

Publications — Over 100 years of publishing excellence
- Book Author Resources
- Submit a Book Proposal
- AMS Rights, Licensing, and Permissions
- Open Math Notes
- Frequently asked questions
- Member Journals
- Research Journals
- Translation Journals
- Distributed Journals
- Open Access Journals
- Guidelines and Policies
- Journal Author Resources
Librarian Resources
- eBook Collections
- COUNTER Usage Statistics
- My Subscriptions
- Subscription Information
- Licensing Information
Mathematical Reviews/MathSciNet®
- MathSciNet ®
- Reviewer Home
- MathSciNet ® Subscriptions
Membership — Welcome to your membership center
Join the ams, renew your membership, give a membership, individual membership.
- Member Benefits
- Member Directory
- Reciprocating Societies
- Members in Developing Countries
Institutional Membership
- Domestic Institutions
- International Institutions
- Two-Year Institutions
- Graduate Student Chapter Program
Other Member Types
- Corporate Memberships
- Associate Memberships
Meetings & Conferences — Engage with colleagues and the latest research
National meetings.
- Joint Mathematics Meetings
- Upcoming JMMs
- Previous JMMs
- Special Lectures
- Professional Enhancement Programs (PEPs)
Sectional Meetings
- Upcoming Sectionals
- Previous Sectionals
- Presenting Papers
- Hosting Sectionals
Other Meetings, Conferences & Workshops
- Mathematics Research Communities
- Education Mini-conference
- International Meetings
- Mathematics Calendar
- Short Courses
- Workshop for Department Chairs and Leaders
Meetings Resources
- Suggest a Speaker
- AMS Meetings Grants
- Submitting Abstracts
- Welcoming Environment Policy
- MathSafe – supporting safe meetings
News & Outreach — Explore news, images, posters, and mathematical essays
News from the ams.
- AMS News Releases
- Feature Stories
- Information for Journalists
- In Memory Of
Math Voices
- Feature Column
- Math in the Media
- Column on Teaching and Learning
Explorations
- Recognizing Diverse Mathematicians
- AMS Posters
- Mathematics & Music
- Mathematical Imagery
- Mathematical Moments
Professional Programs — Resources and opportunities to further your mathematical pursuits
Professional development.
- Employment Services
- Mathjobs.org
- BEGIN Career Initiative
- Mathprograms.org
- Mathematical Opportunities Database
- Research Seminars
Institutional Information and Data
- Annual Survey of the Mathematical and Statistical Sciences
- CBMS Survey
- Other Sources of Data
- Directory of Institutions in the Mathematical Sciences
- Professional Directory
Grants & Support
- AMS-Simons Grants for PUI Faculty
- Travel Grants
- Fellowships & Scholarships
- Epsilon Fund
- Child Care Grants
Awards & Recognition
- AMS Prizes & Awards
- Fellows of the AMS
Education — Resources to support advanced mathematics teaching and learning
For students.
- Information for Undergraduate and High School Students
- Research Experiences for Undergraduates (REUs)
- Considering Grad School
- Find Grad Programs
- Applying to Grad School
- What do Mathematicians Do?
For Teachers
- Teaching Online
- Teaching Resources
- Inclusive Classrooms
- Assessing Student Learning
- Education Webinars
For Department Leaders & Mentors
- Information for Department Leaders
- paraDIGMS (Diversity in Graduate Mathematical Sciences)
Government Relations — Advocating for the mathematical sciences
Elevating mathematics in congress.
- Our Mission
- Letters, Statements, & Legislation
- Congressional Briefings
Legislative Priorities
- Federal Issues of Concern
- Federal Budget Process
Get Involved
- Advocacy Resources
- Take Action
DC-Based Fellowships
- Congressional Fellowship
- Mass Media Fellowship
- Catalyzing Advocacy in Science & Engineering (CASE) Fellowship
Giving to the AMS — Your gifts make great things happen for mathematics Make a Gift
What you can support.
- The 2020 Fund
- Next Generation Fund
- Birman Fellowship for Women Scholars
- JMM Child Care Grants
- MathSciNet for Developing Countries
Create a Legacy
- Make a Tribute Gift
- Create a Permanent Fund
- Establish a Prize, Award or Fellowship
- Bequests and Charitable Estate Planning
Honoring Your Gift
- Donor Stories
- Donor Wall of Honor
- Thomas S. Fiske Society
- AMS Contributors Society
- AMS Gardens
Giving Resources
- AMS Development Committee
- AMS Gift Acceptance Policy
About the AMS — Advancing research. Connecting the mathematics community.
Our organization.
- Executive Staff
- Equity, Diversity, & Inclusion
- Jobs at AMS
- Customer Service
Our Governance
- Board of Trustees
- Executive Committee
Governance Operations
- Calendar of Meetings
- Policy Statements & Guidelines
Welcome to the Feature Column
Mathematics and chemistry: partners in understanding our world.

Introduction
Which came first, the chicken or the egg? Trying to answer this question is a bit like trying to ask for mathematics, which came first, theoretical mathematics or applied mathematics? In a series of columns I plan to demonstrate that theory and applications of mathematics are, and have always been, linked together. Typically mathematics generated seemingly only for intellectual curiosity eventually finds use outside of mathematics itself. Moreover, questions born outside of mathematics usually spark new theory. Society and mathematics are both better off because of this partnership. Here I will give a sketch of some of the issues arising from using mathematics in fields outside of mathematics, but for this column I will use examples from the area of chemistry. Not surprisingly as mathematics has produced more tools for chemists to put to use, the chemists have done so. For example, chemists use group theory to study aspects of their field, but this as a mathematical tool came much later than the first work that made chemistry more "mathematical." Of course, it was a revolution just to develop an experimental approach to chemistry - carrying out procedures under controlled circumstances many times and seeing if one got repeatable results. Controlled experiments open the door to using mathematical tools from statistics and from the field of experimental design.
When people think about theoretical mathematics they often think of the Pythagoreans and Euclid's Elements . For example, the Pythagoreans are reputed to have been the earliest to think about the issue of irrational numbers. A number which is the ratio of two integers a and b , where b is not zero, is called rational. For measuring lengths, areas, and volumes in practical terms the way a chemist might need to, one can get away with only rational numbers. Despite the fact that one can conceptualize about numbers, the real numbers, which are not all rational, these numbers and lengths can be approximated "arbitrarily" close using only rational quantities. Famous examples of irrational numbers are √ 2 , π, and e (the base of the natural logarithm system -although base 2 and 10 are used more often). A chemist might be interested in knowing the volume of the region in space bounded by a Euclidean sphere whose exact value is (4/3)πr 3 , but for any particular radius it is good enough to work with rational numbers. Chemists also don't particularly care if one represents numbers in base 10 or with some other system. The units for measurements that are chosen vary from the metrical system to the one still used, with many disadvantages, in the United States.
The world of chemistry involves the creation of molecules from the atoms that occur naturally in the world. There are 92 such elements and they have complex properties - some are gases, some are liquid, and some are solid at "room" temperature (although there is some debate about the number of naturally occurring elements , 92 is the number most often cited). From the very beginnings of chemistry, mathematics was used to create quantitative and qualitative models for helping comprehend the world of chemistry by understanding the elements that make up molecules. An atom is made up of particles which are known as protons, neutrons, and electrons. Measurement issues concerning these particles are a big part of what chemistry is about. Protons, neutrons, and electrons have mass and they have electrical charge (or the lack thereof) and mass and charge can be measured. Patterns in the mass and charge of atomic particles helped chemists get insight into the nature of atoms and the molecules these atoms can form. We take for granted today that molecules are made from elements of different kinds but this insight is relatively new. We now know there are 92 naturally occurring types of atoms, though some of these elements come in different forms, where the atoms differ in their atomic weight. Numbers have been used to understand the structure of these elements and to classify them into families with similar kinds of chemical properties. This leads to the notion of the periodic table - to try to organize the elements into "clusters" which have similar properties, and then use this information to understand the properties of these elements. While history has governed the form used to display the "periodic" nature of the elements, various new proposals have been made that some suggest would be more insightful than the usual periodic table . After all chemists, too, look for patterns just the way mathematicians do! While not surprisingly those who contribute to chemistry have training in chemistry, they can still put to use the mathematics they learn in support of their research into chemistry. However, it is also true that people who start as mathematics majors can become very distinguished research chemists. One dramatic example is the career of Herbert Hauptman , who started out as a mathematics major (at City College in New York) but eventually went on to win the Nobel Prize in Chemistry. .

Herbert Aaron Hauptman (1917-2011) (Courtesy of Wikipedia)
One interaction of mathematics and chemistry that is so familiar now that it is taken for granted, is the way certain quantities are balanced or preserved when a chemical reaction occurs. In a chemical reaction the masses of the inputs to the reaction must be the same as the masses of the products produced by the reaction. A good example of the complex ways mathematics helps one grasp chemistry issues is the recent concern with rising levels of carbon dioxide in the atmosphere, and the pollution that comes from using coal as a source of energy. One way to deal with the issue of the negative aspects of burning coal is to use more natural gas, whenever possible, as a source of energy. One of the major components of natural gas is methane. Methane is an example of a hydrocarbon , a molecule made up of hydrogen and carbon atoms. Methane when ignited undergoes a chemical reaction that releases energy. The energy is in part stored in the bonds that keep the molecule from separating into the components that make up methane - the hydrogen and carbon atoms. In Figure 1, if one knows that methane and oxygen mix to produce carbon dioxide and water, the question is how much of each of the "reagents" are involved? Where do the coefficient numbers shown for the chemical reaction below, the 1, 2, 1, 2 respectively, come from?

