
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.


Clinical Research in Primary Care
Research opportunity announcement: integrating clinical research into primary care settings through network research hubs – a pilot (ot2).
Research Opportunity Announcement Overview
Purpose and Scope
Phased Approach
Definitions
Eligibility
Application Responsiveness
Application Requirements
Objective Review
Special Award Terms
Research Opportunity Announcement Overview #announcementoverview
ROA Number: OTA-24-016
- ROA Posting: May 6, 2024
- Technical Webinar 1: 12pm EDT on May 14, 2024
- Technical Webinar 2: 12pm EDT on May 22, 2024
- Submission Deadline: Complete applications must be submitted under OTA-24-016 via NIH eRA Commons ASSIST no later than 5:00pm EDT on June 14, 2024. Late applications submitted to this ROA will not be accepted.
Brief Overview of the Research Opportunity: The purpose of this limited competition research opportunity announcement (ROA) is to invite applications by organizations currently affiliated with and participating in specific existing clinical research networks --to serve as “Network Research Hubs” and establish the infrastructure to conduct clinical research in primary care settings. This opportunity is limited to organizations that focus on serving rural communities and are part of or funded by: NIH Institutional Development Award Clinical and Translational Research (IDeA-CTR) awards, the NIH Clinical and Translational Science Award (CTSA) Program, and/or the Patient-Centered Outcomes Research Institute’s (PCORI) Patient-Centered Clinical Research Network (PCORnet).
Award Mechanism: This funding opportunity will use the Other Transactions Authority (OTA) governed by 42 U.S. Code § 282 (n)(1)(b) to issue Other Transaction (OT) awards. OT awards are not grants, cooperative agreements, or contracts and use an OTA, provided by law. Policies and terms for individual OTs may vary between awards. Each award is therefore issued with a specific agreement which is negotiated with the recipient, and which may be expanded, modified, partnered, not supported, or later discontinued based on program needs, changing research landscape, performance and or availability of funds.
Anticipated Awards and Budget: NIH anticipates 2-5 awards will be issued through this ROA in FY24. NIH intends to allocate a total of approximately $5M in FY24 and approximately $20M in FY25 to fund Network Research Hubs during the two-year pilot phase, contingent on programmatic objectives, performance and availability of funds. After the two-year pilot phase, individual awards may be terminated, extended, or curtailed based on programmatic objectives, performance, and availability of funds.
Contact Information: [email protected]
Background #background
In recent years, the U.S. has experienced trending declines in health that are disproportionately occurring in medically underserved and disadvantaged populations. Many of these sectors are also often underrepresented in clinical research. When study demographics don’t match the demographics of those impacted by the illness or condition under investigation, the results may have limited generalizability, leading to evidence gaps and further compounding health disparities. A major barrier to participating in clinical research is lack of access to or availability of clinical studies. In addition, the majority of Americans have never talked to their doctor about participating in research. There is a critical need to extend research participation opportunities to communities often underrepresented in clinical research, and to integrate those opportunities into settings where people seek care.
Therefore, the National Institutes of Health (NIH) is planning to establish a coordinated infrastructure that integrates innovative research into routine clinical care in primary care settings. Through this effort, NIH will:
- Pilot and implement infrastructure to support primary care-based clinical research in mission areas across all NIH Institutes and Centers (ICs) spanning prevention and treatment and with a focus on health equity and whole person health;
- Establish a foundation for sustained engagement with communities underrepresented in clinical research (e.g., individuals who live in rural environments, racial and ethnic minority groups, older adults, persons experiencing challenging social determinants of health and related experiences);
- Implement innovative study designs that address common health issues, including disease prevention; and
- Utilize a full range of clinical research designs as appropriate, including dissemination and implementation research, to inform clinical practice
NIH envisions this infrastructure will facilitate and accelerate research advances for adoption and implementation into everyday clinical care, improving health outcomes, and advancing health equity for all Americans.
The infrastructure NIH will pilot and implement is anticipated to include the following components:
- Providing oversight of the studies/protocols and site and study selection
- Providing statistical and data management support
- Developing innovative clinical study designs and implementation strategies to minimize burden on participants and providers in primary care settings
- Operations Center – conducting site feasibility assessments, site agreements/contracting, and coordination of study operations (protocol development; compliance with Food and Drug Administration (FDA) and Office of Human Research Protections (OHRP) regulatory and participant protection requirements; communications; training; auditing; quality assurance; and data monitoring)
- Independent Review and Monitoring Boards - including Data and Safety Monitoring Board (DSMB), Observational Study Monitoring Board (OSMB), and the Central Institutional Review Board (IRB)
- Network Research Hubs – leveraging existing research networks and partnerships with Clinical Sites to conduct clinical research in primary care settings
- Community Engagement – providing support, advice, and resources, in part through partnerships with existing entities, to facilitate sustained participant and community engagement, community-driven research, and integration of studies in primary and community care settings
- Industry Partnerships – engaging for-profit partners for collaborative knowledge sharing and potential participation in/use of the infrastructure
Purpose and Scope #purposeandscope
The purpose of this research opportunity announcement (ROA) is to invite applications by existing clinical research networks – as well organizations currently affiliated with and participating in specific existing clinical research networks – to serve as “Network Research Hubs” as part of a larger infrastructure (comprising the components described above) supporting research in primary care settings. These Network Research Hubs must be actively part of (i.e., active funding/award) one or more of the following: NIH Institutional Development Award Clinical and Translational Research (IDeA-CTR) awards, the NIH Clinical and Translational Science Award (CTSA) Program, and/or the Patient-Centered Outcomes Research Institute’s (PCORI) Patient-Centered Clinical Research Network (PCORnet). The Network Research Hubs will serve to expand accrual efforts of select existing NIH-funded studies and develop and conduct new studies with a focus on engaging underrepresented populations, particularly those in rural or underserved areas, and enhancing study inclusivity. Bringing clinical research studies to individuals in their own communities, informed by those communities, and improving clinical research inclusivity will facilitate the generation of a more broadly applicable evidence base that contributes to improved patient outcomes and health equity for all Americans.
Objectives #objectives
Network Research Hubs will be responsible for:
- Provide clinical research leadership and oversight for clinical studies at all sites supported by and/or partnering with the Network Research Hub, including Clinical Sites.
- Participate in select existing studies/trials conducted by NIH-funded investigators. Identify and recruit primary care-providing organizations to serve as Clinical Sites for study accrual, supporting the particular needs of each site (e.g., assembling and/or mentoring local research team(s), training providers and clinical staff, assuring protocol adherence, ensuring adequacy of human subjects protections) in collaboration with the Operations Center.
- Accurately identify, screen, recruit and enroll eligible participants for clinical research studies, meeting or exceeding demographic representation and study inclusivity targets as agreed upon with the Scientific and Medical Director and/or the Scientific Committee.
- Implement strategies for culturally appropriate and inclusive study participation, including ensuring study interventions and measures are clinically meaningful and adapted for different populations, as appropriate.
- Provide complete, accurate, and timely collection and entry of high-quality data and biosamples into data management system and repositories as required by the protocol, Scientific Medical Director, and/or Scientific Committee.
- Track and report trial and performance data (e.g., recruitment, retention, adverse events) by site on a regular and frequent basis as required by the clinical studies, the Scientific and Medical Director, Scientific Committee, and/or data and safety monitoring plans.
- If successful with enhancing accrual of an existing NIH-funded study, develop new study ideas (interventional or observational) for consideration by the Scientific and Medical Director, Scientific Committee, and Operations Center that reflect the clinical needs and priorities of the applicant’s community base. These ideas for new studies will require external funding sources to cover study costs not supported by the components of the coordinated infrastructure.
- Respond to information requests for study feasibility assessments (e.g., accessible study population, enrollment estimates, resources available) during research planning and protocol development.
- Identify health disparities and care disparities and needs of the local population of clinical sites for research planning and prioritization.
- Participate in data analysis, development of results, and dissemination efforts as appropriate.
- Ensure that aggregate research results and/or final study findings are shared with study participants through effective communications.
- Work collaboratively with and through individuals and communities on a continuum of practices from outreach to shared decision making to build trust, foster meaningful bi-directional relationships, and identify and address the health needs and priorities of those individuals and communities.
- Increase and sustain the involvement of research participants, patients, patient advocates, and community organizations as partners in research, including research planning and prioritization.
- Operationalize and sustain engagement from the onset of research activities and through various culturally appropriate approaches to create awareness, provide education, develop and perform targeted recruitment and enrollment activities, and mobilize knowledge of the benefits of the research.
- Leverage existing resources and expand community partnerships (e.g., safety-net health systems, other health systems, grassroots organizations, public health departments, community and faith-based organizations, schools or childcare settings, Tribal organizations and agencies) to increase access to clinical studies.
- Manage unintended consequences and/or breaks in community relationships.
- Evaluate engagement efforts for continuous improvement and sustainability.
- Share approaches and strategies for effective community engagement and build a community of practice.
- In coordination with the Scientific Committee and Scientific and Medical Director, apply and execute innovations across the landscape of clinical research to minimize burden of research on participants and clinical staff. Innovations may include activities such as: leveraging electronic health records for recruitment, randomization, and data collection; leveraging digital health technologies to reduce research burden and facilitate incorporation of diverse precision measurements into participant monitoring and clinical outcomes assessments; aligning clinical care and research workflows; implementing point-of-care trial, pragmatic, and decentralized approaches in study designs.
- Optimize study designs to increase research equity and accessibility in real world health care settings while maintaining scientific rigor.
- Through alignment of research and clinical workflows, facilitate clinical sites movement towards a learning healthcare system, accelerating the adoption and implementation of evidence into clinical practice.
- Accept and implement policies and procedures established and/or approved by the Scientific and Medical Director, Scientific Committee, Operations Director, and/or additional governance structures established as the infrastructure is further developed.
- Contribute to the development of policies and procedures by participating in Working Groups or Sub-Committees of the Scientific Committee and other governing bodies established as the infrastructure is further developed.
- Actively participate and cooperate with quality assurance, study oversight, and study monitoring efforts.
- Interact and collaborate with other federal and non-federal primary care and clinical research networks and entities (e.g., the NIH Community Engagement Alliance (CEAL), Federally Qualified Healthcare Centers (FQHCs), the NIH Collaboratory, IDeA-CTRs, CTSAs, PCORnet), to leverage existing resources and partnerships, as appropriate.
- Work collaboratively with all other components of the coordinated infrastructure.
- Participate in cross-site and cross-component/cross-effort meetings to foster relationship building and enhance partnerships.
- Identify, track, and consolidate challenges and successes and share best/promising practices to integrating research in primary care settings for scalability and sustainability.
Phased Approach to Launching Studies and Building the Infrastructure #phasedapproach
NIH is planning to launch this effort as a two-year pilot. The first year of the pilot will involve selecting and funding Network Research Hubs through this ROA to support participation in select existing NIH-funded studies that are agreed upon between the applicant and NIH (in coordination with NIH-funded investigators as needed) during negotiations of a potential award. These initial studies may be interventional or observational and are expected to be reasonably suitable for primary care settings. It is expected that the select existing NIH-funded studies will have infrastructure to support operational aspects (e.g., central IRB, data management) for new sites, but resources available and needed will be negotiated prior to award.
If the Network Research Hub is successful in enhancing participant accrual into an existing study, they may potentially expand in year two with new research in coordination with the other components of the infrastructure. In order to leverage the coordinated research infrastructure described above, these new research concepts will require approval by the Scientific and Medical Director, and Operations Director and funding from external sources for all study aspects not covered by the coordinated infrastructure described above (e.g., specific interventions, additional clinical research staff). Before ramping up to an implementation phase in year three, NIH will conduct an evaluation of the program to assess which approaches and efforts are working. NIH may expand, pivot, and/or sunset awards and/or components based on the results.
Definitions #definitions
This announcement follows the definitions for Clinical Trial-Related Terms below in addition to those in the NIH glossary for clinical trial-related terms: https://grants.nih.gov/policy/clinical-trials/glossary-ct.htm.
Additional key terms are defined below:
Central Institutional Review Board (Central IRB) : A centralized approach to human subject protection through a process that streamlines IRB review of selected NIH-sponsored trials for institutions across the country by relying on national experts to ensure trials are reviewed efficiently and with the highest ethical and quality standards.
Clinical Research Network : Collaborative groups of researchers and/or clinicians and that come together in partnership with healthcare systems to identify important clinical questions and design clinical studies to answer them, with coordinated support to manage regulatory, financial, scientific, and/or operational aspects of the research.
Clinical Site : A primary care practice, community health center, hospital, or other health services institution where participants are identified, screened, recruited, and/or enrolled in research conducted by the Network Research Hub.
Federally Qualified Health Centers (FQHC) : As defined by the Health Resources and Services Administration (HRSA), public and private non-profit health care organizations that meet certain criteria under the Medicare and Medicaid Programs. FQHCs include:
- Nonprofit entities that receive a grant, or funding from a grant, under section 330 of the Public Health Service Act to provide primary health services and other related services to a population that is medically underserved;
- FQHC “Look-Alikes” – nonprofit entities certified by the Secretary of the U.S. Department of Health and Human Services as meeting the requirements for receiving a grant under section 330 of the Public Health Service Act but are not grantees; and
- Outpatient health programs or facilities operated by a Tribe or Tribal organization under the Indian Self-Determination Act or by an urban Indian organization receiving funds under Title V of the Indian Health Care Improvement Act.
Medically Underserved Area/Population (MUA/P) : As defined by HRSA, MUAs may be a whole county or a group of contiguous counties, a group of county or civil divisions or a group of urban census tracts in which residents have a shortage of personal health services; MUPs may include groups of persons who face economic, cultural or linguistic barriers to health care.
Milestones : Objective, measurable events that are indicative of project progress occurring as proposed in the application.
Network Research Hub : An institution/organization with an established organizational structure and scientific and statistical leadership for developing, implementing, and analyzing multi-institutional clinical studies/trials.
Other Transactions Authority (OTA) : A unique type of authority that allows an agency to enter into a legal agreement with a recipient organization that is not a contract, grant, or cooperative agreement (Learn more about OTAs on the NIH website) .
Partnership : An association of two or more individuals or entities with a commitment to an ongoing relationship to work toward common goals as established.
Primary Care: As defined by HRSA, the provision of integrated, accessible health services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained partnership with patients, and practicing in the context of family and community.
Primary Care Setting : As defined by HRSA, a setting with integrated, accessible health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained partnership with patients, and practicing in the context of family and community. These don’t meet the criteria:
- Emergency departments
- Inpatient hospital settings
- Ambulatory surgical centers
- Independent diagnostic testing facilities
- Skilled nursing facilities
- Inpatient rehabilitation facilities
Research Opportunity Announcement (ROA) : Used to solicit applications for programs using Other Transactions Authority.
Rural: For the purposes of this ROA, rural areas are defined according to the Office of Management and Budget and Federal Office of Rural Health Policy (FORHP) definitions, where primary Rural-Urban Commuting Area (RUCA) codes between 4 and 10 correspond to rural areas and primary RUCA codes 1-3 correspond to urban areas.
Rural Health Clinic: An entity certified by the Centers for Medicare & Medicaid Services. A rural health clinic provides outpatient services to a non-urban area with an insufficient number of health care practitioners.
Social Determinants of Health (SDOH) : As defined by the CDC, SDOH are the nonmedical factors that influence health outcomes. They are the conditions in which people are born, grow, work, live and age, and the wider set of forces and systems (e.g., economic policies and systems, development agendas, social norms, social policies, racism, climate change, and political systems) shaping the conditions of daily life.
Eligibility #eligibility
Eligible applicants are limited to organizations that are lead or funded partner organizations of one or more of the following clinical research networks: the NIH IDeA-CTRs, NIH CTSA, and/or PCORI PCORnet. For the purposes of this ROA, organizations with IDeA-CTR and CTSA awards or sub-awards in no-cost-extensions are eligible to apply. In addition, applicant organizations must be located in a state/jurisdiction where at least 25% of its census tracts are defined as rural using the Revised 2010 RUCA Codes.
Non-domestic (non-U.S.) Entities (Foreign Institutions) are not eligible to apply. Non-domestic (non-U.S.) components of U.S. Organizations are not eligible to apply. Foreign components, as defined in the NIH Grants Policy Statement, are not allowed.
Higher Education Institutions
- Public/State Controlled Institutions of Higher Education
- Private Institutions of Higher Education
- Hispanic-Serving Institutions (HSIs)
- Historically Black Colleges and Universities (HBCUs)
- Tribally Controlled Colleges and Universities (TCCUs)
- Alaska Native and Native Hawaiian Serving Institutions
- Asian American Native American Pacific Islander Serving Institutions (AANAPISIs)
Nonprofits Other Than Institutions of Higher Education
- Nonprofits with 501(c)(3) IRS Status (Other than Institutions of Higher Education)
- Nonprofits without 501(c)(3) IRS Status (Other than Institutions of Higher Education)
For-Profit Organizations
- Small Businesses
- For-Profit Organizations (Other than Small Businesses)
Governments
- State Governments
- County Governments
- City or Township Governments
- Special District Governments
- American Indian/Native American Tribal Governments (Federally Recognized)
- American Indian/Native American Tribal Governments (Other than Federally Recognized)
- Independent School Districts
- Public Housing Authorities/American Indian Housing Authorities
- Native American Tribal Organizations (Other than Federally recognized tribal governments)
- Faith-based or Community-based Organizations
- Regional Organizations
Application Responsiveness #responsiveness
Applications will undergo a responsiveness screening conducted by NIH program staff. Applications that are deemed nonresponsive will be withdrawn and will no longer be in consideration for funding. Examples of projects that will be considered unresponsive to this announcement include the following:
- Applications that do not meet the “Eligibility” requirements specified above.
- Applications from organizations that are not actively participating as lead or a funded partner in one or more of the following clinical research networks: IDeA-CTRs, CTSA, and/or PCORnet in the United States,
- Applications proposing partnerships only with Clinical Sites that do not meet the definition of primary care settings.
- Applications proposing support for routine patient care unrelated to human subjects research.
- Applications proposing animal or in vitro research.
Application Requirements #applicationrequirements
All application components should be uploaded in eRA Commons in searchable PDF format with a font size of 11 or 12 point and font type of Calibri, Aptos, Arial, or Times New Roman. Margins must be 1-inch wide (top, bottom, left, and right). The components of the application should be loaded as separate attachments and should be titled as specified in each section below (title included in parentheses following each section). Guidance for OT application submission can be found on the NIH website.
Cover ( “Cover.pdf”, 2 page maximum )
- Number and title of this Research Opportunity Announcement
- Application Title
- Principal Investigator(s) (PI) first and last name, title, organization, mailing address, email address and phone number. If multiple PIs are named, the Contact PI is clearly identified.
- Name and address of the partnering Clinical Sites with a contact for each (full name, email address)
- Recipient Business Official/Signing Official first and last name, title, organization, mailing address, email address and phone number
- First and last name of other key personnel, their title, institutional affiliation, and email address
Abstract ( “Abstract.pdf”, no more than 250 words )
A brief summary of the application.
Specific Aims ( “Specific Aims.pdf”, 1 page maximum )
Provide a cogent overview, at a high level, of the capabilities and proposed plans to carry out the objectives of a Network Research Hub as part of a coordinated infrastructure supporting clinical research in primary care settings. Include how the work of the Network Research Hub will increase the accessibility of clinical research and improve health equity.
Project Plan ( “Project Plan.pdf”, 16 pages maximum )
Applications must include a Project Plan that clearly and fully demonstrates the applicant’s capabilities, understanding, and experience to accomplish the objectives of the Network Research Hubs.
Technical Approach ( 6 pages maximum )
Section A: Overview and Organization
- Outline the overall organization of the Network Research Hub and partnering Clinical Sites, and briefly describe the collective strengths of the team. Include how the Network Research Hub is actively participating in and/ or affiliated with one of the following clinical research networks: NIH IDeA-CTRs, NIH CTSA, PCORI’s PCORnet.
- Provide a diagram (and/or map) showing the geographical relationships between all entities included in the application. The rural participant catchment area is not limited to the applicant organization’s state.
- List the underrepresented populations that could be served by the Network Research Hub. Define the community or communities the Network Research Hub will serve.
- Describe plans to collaborate with NIH-funded investigators, other Network Research Hubs, and the other components of the coordinated infrastructure. Share how the Network Research Hub plans to contribute to the development of policies and procedures and abide by them as they are established.
Subsection B. Developing and Implementing Studies and Use of Innovative Designs
- Identify areas of research that the Network Research Hub would like to pursue if successful with implementing expansion of an initial, existing NIH-funded study. Include a description of the criteria to be used and process by which studies will be identified that are priorities of and/or co-developed by their community.
- Explain how the Network Research Hub’s research interests and/or proposed research agenda will improve scientific knowledge, develop a more broadly applicable clinical evidence base, and improve clinical practice.
- Describe innovative/novel solutions to address challenges with integrating research into primary care settings and to minimize the burden of research on participants and clinical staff. Share the team’s expertise and experience with operationalizing these solutions.
- Briefly describe processes the applicant will use to ensure compliance with regulations for research involving human subjects, and that study teams obtain and maintain sufficient proficiency level regarding the conduct of clinical research in coordination with the Operations Center.
Subsection C. Participant and Community Engagement
- Illustrate the team’s expertise in implementing related plans through recent examples and experiences.
- Clearly describe how the Network Research Hub will solicit and understand the challenges and health needs of their community.
- Highlight flexibilities in study designs, features, and engagement plans that can be adapted to meet the needs of a variety of participant groups and community partners, including those that are underrepresented in research.
- Describe how the Network Research Hub will contribute to the development of a community of practice in community engagement.
Primary Care Research Experience (6 pages maximum)
- Study title, type/design, research question(s) addressed, and target and actual total enrollment over time
- Partnerships with organizations providing care in primary care settings.
- Total enrollment by site
- Demographic breakdown of enrolled participants by race/ethnicity, sex, age, rural vs non-rural, and at least one other measure of social determinants of health (SDOH)
- How the data and/or results were shared and disseminated (including if shared with research participants)
- List additional previous relationships and/or partnerships with the proposed Clinical Sites to provide evidence supporting the likelihood of successful collaborations on future research.
Environment and Resources (3 pages maximum)
List and describe the salient features of the facilities and other resources available for use by the proposed Network Research Hub.
- Describe the resources available to facilitate carrying out the objectives of a Network Research Hub.
- Characterize the proposed Clinical Sites: describe the collective catchment area of the potential research participants through geographic boundaries, quantify the proportion of the catchment area as being rural vs urban in alignment with primary RUCA codes between 1-3 as defining urban areas and 4-10 as defining rural areas, and share any unique features to facilitate accrual of populations underrepresented in research.
Leadership Plan (1 pages maximum)
A brief leadership plan should be presented which identifies and describes the governance of the Research and Clinical Sites, chain of responsibility for decision making, and process for conflict resolution. The plan should describe how the leadership will contribute to the success of and collaboration within the infrastructure and the implementation of clinical studies with a focus on underrepresented populations. A succession plan with identification of a substitute/back-up lead investigator candidate should be included, if possible, to assure programmatic continuity.
Budget ( “Budget.pdf”, no page limitations )
The Budget must demonstrate estimated baseline costs of adding a select and limited number of primary care Clinical Sites to existing studies conducting clinical research in primary care settings, where the operational aspects of the study are supported by other NIH awards. Budgets are expected to be negotiated as the initial study is selected in coordination with NIH before an award is issued. Cost sharing is allowable.
Applicants shall assume a budget period of 12 months initially, and an additional 12-month option period during the 2-year pilot of this infrastructure for research in primary care settings. Funding for core support will be reimbursable, but costs per participant is expected to be funded based on study accrual milestones. Towards the end of the initial budget period, NIH will conduct an evaluation of the program to assess which approaches and efforts are working; NIH may continue, expand, pivot, and/or sunset awards and/or award components based on the results, infrastructure needs and/or congressional appropriations.
Study budgets should include funds for the community partners to be fully engaged and successfully participate in research prioritization, design and implementation.
Provide the overall expected cost for expanding research enrollment in primary care settings including but not limited to each of the following categories:
- Personnel
- Equipment
- Travel
- Subawards/subcontracts/consultants
- Other direct costs
- Total cost (with indirect costs included)
- Proposed Cost Share contribution (if applicable)
Applicants must provide a budget justification for all budget items. Subrecipients/subaward budgets must include a breakdown of costs and a budget justification. Applicants should provide one budget and budget justification per institution or organization in the application.
Additional information to include in the submission
List of Key Personnel (“Key Personnel.pdf”, 1 page maximum)
Provide a list of key personnel that will significantly contribute to the objectives of the Network Research Hub. Provide their first name, last name, title, institutional affiliation, and email address.
Biosketches of Key Personnel (“Biosketch.pdf”, 3 page maximum per individual)
Provide a biosketch of each named key individual appearing in the Key Personnel List. The information in the biosketch should include the name and position title, education/training (including institution, degree, date (or expected date) of degree, and field; list of positions and employment in chronological order (including dates); list of relevant publications, proposed level of effort and a personal statement that briefly describes the individual’s role in the project and why they are well-suited for this role. Providing successful examples from past work on similar infrastructure building projects as appropriate to illustrate the relevant experience is desired. The format used for an NIH grant application is acceptable: https://grants.nih.gov/grants/forms/biosketch.htm .
Letters of Interest/Support (1 page maximum per institution or organization)
A letter from the applicant's current affiliated clinical research network(s) (e.g., the director of the network coordinating center, administrative core, other authorized representative) should provide assurance that the proposed Network Research Hub is active and in good standing with the affiliated clinical research network. In addition, letters of interest and support should be provided from an authorized official from each of the proposed Clinical Sites and should include references to or direct evidence of prior research partnership or relationship with the Network Research Hub.
Appendix of Data Characterizing the Research and Clinical Sites’ Catchment Area (no page limit)
Data used to characterize the catchment area as rural or non-rural may be included to verify the catchment area descriptions in the Project Plan.
Objective Review #objectivereview
The intent of the objective review is to evaluate the strengths and the weaknesses of the proposed Network Research Hub and how well they would meet the objectives of a Network Research Hub. Applications will not be evaluated against each other during the review process but rather on their own individual merit.
Objective review will involve the submission of written critiques by subject matter experts documenting the strengths and weaknesses of responsive applications against the Review Criteria described below and interactive individual discussions between those experts and NIH program staff. The subject matter experts will include NIH staff and/or other federal staff.
Applications may be triaged for review based on the proposed catchment area of potential research participants, with applications with catchment areas characterized by >50% of the population being from rural areas, as defined by primary RUCA codes between 4-10 , receiving priority for review.
NIH will NOT provide feedback on applications, except as a part of follow-up on an as-needed basis as time permits. NIH will not accept an appeal of the objective review or funding decision outcomes.
Review Criteria:
Potential Contribution to the Coordinated Infrastructure for Supporting Research in Primary Care Settings
The approach of proposed Research Site has a high likelihood of meeting the objectives of a Network Research Hub. The applicant has described adequate plans for collaboration with other components of the coordinated infrastructure. The proposed activities involve engagement following a comprehensive framework, facilitating early and sustained engagement with a diverse group of individuals and communities, especially those that are underrepresented in research. Plans to engage the community they serve will successfully support a community-driven research agenda. Leadership plans, support from existing clinical research networks, and support from Clinical Site partners demonstrate a high commitment to the success of the proposed Network Research Hub.
Capabilities and Experience
The applicant has a demonstrated track record of successfully implementing research in primary care settings in the recent past. Prior experience showcases the ability to access and partner with underrepresented individuals in clinical research. Evidence of prior partnerships or relationships with proposed Clinical Sites has been provided. Key personnel have sufficient and relevant expertise to support the activities of the Network Research Hub.
Resources and Environment
The Network Research Hub is currently participating in and affiliated with one or more clinical research networks (NIH IDeA-CTRs, NIH CTSA, and/or PCORI PCORnet), that will increase site readiness to rapidly launch as a new site for existing NIH-funded studies. The catchment area of the Network Research Hub and its Clinical Sites will provide a high likelihood of facilitating engagement and study accrual of populations underrepresented in research (with a priority on individuals in rural communities).
Award Negotiation and Selection Information
Based on the identified strengths and weaknesses, NIH will determine whether an application will be selected for negotiation and/or award. NIH may select up to six viable applications to move forward in negotiations for a potential award, based on the objective review. Negotiations will involve identification of the initial study already funded by NIH to which the Network Research Hub will serve as an additional enrollment site. Coordination with the NIH-funded investigators and/or NIH program staff overseeing those studies will be required in order to understand the needs and existing support for the studies and to develop a final budget and milestone plan for the Network Research Hub. Final award selection will involve assessment of applications with successful negotiations and review and approval by NIH leadership and development of objective milestones as agreed upon by NIH and the applicant.
The level of funding for any award(s) made will depend on the negotiated studies and milestones and availability of funds.
Special Award Terms #Special Award Terms
The complete terms and conditions of each OT award issued under this ROA are subject to negotiation and will be contained in the Agreement entered between NIH and award recipient. This Special Award Terms section is provided for informational purposes only in order to provide prospective applicants with an understanding of key expectations and terms that may differ from traditional NIH award mechanisms. All terms and conditions of award will flow down to any partners (e.g., subrecipients, collaborators) participating in the OT award.
Lower Tier Agreements
Award recipients will be expected to issue sub-awards to entities identified in their applications and approved by NIH under this ROA. Any changes to sub-awards must be in consultation with NIH prior to adding or removing partners.
Milestone-Based Workplan
A milestone-based workplan will be requested and negotiated prior to award for inclusion in the OT Agreement. The workplan should include a description of operational milestones, completion criteria, and expected start and completion dates.
Enhancing Diversity, Equity, Inclusivity, and Accessibility in the Research Community
Award recipients will be encouraged to diversify their staff populations to facilitate engagement with diverse research partners and to enhance the participation of individuals from groups that are underrepresented in the biomedical, clinical, behavioral and social sciences, such as those defined in Notice of NIH’s Interest in Diversity ( https://grants.nih.gov/grants/guide/notice-files/NOT-OD-20-031.html ).
Program Governance The Network Research Hubs will be part of a program consisting of a coordinated infrastructure involving the following governance and components:
- establishing the vision, mission, strategic objectives, and goals of the coordinated infrastructure to be carried out by a Scientific and Medical Director (SMD) and Operations Director.
- ensuring the supported work appropriately and equitably supports and prioritizes the needs of all NIH ICs.
- monitoring and evaluating the progress of the infrastructure, its efficiency and effectiveness, and that its outputs align with the vision and mission.
- providing oversight of the studies/protocols and site and study selection,
- managing/coordinating the Central Institutional Review Board (IRB),
- providing statistical and data management support,
- collaborating with sites to develop innovations on clinical study design and implementation to minimize burden on participants and providers in primary care settings,
- facilitating sustained engagement with key partners through a community advisory board, and
- other functions as the governance and structure of the Clinical Science Center is further developed.
- conducting site feasibility assessments
- facilitating Clinical Site agreements/contracting,
- coordination of study operations (protocol development; compliance with Food and Drug Administration (FDA) and Office of Human Research Protections (OHRP) regulatory and participant protection requirements,
- quality assurance, and
- data monitoring.
OT Agreement Governance
Other Transactions (OT) are a special type of legal instrument other than contracts, grants or cooperative agreements. Generally, these awarding instruments are not subject to the Federal Acquisition Regulation (FAR ), nor to grant regulations unless otherwise noted for certain provisions in the terms and conditions of award. They are, however, subject to the OT authorities that govern the initiative and/or programs as well as applicable legislative mandates. NIH and its components, including the Office of Strategic Coordination (OSC), have been authorized by Congress to use them. They provide considerable flexibility to the government to establish policies for the awards, so the policies and terms for individual OT awards may vary between awards. Each award is therefore issued with a specific Agreement, which is negotiated with the recipient and details terms and conditions for that specific award. Program and administrative policies and the terms and conditions of individual awards are intended to supplement, rather than substitute for, governing statutory and regulatory requirements. Awards or a specified subset of awards also may be subject to additional requirements, such as those included in executive orders and appropriations acts (including the other transaction legislation cited in the Agreement), as well as all terms and conditions cited in the Agreement and its attachments, conditions on activities and expenditure of funds in other statutory or regulatory requirements, including any revisions in effect as of the beginning date of the next funding segment. The terms and conditions of the resulting OT awards are intended to be compliant with governing statutes.
For the awards funded under this ROA, NIH will engage in negotiations and all agreed upon terms and conditions will be incorporated into the Agreement. Either a bilateral agreement or a Notice of Award (NoA) will be used as the official Agreement. The signature of the Signing Official in the application certifies that the organization complies, or intends to comply, with all applicable terms and conditions, policies, certifications, and assurances referenced (and, in some cases, included) in the application instructions.
Award Administration Roles and Responsibilities
Other Transactions Agreements Officer (OTAO)
- is responsible for legally committing funds on behalf of the Federal government and that OT actions taken are in the best interest of the government
- administers, manages, and closes out awards
- oversees the management of award records
- receives and acts on requests for NIH approval; the only NIH official authorized to change the funding, duration, or other terms and conditions of award
Other Transactions Agreements Specialist (OTAS)
- serves as the first line contact for OT correspondence with applicants/recipients for administrative and financial aspects of the award
Other Transactions Program Official (OTPO)
- provides the day-to-day programmatic oversight of individual awards
- seeks guidance and advice as appropriate from subject matter experts for various disease areas and/or clinical trial oversight (e.g., medical monitoring)
- documents programmatic decisions related to an OT
- upholds government regulations on the appropriate use of federal funds
- conducts timely review of reports, inspection of deliverables, and other mechanisms to monitor and evaluate performance of the OT recipients
- serves on the OT Team, which includes developing ROAs and contributing to the development of OT award terms and conditions
- maintains certifications to serve as OTPO
- coordinates with other NIH Program Officers when partnering on other NIH-funded projects
Subject Matter Experts
- assist the OTPO in scientific and technical discussions with awardees
- review reports and discuss progress towards milestones and deliverables
- provide recommendations to the OTPO based on progress reviews
- attend face-to-face awardee meetings, as necessary
- attend site visits, as necessary
The terms and conditions of each award will address this criterion as appropriate based upon the final negotiated terms and agreed upon budget.
Human Subjects Research
All applications for work that will involve engagement in Human Subjects Research (as defined in 45 CFR § 46)( https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46 ) must provide documentation of one or more current Assurance of Compliance with federal regulations for human subject protection, including at least a Department of Health and Human Services (HHS), Office of Human Research Protection (OHRP), Federal Wide Assurance ( https://www.hhs.gov/ohrp/index.html ). All research involving Human Subjects must be reviewed and approved by an Institutional Review Board (IRB), as applicable under 45 CFR § 46 ( https://www.ecfr.gov/current/title-45/subtitleA/subchapter-A/part-46 ) and/or 21 CFR § 56 ( https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-56 ).
The entity’s Human Subjects Research protocol must include a detailed description of the research plan, study population, risks and benefits of study participation, recruitment and consent process, data collection, and data analysis. Award recipients must comply with all applicable laws, regulations, and policies for NIH-funded work. This includes, but is not limited to, laws, regulations, and policies regarding the conduct of Human Subjects research, such as the U.S. federal regulations protecting human subjects in research (e.g., 45 CFR § 46, 21 CFR § 50, § 56, § 312, § 812) and any other equivalent requirements of the applicable jurisdiction.
The informed consent document utilized in human subject research funded by NIH must comply with all applicable laws, regulations, and policies, including but not limited to U.S. federal regulations protecting human subjects in research (45 CFR§ 46 ( https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46 ) and, as applicable, 21 CFR § 50 ( https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-50 ). The protocol package submitted to the IRB must contain evidence of completion of appropriate Human Subject Research training by all investigators and key personnel who will be involved in the design or conduct of NIH funded human subject research. Funding cannot be used toward human subject research until all approvals are granted.
Intellectual Property
Specific terms with respect to intellectual property will be negotiated at the time of award; however, any negotiation will consider other laws (as relevant) that affect the government’s issue and handling of intellectual property, such as the Bayh-Dole Act (35.U.S.C. 200-212); the Trade Secrets Act (18U.S.C. 1905) the Freedom of Information Act (5 U.S.C. 552); 10 U.S.C. 130; 28 U.S.C. 1498; 35 U.S.C. 205 and 207-209; and the Lanham Act, partially codified at 15 U.S.C.1114 and 1122.
Payment
The OT award will use the Payment Management System (PMS) operated by the DHHS Program Support Center. Payments by PMS are made on a reimbursement basis unless otherwise specified in the terms of the Agreement.
Management Systems and Procedures
Recipient organizations are expected to have systems, policies, and procedures in place by which they manage funds and activities. Recipients may use their existing systems to manage OT award funds and activities as long as they are consistently applied regardless of the source of funds and across their business functions. To ensure that an organization is committed to compliance, recipient organizations are expected to have in use clearly delineated roles and responsibilities for their organization’s staff, both programmatic and administrative; written policies and procedures; training; management controls and other internal controls; performance assessment; administrative simplifications; and information sharing.
Financial Management System Standards
Recipients must have in place accounting and internal control systems that provide for appropriate monitoring of other transaction accounts to ensure that obligations and expenditures are congruent with programmatic needs and are reasonable, allocable, and allowable. A list of unallowable costs will be included in the terms and conditions of the award. In addition, the systems must be able to identify unobligated balances, accelerated expenditures, inappropriate cost transfers, and other inappropriate obligation and expenditure of funds, and recipients must notify NIH when problems are identified. A recipient’s failure to establish adequate control systems constitutes a material violation of the terms of the award.
Property Management System Standards
Recipients may use their own property management policies and procedures for property purchased, constructed, or fabricated as a direct cost using NIH OT award funds. The terms and conditions of award will address this criterion as appropriate based upon the final negotiated and agreed upon budget. Procurement System Standards and Requirements Recipients may acquire a variety of goods or services in connection with an OT award-supported project, ranging from those that are routinely purchased goods or services to those that involve substantive programmatic work. Recipients must acquire goods and services under OT awards in compliance with the organizations established policies and procedures. The terms and conditions of each award will address this criterion as appropriate based on the final negotiated and agreed upon budget.
Organizational Conflicts of Interest (OCIs)
Applicants are required to identify and disclose all facts relevant to potential OCIs involving subrecipients, consultants, etc. Under this section, the proposer is responsible for providing this disclosure with each Detailed Plan. The disclosure must include the PI/Collaborators’, and as applicable, proposed members’ OCI mitigation plan. The OCI mitigation plan must include a description of the actions the proposer has taken, or intends to take, to prevent the existence of conflicting roles that might bias the proposer’s judgment and to prevent the proposer from having an unfair competitive advantage. The government will evaluate OCI mitigation plans to avoid, neutralize, or mitigate potential OCI issues before award issuance and to determine whether it is in the government’s interest to grant a waiver.
The government will only evaluate OCI mitigation plans for proposals that are determined selectable. The government may require applicants to provide additional information to assist the government in evaluating the proposer’s OCI mitigation plan. If the government determines that a proposer failed to fully disclose an OCI or failed to reasonably provide additional information requested by the government to assist in evaluating the proposer’s OCI mitigation plan, the government may reject the Detailed Plan and withdraw it from consideration for award.
Monitoring
Recipients are responsible for managing the day-to-day operations of OT award-supported activities using their established controls and policies. However, to fulfill their role in regard to the stewardship of federal funds, the program team will monitor their OT awards to identify potential problems and areas where technical assistance might be necessary. This active monitoring is accomplished through review of reports and correspondence, audit reports, site visits and other information, which may be requested of the recipient. The names and contact information of the individuals responsible for monitoring the programmatic and business management aspects of awards will be provided to the recipient at the time of award.
Monitoring of a project or activity will continue for as long as NIH retains a financial interest in the project or activity as a result of property accountability, audit, and other requirements that may continue for a period of time after the OT award is administratively closed out and NIH is no longer providing active OT award support.
Audit
NIH OT recipients for the Program are subject to the audit requirements of OMB 2 CFR 200, Subpart F- Audit Requirements, as implemented by DHHS 45 CFR Subpart F. In general, 45 CFR 75, Subpart F-Audit Requirements requires a state government, local government, or non-profit organization (including institutions of higher education).
For-profit organizations have two options regarding the type of audit that will satisfy the audit requirements. The recipient either may have (1) a financial-related audit (as defined in, and in accordance with, the Government Auditing Standards (commonly known as the “Yellow Book”), GPO stock 020-000-00-265-4, of a particular award in accordance with Government Auditing Standards, in those cases where the recipient receives awards under only one DHHS program, or (2) an audit that meets the requirements of 45 CFR 75, Subpart F-Audit Requirements.
This page last reviewed on May 6, 2024
The Research Whisperer
Just like the thesis whisperer – but with more money, how do you start a research network.

