Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED TOPICS
INTRODUCTION
Issues related to clinical manifestations and diagnosis of malaria will be reviewed here. Technical aspects of laboratory tools for diagnosis of malaria are discussed further separately.
The epidemiology, pathogenesis, diagnosis, and treatment of malaria are discussed separately:
● (See "Malaria: Epidemiology, prevention, and control" .)
● (See "Treatment of uncomplicated falciparum malaria in nonpregnant adults and children" .)

S. DAVID SHAHBODAGHI, MD, MPH, AND NICHOLAS A. RATHJEN, DO
Editor's Note: This article has been updated to incorporate the February 2023 guidance update from the Centers for Disease Control and Prevention.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2022;106(3):270-278
Author disclosure: No relevant financial relationships.
Each year, malaria causes an estimated 500,000 deaths worldwide. Most of these deaths occur in Africa and disproportionally affect children younger than five years worldwide. Human malarial disease is caused by protozoan parasites of the genus Plasmodium . The primary means of infection is through the bite of a female Anopheles mosquito. The incidence of malaria in the United States has increased since 2011, in conjunction with the increase in worldwide travel. An estimated 2,000 cases of malaria occur annually in the United States. All travelers to malaria-endemic regions should be prescribed prophylaxis. Malaria has a broad range of clinical presentations. Travelers who have symptoms of malaria should seek medical attention as soon as possible. All febrile travelers who have recently returned from a malarious area should be evaluated for malaria. The accurate, timely, and species-specific diagnosis of malaria is essential for successful treatment. Direct microscopy of Giemsa-stained blood smears is the reference standard for laboratory diagnosis. Rapid testing for malaria has emerged as an important adjunctive diagnostic modality. Malaria treatment is determined by individual patient factors and geography. The World Health Organization recommends treating uncomplicated cases of malaria with artemisinin combination therapy. [corrected] Severe malaria is mainly caused by Plasmodium falciparum . Children, pregnant patients, and people who are not from endemic regions are at highest risk of severe malaria. Intravenous artesunate is the treatment of choice for severe malaria.
Malaria has infected humans since the beginning of recorded history. 1 Some estimates place its total mortality burden at one-half of all people who have ever lived. 2 Each year, the disease continues to cause an estimated 500,000 deaths worldwide. 2 Most of these deaths occur in Africa and disproportionally affect children younger than five years worldwide. 3
Human malarial disease is caused by protozoan parasites of the genus Plasmodium , which has five known species: P. falciparum , P. vivax , P. ovale , P. malariae , and the emerging zoonotic parasite P. knowlesi . Most deaths are caused by P. falciparum . 4 The primary means of human infection is through the bite of a female Anopheles mosquito.
Malaria poses a threat to one-half of the world's population. 5 The incidence of malaria in the United States has continued to increase annually since 2011, in conjunction with the increase in worldwide travel. 6 Malaria, formerly endemic to the United States, was successfully eradicated in the country during the mid-20th century. 7 In the United States today, malaria is almost exclusively found in travelers to and immigrants from endemic regions of the world. 7 However, transmission can rarely occur via other means, such as exposure to infected blood products, congenital transmission, or local mosquito-borne outbreaks. 8 In the United States, an estimated 2,000 cases of malaria occur annually. 7
Before a patient travels internationally, the physician should conduct a personalized risk assessment, including travel location, the season of travel, and the proposed itinerary. The regions with the highest rates of malaria transmission are sub-Saharan Africa, the Indian subcontinent, and Southeast Asia. The risk of contracting malaria varies seasonally, with the highest risk occurring during and just after the rainy season, typically between May and December. 9
The primary method of malaria prevention is avoiding mosquito bites. Anopheles mosquitoes primarily feed at night; most malaria transmission occurs between dusk and dawn. Prevention strategies include personal protective measures such as using insecticide-treated bed nets, wearing clothes that minimize exposed skin, and applying mosquito-repelling chemicals. The most effective insect repellents contain 20% to 30% N , N -diethyl- m -toluamide (DEET) or 20% picaridin. 10 Higher concentrations are not associated with greater protection. Applying permethrin to clothing increases protection against penetrating insect bites. 11
All travelers to malaria-endemic regions should be prescribed prophylaxis. 9 The choice of agent should be based on location and duration of travel, malarial resistance patterns, and the patient's medical history ( Table 1 12 , 13 ) . All prevention regimens involve beginning the medication before departure, taking the medication while in the high-risk area, and continuing the medication for a defined period after travel has ended. The use of antimalarial agents does not negate the need for personal protective measures. The Centers for Disease Control and Prevention (CDC) provides country-specific prophylaxis recommendations at http://www.cdc.gov/malaria/travelers/country_table/a.html .
In 2021, the first malaria vaccine approved for widespread use was recommended by the World Health Organization for the prevention of P. falciparum malaria in children living in endemic areas. The vaccine has been administered to more than 1 million children in Ghana, Malawi, and Kenya. 14 , 15
Clinical Presentation
The clinical presentation of malaria ranges from asymptomatic parasitemia or uncomplicated disease to severe disease or death. The differential diagnosis of malaria is summarized in Table 2 . 16 Symptoms of malaria can develop within six to seven days of exposure, but the presentation may be delayed for several months after leaving an endemic region. 17 Symptomatic malaria is characterized by fevers, chills, headaches, myalgias, and malaise. It may also present as fever without a specific or obvious cause or as gastrointestinal symptoms in children. There are no typical features of malaria. 10 , 17
In the absence of a detailed travel history, malaria is often misdiagnosed as a nonspecific viral illness. 18 Travelers who have symptoms of malaria should seek medical attention as soon as possible, regardless of whether prophylaxis or preventive measures were used. All febrile travelers who have recently returned from a malarious area should be evaluated for malaria. 19 Suspicion of P. falciparum malaria is a medical emergency. Physicians should use only laboratory-based diagnostic methods. 18 Because most patients with malaria have no specific fever pattern, a pattern should not be considered in the diagnosis. 17 Clinical deterioration or death can occur within 24 to 36 hours in a malaria-naive patient. 7
Diagnostic Testing
The accurate, timely, and species-specific diagnosis of malaria is essential for successful treatment. Microscopic examination of Giemsa-stained blood smears is the reference standard for laboratory diagnosis. Thick blood smears are used to detect the presence of malarial parasites, and thin blood smears are used to determine the species and quantify parasitemia. 18 , 20 When malaria is suspected, urgent microscopy should be performed by an individual with expertise in examining blood smears and diagnosing malaria. 17 Multiple blood smears may be needed to produce a positive result. Three negative results, 12 hours apart, are needed to rule out malaria. 21
Rapid testing for malaria has emerged as an important adjunctive diagnostic modality. Rapid diagnostic tests have excellent sensitivity and negative predictive value with results available in five to 20 minutes. 22 , 23 Rapid diagnostic tests for malaria are simple to use, do not require laboratory facilities or diagnostic expertise, and enable prompt diagnosis. 24 However, rapid diagnostic tests can detect only P. falciparum and P. vivax , and they do not provide data regarding parasite density. 17 , 23 , 24 In the United States, rapid diagnostic tests for malaria should be used only in conjunction with thick and thin blood smears. 23 , 24 The usefulness of these rapid tests ends with diagnosis because further testing and monitoring must be completed via microscopy. 23 Binax-NOW is the only rapid diagnostic test approved by the U.S. Food and Drug Administration for malaria, 25 but a variety of other assays are available worldwide.
The CDC-recommended treatment of malaria is based on four variables: the clinical status of the patient (uncomplicated vs. severe disease), the species involved, the patient's history of prophylaxis, and the geographic region where the infection occurred. 25 Under certain circumstances, laboratory testing may not be readily available. If clinical suspicion for malaria is high, empiric treatment should be initiated promptly, especially in the setting of severe disease. Patients who used prophylaxis should be treated with different antimalarial medications than those used for prophylaxis. 13 , 25 , 26
Patients who are immunocompromised, patients with no previous malarial immunity, children, pregnant patients, and patients with signs of severe disease should be hospitalized. Severe disease is defined as the presence of at least one of the following: impaired consciousness (Glasgow Coma Scale score less than 11), convulsions, severe anemia (hemoglobin less than 7 g per dL [70 g per L] in adults or less than 5 g per dL [50 g per L] in children younger than 12 years), acute kidney injury, hypoglycemia, acute respiratory distress syndrome, shock, disseminated intravascular coagulation, acidosis, coma, liver dysfunction, or parasite density greater than 5%. 25
Hospitalized patients should receive standard supportive care, including intravenous fluids, antipyretics, and antiemetics. Outpatient treatment with close clinical follow-up can be considered in patients without an indication for hospitalization. Malaria specialists are available 24 hours a day, seven days a week to aid physicians with diagnosis and treatment ( Table 3 ) .
UNCOMPLICATED MALARIA
The World Health Organization recommends treating uncomplicated cases of malaria with artemisinin combination therapy (ACT), which comprises an artemisinin derivative and a partner drug. However, artemisinin should not be used in the first trimester of pregnancy with the exception of artemether/lumefantrine (Coartem), which is acceptable for all trimesters ( https://www.cdc.gov/malaria/new_info/2023/Coartem.html ). 26 [ corrected] ACTs are well tolerated and highly effective against all Plasmodium species. Patients should be informed that counterfeit and substandard antimalarials are widespread in resource-limited and lower-income countries.
Malaria Caused by Plasmodium falciparum or Unknown Species . If ACT is not available and the infection likely occurred in an area with chloroquine-sensitivity, chloroquine or hydroxychloroquine (Plaquenil) may be used. If ACT is unavailable and the infection occurred in an area with chloroquine resistance, atovaquone/proguanil (Malarone), a combination of quinine (Qualaquin) plus tetracycline, doxycycline, or clindamycin (Cleocin) should be used. Mefloquine is a treatment of last resort. Table 4 summarizes treatment options for acute uncomplicated malaria. 25 , 26
Malaria Caused by Plasmodium ovale or Plasmodium vivax. Initial treatment is the same as for uncomplicated malaria due to P. falciparum or unknown species, as described previously. In addition, patients infected with P. ovale or P. vivax require treatment against hypnozoites (dormant forms), which are responsible for relapsing infections. Patients should be tested for glucose-6-phosphate dehydrogenase (G6PD) deficiency because the drugs of choice, primaquine and tafenoquine, are associated with hemolytic anemia in people with G6PD deficiency. Tafenoquine should not be used in patients younger than 16 years or in patients with neuropsychiatric disorders. Tafenoquine is used only if chloroquine or hydroxychloroquine was used for the acute infection.
For people with G6PD deficiency who cannot tolerate primaquine or tafenoquine, chloroquine prophylaxis should be continued for one year. In those with intermediate G6PD deficiency, primaquine may be considered in close consultation with infectious disease or tropical medicine specialists. Table 5 summarizes antirelapse treatment options. 25 , 26
Malaria Caused by Plasmodium malariae or Plasmodium knowlesi. Although resistance to chloroquines is not widely documented with P. malariae or P. knowlesi , the World Health Organization recommends the use of ACT, regardless of geographic region of infection. 26 P. knowlesi is associated with severe disease, and patients should be hospitalized if this species is isolated. If ACT is not available and the infection is likely from a chloroquine-sensitive area, chloroquine or hydroxychloroquine may be used. 25 , 26
SEVERE MALARIA
P. falciparum and, to a lesser degree, P. knowlesi cause almost all cases of severe malaria. 26 Children, pregnant patients, and people who are not from endemic regions are at highest risk of severe malaria. Intravenous artesunate is the treatment of choice for severe disease and should be initiated as soon as possible ( Table 6 ) . 25 , 26 The dosage for adults and children is 2.4 mg per kg at 0, 12, and 24 hours. Blood smears should be obtained every 12 hours. If the parasite density is less than 1% at least four hours after the third dose, the patient should be transitioned to a full course of an oral medication, ideally ACT. If the parasite density is greater than 1% after the third artesunate dose, artesunate should be continued as a single daily dose until parasitemia is less than 1%, not to exceed seven days. Artesunate is well tolerated, and allergy to artemisinins is the only absolute contraindication. 25 , 26
If artesunate is not immediately available, the preferred oral medication for severe disease is artemether/lumefantrine (Coartem). Other options also include atovaquone/proguanil, quinine, and mefloquine. Tetracyclines and clindamycin should not be used because of their delayed onset of action. Once intravenous artesunate therapy becomes available, the oral medication should be discontinued.
The CDC no longer recommends the use of exchange transfusions as an adjunctive therapy for severe malaria. 25 All patients treated for severe malaria should be evaluated for hemolytic anemia within 30 days after completing treatment.
PREGNANT PATIENTS
Malaria is associated with significant morbidity and mortality in pregnant patients. ACTs may be used in the second and third trimesters except for artemether/lumefantrine, which may be used in the first trimester as well. [corrected] Chloroquine, hydroxychloroquine, and quinine with clindamycin or mefloquine may be used throughout pregnancy. Artemether/lumefantrine may be used in the first trimester if no other options are available. Primaquine should not be used during pregnancy. Tafenoquine should not be used in patients who are pregnant or breastfeeding.
Infants born to mothers who had P. vivax or P. ovale infection during pregnancy should be tested for G6PD deficiency. If results are normal, the mother should be treated with primaquine while breastfeeding. If G6PD deficiency is diagnosed, chloroquine should be used for one year after the initial treatment to prevent relapse. 25 , 26
This article updates previous articles on this topic by Johnson and Kalra , 8 Lo Re and Gluckman , 27 and Juckett . 28
Data Sources: PubMed was searched using the key words prevention, diagnosis, treatment, malaria, surveillance, travel medicine, chemoprophylaxis, and malaria treatment. The search was limited to English-language studies published since 2000. Secondary references from the key articles identified by the search were also used. Search dates: January 2018, October 2021, June 2022.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army at large.
Centers for Disease Control and Prevention. The history of malaria, an ancient disease. Last reviewed November 14, 2018. Accessed October 23, 2021. https://www.cdc.gov/malaria/about/history
Whitfield J. Portrait of a serial killer [published online October 3, 2002]. Nature . Accessed October 23, 2021. https://www.nature.com/articles/news021001-6
World Health Organization. World malaria report 2021. Accessed May 20, 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021
- Foster WA, Walker ED. Mosquitoes ( Culicidae ). In: Mullen GR, Durden LA, eds. Medical and Veterinary Entomology . 3rd ed. Elsevier; 2019:261–325.
