
< Go back to Login
Forgot Password
Please enter your registered email ID. You will receive an email message with instructions on how to reset your password.


Meeting PowerPoint Templates
Our extensive collection of Meeting PPT templates promote productivity and convenience when it comes to its applications. These templates have been thoughtfully designed to ensure maximum visual impact. Explore our collection of Meeting presentation templates and download the perfect template to take your presentation to new heights!
- Conference-Meeting-Agenda - 4x3 – $4.99
- Conference-Meeting-Agenda - 16x9 – $4.99
Conference Meeting Agenda PowerPoint Template
The Conference Meeting Agenda PowerPoint Template provides a contemporary way to outline the discussion points for any business meeting. The slid....
- PowerPoint-Meeting-Agenda-Template - 4x3 – $4.99
- PowerPoint-Meeting-Agenda-Template - 16x9 – $4.99

PowerPoint Meeting Agenda Template
PowerPoint Meeting Agenda Template A company’s main agenda is its growth and development, striving to be the best in the market. We bring y....
- Table-of-Contents-PPT-Template - 4x3 – $4.99
- Table-of-Contents-PPT-Template - 16x9 – $4.99

Table of Contents PPT Template
Table of Contents Presentation Template Use this Table of Contents PowerPoint template to create visually appealing presentations in any professi....
- Six-Thinking-Hats-PowerPoint-Template - 4x3 – $4.99
- Six-Thinking-Hats-PowerPoint-Template - 16x9 – $4.99

Six Thinking Hats PowerPoint Template
Six Thinking Hats Presentation Template Use this Six Thinking Hats PowerPoint template to create visually appealing presentations in any professi....
- Planning-Isometric-PowerPoint-Template - 4x3 – $4.99
- Planning-Isometric-PowerPoint-Template - 16x9 – $4.99

Planning Isometric PowerPoint Template
Planning Isometric Presentation Template Use this Planning Isometric PowerPoint template to create visually appealing presentations in any profes....
- Problem-Solving-Isometric-PowerPoint-Template - 4x3 – $4.99
- Problem-Solving-Isometric-PowerPoint-Template - 16x9 – $4.99

Problem Solving Isometric PowerPoint Template
Problem Solving Isometric Presentation Template Use this Problem Solving Isometric PowerPoint template to create visually appealing presentations....
- Teamwork-Isometric-PowerPoint-Template - 4x3 – $4.99
- Teamwork-Isometric-PowerPoint-Template - 16x9 – $4.99

Teamwork Isometric PPT Template
Use this Teamwork Isometric PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design a....
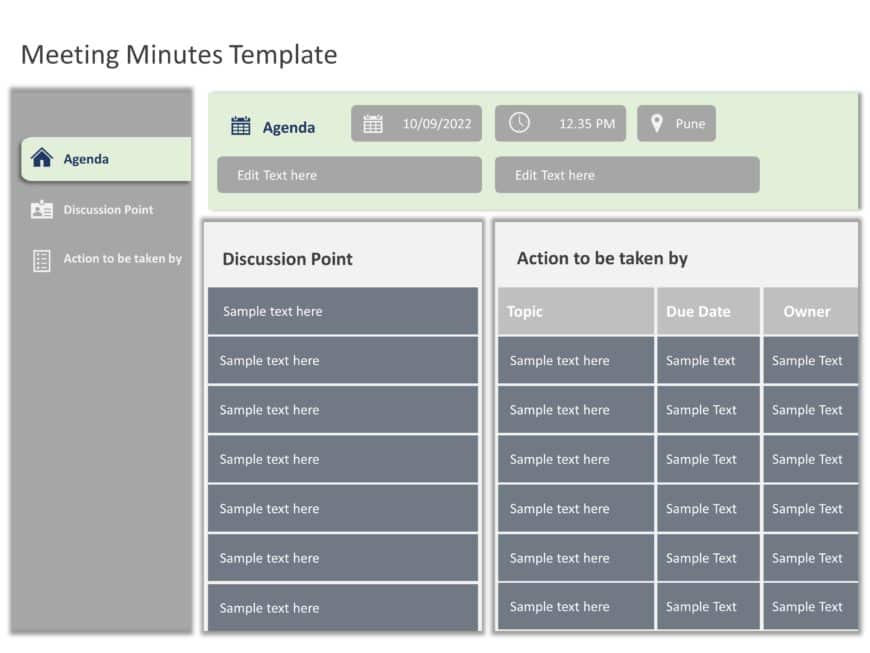
- Meeting-Minutes-PowerPoint-Template - 4x3 – $4.99
- Meeting-Minutes-PowerPoint-Template - 16x9 – $4.99

Meeting Minutes PowerPoint Template
Meeting Minutes Presentation Template Use this Meeting Minutes PowerPoint template to create visually appealing presentations in any professional....
- Minutes-of-Meeting-PowerPoint-Template - 4x3 – $4.99
- Minutes-of-Meeting-PowerPoint-Template - 16x9 – $4.99
Minutes of Meeting PowerPoint Template
Minutes of Meeting Presentation Template Use this Minutes of Meeting PowerPoint template to create visually appealing presentations in any profes....
- Business-Presentation-Isometric-PowerPoint-Template - 4x3 – $4.99
- Business-Presentation-Isometric-PowerPoint-Template - 16x9 – $4.99

Business Presentation Isometric PowerPoint Template
Business Presentation Isometric Presentation Template Use this Business Presentation Isometric PowerPoint template to create visually appealing p....
- Business-Review-Isometric-PowerPoint-Template - 4x3 – $5.99
- Business-Review-Isometric-PowerPoint-Template - 16x9 – $5.99
Business Review Isometric PowerPoint Template
Business Review Isometric Presentation Template Use this Business Review Isometric PowerPoint template to create visually appealing presentations....
- Team-Discussion-Isometric-PowerPoint-Template - 4x3 – $5.99
- Team-Discussion-Isometric-PowerPoint-Template - 16x9 – $5.99

Team Discussion Isometric PowerPoint Template
About Team Discussion Isometric PowerPoint Template The Team Discussion Isometric PowerPoint Template is a dynamic and visually appealing tool de....
Related Presentations
17 templates >
36 templates >
14 templates >
157 templates >
Minutes Of Meeting
11 templates >
Meeting PowerPoint Templates For Presentations:
The Meeting PowerPoint templates go beyond traditional static slides to make your professional presentations stand out. Given the sleek design and customized features, they can be used as PowerPoint as well as Google Slides templates . Inculcated with visually appealing unique and creative designs, the templates will double your presentation value in front of your audience. You can browse through a vast library of Meeting Google Slides templates, PowerPoint themes and backgrounds to stand out in your next presentation.
Product Pricing
What is a meeting powerpoint template.
A Meeting PowerPoint template is a ready-made presentation template that provides a structured framework for creating professional Meeting presentations. The Meeting PPT presentation template includes design elements, layouts, and fonts that you can customize to fit your content and brand.
How To Choose The Best Meeting Presentation Templates?
Keep the following points in mind while choosing a Meeting Presentation template for PowerPoint (PPT) or Google Slides:
- Understand your presentation goals and objectives.
- Make sure the Meeting template aligns with your visual needs and appeal.
- Ensure the template is versatile enough to adapt to various types of content.
- Ensure the template is easily customizable.
Are Meeting PowerPoint Templates Compatible With Google Slides?
Yes, all our Meeting presentation templates are compatible and can be used as Meeting Google Slides templates.
What Are The Advantages Of Meeting Presentation Templates?
Meeting PPT presentation templates can be beneficial because they:
- Add multiple visual and aesthetic layers to your slides.
- Ensure that complex information, insights and data is presented in a simplistic way.
- Enhance the overall visual appeal of the content.
- Save you a lot of time as you don’t have to start editing from scratch.
- Improve the professional outlook of your presentation.
Can I Edit The Elements In Meeting PowerPoint Templates?
Yes, our Meeting PowerPoint and Google Slides templates are fully editable. You can easily modify the individual elements including icons, fonts, colors, etc. while making your presentations using professional PowerPoint templates .
How To Download Meeting PowerPoint Templates For Presentations?
To download Meeting presentation templates, you can follow these steps:
- Select the resolution (16*9 or 4*3).
- Select the format you want to download the Meeting template in (Google Slides or PowerPoint).
- Make the payment (SlideUpLift has a collection of paid as well as free Meeting PowerPoint templates).
- You can download the file or open it in Google Slides.
Forgot Password?
Privacy Overview
Necessary cookies are absolutely essential for the website to function properly. This category only includes cookies that ensures basic functionalities and security features of the website. These cookies do not store any personal information
Any cookies that may not be particularly necessary for the website to function and is used specifically to collect user personal data via ads, other embedded contents are termed as non-necessary cookies. It is mandatory to procure user consent prior to running these cookies on your website.
7 Useful PowerPoint Templates for More Efficient Meetings
Spending too much time in meetings? Use these handy PowerPoint templates to prepare and make your meetings more efficient.
Statistics indicate people spend up to 35-50 percent of their work time in meetings. It's no wonder many individuals grumble at the mere idea of meeting with colleagues during the workday.
Fortunately, tools like Microsoft PowerPoint make meetings less miserable. They can serve as aids and keep the pace of meetings flowing.
Even better, PowerPoint templates can shorten the time you have to spend putting your slides together. Below, you can explore several business, staff, and team meeting PowerPoint templates. Each template can be customized to suit the unique needs of your next presentation.
1. General Business Meeting Template
A company meeting is an excellent way to bring all your employees together. It helps to get them on the same page about new procedures, plans for the future, and future milestones. This Company Meeting PowerPoint template is a fine choice for any employee meeting on your agenda.
Made with a gray background, the template includes a crisp, easy-to-read font in black and dark blue. The good visibility of the lettering, combined with the contrasting color scheme, makes it simple for people to read the slides, even from the back of a large room. That reduces the likelihood of having to go over points repeatedly.
Also, you can customize the slides with other colors. This could help if you want to reflect your company's branding or if your meeting is about the changing look of your business.
A design tab within the template allows you to change things such as the font styles. That feature could be extremely useful when you want to give your audience visual cues that you are transitioning into a new segment of the meeting.
You can also use the customizable slides that come with themed titles. There is one for Revenue and Profit, another for Critical Success Factors, and a custom slide for an Organizational Overview.
You'll find 12 of these slides. Use them to give your presentation a polished and cohesive look.
2. New Hire Onboarding Template
The corporate onboarding process is essential for helping newly hired team members feel well equipped and at ease in their new workplace. However, it can also be very time-consuming when not done properly.
This Hello 2 PowerPoint template is great to use for employee orientations. It is so diverse that you can easily depend on it for other types of meetings too.
Choose from over 500 unique slides and build a presentation that skillfully gives new hires the need-to-know information about your company's history.
Slides include graphic-rich title slides, slides featuring smartphones --- great for explaining how to use an app you've made to acquaint employees with how things work --- and a title slide.
The latter features a mountaintop design that may work well if you are discussing things like advancement opportunities and continuing education for workers.
Behance's New Hire Onboarding Template is the only paid template on our list. It's just $15 and available directly from their website.
3. Company Meeting Template
This Company Meeting Template has everything you need for your next meeting slideshow. You have slides for a table of contents, objectives, and upcoming events. If your meeting is related to a project, you'll like the slides for the project schedule, timeline, and status report .
You can easily swap out the graphs and charts for your own. Plus, all other elements in the presentation are editable and ready for your company data.
The slideshow theme is for a company meeting but offers an attractive and airy nature background. There's also a slide formatted for an inspiring quote to get everyone in the room motivated.
The Company Meeting Template gives you 15 slides for the presentation and another 15 that include icons and graphics to spruce up your slideshow.
4. Timeline Meeting Template
When discussing things about your company's upcoming anniversary, a planned open house event for customers, or guidelines about how employees should ask for time off, a calendar-themed PowerPoint template is a smart option.
Consider this simple and straight-to-the-point template from Slide Hunter. It includes a red- or blue-themed calendar slide, which helps you get right to the point. Encourage continual focus on a chosen date by customizing the numerical text in each image so your team members know exactly what deadline you're talking about.
You can write subtitles made from white text inside a blue or red box, depending on the initial color scheme used. The high level of contrast between the text and background promotes quick and effective information retention.
Be sure to check out our PowerPoint tips for creating professional presentations .
5. New Property Meeting Template
In many cases, a company-wide meeting is the easiest way to inform employees about new building acquisitions or office space. That's when this business meeting PowerPoint template comes in.
The City Skyline Template from Presentation Load is a flexible template that makes it simple to get people excited about and in full support of an upcoming move to a new office building or news about an additional location opening soon.
Begin customizing the template by picking a 16:9 or 4:3 aspect ratio for ideal, properly scaled visual results.
Then, select from three appealing color schemes that are easy on the eyes and maintain a theme by showing various buildings set against a straightforward backdrop of the sky. Arrange content into bulleted lists and move it into one or two columns to showcase necessary information strategically.
6. Weekly Meeting Template
For companies with weekly meetings, this Weekly Meeting Template is the perfect tool. It has a nice, clean appearance with simple colors and well-structured elements.
You can use all of the slides or just those that pertain to your company or specific recurring meeting, such as a project update. Edit the slide elements quickly for your own table of contents, meeting objectives, and project status.
Slidesgo provides this template like the Company Meeting template, so you'll receive the slides for the presentation along with those extra images.
7. Monthly Meeting Template
If your business meetings take place monthly instead of weekly, check out this Monthly Meeting Template, also from Slidesgo. The slides offer a blue and white color scheme with a casual appearance using text bubbles and callouts, staggered lines, and hand-drawn graphics.
Slides include a table of contents, meeting objectives, a checklist, and project-related options. Like the other templates, it's super easy to switch the charts, graphs, and other elements for your own or simply edit them.
And you'll also receive that set of alternative resources like icons and graphics to match your type of business.
Try Out These Business Meeting PowerPoint Templates
Getting meetings to run smoothly and efficiently is a skill that even a seasoned professional can struggle with. Using these business, staff, and team meeting PowerPoint templates, you'll be able to create streamlined presentations that keep you and your talking points on track, without distracting your listeners.
If your business is in the education field, take a look at these PowerPoint templates specifically for education .
Image Credits: Rawpixel.com/Shutterstock

- Search Search Search …
Parker, meeting presentation template.

ADVERTISEMENT
Free PowerPoint template and Google Slides theme.
Professional slide perfect for meetings. Simple and organized style, with focus on the content, which will make your meetings more efficient. This slide is available for PowerPoint and Google Slides.
#Business #Budget #Finance #Quotation #Presentations #Company #Elevator Pitch #Meetings
You may also like

Cute Monkeys, mini theme and subtraction drag and drop activity.
Unleash the wild fun in your classroom with this FREE PowerPoint Template and Google Slides Theme. Liven up your classroom with a touch […]

Amelia free PowerPoint and Google Slides template with floral backgrounds.
Elegant and classy with flowers and leaves backgrounds free PowerPoint Template and Google Slides Theme Amelia is a free PowerPoint and Google […]

Cinema choice board and daily activities, a fun slides theme.
Free PowerPoint template and Google Slides theme. Daily Activities Template for online lessons Or choice board template It’s showtime! Add your activities […]

Chapman Free Presentation template for Google Slides or PowerPoint
Free Template for PowerPoint and Google Slides Presentations Chapman Chapman is a simple theme with fresh colors that fit different presentation topics. It […]
Home Collections Strategy / Business Plan Team meeting
Team Meeting Presentation Templates
Revamp your team meetings with our team meeting presentation templates engage your team with professional slides and 100% customizable designs. download our free google slides themes and powerpoint templates today.
Elevate Your Team Meetings with Dynamic Free Team Meeting PowerPoint Templates and Google Slides Themes!
Team meetings are like assembling a puzzle; each piece contributes to the bigger picture of success. Presentations play a vital role in these gatherings, acting as the glue that binds ideas and discussions together. In our team meeting presentation templates category, we offer a pack of premade slides to make your meetings impactful and engaging.
What We Offer:
Our collection covers a spectrum of themes to suit every occasion, ensuring that your presentations are always on point and engaging. Whether it's a virtual company meeting, a town hall discussion, a video conferencing, a team learning program, or a performance review, we've slides here.
Why Choose Our Meeting PPT Slides:
Our slides stand out from the crowd with their captivating designs and user-friendly features. With these templates, you can effortlessly convey your message while keeping your audience hooked from start to finish.
Who Can Benefit:
Our templates are designed for everyone, from seasoned professionals to budding entrepreneurs. Whether you're leading a team, presenting to clients, or sharing insights with stakeholders, our slides will help you shine. No matter where you are or who you're presenting to, our templates are your companion for success.
Where to Use Them:
Our templates are highly versatile. Whether you're in the boardroom, the classroom, or the comfort of your own home, our slides adapt seamlessly to any environment. With these slides, you can create stunning presentations that leave a lasting impression, no matter the setting.
Features and Benefits:
- Royalty-free: No need to worry about licensing fees or copyright issues.
- 100% editable: Customize your presentations to suit your unique style and preferences.
- Multiple formats and orientations: Choose from 4:3 or 16:9 aspect ratios, and portrait or landscape orientations, to create presentations that fit your needs perfectly.
- Free slides available: Try before you buy with our selection of free team meeting slides, giving you a taste of what's possible with our templates.
Elevate your team meetings to new heights with our dynamic presentation templates. With their engaging designs, user-friendly features, and unmatched versatility, our slides are the perfect partner for any occasion. Start creating your presentations today!
We're here to help you!
What are team meeting powerpoint templates.
Team Meeting PowerPoint Templates will help you to make your team meeting more effective. Using these templates, you can show your team projects, work progress, and ideas more creatively to your team members.
Where can we use these Team Meeting PPT Slides?
We can use these Team Meeting PPT Slides in every team meeting for meeting invitations, talent reviews, video conferencing, team overviews, board meetings, business consulting, team learning, and so on.
How can I make Team Meeting Slides in a presentation?
You can make Team Meeting Slides with your team introduction along with your team member's photographs. Find a pre-designed slide online with editable images and text placeholders to quickly make team slides. Our tricks and tips tutorials will also guide you through the proper steps to make these slides yourself.
Who can use these Team Meeting PPT Templates?
Team meeting organizers and team leads can use these Team Meeting PPT Templates to make your session livelier.
Why do we need Team Meeting PowerPoint Templates?
Team Meeting PowerPoint Templates help us organize the team meetings more effectively with a perfect slideshow to engage the team members throughout the session.
Where can I find Free Team Meeting PowerPoint Templates?
It is easy to find Free Team Meeting PowerPoint Templates on the internet, as it is the main source of finding the templates. Slide Egg also offers 73+ eye-grabbing, professional-looking Team Meeting Slides.
Board Meeting Presentation Template
Preparing for your next board meeting? Make an impression by putting together an impactful presentation with Beautiful.ai’s customizable board meeting presentation template. Our template will help you host a productive, efficient board meeting that reflects on company progress, refines goals, and celebrates company wins.
Use a board meeting presentation to:
- Align on key company initiatives
- Share updates with the board
- Evaluate campaigns or goals
Board Meeting Presentation Sample
Customize your board meeting presentation. Make an impact with your presentation by using graphs, charts, timelines, diagrams, and sales funnels. Each of these graphics can easily be added to your board meeting presentation template with just one click. Some potential slides to include are:

Pro Tips for Your Board Meeting Slides
Consider these tips when building your board meeting slides.
You need an agenda to outline your presentation, but you also need objectives. Explain what you want to get out of the board meeting by stating your objectives up front.
Include brief summaries for each department or section in your presentation. Summaries ensure that your info sticks with the board.
Acknowledge your team members’ hard work. It reminds your board that the people in the company are the most valuable asset in the business.
Your board is busy, and no one likes to have their time wasted. Keep your presentation as concise as possible.
More Popular Templates

Event Marketing Plan Presentation
Make your next company event a success. Use an event marketing plan template to promote, organize and evaluate your event.

Business Plan Presentation Template
Whether you’re looking to fund your own small business, or looking to raise money from investors, this business plan template will give you a headstart

Press Kit Presentation Template
Beautiful.ai’s press kit template helps you compile your company information that a media contact may request if they were to cover you in their publication or news outlet.

Reddit Presentation Template
The popular site Reddit helps users engage with communities and conversations of varying topics so we’ve revamped their presentation deck to help them create more engagement with their presentations.

OKR Presentation Template
Learn how Beautiful.ai’s OKR template can help leadership set goals and expectations to improve team processes.


Recruitment Presentation
Bring top talent to your organization with a compelling, informative recruitment presentation.
Home PowerPoint Templates PowerPoint Templates Team Meeting PowerPoint Template
Team Meeting PowerPoint Template
The Team Meeting PowerPoint Template is a set of 10 slides for team collaboration sessions. It contains slides of new team member introductions, meeting schedules, special topics, and weekly planner templates. These templates are suitable for periodic progress updates and planners to keep track of activities. The questions and let’s go slides at the end of the presentation encourages feedback in an office setting. Weekly team meetings are crucial to the project’s success because they address action items, problems, and questions. Supervising managers are responsible for arranging regular staff meetings. This activity lets team members collaborate on assigned tasks. The template of team meetings will help managers and supervisors to make meetings and sessions more interesting and productive.
The team meeting templates offer a range of valuable layouts including time planners, introductions, and work allocation charts. These templates are an excellent alternative to weekly or monthly progress reports for a collaborative approach. However, users can apply these templates to illustrate several other meeting situations in PowerPoint. You can prepare presentations around skill training, project planning, change management, and project reviews.
The Team Meeting PowerPoint Template is a resourceful tool for team management and effective communication strategies. As a pre-design team meeting template, it fits all types of businesses and industries. Tailor the design template according to your presentation requirements brand theme. The template includes readymade graphics such as human figures and picture placeholders. Users can replace the existing pictures with photos of team members. These PowerPoint objects will benefit users in their upcoming staff meeting sessions.
Alternatively, you can check our gallery of Team Slide templates for PowerPoint .

You must be logged in to download this file.
Favorite Add to Collection
Details (10 slides)

Supported Versions:
Subscribe today and get immediate access to download our PowerPoint templates.
Related PowerPoint Templates

Training Session PowerPoint Template

Annual Report PowerPoint Template

Board Deck PowerPoint Template
Product Management Skills PowerPoint Diagram
Presentation templates
Captivate your audience with customizable business presentation templates. whether you're pitching clients, wooing investors, or showing off your latest wins, there are presentation templates that'll suit your next meeting..

Free slide templates for presentations
Presentation decks can make or break your speech—don't risk boring or unprofessional slides distracting from your message. Set yourself up for success with free, eye-catching presentation templates that don't require graphic design skills to use. Whether you're pitching to investors or sharing a class project, using presentation templates allows you to focus on the content of your work without worrying about the design. Explore presentation templates for pitch decks, annual reviews, and conference slides, and get ready to wow your audience. Choose a presentation template and customize it with your business's branding and logo. If you work in a creative field, don't shy away from bold designs and vivid colors . Presentation templates offer versatile options for personalizing—get creative by customizing your template or opt for adding your own text to existing designs. When you use a template at your next meeting, you'll turn a simple presentation into an opportunity to impress. To make presenting even easier, you can download your template in a variety of formats, like PowerPoint and PDF, or share it digitally with your colleagues.

Home Powerpoint Infographics Board Meeting Presentation Template
Board Meeting Presentation Template
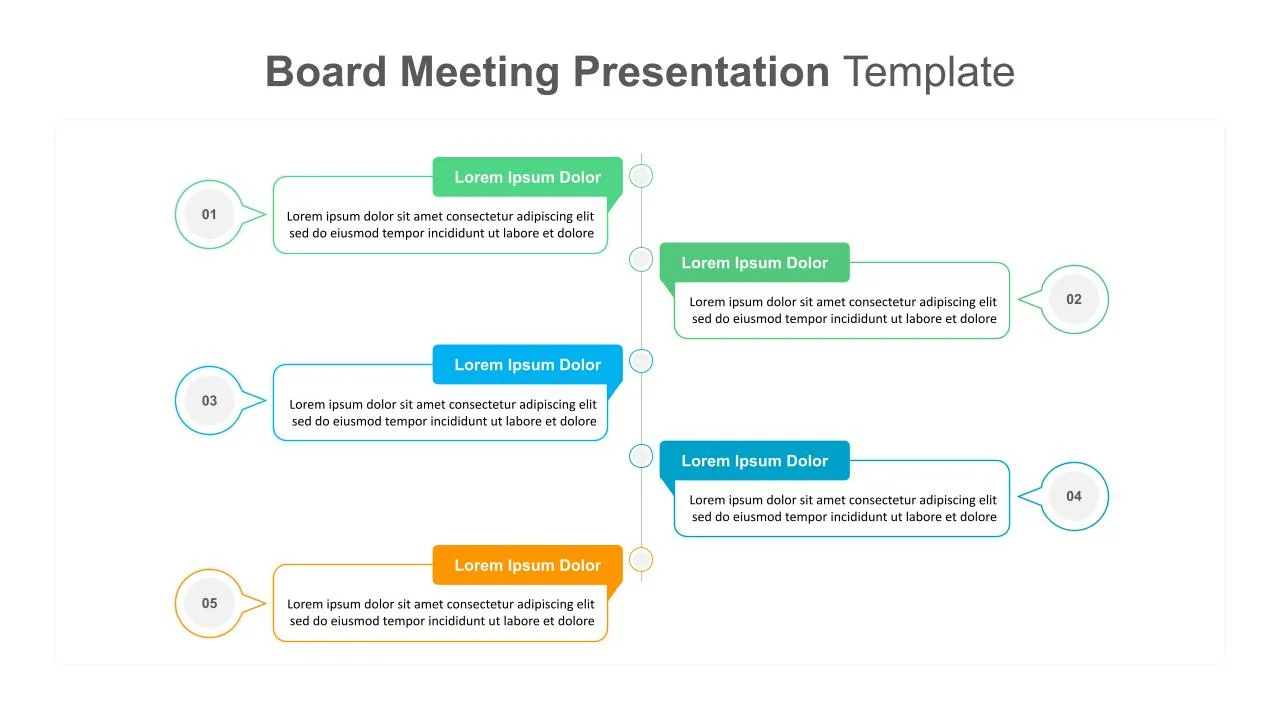
Board Meeting Presentation will cover agenda presentation in a simple google slide design. The timeline designs in the slides enable step-by-step presentation with modern meeting styles. In addition, it has six slides similar to a process flowchart with connected diagrams. It also includes horizontal and vertical agenda slide designs in different column color sections.
The board meeting presentation template showcases four to six-stage presentation designs with an easily understandable poster design format. For example, the six-step presentation, as in the hub and spoke model infographic, supports presenters to put the main heading on the nucleus point, and its sub-elements can display in the six square templates on the brinks. You can also use these google slides templates for company agenda presentations.
Like this template
Get access to this template
No. of Slides
Aspect Ratio
Can I customize the PowerPoint templates to match my branding?
Yes, all our PowerPoint templates are fully customizable, allowing you to edit colors, fonts, and content to align with your branding and messaging needs.
Will your templates work with my version of PowerPoint?
Yes, our templates are compatible with various versions of Microsoft PowerPoint, ensuring smooth usage regardless of your software version.
What software are these templates compatible with?
Our templates work smoothly with Microsoft PowerPoint and Google Slides. Moreover, they’re compatible with Apple Keynote, LibreOffice Impress, Zoho Show, and more, ensuring flexibility across various presentation software platforms.
Agenda Template For Powerpoint
Creative Agenda Template For Powerpoint
6 Items Ribbon Powerpoint Agenda Slide Template

Agenda Powerpoint Presentation Template

Meeting Agenda Presentation Slide
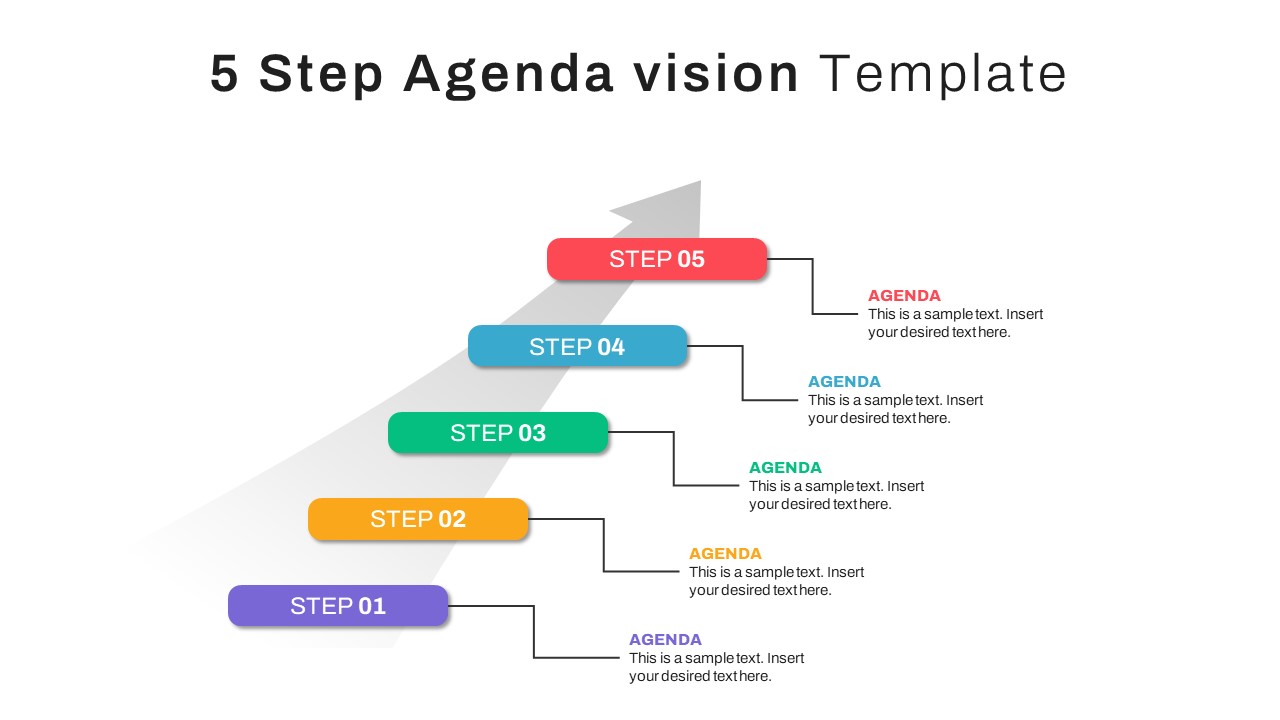
5 Step Agenda Vision Template For Powerpoint
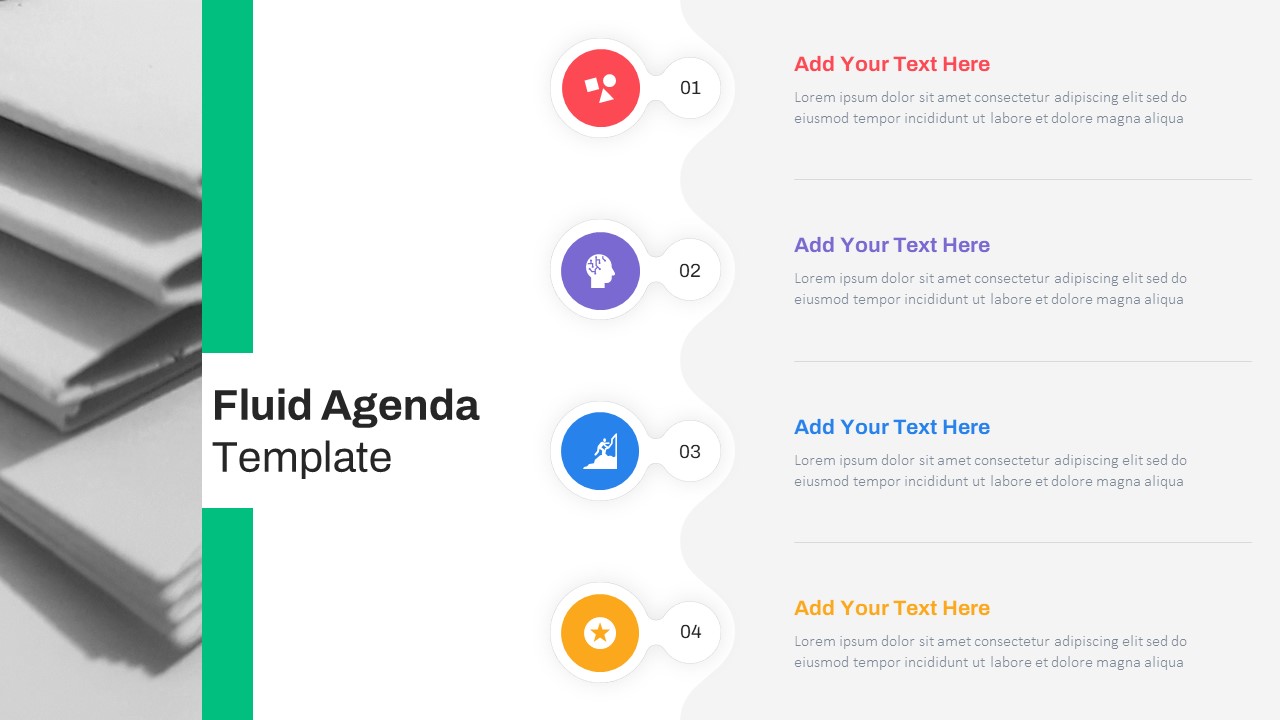
Fluid Agenda Slide Template
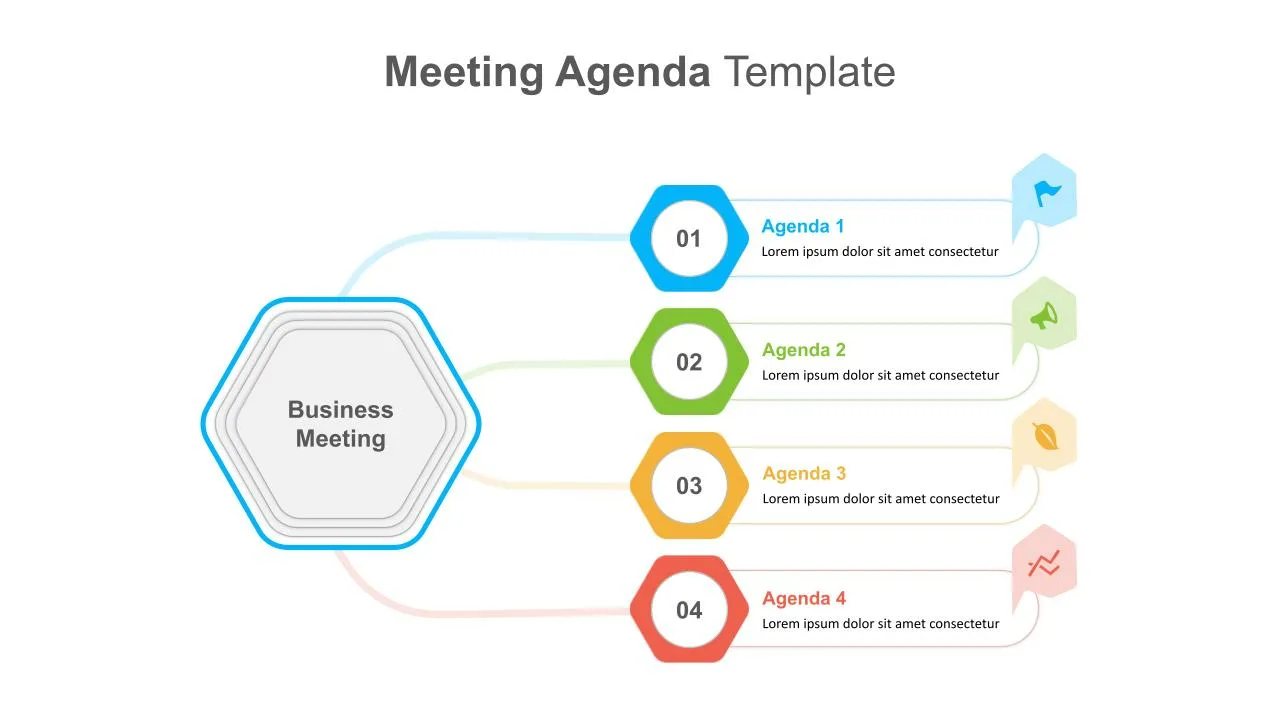
Cool Agenda Presentation Slide

