
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


Case Study: Cystic Fibrosis - CER
- Last updated
- Save as PDF
- Page ID 26446
This page is a draft and is under active development.
Part I: A Case of Cystic Fibrosis
Dr. Weyland examined a six month old infant that had been admitted to University Hospital earlier in the day. The baby's parents had brought young Zoey to the emergency room because she had been suffering from a chronic cough. In addition, they said that Zoey sometimes would "wheeze" a lot more than they thought was normal for a child with a cold. Upon arriving at the emergency room, the attending pediatrician noted that salt crystals were present on Zoey's skin and called Dr. Weyland, a pediatric pulmonologist. Dr. Weyland suspects that baby Zoey may be suffering from cystic fibrosis.
CF affects more than 30,000 kids and young adults in the United States. It disrupts the normal function of epithelial cells — cells that make up the sweat glands in the skin and that also line passageways inside the lungs, pancreas, and digestive and reproductive systems.
The inherited CF gene directs the body's epithelial cells to produce a defective form of a protein called CFTR (or cystic fibrosis transmembrane conductance regulator) found in cells that line the lungs, digestive tract, sweat glands, and genitourinary system.
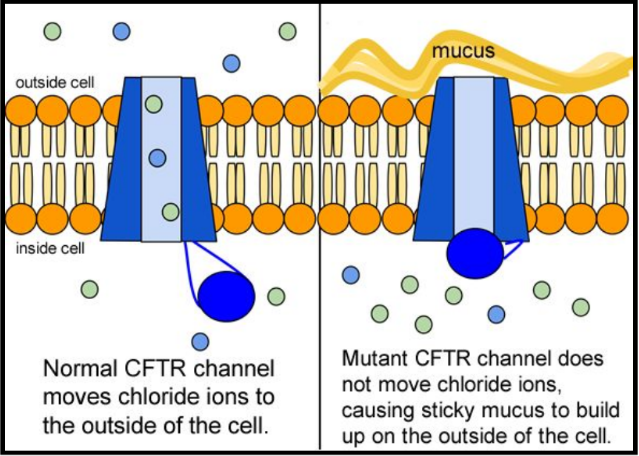
When the CFTR protein is defective, epithelial cells can't regulate the way that chloride ions pass across cell membranes. This disrupts the balance of salt and water needed to maintain a normal thin coating of mucus inside the lungs and other passageways. The mucus becomes thick, sticky, and hard to move, and can result in infections from bacterial colonization.
- "Woe to that child which when kissed on the forehead tastes salty. He is bewitched and soon will die" This is an old saying from the eighteenth century and describes one of the symptoms of CF (salty skin). Why do you think babies in the modern age have a better chance of survival than babies in the 18th century?
- What symptoms lead Dr. Weyland to his initial diagnosis?
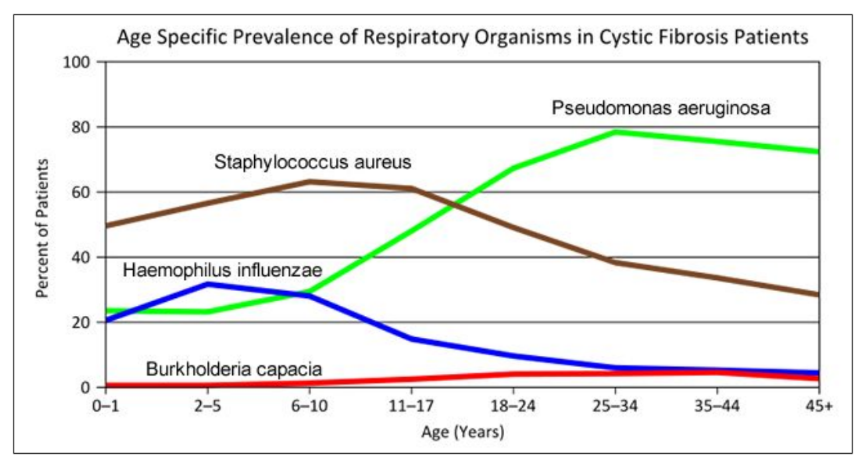
- Consider the graph of infections, which organism stays relatively constant in numbers over a lifetime. What organism is most likely affecting baby Zoey?
- What do you think is the most dangerous time period for a patient with CF? Justify your answer.
Part II: CF is a disorder of the cell membrane.
Imagine a door with key and combination locks on both sides, back and front. Now imagine trying to unlock that door blind-folded. This is the challenge faced by David Gadsby, Ph.D., who for years struggled to understand the highly intricate and unusual cystic fibrosis chloride channel – a cellular doorway for salt ions that is defective in people with cystic fibrosis.
His findings, reported in a series of three recent papers in the Journal of General Physiology, detail the type and order of molecular events required to open and close the gates of the cystic fibrosis chloride channel, or as scientists call it, the cystic fibrosis transmembrane conductance regulator (CFTR).
Ultimately, the research may have medical applications, though ironically not likely for most cystic fibrosis patients. Because two-thirds of cystic fibrosis patients fail to produce the cystic fibrosis channel altogether, a cure for most is expected to result from research focused on replacing the lost channel.
5. Suggest a molecular fix for a mutated CFTR channel. How would you correct it if you had the ability to tinker with it on a molecular level?
6. Why would treatment that targets the CFTR channel not be effective for 2⁄3 of those with cystic fibrosis?
7. Sweat glands cool the body by releasing perspiration (sweat) from the lower layers of the skin onto the surface. Sodium and chloride (salt) help carry water to the skin's surface and are then reabsorbed into the body. Why does a person with cystic fibrosis have salty tasting skin?
Part III: No cell is an island
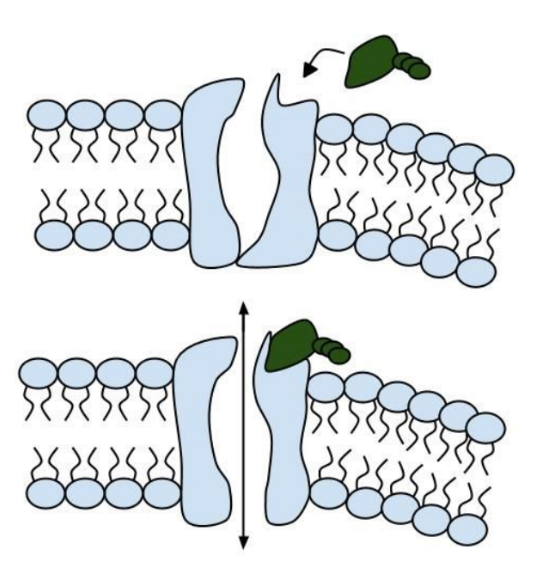
Like people, cells need to communicate and interact with their environment to survive. One way they go about this is through pores in their outer membranes, called ion channels, which provide charged ions, such as chloride or potassium, with their own personalized cellular doorways. But, ion channels are not like open doors; instead, they are more like gateways with high-security locks that are opened and closed to carefully control the passage of their respective ions.
In the case of CFTR, chloride ions travel in and out of the cell through the channel’s guarded pore as a means to control the flow of water in and out of cells. In cystic fibrosis patients, this delicate salt/water balance is disturbed, most prominently in the lungs, resulting in thick coats of mucus that eventually spur life-threatening infections. Shown below are several mutations linked to CFTR:
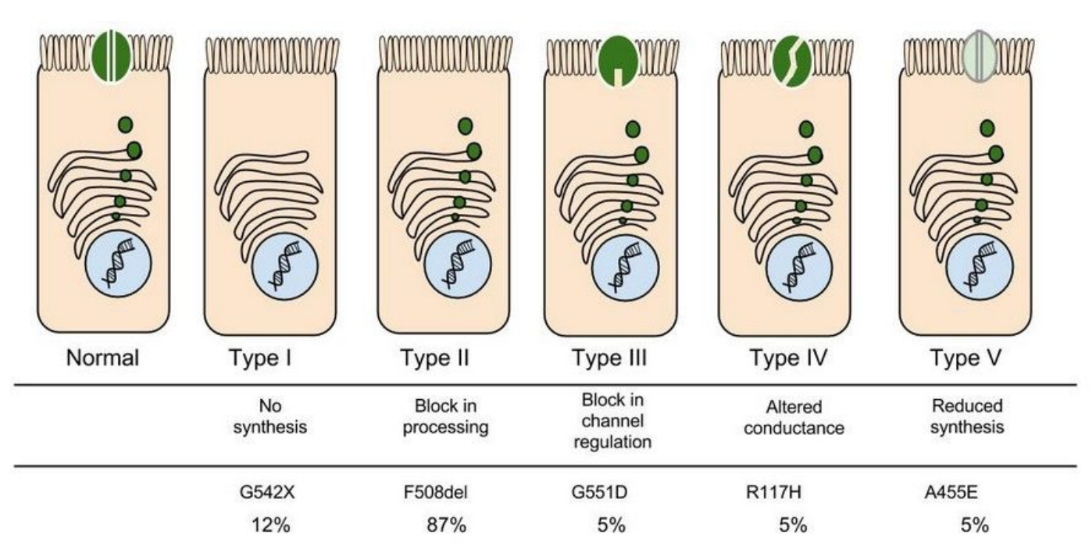
8. Which mutation do you think would be easiest to correct. Justify your answer. 9. Consider what you know about proteins, why does the “folding” of the protein matter?
Part IV: Open sesame
Among the numerous ion channels in cell membranes, there are two principal types: voltage-gated and ligand-gated. Voltage-gated channels are triggered to open and shut their doors by changes in the electric potential difference across the membrane. Ligand-gated channels, in contrast, require a special “key” to unlock their doors, which usually comes in the form of a small molecule.
CFTR is a ligand-gated channel, but it’s an unusual one. Its “key” is ATP, a small molecule that plays a critical role in the storage and release of energy within cells in the body. In addition to binding the ATP, the CFTR channel must snip a phosphate group – one of three “P’s” – off the ATP molecule to function. But when, where and how often this crucial event takes place has remains obscure.
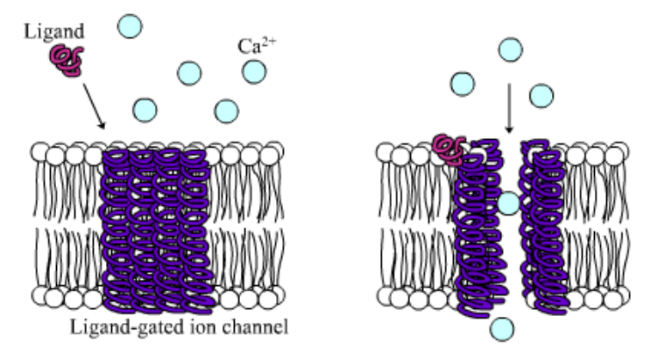
10. Compare the action of the ligand-gated channel to how an enzyme works.
11. Consider the model of the membrane channel, What could go wrong to prevent the channel from opening?
12. Where is ATP generated in the cell? How might ATP production affect the symptoms of cystic fibrosis?
13. Label the image below to show how the ligand-gated channel for CFTR works. Include a summary.
Part V: Can a Drug Treat Zoey’s Condition?
Dr. Weyland confirmed that Zoey does have cystic fibrosis and called the parents in to talk about potential treatments. “Good news, there are two experimental drugs that have shown promise in CF patients. These drugs can help Zoey clear the mucus from his lungs. Unfortunately, the drugs do not work in all cases.” The doctor gave the parents literature about the drugs and asked them to consider signing Zoey up for trials.
The Experimental Drugs
Ivacaftor TM is a potentiator that increases CFTR channel opening time. We know from the cell culture studies that this increases chloride transport by as much as 50% from baseline and restores it closer to what we would expect to observe in wild type CFTR. Basically, the drug increases CFTR activity by unlocking the gate that allows for the normal flow of salt and fluids.
In early trials, 144 patients all of whom were age over the age of 12 were treated with 150 mg of Ivacaftor twice daily. The total length of treatment was 48 weeks. Graph A shows changes in FEV (forced expiratory volume) with individuals using the drug versus a placebo. Graph B shows concentrations of chloride in patient’s sweat.
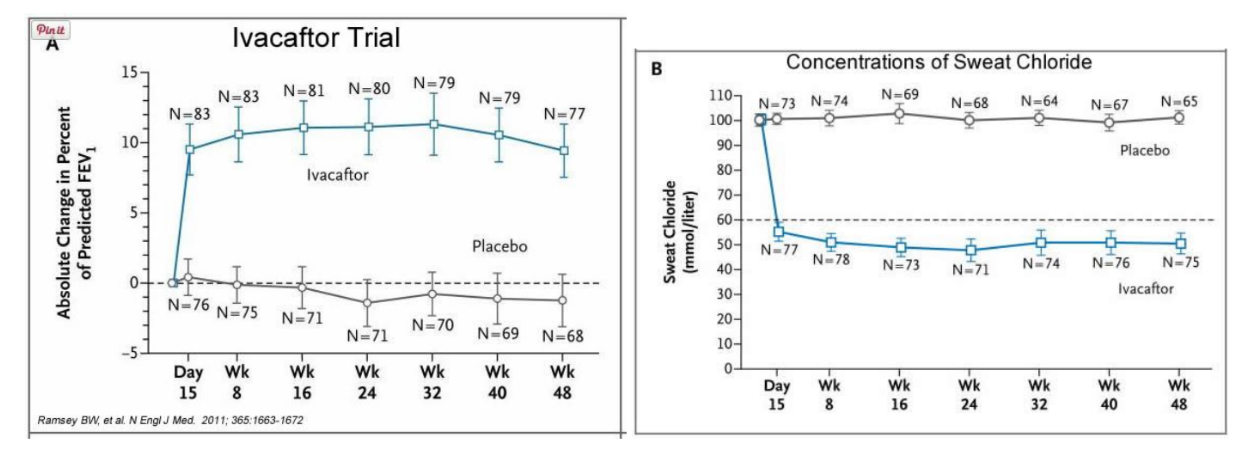
14. What is FEV? Describe a way that a doctor could take a measurement of FEV.
15. Why do you think it was important to have placebos in both of these studies?
16. Which graph do you think provides the most compelling evidence for the effectiveness of Ivacafor? Defend your choice.
17. Take a look at the mutations that can occur in the cell membrane proteins from Part III. For which mutation do you think Ivacaftor will be most effective? Justify your answer.
18. Would you sign Zoey up for clinical trials based on the evidence? What concerns would a parent have before considering an experimental drug?
Part VI: Zoey’s Mutation
Dr. Weyland calls a week later to inform the parents that genetic tests show that Zoey chromosomes show that she has two copies of the F508del mutation. This mutation, while the most common type of CF mutation, is also one that is difficult to treat with just Ivacaftor. There are still some options for treatment.
In people with the most common CF mutation, F508del, a series of problems prevents the CFTR protein from taking its correct shape and reaching its proper place on the cell surface. The cell recognizes the protein as not normal and targets it for degradation before it makes it to the cell surface. In order to treat this problem, we need to do two things: first, an agent to get the protein to the surface, and then ivacaftor (VX-770) to open up the channel and increase chloride transport. VX-809 has been identified as a way to help with the trafficking of the protein to the cell surface. When added VX-809 is added to ivacaftor (now called Lumacaftor,) the protein gets to the surface and also increases in chloride transport by increasing channel opening time.
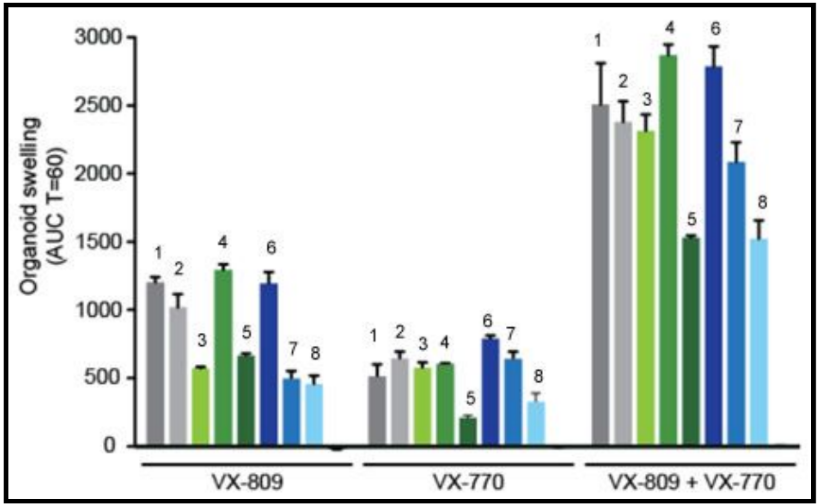
In early trials, experiments were done in-vitro, where studies were done on cell cultures to see if the drugs would affect the proteins made by the cell. General observations can be made from the cells, but drugs may not work on an individual’s phenotype. A new type of research uses ex-vivo experiments, where rectal organoids (mini-guts) were grown from rectal biopsies of the patient that would be treated with the drug. Ex-vivo experiments are personalized medicine, each person may have different correctors and potentiators evaluated using their own rectal organoids. The graph below shows how each drug works for 8 different patients (#1-#8)
19. Compare ex-vivo trials to in-vitro trials.
20. One the graph, label the group that represents Ivacaftor and Lumacaftor. What is the difference between these two drugs?
21. Complete a CER Chart. If the profile labeled #7 is Zoey, rank the possible drug treatments in order of their effectiveness for her mutation. This is your CLAIM. Provide EVIDENCE to support your claim. Provide REASONING that explains why this treatment would be more effective than other treatments and why what works for Zoey may not work for other patients. This is where you tie the graph above to everything you have learned in this case. Attach a page.
- Case report
- Open access
- Published: 19 January 2022
Malignancies in patients with cystic fibrosis: a case series
- Dorothea Appelt 1 ,
- Teresa Fuchs 1 ,
- Gratiana Steinkamp 1 , 2 &
- Helmut Ellemunter 1
Journal of Medical Case Reports volume 16 , Article number: 27 ( 2022 ) Cite this article
3413 Accesses
11 Citations
1 Altmetric
Metrics details
Previous reports have shown an increased number of colorectal cancers in patients with cystic fibrosis. We assessed the database of our cystic fibrosis center to identify patients with all kinds of cancer retrospectively. All patients visiting the Cystic Fibrosis Centre Innsbruck between 1995 and 2019 were included.
Case presentation
Among 229 patients with cystic fibrosis treated at the Cystic Fibrosis Centre in Innsbruck between 1995 and 2019, 11 subjects were diagnosed with a malignant disease. The median age at diagnosis was 25.2 years (mean 24.3 years). There were four gynecological malignancies (cervical intraepithelial neoplasia and cervical cancer), two hematological malignancies (acute lymphocytic leukemia), one gastrointestinal malignancy (peritoneal mesothelioma), and four malignancies from other origins (malignant melanoma, neuroblastoma, adrenocortical carcinoma, and thyroid cancer). One malignancy occurred after lung transplantation. There was a strong preponderance of females, with 10 of the 11 cases occurring in women. Six deaths were attributed to cancer.
Conclusions
Most diagnoses were made below 30 years of age, and half of the subjects died from the malignant disease. Awareness of a possible malignancy is needed in patients with atypical symptoms. Regular screenings for cancer should also be considered, not only for gastrointestinal tumors.
Peer Review reports
Improvements in the management of cystic fibrosis (CF) have resulted in better survival of patients, with increasing numbers of patients reaching adulthood. It also seems to be more common that patients with CF suffer from cancer [ 1 , 2 ]. An increased risk of gastrointestinal cancers among CF patients is known [ 3 , 4 , 5 , 6 ], whereas other types of cancer have rarely been reported. A few studies showed differing results with similar [ 7 , 8 ] or higher risk of cancer [ 3 , 9 ] compared with non-CF cohorts. To assess the prevalence of malignant disease in our patients, we collected data from the patient database at the CF Centre Innsbruck from 1995 to 2019, including diagnosis, treatment, and outcome of the malignant condition. Among 229 patients with CF, we observed 11 cases with cancer over a period of 24 years.
A 9-month-old Caucasion girl, who had been diagnosed with CF at the age of 1 month with an abnormal newborn screening, had a routine abdominal ultrasound, where a neuroblastoma stage 4s was diagnosed. At the time of diagnosis, no symptoms were present. The entire tumor was surgically removed, and she received chemotherapy according to the European HR-NBL-1/ESIOP protocol followed by an autologous bone-marrow stem-cell transplantation. At 6 years, the patient presented with pain in the left proximal tibia. Osteomyelitis was suspected, but antibiotic treatment showed no improvement in symptoms. The suspicion of a systemic relapse of the neuroblastoma was confirmed histologically. Chemotherapy according to protocol ESIOP TVD was started. After the fourth cycle, the tumor cells showed resistance to treatment and the disease progressed with changes in the bone marrow. Therapy was intensified with chemotherapy, radiation therapy, and allogeneic stem cell transplantation. Under this therapy, the patient was clinically stable with recurring aplasia, thrombocytopenia, and anemia. Just after her ninth birthday, she presented with a pulmonary exacerbation, which improved only after discontinuation of immunosuppression. The patient and her parents decided to continue with palliative therapy with fractionated low-dose 131I-metaiodobenzylguanidine. Three years after stem cell transplantation, the patient died at home surrounded by family.
A 14-year-old Caucasion girl with CF presented with fever, urticaria, joint pain, fatigue, and reduced general condition. She was diagnosed with c-ALL B-II and admitted for treatment. Chemotherapy was started under protocol AIEOP-BFM ALL 2000. Three weeks after diagnosis, she was discharged. Two days later, she presented with symptoms of a distal intestinal obstruction syndrome (DIOS) with constipation, weakness, hypoglycemia, and hypotonic dehydration. Her condition improved slightly after enema and antibiotic treatment, but she soon developed fever. Chest x-ray showed several peripheral infiltrates in the lungs, so antifungal therapy with amphotericin B was started according to local standard procedures. Despite decreasing inflammatory parameters, her general condition worsened with dyspnea, vertigo, and scintillating scotoma. A head CT scan showed seven brain abscesses. The girl died 1 month and 4 days after diagnosis of c-ALL. The autopsy showed endocarditis with septic abscesses in the brain, lungs, liver, kidney, and spleen. Microbiological examination of blood detected Saccharomyces cerevisiae , sensitive to itraconazole and resistant to amphotericin B.
A 17-year-old Caucasion girl with CF was admitted to the intensive care unit (ICU) with strong suspicion of leukemia. She was diagnosed with acute lymphocytic leukemia (ALL) B-II without central nervous system (CNS) involvement. She received chemotherapy under protocol AIEOP-BFM 2009. The ALL was classified as “intermediate risk.” For aplasia, the patient received antibiotic and antifungal prophylaxis and erythrocyte and platelet concentrates. She developed abdominal pain due to Clostridium difficile -associated colitis and considerable accumulation of ascites. With abdominal paracentesis, 6 L of ascites was removed. Then she developed constipation that did not improve with Macrogol therapy. Endoscopic stool removal was performed. Two months after beginning treatment, the patient had peritonitis with Staphylococcus hominis . Antibiotic treatment was started. Chemotherapy was discontinued due to the high risk of infection. She then developed hepatorenal syndrome with a known liver fibrosis and decreasing urine output. Intermittent hemodiafiltration and hemodialysis were necessary. In addition, the patient had deteriorating liver function values. A bone marrow biopsy showed no progression of the leukemia. Infection parameters increased nonetheless. There were multiple possible foci such as colitis with Clostridium difficile , peritonitis with Staphylococcus hominis , detection of atypical nontuberculosis mycobacteria in sputum, and a local infection of the central venous catheter. Two months and 9 days after diagnosis of ALL, the patient developed multiple organ dysfunction syndrome and died.
A 21-year-old Caucasion woman had an excision of a malign melanoma on her back. Histology showed a Breslow thickness of 1.9 mm and Clark level IV. A re-excision with 1 cm safety margin and a biopsy of sentinel lymph nodes were performed, showing no sign of metastases. Seven years after diagnosis of the melanoma, the patient remains tumor free.
A 22-year-old Caucasion woman presented with Cushing’s syndrome. Further investigations showed an adrenal carcinoma. The patient underwent surgery where the entire tumor was removed. The staging showed no metastases, so chemotherapy was not added to the treatment. Ten months after the first symptoms, the woman had a local tumor relapse with metastases in the lung, femur, and tibia. The tumor was unresectable, and the patient refused further chemotherapy. She started palliative therapy with radiation of the painful metastases in the femur and tibia. The disease progressed, and the patient showed psychological alterations. Airway clearance therapy became more difficult and less effective, and the respiratory status of the patient worsened. Fifteen months after diagnosis, the patient died due to respiratory insufficiency.