- Dye-Braumuller KC, Kanyangarara M. Malaria in the USA: how vulnerable are we to future outbreaks? Curr Trop Med Rep. 2021;8(1):43-51.
Cullen KA, Mace KE, Arguin PM Centers for Disease Control and Prevention (CDC). Malaria surveillance—United States, 2013. MMWR Surveill Summ. 2016;65(2):1-22.
Centers for Disease Control and Prevention. About malaria. Accessed October 23, 2021. https://www.cdc.gov/malaria/about/index.html
- Johnson BA, Kalra MG. Prevention of malaria in travelers [published correction appears in Am Fam Physician . 2012;86(3):222]. Am Fam Physician. 2012;85(10):973-977.
Briët OJ, Vounatsou P, Gunawardena DM, et al. Temporal correlation between malaria and rainfall in Sri Lanka. Malar J. 2008;7:77.
Sanford C, McConnell A, Osborn J. The pretravel consultation. Am Fam Physician. 2016;94(8):620-627.
Banks SD, Murray N, Wilder-Smith A, et al. Insecticide-treated clothes for the control of vector-borne diseases: a review on effectiveness and safety. Med Vet Entomol. 2014;28(suppl 1):14-25.
Bazemore AW, Huntington M. The pretravel consultation. Am Fam Physician. 2009;80(6):583-590.
Centers for Disease Control and Prevention. Choosing a drug to prevent malaria. Last reviewed November 15, 2018. Accessed December 4, 2021. https://www.cdc.gov/malaria/travelers/drugs.html
World Health Organization. Malaria vaccine implementation programme. Accessed March 9, 2022. https://www.who.int/initiatives/malaria-vaccine-implementation-programme
Alonso PL, O'Brien KL. A malaria vaccine for Africa—an important step in a century-long quest. N Engl J Med. 2022;386(11):1005-1007.
Centers for Disease Control and Prevention. CDC Yellow Book 2020: Health Information for International Travel . Oxford University Press; 2019. October 23, 2021. https://wwwnc.cdc.gov/travel/page/yellowbook-home-2020
Lalloo DG, Shingadia D, Bell DJ, et al.; PHE Advisory Committee on Malaria Prevention in UK Travellers. UK malaria treatment guidelines 2016. J Infect. 2016;72(6):635-649.
Amir A, Cheong F-W, De Silva JR, et al. Diagnostic tools in childhood malaria. Parasit Vectors. 2018;11(1):53.
Plewes K, Leopold SJ, Kingston HWF, et al. Malaria: what's new in the management of malaria?. Infect Dis Clin North Am. 2019;33(1):39-60.
Feder HM, Mansilla-Rivera K. Fever in returning travelers: a case-based approach. Am Fam Physician. 2013;88(8):524-530.
Mbakilwa H, Manga C, Kibona S, et al. Quality of malaria microscopy in 12 district hospital laboratories in Tanzania. Pathog Glob Health. 2012;106(6):330-334.
World Health Organization. Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 6 (2014–2015). Accessed March 9, 2022. https://apps.who.int/iris/bitstream/handle/10665/204118/9789241510035_eng.pdf
Enane LA, Sullivan KV, Spyridakis E, et al. Clinical impact of malaria rapid diagnostic testing at a US children's hospital. J Pediatric Infect Dis Soc. 2020;9(3):298-304.
Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in travel medicine. Clin Microbiol Infect. 2013;19(5):408-415.
Centers for Disease Control and Prevention. Treatment of malaria: guidelines for clinicians (United States). Last reviewed November 2, 2020. Accessed October 23, 2021. https://www.cdc.gov/malaria/diagnosis_treatment/clinicians1.html
World Health Organization. WHO guidelines for malaria. February 16, 2021. Accessed October 23, 2021. https://reliefweb.int/report/world/who-guidelines-malaria
- Lo Re V III, Gluckman SJ. Prevention of malaria in travelers. Am Fam Physician . 2003;68(3):509–514.
Juckett G. Malaria prevention in travelers. Am Fam Physician. 1999;59(9):2523-2530.
Continue Reading
More in AFP
More in pubmed.
Copyright © 2022 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Pathogenesis and Clinical Features of Malaria
- First Online: 30 September 2023
Cite this chapter

- Huilong Chen 5
Part of the book series: Parasitology Research Monographs ((Parasitology Res. Monogr.,volume 18))
85 Accesses
Understanding the pathogenesis of malaria requires a detailed investigation of the mechanisms of Plasmodium invasion, Plasmodium biology, and host defense, and by understanding the life history of Plasmodium , the impact of Plasmodium and host interactions on each other can be elucidated. Plasmodium falciparum infection causes the most severe clinical manifestations, and its pathogenesis is the best studied. The clinical presentation of patients with malaria is closely related to the malaria parasites and varies according to local malaria epidemiology, the patient's immune status and age. Populations at high risk of malaria include infants and young children (6–59 months), where severe malaria can occur, and pregnant women, where anemia and low birth weight neonates may occur. In areas where malaria is transmitted year-round, older children and adults develop partial immunity after repeated infections and are therefore at relatively low risk of severe malaria. Treatment of malaria includes antimalarial treatment and symptomatic management, which is difficult given the pathophysiological changes in multiple organ systems involved in severe malaria. This topic will discuss in detail the pathogenesis and clinical features of malaria.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Aikawa M (1988) Morphological changes in erythrocytes induced by malarial parasites. Biol Cell 64(2):173–181
Article CAS PubMed Google Scholar
Allen SJ, O'Donnell A, Alexander ND et al (1997) alpha+-Thalassemia protects children against disease caused by other infections as well as malaria. Proc Natl Acad Sci U S A 94(26):14736–14741
Article CAS PubMed PubMed Central Google Scholar
Angchaisuksiri P (2014) Coagulopathy in malaria. Thromb Res 133(1):5–9
Ayi K, Turrini F, Piga A, Arese P (2004) Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood 104(10):3364–3371
Boele van Hensbroek M, Calis JC, Phiri KS et al (2010) Pathophysiological mechanisms of severe anaemia in Malawian children. PLoS One 5(9):e12589
Article PubMed PubMed Central Google Scholar
Bunn HF (2013) The triumph of good over evil: protection by the sickle gene against malaria. Blood 121(1):20–25
Bunyaratvej A, Butthep P, Yuthavong Y et al (1986) Increased phagocytosis of Plasmodium falciparum-infected erythrocytes with haemoglobin E by peripheral blood monocytes. Acta Haematol 76(2-3):155–158
Chaiyaroj SC, Angkasekwinai P, Buranakiti A, Looareesuwan S, Rogerson SJ, Brown GV (1996) Cytoadherence characteristics of Plasmodium falciparum isolates from Thailand: evidence for chondroitin sulfate a as a cytoadherence receptor. Am J Trop Med Hyg 55(1):76–80
Chen Q, Schlichtherle M, Wahlgren M (2000) Molecular aspects of severe malaria. Clin Microbiol Rev 13(3):439–450
Chookajorn T, Ponsuwanna P, Cui L (2008) Mutually exclusive var gene expression in the malaria parasite: multiple layers of regulation. Trends Parasitol 24(10):455–461
Cohee LM, Kalilani-Phiri L, Mawindo P et al (2016) Parasite dynamics in the peripheral blood and the placenta during pregnancy-associated malaria infection. Malar J 15(1):483
Conroy AL, Hawkes M, Elphinstone RE et al (2016) Acute kidney injury is common in pediatric severe malaria and is associated with increased mortality. Open Forum Infect Dis 3(2):ofw046
D’Ombrain MC, Robinson LJ, Stanisic DI et al (2008) Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 47(11):1380–1387
Article PubMed Google Scholar
Das BS (2008) Renal failure in malaria. J Vector Borne Dis 45(2):83–97
CAS PubMed Google Scholar
Devarbhavi H, Alvares JF, Kumar KS (2005) Severe falciparum malaria simulating fulminant hepatic failure. Mayo Clin Proc 80(3):355–358
Dondorp AM (2008) Clinical significance of sequestration in adults with severe malaria. Transfus Clin Biol 15(1-2):56–57
Dondorp AM, Lee SJ, Faiz MA et al (2008) The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis 47(2):151–157
Doolan DL, Dobaño C, Baird JK (2009) Acquired immunity to malaria. Clin Microbiol Rev 22(1):13–36, Table of Contents.
Espinoza E, Hidalgo L, Chedraui P (2005) The effect of malarial infection on maternal-fetal outcome in Ecuador. J Matern Fetal Neonatal Med 18(2):101–105
Fried M, Kurtis JD, Swihart B et al (2017) Systemic inflammatory response to malaria during pregnancy is associated with pregnancy loss and preterm delivery. Clin Infect Dis 65(10):1729–1735
Gong L, Maiteki-Sebuguzi C, Rosenthal PJ et al (2012) Evidence for both innate and acquired mechanisms of protection from Plasmodium falciparum in children with sickle cell trait. Blood 119(16):3808–3814
Grigg MJ, Snounou G (2017) Plasmodium simium: a Brazilian focus of anthropozoonotic vivax malaria? Lancet Glob Health 5(10):e961–e9e2
Hochman SE, Madaline TF, Wassmer SC et al (2015) Fatal pediatric cerebral malaria is associated with intravascular monocytes and platelets that are increased with HIV coinfection. mBio 6(5):e01390–e01315
Idro R, Ndiritu M, Ogutu B et al (2007) Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA 297(20):2232–2240
Kreuels B, Kreuzberg C, Kobbe R et al (2010) Differing effects of HbS and HbC traits on uncomplicated falciparum malaria, anemia, and child growth. Blood 115(22):4551–4558
Lampah DA, Yeo TW, Malloy M et al (2015) Severe malarial thrombocytopenia: a risk factor for mortality in Papua, Indonesia. J Infect Dis 211(4):623–634
Leoratti FM, Durlacher RR, Lacerda MV et al (2008) Pattern of humoral immune response to Plasmodium falciparum blood stages in individuals presenting different clinical expressions of malaria. Malar J 7:186
Liehl P, Zuzarte-Luís V, Chan J et al (2014) Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med 20(1):47–53
Lin MJ, Nagel RL, Hirsch RE (1989) Acceleration of hemoglobin C crystallization by hemoglobin S. Blood 74(5):1823–1825
Maubert B, Fievet N, Tami G, Boudin C, Deloron P (2000) Cytoadherence of Plasmodium falciparum-infected erythrocytes in the human placenta. Parasite Immunol 22(4):191–199
Maubert B, Guilbert LJ, Deloron P (1997) Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect Immun 65(4):1251–1257
McGready R, Wongsaen K, Chu CS et al (2014) Uncomplicated Plasmodium vivax malaria in pregnancy associated with mortality from acute respiratory distress syndrome. Malar J 13:191
Mung'Ala-Odera V, Snow RW, Newton CR (2004) The burden of the neurocognitive impairment associated with Plasmodium falciparum malaria in sub-saharan Africa. Am J Trop Med Hyg 71(2 Suppl):64–70
Nagel RL, Raventos-Suarez C, Fabry ME, Tanowitz H, Sicard D, Labie D (1981) Impairment of the growth of Plasmodium falciparum in HbEE erythrocytes. J Clin Invest 68(1):303–305
Newbold C, Craig A, Kyes S, Rowe A, Fernandez-Reyes D, Fagan T (1999) Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. Int J Parasitol 29(6):927–937
Oh SS, Chishti AH, Palek J, Liu SC (1997) Erythrocyte membrane alterations in Plasmodium falciparum malaria sequestration. Curr Opin Hematol 4(2):148–154
Olliaro P, Nevill C, LeBras J et al (1996) Systematic review of amodiaquine treatment in uncomplicated malaria. Lancet 348(9036):1196–1201
Pasvol G, Weatherall DJ, Wilson RJ (1977) Effects of foetal haemoglobin on susceptibility of red cells to Plasmodium falciparum. Nature 270(5633):171–173
Ponsford MJ, Medana IM, Prapansilp P et al (2012) Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis 205(4):663–671
Ranque S, Safeukui I, Poudiougou B et al (2005) Familial aggregation of cerebral malaria and severe malarial anemia. J Infect Dis 191(5):799–804
Rénia L, Howland SW, Claser C et al (2012) Cerebral malaria: mysteries at the blood-brain barrier. Virulence 3(2):193–201
Reyburn H, Mbatia R, Drakeley C et al (2005) Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA 293(12):1461–1470
Roberts DJ, Casals-Pascual C, Weatherall DJ (2005) The clinical and pathophysiological features of malarial anaemia. Curr Top Microbiol Immunol 295:137–167
Rogerson SJ, Tembenu R, Dobaño C, Plitt S, Taylor TE, Molyneux ME (1999) Cytoadherence characteristics of Plasmodium falciparum-infected erythrocytes from Malawian children with severe and uncomplicated malaria. Am J Trop Med Hyg 61(3):467–472
Schwartz E, Parise M, Kozarsky P, Cetron M (2003) Delayed onset of malaria—implications for chemoprophylaxis in travelers. N Engl J Med 349(16):1510–1516
Sharma YD (1991) Knobs, knob proteins and cytoadherence in falciparum malaria. Int J Biochem 23(9):775–789
Sharma YD (1997) Knob proteins in falciparum malaria. Indian J Med Res 106:53–62
Steketee RW, Nahlen BL, Parise ME, Menendez C (2001) The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 64(1-2 Suppl):28–35
Stevenson MM, Riley EM (2004) Innate immunity to malaria. Nat Rev Immunol 4(3):169–180
Svenson JE, MacLean JD, Gyorkos TW, Keystone J (1995) Imported malaria. Clinical presentation and examination of symptomatic travelers. Arch Intern Med 155(8):861–868
Taylor WRJ, Hanson J, Turner GDH, White NJ, Dondorp AM (2012) Respiratory manifestations of malaria. Chest 142(2):492–505
Tiffert T, Lew VL, Ginsburg H, Krugliak M, Croisille L, Mohandas N (2005) The hydration state of human red blood cells and their susceptibility to invasion by Plasmodium falciparum. Blood 105(12):4853–4860
(1990) Severe and complicated malaria. World Health Organization, Division of Control of Tropical Diseases. Trans R Soc Trop Med Hyg 84(Suppl 2):1–65
Google Scholar
Travassos MA, Coulibaly D, Laurens MB et al (2015) Hemoglobin C trait provides protection from clinical falciparum malaria in malian children. J Infect Dis 212(11):1778–1786
(2014) Severe malaria. Trop Med Int Health 19(Suppl 1):7–131
Viebig NK, Wulbrand U, Förster R, Andrews KT, Lanzer M, Knolle PA (2005) Direct activation of human endothelial cells by Plasmodium falciparum-infected erythrocytes. Infect Immun 73(6):3271–3277
White NJ (1996) The treatment of malaria. N Engl J Med 335(11):800–806
Willcox M, Björkman A, Brohult J (1983) Falciparum malaria and beta-thalassaemia trait in northern Liberia. Ann Trop Med Parasitol 77(4):335–347
Woodford J, Shanks GD, Griffin P, Chalon S, McCarthy JS (2018) The dynamics of liver function test abnormalities after malaria infection: a retrospective observational study. Am J Trop Med Hyg 98(4):1113–1119
World Health Organization (2015) WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for the treatment of malaria. World Health Organization, Geneva. Copyright © World Health Organization 2015.