We had a question recently from Ely asking for pragmatic advice on starting an international research network. Alyssa Sbisa and Sally Grace wrote “ Setting up a professional network ” a while back and that post has heaps of relevant good advice that I’d strongly encourage you to check out!
I’d written previously on building a research network on a shoestring , and much of that still applies. I realise now, however, that the earlier post presumed a network that needed cohering and development.
I think Ely is after something that addresses a much earlier step: how do you even get a research network started?
This post aims to tackle this, and would welcome others’ input on the topic. I’m speaking very much from my HaSS (Humanities/Social Sciences) point of view, and realise that other areas may have quite different contexts and ways of doing things. One of the things I should make clear from the start is that I’m talking about how to start a research network with few to zero resources . I’m not talking about setting something up with a ready cache of funding, or the need to access such a cache.
These are the key things you need if you want to start a research network:
OK, so you may be thinking ‘what the heck?’ with this one, but bear with me. Having a personal network (scholarly or otherwise) and forming a research network are two different things. But creating a research network requires you to have a strong personal network.
A big part of being the person, or group, that starts a research network is that the most effective first step is to reach out to your immediate circle of colleagues to spread the word for you about this shiny new endeavour. They may not be the actual people who will join the network but they will be the most likely to share your stuff around and lend it their support (and I would include scholarly or professional associations and societies in this – they may not become core participants but they may give you access to the kind of people or other organisations who will be). This kind of advocacy is based on your reputation in various circles and does not exist if you haven’t been developing good connections early and often in your career. We’ve published quite a lot about networking that (hopefully) won’t make you cringe – here’s a selection across various modes so that you can see what ‘networking’ can mean:
- Creating and growing a personal industry group (Jonathan O’Donnell)
- Networking that works (Tseen Khoo)
- The surprising benefits of a read-aloud reading group (Matilda Keynes and Nikita Vanderbyl)
- 3 reasons why you’d livetweet (Tseen Khoo)
- Starting/participating in a ‘Shut up and write’ group – Part 1 and Part 2 (compiled by Tseen Khoo)
RW features a stack of stuff on Twitter and its networking benefits so that’s well worth browsing, too, if this is going to be one of the main ways you connect with colleagues.
So, if you’re thinking of setting up a research network, fingers crossed that you have already been doing these types of things. Investing more time in it straight away would also work well if you’re starting your research network in the near future. Trying to create an instant and meaningful personal network however…that’s a tough row to hoe.
If you’re a PhD researcher, and there’s opportunity for it, I’d highly recommend starting a postgraduate group to cut your teeth in scholarly network and general group management!
Enough momentum and clarity of purpose
An essential part of any successful research network is having the momentum and clarity of purpose to establish and keep growing. What is the group for and does it already exist? Are you bringing together researchers in new and exciting ways who wouldn’t otherwise be in the same group? Is it a growing new area that could do with concentrated energy?
Do your research! Do not set up a snazzy ‘international research network’ for something that others are already doing. If there are very strong national networks that happen to have good international membership, should you be saying that yours is the ‘international network for such-and-such’? Chances are that you’d be targeting the same scholars for membership and you’ll just annoy colleagues who’ve already put in the work to establish those spaces.
- If you are truly setting up the only thing like it in the world and welcome international scholars in authentic ways, then go for it!
- If you can see a way to bring several smaller groups together to gain better profile for all and generate wonderful intellectual fizz, do that!
- If there’s a research network out there that covers what you’re wanting to do, think about participating more strongly in that one rather than starting your own. If you’re set on starting your own even though there’s something already out there that’s very similar, think about your reasons for doing so. There may be good and valid reasons, but you need to think them through because you’ll be trying to draw scholars to yours in particular ways.
Here are some ways to test the waters for whether there’s enough interest in the area to warrant a new research network – if you’re struggling to find enough people or interest, it’s good to know early rather than after you’ve tried to start a new network:
- See if you can convene a good stream on the area through an existing conference
- Hold a focused symposium and see what the response is like during the call for papers/interest
- Again, using an existing conference or association gathering, gauge interest in a ‘special interest group’ or ‘research focus cluster’ in that area and get people together to have a chat about it.
- If you have a large personal network through which you’re discerning strong threads of interest, find a way to bring those people together to talk further.
Down the track, it would be good for anyone you’d recruit to be core members of your research network to be collegial fellow-travellers in the area already. You’re going to have to work quite closely with them to create and grow this network so it’d be handy to get along! You don’t all have to have exactly the same opinions in the area but it helps to have aligned, sympathetic aims.
Basic infrastructure
To get a good foundation for building a research network’s profile, I’d suggest a combination of these basic digital presences:
- Website (essential) – recommended pages for the website: About | News | Membership | Resources | Contact >> would also recommend “Meetings” (or Talks or Conferences) page if there are regular ways that people in the network can gather.
- Facebook group or page
- Twitter account
- Social accounts and strong profiles for core network members
Skills : You need people who are willing and able to manage the social media faces of the research network – they need to be social media savvy and good content creators . If you’re running without a budget, this will be the major way people get to know and relate to your group so it is extremely important to make it a strong, engaging presence.
As democratic as you’d like your research network to be, the group must have strong decision-making skills , whether this is one ultimate decision-maker (designated leader) or a very efficient core group.
It would be ideal to have someone (or several people) on board who are good negotiators and adept at approaching potential sponsors (in academia and beyond). You can run mostly on a shoestring but you will occasionally need to make a bigger splash or invite sparkly people in. For this, you need colleagues who are either influential enough to be in there advocating for the network to get funding for its initiatives, or good at creating advocates among those who make the financial decisions.
Meetings ? I’m not a fan of meetings in general but I acknowledge the importance of research network meetings to ensure the smooth working and growth of the group. Meeting regularly and planning the next big things (whatever those things are) are essential tasks. It’s very easy for time to lapse between chance or hastily arranged meetings. It’s MUCH better to have, at least, monthly or quarterly discussions to ensure things are moving along and the research network is active and growing.
——————-
I’ve missed plenty in this post, I know. Creating a research network is a big undertaking and doing it successfully is all in the planning. It would be great to hear about your experiences or tips on this topic – comment below!
Share this:
I forgot to comment when this came out… but I wanted to say that I’ve run research centres before, started or been a member of research groups, I’ve been a researcher for a long time… but you raise things here (and map a pathway) that I intend to use as a model for the development of the new research group I’m starting. I often say this to Research Whisperer, but I’m so grateful for your knowledge and sharing around the development of research culture and thinking.
Like Liked by 1 person
Better late than never, right Sandy?! (I’ve only just seen your comment here)
Really appreciate your supportive words here and I’m curious to know how that research group you started is going, and whether this model of getting started worked for you!
The stakes for establishing a research centre that’s part of formal institutional infrastructure are usually high (esp the element of being ‘self-sustaining’), and it can be hard for emerging areas to gain that traction. A research network or group is a good way to test the viability of a particular focus, and to road-test potential colleagues as collaborators, too.
[…] I have engaged with has been wonderful. It has helped that I have had great people around me who advocate and support what I do. […]
Leave a comment Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

Innovation Anywhere, Opportunity Everywhere
Get the latest news on topics you choose, right in your inbox..
What's new

Celebrating 74 years of discovery and innovation

Statement by Director Sethuraman Panchanathan on NSF ranking in Best Places to Work in the Federal Government
Nsf planning major infrastructure overhaul to support future research in south pole.

NSF announces pilot phase and anticipated opening date of Arecibo C3 at the site of the NSF Arecibo Observatory Historic District in Puerto Rico
Our priorities.

America's economic and national security depend on our ability to invest heavily in the technologies of today while making the discoveries that are the foundation for the technologies of tomorrow.
NSF by the numbers
NSF’s Fiscal Year 2023 enacted budget
Percent of budget supporting research, education and related activities
Organizations supported by NSF across every state and U.S. territory
Researchers, entrepreneurs, students and teachers supported by NSF
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Thursday, October 3, 2019
NIH funding bolsters rare diseases research collaborations
New grants aimed at better understanding diseases, moving potential treatments closer to the clinic.
Of an estimated 6,500 to 7,000 known rare diseases, only a fraction – maybe 5% – have U.S. Food and Drug Administration-approved treatments. To increase that percentage, the National Institutes of Health has awarded approximately $31 million in grants in fiscal year 2019 to 20 teams – including five new groups -- of scientists, clinicians, patients, families and patient advocates to study a wide range of rare diseases. An additional $7 million has been awarded to a separate data coordinating center to support these research efforts.
The grants, which support consortia that together form the Rare Diseases Clinical Research Network (RDCRN) , are aimed at fostering collaborative research among scientists to better understand how rare diseases progress and to develop improved approaches for diagnosis and treatment. This is the fourth five-year funding cycle for the RDCRN, which is supported by multiple NIH Institutes and Centers and led by NIH’s National Center for Advancing Translational Sciences (NCATS) and the NCATS Office of Rare Diseases Research.
Individually, most rare diseases affect only a few hundred to several thousand people; collectively, rare diseases affect more than 25 million Americans. Many rare diseases are life-threatening and about half of those affected are children.
Because rare diseases affect a small number of people, they can be extremely challenging to study. Scientists often lack basic information about a rare disease’s symptoms and biology, and the ways a disease can affect people over time. Research funding can be scarce.
“Over the years, RDCRN scientists have partnered with patients and advocates to develop new insights into the causes and progression of – and potential therapies for – rare diseases that were simply not receiving the attention they deserved,” said NCATS Director Christopher Austin, M.D. “Their pioneering work in discerning underlying clinical differences and commonalities in hundreds of rare conditions has already changed the rare disease landscape in immeasurable ways.”
Established by Congress under the Rare Diseases Act in 2002, the RDCRN has included more than 350 sites in the United States and more than 50 in 22 other countries. To date, they have encompassed 237 research protocols and included more than 56,000 participants in studies ranging from immune system disorders and rare cancers to heart and lung disorders, brain development diseases and more.
Each RDCRN member is a consortium of clinical and scientific experts and patient groups who study a group of rare diseases. Each consortium must study three or more diseases, partner with rare disease patient advocacy groups, provide rare disease research training to investigators and perform natural history studies that chart the course and progression of diseases. The primary focus of the RDCRN is clinical research, and the network does not generally support clinical care outside of research activities.
A key component of the RDCRN is the Data Management and Coordinating Center (DMCC), which was awarded to the Cincinnati Children’s Hospital Medical Center. The DMCC manages shared resources and data from the RDCRN research studies. The DMCC emphasizes the standardization of data, increased data sharing and broad dissemination of research findings.
The RDCRN consortia have a rich history of accomplishment. For example, Lysosomal Disease Network scientists led crucial natural history studies and gene editing research that provided a foundation for first-in-human genome editing clinical studies for a rare metabolic disease. Primary Immune Deficiency Treatment Consortium members showed the advantage of early stem cell transplants for patients with a rare immune system disorder, severe combined immunodeficiency, and the group’s work contributed to advances in gene therapy-based treatments for the disease.
New groups, new emphasis
The five new consortia are:
- The Global Leukodystrophy Initiative Clinical Trials Network . Lead: Children’s Hospital of Philadelphia
- Congenital and Perinatal Infections Rare Diseases Clinical Research Consortium . Lead: University of Alabama at Birmingham
- Frontiers in Congenital Disorders of Glycosylation . Lead: Mayo Clinic, Rochester, Minnesota
- Hyperphenylalaninemia Disorders Consortium . Lead: Oregon Health and Science University, Portland
- Myasthenia Gravis Rare Disease Network . Lead: George Washington University, Washington, D.C.
According to ORDR director Anne Pariser, M.D., an important focus of the latest group of awards is on clinical trial readiness.
“Some of the RDCRN research groups have been working together for 10 or 15 years and have gathered important data and developed a good understanding of the diseases they study, in addition to new potential therapies. We’re emphasizing the need to be prepared to conduct clinical trials,” Pariser said.
“We’re trying to get the drug candidates closer to be ready for clinical testing and de-risk the processes that lead to a successful clinical trial,” said RDCRN program officer Tiina Urv, Ph.D. “To get funding to conduct trials, they need to have strong natural history studies that show how the disease progresses, ways to measure outcomes of treatments and biomarker studies that provide indicators of how a drug is working in patients.”
Collaboration is key. Consortia can involve numerous partner research teams from different sites, along with rare disease patients and advocacy groups. Scientists from different institutions come together to pool patients, data, experience and resources.
“Scientists can’t work alone. They wouldn’t have enough patients, and they wouldn’t have adequate resources and information about the diseases,” Urv said. “Patients and families help scientists decide what is important to study, test and treat.”
To read more about the five new consortia, 15 continuing consortia and the DMCC, see: https://ncats.nih.gov/rdcrn/consortia
In addition to NCATS, other NIH funding support comes from the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Neurological Disorders and Stroke, the National Heart, Lung, and Blood Institute, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Dental and Craniofacial Research, the National Institute of Mental Health and the Office of Dietary Supplements.
About the National Center for Advancing Translational Sciences (NCATS): NCATS conducts and supports research on the science and operation of translation — the process by which interventions to improve health are developed and implemented — to allow more treatments to get to more patients more quickly. For more information about how NCATS is improving health through smarter science, visit https://ncats.nih.gov .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Croat Med J
- v.55(3); 2014 Jun
Significance of research networking for enhancing collaboration and research productivity
Livia puljak.
1 University of Split School of Medicine, Department of Anatomy, Histology and Embryology, Split, Croatia
Sandor G. Vari
2 International Research and Innovation Management Program, Cedars-Sinai Medical Center, Los Angeles, CA, USA
International collaboration is growing exponentially ( 1 ) and researchers from different institutions and countries increasingly work together as consortia focused around specific research questions. Such consortia are especially valuable for international health research because they offer interdisciplinary expertise and allow recruitment of patients in different settings ( 2 ). Establishing research networks and collaborations in the form of non-governmental organizations (NGO) and non-profit, voluntary participants' groups provides the necessary flexibility to adapt to a wide spectrum of arising challenges. It enables shared learning, new research opportunities, establishing new research projects, joint applications for funds, and technology transfer ( 3 ). The collaborations increase citations of research manuscripts, especially if there is an international team of authors involved ( 4 ). Building research networks is particularly important for Central and Eastern Europe (CEE) countries, which have fragmented scientific community, small research groups, and scarce financing.
Recognizing the value of research networking, Cedars-Sinai Medical Center (CSMC) started building in 2002 a regional research organization in CEE by establishing International Research and Innovation Management program. During the first four years of the program, CSMC established partnerships with research institutions in Croatia, Czech Republic, Hungary, Romania, Slovakia, and Ukraine. CMSC provided training on research and innovation management, technology transfer infrastructure, and management capacity. In 2006, it formed the Regional Cooperation for Health, Science and Technology (RECOOP HST) Consortium with eleven CEE universities and research organizations from the following countries: Croatia, Czech Republic, Hungary, Romania, Slovakia, Ukraine, and the USA ( 5 ).
In 2012, CSMC registered with the Consortium the Association for Regional Cooperation in the Fields of Health, Science and Technology at the Court of Debrecen in Hungary. The newly established Association included members from 8 countries, adding Denmark and Poland to the consortium, and involving 14 higher education or research organizations.
The Association promotes creative thinking and helps its members to make decision whether they want to “publish and disclose” or “protect and publish.” Special attention is paid to young scientists and their education on scientific communication and technology transfer. In 2012, it carried out several integrated multidisciplinary, multicenter research studies within the joint RECOOP Life Science Research Platform and formed 18 CSMC RECOOP Research Centers (CRRC) in 7 countries (Croatia, Czech Republic, Hungary, Poland, Romania, Slovakia, and Ukraine) to facilitate the cooperation on translational and clinical research in the field of genomics-proteomics, epigenetics, metagenomics, molecular biology, metabolomics, and nano-biotechnology. Besides that, among its highest priorities is the involvement of medical and PhD students into the CRRCs’ research programs.
RECOOP Life Science Research Platform already completed a number of short term (1-2 years) pilot research studies, which are now being converted to midterm (5 years) projects. The clinical studies are planned to continue for a minimum of 20-30 years and follow up the women, men and newborns registered in the Electronic Data Entry Forms, started in 2011 ( 6 ).
The RECOOP HST Association annually organizes the Bridges in Life Sciences Conferences to review the scientific progress in the Association. At the annual meeting, the Scientific Advisory Board selects the top ten young scientists, for whom the Association covers the attendance costs of summer schools and workshops on manuscript writing and intellectual property protection.
RECOOP plays a significant role in the integration of life science research activities in member countries of Visegrad Four European Macro-Region (Czech Republic, Hungary, Poland, and Slovakia) and their neighboring countries (Belarus, Croatia, Romania, and Ukraine). The Association paves the way for GLOBAL Research Programs of the National Institutes of Health and the World Health Organization, and private funds such as Bill & Melinda Gates Foundation and Clinton Foundation ( 7 ).
In Croatia, the RECOOP HST Association includes researchers from the J. J. Strossmayer University School of Medicine in Osijek and University of Split School of Medicine. Professor Ana Marušić from the University of Split School of Medicine, who has extensive experience in teaching and studying research methodology ( 8 , 9 ), continuously organizes education programs for young researchers within the RECOOP HST Association. University of Split School of Medicine has proven its capacity for international collaboration by establishing a branch of The Cochrane Collaboration in 2008 ( 10 ) and promoting evidence-based medicine in medical education and practice ( 11 , 12 ). This, together with its continuously increasing research output ( 13 , 14 ), is making the School a desirable partner in international networking scheme.
Since CEE countries are considered a scientific periphery, there must be an even stronger impetus to increase their participation in international research consortia. Sustained engagement in training programs and joint applications to research funding can stimulate research network development ( 15 ). RECOOP HST Association is a platform that offers such research networking opportunities. Benefits of participation in such consortium can be fully appreciated in the long-term, but its positive impact is already visible, judging by the number of joint publications and research proposals that are being developed.
As part of the ongoing process of European integration ( 16 ), the RECOOP HST Association contributes to the establishment of the “European knowledge society” and increases the competitiveness of CEE. Enhancing international collaboration, possibly using RECOOP HST Association as a model, should be the goal of every researcher and research institution, because such collaboration enables capacity building and offers multiple opportunities to surpass limitations that arise within a single institution and due to scarce resources.
Cancer Screening Research Network Funding Opportunities Released
Date Posted Monday, November 21, 2022

The National Cancer Institute Division of Cancer Prevention has released three Funding Opportunity Announcements to accept applications to create the Cancer Screening Research Network (CSRN). The CSRN will conduct rigorous, multi-center cancer screening trials and studies to evaluate emerging technologies and strategies for the purpose of cancer screening. Cancer screening provides the opportunity to diagnose cancers and precancerous lesions before symptoms develop. Multi-Cancer Detection assays (MCDs) will be evaluated through this network.
*--- We encourage you to share these Funding Opportunity Announcements with others who may also be interested. ---*
The Cancer Screening Research Network will have three different components for an integrated network:
- NCI Cancer Screening Research Network: ACCrual, Enrollment, and Screening Sites (ACCESS) Hub (UG1 Clinical Trial Required) https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-23-020.html
- NCI Cancer Screening Research Network: Statistics and Data Management Center (UG1 Clinical Trial Required) https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-23-021.html
- NCI Cancer Screening Research Network: Coordinating and Communication Center (UG1 Clinical Trial Required) https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-23-022.html
Informational pre-application webinars are being scheduled and will be announced soon.
Questions can be directed to this email: [email protected]
Please check the Cancer Screening Research Network web page for updates and further announcements at: https://prevention.cancer.gov/CSRN

U.S. Government Accountability Office
Federal Research and Development: Funding Has Grown since 2012 and Is Concentrated within a Few Agencies
Innovation is critical to U.S. competitiveness, prosperity, and security. In the last 10 years, the federal government has increased funding for research and development (R&D)—investing $179.5 billion in FY 2021.
DOD and the Department of Health and Human Services received 77% of the FY 2021 funding. COVID-19 stimulus funding led to large R&D increases for HHS. For example, an HHS agency that helps develop vaccines saw increased spending from $736 million in FY 2019 to $16 billion in FY 2020.
Some funding supports multi-agency initiatives in complex areas of strategic national importance—such as nanotechnology and artificial intelligence.
Federal Research and Development Investments, FYs 2012-2021
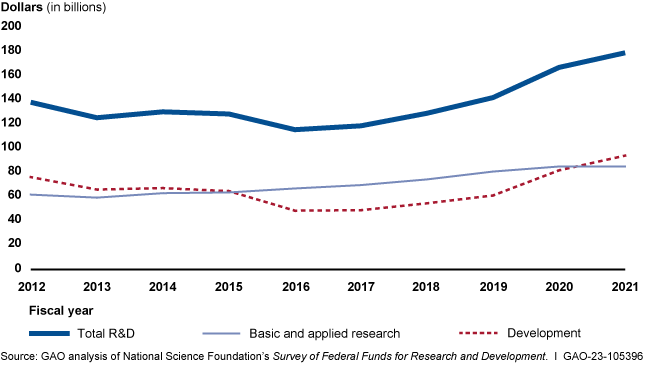
What GAO Found
Federal research and development (R&D) funding has increased since 2012—most recently because of COVID-19 stimulus funding. Five agencies obligated the majority of federal R&D funding with the Departments of Defense (DOD) and Health and Human Services (HHS) accounting for nearly 80 percent in fiscal year 2021 (see figure). HHS has mainly funded research, while DOD mainly funds development. However, HHS has become a major funder of development in recent years because of COVID-19 stimulus funding. HHS averaged less than 1 percent in development funding through fiscal year 2019 but reported 37 percent of its R&D obligations were for development in fiscal year 2021. Of the estimated $179.5 billion in federal R&D obligations in fiscal year 2021, about two-thirds went to organizations outside the federal government. In fiscal year 2021, industry, universities, and colleges received the majority of these external R&D obligations—almost $90 billion.
Federal Research and Development Obligations, Fiscal Year 2021
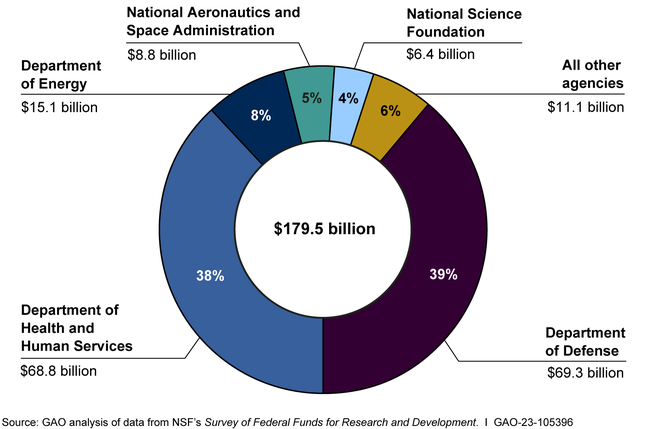
Note: FY 2021 data are estimates provided by federal agencies to the National Science Foundation.
Federal funding also includes four multi-agency initiatives in areas identified as having long-term national importance, such as quantum information science and nanotechnology. These initiatives coordinate activities in areas that are too broad or complex to be addressed by one agency alone. For example, more than 60 agencies participate in an initiative on network and information technology, which includes investments in artificial intelligence and machine learning. Not all participating agencies contribute funding to such initiatives. Funding for these initiatives increased over the previous decade, and accounted for roughly $14 billion in fiscal year 2020, just under 9 percent of the total federal R&D budget.
Why GAO Did This Study
Scientific and technological innovation are critical to long-term U.S. economic competitiveness, prosperity, and national security. The U.S. has long been a global leader in advancing the frontiers of science and technology. Increased competition from other countries has led some experts to express concern that the U.S. may be losing its competitive edge in certain technologies. Agencies are investing in various R&D initiatives, including those that are of strategic national importance, such as network and information technology, nanotechnology, quantum information science, and global environmental changes.
This report describes (1) trends in federal R&D funding over the last 10 years and (2) the funding and organization for selected multi-agency R&D initiatives, among other objectives.
To address these objectives, GAO analyzed data published by the National Science Foundation on annual R&D expenditures and examined Office of Management and Budget (OMB) data. GAO also reviewed agency documentation and collected written responses to structured questions on federal R&D from the Chief Financial Officer or budget office from the five agencies that fund most R&D.
In addition, GAO interviewed officials from OMB and the Office of Science and Technology Policy, including the Directors of the National Coordination Offices for selected multi-agency R&D initiatives, which are coordinated under the auspices of the National Science and Technology Council.
For more information, contact Candice N. Wright at (202) 512-6888 or [email protected] .
Full Report
Gao contacts.
Candice N. Wright Director [email protected] (202) 512-6888
Office of Public Affairs
Chuck Young Managing Director [email protected] (202) 512-4800

Prepare & Submit Proposals
Prepare, submit and check status of proposals
- Letters of Intent and Proposals
- Demo Site: Prepare Proposals
- Check Proposal Status
Proposal/ Panel Review
Review proposals, participate in panels
- Proposal Review
- Panelist Functions
Awards & Reporting
Submit project reports, notifications & requests, and supplemental funding requests
- Project Reports
- Demo Site: Project Reports (Training)
- Notifications & Requests
- Deposit Public Access Publication
- Award Documents
- Supplemental Funding Requests (including Career-Life Balance)
- Demo Site: Supplement Funding Requests (Training)
- Continuing Grant Increments Reports
Fellowships & Honorary Awards
Nominate colleagues, apply for awards
- Graduate Research Fellowship Program (GRFP)
- Honorary Awards
- Manage Reference Letters (Writers)
Manage Financials
View or submit cash requests, reports, and individual banking information
- Submit or manage payment transactions
- More about ACM$
- Foreign Financial Disclosure Report (FFDR)
- Program Income Reporting
- More about Individual Banking
Administration
Manage your account and user roles
- User Management
NSF Award Highlights
- Explore Scholarly publications in the NSF Public Access Repository (NSF-PAR)
- Search awards going back to 1994
Internet Explorer is no longer supported by Microsoft. To browse the NIHR site please use a modern, secure browser like Google Chrome, Mozilla Firefox, or Microsoft Edge.

Clinical Research Network

The NIHR Clinical Research Network (CRN) supports patients, the public and health and care organisations across England to participate in high-quality research, thereby advancing knowledge and improving care. The CRN supports research being delivered through 30 specialty therapy areas and 15 Local Clinical Research Networks . These provide a network of research expertise and clinical leadership to deliver research studies on the NIHR CRN Portfolio of studies . Find out more about our performance and key statistics relating to our activity.
From April 2024, the CRN will transition to a new organisation, the NIHR Research Delivery Network (RDN).
Research Delivery Network
The NIHR RDN is being established to support the delivery of high quality research that enables the best care for our population. The new organisation will build on the success of the CRN to support the country's world-class research system to deliver for the future.
The RDN will operate as one organisation across England and will play a critical and active role in implementing government policy, including:
- the Life Sciences Vision
- the Future of UK Clinical Research Delivery vision
- policy for life sciences research and development
- responding to the findings of the Lord O'Shaughnessy review
The RDN will support:
- clinical trials and other well-designed health and social care research studies (including studies that are delivered outside of an NHS setting)
- public health studies that require the recruitment of individuals within an NHS setting - this may include acute, ambulance, mental health, community or primary care
Growing the amount of commercial clinical research will be a key strategic ambition for the new network. The RDN will play a critical role in supporting the implementation of the Government's response to the Lord O'Shaughnessy report, which set out a clear blueprint for how the UK can return to its global leadership role in the support of commercial clinical trials and world-class research.
How will the RDN work in a different way to the CRN?
- The RDN will operate as one organisation across England. The RDN will have a shared vision and purpose, delivering a consistent experience for the research and healthcare communities. Innovations in one region will be shared and replicated across the country. It will be rooted in the local experience and needs of the research system and the populations it serves.
- The 12 Regional Research Delivery Networks will work as one with the Coordinating Centre and the Department of Health and Social Care to deliver a consistent, high quality and responsive service. The RDN will draw strength from its collaborative leadership model, with joint leadership composed from regional leaders, Coordinating Centre and DHSC colleagues. Regional context, expertise and relationships will enhance the quality of national decision making, leadership and coordination.
- The RDN will provide a range of services and support for research delivery, listening and addressing the needs of customers and working in collaboration with R&D teams. It will also support research sites with recruitment resources, tools to support research delivery, analysis and advice, and workforce development.
- The RDN will develop the strategic capacity and capability of the research system. The work of the RDN will build capacity for delivery partners to conduct research, with more researchers, sites, studies and participants involved in research. It will continue to bring research to under-served regions and communities with major health and care needs, considering the need for research inclusion and for the benefits of research to be spread across the whole country.
- The RDN will continuously learn and adapt to the changing domestic and global environment for health and care, life sciences and health research. It will work in collaboration with partners across UK health and care research to help drive improved efficiency across the system.
Regional Research Delivery Networks
NIHR Regional Research Delivery Networks (RRDNs) will develop excellent relationships with organisations commissioning and providing health and social care across their regions. They will help support research and promote the value of research in meeting the needs of local populations.
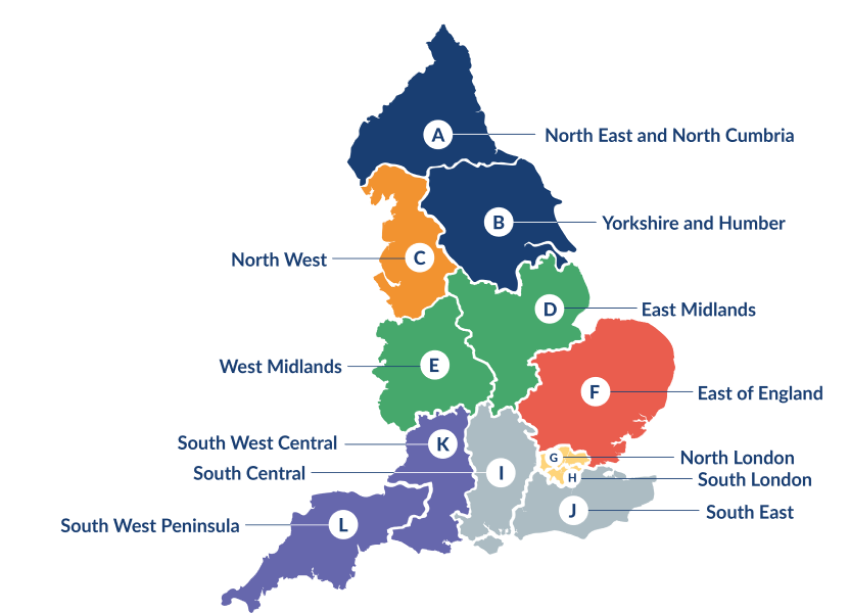
The NIHR RRDN map (pictured above) shows the geography of the 12 RRDNs that will make up the NIHR RDN from October 2024. These 12 RRDNs and their host organisations are listed below:
- North East and North Cumbria - hosted by The Newcastle upon Tyne Hospitals NHS Foundation Trust- Morag Burton, Director
- Yorkshire and Humber - hosted by Leeds Teaching Hospitals NHS Trust- Amber O'Malley, Director
- North West- hosted by Manchester University NHS Foundation Trust- Andy Ustianowski, Director
- East Midlands - hosted by University Hospitals of Leicester NHS Trust- Elizabeth Moss, Director
- West Midlands - hosted by The Royal Wolverhampton NHS Trust- Matt Brookes, Director
- East of England - hosted by Norfolk and Norwich University NHS Foundation Trust- Helen Macdonald, Director
- North London - hosted by Barts Health NHS Trust- Sharon Barrett, Director
- South London - hosted by Guy's & St Thomas' NHS Foundation Trust- James Lyddiard, Director
- South Central - hosted by University Hospital Southampton NHS Foundation Trust- Clare Rook, Director
- South East - hosted by Royal Surrey NHS Foundation Trust- Stephen Barnett, Director
- South West Central - hosted by University Hospitals Bristol and Weston NHS Foundation Trust- Kyla Thomas, Director
- South West Peninsula - hosted by Royal Devon University Healthcare NHS Foundation Trust- To be announced
The RDN Coordinating Centre will be hosted by the University of Leeds.

