

PDO MISSION: TURNING INNOVATIVE IDEAS INTO IMPACTFUL PROPOSALS
Grant templates and guides, nih research grant templates.
Depending on the scientific content and administrative structure of your grant, you may be required to submit some or all of the documents listed below. If you need help determining which documents are required for your application, please reach out to our team. The PDO has prepared these templates to serve as a helpful starting point for developing your NIH grant application. All documents should be tailored to meet the specific requirements of your grant. We update these documents as needed and the date of last update is in the header of each document.
Beginning on January 25, 2023, grant applications to NIH must use FORMS-H application packages. The templates below comply with the FORMS-H instructions.
Research Plan Form:
- Specific Aims and Research Strategy (Lab-based Proposal)
- Specific Aims and Research Strategy (Clinical Trial)
- Introduction
- Vertebrate Animals
- Select Agent Research
- Multiple PD/PI Leadership Plan
- Consortium/Contractual Arrangements
- Resource Sharing Plan(s)
- Data Management and Sharing Plan
- Authentication of Key Biological and/or Chemical Resources
- Plan for Enhancing Diverse Perspectives
Other Project Information Form:
- Project Summary/Abstract
- Project Narrative
- Facilities and Other Resources
Human Subjects and Clinical Trials Information Form:
Effective January 2018, all grants proposing human subjects research must include a Human Subjects and Clinical Trials Information Form. A PDF copy of this form is included below.
- Human Subjects and Clinical Trials Information Form
- Study Record: Human Subjects and Clinical Trials Information Form
- Annotated Human Subjects Form
*Note that beginning May 25, 2020, a Single IRB plan is no longer required for NIH applications. Single IRB plans are only required for applications to AHRQ.
- Non-Human Subjects Research Justification
- Delayed Onset Study Justification
- Inclusion of Individuals Across the Lifespan
- Inclusion of Women and Minorities
- Recruitment and Retention Plan
- Study Timeline
- Protection of Human Subjects
- Data and Safety Monitoring Plan
- Overall Structure of the Study Team
- Statistical Design and Power
- IND/IDE Status
- Dissemination Plan
NIH Program and Center Grant Resources
- Common Program and Center Grant Critiques to Avoid
Additional Stanford Resources
Our Stanford colleagues have also developed valuable grant development resources that are available to Stanford affiliates:
- RMG Tools and Templates
- Grant Writing Academy's K Award Workbook
- OFDD R01 Countdown: Tools for Writing Concise and Compelling Grants

- About Grants
- How to Apply - Application Guide
Samples: Applications, Attachments, and Other Documents
As you learn about grantsmanship and write your own applications and progress reports, examples of how others presented their ideas can help. NIH also provides attachment format examples, sample language, and more resources below.
On This Page:
Sample Grant Applications
Nih formats, sample language, and other examples.
With the gracious permission of successful investigators, some NIH institutes have provided samples of funded applications, summary statements, and more. When referencing these examples, it is important to remember:
- The applications below used the form version and instructions that were in effect at the time of their submission. Forms and instructions change regularly. Read and carefully follow the instructions in your chosen funding opportunity and the Application Guide .
- The best way to present your science may differ substantially from the approaches used in these examples. Seek feedback on your draft application from mentors and others.
- Talk to an NIH program officer in your area of science for advice about which grant program would be a good fit for you and the Institute or Center that might be interested in your idea.
- Samples are not available for all grant programs. Because many programs have common elements, the available samples can still provide helpful information and demonstrate effective ways to present information.
National Institute of Allergy and Infectious Diseases (NIAID)
- Sample Applications and Summary Statements (R01, R03, R15, R21, R33, U01, SBIR, STTR, G11, K, and F)
- NIAID Sample Forms, Plans, Letters, Emails, and More
National Cancer Institute (NCI)
- Behavioral Research Grant Applications (R01, R21, R03)
- Cancer Epidemiology Grant Applications (R01, R21, R03, R37)
- Cancer Control and Population Sciences Grant Applications (R01, R21, R37)
- Healthcare Delivery Research Grant Applications (R01, R03, R21, R50)
National Human Genome Research Institute (NHGRI)
- ELSI Applications and Summary Statements and biosketches (K99/R00, K01, R01, R03, and R21)
- NHGRI Sample Consent Forms
National Institute on Aging (NIA)
- K99/R00: Pathway to Independence Awards Sample Applications
- NIA Small Business Sample Applications (SBIR and STTR Phase 1, Phase 2, and Fast-Track)
National Institute on Deafness and Other Communication Disorders (NIDCD)
- Research Project Grants (R01) Sample Applications and Summary Statements
- Early Career Research (ECR) R21 Sample Applications and Summary Statements
- Exploratory/Developmental Research Grant (R21) Sample Applications and Summary Statements
NIH provides additional examples of completed forms, templates, plans, and other sample language for reference. Your chosen approach must follow the instructions in your funding opportunity and the Application Guide .
- Application Format Pages
- Annotated Form Sets
- Animal Document Samples from Office of Laboratory Animal Welfare (OLAW) for animal welfare assurances, study proposals, Memorandum of Understanding , and more
- Allowable Appendix Materials Examples
- Authentication of Key Biological and/or Chemical Resources Plan Examples
- Biosketch Format Pages, Instructions, and Samples
- Data Management and Sharing (DMS) Plan Samples
- Informed Consent Example for Certificates of Confidentiality
- Informed Consent Sample Language for secondary research with data and biospecimens and for genomic research
- Model Organism Sharing Plans
- Multiple PI Leadership Plan Examples
- Other Support format page, samples, and instructions
- Scientific Rigor Examples
- Person Months FAQ with example calculations
- Plain Language Examples for application title, abstract, and public health relevance statements
- Project Outcome Description Examples for interim or final Research Performance Progress Report (RPPR)
This page last updated on: February 7, 2024
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health
- You are here:
- HelpGoAbroad Ltd. /
How to Write a Successful Biomedical Grant Application
The problem.
A research proposal is basically a plan for work required to test a research hypothesis/set of hypotheses. The most important thing to keep in mind is that, when drafting a biomedical research grant proposal, you are required to tailor it to suit a specific audience (i.e., grant application reviewers).
In academic research settings, success and promotion mainly depend on the quantity and quality of the grants received, as grant monies bring notoriety and prestige to the investigator, and the institution they are affiliated with. However, knowing how to write a successful grant proposal can be quite a challenge, especially if you are an inexperienced writer. Most research-funding agencies, such as the National Institutes of Health and the National Science Foundation (both of which provide both academic and small business grants), are reducing their budgets, while more and more researchers are competing for it, thus creating an enormous demand vs. supply. Consequently, it is becoming increasingly important to effectively write high-quality grant proposals.
The strategy
In this article, we will guide you in assembling a research proposal that will have maximal chances of being noticed, and well-received, by reviewers (to optimize your chances of obtaining funding). Here, we mainly focus on writing grant proposals for biomedical research studies, although these suggestions are applicable to most any type of application.
- Get started . Any quality research study worth funding must start with a good idea. Start by clearly defining the problem you intend to solve, and formulate a hypothesis or research question. Most importantly, you should find out if that question has already been addressed (or perhaps even answered). Thus, you must perform a very thorough literature review, because many times you will find you're not the first one to come up with that idea. If this is the case, you must clearly acknowledge all previous work, and decide whether you should spend a lot of energy and time on a similar research project. You should realize that the reviewers will likely find previously done work on your proposed hypothesis, and if you have not acknowledged those previous studies, they will severely criticize the application.
- Finalize your hypothesis, and formulate it in writing . Other than knowing how much is already published about your research topic, you need to assess the quality of the available preliminary evidence (including your own). In bioscience, questions rarely have definitive answers. For example, if there exist multiple studies already published about the topic, you might decide to do a comparative study. After finalizing your study topic, determine how many study subjects will be required (for maximal statistical significance), how much money you will need, if your facilities ("environment") are adequate for the research, and who your collaborators will be. You must also clearly make a case for the innovation of your idea; for example, how it will advance current knowledge. Moreover, for your grant application to be successful, you will need to collect convincing data (your own or others'), and convince the funding agency's reviewers that you are capable of carrying out the project as you have proposed it.
- Specific aims : This section clearly describes the question your proposed study will be answering. State the hypothesis of the study as well as the primary and secondary objectives. For a longer grant period (e.g., 4 – 5 years), you may wish to propose 3 or 4 specific aims. For shorter durations (0.5 – 1 year), you might want only two, or even one, specific aims. Precisely write out this section, limited to one page, as this will be the guide for writing the entirety of the application.
- The overall application . Most biomedical grant applications require several distinct, key elements, including the executive summary, background and significance, specific aims, innovation, research design and methods, and preliminary results. Separate pages are reserved for the environment, budget, and budget justification. You should describe the research methodology and design used, in detail, and include any prior work relevant to the research project. You also must clearly describe the statistical analyses of the data, and decide whether you should hire a professional statistician as a consultant.
- Executive summary (abstract) . This is the first page that the reviewers will read, which makes it extremely important to your proposal. Unfortunately, many reviewers will make their opinion based on the executive summary alone. Thus, your abstract needs to succinctly describe every key element of your proposed study, including its significance, feasibility, and innovation.
- Background and significance . This section must outline the rationale for your proposed study and its innovation, and also summarize literature that is relevant to your project. You will need to describe the magnitude of the problem you're trying to address. For instance, you should indicate the population of patients suffering from a disease you wish to investigate, the incidence of the problem, and the likelihood of it recurring or increasing in the future. You're essentially trying to justify your study proposal here. So you will also need to describe how the study results will benefit society and hence, convince the granting agency that this is worth their money.
- Study design . Addressing your hypothesis/research question correctly requires you to choose a suitable study design. The most common clinical study designs are observational studies, diagnostic studies, and interventional studies. The type of study design you choose should be the one that gives the highest quality of results, statistical significance, is most feasible within the timeframe and budget, and concisely answers your research question. Seek advice at an early stage. Before even going too far with your writing, create a collaborative network, within your institution, and beyond (including paid consultants). Talk to your colleagues and mentors who have been on funding panels. Here it may be quite helpful to hire a privately held, grant-consulting/preparation firm. Tell a compelling story.
Stay focused . You're essentially selling an idea to an audience, so make sure the idea sounds exciting and is meant to tackle a serious challenge. You need to identify the hook or key feature that your study proposal hangs off, and tell a compelling narrative that links each specific aim to each other, and your main objectives. Have your proposal reviewed internally or externally (private grant-consulting firm). In particular, spell check, proofread, and stick to the specified format. All the little things count– grammar, punctuation, "flow", and presentation set the tone for how reviewers will feel about your proposed work.
Grant Writing Conclusion
Grants are important for success in both private and academic research. The key to a successful grant proposal application, whether it is for an NIH, NSF, or SBIR grant, you must "sell" to reviewers that it is a good idea, innovative, and that you have the resources to successfully complete it. For you to properly sell your idea, thorough background research, a suitable study design, as well as a well thought-out research methodology, are imperative. If you're too busy to fully commit your mind to it or just starting out and find grants application proposal quite overwhelming, you can always seek help from grant-writing service providers. They will provide all the professional support you need, do research on your behalf, and draft a proposal that will catch the attention of even the most ruthless reviewers.
Dr. Curt Balch has over 20 years of experience in the biomedical and health-research disciplines. His past work included both direct laboratory research as well as the compilation of biomedical research for publication.
This expertise makes Curt and Bioscience Advising a fiscally prudent and productive means of writing grants, getting journal manuscripts, research funding applications, book chapters, and abstracts "out the door" so that you can start reaping the benefits of your hard work.
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.
About the author
HelpGoAbroad Ltd.
HelpGoAbroad - an online directory of opportunities to study abroad, teach abroad, volunteer abroad, and internships abroad, including reviews from past program alumni.
Related Posts
How nih & ro1 grants have contributed medical advancements, nih-sbir grants support neuron reconstruction and 3-d brain mapping, nih and ro1 grants and their contribution to medical advancements.
About HelpGoAbroad
We showcase the best programs, countries and institutions in the world, so whether you are interested in interning abroad, studying abroad, working abroad or simply traveling or living abroad, we have your back.
Subscribe to the Newsletter
Find helpgoabroad on:.
- Study Abroad
- Language Schools
- Degrees Abroad
- High School Study Abroad
- Internships Abroad
- Graduate Schemes
- Jobs Abroad
- Teach Abroad
- Work & Travel/Gap Year
- TEFL Certificates
- Volunteer Abroad
- Forgot your password?
- Forgot your username?
- About Research & Innovation
- Advanced Cardiovascular Care
- Health Equity
- Inflammation
- Maternal and Fetal Medicine
- Musculoskeletal Care
- Neuroscience
- Opioid & Pain
- Research Centers
- Departments
- About the Office of Research
- Funding & Proposal Development
- Regulatory Review & Compliance
- Research Project Management
- Cores & Resources
- Education & Training
- Antiphospholipid Syndrome Research Labs
- J. Michelle Kahlenberg Lab
- John Varga Lab (ScleroLab)
- Mulholland Lab
- Raghavendran Lab
- ALS Center of Excellence
- Institute for Heart & Brain Health
- Center for Basic & Translational Science
- Center for Bioethics and Social Sciences in Medicine
- Biomedical Research Core Facilities
- IT Route Map
- Clinical Research Route Map
- Commercialization Route Map
- Great Minds, Greater Discoveries
- Research Scouts
- Meet the ROMS Team
- ROMS Fellowship Application Information
- Working with a ROMS Fellow
- Pandemic Research Recovery
- Research Climate Council
- Support for Outstanding Research
- Research News Trivia
- Frequently Asked Questions (FAQ)
From an initial funding search to the grant writing process to developing successful proposals, explore these helpful funding and proposal resources from the Medical School Office of Research and across campus.