Wu X, Gowda N, Gowda D (2015) Phagosomal acidification prevents macrophage inflammatory cytokine production to malaria, and dendritic cells are the major source at the early stages of infection: IMPLICATION FOR MALARIA PROTECTIVE IMMUNITY DEVELOPMENT. J Biol Chem 290(38):23135–23147
Download references
Author information
Authors and affiliations.
Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Huilong Chen
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Department of Parasitology, Heinrich Heine University, Düsseldorf, Germany
Heinz Mehlhorn
School of Basic Medical Sciences, Hubei University of Medicine, Shiyan City, China
Wuhan Center for Disease Control and Prevention, Wuhan City, China
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Chen, H. (2023). Pathogenesis and Clinical Features of Malaria. In: Mehlhorn, H., Li, J., Wu, K. (eds) Malaria Control and Elimination in China. Parasitology Research Monographs, vol 18. Springer, Cham. https://doi.org/10.1007/978-3-031-32902-9_5
Download citation
DOI : https://doi.org/10.1007/978-3-031-32902-9_5
Published : 30 September 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-32901-2
Online ISBN : 978-3-031-32902-9
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
On This Page
- Incubation Period
- Uncomplicated Malaria
- Severe Malaria
- Malaria Relapses
- Other Manifestations of Malaria
Infection with malaria parasites may result in a wide variety of symptoms, ranging from absent or very mild symptoms to severe disease and even death. Malaria disease can be categorized as uncomplicated or severe (complicated) . In general, malaria is a curable disease if diagnosed and treated promptly and correctly.
All the clinical symptoms associated with malaria are caused by the asexual erythrocytic or blood stage parasites. When the parasite develops in the erythrocyte, numerous known and unknown waste substances such as hemozoin pigment and other toxic factors accumulate in the infected red blood cell. These are dumped into the bloodstream when the infected cells lyse and release invasive merozoites. The hemozoin and other toxic factors such as glucose phosphate isomerase (GPI) stimulate macrophages and other cells to produce cytokines and other soluble factors which act to produce fever and rigors and probably influence other severe pathophysiology associated with malaria.
Plasmodium falciparum- infected erythrocytes, particularly those with mature trophozoites, adhere to the vascular endothelium of venular blood vessel walls and do not freely circulate in the blood. When this sequestration of infected erythrocytes occurs in the vessels of the brain it is believed to be a factor in causing the severe disease syndrome known as cerebral malaria, which is associated with high mortality.
Following the infective bite by the Anopheles mosquito , a period of time (the “incubation period”) goes by before the first symptoms appear. The incubation period in most cases varies from 7 to 30 days. The shorter periods are observed most frequently with P. falciparum and the longer ones with P. malariae .
Antimalarial drugs taken for prophylaxis by travelers can delay the appearance of malaria symptoms by weeks or months, long after the traveler has left the malaria-endemic area. (This can happen particularly with P. vivax and P. ovale , both of which can produce dormant liver stage parasites; the liver stages may reactivate and cause disease months after the infective mosquito bite.)
Such long delays between exposure and development of symptoms can result in misdiagnosis or delayed diagnosis because of reduced clinical suspicion by the health-care provider. Returned travelers should always remind their health-care providers of any travel in areas where malaria occurs during the past 12 months.
Top of Page
The classical (but rarely observed) malaria attack lasts 6–10 hours. It consists of
- A cold stage (sensation of cold, shivering)
- A hot stage (fever, headaches, vomiting; seizures in young children); and
- Finally a sweating stage (sweats, return to normal temperature, tiredness).
Classically (but infrequently observed) the attacks occur every second day with the “tertian” parasites ( P. falciparum , P. vivax , and P. ovale ) and every third day with the “quartan” parasite ( P. malariae ).
More commonly, the patient presents with a combination of the following symptoms:
- Nausea and vomiting
- General malaise
In countries where cases of malaria are infrequent, these symptoms may be attributed to influenza, a cold, or other common infections, especially if malaria is not suspected. Conversely, in countries where malaria is frequent, residents often recognize the symptoms as malaria and treat themselves without seeking diagnostic confirmation (“presumptive treatment”). Physical findings may include the following:
- Elevated temperatures
- Perspiration
- Enlarged spleen
- Mild jaundice
- Enlargement of the liver
- Increased respiratory rate
Diagnosis of malaria depends on the demonstration of parasites in the blood, usually by microscopy. Additional laboratory findings may include mild anemia, mild decrease in blood platelets (thrombocytopenia), elevation of bilirubin, and elevation of aminotransferases. Top of Page
Severe malaria occurs when infections are complicated by serious organ failures or abnormalities in the patient’s blood or metabolism. The manifestations of severe malaria include the following:
- Cerebral malaria, with abnormal behavior, impairment of consciousness, seizures, coma, or other neurologic abnormalities
- Severe anemia due to hemolysis (destruction of the red blood cells)
- Hemoglobinuria (hemoglobin in the urine) due to hemolysis
- Acute respiratory distress syndrome (ARDS), an inflammatory reaction in the lungs that inhibits oxygen exchange, which may occur even after the parasite counts have decreased in response to treatment
- Abnormalities in blood coagulation
- Low blood pressure caused by cardiovascular collapse
- Acute kidney injury
- Hyperparasitemia, where more than 5% of the red blood cells are infected by malaria parasites
- Metabolic acidosis (excessive acidity in the blood and tissue fluids), often in association with hypoglycemia
Severe malaria is a medical emergency and should be treated urgently and aggressively.
In P. vivax and P. ovale infections, patients having recovered from the first episode of illness may suffer several additional attacks (“relapses”) after months or even years without symptoms. Relapses occur because P. vivax and P. ovale have dormant liver stage parasites ( “hypnozoites” ) that may reactivate. Treatment to reduce the chance of such relapses is available and should follow treatment of the first attack.
- Neurologic defects may occasionally persist following cerebral malaria, especially in children. Such defects include trouble with movements (ataxia), palsies, speech difficulties, deafness, and blindness.
- Recurrent infections with P. falciparum may result in severe anemia. This occurs especially in young children in tropical Africa with frequent infections that are inadequately treated.
- Malaria during pregnancy (especially P. falciparum ) may cause severe disease in the mother, and may lead to premature delivery or delivery of a low-birth-weight baby.
- On rare occasions, P. vivax malaria can cause rupture of the spleen.
- Nephrotic syndrome (a chronic, severe kidney disease) can result from chronic or repeated infections with P. malariae .
- Hyperreactive malarial splenomegaly (also called “tropical splenomegaly syndrome”) occurs infrequently and is attributed to an abnormal immune response to repeated malarial infections. The disease is marked by a very enlarged spleen and liver, abnormal immunologic findings, anemia, and a susceptibility to other infections (such as skin or respiratory infections).
To receive email updates about this page, enter your email address:
New! Locally Acquired Cases of Malaria in Florida, Texas, Maryland, and Arkansas
New! Update to Guidance for use of Artemether-Lumefantrine (Coartem®) in Pregnancy for Uncomplicated Malaria New! Discontinuation of CDC’s Distribution of Intravenous Artesunate as Commercial Drug Guidance for Malaria Diagnosis in Patients Suspected of Ebola Infection in the United States -->
See all Malaria Notices
- New! Malaria is a Serious Disease
- New! La malaria (paludismo) es una enfermedad grave
- How to Report a Case of Malaria
- CDC Yellow Book
- Red Pages: Malaria-endemic areas by country
- Drugs for Prevention
- Choosing a Drug to Prevent Malaria
- Drugs for Treatment in the U.S.
- Frequently Asked Questions (FAQs)
- Blood Banks
Click here for contact information
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Skip to content
- Accessibility help
Malaria: When should I suspect malaria?
Last revised in January 2024
When should I suspect malaria?
- Almost all cases of P. falciparum malaria present within 6 months of exposure and most within 2–3 months.
- Infection with other malaria species, such as P. ovale and P. vivax may present months or years after exposure due to reactivation of hypnozoites (a dormant liver stage).
- Presentation may be delayed in people who have taken chemoprophylaxis.
- Most missed cases of malaria are wrongly diagnosed as non-specific viral infections, influenza, gastroenteritis, and hepatitis.
- Fever (often 39°C or higher), sweats, and/or chills — absence of fever should not remove the suspicion of malaria.
- General malaise, lethargy, and fatigue — somnolence is more common in children than in adults.
- Anorexia, gastrointestinal disturbance (such as nausea, abdominal pain, vomiting, diarrhoea), and jaundice.
- Poor feeding in children.
- Myalgia and arthralgia.
- Sore throat, cough, lower respiratory tract symptoms, and respiratory distress.
- Hepatomegaly, splenomegaly, and somnolence are more common on examination in children.
- Cerebral malaria — impaired conscious level ( Glasgow coma score less than 11) or seizures.
- Renal impairment (may present with oliguria).
- Acidosis (may present with acidotic breathing).
- Hypoglycaemia (< 2.2 mmol/L) — common in pregnant women.
- Respiratory distress which may be due to pulmonary oedema or acute respiratory distress syndrome (ARDS) — common in pregnant women.
- Severe anaemia (may present with pallor).
- Spontaneous bleeding/disseminated intravascular coagulation.
- Shock (BP < 90/60 mmHg).
- Sepsis — more common in pregnant women.
- Haemoglobinuria — P. falciparum can cause severe haemolysis with dark red urine (‘blackwater fever’).
- Parasitaemia > 10%.
- Cerebral malaria — impaired conscious level ( Glasgow coma score less than 11 or Blantyre coma score less than 3), seizures, altered respiration, and posturing (decorticate or decerebrate).
- Respiratory distress or acidosis (may present with acidotic breathing).
- Hypoglycaemia (< 2.2 mmol/L).
- Prostration (inability to stand or sit).
- Parasitaemia > 2% red blood cells parasitized.
[ Lalloo, 2016 ; CDC, 2020 ; Behrens, 2021 ]
Basis for recommendation
The recommendations on when to suspect malaria are based on the clinical guidelines U K malaria treatment guidelines 2016 [ Lalloo, 2016 ], CDC guidance Malaria treatment [ CDC, 2020 ], and expert opinion in BMJ Best Practice review Malaria infection [ Behrens, 2021 ].
Suspect malaria in all returning travellers who are unwell, have a fever, or history of fever
- A high degree of suspicion is needed in returning travellers with non-specific symptoms — misdiagnosis and delay of treatment are the most common reasons cited for death from malaria in Europe and the USA.
- A retrospective observational study of malaria-related deaths in the UK (n = 191) between 1987 and 2006 found case fatality to be inversely related to malaria incidence and suggested that malaria is more easily missed if clinicians are unused to seeing it [ Checkley, 2012 ].
- A retrospective study of malaria in Sheffield between 2000 and 2005 (n = 39) found that eight people presenting to healthcare professionals with malaria symptoms were not immediately referred for diagnosis or treatment suggesting that malaria was not considered in the initial differential [ Green, 2009 ].
Clinical features of malaria are non-specific
- Malaria in children (and sometimes in adults) can present with non-specific or misleading symptoms — a high degree of suspicion is needed [ Lalloo, 2016 ].
- Most missed malaria infections are incorrectly diagnosed as non-specific viral infections, influenza, gastroenteritis, or hepatitis [ Lalloo, 2016 ].
Absence of fever does not rule out malaria
- Fever is not always present in malaria — absence of fever should not remove the suspicion of malaria in an ill person [ Lalloo, 2016 ].
The content on the NICE Clinical Knowledge Summaries site (CKS) is the copyright of Clarity Informatics Limited (trading as Agilio Software Primary Care) . By using CKS, you agree to the licence set out in the CKS End User Licence Agreement .
- Search Menu
- Volume 2024, Issue 4, April 2024 (In Progress)
- Volume 2024, Issue 3, March 2024
- Case of the Year
- MSF Case Reports
- Audiovestibular medicine
- Cardiology and cardiovascular systems
- Critical care medicine
- Dermatology
- Emergency medicine
- Endocrinology and metabolism
- Gastroenterology and hepatology
- Geriatrics and gerontology
- Haematology
- Infectious diseases and tropical medicine
- Medical ophthalmology
- Medical disorders in pregnancy
- Paediatrics
- Palliative medicine
- Pharmacology and pharmacy
- Radiology, nuclear medicine, and medical imaging
- Respiratory disorders
- Rheumatology
- Sexual and reproductive health
- Sports medicine
- Substance abuse
- Author Guidelines
- Submission Site
- Open Access
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, conflict of interest statement, ethical approval.
- < Previous
Email alerts
Citing articles via, affiliations.
- Online ISSN 2053-8855
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
COVID-19 and malaria co-infection: a systematic review of clinical outcomes in endemic areas
Affiliations.
- 1 Jazan University, Jazan, Saudi Arabia.
- 2 Univ Lille, CHU Lille Laboratoire de Virologie ULR3610, Lille, France.
- PMID: 38646476
- PMCID: PMC11032658
- DOI: 10.7717/peerj.17160
Background: COVID-19 and malaria cause significant morbidity and mortality globally. Co-infection of these diseases can worsen their impact on public health. This review aims to synthesize literature on the clinical outcomes of COVID-19 and malaria co-infection to develop effective prevention and treatment strategies.
Methods: A comprehensive literature search was conducted using MeSH terms and keywords from the start of the COVID-19 pandemic to January 2023. The review included original articles on COVID-19 and malaria co-infection, evaluating their methodological quality and certainty of evidence. It was registered in PROSPERO (CRD42023393562).