A 25-year-old Caucasion woman had an abnormal Pap smear (PAP IV) followed by a cervical conization and fractional abrasion. Histology showed a cervical glandular intraepithelial neoplasia (CGIN II) with a positive resection margin. The patient refused a hysterectomy at that time. The gynecological follow-ups with Pap smear and abrasion showed no residuum. Over the years, the pulmonary condition of the woman constantly declined with severe pulmonary hemorrhage at age 29. An angiographic coiling was performed that stabilized the condition for some time. She had to be ventilated and received extracorporeal membrane oxygenation but, despite an emergency lung transplantation, died due to organ failure.
A 28-year-old Caucasion woman with CF liver disease had a decompensation with increasing amounts of ascites. Diuretic therapy was unsatisfactory, so an ascites puncture was done. Laboratory investigation of the ascites showed a high cell count and high protein concentrations suggestive of an inflammatory cause with no sign of malignancy. In addition, the bacteriological cultures of ascites, blood, and urine were sterile. Only the sputum showed a known infection of Pseudomonas aeruginosa . An intravenous suppressive antibiotic treatment was started. A positron emission tomography–computed tomography (PET-CT) scan showed changes in the lungs consistent with CF and liver cirrhosis with portal hypertension and severe peritonitis with mesentery thickening.
The patient developed severe hypoglycemia and increasing anemia. Gastroscopy showed small esophageal varices without any sign of bleeding. She received intravenous glucose and erythrocyte concentrates. An X-ray of the lung showed growing consolidations in the inferior lobes of the lung. She was transferred to the intensive care unit. Hemofiltration was started because of increasing metabolic acidosis and anuria. The antibiotic treatment was adapted several times, but inflammatory parameters did not improve. No other focus except for the known pulmonary infection could be found. About 1 month after admission to hospital, the patient had more abdominal pain despite permanent analgesic therapy. A CT scan of the abdomen showed a toxic mega colon and a massive growth of the mesentery bulk. A fine-needle biopsy showed a malignant deciduoid mesothelioma. Based on the diagnosis, a multidisciplinary team suggested palliative care, to which the patient and her family agreed. Six weeks after admission to the hospital, the patient died.
On a screening examination in the 15th week of pregnancy, a 28-year-old Caucasion woman had an abnormal Pap smear (PAP IV). Cervical conization showed an adenocarcinoma that had a positive resection margin histologically. The patient had two more conizations, which had a positive resection margin again in histology, and a Shirodkar cerclage was placed. At 32 weeks of pregnancy, fetal lung maturity was induced and a healthy child was born via cesarean section. In the same operation, a radical Wertheim hysterectomy was performed. Histology showed an adenocarcinoma grade 2 with negative resection margins and micrometastases in one of 22 examined lymph nodes. Combined treatment with chemotherapy (cisplatin) and radiation was performed. Six years after diagnosis, the patient is still in remission.
Due to an abnormal Pap smear (PAP IV), a 31-year-old Caucasion woman had a cervical conization and fractional abrasion with CGIN grade II. All resection margins were negative. The patient then developed menometrorrhagia and dyspareunia. A hysterectomy was performed 4 years after diagnosis because of discomfort. The patient is still in remission 15 years after diagnosis.
A 36-year-old Caucasion woman presented with recurring vaginal bleeding. Further gynecological examination with a biopsy showed a cervix carcinoma grade II b. The histology after laparoscopic lymphadenectomy was tumor free. Two weeks after diagnosis, radiochemotherapy was initiated. Five months after diagnosis, a biopsy of the cervix showed mostly necrotic tumor tissue with 10% vital tissue. The woman had surgery with hysterectomy, salpingectomy, and iliac lymphadenectomy. Histology showed an invasive, low-differentiated, nonkeratinized squamous cell carcinoma of the cervix and three of five lymph nodes with metastases. After surgery, a PET-CT scan showed complete remission. Seven months after diagnosis, the woman presented with fever and pain in the groin. A CT scan showed a lymphocele that was punctured, streptococcus was detected, and treatment with antibiotics was started. The check-up examination 12 months after diagnosis with magnetic resonance imaging (MRI) and PET-CT scans showed an extensive vaginal stump relapse. Chemotherapy was started, but the disease progressed despite therapy. A multidisciplinary team suggested to continue with palliative care, to which the patient and her family agreed. Seventeen months after diagnosis, the patient died.
A 40-year-old Caucasion man with CF who had a lung transplant at 23, was admitted with a multinodular goiter for an operation. During the operation, an immediately frozen section showed bilateral papillary thyroid cancer (right 0.9 cm, left 1 cm). A total thyroidectomy with excision of local lymph nodes was performed. After the operation, a PET-CT-scan showed multiple glucose-metabolizing lesions in lymph nodes from neck to mediastinum. A neck dissection was performed. Histology showed multiple metastases in the lymph nodes. Thyroid hormones as suppression therapy were administered. Three months after diagnosis, therapy with radioactive iodine was started. In total, the man received five cycles with radioactive iodine. Five years after diagnosis, the patient still has a stable disease. The last PET-CT scan showed no glucose-metabolizing lesions.
Malignancies in patients with Cystic Fibrosis at the CF Centre Innsbruck
- n number, CFTR cystic fibrosis transmembrane regulator, ppFEV1 percent predicted forced expiratory volume in one second, CIN Cervical intraepithelial neoplasia
In total, we report ten female patients and one male patient with CF and cancer. The patients had a mean age of 24.3 years, about half of the patients were phe508del homozygous, and more than half had died by the end of our observation. The mean of the most recent lung function before cancer diagnosis showed a forced expiratory volume in one second (FEV 1 ) of 78.6% of predicted normal value.
Among 229 patients with cystic fibrosis visiting our CF Centre between 1995 and 2019, 11 cases of cancer were diagnosed, mainly in the third decade of life. Ten patients were female, four of whom had cervical cancer or cervical intraepithelial neoplasia. Six women died from cancer. Cancer screening could potentially prevent early deaths and should be included in routine diagnostics for adults with cystic fibrosis.
This is the largest case series of CF patients with cancer diagnoses. Previous reports show that patients with CF carry an increased risk for gastrointestinal cancer, and they appear to have an earlier onset of cancer than otherwise healthy adults [ 6 , 10 , 11 , 12 , 13 , 14 , 15 ]. The patients in our case series had a mean age of only 24.3 years at cancer diagnosis. For cervical cancer, the average age at diagnosis worldwide in 2018 was 53 years, while our four patients were much younger when diagnosed at 25–39 years of age [ 16 ].
The lung function of the patients before malignancy diagnosis was mostly normal despite cystic fibrosis, with a mean forced expiratory volume in one second (FEV 1 ) of 78.6 % of the predicted normal value. According to recent European CF Registry data, mean FEV 1 % predicted for patients aged 18 years or older without a transplant is 68.5% [ 17 ]. Only one death (case 6) was associated with severe CF lung disease and pulmonary hemorrhage. Thus, cancer shortened life considerably in half of the patients.
The Cystic Fibrosis Foundation recommends to start colonoscopy as a screening for colorectal cancer in patients with CF starting at the age of 40 years [ 18 ]. In our patient group, only one patient was diagnosed with gastrointestinal cancer (peritoneal mesothelioma, case 7), while more patients had gynecological or hematological malignancies. The low number of colorectal cancers in our subgroup may be due to the screening programme at our center, with colonoscopy and therapeutic polypectomy on a regular basis starting at the age of 40 years.
From a total of 11 cases, we report 4 cases of cervical cancer or cervical intraepithelial neoplasia in 229 CF patients over a period of 24 years (mean age 30.6 years). This corresponds to a rate of 0.073% of patients per year. The incidence rate of cervical cancer per year (mean of 1995–2018) in the general population in Tyrol, Austria, where the CF Centre Innsbruck is located, is 0.0015% for the same age range [ 19 ]. This is a 49-times-higher incidence of cervical cancer in our collective compared with the general population in the same region for the same age range. Significant expression of cystic fibrosis transmembrane conductance regulator (CFTR) gene in the cervical epithelium has been reported [ 20 ]. An analysis of Pap smear tests in women with CF also showed a high proportion of abnormal tests and cervical dysplasia [ 21 ]. Considerably higher expression of CFTR in ovarian cancer was seen in vitro and in vivo , so downregulation of CFTR or dysfunctional CFTR should suppress aggressive malignant biological behaviors of ovarian cancer cells [ 22 ]. However, compared with cells from normal endometrium, the expression of CFTR was significantly upregulated in endometrial carcinoma cells, which could result in increased proliferation and transfer of endometrial carcinoma cells [ 23 ]. Due to CFTR dysfunction in the cervix, women with CF have an abnormally thick and dense cervical mucus [ 24 ]. The squamocolumnar junction is prone to human papillomavirus (HPV) infection and cell dysfunction [ 24 ].
The CFTR plays a role in multiple cellular processes, such as development, epithelial differentiation and polarization, regeneration, migration, proliferation, and epithelial–mesenchymal transition. Several studies suggest that CFTR exerts variable effects in different tissues and in different cancer types. Especially downregulation of CFTR seems to lead to tumorigenesis and invasiveness of tumors, but the exact mechanisms are still unknown [ 25 ]. The correlation between CF and a higher risk for gastrointestinal cancer may be explained by chronic inflammation, dysmotility, and altered fecal microbiome in the gut [ 7 , 15 , 26 ].
Another potential risk factor for cancers in cystic fibrosis is transplantation. After organ replacement, CF patients were shown to have an overall increased risk of cancer and specifically an elevated risk of gastrointestinal malignancy and lymphoma. This has largely been attributed to the intense, long-term immunosuppressive medication [ 5 ]. In our case series, only 1 of 11 patients had received a lung transplant.
Frequent exposure to radiation could be an additional reason for increased cancer risk. In most centers, patients receive chest X-rays on a yearly basis and further imaging in case of pulmonary exacerbations. At our center, we perform ultra-low-dose high-resolution chest tomography to document any progression of lung disease. Nevertheless, we are very aware of the fact that routine use of computed tomography needs to be done with great care for future implications [ 27 ]. In the future, CT may be replaced by MRI imaging for monitoring of pulmonary structural changes and inflammation [ 28 ].
Only a few studies record the occurrence of cancer in patients with CF over a longer period of time, and through our database we had access to 24 years of patient data. However, this is a retrospective study with a small patient collective, during a time where the treatment of CF is constantly developing. Since the association of some cancer types such as gastrointestinal cancer with CF is clear, other cancers such as neuroblastoma or leukemia may appear coincidental. No association between neuroblastoma and CF is known; only three cases of CF and neuroblastoma have been reported in literature [ 29 , 30 ], and no causality is assumed.
Further studies about cancer risk in patients with CF and a larger patient collective are needed. Ideally, the data could be obtained from national patient registries. Improvement of life expectancy in patients with CF increases the expected absolute risk of malignancies; therefore, it is necessary to integrate screening in the care of patients with CF.
Our data suggest an increased risk for gynecological malignancies. Considering the role of CFTR in endometrial carcinoma cells [ 31 ] and in the cervical epithelium, intensified screening for women with CF, compared with healthy women, may be reasonable, as well as routine HPV vaccination. In light of this, we recommend screening for gastrointestinal and gynecological malignancies. Special alertness for malignant diseases is obviously needed in patients after transplantation due to long-term immunosuppression. Screening for other types of malignant tumors should be included in the regular follow-up of adult patients. The role of CFTR in risk of cancer may change considering the effect of new CFTR modulator therapies on CFTR, and this needs to be investigated in further studies.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Acute lymphocytic leukemia
Common acute lymphoblastic leucemia Type II B
- Cystic fibrosis
Cystic fibrosis transmembrane conductance regulator
Cervical glandular intraepithelial neoplasia
Central nervous system
Computer tomography
Distal intestinal obstruction syndrome
Human papillomavirus
Intensive care unit
Magnetic resonance imaging
Positron emission tomography
Parkins MD, Parkins VM, Rendall JC, Elborn S. Changing epidemiology and clinical issues arising in an ageing cystic fibrosis population. Ther Adv Respir Dis. 2011;5(2):105–19.
Article Google Scholar
Neglia JP, Wielinski CL, Warwick WJ. Cancer risk among patients with cystic fibrosis. J Pediatr. 1991;119(5):764–6.
Article CAS Google Scholar
Johannesson M, Askling J, Montgomery SM, Ekbom A, Bahmanyar S. Cancer risk among patients with cystic fibrosis and their first-degree relatives. Int J Cancer. 2009;125(12):2953–6.
Schöni MH, Maisonneuve P, Schöni-Affolter F, Lowenfels AB. Cancer risk in patients with cystic fibrosis: the European data. CF/CSG Group. J R Soc Med. 1996;89(Suppl 27):38–43.
PubMed PubMed Central Google Scholar
Maisonneuve P, FitzSimmons SC, Neglia JP, Campbell PW, Lowenfels AB. Cancer risk in nontransplanted and transplanted cystic fibrosis patients: a 10-year study. J Natl Cancer Inst. 2003;95(5):381–7.
Hough NE, Chapman SJ, Flight WG. Gastrointestinal malignancy in cystic fibrosis. Paediatr Respir Rev. 2020;35:90–2.
PubMed Google Scholar
Maisonneuve P, Marshall BC, Knapp EA, Lowenfels AB. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst. 2013;105(2):122–9.
Neglia JP, FitzSimmons SC, Maisonneuve P, Schöni MH, Schöni-Affolter F, Corey M, et al. The risk of cancer among patients with cystic fibrosis. Cystic Fibrosis and Cancer Study Group. N Engl J Med. 1995;332(8):494–9.
Sheldon CD, Hodson ME, Carpenter LM, Swerdlow AJ. A cohort study of cystic fibrosis and malignancy. Br J Cancer. 1993;68(5):1025–8.
Abraham JM, Taylor CJ. Cystic fibrosis & disorders of the large intestine: DIOS, constipation, and colorectal cancer. J Cyst Fibros. 2017;16(Suppl 2):S40–9.
Garg M, Ooi CY. The enigmatic gut in cystic fibrosis: linking inflammation, dysbiosis, and the increased risk of malignancy. Curr Gastroenterol Rep. 2017;19(2):6.
Hegagi M, Aaron SD, James P, Goel R, Chatterjee A. Increased prevalence of colonic adenomas in patients with cystic fibrosis. J Cyst Fibros. 2017;16(6):759–62.
McKenna PB, Mulcahy E, Waldron D. Early onset of colonic adenocarcinoma associated with cystic fibrosis—a case report. Ir Med J. 2006;99(10):310–1.
CAS PubMed Google Scholar
Meyer KC, Francois ML, Thomas HK, Radford KL, Hawes DS, Mack TL, et al. Colon cancer in lung transplant recipients with CF: increased risk and results of screening. J Cyst Fibros. 2011;10(5):366–9.
Yamada A, Komaki Y, Komaki F, Micic D, Zullow S, Sakuraba A. Risk of gastrointestinal cancers in patients with cystic fibrosis: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):758–67.
Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–203.
Zolin A, Orenti A, Naehrlich L, Jung A, van Rens J, et al. ECFSPR Annual Report 2018; 2020.
Hadjiliadis D, Khoruts A, Zauber AG, Hempstead SE, Maisonneuve P, Lowenfels AB. Cystic fibrosis colorectal cancer screening consensus recommendations. Gastroenterology. 2018;154(3):736-745.e14.
Statistik Austria. Österreichisches Krebsregister (Stand:17.12.2021) [cited 2021 Mar 22]. https://www.statistik.at .
Tizzano EF, Silver MM, Chitayat D, Benichou JC, Buchwald M. Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues. Clues for the infertility in patients with cystic fibrosis. Am J Pathol. 1994;144(5):906–14.
CAS PubMed PubMed Central Google Scholar
Rousset-Jablonski C, Reynaud Q, Nove-Josserand R, Ray-Coquard I, Mekki Y, Golfier F, et al. High proportion of abnormal pap smear tests and cervical dysplasia in women with cystic fibrosis. Eur J Obstet Gynecol Reprod Biol. 2018;221:40–5.
Wu Z, Peng X, Li J, Zhang Y, Hu L. Constitutive activation of nuclear factor κB contributes to cystic fibrosis transmembrane conductance regulator expression and promotes human cervical cancer progression and poor prognosis. Int J Gynecol Cancer. 2013;23(5):906–15.
Xia X, Wang J, Liu Y, Yue M. Lower cystic fibrosis transmembrane conductance regulator (CFTR) promotes the proliferation and migration of endometrial carcinoma. Med Sci Monit. 2017;23:966–74.
Edenborough FP. Women with cystic fibrosis and their potential for reproduction. Thorax. 2001;56(8):649–55.
Amaral MD, Quaresma MC, Pankonien I. What role does CFTR play in development, differentiation, regeneration and cancer? Int J Mol Sci. 2020;21(9):3133.
Slae M, Wilschanski M. Cystic fibrosis: a gastrointestinal cancer syndrome. Lancet Oncol. 2018;19(6):719–20.
de González BA, Mahesh M, Kim K-P, Bhargavan M, Lewis R, Mettler F, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071–7.
Woods JC, Wild JM, Wielpütz MO, Clancy JP, Hatabu H, Kauczor H-U, et al. Current state of the art MRI for the longitudinal assessment of cystic fibrosis. J Magn Reson Imag. 2019;52:1306–20.
