Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Coordination chemistry articles from across Nature Portfolio
Coordination chemistry is the study of compounds that have a central atom (often metallic) surrounded by molecules or anions, known as ligands. The ligands are attached to the central atom by dative bonds, also known as coordinate bonds, in which both electrons in the bond are supplied by the same atom on the ligand.
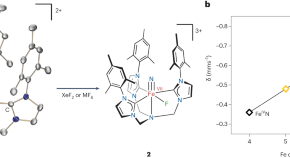
Lifting iron higher and higher
Biological and synthetic catalysts often utilize iron in high oxidation states (+IV and greater) to perform challenging molecular transformations. A coordination complex featuring an Fe(VII) ion has now been synthesized through sequential oxidations of nonheme iron–nitrido precursors.
- Adam T. Fiedler
- Laxmi Devkota
Related Subjects
- Organometallic chemistry
Latest Research and Reviews
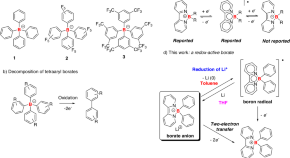
Reduction of Li + within a borate anion
Chemical reduction of alkali cations to their metals is extremely challenging. Here, the authors synthesized a series of redox-active borate anions stabilized by bipyridine ligands which can reduce lithium ions generating elemental lithium metal and borate radicals.
- Jiachen Yao
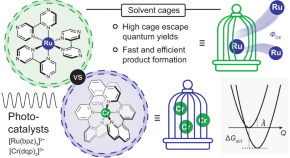
Cage escape governs photoredox reaction rates and quantum yields
The spontaneous recombination of photogenerated radicals surrounded by solvent molecules is an important energy-wasting elementary step in photoredox reactions. Now the decisive role that cage escape plays in these reactions is shown in three benchmark photocatalytic reactions, with quantitative correlations observed between photoredox product formation rates and cage escape quantum yields.
- Oliver S. Wenger
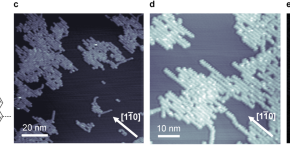
Kinetic control over the chiral-selectivity in the formation of organometallic polymers on a Ag(110) surface
The effective control of chirality on surfaces is crucial for applications such as enantioselective heterogeneous catalysis and nonlinear optics. Here, the authors study the on-surface synthesis of organometallic polymers and their chiral expression on Ag(110), demonstrating that kinetic effects play an important role in determining polymer chirality.
- R. S. Koen Houtsma
- Floris van Nyendaal
- Meike Stöhr
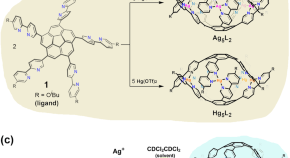
Dual-controlled guest release from coordination cages
Dual-controlled molecular release is of importance in the construction of controlled delivery systems with high anti-interference capabilities, but has been underexplored in the context of guest release from supramolecular entities. Here, the authors report a coordination cage whereby changes in both the coordinative metal cation and the solvent system are needed to induce guest release.
- Chengyuan Shao
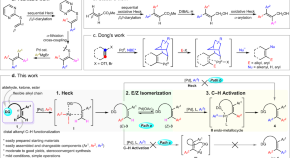
Remote-carbonyl-directed sequential Heck/isomerization/C(sp 2 )–H arylation of alkenes for modular synthesis of stereodefined tetrasubstituted olefins
The synthesis of highly substituted olefins has been pursued for decades. Here, the authors show a carbonyl-directed sequential Heck/isomerization/C–H arylation of alkenes for modular synthesis of stereodefined all-carbon tetrasubstituted olefins.
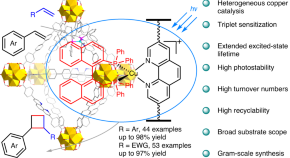
Visible light-mediated intermolecular crossed [2+2] cycloadditions using a MOF-supported copper triplet photosensitizer
[Cu(phen)(binap)] + features a relatively high photocatalytic activity, but its low photostability hinders its use in organic chemistry. Now immobilization of this motif on a metal–organic framework matrix enhances its stability and excited-state lifetime, enabling the promotion of [2+2] cycloadditions of styrenes with a variety of olefins, including electron-deficient alkenes.
News and Comment
Cyclopropanes from iron carbenes
- Peter W. Seavill
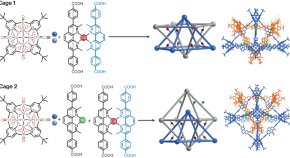
Picking an inter-locked cage
A metal templating approach can be used to generate homo- and heterointerlocked cage structures.
- Stephanie Greed
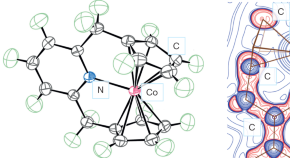
A 21-electron cobalt sandwich
- Joan Serrano-Plana
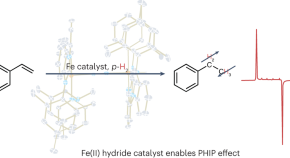
Benign hyperpolarization
- Davide Esposito
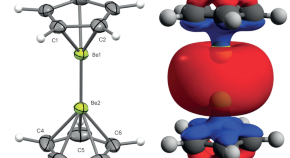
The elusive Be–Be bond
- Thomas West
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- Coordination Chemistry
Basics and Current Trends
- © 2023
- Birgit Weber 0
Anorganische Chemie IV, Universität Bayreuth, Bayreuth, Germany
You can also search for this author in PubMed Google Scholar
- Comprehensive textbook on coordination chemistry
- Based on proven lecture manuscripts
- Outlook into current research areas
6378 Accesses
1 Citations
- Table of contents
About this book
Authors and affiliations, about the author, bibliographic information.
- Publish with us
This is a preview of subscription content, log in via an institution to check access.
Access this book
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
Table of contents(13 chapters)
Front matter, what are complexes.
Birgit Weber
Structure and Nomenclature
What are organometallic compounds, bond theories, color of coordination compounds, stability of coordination compounds, redox reactions of coordination compounds, supramolecular coordination chemistry, metal-metal bond, luminescence of metal complexes, bioinorganic chemistry, back matter.
- Inorganic chemistry
- Complex Theory
- Complex compounds
- Ligand Field Theory
- Organometallic chemistry
The Chemistry of Complex Compounds is ideally prepared in this textbook for undergraduate chemistry students, providing both an easy and comprehensive introduction to the subject, which is relevant to examinations. It is based on proven lecture notes and assumes no basic knowledge. In addition to basic questions such as "what are complexes" and "what are organometallic compounds", the common bonding models are presented and the colour and stability of coordination compounds are explained, among other things. Other chapters cover redox reactions in complexes, the metal-metal bond, molecular magnetism, supramolecular chemistry, and bioinorganic chemistry. As a conclusion, the book gives an outlook into current research areas and trends in coordination chemistry, so that students of higher semesters and PhD students will also benefit from reading it. This includes the luminescence of complexes and selected examples of reactions catalyzed by complexes. Birgit Weber is a professor of inorganic chemistry at the University of Bayreuth. Her research focuses on coordination chemistry and ligand design for multifunctional switchable complexes.
Birgit Weber is Professor of Inorganic Chemistry at the University of Bayreuth. Her research focuses on coordination chemistry and ligand design for multifunctional switchable complexes.
Book Title : Coordination Chemistry
Book Subtitle : Basics and Current Trends
Authors : Birgit Weber
DOI : https://doi.org/10.1007/978-3-662-66441-4
Publisher : Springer Spektrum Berlin, Heidelberg
eBook Packages : Chemistry and Materials Science , Chemistry and Material Science (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer-Verlag GmbH, DE, part of Springer Nature 2023
Softcover ISBN : 978-3-662-66440-7 Published: 28 March 2023
eBook ISBN : 978-3-662-66441-4 Published: 27 March 2023
Edition Number : 1
Number of Pages : XVII, 266
Number of Illustrations : 135 b/w illustrations, 57 illustrations in colour
Topics : Inorganic Chemistry , Chemistry/Food Science, general , Chemistry/Food Science, general
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Application of Coordination Compounds with Transition Metal Ions in the Chemical Industry—A Review
Jacek malinowski.
1 Faculty of Chemistry, University of Gdańsk, Wita Stwosza 63, 80-308 Gdańsk, Poland; [email protected] (J.M.); lp.pw@79hcyzakinimod (D.Z.); [email protected] (D.J.); [email protected] (J.D.)
Dominika Zych
Dagmara jacewicz, barbara gawdzik.
2 Institute of Chemistry, Jan Kochanowski University, Świętokrzyska 15 G, 25-406 Kielce, Poland
Joanna Drzeżdżon
This publication presents the new trends and opportunities for further development of coordination compounds used in the chemical industry. The review describes the influence of various physicochemical factors regarding the coordination relationship (for example, steric hindrance, electron density, complex geometry, ligand), which condition technological processes. Coordination compounds are catalysts in technological processes used during organic synthesis, for example: Oxidation reactions, hydroformylation process, hydrogenation reaction, hydrocyanation process. In this article, we pointed out the possibilities of using complex compounds in catalysis, and we noticed what further research should be undertaken for this purpose.
1. Introduction
The use of complex compounds in processes employed in the broadly understood chemical industry is a very important aspect of the work of scientists around the world. Processes carried out on an industrial scale and conducted by scientists are enjoying the growing recognition of global corporations dealing in the production of various polymeric materials used in many aspects of our lives [ 1 , 2 , 3 , 4 ].
In order to understand the important function of coordination compounds in catalytic reactions during industrial processes carried out on an industrial scale, it is necessary to learn the exact mechanisms of the reactions carried out. Delving into the mechanism of an exemplary process will allow us to conclude that the most important changes occur in the coordination sphere of the comprehensive relationship. This means that many factors related to geometry, steric hindrance, or the used central atom and attached ligands of the complex compound affect the catalytic reaction.
In recent years, the focus has been on improving the efficiency and selectivity of processes. It has been confirmed that the selection of appropriate ligands in the metal coordination sphere allows a catalyst with properties that have been planned to be obtained. We can therefore conclude that the catalytic properties of the complex compound used depend to the greatest extent on the structure of the catalyst [ 5 , 6 ].
A very important aspect of work on chemical technologies is the smallest possible pollution of the environment, i.e., the application of the principles of green chemistry. Conducting reactions in organic solvents is replaced by supercritical liquids or ionic liquids. Developing the implement of biotechnological and organocatalytic methods allows environmentally friendly chemical technologies to be obtained [ 7 , 8 ].
In this publication, we present the latest methods of using coordination compounds in catalytic reactions used in the chemical industry. We present the influence of coordination compounds on the course of reactions taking place in organic synthesis (for example: Hydroformylation process, hydrogenation reaction, oxidation processes, olefin polymerization processes). We also describe the application of green chemistry principles in a catalysis with the participation of complex compounds.
2. Oxidation Processes
2.1. wacker process.
The Wacker process was developed in cooperation of two concerns, Wacker and Hoechst, and then published in 1959. The process is a well-known reaction for obtaining acetaldehyde by oxidation of ethene. The reaction is carried out in an aqueous environment using a homogeneous catalyst (PdCl 2 · CuCl 2 ). The synthesis was recognized by the chemical industry, due to the fact that it was an alternative to the hydroformylation reaction. The acetaldehyde obtained in the synthesis is widely used. It is mainly applied for production of acetic acid, acetic anhydride or chloroform. In addition, it can form synthetic resins in condensation reactions with phenols and amines. The Wacker process is shown in Figure 1 , in which stoichiometric reactions combine in one catalytic cycle. In the first stage, in an aqueous environment, it reacts with palladium(II) chloride, forming a stoichiometric amount of acetaldehyde, and palladium(II) is reduced to palladium(0). In the next stage, palladium(0) undergoes reoxidation in the presence of copper(II) compounds. Copper(I) is formed as the product of this reaction while in the last stage copper(I) is oxidized with oxygen to copper(II). Reaction 4 in Figure 1 represents the overall reaction of the process [ 9 , 10 ].

Reactions forming the catalytic cycle of the Wacker process [ 1 ].
According to research recommendations, the oxidation stage is actually catalyzed by [PdCl 4 ] 2− , which is formed under the conditions of water and Cl − ions. In the analyzed cycle, two chloride ligands are replaced by ethene and water. In the next stage, there is a nucleophilic attack of water on ethene in the complex, followed by substitution of the chloride ligand by another water molecule. β-elimination of a hydrogen atom leads to the formation of vinyl alcohol. This stage is followed by a series of processes leading to the production of acetaldehyde and palladium(II) hydride. Due to the fact that palladium(II) hydride is unstable, Pd(0) and HCl are formed as a result of the reduction reaction. The cycle is closed. Palladium(0) is oxidized to palladium(II) with CuCl 2 [ 11 , 12 ].
In [ 13 ] the researchers carried out quantum mechanical tests and they concluded that the anti-nucleophilic attack is the step determining the Wacker process under standard conditions. Unfortunately, experimental studies did not confirm the results obtained by calculation methods. Keith et al. report that the Wacker process is determined by a syn-nucleophilic attack [ 14 ].
The Wacker oxidation usually gives ketones as reaction products, but the literature reports that one can carry out the Wacker process that will give 99% selectivity in obtaining aldehydes, e.g., using 1,4-benzoquinone, t-BuOH, and PdCl 2 (MeCN) 2 [ 15 , 16 , 17 , 18 ]. Regarding the selectivity of the Wacker process, products with high selectivity are also obtained using styrene derivatives as substrates with the reaction carried out under mild conditions [ 19 ].
Studies conducted over the years proved that the Wacker process produces environmentally hazardous, chlorinated by-products, as well as harmful copper waste. According to the principles of green chemistry, solutions are being sought that affect the protection of ecosystems and the whole world. Researchers at the University of Pune proposed the Pd(0)/C system as a heterogeneous catalyst that can be recycled. This allowed the elimination of pure CuCl 2 . In addition, they used potassium bromate KBrO 3 as an oxidizing agent instead of molecular oxygen. By that, they developed a method using inexpensive substrates and achieved high process efficiency [ 20 ].
One may conclude that the by-products formed during the Wacker process are a major disadvantage of this cycle. Chlorine derivatives are environmental pollutants. Therefore, scientists should attempt to deactivate the resulting by-products.
2.2. Hydroperoxide Epoxidation Reaction of Olefins Catalyzed by Mo(VI) Complex
Epoxides are olefin oxides. Their structure contains a three-membered ring made of two carbon and oxygen atoms. They are the heterocyclic compounds. These compounds are characterized by high reactivity. For example, they undergo reactions that are accompanied by ring opening and closing followed by attachment of the nucleophile [ 21 , 22 ]. These reactions are used in the production of hypertension medications, antibiotics, and steroids. Hydrogen peroxides are the most common oxidants in the olefin epoxidation processes; H 2 O 2 is rarely availed because it poorly dissolves in hydrocarbons. The course of the process is catalyzed by metal oxides of groups 4–6, e.g., Ti, V, Mo, W. There are many methods of epoxide synthesis, but the most well-known is the propene epoxidation reaction. According to data, propene oxide production in the world is close to 6 million tons per year [ 12 ].
One of the epoxidation methods, which includes peroxide metal compounds, is the reaction of olefin epoxidation with hydroperoxide catalyzed by the Mo(VI) complex with a peroxide ligand. Olefin is attached to the active form of the catalyst, namely the ROO group. Olefin binds to the catalyst through an oxygen atom, which is a characteristic feature of this reaction mechanism. No bond is formed by the metal atom; however, molybdenum in the 6th degree of oxidation will have a positive effect on the breaking of the O–O bond and restoration of the catalyst [ 11 , 12 ].
The epoxy—propene oxide—formed in the reaction is commonly used for the production of polyether and polyhydric alcohols, ethers, or propylene glycols.
Studies on epoxidation using molybdenum(VI) compounds have contributed to the development of significant stereoselectivity and efficiency of conducted reactions. Unfortunately, the use of solvent-free epoxidation reactions remains to be explored.
2.3. Sharpless System for Asymmetric Epoxidation of Olefins with Hydroperoxide with Dimeric Titanium Complex as a Catalytically Active Form
The development of the chemistry of organometallic compounds contributed to the growing interest in the utilize of newly created systems. Sharpless conducted research on the use of organometallic catalysts in the asymmetric epoxidation reaction [ 23 ]. Allyl alcohol epoxidation is carried out with the participation of a system consisting of titanium(IV) isopropoxide, tartaric acid derivatives, and an oxidant in the form of tert-butyl hydroperoxide ( Figure 2 ). Ti(O- i Pr) 4 acting as a catalyst is a dimeric complex of titanium with tartaric ligands [ 21 , 24 ]. The tartaric acid ethyl ester used affects the stereochemistry of the reaction product. The ester interacting with the metal will form a complex. The resulting system undergoes the oxidation of hydrogen peroxide and the bond breaks on the appropriate side of the hydrogen bond depending on the chirality of the tartrate derivative [ 25 , 26 ].

