STP, regardless of type of drug use.
Group Treatments for SUD and Co-Occurring Psychiatric Problems
Individuals with psychiatric distress are at high risk for comorbid SUD [ 90 ]. Ten randomized controlled studies meeting our inclusion criteria examined the efficacy of group therapy for SUD and co-occurring psychiatric problems (see Table 7 ). Three studies described group treatment of SUD and co-occurring DSM-IV Axis II disorders [ 18 , 91 , 96 ], three studies examined group treatment of drug abuse and co-occurring DSM-IV classified Axis I disorders [ 92 , 93 , 99 ], one study explored group drug abuse treatment and co-occurring psychiatric problems among homeless individuals without limiting to DSM-IV Axis I or Axis II diagnoses [ 97 ], and one study focused on group drug treatment among individuals testing positive for HIV [ 98 ]. Within this diverse set of RCTs, participants generally included individuals diagnosed with any form of SUD; however, some studies focused specifically on individuals using cocaine [ 91 , 97 ] or cocaine/opioids [ 98 ].
A range of group treatment approaches are represented, including group psychoeducational therapy, group CBT approaches, group DBT, Seeking Safety and CM. DiNitto and colleagues [ 92 ] evaluated the efficacy of adding a group-based psychoeducational program entitled “Good Chemistry Groups” to standard inpatient SUD treatment services among 97 individuals with a dual diagnosis of SUD and a DSM-IV Axis I psychological disorder. The nine 60-min Good Chemistry Group sessions were offered 3 times per week for 3 weeks. When compared to standard inpatient treatment, the addition of the psychoeducational group was not associated with any changes in medical, legal, alcohol, drug, psychiatric or family/social problems among participants.
The efficacy of adding a psychoeducational group treatment to standard individual therapy to address HIV risk among cocaine users has also been examined [ 91 ]. Participants were randomly assigned to complete the following: 1) individually-administered Standard Intervention developed by the NIDA Cooperative Agreement Final Cohort sites [ 100 ] including HIV testing, and pre- and post-HIV testing counseling on risks relating to cocaine use, transmission of STDs/HIV, condom use, cleaning injection equipment, and the benefits of treatment; or) Standard Intervention plus four 2-h peer-delivered psychoeducational groups addressing stress management, drug awareness, risk reduction strategies, HIV education and AIDS. Among the sample of 966 individuals completing the 3-month follow-up, the group psychoeducational treatment was not differentially effective in reducing drug use and HIV risk behavior in comparison to standard treatment alone at 3-months post-baseline, regardless of treatment type, individuals with antisocial personality disorder (ASPD) demonstrated less improvement in crack cocaine use compared to individuals without ASPD or depression.
The following types of group CBT have sustained research evaluation meeting our inclusion criteria to address co-occurring SUD and Axis I or Axis II disorders: 1) group behavioral skills training; 2) group cognitive behavioral therapy; 3) group-based Seeking Safety [ 51 ], and 4) group dialectical behavioral therapy. Specifically, Jerrell and Ridgely [ 93 ] examined the efficacy of group behavioral skills (BS) training, group-based 12-step facilitation (TS) treatment, and intensive case management among 132 individuals with a dual diagnosis of SUD and another Axis I psychiatric problem over the course of 24-months. Based on the Social and Independent Living Skills program [ 101 ], the BS group included one group per week addressing self-management skills designed to enhance abstinence, including medication management, relapse prevention, social skills, leisure activities and symptom monitoring. Relative to participants in TS groups, participants in the BS groups evidenced increased psychosocial functioning and decreased psychiatric symptoms (i.e., schizophrenia, depressive symptoms, mania, drug use and alcohol use) across the 6-, 12- and 18-month follow-up assessments after treatment entry.
Lehman and colleagues’ [ 95 ] examination of the efficacy of group CBT for substance abuse compared to TAU among 54 individuals with SUD and either schizophrenia or a major affective disorder revealed no differences between treatment groups over the course of a 1-year follow-up period. More promising findings were reported in Fisher and Bentley’s [ 18 ] evaluation of a group CBT and group therapy based in the disease and recovery model (DRM) among 38 individuals with dual diagnosis of SUD and a personality disorder. Groups met three times per week for 12 weeks and were compared to TAU. Individuals in group CBT and group DRM indicated improved social and family functioning compared to TAU, and among those who completed the group in an outpatient setting, CBT was more effective in reducing alcohol use, enhancing psychological functioning and improving social and family functioning compared to DRM and TAU.
Group behavioral therapy plus abstinence contingent housing and work administered in the context of a day treatment program was compared to behavioral group treatment alone among individuals with cocaine abuse/dependence, non-psychotic psychiatric conditions, and homelessness [ 97 , 102 ]. The group behavioral therapy included 8 weeks of daily treatment (4 h and 50 min per day) of groups addressing relapse prevention training, assertiveness training, AIDS education, 12-step facilitation, relaxation, recreation development, goal setting, and goal planning. Participants also engaged in a process-oriented group as well as individual counseling and urine monitoring and engaged in a weekly 90 min psychoeducational group therapy during months 3–6 following treatment enrollment. Individuals who received contingency-based work and housing were provided with rent-free housing and employment in construction or food service industries after 2 consecutive weeks of abstinence [ 103 ]. Relative to BS groups alone, group behavioral day treatment plus contingency management was associated with greater abstinence at 2- and 6-month follow-ups [ 102 ] and were less likely to relapse [ 97 ], although gains were not maintained at 12-months [ 104 ]. Both groups evidenced positive changes in drug use overtime compared to baseline [ 104 ].
Zlotnick, Johnston and Najavits [ 99 ] evaluated the efficacy of Seeking Safety (SS), in comparison to treatment as usual (TAU) among 49 incarcerated women with substance use disorder (SUD) and full or subthreshold posttraumatic stress disorder (PTSD). SS aims to decrease PTSD and SUD through psychoeducational and present-focused and empowerment-based instruction on coping skills that emphasize abstinence and safety [ 51 ]. The SS group treatment included 90-min group sessions held three times per week, that were completed in addition to the 180 to 240 h of group and individual therapy provided in TAU. All participants showed similar improvement on assessments of PSTD, SUD, legal problems and other psychiatric concerns at 12-week, 3- and 6-month follow-ups following prison release. Nonetheless, there was a trend for improved PTSD and continued improvements in psychiatric symptoms at follow-up among participants completing SS compared to TAU. Greater completion of SS sessions was associated with increased improvement in PTSD as well as drug use among women [ 99 ].
Dialectical behavioral group therapy (DBT), a CBT-focused treatment for individuals with borderline personality disorder (BPD), has also been evaluated in comparison to TAU among individuals with BPD and co-occurring SUD [ 96 ]. Core elements of DBT are manualized [ 105 ], and have been evaluated in prior research [ 106 , 107 , 108 ]. Techniques center on providing the participant with acceptance and validation while maintaining a continual focus on behavior change, and include the following: mindfulness skills training, behavioral analysis of dysfunctional behavior, cognitive restructuring, coping skills training, exposure-based strategies addressing maladaptive emotions, and behavioral management skills training. DBT was administered through 2 ¼ hour weekly group sessions administered in combination with 60 min of weekly individual therapy and the opportunity for skills-coaching phone calls. Relative to TAU, participants randomly assigned to DBT demonstrated greater reductions in drug use during the 12-month treatment and at the 16-month follow-up assessment, as well as greater gains in adjustment at the 16-month follow-up assessment.
Although contingency management is commonly administered individually, Petry and colleagues [ 98 ] examined the efficacy of weekly 60-min group-based contingency management (CM) for reinforcing health behaviors and HIV-positive individuals with cocaine or opioid disorders ( N = 170) in comparison to 12-step facilitation (TS) over the course of a 24-week period. Overall, participants in CM were more likely than those in TS to submit consecutive drug-free urine specimens, although the overall proportion of drug-free specimens did not vary between groups during treatment or over the follow-up period. Notably, during treatment, group CM was associated with greater reductions in HIV-risk behaviors as well as overall viral load compared to TS; although effects were not maintained over the follow-up period.
Across these studies, many trials showed positive gains for both group treatments examined [ 18 , 97 , 98 ], or no difference between groups when examining the benefit of adding group treatment to existing TAU [ 91 , 92 , 95 , 99 ]. However, one study demonstrated greater reductions in drug use among individuals with BPD and SUD who completed group DBT in comparison to TAU [ 96 ]. Further, BS groups were more effective than TS groups in improving psychosocial functioning and decreasing substance use [ 93 ]. Finally, CBT was more effective than DRM in reducing alcohol use, enhancing psychological functioning and improving social and family functioning compared to DRM and TAU among individuals dually diagnosed with SUD and a personality disorder [ 18 ].
Factors associated with treatment efficacy
Gender and treatment efficacy.
Five of the studies included in the present review examined whether treatment was differentially effective for men and women. Although Jarrell and Ridgely’s [ 93 ] evaluation of group BS, group TS and individual case management for individuals with SUD and co-occurring Axis I disorders did not examine whether group treatment types were differentially effective for men and women, data indicated that women—regardless of treatment group—reported higher role functioning (i.e.., independent living, work productivity, as well as immediate and extended social relationships), increased psychiatric symptomatology (depression, mania, drug use, alcohol use) across the follow-up periods compared to men.
Race and ethnicity and treatment efficacy
Among the studies included in the present review, only three examined whether treatment efficacy varied as a function of race and ethnicity. A secondary examination of the efficacy of group BS in comparison to group TS and individual case management [ 93 ] suggested that outcomes in each group treatment among ethnic and racial minority clients were equivalent to White participants during the 6-month follow [ 94 ]. The initial evaluation indicated that—regardless of group treatment type—racial/ethnic minority participants reported lower scores in personal well-being, lower life satisfaction (i.e., satisfaction with living), worse role functioning (i.e., independent living, work productivity, immediate and extended social relationships) over the follow-up periods compared to White participants [ 93 ].
Conclusions
In general, participants in group treatment for drug use disorders exhibit more improvement on typical measures of outcome (e.g., abstinence & use rates, objective measures, urinalysis) when compared to standard care without group [ 18 , 109 ] and those who refuse or drop out of treatment [ 110 ]. Specifically, CBT and CM appear to be more effective at reducing cocaine use than TAU groups. CM is effective in increasing periods of abstinence among users of methamphetamine. Both relapse prevention and social support group therapy were effective for marijuana use although relapse prevention was more helpful for men than for women. Brief MI and relapse prevention were both effective at reducing marijuana use. CBT and CBT-related treatments (including the matrix model) when added to pharmacotherapy were more effective for opioid use disorder than pharmacotherapy alone. Effective treatments for Mixed SUD include group CBT, CM, and women’s recovery group. Longer relapse prevention periods appear to be more helpful in reducing mixed SUD. Behavioral skills and behavioral skills plus contingency management helped decreased psychiatric symptoms and drug use behaviors. Psychoeducation groups alone, a commonly used intervention, were not effective at addressing SUD and co-occurring psychiatric problems. Additionally, it is important to note that there is potential for risk of bias in the studies included across four domains: participants, predictors, outcome, and analysis [ 111 ]. The current study did not comprehensively assess for risk of bias and this is a study limitation. Future research could assess for risk of bias by following the guidelines suggested by the Cochrane Handbook [ 112 ].
The current literature offers a wide variety of group treatments with varying goals and based on varying formal change theories. Overall, studies that reported between-group effect size ( n = 7) reported small to medium effect sizes potentially suggesting differences were moderate but of potential theoretical interest. Of those seven studies, only two studies reported large effect sizes (both comparing an active treatment to a delayed treatment/untreated condition). In order to better characterize magnitude of intervention effects, future studies should report effect sizes and their confidence intervals [ 113 , 114 ]. Moreover, groups based on cognitive-behavioral theory [ 35 ], motivational enhancement theory [ 43 ], stages of change theory [ 115 ], 12-step theory [ 41 ] and psychoeducational group models [ 116 ] have all been the subject of recent studies. Steps of treatment have also been used to classify groups for acutely ill individuals with SUD versus middle stage (recovering) or after care groups, with the latter mainly focusing on relapse prevention. Group therapy is provided – at least as an augment to multimodal interventions – in most of the outpatient and inpatient programs in English speaking and European countries [ 17 , 117 ]. Therefore, continued efforts to implement and scale up group-based treatments for SUD known to be effective are needed. CM appears to be effective at addressing various drug use problems and further research should evaluate whether it would also be useful for marijuana use.
Future Research Questions
Studies of other group treatments for SUD that use rigorous, interview-based diagnosis, use control groups, randomly assign participants to condition, report the ethnic and racial composition of the sample, are adequately powered, implement a treatment manual, and compare outcomes to individual treatment as well are necessary.
Little is known regarding the possible mediators and moderators of treatment outcome in group interventions for SUD
Key Learning Objectives
Group treatment approaches are widely utilized and are often less costly to implement than individual treatments, currently we know very little whether one group approach is superior to another in the treatment of SUD.
Group treatment approaches seem to be more effective at improving positive outcomes (e.g., abstinence, use rates, objective measures, urinalysis) when compared to standard care without group [ 18 , 109 ], and those who refuse and drop out of treatment
More thorough randomized controlled trials of group SUD treatments are needed [ 110 ].
Availability of data and materials
Not applicable. The present study does not include original data. However, the authors of the study have listed all articles reviewed in this study in the reference section.
Abbreviations
Twelve Step Facilitation Group Therapy
Alcohol Dependence
Acquired Immunodeficiency Syndrome
Addiction Severity Index
Antisocial Personality Disorder
Abbreviated Program
Behavioral Skills
Borderline Personality Disorder
Cognitive Behavioral Therapy
Cocaine Dependence
Composite Diagnostic Interview Schedule
Contingency Management
Community Reinforcement Approach
Coping Skills
Cognitive Therapy
Dialectical Behavioral Therapy
Day Treatment
Drug Counseling
Diagnostic Interview Schedule
Diagnostic and Statistical Manual
Disease and Recovery Model
Delayed to Control
Evidence-Based Practice
Evidence-Based Treatment Practice
Enhanced Group Condition
Enhanced Treatment Program
Family Therapy
Group Drug Counseling
Human Immunodeficiency Virus
HIV Harm Reduction
Harm Reduction
Intensive Group Therapy
Individual Therapy
Motivational Interviewing
Matrix Model
Methadone Maintenance Therapy
National Institute of Drug Abuse
Psychoeducational Therapy Group
Pre-Post with Comparison Group (matched or otherwise)
Post Traumatic Stress Disorder
Random Assignment with Control
Relapse Prevention
Recovery Training
Random Assignment to Active Treatment
Relational Psychotherapy Mothers’ Group
Structured Clinical Interview for Diagnosis
Social Support
Standard Group Therapy
Substance Use Disorder
Seeking Safety
Standard Group Counseling
Standard Treatment Program
Treatment as Usual
Phone Monitoring and Counseling, with Support Group
Therapeutic Community Program
Twelve Step
Women’s Recovery Group
Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, et al. Epidemiology of DSM-5 drug use disorder: results from the National Epidemiologic Survey on alcohol and related conditions-III. JAMA Psychiatry. 2016;73(1):39–47.
Article PubMed PubMed Central Google Scholar
Edition F. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th ed; 2013.
Google Scholar
Rehm J, Marmet S, Anderson P, Gual A, Kraus L, Nutt DJ, et al. Defining substance use disorders: do we really need more than heavy use? Alcohol Alcohol. 2013;48(6):633–40.
Article CAS PubMed Google Scholar
Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12(4):657–67.
Article PubMed Google Scholar
Center NDI. National Drug Threat Assessment. Washington: United States Department of Justice; 2011.
Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901–6.
Kamal R, Cox C, Rousseau D, Foundation ftKF. Costs and outcomes of mental health and substance use disorders in the US. JAMA. 2017;318(5):415.
Hall WD, Patton G, Stockings E, Weier M, Lynskey M, Morley KI, et al. Why young people's substance use matters for global health. Lancet Psychiatry. 2016;3(3):265–79.
Khalsa JH, Treisman G, McCance-Katz E, Tedaldi E. Medical consequences of drug abuse and co-occurring infections: research at the National Institute on Drug Abuse. Subst Abus. 2008;29(3):5–16.
McCoy CB, Lai S, Metsch LR, Messiah SE, Zhao W. Injection drug use and crack cocaine smoking: independent and dual risk behaviors for HIV infection. Ann Epidemiol. 2004;14(8):535–42.
Contoreggi, md C, Rexroad, rph VE, Lange, md, et al. Current Management of Infectious Complications in the injecting drug user. J Subst Abus Treat. 1998;15(2):95–106.
Article Google Scholar
Sackett DL, Rosenberg WMC, Gray JAM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312(7023):71–2.
Article CAS PubMed PubMed Central Google Scholar
American Psychological Association PTFoE-BP. Evidence-based practice in psychology. Am Psychol. 2006;61:271–85.
Pope C. Resisting evidence: the study of evidence-based medicine as a contemporary social movement. Health. 2003;7(3):267–82.
Wendt D. Group therapy for substance use disorders: a survey of clinician practices. J Groups Addict Recover. 2017;12(4):243 EOA.
Johnson J. Using research-supported group treatments. J Clin Psychol In Session. 2008;64(11):1206–24.
Stinchfield RD, Owen P, Winters K. Group therapy for substance abuse: A review of the empirical research. In: Burlingame AFG, editor. Handbook of group psychotherapy. New York: Wiley; 1994. p. 458–88.
Fisher MS Sr, Bentley KJ. Two group therapy models for clients with a dual diagnosis of substance abuse and personality disorder. Psychiatr Serv. 1996;47(11):1244–50.
Kaminer Y, Burleson JA, Blitz C, Sussman J, Rounsaville BJ. Psychotherapies for adolescent substance abusers: a pilot study. J Nerv Ment Dis. 1998;186(11):684–90.
McKay JR, Alterman AI, Cacciola JS, O'Brien CP, Koppenhaver JM, Shepard DS. Continuing care for cocaine dependence: comprehensive 2-year outcomes. J Consult Clin Psychol. 1999;67(3):420–7.
McKay JR, Alterman AI, Cacciola JS, Rutherford MJ, O'Brien CP, Koppenhaver J. Group counseling versus individualized relapse prevention aftercare following intensive outpatient treatment for cocaine dependence: initial results. J Consult Clin Psychol. 1997;65(5):778–88.
Shaffer HJ, LaSalvia TA, Stein JP. Comparing hatha yoga with dynamic group psychotherapy for enhancing methadone maintenance treatment: a randomized clinical trial. Altern Ther Health Med. 1997;3(4):57–66.
CAS PubMed Google Scholar
Morgan-Lopez AA, Fals-Stewart W. Analytic methods for modeling longitudinal data from rolling therapy groups with membership turnover. J Consult Clin Psychol. 2007;75(4):580–93.
Weiss RD. Treating patients with bipolar disorder and substance dependence: lessons learned. J Subst Abus Treat. 2004;27(4):307–12.
Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke BL. A meta-analysis of motivational interviewing: twenty-five years of empirical studies. Res Soc Work Pract. 2010;20(2):137–60.
Weiss RD, Jaffee WB, de Menil VP, Cogley CB. Group therapy for substance use disorders: what do we know? Harvard Rev Psychiatry. 2004;12(6):339–50.
Burlingame GM, MacKenzie KR, Strauss B. Bergin & Garfields Handbook Of Psychotherapy And Behavior Change. Small group treatment: Evidence for effectiveness and mechanisms of change. In: Lambert M, editor. Handbook of psychotherapy and behavior change. 5th ed. New York: Wiley; 2003. p. 647–96.
Beck AT. Cognitive therapy: nature and relation to behavior therapy. Behav Ther. 1970;1(2):184–200.
Guydish J, Sorensen JL, Chan M, Werdegar D, Bostrom A, Acampora A. A randomized trial comparing day and residential drug abuse treatment: 18-month outcomes. J Consult Clin Psychol. 1999;67(3):428–34.
Guydish J, Werdegar D, Sorensen JL, Clark W, Acampora A. Drug abuse day treatment: a randomized clinical trial comparing day and residential treatment programs. J Consult Clin Psychol. 1998;66(2):280–9.
Avants SK, Margolin A, Sindelar JL, Rounsaville BJ, Schottenfeld R, Stine S, et al. Day treatment versus enhanced standard methadone services for opioid-dependent patients: a comparison of clinical efficacy and cost. Am J Psychiatry. 1999;156(1):27–33.
Avants SK, Margolin A, Usubiaga MH, Doebrick C. Targeting HIV-related outcomes with intravenous drug users maintained on methadone: a randomized clinical trial of a harm reduction group therapy. J Subst Abus Treat. 2004;26(2):67–78.
Maude-Griffin PM, Hohenstein JM, Humfleet GL, Reilly PM, Tusel DJ, Hall SM. Superior efficacy of cognitive-behavioral therapy for urban crack cocaine abusers: main and matching effects. J Consult Clin Psychol. 1998;66(5):832–7.
Rohsenow DJ, Monti PM, Martin RA, Michalec E, Abrams DB. Brief coping skills treatment for cocaine abuse: 12-month substance use outcomes. J Consult Clin Psychol. 2000;68(3):515–20.
Beck ATW, F. D.; Newman, C. F.; Liese, B. S. Cognitive therapy of substance abuse. New York: Guilford; 1993.
McAuliffe WE. A randomized controlled trial of recovery training and self-help for opioid addicts in New England and Hong Kong. J Psychoactive Drugs. 1990;22(2):197–209.
Czuchry M, Dansereau DF. Node-link mapping and psychological problems. Perceptions of a residential drug abuse treatment program for probationers. J Subst Abus Treat. 1999;17(4):321–9.
Article CAS Google Scholar
McCusker J, Bigelow C, Frost R, Garfield F, Hindin R, Vickers-Lahti M, et al. The effects of planned duration of residential drug abuse treatment on recovery and HIV risk behavior. Am J Public Health. 1997;87(10):1637–44.
Rosenblum A, Magura S, Kayman DJ, Fong C. Motivationally enhanced group counseling for substance users in a soup kitchen: a randomized clinical trial. Drug Alcohol Depend. 2005;80(1):91–103.
Marlatt GA, Donovan DM. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. 2nd ed. New York: The Guilford Press; 2005. p. xiv, 416-xiv
Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, et al. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse collaborative cocaine treatment study. Arch Gen Psychiatry. 1999;56(6):493–502.
Luthar SSS, N.E.; Altomare, M. Relational psychotherapy Mother’s group: A randomized clinical trial for substance abusing mothers. Dev Psychopathol. 2007;19:243–61.
Miller WRR, S. Motivational interviewing: preparing people to change addictive behavior. New York: Guilford Press; 1991.
Hoffman JA, Caudill BD, Koman JJ 3rd, Luckey JW, Flynn PM, Mayo DW. Psychosocial treatments for cocaine abuse. 12-month treatment outcomes. J Subst Abus Treat. 1996;13(1):3–11.
Kaminer Y, Burleson JA, Goldberger R. Cognitive-behavioral coping skills and psychoeducation therapies for adolescent substance abuse. J Nerv Ment Dis. 2002;190(11):737–45.
Linehan M. DBT skills training manual. 2nd ed. London: The Guilford Press; 2015.
Petry NM. Contingency management: what it is and why psychiatrists should want to use it. Psychiatrist. 2011;35(5):161–3.
Logan DE, Marlatt GA. Harm reduction therapy: a practice-friendly review of research. J Clin Psychol. 2010;66(2):201–14.
PubMed PubMed Central Google Scholar
Ellis B, Bernichon T, Yu P, Roberts T, Herrell JM. Effect of social support on substance abuse relapse in a residential treatment setting for women. Eval Program Plan. 2004;27(2):213–21.
Nowinski J, Baker S. The twelve-step facilitation handbook: A systematic approach to early recovery from alcoholism and addiction. San Francisco: Jossey-Bass; 1992. p. xxii. 215-xxii
Najavits LM. Seeking safety: a treatment manual for PTSD and substance abuse. New York: Guilford; 2002.
Greenfield SF, Trucco EM, McHugh RK, Lincoln M, Gallop RJ. The Women's recovery group study: a stage I trial of women-focused group therapy for substance use disorders versus mixed-gender group drug counseling. Drug Alcohol Depend. 2007;90(1):39–47.
Liddle HA, Dakof GA, Parker K, Diamond GS, Barrett K, Tejeda M. Multidimensional family therapy for adolescent drug abuse: results of a randomized clinical trial. Am J Drug Alcohol Abuse. 2001;27(4):651–88.
Coviello DM, Alterman AI, Rutherford MJ, Cacciola JS, McKay JR, Zanis DA. The effectiveness of two intensities of psychosocial treatment for cocaine dependence. Drug Alcohol Depend. 2001;61(2):145–54.
Crits-Christoph P, Siqueland L, McCalmont E, Weiss RD, Gastfriend DR, Frank A, et al. Impact of psychosocial treatments on associated problems of cocaine-dependent patients. J Consult Clin Psychol. 2001;69(5):825–30.
Siqueland L, Crits-Christoph P, Gallop R, Barber JP, Griffin ML, Thase ME, et al. Retention in psychosocial treatment of cocaine dependence: predictors and impact on outcome. Am J Addict. 2002;11(1):24–40.
Mercer D, Carpenter G, Daley D. The adherence and competence scale for group addiction counseling for TCACS: Center for Psychotherapy Research, Department of Psychiatry, University of Pennsylvania Medical School; 1994.
Luborsky L. Principles of psychoanalytic psychotherapy a manual for supportive-expressive treatment; 1984.
Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: findings during treatment and across 12-month follow-up. Psychol Addict Behav. 2003;17(1):73–82.
Magura S, Rosenblum A, Lovejoy M, Handelsman L, Foote J, Stimmel B. Neurobehavioral treatment for cocaine-using methadone patients: a preliminary report. J Addict Dis. 1994;13(4):143–60.
Petry NM, Alessi SM, Hanson T. Contingency management improves abstinence and quality of life in cocaine abusers. J Consult Clin Psychol. 2007;75(2):307–15.
Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59(9):817–24.
Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, et al. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction. 2001;96(8):1161–74.
Rosenblum A, Magura S, Foote J, Palij M, Handelsman L, Lovejoy M, et al. Treatment intensity and reduction in drug use for cocaine-dependent methadone patients: a dose-response relationship. J Psychoactive Drugs. 1995;27(2):151–9.
Rosenblum A, Magura S, Palij M, Foote J, Handelsman L, Stimmel B. Enhanced treatment outcomes for cocaine-using methadone patients. Drug Alcohol Depend. 1999;54(3):207–18.
Volpicelli JR, Markman I, Monterosso J, Filing J, O'Brien CP. Psychosocially enhanced treatment for cocaine-dependent mothers: evidence of efficacy. J Subst Abus Treat. 2000;18(1):41–9.
Weinstein SP, Gottheil E, Sterling RC. Randomized comparison of intensive outpatient vs. individual therapy for cocaine abusers. J Addict Dis. 1997;16(2):41–56.
Gottheil E, Weinstein SP, Sterling RC, Lundy A, Serota RD. A randomized controlled study of the effectiveness of intensive outpatient treatment for cocaine dependence. Psychiatr Serv. 1998;49(6):782–7.
Magura S, Rosenblum A, Fong C, Villano C, Richman B. Treating cocaine-using methadone patients: predictors of outcomes in a psychosocial clinical trial. Subst Use Misuse. 2002;37(14):1927–55.
Jaffe A, Shoptaw S, Stein J, Reback CJ, Rotheram-Fuller E. Depression ratings, reported sexual risk behaviors, and methamphetamine use: latent growth curve models of positive change among gay and bisexual men in an outpatient treatment program. Exp Clin Psychopharmacol. 2007;15(3):301–7.
Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708–17.
Roll JM, Chudzynski J, Cameron JM, Howell DN, McPherson S. Duration effects in contingency management treatment of methamphetamine disorders. Addict Behav. 2013;38(9):2455–62.
Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff D, et al. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85(1):12–8.
Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68(5):898–908.
Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: a test of the relapse prevention model. J Consult Clin Psychol. 1994;62(1):92–9.
Marlatt GAG, J.R. Relapse prevention (unpublished manuscript). In: Luther SSS, Boltas D, editors. Relational parenting Mother’s group: A therapist’s manual. New York: Guilford Press; 1985.
Miller WR, Benefield RG, Tonigan JS. Enhancing motivation for change in problem drinking: a controlled comparison of two therapist styles. J Consult Clin Psychol. 1993;61(3):455–61.
Des Jarlais DC, Casriel C, Friedman SR, Rosenblum A. AIDS and the transition to illicit drug injection--results of a randomized trial prevention program. Br J Addict. 1992;87(3):493–8.
Rawson RA, McCann MJ, Shoptaw SJ, Miotto KA, Frosch DL, Obert JL, et al. Naltrexone for opioid dependence: evaluation of a manualized psychosocial protocol to enhance treatment response. Drug Alcohol Rev. 2001;20(1):67–78.
Scherbaum N, Kluwig J, Specka M, Krause D, Merget B, Finkbeiner T, et al. Group psychotherapy for opiate addicts in methadone maintenance treatment--a controlled trial. Eur Addict Res. 2005;11(4):163–71.
Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283(10):1303–10.
Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Exp Clin Psychopharmacol. 2000;8(2):176–84.
Margolin A, Avants SK, Warburton LA, Hawkins KA, Shi J. A randomized clinical trial of a manual-guided risk reduction intervention for HIV-positive injection drug users. Health Psychol. 2003;22(2):223–8.
Marques AC, Formigoni ML. Comparison of individual and group cognitive-behavioral therapy for alcohol and/or drug-dependent patients. Addiction. 2001;96(6):835–46.
McKay JR, Lynch KG, Shepard DS, Morgenstern J, Forman RF, Pettinati HM. Do patient characteristics and initial progress in treatment moderate the effectiveness of telephone-based continuing care for substance use disorders? Addiction. 2005;100(2):216–26.
Nemes S, Wish ED, Messina N. Comparing the impact of standard and abbreviated treatment in a therapeutic community. Findings from the district of Columbia treatment initiative experiment. J Subst Abus Treat. 1999;17(4):339–47.
Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267–74.
Schottenfeld RS, Pantalon MV, Chawarski MC, Pakes J. Community reinforcement approach for combined opioid and cocaine dependence. Patterns of engagement in alternate activities. J Subst Abus Treat. 2000;18(3):255–61.
Smith TL, Volpe FR, Hashima JN, Schuckit MA. Impact of a stimulant-focused enhanced program on the outcome of alcohol- and/or stimulant-dependent men. Alcohol Clin Exp Res. 1999;23(11):1772–9.
Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse: results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511–8.
Compton WM, Cottler LB, Ben-Abdallah A, Cunningham-Williams R, Spitznagel EL. The effects of psychiatric comorbidity on response to an HIV prevention intervention. Drug Alcohol Depend. 2000;58(3):247–57.
DiNitto DM, Webb DK, Rubin A. Gender differences in dually-diagnosed clients receiving chemical dependency treatment. J Psychoactive Drugs. 2002;34(1):105–17.
Jerrell JM, Ridgely MS. Comparative effectiveness of three approaches to serving people with severe mental illness and substance abuse disorders. J Nerv Ment Dis. 1995;183(9):566–76.
Jerrell JM, Wilson JL. Ethnic differences in the treatment of dual mental and substance disorders. A preliminary analysis. J Subst Abus Treat. 1997;14(2):133–40.
Lehman AF, Herron JD, Schwartz RP, Myers CP. Rehabilitation for adults with severe mental illness and substance use disorders. A clinical trial. J Nerv Ment Dis. 1993;181(2):86–90.
Linehan MM, Schmidt H 3rd, Dimeff LA, Craft JC, Kanter J, Comtois KA. Dialectical behavior therapy for patients with borderline personality disorder and drug-dependence. Am J Addict. 1999;8(4):279–92.
Milby JB, Schumacher JE, Vuchinich RE, Wallace D, Plant MA, Freedman MJ, et al. Transitions during effective treatment for cocaine-abusing homeless persons: establishing abstinence, lapse, and relapse, and reestablishing abstinence. Psychol Addict Behav. 2004;18(3):250–6.
Petry NM, Weinstock J, Alessi SM, Lewis MW, Dieckhaus K. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol. 2010;78(1):89–97.
Zlotnick C, Johnson J, Najavits LM. Randomized controlled pilot study of cognitive-behavioral therapy in a sample of incarcerated women with substance use disorder and PTSD. Behav Ther. 2009;40(4):325–36.
Wechsberg WM, MacDonald BR, Dennis ML, Inciardi JA, Surratt HL, Leukefeld CG, et al. The standard intervention for reduction in HIV risk behavior; protocol changes suggested by the continuing HIV/AIDS epidemic. Bloomington: Lighthouse Institute; 1997.
Liberman RP, Massel HK, Mosk MD, Wong SE. Social skills training for chronic mental patients. Hosp Community Psychiatry. 1985;36(4):396–403.
Milby JB, Schumacher JE, McNamara C, Wallace D, Usdan S, McGill T, et al. Initiating abstinence in cocaine abusing dually diagnosed homeless persons. Drug Alcohol Depend. 2000;60(1):55–67.
Milby JB, Schumacher JE, Raczynski JM, Caldwell E, Engle M, Michael M, et al. Sufficient conditions for effective treatment of substance abusing homeless persons. Drug Alcohol Depend. 1996;43(1–2):39–47.
Milby JB, Schumacher JE, Wallace D, Frison S, McNamara C, Usdan S, et al. Day treatment with contingency management for cocaine abuse in homeless persons: 12-month follow-up. J Consult Clin Psychol. 2003;71(3):619–21.
Linehan MMD, L.A. Dialectical behavior therapy manual of treatment interventions for drug abusers with borderline personality disorder. Seattle: University of Washington; 1997.
Linehan MM, Heard HL, Armstrong HE. Naturalistic follow-up of a behavioral treatment for chronically parasuicidal borderline patients. Arch Gen Psychiatry. 1993;50(12):971–4.
Linehan MM, Tutek DA, Heard HL, Armstrong HE. Interpersonal outcome of cognitive behavioral treatment for chronically suicidal borderline patients. Am J Psychiatry. 1994;151(12):1771–6.
Linehan MM, Armstrong HE, Suarez A, Allmon D, Heard HL. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry. 1991;48(12):1060–4.
Burtscheidt W, Schwarz R, Redner C, Gaebel W. Behavioral therapeutic methods in ambulatory treatment of alcoholism. Early results of an experimental study. Fortschr Neurol Psychiatr. 1999;67(6):274–80.
Sandahl C, Herlitz K, Ahlin G, Rönnberg S. Time-limited group psychotherapy for moderately alcohol dependent patients: A randomized controlled clinical trial. Psychother Res. 1998;8(4):361–78.
Wolff RF, Moons KG, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. 2019;170(1):51–8.
Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. In: Cochrane handbook for systematic reviews of interventions; 2019. p. 205–28.
Chapter Google Scholar
Diener MJ. The Corsini Encyclopedia of Psychology; 2010. p. 1.
Book Google Scholar
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):1–8.
Prochaska JO, DiClemente CC. Stages of change in the modification of problem behaviors. Prog Behav Modif. 1992;28:183–218.
Drake RE, McLaughlin P, Pepper B, Minkoff K. Dual diagnosis of major mental illness and substance disorder: an overview. New Dir Ment Health Serv. 1991;1991(50):3–12.
Kunzke D, Strauss B, Burtscheidt W. The effectiveness of psychodynamic group therapy in the treatment of alcohol abuse. A review. Gruppenpsychotherapie und Gruppendynamik. 2002;38:53–70.
Download references
Acknowledgements
The authors have no acknowledgements.
The study is not supported through funding.
Author information
Authors and affiliations.