Naselli A, Cresta F, Favilli F, Casciaro R. A long-term follow-up of residual mass neuroblastoma in a patient with cystic fibrosis. BMJ Case Rep 2015; 2015.
Moss RB, Blessing-Moore J, Bender SW, Weibel A. Cystic fibrosis and neuroblastoma. Pediatrics. 1985;76(5):814–7.
Xu J, Yong M, Li J, Dong X, Yu T, Fu X, et al. High level of CFTR expression is associated with tumor aggression and knockdown of CFTR suppresses proliferation of ovarian cancer in vitro and in vivo . Oncol Rep. 2015;33(5):2227–34.
Download references
Acknowledgements
The authors thank Johannes Eder, M.D. for assistance with data collection, Katharina Niedermayr, M.D. for reviewing the manuscript, and Nikelwa Theileis, M.A. for proofreading.
There were no sources of funding for this manuscript.
Author information
Authors and affiliations.
Medical University of Innsbruck, Cystic Fibrosis Centre Innsbruck, 6020, Innsbruck, Austria
Dorothea Appelt, Teresa Fuchs, Gratiana Steinkamp & Helmut Ellemunter
Clinical Research and Medical Scientific Writing, Schwerin, Germany
Gratiana Steinkamp
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Dorothea Appelt .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Written informed consent was obtained from the patient's legal guardian or next of kin for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Appelt, D., Fuchs, T., Steinkamp, G. et al. Malignancies in patients with cystic fibrosis: a case series. J Med Case Reports 16 , 27 (2022). https://doi.org/10.1186/s13256-021-03234-1
Download citation
Received : 05 February 2021
Accepted : 19 December 2021
Published : 19 January 2022
DOI : https://doi.org/10.1186/s13256-021-03234-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mucoviscidosis
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 14, Issue 11
- Cystic fibrosis: a diagnosis in an adolescent
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-9674-0879 Monica Bennett 1 ,
- Andreia Filipa Nogueira 1 ,
- Maria Manuel Flores 2 and
- Teresa Reis Silva 1
- 1 Pediatric , Centro Hospitalar e Universitario de Coimbra EPE , Coimbra , Portugal
- 2 Pediatric , Centro Hospitalar do Baixo Vouga EPE , Aveiro , Aveiro , Portugal
- Correspondence to Dr Monica Bennett; acinomaicila{at}gmail.com
Most patients with cystic fibrosis (CF) develop multisystemic clinical manifestations, the minority having mild or atypical symptoms. We describe an adolescent with chronic cough and purulent rhinorrhoea since the first year of life, with diagnoses of asthma, allergic rhinitis and chronic rhinosinusitis. Under therapy with long-acting bronchodilators, antihistamines, inhaled corticosteroids, antileukotrienes and several courses of empirical oral antibiotic therapy, there was no clinical improvement. There was no reference to gastrointestinal symptoms. Due to clinical worsening, extended investigations were initiated, which revealed Pseudomonas aeruginosa in sputum culture, sweat test with a positive result and heterozygosity for F508del and R334W mutations in genetic study which allowed to confirm the diagnosis of CF. In this case, heterozygosity with a class IV mutation can explain the atypical clinical presentation. It is very important to consider this diagnosis when chronic symptoms persist, despite optimised therapy for other respiratory pathologies and in case of isolation of atypical bacterial agents.
- cystic fibrosis
- pneumonia (respiratory medicine)
https://doi.org/10.1136/bcr-2021-245971
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
A high degree of diagnostic suspicion is of fundamental importance when chronic symptoms persist, despite optimised therapy for previous diagnoses and in case of isolation of atypical bacterial agents in microbiological studies.
This case describes an adolescent with a chronic cough since the first year of life, adequate weight gain and normal pubertal development, without improvement with optimised therapy for other respiratory pathologies. There was no reference to gastrointestinal symptoms. There was clinical worsening at 13 years of age and isolation of Pseudomonas aeruginosa in sputum culture. After extensive investigation, including sweat test and genetic study, it was possible to confirm the diagnosis of cystic fibrosis (CF).
Case presentation
A 13-year-old female teenager presented with chronic cough and purulent rhinorrhoea with periods of intermittent clinical worsening with associated fever since the first year of life. This was accompanied by various medical specialties, with diagnoses of asthma, allergic rhinitis and chronic rhinosinusitis. She was under therapy with long-acting bronchodilators, antihistamines, inhaled corticosteroids, and antileukotrienes and submitted to several courses of empirical oral antibiotic therapy, without sustained and effective clinical improvement. She presented an adequate height–weight evolution, with a body mass index (BMI) at 50th−85th percentile and normal pubertal development, no reference to gastrointestinal symptoms or previous hospitalisations. Her family background was irrelevant. Due to clinical worsening, with emetising cough associated with intermittent fever and night sweats, a pulmonary CT scan was performed, which revealed parenchymal densification, air bronchogram, thickened bronchi, mucoid impaction and mediastinal adenopathies. Observed in the emergency department, the objective examination highlighted bibasal crackles on pulmonary auscultation, without other alterations. She was treated with clarithromycin, later associated with co-amoxiclav. An extended investigation was initiated, which revealed erythrocyte sedimentation rate of 52 mm/hour, C reactive protein test of 4.10 mg/dL, negative BK and interferon gamma release assay test, and isolation of P. aeruginosa in sputum culture. The antibiotic therapy was changed to ciprofloxacin and sweat tests were performed with positive results on two occasions (102 and 110 mmol/L). Later, a genetic study revealed heterozygosity for the F508del and R334W mutations, which confirmed the diagnosis of CF. Faecal elastase was performed, and the result was normal (>500 µg/g).
After antimicrobial therapy with ciprofloxacin, she maintained P. aeruginosa, and methicillin-sensitive Staphylococcus aureus (MSSA) was now discovered in the sputum. For this reason, she was hospitalised for intravenous eradication. After 2 weeks of antibiotic therapy with meropenem, gentamicin and teicoplanin, P. aeruginosa was eradicated but not MSSA. Linezulide was prescribed for 2 weeks, with a good response, and the microbiological study was negative.
Outcome and follow-up
During the follow-up period (2 years), she continued having frequent respiratory infections, with isolation of P. aeruginosa and MSSA in respiratory secretions intermittently, requiring the need for several courses of antibiotic therapy. The antibiogram of P. aeruginosa has remained sensible. Currently, she continues follow-up in a specialised fibrosis cystic centre, under inhaled therapy with colistin/tobramycin, hypertonic saline, salbutamol, dornase alfa, budesonide/formoterol, chest physiotherapy and oral azithromycin prophylaxis. Her pulmonary function is normal with a currently forced expiratory volume in 1 s of 87% and she shows adequate height−weight evolution, with BMI maintained at P50–85. The sweat chloride test was not repeated after confirmed diagnosis.
CF is one of the most commonly diagnosed genetic disorders 1 and the most common life-shortening autosomal recessive disease among Caucasian populations, with a frequency of 1 in 2000–3000 live births. 2 CF is caused by mutations in a single large gene on chromosome 7 that encodes the cystic fibrosis transmembrane conductance regulator ( CFTR ) protein.
There are more than 2000 mutations/variations of the CFTR gene reported and listed in the CFTR mutation database. A small subset are CF disease-causing mutations, of which the majority are associated with pancreatic insufficiency and a smaller subset are associated with pancreatic sufficiency. Most of the known mutations/variations related to CF are described in the CFTR2 database (Clinical and Functional Translation of CFTR). This website provides information about what is currently known about specific genetic variants or variant combination and is a useful resource to correlate clinical measures to the large number of variants identified to date. 3 4
Clinical disease requires disease-causing mutations in both copies of the CFTR gene. Mutations of the CFTR gene have been divided into five different classes. The most common mutation is F508del which is included in category class II mutations—defective protein processing. Approximately 50% of patients with CF are homozygous for this mutation, and 90% will carry at least one copy of this gene. In general, mutations in classes I−III cause more severe disease than those in classes IV and V. Class IV and V mutations are associated with moderate phenotypes and pancreatic sufficiency. 5 The R334W is a rare mutation included in class IV—defective conduction and associated with pancreatic sufficiency. 5 6 Those with less severe mutations present with pancreatic sufficiency and single organ manifestations of CF. Some of these patients would fulfil the diagnostic criteria for CF and some would be classified as having a CFTR-related disorder if the diagnosis of CF cannot be fulfilled. 7
The phenotypic expression of disease varies widely, based on CFTR-related (genotype-related) and non-CFTR-related factors (environmental and other genetic modifiers). Genotype–phenotype correlations are weak for pulmonary disease in CF and somewhat stronger for the pancreatic insufficiency phenotype. 5
Many studies in different individuals heterozygous for CFTR gene mutation have been performed to find out the association of CFTR gene mutation with asthma. The results are inconclusive, as some of the studies have shown positive association, whereas other could find either protective or no association. 8 Also, at this time, there is no evidence for a specific association between CFTR gene mutation and other allergic manifestations.
Clinical manifestations are multisystemic and heterogeneous. 9 The first symptoms of the disease usually appear in the first years of life, and most patients develop a multisystem disease, with predominantly respiratory and digestive symptoms. 2 5 10 The usual presenting symptoms and signs include persistent pulmonary infection, pancreatic insufficiency and elevated sweat chloride levels. However, many patients demonstrate mild or atypical symptoms, and clinicians should remain alert to the possibility of CF even when only a few of the usual features are present. 2 Progressive pulmonary involvement is the main cause of morbidity and mortality. Clinically significant pancreatic insufficiency eventually develops in approximately 85% of individuals with CF. The remaining 10%–15% of patients with CF remain pancreatic sufficient throughout childhood and early adulthood, but these individuals are at risk of pancreatitis. Pancreatic exocrine function may be evaluated indirectly by measurement of faecal elastase, which is clinically practical but has limited accuracy. Low levels of faecal elastase suggest pancreatic insufficiency and support a diagnosis of CF. 2 5 11–13
The diagnosis of CF is based on compatible clinical findings with biochemical or genetic confirmation. The sweat chloride test is the mainstay of laboratory confirmation, although tests for specific mutations, nasal potential difference (NPD), immunoreactive trypsinogen, stool faecal fat or pancreatic enzyme secretion may also be useful in some cases.
Both of the following criteria must be met to diagnose CF: (1) clinical symptoms consistent with CF in at least one organ system, or positive newborn screen or having a sibling with CF; and (2) evidence of cystic CFTR dysfunction (any of the following): elevated sweat chloride ≥60 mmol/L; presence of two disease-causing mutations in the CFTR gene, one from each parental allele; abnormal NPD.
Sweat chloride test ≥60 mmol/L is considered abnormal. If confirmed on a second occasion, this is sufficient to confirm the diagnosis of CF in patients with clinical symptoms of CF. Positive results of sweat testing should be further evaluated by CFTR sequencing. Determining the CFTR genotype is important because the results may affect treatment choices as well as confirm the diagnosis. For patients with inconclusive results of sweat chloride and DNA testing, measurement of NPD can be used to further evaluate for CFTR dysfunction. 5 14
Newborn screening programmes for CF are now performed routinely in several countries, which contributed to a dramatic increase in the number of CF cases identified before presenting with symptoms. The rationale for this screening is that early detection of CF may lead to earlier intervention and improved outcomes because the affected individuals are diagnosed, referred and treated earlier in life compared with individuals who are diagnosed after presenting with symptomatic CF. In Portugal and some other European countries, this programme was implemented less than 10 years ago, contributing to a late diagnosis in older children.
There are different neonatal screening programmes that include biochemical screening and/or DNA assays with panels to test for the most common CFTR mutations in the local population. Most programmes test for between 23 and 40 mutations, and some programmes even perform adjunctive full gene sequencing. Screening for a greater number of mutations increases the likelihood of identifying infants with CF and also increases the identification of rare or unique sequence mutations, making interpretation of the result more complicated. As only a limited number of mutations are evaluated on the genetic screens, it is possible to miss the diagnosis. Thus, it is important to follow such children closely, with particular attention to weight gain and recurrent respiratory infections. Clinicians should consider CF in individuals with suggestive symptoms, even when results of the newborn screen are negative or equivocal. 5 14
In the case described here, heterozygosity with a class IV mutation, usually associated with an intermediate phenotype and pancreatic sufficiency, may explain the atypical clinical presentation and consequent diagnosis only in adolescents. We also hypothesise that this child’s allergic manifestations may have delayed the diagnosis.
As the spectrum of clinical presentation is very variable, it is very important for clinicians from multiple specialties to be vigilant and suspect this diagnosis in conditions such as recurrent pulmonary infection, male infertility, pancreatitis, nasal polyposis and malabsorption even in patients with negative newborn screening. 2 10 13
Learning points
There is a wide spectrum of manifestations of cystic fibrosis (CF). These variations and wide spectrum are based on cystic fibrosis transmembrane conductance regulator (CFTR)-related (genotype-related) and non-CFTR-related factors (environmental and other genetic modifiers).
Most patients with CF develop multisystemic and heterogeneous clinical manifestations, with predominantly respiratory and digestive symptoms.
A minority have mild or atypical symptoms.
Heterozygosity with a class IV mutation usually is associated with an intermediate phenotype and pancreatic sufficiency and can explain the atypical clinical presentation.
It is very important to consider this diagnosis when chronic symptoms persist, despite optimised therapy for other respiratory pathologies and in case of isolation of atypical bacterial agents in microbiological studies.
Ethics statements
Patient consent for publication.
Consent obtained from parent(s)/guardian(s)
- Dickinson KM ,
- ↵ Cystic fibrosis mutation database . Available: http://www.genet.sickkids.on.ca/Home.html
- ↵ Clinical and functional translation of CFTR . Available: https://cftr2.org/
- Ellis L , et al
- Awasthi S ,
- Gartner S ,
- Salcedo Posadas A ,
- García Hernández G
- Castellani C ,
- Linnane B ,
- Pranke I , et al
- Farrell PM ,
- Ren CL , et al
- Kharrazi M ,
- Bishop T , et al
Contributors MB cared for study patient, planned and wrote the article. AFN collected data. MMF provided and cared for study patient, served as scientific advisors and critically reviewed the study proposal. TRS cared for study patient, served as scientific advisors and critically reviewed the study proposal.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 19: Case Study: Cystic Fibrosis
Julie M. Skrzat; Carole A. Tucker
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Introduction.
- Examination: Age 2 Months
- Evaluation, Diagnosis, and Prognosis
- Intervention
- Conclusion of Care
- Examination: Age 8 Years
- Examination: Age 16 Years
- Recommended Readings
- Full Chapter
- Supplementary Content
C ystic fibrosis (CF) is an autosomal recessive condition affecting approximately 30,000 Americans and 70,000 people worldwide. According to the Cystic Fibrosis Foundation ( Cystic Fibrosis Foundation, 2019a ), approximately 1,000 new cases are diagnosed yearly in the United States, with a known incidence of 1 per 3,900 live births. The disease prevalence varies greatly by ethnicity, with the highest prevalence occurring in Western European descendants and within the Ashkenazi Jewish population.
The CF gene, located on chromosome 7, was first identified in 1989. The disease process is caused by a mutation to the gene that encodes for the CF transmembrane conductance regulator (CFTR) protein. This mutation alters the production, structure, and function of cyclic adenosine monophosphate (cAMP), a dependent transmembrane chloride channel carrier protein found in the exocrine mucus glands throughout the body. The mutated carrier protein is unable to transport chloride across the cell membrane, resulting in an electrolyte and charge imbalance. Diffusion of water across the cell membrane is thus impaired, resulting in the development of a viscous layer of mucus. The thick mucus obstructs the cell membranes, traps nearby bacteria, and incites a local inflammatory response. Subsequent bacterial colonization occurs at an early age and ultimately this repetitive infectious process leads to progressive inflammatory damage to the organs involved in individuals with CF.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
- Reference Manager
- Simple TEXT file
People also looked at
Case report article, case report: cystic fibrosis with kwashiorkor: a rare presentation in the era of universal newborn screening.

