Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology of Xanthium strumarium L.: A Review
Affiliations.
- 1 School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. [email protected].
- 2 School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. [email protected].
- 3 School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. [email protected].
- 4 School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. [email protected].
- 5 School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. [email protected].
- 6 School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. [email protected].
- 7 School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. [email protected].
- 8 School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. [email protected].
- 9 Sichuan Neautus Traditional Chinese Herb Limited Company, Chengdu 611731, China. [email protected].
- 10 School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. [email protected].
- 11 School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. [email protected].
- PMID: 30669496
- PMCID: PMC6359306
- DOI: 10.3390/molecules24020359
Xanthium strumarium L. (Asteraceae) is a common and well-known traditional Chinese herbal medicine usually named Cang-Er-Zi, and has been used for thousands of years in China. The purpose of this paper is to summarize the progress of modern research, and provide a systematic review on the traditional usages, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology of the X. strumarium . Moreover, an in-depth discussion of some valuable issues and possible development for future research on this plant is also given. X. strumarium , as a traditional herbal medicine, has been extensively applied to treat many diseases, such as rhinitis, nasal sinusitis, headache, gastric ulcer, urticaria, rheumatism bacterial, fungal infections and arthritis. Up to now, more than 170 chemical constituents have been isolated and identified from X. strumarium , including sesquiterpenoids, phenylpropenoids, lignanoids, coumarins, steroids, glycosides, flavonoids, thiazides, anthraquinones, naphthoquinones and other compounds. Modern research shows that the extracts and compounds from X. strumarium possess wide-ranging pharmacological effects, including anti- allergic rhinitis (AR) effects, anti-tumor effects, anti-inflammatory and analgesic effects, insecticide and antiparasitic effects, antioxidant effects, antibacterial and antifungal effects, antidiabetic effects, antilipidemic effects and antiviral effects. However, further research should focus on investigating bioactive compounds and demonstrate the mechanism of its detoxification, and more reasonable quality control standards for X. strumarium should also be established.
Keywords: Xanthium strumarium L.; botany; pharmacokinetics; pharmacology; phytochemistry; toxicology; traditional usages.

Publication types
- Anti-Allergic Agents / therapeutic use
- Anti-Inflammatory Agents / therapeutic use
- Antineoplastic Agents / therapeutic use
- Antioxidants / therapeutic use
- Antiparasitic Agents / therapeutic use
- Drugs, Chinese Herbal / pharmacology*
- Glycosides / chemistry
- Molecular Structure
- Phytotherapy
- Plant Extracts / pharmacokinetics*
- Plant Extracts / pharmacology*
- Plants, Medicinal / chemistry
- Xanthium / chemistry*
- Anti-Allergic Agents
- Anti-Inflammatory Agents
- Antineoplastic Agents
- Antioxidants
- Antiparasitic Agents
- Drugs, Chinese Herbal
- Plant Extracts
Comparative Assessment of Remediation Potential of Xanthium strumarium Ecotypes in NaCl-Affected Root Zone
- Published: 01 December 2022
- Volume 233 , article number 509 , ( 2022 )
Cite this article

- Noreen Akhter 1 na1 ,
- Muhammad Aqeel ORCID: orcid.org/0000-0003-0690-4783 2 na1 ,
- Muhammad Faisal Maqsood 3 ,
- Saher Nawaz 4 ,
- Muhammad Muslim Shahnaz 5 ,
- Noreen Khalid 6 ,
- Mohammed A. Basahi 7 ,
- Omar Mahmoud Al-Zoubi 8 ,
- Talaat Habeeb 8 ,
- Romina Alina Marc 9 ,
- Muhammad Kashif Irshad 10 &
- Ali Noman 11
302 Accesses
2 Citations
Explore all metrics
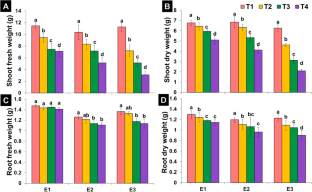
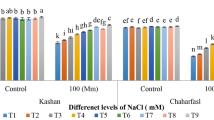
In the present study, three ecotypes of Xanthium strumarium L. were collected from different ecological regions, i.e., Uchalli (E 1 ), Sargodha (E 2 ), and Samundri (E 3 ) in Punjab province, Pakistan. All ecotypes were assessed for their salt resistance and remediation capacity at different NaCl levels (T 1 (control), T 2 (50 mM), T 3 (100 mM), and T 4 (150 mM)). Xanthium responses to varied NaCl levels were investigated for growth, anatomical, and physio-biochemical attributes. A comparative account of different attributes revealed a performance difference between three ecotypes. Biomass of all ecotypes was reduced, but the minimum reduction in biomass was noticed in E 1 as compared to other ecotypes. Comparison of control and NaCl-treated Xanthium plants indicated highest reduction (96.3%) for both chlorophyll a and chlorophyll b observed under T 4 in E 3 as compared to E 1 and E 2 . Maximum increase in carotenoids was observed in E 2 (82.2%) under T 4 . Shoot Na + correspondingly increased in all ecotypes with NaCl levels and maximum Na + in E 3 . Minimum absorption of Cl − ion was observed in E 1 . Osmoprotectants in three ecotypes were much higher under elevated NaCl levels than that of control plants. A significant increase in total soluble sugars was recorded in E1 (76.82%) and E2 (45.35%) as compared to E3. Additionally, significant anatomical changes in stem, leaf, and root of X. strumarium L. were observed in all three ecotypes grown under NaCl conditions. E1 ecotype showed large vascular bundle cell area (119.23%), (110.95%), (56.34%), epidermal thickness (152.21%), (187.40%), (117.28%) of leaf, root, and stem, respectively, as compared to E2 and E3 ecotypes. Similarly, E1 ecotypes showed the highest percentage increase in root sclerenchyma cell area (93.46%), stem epidermal cell area (246.66%), stem cortical cell area (114.23%), and stem sclerenchyma cell area (92.5%) as compared to E2 and E3 ecotypes. These findings endorse differential capabilities of different ecotypes of the same plant with reference to the changes in studied attributes. Overall, results indicate that X. strumarium L. withstood high NaCl stress (150 mM) and can be an eco-friendly source for the phytoremediation of saline soils. It could serve as a foundation for future research on the plant adaptability. We recommend use of this plant for restoring saline-degraded and marginal soils.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Comparative physiology of Arthrocnemum indicum and Tamarix gallica under aluminum alone or combined with NaCl

Foliar Application of Nano-zinc and Iron Affects Physiological Attributes of Rosmarinus officinalis and Quietens NaCl Salinity Depression
Nano-silicone and Ascophyllum nodosum-based biostimulant down-regulates the negative effect of in vitro induced-salinity in Rosa damascena
Data availability.
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abouelsaad, I., & Renault, S. (2018). Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. Journal of Plant Physiology, 226 , 136–144.