Vision Board Presentation Templates
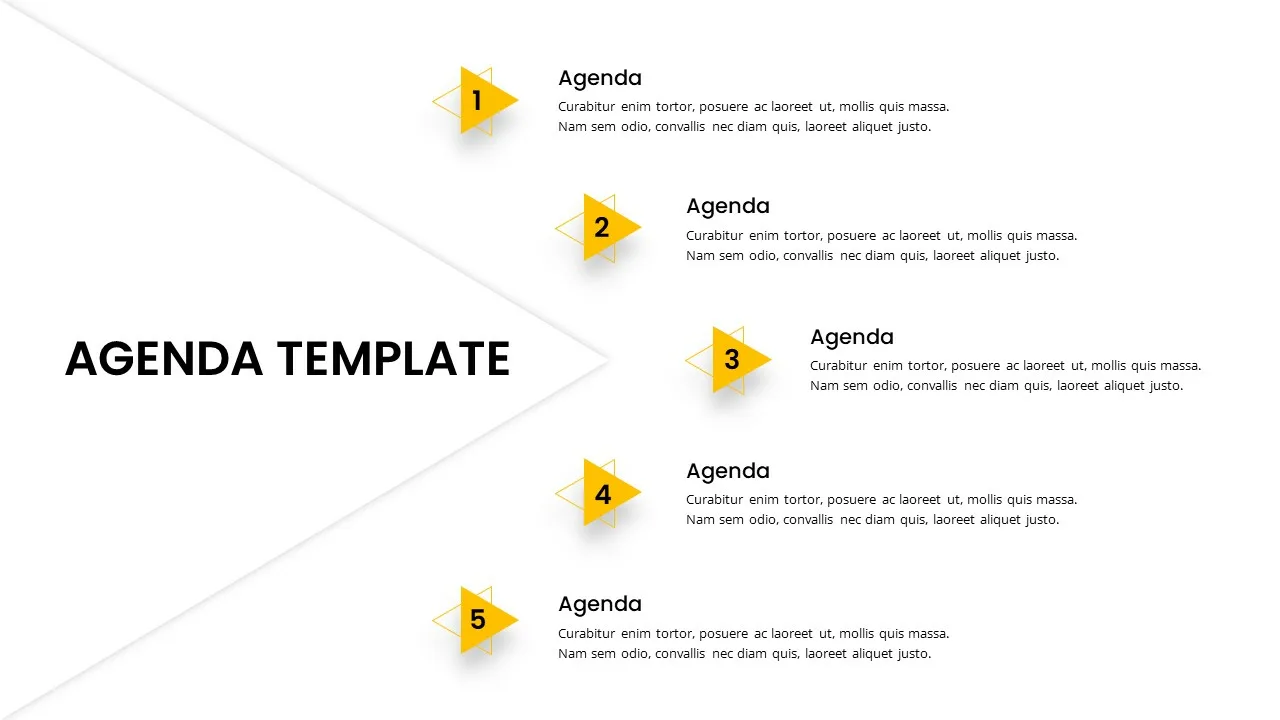
5 Step Agenda Presentation Template
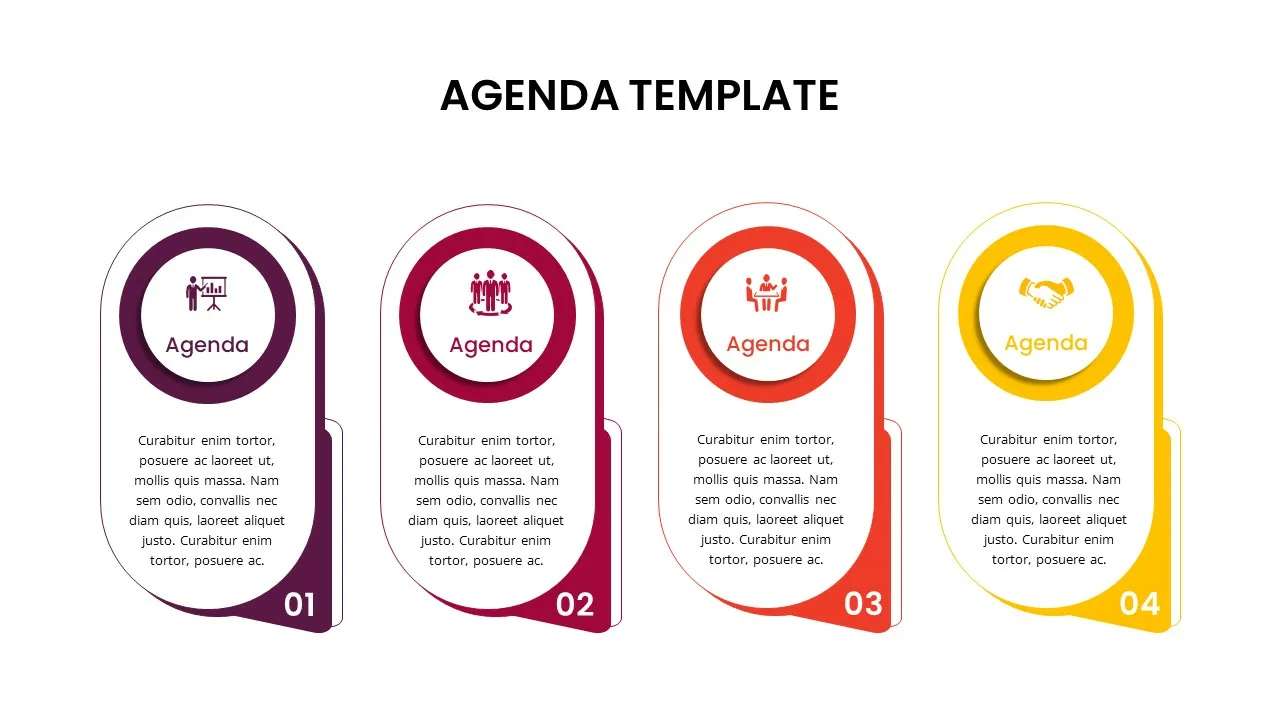
4 Point Agenda Presentation Slide
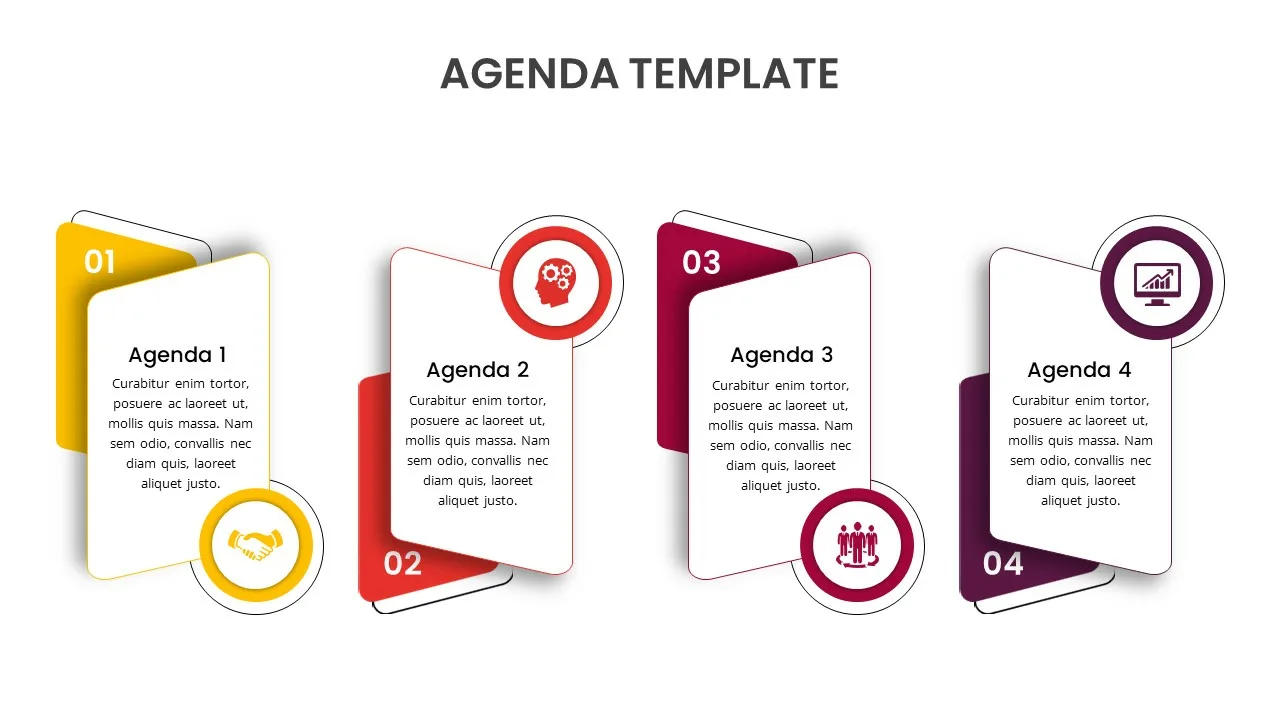
Agenda Presentation Template
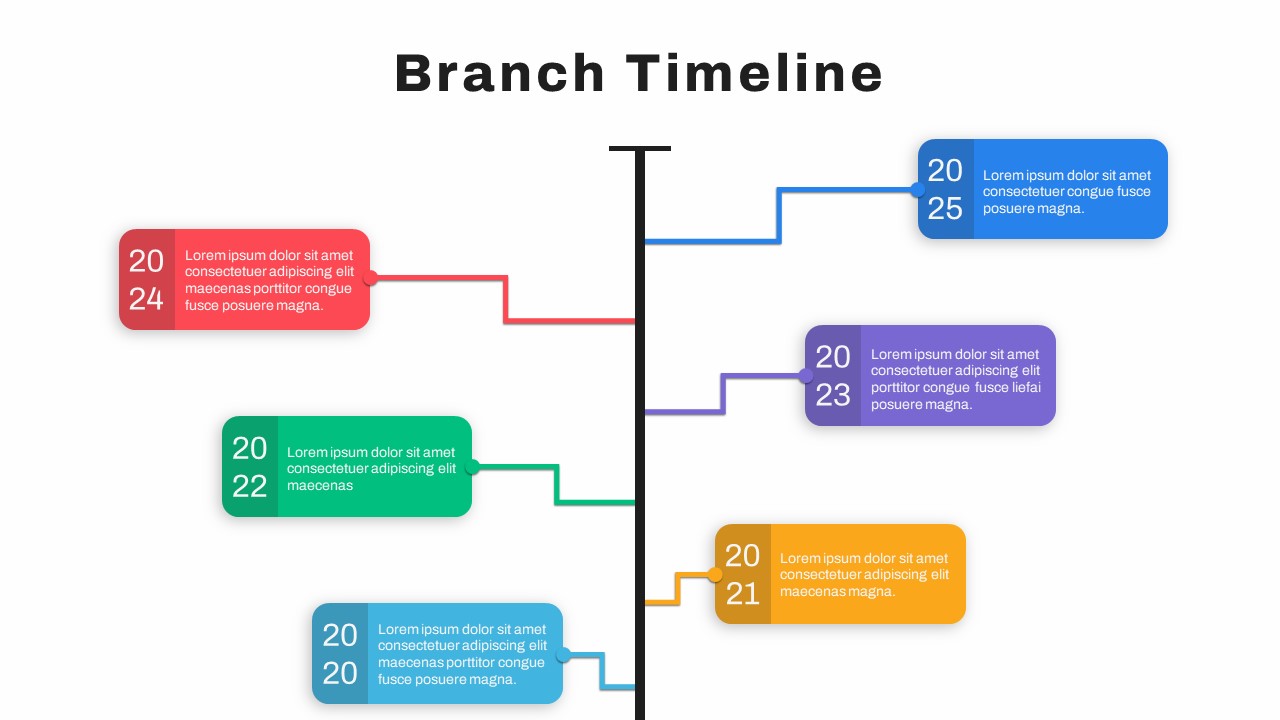
Google Slide Branch Timeline Template
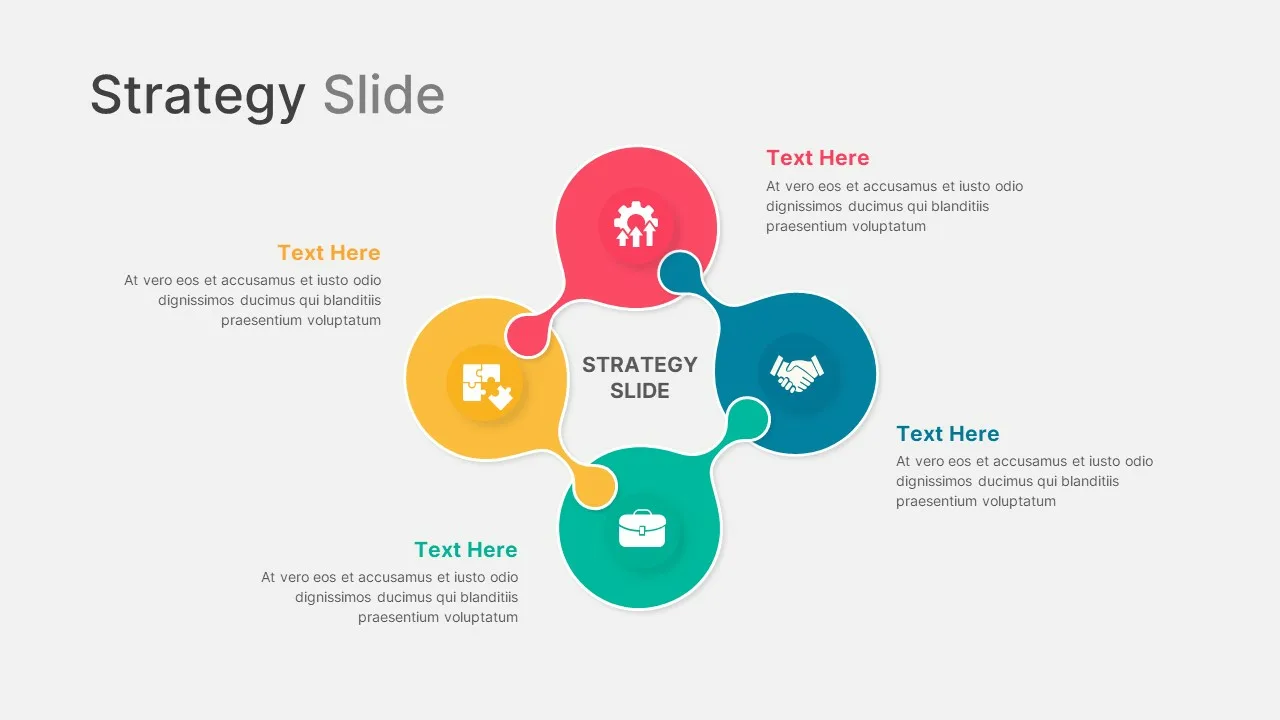
Strategy Presentation Slide Templates
6 Point Presentation Agenda Slide
Hexagon Infographic Slide Template

To-Do-List & Task List Slide Templates
Business Timeline Slide

Horizontal Infographic Slide Templates
Career Timeline Slide Template
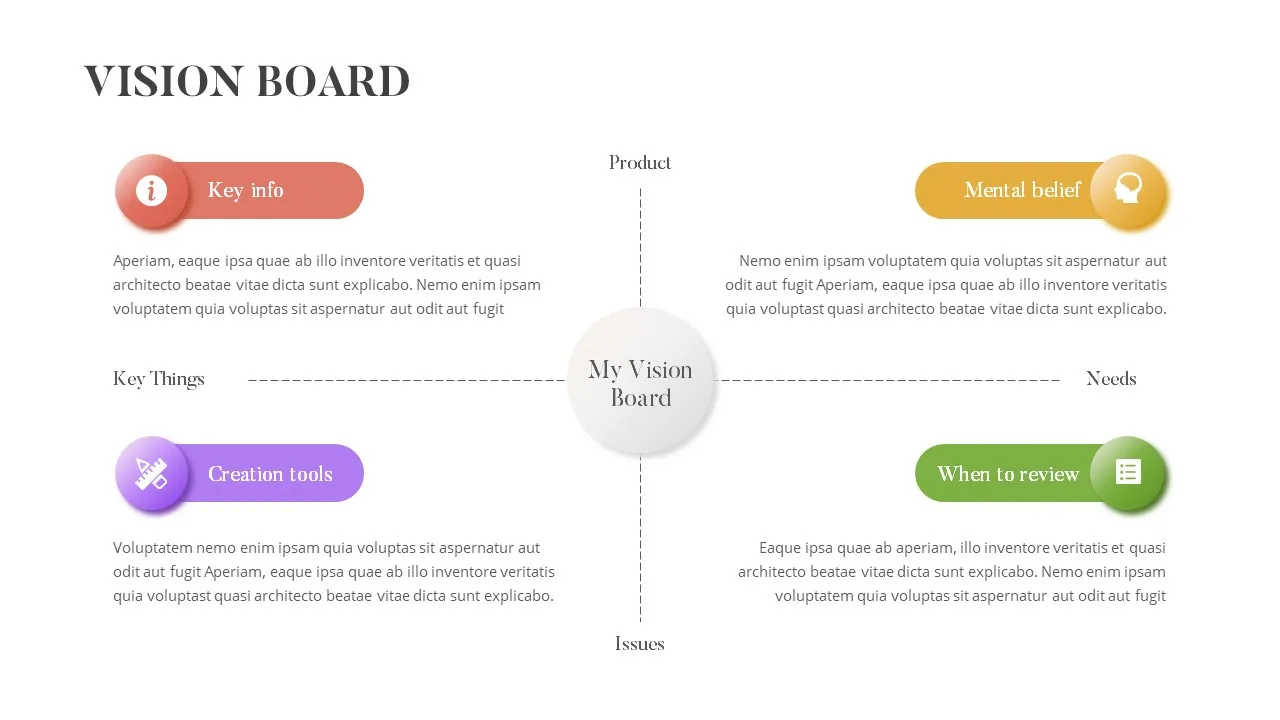
Vision Board Template
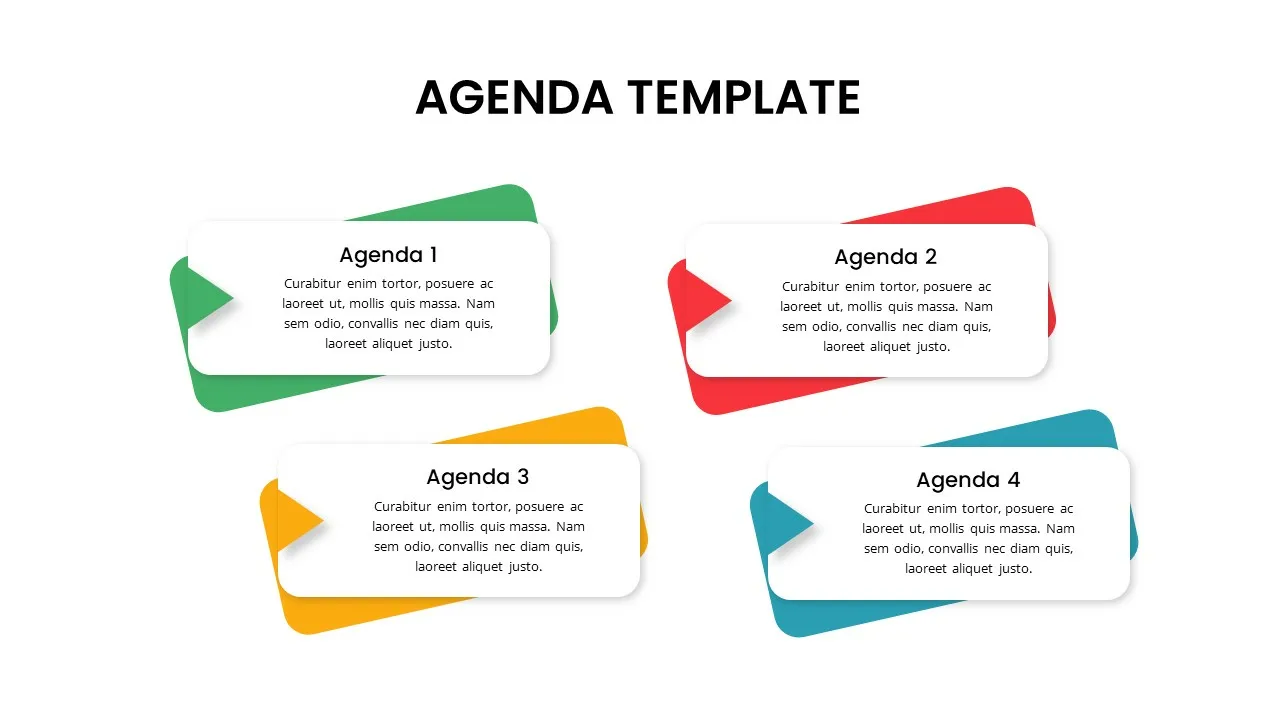
4 Point Agenda Presentation Slides
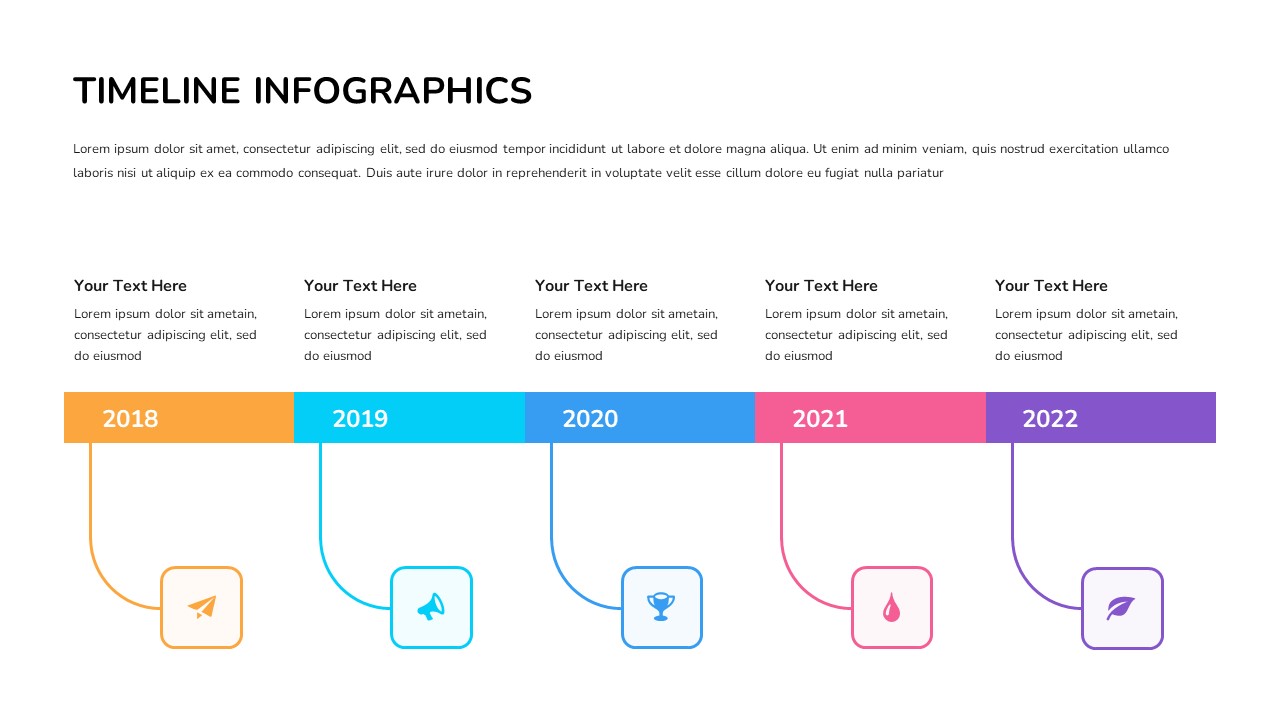
Simple Timeline Template For Powerpoint
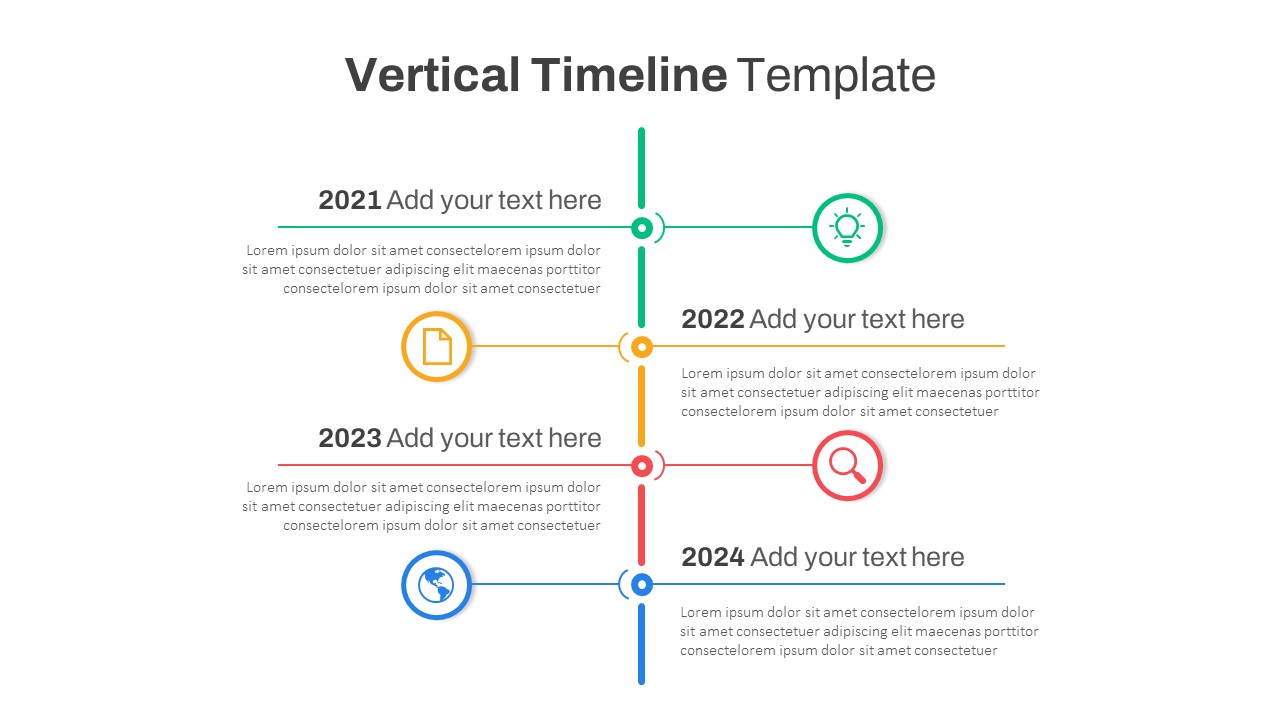
Vertical Timeline Powerpoint Template

Negotiation Powerpoint Presentation Template

Conference Powerpoint Presentation Template
Paper Strip Process Timeline Powerpoint Template

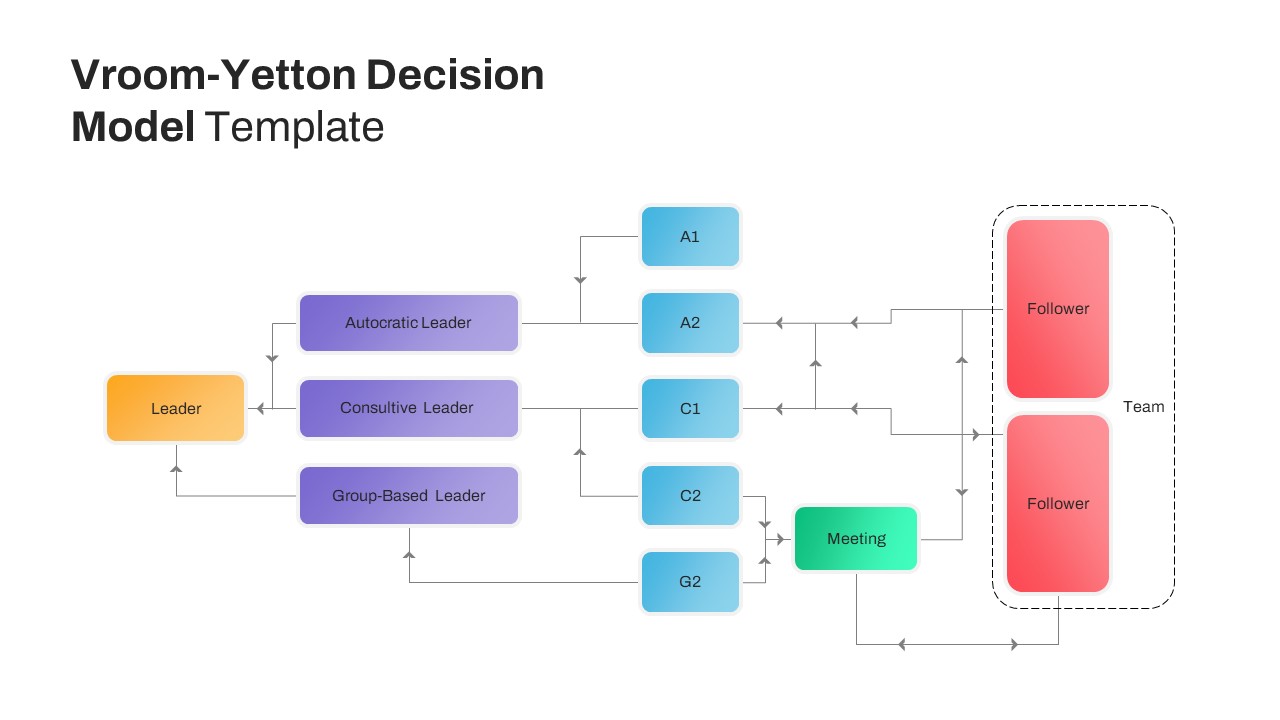
Vroom Yetton Model Google Slide Template
Box Timeline Slide Presentation Template

Free Vintage Powerpoint Themes
Project Timeline Slide Template
Block Timeline Slide Template

Watercolour Powerpoint Template
Animated Curved Timeline Powerpoint Template
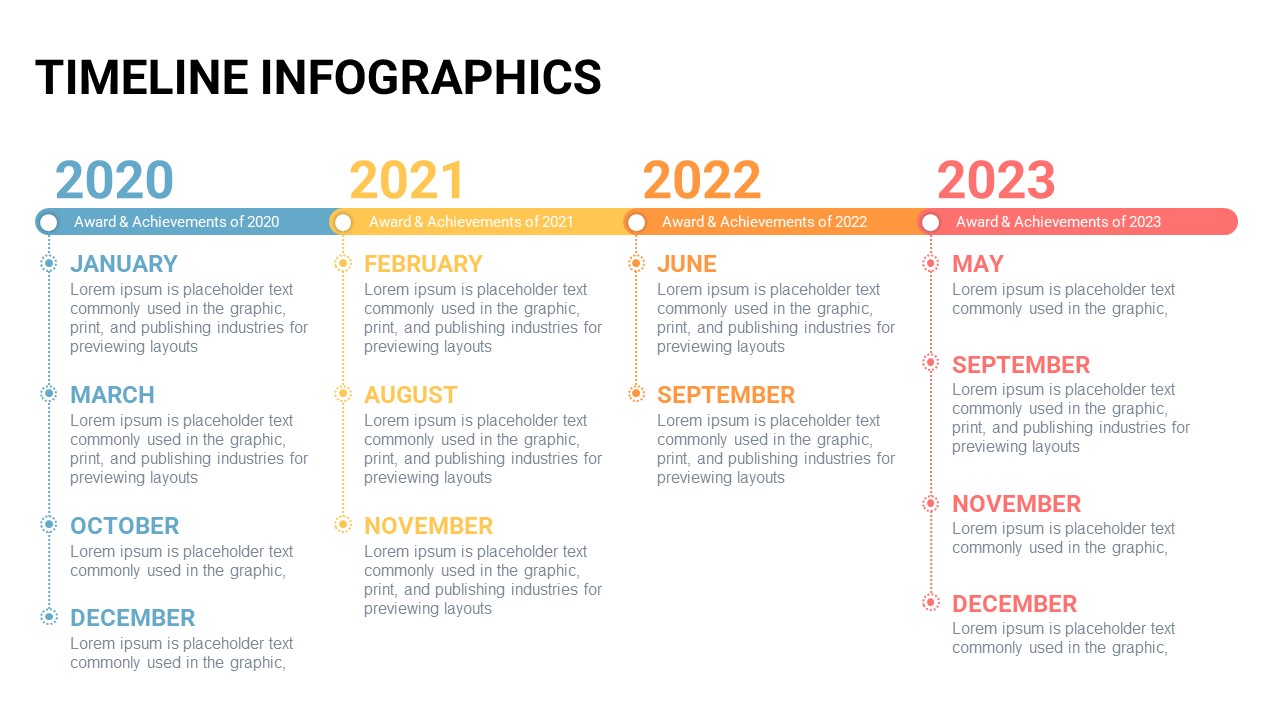
Timeline Template For Power Point

Marketing Plan Presentation Templates
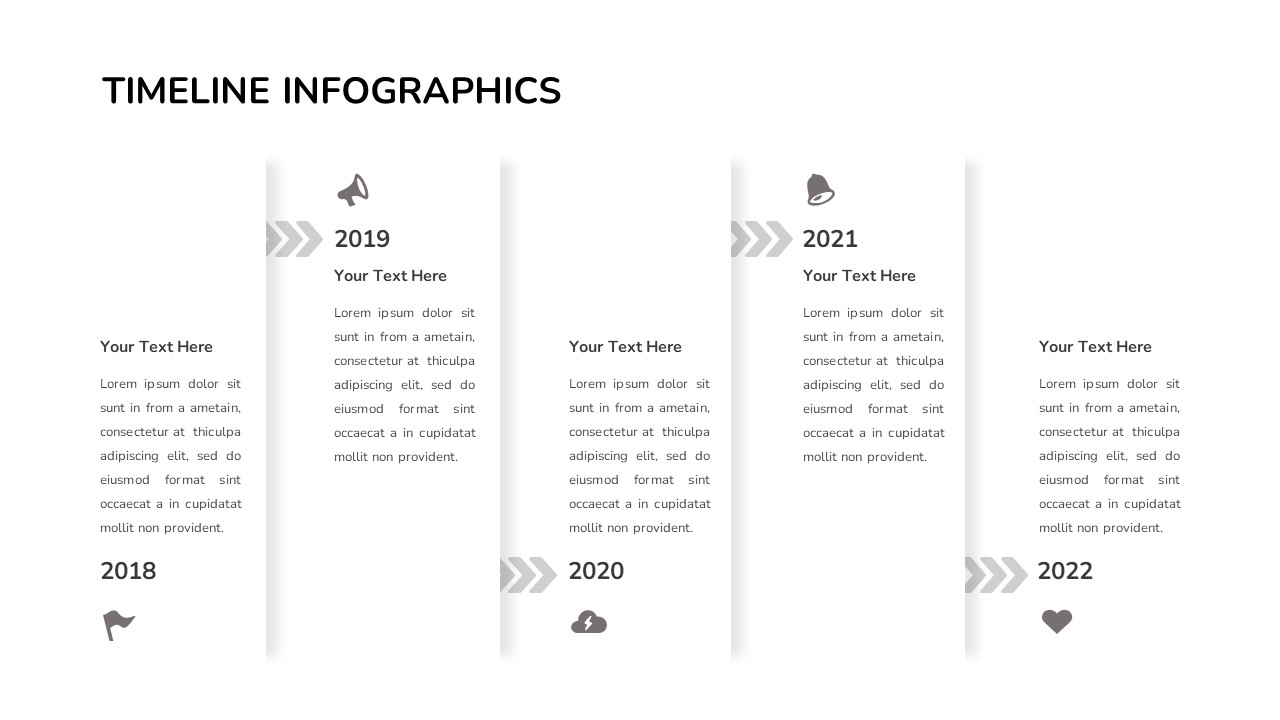
Creative Timeline Powerpoint Presentation Template

Theater Theme Google Slides
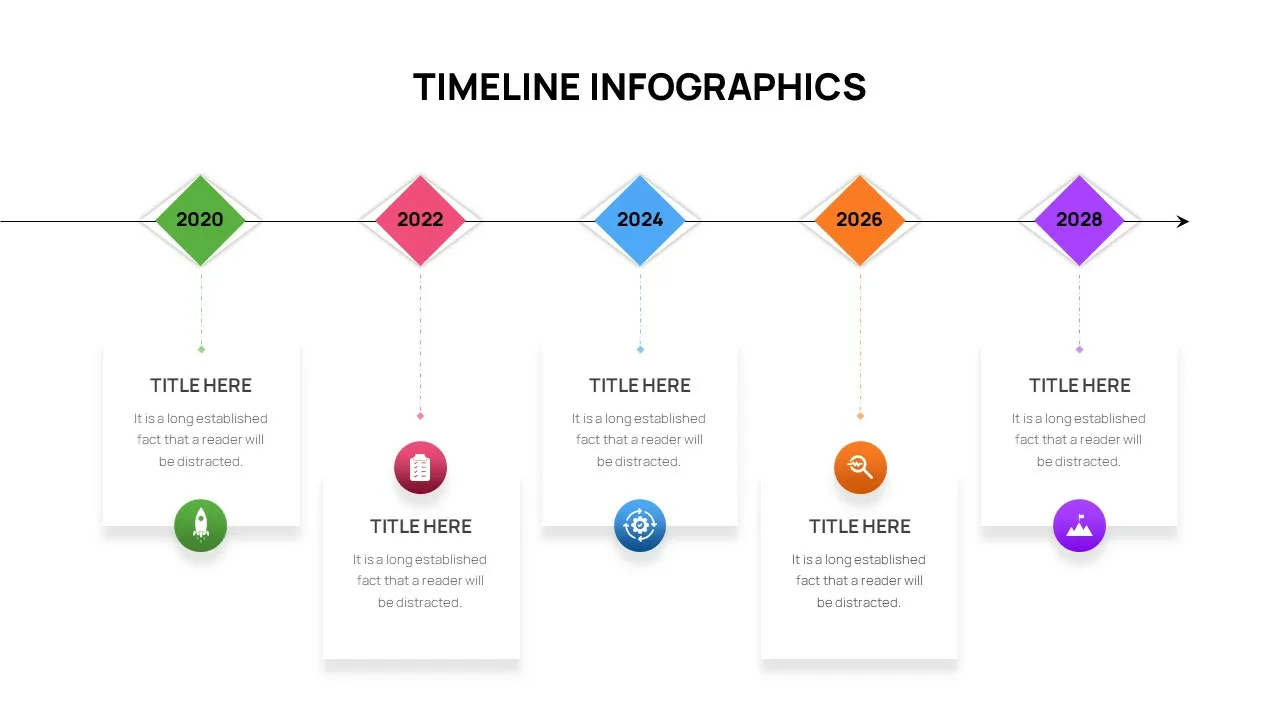
Editable Timeline Slide Template
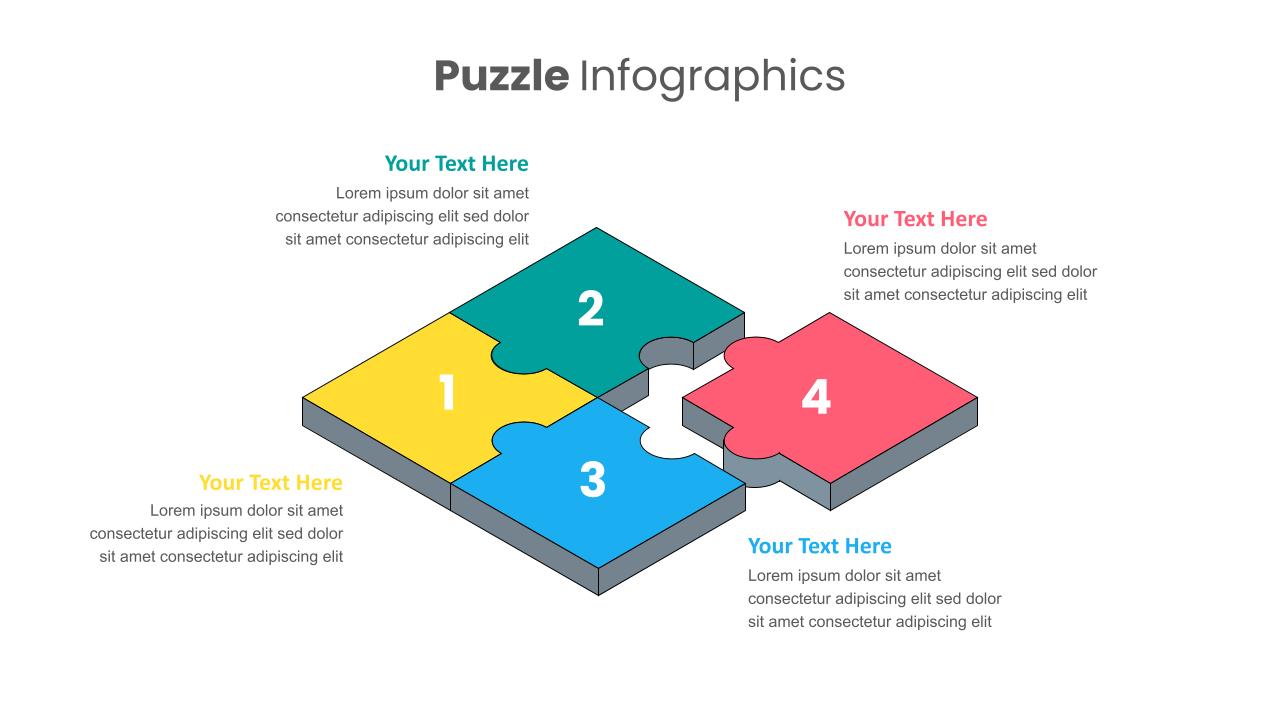
Puzzle Pieces Infographics Presentation Slides