- Department of Pediatrics, University of Colorado Denver Anschutz Medical Campus, Aurora, CO, United States
Background: Universal newborn screening changed the way medical providers think about the presentation of cystic fibrosis (CF). Before implementation of universal screening, it was common for children with CF to present with failure to thrive, nutritional deficiencies, and recurrent infections. Now, nearly all cases of CF are diagnosed by newborn screening shortly after birth before significant symptoms develop. Therefore, providers often do not consider this illness in the setting of a normal newborn screen. Newborn screening significantly decreases the risk of complications in early childhood, yet definitive testing should be pursued if a patient with negative newborn screening presents with symptoms consistent with CF, including severe failure to thrive, metabolic alkalosis due to significant salt losses, or recurrent respiratory infections.
Case presentation: We present a case of a 6-month-old infant male with kwashiorkor, severe edema, multiple vitamin deficiencies, hematemesis secondary to coagulopathy, and diffuse erythematous rash, all secondary to severe pancreatic insufficiency. His first newborn screen had an immunoreactive trypsinogen (IRT) value below the state cut-off value, so additional testing was not performed, and his growth trajectory appeared reassuring. He was ultimately diagnosed with CF by genetic testing and confirmatory sweat chloride testing, in the setting of his parents being known CF carriers and his severe presentation being clinically consistent with CF. Acutely, management with supplemental albumin, furosemide, potassium, and vitamin K was initiated to correct the presenting hypoalbuminemia, edema, and coagulopathy. Later, pancreatic enzyme supplementation and additional vitamins and minerals were added to manage ongoing deficiencies from pancreatic insufficiency. With appropriate treatment, his vitamin deficiencies and edema resolved, and his growth improved.
Conclusion: Due to universal newborn screening, symptomatic presentation of CF is rare and presentation with kwashiorkor is extremely rare in resource-rich communities. The diagnosis of CF was delayed in our patient because of a normal newborn screen and falsely reassuring growth, which after diagnosis was determined to be secondary to severe edematous malnutrition. This case highlights that newborn screening is a useful but imperfect tool. Clinicians should continue to have suspicion for CF in the right clinical context, even in the setting of normal newborn screen results.
Introduction
Newborn screening for cystic fibrosis (CF) in the United States was adopted in the early 1980s and became standard in all fifty states by 2010 ( 1 , 2 ). Prior to the implementation of universal newborn screening and in countries where newborn screening is not performed, many children were diagnosed with CF after developing symptoms including malnutrition, growth faltering, chronic cough, recurrent respiratory infections/pneumonias, rectal prolapse, and/or electrolyte and other nutritional abnormalities ( 1 ). Due to the success of newborn screening, clinicians in high resource settings have never seen a child present with symptomatic CF and may not consider CF when these symptoms occur.
Early diagnosis of CF has been shown to improve outcomes due to optimized nutrition and targeted interventions ( 3 ). Numerous studies over the years continually reconfirm that patients do better when diagnosis happens earlier ( 3 – 5 ). Improved growth, presumably secondary to early initiation of pancreatic enzyme replacement therapy and other vitamin supplementation, leads to better pulmonary function throughout life as well as improved neurological outcomes into adolescence. Malnutrition in children with CF tends to lead to increased lung disease ( 3 ). Seventy percent of infants that were symptomatic at presentation had more hospitalizations in the first year of life and more complications, including growth faltering, positive Pseudomonas aeruginosa culture results, and electrolyte abnormalities when compared to patients diagnosed prenatally or via newborn screening ( 6 , 7 ). Decreased chronic infection with Pseudomonas aeruginosa has also been observed since implementation of universal newborn screening ( 1 ) which leads to improved lung function.
Newborn screening is performed with measurements of immunoreactive trypsinogen (IRT) and genetic testing but testing protocols vary from state to state ( 8 ) ( Figure 1 ). IRT is a pancreatic precursor enzyme that is elevated in patients with CF due to pancreatic duct blockage ( 9 ). An elevated IRT is not specific to CF and can be elevated in the absence of CF, particularly when infants are born premature, have low Apgar scores, or experience perinatal stress ( 3 ). In all states, an IRT level above a certain threshold is indicative of a positive screen; however, the cutoff varies and is not specified in the American College of Medical Genetics and Genomics guidelines ( 10 ), allowing states to set their own threshold. Different procedures for newborn screening for CF include IRT-only, IRT-IRT, IRT-DNA, and IRT-IRT-DNA ( 8 , 9 , 11 ). IRT-only states measure IRT levels in blood samples from newborns collected around 2 weeks of life and if elevated, proceed directly to sweat testing. In IRT-IRT states, the first IRT sample is collected in the first few days of life and if abnormal, a repeat IRT will be collected. If the repeat IRT remains elevated, the next step is sweat chloride testing ( 11 ). In IRT-DNA states, the sample is typically collected in the first days of life and the sample is reflexively sent for DNA testing if the IRT level is above a particular threshold. In IRT-IRT-DNA states, the first sample is collected within 24 h of life, and if negative, no further testing is done. If the initial IRT is elevated in these states, IRT testing will be repeated on the second screen, sent around 2 weeks of life. If the IRT remains elevated, then reflex testing for mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene occurs, either with a panel of common genetic variants seen in that state or full genetic sequencing ( 12 ). For all states, patients flagged with abnormal newborn screens have a protocol for confirmatory testing with sweat chloride testing, which remains the gold standard for diagnosing cystic fibrosis ( 9 ). All states have varying, non-zero false negative rates, meaning that all states will intermittently miss true cases of CF on the screen. It is estimated that newborn screening with any IRT process identifies approximately 95%–99% of newborns with CF ( 13 ).
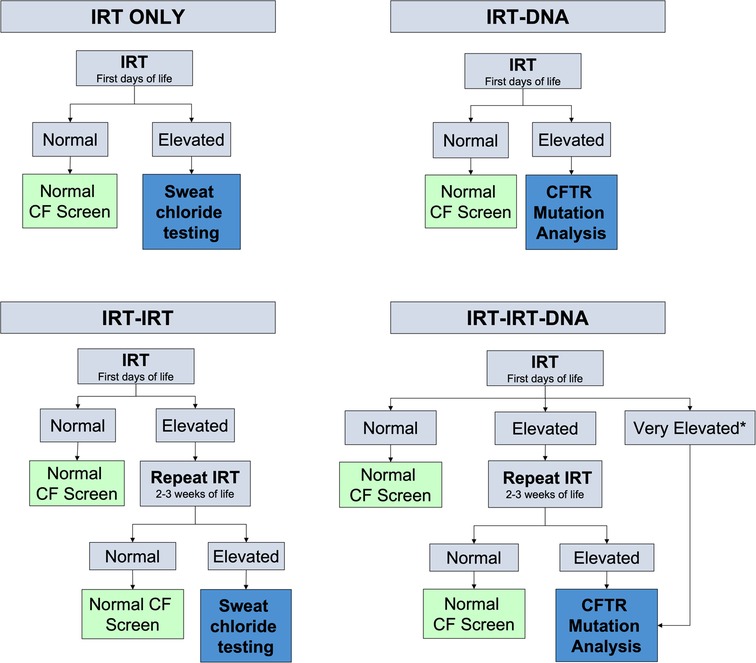
Figure 1 . Different algorithms for newborn screening processes measuring newborn IRT levels in blood spot testing. Levels for normal or elevated also vary depending on the state. *Some states have automatic CFTR mutation analysis if the IRT level is above a certain threshold.
Case presentation
A 6-month-old Caucasian male presented with 2 weeks of fussiness, fever, and decreased activity and one episode of hematochezia and hematemesis in the setting of 1 month of a progressively worsening rash that started in his diaper area and spread to the extremities, neck, and trunk ( Figure 2 ). There was no change in his rash with emollients or topical steroid use.
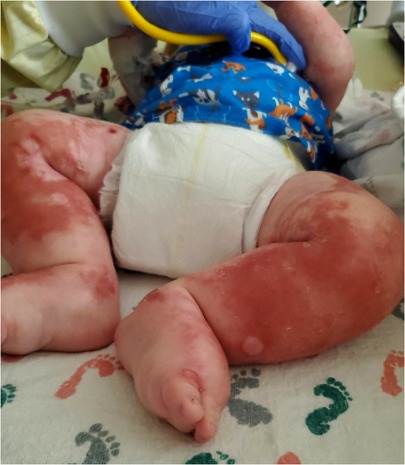
Figure 2 . Patient's lower extremities on presentation demonstrating edema and diffuse rash with scaly plaques and bullous areas.
He was born full term without complications, passed meconium in the first 24 h of life, and had a normal newborn screen. His initial IRT was 59.3 ng/ml (normal <60 ng/ml), so no further testing was performed according to the state protocol. Parents were both identified as CF carriers during the pregnancy. His weight dropped from the 85th percentile at 2 months of age to the 33rd percentile at 4 months of age which coincided with a transition from exclusive direct breastfeeding to mostly bottle feeding of expressed breast milk. Weight gain from that point forward was consistent around the 35th percentile. His length, however, dropped from the 73rd percentile at 2 months of age to less than 3rd percentile at presentation at 6 months of age ( Figure 3 ). He was taking adequate volumes of breastmilk on demand about every 3–4 h with occasional reflux episodes. He was taking minimal solid foods at the time of admission. Vitamin D supplementation was inconsistent. His stooling pattern was reported as 3–4 times per day, soft consistency without malodor, discoloration, or oil droplets except for one episode of bright red blood per rectum immediately prior to admission. His mother reported she had concerns about constipation due to crying and fussiness with stooling. He had no respiratory symptoms such as cough or history of pneumonia.
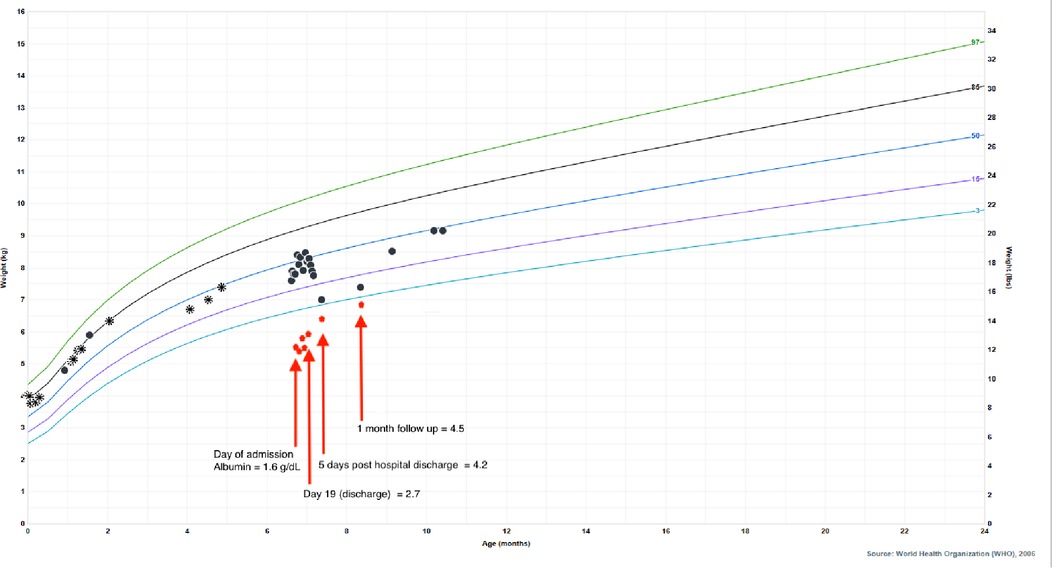
Figure 3 . Patient's growth chart for weight from birth to present day with superimposed albumin values to highlight the impact of hypoalbuminemia on his falsely reassuring growth curve. Circle points indicate hospital system measurements, asterisk points indicate outside of hospital measurements. Albumin normal lab value range: 3.4–4.2 g/dl.
Physical examination on admission was remarkable for generalized edema (facial, periorbital, peripheral) with a diffuse, reddish-brown in color, serpiginous, rash with scaly plaques at his extremities and perineal area ( Figure 2 ). The rash was most prominent in the flexural surfaces with darker purple appearance in the intertriginous areas. There was an approximately three centimeter left inguinal mass with overlying purpura. His breathing was comfortable without any adventitious breath sounds, and his abdomen was soft, nontender, and without appreciable organomegaly, though this was difficult to assess due to his anasarca. His hair was thin and reddish in color.
Labs were notable for coagulopathy, hypoalbuminemia, hyponatremia, elevated liver enzymes, elevated ferritin, elevated lactate dehydrogenase (LDH), elevated c-reactive protein, decreased 25-hydroxy vitamin D level, decreased retinol/vitamin A level, decreased vitamin E alpha tocopherol level, and decreased zinc level ( Table 1 ). Stool was positive for occult blood. Due to multiple fat-soluble vitamin deficiencies, fecal pancreatic elastase was assessed and was low. His potassium was initially normal, but quickly dropped to 2.1 mmol/L on the second day of his hospitalization. He also tested positive for SARS-CoV-2 on admission. Of note, his father had tested positive for SARS-CoV-2 approximately 1 week prior to admission.
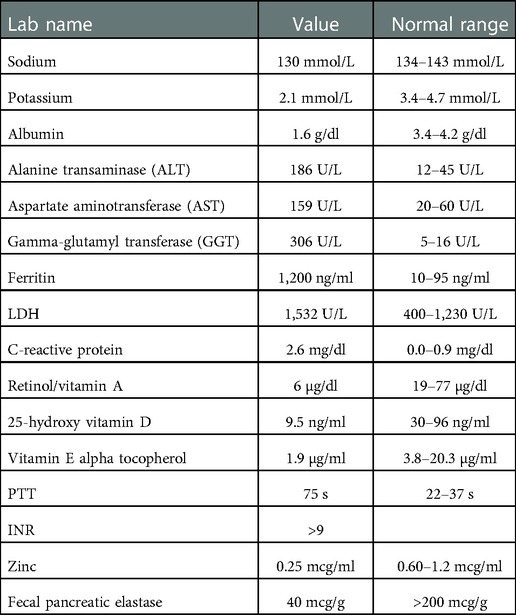
Table 1 . Labs values at the time of presentation with normal reference ranges.
He was admitted and diagnosed with kwashiorkor with prompt and cautious initiation of treatment for severe malnutrition, electrolyte derangements, and coagulopathy. Consultants involved included gastroenterology, hepatology, genetics/metabolism, allergy/immunology, cardiology, hematology, nutrition physicians, pulmonology, infectious disease, and dermatology. He received intravenous vitamin K and was initially started on continuous nasogastric feeds with breastmilk fortified with a high-protein extensively hydrolyzed formula. He received intravenous antibiotics for inguinal lymphadenopathy and cellulitis with intravenous antibiotics. Based on his very low fecal elastase measurements without diarrhea and his persistent edema and electrolyte derangements, he was started on empiric pancreatic enzymes on hospital day 10 pending a unifying diagnosis. Given the severity of his presentation and the broad differential diagnosis, rapid whole exome sequencing was pursued which identified two mutations in CFTR: F508del and 1717-1G->A, leading to a diagnosis of CF. No other genetic mutations were identified. A sweat chloride test was not obtained during his hospitalization due to the severity of his rash and electrolyte derangements but was performed about 1 month after hospitalization. One month after discharge, his sweat chloride levels were elevated at 86 and 90 mmol/L (normal <60 mmol/L) on his right and left arms respectively, confirming a diagnosis of CF.
Ultimately, his presenting diagnoses of kwashiorkor and coagulopathy were considered secondary to chronic CF-associated pancreatic insufficiency and malabsorption. Kwashiorkor explained his edema, hypoalbuminemia, elevated liver enzymes, hypoglycemia, malabsorption, thin and reddish hair, and rash. He demonstrated improvement with continuous nasogastric feeds of fortified breastmilk, supplemental fat-soluble vitamins, pancreatic enzymes, and electrolyte replacements. He was discharged home on 24 kcal/oz fortified oral and nasogastric feeds, DEKA vitamins, supplemental vitamin D3 (cholecalciferol) and zinc, and pancreatic enzymes. Additionally, he was started on twice daily respiratory treatments with albuterol and chest physiotherapy. After diuresis, his weight dropped to the 4th percentile, which correlated with normalization of his albumin ( Figure 3 ), indicating that the appearance of his reassuring growth prior to presentation was weight gain secondary to edema and not true growth. After interventions, his weight appropriately increased and his stunted length began to normalize, signaling improved absorption and adequate nutrition. Growth measurements 5 months after hospitalization demonstrate significant recovery with weight at the 45th percentile, length at the 11th percentile, and weight-for-length at the 75th percentile.
Discussion and conclusion
Prior to newborn screening for CF, the average age at diagnosis was 2.9 years (in 1995) and children typically presented with malnutrition and growth faltering ( 14 ). Additionally, a symptomatic presentation could include dehydration, steatorrhea or abnormal stools, electrolyte abnormalities, recurrent respiratory infections and sinus disease, or meconium ileus at birth. Electrolyte derangements most commonly included hyponatremic, hypochloremic, and hypokalemic metabolic alkalosis along with hypoproteinemia and edema ( 15 ). Fat soluble vitamin deficiencies were common due to pancreatic insufficiency. It is now well-known that CF outcomes are improved in patients who are diagnosed earlier in life ( 1 , 14 ). In the early 1990s, Farrell et al. demonstrated that patients diagnosed earlier had significantly higher height and weight percentiles not only at the time of diagnosis, but also during the 10-year follow up period. They also found that patients diagnosed by newborn screening rather than symptoms had less severe lung disease during childhood ( 14 ). Today, most individuals with CF are diagnosed through newborn screening and subsequent confirmatory testing. The 2021 United States Cystic Fibrosis Foundation Registry Annual Data Report, as expected, reports that the majority of those diagnosed with CF in the first year of life are asymptomatic or mildly symptomatic due to newborn screening, DNA analysis, and prenatal testing ( 16 ). This means that the current generation of providers, particularly general practitioners, are not familiar with symptomatic presentation. Being unable to recognize symptomatic presentation of CF means that questionable symptoms may go unnoticed for longer in the setting of a normal newborn screen. Cases of CF presenting with kwashiorkor secondary to severe pancreatic insufficiency were documented prior to newborn screening and continue to be reported occasionally in low-resource communities ( 17 – 19 ). Kwashiorkor is a form of severe protein energy malnutrition seen in infants and young children and is more prevalent in low resource communities ( 20 , 21 ). Manifestations can involve all body systems and are notable for peripheral pitting edema, marked muscle atrophy, depletion of fat stores, low weight-for-height, reduced mid-upper arm circumference, thin and dry skin, rash, dry hypopigmented hair, hepatomegaly from fatty liver infiltrates with abdominal distention, bradycardia, hypotension, and hypothermia ( 20 ). Other less commonly seen features include elevated liver enzymes, low serum concentrations of trace metals, and elevated ferritin concentrations ( 20 ). With implementation of universal newborn screening in the United States, CF presenting with kwashiorkor is now rarely seen, and as such, providers may not consider CF on the differential. According to the 2021 CF Registry Annual Data Report, no infants with CF have presented with edema at the time of diagnosis, highlighting the rarity of kwashiorkor ( 16 ). Our patient was receiving adequate intake by volume as assessed by parent report, leading to the concern that his kwashiorkor had an underlying cause rather than chronic inadequate intake.
Many patients with CF-related kwashiorkor have the characteristic kwashiorkor dermatosis: a rash with diffuse erythematous plaques, desquamation, and hyperpigmentation followed by peeling ( 18 , 19 , 22 , 23 ). Similar appearing rashes can occur in patients with essential fatty acid deficiency ( 24 ) or severe zinc deficiency, particularly, acrodermatitis enteropathica, a rare genetic condition where intestinal zinc absorption is impaired ( 23 , 25 , 26 ). These diagnoses should also be considered, and a thorough dietary history is extremely important.
Newborn screening has resulted in identification of CF early in life, although not without false negatives. Children who are diagnosed with CF after a false negative newborn screen tend to have worse respiratory outcomes ( 7 ). Our patient's first newborn screen, obtained at 24 h of life, was reported as normal due to an IRT value of 59.3 ng/ml. He was born in an IRT-IRT-DNA state with a fixed cutoff of 60 ng/ml. Because his initial screen fell below the fixed cutoff, it was reported as normal, and so did not trigger repeat IRT or CFTR gene sequencing. His falsely normal newborn screen resulted in delayed consideration of CF as the cause for his rash, edema, and linear growth faltering. His substantial edema made his weight appear normal, providing further false reassurance due to what appeared to be adequate weight gain.
To attempt to limit the number of false negative cases and increase the sensitivity of screening, the cutoff values for newborn screening in our state have been adjusted ( 27 ). Instead of strict single cutoff value, the newborn screen process has been modified to have a floating cutoff calculated each day based on percentiles from daily IRT samples for the initial sample and fixed IRT cutoff of 50 ng/ml on the second sample. Variation in daily average IRT values have been observed with changes in seasons and reagents in the bloodspot kits, which supports the use of a floating cutoff ( 28 ). This also highlights that newborn screening programs should periodically re-evaluate their algorithm to identify areas for potential improvement. Areas for improvement include education, communication, and accuracy ( 2 ). Our case also emphasized the fact that the newborn screen is only a screen and not diagnostic; a reportedly normal value so close to the cutoff may warrant further evaluation, especially in children of parents who are known carriers. Clinicians should maintain a high index of suspicion for CF when infants present with pancreatic insufficiency, severe edematous malnutrition, and/or persistent respiratory infections, or when both parents are CF carriers, even in the setting of a negative newborn screen. Any child with symptoms consistent with CF should undergo diagnostic sweat chloride testing and subsequent genetic testing, if indicated.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors made substantial contributions to the conception and design and have approved the final manuscript and agree to be personally accountable for contributions. AW is the first author and made substantial contributions to conception and design, drafted and revised the manuscript. SG made substantial contributions to conception and design, and revised the manuscript. SW made substantial contributions to conception and design and revised the manuscript. CO made substantial contributions to conception and design, supervised and revised the manuscript. EH made substantial contributions to conception and design and revised the manuscript. KW made substantial contributions to conception and design and revised the manuscript. LG made substantial contributions to conception and design and revised the manuscript. MH made substantial contributions to conception and design, supervised, and revised the manuscript. JH made substantial contributions to conception and design, acquisition and analysis of data, supervised, and revised the manuscript and is the senior author. All authors contributed to the article and approved the submitted version.
SG and SW were supported by NIDDK grant no. T32-DK07658.
Acknowledgments
The authors would like to acknowledge the Children's Hospital Colorado Cystic Fibrosis team.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; IRT, immunoreactive trypsinogen.
1. Hoch H, Sontag MK, Scarbro S, Juarez-Colunga E, McLean C, Kempe A, et al. Clinical outcomes in U.S. Infants with cystic fibrosis from 2001 to 2012. Pediatr Pulmonol . (2018) 53(11):1492–7. doi: 10.1002/ppul.24165
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Sontag MK, Miller JI, McKasson S, Gaviglio A, Martiniano SL, West R, et al. Newborn screening for cystic fibrosis: a qualitative study of successes and challenges from universal screening in the United States. Int J Neonatal Screen . (2022) 8(3):1–14. doi: 10.3390/ijns8030038
CrossRef Full Text | Google Scholar
3. Coverstone AM, Ferkol TW. Early diagnosis and intervention in cystic fibrosis: imagining the unimaginable. Front Pediatr . (2020) 8:608821. doi: 10.3389/fped.2020.608821
4. Tridello G, Castellani C, Meneghelli I, Tamanini A, Assael BM. Early diagnosis from newborn screening maximises survival in severe cystic fibrosis. ERJ Open Res . (2018) 4(2):1–8. doi: 10.1183/23120541.00109-2017
5. Dijk FN, McKay K, Barzi F, Gaskin KJ, Fitzgerald DA. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same centre. Arch Dis Child . (2011) 96(12):1118–23. doi: 10.1136/archdischild-2011-300449
6. Accurso FJ, Sontag MK, Wagener JS. Complications associated with symptomatic diagnosis in infants with cystic fibrosis. J Pediatr . (2005) 147(3 Suppl):S37–41. doi: 10.1016/j.jpeds.2005.08.034
7. Coffey MJ, Whitaker V, Gentin N, Junek R, Shalhoub C, Nightingale S, et al. Differences in outcomes between early and late diagnosis of cystic fibrosis in the newborn screening era. J Pediatr . (2017) 181:137–45.e1. doi: 10.1016/j.jpeds.2016.10.045
8. Ross LF. Newborn screening for cystic fibrosis: a lesson in public health disparities. J Pediatr . (2008) 153(3):308–13. doi: 10.1016/j.jpeds.2008.04.061
9. Martiniano SL, Hoppe JE, Sagel SD, Zemanick ET. Advances in the diagnosis and treatment of cystic fibrosis. Adv Pediatr . (2014) 61(1):225–43. doi: 10.1016/j.yapd.2014.03.002
10. American College of Medical Genetics and Genomics. ACT sheets and algorithms 2006. Available at: https://www.acmg.net/ACMG/Medical-Genetics-Practice-Resources/ACT_Sheets_and_Algorithms.aspx .
11. Price JF. Newborn screening for cystic fibrosis: do we need a second IRT? Arch Dis Child . (2006) 91(3):209–10. doi: 10.1136/adc.2005.085084
12. The New York-Mid-Atlantic Consortium for Genetic Newborn Screening Services. Genetic alliance monographs and guides. In: Lisa Wise MA, editor. Understanding genetics: A New York, mid-atlantic guide for patients and health professionals . Washington (DC): Genetic Alliance (2009). p. 19–22.
13. Lumertz MS, Rispoli T, Rosa KMD, Pinto LA. False-negative newborn screening result for immunoreactive trypsinogen: a major problem in children with chronic lung disease. J Bras Pneumol . (2019) 45(3):e20180062. doi: 10.1590/1806-3713/e20180062
14. Farrell PM, Kosorok MR, Laxova A, Shen G, Koscik RE, Bruns WT, et al. Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin cystic fibrosis neonatal screening study group. N Engl J Med . (1997) 337(14):963–9. doi: 10.1056/NEJM199710023371403
15. Scurati-Manzoni E, Fossali EF, Agostoni C, Riva E, Simonetti GD, Zanolari-Calderari M, et al. Electrolyte abnormalities in cystic fibrosis: systematic review of the literature. Pediatr Nephrol . (2014) 29(6):1015–23. doi: 10.1007/s00467-013-2712-4
16. Cystic fibrosis foundation patient registry 2021 annual data report Bethesda, Maryland ©2022 Cystic Fibrosis Foundation.
17. Mei-Zahav M, Solomon M, Kawamura A, Coates A, Durie P. Cystic fibrosis presenting as kwashiorkor in a Sri Lankan infant. Arch Dis Child . (2003) 88(8):724–5. doi: 10.1136/adc.88.8.724
18. Sandy NS, Nogueira RJN. Nutritional treatment of a young infant with cystic fibrosis presenting with severe kwashiorkor dermatosis. J Trop Pediatr . (2019) 65(6):634–7. doi: 10.1093/tropej/fmz008
19. Shajil C, Sathishkumar D, Chiramel MJ, Kumar M, Varkki S, Rose W, et al. Kwashiorkor-like dermatosis: a rare presentation of cystic fibrosis. Clin Exp Dermatol . (2021) 46(1):213–5. doi: 10.1111/ced.14438
20. Benjamin O, Lappin SL. Kwashiorkor. In: Statpearls . Treasure Island (FL): StatPearls Publishing (2021). Available at: https://www.ncbi.nlm.nih.gov/books/NBK507876/
21. Bhutta ZA, Berkley JA, Bandsma RHJ, Kerac M, Trehan I, Briend A. Severe childhood malnutrition. Nat Rev Dis Primers . (2017) 3:17067. doi: 10.1038/nrdp.2017.67
22. O'Regan GM, Canny G, Irvine AD. ‘Peeling paint’ dermatitis as a presenting sign of cystic fibrosis. J Cyst Fibros . (2006) 5(4):257–9. doi: 10.1016/j.jcf.2006.05.003
23. Boos MD, Briggs S. Scaly dermatitis and edema in an irritable child. JAMA . (2021) 325(4):393–4. doi: 10.1001/jama.2020.10429
24. Roongpisuthipong W, Phanachet P, Roongpisuthipong C, Rajatanavin N. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition. BMJ Case Rep . (2012) 2012:1–4. doi: 10.1136/bcr.07.2011.4475
25. Crone J, Huber WD, Eichler I, Granditsch G. Acrodermatitis enteropathica-like eruption as the presenting sign of cystic fibrosis–case report and review of the literature. Eur J Pediatr . (2002) 161(9):475–8. doi: 10.1007/s00431-002-0982-0
26. Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab . (2013) 62(Suppl 1):19–29. doi: 10.1159/000348261
27. Martiniano SL, Croak K, Bonn G, Sontag MK, Sagel SD. Improving outcomes for Colorado's IRT-IRT-DNA cystic fibrosis newborn screening algorithm by implementing floating cutoffs. Mol Genet Metab . (2021) 134(1-2):65–7. doi: 10.1016/j.ymgme.2021.08.005
28. Kay DM, Maloney B, Hamel R, Pearce M, DeMartino L, McMahon R, et al. Screening for cystic fibrosis in New York state: considerations for algorithm improvements. Eur J Pediatr . (2016) 175(2):181–93. doi: 10.1007/s00431-015-2616-3
Keywords: cystic fibrosis - CF, kwashiorkor, newborn screening (NBS), pancreatic insufficiency, missed diagnosis
Citation: Wolfe AG, Gilley SP, Waldrop SW, Olson C, Harding E, Widmer K, Gumer LB, Haemer M and Hoppe JE (2023) Case report: Cystic fibrosis with kwashiorkor: A rare presentation in the era of universal newborn screening. Front. Pediatr. 10:1083155. doi: 10.3389/fped.2022.1083155
Received: 28 October 2022; Accepted: 9 December 2022; Published: 6 January 2023.
Reviewed by:
© 2023 Wolfe, Gilley, Waldrop, Olson, Harding, Widmer, Gumer, Haemer and Hoppe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Annemarie G. Wolfe [email protected]
Specialty Section: This article was submitted to Pediatric Pulmonology, a section of the journal Frontiers in Pediatrics
This article is part of the Research Topic
New Insights into Caring for Pediatric Patients with Cystic Fibrosis