The Funding & Proposal Development Route Map Green Line provides a snapshot of the resources available to research project teams at Michigan Medicine, and Grant Services & Analysis is committed to providing personal service that helps you navigate your own successful path. CLICK HERE to step back one level to the Project At-A-Glance Blue Line.
In partnership with the Medical School Office of Research, the U-M Office of Research & Sponsored Projects, U-M Foundation Relations, and Michigan Medicine Corporate & Foundation Relations, the U-M Library maintains the Research Funding & Grants Guide , a one-stop-shop for research funding resources. The site is a central, campus-wide portal for finding internal and external funding opportunities and for consultations on how to personalize your search and stay informed.
- Internal Funding
- External Funding
- Search Funding Databases
Need additional help? Schedule a funding search consultation .
The U-M Medical School Competition Space is an innovative online platform that streamlines the process of finding, and applying for, funding opportunities through the U-M Medical School.
The following opportunities are posted, and can be applied for, through UMMS Competition Space:
- Bridging Support
- External Limited Submissions
- Other Opportunities
- Pilot Grants
The Michigan Medicine Corporate and Foundation Relations team can assist in connecting faculty to private funding opportunities, liaising between sponsors and researchers, guiding faculty on proposal approaches, providing examples of previously funded U-M proposals, and answering general faculty questions. For a wide range of Foundation and Corporate funding opportunities, visit their Competition Space or contact Joe Piffaretti, Senior Director, at [email protected] .
The Michigan Institute for Clinical & Health Research (MICHR) enables and enhances clinical & translational research at U-M by offering many funding opportunities throughout the year. For more information, visit their website .
Fast Forward Medical Innovation helps biomedical researchers navigate the wide variety of funding opportunities available at U-M that will help move their research down a successful commercialization path, including:
- The statewide MTRAC for Life Sciences Innovation Hub
- Frankel Innovation Initiative
- Michigan Biomedical Venture Fund
- Idea Consultations
The Bridging Support program provides funding to continue federally funded biomedical research projects that demonstrate a likelihood of successfully competing for federal funding upon resubmission of A0 or A1 proposals. Bridging applications must address current NIH grant critiques or other sponsors’ (e.g., VA Merit) written comments and explain planned use of bridging funds. Priority will focus on financial need to maintain a defined research project and to retain key research personnel. Amounts of other support, internal resources, and start-up funds will be considered. For more information about the application process, visit the website .
Limited Submissions are funding opportunities where sponsors invite institutions to nominate a proposal (the number of proposals is predetermined by the Sponsor/Foundation - typically one to two). In order to select these proposals/or institutional nominees, the University coordinates internal competitions, and invites all eligible applicants to apply. If selected, the institutional nominee/s are invited to submit their grant application to the sponsor to compete (together with nominees from other institutions) for the award.
Limited Submission opportunities/competitions are managed by both the Medical School Office of Research and the U-M Office of the Vice President for Research.
Managing the business aspects of research takes a team of experts, and your first point of contact should always be your department Research Administrator (RA). Your RA can help with policies, processes, system, forms, and many other aspects of your research proposal.
- The Office of Research & Sponsored Projects (ORSP) can connect you with your department RA through their Blue Pages - a searchable form that identifies RAs by department.
- The Grant Services & Analysis unit in the Medical School Office of Research also maintains a list of department contacts .
Michigan Experts is a searchable database of research expertise across disciplines, offering a rich representation of the research knowledge and talent of over 5,000 faculty members across the University of Michigan.
Use the database to find researchers with specific areas of expertise for collaboration or mentoring, explore co-author networks, and connect with colleagues across the preeminent U-M research enterprise to expand your research network.
Research Development team members within the Medical School Office of Research design and implement services that help faculty become successful in obtaining and maintaining extramural funding and — once successful — share their expertise to help their colleagues do the same. Faculty members are encouraged to contact them for assistance with pre-award activities related to finding funding opportunities and to proposal development and editing. For more information, visit the Proposal Development page.
The Medical School Grant Proposal Sampler is a repository of successfully funded grant proposals provided by Medical School faculty members for NIH, federal, and foundation awards. The examples offer insight into proposal development, including writing (e.g. organization, detail), responding to reviewers’ comments, sample sections (biosketch, budget), etc. The platform also contains resources to assist in the development of NIH Data Management and Sharing Plans, as well as features lists of Medical School faculty members who currently serve or have recently served on NIH Study Sections. The site requires level-2 password access.
The Michigan Institute for Clinical & Health Research (MICHR) offers many grant writing services including:
- Research Development Consultations - Free in-person consultations offer personalized support and advice for your grant proposals or research ideas and are available to any U-M investigator.
- Grant Editing - MICHR's editor will carefully review your grant to identify red flags in the content; ensure logic, flow and clarity of ideas; eliminate jargon; improve sentence structure and grammar; and comment on unclear text.
- Letters of Support - Grant proposals may be strengthened by a letter of support detailing MICHR services you will use to meet the research goals outlined in your proposal.
The Office of Graduate & Postdoctoral Studies provides information and resources for the preparation of NIH Training Grant applications (e.g. T32, T35, R25, K12), along with resources for Training Program administration (Diversity, Responsible Conduct of Research, Rigor and Transparency in Research, and Professional Development). With almost 70 NIH-supported Institutional Training Grants at the University of Michigan, these grants provide resources for training programs, which include committed participating faculty, competitively appointed trainees, and special events and activities for optimal training in specialized areas of biomedical research. Learn more .
The U-M Medical School Facility & Resource Profile Templates contain descriptions of units and resources at Michigan Medicine and elsewhere on campus. These boilerplate descriptions of U-M facilities and resources are intended to be copied, pasted, and edited as needed for grant proposals or marketing/recruiting materials.
The goal of the Proposal Preparation Funding Program is to provide funds to support the submission of large-scale, multi-investigator grants; this program is not designed to provide support for submission of individual R01-type grants. The funds may be used to offset the costs of hiring expert consultants or editors; coordinating and hosting brainstorming sessions; paying reviewers; or hiring temporary administrative and/or other services required to assemble and submit large proposals.
The Academic Writing & Research Development Series (AWARD) consists of articles published in UMMS Research News, the Medical School Office of Research's monthly newsletter. Topics cover proposal planning, proposal development and funding agency specifics.
The Grant Services & Analysis staff have experienced interdisciplinary proposal editors who will, as time permits, provide help editing external grant applications for grammar and grantsmanship issues. You may contact the staff by email ( [email protected] ) or phone (734-763-4272).
The Michigan Institute for Clinical & Health Research (MICHR) provides personalized support and advice for your grant proposals or research ideas. Free, one-hour consultations can help you improve your research and funding success and are available to any researcher at the University of Michigan.
The Medical School Office of Research maintains a list of freelance editors and freelance graphics specialists who may be available to help prepare various documents, e.g., grant proposals, manuscripts, dissertations.
Grant Services & Analysis in the Medical School requests that all proposals be submitted to the office ten business days prior to the sponsor’s proposal deadline . This includes the cumulative time needed for both the Medical School GS&A Office to check the application and to meet the ORSP Deadline Policy for required review. CLICK HERE to learn more about grant routing deadlines.
Often proposals submitted to sponsors can be strengthened with a letter of support or a letter of commitment for cost sharing from the leadership of Michigan Medicine or the Medical School. In order to facilitate these requests, the Executive Vice President for Medical Affairs (EVPMA) will sign the letter on behalf of Michigan Medicine, and the Senior Associate Dean for Research will sign the letter on behalf of the Medical School.
The budgeting/costs resources provided by Grant Services & Analysis offer a variety of information on budgeting various types of proposals and includes a variety of topics from budgeting personnel to budgeting industry studies and clinical trials. They also provide budget templates to facilitate budgeting and translation of the budget into sponsor forms.
Grant Services & Analysis has curated information on many NIH topics such as preparing a Biosketch, Resubmission, Training (T) Grant, or Cover Letter. For more information, visit the NIH Specifics, Tips & Tricks page.
Grant Services & Analysis provides a list of definitions and explanations of how to use the Proposal Approval Form (PAF), Clinical Trial Routing Form (CTRF), and Unfunded Agreement (UFA) in eRPM. For more information, visit the Using eResearch Proposal Management (eRPM): PAFs, CTRFs & UFAs page.
REMEMBER: Your grant routing deadline is before the sponsor deadline. Use Grant Services & Analysis' Deadline Calculator to determine your school deadline.
ORSP requires proposals no later than four (4) business days in advance of the sponsor’s deadline date and time, and the Medical School Submission Policy requests all proposals arrive to Grant Services & Analysis seven (7) business days prior to the project being submitted by campus. This means that the Medical School deadline that should be added to the PAF is a total of ten (10) business days prior to the sponsor’s deadline.
For additional information on Streamlined Review Services, Proposal Review Checklists, Required Proposal Components, and Medical School Department Contacts & Authorized Signers, visit the Pre-Award page.
The Proposal Review Checklists can be used to verify the completeness of the documents required in a grant application prior to routing the proposal.
The Proposal Finalization Checklist provides you with key items to check before finalizing a proposal (reflective of issues commonly found prior to submission).
For more information, visit the Grant Services & Analysis Pre-Award page.
A Project Award Notice (PAN) is prepared and distributed by ORSP via eResearch to the Principal Investigator and all signers off on a PAF serves to establish the project/grant number (PGN) for the project. For more information, visit ORSP's Project Award Notice page.
Data Management and Sharing plans are required by the NIH. CLICK HERE to learn more about this requirement, including links to plan examples.

We transform lives through bold discovery, compassionate care and innovative education.
- Diversity, Equity & Inclusion
- News & Stories
- Find a Doctor
- Conditions & Treatments
- Patient & Visitor Guide
- Patient Portal
- Clinical Trials
- Research Labs
- Cores and Resources
- Programs & Admissions
- Our Community
- Departments, Centers & Offices
- About the Medical School
Global Footer Secondary Navigation

RELATED PRODUCTS
Related tags and topics, related content, how to write a grant proposal.

Grant writing is a critical part of an investigator’s portfolio. It is a process by which a grant writer requests funding to test/study a novel idea to advance human health.
Funding for research is a highly competitive process. For example, over the last 2-decades, the National Institute of Health (NIH) grant acceptance rate has dipped from 31% in 2002 (30,068 applications / 9,396 awarded) to 19% in 2022 (58,872 applications / 11,229 awarded). 1 To increase the odds of funding, a compelling storyline invoking grant makers' immediate attention is necessary.
This document will provide high-level information on the dos and don’ts of grant writing. Essential features increasing the probability of grant award are discussed. This report would be a beneficial read for investigators in the healthcare sector, especially those performing basic and applied research in life science or biomedical sciences.
2. Know the Grant Proposal Guideline
Before putting pen to paper, the investigator needs to inquire if the grantor and the grantee have a common goal. For example, NIH, the largest funding agency in the world, has around 24 institutes and centers that award grant funding. NIH centers include National Cancer Institute, National Heart, Lung, and Blood Institute, National Human Genome Research Institute, etc. Each center has its federal appropriation and specific requisites.
Always keep a regular check on the funding opportunity announcement by the funder. Before initiating the grant proposal, the investigator must thoroughly go through the research grant application to maximize the chances of success. 2
There are several types of research grants that are funded by various agencies. These may include research grants, research career, development grants, small business grants, etc. 3 It is necessary for the grantee to perform thorough research on finalizing the type of grant idea pitched to the reviewers. Therefore, visiting the guidelines and requisites of the grant advertisement is highly recommended.
3. Start Early
One should start planning for the grant proposal around 6-8 months before submission date. This will give the writer time to discuss the grant content with peers, collaborators, and experts. Starting early will give you a cushion for giving thought to the idea, performing due diligence by sifting through the related literature, and eventually getting a broad understanding of the problem statement.
3.1 Regular connection with the program official
Each grant is assigned to a program officer. A grant program officer can provide insightful feedback to the investigator in the context of the content, the overall flow of the idea, etc. This exercise can give a clear understanding to the investigator of whether the research grant proposal is moving in the right direction. Providing a grant summary to the program officer, including high-level information on the specific aims, can help refine the grant and get continued alignment with the funder’s requirements.
4. Know your Audience
Before initiating the grant writing process, identify the appropriate audience/funding department having a common mission/problem statement. Some tools can help direct the grantee to an appropriate funding agency. For example, NIH’s website uses NIH Reporter that matches the investigator’s proposed abstract to the department most likely to provide the necessary grant aid. 4 Once a pertinent institute is identified, a grantee can tailor the relevant information per the funder’s mission statement.
4.1 Succinct biosketch
A biosketch/CV describing an investigator’s qualification and background helps reviewers to better understand the grant writer’s experience and expertise related to the project. Periodically browse the funder's website and format the CV per the latest guidance. A well-crafted biosketch can make a significant impact on the reviewers.
5. Well-Structured Research Grant
A grant should tell a crisp story. Clear emphasis should be provided on the gap in the field and how your effort will close this gap. Finer details must be discussed to the extent that the message should not get redundant or boring. Additionally, from start to finish, one should not lose track of the central hypothesis/primary message.
5.1 Clear aims
Goals and aims should be well-defined. This section is central to your grant. 5 The hypothesis-driven aims must be clearly presented. The aims hypothesis should be tested by reliable methods. Always ensure that you design SMART (specific, measurable, actionable, relevant, and time-based) goals. Also, a holistic approach should be presented to the reviewer entailing the scenarios where the hypothesis both succeeds or fails. It is generally recommended that the project aims are independent of each other. This way if the first goal is not accomplished, you can still test your second goal.
Consideration must be given to the fact that the research aims are more important than the broader research area itself. For example, working on cutting-edge research aims is far more important than investigating critical research area such as cancer with redundant specific aims.
5.2 Balanced approach
A grant should have a coherent description to the approach for achieving specific aims. Additionally, details should be provided on the alternate aim in case the primary one is not achieved. Discussing the issues that may arise in the primary goal, corresponding potential pitfalls, and a robust alternate strategy substantially improves the probability of funding for research. The central hypothesis must not be overambitious (especially for early-stage/new investigators). The problem description should include a background section comprising state-of-the-art information. 5 The background, with literature-backed evidence, should discuss the central problem and how your research would contribute to the advancement of the field. Also, an extensive literature search will aid in understanding the type and amount of work done in the field. It may also confirm whether your work is novel. If it’s otherwise, you may save time writing the grant further and perhaps go back to the drawing board to re-analyze other important topics.
5.3 Statistical rigor
The supportive data should be backed up by sound statistical inference. Study data can only be conclusive if it undergoes robust statistical analyses, The grant will probably fare poorly if the experiment analysis is not sufficiently powered. For example, if you think that an oncogene is over-expressed in lung cancer tissues, the statement must be buttressed by an appropriate statistical approach.
5.4 Reasonable Budget and Timeline
Provide a clear description of both the direct and indirect project costs. The direct cost would include funding to the scientific staff, reagents, equipment, etc., while the indirect cost may cover institute's sustainability (for example, supporting the administration, upkeep of the building, etc.). The itemized budget must be clearly defined as it highlights the investigator’s vision of successfully running the project. Connect with your institute’s sponsored research office that will assist in developing the budget. 3
The grant must also mention the year-by-year timeline of how each specific aim will progress.
6. Rigorous Review of the Content
Proofread the content several times. Ensure that the figure, table, and overall presentation are clear, concise, and devoid of redundancy. If the funding proposal is sloppy with poorly labeled legends, typographical errors, etc., then there are high chances that the reviewer may lose interest and may not even go through the full content.
Provide the draft to a peer/colleague for editing. If necessary, run it through a typing assistant program such as Grammarly. Regularly align with subject matter experts. This activity will allow you to draft a high-quality impactful grant. For example, if a pathologist is drafting a grant on diagnosing breast cancer using artificial intelligence, then collaborating with an expert computational biologist will improve the chances of funding.
7. Be Persistent
Industry, foundation, and government-funded grants are advertised regularly. Hence, keep looking for these opportunities. In general, most applicants try more than once to get their grant funded. Therefore, if unsuccessful, consistently refine your grant and apply again with a better than before version.
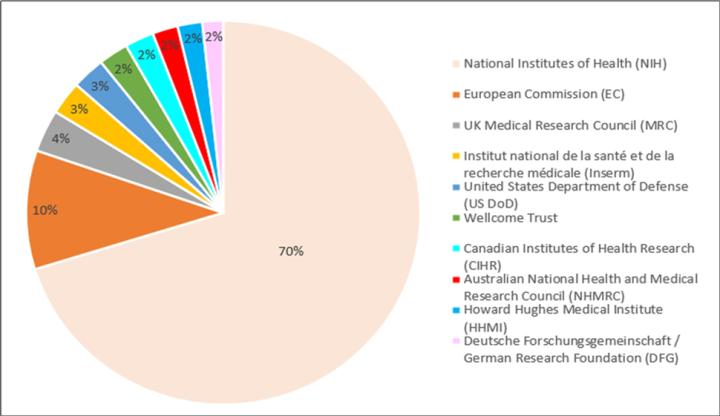
In addition to the NIH, other organizations such as the National Science Foundation, Department of Defense (DoD), Centers for Disease Control and Prevention (CDC), Wellcome trust, World health organization, etc., sponsor large and small grants. Also, it always adds value to a grant proposal if you can show funding from other organization(s) as well. Table 1 and Table 2 captures the annual research expenditures by top donors in almost a decade. 6,7 A corresponding percent funding breakdown is described in Figure 1 and Figure 2 .
Table 1. Top donors in the year 2013
Figure 1. top donors’ percent funding disbursement for the year 2013.
- Langson Library
- Science Library
- Grunigen Medical Library
- Law Library
- Connect From Off-Campus
- Accessibility
- Gateway Study Center