Results: Out of 1,596 screened articles, 19 met the inclusion criteria. These studies involved 2,810 patients, 618 of whom had COVID-19 and malaria co-infection. Plasmodium falciparum and vivax were identified as causative organisms in six studies. Hospital admission ranged from three to 18 days. Nine studies associated co-infection with severe disease, ICU admission, assisted ventilation, and related complications. One study reported 6% ICU admission, and mortality rates of 3%, 9.4%, and 40.4% were observed in four studies. Estimated crude mortality rates were 10.71 and 5.87 per 1,000 person-days for patients with and without concurrent malaria, respectively. Common co-morbidities included Diabetes mellitus, hypertension, cardiovascular diseases, and respiratory disorders.
Conclusion: Most patients with COVID-19 and malaria co-infection experienced short-term hospitalization and mild to moderate disease severity. However, at presentation, co-morbidities and severe malaria were significantly associated with higher mortality or worse clinical outcomes. These findings emphasize the importance of early detection, prompt treatment, and close monitoring of patients with COVID-19 and malaria co-infection.
Keywords: COVID-19; Clinical outcome; Co-infection; Infection; Malaria; Morbidity; Mortality.
© 2024 Mohamed et al.
Publication types
- Systematic Review
- COVID-19* / complications
- COVID-19* / epidemiology
- COVID-19* / mortality
- Coinfection* / epidemiology
- Comorbidity
- Hospitalization / statistics & numerical data
- Malaria* / epidemiology
- Malaria, Falciparum / complications
- Malaria, Falciparum / epidemiology
- SARS-CoV-2*
Grants and funding
- Open access
- Published: 29 April 2024
Blood count changes in malaria patients according to blood groups (ABO/Rh) and sickle cell trait
- Euclides N. M. Sacomboio 1 , 2 , 3 ,
- Santo D. Zua 1 ,
- Adelino T. Tchivango 4 ,
- António D. Pululu 1 ,
- Adilson C. D. Caumba 1 ,
- Adelina B. M. Paciência 1 ,
- Danilson V. Sati 1 ,
- Sabina G. Agostinho 1 ,
- Yolanda S. Agostinho 1 ,
- Fernando G. Mazanga 1 ,
- Neusa B. Ntambo 1 ,
- Cruz S. Sebastião 1 , 5 , 6 ,
- Joana P. Paixão 5 , 6 &
- Joana Morais 5 , 7
Malaria Journal volume 23 , Article number: 126 ( 2024 ) Cite this article
Metrics details
Introduction
Introduction: Malaria continues to be the leading cause of hospitalization and death in Angola, a country in sub- Saharan Africa. In 2023, in the first quarter, 2,744,682 cases were registered, and of these 2,673 patients died due to malaria disease. Previous studies have shown that the ABO blood group can affect the progression of malaria to severe conditions after P. falciparum infection, while the sickle cell gene offers relative protection.
We investigated changes in the blood count according to blood groups (ABO/Rh) and sickle cell trait in patients with malaria in Luanda, capital of Angola.
Methodology
This was a longitudinal, prospective and observational study with 198 patients hospitalized for malaria.
Of the 198 patients studied, 13(6.6%) were ABRh(+), 4(2.0%) were ARh(-), 49(24.7%) were ARh(+), 42(21, 2%) were BRh (+), 5(2.5%) were ORh(-) and 85(42.9%) were ORh(+). For sickle cell trait, 145(73.2%) were AA, 37(18.7%) were AS and 16(8.1%) were SS. No statistical relationship was observed between age group, sex, parasitemia, clinical picture, hematocrit, MCV, HCM, MCHC, leukocytes, NEUT, LINF and PTL values with blood groups (p<0.05), but there was a relationship between values of hemoglobin and ABO/Rh blood groups (p>0.05). There was no relationship between age, parasitemia, clinical condition, MCV, HCM and MCHC values, leukocytes, NEUT and LINF with sickle cell trait (p<0.05), but there was a relationship between sex, hemoglobin and PTL and sickle cell values. sickle cell trait (p>0.05).
It is imperative to differentiate patients with malaria based on blood groups and sickle cell trait, taking into account mainly the blood count parameters that demonstrate that there are patients who, depending on blood group or sickle cell trait, may react weakly to malaria infection regardless of the degree of parasitemia and medical prognosis.
Malaria remains the leading cause of hospitalization and death in Angola. In 2022, around 9,211,346 cases of the disease were reported and 12,485 patients died from the disease, which is an increase of 41,886 cases compared to the year 2011 when there were fewer records of cases. It is suspected that because of attention to COVID-19, many cases ended up being underreported. In the first quarter of 2023, 2,744,682 cases were registered and of these 2673 patients died due to the disease [ 1 , 2 ].
Several genetic variants or polymorphisms of red blood cells have been identified as cofactors that can make humans relatively more susceptible or resistant to Plasmodium falciparum and affect clinical outcomes. Many studies have shown that the interaction between the ABO blood group and the infection by P. falciparum can increase or decrease the severity of the disease, where it seems that individuals from blood groups A, B, and AB are more susceptible to the severity of malaria compared to those from blood group O [ 3 , 4 , 5 ].
Studies published so far demonstrate that the ABO blood group can affect the progression of malaria to serious situations after infection with P. falciparum , since some groups, such as the ABO blood group, delay the clearance of parasitized red blood cells (pRBCs) promoting the formation of rosettes and cytoadhesion, while other groups such as blood group O increase the clearance of pGVs red blood cells by reducing rosette formation and cytoadhesion [ 6 , 7 , 8 ].
Sickle cell anaemia is a serious public health problem, mainly present in tropical countries, especially in sub-Saharan Africa, and the World Health Organization (WHO) estimates that 300,000 children are born with sickle cell anaemia each year, 75% of which are in sub-Saharan Africa [ 9 ].
Other studies proved that the (SS) gene does not protect against infection by the malaria parasite, but prevents the establishment of the disease after infection, which demonstrates that the sickle cell gene offers relative protection against malaria, it can be expected that the protection is at least as effective in the homozygous state (SS) [ 10 ], however, clinical experience has shown that it is more dangerous, as malaria not only worsens pre-existing anaemia in SS patients to the point of becoming life-threatening but also abnormal splenic function in patients with sickle cell anaemia which makes it difficult to eliminate parasitized red blood cells and for this reason in African countries, malaria contributes substantially to the early mortality of patients with sickle cell anaemia [ 10 , 11 ].
Plasmodium falciparum infection is usually fatal in individuals with sickle cell anaemia (HbSS), as protection against infection appears to operate in a dose-dependent manner with HbS, therefore, individuals with HbSS have an even lower risk of infection than those with HbAS [ 11 ]. Although sickle cell disease (SCD) is primarily a disease of red blood cells, both leukocytes and thrombocytes are equally affected, as in malarial infection, and are known to cause sickle cell crises through vaso-occlusion [ 12 ].
Studies developed by our research team in Angola have shown that non-O blood groups appear to be important biological factors for SARS-CoV-2 infection and the risk of developing cardiovascular disease after or during exposure to SARS-CoV-2 [ 13 ]. HIV infection seems to be common in ORh + individuals, where alterations in the blood count occur moderately in individuals from groups O and A and biochemical alterations in individuals A, B, and O [ 14 ]. Leprosy seems to be common in ORh + individuals, where changes in the blood count are greater in non-O individuals [ 15 ].
Chronic kidney disease seems to be more frequent in ORh+ blood group patients, followed by ARh+ and BRh+ , who resided in urbanized and rural areas born in the north of Angola [ 16 ]. Patients with nephrotic syndrome (NS) and sickle cell anaemia, found that the majority of the population belonged to the ORh + group, followed by patients from the ABRh+ , ARh+ , and BRh+ groups [ 17 ]. In hypertensive individuals, most patients had blood group B, blood group O, and, Rh+ [ 18 ], and the incidence of sickle cell trait was found to be high among individuals from the ORh+ and ABRh+ group [ 19 ].
It was found that studies are showing that genetic factors such as red blood cell polymorphisms and sickle cell anaemia may have influenced the severity of the disease due to P. falciparum infection, however, there is a lack of information about the role of host genetic factors (such as ABO/Rh blood group and sickle cell trait) in changing the blood count of patients with malaria. In this study, we investigated changes in the blood count in patients with malaria according to blood groups (ABO/Rh) and sickle cell trait admitted to a tertiary hospital in Luanda, the capital city of Angola.
Study design and setting
A longitudinal, prospective, and observational study was performed with 217 patients hospitalized due to malaria in Josina Machel, a tertiary Hospital, from March to August 2023. The patients were invited and freely consented to participate in the study, those patients who were unable to provide blood samples or to give their informed consent were excluded from the study population. A total of 198 patients fulfilled the inclusion criteria and were enrolled in the study. The study protocol was revised and approved by the scientific council of the Institute of Health Sciences of Agostinho Neto University (118/GD/ICISA/UAN/2021) and by the clinical management of Josina Machel Hospital (36/DPC/HJM/2023).
Patient’s enrolment criteria and sample collection
Malaria diagnosis were performed by Josina Machel Hospital professionals using rapid malaria antigen tests (SD-Bioline Malaria AG Pf/PAN) and confirmed with microscopy technique of direct visualization of the parasite by Giemsa-stained peripheral blood thick films. Patients who presented parasitaemia less than or equal to 1000 p/mm 3 were classified as moderate parasitaemia whilst patients who presented parasitaemia above 1000 p/mm 3 were classified as high parasitaemia [ 20 ]. For the clinical data presented in the article, a blood sample was taken from the patients in test tubes containing EDTA (ethylenediaminetetraacetic acid) anticoagulant specific for the ABO and Rh blood group phenotyping tests and for the electrophoresis examination. Haemoglobin electrophoresis was performed using a device called Sebia brand Minicap, the Hb (E) minicab kit was developed to separate normal haemoglobins (A, F, and A2) and to detect and quantify variant haemoglobins (including S, C, E, D). Blood group determination was performed by the microplate technique, which is an agglutination test between patient serum and Anti A, Anti B, and Anti D reagents in each of the wells for phenotypic identification of blood groups (ABO and Rh) [ 21 ]. The samples were placed in three wells and the posterior was associated with anti-A, anti-B, and anti-D reagents (Immucor, Portugal).
The erythrogram and white blood cell data were evaluated on the admission of patients before starting treatment, complete blood count or haemogram was determined using the Automated Hematology Analyzer SYSMEX XT-4000i (Sysmex Europe SE, Germany). For the erythrogram data, haemoglobin (Hb), red blood cell count (RBCs), haematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), and mean corpuscular haemoglobin concentration (MCHC) were evaluated. For the leukocyte count data, the lymphocytes, platelets, neutrophils, and leukocyte counts were evaluated [ 22 ]. The study did not include monocyte count, eosinophil count, neutrophil-to-lymphocyte ratio (NLR), and monocyte-to-lymphocyte ratio (MLR) due to the devices that had some problems in reading these exams in some of the patients, they did not obtain data from all patients included in the study, these data were not analyzed in the study because they were not complete for all patients. In cases where the reference values of blood cell count components were different between men and women, the reference values were adjusted for the whole group based on the minimum value for women and the maximum value for men, in addition, adjustment was performed according to the references according to the specific characteristics of the Angolan population, as can be seen in the presentation of the results. All blood count results were classified as altered and clinically significant when they were higher or lower than 10% of the reference value for the test.
Statistical analysis
Descriptive statistics were calculated using the statistical program SPSS v20.0 (IBM SPSS Statistics, USA), and the results presented in graphs were developed using Sigmaplot 12.0 (Systat Software, Inc.). The descriptive analysis was presented with frequencies and percentages. The normal distribution of data was presented as mean and standard deviation (SD). The Chi-square (X 2 ) test was used to assess the relationship between categorical variables. All reported p-values are two-tailed and deemed significant when p < 0.05.

Clinical data and distribution of ABO/Rh blood groups and sickle cell trait
Clinical data are presented in Table 1 , where it was found that in terms of age groups, most patients included in the study were young (75.8%, n = 150/198) aged between 20 and 40 years. It was in this group where only individuals with the ARh(−) blood group and it was in this group where the majority of patients with sickle cell anaemia (75%) and with sickle cell trait (89.2%) were identified. As for gender, it was found that women constituted the majority in this study group (61.6%, n = 122/198) and they also represented the majority of patients with sickle cell anaemia (75%) and with the sickle cell trait (78.4%), in all blood groups women had 50% or more, in groups ABRh (+), ARh(+) and ORh(+), men represented less than 40% of the studied population. In assessing the degree of parasitaemia, it was noticed that most patients had low parasitaemia on hospital admission (less than 51 parasites/mm3) and among individuals with sickle cell anaemia, 93.8% had low parasitaemia, high parasitaemia was observed mostly in individuals of the ABRh(+), BRh(+) and ORh(+) group, which presented a percentage ranged between 25% and 38%. As for the clinical condition, most patients had a clinical picture considered moderate (53.5%, n = 106/198) and this picture was also verified concerning sickle cell trait, but it was found that 46.5% of ABRh patients (+) had a severe clinical picture, patients in the ARh(+), BRh(+) and ORh(−) group also had a severe clinical picture equal to or greater than 20%. Statistical analysis showed no statistical relationship between age group, gender, parasitaemia, and clinical condition with blood groups (p < 0.05). There was no relationship between age, parasitaemia, and clinical condition with sickle cell trait (p < 0.05), but there was a relationship between gender and sickle cell trait (p > 0.05).