Article CAS Google Scholar
Aghaleh, M., Niknam, V., Ebrahimzadeh, H., & Razavi, K. (2011). Effect of salt stress on physiological and antioxidative responses in two species of Salicornia (S persica and S europaea). Acta Physiologiae Plantarum, 33 (4), 1261–1270.
Akcin, A., & Yalcin, E. (2016). Effect of salinity stress on chlorophyll, carotenoid content, and proline in Salicornia prostrata Pall and Suaeda prostrata Pall subsp prostrata (Amaranthaceae). Brazilian Journal of Botany, 39 (1), 101–106.
Article Google Scholar
Akhter, N., Aqeel, M., Shahnaz, M. M., Alnusairi, G. S., Alghanem, S. M., Kousar, A., Hashem, M., Kanwal, H., Alamri, S., & Ilyas, A. (2021). Physiological homeostasis for ecological success of Typha (Typha domingensis Pers) populations in saline soils. Physiology and Molecular Biology of Plants, 27 (4), 687–701.
Akhter, N., Habiba, O., Hina, M., Shahnaz, M. M., Alzuaibr, F. M., Alamri, S., Hashem, M., Khalid, N., Aqeel, M., & Noman, A. (2022). Structural, Biochemical, and physiological adjustments for toxicity management, accumulation, and remediation of cadmium in wetland ecosystems by typha domingensis pers. Water, Air, & Soil Pollution, 233 (5), 1–23.
Akram, M., Akhtar, S., Javed, I., Wahid, A., & Rasul, E. (2002). Anatomical attributes of different wheat (Triticum aestivum ) accessions/varieties to NaCl salinity. International Journal of Agriculture and Biology, 4 , 166–168.
Google Scholar
Akram, S., Siddiqui, M., Hussain, B., Al Bari, M., Mostofa, M. G., Hossain, M. A., & Tran, L.-S.P. (2017). Exogenous glutathione modulates salinity tolerance of soybean (Glycine max (L) Merrill) at reproductive stage. Journal of Plant Growth Regulation, 36 (4), 877–888.
Alam, M., Juraimi, A. S., Rafii, M. & Abdul Hamid, A. (2015). Effect of salinity on biomass yield and physiological and stem-root anatomical characteristics of purslane ( Portulaca oleracea L) accessions. BioMed research international, 2015.
Alorfi, H. S., Alshehry, A. A., Ghandourah, M. A., Bawakid, N. O., Elfaky, M. A., Ali, A. M., & Alarif, W. M. (2020). Cytotoxic isoprenoids from Xanthium strumarium linn. Pharmacognosy Magazine, 16 (70), 391.
Aqeel, M., Khalid, N., Tufail, A., Ahmad, R. Z., Akhter, M. S., Luqman, M., Javed, M. T., Irshad, M. K., Alamri, S., Hashem, M. & Noman, A. (2021). Elucidating the distinct interactive impact of cadmium and nickel on growth, photosynthesis, metal-homeostasis, and yield responses of mung bean ( Vigna radiata L) varieties. Environmental Science and Pollution Research, 1–15.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiology, 24 (1), 1–15.
Awasthi, O., Pathak, R., & Pandey, S. (1999). Anatomical variation in leaf lamina of ber seedling and budded plants grown at different sodicity levels. Indian Journal of Horticulture, 56 (1), 29–33.
Barhoumi, Z., Djebali, W., Chaïbi, W., Abdelly, C., & Smaoui, A. (2007). Salt impact on photosynthesis and leaf ultrastructure of Aeluropus littoralis . Journal of Plant Research, 120 (4), 529–537.
Beinsan, C., Camen, D., Sumalan, R. & Babau, M. (2009). 'Study concerning salt stress effect on leaf area dynamics and chlorophyll content in four bean local landraces from Banat area', 44th Croatian & 4th International Symposium on Agriculture, Opatija, Bosnia and Herzegovina.
Bhattarai, S., Biswas, D., Fu, Y.-B., & Biligetu, B. (2020). Morphological, physiological, and genetic responses to salt stress in alfalfa: A review. Agronomy, 10 (4), 577.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72 (1–2), 248–254.
Devi, S., Nandwal, A., Angrish, R., Arya, S., Kumar, N., & Sharma, S. (2016). Phytoremediation potential of some halophytic species for soil salinity. International Journal of Phytoremediation, 18 (7), 693–696.
Ditta, A., Imtiaz, M., Mehmood, S., Rizwan, M. S., Mubeen, F., Aziz, O., Qian, Z., Ijaz, R., & Tu, S. (2018). Rock phosphate-enriched organic fertilizer with phosphate-solubilizing microorganisms improves nodulation, growth, and yield of legumes. Communications in Soil Science and Plant Analysis, 49 (21), 2715–2725.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P., & t. & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28 (3), 350–356.
FAO. (2009). How to feed the world in 2050. Population and Development Review, 35 , 837–839.
Farhangi-Abriz, S., & Torabian, S. (2017). Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicology and Environmental Safety, 137 , 64–70.
Flowers, T. J., Galal, H. K., & Bromham, L. (2010). Evolution of halophytes: Multiple origins of salt tolerance in land plants. Functional Plant Biology, 37 (7), 604–612.
Flowers, T. J. & Colmer, T. D. (2008). Salinity tolerance in halophytes. New Phytologist, 945–963.
Freire, M. B. G. S., Freire, F. J., Pessoa, L. G. M., Souza, E. R. d. & Gheyi, H. R. (2021). 'Salt affected soils in the Brazilian semiarid and phytoremediation as a reclamation alternative', Saline and Alkaline Soils in Latin America, Springer, pp. 119–139.
Gupta, B. & Huang, B. (2014). Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. International Journal of Genomics, 2014.
Haque, M. I., Siddiqui, S. A., Jha, B. & Rathore, M. S. (2021). Interactive effects of abiotic stress and elevated CO2 on physio-chemical and photosynthetic responses in Suaeda species. Journal of Plant Growth Regulation, 1–19.
He, Y., & Ma, M. (2018). Responses of seed germination of the invasive plant Xanthium italicum to environmental factors. Acta Ecology Sinica, 38 , 1226–1234.
CAS Google Scholar
Hu, L., Chen, L., Liu, L., Lou, Y., Amombo, E., & Fu, J. (2015). Metabolic acclimation of source and sink tissues to salinity stress in bermudagrass ( Cynodon dactylon ). Physiologia Plantarum, 155 (2), 166–179.
Jackson, M. L. (1962). Soil chemical analysis-Advanced Course, Madison, Wisconsin Dept. of Soil Science, Univ. of Wisconsin
Jiang, J.-L., Tian, Y., Li, L., Yu, M., Hou, R.-P., & Ren, X.-M. (2019). H 2 S alleviates salinity stress in cucumber by maintaining the Na + /K + balance and regulating H 2 S metabolism and oxidative stress response. Frontiers in Plant Science, 10 , 678.