Figure 1 (Balancing equations of molecular reactions) (Courtesy of Wikipedia)
It turns out that one can use ideas from the algebra learned in high school (or in a linear algebra class) to use systems of linear equations to "balance" chemical reactions in this spirit. The idea is to introduce letters for the molecules involved and then to use chemical principles for producing these equations. For example, in the equation above we have four molecules CH 4 , O 2 , CO 2 , and H 2 O. Suppose we use the letters x, y, z, w for the numbers of these molecules, respectively. Can we deduce relationships between the values of x, y, z and w ? Since C appears on the left and right of the "equation" (bottom of Figure 1) we can say that x = z . What about oxygen? We have 2 y = 2 z + w because there are two oxygen atoms on the left, and two atoms in the carbon dioxide and one in the water. Finally for H, we have 4 x = 2 w . Notice here we have more "unknowns" than equations and if there is a way to balance the this reaction we will need to use nonnegative integers as the values for x, y, and z . Why not try a solution where we find the unknowns, y, z, and w in terms of x ? Using some simple algebra we see that: y = 2 x z = x w = 2 x The smallest value x can have is 1. What does this mean for the other values? if x = 1, then y must be 2, z must be 1, and w must be 2. Thus, we get exactly the values for the coefficients of CH 4 , O 2 , CO 2 , and H 2 O we see in Figure 1. In some cases the linear equations that we get have no positive integer solutions. Either by predicting the amounts of reagents in a chemical reaction or showing that the reagents can't combine in a "legal" way, linear algebra is a tool for insight.
Visual Tools
From early on chemists have used diagrams to help them think through issues involving molecules. For example, here is the way a methane molecule might be drawn.
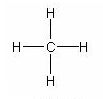
Figure 2 (Diagram of methane molecule. Courtesy of Wikipedia)
Other diagrams might give more of a sense that the methane molecule is three-dimensional, and give a more accurate feel for the molecule because the labels give information such as bond length or angles between bonds. In Figure 3 the drawing is designed to show the "tetrahedral" nature of the methane molecule, which is harder to see in Figure 2.

Figure 3 (Diagram which tries to show 3-dimensional information about the methane molecule. Courtesy of Wikipedia)
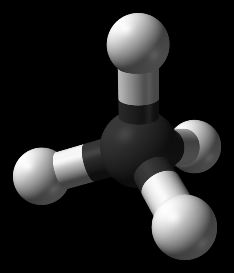
Figure 4 (Representation of the methane molecule. Courtesy of Wikipedia)
While the material I am about to discuss is probably less important in the grand scheme of things to chemistry than some other ways mathematics is helping the subject, it has the advantage of being accessible with less mathematical background. One of the things mathematics typically does is formalize "ad hoc" tools and use the general theory that evolves from the mathematical approach to advantage. Diagrams such as the one chemists might draw can be considered in the framework of geometrical diagrams called graphs . Graph theory involves studying the properties of diagrams that make use of dots and line segments. The dots of a graph are called vertices and the line segments are called edges . When chemists use these diagrams they are usually called molecular graphs or structural formulas . A structural formula for methane is shown in Figure 5. The dots represent atoms, labeled to show what kind of atom is involved, and the edges represent bonds formed between the atoms. Different authors use the same or similar terms in different ways. Here the diagram does not have metric (distance) information, and the angles between bonds and the lengths of bonds is not part of the information that the diagram is conveying. So graph theory is a kind of geometry but geometry that does not use "metrical" information directly.
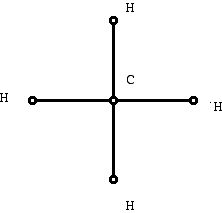
Figure 5 (Graph representing methane)
While it may seem like a small step to use Figure 5 instead of Figure 2, it is larger than appears at first glance. Using the mathematical results and ideas from the mathematics of "graph theory" opens many new perspectives for how to think about the chemistry of molecules which might not have occurred otherwise. It is natural to object that diagram in Figure 2 has been drawn in the plane, surely the methane molecule exists in three dimensional space, that it might be helpful to use a more "accurate" diagram. However, what mathematical modeling does is to make simplifying assumptions and in this case it is very helpful to work with a plane diagram even though it loses information. Notice that even if the vertices are not labeled, if one knows that this molecule is representing a molecule consisting of hydrogen and carbon, one can recover the location of the carbon and hydrogen atoms. This is because of the notation of "valence." Different atoms "bond" with other atoms in a particular way, and this knowledge from chemistry guides what one does with graphs of chemical molecules. Here are two other structural diagrams:
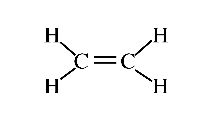
Figure 6 (Diagram showing double bond. Courtesy of Wikipedia)
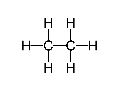
Figure 7 (Hydrocarbon with a tree structure. Courtesy of Wikipedia)
While they differ in the size of the print and the lengths of the line segments shown, they share the feature that only carbon and hydrogen atoms are present, and, thus, such molecules are known as hydrocarbons. Also notice that one of the diagrams indicates that two carbon atoms are joined with two bonds though the two molecules have the same number of carbon atoms, they differ in the number of hydrogen atoms. Thus, you will not be surprised if these molecules had different chemical and physical properties. What aspects of such diagrams make them "legal" from the point of view of a chemist? What about the diagram:
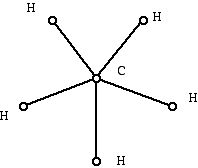
Figure 8 (Graph which can't represent a hydrocarbon)
Chemists know that the diagram in Figure 8 cannot be the structural diagram for a hydrocarbon. On the other hand, the diagram below (Figure 9):
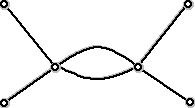
Figure 9 (Graph used to represent a hydrocarbon with double bond)
can be labeled with C's and H's so that the result is a "legal" hydrocarbon - one with two carbon atoms and four hydrogen atoms. Thus, this is a graph theory version of the diagram in Figure 6. Notice that if we count the number of edges at each vertex there are two vertices which have four edges at them and 4 vertices that have one edge at them. If we add up these numbers we get 4 + 4 + 1 + 1 + 1 + 1 = 12, which is twice the number of edges. This is a general fact, that since each edge has exactly two end points, adding up the number of edges at a vertex will always yield twice the number of edges.
What governs such diagrams from a chemist's point of view is that different kinds of atoms can form only specific allowable numbers of "bonds" with other atoms. Use of the word valence in graph theory and the theory of polytopes is "borrowed" from the chemical notation of valence. Graph theorists capture the idea of "bonding" between atoms with the concept of degree or valence of a vertex of a graph. Consider the graph below (Figure 10). It has 6 vertices and 18 edges. However, some of these edges join a vertex to itself, called a loop or self-loop, and for some vertices there is more than one pair of edges that connect the same pair of vertices. When the graph has such vertices, joined by several edges, the edges involved are called multiple edges. Some definitions of graphs don't allow for loops and multiple edges. (Some authors call graphs where loops as well as multiple edges are allowed pseudographs, while the term multigraph is reserved for the case where there are no loops but multiple edges are allowed.) But for the kind of models we want to use here, chemistry applications, we definitely want to allow multiple edges because structural formulas with "double bonds" are not uncommon. While some authors restrict the word graph to mean dot-line diagrams without loops or multiples edges, here we will allow both.

Figure 10 (Diagram of a graph with loops and multiple edges. Courtesy of Wikipedia)
In mathematics one has the "freedom" to define terms so that they capture important applications ideas (like valence in chemistry) or because they have mathematical appeal. It would be tempting to define the valence or degree of the vertices in the graph in Figure 10 as the number of edges at a vertex. However, when one does this, it is not true that the sum of the valences of the vertices of the graph adds to twice the number of edges. The "culprits" are the loops, which contribute not one edge at a vertex but two "ends" at a vertex. With this in mind we will use the following definition of valence or degree of a vertex. Given a vertex v of a graph G the degree of the vertex is the number of local line segments at the vertex. This means that in Figure 10 we have the following valences for the vertices: valence( v ) = 4 valence( w ) = 6 valence( x ) = 6 valence( y ) = 5 valence( u ) = 6 valence( z ) = 9 Blue edges contribute 2 to the valence of vertices they are present at, red edges (those that are multiple edges) contribute 1, while gray edges contribute 1 as well. Note that some vertices have odd valence (odd number as the valence) while some have even valence (even number as the valence). If we add the numbers 4 + 6 + 6 + 5 + 6 + 9 we get 36, which is twice the number edges, so we have 18 edges. Theorem: The degrees or valences of a graph sum to twice the number of edges. Corollary: The number of vertices of a graph of odd valence is even. Proof: The sum of an odd number of odd numbers is odd, and so to have an even sum for the valences, we have to have an even number of odd-valent vertices.
Graph theory in chemistry
The value of graph theory to chemistry started to become apparent in the 19th century. Work by two British mathematicians, Arthur Cayley (1821-1895) and James Joseph Sylvester (1814 -1897), laid the ground for a long tradition of successful use of graph-theoretical ideas in chemistry.

