- International edition
- Australia edition
- Europe edition


‘We’re not making new hairs, we’re rescuing’: could scientists reverse male pattern baldness?
The onset of hair loss can be devastating for some men. But research into experimental cell therapy is offering hope of keeping baldness at bay
P aul Kemp’s introduction to the issue of baldness came as a rude awakening while getting a haircut at the age of 20. “I remember my hairdresser looking down and saying ‘Oh my God, you’re going bald on top’.”
Kemp, now in his 60s, was dismayed at the discovery, but it also marked the beginning of a lifelong professional interest in the science of hair loss and how to stop it. Kemp is the co-founder and chief executive officer of HairClone, a company that is developing an experimental cell therapy treatment for male pattern baldness and whose tagline is “making hair loss history”.
Male pattern baldness affects around 85% of men by the age of 50 and losing hair can be a source of anxiety and low self-esteem . There are drugs that can slow hair loss, transplants to redistribute hair around the head, or strategic hairstyles to disguise receding hairlines and bald spots. Nothing exists to reverse the process, but that could be set to change.
In recent years, scientists have discovered that baldness has its origin in the loss of specialised skin cells, called dermal papillae, that line the base of hair follicles. These cells are crucial for regulating hair thickness, growth, texture and possibly even colour. But in some men, these cells are progressively killed off by dihydrotestosterone, the hormone that causes the male body to mature during puberty.
“You have about 1,000 of these dermal papilla cells per hair,” says Kemp. “The more dermal papilla cells, the thicker the hair shaft. When you get down to around half that number, you’ll notice the hair thinning.”
Baldness implies a lack of hair, but, technically, bald heads are not hairless. As dermal papilla cells are lost, the follicle shrinks and the hair shaft it produces becomes finer and spends longer in the dormant state. Eventually, the hairs become so fine and grow so slowly that they are effectively invisible.
HairClone is aiming to reverse this so-called miniaturisation process by allowing people to bank 100 or so youthful follicles or follicles from parts of the head that still have hair. After being taken from a patient’s head, the follicles are placed in a deep freeze container at -150C. Then, as and when required, the hair can be thawed and the dermal papilla cells can be cloned and multiplied in the laboratory to provide an almost unlimited supply.
The hope, based on experiments in mice and previous tests in people by an earlier company, is that injecting the cells back into the scalp will plumpen the follicles and return hair to a more youthful state.
“We’re not making new hairs, we’re rescuing miniaturising hairs,” says Kemp.
Around 200 clients have already banked hair, although none have yet been treated. The efficacy of the approach has not yet been established in a clinical trial, but the company is in the process of establishing quality controls that will allow it to manufacture cells to clinical standards. At that point – and the company is hoping that will be within the next 12-18 months – doctors will be able to offer it on an experimental basis to patients who they think could benefit.
One of those to have banked his hair is Tommy Smith, a 65-year-old planning consultant, who lives in Red Oak, North Carolina. Smith initially began losing hair in his 20s, possibly as a side-effect of powerful acne medications, including high dosages of prescription vitamin A.
He had a hair transplant in 1988, aged 30, which he says had “outstanding” results until he began to lose both his transplanted hair and original hair in 2015. He sees hair cloning as an insurance against further hair loss. “The concept of having hair follicles stored to address the potential of additional hair loss in the future is very encouraging,” he says. “I also think this could provide young men who have hair loss history in their family the opportunity to address future hair loss in a much less painful and complicated manner.”
Smith’s experience highlights the dilemma that hair surgeons and their patients are faced with when planning a transplant. Once transplanted, follicles retain their original identity and so need to be taken from a safe zone of the scalp that is not destined to go bald in future. Transplanting before the hairline has stabilised can lead to a “hair island” effect, requiring further transplants – and there’s also a question of whether the hairline will recede so far that there won’t be sufficient hair to spread over the whole head. “You’re chasing a moving target,” says Kemp.
However, scientists believe it may be possible to predict a man’s eventual hairline by analysing genetic markers hidden inside dermal papilla cells, meaning that in future men could make more informed choices about the most appropriate treatment.
“There’s always been this clear pattern of male hair loss, but no one’s really explained why it’s like that,” says Dr Claire Higgins, a lecturer in tissue regeneration at Imperial College London and scientific adviser to HairClone.
In a paper published in May , Higgins described evidence that the hairline of a middle-aged man could be traced back to the earliest stages of embryonic development. Around the third week of an embryo’s life, cells form three layers called the ectoderm, mesoderm and endoderm. Most organs in the body contain cells that derive from just one of these lineages: the endoderm gives rise to the internal organs, the mesoderm becomes the muscle and connective tissues, and the ectoderm becomes the central nervous system. “Normally a tissue is one lineage, but the [skin] is a bit of an enigma,” says Higgins. “The dermis [the skin’s lower layer] on the face is ectoderm and the dermis on your body is mesoderm, but the top of your head is not really known.”
Higgins argues that male pattern baldness traces out the boundary between skin cells that have taken two very different paths during development. This, she says, could explain why the cells on only certain parts of the head are oversensitive to dihydrotestosterone.
Kemp and colleagues are working to develop a test, based on the gene expression of dermal papilla cells, to establish whether the part of the scalp they come from is balding, destined to bald or will always retain its hair. “Ideally you want to be able to map the head,” he says. “We’re finding genetic differences between the hairs and we’re in the preliminary phases of doing that.”
Kemp says that after years of living with hair loss, he is comfortable with his appearance – and treatment is not for everyone. “At this stage, I’m used to looking like this,” he says. “But when I was 20, had they said, there’s something we can do about that, I would’ve done it. Our targets are younger people.”
- How to have healthy skin
- Health & wellbeing
Comments (…)
Most viewed.
Advertisement
Can we finally reverse balding with these new experimental treatments?
Male pattern baldness could soon be a thing of the past, with new hair loss treatments beginning to show tantalising results
By Joshua Howgego
26 September 2023

baytunc/Getty Images
I’LL level with you: a part of me didn’t want to write this story. When I first realised that I was losing my hair, I found it important to mention it often in conversation. I was so embarrassed about it that I was trying some sort of reverse psychology. But I soon realised that if there was one thing less attractive than my balding head, it was how much I was talking about it. I am joking, of course: there is nothing wrong with being bald. Still, for me, the prospect is terrifying. My hair is a big part of my identity, so to lose it is crushing.
I’m not alone. By the age of 50, between 30 and 50 per cent of men have begun to experience male pattern baldness . Despite there being plenty of handsome hairless men out there – I’m looking at you, Thierry Henry – studies suggest that people tend to perceive bald men as less attractive and less friendly . And we don’t need science to tell us that this can be deeply upsetting.
So although I have dialled down the discussion of my growing bald patch, I have been quietly digging into the science of hair loss – and what I found is worth shouting about. It is common knowledge that some treatments can slow hair loss. What is less known is that as we are coming to understand the reasons why male pattern baldness causes people to lose their hair, we are finding new strategies to restore it. There may soon be a way to not just slow balding, but reverse it.
In a field…
Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
To continue reading, subscribe today with our introductory offers
No commitment, cancel anytime*
Offer ends 2nd of July 2024.
*Cancel anytime within 14 days of payment to receive a refund on unserved issues.
Inclusive of applicable taxes (VAT)
Existing subscribers
More from New Scientist
Explore the latest news, articles and features
Physicists have worked out how to melt any material
Subscriber-only
Environment
Heatwaves now last much longer than they did in the 1980s, unprecedented gps jamming attack affects 1600 aircraft over europe, tooth loss linked to early signs of alzheimer’s disease, popular articles.
Trending New Scientist articles
Stay up to date with notifications from The Independent
Notifications can be managed in browser preferences.
UK Edition Change
- UK Politics
- News Videos
- Paris 2024 Olympics
- Rugby Union
- Sport Videos
- John Rentoul
- Mary Dejevsky
- Andrew Grice
- Sean O’Grady
- Photography
- Theatre & Dance
- Culture Videos
- Food & Drink
- Health & Families
- Royal Family
- Electric Vehicles
- Lifestyle Videos
- UK Hotel Reviews
- News & Advice
- Simon Calder
- Australia & New Zealand
- South America
- C. America & Caribbean
- Middle East
- Politics Explained
- News Analysis
- Today’s Edition
- Home & Garden
- Fashion & Beauty
- Travel & Outdoors
- Sports & Fitness
- Sustainable Living
- Climate Videos
- Behind The Headlines
- On The Ground
- Decomplicated
- You Ask The Questions
- Binge Watch
- Travel Smart
- Watch on your TV
- Crosswords & Puzzles
- Most Commented
- Newsletters
- Ask Me Anything
- Virtual Events
- Betting Sites
- Online Casinos
- Wine Offers
Thank you for registering
Please refresh the page or navigate to another page on the site to be automatically logged in Please refresh your browser to be logged in
Scientists find potential ‘cure’ for baldness
New research pins baldness to a single chemical, article bookmarked.
Find your bookmarks in your Independent Premium section, under my profile

Sign up to our free Living Well email for advice on living a happier, healthier and longer life
Live your life healthier and happier with our free weekly living well newsletter, thanks for signing up to the living well email.
A single chemical could be responsible for whether people go bald or not, a new study has found.
In the UK, approximately two thirds of men will face male pattern baldness . The study says the discovery of the chemical could “not only treat baldness, but ultimately speed wound healing”.
Researchers at the University of California, Riverside, found that a sole chemical is responsible for hair follicles dividing and dying.
In the study published in the Biophysical Journal , study co-author Qixuan Wang said: “In science fiction when characters heal quickly from injuries, the idea is that stem cells allowed it.
“In real life, our new research gets us closer to understanding stem cell behavior, so that we can control it and promote wound healing.”
- Covid: Professor warns of two new ‘telltale’ symptoms as cases rise
- Student gives birth in toilet while preparing for night out
The team looked at hair follicles as these are the only human organ that regenerates regularly and automatically, and discovered that a type of protein called TGF-beta controls how the stem cells in hair follicles divide and why some can die off.
Wang explained: “TGF-beta has two opposite roles. It helps activate some hair follicle cells to produce new life, and later, it helps orchestrate apoptosis, the process of cell death.
“Even when a hair follicle kills itself, it never kills its stem cell reservoir. When the surviving stem cells receive the signal to regenerate, they divide, make new cell and develop into a new follicle.”
However, the scientists found that when a hair follicle dies, the stem cell reservoir still remains.
“When the surviving stem cells receive the signal to regenerate, they divide, make new cells and develop into a new follicle,” Wang said
The study authors added that it may be possible to stimulate hair growth by activating follicle stem cells, but more research on the subject needs to be done.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.
New to The Independent?
Or if you would prefer:
Want an ad-free experience?
Hi {{indy.fullName}}
- My Independent Premium
- Account details
- Help centre
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 07 November 2022
Genetic prediction of male pattern baldness based on large independent datasets
- Yan Chen 1 , 2 , 3 ,
- Pirro Hysi ORCID: orcid.org/0000-0001-5752-2510 4 ,
- Carlo Maj 5 ,
- Stefanie Heilmann-Heimbach 6 ,
- Timothy D. Spector 4 ,
- Fan Liu ORCID: orcid.org/0000-0001-9241-8161 1 , 2 , 3 &
- Manfred Kayser ORCID: orcid.org/0000-0002-4958-847X 1
European Journal of Human Genetics volume 31 , pages 321–328 ( 2023 ) Cite this article
7565 Accesses
4 Citations
3 Altmetric
Metrics details
- Genetic markers
Genetic prediction of male pattern baldness (MPB) is important in science and society. Previous genetic MPB prediction models were limited by sparse marker coverage, small sample size, and/or data dependency in the different analytical steps. Here, we present novel models for genetic prediction of MPB based on a large set of markers and large independent subsample sets drawn among 187,435 European subjects. We selected 117 SNP predictors within 85 distinct loci from a list of 270 previously MPB-associated SNPs in 55,573 males of the UK Biobank Study (UKBB). Based on these 117 SNPs with and without age as additional predictor, we trained, by use of different methods, prediction models in a non-overlapping subset of 104,694 UKBB males and tested them in a non-overlapping subset of 26,177 UKBB males. Estimates of prediction accuracy were similar between methods with AUC ranges of 0.725–0.728 for severe, 0.631–0.635 for moderate, 0.598–0.602 for slight, and 0.708–0.711 for no hair loss with age, and slightly lower without, while prediction of any versus no hair loss gave 0.690–0.711 with age and slightly lower without. External validation in an early-onset enriched MPB dataset from the Bonn Study ( N = 991) showed improved prediction accuracy without considering age such as AUC of 0.830 for no vs. any hair loss. Because of the large number of markers and the large independent datasets used for the different analytical steps, the newly presented genetic prediction models are the most reliable ones currently available for MPB or any other human appearance trait.
Similar content being viewed by others

Improved polygenic prediction by Bayesian multiple regression on summary statistics
Luke R. Lloyd-Jones, Jian Zeng, … Peter M. Visscher

GWAS of allometric body-shape indices in UK Biobank identifies loci suggesting associations with morphogenesis, organogenesis, adrenal cell renewal and cancer
Sofia Christakoudi, Evangelos Evangelou, … Konstantinos K. Tsilidis

