Essay on Smoking
500 words essay on smoking.
One of the most common problems we are facing in today’s world which is killing people is smoking. A lot of people pick up this habit because of stress , personal issues and more. In fact, some even begin showing it off. When someone smokes a cigarette, they not only hurt themselves but everyone around them. It has many ill-effects on the human body which we will go through in the essay on smoking.


Ill-Effects of Smoking
Tobacco can have a disastrous impact on our health. Nonetheless, people consume it daily for a long period of time till it’s too late. Nearly one billion people in the whole world smoke. It is a shocking figure as that 1 billion puts millions of people at risk along with themselves.
Cigarettes have a major impact on the lungs. Around a third of all cancer cases happen due to smoking. For instance, it can affect breathing and causes shortness of breath and coughing. Further, it also increases the risk of respiratory tract infection which ultimately reduces the quality of life.
In addition to these serious health consequences, smoking impacts the well-being of a person as well. It alters the sense of smell and taste. Further, it also reduces the ability to perform physical exercises.
It also hampers your physical appearances like giving yellow teeth and aged skin. You also get a greater risk of depression or anxiety . Smoking also affects our relationship with our family, friends and colleagues.
Most importantly, it is also an expensive habit. In other words, it entails heavy financial costs. Even though some people don’t have money to get by, they waste it on cigarettes because of their addiction.
How to Quit Smoking?
There are many ways through which one can quit smoking. The first one is preparing for the day when you will quit. It is not easy to quit a habit abruptly, so set a date to give yourself time to prepare mentally.
Further, you can also use NRTs for your nicotine dependence. They can reduce your craving and withdrawal symptoms. NRTs like skin patches, chewing gums, lozenges, nasal spray and inhalers can help greatly.
Moreover, you can also consider non-nicotine medications. They require a prescription so it is essential to talk to your doctor to get access to it. Most importantly, seek behavioural support. To tackle your dependence on nicotine, it is essential to get counselling services, self-materials or more to get through this phase.
One can also try alternative therapies if they want to try them. There is no harm in trying as long as you are determined to quit smoking. For instance, filters, smoking deterrents, e-cigarettes, acupuncture, cold laser therapy, yoga and more can work for some people.
Always remember that you cannot quit smoking instantly as it will be bad for you as well. Try cutting down on it and then slowly and steadily give it up altogether.
Get the huge list of more than 500 Essay Topics and Ideas
Conclusion of the Essay on Smoking
Thus, if anyone is a slave to cigarettes, it is essential for them to understand that it is never too late to stop smoking. With the help and a good action plan, anyone can quit it for good. Moreover, the benefits will be evident within a few days of quitting.
FAQ of Essay on Smoking
Question 1: What are the effects of smoking?
Answer 1: Smoking has major effects like cancer, heart disease, stroke, lung diseases, diabetes, and more. It also increases the risk for tuberculosis, certain eye diseases, and problems with the immune system .
Question 2: Why should we avoid smoking?
Answer 2: We must avoid smoking as it can lengthen your life expectancy. Moreover, by not smoking, you decrease your risk of disease which includes lung cancer, throat cancer, heart disease, high blood pressure, and more.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App