Center for Alcohol and Addiction Studies, Brown University, Providence, RI, 02912, USA
Gabriela López
Alpert Medical School of Brown University, Department of Psychiatry and Human Behavior, Providence, RI, 02904, USA
Lindsay M. Orchowski
Walter Reed Army Institute of Research, Silver Spring, MD, 20910, USA
Madhavi K. Reddy
Massachusetts General Hospital, Harvard Medical School, Boston, MA, 02115, USA
Jessica Nargiso
Division of Public Health, Michigan State University, Flint, MI, 48502, USA
Jennifer E. Johnson
You can also search for this author in PubMed Google Scholar
Contributions
All authors participated in the review of manuscripts included in the study, writing of sections of the manuscript, as well as final editing of the paper. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Lindsay M. Orchowski .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
López, G., Orchowski, L.M., Reddy, M.K. et al. A review of research-supported group treatments for drug use disorders. Subst Abuse Treat Prev Policy 16 , 51 (2021). https://doi.org/10.1186/s13011-021-00371-0
Download citation
Accepted : 03 April 2021
Published : 21 June 2021
DOI : https://doi.org/10.1186/s13011-021-00371-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Substance use disorders
- Group therapy
Substance Abuse Treatment, Prevention, and Policy
ISSN: 1747-597X
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 22 February 2021
Addiction as a brain disease revised: why it still matters, and the need for consilience
- Markus Heilig 1 ,
- James MacKillop ORCID: orcid.org/0000-0003-4118-9500 2 , 3 ,
- Diana Martinez 4 ,
- Jürgen Rehm ORCID: orcid.org/0000-0001-5665-0385 5 , 6 , 7 , 8 ,
- Lorenzo Leggio ORCID: orcid.org/0000-0001-7284-8754 9 &
- Louk J. M. J. Vanderschuren ORCID: orcid.org/0000-0002-5379-0363 10
Neuropsychopharmacology volume 46 , pages 1715–1723 ( 2021 ) Cite this article
83k Accesses
93 Citations
321 Altmetric
Metrics details
The view that substance addiction is a brain disease, although widely accepted in the neuroscience community, has become subject to acerbic criticism in recent years. These criticisms state that the brain disease view is deterministic, fails to account for heterogeneity in remission and recovery, places too much emphasis on a compulsive dimension of addiction, and that a specific neural signature of addiction has not been identified. We acknowledge that some of these criticisms have merit, but assert that the foundational premise that addiction has a neurobiological basis is fundamentally sound. We also emphasize that denying that addiction is a brain disease is a harmful standpoint since it contributes to reducing access to healthcare and treatment, the consequences of which are catastrophic. Here, we therefore address these criticisms, and in doing so provide a contemporary update of the brain disease view of addiction. We provide arguments to support this view, discuss why apparently spontaneous remission does not negate it, and how seemingly compulsive behaviors can co-exist with the sensitivity to alternative reinforcement in addiction. Most importantly, we argue that the brain is the biological substrate from which both addiction and the capacity for behavior change arise, arguing for an intensified neuroscientific study of recovery. More broadly, we propose that these disagreements reveal the need for multidisciplinary research that integrates neuroscientific, behavioral, clinical, and sociocultural perspectives.
Similar content being viewed by others
Subtypes in addiction and their neurobehavioral profiles across three functional domains
Gunner Drossel, Leyla R. Brucar, … Anna Zilverstand
Drug addiction: from bench to bedside
Julian Cheron & Alban de Kerchove d’Exaerde
The neurobiology of drug addiction: cross-species insights into the dysfunction and recovery of the prefrontal cortex
Ahmet O. Ceceli, Charles W. Bradberry & Rita Z. Goldstein
Introduction
Close to a quarter of a century ago, then director of the US National Institute on Drug Abuse Alan Leshner famously asserted that “addiction is a brain disease”, articulated a set of implications of this position, and outlined an agenda for realizing its promise [ 1 ]. The paper, now cited almost 2000 times, put forward a position that has been highly influential in guiding the efforts of researchers, and resource allocation by funding agencies. A subsequent 2000 paper by McLellan et al. [ 2 ] examined whether data justify distinguishing addiction from other conditions for which a disease label is rarely questioned, such as diabetes, hypertension or asthma. It concluded that neither genetic risk, the role of personal choices, nor the influence of environmental factors differentiated addiction in a manner that would warrant viewing it differently; neither did relapse rates, nor compliance with treatment. The authors outlined an agenda closely related to that put forward by Leshner, but with a more clinical focus. Their conclusion was that addiction should be insured, treated, and evaluated like other diseases. This paper, too, has been exceptionally influential by academic standards, as witnessed by its ~3000 citations to date. What may be less appreciated among scientists is that its impact in the real world of addiction treatment has remained more limited, with large numbers of patients still not receiving evidence-based treatments.
In recent years, the conceptualization of addiction as a brain disease has come under increasing criticism. When first put forward, the brain disease view was mainly an attempt to articulate an effective response to prevailing nonscientific, moralizing, and stigmatizing attitudes to addiction. According to these attitudes, addiction was simply the result of a person’s moral failing or weakness of character, rather than a “real” disease [ 3 ]. These attitudes created barriers for people with substance use problems to access evidence-based treatments, both those available at the time, such as opioid agonist maintenance, cognitive behavioral therapy-based relapse prevention, community reinforcement or contingency management, and those that could result from research. To promote patient access to treatments, scientists needed to argue that there is a biological basis beneath the challenging behaviors of individuals suffering from addiction. This argument was particularly targeted to the public, policymakers and health care professionals, many of whom held that since addiction was a misery people brought upon themselves, it fell beyond the scope of medicine, and was neither amenable to treatment, nor warranted the use of taxpayer money.
Present-day criticism directed at the conceptualization of addiction as a brain disease is of a very different nature. It originates from within the scientific community itself, and asserts that this conceptualization is neither supported by data, nor helpful for people with substance use problems [ 4 , 5 , 6 , 7 , 8 ]. Addressing these critiques requires a very different perspective, and is the objective of our paper. We readily acknowledge that in some cases, recent critiques of the notion of addiction as a brain disease as postulated originally have merit, and that those critiques require the postulates to be re-assessed and refined. In other cases, we believe the arguments have less validity, but still provide an opportunity to update the position of addiction as a brain disease. Our overarching concern is that questionable arguments against the notion of addiction as a brain disease may harm patients, by impeding access to care, and slowing development of novel treatments.
A premise of our argument is that any useful conceptualization of addiction requires an understanding both of the brains involved, and of environmental factors that interact with those brains [ 9 ]. These environmental factors critically include availability of drugs, but also of healthy alternative rewards and opportunities. As we will show, stating that brain mechanisms are critical for understanding and treating addiction in no way negates the role of psychological, social and socioeconomic processes as both causes and consequences of substance use. To reflect this complex nature of addiction, we have assembled a team with expertise that spans from molecular neuroscience, through animal models of addiction, human brain imaging, clinical addiction medicine, to epidemiology. What brings us together is a passionate commitment to improving the lives of people with substance use problems through science and science-based treatments, with empirical evidence as the guiding principle.
To achieve this goal, we first discuss the nature of the disease concept itself, and why we believe it is important for the science and treatment of addiction. This is followed by a discussion of the main points raised when the notion of addiction as a brain disease has come under criticism. Key among those are claims that spontaneous remission rates are high; that a specific brain pathology is lacking; and that people suffering from addiction, rather than behaving “compulsively”, in fact show a preserved ability to make informed and advantageous choices. In the process of discussing these issues, we also address the common criticism that viewing addiction as a brain disease is a fully deterministic theory of addiction. For our argument, we use the term “addiction” as originally used by Leshner [ 1 ]; in Box 1 , we map out and discuss how this construct may relate to the current diagnostic categories, such as Substance Use Disorder (SUD) and its different levels of severity (Fig. 1) .
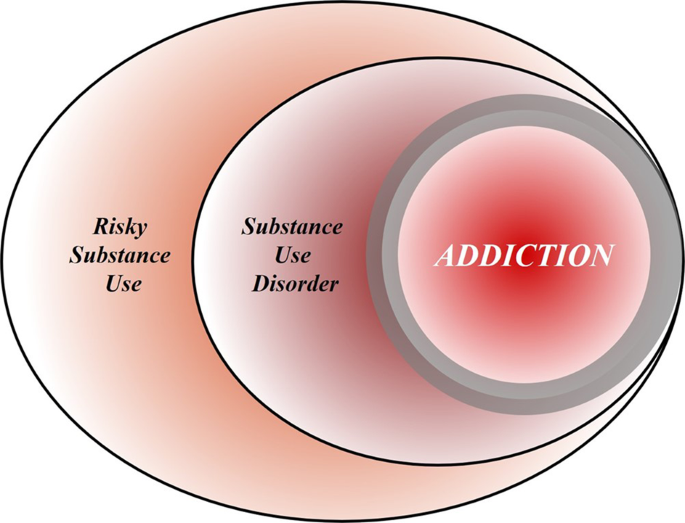
Risky (hazardous) substance use refers to quantity/frequency indicators of consumption; SUD refers to individuals who meet criteria for a DSM-5 diagnosis (mild, moderate, or severe); and addiction refers to individuals who exhibit persistent difficulties with self-regulation of drug consumption. Among high-risk individuals, a subgroup will meet criteria for SUD and, among those who have an SUD, a further subgroup would be considered to be addicted to the drug. However, the boundary for addiction is intentionally blurred to reflect that the dividing line for defining addiction within the category of SUD remains an open empirical question.
Box 1 What’s in a name? Differentiating hazardous use, substance use disorder, and addiction
Although our principal focus is on the brain disease model of addiction, the definition of addiction itself is a source of ambiguity. Here, we provide a perspective on the major forms of terminology in the field.
Hazardous Substance Use
Hazardous (risky) substance use refers to quantitative levels of consumption that increase an individual’s risk for adverse health consequences. In practice, this pertains to alcohol use [ 110 , 111 ]. Clinically, alcohol consumption that exceeds guidelines for moderate drinking has been used to prompt brief interventions or referral for specialist care [ 112 ]. More recently, a reduction in these quantitative levels has been validated as treatment endpoints [ 113 ].
Substance Use Disorder
SUD refers to the DSM-5 diagnosis category that encompasses significant impairment or distress resulting from specific categories of psychoactive drug use. The diagnosis of SUD is operationalized as 2 or more of 11 symptoms over the past year. As a result, the diagnosis is heterogenous, with more than 1100 symptom permutations possible. The diagnosis in DSM-5 is the result of combining two diagnoses from the DSM-IV, abuse and dependence, which proved to be less valid than a single dimensional approach [ 114 ]. Critically, SUD includes three levels of severity: mild (2–3 symptoms), moderate (4–5 symptoms), and severe (6+ symptoms). The International Classification of Diseases (ICD) system retains two diagnoses, harmful use (lower severity) and substance dependence (higher severity).
Addiction is a natural language concept, etymologically meaning enslavement, with the contemporary meaning traceable to the Middle and Late Roman Republic periods [ 115 ]. As a scientific construct, drug addiction can be defined as a state in which an individual exhibits an inability to self-regulate consumption of a substance, although it does not have an operational definition. Regarding clinical diagnosis, as it is typically used in scientific and clinical parlance, addiction is not synonymous with the simple presence of SUD. Nowhere in DSM-5 is it articulated that the diagnostic threshold (or any specific number/type of symptoms) should be interpreted as reflecting addiction, which inherently connotes a high degree of severity. Indeed, concerns were raised about setting the diagnostic standard too low because of the issue of potentially conflating a low-severity SUD with addiction [ 116 ]. In scientific and clinical usage, addiction typically refers to individuals at a moderate or high severity of SUD. This is consistent with the fact that moderate-to-severe SUD has the closest correspondence with the more severe diagnosis in ICD [ 117 , 118 , 119 ]. Nonetheless, akin to the undefined overlap between hazardous use and SUD, the field has not identified the exact thresholds of SUD symptoms above which addiction would be definitively present.
Integration
The ambiguous relationships among these terms contribute to misunderstandings and disagreements. Figure 1 provides a simple working model of how these terms overlap. Fundamentally, we consider that these terms represent successive dimensions of severity, clinical “nesting dolls”. Not all individuals consuming substances at hazardous levels have an SUD, but a subgroup do. Not all individuals with a SUD are addicted to the drug in question, but a subgroup are. At the severe end of the spectrum, these domains converge (heavy consumption, numerous symptoms, the unambiguous presence of addiction), but at low severity, the overlap is more modest. The exact mapping of addiction onto SUD is an open empirical question, warranting systematic study among scientists, clinicians, and patients with lived experience. No less important will be future research situating our definition of SUD using more objective indicators (e.g., [ 55 , 120 ]), brain-based and otherwise, and more precisely in relation to clinical needs [ 121 ]. Finally, such work should ultimately be codified in both the DSM and ICD systems to demarcate clearly where the attribution of addiction belongs within the clinical nosology, and to foster greater clarity and specificity in scientific discourse.
What is a disease?
In his classic 1960 book “The Disease Concept of Alcoholism”, Jellinek noted that in the alcohol field, the debate over the disease concept was plagued by too many definitions of “alcoholism” and too few definitions of “disease” [ 10 ]. He suggested that the addiction field needed to follow the rest of medicine in moving away from viewing disease as an “entity”, i.e., something that has “its own independent existence, apart from other things” [ 11 ]. To modern medicine, he pointed out, a disease is simply a label that is agreed upon to describe a cluster of substantial, deteriorating changes in the structure or function of the human body, and the accompanying deterioration in biopsychosocial functioning. Thus, he concluded that alcoholism can simply be defined as changes in structure or function of the body due to drinking that cause disability or death. A disease label is useful to identify groups of people with commonly co-occurring constellations of problems—syndromes—that significantly impair function, and that lead to clinically significant distress, harm, or both. This convention allows a systematic study of the condition, and of whether group members benefit from a specific intervention.
It is not trivial to delineate the exact category of harmful substance use for which a label such as addiction is warranted (See Box 1 ). Challenges to diagnostic categorization are not unique to addiction, however. Throughout clinical medicine, diagnostic cut-offs are set by consensus, commonly based on an evolving understanding of thresholds above which people tend to benefit from available interventions. Because assessing benefits in large patient groups over time is difficult, diagnostic thresholds are always subject to debate and adjustments. It can be debated whether diagnostic thresholds “merely” capture the extreme of a single underlying population, or actually identify a subpopulation that is at some level distinct. Resolving this issue remains challenging in addiction, but once again, this is not different from other areas of medicine [see e.g., [ 12 ] for type 2 diabetes]. Longitudinal studies that track patient trajectories over time may have a better ability to identify subpopulations than cross-sectional assessments [ 13 ].
By this pragmatic, clinical understanding of the disease concept, it is difficult to argue that “addiction” is unjustified as a disease label. Among people who use drugs or alcohol, some progress to using with a quantity and frequency that results in impaired function and often death, making substance use a major cause of global disease burden [ 14 ]. In these people, use occurs with a pattern that in milder forms may be challenging to capture by current diagnostic criteria (See Box 1 ), but is readily recognized by patients, their families and treatment providers when it reaches a severity that is clinically significant [see [ 15 ] for a classical discussion]. In some cases, such as opioid addiction, those who receive the diagnosis stand to obtain some of the greatest benefits from medical treatments in all of clinical medicine [ 16 , 17 ]. Although effect sizes of available treatments are more modest in nicotine [ 18 ] and alcohol addiction [ 19 ], the evidence supporting their efficacy is also indisputable. A view of addiction as a disease is justified, because it is beneficial: a failure to diagnose addiction drastically increases the risk of a failure to treat it [ 20 ].
Of course, establishing a diagnosis is not a requirement for interventions to be meaningful. People with hazardous or harmful substance use who have not (yet) developed addiction should also be identified, and interventions should be initiated to address their substance-related risks. This is particularly relevant for alcohol, where even in the absence of addiction, use is frequently associated with risks or harm to self, e.g., through cardiovascular disease, liver disease or cancer, and to others, e.g., through accidents or violence [ 21 ]. Interventions to reduce hazardous or harmful substance use in people who have not developed addiction are in fact particularly appealing. In these individuals, limited interventions are able to achieve robust and meaningful benefits [ 22 ], presumably because patterns of misuse have not yet become entrenched.
Thus, as originally pointed out by McLellan and colleagues, most of the criticisms of addiction as a disease could equally be applied to other medical conditions [ 2 ]. This type of criticism could also be applied to other psychiatric disorders, and that has indeed been the case historically [ 23 , 24 ]. Today, there is broad consensus that those criticisms were misguided. Few, if any healthcare professionals continue to maintain that schizophrenia, rather than being a disease, is a normal response to societal conditions. Why, then, do people continue to question if addiction is a disease, but not whether schizophrenia, major depressive disorder or post-traumatic stress disorder are diseases? This is particularly troubling given the decades of data showing high co-morbidity of addiction with these conditions [ 25 , 26 ]. We argue that it comes down to stigma. Dysregulated substance use continues to be perceived as a self-inflicted condition characterized by a lack of willpower, thus falling outside the scope of medicine and into that of morality [ 3 ].
Chronic and relapsing, developmentally-limited, or spontaneously remitting?
Much of the critique targeted at the conceptualization of addiction as a brain disease focuses on its original assertion that addiction is a chronic and relapsing condition. Epidemiological data are cited in support of the notion that large proportions of individuals achieve remission [ 27 ], frequently without any formal treatment [ 28 , 29 ] and in some cases resuming low risk substance use [ 30 ]. For instance, based on data from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) study [ 27 ], it has been pointed out that a significant proportion of people with an addictive disorder quit each year, and that most afflicted individuals ultimately remit. These spontaneous remission rates are argued to invalidate the concept of a chronic, relapsing disease [ 4 ].
Interpreting these and similar data is complicated by several methodological and conceptual issues. First, people may appear to remit spontaneously because they actually do, but also because of limited test–retest reliability of the diagnosis [ 31 ]. For instance, using a validated diagnostic interview and trained interviewers, the Collaborative Studies on Genetics of Alcoholism examined the likelihood that an individual diagnosed with a lifetime history of substance dependence would retain this classification after 5 years. This is obviously a diagnosis that, once met, by definition cannot truly remit. Lifetime alcohol dependence was indeed stable in individuals recruited from addiction treatment units, ~90% for women, and 95% for men. In contrast, in a community-based sample similar to that used in the NESARC [ 27 ], stability was only ~30% and 65% for women and men, respectively. The most important characteristic that determined diagnostic stability was severity. Diagnosis was stable in severe, treatment-seeking cases, but not in general population cases of alcohol dependence.
These data suggest that commonly used diagnostic criteria alone are simply over-inclusive for a reliable, clinically meaningful diagnosis of addiction. They do identify a core group of treatment seeking individuals with a reliable diagnosis, but, if applied to nonclinical populations, also flag as “cases” a considerable halo of individuals for whom the diagnostic categorization is unreliable. Any meaningful discussion of remission rates needs to take this into account, and specify which of these two populations that is being discussed. Unfortunately, the DSM-5 has not made this task easier. With only 2 out of 11 symptoms being sufficient for a diagnosis of SUD, it captures under a single diagnostic label individuals in a “mild” category, whose diagnosis is likely to have very low test–retest reliability, and who are unlikely to exhibit a chronic relapsing course, together with people at the severe end of the spectrum, whose diagnosis is reliable, many of whom do show a chronic relapsing course.
The NESARC data nevertheless show that close to 10% of people in the general population who are diagnosed with alcohol addiction (here equated with DSM-IV “dependence” used in the NESARC study) never remitted throughout their participation in the survey. The base life-time prevalence of alcohol dependence in NESARC was 12.5% [ 32 ]. Thus, the data cited against the concept of addiction as a chronic relapsing disease in fact indicate that over 1% of the US population develops an alcohol-related condition that is associated with high morbidity and mortality, and whose chronic and/or relapsing nature cannot be disputed, since it does not remit.
Secondly, the analysis of NESARC data [ 4 , 27 ] omits opioid addiction, which, together with alcohol and tobacco, is the largest addiction-related public health problem in the US [ 33 ]. This is probably the addictive condition where an analysis of cumulative evidence most strikingly supports the notion of a chronic disorder with frequent relapses in a large proportion of people affected [ 34 ]. Of course, a large number of people with opioid addiction are unable to express the chronic, relapsing course of their disease, because over the long term, their mortality rate is about 15 times greater than that of the general population [ 35 ]. However, even among those who remain alive, the prevalence of stable abstinence from opioid use after 10–30 years of observation is <30%. Remission may not always require abstinence, for instance in the case of alcohol addiction, but is a reasonable proxy for remission with opioids, where return to controlled use is rare. Embedded in these data is a message of literally vital importance: when opioid addiction is diagnosed and treated as a chronic relapsing disease, outcomes are markedly improved, and retention in treatment is associated with a greater likelihood of abstinence.
The fact that significant numbers of individuals exhibit a chronic relapsing course does not negate that even larger numbers of individuals with SUD according to current diagnostic criteria do not. For instance, in many countries, the highest prevalence of substance use problems is found among young adults, aged 18–25 [ 36 ], and a majority of these ‘age out’ of excessive substance use [ 37 ]. It is also well documented that many individuals with SUD achieve longstanding remission, in many cases without any formal treatment (see e.g., [ 27 , 30 , 38 ]).
Collectively, the data show that the course of SUD, as defined by current diagnostic criteria, is highly heterogeneous. Accordingly, we do not maintain that a chronic relapsing course is a defining feature of SUD. When present in a patient, however, such as course is of clinical significance, because it identifies a need for long-term disease management [ 2 ], rather than expectations of a recovery that may not be within the individual’s reach [ 39 ]. From a conceptual standpoint, however, a chronic relapsing course is neither necessary nor implied in a view that addiction is a brain disease. This view also does not mean that it is irreversible and hopeless. Human neuroscience documents restoration of functioning after abstinence [ 40 , 41 ] and reveals predictors of clinical success [ 42 ]. If anything, this evidence suggests a need to increase efforts devoted to neuroscientific research on addiction recovery [ 40 , 43 ].
Lessons from genetics
For alcohol addiction, meta-analysis of twin and adoption studies has estimated heritability at ~50%, while estimates for opioid addiction are even higher [ 44 , 45 ]. Genetic risk factors are to a large extent shared across substances [ 46 ]. It has been argued that a genetic contribution cannot support a disease view of a behavior, because most behavioral traits, including religious and political inclinations, have a genetic contribution [ 4 ]. This statement, while correct in pointing out broad heritability of behavioral traits, misses a fundamental point. Genetic architecture is much like organ structure. The fact that normal anatomy shapes healthy organ function does not negate that an altered structure can contribute to pathophysiology of disease. The structure of the genetic landscape is no different. Critics further state that a “genetic predisposition is not a recipe for compulsion”, but no neuroscientist or geneticist would claim that genetic risk is “a recipe for compulsion”. Genetic risk is probabilistic, not deterministic. However, as we will see below, in the case of addiction, it contributes to large, consistent probability shifts towards maladaptive behavior.
In dismissing the relevance of genetic risk for addiction, Hall writes that “a large number of alleles are involved in the genetic susceptibility to addiction and individually these alleles might very weakly predict a risk of addiction”. He goes on to conclude that “generally, genetic prediction of the risk of disease (even with whole-genome sequencing data) is unlikely to be informative for most people who have a so-called average risk of developing an addiction disorder” [ 7 ]. This reflects a fundamental misunderstanding of polygenic risk. It is true that a large number of risk alleles are involved, and that the explanatory power of currently available polygenic risk scores for addictive disorders lags behind those for e.g., schizophrenia or major depression [ 47 , 48 ]. The only implication of this, however, is that low average effect sizes of risk alleles in addiction necessitate larger study samples to construct polygenic scores that account for a large proportion of the known heritability.
However, a heritability of addiction of ~50% indicates that DNA sequence variation accounts for 50% of the risk for this condition. Once whole genome sequencing is readily available, it is likely that it will be possible to identify most of that DNA variation. For clinical purposes, those polygenic scores will of course not replace an understanding of the intricate web of biological and social factors that promote or prevent expression of addiction in an individual case; rather, they will add to it [ 49 ]. Meanwhile, however, genome-wide association studies in addiction have already provided important information. For instance, they have established that the genetic underpinnings of alcohol addiction only partially overlap with those for alcohol consumption, underscoring the genetic distinction between pathological and nonpathological drinking behaviors [ 50 ].
It thus seems that, rather than negating a rationale for a disease view of addiction, the important implication of the polygenic nature of addiction risk is a very different one. Genome-wide association studies of complex traits have largely confirmed the century old “infinitisemal model” in which Fisher reconciled Mendelian and polygenic traits [ 51 ]. A key implication of this model is that genetic susceptibility for a complex, polygenic trait is continuously distributed in the population. This may seem antithetical to a view of addiction as a distinct disease category, but the contradiction is only apparent, and one that has long been familiar to quantitative genetics. Viewing addiction susceptibility as a polygenic quantitative trait, and addiction as a disease category is entirely in line with Falconer’s theorem, according to which, in a given set of environmental conditions, a certain level of genetic susceptibility will determine a threshold above which disease will arise.
A brain disease? Then show me the brain lesion!
The notion of addiction as a brain disease is commonly criticized with the argument that a specific pathognomonic brain lesion has not been identified. Indeed, brain imaging findings in addiction (perhaps with the exception of extensive neurotoxic gray matter loss in advanced alcohol addiction) are nowhere near the level of specificity and sensitivity required of clinical diagnostic tests. However, this criticism neglects the fact that neuroimaging is not used to diagnose many neurologic and psychiatric disorders, including epilepsy, ALS, migraine, Huntington’s disease, bipolar disorder, or schizophrenia. Even among conditions where signs of disease can be detected using brain imaging, such as Alzheimer’s and Parkinson’s disease, a scan is best used in conjunction with clinical acumen when making the diagnosis. Thus, the requirement that addiction be detectable with a brain scan in order to be classified as a disease does not recognize the role of neuroimaging in the clinic.
For the foreseeable future, the main objective of imaging in addiction research is not to diagnose addiction, but rather to improve our understanding of mechanisms that underlie it. The hope is that mechanistic insights will help bring forward new treatments, by identifying candidate targets for them, by pointing to treatment-responsive biomarkers, or both [ 52 ]. Developing innovative treatments is essential to address unmet treatment needs, in particular in stimulant and cannabis addiction, where no approved medications are currently available. Although the task to develop novel treatments is challenging, promising candidates await evaluation [ 53 ]. A particular opportunity for imaging-based research is related to the complex and heterogeneous nature of addictive disorders. Imaging-based biomarkers hold the promise of allowing this complexity to be deconstructed into specific functional domains, as proposed by the RDoC initiative [ 54 ] and its application to addiction [ 55 , 56 ]. This can ultimately guide the development of personalized medicine strategies to addiction treatment.
Countless imaging studies have reported differences in brain structure and function between people with addictive disorders and those without them. Meta-analyses of structural data show that alcohol addiction is associated with gray matter losses in the prefrontal cortex, dorsal striatum, insula, and posterior cingulate cortex [ 57 ], and similar results have been obtained in stimulant-addicted individuals [ 58 ]. Meta-analysis of functional imaging studies has demonstrated common alterations in dorsal striatal, and frontal circuits engaged in reward and salience processing, habit formation, and executive control, across different substances and task-paradigms [ 59 ]. Molecular imaging studies have shown that large and fast increases in dopamine are associated with the reinforcing effects of drugs of abuse, but that after chronic drug use and during withdrawal, brain dopamine function is markedly decreased and that these decreases are associated with dysfunction of prefrontal regions [ 60 ]. Collectively, these findings have given rise to a widely held view of addiction as a disorder of fronto-striatal circuitry that mediates top-down regulation of behavior [ 61 ].
Critics reply that none of the brain imaging findings are sufficiently specific to distinguish between addiction and its absence, and that they are typically obtained in cross-sectional studies that can at best establish correlative rather than causal links. In this, they are largely right, and an updated version of a conceptualization of addiction as a brain disease needs to acknowledge this. Many of the structural brain findings reported are not specific for addiction, but rather shared across psychiatric disorders [ 62 ]. Also, for now, the most sophisticated tools of human brain imaging remain crude in face of complex neural circuit function. Importantly however, a vast literature from animal studies also documents functional changes in fronto-striatal circuits, as well their limbic and midbrain inputs, associated with addictive behaviors [ 63 , 64 , 65 , 66 , 67 , 68 ]. These are circuits akin to those identified by neuroimaging studies in humans, implicated in positive and negative emotions, learning processes and executive functions, altered function of which is thought to underlie addiction. These animal studies, by virtue of their cellular and molecular level resolution, and their ability to establish causality under experimental control, are therefore an important complement to human neuroimaging work.
Nevertheless, factors that seem remote from the activity of brain circuits, such as policies, substance availability and cost, as well as socioeconomic factors, also are critically important determinants of substance use. In this complex landscape, is the brain really a defensible focal point for research and treatment? The answer is “yes”. As powerfully articulated by Francis Crick [ 69 ], “You, your joys and your sorrows, your memories and your ambitions, your sense of personal identity and free will, are in fact no more than the behavior of a vast assembly of nerve cells and their associated molecules”. Social and interpersonal factors are critically important in addiction, but they can only exert their influences by impacting neural processes. They must be encoded as sensory data, represented together with memories of the past and predictions about the future, and combined with representations of interoceptive and other influences to provide inputs to the valuation machinery of the brain. Collectively, these inputs drive action selection and execution of behavior—say, to drink or not to drink, and then, within an episode, to stop drinking or keep drinking. Stating that the pathophysiology of addiction is largely about the brain does not ignore the role of other influences. It is just the opposite: it is attempting to understand how those important influences contribute to drug seeking and taking in the context of the brain, and vice versa.
But if the criticism is one of emphasis rather than of principle—i.e., too much brain, too little social and environmental factors – then neuroscientists need to acknowledge that they are in part guilty as charged. Brain-centric accounts of addiction have for a long time failed to pay enough attention to the inputs that social factors provide to neural processing behind drug seeking and taking [ 9 ]. This landscape is, however, rapidly changing. For instance, using animal models, scientists are finding that lack of social play early in life increases the motivation to take addictive substances in adulthood [ 70 ]. Others find that the opportunity to interact with a fellow rat is protective against addiction-like behaviors [ 71 ]. In humans, a relationship has been found between perceived social support, socioeconomic status, and the availability of dopamine D2 receptors [ 72 , 73 ], a biological marker of addiction vulnerability. Those findings in turn provided translation of data from nonhuman primates, which showed that D2 receptor availability can be altered by changes in social hierarchy, and that these changes are associated with the motivation to obtain cocaine [ 74 ].
Epidemiologically, it is well established that social determinants of health, including major racial and ethnic disparities, play a significant role in the risk for addiction [ 75 , 76 ]. Contemporary neuroscience is illuminating how those factors penetrate the brain [ 77 ] and, in some cases, reveals pathways of resilience [ 78 ] and how evidence-based prevention can interrupt those adverse consequences [ 79 , 80 ]. In other words, from our perspective, viewing addiction as a brain disease in no way negates the importance of social determinants of health or societal inequalities as critical influences. In fact, as shown by the studies correlating dopamine receptors with social experience, imaging is capable of capturing the impact of the social environment on brain function. This provides a platform for understanding how those influences become embedded in the biology of the brain, which provides a biological roadmap for prevention and intervention.
We therefore argue that a contemporary view of addiction as a brain disease does not deny the influence of social, environmental, developmental, or socioeconomic processes, but rather proposes that the brain is the underlying material substrate upon which those factors impinge and from which the responses originate. Because of this, neurobiology is a critical level of analysis for understanding addiction, although certainly not the only one. It is recognized throughout modern medicine that a host of biological and non-biological factors give rise to disease; understanding the biological pathophysiology is critical for understanding etiology and informing treatment.
Is a view of addiction as a brain disease deterministic?
A common criticism of the notion that addiction is a brain disease is that it is reductionist and in the end therefore deterministic [ 81 , 82 ]. This is a fundamental misrepresentation. As indicated above, viewing addiction as a brain disease simply states that neurobiology is an undeniable component of addiction. A reason for deterministic interpretations may be that modern neuroscience emphasizes an understanding of proximal causality within research designs (e.g., whether an observed link between biological processes is mediated by a specific mechanism). That does not in any way reflect a superordinate assumption that neuroscience will achieve global causality. On the contrary, since we realize that addiction involves interactions between biology, environment and society, ultimate (complete) prediction of behavior based on an understanding of neural processes alone is neither expected, nor a goal.
A fairer representation of a contemporary neuroscience view is that it believes insights from neurobiology allow useful probabilistic models to be developed of the inherently stochastic processes involved in behavior [see [ 83 ] for an elegant recent example]. Changes in brain function and structure in addiction exert a powerful probabilistic influence over a person’s behavior, but one that is highly multifactorial, variable, and thus stochastic. Philosophically, this is best understood as being aligned with indeterminism, a perspective that has a deep history in philosophy and psychology [ 84 ]. In modern neuroscience, it refers to the position that the dynamic complexity of the brain, given the probabilistic threshold-gated nature of its biology (e.g., action potential depolarization, ion channel gating), means that behavior cannot be definitively predicted in any individual instance [ 85 , 86 ].
Driven by compulsion, or free to choose?
A major criticism of the brain disease view of addiction, and one that is related to the issue of determinism vs indeterminism, centers around the term “compulsivity” [ 6 , 87 , 88 , 89 , 90 ] and the different meanings it is given. Prominent addiction theories state that addiction is characterized by a transition from controlled to “compulsive” drug seeking and taking [ 91 , 92 , 93 , 94 , 95 ], but allocate somewhat different meanings to “compulsivity”. By some accounts, compulsive substance use is habitual and insensitive to its outcomes [ 92 , 94 , 96 ]. Others refer to compulsive use as a result of increasing incentive value of drug associated cues [ 97 ], while others view it as driven by a recruitment of systems that encode negative affective states [ 95 , 98 ].
The prototype for compulsive behavior is provided by obsessive-compulsive disorder (OCD), where compulsion refers to repeatedly and stereotypically carrying out actions that in themselves may be meaningful, but lose their purpose and become harmful when performed in excess, such as persistent handwashing until skin injuries result. Crucially, this happens despite a conscious desire to do otherwise. Attempts to resist these compulsions result in increasing and ultimately intractable anxiety [ 99 ]. This is in important ways different from the meaning of compulsivity as commonly used in addiction theories. In the addiction field, compulsive drug use typically refers to inflexible, drug-centered behavior in which substance use is insensitive to adverse consequences [ 100 ]. Although this phenomenon is not necessarily present in every patient, it reflects important symptoms of clinical addiction, and is captured by several DSM-5 criteria for SUD [ 101 ]. Examples are needle-sharing despite knowledge of a risk to contract HIV or Hepatitis C, drinking despite a knowledge of having liver cirrhosis, but also the neglect of social and professional activities that previously were more important than substance use. While these behaviors do show similarities with the compulsions of OCD, there are also important differences. For example, “compulsive” substance use is not necessarily accompanied by a conscious desire to withhold the behavior, nor is addictive behavior consistently impervious to change.
Critics question the existence of compulsivity in addiction altogether [ 5 , 6 , 7 , 89 ], typically using a literal interpretation, i.e., that a person who uses alcohol or drugs simply can not do otherwise. Were that the intended meaning in theories of addiction—which it is not—it would clearly be invalidated by observations of preserved sensitivity of behavior to contingencies in addiction. Indeed, substance use is influenced both by the availability of alternative reinforcers, and the state of the organism. The roots of this insight date back to 1940, when Spragg found that chimpanzees would normally choose a banana over morphine. However, when physically dependent and in a state of withdrawal, their choice preference would reverse [ 102 ]. The critical role of alternative reinforcers was elegantly brought into modern neuroscience by Ahmed et al., who showed that rats extensively trained to self-administer cocaine would readily forego the drug if offered a sweet solution as an alternative [ 103 ]. This was later also found to be the case for heroin [ 103 ], methamphetamine [ 104 ] and alcohol [ 105 ]. Early residential laboratory studies on alcohol use disorder indeed revealed orderly operant control over alcohol consumption [ 106 ]. Furthermore, efficacy of treatment approaches such as contingency management, which provides systematic incentives for abstinence [ 107 ], supports the notion that behavioral choices in patients with addictions remain sensitive to reward contingencies.