Improved genetic prediction of complex traits from individual-level data or summary statistics
Qianqian Zhang, Florian Privé, … Doug Speed
Introduction
Male pattern baldness (MPB), or androgenetic alopecia, is a highly heritable, age independent appearance trait [ 1 , 2 ]. MPB has socio-psychological consequences on a large proportion of the world’s male population. MPB is of interest to various fields of scientific research and applications, such as in medicine, anthropology, evolutionary biology, and forensics. Availability of accurate and reliable genetic prediction of MPB would allow addressing several concerns. Genetic MPB prediction could enable early treatment of MPB before it becomes externally manifest, which may result in better therapeutic outcomes alleviating socio-psychological problems. MPB prediction from old or modern DNA would help describing the appearance of historical persons from skeletal remains or DNA found at crime scenes, as previously done for pigmentation traits [ 3 , 4 ]. MPB prediction from ancient DNA would allow reconstructing what ancestors of modern humans looked like and help understanding when and where MPB first arose in time and space, as previously shown for pigmentation traits [ 5 , 6 ]. Additionally, as MPB was discussed as marker of several later-onset health disorders (e.g., metabolic and cardiovascular diseases), genetic MPB prediction may contribute to better management of age-dependent human diseases.
A key requirement for the development of accurate genetic prediction models for genetically complex traits like MPB is the availability of sufficiently large number of DNA predictors. To date, four sufficiently powered GWASs for MPB have identified hundreds of MPB-associated SNPs, available for MPB prediction modelling. Heilmann-Heimbach et al. [ 7 ] reported a GWAS meta-analysis of 10,846 early-onset MPB cases and 11,672 controls from eight independent cohorts and identified 63 distinct genetic loci. Hagenaars et al. [ 8 ] identified 287 genetic loci in 52,874 UK Biobank (UKBB) male participants. Pirastu et al. [ 9 ] described 71 genetic loci in 43,590 UKBB male participants, follow by an independent replication study in 31,112 men. Recently, Yap et al. [ 2 ] identified 624 genetic loci from what currently is the largest GWAS on MPB, involving 205,327 UKBB males.
A key requirement for developing reliable genetic prediction models for MPB and any other trait are sufficiently large and independent datasets needed for the different analytical steps. The genetic prediction models by Hagenaars et al. [ 8 ], which with 331 SNPs currently include the largest number of DNA predictors, discriminated between subjects with no hair loss from those with severe hair loss with an AUC 0.78 (sensitivity = 0.74, specificity = 0.69, PPV = 0.59, NPV = 0.82). Although these models were tested in independent dataset of 12,874 UKBB samples, GWAS-based marker discovery and prediction model training were performed in the very same dataset of 40,000 UKBB samples, and no external model validation was reported. It remains unclear, if and to what degree the non-independence of datasets for marker discovery and model training induces bias and how they would perform in the external validation in different datasets.
Aiming to overcome limitations of previously reported models, we developed new genetic prediction models for MPB based on a large number of DNA markers and four large independent datasets for the four analytical steps i.e., i) 55,573 UKBB samples for prediction marker ascertainment, ii) 104,694 UKBB samples for prediction model training, iii) 26,177 UKBB samples for prediction model testing, and iv) 991 samples from the early-onset MPB enriched Bonn Study for external model validation.
Materials and methods
Uk biobank study dataset.
The UK Biobank Study ( http://www.ukbiobank.ac.uk ) is a large, population-based genetic epidemiology cohort of volunteers living in the UK. In response to UK Biobank question 2395 [ 8 ], male participants chose one answer that most closely matched their head hair coverage from four given patterns adapted from the Hamilton-Norwood scale [ 10 , 11 ] (Supplementary Fig. 2 ), i.e., no, slight, moderate, and severe hair loss (Supplementary Fig. 2 ). Details on microarray genotyping and imputation have been described previously [ 12 ]. After genomic and phenotypic quality controls, to avert issues of population admixture and stratification, the current study included a total of 186,444 UKBB males of European descent, divided into three independent subsets for: 1) prediction marker ascertainment ( N = 55,573), 2) model training ( N = 104,694), and 3) model testing ( N = 26,177) (Supplementary Fig. 1 ). The 80–20% data split into model training and model testing datasets resulted in the same proportions of each of the four baldness phenotype categories observed over the combined dataset (Table 1 ). Our prediction marker ascertainment dataset used for feature selection likely included all the 52,874 UKBB men previously used in the GWAS by Hagenaars et al. [ 8 ]. We initially considered a total of 270 out of the original Hagenaars et al. [ 8 ] 287 MPB-associated SNPs at 85 genetic loci which were carried forward in our feature selection analysis (Supplementary Table 1 ). The rest were removed due to our quality control procedures. This loss is due to an up todate genotypic and imputated SNPs.
Bonn Study dataset
The Bonn Study is a case-control study designed to investigate the genetic basis of early-onset MPB. This study includes 991 males of European descent from Germany i.e., 582 early-onset MPB cases representing the 20% most severely affected in the respective age-groups (<30 years; <40 years), 268 “unaffected” controls ≥60 years with no signs of baldness representing the 10% least affected in their age-group, and 141 samples from the population-based Heinz Nixdorf Recall cohort. Hair loss status was ascertained by expert dermatologists according to the Hamilton/Norwood (HN) classification [ 11 , 13 ]. Baldness status was aligned to the same categories as done in the UKBB Study (Supplementary Fig. 2 ). Details on microarray genotyping and SNP imputation have been described previously [ 7 ]. Of the 117 SNP predictors we identified via feature selection in UKBB, 107 were available in the Bonn dataset. The 10 SNPs missing in Bonn were neither present in the genotyping array dataset nor could they be properly imputed. These data were used as external model validation dataset to additionally test the MPB prediction models trained and tested in UKBB data.
Multinomial and binomial logistic regression
The multinomial logistic regression (MLR) and binomial logistic regression (BLR) modelling was implemented via multinom function in ‘ nnet’ package of R ( www.r-project.org ). The modelling specifications were as following. For MLR, consider baldness pattern, y, to be four categories: i) no hair loss, ii) slight hair loss, iii) moderate hair loss and iv) severe hair loss, which are determined by the genotype, x , of k SNPs, where x represents the number of minor alleles. If π 1 , π 2 , π 3 , and π 4 denote the probability of the four MPB categories, respectively, the MLR can be written as
where α and β can be derived in the modelling set. Baldness pattern of each individual in the testing set can be probabilistically predicted based on the genotypes and the derived α and β,
The baldness category with the max( π 1 , π 2 , π 3 , π 4 ) was considered as the predicted baldness pattern.
For binomial logistic regression (BLR), consider baldness pattern, y, to be two categories any hair loss and no hair loss, which are determined by the genotype, x , of k SNPs, where x represents the number of minor alleles. Let p denote the probability of any hair loss, and 1 − p is the probability of no hair loss. The BLR can be written as
where α and β can be derived in the modelling set. Baldness pattern of each individual in the testing set can be probabilistically predicted based on his or her genotypes and the derived α and β,
The MPB category with the max( p , 1 − p ) was considered as the predicted baldness pattern.
Support vector machine and Artificial neural network
Next to logistic regression analyses, two types of machine learning (ML) algorithms were additionally applied for prediction modelling: support vector machine (SVM) and artificial neural networks (ANN). SVM was used via svm function in the e1071 package of R with the nonlinear radial basis function as the kernel and 2 -4 as the cost of constraints violation set. A “one against one” strategy was applied for classification of both 4-category and 2-category baldness pattern, and posterior probability estimates for each baldness category were obtained via predict.svm function in the e1071 R package. A feed-forward ANN was implemented in Tensorflow [ 14 ] and Keras ( https://github.com/fchollet/keras ). The ANN model consisted of three layers with a hidden layer of ten neurons using the Rectified Linear Unit (ReLU) function in hidden layer and softmax activation function in output respectively. The output layer contained four neurons for 4-category baldness pattern and two neurons for binary baldness pattern, each represents yes-no for one baldness type. The training algorithm of the neural network was performed using Adam optimizer [ 15 ]. ANN model training and testing were conducted in Python version 3.6.1 using the package keras. As for the hyper parameter tuning, we used grid search and chosen hyper parameter with the best prediction performance for both ANN and SVM. For ANN model, we considered activation function of tanh, relu and sigmoid, the number of neurons in hidden layer ranging from 10 to 117 (including 10, 20, 30, 40, 50, 60 and 117), and patience of 25, 50 and 100. For SVM, we considered kernel of linear, polynomial, radial and sigmoid, cost of 2 −5 , 2 −4 , 2 −3 , 2 −2 , 2 −1 , 1, 2, 2 2 , 2 3 and 2 4 . We got the best hyper parameters and built best model by using these best hyper parameters, that is, ANN model using activation function of relu, 10 neurons, and patience of 100, and best SVM model using kernel of radial and cost of 2 −4 .
For both SVM and ANN models, the input data included 118 features (age and genotypes at the 117 SNP marker sites) and output was the probability of being classified into each baldness pattern category. The same 80% training and 20% testing datasets as applied for regression analyses were also used for SVM and ANN analyses. The derived SVM and ANN models were subsequently used to predict baldness pattern categories in the model testing dataset, returning 4-element and 2-element probability vectors for 4-category and binary baldness pattern with values between 0 and 1, respectively. The baldness category with the maximal value was considered as the predicted category.
Feature selection, prediction model training, and model testing
We conducted a Forward Stepwise Regression (FSR) analysis in the marker ascertainment dataset of UKBB by iteratively including the next largest contributor to the model according to Akaike Information Criterion (AIC), \({{{\rm{AIC}}}} \sim 2k + n{\rm ln} (\mathop{\sum}\limits^{n}_{i=1} (y_i-E(y_i))^2)\) , where k is the number of markers, n is the sample size, and \(E\left( {y_i} \right)\) is the fitted value. FSR was performed on 4-category MPB phenotypes using MLR. The marker set proposed by FSR with the minimal AIC value was considered as the theoretically optimal subset for prediction analysis. The rank of the SNP predictors was determined by their rank in FSR analysis or their association significance.
Prediction models were built in the prediction model training dataset of UKBB using MLR and BLR considering MPB as a 4-category as well as as binary trait by joining all samples into two categories of no hair loss and any hair loss. Additionally, SVM and ANN models also were built in the same datasets and using the same four and two categories, respectively. The developed prediction models were assessed for their accuracy in the model testing dataset of UKBB and additionally externally validated in the Bonn Study dataset.
Prediction accuracy was evaluated by estimating the following parameters including the Area Under the Receiver Operating Characteristic (ROC) Curves (AUC), sensitivity, specificity, positive prediction value (PPV), and negative prediction value (NPV). AUC is the integral of ROC curves which ranges from 0.5 representing random prediction to 1.0 representing perfect prediction. Sensitivities and specificities, PPVs and NPVs were calculated using confusion matrices considering the highest predicted probability as the predicted baldness pattern for 4-category prediction, while for binary prediction considering the predicted probability >0.5 as the predicted baldness pattern. Statistical analyses and result visualisation were conducted in R version 3.6.1 using nnet, e1071, pscl, and pROC packages.
Phenotypic characteristics in UKBB and Bonn Study
Individual phenotype and genotype data of 187,435 men from two European countries were included i.e., 186,444 males from the UKBB (32% no, 23% slight, 27% moderate, and 18% severe hair loss; 57.05 ± 8.10 years of age; age range 39–73) and 991 males from the Bonn Study (2% slight, 16% moderate, and 50% severe hair loss; 46 ± 16 years of age; age range 18–70; Table 1 , Supplementary Figs. 1 and 2 ). In the UKBB, age was significantly associated with increased MPB severity (Supplementary Fig. 3 ). In the Bonn Study, lower mean ages were observed across the four MPB categories, because a large proportion (58.7%) of the participants were early-onset MPB (Supplementary Fig. 3 ).
Feature selection, prediction model training, and model testing in UKBB data
Feature selection (or feature thinning) of 270 MPB-associated SNPs from 85 genetic loci previously discovered in the study of Hagenaars et al. [ 8 ] was carried out in 55,573 UKBB samples using FSR. Notably, this sample set likely includes all the 52,874 UKBB men previously used by Hagenaars et al. (Supplementary Table 1 ) for GWAS-based discovery of these 270 associated SNPs. This analysis identified 101 SNPs from 69 distinct loci, which in multinomial logistic combination achieved the maximal model fitting. To this FSR-selected prediction marker set, we additionally added the top-associated SNP from each of the remaining 16 genetic loci (Supplementary Table 1 ), resulting in a total set of 117 SNP predictors from all 85 distinct loci (Pseudo Nagelkerke- R 2 = 0.22). The remaining 153 SNPs from these loci that were removed from our models by FSR did not independently contribute to the MBP prediction (Fig. 1 ), justifying their exclusion.
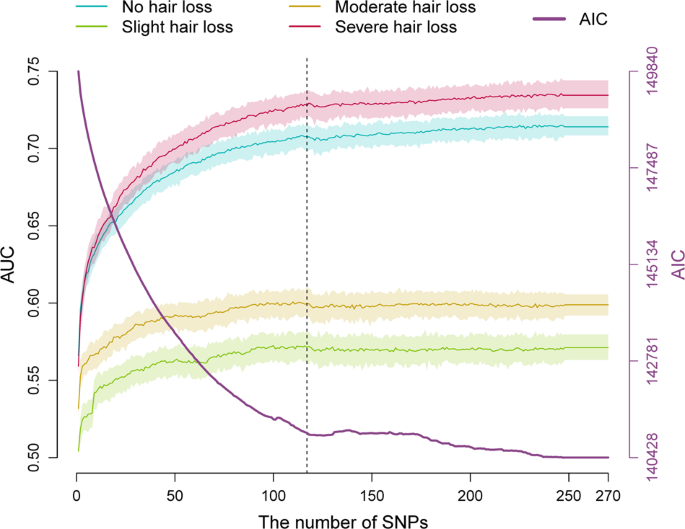
This dataset includes the 52,874 UKBB men previously applied for discovering the MPB-association of the 270 SNPs [ 8 ] used here for feature selection. Based on this dataset, a MLR model was fit in a randomly selected 80% prediction model training subset and applied in the remaining 20% prediction model testing subset with 100 replicates. The estimated mean AUCs are depicted as coloured line and the 5–95% boundary as respective light colour shade for the four predicted MPB categories. The dashed line marks the cut-off at 117 SNPs that were used as best-fit prediction marker set in all subsequent prediction analyses.
We then used these 117 SNP predictors with and without considering age as additional predictor to build 4-category (no, slight, moderate, and severe hair loss) MLR models as well as 2-category (no versus [vs.] any hair loss) BLR models in the model training subset of 104,694 UKBB males. Subsequently, these models were validated in the independent model testing subset of 26,177 UKBB males and prediction accuracy parameters were estimated (Supplementary Table 2 ). Accumulative AUC analysis in the model testing subset showed a fast to slow increase according to the ranking of the predictors (Fig. 2 ). Notably, AUC increased at faster rates for comparisons of no hair loss with increasingly severe hair loss categories. As expected for an age-dependent trait, age was the strongest single predictor, alone resulting in an AUC of 0.560 for any vs. no hair loss, followed by the 101 SNPs selected by the FSR analysis, then followed by the extra 16 SNPs. The most predictive SNP was rs12558842 on the X chromosome, which is located ~280 kbp upstream of the androgen receptor (AR) gene. Alongside age, rs12558842 provided an AUC of 0.608 for any vs. no hair loss. The MBP-association of rs12558842 was stronger than that of rs2497938 in the study of Hagenaars et al., and the later was the highest ranking MPB predictor in Liu et al. [ 16 ]. Rs2497938 is in perfect linkage equilibrium with rs2207081, which fully masks the predictive power of the polyglycine GGN triplet repeat of AR [ 17 ].
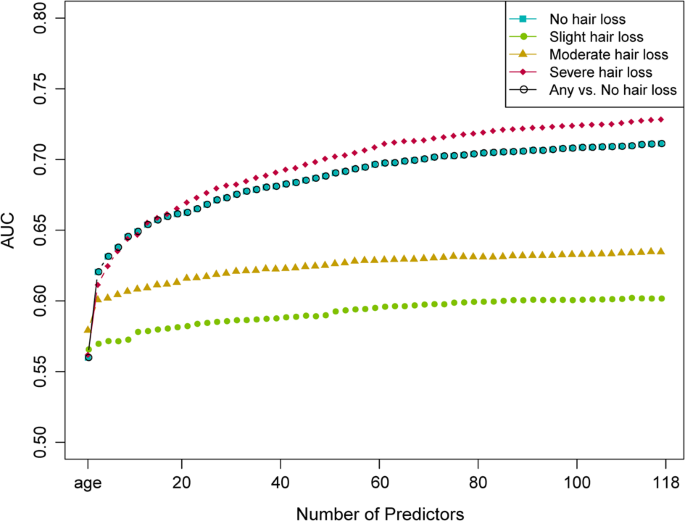
These prediction models were built in the prediction model training dataset of 104,694 UKKBB men.