Consultant Presentation Pitch Deck Templates
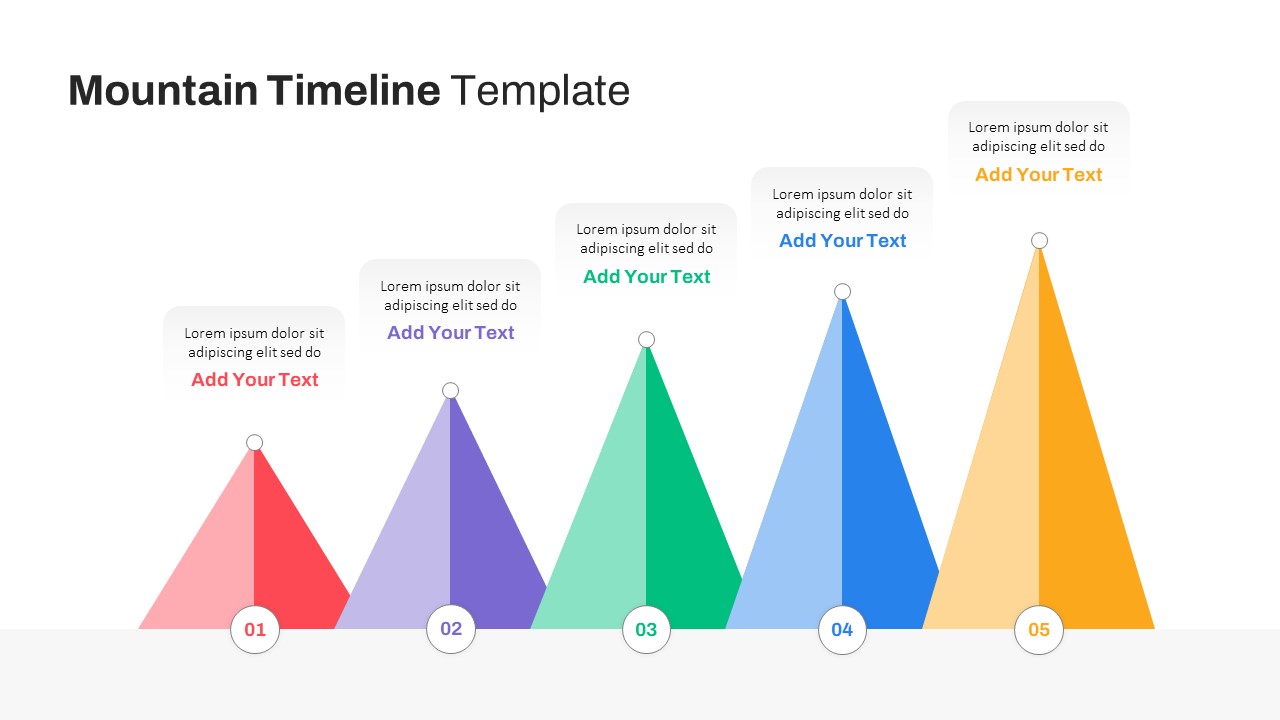
Mountain Timeline Slides Template
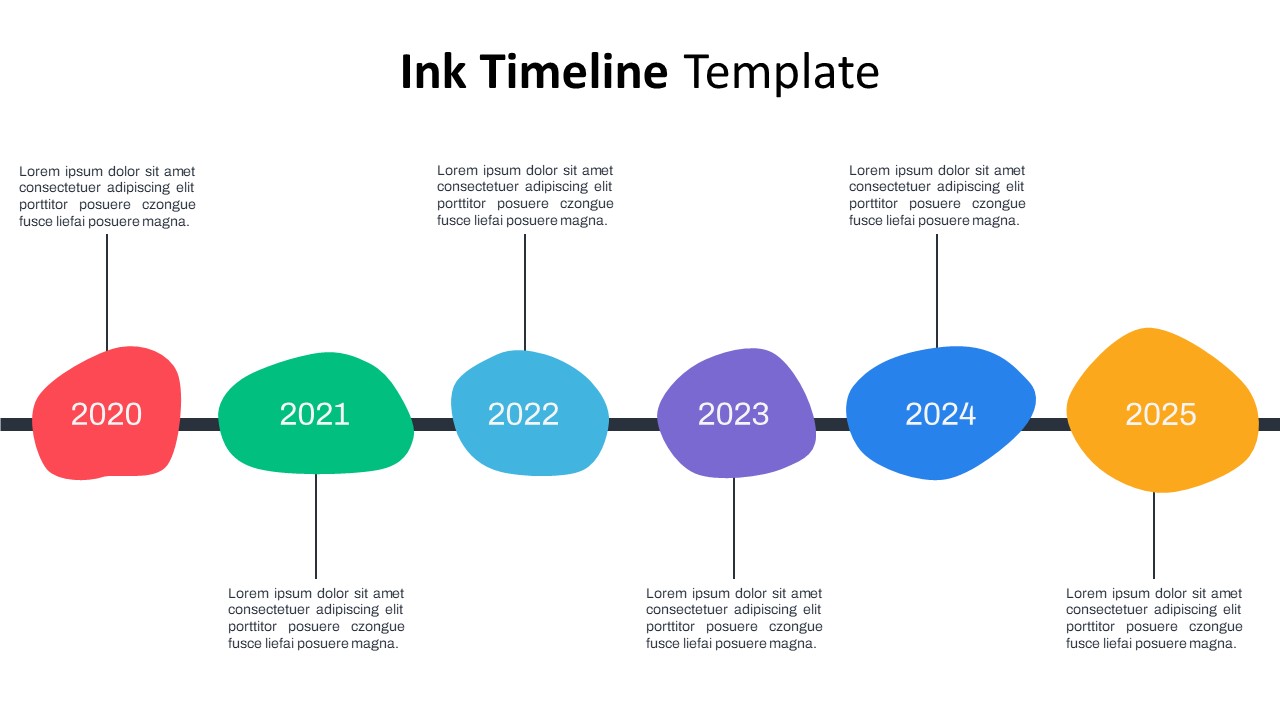
Ink Timeline Presentation Slide

Education Timeline Presentation Template
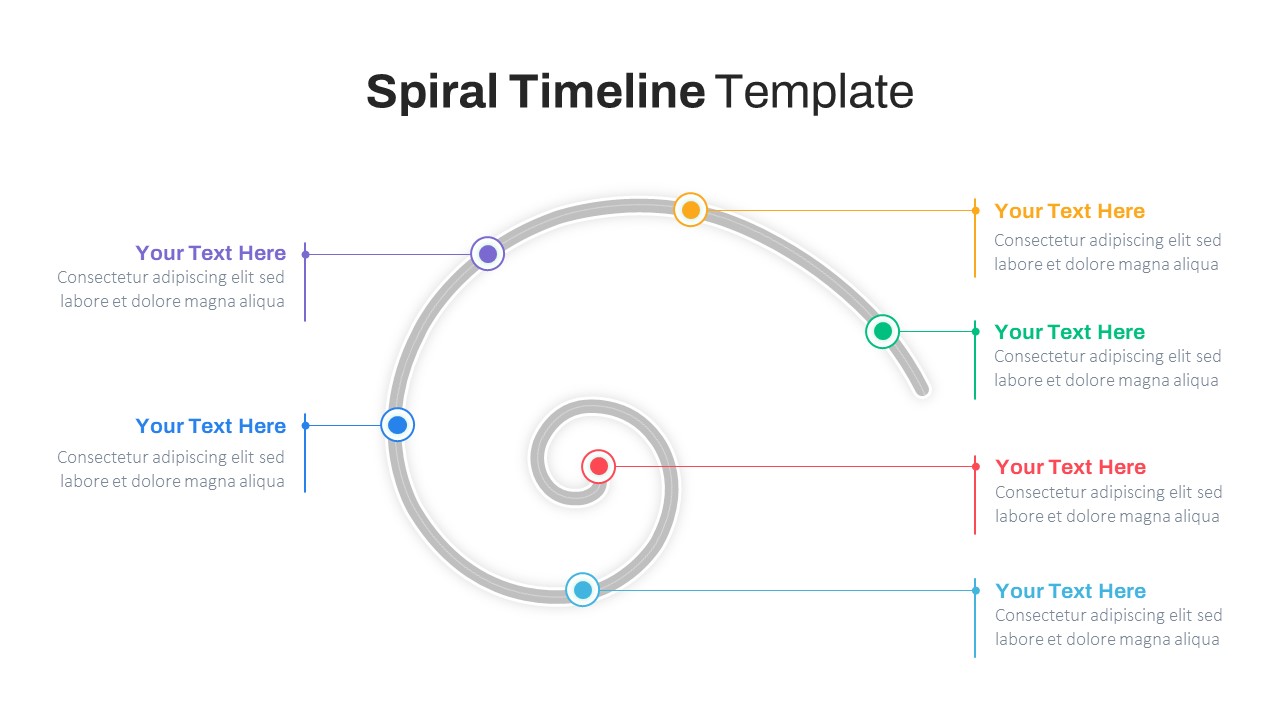
Spiral Timeline Template

Digital Marketing Presentation Slide

Free Sales Funnel Presentation Template

Modern Google Slides Presentation Template
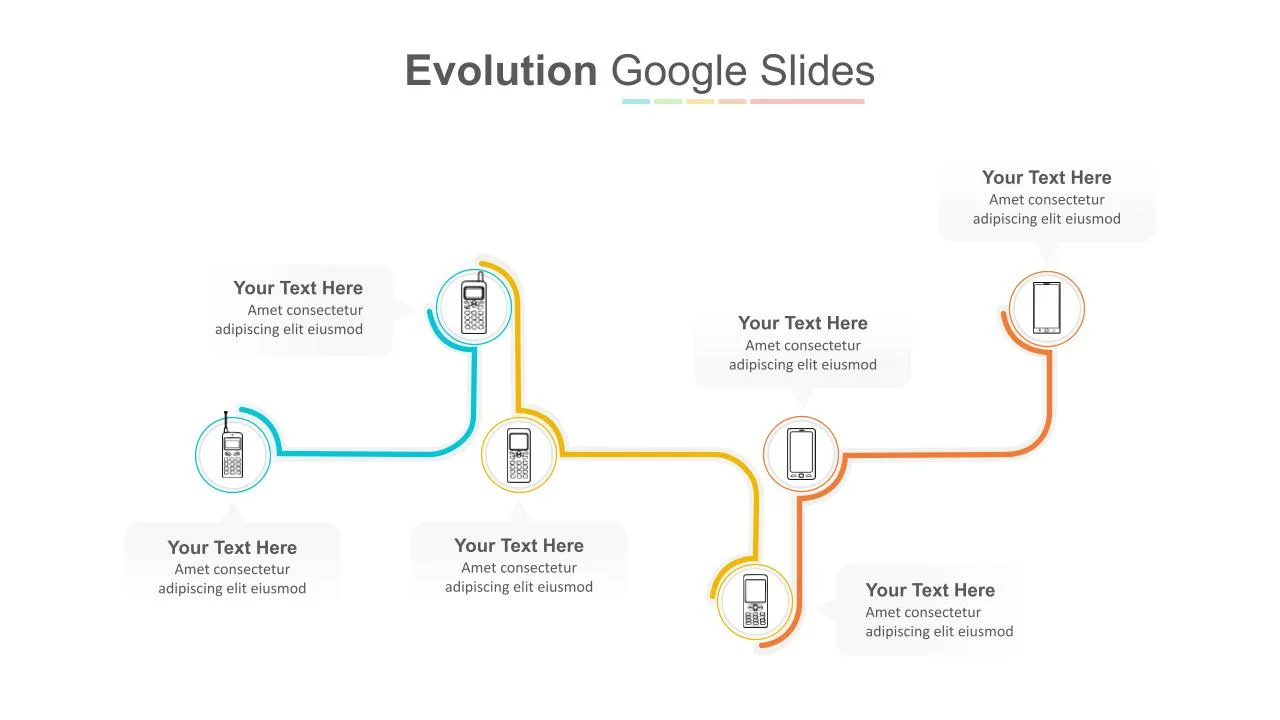
Multi-Step Evolution Slide Template
Funnel Slide Template
Simple Google Slide Background

Free Back-To-School Slides, Themes & Templates
Milestone Timeline Powerpoint Template

Free Science Presentation Template

Animated Professional Deck Presentation Template

Retro Presentation Slides

Animated Technology Presentation Template

Restaurant Google Slides Template

Purple Theme Presentation Template

Awesome Cartoon Slides
Customized Strategy Presentation Template
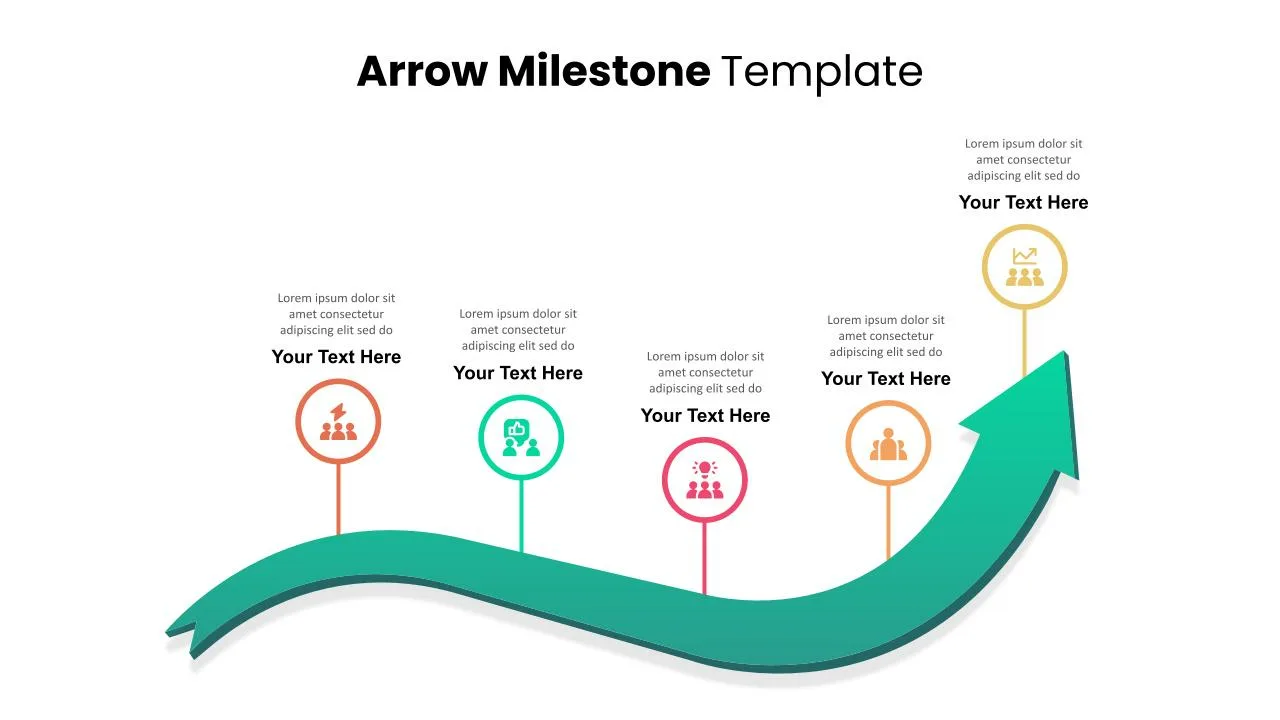
Timeline Arrow Presentation
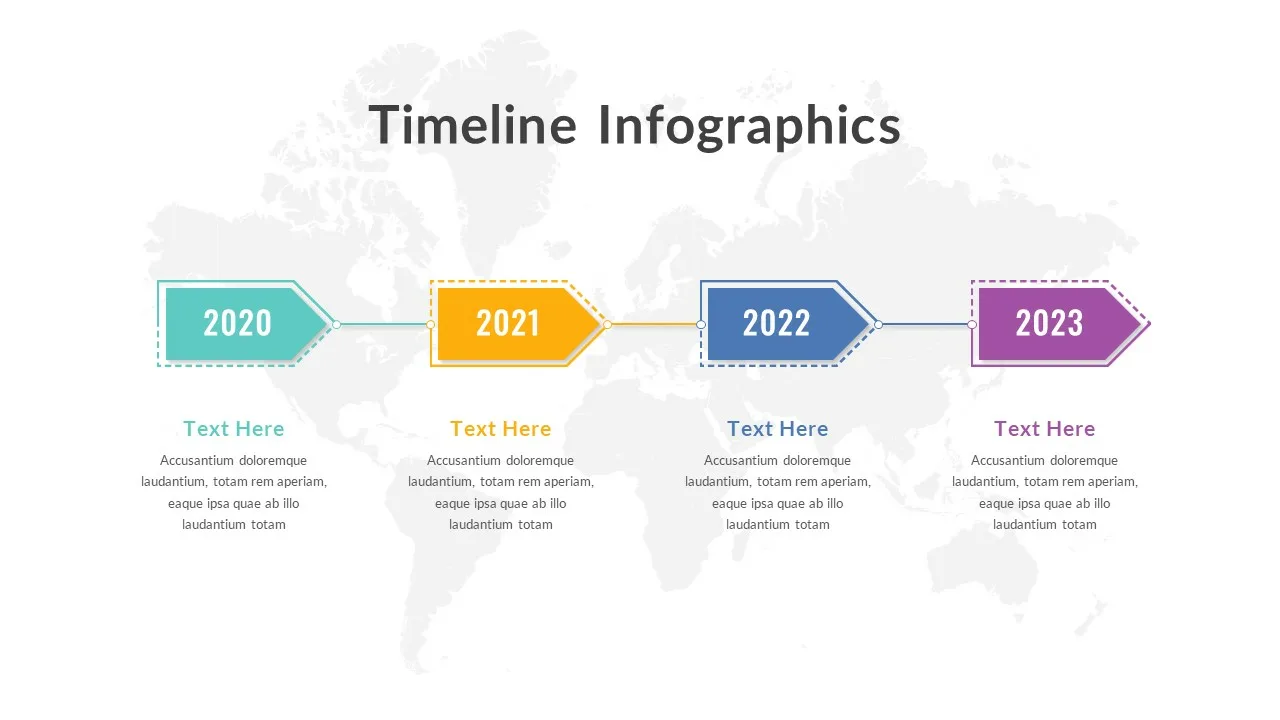
4 Stage Timeline Presentation Slide
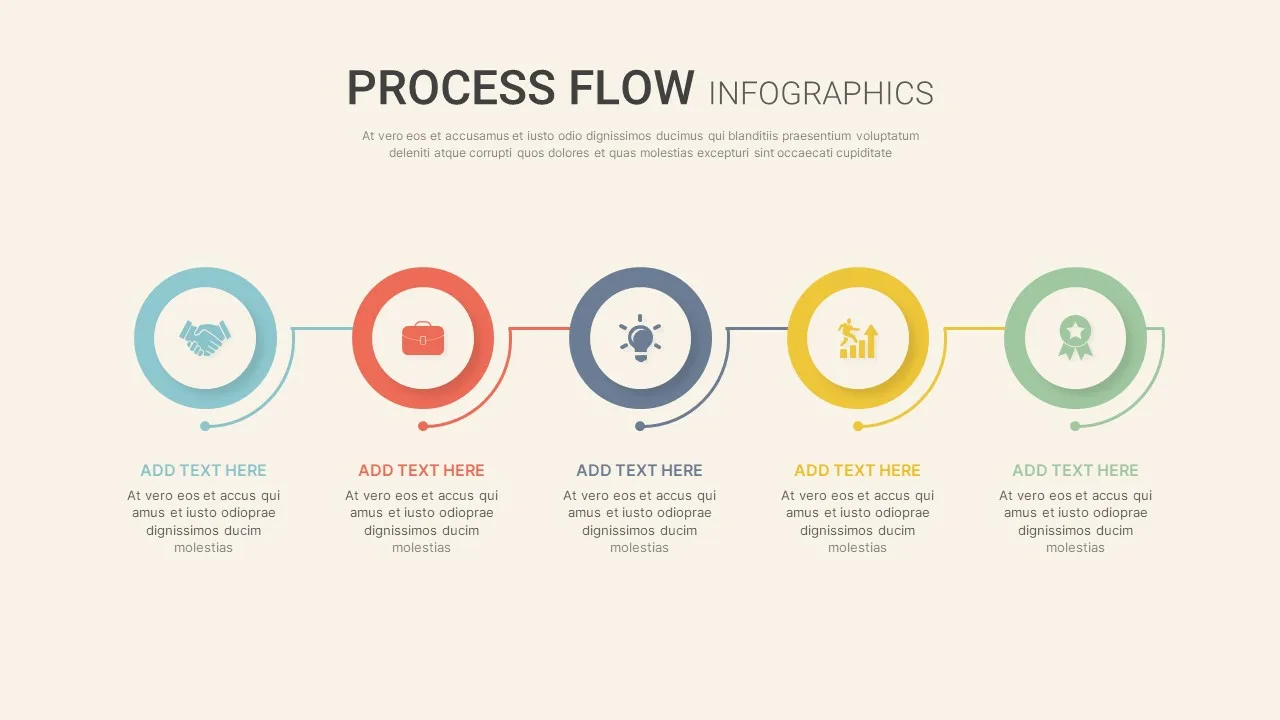
Linear Process Flow Template

2024 Calendar Presentation Template
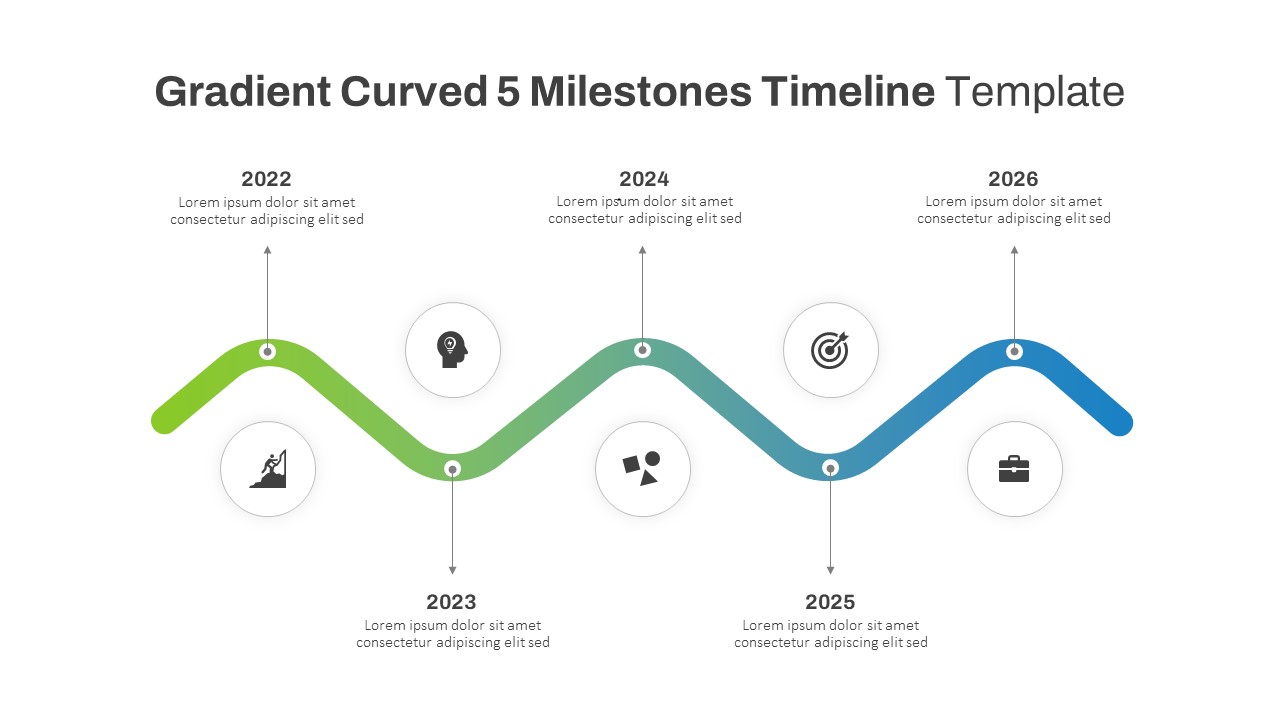
Gradient Curved 5 Milestone Powerpoint Slide

Start Stop Continue Slide Template
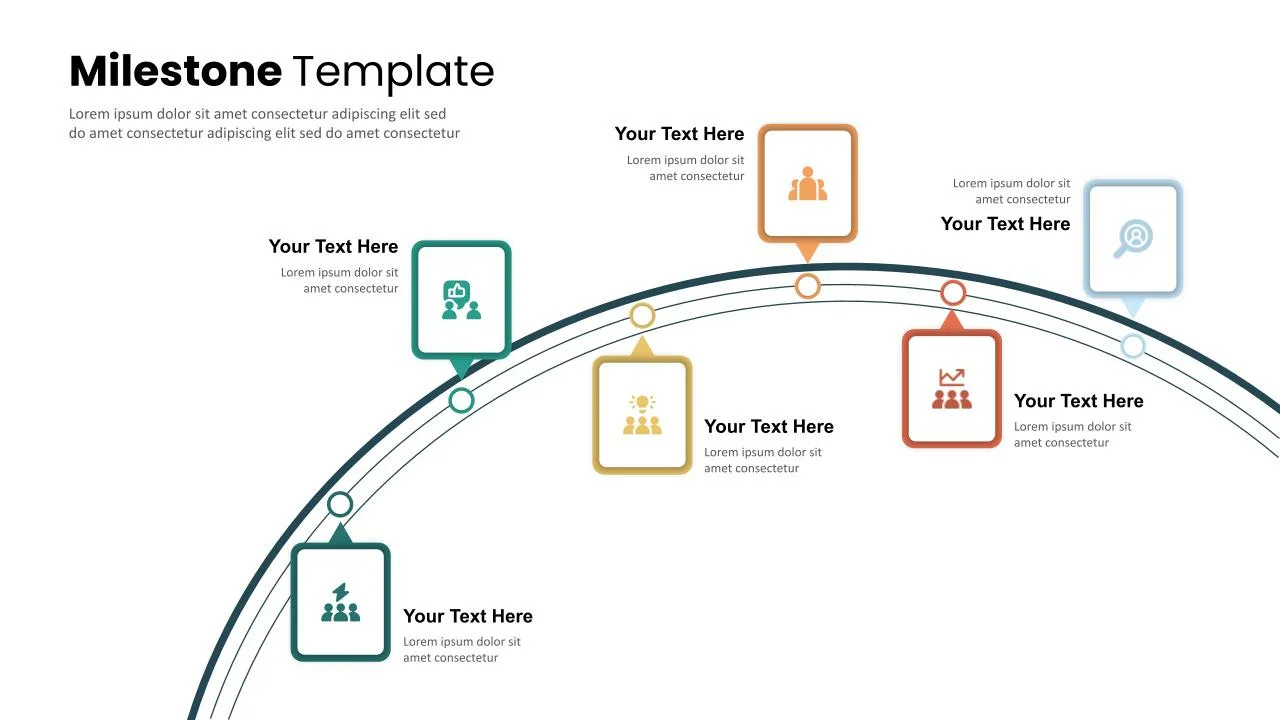
6 Step Milestones Presentation Slide

Finance Theme Powerpoint Templates

Free Movie Presentation Slides & Templates
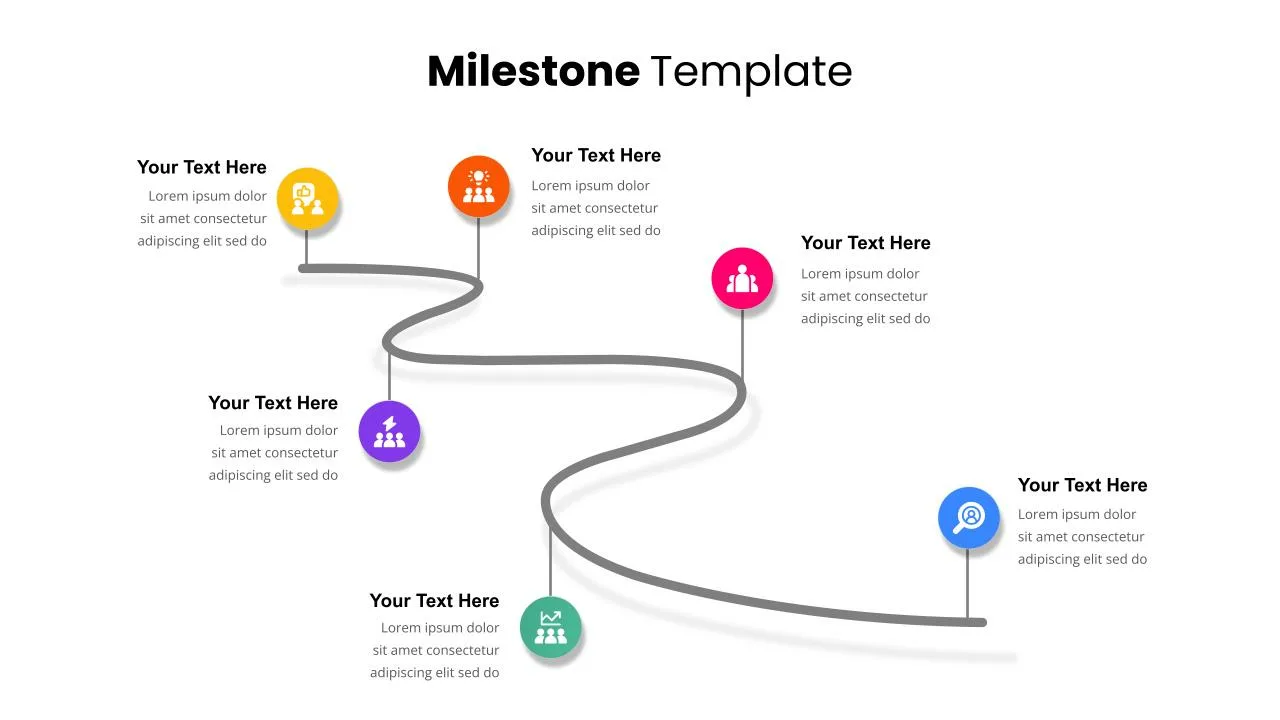
6 Point Milestones Slide Template
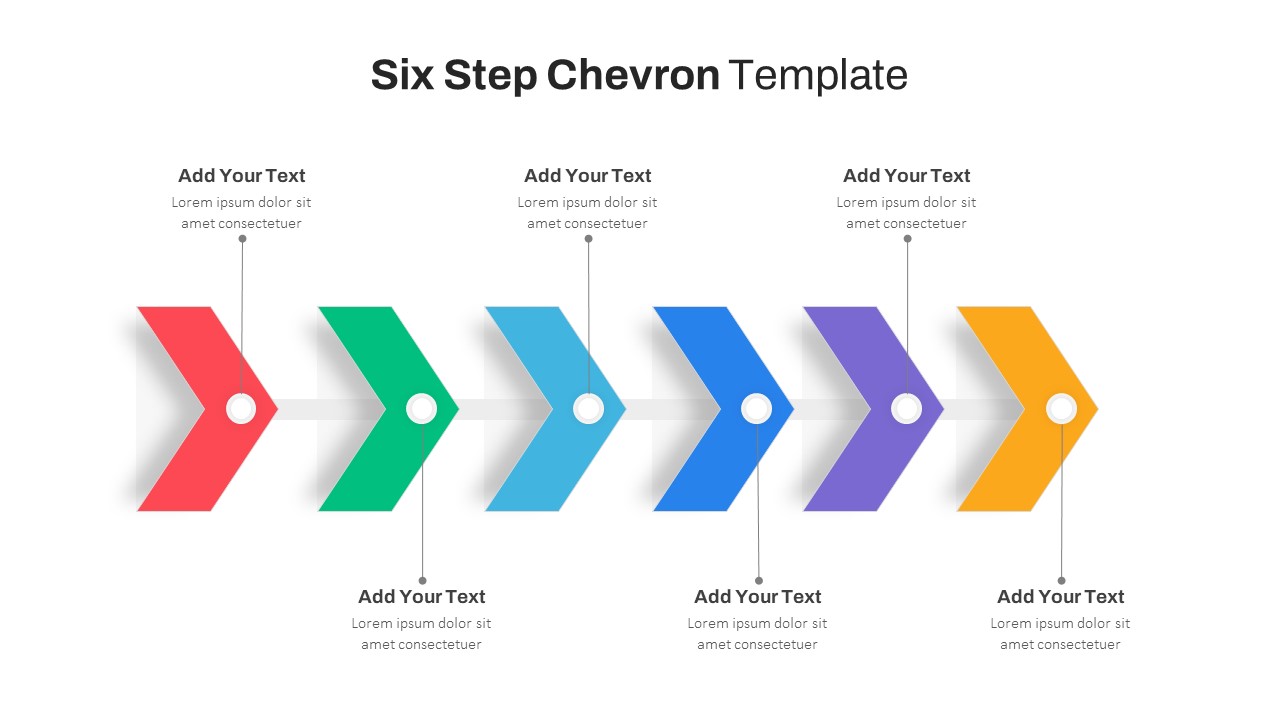
Six Step Chevron Slide Template
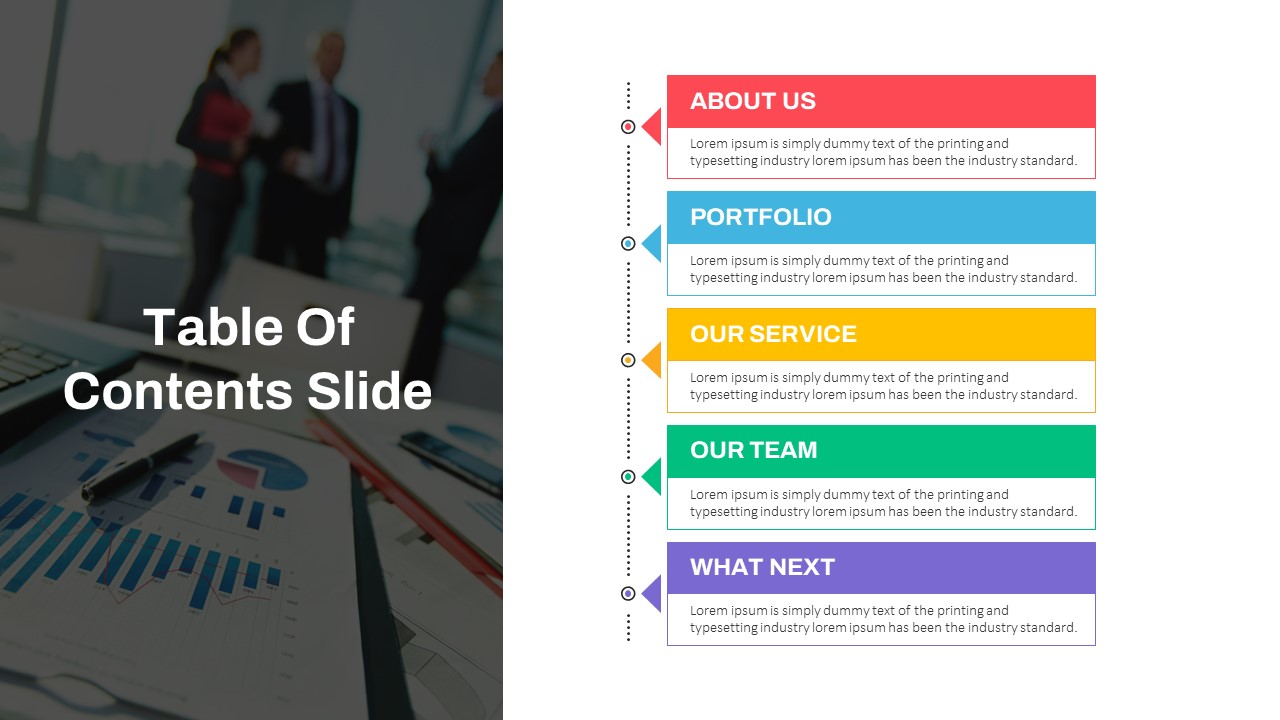
Table Of Contents Ppt Template
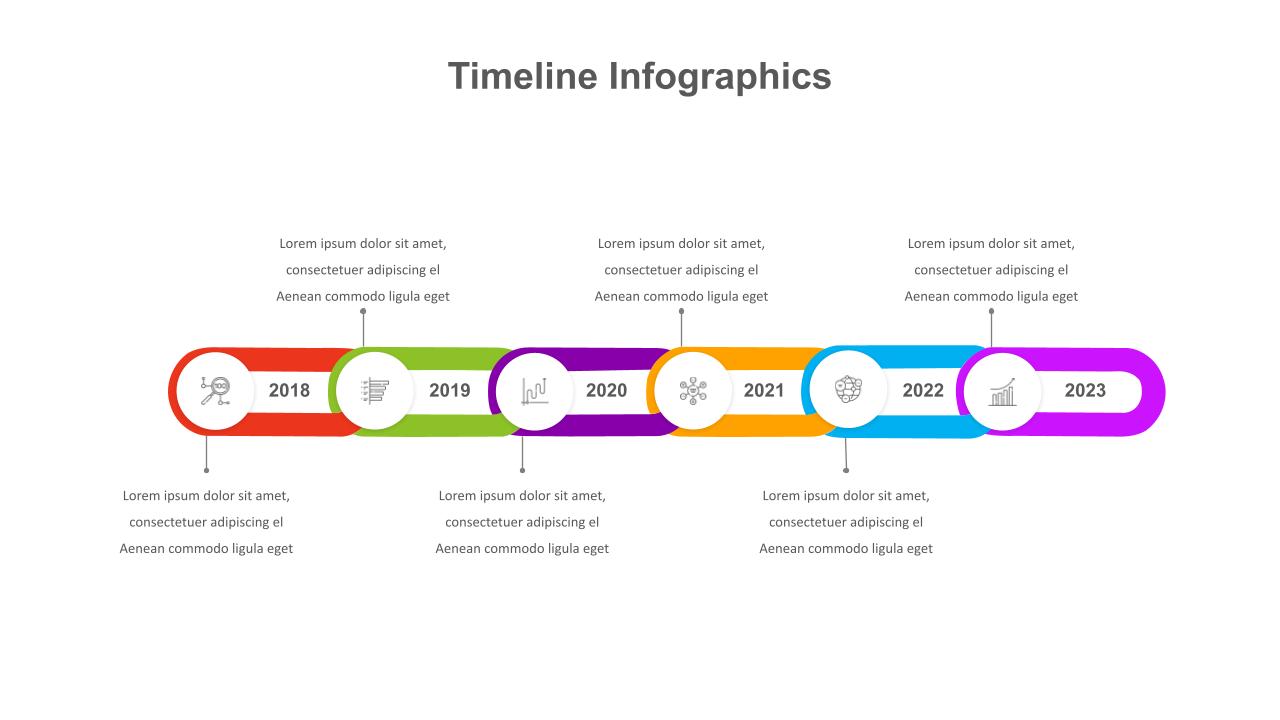
Powerpoint Timeline Template
Free Vertical PowerPoint Templates