Karakas, S., Dikilitas, M. & Tıpırdamaz, R. (2020). Phytoremediation of salt-affected soils using halophytes. Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture, 1–18.
Katschnig, D., Broekman, R., & Rozema, J. (2013). Salt tolerance in the halophyte Salicornia dolichostachya Moss: Growth, morphology and physiology. Environmental and Experimental Botany, 92 , 32–42.
Kilic, S., Cavusoglu, K., & Kabar, K. (2007). Effects of 24-epibrassinolide on salinity stress induced inhibition of seed germination, seedling growth and leaf anatomy of barley. Süleyman Demirel Üniversitesi Fen Edebiyat Fakültesi Fen Dergisi, 2 (1), 41–52.
Kirk, J., & Allen, R. (1965). Dependence of chloroplast pigments synthesis on protein effects on actilione. Biochem. Biph. Res. Cann, 27 , 523–530.
Kumar, S., Beena, A., Awana, M., & Singh, A. (2017). Physiological, biochemical, epigenetic and molecular analyses of wheat ( Triticum aestivum ) genotypes with contrasting salt tolerance. Frontiers in Plant Science, 8 , 1151.
Moore, S., & Stein, W. H. (1948). Photometric ninhydrin method for use in the chromatography of amino acids. Journal of Biological Chemistry, 176 , 367–388.
Naheed, R., Zahid, M., Aqeel, M., Maqsood, M. F., Kanwal, H., Khalid, N., Hashem, M., Alamri, S. & Noman, A. (2022). Mediation of growth and metabolism of Pisum sativum in salt stress potentially be credited to thiamine. Journal of Soil Science and Plant Nutrition, 1–14.
Nasir, F. A., Batarseh, M., Abdel‐Ghani, A. H. & Jiries, A. (2010). Free amino acids content in some halophytes under salinity stress in arid environment Jordan. CLEAN–Soil Air Water 38 (7) 592–600.
Niknam, V., Sharifizadeh, B., Ebrahimzadeh, H., Zarre, S., & Izadpanah, M. (2004). Comparative study of proteins in seeds of some species of Trigonella from Iran. Iranian International Journal of Science, 5 (1), 1–11.
Noreen, S., Faiz, S., Akhter, M. S., & Shah, K. H. (2019). Influence of foliar application of osmoprotectants to ameliorate salt stress in sunflower (Helianthus annuus L). Sarhad Journal of Agriculture, 35 (4), 1316–1325.
Nurzhanova, A., Mukasheva, T., Berzhanova, R., Kalugin, S., Omirbekova, A., & Mikolasch, A. (2021). Optimization of microbial assisted phytoremediation of soils contaminated with pesticides. International Journal of Phytoremediation, 23 (5), 482–491.
Ola, H. A. E., Reham, E. F., Eisa, S., & Habib, S. (2012). Morpho-anatomical changes in salt stressed kallar grass (Leptochloa fusca L Kunth). Research Journal of Agriculture and Biological Sciences, 8 (2), 158–166.
Pan, G., Liu, W., Zhang, H., & Liu, P. (2018). Morphophysiological responses and tolerance mechanisms of Xanthium strumarium to manganese stress. Ecotoxicology and Environmental Safety, 165 , 654–661.
Pan, G., Zhang, H., Liu, P., Xiao, Z., Li, X., & Liu, W. (2019). Effects of manganese stress on phenology and biomass allocation in Xanthium strumarium from metalliferous and non-metalliferous sites. Ecotoxicology and Environmental Safety, 172 , 308–316.
Parida, A. K., Veerabathini, S. K., Kumari, A., & Agarwal, P. K. (2016). Physiological, anatomical and metabolic implications of salt tolerance in the halophyte Salvadora persica under hydroponic culture condition. Frontiers in Plant Science, 7 , 351.
Parihar, P., Singh, S., Singh, R., Singh, V. P., & Prasad, S. M. (2015). Effect of salinity stress on plants and its tolerance strategies: A review. Environmental Science and Pollution Research, 22 (6), 4056–4075.
Qadir, M., Schubert, S., Ghafoor, A., & Murtaza, G. (2001). Amelioration strategies for sodic soils: A review. Land Degradation & Development, 12 (4), 357–386.
Rabhi, M., Hafsi, C., Lakhdar, A., Hajji, S., Barhoumi, Z., Hamrouni, M. H., Abdelly, C., & Smaoui, A. (2009). Evaluation of the capacity of three halophytes to desalinize their rhizosphere as grown on saline soils under nonleaching conditions. African Journal of Ecology, 47 (4), 463–468.
Saule, A., Akmaral, N., Subhash, M., Aygul, A., Saule, K., Saule, A., Asil, N., Anar, Z., Saltanat, A., & Ravilya, A. (2013). The effect of salinity on growth and anatomical attributes of barley seedling (Hordeum vulgare L). African Journal of Biotechnology, 12 (18), 2366–2377.
Shabala, S., Hariadi, Y., & Jacobsen, S.-E. (2013). Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na + loading and stomatal density. Journal of Plant Physiology, 170 (10), 906–914.
Shabani, M. (2016). CO 2 bio-sequestration by Chlorella vulgaris and Spirulina platensis in response to different levels of salinity and CO 2 . Proceedings of the International Academy of Ecology and Environmental Sciences, 6 (2), 53.
Shabani, N., & Sayadi, M. (2012). Evaluation of heavy metals accumulation by two emergent macrophytes from the polluted soil: An experimental study. The Environmentalist, 32 (1), 91–98.
Shahzad, R., Khan, A. L., Bilal, S., Waqas, M., Kang, S.-M., & Lee, I.-J. (2017). Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa . Environmental and Experimental Botany, 136 , 68–77.
Silva, B., Batista, B., & Lobato, A. (2021). Anatomical changes in stem and root of soybean plants submitted to salt stress. Plant Biology, 23 (1), 57–65.
Singh, J., Singh, V., & Sharma, P. (2018). Elucidating the role of osmotic, ionic and major salt responsive transcript components towards salinity tolerance in contrasting chickpea (Cicer arietinum L) genotypes. Physiology and Molecular Biology of Plants, 24 (3), 441–453.
Slama, I., Abdelly, C., Bouchereau, A., Flowers, T., & Savouré, A. (2015). Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Annals of Botany, 115 (3), 433–447.
Steel, R. G., Torrie, J. H., & Dickey, D. A. (1997). Principles and procedures of statistics (pp. 4–7). McGraw-Hill.
Stirzaker, R., Cook, F., & Knight, J. (1999). Where to plant trees on cropping land for control of dryland salinity: Some approximate solutions. Agricultural Water Management, 39 (2–3), 115–133.
Tavakkoli, E., Rengasamy, P., & McDonald, G. K. (2010). High concentrations of Na + and Cl – ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. Journal of Experimental Botany, 61 (15), 4449–4459.
Ventura, Y., Myrzabayeva, M., Alikulov, Z., Omarov, R., Khozin-Goldberg, I. & Sagi, M. (2014). Effects of salinity on flowering, morphology, biomass accumulation and leaf metabolites in an edible halophyte. AoB Plants, 6.