The 4-category MLR models without considering age predicted with considerably higher accuracies for severe (AUC = 0.718) and no hair loss (0.702) than for moderate (0.607) and slight (0.574) hair loss (see Table 2 for additional prediction accuracy metrics). Inclusion of age in the MLR modelling improved the estimates of accuracy to AUC of 0.728 for severe hair loss, 0.711 for no hair loss, 0.635 for moderate hair loss, and 0.602 for slight hair loss (Table 2 ). For the 2-category BLR model of predicting any vs. no hair loss, we obtained an AUC of 0.702 without age and a slightly higher AUC of 0.711 with age in the model (Table 2 ). This model provided informative prediction for about 26.2% of the 26,177 males tested, that is, individuals with predicted probabilities of any hair loss <0.2 or >0.8 (Fig. 3A ).
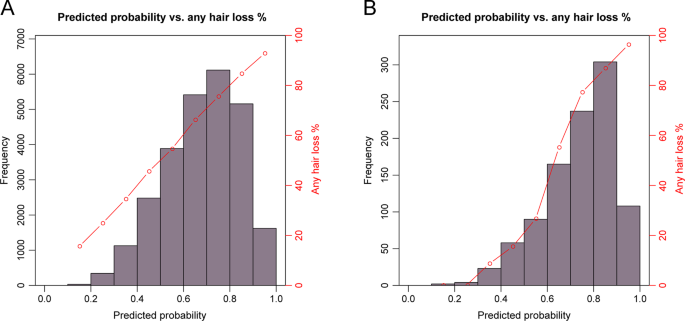
A The probability was derived from MPB prediction model with 117 SNPs and age as predictors in the prediction model testing dataset of 26,177 UKBB men. These prediction results were practically informative for about 26.2% of the tested males (0.4% <0.2 and 25.8% >0.8). B The probability was derived from MPB prediction model with 107 SNPs as predictors without age in the external validation dataset of 991 men from the Bonn Study. These prediction results were practically informative for about 41.8% of the tested males (0.2% <0.2 and 41.6% >0.8). These 117-SNP ( A ) and the 107-SNP ( B ) MPB prediction models were built in the prediction model training dataset of 104,694 UKBB men.
We further investigated the performance of the MLR models over six age groups of the model testing subset, i.e. in men 39–45, 46–50, 51–55, 56–60, 61–65, and 66–72 years of age. Overall, the performance of the predictive models with and without age was similar across these six age groups and the four different MPB categories (Supplementary Fig. 4 ). Slight differences were seen for some MPB categories and some age groups, such as better performance of the model with age for no hair loss in 39–45 years old and for moderate hair loss in 39–45 and 46–50 years old men.
The SVM and ANN machine-learning models provided similar prediction accuracy estimates compared to the M/BLR models (Table 2 ). As with M/BLR models, we also saw for SVM and ANN models that when including age, the prediction accuracy estimates were higher than without age in the models. Notably, seeing similar prediction estimates of accuracy from M/BLR and SVM/ANN methods in such large datasets may imply a lack of epistasis or nonlinear associations. It is unclear if this finding is a reflection of the true genetic architecture of MPB, or may be the consequence of the additive, univariate model used in the SNP discovery GWAS, whose results were carried forward into the ascertainment of the SNP predictors used in the models.
External model validation in Bonn Study data
External validation was conducted in 991 males from the Bonn Study (58.7% early-onset cases, 27% unaffected controls, and 14.2% were population-based). Due to differences in genotyping platforms and quality control analyses, 10 of the 117 SNPs (Supplementary Table 3 ) were unavailable in the Bonn Study. Therefore, we first trained the models based on the 107 SNPs available in Bonn by using the UKBB training subset and validated them in UKBB testing subset. This demonstrated only marginally lower performance of the 107-SNP models compared to the full 117-SNP models in UKBB (AUC loss 0.002–0.006, Table 3 ).
In the Bonn Study, the 107-SNP models that did not include age as predictor, achieved considerably higher prediction accuracy estimates compared to UKBB i.e., 0.830 in Bonn vs. 0.696 in UKBB for no hair loss, 0.775 vs. 0.712 for severe hair loss, and 0.830 vs. 0.696 for any vs. no hair loss. For the slight hair loss phenotype, the AUC in Bonn was slightly higher than in UKBB with 0.599 vs. 0.573, while for moderate hair loss it was slightly lower with 0.591 vs. 0.602 (Table 3 ). For any vs. no hair loss prediction, the BLR model without considering age in Bonn provides accurate prediction for about 41.8% of males, that is, individuals with predicted probabilities of any hair loss <0.2 or >0.8 (0.2% <0.2 and 41.6% >0.8, Fig. 3B ).
For the 107-SNP models that included age as predictor, we achieved considerably less accurate prediction performances in the Bonn Study compared to UKBB (Table 3 ) i.e., AUC of 0.559 in Bonn vs. 0.705 in UKBB for no hair loss, 0.539 vs. 0.722 for severe hair loss, 0.550 vs. 0.705 for any vs. no hair loss, and 0.505 vs. 0.631 for moderate hair loss. For slight hair loss, however, it was higher in Bonn than in UKBB with 0.694 vs. 0.600. For the BLR model predicting any vs. no hair loss, the sensitivity was similarly high with (0.928) and without (0.984) age in Bonn and higher in Bonn than in UKBB (0.915 and 0.919) (Table 3 ). The relative drop in performance of age-inclusive models in the Bonn Study is likely a consequence of age confounding which arises from its case-control design. The deliberate focus in the recruitment of early MBP cases and relatively old super-controls results in a very different age distribution and a negative correlation between MBP and age in this cohort, as opposed to the expected positive correlations expected in the wider population as seen in UKBB.
Reliable genetic predictions for MPB can contribute to different disciplines of scientific research and science applications, including in medicine, evolutionary biology, anthropology, human history, and forensics. Previously reported genetic prediction models for MPB were limited by sparsity of genetic marker coverage, low-powered sample size, and non-independence of data used in the different analytical steps of prediction modelling. Here, we have sought to overcome these previous limitations by developing and validating new genetic MPB prediction models based on a large number of DNA predictors and large and fully independent datasets in the different analytical steps. These new models achieve more accurate and more reliable genetic MPB prediction than previous models.
Previously, Marcińska et al. [ 18 ] applied 20 SNPs from only 10 MPB-associated loci and achieved an AUC of 0.66 to discriminate between male subjects with vs. without significant baldness, in a model that did not include age as a predictor. The prediction accuracy we achieved here with our 117-SNP model without age for any vs. no hair loss was considerably higher (AUC = 0.702) than that reported by Marcińska et al. [ 18 ]. This improvement is likely due to the effect of the largely increased number of SNP predictors that we included in our models by taking advantage of recently published GWAS-based marker knowledge.
For another previous genetic MPB prediction model based on 11 SNPs from only 7 genetic loci plus age, Liu et al. [ 16 ] reported an AUC of 0.711 for no hair loss vs. any hair loss, which surprisingly was the same as what we achieved with our 117-SNP model with age (Table 2 ). Aiming to understand this apparent similarity of AUC while using more than 100 additional DNA preditors, we build a MPB prediction model in the UKBB model training subset based on 10 available of the 11 SNPs used by Liu et al. [ 16 ]. Considering age as additional predictor, this 10-SNP model achieved an AUC of 0.611 in the UKBB model testing subset (Supplementary Table 4 ), considerably lower than the AUC = 0.711 reported by Liu et al. for all 11 SNPs [ 16 ]. This 0.1 difference in AUC cannot be explained by the one missing SNP duplicated? in this comparative analysis. Rather, this may be explained by the larger effect of age on MPB prediction in the Rotterdam Study [ 16 ] (a contribution of 0.171 to the AUC), an ageing cohort with mean age of 68 years, considerably older than the UKBB (mean age 57) where age contributes only 0.06 to the AUC. Alternatively, higher prediction accuracies may result from the better investigator-conducted phenotyping in the Rotterdam Study compared with the self-reported UKBB phenotyping.
The recently reported genetic prediction model for MPB by Hagenaars et al. based on 331 SNPs [ 8 ], including the 270 SNPs we used in our feature selection analysis (the identity of only these 270 SNP predictors out of the 331 was publicly available at the time of writing) was built in 40,000 UKBB participants' samples that were also used for GWAS-based marker discovery. This model was internally validated in 12,874 additional UKBB samples by Hagenaars et al. and achieved AUCs of 0.78 for no vs. severe hair loss, 0.68 for no vs. moderate hair loss, and 0.61 for no vs. slight hair loss without considering age, and 0.79, 0.70 and 0.61, respectively, for models that additionally included age [ 8 ]. A direct comparison of these AUC estimates with the AUCs from our 4-category approach is difficult. In our analysis, we considered all individuals from all four categories to estimate AUC per each category, while Hagenaars et al. left out data that did not fit the predicted categories. Although we believe that using all data is the better way, we attempted a direct comparison by estimating the prediction accuracy of a model based on our 117 SNP predictors using the same analytical approach as Hagenaars et al. [ 8 ]. In our UKBB model testing subset, this analysis revealed AUC values of 0.81 for no vs. severe hair loss, 0.73 for no vs. moderate hair loss, and 0.63 for no vs. slight hair loss when age is included as a predictor, which were higher than those previously reported by Hagenaars et al. This different prediction performance may be explained by the larger model training dataset we used here, which generally increases the predictive performance of statistical models. These results, however, provide no evidence that the Hagenaars et al. approach of performing marker discovery and model training in the very same dataset led to over-inflated prediction accuracy.
Our study also demonstrates that it still is a long way towards highly accurate genetic prediction of MPB such as already available for eye colour with AUCs of 0.94 [ 3 , 4 ]. As next further step, all of the 624 MPB-associated loci identified in the entire UKBB data resource of >200,000 male subjects [ 2 ] shall be used for prediction modelling. This, however, can only be done once sufficiently large independent datasets for model training, testing, and external validation become available in the future. It is possible though, as implied by the results of our feature selection analysis, that inclusion of more SNPs may not necessarily lead to the envisioned significant improvement of MPB prediction accuracy, unless perhaps a large number is added. This is because GWASs with larger sample size are known to discover associated SNPs with smaller allelic effect sizes, which are expected to have smaller predictive value compared to SNPs previously identified in smaller-sized GWASs included in the current models. Although the new prediction models that we introduce here can reliably predict a good proportion of MPB risk in subjects of European ancestry and thus have good potential for practical use, future models may benefit from additional predicting biomarkers, for which also other types of biomarkers, such as epigenomic and metabolomic biomarkers, should be investigated.
In summary, we introduce new genetic prediction models for MPB that overcome limitations of previous models by including a large number of genetic predictors and utilizing large and independent datasets for the different analytical steps. Therefore our new models can be considered the most reliable genetic prediction models available to date for MPB and any other human appearance trait.
Data availability
The UK Biobank data are available from the UK Biobank ( https://www.ukbiobank.ac.uk/ ).
Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57:9–17.
Article CAS PubMed Google Scholar
Yap CX, Sidorenko J, Wu Y, Kemper KE, Yang J, Wray NR, et al. Dissection of genetic variation and evidence for pleiotropy in male pattern baldness. Nat Commun. 2018;9:5407.
Article CAS PubMed PubMed Central Google Scholar
King TE, Fortes GG, Balaresque P, Thomas MG, Balding D, Delser PM, et al. Identification of the remains of King Richard III. Nat Commun. 2014;5:5631.
Walsh S, Liu F, Wollstein A, Kovatsi L, Ralf A, Kosiniak-Kamysz A, et al. The HIrisPlex system for simultaneous prediction of hair and eye colour from DNA. Forensic Sci Int Genet. 2013;7:98–115.
Wilde S, Timpson A, Kirsanow K, Kaiser E, Kayser M, Unterlander M, et al. Direct evidence for positive selection of skin, hair, and eye pigmentation in Europeans during the last 5,000 y. Proc Natl Acad Sci USA. 2014;111:4832–7.
Brace S, Diekmann Y, Booth TJ, van Dorp L, Faltyskova Z, Rohland N, et al. Ancient genomes indicate population replacement in Early Neolithic Britain. Nat Ecol Evol. 2019;3:765–71.
Article PubMed PubMed Central Google Scholar
Heilmann-Heimbach S, Herold C, Hochfeld LM, Hillmer AM, Nyholt DR, Hecker J, et al. Meta-analysis identifies novel risk loci and yields systematic insights into the biology of male-pattern baldness. Nat Commun. 2017;8:14694.
Hagenaars SP, Hill WD, Harris SE, Ritchie SJ, Davies G, Liewald DC, et al. Genetic prediction of male pattern baldness. PLoS Genet. 2017;13:e1006594.
Pirastu N, Joshi PK, de Vries PS, Cornelis MC, McKeigue PM, Keum N, et al. GWAS for male-pattern baldness identifies 71 susceptibility loci explaining 38% of the risk. Nat Commun. 2017;8:1584.
Giles GG, Severi G, Sinclair R, English DR, McCredie MR, Johnson W, et al. Androgenetic alopecia and prostate cancer: findings from an Australian case-control study. Cancer Epidemiol, Biomark Prev. 2002;11:549–53.
Google Scholar
Norwood OT. Male pattern baldness: classification and incidence. South Med J. 1975;68:1359–65.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9.
Hamilton JB. Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. 1951;53:708–28.
Abadi M. TensorFlow: learning functions at scale. Acm Sigplan Not. 2016;51:1–1.
Article Google Scholar
Kingma DP, Ba J: Adam: A method for stochastic optimization. Preprint at https://arxiv.org/abs/1412.6980 .
Liu F, Hamer MA, Heilmann S, Herold C, Moebus S, Hofman A, et al. Prediction of male-pattern baldness from genotypes. Eur J Hum Genet. 2016;24:895–902.
Article PubMed Google Scholar
Hillmer AM, Hanneken S, Ritzmann S, Becker T, Freudenberg J, Brockschmidt FF, et al. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Am J Hum Genet. 2005;77:140–8.
Marcinska M, Pospiech E, Abidi S, Andersen JD, van den Berge M, Carracedo A, et al. Evaluation of DNA variants associated with androgenetic alopecia and their potential to predict male pattern baldness. PLoS One. 2015;10:e0127852.
Download references
Acknowledgements
The authors are grateful to all participants of the UKBB Study and the Bonn Study for their participation. This study received support from the European Union’s Horizon 2020 Research and Innovation programme under grant agreement No 740580 within the framework of the Visible Attributes through Genomics (VISAGE) Project. YC was supported by the University of Chinese Academy of Science (UCAS) Joint PhD Training Program. None of the funding organisations had any influence on the design, conduct or conclusions of the study.
Author information
Authors and affiliations.
Department of Genetic Identification, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands
Yan Chen, Fan Liu & Manfred Kayser
Key Laboratory of Genomic and Precision Medicine, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing, China
Yan Chen & Fan Liu
University of Chinese Academy of Sciences, Beijing, China
Department of Twin Research and Genetic Epidemiology, King’s College London, London, United Kingdom
Pirro Hysi & Timothy D. Spector
Institute for Genomic Statistics and Bioinformatics (IGSB), University of Bonn, School of Medicine & University Hospital Bonn, Bonn, Germany
Institute of Human Genetics, University of Bonn, School of Medicine & University Hospital Bonn, Bonn, Germany
Stefanie Heilmann-Heimbach
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualisation: FL, MK; data curation: PH, YC, CM, SH-H, TDS; formal analysis: YC, CM; result visualisation: YC; funding acquisition: YC, MK; resources: PH, TDS, SH-H, MK; supervision: PH, SH-H, TDS, FL, MK; writing – first draft: YC, PH, FL, MK; writing – revised: YC, PH, FL, CM, MK; writing – final: YC, PH, FL, MK; final approval: YC, PH, CM, SH-H, TDS, FL, MK.
Corresponding author
Correspondence to Manfred Kayser .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethical approval
The UKBB Study was approved by the North West Multi-center Research Ethics Committee (MREC) with details provided elsewhere ( https://www.ukbiobank.ac.uk/the-ethics-and-governance-council ). The Bonn Study was approved by the Ethics Committees of the Universities of Bonn, Düsseldorf and Essen, and informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Chen, Y., Hysi, P., Maj, C. et al. Genetic prediction of male pattern baldness based on large independent datasets. Eur J Hum Genet 31 , 321–328 (2023). https://doi.org/10.1038/s41431-022-01201-y
Download citation
Received : 03 May 2022
Revised : 15 September 2022
Accepted : 20 September 2022
Published : 07 November 2022
Issue Date : March 2023
DOI : https://doi.org/10.1038/s41431-022-01201-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Genes=disease ().
- Alisdair McNeill
European Journal of Human Genetics (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
MIT Technology Review
- Newsletters
Going bald? Lab-grown hair cells could be on the way
These biotech companies are reprogramming cells to treat baldness, but it’s still early days.
- Antonio Regalado archive page