Arthur Cayley

James Joseph Sylvester
These two individuals, very different in their backgrounds and temperaments, were also remarkable for having been good friends. Sylvester never married but Cayley together with his wife came to America to visit Sylvester during his stay at Johns Hopkins University in Baltimore. For better or worse, when the term graph is used in mathematics it can mean "graph" of a function like y = x 2 or a dots and lines diagram such as that in Figure 10. It was Sylvester that we have to thank for introducing this second sense of the word "graph." Perhaps surprisingly, the first use of the word "graph" for a curve is relatively recent, too. It is Cayley's work in using what are today called graphs in chemistry that is quite significant in terms of what he accomplished and what it inspired people who followed him to do. Cayley, pioneer that he was here, had to develop his own terminology. He used the terms plerograms and kenograms for the two kinds of graphs that one might consider when studying hydrocarbons which are trees (graphs in one piece, with no circuits) when drawn as graphs. The plerogram would show all of the atoms of the molecule involved, hydrogen as well as carbon atoms. The kenogram would show only the carbon atoms. One of the early discoveries of chemists was that molecules with the same number of carbon and hydrogen atoms (by way of an example) could have different physical or chemical properties. Chemical properties involve such things as such as which other substances a substance will react with. Physical properties include things like boiling point. Molecules which are the same chemically but different in physical terms are called isomers of each other. When molecules are represented by graph diagrams these isomers correspond to the fact that the graphs associated with them are not isomorphic (they have different structures). Graph theory is a part of combinatorial geometry (how things fit together) in the sense that its basic ideas are not rooted in metrical (area, angles, distance, etc.) but rather in structural properties. However, with some ingenuity one can obtain surprisingly large amounts of information from graphs by looking at properties that they have. We will now explore a bit of these ideas. Consider the graph in Figure 11 . What properties does it have?

Figure 11 (A connected graph used to model a hydrocarbon)
Graph theorists have developed terms for how to move around in graphs to get from one vertex to another by moving along edges. For example there are several paths from vertex e to vertex i in graph H in Figure 11. Ecbfglhi, ecflghi , and ecbglhi are all examples of different paths. These paths have lengths 7, 6, and 6 respectively because they use this number of edges in getting from e to i . However, there are "shorter" paths from e to i in the sense that they use fewer edges. Both ecbghi and ecflhi have length 5. We can define the distance between two vertices in a connected graph, (a graph having one piece) as the length of the shortest path between them. The distance between vertices e and k is 4. There is a unique path which has this distance, while the distance between e and g is 3 but there are two that give this shortest distance, ecfg and ecbg . Note that we can interpret this diagram as that of a hydrocarbon, since all its vertices are either 4-valent or 1-valent. Some connected graphs have only one path between every pair of vertices (Figure 13). Such graphs cannot have any circuits ( cbgfc and fglf are examples of circuits in the graph above). Given two vertices in a circuit, there are two paths between them along the two parts of the circuit that connect the vertices. A graph without circuits is known as a tree and trees have many nice properties , including the fact that except for the tree with a single vertex, they have at least two 1-valent vertices. Another nifty property of a tree is this: Theorem 1: If T is a tree where the number of vertices of T is n , then the number of edges of T is n - 1. Perhaps even more remarkable is that the converse of this theorem is also true: Theorem 2: If one has a connected graph T which has n vertices and n - 1 edges, then the graph T must be a tree! One of the accomplishments of Cayley was showing that trees play a particularly important role in getting insight into problems in mathematical chemistry. Sometimes one knows for chemical reasons the numbers of hydrogen and carbon atoms that are part of a series of chemical molecules. For example, the alkanes are a family of hydrocarbons where, if there are n carbon atoms, there are 2 n + 2 hydrogen atoms, so the alkanes obey: C n H 2 n +2 . What will the graphs of these molecules look like? Using some simple graph theory we can see that these molecules must have a tree structure! Since chemical molecules are connected, this means that an alkane has a total of n (carbons) + 2 n + 2 (hydrogen) atoms. So in a graph of such a molecule we have 3 n + 2 vertices. However, carbon is 4-valent in such a diagram while hydrogen is 1-valent. Thus we have that 4 n (4 times the number of carbon molecules) + 1(2 n +2) (1 times the number of hydrogen atoms) = 4 n + 2 n +2 = 2(3 n +1), which is twice the number of bonds in the molecule. So the number of edges (bonds) in the graph of the molecule is 3 n +1. Since the number of vertices 3 n + 2 is one more than the number of edges, 3 n +1, we can conclude by Theorem 2 that the diagrams for alkanes are always trees! We can put together two aspects of graphs to get a fuller understanding that is useful to chemists. Given a connected graph, where are the vertices that are "in the center" or "towards the middle" of the graph? Questions of this kind date back to the French mathematician Camille Jordan, who is best known for the " Jordan Curve Theorem ."

Camille Jordan
One approach to explaining in the "center" of a graph is to use these definitions. A vertex u of a (connected) graph is called central if the eccentricity of u is as small as possible. The eccentricity of a vertex u is the farthest distance any vertex can be from u .
Remember that the distance between two vertices is the length of a shortest path between them. In Figure 11, for example, vertices d, e, i and j have eccentricity 5, vertices a, c, h and k have eccentricity 4, and vertices b, f, g and l have eccentricity 3. The central vertices in Figure 11 are b, f, g, and l . The center C of a connected graph G consists of all its central vertices together with those edges that are present in G that join vertices of C. (Sometimes this is described by saying the center of a connected graph is the subgraph of G "induced" or "generated" by the central vertices.) In Figure 11 the center consists of 4 vertices ( b, f, g, and l ) together with the 5 edges, bf, bg, fl, gl , and fg . While these definitions are intuitively appealing, for some graphs they don't give much "useful" information in a sense because every vertex can be central and thus the center of the graph is the graph itself. An example of this kind is the 3-dimensional cube, shown in two different but isomorphic drawings in Figure 12. For the cube every vertex has eccentricity 3. Cubes have lots of symmetry and since all of the vertices are alike, they all wind up as central vertices.
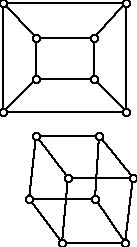
Figure 12 (Two different representations of a 3-dimensional cube)
However, the special insight here is that for trees with at least 3 vertices not every vertex can be in the center of a tree.
Theorem: If T is a tree then the center of T is either a single vertex or a pair of vertices joined by an edge. Here is an example of a tree (which represents a hydrocarbon molecule) whose center is a single edge, shown in red.
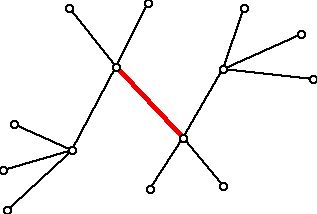
Figure 13 (Tree with a center consisting of an edge)
Another remarkable property of trees and their centers is that if one has a tree T with at least 3 vertices and removes the 1-valent vertices from the tree, the new tree T* has the same center as the original tree. For example, the tree below has the red graph left after its 1-valent vertices are "pruned." The resulting red tree has as its center a single vertex, which is the same as the center of the unpruned tree.

Figure 14 (Pruned hydrocarbon tree in red; center a single vertex)
When Cayley first began doing his work on isomers of hydrocarbons which were trees, he had to contend with making a decision about what graph theory model to use for a molecule. Tree molecule hydrocarbons have 4-valent and 1-valent vertices and we have just seen that the center of such a tree molecule is the same for the graph where the 1-valent vertices are deleted. Cayley looked at two different approaches. In one he drew the diagram he called a plerogram which includes the 1-valent and 4-valent vertices, and in the other, he drew the graph which only consisted of the carbon atoms - the structure he called a kenogram. Butane C 4 H 10 has two isomers and the kenographs for these are n -butane (4-vertex path) and isobutane. These two "kerograms" are shown in Figure 15.