Email this link
Biomedical engineering.
- Find Articles
- Reference Sources
- Patent Information
- Professional & Career Information
- Bibliographic Management Software
- UC Irvine Departments & Sites
Research Grants & Funding Resources
- Dissertation/Thesis Preparation
- Media Resources Center
- Henry Stewart Talks: The Biomedical & Life Sciences Collection
- Engineering Ethics
- Things To Do & Things to Know
* Indicates UCI Access Only
- GrantForward GrantForward is a funding opportunity database and recommendation service built by academics for researchers
- SPIN - Sponsored Projects Information Network* SPIN is a searchable database made available by the UCI Office of Research that indexes approximately 8,000 opportunities. SPIN and IRIS include some overlap. Spin Userguide
- CORDIS - Community Research and Development Information Service This site provides information on current research projects in the European Union and gives access to documents concerning the EU Framework programmes as well as to other EU sites.
- CRISP - Computer Retrieval of Information on Scientific Projects Contains information on federally funded biomedical research projects. The database is maintained by the Office of Extramural Research at the National Institutes of Health.
- D&B Million Dollar Directory*
- Grants.gov Grants.gov is a central storehouse for information on over 1,000 U.S. Government grant programs offered by the 26 federal grant-making agencies and provides access to approximately $500 billion in annual awards.
- NIH Guide for Grants and Contracts NIH Funding Opportunities (NIH Guide for Grants and Contracts)
- NSF (National Science Foundation) A-Z Index of Funding Opportunities
- UROP Off-campus: Biomedical sciences For Undergraduates-These listings are identified by the program name, which is linked to a summary of the program and the program's official Web site.
- UROP Off-campus: Engineering, Computer Science, and Applied Sciences For Undergraduates-These listings are identified by the program name, which is linked to a summary of the program and the program's official Web site.
- UROP Off-campus: Physical Sciences For Undergraduates-These listings are identified by the program name, which is linked to a summary of the program and the program's official Web site.
- Annual register of grant support LB 2336 A5
- Grants Register
- Directory of research grants LB 2338 D5
- Fastweb Fastweb and the College Board offers scholarship searches for parents and students.
- FinAid: The Smart Student Guide to Financial Aid Resources on loans, scholarships, and financial aid of every kind; also on calculating needs, application forms, etc.
- Federal Students Aid Provides information on federal loans.
- Campus Grotto Offers a tool that lets students and parents compare loan options.
- PLUS Loans Explains how parents van borrow for their childrens's education.
- Research Grants & Resources
Proposal Preparation
- Developing And Writing Grant Proposals Useful for applying for federal grants. By the staff of the Catalog of Federal Domestic Assistance.
- EPA Grantwriting Tutorial Online tutorial from Purdue University in collaboration with the U.S. Environmental Protection Agency.
- Grants.gov: Applicant Resources Includes Developing a Grant Proposal, terminology proposal writing links, links to application packages, grantmaking agencies, types of grants, and more.
- All About Grants Tutorials For biomedical investigators applying for NIH research project grants. Maintained by its National Institute of Allergy and Infectious Diseases.
- Guide for Writing a Funding Proposal Written by S. Joseph Levine of Michigan State University. Offers excellent advice on all parts of the proposal. Includes a sample proposal and links to other proposal writing sites.
- Proposal Writer's Guide An excellent outline by Donald Thackrey for academic faculty and staff. Especially useful. A site maintained by the University of Michigan's Division of Research and Development Administration.
- Proposal Preparation and Submission A site maintained by the University of Michigan's Division of Research and Development Administration.
- Proposal Writing: Selected Web Sites Outstanding. Provides essential links for university scholars and researchers needing more than here on research funding in particular.
- Writing Winning Proposals: An Introduction Excellent advice from the ASME: American Society of Mechanical Engineers.
- Grant Proposal Writing Tips Some good, clearly-stated tips from the Corporation for Public Broadcasting, which reviews hundreds of proposals a year.
- GrantProposal.com Well organized site on proposal writing. Includes an overview, inquiry and cover letters, standard components of a proposal, a sample proposal, advice from funders, and more. An excellent section on researching funding opportunities is included also.
- How to Write a Mission Statement Outline by Janet M. Radtke on writing a mission statement for an organization. A site of the Los-Angeles based Grantsmanship Center.
- National Network of Grantmakers: NNG Common Grant Application This common grant application form is accepted by a number of foundations. It can also be useful in organizing the information needed in a project proposal. (pdf)
- Grants for Nonprofits Information for how to write specific grants for nonprofits, how to find appropriate funding sources for nonprofits and how to get grants from nonprofit organizations. Lots of easy hints.
- The Foundation Center's Proposal Writing Short Course Free, online course.
- The Foundation Center's Proposal Budgeting Basics Free, online course.
- The Foundation Center Webinars on Proposal Writing Basics Free; must register in advance.
- The Foundation Center's Guide to Proposal Writing, 5th ed. - An Audio Book! Free audio book from the Foundation Center. Listen online now or download for later listening.
- Sample Grant Proposals Proposals from the Idea Bank's Grant Writing Course. Most are successfully funded, and are for funding for fire departments.
- School Grants Offers a number of education-focused, successful, sample proposals. Most are directed to corporate or government funding sources and are downloadable in PDF format.
- Winning Grant Proposals Online Provides funded grant proposals for sale in a variety of categories. Particularly well-stocked with federal grant proposals. A site from the Grantsmanship Center, a Los-Angeles based proposal training center.
- Writing a Successful Grant Proposal An excellent and thorough guide for writing grant proposals, from the Minnesota Council on Foundations. FAQs include pros and cons of hiring a professional grantwriter and what to do if a proposal is funded.
Sources for Funding - Databases
There are several major databases to consult for seeking funding - the two largest are Pivot & COS. On the left sidebar, is a long list of resources that source funding opportunities for travel, research, grants & contracts.
- COS - Community of Science Funding Opportunities Funded at UCI by the UCI Libraries. Provides a user-friendly interface to a worldwide database of more than 25,000 records.
- COS Userguide
- COS Funding News
- COS Top Ten Funding Opportunities Of the more than 25,000 records, the records most viewed last week.
- << Previous: UC Irvine Departments & Sites
- Next: Dissertation/Thesis Preparation >>
- Last Updated: Apr 4, 2024 9:24 AM
- URL: https://guides.lib.uci.edu/engr_biomed
Off-campus? Please use the Software VPN and choose the group UCIFull to access licensed content. For more information, please Click here
Software VPN is not available for guests, so they may not have access to some content when connecting from off-campus.
ANNOTATED SAMPLE GRANT PROPOSALS
How to Use Annotated Sample Grants
Are these real grants written by real students.
Yes! While each proposal represents a successfully funded application, there are two things to keep in mind: 1) The proposals below are final products; no student started out with a polished proposal. The proposal writing process requires stages of editing while a student formulates their project and works on best representing that project in writing. 2) The samples reflect a wide range of project types, but they are not exhaustive . URGs can be on any topic in any field, but all must make a successful argument for why their project should be done/can be done by the person proposing to do it. See our proposal writing guides for more advice. The best way to utilize these proposals is to pay attention to the proposal strengths and areas for improvement on each cover page to guide your reading.
How do I decide which sample grants to read?
When students first look through the database, they are usually compelled to read an example from their major (Therefore, we often hear complaints that there is not a sample proposal for every major). However, this is not the best approach because there can be many different kinds of methodologies within a single subject area, and similar research methods can be used across fields.
- Read through the Methodology Definitions and Proposal Features to identify which methodolog(ies) are most similar to your proposed project.
- Use the Annotated Sample Grant Database ( scroll below the definitions and features) filters or search for this methodology to identify relevant proposals and begin reading!
It does not matter whether the samples you read are summer grants (SURGs) or academic year grants (AYURGs). The main difference between the two grant types is that academic year proposals (AYURG) require a budget to explain how the $1,000 will be used towards research materials, while summer proposals (SURG) do not require a budget (the money is a living stipend that goes directly to the student awardee) and SURGs have a bigger project scope since they reflect a project that will take 8 weeks of full time research to complete. The overall format and style is the same across both grant cycles, so they are relevant examples for you to review, regardless of which grant cycle you are planning to apply.
How do I get my proposal to look like these sample grants?
Do not submit a first draft: These sample proposals went through multiple rounds of revisions with feedback from both Office of Undergraduate Research advisors and the student’s faculty mentor. First, it helps to learn about grant structure and proposal writing techniques before you get started. Then, when you begin drafting, it’s normal to make lots of changes as the grant evolves. You will learn a lot about your project during the editing and revision process, and you typically end up with a better project by working through several drafts of a proposal.
Work with an advisor: Students who work with an Office of Undergraduate Research Advisor have higher success rates than students who do not. We encourage students to meet with advisors well in advance of the deadline (and feel free to send us drafts of your proposal prior to our advising appointment, no matter how rough your draft is!), so we can help you polish and refine your proposal.
Review final proposal checklists prior to submission: the expectation is a two-page, single-spaced research grant proposal (1″ margins, Times New Roman 12 or Arial 11), and proposals that do not meet these formatting expectations will not be considered by the review committee. Your bibliography does not count towards this page limit.
Academic Year URG Submission Checklist
Summer URG Application Checklist
METHODOLOGY DEFINITIONS & PROPOSAL FEATURES
Research methodologies.
The proposed project involves collecting primary sources held in archives, a Special Collections library, or other repository. Archival sources might include manuscripts, documents, records, objects, sound and audiovisual materials, etc. If a student proposes a trip to collect such sources, the student should address a clear plan of what will be collected from which archives, and should address availability and access (ie these sources are not available online, and the student has permission to access the archive).
Computational/Mathematical Modeling
The proposed project involves developing models to numerically study the behavior of system(s), often through computer simulation. Students should specify what modeling tool they will be using (i.e., an off-the-shelf product, a lab-specific codebase), what experience they have with it, and what resources they have when they get stuck with the tool (especially if the advisor is not a modeler). Models often involve iterations of improvements, so much like a Design/Build project, the proposal should clearly define parameters for a “successful” model with indication of how the student will assess if the model meets these minimum qualifications.
Creative Output
The proposed project has a creative output such playwriting, play production, documentary, music composition, poetry, creative writing, or other art. Just like all other proposals, the project centers on an answerable question, and the student must show the question and method associated with the research and generation of that project. The artist also must justify their work and make an argument for why this art is needed and/or how it will add to important conversations .
Design/Build
The proposed project’s output centers around a final product or tool. The student clearly defines parameters for a “successful” project with indication of how they will assess if the product meets these minimum qualifications.
The project takes place in a lab or research group environment, though the methodology within the lab or research group vary widely by field. The project often fits within the larger goals/or project of the research group, but the proposal still has a clearly identified research question that the student is working independently to answer.
Literary/Composition Analysis
The project studies, evaluates, and interprets literature or composition. The methods are likely influenced by theory within the field of study. In the proposal, the student has clearly defined which pieces will be studied and will justify why these pieces were selected. Context will be given that provides a framework for how the pieces will be analyzed or interpreted.
Qualitative Data Analysis
The project proposes to analyze data from non-numeric information such as interview transcripts, notes, video and audio recordings, images, and text documents. The proposal clearly defines how the student will examine and interpret patterns and themes in the data and how this methodology will help to answer the defined research question.
Quantitative Data Analysis
The project proposes to analyze data from numeric sources. The proposal clearly defines variables to be compared and provides insight as to the kinds of statistical tests that will be used to evaluate the significance of the data.
The proposed project will collect data through survey(s). The proposal should clearly defined who will be asked to complete the survey, how these participants will be recruited, and/or proof of support from contacts. The proposal should include the survey(s) in an appendix. The proposal should articulate how the results from these survey(s) will be analyzed.
The proposed project will use theoretical frameworks within their proposed area of research to explain, predict, and/or challenge and extend existing knowledge. The conceptual framework serves as a lens through which the student will evaluate the research project and research question(s); it will likely contain a set of assumptions and concepts that form the basis of this lens.
Proposal Features
Group project.
A group project is proposed by two or more students; these proposals receive one additional page for each additional student beyond the two page maximum. Group projects must clearly articulate the unique role of each student researcher. While the uploaded grant proposal is the same, each student researcher must submit their own application into the system for the review.
International Travel
Projects may take place internationally. If the proposed country is not the student’s place of permanent residence, the student can additionally apply for funding to cover half the cost of an international plane ticket. Proposals with international travel should likely include travel itineraries and/or proof of support from in-country contacts in the appendix.
Non-English Language Proficiency
Projects may be conducted in a non-English language. If you have proficiency in the proposed language, you should include context (such as bilingual, heritage speaker, or by referencing coursework etc.) If you are not proficient and the project requires language proficiency, you should include a plan for translation or proof of contacts in the country who can support your research in English.
DATABASE OF ANNOTATED SAMPLE GRANTS

- Sample Grant Applications
- Implementation Science
- Funding Opportunities
Several investigators and their organizations agreed to post part of their dissemination and implementation grant applications online. We are grateful to them for letting us provide this resource to the community.
R37: Sustainability Determinants of an Intervention to Identify Clinical Deterioration and Improve Childhood Cancer Survival in Low-Resource Hospitals
Principal investigator.

Asya Agulnik, MD, MPH ST. JUDE CHILDREN'S RESEARCH HOSPITAL*

Virginia McKay, MA, PhD RESEARCH ASSISTANT PROFESSOR*
Award Number
1R37CA276215-01
R21: Policy Implementation Research on Health Benefit Mandates for Fertility Preservation Services to Improve Access to Care in Young Cancer Survivors

Hui-Chun Irene Su, MD, MSCE UNIVERSITY OF CALIFORNIA SAN DIEGO HEALTH*
R21#CA271184-01A1
R01: Establishing Smoke-Free Homes with Families Involved in Child Protective Services: An Effectiveness-Implementation Trial of an Integrated Program

Shannon R. Self-Brown, PhD Georgia State University School of Public Health*
R01#CA248551-01A1
R37: Testing an Adaptive Implementation Strategy to Optimize Delivery of Obesity Prevention Practices in Early Care and Education Settings

Taren Swindle, PhD University of Arkansas for Medical Sciences*
R37#CA252113-01A1
R37: De-implementation of low value castration for men with prostate cancer

Ted Skolarus, MD, MPH, FACS University of Michigan at Ann Arbor*
R37#CA222885-01
R21: Improving HPV Vaccination Using Implementation Strategies in Community Pharmacies

Benjamin Teeter, PhD, MS University of Arkansas for Medical Sciences*
R21#CA231180-01A1
R01: Using Technology to Scale-Up an Occupational Sun Protection Policy Program

David B. Buller, PhD Klein Buendel Inc.*
R01#CA210259-01A1
R01: Implementing Universal Lynch Syndrome Screening across Multiple Healthcare Systems: Identifying Strategies to Facilitate and Maintain Programs in Different Organizational Contexts

Alanna Rahm, PhD Geisinger Clinic*
R01#CA211723-01A1
R01: Implementing Cancer Prevention Using Patient - Provider Clinical Decision Support

Thomas E. Elliott, MD HealthPartners Institute*
R01#CA193396-01A1
R21: Effective Training Models for Implementing Health-Promoting Practices Afterschool

Rebekka Mairghread Lee, ScD Harvard School of Public Health*
R21#CA201567-01A1
R01: Increasing Implementation of Evidence-Based Interventions at Low-Wage Worksites