Biologists at several startups are applying the latest advances in genetic engineering to the age-old problem of baldness, creating new hair-forming cells that could restore a person’s ability to grow hair.
Some researchers tell MIT Technology Review they are using the techniques to grow human hair cells in their labs and even on animals. A startup called dNovo sent us a photograph of a mouse sprouting a dense clump of human hair—the result of a transplant of what the company says are human hair stem cells.
The company’s founder is Ernesto Lujan, a Stanford University–trained biologist. He says his company can produce the components of hair follicles by genetically “reprogramming” ordinary cells, like blood or fat cells. More work needs to be done, but Lujan is hopeful that the technology could eventually treat “the underlying cause of hair loss.”
We’re born with all the hair follicles we’ll ever have—but aging, cancer, testosterone, bad genetic luck, even covid-19 can kill the stem cells inside them that make hair. Once these stem cells are gone, so is your hair. Lujan says his company can convert any cell directly into a hair stem cell by changing the patterns of genes active in it.
In biology, we “now understand cells as a ‘state’” rather than a fixed identity, says Lujan. “And we can push cells from one state to another.”
Reprogramming cells
The chance of replacing hair is one corner in a wider exploration of whether reprogramming technology can defeat the symptoms of aging. In August, MIT Technology Review reported on a stealthy company, Altos Labs , that plans to explore whether people can be rejuvenated using reprogramming. Another startup, Conception , is trying to extend fertility by converting blood cells into human eggs.
A key breakthrough came in the early 2000s, when Japanese researchers hit on a simple formula to turn any type of tissue into powerful stem cells, similar to ones in an embryo. Imaginations ran wild. Scientists realized they could potentially manufacture limitless supplies of nearly any type of cell—say, nerves or heart muscle.
In practice, though, the formula for producing specific cell types can prove elusive, and then there’s the problem of getting lab-grown cells back into the body. So far, there have been only a few demonstrations of reprogramming as a way to treat patients. Researchers in Japan tried transplanting retina cells into blind people. Then, last November, a US company, Vertex Pharmaceuticals, said it might have cured a man’s type 1 diabetes after an infusion of programmed beta cells, the kind that respond to insulin.
The concept startups are pursuing is to collect ordinary cells such as skin cells from patients and then convert these into hair-forming cells. In addition to dNovo, a company called Stemson (its name is a portmanteau of “stem cell” and “Samson”) has raised $22.5 million from funders including from the drug company AbbVie. Cofounder and CEO Geoff Hamilton says his company is transplanting reprogrammed cells onto the skin of mice and pigs to test the technology.
Both Hamilton and Lujan think there is a substantial market. About half of men undergo male-pattern baldness, some starting in their 20s. When women lose hair, it’s often a more general thinning, but it’s no less a blow to self-image.
These companies are bringing high-tech biology to an industry known for illusions. There are plenty of bogus claims about both hair-loss remedies and the potential of stem cells. “You’ve got to be aware of scam offerings,” Paul Knoepfler, a stem-cell biologist at UC Davis, wrote in November .
Tricky business
So is stem-cell technology going to cure baldness or become the next false hope? Hamilton, who was invited to give the keynote at this year’s Global Hair Loss Summit , says he tried to emphasize that the company still has plenty of research ahead of it. “We have seen so many [people] come in and say they have a solution. That has happened a lot in hair, and so I have to address that,” he says. “We’re trying to project to the world that we are real scientists and that it's risky to the point I can’t guarantee it’s going to work.”
Right now, there are some approved drugs for hair loss, like Propecia and Rogaine, but they’re of limited use. Another procedure involves cutting strips of skin from someplace where a person still has hair and surgically transplanting those follicles onto a bald spot. Lujan says in the future, hair-forming cells grown in the lab could be added to a person’s head with a similar surgery.
“I think people will go pretty far to get their hair back. But at first it will be a bespoke process and very costly,” says Karl Koehler, a professor at Harvard University.
Hair follicles are surprisingly complicated organs that arise through the molecular crosstalk between several cell types. And Koehler says pictures of mice growing human hair aren't new. “Anytime you see these images,” says Koehler, “there is always a trick, and some drawback to translating it to humans.”
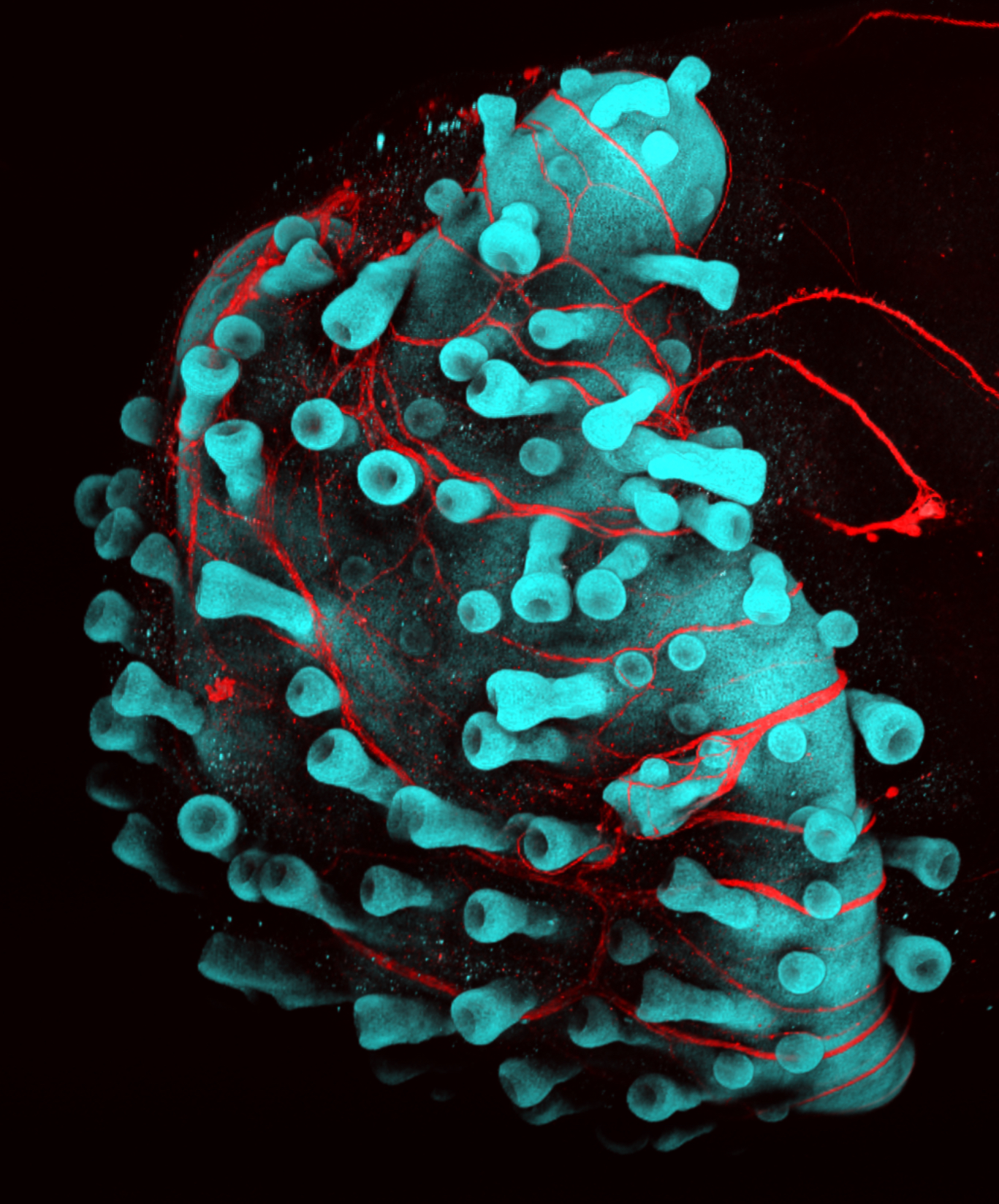
Koehler’s lab makes hair shafts in an entirely different way—by growing organoids. Organoids are small blobs of cells that self-organize in a petri dish. Koehler says he originally was studying deafness cures and wanted to grow the hair-like cells of the inner ear. But his organoids ended up becoming skin instead, complete with hair follicles.
Koehler embraced the accident and now creates spherical skin organoids that grow for about 150 days, until they are around two millimeters across. The tube-like hair follicles are clearly visible; he says they are the equivalent of the downy hair that covers a fetus.
One surprise is that the organoids grow backwards, with the hairs pointing in. “You can see a beautiful architecture, although why they grow inside out is a big question,” says Koehler.
Biotechnology and health
An ai-driven “factory of drugs” claims to have hit a big milestone.
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
- Cassandra Willyard archive page
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
- Jessica Hamzelou archive page
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from mit technology review.
Discover special offers, top stories, upcoming events, and more.
Thank you for submitting your email!
It looks like something went wrong.
We’re having trouble saving your preferences. Try refreshing this page and updating them one more time. If you continue to get this message, reach out to us at [email protected] with a list of newsletters you’d like to receive.
Skip to content
Explore CUIMC
Diversity, equity, and inclusion.
At CUIMC, we are committed to continuous improvement in providing culturally inclusive medical education and clinical care.
Columbia Vagelos College of Physicians and Surgeons is dedicated to developing the next generation of leaders in medicine
Patient Care
- Find a Doctor
Search for a provider by specialty, expertise, location and insurance. Schedule an appointment online.
Read the latest news stories about CUIMC faculty, research, and events
The Key to Locks: Columbia Team’s Breakthrough Led to Hair Loss Treatment
Share this page.
- Share on Facebook
- Share on X (formerly Twitter)
- Share on LinkedIn
- Share by email
For over a decade, Columbia geneticist Angela Christiano , PhD, has attended the annual meeting of the National Alopecia Areata Foundation, where hundreds of individuals affected by the hair loss disorder gather to support one another and learn about the latest scientific research. The meeting is a safe space where patients with alopecia, many of whom have lost all their hair, joyfully remove their wigs and head coverings for the three-day celebration, without fear of shame or judgment.
But this year’s meeting was a bit different. Christiano had trouble recognizing conference attendees she’s known and worked with for years, because many of them now have full heads of hair.
For people with alopecia areata, an autoimmune disease that can cause hair loss so complete that people even lose their eyebrows, the change in appearance was dramatic.
drug_restores_hair_growth_in_patients_with_alopecia_areata
It was also a direct result of Christiano’s groundbreaking research on the condition, which led the FDA in June to approve the first systemic treatment specifically developed for severe alopecia areata.
“It’s a strange feeling. It’s what every geneticist dreams of, to find the genes for a condition and develop a treatment that can directly benefit patients. But it’s extremely rare that it actually works out that way,” says Christiano, who has studied alopecia areata for more than 20 years, motivated by her own bout with the disease.
Mysterious origins
Unlike hormone-driven male pattern baldness, alopecia areata is an autoimmune disease in which the body’s own immune system mistakenly attacks the hair follicle and shuts down hair production.
When Christiano began working on it, though, nobody knew exactly what caused the problem.
Starting with a series of basic research studies on the genetics and cell biology of hair growth, Christiano and a multidisciplinary team of collaborators produced a steady stream of advances, first in the lab and then in the clinic.
The first major clue came in 2010, from a study led by Christiano’s team that looked through the genomes of a thousand patients. The study, published in Nature, uncovered a gene that, when abnormally expressed, produces a known "danger signal" that causes the body to recognize the hair follicle as foreign.
The genome study was also crucial since the findings also explained why previous efforts to treat the condition hadn’t worked.
“Drugs for other autoimmune skin diseases had been tested in alopecia, but they had largely failed,” Christiano says.
“At that point, we realized that was because alopecia doesn’t share genetic pathways with other autoimmune skin diseases.”
Taming killer T cells
The genome study led the team to focus on investigating a particular kind of "killer" T cell recruited by the danger signal, which became central to understanding the mechanism of hair follicle destruction.
Columbia alopecia team . Dermatologist Julian Mackay-Wiggan (left), immunologist Raphael Clynes (center), and geneticist Angela Christiano (right) made key discoveries that have led to a new drug for alopecia areata, an autoimmune disease that can cause severe hair loss. Photo: Columbia University Irving Medical Center.
Christiano is not an immunologist, so she needed to enlist an expert to help make inroads into understanding the behavior of these cells. She approached Raphael Clynes, MD, PhD, at that time a faculty member in the Department of Medicine, who was an expert in studying the same kinds of killer T cells in type 1 diabetes and in cancer.
Clynes looked at the list of genes from the genome study and images of the "swarm" of killer T cells surrounding the hair follicle and suggested that inhibiting enzymes known as JAK kinases might be one way to treat the disease.
The team showed that small molecule drugs called JAK inhibitors could shut down signaling inside the killer T cells. Amazingly, by inhibiting the JAK pathway, the team found they could reverse alopecia areata in a mouse model of the disease.
Dramatic regrowth of hair
Armed with photos of mice with alopecia that had regrown all their hair, Christiano next approached her colleague Julian Mackay-Wiggan, MD, a Columbia dermatologist who specialized in hair disorders and had an interest in early-stage clinical research.
Excited by the early results in the mice, Mackay-Wiggan began treating a few patients with alopecia areata using JAK inhibitors that were already FDA-approved for other disorders. The first few patients experienced dramatic regrowth of their hair, just as the researchers had observed in the mice. Christiano’s team reported these groundbreaking studies in 2014.
Building on these early results, Mackay-Wiggan conducted additional Columbia clinical studies that showed that 75% of patients experienced significant hair regrowth after treatment with two different JAK inhibitors.
Pharma attention
Soon after the Columbia team reported its findings, additional case reports began appearing in the published literature that replicated the results in alopecia patients from around the world.
Because there were no FDA-approved drugs for alopecia when their work began, it didn’t take long for pharmaceutical companies to turn their attention to developing JAK inhibitors specifically for alopecia treatment. These efforts led to newly approved Olumiant from Eli Lilly, Incyte (previously approved for rheumatoid arthritis and hospitalized patients with COVID-19), and two additional JAK inhibitors being developed by Pfizer and Concert Pharmaceuticals and now in late-stage clinical trials.
Christiano welcomes the pharmaceutical companies’ new attention to alopecia areata after the condition had long been neglected and was frequently dismissed as a cosmetic problem.
For patients with complete hair loss, the barrage of stares and intrusive questions can be demoralizing and psychologically devastating. “It’s the stigma of unwanted attention; how do you quantify that?” says Christiano. “The impact of this treatment on patients has been truly transformative.”
Male pattern baldness next?
While Olumiant and other new JAK inhibitors are often life-changing for patients who respond well to them, the treatments are still far from perfect.
“These are potent immunosuppressive drugs, so there are safety considerations to be taken into account when assessing the risk/benefit ratio for individual patients,” says Christiano. After the treatment ends, some patients’ alopecia relapses for reasons the researchers don’t fully understand. In addition, about a third of patients don’t respond to the drugs.
Fortunately, Christiano has no intention of resting on her laurels. Her lab is hoping to understand what causes the condition to relapse after JAK inhibitor treatment.
And by continuing to investigate alopecia areata with new tools and techniques, her team is developing both new biological insights and more potential ways to attack the disease process. “We’re now looking upstream of the JAK signaling pathway to see if other mechanisms can lead to the common endpoint of alopecia areata,” she says.
Christiano’s team also hopes to extend these insights and apply the same approaches to treat other types of hair loss.
Hair follicles grown in a dish in the Christiano lab. Made possible with 3D printing technology, engineered human hair follicles created in this way could generate an unlimited source of new hair follicles for patients undergoing robotic hair restoration surgery. Read more .
In one recent study, for example, Christiano’s team found that JAK inhibitors also reawaken dormant hair follicles , a problem common to male and female pattern baldness.
They also discovered a previously unknown type of immune cell that puts hair follicles into a dormant state by secreting a substance called oncostatin M and that the hair cycle can be reactivated by blocking this pathway.
While translating these results into effective treatments for hair loss will likely take years, it’s a process Christiano now knows well.
More information
Angela M. Christiano, PhD, is the Richard and Mildred Rhodebeck Professor of Dermatology, vice chair of research in the Department of Dermatology, and professor of genetics & development at Columbia University Vagelos College of Physicians and Surgeons. She also serves as an Advisory Dean for Basic Science Faculty. In 2020, Christiano was elected to the National Academy of Sciences.
Raphael Clynes, MD, PhD is currently vice president of translational biology at Xencor Inc.
Julian Mackay-Wiggan, MD, MPH, is currently in practice in the Siperstein Dermatology Group.
Angela Christiano and Raphael Clynes are co-inventors on several patents filed by Columbia University on the use of JAK inhibitors in treating hair loss disorders, which have been licensed to Aclaris Therapeutics, Inc. Angela Christiano is a shareholder of Aclaris Therapeutics, Inc. and has served as a consultant/scientific advisor for Arcutis Biotherapeutics, Inc., Almirall, S.A., Aclaris Therapeutics, Inc., Bioniz Therapeutics, Inc., Dermira, Inc., Intrinsic Medicine, Inc., Janssen Pharmaceuticals, Inc., and Pfizer, Inc. She is a shareholder of Intrinsic Medicine, Inc., has received research grant support from Bristol-Myers Squibb, Inc., Pfizer, Inc., and Sanofi Genzyme S.A. and serves on/chairs the scientific advisory boards for the Dystrophic EB Research Association of America and the National Alopecia Areata Foundation. She previously served as president of the Society for Investigative Dermatology and currently serves as president of the American Hair Research Society. She is a scientific co-founder of Rapunzel Bioscience.
Hair Loss News
Top headlines, latest headlines.
- Gel to Promote Hair Follicle Growth
- How Humans Lost Their Body Hair
- Microneedle Patches Restore Hair in Balding Mice
- Preventing Hearing Loss Caused by Antibiotics
- Discovery Could Help End Baldness, Speed Healing
- Molecule Potently Stimulates Hair Growth
- Surprising Link Between Immune System, Hair ...
- Shedding Light On Early Human Hair Evolution
- Eating Less Fat May Save Your Hair
- Treating the ‘Root’ Cause of Baldness
Earlier Headlines
- LATEST NEWS
- Health & Medicine
- Diseases & Conditions
- Alzheimer's Research
- Amyotrophic Lateral Sclerosis
- Attention Deficit Disorder
- Back and Neck Pain
- Birth Defects
- Bladder Disorders
- Blood Clots
- COVID and SARS
- Cervical Cancer
- Bladder Cancer
- Multiple Myeloma
- Pancreatic Cancer
- Brain Tumor
- Colon Cancer
- Breast Cancer
- Ovarian Cancer
- Lung Cancer
- Mesothelioma
- Skin Cancer
- Prostate Cancer
- Cerebral Palsy
- Chikungunya
- Chronic Fatigue Syndrome
- Cold and Flu
- Crohn's Disease
- Cystic Fibrosis
- Dengue Fever
- Down Syndrome
- Eating Disorder Research
- Encephalitis
- Epilepsy Research
- Erectile Dysfunction
- Fibromyalgia
- Gastrointestinal Problems
- HIV and AIDS
- Headache Research
- Hearing Loss
- Heart Health
- Cholesterol
- Stroke Prevention
- Heart Disease
- Hormone Disorders
- Hypertension
- Infectious Diseases
- Insomnia Research
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Lung Disease
- Lyme Disease
- Mental Health Research
- Multiple Sclerosis Research
- Mumps, Measles, Rubella
- Muscular Dystrophy
- Osteoporosis
- Parkinson's Research
- Prostate Health
- Restless Leg Syndrome
- Sickle Cell Anemia
- Sleep Disorder Research
- Thyroid Disease
- Triglycerides
- Tuberculosis
- Medical Topics
- Accident and Trauma
- Alternative Medicine
- Birth Control
- Bone and Spine
- Chronic Illness
- Controlled Substances
- Dietary Supplements and Minerals
- Epigenetics
- Food Additives
- Foodborne Illness
- Foot Health
- Gene Therapy
- Health Policy
- Human Biology
- Immune System
- Joint Health
- Medical Imaging
- Nervous System
- Pain Control
- Personalized Medicine
- Pharmacology
- Psychology Research
- Wounds and Healing
- PHYSICAL/TECH
- ENVIRONMENT
- SOCIETY & EDUCATION
- Illuminating Oxygen's Journey in the Brain
- DNA Study IDs Descendants of George Washington
- Heart Disease Risk: More Than One Drink a Day
- Unlocking Supernova Stardust Secrets
- Why Do Some Memories Become Longterm?
- Cell Division Quality Control 'Stopwatch'
- What Controls Sun's Differential Rotation?
- Robot, Can You Say 'Cheese'?
- Researchers Turn Back the Clock On Cancer Cells
- Making Long-Term Memories: Nerve-Cell Damage