Condition of work and ABO/Rh blood groups and sickle cell trait
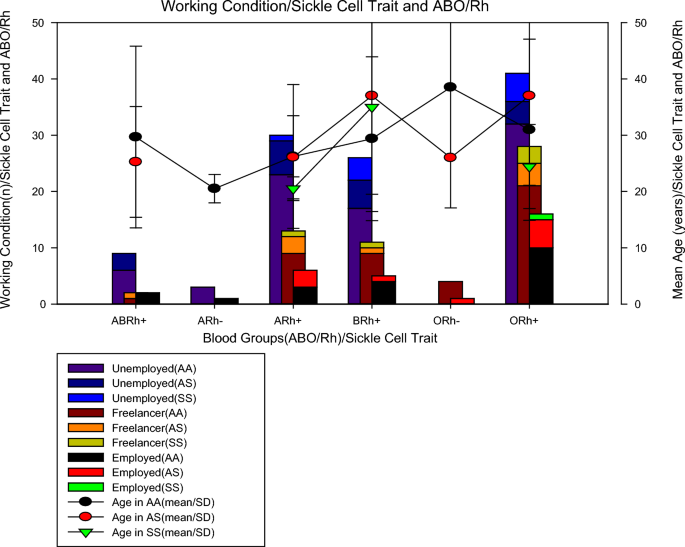
In Fig. 1 , it can be seen that of the 198 patients studied, regarding blood groups, 13 (6.6%) were from the ABRh(+) group, 4 (2.0%) were from the ARh(−) group, 49 (24.7%) were from the ARh(+) group, 42 (21.2%) were from the BRh(+) group, 5 (2.5%) were from the ORh(−) group and 85 (42.9%) were from the ORh(+) group. As for the sickle cell trait, we found that 145 (73.2%) were AA, 37 (18.7%) were AS and 16 (8.1%) were SS. Of the 16 (8.1%) patients with sickle cell anaemia, 2 were in the anaemia+) group, 5 in the BRh(+) group, and 9 in the ORh(+) group, no Rh(-) patients with sickle cell trait were found. One piece of information that aroused the most interest was to verify the work status of patients with sickle cell anaemia and it was found that among individuals with sickle cell trait, most were unemployed (62.5%, n = 10/16), others were self-employed (31, 25%, n = 5/16) and only 6.25% (1/16) work formally. To rule out the possibility that unemployment was due to age, it was calculated the mean age of the individuals studied in those studied blood groups and sickle cell traits and it was noted that all groups of individuals with sickle cell anaemia belonging to the ARh(+) groups, BRh(+) and ORh(+) had a mean age greater than 20 years old, which means that age was not the limiting factor, more possibly the health condition.
Evaluation of the erythrogram according to the ABO/Rh blood groups and sickle cell trait
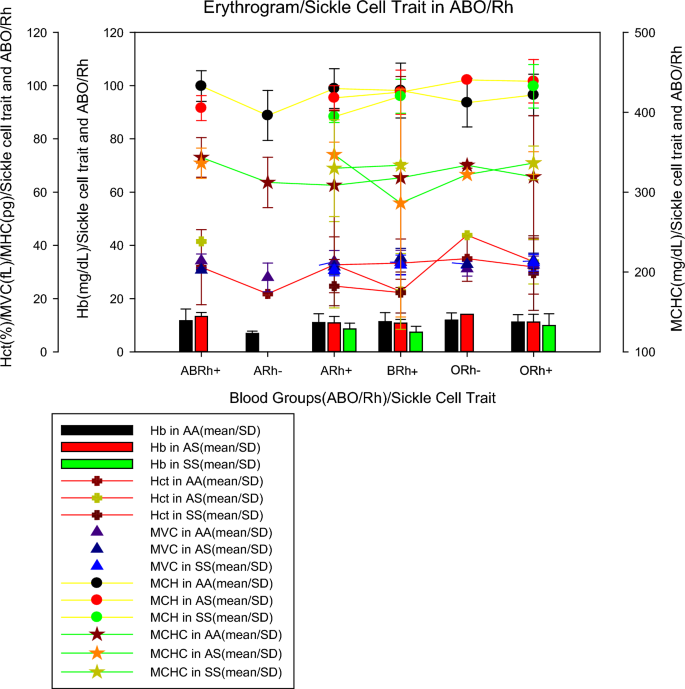
In evaluating the erythrogram (Table 2 ), most patients were found to have normal haemoglobin values (above 10 mg/dL), however, it was found that all patients in the ARh(−) blood group had low haemoglobin, while in the other blood groups individuals with low haemoglobin represented percentages below 32%, while patients with sickle cell anaemia were the only ones in which 50% had low haemoglobin, in normal individuals and with sickle cell trait the percentages of individuals with low haemoglobin were less than 25%. In the evaluation of haematocrit, it was found that individuals with hematocrit that individuals with haematocrit results below the reference values (39–55%) giving a margin of 10% more than the reference values, were mostly from the ARh(−) blood group in 75% and BRh(+) in 50%, it was found that among individuals with sickle cell anaemia, individuals with low haematocrits represented about 62% of the population, while for normal individuals (AA) and with sickle cell trait (AS) these percentages were below 41%.
The evaluation of the mean corpuscular volume (MCV), verified that the majority (67.7%, n = 134/198) of the individuals presented normal values (between 71 and 100 fL), however, all the patients of the blood group ARh(-) showed low MCV, while in the other blood groups individuals with low haemoglobin represented percentages below 38%, patients with sickle cell anaemia were the only ones in which 50% of them had low MCV, in normal individuals and with trait sickle cell, the percentages of individuals with low MCH were less than 38%.
The assessment of mean corpuscular haemoglobin (MCH), found that most individuals (68.9%, n = 137/198) of the patients studied who had normal MCH results (24.1–32.6 pg), all (100%) individuals from the ARh(-) blood group had MCH below the reference values. In other groups, the percentage of patients with low MCH did not exceed 38%, and the MCH did not show many differences between individuals with sickle cell anaemia, or normal individuals (AA) and individuals with sickle cell trait (AS), since the percentages for all cases were between 37.8% to 27.3%.
The assessment of mean corpuscular haemoglobin concentration (MCHC), showed that regardless of blood group or sickle cell trait, most patients (above 90% in all groups) had high MCHC (above 39.6 mg/dL). Statistical analysis showed a relationship between haemoglobin values and ABO/Rh blood groups (p < 0.05), but there was no relationship between haematocrit, VCM, MCH, and MCHC values in blood groups (p > 0.05). Also, there was a relationship between haemoglobin values and sickle cell trait (p < 0.05), but there was no relationship between haematocrit, VCM, MCH, and MCHC values with sickle cell trait (p > 0.05).
Erythrogram parameters in ABO/Rh blood groups and sickle cell trait
Figure 2 shows the mean values of the erythrogram parameters (mean ± SD) by blood group and sickle cell trait, where it can be seen that individuals from the ARh(−) blood group, without sickle cell trait (AA), had lower haemoglobinin values (6.95 ± 1.7 mg/dL) than individuals with sickle cell anaemia in groups A (8.6 ± 2.2 mg/dL), B (7.4 ± 2.2 mg/dL) and O (9, 9 ± 4.4 mg/dL) all Rh(+) as previously described, ARh(−) patients showed the lowest mean erythrocyte values (21.8 ± 0.1%), MCV (23.3 ± 4.5 fL), MCH (74.0 ± 7.0 pg) except for MCHC which was increased in all patients, including patients with RA(−). For sickle cell traits, we noticed that individuals with sickle cell anaemia (AA) belonging to the ARh(+), BRh(+), and ORh(+) groups had an average erythrocyte smaller than normal (AA) and heterozygous (AS) individuals, however, for tests such as VCM, MCH, and MCHC, in most cases, individuals with sickle cell anaemia showed values close to or higher than normal (AA) and heterozygous (AS) individuals.
Evaluation of the leucocyte count according to the ABO/Rh blood groups and sickle cell trait
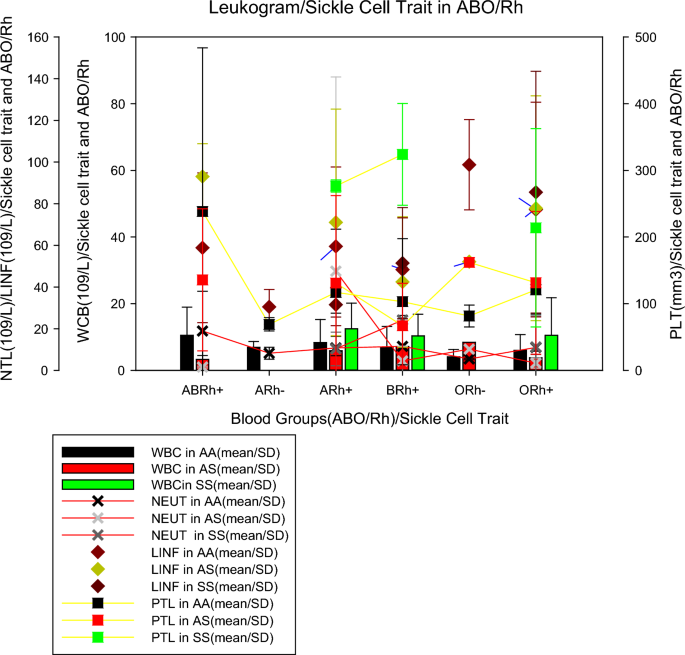
The evaluation of the leucocyte count (Table 3 ), showed that more than half of the patients had normal leukocyte (WBC) values (1–4.8 × 10 9 /L), however, more than 30% of patients from all blood groups had altered (increased) WBC, except patients in the ARh(−) group, all had normal WBC values, for sickle cell trait, it was found that more in all groups (AA, AS, SS) more than 40% of individuals who had altered (increased) WBC values. The evaluation of neutrophils (NEUT) found that a little more than half of the patients had NEUT results between the reference values (1.8–7.7 × 10 9 /L), patients of the ABRh(+) blood group, ARh(−) and Brh(+) presented alterations in neutrophils (low) ≤ 50%. Among individuals with sickle cell anaemia, patients with altered NEUT (low) represented about 50% of the population, while for normal individuals (AA) and with sickle cell trait (AS) these percentages were below 46%.
The lymphocyte evaluation (LINF) verified that the majority (70.4%, n = 140/198) of the individuals presented values above the reference values (1 to 4.8 × 10 9 /L), however, in all blood groups studied there was an alteration (increase) in the leukocyte value in more than 60%, however, among patients in the ARh(−) group, these alterations occurred in only 25% of the patients, for the sickle cell trait, it was found that in all groups (AA, AS, SS) changes were greater than 66%. In the assessment of platelets (PTL), it was discovered that in the majority ty (64.2%, n = 131/198) of the patients studied who had PTL results below the reference values (150,000 and 450,000 mm 3 ), in all groups the reduction was greater than 46% of platelets in all (100%) of the individuals of the different groups, it was verified for sickle cell trait, individuals with sickle cell anaemia (SS), more than 60% had normal PTL, while in the other groups, this percentage did not exceed 36%. Statistical analysis showed no relationship between WBC, NEUT, LINF, and PTL values with blood groups (p > 0.05). There was no statistically significant relationship between WBC, NEUT, and LINF values with sickle cell trait (p > 0.05), but there was a relationship between PTL and sickle cell trait (p < 0.05).
Figure 3 shows the mean values of the leucocyte count parameters (mean ± SD) by blood group and sickle cell trait. This shows that individuals from the blood group ABRh(+) with sickle cell trait (AS), ORh(-) without sickle cell trait (AA) and ORh(+) with sickle cell trait showed lower WBC values (3.28 ± 1.19 10 9 /L, 4.05 ± 2.24 10 9 /L and 3.76 ± 1.98 10 9 /L, respectively). The mean NEUT in individuals with sickle cell trait (AS) in the ABRh(+) group and without sickle cell trait (AS) in the ARh(−) and ORh(−) groups were lower (1.60 ± 0.41 10 9 /L, 8.25 ± 2.90 10 9 /L and 5.48 ± 0.35 10 9 /L, respectively) when compared to individuals of other blood groups. The mean number of lymphocytes in individuals with ARh(−) without trait sickle cell and ARh(+) with sickle cell anaemia were the lowest (3.33 ± 3.65 10 9 /L and 3.78 ± 2.58 10 9 /L) when compared to individuals from other groups. Mean PLT in individuals without trait sickle cell(AS) from the ARh(−) and ORh(−) groups were lower (69.0 ± 10.0 10 9 /L and 81.5 ± 16.34 10 9 /L, respectively) when compared to individuals from other groups.
Leucogram parameters in ABO/Rh blood groups and sickle cell trait
In the present study, the O blood group represented about 46% of the entire population, followed by groups A (26.7%), B (21.2), and AB (6.6%). Regarding the sickle cell trait, AS and SS represented 18.7% and 8.1%, respectively. Similar data were observed in a study carried out in Nigeria, where group O was the most frequent (47.7%), followed by blood group B (25.2%), A (22.5%), and AB (4.6%), also, the frequencies of HbAS and HbAC were 14.4% and 5.8%, respectively [ 23 ]. In the present study, women were the majority (68.6%) in all blood groups, except for the ARh(-), and also represented 75% of the SS individuals. A statistical relationship was observed between sickle cell trait and gender (p < 0.05), which seems to be similar to that reported in Ghana with children, where blood group O was the most prevalent (41.4%), followed by blood groups A (29.6%) and B (23.3%), while AB (5.7%) had the lowest frequency. The authors also showed that the prevalence rates of sickle cell trait (HbAS and HbSC) and sickle cell disease (HbSS) were 17.5% and 0.5%, respectively [ 25 ].
High parasitaemia was mostly frequent in individuals with ABRh(+) and BRh(+) blood groups representing more than 30%. The ARh(+) blood group presents the highest number of severe cases of malaria (46.2%), followed by the BRh (28.6%) and ARh (22.4%). However, both for high parasitaemia and severe malaria, individuals with AS and SS had a lower prevalence than AA individuals, which corroborates the Nigerian study where was showed that HbAS was associated with a reduced risk of severe malaria [OR = 0.46 (95% CI 0.27–0.77)]. Moreover, among individuals with severe malaria, HbAS was associated with significantly lower parasite densities. The protective effect of blood group O was demonstrated with a low risk of severe malaria [OR = 0.74 (95% CI 0.56–0.97)]. Blood group B was associated with an increased risk of severe malaria [OR = 1.63 (95% CI 1.12–2.38)] [ 23 ]. The study results found (Table 1 ) differ from a study conducted in Nigeria, which showed that individuals with sickle cell disease (HbSS, HbSC) had the highest prevalence of malaria parasitaemia and severe malaria. In addition, individuals with blood group O also had a higher prevalence of malaria parasitaemia, but a lower prevalence of severe malaria when compared with non-O blood groups, which confirmed the protective role of the O antigen in impairing the formation of rosettes and the vascular cytoadhesion of parasitized red blood cells, making it more susceptible to malaria infection than non-O antigens, but less susceptible to severe disease [ 24 ].
In the present study (Fig. 1 ), It was found that individuals with sickle cell anaemia were only from the ARh(+), BRh(+), and ORh(+) groups, with an average age of over 20 years old (Table 1 ). A study conducted with 300 pregnant women from Nigeria found that 73.7% were HbAA, 22.3% were HbAS, 3.7% were HbAC and 0.3% were HbSC, with HbAA being more prevalent among subjects in group O followed by A, B, and AB, but HbAS was more prevalent among group A followed by B, O, and AB while HbAC was more prevalent between groups A and O, followed by B and AB, whereas the prevalence of HbSC was concentrated in the blood group and even so there were no homozygous haemoglobinopathies S and C [ 26 , 27 ].