Vijayan, K., Chakraborti, S. P., Ercisli, S., & Ghosh, P. D. (2008). NaCl induced morpho-biochemical and anatomical changes in mulberry (Morus spp). Plant Growth Regulation, 56 (1), 61–69.
Voltolini, C. H., Reis, A. & Santos, M. (2009). Leaf morphoanatomy of rheophyte Dyckia distachya Hassler (Bromeliaceae). Revista Brasileira de Biociências , 7 (4).
Wasim, M. & Naz, N. (2020). Anatomical adaptations of tolerance to salt stress in Cenchrus ciliaris L., a saline desert grass. JAPS: Journal of Animal & Plant Sciences, 30 (6).
Wolf, B. (1982). A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Communications in Soil Science and Plant Analysis, 13 (12), 1035–1059.
Xue, C., Gao, Y., Qu, B., Tai, P., Guo, C., Chang, W. & Zhao, G. (2021). Hybridization with an invasive plant of Xanthium strumarium improves the tolerance of its native congener X. sibiricum to cadmium. Frontiers in Plant Science, 1543.
Yadav, S. P., Bharadwaj, R., Nayak, H., Mahto, R., Singh, R. K., & Prasad, S. K. (2019). Impact of salt stress on growth, productivity and physicochemical properties of plants: A Review. International Journal of Chemical Studies, 7 (2), 1793–1798.
Zheng, H., & Li, J. (1999). Saline plants in Songnen Plain and restoration of alkaline-saline grass (pp. 179–206). China, Science Press of China.
Zojaji, F., Hassani, A., & Sayadi, M. (2015). A comparative study on heavy metal content of plants irrigated with tap and wastewater. International Journal of Environmental Science and Technology, 12 (3), 865–870.
Download references
Acknowledgements
The authors are highly thankful to the Government College for Women University, Faisalabad, Pakistan, for providing experimental station and lab facilities.
Author information
Noreen Akhter and Muhammad Aqeel contributed equally to this publication work.
Authors and Affiliations
Department of Botany, Government College Women University, Faisalabad, 38000, Pakistan
Noreen Akhter
State Key Laboratory of Grassland Agro-Ecosystems, College of Ecology, Lanzhou University, Lanzhou, 730000, Gansu, People’s Republic of China
Muhammad Aqeel
Department of Botany, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
Muhammad Faisal Maqsood
Department of Botany, Ghazi University, Dera Ghazi Khan, Pakistan
Saher Nawaz
Department of Botany, Govt. Post-Graduate College of Science, Faisalabad, Pakistan
Muhammad Muslim Shahnaz
Department of Botany, Government College Women University, Sialkot, Pakistan
Noreen Khalid
Shaqra University, College of Science and Arts Sajir, P.O. Box 33, Shaqra, 11961, Saudi Arabia
Mohammed A. Basahi
Biology Department, Faculty of Science Yanbu, Taibah University, Yanbu Al-Bahr, 46423, Saudi Arabia
Omar Mahmoud Al-Zoubi & Talaat Habeeb
Faculty of Food Science and Technology, Food Engineering Department, University of Agricultural Sciences and Veterinary Medicine, 400372, Cluj-Napoca-Napoca, Romania
Romina Alina Marc
Department of Environmental Sciences, Government College University, Faisalabad, Pakistan
Muhammad Kashif Irshad
Department of Botany, Government College University, Faisalabad, Pakistan
You can also search for this author in PubMed Google Scholar
Contributions
NA, AN: Conceived the idea and designed the experiment. NA, MA, MFI, SN, and AN: Wrote first draft. MA, MKI, and AN: Writing, review, editing. NA, MMS: Performed experiment, investigation. NA, MA: Statistical analysis. MA, RAM, OMA, TH, and AN: Critical revision of the manuscript.
Corresponding author
Correspondence to Ali Noman .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 42 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Akhter, N., Aqeel, M., Maqsood, M.F. et al. Comparative Assessment of Remediation Potential of Xanthium strumarium Ecotypes in NaCl-Affected Root Zone. Water Air Soil Pollut 233 , 509 (2022). https://doi.org/10.1007/s11270-022-05990-2
Download citation
Received : 23 September 2022
Accepted : 23 November 2022
Published : 01 December 2022
DOI : https://doi.org/10.1007/s11270-022-05990-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Phytoremediation
- Osmoprotectants
- Anatomical alterations
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Phytochemical Compositions and Biological Activities of Essential Oil from Xanthium strumarium L.
Javad sharifi-rad.
1 Zabol Medicinal Plants Research Center, Zabol University of Medical Sciences, Zabol 61615-585, Iran; E-Mail: [email protected]
2 Department of Pharmacognosy, Faculty of Pharmacy, Zabol University of Medical Sciences, Zabol 61615-585, Iran

Seyedeh Mahsan Hoseini-Alfatemi
3 Pediatric Infections Research Center, Mofid Children Hospital, Shahid Beheshti University of Medical Sciences, Tehran 15468-15514, Iran; E-Mail: [email protected]
Majid Sharifi-Rad
4 Department of Range and Watershed Management, Faculty of Natural Resources, University of Zabol, Zabol 98615-538, Iran; E-Mail: [email protected]
Mehdi Sharifi-Rad
5 Zabol University of Medical Sciences, Zabol 61663335, Iran; E-Mail: moc.oohay@darifirahs_idhem
Marcello Iriti
6 Department of Agricultural and Environmental Sciences, Milan State University, via G. Celoria 2, Milan 20133, Italy
Marzieh Sharifi-Rad
7 Department of Chemistry, Faculty of Science, University of Zabol, Zabol 98615-538, Iran; E-Mail: [email protected]
Razieh Sharifi-Rad
8 Department of Biology, Faculty of Science, University of Sistan and Baluchestan, Zahedan 33431063, Iran; E-Mail: [email protected]
Sara Raeisi
9 Department of Fishery, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan 49138-15739, Iran; E-Mail: [email protected]
The chemical composition of the essential oil (EO) from fresh cocklebur ( Xanthium strumarium L.) leaves was investigated by GC-MS. The antimicrobial activity of the EO was tested against Gram-positive and Gram-negative bacteria and fungi. Scolicidal activity was assayed against Echinococcus granulosus protoscolices. In total, 34 compounds were identified, accounting for 98.96% of the EO. The main compounds in the EO were cis -β-guaiene (34.2%), limonene (20.3%), borneol (11.6%), bornyl acetate (4.5%), β-cubebene (3.8%), sabinene (3.6%), phytol (3.1%), β-selinene (2.8%), camphene (2.2%), α-cubebene (2.4%), β-caryophyllene (1.9%), α-pinene (1.8%) and xanthinin (1.04%). The antibacterial and antifungal screening of the EO showed that all assayed concentrations significantly inhibited the growth of Staphylococcus aureus , Bacillus subtilis , Klebsiella pneumoniae , Pseudomonas aeruginosa , Candida albicans and Aspergillus niger (MIC = 0.5 ± 0.1, 1.3 ± 0.0, 4.8 ± 0.0, 20.5 ± 0.3, 55.2 ± 0.0 and 34.3 ± 0.0 µg/mL, respectively). The scolicidal assay indicated that the EO exhibited a significant activity against E. granulosus protoscolices. To the best of our knowledge, this is the first report on the scolicidal activity of X. strumarium . Because of the emergence of antimicrobial drug resistance, the study of new effective natural chemotherapeutic agents, such as the X. strumarium EO, possibly with low side effects, represents a very promising approach in biomedical research.