Trending Topics
Strange & offbeat.
Loading metrics
Open Access
Peer-reviewed
Research Article
Genetic prediction of male pattern baldness
Contributed equally to this work with: Saskia P. Hagenaars, W. David Hill
Affiliations Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, United Kingdom, Department of Psychology, University of Edinburgh, Edinburgh, United Kingdom, Division of Psychiatry, University of Edinburgh, Edinburgh, United Kingdom
Affiliations Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, United Kingdom, Department of Psychology, University of Edinburgh, Edinburgh, United Kingdom
Affiliations Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, United Kingdom, Centre for Genomic and Experimental Medicine, Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, United Kingdom
Affiliation Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, United Kingdom
Affiliations Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, United Kingdom, Department of Psychology, University of Edinburgh, Edinburgh, United Kingdom, Medical Research Council Lifecourse Epidemiology Unit, University of Southampton, Southampton, United Kingdom
* E-mail: [email protected]
- Saskia P. Hagenaars,
- W. David Hill,
- Sarah E. Harris,
- Stuart J. Ritchie,
- Gail Davies,
- David C. Liewald,
- Catharine R. Gale,
- David J. Porteous,
- Ian J. Deary,
- Riccardo E. Marioni

- Published: February 14, 2017
- https://doi.org/10.1371/journal.pgen.1006594
- See the preprint
- Reader Comments
Male pattern baldness can have substantial psychosocial effects, and it has been phenotypically linked to adverse health outcomes such as prostate cancer and cardiovascular disease. We explored the genetic architecture of the trait using data from over 52,000 male participants of UK Biobank, aged 40–69 years. We identified over 250 independent genetic loci associated with severe hair loss (P<5x10 -8 ). By splitting the cohort into a discovery sample of 40,000 and target sample of 12,000, we developed a prediction algorithm based entirely on common genetic variants that discriminated (AUC = 0.78, sensitivity = 0.74, specificity = 0.69, PPV = 59%, NPV = 82%) those with no hair loss from those with severe hair loss. The results of this study might help identify those at greatest risk of hair loss, and also potential genetic targets for intervention.
Author summary
Living with male pattern baldness can be stressful and embarrassing. Previous studies have shown baldness to have a complex genetic architecture, with particularly strong signals on the X chromosome. However, these studies have been limited by small sample sizes. Here, we present the largest genome-wide study of baldness to date, using data from over 52,000 male participants in the UK Biobank study. We identify over 200 novel findings. We also split our dataset in two to build and apply a genetic predictor of baldness. Of those with a polygenic score below the median, 14% had severe hair loss and 39% no hair loss. By contrast, of those with a polygenic score in the top 10%, 58% reported moderate-to-severe hair loss.
Citation: Hagenaars SP, Hill WD, Harris SE, Ritchie SJ, Davies G, Liewald DC, et al. (2017) Genetic prediction of male pattern baldness. PLoS Genet 13(2): e1006594. https://doi.org/10.1371/journal.pgen.1006594
Editor: Markus M. Noethen, University of Bonn, GERMANY
Received: August 18, 2016; Accepted: January 21, 2017; Published: February 14, 2017
Copyright: © 2017 Hagenaars et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Summary data from this paper can be downloaded from the Centre for Cognitive Ageing and Cognitive Epidemiology website ( http://www.ccace.ed.ac.uk/node/335 ).
Funding: This research was conducted, using the UK Biobank Resource, in The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross-council Lifelong Health and Wellbeing Initiative (MR/K026992/1). Funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and Medical Research Council (MRC) is gratefully acknowledged. WDH is supported by a grant from Age UK (Disconnected Mind Project). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: I have read the journal's policy and the authors of this manuscript have the following competing interests: IJD and DJP are participants in UK Biobank.
Introduction
Male pattern baldness affects around 80% of men by the age of 80 years [ 1 ], and it can have substantial psychosocial impacts via changes in self-consciousness and social perceptions [ 2 , 3 ]. In addition to alterations in physical appearance, some, but not all, studies have identified negative health outcomes associated with baldness including increased risk of prostate cancer [ 4 – 6 ] and cardiovascular disease [ 7 – 9 ]. Baldness is known to be substantially heritable [ 10 ]. Here, we use a large, population-based dataset to identify many of the genes linked to variation in baldness, and build a genetic score to improve the prediction of severe hair loss.
The total proportion of variance in male pattern baldness that can be attributed to genetic factors has been estimated in twin studies to be approximately 80% for both early- and late-onset hair loss [ 11 , 12 ]. Newer molecular-genetic methods have estimated the single-nucleotide polymorphism (SNP)-based, common-variant heritability of baldness at around 50% [ 13 ]. Molecular methods also indicate a degree of overlap between genetic variants linked to baldness and those linked to phenotypes such as height, waist-hip ratio, age at voice drop in males, age at menarche in females, and presence of a unibrow [ 14 ].
A number of studies have identified specific genetic variants linked to variations in baldness, usually with the AR gene showing the strongest association. The largest published genome-wide association study (GWAS) to date highlighted eight independent genetic loci that were linked to baldness; the top AR SNP yielded an odds ratio of 2.2 in a case-control meta-analysis of 12,806 individuals of European ancestry [ 15 ]. One of the autosomal hits identified in that study was found to be in a gene linked to Parkinson’s disease. More recently, a review paper highlighted fifteen loci from six studies that have been associated at genome-wide significance (P<5x10 -8 ) with baldness; two of these were located on the X chromosome [ 16 ].
Several attempts have been made to build predictors of male pattern baldness using polygenic risk scores. Heilmann et al. found, using a case-control design with ~600 per arm, that a predictor based on 34,186 SNPs explained 4.5% of the variance on the liability scale [ 17 ]. Marcińska et al. used candidate genes to build 5-SNP and 20-SNP polygenic predictors, which performed well when considering prediction of early-onset male pattern baldness, but poorly when considering those with no baldness versus those with severe baldness across all ages [ 18 ]. Most recently, a 20-SNP predictor was assessed in three European studies [ 13 ]. It achieved a maximum Area Under the Curve (AUC) prediction of 0.74 in an early-onset cohort, but weaker estimates in the other two, late-onset cohorts (AUC = 0.69 and 0.71). These values correspond to poor-to-fair predictions of baldness. In addition, in that study, age was included in the predictor, explaining the bulk of the differences. A meta-analysis of the three cohorts’ GWAS studies identified a novel locus on chromosome 6. The study also estimated the SNP-based heritability of early-onset (56% (SE 22%) from the autosomes, 23% (SE 1.1%) from the X chromosome) and late-onset baldness (42% (SE 23%) from the autosomes, 10% (SE 5%) from the X chromosome).
The present study
The UK Biobank study [ 19 ] ( http://www.ukbiobank.ac.uk ) is a large, population-based genetic epidemiology cohort. At its baseline assessment (2006–2010), around 500,000 individuals aged between 40 and 70 years and living in the UK completed health and lifestyle questionnaires and provided biological samples for research.
The present study reports a GWAS of male pattern baldness in the UK Biobank cohort, which is over four times the size of the previously-largest meta-analytic study [ 15 ]. After completing the GWAS, we split the cohort into a large ‘discovery’ sample of 40,000 participants in which the GWAS was re-run. The regression weights from this GWAS were used to perform a prediction analysis in the sub-sample of 12,000 participants who did not contribute to the GWAS. We determined the accuracy of the polygenic profile score by discriminating between those with severe hair loss and those with no hair loss.
The mean age of the 52,874 men was 57.2 years (SD 8.0). 16,724 (31.6%) reported no hair loss, 12,135 (23.0%) had slight hair loss, 14,234 (26.9%) had moderate hair loss, and 9,781 (18.5%) had severe hair loss.
The genome-wide association study of the four-category self-reported baldness measure in 52,874 White British men from UK Biobank yielded 13,029 autosomal hits from the imputed data (P<5x10 -8 ), in addition to 117 hits (out of 14,350 genotyped SNPs) on the X chromosome ( Fig 1 ). The QQ plot for the autosomal GWAS is shown in S1 Fig . An LD clumping analysis indicated that these hits can be attributed to 247 independent autosomal regions. All previously reported autosomal hits [ 10 , 13 – 16 ] that mapped to SNPs in our study (62 out of 68 SNPs) replicated with a maximum P-value of 0.006 (54 out of 62 lookups had P<5x10 -8 , S1 Table ). The previously reported X chromosome variant from Li et al. [ 15 ] and the variant from Richards et al. [ 10 ] also replicated with P-values that were effectively zero ( S1 Table ). The chromosome 6 hit (rs4959410) from Liu et al. [ 13 ], which was not supported by additional SNPs in the region, failed to replicate (P = 0.37). All other hits from Liu et al. [ 13 ] had been previously reported in the literature. A list of the top 20 independent autosomal hits are presented in Table 1 . The top 10 independent X chromosome hits are presented in Table 2 ; rs140488081 and rs7061504 are intronic SNPs in the OPHN1 gene. After conditioning on the top SNP (rs73221556), 47 SNPs (including the two lead X chromosome SNPs from the literature: rs2497938 and rs6625163) remained significant at P<5x10 -8 . In the UK Biobank data, the two lead SNPs from the literature were in very high LD (R 2 = 0.98). Summary output for all of the SNPs is available at the following URL: http://www.ccace.ed.ac.uk/node/335 . A list of the 287 independent loci are reported in S2 Table .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pgen.1006594.g001
https://doi.org/10.1371/journal.pgen.1006594.t001
https://doi.org/10.1371/journal.pgen.1006594.t002
The gene-based analysis identified 112 autosomal genes and 13 X chromosome genes that were associated with baldness after a Bonferroni correction (P<0.05/18,061 and P<0.05/567, respectively). The top gene-based hit was, as expected, the androgen receptor on the X chromosome (P = 2.0x10 -269 ). A full list of the autosomal significant gene-based hits is provided in S3 Table and significant genes on the X chromosome are shown in S4 Table . A significant enrichment (FDR <0.05) was found for 143 gene sets; the full results are presented in S5 Table .
Using common genetic variants with a minor allele frequency of at least 1%, GCTA-GREML analysis found that 47.3% (SE 1.3%) of the variance in baldness can be explained by common autosomal genetic variants, while 4.6% (SE 0.3%) can be explained by common X chromosome variants.
Genetic correlations were examined between male pattern baldness and 24 cognitive, health, and anthropometric traits using LD Score regression. No significant associations were found; all estimates were close to zero ( S6 Table ).
The GWAS for self-reported baldness was re-run on a sub-sample of 40,000 individuals—retaining an equal proportion of each of the four baldness patterns as observed in the full cohort—to allow a polygenic prediction score to be built and applied to the remaining, independent sample of 12,874 individuals. The most powerful predictions from comparing the extreme phenotype groups were observed at the P<1x10 -5 threshold for both the autosomal and X chromosome polygenic scores ( Table 3 ). The optimal autosomal polygenic score yielded an AUC of 0.75 for discriminating between those with no hair loss (n = 4,123) and those with severe hair loss (n = 2,456). The corresponding AUC for the optimal X chromosome polygenic score was 0.62. An additive combination of the autosomal and X chromosome polygenic scores gave an AUC of 0.78 (sensitivity = 0.74, specificity = 0.69, PPV = 0.59, NPV = 0.82) for severe hair loss, 0.68 (sensitivity = 0.66, specificity = 0.61, PPV = 0.58, NPV = 0.68) for moderate hair loss, and 0.61 (sensitivity = 0.64, specificity = 0.53, PPV = 0.49, NPV = 0.68) for slight hair loss ( Fig 2 ). Adding age as a covariate boosted the AUC to 0.79 for severe hair loss (P<2x10 -16 ), 0.70 for moderate hair loss (P<2x10 -16 ), and 0.61 for slight hair loss (P = 0.019). Fig 3 shows the proportion of participants in the four baldness groups for each polygenic risk decile of male pattern baldness. Of those with a baldness polygenic score below the median, 14% reported severe hair loss and 39% no hair loss. By contrast, of those with a polygenic score in the top 10%, 58% reported moderate-to-severe hair loss.
https://doi.org/10.1371/journal.pgen.1006594.g002
https://doi.org/10.1371/journal.pgen.1006594.g003
https://doi.org/10.1371/journal.pgen.1006594.t003
The results of the partitioned heritability analysis indicated that 27 of the functional annotations from the baseline model were statistically significant ( S2 Fig and S7 Table ). These significant annotations included a broad array of functional elements including histone marks, enhancer regions, conserved regions, and DNaseI hypersensitivity sites (DHS). The ten tissue types were then tested for significance after controlling for the baseline model. Following correction for multiple testing, all ten of the tissue groups showed significant enrichment ( S3 Fig and S7 Table ).
In this large GWAS study of male pattern baldness, we identified 287 independent genetic signals that were linked to differences in the trait, a substantial advance over the previous largest GWAS meta-analysis, which identified eight independent signals [ 15 ]. We showed—in line with a previous study [ 13 ], but with much greater precision—that a substantial proportion of individual differences in hair loss patterns can be explained by common genetic variants on the autosomes as well as on the X chromosome. However, the variance explained by X chromosome variants is much lower for late-onset compared to early-onset male pattern baldness [ 13 ]. Finally, by splitting our cohort into a discovery and a prediction sample, we showed a predictive discrimination (AUC = 0.78) between those with no hair loss and those with severe hair loss.
Despite there being genetic overlap for SNP hits associated with baldness and Parkinson’s Disease—first noted in Li et al. [ 15 ] and replicated here—we observed no statistically significant genetic correlations after correcting for multiple testing between baldness and any of the health, cognitive, or anthropometric outcomes we studied. There were very small (maximum absolute genetic correlation of 0.13) but nominally-significant associations with height, bipolar disorder, number of children born, and age at menarche, such that the genes associated with more hair loss were linked to shorter stature, younger age at menarche, fewer offspring, and a lower risk of bipolar disorder (all P<0.05). The local but not global overlap of SNPs associated with baldness and other traits, such as Parkinson's Disease, might be explained by chance due to the large number of hits for baldness, or pleiotropy at single sites with no systemic overlap. The point estimates for the genetic correlations were all near zero, suggesting true null associations as opposed to a lack of statistical power to detect modest-sized correlations.
As mentioned above, the GWAS identified 247 independent autosomal loci and 40 independent X chromosome loci. The top 20 hits from the autosomes were located in or near to genes that have been associated with, for example, hair growth/length in mice ( FGF5 ) [ 20 ], grey hair ( IRF4 ) [ 21 ], cancer (breast: MEMO1 [ 22 ], bladder: SLC14A2 [ 23 ]), histone acetylation ( HDAC9 ), and frontotemporal dementia ( MAPT ) [ 24 ]. A previous GWAS showed an association of IRF4 with both hair colour and hair greying, but not with male pattern baldness [ 21 ]. HDAC9 has been identified as a baldness susceptibility gene in a previous study [ 25 ]. Two of the top 10 X chromosome SNPs were located in OPHN1 , a gene previously associated with X-linked mental retardation [ 26 ].
Of the top autosomal gene-based findings (maximum P = 3.1x10 -15 ), RSPO2 has been linked to hair growth in dogs. PGDFA has been linked to hair follicle development [ 27 ]; EBF1 is expressed in dermal papillae in mature hair follicles [ 28 ]; PRR23B is proximal to a GWAS hit for eyebrow thickness [ 21 ]; and WNT10A has been linked to both straight hair [ 29 ] and dry hair [ 30 ]. The WNT signaling pathway is involved in the activation of β-catenin, which regulates the differentiation of follicular keratinocytes, which form the hair follicle [ 31 ].
The top X chromosome gene-based findings included the androgen receptor ( AR ), which has been well established as a baldness associated gene [ 32 ], along with its upstream ( EDA2R ) and downstream ( OPHN1 ) genes. EDA2R plays a role in the maintenance of hair and teeth as part of the tumor necrosis factor receptor. Onset of male pattern baldness could be influenced by EDA2R via activation of nuclear proto-oncoprotein c-Jun , which is linked to transcription activation of AR [ 33 ]. Two other genes included in the gene-based findings, OPHN1 and ZC4H2 , have previously been associated with X-linked mental retardation [ 26 , 34 ]. One limitation of our X chromosome analysis was that it contained genotyped SNPs only. The imputed X chromosome SNP data for UK Biobank have not yet been released but will likely provide further clues about the genetic architecture of male pattern baldness.
Many of the genes identified are associated with hair structure and development, which may be critical for the process of hair loss. For example, animal models indicate that FGF5 is critical for the inhibition of hair growth and mutations in FGF5 are associated with excessively long eyelashes in humans [ 35 ]. It is possible that genetic variants leading to higher levels of expression of this gene result in greater inhibition of hair growth, leading to male pattern baldness. As a second example, the RSPO2 gene is associated with hair growth in dogs [ 36 ]. It is part of the Wnt signalling pathway needed for the establishment of hair follicles [ 37 ]. Variation in the activity of this pathway caused by genetic variants within PSPO2 may lead to differences in levels of hair growth in men and may contribute to male pattern baldness. The inclusion of hits on the X chromosome, specifically the Androgen Receptor, suggests that hormonal mechanisms are also involved in hair loss. It is possible that the hair structure proteins interact biologically with sex hormones, leading to a higher prevalence of baldness.
The results of the gene set analysis indicated that the genomic regions with the greatest evidence for association with male pattern baldness are united by a shared biological theme. In particular, these associated regions appear to converge on the transcription factor complex, and transcription factor binding gene sets.
The most significant gene set, GO:0005667, corresponded to the transcription factor complex gene set, which includes the gene ALX4 . ALX4 was found to be mutated in a patient with frontonasal dysplasia, presenting with alopecia [ 38 ]. Of the other genome-wide significant gene sets, ENSG00000141027 (NCOR1 subnetwork), includes members of the histone deacetylase (HDAC) family [ 39 ]. HDAC9 is associated with male pattern baldness (the present paper and Li et al. [ 15 ]). GO:0003682 (transcription factor binding), includes the murine gene Cux1 that is important for, amongst other things, hair growth [ 40 ]. GO:0003712 (transcription cofactor activity), includes the gene AIRE , which is associated with alopecia [ 41 ]. MP:0000097, (short maxilla), and GO:0044212 (transcription regulatory region DNA binding), both include the murine gene Grhl1 . Grhl1-null mice suffer from a delay in coat growth and later hair loss [ 42 ]. It is important to note that, as with all pathway analyses, the results are dependent on the gene sets defined in the databases used. These rely on accurate functional annotations, which are continually updated.
The main strength of this study is its large sample size and phenotypic homogeneity. Many meta-analytic studies of complex traits are weakened by different cohorts collecting data at different time-points, under different protocols, in different populations. The present study replicated all of the previously identified autosomal hits for baldness from Li et al. [ 15 ] and Heilmann-Heimbach et al.,[ 16 ] suggesting a degree of robustness in phenotypic measurement, which was briefer here than in previous studies of male pattern baldness. Whereas the genomic inflation factor from the GWAS was large (1.09, Q-Q plot in S1 Fig ), this is likely to be a result of genuine polygenic effects. We have used identical analysis protocols for other traits with far lower SNP-based heritabilities in the same UK Biobank cohort and observed no evidence of inflation [ 43 ].
We identified over two hundred independent, novel genetic correlates of male pattern baldness—an order of magnitude greater than the list of previous genome-wide hits. Our top SNP and gene-based hits were in genes that have previously been associated with hair growth and development. We also generated a polygenic predictor that discriminated between those with no hair loss and those with severe hair loss. Whereas accurate predictions for an individual are still relatively crude, of those with a genetic score in the top 10% of the distribution, 58% reported moderate-to-severe hair loss. The release of genetic data on the full UK Biobank cohort will further refine these predictions and increase our understanding of the genetic architecture of male pattern baldness.
Data came from the first release of genetic data of the UK Biobank study and analyses were performed under the data application 10279. Ethical approval for UK Biobank was granted by the Research Ethics Committee (11/NW/0382).
Genotyping information
Genotyping details including quality control steps have been reported previously [ 43 ]. Briefly, from the sample with genetic data available as of June 2015, 112,151 participants remained after the following exclusion criteria were applied: SNP missingness, relatedness, gender mismatch, non-British ancestry, and failed quality control for the UK BiLEVE study [ 43 ]. For the current analysis, an imputed dataset was used for the autosomes (reference set panel combination of the UK10K haplotype and 1000 Genomes Phase 3 panels: http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=157020 ). Imputed data were not available for the X chromosome, hence only genotyped variants were considered. X chromosome quality control steps included a minor allele frequency cut-off of 1% and a genotyping call rate cut-off of 98% [ 44 ]. For the imputed autosomal data, we restricted the analyses to variants with a minor allele frequency >0.1% and an imputation quality score >0.1.
Male pattern baldness phenotype
From the sample of 112,151 unrelated White British participants with genetic data, we identified 52,874 men with a self-reported response to UK Biobank question 2395, which was adapted from the Hamilton-Norwood scale [ 45 , 46 ]. These men were asked to choose, from four patterns (no loss; slight loss; moderate loss; severe loss), the one that matched their hair coverage most closely. Fig 4 shows a screenshot of the four options.
https://doi.org/10.1371/journal.pgen.1006594.g004
GWAS of male pattern baldness on the whole sample
A genome-wide association study was conducted using baldness pattern residuals as the dependent variable. The residuals were obtained from a linear regression model of baldness pattern on age, assessment centre, genotyping batch and array, and 10 principal components to correct for population stratification.
The GWAS for the imputed autosomal dataset was performed in SNPTest v2.5.1 [ 47 ] via an additive model, using genotype probability scores. The GWAS for the X chromosome was performed in Plink [ 48 , 49 ].
Identification of independent GWAS signals
The number of independent signals from the GWAS was determined using LD-clumping [ 48 , 49 ] based on the LD structure annotated in the 1000 genomes project [ 50 ]. Index SNPs were identified (P<5x10 -8 ) and clumps were formed for SNPs with P<1x10 -5 that were in LD (R 2 >0.1) and within 500kb of the index SNP. SNPs were assigned to no more than one clump.
Lookup of published male pattern baldness hits
GWAS lookups were performed for the top hits reported in Richards et al. [ 10 ], Li et al. [ 15 ], Heilmann-Heimbach et al. [ 16 ], Liu et al. [ 13 ] and Pickrell et al. [ 14 ].
Gene-based correlates of male pattern baldness
Gene-based analyses were performed using MAGMA [ 51 ]. SNPs were mapped to genes according to their position in the NCBI 37.3 build map. No additional boundary was added beyond the genes start and stop site. For the autosomal genes the summary statistics from the imputed GWAS were used to derive gene-based statistics using the 1000 genomes (phase 1, release 3) to model linkage disequilibrium. For genes on the X chromosome the genotype data from UK Biobank was used and the gene-based statistic was derived using each participant’s phenotype score. Gene-set pathway analyses were carried out in DEPICT [ 52 ] using the genome-wide significant autosomal SNPs as input.
GWAS of male pattern baldness on a sub-sample of 40,000 and trait prediction in the residual sample of 12,874 participants
For the prediction analysis, the GWAS was re-run on a randomly selected cohort of 40,000 individuals to give regression weights for prediction, leaving an independent cohort of 12,874 in which to test the polygenic predictor. The methods for the GWAS were identical to those reported for the full sample. The regression weights from the GWAS on the 40,000 cohort were used to construct polygenic scores in the target dataset at P value thresholds of <1x10 -20 , <5x10 -8 , <1x10 -5 , <0.01, <0.05, <0.1, <0.2, <0.5, <1 using PRSice software [ 53 ]. PRSice creates polygenic scores by calculating the sum of alleles associated with male pattern baldness across many genetic loci, weighted by their effect sizes estimated from the male pattern baldness GWAS. Prior to calculating the scores, SNPs in the prediction dataset were clumped across 250kb sliding windows at an R 2 >0.25. Thereafter, each threshold was used to discriminate between those with no hair loss and those with severe hair loss via logistic regression with results being reported for the optimal predictor only. A predictor for both the autosomes and X chromosome were built and assessed independently and additively. Receiver operator characteristic (ROC) curves were plotted and areas under the curve (AUC) were calculated using the pROC package in R [ 54 , 55 ].
Heritability of male pattern baldness
SNP-based heritability of baldness was estimated using GCTA-GREML [ 56 ] after applying a relatedness cut-off of >0.025 in the generation of the autosomal (but not X chromosome) genetic relationship matrix.
Genetic correlations with male pattern baldness
Linkage disequilibrium score (LDS) regression analyses [ 57 ] were used to generate genetic correlations between baldness and 24 cognitive, anthropometric, and health outcomes, where phenotypic correlations or evidence of shared genetic architecture have been found ( S7 Table ). Due to the large effects in the APOE region for Alzheimer's disease, 500kb was removed from around each side of this region and the analysis was repeated for the Alzheimer's—male pattern baldness analysis. The Alzheimer’s data set without this region is referred to as 'Alzheimer’s 500kb'. In total, we carried out 25 hypothesis tests. Multiple testing was controlled for using a false discovery rate (FDR) correction [ 58 ]. An overview of the GWAS summary data for the anthropometric and health outcomes is provided in S1 Appendix .
Partitioned heritability of male pattern baldness
Stratified linkage disequilibrium score (SLDS) regression [ 59 ] was used to determine if a specific group of SNPs made a greater contribution to the heritability of male pattern baldness than would be expected by the size of the SNP set. Firstly, a baseline model was derived using 52 overlapping, functional categories. Secondly, a cell-specific model was constructed by adding each of the 10 cell-specific functional groups to the baseline model and the level of enrichment was obtained. Multiple testing was controlled for using FDR correction [ 58 ] in both the functional category and cell-specific analysis.
Supporting information
S1 table. lookup of gwas hits from richards et al. [ 10 ], pickrell et al. [ 14 ], li et al. [ 15 ], and heilmann-heimbach et al. [ 16 ]..
https://doi.org/10.1371/journal.pgen.1006594.s001
S2 Table. Summary information for the 287 independent loci associated with male pattern baldness.
https://doi.org/10.1371/journal.pgen.1006594.s002
S3 Table. Genome-wide significant autosomal gene-based hits (Bonferroni correction of α < 2.769x10 -6 ) in the MAGMA gene-based analysis for male pattern baldness.
NSNPS is the number of SNPs in the gene.
https://doi.org/10.1371/journal.pgen.1006594.s003
S4 Table. List of genome-wide significant gene-based hits (Bonferroni correction of α < 8.818x10 -5 ) in the MAGMA gene-based analysis for male pattern baldness, performed on the X chromosome.
https://doi.org/10.1371/journal.pgen.1006594.s004
S5 Table. Results of the Gene-Set Analysis performed in DEPICT.
https://doi.org/10.1371/journal.pgen.1006594.s005
S6 Table. Genetic correlations between baldness and the 24 cognitive, health, psychiatric, and anthropometric variables.
The heritability Z-score and the mean χ 2 indicate the level of power to detect association where a heritability Z-score of >4 and a mean χ 2 >1.02 being considered well powered [ 57 ]. None of the 25 tests performed survived FDR control for multiple comparisons. Nominally significant genetic correlations highlighted in bold. ADHD, attention deficit hyperactivity disorder; MDD, major depressive disorder.
https://doi.org/10.1371/journal.pgen.1006594.s006
S7 Table. Showing the full output of the partitioned heritability analysis for male pattern baldness.
Prop._SNPs refers to the proportion of SNPs from the data set that were a part of the corresponding functional annotation. Statistical significance indicated in bold. Tissue groups are listed in the first ten rows followed by the functional annotation groups.
https://doi.org/10.1371/journal.pgen.1006594.s007
S8 Table. The 24 health-related phenotypes included in the genetic correlation analysis with male pattern baldness.
Verbal-numerical reasoning and childhood intelligence were examined as educational attainment (genetic association with baldness reported by Pickrell et al. 2016 [ 14 ]) can be used as a proxy phenotype for general cognitive ability. Metabolic traits were included as metabolic disease has been associated with baldness (references noted in the review paper by Heilmann-Heimbach et al. 2016 [ 16 ]). Psychiatric disorders were included due to the association between baldness and neurological conditions such as Parkinson’s disease. Genetic correlations have been observed between baldness and the listed anthropometric and developmental traits [ 14 ]. Fertility traits [ 60 ] were selected due to the published associations between baldness and the androgen receptor.
https://doi.org/10.1371/journal.pgen.1006594.s008
S1 Appendix. Sources of GWAS summary results from genome-wide association consortia.
https://doi.org/10.1371/journal.pgen.1006594.s009
S1 Fig. Male pattern baldness QQ Plot for imputed GWAS of autosomal variants (p-values truncated at 1x10 -30 ).
https://doi.org/10.1371/journal.pgen.1006594.s010
S2 Fig. Enrichment analysis for male pattern baldness using the 52 functional categories in 52,874 individuals.
The enrichment statistic is the proportion of heritability found in each functional group divided by the proportion of SNPs in each group (Pr(h 2 )/Pr(SNPs)). Error bars are jackknife standard errors around the estimate of enrichment. The dashed line indicates no enrichment found when Pr(h 2 )/Pr(SNPs) = 1. FDR correction indicated significance at P = 0.011 indicated by asterisk
https://doi.org/10.1371/journal.pgen.1006594.s011
S3 Fig. Enrichment analysis for male pattern baldness using the 10 cell specific functional The enrichment statistic is the proportion of heritability found in 52,874 individuals.
In each functional group divided by the proportion of SNPs in each group (Pr(h 2 )/Pr(SNPs). Error bars are jackknife standard errors around the estimate of enrichment. The dashed line indicates no enrichment found when Pr(h 2 )/Pr(SNPs) = 1. FDR correction indicated significance at P = 0.037 indicated by asterisk.
https://doi.org/10.1371/journal.pgen.1006594.s012
Author Contributions
- Conceptualization: REM.
- Data curation: GD DCL.
- Formal analysis: SPH WDH REM.
- Funding acquisition: DJP CRG IJD.
- Supervision: REM.
- Writing – original draft: REM.
- Writing – review & editing: SPH WDH SEH SJR DJP CRG IJD REM.
- View Article
- PubMed/NCBI
- Google Scholar
- 49. Purcell S, Chang CC. PLINK v1.90b3i. Available from: https://www.cog-genomics.org/plink2 .
- 54. R Core Team. R: A language and environment for statistical computing. 2013.
Starting to thin out? Hair loss doesn’t have to lead to baldness
Professor of Dermatology, The University of Melbourne
Disclosure statement
Rodney Sinclair is principal investigator in a pharmaceutical company sponsored clinical trial investigating new treatments for male pattern baldness
University of Melbourne provides funding as a founding partner of The Conversation AU.
View all partners