Margaret Hannon, PhD University of Washington*
R01#CA160217-01A1
Additional Information
We redact some information from these documents, such as budgets, social security numbers, home addresses, and introductions to revised applications. We also only include a copy of the SF 424 R&R Face Page, Project Summary/Abstract (Description), Project Narrative, Specific Aims, and Research Strategy. We do not include other SF 424 (R&R) forms or basic information found in full grant applications, such as performance sites, key personnel, or biographical sketches.
*Institution at time of grant award. **Notice(s) of Funding Opportunities (NOFOs) at the time of grant award that have since expired.
The text of the grant applications is copyrighted. Investigators and others may use the text from these applications only for nonprofit educational purposes provided that the content remains unchanged and that the Principal Investigator(s), their organization(s), and NCI are credited.
Individuals using assistive technology (e.g., screen reader, Braille reader, etc.) who experience difficulty accessing any information should send an email to the IS team ( [email protected] ).
Other Sample Grants
See examples of successfully funded grant applications.
Current Implementation Science Funding Opportunities
Find resources and learn about implementation science funding opportunities such as Dissemination and Implementation Research in Health.
- Public Goods
- Eligibility Requirements
- Application Procedure
- Frequently Asked Questions
- Outcomes of the 2023 CCIS Meeting
- Outcomes of the 2022 CCIS Meeting
- Outcomes of the 2021 CCIS Meeting
- Outcomes of the 2020 ISCC Meeting
- Outcomes of the 2019 ISCC Meeting
- CCIS Action Groups
- Implementation Science Centers in Cancer Control (ISC3)
- Training in Cancer
- Research Tools
- Practice Tools
- Research-Practice Partnerships
- Ali Abazeed, MPH, MPP
- Annabelle Uy, MS
- Antoinette Percy-Laurry, DrPH, MSPH
- Cynthia A. Vinson, PhD, MPA
- David Chambers, DPhil
- Gila Neta, PhD, MPP
- Laurie Hursting, MA
- Mindy Clyne, MHS, CGC
- Pete DelNero, PhD, MPH
- Sarah Bruce Bernal, MA
- Wynne E. Norton, PhD
Sample Healthcare Delivery Research Grant Applications
The National Cancer Institute (NCI) frequently receives requests for examples of funded grant applications. Several investigators and their organizations agreed to let the Healthcare Delivery Research Program (HDRP) post excerpts of their healthcare delivery research grant applications online.
We are grateful to the investigators and their institutions for allowing us to provide this important resource to the community. We include a copy of the SF 424 R&R Face Page, Project Summary/Abstract (Description), Project Narrative, Specific Aims, and Research Strategy; we do not include other SF 424 (R&R) forms or requisite information found in the full grant application (e.g., performance sites, key personnel, biographical sketches). To maintain confidentiality, we have redacted some information from these documents (e.g., budgets, social security numbers, home addresses, introduction to revised application).
Sample Applications
R01: personalized screening for lung cancer: the importance of co-existing chronic conditions to clinical practice and policy, principal investigator.

Grant Mechanism & Award Number
R01CA249506-01
R01: Predicting and Addressing Colonoscopy Non-adherence in Community Settings

R01CA218923-01A1
R01: Using MOST to EMPOWER: Optimizing an Emotional Regulation Intervention to Enhance Well-being Among Young Adult Cancer Survivors
Principal investigators.

R01CA242849-01
R01: Improving Informal Caregivers' and Cancer Survivors' Psychological Distress, Symptom Management and Health Care Use

R01CA224282-01A1
R03: Statewide Assessment of HPV Vaccination Among Childhood Cancer Survivors

1R03CA216174-01A1
R03: Multi-center Evaluation of Digital Breast Tomosynthesis with Synthesized Two-dimensional Mammography for Breast Cancer Screening

R03CA223725-01
R21: Improving Transition Readiness in Adolescent and Young Adult (AYA) Survivors of Childhood Cancer

R21CA222936-01A1
R50: Natural History of Lung Cancer Diagnosed Within and Across Diverse Health Systems Implementing Lung Cancer Screening

R50CA251966-01
Additional Details
Copyright information.
The text of the grant applications is copyrighted. Investigators and others may use the text from these applications only for nonprofit educational purposes provided that the content remains unchanged and that the Principal Investigator(s), their organization(s), and NCI are credited.
Accessibility
Individuals using assistive technology (e.g., screen reader, Braille reader, etc.) who experience difficulty accessing any information should send an email to the HDRP team ( [email protected] ).
Other Sample Grants
See examples of successfully funded grant applications.
Currently Open Notice of Funding Opportunities Relevant to HDRP
See the currently open notice of funding opportunities (NOFOs) sponsored or co-sponsored by HDRP; other NOFOs relevant to HDRP; and NIH and NCI Parent and Omnibus NOFOs for investigator-initiated research.

- Research Process
Writing a Scientific Research Project Proposal
- 5 minute read
- 95.8K views
Table of Contents
The importance of a well-written research proposal cannot be underestimated. Your research really is only as good as your proposal. A poorly written, or poorly conceived research proposal will doom even an otherwise worthy project. On the other hand, a well-written, high-quality proposal will increase your chances for success.
In this article, we’ll outline the basics of writing an effective scientific research proposal, including the differences between research proposals, grants and cover letters. We’ll also touch on common mistakes made when submitting research proposals, as well as a simple example or template that you can follow.

What is a scientific research proposal?
The main purpose of a scientific research proposal is to convince your audience that your project is worthwhile, and that you have the expertise and wherewithal to complete it. The elements of an effective research proposal mirror those of the research process itself, which we’ll outline below. Essentially, the research proposal should include enough information for the reader to determine if your proposed study is worth pursuing.
It is not an uncommon misunderstanding to think that a research proposal and a cover letter are the same things. However, they are different. The main difference between a research proposal vs cover letter content is distinct. Whereas the research proposal summarizes the proposal for future research, the cover letter connects you to the research, and how you are the right person to complete the proposed research.
There is also sometimes confusion around a research proposal vs grant application. Whereas a research proposal is a statement of intent, related to answering a research question, a grant application is a specific request for funding to complete the research proposed. Of course, there are elements of overlap between the two documents; it’s the purpose of the document that defines one or the other.
Scientific Research Proposal Format
Although there is no one way to write a scientific research proposal, there are specific guidelines. A lot depends on which journal you’re submitting your research proposal to, so you may need to follow their scientific research proposal template.
In general, however, there are fairly universal sections to every scientific research proposal. These include:
- Title: Make sure the title of your proposal is descriptive and concise. Make it catch and informative at the same time, avoiding dry phrases like, “An investigation…” Your title should pique the interest of the reader.
- Abstract: This is a brief (300-500 words) summary that includes the research question, your rationale for the study, and any applicable hypothesis. You should also include a brief description of your methodology, including procedures, samples, instruments, etc.
- Introduction: The opening paragraph of your research proposal is, perhaps, the most important. Here you want to introduce the research problem in a creative way, and demonstrate your understanding of the need for the research. You want the reader to think that your proposed research is current, important and relevant.
- Background: Include a brief history of the topic and link it to a contemporary context to show its relevance for today. Identify key researchers and institutions also looking at the problem
- Literature Review: This is the section that may take the longest amount of time to assemble. Here you want to synthesize prior research, and place your proposed research into the larger picture of what’s been studied in the past. You want to show your reader that your work is original, and adds to the current knowledge.
- Research Design and Methodology: This section should be very clearly and logically written and organized. You are letting your reader know that you know what you are going to do, and how. The reader should feel confident that you have the skills and knowledge needed to get the project done.
- Preliminary Implications: Here you’ll be outlining how you anticipate your research will extend current knowledge in your field. You might also want to discuss how your findings will impact future research needs.
- Conclusion: This section reinforces the significance and importance of your proposed research, and summarizes the entire proposal.
- References/Citations: Of course, you need to include a full and accurate list of any and all sources you used to write your research proposal.
Common Mistakes in Writing a Scientific Research Project Proposal
Remember, the best research proposal can be rejected if it’s not well written or is ill-conceived. The most common mistakes made include:
- Not providing the proper context for your research question or the problem
- Failing to reference landmark/key studies
- Losing focus of the research question or problem
- Not accurately presenting contributions by other researchers and institutions
- Incompletely developing a persuasive argument for the research that is being proposed
- Misplaced attention on minor points and/or not enough detail on major issues
- Sloppy, low-quality writing without effective logic and flow
- Incorrect or lapses in references and citations, and/or references not in proper format
- The proposal is too long – or too short
Scientific Research Proposal Example
There are countless examples that you can find for successful research proposals. In addition, you can also find examples of unsuccessful research proposals. Search for successful research proposals in your field, and even for your target journal, to get a good idea on what specifically your audience may be looking for.
While there’s no one example that will show you everything you need to know, looking at a few will give you a good idea of what you need to include in your own research proposal. Talk, also, to colleagues in your field, especially if you are a student or a new researcher. We can often learn from the mistakes of others. The more prepared and knowledgeable you are prior to writing your research proposal, the more likely you are to succeed.
Language Editing Services
One of the top reasons scientific research proposals are rejected is due to poor logic and flow. Check out our Language Editing Services to ensure a great proposal , that’s clear and concise, and properly referenced. Check our video for more information, and get started today.

- Manuscript Review
Research Fraud: Falsification and Fabrication in Research Data

Research Team Structure
You may also like.

Descriptive Research Design and Its Myriad Uses

Five Common Mistakes to Avoid When Writing a Biomedical Research Paper

Making Technical Writing in Environmental Engineering Accessible

To Err is Not Human: The Dangers of AI-assisted Academic Writing

When Data Speak, Listen: Importance of Data Collection and Analysis Methods

Choosing the Right Research Methodology: A Guide for Researchers

Why is data validation important in research?

Writing a good review article
Input your search keywords and press Enter.
Preparing a Grant Proposal for Medical Research in the UK
- First Online: 12 January 2023
Cite this chapter

- Tae-kyung Park 3 ,
- Khaled Altarrah 4 , 5 &
- Rajive Mathew Jose 6
343 Accesses
Finding the money for research is not easy and the process of applying for grants can be tedious and often frustrating. There are several organizations that fund medical research in the UK. There are government organizations including charities, governmental bodies, pharmaceutical companies, and Royal colleges. The money offered through the grants from these bodies can vary from pump-priming grants of a few thousand to millions of pounds.
If I should die, think only this of me: that there is some corner of a foreign field that is forever England Rupert Brooke
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Jonker L, Fisher SJ. The correlation between National Health Service Trusts’ clinical trial activity and both mortality rates and care quality commission ratings: a retrospective cross-sectional study. Public Health. 2018;157:1–6. https://doi.org/10.1016/j.puhe.2017.12.022 .
Article CAS Google Scholar
NIHR Research Design Service London: how to find funding. https://www.rds-london.nihr.ac.uk/resources/how-to-find-funding/ .
Arthurs OJ. Think it through first: questions to consider in writing a successful grant application. Pediatr Radiol. 2014;44(12):1507–11. https://doi.org/10.1007/s00247-014-3053-6 .
Article Google Scholar
Morgan B, Yu LM, Solomon T, Ziebland S. Assessing health research grant applications: a retrospective comparative review of a one-stage versus a two-stage application assessment process. PLoS One. 2020;15(3):e0230118. https://doi.org/10.1371/journal.pone.0230118 .
Wilkinson E. Wellcome Trust to fund people not projects. Lancet. 2010;375:185–6.
Herbert DL, Barnett AG, Clarke P, et al. On the time spent preparing grant proposals: an observational study of Australian researchers. BMJ Open. 2013;3:e002800. https://doi.org/10.1136/bmjopen-2013-002800 .
Download references
Author information
Authors and affiliations.
Medical Education, University Hospital Birmingham, Birmingham, UK
Tae-kyung Park
Faculty of Medicine, University of Birmingham, Birmingham, UK
Khaled Altarrah
Ibn Sina Specialist Hospital, Ministry of Health, Kuwait City, Kuwait
University Hospital Birmingham, Birmingham, UK
Rajive Mathew Jose
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Sri Balaji Vidyapeeth University, Pondicherry, India
Subhash Chandra Parija
Department of General and Gastrointestinal surgery, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India
Vikram Kate
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Park, Tk., Altarrah, K., Jose, R.M. (2023). Preparing a Grant Proposal for Medical Research in the UK. In: Parija, S.C., Kate, V. (eds) Grant writing for medical and healthcare professionals. Springer, Singapore. https://doi.org/10.1007/978-981-19-7018-4_17
Download citation
DOI : https://doi.org/10.1007/978-981-19-7018-4_17
Published : 12 January 2023
Publisher Name : Springer, Singapore
Print ISBN : 978-981-19-7017-7
Online ISBN : 978-981-19-7018-4
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Grants & funding.
The National Institutes of Health is the largest public funder of biomedical research in the world. In fiscal year 2022, NIH invested most of its $45 billion appropriations in research seeking to enhance life, and to reduce illness and disability. NIH-funded research has led to breakthroughs and new treatments helping people live longer, healthier lives, and building the research foundation that drives discovery.
three-scientists-goggles-test-tube.jpg

Grants Home Page
NIH’s central resource for grants and funding information.
lab-glassware-with-colorful-liquid-square.jpg

Find Funding
NIH offers funding for many types of grants, contracts, and even programs that help repay loans for researchers.
calendar-page-square.jpg

Grant applications and associated documents (e.g., reference letters) are due by 5:00 PM local time of application organization on the specified due date.
submit-key-red-square.jpg

How to Apply
Instructions for submitting a grant application to NIH and other Public Health Service agencies.
female-researcher-in-lab-square.jpg

About Grants
An orientation to NIH funding, grant programs, how the grants process works, and how to apply.
binder-with-papers-on-office-desk-square.jpg

Policy & Compliance
By accepting a grant award, recipients agree to comply with the requirements in the NIH Grants Policy Statement unless the notice of award states otherwise.
blog-key-blue-square.jpg

Grants News/Blog
News, updates, and blog posts on NIH extramural grant policies, processes, events, and resources.
scientist-flipping-through-report-square.jpg

Explore opportunities at NIH for research and development contract funding.
smiling-female-researcher-square.jpg

