

Clinical Research Certification
Clinical research training.
Our clinical research training program s have supported over 22,000 members in the past 7 years. CCRPS clinical research courses are used by students at 1,200+ organizations, 6 governmental agenies, and 308 universities. Graduates of our clinical research certification have worked at over 1,600 different organizations including all major known CROs and 23% of our graduates have obtained managerial or higher level roles (2024 CCRPS LinkedIn Graduate Survey). We offer personalized clinical research career coaching after clinical research course completion. See April 2024 Graduate Case Studies .
We have major clinical research training accreditations including Transcelerate Biopharma, ACCRE, and offer CME for physicians, nurses, and pharmacists through AMA, ANCC, and ACPE. We are a candidate to become a federally-qualified institution with MSA-CESS.
We are dedicated to our evidence-based and practice-based education philosophy to help you learn clinical research through simply great content unlike what you may have seen before. Clinical research training is self-paced, online, on-demand so you can start today and finish on the go with our mobile app. Speak to our 24/7 chat team or book with a course advisor to see which path is best for you.
Speak to Course and Career Adviser Liz
CCRPS Course Catalog

Advanced Clinical Research Coordinator Certification (ACRCC)
Advanced Clinical Research Coordinator Certification (ACRCC) - Triple-Accredited I 200 Hours I Online I Instant Enrollment I Dual ICH GCP w/ E6 and Advanced CRC Certification I 1+ Week Certification I Tuition with 2-4 Month Payment Plans

Advanced Clinical Research Associate Certification (ACRAC)
A Clinical Research Associate (CRA) assists in the monitoring and compliance of clinical trials. Clinical research associate certification is the leading, accredited, internationally-recognized CRA certification program available online.

Advanced International Pharmacovigilance and Argus Safety Certification (APVASC)
Pharmacovigilance Certification - Advanced Pharmacovigilance & Argus Safety Certification (APVASC) ACCRE Accredited I 180 Hours I Online I Instant Enrollment I I I Globally Recognized I 1 Wk Certification

Advanced ICH GCP Certification (AGCPC)
ICH GCP Certification - The most advanced ICH GCP training available I 50 Hours I On-Demand I Biopharma Recognized I 15+ Modules I Instant Enrollment I 1+ Day Certification I

Advanced Clinical Trial Assistant Certification (ACTAC)
Advanced Clinical Trials Assistant Certification (ACTAC) I Industry-Recognized CTA Training I 100 Hours I On-Demand I Accredited I 25+ Modules I GCP & E6 R2 Complaint I Instant Enrollment I 1+Wk Certification I

Advanced Clinical Research Project Manager Certification (ACRPMC)
Advanced Clinical Research Project Manager Certification provides 150 hours of advanced clinical trials and project management training for clinical research professionals looking to get into managerial roles in the industry.

Advanced Principal Investigator Physician Certification (APIPC)
Industry-Recognized PI Training I Modifiable courses based on prior experience I On-Demand I 17.5 CME I 100+ Modules (only review those you don't know) I GCP Complaint I Instant Enrollment I 1D-2 Wks for Certification

Advanced Physician Medical Monitor Certification (APMMC)
Advanced Physician Medical Monitor Certification (APMMC) Triple-Accredited I Written by Physicians I 17.5 CME AMA/ACCME I 250 Hours I 100+ Modules I GCP Complaint I Instant Enrollment I 2+Wk Certification I

