- Key Personnel

Sponsor Specific Guidance
Frequently asked questions.
- PIs are discouraged from listing project participants other than the PI and Co-PI as “Key” since named “Key Persons” incur additional burden and audit risk.
- RAS staff are the only personnel to maintain key persons in an Award in the KC system. When reviewing your Award, if Key Person(s) should be deleted or added to an Award, please contact your RAS Contract Administrator for further review.
- No changes should be made to the PI, Co-PI or Key Persons on a project before first checking with RAS.
- Annual Conflict of Interests disclosures and effort tracking are required for named Key Persons by some Sponsors.
Key personnel are those people who are essential to carrying out the work of a project, typically those responsible for the design, conduct and reporting of the research. Key personnel includes: PIs, Co-PIs, and a third category known as “Key Persons”.
MIT’s policy is that the PI and any Co-PIs for a project, as well as fellows on any Fellowship proposal, must be included as key personnel. PIs are discouraged from listing other people as Key Persons at proposal stage due to the increased administrative burdens imposed by the sponsor and the additional audit risk to MIT.
Sponsor requirements for tracking key people vary widely – see the sponsor-specific guidance below. Most federal sponsors require that we track effort for key personnel and notify sponsors when there is a significant reduction in effort (25% or more) or when one of the key people leave the project. Several federal and foundation sponsors require that key personnel keep current on conflict of interest disclosures of financial interest and conflict of interest training.
PIs and DLCs are responsible for tracking effort for key personnel and notifying the sponsor of significant changes. Such requests should be sent in writing to RAS for review and approval before they are submitted to the sponsor.
Sponsor guidelines spell out who MUST be named as “key” at the proposal stage. MIT guidelines spell out which titles are equivalent to a PI or Co-PI role. MIT recommends that additional people not be listed as “Key Persons” on proposals – this does not diminish the individual’s role or the importance of the individual to the project.
For NIH/PHS proposals, Kuali Coeus (KC) will recognize those Key Persons that have a role requiring certification on the proposal, and the system will prevent routing until this step is completed. More information and instructions may be found on the “ Kuali Coeus: Investigators & Key Persons Certifications NIH/PHS Sponsor Proposal Quick Reference Card at the COI website [website] ”
For non-NIH/PHS proposals, Sponsors generally do not require certification of key personnel other than the PI and co-PI. However, if a specific solicitation requires all named Key Persons to complete a COI disclosure, the Aggregator must check the “PCK” flag on that proposal (in the “Supplemental Information” section). Detailed information and instructions for selecting the “PCK” flag at proposal stage is found on the “ Kuali Coeus Certifying Key Persons and Fellows Quick Reference Card at the COI website [website] ”
For Fellowship proposals , most fellowships require the faculty mentor to be the named PI and the graduate or postdoctoral fellow to be named in the role of Key Person to reflect the fellow’s responsibility for conducting his/her research. Fellowship proposal processing guidelines are found at the VPR website [website] .
RAS staff will maintain key persons in an Award in the KC system, based upon the individuals listed in the development proposal and those named in the Sponsor award. PI/DLC should verify the Notice of Award, and contact your RAS Contract Administrator if changes are needed.
For NIH/PHS awards, or where the “PCK” flag was checked on the proposal: The KC system will check that the COI disclosures and COI training requirements have been completed for the Key Person(s) named in the NOA. If Key Person(s) need to be removed or added to the Award, please contact your RAS Contract Administrator for further review.
Refer to the sponsor guidelines and the Notice of Award for more information. RAS can help interpret the Sponsor’s policies.
Remember – if you plan to reduce the effort of key personnel named in a Notice of Award by 25% or more of their previously approved effort, you must notify RAS and you may need to write to your program officer to request prior approval of the reduction. Such requests should be sent in writing to RAS for review and approval before they are submitted to the sponsor.
If the PI has discussed the intended change with the sponsor, please provide any relevant correspondence to your RAS representative. In some cases, the change may need to be submitted to RAS as a Supplement proposal in Kuali Coeus so that any new personnel can complete a certification. Any reduction in effort that is less than 25% of the previously approved effort may be reported in your next progress report, if required. Your RAS representative can advise you on what is needed.
In addition, DLC administrators should consider whether the intended change will have an impact on cost sharing commitments, if any, and should work with PIs to ensure that all required progress reports have been submitted to the sponsor prior to the departure of project personnel.
DOD solicitations indicate whether or not to name key people in grant proposals. Please review the request for proposal for more information. Key people named in the proposal may also be named in the award – refer to the award document. Reductions in effort of 25% or more or substitutions of key personnel must be approved by the sponsor in advance. Awards are not flagged as PCK in KC.
For DOD grants:
- Policy Reference: For grants that incorporate OMB’s Uniform Guidance, the reference would be 2CFR 200.308 (c) (3).
For DOD contracts:
- Normally, DOD contracts include a clause that names specific individuals and describes the requirements for requesting sponsor approval for any change in key personnel.
Per NIH guidance, at the time of proposal, senior/key personnel are identified in the application. The NIH grant Policy Statement (GPS) defines senior/key personnel as:
The PD/PI and other individuals who contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they receive salaries or compensation under the grant. Typically these individuals have doctoral or other professional degrees, although individuals at the masters or baccalaureate level may be considered senior/key personnel if their involvement meets this definition. Consultants and those with a postdoctoral role also may be considered senior/key personnel if they meet this definition. Senior/key personnel must devote measurable effort to the project whether or not salaries or compensation are requested. "Zero percent" effort or "as needed" are not acceptable levels of involvement for those designated as Senior/Key Personnel.
If awarded, those named at proposal stage must continue to track COI disclosures and training for as long as they are participating on the award. However, the prior approval requirement for changes in personnel effort of 25% or greater applies only to those senior/key personnel named in the Notice of Award (NoA). (See NIH GPS 8.1.2.6 ) Note, NIH program officials may identify certain senior/key personnel other than the PD/PI(s) in the NoA they consider critical to the project, i.e., their absence from the project would have a significant impact on the approved scope of the project. NIH points out that limiting the number of individuals named in the NoA does not diminish their scientific contribution; it only reduces the number of individuals subject to this requirement.
Policy reference: NIH Grants Policy Statement 8.1.2.6 [website]
NSF uses the titles “Senior Personnel” and “Non Co-PI Senior Personnel”, for those responsible for the scientific/technical direction of the project and reporting. We must assume that both are “key personnel” for NSF projects. Note that on occasion, NSF may have additional solicitation-specific guidance for key personnel. Changes in effort of key personnel of 25% or more must receive prior approval from the sponsor. Key personnel are responsible for keeping current on COI disclosures for the life of the award.
Policy references:
- Definition of Senior Personnel Exhibit II-7 [website]
- Footnote 27 regarding Senior Personnel responsibility [website]
- Prior approval for Changes of PI or Co-PI: Ch VII.B.2 [website]
- COI Policy; Ch IX.A [website]
Grants: NASA policy strongly encourages one PI as the lead, only. Co-Investigators “serve under the direction of the PI”, so MIT does not require PI status approval for Co-I’s or others named, since they are all under the direction of the PI.
NASA does not use the term “Key Person” related to grants, however, several individuals may be named on the proposal and their participation must be verified through NSPIRES – this does not make them “key”.
Contracts: People named in the key personnel clause of the award are considered “key” for the award and must be tracked for effort purposes, but not for COI disclosures or training. NASA policy states that before removing, replacing, or diverting any of the listed or specified personnel or facilities, MIT must notify the sponsor “reasonably in advance” of the change, and submit a justification (including proposed substitutions) in sufficient detail for NASA to evaluate the impact on this contract.
Policy Reference: NASA FAR Supplement 1852.235-71 [website]
“Senior key person” is referred to in the Funding Opportunity Announcement but not always defined, though we must assume that these are the same as “key personnel”. People named in the proposal as key personnel are considered “key” for the life of the project. Any changes in key personnel require DOE prior approval. Effort for key personnel must be tracked but COI disclosures or training are not required unless specified in the FOA.
Policy Reference: DOE Assistance Regulations, 2 CFR Part 200 as amended by 2 CFR Part 910 at http://www.eCFR.gov [website]
NOAA typically only recognizes the PI as a key person. However, any person named in the proposal as key may also be named in the award. If named in the award as key, prior approval will be required to reduce their effort by 25% percent or more and/or to remove them from the project. A change in the project director or principal investigator shall always require approval unless waived by the agency.
Policy Reference: NOAA Administrative Standard Award Conditions [website]
Simons Foundation
Key personnel are those named on the contact sheet, and who dedicate more than 25% of their time to the project. Reduction in effort of 25% or more or removal from the project must be approved by the sponsor in advance for named key people. The sponsor requires we track effort for key personnel but not COI disclosures or training.
Policy references: Simons Foundation Policies and Procedures [website]
Related terms: Key Person, Key Persons
The Kuali Coeus (KC) system keeps track of individuals in the Key Person(s) role in the Award module, for those individuals named a Key Person on an NIH/PHS proposal or those proposals where the “PCK” flag was selected.
Remember – if you are reducing effort of key personnel name in a Notice of Award by 25% or more of their previously approved effort, you must notify RAS and write to your program officer and request prior approval of the reduction. Any other reduction in effort may be reported in your next progress report
RAS staff are the only personnel to maintain key persons in an Award in the KC system. If Key Person(s) should be deleted or added to an Award, please contact your RAS Contract Administrator for further review.
PIs/DLCs are responsible for reviewing key personnel on new awards and for tracking effort for key personnel throughout the project period. When reviewing a new award, if there are questions about key personnel listed (or not listed), please contact your RAS Contract Administrator for further review.
PIs/DLCs are responsible for tracking effort of key personnel throughout the project period. If, during the life of an award, any of the key personnel reduce their effort by 25% or more, or leave the institute and/or new key personnel are added to the award, see above: Managing Key Personnel for the Life of an Award.
If you have additional questions, contact your RAS Contract Administrator.
- Uniform Guidance Fixed Rate Requirements
- F&A Methodology
- F&A Components
- MIT Use of a de minimis Rate
- Fund Account Overhead Rates
- Allocation Rates
- Determination of On-Campus and Off-Campus Rates
- Employee Benefits (EB) Rates
- Vacation Accrual Rates
- Graduate Research Assistant Tuition Subsidy
- Historical RA Salary Levels
- MIT Facts and Profile Information
- Classification of Sponsored Projects
- Types of Sponsored Awards
- How Are Sponsored Projects Generated?
- Cost Principles and Unallowable Costs
- Direct and Indirect Costs
- Pre-Proposals / Letters of Intent
- MIT Investigator Status
- Components of a Proposal
- Special Reviews
- Applying Through Workspace
- Proposal Preparation Checklist
- Proposals and Confidential Information
- Personnel Costs
- Subcontracts and Consultants
- Annotated Budget Justification - Federal Research
- Annotated Budget Justification - Non-Federal Research
- Annotated Budget Justification - Federal Non-Research
- Annotated Budget Justification - Non-Federal Non-Research
- Kuali Coeus Approval Mapping
- Roles and Responsibilities
- Submission of Revised Budgets
- Standard Contract Terms and Conditions
- Contractual Obligations and Problematic Terms and Conditions
- Review and Negotiation of Federal Contract and Grant Terms and Conditions
- Industrial Collaboration
- International Activities
- MIT Export Control - Export Policies
- Nondisclosure and Confidentiality Agreements
- Negative Confirmation On Award Notices
- Routing and Acceptance of the Award Notice
- COI and Special Review Hold Notice Definitions
- Limiting Long-Term WBS Account Structures
- SAP Project WBS Element Conditions
- Kuali Coeus Electronic Document Storage (EDS)
- Billing Agreements
- PI Absence from Project
- Cost Transfers
- Equipment Threshold
- Uniform Guidance and the FAR
- MIT Standard Terms and Policies
- Guidelines for Charging Faculty Summer Salary
- Limitations on Funds - Federal Contracts
- Managing Salary Costs
- Monitoring Project Budgets
- Monthly Reconciliation and Review
- No-Cost Extensions
- Reporting Requirements
- Return of Unexpended Funds to Foundations
- Determining the Sponsor Approved Budget (SAB)
- Working With the Sponsor Approved Budget (SAB)
- Sponsor Approved Budget (SAB) and Child Account Budgets
- Sponsor Approved Budget (SAB) and Prior Approvals
- Submitting an SAB Change Request
- When a PI Leaves MIT
- Research Performance Progress Reports
- Closing Out Fixed Price Awards
- Closeout of Subawards
- Record Retention
- Early Termination
- Reporting FAQs
- Using SciENcv
- AFOSR No-Cost Extension Process
- Terms and Conditions
- New ONR Account Set Ups
- Department of Defense Disclosure Guidance
- Department of Energy / Office of Science Disclosure Guidance
- Introduction to Industrial Sponsors
- General Considerations for Industrial Proposals
- SRC Guidance to Faculty Considering Applying for SRC Funding
- Find Specific RFP Information
- Industrial Proposal Checklist
- Proposal Formats
- Special Requirements
- Deadline Cycles
- Model Proposals
- Non-Competitive Industrial Proposals
- Master and Alliance Agreements With Non-Standard Proposal Processes
- Template Agreements
- New Consortium Agreements
- Competitive Industrial Proposals
- Collaborative (No-cost) Research Agreements
- National Aeronautics and Space Administration Disclosure Guidance
- NASA Graduate Research Fellowship Programs
- NASA PI Status and Definitions
- NIH Checklists and Preparation Guides
- National Institutes of Health Disclosure Guidance
- Human Subjects and NIH Proposals
- NIH Data Management and Sharing
- NIH Research Performance Progress Reports
- Grant Opportunities for Academic Liaison with Industry (GOALI) proposals
- MIT Guidance Regarding the NSF CAREER Program
- Research Experiences for Undergraduates (REU) Supplements
- National Science Foundation Disclosure Guidance
- NSF Proposals: Administrative Review Stage
- NSF Collaborations
- NSF Pre-Award and Post-Award Actions
- NSF Reporting
- NSF Frequently Asked Questions
- NSF Safe and Inclusive Working Environment
- Process, Roles and Responsibilities
- What Is Allowable/Eligible Cost Sharing?
- MIT’s Preferred Cost Sharing Funds
- Third-Party Cost Sharing
- Showing Cost Sharing in a Proposal Budget
- Sponsor Specific Instructions Regarding Location in the Proposal
- Funding F&A Costs as Cost Sharing
- Using Faculty Effort for Cost Sharing
- Information about Completing the Cost Sharing Template
- NSF Cost Sharing Policy
- Tracking/Reporting Cost Sharing
- Special Cost Sharing Topics
- International Activities Examples
- Rubicon Fellowships
- Marie Skłodowska-Curie Fellowships
- Criteria for Subrecipients
- Subawards at Proposal
- Requesting New Subawards
- Managing Subawards
- RAS Subaward Team Contacts
- Funding and Approval
- Proposal Phase
- Award Set-up
- Monitoring Research During Project Period
- Closeout Phase
- Voluntary Cost Sharing
- Sponsor-Specific Guidance
- Audits and Auditors
- Upcoming Trainings and Events
- Research Administration Practices (RAP)
- NCURA Virtual Workshops and Webinars
- Guide to RA Resources and Training
- Career Paths
- Newsletters
- Tools and Systems
- Award Closeout & Audits
- Award Setup
- Cost Sharing
- Export Control
- Financial Conflict of Interest
- Kuali Coeus
- Project Monitoring
- Proposal Preparation & Submission
- Research Sub Awards
- Research Administration Email Lists
- RAS Operations
- VPR Research Administration Organization Chart
- By department
- By administrator
- Research Administrator Day
- News & Announcements
- Onsite searching on the VPR public websites