Art Deco Presentation Template

Mergers And Acquisitions Slide

Google Slides Game Theme Templates

T-Shirt Business Powerpoint Presentation Template
Branch Timeline Slide
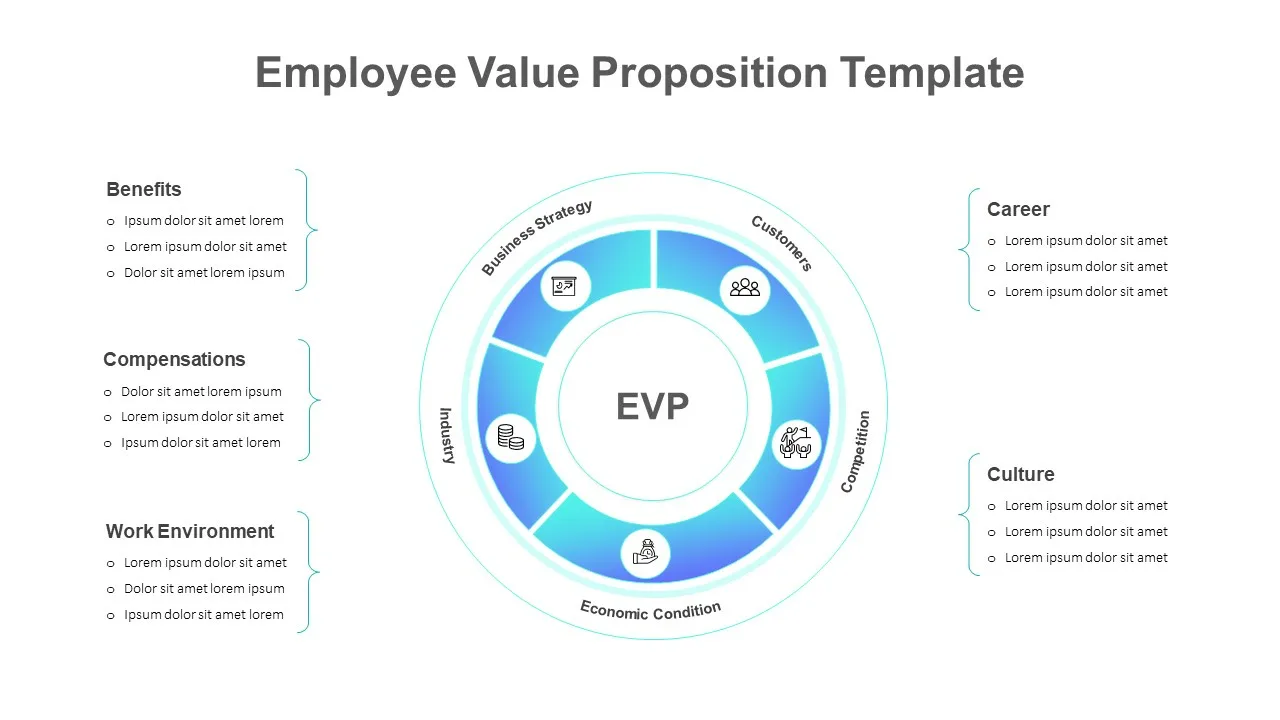
Employee Value Proposition Template

Minimalist Presentation Background Template

Agenda Template Power Point and Google Slides
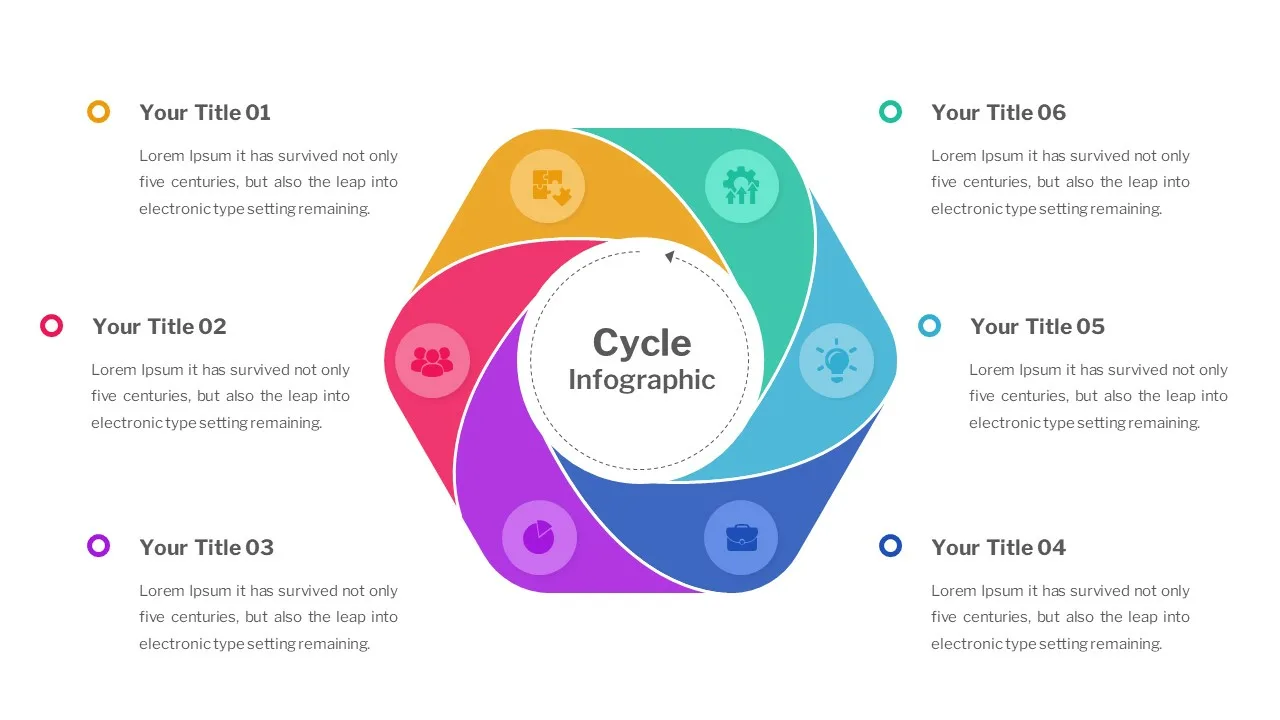
Cycle Slide Template

Orange, Blue & White Theme Templates
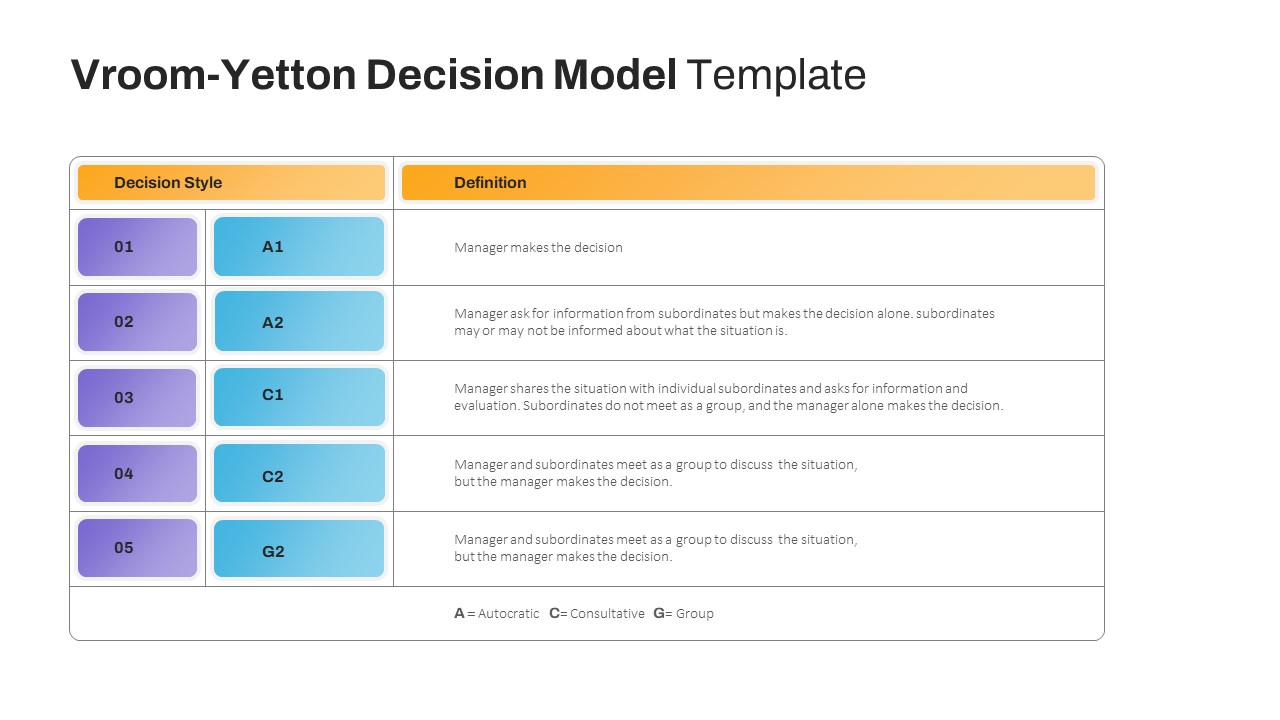
Vroom Yetton Decision Making Model Slides

Marketing Plan Template Slides

Colorful Theme Presentation Template
Weekly Timeline Powerpoint Presentation Template

Employee Of The Month Powerpoint Template

Technology Presentation Templates
Best Summary Google Slides & PowerPoint Templates

Purple And Yellow Theme Slides Template
Editable Budget Presentation Slides
Self Introduction Ppt Templates
Spaghetti Process Flow Slide Template
Travel Slide Template

Daily & Monthly Planner Calendar Ppt Template
Attractive Roadmap Template Slides
Vroom-Yetton Decision Model Template

Agriculture Presentation Template
Customized 3D Presentation Template
Mathematics Powerpoint Templates

Attractive Green Theme Presentation Template
Strategic Planning Presentation Template

Pastel Google Slides Presentation Theme

Free Carnival Slides for Powerpoint and Google Slides

Professional Pitch Deck Template

Creative Pastel Themes & Templates

Product Pitch Powerpoint Templates
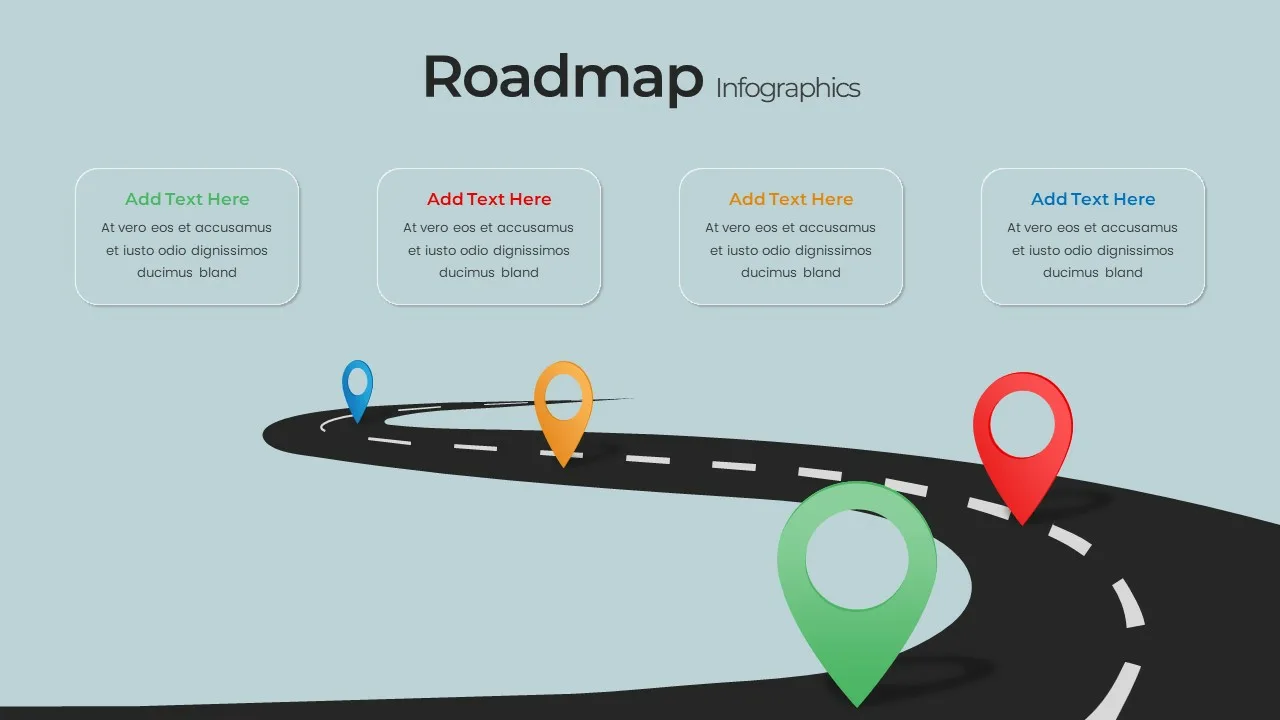
Editable Road Map Presentation Template
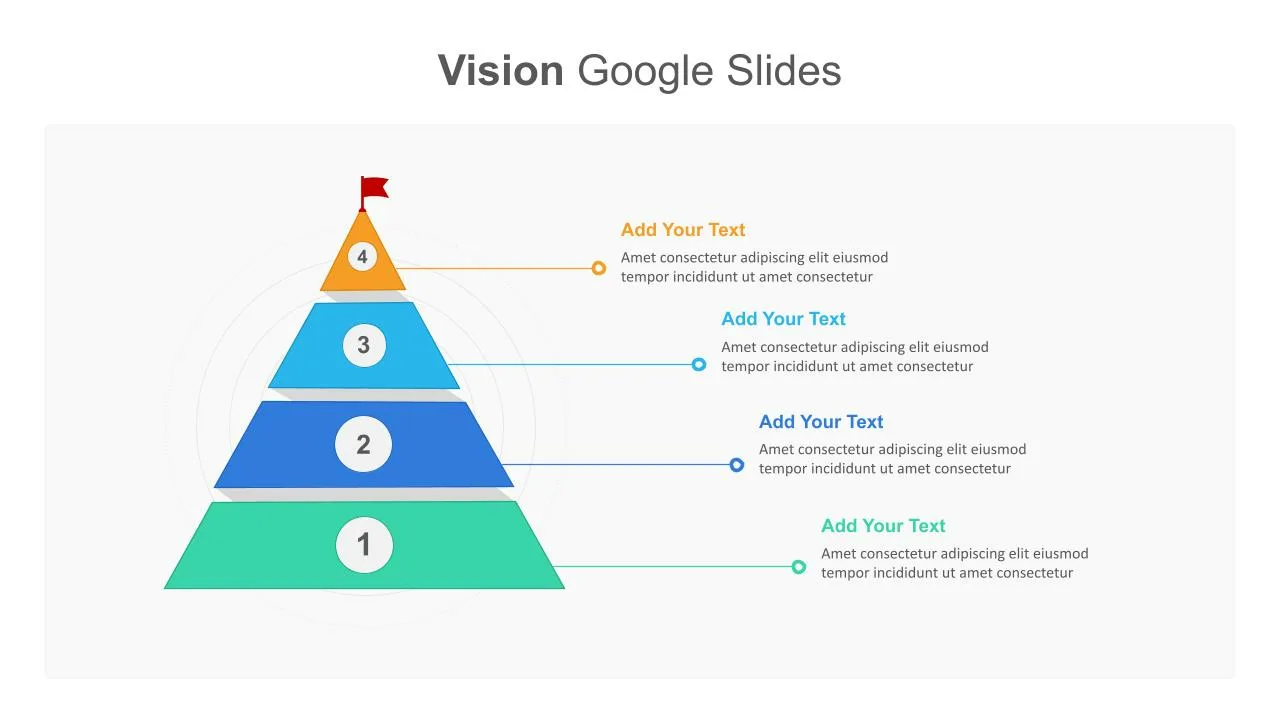
Business Vision Presentation Slides

Free Jeopardy Game Theme Slides & Templates
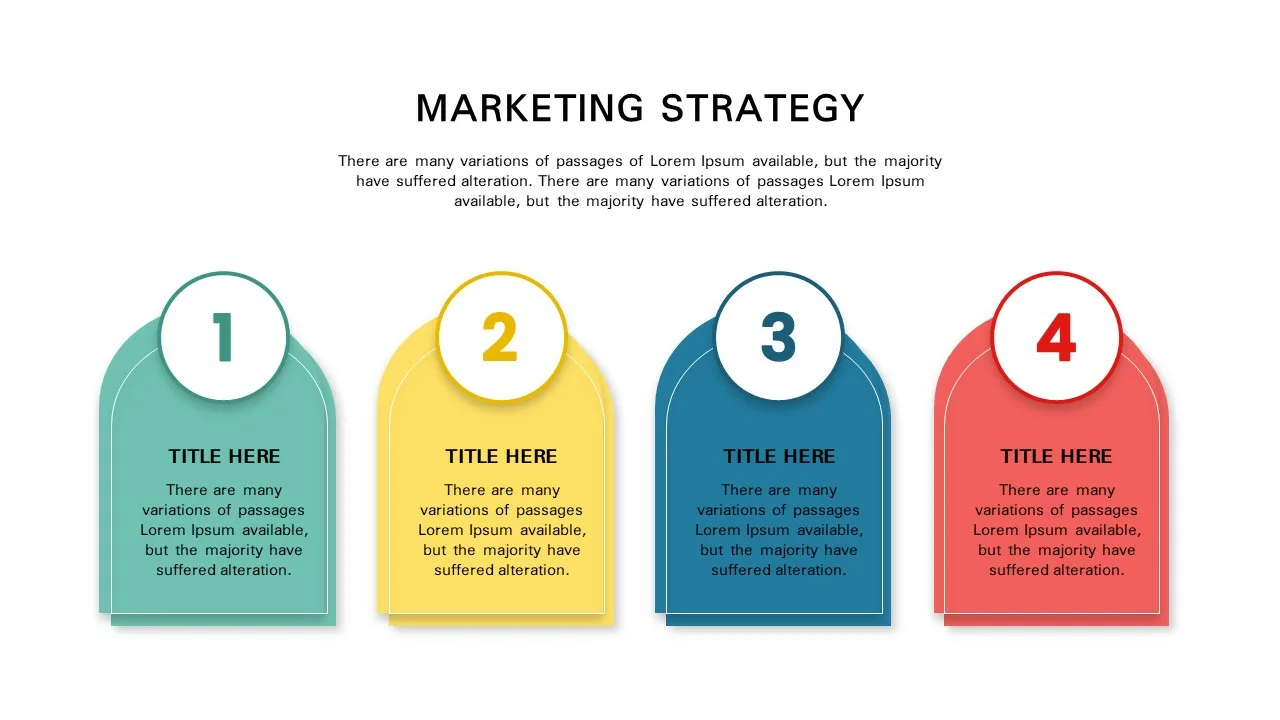
Marketing Strategy Presentation Template

Food Startup Pitch Deck Templates
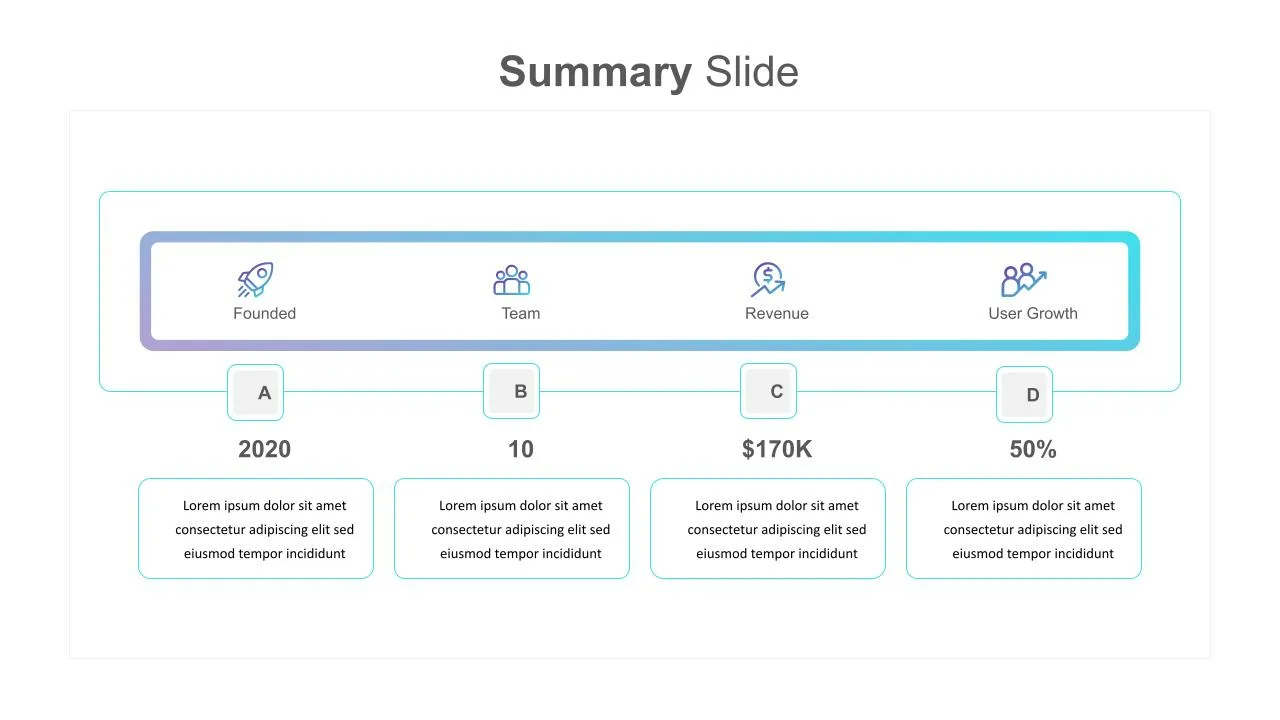
Summary Slide Templates
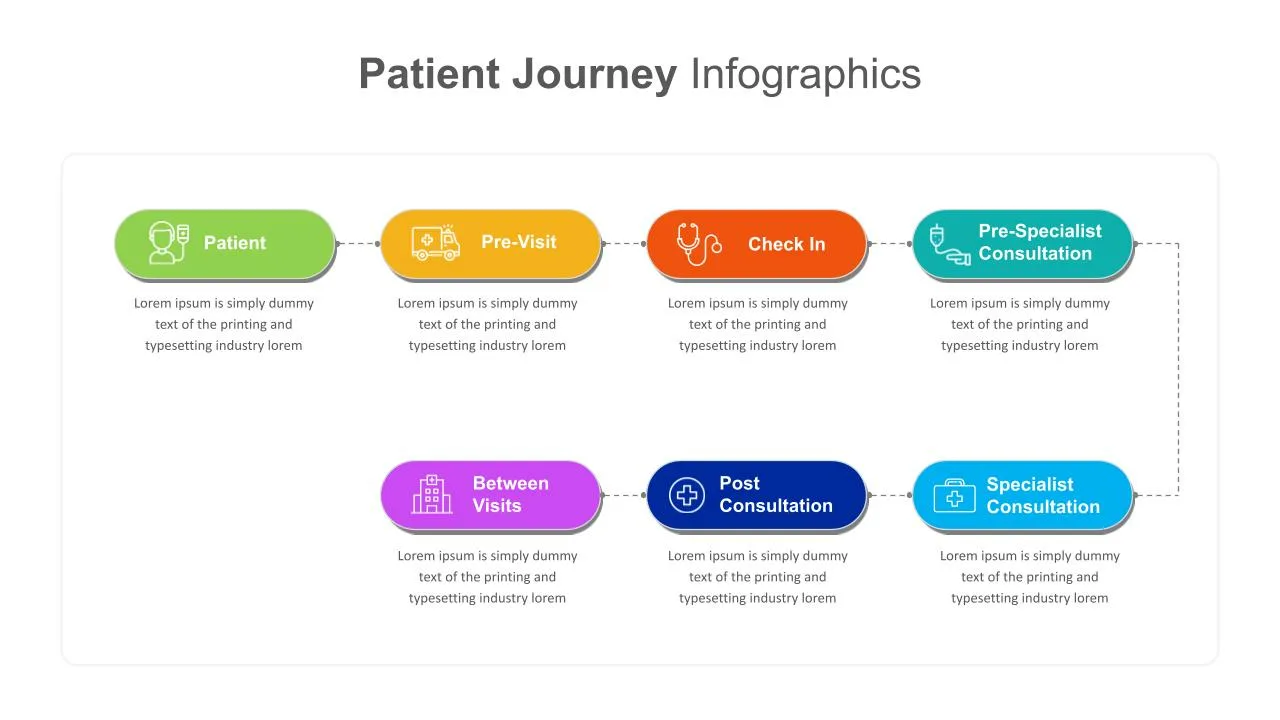
Patient Journey Slides & PowerPoint Templates

Free Earth Day Presentation Slides
Company Profile Slide Template
5 Step Globe Timeline Slide Google Slides

Classroom Slide Background Template
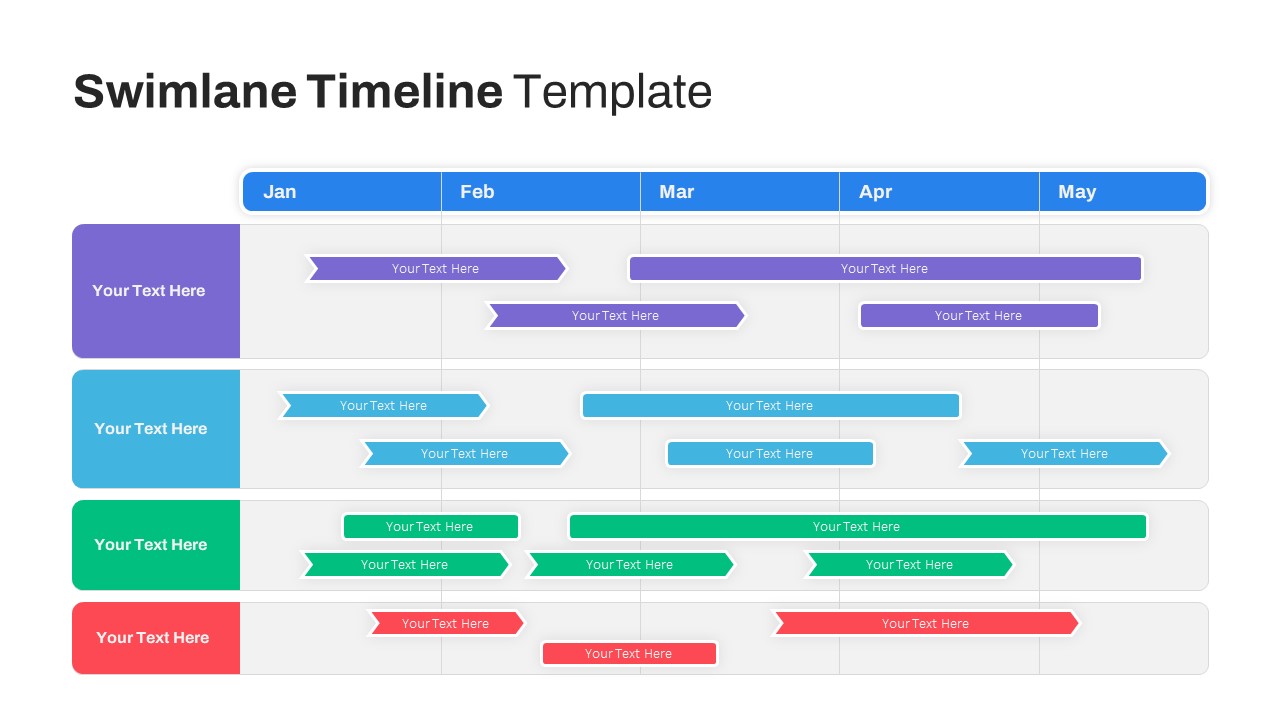
Swimlane Timeline Slide Template
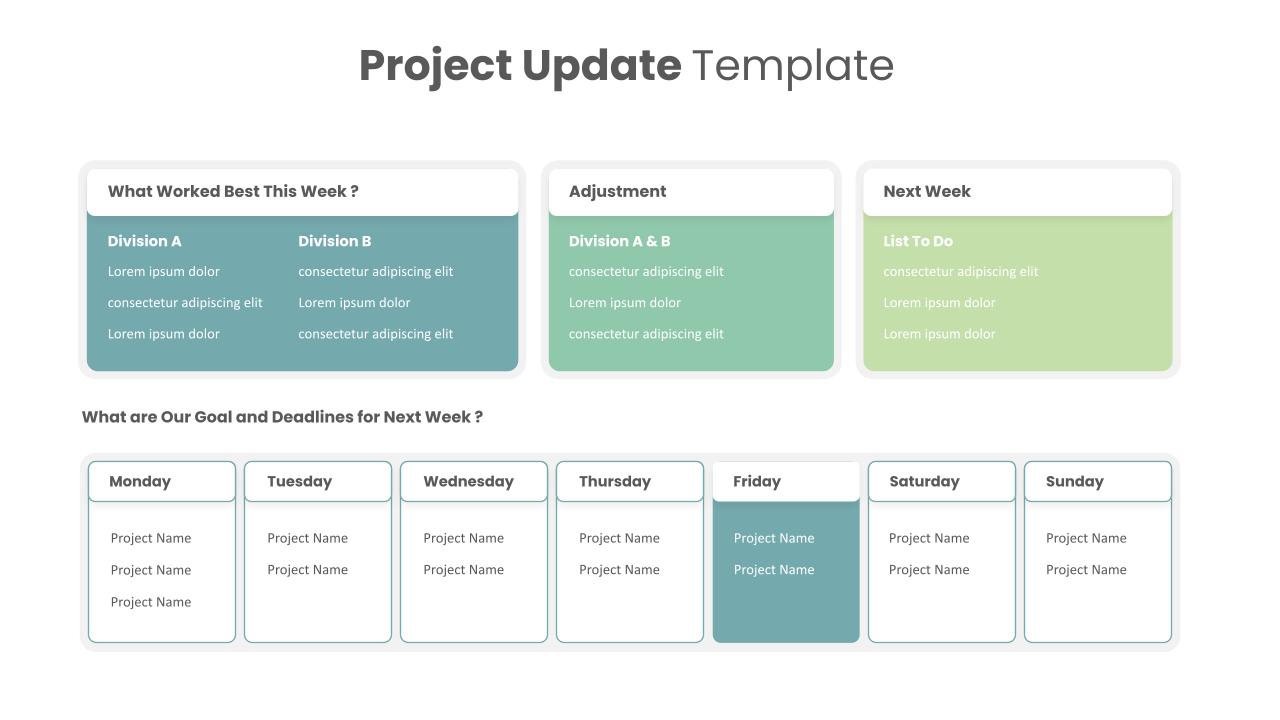
Editable Project Status Presentation Template
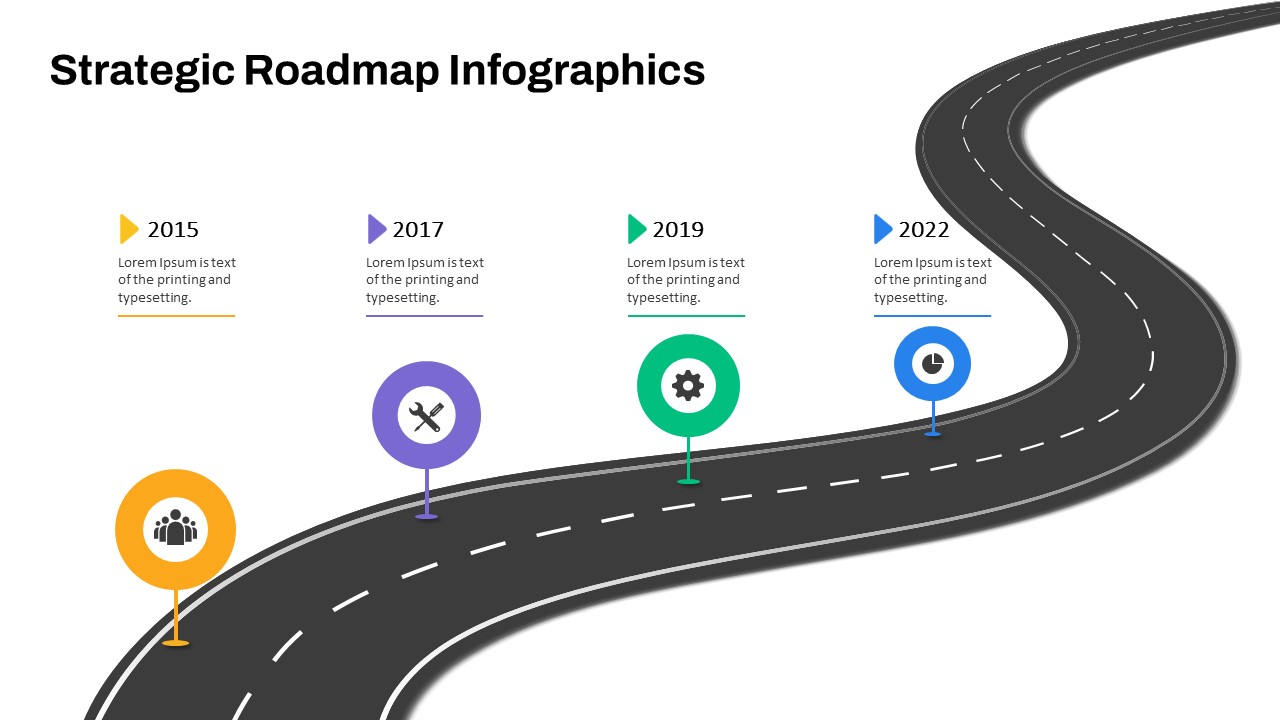
Strategic Roadmap PowerPoint Template
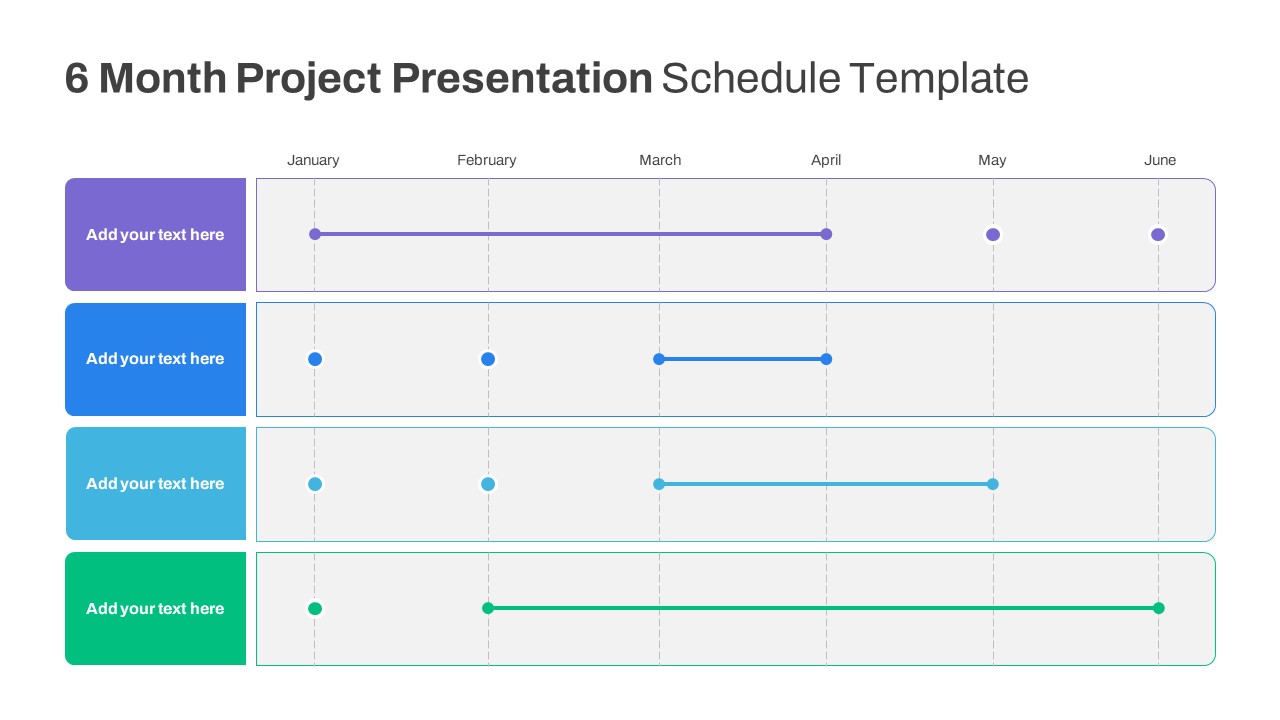
6 Month Project Presentation Slide Template
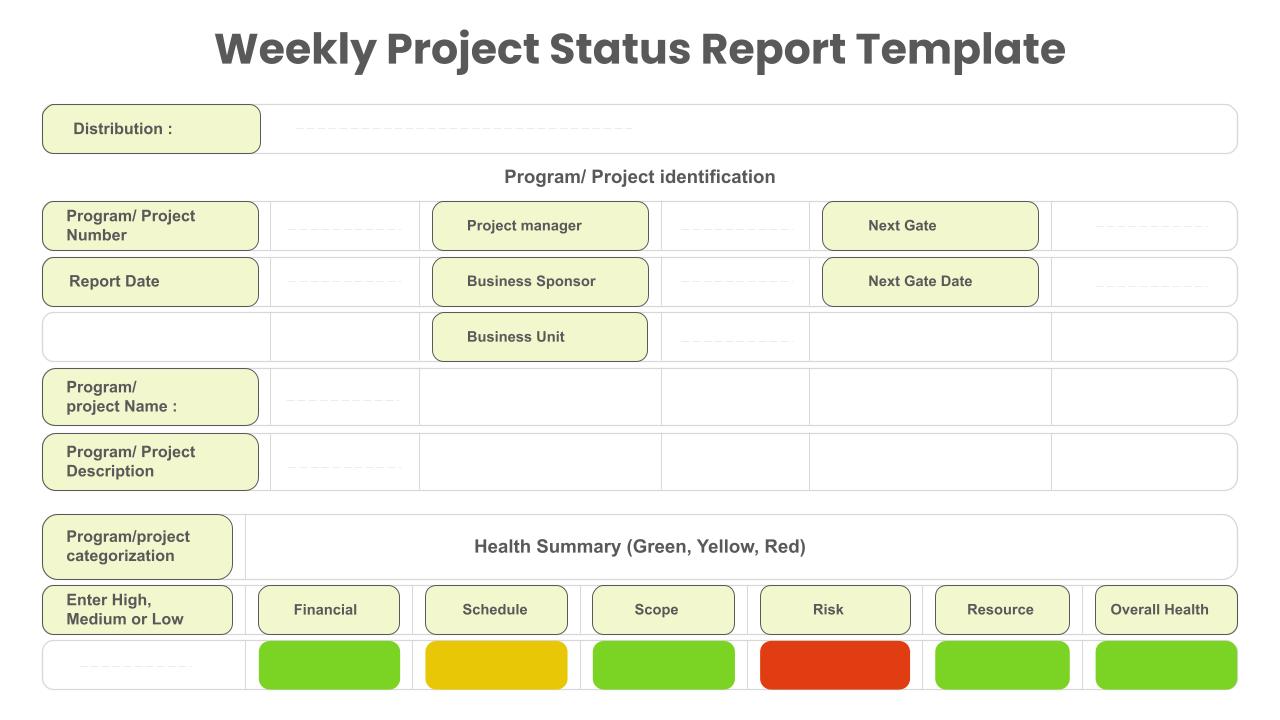
Project Status Report Presentation