The present study (Table 2 ) found a relationship between the haemoglobin values with the blood type and with the sickle cell trait (p < 0.05), where all individuals in the ARh(−) group showed alterations and individuals in the group ORh(+) had lower percentages of low haemoglobin (18.8%), however, more than 50% of SS individuals had low haemoglobin. A study carried out in Cameroon showed that the mean parasite density was significantly lower among children with SCD than among children without SCD (22,875 vs 57,053 parasites/µL, p = 0.002), where the mean haemoglobin concentration was lower in SCD compared to non-SCD (5.7 g/L vs 7.4 g/L, p ≤ 0.001) and the mean haemoglobin concentration was significantly lower among patients with SCD in the admission compared to patients without SCD (5.7 g/dL vs 7.4 g/dL, p < 0.001) [ 28 ].
In the present study, it was not observed a relationship between blood groups and SCD (Table 2 ), however, the blood group ARh(−) showed a different behavior from all blood groups in the results of the erythrogram, and similar data were recorded in a study carried out in Nigeria among patients with malaria, The haemoglobin (Hb), [median (IQR); 7.3 (1.3), p = 0.001], haematocrit (HCT), [median (IQR); 26.4 (4.4), p = 0.009], red blood cells (RBC), [median (IQR); 3.2 (1.7), p = 0.048] were markedly reduced in HbSS, however, red cell distribution with (RDW) [median (IQR); 14.9 (3.3), p = 0.030] increased in malaria-infected children with HbSS and severe anaemia was higher in HbSS (23.1%), followed by HbAA (8.6%) and HbAS (7.1%) [ 23 ]. Another study found that blood group B was associated with an increased risk of severe malaria [OR = 1.638 (95% CI 1.128–2.380)] and after adjusting for age, gender, haematocrit, parasite density, and Hb genotype, we confirm from this large study in Nigerian children that the greater protective effect of the heterozygous sickle cell state against cerebral malaria and severe malarial anaemia) [ 11 , 12 ].
Individuals from the ARh(−) blood group, without sickle cell trait (AA), had lower haemoglobin values (6.95 ± 1.7 mg/dL) than individuals with sickle cell anaemia from groups A (8.6 ± 2.2 mg/dL), B(7.4 ± 2.2 mg/dL) and O (9.9 ± 4.4 mg/dL). We observed that all Rh(+) patients with sickle cell anaemia belonging to the ARh(+), BRh(+), and, ORh(+) had a mean number of erythrocytes smaller than normal (AA) and heterozygous (AS) individuals (Fig. 2 ). It seems that the very condition of infection by P. falciparum can promote haematological abnormalities due to the life cycle of the parasite. Previous studies concluded that haematological markers for anaemia, that is haemoglobin, haematocrit, and red blood cell count were higher in those infected with P. falciparum malaria, as well as the distribution of red blood cells, MCV, MCH, and MCHC also differ when comparing individuals with and without infection [ 23 ].
Leucocyte count (64.2%, n = 131/198) of the patients studied had platelets (PTL) below the reference values (150,000 and 450,000 mm 3 ) and there was a significant relationship between PTL and sickle cell trait (p < 0.05). A previous study conducted in Cameroon showed that the mean PLT count was significantly higher in patients with SCD compared to patients without SCD (274,014 vs 177,668 10 9 /L; p < 0.001) and that the mean white blood cell count was lower in patients with sickle cell disease compared to patients without sickle cell disease (20.3 vs 9.8 × 10 9 /L), although this difference was not statistically significant (p = 0.394) [ 28 ]. On the other hand, a study carried out in Nigeria, showed no differences in PLT count (p = 0.399), therefore, no severe thrombocytopenia between genotypes and identified that leukocytosis was higher in HbSS (69.2%), HbAS (42%) and HbAA (31%) [ 11 ].
It was noticed that individuals from the blood group ABRh(+) with sickle cell trait (AS), ORh(−) without sickle cell trait (AA), and ORh(+) with sickle cell trait had lower WBC values, as well as the mean NEUT in individuals with sickle cell trait(AS) from the ABRh(+) group and without sickle cell trait(AS) from the ARh(−) and ORh(−) groups were lower when compared to individuals of other blood groups (Fig. 3 ). A study conducted in Nigeria also reported that the mean concentration of WBC, lymphocytes, monocytes and granulocyte was lower in individuals with SCD compared to individuals without SCD. Moreover, other studies revealed that PLT counts were lower in the malaria-infected group [median (IQR), 236 (129.5) vs. uninfected group [median (IQR), 278 (112.8), p = 0.001] [ 11 ]. Previous studies reported that monocytosis is associated with hemolysis and inflammation in sickle cell anaemia, however, the influence of carrying different HBB genotypes (6GAG > 6G TG) on the severity of major haematological abnormalities and correlation of haematological parameters with HBB genotypes (6G AG > 6G TG) [12,29]. Future studies exploring the demographic, genetic, clinical, and laboratory determinants related to sickle cell anaemia should be carried out in patients with Malaria in Angola.
With the results of the study, it can be concluded that individuals from the ORh + blood group, followed by the ARh(+) and BRh(+) groups seem to be the majority as well in malaria infection among Angolan patients. However, the ARh(−) group seems to be the one that most suffers the impact of the disease both in the erythrocyte and leukocyte responses, even in the low parasitaemia and moderate disease, which is different in the ABRh(+) group, which seems to be the most susceptible to severe disease and which individuals with sickle cell anaemia seem to have a poor response in the replacement of the erythrocyte response and some leucocytic parameters. The study results highlight the importance of differentiated care for malaria patients according to ABO/RH blood groups and the sickle cell trait, taking into account the haemogram parameters that demonstrate how patients can react to infection regardless of the degree of parasitaemia and medical prognosis.
Availability of data and materials
All relevant data are within the paper.
Ministry of Health. Angola reports more cases of malaria in 2022, but mortality decreases by 10 percent. Lusa-Verangola, 2023. Accessed 08/21/2023. Available at: https://www.verangola.net/va/pt/042023/Saude/35323/Angola-notifica-mais-casos-de-mal%C3%A1ria-em-2022-mas-mortalidade-diminui-10-percent.htm .
Jornal de Angola. Angola prepares entry of the vaccine to reduce deaths from malaria. 2023. Accessed 08/21/2023. Available at: https://www.jornaldeangola.ao/ao/noticias/angola-prepara-entrada-da-vacina-para-reduzir-mortes-por-malaria/ .
Driss A, Hibbert JM, Wilson NO, Iqbal SA, Adamkiewicz TV, Stiles JK. Genetic polymorphisms associated with susceptibility to malaria. Malar J. 2011;10:271.
Article PubMed PubMed Central Google Scholar
Wahlgren M, Goel S, Akhouri RR. Plasmodium falciparum variant surface antigens and their roles in severe malaria. Nat Rev Microbiol. 2017;15:479–91.
Article CAS PubMed Google Scholar
Degarege A, Gebrezgi MT, Ibaneza G, Wahlgren M, Madhivanan P. Effect of ABO blood group on susceptibility to severe malaria: a systematic review and meta-analysis. Blood Rev. 2018;33:53–62.
Article PubMed Google Scholar
Cserti-Gazdewich CM, Mayr WR, Dzik WH. Plasmodium falciparum malaria and immunogenetics of ABO, HLA and CD36 (platelet glycoprotein IV) Vox Sang. 2011;100:99–111.
Moll K, Palmkvist M, Ch’ng J, Kiwuwa MS, Wahlgren M. Plasmodium falciparum immunity evasion: blood group a rosettes impair PfEMP1 recognition. PLoS ONE. 2015;10: e0145120.
Goel S, Palmkvist M, Moll K, Joannin N, Lara P, Akhouri RR, et al. RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nat Med. 2015;21:314–7.
Roucher C, Rogier C, Dieye-Ba F, Sokhna C, Tall A, Trape JF. Changing malaria epidemiology and diagnostic criteria for Plasmodium falciparum clinical malaria. PLoS ONE. 2012;7: e46188.
Article CAS PubMed PubMed Central Google Scholar
Luzzatto L. Sickle cell anaemia and malaria. Mediterr J Hematol Infect Dis. 2012;4: e2012065.
Kosiyo P, Otieno W, Gitaka J, Munde EO, Ouma C. Haematological abnormalities in children with sickle cell disease and non-severe malaria infection in western Kenya. BMC Infect Dis. 2021;21:329.
Wonkam A, Mnika K, Ngo Bitoungui VJ, ChetchaChemegni B, Chimusa ER, Dandara C, et al. Clinical and genetic factors are associated with pain and hospitalisation rates in sickle cell anaemia in Cameroon. Br J Haematol. 2018;180:134–46.
Cruz SS, Sacomboio E, Francisco NM, Cassinela EK, Mateus A, David Z, et al. Blood pressure pattern among blood donors exposed to SARS-CoV-2 in Luanda, Angola: a retrospective study. Health Sci Rep. 2023;6: e1498.
Article Google Scholar
Sacomboio ENM, Agostinho EMF, Tchivango AT, Cassinela EK, Rocha Silveira SD, da Costa M, et al. Sociodemographic clinical and blood group (ABO/Rh) profile of Angolan individuals with HIV. J Clin Cell Immunol. 2022;13:675.
Google Scholar
Sacomboio ENM, Muhongo TO, Tchivango AT, Cassinela EK, Silveira SDR, Da Costa M, et al. Blood groups (ABO/Rh) and sociodemographic and clinical profile among patients with leprosy in Angola. Preprints. 2022:2022090299.
Sacomboio ENM. ABO/Rh blood groups and chronic diseases in Angolan patients. Am J Biomed Sci Res. 2021;13:1.
Sacomboio ENM, Sassoke JL, Hungulo OFS, Ekundi-Valentim E, Cassinela EK, et al. Frequency of ABO/Rh blood groups and social condition of hypertensive patients in Luanda. J Blood Disord Med. 2021;4:1–5.
Sacomboio E, Sebastião D, Sacomboio F. Sickle cell trait and blood groups (ABO and Rh) in Angolans submitted to hemoglobin electrophoresis. Hematol Oncol Curr Res. 2020;7:605–16.
Sacomboio ENM, Neto CR, Hungulo OFS, Valentim EE. Blood group (ABO/Rh) and Clinical Conditions Common in Children with Nephrotic Syndrome and Sickle Cell Anemia in Angola. J Blood Disord Med. 2021;4.
Zua SS, Filho-Sacomboio FC, Ekundi-Valentim E, Sacomboio ENM. Social and clinical factors affecting the uremic condition of Angolan patients with malaria. Int J Adv Res. 2020;8:986–98.
Sacomboio ENM, Sebastião CS, Tchivango AT, Pecoits-Filho R, Calice-Silva V. Does parasitemia level increase the risk of acute kidney injury in patients with malaria? Results from an observational study in Angola. Scientific African. 2020;7: e00232.
Sacomboio ENM, dos Santos Sebastião C, Salvador STDC, João JA, Bapolo DVS, Francisco NM, et al. Evaluation of blood cell count parameters as predictors of treatment failure of malaria in Angola: an observational study. PLoS One. 2022;17: e0267671.
Amodu OK, Olaniyan SA, Adeyemo AA, Troye-Blomberg M, Olumese PE, Omotade OO. Association of the sickle cell trait and the ABO blood group with clinical severity of malaria in southwest Nigeria. Acta Trop. 2012;123(2):72–7.
Akhigbe RE, Ige SF, Adegunlola GJ, Adewumi MO, Azeez MO. Malaria, haemoglobin genotypes and abo blood groups in Ogbomoso, Nigeria. Int J Trop Med. 2011;6:73–6.
Tchum SK, Sakyi SA, Arthur F, Adu B, Abubakar LA, Oppong FB, et al. Effect of iron fortification on anaemia and risk of malaria among Ghanaian pre-school children with haemoglobinopathies and different ABO blood groups. BMC Nutr. 2023;9:56.
Buseri FI, Okonkwo C. Abnormal hemoglobin genotypes and ABO and rhesus blood groups associated with HIV infection among HIV-exposed infants in North Western Nigeria. Pathol Lab Med Int. 2014;6:15–20.
Udomah FP, Isaac IZ, Aliyu N, Erhabor O, Ahmed MH, Abdullahi N, et al. Haemoglobin electrophoretic patterns, ABO and Rhesus D blood groups distribution among antenatal women in Sokoto. Nigeria Obstet Gynecol Cases Rev. 2015;2:34.
Eleonore NLE, Cumber S, Charlotte EE, Lucas EE, Edgar MML, Nkfusai CN, et al. Malaria in patients with sickle cell anaemia: burden, risk factors and outcome at the Laquintinie hospital. Cameroon BMC Infect Dis. 2020;20:40.
Download references
Acknowledgements
The authors would like to thank the patients for their participation and the management and the workers from the Josina Machel Hospital for institutional support.
This project was funded by the European Union and the African Union through the 2022 ARISE-PP-13 Project, which is coordinated by the African Academy of Sciences.
Author information
Authors and affiliations.
Instituto de Ciências de Saúde da Universidade Agostinho Neto (ICISA/UAN), Luanda, Angola
Euclides N. M. Sacomboio, Santo D. Zua, António D. Pululu, Adilson C. D. Caumba, Adelina B. M. Paciência, Danilson V. Sati, Sabina G. Agostinho, Yolanda S. Agostinho, Fernando G. Mazanga, Neusa B. Ntambo & Cruz S. Sebastião
Instituto Superior de Ciências de Saúde/Universidade Católica de Angola (ISCS/UCAN), Luanda, Angola
Euclides N. M. Sacomboio
Centro de Formação em Saúde (CFS) da Clinica Multiperfil, Luanda, Angola
Instituto Politécnico de Malanje da Universidade Rainha Njinga A Mbande (IPM/URNM), Malanje, Angola
Adelino T. Tchivango
Instituto Nacional de Investigação em Saúde (INIS), Luanda, Angola
Cruz S. Sebastião, Joana P. Paixão & Joana Morais
Centro de Investigação em Saúde de Angola (CISA), Caxito, Angola
Cruz S. Sebastião & Joana P. Paixão
Faculdade de Medicina, Universidade Agostinho Neto, Luanda, Angola
Joana Morais
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: ENMS. Data curation: ENMS, ATT, ADP, CC, ABP, DVS, SGA, YSA, FGM, NBN, JPP, JM, CSS. Formal analysis: ENMS, JM, and CSS. Investigation: ENMS, ATT, ADP, CC, ABP, DVS, SGA, YSA, FGM, NBN. Project administration: ENMS, JM, CSS, JM, ATT. Supervision: ENMS. Validation: ENMS, ATT, ADP, CC, ABP, DVS, SGA, YSA, FGM, NBN, JPP, JM, CSS. Writing—original draft: ENMS, and CSS. Writing—review and editing: ENMS, ATT, ADP, CC, ABP, DVS, SGA, YSA, FGM, NBN, JPP, JM, and CSS. All authors have seen and approved the submitted version of this manuscript.