- Proposal Preparation Data
- Find Your OSP Contact
- Find Funding
- Prepare Proposals
- Negotiations
- Manage Awards & Agreements
- Award Modifications
- Account Activation
- Frequently Asked Questions
- Contract Basics
- Team Assignments
- Basics of Federal Contracting
- Ancillary Agreements
- Fee for Service
- Information Services
- About Research Development
- Pitt Momentum Funds FAQs
- Arts & Humanities Microgrants
- Priming Grants
- Teaming Grants
- Scaling Grants
- Limited Submissions
- Honorific Awards Bootcamp
- Receive Honorific Awards Updates
- Learn About Honorific Awards
- Internal Funding
- External Funding
- NEH Proposal Development Resources
- NIH Proposal Development Resources
- NSF Proposal Development Resources
- Proposal Writing Resources
- Proposal Boilerplate & Professional Development Templates
- Big Proposal Bootcamp
- Fulbright Scholars
- General Resources
- Forms & Waivers
- Faculty Resources
- Pitt Research Concierge Program
- Pitt Research Navigator
- General Helpful Links
- COVID-19 Information for Sponsored Programs
- PERIS™ Project
How does the NIH define Senior/Key Personnel?
The National Institutes of Health (NIH) define senior and key personnel to be:
The program director/principal investigator (PD/PI) and other individuals who contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they request salaries or compensation.
The prior approval requirement for changes in status of personnel applies only to those senior/key personnel named in the Notice of Award (NoA).
For more information about NIH personnel definitions and examples, review the related NIH Frequently Asked Questions (FAQs) on senior/key personnel .
- Skip to Content
- Skip to Main Navigation
- Skip to Search