CCRPS Clinical Research Community
CCRPS Reviews
CCRPS Graduate Case Studies April 2024
We’re thrilled to share more graduate case studies from graduates of our program. We value full transparency so full case study transcripts and videos will be available at your request. Please email our case study interviewer Courtney Fulkerson, a clinical research project manager herself, at [email protected] if you have any questions about this process.
Need help deciding on a course or motivating yourself to finish your current course? We want to help you succeed. Speak with our course advisor in clinical research field herself, Liz. Schedule course and career advising session today (email [email protected] your resume before meeting).
1. From IMG to Clinical Research Coordinator at Columbia University: “ This course not only met but exceeded my expectations with its thorough curriculum and insightful modules.” -Lisa-Pierre ( view full case study )
2. From IMG to Clinical Research Coordinator “The hands-on activities integrated throughout the course really helped solidify my understanding of complex concepts.” - Unber Mahmood ( case study summary )
3. Promoted to Senior Startup Specialist in Clinical Trials : “I appreciate how the course was structured—very interactive and engaging from start to finish.” - Justin Scott Brathwaite ( transcript summary )
4. From Physical Therapist to Clinical Researcher: “The in-depth content and expert instructors provided me with invaluable insights into the field.” - Celia Moon ( case study summary )
5. ICH GCP Usability Confidence : “Thanks to this course, I feel more competent and confident in my role.” - Stephanie ( case study summary )
6. Enjoyed Clinical Research Training through Examples “The real-world examples used throughout the course were incredibly useful for applying theory to practice.” -Marta Marszalek ( view full case study )
7. From Clinical Research Receptionist to Certified Study Coordinator with CCRPS: “I highly recommend this course for its comprehensive approach and practical applications.” - Katie Decker ( view full case study )
8. From International CRC to U.S. Lead CRC and CRA: “The flexible online format allowed me to balance my studies with my professional commitments seamlessly.” - Aishwarya Sukumar ( view full case study )
9. Learning to Lead Safety Associate: "The course materials were clear, well-organized, and directly applicable to my work.” - Renata Noronha ( view full case study )
10. From IMG to securing roles as a CRC, CRA, and now a project manager: “Joining this course was a pivotal step in my career advancement.” - Dr. Vrushali Borawak ( view full case study )
11. From Physician to Confident Drug Safety Specialist: “The course provided a robust foundation in the field, which was critical for my professional development.” - Rabiea Bilal ( view full case study )
12. From plant biologist to clinical recruitment administrative coordinator : “This program is a gateway to extensive knowledge and skills in a supportive learning environment.” -Olajumoke Owati ( view full case study )
13. From International PV Roles To North American Market Success: “The detailed modules prepared me excellently for real-world applications.” - John Vinil ( view full case study )
14. From Educational Research to Clinical Trials Project Manager: “I was able to immediately apply what I learned in the course to my job. ” - Rose Hyson ( view full case study )
15. From Masters in Health Safety to Clinical Researcher: " I will say quality of delivery, quality of the materials. - Ossai Opene ( view full case study )
16. ICH GCP made her more confident in research: "this course just overall did a really good job going in depth, which I feel like wasn't just, it wasn't just covered just for the sake of covering content" - Aastha Shah (view full transcript)
17. From Grant Program Manager to Leading Clinical Trials at UCSF : "it really did a great job of the full scope of clinical research from start to finish. Since completing the course, I've received a promotion at work. " -Hannah Fischer (view HF clinical research training case study)
18. From Clinical Research Intern to Regulatory Affairs Associate at UPenn: "I would say since then. I've completed this course. It's helped me get my job in regulatory affairs at a clinical research site." - Scott Boyle (view SB full case study)
19. From Clinical Research Monitor to Chief Medical Officer for CRO: " And CCRPS has a a complete, you have a really, really good approach to that. Because that is what we offer to our sponsors, quality and safety, because we are all physicians." - Maria Lopez (full case study report pending)
18. From International Pharmacist to Pharmacovigilance: The pharmacist detailed challenges becoming a pharmacist in the US and choosing CCRPS for flexibility. Certification benefits were gaining clarity on research topics and standing out for clinical research roles. - Ijeoma Osunwa (full case study report pending)
CRTI Online LLC | Houston, TX 77079 | 1-866-378-8206
Privacy Policy
Student Login
The CRA Training Institute

Training & Onboarding Clinical Research Professionals since 1989
Online cra, crc & cdm clinical research certification courses .
Receive your Accredited Certificate and be
job-ready in as little as 3-4 Weeks!
CRA Certificate Course
COURSE FEE: $49 9.00
DURATION: 3-4 Weeks Average
No Time Limit
DELIVERY: 100% online
Self-Paced Stu dy
START DATE: Immediately
CRC Certificate Course
COURSE FEE: $44 9.00
DURATION: 2-4 Weeks Average
Self-Paced Study
CDM Certificate Course
COURSE FEE: $3 9 9.00
DURATION: 2-3 Weeks
DELIVERY: 100% o nline
Trusted by CROs & Biotech Companies Globally

Curavit Clinical Research

Accreditation
The cra training institute and its online clinical research courses are accredited by the accreditation council for clinical research education (accre)..
Global Program Code
463-04-112-GPC02
www.accre-accredit.org 1840 W Whittier Blvd #4320 La Habra, CA 90631 USA Phone: 1-(888) 512-6760

Student Testimonials
Got hired as a pharmacovigilance officer for IQVIA working with Moderna on their Phase III mRNA vaccine candidate! Thanks so much for your help.
S. Ray, MD, MSc, CRA - Georgia
Job placement assistance.