Funding opportunity: AHRC research networking scheme
The grant aims to stimulate new debate and exchange of ideas between researchers and stakeholders on a specified thematic area or issue, by covering the costs for workshops, seminars, networking activities or other events.
You can apply for up to £30,000 for a period of up to two years, at full economic cost.
Additional funding up to £15,000 is available to cover costs of any international activities or participants. UK costs must be accounted for within the initial £30,000 funding.
Please note the announcement outlining changes to this scheme. Notably the closure of the scheme on 29 June 2023 and the aims and objectives of this scheme will transfer to and will be incorporated within the new curiosity awards opportunity.
In 2023 UK Research and Innovation will introduce a new grants system, the Funding Service, to replace the Joint Electronic Submission (Je-S) system. This will impact how you apply to responsive mode opportunities. Find out more about the transition timeline and our pathway for change .
Who can apply
You can apply if you are a principal investigator at an eligible research organisation. This is any UK higher education institution that receives grant funding from one of the UK higher education funding bodies, or a UKRI-recognised research institute or organisation.
Proposals have to be submitted by an eligible research organisation but must involve collaboration with at least one other organisation, as well as having clear impact for beneficiaries in the UK.
Only one co-investigator can be included in the proposal, and international co-investigators are allowed.
Research assistants are not eligible under this scheme.
What we're looking for
AHRC oversees a diverse range of subject disciplines within arts and humanities, from history to literature, design to dance, linguistics to archaeology. Your application must be arts and humanities-led, with a majority of the methodologies, research questions and outputs falling within AHRC’s subject remit. We also welcome interdisciplinary research proposals, provided that arts and humanities are the main focus.
Research networking grants cover the costs of networking activities that support the exchange of ideas across boundaries – primarily between researchers in the arts and humanities, but also with colleagues in other disciplines and sectors.
Your proposal should explore new areas, include other user communities, and can include innovative approaches.
You must justify the chosen approach and explain the added value of bringing the network participants together.
Activities connected to existing networks are also included under this funding scheme, provided the activities are focusing on a new area.
The grant will cover:
- the salary costs for you and one co-investigator for the time spent overseeing and providing intellectual input to the activities
- the cost of setting up and coordinating the activities, along with related indirect and estates costs (UK researchers only).
Time spent on coordinating activities should not make up the majority of the cost in the proposal.
Proposals for the development of European collaborative networks or consortia that might support the development of applications to the EU under Horizon Europe or other EU funding opportunities are also welcome.
The grant does not cover:
- standalone events that are not part of the research process
- activities that would have taken place regardless (for instance, connected to an existing conference)
- research assistants’ salary costs
The proposed start date of your project must be at least six months from the date you send your proposal.
You can apply for an extra £15,000 (full economic cost) to cover the costs of any international participants or activities. UK costs must be accounted for within the initial £30,000 funding.
We strongly encourage you to follow the principles of The Concordat to Support the Career Development of Researchers .
How to apply
You must apply using the Joint Electronic Submission system (Je-S) .
We recommend you start your application early, you can save completed details in Je-S at any time and return to continue your application later.
When applying select ‘New document’ then:
- council: AHRC
- document type: Standard proposal
- scheme: Research networking
- call/type/mode: Research networking (open call)
Please read the research funding guide (pages 22-26) for further details and help in applying.
You can find advice on completing your application in the Je-S handbook .
If you need further help, you can contact the Je-S help desk on 01793 444164 or by email [email protected] .
Your host organisation will be able to provide advice and guidance on completing your application.
You must include the following with your application:
- case for support
- justification of resources
You must itemise all costs separately within the budget breakdown section of the application, and clearly indicate the costs of any international collaboration. In addition, all costs must be justified in the justification of resources attachment.
The case for support attachment must outline clearly the rationale for the activities, approach and the research context by answering the following questions:
- what is the central theme of the proposed activity?
- why is it important that this theme is explored?
- what is innovative about the network?
- how will the questions be addressed?
- how will the proposed activities create genuine interaction across boundaries and advance understanding?
You should also give details of the aims and objectives, the timetable for any proposed activities, proposed participants and key speakers, and plans for management and coordination, including the membership of any proposed advisory group or steering committee.
How we will assess your application
We will assess your application based on:
- the quality of the research process, including research agenda, participants, sustainability and whether research methods foster interactions
- the level of genuine collaboration that’s proposed across boundaries, and the value that this will add to the development of research in that area
- the significance and importance of the proposed thematic area
- how much the proposed activities will create genuine and productive interaction across boundaries (for example, disciplinary, conceptual, theoretical or methodological), including the potential to advance knowledge and understanding, or lead to new cross-disciplinary research projects
- the level of involvement from different organisations and interaction between participants – we welcome creative techniques for fostering interactions
For proposals requesting additional funds for international collaboration we will also consider:
- how much the proposed activities will build strong academic links between the UK and researchers in other countries, and the value that this adds to the research area
Proposals will be sent to a peer review panel for assessment. The principal investigator will not be offered a response to comments. The proposal and peer reviews will be moderated by a third member of the peer review college, who will consider the reviewers’ comments and allocate a final grade, ranking applications by priority for funding.
Final funding decisions will be made by AHRC.
We aim to inform you of the outcome of your proposal within five months from the date of submission.
Additional info
Research funding guide
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services .
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Extramural Programs
- Funding Opportunities
- Performance Data
- Research and Training Highlights
- News and Notices
Research Grant Funding
- NIOSH funds four types of research grants (R01, R03, R21, and K01).
- Applications are accepted three times per year.
- Researchers are encouraged to develop proposed research around NIOSH's priority goals.

NIOSH funds many types of research. This includes:
- Identification and investigation of the relationships between hazardous working conditions and associated occupational diseases and injuries;
- Development of more sensitive means of evaluating hazards at work sites;
- Development of methods for measuring early markers of adverse health effects and injuries;
- Development of new protective equipment and engineering control technology to reduce work-related illnesses and injuries;
- Development of work practices that reduce the risks of occupational hazards; and
- Evaluation of the technical feasibility or application of a new or improved occupational safety and health procedure, method, technique, or system.
Funding by category
Large osh research grants (r01).
The purpose of RO1 research grants is to
- Develop an understanding of the risks and conditions that are associated with occupational diseases and injuries.
- Explore methods for reducing risks and for preventing or minimizing exposure to hazardous conditions in the workplace.
- Translate significant scientific findings into prevention practices and products that will effectively reduce work-related illnesses and injuries.
Small Research Grants (R03)
R03 grants support small research projects that can be carried out in a short period of time with limited resources. Potential projects include:
- Pilot and feasibility studies
- Secondary analysis of existing data
- Small, self-contained research projects
- Development of research methodology; and
- Development of new research technology
Exploratory Grant Program (R21)
R21 grants are intended to encourage new exploratory and developmental research projects. These studies may involve considerable risk but may lead to a breakthrough in a particular area. They may also lead to the development of novel techniques, agents, methodologies, models, or applications. These innovations could have a major impact on a field of biomedical, behavioral, or clinical research.
Applications for R21 awards should describe projects distinct from those supported through the traditional R01 mechanism. Projects of limited cost or scope that use widely accepted approaches and methods within well-established fields are better suited for the R03 small grant mechanism.
Mentored Research Scientist Career Development (K01)
KO1 grants are intended to prepare the next generation of occupational safety and health researchers and educators. Emphasis for funding is placed on projects that specifically address the priority goals of NIOSH Strategic Plan . Research training supported by this announcement may include a wide range of training modalities reflecting the diverse approaches needed to effectively address occupational safety and health problems effectively.

Ongoing funding opportunities
The announcements listed below are published in the NIH Guide to Grants and Contracts. Each announcement specifies the research priorities, type of grant activity supported, and information needed to submit a grant application. Applications are accepted three times a year on standard due dates .
Occupational Safety and Health Research (RO1)
NIOSH Small Research Grant Program (R03)
NIOSH Exploratory and/or Developmental Grant Program (R21)
Mentored Research Scientist Development Award (K01)
Previous funding opportunities
These funding announcements have expired and been reissued. The links are included here as a reference.