1. Introduction
For a long time, aromatic and medicinal plants have played an important role as (phyto) therapeutic agents of both pharmacological and economic relevance [ 1 , 2 , 3 , 4 ]. In developing countries, due to economic constraints, nearly 80% of the population still depends on plant extracts as a source of natural remedies. Noteworthily, the excessive and repeated use of pharmaceuticals in modern medicine has caused the selection of antibiotic resistant microbial strains, thus reducing the number of antibiotics available to treat clinical infections [ 5 , 6 , 7 , 8 , 9 , 10 ], therefore, the use of medicinal and aromatic plants as a source of new therapeutic agents continues to be a pivotal element in traditional health care systems [ 10 ]. In addition, phytochemicals from these plants may also serve as precursors or lead compounds for the development of new pharmaceuticals [ 3 , 11 , 12 ].
Cocklebur ( Xanthium strumarium L.) is an annual plant species belonging to the Asteraceae family. In Iran, X. strumarium is available between August and September, where it competes with a number of agronomic crops. In many countries, different plant organs, especially fruits and roots, are used as remedies [ 13 ]. Extracts from these plant organs were found to possess antifungal [ 14 ], anti-inflammatory [ 15 , 16 ], antileishmanial [ 14 ], antitrypanosomal [ 17 ], hypoglycemic [ 18 ], anthelmintic [ 19 ], antiulcerogenic [ 20 ], diuretic [ 21 ] and anticancer [ 22 ] activities.
Essential oils are complex mixtures of lipophilic, volatile and aromatic plant secondary metabolites. The principal constitutes of essential oils include mono- and sesquiterpenes, arising from the isoprenoid pathway, and their oxygenated derivatives such as ketones, alcohols, aldehydes, esters, oxides and phenols [ 23 ]. Several studies have reported the biocide activity of essential oils against many different agents, including clinically relevance pathogens [ 24 , 25 , 26 ].
The most important chemical constituents of X. strumarium include phenolic compounds as thiazolidinediones, chlorogenic acids, ferulic acids [ 27 ], 1,3,5-tri- O -caffeoyl quinic acid, 1,5-di- O -caffeoyl quinic acid, caffeic acid [ 28 ], as well as isoprenoids such as strumasterol, β-sitosterol [ 29 ], monoterpene and sesquiterpene hydrocarbons [ 30 ], triterpenoid saponins [ 29 ] and xanthanolide sesquiterpene lactones [ 31 ]. Based on these premises, the main aim of the this study was to carry out in vitro assays to estimate the antimicrobial and scolicidal activities of essential oil extracted from leaves of X. strumarium grown in Iran.
2. Results and Discussion
2.1. chemical composition of x. strumarium leaf essential oil.
The chemical composition of essential oil extracted from the leaves of X. strumarium is shown in Table 1 .
Phytochemical composition of Xanthium strumarium L. leaf essential oil.
* RI: retention index; t: traces, concentration less than 0.05%.
GC-MS analysis revealed that the main components of the essential oil were cis -β-guaiene (34.2%), limonene (20.3%), borneol (11.6%), bornyl acetate (4.5%), β-cubebene (3.8%), sabinene (3.6%), phytol (3.1%), β-selinene (2.8%), camphene (2.2%) α-cubebene (2.4%), β-caryophyllene (1.9%), α-pinene (1.8%) and xanthinin (1.04%). Scherer et al . [ 32 ] studied the X. strumarium leaf essential oil from São Paulo, Brazil: among the 24 components identified in that work, β-guaiene was the most abundant (79.6%). Esmaeili et al . [ 33 ] collected X. strumarium plants at full flowering stage, from Khoramabad, Lurestan Province, Iran, and obtained the essential oil from stems and leaves. They reported that 22 compounds (86.4%) were identified in the stem essential oil, among which bornyl acetate (19.5%), limonene (15.0%) and β-selinene (10.1%) were the most abundant. In the leaf essential oil, 28 components were identified (85.2%), characterized by higher amounts of limonene (24.7%) and borneol (10.6%). Our results are in agreement with previous studies: no significant qualitative difference was observed in the essential oil composition, whereas any quantitative differences may be due to genetic, environmental and ecological factors.
2.2. Antibacterial, Antifungal and Scolicidal Activities
The antibacterial and antifungal activity results are summarized in Table 2 and Table 3 , respectively. X. strumarium essential oil significantly inhibited the growth of Gram-positive ( S. aureus and B. subtilis ) and Gram-negative ( K. pneumoniae ) bacteria ( p < 0.05). MIC for S. aureus , B. subtilis and K. pneumoniae were 0.5 ± 0.1, 1.3 ± 0.0 and 4.8 ± 0.0 µg/mL of essential oil, respectively. S. aureus was the most sensitive microorganism, because of its very low MIC. P. aeruginosa was slightly inhibited in the disc diffusion assay, and its MIC was 20.5 ± 0.3 μg/mL of essential oil in the broth dilution assay. In addition, the essential oil significantly inhibited C. albicans and A. niger ( p < 0.05), at all the assayed concentrations. MIC for C. albicans and A. niger were 55.2 ± 0.0 and 34.3 ± 0.0 µg/mL of essential oil, respectively.
Antibacterial activity of Xanthium strumarium L. leaf essential oil against gram-positive and gram-negative bacterial strains.
§ Data are expressed as mean ± SD of inhibition zone diameter (mm) for different concentrations of essential oil, controls and minimum inhibitory concentration (MIC) (µg/mL); the values with different letters within a column are significantly different ( p < 0.05; LSD); * DMSO: dimethyl sulfoxide.
Antifungal activity of Xanthium strumarium L. leaf essential oil against fungal strains.
The mortality rates of E. granulosus protoscolices after treatment with different concentrations of X. strumarium leaf essential oil are reported in Table 4 . As exposure time and essential oil concentration increased, percentage mortality rised. Therefore, exposure to the essential oil for 60 min, at 2.5, 5, 10 and 20 mg/mL resulted in 58.7%, 64.48%, 68.48% and 79.22% inhibition, respectively. After 60 min, the mortality in the control was 43.56%.
Scolicidal activity of Xanthium strumarium leaf essential oil against Echinococcus granulosus .