Figure 15 (Two isomers of butane, carbon atoms only)
Can you draw the plerograms for these two isomers? Long after Cayley, Sylvester and Jordan showed that graph theory ideas were of interest to mathematicians and to researchers outside of mathematics, a chemist invigorated the use of graph theory in chemistry by inventing the Wiener Index, an invariant of a graph that is now named for him. The issue for chemists and mathematicians interested in chemistry is whether information about the graph of a molecule allows one to "predict," perhaps even approximately, different chemical or physical properties of a molecule. For molecules that form liquids at room temperature such properties might include boiling point, density, viscosity, etc. This has proven to be true.
Wiener Index
Harry Wiener was a more recent pioneer in trying to develop an "index" for the graphs of chemical molecules which would help one grasp the physical properties of a molecule by computing information from the graph of the molecule. Wiener's idea was to look at the distances between the vertices of the graph that represented the molecule. If the molecule is a tree, what graph would best capture the properties of the molecule? One could use the graph of the connections between the carbon molecules only (kenogram), or one could use the full graph with carbons and hydrogen atoms (plerogram). For the original Wiener Index (a term used after Wiener's work) Wiener chose to use the graph of the carbon atoms only. So the Wiener Index of a hydrocarbon is the sum of the lengths of all of the shortest paths between the vertices of the graph of the hydrocarbon consisting only of the carbon molecules. For example, let us compute the Wiener Index for the two graphs in Figure 15. Each of these graphs has 4 vertices, so we will have to add up 6 (the number of ways of selecting 2 vertices from 4) distances. For the top graph in Figure 15 we have: 1 + 2 + 3 + 1 + 2 + 1 = 10 while for the lower graph we have 1 + 2 + 2 +1 + 1 + 2 = 9. While often the Wiener Index for non-isomorphic graphs is different, there are also non-isomorphic graphs which have the same Wiener Index. This is not surprising. Mathematicians have been trying for a long time to find an easy way to tell that two graphs are isomorphic, some collection of numbers which, if these numbers are different, would guarantee that two graphs were non-isomorphic. No such "invariants" have been found nor are likely to be found. In fact, the exact complexity of the "Graph Isomorphism Problem" in the computer science sense - how much "work" is required for two graphs with n vertices to be shown to be isomorphic - is still an unsolved question. Many variants of the Wiener Index now have been explored, including not only using all of the atoms in the graph of the molecule, but when there are 1-valent vertices using only the distances between 1-valent vertices. The Wiener Index also inspires graph theory problems which may have little relation to chemistry. For example, given an integer s , is there a graph whose Wiener Index is s ? Other individuals who have tried to find graph invariants of value in chemistry, as well as working on other questions in mathematical chemistry , include Alexandru Balaban, Danail Bonchev, Ante Graovac, Ivan Gutman, Haruo Hosoya, Milan Randic, D.H. Rouvray, and Nenad Trinajstic.
Other mathematics used in chemistry
Sometimes issues of "mathematical appeal" govern attempts to discover molecules with what might have valuable properties or whose synthesis will develop new chemical "assembly" methods. For example three of the platonic solids , the tetrahedron, the cube, and the dodecahedron have vertices which are 3-valent. Can one construct hydrocarbons which have their carbon atoms at the vertices of these solids, and additional hydrogen atoms to achieve 4-valence at all of the vertices of the original solid? In particular, Figure 12 shows a 3-cube, but the right angles at the vertices made chemists wonder if such angles could be achieved for the carbon atoms of an actual molecule. Responding to this challenge, Philip Eaton and Thomas Cole constructed cubane and related compounds. Similarly, dodecahedrane has also been synthethesized but "tetrahedrane" has still not been made! Many important applications of mathematics to chemistry involve spectra. The idea is that when a molecule moves from one energy state to another, some "signature" of the molecule can be determined, i.e. different molecules can be identified because the measurements in energy changes are characteristic of specific molecules. This work involves the idea of eigenvalues of matrices (array or table of numbers) and other topics in linear algebra and operator theory. One goal of this work is to be able to see what molecules are present in different parts of the visible universe. Molecules of different kinds far away from Earth can be determined by a technique called spectroscopy. It is not surprising that it is easier to verify the existence of molecules with smaller clusters of atoms (molecules with few atoms) than molecules with many atoms. However, it has now been verified that there are a variety of hydrocarbons in parts of the universe that are very far from our solar system. Part of the interest in these questions is the issue of whether there is life in other parts of the universe. Human life is based on carbon chemistry molecules and so the fact that there are complex carbon molecules elsewhere in the universe raises the possibility that there are other star systems different from the Sun that have life. Mathematics has played a big role in helping with spectroscopy . Quantum mechanics has proved to be exceedingly successful in understanding the physical world. Chemistry, as much as physics, has benefited from insights from quantum mechanics, and mathematics plays an important role in quantum mechanics . Mathematics is in many regards unlike any other subject. In particular, after the work on DNA that resulted in understanding its role in inheritance, many facts in biology text books had to be discarded. At one time people regarded atoms as "miniature solar systems" with "planetary" electrons moving around the nucleus. Today, due to the work of Linus Pauling and others, the texts had to be altered. However, mathematical theorems stay theorems for ever! This creates a challenge for what to teach in K-12 as mathematics. It might seem that mathematical progress is mostly in areas which are too technical to treat in lower grades. However, this is not true. The very technical parts of mathematics are vibrant and healthy, but there are also recent quite easy-to- understand parts of mathematics. Not surprisingly, as the tools of mathematics have grown, its ability to be of service to other disciplines - chemistry, physics, engineering, art, etc. has expanded. While it has been known for a long time that there are 17 types of wallpaper groups for the plane, it was not until relatively recently that William Thurston and John Conway found a way of "explaining" where the 17 came from in a more elementary way than had been done in the past. In a sense this discovery has its roots in graph theory, too, since at its heart is the fact that for a plane connected graph V (vertices) + F (faces) - E (edges) = 2. I have just scratched the surface here of how the interaction of chemistry and mathematics has enriched chemistry and the two faces of mathematics, its theoretical and applied sides.
Cayley, A., On the mathematical theory of isomers, Phil. Mag., 67 (1874), 444–446. Crilly, T. Arthur Cayley: Mathematician Laureate of the Victorian World, Johns Hopkins University Press, Baltimore, 2006. Dias, Jerry R., Molecular Orbital Calculations Using Chemical Graph Theory, Springer 1993 Flapan, E., When Topology Meets Chemistry, Cambridge U. Press, New York 2000. Gutman, I. and L. Popovi, Graph representation of organic molecules, Cayley's plerograms vs. his kenograms, J. of Chem. Soc., Faraday Transactions, 94 (1998) 857-860. Gutman, I. and M. Essalth, M. El Marraki, B. Furtula, Why plerograms are not used in chemical graph theory? The case of terminal-Wiener index, Chemical Physics Letters, 568-56(2013) 195-197. Hansen, P., and P. Fowler, M. Zheng, (eds.), Discrete Mathematical Chemistry, DIMACS Volume 51, American Mathematical Society, Providence, 1998. Klein, Douglas J., Mathematical Chemistry! Is It? And if so, What Is It?, HYLE – International Journal for Philosophy of Chemistry, Vol. 19 (2013), No. 1, 35-85. Parshall, K., James Joseph Sylvester: Jewish Mathematician in a Victorian World., Johns Hopkins University Press, Baltimore, 2006. Mohar, B. and T. Pisanski, How to compute the Wiener index of a graph. Journal of Mathematical Chemistry, 2 (1988) 267-277. Rouvray, D., The rich legacy of half a century of the Wiener index, in Rouvray, Dennis H.; King, Robert Bruce, Topology in Chemistry: Discrete Mathematics of Molecules, 2002, Horwood Publishing, pp. 16–37. Wang, H. and G. Yu, All but 49 numbers are Wiener indices of trees, Acta Applicandae Mathematicae 92 (2006): 15–20. Wiener, H. Influence of Interatomic Forces on Paraffin Properties. J. Chem. Phys., 15 (1947) 766. Wiener, H. Structural determination of paraffin boiling points, Journal of the American Chemical Society 69 (1947): 17-20. Wilson, R. and L. Beineke, (eds.), Applications of Graph Theory, Academic Press, New York, 1979. Publishers of chemistry education books Those who can access JSTOR can find some of the papers mentioned above there. For those with access, the American Mathematical Society's MathSciNet can be used to get additional bibliographic information and reviews of some these materials. Some of the items above can be found via the ACM Portal , which also provides bibliographic services.
Welcome to the Feature Column!
These web essays are designed for those who have already discovered the joys of mathematics as well as for those who may be uncomfortable with mathematics. Read more . . .
Search Feature Column
Feature Column at a glance
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Role of Mathematics in Chemistry and its future