Sharpless system diagram for asymmetric olefin epoxidation [ 21 ].
The problem in the Sharpless system for asymmetric epoxidation of olefins with hydroperoxide with the dimeric titanium complex as a catalytically active form is the use of chlorinated solvents. For this reason, this reaction creates many problems in industrial production.
2.4. Cyclohexyl Hydroperoxide Decomposition Reaction Catalyzed by Cobalt(II) and Cobalt(III) Complex Compounds
Improving the technology of obtaining polymer fibers is the subject of research in numerous research centers. Caprolactam, which is used for the production of polyamide fibers and plastics, is particularly important in great chemical synthesis. According to statistical data, caprolactam production capacity is 5.2 million tons per year. It is worth noting that the cyclohexanol–cyclohexanone mixture from hydrogenated benzene has a huge share in caprolactam production in Poland and worldwide. It is estimated that 90% of the world’s production of this raw material uses this technology [ 27 , 28 , 29 ]. In Poland, the leading production process for caprolactam is CYCLOPOL technology developed at the Industrial Chemistry Research Institute in cooperation with Zakłady Azotowe in Tarnów and Puławy. CYCLOPOL technology has also been implemented in other countries, i.e., Russia, India, Spain, Italy, and Slovakia. In the first stage of the process, the hydrogenation of benzene to cyclohexane occurs. The cyclohexane oxidation process takes place in a bubble reactor. It is carried out in the liquid phase at a temperature in the range 155–165 °C, under pressure from 0.8 to 1.05 MPa. The technology implies that air or air enriched with oxygen should be an oxidant. A mixture of cobalt and chromium catalysts that support cyclohexane oxidation to a mixture of cyclohexanol and cyclohexanone and decomposition of hydroperoxides is also used. The next stage is the distillation of a mixture of cyclohexanols and cyclohexanone, followed by their dehydrogenation [ 12 ]. The key step is the cyclohexane oxidation reaction. The various cobalt compounds, e.g., naphthenate, play the catalyst role in this step. An important reaction step is the decomposition of cyclohexyl hydroperoxide. The reaction takes place according to radical mechanism [ 4 ].
Cobalt(II) and cobalt(III) compounds are described in the literature as catalysts in this reaction. The mechanism of catalysis is described by the Haber–Weiss reaction [ 4 ].
Ongoing work on improving the cyclohexane oxidation reaction node at the Tarnów plant has resulted in significant technological changes. The new CYCLOPOL–bis process consists of two stages. The process of synthesis of cyclohexyl hydroperoxides was separated from the process of their selective decomposition. In addition, optimal aeration, pressure, and temperature parameters were selected for these separate oxidation phases. The modernized CYCLOPOL–bis technology has allowed production costs to be minimized, the quality of products to be improved, and the effects on the environment to become less toxic [ 12 ].
Research to improve the selectivity of decomposition reactions is still needed. It should be emphasized that the CYCLOPOL–bis technology is close to the principle of green chemistry.
3. The Hydrocyanation Reaction
Hydrocyanation is used in the industry in the production of adipic acid nitrile. It is the basic raw material for the production of nylon 6,6, the reaction of which consists of attaching HCN to the olefin. DuPont company processes possess an economic significance, where adipic acid nitrile is obtained in the process of butadiene hydrocyanation. The process uses the Ni(0) catalyst with phosphate ligands [ 20 ].
The process consists of two stages. The catalyst plays a key role in the whole process. At first, the oxidative attachment of HCN to the NiL 4 catalyst occurs and the hydrocyanide complex is formed. At this stage, the selection of a suitable ligand (L), e.g., a phosphorus ligand, is particularly important. Electronic and steric properties are taken into account, which have an impact on the course of individual stages of the hydrocyanation reaction. On the one hand, more basic ligands favor the addition of HCN. On the other hand, less basic ligands, which are characterized by high steric hindrance, are more popular [ 12 , 30 ]. Representative of this group is o-methylphenyl phosphate P(O-o-MeC 6 H 4 ) 3 , which facilitates the reductive elimination of the product (3-pentenenitrile). According to the research, steric effects in the case of phosphine and phosphite ligands have a significant impact on the stability of Ni(0) complexes [ 31 , 32 ]. IR analyses determining changes of carbonyl vibration frequency (νCO) in [Ni(CO) 3 L] (where L denotes a broad variety of phosphorus ligands) complexes have shown that the nature of the ligand π acceptor increases the stability of Ni(0) complexes [ 33 ]. The steric properties of the ligands are represented using the Tolman cone angle. For ligands with a small cone angle of approximately 109°, dissociation will not occur even if we use high dilution solutions [ 31 , 32 ]. An example of such a complex is [Ni[P(OEt) 3 ] 4 ]. 16-electron complexes, e.g., [Ni[P(O-o-tolyl) 3 ]], whose Tolman cone angle was 141°, and alkene complexes [Ni(alkene)L 2 ] were also examined. Subsequent studies have determined that the increase in metal–alkene bond strength is affected by the replacement of the H-alkene atom by a more electronegative CN. The electron effect is visible here, which results in higher stability of the acrylonitrile complex. Tolman also presented in his researches 1 H and 31 P NMR studies of the complex [HNi(CN)L 3 ] (where L is various phosphorus ligands) [ 34 , 35 ]. The addition of hydrogen cyanide to nickel(0) complexes is represented by the following reactions, where protonation is preceded by the dissociation of the ligand. The scientist pointed out that the addition of excess HCN may result in the formation of an undesirable complex [Ni(CN) 2 L 2 ] (where L is a phosphorus donor ligand), which deactivates the catalyst. For this reason, [Ni(CN) 2 L 2 ] is not active in the process of hydrocyanation [ 36 , 37 , 38 ].
Research concerns the HCN addition to butadiene at the first stage and the reaction taking place in the presence of a NiL 4 catalyst ( Figure 3 ). The reaction products are 3-pentenenitrile and 2-methyl-3-butenenitrile. The resulting isomer in branched form is not a substrate in the reaction of obtaining adipic acid nitrile; therefore, it is subjected to the process of isomerization to 3-pentenenitrile. The next stage takes place using the same catalyst with addition of Lewis acid. The Lewis acids used can be ZnCl 2 , ZnBr 2 , AlCl 3 , BPh 3 . It works with a free electron pair on the nitrogen of the CN group, which facilitates the creation of C–C connections. Isomerization of the double bond occurs and the 3-pentenenitrile is structured to an alkene (4-pentenitrile). The second CN group is joined in this reaction. Secondary by-products include 2-methylglutaronitrile (MGN), ethyl succinonitrile (ESN), and 2-pentenenitrile (AdN). Studies show that the hydrocyanation rate relative to isomerization is the highest for AlCl 3 and decreases in the order of AlCl 3 > ZnCl 2 > BPh 3 [ 12 , 29 , 30 , 38 ].

Mechanism of butadiene hydrocyanation [ 38 ].
In 2019 it was confirmed that hydrocyanation can run without cyanide [ 39 ]. The mentioned reaction was asymmetric olefin hydrocyanation. In the alkenic hydrocyanation process, the use of bident phosphorus-based ligands is of great importance, which contributes to increasing the stability of the products obtained during hydrocyanation [ 40 ]. In addition to nickel complexes, ruthenium(I) complexes for hydrocyanation are used [ 41 , 42 , 43 ].
The important issue regarding the hydrocyanation reaction is alkene isomerization. Low reaction yields are a disadvantage of hydrocyanation reactions. These issues remain to be improved.
4. Metathesis Reaction
The first studies on the metathesis reaction date back to the 1930s. Schneider and Frolich described the pyrolytic conversion of propene to ethene and butene, which took place without the use of a catalyst at a high temperature of about 852 °C [ 44 ]. Metathesis has been a major course in academic and industrial research for years. Banks and Baile’s research, conducted in 1964, on the alkylation of isoalkenes with olefin on heterogeneous catalysts [Mo(CO) 6 /Al 2 O 3 , WO 3 /SiO 2 , Re 2 O 7 /Al 2 O 3 ] at 160 °C turned out to be a breakthrough. These studies were aimed at obtaining fuels with a higher octane number, and also brought new information on the disproportion of linear olefins. Based on the developed technology, Philips Petroleum began production of high purity ethylene and but-2-ene from propylene [ 45 ]. Similar studies were conducted by Natta, who proved that the presence of homogeneous catalyst [TiCl 4 /AlEt 3 , MoCl 5 /AlEt 3 , WCl 6 /AlEt 3 ] cyclobutenes and cyclopentenes allows for the polymerization of the ring opening metathesis polymerization (ROMP) type already at low temperature. His work concerned the copolymerization of ethylene with both linear and cyclic olefins and the polymerization of cyclic alkenes (ring opening metathesis polymerization; ROMP). Catalytic systems based on metal oxides on a solid support were also known. Due to the low cost of their production, and thus their wide availability, they were used in the Shell higher olefin process (SHOP) and in further exploitation of advanced Shell technology (FEAST) [ 46 , 47 ].
At the same time, numerous studies were being conducted, each of which was characterized by the use of compounds containing multiple bonds. In addition, the reactions mentioned took place under the influence of catalysts. The research aimed to find out the exact reaction mechanism that could explain the experimental results obtained. Researchers tried to describe the individual reactions with understanding of the structure of the transition state. They strived to characterize the structure of the catalytic center and determine which homo- or heterogeneous catalysts affect the conversion of compounds containing multiple bonds.
The creator of the term “metathesis” is Calderon, who used the synthesized heterogeneous catalytic system WCl 6 /EtAlCl 2 /EtOH to synthesize but-2-ene and hex-3-ene from pent-2-ene [ 48 , 49 ].
The exact definition of metathesis was provided by the Nobel Prize winners in chemistry: Grubbs, Schrock, and Chauvin (2005). Metathesis is a catalytic reaction accompanied by an exchange of double bonds between carbon atoms [ 12 , 50 ]. This reaction can be applied to various chemical compounds, for example: Internal, terminal, and cyclic olefins, as well as dienes and podiums. Metathesis reactions usually do not require high temperatures and pressure. In addition, all atoms are used in metathesis processes, and the resulting by-products can be processed or reused. Therefore, the reactions are reversible and meet the requirements of green chemistry [ 51 ].
The olefin metathesis process is considered to be an especially important technology for the production and processing of olefins. Considering the type of substrate used, we distinguish the following types of metathesis [ 51 ]:

- Ring Closing Metathesis—RCM

- Ring Opening Metathesis Polymerization—ROMP
- Acyclic Diene Metathesis—ADMET
Chauvin worked on the metathesis process with his student, Herisson. The mechanism of metathesis proposed by these colleagues is shown in Figure 4 . They assumed that the metathesis reaction was catalyzed by alkylidene metal complexes. The first stage of the process is the production of metallycyclobutane II by reacting the R 1 -CH=CH 2 substrate with the complex I . The resulting complex degrades to the alkene, in this case, ethene, and to the corresponding metallocarbene. The resulting metallocarbene III reacts with the olefin R 2 -CH=CH 2 , and the subsequent decomposition of IV leads to the formation of carbene I . Carbene as an alkylidene complex is a proper reaction catalyst [ 52 , 53 ].

The mechanism of metathesis reaction.
Scientists in their research present homogeneous and heterogeneous catalytic systems. The metals used in the design of carbene complexes are transition metals with incompletely filled 3d, 4d, or 5d coatings [ 53 ]. Ruthenium, tungsten, molybdenum, and rhenium are most commonly applied. One of the first catalysts used in the described reaction was one-component tungsten carbide Ph 2 C=W(CO) 5 . The next step in the development of research on alkylidene catalysts was Schrock’s synthesis of a molybdenum complex ( Figure 5 ). This complex showed high catalytic activity but was unstable. It was one of the first complexes that could be used without the activators, e.g., Lewis acids. It is worth noting that it is applicable for the synthesis of sterically crowded compounds. Disadvantages of this catalyst were sensitivity to oxygen, moisture, and polar groups. Complex compounds based on ruthenium are of particular interest. They show high reactivity towards olefins (C–C bonds) and tolerance to the functional groups of the substrates [ 53 , 54 , 55 ]. In addition, they are stable and do not react negatively to oxygen and moisture that may be present in the reaction system. An example of such a complex is the first-generation Grubbs catalyst ( Figure 5 ). The catalyst synthesis is shown in Figure 5 . The resulting complex, in addition to the above-mentioned features, is easy to store. It is one of the most commonly used complexes in metathesis reactions due to the fact that it can act toward obtaining carbo- and heterocyclic ring compounds, as well as macrocyclic compounds [ 54 ]. In addition, it catalyzes the metathesis cyclization reaction of enines and the alkene–alkyne metathesis. It is often used in polymerization reactions: ADMET, ROMP, and living polymerization of norbornenes [ 54 ]. It does not catalyze cross metathesis with α, β-unsaturated compounds, which is its drawback. Catalysts that contain imidazole derivatives are also noteworthy. N-heterocyclyl carbenes are obtained by reacting a first-generation Grubbs catalyst with a compound that is a source of stable carbene, e.g., the imidazolidine salt ( Figure 6 ). In comparison to the first-generation catalyst, it takes part in reactions with α, β-unsaturated olefins, catalyzes ROMP cycloolefins with low ring stresses, and shows much higher activity.

Metathesis catalysts, from the left: ( A ) Schrock’s molybdenum complex, ( B ) first-generation Grubbs catalyst, ( C ) second-generation catalyst.

Grubbs catalyst preparation reaction.
Nickel complexes with chelate ligands have been used in metathesis reactions in a multistage SHOP process [ 54 , 55 ]. Initial material in this reaction is ethene. With the participation of a nickel catalyst, ethene is oligomerized and C 4 -C 40 olefins are formed. Light < C 6 and heavy > C 18 olefins in the isomerization process are converted into appropriate internal olefins. In the next stage of the metathesis process, olefins of medium length C 11 –C 14 are formed from them. The obtained internal olefins are used in the production of surfactants. In addition, the olefins subjected to a carbonylation process and hydrogenated to alcohols are the substrates in the synthesis of alkyl-benzenes. The separated C 6 –C 18 fraction is used for the production of plasticizers, detergents, and also lubricants. The oligomerization catalysts are the nickel complexes mentioned above. Chelate ligands are coordinated by oxygen and phosphorus atoms. Scientists pay particular attention to the hemilability of metal-coordinated ligands. Such ligands have the ability to open and close the ring, which is possible due to the different strength of coordination of atoms that act as donors. This property allows transitions between cis- and trans-forms, and the change in symmetry affects the selectivity of the reaction. Due to the selectivity, scientists are conducting numerous studies on the synthesis of catalysts, affecting the preparation of sterically crowded olefins and catalysts, which control the stereoselectivity of reactions [ 55 ].
The following complexes are olefin metathesis catalysts: Heterocyclic carbene-coordinated compounds of ruthenium, salamo-type bisoxime complexes of Co (II) and Ni (II), carboxylate, phenolate, hydroxycarboxylate, and catecholate derivatives of Ti (IV) [ 56 , 57 , 58 ]. It should also be noted that FeCl 3 also catalyzes the metathesis reaction [ 59 , 60 ].
In our view, the metathesis reaction provides many opportunities for the creation of various chemical compounds. It has application in refining industry and chemical studies. It is a powerful method of synthesis. For this reason, it is worth looking for new catalysts that meet the principles of green chemistry that can be used in metathesis.
5. Hydrosilylation Reaction
5.1. reduction pt(ii) to pt(0) in reaction with silane hsi(oet) 3.
Silicon compounds are widely used in the modern world. They are applied as additives to cleaning products, cosmetics, and even food products. In addition, they increasingly have found application in electronics and the automotive industry. For example, organosilicon polymers, polycarbosilanes, and polycarbosiloxanes are used as precursors of ceramic materials and are resistant to chemical agents. Some of these materials exhibit optoelectronic, photoconductive, or electroluminescent properties. The synthesis of silicon derivative compounds, necessary for the production of the above materials, is based on the hydrosilylation reaction. Hydrosilylation is the catalytic addition reaction of organic and inorganic hydrogen silanes to unsaturated compounds. Depending on the substrate used in the synthesis, new Si-C, Si-heteroatom bonds are formed. The most commonly used hydrosilylation catalysts are platinum(II) complexes; however, other transition metal coordination compounds are also known, e.g., Pd(II), Co(II), Ni(II), Ir(I), Rh(II), and Ru(I). Scientist reports inform about numerous homogeneous catalysts active in the hydrosilylation reaction. They are [PtCl 2 (cod)], [Pt(CH 2 =CHSiMe 2 ) 2 O 3 ], [RhCl(PPh 3 ) 3 ], [Pd 2 (dba) 3 ], where cod—cyclooctadiene, dba—dibenzoylacetone [ 61 ].
As an example of the reaction of reducing platinum(II) to platinum(0) is the reaction of [PtCl 2 (cod)] with (EtO) 3 SiH ( Figure 7 ). The metal colloid formed in the reaction can also be used in the hydrosilylation process.

Example of the reduction of platinum(II) to platinum(0).
It should be noted that the hydroformylation reaction is commonly used in the industry for siloxane and silanes production. Chemoselectivity of hydroformylation means that its applicability for the synthesis of new compounds is high. Currently, hydroformylation catalysts are not based on platinum due to the reduction of catalyst costs. The use of new catalysts in hydroformylation opens up the possibility of designing new transition metal compounds with catalytic properties in this process.
5.2. Karstedt’s Catalyst
Platinum complexes are most commonly used in hydrosilylation of carbon–carbon double bonds. Karstedt’s catalyst was described in 1973 as a compound of platinum(0) with divinylsilaxane ligands. It is formed in the reaction of divinyltetramethyldisiloxane with chloroplatinic acid, and it is applicable in numerous reactions due to its properties, i.e., high catalytic activity and good solubility in polysiloxane systems ( Figure 8 ) [ 61 , 62 ].