Indiana University Indiana University IU

- Get Started
- Internal Funding Opportunities
- Critical Contact List
- Engaging Corporations & Foundations
- Funding Opportunities List
- Limited Submissions
- Proposal Development Services
- Proposal Tools & Resources
- Institutional Information
- IU Guidelines
- Agency Guidelines
- Uniform Guidance
- IU Rates (Fringe, Facility & Admin)
- Costs and Cost Sharing
- NIH Other Support Requirements
- IU Implementation
- Proposal Routing & Submission
- Pre-Award Requests from Sponsors
- Subrecipient Vs. Vendor
- Other Research Agreements
- Commonly Negotiated Terms
- Advanced Accounts
- Reports and Resources
- Best Practices and Requirements
- Allowable Costs
- Program Income
- Cost Transfers
- No Cost Extensions & Sponsor Prior Approvals
- Risk Assessment
- Monitoring Procedures
- Report Submission Requirements
- Final Financial Reports
- Residual Balances & Refunds
- Account Expiration & Reporting
- Record Retention
- Single Audit
- External Audit & Financial Reviews
- Create a New Study
- Reviews & Renewals
- Inspections
- Protocol Amendments
- Safety & Injuries
- New Protocols
- Annual Review
- Clinical Trials
- Renewals & Protocol Closure
- Reportable Events
- Get started
- Employee Status Change
- Removal of Radionuclide Labs
- Information for Participants
- Levels of Review
- New Studies
- Reliance Requests
- Study Closure
- Managing Study Documents After Approval
- Clinicaltrials.gov Program
- Sponsor-Investigator Program
- Clinical Research Billing Compliance
- Federalwide Assurances
- Fee Schedule
- Complete a Disclosure
- Disclosure Review Process
- Resolving Conflicts
- Request Disclosure Information
- Restricted Party Screening Guidance and Procedures
- When to Contact the Export Control Office
- International Travel and Activities
- International shipping and exports
- International Visitors
- Research Misconduct
- Responsible Conduct of Research
- IU Technologies
- Navigating the Commercialization Process
- Invention Disclosures
- Policies and Procedures
- Open Source Licensing
- Partnerships
- For Entrepreneurs
- For Investors
- Innovation & Commercialization Staff
- ICO Office Hours
- Quick Guides
- Required Training
- Office for Research Administration (ORA) Training Videos
- IU Research leadership team
- Associate Deans for Research
- Research Units
- Indirect Cost Recovery (ICR) Allocation Formula
- Centers, Institutes, & Museums Search
- Establishing a New Institute
- Research Scientists
- Sponsored Activity Reports
- Predefined Reports
- Compliance Reports
- Financial Audit Reports
- Service Survey Reports
- Annual Report
- Big 10 Data
- Announcements
- Research Compliance Quarterly
- Research Impact Newsletter
- IU Bloomington Research Events
- IUPUI Research Events
- Institutional Biosafety Committee
- Submit News & Events
- People Directory
- Report a Concern
- Contact Form
- News & Events
- Training & Workshops
- Human Subjects & Institutional Review Boards
Research Personnel
Quick guide: research personnel.
Research personnel, i.e. individuals engaged in human subjects research, specifically, individuals who intervene or interact with human subjects or access identifiable information for research purposes, must obtain IRB approval for their participation.
Words to know
- Co-PI : Research personnel who share responsibility of the design and/or conduct of the research with the Principal Investigator. This may include a student, fellow, or resident under the mentorship of a PI in order to complete an education requirement.
- Investigator : In research subject to FDA regulations, an individual who actually conducts a clinical investigation, i.e., under whose immediate direction the test article is administered or dispensed to, or used involving a subject, or, in the event of an investigation conducted by a team of individuals, is the responsible leader of that team.
- IU-affiliated research personnel : research personnel who are Indiana University faculty, staff, and students or employees and staff of IU affiliate institutions that have contracted with the IU IRBs for review and oversight of human subjects research. IU affiliate institutions include Eskenazi Health, IU Health, Purdue Pharmacy Practice (when research is occurring at IU or an IU-affiliated health care system), Regenstrief Institute, Rehabilitation Hospital of Indiana, and Roudebush VAMC.
- Individuals making critical decisions regarding eligibility of subjects
- Individuals obtaining consent for a study that is greater than minimal risk
- Individuals listed on Form FDA 1572 or the investigator agreement
- Non-affiliated research personnel : research personnel who are not faculty, staff, or students of IU, or employees or staff of IU affiliate institutions that have contracted with the IU IRBs
- Other research staff : research personnel conducting research procedures under the direction of the PI or key personnel but are not considered responsible for the conduct and/or reporting of research.
- Principal investigator (PI) : Responsible leader of a team of research personnel who has the ultimate responsibility for the conduct of the research.
- Site-specific PI : When the IU IRB is providing review and oversight for an external research site, the responsible leader of a team of research personnel at that site who has the ultimate responsibility for the conduct of the research only at that site.
How it works
Step 1: ensure the investigator is engaged in research.
- Individuals who intervene or interact with human subjects, or access subjects’ identifiable data, are considered engaged in research and must obtain IRB approval for their participation.
- Individuals who are receiving only deidentified samples or data
- Individuals who access identifiable data for auditing or monitoring
- Individuals who facilitate recruitment by informing potential subjects about the research, sharing recruitment materials or other information about the research with potential subjects, direct potential subjects to the study team, or seek or obtain potential subjects' permission for the study team to contact them.
- Individuals who perform commercial or other services for investigators, when (1) services performed do not merit professional recognition or publication privileges, (2) services performed are typically performed by those institutions/individuals for non-research purposes; and (3) the individuals do not administer the study intervention being tested or evaluated under the protocol.
- Individuals at an institution not selected as a research site who perform protocol-dictated services/procedures which would typically be performed as part of routine clinical monitoring or follow-up of subjects enrolled at a study site. Please contact the HRPP for details and specific requirements.
- Investigator(s) at an institution not selected as a research site who administer the study interventions being evaluated under the protocol on a one-time or short-term basis. Please contact the HRPP for details and specific requirements.
Step 2: Complete training requirements
- Individual serving as PI must be eligible. See PI eligibility requirements.
- Research Personnel must complete training requirements .
- Research Personnel in the following roles must complete a conflict of interest disclosure: PI, Co-PI, Key Personnel.
Step 3: Obtain IRB approval for investigator’s participation
- Research personnel should be listed in Kuali Protocols and assigned the appropriate role as defined above. The study team determines the most appropriate role based on the individual’s protocol-specific responsibilities.
- If you need to make changes to PI, Co-PI, Key Personnel, Site-Specific PI or Other Research Staff, an amendment must be submitted to and approved by the IRB prior to participation in research.
- Adding non-affiliated research personnel or external sites must be done by submitting an amendment. When IU is providing approval for an external site, only the Site-specific PI should be listed in Kuali Protocols. For more information, see the Quick Guide on IU IRB Review for Non-Affiliated Research Personnel or External Sites .
- If IU-affiliated research personnel wish to obtain approval from an external IRB, see the Quick Guide on Reliance on external IRBs .
Who to contact
For questions about investigator engagement or roles, or obtaining IRB review, contact the HRPP at irb@iu.edu or 317-274-8289.
- Contact information
- Information for research participants
- Information for media
- Information for new researchers
- Centers & Institutes
- Report a concern
- Collaborative Institutional Training Initiative (CITI)
- Kuali COI Disclosure system
- Kuali Coeus Grants system
- Kuali Protocols IRB system
- Grants Management Toolkit (GMT)
- Fiscal Officer Lookup
- Research Equipment & Tools Database
FORMS & POLICIES
- Conflict of interest
- Intellectual property
- Research misconduct
- Research policies
- NIH Data Management & Sharing Policy
DATA & REPORTS
- Institutional information
- IU rates (Fringe, F & A)
- Uniform guidance
- Sponsored activity reports
- Predefined reports
- Research administration dashboards
- Big 10 data
- Compliance reports
AWARD RESOURCES
- Research agreements
- Advanced accounts
senior/key personnel (per NIH)
The Principal Investigator (PI) and other individuals so designated on an NIH funding proposal. They contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they receive salaries or compensation under the grant. They typically have doctoral or other professional degrees, although individuals at the masters or baccalaureate level may be considered senior/key personnel if their involvement meets the definition. Consultants also may be considered senior/key personnel if they meet the definition.
- NIH: Senior/Key Personnel FAQ
- Who is considered an Investigator and needs to disclose Significant Financial Interest?
University of Washington Office of Research
Or support offices.
- Human Subjects Division (HSD)
- Office of Animal Welfare (OAW)
- Office of Research (OR)
- Office of Research Information Services (ORIS)
- Office of Sponsored Programs (OSP)
OR Research Units
- Applied Physics Laboratory (APL-UW)
- WA National Primate Research Center (WaNPRC)
Research Partner Offices
- Corporate and Foundation Relations (CFR)
- Enivronmental Health and Safety (EH&S)
- Grant and Contract Accounting (GCA)
- Institute of Translational Health Sciences (ITHS)
- Management Accounting and Analysis (MAA)
- Post Award Fiscal Compliance (PAFC)
Collaboration
- Centers and Institutes
- Collaborative Proposal Development Resources
- Research Fact Sheet
- Research Annual Report
- Stats and Rankings
- Honors and Awards
- Office of Research
© 2024 University of Washington | Seattle, WA
DUHS Nursing Research + Evidence-Based Practice
Home » Key Personnel
Key Personnel
Who are the key personnel?
Participating in nursing research:
Key Personnel are individuals who have access to Protected Health Information (PHI) and generate data either through direct interactions with subjects or through access to their medical records. Key Personnel and their study roles must be listed on an IRB submission.
In accordance with Duke IRB policy, individuals who are not Key Personnel are individuals who will be interacting with research subjects during the course of a research study, but only in his/her regular non-research employment capacities, such as a clinic receptionist, nurse, or phlebotomist, or a radiologist or radiology technician. These individuals should not be listed as Key Personnel for the study if the person will perform only genuinely non-collaborative services meriting neither professional recognition nor publication privileges, and not associated with individual financial gain, and will not contribute to the design, governance, and/or analysis of the study.
All Key Personnel must complete Duke Health Biomedical Research CITI (ethics) training.
Assure Key Personnel Qualifications