Related Papers
International Research Journal Commerce arts science
Many-body problems are ubiquitous in physics, chemistry, biology, and materials science. Simulations provide a very powerful way of solving these problems, enabling one to go directly from a microscopic Hamiltonian to macroscopic properties measured in experiment, essentially without approximation. We hope to convince you of this! Some of the reasons why simulations have become so important are: Other general methods for many-body problems involve approximations. In the continuum, a two body problem can be solved analytically. There is no exact solution for more than two bodies. Uncontrolled approximations always breakdown somewhere. One needs a method to test out or benchmark, approximate methods to find their range of validity. This implies that the exactness of simulation is very important, otherwise we're wasting computer and human time.
Chemical dynamics is a field in which scientists study the rates and mechanisms of chemical reactions. It also involves the study of how energy is transferred among molecules as they undergo collisions in gas-phase or condensed-phase environments. Therefore, the experimental and theoretical tools used to probe chemical dynamics must be capable of monitoring the chemical identity and energy content (i.e., electronic, vibrational, and rotational state populations) of the reacting species. Moreover, because the rates of chemical reactions and energy transfer are of utmost importance, these tools must be capable of doing so on time scales over which these processes, which are often very fast, take place. History In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances. Van 't Hoff studied chemical dynamics and published in 1884 his famous "Etudes de dynamique chimique". In 1901 he was awarded by the first Nobel Prize in Chemistry "in recognition of the extraordinary services he has rendered by the discovery of the laws of chemical dynamics and osmotic pressure in solutions".After van 't Hoff, chemical kinetics deals with the experimental determination of reaction rates from which rate laws and rate constants are derived. Relatively simple rate laws exist for zero order reactions (for which reaction rates are independent of concentration), first order reactions, and second order reactions, and can be derived for others. Elementary reactions follow the law of mass action, but the rate law of stepwise reactions has to be derived by combining the rate laws of the various elementary steps, and can become rather complex. In consecutive reactions, the rate-determining step often determines the kinetics. In consecutive first order reactions, a steady state approximation can simplify the rate law. The activation energy for a reaction is experimentally determined through the Arrhenius equation and the Eyring equation. The main factors that influence the reaction rate include: the physical state of the reactants, the
Interal Res journa Managt Sci Tech
The term symmetry implies a structure in which the parts are similar both to each other as well as to the whole structure i.e. the structure is proportional as well as balanced. In our day-to-day life, we find symmetry in many things though we usually don‟t notice it. It is easily noticeable in various arts, buildings, and monuments. Nature uses symmetry to make things beautiful. The objects, images etc. look more beautiful due to symmetry. For example, the beauty of a butterfly lies in its symmetry
Green Chemistry Today in order to protect our environment, the challenge facing organic chemist is to design syntheses that use reactants and generate products that cause little or no toxicity to the environment. Preventing pollution at the molecular level is known as green chemistry. Green Chemistry is a proactive approach to pollution prevention. It targets pollution at the design stage, before it even begins. If chemists aretargets to develop products and materials in a manner that does not use hazardous substances, than much waste, hazards and cost can be avoided. Green Chemistry is designing chemical products and processes that reduce or eliminate the use and or the generation of hazardous substances. Think about the simple equation of risk, Risk = Hazard x Exposure. Traditional approaches to pollution prevention focus on mitigating the hazards or end of pipe pollution prevention controls. These traditional technologies focus on limiting the exposure of a hazardous material. Unfortunately, exposure precautions can and will fall. Green Chemistry goes to the root of the problem and aim to eliminate the hazards itself. Green chemistry is the onlyscience that focuses on the intrinsic hazard of a chemical or chemical process .It seeks to minimize or eliminate that hazard so that we do not have to worry about exposure. Green Chemistry is not environmental chemistry. Green chemistry targets pollution prevention at the source during the design stage of a chemical product or process and thus prevents pollution before it begins. Green chemistry basically based on twelve principles, they are a set of guidelines that chemist use in order to perform chemistry in a better way. As you take a closer look at them, you will find they are intuitive and simply good practice.The 12 principles of green chemistry are as following 1.Prevention-This principle is the most obvious and most important than the other principles. It goes back to the old phrase " an ounce of prevention is better worth a pound of cure ". It is better to prevent waste than clean it up after the fact. Throughout history there have been many cases of environmental disasters that demonstrated this. For eg. In NiagaraFalls, New York a chemical and plastic company had used an old canal bed as a chemicaldump from 1930 to 1950's. The land was then used for a new school and housing track. The chemical leaked through a clay cap that sealed the dump. It was contaminated with a least 82 chemicals (benzene, chlorinated hydrocarbons, dioxin). These effects the health of the people living there included high birth defectincident and inducing nervous diseases among the children. 2. Atom Economy-This principle gets into the actual chemistry of how products are made. As Chemist, atoms are assembled to make molecules. The molecules are assembled together to make materials. This principle states that it is best use all the atoms in a process and those atoms that are
Infrared spectroscopy exploits the fact that molecules absorb specific frequencies that are characteristic of their structure. These absorptions are resonant frequencies, i.e. the frequency of the absorbed radiation matches the transition energy of the bond or group that vibrates. The energies are determined by the shape of the molecular potential energy surfaces, the masses of the atoms, and the associated vibronic coupling. (IR spectroscopy) is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic techniques, it can be used to identify and study chemicals. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer.
Interal Res journa Managt Sci Tech , DR PRAVEEN BHATT
A B S T R A C T :-We have made a study of the total and partial ionization cross-sections of a Sodium atom caused by electron impact for a single ionization. Electron-impact ionization cross-sections (EIICS) have been calculated from threshold ionization energy to 2240 eV. The theoretical model, developed by Jain and Khare, has been used to calculate the EIICS for Sodium. We observed the following product ions: Na + , Na 2+ , Na 3+ , Na 4+ , Na 5+ , Na 6+, Na 7+ and Total. The predicted EIICS of Potassium for all seven fragment ions are compared with other theoretical and experimental data. The present model shows a good agreement with the previously reported data. * (2017) All right reserved. K E Y WO R D S : Ionization Cross Section, Electron impact ionization
Quantum Nano science is the research area and the branch of nanotechnology and physics that uses methods of quantum mechanics to the design of new types of Nano devices and Nano scale materials, where functionality and structure of quantum Nano devices are described through quantum phenomena and principles such as discretization, superposition and entanglement
A B S T R A C T :-The Partial and total electron scattering cross section of Boron atom have been obtained form 0–1100 eV energy by semi empirical formula. The present cross sections are compared to existing experimental cross sections as well as to theoretical predictions. The correlation between the total electron scattering cross section and the number of target-molecule electrons is discussed. * (2017) All right reserved. K E Y WO R D S : Ionization Cross Section, Electron impact ionization
Tinospora cordifolia, which is known by the common names heart-leaved moonseed, guduchi and giloy, is an herbaceous vine of the family Menispermaceae indigenous to the tropical areas of India, Myanmar and Sri Lanka. It is a large, deciduous extensively spreading climbing shrub with several elongated twining branches. Leaves simple, alternate, exstipulate, long petioles up to 15 cm long, roundish, pulvinate, both at the base and apex with the basal one longer and twisted partially and half way around. Lamina broadly ovate or ovate cordate, 10–20 cm long or 8– 15 cm broad, 7 nerved and deeply cordate at base, membranous, pubescent above, whitish tomentose with a prominent reticulum beneath. Flowers unisexual, small on separate plants and appearing when plant is leafless, greenish yellow on axillary and terminal racemes. Male flowers clustered, female usually solitary. Sepals 6, free in two series of three each, the outer ones are smaller than the inner. Petals 6 free smaller than sepals, obovate and membranous. Fruits aggregate of 1-3, ovoid smooth drupelets on thick stalk with sub terminal style scars, scarlet or orange coloured.
There is an increasing commercial demand for nanoparticles due to their wide applicability in various areas such as electronics, catalysis, chemistry, energy, and medicine. Metallic nanoparticles are traditionally synthesized by wet chemical techniques, where the chemicals used are quite often toxic and flammable. So the exploitation of various plant materials for the biosynthesis of nanoparticles is considered a green technology because it does not involve any harmful chemicals. The present study reported the green synthesis of silver nanoparticles using Acacia catechu leaves. Green synthesis of silver oxide nanoparticles (AgONps) was carried out using the aqueous extract of Acacia catechu leaves. The UV-Vis spectrum was recorded to monitor the formation of the nanoparticles, which exhibited a blue shifted absorption peak at 490 nm. The morphology structure and stability of the synthesized AgO nanoparticles were studied using scanning electron microscope (SEM). Further these biologically synthesized nanoparticles exhibited antibacterial activity against E.coli and Staphylococcus aureus. We cheked for anti bacterial activity which were found to be exhibiting
RELATED PAPERS
Interal Res journa Managt Sci Tech , Madhura Bhalerao
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Search Menu
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Literature
- Classical Reception
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Papyrology
- Greek and Roman Epigraphy
- Greek and Roman Law
- Greek and Roman Archaeology
- Late Antiquity
- Religion in the Ancient World
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Emotions
- History of Agriculture
- History of Education
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Evolution
- Language Reference
- Language Acquisition
- Language Variation
- Language Families
- Lexicography
- Linguistic Anthropology
- Linguistic Theories
- Linguistic Typology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies (Modernism)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Media
- Music and Religion
- Music and Culture
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Science
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Lifestyle, Home, and Garden
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Politics
- Law and Society
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Neuroanaesthesia
- Clinical Neuroscience
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Toxicology
- Medical Oncology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Medical Ethics
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Security
- Computer Games
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Psychology
- Cognitive Neuroscience
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Ethics
- Business Strategy
- Business History
- Business and Technology
- Business and Government
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic History
- Economic Systems
- Economic Methodology
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Natural Disasters (Environment)
- Social Impact of Environmental Issues (Social Science)
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Behaviour
- Political Economy
- Political Institutions
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Political Theory
- Politics and Law
- Public Policy
- Public Administration
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Developmental and Physical Disabilities Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous chapter
- Next chapter >
332CHAPTER 15 Mathematical Chemistry, a New Discipline
- Published: July 2016
- Cite Icon Cite
- Permissions Icon Permissions
THE AIM OF THIS chapter is to ponder and discuss the relationship between chemistry and mathematics, taking into account some early research we have performed on the subject (Restrepo and Schummer 2014; Restrepo and Villaveces 2012, 2013; Restrepo 2013). In those works we have discussed some criticism and some support throughout history regarding the relationship. We analyzed the opinions of scholars ranging from Venel and Denis Diderot (eighteenth century) to Pierre Laszlo (twentieth century), all of whom are critical of mathematical chemistry. We also analyzed opinions by Brown and Paul Dirac (nineteenth and twentieth centuries, respectively), who sought a fruitful relationship between mathematics and chemistry. We discussed Kant and his double opinion regarding such a relationship as well. (In summary, Kant initially did not consider chemistry to be a science because of its apparent lack of mathematization, an idea Kant supported in the apparent a priori background of mathematics and in the a posteriori one of chemistry. Kant’s revised opinion about the relationship between mathematics and chemistry is totally different, Kant now thinks chemistry contains elements of mathematics.) We have also analyzed Comte’s opinions on the necessity of mathematics for chemistry and for the advancement of the latter (Restrepo 2013). Our work on the philosophy and history of the relationship between mathematics and chemistry is driven by the attention research on the field has gained between the 1960s and the present. A wealth of knowledge in the border between the two sciences has been generated but little attention has been paid to the philosophy and history of the subject (Restrepo and Schummer 2012). Thus in recent years scholars (Balaban 2005, 2013; Basak 2013; Deltete 2012; Gavroglu and Simões 2012; Restrepo and Villaveces 2012, 2013; Restrepo 2013; Schummer 2012; Hosoya 2013; Klein 2013), including the author of the present chapter, have decided to study such a relationship from both a historical and a philosophical viewpoint. One example of the increased interest are the special issues of Hyle, which were dedicated to mathematical chemistry.
Signed in as
Institutional accounts.
- Google Scholar Indexing
- GoogleCrawler [DO NOT DELETE]
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
Institutional access
- Sign in with a library card Sign in with username/password Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Sign in through your institution
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Sign in with a library card
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:

Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Home / Blog / Math / Math in Chemistry: What Kind of Math is Used in Chemistry?