Corresponding author
Correspondence to Euclides N. M. Sacomboio .
Ethics declarations
Consent for publication.
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sacomboio, E.N.M., Zua, S.D., Tchivango, A.T. et al. Blood count changes in malaria patients according to blood groups (ABO/Rh) and sickle cell trait. Malar J 23 , 126 (2024). https://doi.org/10.1186/s12936-024-04886-2
Download citation
Received : 05 September 2023
Accepted : 20 February 2024
Published : 29 April 2024
DOI : https://doi.org/10.1186/s12936-024-04886-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Blood count
- Blood group
- Sickle cell trait
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]

- April 29, 2024 | From Theory to Therapy: MIT’s Computational Breakthrough in Protein Optimization
- April 29, 2024 | Energy Scientists Have Unraveled the Mystery of Gold’s Glow
- April 29, 2024 | Micrometeoroid Bombardment and Magnetic Fields: Decoding the Effects of Interplanetary Space on Asteroid Ryugu
- April 29, 2024 | New Alloy Shocks Scientists With Its Nearly Impossible Strength and Toughness
- April 29, 2024 | Spiraling Secrets: Unveiling the X-Ray Mysteries of Dwarf Galaxy IC 776
Experimental NIH Antibody Protects Children From Malaria in Clinical Trial
By National Institute of Allergy and Infectious Diseases April 26, 2024
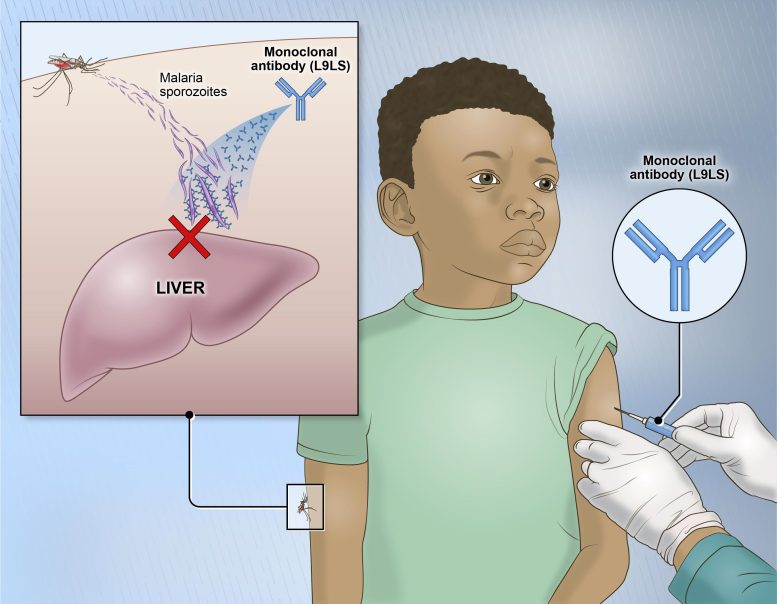
A single injection of an experimental monoclonal antibody called L9LS prevented malaria infection in children in Mali. L9LS binds to and neutralizes “sporozoites,” the form of the malaria parasite transmitted by mosquitoes that invades the liver to initiate infection. Credit: NIH
A new study reports a single dose of a malaria antibody was 77% effective in protecting children in Mali from malaria, marking a significant step in malaria prevention efforts.
One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH). The results will be published today (April 26) in The New England Journal of Medicine .
“A long-acting monoclonal antibody delivered at a single health care visit that rapidly provides high-level protection against malaria in these vulnerable populations would fulfill an unmet public health need,” said Dr. Jeanne Marrazzo, director of the National Institute of Allergy and Infectious Diseases, part of NIH.
In 2022, the P. falciparum parasite caused a majority of the nearly 250 million estimated cases of malaria globally and most of the more than 600,000 malaria deaths, according to the World Health Organization. Most malaria cases and deaths are among children in Africa. Malaria parasites such as P. falciparum are transmitted to people by mosquito bites.
Clinical Trial Results
The clinical trial assessed two dose levels, with 19% of the 300mg-dose group and 28% of the 150mg-dose group developing symptomatic malaria, providing protective efficacy of 77% and 67% against symptomatic malaria, respectively. Among children who received placebo, 81% became infected with Plasmodium falciparum , and 59% had symptomatic malaria during the six-month study period.
The authors note that the trial demonstrated for the first time that a single dose of a monoclonal antibody given by subcutaneous injection can provide high-level protection against malaria in children in an area of intense malaria transmission.
Development of the Antibody
In 2020, scientists at NIAID’s Vaccine Research Center reported that they had isolated the antibody from a volunteer who had been vaccinated with an experimental malaria vaccine. The antibody was modified with a mutation that prolonged its durability in the bloodstream following administration. In an earlier study, conducted in Mali by the same research group, a previously discovered antibody was highly protective against P. falciparum infection in adults when given intravenously. However, the new antibody was shown to be more potent in animal studies and was manufactured at a higher concentration than CIS43LS, allowing it to be given by subcutaneous injection.
The trial in Mali took place in two parts, first to assess safety in a small number of adults and children, and then in a larger clinical efficacy trial involving 225 children. The efficacy trial took place from July 2022 to January 2023 and included healthy children 6 to 10 years of age, 75 of whom received a 300 mg dose, 75 a 150 mg dose, and 75 of whom received a placebo.
Future Research Directions
The researchers are continuing clinical development of the experimental antibody, focusing on other high-risk populations, such as infants and young children, children hospitalized with severe anemia, and pregnant women. An ongoing clinical trial in Kenya is assessing the efficacy of the antibody in children 5 months to 5 years of age over a 12-month study period, and scientists are also conducting a clinical trial in Mali to assess the antibody in women of childbearing potential to prepare to test the antibody in pregnancy.
Reference: “Subcutaneous Administration of a Monoclonal Antibody to Prevent Malaria” by Kassoum Kayentao, Aissata Ongoiba, Anne C. Preston, Sara A. Healy, Zonghui Hu, Jeff Skinner, Safiatou Doumbo, Jing Wang, Hamidou Cisse, Didier Doumtabe, Abdrahamane Traore, Hamadi Traore, Adama Djiguiba, Shanping Li, Mary E. Peterson, Shinyi Telscher, Azza H. Idris, William C. Adams, Adrian B. McDermott, Sandeep Narpala, Bob C. Lin, Leonid Serebryannyy, Somia P. Hickman, Andrew J. McDougal, Sandra Vazquez, Matthew Reiber, Judy A. Stein, Jason G. Gall, Kevin Carlton, Philipp Schwabl, Siriman Traore, Mamadou Keita, Amatigué Zéguimé, Adama Ouattara, M’Bouye Doucoure, Amagana Dolo, Sean C. Murphy, Daniel E. Neafsey, Silvia Portugal, Abdoulaye Djimdé, Boubacar Traore, Robert A. Seder and Peter D. Crompton, 1 May 2024, New England Journal of Medicine . DOI: 10.1056/NEJMoa2312775
NIAID led the clinical trial in conjunction with the University of Sciences, Techniques and Technologies of Bamako, Mali, through NIAID’s Division of Intramural Research International Centers of Excellence in Research (ICER) program. For more details about the clinical trial, see ClinicalTrials.gov using identifier NCT05304611 .
More on SciTechDaily

Micrometeoroid Bombardment and Magnetic Fields: Decoding the Effects of Interplanetary Space on Asteroid Ryugu
Lung Cancer: Breakthrough Offers Hope for Increasing Immunotherapy Efficacy

Evolution May Be to Blame for High Risk of Advanced Cancers in Humans – “Gene Has Gone Rogue”
Covid-19 mutation: sars-cov-2 virus can find alternate route to infect cells.

Game-Changer in Future Solar Technology: New Perovskite Solar Modules With Greater Size, Power and Stability

Scientists Shatter Record for the Amount of Energy Produced During a Controlled, Sustained Fusion Reaction

Four New Bat Species Discovered — Cousins of the Suspected COVID-19 Bats

Unexpected Electrical Current Discovered That Could Stabilize Fusion Reactions – Bringing the Fusion Energy That Drives the Sun to Earth
Be the first to comment on "experimental nih antibody protects children from malaria in clinical trial", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Friday, April 26, 2024
Experimental NIH malaria monoclonal antibody protective in Malian children
Mid-stage trial shows treatment prevents infection, disease.

One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH), and results appear in The New England Journal of Medicine.
“A long-acting monoclonal antibody delivered at a single health care visit that rapidly provides high-level protection against malaria in these vulnerable populations would fulfill an unmet public health need,” said Dr. Jeanne Marrazzo, director of the National Institute of Allergy and Infectious Diseases, part of NIH.
The clinical trial assessed two dose levels, with 19% of the 300mg-dose group and 28% of the 150mg-dose group developing symptomatic malaria, providing protective efficacy of 77% and 67% against symptomatic malaria, respectively. Among children who received placebo, 81% became infected with Plasmodium falciparum , and 59% had symptomatic malaria during the six-month study period. The authors note that the trial demonstrated for the first time that a single dose of a monoclonal antibody given by subcutaneous injection can provide high-level protection against malaria in children in an area of intense malaria transmission.
In 2022, the P. falciparum parasite caused a majority of the nearly 250 million estimated cases of malaria globally and most of the more than 600,000 malaria deaths, according to the World Health Organization. Most malaria cases and deaths are among children in Africa. Malaria parasites such as P. falciparum are transmitted to people by mosquito bites.
In 2020, scientists at NIAID’s Vaccine Research Center reported that they had isolated the antibody from a volunteer who had been vaccinated with an experimental malaria vaccine. The antibody was modified with a mutation that prolonged its durability in the bloodstream following administration. In an earlier study, conducted in Mali by the same research group, a previously discovered antibody was highly protective against P. falciparum infection in adults when given intravenously. However, the new antibody was shown to be more potent in animal studies and was manufactured at a higher concentration than CIS43LS, allowing it to be given by subcutaneous injection.
The trial in Mali took place in two parts, first to assess safety in a small number of adults and children, and then in a larger clinical efficacy trial involving 225 children. The efficacy trial took place from July 2022 to January 2023 and included healthy children 6 to 10 years of age, 75 of whom received a 300 mg dose, 75 a 150 mg dose, and 75 of whom received a placebo.
The researchers are continuing clinical development of the experimental antibody, focusing on other high-risk populations, such as infants and young children, children hospitalized with severe anemia, and pregnant women. An ongoing clinical trial in Kenya is assessing the efficacy of the antibody in children 5 months to 5 years of age over a 12-month study period, and scientists are also conducting a clinical trial in Mali to assess the antibody in women of childbearing potential to prepare to test the antibody in pregnancy.
NIAID led the clinical trial in conjunction with the University of Sciences, Techniques and Technologies of Bamako, Mali, through NIAID’s Division of Intramural Research International Centers of Excellence in Research (ICER) program. For more details about the clinical trial, see clinicaltrials.gov using identifier NCT05304611 .
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
K Kayentao et al . Subcutaneous Administration of a Monoclonal Antibody to Prevent Malaria . The New England Journal of Medicine DOI: 10.1056/NEJMoa2312775 (2024).
Connect with Us
- More Social Media from NIH
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.16(3); 2024 Mar
- PMC11015937

Plasmodium falciparum Malaria Presenting as a Thrombotic Thrombocytopenic Purpura (TTP) Mimic: A Case Report
Kalendra kunwar.
1 Internal Medicine, One Brooklyn Health/Interfaith Medical Center, Brooklyn, USA
Sailesh Karki
Monika jain, sushma edara, james y rixey.
2 Internal Medicine, Brookdale University Hospital and Medical Center, Brooklyn, USA
Frances Schmidt
3 Pulmonary Medicine, Interfaith Medical Center, Brooklyn, USA
Malaria can present with clinical manifestations overlapping with thrombotic thrombocytopenic purpura (TTP). We present the case of a 55-year-old female who presented with abdominal pain, fever, confusion, dehydration, and recent travel to Nigeria. Laboratory investigations were remarkable for low hemoglobin, decreased platelets, and elevated lactate. Suspicion for TTP occurred when the patient’s platelet count and hemoglobin progressively decreased along with acute kidney injury and confusion. There was an elevated ADAMTS13 antibody level and mildly reduced ADAMTS13 activity suggesting possible TTP. However, Plasmodium falciparum was seen on peripheral blood smears. Treatment with artemether-lumefantrine was initiated which led to improvement in parasitemia, platelet count, and anemia. The similarity between malaria and TTP is mostly explained by thrombotic microangiopathic anemia (TMA) present in both diseases. Awareness of the common pathogenesis of TMA in both diseases and clinical judgment are pivotal in determining the timely initiation of appropriate treatment.
Introduction
Malaria is a life-threatening condition and remains a major killer in tropical countries. Malaria is caused by an infection of the parasite belonging to the genus Plasmodium . The most virulent is Plasmodium falciparum , causing one-third of the deaths associated with the disease [ 1 ]. Anemia in malaria is multifactorial [ 2 ], and quite often the presentation is that of microangiopathic hemolytic anemia with direct destruction of the red blood cells (RBCs) [ 3 ]. Plasmodium falciparum infection, responsible for severe malaria, leads to the adhesion of infected RBCs to blood vessel walls, causing endothelial cell activation, platelets, and white blood cell sequestration [ 4 ]. Thrombotic thrombocytopenic purpura (TTP) is caused by a deficiency of plasma metalloproteinase and ADAMTS13 and an increase in von Willebrand factor (VWF), leading to extensive microvascular thrombosis creating exceedingly high levels of shear stress and microangiopathic hemolysis [ 5 ]. Malaria and TTP are two serious medical conditions that can have similar symptoms, including fever, microangiopathic hemolytic anemia, thrombocytopenia, acute kidney injury, and changes in mental status [ 6 , 7 ]. This may potentially lead to misdiagnosis and delayed treatment. Similarities in the pathogenesis of thrombotic microangiopathic anemia (TMA) in both diseases have been reported previously in the literature. We present a case of Plasmodium falciparum malaria with microangiopathic hemolysis closely mimicking the presentation of TTP.