Karstedt’s catalyst.
Therefore, the high activity and selectivity of Karstedt’s catalyst causes it to be successfully used in industry. It will be difficult to find a replacement for this hydroformylation catalyst.
5.3. Hydrosilylation Mechanism Proposed by Chalk and Harrod and Modified Mechanism of Chal–Harrod—Catalyzed by Rhodium Complex Compounds
Reactions entering the cycle of the hydrosilylation process may take place with the participation of free radicals or in the presence of catalysts in the form of transition metal complex compounds. It is worth paying attention to the mechanisms of the hydrosilylation process of carbon–carbon multiple bonds in the presence of transition metal complexes. The use of transition metal complexes as catalysts affects the selectivity and speed of individual reactions. The hydrosilylation process is widely used in the synthesis of organosilicon compounds. In the 1960s, Chalk and Harrod introduced the first concept of a hydrosilylation mechanism [ 61 ]. In their research, they used hexachloroplatinic acid H 2 [PtCl 6 ] as a homogeneous reaction catalyst. In the first stage, silane is attached to the catalyst metal. The configuration of the transition metal used in the catalyst is usually d 8 or d 10 [ 61 ]. The second stage leads to a stable alkene complex. Olefin coordination will only occur when the electron cloud transfer from the double bond to the hybridized metal orbit occurs. It is important to use excess substrate (olefin). As a result of the oxidative addition of HSiR 3 to the complex, an M-H and M-Si bond is formed. The rearrangement of π–σ is considered a key stage. The migratory insertion of olefin for M–H binding generates a silyl–alkyl complex. The reaction product is formed by reducing the elimination of the alkyl complex [ 61 , 62 , 63 ].
Research on the formation of unsaturated products in the hydrosilylation process was also undertaken. The explanation for this problem is the modified Chalk–Harrod mechanism, catalyzed by rhodium complex compounds. Although the product obtained in both processes is the same and does not differ in any way, the process itself changes. This is visible at the key stage which is alkene insertion. In the new process, it occurs to the M–Si bond, not to the M–H bond. The final stage proceeds in the same way by the elimination of alkenylsilane. Both processes are shown in the diagram ( Figure 9 ) [ 61 , 62 , 63 ].

Hydrosilylation mechanism and modified mechanism proposed by Chalk and Harrod [ 63 ].
The hydrosilylation of alkenes with tertiary silanes can run without the use of a catalytic activator but with the use of the catalyst itself, i.e., cobalt(II) amide coordination compounds [ 64 , 65 , 66 , 67 ]. It is worth noting that alkene hydrosilylation can be successfully catalyzed by earth-abundant transition metal compounds [ 68 ].
The regioselectivity and stereoselectivity of the hydroformylation reaction depends on many factors e.g., solvent, catalyst, or temperature. Thus, it should be noted that the characteristic of the actual structure obtained product is difficult.
6. Hydroformylation Reaction
Hydroformylation is the reaction of olefins with hydrogen and carbon monoxide(II), where the products are straight chain (n) and branched chain (iso) aldehydes. This process occurs under the influence of the catalyst present during the reaction [ 69 , 70 ]. The products obtained in the form of aldehydes can be converted into alcohols, carboxylic acids, acetals, diols, or aldols under appropriate conditions. Most often, however, the intention of the reaction is to obtain an unbranched chain aldehyde. The catalytic system is most often characterized by the n/iso ratio. As we can see, the higher the numerical value of the ratio, the better the catalytic system used during the hydroformylation. The hydroformylation reaction scheme is shown below.
R-CH=CH 2 + CO + H 2 → R-CH 2 -CH 2 -CHO + R-CH(CH 3 )-CHO
The hydroformylation process was discovered by German chemist, Roelen, in 1938. He made the discovery during the Fischer–Tropsch reaction [ 71 ]. The first catalyst used during the hydroformylation reaction was hydridotetracarbonyl—[HCo(CO) 4 ]. Then, the addition of phosphines to cobalt catalysts was applied, which resulted in a higher n/iso ratio and the hydroformylation process was carried out at a lower pressure and temperature.
A great breakthrough in the search for catalytic systems was made thanks to the work of the English Nobel Prize winner Sir Geoffrey Wilkinson. They allowed the discovery of compounds based on rhodium atoms that showed a thousand-fold higher catalytic activity in the hydroformylation reaction compared to cobalt catalysts [ 72 , 73 ].
Rhodium compounds, despite a higher price, are commonly used as catalysts for hydroformylation reactions. Union Carbide Davy Powergass Johnson–Matthey LPO—a British chemical company—uses a hydroformylation process using a precursor of formula [(acac)Rh(CO) 2 ] and a modifying ligand, phosphine (PPh 3 ). The addition of approximately 10% by weight PPh 3 produces the active catalyst form—[(acac)Rh(CO)(PPh 3 )]. The hydroformylation process is carried out under the following conditions: At a temperature from 60 °C to 120 °C, pressure in the range of 10–50 bar. This process has a n/iso ratio of around 1:5. The hydroformylation process in Kędzierzyn–Koźle at Zakłady Azotowe is used in Poland [ 74 , 75 ].
Kuntz’s discovery of the industrial conduct of the hydroformylation process and its commercialization by Ruhrchemie/Rhone-Poulenc is based on the use of a two-phase catalytic system based on a rhodium complex compound. Tris(3-sulfonylphenyl)phosphine sodium (TPPS) was used in the hydroformylation process ( Figure 10 ).

Simplified formula of sodium salt (tris(3-sulfonylphenyl)phosphine).
The use of the aforementioned catalyst system allows easy separation of the catalyst from the desired products (aldehydes) because it is in the polar phase. Therefore, the catalyst is used in a continuous process. Conducting the hydroformylation process using a two-phase catalytic system allows for high selectivity and activity for the production of a linear product to be maintained [ 76 ].
The electron and steric properties of ligands attached to the rhodium atom influence the selectivity of the obtained product in the hydroformylation reaction. A larger amount of the linear product in the olefin hydroformylation reaction is obtained by using ligands with weaker basic properties—for example, pyrrolylphosphines or phosphites [ 77 , 78 , 79 ].
The steric properties of the ligands are evaluated based on the size of the conic angle, while for chelate ligands they are based on the grip angle, which determines the value of the angle between the P–M–P bonds present in the complex with the ligand. Studies on the impact of the grip angle on the selectivity of the reaction have shown that the equatorial–equatorial location of donor atoms in chelating ligands found in rhodium atom-based catalyst systems results in obtaining the best n/iso values. This means that the geometry of the respective rhodium complexes, in this case, for grip angles, is about 120°, exhibiting the geometry of the trigonal bipyramid.
The newest reports from scientists inform that the use of carbene and phosphorus ligand catalysts simultaneously attached to the rhodium atom in the coordination sphere results in obtaining a much better n/iso ratio. [RhH(NHC)(CO)P(OPh) 3 ) 2 ], where NHC denotes heterocyclic carbene, is an examples of such a catalyst [ 80 , 81 , 82 , 83 ].
The catalysis of the hydroformylation process is constantly evolving with regard to the use of rhodium compounds as catalysts [ 84 ]. Even a rhodium single-atom exhibits catalytic activity in hydroformylation similar to the RhCl(PPh 3 ) 3 complex [ 85 ]. Rhodium particles also have catalytic properties in hydroformylation [ 86 , 87 ]. In 2018 it was published that Fe(II) has the ability to catalyze hydroformylation of alkenes under mild conditions [ 88 ].
The most important advantage of the hydroformylation reaction is the ease of separation of the resulting product and catalyst. It is very important when receiving new materials.
7. Carbonylation Reaction
The carbonylation reaction is the process of attaching a carbon monoxide(II) molecule to an organic compound [ 89 , 90 , 91 ]. Most often, this process is used to produce acetic acid from methanol or an anhydrous derivative of the acid from the ester—methyl acetate. This reaction is carried out in the presence of transition metals, specifically metals in group 9, cobalt, rhodium, and iridium are used primarily. In addition, an iodide co-catalyst is needed during the process to activate methanol. This reaction produces acetyl iodide, which in subsequent hydrolysis produces a mixture of acetic acid and hydroiodic acid.
BASF carried out the first commercially conducted process of carbonylation of methyl alcohol with a cobalt catalyst. Then, industrial scale carbonylation of methanol was started by Monstanto, which applied a rhodium catalyst. The conditions operated by Monstanto compared to BASF differed in the use of lower carbon monoxide pressure and temperature. This process was also carried out with much better selectivity for methyl alcohol.
Studies on the mechanism of methanol carbonylation were conducted very thoroughly. Maitlis’ group examined the reaction using infrared spectroscopy and found that the mechanism of methanol carbonylation contains two catalytic cycles. The first cycle, rhodium, involves organometallic compounds and an iodide cycle containing organic reactions. The catalyst for this carbonylation reaction was the rhodium complex compound, cis-[Rh(CO) 2 I 2 ] − [ 92 ].
The methanol carbonylation process proposed by BP Chemicals, called the Cativa process, was carried out using an alternative iridium catalyst. The main difference of this process compared to the mechanism proposed by Monstanto is that it has about 100 times faster oxidation caused by the addition of CH 3 I to [Ir(CO) 2 I 2 ] − than to [Rh(CO) 2 I 2 ] − .
However, a different type of catalyst is used when carbonylation of higher alcohols is carried out. This is caused by too slow a reaction using a rhodium catalyst. Therefore, palladium catalysts are applied that simultaneously also catalyze the olefin hydroxycarbonylation.
The palladium complex compound-PdCl 2 (PPh 3 ) 2 is used in the carbonylation reaction, where the product is the known anti-inflammatory drug, ibuprofen. This synthesis is carried out by Boots–Hoechst–Celanese. Below, Figure 11 shows a simplified formula of this catalyst.

Simplified formula of the palladium complex used during ibuprofen synthesis.
Naproxen is another very well-known remedy in which production palladium complexes are used as catalysts. This drug belongs to non-steroidal inflammatory drugs, which are obtained in a multistage synthesis. The palladium complex is a catalyst during stage 2—Heck reaction, and during stage 3—hydroxycarbonylation.
Both ibuprofen and naproxen synthesis can be carried out in the presence of chiral phosphine. Reactions carried out in ionic liquids, using a ruthenium coordination compound as a catalyst, and with the introduction of a chiral phosphine allows us to obtain the appropriate optical isomer. Below, Figure 12 shows the catalyst used to obtain (S)-ibuprofen [ 93 ].

Ruthenium catalyst from S-BINAP used for the synthesis of S-ibuprofen.
Hydroaminocarbonylation of olefins, aminocarbonylation of aryl iodides, and oxidative carbonylation of amines occurs with the application of bulk Pd catalyst without the use of organic ligands [ 94 , 95 ]. Iridium and immobilized-rhodium catalyze the methanol carbonylation process, leading to the formation acetic acid [ 96 ]. An interesting fact is that membranes, e.g., chitosan, polyamide, polyvinyl alcohol, are used to catalyze carbonylation reactions [ 97 ]. Membranes take part in the product separation of carbonylation. In 2019 K 2 [Fe(CO) 4 ] as a catalyst promoting the formal insertion of CO was published [ 98 ].
Therefore, in the carbonylation reaction, iron(II) and platinum(II) complexes should be tested as catalysts. Palladium(II) complexes satisfactorily perform the role of catalyst carbonylation reaction; therefore, due to the similarity, analogous platinum(II) complexes should be tested.
8. Olefin Polymerization
Polymerization of olefins is a process that is used commercially around the world on a very large scale in the chemical industry. This is evidenced by the fact that the sum of the annual production of polyethylene and polypropylene is about 15 million tons. Another important aspect of using complex compounds such as olefin polymerization catalysts is the fact that it is economically cheap and environmentally friendly [ 99 ].
The newest version of the Ziegler–Natta catalysts used in the olefin polymerization process is the deposition of titanium(IV) tetrachloride on magnesium chloride. The MgCl 2 , the carrier, has a grain diameter of about 50 µm. A carrier with such grain diameter characteristics is obtained by precipitation from a soluble precursor solution with the addition of a Lewis base or by mechanical milling. The second stage is the addition of phthalic esters or silyl ethers. Magnesium(II) ions and titanium(III) ions have similar ionic rays, which make it possible to obtain a third-generation catalyst. This synthesis consists of adding titanium(I) tetrachloride to a solution of alkoxide, sulfide, or carboxylate containing magnesium(II) ions. Then the addition of TiCl 4 and phthalates activates the catalyst [ 100 ].
Atom transfer radical polymerization (ATRP) is a technique that allows the production of polymers and co-polymers used commercially around the world. Polish chemist Matyjaszewski, working in the United States, discovered and developed this method of polymerization. The ATRP technique is cheap and does not pollute the environment in the production of polymeric materials on an industrial scale.
The application of radical transfer polymerization techniques allows for polymers with the appropriate structure and morphology to be obtained. Thanks to such control possibilities of the process being carried out, its application in various industrial technologies is becoming wider.
Copper and iron coordination compounds are used as catalysts during radical atomization polymerization. Application of copper compounds during the process determines the necessity of the presence of reducing agents. The polymerization process carried out in water can be in homogeneous conditions or heterogeneous. Below, Figure 13 shows an example of a complex which is a catalyst for the ATPR process.

An example of a copper complex used as a catalyst in atom transfer radical polymerization.
The technique of radical polymerization with atom transfer allows for the production of polymers that are components of moisturizing agents, surfactants, paints, and surfactants. It is also used in the synthesis of polar thermoplastic elastomers. It also allows the improvement of hydrophilic, antibacterial, or conductivity properties in the polymer materials produced.
Radical polymerization using organometallic compounds (OMRP) is another technique used in polymerization. This method was initially proposed for the polymerization of acrylates. Acrylic acid, vinyl acetate, or other substrates are the monomers. Titanium, cobalt, iron, and chromium compounds have found application in this technique as catalysts. Figure 14 shows examples of ruthenium complexes used during OMRP [ 101 , 102 ].

Titanium compounds used as catalysts in radical polymerization using organometallic compounds.
In our view, olefin polymerization is a wide field for research, because nowadays there are reports of ever-increasing catalysts of this reaction being non-metallocene transition metal complexes [ 103 , 104 , 105 , 106 , 107 , 108 ]. The new complex compounds have higher values of catalytic activities than those traditionally used, and their synthesis is simple and cheap. New catalysts are chromium(III), oxovanadium(IV), and cobalt(II) complexes.
Late transition-metal catalysts are used in olefin polymerization. However, one should bear in mind that most of them cause the formation of amorphous and atactic polymers [ 109 , 110 ]. [Cu(2,3-pydc)(bpp)]·2.5H 2 O, [Zn(2,3-pydc)(bpp)]·2.5H 2 O, and [Cd(2,3-pydc)(bpp)(H 2 O)]·3H 2 O (2,3-pydc denotes pyridine-2,3-dicarboxyliate, bpp = 1,3-bis(4-pyridyl)propane) are metal–organic frameworks which are applied in heterogeneous catalysis [ 111 ]. Tridentate ligands based on N-, P-, and S-donors are used to synthesize the chromium(III) catalyst for ethylene trimerization and tetramerization [ 112 , 113 ].
One may conclude that this field must be developed because new catalysts can successfully replace traditionally used ones, which, unlike new catalysts, unfortunately did not meet the principles of green chemistry.
9. Hydrogenation Reaction
The hydrogenation reaction is a very important organic chemistry reaction. Hydrogen, one of the most important elements in the chemical industry, is used in almost 4% of hydrogenate carbon and hydrogen compounds. The mechanism of this reaction requires the presence of a catalyst, which is most often transition metal compounds. This process is carried out to create new bonds between carbon and hydrogen [ 114 , 115 ].
Heterogeneous catalysts are most often used during the hydrogenation reaction. The reason for their application is the ease of separation from organic products. However, the exploit of heterogeneous catalysts during the hydrogenation reaction is not possible in the asymmetric hydrogenation reaction. In addition, homogeneous catalysts, i.e., those made of complex compounds that are based on transition metals, increase chemoselectivity and regioselectivity of the reaction.
If we use a metal complex as a catalyst during the hydrogenation reaction, this gives the opportunity to create a spatial and electronic structure of the compound. This can be achieved by choosing the right ligand. An example of the best-known hydrogenation reaction catalyst is the Wilkinson catalyst—[RhCl(PPh 3 ) 3 ] ( Figure 15 ).

Simplified formula of Wilkinson’s catalyst.
A very important enantioselective synthesis, during which the L-3,4-dihydroxylphenylalanine compound is obtained (L-DOPA), in one of the stages uses an asymmetric hydrogenation reaction. L-DOPA is a medicine applied during therapy against Parkinson’s disease. The need to obtain the appropriate product enantiomer informs us that the catalytic system of the hydrogenation mechanism must contain a chiral form of phosphine. During the synthesis reaction of L-3,4-dihydroxylphenylalanine, the catalytic system of the rhodium complex with 1,2-bis[(2-methoxyphenyl)phenylphosphine]ethane (DIPAMP) is used. Figure 16 shows a simplified formula of the chiral form of phosphine, which is a ligand in the rhodium complex [ 116 ].