Math in Chemistry: What Kind of Math is Used in Chemistry?

Math is an essential component of all scientific disciplines, 1 and its applications extend beyond the realm of math.
There are numerous widespread applications of math in multiple disciplines of study as well as in everyday life. According to the Mathematical Association of America, “the future of math education and the discipline of math should not be considered in a vacuum but as an important tool for all science.” 2
When we talk about math, we include both theoretical and applied math, which are equally important as theory and applications of math go hand in hand and have always been linked together.
One such area where math is widely used and is absolutely necessary to explore important concepts is chemistry. Without basic math, making calculations within chemistry can be extremely difficult. 1
Most of us would have probably come across the use of math in chemistry, such as using ratios for mixing solutions and taking measurements for making dilutions. As children progress through their academic learning for higher grades, they will see the applications of math being used increasingly to explain chemistry concepts in more sophisticated ways, like using vectors to understand the structures of crystals. Therefore, the ability to apply math can be greatly helpful to children when learning chemistry. Math is present in almost all areas of chemistry, such as inorganic chemistry, organic chemistry, physical chemistry, biochemistry, analytical, and environmental chemistry, etc. 1
Let’s now look at how different math concepts are used within chemistry.
The Basic Mathematical Foundation
While most of you are aware of the concepts of addition, subtraction, multiplication, and division, how many of you are aware that they are also used extensively in chemistry? Calculating the molecular mass of components, volume, and other concepts, for example, necessitates these calculations. 1
Elementary Topics Used in General Chemistry
In order to excel in chemistry, you must understand unit conversions, significant figures, summation notations, probability and statistics, exponents and logarithms, proportions, and concentrations. In most cases, these subjects overlap, so they are frequently discussed together. 2
Chemistry Math Problems
These are the topics for which one cannot be prepared without a foundation in chemistry. Taking measurements, performing dimensional analysis, determining temperature, density, and so on are all things that cannot be done without math. 3 Most of you are probably aware that scientists use a variety of equipment to measure matter, and these measurements are crucial because minor errors in measurements can have a significant impact on the research they are conducting. Converting units to other units requires the use of conversion factors, which are critical for constructing equality and relating to different units.The density of a substance is a commonly measured property that is required in most chemistry calculations, and temperature is one of the most basic quantities in science, with applications ranging from weather forecasts to advanced medical facilities. 3
Applied Math
These are concepts and techniques that science students, particularly those studying chemistry, can use to apply to various scientific concepts. Basic trigonometry, algebra, graphing, calculus, and geometry are all concepts that can help with understanding and working on various chemistry concepts. 2 It improves quantitative skills while also assisting with the conceptual understanding of derivatives and integrals, which are used in a variety of chemistry studies. While concepts like these are not a formal prerequisite for chemistry, learning topics like three-dimensional geometry can help students learn chemistry a great deal, even if they have rudimentary knowledge or experience with visualizing graphs in three-dimensional spaces. 2 Applied math is crucial in both organic and physical chemistry, and it extends far beyond general chemistry.
Various operators, such as the Delta, Sigma, and Pi operators, are commonly used in chemistry to perform various calculations. For example, if the optical absorbance of a reaction must be calculated, the Delta operators must be calculated. 1
These are just a few examples of how math is used in chemistry and what types of math are used to learn various chemistry concepts. Math plays a fundamental role in fundamental science, particularly chemistry, as was emphasized throughout this blog. The kind of math background that children have will give them an edge in graduate programs in math, chemistry, applied math, statistics, crystallography, biochemistry, and molecular biology, among others.
Interesting, isn’t it? Please leave your comments to share your thoughts about this blog and visit BYJU’S FutureSchool’s blog to learn more about the applications of math in other fields.
References:
- Cunningham, A., Whelan, R., Grove, M., Kyle, J., & Pugh, S. (2014). Maths for Chemists .
- Flapan, E., Hemkin, S., Jorgensen, A., Robinson, M., Schrier, J., Seeman, N. C., & Simon, J. (n.d.). Mathematics and Chemistry .
- 2: The Mathematics of Chemistry – Chemistry LibreTexts . (n.d.). Retrieved May 26, 2022, from https://chem.libretexts.org/Courses/Furman_University/CHM101%3A_Chemistry_and_Global_Awareness_(Gordon)/02%3A_The_Mathematics_of_Chemistry
Share Article:
You might also like
Josiah willard gibbs, the math genius behind gibbs free energy, a brief introduction to george dantzig, the father of linear programming, lotfi zadeh: the man behind fuzzy logic, how to create a mobile math game, how effective is high school math in solving real-life problems, what is the importance of math in computer science, what type of math is used in gaming, what are the uses of math in everyday life.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More from navigation items
Maths skills for chemistry
- 2 Using ratios in chemistry
- 3 Improving fluency in standard form
- 4 Solving algebra in chemistry
- 5 Practise rounding and significant figures in chemistry
- 6 Improving students’ geometry skills
- 7 Improve graph skills for chemistry
- Five out of five
- No comments
Target the maths skills your students need to succeed in their chemistry course
This collection of resources will help your learners apply what they have learnt in their maths lessons in the science lab. Each resource focusses on a key area of maths that chemistry learners need to be confident with and will support their learning in other science subjects too.
These resources support the maths series from Education in Chemistry magazine. Each article offers more ideas and support for teaching each of these maths topics in a chemistry context.

Using ratios in chemistry

Improving fluency in standard form

Solving algebra in chemistry

Practise rounding and significant figures in chemistry

Improving students’ geometry skills

Improve graph skills for chemistry
- Maths skills
Related articles

Boost maths skills to improve chemistry learning
2024-01-18T08:00:00Z By Fraser Scott
Use these evidence-based tips to help your learners get ahead with chemical calculations

Enthalpy change of combustion of ethanol | practical videos | 14–16 years
By Karen Marshall and Sandrine Bouchelkia
Video and resources investigating the heat energy change of combustion of ethanol
Electrochemical cells misconception buster | 16–18 years
By Louise Hussein
Probe learners’ knowledge of setting up electrochemical cells, redox equations and calculations
No comments yet
Only registered users can comment on this article., more from resources.

Chromatography challenge | 16–18 years
By Andy Markwick
Explore analytical techniques and their applications with a chromatography investigation and research activity

Metallic bonding | Structure strip | 14–16
By Kristy Turner
Describe the metallic bonding model and explain how this leads to particular properties in metals, with this scaffolded writing activity

Ionic bonding | Structure strip | 14–16
Understand the models and diagrams used to represent ionic bonding and their limitations, with this scaffolded writing activity
- Contributors
- Email alerts
Site powered by Webvision Cloud
Skip to Content
Regents approve plans for new chemistry and applied mathematics facility
- Share via Twitter
- Share via Facebook
- Share via LinkedIn
- Share via E-mail

Rendering of the newly approved chemistry and applied mathematics facility. This is a draft rendering, subject to change.
The University of Colorado Board of Regents today approved plans to construct a new academic and research facility aimed at advancing research and educational opportunities in the fields of chemistry and applied mathematics. The proposed facility, spanning approximately 79,200 square feet, will be situated on the southeast side of Business Field on Main Campus.
The project, with an estimated cost of $175.43 million, will be funded primarily through a combination of campus cash reserves and debt.
Key features
- Shelled space for future specialized quantum research laboratories and offices.
- A nuclear magnetic resonance (NMR) spectroscopic core facility
- Modern classroom, office, meeting and research lab space
- A centrally scheduled 200-seat auditorium
- Student study areas
- Upper-level roof terrace
Academic goals
- The facility will provide modern research laboratories to promote the Department of Chemistry ’s research mission, improve recruitment and strengthen retention. Foundational and interdisciplinary research fields include: analytical spectroscopy, environmental chemistry, experimental and theoretical physical/ biophysical chemistry, inorganic and materials chemistry, synthetic and physical organic/bio-organic chemistry.
- The facility will support the Department of Applied Mathematics ’ efforts to continue to provide a major research presence in computational and physical/biological mathematics and the statistical sciences, enhancing the department’s effort to be a leading program of applied mathematics in the United States.
- The facility will provide shelled spaces to be completed in the future for the growing field of quantum-chemistry research.
- It will serve as a central hub for cutting-edge research, student learning outcomes and sustainable innovation, and it will foster interdisciplinary collaboration among students and researchers.
- It will provide training opportunities for students and researchers, fostering interdisciplinary collaboration and innovation.
Sustainability considerations
- The chemistry and applied math building seeks to be one of the most energy-efficient research buildings in the history of CU Boulder by achieving an Energy Use Intensity (EUI) of approximately 100 kilo British thermal units (kBtu) per gross square feet of the building (kBtu/ft2), according to current modeling.
- Construction will follow Buy Clean Colorado Act guidelines, using eligible materials that do not exceed the maximum allowable global warming potential limit in each construction category.
- The building’s mechanical system is being designed to use low-temperature hot water in preparation for the eventual connection to a future district energy loop heated by electricity, in support of the university’s overarching decarbonization plans.
- It will be the first building on campus to implement the use of cross-laminated timber (CLT) construction. The project will use CLT instead of concrete for the structure in non-research areas of the building. This will reduce the carbon impact of the structure.
What they’re saying
“The approval of the new chemistry and applied mathematics facility marks a significant milestone for our campus,” CU Boulder Chancellor Phil DiStefano said.
“This project underscores our commitment to advancing research and fostering interdisciplinary collaboration. It will not only provide state-of-the-art resources for our scholars but also pave the way for groundbreaking discoveries that will benefit both our campus community and society at large."
What’s next
Construction is slated to begin in October, with occupancy planned for late 2026/early 2027.
Vacated spaces within Cristol Chemistry & Biochemistry are anticipated to be assigned to the College of Media, Communications and Information (CMCI), while spaces vacated by Applied Mathematics within the Engineering Center will benefit the College of Engineering and Applied Science.
- Administration
Campus Community
Related articles.