Free Meet Your Teacher Presentation Template
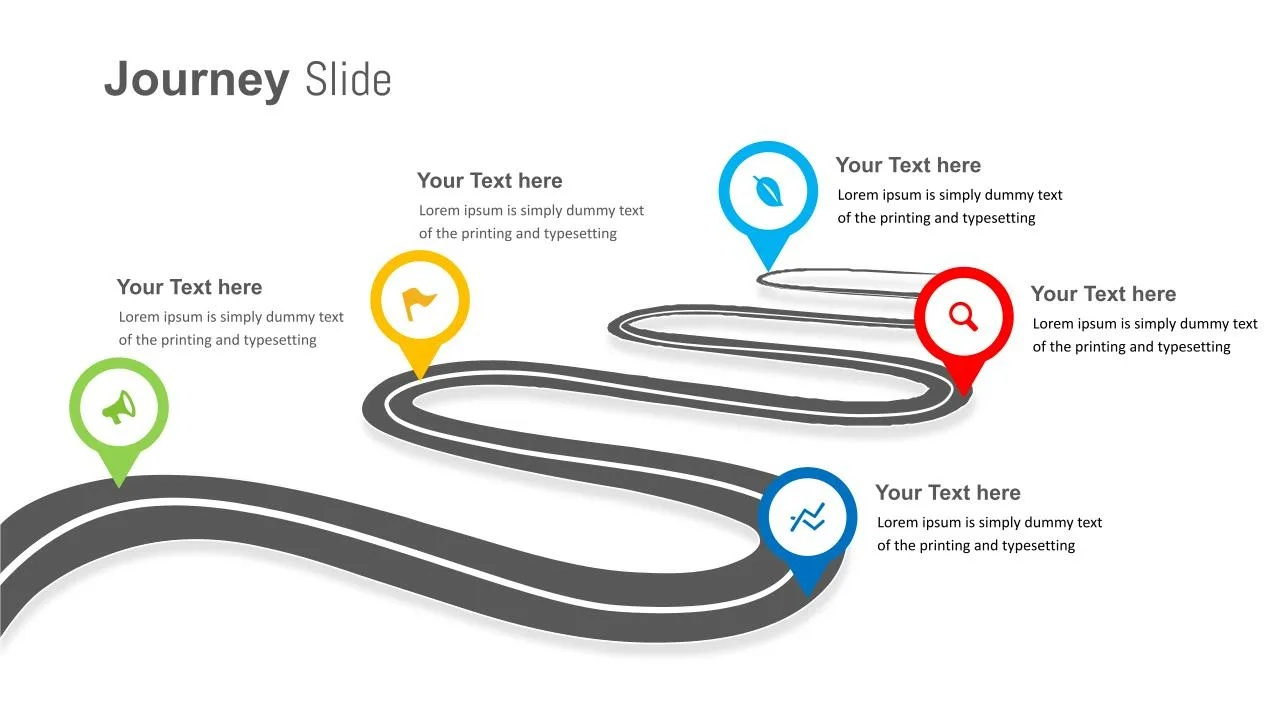
Journey Slide Templates
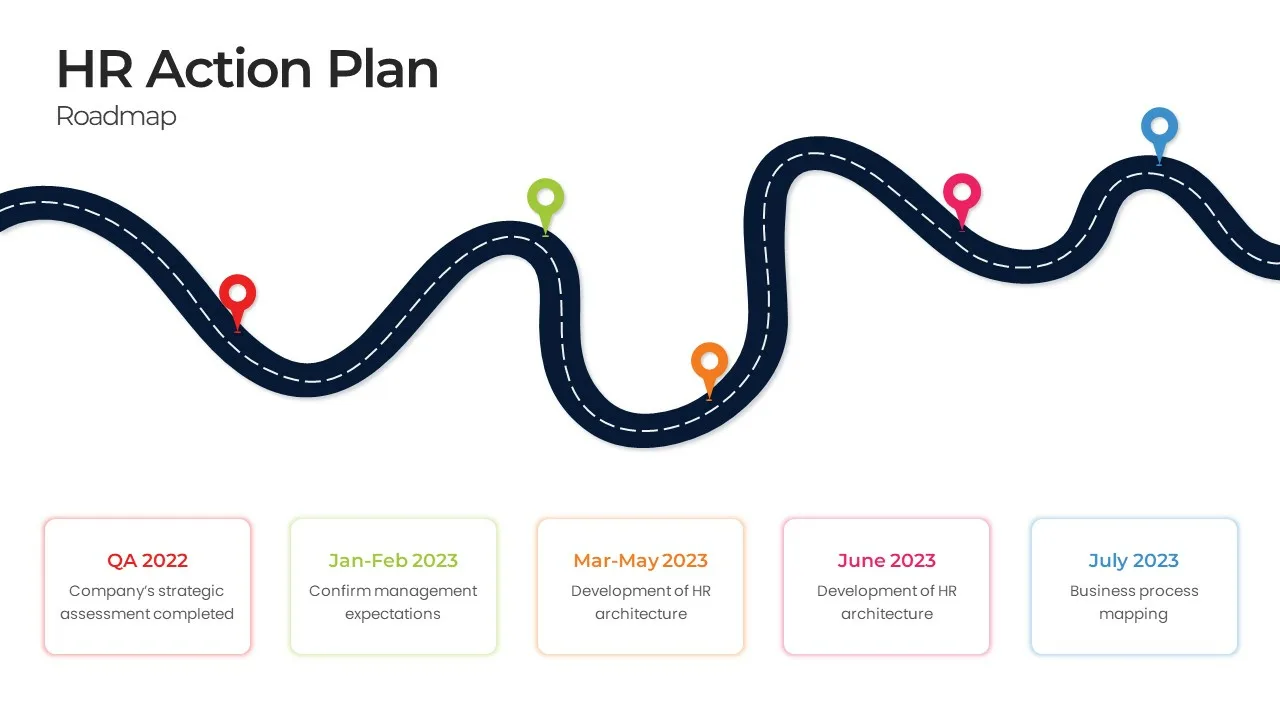
HR Roadmap Presentation

Checklist Slide Template
Circle Diagrams Presentation Template

Dark Blue Theme Nft Powerpoint Template
Strategic Plan Presentation Template

Abstract Presentation Template
Five Ws -Who What When Where Why Slide

Fitness Slide Template

Nature Presentation Template

Teacher Slide Template
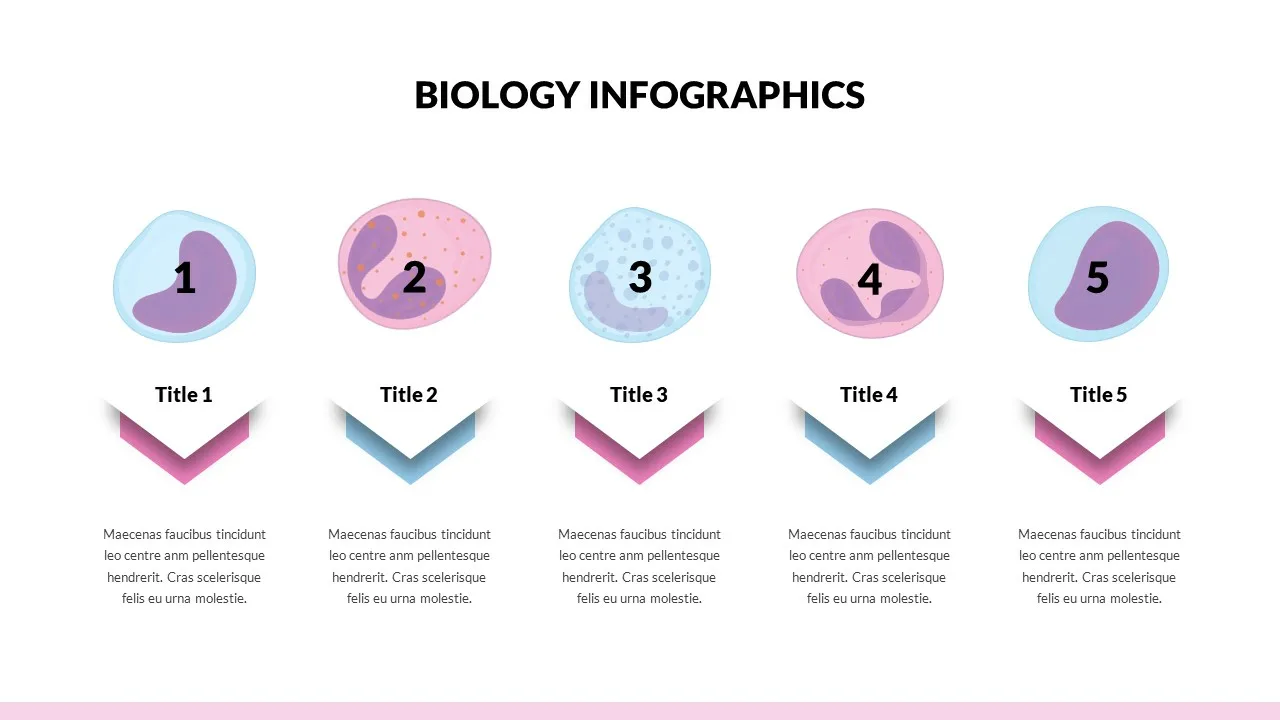
Biology Presentation Template
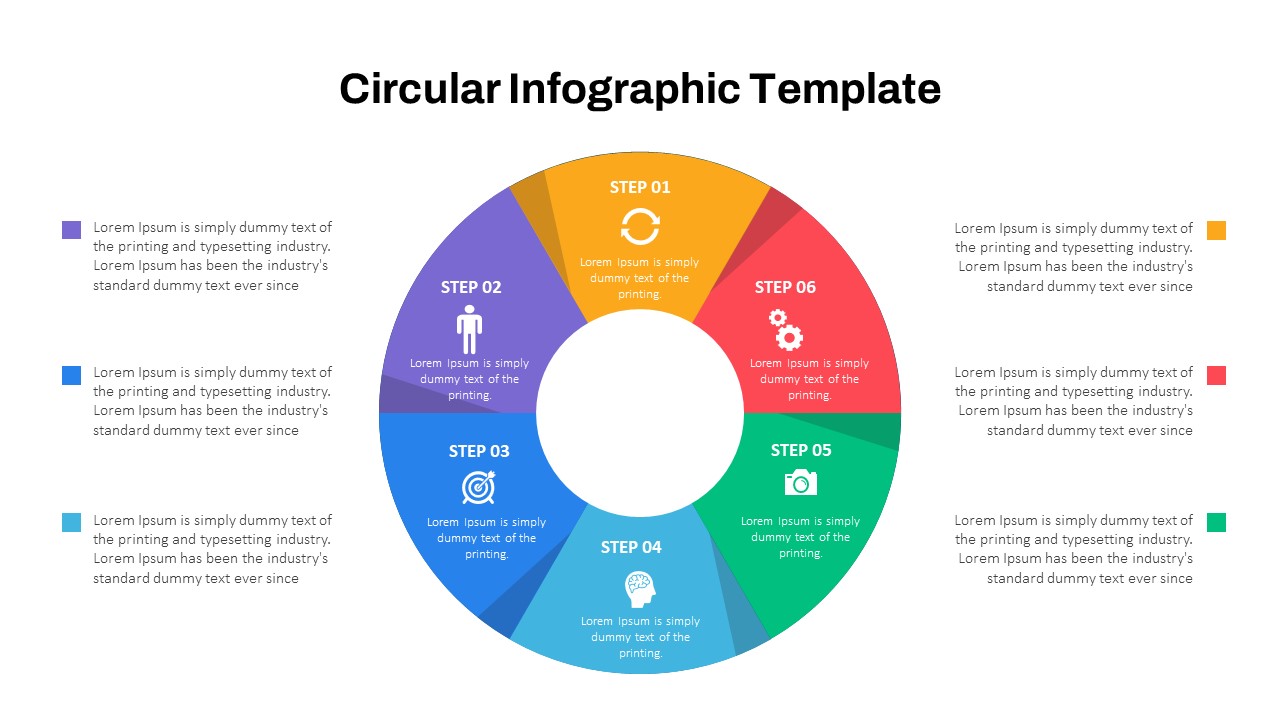
Circular Infographic Template
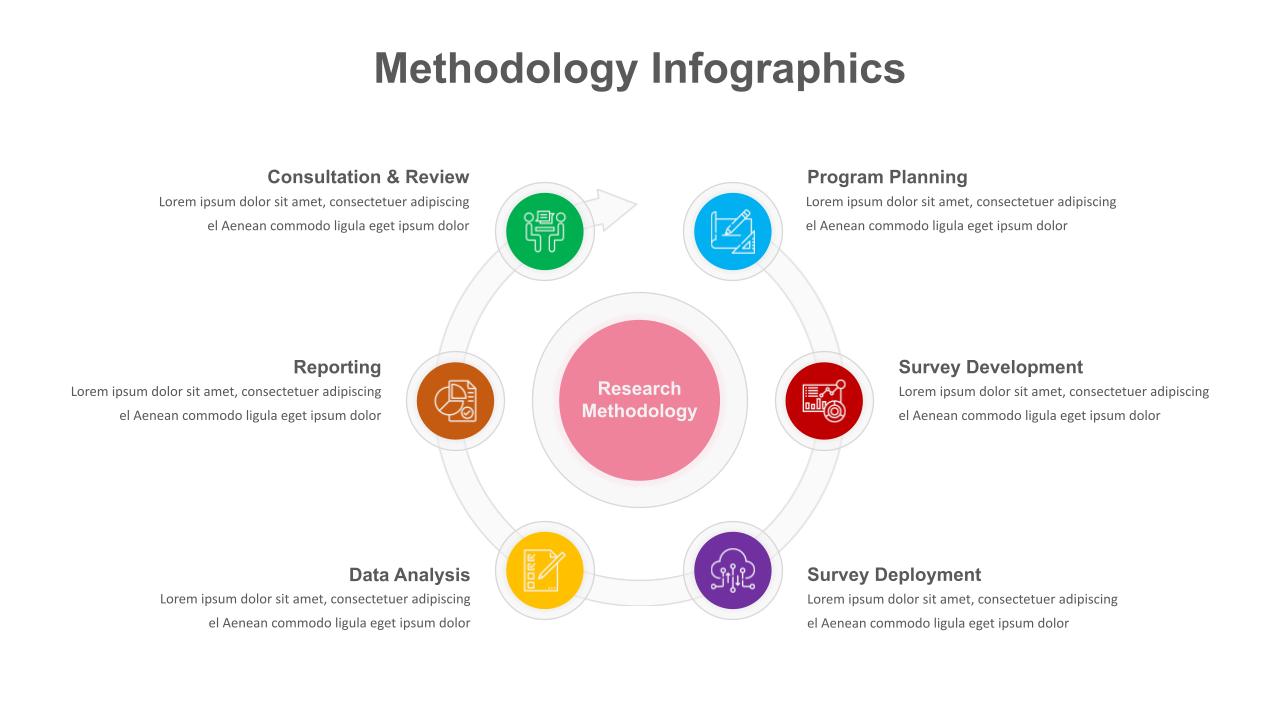
6 Point Research Methodology Powerpoint Template

Medical Powerpoint Presentation Templates

Construction Business Template for PowerPoint

Awesome Game Templates
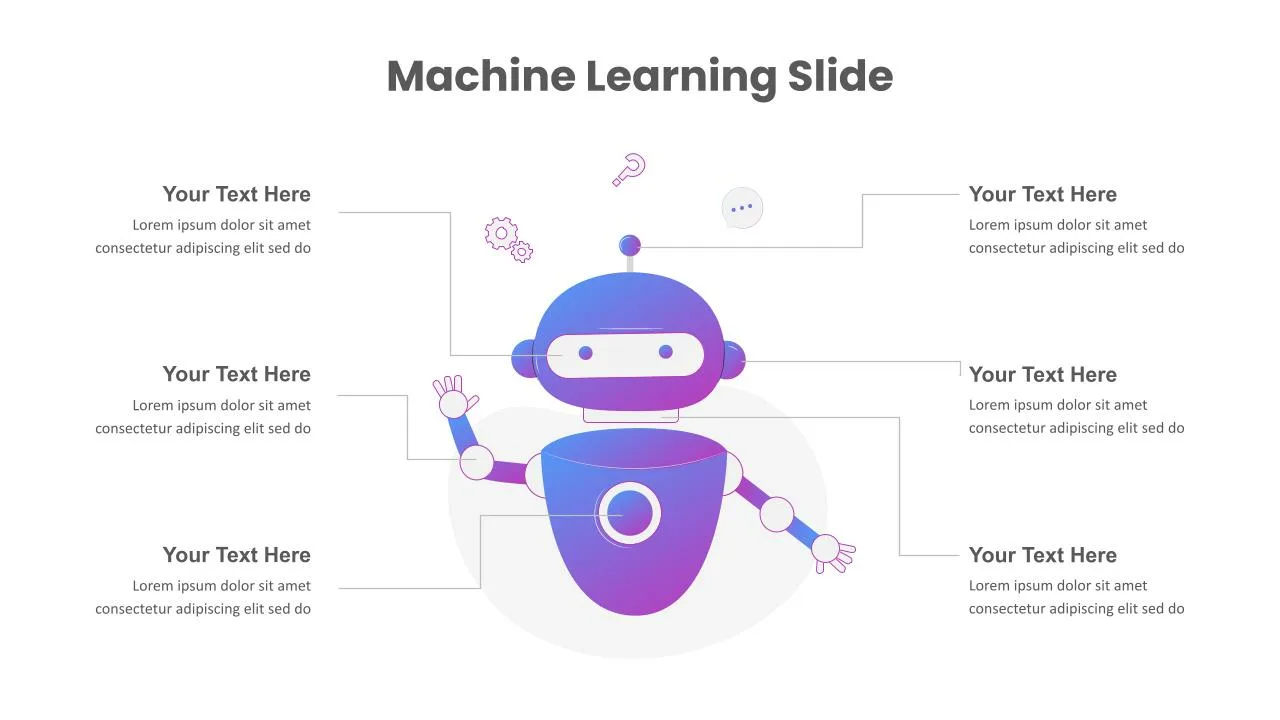
Machine Learning Slides

Powerpoint Template For Marketing

Mathematics Powerpoint Template
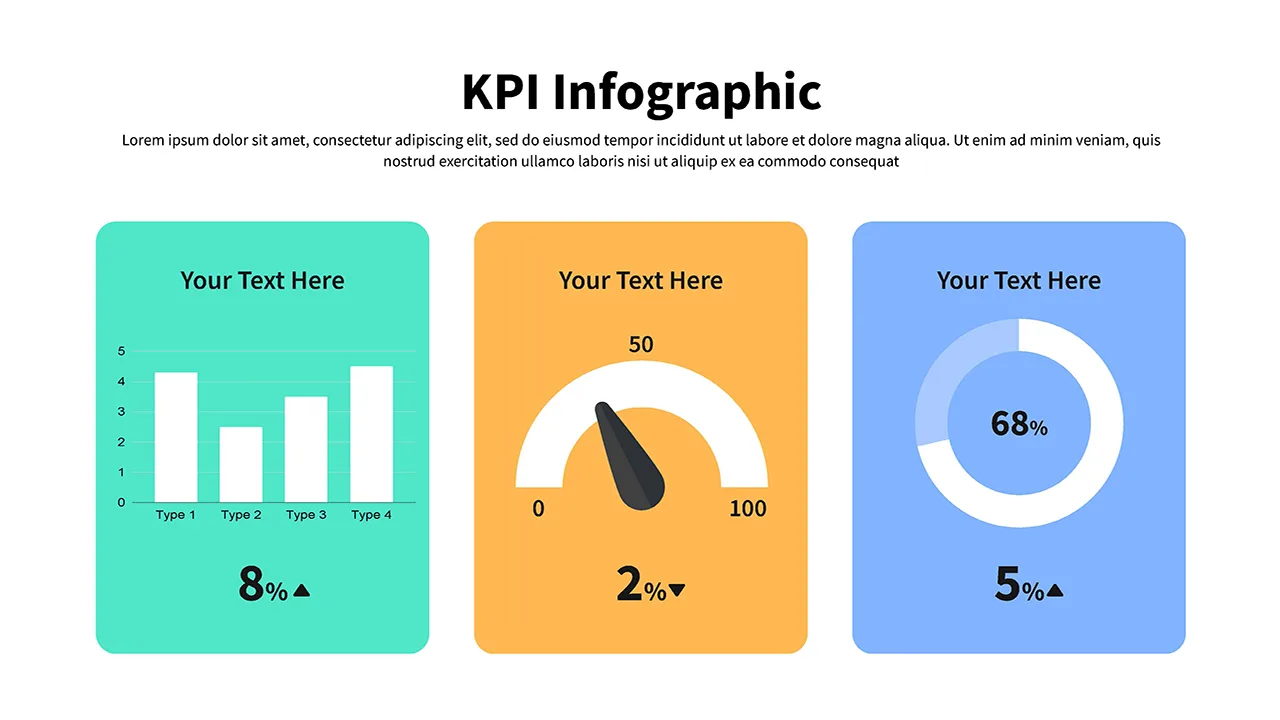
KPI Presentation Google Slides & PowerPoint Templates
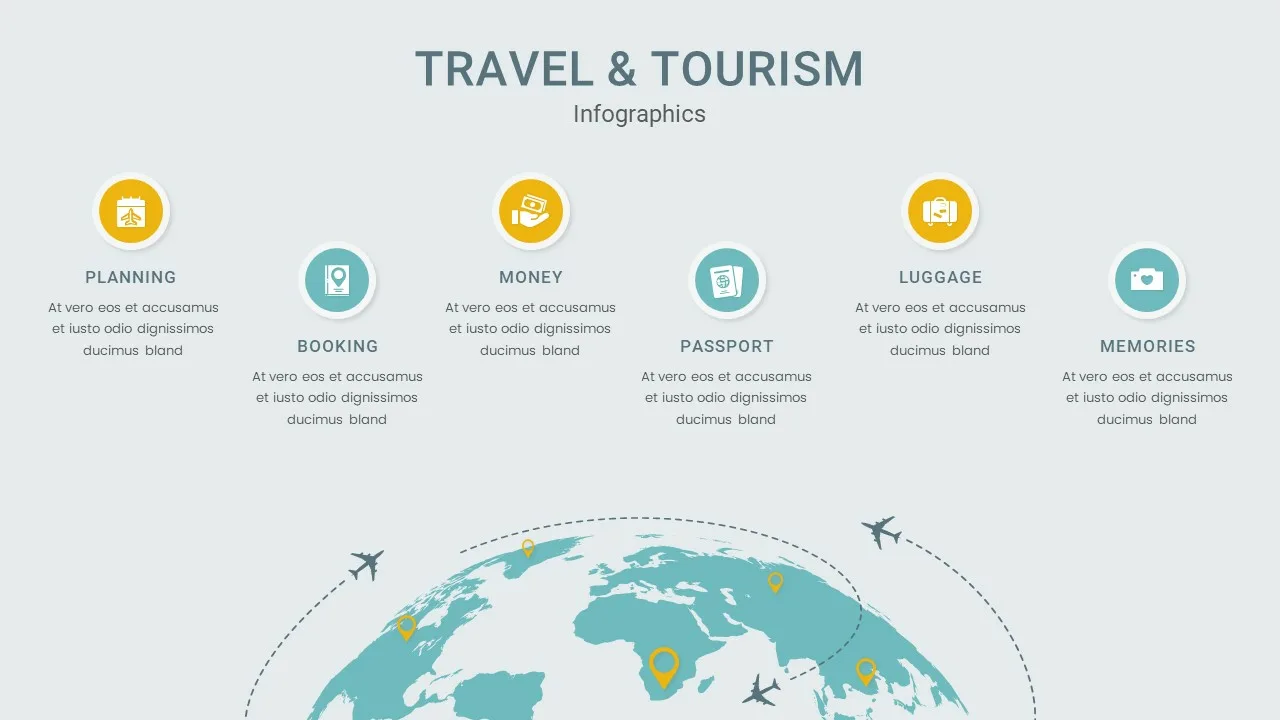
Travel & Tourism Package Presentation Template
Welcome Back!
Please sign in to continue.
Don't you have an account?
Filter by Keywords
10 Project Kickoff Templates for Meetings in Docs & PPT
Praburam Srinivasan
Growth Marketing Manager
April 3, 2024
There are times in life and business when making a decision without a second thought is the best course of action. Still, when it comes to projects, starting with a strong foundation often leads to better outcomes. Project kickoff meetings with other project-related discussions offer significant advantages to your team as they establish the framework for the project timeline and goals.
Stay with us as we examine the crucial elements of the top 10 project kickoff templates and show you how to use their potential. These templates will come in handy for laying the bedrock for success throughout different project phases. 🚀
What is a Project Kickoff Template?
What makes a good project kickoff template, 1. clickup project kickoff template, 2. clickup project implementation plan template, 3. clickup example project plan template, 4. clickup high-level project plan template, 5. clickup planning a project template, 6. clickup meeting agenda template, 7. clickup project deliverables template, 8. clickup statement of work template, 9. powerpoint project kickoff meeting agenda template by slideteam, 10. powerpoint corporate project kickoff template by slideteam.
A project kickoff template provides project managers and their teams a structured framework that fosters alignment and clarity during and after a project launch. By incorporating several important elements, this tool plays a pivotal role in supporting seamless processes, including:
- Defining expectations : It establishes a clear understanding of project goals , scope, and success criteria from the get-go, preventing misunderstandings and misalignments down the road
- Outlining crucial steps : Within the template, a well-structured roadmap delineates the sequential steps and phases of the project. It offers a strategic perspective, ensuring that tasks are organized logically and in alignment with the project’s overarching vision
- Identifying task owners : The template simplifies the assignment of specific tasks to respective team members, ensuring that ownership is clearly established
A good project kickoff template should encapsulate essential information, establish clear expectations, and foster effective communication among team members. Here are key features to look for:
- Clear objectives and scope : Outlining the project’s objectives and parameters allows team members to share a common understanding
- Roles and responsibilities : Determining clear roles and responsibilities before the project begins ensures accountability and eliminates confusion regarding who is in charge of each task
- Project timeline : Including significant checkpoints and due dates offers a visual map that helps the team monitor project development and adhere to the schedule
- Communication plan : Setting steady communication channels for prompt updates, involving all stakeholders promotes smooth information flow and collaboration
- Risk assessment and mitigation : Identifying potential risks and mitigation strategies prepares the team for challenges and enables proactive problem-solving
10 Project Kickoff Templates to Use in 2024
You are prepared to begin a new project, and now is the time to meet with your team to discuss how to carry it out effectively. All participants should leave the project kickoff meeting with a clear understanding of the project’s scope and the deliverables required for its successful completion. So, you need a project kickoff template to ensure the initial session is productive.
With so many options on the market, how do you choose the right one? Check out our recommendations for the best templates to launch your project successfully! 🔝
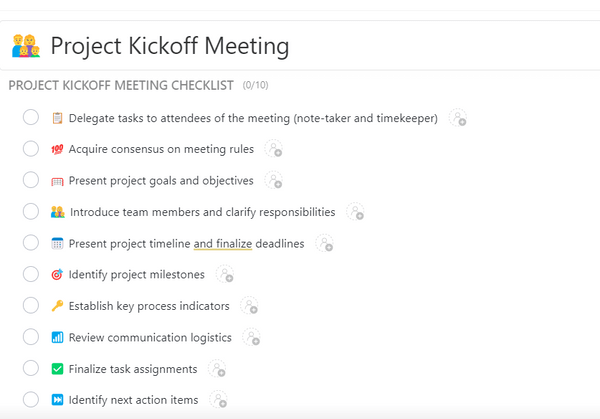
Any project’s success depends on the success of the kickoff meeting. It provides a framework for setting expectations, discussing roles, assigning tasks, and understanding project schedules. The importance of these meetings highlights the need for effective tools to prepare for the session. ClickUp’s Project Kickoff Template does exactly that by speeding up the planning process.
This template helps you organize and manage important project meetings . It includes custom statuses, fields, and views for better tracking. With features like comment reactions, nested subtasks, and multiple assignees, you’ll have more flexibility and control over how you manage your project meetings.
With the help of this template, you can:
- Precisely convey project objectives
- Define attainable goals and timelines
- Maintain methodical planning and alignment throughout the project’s lifecycle ♻️
By using this template, you can easily assign roles and responsibilities to team members and clearly state the project’s objectives and goals to foster a shared vision. You can then carefully document important decisions and action items , keeping a methodically organized and forward-moving project direction. This comprehensive integration of crucial elements opens a clear path to success, enhancing the efficacy of your project management initiatives.
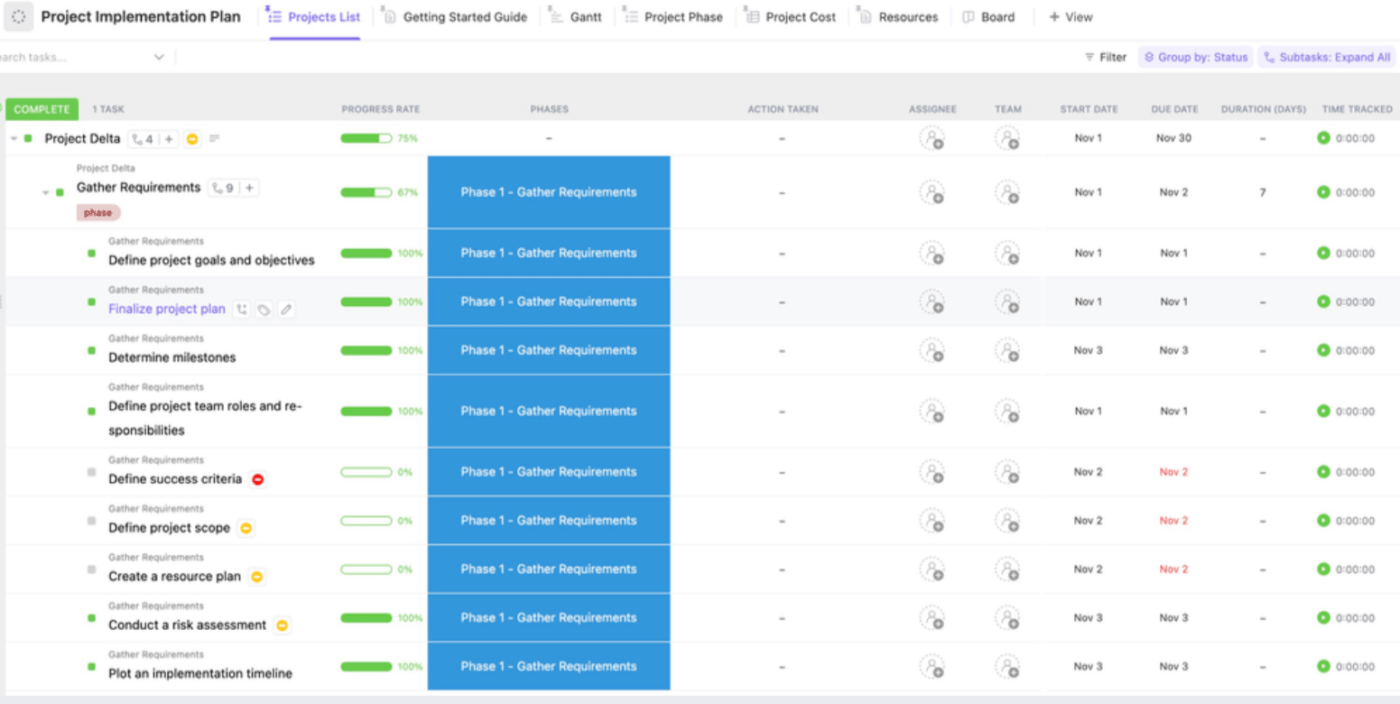
Crafting an adept project implementation plan helps define the project scope, establish precise timelines, outline deliverables, and more. ClickUp’s Project Implementation Plan Template makes it easier to monitor a project’s development with Custom Statuses , Custom Fields , Custom Views , and Project Management .
Using this template, you can seamlessly devise a comprehensive scheme that spans from project initiation to its culmination. Visualizing essential tasks, deadlines, and interdependencies becomes effortless within this singular, organized hub. The transparency enables real-time monitoring of progress and resource adaptation for a seamless execution process. ⏳
In order to guarantee the project’s timely completion, this template assists in supervising essential elements like resource allocation, budget planning, and timeline coordination. Consider these six steps to create a strong project implementation plan with this ClickUp template :
- Collaborate on creating a shared Doc in ClickUp to ensure the team’s understanding of project goals
- Use the Gantt Chart feature to visually outline the project’s timeline and track progress
- Assign tasks using ClickUp to ensure team members understand their responsibilities
- Leverage ClickUp’s custom fields to input and oversee budget details and expenditures
- Strategize against potential risks and devise preparatory measures using Board View
- Facilitate progress check-ins and stay within the timeline by configuring recurring tasks in ClickUp
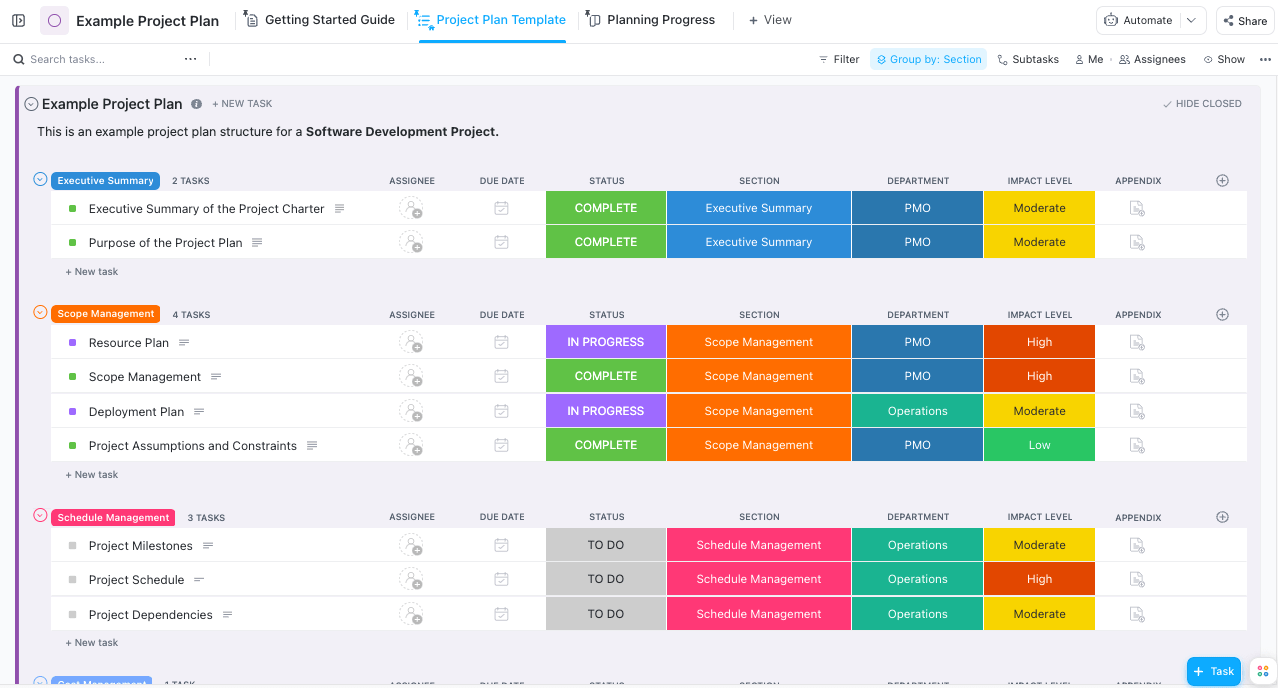
It can be difficult to navigate the complexities of project planning, and the solution lies in a seamlessly adaptable template that streamlines the process, fostering efficiency and transparency. ClickUp’s Example Project Plan Template combines all essential planning elements in a single platform .
You can create detailed plans with intuitive visual aids that improve overall comprehension, effectively arrange tasks, encourage smooth communication across multifaceted teams, and track progress against predetermined objectives, ensuring the project gets completed on schedule.
With this template, creating a clear timetable becomes a breeze. You can map out tasks and important milestones with ease, ensuring your project stays on track. The template also helps you identify task relationships so you can plan more effectively and avoid disruptions . Once you share this detailed plan with your team, it brings everyone on the same page, promoting agreement and better understanding among team members. 👥