Professional Resume preparation

Interview preparation

Connecting with Employers
Our Alumni have either found employment with or have been referred from a wide cross-section of hospitals, clinics, CROs, biotech and pharmaceutical companies across North America and globally.
With the knowledge and job skills gained as a result of your study, you can help to meet the ever-growing demand for trained CRAs, CRCs or CDMs to develop exciting new therapeutics for the 21st century in the biotechnology, pharmaceutical and medical device industries.
Clinical Trials in a Nutshell
.png)
Our Accredited Interactive-Online Clinical Research Courses are designed for persons with or without prior on-site clinical trials monitoring experience, seeking qualifications and/or current practical knowledge to effectively work as a Clinical Research Associate (CRA), Clinical Research Coordinator (CRC), or Clinical Data Manager (CDM) in the monitoring of clinical trials globally.
Online CRA, CRC & CDM Clinical Research Courses
ACRP Certification
With a 30-year legacy, acrp certification is the most reputable credentialing program in clinical research. since 1992, more than 40,000 professionals and their employers have come to trust acrp certification as the mark of excellence in clinical research., “joining acrp and becoming certified was the best thing i ever did to jumpstart my career in research and has opened many doors for me.”, jeri burr, ms, rn, ped-bc, ccra, facrp acrp certified since 1999, benefits of certification >, start your next career journey by exploring acrp’s flagship certification and subspecialty credential programs below. each program provides specific information and resources on exam eligibility, exam content, how to apply, scheduling & testing, how to prepare, and after your test. if you have questions, explore the faq page or the acrp certification handbook ., acrp certified professional, acrp-cp ® is a credential formally recognizing clinical research professionals of all types, regardless of their roles or functional activities on the clinical study team., learn more >, certified clinical research associate, ccra ® is a credential formally recognizing clinical research professionals with experience monitoring and supervising the conduct and progress of clinical trials on behalf of a sponsor., certified clinical research coordinator, ccrc ® is a credential formally recognizing clinical research professionals with experience coordinating and facilitating clinical trial activities in adherence to gcp, under the direction of a principal investigator., certified principal investigator, cpi ® is a credential formally recognizing clinical research professionals with experience as a principal investigator or sub investigator on multiple studies., acrp medical device professional, acrp-mdp ® is a credential formally recognizing clinical research professionals with specialized knowledge in medical device clinical trials. candidates must be acrp certified to sit for this exam., acrp project manager, acrp-pm ® is a credential formally recognizing clinical research professionals with specialized knowledge in project management. candidates must be acrp certified to sit for this exam., the academy’s acrp-cp, ccrc, ccra, and cpi programs are accredited by the national commission for certifying agencies (ncca), which sets internationally recognized standards for the development and operation of certification programs. the standards assure that a program is valid, reflects current practice, and treats candidates fairly and are based on the established processes for developing certification exams., the most respected certification thought leaders in the country agree that ncca accreditation is the gold standard when it comes to accreditation of programs that certify professionals working in healthcare—including the clinical research industry. when you’re searching for certification programs, make sure you always look for the ncca-accredited program seal., bring certification to your team —acrp certification is an ideal solution for managers looking to enhance team knowledge, grow leaders, and signify to others that their study teams are among the best of the best. learn more >.
Foundations of Clinical Research
This Harvard Medical School six-month, application-based certificate program provides the essential skill sets and fundamental knowledge required to begin or expand your clinical research career.

Associated Schools

Harvard Medical School
What you'll learn.
Understand and apply the foundational concepts of biostatistics and epidemiology
Develop a research question and formulate a testable hypothesis
Design and begin to implement a clinical research study
Cultivate the skills required to present a clinical research study
Critically evaluate the research findings in medical literature
Synthesize crucial statistical analyses using Stata software
Course description
The Foundations of Clinical Research program is rooted in the belief that clinical research training is critical to professional development in health care. Clinical research training not only creates potential independent investigators, but also enables clinicians to advance their careers through a greater understanding of research evidence. Designed to provide learners with the foundational knowledge and skill sets required to produce high-quality clinical research, our program will lay the fundamental groundwork in epidemiology and biostatistics required for a multifaceted career in clinical research.
The overarching goal of the Foundations of Clinical Research program is to equip the next generation of researchers with the skill sets essential to evaluating evidence, understanding biostatistics, and beginning their clinical research careers. Our aim is to ensure that learners develop a strong foundation in the design, implementation, analysis and interpretation of clinical research studies.
During the program, our innovative active learning approach emphasizes the traditional tutorial system with weekly live video tutorials, seminars and symposia anchored by 3 live intense weekend online workshops. The Foundations of Clinical Research program’s six-month online curriculum emphasizes real-time skill-based learning.
Participants will be eligible for Associate Alumni status upon successful completion of the program. Early tuition and need-based tuition reductions may be available.
Course Outline
Live Workshops
The interactive workshop curriculum will focus on hands-on skill development through active learning. To that end, the intensive schedule is designed to accelerate the growth of high-yield clinical research skills via individual and team-based workshop exercises. Students will be immersed in a dynamic learning environment that encourages collaboration and collegial networking with faculty and peers.
Essential elements of the workshop include instruction and practical exercises in the core concepts of biostatistics, epidemiology and research question development, as well as critical assessment of the medical literature and practical training in statistical software using real-life datasets. In addition to providing training in mentorship, academic career development and leadership, we create a supportive and active learning environment where opportunities for knowledge retention and networking abound.
Live Symposia, Tutorials and Seminars
Symposia, tutorials and seminars are mandatory and will be delivered live online and organized according to eight specific clinical research topics.
Eight 3-Hour Symposia
- Instruction on a specific clinical research topic (e.g., cohort study design and interpretation)
- In-depth discussion on a related epidemiology concept (e.g., odds ratio)
- Hands-on guidance for implementing the related analysis with statistical programming in Stata
Eight 1-Hour Tutorials
- Interpret and report on papers related to the specific clinical research topic
Eight 1-Hour Special-Topic Seminars
- The biostatistical and epidemiological concepts to specific clinical research topics with concrete examples
Assignments
All students will be expected to complete all assignments by the due dates. Assignments will be graded as either “pass” or “fail.”
Individual Assignment 1
Individual Research Question and Study Design
- Generate a novel research question in the evidence-based PICO format
- Receive expert faculty review
Individual Assignment 2
Design, Implement and Present an Original Abstract
- Design and implement a clinical research study based on a publicly available dataset
- Analyze and create data visualizations via a user-friendly R Shiny web app
- Write a formal 350-word abstract suitable for submission to an international conference
- Present a digital poster to faculty at Workshop 3
Online Lectures
Research Study Introduction
- Designing a Clinical Research Study I–III
- Introduction to Evidence-Based Medicine, Systematic Review and Meta-Analysis
- Study Design 1 – Observational
- Study Design 2 – Randomized Controlled Trials
- Study Design 3 – Quasi-Experimental Studies
- Introduction to Biostatistics
- An Investigator’s Responsibility for Protection of Research Subjects
- How to Search PubMed
- Overview of Evidence-Based Medicine
Statistical Programming in Stata
- Loading Data
- Basic Programming Commands
- Data Cleansing
- Data Analytics I – Central Tendency
- Data Analytics II – Statistical Testing
- Data Analytics III – Regression Testing
Instructors