Evidence that a capacity for choosing advantageously is preserved in addiction provides a valid argument against a narrow concept of “compulsivity” as rigid, immutable behavior that applies to all patients. It does not, however, provide an argument against addiction as a brain disease. If not from the brain, from where do the healthy and unhealthy choices people make originate? The critical question is whether addictive behaviors—for the most part—result from healthy brains responding normally to externally determined contingencies; or rather from a pathology of brain circuits that, through probabilistic shifts, promotes the likelihood of maladaptive choices even when reward contingencies are within a normal range. To resolve this question, it is critical to understand that the ability to choose advantageously is not an all-or-nothing phenomenon, but rather is about probabilities and their shifts, multiple faculties within human cognition, and their interaction. Yes, it is clear that most people whom we would consider to suffer from addiction remain able to choose advantageously much, if not most, of the time. However, it is also clear that the probability of them choosing to their own disadvantage, even when more salutary options are available and sometimes at the expense of losing their life, is systematically and quantifiably increased. There is a freedom of choice, yet there is a shift of prevailing choices that nevertheless can kill.
Synthesized, the notion of addiction as a disease of choice and addiction as a brain disease can be understood as two sides of the same coin. Both of these perspectives are informative, and they are complementary. Viewed this way, addiction is a brain disease in which a person’s choice faculties become profoundly compromised. To articulate it more specifically, embedded in and principally executed by the central nervous system, addiction can be understood as a disorder of choice preferences, preferences that overvalue immediate reinforcement (both positive and negative), preferences for drug-reinforcement in spite of costs, and preferences that are unstable ( “I’ll never drink like that again;” “this will be my last cigarette” ), prone to reversals in the form of lapses and relapse. From a contemporary neuroscience perspective, pre-existing vulnerabilities and persistent drug use lead to a vicious circle of substantive disruptions in the brain that impair and undermine choice capacities for adaptive behavior, but do not annihilate them. Evidence of generally intact decision making does not fundamentally contradict addiction as a brain disease.
Conclusions
The present paper is a response to the increasing number of criticisms of the view that addiction is a chronic relapsing brain disease. In many cases, we show that those criticisms target tenets that are neither needed nor held by a contemporary version of this view. Common themes are that viewing addiction as a brain disease is criticized for being both too narrow (addiction is only a brain disease; no other perspectives or factors are important) or too far reaching (it purports to discover the final causes of addiction). With regard to disease course, we propose that viewing addiction as a chronic relapsing disease is appropriate for some populations, and much less so for others, simply necessitating better ways of delineating the populations being discussed. We argue that when considering addiction as a disease, the lens of neurobiology is valuable to use. It is not the only lens, and it does not have supremacy over other scientific approaches. We agree that critiques of neuroscience are warranted [ 108 ] and that critical thinking is essential to avoid deterministic language and scientific overreach.
Beyond making the case for a view of addiction as a brain disease, perhaps the more important question is when a specific level of analysis is most useful. For understanding the biology of addiction and designing biological interventions, a neurobiological view is almost certainly the most appropriate level of analysis, in particular when informed by an understanding of the behavioral manifestations. In contrast, for understanding the psychology of addiction and designing psychological interventions, behavioral science is the natural realm, but one that can often benefit from an understanding of the underlying neurobiology. For designing policies, such as taxation and regulation of access, economics and public administration provide the most pertinent perspectives, but these also benefit from biological and behavioral science insights.
Finally, we argue that progress would come from integration of these scientific perspectives and traditions. E.O. Wilson has argued more broadly for greater consilience [ 109 ], unity of knowledge, in science. We believe that addiction is among the areas where consilience is most needed. A plurality of disciplines brings important and trenchant insights to bear on this condition; it is the exclusive remit of no single perspective or field. Addiction inherently and necessarily requires multidisciplinary examination. Moreover, those who suffer from addiction will benefit most from the application of the full armamentarium of scientific perspectives.
Funding and disclosures
Supported by the Swedish Research Council grants 2013-07434, 2019-01138 (MH); Netherlands Organisation for Health Research and Development (ZonMw) under project number 912.14.093 (LJMJV); NIDA and NIAAA intramural research programs (LL; the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health); the Peter Boris Chair in Addictions Research, Homewood Research Institute, and the National Institute on Alcohol Abuse and Alcoholism grants AA025911, AA024930, AA025849, AA027679 (JM; the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health).
MH has received consulting fees, research support or other compensation from Indivior, Camurus, BrainsWay, Aelis Farma, and Janssen Pharmaceuticals. JM is a Principal and Senior Scientist at BEAM Diagnostics, Inc. DM, JR, LL, and LJMJV declare no conflict of interest.
Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–7.
Article CAS PubMed Google Scholar
McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–95.
Schomerus G, Lucht M, Holzinger A, Matschinger H, Carta MG, Angermeyer MC. The stigma of alcohol dependence compared with other mental disorders: a review of population studies. Alcohol Alcohol. 2011;46:105–12.
Heyman GM. Addiction and choice: theory and new data. Front Psychiatry. 2013;4:31.
Article PubMed PubMed Central Google Scholar
Heather N, Best D, Kawalek A, Field M, Lewis M, Rotgers F, et al. Challenging the brain disease model of addiction: European launch of the addiction theory network. Addict Res Theory. 2018;26:249–55.
Article Google Scholar
Pickard H, Ahmed SH, Foddy B. Alternative models of addiction. Front Psychiatr.y 2015;6:20.
Google Scholar
Hall W, Carter A, Forlini C. The brain disease model of addiction: is it supported by the evidence and has it delivered on its promises? Lancet Psychiatr. 2015;2:105–10.
Hart CL. Viewing addiction as a brain disease promotes social injustice. Nat Hum Behav. 2017;1:0055.
Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosc.i 2016;17:592–9.
Article CAS Google Scholar
Jellinek EM. The disease concept of alcoholism. Hillhouse Press on behalf of the Christopher J. Smithers Foundation: New Haven, CT; 1960.
Stevenson A. Oxford dictionary of English. 3 ed. New York, NY: Oxford University Press; 2010.
Fan J, May SJ, Zhou Y, Barrett-Connor E. Bimodality of 2-h plasma glucose distributions in whites: the Rancho Bernardo study. Diabetes Care 2005;28:1451–6.
King AC, Vena A, Hasin D, De Wit D, O’Connor CJ, Cao D. Subjective responses to alcohol in the development and maintenance of alcohol use disorder (AUD). Am J Psychiatry. 2021. https://doi.org/10.1176/appi.ajp.2020.20030247 .
GBD. 2016 Alcohol and Drug Use Collaborators. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5:987–1012.
Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. Br Med J. 1976;1:1058–61.
Article CAS PubMed PubMed Central Google Scholar
Epstein DH, Heilig M, Shaham Y. Science-based actions can help address the opioid crisis. Trends Pharm Sci. 2018;39:911–16.
Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abus Treat. 2005;28:321–9.
Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Datab System Rev. 2013;5:CD009329.
Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings a systematic review and meta-analysis. JAMA. 2014;311:1889–900.
Article PubMed CAS Google Scholar
Mark TL, Kranzler HR, Song X. Understanding US addiction physicians’ low rate of naltrexone prescription. Drug Alcohol Depend. 2003;71:219–28.
Article PubMed Google Scholar
Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–65.
Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Intern Med. 1997;12:274–83.
Laing RD. The divided self; a study of sanity and madness. London: Tavistock Publications; 1960.
Foucault M, Khalfa J. History of madness. New York: Routledge; 2006.
Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL. et al.Comorbidity of mental disorders with alcohol and other drug abuse. Results Epidemiologic Catchment Area (ECA) study.JAMA. 1990;264:2511–8.
Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–16.
Lopez-Quintero C, Hasin DS, de Los Cobos JP, Pines A, Wang S, Grant BF, et al. Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction. 2011;106:657–69.
Humphreys K. Addiction treatment professionals are not the gatekeepers of recovery. Subst Use Misuse. 2015;50:1024–7.
Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2007;86:214–21.
Sobell LC, Cunningham JA, Sobell MB. Recovery from alcohol problems with and without treatment: prevalence in two population surveys. Am J Public Health. 1996;86:966–72.
Culverhouse R, Bucholz KK, Crowe RR, Hesselbrock V, Nurnberger JI Jr, Porjesz B, et al. Long-term stability of alcohol and other substance dependence diagnoses and habitual smoking: an evaluation after 5 years. Arch Gen Psychiatry. 2005;62:753–60.
Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42.
Skolnick P. The opioid epidemic: crisis and solutions. Annu Rev Pharm Toxicol. 2018;58:143–59.
Hser YI, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harv Rev Psychiatry. 2015;23:76–89.
Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:102–23.
Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66.
Article PubMed PubMed Central CAS Google Scholar
Lee MR, Sher KJ. “Maturing Out” of binge and problem drinking. Alcohol Res: Curr Rev. 2018;39:31–42.
Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ. Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction. 2005;100:281–92.
Berridge V. The rise, fall, and revival of recovery in drug policy. Lancet. 2012;379:22–23.
Parvaz MA, Moeller SJ, d’Oleire Uquillas F, Pflumm A, Maloney T, Alia-Klein N, et al. Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict Biol. 2017;22:1391–401.
Korponay C, Kosson DS, Decety J, Kiehl KA, Koenigs M. Brain volume correlates with duration of abstinence from substance abuse in a region-specific and substance-specific manner. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:626–35.
PubMed PubMed Central Google Scholar
Janes AC, Datko M, Roy A, Barton B, Druker S, Neal C, et al. Quitting starts in the brain: a randomized controlled trial of app-based mindfulness shows decreases in neural responses to smoking cues that predict reductions in smoking. Neuropsychopharmacology. 2019;44:1631–38.
Humphreys K, Bickel WK. Toward a neuroscience of long-term recovery from addiction. JAMA Psychiatry. 2018;75:875–76.
Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015;45:1061–72.
Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–32.
Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. AJ Psychiatry. 2003;160:687–95.
Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.
Wray NR, Lin T, Austin J, McGrath JJ, Hickie IB, Murray GK, et al. From basic science to clinical application of polygenic risk scores: a primer. JAMA Psychiatry. 2021;78:101–9.
Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21:1656–69.
Visscher PM, Wray NR. Concepts and misconceptions about the polygenic additive model applied to disease. Hum Hered. 2015;80:165–70.
Heilig M, Leggio L. What the alcohol doctor ordered from the neuroscientist: theragnostic biomarkers for personalized treatments. Prog Brain Res. 2016;224:401–18.
Rasmussen K, White DA, Acri JB. NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacology. 2019;44:657–59.
Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. AJ Psychiatry. 2010;167:748–51.
Kwako LE, Schwandt ML, Ramchandani VA, Diazgranados N, Koob GF, Volkow ND, et al. Neurofunctional domains derived from deep behavioral phenotyping in alcohol use disorder. AJ Psychiatry. 2019;176:744–53.
Kwako LE, Bickel WK, Goldman D. Addiction biomarkers: dimensional approaches to understanding addiction. Trends Mol Med. 2018;24:121–28.
Xiao P, Dai Z, Zhong J, Zhu Y, Shi H, Pan P. Regional gray matter deficits in alcohol dependence: a meta-analysis of voxel-based morphometry studies. Drug Alcohol Depend. 2015;153:22–8.
Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23:615–24.
Klugah-Brown B, Di X, Zweerings J, Mathiak K, Becker B, Biswal B. Common and separable neural alterations in substance use disorders: a coordinate-based meta-analyses of functional neuroimaging studies in humans. Hum Brain Mapp. 2020;41:4459–77.
Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Investig. 2003;111:1444–51.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69.
Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15.
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharm Rev. 2016;68:816–71.
Korpi ER, den Hollander B, Farooq U, Vashchinkina E, Rajkumar R, Nutt DJ, et al. Mechanisms of action and persistent neuroplasticity by drugs of abuse. Pharm Rev. 2015;67:872–1004.
Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63.
Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories–indications for novel treatments of addiction. Eur J Neurosci. 2014;40:2163–82.
Lesscher HM, Vanderschuren LJ. Compulsive drug use and its neural substrates. Rev Neurosci. 2012;23:731–45.
Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, et al. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–54.
Crick F. The astonishing hypothesis: the scientific search for the soul. Scribner; Maxwell Macmillan International: New York, NY; 1994.
Vanderschuren LJ, Achterberg EJ, Trezza V. The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev. 2016;70:86–105.
Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018;21:1520–29.
Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, et al. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry. 2010;67:275–8.
Wiers CE, Shokri-Kojori E, Cabrera E, Cunningham S, Wong C, Tomasi D, et al. Socioeconomic status is associated with striatal dopamine D2/D3 receptors in healthy volunteers but not in cocaine abusers. Neurosci Lett. 2016;617:27–31.
Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74.
Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e66.
Gilbert PA, Zemore SE. Discrimination and drinking: a systematic review of the evidence. Soc Sci Med 2016;161:178–94.
Oshri A, Gray JC, Owens MM, Liu S, Duprey EB, Sweet LH, et al. Adverse childhood experiences and amygdalar reduction: high-resolution segmentation reveals associations with subnuclei and psychiatric outcomes. Child Maltreat. 2019;24:400–10.
Holmes CJ, Barton AW, MacKillop J, Galván A, Owens MM, McCormick MJ, et al. Parenting and salience network connectivity among African Americans: a protective pathway for health-risk behaviors. Biol Psychiatry. 2018;84:365–71.
Brody GH, Gray JC, Yu T, Barton AW, Beach SR, Galván A, et al. Protective prevention effects on the association of poverty with brain development. JAMA Pediatr. 2017;171:46–52.
Hanson JL, Gillmore AD, Yu T, Holmes CJ, Hallowell ES, Barton AW, et al. A family focused intervention influences hippocampal-prefrontal connectivity through gains in self-regulation. Child Dev. 2019;90:1389–401.
Borsboom D, Cramer A, Kalis A. Brain disorders? Not really… why network structures block reductionism in psychopathology research. Behav Brain Sci. 2018;42:1–54.
Field M, Heather N, Wiers RW. Indeed, not really a brain disorder: Implications for reductionist accounts of addiction. Behav Brain Sci. 2019;42:e9.
Pascoli V, Hiver A, Van Zessen R, Loureiro M, Achargui R, Harada M, et al. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature. 2018;564:366–71.
James W. The dilemma of determinism. Whitefish, MT: Kessinger Publishing; 2005.
Gessell B. Indeterminism in the brain. Biol Philos. 2017;32:1205–23.
Jedlicka P. Revisiting the quantum brain hypothesis: toward quantum (neuro)biology? Front Mol Neurosci. 2017;10:366.
Heyman GM. Addiction: a disorder of choice. Cambridge, MA: Harvard University Press; 2010.
Heather NQ. Is addiction a brain disease or a moral failing? A: Neither. Neuroethics. 2017;10:115–24.
Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–7.
Hogarth L, Lam-Cassettari C, Pacitti H, Currah T, Mahlberg J, Hartley L, et al. Intact goal-directed control in treatment-seeking drug users indexed by outcome-devaluation and Pavlovian to instrumental transfer: critique of habit theory. Eur J Neurosci. 2019;50:2513–25.
Mathis V, Kenny PJ. From controlled to compulsive drug-taking: the role of the habenula in addiction. Neurosci Biobehav Rev. 2019;106:102–11.
Luscher C, Robbins TW, Everitt BJ. The transition to compulsion in addiction. Nat Rev Neurosci. 2020;21:247–63.
Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53.
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–89.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–68.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91.
Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–4.
Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive-compulsive disorder. Nat Rev Dis Prim. 2019;5:52.
Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 2004;305:1017–9.
American_Psychiatric_Association. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th ed. Arlington, VA, US: American Psychiatric Publishing, Inc; 2013.
Book Google Scholar
Spragg SDS. Morphine addiction in chimpanzees. Comp Psychol Monogr. 1940;15:132–32.
Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20.
Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 2015;78:463–73.
Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, et al. A molecular mechanism for choosing alcohol over an alternative reward. Science. 2018;360:1321–26.
Bigelow GE. An operant behavioral perspective on alcohol abuse and dependence. In: Heather N, Peters TJ, Stockwell T, editors. International handbook of alcohol dependence and problems. John Wiley & Sons Ltd; 2001. p. 299–315.
Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annu Rev Psychol. 2004;55:431–61.
Satel S, Lilienfeld SO. Brainwashed: the seductive appeal of mindless neuroscience. New York, NY: Basic Books; 2015.
Wilson EO. Consilience: the unity of knowledge. New York, NY: Vintage Books; 1999.
Saunders JB, Degenhardt L, Reed GM, Poznyak V. Alcohol use disorders in ICD-11: past, present, and future. Alcohol Clin Exp Res 2019;43:1617–31.
Organization. WH. ICD-11 for mortality and morbidity statistics. 2018. https://icd.who.int/browse11/l-m/en . Accessed 21 Oct 2020.
Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, brief intervention, and referral to treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus. 2007;28:7–30.
Witkiewitz K, Hallgren KA, Kranzler HR, Mann KF, Hasin DS, Falk DE, et al. Clinical validation of reduced alcohol consumption after treatment for alcohol dependence using the World Health Organization risk drinking levels. Alcohol Clin Exp Res 2017;41:179–86.
Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. AJ Psychiatry. 2013;170:834–51.
Rosenthal RJ, Faris SB. The etymology and early history of ‘addiction’. Addict Res Theory. 2019;27:437–49.
Martin CS, Steinley DL, Verges A, Sher KJ. The proposed 2/11 symptom algorithm for DSM-5 substance-use disorders is too lenient. Psychol Med. 2011;41:2008–10.
Degenhardt L, Bharat C, Bruno R, Glantz MD, Sampson NA, Lago L, et al. Concordance between the diagnostic guidelines for alcohol and cannabis use disorders in the draft ICD-11 and other classification systems: analysis of data from the WHO’s World Mental Health Surveys. Addiction. 2019;114:534–52.
PubMed Google Scholar
Lago L, Bruno R, Degenhardt L. Concordance of ICD-11 and DSM-5 definitions of alcohol and cannabis use disorders: a population survey. Lancet Psychiatry. 2016;3:673–84.
Lundin A, Hallgren M, Forsman M, Forsell Y. Comparison of DSM-5 classifications of alcohol use disorders with those of DSM-IV, DSM-III-R, and ICD-10 in a general population sample in Sweden. J Stud Alcohol Drugs. 2015;76:773–80.
Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions neuroclinical assessment: a neuroscience-based framework for addictive disorders. Biol Psychiatry. 2016;80:179–89.
Rehm J, Heilig M, Gual A. ICD-11 for alcohol use disorders: not a convincing answer to the challenges. Alcohol Clin Exp Res. 2019;43:2296–300.
Download references
Acknowledgements
The authors want to acknowledge comments by Drs. David Epstein, Kenneth Kendler and Naomi Wray.
Author information
Authors and affiliations.
Center for Social and Affective Neuroscience, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden
Markus Heilig
Peter Boris Centre for Addictions Research, McMaster University and St. Joseph’s Healthcare Hamilton, Hamilton, ON, Canada
- James MacKillop
Homewood Research Institute, Guelph, ON, Canada
New York State Psychiatric Institute and Columbia University Irving Medical Center, New York, NY, USA
Diana Martinez
Institute for Mental Health Policy Research & Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health (CAMH), Toronto, ON, Canada
Jürgen Rehm
Dalla Lana School of Public Health and Department of Psychiatry, University of Toronto (UofT), Toronto, ON, Canada
Klinische Psychologie & Psychotherapie, Technische Universität Dresden, Dresden, Germany
Department of International Health Projects, Institute for Leadership and Health Management, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, Translational Addiction Medicine Branch, National Institute on Drug Abuse Intramural Research Program and National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research, National Institutes of Health, Baltimore and Bethesda, MD, USA
Lorenzo Leggio
Department of Population Health Sciences, Unit Animals in Science and Society, Faculty of Veterinary Medicine, Utrecht University, Utrecht, the Netherlands
Louk J. M. J. Vanderschuren
You can also search for this author in PubMed Google Scholar
Contributions
All authors jointly drafted the paper.
Corresponding author
Correspondence to Markus Heilig .
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Heilig, M., MacKillop, J., Martinez, D. et al. Addiction as a brain disease revised: why it still matters, and the need for consilience. Neuropsychopharmacol. 46 , 1715–1723 (2021). https://doi.org/10.1038/s41386-020-00950-y
Download citation
Received : 10 November 2020
Revised : 11 December 2020
Accepted : 14 December 2020
Published : 22 February 2021
Issue Date : September 2021
DOI : https://doi.org/10.1038/s41386-020-00950-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Persistent impacts of smoking on resting-state eeg in male chronic smokers and past-smokers with 20 years of abstinence.
- Yoonji Jeon
- Dongil Chung
Scientific Reports (2023)
A contextualized reinforcer pathology approach to addiction
- Samuel F. Acuff
- James G. Murphy
Nature Reviews Psychology (2023)
Functional genomic mechanisms of opioid action and opioid use disorder: a systematic review of animal models and human studies
- Camille Falconnier
- Alba Caparros-Roissard
- Pierre-Eric Lutz
Molecular Psychiatry (2023)
A neuromarker for drug and food craving distinguishes drug users from non-users
- Leonie Koban
- Tor D. Wager
Nature Neuroscience (2023)
Does a Survivorship Model of Opioid Use Disorder Improve Public Stigma or Policy Support? A General Population Randomized Experiment
- Jarratt D. Pytell
- Geetanjali Chander
- Emma E. McGinty
Journal of General Internal Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
A systematic review of substance use and substance use disorder research in Kenya
Roles Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Mental Health, Moi Teaching & Referral Hospital, Eldoret, Kenya
Roles Conceptualization, Methodology, Supervision, Writing – review & editing
Affiliation Department of Mental Health, Mbagathi Hospital, Nairobi, Kenya
Roles Formal analysis, Validation, Writing – review & editing
Affiliation Department of Mental Health & Behavioral Sciences, Moi University School of Medicine, Eldoret, Kenya
Affiliation Population Health, Academic Model Providing Access to Healthcare, Eldoret, Kenya
Roles Formal analysis, Writing – review & editing
Affiliation Department of Mental Health, Gilgil Sub-County Hospital, Gilgil, Kenya
Affiliation Intensive Care Unit, Aga Khan University Hospital, Nairobi, Kenya
Roles Formal analysis, Supervision, Writing – review & editing
- Florence Jaguga,
- Sarah Kanana Kiburi,
- Eunice Temet,
- Julius Barasa,
- Serah Karanja,
- Lizz Kinyua,
- Edith Kamaru Kwobah

- Published: June 9, 2022
- https://doi.org/10.1371/journal.pone.0269340
- Peer Review
- Reader Comments
The burden of substance use in Kenya is significant. The objective of this study was to systematically summarize existing literature on substance use in Kenya, identify research gaps, and provide directions for future research.
This systematic review was conducted in line with the PRISMA guidelines. We conducted a search of 5 bibliographic databases (PubMed, PsychINFO, Web of Science, Cumulative Index of Nursing and Allied Professionals (CINAHL) and Cochrane Library) from inception until 20 August 2020. In addition, we searched all the volumes of the official journal of the National Authority for the Campaign Against Alcohol & Drug Abuse (the African Journal of Alcohol and Drug Abuse). The results of eligible studies have been summarized descriptively and organized by three broad categories including: studies evaluating the epidemiology of substance use, studies evaluating interventions and programs, and qualitative studies exploring various themes on substance use other than interventions. The quality of the included studies was assessed with the Quality Assessment Tool for Studies with Diverse Designs.
Of the 185 studies that were eligible for inclusion, 144 investigated the epidemiology of substance use, 23 qualitatively explored various substance use related themes, and 18 evaluated substance use interventions and programs. Key evidence gaps emerged. Few studies had explored the epidemiology of hallucinogen, prescription medication, ecstasy, injecting drug use, and emerging substance use. Vulnerable populations such as pregnant women, and persons with physical disability had been under-represented within the epidemiological and qualitative work. No intervention study had been conducted among children and adolescents. Most interventions had focused on alcohol to the exclusion of other prevalent substances such as tobacco and cannabis. Little had been done to evaluate digital and population-level interventions.
The results of this systematic review provide important directions for future substance use research in Kenya.
Systematic review registration
PROSPERO: CRD42020203717.
Citation: Jaguga F, Kiburi SK, Temet E, Barasa J, Karanja S, Kinyua L, et al. (2022) A systematic review of substance use and substance use disorder research in Kenya. PLoS ONE 17(6): e0269340. https://doi.org/10.1371/journal.pone.0269340
Editor: Judith I. Tsui, University of Washington, UNITED STATES
Received: January 8, 2022; Accepted: May 18, 2022; Published: June 9, 2022
Copyright: © 2022 Jaguga et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: ASI, Addiction Severity Index; ASSIST, Alcohol Smoking and Substance Involvement Screening Test; AUD, Alcohol Use Disorder; AUDIT, Alcohol Use Identification Test; AUDIT-C, Alcohol Use Identification Test–Concise; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; BHS, Behavioral Health Screen; BMI, Body Mass index; BSIS, Beck Suicidal Intent Scale; CAD, Coronary Artery Disease; CAGE, Cut, Annoyed, Guilty, Eye-opener; CIDI, Composite International Diagnostic Interview; CINAHL, Cumulative Index of Nursing and Allied Professionals; CRAFFT, Car, Relax, Alone, Forget, Friends, Trouble; DAST, Drug Abuse Screening Test; DSM-III, Diagnostic & Statistical Manual Third Edition; DSM-III R, Diagnostic & Statistical Manual Third Edition Revised; DSM-IV, Diagnostic & Statistical Manual Fourth Edition; DSM-V, Diagnostic & Statistical Manual Fifth Edition; DUSI-R, Drug Use Screening Inventory—Revised; FGD, Focus Group Discussion; FSW, Female Sex Workers; GSHS, Global School-based Health Survey; HCV, Hepatitis C Virus; HCW, Healthcare worker; HIC, High Income Country; HIV, Human Immunodeficiency Virus; ICD, International Classification of Disease; IDI, In-depth Interviews; IDP, Internally Displaced Persons; IPV, Intimate Partner Violence; KIIs, Key Informant Interviews; K-SADS, Kiddie-Schedule for Affective Disorders; LGBTQ, Lesbian, Gay, Bisexual, Transgender, Queer; LMIC, Low and Middle Income Country; MAST, Michigan Alcohol Screening Test; MI, Motivational Interviewing; MINI, Mini International Neuropsychiatric Interview; MMT, Methadone Maintenance Therapy; MPBI, Multiple Problem Behavior Inventory; MSM, Men who have Sex with Men; MSME, Men who have Sex with Men Exclusively; MSMW, Men who have Sex with Men & Women; NIH, National Institute of Health; NSP, Needle Syringe Program; OST, Opioid Substitution Therapy; PLHIV, People Living with HIV; PrEP, Pre-exposure Prophylaxis; PTSD, Post-Traumatic Stress Disorder; PWID, People Who Inject Drugs; QATSDD, Quality Assessment Tool for Studies with Diverse Designs; RCT, Randomized controlled trial; RTAs, Road Traffic Accidents; SCID, Structured Clinical interview for DSM; SES, Socio-economic Status; SSA, Sub-Saharan Africa; TB, Tuberculosis; UNODC, United Nations Office on Drugs and Crime; VCT, Voluntary Counseling & Testing; WOTC, Wisdom of the Crowds
Introduction
Globally, substance use is associated with significant morbidity and mortality. In the 2017 Global Burden of Disease (GBD) study, substance use disorders (SUDs) were the second leading cause of disability among the mental disorders with 31,052,000 (25%) Years Lived with Disability (YLD) attributed to them [ 1 ]. In 2016, harmful alcohol use resulted in 3 million deaths (5.3% of all deaths) worldwide and 132.6 (5.1%) million disability-adjusted life years (DALYs) [ 2 ]. Tobacco use, the leading cause of preventable death, kills more than 8 million people worldwide annually [ 3 ]. Alcohol and tobacco use are leading risk factors for non-communicable diseases for example cardiovascular disease, cancer, and liver disease [ 3 , 4 ]. Even though the prevalence rate of opioid use is small compared to that of tobacco and alcohol use, opioid use disorder contributes to 76% of all deaths from SUDs [ 4 ]. Other psychoactive substances such as cannabis and amphetamines are associated with mental health consequences including increased risk of suicidality, depression, anxiety and psychosis [ 5 , 6 ]. In addition to the effect on health, substance use is associated with significant socio-economic costs arising from its impact on health and criminal justice systems [ 7 ].
Low- and middle-income countries (LMICs) bear the burden of substance use. Over 80% of the 1.3 billion tobacco users worldwide live in LMICs [ 3 ]. In 2016, the alcohol-attributable disease burden was highest in LMICs compared to upper-middle-income and high-income countries (HICs) [ 2 ]. In Kenya, a nationwide survey conducted in 2017 reported that over 10% of Kenyans between the ages of 15 to 65 years had a SUD [ 8 ]. In another survey, 20% of primary school children had ever used at least one substance in their lifetime [ 9 ]. Moreover, Kenya has the third highest total DALYs (54,000) from alcohol use disorders (AUD) in Africa [ 4 ] Unfortunately, empirical work on substance use in LMICs is limited [ 10 , 11 ]. In a global mapping of SUD research, majority of the work had been conducted in upper-middle income and HICs (HICs) [ 11 ]. In a study whose aim was to document the existing work on mental health in Botswana, only 7 studies had focused on substance use [ 10 ]. Information upon which policy and interventions could be developed is therefore lacking in low-and-middle income settings.
Since the early 1980s, scholars in Kenya began engaging in research to document the burden and patterns of substance use [ 12 ]. In 2001 the National Authority for the Campaign Against Alcohol and Drug Abuse (NACADA) was established in response to the rising cases of harmful substance use in the country particularly among the youth. The mandate of the Authority was to educate the public on the harms associated with substance use [ 13 ]. In addition to prevention work, NACADA contributes to research by conducting general population prevalence surveys every 5 years and recently launched its journal, the African Journal of Alcohol and Drug Abuse (AJADA) [ 14 ]. The amount of empirical work done on substance use in Kenya has expanded since these early years but has not been systematically summarized. The evidence gaps therefore remain unclear.
In order to guide future research efforts and adequately address the substance use scourge in Kenya, there is need to document the scope and breadth of available scientific literature. The aim of this systematic review is therefore: (i) to describe the characteristics of research studies conducted on substance use and SUD in Kenya; (ii) to assess the methodological quality of the studies; (iii) to identify areas where there is limited research evidence and; (iv) to make recommendations for future research. This paper is in line the Vision 2030 [ 15 ], Kenya’s national development policy framework, which directs that the government implements substance use treatment and prevention projects and programs, and target 3.5 of the Sustainable Development Goals (SDGs) which requires that countries strengthen the treatment and prevention for SUDs [ 16 ].
Materials and methods
Protocol and registration.
In conducting this systematic review we adhered to the recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [ 17 ]. A 27-item PRISMA checklist is available as an additional file to this protocol ( S1 Checklist ). Our protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO): CRD42020203717.

Search strategy
A search was carried out in five electronic databases on 20 th August 2020: PubMed, PsychINFO, Web of Science, Cumulative Index of Nursing and Allied Professionals (CINAHL) and Cochrane Library. The full search strategy can be found in S1 File and takes the following form: (terms for substance use) and (terms for substance use outcomes of interest) and (terms for region) . The searches spanned the period from inception to date. No filter was applied. A manual search was done in Volumes 1, 2 and 3 (all published volumes by the time of the search) of the recently launched AJADA journal by NACADA, and additional articles identified.
[ 14 , 18 , 19 ].
Study selection
Following the initial search, all articles were loaded onto Mendeley reference manager where initial duplicate screening and removal was done. After duplicate removal, the articles were loaded onto Rayyan, a soft-ware for screening and selecting studies during the conduct of systematic reviews [ 20 ]. The abstract and titles of retrieved articles were independently screened by two authors based on a set of pre-determined eligibility criteria. A second screening of full text articles was also done independently by two authors and resulted in an 88.7% agreement. Disagreements during each stage of the screening were resolved through discussion and consensus.
Inclusion criteria
Since we sought to map existing literature on the subject, our inclusion criteria were broad. We included articles on substance use if (i) the sample or part of the sample was from Kenya, (ii) they were original research articles, (iii) they had a substance use or SUD exposure, (iv) they had a substance use or SUD related outcome such as prevalence, pattern of use, prevention and treatment, and (iv) they were published in English or had an English translation available. We included studies conducted among all age groups and studies that used all designs including quantitative, qualitative and mixed methods.
Exclusion criteria
Studies were excluded if: (i) they were cross-national and did not report country specific results (ii) they did not report substance use or SUD as an exposure, and did not have substance use or SUD related outcomes or as part of the outcomes, (iii) they were review articles, dissertations, conference presentations or abstracts, commentaries or editorials, (iv) and the full text articles were not available.
Data extraction
We prepared 3 data extraction forms based on three emerging categories of studies i.e.:
- Studies reporting on the epidemiology of substance use or SUD
- Studies evaluating substance use or SUD interventions and programs
- Studies qualitatively exploring various themes on substance use or SUD (but not evaluating interventions or programs)
The forms were piloted by F.J. and S.K. and adjustments made to the content. Data extraction was then done using the final form by all authors and double checked by F.J. for completeness and accuracy. Discrepancies were resolved by discussion with S.K. and E.T. until consensus was achieved. The following data was extracted for each study category:
- Studies reporting on the epidemiology of substance use or SUD: study design, study population characteristics, study setting, sample size, age and gender distribution, substance(s) assessed, standardized tool or criteria used, main findings (prevalence, risk factors, other key findings).
- Studies evaluating substance use or SUD interventions and programs: study design, study objective, sample size, name of the intervention or program, person delivering intervention, outcomes and measures, and main findings.
- Studies qualitatively exploring various aspects of substance use or SUD other than programs and interventions: study objective, methods of data collection, study setting, study population, age and gender distribution, theoretical framework used, and main findings.
Data synthesis
The results have been summarized descriptively and organized by the three categories above. Within each category, a general description of the study characteristics has been provided followed by a narrative synthesis of findings organized by sub-themes inductively derived from the data. The sub-themes within each category are as follows:
- Studies reporting on the epidemiology of substance use or SUD : Epidemiology of alcohol use, epidemiology of tobacco use, epidemiology of khat use, epidemiology of cannabis use, epidemiology of opioid and cocaine use, epidemiology of other substance use (sedatives, inhalants, hallucinogens, prescription medication, emerging drugs, ecstasy).
- Studies evaluating substance use or SUD interventions and programs: Individual level interventions (Individual-level interventions for harmful alcohol use, individual-level interventions for khat use, individual level intervention for substance use in general); Programs (Methadone programs, needle-syringe programs, tobacco cessation programs, out-patient SUD treatment programs); Population-level interventions : Population-level tobacco interventions, population-level alcohol interventions.
- Studies qualitatively exploring various aspects of substance use or SUD other than programs and interventions : Injecting drug use and heroin use, alcohol use, substance use among youth and adolescents, other topics.
Quality assessment of the studies
Quality assessment was conducted by S.K. using the Quality Assessment Tool for Studies with Diverse Designs (QATSDD) [ 21 ]. F.J. & J.B. double checked the scores for completeness and accuracy. Any disagreements were discussed and resolved by consensus. We had initially planned to use the National Institute of Health (NIH) set of quality assessment tools but due to the diverse nature of study designs, the authors agreed to use the QATSDD tool. The QATSDD is a 16-item tool for both qualitative and quantitative studies. Each item is scored on a 4-point scale (0–3), with a total of 14 criteria for each study design and 16 for studies with mixed methods. Scoring relies on guidance notes provided as well as judgment and expertise from the reviewers. The criteria used are: (i) theoretical framework; (ii) statement of aims or objectives; (iii) description of research setting; (iv) sample size consideration; (v) representative sample of target group (vi) data collection procedure description; (vii) rationale for choice of data collection tool(s); (viii) detailed recruitment data; (ix) statistical assessment of reliability and validity of measurement tools (quantitative only); (x) fit between research question and method of data collection (quantitative only); (xi) fit between research question and format and content data collection (qualitative only); (xii) fit between research question and method of analysis; (xiii) justification of analytical method; (xiv) assessment of reliability of analytical process (qualitative only); (xv) user involvement in design and (xvi) discussion on strengths and limitations[ 21 ]. Scores are awarded for each criterion as follows: 0 = no mention at all; 1 = very brief description; 2 = moderate description; and 3 = complete description. The scores of each criterion are then summed up with a maximum score of 48 for mixed methods studies and 42 for studies using either qualitative only or quantitative only designs. For ease of interpretation, the scores were converted to percentages and classified as low (<50%), medium (50%–80%) or high (>80%) quality of evidence [ 22 ].