Whether a small project or a big venture, ClickUp’s High-Level Project Plan Template makes planning and managing any enterprise easier, regardless of its scope or complexity.
It lets you:
- Create a big-picture view of your project
- Organize tasks and deadlines for each project phase
- Set clear goals for your team at every stage 🎯
Using this template entails fantastic perks:
- You’ll get a clear view of your project’s progress
- It helps you plan ahead with a straightforward timeline
- Everyone works better together because the responsibilities and assignments are visible to all team members
- You can set achievable goals that keep your team motivated and on track
- Keeping track of project progress and indicating task statuses like Deployed , In Progress , and To Do
- You can use five custom characteristics, including Copy Stage , Approver , Project Team , Completion , and Design Stage, to preserve important project information and see progress
- Five distinct views in various ClickUp configurations, such as the Deliverables List , Copywriter Board , Graphic Designer Board , Timeline , and Getting Started Guide , make all the information accessible and arranged
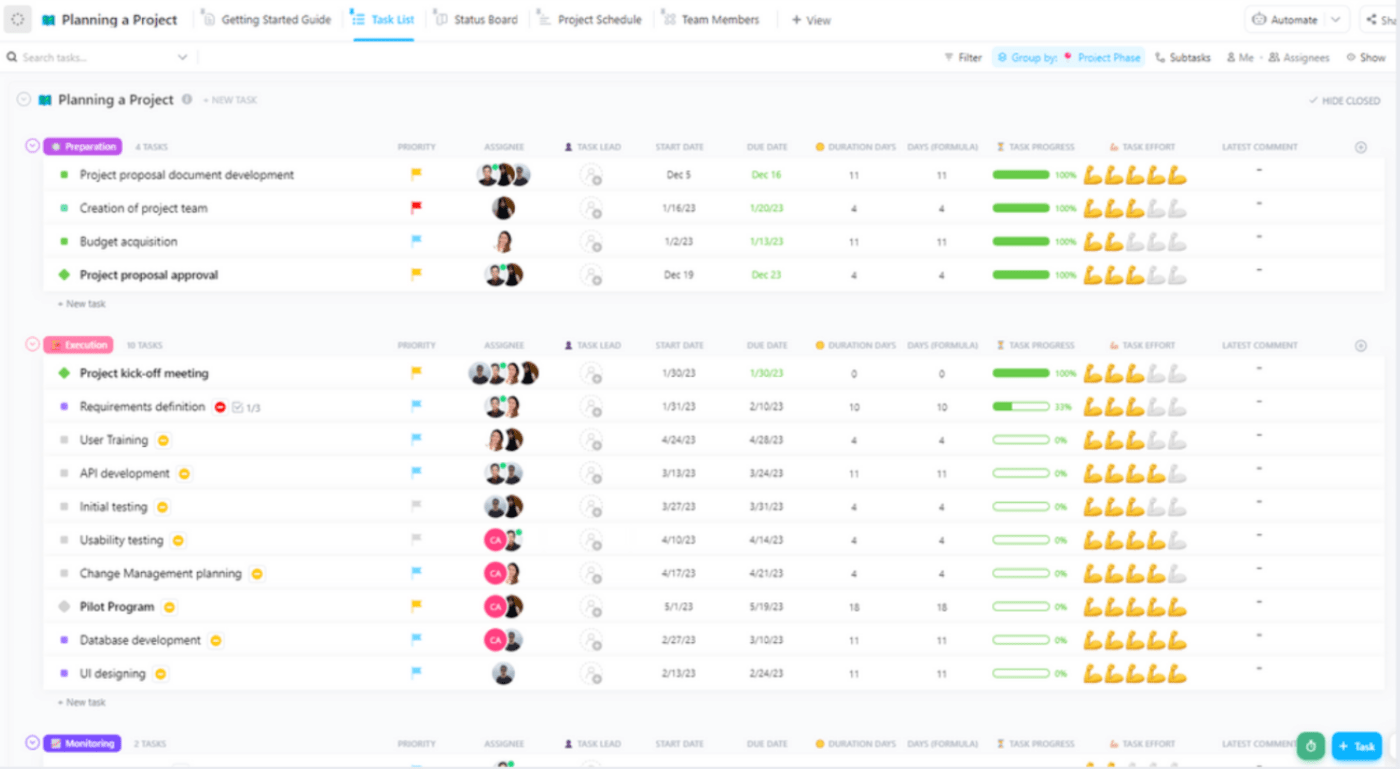
Project templates are invaluable for saving time and resources during planning . They provide a structured foundation, ensuring all vital steps are incorporated into the plan and fostering clear communication among team members and stakeholders.
This approach empowers teams to focus on the core project intricacies. ClickUp Planning a Project Template is your ally in streamlining project management . It guides you through each project stage, enabling you to grasp the project core, tailor plans to your needs, organize tasks effectively, and align team members for seamless execution.
Let’s see how you can make the most of this template:
- Use the Project Schedule View to plan when tasks should be done
- Assign tasks in the Team Members View and track progress
- Visualize the progress with the Status Board View
- Stay organized using the Task List View for tracking
- Access the Getting Started Guide View for help
- Sort tasks into statuses ( Complete, Stuck, To Do, In Progress ) for monitoring
- Keep stakeholders updated by changing statuses as tasks move forward
- Monitor and analyze tasks for maximum productivity 🔍
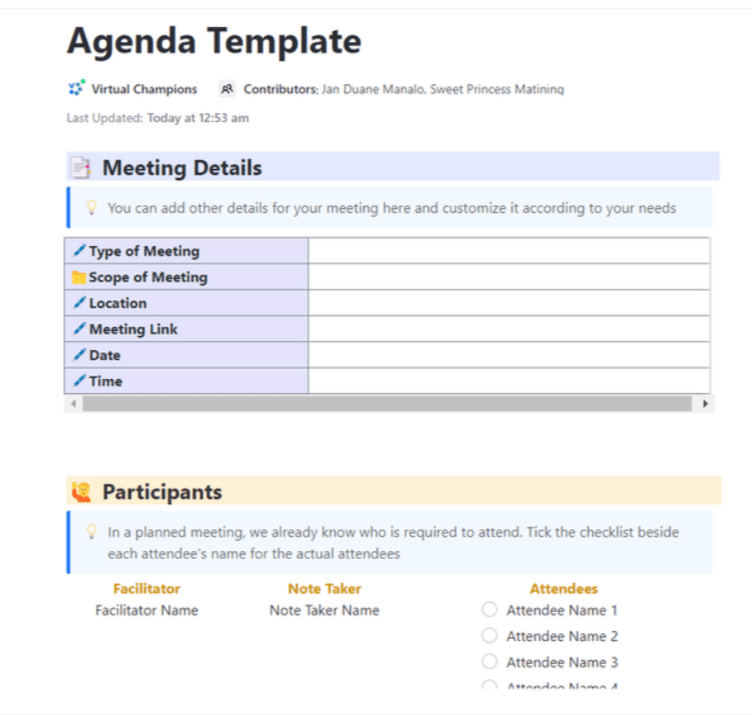
Meetings hold the potential to boost collaboration and productivity, yet without a clear plan, they can become a waste of time. ClickUp’s Meeting Agenda Template steps in as your meeting ally, ensuring that every gathering is purposeful and efficient. This template helps you:
- Outline meeting topics, objectives, and goals
- Break down tasks and action items for clarity
- Assign responsibilities to team members
The beauty of it lies in its versatility. It’s suitable for any type of meeting or event.
ClickUp’s Meeting Agenda Template ensures that every discussion topic is given proper attention, leaving no subject overlooked. It provides participants with a thorough overview of the meeting’s key points , assisting in effective preparation. The meeting also gets a sense of structure and order, which significantly increases its overall effectiveness.
Most importantly, an agenda template promotes active participation from all attendees , leading to more interesting and fruitful discussions. ✨
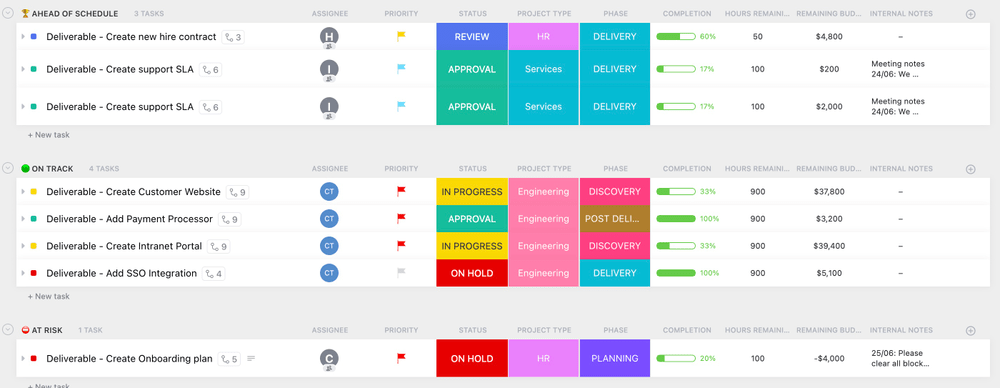
The main objective of project, resource, and operations managers is to ensure that projects follow schedules and produce successful results. No matter how carefully a project is planned, timely completion with positive results can be challenging.
ClickUp’s Project Deliverables Template defines project phases and milestones comprehensively to offer a clear picture of each team member’s role and set attainable deadlines. Each deliverable, whether a task, product, or service, gets its timeline, associated responsibilities, and anticipated outcomes. The template extends its value to encompass budget allocation , essential milestones, and a contingency strategy should challenges arise.
Having these components at your disposal empowers you to ensure accountability in your team, enabling meeting deadlines and achieving successful outcomes.
The Project Deliverables Template elevates project management by aligning expectations, managing resources, and steering projects in the right direction . 🏆
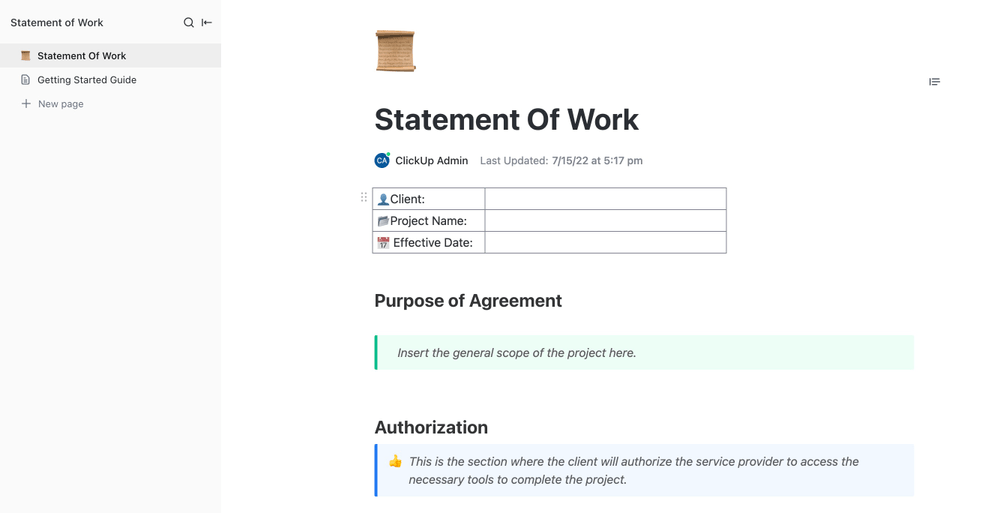
When it comes to efficient project management, having a well-crafted Statement of Work (SOW) is a game-changer. The SOW outlines project goals, timelines, and deliverables, creating a shared understanding between your company and clients or contractors.
ClickUp’s Statement of Work Template helps you effortlessly create a comprehensive SOW , define project specifics with precision, and keep all stakeholders aligned throughout the project journey.
The project’s scope and each participant’s role are clearly laid out, setting realistic expectations. It also determines a clear timeline regarding the completion, which helps with planning. By making responsibilities clear from the beginning, you can avoid misunderstandings among team members. Most importantly, it helps lower the chances of things going off track or not getting done right, which makes the whole project more likely to succeed.
You can take advantage of this template by:
- Creating tasks and assigning them to team members
- Using Goals to gauge and monitor progress toward objectives
- Employing the Gantt Chart to craft deliverable timelines, ensuring punctual completion
- Leveraging Milestones for tracking progress and setting payment deadlines 🚩
With ClickUp’s Statement of Work Template, you can ensure projects are completed on time, within budget, and with the desired outcomes.
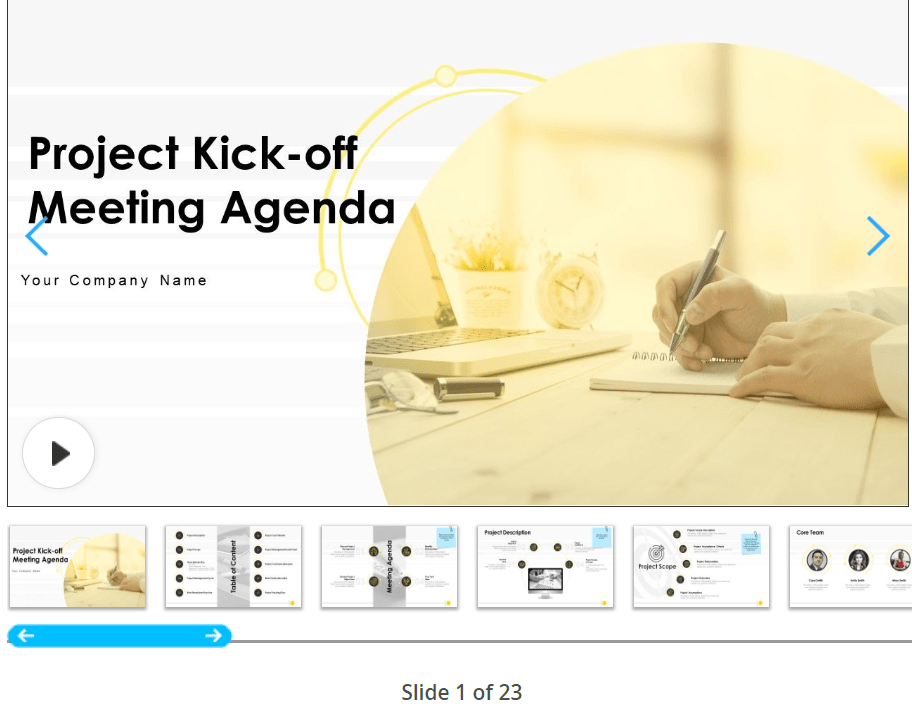
The comprehensive PowerPoint Project Kickoff Meeting Agenda Template by SlideTeam is a robust collection of 23 slides designed to facilitate the initiation of new projects . The deck encompasses vital elements such as project description and scope, core team details, project management team composition, rapid communication plan (RACI), project management cycle, work breakdown structure, project cost estimate, Gantt chart , communication plan, and tracking plan, among others.
This template empowers users to effortlessly build a presentation that covers all aspects of launching a new project. Its versatile nature allows easy customization of every element, ensuring presentations can be customized quickly to specific project requirements.
The template expands its usefulness to project agenda presentations , making it easier to communicate the project core, methodology, and organizational structure. It helps in outlining milestones, the project’s history, its business requirements, and its importance to stakeholders and the company. 💼
Essentially, this template acts as a complete toolkit for project managers and teams , enabling them to clearly communicate project details, involve stakeholders, and prepare for productive meetings.
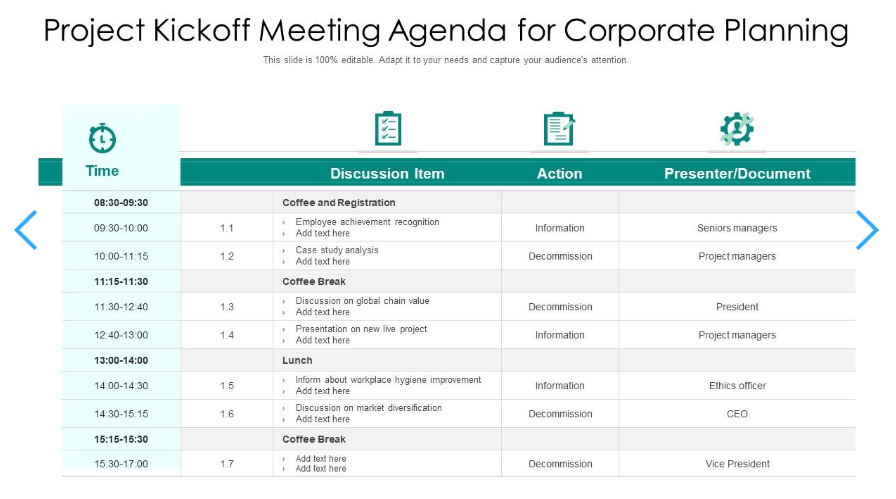
If you want to streamline your corporate planning efforts, the PowerPoint Corporate Project Kickoff Template by SlideTeam can help you with it. This presentation template comes with two pre-designed slides that seamlessly integrate into any presentation format, allowing you to make the most of your valuable time. The pre-made slides are a simple way to organize the agenda for your project kickoff meeting.
With this template, you can effortlessly polish your presentation, engage your audience, and ensure a successful corporate planning kickoff. This presentation covers all key components of a project kickoff meeting agenda designed for corporate planning.
It seamlessly opens in PowerPoint , offering you the flexibility to tailor it to your exact requirements. Easily eliminate any unnecessary text and replace it with your desired content. Modify colors and adjust layouts in a few clicks. 🙌
Your Project Kickoff Toolkit for Seamless Starts
Planning complex projects requires thorough preparation and close collaboration with your team members and clients. These templates provide the complete toolkit for team leaders organizing a project launch or kickoff meeting. By leveraging these tools, you can set clear objectives, boost effective teamwork, and reach positive results, regardless of your project’s scope and demands. 💫
Questions? Comments? Visit our Help Center for support.
Receive the latest WriteClick Newsletter updates.
Thanks for subscribing to our blog!
Please enter a valid email
- Free training & 24-hour support
- Serious about security & privacy
- 99.99% uptime the last 12 months
Get the mobile app for the best Kahoot! experience!

Back to blog
Kahoot! stands with Ukraine
Kahoot! is committed to supporting Ukrainian educators and learners affected by the current crisis. To protect the integrity of our platform and our users, we will suspend offering Kahoot!’s services in Russia, with the exception of self-study.

Ukrainian educators and learners need our support
We are deeply troubled and concerned by the violence and loss of life resulting from the Russian invasion of Ukraine. We stand with the people of Ukraine and we hope for the swiftest and most peaceful possible end to the current crisis.
Kahoot! has received a number of requests from schools and educators in Ukraine requesting the help of our services to continue teaching despite the disruption of the war. We have supported each of these and we are now offering Kahoot! EDU solutions for free for both K-12 and higher education institutions for one year to Ukrainian schools in need. In addition, we are fast-tracking translation and localization of the Kahoot! platform into Ukrainian.
Suspending commercial services and sales in Russia
Our commercial footprint in the Russian market is very limited. We do not have offices or representation in the country, nor do we have any physical operations or data services there. The overwhelming majority of our users in Russia are teachers and students using our free service.
Kahoot! is abiding by the international sanctions regime, and does not allow sales to sanctioned individuals or entities in Russia. Shortly after the Russian invasion of Ukraine, Kahoot! initiated a process to suspend offering of all commercial services in Russia. This includes but is not limited to online sales, assisted sales, app store sales and prohibiting sales to Russian corporations and organizations.
Prioritizing safe and secure use of the Kahoot! platform
As part of our mission to make learning awesome, and as education remains a fundamental human right, we offer teachers, students and personal users free access to our platform. We do this in more than 200 countries and regions in a spirit similar to public commons services, such as Wikipedia.
Similarly, inclusivity is one of Kahoot!’s overarching values. As such, our aim is to, whenever and wherever possible, offer children, schools and others the opportunity to use digital tools for impactful education and learning, irrespective of their background or location. This has been our guiding principle also for offering our service in Russia.
Among our first responses to the crisis was to swiftly expand our global moderation team’s monitoring on all Russia-related content to safeguard the integrity of the platform.
However, as the situation continues to escalate, it is vital that we are able to ensure that our platform is used according to our own guidelines and standards. Therefore, in addition to suspending sales, we will be taking all possible and necessary steps to suspend access to Kahoot! services in Russia, with the eventual exception of self-study mode which will feature only content verified by Kahoot!.
This will enable students, school children and other individual users to continue their learning journeys both safely and responsibly. We will continue to assess ways in which our services can be offered safely and responsibly to support all learners and educators, also those based in Russia.
Supporting our employees
At Kahoot!, we are not just a team in name, we are a team in practice. As such, we are committed to the well-being of our employees, especially those with ties to Ukraine, or those that in other ways are particularly affected by the war. We are providing these colleagues with any support we can.
Acknowledging the current situation, the Kahoot! Group made an emergency aid donation to Save the Children and the Norwegian Refugee Council. This is a contribution to support life-saving assistance and protection for innocent Ukrainian children, families and refugees.
As the situation in Ukraine continues to develop our teams across the company are actively monitoring the crisis so that we can respond in the most responsible and supportive way possible.
Our hearts go out to the people of Ukraine, their loved ones, and anyone affected by this crisis.
Related articles

Deliver interactive presentations that keep everyone energized with K...
Turn any presentation into an interactive learning experience! Read how our add-in for PowerPoint helps you boost engagement and makes key points stick.

Creating global impact and empowering learners: Kahoot! named among t...
In this first ranking by TIME and Statista, Kahoot! is recognized among the top EdTech companies in the world, helping to reimagine the future...

Bring fun to fractions with Snoopy Fractions, our new ready-to-play c...
Motivate young learners to master the fundamentals of fractions through engaging level-based gameplay.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Advisory Committees
- Advisory Committee Calendar
- UPDATED MEETING TIME AND PUBLIC PARTICIPATION INFORMATION: June 4, 2024: Meeting of the Psychopharmacologic Drugs Advisory Committee Meeting Announcement - 06/04/2024
Advisory Committee Meeting | Mixed
Event Title UPDATED MEETING TIME AND PUBLIC PARTICIPATION INFORMATION: June 4, 2024: Meeting of the Psychopharmacologic Drugs Advisory Committee Meeting Announcement June 4, 2024
What is an advisory committee.
Advisory committees provide independent expert advice to the FDA on broad scientific topics or on certain products to help the agency make sound decisions based on the available science. Advisory committees make non-binding recommendations to the FDA, which generally follows the recommendations but is not legally bound to do so. Please see, " Advisory Committees Give FDA Critical Advice and the Public a Voice ," for more information.
YouTube Broadcast of the Meeting: Psychopharmacologic Drugs Advisory Committee (PDAC) Live Video
YouTube live video link
UPDATED INFORMATION (as of May 28, 2024):
The meeting time has been changed for the June 4, 2024 meeting of the Psychopharmacologic Drugs Advisory Committee. The meeting time has changed from 8:30 a.m. to 4:30 p.m. to 8:30 a.m. to 5:30 p.m. Eastern Time .
Additionally, the public participation information has been changed. The time for oral presentations from the public has changed from approximately 2 p.m. to 3 p.m. to 2 p.m. to 3:45 p.m. Eastern Time .
All other information remains the same.
UPDATED INFORMATION (as of May 15, 2024):
The public participation information has been changed for the June 4, 2024, meeting of the Psychopharmacologic Drugs Advisory Committee. The deadline for making formal oral presentation requests has been extended from Friday, May 17, 2024 to Tuesday, May 21, 2024 . The contact person will notify interested persons regarding their request to speak by May 22, 2024 .
ORIGINAL INFORMATION:
Center: Center for Drug Evaluation and Research
Location: FDA and invited participants may attend the meeting at FDA White Oak Campus, 10903 New Hampshire Ave., Bldg. 31 Conference Center, the Great Room (Rm. 1503), Silver Spring, MD 20993-0002. The public (including the media) will have the option to participate via an online teleconferencing and/or video conferencing platform, and the advisory committee meeting will be heard, viewed, captioned, and recorded through an online teleconferencing and/or video conferencing platform.
The meeting presentations will be heard, viewed, captioned, and recorded through an online teleconferencing and/or video conferencing platform. The Committee will discuss new drug application 215455, for midomafetamine (MDMA) capsules, submitted by Lykos Therapeutics, for the proposed indication of treatment of post-traumatic stress disorder. The Committee will be asked to discuss the overall benefit-risk profile of the product, including the potential public health impact.
Meeting Materials
FDA intends to make background material and the link to the live webcast available to the public no later than two (2) business days before the meeting in the Event Materials section of this web page. If FDA is unable to post the background material on its website prior to the meeting, the background material will be made publicly available on FDA’s website at the time of the advisory committee meeting. The meeting will include slide presentations with audio and video components to allow the presentation of materials for online participants in a manner that most closely resembles an in-person advisory committee meeting.
Public Participation Information
Interested persons may present data, information, or views, orally or in writing, on issues pending before the committee.
FDA is establishing a docket for public comment on this meeting. The docket number is FDA-2024-N-1938 . Please note that late, untimely filed comments will not be considered. The docket will close on June 3, 2024. The https://www.regulations.gov electronic filing system will accept comments until 11:59 p.m. Eastern Time at the end of June 3, 2024. Comments received by mail/hand delivery/courier (for written/paper submissions) will be considered timely if they are received on or before that date.
Comments received on or before May 23, 2024 will be provided to the Committee. Comments received after that date will be taken into consideration by FDA. In the event that the meeting is cancelled, FDA will continue to evaluate any relevant applications or information, and consider any comments submitted to the docket, as appropriate. You may submit comments as follows:
Electronic Submissions
Submit electronic comments in the following way:
- Federal eRulemaking Portal: https://www.regulations.gov . Follow the instructions for submitting comments. Comments submitted electronically, including attachments, to https://www.regulations.gov will be posted to the docket unchanged. Because your comment will be made public, you are solely responsible for ensuring that your comment does not include any confidential information that you or a third party may not wish to be posted, such as medical information, your or anyone else’s Social Security number, or confidential business information, such as a manufacturing process. Please note that if you include your name, contact information, or other information that identifies you in the body of your comments, that information will be posted on https://www.regulations.gov .
- If you want to submit a comment with confidential information that you do not wish to be made available to the public, submit the comment as a written/paper submission and in the manner detailed (see “Written/Paper Submissions” and “Instructions”).
Written/Paper Submissions
Submit written/paper submissions as follows:
- Mail/Hand delivery/Courier (for written/paper submissions): Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852.
- For written/paper comments submitted to the Dockets Management Staff, FDA will post your comment, as well as any attachments, except for information submitted, marked and identified, as confidential, if submitted as detailed in “Instructions.”
Instructions: All submissions received must include the Docket No. FDA-2024-N-1938 for “Psychopharmacologic Drugs Advisory Committee; Notice of Meeting; Establishment of a Public Docket; Request for Comments-- midomafetamine (MDMA) capsules.” Received comments, those filed in a timely manner, will be placed in the docket and, except for those submitted as “Confidential Submissions,” publicly viewable at https://www.regulations.gov or at the Dockets Management Staff between 9 a.m. and 4 p.m., Monday through Friday, 240-402-7500.
- Confidential Submissions--To submit a comment with confidential information that you do not wish to be made publicly available, submit your comments only as a written/paper submission. You should submit two copies total. One copy will include the information you claim to be confidential with a heading or cover note that states “THIS DOCUMENT CONTAINS CONFIDENTIAL INFORMATION.” FDA will review this copy, including the claimed confidential information, in its consideration of comments. The second copy, which will have the claimed confidential information redacted/blacked out, will be available for public viewing and posted on https://www.regulations.gov . Submit both copies to the Dockets Management Staff. If you do not wish your name and contact information be made publicly available, you can provide this information on the cover sheet and not in the body of your comments and you must identify the information as “confidential.” Any information marked as “confidential” will not be disclosed except in accordance with 21 CFR 10.20 and other applicable disclosure law. For more information about FDA’s posting of comments to public dockets, see 80 FR 56469, September 18, 2015, or access the information at: https://www.gpo.gov/fdsys/pkg/FR-2015-09-18/pdf/2015-23389.pdf .
Docket: For access to the docket to read background documents or the electronic and written/paper comments received, go to https://www.regulations.gov and insert the docket number, found in brackets in the heading of this document, into the “Search” box and follow the prompts and/or go to the Dockets Management Staff, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852, 240-402-7500.
Oral Presentations
Oral presentations from the public will be scheduled between approximately 2 p.m. and 3.p.m Eastern Time and will take place entirely through an online meeting platform. Those individuals interested in making formal oral presentations should notify the contact person and submit a brief statement of the general nature of the evidence or arguments they wish to present, the names and addresses of proposed participants, and an indication of the approximate time requested to make their presentation on or before May 17, 2024.
Time allotted for each presentation may be limited. If the number of registrants requesting to speak is greater than can be reasonably accommodated during the scheduled open public hearing session, FDA may conduct a lottery to determine the speakers for the scheduled open public hearing session. The contact person will notify interested persons regarding their request to speak by May 20, 2024.
Webcast Information
CDER plans to provide a free of charge, live webcast of the upcoming advisory committee meeting. If there are instances where the webcast transmission is not successful, staff will work to re-establish the transmission as soon as possible. Further information regarding the webcast, including the web address for the webcast, will be made available no later than two (2) business days before the meeting in the Event Materials section of this web page.
CDER plans to post archived webcasts after the meeting, however, in cases where transmission was not successful, archived webcasts will not be available.
Contact Information
- Joyce Frimpong, PharmD Center for Drug Evaluation and Research Food and Drug Administration 10903 New Hampshire Avenue WO31-2417 Silver Spring, MD 20993-0002 Phone: 301-796-7973 Email: [email protected]
- FDA Advisory Committee Information Line 1-800-741-8138 (301-443-0572 in the Washington DC area) Please call the Information Line for up-to-date information on this meeting.
- For press inquiries, please contact the Office of Media Affairs at [email protected] or 301–796–4540.
A notice in the Federal Register about last minute modifications that impact a previously announced advisory committee meeting cannot always be published quickly enough to provide timely notice. Therefore, you should always check the agency’s website or call the committee’s Designated Federal Officer (see Contact Information) to learn about possible modifications before coming to the meeting.
Persons attending FDA’s advisory committee meetings are advised that the agency is not responsible for providing access to electrical outlets. FDA welcomes the attendance of the public at its advisory committee meetings and will make every effort to accommodate persons with disabilities. If you require accommodations due to a disability, please contact the committee’s Designated Federal Officer (see Contact Information) at least 7 days in advance of the meeting.
Answers to commonly asked questions including information regarding special accommodations due to a disability may be accessed at: Common Questions and Answers about FDA Advisory Committee Meetings .
FDA is committed to the orderly conduct of its advisory committee meetings. Please visit our Web site at Public Conduct During FDA Advisory Committee Meetings for procedures on public conduct during advisory committee meetings.
Notice of this meeting is given under the Federal Advisory Committee Act (5 U.S.C. app.2).
Event Materials

News Release
Lilly announces details of presentations at 2024 american society of clinical oncology (asco) annual meeting.
INDIANAPOLIS , May 23, 2024 /PRNewswire/ -- Eli Lilly and Company (NYSE: LLY) today announced that data from studies of Verzenio ® (abemaciclib; a CDK4/6 inhibitor), Retevmo ® (selpercatinib; a rearranged during transfection [ RET ] inhibitor), olomorasib (an investigational KRAS G12C inhibitor) and imlunestrant (an investigational oral selective estrogen receptor degrader [SERD]) will be presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting taking place May 31 – June 4 in Chicago .