Jamie Robertson

Djøra Soeteman
You may also like.

Global Clinical Scholars Research Training
This Harvard Medical School one-year, application-based certificate program provides advanced training in health care research and methods.

Clinical Drug Development
Learning about the process of clinical drug development has important implications for anyone working in health care and related sectors.
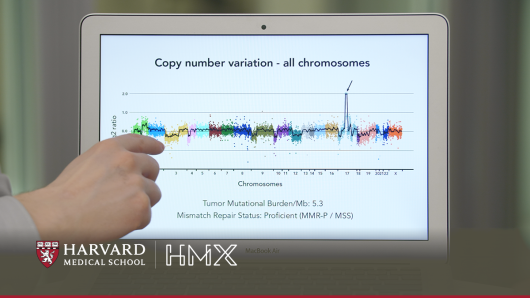

Cancer Genomics and Precision Oncology
Learn how cancer treatment is evolving due to advances in genetics..
Society of Clinical Research Associates
- Courses & Conferences
Become a Member
- Find a Local Chapter
Upcoming Conferences and Courses
Clinical research never stops advancing, neither should you..
In Person Conferences + Workshops
Canadian Regulatory Conference June 13 & 14, 2024 | Toronto, ON, Canada
Project / Program Management October 17 & 18, 2024 | Philadelphia, PA
FDA Clinical Trial Requirements, Regulations, Compliance and GCP Conference November 6 & 7, 2024 I New Orleans, LA
Emergency Clinical Research Symposium December 5 & 6, 2024 I Orlando, FL
Clinical Research Nursing Conference December 5 & 6, 2024 I Orlando, FL
See All Upcoming Events
Featured Courses
Fda clinical trial requirements regulations, compliance and gcp conference.
New Orleans, LA November 6 & 7, 2024
This two-day conference is intended to share information among FDA representatives and the regulated community, to facilitate the understanding of regulations, guidelines and practices, and to suggest methods and opportunities to enhance the research professional’s product development experience. The program will focus on the relationships among the FDA and clinical trial staff, investigators and IRBs.
Quality Management Conference
Chicago, IL October 24 & 25, 2024
This conference provides attendees with new information, tools, and real life examples to help participants navigate the components of quality management in clinical research - quality planning, quality control, quality assurance, and quality improvement.
Belonging to SOCRA provides you with the information and resources you need to advance your career and enhance your professionalism.
- 15,100+ Members
- 11,300+ "CCRP" Certified Clinical Research Professionals
- 34 education programs held in 2022
Learn More About Membership
Recent Blogs
Clinical trials day 2024: a call to reflect, engage & inspire.
Clinical Trials Day is a moving reminder of the clinical research community’s pivotal role in shaping the future of the... Read More >>
Celebrating Chapter Volunteers on Clinical Trials Day 2024
As Clinical Trials Day 2024 approaches on May 20, we're spotlighting the incredible work of our SOCRA chapter volunteers. The post... Read More >>
17th Annual Device Research & Regulatory Conference: Navigating Innovation and Compliance
The 17th Annual Device Research & Regulatory Conference is just around the corner, and it promises to be an enlightening... Read More >>
Our Mission
In order to promote quality clinical research, to protect the welfare of research participants, and improve global health, SOCRA's mission is:
To establish educational programming and provide continuing education for clinical research professionals To establish an internationally recognized certification program for clinical research professionals (CCRP®) To foster the professional development and peer recognition of clinical research professionals
SOCRA's Vision That all clinical research fully protects every research participant and is accomplished with excellence, quality, and professionalism.

- Core Curriculum
- Web Seminars
- On-Boarding Programs
- On-Site Training
- On-Demand eLearning Courses
- Web Seminar Archives
- CCAD Programs
- GCP Training Assessments
- Mock Audits Findings Based Training
- Virtual Meetings Support Services
- Customized eLearning Solutions
- Licensing of Barnett's Content
- SOP Development Training
- Acquisition Integration
- Training Subscription
- Publications
- Course Catalog

- Training Courses
- GCP Training & Assessments
- Audits and Mock Inspections
- Licensing of Barnett's Content
- SOP Development & Training

10-Week CRA & CRC Beginner Program
Upcoming Courses
Wednesday Evenings.
Resume support is available as an add-on option! Click here for more details.
Wednesday Afternoons.
Wednesday Evenings. No class December 24 December 31.
Thursday Afternoons.
Modal body text goes here.