Powered by WordPress / Academica WordPress Theme by WPZOOM
- NIH FAQs about Senior/Key Personnel
The PHS Conflict of Interest regulations apply to all Investigators. MIT policy defines Investigators as those individuals who are independently responsible for the design, conduct and reporting of research. The definition of senior/key person and Investigator are closely related. When determining whether an individual should be listed as a senior/key person, PIs should consult the guidance provided in the MIT Investigator Matrix .
- Policy Statement
- Definitions
- Guiding Principles
- Application of Guiding Principles
- Disclosure Requirements
- Review, Evaluation and Resolution
- Disclosure to Third Parties
- Disciplinary Action
- Record Retention
- PHS Addendum
- Summary of Updates to the MIT Policies and Procedures on Conflicts of Interest in Research
- Sponsored Research with SFI Entity Questionnaire
- Who is an Investigator
- Change in Status or Absence of PI or Key Personnel
- National Science Foundation (NSF)
- Helpful Hints About SBIR/STTR Phase 1 Awards
- Training Requirements
- What do I need to know before starting my COI Disclosure?
- COI vs. OPA: What You Need to Know
- Screening Result
- Create an SFI Entity
- Entity Definition Section
- Maintaining the Relationship Details Grid
- Overview of Relationships Screen
- Relating Projects to SFIs
- Why Can’t I Save & Continue a Project Relationship Page?
- What Happens If I Skip A Project?
- Step 4: Certify
- Revise or Update an Initial Disclosure in Progress
- Revise or Update a Revision in Progress
- View SFI Entity (make Inactive)
- Create Travel Disclosure
- Revise Travel Disclosure(s) in Progress
- View Travel Disclosure(s)
- Prepare Your Proposal Disclosure
- Questionnaire Screening Result – No Conflicts
- Questionnaire Screening Result – Potential Conflict
- Step 1: Proposal Certification Questionnaire
- Step 2: Significant Financial Interest
- Step 3: Relationships
- Step 1: Screening Questions
- Modifying Your Proposal Certification Questionnaire Answers
- Step 2: Significant Financial Interests
- Integration with Proposal Certification
- View and Print Your Master Disclosure
- Why can’t I edit my Disclosure?
- FCOI Public Information Request Form
- COI Training Requirement
- Investigator Self Certification
- Key Persons
- NIH Individual Fellowships
- NIH Travel Disclosures
- Outside Professional Activities
- Significant Financial Interest

Research Team Training & Education
Education is a key component in the protection of human subjects in research. It is essential that all key personnel engaged in human subjects research understand the regulations that govern research that involve the use of information and specimens obtained from human subjects. All WCM investigators, research coordinators, and research staff who are engaged in research involving living human subjects, human tissue samples, or identifiable private information must complete the required Human Subjects Protection (HSP) training mandated by the terms of our Federal Wide Assurance before they can submit their protocols in WRG-HS.
- Please refer to the CITI Access Information Page for instructions on how to access the required courses.
- When logging into CITI, use the Log In Through My Organization sign in page.
What constitutes "key personnel"?
Key Personnel includes any individual who is "engaged in research with human subjects". Key Personnel includes the Primary Investigator(s) and can be WCM faculty, staff, or WCMC students who:
- Enroll individuals;
- Obtain subjects' informed consent by engaging in the consent process ("process" involves more than simply handing out or collecting forms, or providing contact information for available research studies);
- Intervene or interact with subjects (this includes both non-invasive AND invasive procedures);
- Collect data/identifiable private information from or follow-up with subjects;
- Have access to information that links subject names or other identifiers with their data;
- Act as an authorized representative of the PI(s).
Human Subjects Protections Training
Weill Cornell Medicine requires that all researchers and research staff engaged in human subjects research complete specialized training in human subjects protection. A notice of completion must be recorded by WRG-HS for all members of a research team before a submission can be processed.
HSP training must be renewed every three (3) years to maintain your credentials
What HSP training must I complete?
- Research Investigators and Staff : Biomedical Research Investigators and Key Personnel course (ID 1407)
Please refer to the CITI Access Information Page for instructions on how to access the required courses. You can log into CITI by going to CITI's Log In Through My Organization page.