Previous announcements
Occupational Safety and Health Research (RO1) - Active 2013-2018
NIOSH Small Research Grant Program (R03) - Active 2012-2018
NIOSH Exploratory and/or Developmental Grant Program (R21) - Active 2012-2018
Mentored Research Scientist Development Award (K01) - Active 2012-2018
NIOSH has identified research priorities in its strategic plan . NIOSH Sector and Cross-Sector programs have identified priority goals for extramural research to fill important research gaps. These are issues that NIOSH intramural (i.e., internal) projects are not addressing. Researchers should consider developing their research concept around one or more priority goals. Researchers are also encouraged to identify priority goals for extramural research as a part of their application.
Resource
Funding data.
Learn more about:
- Success rates for research grants
- Current funding levels for research grants and other awards
- Repository of all active awards
National Institute for Occupational Safety and Health (NIOSH)
The Occupational Safety and Health Act of 1970 established NIOSH as a research agency focused on the study of worker safety and health, and empowering employers and workers to create safe and healthy workplaces.
NIOSH Extramural Research and Training
Part 1. Overview Information
National Institutes of Health ( NIH )
National Heart, Lung, and Blood Institute ( NHLBI )
National Human Genome Research Institute ( NHGRI )
National Institute on Aging ( NIA )
National Institute of Allergy and Infectious Diseases ( NIAID )
National Institute of Arthritis and Musculoskeletal and Skin Diseases ( NIAMS )
Eunice Kennedy Shriver National Institute of Child Health and Human Development ( NICHD )
National Institute on Deafness and Other Communication Disorders ( NIDCD )
National Institute of Dental and Craniofacial Research ( NIDCR )
National Institute of Diabetes and Digestive and Kidney Diseases ( NIDDK )
National Institute of Neurological Disorders and Stroke ( NINDS )
U54 Specialized Center- Cooperative Agreements
- May 1, 2024 - Notice of Information: Technical Assistance Webinars for PAR-24-206: Rare Diseases Clinical Research Consortia (RDCRC) for the Rare Diseases Clinical Research Network (RDCRN) (U54 Clinical Trial Optional). See Notice PAR-24-206
- May 3, 2023 - Notice of Intent to Publish a Notice of Funding Opportunity (NOFO) for the Rare Diseases Clinical Research Consortia (RDCRC) for the Rare Diseases Clinical Research Network (RDCRN). See Notice NOT-TR-23-015 .
- August 31, 2022 - Implementation Changes for Genomic Data Sharing Plans Included with Applications Due on or after January 25, 2023. See Notice NOT-OD-22-198 .
- August 5, 2022 - Implementation Details for the NIH Data Management and Sharing Policy. See Notice NOT-OD-22-189 .
- Mar 2, 2022 - Notice of Intent to Publish a Funding Opportunity Announcement for Data Management and Coordinating Center (DMCC) for Rare Diseases Clinical Research Network (RDCRN) (U2C Clinical Trial Not Allowed). See Notice NOT-TR-22-016 .
- Feb 25, 2022 - RESCINDED - Notice of Intent to Publish a Funding Opportunity Announcement for the Rare Diseases Clinical Research Consortia (RDCRC) for the Rare Diseases Clinical Research Network (RDCRN). See Notice NOT-TR-22-017 .
See Section III. 3. Additional Information on Eligibility .
The objective of this Notice of Funding Opportunity (NOFO) is to invite new and renewal applications for the Rare Diseases Clinical Research Consortia (RDCRC) that comprise the Rare Diseases Clinical Research Network (RDCRN). The RDCRCs are intended to advance and improve diagnosis, management, and treatment of numerous, diverse rare diseases through highly collaborative, multi-site, patient-centric, translational, and clinical research. Special emphasis will be placed on the early and timely identification of individuals with rare diseases and clinical trial readiness.
30 days prior to the application due date
All applications are due by 5:00 PM local time of applicant organization.
Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date.
Not Applicable
It is critical that applicants follow the Multi-Project (M) Instructions in the How to Apply - Application Guide , except where instructed to do otherwise (in this NOFO or in a Notice from the NIH Guide for Grants and Contracts ). Conformance to all requirements (both in the How to Apply - Application Guide and the NOFO) is required and strictly enforced. Applicants must read and follow all application instructions in the How to Apply - Application Guide as well as any program-specific instructions noted in Section IV. When the program-specific instructions deviate from those in the How to Apply - Application Guide , follow the program-specific instructions. Applications that do not comply with these instructions may be delayed or not accepted for review.
There are several options available to submit your application through Grants.gov to NIH and Department of Health and Human Services partners. You must use one of these submission options to access the application forms for this opportunity.
- Use the NIH ASSIST system to prepare, submit and track your application online.
- Use an institutional system-to-system (S2S) solution to prepare and submit your application to Grants.gov and eRA Commons to track your application. Check with your institutional officials regarding availability.
Part 2. Full Text of Announcement
Section i. notice of funding opportunity description.
The Division of Rare Diseases Research Innovation (DRDRI), within the National Center for Advancing Translational Sciences (NCATS), along with the Institutes, Centers, and Offices (ICOs) listed in Part I at the National Institutes of Health (NIH), invites applications in response to this Notice of Funding Opportunity (NOFO) for the Rare Diseases Clinical Research Consortia (RDCRC) component of the Rare Diseases Research Network (RDCRN). The RDCRCs were established with the intent of advancing the diagnosis, management, and treatment of rare diseases. Each RDCRC will promote highly collaborative, multi-site, and patient-centric translational and clinical research. The lived perspective of patients, and their needs and priorities, must be meaningfully incorporated into decisions and activities of the proposed RDCRC, with related patient advocacy groups having an active partnership role within the RDCRC. Each RDCRC must address specific unmet clinical research needs that will move the field of research forward from its current state of knowledge. This includes activities such as earlier diagnosis of individuals with targeted rare diseases and facilitating more rapid development of new treatments through a program in clinical trial readiness.
The previous iteration of the RDCRC ( RFA-TR-18-020 ) indicated that it is the intent of the NIH to support individual RDCRCs for no more than three award cycles; that is, RDCRCs that have successfully competed for RDCRC funding with the same focus three times. New applications should not replicate the research project or aims of the previously funded RDCRC. The NIH will continue to support RDCRCs funded under previous, current, or future funding opportunities for no more than three award cycles. All new projects must address the specific interests of one or more NIH ICO. Prospective applicants are strongly encouraged to consult with the Scientific/Research Contacts of the NIH early in the preparation of the application (see Section VII. Agency Contacts).
The Rare Diseases Act of 2002 (Public Law 107-280 ( https://www.gpo.gov/fdsys/pkg/PLAW-107publ280/content-detail.html )) defines a rare disease as a condition affecting fewer than 200,000 individuals in the United States. Collectively there are estimated to be over 10,000 diseases or conditions that fall into this category; cumulatively, there are approximately 25 – 30 million people in the United States who are affected by rare diseases or conditions. Many of these disorders lead to significant morbidity and mortality. These facts highlight that rare diseases are a significant public health concern.
Despite advances in our understanding of the causes and mechanisms of many diseases, effective treatments are available for fewer than 5% of rare diseases. Although advances in technologies such as gene-based therapies have recently led to promising and potentially transformative treatments, these are currently limited to a few rare diseases.
Bringing effective treatments to more people living with rare diseases is a risk-filled venture for numerous reasons. First, making a diagnosis can be challenging with many patients experiencing a "diagnostic odyssey" of years because of limited knowledge of the range of disease manifestations and of genotype-phenotype correlations. Second, many rare diseases have a spectrum of phenotypes, and there are often no high-quality natural history datasets documenting how the disease affects patients' functioning and how it progresses over time. Third, the relatively small number of patients and clinicians caring for them leads to challenges in the design and implementation of clinical trials. Fourth, there are often no sensitive and specific outcome measures or biomarkers that are appropriate for use in clinical trials or that are clinically relevant to patient needs. Fifth, the data quality and the rigor of scientific studies are often lacking and usage of data standards and adherence to FAIR principles for data management vary across rare disease research making collaborations difficult. Finally, the resources available for therapeutics development are limited, making it critical to find frameworks for leveraging partnerships among patient groups, industry, academic investigators, and federal funding agencies. The global burden associated with rare diseases necessitates international coordination and collaboration.
To facilitate progress in addressing these challenges, the Rare Diseases Act of 2002 directed the DRDRI to support regional "RDCRCs of Excellence" for clinical research, career enhancement, and demonstration of diagnostic, prevention, control, and treatment methods for rare diseases. Since the inception of this legislation, numerous NIH ICOs (NCATS, NCRR, OD, NICHD, NINDS, NIAMS, NHLBI, NIDDK, NIDCR, NIMH, NCI, NIAID and NEI), led by the DRDRI at NCATS, have partnered to support the RDCRN. The RDCRN was established to be a collaborative and coordinated network of investigators and patient groups committed to the investigation of rare diseases working in partnership with leaders in technology to enhance communication and sharing of resources through a multidisciplinary approach.
To date, this program has successfully supported 33 individual RDCRCs that have conducted research on nearly 250 individual disorders. The research conducted within the RDCRN has contributed to the Food and Drug Administration (FDA) approval for eleven treatments for rare diseases.
Organization of the RDCRN
The RDCRN is a cooperative network composed of multiple Consortia (RDCRCs) and a Data Management and Coordinating Center (DMCC) established to coordinate RDCRN activities and to support and facilitate clinical research in rare diseases conducted by the RDCRCs. The RDCRN functions as a collaborative network with each RDCRC establishing and maintaining an environment that fosters collaborative, patient-oriented, multi-site, multi-disciplinary research collaborations and career enhancement.
The activities of the RDCRN are coordinated, facilitated, and supported by the DMCC. Patient advocacy group partners specific to the RDCRC participate in individual RDCRC activities as well as participating in Coalition of Patient Advocacy Group (CPAG) activities. The RDCRN governance consists of three committees: the Network Steering Committee (SC), the CPAG SC and the Joint Leadership Team (JLT). These committees are described in detail in the "Governance" section. The committees consist of the PIs of each funded RDCRC, their affiliated patient advocacy groups, the DMCC, and NIH program representatives including the NCATS RDCRN Program Director, Program Officials (PO) and Project Scientists (PS).
All recipients of an RDCRC and their teams are members of the RDCRN, and as such are part of a national rare diseases research resource. Each RDCRC will be expected to actively participate in network activities, including meetings of the Steering committee, biennial RDCRC meetings, and various relevant workgroups (e.g., bioinformatics, engagement, dissemination of information).
Each RDCRC must be willing to work towards being a member of a network that supports common data standards, FAIR data practices across sites; encourage the use of common data elements (CDEs); commit to community engagement; and agree to share data and other resources to the network, the broader scientific research community, and the general public.
RDCRC Elements:
Each RDCRC must focus on rare diseases. In this document, a rare disease is defined as one that affects fewer than 200,000 people in the United States (per the Rare Diseases Act 2002).
Each RDCRC application must indicate at least three different rare diseases that may share, but are not limited to, common pathways/mechanisms of action/organ system, and may be defined as:
- Conditions - a particular state of being that limits/restricts something else.
- Disorders - abnormal physical or mental conditions or ailments.
- Syndromes - a group of symptoms that occur together, or a condition characterized by a set of associated symptoms.
- Diseases - a disorder of structure or function that affects a specific location and is not simply a result of physical injury.
Each RDCRC is required to have one natural history or longitudinal study. Research studies must all be conducted at multiple sites; however, pilot studies may be conducted at a single site.
Applicants are encouraged to emphasize new ideas, novel approaches, and state-of-the-art technologies to address the needs for early diagnosis and effective treatments along with other strategies to improve the lives of individuals with rare diseases.
Applicants with research agendas at varying stages of scientific development within the research program are encouraged to apply. This includes groups that would be considered early-stage RDCRC with many knowledge gaps that need to be addressed (e.g., groups that do not yet have established registries, groups with poorly defined natural history data that would benefit from an RDCRC effort).
Each RDCRC must form partnerships with patient advocacy groups. These groups must participate as active members of the RDCRC with meaningful roles within the RDCRC and as members of the RDCRN CPAG.
DMCC Structure
The platform for the DMCC resides within the NCATS Information Technology Resources Branch (ITRB) provided cloud resource. All software licenses belong to NCATS. This is an NCATS-furnished infrastructure that the award recipient will use as a platform on which to build the data management and coordination center. This protects the integrity of the data and allows the RDCRN DMCC system to stay in the same location regardless of future DMCC award recipients.
Overall, the goal of the DMCC is to provide support to each individual RDCRC, and to coordinate and support the activities of the RDCRN as a whole. The structural requirements of the DMCC are:
- Administrative Core to facilitate network operation, governance, and communication.
- Data Management Core to build and maintain a robust, secure data infrastructure.
- Clinical Research Core to provide guidance in developing best practices in clinical research, protocol development and good data practices using the FAIR principles.
- Engagement and Dissemination Core to promote patient engagement and broad research dissemination.
It is the responsibility of the investigative teams of the DMCC cores (Administrative Core, Data Management Core, Clinical Research Core, and Engagement and Dissemination Core) to work collaboratively with each RDCRC to coordinate and support both RDCRC activities and RDCRN-wide efforts (e.g., develop and monitor best practices for clinical and research data handling and use as well as data sharing).
The RDCRCs will collaborate with the DMCC to establish and promulgate coordinated RDCRN data management and data sharing standards and policies within the RDCRC.
It is imperative that RDCRC applicants carefully review the DMCC NOFO to fully understand the resources and services that will be provided to the network participants by the DMCC. Applicants should ensure planned activities involve coordination with the DMCC and do not replicate efforts.
Coalition of Patient Advocacy Groups (CPAG)
The RDCRN CPAG was established to promote collaboration between rare disease patient and stakeholder organizations and the RDCRN to facilitate better access to, and earlier benefit from, research conducted on rare diseases. Each RDCRC must be actively engaged with the CPAG.
Any RDCRC patient advocacy group members may participate in RDCRN activities. One patient advocacy group member from each RDCRC will be identified by the RDCRC to represent the consortia on the CPAG steering committee. As the patient advocacy arm of the RDCRN, CPAG members will use their position to advance the cause of rare disease research and improved patient outcomes through the network.
RDCRC Structure
The structural requirements of an RDCRC under this NOFO are:
Administrative Core
Pilot/Feasibility Governance Core
- Career Enhancement Core
- 2-4 Clinical Research Projects
Personnel An individual with demonstrated experience in the management of multisite clinical research programs, along with rare disease research should be identified as PI for the Administrative Core.
Administrative Coordinator - with appropriate skills and experience to assist the RDCRC Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) and Administrative Core PI with the day-to-day administrative details and program coordination.
Each Administrative Core should have identified statistical and bioinformatics team members to work collaboratively with the DMCC to coordinate and leverage DMCC core support and meet the needs of the RDCRC that extend beyond the scope of the DMCC:
- Biostatistician - Individuals with demonstrated strong statistical experience working with rare diseases research as well as clinical trial design and implementation.
- Bioinformatics - Individuals with demonstrated skills in managing RDCRN established data standards, FAIR data principles, and biological data, especially when the data sets are small and complex.
The Administrative Core of each RDCRC will be responsible for the following activities:
- Administrative Support for RDCRC
- Management of RDCRC Agreements and Regulatory and Clinical Documentation
Coordination of Activities with DMCC
- Management and Sharing of Data and Biospecimens
- Collaborating with the DMCC in the Engagement and Dissemination of Information
- Coordination of Patient Advocacy Groups Participation
Administrative Support for RDCRC The Administrative Core is responsible for the overall management of day-to-day program activities for the RDCRC.
Clear policies and procedures should be established for the RDCRC that dovetail with those of the RDCRN. The core is responsible for funds allocation and establishing contracts with all participating RDCRC sites. The Administrative Core will facilitate the process of monitoring the progress of both the RDCRC and RDCRN program milestones that will be negotiated at the time of award.
All RDCRC-related meetings and calls will be managed by the Administrative Core along with coordination of the activities of the external advisory group. The Administrative Core is responsible for coordinating patient advocacy group involvement across RDCRC activities. The Administrative Core will also serve as the primary touchpoint for coordinating collaborations with the DMCC.
Management of RDCRC Agreements and Regulatory and Clinical Documentation The Administrative Core will coordinate and manage RDCRC agreements that regulate RDCRC activities. The Administrative Core will coordinate and manage all regulatory and clinical documents in collaboration with the NIH and the DMCC (e.g., Investigational New Drug Application (IND), Institutional Review Board (IRB), clinical protocols, consent forms). The Administrative Core will be responsible for coordinating the required single IRB.
The Administrative Core, in consultation with the DMCC, will establish data sharing and data use agreements within the RDCRC, for all clinical sites, and between the primary recipient institution and the DMCC.
The RDCRC must not replicate services provided by the DMCC. The Administrative Core will work collaboratively with the DMCC Cores (Administrative Core, Data Management Core, Clinical Research Core, and Engagement and Dissemination Core) to coordinate and support both RDCRC activities and RDCRN-wide efforts (e.g., develop and monitor best practices for clinical and research data handling and use as well as data sharing). This includes the development and utilization of clinical trial readiness metrics for both regulatory requirements (e.g., protocols, IRBs) and clinical research components (e.g., natural history studies outcome measures, biomarkers) to evaluate their progress in clinical trial readiness.
They will also collaborate with the DMCC to establish and promulgate coordinated RDCRN data management and data sharing standards and policies within the RDCRC. A plan should be developed to ensure effective use of the DMCC’s shared resources.
It is important that the RDCRC is prepared to identify a point of contact for communications with DMCC and share the following information with the DMCC:
- Key contacts for individual research studies
- Patient advocacy group contacts
- Protocols and schedule of events
- List of diseases/disorders studied
- Documents and data use limitations
- List of data elements and data modalities
- List of sites and personnel
Management and Sharing of Data and Biospecimens The Administrative Core will coordinate data sharing and biospecimen sharing, storage and tracking information across all clinical sites within the RDCRC and externally with appropriate agreements. The DMCC will provide resources for tracking specimens and, via a “virtual repository,” indexing biospecimens.
Engagement and Dissemination of Information The dissemination of information is an important component of the RDCRN. Outreach beyond academic activities is expected; this may include, but is not limited to, dissemination through social media, information and education for the patient community, and collaboration with the patient advocacy group(s) and the DMCC. The RDCRC will collaborate with the DMCC to create and manage a RDCRC-specific website. The RDCRC will provide information including:
- Diseases studied, disease definitions, publications, clinical sites, studies, resources, funding opportunities, etc.
- Design (determine logo, colors, photos, site layout, etc.)
- Review (attend regular meetings and reviews with team to build and finalize site)
- Collaborate with DMCC to promote new RDCRC via RDCRN blog, podcast, newsletter, social media.
- Highlight new publications
- Highlight ongoing studies
Coordination of Patient Advocacy Group Participation The Administrative Core will establish communication and maintain engagement with all partnering patient advocacy groups.
A Pilot/Feasibility Governance Core should be established to enable future innovative single- or multi-site pilot studies aimed at advancing the diagnosis, clinical trial readiness, management, and/or treatment of rare diseases. Pilot projects that extend RDCRC research collaborations beyond the RDCRN are allowed. Pilot projects may be awarded to institutions that are not already RDCRC members; however, execution of new subawards may be required.
The core should define the process by which pilot studies will be encouraged, assessed, and managed.
Specific pilot/feasibility projects must not be described in the current application and if described will not be considered in the review of the application. However, a plan for how pilots will be selected and how results will be disseminated must be included. Pilot projects may be clinical trials only with prior NIH institute approval, and policies may vary by institute.
The selection and initiation of future pilot/feasibility projects are contingent upon approval by the administering NIH institute and are subject to NIH clinical research regulations; see Prior NIH Approval of Human Subjects Research in Active Awards Initially Submitted without Definitive Plans for Human Subjects Involvement (Delayed Onset Awards): See https://grants.nih.gov/policy/nihgps/index.htm .
All pilot/feasibility projects must be documented within the DMCC’s network-tracking system once an award is made.
Career Enhancement Core The RDCRCs are expected to play a leadership role in career enhancement; attract new researchers for the rare diseases field; foster diversity, equitability, inclusivity, and accessibility in the rare diseases research community; and contribute to the development of future research leaders. Each RDCRC should provide a Career Enhancement Program to provide support for career enhancement-related activities, as well as support for development of the institution's environment for the education of diverse Career Enhancement candidates in rare diseases research. Career Enhancement plans aimed at fostering diverse scholars and investigators from populations traditionally underrepresented in research careers, such as race, ethnicity, geography (e.g., rural, urban), gender, and socioeconomic status, are especially welcome ( https://grants.nih.gov/grants/guide/notice-files/NOT-OD-22-019.html ), as are Career Enhancement Programs at minority serving institutions. Examples of Career Enhancement candidates include, but are not limited to, the following:
- Doctoral/Medical students
- Postdoctoral fellow/researchers
- Clinical fellows
- Early-stage investigators
- Investigators new to the rare disease field
Leveraging existing career enhancement programs and exploring fellowship opportunities from other public and private funding organizations is encouraged. This program may propose activities that expand the career enhancement environment through, for example, specialized coursework, a seminar program, retreats for presentation of Career Enhancement candidates’ research, mentoring programs, journal clubs, professional development programs, or other activities that contribute to the preparation of scholars and junior investigators for careers in rare diseases research. Exposure to research at other RDCRCs is also encouraged through exchange programs or visits to learn new research approaches. The leads of the Career Enhancement program should collaborate with the DMCC to develop disease agnostic, rare disease specific activities that would benefit participants of the Career Enhancement program. Results of projects supported by the core should be shared with the RDCRN.
The RDCRC must coordinate with the DMCC to track Career Enhancement recipients and activities within the RDCRN.
Clinical Research Projects The RDCRCs are intended to advance the diagnosis, management, and treatment of rare diseases with a focus on clinical trial readiness and early and timely diagnosis. A minimum of two but no more than four multi-site clinical research projects are required. One of the projects must be longitudinal in nature with the intent of understanding the clinical course of the disease and helping inform future clinical trials (e.g., natural history studies). Clinical research supported under this NOFO includes mechanistic studies designed to understand a biological or behavioral process, the pathophysiology of a disease, or the mechanism of action of an intervention. Epidemiological, behavioral, and health outcomes research studies in rare diseases are also encouraged. Projects should address any relevant ethical, legal or social implications as needed.
Each of the proposed clinical research projects should address problems that require a substantial collaborative research effort as well as a multi-site RDCRC environment to solve and can benefit from NIH programmatic input. The research projects should be more substantial than what can be accomplished in a single site project. Success of each project should move the field closer to the design and/or implementation of pivotal clinical trials or earlier diagnosis.
Collectively, the projects should involve collaborative teams with experience in rare diseases, the RDCRC’s specific disease(s)/disorder(s)/syndrome(s)/condition(s), clinical research, biostatistics, and other requisite complementary expertise. Collaborations should ensure that input from all patients and partners (parents, caregivers, support, and advocacy groups) is sought at all stages in the clinical research process.
Any clinical trials proposed as part of a RDCRC must meet ICO-specific rules for clinical trials. Consultation with relevant ICO contact prior to proposing a clinical trial is highly encouraged . Such clinical trials should be designed to provide specific data that will be necessary to design a subsequent definitive efficacy trial. The proposed clinical trial must address questions that, when answered, will optimize the design of a subsequent definitive clinical trial rather than simply address the clinical question with lower power.
Small targeted clinical trials may be proposed if the project meets the criteria listed in the "Clinical Research - definitions and requirements" section. Knowledge from such clinical studies should be essential to direct subsequent clinical trials and can be invaluable for the targeted rare diseases. See Section IV.2 for additional guidance on projects that propose a clinical trial. Although clinical trials can be supported through an RDCRC, clinical trials are NOT a required component of an RDCRC application.
Examples of research areas that propose to advance rare disease research include but are not limited to:
- Characterization of the rare disease cohort being studied, including demographic, genetic, phenotypic, imaging and laboratory data to identify disease subtypes, i.e., differentiate patients with similar morphological phenotypes but different genetic mutations and severity of outcomes.
- Clinical studies aimed at clinical trial preparation, including natural history studies.
- Development of algorithms connecting clinical and patient-reported outcomes with laboratory, imaging, environmental and -omics data to aid decision-making for clinical management of the rare diseases being studied.
- Early-stage clinical trials.
- Elucidation of genotype-phenotype interactions and multisystem phenotyping to develop reliable and valid predictive tools to determine who will respond to which treatments and when to intervene.
- Partnering with clinicians and patient communities to understand barriers to and facilitators of implementation of known treatments and interventions for rare diseases.
- Study of implementation strategies to increase adoption, feasibility, and sustainability of known effective evidence-based interventions for rare diseases.
- Strategies to understand how to disseminate evidence-based assessments or diagnostics and clinical guidelines to relevant rare disease populations.
- Encouragement of innovative methods such as use of telemedicine and community-engagement approaches to improve inclusion of participants with rare diseases located in remote locations or facing other barriers to participating in research.
- Evaluation of the contribution of lifestyle factors to racial/ethnic and other demographic differences in rare-disease risk, severity, and treatment response.
- Generation of data (laboratory, clinical and imaging data) to improve diagnosis and to distinguish subtypes of the rare disease being studied.
- Identification of molecular pathways that can lead to therapeutic targets.
- Natural history of progression of rare disease being studied and clinical outcomes.
- New treatment modalities.
- Prevention studies.
- Repurposing of drugs.
- Studies that will address evidence gaps that are required for a successful Recommended Uniform Screening Panel nomination for newborn screening.
- Utilization of data science and other novel analytic techniques to study rare-disease risk, severity, and treatment response among specific populations (e.g., pregnant women, obese or low-weight patients, elderly patients).
- Validation of biomarkers that meet FDA requirements as a drug development tool for use in future Phase III Randomized Clinical Trials.
Clinical Research - Definitions and requirements
Clinical Trial Readiness For this NOFO, clinical trial readiness is the state of having validated clinical research tools and sufficient knowledge of disease natural history to design efficient clinical trials. Validated clinical research tools can include biomarkers or clinical outcome assessment measures that are fit-for-purpose within a defined context of use relevant to the clinical trials. Knowledge of disease natural history necessary for clinical trial design can include, but is not limited to, characteristics for stratification or determining inclusion and exclusion criteria, the stage of disease progression that may be responsive to treatment, and data needed for determining sample size through power calculations.
Clinical Research Clinical research, as it is defined by the NIH ( https://grants.nih.gov/grants/glossary.htm#ClinicalResearch ) is research with human subjects that is either:
- Patient-oriented research. Research conducted with human subjects (or on material of human origin such as tissues, specimens, and cognitive phenomena) for which an investigator (or colleague) directly interacts with human subjects. Excluded from this definition are in vitro studies that utilize human tissues that cannot be linked to a living individual. This definition includes (a) mechanisms of human disease, (b) therapeutic interventions, (c) clinical trials, or (d) development of new technologies.
- Epidemiological and behavioral studies.
- Outcomes research and health services research.
In vitro studies with human biospecimens must be within the context of NIH-defined Clinical Research and relevant to clinical endpoints.
Studies based entirely on publicly available or deidentified data or specimens (thus falling under 45 CFR 46.101(b) ( https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html ), Exemption 4) are not considered clinical research by this definition. However, these types of studies can be included in pilot studies or as a part of larger clinical research projects.
Clinical Trials A clinical trial, as defined by the NIH, is a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. NIH provides a decision tool to determine whether a study meets the NIH definition of a clinical trial ( https://grants.nih.gov/ct-decision/index.htm ).
If a clinical trial is included in the application, it must demonstrate potential value of the trial and the feasibility of successfully completing the trial within the duration of the award, including the preclinical rationale, if applicable. The preclinical rationale should provide evidence that the rigor of preclinical efficacy studies and the level of effect of the agent are both sufficient to warrant clinical testing of the agent (for guidance, see https://grants.nih.gov/policy/reproducibility/index.htm ).
Clinical trials involving the testing of new investigational therapeutics, new indications for FDA-approved drugs, or other medical interventions under a research protocol should be performed under an IND or Investigational Device Exemption (IDE), unless otherwise agreed upon by the FDA. Unless the investigational product or device is exempted from the FDA IND/IDE requirements, the applicant must provide the NIH with the name and organization of the IND/IDE holder, the date the IND/IDE was filed with the FDA, the FDA IND/IDE number, and any comments from the FDA regarding this protocol. Studies will not be funded unless necessary regulatory approval has first been obtained; regulatory approval at the time of application is preferred.
Examples of relevant clinical trials include, but are not limited to, the following:
- Studies to optimize the intervention strategy: for example, studies designed to investigate dose-concentration, dose-response, or concentration-response relationships that may inform optimal dosage selection for definitive trials.
- Studies to assess the appropriate delivery system or parameter settings of an electronic device or surgical technique.
- Studies to assess the safety and tolerability of various doses or concentrations of a specific intervention.
- Studies designed to evaluate whether an intervention produces sufficient evidence of short-term activity (e.g., biomarker activity) in humans to justify an efficacy trial.
- Studies designed to select the best of two or more potential interventions or dosing regimens to evaluate in a subsequent definitive trial, based on tolerability or evidence of biological activity.
Although some clinical trials can be supported through the RDCRC, clinical trials are NOT a required component of a RDCRC application. RDCRC award recipients are encouraged to leverage the RDCRC infrastructure to support clinical trials funded through other sources. Award recipients that desire to seek support for projects that are single-site, basic, translational, or clinical trials outside of the scope of this NOFO should contact the Scientific/Research Contact listed in Section VII. Agency Contacts below for guidance on appropriate funding opportunities. Any clinical trial using the RDCRC resources must receive prior approval from the NIH awarding institute/center and be managed through an appropriate agreement between the RDCRC and funding source which requires all parties to adhere to NIH clinical trial policies and regulations.
Enhancing Reproducibility through Rigor and Transparency The NIH is committed to promoting rigorous and transparent research in all areas of science it funds ( https://grants.nih.gov/policy/reproducibility/index.htm ). The basic principles of rigor and transparency are: 1) rigor of the prior research; 2) rigorous experimental design for robust and unbiased results; 3) consideration of relevant biological variables, and 4) authentication of key biological and/or chemical resources ( https://grants.nih.gov/policy/reproducibility/guidance.htm ). Investigators should consider how all four areas apply to their proposed research.
Additional Support Applicants are strongly encouraged to leverage other existing NIH-supported research resources, such as the SMART IRB ( https://smartirb.org/ ), Clinical and Translational Science Award (CTSA) hubs ( https://ncats.nih.gov/ctsa ) and the Trial Innovation Network ( https://trialinnovationnetwork.org/ ). Projects that leverage the resources of ongoing clinical trials or longitudinal studies supported through other Federal or private funds are also encouraged.
Funds from other sources are acceptable, such as support from advocacy groups, private research foundations, academic institutions, industry, and other government agencies. Memorandum of Understanding agreements between parties need to ensure transparency, prevent misuse of federal funds, and ensure that NIH policies with respect to sharing of data and resources, academic freedom, and publication rights are not violated. Any activity conducted via the Memorandum of Understanding must comply with NIH policies and regulations for conducting human subject's research.
Data Management and Coordinating Center (DMCC) Support
Advancing rare disease research by freely sharing high-value data is a critical goal of the program. Deidentified data collected within this network will become a resource for the greater rare disease research community and will be made available to the scientific community, stakeholders and other relevant partners in a timely manner that meets all NIH human subject's protection, data safety and data sharing requirement.
RDCRC participants are required to work collaboratively within the RDCRN DMCC cloud environment and to ultimately share their data within RDCRN Data Repository (RDCRN-DR).
The DMCC will support the consortia by maintaining a robust secure data infrastructure for the RDCRN. The DMCC will work closely with NCATS to provide Cloud Computing Services and Engineering Support provisioned by the ITRB at NCATS. The DMCC will provide:
RDCRN Cloud-Based Working Environment
The DMCC will provide:
- A management system for collection, storage, and quality control of clinical research data, including a web-based platform that allows for real-time tracking of data quality and completeness and that facilitates remote monitoring.
- A management system that provides a "virtual repository" that indexes existing biosamples and other resources within the network.
- A portal and tools establishing a "tool garden" of shared research tools for research scientists and clinicians to access and manage their own data. Tools provided by NCATS are based on need and use and include, but are not limited to, Amazon Web Services, Atlassian, CODECOV, Facebook, Github, MAILCHIMP, Pantheon, Shippo, SHUTTERSTOCK, TWILIO, VIMEO PRO, Seven Bridges, Box.com.
Data Management Services
The investigative team of the Data Management Core will also manage and facilitate use of Cloud Computing Services and Engineering Support provisioned by the ITRB, NCATS.
NCATS ITRB will provide access to various public cloud services and high-performance computing services for the needs of the RDCRN. The NCATS ITRB works directly with the DMCC. The DMCC is responsible for working with each individual RDCRC however, there may be special instances where the ITRB will work collaboratively with the DMCC and RDCRC (e.g., establishing use of a new tool in the cloud environment). This enables the DMCC to provide the RDCRCs systems, projects, and research in a secure environment with simplified implementation, deployment and operational reliability. Through these services, NCATS ITRB will prepare the recipients to gain a self-service capability.
The DMCC will provide RDCRCs access to the cloud instance through the NCATS' Federated Authorization Services and will have complete rights to all services provided by the Cloud Service Provider.
The DMCC will coordinate and support efforts, in collaboration with representatives of the RDCRC, to develop and monitor Good Data Practices (GDP) of clinical and research data and will assist in facilitating the use of the FAIR principles for data management. The DMCC will also facilitate and coordinate data standards across the network.
Investigators who participated in the RDCRN and shared data in the current RDCRN data environment will be provided by NCATS access to account management, tools, and data storage (provisioned by the DMCC) for their RDCRC data for up to five years post-award. Any custom development, setup, or data management will not be included; if needed, it can be provided by the former RDRCR, or a third party paid for by the former RDCRC. Access to this resource will be provided based upon application and NCATS approval.
Data Sharing Environment - The RDCRN-Data Repository (RDCRN-DR)
The RDCRN-DR will serve as the central repository for RDCRN-generated clinical research data. It will host consented research data from RDCRC-initiated natural history studies, interventional studies and trials, patient reported outcomes and other modalities that were obtained under RDCRN protocols. The RDCRN-DR will also function as a central indexing site for RDCRN data that may be required to reside in other NIH-based data repositories, such as the database of Genotypes and Phenotypes (dbGaP https://www.ncbi.nlm.nih.gov/gap/) , National Database for Autism Research (NDAR https://nda.nih.gov/) .
Once the data has been transferred to the RDCRN-DR NCATS becomes responsible for regulating access to the RDCRN-DR through a Data Access Committee (DAC) made up of Federal representatives. The DAC will meet regularly to adjudicate access requests and ensure that data is only made accessible if the requests are compatible with data use limitations for the data in question. NCATS hosts this cloud-based platform and ensures its long-term sustainability.
Deidentified data collected within this network and housed within cloud services, provisioned by NCATS, will become a resource for the greater rare disease research community and will be made available, via controlled access, to the scientific community, stakeholders and other relevant partners in a manner that meets all NIH human subject's protection, data safety and data sharing requirements.
Milestones Both RDCRN and RDCRC milestones should inform annual evaluations of progress of the network. When applicable, any milestone report should describe how measurable outcomes will be collected using rigorous and transparent experimental approaches. These approaches include, but are not limited to, randomization, blinding, use of statistically adequate sample sizes with biologically relevant effect sizes, minimization of potential bias, independent replication, and adequate reporting of experimental details and results as described at https://www.ninds.nih.gov/Funding/grant_policy .
Prior to funding an application, the NIH PO will contact the applicant to discuss the proposed milestones and any concerns raised by the review panel or program staff. A final set of NIH-approved RDCRC and RDCRN milestones will be agreed upon prior to award.
RDCRN milestones will include, but are not limited to:
- Key contacts for individual research studies
- Key Patient Advocacy Group contacts
- Consent documents and data use limitations
- Protocols and schedule of events for all studies
- List of participating sites and personnel
- Anticipated date of enrollment of the first participant
- Completion of data collection
- Information regarding pilot studies
- Information regarding Career Enhancement Core beneficiaries
- Establish a plan for data management and data coordination-including the use of data standards and data sharing best practices.
- Establish a plan for effectively using RDCRN shared resources.
- Establish a plan track project biospecimens in a virtual repository that indexes samples.
- Establish a RDCRC-specific website.
- Establish all required data use and data sharing agreements with the DMCC.
- Establish data use and data sharing agreements across all sites within the RDCRC that correspond to network wide agreements.
Finalization of milestones will be negotiated at the time of the award, as appropriate. Future year support is contingent on satisfactory achievement of performance milestones. If milestones are not achieved fully, NIH POs may request development of a remedial plan and more frequent monitoring of progress or take other remedial action.
RDCRN Governance (Network-specific) The awards funded under this NOFO will be cooperative agreements (see Section VI.2. Cooperative Agreement Terms and Conditions of Award).
The RDCRN will consist of all the awarded RDCRCs, their affiliated patient advocacy group(s), the DMCC, and NIH program representatives. The RDCRN governance consists of the NCATS RDCRN Program Director, JLT, Network SC, and the CPAG SC.
NCATS RDCRN Program Director
- Provide coordination for network activities.
- In consultation with NIH staff, other federal agency staff, and non-NIH experts in the field, provide feedback and guidance on program activities.
- Coordinate with the DMCC to interact with PD(s)/PI(s) on a regular basis to facilitate access to NIH resources.
- Help organize the annual Network SC Meeting.
- Help coordinate collaborative efforts within the RDCRN.
- Help facilitate collaborative efforts with external partners (e.g., National Organization for Rare Disorders (NORD), European Rare Disease Research Coordination and Support Action consortium (ERICA), Chan Zuckerberg Initiative (CZI)).
- Be the vote casting NIH member of the Network SC (the NIH will have only one vote – that will be a consensus decision across PO).
- Advise the SC on drafting and applying operating policies that require coordinated action across the RDCRN.
- Work collaboratively with all PO and PS.
- Have responsibility for negotiating and approving RDCRN Milestones that are quantifiable for measuring success in meeting the goals of RDCRN membership. Each RDCRN milestone should have quantitative criteria associated with them.
RDCRN Joint Leadership Team
The RDCRN JLT will serve as the Executive Committee of the RDCRN. The JLT will meet on a monthly basis to hold strategic discussions regarding the RDCRN functioning and will make executive decisions related to RDCRN activities. The JLT will screen any potential cross-network research projects from external or internal collaborators and make recommendations to the Network SC. In addition, the JLT will discuss any issues in need of consensus or resolution and will identify solutions to be discussed with the RDCRN SC and/or the CPAG SC.
The JLT is composed of:
- Network SC Chairs
- CPAG SC Chairs
- One DMCC PD/PI representative
- NCATS RDCRN Program Director
RDCRN Network Steering Committee
The Network SC will identify scientific and policy issues that need to be addressed at the network level and review and approve all RDCRN-wide policies. The Network SC will identify broad issues in the field of rare diseases research that can be addressed by the network. It will also ensure dissemination of program data, career enhancement schedules and other materials to the wider scientific community. The Network SC may establish subcommittees and working groups to facilitate development, implementation, and monitoring of specific network functions as needed.
The Network SC is composed of:
- The RDCRC PD(s)/PI(s)
- The PD(s)/PI(s) of the DMCC
- NIH PO(s)/PS(s) from participating ICOs
RDCRN Coalition of Patient Advocacy Group Steering Committee
The CPAG SC will identify scientific and policy issues that need to be addressed at the network level, as well as broad issues in the field of rare diseases research that are specifically relevant to patients.
The CPAG SC is composed of:
- One patient advocacy group representative from each RDCRC
- Two PD(s)/PI(s) representatives
- NIH PO(s)/PS(s) from participating ICO
RDCRC Governance (Consortium-specific)
Each RDCRC will establish an External Advisory Committee (EAC) consisting of scientific, clinical, and patient group representation that will be composed of at least five members. The EAC serves in an advisory capacity to the RDCRC by providing an annual review and critique of the scientific progress of the RDCRC. The EAC should meet in-person or electronically at least once a year, beginning in the first or second year of the award.
Regulatory Requirements
Single IRB (sIRB) for Multisite Projects Single IRBs are required for all multisite projects. Investigators are strongly encouraged to use SMARTIRB and its reliance agreements. https://smartirb.org /.
Genomic Sequencing Due to regulatory requirements for using research results in clinical care, some sequencing data will be expected to be in compliance with the Clinical Laboratory Improvement Amendments (CLIA) when the results of genomic sequencing will be returned to participants or used for clinical decision-making purposes. In addition, an FDA IDE may be needed for new sequencing methods used in clinical care, separate from the requirement for the test to have been conducted within a CLIA-certified environment. Investigators must be prepared to discuss the possible need for an IDE with their IRBs and document the outcome of those discussions, and to subsequently engage in pre-submission discussions with the FDA. These typically involve submission of actual study protocols, including the entire sequencing pipeline from sample preparation to return of results. Applicants may wish to consult the following "Points to Consider in Assessing When an Investigational Device Exemption (IDE) Might be Needed" ( http://www.genome.gov/27561291 ).
Institute Specific Research Interests and Priorities
NOTE: Prospective applicants are urged to consult with the Scientific/Research Contacts of the NIH early in the preparation of the application (see Section VII. Agency Contacts).
The NHLBI seeks applications that will establish longitudinal cohorts in rare Heart, Lung, Blood, or Sleep (HLBS) diseases to investigate unaddressed research questions using epidemiologic study designs and methods that are appropriate for HLBS conditions affecting fewer than 200,000 persons in the US. These observational cohort studies should be designed to provide an evidence base for future interventional studies, including clinical trials; for developing better diagnostics than those that are currently available; for answering early translational questions; or for broader implementation of guidelines for managing these diseases. NHLBI encourages consortia study rare or related rare diseases, disorders, conditions, or syndromes together based on pathogenesis, affected biochemical, cellular, or physiological features or organ system involvement. Studying related rare diseases within the same cohort could help understand the nuances, and knowledge gained from one disease could accelerate the advances in related diseases.
Examples of research areas that propose to advance rare disease research include but are not limited to:
- Natural history of progression of HLBS rare disease being studied and clinical outcomes;
- Generation of data (laboratory, clinical and imaging data) to improve diagnosis and to distinguish subtypes of the rare disease being studied;
- Development of algorithms clinical and patient-reported outcomes with laboratory, imaging, environmental and -omics data to aid decision-making for clinical management of the rare diseases being studied;
- Elucidate genotype-phenotype interactions and multisystem phenotyping to develop reliable and valid predictive tools to determine who will respond to which treatments and when to intervene;
- Encourage innovative methods such as telemedicine and community-engagement approaches to include participants with rare diseases located in remote locations;
- Characterization of the rare disease cohort being studied, including genetic, phenotypic, imaging and laboratory data to identify disease subtypes i.e., differentiate patients with same morphological phenotypes but different genetic mutations and severity of outcomes;
- Validation of biomarkers that meet FDA requirements as a drug development tool for use in future Phase III Randomized Clinical Trials
- Identification of molecular pathways that can lead to therapeutic targets;
- Comparative studies of newborn-screened cohorts diagnosed with an HLBS disease that receive prescribed standards of care versus those who do not and the factors (e.g., socioeconomic, geographic) that contribute to their health outcomes
- partnering with clinicians and patient communities to understand barriers to and facilitators of implementation of known treatments and interventions for rare diseases;
- study of implementation strategies to increase adoption, feasibility, and sustainability of known effective evidence-based interventions for rare diseases;
- strategies to understand how to disseminate evidence-based assessments or diagnostics and clinical guidelines to relevant rare disease populations
- Evaluate the contribution of lifestyle factors to racial/ethnic differences in rare-disease risk, severity, and treatment response
- Utilize data science and other novel analytic techniques to study rare-disease risk, severity, and treatment response among specific populations (e.g., pregnant women, obese or low-weight patients, elderly patients).
- Proposals are encouraged to include projects with significant unmet clinical and research needs which utilize teams from different clinical sub-specialties in HLBS mission areas, e.g. dedicated projects which study pulmonary and cardiovascular complications of sickle cell disease.
Research supported by this NOFO should fall within the NHLBI mission of (HLBS)-related disorders. Some examples of eligible rare HLBS diseases underrepresented in the current NHLBI portfolio include:
Rare Blood Diseases
Acquired aplastic anemia, antiphospholipid syndrome, hemophagocytic lymphohistiocytosis, hemophilias, hereditary hemorrhagic telangiectasia, heparin-induced thrombocytopenia, paroxysmal nocturnal hemoglobinuria, rare bleeding disorders, rare nutritional anemias, rare thrombotic disorders, rare hemolytic anemias, sickle cell disease, thrombocytopenias of different etiologies, thrombotic thrombocytopenic purpura, inherited bone marrow failure syndromes (IBMFS) that encompass a group of rare genetic blood disorders in which there is usually some form of aplastic anemia (failure of the bone marrow to produce blood), associated with a family history of the same disorder such as Fanconi Anemia, Diamond Blackfan Anemia (DBA), Dyskeratosis Congenita (DC) and Severe congenital Neutropenias (SCN), thalassemias (e.g. Cooley’s Anemia).
Rare Lung Diseases
Alpha-1-antitrypsin deficiency, Birt-Hogg-Dubé Syndrome, Hermansky-Pudlak Syndrome, pediatric interstitial lung diseases, primary ciliary dyskinesia, pulmonary alveolar microlithiasis, pulmonary alveolar proteinosis, pulmonary arterial hypertension, pulmonary Langerhans cell histiocytosis, sarcoidosis, lymphangioleiomyomatosis, studies of systemic autoimmune diseases that focus on pulmonary manifestations i.e. Systemic Sclerosis, primary Sjogren syndrome, Systemic lupus erythematosus (SLE), Dermatomyositis (DM) and polymyositis (PM), systemic (ANCA-associated) vasculitis and mixed connective tissue disease.
Rare Cardiovascular Diseases
Alagille Syndrome, Ehlers Danlos Syndrome, DiGeorge Syndrome, dysbetaliproproteinemia, familial hypercholesterolemia, Hutchinson-Gilford progeria syndrome, inherited channelopathies (Long-QT Syndrome, Brugada Syndrome), Kawasaki Disease, Klippel-Trenaunay-Weber Syndrome, Loeys Dietz Syndrome, Marfan Syndrome, Peripartum Cardiomyopathy, Pompe Disease, rare inherited cardiomyopathies (such as arrhythmogenic right ventricular dysplasia, Danon Disease, LEOPARD Syndrome, Noonan Syndrome), Tangier Disease, Turner Syndrome, Vascular malformations including capillary, venous, lymphatic, and rare arteriovenous malformations including but not limited to, pulmonary arteriovenous malformation, Sturge-Weber syndrome, Gorham-Stout syndrome, hereditary hemorrhagic telangiectasia, kaposiform lymphangiomatosis.
Rare Sleep Disorders
Advanced sleep phase circadian disorder, narcolepsy, Klein-Levine syndrome, sleep-related eating disorder, fatal familial insomnia, exploding head syndrome (episodic cranial sensory shock), central hypoventilation syndrome.
Prior to submission of an application in response to this NOFO, applicants are strongly encouraged to consult with the Scientific Research Contacts. Early contact is encouraged, as this provides an opportunity for NHLBI staff to provide information and guidance to a potential applicant.
NIAID research activities on rare diseases are classified into four areas: infectious diseases, primary immunodeficiency diseases, autoimmune diseases, and allergic diseases.
- Infectious diseases include diseases caused by bacteria, parasites, viruses, fungi, and other pathogens. Research on rare infectious diseases is aimed at delineating mechanisms of disease pathogenesis and developing more effective diagnostic, treatment, and prevention strategies.
- Primary immunodeficiency diseases are hereditary disorders caused by intrinsic defects in the cells of the immune system and are characterized by unusual susceptibility to infection. NIAID research is focused on the identification of gene defects and immunologic abnormalities that lead to defective function, and the development of new approaches for the diagnosis and treatment of primary immunodeficiency disease, including gene transfer as an effective and curative therapy.
- Autoimmune diseases are diseases in which the immune system mistakenly attacks and damages the body's own cells and tissues. NIAID research is focused on the identification of mechanisms of pathogenesis and the development of new approaches to prevention and treatment.
- Allergies are inappropriate or exaggerated reactions of the immune system to substances that cause no symptoms in the majority of people.
NIAID welcomes applications focused on rare diseases that fall within the four areas above and NIAID staff encourages prospective investigators to contact them regarding the fit of their research for this funding opportunity. For applications that propose clinical trials, NIAID strongly encourages applicants to contact NIAID staff early in the planning stage to assist applicants in meeting NIAID clinical trial requirements.
Many arthritic, rheumatic, musculoskeletal and skin diseases affecting adults and children that are of importance to NIAMS are considered rare. NIAMS is interested in supporting research into the causes, treatment and prevention of these rare diseases.
Applicants are strongly encouraged to contact NICHD staff to discuss potential applications and disease areas of interest.
The NICHD conducts and supports research on topics related to health of children, adolescents, adults, families, and populations. NICHD also supports research on populations that are often excluded from research, such as pregnant and lactating people or people with intellectual, developmental, and/or physical disabilities. Many disorders that affect children and their families are rare and/or neglected diseases. Many of these diseases have a genetic basis, and many affect neurodevelopment. NICHD is especially interested in research proposals that include both underrepresented and underserved patient populations and whose team members represent diverse backgrounds. NICHD encourages applications in rare disorders, including (but not limited to):
- This includes, but is not limited to, conditions currently on or with the potential to be added to the Recommended Uniform Screening Panel (RUSP; https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp)
- NICHD is especially interested in supporting natural history studies and longitudinal follow-up studies of newborn screenable conditions
- Metabolic disorders (e.g., organic acidemias, amino acidopathies, fatty acid oxidation disorders, neurotransmitter disorders)
- Storage diseases (e.g., gangliosidoses, mucopolysaccharidoses, mucolipidoses, peroxisomal disorders, glycogen storage disorders, other lysosomal storage diseases)
- Congenital anomalies and other structural malformations
- Angelman, Fragile X, Prader-Willi, Williams, Smith-Magenis, Cornelia de Lange, and other syndromes
- Copy number variation disorders, such as 22q11.2 deletion syndrome, 18q, 1p36, and other microdeletion or microduplication disorders
- Sotos, Weaver, and Kabuki syndromes, and other Mendelian disorders of the epigenetic or chromatin remodeling machinery
- Sex chromosome disorders (e.g., Turner syndrome, Klinefelter syndrome, XXYY, XXXY, XXX, XYY)
- Disorders of sex development and other rare disorders of the renal and genitourinary systems
- Other rare, pediatric-onset disorders
NIDCD is interested in supporting research that examines hearing, balance, taste, smell, voice, speech, or language in individuals with rare diseases.
The NIDCR seeks to support clinical research on rare dental, oral, and craniofacial diseases and conditions. NIDCR is also interested in oral manifestations of rare diseases or conditions that severely impact oral health.
Interests include, but are not limited to, the conditions listed below. Applicants are strongly encouraged to contact NIDCR to discuss potential applications and areas of interest.
Examples include:
- Diseases associated with craniofacial or alveolar bone loss, early tooth loss, severe dental caries, and enamel and dentin defects;
- Diseases of the gingiva, periodontium, and other oral soft and hard tissues that may or may not be associated with metabolic, structural, or immune defects;
- Dental and craniofacial anomalies, syndromes, and disorders resulting from immunodeficiencies, inborn errors of metabolism, environmental factors, and other genetic defects;
- Dentofacial malformations and birth defects including hypodontia;
- Infantile to adult-onset forms of rare diseases or disorders that manifest in the oral cavity or craniofacial skeleton such as hypomineralization and hypophosphatasia;
- Rare tumors of the head and neck, including salivary gland cancers; and
- Oral diseases or conditions that are the result of head and neck radiotherapy or medication treatment for cancers such as medication-induced osteonecrosis of the jaw and osteoradionecrosis of the jaw.
The NIDDK supports research on selected rare diseases and conditions resulting in endocrine, metabolic, digestive, hematologic, urologic, and kidney disorders. Below are some examples of rare diseases that may be appropriate. Examples of rare liver diseases include biliary atresia or monogenic forms of severe liver diseases in children; diseases of the pancreas include hereditary recurrent acute and chronic pancreatitis; diseases of the alimentary tract include rare forms of intestinal failure such as tufting enteropathy. Examples of rare endocrine diseases include acromegaly, hypoparathyroidism, pseudohypoparathyroidism, familial hypocalciuric hypercalcemia, thyroid hormone resistance. Examples of rare metabolic diseases include aminoacidopathies, cystic fibrosis, lysosomal storage diseases, lipodystrophy, monogenic diabetes, monogenic obesity, fatty acid oxidation and urea cycle defects. Examples of rare hematologic, urologic, and kidney diseases include congenital dyserythropoietic anemias, inherited bone marrow failure syndromes or hemoglobinopathies, inherited and acquired systemic amyloidosis, autosomal recessive polycystic kidney disease, and renal disease associated with cystinosis or tuberous sclerosis.
NHGRI encourages the sharing of aggregate sequence variant interpretations to ClinVar; leveraging ClinGen's patient registry, GenomeConnect ; and participating in relevant ClinGen gene and variant curation activities. More information can be found on at clinicalgenome.org . In addition, NHGRI is interested in supporting pharmacogenomic research and studies to advance the treatment of rare diseases/conditions through genetic/genomic testing to improve drug efficacy and effectiveness and to reduce drug adverse effects in patients of diverse populations. More details about the institute’s vision and priority areas can be found in the 2020 NHGRI Strategic Vision ( https://www.genome.gov/2020SV ).
The NIA, Division of Aging Biology (DAB) is interested in studying aging in the context of rare diseases and geroscience-based interventions. Areas of research could include, but are not limited to:
- Biology of aging in rare diseases such as understanding the aging process in different rare diseases; identification and in-depth investigation of disease-specific biomarkers and the epigenetic clock that could provide insights into the predictors of accelerated aging in rare diseases.
- Functional impact of aging phenotypes such as frailty, sarcopenia, and cognitive decline in rare diseases.
- Interventions (nutrition, physical activity, use of geroprotectants) to delay aging in patients with rare diseases in different stages of life.
- Genetic interaction between rare disease alleles and hallmarks of aging.
The NIA, Division of Geriatrics and Clinical Gerontology (DGCG) is interested in studying topics related to the following progeroid syndromes, but are not limited to:
- Down Syndrome
- Werner Syndrome
- Cockayne Syndrome
- Wiedemann-Rautenstrauch Syndrome
The NIA, Division of Neuroscience (DN) is interested in studying basic and clinical research including genetic determinants of rare diseases in the aging population; specific areas of interest include but are not limited to:
- Rare heterogeneous premature-ageing diseases, including telomere biology disorders (TBDs).
- Rare age-related genetically driven circadian dysregulation and expression changes.
- Cardiac amyloidosis caused by rare genetic factors and their relationship to amyloidosis found in Alzheimer’s Disease and Related Dementias.
- Rare age-related mitochondrial disorders.
- Rare age-related neurotransmitter disorders.
- Rare age-related genetically driven frailty factors including the identification of biomarkers that may reveal therapeutic targets for age-related diseases.
- Rare age-related genetic resilience factors found in the oldest old that may reveal therapeutic approaches to age-related diseases.
- Rare cytoskeleton disorders that impact the central nervous system.
- Rare age-related metabolic, endocrine, digestive, hematologic, kidney, and storage disorders that impact the central nervous system.
- Rare arthritic, rheumatic, musculoskeletal, skin, and neuromuscular disorders that may impact the central nervous system.
- Rare age-related resilience factors in genetically predisposed cohorts such as frontotemporal dementias and other rare Alzheimer’s disease-related dementias.
The NINDS seeks to support clinical research on rare neurological and neuromuscular conditions. Examples include (but are not limited to): cerebrovascular disorders, neurometabolic disorders, neuromuscular and neurodegenerative disorders, movement disorders, epilepsies and paroxysmal disorders, channelopathies, mitochondrial diseases, and childhood developmental and/or genetic syndromes, including those that involve rare genetic forms of autism or neurodevelopmental disabilities. Applicants proposing a clinical trial should contact the Scientific/Research Contact listed in Section VII. Agency Contacts below for guidance to meet NS-specific policies for clinical trials. Applicants seeking support for projects that are outside of the scope of the NOFO (e.g., single-site, basic, translational, clinical studies or Phase III clinical trials) should contact the Scientific/Research Contact listed in Section VII. Agency Contacts below for guidance on other, more appropriate funding opportunities.
Applications Not Responsive to this NOFO:
The following types of studies are not responsive to this NOFO. Applications proposing such studies will be considered non-responsive, will be withdrawn from review, and not considered for funding.
- Single site clinical studies
- Phase III Clinical Trials as part of Clinical Research Projects
- There are fewer than three rare diseases included
- There is not at least one longitudinal study
- There are either less than two or more than four research projects submitted
- There is no patient advocacy group involved
- Basic sciences studies
- Applications that propose any type of animal studies within the RDCRC. The use of in vitro models must be relevant to clinical endpoints (i.e., testing drugs, validating biomarkers versus more basic research)
Pre-application Information Session
All applicants are strongly encouraged to contact NIH ICO staff to discuss the alignment of their proposed work with the goals of this NOFO and the RDCRN. A technical assistance teleconference will be held for potential applicants. NIH staff will be available to answer questions related to this and the associated DMCC NOFO. Time, date, and dial-in information for the call will be announced in an NIH Guide Notice.
Note: For more information, please refer to the Questions and Answers website for this NOFO ( https://ncats.nih.gov/research/research-activities/RDCRN/applicant-information ).
See Section VIII. Other Information for award authorities and regulations.
Investigators proposing NIH-defined clinical trials may refer to the Research Methods Resources website for information about developing statistical methods and study designs.
Section II. Award Information
Cooperative Agreement: A financial assistance mechanism used when there will be substantial Federal scientific or programmatic involvement. Substantial involvement means that, after award, NIH scientific or program staff will assist, guide, coordinate, or participate in project activities. See Section VI.2 for additional information about the substantial involvement for this NOFO.
The OER Glossary and the How to Apply - Application Guide provides details on these application types. Only those application types listed here are allowed for this NOFO.
Optional: Accepting applications that either propose or do not propose clinical trial(s).
The number of awards is contingent upon NIH appropriations and the submission of a sufficient number of meritorious applications.
Applicants may request up to $1 million in direct costs/year. All costs must be well justified in accordance with the activity proposed.
Applicants may request up to 5 years of support.
NIH grants policies as described in the NIH Grants Policy Statement will apply to the applications submitted and awards made from this NOFO.
Section III. Eligibility Information
1. Eligible Applicants
Higher Education Institutions
- Public/State Controlled Institutions of Higher Education
- Private Institutions of Higher Education
The following types of Higher Education Institutions are always encouraged to apply for NIH support as Public or Private Institutions of Higher Education:
- Hispanic-serving Institutions
- Historically Black Colleges and Universities (HBCUs)
- Tribally Controlled Colleges and Universities (TCCUs)
- Alaska Native and Native Hawaiian Serving Institutions
- Asian American Native American Pacific Islander Serving Institutions (AANAPISIs)
Nonprofits Other Than Institutions of Higher Education
- Nonprofits with 501(c)(3) IRS Status (Other than Institutions of Higher Education)
- Nonprofits without 501(c)(3) IRS Status (Other than Institutions of Higher Education)
For-Profit Organizations
- Small Businesses
- For-Profit Organizations (Other than Small Businesses)
Local Governments
- State Governments
- County Governments
- City or Township Governments
- Special District Governments
- Indian/Native American Tribal Governments (Federally Recognized)
- Indian/Native American Tribal Governments (Other than Federally Recognized)
Federal Governments
- Eligible Agencies of the Federal Government
- U.S. Territory or Possession
- Independent School Districts
- Public Housing Authorities/Indian Housing Authorities
- Native American Tribal Organizations (other than Federally recognized tribal governments)
- Faith-based or Community-based Organizations
- Regional Organizations
Non-domestic (non-U.S.) Entities (Foreign Organization) are not eligible to apply.
Non-domestic (non-U.S.) components of U.S. Organizations are not eligible to apply.
Foreign components, as defined in the NIH Grants Policy Statement , are allowed.
Applicant organizations
Applicant organizations must complete and maintain the following registrations as described in the How to Apply- Application Guide to be eligible to apply for or receive an award. All registrations must be completed prior to the application being submitted. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. Failure to complete registrations in advance of a due date is not a valid reason for a late submission, please reference NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications for additional information.
- NATO Commercial and Government Entity (NCAGE) Code – Foreign organizations must obtain an NCAGE code (in lieu of a CAGE code) in order to register in SAM.
- Unique Entity Identifier (UEI) - A UEI is issued as part of the SAM.gov registration process. The same UEI must be used for all registrations, as well as on the grant application.
- eRA Commons - Once the unique organization identifier is established, organizations can register with eRA Commons in tandem with completing their Grants.gov registration; all registrations must be in place by time of submission. eRA Commons requires organizations to identify at least one Signing Official (SO) and at least one Program Director/Principal Investigator (PD/PI) account in order to submit an application.
- Grants.gov – Applicants must have an active SAM registration in order to complete the Grants.gov registration.
Program Directors/Principal Investigators (PD(s)/PI(s))
All PD(s)/PI(s) must have an eRA Commons account. PD(s)/PI(s) should work with their organizational officials to either create a new account or to affiliate their existing account with the applicant organization in eRA Commons. If the PD/PI is also the organizational Signing Official, they must have two distinct eRA Commons accounts, one for each role. Obtaining an eRA Commons account can take up to 2 weeks.
Any individual(s) with the skills, knowledge, and resources necessary to carry out the proposed research as the Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) is invited to work with his/her organization to develop an application for support. Individuals from diverse backgrounds, including underrepresented racial and ethnic groups, individuals with disabilities, and women are always encouraged to apply for NIH support. See, Reminder: Notice of NIH's Encouragement of Applications Supporting Individuals from Underrepresented Ethnic and Racial Groups as well as Individuals with Disabilities, NOT-OD-22-019 .
For institutions/organizations proposing multiple PDs/PIs, visit the Multiple Program Director/Principal Investigator Policy and submission details in the Senior/Key Person Profile (Expanded) Component of the How to Apply - Application Guide .
The RDCRC PD/PI(s) should have documented experience in conducting research on a disorder that qualifies as a rare disease (within the definition of this NOFO) and must have demonstrated experience in managing large multi-component clinical research programs. The RDCRC PD(s)/PI(s) cannot serve as the PD/PI of a project in another active RDCRC at the time of award, however collaborations among RDCRCs are encouraged. The minimum effort of the PD(s)/PI(s) across RDCRC projects and/or cores should be at least 2.0 person months per year. If there are multiple PDs/PIs each individual must meet the minimum requirement.
2. Cost Sharing
This NOFO does not require cost sharing as defined in the NIH Grants Policy Statement Section 1.2- Definitions of Terms.
3. Additional Information on Eligibility
Number of Applications
Applicant organizations may submit more than one application, provided that each application is scientifically distinct.
The NIH will not accept duplicate or highly overlapping applications under review at the same time per NIH Grants Policy Statement Section 2.3.7.4 Submission of Resubmission Application . This means that the NIH will not accept:
- A new (A0) application that is submitted before issuance of the summary statement from the review of an overlapping new (A0) or resubmission (A1) application.
- A resubmission (A1) application that is submitted before issuance of the summary statement from the review of the previous new (A0) application.
- An application that has substantial overlap with another application pending appeal of initial peer review (see NIH Grants Policy Statement 2.3.9.4 Similar, Essentially Identical, or Identical Applications ).
Section IV. Application and Submission Information
1. Requesting an Application Package
The application forms package specific to this opportunity must be accessed through ASSIST or an institutional system-to-system solution. A button to apply using ASSIST is available in Part 1 of this NOFO. See the administrative office for instructions if planning to use an institutional system-to-system solution.
2. Content and Form of Application Submission
It is critical that applicants follow the Multi-Project (M) Instructions in the How to Apply - Application Guide , except where instructed in this notice of funding opportunity to do otherwise and where instructions in the How to Apply - Application Guide are directly related to the Grants.gov downloadable forms currently used with most NIH opportunities. Conformance to the requirements in the How to Apply - Application Guide is required and strictly enforced. Applications that are out of compliance with these instructions may be delayed or not accepted for review.
Letter of Intent
Although a letter of intent is not required, is not binding, and does not enter into the review of a subsequent application, the information that it contains allows IC staff to estimate the potential review workload and plan the review.
By the date listed in Part 1. Overview Information , prospective applicants are asked to submit a letter of intent that includes the following information:
- Descriptive title of proposed activity
- Name(s), address(es), and telephone number(s) of the PD(s)/PI(s)
- Names of other key personnel
- Participating institution(s)
- Number and title of this funding opportunity
The letter of intent should be sent to:
NCATS Letters of Intent Telephone: 301-827-9549 Email: [email protected]
Page Limitations
All page limitations described in the How to Apply- Application Guide and the Table of Page Limits must be followed.
Instructions for the Submission of Multi-Component Applications
The following section supplements the instructions found in How to Apply- Application Guide and should be used for preparing a multi-component application.
The application should consist of the following components:
- Overall: required
- Administrative Core: required; maximum of 1
- Pilot/Feasibility Governance Core: required; maximum of 1
- Career Enhancement Core: required; maximum of 1
- Clinical Research Projects: required; minimum of 2, maximum of 4
Overall Component
When preparing the application, use Component Type ‘Overall’.
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions, as noted.
SF424(R&R) Cover (Overall)
Complete entire form.
PHS 398 Cover Page Supplement (Overall)
Note: Human Embryonic Stem Cell lines from other components should be repeated in cell line table in Overall component.
Research & Related Other Project Information (Overall)
Follow standard instructions.
Other Attachments:
Justification for Rare Disease Status (Required)
- The Rare Disease Status attachment may be no more than 3 pages in length and must include all targeted diseases/conditions.
- In an "Other Attachment" entitled "Rare Disease Status", all applicants must include a justification that the diseases/conditions being studied are rare in the U.S.
- This section may include one or more references confirming that the prevalence of the diseases/conditions that are the primary focus of the research application is 200,000 or fewer patients in the U.S., as defined by The Rare Diseases Act of 2002 (Public Law 107-280). If the diseases/conditions have been granted orphan status by the FDA, provide this information in the justification.
- If it is a rare variant or subset of a more common condition, provide a justification for including this variant in the RDCRC. Describe the scientific basis for separating biomarker/clinical outcome assessment (COA) validation for this rare variant or subset from that of the common condition.
Applications missing a Justification for Rare Disease Status may be deemed incomplete and not sent forward for review.
Project/Performance Site Locations (Overall)
Enter primary site only.
A summary of Project/Performance Sites in the Overall section of the assembled application image in eRA Commons compiled from data collected in the other components will be generated upon submission.
Research and Related Senior/Key Person Profile (Overall)
Include only the Project Director/Principal Investigator (PD/PI) and any multi-PDs/PIs (if applicable to this NOFO) for the entire application.
A summary of Senior/Key Persons followed by their Biographical Sketches in the Overall section of the assembled application image in eRA Commons will be generated upon submission.
Budget (Overall)
The only budget information included in the Overall component is the Estimated Project Funding section of the SF424 (R&R) Cover.
A budget summary in the Overall section of the assembled application image in eRA Commons compiled from detailed budget data collected in the other components will be generated upon submission.
PHS 398 Research Plan (Overall)
Introduction to Application: For Resubmission and Revision applications, an Introduction to Application is required in the Overall component.
Specific Aims: Describe the overall goals of the RDCRC for the performance period of the grant. Describe the research objectives of the RDCRC for promoting understanding of rare diseases, facilitating early and timely diagnosis and establishing clinical trial readiness.
Research Strategy: This section should describe the major theme of the RDCRC, its goals and objectives, background information, the overall importance of the research and provide a sense of the overall significance of the RDCRC, i.e., how the RDCRC infrastructure and any results and resources it generates will impact the encompassed rare diseases in the near- and long-term if the goals and objectives are achieved.
- Describe the rationale for the overall proposed program including the rationale for why these rare diseases were chosen for study (minimum of three).
- Briefly describe the clinical research projects and cores and provide a figure of the organizational structure of the RDCRC.
- Clearly state the unmet research/clinical needs these projects address, and how the RDCRC will accelerate progress toward effective treatments or other improvements in the lives of individuals with the targeted rare diseases through coordinated and collaborative research and infrastructure activities.
- Describe how the projects and cores contribute to the overall goals and objectives of the RDCRN in advancing understanding of the diseases, facilitating early diagnosis, improving clinical trial readiness, developing and testing therapies, advancing patient care and reducing disease burden.
- Describe the justification for why the RDCRN infrastructure is needed to advance research for the selected rare diseases and why standard NIH grant support mechanisms (e.g., R01s) are insufficient.
- Describe the RDCRC PD(s)/PI(s) expertise in rare diseases and experience managing large multi-component clinical research programs.
- For multi-PD/PI applications, describe their complementary and integrated expertise, and comment on their leadership approach, governance, and organizational structure.
- Indicate prior collaborative relationships among proposed senior key personnel at the various sites and across the RDCRC.
- Describe the leaders and collective teams for each core and how their skills complement each other to contribute to the overall team.
- Describe the nature of the ongoing relationships with patient advocacy group(s) and the approach of patient or stakeholder participation across the planned objectives (e.g., in addressing clinical design, recruitment, and education). Describe how patient and stakeholder experiences, perspectives, needs, and priorities will be meaningfully incorporated as partners into decisions and activities of the RDCRC.
- Describe how the RDCRC will work with the DMCC and leverage the shared network tools provided by NIH.
- Describe plans for the RDCRC to collaborate and otherwise contribute to the RDCRN, through participating in network-wide committees, workshops, meetings, career enhancement, collaborative efforts, or other RDCRN-wide activities and initiatives.
- Describe the rationale for choosing clinical sites to participate in the network.
- Describe any relevant existing partnerships with other rare disease organizations or industry.
Renewal applications from existing RDCRCs:
- For RDCRC that have been previously funded, document achievement of the goals of the prior funding period.
- For RDCRC that have been previously funded, justify the need for continued funding for the RDCRC and the value of additional knowledge to be gained. Specifically, the value of continuing ongoing natural history studies with a focus on clinical trial readiness should be addressed.
- Describe inclusion of patient or stakeholder groups by providing examples of previous and/or ongoing collaborations.
- For RDCRC that have been previously funded, a sustainability plan should be proposed. The plan should address maintenance of the critical functions of the RDCRC and preparing for the end of NIH funding. Applicants are encouraged to creatively engage in the scientific and operational problems that need to be addressed for the RDCRC to be a sustainable success. This includes plans for continued relationships with patient and family groups, infrastructure support for critical components of the RDCRC, and data sharing (for current data and beyond). A timeline should be included that illustrates the transition process and out years beyond the NIH funding period.
Letters of Support:
Applicants must provide letters from the appropriate high-ranking institutional official(s) from the lead institution and partnering institutions that:
- Commit the institution(s) to the RDCRC goals, indicating that the program will be integral to their broad vision of clinical research in rare diseases.
- Defines the position, authority, and reporting responsibility (on the institution's organizational chart) for the RDCRC PD(s)/PI(s).
- Defines the financial and other resource support for the RDCRC that will be provided by the applicant institution(s). There are no dollar requirements, but specific commitment is required. Some examples include financial support, adequate space, release time agreements, tenured or tenure-track positions for clinical/translational faculty, FTEs for clinical support or ancillary personnel, core consolidation, and maintenance. Specific commitments to cores and other components can be summarized in a table.
- Defines the authority or influence that the RDCRC PD(s)/PI(s) has, and/or will have over the different components of the Center, facilities, and space, as well as decision-making authority for hiring and/or approving new faculty and support personnel.
- Commit to notify NIH program staff of adverse events in all clinical studies supported by the Center that are serious, unexpected, and related to participation in research. A reiteration to notify NIH program staff of all unanticipated problems as outlined in OHRP guidance ( http://www.hhs.gov/ohrp/policy/advevntguid.html ) on studies supported by RDCRC resources would satisfy this requirement. These notifications include all adverse events as noted above as well as other reportable unanticipated problems.
Applicants should provide letters from the appropriate high-ranking institutional official(s) from the partnering institutions that:
- Leveraging funds from other sources (including industry) is encouraged, as long as these funds do not limit faculty research, communications, and implementation at any point and there are methods in place to ensure transparency, prevent misuse of federal funds, and ensure that NIH policies with respect to sharing of data and resources, academic freedom, and publication rights are not violated. Any activity conducted with an external collaborator must meet NIH requirements for conducting human subject’s research.
- For the RDCRC, the institution that submits the U54 application should receive a formal written agreement(s) from the other participant organization(s) and submit them with the application. This agreement should clearly delineate the institutional commitment of the participating organization(s) (in the ways outlined above) to the RDCRC Program.
- These letters should be clear expressions of commitment consistent with achieving the goals of the program.
Applicants may provide letters of support specific to the cores or projects if they do not duplicate other letters of support. Please label each core- or project- specific letters of support with the subcomponent it is supporting.
Also, only include letters of collaboration from individuals and groups who will contribute in a substantive, meaningful way to the scientific development or execution of the overall RDCRC, whether or not salaries are requested. Supporting letters from patient or stakeholder groups should be included.
Resource Sharing Plan : Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the How to Apply - Application Guide .
Other Plan(s):
All instructions in the How to Apply- Application Guide must be followed, with the following additional instructions:
- All applicants planning research (funded or conducted in whole or in part by NIH) that results in the generation of scientific data are required to comply with the instructions for the Data Management and Sharing Plan. All applications, regardless of the amount of direct costs requested for any one year, must address a Data Management and Sharing Plan. . The Data Management and Sharing (DMS) Plan must be provided in the Overall component. Applicants should develop the DMS Plan taking the following into consideration:
NIH expects that datasets from the RDCRN will be widely shared with the scientific community for research, while carefully observing standards of patient privacy, confidentiality, and management of health information. Information such as study protocols, descriptions, bioinformatics tools, and publications are expected to be made available through an open access section of a database such as the RDCRN and other public web sites, and publication in the scientific literature.
In addition, the RDCRN DMCC will work with each RDCRC to ensure that data to be shared meets all quality control standards, uses RDCRN-wide data standards, provides all appropriate metadata, and identifies all data use limitations. Individual RDCRCs will then be responsible for submitting the data into the NCATS Governed RDCRN-DR, along with a university signing official signature, within a time frame dictated by the applicable NIH data sharing policies, their funding IC, and NCATS. The investigative team of the RDCRC Administrative Core has the responsibility for certifying that submitted data is compliant with regulations and was obtained under the informed consent of the participants.
Only limited items are allowed in the Appendix. Follow all instructions for the Appendix as described in How to Apply- Application Guide ; any instructions provided here are in addition to the How to Apply - Application Guide instructions.
PHS Human Subjects and Clinical Trials Information (Overall)
When involving human subjects research, clinical research, and/or NIH-defined clinical trials follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the How to Apply - Application Guide , with the following additional instructions:
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R&R Other Project Information form, there must be at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or a Delayed Onset Study record within the application. The study record(s) must be included in the component(s) where the work is being done, unless the same study spans multiple components. To avoid the creation of duplicate study records, a single study record with sufficient information for all involved components must be included in the Overall component when the same study spans multiple components.
Study Record: PHS Human Subjects and Clinical Trials Information
All instructions in the How to Apply - Application Guide must be followed.
All applications must follow the instructions G.500 PHS Human Subjects and Clinical Trials Information with additional instructions specific to this NOFO.
Section 2 - Study Population Characteristics
A goal of this initiative is to support studies that lead to findings that are applicable to all people affected by the rare disease being studied. Therefore, it is important that the sex/gender, race, ethnicity, and age of the participants appropriately represent the population of people living with the studied condition in the U.S. Applications may request personnel effort, support for study participant travel/meals and other budget items within the overall budget cap to ensure that this goal of appropriate inclusion is met.
In sections 2.4 Inclusion of Women and Minorities and 2.5 Recruitment and Retention Plan of the Human Subject form applications must address the following points in addition to the G.500 instructions.
2.4 Inclusion of Women and Minorities
- Provide data if available on the demographics of individuals affected by the condition under study in the catchment area for the clinic sites proposed in the application.
- Provide annual targets for enrollment including numbers by sex/gender, race, and ethnicity.
- Identify the person/people in the research team that will carry out the proposed outreach and their qualifications or relevant abilities such as fluency in languages other than English and/or cultural sensitivity.
2.5 Recruitment and Retention Plan
- Broaden the eligibility criteria as appropriate so as not to exclude potential participants for reasons that are unlikely to affect the outcomes of the study.
- Minimize the burden of participating in the study by reducing the frequency and/or duration of clinic visits and overall time required.
- Select study sites with ample numbers and diversity of potential study participants.
- Select study sites that minimize the travel of study participants.
- Provide support for study participant transportation, accommodations and parking as needed.
- Provide daycare for family members during study visits.
- Allow for clinic visits in evenings or during weekends.
- Integrate remote data collection such as smartphone apps or wearables into the study design while also taking into consideration the need for access to broadband communication networks in rural areas.
- Establish recruitment, enrollment and/or data collection sites in the community at locations that are convenient, familiar, and trusted by potential study participants.
- Have validated translations of consent forms and other relevant study documents available in languages that help ensure achievement of the planned enrollment.
- Include study personnel who are bilingual and culturally sensitive to the planned enrollment population. Consider enlisting the help of community ambassadors to build trust in the communities of potential study participants.
- In addition to the primary plan for recruiting sex/gender, racial, and ethnic group members, provide alternative/back-up strategies to be used if enrollment significantly deviates (more than 20% of any category for each annual milestone) from the planned numbers according to sex/gender, race, or ethnicity. Back-up plans may, for example, propose to add research sites with access to additional individuals that are understudied in the enrollment.
- Describe what other research studies may be competing for recruitment of the same patient population at the same clinic sites that are proposed in the application. Describe plans for communicating to potential study participants the options available to them.
Section 4 - Protocol Synopsis
4.3 Statistical Design and Power
Applicants should provide a Statistical Analysis Plan (SAP) for the proposed research project(s). This may include details on the analyses specified in the study protocol, including a description of how the statistical analysis of the primary, secondary and other endpoints will be performed, how the sample size was determined, how missing data will be handled, plans for interim analyses for safety, etc.
Delayed Onset Study
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start). All instructions in the How to Apply- Application Guide must be followed.
PHS Assignment Request Form (Overall)
All instructions in the How to Apply- Application Guide must be followed.
Administrative Core
When preparing your application, use Component Type ‘Admin Core.’
All instructions in the SF424 (R&R) Application Guide must be followed, with the following additional instructions, as noted.
SF424 (R&R) Cover (Administrative Core)
Complete only the following fields:
- Applicant Information
- Type of Applicant (optional)
- Descriptive Title of Applicant’s Project
- Proposed Project Start/Ending Dates
PHS 398 Cover Page Supplement (Administrative Core)
Enter Human Embryonic Stem Cells in each relevant component.
Research & Related Other Project Information (Administrative Core)
Human Subjects: Answer only the ‘Are Human Subjects Involved?’ and 'Is the Project Exempt from Federal regulations?’ questions.
Vertebrate Animals: Answer only the ‘Are Vertebrate Animals Used?’ question.
Project Narrative: Do not complete. Note: ASSIST screens will show an asterisk for this attachment indicating it is required. However, eRA systems only enforce this requirement in the Overall component and applications will not receive an error if omitted in other components.
Project /Performance Site Location(s) (Administrative Core)
List all performance sites that apply to the specific component.
Note: The Project Performance Site form allows up to 300 sites, prior to using additional attachment for additional entries.
Research & Related Senior/Key Person Profile (Administrative Core)
- In the Project Director/Principal Investigator section of the form, use Project Role of ‘Other’ with Category of ‘Project Lead’ and provide a valid eRA Commons ID in the Credential field.
- In the additional Senior/Key Profiles section, list Senior/Key persons that are working in the component.
- Include a single Biographical Sketch for each Senior/Key person listed in the application regardless of the number of components in which they participate. When a Senior/Key person is listed in multiple components, the Biographical Sketch can be included in any one component.
- If more than 100 Senior/Key persons are included in a component, the Additional Senior Key Person attachments should be used.
Budget (Administrative Core)
Budget forms appropriate for the specific component will be included in the application package.
The Administrative Core budget should include travel for the RDCRC PD(s)/PI(s) and one Scholar to attend an annual two-day in-person meeting in the Washington DC metro area.
Note: The R&R Budget form included in many of the component types allows for up to 100 Senior/Key Persons in section A and 100 Equipment Items in section C prior to using attachments for additional entries. All other SF424 (R&R) instructions apply.
PHS 398 Research Plan (Administrative Core)
Specific Aims: The RDCRC Administrative Core is responsible for the overall administration of the RDCRC (including policy, procedure, and funds allocation).
Research Strategy:
- Describe the Administrative Core Team's leadership experience with multisite clinical research programs and how it will adequately provide for administrative management.
- Applicants are encouraged to form the strongest teams possible to promote strategies to ensure a robust scientific approach, which can include both domestic and international sites and investigators. Because close partnership with the patients and stakeholders has been shown to be critical to the success of the RDCRCs, applicants are encouraged to include patient advocates in the leadership team of the Administrative Core.
- Describe plans for the organization and administrative management of the RDCRC. The plan should include management of day-to-day activities, establishing policies and procedures that dovetail with those of the RDCRN, funds allocation, and establishing contracts with all participating RDCRC sites, monitoring progress of RDCRC program milestones.
- Describe the skills and experience of the Administrative Core PI and how it is related to the management of multisite clinical research programs and rare disease research.
- Describe the skills and experience of the Administrative Coordinator to assist the RDCRC PD(s)/PI(s) and Administrative Core PI with the day-to-day administrative details and program coordination.
- Describe a plan for communication (meetings, conference calls etc.) and participation of all personnel within the RDCRC. Provide details of how the activities and contribution of the collaborating investigators, institutions and patient advocacy groups will be coordinated.
- Describe how the Administrative Core will work collaboratively with the DMCC Cores (Administrative Core, Data Management Core, Clinical Research Core, and Engagement and Dissemination Core) to coordinate and support both RDCRC activities and RDCRN-wide efforts (e.g., develop and monitor best practices for clinical and research data handling and use as well as data sharing).
- Describe how the Administrative Core will coordinate and manage regulatory and clinical documents in collaboration with the NIH and the DMCC (e.g., Investigational New Drug Application (IND), single Institutional Review Board (IRB), clinical protocols, consent forms). Describe how the Administrative Core will manage and monitor quality control of ongoing research projects.
- Describe how the Administrative Core will work collaboratively with the DMCC to develop and utilize clinical trial readiness metrics for both regulatory requirements and clinical research components to evaluate their progress in clinical trial readiness.
- Describe how the Administrative Core will coordinate data and biospecimen sharing and storage across all clinical sites.
- Describe how the Administrative Core will coordinate with the DMCC to develop and maintain a RDCRC communication plan.
- Describe how the Administrative Core will establish data sharing and data use agreements within the RDCRC for all clinical sites, and between the primary recipient institute and the DMCC.
- Describe how the Administrative Core will collaborate with the DMCC to establish and promulgate RDCRN data management and sharing standards and policies within the RDCRC.
- Describe how the Administrative Core will collaborate with the DMCC to index biosamples and other resources within the RDCRC in a "virtual repository" management system.
- Identify the individual(s) with statistical expertise in rare disease research (e.g., small sample size power analysis, analysis of longitudinal data, qualitative studies, innovative study design, platform clinical trials) who will coordinate and leverage DMCC core support.
- Identify the individual(s) with bioinformatic expertise who will collaborate with the DMCC to facilitate the use of research tools (e.g., RedCap, Ambra, Complion) within the RDCRC.
- Describe the plan for promoting awareness of disorders within the RDCRC research program to the scientific, clinical, and patient/stakeholder communities in collaboration with the RDCRN.
- If working groups will be established, indicate their specific functions, composition and to whom they report.
- Describe how an EAC consisting of scientific, clinical, and patient group representation that will be composed of at least five members will be established and will function. Applicants without an existing EAC should describe their plans for constituting an EAC but should not specify names and should not contact potential EAC members in advance of review of the application.
Letters of Support: Any letters of support should be included in the Overall Component.
Appendix:
Only limited items are allowed in the Appendix. Follow all instructions for the Appendix as described in the SF424 (R&R) Application Guide; any instructions provided here are in addition to the SF424 (R&R) Application Guide instructions.
PHS Human Subjects and Clinical Trials Information (Administrative Core)
When involving human subjects research, clinical research, and/or NIH-defined clinical trials follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the SF424 (R&R) Application Guide, with the following additional instructions:
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R&R Other Project Information form, you must include at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or a Delayed Onset Study record.
Study Record: PHS Human Subjects and Clinical Trials Information
All instructions in the SF424 (R&R) Application Guide must be followed.
Delayed Onset Study
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start).All instructions in the SF424 (R&R) Application Guide must be followed.
Pilot/Feasibility Governance Core
When preparing your application, use Component Type ‘Pilot/Feasibility.’
SF424 (R&R) Cover (Pilot/Feasibility Governance Core)
PHS 398 Cover Page Supplement (Pilot/Feasibility Governance Core)
Research & Related Other Project Information (Pilot/Feasibility Governance Core)
Project Narrative: Do not complete. Note: ASSIST screens will show an asterisk for this attachment indicating it is required. However, eRA systems only enforce this requirement in the Overall component and applications will not receive an error if omitted in other components.
Project /Performance Site Location(s) (Pilot/Feasibility Governance Core)
Research & Related Senior/Key Person Profile (Pilot/Feasibility Governance Core)
Budget (Pilot/Feasibility Governance Core
PHS 398 Research Plan (Pilot/Feasibility Governance Core)
Specific Aims: State the goals of the Pilot/Feasibility Governance Core concisely and summarize the expected outcome(s).
- Describe in detail how the pilot/feasibility core will organize and administer the process for selecting pilot and/or feasibility project establishment. Specific pilot/feasibility projects must not be described in the current application and if described will not be reviewed.
- Pilot projects should be at least 1 year but not more than 2 years in duration. If 2-year awards are allowed, the second year must be contingent on year 1 progress. No new pilot/feasibility projects may be initiated in the final year of the award.
- Clearly describe the solicitation processes, including multiple avenues of outreach for projects. Describe the eligibility criteria for the investigators and the scope of projects that are being sought.
- Have scientific merit.
- Are related to the overall goals and activities of the RDCRC.
- Have the potential to advance the field of research.
- Leverage existing resources and infrastructure within the RDCRC and RDCRN and the corresponding rare disease community, if available.
- Describe funding caps for individual pilot/feasibility projects, including any planned caps on F&A costs.
- Describe how diversity will be addressed in the review process.
- Describe the selection process, including any involvement of the assigned NIH PS.
- The process for ensuring that NIH prior approval requirements are met.
- The process for issuing new subawards if pilot recipients are not already at a RDCRC institution.
- Reporting pilot awards to the DMCC.
- How the progress of the pilot/feasibility projects will be evaluated for consideration for a second year of support (if applicable).
Letters of Support : Any letters of support should be included in the Overall Component.
Only limited items are allowed in the Appendix. Follow all instructions for the Appendix as described in the SF424 (R&R) Application Guide; any instructions provided here are in addition to the SF424 (R&R) Application Guide instructions.
PHS Human Subjects and Clinical Trials Information (Pilot/Feasibility Governance Core)
Career Enhancement Core
When preparing your application, use Component Type ‘Career Enhancement.’
SF424 (R&R) Cover (Career Enhancement Core)
PHS 398 Cover Page Supplement (Career Enhancement Core)
Research & Related Other Project Information (Career Enhancement Core)
Project Narrative: Do not complete. Note: ASSIST screens will show an asterisk for this attachment indicating it is required. However, eRA systems only enforce this requirement in the Overall component and applications will not receive an error if omitted in other components.
Project /Performance Site Location(s) (Career Enhancement Core)
Research & Related Senior/Key Person Profile (Career Enhancement Core)
Budget (Career Enhancement Core)
The Career Enhancement Core is limited to $100,000 direct costs per year. Award recipients may choose to leverage other sources for additional support; however, NIH will not provide support greater than $100,000 direct costs per year for this activity.
PHS 398 Research Plan (Career Enhancement Core)
Specific Aims: State the goals of the proposed Career Enhancement Core concisely and summarize the expected outcome(s).
Program Leadership and Administration:
- Describe the strengths, leadership and administrative skills, career enhancement experience, scientific expertise, and active research of the Career Enhancement Core Leader. Describe the Core Leader’s commitment and track record of training and career development of women, under-represented minorities, and people with disabilities. Relate these strengths to the proposed management of the Career Enhancement Program.
- Describe the planned strategy and administrative structure to be used to oversee and monitor the Career Enhancement Program.
Program Faculty:
- Describe the RDCRC faculty who will be available to serve as preceptors/mentors and provide guidance and expertise appropriate to the level of Career Enhancement candidates proposed in the application.
- Describe the complementary expertise and experiences of the proposed RDCRC Faculty, including active research and other scholarly activities in which the faculty are engaged, as well as experience mentoring and career enhancement individuals at the proposed career stage(s).
Proposed Career Enhancements:
- Provide an overview (objectives, design, and direction) of the proposed Career Enhancement Program aimed at the career enhancement of new researchers for the rare diseases field and development of future research leaders. Career enhancement activities that go beyond the RDCRC to enhance the experiences of Career Enhancement candidate(s) in other laboratories or at other institutions are also encouraged.
- Describe how the Career Enhancement candidate(s) will be prepared to address technical challenges unique to clinical research with rare diseases, such as partnering with patients, stakeholders, and/or multidisciplinary teams to leverage existing resources. Describe training in clinical trial design and statistical approaches that address the challenges of small sample sizes common to rare diseases.
- Describe program activities intended to develop the working knowledge needed for Career Enhancement candidate(s) to select among and prepare for the next step in varied career options available in the rare disease workforce.
- The Career Enhancement Program should promote support of scholars and fellows from other public or private funding organizations. Describe strategies to help publicize the availability of fellowships, train junior/new investigators to apply for fellowships and provide feedback on their applications. Track the success of fellowship applications and report annual progress on these activities.
- The Career Enhancement Program may propose activities that enhance the environment through specialized coursework, a seminar program, retreats for presentation of Career Enhancement candidate(s) research, journal clubs or other activities that contribute to the preparation of investigators for careers in rare diseases research. Explore ways to publicize and provide access to these activities to scholars and fellows within the RDCRC’s institutions, at other RDCRCs and other institutions. Exposure to research at other RDCRCs is also encouraged through exchange programs, short-term career enhancement opportunities or visits to learn new research approaches.
Career Enhancement Candidates:
- The Career Enhancement Core could provide direct support for scholars/fellows, leverage existing training programs (e.g., Institutional Training or Career Development Awards; T32, T35, K12) and/or facilitate funding of fellowships from other public or private funding organizations. If direct support is proposed, describe the nomination and selection process to be used to select candidates who would be supported by funds from the Career Enhancement Core.
- Applicants are encouraged to place a high priority on the recruitment of Career Enhancement candidate(s) with clinical expertise due to the significant need for well-trained clinical researchers in the rare diseases field. Describe strategies to recruit students/fellows with clinical training; for example, outreach/presentations to medical school classes or summer fellowship opportunities. Do not name prospective Career Enhancement candidate(s) in the application or contact them in advance of the review.
- Describe for whom the Career Enhancement Program is intended, including the career enhancement level(s) of the Career Enhancement candidate(s) the academic and research background needed to pursue the proposed career enhancement, and, as appropriate, plans to accommodate differences in preparation among Career Enhancement candidate(s).
Institutional Environment:
- The sponsoring institution must assure support for the career enhancement environment of the RDCRC as proposed in the Career Enhancement Core, including assurance that sufficient time will be allowed for the Career Enhancement Core Leader and other RDCRC Faculty to contribute to the proposed career enhancement.
- Describe existing research career enhancement, student development, or career development programs in which the RDCRC faculty are eligible to participate as mentors. Differentiate the proposed Career Enhancement Program from existing career enhancement programs at the same Career Enhancement candidate(s) level, and explain how the programs will synergize, if applicable, whether Career Enhancement candidate(s) are expected to transition from one support program to another, and how the career enhancement faculty, pool of potential Career Enhancement candidate(s), and resources are sufficiently robust to support the proposed Career Enhancement Program in addition to existing career enhancement programs.
Program Evaluation:
- Describe a plan to review and determine the quality and effectiveness of the Career Enhancement Program.
For renewal applications:
- Provide aggregate data that demonstrates the impact of Career Enhancement activities on participant's professional development. Aggregate data should address groups identified as underrepresented in the biomedical and clinical sciences, examples of their accomplishments while supported by the RDCRC and how RDCRC support has enhanced their careers in rare disease research.
- Highlight how Career Enhancement activities have evolved in response to changes in relevant scientific and technical knowledge, educational practices, and to evaluation of the Career Enhancement Program.
Letters of Support: Any letters of support should be included in the Overall Component.
PHS Human Subjects and Clinical Trials Information (Career Enhancement Core)
Clinical Research Projects
When preparing your application, use Component Type ‘Clinical Research Projects.’
SF424 (R&R) Cover (Clinical Research Projects)
PHS 398 Cover Page Supplement (Clinical Research Projects)
Research & Related Other Project Information (Clinical Research Projects)
Project /Performance Site Location(s) (Clinical Research Projects)
Research & Related Senior/Key Person Profile (Clinical Research Projects)
Budget (Clinical Research Projects)
PHS 398 Research Plan (Clinical Research Projects)
Specific Aims: State the goals of the proposed Clinical Research Projects concisely and summarize the expected outcome(s).
- Clearly describe the hypothesis or hypotheses to be tested for the project and explain its relevance to the central theme of the RDCRC.
- Describe how the individual clinical research project's achievement will address the central objectives of the RDCRC.
- Highlight why the implementation of the project will be facilitated by a RDCRC environment.
- Specify the overall biomedical significance of the work proposed.
- Specify the gaps filled by each project in the long-term goal of advancing early diagnosis and treatments for the targeted rare disease/disorder/syndrome/condition.
- Describe how the perspectives of patients and stakeholders will be included in the work proposed.
- Describe the source of common data elements that will be used in the study and how they will ensure comparability with other clinical research and clinical trials (e.g., NIH CDE ( https://cde.nlm.nih.gov/home ), Longitudinal Pediatric Data Resource, NINDS CDEs ( https://commondataelements.ninds.nih.gov/ )).
If a clinical research project includes an NIH-defined clinical trial:
- Describe the potential value of the study and the feasibility of successfully completing the study within the duration of the award, including the preclinical rationale.
- Provide evidence that the rigor of preclinical efficacy studies and the level of effect of the agent are both sufficient to warrant clinical testing of the agent.
- Describe how regulatory requirements will be met in a timely manner.
- Indicate drug/biologic availability for use in a trial document agreement of all participating clinical/corporate partners.
If clinical trials involving the testing of new investigational therapeutics, new indications for FDA-approved drugs, or other medical interventions under a research protocol will be performed indicate:
- Status of IND/IDE or timeline for obtaining an IND
- Name and organization of the IND/IDE holder
- Date the IND/IDE was filed with the FDA
- FDA IND/IDE number
- Any comments from the FDA regarding this protocol
Appendix :
PHS Human Subjects and Clinical Trials Information (Clinical Research Projects)
All instructions in the SF424 (R&R) Application Guide must be followed
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start).All instructions in the SF424 (R&R) Application Guide must be followed
Foreign Institutions
Foreign (non-U.S.) institutions must follow policies described in the NIH Grants Policy Statement , and procedures for foreign institutions described throughout the SF424 (R&R) Application Guide.
3. Unique Entity Identifier and System for Award Management (SAM)
See Part 2. Section III.1 for information regarding the requirement for obtaining a unique entity identifier and for completing and maintaining active registrations in System for Award Management (SAM), NATO Commercial and Government Entity (NCAGE) Code (if applicable), eRA Commons, and Grants.gov
4. Submission Dates and Times
Part I. contains information about Key Dates and times. Applicants are encouraged to submit applications before the due date to ensure they have time to make any application corrections that might be necessary for successful submission. When a submission date falls on a weekend or Federal holiday , the application deadline is automatically extended to the next business day.
Organizations must submit applications to Grants.gov (the online portal to find and apply for grants across all Federal agencies) using ASSIST or other electronic submission systems. Applicants must then complete the submission process by tracking the status of the application in the eRA Commons , NIH’s electronic system for grants administration. NIH and Grants.gov systems check the application against many of the application instructions upon submission. Errors must be corrected and a changed/corrected application must be submitted to Grants.gov on or before the application due date and time. If a Changed/Corrected application is submitted after the deadline, the application will be considered late. Applications that miss the due date and time are subjected to the NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications .
Applicants are responsible for viewing their application before the due date in the eRA Commons to ensure accurate and successful submission.
Information on the submission process and a definition of on-time submission are provided in How to Apply- Application Guide.
5. Intergovernmental Review (E.O. 12372)
This initiative is not subject to intergovernmental review .
6. Funding Restrictions
All NIH awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Pre-award costs are allowable only as described in the NIH Grants Policy Statement Section 7.9.1 Selected Items of Cost.
Clinical trials involving the testing of new investigational therapeutics, new indications for FDA-approved drugs, or other medical interventions under a research protocol should be performed under an IND, unless otherwise agreed upon by the FDA. See Section IV.2 for additional guidance on projects that propose a clinical trial. If not exempt, the applicant must provide the NIH with the name and organization of the IND/IDE holder, the date the IND/IDE was filed with the FDA, the FDA IND/IDE number, and any comments from the FDA regarding this protocol. Studies will not be funded unless necessary regulatory approval has first been obtained; regulatory approval at the time of application is preferred.
7. Other Submission Requirements and Information
Applications must be submitted electronically following the instructions described in the How to Apply - Application Guide . Paper applications will not be accepted.
For information on how applications will be automatically assembled for review and funding consideration after submission, refer to: http://grants.nih.gov/grants/ElectronicReceipt/files/Electronic_Multi-project_Application_Image_Assembly.pdf .
Applicants must complete all required registrations before the application due date. Section III. Eligibility Information contains information about registration.
For assistance with your electronic application or for more information on the electronic submission process, visit How to Apply - Application Guide . If you encounter a system issue beyond your control that threatens your ability to complete the submission process on-time, you must follow the Dealing with System Issues guidance. For assistance with application submission, contact the Application Submission Contacts in Section VII.
Important reminders:
All PD(s)/PI(s) and component Project Leads must include their eRA Commons ID in the Credential field of the Senior/Key Person Profile form . Failure to register in the Commons and to include a valid PD/PI Commons ID in the credential field will prevent the successful submission of an electronic application to NIH.
The applicant organization must ensure that the unique entity identifier provided on the application is the same identifier used in the organization’s profile in the eRA Commons and for the System for Award Management. Additional information may be found in How to Apply - Application Guide .
See more tips for avoiding common errors.
Upon receipt, applications will be evaluated for completeness and compliance with application instructions by the Center for Scientific Review and responsiveness by components of participating organizations, NIH. Applications that are incomplete, non-compliant and/or nonresponsive will not be reviewed.
In order to expedite review, applicants are requested to notify the NCATS Referral Office by email at [email protected] when the application has been submitted. Please include the NOFO number and title, PD/PI name, and title of the application
Applications Involving the NIH Intramural Research Program
The requests by NIH intramural scientists will be limited to the incremental costs required for participation. As such, these requests will not include any salary and related fringe benefits for career, career conditional or other Federal employees (civilian or uniformed service) with permanent appointments under existing position ceilings or any costs related to administrative or facilities support (equivalent to Facilities and Administrative or F&A costs). These costs may include salary for staff to be specifically hired under a temporary appointment for the project, consultant costs, equipment, supplies, travel, and other items typically listed under Other Expenses. Applicants should indicate the number of person-months devoted to the project, even if no funds are requested for salary and fringe benefits.
If selected, appropriate funding will be provided by the NIH Intramural Program. NIH intramural scientists will participate in this program as PDs/PIs in accord with the Terms and Conditions provided in this NOFO. Intellectual property will be managed in accord with established policy of NIH in compliance with Executive Order 10096, as amended, 45 CFR Part 7; patent rights for inventions developed in NIH facilities are NIH property unless NIH waives its rights.
Should an extramural application include the collaboration with an intramural scientist, no funds for the support of the intramural scientist may be requested in the application. The intramural scientist may submit a separate request for intramural funding as described above.
Use of Common Data Elements in NIH-funded Research
Many NIH ICs encourage the use of common data elements (CDEs) in basic, clinical, and applied research, patient registries, and other human subject research to facilitate broader and more effective use of data and advance research across studies. CDEs are data elements that have been identified and defined for use in multiple data sets across different studies. Use of CDEs can facilitate data sharing and standardization to improve data quality and enable data integration from multiple studies and sources, including electronic health records. NIH ICs have identified CDEs for many clinical domains (e.g., neurological disease), types of studies (e.g., genome-wide association studies (GWAS)), types of outcomes (e.g., patient-reported outcomes), and patient registries (e.g., the Global Rare Diseases Patient Registry and Data Repository). NIH has established the NIH CDE Repository to assist investigators in identifying NIH-supported CDEs when developing protocols, case report forms, and other instruments for data collection. The Portal provides guidance about and access to NIH-supported CDE initiatives and other tools and resources for the appropriate use of CDEs and data standards in NIH-funded research. Investigators are encouraged to consult the Portal and describe in their applications any use they will make of NIH-supported CDEs in their projects.
Prior Consultation with Scientific/Research Staff
Consultation with the appropriate Institute or Center (IC) staff at least 10 weeks prior to the application due date is strongly encouraged for all applicants considering submission of the Rare Diseases Clinical Research Consortia (RDCRC) for Rare Diseases Clinical Research Network (U54 Clinical Trials Optional) application, including new applications. If requested, IC staff will consider whether the proposed clinical trial meets the goals and mission of the Institute/Center, and whether it addresses one or more high priority research areas. IC staff will not evaluate the technical and scientific merit of the proposed project; technical and scientific merit will be determined during peer review using the review criteria indicated in this NOFO. During the consultation phase, if the proposed RDCRC does not meet an IC's programmatic needs, applicants will be strongly encouraged to consider other funding opportunities.
Post Submission Materials
Applicants are required to follow the instructions for post-submission materials, as described in the policy . Any instructions provided here are in addition to the instructions in the policy.
Section V. Application Review Information
1. Criteria
Only the review criteria described below will be considered in the review process. Applications submitted to NIH in support of the NIH mission are evaluated for scientific and technical merit through the NIH peer review system.
A proposed Clinical Trial application may include study design, methods, and intervention that are not by themselves innovative but address important questions or unmet needs. Additionally, the results of the clinical trial may indicate that further clinical development of the intervention is unwarranted or lead to new avenues of scientific investigation.
Reviewers will provide an overall impact score to reflect their assessment of the likelihood for the project to exert a sustained, powerful influence on the research field(s) involved, in consideration of the following review criteria and additional review criteria (as applicable for the project proposed).
Reviewers will consider each of the review criteria below in the determination of scientific merit and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact. For example, a project that by its nature is not innovative may be essential to advance a field.
Significance
Does the project address an important problem or a critical barrier to progress in the field? Is the prior research that serves as the key support for the proposed project rigorous? If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field?
Specific to this NOFO:
- To what extent is there sufficient rationale provided for the rare diseases chosen? Consider whether these rare diseases can be reasonably grouped together to move the individual fields forward.
- To what extent do the clinical projects and cores demonstrate a focus on unmet research/clinical needs for the targeted rare diseases?
- To what extent will the proposed RDCRC accelerate progress toward effective treatments or other improvements in the lives of individuals with the targeted rare diseases through coordinated and collaborative research and infrastructure activities?
- To what extent are the clinical projects and cores individually meritorious and complementary to the overarching goals of the RDCRN? If successful, how might the proposed RDCRN advance understanding of the diseases, facilitating early diagnosis, improving clinical trial readiness, developing and testing therapies, advancing patient care and reducing disease burden?
- To what extent is there sufficient justification for the need for the RDCRN center infrastructure over completing the research via traditional grant mechanisms?
In addition, for applications involving clinical trials
Are the scientific rationale and need for a clinical trial to test the proposed hypothesis or intervention well supported by preliminary data, clinical and/or preclinical studies, or information in the literature or knowledge of biological mechanisms? For trials focusing on clinical or public health endpoints, is this clinical trial necessary for testing the safety, efficacy or effectiveness of an intervention that could lead to a change in clinical practice, community behaviors or health care policy? For trials focusing on mechanistic, behavioral, physiological, biochemical, or other biomedical endpoints, is this trial needed to advance scientific understanding?
Investigator(s)
Are the PD(s)/PI(s), collaborators, and other researchers well suited to the project? If Early Stage Investigators or those in the early stages of independent careers, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If the project is collaborative or multi-PD/PI, do the investigators have complementary and integrated expertise; are their leadership approach, governance and organizational structure appropriate for the project?
- To what extent is the PD(s)/PI(s) rare disease expertise and experience appropriate for managing large multi-component clinical research programs in rare diseases addressed?
With regard to the proposed leadership for the project, do the PD/PI(s) and key personnel have the expertise, experience, and ability to organize, manage and implement the proposed clinical trial and meet milestones and timelines? Do they have appropriate expertise in study coordination, data management and statistics? For a multicenter trial, is the organizational structure appropriate and does the application identify a core of potential center investigators and staffing for a coordinating center?
Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation, or interventions? Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense? Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, instrumentation, or interventions proposed?
Does the design/research plan include innovative elements, as appropriate, that enhance its sensitivity, potential for information or potential to advance scientific knowledge or clinical practice?
Are the overall strategy, methodology, and analyses well-reasoned and appropriate to accomplish the specific aims of the project? Have the investigators included plans to address weaknesses in the rigor of prior research that serves as the key support for the proposed project? Have the investigators presented strategies to ensure a robust and unbiased approach, as appropriate for the work proposed? Are potential problems, alternative strategies, and benchmarks for success presented? If the project is in the early stages of development, will the strategy establish feasibility and will particularly risky aspects be managed? Have the investigators presented adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects?
If the project involves human subjects and/or NIH-defined clinical research, are the plans to address:
1) the protection of human subjects from research risks, and 2) inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion or exclusion of individuals of all ages (including children and older adults), justified in terms of the scientific goals and research strategy proposed?
- To what extent are patient and stakeholder experiences, needs and priorities meaningfully incorporated as partners into decisions and activities of the RDCRC?
- To what extent are the plans to work with the DMCC and leverage the shared network tools provided by the NIH appropriate?
- To what extent are the plans for the RDCRC to collaborate and otherwise contribute to the RDCRN meaningful?
- To what extent are the plans adequate to ensure and maintain collaborative relationships across multiple research sites and the RDCRC?
Does the application adequately address the following, if applicable
Study Design
Is the study design justified and appropriate to address primary and secondary outcome variable(s)/endpoints that will be clear, informative and relevant to the hypothesis being tested? Is the scientific rationale/premise of the study based on previously well-designed preclinical and/or clinical research? Given the methods used to assign participants and deliver interventions, is the study design adequately powered to answer the research question(s), test the proposed hypothesis/hypotheses, and provide interpretable results? Is the trial appropriately designed to conduct the research efficiently? Are the study populations (size, gender, age, demographic group), proposed intervention arms/dose, and duration of the trial, appropriate and well justified?
Are potential ethical issues adequately addressed? Is the process for obtaining informed consent or assent appropriate? Is the eligible population available? Are the plans for recruitment outreach, enrollment, retention, handling dropouts, missed visits, and losses to follow-up appropriate to ensure robust data collection? Are the planned recruitment timelines feasible and is the plan to monitor accrual adequate? Has the need for randomization (or not), masking (if appropriate), controls, and inclusion/exclusion criteria been addressed? Are differences addressed, if applicable, in the intervention effect due to sex/gender and race/ethnicity?
Are the plans to standardize, assure quality of, and monitor adherence to, the trial protocol and data collection or distribution guidelines appropriate? Is there a plan to obtain required study agent(s)? Does the application propose to use existing available resources, as applicable?
Data Management and Statistical Analysis
Are planned analyses and statistical approach appropriate for the proposed study design and methods used to assign participants and deliver interventions? Are the procedures for data management and quality control of data adequate at clinical site(s) or at center laboratories, as applicable? Have the methods for standardization of procedures for data management to assess the effect of the intervention and quality control been addressed? Is there a plan to complete data analysis within the proposed period of the award?
Environment
Will the scientific environment in which the work will be done contribute to the probability of success? Are the institutional support, equipment and other physical resources available to the investigators adequate for the project proposed? Will the project benefit from unique features of the scientific environment, subject populations, or collaborative arrangements?
- To what extent is the rationale for choosing clinical sites adequate and justified? To what extent does the overall RDCRC include a diverse set of sites?
- To what extent is the institutional commitment appropriate for supporting a rare diseases research center?
If proposed, are the administrative, data coordinating, enrollment and laboratory/testing centers, appropriate for the trial proposed?
Does the application adequately address the capability and ability to conduct the trial at the proposed site(s) or centers? Are the plans to add or drop enrollment centers, as needed, appropriate?
If international site(s) is/are proposed, does the application adequately address the complexity of executing the clinical trial?
If multi-sites/centers, is there evidence of the ability of the individual site or center to: (1) enroll the proposed numbers; (2) adhere to the protocol; (3) collect and transmit data in an accurate and timely fashion; and, (4) operate within the proposed organizational structure?
Scored Review Criteria - CORES
As applicable for the Pilot/Feasibility Governance and Career Enhancement Cores, reviewers will provide an assessment of its strengths and weaknesses and provide a score of acceptable, acceptable with concerns, or unacceptable. The following items should be evaluated while determining scientific and technical merit, and in providing an overall Impact Score for the Core.
Administrative Core
Review Criteria for the Administrative Core
Significance:
- To what extent will the Administrative Core promote strategies to ensure robust and impactful scientific outcomes for the RDCRC?
Team:
- To what extent do the skills and experience of the Administrative Core PI provide the needed knowledge and experience to facilitate the requirements of the Administrative Core?
- To what extent do the skills and experience of the proposed Administrative Coordinator provide them with the ability to assist the RDCRC PD(s)/PI(s) and the Administrative Core PI with day-to-day administrative details and program coordination?
- To what extent does the leadership experience and expertise provide the Administrative Core with the management and coordination skills needed for success for a large RDCRC?
- To what extent is there sufficient collaboration between the bioinformatics and statistical staff, and the DMCC, to ensure appropriate access to the DMCC resources described?
- To what extent is there appropriate effort and expertise for the Administrative Core team to support the RDCRC, including statistical and informatics expertise in rare disease research?
Communication:
- To what extent is there an appropriate plan for promoting awareness of disorders within the RDCRC to the scientific, clinical, and patient/stakeholder communities?
- To what extent will there be coordination with the DMCC for coordinating outreach and communication?
- How effectively will the communication plan ensure participation and coordination between the collaborating investigators, institutions, patient advocacy group(s) and the DMCC?
Approach:
- To what extent does the Administrative Core make adequate provisions to meet the needs of the RDCRC and to work collaboratively with the RDCRN DMCC?
- The management of day-to-day activities.
- Establishing policies and procedures that dovetail with those of the RDCRN.
- Funds allocation and establishing contracts with all participating RDCRC sites.
- Monitoring progress of RDCRC program milestones.
- Considering both RDCRC activities and RDCRN activities, to what extent is the collaboration with the DMCC Cores sufficient to integrate the RDCRC into the network?
- To what extent will the Administrative Core collaborate with the DMCC on the management and coordination of regulatory and clinical documents? Is the proposed timeline for having all agreements with all RDCRC clinical sites in place acceptable and timely? Is there an appropriate timeline for having data use and data sharing agreements in place with the DMCC and is the plan acceptable and timely? Are there appropriate plans for monitoring the quality of ongoing research?
- To what extent will the Administrative Core collaborate with the DMCC to develop and utilize clinical trial readiness metrics for both regulatory requirements and clinical research components to evaluate their progress in clinical trial readiness?
- Facilitate an environment that provides equitable access to information and resources to all participants?
- Promulgate data management and sharing standards and policies across the RDCRC?
- Include the use of RDCRN standardized data sharing and data use agreements?
- Index biosamples and other resources within the RDCRC in a "virtual repository" management system?
- To what extent is the institutional environment, including institutional support, appropriate?
Pilot/Feasibility Governance Core
Review Criteria for the Pilot/Feasibility Governance Core
- To what extent is the strategy for soliciting and selecting pilot/feasibility projects clearly described? How will the applicant ensure that the solicitation, review, and selection processes are rigorous and unbiased?
- To what extent does the application adequately describe the plan for ensuring that the proposed projects have scientific merit, relationship to the overall goals and activities of the RDCRC, potential to advance the field of research, leverage of existing resources and infrastructure, address diversity and the selection process?
- To what extent does the application adequately address how recipients will award and oversee pilot/feasibility projects, including how they will ensure that NIH prior approval requirements are met, how new subawards will be issued if needed, how awards will be reported to the DMCC, and how the progress of projects will be evaluated for a second year of funding (if applicable)?
Career Enhancement Core
Review Criteria for the Career Enhancement Core
- To what extent is the Career Enhancement Program administration, including the qualifications of the core leader and the proposed oversight and monitoring, appropriate?
- To what extent are the proposed faculty appropriate for the Career Enhancement candidate(s) and do they have a strong record of research and mentoring?
- To what extent are the proposed career enhancement activities, including those unique to clinical research with rare diseases and those focused on workforce development, appropriate?
- If strategies are proposed to promote fellowship funding from other public/provide funding organizations, are the plans for facilitating grant writing, refining applications, tracking, and reporting on fellowship success well considered and described?
- If direct support for Career Enhancement candidate(s) is proposed, to what extent is the nomination and selection process appropriate for the proposed Career Enhancement Program?
- To what extent is the institutional environment, including the institutional support and the proposed interaction with existing Career Enhancement Programs, appropriate?
- To what extent is the plan to review and determine the quality and effectiveness of the Career Enhancement Program adequate?
- For renewal applications, to what extent has the program been successful in meeting the goals of the Career Enhancement Program?
Scored Review Criteria - Clinical Research Projects
Reviewers will consider each of the review criteria below in the determination of scientific merit and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact. For example, a project that by its nature is not innovative may be essential to advance a field.
Significance Does the project address an important problem or a critical barrier to progress in the field? Is the prior research that serves as the key support for the proposed project rigorous? If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field?
Specific to this NOFO:
- To what extent does the research project target gaps to be filled for advancing early diagnosis and treatments for the targeted rare disease/disorder/syndrome/condition?
In addition, for applications involving clinical trials Are the scientific rationale and need for a clinical trial to test the proposed hypothesis or intervention well supported by preliminary data, clinical and/or preclinical studies, or information in the literature or knowledge of biological mechanisms? For trials focusing on clinical or public health endpoints, is this clinical trial necessary for testing the safety, efficacy or effectiveness of an intervention that could lead to a change in clinical practice, community behaviors or health care policy? For trials focusing on mechanistic, behavioral, physiological, biochemical, or other biomedical endpoints, is this trial needed to advance scientific understanding?
Investigator(s) Are the PD(s)/PI(s), collaborators, and other researchers well suited to the project? If Early-Stage Investigators or those in the early stages of independent careers, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If the project is collaborative or multi-PD/PI, do the investigators have complementary and integrated expertise; are their leadership approach, governance, and organizational structure appropriate for the project?
- To what extent does the investigative team at each clinical site have appropriate experience in the field of rare disease research and expertise required to conduct the proposed research?
In addition, for applications involving clinical trials With regard to the proposed leadership for the project, do the PD/PI(s) and key personnel have the expertise, experience, and ability to organize, manage and implement the proposed clinical trial and meet trial specific milestones and timelines? Do they have appropriate expertise in study coordination, data management and statistics? For a multicenter trial, is the organizational structure appropriate and does the application identify a core of potential center investigators and staffing for a coordinating center?
Innovation Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation, or interventions? Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense? Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, instrumentation, or interventions proposed?
In addition, for applications involving clinical trials Does the design/research plan include innovative elements, as appropriate, that enhance its sensitivity, potential for information or potential to advance scientific knowledge or clinical practice?
Approach Are the overall strategy, methodology, and analyses well-reasoned and appropriate to accomplish the specific aims of the project? Have investigators included plans to address weaknesses in the rigor of prior research that serves as the key support for the proposed project? Have the investigators presented strategies to ensure a robust and unbiased approach, as appropriate for the work proposed? Are potential problems, alternative strategies, and benchmarks for success presented? If the project is in the early stages of development, will the strategy establish feasibility, and will particularly risky aspects be managed? Have the investigators presented adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects?
If the project involves human subjects and/or NIH-defined clinical research, are the plans to address: 1) the protection of human subjects from research risks, and 2) inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion or exclusion of individuals of all ages (including children and older adults), justified in terms of the scientific goals and research strategy proposed?
- To what extent are the approaches proposed appropriate for rare diseases research?
- To what extent is the study design(s) appropriate for rare diseases research?
- If applicable, to what extent are plans for statistical analysis appropriate for rare diseases research?
In addition, for applications involving clinical trials Does the application adequately address the following, if applicable? Study Design Is the study design justified and appropriate to address primary and secondary outcome variable(s)/endpoints that will be clear, informative, and relevant to the hypothesis being tested? Is the scientific rationale/premise of the study based on previously well-designed preclinical and/or clinical research? Given the methods used to assign participants and deliver interventions, is the study design adequately powered to answer the research question(s), test the proposed hypothesis/hypotheses, and provide interpretable results? Is the trial appropriately designed to conduct the research efficiently? Are the study populations (size, gender, age, demographic group), proposed intervention arms/dose, and duration of the trial, appropriate and well justified?
Are potential ethical issues adequately addressed? Is the process for obtaining informed consent or assent appropriate? Is the eligible population available? Are the plans for recruitment outreach, enrollment, retention, handling dropouts, missed visits, and losses to follow-up appropriate to ensure robust data collection? Are the planned recruitment timelines feasible and is the plan to monitor accrual adequate? Has the need for randomization (or not), masking (if appropriate), controls, and inclusion/exclusion criteria been addressed? Are differences addressed, if applicable, in the intervention effect due to sex/gender and race/ethnicity?
Are the plans to standardize, assure quality of, and monitor adherence to, the trial protocol and data collection or distribution guidelines appropriate? Is there a plan to obtain required study agent(s)? Does the application propose to use existing available resources, as applicable?
Data Management and Statistical Analysis Are planned analyses and statistical approach appropriate for the proposed study design and methods used to assign participants and deliver interventions? Are the procedures for data management and quality control of data adequate at clinical site(s) or at center laboratories, as applicable? Have the methods for standardization of procedures for data management to assess the effect of the intervention and quality control been addressed? Is there a plan to complete data analysis within the proposed period of the award?
Environment Will the scientific environment in which the work will be done contribute to the probability of success? Are the institutional support, equipment, and other physical resources available to the investigators adequate for the project proposed? Will the project benefit from unique features of the scientific environment, subject populations, or collaborative arrangements?
- Is the environment for the project conducive to completing the project in terms of access to the populations?
In addition, for applications involving clinical trials If proposed, are the administrative, data coordinating, enrollment, and laboratory/testing centers, appropriate for the trial proposed?
Does the application adequately address the capability and ability to conduct the trial at the proposed site(s) or centers? Are the plans to add or drop enrollment centers, as needed, appropriate?
If international site(s) is/are proposed, does the application adequately address the complexity of executing the clinical trial?
If multi-sites/centers, is there evidence of the ability of the individual site or center to: (1) enroll the proposed numbers; (2) adhere to the protocol; (3) collect and transmit data in an accurate and timely fashion; and (4) operate within the proposed organizational structure?
Additional Review Criteria - Overall, Clinical Research Projects, and Cores
As applicable for the project proposed, reviewers will evaluate the following additional items while determining scientific and technical merit, and in providing an overall impact score, but will not give separate scores for these items.
Study Timeline
Specific to applications involving clinical trials
Is the study timeline described in detail, taking into account start-up activities, the anticipated rate of enrollment, and planned follow-up assessment? Is the projected timeline feasible and well justified? Does the project incorporate efficiencies and utilize existing resources (e.g., CTSAs, practice-based research networks, electronic medical records, administrative database, or patient registries) to increase the efficiency of participant enrollment and data collection, as appropriate?
Are potential challenges and corresponding solutions discussed (e.g., strategies that can be implemented in the event of enrollment shortfalls)?
Protections for Human Subjects
For research that involves human subjects but does not involve one of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate the justification for involvement of human subjects and the proposed protections from research risk relating to their participation according to the following five review criteria: 1) risk to subjects, 2) adequacy of protection against risks, 3) potential benefits to the subjects and others, 4) importance of the knowledge to be gained, and 5) data and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or more of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials. For additional information on review of the Human Subjects section, please refer to the Guidelines for the Review of Human Subjects .
Inclusion of Women, Minorities, and Individuals Across the Lifespan
When the proposed project involves human subjects and/or NIH-defined clinical research, the committee will evaluate the proposed plans for the inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion (or exclusion) of individuals of all ages (including children and older adults) to determine if it is justified in terms of the scientific goals and research strategy proposed. For additional information on review of the Inclusion section, please refer to the Guidelines for the Review of Inclusion in Clinical Research .
Vertebrate Animals
The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following three points: (1) a complete description of all proposed procedures including the species, strains, ages, sex, and total numbers of animals to be used; (2) justifications that the species is appropriate for the proposed research and why the research goals cannot be accomplished using an alternative non-animal model; and (3) interventions including analgesia, anesthesia, sedation, palliative care, and humane endpoints that will be used to limit any unavoidable discomfort, distress, pain and injury in the conduct of scientifically valuable research. Methods of euthanasia and justification for selected methods, if NOT consistent with the AVMA Guidelines for the Euthanasia of Animals, is also required but is found in a separate section of the application. For additional information on review of the Vertebrate Animals Section, please refer to the Worksheet for Review of the Vertebrate Animals Section.
Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
Resubmissions
For Renewals, the committee will consider the progress made in the last funding period. For consortia that have been previously funded, how well were the goals of the prior funding period achieved? How strong is the justification for continued support? What is the value of continuing ongoing natural history studies, and will these studies facilitate clinical trial readiness? How well have patient or stakeholder groups been included in previous and/or ongoing collaborations?
Additional Review Considerations - Overall, Clinical Research Projects and Cores
As applicable for the project proposed, reviewers will consider each of the following items, but will not give scores for these items, and should not consider them in providing an overall impact score.
Applications from Foreign Organizations
Select Agent Research
Reviewers will assess the information provided in this section of the application, including 1) the Select Agent(s) to be used in the proposed research, 2) the registration status of all entities where Select Agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of Select Agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the Select Agent(s).
Resource Sharing Plans
Reviewers will comment on whether the Resource Sharing Plan(s) (e.g., Sharing Model Organisms ) or the rationale for not sharing the resources, is reasonable.
Authentication of Key Biological and/or Chemical Resources:
For projects involving key biological and/or chemical resources, reviewers will comment on the brief plans proposed for identifying and ensuring the validity of those resources.
Budget and Period of Support
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
2. Review and Selection Process
Applications will be evaluated for scientific and technical merit by (an) appropriate Scientific Review Group(s) convened by the National Center for Advancing Translational Sciences, in accordance with NIH peer review policy and procedures , using the stated review criteria. Assignment to a Scientific Review Group will be shown in the eRA Commons.
As part of the scientific peer review, all applications will receive a written critique.
Applications may undergo a selection process in which only those applications deemed to have the highest scientific and technical merit (generally the top half of applications under review) will be discussed and assigned an overall impact score.
Applications will be assigned on the basis of established PHS referral guidelines to the appropriate NIH Institute or Center. Applications will compete for available funds with all other recommended applications submitted. Following initial peer review, recommended applications will receive a second level of review by the appropriate national Advisory Council or Board. The following will be considered in making funding decisions:
- Scientific and technical merit of the proposed project as determined by scientific peer review.
- Availability of funds.
- Relevance of the proposed project to program priorities.
3. Anticipated Announcement and Award Dates
After the peer review of the application is completed, the PD/PI will be able to access their Summary Statement (written critique) via the eRA Commons . Refer to Part 1 for dates for peer review, advisory council review, and earliest start date.
Information regarding the disposition of applications is available in the NIH Grants Policy Statement Section 2.4.4 Disposition of Applications .
Section VI. Award Administration Information
1. Award Notices
If the application is under consideration for funding, NIH will request "just-in-time" information from the applicant as described in the NIH Grants Policy Statement . This request is not a Notice of Award nor should it be construed to be an indicator of possible funding.
A formal notification in the form of a Notice of Award (NoA) will be provided to the applicant organization for successful applications. The NoA signed by the grants management officer is the authorizing document and will be sent via email to the recipient's business official.
Recipients must comply with any funding restrictions described in Section IV.6. Funding Restrictions. Selection of an application for award is not an authorization to begin performance. Any costs incurred before receipt of the NoA are at the recipient's risk. These costs may be reimbursed only to the extent considered allowable pre-award costs.
Any application awarded in response to this NOFO will be subject to terms and conditions found on the Award Conditions and Information for NIH Grants website. This includes any recent legislation and policy applicable to awards that is highlighted on this website.
Individual awards are based on the application submitted to, and as approved by, the NIH and are subject to the IC-specific terms and conditions identified in the NoA.
ClinicalTrials.gov: If an award provides for one or more clinical trials. By law (Title VIII, Section 801 of Public Law 110-85), the "responsible party" must register and submit results information for certain “applicable clinical trials” on the ClinicalTrials.gov Protocol Registration and Results System Information Website ( https://register.clinicaltrials.gov ). NIH expects registration and results reporting of all trials whether required under the law or not. For more information, see https://grants.nih.gov/policy/clinical-trials/reporting/index.htm
Institutional Review Board or Independent Ethics Committee Approval: Grantee institutions must ensure that all protocols are reviewed by their IRB or IEC. To help ensure the safety of participants enrolled in NIH-funded studies, the recipient must provide NIH copies of documents related to all major changes in the status of ongoing protocols.
Data and Safety Monitoring Requirements: The NIH policy for data and safety monitoring requires oversight and monitoring of all NIH-conducted or -supported human biomedical and behavioral intervention studies (clinical trials) to ensure the safety of participants and the validity and integrity of the data. Further information concerning these requirements is found at http://grants.nih.gov/grants/policy/hs/data_safety.htm and in the application instructions (SF424 (R&R) and PHS 398).
Investigational New Drug or Investigational Device Exemption Requirements: Consistent with federal regulations, clinical research projects involving the use of investigational therapeutics, vaccines, or other medical interventions (including licensed products and devices for a purpose other than that for which they were licensed) in humans under a research protocol must be performed under a Food and Drug Administration (FDA) investigational new drug (IND) or investigational device exemption (IDE).
Prior Approval of Pilot Projects
Recipient-selected projects that involve clinical trials or studies involving human subjects require prior approval by NIH prior to initiation.
- The recipient institution will comply with the NIH Guidance on Changes That Involve Human Subjects in Active Awards and That Will Require Prior NIH Approval .
- The recipient institution will provide NIH with specific plans for data and safety monitoring, and will notify the IRB and NIH of serious adverse events and unanticipated problems, consistent with NIH DSMP policies .
All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the NoA. For these terms of award, see the NIH Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards, Subpart A: General and Part II: Terms and Conditions of NIH Grant Awards, Subpart B: Terms and Conditions for Specific Types of Grants, Recipients, and Activities , including of note, but not limited to:
- Federal-wide Standard Terms and Conditions for Research Grants
- Prohibition on Certain Telecommunications and Video Surveillance Services or Equipment
- Acknowledgment of Federal Funding
If a recipient is successful and receives a Notice of Award, in accepting the award, the recipient agrees that any activities under the award are subject to all provisions currently in effect or implemented during the period of the award, other Department regulations and policies in effect at the time of the award, and applicable statutory provisions.
If a recipient receives and award, the recipient must follow all applicable nondiscrimination laws. The recipient agrees to this when registering in SAM.gov. The recipient must also submit an Assurance of Compliance ( HHS-690 ). To learn more, see the Laws and Regulations Enforced by the HHS Office for Civil Rights website .
HHS recognizes that NIH research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator’s scientific interest, funding limitations, recruitment requirements, and other considerations. Thus, criteria in research protocols that target or exclude certain populations are warranted where nondiscriminatory justifications establish that such criteria are appropriate with respect to the health or safety of the subjects, the scientific study design, or the purpose of the research. For additional guidance regarding how the provisions apply to NIH grant programs, please contact the Scientific/Research Contact that is identified in Section VII under Agency Contacts of this NOFO.
In accordance with the statutory provisions contained in Section 872 of the Duncan Hunter National Defense Authorization Act of Fiscal Year 2009 (Public Law 110-417), NIH awards will be subject to System for Award Management (SAM.gov) requirements. SAM.gov requires Federal agencies to review and consider information about an applicant in the designated integrity and performance system (currently SAM.gov) prior to making an award. An applicant can review and comment on any information in the responsibility/qualification records available in SAM.gov. NIH will consider any comments by the applicant, in addition to the information available in the responsibility/qualification records in SAM.gov, in making a judgement about the applicant’s integrity, business ethics, and record of performance under Federal awards when completing the review of risk posed by applicants as described in 2 CFR Part 200.206 “Federal awarding agency review of risk posed by applicants.” This provision will apply to all NIH grants and cooperative agreements except fellowships.
The following special terms of award are in addition to, and not in lieu of, otherwise applicable U.S. Office of Management and Budget (OMB) administrative guidelines, U.S. Department of Health and Human Services (HHS) grant administration regulations at 2 CFR Part 200, and other HHS, PHS, and NIH grant administration policies.
The administrative and funding instrument used for this program will be the cooperative agreement, an "assistance" mechanism (rather than an "acquisition" mechanism), in which substantial NIH programmatic involvement with the recipients is anticipated during the performance of the activities. Under the cooperative agreement, the NIH purpose is to support and stimulate the recipients' activities by involvement in and otherwise working jointly with the recipients in a partnership role; it is not to assume direction, prime responsibility, or a dominant role in the activities. Consistent with this concept, the dominant role and prime responsibility resides with the recipients for the project as a whole, although specific tasks and activities may be shared among the recipients and NIH as defined below.
The PD(s)/PI(s) will have the primary responsibility for:
- Defining objectives and approaches to plan, conduct, analyze, and publish results, interpretations, and conclusions of their studies.
- Serving as RDCRC Director(s) and responsible for the integration and management of activities within the RDCRC including ensuring data sharing and data use agreements are aligned with RDCRN requirements.
- Implementing a Local Executive Committee for day-to-day management of the RDCRC, and an External Advisory Committee, with scientific, clinical, and patient or stakeholder representation.
- Partnering with patient advocacy group(s) and ensuring that a representative from the patient advocacy group(s) represents the RDCRC on the CPAG steering committee.
- Attending two RDCRN meetings per year (one in-person and one virtual).
- Collaborating with the DMCC to use network resources and data standards.
- Ensuring clinical trials using RDCRC resources receive prior approval from the NIH awarding institute/center and are managed through an appropriate agreement between the RDCRC and funding source which requires all parties to adhere to NIH clinical trial policies and regulations.
- Participating in the overall coordination of NIH research efforts in Rare Diseases including collaboration and consultation with other NIH recipients, the appropriate sharing of information, data, and research materials, and participating in NIH efforts to standardize and harmonize pre-clinical and clinical data collection.
- Ensuring results of the research projects are published in a timely manner.
- Retaining custody of and having primary rights to the data and software developed under these awards, subject to Government rights of access consistent with current DHHS, PHS, and NIH policies.
NIH staff have substantial programmatic involvement that is above and beyond the normal stewardship role in awards, as described below:
One or more designated NIH program staff members will have substantial involvement as Project Scientists in the awards under this NOFO. The specific roles of the substantially involved NIH staff members include the following activities:
- Overseeing the activities of the RDCRC to ensure studies are properly conducted and completed in a timely manner.
- Serving as a non-voting member of the Network SC and/or CPAG SC.
- Providing status updates on other RDCRN activities that may be relevant to the individual RDCRC study goals or milestones.
- Assisting the NCATS RDCRN Program Director as well as the Network SC in avoiding unwarranted duplication of effort across the RDCRN.
- Monitoring the operations of the RDCRC for progress towards milestones and making recommendations on overall project directions.
- Informing the NCATS RDCRN Program Director as well as the Network SC of any challenges or delays in project progress.
- Ensuring proper collaboration between the RDCRC and the other members of the RDCRN.
- Enlisting additional technical experts as necessary from within the NIH, and other government agencies, to review scientific progress and administrative requirements to ensure compliance with NIH policies and procedures.
An agency PO will be responsible for the scientific and programmatic stewardship of the award and will be named in the award notice. The PO will be responsible for negotiating the final set of approved RDCRC award milestones and coordinating with the NCATS RDCRN Program Director on the RDCRN milestones.
Areas of Joint Responsibility include:
The RDCRN will consist of all the awarded RDCRCs, their affiliated patient advocacy groups, the DMCC, and NIH program representatives. All responsibilities are divided between recipients and NIH staff as described above.
Dispute Resolution:
Any disagreements that may arise in scientific or programmatic matters (within the scope of the award) between recipients and NIH may be brought to Dispute Resolution. A Dispute Resolution Panel composed of three members will be convened: a designee of the Steering Committee chosen without NIH staff voting, one NIH designee, and a third designee with expertise in the relevant area who is chosen by the other two; in the case of individual disagreement, the first member may be chosen by the individual recipient. This special dispute resolution procedure does not alter the recipient's right to appeal an adverse action that is otherwise appealable in accordance with PHS regulation 42 CFR Part 50, Subpart D and HHS regulation 45 CFR Part 16.
External Collaborations:
External collaborations may entail providing financial support; participating directly in a study; supplying study resources; or receiving special access to study results, data, findings, or intellectual property. External collaborations may concern only the original scope of the study or may extend to enlargements of the scope or ancillary additions through further procedures, data collection, or analyses, and may involve one, some, or all centers in a collaborative clinical trial.
Examples of types and mechanisms of external collaborations include:
- In-kind support of materials, e.g., drugs, devices, reagents.
- In-kind support of services, e.g., laboratory services.
- Financial support through a conditional gift to an NIH Gift Fund.
- Financial support through a charitable third party that works in collaboration with the NIH ICO and that manages, accounts for, and distributes funds to study investigators in accordance with ICO program plans.
- Financial support provided directly to an ICO supported investigator for use in accordance with study plans.
Any external collaboration must be governed by a research collaboration agreement (e.g., Clinical Trials Agreement (CTA), Research Collaboration Agreement (RCA), Memorandum of Understanding (MOU), etc.) with terms that ensure the collaboration is conducted in accordance with the Cooperative Agreement, applicable NIH policies and procedures.
Any external collaborator in the study, including but not limited to, access to any study data; study results; using the name of the study, is permitted only after concurrence by the PO who may consult with others at NIH including the Technology Advancement Office.
3. Data Management and Sharing
Consistent with the 2023 NIH Policy for Data Management and Sharing, when data management and sharing is applicable to the award, recipients will be required to adhere to the Data Management and Sharing requirements as outlined in the NIH Grants Policy Statement . Upon the approval of a Data Management and Sharing Plan, it is required for recipients to implement the plan as described.
Sharing Human Data via the RDCRN Data Repository (RDCRN-DR) To advance rare disease research as broadly and effectively as possible, investigators funded under this NOFO who are collecting data from humans are expected to share that data via the RDCRN Data Repository (RDCRN-DR). Fulfilling this expectation by the recipient will be among the terms and conditions of the award. Established by NCATS, and supported by other NIH Institutes, the RDCRN-DR is a secure informatics platform for scientific collaboration and data-sharing that enables the effective communication of detailed research data, results, tools, and supporting documentation. All human subject's data collected and/or derived for a project funded under this NOFO are expected to be submitted to the RDCRN-DR. Investigators funded under this NOFO are expected to use RDCRN technologies to submit data in accordance with the RDCRN-DR Data Sharing Terms and Conditions, outlined in the following checklist:
This checklist outlines the basic elements that need to be incorporated in the informed consent language, the contractual language established between the RDCRC Administrative Core and its sites, and the RDCRC policies that govern data sharing, use, and publication within the RDCRC and with external users.
- Sharing data with the Administrative Core of the RDCRC as a limited data set.
- Sharing data with the DMCC.
- Sharing data with other researchers for future studies.
- Allowing the transfer of data, without identifiers, to a federal data repository maintained by the NCATS.
- Options for restricting data sharing that the investigators deem necessary to offer the participant (e.g., as mandated by the IRB).
- Makes explicit that each site agrees to enter data in a data capture system maintained by the DMCC unless otherwise specified by the sponsoring NIH institute, acknowledging that the data entered into the DMCC may meet the definition of a limited data set.
- Allows the AC to collate participant data in the form of a limited data set from all protocols that the site participates in.
- Gives the AC the authority to distribute and share data with the DMCC and the NIH.
- Gives the AC the authority to share the data with other third parties as governed by specific data use agreements.
- Gives the AC the authority to transfer the data, without direct identifiers, to a Federal data repository maintained by the NIH.
- References the Data Management & Sharing Plan as written in the grant application and any Data Sharing Policy between RDCRC sites and the Administrative Core. Suggested language: Pursuant to this agreement, as outlined in the Data Management & Sharing Plan of award [NIH award number] [Site] will enter [RDCRC] data in a data capture system hosted in the NCATS cloud and maintained by the DMCC on behalf of the [RDCRC] unless otherwise specified by the sponsoring NIH institute. The Administrative Core of [RDCRC] has the authority to collect information from [Site] in the form of a limited data set for the purpose of combining the data across the sites of each protocol. The Administrative Core has the authority to manage the data with the support of the RDCRN DMCC and further share the data with third parties as governed by data use agreements that the Administrative Core will prepare and execute according to the policies of the [RDCRC]. Further, the Administrative Core has the authority to transfer [RDCRC] data to Federal data repositories to fulfill their obligation to the sponsor(s), in doing so, the Administrative Core will utilize the services of the RDCRN DMCC.
- A Data Sharing Policy between all participating sites of an RDCRC should be consistent with the RDCRN Template Document (linked above) and guidelines published by the RDCRN and the NIH. This policy should facilitate data sharing and outline the mechanisms of data sharing within the RDCRC and with external partners. The policy should clearly state the intention to transfer patient-level information, stripped of identifiers, to a federal data repository.
- A Data Use Policy, consistent with the template and guidelines published by the RDCRN, that includes a publication policy outlining how data collected in RDCRC studies is to be used, disseminated, and attributed.
A Data Management and Sharing Plan, formulated in accordance with these RDCRN Data Sharing Terms and Conditions, must be included in the grant application. It is expected that the investigator’s data sharing plan will specify the following elements: (1) description of what data will be collected including clinical data, diagnostic data, and physiological measurements such as MRI, (2) description of what biospecimens will be collected, (3) description of the data that will be derived from the biospecimens such as genotyping, sequence, metabolomic measures, proteomic measures, etc., (4) what data and/or biospecimens will be made available for deposit in databases or in a repository accessible to the research community, (5) a timetable for deposition of the data and/or biomaterials, and a specified time interval after which those data and materials can be released to the research community.
When multiple years are involved, recipients will be required to submit the Research Performance Progress Report (RPPR) annually and financial statements as required in the NIH Grants Policy Statement.
Progress reports should briefly describe status of pilot projects, including data and safety monitoring, and should notify NIH of serious adverse events and unanticipated problems.
A final RPPR, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the NIH Grants Policy Statement . NIH NOFOs outline intended research goals and objectives. Post award, NIH will review and measure performance based on the details and outcomes that are shared within the RPPR, as described at 2 CFR Part 200.301.
The Federal Funding Accountability and Transparency Act of 2006 as amended (FFATA), includes a requirement for recipients of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All recipients of applicable NIH grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over the threshold. See the NIH Grants Policy Statement for additional information on this reporting requirement.
In accordance with the regulatory requirements provided at 2 CFR Part 200.113 and Appendix XII to 2 CFR Part 200, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts from all Federal awarding agencies with a cumulative total value greater than $10,000,000 for any period of time during the period of performance of a Federal award, must report and maintain the currency of information reported in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (Responsibility/Qualification in SAM.gov, formerly FAPIIS). This is a statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313). As required by section 3010 of Public Law 111-212, all information posted in the designated integrity and performance system on or after April 15, 2011, except past performance reviews required for Federal procurement contracts, will be publicly available. Full reporting requirements and procedures are found in Appendix XII to 2 CFR Part 200 – Award Term and Condition for Recipient Integrity and Performance Matters.
Section VII. Agency Contacts
We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants.
eRA Service Desk (Questions regarding ASSIST, eRA Commons, application errors and warnings, documenting system problems that threaten submission by the due date, and post-submission issues)
Finding Help Online: https://www.era.nih.gov/need-help (preferred method of contact) Telephone: 301-402-7469 or 866-504-9552 (Toll Free)
General Grants Information (Questions regarding application instructions, application processes, and NIH grant resources) Email: [email protected] (preferred method of contact) Telephone: 301-480-7075
Grants.gov Customer Support (Questions regarding Grants.gov registration and Workspace) Contact Center Telephone: 800-518-4726 Email: [email protected]
Tiina K. Urv, Ph.D. National Center for Advancing Translational Sciences (NCATS) Telephone: 301-827-2746 Email: [email protected]
Jill A. Morris, Ph.D. National Institute of Neurological Disorders and Stroke (NINDS) Telephone: 301-496-5745 Email: [email protected]
Marilyn Miller, Ph.D. National Institute on Aging (NIA) Phone: (301)496-9350 E-mail: [email protected]
Melissa Parisi, MD, PhD Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Telephone: 301-435-6880 Email: [email protected]
Marrah Lachowicz-Scroggins, PhD National Heart, Lung, and Blood Institute (NHLBI) Telephone: 301-827-8229 Email: [email protected]
Faye H Chen, Ph.D. NIAMS - NATIONAL INSTITUTE OF ARTHRITIS AND MUSCULOSKELETAL AND SKIN DISEASES Phone: 301-594-5055 E-mail: [email protected]
Zubaida Saifudeen, PhD NIDCR - NATIONAL INSTITUTE OF DENTAL & CRANIOFACIAL RESEARCH Phone: (301) 827-3029 E-mail: [email protected]
Ruth Florese, Ph.D. National Institute of Allergy and Infectious Diseases (NIAID) Telephone: 301-761-6284 Email: [email protected]
Cindy Roy, Ph.D NIDDK - NATIONAL INSTITUTE OF DIABETES AND DIGESTIVE AND KIDNEY DISEASES Phone: 301-594-8805 E-mail: [email protected]
Rongling Li NHGRI - NATIONAL HUMAN GENOME RESEARCH INSTITUTE Phone: 301.480.2487 E-mail: [email protected]
Holly Lynn Storkel NIDCD - NATIONAL INSTITUTE ON DEAFNESS AND OTHER COMMUNICATION DISORDERS Phone: 301.451.6842 E-mail: [email protected]
Marilyn Moore-Hoon, Ph.D. National Center for Advancing Translational Sciences (NCATS) Telephone: 301-827-9549 Email: [email protected]
Steve Elsberg National Center for Advancing Translational Sciences (NCATS) Telephone: 301-435-0528 Email: [email protected]
Chief Grants Management Officer National Institute of Neurological Disorders and Stroke (NINDS) Email: [email protected]
Jeni Smits National Institute on Aging (NIA) Phone: none E-mail: [email protected]
Margaret Young Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Telephone: 301-642-4552 Email: [email protected]
Taylor Svilar National Heart, Lung, and Blood Institute (NHLBI) Telephone: 301-402-8545 Email: [email protected]
Erik Edgerton NIAMS - NATIONAL INSTITUTE OF ARTHRITIS AND MUSCULOSKELETAL AND SKIN DISEASES Phone: 301-594-7760 E-mail: [email protected]
Gabriel Hidalgo, MBA NIDCR - NATIONAL INSTITUTE OF DENTAL & CRANIOFACIAL RESEARCH Phone: 301-827-4630 E-mail: [email protected]
Sam Ashe National Institute of Allergy and Infectious Diseases (NIAID) Telephone: 301-435-4799 Email: [email protected]
Pamela Love NIDDK - NATIONAL INSTITUTE OF DIABETES AND DIGESTIVE AND KIDNEY DISEASES Phone: (301) 435-6198 E-mail: [email protected]
Deanna L Ingersoll NHGRI - NATIONAL HUMAN GENOME RESEARCH INSTITUTE Phone: 301-435-7858 E-mail: [email protected]
Christopher Myers NIDCD - NATIONAL INSTITUTE ON DEAFNESS AND OTHER COMMUNICATION DISORDERS Phone: (301) 435-0713 E-mail: [email protected]
Section VIII. Other Information
Recently issued trans-NIH policy notices may affect your application submission. A full list of policy notices published by NIH is provided in the NIH Guide for Grants and Contracts . All awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Awards are made under the authorization of Sections 301 and 405 of the Public Health Service Act as amended (42 USC 241 and 284) and under Federal Regulations 42 CFR Part 52 and 2 CFR Part 200.