Course Description
The online 10-Week CRA & CRC Beginner Program provides a comprehensive introduction to clinical research and the job functions of the Clinical Research Associate (CRA) and Clinical Research Coordinator (CRC) for drug, biologic, and device trials. This program is geared toward individuals seeking a new career or career change into clinical research, but haven’t decided which job track to pursue. Case studies and industry best practices are presented to emphasize how the learning objectives apply directly to the responsibilities of the CRA and CRC.
The resources required to take this on-line course are already at your fingertips - an Internet connection and a phone. After registering, you will receive an email confirmation that provides you with the Web Seminar link and audio connection information.
Prior to the start of the course, participants will receive Module 1 materials. Course materials for subsequent modules will be sent weekly prior to class. Come to class prepared to interact – you will be able to ask questions, provide feedback and participate in discussions and group work. Upon course completion, participants will be provided training certificates. In order to receive accreditation CEUs, participants are required to pass both a mid-term and final exam. Upon completion of the exams, CEU certificates will be provided.
Learning Objectives
- Describe and discuss the investigational product development process, including FDA regulations, ICH guidelines, and Good Clinical Practice (GCP)
- Explain the roles and responsibilities of a CRA and CRC
- Describe the four types of monitoring visits, including the responsibilities of the CRA and CRC in preparation, activities, and follow-up
- Explain the Key Pre-Study Concepts: Role of the Principal Investigator, Site Selection, Clinical Trial Agreement and Budget Negotiation
- Discuss the role of the Institutional Review Board in clinical trials, define informed consent requirements, and discuss the informed consent process
- Discuss the study site initiation, interim monitoring activities, and data management
- Define safety definitions and reporting requirements for both drugs and devices
- Examine accountability for the investigational product and study closeout visits
- Discuss regulatory compliance and quality assurance as it relates to audits and inspections
Course Outline
- Module 1: Investigational Product Development, the FDA, and Good Clinical Practice Guidelines
- Module 2: Clinical Research Team: Roles and Responsibilities
- Module 3: The Principal Investigator, Site Selection, and Budget Negotiation
- Module 4: Clinical Study Protocol Elements
- Module 5: Institutional Review Boards, the Consent of Human Volunteers, and HIPAA
- Module 6: Study Monitoring, Data Management, and Study Initiation Visit
- Module 7: Safety Reporting: Definitions and Reporting Requirements
- Module 8: Accountability for the Test Article and Trial Termination Visits
- Module 9: Regulatory Compliance and Quality Assurance: Audits and Inspections
- Module 10: Managing Your Time and Preparing for the Interview
Who Should Attend
- Aspiring Clinical Research Associates and Clinical Research Coordinators (This course is also appropriate for Clinical Research Associates and Clinical Research Coordinators with less than six months of experience)
- College Students and New Graduates in a Scientific Field
NOTE: This course is for individual registrants only and does not allow for group training.
What participants say about Barnett's 10-Week courses ...
“Great course! Instruction is expertly led and engaging. I will recommend Barnett to any colleague and will seek out topics for my own future training and professional development needs.”
"The course has been so incredibly helpful thus far...I look forward to Thursday evenings!"
“This class exceeded my expectations of an online learning experience. The instructor was knowledgeable, came equipped with great examples to keep the class interesting and is a strong presenter. Thank you!”
The course will be led by one of the following instructors:
Nikki Christison, B.S., C.C.R.A., T.I.A.C.R.
Susan Torchio, R.N., B.S.N.
Elizabeth Weeks-Rowe, LVN, C.C.R.A.
Click here for complete trainer biographies
Course Length and Time
10 weeks for 3 hours each week.
Registration Fees
$1,795 by Early Bird Deadline
$1,995 after Early Bird Deadline
This course is for individual registrants only and does not allow for group training.
All participants are eligible for “Certificates of Attendance,” and accreditation, provided that accreditation requirements are met.
Accreditation Information
Barnett International is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Participants will receive 30 hours (3.0 CEUs) of continuing education credit for full participation, including the completion of a mid-term exam, final exam, and program evaluation. Barnett International will issue a receipt of completion for earned CEUs within three weeks of program completion. ACPE#: 0778-0000-23-002-L99-P. Released: 1/23.
Hold this course at your company! For more information, contact Naila Ganatra at +1 215.413.2471.
Get extra -20% OFF THE ALL-IN-ONE COURSE with "EXTRA20"
SAVE UP TO -50%
ON YOUR FIRST PURCHASE
BECOME the BEST Candidate for your Clinical Research Dream Job
9,000+ talents grow their career across 120 countries with VIARES
1,500+ VIARES graduates with new jobs in the industry since 2020
85% with new job within 6 months after starting with VIARES
TRAINING – CERTIFICATION – CAREER SERVICES – JOBS