§ Values are mean ± SD of three replicates; * in the control, protoscolices were treated only with saline + Tween-80 solution.
According to Scherer et al . [ 32 ], leaves of X. strumarium exhibited powerful antimicrobial activity against S. aureus , Escherichia coli , Salmonella thyphimurium , P. aeruginosa and Clostridium perfringens. In addition, they showed that S. aureus was the most susceptible microorganism followed by E. coli and P. aeruginosa , while S. typhimurium and C. perfringens were the most resistant to the X. strumarium essential oil. Rad et al . [ 13 ] investigated the antibacterial activity of X. strumarium on methicillin-susceptible (MSSA) and methicillin-resistant S. aureus (MRSA), showing that the plant extracts were effective on both strains, though their antibacterial activity was higher on the MSSA one. Similarly, Jawad et al. [ 34 ] reported that X. strumarium extract exhibited antimicrobial activity against S. aureus , B. subtilis , Proteus vulgaris , Candida pseudotropicalis and C. albicans . Gautam et al . [ 35 ] investigated X. strumarium extracts for in vitro antimycobacterium activity, and found that the ethylacetate and MeOH-petroleum ether extracts were effective against Mycobacterium smegmatis and M. tuberculosis . Amerjothy et al . [ 36 ] studied the hexane, alcoholic and ethylacetate extracts of Xanthium indicum Koen leaves for their antimicrobial activity. Hexane extract showed significant inhibition against P. aeruginosa, S. aureus, Aspergillus niger and C. albicans ; ethylacetate extract inhibited S. aureus, A. niger and E. coli ; alcoholic extract was active only against S. aureus . Antifungal activity of X. strumarium was also documented against both pathogenic and non-pathogenic fungi by Bisht and Singh [ 37 ], due to the presence of terpenes, limonene and carveol.
Among the most representative constituents found in our essential oil, the sesquiterpene β-caryophyllene was extensively investigated because of its several biological activities, including antimicrobial [ 38 , 39 ], insecticidal [ 40 , 41 ], anti-inflammatory [ 42 , 43 ], anticarcinogenic [ 44 , 45 , 46 , 47 , 48 ] and local anaesthetic [ 49 ] activities.
Similarly, many studies showed the antimicrobial activity of α-pinene and eugenol on Gram-positive bacterial strains ( S. aureus , Streptococcus pyogenes , S. epidermidis and Streptococcus pneumoniae ) and fungi ( Cryptococcus neoformans and C. albicans ) [ 23 , 50 , 51 ]. In our study, both α-pinene (1.8%) and eugenol (trace amount) were detected in X. strumarium essential oil, as well as limonene (20.3%) and linalool (0.9%) ( Table 1 ) [ 52 ]. Aggarwal et al. [ 53 ] reported that limonene was particularly efficient in inhibiting the proliferation of a variety of microorganisms that cause food spoilage. Özek et al. [ 54 ] demonstrated that linalool enantiomers possessed the same antimicrobial activity against several microorganisms, specifically against the protozoan Plasmodium falciparum and the fungus Botrytis cinerea .
Mulyaningsih et al. [ 55 ] studied antibacterial activity of Kadsuralongi pedunculata essential oil and its major constituents against MRSA and vancomycin-resistant Enterococcus faecalis . Fifty compounds were identified, including δ-cadinene (21.79%), camphene (7.27%), borneol (6.05%), cubenol (5.12%) and δ-cadinol (5.11%), and the authors reported that camphene and borneol exhibited antimicrobial activity. Borneol (11.6%), camphene (2.2%), δ-cadinene (0.2%) and α-cadinol (trace amount) were found in our X. strumarium essential oil ( Table 1 ). δ-Cadinene inhibited the growth of Propionibacterium acnes and S. mutans [ 56 ]. Pérez-Lopez et al. [ 57 ] essayed the essential oil obtained from the fruit of Schinus molle against S. pneumonia resistant to antibiotics, and identified δ-cadinene as the principal active ingredient.
Xanthinin (1.04%) was found in X. strumarium essential oil ( Table 1 ). This compound was previously isolated from the extracts of X. spinosum and was active against Colletotrichum gloesporoides , Trichothecium roseum , Bacillus cereus and Staphylococcus aureus [ 58 ]. Little et al . [ 59 ] reported that alcoholic extract of xanthinin in concentration of 0.01%–0.1% showed high antimicrobial activity against fungi and gram-negative bacteria.
Inoue et al. [ 60 ] examined the bactericidal activity of three diterpenes, i.e. phytol, terpenone and geranylgeraniol, showing that these compounds were effective against S. aureus . Similarly, Pejin et al . [ 61 ] investigated the antimicrobial activity of phytol against eight bacterial and eight fungal strains. It was proven phytol to be active against all tested bacteria and fungi. The amount of phytol in X. strumarium essential oils was 3.1% ( Table 1 ).
Maggiore et al . [ 62 ] reported the efficacy of Thymus vulgaris and Origanum vulgare essential oils and thymol on E. granulosus protoscoleces and cysts [ 63 ]. Mahmoudvand et al . [ 64 ] studied scolicidal activity of black cumin seed ( Nigella sativa ) essential oil on hydatid cysts, and thymoquinone, p -cymene, carvacrol and longifolene were found to be the main components of the essential oil. To the best of our knowledge, this is the first report on the scolicidal activity of X. strumarium .
3. Experimental Section
3.1. plant material.
The Xanthium strumarium L. leaves were collected between August-September 2013 from area of Hamun Lake of Zabol (31°1'43'' N, 61°30'4'' E), Sistan and Baluchestan Province, Iran. The plant was taxonomically identified at the Department of Botany of Shahid Beheshti University of Medical Sciences, Tehran, Iran, where a voucher specimen was conserved.
3.2. Essential Oils Extraction
Fresh leaves (1 kg) were detached from the stem and dried in the shade for 96 h. Then, they were chopped and hydro-distilled for 3 h utilizing an all-glass Clevenger-type apparatus. The distillate was saturated with sodium chloride (NaCl) (Merck, Darmstadt, Germany) and the oil was extracted with n -hexane (Merck) and dichloromethane (Merck). The essential oil obtained was dried over anhydrous sodium sulphate (Sigma-Aldrich, St. Louis, MO, USA) and stored at 4 °C before gas chromatography coupled to mass spectrometry (GC-MS) analysis and bioassays.
3.3. Identification of Essential Oil Constituents
The leaf essential oil was analyzed by GC-MS. A Shimadzu 17A gas chromatograph coupled with a Shimadzu QP-5000 quadrupole mass spectrometer and Varian 3800 gas chromatograph coupled with FID detector was used. The extracted compounds were separated on DB-5 fused silica capillary column (30 m × 0.25 mm × 0.25 µm film thickness). Helium was used as carrier gas with a 1.0 mL/min flow rate. The analyses were carried out by a splitless injection (1 µL), with the injector set at 230 °C. The oven temperature program used was 60–240 °C at 3 °C /min and the final temperature was held for 8 min. The GC/MS interface and FID detector were sustained at 240 °C and 250 °C, respectively. Retention indices for all constituents were determined based on the method using n -alkanes as standard. Retention indices were determined using retention times of n -alkanes that were injected after the essential oil under the same chromatographic conditions. All data were acquired by collecting the full-scan mass spectra within the scan range 50–550 amu. Compounds were recognized using comparison of their mass spectra with the Wiley GC-MS Library and Adams Library [ 65 , 66 ].