Search results
The search from the five electronic databases yielded 1535 results: 950 from PubMed, 173 from PsychINFO, 210 from web of science, 123 from CINAHL and 79 from Cochrane library. Thirteen additional studies were identified through a manual search of the AJADA journals (Volumes 1, 2 and 3). Studies were assessed for duplicates and 1154 articles remained after removal of duplicates. The 1154 studies underwent an initial screening based on abstracts and titles, and 946 articles were excluded. A second screen of full text articles was done for the 208 studies that were potentially eligible for the review. Twenty three studies were excluded as follows: 21 did not meet the eligibility criteria and 2 had duplicated results. A total of 185 studies were found to meet the inclusion criteria and were included in the review ( Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0269340.g001
General characteristics of the studies
Of the 185 studies included in this review, 144 (77.8%) investigated the epidemiology of substance use or SUD, 18 (9.7%) evaluated substance use or SUD interventions and programs, and 23 (12.4%) were qualitative studies exploring perceptions on various substance use or SUD topics other than interventions and programs (Table 4). The studies were published between 1982 and 2020. The number of studies published has gradually increased in number over the years, particularly in the past decade. Fig 2 shows the publication trends for substance use research in Kenya.
https://doi.org/10.1371/journal.pone.0269340.g002
Quality assessment
The QATSDD scores ranged from 28.6% [ 23 ] to 92.9% [ 24 ]. Only 14 studies [ 12 , 23 , 25 – 36 ] (all quantitative) had scores of less than 50%. Of these, the main items driving low quality were: no mention of user involvement in study design (n = 14) [ 12 , 23 , 25 – 36 ], no explicit mention of a theoretical framework (n = 10) [ 12 , 23 , 25 – 28 , 30 , 33 , 35 , 36 ] and a lack of a statistical assessment of reliability and validity of measurement tools (n = 10) [ 12 , 23 , 25 , 28 , 30 – 33 , 35 , 36 ] Table 1 .
https://doi.org/10.1371/journal.pone.0269340.t001
Studies examining the epidemiology of substance use or SUD
General description of epidemiological studies..
One hundred and forty-four studies examined the prevalence and or risk factors for various substances. The studies were published between 1982 and 2020. The four main study designs used were cross-sectional (n = 126), cohort (n = 5), case-control (n = 10), and mixed methods (n = 2). One study used a combination of the multiplier method, Wisdom of the Crowds (WOTC) method, and a published literature review to document the size of key populations [ 164 ]. The sample size for this category of studies ranged from 42 [ 130 ] to 72292 [ 128 ].
The studies were conducted in diverse settings including the community (n = 72), hospitals (n = 40), institutions of learning (n = 24), streets (n = 5), prisons and courts (n = 3), charitable institutions (n = 1), methadone maintenance therapy (MMT) clinics (n = 1), and in needle-syringe program (NSP) sites (n = 1). Of the studies conducted within the community, 12 were conducted in informal settlements. The study populations were similarly diverse as follows: general population adults & adolescents (n = 39), persons with NCDs (n = 11), primary and secondary school students (n = 15), people who inject drugs (PWID) (n = 11), general patients (n = 5), men who have sex with men (MSM) (n = 8), university and college students (n = 9), commercial sex workers (n = 7), psychiatric patients (n = 6), orphans and street connected children and youth (n = 6), people living with HIV (PLHIV) (n = 6), healthcare workers (n = 3), law offenders (n = 3), military (n = 1), and teachers (n = 1). Only one study was conducted among pregnant women [ 131 ].
Sixty-nine studies (47.6%) used a standardized diagnostic tool to assess for substance use. The Alcohol Use Disorder Identification Test (AUDIT) (n = 21) and the Alcohol, Smoking & Substance Use Involvement Screening Test (ASSIST) questionnaire (n = 10) were the most frequently used tools. Most papers assessed for alcohol ( n = 109) and tobacco use ( n = 80). Other substances assessed included khat (n = 34), opioids (n = 21), sedatives (n = 19), cocaine (n = 19), inhalants (n = 16), cannabis (n = 14), hallucinogens (n = 7), prescription medication (n = 4), emerging drugs (n = 1) and ecstasy (n = 1). Most studies (n = 93) assessed for more than one substance.
Epidemiology of alcohol use.
One hundred and nine papers assessed for the prevalence and or risk factors for alcohol use. Using the AUDIT, the 12-month prevalence rate for hazardous alcohol use ranged from 2.9% among adults drawn from the community [ 97 ] to 64.6% among female sex workers (FSW) [ 77 ]. Based on the same tool, the lowest and highest 12-month prevalence rates for harmful alcohol use were both reported among FSWs i.e. 9.3% [ 80 ] and 64.0% [ 174 ] respectively, while the prevalence of alcohol dependence ranged from 8% among FSWs living with HIV [ 203 ] to 33% among MSM who were commercial sex workers [ 144 ]. The highest lifetime prevalence rate for alcohol use was reported by Ndegwa & Waiyaki [ 151 ]. The authors found that 95.7% of undergraduate students had ever used alcohol.
Alcohol use, was associated with several socio-demographic factors including being male [ 50 , 112 , 114 , 140 , 158 , 168 , 182 , 191 ], being unemployed [ 114 ], being self-employed [ 97 ], having a lower socio-economic status (SES) [ 128 ], being single or separated, living in larger households [ 97 ], having a family member struggling with alcohol use, and alcohol being brewed in the home [ 143 ]. Alcohol use was linked to various health factors including glucose intolerance [ 81 ], poor cardiovascular risk factor control [ 111 ], having a diagnosis of diabetes mellitus [ 134 ], hypertension [ 112 , 139 ], default from tuberculosis (TB) treatment [ 148 ], depression [ 113 ], psychological Intimate Partner Violence (IPV) [ 205 ], tobacco use [ 182 , 205 ], and increased risk of esophageal cancer [ 137 , 179 ]. Finally, alcohol use was associated with involvement in Road Traffic Accidents (RTAs) [ 88 ], and having injuries [ 88 , 171 ] and suicidal behavior [ 109 ].
Epidemiology of tobacco use.
Eighty papers assessed for the prevalence and risk factors for tobacco use. The lifetime prevalence of tobacco use ranged from 23.5% among healthcare workers (HCWs) [ 140 ] to 84.3% among psychiatric patients [ 110 ]. The highest lifetime prevalence rate for tobacco use was reported by Ndegwa & Waiyaki [ 151 ]. The authors found that 95.7% of undergraduate students had ever used tobacco.
Tobacco use was associated with socio-demographic factors such as being male [ 112 , 140 , 168 ] and living in urban areas [ 163 ]. Several health factors were linked to tobacco use including hypertension [ 112 ], development of oral leukoplakia [ 32 ], pneumonia [ 146 ], increased odds of laryngeal cancer [ 136 ], ischemic stroke [ 100 ] and diabetes mellitus [ 134 ]. In addition, tobacco use was associated with having had an injury in the last 12 months [ 171 ], emotional abuse [ 110 ], and psychological IPV [ 205 ]. Longer duration of smoking was associated with a diagnosis of diabetes mellitus [ 73 ], lower SES [ 128 ], and hypertension [ 98 , 142 ]. Peltzer et al. [ 181 ] reported that early smoking initiation among boys was associated with ever drunk from alcohol use, ever used substances, and ever had sex. Among girls, the authors found that early smoking initiation was associated with higher education, ever drunk from alcohol use, parental or guardian tobacco use, and suicide ideation.
Epidemiology of khat use.
The epidemiology of khat use was investigated by 34 studies. The lifetime prevalence rate for khat use ranged from 10.7% among general hospital patients [ 168 ] to 88% among a community sample [ 23 ]. Khat use was associated with being male [ 114 , 168 ]; unemployment [ 114 ]; being employed [ 25 ]; younger age (less than 35 years), higher level of income, comorbid alcohol and tobacco use [ 166 ] and age at first paid sex of less than 20 years among FSWs [ 195 ]. Further, khat use was associated with increased odds of negative health outcomes [ 130 , 146 , 166 , 201 ].
Higher odds of reporting psychotic [ 166 , 201 ], and PTSD (Post-Traumatic Stress Disorder) symptoms [ 201 ], having thicker oral epithelium [ 130 ], and pneumonia [ 146 ], were reported among khat users compared to non-users.
Epidemiology of cannabis use.
Fourteen studies evaluated the prevalence of cannabis use. The lifetime prevalence rate of cannabis use ranged from 21.3% among persons with AUD [ 120 ] to 64.2% among psychiatric patients [ 110 ]. Cannabis use was associated with being male [ 140 , 168 ], and with childhood exposure to physical abuse [ 110 ].
Epidemiology of opioid and cocaine use.
Twenty-one studies investigated the prevalence of opioid use. The lifetime prevalence rate of opioid use ranged from 1.1% among PLHIV [ 132 ] to 8.2% among psychiatric patients [ 110 ].
Nineteen studies assessed for the prevalence of cocaine use. The highest reported prevalence rates were 76.2% among PWID use (current use) [ 190 ]; 8.8% among healthcare workers (lifetime use) [ 140 ]; and 6.7% among PLHIV (lifetime use) [ 132 ].
Epidemiology of IDU.
One study assessed the prevalence for IDU. Key population size estimates for PWID use was reported as 6107 for Nairobi [ 164 ]. IDU was associated with depression, risky sexual behavior [ 149 ], Hepatitis-C Virus (HCV) infection [ 173 ], and HIV-HCV co-infection [ 68 ].
Epidemiology of other substance use (sedatives, inhalants, hallucinogens and prescription medication, emerging drugs, ecstasy).
The epidemiology of sedative use was investigated by 19 studies, inhalant use by 16 studies, hallucinogen use by 7 studies, prescription medication by 4 studies, and emerging drugs and ecstasy by one study each. The highest lifetime prevalence rate for sedative use was reported as 71.4% among a sample of psychiatric patients [ 28 ], while the highest prevalence rate for inhalant use was 67% among children living in the streets [ 86 ]. The lifetime prevalence rates for hallucinogen use ranged from 1.4% among university students [ 160 ] to 3.7% among psychiatric patients [ 110 ]. The highest prevalence rate for the use of prescription medication was reported as 21.2% among PWID [ 190 ]. One study each reported on the prevalence of emerging drugs [ 122 ] and ecstasy [ 153 ]. The studies were both conducted among adolescents and youth. The authors found the lifetime prevalence rates for the two substances to be 11.8% [ 122 ] and 4.0% [ 153 ] respectively.
Other topics explored by the epidemiology studies.
In addition to prevalence and associated factors, the epidemiological studies explored other topics.
Papas et al. [ 176 ] explored the agreement between self-reported alcohol use and the biomarker phosphatidyl ethanol and reported a lack of agreement between self-reported alcohol use and the biomarker phosphatidyl ethanol among PLHIV with AUD.
One study investigated the self-efficacy of primary HCWs for SUD management and reported that self-efficacy for SUD management was lower in those practicing in public facilities and among those perceiving a need for AUD training. Higher self-efficacy was associated with attending to a higher proportion of patients with AUD, and the belief that AUD is manageable in outpatient settings [ 196 ].
Five studies investigated the reasons for substance use. Common reasons for substance use included leisure, stress and peer pressure among psychiatric patients[ 28 ], curiosity, fun, and peer influence among college students [ 123 ], peer influence, idleness, easy access, and curiosity among adults in the community [ 25 ], and peer pressure, to get drunk, to feel better and to feel warm among street children [ 74 ]. Atwoli et al. 2011 [ 72 ] reported that most students were introduced to substances by friends.
Kaai et al. [ 99 ] conducted a study regarding quit intentions for tobacco use and reported that 28% had tried to quit in the past 12 months, 60.9% had never tried to quit, and only 13.8% had ever heard of smoking cessation medication. Intention to quit smoking was associated with being younger, having tried to quit previously, perceiving that quitting smoking was beneficial to health, worrying about future health consequences of smoking, and being low in nicotine dependence. A complete description of the prevalence studies has been provided in Table 2 .
https://doi.org/10.1371/journal.pone.0269340.t002
Studies evaluating substance use or SUD programs and interventions
General description of studies evaluating programs and interventions..
A total of eighteen studies evaluated specific interventions or programs for the treatment and prevention of substance use. These were carried out between 2009 and 2020. Eleven studies focused on individual-level interventions, 5 studies evaluated programs, and 2 studies evaluated population-level interventions. The studies used various approaches including randomized control trials (RCT) (n = 7), mixed methods (n = 3), non-concurrent multiple baseline design (n = 1), quasi experimental (n = 1), cross-sectional (n = 2), and qualitative (n = 3). One study employed a combination of qualitative methods and mathematical modeling.
Individual-level interventions.
Individual-level interventions for harmful alcohol use . Nine studies evaluated either feasibility, acceptability, and or efficacy for individual-level interventions for harmful alcohol use [ 38 , 40 , 90 , 94 , 127 , 141 , 175 , 178 , 193 ]. All the interventions were tested among adult populations including persons attending a Voluntary Counseling & Testing (VCT) center (38), PLHIV [ 40 , 175 ], and adult males and females drawn from the community [ 94 , 141 ] and FSWs [ 127 , 178 ].
Two studies evaluated a six session CBT intervention for harmful alcohol use among PLHIV. The intervention was reported as feasible, acceptable [ 40 ] and efficacious [ 175 ] in reducing alcohol consumption among PLHIV. The intervention was delivered by trained lay providers.
Giusto et al [ 90 ] evaluated the preliminary efficacy of an intervention aimed at reducing men’s alcohol use and improving family outcomes. The intervention was delivered in 5 sessions by trained lay-providers, and utilized a combination of behavioral activation, motivational interviewing (MI) and gender norm transformative strategies. The intervention showed preliminary efficacy for addressing alcohol use and family related problems.
Five studies evaluated brief interventions that ranged from 1 to 6 sessions and were delivered by primary HCWs, lay providers and specialist mental health professionals [ 38 , 94 , 127 , 178 , 193 ]. The brief interventions were reported as feasible, acceptable [ 38 ], and efficacious in reducing alcohol consumption [ 94 , 127 , 178 , 193 ]. The brief interventions additionally resulted in reductions to IPV, participation in sex work [ 178 ], and risky sexual behavior [ 127 ].
One study evaluated the efficacy of a mobile delivered MI intervention and found that at 1 month, AUDIT-C scores were significantly higher for waiting-list controls compared to those who received the mobile MI [ 94 ].
Moscoe at al. [ 141 ] found no effect of a prize-linked savings account on alcohol, gambling and transactional sex expenditures among men.
Individual-level interventions for khat use . One study utilized a randomized control trial (RCT) approach to evaluate the effect of a three-session brief intervention for khat use on comorbid psychopathology (depression, PTSD, khat induced psychotic symptoms) and everyday functioning. The intervention was delivered by trained college graduates and was found to result in reduced khat use and increased functioning levels, but had no benefit for comorbidity symptoms (compared to assessments only) [ 202 ].
Individual level intervention for any substance use . One study evaluated the efficacy of a four-session psychoeducation intervention using an RCT approach. The study found that the intervention was effective in reducing the severity of symptoms of any substance abuse at 6 months compared to no intervention. The intervention was additionally effective in reducing symptoms for depression, hopelessness, suicidality, and anxiety [ 145 ].
Methadone programs . Two studies utilized qualitative methods to evaluate the perceptions of persons receiving methadone on the benefits of the programs [ 61 , 62 ]. The methadone programs were perceived as having potential to aid in recovery from opioid use and to reduce HIV transmission among PWID [ 61 , 62 ].
Needle-syringe programs (NSPs) . One paper explored the impact of NSPs programs on needle and syringe sharing among PWID. The study reported that the introduction of NSPs led to significant reductions in needle and syringe sharing [ 56 ].
Tobacco cessation programs . One study evaluated HCWs knowledge and practices on tobacco cessation and found that the knowledge and practice on tobacco cessation was inadequate [ 89 ].
Out-patient SUD treatment programs . One paper investigated the impact of community based outpatient SUD treatment services and reported a 42% substance use abstinence rate 0–36 months following treatment termination [ 84 ].
Population-level interventions.
Population-level tobacco interventions . One study evaluated the appropriateness and effectiveness of HIC anti-tobacco adverts in the African context and found the adverts to be effective and appropriate [ 183 ].
Population-level alcohol interventions . One paper examined community members’ perspectives on the impact of the government’s public education messages on alcohol abuse and reported that the messages were ineffective and unpersuasive [ 55 ].
A complete description of studies investigating programs and interventions is in Table 3 .
https://doi.org/10.1371/journal.pone.0269340.t003
Studies qualitatively exploring various substance use or SUD topics (other than interventions)
General description of qualitative studies..
There were 23 qualitative studies included in our review. The studies were conducted between 2004 and 2020. Data was collected using several approaches including in-depth interviews (IDIs) only (n = 6), focus group discussions (FGDs) only (n = 2), a combination of FGDs and IDIs (n = 10), a combination of observation and individual IDIs (n = 2), a combination of observation, IDIs and FGDs (n = 1), a combination of literature review, observation, IDIs and FGDs (n = 1). One study utilized the participatory research and action approach [ 60 ]. The target populations for the qualitative studies included persons using heroin (n = 3), males and females with IDU (n = 11) adolescents and youth (n = 3), FSWs (n = 2), refugees and Internally Displaced Persons (IDPs) (n = 1), and PLHIV (n = 2).
Injecting drug use and heroin use.
Thirteen studies explored various themes related to IDU and heroin use with most of them (n = 8) focusing on issues related to women. Three studies explored the drivers of IDU among women and found them to include influence of intimate partners [ 48 , 49 ], stress of unexpected pregnancies [ 49 ], gender inequality, and social suffering [ 67 ]. One study found that IDU among women interfered with utilization of antenatal and maternal and child health services [ 57 ], while another reported that women who inject drugs linked IDU to amenorrhea hence did not perceive the need for contraception [ 51 ].
Mburu et al [ 47 ] explored the social contexts of women who inject drugs and found that these women experienced internal and external stigma of being injecting drug users, and external gender-related stigma of being female injecting drug users. Using a socio-ecological approach, Mburu et al [ 50 ] reported that IDU during sex work was an important HIV risk behavior. In another study, FSWs reported that they used heroin to boost courage to engage in sex work [ 65 ].
Other than IDU and heroin use among women, five studies investigated other themes. One study explored the experiences of injecting heroin users and found that the participants perceived heroin injection as cool [ 42 ]. Guise et al. 2015 [ 44 ] conducted a study to explore transitions from smoking to injecting and reported that transitions from smoking to IDU were experienced as a process of managing resource constraints, or of curiosity, or search for pleasure. One study explored the experiences of persons on MMT as regards integration of MMT with HIV treatment. The study was guided by the material perspective in sociology theory and Annmarie’s Mol’s analysis of logic of care. Persons on MMT preferred that they have choice over whether to seek care for HIV and MMT in a single, or in separate settings.
Alcohol use.
Six studies focused on alcohol use. Three studies explored perceptions of service providers and communities on the effects of alcohol use. Alcohol use was perceived as having a negative impact on sexual and reproductive health [ 53 , 54 ] and on socio-economic status [ 43 , 46 ]. One study explored the reasons for alcohol use among PLHIV and found that reasons for alcohol use included stigma and psychological problems, perceived medicinal value, and poverty [ 60 ].
Youth and adolescent substance use.
Three studies focused on substance use among youth and adolescents. In one study, the adolescents perceived that substance use contributed to risky sexual behavior including unprotected sex, transactional sex, and multiple partner sex [ 58 ]. The youth identified porn video shows and local brew dens as places where risky sexual encounters between adolescents occurred [ 59 ]. Ssewanyana et al. [ 63 ] utilized the socio-ecological model to explore perceptions of adolescents and stakeholders on the factors predisposing and contributing to substance use. Substance use among adolescents was perceived to be common and to be due to several socio-cultural factors e.g. access to disposable income, idleness, academic pressure, low self-esteem etc.
Other topics.
Utilizing the syndemic theory, one study explored how substance use, violence and HIV risk affect PrEP (Pre-exposure prophylaxis) acceptability, access and intervention needs among male and female sex workers. The study found that co-occurring substance use, and violence experienced by sex workers posed important barriers to PrEP access [ 41 ].
A complete description of included qualitative studies is in Table 4 .
https://doi.org/10.1371/journal.pone.0269340.t004
This is to our knowledge, the first study to summarize empirical work done on substance use and SUDs in Kenya. More than half (77.8%) of the reviewed studies investigated the area of prevalence and risk factors for substance use. Less common were qualitative studies exploring various themes (12.4%) and studies evaluating interventions and programs (9.7%). The first study was conducted in 1982 and since then the number of publications has gradually risen. Most of the research papers (92.4%) were of moderate to high quality. In comparison to two recent scoping reviews conducted in South Africa and Botswana, more research work has been done on substance use in Kenya. Our study found that 185 papers on substance use among Kenyans had been published by the time of the search while Opondo et al. [ 11 ] and Tran et al. [ 10 ] reported that only 53 and 7 papers focusing on substance use had been published in South Africa (between 1971 and 2017) and in Botswana (between 1983 and 2020) respectively.
Epidemiology of substance use or SUD
Studies investigating the prevalence, and risk factors for substance use dominated the literature. The studies, which were conducted across a broad range of settings and populations, focused on various substances including alcohol, tobacco, cannabis, opioids, cocaine, sedatives, inhalants, hallucinogens, prescription medication, and ecstasy. In addition, a wide range of important health and socio-demographic factors were examined for their association with substance use. Most studies had robust sample sizes and were conducted using diverse designs including cross-sectional, case-control and cohort. The studies showed a significant burden of substance use among both adults and children and adolescents. In addition, substance use increased the odds of negative mental and physical health outcomes consistent with findings documented in global reports [ 2 , 3 ]. These findings highlight the importance of making the treatment and prevention for substance use and SUDs of high priority in Kenya.
- Two main evidence gaps were identified within this category: The prevalence and risk factors for substance use among certain vulnerable populations for whom substance use can have severe negative consequences, had not been investigated. For example, no study had included police officers or persons with physical disability, only one study had its participants as pregnant women [ 113 ], and only 2 studies had been conducted among HCWs [ 140 , 196 ].
- Few studies had explored the epidemiology of hallucinogens, prescription medication, ecstasy, IDU, and emerging substances e.g. synthetic cannabinoids. These substances are a public health threat globally [ 207 , 208 ] yet their use remains poorly documented in Kenya.
Interventions and programs
Given the significant documented burden of substance use and SUDs in Kenya, it was surprising that few studies had focused on developing and testing treatment and prevention interventions for SUDs. A possible reason for this is limited expertise in the area of intervention development and testing. For example, research capacity in implementation science has been shown to be limited in resource-poor settings such as ours [ 209 ].
Of note is that most of the tested interventions had been delivered by lay providers [ 40 , 90 , 175 ] and primary HCWs [ 38 , 127 , 178 ] indicating a recognition of task-shifting as a strategy for filling the mental health human resource gap in Kenya.
Several research gaps were identified within this category.
- Out of the 11 individual-level interventions tested, nine had targeted harmful alcohol use except one which focused on khat [ 202 ] and another that targeted several substances [ 145 ]. No studies had evaluated individual-level interventions targeting tobacco and cannabis use, despite the two being the second and third most commonly used substances in Kenya [ 8 ]. Further, no individual-level interventions had focused on other important SUDs like opioid, sedative and cocaine use disorders.
- Few studies had evaluated the impact of substance use population-level interventions [ 55 , 183 ]. Several cost-effective population-level interventions have been recommended by WHO e.g. mass media education and national toll free quit line services for tobacco use, and brief interventions integrated into all levels of primary care for harmful alcohol use [ 210 ]. Such strategies need to be tested for scaling up in Kenya.
- None of the interventions had been tested among important vulnerable populations for whom local research already shows a significant burden e.g. children and adolescents, the Lesbian Gay Bisexual Transgender & Queer (LGBTQ) community, HCWs, prisoners, refugees, and IDPs. In addition, no interventions had been tested for police officers and pregnant women, and no studies had evaluated interventions to curb workplace substance use.
- Only one study evaluated digital strategies for delivering substance use interventions [ 94 ] yet the feasibility of such strategies has been demonstrated for other mental health disorders in Kenya [ 211 ]. Moreover, the time is ripe for adopting such an approach to substance use treatment given the fact that the country currently has a mobile subscriptions penetration of greater than 90% [ 212 ].
- No studies had evaluated the impact of other interventions such as mindfulness and physical exercise. Meta-analytic evidence suggests that such strategies hold promise for reducing the frequency and severity of substance use and craving [ 213 , 214 ].
Qualitative studies
The qualitative studies focused on a broad range of themes including drivers and impact of substance use, drug markets, patterns of substance use, stigma, and access to treatment. Most of the work however focused on PWID and heroin users. Future qualitative work should explore issues relating to other populations for example persons with other mental disorders, persons with physical disabilities, police officers, and persons using other commonly used substances such as tobacco, khat, and cannabis.
Limitations
The aim of this systematic review was to provide an overview of the existing literature on substance use and SUD research in Kenya. We therefore did not undertake a meta-analysis and detailed synthesis of the findings of studies included in this review. In addition, variability in measurements of substance use outcomes precluded our ability to more comprehensively summarize the study findings. For quality assessment, detailed assessments using design specific tools were not possible given the diverse methodological approaches utilized in the studies. We therefore used a single tool for the quality assessment of all studies. The results of the quality assessment are therefore to be interpreted with caution. Nonetheless this review describes for the first time the breadth of existing literature on substance use and SUDs in Kenya, identifies research gaps, and provides important directions for future research.
The purpose of this systematic review was to map the research that has been undertaken on substance use and SUDs in Kenya. Epidemiological studies dominated the literature and indicated a significant burden of substance use among both adults and adolescents. Our findings indicate that there is a dearth of literature regarding interventions for substance use and we are calling for further research in this area. Specifically, interventions ought to be tested not just for alcohol but for other substances as well, and among important at risk populations. In addition, future research ought to explore the feasibility of delivering substance use interventions using digital means, and the benefit of other interventions such as mindfulness and physical exercise. Future qualitative work should aim at providing in-depth perspectives on substance use among populations excluded from existing literature e.g. police officers, persons using other substances such as tobacco, cannabis and khat, and persons with physical disability.
Supporting information
S1 checklist. prisma checklist..
https://doi.org/10.1371/journal.pone.0269340.s001
S1 File. Search terms for PsychINFO.
https://doi.org/10.1371/journal.pone.0269340.s002
- View Article
- PubMed/NCBI
- Google Scholar
- 2. Hammer JH, Parent MC, Spiker DA, World Health Organization. Global status report on alcohol and health 2018. https://apps.who.int/iris/bitstream/handle/10665/274603/9789241565639-eng.pdf?ua=1 . Accessed 12 June 2021
- 3. World Health Organisation. Tobacco [Internet]. 2020. https://www.who.int/news-room/fact-sheets/detail/tobacco . Accessed 12 June 2021
- 4. World Health Organization. Alcohol and drug use disorders: Global health estimates. 2017. http://www.who.int/substance_abuse/activities/fadab/msb_adab_2017_GHE_23June2017.pdf . Accessed 12 June 2021
- 7. International Narcotics Control Board. Chapter 1: Economic consequences of drug abuse. 2013. https://www.incb.org/documents/Publications/AnnualReports/AR2013/English/AR_2013_E_Chapter_I.pdf . Accessed 12 June 2021
- 8. National Authority for the Campaign Against alcohol and Drug Abuse. Rapid Situation Assessment of Drugs abd Substance Abuse in Kenya. 2017. https://nacada.go.ke/sites/default/files/2019-10/National%20ADA%20Survey%20Report%202017_2_2.pdf . Accessed 12 June 2021
- 9. National Authority for the Campaign Against alcohol and Drug Abuse, Kenya Institute for Public Policy Research and Analysis. Status of Drugs and Substance Abuse among Primary School Pupils in Kenya. 2019. https://nacada.go.ke/sites/default/files/2019-10/Report%20on%20the%20Status%20of%20Drugs%20and%20Substance%20Abuse%20among%20Primary%20School%20Pupils%20in%20Kenya.pdf . Accessed 12 June 2021
- 13. The National Authority for the Campaign aganist Alcohol and Drug Abuse [Internet]. http://www.nacada.go.ke . Accessed 12 June 2021
- 14. The National Authority for the Campaign Aganist Alcohol and Drug Abuse. African Journal of Alcohol & Drug Abuse (Volume 2). https://nacada.go.ke/sites/default/files/AJADA/AJADA%202%20ammended/AJADA%20Volume%20II%20(Full%20Booklet).pdf . Accessed 12 june 2021
- 15. Republic of Kenya. The National Treasury and Planning. Third Medium Term Plan 2018–2022. Kenya Vision 2030. https://planning.go.ke/wp-content/uploads/2018/12/THIRD-MEDIUM-TERM-PLAN-2018-2022.pdf . Accessed 20 June 2020
- 16. United Nations Development Programme. The 2030 Agenda for Sustainable Development, A/RES/70/1. Undp. 2015.
- 18. The National Authority for the Campaign Aganist Alcohol and Drug Abuse. African Journal of Alcohol & Drug Abuse (Volume 3). https://nacada.go.ke/sites/default/files/AJADA/AJADA%203/NACADA%20AJADA%20Vol%203-%20Full%20Booket.pdf . Accessed 12 june 2021
- 19. The National Authority for the Campaign Aganist Alcohol and Drug Abuse. African Journal of Alcohol & Drug Abuse (Volume 1). https://nacada.go.ke/sites/default/files/AJADA/AJADA%201%20ammended/AJADA%20Volume%20I%20(Full%20Booklet).pdf . Accessed 12 june 2021
- 207. United Nations Office on Drugs and Crime. New psychoactive substances.2020. https://www.unodc.org/documents/scientific/NPS-Leaflet_WEB_2020.pdf . Accessed 12 June 2021
- 208. UNODC World Drug Report 2020: Global drug use rising; while COVID-19 has far reaching impact on global drug markets. 2020. https://www.unodc.org/unodc/press/releases/2020/June/media-advisory—global-launch-of-the-2020-world-drug-report.html
- 210. World Health Organisation 2017. ‘ Best Buys ‘ and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases; “the updated Appendix 3 of the WHO Global NCD Action Plan 2013–2020. https://www.who.int/ncds/management/WHO_Appendix_BestBuys.pdf . Accessed 12 June 2021
- 212. Business Today. Kenya leads Africa in smartphone usage [Internet]. 2020. https://businesstoday.co.ke/kenya-leads-africa-smartphone-usage/ . Accessed 12 June 2021.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Center for Substance Abuse Treatment. Incorporating Alcohol Pharmacotherapies Into Medical Practice: A Review of the Literature. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2009. (Treatment Improvement Protocol (TIP) Series, No. 49s.)

Incorporating Alcohol Pharmacotherapies Into Medical Practice: A Review of the Literature.
1 a review of the literature.
This literature review is part of the Substance Abuse and Mental Health Services Administration’s (SAMHSA’s) Treatment Improvement Protocol (TIP) 49, Incorporating Alcohol Pharmacotherapies Into Medical Practice . Developed by a panel of experts for SAMHSA’s Center for Substance Abuse Treatment (CSAT), the TIP can assist physicians and other medical professionals in providing pharmacologic treatment, combined with psychosocial therapy, for patients who are alcohol dependent, both in primary care settings and in specialized substance abuse treatment settings.
TIP 49 focuses on the best currently recognized clinical practices for the medical maintenance of patients with alcohol use disorders (AUDs), using the four medications (disulfiram, oral naltrexone, injectable naltrexone, and acamprosate) approved by the U.S. Food and Drug Administration (FDA) for this purpose. The TIP presents best practices according to the scientific literature and the clinical experts who developed the TIP. This literature review emphasizes recent research published from 2000 to 2007 but also includes classic research studies published before 2000.
- Introduction
Recently, much new scientific knowledge has emerged concerning how pharmacotherapy can treat individuals who are alcohol dependent. Physicians can prescribe four FDA-approved medications to dampen craving, reduce heavy drinking, or promote abstinence. These medications have a mild to moderate effect and do not work for all individuals, but this first wave of effective, evidence-based medication treatments for alcoholism is at the forefront of a Government push to develop even more powerful medications. Research on pharmacotherapies for alcohol dependence is a top priority of the National Institutes of Health, which funds 68 grants totaling more than $26 million annually on medications that target the multiple neurotransmitter systems implicated in alcohol addiction ( Johnson et al., 2005 ). Several promising drugs are in development, supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
For years, medications have been used primarily as an adjunct to psychosocial treatment in specialized treatment settings. With newer medications now available (naltrexone and acamprosate), interest is increasing in whether primary care physicians in routine medical practice can successfully treat with FDA-approved medications individuals who are alcohol dependent. Recent research indicates that such treatment by mainstream medical practitioners appears promising. Project COMBINE (Combining Medications and Behavioral Interventions), a recent comprehensive, well-designed NIAAA clinical trial, was carried out at 11 academic sites in the United States with nearly 1,400 patients with alcohol dependence; this project explored a variety of treatment methods—alone and in combination—in the context of low-intensity medical management ( Anton et al., 2006 ). Alcohol consumption decreased by 80 percent over a 4-month treatment period, which suggests that medical management by primary physicians in routine practice can be a benefit in treating AUDs ( Kranzler, 2006 ).
Emerging developments in the pharmacologic treatment of AUDs offer a significant opportunity for physicians to integrate the management of substance use disorders into primary health care. Practitioners in medical settings can add pharmacotherapies to their interventions with patients who drink heavily or are dependent on alcohol. This literature review provides resources for practitioners. Using medications to treat AUDs concerns practitioners for the following reasons:
- Up to one-half of patients with AUDs relapse shortly after detoxification and psychosocial or behavioral treatment ( Johnson & Ait-Daoud, 2000 ). Research shows that existing approved pharmacotherapies reduce craving and help from 20 to 50 percent of patients reduce their heavy drinking and promote abstinence. When combined with primary care and psychosocial therapy, medications can effectively help many patients reduce their substance use or attain abstinence.
- Only 3 to 13 percent of patients in treatment receive naltrexone ( Mark et al., 2003a ). Pharmacologic treatment is grossly underused because few physicians know about these medications—or about the research showing their efficacy. The information in this literature review can assist mainstream physicians in learning about the efficacy of medications and may promote the medications’ effective use in patients who could benefit from pharmacotherapy.
Practitioners should note that patients need some level of psychosocial intervention in addition to pharmacotherapy—a consistent finding across both European and U.S. research.
- Recent Pharmacological Advances
Advances in the neurobiology of addiction and improved methodology for clinical trials have recently increased the state of knowledge about pharmacotherapies for addiction. Every 3 years, the Mesa Grande project reviews clinical trials on a variety of approaches for treating AUDs. In the 2002 update, this project added 59 new controlled trials. Based on clinical trials, this review lists the most effective treatment approaches. In 2002, for the first time, two pharmacologic therapies appeared on this list. Therapies with acamprosate and naltrexone were rated third and fourth in effectiveness among 46 treatment modalities that had 3 or more studies; these pharmacotherapies were rated behind only brief intervention and motivational enhancement, which were rated first and second ( Miller & Wilbourne, 2002 ).