Hair loss is no longer an inevitable march to baldness. Medical advances over recent decades mean male hair loss can be treated. And it need not break the bank.
The cause of male pattern baldness is well established as an act of nature not nurture. Identical twins go bald at the same age, rate and pattern irrespective of diet, lifestyle or stress levels.
Baldness is a complex polygenic trait: up to five genes are involved, and it is the interplay between these genes , not unlike the interaction between the cards in a poker hand, that determine the specifics of male pattern hair loss.
So, what are the treatment options?
Finasteride
Finasteride is a Therapeutic Goods Administration-approved drug that dermatologists and general practitioners have been prescribing to treat hair loss for around 15 years.
It works by stopping the conversion of testosterone to dihydrotestosterone in the prostate, by blocking an enzyme called 5-alpha reductase .
You’ll need a prescription for finasteride and your doctor will explain the benefits and risks of the drug before you decide to proceed.
The benefits are clear: men who start taking daily finasteride at the first signs of hair loss will not go bald. Plus there are no interactions with other drugs.
But there is a small risk of sexual side effects such as such as erectile dysfunction, ejaculatory problems and reduced libido, which require careful management by an experienced doctor.
An added bonus is that finasteride reduces a man’s future chance of developing of prostate cancer. Though it does not increase longevity , as the reduction in risk is exclusively for low-risk prostate cancer which are not the type men generally die from.
The cost for a year’s supply of tablets starts at around as low as A$200 (plus the extra you will need to put aside for haircuts!).
Developed in the 1950s as a tablet to treat high blood pressure, minoxidil was an abject failure due to unwanted hair growth. Re-invented as a lotion applied directly to the scalp, it was launched in the United States in 1988 and soon became popular around the world.
Minoxidil is also TGA-approved and available over the counter in pharmacies for between A$15 and A$60 per month, depending on the brand.
No prescription or trip to the doctor is required, but talk to your pharmacist if you are taking any other medicines, especially high-blood-pressure medication.
Hair removal laser has led to a renaissance in the use of minoxidil tablets, as doctors can now effectively manage the unwanted hair that was previously a deal breaker. Low doses of minoxidil, as low as one-fortieth of a tablet, can still grow hair with minimal effect on blood pressure and no need to put anything in your hair.
How they compare
While finasteride works best at arresting progression of genetically programmed hair loss, some men will achieve partial regrowth. The sooner you begin treatment, the more hair there is to protect.
Minoxidil is better at stimulating new growth rather than preventing progression. A bit like the bank manager who lends an umbrella when the sun is shining, and wants it back when it starts to rain, minoxidil works better before you go bald. Men who still have hair regrow more hair than men who have lost it all.
When used together, the combination of minoxidil and finasteride is a man’s best chance to both arrest hair loss progression and maximise hair regrowth.
Hair transplant surgery
Men whose hair is too far gone to be saved by tablets and lotions might consider hair transplantation surgery , a cosmetic surgical procedure that has improved dramatically in recent years.
Various techniques are available, but all involve taking hair-bearing skin from one part of the scalp and grafting these pieces of skin onto bald or thinning areas of the scalp.
The main down side, apart from the cost (A$5,000 to A$10,000, depending on the number of grafts required) is the linear scar left behind on the back of the head locking men into long hair styles.
Another option is follicular unit extraction . Rather than a strip of skin and a scar, donor tissue comprises multiple tiny punch grafts that heal invisibly by wound contraction. Further dissection of these grafts releases single hair follicle units that can be implanted one by one into the bald scalp to recreate a natural hair line.
The only limiting factor for follicular unit extraction is whether there is enough remaining donor hair to transplant. While chest and body hair have been used when donor hair is poor, artificial hair implants, also TGA-approved, remain the last resort. Artificial hairs cover the scalp, but do not grow and grey and regenerate like hair transplants.
Alternative therapies
What about other suggested treatments such as vitamins, dietary supplements, lasers, stem-cell therapy and platelet rich plasma? At this stage the most generous interpretation of the available data is that they are unproven, expensive and poor value compared with the TGA-approved treatments.
The take home message for boys staring at their bald fathers’ scalp and wondering what their genes have in store for them is, don’t panic, but don’t leave it too late.
- Testosterone
- Cosmetic surgery
- Men's health
- Health Explainer

Biocloud Project Manager - Australian Biocommons

Director, Defence and Security

Opportunities with the new CIEHF

School of Social Sciences – Public Policy and International Relations opportunities