Essay on Negative Effects Of Smoking
Students are often asked to write an essay on Negative Effects Of Smoking in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Negative Effects Of Smoking
Introduction to smoking.
Smoking is a bad habit that harms our body. Many people smoke cigarettes, cigars, or pipes. Some people also chew tobacco. These things contain nicotine, a harmful chemical. It’s highly addictive, meaning once you start smoking, it’s very hard to stop.
Health Problems Caused by Smoking
Smoking can cause many health problems. It can lead to lung cancer, heart disease, and stroke. It can also cause other types of cancer, like mouth and throat cancer. Smoking can make it hard to breathe and can cause chronic coughing.
Smoking and Secondhand Smoke
Not only does smoking hurt the smoker, but it also harms others around them. This is called secondhand smoke. It can cause the same health problems in non-smokers. Children exposed to secondhand smoke can get sick more often.
Smoking and Appearance
Smoking can also affect how you look. It can cause yellow teeth and bad breath. It can also cause your skin to age faster, leading to wrinkles. Smoking can even cause hair loss and turn your fingers yellow.
In conclusion, smoking is very harmful. It can cause many health problems and can even harm others around you. It’s best to avoid this bad habit. If you or someone you know smokes, try to quit. Your body will thank you.
250 Words Essay on Negative Effects Of Smoking
Introduction.
Smoking is a harmful habit that many people around the world have. It is bad for our health and the environment. This essay will talk about the negative effects of smoking.
Damages to Health
Smoking hurts our bodies in many ways. It is the main cause of lung cancer. This is a very serious disease that can lead to death. Other than lung cancer, smoking can also cause heart disease. This is because the smoke makes it harder for the heart to pump blood.
Problems for the Environment
Smoking is not just bad for our health, but also for our environment. Cigarette butts are often thrown on the ground, causing pollution. Also, the smoke from cigarettes adds to air pollution. This is bad for all living things, not just humans.
Effects on Others
Smoking is not only harmful to the person who smokes, but also to the people around them. This is called second-hand smoke. It can cause the same health problems as smoking does. This means that even if you do not smoke, you can still get sick from being around someone who does.
In conclusion, smoking is a harmful habit with many negative effects. It causes health problems, harms the environment, and can even make others sick. It is important to avoid smoking for a healthier and safer world.
500 Words Essay on Negative Effects Of Smoking
Smoking is a habit that many people pick up due to various reasons, such as stress, peer pressure, or even out of curiosity. Despite its popularity, smoking has many negative effects on our health and the environment. This essay will discuss these harmful effects in simple terms.
Effects on Personal Health
Firstly, let’s talk about how smoking harms our own health. When you smoke, you inhale many dangerous chemicals. These chemicals can harm nearly every organ in your body. The most commonly known health problem caused by smoking is lung cancer. But that’s not all. Smoking can also lead to other types of cancer, such as mouth cancer and throat cancer.
Apart from cancer, smoking can cause heart disease. The chemicals in smoke make it harder for your heart to work properly. This can lead to heart attacks. Smoking also harms your lungs, making it difficult to breathe. This can lead to diseases like bronchitis and emphysema.
Effects on Others’ Health
Smoking is not only harmful to the smoker but also to those around them. This is called secondhand smoke. When you smoke, the people around you also breathe in the harmful chemicals. This can lead to the same health problems that smokers face. Children are particularly at risk. They can suffer from problems like asthma, ear infections, and even sudden infant death syndrome.
Effects on the Environment
Smoking also hurts our environment. Cigarette butts, which are often thrown away carelessly, are a form of litter. They can take many years to break down and are harmful to wildlife. The smoke from cigarettes also adds to air pollution. This can harm the air we all breathe and contribute to climate change.
Effects on Personal Life
Lastly, smoking can affect your personal life. It can make your clothes and breath smell bad, which can affect your relationships with others. It can also be a costly habit. The money spent on cigarettes could be used for other things like education, hobbies, or saving for the future.
In conclusion, smoking has many negative effects. It harms our health, the health of those around us, our environment, and our personal lives. It’s important to understand these effects and to make healthy choices for ourselves and our communities. Remember, it’s never too late to quit smoking and start living a healthier life.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Negative Effects Of Religion
- Essay on Negative Effects Of Music
- Essay on Negative Effects Of Mobile Phones
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Persuasive Essay Guide
Persuasive Essay About Smoking
Persuasive Essay About Smoking - Making a Powerful Argument with Examples
People also read
A Comprehensive Guide to Writing an Effective Persuasive Essay
200+ Persuasive Essay Topics to Help You Out
Learn How to Create a Persuasive Essay Outline
30+ Free Persuasive Essay Examples To Get You Started
Read Excellent Examples of Persuasive Essay About Gun Control
How to Write a Persuasive Essay About Covid19 | Examples & Tips
Crafting a Convincing Persuasive Essay About Abortion
Learn to Write Persuasive Essay About Business With Examples and Tips
Check Out 12 Persuasive Essay About Online Education Examples
Are you wondering how to write your next persuasive essay about smoking?
Smoking has been one of the most controversial topics in our society for years. It is associated with many health risks and can be seen as a danger to both individuals and communities.
Writing an effective persuasive essay about smoking can help sway public opinion. It can also encourage people to make healthier choices and stop smoking.
But where do you begin?
In this blog, we’ll provide some examples to get you started. So read on to get inspired!
- 1. What You Need To Know About Persuasive Essay
- 2. Persuasive Essay Examples About Smoking
- 3. Argumentative Essay About Smoking Examples
- 4. Tips for Writing a Persuasive Essay About Smoking
What You Need To Know About Persuasive Essay
A persuasive essay is a type of writing that aims to convince its readers to take a certain stance or action. It often uses logical arguments and evidence to back up its argument in order to persuade readers.
It also utilizes rhetorical techniques such as ethos, pathos, and logos to make the argument more convincing. In other words, persuasive essays use facts and evidence as well as emotion to make their points.
A persuasive essay about smoking would use these techniques to convince its readers about any point about smoking. Check out an example below:
Simple persuasive essay about smoking