The effectiveness of the new medications reflects the findings from preclinical studies, which have exploded our knowledge about the behavioral and biological underpinnings of alcoholism ( Johnson & Ait-Daoud, 2000 ). There have been numerous advances during the past two decades in understanding the mechanisms underlying substance dependence disorders. It is now known that alcohol-seeking behavior and drinking are influenced by multiple neurotransmitter systems, as well as by neuromodulators, hormones, and various intercellular networks ( Litten, Fertig, Mattson, & Egli, 2005 ). The multiple neurotransmitter systems implicated in addiction include dopamine, serotonin, gamma‐aminobutyric acid (GABA), glutamate, and opiate systems. This means that researchers, in focusing on the biological systems underlying the disease, now have multiple potential targets for developing medications to treat substance dependence. Some promising directions of current research include the following:
- Applying pharmacogenetic techniques to the field of addiction. Some patients may possess a biological predisposition to the disease. These biologically vulnerable people may benefit from specific medications targeted toward ameliorating or correcting the underlying abnormalities.
- Learning about which subgroups of people may respond most positively to particular medications.
- Determining optimal dosage ranges and combinations of treatments—both combinations of medications and types and levels of psychosocial treatment—most likely to benefit particular groups based on their different biologies. Because medications are aimed at different neurotransmitter targets associated with addiction, combinations of pharmacological agents may have a synergistic effect.
Over the last decade, pharmaceutical companies have invested significant resources in studying and developing new formulations ( Kenna, McGeary, & Swift, 2004b ). In 1994, naltrexone became available for AUD treatment in the United States, and acamprosate became available in 2004. In addition, extended-release injectable naltrexone was approved by FDA on April 13, 2006, and became commercially available on June 13, 2006. Several review articles summarize the pharmacotherapy underlying these new medications and future therapies ( Johnson & Ait-Daoud, 2000 ; Kenna, McGeary, & Swift, 2004a , 2004b ; Kreek, LaForge, & Butelman, 2002 ; Mann, 2004 ; Myrick & Anton, 2004 ).
- Updated Findings From the Literature, October 2007
This online pharmacotherapy literature review, which covers articles published between 2000 and April 2007, is updated at 6-month intervals. This section describes findings from the latest update and primarily covers materials published from May through October 2007. This update identified 7 additional articles on alcohol pharmacotherapy published in 2006, as well as 37 new research articles published in 2007. Many of these articles make a significant contribution to existing knowledge. Three predominant themes are evident in this latest update of the literature:
- The search found an unusually large number of comprehensive reviews on the current state of knowledge regarding pharmacotherapy for AUDs. The purpose of many of these reviews is to educate primary care physicians about current pharmacotherapies and to stimulate their interest in treating patients with AUDs. A recent editorial in JAMA reflects this effort to convince psychiatrists and other physicians to add medications for alcohol dependence to their continuum of care ( Willenbring, 2007 ).
- Several articles reflect the effort to fill in gaps in existing research knowledge—especially to cast light on which subgroups of patients will benefit most from which combinations of therapies. Some of these articles present a secondary analysis of data from large randomized controlled studies, in which the new analysis shows medication effectiveness with specific subgroups of patients in the trials.
- The latest literature also demonstrates the ongoing surge in the number of clinical trials aimed at testing promising new pharmacotherapies for the treatment of alcohol dependence, as well as refining the use of current medications.
Much work is still needed to identify which subtypes of individuals with alcohol dependence will benefit most from a particular type of medication. Furthermore, the subtype response to a particular medication may also depend on the stage of illness at which such a person enters treatment ( Ait-Daoud, Malcolm, & Johnson, 2006 ).
Typologies of individuals with alcohol dependence. One approach used by researchers to improve medication efficacy is to identify alcohol-dependent subtypes who may respond preferentially to a particular medication. Although a standardized typology has not been established, two frequently used typologies are (1) early- versus late-onset alcohol dependence and (2) Type A versus Type B groupings. The Type B alcohol-dependent subgroup, as characterized by Babor and colleagues (1992) , includes an early age of onset of alcohol problems, high severity of dependence, polydrug use, a high degree of concomitant psychopathology, and a poor prognosis after alcohol treatment. In contrast, Type A individuals with alcohol dependence can be characterized by a late onset of problem drinking and such features as few childhood risk factors, low severity of alcohol dependence, little drug use, few alcohol-related problems, little concomitant psychopathology, and a relatively promising prognosis with traditional alcohol treatment. The Type A group is highly heterogeneous and is therefore susceptible to further subdivisions based on other features.
Genetic subtypes. Another rich current area of research aims to identify genetic variants that may modify the effects of various medications in specific subgroups of people with alcohol dependence. The gene coding for the μ opiate receptor (i.e., OPRM1) gene is a current target of interest, primarily to identify a genetic marker for subgroups that are most likely to respond to naltrexone treatment.
New Review Articles on Pharmacotherapy for Alcohol Dependence
The recent literature includes a number of review articles that may be helpful to practitioners. In “A Rational Approach to the Pharmacotherapy of Alcohol Dependence,” Petrakis (2006) reviews the neurobiology of alcohol dependence and relates this understanding to how pharmacologic interventions can effectively address three important clinical stages in the development and maintenance of alcohol dependence. These three stages are (1) the transition between initiation of alcohol use and the start of heavy drinking, (2) the cessation of heavy drinking in individuals who want to quit, and (3) the prevention of relapse in individuals who have initiated abstinence but struggle with craving or the desire to resume alcohol use. Petrakis (2006) concludes that the best strategy in the pharmacotherapy of alcohol dependence ultimately may be based on the targeted use of medications that act on the various neurotransmitters associated with different stages of alcohol dependence.
A second overview article discusses the preclinical and clinical pharmacology of alcohol dependence, covering the most recent developments in alcohol pharmacology ( Tambour & Quertemont, 2007 ). This article focuses on the neurobiological basis of medications for treating alcohol dependence, including promising drugs now in preliminary clinical studies.
In addition, four other reviews cover specific aspects of the pharmacotherapy of alcohol dependence. These articles include the following:
- A review of key studies on treating alcohol dependence published between 2005 and 2006, particularly randomized controlled trials ( Assanangkornchai & Srisurapanont, 2007 ). In terms of pharmacotherapies, the authors conclude that (1) recent studies show naltrexone has the most consistent effect in reducing alcohol consumption in the context of behavioral therapy and (2) topiramate is the only new medication on the horizon that has demonstrated effectiveness for treating alcohol dependence ( Assanangkornchai & Srisurapanont, 2007 ).
- A summary and review of the clinical experiences reported in the literature on the four current FDA-approved medications for treating alcohol dependence —disulfiram, naltrexone (oral and injectable forms), and acamprosate ( Rosenthal, 2006 ). This article describes the clinical use and evidence of clinical efficacy for each medication and discusses more recent trends in combination therapies. Rosenthal (2006) also briefly discusses promising future pharmacotherapies for treating alcohol dependence, including serotonergic medications, anticonvulsants, and antipsychotics.
- A comprehensive review of the research on medication and psychosocial treatments for those dually diagnosed with a substance-related disorder and one of the following: depression, anxiety disorder, schizophrenia, bipolar disorder, severe mental illness, or nonspecific mental illness ( Tiet & Mausbach, 2007 ). The authors identified 59 studies, including 36 randomized controlled trials, and found existing treatments that effectively reduce substance use also decrease substance use in patients who are dually diagnosed. Tiet and Mausbach (2007) also concluded that research is urgently needed on the topic of alcohol dependence and co-occurring mental disorders, because the current status of the literature is so poor.
- A comprehensive review of research into drug pharmacotherapies, particularly single-drug therapies, for treating common dual substance abuse and dependence disorders ( Kenna, Nielsen, Mello, Schiesl, & Swift, 2007 ) This article covers the neurobiology and existing research on numerous approved and off-label medications for treating alcohol dependence combined with cocaine, nicotine, and opioid use disorders. The review finds strongest support for the use of disulfiram to treat co-occurring alcohol and cocaine dependence and for topiramate to treat co-occurring alcohol, nicotine, and cocaine dependence ( Kenna et al., 2007 ).
New Findings on Disulfiram
Two new studies report finding that disulfiram compares favorably in effectiveness with other pharmacotherapies for patients with alcohol dependence. Both studies involve subjects who were voluntarily seeking treatment.
Medication combined with brief, manual-based intervention therapy
Researchers in Finland have found disulfiram to be superior to both naltrexone and acamprosate in the first randomized comparison of disulfiram, naltrexone, and acamprosate with brief, manual-based intervention therapy ( Laaksonen, Koski-Jännes, Salaspuro, Ahtinen, & Alho, 2007 ). This open-label, naturalistic trial, which took place at six different alcohol treatment and healthcare units, involved 243 Caucasian subjects who met the International Statistical Classification of Diseases and Related Health criteria for alcohol dependence; the subjects were voluntarily seeking treatment. Patients visited a physician at scheduled intervals during two phases that lasted 52 weeks; about 67 weeks after completing the study, patients were contacted for followup information—a total of 2.5 years after starting the study. This study experienced a low rate of dropout (25.1 percent after 12 weeks and 51.8 percent by the end of the 52-week study period).
During the Phase 1 continuous medication period (weeks 1–12), patients designated a contact person to be responsible for supervising and controlling their daily study medication. Patients were randomly assigned to receive (1) 50 mg of naltrexone once a day, (2) 666 mg of acamprosate 3 times a day or 1,333 mg/day for those weighing less than 60 kg, or (3) 100–200 mg of disulfiram once a day or 2 tablets (400 mg) twice a week. At regular visits throughout the 52 weeks, patients brought in a diary of their alcohol consumption and medication intake and also used a manual with homework based on cognitive-behavioral principles. During the Phase 2 targeted medication period (weeks 13–52), patients were asked to take a daily medication dose in any “craving situation” when they perceived their propensity to drink was high. During this targeted medication phase, the study center no longer provided free medication.
The main conclusion of this study was that all three medications—disulfiram, naltrexone, and acamprosate, combined with brief manual-based intervention extended over time—significantly reduce heavy drinking, reduce craving for alcohol, and increase the quality of life. However, disulfiram was superior to the other medications, especially during the continuous medication period. The study data show that, during Phase 1, disulfiram was significantly better than naltrexone and acamprosate as follows:
- In time to first heavy drinking day—46.6±27.5 days for disulfiram versus 22.0±22.0 for naltrexone and 17.6±22.0 for acamprosate:
- In time to first drink—30.4±27.8 days for disulfiram versus 16.2±20.2 for naltrexone and 11.4±17.0 for acamprosate
- In average weekly alcohol intake (g/ethanol per week)—52.0±90.7 for disulfiram versus 183.7±174.1 for naltrexone and 203.2±180.2 for acamprosate.
During Phase 2, the targeted medication period, there were no significant differences among the three medication groups on time to first heavy drinking day or in days to first drinking. Average alcohol consumption in all groups remained significantly below the baseline. However, those in the disulfiram group had significantly more abstinence days than those in the other two groups.
Medication for patients with co-occurring alcohol dependence and depression
Petrakis and colleagues (2007) conducted a secondary analysis of 139 male veterans with alcohol dependence and current major depression, assessing the effectiveness of naltrexone and disulfiram in this population. As in their original large-scale study comparing naltrexone and disulfiram among 254 male veterans who had alcohol dependence and comorbid mental disorders ( Petrakis et al., 2005 ), they found no advantage of one medication over the other. In comparison with outcomes found in the original study, a person’s having a diagnosis of co-occurring depression had no significant effect on retention in treatment or on drinking outcomes, including maximum consecutive days of abstinence, percentage of heavy drinking days, or abstinence throughout the entire study period. Petrakis and colleagues (2007) concluded that both disulfiram and naltrexone are safe pharmacotherapeutic agents for treating alcohol dependence in dually diagnosed individuals with depression.
As in the original study, an unexpected finding was that patients with depression who received disulfiram reported lower craving over time than subjects with depression who received naltrexone ( Petrakis et al., 2007 ). (For additional information on this study, see New Findings on Combined Medication Therapy below.)
New Findings on Oral Naltrexone
Effects of patient compliance on outcomes.
Research suggests that the effectiveness of naltrexone in clinical trials—and probably also in clinical treatment—can be greatly influenced by the subjects’ adherence to the medication. A new study reanalyzed data, expanding the variable drinking outcomes reviewed, from an alcohol treatment trial involving 160 participants ( Anton et al., 2005 ). This reanalysis looked specifically at how much patient compliance with naltrexone influenced the outcomes and compared two methods for measuring compliance ( Baros, Latham, Moak, Voronin, & Anton, 2007 ). The researchers conclude that, because patient adherence to naltrexone has such a large influence on treatment outcomes, practitioners need to give utmost attention to methods for enhancing their patients’ compliance. The article also summarizes evidence from the literature on strategies to use for improving compliance ( Baros et al., 2007 ).
This study evaluated outcomes for 137 randomized patients with alcohol dependence who completed 12 weeks of naltrexone or placebo, combined with either cognitive-behavioral therapy (CBT) or motivational enhancement therapy (MET). Compliance was monitored and compared using urine riboflavin measurements during study weeks 2, 6, and 12, as well as a medication event monitoring system (MEMS) that provided a detailed computerized record of when patients opened their medication bottles. Findings included the following:
- Accounting for adherence and compliance with naltrexone changed the outcomes (not significant in the original study) to demonstrate a significant drug therapy interaction for percentage of days abstinent, number of heavy drinking days, or total standard drinks.
- MEMS and urine riboflavin measures of compliance provided similar estimates of treatment effectiveness, although combining these two measures yields the most conservative, stringent index of medication compliance.
- The size of the treatment effect approximately doubled in the most compliant individuals.
- Patients treated with naltrexone and CBT showed more days of abstinence, less relapse to heavy drinking days, and fewer total drinks than the other groups (those receiving naltrexone plus MET or placebo plus psychotherapy).
- Older age predicted pill-taking compliance.
Effects of naltrexone on specific subgroups or populations
The treatment effects of naltrexone have recently been examined in several alcohol-dependent subgroups, including (1) those with a family history of alcohol problems and/or antisocial traits, (2) individuals with at least one copy of the G allele of the OPRM1 gene, and (3) women, including those with a comorbid eating disorder.
- Having a high percentage (at least 20 percent) of first- and second-degree family members with problem drinking significantly affected naltrexone’s effects, resulting in lower drinking rates among those patients
- Having more antisocial traits resulted in less heavy drinking on naltrexone than on placebo when the patient took at least 70 percent of the medication, whereas more socialized patients had no benefit from naltrexone regardless of compliance (degree of socialization was measured using the California Personality Inventory Socialization scale).
- Age of onset of alcohol problems and comorbid cocaine or marijuana use had no interaction effect with the medication.
- This study suggests that a meaningful (and inexpensive) way to match patients to naltrexone is by identifying those who have 20 percent or more relatives with alcohol problems and/or have high antisocial scores. Patients with alcohol dependence who use marijuana or cocaine can also be benefited by naltrexone.
- Naltrexone was differentially effective based on the individual’s genotype; it significantly reduced the self-reported, alcohol-induced high in participants with at least one copy of the G allele but had no effect on participants who were homozygous for the A allele.
- After taking naltrexone, subjects with the G allele demonstrated greater blunting of the alcohol-induced high when the breath alcohol concentration (BrAC) reached 0.06 mg/L, with greatest effects at highest BrAC. This suggests that the effects of naltrexone may be alcohol-dose dependent ( Ray & Hutchison, 2007 ).
- Naltrexone significantly delayed the time to second and third drinking days for women who did not maintain abstinence from alcohol.
- Symptoms of eating pathology decreased during treatment among all groups (e.g., the frequency of binge eating decreased by almost 70 percent). This suggests that treatment for alcohol dependence may be associated with improvements in eating pathology ( O’Malley et al., 2007b ).
- The outcomes of this study have been reexamined in a reanalysis of the data, described in the following study.
Reanalysis of Negative Trials of Naltrexone
Novel approaches to data analysis may help resolve the current heterogeneity of clinical findings about naltrexone’s efficacy in treating alcohol dependence. Most clinical trials show naltrexone to be effective in delaying relapse to heavy drinking, reducing the intensity of drinking, or increasing the percentage of days abstinent—usually with a small to moderate effect size. However, several randomized trials have found no significant benefit associated with naltrexone treatment. Gueorguieva and colleagues (2007) reanalyzed two such negative trials: a large Veterans Affairs (VA) clinical trial ( Krystal, Cramer, Krol, Kirk, & Rosenheck, 2001 ) and the Women’s Naltrexone Study described above ( O’Malley et al., 2007b ). The researchers used a trajectory-based approach—previously untried in alcohol treatment studies—to look at naltrexone efficacy by evaluating patterns of drinking rather than single events or summary measures. Findings were as follows:
- Based on the data, three distinct trajectories of daily drinking over time (both for any drinking and for heavy drinking) could be modeled using a semiparametric group-based approach. These trajectories were similar for both studies and consisted of (1) “abstainer,” (2) “sporadic drinker,” and (3) “consistent drinker.”
- Compared with those on placebo, subjects on naltrexone were significantly more likely to be abstainers or sporadic drinkers rather than consistent drinkers.
- Naltrexone doubled the odds of following the “abstainer” trajectory instead of the “consistent drinker” trajectory.
- Medication compliance had a significant effect on the trends over time, decreasing the odds of drinking in all trajectories.
The authors suggest that trajectory-based statistical methods could play a role in the future analysis of clinical trials. This method can be used to estimate empirically the heterogeneity in the study population and to identify subgroups with similar response patterns for whom treatment is effective.
New Findings on Extended-Release Injectable Naltrexone
Researchers have conducted a secondary analysis to show the efficacy of extended-release, injectable naltrexone (XR-NTX) among a subgroup of patients enrolled in a 6-month, multicenter, randomized, double-blind, placebo-controlled trial that was previously reported in the literature ( O’Malley, Garbutt, Gastfriend, Dong, & Kranzler, 2007a ). The original study ( Garbutt et al., 2005 ) found that 380 mg (the approved dose) per day of XR-NTX, combined with 12 sessions of psychosocial therapy, was significantly more effective than placebo in reducing the rate of heavy drinking among patients with alcohol dependence who had been abstinent for 7 or more days before receiving their first injection.
The question addressed in this new data analysis is, “How effective is XR-NTX among patients who had been abstinent for as few as 4 days before receiving the first injection?”—a practical issue in U.S. detoxification settings, where detoxification commonly takes 4 days ( O’Malley et al., 2007a ). Of 624 patients with alcohol dependence in the original study, 82 patients—the subjects of this analysis—had been voluntarily abstinent for 4 days or more before treatment started. To be eligible for the study, patients had to have had at least two episodes per week of heavy drinking (5 or more drinks per day for men and 4 or more drinks for women) in the 30 days before enrollment. O’Malley and colleagues (2007a) , who analyzed the data on a wide range of drinking-related outcomes, concluded that a long period of pretreatment abstinence is not required to achieve positive outcomes among patients receiving monthly 380 mg injections of XR-NTX. The data showed that XR-NTX prolongs abstinence and reduces the number of both drinking days and heavy drinking days in patients who are abstinent for as few as 4 days before starting treatment. For these patients, the analysis showed the following:
- Their rate of abstinence throughout the entire 6-month study was nearly 3 times higher than in patients on placebo.
- Their median time to first drink was 41 days compared with 12 days for those on placebo.
- Their rate of continuous abstinence at the end of the study was 32 percent, compared with 11 percent for those on placebo.
- Their median time to first heavy drinking event was 9 times longer than median time for those on placebo (more than 180 days compared with 20 days).
- Their median number of drinking days per month decreased by 90 percent, totaling 0.7 days per month versus 2.9 days for those on placebo.
- Their median number of heavy drinking days per month decreased by 93 percent, totaling 0.2 days versus 2.9 days for those on placebo.
Responders to treatment were defined as patients who had no more than 2 heavy drinking days in any consecutive 28-day period. Among patients who abstained for as few as 4 days before receiving 380 mg of XR-NTX, 70 percent were classified as responders, more than twice as many as the 30 percent of responders on placebo. Consistent with these observed reductions in drinking, the XR-NTX treatment was associated with greater reductions in gamma glutamyltransferase (GGT) levels over time compared with placebo.
The analysis also looked at patients who received a 190 mg dose of XR-NTX. Their drinking-related outcomes generally fell in an intermediate range between those of patients receiving a 380 mg dose and those on placebo, suggesting a dose-response effect.
New Findings on Acamprosate
A brief article on acamprosate reported on a previously unknown finding—three case studies of patients with long-standing alcohol dependence who had primitive reflexes that continued throughout detoxification but were completely resolved within 24 hours after initiation of acamprosate ( Guzik, Bankes, & Brown, 2007 ). These male patients presented with the primitive snout (sucking motion) reflex and/or the grasp reflex, both of which are highly unusual in healthy adults. Primitive brain-stem reflexes are suppressed after infancy; the presence of these reflexes in adults connotes systemic, metabolic, or neurologic disease that impairs the brain’s ability to suppress them. Guzik and colleagues (2007) suggest that acamprosate (at 666 mg 3 times daily) may resolve the primitive snout and grasp reflexes—a neurological finding that suggests cognitive impairment—among patients with alcohol dependence.
Primitive reflexes can be readily identified in a physical examination, and their potential value in staging various illnesses and assessing prognosis is just beginning to be studied ( Guzik et al., 2007 ). Research is needed to determine whether monitoring primitive reflexes in patients with alcohol dependence before and after initiation of therapy with acamprosate could help identify their potential treatment responses and prognoses. This study also suggests that acamprosate may reduce the cognitive impairment that interferes with early-stage recovery for some patients who are alcohol dependent.
New Findings on Combined Medication Therapy
Combining naltrexone and disulfiram for patients with co-occurring alcohol dependence and depression.
Petrakis and colleagues (2007) concluded that the combined use of naltrexone and disulfiram offered no advantage over either medication alone for subjects who are alcohol dependent with co-occurring major depression. This secondary analysis of data from a large randomized controlled trial looked at 139 male veterans who were alcohol dependent with a Diagnostic and Statistical Manual of Mental Disorders ( American Psychiatric Association, 1994 ) diagnosis of current major depression. These subjects represented 54.7 percent of the 254 participants in the original study of veterans with alcohol dependence and comorbid mental disorders ( Petrakis et al., 2005 ).
This secondary analysis showed that subjects with current co-occurring depression achieved positive outcomes comparable to all subjects in the trial—a trial in which almost 70 percent of subjects achieved complete abstinence during the 12-week study period. In addition, on the Hamilton Depression Rating Scale, these subjects with depression also showed a significant decrease in depression from baseline to posttreatment. Side effects of the combined medications, as well as naltrexone and disulfiram alone, were tolerated and consistent with those seen in patients who do not have a dual diagnosis. Because there was no advantage to the combined medication or to one pharmacotherapeutic agent over another, the choice of medication to treat AUDs in patients with depression can depend on such factors as patient preference ( Petrakis et al., 2007 ).
Combining medication with psychosocial treatment
In a review article designed for physicians, Weiss and Kueppenbender (2006) describe the significant advances made in the development, standardization, and rigorous testing of the psychotherapeutic approaches used to treat alcohol dependence. Medical management interventions are available to physicians, as are strategies for improving medication adherence. The authors discuss the evidence from the literature since 1984, particularly clinical trials, on the interactions and efficacy of disulfiram, oral naltrexone, and acamprosate with particular psychosocial treatments. Weiss and Kueppenbender (2006) recommend that physicians use these medical management techniques when prescribing pharmacologic agents to patients with alcohol dependence. They also recommend that physicians become knowledgeable about the various psychotherapies as background for referring their patients who are alcohol dependent to concurrent psychosocial treatment. Some of the conclusions made by Weiss and Kueppenbender (2006) , based on their review of the literature, include the following:
- Adding medical management therapy and pills to a specialty psychosocial therapy improves outcomes for patients who are alcohol dependent.
- Psychosocial interventions, ranging from brief medical management to more intensive manual-based psychotherapies, have all been shown to produce positive outcomes in certain studies, depending on the specific medication and the study context.
- No evidence suggests that one single form of psychosocial treatment is a criterion standard for patients with alcohol dependence who receive pharmacotherapy.
- For disulfiram, a successful and promising adjunctive approach is behavioral marital therapy augmented with a disulfiram contract by the couple.
- For naltrexone, the evidence suggests (although not conclusively) that CBT may be particularly effective as adjunct therapy.
- For acamprosate, few studies have been done in combination with structured, controlled psychosocial interventions. The limited evidence suggests that acamprosate may be used equally effectively with a variety of psychosocial treatments and that little psychosocial treatment may be needed beyond medical management.
Building on the existing literature about psychosocial approaches combined with pharmacotherapy, two recent articles describe additional possible psychotherapy approaches to augment the medication. The proposed therapies, described below, include (1) the trial of a second-generation CBT combined with oral naltrexone and (2) the use of contingency management (CM) with medications for treating substance abuse.
Broad-spectrum treatment (BST). A 3-month, randomized controlled trial explored whether a broad-spectrum CBT would be more effective than MET for patients who are alcohol dependent treated with naltrexone ( Davidson, Gulliver, Longabaugh, Wirtz, & Swift, 2007 ). This initial trial suggests that, at least when combined with naltrexone, a second-generation CBT may have a meaningful clinical advantage over brief interventions such as MET ( Davidson et al., 2007 ).
This research group developed a unique CBT manual-based protocol for alcohol dependence that combined components of the three psychotherapies demonstrated to be effective in NIAAA’s Project MATCH: CBT, 12-Step facilitation, and MET. This new BST approach incorporates such content material as cognitive restructuring, drink refusal, and assertiveness training with a patient-specific selection of session modules. The treatment matching uses a decision tree, with modules tailored for the individual through a psychometric assessment of each patient’s need.
In this study, 149 patients with alcohol dependence were randomly assigned to receive either BST and naltrexone or MET and naltrexone. Patients who received BST had a significantly higher percentage of days abstinent than patients receiving MET. Treatment was tailored in response to an assessment of the patients’ psychosocial resources, and the differential advantage for BST was most marked for those patients with social networks that supported drinking.
Contingency management. Clinical trials have demonstrated the effectiveness of CM procedures—an approach in which patients receive concrete rewards or reinforcers for discrete targeted behaviors. However, few trials have assessed the value of CM procedures when combined with pharmacotherapy for alcohol dependence. Carroll and Rounsaville (2007) review the existing evidence and suggest that CM would be an ideal platform for addressing the weaknesses of many pharmacotherapies used to treat drug abuse. CM can directly reinforce medication adherence, which may substantially improve compliance in treatment where unpleasant side effects must be overcome or where compliance is not strongly reinforced by rapid benefits from the treatment itself. The authors describe a variety of CM strategies used to improve compliance with disulfiram among patients with alcohol dependence.
A recent pilot double-blind trial of memantine—a selective noncompetitive N- methyl-Daspartate (NMDA) receptor antagonist—among 34 individuals with alcohol dependence did not support the use of memantine for treating patients who are actively drinking. However, this study did support the use of voucher incentives to facilitate retention. With voucher incentives for clinic attendance, 80 percent of subjects completed the 16-week trial ( Evans, Levin, Brooks, & Garawi, 2007 ).
New Findings on Promising Drugs
In a review of the evidence supporting use of medications for alcohol withdrawal and dependence, Ait-Daoud, Malcolm, and Johnson (2006) discuss clinical trial findings on naltrexone and acamprosate but focus particularly on anticonvulsants. The article presents the neurochemical rationale and research evidence supporting use of anticonvulsants, particularly carbamazepine, valproate, and topiramate, for treating alcohol dependence. On the basis of controlled trials to date, the authors conclude:
- Valproate may be a promising medication for treating patients who are alcohol dependent with a comorbid bipolar disorder.
- Topiramate, a potent novel anticonvulsant, offers promising evidence of being a safe and effective option for the pharmacological treatment of alcohol dependence, warranting further study.
- Anticonvulsants such as valproate and topiramate may offer the advantage of being single medications that can be used from detoxification through the treatment process—being used first to treat the acute withdrawal symptoms and then, once abstinence has been achieved, to prevent relapse by modulating postcessation craving and affective disturbance ( Ait-Daoud, Malcolm, & Johnson, 2006 ).
Recent research shows that topiramate, a drug with complex actions that include activity at the GABA and glutamate receptors, is a promising treatment for alcohol dependence. Although only two major studies have been conducted, the consistency and size of topiramate’s clinical efficacy suggest the need for further research, particularly on the most efficacious ceiling dose, the impact of longer periods of treatment, and the subtypes of alcoholism most benefited by treatment with topiramate ( Johnson et al., 2007 ).
A 17-site, double-blind, placebo-controlled trial with 371 men and women who were alcohol dependent found that up to 300 mg per day of topiramate reduced the percentage of heavy drinking days from baseline to week 14 and produced significant and meaningful improvement in a wide variety of self-reported drinking outcomes ( Johnson et al., 2007 ). Topiramate compared with placebo treatment was associated with a significantly higher rate of achieving 28 or more days of continuous nonheavy drinking and 28 or more days of continuous abstinence. Furthermore, using two different analytic approaches, the topiramate group reached 28 or more days of continuous abstinence significantly faster than the placebo group ( Johnson et al., 2007 ). Topiramate also decreased plasma GGT in the heterogeneous and graphically diverse population. These positive findings replicated the results of a smaller, randomized controlled trial ( Johnson et al., 2003b ). Topiramate’s therapeutic effect was evident no later than week 4. At the end of the 14-week trial, differences between topiramate and placebo were still increasing, suggesting that even more improvement may occur with longer administration ( Willenbring, 2007 ).
Two additional factors make topiramate seem particularly promising for treating alcohol dependence in primary care settings. First, topiramate proved effective with patients who were actively drinking rather than abstinent at the time medication was started. Patients in the multisite study were drinking heavily at the time of enrollment and study randomization (men were drinking 35 or more and women 28 or more standard drinks per week). These patients were not required to stop drinking before entering the study, although they had to express a desire to stop or reduce their consumption of alcohol with the possible long-term goal of abstinence. Second, the study provided only minimal behavioral support that focused on enhancing medication compliance and encouraging abstinence—a brief intervention that could be provided by nonspecialist health practitioners ( Johnson et al., 2007 ).
At least 10 percent of participants reported adverse events; the events reported most by those on topiramate, as compared with placebo, included paresthesia, taste perversion, anorexia, difficulty with concentration and attention, and pruritus ( Johnson et al., 2007 ). In the multisite study, where topiramate was titrated over a 6-week period, the attrition that was due to adverse events was 18.6 percent for the topiramate group and 4.3 percent for controls. In the earlier study ( Johnson et al., 2003a ), where adverse events were similar but titration occurred over a longer, 8-week period, retention rates for the topiramate and control groups were similar. However, in the multisite study, the researchers found that completion rates approached 90 percent among practitioners experienced in administering topiramate, whereas less experienced practitioners had more difficulty with retention. To enhance adherence, the authors advise clinicians to use a slow titration schedule over 8 weeks and to provide focused education for patients on how to manage emergent adverse events ( Johnson et al., 2007 ).
The efficacy of topiramate was also supported in the following two small studies:
- Topiramate as add-on therapy for patients with alcohol dependence who do not respond to standard treatment. In an observational open-label, multisite study in Spain, 64 patients who were alcohol dependent with poor outcomes in standard treatment were provided with a mean dose of almost 200 mg per day of topiramate and monitored over a 12-month period ( Fernández Miranda et al., 2007 ). The addition of topiramate resulted in a significant decrease in all outcomes measured—number of drinking days per month and standard drinking units consumed per day, craving, priming, dependence intensity scales, and serum transaminase levels.
- Ability of topiramate to increase periods of continuous “safe” drinking (defined by NIAAA as 1 or fewer standard drinks per day for women and 2 or fewer standard drinks per day for men). Some patients with alcohol dependence do not achieve abstinence during treatment. Researchers carried out a secondary analysis of data from a double-blind, randomized, controlled 12-week trial ( Johnson et al., 2003b ) to determine whether topiramate recipients were able to achieve longer continuous periods of “safe” drinking than those on placebo ( Ma, Ait-Daoud, & Johnson, 2006 ). The analysis found that soon after topiramate was administered, recipients began to achieve increasing lengths of “safe” drinking relative to placebo. Furthermore, these early treatment gains appear to be predictive of continuing improvement as the length of time in treatment increases ( Ma et al., 2006 ).
Other medications under development
In addition to topiramate, the 2007 literature search identified positive reports on controlled clinical trials of two potential medications for treating alcohol dependence: (1) nalmefene, an opioid antagonist, and (2) quetiapine, an atypical antipsychotic that targets both dopamine and serotonin receptors. Findings were as follows:
- Targeted nalmefene. A multisite, randomized double-blind study in Finland found that 10 to 40 mg doses of nalmefene were safe and reduced heavy drinking among 242 subjects with self-identified drinking problems ( Karhuvaara et al., 2007 ). Subjects received minimal psychosocial intervention and took nalmefene only when drinking seemed imminent. After 28 weeks, 57 subjects on nalmefene continued into a 24-week extension period with randomization to continued nalmefene or placebo. Decrease in drinking was significantly greater for subjects on nalmefene than on placebo, which was corroborated by significant decreases in alanine aminotransferase and GGT. During this randomized withdrawal period, subjects remaining on nalmefene maintained the drinking level achieved in the initial 28 weeks, whereas those switched to placebo seemed to return to more frequent heavy drinking.
- Quetiapine. According to a double-blind, placebo-controlled 12-week trial among 61 subjects who were alcohol dependent, quetiapine (400 mg per day) may be more effective in treating people with the more severely affected Type B alcoholism compared with those with Type A alcoholism. This small study found a significant interaction between quetiapine and alcoholic subtype ( Kampman et al., 2007 ). As predicted, Type B subjects treated with quetiapine had significantly fewer days of drinking and fewer days of heavy drinking than Type B subjects on placebo. Compared with those on placebo, people with Type B alcoholism who were treated with quetiapine had alcohol craving significantly reduced. Among the patients with Type A alcoholism, quetiapine offered no advantage over placebo in improving drinking outcomes. Nine patients treated with quetiapine (31 percent) maintained complete abstinence compared with two patients on placebo (6 percent).
New Findings on Pharmacotherapy Use by Medical Care Providers
Relatively few specialized addiction treatment programs use pharmacotherapies for alcohol dependence, according to recent data collected from large samples of specialty programs in the public and private sectors ( Ducharme, Knudsen, & Roman, 2006 ). Even as evidence for the efficacy of these medications has increased, the longitudinal data in this study suggest that the proportion of treatment programs using pharmacotherapies has actually been declining over time and the number of patients who receive medications remains low. Ducharme and colleagues (2006) discuss historical patterns among addiction treatment programs, as well as the numerous structural and philosophical barriers that impede the adoption of pharmacotherapies by the specialty treatment system. This article suggests a wide range of specific environmental, funding, regulatory, and linking structures and strategies that could help reduce resistance and promote the adoption of medications in addiction treatment ( Ducharme et al., 2006 ).
Attitudes of professional alcohol counselors
Community-based addiction treatment centers rarely use pharmacotherapies for treating their patients who are alcohol dependent ( Thomas, Wallack, Lee, McCarty, & Swift, 2003 ). Before initiating pharmacotherapy education at six community-based addiction treatment centers, Thomas and Miller (2007) collected baseline data on the knowledge and attitudes of 84 counselors and administrators attending a staff education project. Respondents came only from centers that had no on-staff medical provider. The data showed the following:
- These counselors and administrators, with just one exception, had very little or no knowledge about naltrexone.
- Most believed that adjunctive pharmacotherapy is ineffectual in treating alcohol dependence.
The authors concluded that lack of knowledge and confidence about pharmacotherapy by counselors is a barrier to more widespread referral and use of pharmacotherapies in alcohol treatment centers. Focused education will be needed for both counselors and administrators.
The study suggests that educational efforts do not need to overcome negative opinions about adjunctive pharmacotherapies. Instead, the intent should be to convey accurate and empirically supported information about the value of current medications. The respondents’ personal recovery status from addiction did not appear related to their valuation of pharmacotherapies. The most senior addiction professionals—those with more than 10 years of experience in the addiction field—were generally more positive in their valuation of adjunctive pharmacotherapy ( Thomas & Miller, 2007 ).