Clinical Research Academy Courses & Certificates
Viares academy all-in-one.
€ 399,90 Original price was: € 399,90. € 199,90 Current price is: € 199,90.
Clinical Research Associate Academy
€ 199,90 Original price was: € 199,90. € 99,90 Current price is: € 99,90.
Clinical Research Project Manager Academy
Clinical study coordinator academy, clinical trials assistant academy, navigating ich gcp e6(r3).
€ 59,90 Original price was: € 59,90. € 29,90 Current price is: € 29,90.
A few Stats on our Graduates
VIARES is your solid step stone to a career in clinical research. We make recent life-science graduates, and those looking to upgrade their employment, job ready.
WE BELIEVE IN YOU TO BE THE FUTURE OF CLINICAL RESEARCH
Clinical research is how new medical treatments are discovered. Different jobs are needed to conduct trials, including those who monitor progress and others who help clean, report, and analyze data. There are more job openings than talents available, making clinical research a great career opportunity also for you. Most jobs only require bachelor-level degrees or patient-facing work experience. The challenge is getting your first job in the industry. This is where VIARES supports you as we did with hundreds of previous graduates.
Clinical Research Training Courses
We offer industry leading training courses to make you the best candidate for your dream job!
Industry recognized Certificates
Upon completion of your course you will get an industry certificate. 98% got certified by VIARES.
Application coaching
Boost your Job Search and become the strongest candidate and find your Clinical Research Dream Job
Global Jobs
Access hundreds of daily updated clinical research job offers. Your dream job at your fingertips.
Meet our Graduates - TALENTS LIKE YOU
VIARES already supported 9,000+ talents from 120+ countries on all continents. Here is a Snapshot of Graduate Experience and how they benefited from our Clinical Research Training Courses and Job Support.
I wanted to progress in my career, as I already was working in the field of clinical research. For me the course was a great experience, I learned a lot. I was able to broaden my knowledge about clinical trials and now
I feel prepared for the interviews and career steps to come! The teachers were extremely good trained and it was a pleasure to learn from them.
Proud to announce that I have passed the Final Exam on the VIARES Clinical Research Talent Program. I strongly recommend it to all people interested in the field of clinical trials. Of course many thanks to Dietmar Eglhofer for creating this program
and all the great tutors: Eric Klaver, Gavin Chait and Gabriele Disselhoff.
The VIARES Clinical Research Talent Program provided me a great opportunity to refresh and rebuild my clinical research skills and competencies. The program was well structured and covered the key aspects related to clinical research, drug
development, ICH guidelines, and clinical research regulations. The self-learning modules, followed by live webinars led by subject matter experts, provided the participants a platform to understand the key concepts and engage in great dialogue and discussions. I thank VIARES for giving me this opportunity and highly recommend this program to professionals looking to start a career in clinical research.
VIARES Academy supported me at every step in my course and answered my questions instantly. Moreover the platform is very organized and I could find my way around very fast.
The course content provided by the courses at VIARES have helped me to improve my knowledge at my current job as a clinical trials assistant.
Find The Right Course For Your Career
Explore our courses and find the skills for your interests.
VIARES is your place to start and grow your career in clinical research. We provide industry relevant online competency training courses, support you with applying for jobs and get you access to our jobs-list today.
You can get trained for key jobs in clinical research, improve your application documents and approach with our experts and search from our curated job-list today.
Our training courses are built to support new talents for the industry and those who already know a bit about clinical research. Our students typically work in the following jobs:
medical assistant, dentist, researcher, physician, nurse, med technician, pharmacist, site study coordinator, physiologist, postdoc, pharmacy technician, formulation analyst, chemical engineer, health care specialist, medical receptionist, lab assistant, regulatory compliance analyst, quality assurance specialist, clinical data coordinator, veterinary, ophthalmologist, radiographer and many more…
VIARES is a global online platform and we have students from all continents. You can learn from any location.
Our trainings are recognized by the global CRO industry.
Just sign up today and get started immediately.
Please check with our VIARES Individual Recommendation Builder™ below.
Access our online courses, improve your application documents and approach and find your jobs from our curated jobs-list today. Check our course fees here .
Get new job posts and all the news about our VIARES clinical research courses in your inbox!
- All Courses
- Verify Certificate
- Privacy Policy
- Term Of Service
- VIARES Trainer
- Contract Sales Specialist
Our support and sales team is available 24 /7 to answer your queries.
Clinical Research Training courses all prices incl. VAT