Simplified formula of the chiral form of phosphine-1,2-bis[(2-methoxyphenyl)phenylphosphine] ethane.
It can be concluded that the use of phosphines as ligands in catalysts creates many opportunities to design new complexes as catalysts. Hydrogenation of esters and amides are catalyzed by these types of compounds. It is worth trying to synthesize new complexes containing other organophosphorus compounds as ligands, as well as trying to check the catalytic activity of phosphine-containing dual-core complexes.
10. Catalysis and Green Chemistry
10.1. ionic liquids—catalytic reactions.
In modern catalysis, particular attention is paid to the principles of green chemistry. Technological processes must be as environmentally friendly as possible. Ethylammonium nitrate is a chemical compound recognized as one of the first ionic liquids. This chemical compound was obtained in 1914 by Walden as a result of the neutralization of ethylamine with the help of concentrated nitric acid(V) [ 117 ]. Ionic liquids are characterized by very low vapor pressure and low melting points. The low volatility of ionic liquids is a property that caused these chemicals to be classified as green solvents. The group of compounds called ionic liquids includes: Pyridinium, tetraalkylammonium, imidazolium, and phosphonium salts. Ionic liquids can be used as solvents friendly to the environment because it does not cause environmental load. The biodegradability and ecotoxicity of ionic liquids are currently studied. Ionic liquids are involved in the transport of metals and chemical compounds in soil [ 118 ].
Imidazolium salts can be used in two-phase systems. For this reason, they are the most commonly applied ionic liquids as a catalytic reaction medium. Ionic liquids are implemented in the technological process of obtaining S-Naproxen and S-Ibuprofen anti-inflammatory drugs. Asymmetric hydrogenation is catalyzed by ruthenium complexes with S-Binap chiral phosphine carried out in systems containing ionic liquid and alcohol, e.g., methanol.
Pyridinium ionic liquids are a very good medium for the methoxycarbonylation reaction of iodobenzene catalyzed by [PdCl 2 (cod)] or Pd(0)/PVP [ 119 ]. In the Sonogashira reaction, ionic liquids were used, and thus effective catalyst recycling was possible [PdCl 2 (P(OPh) 3 ) 2 ] [ 120 ]. Processes based on the Sonogashira reaction that applies in industries are the synthesis of alkyl derivatives. These types of reactions are used in the pharmaceutical industry. In this type of reaction tetraalkylphosphonium salts are the best reaction medium. The resulting product is separated from the ionic liquid containing the catalyst using hexane extraction. By-products are removed by washing with water.
Palladium precursors with triphenylphosphine in imidazolium ionic liquids are used in the Suzuki reaction to which bromobenzene undergoes [ 121 ]. In the Suzuki reaction, the carbene complex is the active form of the catalyst. The formation of carbene complexes is characteristic of ionic liquids with particular regard to imidazolium halides. Complex compounds of ruthenium(I) and palladium(II) undergo such reactions. During the reaction, the C(2) carbon of the imidazolium ring is deprotonated and an M–C bond is formed [ 122 , 123 ].
Carbene complexes are formed in situ if the ionic liquid is a solvent in the catalytic reaction. The reactivity of carbene complexes often differs from the catalyst precursor. For this reason, there are cases where the ionic liquid is an inhibitor of catalytic activity [ 123 ]. Iodobenzene methoxycarbonylation is an example of such a reaction. It can be concluded that ionic liquids are not only solvents, but are also active components of the reaction system. Ionic liquids take part in the formation of [IL] 2 [PdX 4 ], where IL is the cation of the ionic liquid and X is the halide. [IL] 2 [PdX 4 ] complex compounds are more active in the Suzuki reaction than in the [PdCl 2 (cod)] complex [ 124 , 125 ].
C–C coupling reactions catalyzed by palladium compounds in an ammonium salt environment, i.e., [Bu 4 N]Br, show that the use of ionic liquids increases the efficiency of the reaction [ 126 ].
The application of catalysts which are coordination compounds containing ionic liquids (as ligands) was implemented in the technological process of obtaining S-naproxen and S-ibuprofen. Therefore, we conclude that it is necessary to examine the influence of geometric isomerism of catalysts on their catalytic activity and the type of reaction product obtained.
10.2. Catalysts of Organic Reactions—Metal Nanoparticles
Metal nanoparticles have diameters in the range from 1 to 50 nm. The size of the nanotube has a very large impact on their properties. First information on the use of metal nanoparticles of Pd in Heck’s reaction comes from the 90s [ 127 ]. This concerned reactions between aryl bromides and styrene, or butyl acrylate and iodobenzene [ 127 ]. Metal nanoparticles obtained by reducing metal chloride salts in the presence of tetra-N-alkylammonium cations are electrostatically stabilized [ 127 ]. The application of nanoparticles and metal complexes in various catalytic reactions is presented in Figure 17 .

The application of exemplary metal nanoparticles in various catalytic reactions.
Metal nanoparticles have some features of both homogeneous and heterogeneous catalysts. Nanoparticles can be a source of soluble metal complexes formed during a given reaction.
The complex compound undergoing a reduction reaction generates nanoparticles ( Figure 18 ). Then the nanoparticles form a metallic structure because they agglomerate. The reverse process is also possible. This happens in the case of catalytic reactions during which nanoparticles are dissolved, which in turn contributes to the formation of complex compounds. There are known cases of equilibrium between nanoparticles and palladium complexes in the catalytic system. The equilibrium is particularly common when phosphorus ligands are present in the catalytic system [ 128 ]. Metal nanoparticles are formed as a result of a reduction of complex compounds. Reduction may occur during the catalytic reaction, but it is also possible before catalysis [ 129 ].

Transformations of palladium catalysts from monomolecular to nanoparticles. This figure is adapted from [ 129 ].
Among the nanoparticles, monometallic nanoparticles are the most popular. However, bimetallic nanoparticles are becoming the subject of more and more frequent scientific research. Cheaper base metals are part of the inner sphere of bimetallic nanoparticles [ 130 ]. The base metals are covered with a layer of precious metals, e.g., platinum, palladium, and ruthenium.
Catalysis with palladium and copper nanoparticles is applied to form C–C and C–S bonds [ 131 ]. The palladium nanoparticles cause coupling of vicinal–diiodoalkenes and acrylic esters and nitriles. The copper nanoparticles are used in chemoselective reduction of N-aromatic compounds.
It can be stated that the future research approach is the application of metal nanoparticles to identify catalytic sites [ 132 ]. The identification of catalytic sites for oxygen reduction reactions is extremely important when designing new highly active catalysts containing base metals.
11. Conclusions
This article describes many chemical technology processes that involve coordination compounds as catalysts. Many organic synthesis processes in the chemical industry, including the olefin polymerization process and atom transfer radical polymerization (ATRP) process, are used to produce polymer materials. The resulting products are used in many areas of our lives in the pharmaceutical industry—for the synthesis of drugs, polyethylene production, or for the synthesis of complex organic compounds. A very important aspect in chemical technologies is the widest possible application of the principles of green chemistry—in the case of catalytic reactions involving coordination compounds, biotechnological, or organocatalytic methods are applied. An example of the described principle can be use of ultrasound to better dissolve solutions or microwave radiation for heating.
The main conclusions of this review are following:
- PdCl 2 taking part in the Wacker process forming a stoichiometric amount of acetaldehyde;
- Modernized CYCLOPOL-bis technology for caprolactam production allows for production costs to be minimized and the quality of products to be improved;
- Ni complexes with bidentate chelate ligands applied as catalysts in SHOP allow a separated C 6 –C 18 fraction to be obtained, which is used for the production of plasticizers and detergents;
- [PtCl 2 (cod)], [Pt(CH 2 =CHSiMe 2 ) 2 O 3 ], [RhCl(PPh 3 ) 3 ], [Pd 2 (dba) 3 ] are the hydrosilylation catalysts;
- Cu(I) and Fe(II) complexes with ligands such as halides, 2,2′-bipyridine, or tris(2-pyridylmethyl)amine are the catalysts in ATRP;
- [PdCl 2 (cod)] and Pd(0)/PVP are catalysts for the methoxycarbonylation reaction;
- K 2 [Fe(CO) 4 ] catalyse carbonylation;
- [Cu(2,3-pydc)(bpp)]·2.5H 2 O, [Zn(2,3-pydc)(bpp)]·2.5H 2 O, and [Cd(2,3-pydc) (bpp)(H 2 O)]·3H 2 O are metal–organic frameworks which are applied in heterogeneous catalysis;
- [IL] 2 [PdX 4 ] complex is more active than [PdCl 2 (cod)] in the Suzuki reaction.
In recent years, the development of research on the use of complex noble metal compounds as photoredox catalysts has been observed. This trend will continue for a long time because photoredox catalysis is a very useful method for activating small molecules.
Acknowledgments
Supported by the Foundation for Polish Science (FNP).
Author Contributions
Writing—original draft preparation: J.M., D.Z., J.D., and D.J.; writing—review and editing: J.M., D.Z., J.D., D.J., and B.G.; funding acquisition: J.D. and B.G. All authors have read and agreed to the published version of the manuscript.
This research was funded by National Science Centre, Poland under grant number 2015/19/N/ST5/00276.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.1: Introduction to Coordination Chemistry
- Last updated
- Save as PDF
- Page ID 296074
Coordination chemistry is the study of the compounds that form between metals and ligands , where a ligand is any molecule or ion that binds to the metal. A metal complex is the unit containing the metal bound to its ligands. For example, [PtCl 2 (NH 3 ) 2 ] is the neutral metal complex where the Pt(II) metal is bound to two Cl ligands and two NH 3 ligands. If a complex is charged, it is called a complex ion (ex. [Pt(NH 3 ) 4 ] +2 is a complex cation). A complex ion is stabilized by formation of a coordination compound with ions of opposite charge (ex. [Pt(NH 3 ) 4 ]Cl 2 ). It is convention to write the formula of a complex or complex ion inside of square brackets, while counterions are written outside of the brackets. In this convention, it is understood that ligands inside the brackets are bound directly to the metal, in the metal's first coordination sphere (a.k.a inner coordination sphere). Ions written outside of the brackets are assumed to be in the second coordination sphere , and they are not directly bound to the metal.
- Neutral Complex: [CoCl 3 (NH 3 ) 3 ]
- Complex Cation: [Co(NH 3 ) 6 ] 3+
- Complex Anion: [CoCl 4 (NH 3 ) 2 ] -
- Coordination Compound: K 4 [Fe(CN) 6 ]
A common metal complex is [Ag(NH 3 ) 2 ] + formed when Ag + ions are mixed with neutral ammonia molecules.
\[Ag^+ + 2 NH_3 \rightarrow [Ag(NH_3)_2]^+\]
A complex [Ag(S 2 O 3 ) 2 ] 3- is formed between silver ions and negative thiosulfate ions:
\[Ag^+ + 2 S_2O_3^{2-} \rightarrow [Ag(S_2O_3)_2]^{3-}\]
How did the study of coordination compounds started?
The coordination chemistry was pioneered by Nobel Prize winner Alfred Werner (1866-1919). He received the Nobel Prize in 1913 for his coordination theory of transition metal-amine complexes. At the start of the 20th century, inorganic chemistry was not a prominant field until Werner studied the metal-amine complexes of cobalt. Werner recognized the existence of several forms of cobalt-ammonia chloride. These compounds have different color and other characteristics. The chemical formula has three chloride ions per mole, but the number of chloride ions that precipitate with Ag + ions per formula was not always three. He thought only ionized chloride ions will form precipitate with silver ion. In the following table, the number below the Ionized Cl - is the number of ionized chloride ions per formula. To distinguish ionized chloride from the coordinated chloride, Werner formulated the Complex formula and explained structure of the cobalt complexes.
The structures of the complexes were proposed based on a coordination number of 6. The six ligands can be ammonia or chloride. Two different structures were proposed for the last two compounds, the trans- compound has two chloride ions on opposite vertices of an octahedral, whereas the the two chloride ions are adjacent to each other in the cis- compound. The cis- and trans- compounds are known as geometric isomers. Isomers will be the focus of a later section.
Classification of ligands
The discussion of the cobalt complexes above suggests that there are two types of chlorides in the [Co(NH 3 ) 5 Cl]Cl 2 and cis- / trans- [Co(NH 3 ) 4 Cl 2 ]Cl complexes. In comparing the formula and the amounts of ionized Cl - listed in Table \(\sf{\PageIndex{1}}\), the outer sphere chlorides must be the ionizable ones. The reason the inner sphere ligands are not ionizable is because there is significant covalent character in the bond with the cobalt. Even though we call these inner sphere ligands chloride (or more properly chloro) ligands, they are not Cl - , but rather better thought of as being Co-Cl.
In order to account for this difference, we will use the Covalent Bond Classification (CBC) method for thinking about ligands. In this system, ligands are classified as either being X-type (one-electron donors) or L-type (two-electron donors). In order to determine if a ligand is an X-type or L-type ligand, perform a thought experiment in which you remove the ligand from the metal as a neutral atom or molecule.
Example \(\PageIndex{1}\)
Consider the complex cation, [Co(NH 3 ) 5 Cl] 2+ . What types of ligands are NH 3 and Cl?
There are three possible ways you could think of breaking the Co-N bond. One could consider homolytic cleavage (one electron goes with Co and one with N) or heterolytic cleavage (one atom takes both electrons). The homolytic cleavage option is shown in the middle results in the formation of NH 3 + . Our goal is to remove the ligand as a neutral species, so this is not the correct option. If both electrons in the bond go with the nitrogen, there is no charge on the outgoing ligand. When considering the two heterolytic cleavage options, the pair of electrons can either go with the nitrogen (left) or Co (right). Only one of these options (the one on the left) results in the formation of the neutral NH 3 molecule. As such, NH 3 is a two-electron donor and therefore a L-type ligand.
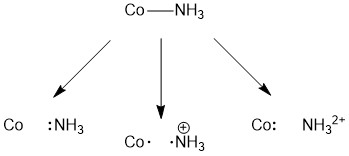
It is important to note two things before we move on to our consideration of chloride. First, even though there are four bonds to the nitrogen atom in the initial Co-NH 3 , we do not put a positive charge on the nitrogen. While the nitrogen atom bound to cobalt is certainly less electron rich than the nitrogen atom in an uncoordinated ammonia molecule, if we think back on our discussion of the acidity of hexaaqua species, the charge is difficult to localize and so we ignore it. This will be true for all metal complexes. Second, no charge is shown on any of the cobalt species as accounting for charge on the ligand will likely be more straightforward.
Similar to our treatment of NH 3 , there are three ways to consider removing the chloride. In this case, both heterolytic cleavage options (left and right) result in a charged chlorine species so these are not the correct option. However homolytic cleavage (center) of the Co-Cl bond results in a neutral chlorine atom. So the chloride ligand is a X-type ligand.
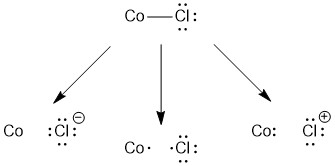
Why do we need to classify the different ligand types? Doing so is going to help us in determining the valence of the metal. Valence is somewhat similar to oxidation state, but there are subtle differences. The main difference is that valence accounts for both the overall charge on the complex and the number of X-type ligands in the complex. The emphasis with valence is once again on the idea that the metal needs to provide electrons to make covalent bonds to some ligands. To determine the valence of a metal use the following equation:
valence - number of X-type ligands = overall charge on the complex
Example \(\PageIndex{2}\)
Determine the valence of cobalt in [Co(NH 3 ) 5 Cl] 2+ .
Previously we determined that the cobalt in this complex has one X-type ligand, the chloride. The complex has an overall +2 charge. Based on the equation above, the valence of the cobalt would be +3 or Co(III).
Had you followed traditional oxidation state rules, you should have arrived at the same conclusion. These rules would have treated the chloride as Cl - and accounted for the remainder of the charge by oxidizing the cobalt. While a subtle difference, the results of experiments in which Ag + is added to [Co(NH 3 ) 5 Cl] 2+ clearly indicate that the chloride is not ionizable and therefore should not be treated as Cl - . While similar, the idea of valence emphasizes the covalent nature of the Co-Cl bond.
Exercise \(\PageIndex{1}\)
Determine the valence of cobalt in [Co(NH 3 ) 4 Cl 2 ]Cl.
While the amounts have changed, the NH 3 ligands are still L-type and the Cl ligands are X-type.
The valence on cobalt is (valence - 2 = +1) or Co(III) just like in the previous example.
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)
Comprehensive Coordination Chemistry III
- Chemistry (Twin Cities)
Research output : Book/Report › Book
Comprehensive Coordination Chemistry III, Nine Volume Set describes the fundamentals of metal-ligand interactions, provides an overview of the systematic chemistry of this class of compounds, and details their importance in life processes, medicine, industry and materials science. This new edition spans across 9 volumes, 185 entries and 6600 printed pages. Comprehensive Coordination Chemistry III is not just an update of the second edition, it includes a significant amount of new content. In the descriptive sections 3-6, emphasis is placed upon material that has appeared in primary and secondary review literature since the previous edition published. The material in other sections is newly written, with an emphasis on modern aspects of coordination chemistry and the latest developments. The metal-ligand interaction is the link between the award of the 1913 Nobel Prize in Chemistry to Alfred Werner, the father of Coordination Chemistry, the 1987 prize for supramolecular chemistry and the 2016 award for molecular machines. The key role of coordination chemistry in the assembly of hierarchical nano- and micro-dimensioned structures lies at the core of these applications and so this Major Reference Work bridges several sub-disciplines of chemistry, thus targeting a truly interdisciplinary audience.
Bibliographical note
Other files and links.
- Link to publication in Scopus
- Link to the citations in Scopus
Fingerprint
- Coordination Chemistry Chemistry 100%
- Chemistry Chemistry 50%
- Metal Chemistry 33%
- Ligand Chemistry 33%
- Volume Chemistry 33%
- Application Chemistry 16%
- Structure Chemistry 16%
- Amount Chemistry 16%
T1 - Comprehensive Coordination Chemistry III
AU - Constable, Edwin C.
AU - Parkin, Gerard
AU - Que, Lawrence
N1 - Publisher Copyright: © 2021 Elsevier Ltd. All rights reserved.
PY - 2021/7/21
Y1 - 2021/7/21
N2 - Comprehensive Coordination Chemistry III, Nine Volume Set describes the fundamentals of metal-ligand interactions, provides an overview of the systematic chemistry of this class of compounds, and details their importance in life processes, medicine, industry and materials science. This new edition spans across 9 volumes, 185 entries and 6600 printed pages. Comprehensive Coordination Chemistry III is not just an update of the second edition, it includes a significant amount of new content. In the descriptive sections 3-6, emphasis is placed upon material that has appeared in primary and secondary review literature since the previous edition published. The material in other sections is newly written, with an emphasis on modern aspects of coordination chemistry and the latest developments. The metal-ligand interaction is the link between the award of the 1913 Nobel Prize in Chemistry to Alfred Werner, the father of Coordination Chemistry, the 1987 prize for supramolecular chemistry and the 2016 award for molecular machines. The key role of coordination chemistry in the assembly of hierarchical nano- and micro-dimensioned structures lies at the core of these applications and so this Major Reference Work bridges several sub-disciplines of chemistry, thus targeting a truly interdisciplinary audience.
AB - Comprehensive Coordination Chemistry III, Nine Volume Set describes the fundamentals of metal-ligand interactions, provides an overview of the systematic chemistry of this class of compounds, and details their importance in life processes, medicine, industry and materials science. This new edition spans across 9 volumes, 185 entries and 6600 printed pages. Comprehensive Coordination Chemistry III is not just an update of the second edition, it includes a significant amount of new content. In the descriptive sections 3-6, emphasis is placed upon material that has appeared in primary and secondary review literature since the previous edition published. The material in other sections is newly written, with an emphasis on modern aspects of coordination chemistry and the latest developments. The metal-ligand interaction is the link between the award of the 1913 Nobel Prize in Chemistry to Alfred Werner, the father of Coordination Chemistry, the 1987 prize for supramolecular chemistry and the 2016 award for molecular machines. The key role of coordination chemistry in the assembly of hierarchical nano- and micro-dimensioned structures lies at the core of these applications and so this Major Reference Work bridges several sub-disciplines of chemistry, thus targeting a truly interdisciplinary audience.
UR - http://www.scopus.com/inward/record.url?scp=85129922323&partnerID=8YFLogxK
UR - http://www.scopus.com/inward/citedby.url?scp=85129922323&partnerID=8YFLogxK
AN - SCOPUS:85129922323
SN - 9780081026892
BT - Comprehensive Coordination Chemistry III
PB - Elsevier
Daly Research Group