Regents consider tuition, compensation, concealed carry policies and more

Chancellor Philip DiStefano addresses faculty assembly

Campus Sustainability Executive Council to add student government representatives—March meeting summary
News headlines.
- Arts & Humanities
- Business & Entrepreneurship
- Climate & Environment
- Education & Outreach
- Health & Society
- Law & Politics
- Science & Technology
- Announcements & Deadlines
- Career Development
- Getting Involved
- Mind & Body
Events & Exhibits
- Arts & Culture
- Conferences
- Lectures & Presentations
- Performances & Concerts
- Sports & Recreation
- Workshops & Seminars
Subscribe to CUBT
Sign up for Alerts
Administrative eMemos
Buff Bulletin Board
Events Calendar

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

2: The Mathematics of Chemistry
- Last updated
- Save as PDF
- Page ID 441150

- Elizabeth Gordon
- Furman University
Mathematics is used widely in chemistry and are absolutely necessary to explore important concepts in chemistry. Without some basic mathematics skills, these calculations, and therefore chemistry itself, will be extremely difficult. However, with a basic knowledge of some of the mathematics that will be used in your chemistry course, you will be well prepared to deal with the concepts and theories of chemistry.
- 2.1: Measuring Matter Scientists use all kinds of equipment to measure matter. Balances are used to measure mass while pipettes are used to measure volume. Errors in measurements can be made if the scientist does not know how to properly use the equipment or if the equipment is damaged. It is important that you know the types of equipment a scientists uses and the basic units that are found on the equipment. In addition, memorize and be able to apply the necessary conversion factors for this course.
- 2.2: Dimensional Analysis Units can be converted to other units using the proper conversion factors. Conversion factors are constructed from equalities that relate two different units. Conversions can be a single step or multistep. Unit conversion is a powerful mathematical technique in chemistry that must be mastered.
- 2.3: Density Density is a physical property that is defined as a substance’s mass divided by its volume. Density is usually a measured property of a substance, so its numerical value affects the significant figures in a calculation. Notice that density is defined in terms of two dissimilar units, mass and volume. That means that density overall has derived units, just like velocity.
- 2.4: Temperature One of the fundamental quantities in science is temperature. Temperature is a measure of the average amount of energy of motion, or kinetic energy, a system contains. Temperatures are expressed using scales that use units called degrees, and there are several temperature scales in use. In the United States, the commonly used temperature scale is the Fahrenheit scale (symbolized by °F and spoken as “degrees Fahrenheit”).
- 2.E: The Mathematics of Chemistry (Exercises) These are homework exercises to accompany Chapter 2 of the Furman University's LibreText for CHE 101 - Chemistry and Global Awareness.
Maintenance work is planned for Wednesday 1st May 2024 from 9:00am to 11:00am (BST).
During this time, the performance of our website may be affected - searches may run slowly and some pages may be temporarily unavailable. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.

Journal of Materials Chemistry C
Enhanced electromagnetic absorption properties of the double-layer ti 3 c 2 t x mxene absorber with orthogonal microstructures †.

* Corresponding authors
a National Key Laboratory of Science and Technology on Advanced Composites in Special Environments, Harbin Institute of Technology, Harbin 150080, P. R. China E-mail: [email protected]
b Center for Composite Materials and Structures, Harbin Institute of Technology, Harbin 150080, P. R. China
c School of Materials Science and Engineering, Beihang University, Beijing 100081, P. R. China E-mail: [email protected]
Transition metal carbides and nitrides (MXenes) with excellent properties have broad prospects in electromagnetic wave absorption (EMA). In this paper, a unidirectional freeze-casting method was utilized to design a double-layer MXene absorber with orthogonal microstructures. The perpendicular and parallel samples (associated with the unidirectional freeze-casting direction), perform their respective roles in the designed double-layer absorber. The perpendicular sample with various attenuation techniques is employed as an electromagnetic wave attenuation layer, while the parallel sample with good impedance matching conditions is utilized as an impedance matching layer. Consequently, the entire absorber exhibits favorable EMA characteristics. Upon incidence of electromagnetic waves onto the parallel terminus, it is observed that the sample measuring 4.6 mm in thickness exhibits a minimal reflection loss (RL min ) of −60.8 dB at a frequency of 6.63 GHz, with a parallel end thickness accounting for 25%. In addition, the sample with a thickness of 2.6 mm displays a noteworthy maximal effective absorption bandwidth (EAB) amounting to 9.4 GHz, given a parallel end thickness of 20%. A thorough analysis of the impedance matching conditions and electromagnetic wave attenuation mechanisms of each sample reveals that the double-layer MXene absorbers with orthogonal microstructures can significantly increase the viability of MXenes in EMA.
- This article is part of the themed collection: Journal of Materials Chemistry C HOT Papers
Supplementary files
- Supplementary information PDF (2611K)
Article information
Download citation, permissions.
Enhanced electromagnetic absorption properties of the double-layer Ti 3 C 2 T x MXene absorber with orthogonal microstructures
Y. Ning, S. Yang, X. Sun, S. Wang, L. Liang, Y. Cheng, Y. Yuan and Y. Li, J. Mater. Chem. C , 2024, Advance Article , DOI: 10.1039/D3TC03973K
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
- Bihar Board
SRM University
Jac 10th result.
- UP Board 10th Result 2024
- UP Board 12th Result 2024
- Punjab Board Result 2024
- JAC Board Result 2024
- Rajasthan Board Result 2024
- Karnataka Board Result
- Shiv Khera Special
- Education News
- Web Stories
- Current Affairs
- नए भारत का नया उत्तर प्रदेश
- School & Boards
- College Admission
- Govt Jobs Alert & Prep
- GK & Aptitude
CUET UG Syllabus For Science Students 2024: Download Subject-wise PDF, Check Exam Pattern
Cuet ug science subject syllabus 2024: cuet ug 2024 syllabus has been released on the official website. candidates need to familiarize themselves with it to prepare effectively. this article provides the detailed subject-wise cuet ug syllabus for science students along with exam pattern, marking scheme, syllabus pdf download link, and preparation tips..

Download CUET UG Syllabus 2024 PDF
Cuet syllabus 2024 for science students pdf download , cuet ug 2024 exam pattern .
The NTA conducts CUET UG 2024 exam for the students who wish to get admission into Postgraduate Programmes in Central and other participating Universities/Institutions.
What CUET Courses Are There for Science Students?
- Bachelor of Science (BSc) in Physics, Chemistry, Mathematics, Biology and Environmental Studies.
- Bachelor of Computer Applications (BCA) in Computer Science and Technology.
How to Prepare the CUET Syllabus for Science?
- Understand the Syllabus : The candidates must carefully go through the complete CUET UG syllabus. Note down the important topics, giving priority to those that require more attention. Create a study plan around these requirements.
- Create a Study Schedule : Once you analyse the syllabus, make an extensive study plan that covers all the topics mentioned in the syllabus. As per your requirements allocate ample time to each topic.
- Focus on Fundamental Understanding: Always focus on understanding the core principles of each topic. Only memorising things will not be enough for this exam.
- Create Revision Notes: Create short revision notes with important formulas, concepts, and important points for quick last-minute review.
- Practice Previous Year Papers: The candidates must solve previous years' papers to understand the exam pattern and question types asked in the exam. This will give an idea about important topics and also help in identifying the areas that require improvement.
Best Books to Prepare CUET UG Science Subject Syllabus 2024
- CBSE All-In-One Chemistry Class 12
- Mathematics for Class 12 (Set of 2 Volumes)
- S. Chand’s Biology for Class XII
- Concept of Physics Vol 1 & 2 by H.C. Verma
- CUET-UG: Agriculture by RPH Editorial Board
- Go To Guide for CUET (UG) Computer Science/ Informatics Practices
- NCERT Books of Class 11 and 12
Get here latest School , CBSE and Govt Jobs notification in English and Hindi for Sarkari Naukari and Sarkari Result . Download the Jagran Josh Sarkari Naukri App . Check Board Result 2024 for Class 10 and Class 12 like CBSE Board Result , UP Board Result , Bihar Board Result , MP Board Result , Rajasthan Board Result and Other States Boards.
- How to download the CUET UG 2024 syllabus PDF for Science students? + The NTA has released the syllabus of CUET UG 2024 syllabus. The candidates can download it from their official website. We have also shared the direct link to download the CUET UG 2024 syllabus in this article.
- What is the mode of CUET UG 2024 exam? + This year the CUET UG exam will be conducted in Hybrid mode which means the exam will be conducted in both online and offline mode.
- PSEB 10th Result 2024
- PSEB Class 10th Result 2024
- Punjab Board 10th Result 2024
- pseb.ac.in 10th Result 2024
- Punjab Board Class 10th Result 2024
- ਪੀਐਸਈਬੀ 10ਵੀਂ ਦਾ ਨਤੀਜਾ 2024
- PSEB १०थ रिजल्ट २०२४
- पंजाब बोर्ड १०थ टॉपर्स लिस्ट 2024
- PSEB 10th Topper List 2024
Latest Education News
Purple Cap in IPL 2024: Top Players List with Most Wickets in TATA IPL
[Current] Orange Cap and Purple Cap Holders in IPL 2024
Orange Cap in IPL 2024: Top Players List with Most Runs in TATA IPL
Jharkhand Board 10th Result 2024: आज 11:30 बजे जारी होगा झारखण्ड बोर्ड 10वीं का रिजल्ट, jac.jharkhand.gov.in पर मिलेगा डायरेक्ट रिजल्ट लिंक
UP Board Result Date 2024: यूपी बोर्ड में पास होने के लिए चाहिए बस इतने अंक, रिजल्ट से जुड़ी लेटेस्ट अपडेट यहां देखें
[Today] IPL 2024 Points Table: Team Rankings and Net Run Rate
Most Runs In IPL 2024: दिलचस्प हो गयी है ऑरेंज कैप की रेस, ये युवा बल्लेबाज रेस में है शामिल
Most wickets in ipl 2024: इन पांच गेंदबाजों में है पर्पल कैप की रेस, कौन निकलेगा सबसे आगे?
IPL Points Table 2024: आईपीएल 2024 अपडेटेड पॉइंट टेबल यहां देखें, राजस्थान टॉप पर
T20 World Cup 2024: कौन-कौन से खिलाड़ी भारतीय स्क्वॉड के है प्रबल दावेदार?
Who Won Yesterday IPL Match: MI vs PBKS, Match 33, Check All Details and Latest Points Table
JAC 10th Result 2024 Live Updates: Jharkhand Board Class 10 Results to be Released on April 19 at 11:30 AM, Check Online by Roll Number
JAC 10th Result 2024: झारखंड बोर्ड मैट्रिक के नतीजे कब, कहां और कैसे ऑनलाइन और एसएमएस से चेक करें
JAC 10th Result 2024 Live Updates: झारखंड बोर्ड 10वीं का रिजल्ट jacresults.com पर होगा जारी, बिना इंटरनेट मोबाइल पर ऐसे चेक करें स्कोर
JAC Board Result 2024: Check झारखंड बोर्ड रिजल्ट at Jagran Josh, jacresults.com and jac.jharkhand.gov.in
JAC Board 10th Result 2024: Check झारखंड 10वीं रिजल्ट at Jagran Josh, jacresults.com
PSEB 10th Result 2024 Released: Aditi, Alisha and Karmanpreet got 1st, 2nd and 3rd Position, 97.2% Students Passed and Live Link Tomorrow
Genius IQ Test: Can you solve the math puzzle in 5 seconds?
Picture Puzzle IQ Test: Only Highly Observant Can Spot The Eyeglasses In 8 Seconds!
NEET Exam Pattern 2024: Check Question Paper Format and Marking Scheme