Cystic Fibrosis Research Center
The Willard A. Bernbaum Cystic Fibrosis Research Center at Case Western Reserve University School of Medicine is dedicated to devising new therapeutics to treat this disease and its complications.
The Willard A. Bernbaum Cystic Fibrosis Research Center dates back to 1964 when it was initially funded by the National Institutes of Health.

Discover presentations, grants and manuscripts that demonstrate the impact of our work.

Student Programs
Our center provides multiple avenues for young scientists to experience, and become engaged with, research related to cystic fibrosis.
Disclaimer » Advertising
- HealthyChildren.org
Recommendations
Genetic testing, communication and psychosocial issues, no consensus, limitations and areas for future research, cystic fibrosis foundation evidence-based guideline for the management of crms/cfspid.
Contributed equally as co-senior authors.
FUNDING: This guideline was supported by the Cystic Fibrosis Foundation. The sponsor assisted with the application of the committee members and resources for weekly and monthly virtual meetings of the committee members.
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no potential conflicts of interest relevant to this article to disclose.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Deanna M. Green , Thomas Lahiri , Karen S. Raraigh , Fadel Ruiz , Jacquelyn Spano , Nicholas Antos , Lynn Bonitz , Lillian Christon , Myrtha Gregoire-Bottex , Jaime E. Hale , Elinor Langfelder-Schwind , Álvaro La Parra Perez , Karen Maguiness , John Massie , Erin McElroy-Barker , Meghan E. McGarry , Angelique Mercier , Anne Munck , Kathryn E. Oliver , Staci Self , Kathryn Singh , Michael Smiley , Steven Snodgrass , Audrey Tluczek , Pamela Tuley , Paula Lomas , Elise Wong , Sarah E. Hempstead , Albert Faro , Clement L. Ren; Cystic Fibrosis Foundation Evidence-Based Guideline for the Management of CRMS/CFSPID. Pediatrics 2024; e2023064657. 10.1542/peds.2023-064657
Download citation file:
- Ris (Zotero)
- Reference Manager
Video Abstract
A multidisciplinary committee developed evidence-based guidelines for the management of cystic fibrosis transmembrane conductance regulator-related metabolic syndrome/cystic fibrosis screen-positive, inconclusive diagnosis (CRMS/CFSPID). A total of 24 patient, intervention, comparison, and outcome questions were generated based on surveys sent to people with CRMS/CFSPID and clinicians caring for these individuals, previous recommendations, and expert committee input. Four a priori working groups (genetic testing, monitoring, treatment, and psychosocial/communication issues) were used to provide structure to the committee. A systematic review of the evidence was conducted, and found numerous case series and cohort studies, but no randomized clinical trials. A total of 30 recommendations were graded using the US Preventive Services Task Force methodology. Recommendations that received ≥80% consensus among the entire committee were approved. The resulting recommendations were of moderate to low certainty for the majority of the statements because of the low quality of the evidence. Highlights of the recommendations include thorough evaluation with genetic sequencing, deletion/duplication analysis if <2 disease-causing variants were noted in newborn screening; repeat sweat testing until at least age 8 but limiting further laboratory testing, including microbiology, radiology, and pulmonary function testing; minimal use of medications, which when suggested, should lead to shared decision-making with families; and providing communication with emphasis on social determinants of health and shared decision-making to minimize barriers which may affect processing and understanding of this complex designation. Future research will be needed regarding medication use, antibiotic therapy, and the use of chest imaging for monitoring the development of lung disease.
Cystic fibrosis (CF) newborn screening (NBS) has been offered in many countries around the world and in every US state since 2010. 1 An unintended consequence of CF NBS is the detection of infants with an abnormal CF NBS result but inconclusive diagnostic testing, which has been termed CF transmembrane conductance regulator ( CFTR )-related metabolic syndrome (CRMS) in the United States and CF screen-positive, inconclusive diagnosis (CFSPID) in other parts of the world. 2 Many people with CRMS/CFSPID are healthy, but a small proportion (<10%) will be reclassified as CF because of an updated annotation of their CFTR variants as CF-causing or an increase in sweat chloride concentration (sweat [Cl - ]) to ≥60 mmol/L(2). Approximately 10% will also develop clinical features that are concerning for CF (eg, pulmonary disease, Pseudomonas aeruginosa [ Pa ] in a respiratory culture). 2
In 2009, the Cystic Fibrosis Foundation (CFF) convened an expert panel to develop consensus recommendations for the management of infants with CRMS. 3 There have been multiple studies of CRMS/CFSPID outcomes and genetics 2 , 4 since then; thus, a diverse committee of CF providers and parents of people with CRMS/CFSPID was assembled in 2021 to develop an up-to-date, evidence-based guideline for the management and care of people with CRMS/CFSPID.
This guideline is intended to be used by both CF specialists and primary care providers (PCPs) who care for people with CRMS/CFSPID and their families. It should supplement the standard care provided in primary care. The guideline will not address how CRMS/CFSPID is diagnosed, nor the criteria for reclassifying people with CRMS/CFSPID as people with CF (pwCF) because these recommendations already exist; 2 , 5 however, it will address genetic testing to better refine the diagnosis. The European Cystic Fibrosis Society (ECFS) recently published a consensus guidance document concerning the management of children with CRMS/CFSPID. 6 The current guideline is evidence-based and is intended to complement the ECFS paper.
CFF intends for this guideline to summarize data and provide reasonable clinical recommendations to clinicians, patients, and other stakeholders. The application of these recommendations should not be mandated. Care decisions regarding individual patients should be made by using a combination of these recommendations, an associated benefit–risk assessment of the treatment options, the patient’s individual and unique circumstances, and the goals and preferences of the patients and families that the team serves as a part of shared decision-making (SDM) between the patient and clinician.
CFF sponsored the creation of the committee. The committee defined people with CRMS/CFSPID as people with an abnormal CF NBS result and (1) a sweat [Cl - ] of <30 mmol/l (normal) and 2 CFTR variants, at least 1 of which with unclear phenotypic consequences, or (2) sweat [Cl - ] of ≥30 to 59 mmol/L (intermediate value) and 1 or no CF-causing variants. 2 An online survey was sent to CF care centers (CFCC) and the families of people with CRMS/CFSPID to identify high-priority issues for both groups. Based on survey results, input from the committee, and areas of further research identified in previous guidelines, 24 questions were written in patient, intervention, comparison, outcome (PICO) format. 7 A systematic review was performed by using PubMed and the Cumulative Index to Nursing and Allied Health Literature databases. Literature review and evidence grading were performed by 4 working groups: genetic testing, monitoring, treatment, and psychosocial and communication issues. The groups generated recommendations that were graded by using the US Preventive Services Task Force (USPSTF) definitions ( Table 1 ). 8 The committee adhered to the USPSTF Procedure Manual 9 in generating and reviewing 31 specific recommendations. Statements that received ≥80% consensus among the committee were approved, resulting in 30 final recommendations ( Table 2 ) and 1 non-consensus statement ( Table 3 ). Details of the committee selection, PICO framework, search terms, and recommendation statements are available in the Supplemental Information and Supplementary Table 5 .
USPSTF Guideline Recommendations 8
Consensus Recommendation Statements: 30 Statements That Made Consensus
Non-Consensus Recommendation Statement: 1 Statement That Did Not Make Consensus