Note: For help accessing PDF, RTF, MS Word, Excel, PowerPoint, Audio or Video files, see Help Downloading Files .
ERJN Seed Funding
The Environmental and Racial Justice Network (ERJN) provides seed funding for projects that address issues at the intersection of environmental and racial justice. Funding of up to $2,000 is available for qualifying projects led by undergraduate or graduate students. Students are encouraged to form teams that include other students, staff, and faculty across different schools or campuses, and are required to have a faculty advisor.
Along with the funds, awardees will also receive two workshops to ensure long-term impact of their project along with one-on-one check-ins with the Office of Sustainability and Office of Global Inclusion. Awardees are expected to provide a mid-year update as well as a final presentation at the end of their year-long grant period.
Complete the ERJN Expression of Interest (EOI) form . After the EOI is reviewed, you will be contacted to schedule a project consultation. You may submit a project application only after you submit an EOI and undergo a consultation.
Applicants must be undergraduate or graduate students enrolled in any degree-granting program planning to graduate in May 2025 or later. Applicants may be affiliated with any NYU school or based at any global site.
- Faculty Advisor
- Project teams of two or more people
- Project details, including: summary, background/rationale, process, and impact (which considers the evaluation criteria below)
- Project timeline (based on completion within the 2022 calendar year)
- Environmental Impact Statement
- Projected Budget with cost breakdown (finalists will be expected to submit a finalized budget and proof of expenditures)
Project Criteria
Applications will be evaluated based on the following criteria:
- Project is solution-driven and takes an innovative approach to address inequality or real problem
- Project centers and advances environmental and racial justice from an intersectional approach and addresses a concrete gap
- Project draws on interdisciplinary and cross-disciplinary research
- Project is based on a collaborative approach and includes multiple team members or collaborators
- Project proposal is detailed, thorough, and meets all application requirement
- Grade school programs teaching about environmental and racial justice
- Identifying a new waste route to support equity in waste transport and storage
- Initiatives to garner investment and/or governmental support for businesses in impacted communities
- Supporting skill building and/or mentorship for ERJ organizations
- Strategic Partnership program development with environmental justice organizations
- Research on an environmental issue impacting communities of color
- A new campus-based global educational initiative