Lilly will also host an investor event to provide an update on its oncology strategy and pipeline. The event will take place on Sunday, June 2 , at 7:30 p.m. CDT and will be available via a live webcast on the "Webcasts & Presentations" section of Lilly's investor website . A replay will also be available on the website following the event.
Presentation Highlights
Verzenio (abemaciclib) In a late-breaking oral presentation, Lilly will report outcome data from the pivotal Phase 3 postMONARCH study evaluating Verzenio in combination with fulvestrant compared to placebo plus fulvestrant for patients with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) advanced or metastatic breast cancer with disease recurrence or progression on a prior CDK4/6i plus endocrine therapy.
Retevmo (selpercatinib) In a rapid oral abstract presentation, Lilly will report results from the Phase 1/2 LIBRETTO-121 study evaluating the safety and efficacy of Retevmo in pediatric and adolescent patients with advanced solid tumors harboring an activating RET alteration.
Olomorasib (investigational KRAS G12C inhibitor): In two oral presentations, Lilly will report updated results from the Phase 1/2 study evaluating the safety and efficacy of olomorasib (LY3537982), a potent and highly selective second-generation inhibitor of KRAS G12C, in combination with pembrolizumab in patients with KRAS G12C - mutant advanced non-small cell lung cancer (NSCLC), and updated results for olomorasib as a monotherapy in patients with KRAS G12C - mutant advanced solid tumors. Submitted abstracts utilized an October 30, 2023 data cut-off date, and the presentations will utilize a March 18, 2024 data cut-off date.
A full list of abstract titles and viewing details are listed below:
Verzenio (abemaciclib): Presentation Title: Abemaciclib plus fulvestrant vs fulvestrant alone for HR+, HER2- advanced breast cancer following progression on a prior CDK4/6 inhibitor plus endocrine therapy: Primary outcome of the phase 3 postMONARCH trial Abstract Number: LBA1001 Presentation Date & Time: Saturday, June 1, 3:00 p.m. – 3:12 p.m. CDT Location: Hall B1 (Live Stream) Presenter: Kevin Kalinsky
Presentation Title: CYCLONE 2: A phase 3 study of abemaciclib with abiraterone in patients with metastatic castration-resistant prostate cancer Abstract Number: 5001 Presentation Date & Time: Saturday, June 1, 3:12 p.m. – 3:24 p.m. CDT Location: Arie Crown Theater (Live Stream) Presenter: Matthew Smith
Presentation Title: Prognostic utility of ctDNA detection in the monarchE trial of adjuvant abemaciclib plus endocrine therapy (ET) in HR+, HER2-, node-positive, high-risk early breast cancer (EBC) Abstract Number: LBA507 Presentation Date & Time: Monday, June 3, 5:12 p.m. – 5:24 p.m. CDT Location: Hall B1 (Live Stream) Presenter: Sherene Loi
Retevmo (selpercatinib) : Presentation Title: Safety and efficacy of selpercatinib in pediatric patients with RET-altered solid tumors: Updated results from LIBRETTO-121 Abstract Number: 10022 Presentation Date & Time: Sunday, June 2, 5:06 p.m. – 5:12 p.m. CDT Location: S504 (On Demand) Presenter: Daniel Morgenstern
Presentation Title: Intracranial outcomes of 1L selpercatinib in advanced RET fusion-positive NSCLC: LIBRETTO-431 study Abstract Number: 8547 Presentation Date & Time: Monday, June 3, 1:30 p.m. – 4:30 p.m. CDT Location: Hall A (On Demand) Presenter: Maurice Perol
Presentation Title: Selpercatinib in non-MTC, RET-mutated tumors: Efficacy in MEN-associated and other tumors Abstract Number: 3150 Presentation Date & Time: Saturday, June 1 , 9:00 a.m. – 12:00 p.m. CDT Location: Hall A (On Demand) Presenter: Philippe Cassier
Presentation Title: Health-related quality of life (HRQoL) and symptoms in LIBRETTO-431 patients with RET fusion-positive advanced non-small-cell lung cancer (NSCLC) Abstract Number: 11068 Presentation Date & Time: Monday, June 3, 9:00 a.m. – 12:00 p.m. CDT Location: Hall A (On Demand) Presenter: Caicun Zhou
Presentation Title: Comparative patient-reported tolerability (PRT): A multiplicity-controlled analysis of LIBRETTO-531, a randomized controlled trial (RCT) in medullary thyroid cancer (MTC) Abstract Number: 11111 Presentation Date & Time: Monday, June 3, 9:00 a.m. – 12:00 p.m. CDT Location: Hall A (On Demand) Presenter: Marcia Brose
Imlunestrant (investigational oral SERD): Presentation Title: Imlunestrant, an oral selective estrogen receptor degrader (SERD), in combination with human epidermal growth factor receptor 2 (HER2) directed therapy, with or without abemaciclib, in estrogen receptor (ER) positive, HER2 positive advanced breast cancer (aBC): EMBER phase 1a/1b study Abstract Number: 1027 Presentation Date & Time: Sunday, June 2, 9:00 a.m. – 12:00 p.m. CDT Location: Hall A (on Demand) Presenter: Manali Bhave
Presentation Title: Imlunestrant, an oral selective estrogen receptor degrader (SERD), as monotherapy and in combination with abemaciclib, in endometrioid endometrial cancer (EEC): Results from the EMBER phase 1a/1b study Abstract Number: 5589 Presentation Date & Time: Monday, June 3, 9:00 a.m. – 12:00 p.m. CDT Location: Hall A (on Demand) Presenter: Kan Yonemori
Olomorasib (investigational KRAS G12C inhibitor): Presentation Title: Efficacy and safety of olomorasib (LY3537982), a second-generation KRAS G12C inhibitor (G12Ci), in combination with pembrolizumab in patients with KRAS G12C-mutant advanced NSCLC Abstract Number: 8510 Presentation Date & Time: Saturday, June 1, 1:39 p.m. – 1:51 p.m. CDT Location: Hall B1 (Live Stream) Presenter: Timothy Burns
Presentation Title: Pan-tumor activity of olomorasib (LY3537982), a second-generation KRAS G12C inhibitor (G12Ci), in patients with KRAS G12C-mutant advanced solid tumors Abstract Number: 3007 Presentation Date & Time: Saturday, June 1 , 5:00 p.m. – 5:12 p.m. CDT Location: Hall D1 (Live Stream) Presenter: Rebecca Suk Heist
About Verzenio ® (abemaciclib) Verzenio ® (abemaciclib) is approved to treat people with certain HR+, HER2- breast cancers in the adjuvant and advanced or metastatic setting. Verzenio is the first and only CDK4/6 inhibitor approved to treat node-positive, high risk early breast cancer (EBC) patients. The National Comprehensive Cancer Network ® (NCCN ® ) recommends consideration of two years of abemaciclib (Verzenio) added to endocrine therapy as a Category 1 treatment option in the adjuvant setting. 1 NCCN ® also includes Verzenio plus endocrine therapy as a preferred treatment option for metastatic breast cancer. 2
The collective results of Lilly's clinical development program continue to differentiate Verzenio as a CDK4/6 inhibitor. In high risk EBC, Verzenio has shown a persistent and deepening benefit beyond the two-year treatment period in the monarchE trial, the only adjuvant study designed specifically to investigate a CDK4/6 inhibitor in a high risk population. 2 In metastatic breast cancer, Verzenio has demonstrated statistically significant OS in the Phase 3 MONARCH 2 study. 3 Verzenio has shown a consistent and generally manageable safety profile across clinical trials.
Verzenio is an oral tablet taken twice daily and available in strengths of 50 mg, 100 mg, 150 mg, and 200 mg. Discovered and developed by Lilly researchers, Verzenio was first approved in 2017 and is currently authorized for use in more than 90 counties around the world. For full details on indicated uses of Verzenio in HR+, HER2- breast cancer, please see full Prescribing Information , available at www.Verzenio.com .
INDICATIONS FOR VERZENIO ® VERZENIO ® is a kinase inhibitor indicated:
- in combination with endocrine therapy (tamoxifen or an aromatase inhibitor) for the adjuvant treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive, early breast cancer at high risk of recurrence.
- in combination with an aromatase inhibitor as initial endocrine-based therapy for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer.
- in combination with fulvestrant for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer with disease progression following endocrine therapy.
- as monotherapy for the treatment of adult patients with HR-positive, HER2-negative advanced or metastatic breast cancer with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting.
IMPORTANT SAFETY INFORMATION FOR VERZENIO (abemaciclib) Severe diarrhea associated with dehydration and infection occurred in patients treated with Verzenio. Across four clinical trials in 3691 patients, diarrhea occurred in 81 to 90% of patients who received Verzenio. Grade 3 diarrhea occurred in 8 to 20% of patients receiving Verzenio. Most patients experienced diarrhea during the first month of Verzenio treatment. The median time to onset of diarrhea ranged from 6 to 8 days; and the median duration of Grade 2 and Grade 3 diarrhea ranged from 6 to 11 days and 5 to 8 days, respectively. Across trials, 19 to 26% of patients with diarrhea required a Verzenio dose interruption and 13 to 23% required a dose reduction.
Instruct patients to start antidiarrheal therapy, such as loperamide, at the first sign of loose stools, increase oral fluids, and notify their healthcare provider for further instructions and appropriate follow-up. For Grade 3 or 4 diarrhea, or diarrhea that requires hospitalization, discontinue Verzenio until toxicity resolves to ≤Grade 1, and then resume Verzenio at the next lower dose.
Neutropenia, including febrile neutropenia and fatal neutropenic sepsis, occurred in patients treated with Verzenio. Across four clinical trials in 3691 patients, neutropenia occurred in 37 to 46% of patients receiving Verzenio. A Grade ≥3 decrease in neutrophil count (based on laboratory findings) occurred in 19 to 32% of patients receiving Verzenio. Across trials, the median time to first episode of Grade ≥3 neutropenia ranged from 29 to 33 days, and the median duration of Grade ≥3 neutropenia ranged from 11 to 16 days. Febrile neutropenia has been reported in <1% of patients exposed to Verzenio across trials. Two deaths due to neutropenic sepsis were observed in MONARCH 2. Inform patients to promptly report any episodes of fever to their healthcare provider.
Monitor complete blood counts prior to the start of Verzenio therapy, every 2 weeks for the first 2 months, monthly for the next 2 months, and as clinically indicated. Dose interruption, dose reduction, or delay in starting treatment cycles is recommended for patients who develop Grade 3 or 4 neutropenia.
Severe, life-threatening, or fatal interstitial lung disease (ILD) or pneumonitis can occur in patients treated with Verzenio and other CDK4/6 inhibitors. In Verzenio-treated patients in EBC (monarchE), 3% of patients experienced ILD or pneumonitis of any grade: 0.4% were Grade 3 or 4 and there was one fatality (0.1%). In Verzenio-treated patients in MBC (MONARCH 1, MONARCH 2, MONARCH 3), 3.3% of Verzenio-treated patients had ILD or pneumonitis of any grade: 0.6% had Grade 3 or 4, and 0.4% had fatal outcomes. Additional cases of ILD or pneumonitis have been observed in the postmarketing setting, with fatalities reported.
Monitor patients for pulmonary symptoms indicative of ILD or pneumonitis. Symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams. Infectious, neoplastic, and other causes for such symptoms should be excluded by means of appropriate investigations. Dose interruption or dose reduction is recommended in patients who develop persistent or recurrent Grade 2 ILD or pneumonitis. Permanently discontinue Verzenio in all patients with Grade 3 or 4 ILD or pneumonitis.
Grade ≥3 increases in alanine aminotransferase (ALT) (2 to 6%) and aspartate aminotransferase (AST) (2 to 3%) were reported in patients receiving Verzenio. Across three clinical trials in 3559 patients (monarchE, MONARCH 2, MONARCH 3), the median time to onset of Grade ≥3 ALT increases ranged from 57 to 87 days and the median time to resolution to Grade <3 was 13 to 14 days. The median time to onset of Grade ≥3 AST increases ranged from 71 to 185 days and the median time to resolution to Grade <3 ranged from 11 to 15 days.
Monitor liver function tests (LFTs) prior to the start of Verzenio therapy, every 2 weeks for the first 2 months, monthly for the next 2 months, and as clinically indicated. Dose interruption, dose reduction, dose discontinuation, or delay in starting treatment cycles is recommended for patients who develop persistent or recurrent Grade 2, or any Grade 3 or 4 hepatic transaminase elevation.
Venous thromboembolic events (VTE) were reported in 2 to 5% of patients across three clinical trials in 3559 patients treated with Verzenio (monarchE, MONARCH 2, MONARCH 3). VTE included deep vein thrombosis, pulmonary embolism, pelvic venous thrombosis, cerebral venous sinus thrombosis, subclavian and axillary vein thrombosis, and inferior vena cava thrombosis. In clinical trials, deaths due to VTE have been reported in patients treated with Verzenio.
Verzenio has not been studied in patients with early breast cancer who had a history of VTE. Monitor patients for signs and symptoms of venous thrombosis and pulmonary embolism and treat as medically appropriate. Dose interruption is recommended for EBC patients with any grade VTE and for MBC patients with a Grade 3 or 4 VTE.
Verzenio can cause fetal harm when administered to a pregnant woman, based on findings from animal studies and the mechanism of action. In animal reproduction studies, administration of abemaciclib to pregnant rats during the period of organogenesis caused teratogenicity and decreased fetal weight at maternal exposures that were similar to the human clinical exposure based on area under the curve (AUC) at the maximum recommended human dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Verzenio and for 3 weeks after the last dose. Based on findings in animals, Verzenio may impair fertility in males of reproductive potential. There are no data on the presence of Verzenio in human milk or its effects on the breastfed child or on milk production. Advise lactating women not to breastfeed during Verzenio treatment and for at least 3 weeks after the last dose because of the potential for serious adverse reactions in breastfed infants.
The most common adverse reactions (all grades, ≥10%) observed in monarchE for Verzenio plus tamoxifen or an aromatase inhibitor vs tamoxifen or an aromatase inhibitor, with a difference between arms of ≥2%, were diarrhea (84% vs 9%), infections (51% vs 39%), neutropenia (46% vs 6%), fatigue (41% vs 18%), leukopenia (38% vs 7%), nausea (30% vs 9%), anemia (24% vs 4%), headache (20% vs 15%), vomiting (18% vs 4.6%), stomatitis (14% vs 5%), lymphopenia (14% vs 3%), thrombocytopenia (13% vs 2%), decreased appetite (12% vs 2.4%), ALT increased (12% vs 6%), AST increased (12% vs 5%), dizziness (11% vs 7%), rash (11% vs 4.5%), and alopecia (11% vs 2.7 %).
The most frequently reported ≥5% Grade 3 or 4 adverse reaction that occurred in the Verzenio arm vs the tamoxifen or an aromatase inhibitor arm of monarchE were neutropenia (19.6% vs 1%), leukopenia (11% vs <1%), diarrhea (8% vs 0.2%), and lymphopenia (5% vs <1%).
Lab abnormalities (all grades; Grade 3 or 4) for monarchE in ≥10% for Verzenio plus tamoxifen or an aromatase inhibitor with a difference between arms of ≥2% were increased serum creatinine (99% vs 91%; .5% vs <.1%), decreased white blood cells (89% vs 28%; 19.1% vs 1.1%), decreased neutrophil count (84% vs 23%; 18.7% vs 1.9%), anemia (68% vs 17%; 1% vs .1%), decreased lymphocyte count (59% vs 24%; 13.2 % vs 2.5%), decreased platelet count (37% vs 10%; .9% vs .2%), increased ALT (37% vs 24%; 2.6% vs 1.2%), increased AST (31% vs 18%; 1.6% vs .9%), and hypokalemia (11% vs 3.8%; 1.3% vs 0.2%).
The most common adverse reactions (all grades, ≥10%) observed in MONARCH 3 for Verzenio plus anastrozole or letrozole vs anastrozole or letrozole, with a difference between arms of ≥2%, were diarrhea (81% vs 30%), fatigue (40% vs 32%), neutropenia (41% vs 2%), infections (39% vs 29%), nausea (39% vs 20%), abdominal pain (29% vs 12%), vomiting (28% vs 12%), anemia (28% vs 5%), alopecia (27% vs 11%), decreased appetite (24% vs 9%), leukopenia (21% vs 2%), creatinine increased (19% vs 4%), constipation (16% vs 12%), ALT increased (16% vs 7%), AST increased (15% vs 7%), rash (14% vs 5%), pruritus (13% vs 9%), cough (13% vs 9%), dyspnea (12% vs 6%), dizziness (11% vs 9%), weight decreased (10% vs 3.1%), influenza-like illness (10% vs 8%), and thrombocytopenia (10% vs 2%).
The most frequently reported ≥5% Grade 3 or 4 adverse reactions that occurred in the Verzenio arm vs the placebo arm of MONARCH 3 were neutropenia (22% vs 1%), diarrhea (9% vs 1.2%), leukopenia (7% vs <1%)), increased ALT (6% vs 2%), and anemia (6% vs 1%).
Lab abnormalities (all grades; Grade 3 or 4) for MONARCH 3 in ≥10% for Verzenio plus anastrozole or letrozole with a difference between arms of ≥2% were increased serum creatinine (98% vs 84%; 2.2% vs 0%), decreased white blood cells (82% vs 27%; 13% vs 0.6%), anemia (82% vs 28%; 1.6% vs 0%), decreased neutrophil count (80% vs 21%; 21.9% vs 2.6%), decreased lymphocyte count (53% vs 26%; 7.6% vs 1.9%), decreased platelet count (36% vs 12%; 1.9% vs 0.6%), increased ALT (48% vs 25%; 6.6% vs 1.9%), and increased AST (37% vs 23%; 3.8% vs 0.6%).
The most common adverse reactions (all grades, ≥10%) observed in MONARCH 2 for Verzenio plus fulvestrant vs fulvestrant, with a difference between arms of ≥2%, were diarrhea (86% vs 25%), neutropenia (46% vs 4%), fatigue (46% vs 32%), nausea (45% vs 23%), infections (43% vs 25%), abdominal pain (35% vs 16%), anemia (29% vs 4%), leukopenia (28% vs 2%), decreased appetite (27% vs 12%), vomiting (26% vs 10%), headache (20% vs 15%), dysgeusia (18% vs 2.7%), thrombocytopenia (16% vs 3%), alopecia (16% vs 1.8%), stomatitis (15% vs 10%), ALT increased (13% vs 5%), pruritus (13% vs 6%), cough (13% vs 11%), dizziness (12% vs 6%), AST increased (12% vs 7%), peripheral edema (12% vs 7%), creatinine increased (12% vs <1%), rash (11% vs 4.5%), pyrexia (11% vs 6%), and weight decreased (10% vs 2.2%).
The most frequently reported ≥5% Grade 3 or 4 adverse reactions that occurred in the Verzenio arm vs the placebo arm of MONARCH 2 were neutropenia (25% vs 1%), diarrhea (13% vs 0.4%), leukopenia (9% vs 0%), anemia (7% vs 1%), and infections (5.7% vs 3.5%).
Lab abnormalities (all grades; Grade 3 or 4) for MONARCH 2 in ≥10% for Verzenio plus fulvestrant with a difference between arms of ≥2% were increased serum creatinine (98% vs 74%; 1.2% vs 0%), decreased white blood cells (90% vs 33%; 23.7% vs .9%), decreased neutrophil count (87% vs 30%; 32.5% vs 4.2%), anemia (84% vs 34%; 2.6% vs .5%), decreased lymphocyte count (63% vs 32%; 12.2% vs 1.8%), decreased platelet count (53% vs 15%; 2.1% vs 0%), increased ALT (41% vs 32%; 4.6% vs 1.4%), and increased AST (37% vs 25%; 3.9% vs 4.2%).
The most common adverse reactions (all grades, ≥10%) observed in MONARCH 1 with Verzenio were diarrhea (90%), fatigue (65%), nausea (64%), decreased appetite (45%), abdominal pain (39%), neutropenia (37%), vomiting (35%), infections (31%), anemia (25%), thrombocytopenia (20%), headache (20%), cough (19%), constipation (17%), leukopenia (17%), arthralgia (15%), dry mouth (14%), weight decreased (14%), stomatitis (14%), creatinine increased (13%), alopecia (12%), dysgeusia (12%), pyrexia (11%), dizziness (11%), and dehydration (10%).
The most frequently reported ≥5% Grade 3 or 4 adverse reactions from MONARCH 1 with Verzenio were diarrhea (20%), neutropenia (24%), fatigue (13%), and leukopenia (5%).
Lab abnormalities (all grades; Grade 3 or 4) for MONARCH 1 with Verzenio were increased serum creatinine (99%; .8%), decreased white blood cells (91%; 28%), decreased neutrophil count (88%; 26.6%), anemia (69%; 0%), decreased lymphocyte count (42%; 13.8%), decreased platelet count (41%; 2.3%), increased ALT (31%; 3.1%), and increased AST (30%; 3.8%).
Strong and moderate CYP3A inhibitors increased the exposure of abemaciclib plus its active metabolites to a clinically meaningful extent and may lead to increased toxicity. Avoid concomitant use of ketoconazole. Ketoconazole is predicted to increase the AUC of abemaciclib by up to 16-fold. In patients with recommended starting doses of 200 mg twice daily or 150 mg twice daily, reduce the Verzenio dose to 100 mg twice daily with concomitant use of strong CYP3A inhibitors other than ketoconazole. In patients who have had a dose reduction to 100 mg twice daily due to adverse reactions, further reduce the Verzenio dose to 50 mg twice daily with concomitant use of strong CYP3A inhibitors. If a patient taking Verzenio discontinues a strong CYP3A inhibitor, increase the Verzenio dose (after 3 to 5 half-lives of the inhibitor) to the dose that was used before starting the inhibitor. With concomitant use of moderate CYP3A inhibitors, monitor for adverse reactions and consider reducing the Verzenio dose in 50 mg decrements. Patients should avoid grapefruit products.
Avoid concomitant use of strong or moderate CYP3A inducers and consider alternative agents. Coadministration of strong or moderate CYP3A inducers decreased the plasma concentrations of abemaciclib plus its active metabolites and may lead to reduced activity.
With severe hepatic impairment (Child-Pugh C), reduce the Verzenio dosing frequency to once daily. The pharmacokinetics of Verzenio in patients with severe renal impairment (CLcr <30 mL/min), end stage renal disease, or in patients on dialysis is unknown. No dosage adjustments are necessary in patients with mild or moderate hepatic (Child-Pugh A or B) and/or renal impairment (CLcr ≥30-89 mL/min).
Please see full Prescribing Information and Patient Information for Verzenio.
AL HCP ISI 12OCT2021
About Retevmo ® (selpercatinib, 40 mg & 80 mg capsules) Retevmo (selpercatinib, formerly known as LOXO-292) (pronounced reh-TEHV-moh) is a highly selective and potent RET kinase inhibitor with central nervous system (CNS) activity. Retevmo may affect both tumor cells and healthy cells, which can result in side effects. RET-driver alterations are predominantly mutually exclusive from other oncogenic drivers. Retevmo is a U.S. FDA-approved oral prescription medicine, 120 mg or 160 mg dependent on weight (<50 kg or ≥50 kg, respectively), taken twice daily until disease progression or unacceptable toxicity. 4
INDICATIONS FOR RETEVMO ® Retevmo ® is kinase inhibitor indicated for the treatment of:
- Adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with a rearranged during transfection ( RET ) gene fusion, as detected by an FDA-approved test.
- Adult and pediatric patients 12 years of age and older with advanced or metastatic medullary thyroid cancer (MTC) with a RET mutation, as detected by an FDA-approved test, who require systemic therapy. 1
- Adult and pediatric patients 12 years of age and older with advanced or metastatic thyroid cancer with a RET gene fusion, as detected by an FDA-approved test, who require systemic therapy and who are radioactive iodine-refractory (if radioactive iodine is appropriate). 1
- Adult patients with locally advanced or metastatic solid tumors with a RET gene fusion that have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options. 1
This indication is approved under accelerated approval based on overall response rate and duration of response. 1 Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
IMPORTANT SAFETY INFORMATION FOR RETEVMO ® (selpercatinib)
Hepatotoxicity: Serious hepatic adverse reactions occurred in 3% of patients treated with Retevmo. Increased aspartate aminotransferase (AST) occurred in 59% of patients, including Grade 3 or 4 events in 11% and increased alanine aminotransferase (ALT) occurred in 55% of patients, including Grade 3 or 4 events in 12%. Monitor ALT and AST prior to initiating Retevmo, every 2 weeks during the first 3 months, then monthly thereafter and as clinically indicated. Withhold, reduce dose, or permanently discontinue Retevmo based on the severity.
Severe, life-threatening, and fatal interstitial lung disease (ILD)/pneumonitis can occur in patients treated with Retevmo. ILD/pneumonitis occurred in 1.8% of patients who received Retevmo, including 0.3% with Grade 3 or 4 events, and 0.3% with fatal reactions. Monitor for pulmonary symptoms indicative of ILD/pneumonitis. Withhold Retevmo and promptly investigate for ILD in any patient who presents with acute or worsening of respiratory symptoms which may be indicative of ILD (e.g., dyspnea, cough, and fever). Withhold, reduce dose, or permanently discontinue Retevmo based on severity of confirmed ILD.
Hypertension occurred in 41% of patients, including Grade 3 hypertension in 20% and Grade 4 in one (0.1%) patient. Overall, 6.3% had their dose interrupted and 1.3% had their dose reduced for hypertension. Treatment-emergent hypertension was most commonly managed with anti-hypertension medications. Do not initiate Retevmo in patients with uncontrolled hypertension. Optimize blood pressure prior to initiating Retevmo. Monitor blood pressure after 1 week, at least monthly thereafter, and as clinically indicated. Initiate or adjust anti-hypertensive therapy as appropriate. Withhold, reduce dose, or permanently discontinue Retevmo based on the severity.
Retevmo can cause concentration-dependent QT interval prolongation . An increase in QTcF interval to >500 ms was measured in 7% of patients and an increase in the QTcF interval of at least 60 ms over baseline was measured in 20% of patients. Retevmo has not been studied in patients with clinically significant active cardiovascular disease or recent myocardial infarction. Monitor patients who are at significant risk of developing QTc prolongation, including patients with known long QT syndromes, clinically significant bradyarrhythmias, and severe or uncontrolled heart failure. Assess QT interval, electrolytes, and thyroid-stimulating hormone (TSH) at baseline and periodically during treatment, adjusting frequency based upon risk factors including diarrhea. Correct hypokalemia, hypomagnesemia, and hypocalcemia prior to initiating Retevmo and during treatment. Monitor the QT interval more frequently when Retevmo is concomitantly administered with strong and moderate CYP3A inhibitors or drugs known to prolong QTc interval. Withhold and dose reduce or permanently discontinue Retevmo based on the severity.
Serious, including fatal, hemorrhagic events can occur with Retevmo. Grade ≥3 hemorrhagic events occurred in 3.1% of patients treated with Retevmo including 4 (0.5%) patients with fatal hemorrhagic events, including cerebral hemorrhage (n=2), tracheostomy site hemorrhage (n=1), and hemoptysis (n=1). Permanently discontinue Retevmo in patients with severe or life-threatening hemorrhage.
Hypersensitivity occurred in 6% of patients receiving Retevmo, including Grade 3 hypersensitivity in 1.9%. The median time to onset was 1.9 weeks (range: 5 days to 2 years). Signs and symptoms of hypersensitivity included fever, rash and arthralgias or myalgias with concurrent decreased platelets or transaminitis. If hypersensitivity occurs, withhold Retevmo and begin corticosteroids at a dose of 1 mg/kg prednisone (or equivalent). Upon resolution of the event, resume Retevmo at a reduced dose and increase the dose of Retevmo by 1 dose level each week as tolerated until reaching the dose taken prior to onset of hypersensitivity. Continue steroids until patient reaches target dose and then taper. Permanently discontinue Retevmo for recurrent hypersensitivity.
Tumor lysis syndrome (TLS) occurred in 0.6% of patients with medullary thyroid carcinoma receiving Retevmo. Patients may be at risk of TLS if they have rapidly growing tumors, a high tumor burden, renal dysfunction, or dehydration. Closely monitor patients at risk, consider appropriate prophylaxis including hydration, and treat as clinically indicated.
Impaired wound healing can occur in patients who receive drugs that inhibit the vascular endothelial growth factor (VEGF) signaling pathway. Therefore, Retevmo has the potential to adversely affect wound healing. Withhold Retevmo for at least 7 days prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of Retevmo after resolution of wound healing complications has not been established.
Retevmo can cause hypothyroidism . Hypothyroidism occurred in 13% of patients treated with Retevmo; all reactions were Grade 1 or 2. Hypothyroidism occurred in 13% of patients (50/373) with thyroid cancer and 13% of patients (53/423) with other solid tumors including NSCLC. Monitor thyroid function before treatment with Retevmo and periodically during treatment. Treat with thyroid hormone replacement as clinically indicated. Withhold Retevmo until clinically stable or permanently discontinue Retevmo based on severity.
Based on data from animal reproduction studies and its mechanism of action, Retevmo can cause fetal harm when administered to a pregnant woman. Administration of selpercatinib to pregnant rats during organogenesis at maternal exposures that were approximately equal to those observed at the recommended human dose of 160 mg twice daily resulted in embryolethality and malformations. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with Retevmo and for 1 week after the last dose. There are no data on the presence of selpercatinib or its metabolites in human milk or on their effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with Retevmo and for 1 week after the last dose.
Severe adverse reactions (Grade 3-4) occurring in ≥20% of patients who received Retevmo in LIBRETTO-001 , were hypertension (20%), diarrhea (5%), prolonged QT interval (4.8%), dyspnea (3.1%), fatigue (3.1%), hemorrhage (2.6%), abdominal pain (2.5%), vomiting (1.8%), headache (1.4%), nausea (1.1%), constipation (0.8%), edema (0.8%), rash (0.6%), and arthralgia (0.3%).
Serious adverse reactions occurred in 44% of patients who received Retevmo. The most frequently reported serious adverse reactions (in ≥2% of patients) were pneumonia, pleural effusion, abdominal pain, hemorrhage, hypersensitivity, dyspnea, and hyponatremia.
Fatal adverse reactions occurred in 3% of patients ; fatal adverse reactions included sepsis (n=6), respiratory failure (n=5), hemorrhage (n=4), pneumonia (n=3), pneumonitis (n=2), cardiac arrest (n=2), sudden death (n=1), and cardiac failure (n=1).
Common adverse reactions (all grades) occurring in ≥20% of patients who received Retevmo in LIBRETTO-001 , were edema (49%), diarrhea (47%), fatigue (46%), dry mouth (43%), hypertension (41%), abdominal pain (34%), rash (33%), constipation (33%), nausea (31%), headache (28%), cough (24%), vomiting (22%), dyspnea (22%), hemorrhage (22%), arthralgia (21%), and prolonged QT interval (21%).
Laboratory abnormalities (all grades ≥20%; Grade 3-4) worsening from baseline in patients who received Retevmo in LIBRETTO-001 , were increased AST (59%; 11%), decreased calcium (59%; 5.7%), increased ALT (56%; 12%), decreased albumin (56%; 2.3%), increased glucose (53%; 2.8%), decreased lymphocytes (52%; 20%), increased creatinine (47%; 2.4%), decreased sodium (42%; 11%), increased alkaline phosphatase (40%; 3.4%), decreased platelets (37%; 3.2%), increased total cholesterol (35%; 1.7%), increased potassium (34%; 2.7%), decreased glucose (34%; 1.0%), decreased magnesium (33%; 0.6%), increased bilirubin (30%; 2.8%), decreased hemoglobin (28%; 3.5%), and decreased neutrophils (25%; 3.2%).
Concomitant use of acid-reducing agents decreases selpercatinib plasma concentrations which may reduce Retevmo antitumor activity. Avoid concomitant use of proton-pump inhibitors (PPIs), histamine-2 (H2) receptor antagonists, and locally-acting antacids with Retevmo. If coadministration cannot be avoided, take Retevmo with food (with a PPI) or modify its administration time (with a H2 receptor antagonist or a locally-acting antacid).
Concomitant use of strong and moderate CYP3A inhibitors increases selpercatinib plasma concentrations which may increase the risk of Retevmo adverse reactions including QTc interval prolongation. Avoid concomitant use of strong and moderate CYP3A inhibitors with Retevmo. If concomitant use of a strong or moderate CYP3A inhibitor cannot be avoided, reduce the Retevmo dosage as recommended and monitor the QT interval with ECGs more frequently.
Concomitant use of strong and moderate CYP3A inducers decreases selpercatinib plasma concentrations which may reduce Retevmo anti-tumor activity. Avoid coadministration of Retevmo with strong and moderate CYP3A inducers.
Concomitant use of Retevmo with CYP2C8 and CYP3A substrates increases their plasma concentrations which may increase the risk of adverse reactions related to these substrates. Avoid coadministration of Retevmo with CYP2C8 and CYP3A substrates where minimal concentration changes may lead to increased adverse reactions. If coadministration cannot be avoided, follow recommendations for CYP2C8 and CYP3A substrates provided in their approved product labeling.
Retevmo is a P-glycoprotein (P-gp) inhibitor. Concomitant use of Retevmo with P-gp substrates increases their plasma concentrations, which may increase the risk of adverse reactions related to these substrates. Avoid coadministration of Retevmo with P-gp substrates where minimal concentration changes may lead to increased adverse reactions. If coadministration cannot be avoided, follow recommendations for P-gp substrates provided in their approved product labeling.
The safety and effectiveness of Retevmo have not been established in pediatric patients less than 12 years of age . The safety and effectiveness of Retevmo have been established in pediatric patients aged 12 years and older for medullary thyroid cancer (MTC) who require systemic therapy and for advanced RET fusion-positive thyroid cancer who require systemic therapy and are radioactive iodine-refractory (if radioactive iodine is appropriate). Use of Retevmo for these indications is supported by evidence from adequate and well-controlled studies in adults with additional pharmacokinetic and safety data in pediatric patients aged 12 years and older. Monitor open growth plates in adolescent patients . Consider interrupting or discontinuing Retevmo if abnormalities occur.
No dosage modification is recommended for patients with mild to severe renal impairment (estimated Glomerular Filtration Rate [eGFR] ≥15 to 89 mL/min, estimated by Modification of Diet in Renal Disease [MDRD] equation). A recommended dosage has not been established for patients with end-stage renal disease.
Reduce the dose when administering Retevmo to patients with severe hepatic impairment (total bilirubin greater than 3 to 10 times upper limit of normal [ULN] and any AST). No dosage modification is recommended for patients with mild or moderate hepatic impairment. Monitor for Retevmo-related adverse reactions in patients with hepatic impairment.
Please see full Prescribing Information for Retevmo.
SE HCP ISI All_21SEP22
About Imlunestrant Imlunestrant (LY3484356) is an investigational, next-generation oral selective estrogen receptor degrader (SERD) with pure antagonistic properties. The estrogen receptor (ER) is the key therapeutic target for patients with ER+/HER2- breast cancer. Novel degraders of ER may overcome endocrine therapy resistance while providing consistent oral pharmacology and convenience of administration. Imlunestrant was specifically designed to deliver continuous estrogen receptor target inhibition throughout the dosing period and regardless of ESR1 mutational status. Imlunestrant is currently being studied in several clinical trials.
About Olomorasib Olomorasib (LY3537982) is an investigational, oral, potent, and highly selective second-generation inhibitor of the KRAS G12C protein. KRAS is the most common oncogene across all tumor types, and KRAS G12C mutations occur in 13% of patients with non-small cell lung cancer (NSCLC), and 1-3% of patients with other solid tumors. 5 Olomorasib was specifically designed to target KRAS G12C mutations and has pharmacokinetic properties which allow for high predicted target occupancy and high potency when used as monotherapy or in combination. 6
Olomorasib is currently being studied in the LOXO-RAS-20001 Phase 1/2 trial (NCT04956640) in patients with KRAS G12C-mutant NSCLC, and other advanced solid tumors and in the pivotal, registrational SUNRAY-01 global study (NCT06119581) investigating olomorasib in combination with pembrolizumab with or without chemotherapy for first-line treatment of KRAS G12C-mutant advanced NSCLC. For additional information about olomorasib clinical trials, please refer to clinicaltrials.gov .
About Lilly Lilly is a medicine company turning science into healing to make life better for people around the world. We've been pioneering life-changing discoveries for nearly 150 years, and today our medicines help more than 51 million people across the globe. Harnessing the power of biotechnology, chemistry and genetic medicine, our scientists are urgently advancing new discoveries to solve some of the world's most significant health challenges: redefining diabetes care; treating obesity and curtailing its most devastating long-term effects; advancing the fight against Alzheimer's disease; providing solutions to some of the most debilitating immune system disorders; and transforming the most difficult-to-treat cancers into manageable diseases. With each step toward a healthier world, we're motivated by one thing: making life better for millions more people. That includes delivering innovative clinical trials that reflect the diversity of our world and working to ensure our medicines are accessible and affordable. To learn more, visit Lilly.com and Lilly.com/news , or follow us on Facebook , Instagram and LinkedIn . P-LLY
Retevmo ® is a registered trademark owned by or licensed to Eli Lilly and Company , its subsidiaries, or affiliates.
Verzenio ® is a registered trademark owned or licensed by Eli Lilly and Company , its subsidiaries, or affiliates.
© Lilly USA , LLC 2024. ALL RIGHTS RESERVED.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements (as that term is defined in the Private Securities Litigation Reform Act of 1995) about Verzenio ® (abemaciclib) as a potential treatment for people with certain types of early breast cancer, Retevmo ® (selpercatinib) as a potential treatment for people with locally advanced and metastatic RET fusion-positive NSCLC, RET -mutant MTC, and RET -activated thyroid cancer, imlunestrant as a potential treatment for people with certain types of breast cancer and olomorasib as a potential treatment for certain KRAS G12C-mutant advanced solid tumors, and reflects Lilly's current beliefs and expectations. However, as with any pharmaceutical product, there are substantial risks and uncertainties in the process of drug research, development, and commercialization. Among other things, there can be no guarantee that studies will be initiated or completed as planned, that future study results will be consistent with the results to date, or that Verzenio, Retevmo, imlunestrant, or olomorasib will receive initial regulatory approvals or approvals for additional indications, as applicable, or be commercially successful. For further discussion of these and other risks and uncertainties that could cause actual results to differ from Lilly's expectations, see Lilly's Form 10-K and Form 10-Q filings with the United States Securities and Exchange Commission . Except as required by law, Lilly undertakes no duty to update forward-looking statements to reflect events after the date of this release.
1 Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ® ) for Breast Cancer V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed May 9, 2024. To view the most recent and complete version of the guidelines, go online to NCCN.org . NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 2 Johnston SRD, Toi M, O'Shaughnessy J, Rastogi P, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol . 2023 Jan;24(1):77-90. 3 Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2–negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol . 2020;6(1):116-124. doi:10.1001/jamaoncol. 2019.4782. 4 Retevmo. Prescribing information. Lilly USA, LLC . 5 Ji J, Wang C, Fakih M. Targeting KRASG12C-mutated advanced colorectal cancer: Research and clinical developments. OncoTargets and Therapy . 2022;Volume 15:747-756. doi:10.2147/ott.s340392 6 Peng S-B, Si C, Zhang Y, et al. Abstract 1259: Preclinical characterization of Ly3537982, a novel, highly selective and potent KRAS-G12C inhibitor. Cancer Research . 2021;81(13_Supplement):1259-1259. doi:10.1158/1538-7445.am2021-1259
SOURCE Eli Lilly and Company
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Premium News
- Biden Economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Student Loans
- Personal Loans
- Car Insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Asking for a Trend
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Lilly announces details of presentations at 2024 american society of clinical oncology (asco) annual meeting.
INDIANAPOLIS , May 23, 2024 /PRNewswire/ -- Eli Lilly and Company (NYSE: LLY) today announced that data from studies of Verzenio ® (abemaciclib; a CDK4/6 inhibitor), Retevmo ® (selpercatinib; a rearranged during transfection [ RET ] inhibitor), olomorasib (an investigational KRAS G12C inhibitor) and imlunestrant (an investigational oral selective estrogen receptor degrader [SERD]) will be presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting taking place May 31 – June 4 in Chicago .
Lilly will also host an investor event to provide an update on its oncology strategy and pipeline. The event will take place on Sunday, June 2 , at 7:30 p.m. CDT and will be available via a live webcast on the "Webcasts & Presentations" section of Lilly's investor website . A replay will also be available on the website following the event.
Presentation Highlights
Verzenio (abemaciclib) In a late-breaking oral presentation, Lilly will report outcome data from the pivotal Phase 3 postMONARCH study evaluating Verzenio in combination with fulvestrant compared to placebo plus fulvestrant for patients with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) advanced or metastatic breast cancer with disease recurrence or progression on a prior CDK4/6i plus endocrine therapy.
Retevmo (selpercatinib) In a rapid oral abstract presentation, Lilly will report results from the Phase 1/2 LIBRETTO-121 study evaluating the safety and efficacy of Retevmo in pediatric and adolescent patients with advanced solid tumors harboring an activating RET alteration.
Olomorasib (investigational KRAS G12C inhibitor): In two oral presentations, Lilly will report updated results from the Phase 1/2 study evaluating the safety and efficacy of olomorasib (LY3537982), a potent and highly selective second-generation inhibitor of KRAS G12C, in combination with pembrolizumab in patients with KRAS G12C - mutant advanced non-small cell lung cancer (NSCLC), and updated results for olomorasib as a monotherapy in patients with KRAS G12C - mutant advanced solid tumors. Submitted abstracts utilized an October 30, 2023 data cut-off date, and the presentations will utilize a March 18, 2024 data cut-off date.
A full list of abstract titles and viewing details are listed below:
Verzenio (abemaciclib): Presentation Title: Abemaciclib plus fulvestrant vs fulvestrant alone for HR+, HER2- advanced breast cancer following progression on a prior CDK4/6 inhibitor plus endocrine therapy: Primary outcome of the phase 3 postMONARCH trial Abstract Number: LBA1001 Presentation Date & Time: Saturday, June 1, 3:00 p.m. – 3:12 p.m. CDT Location: Hall B1 (Live Stream) Presenter: Kevin Kalinsky
Presentation Title: CYCLONE 2: A phase 3 study of abemaciclib with abiraterone in patients with metastatic castration-resistant prostate cancer Abstract Number: 5001 Presentation Date & Time: Saturday, June 1, 3:12 p.m. – 3:24 p.m. CDT Location: Arie Crown Theater (Live Stream) Presenter: Matthew Smith
Presentation Title: Prognostic utility of ctDNA detection in the monarchE trial of adjuvant abemaciclib plus endocrine therapy (ET) in HR+, HER2-, node-positive, high-risk early breast cancer (EBC) Abstract Number: LBA507 Presentation Date & Time: Monday, June 3, 5:12 p.m. – 5:24 p.m. CDT Location: Hall B1 (Live Stream) Presenter: Sherene Loi
Retevmo (selpercatinib) : Presentation Title: Safety and efficacy of selpercatinib in pediatric patients with RET-altered solid tumors: Updated results from LIBRETTO-121 Abstract Number: 10022 Presentation Date & Time: Sunday, June 2, 5:06 p.m. – 5:12 p.m. CDT Location: S504 (On Demand) Presenter: Daniel Morgenstern
Presentation Title: Intracranial outcomes of 1L selpercatinib in advanced RET fusion-positive NSCLC: LIBRETTO-431 study Abstract Number: 8547 Presentation Date & Time: Monday, June 3, 1:30 p.m. – 4:30 p.m. CDT Location: Hall A (On Demand) Presenter: Maurice Perol
Presentation Title: Selpercatinib in non-MTC, RET-mutated tumors: Efficacy in MEN-associated and other tumors Abstract Number: 3150 Presentation Date & Time: Saturday, June 1 , 9:00 a.m. – 12:00 p.m. CDT Location: Hall A (On Demand) Presenter: Philippe Cassier
Presentation Title: Health-related quality of life (HRQoL) and symptoms in LIBRETTO-431 patients with RET fusion-positive advanced non-small-cell lung cancer (NSCLC) Abstract Number: 11068 Presentation Date & Time: Monday, June 3, 9:00 a.m. – 12:00 p.m. CDT Location: Hall A (On Demand) Presenter: Caicun Zhou
Presentation Title: Comparative patient-reported tolerability (PRT): A multiplicity-controlled analysis of LIBRETTO-531, a randomized controlled trial (RCT) in medullary thyroid cancer (MTC) Abstract Number: 11111 Presentation Date & Time: Monday, June 3, 9:00 a.m. – 12:00 p.m. CDT Location: Hall A (On Demand) Presenter: Marcia Brose
Imlunestrant (investigational oral SERD): Presentation Title: Imlunestrant, an oral selective estrogen receptor degrader (SERD), in combination with human epidermal growth factor receptor 2 (HER2) directed therapy, with or without abemaciclib, in estrogen receptor (ER) positive, HER2 positive advanced breast cancer (aBC): EMBER phase 1a/ 1b study Abstract Number: 1027 Presentation Date & Time: Sunday, June 2, 9:00 a.m. – 12:00 p.m. CDT Location: Hall A (on Demand) Presenter: Manali Bhave
Presentation Title: Imlunestrant, an oral selective estrogen receptor degrader (SERD), as monotherapy and in combination with abemaciclib, in endometrioid endometrial cancer (EEC): Results from the EMBER phase 1a/ 1b study Abstract Number: 5589 Presentation Date & Time: Monday, June 3, 9:00 a.m. – 12:00 p.m. CDT Location: Hall A (on Demand) Presenter: Kan Yonemori
Olomorasib (investigational KRAS G12C inhibitor): Presentation Title: Efficacy and safety of olomorasib (LY3537982), a second-generation KRAS G12C inhibitor (G12Ci), in combination with pembrolizumab in patients with KRAS G12C-mutant advanced NSCLC Abstract Number: 8510 Presentation Date & Time: Saturday, June 1, 1:39 p.m. – 1:51 p.m. CDT Location: Hall B1 (Live Stream) Presenter: Timothy Burns
Presentation Title: Pan-tumor activity of olomorasib (LY3537982), a second-generation KRAS G12C inhibitor (G12Ci), in patients with KRAS G12C-mutant advanced solid tumors Abstract Number: 3007 Presentation Date & Time: Saturday, June 1 , 5:00 p.m. – 5:12 p.m. CDT Location: Hall D1 (Live Stream) Presenter: Rebecca Suk Heist
About Verzenio ® (abemaciclib) Verzenio ® (abemaciclib) is approved to treat people with certain HR+, HER2- breast cancers in the adjuvant and advanced or metastatic setting. Verzenio is the first and only CDK4/6 inhibitor approved to treat node-positive, high risk early breast cancer (EBC) patients. The National Comprehensive Cancer Network ® (NCCN ® ) recommends consideration of two years of abemaciclib (Verzenio) added to endocrine therapy as a Category 1 treatment option in the adjuvant setting. 1 NCCN ® also includes Verzenio plus endocrine therapy as a preferred treatment option for metastatic breast cancer. 2
The collective results of Lilly's clinical development program continue to differentiate Verzenio as a CDK4/6 inhibitor. In high risk EBC, Verzenio has shown a persistent and deepening benefit beyond the two-year treatment period in the monarchE trial, the only adjuvant study designed specifically to investigate a CDK4/6 inhibitor in a high risk population. 2 In metastatic breast cancer, Verzenio has demonstrated statistically significant OS in the Phase 3 MONARCH 2 study. 3 Verzenio has shown a consistent and generally manageable safety profile across clinical trials.
Verzenio is an oral tablet taken twice daily and available in strengths of 50 mg, 100 mg, 150 mg, and 200 mg. Discovered and developed by Lilly researchers, Verzenio was first approved in 2017 and is currently authorized for use in more than 90 counties around the world. For full details on indicated uses of Verzenio in HR+, HER2- breast cancer, please see full Prescribing Information , available at www.Verzenio.com .
INDICATIONS FOR VERZENIO ® VERZENIO ® is a kinase inhibitor indicated:
in combination with endocrine therapy (tamoxifen or an aromatase inhibitor) for the adjuvant treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive, early breast cancer at high risk of recurrence.
in combination with an aromatase inhibitor as initial endocrine-based therapy for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer.
in combination with fulvestrant for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer with disease progression following endocrine therapy.
as monotherapy for the treatment of adult patients with HR-positive, HER2-negative advanced or metastatic breast cancer with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting.
IMPORTANT SAFETY INFORMATION FOR VERZENIO (abemaciclib) Severe diarrhea associated with dehydration and infection occurred in patients treated with Verzenio. Across four clinical trials in 3691 patients, diarrhea occurred in 81 to 90% of patients who received Verzenio. Grade 3 diarrhea occurred in 8 to 20% of patients receiving Verzenio. Most patients experienced diarrhea during the first month of Verzenio treatment. The median time to onset of diarrhea ranged from 6 to 8 days; and the median duration of Grade 2 and Grade 3 diarrhea ranged from 6 to 11 days and 5 to 8 days, respectively. Across trials, 19 to 26% of patients with diarrhea required a Verzenio dose interruption and 13 to 23% required a dose reduction.
Instruct patients to start antidiarrheal therapy, such as loperamide, at the first sign of loose stools, increase oral fluids, and notify their healthcare provider for further instructions and appropriate follow-up. For Grade 3 or 4 diarrhea, or diarrhea that requires hospitalization, discontinue Verzenio until toxicity resolves to ≤Grade 1, and then resume Verzenio at the next lower dose.
Neutropenia, including febrile neutropenia and fatal neutropenic sepsis, occurred in patients treated with Verzenio. Across four clinical trials in 3691 patients, neutropenia occurred in 37 to 46% of patients receiving Verzenio. A Grade ≥3 decrease in neutrophil count (based on laboratory findings) occurred in 19 to 32% of patients receiving Verzenio. Across trials, the median time to first episode of Grade ≥3 neutropenia ranged from 29 to 33 days, and the median duration of Grade ≥3 neutropenia ranged from 11 to 16 days. Febrile neutropenia has been reported in <1% of patients exposed to Verzenio across trials. Two deaths due to neutropenic sepsis were observed in MONARCH 2. Inform patients to promptly report any episodes of fever to their healthcare provider.
Monitor complete blood counts prior to the start of Verzenio therapy, every 2 weeks for the first 2 months, monthly for the next 2 months, and as clinically indicated. Dose interruption, dose reduction, or delay in starting treatment cycles is recommended for patients who develop Grade 3 or 4 neutropenia.
Severe, life-threatening, or fatal interstitial lung disease (ILD) or pneumonitis can occur in patients treated with Verzenio and other CDK4/6 inhibitors. In Verzenio-treated patients in EBC (monarchE), 3% of patients experienced ILD or pneumonitis of any grade: 0.4% were Grade 3 or 4 and there was one fatality (0.1%). In Verzenio-treated patients in MBC (MONARCH 1, MONARCH 2, MONARCH 3), 3.3% of Verzenio-treated patients had ILD or pneumonitis of any grade: 0.6% had Grade 3 or 4, and 0.4% had fatal outcomes. Additional cases of ILD or pneumonitis have been observed in the postmarketing setting, with fatalities reported.
Monitor patients for pulmonary symptoms indicative of ILD or pneumonitis. Symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams. Infectious, neoplastic, and other causes for such symptoms should be excluded by means of appropriate investigations. Dose interruption or dose reduction is recommended in patients who develop persistent or recurrent Grade 2 ILD or pneumonitis. Permanently discontinue Verzenio in all patients with Grade 3 or 4 ILD or pneumonitis.
Grade ≥3 increases in alanine aminotransferase (ALT) (2 to 6%) and aspartate aminotransferase (AST) (2 to 3%) were reported in patients receiving Verzenio. Across three clinical trials in 3559 patients (monarchE, MONARCH 2, MONARCH 3), the median time to onset of Grade ≥3 ALT increases ranged from 57 to 87 days and the median time to resolution to Grade <3 was 13 to 14 days. The median time to onset of Grade ≥3 AST increases ranged from 71 to 185 days and the median time to resolution to Grade <3 ranged from 11 to 15 days.
Monitor liver function tests (LFTs) prior to the start of Verzenio therapy, every 2 weeks for the first 2 months, monthly for the next 2 months, and as clinically indicated. Dose interruption, dose reduction, dose discontinuation, or delay in starting treatment cycles is recommended for patients who develop persistent or recurrent Grade 2, or any Grade 3 or 4 hepatic transaminase elevation.
Venous thromboembolic events (VTE) were reported in 2 to 5% of patients across three clinical trials in 3559 patients treated with Verzenio (monarchE, MONARCH 2, MONARCH 3). VTE included deep vein thrombosis, pulmonary embolism, pelvic venous thrombosis, cerebral venous sinus thrombosis, subclavian and axillary vein thrombosis, and inferior vena cava thrombosis. In clinical trials, deaths due to VTE have been reported in patients treated with Verzenio.
Verzenio has not been studied in patients with early breast cancer who had a history of VTE. Monitor patients for signs and symptoms of venous thrombosis and pulmonary embolism and treat as medically appropriate. Dose interruption is recommended for EBC patients with any grade VTE and for MBC patients with a Grade 3 or 4 VTE.
Verzenio can cause fetal harm when administered to a pregnant woman, based on findings from animal studies and the mechanism of action. In animal reproduction studies, administration of abemaciclib to pregnant rats during the period of organogenesis caused teratogenicity and decreased fetal weight at maternal exposures that were similar to the human clinical exposure based on area under the curve (AUC) at the maximum recommended human dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Verzenio and for 3 weeks after the last dose. Based on findings in animals, Verzenio may impair fertility in males of reproductive potential. There are no data on the presence of Verzenio in human milk or its effects on the breastfed child or on milk production. Advise lactating women not to breastfeed during Verzenio treatment and for at least 3 weeks after the last dose because of the potential for serious adverse reactions in breastfed infants.
The most common adverse reactions (all grades, ≥10%) observed in monarchE for Verzenio plus tamoxifen or an aromatase inhibitor vs tamoxifen or an aromatase inhibitor, with a difference between arms of ≥2%, were diarrhea (84% vs 9%), infections (51% vs 39%), neutropenia (46% vs 6%), fatigue (41% vs 18%), leukopenia (38% vs 7%), nausea (30% vs 9%), anemia (24% vs 4%), headache (20% vs 15%), vomiting (18% vs 4.6%), stomatitis (14% vs 5%), lymphopenia (14% vs 3%), thrombocytopenia (13% vs 2%), decreased appetite (12% vs 2.4%), ALT increased (12% vs 6%), AST increased (12% vs 5%), dizziness (11% vs 7%), rash (11% vs 4.5%), and alopecia (11% vs 2.7 %).
The most frequently reported ≥5% Grade 3 or 4 adverse reaction that occurred in the Verzenio arm vs the tamoxifen or an aromatase inhibitor arm of monarchE were neutropenia (19.6% vs 1%), leukopenia (11% vs <1%), diarrhea (8% vs 0.2%), and lymphopenia (5% vs <1%).
Lab abnormalities (all grades; Grade 3 or 4) for monarchE in ≥10% for Verzenio plus tamoxifen or an aromatase inhibitor with a difference between arms of ≥2% were increased serum creatinine (99% vs 91%; .5% vs <.1%), decreased white blood cells (89% vs 28%; 19.1% vs 1.1%), decreased neutrophil count (84% vs 23%; 18.7% vs 1.9%), anemia (68% vs 17%; 1% vs .1%), decreased lymphocyte count (59% vs 24%; 13.2 % vs 2.5%), decreased platelet count (37% vs 10%; .9% vs .2%), increased ALT (37% vs 24%; 2.6% vs 1.2%), increased AST (31% vs 18%; 1.6% vs .9%), and hypokalemia (11% vs 3.8%; 1.3% vs 0.2%).
The most common adverse reactions (all grades, ≥10%) observed in MONARCH 3 for Verzenio plus anastrozole or letrozole vs anastrozole or letrozole, with a difference between arms of ≥2%, were diarrhea (81% vs 30%), fatigue (40% vs 32%), neutropenia (41% vs 2%), infections (39% vs 29%), nausea (39% vs 20%), abdominal pain (29% vs 12%), vomiting (28% vs 12%), anemia (28% vs 5%), alopecia (27% vs 11%), decreased appetite (24% vs 9%), leukopenia (21% vs 2%), creatinine increased (19% vs 4%), constipation (16% vs 12%), ALT increased (16% vs 7%), AST increased (15% vs 7%), rash (14% vs 5%), pruritus (13% vs 9%), cough (13% vs 9%), dyspnea (12% vs 6%), dizziness (11% vs 9%), weight decreased (10% vs 3.1%), influenza-like illness (10% vs 8%), and thrombocytopenia (10% vs 2%).
The most frequently reported ≥5% Grade 3 or 4 adverse reactions that occurred in the Verzenio arm vs the placebo arm of MONARCH 3 were neutropenia (22% vs 1%), diarrhea (9% vs 1.2%), leukopenia (7% vs <1%)), increased ALT (6% vs 2%), and anemia (6% vs 1%).
Lab abnormalities (all grades; Grade 3 or 4) for MONARCH 3 in ≥10% for Verzenio plus anastrozole or letrozole with a difference between arms of ≥2% were increased serum creatinine (98% vs 84%; 2.2% vs 0%), decreased white blood cells (82% vs 27%; 13% vs 0.6%), anemia (82% vs 28%; 1.6% vs 0%), decreased neutrophil count (80% vs 21%; 21.9% vs 2.6%), decreased lymphocyte count (53% vs 26%; 7.6% vs 1.9%), decreased platelet count (36% vs 12%; 1.9% vs 0.6%), increased ALT (48% vs 25%; 6.6% vs 1.9%), and increased AST (37% vs 23%; 3.8% vs 0.6%).
The most common adverse reactions (all grades, ≥10%) observed in MONARCH 2 for Verzenio plus fulvestrant vs fulvestrant, with a difference between arms of ≥2%, were diarrhea (86% vs 25%), neutropenia (46% vs 4%), fatigue (46% vs 32%), nausea (45% vs 23%), infections (43% vs 25%), abdominal pain (35% vs 16%), anemia (29% vs 4%), leukopenia (28% vs 2%), decreased appetite (27% vs 12%), vomiting (26% vs 10%), headache (20% vs 15%), dysgeusia (18% vs 2.7%), thrombocytopenia (16% vs 3%), alopecia (16% vs 1.8%), stomatitis (15% vs 10%), ALT increased (13% vs 5%), pruritus (13% vs 6%), cough (13% vs 11%), dizziness (12% vs 6%), AST increased (12% vs 7%), peripheral edema (12% vs 7%), creatinine increased (12% vs <1%), rash (11% vs 4.5%), pyrexia (11% vs 6%), and weight decreased (10% vs 2.2%).
The most frequently reported ≥5% Grade 3 or 4 adverse reactions that occurred in the Verzenio arm vs the placebo arm of MONARCH 2 were neutropenia (25% vs 1%), diarrhea (13% vs 0.4%), leukopenia (9% vs 0%), anemia (7% vs 1%), and infections (5.7% vs 3.5%).
Lab abnormalities (all grades; Grade 3 or 4) for MONARCH 2 in ≥10% for Verzenio plus fulvestrant with a difference between arms of ≥2% were increased serum creatinine (98% vs 74%; 1.2% vs 0%), decreased white blood cells (90% vs 33%; 23.7% vs .9%), decreased neutrophil count (87% vs 30%; 32.5% vs 4.2%), anemia (84% vs 34%; 2.6% vs .5%), decreased lymphocyte count (63% vs 32%; 12.2% vs 1.8%), decreased platelet count (53% vs 15%; 2.1% vs 0%), increased ALT (41% vs 32%; 4.6% vs 1.4%), and increased AST (37% vs 25%; 3.9% vs 4.2%).
The most common adverse reactions (all grades, ≥10%) observed in MONARCH 1 with Verzenio were diarrhea (90%), fatigue (65%), nausea (64%), decreased appetite (45%), abdominal pain (39%), neutropenia (37%), vomiting (35%), infections (31%), anemia (25%), thrombocytopenia (20%), headache (20%), cough (19%), constipation (17%), leukopenia (17%), arthralgia (15%), dry mouth (14%), weight decreased (14%), stomatitis (14%), creatinine increased (13%), alopecia (12%), dysgeusia (12%), pyrexia (11%), dizziness (11%), and dehydration (10%).
The most frequently reported ≥5% Grade 3 or 4 adverse reactions from MONARCH 1 with Verzenio were diarrhea (20%), neutropenia (24%), fatigue (13%), and leukopenia (5%).
Lab abnormalities (all grades; Grade 3 or 4) for MONARCH 1 with Verzenio were increased serum creatinine (99%; .8%), decreased white blood cells (91%; 28%), decreased neutrophil count (88%; 26.6%), anemia (69%; 0%), decreased lymphocyte count (42%; 13.8%), decreased platelet count (41%; 2.3%), increased ALT (31%; 3.1%), and increased AST (30%; 3.8%).
Strong and moderate CYP3A inhibitors increased the exposure of abemaciclib plus its active metabolites to a clinically meaningful extent and may lead to increased toxicity. Avoid concomitant use of ketoconazole. Ketoconazole is predicted to increase the AUC of abemaciclib by up to 16-fold. In patients with recommended starting doses of 200 mg twice daily or 150 mg twice daily, reduce the Verzenio dose to 100 mg twice daily with concomitant use of strong CYP3A inhibitors other than ketoconazole. In patients who have had a dose reduction to 100 mg twice daily due to adverse reactions, further reduce the Verzenio dose to 50 mg twice daily with concomitant use of strong CYP3A inhibitors. If a patient taking Verzenio discontinues a strong CYP3A inhibitor, increase the Verzenio dose (after 3 to 5 half-lives of the inhibitor) to the dose that was used before starting the inhibitor. With concomitant use of moderate CYP3A inhibitors, monitor for adverse reactions and consider reducing the Verzenio dose in 50 mg decrements. Patients should avoid grapefruit products.
Avoid concomitant use of strong or moderate CYP3A inducers and consider alternative agents. Coadministration of strong or moderate CYP3A inducers decreased the plasma concentrations of abemaciclib plus its active metabolites and may lead to reduced activity.
With severe hepatic impairment (Child-Pugh C), reduce the Verzenio dosing frequency to once daily. The pharmacokinetics of Verzenio in patients with severe renal impairment (CLcr <30 mL/min), end stage renal disease, or in patients on dialysis is unknown. No dosage adjustments are necessary in patients with mild or moderate hepatic (Child-Pugh A or B) and/or renal impairment (CLcr ≥30-89 mL/min).
Please see full Prescribing Information and Patient Information for Verzenio.
AL HCP ISI 12OCT2021
About Retevmo ® (selpercatinib, 40 mg & 80 mg capsules) Retevmo (selpercatinib, formerly known as LOXO-292) (pronounced reh-TEHV-moh) is a highly selective and potent RET kinase inhibitor with central nervous system (CNS) activity. Retevmo may affect both tumor cells and healthy cells, which can result in side effects. RET-driver alterations are predominantly mutually exclusive from other oncogenic drivers. Retevmo is a U.S. FDA-approved oral prescription medicine, 120 mg or 160 mg dependent on weight (<50 kg or ≥50 kg, respectively), taken twice daily until disease progression or unacceptable toxicity. 4
INDICATIONS FOR RETEVMO ® Retevmo ® is kinase inhibitor indicated for the treatment of:
Adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with a rearranged during transfection ( RET ) gene fusion, as detected by an FDA-approved test.
Adult and pediatric patients 12 years of age and older with advanced or metastatic medullary thyroid cancer (MTC) with a RET mutation, as detected by an FDA-approved test, who require systemic therapy. 1
Adult and pediatric patients 12 years of age and older with advanced or metastatic thyroid cancer with a RET gene fusion, as detected by an FDA-approved test, who require systemic therapy and who are radioactive iodine-refractory (if radioactive iodine is appropriate). 1
Adult patients with locally advanced or metastatic solid tumors with a RET gene fusion that have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options. 1
This indication is approved under accelerated approval based on overall response rate and duration of response. 1 Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
IMPORTANT SAFETY INFORMATION FOR RETEVMO ® (selpercatinib)
Hepatotoxicity: Serious hepatic adverse reactions occurred in 3% of patients treated with Retevmo. Increased aspartate aminotransferase (AST) occurred in 59% of patients, including Grade 3 or 4 events in 11% and increased alanine aminotransferase (ALT) occurred in 55% of patients, including Grade 3 or 4 events in 12%. Monitor ALT and AST prior to initiating Retevmo, every 2 weeks during the first 3 months, then monthly thereafter and as clinically indicated. Withhold, reduce dose, or permanently discontinue Retevmo based on the severity.
Severe, life-threatening, and fatal interstitial lung disease (ILD)/pneumonitis can occur in patients treated with Retevmo. ILD/pneumonitis occurred in 1.8% of patients who received Retevmo, including 0.3% with Grade 3 or 4 events, and 0.3% with fatal reactions. Monitor for pulmonary symptoms indicative of ILD/pneumonitis. Withhold Retevmo and promptly investigate for ILD in any patient who presents with acute or worsening of respiratory symptoms which may be indicative of ILD (e.g., dyspnea, cough, and fever). Withhold, reduce dose, or permanently discontinue Retevmo based on severity of confirmed ILD.
Hypertension occurred in 41% of patients, including Grade 3 hypertension in 20% and Grade 4 in one (0.1%) patient. Overall, 6.3% had their dose interrupted and 1.3% had their dose reduced for hypertension. Treatment-emergent hypertension was most commonly managed with anti-hypertension medications. Do not initiate Retevmo in patients with uncontrolled hypertension. Optimize blood pressure prior to initiating Retevmo. Monitor blood pressure after 1 week, at least monthly thereafter, and as clinically indicated. Initiate or adjust anti-hypertensive therapy as appropriate. Withhold, reduce dose, or permanently discontinue Retevmo based on the severity.
Retevmo can cause concentration-dependent QT interval prolongation . An increase in QTcF interval to >500 ms was measured in 7% of patients and an increase in the QTcF interval of at least 60 ms over baseline was measured in 20% of patients. Retevmo has not been studied in patients with clinically significant active cardiovascular disease or recent myocardial infarction. Monitor patients who are at significant risk of developing QTc prolongation, including patients with known long QT syndromes, clinically significant bradyarrhythmias, and severe or uncontrolled heart failure. Assess QT interval, electrolytes, and thyroid-stimulating hormone (TSH) at baseline and periodically during treatment, adjusting frequency based upon risk factors including diarrhea. Correct hypokalemia, hypomagnesemia, and hypocalcemia prior to initiating Retevmo and during treatment. Monitor the QT interval more frequently when Retevmo is concomitantly administered with strong and moderate CYP3A inhibitors or drugs known to prolong QTc interval. Withhold and dose reduce or permanently discontinue Retevmo based on the severity.
Serious, including fatal, hemorrhagic events can occur with Retevmo. Grade ≥3 hemorrhagic events occurred in 3.1% of patients treated with Retevmo including 4 (0.5%) patients with fatal hemorrhagic events, including cerebral hemorrhage (n=2), tracheostomy site hemorrhage (n=1), and hemoptysis (n=1). Permanently discontinue Retevmo in patients with severe or life-threatening hemorrhage.
Hypersensitivity occurred in 6% of patients receiving Retevmo, including Grade 3 hypersensitivity in 1.9%. The median time to onset was 1.9 weeks (range: 5 days to 2 years). Signs and symptoms of hypersensitivity included fever, rash and arthralgias or myalgias with concurrent decreased platelets or transaminitis. If hypersensitivity occurs, withhold Retevmo and begin corticosteroids at a dose of 1 mg/kg prednisone (or equivalent). Upon resolution of the event, resume Retevmo at a reduced dose and increase the dose of Retevmo by 1 dose level each week as tolerated until reaching the dose taken prior to onset of hypersensitivity. Continue steroids until patient reaches target dose and then taper. Permanently discontinue Retevmo for recurrent hypersensitivity.
Tumor lysis syndrome (TLS) occurred in 0.6% of patients with medullary thyroid carcinoma receiving Retevmo. Patients may be at risk of TLS if they have rapidly growing tumors, a high tumor burden, renal dysfunction, or dehydration. Closely monitor patients at risk, consider appropriate prophylaxis including hydration, and treat as clinically indicated.
Impaired wound healing can occur in patients who receive drugs that inhibit the vascular endothelial growth factor (VEGF) signaling pathway. Therefore, Retevmo has the potential to adversely affect wound healing. Withhold Retevmo for at least 7 days prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of Retevmo after resolution of wound healing complications has not been established.
Retevmo can cause hypothyroidism . Hypothyroidism occurred in 13% of patients treated with Retevmo; all reactions were Grade 1 or 2. Hypothyroidism occurred in 13% of patients (50/373) with thyroid cancer and 13% of patients (53/423) with other solid tumors including NSCLC. Monitor thyroid function before treatment with Retevmo and periodically during treatment. Treat with thyroid hormone replacement as clinically indicated. Withhold Retevmo until clinically stable or permanently discontinue Retevmo based on severity.
Based on data from animal reproduction studies and its mechanism of action, Retevmo can cause fetal harm when administered to a pregnant woman. Administration of selpercatinib to pregnant rats during organogenesis at maternal exposures that were approximately equal to those observed at the recommended human dose of 160 mg twice daily resulted in embryolethality and malformations. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with Retevmo and for 1 week after the last dose. There are no data on the presence of selpercatinib or its metabolites in human milk or on their effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with Retevmo and for 1 week after the last dose.
Severe adverse reactions (Grade 3-4) occurring in ≥20% of patients who received Retevmo in LIBRETTO-001 , were hypertension (20%), diarrhea (5%), prolonged QT interval (4.8%), dyspnea (3.1%), fatigue (3.1%), hemorrhage (2.6%), abdominal pain (2.5%), vomiting (1.8%), headache (1.4%), nausea (1.1%), constipation (0.8%), edema (0.8%), rash (0.6%), and arthralgia (0.3%).
Serious adverse reactions occurred in 44% of patients who received Retevmo. The most frequently reported serious adverse reactions (in ≥2% of patients) were pneumonia, pleural effusion, abdominal pain, hemorrhage, hypersensitivity, dyspnea, and hyponatremia.
Fatal adverse reactions occurred in 3% of patients ; fatal adverse reactions included sepsis (n=6), respiratory failure (n=5), hemorrhage (n=4), pneumonia (n=3), pneumonitis (n=2), cardiac arrest (n=2), sudden death (n=1), and cardiac failure (n=1).
Common adverse reactions (all grades) occurring in ≥20% of patients who received Retevmo in LIBRETTO-001 , were edema (49%), diarrhea (47%), fatigue (46%), dry mouth (43%), hypertension (41%), abdominal pain (34%), rash (33%), constipation (33%), nausea (31%), headache (28%), cough (24%), vomiting (22%), dyspnea (22%), hemorrhage (22%), arthralgia (21%), and prolonged QT interval (21%).
Laboratory abnormalities (all grades ≥20%; Grade 3-4) worsening from baseline in patients who received Retevmo in LIBRETTO-001 , were increased AST (59%; 11%), decreased calcium (59%; 5.7%), increased ALT (56%; 12%), decreased albumin (56%; 2.3%), increased glucose (53%; 2.8%), decreased lymphocytes (52%; 20%), increased creatinine (47%; 2.4%), decreased sodium (42%; 11%), increased alkaline phosphatase (40%; 3.4%), decreased platelets (37%; 3.2%), increased total cholesterol (35%; 1.7%), increased potassium (34%; 2.7%), decreased glucose (34%; 1.0%), decreased magnesium (33%; 0.6%), increased bilirubin (30%; 2.8%), decreased hemoglobin (28%; 3.5%), and decreased neutrophils (25%; 3.2%).
Concomitant use of acid-reducing agents decreases selpercatinib plasma concentrations which may reduce Retevmo antitumor activity. Avoid concomitant use of proton-pump inhibitors (PPIs), histamine-2 (H2) receptor antagonists, and locally-acting antacids with Retevmo. If coadministration cannot be avoided, take Retevmo with food (with a PPI) or modify its administration time (with a H2 receptor antagonist or a locally-acting antacid).
Concomitant use of strong and moderate CYP3A inhibitors increases selpercatinib plasma concentrations which may increase the risk of Retevmo adverse reactions including QTc interval prolongation. Avoid concomitant use of strong and moderate CYP3A inhibitors with Retevmo. If concomitant use of a strong or moderate CYP3A inhibitor cannot be avoided, reduce the Retevmo dosage as recommended and monitor the QT interval with ECGs more frequently.
Concomitant use of strong and moderate CYP3A inducers decreases selpercatinib plasma concentrations which may reduce Retevmo anti-tumor activity. Avoid coadministration of Retevmo with strong and moderate CYP3A inducers.
Concomitant use of Retevmo with CYP2C8 and CYP3A substrates increases their plasma concentrations which may increase the risk of adverse reactions related to these substrates. Avoid coadministration of Retevmo with CYP2C8 and CYP3A substrates where minimal concentration changes may lead to increased adverse reactions. If coadministration cannot be avoided, follow recommendations for CYP2C8 and CYP3A substrates provided in their approved product labeling.
Retevmo is a P-glycoprotein (P-gp) inhibitor. Concomitant use of Retevmo with P-gp substrates increases their plasma concentrations, which may increase the risk of adverse reactions related to these substrates. Avoid coadministration of Retevmo with P-gp substrates where minimal concentration changes may lead to increased adverse reactions. If coadministration cannot be avoided, follow recommendations for P-gp substrates provided in their approved product labeling.
The safety and effectiveness of Retevmo have not been established in pediatric patients less than 12 years of age . The safety and effectiveness of Retevmo have been established in pediatric patients aged 12 years and older for medullary thyroid cancer (MTC) who require systemic therapy and for advanced RET fusion-positive thyroid cancer who require systemic therapy and are radioactive iodine-refractory (if radioactive iodine is appropriate). Use of Retevmo for these indications is supported by evidence from adequate and well-controlled studies in adults with additional pharmacokinetic and safety data in pediatric patients aged 12 years and older. Monitor open growth plates in adolescent patients . Consider interrupting or discontinuing Retevmo if abnormalities occur.
No dosage modification is recommended for patients with mild to severe renal impairment (estimated Glomerular Filtration Rate [eGFR] ≥15 to 89 mL/min, estimated by Modification of Diet in Renal Disease [MDRD] equation). A recommended dosage has not been established for patients with end-stage renal disease.
Reduce the dose when administering Retevmo to patients with severe hepatic impairment (total bilirubin greater than 3 to 10 times upper limit of normal [ULN] and any AST). No dosage modification is recommended for patients with mild or moderate hepatic impairment. Monitor for Retevmo-related adverse reactions in patients with hepatic impairment.
Please see full Prescribing Information for Retevmo.
SE HCP ISI All_21SEP22
About Imlunestrant Imlunestrant (LY3484356) is an investigational, next-generation oral selective estrogen receptor degrader (SERD) with pure antagonistic properties. The estrogen receptor (ER) is the key therapeutic target for patients with ER+/HER2- breast cancer. Novel degraders of ER may overcome endocrine therapy resistance while providing consistent oral pharmacology and convenience of administration. Imlunestrant was specifically designed to deliver continuous estrogen receptor target inhibition throughout the dosing period and regardless of ESR1 mutational status. Imlunestrant is currently being studied in several clinical trials.
About Olomorasib Olomorasib (LY3537982) is an investigational, oral, potent, and highly selective second-generation inhibitor of the KRAS G12C protein. KRAS is the most common oncogene across all tumor types, and KRAS G12C mutations occur in 13% of patients with non-small cell lung cancer (NSCLC), and 1-3% of patients with other solid tumors. 5 Olomorasib was specifically designed to target KRAS G12C mutations and has pharmacokinetic properties which allow for high predicted target occupancy and high potency when used as monotherapy or in combination. 6
Olomorasib is currently being studied in the LOXO-RAS-20001 Phase 1/2 trial (NCT04956640) in patients with KRAS G12C-mutant NSCLC, and other advanced solid tumors and in the pivotal, registrational SUNRAY-01 global study (NCT06119581) investigating olomorasib in combination with pembrolizumab with or without chemotherapy for first-line treatment of KRAS G12C-mutant advanced NSCLC. For additional information about olomorasib clinical trials, please refer to clinicaltrials.gov .
About Lilly Lilly is a medicine company turning science into healing to make life better for people around the world. We've been pioneering life-changing discoveries for nearly 150 years, and today our medicines help more than 51 million people across the globe. Harnessing the power of biotechnology, chemistry and genetic medicine, our scientists are urgently advancing new discoveries to solve some of the world's most significant health challenges: redefining diabetes care; treating obesity and curtailing its most devastating long-term effects; advancing the fight against Alzheimer's disease; providing solutions to some of the most debilitating immune system disorders; and transforming the most difficult-to-treat cancers into manageable diseases. With each step toward a healthier world, we're motivated by one thing: making life better for millions more people. That includes delivering innovative clinical trials that reflect the diversity of our world and working to ensure our medicines are accessible and affordable. To learn more, visit Lilly.com and Lilly.com/news , or follow us on Facebook , Instagram and LinkedIn . P-LLY
Retevmo ® is a registered trademark owned by or licensed to Eli Lilly and Company, its subsidiaries, or affiliates.
Verzenio ® is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates.
© Lilly USA , LLC 2024. ALL RIGHTS RESERVED.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements (as that term is defined in the Private Securities Litigation Reform Act of 1995) about Verzenio ® (abemaciclib) as a potential treatment for people with certain types of early breast cancer, Retevmo ® (selpercatinib) as a potential treatment for people with locally advanced and metastatic RET fusion-positive NSCLC, RET -mutant MTC, and RET -activated thyroid cancer, imlunestrant as a potential treatment for people with certain types of breast cancer and olomorasib as a potential treatment for certain KRAS G12C-mutant advanced solid tumors, and reflects Lilly's current beliefs and expectations. However, as with any pharmaceutical product, there are substantial risks and uncertainties in the process of drug research, development, and commercialization. Among other things, there can be no guarantee that studies will be initiated or completed as planned, that future study results will be consistent with the results to date, or that Verzenio, Retevmo, imlunestrant, or olomorasib will receive initial regulatory approvals or approvals for additional indications, as applicable, or be commercially successful. For further discussion of these and other risks and uncertainties that could cause actual results to differ from Lilly's expectations, see Lilly's Form 10-K and Form 10-Q filings with the United States Securities and Exchange Commission. Except as required by law, Lilly undertakes no duty to update forward-looking statements to reflect events after the date of this release.
1 Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ® ) for Breast Cancer V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed May 9, 2024. To view the most recent and complete version of the guidelines, go online to NCCN.org . NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 2 Johnston SRD, Toi M, O'Shaughnessy J, Rastogi P, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol . 2023 Jan;24(1):77-90. 3 Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2–negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol . 2020;6(1):116-124. doi:10.1001/jamaoncol. 2019.4782. 4 Retevmo. Prescribing information. Lilly USA , LLC. 5 Ji J, Wang C, Fakih M. Targeting KRASG12C-mutated advanced colorectal cancer: Research and clinical developments. OncoTargets and Therapy . 2022;Volume 15:747-756. doi:10.2147/ott.s340392 6 Peng S-B, Si C, Zhang Y, et al. Abstract 1259: Preclinical characterization of Ly3537982, a novel, highly selective and potent KRAS-G12C inhibitor. Cancer Research . 2021;81(13_Supplement):1259-1259. doi:10.1158/1538-7445.am2021-1259
View original content to download multimedia: https://www.prnewswire.com/news-releases/lilly-announces-details-of-presentations-at-2024-american-society-of-clinical-oncology-asco-annual-meeting-302148904.html
SOURCE Eli Lilly and Company
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