Deputy Editor - Technology
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Eur J Hum Genet
- v.31(3); 2023 Mar
Genetic prediction of male pattern baldness based on large independent datasets
1 Department of Genetic Identification, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands
2 Key Laboratory of Genomic and Precision Medicine, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing, China
3 University of Chinese Academy of Sciences, Beijing, China
4 Department of Twin Research and Genetic Epidemiology, King’s College London, London, United Kingdom
5 Institute for Genomic Statistics and Bioinformatics (IGSB), University of Bonn, School of Medicine & University Hospital Bonn, Bonn, Germany
Stefanie Heilmann-Heimbach
6 Institute of Human Genetics, University of Bonn, School of Medicine & University Hospital Bonn, Bonn, Germany
Timothy D. Spector
Manfred kayser, associated data.
The UK Biobank data are available from the UK Biobank ( https://www.ukbiobank.ac.uk/ ).
Genetic prediction of male pattern baldness (MPB) is important in science and society. Previous genetic MPB prediction models were limited by sparse marker coverage, small sample size, and/or data dependency in the different analytical steps. Here, we present novel models for genetic prediction of MPB based on a large set of markers and large independent subsample sets drawn among 187,435 European subjects. We selected 117 SNP predictors within 85 distinct loci from a list of 270 previously MPB-associated SNPs in 55,573 males of the UK Biobank Study (UKBB). Based on these 117 SNPs with and without age as additional predictor, we trained, by use of different methods, prediction models in a non-overlapping subset of 104,694 UKBB males and tested them in a non-overlapping subset of 26,177 UKBB males. Estimates of prediction accuracy were similar between methods with AUC ranges of 0.725–0.728 for severe, 0.631–0.635 for moderate, 0.598–0.602 for slight, and 0.708–0.711 for no hair loss with age, and slightly lower without, while prediction of any versus no hair loss gave 0.690–0.711 with age and slightly lower without. External validation in an early-onset enriched MPB dataset from the Bonn Study ( N = 991) showed improved prediction accuracy without considering age such as AUC of 0.830 for no vs. any hair loss. Because of the large number of markers and the large independent datasets used for the different analytical steps, the newly presented genetic prediction models are the most reliable ones currently available for MPB or any other human appearance trait.
Introduction
Male pattern baldness (MPB), or androgenetic alopecia, is a highly heritable, age independent appearance trait [ 1 , 2 ]. MPB has socio-psychological consequences on a large proportion of the world’s male population. MPB is of interest to various fields of scientific research and applications, such as in medicine, anthropology, evolutionary biology, and forensics. Availability of accurate and reliable genetic prediction of MPB would allow addressing several concerns. Genetic MPB prediction could enable early treatment of MPB before it becomes externally manifest, which may result in better therapeutic outcomes alleviating socio-psychological problems. MPB prediction from old or modern DNA would help describing the appearance of historical persons from skeletal remains or DNA found at crime scenes, as previously done for pigmentation traits [ 3 , 4 ]. MPB prediction from ancient DNA would allow reconstructing what ancestors of modern humans looked like and help understanding when and where MPB first arose in time and space, as previously shown for pigmentation traits [ 5 , 6 ]. Additionally, as MPB was discussed as marker of several later-onset health disorders (e.g., metabolic and cardiovascular diseases), genetic MPB prediction may contribute to better management of age-dependent human diseases.
A key requirement for the development of accurate genetic prediction models for genetically complex traits like MPB is the availability of sufficiently large number of DNA predictors. To date, four sufficiently powered GWASs for MPB have identified hundreds of MPB-associated SNPs, available for MPB prediction modelling. Heilmann-Heimbach et al. [ 7 ] reported a GWAS meta-analysis of 10,846 early-onset MPB cases and 11,672 controls from eight independent cohorts and identified 63 distinct genetic loci. Hagenaars et al. [ 8 ] identified 287 genetic loci in 52,874 UK Biobank (UKBB) male participants. Pirastu et al. [ 9 ] described 71 genetic loci in 43,590 UKBB male participants, follow by an independent replication study in 31,112 men. Recently, Yap et al. [ 2 ] identified 624 genetic loci from what currently is the largest GWAS on MPB, involving 205,327 UKBB males.
A key requirement for developing reliable genetic prediction models for MPB and any other trait are sufficiently large and independent datasets needed for the different analytical steps. The genetic prediction models by Hagenaars et al. [ 8 ], which with 331 SNPs currently include the largest number of DNA predictors, discriminated between subjects with no hair loss from those with severe hair loss with an AUC 0.78 (sensitivity = 0.74, specificity = 0.69, PPV = 0.59, NPV = 0.82). Although these models were tested in independent dataset of 12,874 UKBB samples, GWAS-based marker discovery and prediction model training were performed in the very same dataset of 40,000 UKBB samples, and no external model validation was reported. It remains unclear, if and to what degree the non-independence of datasets for marker discovery and model training induces bias and how they would perform in the external validation in different datasets.
Aiming to overcome limitations of previously reported models, we developed new genetic prediction models for MPB based on a large number of DNA markers and four large independent datasets for the four analytical steps i.e., i) 55,573 UKBB samples for prediction marker ascertainment, ii) 104,694 UKBB samples for prediction model training, iii) 26,177 UKBB samples for prediction model testing, and iv) 991 samples from the early-onset MPB enriched Bonn Study for external model validation.
Materials and methods
Uk biobank study dataset.
The UK Biobank Study ( http://www.ukbiobank.ac.uk ) is a large, population-based genetic epidemiology cohort of volunteers living in the UK. In response to UK Biobank question 2395 [ 8 ], male participants chose one answer that most closely matched their head hair coverage from four given patterns adapted from the Hamilton-Norwood scale [ 10 , 11 ] (Supplementary Fig. 2 ), i.e., no, slight, moderate, and severe hair loss (Supplementary Fig. 2 ). Details on microarray genotyping and imputation have been described previously [ 12 ]. After genomic and phenotypic quality controls, to avert issues of population admixture and stratification, the current study included a total of 186,444 UKBB males of European descent, divided into three independent subsets for: 1) prediction marker ascertainment ( N = 55,573), 2) model training ( N = 104,694), and 3) model testing ( N = 26,177) (Supplementary Fig. 1 ). The 80–20% data split into model training and model testing datasets resulted in the same proportions of each of the four baldness phenotype categories observed over the combined dataset (Table 1 ). Our prediction marker ascertainment dataset used for feature selection likely included all the 52,874 UKBB men previously used in the GWAS by Hagenaars et al. [ 8 ]. We initially considered a total of 270 out of the original Hagenaars et al. [ 8 ] 287 MPB-associated SNPs at 85 genetic loci which were carried forward in our feature selection analysis (Supplementary Table 1 ). The rest were removed due to our quality control procedures. This loss is due to an up todate genotypic and imputated SNPs.
Characteristics of study samples.
Bonn Study dataset
The Bonn Study is a case-control study designed to investigate the genetic basis of early-onset MPB. This study includes 991 males of European descent from Germany i.e., 582 early-onset MPB cases representing the 20% most severely affected in the respective age-groups (<30 years; <40 years), 268 “unaffected” controls ≥60 years with no signs of baldness representing the 10% least affected in their age-group, and 141 samples from the population-based Heinz Nixdorf Recall cohort. Hair loss status was ascertained by expert dermatologists according to the Hamilton/Norwood (HN) classification [ 11 , 13 ]. Baldness status was aligned to the same categories as done in the UKBB Study (Supplementary Fig. 2 ). Details on microarray genotyping and SNP imputation have been described previously [ 7 ]. Of the 117 SNP predictors we identified via feature selection in UKBB, 107 were available in the Bonn dataset. The 10 SNPs missing in Bonn were neither present in the genotyping array dataset nor could they be properly imputed. These data were used as external model validation dataset to additionally test the MPB prediction models trained and tested in UKBB data.
Multinomial and binomial logistic regression
The multinomial logistic regression (MLR) and binomial logistic regression (BLR) modelling was implemented via multinom function in ‘ nnet’ package of R ( www.r-project.org ). The modelling specifications were as following. For MLR, consider baldness pattern, y, to be four categories: i) no hair loss, ii) slight hair loss, iii) moderate hair loss and iv) severe hair loss, which are determined by the genotype, x , of k SNPs, where x represents the number of minor alleles. If π 1 , π 2 , π 3 , and π 4 denote the probability of the four MPB categories, respectively, the MLR can be written as
where α and β can be derived in the modelling set. Baldness pattern of each individual in the testing set can be probabilistically predicted based on the genotypes and the derived α and β, π 1 = exp ( α 1 + ∑ i = 1 k β ( π 1 ) i x i ) 1 + exp α 1 + ∑ i = 1 k β π 1 i x i + exp α 2 + ∑ i = 1 k β π 2 i x i + exp α 3 + ∑ i = 1 k β π 3 i x i π 2 = exp α 2 + ∑ i = 1 k β π 2 i x i 1 + exp α 1 + ∑ i = 1 k β π 1 i x i + exp α 2 + ∑ i = 1 k β π 2 i x i + exp α 3 + ∑ i = 1 k β π 3 i x i
The baldness category with the max( π 1 , π 2 , π 3 , π 4 ) was considered as the predicted baldness pattern.
For binomial logistic regression (BLR), consider baldness pattern, y, to be two categories any hair loss and no hair loss, which are determined by the genotype, x , of k SNPs, where x represents the number of minor alleles. Let p denote the probability of any hair loss, and 1 − p is the probability of no hair loss. The BLR can be written as
where α and β can be derived in the modelling set. Baldness pattern of each individual in the testing set can be probabilistically predicted based on his or her genotypes and the derived α and β,
The MPB category with the max( p , 1 − p ) was considered as the predicted baldness pattern.
Support vector machine and Artificial neural network
Next to logistic regression analyses, two types of machine learning (ML) algorithms were additionally applied for prediction modelling: support vector machine (SVM) and artificial neural networks (ANN). SVM was used via svm function in the e1071 package of R with the nonlinear radial basis function as the kernel and 2 -4 as the cost of constraints violation set. A “one against one” strategy was applied for classification of both 4-category and 2-category baldness pattern, and posterior probability estimates for each baldness category were obtained via predict.svm function in the e1071 R package. A feed-forward ANN was implemented in Tensorflow [ 14 ] and Keras ( https://github.com/fchollet/keras ). The ANN model consisted of three layers with a hidden layer of ten neurons using the Rectified Linear Unit (ReLU) function in hidden layer and softmax activation function in output respectively. The output layer contained four neurons for 4-category baldness pattern and two neurons for binary baldness pattern, each represents yes-no for one baldness type. The training algorithm of the neural network was performed using Adam optimizer [ 15 ]. ANN model training and testing were conducted in Python version 3.6.1 using the package keras. As for the hyper parameter tuning, we used grid search and chosen hyper parameter with the best prediction performance for both ANN and SVM. For ANN model, we considered activation function of tanh, relu and sigmoid, the number of neurons in hidden layer ranging from 10 to 117 (including 10, 20, 30, 40, 50, 60 and 117), and patience of 25, 50 and 100. For SVM, we considered kernel of linear, polynomial, radial and sigmoid, cost of 2 −5 , 2 −4 , 2 −3 , 2 −2 , 2 −1 , 1, 2, 2 2 , 2 3 and 2 4 . We got the best hyper parameters and built best model by using these best hyper parameters, that is, ANN model using activation function of relu, 10 neurons, and patience of 100, and best SVM model using kernel of radial and cost of 2 −4 .
For both SVM and ANN models, the input data included 118 features (age and genotypes at the 117 SNP marker sites) and output was the probability of being classified into each baldness pattern category. The same 80% training and 20% testing datasets as applied for regression analyses were also used for SVM and ANN analyses. The derived SVM and ANN models were subsequently used to predict baldness pattern categories in the model testing dataset, returning 4-element and 2-element probability vectors for 4-category and binary baldness pattern with values between 0 and 1, respectively. The baldness category with the maximal value was considered as the predicted category.
Feature selection, prediction model training, and model testing
We conducted a Forward Stepwise Regression (FSR) analysis in the marker ascertainment dataset of UKBB by iteratively including the next largest contributor to the model according to Akaike Information Criterion (AIC), AIC ~ 2 k + n l n ( ∑ i = 1 n ( y i − E ( y i ) ) 2 ) , where k is the number of markers, n is the sample size, and E y i is the fitted value. FSR was performed on 4-category MPB phenotypes using MLR. The marker set proposed by FSR with the minimal AIC value was considered as the theoretically optimal subset for prediction analysis. The rank of the SNP predictors was determined by their rank in FSR analysis or their association significance.
Prediction models were built in the prediction model training dataset of UKBB using MLR and BLR considering MPB as a 4-category as well as as binary trait by joining all samples into two categories of no hair loss and any hair loss. Additionally, SVM and ANN models also were built in the same datasets and using the same four and two categories, respectively. The developed prediction models were assessed for their accuracy in the model testing dataset of UKBB and additionally externally validated in the Bonn Study dataset.
Prediction accuracy was evaluated by estimating the following parameters including the Area Under the Receiver Operating Characteristic (ROC) Curves (AUC), sensitivity, specificity, positive prediction value (PPV), and negative prediction value (NPV). AUC is the integral of ROC curves which ranges from 0.5 representing random prediction to 1.0 representing perfect prediction. Sensitivities and specificities, PPVs and NPVs were calculated using confusion matrices considering the highest predicted probability as the predicted baldness pattern for 4-category prediction, while for binary prediction considering the predicted probability >0.5 as the predicted baldness pattern. Statistical analyses and result visualisation were conducted in R version 3.6.1 using nnet, e1071, pscl, and pROC packages.
Phenotypic characteristics in UKBB and Bonn Study
Individual phenotype and genotype data of 187,435 men from two European countries were included i.e., 186,444 males from the UKBB (32% no, 23% slight, 27% moderate, and 18% severe hair loss; 57.05 ± 8.10 years of age; age range 39–73) and 991 males from the Bonn Study (2% slight, 16% moderate, and 50% severe hair loss; 46 ± 16 years of age; age range 18–70; Table 1 , Supplementary Figs. 1 and 2 ). In the UKBB, age was significantly associated with increased MPB severity (Supplementary Fig. 3 ). In the Bonn Study, lower mean ages were observed across the four MPB categories, because a large proportion (58.7%) of the participants were early-onset MPB (Supplementary Fig. 3 ).
Feature selection, prediction model training, and model testing in UKBB data
Feature selection (or feature thinning) of 270 MPB-associated SNPs from 85 genetic loci previously discovered in the study of Hagenaars et al. [ 8 ] was carried out in 55,573 UKBB samples using FSR. Notably, this sample set likely includes all the 52,874 UKBB men previously used by Hagenaars et al. (Supplementary Table 1 ) for GWAS-based discovery of these 270 associated SNPs. This analysis identified 101 SNPs from 69 distinct loci, which in multinomial logistic combination achieved the maximal model fitting. To this FSR-selected prediction marker set, we additionally added the top-associated SNP from each of the remaining 16 genetic loci (Supplementary Table 1 ), resulting in a total set of 117 SNP predictors from all 85 distinct loci (Pseudo Nagelkerke- R 2 = 0.22). The remaining 153 SNPs from these loci that were removed from our models by FSR did not independently contribute to the MBP prediction (Fig. 1 ), justifying their exclusion.

This dataset includes the 52,874 UKBB men previously applied for discovering the MPB-association of the 270 SNPs [ 8 ] used here for feature selection. Based on this dataset, a MLR model was fit in a randomly selected 80% prediction model training subset and applied in the remaining 20% prediction model testing subset with 100 replicates. The estimated mean AUCs are depicted as coloured line and the 5–95% boundary as respective light colour shade for the four predicted MPB categories. The dashed line marks the cut-off at 117 SNPs that were used as best-fit prediction marker set in all subsequent prediction analyses.
We then used these 117 SNP predictors with and without considering age as additional predictor to build 4-category (no, slight, moderate, and severe hair loss) MLR models as well as 2-category (no versus [vs.] any hair loss) BLR models in the model training subset of 104,694 UKBB males. Subsequently, these models were validated in the independent model testing subset of 26,177 UKBB males and prediction accuracy parameters were estimated (Supplementary Table 2 ). Accumulative AUC analysis in the model testing subset showed a fast to slow increase according to the ranking of the predictors (Fig. 2 ). Notably, AUC increased at faster rates for comparisons of no hair loss with increasingly severe hair loss categories. As expected for an age-dependent trait, age was the strongest single predictor, alone resulting in an AUC of 0.560 for any vs. no hair loss, followed by the 101 SNPs selected by the FSR analysis, then followed by the extra 16 SNPs. The most predictive SNP was rs12558842 on the X chromosome, which is located ~280 kbp upstream of the androgen receptor (AR) gene. Alongside age, rs12558842 provided an AUC of 0.608 for any vs. no hair loss. The MBP-association of rs12558842 was stronger than that of rs2497938 in the study of Hagenaars et al., and the later was the highest ranking MPB predictor in Liu et al. [ 16 ]. Rs2497938 is in perfect linkage equilibrium with rs2207081, which fully masks the predictive power of the polyglycine GGN triplet repeat of AR [ 17 ].

These prediction models were built in the prediction model training dataset of 104,694 UKKBB men.
The 4-category MLR models without considering age predicted with considerably higher accuracies for severe (AUC = 0.718) and no hair loss (0.702) than for moderate (0.607) and slight (0.574) hair loss (see Table 2 for additional prediction accuracy metrics). Inclusion of age in the MLR modelling improved the estimates of accuracy to AUC of 0.728 for severe hair loss, 0.711 for no hair loss, 0.635 for moderate hair loss, and 0.602 for slight hair loss (Table 2 ). For the 2-category BLR model of predicting any vs. no hair loss, we obtained an AUC of 0.702 without age and a slightly higher AUC of 0.711 with age in the model (Table 2 ). This model provided informative prediction for about 26.2% of the 26,177 males tested, that is, individuals with predicted probabilities of any hair loss <0.2 or >0.8 (Fig. 3A ).
Accuracy estimates of genetic prediction of categorical MPB obtained from the prediction model testing dataset of 26,177 UKBB men using different methods based on 117 SNP predictors with and without age as additional predictor a .
a These models were built in the prediction model training dataset of 104,694 UKBB men.

A The probability was derived from MPB prediction model with 117 SNPs and age as predictors in the prediction model testing dataset of 26,177 UKBB men. These prediction results were practically informative for about 26.2% of the tested males (0.4% <0.2 and 25.8% >0.8). B The probability was derived from MPB prediction model with 107 SNPs as predictors without age in the external validation dataset of 991 men from the Bonn Study. These prediction results were practically informative for about 41.8% of the tested males (0.2% <0.2 and 41.6% >0.8). These 117-SNP ( A ) and the 107-SNP ( B ) MPB prediction models were built in the prediction model training dataset of 104,694 UKBB men.
We further investigated the performance of the MLR models over six age groups of the model testing subset, i.e. in men 39–45, 46–50, 51–55, 56–60, 61–65, and 66–72 years of age. Overall, the performance of the predictive models with and without age was similar across these six age groups and the four different MPB categories (Supplementary Fig. 4 ). Slight differences were seen for some MPB categories and some age groups, such as better performance of the model with age for no hair loss in 39–45 years old and for moderate hair loss in 39–45 and 46–50 years old men.
The SVM and ANN machine-learning models provided similar prediction accuracy estimates compared to the M/BLR models (Table 2 ). As with M/BLR models, we also saw for SVM and ANN models that when including age, the prediction accuracy estimates were higher than without age in the models. Notably, seeing similar prediction estimates of accuracy from M/BLR and SVM/ANN methods in such large datasets may imply a lack of epistasis or nonlinear associations. It is unclear if this finding is a reflection of the true genetic architecture of MPB, or may be the consequence of the additive, univariate model used in the SNP discovery GWAS, whose results were carried forward into the ascertainment of the SNP predictors used in the models.
External model validation in Bonn Study data
External validation was conducted in 991 males from the Bonn Study (58.7% early-onset cases, 27% unaffected controls, and 14.2% were population-based). Due to differences in genotyping platforms and quality control analyses, 10 of the 117 SNPs (Supplementary Table 3 ) were unavailable in the Bonn Study. Therefore, we first trained the models based on the 107 SNPs available in Bonn by using the UKBB training subset and validated them in UKBB testing subset. This demonstrated only marginally lower performance of the 107-SNP models compared to the full 117-SNP models in UKBB (AUC loss 0.002–0.006, Table 3 ).
Accuracy estimates of genetic prediction of categorical MPB obtained from the prediction model testing dataset of 26,177 UKBB men and the external validation dataset of 991 Bonn men with and without age as predictor using Multinomial and Binominal Logistic Regression based on 107 of the 117 SNP predictors available in Bonn a .
In the Bonn Study, the 107-SNP models that did not include age as predictor, achieved considerably higher prediction accuracy estimates compared to UKBB i.e., 0.830 in Bonn vs. 0.696 in UKBB for no hair loss, 0.775 vs. 0.712 for severe hair loss, and 0.830 vs. 0.696 for any vs. no hair loss. For the slight hair loss phenotype, the AUC in Bonn was slightly higher than in UKBB with 0.599 vs. 0.573, while for moderate hair loss it was slightly lower with 0.591 vs. 0.602 (Table 3 ). For any vs. no hair loss prediction, the BLR model without considering age in Bonn provides accurate prediction for about 41.8% of males, that is, individuals with predicted probabilities of any hair loss <0.2 or >0.8 (0.2% <0.2 and 41.6% >0.8, Fig. 3B ).
For the 107-SNP models that included age as predictor, we achieved considerably less accurate prediction performances in the Bonn Study compared to UKBB (Table 3 ) i.e., AUC of 0.559 in Bonn vs. 0.705 in UKBB for no hair loss, 0.539 vs. 0.722 for severe hair loss, 0.550 vs. 0.705 for any vs. no hair loss, and 0.505 vs. 0.631 for moderate hair loss. For slight hair loss, however, it was higher in Bonn than in UKBB with 0.694 vs. 0.600. For the BLR model predicting any vs. no hair loss, the sensitivity was similarly high with (0.928) and without (0.984) age in Bonn and higher in Bonn than in UKBB (0.915 and 0.919) (Table 3 ). The relative drop in performance of age-inclusive models in the Bonn Study is likely a consequence of age confounding which arises from its case-control design. The deliberate focus in the recruitment of early MBP cases and relatively old super-controls results in a very different age distribution and a negative correlation between MBP and age in this cohort, as opposed to the expected positive correlations expected in the wider population as seen in UKBB.
Reliable genetic predictions for MPB can contribute to different disciplines of scientific research and science applications, including in medicine, evolutionary biology, anthropology, human history, and forensics. Previously reported genetic prediction models for MPB were limited by sparsity of genetic marker coverage, low-powered sample size, and non-independence of data used in the different analytical steps of prediction modelling. Here, we have sought to overcome these previous limitations by developing and validating new genetic MPB prediction models based on a large number of DNA predictors and large and fully independent datasets in the different analytical steps. These new models achieve more accurate and more reliable genetic MPB prediction than previous models.
Previously, Marcińska et al. [ 18 ] applied 20 SNPs from only 10 MPB-associated loci and achieved an AUC of 0.66 to discriminate between male subjects with vs. without significant baldness, in a model that did not include age as a predictor. The prediction accuracy we achieved here with our 117-SNP model without age for any vs. no hair loss was considerably higher (AUC = 0.702) than that reported by Marcińska et al. [ 18 ]. This improvement is likely due to the effect of the largely increased number of SNP predictors that we included in our models by taking advantage of recently published GWAS-based marker knowledge.
For another previous genetic MPB prediction model based on 11 SNPs from only 7 genetic loci plus age, Liu et al. [ 16 ] reported an AUC of 0.711 for no hair loss vs. any hair loss, which surprisingly was the same as what we achieved with our 117-SNP model with age (Table 2 ). Aiming to understand this apparent similarity of AUC while using more than 100 additional DNA preditors, we build a MPB prediction model in the UKBB model training subset based on 10 available of the 11 SNPs used by Liu et al. [ 16 ]. Considering age as additional predictor, this 10-SNP model achieved an AUC of 0.611 in the UKBB model testing subset (Supplementary Table 4 ), considerably lower than the AUC = 0.711 reported by Liu et al. for all 11 SNPs [ 16 ]. This 0.1 difference in AUC cannot be explained by the one missing SNP duplicated? in this comparative analysis. Rather, this may be explained by the larger effect of age on MPB prediction in the Rotterdam Study [ 16 ] (a contribution of 0.171 to the AUC), an ageing cohort with mean age of 68 years, considerably older than the UKBB (mean age 57) where age contributes only 0.06 to the AUC. Alternatively, higher prediction accuracies may result from the better investigator-conducted phenotyping in the Rotterdam Study compared with the self-reported UKBB phenotyping.
The recently reported genetic prediction model for MPB by Hagenaars et al. based on 331 SNPs [ 8 ], including the 270 SNPs we used in our feature selection analysis (the identity of only these 270 SNP predictors out of the 331 was publicly available at the time of writing) was built in 40,000 UKBB participants' samples that were also used for GWAS-based marker discovery. This model was internally validated in 12,874 additional UKBB samples by Hagenaars et al. and achieved AUCs of 0.78 for no vs. severe hair loss, 0.68 for no vs. moderate hair loss, and 0.61 for no vs. slight hair loss without considering age, and 0.79, 0.70 and 0.61, respectively, for models that additionally included age [ 8 ]. A direct comparison of these AUC estimates with the AUCs from our 4-category approach is difficult. In our analysis, we considered all individuals from all four categories to estimate AUC per each category, while Hagenaars et al. left out data that did not fit the predicted categories. Although we believe that using all data is the better way, we attempted a direct comparison by estimating the prediction accuracy of a model based on our 117 SNP predictors using the same analytical approach as Hagenaars et al. [ 8 ]. In our UKBB model testing subset, this analysis revealed AUC values of 0.81 for no vs. severe hair loss, 0.73 for no vs. moderate hair loss, and 0.63 for no vs. slight hair loss when age is included as a predictor, which were higher than those previously reported by Hagenaars et al. This different prediction performance may be explained by the larger model training dataset we used here, which generally increases the predictive performance of statistical models. These results, however, provide no evidence that the Hagenaars et al. approach of performing marker discovery and model training in the very same dataset led to over-inflated prediction accuracy.
Our study also demonstrates that it still is a long way towards highly accurate genetic prediction of MPB such as already available for eye colour with AUCs of 0.94 [ 3 , 4 ]. As next further step, all of the 624 MPB-associated loci identified in the entire UKBB data resource of >200,000 male subjects [ 2 ] shall be used for prediction modelling. This, however, can only be done once sufficiently large independent datasets for model training, testing, and external validation become available in the future. It is possible though, as implied by the results of our feature selection analysis, that inclusion of more SNPs may not necessarily lead to the envisioned significant improvement of MPB prediction accuracy, unless perhaps a large number is added. This is because GWASs with larger sample size are known to discover associated SNPs with smaller allelic effect sizes, which are expected to have smaller predictive value compared to SNPs previously identified in smaller-sized GWASs included in the current models. Although the new prediction models that we introduce here can reliably predict a good proportion of MPB risk in subjects of European ancestry and thus have good potential for practical use, future models may benefit from additional predicting biomarkers, for which also other types of biomarkers, such as epigenomic and metabolomic biomarkers, should be investigated.
In summary, we introduce new genetic prediction models for MPB that overcome limitations of previous models by including a large number of genetic predictors and utilizing large and independent datasets for the different analytical steps. Therefore our new models can be considered the most reliable genetic prediction models available to date for MPB and any other human appearance trait.
Supplementary information
Acknowledgements.
The authors are grateful to all participants of the UKBB Study and the Bonn Study for their participation. This study received support from the European Union’s Horizon 2020 Research and Innovation programme under grant agreement No 740580 within the framework of the Visible Attributes through Genomics (VISAGE) Project. YC was supported by the University of Chinese Academy of Science (UCAS) Joint PhD Training Program. None of the funding organisations had any influence on the design, conduct or conclusions of the study.
Author contributions
Conceptualisation: FL, MK; data curation: PH, YC, CM, SH-H, TDS; formal analysis: YC, CM; result visualisation: YC; funding acquisition: YC, MK; resources: PH, TDS, SH-H, MK; supervision: PH, SH-H, TDS, FL, MK; writing – first draft: YC, PH, FL, MK; writing – revised: YC, PH, FL, CM, MK; writing – final: YC, PH, FL, MK; final approval: YC, PH, CM, SH-H, TDS, FL, MK.
Data availability
Competing interests.
The authors declare no competing interests.
Ethical approval
The UKBB Study was approved by the North West Multi-center Research Ethics Committee (MREC) with details provided elsewhere ( https://www.ukbiobank.ac.uk/the-ethics-and-governance-council ). The Bonn Study was approved by the Ethics Committees of the Universities of Bonn, Düsseldorf and Essen, and informed consent was obtained from all participants.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The online version contains supplementary material available at 10.1038/s41431-022-01201-y.
- FUE Hair Transplant
- FUT Hair Transplant
- Beard Transplant
- Eyebrow Transplant
- Female Hair Transplant
- Crown Hair Transplant
- Afro Hair Transplant
- Forehead Reduction Surgery
- How Many Grafts Do I Need
- Hair Transplant Recovery
- Hair Transplant Growth Chart
- Consultation Questions To Ask
- Hair Transplant Costs UK
- Low Level Laser Therapy
- Female Hair Loss Treatment
- Before & After
- Hair Loss Facts & Statistics
- Female Pattern Baldness
- Male Pattern Baldness
- Ludwig Scale
- Norwood Scale
- Minoxidil For Beard
Finasteride
Dutasteride.
- London Harley St
- Our Surgeons
- Quality & Governance
- Our Mission, Vision & Values
- 2 Harley Street, London, W1G 9PA
- [email protected]
- 0207 935 1861