Attitudes of patients toward pharmacotherapy
A second study looked at whether medically hospitalized patients with alcohol dependence are interested in pharmacotherapy and primary care to treat their alcoholism ( Stewart & Connors, 2007 ). This survey covered 50 inpatients identified as alcohol dependent; all were receiving internal medicine services in a university-affiliated public hospital. Most survey participants were socioeconomically disadvantaged males admitted with disorders that heavy alcohol use would typically cause or exacerbate. In the month before being admitted to the hospital, these patients on average had been drinking on 86 percent of days and had averaged 8.4 drinks per drinking day. Their responses suggest that many such patients will be interested in receiving medication for alcoholism:
- 84 percent agreed they needed to stop drinking.
- 50 percent agreed that medication helps prevent drinking.
- 66 percent agreed they would like to receive an effective medication to help prevent drinking.
Interest in receiving effective pharmacotherapy was positively associated with addiction severity, adverse consequences, recognition of the problem, and drinking frequency. The reaction to primary care was mixed; only 32 percent of these patients were interested in primary care treatment for their alcoholism. The authors conclude that primary care followup alone may not adequately address patients’ perceived needs; many patients may also require prompt referral to specialty care after hospitalization ( Stewart & Connors, 2007 ).
- Approved Drugs for Treating Patients Dependent on Alcohol
The following review of the literature covers major research articles published between 2000 and April 2007. The focus is on drugs currently approved by FDA for treating alcohol dependence (disulfiram, oral naltrexone, long-term injectable naltrexone, and acamprosate).
The first medication for alcohol dependence, approved by FDA almost 60 years ago, is an aversive therapy still used today. Disulfiram (Antabuse®) irreversibly inhibits acetaldehyde dehydrogenase, an enzyme involved in alcohol metabolism, which leads to an accumulation of acetaldehyde. This accumulation leads to a severe reaction when alcohol is consumed. Disulfiram also inhibits dopamine β-hydroxylase in the brain and may have a direct effect on brain catecholamines. Disulfiram causes a variety of unpleasant symptoms when a person drinks alcohol, such as nausea, vomiting, hypotension, and facial flushing. Despite these reactions, approximately 15 percent of patients continue to drink alcohol while taking disulfiram ( Myrick, 2002 ). When daily dosages of 1,000–3,000 mg were used, deaths were reported from disulfiram–alcohol reactions ( Fuller & Gordis, 2004 ). The reasonable startup dose today is 250 mg, and, if the patient drinks and does not experience a disulfiram–alcohol reaction, the dose can be increased to 500 mg ( Fuller & Gordis, 2004 ).
Fuller and Gordis (2004) , asking “Does disulfiram have a role in alcoholism treatment today?” respond with a qualified “yes.” They conclude that the field needs to move beyond disulfiram and develop better pharmacotherapies that act on the neurobiological processes underlying alcohol dependence. Fuller and Gordis suggest that physicians do not need to prescribe disulfiram when patients first enter treatment. But if a patient is struggling to maintain sobriety, the supervised use of disulfiram is warranted ( Fuller & Gordis, 2004 ). Side effects are usually minor; serious adverse reactions are uncommon, although the physician needs to monitor for hepatotoxicity.
Research on disulfiram
Research studies and clinical experience over 55 years offer valuable information about the efficacy and safety of disulfiram. Almost 40 years elapsed from the time disulfiram became available before the first multisite, randomized clinical trial covering 605 participants was published ( Fuller et al., 1986 ). Many large double-blind studies of disulfiram show no therapeutic effect compared with placebo (reviewed by Myrick, 2002 ). There is still no unequivocal evidence from randomized, controlled trials to show that disulfiram improves abstinence rates over the long term ( Mann, 2004 ).
Brewer, Meyers, and Johnsen (2000) reviewed all published clinical studies in which there had been attempts to directly supervise the administration of disulfiram at least weekly. Adequate supervision included appropriate training of supervisors and review of their ability to supervise. These researchers found 13 controlled and 5 uncontrolled studies with supervised disulfiram administration and reported positive findings in all but 1 study. In general, the better the supervision, the better the outcomes. Under supervised situations, disulfiram reduced drinking, prolonged remissions, improved treatment retention, and facilitated compliance with psychosocial interventions.
Anton (2001) , in a review of the literature, concluded that the evidence for disulfiram is mixed. According to Anton (2001) , the most reliable study suggests that disulfiram is not better than placebo. In the reviewed studies, Anton (2001) reported that the factors that seem to improve treatment effectiveness with disulfiram include patient motivation, patient monitoring, being an older man, and concomitant treatment with acamprosate.
The most recent comprehensive review of the literature, done by Suh, Pettinati, Kampman, & O’Brien (2006) and covering the literature from 1937 to 2005, concluded that supervised disulfiram can be an effective treatment for alcohol dependence. The reviewers recommended that more research be done on disulfiram combined with other—and especially newer—psychotherapies.
Disulfiram use in primary care
Adverse events. Disulfiram is well tolerated in most patients, with the most common adverse effects being tiredness, headache, and sleepiness ( Chick, 1999 ). Toxicities such as psychotic reactions, confusional states, and neuropathy are rare and appear to be dose related ( Bevilacqua, Diaz, Diaz, Silva, & Fruns, 2002 ; Chick, 1999 ).
Disulfiram hepatitis is a very rare, sometimes fatal complication that particularly affects women ( Brewer & Hardt, 1999 ). A Danish study of adverse reactions to disulfiram over a 22-year period estimated the rate of fatal disulfiram-induced hepatitis to be 1 per 25,000 patients treated per year, with the peak of hepatotoxicity occurring 60 days after the beginning of treatment. Because hepatotoxicity can usually be reversed if disulfiram is stopped before liver disease is clinically evident, Wright, Valfier, and Lake (1988) recommend liver function testing before treatment, at 2-week intervals for 2 months, and at 3- to 6-month intervals thereafter. Chick (1999) recommends informing the patient and the patient’s family and physician of the risk and immediately stopping the drug if adverse effects, such as fever preceding jaundice, are noted. Fuller and Gordis (2004) recommend supervised administration of disulfiram, along with careful monitoring for hepatotoxicity.
Conditions excluding treatment with disulfiram. Patients with cardiovascular or cerebrovascular disease are excluded from treatment because hypotension can occur during a disulfiram–alcohol interaction ( Fuller & Gordis, 2004 ). Disulfiram has been reported to cause fetal abnormalities, so pregnant women should not use it. Disulfiram is also contraindicated in patients who have an idiopathic seizure disorder or cannot understand the risks associated with use of the drug. Disulfiram may influence adversely the pharmacokinetics and, therefore, the effects of medications metabolized by the cytochrome p450 system, such as warfarin, phenytoin, amitriptyline, and benzodiazepines (including chlordiazepoxide and diazepam but not lorazepam and oxazepam). Disulfiram also interferes with the pharmacokinetics of the tricyclic antidepressants. Fuller and Gordis (2004) report that the literature indicates disulfiram is unsafe to use concomitantly with monoamine oxidase inhibitors.
Patients appropriate for disulfiram. Data suggest that disulfiram is most effective in older, motivated individuals and in those who are supervised during daily ingestion. Predictors of efficacy with disulfiram include patients highly motivated for abstinence, people who are married or have a good support system, people with behavioral contracts to take the medication, and people legally compelled to take disulfiram ( Myrick & Anton, 2004 ; O’Farrell, Allen, & Litten, 1995 ). Disulfiram can also support abstinence when people who are alcohol dependent attend events that involve alcohol, such as family celebrations.
In general, disulfiram seems to have limited acceptance in the treatment of alcohol dependence ( Anton & Swift, 2003 ). Several recent, small pilot studies suggest that disulfiram might be safe and useful for the following types of patients:
- Patients who are positive for the hepatitis C virus (HCV). A recent review of the literature recommends monitored disulfiram treatment for patients positive for HCV ( Kulig & Beresford, 2005 ).
- Patients who are court ordered. Martin, Clapp, Alfers, and Beresford (2004) found that compliance with treatment was 61 percent after 18 months for those with court-ordered, supervised disulfiram treatment. This compared with 18-percent compliance among those in a voluntary, supervised disulfiram group.
- Adolescent patients. In a small, preliminary study, Niederhofer and Staffen (2003) compared 13 adolescents ages 16–19 on disulfiram with 13 controls. After 90 days, the mean abstinence duration was significantly greater for the disulfiram group than for the placebo-treated controls (68.5 days [SD 37.5] vs. 29.7 days [SD 19.0]). Disulfiram was well tolerated in adolescents, except for occasional diarrhea.
- Patients with severe mental illness. A preliminary study of 33 patients with severe mental illness and alcohol dependence found that supervised disulfiram treatment was associated with decreases in the number of days hospitalized. Controlled research is needed to evaluate the effects of disulfiram in this population ( Mueser, Noordsy, Fox, & Wolfe, 2003 ).
- Patients codependent on alcohol and cocaine. Disulfiram’s main effects in initiating abstinence in cocaine and alcohol use were still maintained a year after patients with codependence received short-term (12-week) treatment with disulfiram combined with psychotherapy ( Carroll et al., 2000 ). Ninety-six patients who were codependent randomly received CBT either with or without disulfiram, 12Step facilitation with or without disulfiram, or clinical management with disulfiram. Carroll and associates (2000) concluded that this randomized controlled trial supports the efficacy of disulfiram with this challenging codependent population. The findings suggest long-term benefits can result from comparatively brief treatments that facilitate the initiation of abstinence.
Research needs
The effects of disulfiram on craving have not been widely studied, but disulfiram is unlikely to be very powerful in reducing craving, especially if a patient has not achieved sustained abstinence ( Anton & Swift, 2003 ). For better information about the use of disulfiram today, randomized clinical trials need to determine whether supervised ingestion of disulfiram:
- Would be useful to ensure sobriety for such high-risk groups as criminal offenders and those who have failed previous attempts at treatment
- Is better if supervision is performed by a clinic staff member or by a relative
- Would improve treatment outcomes when combined with newer pharmacotherapies ( Fuller & Gordis, 2004 ).
Oral Naltrexone
In 1994, FDA approved naltrexone, an OPRM1 antagonist, as a 50 mg oral tablet for the prevention of relapse to alcohol use. Before its approval for alcohol dependence, naltrexone had been approved by FDA for use in opioid dependence. Adding alcohol treatment as an indicator for use of naltrexone was based on the results of two single-site studies that evaluated the medication as an adjunct to relapse prevention psychotherapy. These studies found that naltrexone reduced drinking frequency and the likelihood of relapses to heavy drinking ( O’Malley et al., 1992 ; Volpicelli, Alterman, Hayashida, & O’Brien, 1992 ).
Naltrexone represented a new era of medications studied specifically to treat AUDs. Disulfiram’s mechanism of action centers on its use as an aversive agent, whereas naltrexone is thought to act directly on the brain as an anticraving compound ( Myrick & Anton, 2004 ). As an opioid antagonist, naltrexone is thought to reduce the reinforcing subjective or behavioral response to alcohol ( Davidson, Palfai, Bird, & Swift, 1999 ; Garbutt et al., 2005 ; McCaul, Wand, Stauffer, Lee, & Rohde, 2001 ). Naltrexone must be prescribed with caution because individuals abusing opioids may experience withdrawal and those receiving opioids for analgesia will find them ineffective during naltrexone treatment. Patients receiving naltrexone should carry an explanatory card to show to healthcare personnel in an emergency.
Research on naltrexone
In the last decade, the efficacy of naltrexone for alcohol dependence has been extensively studied, particularly in the United States. At least 19 published controlled studies of about 3,200 patients have compared the effects of oral naltrexone with placebo; nearly all showed efficacy in the treatment of alcohol dependence ( Garbutt et al., 2005 ). The majority of clinical trials support the hypothesis that naltrexone can reduce the urge to drink, increase the number of days abstinent, and minimize the risk of relapse to heavy drinking in some patients ( O’Malley & Froehlich, 2003 ). However, two recent studies, including a large, multisite VA study, have reported no or minimal effectiveness in reducing drinking behavior as compared with placebo (reviewed by Krystal et al., 2001 ). One reason for this ineffectiveness may be the high rate of noncompliance among patients in the VA study. Lack of compliance with oral naltrexone is a problem that varied greatly across studies, with 40 to 90 percent of subjects completing treatment in the studies.
The lack of consistent findings on the effects of oral naltrexone may be the result, at least in part, of variations in how compliant patients are with the medication. A number of studies indicate that poor compliance with therapy can limit the effectiveness of oral naltrexone ( Johnson & Ait-Daoud, 2000 ; Kranzler, Wesson, & Billot, 2004 ). For example, in a 3-month followup study, Volpicelli and colleagues (1997) found that only patients who took their oral daily dose on at least 90 percent of study days improved their drinking outcomes. No differences were found between the placebo group and those who took naltrexone on fewer than 90 percent of study days on any drinking measure; 50 percent of these subjects relapsed, changing from abstinence to clinically significant drinking during the study. In a large, 1-year collaborative study in the United Kingdom, only patients who took at least 80 percent of their naltrexone tablets experienced better drinking outcomes than those on placebo ( Chick et al., 2000 ). The outcomes in 17 clinical trials of naltrexone at a dose of 50 mg per day are shown in Exhibit 1 ( Mann, 2004 ).
Published Placebo-Controlled Clinical Trials of Naltrexone 50 mg/day in Alcohol Dependence.
The extensive research on oral naltrexone has produced numerous review articles and several meta-analyses of the literature. Three meta-analyses concluded that the effect of naltrexone is significantly greater, on average, than that of placebo ( Kranzler & Van Kirk, 2001 ; Srisurapanont & Jarusuraisin, 2005 ; Streeton & Whelan, 2001 ). The meta-analysis by Streeton and Whelan (2001) found that, after 12 weeks of naltrexone treatment, patients experience significantly fewer episodes of relapse and significantly more remain abstinent compared with subjects on placebo. The meta-analysis by Srisurapanont and Jarusuraisin (2005) , which covered 3,048 subjects in 27 randomized controlled trials from 34 published and unpublished papers, concluded the following:
- Short-term treatment. Naltrexone should be accepted as a short-term treatment for alcoholism. In comparison with placebo, short-term treatment significantly reduces the chance of alcohol relapse for 36 percent of patients, is likely to reduce the chance of returning to drinking for 13 percent, and can lower the risk of withdrawing from treatment for 28 percent of patients.
- Medium-term treatment. Medium-term naltrexone treatment gives no benefit over placebo in terms of relapse prevention, but it does increase the time to first drink and diminishes craving. In this regard, naltrexone plus intensive psychosocial treatment is superior to naltrexone plus a simple psychosocial treatment. Naltrexone is also superior to acamprosate in reducing relapses, number of drinks, and craving.
- Concomitant treatment strategies. To improve treatment adherence and to ensure that real-world treatment is as effective as research findings, some form of psychosocial intervention and management of adverse effects needs to accompany naltrexone therapy.
- Unanswered questions. The existing research is limited because many trials are of short duration and have small sample sizes. Important areas of concern include the lack of data on different psychosocial benefits and on how long patients who respond to naltrexone should continue their treatment. The evidence for longer term (more than 8 months) efficacy of naltrexone remains to be demonstrated ( Mason, 2003 ).
Recently, Pettinati and colleagues (2006) reviewed all published, randomized placebo-controlled trials of naltrexone to resolve inconsistencies in naltrexone’s reported efficacy across trials. Drinking outcomes measured in these studies related to four outcomes: two pertaining to “any drinking” and two pertaining to “heavy or excessive drinking.” This review found an advantage for naltrexone over placebo in 70 percent of clinical trials that measured reductions in “heavy or excessive drinking” but in only 36 percent of trials that measured abstinence or “any drinking.” Pettinati and colleagues (2006) concluded that naltrexone’s therapeutic effects are most related to outcomes pertaining to heavy or excessive drinking.
Naltrexone use in primary care
Of particular interest to physicians in primary healthcare settings is one recent study that looked at whether general internists and primary care physicians, using naltrexone, could treat patients who are alcohol dependent as effectively as addiction specialists can ( O’Malley et al., 2003 ). Results indicated that primary care counseling with naltrexone pharmacotherapy can be effective in select patients. Study subjects were recruited from newspaper ads and required to be abstinent from alcohol for 5 to 30 days before initiating treatment. In this nested sequence of randomized, placebo-controlled trials, patients received the following:
- Phase I—197 patients received 10 weeks of naltrexone and either (1) brief counseling from a primary care physician (an initial 45-minute visit followed by 15-to 20-minute sessions in weeks 1 through 4, 6, 8, and 10) or (2) CBT from an addiction specialist (an initial 1.25-hour session followed by weekly 50-minute sessions for 10 weeks).
- Phase II—Responders from both groups received 24 weeks of continuing maintenance with naltrexone.
In Phase I, the results were comparable in the two groups, with 84.1 percent of primary care patients and 86.5 percent of CBT patients avoiding persistent heavy drinking. Persistent heavy drinking was defined as more than 2 days of heavy drinking (5 or more drinks per day for men and 4 or more drinks per day for women) during the last 28 days of Phase I. In Phase II, the response to naltrexone maintenance was better maintained among those who received primary care than in those with counseling appointments ( O’Malley et al., 2003 ). Monterosso and colleagues (2001) also found a significant advantage of naltrexone use over placebo in patients who received 12 weeks of concurrent primary counseling.
The results are available from NIAAA’s COMBINE study, a multisite, randomized, controlled trial that evaluated medical management with naltrexone, acamprosate, or both, with or without additional specialist treatment (combined behavioral intervention). Participants received interventions over a 4-month period and were evaluated for up to 1 year after treatment. Findings from this study suggest that naltrexone with medical management can be delivered successfully in healthcare settings, which would greatly expand the number of people receiving treatment ( Anton et al., 2006 ). In fact, the COMBINE data suggest that naltrexone can be effective in the context of medical management without specialized behavioral treatment.
In the COMBINE study, participants taking naltrexone received 25 mg on days 1 through 4, 50 mg on days 5 through 7, and 100 mg on days 8 through 112. Doses were chosen based on preliminary evidence that doses higher than those commonly prescribed could be more efficacious and provide better coverage for missed doses; two pilot studies confirmed the tolerability of these doses ( Anton et al., 2006 ). Ongoing or recurrent dose reductions could be made for individual participants and were made in 12.1 percent of patients for naltrexone, compared with 11.9 percent for acamprosate, 20.9 percent for acamprosate plus naltrexone, and 7.8 percent for placebo. On average, 88 mg of naltrexone was taken daily, and the mean medication adherence rate for naltrexone was 85.4 percent—similar to the adherence rates for those receiving acamprosate or combined behavioral interventions. The COMBINE study confirmed the efficacy of naltrexone in reducing drinking among volunteers who were newly abstinent from alcohol. Key findings included the following:
- Participants receiving naltrexone plus medical management had a higher percentage of days abstinent (80.6 percent) than those receiving placebos and medical management only (75.1 percent).
- Naltrexone reduced the risk of a first heavy drinking day over time; the reduction in risk was 0.28, consistent with meta-analyses of other naltrexone trials that used 50 mg per day and included specialist care ( Anton et al., 2006 ).
Other findings from the COMBINE study are detailed in the sections on acamprosate and on combined medication therapy.
Adverse events. Some researchers have attributed the low degree of compliance with naltrexone to poor tolerability and hepatic toxicity ( Volpicelli et al., 1997 ). However, a recent meta-analysis of naltrexone studies concluded that only 10 percent of patients fail to complete treatment because of one or more adverse drug effects and that hepatic toxicity is very unlikely at the current dose of 50 mg of oral naltrexone daily ( Bouza, Magro, Muñoz, & Amate, 2004 ; Yen, Ko, Tang, Lu, & Hong, 2006 ). Yen and colleagues (2006) concluded that naltrexone is not hepatotoxic at the recommended daily dose and may be beneficial for patients with elevated liver enzymes. In the COMBINE study, with its higher naltrexone dosage, only 1 of 70 serious adverse events could have been related to the medication. Of the 601 participants, 12 (primarily those in the naltrexone groups) had treatment-emergent levels of liver enzymes (aspartate aminotransferase or alanine aminotransferase) greater than five times the upper limit of normal; these cases resolved once the medication was discontinued except for two cases (one participant did not return for retesting and the other continued heavy drinking) ( Anton et al., 2006 ).
Conditions excluding treatment with naltrexone. Patients are ineligible for naltrexone if they have poor liver function or a history of liver disease, have recent prescribed or nonprescribed opioid use, and, for women, are pregnant or not using adequate birth control ( Rohsenow, 2004 ). Absolute contraindications to naltrexone use include acute hepatitis, liver failure, active opioid withdrawal, and the current use of methadone or opioid-containing medications prescribed to manage pain and treat serious medical conditions, such as heart disease, severe arthritis, sickle cell anemia, and recurrent congestive heart failure ( CSAT, 1998 ). A relative contraindication applies to patients who have an anticipated need for opioids to treat an identified medical problem, because use of naltrexone can impede the effectiveness of prescription and over-the-counter analgesics, cough medicines, and pain medications that contain opioids ( CSAT, 1998 ).
Patients appropriate for naltrexone. Current research suggests that the patients most likely to benefit from naltrexone are those who have close relatives with alcohol problems, have particularly strong urges to drink, or have limited cognitive abilities ( Rohsenow, 2004 ). Naltrexone is also well tolerated in older patients ( Oslin et al., 1997a ). People with lower blood concentrations of the drug may benefit from a larger dose, and those with good results on naltrexone may benefit from longer maintenance ( Rohsenow, 2004 ).
More research is needed on which subgroups of patients are most likely to respond well to naltrexone, as well as to other pharmacotherapies. In a recent controlled trial in Germany, Kiefer, Helwig, Tarnaske, Otte, and Wiedemann (2005a) looked at the response to naltrexone and acamprosate by patients who had (1) low versus high baseline somatic distress, depression, and anxiety, (2) low versus high baseline craving, and (3) typological differentiation according to the subtypes proposed by Cloninger and Lesch ( Lesch & Walter, 1996 ). A comparison of the course of abstinence rates indicated that naltrexone was effective particularly in patients with high baseline depression, whereas acamprosate was mainly efficacious in patients with low baseline somatic distress. Baseline craving showed no predictive value. Naltrexone revealed best treatment effects in Lesch’s types III and IV typology, whereas acamprosate was mainly effective in type I ( Kiefer et al., 2005a ).
Some researchers hope that it may become possible to choose therapy based on identification of genetic subtypes of the specific molecular targets for drugs. For example, because a family history of alcohol problems is a predictor of naltrexone response, it is hypothesized that a gene variation of the OPRM1 may increase an individual’s susceptibility to substance dependence, as well as increase the response to naltrexone. To test this, a recent randomized study of patients with alcohol dependence examined the association between their treatment outcomes and two specific polymorphisms of the gene encoding the OPRM1. In subjects of European descent, individuals with one or two copies of the Asp40 allele who were treated with naltrexone had significantly lower rates of relapse and a longer time to return to heavy drinking than those homozygous for the Asn40 allele ( Oslin et al., 2003 ). If these results are replicated, then gene testing may be a feasible and cost-effective way to identify individuals who are most likely to respond to naltrexone treatment.
Research by McGeary and colleagues (2006) found that, among non-treatment-seeking heavy drinkers, all of naltrexone’s moderating effects on craving and on a cue-elicited urge to drink could be accounted for by Asn40Asp polymorphisms in the OPRM1 gene. However, in a study of veterans being treated for alcohol dependence, Gelernter and colleagues (2007) found no significant interactions between the OPRM1 Asn40Asp polymorphisms and the response to naltrexone treatment. Oslin, Berrettini, and O’Brien (2006) reviewed the current research agenda and the biological correlates of the receptor genes that have been demonstrated to predict clinical response to naltrexone in individuals who are dependent on alcohol.
Extended-Release Injectable Naltrexone
On April 13, 2006, FDA approved the marketing application of Alkermes, Inc., for its extended-release injectable form of naltrexone with the trade name Vivitrol ® (formerly Vivitrex ® ). Vivitrol is a once-monthly, single-dose 380 mg intramuscular injectable medication that uses a proprietary Medisorb ® drug delivery technology. Vivitrol became commercially available on June 13, 2006, through a limited network of specialty pharmacy providers.
A second company, DrugAbuse Sciences, also has an injectable naltrexone formulation called Naltrel in Stage III clinical trials. A third injectable formulation, Depotrex ® , is under development.
The extended-release injectable formulation of naltrexone was developed to address the problem of compliance with oral naltrexone. The long-acting injectable formulation offers a number of advantages. An intramuscular injection is needed monthly instead of daily, which ensures that patients are exposed to the medication for at least the first month. This monthly, extended-release injection eliminates the need for daily self-dosing and reduces the opportunity for patients to discontinue their medication impulsively. Any discontinuation in therapy would come to the attention of the physician or healthcare provider who is administering the injections. The long-acting formulation also produces a more consistent and predictable drug blood level than oral naltrexone ( Dunbar et al., 2006 ). The injectable form eliminates first-pass metabolism, while reducing the repetitive peak-to‐trough plasma naltrexone levels associated with daily oral naltrexone administration ( Dunbar et al., 2006 ; Johnson et al., 2004 ).
Research on injectable naltrexone
Several formulations of injectable naltrexone have been tested in pilot studies and clinical trials since 1998, and all studies have shown the injectable long-acting formulation to be safe, well tolerated, and effective in reducing heavy drinking days and other measures of problem drinking. Although most studies involved small numbers of subjects, developers of the two investigational formulations have published multicenter, randomized, placebo-controlled clinical trials ( Garbutt et al., 2005 ; Kranzler et al., 2004 ). Studies done to date include the following:
- Preliminary studies. In a 12-week study of an injectable naltrexone formulation (Depotrex) combined with 8 weekly coping skills sessions, the 15 patients on 206 mg of daily naltrexone had fewer drinking days than 5 patients on placebo, supporting continued research on the sustained-release drug ( Kranzler, Modesto-Lowe, & Nuwayser, 1998 ). An 8-week study of the same injectable formulation among 12 subjects who were heroin dependent showed that both low- (192 mg) and high-dose (384 mg) injections were safe and effective and produced long-lasting antagonism to the effects of heroin ( Comer et al., 2002 ). Few adverse effects were reported except for mild discomfort at the injection site, and blood plasma levels remained above 1 ng/ml for 3 to 4 weeks after the injection.
- DrugAbuse Sciences Naltrexone Depot Study Group. This group conducted the first multicenter study of injectable naltrexone (Naltrel) for alcohol dependence, randomly assigning 315 patients either to an intramuscular injection of naltrexone monthly for 3 months or to placebo; all subjects received five sessions of manual- guided MET ( Kranzler et al., 2004 ). The medication was well tolerated, with approximately 74 percent of subjects receiving all injections. For those taking injectable naltrexone, there was a significant advantage over placebo in time to first drinking day, fewer drinking days during treatment, and a significantly greater abstinence rate than for the placebo group (18 vs. 10 percent). Earlier studies, including a 6-week, open-label trial of one 300 mg injection among 16 subjects, combined with weekly individual counseling sessions, found no serious adverse events and a significant reduction in the number of drinks per day, heavy drinking days, and the proportion of drinking days compared with baseline ( Galloway, Koch, Cello, & Smith, 2005 ; Modesto-Lowe, 2002 ).
- Vivitrex ® Study Group. This group conducted a 6-month, double-blind, placebo-controlled trial of long-acting injectable naltrexone, using two different doses, at 24 U.S. public hospitals, private and VA clinics, and tertiary care medical centers. Adults who were actively drinking were randomized to naltrexone treatment or placebo, and 624 received at least one injection. All subjects received 12 sessions of a low-intensity psychosocial intervention. Compared with placebo, the high (380 mg) dose resulted in a 25-percent decrease in the event rate of heavy drinking days, whereas the low (190 mg) dose resulted in a 17-percent decrease. The long-acting naltrexone was well tolerated, and there was no evidence of hepatotoxicity ( Garbutt et al., 2005 ). An earlier 16-week, multisite pilot study had shown the formulation to be both safe and well tolerated ( Johnson et al., 2004 ).
Injectable naltrexone use in primary care
Now that it has been approved for marketing by FDA, injectable naltrexone is available as a treatment option that can be used by primary care practitioners and addiction specialists. In the multisite trial, the efficacy of the 380 mg dose was evident in the first month after the initial injection and was maintained over the 24-week treatment period ( Garbutt et al., 2005 ). Unlike patients in the oral naltrexone trials, the majority of patients were actively drinking when they started injectable naltrexone treatment. However, the FDA Center for Drug Evaluation and Research (CDER) analysis of the study data concluded that injectable naltrexone was effective only in those who were abstinent at baseline. CDER’s analysis emphasized the proportion of patients who did not relapse to heavy drinking (FDA CDER, personal communication, 2008).
Adverse events. In patients using oral naltrexone, high urinary levels of 6-β-naltrexol have been associated with adverse events, such as headache, anxiety, nausea, and spontaneous erection ( King, Volpicelli, Gunduz, O’Brien, & Kreek, 1997 ). In a multicenter study, at least 15 percent of individuals withdrew from oral naltrexone treatment because of adverse events, particularly nausea ( Croop, Faulkner, & Labriola, 1997 ). In contrast to oral naltrexone, plasma levels of extended-release naltrexone remain relatively constant among patients taking the injectable formulation, which may be one reason for its milder adverse effects. The lack of first-pass metabolism with injectable formulations, with reduced levels of 6-β-naltrexol, may also contribute to its improved adverse-event profile ( Johnson, 2006 ). The peak plasma concentration of injectable preparations exceeds that of oral naltrexone during the days immediately following the injection. The higher tolerability of injectable naltrexone may be because such peaks occur daily with oral therapy but only early in treatment with the injectable formulations ( Johnson, 2006 ). The following side effects were observed in the clinical trials of injectable naltrexone:
- Vivitrol. High doses (400 mg) of Vivitrol seemed to be safe and well tolerated in the 16-week clinical trial, with the four most common side effects among 40 subjects being nausea, headaches, nonspecific abdominal pain, and pain at the injection site. Two subjects dropped out from adverse effects—one from induration at the injection site and one from an allergic reaction resulting in angioedema ( Johnson et al., 2004 ). In the longer 24-week clinical trial, subjects who received the high (380 mg) dose of Vivitrol were significantly more likely to report nausea, fatigue, decreased appetite, dizziness, and pain at the injection site than those in the low (190 mg) dose or placebo groups ( Garbutt et al., 2005 ). Among subjects in the high-dose Vivitrol group, 14.1 percent discontinued treatment compared with 6.7 percent of those in both the low-dose and the placebo groups. Two of the high-dose subjects dropped out because of serious adverse events—allergic-type eosinophilic pneumonia and interstitial pneumonia—which resolved following medical treatment. The most frequent reasons for dropping out of the study were nausea, injection site reactions, and headaches ( Garbutt et al., 2005 ).
- Naltrel. In general, the first 12-week clinical trial showed Naltrel to be safe and well tolerated at an initial dose of 300 mg (one 150 mg injection in each buttock), with subsequent doses being only 150 mg ( Kranzler et al., 2004 ). Side effects, including upper abdominal pain, chest pain, and injection site reactions, were significantly more common in the group taking Naltrel than in those taking placebo. Reasons for discontinuing treatment were similar in the Naltrel and placebo groups, although 13 subjects taking Naltrel (8.2 percent) dropped out of treatment, compared with 6 subjects (3.8 percent) in the placebo group. No serious adverse events were reported in a subsequent 6-week, open-label trial. Sixteen subjects, followed for 6 weeks after a single 300 mg dose of Naltrel intramuscularly, reported 198 side effects. The 17 side effects rated as severe included nausea, flatulence, gastrointestinal pain, fatigue, lethargy, somnolence (two reports), depression, irritability, headache (four reports from three participants), back pain, injection site mass, injection site pain, and an elevated GGT level ( Galloway et al., 2005 ).
In light of one diagnosed and one suspected case of eosinophilic pneumonia in the Vivitrol trials, the manufacturer recommends that physicians consider a diagnosis of eosinophilic pneumonia in any patient receiving injectable naltrexone who develops progressive dyspnea and hypoxemia, as well as the possibility of eosinophilic pneumonia in patients who do not respond to antibiotics.
Conditions excluding treatment with injectable naltrexone. More research is needed to determine whether injectable naltrexone is associated with unexpected adverse or allergic reactions because three subjects in the Vivitrol trials had angioedema or pneumonia, an allergic-type reaction rate of 1:218. The Vivitrol manufacturer states that patients should not be actively drinking at the time Vivitrol is initially administered and that the medication is contraindicated in patients who have previously exhibited hypersensitivity to naltrexone, polylactide-co-glycolide, carboxymethylcellulose, or any other components of the diluent.
Injectable naltrexone is a potent opioid antagonist. Contraindications to the oral form of naltrexone also pertain to the injectable formulations, including the following:
- In patients with acute hepatitis or liver failure and patients with active liver disease, injectable naltrexone must be carefully considered, given naltrexone’s hepatotoxic effects. Use of injectable naltrexone should be discontinued in the event of symptoms or signs of acute hepatitis.
- In patients who are receiving opioid analgesics, patients with current physiologic opioid dependence, and patients in acute opioid withdrawal.
- In individuals who have failed the naloxone challenge test or have a positive urine screen for opioids.
There appears to be at most a fivefold margin between a safe dose of naltrexone and a dose that can cause hepatic injury. Injectable naltrexone at recommended doses does not appear to be a hepatotoxin.
Patients appropriate for injectable naltrexone. In reviewing the clinical evidence on injectable naltrexone, Johnson (2006) concludes that injectable naltrexone could benefit individuals who have failed at outpatient treatment using adjunctive medication, as well as other target populations. Such patients with alcohol dependence include the following:
- Patients who show low compliance with medication resulting from nonspecific factors, such as memory impairment
- Patients who experience marked or prolonged side effects from taking oral naltrexone
- Individuals who experience relatively low therapeutic effects from oral naltrexone, suggesting that a trial with an injectable preparation be done to rule out fluctuating blood levels of naltrexone as a possible cause
- Individuals who expect to be in situations where oral naltrexone might be unavailable or difficult to obtain if lost, such as overseas travelers or military personnel on short assignments
- Individuals detoxified in hospitals and awaiting referral to outpatient treatment so that medication can be available to these patients during the hiatus between detoxification and treatment
- Individuals with co-occurring alcohol and psychiatric disorders, for whom the injectable form would reduce the need for additional pills
- Offenders in forensic facilities or drug courts who could be offered the option of imprisonment or supervised treatment with injectable preparations; clear guidelines and protocols must guide the ethical use of injectable naltrexone in forensic settings.
As reported in the section on oral naltrexone, research demonstrates that people with a family history of alcoholism seem to respond best to this medication. The trials on injectable naltrexone have not explored the connection between such characteristics in men and women and the efficacy of the injectable formulation.
Estimates of efficacy—with a small to medium effect size—seem to be comparable in men taking Vivitrol or Naltrel ( Johnson, 2006 ). However, the potential benefit of injectable naltrexone for women is unclear. The multisite clinical trials showed that injectable naltrexone can reduce heavy drinking in men, but no significant effects were shown in women. Injectable naltrexone seems to effectively reduce relapse and promote abstinence among individuals who are alcohol dependent, but the two published trials did not explore the effect of gender on treatment outcomes.
Injectable vs. Oral Naltrexone
No direct studies have compared the efficacies of oral and injectable naltrexone. The decision on which form of naltrexone to prescribe is likely to be driven by patient characteristics, history, and preferences.
Johnson (2006) delineates five important considerations that need to be resolved if injectable naltrexone is to be used fully in the treatment of alcohol dependence:
- Training of healthcare providers. Providers need training in proper administration of the injections, which will reduce the likelihood of local site reactions and of resulting noncompliance by patients.
- Establishment of precedents in psychiatry for initiating an intramuscular rather than an oral medication. Plausible guidelines might include the use of an injectable preparation after a trial of oral naltrexone has failed (presumably because of low compliance) or after a trial of oral naltrexone has shown no untoward side effects or adverse reactions for the patient. To what extent patients in real-world medical clinics will accept voluntary naltrexone injections is unknown.
- Cost arrangements. Uneven insurance coverage across the United States has hindered the widespread use of oral naltrexone and can be a potential problem for injectable naltrexone. Injectable naltrexone could be limited to patients who have private insurance policies or self-pay.