What if I am an external investigator listed on a WCM study protocol?
You will not be required to complete the WCM-specific CITI courses. However, record of training completion in biomedical research human subjects protections, and good clinical practice must be provided. While the WCM IRB does not require external investigators complete the WCM-specific CITI courses, this does not preclude any requirements imposed by regulatory agencies, grantors or sponsors.
Conflict of Interest Training
WCM is dedicated to maintaining the integrity of the research conducted at our institution. Part of this effort is ensuring that all individuals involved in research undergo appropriate training in the regulations for, and identification and reporting responsibilities of, any Conflicts of Interest . Federal regulations requires all research personnel on PHS-funded studies only to complete the Conflict of Training course available on CITI prior to engagement in any research endeavor.
COI training must be renewed every four (4) years to maintain your credentials.
Please visit the COI Office web site for training requirements.
Good Clinical Practice (GCP) Education
The training of investigators and research teams to conduct clinical research is crucial to successful translation of novel drugs, devices and interventions. In order to secure comprehensive competency-based training for clinical research personnel, we will require certification of training in Good Clinical Practices (GCP) for all investigators and personnel directly involved in new and ongoing clinical studies that involve the testing of drugs or devices, including all FDA-registered studies as well as investigator-initiated protocols. This requirement applies to studies reviewed by both the WCM IRB, as well as approved external IRBs (e.g. BRANY, NCI CIRB, etc.).
GCP training must be renewed every three (3) years to maintain your credentials.
What GCP training must I complete?
- Clinical Research Investigator and Staff: CITI Good Clinical Practice (ID: 54618)
- Social-Behavioral Research Investigator and Staff: not required
Please refer to the CITI Access Information Page for instructions on how to access the required courses. You can log into CITI by going to CITI's Log In Through My Organization page.
The WCM IRB will not issue approval for a research protocol if any key personnel has not satisfied the education requirement.
Weill Cornell Medicine Human Research Protections 575 Lexington Avenue New York, NY 10022 Phone: (646) 962-8200
CITI Training for Human Subjects Research
Citi requirements.
The University of Massachusetts-Amherst (UMass-Amherst) IRB requires faculty, staff, and students (graduates and undergraduates) who are conducting or part of a research group to take the research-appropriate course on the CITI Program Webpage and complete certification before submitting the Kuali protocol for initial approval, amendment, renewal to be approved by the IRB.
Training is required of all personnel regardless whether the study qualifies for exempt, expedited or full board review. You must receive a score of 80% or better overall in order to pass this course. If you receive less than 80% you will need to retake one or more of the quizzes to improve your score to 80% overall.
The Human Subjects CITI Online training is divided into two disciplinary categories:
- Group 1: Biomedical research Investigators and Key Personnel - Basic Course. This course is required for medical, physiological or pharmacological studies that includes, but is not limited to, research with drugs, devices or other interventions. (Researchers collaborating with Baystate should complete all modules to be certified for Baystate Collaborative Studies.); or
- Group 2: Social Behavioral and Education Research Investigators and Key Personnel - Basic Course. This course is required for studies on sociological, psychological, anthropological or educational phenomena including observational and survey research and work with population and/or epidemiological studies..
- CITI Courses for these 2 two basic human subjects training modules can be found below under 'CITI Training Module Requirements.'
PLEASE NOTE: The Social and Behavioral Responsible Conduct of Research and the Biomedical Responsible Conduct of Research courses do NOT satisfy the human subjects training requirement.
Refresher Training
All human subjects researchers at UMass are required to complete a CITI Training refresher every 5 years. If CITI training is expired (over 5 years) IRB submissions such as new studies, revisions, renewals will not be processed until the CITI refresher has been completed.
Link to the CITI Home page .
******************************************************************
1. Select Log In. 2. Select "Log in through my Institution." 3. Scroll and click on "University of Massachusetts Amherst." 4. At the UMass NetID login screen enter your campus-wide NetID credentials. 5. On the Main Menu page, click on "University of Massachusetts Amherst Courses." 6. Under "My Learner Tools for University of Massachusetts Amherst" click on "Add a Course." 7. Scroll Down to Question 1 - Human Subjects Research and choose either " Group 1: Biomedical Research Investigators and Key Personnel " OR " Group 2: Social and Behavioral Research Investigators and Key Personnel ." 8. The rest of the courses (#2-8) are not required for Human Subjects research. Please note that you have to answer each question in order to move on to your selected course.
*PLEASE NOTE: The Social and Behavioral Responsible Conduct of Research and the Biomedical Responsible Conduct of Research course do NOT satisfy the human subjects training requirement.
******************************************************************* NOTE: You do not need to complete the course in just one session. You are encouraged to use several "log-on" sessions, but you should take the quizzes immediately after completing each module.
When you have completed all requirements, you will be able to download your Certificate. Under the column "Completion Record" click on "View-Print-Share" for further instructions.
Investigators, including faculty, staff, and students, directly involved in research with human subjects are required to complete an education program in human subjects protection. Completion of required CITI training modules listed below fulfills the UMass requirements for investigator training (NOTE: Investigators taking the training for the first time must take the CITI training basic course. Investigators whose CITI training was first completed over 5 years ago must take the refresher course):
Biomedical Research Investigators and Key Personnel - Basic Course Modules (Group 1)
- Belmont Report and Its Principles
- Recognizing and Reporting Unanticipated Problems involving Risks to Subjects or Others in Biomedical Research
- Populations in Research Requiring Additional Considerations and/or Protections
- History and Ethics of Human Subjects Research
- Basic Institutional Review Board (IRB) Regulations and Review Process
- Informed Consent
- Social and Behavioral Research (SBR) for Biomedical Researchers
- Records Based Research
- Conflicts of Interest in Human Subjects Research
- University of Massachusetts Amherst
In addition, please review other modules appropriate to your area of research.
Social and Behavioral Research Investigators and Key Personnel (Group 2)
- History and Ethical Principles
- Defining Research with Human Subjects - SBE
- The Federal Regulations
- Assessing Risk - SBE
- Informed Consent - SBE
- Privacy and Confidentiality - SBE
- Records-Based Research
- Unanticipated Problems and Reporting Requirements in Social and Behavioral Research
The steps below will assist you if you have mistakenly chosen the wrong institution, your institution has changed or you have an affiliation with more than one institution.
After logging into the CITI Home Page :
- From your main menu click on the link "Affiliate with another institution."
- Choose the correct institution from the appropriate drop down menu.
- Answer the member information questions required by your newly affiliated institution. *Please note that you do not need to provide a UMass email address in the field marked “Institutional email address” if you don’t have one - you can provide any valid email address.
- Enroll in the course(s) required by your newly affiliated institution.
If you would like to receive credit for modules previously taken, consider the following:
- You must choose the same course at both institutions (i.e., Group 1 Basic/same - Biomedical Basic/same - Group 2 Refresher/same) in order to get credit. You will receive automatic credit for any modules duplicated. The modules must be identified as the same module by the unique modules ID number. Because each institution determines which modules are available in their courses not all modules will transfer.
- Each institution determines the time frame in which modules are transferable. This time frame can be between 1 and 10 years. Some institutions opt off of this option and do not allow modules to transfer.
The following information is intended to help researchers determine who is to be included on a protocol involving human subjects. This information is also related to the mandatory training requirement that the UMass Amherst has for involvement in human subjects protection.
A person listed as key study personnel in the IRB application is an individual who is interacting and/or intervening with human subjects or handles the personally identifiable data of a human subject.
Study personnel that are involved in the informed consent process should also be included on the IRB study personnel list.
Addition of new key study personnel to an IRB approved protocol will only be approved if the proposed study personnel have completed the required CITI training. Please note that funding agencies may have their own definition of study personnel as it applies to grant or other funding applications.
Optional/Supplemental modules that are available, include:
- Gender and Sexuality Diversity (GSD) in Human Research
- Consent Tools Used by Researchers
- Consent in the 21st Century
- Avoiding Group Harms - U.S. Research Perspectives
- Avoiding Group Harms - International Research Perspectives
- Cultural Competence in Research
- Genetic Research in Human Populations
- Research with Prisoners - SBE
- Research Involving Prisoners
- Research with Children - SBE
- Research Involving Children
- Research in Public Elementary and Secondary Schools - SBE
- Research Involving Pregnant Women, Fetuses, and Neonates
- International Research - SBE
- International Studies
- Internet-Based Research - SBE
- FDA-Regulated Research
- Research and HIPAA Privacy Protections
- Vulnerable Subjects - Research Involving Workers/Employees
Search form
©2024 University of Massachusetts Amherst • Site Policies • Site Contact

- About Grants
- Forms Directory
Additional Senior/Key Person Profile Format
The R&R Senior/Key Person Profile (Expanded) Form included in NIH application packages allows for the collection of a contact PD/PI and 99 additional other senior/key individuals (including any multi-PD/PIs). If you need to add more Senior/Key Person Profiles than the form allows, prepare a separate file using this format page, convert it to PDF, and upload it in the "Additional Senior/Key Person Profile(s)" attachment field on the R&R Senior/Key Person Profile (Expanded) Form.