26 templates

first day of school
69 templates
18 templates

48 templates

6 templates

great barrier reef
17 templates
Monthly Meeting
It seems that you like this template, monthly meeting presentation, free google slides theme and powerpoint template.
It’s the time of the month again where you’re due to provide a report or update at your company meeting. Slidesgo’s latest business presentation is guaranteed to help calm your nerves and set your audience in a lighter mood. Check it out now!
Having to stand up in front of a crowd for a business presentation can be a daunting experience. We know that. That’s why we’ve created this new adorable template that uses casual typography with a handwriting font for titles and a simple sans-serif for body text. Resources are colored blue to contrast its gray background. The presentation follows a meeting structure and we have incorporated pictures of office settings. Best of all, it is fully adaptable and ideal for project reports.
Features of this template
- An adorable and casual business presentation that’s refreshing and light
- 100% editable and easy to modify
- 24 different slides to impress your audience
- Available in five colors: blue, pink, gray, green, and purple
- Contains easy-to-edit graphics, maps and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the free resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Combines with:
This template can be combined with this other one to create the perfect presentation:

Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Available colors.
Original Color
Related posts on our blog

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access

Register for free and start editing online

IMAGES
VIDEO
COMMENTS
Download the Medical Dentists Students Meeting presentation for PowerPoint or Google Slides. Gone are the days of dreary, unproductive meetings. Check out this sophisticated solution that offers you an innovative approach to planning and implementing meetings! Detailed yet simplified, this template ensures everyone is on the same page ...
Download your presentation as a PowerPoint template or use it online as a Google Slides theme. 100% free, no registration or download limits. Get these meeting templates to streamline your presentations and run efficient meetings. No Download Limits Free for Any Use No Signups.
Free Google Slides theme and PowerPoint template. It's important that everyone in your company should be aware of the current state of the projects during your meetings. To help you achieve the best results, here's an elegant template that won't disappoint you. A minimalist design is always trendy.
Find Free Slide Show Templates that Suit your Needs. Captivate your audience with our collection of professionally-designed PowerPoint and Google Slides templates. Boost your presentations and make a lasting impression!
Premium Google Slides theme, PowerPoint template, and Canva presentation template. Share the details and planification of your next meeting using this presentation. It's minimalist and it includes different sections such as project schedule, project timeline, status and upcoming report. We have also added tables, infographics and many other ...
Our collection of 100% editable Meeting templates for PowerPoint and Google Slides provides presenters with creative slides and diagrams catering to an array of meeting types and objectives. Whether you're hosting a corporate strategy session, conducting a workshop, or delivering a seminar report, our range of meeting PPT templates ensures ...
A Meeting PowerPoint template is a ready-made presentation template that provides a structured framework for creating professional Meeting presentations. The Meeting PPT presentation template includes design elements, layouts, and fonts that you can customize to fit your content and brand.
436 templates. Create a blank Conference Presentation. Blue and White Geometric Modern Leadership During a Crisis Workshop Webinar Keynote Presentation. Presentation by Canva Creative Studio. Event Organizer Presentation. Presentation by Giant Design. Keynote Presentation. Presentation by Canva Creative Studio.
1. General Business Meeting Template. A company meeting is an excellent way to bring all your employees together. It helps to get them on the same page about new procedures, plans for the future, and future milestones. This Company Meeting PowerPoint template is a fine choice for any employee meeting on your agenda.
Free PowerPoint template and Google Slides theme. Professional slide perfect for meetings. Simple and organized style, with focus on the content, which will make your meetings more efficient. This slide is available for PowerPoint and Google Slides. #Business #Budget #Finance #Quotation #Presentations #Company #Elevator Pitch #Meetings. Blue ...
Team meetings are like assembling a puzzle; each piece contributes to the bigger picture of success. Presentations play a vital role in these gatherings, acting as the glue that binds ideas and discussions together. In our team meeting presentation templates category, we offer a pack of premade slides to make your meetings impactful and engaging.
Free Canva presentation template. Introduce an impressive visual narrative to your business meetings with our Blue and White Minimalist Corporate Slide Template. Ideal for Google Slides and PowerPoint, this sleek, modern toolkit is perfect for professionals eager to communicate with clarity and charisma. Whether laying out a business agenda or ...
Board Meeting Presentation Sample. Customize your board meeting presentation. Make an impact with your presentation by using graphs, charts, timelines, diagrams, and sales funnels. Each of these graphics can easily be added to your board meeting presentation template with just one click. Some potential slides to include are: TITLE SLIDE. AGENDA.
You can prepare presentations around skill training, project planning, change management, and project reviews. The Team Meeting PowerPoint Template is a resourceful tool for team management and effective communication strategies. As a pre-design team meeting template, it fits all types of businesses and industries.
Presentation templates offer versatile options for personalizing—get creative by customizing your template or opt for adding your own text to existing designs. When you use a template at your next meeting, you'll turn a simple presentation into an opportunity to impress. To make presenting even easier, you can download your template in a ...
Premium Google Slides theme, PowerPoint template, and Canva presentation template. Meetings, whether they're held daily, weekly, or monthly, are important for all businesses. They keep company employees on track, introduce new products, provide updates, and more. If you're looking for a template to do that, look no further.
6 Build your confidence. Practice, practice, practice. Imagine your living room is your meeting space, and practice giving your presentation aloud with no one present. The more you practice and present, the more confident you'll become. And sure, it's understandable to feel nervous before a presentation.
The board meeting presentation template showcases four to six-stage presentation designs with an easily understandable poster design format. For example, the six-step presentation, as in the hub and spoke model infographic, supports presenters to put the main heading on the nucleus point, and its sub-elements can display in the six square ...
10. PowerPoint Corporate Project Kickoff Template by SlideTeam. Organize your kickoff meeting agenda with SlideTeam's PowerPoint Corporate Project Kickoff Template. If you want to streamline your corporate planning efforts, the PowerPoint Corporate Project Kickoff Template by SlideTeam can help you with it.
Learn more about puzzle questions to create your own version of this energizing icebreaker. 6. Fun kahoot on your topic of choice (flags is a good one!) Sitting in meetings and presentations can get dull. Captivate your audience's attention from the start with a fun, and perhaps a bit random, trivia-like game.
The meeting presentations will be heard, viewed, captioned, and recorded through an online teleconferencing and/or video conferencing platform. The Committee will discuss new drug application ...
Free Google Slides theme, PowerPoint template, and Canva presentation template. Team meetings, if well planned, are the perfect time to advance work with your colleagues. To help you with this task we have created this colorful template, which combines purple and orange backgrounds with green, yellow, orange and purple circles. In addition, its ...
C: 267-693-6224. [email protected]. For Patients and the General Public: 1-800-789-7366. For Media Queries & Requests (24/7): 215-662-2560. Researchers from Penn Medicine's Abramson Cancer Center will present the latest advances in clinical cancer research at the 2024 ASCO Annual Meeting.
Make your morning meetings more engaging with this morning meeting slides template. Perfect for teachers, students, and business professionals, these templates can help you create a dynamic and interactive presentation. With a range of customizable slides, you can easily manage your lessons, brainstorm ideas, or give an inspiring presentation.
Follow. NEW YORK, May 31, 2024 (GLOBE NEWSWIRE) -- TG Therapeutics, Inc. (NASDAQ: TGTX), today announced presentations highlighting study designs for post-marketing studies being undertaken for ...
Lilly will also host an investor event to provide an update on its oncology strategy and pipeline. The event will take place on Sunday, June 2, at 7:30 p.m. CDT and will be available via a live webcast on the "Webcasts & Presentations" section of Lilly's investor website.A replay will also be available on the website following the event.
2024 EPSCoR Annual PI Meeting Presentations; 2024 EPSCoR Annual PI Meeting Presentations. Established Program to Stimulate Competitive Research (EPSCoR) ... Day 2 presentations Day 2 Welcome Remarks (PDF, 2.51 MB) Sandra Richardson, Research Capacity and Competitiveness, Section Head ...
Free Google Slides theme, PowerPoint template, and Canva presentation template. Teleworking is here to stay. And that implies that we need to adapt to new ways of work and communication. Virtual meetings are still important, as is optimising time. That is why it is recommended to plan them in advance and prepare them well.
Presentation Title: Selpercatinib in non-MTC, RET-mutated tumors: Efficacy in MEN-associated and other tumors Abstract Number: 3150 Presentation Date & Time: Saturday, June 1, 9:00 a.m. - 12:00 ...
Free Google Slides theme and PowerPoint template. It's the time of the month again where you're due to provide a report or update at your company meeting. Slidesgo's latest business presentation is guaranteed to help calm your nerves and set your audience in a lighter mood. Check it out now!