Tough Essay Due? Hire Tough Writers!
Persuasive Essay Examples About Smoking
Smoking is one of the leading causes of preventable death in the world. It leads to adverse health effects, including lung cancer, heart disease, and damage to the respiratory tract. However, the number of people who smoke cigarettes has been on the rise globally.
A lot has been written on topics related to the effects of smoking. Reading essays about it can help you get an idea of what makes a good persuasive essay.
Here are some sample persuasive essays about smoking that you can use as inspiration for your own writing:
Persuasive speech on smoking outline
Persuasive essay about smoking should be banned
Persuasive essay about smoking pdf
Persuasive essay about smoking cannot relieve stress
Persuasive essay about smoking in public places
Speech about smoking is dangerous
Persuasive Essay About Smoking Introduction
Persuasive Essay About Stop Smoking
Short Persuasive Essay About Smoking
Stop Smoking Persuasive Speech
Check out some more persuasive essay examples on various other topics.
Argumentative Essay About Smoking Examples
An argumentative essay is a type of essay that uses facts and logical arguments to back up a point. It is similar to a persuasive essay but differs in that it utilizes more evidence than emotion.
If you’re looking to write an argumentative essay about smoking, here are some examples to get you started on the arguments of why you should not smoke.
Argumentative essay about smoking pdf
Argumentative essay about smoking in public places
Argumentative essay about smoking introduction
Check out the video below to find useful arguments against smoking:
Tips for Writing a Persuasive Essay About Smoking
You have read some examples of persuasive and argumentative essays about smoking. Now here are some tips that will help you craft a powerful essay on this topic.
Choose a Specific Angle
Select a particular perspective on the issue that you can use to form your argument. When talking about smoking, you can focus on any aspect such as the health risks, economic costs, or environmental impact.
Think about how you want to approach the topic. For instance, you could write about why smoking should be banned.
Check out the list of persuasive essay topics to help you while you are thinking of an angle to choose!
Research the Facts
Before writing your essay, make sure to research the facts about smoking. This will give you reliable information to use in your arguments and evidence for why people should avoid smoking.
You can find and use credible data and information from reputable sources such as government websites, health organizations, and scientific studies.
For instance, you should gather facts about health issues and negative effects of tobacco if arguing against smoking. Moreover, you should use and cite sources carefully.
Paper Due? Why Suffer? That's our Job!
Make an Outline
The next step is to create an outline for your essay. This will help you organize your thoughts and make sure that all the points in your essay flow together logically.
Your outline should include the introduction, body paragraphs, and conclusion. This will help ensure that your essay has a clear structure and argument.
Use Persuasive Language
When writing your essay, make sure to use persuasive language such as “it is necessary” or “people must be aware”. This will help you convey your message more effectively and emphasize the importance of your point.
Also, don’t forget to use rhetorical devices such as ethos, pathos, and logos to make your arguments more convincing. That is, you should incorporate emotion, personal experience, and logic into your arguments.
Introduce Opposing Arguments
Another important tip when writing a persuasive essay on smoking is to introduce opposing arguments. It will show that you are aware of the counterarguments and can provide evidence to refute them. This will help you strengthen your argument.
By doing this, your essay will come off as more balanced and objective, making it more convincing.
Finish Strong
Finally, make sure to finish your essay with a powerful conclusion. This will help you leave a lasting impression on your readers and reinforce the main points of your argument. You can end by summarizing the key points or giving some advice to the reader.
A powerful conclusion could either include food for thought or a call to action. So be sure to use persuasive language and make your conclusion strong.
To conclude,
By following these tips, you can write an effective and persuasive essay on smoking. Remember to research the facts, make an outline, and use persuasive language.
However, don't stress if you need expert help to write your essay! We're the best essay writing service for you!
Our persuasive essay writing service is fast, affordable, and trustworthy.
Try it out today!
Write Essay Within 60 Seconds!