General instructions for completing the "Additional Senior/Key Person Profile(s)" attachment field on the R&R Senior/Key Person Profile (Expanded) Form are found in the Form Instructions section of the How to Apply – Application Guide . Also check Section IV. Application Information of the funding opportunity for any opportunity-specific instructions.
See R&R Senior/Key Person Profile (Expanded) Form for a reference copy of the data collection used for the contact PD/PI and 99 additional other senior/key individuals.
This page last updated on: April 27, 2024
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health

International Engagement
Assessing and managing international collaborations in research.
Know Your International Partners Establish and Understand the Parameters of the Engagement Prohibition of Engagement in a Malign Foreign Talent Recruitment Program Moving Forward with an International Collaboration
Know Your International Partners
When engaging in international research and other professional activities, whether as part of your U-M role or external to U-M (e.g., outside activities), it is important to assess your international partners. An initial consideration is the country where the partner (either entity or individual(s)) is located.
- Is the country comprehensively embargoed (i.e., Cuba, Iran, North Korea, Syria, and the Crimean, Donetsk, and Luhansk Regions of Ukraine)? You should contact and work with the U-M Export Controls Office early in the process as most activities and collaborations with these countries, associated entities, or individuals from these countries require an export control license .
- Is the entity or individual(s) on a U.S. restricted party list prohibiting receipt of export-controlled items, information, or technology? Ask your department to conduct restricted party screening on the entity and individual(s) you are considering engaging with at the outset to determine if they are subject to restrictions. If you have questions, contact [email protected] .
Another consideration is whether the country is designated as a “Foreign Country of Concern” or “FCOC” (i.e., Iran, Russia, China, and North Korea) in the CHIPS and Science Act of 2022. Research and other professional activities (e.g., foreign institution affiliations) with entities or collaborators in a FCOC may garner additional scrutiny by some federal agencies when considering funding an award (e.g., in areas involving critical and emerging technologies or military or dual use research). Senior/key personnel with affiliations that are considered high risk, where risks cannot be satisfactorily mitigated by the PI and U-M (e.g., through training or other measures to reduce identified risks), may be requested to be removed from a federal agreement by the sponsor. If you have questions about a research or professional international activity, including outside activities and contracts, contact [email protected] .
Establish and Understand the Parameters of the Engagement
When assessing potential collaborations, whether foreign or domestic, it is important to understand and establish the parameters of the engagement. The following are questions to consider, many derived from a 2019 JASON advisory group report, Fundamental Research Security , commissioned by the National Science Foundation.
- Are the terms of the engagement made clear in writing? Are all participants known?
- Are there any aspects of the engagement that are not to be disclosed to any of the participants? If so, what is the reason?
- Is there any aspect of the engagement that seems unusual, unnecessary, or poorly specified?
- Where do the funding and other resources needed for the activity come from? Is it clear what each party is providing?
- Are tangible assets of the engagement, existing or to be generated, known? How will they be shared? Who decides how they are allocated?
- Has ownership of any intellectual property that results from the research or activities been established?
- How does a participant end their engagement?
- Are scholars expected to reside away from their home institution as a part of the engagement? Are the terms consistent with U-M’s/your school and department’s policy for outside activities?
- What are the reporting requirements back to sponsoring institutions or organizations?
- Who will control the dissemination of the resulting research? Will the results be publishable? What are the plans for publication?
- Is there relevant expertise on both sides? Are both sides contributing meaningfully to the research? Will both sides benefit intellectually?
- Does the collaboration involve specific sponsors, research topics, or types of interaction that should be ruled out a priori, e.g., due to potential military applications or adverse human rights consequences?
Prohibition of Engagement in Malign Foreign Talent Recruitment Programs
Ensure that outside activities and associated agreements do not include terms deemed of risk by the U.S., whether part of an official foreign talent recruitment program (FTRP) or employment or other outside agreement. Central concerns include overlap or duplication of U.S. funded research, unauthorized transfer of unpublished U.S. research data, methodology, and intellectual property, and overcommitment on U.S. funded projects due to engagement in undisclosed international activities.

Read the U-M policy prohibiting participation in Malign Foreign Talent Recruitment Programs.
Chips and science act prohibition on malign ftrps.
The CHIPS and Science Act of August 9, 2022 requires federal research funding agencies to establish a policy requiring:
- Certification by senior/key personnel at proposal, and annually for the duration of the award, that they are not a party to a malign FTRP as defined in the Act.
- Senior/key personnel to disclose participation in FTRP contracts, agreements, or other arrangements.
- Certification by the institution that such individuals have been made aware of the requirement.
Agencies are required to have these policies in place by August 09, 2024. The Act also directs the White House to develop guidelines for research funding agencies that:
- Prohibit R&D awards from being made to senior/key personnel participating in malign FTRPs and,
- Requires recipient institutions [U-M] to prohibit these individuals from working on projects supported by federal R&D awards.
Department of Defense (DoD) Prohibition on Malign FTRPs
In June 2023, DoD released Countering Unwanted Foreign Influence in Department-funded Research at Institutions of Higher Education. The document includes a Policy on Risk-based Security Reviews of Fundamental Research and a Decision Matrix. Per the matrix:
- DoD prohibits department funding after August 9, 2024, if the proposing institution [U-M] does not have a malign FTRP policy/prohibition in place.
The U-M Policy Prohibiting Participation in Malign Foreign Talent Recruitment Programs, which includes the CHIPS and Science Act definition.
Certification that Senior/key Personnel are not a Party in a Malign FTRP
Common disclosure forms intended for federal-wide implementation were finalized in November 2023 and will be adopted by agencies in the coming months:
- Current and Pending (Other) Support Common Form
- Biographical Sketch Common Form
The forms include certification to be completed by each senior/key person at the time of submission and annually thereafter that they are not a party in a malign FTRP.
Moving Forward with an International Collaboration
After assessment of international collaborators and the terms of the collaboration, ensure appropriate written agreements are in place (e.g., data and material transfer and use agreements and/or collaborative agreements) to protect property and unpublished work and to facilitate achievement of goals and objectives. Information on unfunded agreements and how to route them can be found on the Office of Research Sponsored Projects webpage .
Disclosure of the collaboration is also indicated, to both U-M and outside entities (e.g., federal funding agencies).
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
A year later, a look back at public opinion about the U.S. military exit from Afghanistan