Welcome to the Daly Research Group!
We are synthetic inorganic chemists who specialize in metal coordination chemistry and reactive ligand design..
New paper in Dalton Transactions
Our paper describing the synthesis of volatile lanthanide complexes containing heptadentate ligands was published in Dalton! Congrats to Josh, Balaka, and Tommy and our theory collaborators Bess and Jorge!
Daly Group receives NNSA Funding
The Transuranic Chemistry Center of Excellence was established with a five-year grant from the National Nuclear Security Administration! This Center includes the Daly Group and researchers from five other universities, four national laboratories, and two user facilities.

Graham named Tillman Scholar
Former Chemistry Platoon student leader Zachary Graham was selected as a Pat Tillman scholar, one of the most prestigious and competitive scholarships for student veterans.
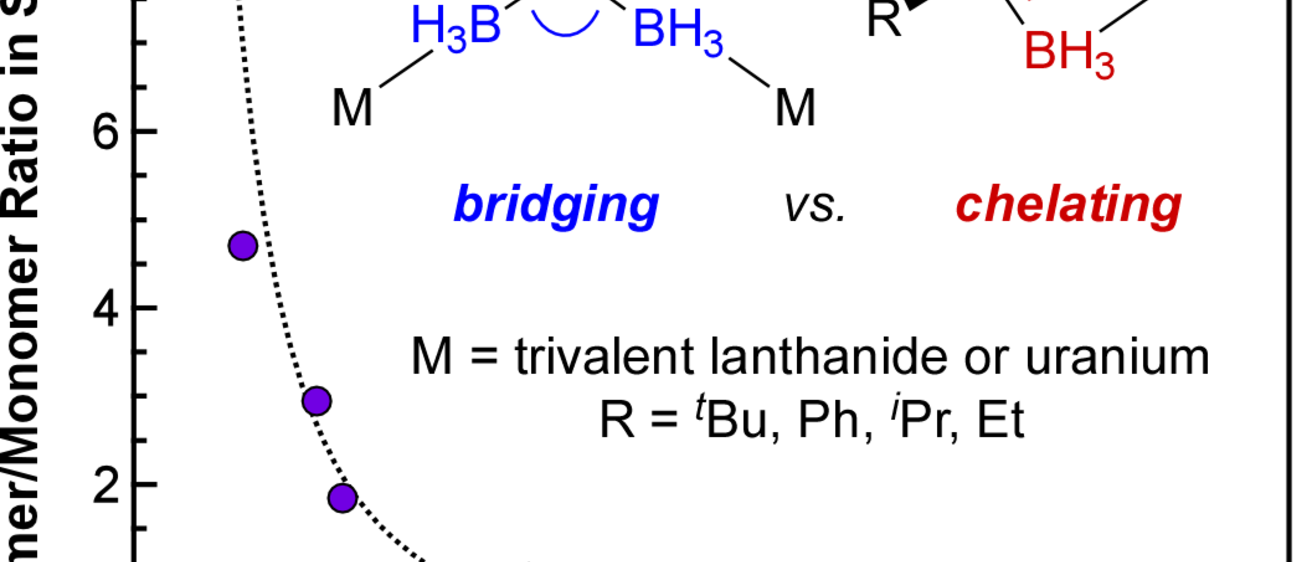
New paper in Inorganic Chemistry
Our paper describing the mechanochemical synthesis and characterization of new uranium and lanthanide phosphinodiboranates has been accepted for publication in Inorganic Chemistry! Congrats to Josh, Fran, Taylor, Rayford, and Anastasia, as well as our awesome theory collaborators Rina and Bess at USD!
Congrats Dr. Zgrabik!
Congrats to Joshua Zgrabik who successfully defended and graduated with his PhD! He is off to start a postdoc position at the Naval Research Laboratory in Washington, DC.
Dunya, Mai Yer, and Darby advance to PhD Candidacy!
Congrats to 2nd-year graduate students Dunya, Mai Yer, and Darby for successfully passing their comprehensive exams! Awesome job!
Dr. Skaria featured in article from International Programs
Former Daly Group postdoc Dr. Manisha Skaria was highlighted in an article for UIowa International Programs entitled " Manisha Skaria balances career, campus involvement, and family ."

Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Organometallic Chemistry
Basic Concepts Viewed from Frontier in Inorganic Coordination Chemistry

Book metrics overview
11,089 Chapter Downloads
Impact of this book and its chapters
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Overall attention for this book and its chapters
Book Citations
Total Chapter Citations
Academic Editor
Tokyo University of Science , Japan
Published 19 December 2018
Doi 10.5772/intechopen.76741
ISBN 978-1-78984-865-6
Print ISBN 978-1-78984-864-9
eBook (PDF) ISBN 978-1-83881-835-7
Copyright year 2018
Number of pages 152
This book is both a review of current research and an undergraduate textbook for inorganic chemistry at university level. In university undergraduate lectures, basic concepts are mainly explained and added examples of frontier research are optional. However, in many cases, frontier research is more interesting for students than basic studies. This book is aimed at undergraduates in inorganic chemi...
This book is both a review of current research and an undergraduate textbook for inorganic chemistry at university level. In university undergraduate lectures, basic concepts are mainly explained and added examples of frontier research are optional. However, in many cases, frontier research is more interesting for students than basic studies. This book is aimed at undergraduates in inorganic chemistry. Each author introduces or reviews "frontier research topics" of inorganic coordination chemistry. Additionally, "basic concepts," as found in textbooks on this subject, indicate application examples of "frontier research topics."
By submitting the form you agree to IntechOpen using your personal information in order to fulfil your library recommendation. In line with our privacy policy we won’t share your details with any third parties and will discard any personal information provided immediately after the recommended institution details are received. For further information on how we protect and process your personal information, please refer to our privacy policy .
Cite this book
There are two ways to cite this book:
Edited Volume and chapters are indexed in
Table of contents.
By Takashiro Akitsu
By Hina Hayat and Muhammad Adnan Iqbal
By Pedro Pedrosa, Andreia Carvalho, Pedro V. Baptista and Alexandra R. Fernandes
By Ataf Ali Altaf, Sumbal Naz and Amin Badshah
By Jia-Syun Lu, Ming-Chung Yang and Ming-Der Su
By Etim Emmanuel, Lawal Usman, Khanal Govinda and Mbakara Idaresit
By Tanja Soldatović
IMPACT OF THIS BOOK AND ITS CHAPTERS
11,089 Total Chapter Downloads
2,150 Total Chapter Views
7 Crossref Citations
20 Web of Science Citations
22 Dimensions Citations
3 Altmetric Score
Order a print copy of this book
Available on

Delivered by
£119 (ex. VAT)*
Hardcover | Printed Full Colour
FREE SHIPPING WORLDWIDE
* Residents of European Union countries need to add a Book Value-Added Tax Rate based on their country of residence. Institutions and companies, registered as VAT taxable entities in their own EU member state, will not pay VAT by providing IntechOpen with their VAT registration number. This is made possible by the EU reverse charge method.
As an IntechOpen contributor, you can buy this book for an Exclusive Author price with discounts from 30% to 50% on retail price.
Log in to your Author Panel to purchase a book at the discounted price.
For any assistance during ordering process, contact us at [email protected]
Related books
Descriptive inorganic chemistry researches of metal compounds.
Edited by Takashiro Akitsu
Symmetry (Group Theory) and Mathematical Treatment in Chemistry
Chirality from molecular electronic states, crystallography, current topics in chirality, schiff base in organic, inorganic and physical chemistry, electrophile and lewis acid, recent progress in organometallic chemistry.
Edited by Mohammed Muzibur Rahman
Chemical Reactions in Inorganic Chemistry
Edited by Chandraleka Saravanan
Infrared Spectroscopy
Edited by Theophile Theophanides
Call for authors
Submit your work to intechopen.

- Privacy Policy
Buy Me a Coffee

Home » 300+ Chemistry Research Topics
300+ Chemistry Research Topics
Table of Contents

Chemistry is a fascinating and complex field that explores the composition, properties, and behavior of matter at the molecular and atomic level. As a result, there are numerous chemistry research topics that can be explored, ranging from the development of new materials and drugs to the study of natural compounds and the environment. In this rapidly evolving field, researchers are constantly uncovering new insights and pushing the boundaries of our understanding of chemistry. Whether you are a student, a professional researcher, or simply curious about the world around you, there is always something new to discover in the field of chemistry. In this post, we will explore some of the exciting and important research topics in chemistry today.
Chemistry Research Topics
Chemistry Research Topics are as follows:
Organic Chemistry Research Topics
Organic Chemistry Research Topics are as follows:
- Development of novel synthetic routes for the production of biologically active natural products
- Investigation of reaction mechanisms and kinetics for organic transformations
- Design and synthesis of new catalysts for asymmetric organic reactions
- Synthesis and characterization of chiral compounds for pharmaceutical applications
- Development of sustainable methods for the synthesis of organic molecules using renewable resources
- Discovery of new reaction pathways for the conversion of biomass into high-value chemicals
- Study of molecular recognition and host-guest interactions for drug design
- Design and synthesis of new materials for energy storage and conversion
- Development of efficient and selective methods for C-H functionalization reactions
- Exploration of the reactivity of reactive intermediates such as radicals and carbenes
- Study of supramolecular chemistry and self-assembly of organic molecules
- Development of new methods for the synthesis of heterocyclic compounds
- Investigation of the biological activities and mechanisms of action of natural products
- Synthesis of polymeric materials with controlled architecture and functionality
- Development of new synthetic methodologies for the preparation of bioconjugates
- Investigation of the mechanisms of enzyme catalysis and the design of enzyme inhibitors
- Synthesis and characterization of novel fluorescent probes for biological imaging
- Development of new synthetic strategies for the preparation of carbohydrates and glycoconjugates
- Study of the properties and reactivity of carbon nanomaterials
- Design and synthesis of novel drugs for the treatment of diseases such as cancer, diabetes, and Alzheimer’s disease.
Inorganic Chemistry Research Topics
Inorganic Chemistry Research Topics are as follows:
- Synthesis and characterization of new metal-organic frameworks (MOFs) for gas storage and separation applications
- Development of new catalysts for sustainable chemical synthesis reactions
- Investigation of the electronic and magnetic properties of transition metal complexes for spintronics applications
- Synthesis and characterization of novel nanomaterials for energy storage applications
- Development of new ligands for metal coordination complexes with potential medical applications
- Investigation of the mechanism of metal-catalyzed reactions using advanced spectroscopic techniques
- Synthesis and characterization of new inorganic materials for photocatalytic water splitting
- Development of new materials for electrochemical carbon dioxide reduction reactions
- Investigation of the properties of transition metal oxides for energy storage and conversion applications
- Synthesis and characterization of new metal chalcogenides for optoelectronic applications
- Development of new methods for the preparation of inorganic nanoparticles with controlled size and shape
- Investigation of the reactivity and catalytic properties of metal clusters
- Synthesis and characterization of new metal-organic polyhedra (MOPs) for gas storage and separation applications
- Development of new methods for the synthesis of metal nanoparticles using environmentally friendly reducing agents
- Investigation of the properties of metal-organic frameworks for gas sensing applications
- Synthesis and characterization of new coordination polymers with potential magnetic and electronic properties
- Development of new materials for electrocatalytic water oxidation reactions
- Investigation of the properties of metal-organic frameworks for carbon capture and storage applications
- Synthesis and characterization of new metal-containing polymers with potential applications in electronics and energy storage
- Development of new methods for the synthesis of metal-organic frameworks using green solvents and renewable resources.
Physical Chemistry Research Topics
Physical Chemistry Research Topics are as follows:
- Investigation of the properties and interactions of ionic liquids in aqueous and non-aqueous solutions.
- Development of advanced analytical techniques for the study of protein structure and dynamics.
- Investigation of the thermodynamic properties of supercritical fluids for use in industrial applications.
- Development of novel nanomaterials for energy storage applications.
- Studies of the surface chemistry of catalysts for the optimization of their performance in chemical reactions.
- Development of new methods for the synthesis of complex organic molecules with improved yields and selectivity.
- Investigation of the molecular mechanisms involved in the catalysis of biochemical reactions.
- Development of new strategies for the controlled release of drugs and other bioactive molecules.
- Studies of the interaction of nanoparticles with biological systems for biomedical applications.
- Investigation of the thermodynamic properties of materials under extreme conditions of temperature and pressure.
- Development of new methods for the characterization of materials at the nanoscale.
- Investigation of the electronic and magnetic properties of materials for use in spintronics.
- Development of new materials for energy conversion and storage.
- Studies of the kinetics and thermodynamics of adsorption processes on surfaces.
- Investigation of the transport properties of ionic liquids for use in energy storage and conversion devices.
- Development of new materials for the capture and sequestration of greenhouse gases.
- Studies of the structure and properties of biomolecules for use in drug design and development.
- Investigation of the dynamics of chemical reactions in solution using time-resolved spectroscopic techniques.
- Development of new approaches for the synthesis of metallic and semiconductor nanoparticles with controlled size and shape.
- Studies of the structure and properties of materials for use in electrochemical energy storage devices.
Analytical Chemistry Research Topics
Analytical Chemistry Research Topics are as follows:
- Development and optimization of analytical techniques for the quantification of trace elements in food and environmental samples.
- Design and synthesis of novel analytical probes for the detection of biomolecules in complex matrices.
- Investigation of the fundamental mechanisms involved in the separation and detection of complex mixtures using chromatographic techniques.
- Development of sensors and biosensors for the detection of chemical and biological species in real-time.
- Investigation of the chemical and structural properties of nanomaterials and their applications in analytical chemistry.
- Development and validation of analytical methods for the quantification of contaminants and pollutants in water, air, and soil.
- Investigation of the molecular mechanisms underlying drug metabolism and toxicity using mass spectrometry.
- Development of analytical tools for the identification and quantification of drugs of abuse in biological matrices.
- Investigation of the chemical composition and properties of natural products and their applications in medicine and food science.
- Development of advanced analytical techniques for the characterization of proteins and peptides.
- Investigation of the chemistry and mechanism of action of antioxidants in foods and their impact on human health.
- Development of analytical methods for the detection and quantification of microorganisms in food and environmental samples.
- Investigation of the molecular mechanisms involved in the biosynthesis and degradation of important biomolecules such as proteins, carbohydrates, and lipids.
- Development of analytical methods for the detection and quantification of environmental toxins and their impact on human health.
- Investigation of the structure and properties of biological membranes and their role in drug delivery and disease.
- Development of analytical techniques for the characterization of complex mixtures such as petroleum and crude oil.
- Investigation of the chemistry and mechanism of action of natural and synthetic dyes.
- Development of analytical techniques for the detection and quantification of pharmaceuticals and personal care products in water and wastewater.
- Investigation of the chemical composition and properties of biopolymers and their applications in biomedicine and biomaterials.
- Development of analytical methods for the identification and quantification of essential nutrients and vitamins in food and dietary supplements.
Biochemistry Research Topics
Biochemistry Research Topics are as follows:
- The role of enzymes in metabolic pathways
- The biochemistry of DNA replication and repair
- Protein folding and misfolding diseases
- Lipid metabolism and the pathogenesis of atherosclerosis
- The role of vitamins and minerals in human metabolism
- Biochemistry of cancer and the development of targeted therapies
- The biochemistry of signal transduction pathways and their regulation
- The mechanisms of antibiotic resistance in bacteria
- The biochemistry of neurotransmitters and their roles in behavior and disease
- The role of oxidative stress in aging and age-related diseases
- The biochemistry of microbial fermentation and its applications in industry
- The biochemistry of the immune system and its response to pathogens
- The biochemistry of plant metabolism and its regulation
- The molecular basis of genetic diseases and gene therapy
- The biochemistry of membrane transport and its role in cell function
- The biochemistry of muscle contraction and its regulation
- The role of lipids in membrane structure and function
- The biochemistry of photosynthesis and its regulation
- The biochemistry of RNA splicing and alternative splicing events
- The biochemistry of epigenetics and its regulation in gene expression.
Environmental Chemistry Research Topics
Environmental Chemistry Research Topics are as follows:
- Investigating the effects of microplastics on aquatic ecosystems and their potential impact on human health.
- Examining the impact of climate change on soil quality and nutrient availability in agricultural systems.
- Developing methods to improve the removal of heavy metals from contaminated soils and waterways.
- Assessing the effectiveness of natural and synthetic antioxidants in mitigating the effects of air pollution on human health.
- Investigating the potential for using algae and other microorganisms to sequester carbon dioxide from the atmosphere.
- Studying the role of biodegradable plastics in reducing plastic waste and their impact on the environment.
- Examining the impact of pesticides and other agricultural chemicals on water quality and the health of aquatic organisms.
- Investigating the effects of ocean acidification on marine organisms and ecosystems.
- Developing new materials and technologies to reduce carbon emissions from industrial processes.
- Evaluating the effectiveness of phytoremediation in cleaning up contaminated soils and waterways.
- Studying the impact of microplastics on terrestrial ecosystems and their potential to enter the food chain.
- Developing sustainable methods for managing and recycling electronic waste.
- Investigating the role of natural processes, such as weathering and erosion, in regulating atmospheric carbon dioxide levels.
- Assessing the impact of urbanization on air quality and developing strategies to mitigate pollution in cities.
- Examining the effects of climate change on the distribution and abundance of species in different ecosystems.
- Investigating the impact of ocean currents on the distribution of pollutants and other environmental contaminants.
- Developing new materials and technologies for renewable energy generation and storage.
- Studying the effects of deforestation on soil quality, water availability, and biodiversity.
- Assessing the potential for using waste materials, such as agricultural residues and municipal solid waste, as sources of renewable energy.
- Investigating the role of natural and synthetic chemicals in regulating ecosystem functions, such as nutrient cycling and carbon sequestration.
Polymer Chemistry Research Topics
Polymer Chemistry Research Topics are as follows:
- Development of new monomers for high-performance polymers
- Synthesis and characterization of biodegradable polymers for sustainable packaging
- Design of stimuli-responsive polymers for drug delivery applications
- Investigation of the properties and applications of conductive polymers
- Development of new catalysts for controlled/living polymerization
- Synthesis of polymers with tailored mechanical properties
- Characterization of the structure-property relationship in polymer nanocomposites
- Study of the impact of polymer architecture on material properties
- Design and synthesis of new polymeric materials for energy storage
- Development of high-throughput methods for polymer synthesis and characterization
- Exploration of new strategies for polymer recycling and upcycling
- Synthesis and characterization of responsive polymer networks for smart textiles
- Design of advanced polymer coatings with self-healing properties
- Investigation of the impact of processing conditions on the morphology and properties of polymer materials
- Study of the interactions between polymers and biological systems
- Development of biocompatible polymers for tissue engineering applications
- Synthesis and characterization of block copolymers for advanced membrane applications
- Exploration of the potential of polymer-based sensors and actuators
- Design of novel polymer electrolytes for advanced batteries and fuel cells
- Study of the behavior of polymers under extreme conditions, such as high pressure or temperature.