Case presentation
A 55-year-old female presented to the emergency department (ED) with abdominal pain, loose stools, nausea, vomiting, and fever for 10 days. She had a recent travel history to Nigeria for four weeks and had returned to the United States two weeks ago. She did not receive any antimalarial prophylaxis during the travel. She had visited the ED a few days ago for similar complaints and was discharged on oral antibiotics. On presentation to the ED, she was ill-looking, pale, and dehydrated. On examination, the patient was confused and the mucous membranes were dry. The abdomen was soft and non-tender. Blood investigations showed low hemoglobin (10.6 mg/dL), low platelets (125,000/µL), lactate (2.3 mmol/L), prothrombin time/international normalized ratio (16.4/1.37), total bilirubin (2.2 mg/dL), lactate dehydrogenase (289 U/L), haptoglobin (<10 mg/dL), blood urea nitrogen/creatinine (35/1.7 mg/dL). Given her recent travel history, malaria, typhoid, and Giardia were the differential diagnoses. In addition, platelet counts and hemoglobin were progressively trending down (Table (Table1) 1 ) raising the possibility of TTP.
MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; LDH: lactate dehydrogenase; NA: not applicable
Blood culture, stool for occult blood, stool culture, Giardia antigen, stool leukocytes, stool for clostridium difficile polymerase chain reaction, and toxins A and B were all negative. Chest X-ray and a CT scan of the abdomen and pelvis were negative. The PLASMIC score was 6 which was suggestive of a high probability of TTP. Thick and thin peripheral blood smear showed Plasmodium falciparum and occasional schistocytes. ADAMTS13 antibody was elevated at 22 U/mL (normal value: <12 U/mL) and reflex ADAMTS13 activity was 49.1 (normal value: >66.8; less than 10% relatively specific for TTP). She was managed with an artemether-lumefantrine combination for four days. With antimalarial medications, platelet counts gradually improved and normalized (Figure (Figure1), 1 ), and the percentage of RBCs infected with Plasmodium falciparum progressively decreased (Figure (Figure2 2 ).

The patient remained afebrile after the second day of hospital admission. She reported gradual improvement in nausea, vomiting, and abdominal pain, with symptoms resolving on day five. She was able to tolerate food. The patient received two units of packed RBC transfusion during the hospital stay. After an improvement in her symptoms and normalization of her platelet count, the patient was discharged home. The patient denied any symptoms on follow-up after one month in the clinic and her laboratory investigations revealed normalization of hemoglobin (11.6 g/dL) and hematocrit (36.7%).
The similarity in clinical and laboratory presentation between malaria and TTP might be related to the presence of thrombotic microangiopathy in both diseases. Sinha et al. showed histological evidence of TMA in malaria in an observational study [ 8 ]. Similarities between the pathogenesis of thrombotic microangiopathy in malaria and TTP are evidenced by increased levels of VWF antibodies and decreased levels of ADAMTS13 antigen in severe malaria [ 9 , 10 ]. The reduction in ADAMTS13 activity might be caused by an unidentified inhibitor in malarial plasma [ 11 ]. The mildly attenuated ADAMTS13 activity in our patient might be related to a similar pathogenic mechanism.
TTP associated with malaria was initially reported by Nemie et al. in 2018. The patient was initially treated for Plasmodium vivax with artesunate and was managed with plasma exchange after TTP was suspected [ 12 ]. Subsequently, Ghadge et al. reported Plasmodium falciparum malaria associated with TTP in 2020. The patient had persistent altered mental status despite treatment for malaria and the mental status improved only after plasmapheresis [ 13 ]. However, in both cases, the diagnosis of TTP was made on clinical grounds after improvement with plasmapheresis, and ADAMTS13 testing was not done.
Similar to our case, Kurek et al. described a patient in 2023 who was initially suspected and treated for TTP. However, the confirmatory test for TTP was found to be negative, the patient was found to have malaria, and improvement was seen after initiation of intravenous artesunate [ 14 ]. This underscores the importance of a high index of suspicion of malaria, especially in patients with a travel history to endemic zones.
Positive antibodies to ADAMTS13 are a common finding in TTP as found in the UK TTP registry [ 15 ]. However, positive antibodies are found in many healthy individuals as well and are not specific to TTP. False-positive antibodies are more common in patients with systemic lupus erythematosus and antiphospholipid syndrome [ 16 ]. ADAMTS13 activity levels of less than 10% are used to confirm the diagnosis in appropriate clinical settings [ 17 , 18 ]. In our patient, ADAMTS13 activity was greater than 10% despite positive ADAMTS13 antibody levels which made TTP unlikely. The clinical presentation in our case was compatible with malaria as well as with TTP; however, our patient did not undergo plasmapheresis and she responded well to antimalarial medications.
Conclusions
The striking clinical and laboratory resemblances between malaria and TTP with the presence of TMA suggest a possibility of shared pathogenic mechanisms involving VWF and ADAMTS13 in both diseases. ADAMTS13 should be monitored, especially in severe malaria cases with TMA. Decreased ADAMTS13 activity in combination with increased VWF may play a role in the complications of malaria. It is unknown if patients with malaria should be treated for TTP only if ADAMTS13 activity level is less than 10% or if there is no clinical improvement despite antimalarial treatment and resolved parasitemia when ADAMTS13 is lower than normal. ADAMTS13 in malaria might be the result of an unidentified inhibitor and the pathogenesis of TMA may be identical in both diseases. Awareness of the association of malaria with TTP may lead to early initiation of lifesaving treatment in appropriate patients. Clinical judgment and ADAMTS13 activity level remain the cornerstone in deciding on the need for plasmapheresis.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Kalendra Kunwar, Monika Jain, James Y. Rixey, Frances Schmidt, Sailesh Karki, Sushma Edara
Acquisition, analysis, or interpretation of data: Kalendra Kunwar, Monika Jain, James Y. Rixey, Frances Schmidt, Sailesh Karki, Sushma Edara
Drafting of the manuscript: Kalendra Kunwar, Monika Jain, Frances Schmidt, Sailesh Karki, Sushma Edara
Critical review of the manuscript for important intellectual content: Kalendra Kunwar, James Y. Rixey, Frances Schmidt, Sailesh Karki, Sushma Edara
Supervision: Kalendra Kunwar, Frances Schmidt, Sailesh Karki
Human Ethics
Consent was obtained or waived by all participants in this study

IMAGES
VIDEO
COMMENTS
The comprehensive list of clinical features associated with severe malaria is shown in Table 1 - Diagnostic criteria for severe P. falciparum malaria. Children. Children are more likely to present with non-specific and gastrointestinal symptoms such as fever, lethargy, malaise, nausea, vomiting, abdominal cramps, and somnolence. They are more ...
Cerebral malaria is the most common clinical presentation and cause of death in adults with severe malaria. The onset may be dramatic with a generalized convulsion, or gradual with initial drowsiness and confusion, followed by coma lasting from several hours to several days. ... When patients with severe falciparum malaria show significant ...
The clinical manifestations of malaria vary with parasite species, epidemiology, immunity, and age. Issues related to clinical manifestations and diagnosis of malaria will be reviewed here. Technical aspects of laboratory tools for diagnosis of malaria are discussed further separately. The epidemiology, pathogenesis, diagnosis, and treatment of ...
Severe falciparum malaria results from the extensive sequestration of erythrocytes containing these mature parasite forms in the ... clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr Opin Infect Dis. 2018; 31:69-77. doi: 10.1097/QCO.0000000000000419. [PMC free article ...
AKI in severe falciparum malaria is caused by acute tubular necrosis and defined as a creatinine more than 265 μmol/l or urea more than 20 mmol/l . In adults with severe malaria, AKI develops in up to 40% of patients, whereas in children, the incidence is historically reported at approximately 10% . As the WHO definition does not define AKI ...
Treatment of Severe Malaria ... with P. falciparum infection to monitor clinical response and check parasite density every 12 -24 hours. Once . clinical presentation improves and a decrease in parasite density becomes apparent, treating clinicians can consider outpatient completion of treatment.
Due to the risk of progression to severe disease in patients with P. falciparum infection, patients should be hospitalized to monitor clinical response and check parasite density every 12-24 hours until clinical presentation improves and a decrease in parasite density becomes apparent. Then, treating clinicians can consider outpatient ...
The clinical presentation can vary substantially depending on the infecting species, the level of parasitemia, and the immune status of the patient. Infections caused by P. falciparum are the most likely to progress to severe, potentially fatal forms with central nervous system involvement (cerebral malaria), acute renal failure, severe anemia ...
The presentation of uncomplicated P. falciparum malaria is highly variable and mimics that of many other diseases. Although fever is common, it is often intermittent and may even be absent in some cases. The fever is typically irregular initially and commonly associated with chills. True rigors are unusual in acute falciparum malaria.
Severe malaria is mainly caused by Plasmodium falciparum. ... Organization for the prevention of Plasmodium falciparum malaria in children ... The clinical presentation of malaria ranges from ...
Clinical features and presentation. Malaria is an important cause of fever, particularly in tropical regions. ... Umulisa N, Gesase S, Day NP, Dondorp AM, Dondorp AM (2012) Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis 54(8):1080-1090 ...
Importantly, virtually all patients with malaria present with headache. Clinical symptoms include the following: Cough. Fatigue. Malaise. Shaking chills. Arthralgia. Myalgia. Patients experience a paroxysm of fever, shaking chills, and sweats (every 48 or 72 h, depending on species).
Artesunate for the Treatment of Severe Falciparum Malaria. Author: Philip J. Rosenthal, M.D. Author Info & Affiliations. Published April 24, 2008. N Engl J Med 2008;358: 1829 - 1836. DOI: 10.1056 ...
Purpose of review: Cerebral impairment and acute kidney injury (AKI) are independent predictors of mortality in both adults and children with severe falciparum malaria. In this review, we present recent advances in understanding the pathophysiology, clinical features, and management of these complications of severe malaria, and discuss future areas of research.
Clinical presentation of malaria varies from asymptomatic infection to benign/uncomplicated disease and even severe/fatal cases depending on host and environmental factors.5 While P.vivax was considered to cause uncomplicated malaria previously, ... Severe falciparum malaria was defined as one or more of the clinical or laboratory parameters ...
Plasmodium falciparum infection causes the most severe clinical manifestations, and its pathogenesis is the best studied. The clinical presentation of patients with malaria is closely related to the malaria parasites and varies according to local malaria epidemiology, the patient's immune status and age.
Background Malaria still represents a major health threat, in terms of both morbidity and mortality. Complications of malaria present a diversified clinical spectrum, with neurological involvement leading to the most serious related-conditions. The authors recently encountered a case of a 60-year old Italian man presenting with confusion, language disturbances and Parkinson-like syndrome 3 ...
Malaria disease can be categorized as uncomplicated or severe (complicated). In general, malaria is a curable disease if diagnosed and treated promptly and correctly. All the clinical symptoms associated with malaria are caused by the asexual erythrocytic or blood stage parasites. When the parasite develops in the erythrocyte, numerous known ...
Almost all cases of P. falciparum malaria present within 6 months of exposure and most within 2-3 months. Infection with other malaria species, such as P. ovale and P. vivax may present months or years after exposure due to reactivation of hypnozoites (a dormant liver stage). Presentation may be delayed in people who have taken chemoprophylaxis.
The first definition of clinical malaria (definition 1) was a body temperature of at least 37.5°C or a history of fever in the previous 24 hours and P. falciparum asexual parasitemia of more than ...
Malaria caused by P. falciparum is a severe, endemic parasitosis, mainly in Africa. In some cases, it can be complicated with ARDS. We present a case of a patient who returned from Nigeria with respiratory symptoms, in which both COVID-19 infection and tropical malaria were proven; with a fatal outcome. ... COVID-19: clinical presentation and ...
Most patients with COVID-19 and malaria co-infection experienced short-term hospitalization and mild to moderate disease severity. However, at presentation, co-morbidities and severe malaria were significantly associated with higher mortality or worse clinical outcomes. These findings emphasize the …
The clinical manifestations of malaria are dependent on the previous immune status of the host. 7 In areas where endemicity of P. falciparum malaria is stable, severe malaria most commonly occurs in children up to 5 years of age, while is less common in older children and adults because of the acquisition of partial immunity. In areas of lower ...
Introduction: Malaria continues to be the leading cause of hospitalization and death in Angola, a country in sub- Saharan Africa. In 2023, in the first quarter, 2,744,682 cases were registered, and of these 2,673 patients died due to malaria disease. Previous studies have shown that the ABO blood group can affect the progression of malaria to severe conditions after P. falciparum infection ...
The malaria vaccine candidate BK-SE36 is based on a recombinant form of the Plasmodium falciparum serine repeat antigen (SERA). The vaccine candidate was selected for clinical development on the following basis: (i)epidemiological studies showing high antibody titers that inversely correlate with malaria symptoms and severe disease; (ii) in vitro studies demonstrating induction of antibodies ...
Each species and its clinical presentation thus represent a particular malaria and are given appropriate terms (e.g., acute uncomplicated ovale malaria, severe vivax malaria [global description of the unwell patient], cerebral malaria due to P. falciparum, P. knowlesi-induced acute respiratory distress syndrome [ARDS]). Therefore clinicians ...
Malaria parasites such as P. falciparum are transmitted to people by mosquito bites. Clinical Trial Results. The clinical trial assessed two dose levels, with 19% of the 300mg-dose group and 28% of the 150mg-dose group developing symptomatic malaria, providing protective efficacy of 77% and 67% against symptomatic malaria, respectively.
The clinical trial assessed two dose levels, with 19% of the 300mg-dose group and 28% of the 150mg-dose group developing symptomatic malaria, providing protective efficacy of 77% and 67% against symptomatic malaria, respectively. ... Malaria parasites such as P. falciparum are transmitted to people by mosquito bites. In 2020, scientists at ...
AKI in severe falciparum malaria is caused by acute tubular necrosis and defined as a creatinine more than 265 μmol/l or urea more than 20 mmol/l . In adults with severe malaria, AKI develops in up to 40% of patients, whereas in children, the incidence is historically reported at approximately 10% [9,10]. As the WHO definition does not define ...
Introduction. Malaria is a life-threatening condition and remains a major killer in tropical countries. Malaria is caused by an infection of the parasite belonging to the genus Plasmodium.The most virulent is Plasmodium falciparum, causing one-third of the deaths associated with the disease [].Anemia in malaria is multifactorial [], and quite often the presentation is that of microangiopathic ...