In August 2021, the United States withdrew the last of its troops from Afghanistan , ending its military presence there after nearly 20 years. The U.S. exit from Afghanistan resulted in the Taliban regaining control of the country and created a refugee crisis as many Afghans fled. It also raised fears that terrorists might use Afghanistan as a safe haven, as was the case with Ayman al-Zawahiri, the al-Qaida leader who was discovered in the nation’s capital, Kabul, and killed in a U.S. drone strike late last month.
A year after the U.S. military exit from Afghanistan, here’s a look back at how people in the United States and other countries have viewed the troop evacuation and its aftermath, as well as their broader attitudes about the war. All findings are based on previously published Pew Research Center surveys.
This Pew Research Center analysis examines Americans’ views of the troop withdrawal from Afghanistan and its aftermath. It is based on recent surveys conducted by the Center. Links to these surveys, including information about the field dates, sample sizes and other methodological details, are available in the text.
At the time of the military evacuation, 54% of Americans said the decision to withdraw U.S. troops from Afghanistan was the right one, according to a survey conducted in August 2021 . Around four-in-ten Americans (42%) said the decision was the wrong one. There was a sharp partisan divide on this topic. While 70% of Democrats and Democratic-leaning independents said the decision to withdraw troops was the right decision, about half as many Republicans and GOP leaners (34%) shared this view. Most Republicans (64%) instead said the decision was wrong.
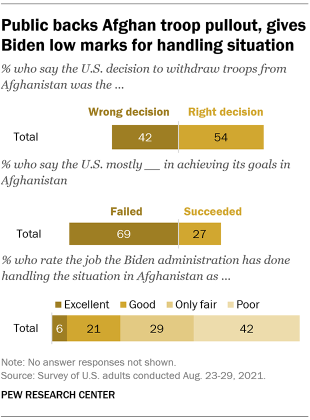
In the same survey, 69% of U.S. adults said the United States mostly failed in achieving its goals in Afghanistan. About a quarter (27%) said the U.S. succeeded. There was partisan agreement on this question: About seven-in-ten in both parties said the U.S. mostly failed to achieve its goals.
Americans harbored doubts about the war in Afghanistan even before the withdrawal of U.S. troops. In a spring 2019 survey , 59% of U.S. adults said that considering the costs versus the benefits to the United States, the war in Afghanistan was not worth fighting, while 36% said it was. The balance of opinion was about the same among U.S. military veterans.
Both during and after the troop withdrawal, large majorities of Americans expressed negative views of the Biden administration’s handling of the situation in Afghanistan. In both August and September 2021, about seven-in-ten or more said that the administration had done an only fair or poor job dealing with the situation there, with around four-in-ten or more saying it had done poorly. In both surveys, fewer Americans said the administration had done an excellent or good job. In the September survey, for instance, only 24% said this.
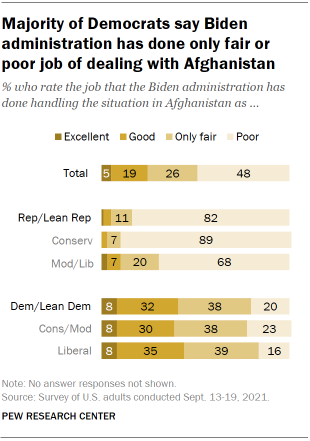
A large majority of Republicans (82%) said in September 2021 that the administration had done a poor job handling the situation in Afghanistan. Conservative Republicans were 21 percentage points more likely than moderate and liberal Republicans to say this (89% vs. 68%).
One-in-five Democrats also said the Biden administration had done a poor job dealing with the Afghanistan situation. About twice as many said the administration had done an only fair job (38%) or an excellent or good job (40%).
Veterans and non-veterans were also divided on this question. While similar shares of veterans (76%) and non-veterans (74%) said in September 2021 that the Biden administration had done an only fair or poor job dealing with the situation in Afghanistan, veterans were more likely than non-veterans to say the administration handled it poorly (60% vs. 47%). Only about a quarter or fewer in either group said the administration had done an excellent or good job, with very few giving it an excellent rating (4% of veterans and 5% of non-veterans). As is the case with the general public, veterans’ views on these issues are deeply divided along party lines.
Last September, a majority of Americans (56%) said they favored admitting thousands of Afghan refugees into the U.S., according to the same survey , which was conducted after the U.S. evacuated thousands of Afghans from the country . About four-in-ten (42%) opposed this move.
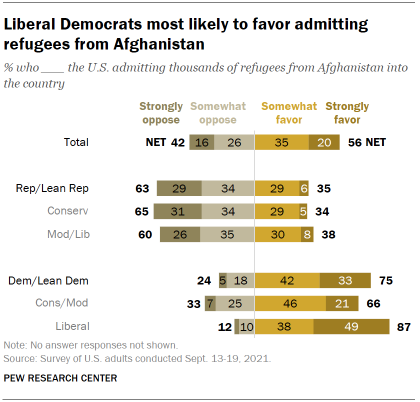
These views were deeply divided by partisanship. At the time, 63% of Republicans either strongly (29%) or somewhat (34%) opposed the U.S. admitting thousands of refugees from Afghanistan into the country. About a third (35%) said they favored admitting these refugees.
By contrast, three-quarters of Democrats were in favor of admitting refugees, including a third who strongly favored it. Liberal Democrats (87%) were more likely than conservative and moderate Democrats (66%) to support this. About half of liberal Democrats (49%) said they strongly favored admitting refugees from Afghanistan.
Despite majority support for admitting refugees, Americans were divided on whether the government was conducting adequate security screenings for those arriving in the U.S. from Afghanistan. About four-in-ten Americans (43%) said they were very or somewhat confident that the government was conducting adequate security screenings, while 55% were not too confident or not at all confident. Democrats were more likely than Republicans to express confidence in the government’s security screenings.
In a spring 2022 survey of 18 countries , people viewed the U.S. decision to withdraw all troops from Afghanistan as the right one, but many said the withdrawal itself was not handled well. A median of 52% across the surveyed countries said the troop pullout was the right choice, compared with a median of 39% who said it was the wrong choice.
Public opinion in these countries was more negative when it came to how the U.S. exit from Afghanistan was handled. A median of 56% said it was not handled well, while a median of 33% said it was. In only two surveyed countries, Poland and Malaysia, did half or more of adults approve of the way the situation in Afghanistan was handled.
Most Americans said in August 2021 that Taliban control of Afghanistan is a threat to the security of the United States. Nearly half (46%) said Taliban control represented a major threat to the U.S., and another 44% saw it as a minor threat. Republicans (61%) were far more likely than Democrats (33%) to view a Taliban-controlled Afghanistan as a major security threat.
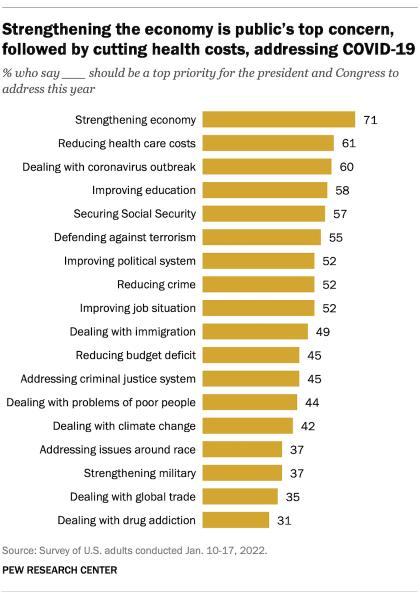
In a January 2022 survey , 55% of Americans said that defending against terrorism should be a top priority for the president and Congress to address this year. Of the 18 issues asked about, defending against terrorism was among the top priorities identified. The survey preceded the U.S. military’s drone strike on al-Qaida leader Ayman al-Zawahiri in Kabul in July.
Americans tend to prioritize the terrorism issue differently based on factors including age and partisanship. About three-quarters of adults ages 65 and older (76%) said that defending against terrorism should be a top priority for the president and Congress, compared with 32% of those under 30. And roughly two-thirds of Republicans (65%) said it should be a top priority, compared with 48% of Democrats.
- Refugees & Asylum Seekers
- War & International Conflict

Katherine Schaeffer is a research analyst at Pew Research Center
How Temporary Protected Status has expanded under the Biden administration
Most americans express support for taking in refugees, but opinions vary by party and other factors, republicans and democrats have different top priorities for u.s. immigration policy, key facts about title 42, the pandemic policy that has reshaped immigration enforcement at u.s.-mexico border, after a month of war, ukrainian refugee crisis ranks among the world’s worst in recent history, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
2-Day Private Sightseeing City Tour of Moscow with Subway Excursion, Tsaritsyno and Novodevichy Monastery and 4-course Traditional Russian Lunch with Vodka Plus Russian Classic Ballet Evening provided by U Visit Russia
- Share full article
Advertisement
Supported by
Russia’s Central Bank Raises Rates to 15 Percent to Curb Inflation
The jump, from 13 percent, would bring a long period of “tight monetary conditions” in order to ease price pressures, the bank said.