Strategically Focused Research Network (SFRN) on Inflammation in Cardiac and Neurovascular Disease
Researchers awarded $15 million to study inflammation’s impact on heart, brain health.
The four-year awards, which started April 1, 2024, include a collaborative research project three groups, called centers. To further the American Heart Association’s commitments to expanding diversity in clinical research, each of the centers will work in conjunction with an academic institution that primarily serves individuals who are underrepresented in science. The research centers and the projects include: Northwestern University Chicago Campus – Led by center director Matthew J. Feinstein, M.D., M.Sc., FAHA, associate professor of medicine (cardiology) and director of the Clinical and Translational ImmunoCardiology Program at Northwestern’s Bluhm Cardiovascular Institute, research teams at Northwestern University, collaborating with a team from Chicago State University, will undertake three different projects focused on inflammation in heart failure with preserved ejection fraction (HFpEF). This type of heart failure is marked by excess inflammation, accounts for more than half of heart failure cases in the U.S. and carries a significant public health burden: 50% of people diagnosed with HFpEF die within 5 years. Unfortunately, few treatments exist for HFpEF, and effective ways to target inflammation in HFpEF are limited. In these complementary projects, the researchers aim to determine how inflammation may be targeted at a cellular level to prevent and treat HFpEF. They will focus on immune cells, which crucially determine whether inflammation persists unchecked or resolves. Specifically, they will study ways that immune cells are primed by metabolic and other stressors to turn problematic inflammation on or off in various contexts, as well as how this impacts heart dysfunction and HFpEF. They will also investigate how existing and novel approaches targeting metabolism and immune cell function affect HFpEF onset and progression. The ultimate goal of these efforts is to develop specific, patient-relevant means of targeting inflammation to curb HFpEF. University of Michigan – Anthony Rosenzweig, M.D., FAHA, director of the Stanley and Judith Frankel Institute for Heart and Brain Health at Michigan Medicine, the academic medical center of the University of Michigan, will serve as the director of a collaborative research effort between Michigan Medicine and Massachusetts General Hospital. These research teams, in collaboration with a team from Oakland University in Rochester Hills, Michigan, will study the driving forces behind inflammatory processes linked to aging and obesity and how to prevent inflammation that could lead to heart failure, dementia and other diseases. They will study mechanisms behind and potential treatments for cell senescence, a process that occurs with aging and unhealthy lifestyles that results in damaged cells. Inflammation from senescent cells can harm other cells, starting a cycle of inflammation that can damage the heart, brain and other organs. They are studying cells called microglia – the brain’s main immune cells – and their counterpart, monocytes and macrophages, in the heart. The combination of aging with an unhealthy lifestyle can lead to dysfunction of these cells and inflammation in both the heart and the brain. These researchers want to understand this process better and learn how to prevent or lessen the inflammatory effects. They are expanding their research of a new investigational treatment that inhibits a pathway regulating metabolism and inflammation, as well as cardiac and skeletal muscle function. Early research suggests that the investigational treatment lessens inflammation, improves immune cell function and improves heart function. The proposal includes a first-ever clinical trial of this inhibitor for patients with unhealthy body weight and heart failure with preserved ejection fraction. University of Pittsburgh – Led by center director Stephen Y. Chan, M.D., Ph.D., FAHA, professor of medicine (cardiology) and director of the Vascular Medicine Institute, the University of Pittsburgh research teams, in collaboration with a team from Prairie View (Texas) A&M University, will conduct three different projects aimed at identifying and treating interrelated conditions of brain and vascular pathology. They will specifically look at how lysosomes, components of cells that contain digestive enzymes and dispose of excess or worn-out cell parts, control inflammation in blood vessel diseases like heart attacks and brain diseases like Alzheimer’s disease. They will also work to develop new lysosomal medications by testing whether changes in a person’s DNA and blood related to the lysosome can predict vascular disease and dementia and response to anti-inflammatory therapy. Additionally, these researchers will study cellular mechanisms that impact memory in hopes of developing new medications to boost brain function for people who have experienced a heart attack.
Link to AHA Newsroom for announcement of the awardees.
The Role of Inflammation in Cardiac and Neurovascular Disease