- Flexible planning for adequate psychosocial support and monitoring of patient care. Injectable naltrexone preparations have been tested only in conjunction with psychosocial support, which will be particularly important for patients coming in monthly for injections. An adequate standard of patient care will require a flexible approach that can provide such features as initial heightened support to establish a firm therapeutic alliance and a safety net in case of relapse.
- Attention to emerging knowledge about combining other medications with injectable naltrexone. Preliminary studies suggest that adding other medications may augment the efficacy of naltrexone. If these studies are confirmed, the injectable form of naltrexone will offer important advantages, such as a lowered risk of kinetic interactions, enhanced patient compliance, and a potential for increased pharmacodynamic response against a platform of stable naltrexone levels in the blood.
The Vivitrol trial, which represents one of the largest samples ever treated with a medication for alcohol dependence, shows that this formulation could improve intervention strategies for alcohol dependence because it can provide a predictable pharmacological foundation for treatment. In addition, it has the clinical benefit of providing a firm basis for combination with other treatments, including psychotherapy, other medications, or both. Additional research will resolve the following issues raised by the multisite trial:
- Better understanding of the effects of injectable naltrexone on women. Treatment effects were highly significant among men taking 380 mg injectable naltrexone but not significant in women. Because only a small number of women were included in the Vivitrol study, they may not be representative of women with alcohol dependence in general. Also, women’s typical heightened response to psychosocial interventions may obscure the medication effects ( Garbutt et al., 2005 ). A recent study found that drinking outcomes with oral naltrexone seemed to be superior for women compared with men ( Kiefer, Jahn, & Wiedemann, 2005b ). In light of this finding, it has been suggested that the injection delivery method may inhibit its effectiveness for women. The injections may have more frequently been delivered subcutaneously rather than intramuscularly in women, thereby slowing absorption ( Johnson, 2006 ). Recent research on the efficacy of medications injected intramuscularly in the buttocks showed that the higher percentage of body fat in women frequently causes injections into fat rather than into muscle, which can be prevented through use of longer needles. More research is needed.
- More knowledge about treatment duration and special populations. Additional research is needed to determine the optimal duration of treatment with long-acting naltrexone, as well as indicators that treatment can be discontinued. The usefulness of injectable naltrexone for special populations, such as people with a major mental disorder or those in the criminal justice system, is yet to be examined.
Acamprosate
Acamprosate calcium delayed-release tablets were approved by FDA on July 29, 2004, for treating AUDs in patients who have completed withdrawal from alcohol. Acamprosate, manufactured by Merck KGaA and marketed by Forest Laboratories, Inc., under the brand name Campral ® , became available to U.S. physicians, patients, and pharmacies on January 11, 2005. The FDA-approved labeling for acamprosate, which is available at the FDA’s Web site ( http://www.fda.gov ), recommends that acamprosate be used in conjunction with “a comprehensive management program that includes psychosocial support.” FDA approval was based on short- and long-term efficacy and safety data from four double-blind, placebo-controlled randomized trials comparing Campral plus psychotherapy with placebo plus psychotherapy ( Forest Laboratories, Inc., 2005 ). In the three 90- to 360-day trials, which required patients to be abstinent before starting the medication, a greater percentage of those taking acamprosate rather than placebo remained abstinent.
The mechanism of action by which acamprosate maintains abstinence from alcohol is not completely understood, but it differs from the modes of naltrexone or disulfiram. Whereas naltrexone blocks the endogenous opioid reward system, acamprosate is believed to act on neurotransmitter systems in the brain that have been altered by alcohol abuse, returning them from a hyperactive to a normal state. Acamprosate has a structure similar to GABA. It is an inhibitory modulator of NMDA-type excitatory amino acid receptors, perhaps acting indirectly via metabotropic glutamate receptors. It is hypothesized that acamprosate interacts with the glutamate neurotransmitter system thereby regulating the glutamatergic system, which reduces symptoms of withdrawal (reviewed by Litten et al., 2005 ; Myrick & Anton, 2004 ). Acamprosate thus may block protracted withdrawal symptoms that could contribute to relapse ( Myrick & Anton, 2004 ). According to De Witte, Littleton, Parot, and Koob (2005) , emerging evidence suggests that acamprosate interacts with excitatory glutamergic neurotransmission in general and as an antagonist of the metabotropic glutamate receptor subtype 5 in particular—which provides a unifying, satisfactory hypothesis to explain the diverse neurochemical effects of this medication.
Because of acamprosate’s poor absorption, the recommended dose of Campral is two 333 mg tablets taken three times daily to provide a daily 2 g dose ( Forest Laboratories, Inc., 2005 ; Sofuoglu & Kosten, 2004 ). Recent U.S. trials have used Campral at an exploratory level of 3 g per day; acamprosate remained safe and well tolerated in a broadly inclusive sample of subjects ( Anton et al., 2006 ; Mason et al., 2006 ).
Research on acamprosate
Over the past 15 years, the safety and efficacy of acamprosate for alcohol dependence have been well established in multiple double-blind, placebo-based trials ( Mason, 2005 ). Overall, in numerous European trials, acamprosate has been consistently associated with greater beneficial effects than placebo on the following measures of alcohol abstinence: greater rates of complete abstinence, longer times to first drink, and/or an increased duration of cumulative abstinence ( Mason, 2005 ). Surprisingly, two recent well-designed, multisite studies of U.S. patients have not shown the level of efficacy for acamprosate that is consistently demonstrated among European patients ( Anton et al., 2006 ; Mason et al., 2006 ). In addition, a recent study in Australia found that use of acamprosate did not further improve the significant change that outpatients reported in their subjective health status and psychological well-being as a result of receiving CBT alone for their alcohol dependence ( Feeney, Connor, Young, Tucker, & McPherson, 2006b ).
Acamprosate has been used in conjunction with psychosocial or behavioral counseling to promote abstinence in 26 countries, producing an extensive body of data. By 2000, acamprosate had been studied in 17 randomized, placebo-controlled clinical trials performed in 11 European countries and South Korea and covering approximately 5,000 male and female outpatients. Reviews and several meta-analyses have been done on these trials, which all support the therapeutic effect of acamprosate ( Mann, 2004 ; Mason, 2001 ; Soyka & Chick, 2003 ). Outcomes of the clinical trials of acamprosate at a dose of 1,998 mg per day are listed in Exhibit 2 ( Mann, 2004 ).
Published Placebo-Controlled Clinical Trials of Acamprosate 1,998 mg/day in Alcohol Dependence.
The European acamprosate studies varied in duration from 3 months to more than a year. In 13 of 15 studies, subjects treated with acamprosate had a higher rate of treatment completion, longer time to first drink, and higher abstinence rates compared with subjects treated with placebo ( Mason, 2001 ). In the combined studies, the abstinence rate at the end of treatment in the acamprosate groups was 35 percent versus 21 percent in the placebo groups.
One review of the clinical data concluded that there was some evidence in three studies (those of Chick, Pelc, and Paille and their colleagues) that acamprosate could reduce craving ( Mann, 2004 ). However, other reviewers consider it inaccurate to refer to acamprosate as an anticraving agent; they believe the evidence supports its efficacy only as a medication to prevent relapse, possibly by blocking prolonged withdrawal symptoms ( Mason, 2001 ). Mann (2004) also concluded that, for three studies that failed to show beneficial effects of acamprosate over placebo, the possible reasons were that patient numbers were too small, a 2-month treatment period was used rather than the longer treatment periods used in the other studies, and acamprosate was not started until 25 days after patients had been weaned from alcohol, by which time many subjects were no longer abstinent.
In a recent meta-analysis of 16 studies, the relative benefit of remaining continually abstinent for 6 months after detoxification was quantified as 1.47 for subjects treated with acamprosate compared with subjects receiving placebo ( Mann, Lehert, & Morgan, 2004 ). This meta-analysis also suggested that the relative benefit attributable to acamprosate may increase over time.
Acamprosate use in primary care
Current data suggest that acamprosate may be equally useful in primary care and in specialized substance abuse treatment settings ( Mann, 2004 ). A number of Phase IV studies of acamprosate have been made under naturalistic practice conditions that basically confirm the abstinence rates found in the placebo-controlled trials ( Pelc et al., 2002 ; Soyka, Preuss, & Schuetz, 2002 ). A pragmatic trial in France compared results when 149 general practitioners, who were accustomed to managing patients in their practice, added acamprosate to standard treatment. A very high percentage of patients successfully completed the 1-year followup period (348 of 422 patients or 82.5 percent). The duration of abstinence compared well with the clinical trials: 0.67 for standard care and 0.81 for acamprosate. In clinical practice, this means that patients taking acamprosate could be expected to remain abstinent for 23 percent longer on average than patients on standard care and to experience about 2 months more abstinence during a 1-year treatment period ( Kiritze-Topor et al., 2004 ). However, the principal finding was that adjunctive therapy with acamprosate was associated with a significantly better outcome in patients’ quality of life, based on social, medical, and economic measures.
The first U.S. study to evaluate the clinical efficacy of acamprosate compared the safety and effects of the standard 2 g dose, an exploratory 3 g dose, or placebo in a double-blind, 6‐month trial conducted among 601 volunteers in 21 outpatient clinics across the United States ( Mason et al., 2006 ). All patients received the drug or placebo plus eight concomitant sessions of brief, manual-guided counseling ( http://www.alcoholfree.info ). The main outcome measure was the percentage of alcohol-free days over the 6-month period. Surprisingly, the percentage of abstinent days did not differ significantly across groups in the a priori analysis (54.3 percent for placebo, 56.1 percent for 2 g acamprosate, and 60.7 percent for 3 g). However, the researchers used standardized assessments to characterize the subjects at baseline according to such potential covariates as baseline goal of total abstinence, alcoholism severity, stage of readiness to change, treatment exposure, and such psychological precedents as psychiatric hospitalizations or suicide attempts. Analysis of these covariates showed that acamprosate was associated with a significantly higher percentage of abstinent days than placebo in the subgroup of patients who had a baseline goal of abstinence (58.1 percent for placebo, 70.0 percent for 2 g acamprosate, and 72.5 percent for 3 g). Researchers concluded that acamprosate has an appreciable treatment effect among patients who have abstinence as a treatment goal.
In the COMBINE study, all groups showed substantial reduction in drinking. This multisite, U.S. study used a 3 g daily dose of acamprosate. However, the study found no evidence of efficacy for acamprosate and no evidence of incremental efficacy for combinations of naltrexone, acamprosate, and combined behavioral intervention ( Anton et al., 2006 ). The lack of acamprosate efficacy was unexpected, given the positive results of many previous trials ( Anton et al., 2006 ). The substantial improvement shown by all COMBINE groups, possibly in part as a result of the attention within the study itself (the “Hawthorne effect”), may have lessened the study’s power to show an impact from the acamprosate.
Adverse events. The international and U.S. clinical trials demonstrated a favorable safety and tolerability profile ( Garbutt, West, Carey, Lohr, & Crews, 1999 ; Mason, 2001 ). Side effects are generally mild, with the most frequent side effect being short-term diarrhea that is dose related and transient ( Boothby & Doering, 2005 ). Very few patients drop out of treatment because of adverse effects. There is no risk of alcohol interactions with acamprosate, and there is no abuse potential ( Mason, 2001 ). Participants in the first U.S. multisite trial experienced no deaths or serious drug-related adverse events ( Mason et al., 2006 ). The COMBINE trial, using acamprosate at a 3 g dosage, found no problems with either adverse events or medication adherence ( Anton et al., 2006 ).
Conditions excluding treatment with acamprosate. Acamprosate is contraindicated in patients with severe renal impairment and requires a dose reduction for patients with moderate renal impairment ( Forest Laboratories, Inc., 2005 ). However, this medication may be particularly useful in patients with hepatic impairment and/or liver disease ( Scott, Figgitt, Keam, & Waugh, 2005 ).
Patients appropriate for acamprosate. Mason and colleagues (2006) suggest that their U.S. multisite trial was “perhaps the most definitive evidence to date that acamprosate is not an effective treatment for alcohol dependence in non-motivated and non-abstinent populations.”
Acamprosate is a proven effective intervention for treatment of alcohol dependence. However, acamprosate prevents lapses or relapses only in a minority of patients. Two important questions, therefore, are (1) whether acamprosate is more effective when combined with particular types of psychosocial treatment and (2) whether specific subgroups of patients respond particularly well to acamprosate.
Three Phase IV studies failed to find any significant differences in outcome among various psychosocial treatment groups, which included individual therapy, group therapy, brief therapy, and CBT. A pooled analysis of seven trials, covering 1,485 patients, was unable to identify a positive predictor of efficacy with acamprosate treatment, suggesting that acamprosate can be considered a potentially effective pharmacotherapy for all patients ( Verheul, Lehert, Geerlings, Koeter, & van den Brink, 2005 ). The variables looked at, none of which predicted efficacy with acamprosate, included family history of alcoholism, late age of onset, female gender, high physiological dependence, serious anxiety symptomatology, and severe craving at baseline.
Soyka and Chick (2003) recommend that patients be given an initial prescription trial of acamprosate and, if they manage to abstain, they should continue receiving the drug for 1 year. Both the European and U.S. studies suggest that treatment needs to be initiated as soon as possible after the period of alcohol withdrawal, once the patient has achieved abstinence ( Soyka & Chick, 2003 ). Acamprosate should not be stopped if the patient lapses because this medication appears to have a small effect in reducing drinking following a relapse ( Chick, Lehert, & Landron, 2003 ).
More research is needed to understand the different outcomes of the international versus the U.S. trials on efficacy of acamprosate. Understanding why the research results are discrepant can have important clinical implications. A number of possible reasons have been postulated for the failure of U.S. studies to show the efficacy for acamprosate shown in nearly all international studies:
- Differences in U.S. and European drinking patterns
- Length of clinical trials (European trials are generally longer than U.S. trials)
- More standardized, manual-based psychosocial treatments in U.S. trials, which may result in more consistently improved patient outcomes that reduce the perceived effect of the added medication ( Mason et al., 2006 )
- Differences in length of pretreatment abstinence preceding the medication (COMBINE required only 4 days of abstinence, achieved primarily on an outpatient basis, whereas most positive studies of acamprosate have a longer pretreatment abstinence period established during inpatient treatment) ( Anton et al., 2006 ).
- Combined Medication Therapy
Currently, much scientific and clinical interest focuses on combining therapeutic agents to treat alcohol dependence. This interest is predicated on the hypothesis that multiple neurochemical pathways may be deranged as either “state” or “trait” effects of the drinking behavior, so combining effective medications that work at different neurotransmitters may produce a synergistic or at least added response ( Johnson & Ait-Daoud, 2000 ). Knowledge is growing about how the various neurotransmitters interact in the brains of people who are alcohol dependent, as well as how this interaction may vary in different stages of the addiction. In the meantime, practical trials are being conducted that combine medications that have some demonstrated effectiveness in clinical settings, such as naltrexone and acamprosate. These trials will help determine the treatment response to combination therapies, as well as delineate the subgroups of patients most positively affected by the various combinations.
The treatment field has considerable interest in the use of therapeutic medications alone or in combination to treat patients who have co-occurring alcohol and mental disorders. The rate of substance use is higher among patients who have psychotic-spectrum mental illnesses, such as schizophrenia, schizoaffective disorder, and bipolar disorder. In a recent review of the small but growing body of literature on the use of disulfiram and naltrexone for alcoholism in patients with co-occurring mental disease, Petrakis, Nich, and Ralevski (2006a) concluded that the literature supports the use of these medications for patients with co-occurring psychotic-spectrum disorders. Recent research on pharmacological treatment for such patients includes the following:
- A 12-week randomized clinical trial of disulfiram and naltrexone each alone and in combination was conducted on individuals with Axis I disorders and alcohol dependence who were receiving intensive psychosocial treatment. Compared with controls on placebo, patients with psychotic-spectrum disorder had better alcohol outcomes on an active medication, but no clear advantage was seen for disulfiram, naltrexone, or the combination. Retention rates and medication compliance were high, exceeding 80 percent ( Petrakis et al., 2006a ).
- Both disulfiram and naltrexone were effective and safe in a subgroup of 93 veteran outpatients in this randomized trial who had posttraumatic stress disorder (PTSD) and co-occurring alcohol dependence. Patients had better alcohol outcomes with naltrexone, disulfiram, or the combination than they did on placebo; their overall PTSD psychiatric symptoms also improved ( Petrakis et al., 2006b ).
- A 16-week, open-label pilot study of naltrexone with 34 outpatients who had bipolar disorder and alcohol dependence found that the medication was well tolerated. Patients showed significant improvement on rating scales for depression and mania, and days of alcohol use and craving decreased significantly ( Brown, Beard, Dobbs, & Rush, 2006 ).
Disulfiram Combined With Acamprosate
Concomitant administration of disulfiram with acamprosate may improve the effectiveness of acamprosate. Besson and colleagues (1998) conducted a double-blind study of 118 patients who were randomly given acamprosate or placebo, with both groups stratified for voluntary, concomitant use of disulfiram. Treatment lasted for 360 days, with a 360-day followup. The subgroup that received both medications had better outcomes with regard to duration of its cumulative abstinence than did the subgroups on one or no medication. No adverse interaction occurred in patients taking concomitant disulfiram and acamprosate, with diarrhea being the only significant treatment-induced effect.
Acamprosate Combined With Naltrexone
Since 1995, an extensive body of clinical trial data indicates that both acamprosate and naltrexone are effective in the treatment of alcohol dependence. Clinical trials with acamprosate demonstrate that this drug significantly increases the proportion of patients who remain abstinent after acute detoxification ( Mann et al., 2004 ; Mason, 2001 ). For naltrexone, the most reproducible finding is that it reduces relapse into heavy drinking ( Kranzler & Van Kirk, 2001 ; Streeton & Whelan, 2001 ). Some studies show that naltrexone reduces craving and the desire to drink in social drinkers and in people with alcohol dependence who are both abstinent and nonabstinent ( Kiefer & Wiedemann, 2004 ).
However, each drug has been shown to be effective in only 20 to 50 percent of unselected patients with alcoholism ( Kreek et al., 2002 ). Because the two drugs have different pharmacological mechanisms of action and appear to act on different behavioral aspects of alcohol dependence, combining these drugs might provide greater benefit than either provide alone.
Kiefer and Wiedemann (2004) reviewed three preclinical and four clinical studies published since 2000 on the pharmacologic aspects of combined treatment. Their meta-analysis concluded that the combination of acamprosate with naltrexone seems to be both safe and effective, with no negative effects on safety or cognitive function ( Kiefer & Wiedemann, 2004 ). The available data show no severe adverse events during the combined treatment, with diarrhea and nausea being the most significant side effects. The clinical data showed that combined treatment was superior to both placebo and therapy with acamprosate alone. The synergistic effect of combined treatment remained after 12 weeks of drug-free followup ( Kiefer & Wiedemann, 2004 ). A recent 12-week, single-site study in Australia found that the combination of acamprosate and naltrexone, with CBT, was superior to either medication alone for alcohol abstinence ( Feeney, Connor, Young, Tucker, & McPherson, 2006a ). Naltrexone alone with CBT was slightly less effective on all measures than the combined medication. Studies suggest the following explanations for a potentiated effect from the combined drugs:
- The administration of acamprosate with naltrexone unexpectedly—and significantly—increased the rate and extent of absorption of acamprosate by about 33 percent ( Johnson et al., 2003b ; Mason et al., 2002 ). This suggests that combination treatment may make acamprosate more available systemically with no decrease in tolerability, which may provide clinical advantages.
- Some patient subgroups may respond preferentially to the anticraving effects of either drug—either being “reward cravers” (naltrexone) or “relief cravers” (acamprosate) or because of other as yet undetermined factors ( Kiefer & Wiedemann, 2004 ). Pharmacological anticraving treatment may also be more effective with patients who have early-onset alcohol dependence ( Johnson, Ait-Daoud, & Prihoda, 2000 ). Larger prospective studies need to evaluate whether any factors can predict a positive response to anticraving treatment.
- The combination of naltrexone and acamprosate may produce a more incisive anticraving effect in patients than either drug alone. Such a synergy could result from two drugs interfering with two distinct biological aspects of the craving process—reward and relief craving. If this is true, then it would be unlikely that distinct groups would respond preferentially to either drug ( Keifer & Wiedemann, 2004 ).
Although more testing is needed, the results to date suggest that some patients could benefit from combined acamprosate–naltrexone therapy ( Kiefer & Wiedemann, 2004 ). The combined treatment could benefit particularly those patients who have had an inadequate response to either naltrexone or acamprosate alone ( Kiefer & Wiedemann, 2004 ).
The COMBINE Clinical Trial
So far, only a limited number of controlled clinical trials have been conducted on combination treatments. Both researchers and practitioners have been eagerly awaiting results from the COMBINE study, NIAAA’s sophisticated, 11-site combination therapy trial with almost 1,400 subjects in the context of primary care and other nonspecialty treatment settings. In addition to comparing the efficacy of naltrexone and acamprosate separately and together for 16 weeks, the study also looked at these pharmacotherapies in combination with different intensities of behavioral interventions. (Note: The behavioral interventions integrate successful elements of those evaluated in NIAAA’s Project MATCH.) The COMBINE study had two pilot studies, which showed the safety and feasibility of the approach ( COMBINE Study Research Group, 2003 ).
This randomized clinical trial was conducted from January 2001 to January 2004 among volunteers who were recently alcohol abstinent (median age 44 years) and who had a Diagnostic and Statistical Manual of Mental Disorders ( American Psychiatric Association, 1994 ) diagnosis of primary alcohol dependence. Eight groups of patients received medical management combined with 16 weeks of naltrexone (100 mg/day), acamprosate (3 g/day), both medications, and/or placebos—both with and without a combined behavioral intervention (CBI). Patients were evaluated at 16 weeks and for up to 1 year after treatment, looking at the percentage of days they were abstinent from alcohol and the time elapsed to their first heavy drinking day. Results were as follows:
- All groups showed substantial reductions in drinking.
- A significant interaction was found in those receiving naltrexone + a behavioral intervention. Patients receiving (1) naltrexone + medical management, (2) CBI + medical management + placebos, or (3) naltrexone + CBI + medical management had a higher percentage of days abstinent (80.6, 79.2, and 77.1, respectively) than the 75.1 percent among those who received placebos and medical management only.
- Naltrexone reduced the risk of a heavy drinking day, which was most evident in those receiving medical management but not CBI.
- Acamprosate, unexpectedly, showed no significant effect on drinking versus placebo, either by itself or with any combination of naltrexone, CBI, or both. This result was unexpected, because the study hypothesis and an earlier COMBINE single-site study had supported the combined use of acamprosate and naltrexone ( Kiefer & Wiedemann, 2004 ). However, because all groups in this study, including the control groups, showed significant reductions in drinking, the power necessary to detect a statistically significant difference between groups may have been lost. As Johnson (2006) points out, pharmacotherapy trials increasingly demonstrate that the greatest treatment effect comes from being enrolled in a study irrespective of the treatment condition; this can make any statistically significant difference between the active medication and placebo groups seem relatively small clinically.
A major finding of the COMBINE study was that patients who received medical management with naltrexone or with behavioral intervention or the combination of both fared better on drinking outcomes than those on acamprosate. Other significant findings included the following:
- Medical management in primary care settings can be an effective means of treating alcohol-dependent populations. Most COMBINE groups received a nine-session medical management intervention that focused on enhancing medication adherence and abstinence, using a model that could be adapted by primary care settings. In the context of medical management, naltrexone yielded outcomes similar to those from behavioral treatment provided by substance abuse treatment specialists. Unexpectedly, the patients who received medical management and placebo showed a positive effect over and above that seen in patients who received only specialist-delivered behavioral therapy.
- At 1 year after treatment, the COMBINE study found that the differential effects of treatment still persisted, but these effects were only marginally significant. These results suggest that a number of individuals require either prolonged or intermittent care. This tends to validate previous research suggesting that useful approaches for those who do well during initial treatment would be (1) continued naltrexone and medical monitoring, (2) the continuation of behavioral intervention, or (3) both continued naltrexone–medical monitoring and behavioral intervention ( Anton et al., 2006 ).
- Research on Promising Drugs
People who develop chronic alcoholism, either because of being genetically at risk or because of sustained, persistent heavy use, ultimately develop brain changes ( Anton, 2002 ). Neuroadaptive changes, or sensitized changes, mean that the brains of people with alcohol dependence are definitely different, both from the brains that they had before they started drinking heavily and from the brains of social drinkers. Through medication, physicians can affect alcoholism in three major areas: (1) reward or reinforcement, (2) protracted withdrawal, and (3) disorder or impulse control ( Anton, 2002 ).
Current research on drugs could potentially be effective in each of these areas. For example, nalmefene and ondansetron are drugs being tested that may work on the patient’s reward system. The selective serotonin reuptake inhibitors (SSRIs) and buspirone work on serotonergic systems, trying to stabilize affective or impulsive conditions that may work, along with the reward mechanisms, to increase a person’s risk of relapse to persistent alcohol use ( Anton, 2002 ). A second rationale for the use of serotonergic drugs in alcohol pharmacotherapy is that studies have clearly shown that serotonin modulates the mesolimbic dopamine transmission. It has been suggested that serotonin-dependent activation of dopaminergic neurons in the ventral tegmental area contributes to the reinforcing effects of alcohol consumption ( Tambour & Quertemont, 2007 ).
Because of the increased understanding of the neurobiology of alcoholism, researchers can study combinations of agents that act on different neurotransmitter systems and can potentially enhance the effect of either medication alone. There is particular interest in potential combinations of naltrexone with other drugs for specific conditions. Some preliminary reports suggest that combining naltrexone with ondansetron may be of some use ( Ait-Daoud, Johnson, Prihoda, & Hargita, 2001 ). Some pharmacologic agents now being studied include the following:
- Opiate receptor antagonists. Nalmefene, which may have a profile similar to naltrexone, shows promising activity in single-site pilot studies ( Mann, 2004 ), but a multisite study found no evidence of superior efficacy outcomes with nalmefene treatment over placebo ( Anton et al., 2004 ). A 2005 meta-analysis of the available research concluded that the evidence on nalmefene was insufficient at that time to warrant use ( Srisurapanont & Jarusuraisin, 2005 ). However, a Finnish multisite, randomized double-blind study recently found that targeted nalmefene was more effective than placebo at reducing heavy drinking among 403 subjects in alcohol treatment centers and private general practices ( Karhuvaara et al., 2007 ). This study is described above in Updated Findings From the Literature, October 2007.
- Serotonergic agents. SSRIs have not proved to have much effect on drinking behavior when used alone, but they might be effective in combination with other drugs. Some studies suggest that SSRIs may be useful in reducing alcohol use among people with Type A alcoholism, as classified by Babor and colleagues ( Myrick & Anton, 2004 ) and that SSRIs may be just as effective for treating depression in people who are alcohol dependent as in those without alcohol problems ( Anton, 2002 ). The serotonin type-3 antagonist ondansetron has shown promise in subjects with early-onset alcohol dependence but needs more extensive study ( Anton & Swift, 2003 ). Some preliminary reports also suggest that combining naltrexone with SSRIs may be of some use.
- Anticonvulsant agents. Double-blind, placebo-controlled trials of carbamazepine, divalproex, and topiramate have shown positive effects on several measures of drinking behavior and craving among patients. In 2007, the anticonvulsant drug topiramate was reported to be a safe, consistent, and efficacious treatment for alcohol dependence in a large, multisite study ( Johnson et al., 2007 ) done to replicate and extend an earlier small, double-blind, placebo-controlled trial. The positive findings of this large, multisite trial of topiramate are described above in Updated Findings from the Literature, October 2007.
- Extent of Pharmacotherapy Use by Medical Care Providers
Only a small percentage of practitioners use the available pharmacotherapies for treating addiction. This pattern of underuse is found in every professional group studied, including general practitioners, family physicians, VA physicians, and addiction psychiatrists ( Petrakis, Leslie, & Rosenheck, 2003 ; Thomas et al., 2003 ). For example, one study found that addiction specialists were prescribing naltrexone to only 3 to 13 percent of their patients ( Mark, Kranzler, & Song, 2003b ). If pharmacotherapies are to be used at an optimum level, then medical administrators and specialty treatment programs will need to address the reasons for physicians’ reluctance to use these medications. Reasons for nonuse tend to be the same across studies and include the following:
- Lack of awareness about the medication (pharmaceutical companies have not provided information about drugs for addiction—to professionals or consumers—as they have for other medications)
- Lack of knowledge about efficacy of the drug in practice, as well as a perceived lack of evidence that the drug would be effective
- The time required for patient management
- Lack of reimbursement and inability of patients to pay for the drug ( Mark et al., 2003a ).
When physicians have more information about a drug, they prescribe it more ( Mark et al., 2003b ). A drug such as naltrexone is used more often when the treatment organization in which physicians work promotes its use ( Thomas et al., 2003 ). In addition, several studies indicate that patients have better outcomes when the physician believes that a medication will be effective. Several studies, particularly those pertaining to methadone or buprenorphine, reported that training of physicians resulted in much more positive attitudes about treating patients who are drug dependent and about the value of pharmacotherapy ( McCarty, Rieckmann, Green, Gallon, & Knudsen, 2004 ).
Although substance use disorders constitute one of the most significant public health issues in the United States, there is evidence that physicians frequently do not appropriately screen, diagnose, provide treatment interventions, or make referrals to specialists for patients with these disorders ( AMA Council on Medical Education, 2007 ). Physicians receive little or no training on treating addictions during medical school. In 2005–2006, just 46 percent of U.S. medical schools offered both required and elective course hours on the topic of substance abuse, and the mean number of course hours required was less than 16 ( AMA Council on Medical Education, 2007 ). It is becoming increasingly important that physicians gain more professional knowledge in this area.
- Ait-Daoud N, Johnson BA, Prihoda TJ, Hargita ID. Combining ondansetron and naltrexone reduces craving among biologically predisposed alcoholics: Preliminary clinical evidence. Psychopharmacology. 2001; 154 :23–27. [ PubMed : 11292002 ]
- Ait-Daoud N, Malcolm RJ, Johnson BA. An overview of medications for the treatment of alcohol withdrawal and alcohol dependence with an emphasis on the use of older and newer anticonvulsants. Addictive Behaviors. 2006; 31 (9):1628–1649. [ PubMed : 16472931 ]
- AMA Council on Medical Education. Report 11: The status of education in substance use disorders in America’s medical schools and residency programs. 2007. Retrieved October 29, 2007, from http://www .ama-assn.org /ama1/pub/upload/mm/377/a-07cmerpt11 .pdf .
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: Author; 1994. 4th ed., text revision.
- Anton R. Opioid antagonists alone and in combination with other medications. Symposium III: Pharmacotherapy of Alcoholism; Presentation at the American Academy of Addiction Psychiatry 13th Annual Meeting and Symposium; Las Vegas, NV. Dec, 2002.
- Anton RF. Pharmacologic approaches to the management of alcoholism. Journal of Clinical Psychiatry. 2001; 62 (Suppl 20):11–17. [ PubMed : 11584870 ]
- Anton RF, Moak DH, Latham P, Waid LR, Myrick H, Voronin K, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. Journal of Clinical Psychopharmacology. 2005; 25 (4):349–357. [ PubMed : 16012278 ]
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: Results of a placebo-controlled trial. American Journal of Psychiatry. 1999; 156 (11):1758–1764. [ PubMed : 10553740 ]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study, A randomized controlled trial. JAMA. 2006; 295 (17):2003–2017. [ PubMed : 16670409 ]
- Anton RF, Pettinati H, Zweben A, Kranzler HR, Johnson B, Bohn MJ, et al. A multisite dose ranging study of nalmefene in the treatment of alcohol dependence. Journal of Clinical Psychopharmacology. 2004; 24 (4):421–428. [ PubMed : 15232334 ]
- Anton RF, Swift RM. Current pharmacotherapies of alcoholism: A U.S. perspective. American Journal on Addictions. 2003; 12 (Suppl 1):S53–S68. [ PubMed : 14972780 ]
- Assanangkornchai S, Srisurapanont M. The treatment of alcohol dependence. Current Opinion in Psychiatry. 2007; 20 :222–227. [ PubMed : 17415073 ]
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, et al. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Archives of General Psychiatry. 1992; 49 (8):599–608. [ PubMed : 1637250 ]
- Balldin J, Berglund M, Borg S, Månsson M, Bendtsen P, Franck J, et al. A 6month controlled naltrexone study: Combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcoholism Clinical Experimental Research. 2003; 27 (7):1142–1149. [ PubMed : 12878920 ]
- Baros AM, Latham PK, Moak DH, Voronin K, Anton RF. What role does measuring medication compliance play in evaluating the efficacy of naltrexone? Alcoholism: Clinical and Experimental Research. 2007; 31 (4):596–603. [ PubMed : 17374038 ]
- Barrias JA, Chabac S, Ferreira L, Fonte A, Potgieter AS, Teixeira de Sousa E. Acamprosate: Multicenter Portuguese efficacy and tolerance evaluation study. Psiquiatria Clinica. 1997; 18 :149–160.
- Besson J, Aeby F, Kasas A, Lehert P, Potgieter A. Combined efficacy of acamprosate and disulfiram in the treatment of alcoholism: A controlled study. Alcoholism: Clinical and Experimental Research. 1998; 22 (3):573–579. [ PubMed : 9622434 ]
- Bevilacqua JA, Diaz M, Diaz V, Silva C, Fruns M. Disulfiram neuropathy. Report of 3 cases. La Revista Médica de Chile. 2002; 130 (9):1037–1042. [ PubMed : 12434653 ]
- Boothby LA, Doering PL. Acamprosate for the treatment of alcohol dependence. Clinical Therapeutics. 2005; 27 (6):695–714. [ PubMed : 16117977 ]
- Bouza C, Magro A, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction. 2004; 99 (7):811–828. [ PubMed : 15200577 ]
- Brewer C, Hardt F. Preventing disulfiram hepatitis in alcohol abusers: Inappropriate guidelines and the significance of nickel allergy. Addiction Biology. 1999; 4 (3):303–308. [ PubMed : 20575796 ]
- Brewer C, Meyers RJ, Johnsen J. Does disulfiram help to prevent relapse in alcohol abuse? CNS Drugs. 2000; 14 (5):329–341.