Clinical Research Coordinator Associate
🔍 school of medicine, stanford, california, united states.
The Division of Cardiovascular Medicine , within Stanford University Department of Medicine, is a dynamic and innovative center dedicated to excellence in research, medical education, and clinical care. Our Division is driven by over 90 faculty members and a cadre of staff who are the pillar of strength in the Division's ongoing efforts into the prevention and treatment of cardiovascular disease.
We are seeking a Clinical Research Coordinator Associate (CRCA) who is passionate about clinical research and wants to deliver results. The CRCA will work with a robust clinical research team, hand in hand with Principal Investigators, Clinical Research Managers, Associates, and other stakeholders in support of cardiac patients.
The Clinical Research Coordinator Associate will be responsible for the overall implementation of an assigned set of research protocols assuring efficiency and regulatory compliance. Other responsibilities will include recruiting, screening, assisting in the informed consent process and enrolling subjects in accordance with good clinical practice guidelines as well as collecting, recording and maintaining complete data files using good clinical practice in accordance with HIPAA regulations. He/she will participate in data retrieval, reporting, and preparation of files and Case Report Forms for the various studies. Other duties may include processing of blood samples, reporting serious adverse events and maintenance of drug accountability, supplies and equipment.
CV Med Clinical Research is a growing, dynamic team who is dedicated to supporting translational medicine and contributing to Stanford Medicine’s mission. If you are eager to quickly achieve lasting results, we invite you to join our team.
Duties include:
- Serve as primary contact with research participants, sponsors, and regulatory agencies. Coordinate studies from start-up through close-out.
- Determine eligibility of and gather consent from study participants according to protocol. Assist in developing recruitment strategies.
- Coordinate collection of study specimens and processing.
- Collect and manage patient and laboratory data for clinical research projects. Manage research project databases, develop flow sheets and other study related documents, and complete study documents/case report forms.
- Ensure compliance with research protocols, and review and audit case report forms for completion and accuracy with source documents. Prepare regulatory submissions, and ensure Institutional Review Board renewals are completed.
- Assemble study kits for study visits, monitor scheduling of procedures and charges, coordinate documents, and attend monitoring meetings with sponsors, acting as primary contact.
- Monitor expenditures and adherence to study budgets and resolve billing issues in collaboration with finance and/or management staff.
- Interact with the principal investigator regularly, ensuring patient safety and adherence to proper study conduct.
- Ensure essential documentation and recording of patient and research data in appropriate files per institutional and regulatory requirements.
- Participate in monitor visits and regulatory audits.
DESIRED QUALIFICATIONS:
- Society of Clinical Research Associates or Association of Clinical Research Professionals certification is preferred.
EDUCATION & EXPERIENCE (REQUIRED):
- Two-year college degree and two years related work experience or a Bachelor's degree in a related field or an equivalent combination of related education and relevant experience.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
- Strong interpersonal skills.
- Proficiency with Microsoft Office.
- Knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
- Working toward certification(s) to perform basic patient measurements and tests, such as phlebotomy and EKG.
PHYSICAL REQUIREMENTS:
- Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
- Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
- Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
WORKING CONDITIONS:
- Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
- May require extended or unusual work hours based on research requirements and business needs.
- Occasional evening and weekend hours.
WORKING STANDARDS:
- Interpersonal Skills: Demonstrates the ability to work well with Stanford colleagues and clients and with external organizations.
- Promote Culture of Safety: Demonstrates commitment to personal responsibility and value for safety; communicates safety concerns; uses and promotes safe behaviors based on training and lessons learned.
- Subject to and expected to comply with all applicable University policies and procedures, including but not limited to the personnel policies and other policies found in the University’s Administrative Guide, http://adminguide.stanford.edu/ .
The expected pay range for this position is $31.73 - $36.54 hourly. Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs.
At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process.
Why Stanford is for You Imagine a world without search engines or social platforms. Consider lives saved through first-ever organ transplants and research to cure illnesses. Stanford University has revolutionized the way we live and enrich the world. Supporting this mission is our diverse and dedicated 17,000 staff. We seek talent driven to impact the future of our legacy. Our culture and unique perks empower you with:
- Freedom to grow. We offer career development programs, tuition reimbursement, or audit a course. Join a TedTalk, film screening, or listen to a renowned author or global leader speak.
- A caring culture. We provide superb retirement plans, generous time-off, and family care resources.
- A healthier you. Climb our rock wall, or choose from hundreds of health or fitness classes at our world-class exercise facilities. We also provide excellent health care benefits.
- Discovery and fun. Stroll through historic sculptures, trails, and museums.
- Enviable resources. Enjoy free commuter programs, ridesharing incentives, discounts and more.
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned.
Consistent with its obligations under the law, the University will provide reasonable accommodations to applicants and employees with disabilities. Applicants requiring a reasonable accommodation for any part of the application or hiring process should contact Stanford University Human Resources at [email protected]. For all other inquiries, please submit a contact form .
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
- Schedule: Full-time
- Job Code: 1013
- Employee Status: Regular
- Requisition ID: 103289
- Work Arrangement : On Site
My Submissions
Track your opportunities.
Similar Listings
School of Medicine, Stanford, California, United States
📁 Research
Post Date: May 07, 2024
Post Date: Jan 29, 2024
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs



IMAGES
VIDEO
COMMENTS
Learn about the role, skills, salary, and certification of a clinical research associate (CRA), who acts as a liaison between research sponsors and clinics. Find out how to get started with a degree, online courses, and job applications.
Here's how to get started as a clinical research associate. 1. Qualify for certification. You can take several paths to becoming a certified CRA in Canada. One path is to earn a high school diploma and clock 3,000 to 3,500 part-time hours of work experience in the field.
Learn about the CCRA (Certified Clinical Research Associate) credential, the exam requirements, and the benefits of ACRP membership. Find out how to apply, prepare, and schedule your CCRA exam with ACRP.
Accreditation and Certification. CCRPS provides online, accredited clinical research training in 1 to 4 weeks utilized by over 22,000 researchers to get hired or promoted within the field. Our clinical research courses are used by students at 1,200+ organizations, 6 government agencies, and 308 universities.
The average annual salary for a CRA was estimated to be $80,000 in 2024. Entry-level clinical research associate salaries can range from $47,000 - $80,000 per year. As a CRA gains more experience and furthers their education, they may be able to negotiate higher salaries due to their valuable knowledge and skills.
Learn how to become a Certified Clinical Research Professional (CCRP) with SOCRA, a US-based society for clinical research. Find out the eligibility, exam, and recertification requirements, and the benefits of CCRP certification.
Advanced Clinical Research Associate Certification (ACRAC) Course. 5.0 (161 reviews) A Clinical Research Associate (CRA) assists in the monitoring and compliance of clinical trials. Clinical research associate certification is the leading, accredited, internationally-recognized CRA certification program available online. $450.
Our Accredited Interactive-Online Clinical Research Courses are designed for persons with or without prior on-site clinical trials monitoring experience, seeking qualifications and/or current practical knowledge to effectively work as a Clinical Research Associate (CRA), Clinical Research Coordinator (CRC), or Clinical Data Manager (CDM) in the ...
Here are some steps you can take to pursue a career as a clinical research associate: 1. Pursue a bachelor's degree in a health science-related field. Most clinical research associate positions require candidates to have a bachelor's degree in a health science-related field. For those interested in a position as a clinical research associate ...
With a 30-year legacy, ACRP Certification is the most reputable credentialing program in clinical research. Since 1992, more than 40,000 professionals and their employers have come to trust ACRP Certification as the mark of excellence in clinical research. "Joining ACRP and becoming certified was the best thing I ever did to jumpstart my ...
Foundations of Clinical Research. This Harvard Medical School six-month, application-based certificate program provides the essential skill sets and fundamental knowledge required to begin or expand your clinical research career. Learn More. September 28, 2024 - April 6, 2025. $6,900 - $7,900.
ISBN 978--12-849905-4. Clinical research associate job requirements Enter the field as a Clinical Research Associate (CRA) with CCRPS's accredited training. Remote roles, $6,500-$12,000 monthly, and 33% annual promotion. 7-day CRA certification for a swift career start.
A Certificate in Clinical Research may help you pursue career paths such as Clinical Research Coordinator (CRC) and eventually Clinical Research Associate (CRA) opportunities. In both of these roles, you'll likely find your skills are in high demand. Reports point to faster than average job growth (10 - 15 percent) by 2026.
Learn Clinical Research, earn certificates with paid and free online courses from Harvard, Stanford, University of Michigan, Johns Hopkins and other top universities around the world. Read reviews to decide if a class is right for you.
Our Mission. In order to promote quality clinical research, to protect the welfare of research participants, and improve global health, SOCRA's mission is: That all clinical research fully protects every research participant and is accomplished with excellence, quality, and professionalism. The Society of Clinical Research Associates (SOCRA) is ...
Hold this course at your company! For more information, contact Naila Ganatra at +1 215.413.2471. Barnett International's 10-Week Online CRA & CRC Beginner Program offers a comprehensive introduction to clinical research roles. Ideal for individuals pursuing a new career or transitioning into CRA or CRC positions.
The Clinical Research Associate Academy is specifically designed for anyone with a life-science education and work experience not yet working in clinical research. This Academy will help you to cross the path from your current job into the clinical research industry. up to 500 contact hours, 50 CEU. 4742 Students.
clinical research associate certification. Online modules and competency exam with 60 day period of completion - Use to enhance knowledge for promotion to CRA, SrCRA, or other management levels in clinical trials. Can be taken by physicians, nurses, and bachelors, masters, and PhD graduates. No clinical research background required for ...
Novartis Compensation and Benefit Summary: The pay range for this position at commencement of employment is expected to be between $112,800- $169,200 annually; however, w hile salary ranges are effective from 1/1/24 through 12/31/24, fluctuations in the job market may necessitate adjustments to pay ranges during this period.
The Division of Cardiovascular Medicine, within Stanford University Department of Medicine, is a dynamic and innovative center dedicated to excellence in research, medical education, and clinical care.Our Division is driven by over 90 faculty members and a cadre of staff who are the pillar of strength in the Division's ongoing efforts into the prevention and treatment of cardiovascular disease.
Welcome to the 628DirtRooster website where you can find video links to Randy McCaffrey's (AKA DirtRooster) YouTube videos, community support and other resources for the Hobby Beekeepers and the official 628DirtRooster online store where you can find 628DirtRooster hats and shirts, local Mississippi honey and whole lot more!
Elektrostal , lit: Electric and Сталь , lit: Steel) is a city in Moscow Oblast, Russia, located 58 kilometers east of Moscow. Population: 155,196 ; 146,294 ...
What time is it in Elektrostal'? Russia (Moscow Oblast): Current local time in & Next time change in Elektrostal', Time Zone Europe/Moscow (UTC+3). Population: 144,387 People
Business, Economics, and Finance. GameStop Moderna Pfizer Johnson & Johnson AstraZeneca Walgreens Best Buy Novavax SpaceX Tesla. Crypto