Materials Chemistry Research Topics
Materials Chemistry Research Topics are as follows:
- Development of new advanced materials for energy storage and conversion
- Synthesis and characterization of nanomaterials for environmental remediation
- Design and fabrication of stimuli-responsive materials for drug delivery
- Investigation of electrocatalytic materials for fuel cells and electrolysis
- Fabrication of flexible and stretchable electronic materials for wearable devices
- Development of novel materials for high-performance electronic devices
- Exploration of organic-inorganic hybrid materials for optoelectronic applications
- Study of corrosion-resistant coatings for metallic materials
- Investigation of biomaterials for tissue engineering and regenerative medicine
- Synthesis and characterization of metal-organic frameworks for gas storage and separation
- Design and fabrication of new materials for water purification
- Investigation of carbon-based materials for supercapacitors and batteries
- Synthesis and characterization of self-healing materials for structural applications
- Development of new materials for catalysis and chemical reactions
- Exploration of magnetic materials for spintronic devices
- Investigation of thermoelectric materials for energy conversion
- Study of 2D materials for electronic and optoelectronic applications
- Development of sustainable and eco-friendly materials for packaging
- Fabrication of advanced materials for sensors and actuators
- Investigation of materials for high-temperature applications such as aerospace and nuclear industries.
Nuclear Chemistry Research Topics
Nuclear Chemistry Research Topics are as follows:
- Nuclear fission and fusion reactions
- Nuclear power plant safety and radiation protection
- Radioactive waste management and disposal
- Nuclear fuel cycle and waste reprocessing
- Nuclear energy and its impact on climate change
- Radiation therapy for cancer treatment
- Radiopharmaceuticals for medical imaging
- Nuclear medicine and its role in diagnostics
- Nuclear forensics and nuclear security
- Isotopic analysis in environmental monitoring and pollution control
- Nuclear magnetic resonance (NMR) spectroscopy
- Nuclear magnetic resonance imaging (MRI)
- Radiation damage in materials and radiation effects on electronic devices
- Nuclear data evaluation and validation
- Nuclear reactors design and optimization
- Nuclear fuel performance and irradiation behavior
- Nuclear energy systems integration and optimization
- Neutron and gamma-ray detection and measurement techniques
- Nuclear astrophysics and cosmology
- Nuclear weapons proliferation and disarmament.
Medicinal Chemistry Research Topics
Medicinal Chemistry Research Topics are as follows:
- Drug discovery and development
- Design and synthesis of novel drugs
- Medicinal chemistry of natural products
- Structure-activity relationships (SAR) of drugs
- Rational drug design using computational methods
- Target identification and validation
- Drug metabolism and pharmacokinetics (DMPK)
- Drug delivery systems
- Development of new antibiotics
- Design of drugs for the treatment of cancer
- Development of drugs for the treatment of neurological disorders
- Medicinal chemistry of peptides and proteins
- Development of drugs for the treatment of infectious diseases
- Discovery of new antiviral agents
- Design of drugs for the treatment of cardiovascular diseases
- Medicinal chemistry of enzyme inhibitors
- Development of drugs for the treatment of inflammatory diseases
- Design of drugs for the treatment of metabolic disorders
- Medicinal chemistry of anti-cancer agents
- Development of drugs for the treatment of rare diseases.
Food Chemistry Research Topics
Food Chemistry Research Topics are as follows:
- Investigating the effect of cooking methods on the nutritional value of food.
- Analyzing the role of antioxidants in preventing food spoilage and degradation.
- Examining the effect of food processing techniques on the nutritional value of fruits and vegetables.
- Studying the chemistry of food additives and their impact on human health.
- Evaluating the role of enzymes in food digestion and processing.
- Investigating the chemical properties and functional uses of food proteins.
- Analyzing the effect of food packaging materials on the quality and safety of food products.
- Examining the chemistry of food flavorings and the impact of flavor on consumer acceptance.
- Studying the role of carbohydrates in food texture and structure.
- Investigating the chemistry of food lipids and their impact on human health.
- Analyzing the chemical properties and functional uses of food gums and emulsifiers.
- Examining the effect of processing on the flavor and aroma of food products.
- Studying the chemistry of food preservatives and their impact on food safety.
- Investigating the chemical properties and functional uses of food fibers.
- Analyzing the effect of food processing on the bioavailability of nutrients.
- Examining the chemistry of food colorants and their impact on consumer acceptance.
- Studying the role of vitamins and minerals in food and their impact on human health.
- Investigating the chemical properties and functional uses of food hydrocolloids.
- Analyzing the effect of food processing on the allergenicity of food products.
- Examining the chemistry of food sweeteners and their impact on human health.
Industrial Chemistry Research Topics
Industrial Chemistry Research Topics are as follows:
- Development of catalysts for selective hydrogenation reactions in the petrochemical industry.
- Green chemistry approaches for the synthesis of biodegradable polymers from renewable sources.
- Optimization of solvent extraction processes for the separation of rare earth elements from ores.
- Development of novel materials for energy storage applications, such as lithium-ion batteries.
- Production of biofuels from non-food sources, such as algae or waste biomass.
- Application of computational chemistry to optimize the design of new catalysts and materials.
- Design and optimization of continuous flow processes for large-scale chemical production.
- Development of new synthetic routes for the production of pharmaceutical intermediates.
- Investigation of the environmental impact of industrial processes and development of sustainable alternatives.
- Development of innovative water treatment technologies for industrial wastewater.
- Synthesis of functionalized nanoparticles for use in drug delivery and other biomedical applications.
- Optimization of processes for the production of high-performance polymers, such as polyamides or polyesters.
- Design and optimization of process control strategies for efficient and safe chemical production.
- Development of new methods for the detection and removal of heavy metal ions from industrial effluents.
- Investigation of the behavior of surfactants in complex mixtures, such as crude oil or food products.
- Development of new materials for catalytic oxidation reactions, such as the removal of volatile organic compounds from air.
- Investigation of the properties and behavior of materials under extreme conditions, such as high pressure or high temperature.
- Development of new processes for the production of chemicals from renewable resources, such as bio-based building blocks.
- Study of the kinetics and mechanism of chemical reactions in complex systems, such as multi-phase reactors.
- Optimization of the production of fine chemicals, such as flavors and fragrances, using biocatalytic processes.
Computational Chemistry Research Topics
Computational Chemistry Research Topics are as follows:
- Development and application of machine learning algorithms for predicting chemical reactions and properties.
- Investigation of the role of solvents in chemical reactions using molecular dynamics simulations.
- Modeling and simulation of protein-ligand interactions to aid drug design.
- Study of the electronic structure and reactivity of catalysts for sustainable energy production.
- Analysis of the thermodynamics and kinetics of complex chemical reactions using quantum chemistry methods.
- Exploration of the mechanism and kinetics of enzyme-catalyzed reactions using molecular dynamics simulations.
- Investigation of the properties and behavior of nanoparticles using computational modeling.
- Development of computational tools for the prediction of chemical toxicity and environmental impact.
- Study of the electronic properties of graphene and other 2D materials for applications in electronics and energy storage.
- Investigation of the mechanisms of protein folding and aggregation using molecular dynamics simulations.
- Development and optimization of computational methods for calculating thermodynamic properties of liquids and solids.
- Study of the properties of supercritical fluids for applications in separation and extraction processes.
- Development of new methods for the calculation of electron transfer rates in complex systems.
- Investigation of the electronic and mechanical properties of carbon nanotubes for applications in nanoelectronics and nanocomposites.
- Development of new approaches for modeling the interaction of biomolecules with biological membranes.
- Study of the mechanisms of charge transfer in molecular and hybrid solar cells.
- Analysis of the structural and mechanical properties of materials under extreme conditions using molecular dynamics simulations.
- Development of new approaches for the calculation of free energy differences in complex systems.
- Investigation of the reaction mechanisms of metalloenzymes using quantum mechanics/molecular mechanics (QM/MM) methods.
- Study of the properties of ionic liquids for applications in catalysis and energy storage.
Theoretical Chemistry Research Topics
Theoretical Chemistry Research Topics are as follows:
- Quantum Chemical Studies of Excited State Processes in Organic Molecules
- Theoretical Investigation of Structure and Reactivity of Metal-Organic Frameworks
- Computational Modeling of Reaction Mechanisms and Kinetics in Enzyme Catalysis
- Theoretical Investigation of Non-Covalent Interactions in Supramolecular Chemistry
- Quantum Chemical Studies of Photochemical Processes in Organic Molecules
- Theoretical Analysis of Charge Transport in Organic and Inorganic Materials
- Computational Modeling of Protein Folding and Dynamics
- Quantum Chemical Investigations of Electron Transfer Processes in Complex Systems
- Theoretical Studies of Surface Chemistry and Catalysis
- Computational Design of Novel Materials for Energy Storage Applications
- Theoretical Analysis of Chemical Bonding and Molecular Orbital Theory
- Quantum Chemical Investigations of Magnetic Properties of Complex Systems
- Computational Modeling of Biological Membranes and Transport Processes
- Theoretical Studies of Nonlinear Optical Properties of Molecules and Materials
- Quantum Chemical Studies of Spectroscopic Properties of Molecules
- Theoretical Investigations of Reaction Mechanisms in Organometallic Chemistry
- Computational Modeling of Heterogeneous Catalysis
- Quantum Chemical Studies of Excited State Dynamics in Photosynthesis
- Theoretical Analysis of Chemical Reaction Networks
- Computational Design of Nanomaterials for Biomedical Applications
Astrochemistry Research Topics
Astrochemistry Research Topics are as follows:
- Investigating the chemical composition of protoplanetary disks and its implications for planet formation
- Examining the role of magnetic fields in the formation of complex organic molecules in space
- Studying the effects of interstellar radiation on the chemical evolution of molecular clouds
- Exploring the chemistry of comets and asteroids to better understand the early solar system
- Investigating the origin and evolution of interstellar dust and its relationship to organic molecules
- Examining the formation and destruction of interstellar molecules in shocked gas
- Studying the chemical processes that occur in the atmospheres of planets and moons in our solar system
- Exploring the possibility of life on other planets through astrobiology and astrochemistry
- Investigating the chemistry of planetary nebulae and their role in the evolution of stars
- Studying the chemical properties of exoplanets and their potential habitability
- Examining the chemical reactions that occur in the interstellar medium
- Investigating the chemical composition of supernova remnants and their impact on the evolution of galaxies
- Studying the chemical composition of interstellar grains and their role in the formation of stars and planets
- Exploring the chemistry of astrocytes and their role in the evolution of galaxies
- Investigating the formation of interstellar ice and its implications for the origin of life
- Examining the chemistry of molecular clouds and its relationship to star formation
- Studying the chemical composition of the interstellar medium in different galaxies and how it varies
- Investigating the role of cosmic rays in the formation of complex organic molecules in space
- Exploring the chemical properties of interstellar filaments and their relationship to star formation
- Studying the chemistry of protostars and the role of turbulence in the formation of stars.
Geochemistry Research Topics
Geochemistry Research Topics are as follows:
- Understanding the role of mineralogical and geochemical factors on metal mobility in contaminated soils
- Investigating the sources and fate of dissolved organic matter in aquatic systems
- Exploring the geochemical signatures of ancient sedimentary rocks to reconstruct Earth’s past atmospheric conditions
- Studying the impacts of land-use change on soil organic matter content and quality
- Investigating the impact of acid mine drainage on water quality and ecosystem health
- Examining the processes controlling the behavior and fate of emerging contaminants in the environment
- Characterizing the organic matter composition of shale gas formations to better understand hydrocarbon storage and migration
- Evaluating the potential for carbon capture and storage in geologic formations
- Investigating the geochemical processes controlling the formation and evolution of ore deposits
- Studying the geochemistry of geothermal systems to better understand energy production potential and environmental impacts
- Exploring the impacts of climate change on the biogeochemistry of terrestrial ecosystems
- Investigating the geochemical cycling of nutrients in coastal marine environments
- Characterizing the isotopic composition of minerals and fluids to understand Earth’s evolution
- Developing new analytical techniques to better understand the chemistry of natural waters
- Studying the impact of anthropogenic activities on the geochemistry of urban soils
- Investigating the role of microbial processes in geochemical cycling of elements in soils and sediments
- Examining the impact of wildfires on soil and water chemistry
- Characterizing the geochemistry of mineral dust and its impact on climate and biogeochemical cycles
- Investigating the geochemical factors controlling the release and transport of contaminants from mine tailings
- Exploring the biogeochemistry of wetlands and their role in carbon sequestration and nutrient cycling.
Electrochemistry Research Topics
Electrochemistry Research Topics are as follows:
- Development of high-performance electrocatalysts for efficient electrochemical conversion of CO2 to fuels and chemicals
- Investigation of electrode-electrolyte interfaces in lithium-ion batteries for enhanced battery performance and durability
- Design and synthesis of novel electrolytes for high-energy-density and stable lithium-sulfur batteries
- Development of advanced electrochemical sensors for the detection of trace-levels of analytes in biological and environmental samples
- Analysis of the electrochemical behavior of new materials and their electrocatalytic properties in fuel cells
- Study of the kinetics of electrochemical reactions and their effect on the efficiency and selectivity of electrochemical processes
- Development of novel strategies for the electrochemical synthesis of value-added chemicals from biomass and waste materials
- Analysis of the electrochemical properties of metal-organic frameworks (MOFs) for energy storage and conversion applications
- Investigation of the electrochemical degradation mechanisms of polymer electrolyte membranes in fuel cells
- Study of the electrochemical properties of 2D materials and their applications in energy storage and conversion devices
- Development of efficient electrochemical systems for desalination and water treatment applications
- Investigation of the electrochemical properties of metal-oxide nanoparticles for energy storage and conversion applications
- Analysis of the electrochemical behavior of redox-active organic molecules and their application in energy storage and conversion devices
- Study of the electrochemical behavior of metal-organic frameworks (MOFs) for the catalytic conversion of CO2 to value-added chemicals
- Development of novel electrode materials for electrochemical capacitors with high energy density and fast charge/discharge rates
- Investigation of the electrochemical properties of perovskite materials for energy storage and conversion applications
- Study of the electrochemical behavior of enzymes and their application in bioelectrochemical systems
- Development of advanced electrochemical techniques for the characterization of interfacial processes in electrochemical systems
- Analysis of the electrochemical behavior of nanocarbons and their application in electrochemical energy storage devices
- Investigation of the electrochemical properties of ionic liquids for energy storage and conversion applications.
Surface Chemistry Research Topics
Surface Chemistry Research Topics are as follows:
- Surface modification of nanoparticles for enhanced catalytic activity
- Investigating the effect of surface roughness on the wetting behavior of materials
- Development of new materials for solar cell applications through surface chemistry techniques
- Surface chemistry of graphene and its applications in electronic devices
- Surface functionalization of biomaterials for biomedical applications
- Characterization of surface defects and their effect on material properties
- Surface modification of carbon nanotubes for energy storage applications
- Developing surface coatings for corrosion protection of metals
- Synthesis of self-assembled monolayers on surfaces for sensor applications
- Surface chemistry of metal-organic frameworks for gas storage and separation
- Investigating the role of surface charge in protein adsorption
- Developing surfaces with superhydrophobic or superoleophobic properties for self-cleaning applications
- Surface functionalization of nanoparticles for drug delivery applications
- Surface chemistry of semiconductors and its effect on photovoltaic properties
- Development of surface-enhanced Raman scattering (SERS) substrates for trace analyte detection
- Surface functionalization of graphene oxide for water purification applications
- Investigating the role of surface tension in emulsion formation and stabilization
- Surface modification of membranes for water desalination and purification
- Synthesis and characterization of metal nanoparticles for catalytic applications
- Development of surfaces with controlled wettability for microfluidic applications.
Atmospheric Chemistry Research Topics
Atmospheric Chemistry Research Topics are as follows:
- The impact of wildfires on atmospheric chemistry
- The role of aerosols in atmospheric chemistry
- The chemistry and physics of ozone depletion in the stratosphere
- The chemistry and dynamics of the upper atmosphere
- The impact of anthropogenic emissions on atmospheric chemistry
- The role of clouds in atmospheric chemistry
- The chemistry of atmospheric particulate matter
- The impact of nitrogen oxides on atmospheric chemistry and air quality
- The effects of climate change on atmospheric chemistry
- The impact of atmospheric chemistry on climate change
- The chemistry and physics of atmospheric mercury cycling
- The impact of volcanic eruptions on atmospheric chemistry
- The chemistry and physics of acid rain formation and effects
- The role of halogen chemistry in the atmosphere
- The chemistry of atmospheric radicals and their impact on air quality and health
- The impact of urbanization on atmospheric chemistry
- The chemistry and physics of stratospheric polar vortex dynamics
- The role of natural sources (e.g. ocean, plants) in atmospheric chemistry
- The impact of atmospheric chemistry on the biosphere
- The chemistry and dynamics of the ozone hole over Antarctica.
Photochemistry Research Topics
Photochemistry Research Topics are as follows:
- Investigating the mechanisms of photoinduced electron transfer reactions in organic photovoltaic materials.
- Developing novel photoredox catalysts for photochemical reactions.
- Understanding the effects of light on DNA and RNA stability and replication.
- Studying the photochemistry of atmospheric pollutants and their impact on air quality.
- Designing new photoresponsive materials for advanced photonic and electronic devices.
- Exploring the photochemistry of metalloporphyrins for potential applications in catalysis.
- Investigating the photochemistry of transition metal complexes and their use as photodynamic therapy agents.
- Developing new photocatalytic systems for sustainable energy production.
- Studying the photochemistry of natural products and their potential pharmaceutical applications.
- Investigating the role of light in the formation and degradation of environmental contaminants.
- Designing new photochromic materials for smart windows and displays.
- Exploring the photochemistry of carbon nanomaterials for energy storage and conversion.
- Developing new light-driven molecular machines for nanotechnology applications.
- Investigating the photochemistry of organic dyes for potential applications in dye-sensitized solar cells.
- Studying the effects of light on the behavior of biological macromolecules.
- Designing new photoresponsive hydrogels for drug delivery applications.
- Exploring the photochemistry of semiconductor nanoparticles for potential applications in quantum computing.
- Investigating the mechanisms of photochemical reactions in ionic liquids.
- Developing new photonic sensors for chemical and biological detection.
- Studying the photochemistry of transition metal complexes for potential applications in water splitting and hydrogen production.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