Is There A Cure For Baldness?

- Share on Twitter
- Share on Facebook
- Share on LinkedIn

Male pattern baldness affects millions of men. It’s estimated that 42% of men under 50 have moderate to extensive baldness, rising to 80% by the age of 70 [1-2].
Lots of male hair loss treatments are now available for male androgenetic alopecia . Unfortunately, despite plenty of ongoing research, there’s still no baldness cure.
In this article, we’ll explore why there’s no cure for baldness yet, as well as the latest developments in hair loss research to determine when a baldness cure might be on the horizon.
Why is there no cure for baldness?
Latest developments in curing baldness, how “caveman genes” made humans hairless, stem cells, hair follicles, and the tgf-beta protein, stress blocks hair growth activation proteins, gene editing for androgenetic alopecia, hair beads can regenerate lost hair cells, when will there be a cure for baldness, the latest hair loss treatments, are you interested in seeking treatment for your hair loss.
It’s difficult to find a cure for baldness because there are so many factors in play. One study found that more than 200 genetic elements contribute to pattern balding — and that’s before we even think about environmental and hormonal factors [4].
Key contributors to male pattern baldness include:
- Dihydrotestosterone (DHT) — this hormone reacts with androgen receptors in the hair follicle, causing irreversible hair shedding .
- Gender — female pattern hair loss is much less common than the male equivalent.
- Genetics — genes can determine your DHT levels and your follicular sensitivity to DHT.
- Age — pattern baldness generally worsens as you get older.
- Ethnicity — white men are more likely to experience pattern baldness than other ethnic groups [3].
- COVID-19 — evidence suggests Covid can make pattern hair loss worse [5].
Male pattern baldness is a complex condition. According to researchers, 79% of male baldness is hereditary and caused by so-called baldness genes [6]. This leaves 21% of cases where the cause is due to “nonshared environmental effects”. As a result, it’s hard to find a cure that addresses every contributing factor to permanently prevent progressive balding.
That said, researchers are continuously investigating hair loss to better understand the condition.

No developments suggest a baldness cure is on the horizon any time soon. However, some scientific discoveries could help other researchers unlock one in the future. Let’s take a look at some of the latest developments.
In January 2023, various UK news outlets reported that a baldness cure could be on the way following research that revealed humans’ so-called “caveman genes”. In this study, researchers discovered several genes that might explain how humans ultimately ended up becoming hairless, unlike most mammals [7].
It’s a bit of a leap to suggest this paves the way for a baldness cure. The research in question doesn’t attempt to find or test a cure for hair loss. But this study helps us better understand the evolutionary impact on human hair, which may ultimately prove useful for finding a baldness cure in future.
Stem cell therapy may eventually unlock the key to a hair loss cure [8]. These adaptable cells help the body regenerate or repair itself after injury by transforming into the required cell type or dividing to produce more stem cells.
Hair follicles, meanwhile, are the only cells in the human body that actively regenerate, regardless of injury or damage. Stem cell researcher Qixuan Wang explains the link between hair follicles and stem cells:
“Even when a hair follicle kills itself, it never kills its stem cell reservoir. When the surviving stem cells receive the signal to regenerate, they divide, make new cells and develop into a new follicle.”
Research published in June 2022 showed that a single protein (TGF-beta ) has a role in both follicle development and follicle death [9]. If future research can pinpoint how this protein activates cell division, it may be possible to artificially activate follicle stem cells to tackle baldness.
Redensyl is a relatively new and largely untested hair loss formula that uses plant compounds to target stem cells for hair growth.
An animal study published in December 2021 found that removing the adrenal glands in mice caused hair growth to improve threefold [10].
The adrenal glands produce cortisol when you’re stressed. Cortisol suppresses an essential hair growth protein known as Gas6. Removing the adrenal glands limits cortisol release, thus allowing Gas6 to activate hair growth.
Human adrenal gland removal isn’t on the cards. Your adrenal glands produce vital hormones that regulate metabolism and the immune system, among other essential bodily functions. But this research is important as it indicates a link between chronic stress and hair loss
Researchers claim:
“Our work identifies corticosterone as a systemic inhibitor of hair follicle stem cell activity [and] demonstrates that the removal of such inhibition drives hair follicle stem cells into frequent regeneration cycles, with no observable defects in the long-term.”
If we can find another way to reduce the impact of cortisol on the hair follicle, we may be able to cure stress-related baldness.

In a 2020 study, researchers used a gene editing technology known as CRISPR/Cas9 to modify the genes that cause hair loss [11]. Tiny ultrasound-activated particles carry CRISPR/Cas9 proteins to the site, where they modify the genes to prevent hair loss.
These results are promising, but this technology has only been tested on animals so far. There are also lots of ethical questions raised by gene editing practices, so a publicly available service is still a long way off.
In 2019, researchers in Japan proposed a new method for generating “hair beads” — a type of collagen-enriched cell aggregate (multiple cells of the same type that have bonded together) [12]. Hair beads can be transplanted into bald patches to regenerate lost hair cells.
They demonstrated that creating hair beads could be scalable — an important factor for cost-effectiveness and high demand. While this technology hasn’t been tested on humans yet, it paves the way for further investigation.
It’s unlikely there will be a cure for baldness any time soon. While scientists continue to conduct invaluable research that helps us better understand the condition, there aren’t yet any groundbreaking inroads towards a permanent cure.
Even when a cure has been touted, it’s likely to undergo several years of testing before it’s approved for widespread public use. Medical authorities need to understand the long-term effectiveness and analyse any adverse effects before licensing a baldness cure.
A baldness cure may not be around the corner, but there are already lots of treatments that are proven to help tackle male pattern baldness. They can’t stop baldness at the source, but they can slow and even reverse hair loss.
Here are some of the most popular baldness treatments recommended to slow hair loss and stimulate hair growth.
Finasteride stops your body from converting testosterone into DHT. It doesn’t desensitise your hair follicles to DHT, but it means there’s a lot less of the hormone reaching your follicles. It’s currently the most recommended treatment among hair transplant practitioners worldwide [13].
Minoxidil dilates the blood vessels around areas of balding or thinning hair, stimulating blood flow to the follicles. With increased blood flow comes increased oxygen and nutrients available to the follicles, which is essential for healthy hair growth.
If you are unhappy about losing hair, the good news is that there is something you can do about it. Treatments for hair loss have come a long way from the days of bad hair plugs. Expert treatment is available for those individuals seeking hair restoration including surgical treatments, hair loss medications, and alternative treatments such as low-level laser therapy.
If you would like to discuss what our hair loss specialists can do for you, then contact us today. Our team is on hand to find the best hair loss treatment for you, designed around your needs and hair restoration goals.
We also specialise in female hair loss treatment where we pride ourselves in providing the very best care to our female patients including diagnosing the cause of their hair loss and recommending suitable treatments to restore hair and prevent further hair loss.
Book a consultation at the Wimpole Clinic to get started.
- Prevalence of male pattern hair loss in 18-49 year old men
- Androgenetic Alopecia
- Male Androgenetic Alopecia
- Genetic prediction of male pattern baldness
- COVID-19 Can Exacerbate Pattern Hair Loss and Trigger Telogen Effluvium – The Role of Proteoglycan Replacement Therapy with Nourkrin® in Clinical Treatment of COVID-19 Associated Hair Loss
- Hair Loss Among Elderly Men: Etiology and Impact on Perceived Age
- Complementary evolution of coding and noncoding sequence underlies mammalian hairlessness
- Advances in Stem Cell-Based Therapy for Hair Loss
- A probabilistic Boolean model on hair follicle cell fate regulation by TGF-β
- Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence
- Ultrasound-activated particles as CRISPR/Cas9 delivery system for androgenic alopecia therapy
- Preparation of hair beads and hair follicle germs for regenerative medicine
- ISHRS Practice Census 2022
- A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia
- Topical ketoconazole for the treatment of androgenetic alopecia: A systematic review
- Caffeine and Its Pharmacological Benefits in the Management of Androgenetic Alopecia: A Review
- Efficacy of a Cosmetic Caffeine Shampoo in Androgenetic Alopecia management. II Note
Blog search

Book a consultation
Simply fill in your details in the form below and we'll get in touch with you shortly.
- Clinic * Choose Clinic Location* London, Harley Street Birmingham Brighton Bristol Leicester Manchester Newcastle Nottingham Oxford Tunbridge Wells Mayfair Hair (VIP)
- I consent to my information being used to contact me regarding my enquiry in accordance with our Privacy Policy
- I would like to receive information, promotions and offers via email

IMAGES
VIDEO
COMMENTS
A receding hairline, a total loss of hair from the crown, and ultimately, the classical horseshoe-shaped pattern of baldness: Previous research into male pattern hair loss, also termed ...
Male pattern baldness affects around 85% of men by the age of 50. Photograph: YAY Media AS/Alamy. In recent years, scientists have discovered that baldness has its origin in the loss of ...
By the age of 50, between 30 and 50 per cent of men have begun to experience male pattern baldness. Despite there being plenty of handsome hairless men out there - I'm looking at you, Thierry ...
Male pattern baldness is one of the most common types of hair loss. Dutasteride does have more severe side effects than several of the treatments, however, including a loss of sexual drive and the ...
The new regenerative and innovative strategies in hair loss. The number of clinical trials evaluating new regenerative and innovative strategies in male and female pattern hair loss have exponentially increased during the last five years. Regarding the innovative strategies, the efficacy of a specific compound obtained from botanical extracts ...
Male-pattern baldness (MPB) is related to dysregulation of androgens such as testosterone. A previously observed relationship between MPB and skin cancer may be due to greater exposure to ...
Genetic prediction of male pattern baldness (MPB) is important in science and society. Previous genetic MPB prediction models were limited by sparse marker coverage, small sample size, and/or data ...
New research pins baldness to a single chemical. A single chemical could be responsible for whether people go bald or not, a new study has found. In the UK, approximately two thirds of men will ...
Male pattern baldness (MPB), or androgenetic alopecia, is a highly heritable, age independent appearance trait [1, 2]. MPB has socio-psychological consequences on a large proportion of the world ...
In male and female pattern baldness, many hair follicles still exist but are dormant. The search for new drugs that reawaken follicles and induce hair growth has been limited by the field's focus on finding drugs that work along the same pathways as finasteride and minoxidil, the only two drugs currently available for men with male pattern baldness.
Scientists from Northwestern University say they've made a breakthrough that could potentially cure male pattern baldness, a common and frustrating condition resulting in hair loss in men. The ...
About half of men undergo male-pattern baldness, some starting in their 20s. When women lose hair, it's often a more general thinning, but it's no less a blow to self-image.
Baldness Breakthrough - Scientists Uncover 5 Key Genes. A study by the University Hospital of Bonn and the University of Bonn has advanced our understanding of male pattern hair loss by identifying the significant role of rare genetic variants. Analyzing data from over 72,000 men, the research identified five key genes, paving the way for ...
In one recent study, for example, Christiano's team found that JAK inhibitors also reawaken dormant hair follicles, a problem common to male and female pattern baldness. They also discovered a previously unknown type of immune cell that puts hair follicles into a dormant state by secreting a substance called oncostatin M and that the hair ...
Learn about hair loss prevention, treatment options for alopecia, male pattern baldness, hair loss in women, and thinning hair in both men and women. Your source for the latest research news
Introduction. Male pattern baldness affects around 80% of men by the age of 80 years [], and it can have substantial psychosocial impacts via changes in self-consciousness and social perceptions [2, 3].In addition to alterations in physical appearance, some, but not all, studies have identified negative health outcomes associated with baldness including increased risk of prostate cancer [4-6 ...
Alopecia areata. In June 2022, the FDA approved the first treatment for a type of hair loss in which the immune system attacks hair follicles, known as alopecia areata. The drug, called ...
A new paper in PLoS Genetics describes discoveries related to the genetics of male pattern baldness, with substantial contributions from the 23andMe community.. Early Onset Baldness. Led by Dr. Brent Richards of McGill University, the study combined genome-wide association data from seven cohorts, comparing men with "early onset" male pattern baldness with older men who had experienced ...
Male pattern baldness is the most common form of hair loss among aging men. New research shows how hair follicles communicate across different areas of the body. Male pattern baldness, also known ...
Other signs of male-pattern baldness include thinning hair and a hairline that moves farther back on your head (receding hairline). There are seven stages of male pattern baldness according to the Hamilton-Norwood scale: Stage 1: There's little or no hair loss or hairline recession. Stage 2: There's slight hair loss near the skin between ...
Hair loss is no longer an inevitable march to baldness. Medical advances over recent decades mean male hair loss can be treated. And it need not break the bank. The cause of male pattern baldness ...
Male pattern baldness (MPB), or androgenetic alopecia, is a highly heritable, ... MPB is of interest to various fields of scientific research and applications, such as in medicine, anthropology, evolutionary biology, and forensics. ... we developed new genetic prediction models for MPB based on a large number of DNA markers and four large ...
Male pattern baldness affects millions of men.It's estimated that 42% of men under 50 have moderate to extensive baldness, rising to 80% by the age of 70 [1-2]. Lots of male hair loss treatments are now available for male androgenetic alopecia.Unfortunately, despite plenty of ongoing research, there's still no baldness cure.