3.4. Microbial Isolates, Antibacterial and Antifungal Activities
All microorganisms were obtained from the Persian Type Culture Collection (PTCC), Tehran, Iran. The essential oil was tested against three gram-negative bacteria: Klebsiella pneumoniae PTCC 1053 (American Type Culture Collection ATCC 10031), Escherichia coli PTCC 1330 (ATCC 8739) and Pseudomonas aeruginosa PTCC 1074 (ATCC 9027); three gram-positive bacteria Staphylococcus aureus PTCC 1112 (ATCC 6538), Staphylococcus epidermis PTCC 1114 (ATCC 12228) and Bacillus subtilis PTCC 1023 (ATCC 6633); and two fungi: Aspergillus niger PTCC 5010 (ATCC 9142) and Candida albicans PTCC 5027 (ATCC 10231).
Different concentrations of essential oil were evaluated against bacteria and fungi by disc diffusion method [ 67 ]. In brief, microorganisms were cultured at 37 °C for 14–24 h and the densities were adjusted to 0.5 McFarland standards at A 530 nm (10 8 CFU/mL). Then, 100 µL of the microbial suspensions (10 8 CFU/mL) were spread on nutrient agar (Merck) plates (100 mm × 15 mm). The discs (6 mm diameter) were separately impregnated with 10 µL of different concentrations of essential oil (10, 20, 40, 60, 80 and 100 µg/mL) and placed on the inoculated agar. All the inoculated plates were incubated at 37 °C for 24 h. Ketoconazole (10 mg/disc), ampicillin (10 mg/disc) and gentamicin (10 mg/disc) were used as positive controls for fungi, gram-positive and gram-negative bacteria, respectively. Dimethyl sulfoxide (DMSO) was used as negative control. Antibacterial and antifungal activities were determined by measuring the zone of inhibition (mm). Minimal inhibitory concentration (MIC) values of the of essential oil versus each investigated microbial strain were determined by the microdilution assay in 96 multi-well microtiter plates, according to the standard procedure of the Clinical and Laboratory Standards Institute [ 68 ]. The bacterial and fungal strains were suspended in Luria-Bertani media and the densities were adjusted to 0.5 McFarland standard at 570 nm (10 8 CFU/mL). Essential oil was dissolved in 50% DMSO to a final concentration of 10 mL. Each strain was assayed with samples that were serially diluted in broth to obtain concentrations ranging from 512.0 to 0.06 µg/mL. Overnight broth cultures of each strain were prepared and the final microorganism concentration in each well was adapted to 10 6 CFU/mL. The optimal incubation conditions were 37 °C for 24 h. Medium without bacteria and fungi was the sterility control, whereas medium with bacteria and fungi, but without essential oil, was the growth control. The growth of bacteria and fungi was compared with that of the controls. The MIC values were visually detected and defined as the lowest essential oil concentrations with >95% growth inhibitory activity to the assessed microorganisms.
3.5. Scolicidal Activity
The Echinococcus granulosus protoscolices were obtained from the infected livers of calves killed in an abattoir used to study scolicidal activity. Animals were ethically treated according to the Helsinki Declaration. In this assay, hydatid fluid was collected together with protoscolices using the Smyth and Barrett method [ 69 ]. Briefly, hydatid fluid was conveyed to a glass cylinder. Protoscolices, settled at the bottom of the cylinder after 40 min, were washed 3 times with normal saline and their viability was confirmed by motility under a light microscope (Nikon Eclipse E200, Tokyo, Japan). Protoscolices were transferred into a dark receptacle containing normal saline and stored at 4 °C. Four concentrations of essential oil (2.5, 5, 10 and 20 mg/mL) were tested for 10, 20, 30 and 60 min. To prepare these concentrations, 25, 50, 100 and 200 µL of essential oil, added to test tubes, were dissolved in 9.7 mL of normal saline supplemented with 0.5 mL of Tween-80 (Merck) under continuous stirring. For each test, one drop of protoscolices-rich solution was added to 3 mL of essential oil solution, mixed slowly, and incubated at 37 °C. After each incubation period (10, 20, 30 and 60 min), the upper phase was gently removed so as not to disturb the protoscolices; then, 1 mL of 0.1% eosin stain was added to the remaining colonized protoscolices and mixed slowly. The supernatant was discarded after incubating for 20 min at 25 °C. The remaining pellet of protoscolices (no centrifugation performed) was smeared on a manually scaled glass slide, covered with a cover glass, and evaluated under a light microscope. The percentage of dead protoscolices was determined after counting a minimum of 600 protoscolices. In the control, protoscolices were treated only with normal saline + Tween-80.
3.6. Statistical Analysis
Essential oil was extracted and tested in triplicate for chemical analysis and bioassays. Data were subjected to analysis of variance (ANOVA) following an entirely randomized design to determine the least significant difference (LSD) at p < 0.05, using statistical software package (SPSS v. 11.5, IBM Corporation, Armonk, NY, USA). All results are expressed as mean ± SD.
4. Conclusions
Our results indicated X. strumarium as a promising source on antimicrobial agents, with potential in biomedical applications. However, in vivo studies on this medicinal plant are needed to determine pharmacokinetics and toxicity of the active components and their side effects. In addition, the antimicrobial, antifungal and scolicidal activities may be increased by purifying active constitutes and determining proper dosages for effective therapies. This would avoid the prescription of inappropriate treatments, a usual practice among many traditional herbal practitioners. Finally, a particular application of X. strumarium plant may involve the field of food hygiene, to reduce the risk of food contamination and to control the food-borne diseases.
Acknowledgments
The authors acknowledge all the colleagues involved in the field of EO research, who inspired their scientific interest.
Author Contributions
Javad Sharifi-Rad and Seyedeh Mahsan Hoseini-Alfatemi designed the study; Javad Sharifi-Rad, Seyedeh Mahsan Hoseini-Alfatemi, Majid Sharifi-Rad, Mehdi Sharifi-Rad, Marzieh Sharifi-Rad, Razieh Sharifi-Rad and Sara Raeisi carried out the experiments and analyzed the results; Javad Sharifi-Rad and Marcello Iriti wrote the paper; Marcello Iriti reviewed critically the manuscript. All the authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability : Samples of the compounds are available from the authors.