200+ Funny Research Topics

500+ Sports Research Topics

300+ American History Research Paper Topics

500+ Cyber Security Research Topics

500+ Environmental Research Topics

500+ Economics Research Topics
This website uses cookies to improve your user experience. By continuing to use the site, you are accepting our use of cookies. Read the ACS privacy policy.
- ACS Publications
Hot Topics in Chemistry at ACS Spring 2024: Part 1
- Mar 18, 2024
From edible bugs to exercise in pill form, we bring you a roundup of hot topics and breakthrough chemistry research presented at ACS Spring 2024.

The spring meeting of the American Chemical Society (ACS), held virtually and in person March 17-21, 2024, features more than 12,000 presentations on a diverse range of science topics. Read on to discover some of the hot topics and research highlights* presented at the meeting—and check back throughout the week for more updates!
1. Seasonal Secrets Unveiled: How Wild Animals' Hair Changes to Beat the Cold
Learn how wild animals have evolved to survive extreme temperatures in the groundbreaking research led by Taylor Millett at Utah Tech University. This study explores the fascinating world of animal hair, revealing for the first time that the inner structure of animals' coats undergoes significant changes with the seasons. Unlike common beliefs that only the color of the hair changes, Millett's research shows microscopic alterations within the hair that are crucial for survival in different weather conditions.
Millett and her colleagues explored the complex hair structures of wild, big-game animals including the pronghorn antelope, mule deer, and Rocky Mountain elk. Using advanced scanning electron microscopy, they discovered the unique 'honeycomb' structure within the hairs, which changes in density and size between seasons to provide essential insulation. Their findings not only shed light on nature's ingenious ways of protecting these animals but also suggest possibilities for other applications, such as synthetic insulation for homes and consumer products.
2. Bugging Out: The Surprisingly Tasty World of Edible Ants
When it comes to keeping with ACS Spring 2024's theme of " Many Flavors of Chemistry ," Changqi Liu and his colleagues understood the assignment. Their latest research uncovers the diverse and rich flavor profiles of edible ants , diving deep into the unique aroma profiles of four distinct ant species. From the acidic and vinegary taste of common black ants to the nutty and woody nuances of chicatana ants, their findings reveal that each species brings its own unique set of tastes and smells to the table, advancing our understanding of these insects' culinary potential.
The study also touches on the potential for incorporating these flavors into new food products, particularly as the world seeks more sustainable and eco-friendly protein alternatives.
Watch the Headline Science video surrounding this research, created by the ACS Science Communications team:

Explore More Bug Science on ACS Axial
Worm Slime: The Key to More Eco-Friendly Plastics? The Secret of Spinning A Bug’s Eye View of Road Safety
3. A Chemist's Guide to Brewing Perfect Kombucha
A team of chemists at Shippensburg University are revolutionizing kombucha brewing , tackling the challenges of inconsistent alcohol levels and flavor profiles in the fermented beverage. Their research reports on innovative ways to control alcohol content, enhance taste, and speed up fermentation, offering new insights for both home and commercial kombucha producers.
First, the team analyzed the fermentation process in different containers, where silicone bags showed superior performance over glass jars in both fermentation speed and increased acid production. They also looked into how different sugars affect kombucha's taste and alcohol content. Using glucose as a starter resulted in higher gluconic acid and lower ethanol levels, while fructose led to sweeter brews with more acetic acid and ethanol. These findings are crucial for brewers aiming to tailor their kombucha to specific flavor profiles and alcohol content.
This is not the team's first foray into the art and science of kombucha brewing. In 2023, they published a study in the Journal of Chemical Education exploring the use of a cost-effective sensor to accurately measure alcohol concentrations in kombucha across a variety of undergraduate chem lab courses. Read the article here .
Watch the Headline Science short surrounding this research, created by the ACS Science Communications team:

Read More Booze & Beverage Chemistry on Axial:
What Gives Red Bordeaux Wine a “Meaty” Aroma? Beyond the Bean: The Science Behind Lab-Grown Coffee Staying Fresh: The Chemistry of Beer Packaging Like a Fine (Sparkling) Wine: How to Age Champagne Without Losing the Bubbles
4. A New Decking Material That Fights Global Warming
In a major stride towards environmentally sustainable construction, David Heldebrant and his team have developed a new composite decking material that is both cheaper than standard options and carbon-negative. It's designed to store more carbon dioxide (CO 2 ) than its manufacturing process emits, providing a viable solution to one of the construction sector's most significant challenges: high carbon emissions.
The new material incorporates low-quality brown coal and lignin as fillers, which are then treated with CO 2 . Not only does this composite meet international building codes for decking materials in terms of strength and durability, but it also offers a significant cost advantage, being 18% cheaper than its conventional counterparts.
This type of composite decking could play a pivotal role in reducing the carbon footprint of the building industry: replacing all U.S. decking with this material could sequester the CO 2 equivalent of the annual emissions from 54,000 cars. As the team works towards commercialization, this carbon-negative decking presents a hopeful glimpse into a more sustainable solution for the construction industry.
Read more of David Hildebrant's research on CO 2 capture published in ACS journals:
Water-Lean Solvents for Post-Combustion CO 2 Capture: Fundamentals, Uncertainties, Opportunities, and Outlook David J. Heldebrant*Orcid, Phillip K. KoechOrcid, Vassiliki-Alexandra GlezakouOrcid, Roger RousseauOrcid, Deepika Malhotra, and David C. Cantu DOI : 10.1021/acs.chemrev.6b00768
In Situ Raman Methodology for Online Analysis of CO 2 and H 2 O Loadings in a Water-Lean Solvent for CO 2 Capture Amanda M. Lines, Dushyant Barpaga*, Richard F. Zheng, James R. Collett, David J. Heldebrant, and Samuel A. Bryan* DOI : 10.1021/acs.analchem.3c02281
Directed Hydrogen Bond Placement: Low Viscosity Amine Solvents for CO2 Capture Deepika Malhotra, David C. Cantu, Phillip K. Koech*, David J. Heldebrant, Abhijeet Karkamkar, Feng Zheng, Mark D. Bearden, Roger Rousseau, and Vassiliki-Alexandra Glezakou* DOI : 10.1021/acssuschemeng.8b05481
5. A Pill to Replace the Gym?
While this may seem too good to be true, researchers have recently identified new compounds that can mimic the physical benefits of exercise—a significant advancement particularly for those unable to engage in regular physical activity.
The team, led by Bahaa Elgendy at Washington University School of Medicine, initially developed SLU-PP-332 , a compound that activates estrogen-related receptors (ERRs), which are crucial in muscle adaptation to exercise. While SLU-PP-332 was a pioneering discovery, it had its limitations—particularly in its inability to cross into the brain.
To improve upon SLU-PP-332, the team engineered new molecules that not only demonstrated a stronger activation of the ERRs but also exhibited attributes like enhanced stability and lower toxicity potential. Their effectiveness was measured through an increase in RNA presence in rat heart muscle cells, suggesting a more robust simulation of exercise effects compared to SLU-PP-332.
A crucial advancement with the new compounds is their ability to penetrate the brain, opening possibilities for treating neurodegenerative disorders like Alzheimer's disease. Elgendy and his team hope to further test these compounds in animal models, potentially leading to new treatments for various medical conditions where exercise-mimicking drugs could be beneficial.
Read more of Bahaa Elgendy's research published in ACS journals:
Synthetic ERRα/β/γ Agonist Induces an ERRα-Dependent Acute Aerobic Exercise Response and Enhances Exercise Capacity Cyrielle Billon, Sadichha Sitaula, Subhashis Banerjee, Ryan Welch, Bahaa Elgendy, Lamees Hegazy, Tae Gyu Oh, Melissa Kazantzis, Arindam Chatterjee, John Chrivia, Matthew E. Hayes, Weiyi Xu, Angelica Hamilton, Janice M. Huss, Lilei Zhang, John K. Walker, Michael Downes, Ronald M. Evans, and Thomas P. Burris* DOI : 10.1021/acschembio.2c00720
Synthesis of 3-Aminoquinazolinones via a SnCl 2 -Mediated ANRORC-like Reductive Rearrangement of 1,3,4-Oxadiazoles Mohamed Elagawany, Lingaiah Maram, and Bahaa Elgendy* DOI : 10.1021/acs.joc.3c01973
Recent Advances in the Medicinal Chemistry of Farnesoid X Receptor Yuanying Fang, Lamees Hegazy, Brian N. Finck, and Bahaa Elgendy* DOI : 10.1021/acs.jmedchem.1c01017
6. Revolutionizing Cancer Research with Artificial Mucus
Led by Jessica Kramer at the University of Utah, this study marks a significant advancement in understanding the role of mucus in tumor formation. By synthesizing mucins, the sugar-coated proteins that mucus primarily consists of, the team discovered that altering the mucins in healthy cells to resemble those in cancer cells can induce cancer-like behaviors in these cells.
Kramer's approach, involving synthetic chemistry and bacterial enzymes, allows for the precise alteration of mucins and reveals how specific changes in their sugar or protein sequences can impact cellular behavior. This methodology led to the observation that healthy epithelial cells with modified, cancer-like mucins cease normal cell extrusion and begin to pile up, a process resembling early tumor formation. While it's still unclear if these cells transform into cancer cells, the findings open new avenues for developing cancer treatments targeting mucins, particularly the sugar groups on these molecules. Beyond cancer, this research could lead to the development of anti-infectives, probiotics, and other health-supporting therapies.