By Ivan Nechepurenko
Russia’s Central Bank on Friday raised its key interest rate by two percentage points to 15 percent, a bigger increase than expected as the bank said it was trying to bring down stubbornly high inflation.
The central bank, which said the annual inflation rate would range from 7 to 7.5 percent this year, predicted a long period of “tight monetary conditions” in order to bring the rate down close to its target of 4 percent.
Driving the price pressures is “steadily rising domestic demand,” the bank said in its statement , spurred by the Kremlin’s decision to inject more money into the economy as it fights a war in Ukraine.
The surge in spending “is increasingly exceeding the capabilities to expand the production of goods and the provision of services,” the bank said.
At a news conference Friday, Elvira Nabiullina, the head of the Central Bank, said that increased government spending was one of the reasons for the interest rate increase. Russia’s defense budget has more than tripled since last year’s invasion of Ukraine, and it is scheduled to reach almost a third of the government’s spending next year.
Russia was largely successful at weathering the immediate storm produced by sanctions aimed at punishing it for the invasion. The restrictions greatly curtailed its lucrative trade with Western countries and largely isolated it from the global financial system.
But as Russia spends vast amounts on its war machine, its industrial production and labor markets are unable to keep up with the increased demand, translating into higher inflation and high levels of borrowing.
Yevgeny Nadorshin, the chief economist at the PF Capital consulting company in Moscow, said the central bank’s effort to slow the economy by raising interest rates could “suffocate the country’s growth.”
“We are in the moment when growth is transforming into a recession,” Mr. Nadorshin said.
He pointed to Russia’s mortgage and consumer borrowing markets, which have experienced rapid expansion.
“People are still tense about the economy, but they feel that in the moment, things are much better than expected,” Mr. Nadorshin said in a phone interview. “People feel that this is a short period that they must take advantage of.”
But Dmitri Polevoy, an economist in Moscow, said that despite high interest rates, he doesn’t see major risks with the Russian economy.
“This story is exclusively about inflation,” Mr. Polevoy said in written comments to questions posed through a messaging service. “Under the current budgetary policy and with the same external conditions,” he said, “the risk of a recession is low.”
After experiencing a nosedive following the invasion of Ukraine, the Russian economy has returned to growth. The International Monetary Fund recently estimated economic output would rise 2.2 percent this year, as oil exports have largely evaded Western sanctions and found new customers in India, China and other countries.
The country has also been able to import Western goods from some former Soviet republics, as well as Turkey and Gulf States. Russian businesses, including banks, have adapted too, serving needs since the departure of many Western companies.
Ivan Nechepurenko has been a Times reporter since 2015, covering politics, economics, sports and culture in Russia and the former Soviet republics. He was raised in St. Petersburg, Russia, and in Piatykhatky, Ukraine. More about Ivan Nechepurenko

IMAGES
VIDEO
COMMENTS
Key Personnel. Key personnel are those people who are essential to carrying out the work of a project, typically those responsible for the design, conduct and reporting of the research. Key personnel includes: PIs, Co-PIs, and a third category known as "Key Persons". MIT's policy is that the PI and any Co-PIs for a project, as well as ...
The National Institutes of Health (NIH) define senior and key personnel to be: The program director/principal investigator (PD/PI) and other individuals who contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they request salaries or compensation. The prior approval requirement for changes in status of personnel applies only to ...
Page 1 of 4 02/2019 Key Personnel Guidance Document Key personnel are persons engaged in the conduct of the research activity such that they directly interact with research participants to obtain consent and/or research data, or will have access to participants' private and identifiable private information during data collection or data analysis
The R&R Senior/Key Person Profile (Expanded) Form is used for all grant applications, and allows the collection of data for all senior/key persons associated with the project. Some information for the PD/PI may be pre-populated from the SF424 (R&R) form. See instructions in G. 200 - SF 424 (R&R) Form if these fields are empty. View larger image.
To determine if they are key personnel, first identify their role on the project, which could include making decisions about the scientific direction of the research or settling disputes between investigators in multiple-PI applications. If they are generally uninvolved in scientific decisions, then they will not be considered key personnel.
Co-investigators should be listed as senior/key personnel. Do not use the term "co-investigator" when you mean a PI on a multiple PI application. Learn more about Multiple Principal Investigators. Collaborators. Collaborators always plays an active role in the research, and the position is sometimes defined interchangeably with co-investigator.
Do proper research Key personnel perform challenging tasks, and it's worth taking time to research an individual before hiring them or promoting them to the position. Check on their online presence and performance reviews from the management to analyze their capabilities. Regardless of how close the employees may be, appoint the key personnel ...
Senior/Key Personnel. Investigator roles mentioned on this page will typically also be considered Senior/Key personnel. Senior/Key Personnel are individuals designated within a research proposal (including the PI) who contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they receive salaries or compensation.
Key Personnel: research personnel, other than the PI, who are responsible for the conduct and/or reporting of research. Such individuals may include, among others: Individuals making critical decisions regarding eligibility of subjects; Individuals obtaining consent for a study that is greater than minimal risk;
senior/key personnel (per NIH) The Principal Investigator (PI) and other individuals so designated on an NIH funding proposal. They contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they receive salaries or compensation under the grant.
Participating in nursing research: Key Personnel are individuals who have access to Protected Health Information (PHI) and generate data either through direct interactions with subjects or through access to their medical records. Key Personnel and their study roles must be listed on an IRB submission. In accordance with Duke IRB policy ...
NIH FAQs about Senior/Key Personnel. The PHS Conflict of Interest regulations apply to all Investigators. MIT policy defines Investigators as those individuals who are independently responsible for the design, conduct and reporting of research. The definition of senior/key person and Investigator are closely related. When determining whether an ...
Key Personnel include the Project Director(s) / Principal Investigator(s) and other individuals who contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they receive salaries or compensation under the grant. Typically these individuals have doctoral or other professional degrees, although individuals at the masters or baccalaureate ...
Summary of NIH Policy Regarding Senior/Key Personnel and Investigator . 09.18.2018 . Stage . Senior/Key Personnel Definition : Who Designates Requirements : Reference : ... • Biosketch for All Key Personnel Research Support Sections: - Ongoing - Completed (last 3 years) Include Project Role • SF424 Pg. G-81 • NIH Senior/Key Personnel FAQs
Research Team Training & Education. Education is a key component in the protection of human subjects in research. It is essential that all key personnel engaged in human subjects research understand the regulations that govern research that involve the use of information and specimens obtained from human subjects.
These individuals are considered to be "Key Personnel" on NIH awards and contracts that include research involving human subjects, this includes the Principal Investigator(s), all individuals responsible for the design or conduct of the study, and those individuals identified as key personnel of consortium participants or alternate performance ...
Scroll Down to Question 1 - Human Subjects Research and choose either "Group 1: Biomedical Research Investigators and Key Personnel" OR "Group 2: Social and Behavioral Research Investigators and Key Personnel." 8. The rest of the courses (#2-8) are not required for Human Subjects research. Please note that you have to answer each question in ...
Policies and Requirements. Outlined below are: NIH policies and requirements that support these efforts; a description of the current reviews NIH takes before an award is made, and NIH's post award management and actions; and important contacts and additional resources for NIH applicants and awardees. On or After July 9, 2022.
The R&R Senior/Key Person Profile (Expanded) Form included in NIH application packages allows for the collection of a contact PD/PI and 99 additional other senior/key individuals (including any multi-PD/PIs). If you need to add more Senior/Key Person Profiles than the form allows, prepare a separate file using this format page, convert it to ...
The key personnel are the founders of the company who started their research programs at VTsIOM and continue in the Levada Center. ... Research by the Levada Center is based on regular Russia-wide public opinion surveys. Completed studies include: [citation needed] Homo Sovieticus (Russian: Советский человек). 5 waves of Russia ...
Research and other professional activities (e.g., foreign institution affiliations) with entities or collaborators in a FCOC may garner additional scrutiny by some federal agencies when considering funding an award (e.g., in areas involving critical and emerging technologies or military or dual use research). Senior/key personnel with ...
In a spring 2022 survey of 18 countries, people viewed the U.S. decision to withdraw all troops from Afghanistan as the right one, but many said the withdrawal itself was not handled well. A median of 52% across the surveyed countries said the troop pullout was the right choice, compared with a median of 39% who said it was the wrong choice.
Thank you for all your efforts and deeds to provide us the key to a magnificent, wonderful and a high artistically show. Above any expectation, both musically and visually. The Bolshoi is a world well-known stage for the highest level of music, opera and ballet show and that is why we wanted to catch a glimpse of that legendary place.
Oct. 27, 2023. Russia's Central Bank on Friday raised its key interest rate by two percentage points to 15 percent, a bigger increase than expected as the bank said it was trying to bring down ...
Key indicators. Dynamics* Key findings > Volume of new supply in Q1 2023 amounted to 26,900 sq m. > Vacancy rate at the end of Q1 2023 increased and reached the level of 13.3% in Class A offices and 8.1% in Class B offices. > Asking rental rates for Q1 2023 amounted to 26,203 roubles/sq m/ year in Class A and 17,408 roubles/sq m/year in Class B.
Abstract. Common issues that arise between the physical education teacher and paraeducators are related to a lack of communication. This article proposes a few strategies to help establish open communication and collaboration between physical education teachers and paraeducators.