IMAGES
VIDEO
COMMENTS
Xanthium strumarium L. (Asteraceae) is a common and well-known traditional Chinese herbal medicine usually named Cang-Er-Zi, and has been used for thousands of years in China. The purpose of this paper is to summarize the progress of modern research, and provide a systematic review on the traditional usages, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology of the X ...
Malaria is a major threat to global health and continues to claim lives of many people each year, especially in developing countries. Xanthium strumarium L., is used by traditional health practitioners in the management of malaria fever in North East India. Bioassay guided fractionation of X. strumarium L. extracts, led to the isolation of five compounds from the aerial part and fruit of ...
herbs, shrubs, trees and climbers [1]. It is a commonly found as a weed in roadsides, rice fields, hedge s. throughout the tropical parts of India. Xanthium strumarium is an annual herb, up t o 1 ...
Abstract Xanthium strumarium and Xanthium spinosum are found in many parts of the world and they are used in traditional medicine in many countries. Several studies have shown some medicinal effects for X. strumarium parts (leaves-seedlings-flowers-roots-fruits), and of the leaves of X. spinosum to treat some diseases. This paper is designed to set standards in determining the quality ...
Introduction. Invasive plants are a major threat to the biodiversity and functioning of ecological systems [1-3].Thus, biological invasions have become one of the hotspots in ecological research [], The invasive plant Xanthium strumarium L. competes with native plant species for soil nutrients, moisture, "shelter", "light", and other resources, severely influencing natural ...
The genus Xanthium is an annual herb belonging to the family Compositae, widely distributed worldwide. The plants of Xanthium have long been used as traditional Chinese medicine for treating fever, rhinitis, headache, sinusitis, tympanitis, scrofula, and arthritis. The secondary metabolites of Xanthium species have been studied since the 1960s. So far, various chemical constituents have been ...
2020. TLDR. Investigation of phytochemical screening and antimicrobial activity of methanol, chloroform, aqueous, and ethanol extracts prepared from leaves of Xanthium strumarium revealed the presence of antibacterial activity of different extracts of this plant against human pathogenic bacteria. Expand.
Xanthium strumarium L. belongs to the Compositae family, is a medicinal plant which widely distributes in the tropical and subtropical regions of southeast Asia [].As a Chinese traditional medicine which be included in the Chinese Pharmacopoeia in 1963, the fruits of X. strumarium, also named as "Xanthii fructus", "Cang-Er-Zi" or "Chang-Er-Cao" in China, were used to treat various ...
Xanthium strumarium (Compositae) is an annual plant widely distributed in eastern China. The fruits of X. strumarium, named as "Cang-Er-Zi" or "Chang-Er-Zi" in China, were traditionally used to treat nasal sinusitis, 1 headache caused by wind cold, 2 urticaria, 2 and arthritis. 3 And X. strumarium also exhibited some useful medicinal properties such as diuretic, 4 anthelmintic, 5 ...
Xanthium Strumarium L. (Family: Asteraceae/ Compositae) a medicinal plant commonly found as a weed, folklore medicine and is ... Finally, research needs quantitation of individual constituents and assessment of their pharmacological activities in humans. WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES SJIF Impact Factor 7.632
A high dose (50 μg/mL) of fruit extracts from Xanthium strumarium and Tribulus terrestris increased the MAP1LC3-II protein level. The net MAP1LC3-II level in cells between extracts treated alone or in combination with BafA1 was decreased compared with the control cells ( Figure 3 C), particularly in XS-(fr)-C- and TT-(fr)-M-treated cells.
Ethnopharmacological relevance. Xanthium strumarium L. fruit (Xanthiu fruit) has been traditionally used as a medicinal herb in China for the treatment of many ailments including rheumatoid arthritis. However, the anti-arthritic activity of Xanthium strumarium fruit has still not been demonstrated. In the present study, we confirmed that the extract of Xanthium strumarium (EXS) prevents ...
Xanthium strumarium is a medicinal plant, generally called. 'cocklebur or bur weed' commonly found as a weed in road-. sides, rice fields, gounds, hedgerows throughout the tropical. parts in ...
Xanthium strumarium L. (Asteraceae) is a common and well-known traditional Chinese herbal medicine usually named Cang-Er-Zi, and has been used for thousands of years in China. The purpose of this paper is to summarize the progress of modern research, and provide a systematic review on the traditional usages, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology of the X ...
One new lignan, fructusol A (1), and one new thiazine derivative, 2-hydroxy-xanthiazone (2), along with eight known ones, were isolated from the seeds of Xanthium strumarium.The structures of new compounds were elucidated on the basis of extensive spectroscopic methods. Meanwhile, compounds 1-3 were tested for their antifungal activities against Candida albicans (ATCC 10231) in vitro.
Its fruits are included in the Chinese Pharmacopeia for treating nasosinusitis, 1 headache caused by cold, 2 pruritus, 2 and rheumatic arthralgia. 3 Recent studies have shown that X. strumarium contains several classes of compounds, including lignans, 4 sesquiterpene lactones, 5 phenolic acids, 6 ent-kauranoid glycosides, 7 and flavonoids. 8 ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... from Xanthium strumarium and ...
In the present study, three ecotypes of Xanthium strumarium L. were collected from different ecological regions, i.e., Uchalli (E1), Sargodha (E2), and Samundri (E3) in Punjab province, Pakistan. All ecotypes were assessed for their salt resistance and remediation capacity at different NaCl levels (T1 (control), T2 (50 mM), T3 (100 mM), and T4 (150 mM)). Xanthium responses to varied NaCl ...
Xanthium strumarium L. is a globally successful invasive herb that has had significant negative ecological, economic and social impacts in many world regions. The present study was therefore conducted to evaluate the invasive potential and spatial distribution patterns of X. strumarium in heavily invaded plant communities of the semiarid regions of northern Pakistan. Investigations were based ...
Xanthium Strumarium L. showed antioxidant, anti-inflammatory, anti-cancer activities as well as various medicinal significances hence it created sufficient interest to investigate phytochemical ...
Introduction. Xanthium strumarium L. (Common cocklebur), a member of the Asteraceae, is an annual weed species propagated by seeds [1, 2].It is native to North America and Argentina [], and regarded as a noxious weed species of corn and soybean crops throughout the world [4-8].Moreover, it produce large amounts of allergenic pollens due to close relatedness of Xanthium and Ambrosia genus [9 ...
Xanthium strumarium L. (Family: Compositae) a medicinal plant commonly found as a weed, is widely distributed in North America, Brazil, China, Malaysia and hotter parts of India. The herb is ...
The Xanthium strumarium L. leaves were collected between August-September 2013 from area of Hamun Lake of Zabol (31°1'43'' N, 61°30'4'' E), Sistan and Baluchestan Province, Iran. The plant was taxonomically identified at the Department of Botany of Shahid Beheshti University of Medical Sciences, Tehran, Iran, where a voucher specimen was ...
The purpose of this paper is to summarize the progress of modern research, and provide a systematic review on the traditional usages, phytochemistry, pharmacology of the X. strumarium.