Loan Repayment
The NIH Loan Repayment Programs repay up to $50,000 annually of a researcher’s qualified educational debt in return for a commitment to engage in NIH mission-relevant research.
Connect with Us
- More Social Media from NIH
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Active funding opportunity
Nsf 24-561: foundations for digital twins as catalyzers of biomedical technological innovation, program solicitation, document information, document history.
- Posted: March 22, 2024
Program Solicitation NSF 24-561
Full Proposal Deadline(s) (due by 5 p.m. submitting organization’s local time):
June 21, 2024
May 05, 2025
First Monday in May, Annually Thereafter
Important Information And Revision Notes
Any proposal submitted in response to this solicitation should be submitted in accordance with the NSF Proposal & Award Policies & Procedures Guide (PAPPG) that is in effect for the relevant due date to which the proposal is being submitted. The NSF PAPPG is regularly revised and it is the responsibility of the proposer to ensure that the proposal meets the requirements specified in this solicitation and the applicable version of the PAPPG. Submitting a proposal prior to a specified deadline does not negate this requirement.
Summary Of Program Requirements
General information.
Program Title:
Foundations for Digital Twins as Catalyzers of Biomedical Technological Innovation (FDT-BioTech)
The Foundations for Digital Twins as Catalyzers of Biomedical Technological Innovation (FDT-BioTech) program supports inherently interdisciplinary research projects that underpin the mathematical and engineering foundations behind the development and use of digital twins and synthetic data in biomedical and healthcare applications, with a particular focus on digital, in silico models used in the evaluation of medical devices and the relevance of the developed models in addressing current and emerging challenges affecting the development and assessment of biomedical technologies. The goal of the FDT-BioTech initiative is to catalyze biomedical technological innovation through new foundational development of methods and algorithms relevant to digital twins and synthetic humans.
Cognizant Program Officer(s):
Please note that the following information is current at the time of publishing. See program website for any updates to the points of contact.
Yulia R. Gel, MPS/DMS, telephone: (703) 292-7888, email: [email protected]
Zhilan J. Feng, MPS/DMS, telephone: (703) 292-7523, email: [email protected]
Stephanie George, ENG/CBET, telephone: (703) 292-7825, email: [email protected]
Varun Chandola, CISE/OAC, telephone: (703) 292-2656, email: [email protected]
Ashok Srinivasan, CISE/OAC, telephone: (703) 292-2122, email: [email protected]
Laura Biven, NIH, telephone: (301)480-4021, email: [email protected]
Fenglou Mao, NIH, telephone: (301)451-9389, email: [email protected]
Aldo Badano, FDA, telephone: (301) 796-2534, email: [email protected]
- 47.049 --- Mathematical and Physical Sciences
- 47.070 --- Computer and Information Science and Engineering
- 93.310 --- NIH Office of Data Science
Award Information
Anticipated Type of Award: Standard Grant or Continuing Grant
Estimated Number of Awards: 6 to 10
The number of awards will depend on the quality of the received proposals and the budget availability.
$4,000,000 to $5,000,000 in FY24, contingent on availability of funds.
The duration of the awards should be up to 3 years. The award size and duration should be consistent with the project scope.
Collaborative projects from multiple organizations are accepted, according to standard NSF procedures. The total budget (direct and indirect cost) for a collaborative project from multiple organizations must not exceed $1,000,000.
Eligibility Information
Who May Submit Proposals:
Proposals may only be submitted by the following: Institutions of Higher Education (IHEs) - Two- and four-year IHEs (including community colleges) accredited in, and having a campus located in the US, acting on behalf of their faculty members. Special Instructions for International Branch Campuses of US IHEs: If the proposal includes funding to be provided to an international branch campus of a US institution of higher education (including through use of subawards and consultant arrangements), the proposer must explain the benefit(s) to the project of performance at the international branch campus, and justify why the project activities cannot be performed at the US campus.
Who May Serve as PI:
There are no restrictions or limits.
Limit on Number of Proposals per Organization:
Limit on Number of Proposals per PI or co-PI: 1
An individual may serve as PI or co-PI on no more than ONE proposal. Participating in a proposal as other senior/key personnel does not count in this limit. Changes in investigator roles post-submission to meet the eligibility limits will not be allowed. It is the responsibility of the submitters to confirm that the entire team is within the eligibility guidelines.
Proposal Preparation and Submission Instructions
A. proposal preparation instructions.
- Letters of Intent: Not required
- Preliminary Proposal Submission: Not required
Full Proposals:
- Full Proposals submitted via Research.gov: NSF Proposal and Award Policies and Procedures Guide (PAPPG) guidelines apply. The complete text of the PAPPG is available electronically on the NSF website at: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg .
- Full Proposals submitted via Grants.gov: NSF Grants.gov Application Guide: A Guide for the Preparation and Submission of NSF Applications via Grants.gov guidelines apply (Note: The NSF Grants.gov Application Guide is available on the Grants.gov website and on the NSF website at: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=grantsgovguide ).
B. Budgetary Information
Cost Sharing Requirements:
Inclusion of voluntary committed cost sharing is prohibited.
Indirect Cost (F&A) Limitations:
Not Applicable
Other Budgetary Limitations:
C. Due Dates
Proposal review information criteria.
Merit Review Criteria:
National Science Board approved criteria. Additional merit review criteria apply. Please see the full text of this solicitation for further information.
Award Administration Information
Award Conditions:
Standard NSF award conditions apply.
Reporting Requirements:
Standard NSF reporting requirements apply.
I. Introduction
Digital twins offer tremendous potential to revolutionize healthcare delivery by enabling data-informed decision-making under uncertainty. The National Academies of Science, Engineering and Medicine (NASEM) published a report in 2023 entitled “Foundational Research Gaps and Future Directions for Digital Twins.” This report defines a digital twin as “a set of virtual information constructs that mimics the structure, context, and behavior of a natural, engineered, or social system (or system-of-systems), is dynamically updated with data from its physical twin, has a predictive capability, and informs decisions that realize value”. In addition, this report recognizes that in the healthcare sciences such virtual representations of human physiology and pathology have the potential to enable novel pathways for the development and evaluation of new biomedical technologies. Achieving this vision requires a convergent research approach that engages disciplines spanning mathematics, statistics, biomedical engineering, and computational sciences to address the broad range of emerging needs for developing foundational concepts behind digital twins. This anticipated paradigm shift hinges on fundamental scientific and engineering breakthroughs by interdisciplinary teams for developing, validating, and sharing human digital twin frameworks, capable of integrating data from individuals, populations, and devices to catalyze new discoveries and innovation in healthcare systems.
The Foundations for Digital Twins as Catalyzers of Biomedical Technological Innovation (FDT-BioTech) program aims to accelerate innovations in biomedical technologies through development of principled mathematical, statistical, and engineering foundations for digital twins and synthetic human models in healthcare applications. The specific focus of FDT-BioTech is on digital, in silico models that could be used in the evaluation of medical devices and to advance regulatory sciences. The work is also expected to contribute more broadly to the development and implementation of human digital twins. This FDT-BioTech program provides an opportunity to form cohesive collaboration teams including mathematicians, statisticians, biomedical engineers, computer scientists, physicians, and experts from other domains. This collaboration will advance our understanding of foundational mechanisms behind computational representations of physiologic systems; verification, validation, and uncertainty quantification in a biomedical context; transferability, generalizability, and robustness; ethics, security, and privacy; and validation and sharing mechanisms, particularly in terms of regulatory relevance.
II. Program Description
This interagency solicitation is a collaboration between the U.S. National Science Foundation (NSF), National Institutes of Health (NIH) and Food and Drug Administration (FDA). The Foundations for Digital Twins as Catalyzers of Biomedical Technological Innovation (FDT-BioTech) supports innovative and transformative research to advance the mathematical, statistical, and engineering approaches underpinning digital twins in biomedical and healthcare domains ultimately enabling unique tools for innovative evaluation of novel emerging technology that can potentially de-risk therapeutic, biologic, and medical device development and accelerate the introduction of safe and effective medical technologies for improved patient outcomes. Additionally, the emerging concepts of digital twins demonstrate a high potential to revolutionize preclinical and clinical research through reliable in silico investigations, as well as transform clinical practice by providing a framework for patient monitoring, management, and optimal decision making.
Furthermore, collectively, digital twins can be used to develop digital cohorts for accelerating innovation in biomedical technology. For instance, ensembles of digital twin humans could allow for on-demand enrollment of digital cohorts and pipelines for development, tuning, testing, and monitoring in the digital world. Digital study populations can display the variability observed in human populations, including under-represented subgroups and rare conditions, thereby addressing the fundamental problem of algorithmic and other biases which remains inaccessible with current paradigms. Ultimately, leveraging digital models of patients, disease processes, and medical devices is an agile modern approach to technological development and represents a paradigm shift in the development and evaluation of medical products and new technologies. However, achieving this vision hinges on fundamental advances in mathematics, statistics, computational sciences, and engineering.
Note: Projects may leverage virtual representations at multiple scales including a single physiologic or pathologic system, multiple systems, whole-humans, or populations; and be patient-specific or synthetically-derived. Virtual representations may include artificial intelligence (AI), first-principles, mechanistic, or empirical models. The virtual representations should be capable of interfacing with medical technologies and thus may include virtual representations of medical devices.
The rationale for FDT-BioTech is the current knowledge gaps that obstruct the development and use of digital twins in biomedical and other domains. Filling this gap requires novel crosscutting interdisciplinary approaches, where mathematical and statistical foundations play a pivotal role. Some examples of new developments in the foundation of digital twins with strong potential to spur new advances in biotechnology include but are not limited to the following:
- Computational representations of physiological systems at appropriate scales: The virtual representation of real-world physiology is at the core of a human digital twin. The human body is a dynamic and complex system whose behaviors are extremely difficult to model and predict. Tools to adequately build computational representations are lacking. New mathematical, statistical and machine learning methods are needed to enable novel computationally efficient pathways for the integration of prior information into the systematic combination of physical data and their digital counterparts. These strategies may include hybrid modeling approaches – combining mechanistic models, machine learning, and data-driven models – and surrogate models – statistical data-fit models, reduced order models, and simplified models. Furthermore, these models must be capable of assimilating dynamic multi-modal data at different spatial and temporal scales, dynamically updating and adapting, coupling multiphysics systems, and operating with limited data or accounting for extrapolation. These requirements may necessitate new model management workflows including assessing model evolution and drift. Understanding the tradeoffs associated with model and computational choices will increase confidence in predictive insights and digital twin-informed decision making. Moreover, digital twins not only integrate data streams from their physical twin but also data and outcomes from similar physical counterparts. New mathematical, statistical and machine learning methods are needed that could enable novel computationally efficient pathways for integration of prior information into the systematic combination of physical data and their digital counterparts.
- Verification, Validation, and Uncertainty Quantification (VVUQ): Appropriate verification, validation, and uncertainty quantification (VVUQ) are essential to build confidence and trust in digital twins. The complexity of the digital twin ecosystem may require new and advanced strategies and workflows that consider VVUQ as a continuous process. New data collection technologies (quality, source, structure) may affect algorithm or solution verification. Further, the state of the physical twin will evolve over time; and new strategies are needed to ensure the virtual representation accurately reflects these changes (i.e., adaptive model validation). The current lack of evidence of digital twin predictive capabilities adversely impacts the use of digital twins in the healthcare domain. There is a critical need for understanding the confidence interval of digital twin outputs while accounting for various types of uncertainties including modeling uncertainties, measurement and data uncertainties, and process uncertainties. One benefit of digital twins is the ability to test what-if scenarios, such as the performance of a therapeutic, biologic, or diagnostic device. However, to harness this potential, the outputs from the digital twin should be representative of the physical twin’s response (i.e. commutable) even when based on unseen data or extrapolation. New approaches, including but not limited to tools for causal inference, covariate adjustment, extreme value analysis, and neural solvers of partial differential equations, are needed for assessment of the digital twin utility in a broad range of settings.
- Transferability, Generalizability and Robustness : Most digital twins are designed with a particular purpose in mind. To leverage these digital twins for new purposes or scenarios (i.e. testing novel medical technologies), new techniques and tools are required to quantify and improve the transferability of digital twin predictions. There is also a need for techniques to advance the generalizability of evaluation findings on synthetic data from digital twin models to findings on patient data, including performance on various population subgroups. Another fundamental question is associated with the analysis of the robustness of the digital twin models, so that the medical devices designed and evaluated using digital twins are ensured to exhibit the expected standards of safety and effectiveness.
- Ethics, Security, and Privacy : Ethics, security, and privacy are critical to the success of digital twin ecosystems; and include fidelity and reliability of the models, security and access to data, recognition that data and models built on that data may be biased, and ethical use of the data and model outputs. The current limited understanding of the sources and types of biases has led to considerable concern in the community that synthetic human models, including digital twins, may inadvertently propagate or even further exacerbate current inequalities in healthcare delivery. For example, bias may be introduced in data measurement technologies, data labeling, data sources (i.e. is the data representative of the population, have rare conditions been included, small data sets); as well as models, algorithms, and decision-making processes based on this data. Understanding, measuring, and minimizing potential latent biases require novel or advanced methods of statistical inference as current approaches are underdeveloped. Further, strategies to ensure protection of privacy of individual’s data used to develop the DT (at various scale), and equitable impacts and distribution of resources within the context of digital twins are needed. Development of such foundational approaches have potential to accelerate the widespread adoption of digital twins not only in biomedical sciences but also numerous application domains.
- Validation and Sharing Mechanisms : There is a critical need to design computational infrastructure, platforms, and best practices for in silico databanks for medical technology evaluation with pre-defined data sequestration provisions. The lack of methods and platforms with broad involvement of the interdisciplinary scientific community substantially impacts the development, validation, and adoption of digital twins in biomedicine. Furthermore, innovative tools are needed for management, maintenance, service, test data reuse, and auditing of banks of digital twins under privacy- and integrity-preserving federated settings. This in turn will allow for a synergistic acceleration of innovation in a wide range of medical technology areas. Finally, the widespread adoption of digital twins and in silico models for human health will only be realized by more collaborative solutions to sharing and validation of models with established protocols between different digital twin sources as opposed to the status of a disconnected, site-specific collection of digital twin data and human in silico models.
The above list of themes provides examples for possible research initiatives that may be supported by the FDT-BioTech solicitation. Proposals with complementary aims, not listed here, will also be considered. Furthermore, these research themes are clearly not mutually exclusive, and a given project may address multiple themes.
Ethical, Legal, and Social Implications (ELSI): It is essential to recognize ethical, legal, and social implications (ELSI) during the development of human digital twins and synthetic humans. A digital twin ecosystem that does not include ESLI at the start will build inequity into core design and implementation principles perpetuating disparities in health, infrastructure, and resources. All proposals must identify potential ELS implications of the proposed work and outline ways to mitigate negative implications.
This program encourages teams to consider the generalizability of their approaches to other systems, populations, or non-biomedical applications.
In addition to the examples described in this solicitation, the program welcomes submissions of proposals that contain outcomes (methods and models) with a clear dissemination plan, made available as practical, open-source tools that industry can utilize in support of the development of new biomedical technologies. Such tools should be of production quality, shared with the research community, and facilitate interoperability with other tools and data infrastructure. Proposals targeting such tools should include project personnel with cyberinfrastructure development expertise. Furthermore, these tools can include innovative science-based approaches including methodologies and datasets and are meant to support innovators in early stages of development as they prepare toward securing premarket authorization by the FDA. Examples of regulatory science tools published by the Office of Science and Engineering Laboratories, Center for Devices and Radiological Health (OSEL/CDRH/FDA) are available in FDA’s catalog of regulatory science tools ( Catalog of Regulatory Science Tools to Help Assess New Medical Devices | FDA ). Furthermore, FDA will offer opportunities to the FDT-BioTech PIs to discuss and submit their software code implementing the developed methods and algorithms and receive feedback on its relevance to current and emerging regulatory science challenges within the precompetitive space.
This program welcomes the submission of proposals that include the participation of the full spectrum of diverse talent in STEM, e.g., as PI, co-PI, senior/key personnel, postdoctoral scholars, graduate or undergraduate students or trainees. This includes historically under-represented or underserved populations. It also includes diverse institutions including Minority-Serving Institutions (MSIs), Primarily Undergraduate Institutions (PUIs), and two-year colleges, as well as major research institutions. Proposals from EPSCoR (Established Program to Stimulate Competitive Research) jurisdictions are especially encouraged.
Successful projects are anticipated to be collaborative in nature and have at least two senior/key personnels, with participation from both the mathematical sciences and at least one of the domain knowledge disciplines such as the biomedical sciences or computer science with cyberinfrastructure development expertise. In particular, interdisciplinary teams with PI and co-PI from the mathematical sciences, biomedical sciences and computer science with cyberinfrastructure development expertise are encouraged. These requirements will help to ensure that the proposals are truly integrative.
III. Award Information
Anticipated Funding Amount: $4,000,000 to $5,000,000
Estimated program budget, number of awards and average award size/duration are subject to the availability of funds.
IV. Eligibility Information
Additional Eligibility Info:
A minimum of two collaborating Senior/Key Personnel, with participation from both the mathematical sciences and at least one of the domain knowledge disciplines such as the biomedical sciences or computer science with cyberinfrastructure development expertise is required. Interdisciplinary teams with PI and co-PI from the mathematical sciences, the biomedical sciences, and computer science with cyberinfrastructure development expertise are encouraged.
V. Proposal Preparation And Submission Instructions
Full Proposal Preparation Instructions : Proposers may opt to submit proposals in response to this Program Solicitation via Research.gov or Grants.gov.
- Full Proposals submitted via Research.gov: Proposals submitted in response to this program solicitation should be prepared and submitted in accordance with the general guidelines contained in the NSF Proposal and Award Policies and Procedures Guide (PAPPG). The complete text of the PAPPG is available electronically on the NSF website at: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg . Paper copies of the PAPPG may be obtained from the NSF Publications Clearinghouse, telephone (703) 292-8134 or by e-mail from [email protected] . The Prepare New Proposal setup will prompt you for the program solicitation number.
- Full proposals submitted via Grants.gov: Proposals submitted in response to this program solicitation via Grants.gov should be prepared and submitted in accordance with the NSF Grants.gov Application Guide: A Guide for the Preparation and Submission of NSF Applications via Grants.gov . The complete text of the NSF Grants.gov Application Guide is available on the Grants.gov website and on the NSF website at: ( https://www.nsf.gov/publications/pub_summ.jsp?ods_key=grantsgovguide ). To obtain copies of the Application Guide and Application Forms Package, click on the Apply tab on the Grants.gov site, then click on the Apply Step 1: Download a Grant Application Package and Application Instructions link and enter the funding opportunity number, (the program solicitation number without the NSF prefix) and press the Download Package button. Paper copies of the Grants.gov Application Guide also may be obtained from the NSF Publications Clearinghouse, telephone (703) 292-8134 or by e-mail from [email protected] .
In determining which method to utilize in the electronic preparation and submission of the proposal, please note the following:
Collaborative Proposals. All collaborative proposals submitted as separate submissions from multiple organizations must be submitted via Research.gov. PAPPG Chapter II.E.3 provides additional information on collaborative proposals.
See PAPPG Chapter II.D.2 for guidance on the required sections of a full research proposal submitted to NSF. Please note that the proposal preparation instructions provided in this program solicitation may deviate from the PAPPG instructions.
The following instructions supplement or deviate from the PAPPG:
Proposal Title : To facilitate timely processing, proposal titles must begin with FDT-BioTech, followed by a colon and the title of the project (i.e. FDT-BioTech: Title). The title of collaborative proposals submitted as separate submissions from multiple organizations should begin with the designation "Collaborative Research: FDT-BioTech:" All proposals in a collaborative project should have the same title. Please note that if submitting via Research.gov, the system will automatically insert the prepended title “Collaborative Research” when the collaborative set of proposals is created.
Project Description : In addition to the requirements specified in the PAPPG, the Project Description should clearly:
- Demonstrate the potential benefits of the proposed work for regulatory sciences.
- Include a separate section with a heading Ethics, Legal, and Social Implications (ELSI) that clearly identifies potential Ethics, Legal, and Social Implications (ELSI) in the proposed work and consider ways to mitigate negative implications.
- Explain how the proposed research effectively integrates diverse fields (e.g. mathematics, statistics, computational sciences, biomedical sciences, computer science, cyberinfrastructure development and engineering) to advance the foundation of digital twins.
- Address how the multidisciplinary group of researchers is appropriate to the project, how the team members provide distinct, complementary expertise to the project, and why all fields of expertise are needed to complete the proposed work represented on the team.
Cost Sharing:
D. Research.gov/Grants.gov Requirements
For Proposals Submitted Via Research.gov:
To prepare and submit a proposal via Research.gov, see detailed technical instructions available at: https://www.research.gov/research-portal/appmanager/base/desktop?_nfpb=true&_pageLabel=research_node_display&_nodePath=/researchGov/Service/Desktop/ProposalPreparationandSubmission.html . For Research.gov user support, call the Research.gov Help Desk at 1-800-381-1532 or e-mail [email protected] . The Research.gov Help Desk answers general technical questions related to the use of the Research.gov system. Specific questions related to this program solicitation should be referred to the NSF program staff contact(s) listed in Section VIII of this funding opportunity.
For Proposals Submitted Via Grants.gov:
Before using Grants.gov for the first time, each organization must register to create an institutional profile. Once registered, the applicant's organization can then apply for any federal grant on the Grants.gov website. Comprehensive information about using Grants.gov is available on the Grants.gov Applicant Resources webpage: https://www.grants.gov/web/grants/applicants.html . In addition, the NSF Grants.gov Application Guide (see link in Section V.A) provides instructions regarding the technical preparation of proposals via Grants.gov. For Grants.gov user support, contact the Grants.gov Contact Center at 1-800-518-4726 or by email: [email protected] . The Grants.gov Contact Center answers general technical questions related to the use of Grants.gov. Specific questions related to this program solicitation should be referred to the NSF program staff contact(s) listed in Section VIII of this solicitation.
Submitting the Proposal: Once all documents have been completed, the Authorized Organizational Representative (AOR) must submit the application to Grants.gov and verify the desired funding opportunity and agency to which the application is submitted. The AOR must then sign and submit the application to Grants.gov. The completed application will be transferred to Research.gov for further processing.
The NSF Grants.gov Proposal Processing in Research.gov informational page provides submission guidance to applicants and links to helpful resources including the NSF Grants.gov Application Guide , Grants.gov Proposal Processing in Research.gov how-to guide , and Grants.gov Submitted Proposals Frequently Asked Questions . Grants.gov proposals must pass all NSF pre-check and post-check validations in order to be accepted by Research.gov at NSF.
When submitting via Grants.gov, NSF strongly recommends applicants initiate proposal submission at least five business days in advance of a deadline to allow adequate time to address NSF compliance errors and resubmissions by 5:00 p.m. submitting organization's local time on the deadline. Please note that some errors cannot be corrected in Grants.gov. Once a proposal passes pre-checks but fails any post-check, an applicant can only correct and submit the in-progress proposal in Research.gov.
Proposers that submitted via Research.gov may use Research.gov to verify the status of their submission to NSF. For proposers that submitted via Grants.gov, until an application has been received and validated by NSF, the Authorized Organizational Representative may check the status of an application on Grants.gov. After proposers have received an e-mail notification from NSF, Research.gov should be used to check the status of an application.
VI. NSF Proposal Processing And Review Procedures
Proposals received by NSF are assigned to the appropriate NSF program for acknowledgement and, if they meet NSF requirements, for review. All proposals are carefully reviewed by a scientist, engineer, or educator serving as an NSF Program Officer, and usually by three to ten other persons outside NSF either as ad hoc reviewers, panelists, or both, who are experts in the particular fields represented by the proposal. These reviewers are selected by Program Officers charged with oversight of the review process. Proposers are invited to suggest names of persons they believe are especially well qualified to review the proposal and/or persons they would prefer not review the proposal. These suggestions may serve as one source in the reviewer selection process at the Program Officer's discretion. Submission of such names, however, is optional. Care is taken to ensure that reviewers have no conflicts of interest with the proposal. In addition, Program Officers may obtain comments from site visits before recommending final action on proposals. Senior NSF staff further review recommendations for awards. A flowchart that depicts the entire NSF proposal and award process (and associated timeline) is included in PAPPG Exhibit III-1.
A comprehensive description of the Foundation's merit review process is available on the NSF website at: https://www.nsf.gov/bfa/dias/policy/merit_review/ .
Proposers should also be aware of core strategies that are essential to the fulfillment of NSF's mission, as articulated in Leading the World in Discovery and Innovation, STEM Talent Development and the Delivery of Benefits from Research - NSF Strategic Plan for Fiscal Years (FY) 2022 - 2026 . These strategies are integrated in the program planning and implementation process, of which proposal review is one part. NSF's mission is particularly well-implemented through the integration of research and education and broadening participation in NSF programs, projects, and activities.
One of the strategic objectives in support of NSF's mission is to foster integration of research and education through the programs, projects, and activities it supports at academic and research institutions. These institutions must recruit, train, and prepare a diverse STEM workforce to advance the frontiers of science and participate in the U.S. technology-based economy. NSF's contribution to the national innovation ecosystem is to provide cutting-edge research under the guidance of the Nation's most creative scientists and engineers. NSF also supports development of a strong science, technology, engineering, and mathematics (STEM) workforce by investing in building the knowledge that informs improvements in STEM teaching and learning.
NSF's mission calls for the broadening of opportunities and expanding participation of groups, institutions, and geographic regions that are underrepresented in STEM disciplines, which is essential to the health and vitality of science and engineering. NSF is committed to this principle of diversity and deems it central to the programs, projects, and activities it considers and supports.
A. Merit Review Principles and Criteria
The National Science Foundation strives to invest in a robust and diverse portfolio of projects that creates new knowledge and enables breakthroughs in understanding across all areas of science and engineering research and education. To identify which projects to support, NSF relies on a merit review process that incorporates consideration of both the technical aspects of a proposed project and its potential to contribute more broadly to advancing NSF's mission "to promote the progress of science; to advance the national health, prosperity, and welfare; to secure the national defense; and for other purposes." NSF makes every effort to conduct a fair, competitive, transparent merit review process for the selection of projects.
1. Merit Review Principles
These principles are to be given due diligence by PIs and organizations when preparing proposals and managing projects, by reviewers when reading and evaluating proposals, and by NSF program staff when determining whether or not to recommend proposals for funding and while overseeing awards. Given that NSF is the primary federal agency charged with nurturing and supporting excellence in basic research and education, the following three principles apply:
- All NSF projects should be of the highest quality and have the potential to advance, if not transform, the frontiers of knowledge.
- NSF projects, in the aggregate, should contribute more broadly to achieving societal goals. These "Broader Impacts" may be accomplished through the research itself, through activities that are directly related to specific research projects, or through activities that are supported by, but are complementary to, the project. The project activities may be based on previously established and/or innovative methods and approaches, but in either case must be well justified.
- Meaningful assessment and evaluation of NSF funded projects should be based on appropriate metrics, keeping in mind the likely correlation between the effect of broader impacts and the resources provided to implement projects. If the size of the activity is limited, evaluation of that activity in isolation is not likely to be meaningful. Thus, assessing the effectiveness of these activities may best be done at a higher, more aggregated, level than the individual project.
With respect to the third principle, even if assessment of Broader Impacts outcomes for particular projects is done at an aggregated level, PIs are expected to be accountable for carrying out the activities described in the funded project. Thus, individual projects should include clearly stated goals, specific descriptions of the activities that the PI intends to do, and a plan in place to document the outputs of those activities.
These three merit review principles provide the basis for the merit review criteria, as well as a context within which the users of the criteria can better understand their intent.
2. Merit Review Criteria
All NSF proposals are evaluated through use of the two National Science Board approved merit review criteria. In some instances, however, NSF will employ additional criteria as required to highlight the specific objectives of certain programs and activities.
The two merit review criteria are listed below. Both criteria are to be given full consideration during the review and decision-making processes; each criterion is necessary but neither, by itself, is sufficient. Therefore, proposers must fully address both criteria. (PAPPG Chapter II.D.2.d(i). contains additional information for use by proposers in development of the Project Description section of the proposal). Reviewers are strongly encouraged to review the criteria, including PAPPG Chapter II.D.2.d(i), prior to the review of a proposal.
When evaluating NSF proposals, reviewers will be asked to consider what the proposers want to do, why they want to do it, how they plan to do it, how they will know if they succeed, and what benefits could accrue if the project is successful. These issues apply both to the technical aspects of the proposal and the way in which the project may make broader contributions. To that end, reviewers will be asked to evaluate all proposals against two criteria:
- Intellectual Merit: The Intellectual Merit criterion encompasses the potential to advance knowledge; and
- Broader Impacts: The Broader Impacts criterion encompasses the potential to benefit society and contribute to the achievement of specific, desired societal outcomes.
The following elements should be considered in the review for both criteria:
- Advance knowledge and understanding within its own field or across different fields (Intellectual Merit); and
- Benefit society or advance desired societal outcomes (Broader Impacts)?
- To what extent do the proposed activities suggest and explore creative, original, or potentially transformative concepts?
- Is the plan for carrying out the proposed activities well-reasoned, well-organized, and based on a sound rationale? Does the plan incorporate a mechanism to assess success?
- How well qualified is the individual, team, or organization to conduct the proposed activities?
- Are there adequate resources available to the PI (either at the home organization or through collaborations) to carry out the proposed activities?
Broader impacts may be accomplished through the research itself, through the activities that are directly related to specific research projects, or through activities that are supported by, but are complementary to, the project. NSF values the advancement of scientific knowledge and activities that contribute to achievement of societally relevant outcomes. Such outcomes include, but are not limited to: full participation of women, persons with disabilities, and other underrepresented groups in science, technology, engineering, and mathematics (STEM); improved STEM education and educator development at any level; increased public scientific literacy and public engagement with science and technology; improved well-being of individuals in society; development of a diverse, globally competitive STEM workforce; increased partnerships between academia, industry, and others; improved national security; increased economic competitiveness of the United States; and enhanced infrastructure for research and education.
Proposers are reminded that reviewers will also be asked to review the Data Management and Sharing Plan and the Mentoring Plan, as appropriate.
Additional Solicitation Specific Review Criteria
Regulatory science tools component for medical device evaluation :
The work to be funded by this solicitation must demonstrate the potential benefits for regulatory sciences. Proposals shall include a detailed section of how the proposed computational methods and tools developed under the awards will impact the early de-risking of new technology and contribute to facilitating regulatory evaluations including, for example, frequent interaction with OSEL/CDRH/FDA during the post-award period.
B. Review and Selection Process
Proposals submitted in response to this program solicitation will be reviewed by Ad hoc Review and/or Panel Review.
NSF will coordinate and manage the review of proposals jointly with NIH and FDA. The representatives from NIH and FDA may serve as panel observers. Relevant information about proposals and reviews of proposals will be shared with NIH and FDA as appropriate.
Reviewers will be asked to evaluate proposals using two National Science Board approved merit review criteria and, if applicable, additional program specific criteria. A summary rating and accompanying narrative will generally be completed and submitted by each reviewer and/or panel. The Program Officer assigned to manage the proposal's review will consider the advice of reviewers and will formulate a recommendation.
After scientific, technical and programmatic review and consideration of appropriate factors, the NSF Program Officer recommends to the cognizant Division Director whether the proposal should be declined or recommended for award. NSF strives to be able to tell proposers whether their proposals have been declined or recommended for funding within six months. Large or particularly complex proposals or proposals from new recipients may require additional review and processing time. The time interval begins on the deadline or target date, or receipt date, whichever is later. The interval ends when the Division Director acts upon the Program Officer's recommendation.
After programmatic approval has been obtained, the proposals recommended for funding will be forwarded to the Division of Grants and Agreements or the Division of Acquisition and Cooperative Support for review of business, financial, and policy implications. After an administrative review has occurred, Grants and Agreements Officers perform the processing and issuance of a grant or other agreement. Proposers are cautioned that only a Grants and Agreements Officer may make commitments, obligations or awards on behalf of NSF or authorize the expenditure of funds. No commitment on the part of NSF should be inferred from technical or budgetary discussions with a NSF Program Officer. A Principal Investigator or organization that makes financial or personnel commitments in the absence of a grant or cooperative agreement signed by the NSF Grants and Agreements Officer does so at their own risk.
Once an award or declination decision has been made, Principal Investigators are provided feedback about their proposals. In all cases, reviews are treated as confidential documents. Verbatim copies of reviews, excluding the names of the reviewers or any reviewer-identifying information, are sent to the Principal Investigator/Project Director by the Program Officer. In addition, the proposer will receive an explanation of the decision to award or decline funding.
VII. Award Administration Information
A. notification of the award.
Notification of the award is made to the submitting organization by an NSF Grants and Agreements Officer. Organizations whose proposals are declined will be advised as promptly as possible by the cognizant NSF Program administering the program. Verbatim copies of reviews, not including the identity of the reviewer, will be provided automatically to the Principal Investigator. (See Section VI.B. for additional information on the review process.)
B. Award Conditions
An NSF award consists of: (1) the award notice, which includes any special provisions applicable to the award and any numbered amendments thereto; (2) the budget, which indicates the amounts, by categories of expense, on which NSF has based its support (or otherwise communicates any specific approvals or disapprovals of proposed expenditures); (3) the proposal referenced in the award notice; (4) the applicable award conditions, such as Grant General Conditions (GC-1)*; or Research Terms and Conditions* and (5) any announcement or other NSF issuance that may be incorporated by reference in the award notice. Cooperative agreements also are administered in accordance with NSF Cooperative Agreement Financial and Administrative Terms and Conditions (CA-FATC) and the applicable Programmatic Terms and Conditions. NSF awards are electronically signed by an NSF Grants and Agreements Officer and transmitted electronically to the organization via e-mail.
*These documents may be accessed electronically on NSF's Website at https://www.nsf.gov/awards/managing/award_conditions.jsp?org=NSF . Paper copies may be obtained from the NSF Publications Clearinghouse, telephone (703) 292-8134 or by e-mail from [email protected] .
More comprehensive information on NSF Award Conditions and other important information on the administration of NSF awards is contained in the NSF Proposal & Award Policies & Procedures Guide (PAPPG) Chapter VII, available electronically on the NSF Website at https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg .
Administrative and National Policy Requirements
Build America, Buy America
As expressed in Executive Order 14005, Ensuring the Future is Made in All of America by All of America’s Workers (86 FR 7475), it is the policy of the executive branch to use terms and conditions of Federal financial assistance awards to maximize, consistent with law, the use of goods, products, and materials produced in, and services offered in, the United States.
Consistent with the requirements of the Build America, Buy America Act (Pub. L. 117-58, Division G, Title IX, Subtitle A, November 15, 2021), no funding made available through this funding opportunity may be obligated for an award unless all iron, steel, manufactured products, and construction materials used in the project are produced in the United States. For additional information, visit NSF’s Build America, Buy America webpage.
C. Reporting Requirements
For all multi-year grants (including both standard and continuing grants), the Principal Investigator must submit an annual project report to the cognizant Program Officer no later than 90 days prior to the end of the current budget period. (Some programs or awards require submission of more frequent project reports). No later than 120 days following expiration of a grant, the PI also is required to submit a final annual project report, and a project outcomes report for the general public.
Failure to provide the required annual or final annual project reports, or the project outcomes report, will delay NSF review and processing of any future funding increments as well as any pending proposals for all identified PIs and co-PIs on a given award. PIs should examine the formats of the required reports in advance to assure availability of required data.
PIs are required to use NSF's electronic project-reporting system, available through Research.gov, for preparation and submission of annual and final annual project reports. Such reports provide information on accomplishments, project participants (individual and organizational), publications, and other specific products and impacts of the project. Submission of the report via Research.gov constitutes certification by the PI that the contents of the report are accurate and complete. The project outcomes report also must be prepared and submitted using Research.gov. This report serves as a brief summary, prepared specifically for the public, of the nature and outcomes of the project. This report will be posted on the NSF website exactly as it is submitted by the PI.
More comprehensive information on NSF Reporting Requirements and other important information on the administration of NSF awards is contained in the NSF Proposal & Award Policies & Procedures Guide (PAPPG) Chapter VII, available electronically on the NSF Website at https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg .
VIII. Agency Contacts
Please note that the program contact information is current at the time of publishing. See program website for any updates to the points of contact.
General inquiries regarding this program should be made to:
For questions related to the use of NSF systems contact:
- NSF Help Desk: 1-800-381-1532
- Research.gov Help Desk e-mail: [email protected]
For questions relating to Grants.gov contact:
Grants.gov Contact Center: If the Authorized Organizational Representatives (AOR) has not received a confirmation message from Grants.gov within 48 hours of submission of application, please contact via telephone: 1-800-518-4726; e-mail: [email protected] .
IX. Other Information
The NSF website provides the most comprehensive source of information on NSF Directorates (including contact information), programs and funding opportunities. Use of this website by potential proposers is strongly encouraged. In addition, "NSF Update" is an information-delivery system designed to keep potential proposers and other interested parties apprised of new NSF funding opportunities and publications, important changes in proposal and award policies and procedures, and upcoming NSF Grants Conferences . Subscribers are informed through e-mail or the user's Web browser each time new publications are issued that match their identified interests. "NSF Update" also is available on NSF's website .
Grants.gov provides an additional electronic capability to search for Federal government-wide grant opportunities. NSF funding opportunities may be accessed via this mechanism. Further information on Grants.gov may be obtained at https://www.grants.gov .
About The National Science Foundation
The National Science Foundation (NSF) is an independent Federal agency created by the National Science Foundation Act of 1950, as amended (42 USC 1861-75). The Act states the purpose of the NSF is "to promote the progress of science; [and] to advance the national health, prosperity, and welfare by supporting research and education in all fields of science and engineering."
NSF funds research and education in most fields of science and engineering. It does this through grants and cooperative agreements to more than 2,000 colleges, universities, K-12 school systems, businesses, informal science organizations and other research organizations throughout the US. The Foundation accounts for about one-fourth of Federal support to academic institutions for basic research.
NSF receives approximately 55,000 proposals each year for research, education and training projects, of which approximately 11,000 are funded. In addition, the Foundation receives several thousand applications for graduate and postdoctoral fellowships. The agency operates no laboratories itself but does support National Research Centers, user facilities, certain oceanographic vessels and Arctic and Antarctic research stations. The Foundation also supports cooperative research between universities and industry, US participation in international scientific and engineering efforts, and educational activities at every academic level.
Facilitation Awards for Scientists and Engineers with Disabilities (FASED) provide funding for special assistance or equipment to enable persons with disabilities to work on NSF-supported projects. See the NSF Proposal & Award Policies & Procedures Guide Chapter II.F.7 for instructions regarding preparation of these types of proposals.
The National Science Foundation has Telephonic Device for the Deaf (TDD) and Federal Information Relay Service (FIRS) capabilities that enable individuals with hearing impairments to communicate with the Foundation about NSF programs, employment or general information. TDD may be accessed at (703) 292-5090 and (800) 281-8749, FIRS at (800) 877-8339.
The National Science Foundation Information Center may be reached at (703) 292-5111.
Privacy Act And Public Burden Statements
The information requested on proposal forms and project reports is solicited under the authority of the National Science Foundation Act of 1950, as amended. The information on proposal forms will be used in connection with the selection of qualified proposals; and project reports submitted by proposers will be used for program evaluation and reporting within the Executive Branch and to Congress. The information requested may be disclosed to qualified reviewers and staff assistants as part of the proposal review process; to proposer institutions/grantees to provide or obtain data regarding the proposal review process, award decisions, or the administration of awards; to government contractors, experts, volunteers and researchers and educators as necessary to complete assigned work; to other government agencies or other entities needing information regarding proposers or nominees as part of a joint application review process, or in order to coordinate programs or policy; and to another Federal agency, court, or party in a court or Federal administrative proceeding if the government is a party. Information about Principal Investigators may be added to the Reviewer file and used to select potential candidates to serve as peer reviewers or advisory committee members. See System of Record Notices , NSF-50 , "Principal Investigator/Proposal File and Associated Records," and NSF-51 , "Reviewer/Proposal File and Associated Records.” Submission of the information is voluntary. Failure to provide full and complete information, however, may reduce the possibility of receiving an award.
An agency may not conduct or sponsor, and a person is not required to respond to, an information collection unless it displays a valid Office of Management and Budget (OMB) control number. The OMB control number for this collection is 3145-0058. Public reporting burden for this collection of information is estimated to average 120 hours per response, including the time for reviewing instructions. Send comments regarding the burden estimate and any other aspect of this collection of information, including suggestions for reducing this burden, to:
Suzanne H. Plimpton Reports Clearance Officer Policy Office, Division of Institution and Award Support Office of Budget, Finance, and Award Management National Science Foundation Alexandria, VA 22314