- Brown ES, Beard L, Dobbs L, Rush AJ. Naltrexone in patients with bipolar disorder and alcohol dependence. Depression and Anxiety. 2006; 23 (8):492–495. [ PubMed : 16841344 ]
- Carroll KM, Nich C, Ball SA, McCance E, Frankforter TL, Rounsaville BJ. One-year follow-up of disulfiram and psychotherapy for cocaine–alcohol users: Sustained effects of treatment. Addiction. 2000; 95 (9):1335–1349. [ PubMed : 11048353 ]
- Carroll KM, Rounsaville BJ. A perfect platform: Combining contingency management with medications for drug abuse. American Journal of Drug and Alcohol Abuse. 2007; 33 (3):343–365. [ PMC free article : PMC2367002 ] [ PubMed : 17613963 ]
- Center for Substance Abuse Treatment. Naltrexone and alcoholism treatment. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1998. Treatment Improvement Protocol Series 28. HHS Publication No. (SMA) 98-3206. [ PubMed : 22514834 ]
- Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Safety. 1999; 20 (5):427–435. [ PubMed : 10348093 ]
- Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, et al. A multicentre, randomised, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol & Alcoholism. 2000; 35 (6):587–593. [ PubMed : 11093966 ]
- Chick J, Lehert P, Landron F. Plinius Maior Society. Does acamprosate improve reduction of drinking as well as aiding abstinence? Journal of Psychopharmacology. 2003; 17 (4):397–402. [ PubMed : 14870951 ]
- COMBINE Study Research Group. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: Rationale and methods. Alcohol: Clinical and Experimental Research. 2003; 27 (7):1107–1122. [ PubMed : 12878917 ]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: Long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002; 159 (4):351–360. [ PMC free article : PMC4079470 ] [ PubMed : 11823887 ]
- Croop RS, Faulkner EB, Labriola DF. the Naltrexone Usage Study Group. The safety profile of naltrexone in the treatment of alcoholism: Results from a multicenter usage study. Archives of General Psychiatry. 1997; 54 :1130–1135. [ PubMed : 9400350 ]
- Davidson D, Gulliver SB, Longabaugh R, Wirtz PW, Swift R. Building better cognitive-behavioral therapy: Is broad-spectrum treatment more effective than motivational-enhancement therapy for alcohol-dependent patients treated with naltrexone? Journal of Studies on Alcohol and Drugs. 2007; 68 (2):238–247. [ PubMed : 17286342 ]
- Davidson D, Palfai T, Bird C, Swift R. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcoholism: Clinical and Experimental Research. 1999; 23 :195–203. [ PubMed : 10069545 ]
- De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: Elucidating the mechanism of action. CNS Drugs. 2005; 19 (6):517–537. [ PubMed : 15963001 ]
- Ducharme LJ, Knudsen HK, Roman PM. Trends in the adoption of medications for alcohol dependence. Journal of Clinical Psychopharmacology. 2006; 26 (Suppl 1):S13–S19. [ PubMed : 17114950 ]
- Dunbar JL, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC. Single- and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcoholism: Clinical and Experimental Research. 2006; 30 (3):480–490. [ PubMed : 16499489 ]
- Evans SM, Levin FR, Brooks DJ, Garawi F. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcoholism: Clinical and Experimental Research. 2007; 31 (5):775–782. [ PubMed : 17378918 ]
- Feeney GF, Connor JP, Young RM, Tucker J, McPherson A. Combined acamprosate and naltrexone with cognitive behavioural therapy is superior to either medication alone for alcohol abstinence: A single centre’s experience with pharmacotherapy. Alcohol & Alcoholism. 2006a; 41 (3):321–327. [ PubMed : 16467406 ]
- Feeney GF, Connor JP, Young RM, Tucker J, McPherson A. Is acamprosate use in alcohol dependence treatment reflected in improved subjective health status outcomes beyond cognitive behavioural therapy alone? Journal of Addictive Diseases. 2006b; 25 (4):49–58. [ PubMed : 17088225 ]
- Fernández Miranda JJ, Marina González PA, Montes Pérez M, Diaz González T, Gutiérrez Cienfuegos E, Antuña Diaz MJ, et al. Topiramate as add-on therapy in non-respondent alcohol dependent patients: A 12 month follow-up study. Actas Españolas de Psiquiatría. 2007; 35 (4):236–242. [ PubMed : 17592785 ]
- Forest Laboratories, Inc. Press release: First new treatment for alcoholism in ten years, now available. New York: Author; January 11, 2005. Retrieved June 16, 2005, from http://www .campral.com .
- Fuller RK, Branchey L, Brightwell DR, Derman RM, Emrick CD, Iber FL, et al. Disulfiram treatment of alcoholism: A Veterans Administration Cooperative Study. Journal of the American Medical Association. 1986; 256 :1449–1455. [ PubMed : 3528541 ]
- Fuller RK, Gordis E. For debate: Does disulfiram have a role in alcoholism treatment today? Addiction. 2004; 99 :21–24. [ PubMed : 14678055 ]
- Galloway GP, Koch M, Cello R, Smith DE. Pharmacokinetics, safety, and tolerability of a depot formulation of naltrexone in alcoholics: An open-label trial. BMC Psychiatry. 2005. pp. 18–28. Retrieved May 27, 2005, from http//:www .biomedcentral .com/1471-244X-5-18 . [ PMC free article : PMC1087493 ] [ PubMed : 15804355 ]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, et al. for the Vivitrex Study Group. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: A randomized controlled trial. JAMA. 2005; 293 (13):1617–1625. [ PubMed : 15811981 ]
- Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: A review of the evidence. JAMA. 1999; 281 :1318–1325. [ PubMed : 10208148 ]
- Gastpar M, Bonnet U, Böning J, Mann K, Schmidt LG, Soyka M, et al. Lack of efficacy of naltrexone in the prevention of alcohol relapse: Results from a German multicenter study. Journal of Clinical Psychopharmacology. 2002; 22 (6):592–598. [ PubMed : 12454559 ]
- Geerlings PJ, Ansoms C, Van Der Brink W. Acamprosate and prevention of relapse in alcoholics. European Addiction Research. 1997; 3 :129–137.
- Gelernter J, Gueorguieva R, Kranzler HR, Zhang H, Cramer J, Rosenheck R, et al. the VA Cooperative Study #425 Study Group. Opioid Receptor Gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: Results from the VA Cooperative Study. Alcoholism: Clinical & Experimental Research. 2007; 31 (4):555–563. [ PubMed : 17374034 ]
- Gual A, Lehert P. Acamprosate during and after acute alcohol withdrawal: A double-blind placebo-controlled study in Spain. Alcohol & Alcoholism. 2001; 36 (5):413–418. [ PubMed : 11524307 ]
- Guardia J, Caso C, Arias F, Gual A, Sanahuja J, Ramírez M, et al. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: Results from a multicenter clinical trial. Alcoholism: Clinical and Experimental Research. 2002; 26 (9):1381–1387. [ PubMed : 12351933 ]
- Gueorguieva R, Wu R, Pittman B, Cramer J, Rosenheck RA, O’Malley SS, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalyses of two negative clinical trials. Biological Psychiatry. 2007; 61 (11):1290–1295. [ PMC free article : PMC1952242 ] [ PubMed : 17224132 ]
- Guzik P, Bankes L, Brown TM. Acamprosate and primitive reflexes. Annals of Pharmacotherapy. 2007; 41 (4):715–718. [ PubMed : 17389668 ]
- Heinälä P, Alho H, Kiianmaa K, Lönnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: A factorial double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology. 2001; 21 (3):287–292. [ PubMed : 11386491 ]
- Hersh D, Van Kirk JR, Kranzler HR. Naltrexone treatment of comorbid alcohol and cocaine use disorders. Psychopharmacology (Berl). 1998; 139 (1–2):44–52. [ PubMed : 9768541 ]
- Johnson BA. A synopsis of the pharmacological rationale, properties, and therapeutic effects of depot preparations of naltrexone for treating alcohol dependence. Expert Opinion on Pharmacotherapy. 2006; 7 (8):1065–1073. [ PubMed : 16722816 ]
- Johnson BA, Ait-Daoud N. Neuropharmacological treatments for alcoholism: Scientific basis and clinical findings. Psychopharmacology. 2000; 149 :327–344. [ PubMed : 10867960 ]
- Johnson BA, Ait-Daoud N, Aubin HJ, van den Brink W, Guzzetta R, Loewy J, et al. A pilot evaluation of the safety and tolerability of repeat dose administration of long-acting injectable naltrexone (Vivitrex) in patients with alcohol dependence. Alcoholism: Clinical and Experimental Research. 2004; 28 (9):1356–1361. [ PubMed : 15365306 ]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: A randomized controlled trial. Lancet. 2003a; 361 (9370):1677–1685. [ PubMed : 12767733 ]
- Johnson BA, Ait-Daoud N, Prihoda TJ. Combing ondansetron and naltrexone effectively treats biological predisposed alcoholics: From hypothesis to preliminary clinical evidence. Alcoholism: Clinical Experimental Research. 2000; 24 (5):737–742. [ PubMed : 10832917 ]
- Johnson BA, Mann K, Willenbring ML, Litten RZ, Swift RM, Lesch OM, et al. Challenges and opportunities for medications development in alcoholism: An international perspective on collaborations between academia and industry. Alcoholism: Clinical and Experimental Research. 2005; 29 (8):1528–1540. [ PubMed : 16156050 ]
- Johnson BA, O’Malley SS, Ciraulo DA, Roache JD, Chambers RA, Sarid-Segal O, et al. Dose-ranging kinetics and behavioral pharmacology of naltrexone and acamprosate, both alone and combined, in alcohol-dependent subjects. Journal of Clinical Psychopharmacology. 2003b; 23 (3):281–293. [ PubMed : 12826990 ]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. for the Topiramate for Alcoholism Advisory Board the Topiramate for Alcoholism Study Group. Topiramate for treating alcohol dependence: A randomized controlled trial. JAMA. 2007; 298 (14):1641–1651. [ PubMed : 17925516 ]
- Kampman KM, Pettinati HM, Lynch KG, Whittingham T, Macfadden W, Dackis C, et al. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. Journal of Clinical Psychopharmacology. 2007; 27 (4):344–351. [ PMC free article : PMC3193934 ] [ PubMed : 17632217 ]
- Karhuvaara S, Simojoki K, Virta A, Rosberg M, Löyttyniemi E, Nurminen T, et al. Targeted nalmefene with simple medical management in the treatment of heavy drinkers: A randomized double-blind placebo-controlled multicenter study. Alcoholism: Clinical and Experimental Research. 2007; 31 (7):1179–1187. [ PubMed : 17451401 ]
- Kenna GA, McGeary JE, Swift RM. Pharmacotherapy, pharmacogenomics, and the future of alcohol dependence treatment, Part 1. American Journal of Health System Pharmacology. 2004a; 61 (21):2272–2279. [ PubMed : 15552634 ]
- Kenna GA, McGeary JE, Swift RM. Pharmacotherapy, pharmacogenomics, and the future of alcohol dependence treatment, Part 2. American Journal of Health System Pharmacology. 2004b; 61 (22):2380–2388. [ PubMed : 15581261 ]
- Kenna GA, Nielsen DM, Mello P, Schiesl A, Swift RM. Pharmacotherapy of dual substance abuse and dependence. CNS Drugs. 2007; 21 (3):213–237. [ PubMed : 17338593 ]
- Kiefer F, Helwig H, Tarnaske T, Otte C, Wiedemann K. Pharmacological relapse prevention of alcoholism: Clinical predictors of outcome. European Addiction Research. 2005a; 11 (2):83–91. [ PubMed : 15785069 ]
- Kiefer F, Jahn H, Wiedemann K. A neuroendocrinological hypothesis on gender effects of naltrexone in relapse prevention treatment. Pharmacopsychiatry. 2005b; 38 :184–186. [ PubMed : 16025425 ]
- Kiefer F, Wiedemann K. Combined therapy: What does acamprosate and naltrexone combination tell us? Alcohol & Alcoholism. 2004; 39 (6):542–547. [ PubMed : 15456690 ]
- King AC, Volpicelli JR, Gunduz M, O’Brien CP, Kreek MJ. Naltrexone biotransformation and incidence of subjective side effects: A preliminary study. Alcoholism: Clinical and Experimental Research. 1997; 21 :906–909. [ PubMed : 9267542 ]
- Kiritze-Topor P, Huas D, Rosenzweig C, Comte S, Paille F, Lehert P. A pragmatic trial of acamprosate in the treatment of alcohol dependence in primary care. Alcohol & Alcoholism. 2004; 39 (6):520–527. [ PubMed : 15304381 ]
- Kranzler HR. Evidence-based treatments for alcohol dependence: New results and new questions. JAMA. 2006; 295 (17):2075–2076. [ PubMed : 16670416 ]
- Kranzler HR, Modesto-Lowe V, Nuwayser ES. Sustained-release naltrexone for alcoholism treatment: A preliminary study. Alcoholism: Clinical and Experimental Research. 1998; 22 :1074–1079. [ PubMed : 9726277 ]
- Kranzler HR, Modesto-Lowe V, Van Kirk J. Naltrexone vs. nefazodone for treatment of alcohol dependence: A placebo-controlled trial. Neuropsychopharmacology. 2000; 22 (5):493–503. [ PubMed : 10731624 ]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: A meta-analysis. Alcoholism: Clinical and Experimental Research. 2001; 25 :1335–1341. [ PubMed : 11584154 ]
- Kranzler HR, Wesson DR, Billot L. for the DrugAbuse Sciences Naltrexone Depot Study Group. Naltrexone depot for treatment of alcohol dependence: A multicenter, randomized, placebo-controlled clinical trial. Alcoholism: Clinical and Experimental Research. 2004; 28 (7):1051–1059. [ PubMed : 15252291 ]
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nature Reviews Drug Discovery. 2002; 1 :710–726. [ PubMed : 12209151 ]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. for the Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. New England Journal of Medicine. 2001; 345 (24):1734–1739. [ PubMed : 11742047 ]
- Kulig CC, Beresford TP. Hepatitis C in alcohol dependence: Drinking versus disulfiram. Journal of Addictive Diseases. 2005; 24 (2):77–89. [ PubMed : 15784525 ]
- Laaksonen E, Koski-Jännes A, Salaspuro M, Ahtinen H, Alho H. A randomized, multicentre, open-label, comparative trial of disulfiram, naltrexone and acamprosate in the treatment of alcohol dependence. 2007. Retrieved December 5, 2007, from http://www .alcalc.oxfordjournals.org . [ PubMed : 17965444 ]
- Ladewig D, Knecht T, Lehert P, Fendl A. Acamprosate: A stabilising factor in long-term withdrawal of alcoholic patients. Therapeutische Umschau. 1993; 50 :182–188. [ PubMed : 8475472 ]
- Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: A randomized controlled trial of effectiveness in a standard clinical setting. Medical Journal of Australia. 2002; 176 :530–534. [ PubMed : 12064984 ]
- Lesch OM, Walter H. Subtypes of alcoholism and their role in therapy. Alcohol & Alcoholism. 1996; 1 (Suppl):63–67. [ PubMed : 9845040 ]
- Lhuintre JP, Daoust M, Moore ND, Chretien P, Saligaut C, Tran G, et al. Ability of calcium bis acetyl homotaurine, a GABA agonist, to prevent relapse in weaned alcoholics. Lancet. 1985; 1 (8436):1014–1016. [ PubMed : 2859465 ]
- Lhuintre JP, Moore N, Tran G, Steru L, Langrenon S, Daoust M, et al. Acamprosate appears to decrease alcohol intake in weaned alcoholics. Alcohol and Alcoholism. 1990; 25 (6):613–622. [ PubMed : 2085344 ]
- Litten RZ, Fertig J, Mattson M, Egli M. Development of medications for alcohol use disorders: Recent advances and ongoing challenges. Expert Opinion on Emerging Drugs. 2005; 10 :323–343. [ PubMed : 15934870 ]
- Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: Implications for public health and primary care. Addiction. 2006; 101 (11):1561–1568. [ PubMed : 17034435 ]
- Mann K. Pharmacotherapy of alcohol dependence: A review of the clinical data. CNS Drugs. 2004; 18 (8):485–504. [ PubMed : 15182219 ]
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: Results of a metaanalysis. Alcoholism: Clinical and Experimental Research. 2004; 28 :51–63. [ PubMed : 14745302 ]
- Mark TL, Kranzler HR, Poole VH, Hagen CA, McLeod C, Crosse S. Barriers to the use of medications to treat alcoholism. American Journal on Addictions. 2003a; 12 (4):281–294. [ PubMed : 14504021 ]
- Mark TL, Kranzler HR, Song X. Understanding U.S. addiction physicians’ low rate of naltrexone prescription. Drug and Alcohol Dependency. 2003b; 71 (3):219–228. [ PubMed : 12957340 ]
- Martin BK, Clapp L, Alfers J, Beresford TP. Adherence to court-ordered disulfiram at fifteen months: A naturalistic study. Journal of Substance Abuse Treatment. 2004; 26 (3):233–236. [ PubMed : 15063918 ]
- Mason BJ. Treatment of alcohol-dependent outpatients with acamprosate: A clinical review. Journal of Clinical Psychiatry. 2001; 62 (Suppl 10):42–48. [ PubMed : 11584875 ]
- Mason BJ. Acamprosate and naltrexone treatment for alcohol dependence: An evidence-based risk-benefits assessment. European Neuropsychopharmacology. 2003; 13 (6):469–475. [ PubMed : 14636963 ]
- Mason BJ. Acamprosate in the treatment of alcohol dependence. Expert Opinions on Pharmacotherapy. 2005; 6 (12):2103–2115. [ PubMed : 16197362 ]
- Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: The role of patient motivation. Journal of Psychiatric Research. 2006; 40 :383–393. [ PubMed : 16546214 ]
- Mason BJ, Goodman AM, Dixon RM, Hameed MH, Hulot T, Wesnes K, et al. A pharmacokinetic and pharmacodynamic drug interaction study of acamprosate and naltrexone. Neuropsychopharmacology. 2002; 27 (4):596–606. [ PubMed : 12377396 ]
- McCarty D, Rieckmann T, Green C, Gallon S, Knudsen J. Training rural practitioners to use buprenorphine: Using The Change Book to facilitate technology transfer. Journal of Substance Abuse Treatment. 2004; 26 (3):203–208. [ PubMed : 15063914 ]
- McCaul ME, Wand GS, Stauffer R, Lee SM, Rohde CA. Naltrexone dampens ethanol-induced cardiovascular and hypothalamic-pituitary-adrenal axis activation. Neuropsychopharmacology. 2001; 25 :537–547. [ PubMed : 11557167 ]
- McGeary JE, Monti PM, Rohsenow DJ, Tidey J, Swift R, Miranda R. Genetic moderators of naltrexone’s effects on alcohol cue reactivity. Alcoholism: Clinical and Experimental Research. 2006; 30 (8):1288–1296. [ PubMed : 16899031 ]
- Miller WR, Wilbourne PL. Mesa Grande: A methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002; 97 (3):265–277. [ PubMed : 11964100 ]
- Modesto-Lowe V. Naltrexone depot—DrugAbuse Sciences. IDrugs. 2002; 5 :835–838. [ PubMed : 12802700 ]
- Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O’Brien CP, et al. Predicting treatment response to naltrexone: The influence of craving and family history. American Journal on Addictions. 2001; 10 (3):258–268. [ PubMed : 11579624 ]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, et al. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcoholism: Clinical and Experimental Research. 1999; 23 (8):1386–1394. [ PubMed : 10470982 ]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, et al. Naltrexone and cue exposure with coping and communication skills training for alcoholics: Treatment process and 1-year outcomes. Alcoholism: Clinical and Experimental Research. 2001; 25 (11):1634–1147. [ PubMed : 11707638 ]
- Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: A randomized controlled trial. Addiction. 2001; 96 (11):1565–1573. [ PubMed : 11784454 ]
- Mueser KT, Noordsy DL, Fox L, Wolfe R. Disulfiram treatment for alcoholism in severe mental illness. American Journal on Addictions. 2003; 12 (3):242–252. [ PubMed : 12851020 ]
- Myrick H. Pharmacotherapy of alcoholism: History and current perspectives. Symposium III: Pharmacotherapy of Alcoholism; Presentation at the American Academy of Addiction Psychiatry 13th Annual Meeting and Symposium; Las Vegas, NV. Dec, 2002.
- Myrick H, Anton R. Recent advances in the pharmacotherapy of alcoholism. Current Psychiatry Reports. 2004; 6 :332–338. [ PubMed : 15355755 ]
- Namkoong K, Lee BO, Lee PG, Choi MJ, Lee E. Acamprosate in Korean alcohol-dependent patients: A multi-centre, randomized, double-blind, placebo-controlled study. Alcohol and Alcoholism. 2003; 38 (2):135–141. [ PubMed : 12634260 ]
- Niederhofer H, Staffen W. Comparison of disulfiram and placebo in treatment of alcohol dependence of adolescents. Drug and Alcohol Review. 2003; 22 (3):295–297. [ PubMed : 15385223 ]
- O’Farrell TJ, Allen JP, Litten RZ. Disulfiram (Antabuse) contracts in treatment of alcoholism. Rockville, MD: National Institute on Drug Abuse; 1995. pp. 65–91. NIDA Research Monograph 150. [ PubMed : 8742773 ]
- O’Malley SS, Froehlich JC. Advances in the use of naltrexone: An integration of preclinical and clinical findings. Recent Developments in Alcoholism. 2003; 16 :217–245. [ PubMed : 12638640 ]
- O’Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. Journal of Clinical Psychopharmacology. 2007a; 27 (5):507–512. [ PubMed : 17873686 ]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence: A controlled study. Archives of General Psychiatry. 1992; 49 :881–887. [ PubMed : 1444726 ]
- O’Malley SS, Rounsaville BJ, Farren C, Namkoong K, Wu R, Robinson J, et al. Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs. specialty care: A nested sequence of 3 randomized trials. Archives of Internal Medicine. 2003; 163 :1695–1704. [ PubMed : 12885685 ]
- O’Malley SS, Sinha R, Grilo CM, Capone C, Farren CK, McKee SA, et al. Naltrexone and cognitive behavioral coping skills therapy for the treatment of alcohol drinking and eating disorder features in alcohol-dependent women: A randomized controlled trial. Alcoholism: Clinical and Experimental Research. 2007b; 31 (4):625–634. [ PubMed : 17374042 ]
- Oslin D, Liberto JG, O’Brien J, Krois S. Tolerability of naltrexone in treating older alcohol-dependent patients. American Journal on Addictions. 1997a; 6 (3):266–270. [ PubMed : 9256993 ]
- Oslin D, Liberto JG, O’Brien J, Krois S, Norbeck J. Naltrexone as an adjunctive treatment for older patients with alcohol dependence. American Journal of Geriatric Psychiatry. 1997b; 5 (4):324–332. [ PubMed : 9363289 ]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003; 28 (8):1546–1552. [ PubMed : 12813472 ]
- Oslin DW, Berrettini WH, O’Brien CP. Targeting treatments for alcohol dependence: The pharmacogenetics of naltrexone. Addiction Biology. 2006; 11 (3/4):397–403. [ PubMed : 16961767 ]
- Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol and Alcoholism. 1995; 30 (2):239–347. [ PubMed : 7662044 ]
- Pelc I, Ansoms C, Lehert P, Fischer F, Fuch WJ, Landron F, et al. The European NEAT program: An integrated approach using acamprosate and psychosocial support for the prevention of relapse in alcohol-dependent patients with a statistical modeling of therapy success prediction. Alcoholism: Clinical and Experimental Research. 2002; 26 :1529–1538. [ PubMed : 12394286 ]
- Pelc I, Le Bon O, Verbanck P, Gavrilovic M, Lion K, Lehert P.1992 Calcium acetyl homotaurinate for maintaining abstinence in weaned alcoholic patients: A placebo controlled double-blind multi-centre study Naranjo C, Sellers E, editors. Novel Pharmacological Interventions for Alcoholism 348–352. New York: Springer Verlag;
- Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. British Journal of Psychiatry. 1997; 170 :73–77. [ PubMed : 9328500 ]
- Petrakis IL. A rational approach to the pharmacotherapy of alcohol dependence. Journal of Clinical Psychopharmacology. 2006; 26 (Suppl 1):S3–S12. [ PubMed : 17114952 ]
- Petrakis IL, Leslie D, Rosenheck R. Use of naltrexone in the treatment of alcoholism nationally in the Department of Veterans Affairs. Alcoholism: Clinical and Experimental Research. 2003; 27 :1780–1784. [ PubMed : 14634494 ]
- Petrakis IL, Nich C, Ralevski E. Psychotic spectrum disorders and alcohol abuse: A review of pharmacotherapeutic strategies and a report on the effectiveness of naltrexone and disulfiram. Schizophrenia Bulletin. 2006a; 32 (4):644–654. [ PMC free article : PMC2632271 ] [ PubMed : 16887890 ]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Ralevski E. Naltrexone and disulfiram in patients with alcohol dependence and comorbid post-traumatic stress disorder. Biological Psychiatry. 2006b; 60 (7):777–783. [ PubMed : 17008146 ]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorder. Biological Psychiatry. 2005; 57 :1128–1137. [ PubMed : 15866552 ]
- Petrakis I, Ralevski E, Nich C, Levinson C, Carroll K, Poling J, Rounsaville B. the VA VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and current depression. Journal of Clinical Psychopharmacology. 2007; 27 (2):160–165. [ PubMed : 17414239 ]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SM, Oslin DW, Kampman KM, et al. The status of naltrexone in the treatment of alcohol dependence: Specific effects on heavy drinking. Journal of Clinical Psychopharmacology. 2006; 26 (6):610–625. [ PubMed : 17110818 ]
- Poldrugo F. Acamprosate treatment in a long-term community-based alcohol rehabilitation programme. Addiction. 1997; 92 (11):537–546. [ PubMed : 9519495 ]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: A double-blind placebo-controlled study. Archives of General Psychiatry. 2007; 64 (9):1069–1077. [ PubMed : 17768272 ]
- Rohsenow DJ. What place does naltrexone have in the treatment of alcoholism? CNS Drugs. 2004; 18 (9):547–560. [ PubMed : 15222772 ]
- Rohsenow DJ, Miranda R, McGeary JE, Monti PM. Family history and antisocial traits moderate naltrexone’s effects on heavy drinking in alcoholics. Experimental and Clinical Psychopharmacology. 2007; 15 (3):272–281. [ PubMed : 17563214 ]
- Rosenthal RN. Current and future drug therapies for alcohol dependence. Journal of Clinical Psychopharmacology. 2006; 26 (Suppl 1):S20–S29.
- Roussaux JP, Hers D, Ferauge M. Does acamprosate diminish the appetite for alcohol in weaned alcoholics? Journal of Pharmacy of Belgium. 1996; 51 (2):65–68. [ PubMed : 8786520 ]
- Sass H, Soyka M, Mann K, Zieglgänsberger W. Relapse prevention by acamprosate: Results from a placebo-controlled study on alcohol dependence. Archives of General Psychiatry. 1996; 53 (8):673–680. [ PubMed : 8694680 ]
- Scott LJ, Figgitt DP, Keam SJ, Waugh J. Acamprosate: A review of its use in the maintenance of abstinence in patients with alcohol dependence. CNS Drugs. 2005; 19 (5):445–464. [ PubMed : 15907154 ]
- Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatric Clinics of North America. 2004; 27 :627–648. [ PubMed : 15550284 ]
- Soyka M, Chick J. Use of acamprosate and opioid agonists in the treatment of alcohol dependence: A European perspective. American Journal on Addictions. 2003; 12 (Suppl 1):S69–S80. [ PubMed : 14972781 ]
- Soyka M, Preuss U, Schuetz C. Use of acamprosate and different kinds of psychosocial support in relapse prevention of alcoholism: Results from a non-blind, multicentre study. Drugs in Research and Development. 2002; 3 :1–12. [ PubMed : 11881521 ]
- Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence (Review). Cochrane Database System Review Issue. 2005; 2 (1):CD001867. [ PubMed : 15674887 ]
- Stewart SH, Connors GJ. Interest in pharmacotherapy and primary care alcoholism treatment among medically hospitalized, alcohol dependent patients. Journal of Addictive Diseases. 2007; 26 (2):63–69. [ PubMed : 17594999 ]
- Streeton C, Whelan G. Naltrexone: A relapse prevention maintenance treatment of alcohol dependence: A meta-analysis of randomized controlled trials. Alcohol & Alcoholism. 2001; 36 :544–552. [ PubMed : 11704620 ]
- Suh JJ, Pettinati HM, Kampman KM, O’Brien CP. The status of disulfiram: A half of a century later. Journal of Clinical Psychopharmacology. 2006; 26 (3):290–302. [ PubMed : 16702894 ]
- Tambour S, Quertemont E. Preclinical and clinical pharmacology of alcohol dependence. Fundamental & Clinical Pharmacology. 2007; 21 :9–28. [ PubMed : 17227441 ]
- Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: A placebo-controlled study. Alcohol and Alcoholism. 2000; 35 (2):202–209. [ PubMed : 10787398 ]
- Thomas CP, Wallack SS, Lee S, McCarty D, Swift R. Research to practice: Adoption of naltrexone in alcoholism treatment. Journal of Substance Abuse Treatment. 2003; 24 (1):1–11. [ PubMed : 12646325 ]
- Thomas SE, Miller PM. Knowledge and attitudes about pharmacotherapy for alcoholism: A survey of counselors and administrators in community-based addiction treatment centres. Alcohol & Alcoholism. 2007; 42 (2):113–118. [ PubMed : 17172258 ]
- Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: A review. Alcoholism: Clinical and Experimental Research. 2007; 31 (4):513–536. [ PubMed : 17374031 ]
- Verheul R, Lehert P, Geerlings PJ, Koeter MW, van den Brink W. Predictors of acamprosate efficacy: Results from a pooled analysis of seven European trials including 1,485 alcohol-dependent patients. Psychopharmacology. 2005; 178 (2–3):167–173. [ PubMed : 15322728 ]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry. 1992; 49 :876–880. [ PubMed : 1345133 ]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence: Role of subject compliance. Archives of General Psychiatry. 1997; 54 :737–742. [ PubMed : 9283509 ]
- Weiss RD, Kueppenbender KD. Combining psychosocial treatment with pharmacotherapy for alcohol dependence. Journal of Clinical Psychopharmacology. 2006; 26 (Suppl 1):S37–S42. [ PubMed : 17114954 ]
- Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, et al. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet. 1996; 347 :1438–1442. [ PubMed : 8676626 ]
- Willenbring ML. Medications to treat alcohol dependence: Adding to the continuum of care. JAMA. 2007; 298 (14):1691–1692. [ PubMed : 17925523 ]
- Wright C IV, Vafier JA, Lake CR. Disulfiram-induced fulminating hepatitis: Guidelines for liver-panel monitoring. Journal of Clinical Psychiatry. 1988; 49 :430–434. [ PubMed : 3053669 ]
- Yen MH, Ko HC, Tang FI, Lu RB, Hong JS. Study of hepatotoxicity of naltrexone in the treatment of alcoholism. Alcohol. 2006; 38 (2):117–120. [ PubMed : 16839858 ]
- Cite this Page Center for Substance Abuse Treatment. Incorporating Alcohol Pharmacotherapies Into Medical Practice: A Review of the Literature. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2009. (Treatment Improvement Protocol (TIP) Series, No. 49s.) 1, A Review of the Literature.
- PDF version of this title (725K)
In this Page
Other titles in these collections.
- SAMHSA/CSAT Treatment Improvement Protocols
- Health Services/Technology Assessment Text (HSTAT)
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- A Review of the Literature - Incorporating Alcohol Pharmacotherapies Into Medica... A Review of the Literature - Incorporating Alcohol Pharmacotherapies Into Medical Practice: A Review of the Literature
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

COMMENTS
Abstract. Substance or Drug abuse is a serious public health problem affecting usually adolescents and young adults. It affects both males and females and it is the major source of crimes in youth ...
The numbers for substance use disorders are large, and we need to pay attention to them. Data from the 2018 National Survey on Drug Use and Health suggest that, over the preceding year, 20.3 million people age 12 or older had substance use disorders, and 14.8 million of these cases were attributed to alcohol.When considering other substances, the report estimated that 4.4 million individuals ...
Dec 2021. Explore the latest full-text research PDFs, articles, conference papers, preprints and more on DRUG ADDICTION. Find methods information, sources, references or conduct a literature ...
Any drug use prevalence was 62.4% Alcohol abuse prevalence was 47.8% associations between major depressive disorders and any drug abuse (OR = 3.40, 95% CI 2.01 to 5.76, p < 0.001), or alcohol use (OR = 3.29, 95% CI 1.94 to 5.57, p < 0.001), khasakala et al. 2013 : Cross-sectional: Youth and biological parents attending a youth clinic (Hospital)
Introduction and background. Drug misuse is a widespread issue; in 2016, 5.6% of people aged 15 to 26 reported using drugs at least once [].Because alcohol and illegal drugs represent significant issues for public health and urgent care, children and adolescents frequently visit emergency rooms [].It is well known that younger people take drugs more often than older adults for most drugs.
Table 1. Definitions of Terms Used in Drug Addiction. Drug addiction is a chronic, relapsing disorder in which compulsive drug-seeking and drug-taking behavior persists despite serious negative ...
This paper reviews methodologically rigorous studies examining group treatments for interview-diagnosed drug use disorders. A total of 50 studies reporting on the efficacy of group drug use disorder treatments for adults met inclusion criteria. Studies examining group treatment for cocaine, methamphetamine, marijuana, opioid, mixed substance, and substance use disorder with co-occurring ...
The view that substance addiction is a brain disease, although widely accepted in the neuroscience community, has become subject to acerbic criticism in recent years. These criticisms state that ...
Abstract. Insight refers to a person's understanding of themselves and the world around them. Recent literature has explored people's insight into their substance use disorder (SUD) and how this is linked to treatment adherence, abstinence rates, and comorbid mental health symptoms. The aim of this systematic review was to synthesise and ...
Objectives The burden of substance use in Kenya is significant. The objective of this study was to systematically summarize existing literature on substance use in Kenya, identify research gaps, and provide directions for future research. Methods This systematic review was conducted in line with the PRISMA guidelines. We conducted a search of 5 bibliographic databases (PubMed, PsychINFO, Web ...
In this section, the findings from the literature review are discussed in 4 parts: definition of concept, attributes, antecedents, consequences, and method of measurement of the concept. ... The consequences of recovery from drug addiction are as follow: Sustained control over substance use: Sustained abstinence is an important consequence of ...
Substance abuse during adolescence. The use of substances by youth is described primarily as intermittent or intensive (binge) drinking and characterized by experimentation and expediency (Degenhardt et al., Citation 2016; Morojele & Ramsoomar, Citation 2016; Romo-Avilés et al., Citation 2016).Intermittent or intensive substance use is linked to the adolescent's need for activities that ...
Literature Review Drug Addiction in Prison. The epidemiology of drug addiction is complicated and the direction of causality can be difficult to tease out, but substance use disorder is statistically correlated with social disadvantage and exclusion (McPhee et al., 2019a).
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on DRUG ABUSE. Find methods information, sources, references or conduct a literature review on DRUG ABUSE
Drug abuse is a serious public health problem that affects almost every community and family in some way. Each year drug abuse causes millions of serious illnesses or injuries among Americans. ... The review of the research literature below outlines the association between substance use disorders (SUD) and the various systems it affects. From ...
This literature review focuses on substance use disorder among youths under 18 and on the utilization of substance use treatment programs. ... Parker, K., Diamond, G.S., Barrett, K., and Tejeda, M. 2001. Multidimensional Family Therapy for adolescent drug abuse: Results of a randomized clinical trial. American Journal of Drug and Alcohol Abuse ...
The National Institute on Drug Abuse (NIDA, 2011) suggests that prevention programs should focus on key transition periods during adolescence, particularly the transition from middle school to high school, when youths are at high risk of experimenting with alcohol and other drugs. ... Literature review. Washington, DC: U.S. Department of ...
INTRODUCTION. Substance abuse is a common phenomenon in the world and has invaded the human society as the most important social damage.[1,2] Substance abuse is a nonadaptive model of drug use, which results in adverse problems and consequences, and includes a set of cognitive, behavioral, and psychological symptoms.[]Iran also, due to its specific human and geographic features, has a ...
Indirect costs such as lost workdays. Single (1998) estimates that the law enforcement costs are 29 percent of the total of all costs incurred due to drug and alcohol abuse. Illicit drug use accounts for approximately 7.4 percent of this cost (40.8 percent due to alcohol and 51.8 percent due to tobacco).
Abstract. Substance abuse is one of the primary concerns of every nation all over the world. The medical revolution has both advantages and disadvantages including the side effects of drug abuse. People try drugs to comfort mood like they are living in the unreal world. Gradually, it modifies their behavior by changing their brain and mental ...
Alcohol is the most commonly used substance among adolescents, with 64% of 18 year olds endorsing lifetime alcohol use, followed by marijuana (45%) and cigarette use (31%) ( Johnston et al., 2017 ). Overall, rates of adolescent substance use have remained relatively stable over the past several years, with a few notable exceptions.
2. REVIEW OF LITERATURE. Alcohol, opium and cannabis have been the traditional drugs of use in India with moderate consumption being ritualised in social gatherings. Associated major health or social problems are not obvious since several informal social controls against abuse are in place. Traditional use continues today (Ganguly et al 1995).
This literature review is part of the Substance Abuse and Mental Health Services Administration's (SAMHSA's) Treatment Improvement Protocol (TIP) 49, Incorporating Alcohol Pharmacotherapies Into Medical Practice. Developed by a panel of experts for SAMHSA's Center for Substance Abuse Treatment (CSAT), the TIP can assist physicians and other medical professionals in providing ...