Additional CFTR Genetic Testing
The CFF recommends that people with CRMS/CFSPID who have <2 disease-causing variants identified by NBS should undergo sequencing of the coding and flanking regions and deletion/duplication (del/dup) analysis of the coding and exon flanking regions of CFTR (Grade B).
Establishing the CFTR genotype of people with CRMS/CFSPID is important for diagnosis, monitoring, and genetic counseling and, should reclassification to CF occur, may also be useful for the approval of CFTR modulators. Genotyping requires a stepwise approach that is dependent on the state’s NBS algorithm. A glossary of genetic terms is available in Supplemental Table 6 . Further genetic analysis is not necessary when 2 CFTR variants identified by NBS are confirmed to be in trans (1 inherited from each parent). However, sequencing of the coding and flanking intronic regions of CFTR, with del/dup analysis to evaluate for large structural variants, such as exon deletions, is useful in the diagnostic workup. 10 It should be performed when people with CRMS/CFSPID have only 1 or no identified disease-causing CFTR variants from NBS.
2. The CFF recommends, for people with CRMS/CFSPID, selectively offering full-gene CFTR sequencing including intronic regions when the CFTR genotype remains incomplete after coding and flanking region sequencing and del/dup (Grade C).
CFTR sequencing that includes all intronic regions has identified putatively causal variants among individuals with CF, CFTR -related disorders, and positive CF NBS results. 18 , – 23 Tests that include many flanking nucleotides and several deep intronic variants are fairly comprehensive; additional testing of all other intronic regions may offer little additional value or sensitivity. Full intronic sequencing should be selectively performed in people with CRMS/CFSPID who demonstrate evidence of potential CFTR dysfunction (eg, sweat [Cl-] of 30–59 mmol/L), or individuals for whom the clinical suspicion of CF development is high and who possess only 1 previously identified causal variant after full sequence and del/dup is provided (see Supplemental Information for further discussion). Availability of full CFTR sequencing is currently limited; this service may become more widely available in the future.
CFTR Testing in Family Members
3. The CFF recommends CFTR genetic evaluation for parents of people with CRMS/CFSPID when phasing the CFTR variants (ie, in cis or trans ) would inform the diagnostic status of the individual by confirming the inheritance pattern (Grade A).
4. The CFF recommends offering CFTR genetic evaluation for siblings of people with CRMS/CFSPID (Grade B).
For people with CRMS/CFSPID who have 2 identified CFTR variants, determining if the variants reside within the same copy (in cis ) or different copies (in trans ) of CFTR is achieved through the genetic testing of at least 1 first-degree relative, which is referred to as “phasing.” CFTR variants in cis can lead to diagnosis of CF carrier instead of CRMS/CFSPID, 21 , 24 which bears health and reproductive implications for the individual and family members.
Evaluating the siblings of people with CRMS/CFSPID can identify at-risk individuals who may also benefit from clinical monitoring and follow-up. 25 , 26 This assessment should be offered to families and promotes SDM with emphasis on the risks and benefits to the individual sibling. 6 In many instances, this may be delayed until childbearing age and at a time the sibling may also share in the decision-making regarding the value of this genetic information.
Genetic Counseling
5. The CFF recommends, for families of people with CRMS/CFSPID, that health care professionals (HCPs) providing genetic counseling should have training or clinical expertise in CF and genetics. A licensed or certified genetic counselor (GC) should be accessible to families of people with CRMS/CFSPID for further support, including discussions regarding future reproductive decision-making (Grade B).
Published recommendations affirm that genetic counseling should be offered to the families of people with CRMS/CFSPID. 6 , 27 , 28 The genetic counseling provider, whether they are a licensed or certified GC or a CF clinician, should have a high level of expertise in both CF genetics and CRMS/CFSPID. 27 Some families feel more comfortable discussing these topics with providers outside the CFCC. 29 , 30 Having access to a trained GC to discuss genetic findings and complement team members in providing psychosocial support promotes understanding 3 , 6 and strengthens long-term retention of genetic knowledge. 31 Genetic counseling at regular intervals throughout the lifespan allows for timely, accurate, supportive, and nondirective information on recurrence risk and reproductive options. 29
Frequency of Follow-Up
6. The CFF recommends, for people with CRMS/CFSPID, at least annual follow-up by a CF clinician and nurse, with an initial assessment to include a social worker, a mental health coordinator (MHC), and/or a genetic counseling provider. Continued follow-up by a social worker, MHC, and/or genetic counselor should be part of the care of CRMS/CFSPID, depending on the needs of that individual and family (Grade B).
The primary goal of monitoring people with CRMS/CFSPID is to detect those individuals who may be reclassified as CF and would benefit from care at a CFCC. Equally important is avoiding the overmedicalization of otherwise healthy individuals, which involves its own set of adverse consequences. Families should be informed about possible outcomes for people with CRMS/CFSPID and plans for future monitoring visits. Communication with PCPs is essential and should highlight reasons for more frequent reassessment by CFCC (eg, persistent cough, constipation, or inadequate weight gain). Routine follow-up should be provided by clinicians with expertise in CF and CFTR genetics to reexamine CFTR variants (eg, periodic assessment of the CFTR2 database) and changes in clinical status. Because most people with CRMS/CFSPID are healthy, evaluation by an entire multidisciplinary team is often unnecessary.
Sweat Chloride Testing (SCT)
7. The CFF recommends for people with CRMS/CFSPID to repeat SCT at 6 months of life and annually, at least until age 8 years (Grade B).
SCT is the mainstay for diagnosing CF and part of the diagnostic criteria for CRMS/CFSPID. One reason for reclassification of CRMS/CFSPID to CF is a sweat [Cl - ] of >60 mmol/L. The authors of multiple studies have reported sweat [Cl - ] elevation above this level after an initial sweat [Cl - ] of <60 mmol/L during the newborn period. 16 , 30 , 32 , – 39 However, evidence is lacking regarding changes in sweat [Cl - ] after 8 years of age, and SDM with parents should be used to determine if SCT should continue past this age. Careful consideration for continued SCT may be given to certain populations, including (1) individuals with initial sweat [Cl - ] of 40 to 59 mmol/L because they are up to 10 times more likely to have a sweat [Cl-] elevation of >59 mmol/L in later childhood 33 , 40 and (2) individuals with sweat [Cl - ] increasing at a high rate over time (>5 mmol/L per year). 16
Respiratory Cultures
8. The CFF recommends, for people with CRMS/CFSPID, selectively offering CF respiratory cultures at each visit (at least until age 8 years) and as clinically indicated for respiratory symptoms (Grade C).
Airway infection with CF-associated microorganisms, specifically Pa , is considered a phenotypic feature supporting a CF diagnosis. Compared with the general population, people with CRMS/CFSPID exhibit a higher prevalence of Pa (10.7% to 78.6%), Stenotrophomonas maltophilia (4.9% to 10%), and Staphylococcus aureus (40% to 85%) in respiratory cultures. 30 , 41 , 42 Some studies have revealed that people with CRMS/CFSPID who reclassify to CF more likely have a positive culture result for Pa than those who do not reclassify (33% vs 10%), whereas other reports have revealed no difference. 30 , 35 However, a positive culture result alone does not warrant reclassification. Some families and physicians may not want to expose the individual to trauma associated with cultures. Respiratory culture data from people with CRMS/CFSPID >8 years of age are currently lacking. If persistent respiratory symptoms are present, obtaining respiratory cultures in people with CRMS/CFSPID may be warranted. Thus, the evidence supports that cultures obtained via throat swab or sputum should be selectively offered after SDM with the family.
Laboratory Testing
9. The CFF recommends, for people with CRMS/CFSPID, that measurement of fecal elastase (FE) should be performed at the initial assessment. Further testing of FE can be provided when clinically appropriate (Grade B).
10. The CFF recommends against routine laboratory evaluations, including fat-soluble vitamin testing, liver function testing, glucose monitoring, and blood counts for people with CRMS/CFSPID (Grade D).
Measuring FE at the initial assessment is important to evaluate for exocrine pancreatic function because pancreatic insufficiency is a clinical feature of CF. FE levels can fluctuate in the first year of life; therefore, a single abnormal FE level should be interpreted cautiously, and repeat testing may be warranted 43 if symptoms of steatorrhea or failure to thrive develop. Most people with CRMS/CFSPID exhibit normal growth and nutrition and are pancreatic sufficient; thus, the presence of pancreatic insufficiency would strongly support reclassification to CF. 35 , 44 , 45
There is no evidence that laboratory results, such as electrolyte concentrations or liver function tests, are abnormal in people with CRMS/CFSPID. 35 Excessive testing leads to overmedicalization and increased cost of care. Therefore, laboratory tests should only be considered when clinically indicated.
Pulmonary Function Testing
11. The CFF recommends against routine pulmonary function testing (PFT; ie, spirometry, multiple-breath washout) for people with CRMS/CFSPID (Grade D).
Spirometry and multiple-breath washout are normal in people with CRMS/CFSPID 4 , 33 , 35 and do not affect reclassification to CF. PFTs should be considered if clinical concern for respiratory disease arises, and if abnormal, may support reclassification to CF.
Radiographic Imaging
12. The CFF recommends against routine chest radiographs for people with CRMS/CFSPID (Grade D).
Chest imaging is normal in people with CRMS/CFSPID within the first years of life 35 and does not inform reclassification to CF. Chest radiographs should be considered if clinical concerns arise.
Infection Prevention and Control
13. The CFF recommends, for people with CRMS/CFSPID, the implementation of standard CF infection prevention and control (IPC) guidelines in health care settings and situations in which there is a high likelihood of being in close contact with multiple pwCF or people with CRMS/CFSPID (Grade B).
The acquisition of Pa may guide reclassification to CF, and there is a risk that exposure to pathogens commonly associated with CF may negatively impact the individual. 46 This risk is most substantial in the health care setting in which close contact could occur among multiple pwCF or people with CRMS/CFSPID. IPC guidelines have been adopted by CF programs with minimal negative impact. 47 The implementation of IPC guidelines in non-health care settings, such as schools, may have significant negative psychological effects that outweigh potential benefits and are not recommended.
Infectious Disease Interventions
14. The CFF recommends, for people with CRMS/CFSPID, selectively offering inhaled antibiotics for the treatment of Pa based on a positive respiratory culture (Grade C).
15. The CFF recommends, for people with CRMS/CFSPID and an unexplained prolonged cough (>2 weeks), selectively offering the use of oral antibiotics (Grade C).
A higher prevalence of Pa is reported in respiratory cultures from people with CRMS/CFSPID compared with those collected from children without CF, 2 , 4 , 41 , 42 and treatment to eradicate incident Pa infection is recommended for pwCF. 48 However, the clinical impact of Pa in respiratory cultures from people with CRMS/CFSPID is unclear. Pa may clear spontaneously, (ie, independent of whether treatment is provided), 49 calling into question the value of Pa eradication therapy. The committee recommends selectively offering this treatment after a SDM process incorporating the potential benefits, risks, and treatment burden of inhaled antibiotic therapy in people with CRMS/CFSPID with positive respiratory culture for Pa . If the decision is to treat, then follow-up cultures are warranted to ensure Pa clearance.
Precedent is established regarding use of oral antibiotics for prolonged cough for individuals with respiratory conditions other than CF, including tracheobronchomalacia, protracted bacterial bronchitis, non-CF bronchiectasis and neuromuscular disease. 50 , – 54 The committee discussed this consideration while weighing the potential benefits and harms of antibiotic therapy. The use of any antibiotic for eradication or treatment may depend on clinical status of the individual, and the risks and benefits of treatment burden should be assessed for each family. For people with CRMS/CFSPID with a cough lasting >2 weeks, other etiologies of persistent cough, such as asthma, should be considered.
Nutritional Interventions
16. The CFF recommends, for people with CRMS/CFSPID with adequate growth, that nutritional management be provided under the direction of the PCP (Grade B).
17. The CFF recommends, for people with CRMS/CFSPID with a downward trajectory of weight-for-age percentile or z-score (eg, crossing percentiles), that screening and evaluation be provided by a dietitian with experience in the management of CRMS/CFSPID and CF (Grade B).
18. The CFF recommends against salt supplementation for people with CRMS/CFSPID (Grade D).
19. The CFF recommends against the use of fat-soluble vitamins for people with CRMS/CFSPID (Grade D).
No evidence exists to support the use of supplemental nutrition, caloric modifications, or CF-specific diets for people with CRMS/CFSPID who are exhibiting normal growth. 55 A downward growth trajectory, as measured by weight-for-age percentile or weight z-score, may be a sign of reclassification to CF. Children who demonstrate this pattern should undergo nutritional screening and appropriate intervention by dietitians with experience in CRMS/CFSPID and CF. Given that people with CRMS/CFSPID have intermediate or normal sweat [Cl - ], excessive salt loss is not expected, and potentially harmful consequences could be conferred by a high-salt diet. 56 , 57 No evidence suggests the need for supplementing fat-soluble vitamins to people with CRMS/CFSPID 35 because deficits in these vitamins have not been observed, and routine laboratory tests are not being monitored.
Pulmonary Interventions
20. The CFF recommends against the routine use of airway clearance therapy (ACT) for people with CRMS/CFSPID (Grade D).
21. The CFF recommends for people with CRMS/CFSPID experiencing new respiratory symptoms selectively offering the use of ACT (Grade C).
22. The CRMS/CFSPID guideline committee recommends against the use of CFTR modulators for people with CRMS/CFSPID (Grade D).
23. The CFF has determined that there is insufficient evidence to recommend for or against the use of medications usually used to treat CF respiratory symptoms for people with CRMS/CFSPID (Grade I).
Routine ACT and its effects on outcomes, such as bronchiectasis, are unclear for people with CRMS/CFSPID; hence, daily use is not recommended. ACT has been recommended for the management of acute respiratory symptoms for pediatric patients with other pulmonary conditions 58 , 59 ; thus, for people with CRMS/CFSPID and new respiratory symptoms, ACT may be considered. Initiation should be discussed with the family because ACT may add a significant burden. The use of cost-effective methods (such as manual percussion) should be attempted.
No studies have been reported in which the authors investigated the outcomes of CFTR modulator therapies among people with CRMS/CFSPID, and potential adverse effects are associated with these medications. 60 , – 62 If people with CRMS/CFSPID develop signs and symptoms warranting the use of CFTR modulators, reclassification to CF may be justified.
Medications that are commonly used for CF lung disease, 63 , 64 (ie, dornase alfa, hypertonic saline, and low-dose azithromycin) have not been evaluated in people with CRMS/CFSPID. The potential benefits of these medications for people with CRMS/CFSPID are unclear because the degree of CFTR dysfunction in CRMS/CFSPID may not necessarily result in significant changes in airway surface liquid. Weighed against the risks of medical complexity, treatment burden, and cost, there is insufficient evidence to either recommend for or against use of these medications. For people with CRMS/CFSPID with chronic respiratory symptoms that would require consideration of therapy, the reassessment of CF is warranted.
Communication With Families
24. The CFF recommends, for people with CRMS/CFSPID, that HCPs assess and consider social determinants of health (SDOH) that can influence the understanding and psychological impact of a CRMS/CFSPID diagnosis and tailor communications appropriately (Grade B).
25. The CFF recommends, for people with CRMS/CFSPID, that HCP tailor communication about CFTR variants based on SDM to minimize psychological, cognitive, and other barriers to processing and understanding genetic information (Grade B).
26. The CFF recommends, for people with CRMS/CFSPID, that clear, concise, consistent, and timely information about the uncertainty related to the CRMS/CFSPID diagnosis is provided using family-centered communication strategies (Grade B).
27. The CFF recommends, for people with CRMS/CFSPID and their families, that gradual, clear, and consistent, verbal and written, developmentally appropriate education about CRMS/CFSPID is provided at diagnosis, at follow-up visits, and at the time of reproductive decision-making (Grade B).
The psychological impact of the uncertainty associated with CRMS/CFSPD is challenging for parents and highlights the need for consistent and clear communication between families, patients, and HCP. 65 , 66 SDOH can influence a persons’ understanding of a diagnosis based on previous knowledge, emotional state, genetic and health literacy, and perceptions of test results. 66 , – 69 Screening for SDOH will identify social needs for which CFCC can provide links to further services. Parental learning preferences should be considered. Qualified medical language interpreters should be involved unless the provider is certified as a proficient interpreter in the primary language of the people with CRMS/CFSPID and their caregivers.
Perceived lack of knowledge of the person communicating NBS results has been linked to parental distress. 70 Clear, concise, and consistent information with plans for future follow-up is necessary for parents managing the uncertainty associated with the diagnosis of CRMS/CFSPID. 2 , 65 Accurate information, with reassurance and without conjecture, is critical for moderating parental response to this diagnosis. 66 , 71 Educational tools have been developed to assist HCP when communicating information to families the uncertainty of other diagnoses. 70 Additional information on this topic is available in the Supplemental Information .
Communication With Primary Care
28. The CFF recommends, for people with CRMS/CFSPID, that the PCPs and other HCPs involved in the care of individuals with CRMS/CFSPID receive accurate and up-to-date education about CRMS/CFSPID, its management, and the state’s NBS program (Grade B).
When communicating with parents and HCPs, it is important to provide information regarding further symptoms that may arise and when to refer to a CFCC. Because NBS algorithms vary by state, 1 CFCCs should serve as a resource with current information for PCPs to help educate and clarify any CFTR results, SCT, or other evaluations that pertain to CRMS/CFSPID. 3 , 6
Screening for Depression and Anxiety
29. The CFF recommends that at least 1 primary caregiver of people with CRMS/CFSPID be offered screening for depression and anxiety annually (Grade B).
30. The CFF recommends, for people with CRMS/CFSPID aged 12 years and older still being followed by CFCC, that screening for depression and anxiety be provided annually (Grade B).
The International Committee on Mental Health in Cystic Fibrosis 72 and the USPSTF screening guidelines 73 , 74 recommend annual screening for depression and anxiety in people aged 12 years and older. To be consistent with guidelines, the committee recommends utilizing the Patient Health Questionnaire-9 and Generalized Anxiety Disorder-7 forms. A team member with expertise and training in mental health should be identified to implement screening, follow-up, and referral. 72 Although there is insufficient evidence to recommend routine screening for anxiety and depression in people with CRMS/CFSPID aged 7 to 11 years, this population should be clinically evaluated for these problems when significant symptoms or behavioral concerns are reported or if caregiver depression or anxiety scores are elevated.
Mothers of people with CRMS/CFSPID exhibit increased rates of anxiety, depression, and postpartum depression that are comparable to rates detected among mothers of pwCF. 75 News of the CRMS/CFSPID designation may create a state of cognitive uncertainty regarding the nature of the diagnosis and prognosis. This mental state contributes to clinically significant distress for parents and caregivers 76 , – 78 like that experienced by parents of pwCF. 66 Discussions should be family-centered and may include asking about the caregiver’s emotional and mental health concerns and SDOH, racism, poverty, and relational health. 73 , 79 , 80
The committee was unable to reach a consensus of 80%, with 20 voting for recommendation (74%) and 7 voting against a recommendation for selectively offering chest computed tomography (CT) scans. There are few reports of chest CT imaging in people with CRMS/CFSPID, 35 , 49 , 81 , 82 and abnormal findings are rare. Many members of the committee felt that chest CTs are not routinely recommended for pwCF and should not be recommended for people with CRMS/CFSPID.
Although numerous studies of CRMS/CFSPID have been published since 2009, most encompass single-center analyses with relatively small cohorts. The CFF Patient Registry (CFFPR) contains many people with CRMS/CFSPID and has been used to study CRMS outcomes. However, there is no requirement for inclusion in the CFFPR, and many people with CRMS/CFSPID are not represented in this database. No randomized clinical trials of people with CRMS/CFSPID have been reported, and it is unlikely that such future studies will be performed, all of which affect the strength of the present recommendations. Enrolling more people with CRMS/CFSPID into CFFPR will be a primary way to develop larger multicenter cohort studies and better guide management.
Genetic testing technology is continually advancing. Tests with limited availability now (eg, compete sequencing of the intronic regions of CFTR ) may become more widely available in the future. Establishing the disease liability of VVCCs and variants of uncertain significance represents an unmet need that may help assess the risk of reclassifying CRMS/CFSPID to CF, as well as guide the selection of CFTR variants in NBS algorithms. Knowledge of state NBS algorithms and access to further genetic testing will be essential because these platforms continue to vary across the United States. 1 People with CFTR -related disorder have CFTR and SCT results similar to people with CRMS/CFSPID, 83 but the likelihood of people with CRMS/CFSPID developing into CFTR -related disorder is currently unknown. Pursuing this area of research is also an important area for future research.
Working closely with PCPs is a mainstay of care for people with CRMS/CFSPID. Table 4 is a suggestion for the continued monitoring and care of people with CRMS/CFSPID and can be employed at both specialty care and primary care. Determining an appropriate time to discharge from care at a specialized center will require SDM with the people with CRMS/CFSPID, family, specialty providers and PCPs. Guidance regarding when to refer back to specialty clinics (eg, change in respiratory symptoms) needs to be developed.
Monitoring and Care for the Person With CRMS/CFSPID
A, annually perform; C, consider; P, perform. —, not needed at this age.
a If still being followed at CFCC.
We present this evidence-based guideline for the management of CRMS. Most of the recommendations were grade C because of the limited data that are currently available. As more people with CRMS/CFSPID are followed for longer periods of time, reassessment of these recommendations will be required. Additionally, research is needed to assess clinical benefits of treating pulmonary symptoms with medications commonly used for CF lung disease, antibiotic therapy for Pa in respiratory cultures, and the use of PFTs or chest imaging for monitoring development of lung disease in people with CRMS/CFSPID.
Drs Green and Ren were co-chairs of the guideline committee, conceptualized the initial survey questions, outlined community concerns and topics to address, authorized and had final selection of committee members, participated in working group meeting and leadership meetings, drafted the initial manuscript, and critically reviewed and revised the manuscript; Drs Lahiri, Ruiz, and Spano and Ms Raraigh were the working group leads, reviewed data, conducted monthly meetings to revise statements, drafted initial sections of the manuscript, and critically reviewed and revised the manuscript; Drs Antos, Christon, Gregoire-Bottex, La Parra Perez, Massie, McGarry, Munck, Oliver, Smiley, and Snodgrass and Ms Bonitz, Ms Hale, Ms Langfelder-Schwind, Ms Maguiness, Ms McElroy-Barker, Ms Mercier, Ms Self, Ms Singh, Ms Tluczek, and Ms Tuley were assigned specific PICO questions to review and abstract data, participated in the final voting meeting for statement inclusion and grading, and critically reviewed and revised the manuscript; Ms Lomas, Ms Wong, Ms Hempstead, and Dr Faro critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The guidelines and recommendations in this article are not American Academy of Pediatrics policy, and publication herein does not imply endorsement.
airway clearance therapy
cystic fibrosis
cystic fibrosis care centers
Cystic Fibrosis Foundation
CFF Patient Registry
cystic fibrosis screen-positive, inconclusive diagnosis
cystic fibrosis transmembrane conductance regulator
CFTR-related metabolic syndrome
computed tomography
deletion/duplication
European Cystic Fibrosis Society
fecal elastase
genetic counselor
health care professional
infection prevention and control
mental health coordinator
newborn screening
Pseudomonas aeruginosa
primary care provider
pulmonary function testing
patient, intervention, comparison, outcome
people with cystic fibrosis
shared decision-making
social determinants of health
sweat chloride concentration
US Preventive Services Task Force
variants of varying clinical consequence
Competing Interests
Supplementary data.
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Pulm Med