Maximizing Access to Research Careers (T34)
The goal of the Maximizing Access to Research Careers (MARC) program is to promote broad participation in the biomedical research workforce by strengthening research training environments and expanding the pool of well-trained students who:
- Complete their baccalaureate degree, and
- Transition into and complete biomedical, research-focused higher degree programs (such as Ph.D. or M.D./Ph.D.).
Specifically, this funding announcement provides support to eligible, domestic organizations to develop and implement effective, evidence-informed approaches to biomedical undergraduate training and mentoring to support the development of a biomedical research workforce that will benefit from the full range of perspectives, experiences and backgrounds needed to advance discovery. NIGMS expects organizations to engage in outreach and recruitment activities to encourage individuals from underrepresented groups to participate in the program. The proposed research training programs will incorporate didactic, research, and career development elements to prepare trainees for careers that will have a significant impact on the health-related research needs of the nation.
This program is limited to applications from baccalaureate degree-granting research-intensive organizations (that is, those with NIH Research Project Grant (RPG) funding averaging greater than or equal to $7.5 million in total costs (direct and F&A/indirect) per year over the last three fiscal years (FY)).
This Notice of Funding Opportunity (NOFO) does not allow appointed Trainees to lead an independent clinical trial, but does allow them to obtain research experience in a clinical trial led by a mentor or co-mentor.
NIGMS will accept only one application per eligible organization.
- Duke Internal: TBA
- Application Due Date(s): May 29, 2024; May 28, 2025; May 27, 2026
PAR-24-138 Expiration Date May 28, 2026
If the internal deadline has passed and you're interested in applying for this opportunity, please email [email protected] .
To promote broad participation among research organizations with cross-disciplinary training programs, NIGMS recognizes separate organizational eligibility tracks:
- Research-intensive, that is, those with an average of NIH research project grant (RPG) funding greater than or equal to $7.5 million total costs per year over the past 3 fiscal years, and
- Research-active, that is, those with an average of RPG funding less than $7.5 million total costs per year over the past 3 fiscal years (RPG data are available through NIH RePORTER ).
For example, applications submitted in FY2024 will use data from FY 2021, FY 2022 and FY 2023.
Organizational eligibility for this NOFO is limited to research-intensive , baccalaureate-degree granting organizations as defined above. Research-active organizations are not eligible to apply for or receive MARC grants but may be eligible to apply for U-RISE grants (see Part 1, Companion Funding Opportunity).
The application must be submitted by the eligible organization with a unique entity identifier (UEI) and a unique NIH eRA Institutional Profile File (IPF) number. For organizations with multiple campuses (e.g., main, satellite, etc.), eligibility can be considered for an individual campus only if a UEI and a unique NIH eRA IPF number are established for the individual campus . For organizations that use one UEI or NIH IPF number for multiple campuses, eligibility is determined for the campuses together.
Organizations funded through this NOFO are eligible for other NIGMS Training programs provided the other eligibility criteria are met. To avoid any potential for overlap, applicants are strongly encouraged to contact the program officers of each program before applying.
The sponsoring organization must assure eligibility and support for the proposed program. Appropriate information about eligibility and organizational commitment to the program should be detailed according to the Letters of Support attachment instructions in Section IV.
As described in the instructions for the Training Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) in Section IV.2 below, NIGMS encourages multiple PDs/PIs, particularly when each brings a unique perspective and skill set that will enhance training. Note the following:
- To provide research training leadership for the program, at least one of the Training PDs/PIs should have a record of using rigorous and transparent methods in experimental design, data collection, analysis, and reporting in a biomedical research field applicable to the program.
- Additional PDs/PIs may be included to strengthen the expertise of the PD/PI team. Examples include individuals such as program directors who regularly interact with students, or individuals with expertise in education, relevant social sciences, program evaluation, mentoring, efforts to promote broad participation in the biomedical sciences, or university administration.
Owing to the sponsor's restriction on the number of applications that may be submitted from Duke, anyone wishing to pursue nomination should submit the following materials as one PDF.
- NIH Biosketch for PI/Applicant
- 1-Page Specific Aims: Propose a feasible academic and research-focused undergraduate training program that will enhance diversity in the biomedical workforce within the mission of NIGMS.
Please submit internal materials through My Research Proposal. (Code: ILN)
Instructions for creating an account (if needed) and submitting your materials: https://ctsi.duke.edu/about-myresearchproposal
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Nepal J Epidemiol
- v.12(4); 2022 Dec
Guidance to applying for health research grants in the UK
Brijesh sathian.
1 Geriatrics and long term care department, Rumailah Hospital, Hamad Medical Corporation, Doha, Qatar
2 Centre for Midwifery, Maternal and Perinatal Health, Bournemouth University, Bournemouth, UK
Edwin van Teijlingen
Indrajit banerjee.
3 Sir Seewoosagur Ramgoolam Medical College, Belle Rive, Mauritius
Russell Kabir
4 School of Allied Health, Anglia Ruskin University, Essex, UK
There is no conflict of interest for any author of this manuscript.
A research proposal is the start and the foundation for many studies, “whether it is to conduct an academic research project or to apply for funding and support for a specific study [ 1 ]. The authors will use their experiences and insights to guide new international researchers who are considering their first research grant application in the UK. The chances of failure in applying for health research funding in the UK should not be underestimated. The typical success rate in the UK in health research varies from one in five [ 2 ] to even lower success rates.
Some funding bodies ask researchers to submit a full proposal in reply to their calls. However, many funders have a two-staged grant application system. First, anybody can submit a general short outline proposal with not too much detail, and once this initial idea has been accepted by the funding panel as having potential, in the second round selected people are invited to submit an in-depth full proposal.
Hundreds of research organizations, funding organizations, and supporting institutions make up the UK research system. These organizations differ in size, emphasis, and internal structure. Whereas this variety is a unique strength for quality of research , but it increases the volume and complexity of research administration, which adds to the competition to get more grants.
Researchers should understand the functions of various organizations, such as funders, authorities, academic institutions, NHS Trusts, and public and non-profit research organizations. An extensive array of research financing options is available to go along with this variety of organizations. Direct government funding, like Quality-Related (QR) funding in Wales, England, and Northern Ireland, the Research Postgraduate Grant and Research Excellence Grant in Scotland, and competitive project funding, like “responsive mode” or challenge-led funding, have very different formats and specifications.
According to the Independent Review of Research Bureaucracy - Final Report, just 20% of research funding applications are generally successful [ 2 ]. As a result, single stage processes that demand applicants to supply all the information up front result in the majority of applicants using this information unproductively and wasting it. Two step application procedures may result in system improvements, but they may also require additional time or resources from funders. UKRI and other organizations are now experimenting similar techniques.
They advised the funders to experiment with application procedures to lighten the load on applicants, such as two-stage procedures where the amount of information needed rises in proportion to the possibility of receiving funding. Funders should cooperate to enhance consistency across their application procedures, including in the language they use and the questions they pose when necessary. In the beginning, UKRI ought to assist Research Councils in this. Funders should consider what modifications to evaluation procedures may be required to account for changes to application models.
This should contain the data required for national security assessments as well as creative strategies, such testing out novel models like randomly allocating funds or using peer reviewer triage to reduce the number of applications requiring complete peer review. Funders need to make sure that the application procedures uphold their pledges to diversity, inclusion, and equality. In most cases, funders should waive the letter of support requirement from applications.
Following the COVID-19 pandemic, the UK clinical research delivery system still faces hurdles in the delivery of research. The pandemic’s continued effects on the backlog in seeing patients, manpower constraints, and the requirement to finish specific COVID-19 research have caused delays in the completion of several studies. As a result, fewer studies are now able to successfully recruit participants and finish on time.
Workload, staffing, and the need to clear the elective backlog due to the combined effects of chronic underfunding and Brexit continue to put strain on the NHS’s ability to support research delivery. Some studies have difficulty in the present context and have limited likelihood of achieving their study endpoints and objectives due to a lack of resources and capacity. For others, the lack of resources and capability makes it difficult to execute studies within reasonable timeframes. The research system must prioritize studies that can be completed given the capability and resources at its disposal, while acknowledging that some studies (such as those involving rare disorders) may require less frequent recruitment of participants.
The Department of Health and Social Care (DHSC) launched the Research Reset programme in response to the ongoing challenges in research delivery with the goal of making portfolio delivery attainable within anticipated timelines (time and target) and sustainable within the resource and capability we currently have in the NHS [ 3 ]. By working with funders and sponsors to promote the evaluation of studies that have already finished or that are unlikely to be able to fulfill their endpoints in the present climate, it seeks to free up capacity throughout the research system.
The programme is overseen by the DHSC with input from an advisory group made up of representatives from medical research charities, industry, NHS Research and Development, research delivery workforce representatives across NHS settings, patient and public representatives, universities, the Royal Colleges, the Medical Research Council (MRC), and NHS regions throughout the UK.
According to the present standards, NIHR will continue to add new research to the portfolio throughout the Research Reset programme.
Building on NIHR achievements and the lessons learned in response to COVID-19, the future of clinical research delivery outlines the vision for a clinical research environment that is more patient-centered, pro-innovation, and digitally enabled. It also aims to maximize the UK’s capacity to profit from cutting-edge innovations across all treatments and technologies, all research phases, and all conditions [ 4 ]. Five key themes underpin the NIHR’s vision are (1) clinical research embedded in the NHS, (2) patient-centred research, (3) streamlined, efficient and innovative research, (4) research enabled by data and digital tools, and (5) a sustainable and supported research workforce.
There have been efforts in the UK to bring various government departments and research funders working in international development together in the UK Collaborative on Development Research (UKCDR). Which is why we have listed in it separately from several of its constituents in Table 1 .
Key funding agencies in the UK
* Relevant to low- and middle-income countries
Two case studies
We offer two case studies to illustrate some of the issues that may occur in applying for grants from Uk organisations. Both examples relate to research conducted in Nepal. The first example is a three-year project is UK-funded under the Health Systems Research Initiative. This study ‘the impact of federalisation on Nepal’s health system: a longitudinal analysis’ is a collaboration between researchers at the University of Sheffield, Bournemouth University and the University of Huddersfield in the UK and Manmohan Memorial Institute of Health Science and PHASE Nepal both in Kathmandu [ 5-7 ]. We first applied for funding in 2018, the committee seemed to like the research idea but were worried about the potential slow progress of the federaisation process in Nepal, the lack academic experts in Nepal and the large proportion of the cost related to the UK. In the 2019 we addressed these issues, obviously to the funders’ satisfaction as we were successful in our resubmission.
The second case study centres on an application for a DelPHE (Round 4), British Council award [ 8-9 ]. Our study ‘Partnership on Improving Access to Research Literature for HE Institutions in Nepal’ (PARI Initiative) was a collaboration between Tribhuvan University (TU), the oldest and largest university in Nepal, and the University of Aberdeen and Bournemouth University in the UK. Our initial application for DelPHE funding with Stupa College in Kathmandu, a smallish not-for-profit college, was unsuccessful. In the feedback from the funder we were advised to collaborate with a larger university, preferably a government university to increase the chance that our intervention/training would be incorporated in future training and curricula. Therefore, we submitted a similar application the next year with a new partner in Nepal, namely the Central Department of Population Studies at Tribhuvan University (TU). This is resubmission was then accepted and funded.
Always consider collaborating with someone in the home country of the funding agency, in this case with an academic at a UK university. Both getting through the first round of a two-staged application and being asked to resubmit means you have had a dummy run. Submitting after feedback from experts on a funding board/panel or from bureaucrats working for the funder offers you the opportunity to improve the application. The improvements can be methodologically, or in the detail of some of the application of your prosed methods, of financially, or ethically, etc. In other words, you can fine tune your new submission and home in on the issues the funders find important. MRC provides 37-page document Guidance for Peer Reviewers [ 10 ].
An innovative and competitive grant proposal will have greater odds to get success. Making connections with researchers with similar research interest to collaborate can help increase your changes of getting funding. Looking for funding opportunities in your own field of expertise or that of your collaboratiors can assist to make sure that the application and the funder are a suitable fit. It is crucial to thoroughly review funding agencies’ focus because funding requirements and eligibility requirements might differ greatly between organisations, but also from year to year for the same funder. The funder’s past awards may serve as a useful indicator of the kinds of studies they are likely to support and the details of winning proposals.
Acknowledgement
Limited Submission Opportunity: Brain Research Foundation 2025 Scientific Innovations Award Grant
URL: https://www.thebrf.org/scientific-innovations-award/
OBJECTIVES :
The objective of the program of the Brain Research Foundation is to support projects that may be too innovative and speculative for traditional funding sources but still have a high likelihood of producing important findings. It is expected that investigations supported by these grants will yield high impact findings and result in major grant applications and funding as well as significant publications in high impact journals.
FUNDING INFORMATION :
Each total award is limited to $150,000 (direct costs) for a two-year grant period. Exact dates will be provided by the Brain Research Foundation upon application approval. The first grant payment of $75,000 will be made upon completion of the SIA Acceptance Form. The final payment of $75,000 will be made contingent upon receipt of a Preliminary Progress and Financial Report. Funds must be utilized within the grant period.
ELIGIBILITY RESTRICTIONS :
To be eligible, the nominee must be a full-time associate professor/full professor working in the area of neuroscience and brain function in health and disease. Current major NIH or other peer-reviewed funding is preferred but evidence of such funding in the past three years is essential.
INTERNAL SELECTION PROCESS:
Interested investigators should submit the following materials via InfoReady Review by DATE: 4/29/2024
- Questions outlined in InfoReady Review application;
- A brief statement (up to 2 pages) by the candidate describing their scientific achievements and proposed project including mention of relevant NIH/peer-reviewed funding;
- A brief budget outline for the proposed research ;
- Up-to-date CV or biosketch.
A faculty committee drawn from both campuses will review internal proposals and select nominees. Foundation Relations will work with the nominees to develop and submit the institutional nomination letter and applicant materials by 6/25/2024.
Internal Materials Due: Monday, April 29, 2024 Sponsor Deadline for Letter of Intent: Tuesday, June 25, 2024
Sponsor’s Deadline for Full Application: Tuesday, October 8, 2024
View all posts
Information For...
Research Teams
Effective research collaboration thrives on a diverse set of expertise and skills. Discover resources that provide support for a vast array of research activities.
Learn how OVPR can support your work at Penn and beyond.
Whether the research unfolds in a lab or a library, on campus or overseas, tapping into the resources at Penn can strengthen any research endeavor.
Quick Links
- Research Stories
- Funding Opportunities
- Centers & Institutes
- Contact the Office
Identify the offices and services that drive research forward.
Discover the right resources and tools to enhance your research experience., managing citations.
Information, support, and workshops on citation management tools
Responsible Conduct of Research (RCR) Seminars (Biomedical Postdoctoral Programs – BPP)
- Postdoctoral Affairs ,
- Research Integrity ,
- Responsible Conduct of Research
Nature Masterclass Course – Experiments from Idea to Design
- Learning & Professional Development ,
- Proposal Preparation
Top FAQs for Research Teams
Reach out to us.
SBIR/STTR Sprint Day Writing Workshop
Jump start your proposal for America’s Seed Fund: SBIR/STTR. Choose a session for National Institutes of Health (NIH), National Science Foundation (NSF), or Department of Defense (DOD). Registration is capped because this is an active, sprint style event in which each participant leaves with:
- A template for writing your Specific Aims (NIH) or Technical Objectives (NSF)
- 1:1 meeting with Consultant on your specific proposal
This event is designed for companies preparing a SBIR/STTR proposal within the next 9 months.
Sessions will be scheduled upon registration.
Register: https://forms.monday.com/forms/13ae7eb331592b44c77702f02190da9c?r=use1
For more information about this event or other programming from Discovery to Impact, contact Andrew Worden at [email protected] .