*Press Release content and videos in this post are brought to you by the ACS Science Communications team. Learn more below.
Want the latest stories delivered to your inbox each month?
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Dear Colleague Letter: Catalyzing human-centered solutions through research and innovation in science, the environment and society
March 28, 2024
Dear Colleagues:
The U.S. National Science Foundation (NSF) seeks to build research capacity and infrastructure to address complex and compounding national and global crises whose solutions require a human-centered approach. To help generate effective and long-lasting solutions, NSF is providing this funding opportunity to inform possible future Centers for Research and Innovation in Science, the Environment and Society (CRISES).
The envisioned centers will catalyze new research and research-based innovations to address seemingly intractable problems that confront society. Research is needed to anticipate how to effectively respond to social, political, economic, and environmental change resulting from systemic disruptions to mitigate and minimize negative impacts on humanity.
This funding opportunity for planning proposals is led by NSF's Social, Behavioral and Economic Sciences Directorate (SBE) with support from NSF's directorates for Geosciences (GEO), Biological Sciences (BIO), Engineering (ENG), Technology, Innovation and Partnerships (TIP), and STEM Education (EDU), as well as the Office of International Science and Engineering (OISE) and the Office of Integrative Activities (OIA). By supporting research to understand the social and behavioral aspects of the rapidly changing world and how these challenges are affected by social, political, economic, and natural environments this DCL aims to advance understandings of fundamental and use-inspired research of people, organizations, and society, while revealing emerging opportunities to address challenges affecting individuals and communities to live healthy and productive lives.
This announcement encourages multi-disciplinary teams led by social or behavioral scientists to develop research programs to advance scientific understanding of critical challenges facing social and environmental systems at local, regional, and global scales.
A deeper, more contextualized understanding is needed to address the many crises facing the world today. Threats to well-being, such as workforce disruptions, governance failures, extreme social and systemic inequities, institutional mistrust, genocides, extremism, wars, decreasing availability and/or quality of natural resources, and the impacts of environmental change, require immediate and innovative solutions and interventions. There are many profound challenges that undermine the success and sustainability of society. In all these cases, human beings and their behavior shaped by society and culture play direct roles in causing crises and responding to severe threats to well-being and even existence.
This DCL seeks to catalyze multi-disciplinary and transdisciplinary research led by social science investigations to improve human livelihoods and support healthy ecosystems by driving discoveries and findings from these areas of research addressing any problems associated with community vulnerability, resource depletion, environmental degradation, group and regional conflict, prejudice, poverty, crime, and violence. Teams of researchers representing diverse disciplinary approaches can develop critical advances and scientific innovations and interventions. Multi-disciplinary teams draw from different theoretical perspectives, varied methodological tools, as well as insight from the communities being served/impacted to drive the context and solution development. This will help to improve the understanding of actions by humans and their institutions and their consequences in more comprehensive ways.
This opportunity supports multi-disciplinary teams, led by researchers in the social, behavioral, and economic sciences, who use empirical methods to grapple with crises that impact individuals, families, communities, organizations, regions, nations, and the planet. The CRISES initiative invites planning proposals as a first step toward facilitating the creation of large-scale interdisciplinary research centers that will address today’s crises and ultimately enhance people’s quality of life. Suitable topics for CRISES may focus entirely on social and behavioral dynamics or address intersections among different components such as economic, political, environmental systems, and the built environment.
Proposal and Award Scope
Through this funding opportunity, NSF seeks to invest in ideas that can potentially serve as the basis for a larger, center-scale activity.
NSF supports a variety of centers that contribute to its mission and goals. Centers leverage research opportunities when the complexity of the research program or the resources needed to solve the problem are of great scope, scale, and duration. Centers require unusually large amounts of equipment, research infrastructure, facilities, and/or people. Centers are a principal means by which NSF fosters interdisciplinary research.
In this call, NSF invites planning proposals for up to $100,000 that will bring together experts across disciplines to seed ideas and help inform the possible full-scale implementation of a CRISES center. As described below, teams are to be led by social scientists and the involvement of researchers from diverse disciplinary perspectives outside the social sciences is encouraged.
A planning proposal is used to support initial conceptualization, planning and collaboration activities that aim to formulate new plans for large-scale projects in emerging research areas for future submission to an NSF program. Planning activities can provide teams with the opportunity to envision structures that would ultimately compose a center. This effort can include forming partnerships with stakeholders and engagement with communities directly impacted by the focus area and outcomes of the research, working as a team to refine the scope and vision for a center, and creating a vision for the potential broader impacts of a center, including diversity, workforce development, and education. Building the framework for a center requires time and investment to strengthen relationships and refine a common vision. Planning proposals are intended to support teams in that process.
Proposals must include the following:
- A lead principal investigator who is a social, behavioral, or economic scientist (with a degree in the SBE sciences or significant publications in SBE journals).
- A focus on at least one program area currently supported by the SBE directorate.
- Identification of the problem(s) the center will address along with a statement of the scope and approach.
- Planned activities that will bring together experts from a range of disciplines to explore the creation of a center to study and develop solutions to one or more pressing societal issues.
Additional principal investigators included in the proposals can be experts in other disciplines. Proposals must demonstrate an interdisciplinary approach beyond that of any single disciplinary program. This DCL encourages the participation of researchers from Minority-Serving Institutions (MSIs), Primarily Undergraduate Institutions (PUIs), eligible institutions in EPSCoR jurisdictions, as well as non-profits and local and state government organizations.
NSF anticipates funding approximately 10-12 awards through this opportunity, subject to the availability of funds and the quality of proposals received.
Proposal Instructions
Planning proposals must be prepared and submitted in accordance with the guidance contained in Chapter II.F.1 of the NSF Proposal & Award Policies & Procedures Guide (PAPPG) . Proposals may be submitted via either Research.gov or Grants.gov.
Prior to submission, potential research teams interested in submitting a planning proposal are required to first send a research concept outline, including project title, team members, institutions involved and a summary of the project concept (up to two pages) by email to [email protected] .
Concept outlines and planning proposals should address the following: (1) Problem Statement, (2) Scientific Approach (e.g., data products and analytical approaches), (3) Planning Activities (e.g. timeline and structure of meetings, workshops, synchronous/asynchronous coordination), and (4) Outcomes and Deliverables (i.e., what would be realized at the completion of the planning endeavor). To ensure proper processing of the Concept Outlines, the subject line of the initial email inquiry should begin with: "Concept Outline: CRISES:" Concept outlines should be submitted by email to [email protected] by May 1, 2024 . NSF program directors will review the concept outlines and will authorize those that fall within the scope of this DCL for submission of a full planning proposal. All PIs will receive notification by May 15, 2024 .
- Planning proposals may only be submitted with NSF approval of a submitted Concept Outline. The email confirming approval to submit must be uploaded in the "Program Officer Concurrence Email" section of Research.gov or as a supplementary document in Grants.gov.
- Proposal titles should start with "CRISES:" and be submitted under the CRISES program description, PD 23-265Y . Please note that if submitting via Research.gov, the system will automatically prepend the title with "Planning" when the proposal is created.
The target date for full planning proposal submissions is by 5 p.m. submitting organization’s local time on July 1, 2024 . and planning proposals will only be accepted if accompanied by the email authorization to submit obtained in response to the research concept outline. Planning proposals submitted without written authorization from an NSF program director will be returned without review.
NSF anticipates that awards will be made in the summer of 2024.
POINT OF CONTACT
Questions about this funding opportunity should be directed to [email protected] .
Sylvia Butterfield Acting Assistant Director Directorate for Social, Behavioral and Economic Sciences Alexandra Isern Assistant Director Directorate for Geosciences Susan Marqusee Assistant Director Directorate for Biological Sciences Susan Margulies Assistant Director Directorate for Engineering Erwin Gianchandani Assistant Director Directorate for Technology, Innovation and Partnerships James Moore Assistant Director Directorate for STEM Education Kendra Sharp Office Head Office of International Science and Engineering Alicia Knoedler Office Head Office of Integrative Activities
share this!
April 1, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
written by researcher(s)
El Niño disasters: Governments know what's coming, but are unprepared. Here's what must change
by Tafadzwanashe Mabhaudhi, The Conversation

Drought disasters in southern Africa are mainly attributed to a lack of preparedness, inadequate response and mitigation and poor risk reduction measures. With little to no preparation for drought disasters , such as the failure of the staple maize crop, the only option after the disaster hits is delayed relief action.
Because of climate change , the El Niño -induced impacts on southern Africa—dry spells, low and erratic rainfall and elevated temperatures, and floods—are becoming more intense and prolonged. These are well-studied and can be mitigated by taking proactive measures .
The looming crises are real and require immediate intervention. But governments in southern Africa often act only when events unfold. They focus on reactive post-disaster recovery, often supported by the international community. This is why impoverished communities in the region are repeatedly exposed to natural disasters .
The current El Niño phase, which has caused drought in the region, was announced at the end of 2022. From the onset , it was predicted by the National Oceanic and Atmospheric Administration to be a strong El Niño with likely impacts on food production, water scarcity and public health. Southern Africa depends heavily on agriculture for food and livelihood options, which makes it highly vulnerable to El Niño. Climate experts urged the region to be prepared .
As a professor of climate change, food systems and health, I believe that the impacts of remaining unprepared for disasters such as those caused by El Niño will be severe for children, women, the elderly and other vulnerable groups. Research has also shown that repeated exposure to disasters by the same vulnerable communities exposes them to mental health problems, such as depression.
The region is poorly prepared because governments do not invest enough in weather monitoring, and they lack comprehensive strategies to prepare for disasters. Government disaster policies are often incoherent and information is not communicated. There is a need to be clearer about who does what and coordinate preparations for disasters better.
Southern Africa's ability to cope with natural disasters
In southern African countries, there are low adaptive capacity and high vulnerability levels. Low adaptive capacity refers to people's or a system's ability to cope and adjust to changes such as those caused by climate change. Poverty and inequality —a feature of the region—leave people less able to cope with climate change impacts and more vulnerable to harm.
Across the region, the number of weather stations has been declining for more than 24 years. Where they exist, they tend to be old and outdated, reducing the region's ability to monitor weather changes. This means there is a lack of real-time and long-term data for developing early warning systems and early action capability, which in turn means that southern African governments react to disasters, such as flash floods, only after they occur.
There are other problems too. Limited proactive disaster risk reduction strategies and the failure of governments to invest in climate change adaptation and mitigation strategies means that southern African countries have less resilience against natural disasters.
Policy incoherence is another problem. Policies meant to achieve similar goals are developed in isolation from each other, with divergent objectives and action plans that are not well implemented. For example, about 54% of surface weather stations in Africa are outdated and unable to capture accurate weather data .
Finally, the countries lack appropriate ways to communicate well in advance to people that floods or droughts are coming. For example, information is often communicated via social media, which is inaccessible to most people in rural areas. A lack of effective response capabilities compounds this, where disaster management officials lack the equipment and trained persons to help affected communities cope with an emergency or a disaster.
How to prepare
The reality of climate change is that the frequency and intensity of extreme weather events are increasing. Given this reality, what can countries do to build preparedness, anticipation, early warning, and action so that they are not always "unprepared?"
El Niño affects water, food and energy supplies. It can cause health and environmental disasters. Therefore, greater coordination and collaboration across water, energy, food, environment and health sectors and across governments is needed. Southern Africa needs integrated proactive disaster response strategies and implementation plans that define the actions to be taken, by whom, and when.
The plans must make it clear who has responsibility for coordinating responses to disasters. Water, energy, food, environment and health sectors need to work together to come up with joint plans and decisions to manage the risk of disasters.
Early warning systems for all are needed. These include sending effective information about the climate changes to everyone involved; proactive disaster response; and disaster management plans from farmer to country level. This also includes providing agricultural advisories to farmers so they can take early action.
To achieve this, governments and the private sector must prioritize climate action in development plans. Together, they will need to allocate enough funding to enable weather offices to monitor, predict disasters and issue early warnings. Additionally, equipment and capacity development is needed to upskill people involved in disaster management, including extension workers, to be able to receive warnings, translate them and help affected communities to manage disasters.
Provided by The Conversation
Explore further
Feedback to editors

82% of EU farm subsidies bolster high emissions foods: Study
33 minutes ago

Leaves of three, let it be? Wide variability among poison ivy plants makes identification more challenging
39 minutes ago

Golfers' risk from pesticides used on turf grass is likely low, studies find

'Frankenstein design' enables 3D printed neutron collimator
44 minutes ago
New antibiotic class effective against multidrug-resistant bacteria discovered
Computational tools fuel reconstruction of new and improved bird family tree

New method reveals hidden activity of life below ground

A frozen chunk of genome rewrites our understanding of bird evolution

Engineers 'symphonize' cleaner ammonia production

Old crystal, new story for enhancing deep ultraviolet laser performance
2 hours ago
Relevant PhysicsForums posts
Iceland warming up again - quakes swarming.
Mar 30, 2024
Unlocking the Secrets of Prof. Verschure's Rosetta Stones
Mar 29, 2024
‘Our clouds take their orders from the stars,’ Henrik Svensmark on cosmic rays controlling cloud cover and thus climate
Mar 27, 2024
Higher Chance to get Lightning Strike by Large Power Consumption?
Mar 20, 2024
A very puzzling rock or a pallasite / mesmosiderite or a nothing burger
Mar 16, 2024
Earth's earliest forest discovered in SW England
Mar 8, 2024
More from Earth Sciences
Related Stories

Faulty warnings, deforestation turned Philippine rains 'deadly': Study
Mar 1, 2024

Effects of climate change such as flooding make existing disadvantages for Indigenous communities so much worse
Nov 15, 2022

Early action vital to stymie climate disasters: report
Oct 13, 2020

Pakistan's floods are a disaster, but they didn't have to be
Sep 21, 2022
Women, low earners 'prone to disaster-linked depression'
May 6, 2022

UN sets 5-year goal to broaden climate early warning systems
Mar 23, 2022
Recommended for you

Simple equations clarify cloud climate conundrum
11 hours ago

Tropical cyclones may be an unlikely ally in the battle against ocean hypoxia
7 hours ago

Atmospheric scientists link Arctic sea loss ice to strong El Niño events
6 hours ago

Study says since 1979 climate change has made heat waves last longer, spike hotter, hurt more people
Largest ice shelf in Antarctica lurches forward once or twice each day
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
Coordination chemistry is the study of compounds that have a central atom (often metallic) surrounded by molecules or anions, known as ligands. The ligands are attached to the central atom by ...
The journal offers rapid publication of research articles, reviews and Short communications on topics of current interest and importance in coordination chemistry.The term coordination chemistry is interpreted broadly, and includes aspects of organometallic, supramolecular, theoretical, and bioinorganic chemistry.The journal also publishes articles on catalysis, materials chemistry and metal ...
The journal offers rapid publication of review articles on topics of current interest and importance in coordination chemistry.The term coordination chemistry is interpreted broadly, and includes aspects of organometallic, supramolecular, theoretical, and bioinorganic chemistry.The journal also publishes review articles on catalysis, materials chemistry and metal-organic frameworks which focus ...
Other chapters cover redox reactions in complexes, the metal-metal bond, molecular magnetism, supramolecular chemistry, and bioinorganic chemistry. As a conclusion, the book gives an outlook into current research areas and trends in coordination chemistry, so that students of higher semesters and PhD students will also benefit from reading it.
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on COORDINATION CHEMISTRY. Find methods information, sources, references or conduct a literature review ...
The Journal of Coordination Chemistry publishes the results of original investigations of coordination complexes, loosely defined as the interactions of organic or inorganic ligands with metal centres. Original investigations may involve syntheses, structures, physical and chemical properties, kinetics and mechanisms of reactions, calculations and applications of coordination compounds.
Coordination chemistry of rare earth elements is an active research theme because of the importance of gadolinium complexes as contrast agents in magnetic resonance imaging, and luminescent europium and terbium complexes as probes in biochemistry. The extremely facile ligand exchange at f element centers makes the isolation of their coordination complexes difficult, particularly from aqueous ...
A milestone in the evolution of coordination chemistry is the revolutionary theory by Alfred Werner in 1893, which laid foundations to modern coordination chemistry. Today, IUPAC defines a coordination compound as any compound that is composed of a central atom, usually that of a metal, to which is attached a surrounding array of other atoms or ...
Thoughtfully organized for academic use, Essentials of Coordination Chemistry: A Simplified. Approach with 3D Visuals encourages interactive learning. Advanced undergraduate and. graduate ...
This publication presents the new trends and opportunities for further development of coordination compounds used in the chemical industry. The review describes the influence of various physicochemical factors regarding the coordination relationship (for example, steric hindrance, electron density, complex geometry, ligand), which condition technological processes.
Description. The journal offers rapid publication of research articles, reviews and Short communications on topics of current interest and importance in coordination chemistry. The term coordination chemistry is interpreted broadly, and includes aspects of organometallic, supramolecular, theoretical, and bioinorganic chemistry.
Introduction to Coordination Chemistry is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Complexes or coordination compounds are molecules that posess a metal center that is bound to ligands (atoms, ions, or molecules that donate electrons to the metal). These complexes can be neutral or ….
Coordination chemistry is the study of the compounds that form between metals and ligands, where a ligand is any molecule or ion that binds to the metal. A metal complex is the unit containing the metal bound to its ligands. For example, [PtCl 2 (NH 3) 2] is the neutral metal complex where the Pt (II) metal is bound to two Cl ligands and two NH ...
Carbon chemistry is, by contrast, comparatively simple, in the sense that essentially all stable carbon compounds have four bonds around each carbon centre. Metals, as a group, can exhibit coordination numbers from two to fourteen, and formal oxidation states that range from negative values to as high as eight.
In solid syntheses, there is a size limit, which is generally higher than 1 nm. After that a) I have doubts and the 2D structure might be true only; b) using a 2:1 molar ratio seems to be too low ...
The key role of coordination chemistry in the assembly of hierarchical nano- and micro-dimensioned structures lies at the core of these applications and so this Major Reference Work bridges several sub-disciplines of chemistry, thus targeting a truly interdisciplinary audience. ... Dive into the research topics of 'Comprehensive Coordination ...
Welcome to the Daly Research Group! We are synthetic inorganic chemists who specialize in metal coordination chemistry and reactive ligand design. Research People Publications Group News Daly Group receives NNSA Funding The Transuranic Chemistry Center of Excellence was established with a five-year grant from the National Nuclear Security ...
The journal offers rapid publication of research articles, reviews and Short communications on topics of current interest and importance in coordination chemistry.The term coordination chemistry is interpreted broadly, and includes aspects of organometallic, supramolecular, theoretical, and …. View full aims & scope
This book is both a review of current research and an undergraduate textbook for inorganic chemistry at university level. In university undergraduate lectures, basic concepts are mainly explained and added examples of frontier research are optional. However, in many cases, frontier research is more interesting for students than basic studies. This book is aimed at undergraduates in inorganic ...
Organic Chemistry Research Topics. Organic Chemistry Research Topics are as follows: Development of novel synthetic routes for the production of biologically active natural products. Investigation of reaction mechanisms and kinetics for organic transformations. Design and synthesis of new catalysts for asymmetric organic reactions.
The spring meeting of the American Chemical Society (ACS), held virtually and in person March 17-21, 2024, features more than 12,000 presentations on a diverse range of science topics. Read on to discover some of the hot topics and research highlights* presented at the meeting—and check back throughout the week for more updates! 1.
Coordination Chemistry and Metal Complexes are for researchers interested in a coordination complex chemistry and the synthesis of metal complexes of carboxylic acid group drugs. Science topics ...
By supporting research to understand the social and behavioral aspects of the rapidly changing world and how these challenges are affected by social, political, economic, and natural environments this DCL aims to advance understandings of fundamental and use-inspired research of people, organizations, and society, while revealing emerging ...
The journal offers rapid publication of review articles on topics of current interest and importance in coordination chemistry. The term coordination chemistry is interpreted broadly, ... APCs are only available for journals that offer the option of publishing your research in gold Open Access. The journals you can find here for comparison ...
Climate experts urged the region to be prepared. As a professor of climate change, food systems and health, I believe that the impacts of remaining unprepared for disasters such as those caused by ...
Territorial spatial planning requires thoughtful consideration of the scientific layout and synergistic control of production, living, and ecological spaces (PLESs). However, research in this field often neglects the human perspective and fails to account for people's demands and behavioral characteristics. This study evaluates the level and spatial characteristics of residents' production ...