The heart and nervous system are also subject to disease with dysregulation of the inflammatory system. Inflammatory myocarditis, characterized by inflammation of the myocardium, is more likely to occur in males compared to females. 2 It is most commonly triggered by viral infection; triggering viruses include adenoviruses, enteroviruses, parvoviruses and coronaviruses (including SARS-CoV-2), among others. 3 Less commonly, myocarditis is caused by bacterial or fungal infection or autoimmune diseases. Of more recent note, it was discovered during the COVID-19 pandemic that vaccines developed against SARS-CoV-2, particularly those using mRNA technology, elicited myocarditis in a subset of vaccine recipients. 4 The highest incidence (approximately 50 / 100,000) was found in men under 40.
Myocarditis can be subclassified based on a number of characteristics. The most prominent symptoms are chest pain and dyspnea, 5 and in many cases, myocarditis may resolve on its own. One notable exception is fulminant myocarditis, a rare and severe form of myocarditis that is responsible for a high proportion of cardiac-related deaths in young individuals. 6 Acute myocarditis is defined as that for which symptoms are of recent onset, generally within a month or so. Inflammatory processes associated with myocarditis, such as infiltration of immune cells, release of pro-inflammatory cytokines, and oxidative stress, can lead to myocyte damage, fibrosis, and impaired contractility. 3,7-8 Myocarditis that is associated with cardiac dysfunction and remodeling of the ventricle is referred to as inflammatory cardiomyopathy, a condition that is typically irreversible. It may result in arrhythmias, ventricular dysfunction or heart failure and requires lifelong therapy and/or heart transplant.
Within the nervous system, inflammation has been implicated in an array of pathologies, such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. 9-10 Inflammation also plays a prominent role in stroke. 11-13 A robust neuroinflammatory response is initiated following an ischemic event. Sex differences are also observed with stroke, with the risk being higher for females than males. 14 The primary cause of this neuroinflammation is the activation of immune cells in the brain, including microglia and astrocytes. These cells are responsible for defending the brain against pathogens and injuries. However, under certain conditions, they can become overactivated and release inflammatory molecules. Neuroinflammation can have both beneficial and detrimental effects. In acute situations, neuroinflammation helps clear pathogens, promote tissue repair, and support the restoration of normal brain function. However, chronic or excessive neuroinflammation can damage neurons, impair synaptic communication, break down the blood-brain barrier, and disrupt the delicate balance of the brain's environment. 11
Cardiotoxicity
Cardiotoxicity describes a condition wherein a decrease in cardiac function results from administration of drugs or other agents. Currently, the term is largely identified with changes in cardiovascular function resulting from treatment with a number of cancer therapies. Whereas a decrease in left ventricular ejection fraction is the cardiac parameter most closely aligned with cardiotoxicity, additional cardiac effects (e.g., left ventricular systolic dysfunction, angina, and acute coronary syndrome) may also be characterized as cardiotoxicity. 15
There are several potential mechanisms underlying cardiotoxicity of chemotherapeutic agents, including inflammation. For example, use of chemotherapeutics of the anthracycline class, widely prescribed because of their efficacy against both solid and hematologic tumors, is associated with a high incidence of cardiotoxicity. 16 Despite this effect of anthracyclines being described decades ago, the mechanism(s) underlying cardiotoxicity are not fully elucidated. Studies in more recent years do suggest, however, that at least part of the cardiotoxic actions of anthracyclines are related to inflammation. 17 In addition, pre-clinical studies assessing effects of anti-inflammatory agents against anthracycline-induced cardiotoxicity have shown favorable results. 18-19 Targeting inflammation thus holds promise for preventing or mitigating cardiotoxic effects of this class of chemotherapeutic.
More contemporary cancer treatments also elicit adverse cardiac effects. Immune checkpoint inhibitors (ICIs), a new and promising class of anti-cancer drugs, may elicit a severe form of myocarditis. 20 Whereas the incidence is relatively low, the mortality rate is high, due in part to the fact that many individuals present with a fulminant-like form of myocarditis. The mechanism underlying ICI-induced myocarditis remains unclear. The promise of this new class of cancer treatment will not be fully realized unless the mechanism is identified, which will facilitate therapeutic strategies to prevent or mitigate this severe adverse effect.
While our understanding of the role inflammation plays in cardiac and brain dysfunction has grown considerably in recent years, several hindrances remain that preclude improved recognition and treatment of these conditions. For instance, significant gaps remain in understanding of the downstream signaling events and potential crosstalk; development of new animal and in vitro models would support these needs. In addition, notable opportunities for optimization of diagnostic capabilities exist, such as identification and assessment of biomarkers with improved specificity and development of improved imaging techniques. Clinical trials designed to assess outcomes more specifically for distinct types and/or stages of inflammatory conditions are also needed.
- Furman et al., Nat Med 25: 1822–1832, 2019
- Fairweather et al., Front Cardiovasc Med 10: 1129348, 2023
- Tschope et al., Nat Rev Cardiol 18:169-193, 2021
- Fairweather et al., Circ Res 132: 1302-1319, 2023
- Ammirati et al., Circ Heart Fail 13: 663-687, 2020
- Kociol et al., Circulation 141: e69–e92, 2020
- Rurik et al, Circ Res 128: 1766-1779, 2021
- Trachtenberg and Hare, Circ Res 121: 803–818, 2017
- Heneka et al., Lancet Neurol 14: 388-405, 2015
- Leibowitz and Yan, Front Mol Neurosci 9: 1-23, 2016
- Candelario-Jalil et al., Stroke 53: 1473-1486, 2022
- Iadecola et al., J Clin Invest 130: 2777-2788, 2020
- Kelly et al., Stroke 52: 2697-2706, 2021
- Rexrode et al., Circ Res 130: 512-538, 2022
- Chung et al., Open Heart 5: e000774
- Cardinale et al., Front Cardiovasc Med 7:26 doi:10.3389
- Fabiano et al., Heart Fail Rev 26: 881-890, 2021
- Peng et al., Arch Biochem Biophys 683: 108238, 2020
- Sheibani et al., Cancer Chemother Pharmacol 85: 563-571, 2020
- Palaskas et al., J Am Heart Assoc 9: e013757, 2020
2024 Holidays

Elon Musk demands charges against Anthony Fauci after NIH comes clean on funding ‘gain-of-function’ research
W ASHINGTON — Elon Musk demanded the arrest and prosecution of Dr. Anthony Fauci on Friday after the National Institutes of Health came clean to Congress and admitted funding risky “gain-of-function” research in Wuhan, China, prior to the outbreak of the COVID-19 pandemic there.
“Prosecute/Fauci,” the billionaire CEO of Tesla and SpaceX and owner of social media network X tweeted, sharing The Post’s Friday front page bearing a photo of Fauci and the headline “SICK LIES.”
NIH principal deputy director Lawrence Tabak confessed Thursday at a House subcommittee hearing that the US government had indeed funded dangerous research at the Wuhan Institute of Virology, which modified bat coronaviruses shortly before the outbreak began in late 2019.
“Dr. Tabak, did NIH fund gain-of-function research at the Wuhan Institute of Virology through [Manhattan-based nonprofit] EcoHealth [Alliance]?” ”asked Rep. Debbie Lesko (R-Ariz.) of the Select Subcommittee on the Coronavirus Pandemic.
“It depends on your definition of gain-of-function research,” Tabak answered. “If you’re speaking about the generic term, yes, we did.”
Tabak’s answer conflicts with Fauci’s several fiery denials.
“The NIH has not ever and does not now fund gain-of-function research in the Wuhan Institute of Virology,” Fauci, now 83, testified to Congress in May 2021.
Musk did not specify what criminal charges Fauci should face, though perjury and lying to Congress are obvious options and have been recommended by congressional Republicans.
Both offenses carry up to five years in prison and generally have five-year statutes of limitations.
It’s unclear if other potential charges could stem from the alleged cover-up of US funding for the Chinese labs suspected of unleashing the lethal respiratory virus, which has killed at least 1,190,546 US residents , according to Centers for Disease Control and Prevention data, in addition to causing enormous economic, social and educational damage across the world.
Fauci served as President Biden’s chief medical adviser in 2021 and 2022 after presenting himself in the early phase of the pandemic as a beacon of reason and good health guidance, though allies of former President Donald Trump noted his often inconsistent tips, including on mask-wearing.
The doctor also led NIH’s National Institute of Allergy and Infectious Diseases from 1984 through the end of 2022.
Documents published in late 2021 by the Intercept revealed that the EcoHealth Alliance used grants from Fauci’s agency to fund Wuhan Institute of Virology experiments that modified three bat coronaviruses distinct from COVID-19.
The research discovered the viruses became much more infectious among “humanized” mice when human-type receptors were added to them.
The Department of Health and Human Services, which includes NIH, on Tuesday barred EcoHealth from receiving federal funding for the next three years.
The origins of COVID-19 remain a mystery due to the Chinese government’s refusal to allow an independent international investigation.
Some parts of the US government, including the FBI and the Energy Department — which includes the US National Laboratories — believe the pandemic emerged via a lab leak in China.
Biden has said very little about determining the origins of the pandemic. Trump, his predecessor and November election challenger, has proposed forcing China to pay $10 trillion in “reparations.”