An adult cystic fibrosis patient presenting with persistent dyspnea: case report
Gary m onady.
1 Medicine-Pediatrics Program, Boonshoft School of Medicine, Wright State University, Suite 500 Elizabeth Place, Dayton, OH, 45408, USA
Catherine L Farinet
2 Piketon Medical Center, 10 Indian Ridge Drive, Suite 1, Piketon, OH 45661, USA
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Persistent dyspnea is a common finding in the cystic fibrosis patient that typically leads to further work up of an alternative pulmonary etiology. Adult cystic fibrosis patients; however, are growing in numbers and they are living into the ages in which coronary artery disease becomes prevalent. Coronary disease should be included in the consideration of diagnostic possibilities.
Case presentation
A 52-year-old white male with cystic fibrosis was evaluated for exertional dyspnea associated with vague chest discomfort. Diagnostic testing revealed normal white blood cell, hemoglobin and platelet count, basic metabolic panel, fasting lipid profile, HbA1c, with chest radiograph confirming chronic cystic findings unchanged from prior radiographs and an electrocardiogram that revealed sinus rhythm with left anterior fascicular block. Stress thallium testing demonstrated a reversible anteroseptal perfusion defect with a 55% left ventricular ejection fraction. Heart catheterization found a 99% occlusion of the left anterior descending artery extending into the two diagonal branches, with 100% obstruction of the left anterior descending artery at the trifurcation and 70% lesion affecting the first posterior lateral branch of the circumflex artery.
This case report represents the first description in the medical literature of a cystic fibrosis patient diagnosed with symptomatic coronary artery disease. Applying a standard clinical practice guide proved useful toward evaluating a differential diagnosis for a cystic fibrosis patient presenting with dyspnea and chest discomfort.
Cystic Fibrosis (CF) is the most common lethal inherited disease in the Caucasian population. It was once considered a childhood disease; however, with advances in health care there is a growing population of adults with CF [ 1 ]. A life expectancy that was only 8 years of age in 1974 had advanced to the age of 21 years in 1994, and today is estimated as high as 40 years of age [ 2 ]. There are patients on the Cystic Fibrosis Foundation Patient Registry in 2003 that are in their eighth decade of life [ 1 ].
With new found longevity comes a new spectrum of disease prevalence associated with aging. Cystic Fibrosis Related Diabetes (43% prevalence) is now the number two chronic illness in the adult CF patient following chronic lung disease and surpassing liver disease (24% prevalence) in patients greater than 30 years of age [ 3 ]. Cardiovascular disease has been essentially isolated to cor pulmonale as a consequence of end stage obstructive pulmonary disease. Hypertension has not been considered a serious problem in this patient population [ 4 ]; however, a 20% prevalence has been observed at our CF adult center. Symptomatic coronary artery disease, one of the most prevalent of diseases in the adult patient population, has never been reported in the CF population from a PubMed literature search to date. Guides for care of health concerns in adult patients with CF were published in a January 2004 Consensus Report to help transition CF health care from pediatrician to internists or other adult care providers [ 3 ]. Many aspects of cardiovascular discussions such as hypertension and forms of heart disease separate from cor pulmonale are missing from this consensus report. The following discussion illustrates the need to continually update and add new information that will lead to optimizing the care of adult CF patients.
A 52-year-old white male with CF presented with persistent exertional dyspnea and cough with scant sputum production. Physical examination demonstrated oxygen saturation of 91%, with normal temperature and vital signs. His weight had fallen from 79.2 to 77.1 kg over 3 months. Cardiovascular exam was entirely normal. Lungs demonstrated coarse breath sounds bilaterally with scattered rales throughout the lung fields. The abdomen was normal, extremities demonstrated significant clubbing and there was no peripheral edema. Forced expiratory volume at 1 second (FEV 1 ) had decreased from 50% to 36%. The chest radiograph resembled baseline findings with no obvious infiltrate or pneumothroax. He received a month-long course of azithromycin, aztreonam and inhaled tobramycin for suspected pulmonary exacerbation of acute super-infection in the setting of underlying chronic CF lung disease.
One month later, follow-up revealed no improvement in dyspnea and no change in the scant sputum production, despite full adherence to the antibiotic regimen and airway clearance techniques. Exertional discomfort located along the sternum and left anterior chest associated with dyspnea without radiation or pressure sensation was further described but was not associated with palpitations, diaphoresis or nausea. This discomfort improved with rest and was not associated with meals. He did report the need to sleep upright in a recliner, but denied paroxysmal nocturnal dyspnea.
CF was diagnosed in this patient by sweat chloride at age 35 after an episode of hemoptysis, with subsequent genetic analysis identifying a ΔF508, 2789+5G>A mutation. He also has gastroesophageal reflux, Barrett's esophagus, azoospermia, and pancreatic insufficiency. He had sinus surgery at age 47. Medications include albuterol, ipratropium, fluticasone, dornase alpha, salmeterol, omeprazole, pancreatin and multivitamin. The patient's father had a myocardial infarction at age 37, his mother had coronary artery bypass surgery at 52 years of age, and a brother underwent coronary bypass at age 61. The patient has never smoked and alcohol intake was minimal.
Diagnostic testing demonstrated normal white blood cell, hemoglobin and platelet count. Electrolytes, albumin, protein and glucose were normal with a 5.1% HbA1c. Total cholesterol was 139 mg/dl, LDL 80 mg/dl, HDL 30 mg/dl and triglycerides at 76 mg/dl. Chronic findings with cystic changes were evident on chest radiograph, but with no obvious consolidation. Electrocardiogram revealed sinus rhythm with a left anterior fascicular block and normal ST findings.
Stress thallium testing was subsequently arranged within a week of the follow up visit, with results positive for moderate anteroseptal area of reversible perfusion with a left ventricular ejection fraction of 55%. Subsequent heart catheterization revealed 99% occlusion of the left anterior descending artery with extension into the two diagonal branches, 100% obstruction of the left anterior descending artery at the trifurcation and 70% lesion affecting the first posterior lateral branch of the circumflex artery. The patient was evaluated for possible coronary artery bypass graft; however, because of his current pulmonary status, angioplasty was elected with successful stenting of the left anterior descending artery. On follow up one year out from stent placement, the patient remained asymptomatic with exercise tolerance and pulmonary function returning to baseline.
This is the first report of a cystic fibrosis patient diagnosed with symptomatic coronary artery disease (CAD) and acknowledges that adult cystic fibrosis patients have indeed survived into the years were coronary artery disease becomes prevalent. Therefore, the likelihood of coronary disease should be included in the diagnostic consideration of persistent dyspnea associated with chest discomfort by applying the same standards used in grading a differential with an anginal presentation in non-CF patients [ 5 ].
The patient presented in this case had a Framingham score estimate that predicted a 10-year cardiovascular risk at 4% [ 6 ]. However, because of the presence of subtle chest discomfort associated with dyspnea, medical decision making includes an active alternative diagnosis of atypical angina based on two of three positive criteria of exertional symptoms with symptom relief upon resting [ 5 ]. Persistent dyspnea may be an occasional finding for a cystic fibrosis patient, and one that typically leads to further work up of a pulmonary etiology; however, cardiovascular disease was additionally considered in this patient.
The typical differential diagnosis of persistent dyspnea in an adult cystic fibrosis patient would include pneumothorax (5% prevalence) [ 7 ], atypical mycobacterial pneumonia (15% prevalence) [ 8 ], allergic bronchopulmonary aspergillosis (30% prevalence) [ 9 ], and cor pulmonale (3% prevalence in association with severe CF pulmonary disease) [ 10 ]. Without a history for hemoptysis, atypical mycobacterium and allergic bronchopulmonary aspergillosis would be less likely. Physical exam and chest radiograph did not support either pneumothorax or congestive heart failure. Symptoms could also be explained by this patient's progression of gastroesophageal reflux and/or Barrett's esophagus.
Atypical angina would be the most prevalent differential diagnosis, at 60%, for our patient's clinical presentation of chest pain in the non-CF male patient at this age [ 5 ]. The risk of CAD is not typically considered as an active alternative diagnosis for the CF patient; yet with aging, even CF patients will be at risk for CAD. This risk may even be greater given higher prevalence of known risk factors such as diabetes mellitus in the CF versus non-CF patient [ 11 ].
Two cases of asymptomatic coronary artery disease have been reported in association with CF with some advanced detail. One case came to diagnosis at autopsy, characterized as generalized atherosclerosis in a 41-year-old female CF patient [homozygous G542X] with diabetes mellitus that died from respiratory failure [ 12 ]. Further review of this case revealed a 200 mg/dl averaged cholesterol level, progressive hypertension with biopsy proven nephrosclerosis by 31-years of age, and a diabetic course complicated by gastrointestinal pseudoparesis, retinopathy and neuropathy. In this case, coronary artery disease was an incidental finding on autopsy as she was asymptomatic for myocardial ischemia during her lifetime.
A second case was described in which segmental hypokinesis with grade 2 systolic function was found as an incidental finding on Doppler echocardiography from a prevalence study looking at pulmonary hypertension that included an adult cystic fibrosis patient population [ 10 ]. This study identified a 40-year-old diabetic male, diagnosed with asymptomatic coronary artery disease after performing a thallium perfusion scan. Further testing by cardiac catheterization was not reported in this study.
What about other coronary risk factors? Cholesterol and hypertension were not identified risk factors present in our patient. The autopsy case report had several risk factors present, most notably, diabetes and hypertension, but with only a borderline elevated cholesterol level. What is the expected lipid level in a CF patient? The largest lipid study conducted on a CF population reported 134 ± 84 mg/dl triacylglycerol and 138 ± 84 mg/dl total cholesterol values [ 14 ]. Only 4% of patients had cholesterol levels >200 mg/dl in this study, with a maximum total cholesterol identified at 240 mg/dl. Serum cholesterol levels can be highly variable in CF patients. Patients with pancreatic insufficiency have low to normal cholesterol levels even with a high fat diet and enzyme supplementation as seen in this case [ 15 ]; however, CF patients with pancreatic sufficiency are likely at the same risk as the general population for complications for hyperlipidemia [ 16 ]. Another study of aortic atherosclerosis in CF patients found that they have less fatty streaking of the aorta than their weight matched counterparts [ 17 ]. Clearly there are other factors that may lead to atherosclerosis than lipid levels; however, applying a clinical practice guide published by the American College of Physicians [ 5 ] proved helpful toward diagnosing CAD in this CF patient presenting with dyspnea associated with a chest discomfort.
Abbreviations
CF – cystic fibrosis; FEV 1 – forced expiratory volume at 1 second; LDL – low density lipoprotein; HDL – high density lipoprotein; CAD – coronary artery disease;
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
GMO contributed to the literature review cited in the discussion and editing of the original manuscript. CLF providing medical care for the patient described in this case presentation, and wrote the original manuscript detailing the clinical findings encountered from the clinical assessment. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/1471-2466/6/9/prepub
Acknowledgements
The authors used no additional resources in preparing this manuscript. Written consent was obtained from the patient for publication of this study.
- Cystic Fibrosis Foundation . Patient registry annual data report 2003. Bethesda MD: Cystic Fibrosis Foundation; 2003. [ Google Scholar ]
- Fogarty A, Hubard R, Britton J. International comparison of median age at death from cystic fibrosis. Chest. 2000; 117 :1656–1660. doi: 10.1378/chest.117.6.1656. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodmann D. Cystic fibrosis adult care consensus conference report. Chest. 2004; 125 [ PubMed ] [ Google Scholar ]
- Super M, Irtiza-Ali A, Roberts SA, Scharz M, Young M, Smith A, Roberts T, Hinks J, Heagerty A. Blood pressure and the cystic fibrosis gene: evidence for lower pressure rises with age in female carriers. Hypertension. 2004; 44 :878–883. doi: 10.1161/01.HYP.0000145901.81989.46. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Panzer RJ. Diagnostic strategies for common medical problems, Coronary Artery Disease. American College of Physicians Press, Philadelphia, PA; 1991. pp. 44–45. [ Google Scholar ]
- Framingham point scores US Department of Health and Human Service. National Institutes of Health, National Heart, Lung and Blood Institute. NIH Publication No 01-3305.
- Kuster P, Bender SW, Posselt HG, Stover B, Wiesemann HG. Spontaneous pneumothorax in cystic fibrosis. Monatsschr Kinderheilkd. 1988; 136 :251–255. [ PubMed ] [ Google Scholar ]
- Pierre-Audigier CP, Feroni A, Sermet-Gaudelus I, Le Bourgeois M, Offredo C, Vu-Thien H, Fauroux B, Mariani P, Munck A, Bingen E, Guillemot D, Quesne G, Vincent V, Berche P, Gaillard JL. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J Clin Micro. 2005; 43 :3467–3470. doi: 10.1128/JCM.43.7.3467-3470.2005. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fink JN, Kelly J. Immunologic aspects of granulomatous and interstitial lung diseases and of cystic fibrosis. JAMA. 1992; 268 :2874–2881. doi: 10.1001/jama.268.20.2874. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fraser KL, Tullis E, Sason Z, Hyland RH, Thornley KS, Hanly PJ. Pulmonary hypertension and cardiac function in adult cystic fobrosis. Chest. 1999; 115 :1321–1328. doi: 10.1378/chest.115.5.1321. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hayes FJ, O'Brien C, FitzGerald MX, McKenna MS. Diabetes mellitus in an adult cystic fibrosis population. Ir Med J. 1994; 87 :59–60. [ PubMed ] [ Google Scholar ]
- Schlesinger DM, Holsclaw DS, Fyfe B. Generalized atherosclerosis in an adult with CF and diabetes mellitus [abstract] Eleventh Annual North American Cystic Fibrosis Conference, Nashville, Tennessee, October 23–26, 1997.
- Vizza CD, Lynch JP, Ochoa LL, Richardson G, Trulock IP. Right and left ventricular dysfunction in patients with severe pulmonary disease. Chest. 1998; 113 :576–583. [ PubMed ] [ Google Scholar ]
- Milla C, Parks EF, Schwarzenberg SJ, Moran A. Abnormal lipid concentrations in cystic fibrosis. Am J Clin Nutr. 2002; 75 :1005–1011. [ PubMed ] [ Google Scholar ]
- Stewart C, Wilson DC, Hanna AK, Corey M. Lipid metabolism in adults with cystic fibrosis [abstract] Eleventh Annual North American Cystic Fibrosis Conference, Nashville, Tennessee, October 23–26, 1997.
- Slesinski MJ, Gloninger MF, Costantino JP, Orenstein DM. Lipid levels in adults with cystic fibrosis. J Am Dietetic Assoc. 1994; 94 :402–408. doi: 10.1016/0002-8223(94)90095-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Holman RL, Blanc W, Anderson D. Decreased aortic atherosclerosis in cystic fibrosis of the pancreas. Pediatrics. 1959; 24 :34–39. [ PubMed ] [ Google Scholar ]
Case Study - What is the Relationship Between the Cell Membrane and Cystic Fibrosis?
CF affects more than 30,000 kids and young adults in the United States. It disrupts the normal function of epithelial cells — cells that make up the sweat glands in the skin and that also line passageways inside the lungs, pancreas, and digestive and reproductive systems.
The inherited CF gene directs the body's epithelial cells to produce a defective form of a protein called CFTR (or cystic fibrosis transmembrane conductance regulator) found in cells that line the lungs, digestive tract, sweat glands, and genitourinary system.
When the CFTR protein is defective, epithelial cells can't regulate the way that chloride ions pass across cell membranes. This disrupts the balance of salt and water needed to maintain a normal thin coating of mucus inside the lungs and other passageways. The mucus becomes thick, sticky, and hard to move, and can result in infections from bacterial colonization.
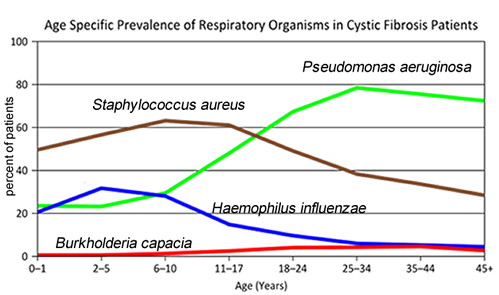
1. "Woe to that child which when kissed on the forehead tastes salty. He is bewitched and soon will die"
This is an old saying from the eighteenth century and describes one of the symptoms of CF (salty skin). Why do you think babies in the modern age have a better chance of survival than babies in the 18th century?
2. What symptoms lead Dr. Weyland to his initial diagnosis?
3. Consider the graph of infections, which organism stays relatively constant in numbers over a lifetime?
What organism is most likely affecting baby Zoey?
4. Explain how the CF gene affects the cell membrane.
5. Consider what you know about TONICITY and the cell membrane. Why is it important to regulate salt in cells?
Part II: CF is a disorder of the cell membrane.
Imagine a door with key and combination locks on both sides, back and front. Now imagine trying to unlock that door blind-folded. This is the challenge faced by David Gadsby, Ph.D., who for years struggled to understand the highly intricate and unusual cystic fibrosis chloride channel – a cellular doorway for salt ions that is defective in people with cystic fibrosis.
His findings, reported in a series of three recent papers in the Journal of General Physiology, detail the type and order of molecular events required to open and close the gates of the cystic fibrosis chloride channel, or as scientists call it, the cystic fibrosis transmembrane conductance regulator (CFTR).
Ultimately, the research may have medical applications, though ironically not likely for most cystic fibrosis patients. Because two-thirds of cystic fibrosis patients fail to produce the cystic fibrosis channel altogether, a cure for most is expected to result from research focused on replacing the lost channel.
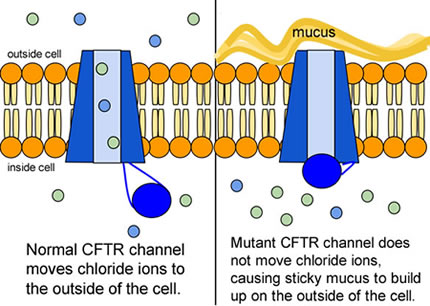
6. Compare the normal and the mutant CFTR protein. How would you correct the mutant protein if you had the ability to tinker with it on a molecular level?
7. Why would treatment that targets the CFTR channel not be effective for ⅔ of those with cystic fibrosis? 8. Sweat glands cool the body by releasing perspiration (sweat) from the lower layers of the skin onto the surface. Sodium and chloride (salt) help carry water to the skin's surface and are then reabsorbed into the body. Why does a person with cystic fibrosis have salty tasting skin?
Part III: No cell is an island
Like people, cells need to communicate and interact with their environment to survive. One way they go about this is through pores in their outer membranes, called ion channels, which provide charged ions, such as chloride or potassium, with their own personalized cellular doorways. But, ion channels are not like open doors; instead, they are more like gateways with high-security locks that are opened and closed to carefully control the passage of their respective ions.
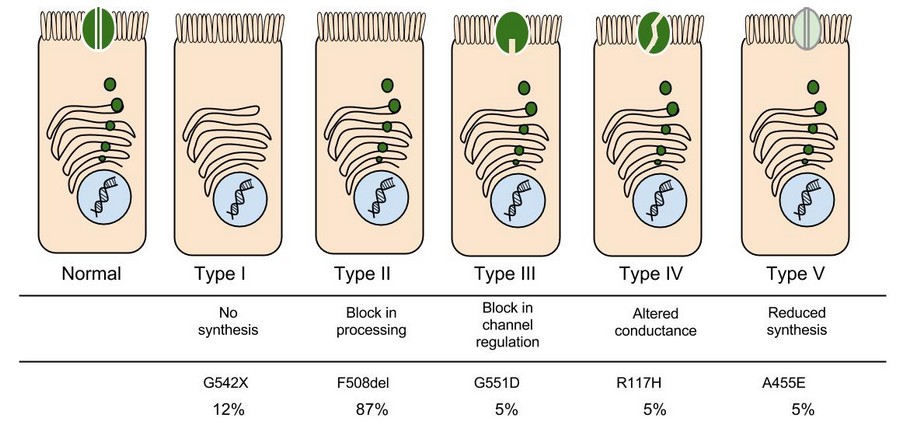
9. Which mutation do you think would be easiest to correct? Justify your answer.
10. Consider what you know about proteins, why does the "folding" of the protein matter?
Part IV: Open Sesame
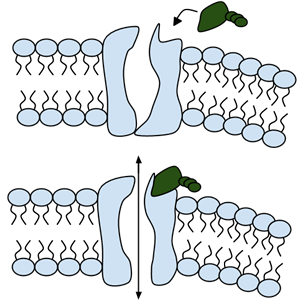
Among the numerous ion channels in cell membranes, there are two principal types: voltage-gated and ligand-gated. Voltage-gated channels are triggered to open and shut their doors by changes in the electric potential difference across the membrane. Ligand-gated channels, in contrast, require a special “key” to unlock their doors, which usually comes in the form of a small molecule.
CFTR is a ligand-gated channel, but it’s an unusual one. Its “key” is ATP, a small molecule that plays a critical role in the storage and release of energy within cells in the body. In addition to binding the ATP, the CFTR channel must snip a phosphate group – one of three “P’s” – off the ATP molecule to function. But when, where and how often this crucial event takes place has remained obscure.
11. Label the image to the right to show how the ligand-gated channel for CFTR works. (Structures: Ligand-gated channel protein, ATP, phospholipids). Summarize how this channels works.
12. Where is ATP generated in the cell? How might ATP production affect the symptoms of cystic fibrosis?
Part V: Can a Drug Treat Zoey's Condition?
Dr. Weyland confirmed that Zoey does have cystic fibrosis and called the parents in to talk about potential treatments. “Good news, there are two experimental drugs that have shown promise in CF patients. These drugs can help Zoey clear the mucus from her lungs. Unfortunately, the drugs do not work in all cases.” The doctor gave the parents literature about the drugs and asked them to consider signing Zoey up for trials.
The Experimental Drugs
Ivacaftor ™ is a potentiator that increases CFTR channel opening time. We know from the cell culture studies that this increases chloride transport by as much as 50% from baseline and restores it closer to what we would expect to observe in wild type CFTR. Basically, the drug increases CFTR activity by unlocking the gate that allows for the normal flow of salt and fluids.
In early trials, 144 patients all of whom were over the age of 12 were treated with 150 mg of Ivacaftor twice daily. The total length of treatment was 48 weeks. Graph A shows changes in FEV (forced expiratory volume) with individuals using the drug versus a placebo. Graph B shows concentrations of chloride in patient’s sweat.
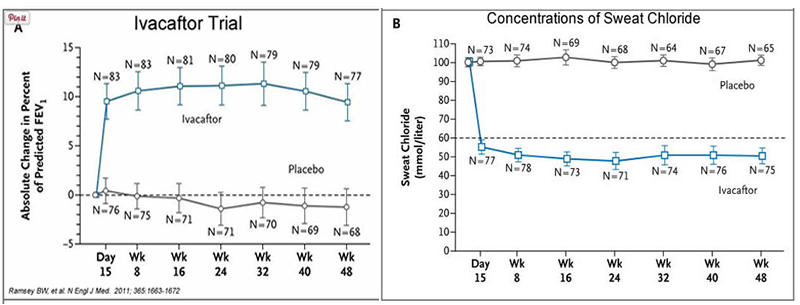
13. What is FEV (if you're not sure, look this one up)? Describe a way that a doctor could take a measurement of FEV.
14. Why do you think it was important to have placebos in both of these studies?
15. Which graph do you think provides the most compelling evidence for the effectiveness of Ivacaftor. Defend your choice.
16. Take a look at the mutations that can occur in the cell membrane protein from Part III. For which mutation do you think Ivacaftor will be most effective. Justify your answer.
17. Would you sign Zoey up for clinical trials based on the evidence? What concerns would a parent have before considering an experimental drug?
Part VI: Zoey's Mutation
Dr. Weyland calls a week later to inform the parents that genetic tests show that Zoey chromosomes show that she has two copies of the F508del mutation. This mutation, while the most common type of CF mutation, is also one that is difficult to treat with just Ivacaftor. There are still some options for treatment.
In people with the most common CF mutation, F508del, a series of problems prevents the CFTR protein from taking its correct shape and reaching its proper place on the cell surface. The cell recognizes the protein as not normal and targets it for degradation before it makes it to the cell surface. In order to treat this problem, we need to do two things: first, an agent to get the protein to the surface, and then ivacaftor (VX-770) to open up the channel and increase chloride transport. VX-809 has been identified as a way to help with the trafficking of the protein to the cell surface. When added VX-809 is added to ivacaftor (now called Lumacaftor,) the protein gets to the surface and also increases in chloride transport by increasing channel opening time.
In early trials, experiments were done in-vitro, where studies were done on cell cultures to see if the drugs would affect the proteins made by the cell. General observations can be made from the cells, but drugs may not work on an individual’s phenotype. A new type of research uses ex-vivo experiments, where rectal organoids (mini-guts) were grown from rectal biopsies of the patient that would be treated with the drug. Ex-vivo experiments are personalized medicine, each person may have different correctors and potentiators evaluated using their own rectal organoids.
The graph below shows how each drug works for 8 different patients (#1-#8). Swelling in the organoid indicates the the channels within the cell membrane are allowing material to pass.
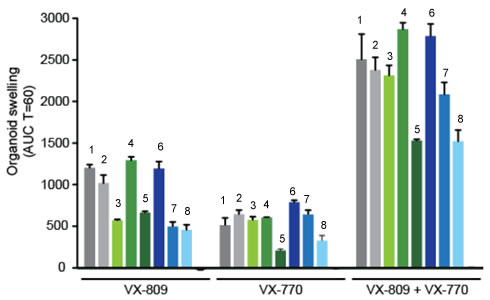
19. . Compare ex-vivo trials to in-vitro trials.
20. One the graph, label the group that represents Ivacaftor and Lumacaftor. What is the difference between these two drugs?
21. Complete a CER Chart.
If the profile labeled #7 is Zoey, rank the possible drug treatments in order of their effectiveness for her mutation. This is your CLAIM. Provide EVIDENCE to support your claim Provide REASONING that explains why this treatment would be more effective than other treatments and why what works for Zoey may not work for other patients. This is where you tie the graph above to everything you have learned in this case. Attach a page.