IMAGES
VIDEO
COMMENTS
Mathematics has played a big role in helping with spectroscopy. Quantum mechanics has proved to be exceedingly successful in understanding the physical world. Chemistry, as much as physics, has benefited from insights from quantum mechanics, and mathematics plays an important role in quantum mechanics.
Introduction Mathematical chemistry is the area of research engaged in novel applications of mathematics to chemistry; it concerns itself principally with the mathematical modeling of chemical phenomena. Mathematical chemistry has also sometimes been called computer chemistry, but should not be confused with computational chemistry.
Mathematical chemistry is the area of research engaged in novel applications of mathematics to chemistry; it concerns itself principally with the mathematical modeling of chemical phenomena. Mathematical chemistry has also sometimes been called computer chemistry, but should not be confused with computational chemistry.. Major areas of research in mathematical chemistry include chemical graph ...
Mathematics is an essential skill for chemistry students to master; the number of chemistry departments either requiring or recommending study of A-level mathematics is but one indicator. However, there exists a mismatch between staff and student understanding of its importance. For example, in the Higher Education Academy student survey, 3 ...
2.E: The Mathematics of Chemistry (Exercises) These are homework exercises to accompany Chapter 2 of the Furman University's LibreText for CHE 101 - Chemistry and Global Awareness. Mathematics is used widely in chemistry and are absolutely necessary to explore important concepts in chemistry. Without some basic mathematics skills, these ...
This page titled Mathematical Methods in Chemistry (Levitus) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Marcia Levitus via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.
Chem- the role of mathematics in chemistry, istry is thus compatible with physical laws but not reduci- ing, conceptually integrated and. ble to them. The really interesting problems in chemistry framework of molecular science, seem to remain fully unresolved in terms of understanding molecular/materials physics and.
THE AIM OF THIS chapter is to ponder and discuss the relationship between chemistry and mathematics, taking into account some early research we have performed on the subject (Restrepo and Schummer 2014; Restrepo and Villaveces 2012, 2013; Restrepo 2013). In those works we have discussed some criticism and some support throughout history ...
The data of this study used the mathematics and chemistry scores of 452 students from 6 third-level grades at a secondary school in the city of Guilin. SPSS22.0 software was used to analyze ...
chemistry courses (such as physical chemistry or computational chemistry) to also be counted as courses in the mathematics major. Chemistry departments should consider doing something similar. For the chemistry part of a double major, students should be expected to take two semesters of
This can be seen in, e.g. Trinajstić and Gutman's and Balaban's papers on mathematical chemistry where they claim that there are several instances of the mathematization of chemistry, by mathematization meaning the application of mathematics to chemistry. Mainzer stated that "chemistry is involved in a growing network of mathematical ...
Four fundamental problems encountered in the education of chemists at the undergraduate and graduate levels are presented, with insightful solutions using mathematics as an important tool. KEYWORDS (Audience): Upper-Division Undergraduate. KEYWORDS (Domain):
The Journal of Mathematical Chemistry publishes original, chemically important mathematical results which use non-routine mathematical methodologies often unfamiliar to the usual audience of mainstream experimental and theoretical chemistry journals.. Publishes papers on novel applications of more familiar mathematical techniques and analyses of chemical problems which indicate the need for ...
These examples show that mathematical chemistry has much more than a century of history. HYLE--International Journal for Philosophy of Chemistry, Vol. 18, No.1 (2012), pp. 3-22. ... (1814-97) was a 19 th-century leading mathematician with an interest in chemistry. He wrote two influential papers related to chemistry in 1878 (Sylvester 1878a, b ...
Words: 536. Page: 1. This essay sample was donated by a student to help the academic community. Papers provided by EduBirdie writers usually outdo students' samples. Cite this essay. Download. I chose to study chemistry as part of a of a science degree with mathematics being my major area of study.
Applied Math. These are concepts and techniques that science students, particularly those studying chemistry, can use to apply to various scientific concepts. Basic trigonometry, algebra, graphing, calculus, and geometry are all concepts that can help with understanding and working on various chemistry concepts. 2 It improves quantitative ...
A very curious fact is that after publishing 19 papers in pure mathematics, Jones published, together with J. R. Bailey, the paper The behavior of 2-phenyl semicarbazones upon oxidation, in the top chemistry journal Journal of the American Chemical Society (Whyburn and Bailey 1928). This paper is entirely dedicated to experimental organic ...
According to this, students' interest in chemistry is a factor in learning chemistry, the teaching method used by the teacher is an essential factor for chemistry, and having enough knowledge of ...
The special issue of this journal, entitled Mathematical Chemistry and dedicated to Professor Milan Randi}, the foremost mathematical chemist of our times, calls for some words to be said about the relationship between mathematics and chemistry. The present article is in part based on an ear-lier essay by one of us (N. T.).1 In that essay, ...
Using and Applying Mathematics in Chemistry | Semantic Scholar. DOI: 10.1021/BK-2019-1316.CH001. Corpus ID: 149958279. How Did We Get Here? Using and Applying Mathematics in Chemistry. M. Towns, K. Bain, Jon-Marc G. Rodriguez. Published in American Chemical Society… 2019. Chemistry, Mathematics.
Each resource focusses on a key area of maths that chemistry learners need to be confident with and will support their learning in other science subjects too. These resources support the maths series from Education in Chemistry magazine. Each article offers more ideas and support for teaching each of these maths topics in a chemistry context.
The chemistry and applied math building seeks to be one of the most energy-efficient research buildings in the history of CU Boulder by achieving an Energy Use Intensity (EUI) of approximately 100 kilo British thermal units (kBtu) per gross square feet of the building (kBtu/ft2), according to current modeling.
2023 Outstanding Papers published in the Environmental Science journals of the Royal Society of Chemistry Zongwei Cai , a Neil Donahue , b Graham Gagnon , c Kevin C. Jones , d Célia Manaia , e Elsie Sunderland f and Peter J. Vikesland g
The present book is an attempt to outline some, certainly not all, mathematical aspects of modern organic chemistry. We have focused our attention on topological, graph-theoretical and group-theoretical features of organic chemistry, Parts A, B and C. The book is directed to all those chemists who use, or who intend to use mathe matics in ...
2.E: The Mathematics of Chemistry (Exercises) These are homework exercises to accompany Chapter 2 of the Furman University's LibreText for CHE 101 - Chemistry and Global Awareness. This page titled 2: The Mathematics of Chemistry is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Elizabeth Gordon.
Transition metal carbides and nitrides (MXenes) with excellent properties have broad prospects in electromagnetic wave absorption (EMA). In this paper, a unidirectional freeze-casting method was utilized to design a double-layer MXene absorber with orthogonal microstructures. The perpendicular and parallel samples Journal of Materials Chemistry C HOT Papers
CUET Syllabus 2024 for Science Students PDF Download. The CUET UG exam for science subjects mainly consists of six subjects i.e., Physics, Chemistry, Biology, Computer Science, Mathematics, and ...