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Archived funding opportunity
Nsf 16-523: international research network connections (irnc) europe and africa, program solicitation, document information, document history.
- Posted: December 18, 2015
- Replaces: NSF 14-554
- Replaced by: NSF 20-535
Program Solicitation NSF 16-523
Full Proposal Deadline(s) (due by 5 p.m. submitter's local time):
March 17, 2016
Important Information And Revision Notes
The previous IRNC solicitation ( NSF 14-554 ) issued in 2014 addressed research and education network connectivity between the U.S. and Asia and the Americas. That solicitation also established award activities supporting open exchange points in the US, centralized network operation center (NOC) activities and advanced measurement. That solicitation explicitly stated that U.S.-Europe/Africa network connectivity community needs would be addressed in a future solicitation. This solicitation targets that area.
Any proposal submitted in response to this solicitation should be submitted in accordance with the revised NSF Proposal & Award Policies & Procedures Guide (PAPPG) ( NSF 16-1 ), which is effective for proposals submitted, or due, on or after January 25, 2016. Please be advised that proposers who opt to submit prior to January 25, 2016, must also follow the guidelines contained in NSF 16-1 .
Summary Of Program Requirements
- General Information
Program Title:
International Research Network Connections (IRNC) Europe and Africa
Synopsis of Program:
The International Research Network Connections (IRNC) program supports high-performance network connectivity required by international science and engineering research and education collaborations involving the NSF research community. NSF expects to make 1-2 awards to link U.S. research networks with peer networks in Europe and Africa and leverage existing international network connectivity. High-performance network connections funded by this program are intended to support science and engineering research and education applications, and preference will be given to solutions that provide the best economy of scale and demonstrate the ability to support the largest communities of interest with the broadest services. Funded projects will assist the U.S. research and education community by enabling state-of-the-art international network services and access to increased collaboration and data services. Through extended international network connections, additional research and production network services will be enabled, complementing those currently offered or planned by domestic research networks.
Cognizant Program Officer(s):
Please note that the following information is current at the time of publishing. See program website for any updates to the points of contact.
Kevin Thompson, CISE/ACI Program Director, telephone: (703) 292-4220, email: [email protected]
- 47.070 --- Computer and Information Science and Engineering
Award Information
Anticipated Type of Award:
Standard Grant or Continuing Grant or Cooperative Agreement
Estimated Number of Awards: 1 to 2
The estimated number of awards is 1-2. Because of the nature and geographic extent of the efforts involved, interested parties are encouraged to form consortia of organizations that can work together to provide the needed services. Consortia may consist of any number of U.S. and foreign, profit and not-for-profit entities. The award(s) resulting from responses to this solicitation will be made to U.S. organizations as cooperative agreements or standard or continuing grants. Any award will be for a maximum of four years.
Anticipated Funding Amount: $3,600,000
The anticipated funding amount is $3,600,000 total for this solicitation, subject to the availability of funds. NSF expects to make 1-2 awards at up to $900,000 total per year for a maximum of 4 years.
Eligibility Information
Who May Submit Proposals:
Proposals may only be submitted by the following: Institutions of Higher Education (IHEs) - Two- and four-year IHEs (including community colleges) accredited in, and having a campus located in the US, acting on behalf of their faculty members. Special Instructions for International Branch Campuses of US IHEs: If the proposal includes funding to be provided to an international branch campus of a US institution of higher education (including through use of subawards and consultant arrangements), the proposer must explain the benefit(s) to the project of performance at the international branch campus, and justify why the project activities cannot be performed at the US campus.
Who May Serve as PI:
There are no restrictions or limits.
Limit on Number of Proposals per Organization:
Limit on Number of Proposals per PI or Co-PI:
Proposal Preparation and Submission Instructions
A. proposal preparation instructions.
- Letters of Intent: Not required
- Preliminary Proposal Submission: Not required
Full Proposals:
- Full Proposals submitted via FastLane: NSF Proposal and Award Policies and Procedures Guide (PAPPG) guidelines apply. The complete text of the PAPPG is available electronically on the NSF website at: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg .
- Full Proposals submitted via Grants.gov: NSF Grants.gov Application Guide: A Guide for the Preparation and Submission of NSF Applications via Grants.gov guidelines apply (Note: The NSF Grants.gov Application Guide is available on the Grants.gov website and on the NSF website at: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=grantsgovguide ).
B. Budgetary Information
Cost Sharing Requirements:
Inclusion of voluntary committed cost sharing is prohibited.
Indirect Cost (F&A) Limitations:
Not Applicable
Other Budgetary Limitations:
C. Due Dates
Proposal review information criteria.
Merit Review Criteria:
National Science Board approved criteria. Additional merit review considerations apply. Please see the full text of this solicitation for further information.
Award Administration Information
Award Conditions:
Additional award conditions apply. Please see the full text of this solicitation for further information.
Reporting Requirements:
Standard NSF reporting requirements apply.
I. Introduction
The United States research and education (R&E) community communicates, cooperates, and collaborates with colleagues in the global community. Members of this community access remote instruments, data, and computational resources located throughout the world, often as part of international collaborations. Similarly, major NSF investments in large-scale science and engineering facilities located both inside and outside the United States are utilized by multi-national research and education collaborations. To support such activities, NSF solicits proposals for International Research Network Connections (IRNC).
NSF made a series of awards in 2014-2015 (pursuant to NSF 14-554 ) supporting continued multi-gigabit high-performance international trans-oceanic network connectivity between the U.S. and other parts of the world, specifically Asia and the Americas. This solicitation addresses science-driven needs for connectivity to Europe and Africa.
NSF expects to make 1-2 awards to link U.S. research networks with peer networks in Europe and Africa and leverage existing international network connectivity. High-performance network connections funded by this program are intended to support science and engineering research and education applications, and preference will be given to solutions that provide the best economies of scale and demonstrate the ability to support the largest communities of interest with the broadest services. The funded project(s) will join other awards made in 2015 in the program in assisting the U.S. research and education community by enabling state-of-the-art international network services and access to increased collaboration and data services. Through extended international network connections, additional research and production network services will be enabled, complementing those currently offered or planned by domestic research networks.
II. Program Description
In 2012 NSF began an effort called the International Research and Education Network Initiative (IREN) Initiative to globally develop and coordinate future strategy in international research and education (R&E) networking. An early result of these efforts in 2013 was a short document, co-developed with the European Commission, that provided a concise set of guiding principles for international networking. The full document can be found at: http://fasterdata.es.net/nsf-iren/ . These principles, paraphrased below, apply in full to this program.
Coordination Statement: Support global research and education communities by funding globally interoperable networked infrastructures, coordinated innovative services and human network community building. This will be achieved through global coordination, planning, execution, and governance through appropriate coordination of governance bodies, and coordinated investments.
Guiding Principles: Open exchange points: "policy-light" operation of open exchange points allowing for bi-lateral peering at all layers. Open shared transit: utilization of the appropriated network capacity will be open to the largest R&E communities possible, including for transit. End-to-end interoperability: end-to-end visibility & interoperability across network links and paths, and extended to end systems, is encouraged. Close partnership with R&E Networks: close coordination and engagement with the community of R&E Networks. Resilience: network resources will be configured and engineered in a way limiting or eliminating single point of failure and ensuring physical and logical path and route diversity. Regional development: commitment to the concept of aggregation of demand at the regional level. Technology agnostic: operators of international R&E network infrastructure are open to different technology architectures. Open innovation: coordinated development and adoption of advanced services at intra-domain, inter-domain and user levels .
The infrastructure and associated services proposed in response to this solicitation must address U.S. research and education needs with respect to international collaboration and communication that advance science and engineering. The science that will be enabled by the proposal should be detailed. Plans for meeting the evolving service needs of the research and education community should also be described.
Because of the nature and geographic extent of the efforts involved, interested parties may choose to form consortia that can work together to provide the needed services. Consortia may consist of any number of U.S. and foreign, profit and not-for-profit entities. Awards resulting from this solicitation will be made to the eligible lead U.S. organizations. Usage of any funded connectivity under this program is required to be dedicated to academic science and engineering communities in support of global research and education activities.
The highest priority is enabling and enhancing communication and collaboration between the U.S. and international science and engineering research and education communities. A key ingredient of networked global scientific collaboration continues to be high-capacity, high-performance network links between the U.S. and other regions of the world. The availability of limited resources means that preference will be given to solutions providing the most efficient economies of scale and demonstrating the ability to link the largest communities of interest with the broadest services. Proposals should describe how this will be accomplished over a four-year period.
NSF is also interested in innovative and forward-looking approaches to promote the development of a rational global network architecture. In this regard, proposals should address the question of how their international links will become an integral component of the global science and engineering research and education network environment and how they will fit into a rational global network architecture. For example, solutions that offer partnering and engineering incentives to foreign connection points to share circuits or encourage the establishment of national or regional distributed exchange points may be considered.
Proposals should describe how the design complements, and improves, existing trans-Atlantic network infrastructure dedicated to the R&E community. If direct submarine cable routes between the U.S. and Africa are either unavailable or unfeasible, proposals should describe the network paths enabled by their designs in support of research and education collaborations with countries and partners in Africa. Proposals should consider the IREN principles listed above in their network designs. In addition to the description of the initial technologies and equipment to be employed, proposals should outline how the proposed IRNC connectivity should evolve and discuss plans for introducing new networking technologies, equipment, and services.
Proposals are encouraged to weigh current network traffic demand and existing R&E network paths and physical connections against future estimated needs and to consider proposing one or more coordinated 100Gbps circuits, depending on geography, availability, and demand.
Proposals must establish and augment high-capacity R&E network connectivity between the U.S. and Europe and/or Africa. Network Operation Center (NOC) and advanced measurement services have been funded separately through 2015 IRNC awards.
- Project Description Elements
The Project Description for all proposals, which can be up to 15 pages in length, should address the following elements:
- scientific research and education needs for the proposed bandwidth;
- a topology including a logical and physical landing point identified on U.S. soil, and how the proposed connectivity complements existing research and education (R&E) networking paths and capacity;
- technologies and services supported in layers 1-3 and interoperability plans;
- how the proposed activities reflect the IREN principles described above; and
- a project plan, including a schedule for capacity availability, network engineering activities in coordination with existing R&E network connections and infrastructure where relevant, and existing IRNC program funded activities, especially NOC and measurement.
NOC services should not be included in proposals. Advanced network measurement services, beyond capabilities required to address impact metrics and normative Simple Network Management Protocol (SNMP)-based passive measurement, should not be included in proposals. 2015 IRNC awards were made in 2015 to support NOC and advanced measurement functions for the IRNC program. Proposals in this solicitation are expected to address plans to work closely, and coordinate with, these other awards.
Letters of commitment, submitted as supplementary documents , should be included for all international partners in a proposal, and are encouraged to specify the partners' views of the relationship with, and value of, the proposed project, and the nature of their interactions. Letters of support from third parties are limited to 8.
Budgets should include pricing information for circuit or wavelength spectrum costs and associated port and cross-connect fees with supporting documentation.
NSF expects to make 1-2 awards at up to $900,000 total per year for up to 4 years, subject to the availability of funds.
Experimental Europe/Africa Open Exchange Point for Network Innovation
Proposals may choose to include an additional element: the design and deployment of an experimental network exchange point serving explicit scientific collaborations between the United States and scientific collaborators in Europe and Africa. This experimental open exchange point represents next-generation integration and provision of limited computational and storage resources embedded in the network path and integrated into the network physically at an exchange point. Proposals including this experimental exchange point element should describe how the work relates to the connectivity described and planned elsewhere in the proposal. Proposals should identify specific U.S.-Africa and/or U.S.-Europe scientific collaborations that they intend to support with this experimental cyberinfrastructure, and the nature of interaction and support for integrated scientific workflows associated with those projects.
III. Award Information
The estimated program budget is a total of $3,600,000 for this solicitation, subject to the availability of funds. NSF expects to make 1-2 awards at up to $900,000 total per year for a maximum of 4 years.
IV. Eligibility Information
Additional Eligibility Info:
V. Proposal Preparation And Submission Instructions
Full Proposal Preparation Instructions : Proposers may opt to submit proposals in response to this Program Solicitation via Grants.gov or via the NSF FastLane system.
- Full proposals submitted via FastLane: Proposals submitted in response to this program solicitation should be prepared and submitted in accordance with the general guidelines contained in the NSF Proposal & Award Policies & Procedures Guide (PAPPG). The complete text of the PAPPG is available electronically on the NSF website at: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg . Paper copies of the PAPPG may be obtained from the NSF Publications Clearinghouse, telephone (703) 292-7827 or by e-mail from [email protected] . Proposers are reminded to identify this program solicitation number in the program solicitation block on the NSF Cover Sheet For Proposal to the National Science Foundation. Compliance with this requirement is critical to determining the relevant proposal processing guidelines. Failure to submit this information may delay processing.
- Full proposals submitted via Grants.gov: Proposals submitted in response to this program solicitation via Grants.gov should be prepared and submitted in accordance with the NSF Grants.gov Application Guide: A Guide for the Preparation and Submission of NSF Applications via Grants.gov . The complete text of the NSF Grants.gov Application Guide is available on the Grants.gov website and on the NSF website at: ( https://www.nsf.gov/publications/pub_summ.jsp?ods_key=grantsgovguide ). To obtain copies of the Application Guide and Application Forms Package, click on the Apply tab on the Grants.gov site, then click on the Apply Step 1: Download a Grant Application Package and Application Instructions link and enter the funding opportunity number, (the program solicitation number without the NSF prefix) and press the Download Package button. Paper copies of the Grants.gov Application Guide also may be obtained from the NSF Publications Clearinghouse, telephone (703) 292-7827 or by e-mail from [email protected] .
In determining which method to utilize in the electronic preparation and submission of the proposal, please note the following:
Collaborative Proposals. All collaborative proposals submitted as separate submissions from multiple organizations must be submitted via the NSF FastLane system. PAPPG Chapter II.D.3 provides additional information on collaborative proposals.
See PAPPG Chapter II.C.2 for guidance on the required sections of a full research proposal submitted to NSF. Please note that the proposal preparation instructions provided in this program solicitation may deviate from the PAPPG instructions.
Refer to Section II, Program Description, for specific proposal preparation information and instructions.
Cost Sharing:
Budget Preparation Instructions:
D. FastLane/Grants.gov Requirements
For Proposals Submitted Via FastLane:
To prepare and submit a proposal via FastLane, see detailed technical instructions available at: https://www.fastlane.nsf.gov/a1/newstan.htm . For FastLane user support, call the FastLane Help Desk at 1-800-673-6188 or e-mail [email protected] . The FastLane Help Desk answers general technical questions related to the use of the FastLane system. Specific questions related to this program solicitation should be referred to the NSF program staff contact(s) listed in Section VIII of this funding opportunity.
For Proposals Submitted Via Grants.gov:
Before using Grants.gov for the first time, each organization must register to create an institutional profile. Once registered, the applicant's organization can then apply for any federal grant on the Grants.gov website. Comprehensive information about using Grants.gov is available on the Grants.gov Applicant Resources webpage: http://www.grants.gov/web/grants/applicants.html . In addition, the NSF Grants.gov Application Guide (see link in Section V.A) provides instructions regarding the technical preparation of proposals via Grants.gov. For Grants.gov user support, contact the Grants.gov Contact Center at 1-800-518-4726 or by email: [email protected] . The Grants.gov Contact Center answers general technical questions related to the use of Grants.gov. Specific questions related to this program solicitation should be referred to the NSF program staff contact(s) listed in Section VIII of this solicitation. Submitting the Proposal: Once all documents have been completed, the Authorized Organizational Representative (AOR) must submit the application to Grants.gov and verify the desired funding opportunity and agency to which the application is submitted. The AOR must then sign and submit the application to Grants.gov. The completed application will be transferred to the NSF FastLane system for further processing.
Proposers that submitted via FastLane are strongly encouraged to use FastLane to verify the status of their submission to NSF. For proposers that submitted via Grants.gov, until an application has been received and validated by NSF, the Authorized Organizational Representative may check the status of an application on Grants.gov. After proposers have received an e-mail notification from NSF, Research.gov should be used to check the status of an application.
VI. NSF Proposal Processing And Review Procedures
Proposals received by NSF are assigned to the appropriate NSF program for acknowledgement and, if they meet NSF requirements, for review. All proposals are carefully reviewed by a scientist, engineer, or educator serving as an NSF Program Officer, and usually by three to ten other persons outside NSF either as ad hoc reviewers, panelists, or both, who are experts in the particular fields represented by the proposal. These reviewers are selected by Program Officers charged with oversight of the review process. Proposers are invited to suggest names of persons they believe are especially well qualified to review the proposal and/or persons they would prefer not review the proposal. These suggestions may serve as one source in the reviewer selection process at the Program Officer's discretion. Submission of such names, however, is optional. Care is taken to ensure that reviewers have no conflicts of interest with the proposal. In addition, Program Officers may obtain comments from site visits before recommending final action on proposals. Senior NSF staff further review recommendations for awards. A flowchart that depicts the entire NSF proposal and award process (and associated timeline) is included in PAPPG Exhibit III-1.
A comprehensive description of the Foundation's merit review process is available on the NSF website at: https://www.nsf.gov/bfa/dias/policy/merit_review/ .
Proposers should also be aware of core strategies that are essential to the fulfillment of NSF's mission, as articulated in Building the Future: Investing in Discovery and Innovation - NSF Strategic Plan for Fiscal Years (FY) 2018 – 2022 . These strategies are integrated in the program planning and implementation process, of which proposal review is one part. NSF's mission is particularly well-implemented through the integration of research and education and broadening participation in NSF programs, projects, and activities.
One of the strategic objectives in support of NSF's mission is to foster integration of research and education through the programs, projects, and activities it supports at academic and research institutions. These institutions must recruit, train, and prepare a diverse STEM workforce to advance the frontiers of science and participate in the U.S. technology-based economy. NSF's contribution to the national innovation ecosystem is to provide cutting-edge research under the guidance of the Nation's most creative scientists and engineers. NSF also supports development of a strong science, technology, engineering, and mathematics (STEM) workforce by investing in building the knowledge that informs improvements in STEM teaching and learning.
NSF's mission calls for the broadening of opportunities and expanding participation of groups, institutions, and geographic regions that are underrepresented in STEM disciplines, which is essential to the health and vitality of science and engineering. NSF is committed to this principle of diversity and deems it central to the programs, projects, and activities it considers and supports.
A. Merit Review Principles and Criteria
The National Science Foundation strives to invest in a robust and diverse portfolio of projects that creates new knowledge and enables breakthroughs in understanding across all areas of science and engineering research and education. To identify which projects to support, NSF relies on a merit review process that incorporates consideration of both the technical aspects of a proposed project and its potential to contribute more broadly to advancing NSF's mission "to promote the progress of science; to advance the national health, prosperity, and welfare; to secure the national defense; and for other purposes." NSF makes every effort to conduct a fair, competitive, transparent merit review process for the selection of projects.
1. Merit Review Principles
These principles are to be given due diligence by PIs and organizations when preparing proposals and managing projects, by reviewers when reading and evaluating proposals, and by NSF program staff when determining whether or not to recommend proposals for funding and while overseeing awards. Given that NSF is the primary federal agency charged with nurturing and supporting excellence in basic research and education, the following three principles apply:
- All NSF projects should be of the highest quality and have the potential to advance, if not transform, the frontiers of knowledge.
- NSF projects, in the aggregate, should contribute more broadly to achieving societal goals. These "Broader Impacts" may be accomplished through the research itself, through activities that are directly related to specific research projects, or through activities that are supported by, but are complementary to, the project. The project activities may be based on previously established and/or innovative methods and approaches, but in either case must be well justified.
- Meaningful assessment and evaluation of NSF funded projects should be based on appropriate metrics, keeping in mind the likely correlation between the effect of broader impacts and the resources provided to implement projects. If the size of the activity is limited, evaluation of that activity in isolation is not likely to be meaningful. Thus, assessing the effectiveness of these activities may best be done at a higher, more aggregated, level than the individual project.
With respect to the third principle, even if assessment of Broader Impacts outcomes for particular projects is done at an aggregated level, PIs are expected to be accountable for carrying out the activities described in the funded project. Thus, individual projects should include clearly stated goals, specific descriptions of the activities that the PI intends to do, and a plan in place to document the outputs of those activities.
These three merit review principles provide the basis for the merit review criteria, as well as a context within which the users of the criteria can better understand their intent.
2. Merit Review Criteria
All NSF proposals are evaluated through use of the two National Science Board approved merit review criteria. In some instances, however, NSF will employ additional criteria as required to highlight the specific objectives of certain programs and activities.
The two merit review criteria are listed below. Both criteria are to be given full consideration during the review and decision-making processes; each criterion is necessary but neither, by itself, is sufficient. Therefore, proposers must fully address both criteria. (PAPPG Chapter II.C.2.d(i). contains additional information for use by proposers in development of the Project Description section of the proposal). Reviewers are strongly encouraged to review the criteria, including PAPPG Chapter II.C.2.d(i), prior to the review of a proposal.
When evaluating NSF proposals, reviewers will be asked to consider what the proposers want to do, why they want to do it, how they plan to do it, how they will know if they succeed, and what benefits could accrue if the project is successful. These issues apply both to the technical aspects of the proposal and the way in which the project may make broader contributions. To that end, reviewers will be asked to evaluate all proposals against two criteria:
- Intellectual Merit: The Intellectual Merit criterion encompasses the potential to advance knowledge; and
- Broader Impacts: The Broader Impacts criterion encompasses the potential to benefit society and contribute to the achievement of specific, desired societal outcomes.
The following elements should be considered in the review for both criteria:
- Advance knowledge and understanding within its own field or across different fields (Intellectual Merit); and
- Benefit society or advance desired societal outcomes (Broader Impacts)?
- To what extent do the proposed activities suggest and explore creative, original, or potentially transformative concepts?
- Is the plan for carrying out the proposed activities well-reasoned, well-organized, and based on a sound rationale? Does the plan incorporate a mechanism to assess success?
- How well qualified is the individual, team, or organization to conduct the proposed activities?
- Are there adequate resources available to the PI (either at the home organization or through collaborations) to carry out the proposed activities?
Broader impacts may be accomplished through the research itself, through the activities that are directly related to specific research projects, or through activities that are supported by, but are complementary to, the project. NSF values the advancement of scientific knowledge and activities that contribute to achievement of societally relevant outcomes. Such outcomes include, but are not limited to: full participation of women, persons with disabilities, and underrepresented minorities in science, technology, engineering, and mathematics (STEM); improved STEM education and educator development at any level; increased public scientific literacy and public engagement with science and technology; improved well-being of individuals in society; development of a diverse, globally competitive STEM workforce; increased partnerships between academia, industry, and others; improved national security; increased economic competitiveness of the United States; and enhanced infrastructure for research and education.
Proposers are reminded that reviewers will also be asked to review the Data Management Plan and the Postdoctoral Researcher Mentoring Plan, as appropriate.
Additional Solicitation Specific Review Criteria
Proposals will be evaluated with careful attention to the following:
- The expected impact of the proposed international networking activities, either directly or indirectly, across the NSF community;
- The expected level of production quality in resulting capabilities made available to the NSF community; and
- The experience and record of the PI team in delivering reliable, robust, dependable, and state-of-the-art capabilities in international R&E networking.
B. Review and Selection Process
Proposals submitted in response to this program solicitation will be reviewed by Ad hoc Review and/or Panel Review.
Reviewers will be asked to evaluate proposals using two National Science Board approved merit review criteria and, if applicable, additional program specific criteria. A summary rating and accompanying narrative will generally be completed and submitted by each reviewer and/or panel. The Program Officer assigned to manage the proposal's review will consider the advice of reviewers and will formulate a recommendation.
After scientific, technical and programmatic review and consideration of appropriate factors, the NSF Program Officer recommends to the cognizant Division Director whether the proposal should be declined or recommended for award. NSF strives to be able to tell applicants whether their proposals have been declined or recommended for funding within six months. Large or particularly complex proposals or proposals from new awardees may require additional review and processing time. The time interval begins on the deadline or target date, or receipt date, whichever is later. The interval ends when the Division Director acts upon the Program Officer's recommendation.
After programmatic approval has been obtained, the proposals recommended for funding will be forwarded to the Division of Grants and Agreements for review of business, financial, and policy implications. After an administrative review has occurred, Grants and Agreements Officers perform the processing and issuance of a grant or other agreement. Proposers are cautioned that only a Grants and Agreements Officer may make commitments, obligations or awards on behalf of NSF or authorize the expenditure of funds. No commitment on the part of NSF should be inferred from technical or budgetary discussions with a NSF Program Officer. A Principal Investigator or organization that makes financial or personnel commitments in the absence of a grant or cooperative agreement signed by the NSF Grants and Agreements Officer does so at their own risk.
Once an award or declination decision has been made, Principal Investigators are provided feedback about their proposals. In all cases, reviews are treated as confidential documents. Verbatim copies of reviews, excluding the names of the reviewers or any reviewer-identifying information, are sent to the Principal Investigator/Project Director by the Program Officer. In addition, the proposer will receive an explanation of the decision to award or decline funding.
VII. Award Administration Information
A. notification of the award.
Notification of the award is made to the submitting organization by a Grants Officer in the Division of Grants and Agreements. Organizations whose proposals are declined will be advised as promptly as possible by the cognizant NSF Program administering the program. Verbatim copies of reviews, not including the identity of the reviewer, will be provided automatically to the Principal Investigator. (See Section VI.B. for additional information on the review process.)
B. Award Conditions
An NSF award consists of: (1) the award notice, which includes any special provisions applicable to the award and any numbered amendments thereto; (2) the budget, which indicates the amounts, by categories of expense, on which NSF has based its support (or otherwise communicates any specific approvals or disapprovals of proposed expenditures); (3) the proposal referenced in the award notice; (4) the applicable award conditions, such as Grant General Conditions (GC-1)*; or Research Terms and Conditions* and (5) any announcement or other NSF issuance that may be incorporated by reference in the award notice. Cooperative agreements also are administered in accordance with NSF Cooperative Agreement Financial and Administrative Terms and Conditions (CA-FATC) and the applicable Programmatic Terms and Conditions. NSF awards are electronically signed by an NSF Grants and Agreements Officer and transmitted electronically to the organization via e-mail.
*These documents may be accessed electronically on NSF's Website at https://www.nsf.gov/awards/managing/award_conditions.jsp?org=NSF . Paper copies may be obtained from the NSF Publications Clearinghouse, telephone (703) 292-7827 or by e-mail from [email protected] .
More comprehensive information on NSF Award Conditions and other important information on the administration of NSF awards is contained in the NSF Proposal & Award Policies & Procedures Guide (PAPPG) Chapter VII, available electronically on the NSF Website at https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg .
Special Award Conditions:
The awardee is responsible for security of all equipment and information systems funded directly or indirectly by this award. The awardee may be required to present to the cognizant NSF Program Officer and Grants and Agreements Officer an IT security plan addressing policies and procedures for review and approval within 60 days of award. The plan should include evaluation criteria that will measure the successful implementation and deployment of the plans, policies and procedures.
Awards with significant software development or application interactions will be subject to the following conditions:
* identification within the 1st year of award the software's open source license to be used.
C. Reporting Requirements
For all multi-year grants (including both standard and continuing grants), the Principal Investigator must submit an annual project report to the cognizant Program Officer no later than 90 days prior to the end of the current budget period. (Some programs or awards require submission of more frequent project reports). No later than 120 days following expiration of a grant, the PI also is required to submit a final project report, and a project outcomes report for the general public.
Failure to provide the required annual or final project reports, or the project outcomes report, will delay NSF review and processing of any future funding increments as well as any pending proposals for all identified PIs and co-PIs on a given award. PIs should examine the formats of the required reports in advance to assure availability of required data.
PIs are required to use NSF's electronic project-reporting system, available through Research.gov, for preparation and submission of annual and final project reports. Such reports provide information on accomplishments, project participants (individual and organizational), publications, and other specific products and impacts of the project. Submission of the report via Research.gov constitutes certification by the PI that the contents of the report are accurate and complete. The project outcomes report also must be prepared and submitted using Research.gov. This report serves as a brief summary, prepared specifically for the public, of the nature and outcomes of the project. This report will be posted on the NSF website exactly as it is submitted by the PI.
More comprehensive information on NSF Reporting Requirements and other important information on the administration of NSF awards is contained in the NSF Award & Administration Guide (AAG) Chapter II, available electronically on the NSF Website at https://www.nsf.gov/publications/pub_summ.jsp?ods_key=aag .
VIII. Agency Contacts
Please note that the program contact information is current at the time of publishing. See program website for any updates to the points of contact.
General inquiries regarding this program should be made to:
For questions related to the use of FastLane, contact:
FastLane Help Desk, telephone: 1-800-673-6188; e-mail: [email protected] .
For questions relating to Grants.gov contact:
Grants.gov Contact Center: If the Authorized Organizational Representatives (AOR) has not received a confirmation message from Grants.gov within 48 hours of submission of application, please contact via telephone: 1-800-518-4726; e-mail: [email protected] .
IX. Other Information
The NSF website provides the most comprehensive source of information on NSF Directorates (including contact information), programs and funding opportunities. Use of this website by potential proposers is strongly encouraged. In addition, "NSF Update" is an information-delivery system designed to keep potential proposers and other interested parties apprised of new NSF funding opportunities and publications, important changes in proposal and award policies and procedures, and upcoming NSF Grants Conferences . Subscribers are informed through e-mail or the user's Web browser each time new publications are issued that match their identified interests. "NSF Update" also is available on NSF's website .
Grants.gov provides an additional electronic capability to search for Federal government-wide grant opportunities. NSF funding opportunities may be accessed via this mechanism. Further information on Grants.gov may be obtained at http://www.grants.gov .
About The National Science Foundation
The National Science Foundation (NSF) is an independent Federal agency created by the National Science Foundation Act of 1950, as amended (42 USC 1861-75). The Act states the purpose of the NSF is "to promote the progress of science; [and] to advance the national health, prosperity, and welfare by supporting research and education in all fields of science and engineering."
NSF funds research and education in most fields of science and engineering. It does this through grants and cooperative agreements to more than 2,000 colleges, universities, K-12 school systems, businesses, informal science organizations and other research organizations throughout the US. The Foundation accounts for about one-fourth of Federal support to academic institutions for basic research.
NSF receives approximately 55,000 proposals each year for research, education and training projects, of which approximately 11,000 are funded. In addition, the Foundation receives several thousand applications for graduate and postdoctoral fellowships. The agency operates no laboratories itself but does support National Research Centers, user facilities, certain oceanographic vessels and Arctic and Antarctic research stations. The Foundation also supports cooperative research between universities and industry, US participation in international scientific and engineering efforts, and educational activities at every academic level.
Facilitation Awards for Scientists and Engineers with Disabilities (FASED) provide funding for special assistance or equipment to enable persons with disabilities to work on NSF-supported projects. See the NSF Proposal & Award Policies & Procedures Guide Chapter II.E.6 for instructions regarding preparation of these types of proposals.
The National Science Foundation has Telephonic Device for the Deaf (TDD) and Federal Information Relay Service (FIRS) capabilities that enable individuals with hearing impairments to communicate with the Foundation about NSF programs, employment or general information. TDD may be accessed at (703) 292-5090 and (800) 281-8749, FIRS at (800) 877-8339.
The National Science Foundation Information Center may be reached at (703) 292-5111.
Privacy Act And Public Burden Statements
The information requested on proposal forms and project reports is solicited under the authority of the National Science Foundation Act of 1950, as amended. The information on proposal forms will be used in connection with the selection of qualified proposals; and project reports submitted by awardees will be used for program evaluation and reporting within the Executive Branch and to Congress. The information requested may be disclosed to qualified reviewers and staff assistants as part of the proposal review process; to proposer institutions/grantees to provide or obtain data regarding the proposal review process, award decisions, or the administration of awards; to government contractors, experts, volunteers and researchers and educators as necessary to complete assigned work; to other government agencies or other entities needing information regarding applicants or nominees as part of a joint application review process, or in order to coordinate programs or policy; and to another Federal agency, court, or party in a court or Federal administrative proceeding if the government is a party. Information about Principal Investigators may be added to the Reviewer file and used to select potential candidates to serve as peer reviewers or advisory committee members. See Systems of Records, NSF-50 , "Principal Investigator/Proposal File and Associated Records," 69 Federal Register 26410 (May 12, 2004), and NSF-51 , "Reviewer/Proposal File and Associated Records," 69 Federal Register 26410 (May 12, 2004). Submission of the information is voluntary. Failure to provide full and complete information, however, may reduce the possibility of receiving an award.
An agency may not conduct or sponsor, and a person is not required to respond to, an information collection unless it displays a valid Office of Management and Budget (OMB) control number. The OMB control number for this collection is 3145-0058. Public reporting burden for this collection of information is estimated to average 120 hours per response, including the time for reviewing instructions. Send comments regarding the burden estimate and any other aspect of this collection of information, including suggestions for reducing this burden, to:
Suzanne H. Plimpton Reports Clearance Officer Office of the General Counsel National Science Foundation Alexandria, VA 22314

Representations and Warranties
Hotwire Funding LLC, Secured Fiber Network Revenue Notes, Series 2024-1 -- Appendix
Fri 17 May, 2024 - 6:37 PM ET
We've detected unusual activity from your computer network
To continue, please click the box below to let us know you're not a robot.
Why did this happen?
Please make sure your browser supports JavaScript and cookies and that you are not blocking them from loading. For more information you can review our Terms of Service and Cookie Policy .
For inquiries related to this message please contact our support team and provide the reference ID below.

IMAGES
COMMENTS
The goal of the RCN program is to advance a field or create new directions in research or education by supporting groups of investigators to communicate and coordinate their research, training and educational activities across disciplinary, organizational, geographic, and international boundaries. The RCN program provides opportunities to ...
These Network Research Hubs must be actively part of (i.e., active funding/award) one or more of the following: NIH Institutional Development Award Clinical and Translational Research (IDeA-CTR) awards, the NIH Clinical and Translational Science Award (CTSA) Program, and/or the Patient-Centered Outcomes Research Institute's (PCORI) Patient ...
Health Equity Research Network (HERN) on Community-Driven Research Approaches, co-funded by the American Heart Association and The Robert Wood Johnson Foundation funding opportunities to strengthen infrastructure to support community-driven health organizations, leading to improvements in upstream health disparities to ultimately help people live longer, healthier lives.
MSPI Research Network Structure. The MSPI Research Network will consist of the awarded UG3/UH3 Research Projects and a Coordinating Center (CC) (see RFA-OD-24-006). The CC and each of the Research Projects will also work collaboratively with one or more Project Scientists and Program Officers from NIH institutes, centers, and offices (ICOs).
But creating a research network requires you to have a strong personal network. A big part of being the person, or group, that starts a research network is that the most effective first step is to reach out to your immediate circle of colleagues to spread the word for you about this shiny new endeavour. They may not be the actual people who ...
The actual number of awards varies across disciplinary research programs. Anticipated Funding Amount: $7,500,000 to $12,500,000. ... A Research Coordination Network is an important opportunity for encouraging the involvement of investigators from underrepresented groups (women, underrepresented minorities, and persons with disabilities), early ...
This funding opportunity announcement (FOA) solicits applications for Technology Research and Development Centers (TRDCs) for the Point of Care Technology Research Network (POCTRN). POCTRN's purpose is to drive the development and application of innovative point of care, home-based, improved clinical laboratory tests and testing technologies.
Advance diversity in science & engineering. For decades, NSF has been one of the largest federal investors in AI across all 50 states, Washington, D.C., and every U.S. territory, accelerating responsible AI innovation, driving cutting-edge research and preparing the next-generation AI workforce — even through AI winters.
Of an estimated 6,500 to 7,000 known rare diseases, only a fraction - maybe 5% - have U.S. Food and Drug Administration-approved treatments. To increase that percentage, the National Institutes of Health has awarded approximately $31 million in grants in fiscal year 2019 to 20 teams - including five new groups -- of scientists, clinicians ...
Sustained engagement in training programs and joint applications to research funding can stimulate research network development . RECOOP HST Association is a platform that offers such research networking opportunities. Benefits of participation in such consortium can be fully appreciated in the long-term, but its positive impact is already ...
R03. NIH Small Grant Program (R03): Provides limited funding for a short period of time to support a variety of types of projects, including pilot or feasibility studies, collection of preliminary data, secondary analysis of existing data, small, self-contained research projects, development of new research technology, etc.
Date PostedMonday, November 21, 2022. The National Cancer Institute Division of Cancer Prevention has released three Funding Opportunity Announcements to accept applications to create the Cancer Screening Research Network (CSRN). The CSRN will conduct rigorous, multi-center cancer screening trials and studies to evaluate emerging technologies ...
In the last 10 years, the federal government has increased funding for research and development (R&D)—investing $179.5 billion in FY 2021. DOD and the Department of Health and Human Services received 77% of the FY 2021 funding. COVID-19 stimulus funding led to large R&D increases for HHS. ... For example, more than 60 agencies participate in ...
Research.gov is a partnership of Federal, ... Supplemental Funding Requests (including Career-Life Balance) Demo Site: Supplement Funding Requests (Training) Continuing Grant Increments Reports . Fellowships & Honorary Awards Nominate colleagues, apply for awards Graduate Research Fellowship Program (GRFP) ...
From 2020-2023, the Research Network Funding is available with the support of the Erasmus+ Programme of the European Union. The European Commission's support for the production of this publication does not constitute an endorsement of the contents, which reflect the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information ...
Funding is intended to cover the operating and support costs of the network and full justification for the sum requested should be included in the proposal. Funding will not be renewed beyond the original length of the grant. Requests for funding may include the following: project lead and co-lead salaries. travel and subsistence.
The NIHR Clinical Research Network (CRN) supports patients, the public and health and care organisations across England to participate in high-quality research, thereby advancing knowledge and improving care. The CRN supports research being delivered through 30 specialty therapy areas and 15 Local Clinical Research Networks.
The research networking scheme supports the exchange of ideas between researchers and stakeholders by funding the costs of workshops, seminars or other networking activities, covering up to £30,000 for a period of up to two years, with an additional £15,000 for international costs.
Funded projects will assist the U.S. research and education community by enabling state-of-the-art international network services and access to increased collaboration and data services. NSF expects to make 3 to 10 awards in production R&E network infrastructure; 1 to 3 awards in international testbeds; and 1 award in Engagement.
R03 grants support small research projects that can be carried out in a short period of time with limited resources. Potential projects include: Pilot and feasibility studies. Secondary analysis of existing data. Small, self-contained research projects. Development of research methodology; and. Development of new research technology
The Division of Rare Diseases Research Innovation (DRDRI), within the National Center for Advancing Translational Sciences (NCATS), along with the Institutes, Centers, and Offices (ICOs) listed in Part I at the National Institutes of Health (NIH), invites applications in response to this Notice of Funding Opportunity (NOFO) for the Rare Diseases Clinical Research Consortia (RDCRC) component of ...
The Environmental and Racial Justice Network (ERJN) provides seed funding for projects that address issues at the intersection of environmental and racial justice. Funding of up to $2,000 is available for qualifying projects led by undergraduate or graduate students. Students are encouraged to form teams that include other students, staff, and ...
The four-year awards, which started April 1, 2024, include a collaborative research project three groups, called centers. To further the American Heart Association's commitments to expanding diversity in clinical research, each of the centers will work in conjunction with an academic institution that primarily serves individuals who are underrepresented in science.
Competing Interest Statement. Acknowledgements A.L.M. is a recipient of post-doctoral funding by AstraZeneca through the Crick/AZ Alliance. D.R.C is supported by the Francis Crick Institute, which receives its core funding form Cancer Research UK (FC001169), the UK Medical Research Council (FC002269), and the Wellcome Trust (FC001169), as well as an NC3Rs training fellowship (NC/S001832/1).
WASHINGTON — Elon Musk demanded the arrest and prosecution of Dr. Anthony Fauci on Friday after the National Institutes of Health came clean to Congress and admitted funding risky "gain-of ...
The International Research Network Connections (IRNC) program supports high-performance network connectivity required by international science and engineering research and education collaborations involving the NSF research community. ... Anticipated Funding Amount: $3,600,000. The anticipated funding amount is $3,600,000 total for this ...
Hotwire Funding LLC, Secured Fiber Network Revenue Notes, Series 2024-1 -- Appendix. Fri 17 May, 2024 - 6:37 PM ET. of 11. This Representations and Warranties Report supplements the Presale Report for Hotwire Funding LLC, Secured Fiber Network Revenue Notes, Series 2024-1. Our Pres.
1:11. Elon Musk 's artificial intelligence startup X.AI Corp. is set to close its funding round at a valuation of about $18 billion as soon as this week, according to people familiar with the ...