Source & Credits
- "CFTR Protein Panels" by Lbudd14 - Own work. Licensed under Creative Commons Attribution-Share Alike 3.0 via Wikimedia Commons
- http://newswire.rockefeller.edu/2003/12/19/scientists-finally-pry-stubborn-cellular-door-ajar/
- http://en.wikipedia.org/wiki/Cystic_fibrosis
- http://www.medscape.org/viewarticle/806649_transcript
- http://www.cff.org/research/clinicalresearch/faqs/combinedkalydeco-vx-809/#Expanded-Access
- Ifacaftor Trial Graph: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3230303/
- Organoid swelling graph: http://www.potentiate.info/?q=trio-clinical-trial-ivacaftor-genistein
- Life expectancy graph: http://www.nationaljewish.org/healthinfo/conditions/cysticfibrosis/life-expectancy/
Follow-up Article: What it's like to have two kids with cystic fibrosis More information at John Hopkins Cystic Fibrosis Center
Other Resources on Cystic Fibrosis
Cystic Fibrosis Mutations Cell Membrane and Transport (Slides)
- News & Analysis on Clinical Trial Services & Contract Research And Development
Landmark study validates Cystic Fibrosis drug for infants as young as four weeks old
11-Apr-2024 - Last updated on 11-Apr-2024 at 12:24 GMT
- Email to a friend

This discovery marks a significant moment for Cystic Fibrosis treatment, with experts hailing it as a breakthrough.
Lead researchers have emphasized the monumental nature of this finding, especially as it pertains to the treatment of Cystic Fibrosis in infants. The drug, Ivacaftor (Kalydeko), originally formulated for adults, has gradually gained approval for use in younger age groups over the years. However, this latest research suggests its effectiveness in infants as young as four weeks, a milestone previously unexplored.
Cystic Fibrosis specialists underscore the importance of early intervention in slowing or halting the progression of the disease in children. With this study's results, there is newfound optimism that eligible newborns could commence treatment immediately following diagnosis, revolutionizing current practices that typically delay treatment until infants reach four months of age.
“This is a huge moment in Cystic Fibrosis,” said Paul McNally, associate professor of paediatrics at RCSI and Consultant in Respiratory Medicine at CHI. McNally is one of the authors of the new study, which was published in the Journal of Cystic Fibrosis.
“Over the years Ivacaftor, or Kalydeko, has been put through clinical trials in younger and younger children. Now, through this study, it has been shown to be safe and effective all the way down to four weeks of age,” he said.
Cystic Fibrosis, a hereditary condition predominantly affecting the lungs and digestive system, poses significant challenges for affected individuals and their families. With Ireland experiencing the highest incidence of the disease globally, the implications of this study resonate deeply within the local community and beyond.
The study's impact extends beyond academic circles, touching the lives of real families like that of siblings Kara and Isaac Moss. Participating in the study through Children’s Health Ireland, both children have defied expectations, experiencing remarkable health despite their diagnosis. Their mother, Debbie, emphasizes the importance of such research endeavors in ensuring timely access to life-changing treatments for children with Cystic Fibrosis.
Siblings Kara, 5, and Isaac Moss, 2, both participated in the study through Children’s Health Ireland. Kara was part of an earlier phase of the study that paved the approval of the drug in older infants and led to the latest trial that Isaac took part in.
Isaac was the first baby with Cystic Fibrosis in the world to be diagnosed from birth and enrolled directly in a trial of these ground-breaking treatments.
“Both Kara and Isaac are doing well and remarkably are not experiencing any of the typical symptoms of Cystic Fibrosis at the moment,” said their mother, Debbie.
“Research studies like this one are so important to ensuring that children get access to the right treatments as early as possible. With the right medications, they can enjoy a healthy childhood and look forward to a brighter future.”
Manufactured by Vertex Pharmaceuticals, the drug Ivacaftor is currently undergoing regulatory review for expanded authorization, potentially reaching infants as young as one month old. This development represents a significant step forward in the fight against Cystic Fibrosis and offers renewed hope for countless families worldwide.
The study, titled ‘Safety and efficacy of Ivacaftor in infants aged one to less than four months with Cystic Fibrosis,’ published in the Journal of Cystic Fibrosis, underscores the collaborative efforts of researchers from RCSI, Children’s Health Ireland, the USA, and the UK in advancing Cystic Fibrosis treatment for the most vulnerable patients—infants.
Related news

Related products

Saama accelerates data review processes
Content provided by Saama | 25-Mar-2024 | Infographic
In this new infographic, learn how Saama accelerates data review processes. Only Saama has AI/ML models trained for life sciences on over 300 million data...

More Data, More Insights, More Progress
Content provided by Saama | 04-Mar-2024 | Case Study
The sponsor’s clinical development team needed a flexible solution to quickly visualize patient and site data in a single location

Generic method approach for determination of residual solvents in active pharmaceutical ingredients by gas chromatography
Content provided by Almac Group | 29-Nov-2023 | White Paper
Solvents are defined as organic chemicals that are used to dissolve components. They are used during the manufacture of drug substances and can also potentially...

Using Define-XML to build more efficient studies
Content provided by Formedix | 14-Nov-2023 | White Paper
It is commonly thought that Define-XML is simply a dataset descriptor: a way to document what datasets look like, including the names and labels of datasets...
Related suppliers
- Saama accelerates data review processes Saama | Download Infographic
- More Data, More Insights, More Progress Saama | Download Case Study
- Ultra Low Temperature Packaging solutions Almac Group | Download Case Study
- Three Ways to Leverage AI in Clinical Trials Right Now Saama | Download Technical / White Paper
- When Every Day Counts Saama | Download Case Study
Upcoming supplier webinars
- 24 Apr 2024 Wed How to reduce crosslinking and ensure fast dissolution in soft caps
Upcoming editorial webinars
- 18 Apr 2024 Thu Webinar Cell and Gene Therapy Manufacturing
On-demand webinars
- Innovation in Drug Delivery Webinar

Promotional Features

Outsourcing-Pharma
- Advertise with us
- Why Register?
- Apply to reuse our content
- Press Releases – Guidelines
- Contact the Editor
- Report a technical problem
- Whitelist our newsletters
- Editorial Calendar
‘Groundbreaking’: Cystic fibrosis drug is safe and effective in newborns, new research shows
Ivacaftor - sold under trade name kalydeko - previously available to babies aged four months or older.
Siblings Kara (5) and Isaac Moss (2), who participated in a landmark study which supports use of a ground-breaking cystic fibrosis drug in infants from four weeks of age.
A “groundbreaking” drug which targets the underlying cause of cystic fibrosis has been shown to be safe and effective in newborns aged four weeks and above.
Until now, Ivacaftor - sold under the trade name Kalydeko - has been available to those aged four months or older.
Ivacaftor is the first drug designed to treat the underlying cause of cystic fibrosis and was originally approved for adults before subsequently being extended to younger age groups.
Ireland has the highest incidence of the inherited disease in the world due to its high prevalence of Celtic genes which are more susceptible to mutations that cause the progressive disease.
O’Gara takes La Rochelle to Cork to prepare for big Champions Cup clash with Leinster
:quality(70)/cloudfront-eu-central-1.images.arcpublishing.com/irishtimes/7273FFGV5LRISLQEKRN5XNBC7Y.jpg)
Hungarian sex abuse scandal hits close to home for Viktor Orban
:quality(70):focal(1909x703:1919x713)/cloudfront-eu-central-1.images.arcpublishing.com/irishtimes/BM2XKDMIZ2MRMRCFWYB6YXO4WQ.jpg)
Is Dunnes breaking consumer law when it comes to returns?
:quality(70):focal(2413x1207:2423x1217)/cloudfront-eu-central-1.images.arcpublishing.com/irishtimes/I4LMLHFIXNOT7AF5PMDFMN45UI.jpg)
John Magnier’s Coolmore pays €8.925m for 591 acres in Tipperary
:quality(70):focal(992x838:1002x848)/cloudfront-eu-central-1.images.arcpublishing.com/irishtimes/7CXEOBWQ3NEW5JSHJEFBZR7QMA.jpg)
The new research carried out by RCSI University of Medicine and Health Sciences and Children’s Health Ireland has been described as a “huge moment” for the condition.
Co-author of the study and Associate Professor of Paediatrics at RCSI and Consultant in Respiratory Medicine at CHI Paul McNally described it as a “huge moment in cystic fibrosis” as it means treatment can be started immediately after diagnosis, once it is approved by the European Medicines Agency.
[ Mothers with cystic fibrosis: ‘We’ve lives we never thought we could as no one thought we’d still be alive’ ]
Though it is known that drugs like Kalydeko improve cystic fibrosis, the “big question” is if they slow down or prevent decline when started earlier in life, he said.
A longer-term study which is tracking the impact and efficacy of earlier intervention among 550 children from Ireland and the UK has recently begun.
However, some early findings in children are promising including a lack of respiratory symptoms and restoration of pancreatic function.
“There’s a lot of stuff that we don’t understand but what we do know I suppose is these drugs hold really amazing promise and what we need is more research,” he said.
With new effective treatments, Prof McNally said the CF adult population across the world is constantly climbing.
“We’re hopefully looking at future generations of children who will have much, much milder disease than previous generations because they started on drugs much earlier,” he said.
About 1,400 children and adults in Ireland live with the condition and more than 30 new cases are diagnosed each year, most commonly at about four weeks of age through the newborn screening programme.
Two-year-old Isaac Moss from Ashbourne, Co Meath was the first baby in the world to be diagnosed with cystic fibrosis from birth through the programme, after which he was enrolled directly onto the trial. His five-year-old sister Kara was part of an earlier phase of the study that paved the approval of the drug in older infants and led to the latest trial.
Their mother Debbie said Kara’s diagnosis through screening was a “huge shock” at the time, and although her husband Wayne has a cousin with cystic fibrosis, none of their nieces or nephews had it.
“Initially, we were devastated, we didn’t know much about CF so we didn’t know what it meant for Kara and what it meant for her life,” she said adding that Kara’s diagnosis meant a one in four risk of their second child having it.
However, Kara had enrolled on a trial of Kalydeko - which is taken twice a day - at nine months of age.
“Because of our experience with Kara and because she was doing so well we were happy to have a second child knowing that the child could be affected.
“We were hopeful that it would be just as successful as it was for Kara and thankfully it has been,” she said.
Because Kara and Isaac have started at such a young age, “they’re the same as any other child,” she said.
“We feel lucky that they have the gene types that respond well to these particular drugs and it gives a lot of hope for the kids’ lives,” she said.
- Listen to our Inside Politics podcast for the best political chat and analysis
- Sign up for push alerts and have the best news, analysis and comment delivered directly to your phone
- Find The Irish Times on WhatsApp and stay up to date
IN THIS SECTION
Menopause support must tread a fine line between awareness and stigma, tony holohan takes up adjunct professor role at trinity’s school of medicine, stronger focus on generic medicines could save hse cash, says industry report, university hospital galway: changes made after review of head injuries to some babies in delivery, developer fails in supreme court bid to save planning permission for 1,593 drumcondra apartments, tánaiste defends state’s payment of up to €1m a month for ukrainian pets, sinn féin is watching the polls and beginning to panic, says stephen donnelly, almost 400 apartments planned for dublin’s grand canal cost-rental scheme, latest stories, the masters: despite a blustery first day, dechambeau will know it is all to play for, ukraine says russian drones damage energy infrastructure in south, banquet room with preserved frescoes unearthed among pompeii ruins, israel’s defence minister has said the country will respond directly to any attack on israel by iran, ‘it is lovely to see a six-year-old child planting a tree. when he is 25, he will have memories with that tree’.
- Terms & Conditions
- Privacy Policy
- Cookie Information
- Cookie Settings
- Community Standards

IMAGES
VIDEO
COMMENTS
1. Introduction. Cystic fibrosis (CF) is a serious and life-shortening genetic disorder affecting approximately 70,000 persons worldwide [].Respiratory failure is the foremost cause of death in CF patients, and lung transplantation is often considered in end-stage CF disease.
To the Editor: The Case Record by Mojica et al. (Dec. 27 issue)1 highlights the importance of considering the diagnosis of cystic fibrosis in adults. We reviewed 842 cases of cystic fibrosis in our...
Introduction. Cystic fibrosis (CF) is a multiorgan disease, caused by autosomal recessive (AR) mutations in the CFTR gene, which regulates the movement of chloride ions across cell membranes [].CF is most commonly diagnosed in Caucasian populations but can affect other ethnic groups as well, with an incidence of one in 4100 live births in the United States, one in 2500-3500 live births in ...
The inherited CF gene directs the body's epithelial cells to produce a defective form of a protein called CFTR (or cystic fibrosis transmembrane conductance regulator) found in cells that line the lungs, digestive tract, sweat glands, and genitourinary system. When the CFTR protein is defective, epithelial cells can't regulate the way that ...
Cystic fibrosis (CF) is the most common genetically inherited condition in European-derived populations. However, it is being increasingly recognised in other populations, including people of Asian, Black African and Caribbean descent. We present a case detailing the diagnosis of CF in a 12-year-old patient of Egyptian background who had been ...
%PDF-1.4 1 0 obj . /Title (þÿ) /Creator (þÿwkhtmltopdf 0.12.6) /Producer (þÿQt 4.8.7) /CreationDate (D:20210429212325Z) >> endobj 3 0 obj /Type /ExtGState /SA true /SM 0.02 /ca 1.0 /CA 1.0 /AIS false /SMask /None>> endobj 4 0 obj [/Pattern /DeviceRGB] endobj 8 0 obj [0 /XYZ 33 776 0] endobj 9 0 obj [0 /XYZ 33 703.250000 0] endobj 10 0 obj /__WKANCHOR_2 8 0 R /__WKANCHOR_4 9 0 R >> endobj ...
Background Previous reports have shown an increased number of colorectal cancers in patients with cystic fibrosis. We assessed the database of our cystic fibrosis center to identify patients with all kinds of cancer retrospectively. All patients visiting the Cystic Fibrosis Centre Innsbruck between 1995 and 2019 were included. Case presentation Among 229 patients with cystic fibrosis treated ...
Most patients with cystic fibrosis (CF) develop multisystemic clinical manifestations, the minority having mild or atypical symptoms. We describe an adolescent with chronic cough and purulent rhinorrhoea since the first year of life, with diagnoses of asthma, allergic rhinitis and chronic rhinosinusitis. Under therapy with long-acting bronchodilators, antihistamines, inhaled corticosteroids ...
Cystic fibrosis (CF) is an autosomal recessive condition affecting approximately 30,000 Americans and 70,000 people worldwide.According to the Cystic Fibrosis Foundation (Cystic Fibrosis Foundation, 2019a), approximately 1,000 new cases are diagnosed yearly in the United States, with a known incidence of 1 per 3,900 live births.The disease prevalence varies greatly by ethnicity, with the ...
The clinical course of patients with cystic fibrosis (CF) is variable and probably determined by many interacting factors. We aimed to examine the influence of early social and clinical factors on long-term survival. A case-control study of adult CF patients was used to compare long-term survivors (aged ≥40 yrs) with patients who died before reaching 30 yrs of age. Each case (n = 78) was ...
Diagnosis of Cystic Fibrosis In most cases the diagnosis of CF is un-equivocal. Patients present with classic clinical symptoms of CF in childhood, and results from standard genetic assays and biochemical tests are confirmatory [13]. The sweat test remains the reference standard for CF diagnosis con-firmation, with a chloride level threshold of 60
BackgroundUniversal newborn screening changed the way medical providers think about the presentation of cystic fibrosis (CF). Before implementation of universal screening, it was common for children with CF to present with failure to thrive, nutritional deficiencies, and recurrent infections. Now, nearly all cases of CF are diagnosed by newborn screening shortly after birth before significant ...
study has estimated that 0.8-13.3% of fetuses with FEB will have CF. 6 When both parents are carriers of pathogenic CFTR variants, there is a one-in-four chance (25%) of CF in the
80,000 PEOPLE LIVE WITH CYSTIC FIBROSIS GLOBALLY. Cystic Fibrosis is a progressive, life-limiting genetic disease that causes severe respiratory and digestive problems as well as other complications such as infections and diabetes. There are over 80,000 people living with Cystic Fibrosis globally, including 10,500 in the UK accounting for 9,500 ...
Background. Cystic fibrosis (CF) is one of the most common autosomal recessive diseases. Factors contributing to disease exacerbations and survival rate of CF patients are type of mutation in the CFTR gene, poor nutritional status, lung failure, and infection development by Pseudomonas aeruginosa.The study aimed to evaluate the relationship between the severity of mutation, nutritional status ...
The Willard A. Bernbaum Cystic Fibrosis Research Center at Case Western Reserve University School of Medicine is dedicated to devising new therapeutics to treat this disease and its complications. ... The Willard A. Bernbaum Cystic Fibrosis Research Center dates back to 1964 when it was initially funded by the National Institutes of Health. ...
This case study is a follow-up to the Cystic Fibrosis Case Study where students explore how changes in transport proteins affects the movement of ions, resulting in a build-up of chloride ions and the symptoms of the disease. Students were introduced to the idea that different mutations can cause differences in the transport proteins, but in ...
A Case of Cystic Fibrosis. Dr. Weyland examined a six month old infant that had been admitted to University Hospital earlier in the day. The baby's parents had brought young Zoey to the emergency room because she had been suffering from a chronic cough. In addition, they said that Zoey sometimes would "wheeze" a lot more than they thought was ...
Case Study - Cystic Fibrosis and the Cell Membrane (CER version).pdf - Google Drive.
10.1542/6347456255112Video AbstractPEDS-VA_2023-0646576347456255112A multidisciplinary committee developed evidence-based guidelines for the management of cystic fibrosis transmembrane conductance regulator-related metabolic syndrome/cystic fibrosis screen-positive, inconclusive diagnosis (CRMS/CFSPID). A total of 24 patient, intervention, comparison, and outcome questions were generated based ...
In this chapter we use the relatively common genetic disease cystic fibrosis (CF) to illustrate many of the concepts covered in the book. CF is inherited in a simple recessive manner. Molecular genetics isolated the CFTR gene and electrical measurements showed the CFTR protein to be a plasma membrane chloride channel. Studies of patients' genes ...
A second case was described in which segmental hypokinesis with grade 2 systolic function was found as an incidental finding on Doppler echocardiography from a prevalence study looking at pulmonary hypertension that included an adult cystic fibrosis patient population . This study identified a 40-year-old diabetic male, diagnosed with ...
This case study asks students to examine a case of cystic fibrosis. As students read the symptoms and gather evidence about membrane proteins, they learn that CF is really a disorder of membrane permeability. Case Study - What is the Relationship Between the Cell Membrane and Cystic Fibrosis? ... Complete a CER Chart.
Case Study - Cystic Fibrosis and the Cell Membrane (CER version) - Free download as PDF File (.pdf), Text File (.txt) or read online for free.
In a groundbreaking revelation, a study conducted by RCSI University of Medicine and Health Sciences and Children's Health Ireland has confirmed the safety and efficacy of a Cystic Fibrosis drug in newborns as young as four weeks old. This discovery marks a significant moment for Cystic Fibrosis treatment, with experts hailing it as a ...
Thu Apr 11 2024 - 17:14. A "groundbreaking" drug which targets the underlying cause of cystic fibrosis has been shown to be safe and effective in newborns aged four weeks and above. Until now ...