COMMENTS
The PDO has prepared these templates to serve as a helpful starting point for developing your NIH grant application. All documents should be tailored to meet the specific requirements of your grant. We update these documents as needed and the date of last update is in the header of each document. Beginning on January 25, 2023, grant ...
research grant proposal. − Receiving relevant and up-to-date information about new research methods. − Establishing collaborative associations with peers. − Constructive feedback on research proposals and throughout the research process. − Assistance in the development of a long-term research and writing plan.
NIAID Sample Forms, Plans, Letters, Emails, and More. National Cancer Institute (NCI) Behavioral Research Grant Applications (R01, R21, R03) Cancer Epidemiology Grant Applications (R01, R21, R03, R37) Cancer Control and Population Sciences Grant Applications (R01, R21, R37) Healthcare Delivery Research Grant Applications (R01, R03, R21, R50)
Research Grants R01 Sample Applications and Summary Statements. The R01 is the NIH standard independent research project grant. An R01 is meant to give you four or five years of support to complete a project, publish, and reapply before the grant ends. Read more at NIAID's Comparing Popular Research Project Grants: R01, R03, and R21.
Research Proposal Template Biomedical Research: General Page 2 Methods . Human Subjects This section describes your proposed study population and, if the study is prospective, how you will identify and enroll participants and obtain their informed consent. The bulleted list gives examples of elements you may need to describe (the first three items
Conceptualising your research idea. Before writing a research grant proposal/application, consider what the research should achieve in the short, medium, and long term, and how the research goals will serve patients, science and society [9, 10].Practical implications of research, policy impact or positive impact on society and active patient/public involvement are highly valued by many ...
The problem. A research proposal is basically a plan for work required to test a research hypothesis/set of hypotheses. The most important thing to keep in mind is that, when drafting a biomedical research grant proposal, you are required to tailor it to suit a specific audience (i.e., grant application reviewers). In academic research settin...
University of Michigan Medical School. Phone: 734-763-4272. Email: [email protected]. 7313 Medical Science I Building. 1301 Catherine Street. Ann Arbor, MI 48109-5624. North Campus Research Complex (NCRC) Building 520, 3rd Floor. 2800 Plymouth Road.
There might be some disagreements between the surgeons and patients perspectives. 1 The purpose of the background and significance chapter is to justify the study you are proposing. Describe how the result of your study will benefit society. You need to convince the granting agencies that it is worth their money.
Biomedical research, grant funding, grant proposals Introduction Obtaining stable funding is one of the most important aspects of running your laboratory. In order to continue your work in the biomedical/cancer field, you need as many tools as possible to secure funding for salaries, cutting-edge equipment, core facility support, and ...
For example, over the last 2-decades, the National Institute of Health (NIH) grant acceptance rate has dipped from 31% in 2002 (30,068 applications / 9,396 awarded) to 19% in 2022 (58,872 applications / 11,229 awarded). 1 To increase the odds of funding, a compelling storyline invoking grant makers' immediate attention is necessary.
For example, over the last 2-decades, the National Institute of Health (NIH) grant acceptance rate has dipped from 31% in 2002 (30,068 applications / 9,396 awarded) to 19% in 2022 (58,872 applications / 11,229 awarded). 1 To increase the odds of funding, a compelling storyline invoking grant makers' immediate attention is necessary.
Budget plan is a key element of a grant application. It demonstrates the required cost for the proposed project. It is a prediction of expenses and serves a plan for funders on how the organization will operate the project, spend the money in a given set of period and where their money will go.
Well organized site on proposal writing. Includes an overview, inquiry and cover letters, standard components of a proposal, a sample proposal, advice from funders, and more. An excellent section on researching funding opportunities is included also.
Review final proposal checklists prior to submission: the expectation is a two-page, single-spaced research grant proposal (1″ margins, Times New Roman 12 or Arial 11), and proposals that do not meet these formatting expectations will not be considered by the review committee. Your bibliography does not count towards this page limit.
We do not include other SF 424 (R&R) forms or basic information found in full grant applications, such as performance sites, key personnel, or biographical sketches. *Institution at time of grant award. **Notice(s) of Funding Opportunities (NOFOs) at the time of grant award that have since expired.
Funding. The National Cancer Institute (NCI) frequently receives requests for examples of funded grant applications. Several investigators and their organizations agreed to let the Healthcare Delivery Research Program (HDRP) post excerpts of their healthcare delivery research grant applications online. We are grateful to the investigators and ...
Objective To quantify randomness and cost when choosing health and medical research projects for funding. Design Retrospective analysis. Setting Grant review panels of the National Health and Medical Research Council of Australia. Participants Panel members' scores for grant proposals submitted in 2009. Main outcome measures The proportion of grant proposals that were always, sometimes, and ...
Abstract: This is a brief (300-500 words) summary that includes the research question, your rationale for the study, and any applicable hypothesis. You should also include a brief description of your methodology, including procedures, samples, instruments, etc. Introduction: The opening paragraph of your research proposal is, perhaps, the most ...
an example of a grant proposal for the research project in 4th year. Module. Research Project (BS41004) 4 Documents. Students shared 4 documents in this course. University ... Practical Techniques in Biomedical Sciences 100% (2) 14. Group project. Introduction to the Life Sciences: ...
The grants applications for substantial funding are extremely competitive and a professionally written grant proposal is the key to securing them. ... including the Clinical Research Network (CRN) and Biomedical Research Centres. ... depending on the funding organization and size of the grant, an example of applying for NIHR which is the ...
Grants & Funding. The National Institutes of Health is the largest public funder of biomedical research in the world. In fiscal year 2022, NIH invested most of its $45 billion appropriations in research seeking to enhance life, and to reduce illness and disability. NIH-funded research has led to breakthroughs and new treatments helping people ...
Busch Biomedical Grant . 2024 Guidelines . Submit Notice of Intent. Submit Proposal . I. Program Overview . The Busch Biomedical Grant program is designed to enhance basic or fundamental biomedical research at the University and to strengthen the competitive position of faculty members who seek external research funds. The Busch Biomedical Grant
Full Proposal Deadline(s) (due by 5 p.m. submitting organization's local time): June 21, 2024. May 05, 2025. First Monday in May, Annually Thereafter. Important Information And Revision Notes. Any proposal submitted in response to this solicitation should be submitted in accordance with the NSF Proposal & Award Policies & Procedures Guide (PAPPG) that is in effect for the relevant due date ...
Research-intensive, that is, those with an average of NIH research project grant (RPG) funding greater than or equal to $7.5 million total costs per year over the past 3 fiscal years, and Research-active, that is, those with an average of RPG funding less than $7.5 million total costs per year over the past 3 fiscal years (RPG data are ...
A research proposal is the start and the foundation for many studies, "whether it is to conduct an academic research project or to apply for funding and support for a specific study [].The authors will use their experiences and insights to guide new international researchers who are considering their first research grant application in the UK.
A brief statement (up to 2 pages) by the candidate describing their scientific achievements and proposed project including mention of relevant NIH/peer-reviewed funding; A brief budget outline for the proposed research; Up-to-date CV or biosketch. A faculty committee drawn from both campuses will review internal proposals and select nominees.
The research being done at the foreign lab is unrelated to the PI's NIH project. This would not qualify as a foreign component of the NIH research, as the foreign work is not part of the NIH-funded project. However, it is a resource made available to the researcher in support of their research. Therefore, it must be reported as Other Support.
Registration is capped because this is an active, sprint style event in which each participant leaves with: A template for writing your Specific Aims (NIH) or Technical Objectives (NSF) 1:1 meeting with Consultant on your specific proposal. This event is designed for companies preparing a SBIR/STTR proposal within the next 9 months. Sessions ...
In 2023, for example, Oklahoma Governor Kevin Stitt (R) signed House Bill 1695. The law allows existing agreements that grant electric utilities access to land (i.e., easements), or access to attach a power line to a pole, to also be applied to the installation of broadband service, instead of requiring that provider restart the process for the ...