Caleb S. has been providing writing services for over five years and has a Masters degree from Oxford University. He is an expert in his craft and takes great pride in helping students achieve their academic goals. Caleb is a dedicated professional who always puts his clients first.

Paper Due? Why Suffer? That’s our Job!
Keep reading

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 10 October 2022
Health effects associated with smoking: a Burden of Proof study
- Xiaochen Dai ORCID: orcid.org/0000-0002-0289-7814 1 , 2 ,
- Gabriela F. Gil 1 ,
- Marissa B. Reitsma 1 ,
- Noah S. Ahmad 1 ,
- Jason A. Anderson 1 ,
- Catherine Bisignano 1 ,
- Sinclair Carr 1 ,
- Rachel Feldman 1 ,
- Simon I. Hay ORCID: orcid.org/0000-0002-0611-7272 1 , 2 ,
- Jiawei He 1 , 2 ,
- Vincent Iannucci 1 ,
- Hilary R. Lawlor 1 ,
- Matthew J. Malloy 1 ,
- Laurie B. Marczak 1 ,
- Susan A. McLaughlin 1 ,
- Larissa Morikawa ORCID: orcid.org/0000-0001-9749-8033 1 ,
- Erin C. Mullany 1 ,
- Sneha I. Nicholson 1 ,
- Erin M. O’Connell 1 ,
- Chukwuma Okereke 1 ,
- Reed J. D. Sorensen 1 ,
- Joanna Whisnant 1 ,
- Aleksandr Y. Aravkin 1 , 3 ,
- Peng Zheng 1 , 2 ,
- Christopher J. L. Murray ORCID: orcid.org/0000-0002-4930-9450 1 , 2 &
- Emmanuela Gakidou ORCID: orcid.org/0000-0002-8992-591X 1 , 2
Nature Medicine volume 28 , pages 2045–2055 ( 2022 ) Cite this article
23k Accesses
34 Citations
132 Altmetric
Metrics details
- Risk factors
Matters Arising to this article was published on 14 April 2023
As a leading behavioral risk factor for numerous health outcomes, smoking is a major ongoing public health challenge. Although evidence on the health effects of smoking has been widely reported, few attempts have evaluated the dose–response relationship between smoking and a diverse range of health outcomes systematically and comprehensively. In the present study, we re-estimated the dose–response relationships between current smoking and 36 health outcomes by conducting systematic reviews up to 31 May 2022, employing a meta-analytic method that incorporates between-study heterogeneity into estimates of uncertainty. Among the 36 selected outcomes, 8 had strong-to-very-strong evidence of an association with smoking, 21 had weak-to-moderate evidence of association and 7 had no evidence of association. By overcoming many of the limitations of traditional meta-analyses, our approach provides comprehensive, up-to-date and easy-to-use estimates of the evidence on the health effects of smoking. These estimates provide important information for tobacco control advocates, policy makers, researchers, physicians, smokers and the public.
Similar content being viewed by others
The Burden of Proof studies: assessing the evidence of risk
Peng Zheng, Ashkan Afshin, … Christopher J. L. Murray
Health effects associated with exposure to secondhand smoke: a Burden of Proof study
Luisa S. Flor, Jason A. Anderson, … Emmanuela Gakidou

Health effects associated with chewing tobacco: a Burden of Proof study
Gabriela F. Gil, Jason A. Anderson, … Emmanuela Gakidou
Among both the public and the health experts, smoking is recognized as a major behavioral risk factor with a leading attributable health burden worldwide. The health risks of smoking were clearly outlined in a canonical study of disease rates (including lung cancer) and smoking habits in British doctors in 1950 and have been further elaborated in detail over the following seven decades 1 , 2 . In 2005, evidence of the health consequences of smoking galvanized the adoption of the first World Health Organization (WHO) treaty, the Framework Convention on Tobacco Control, in an attempt to drive reductions in global tobacco use and second-hand smoke exposure 3 . However, as of 2020, an estimated 1.18 billion individuals globally were current smokers and 7 million deaths and 177 million disability-adjusted life-years were attributed to smoking, reflecting a persistent public health challenge 4 . Quantifying the relationship between smoking and various important health outcomes—in particular, highlighting any significant dose–response relationships—is crucial to understanding the attributable health risk experienced by these individuals and informing responsive public policy.
Existing literature on the relationship between smoking and specific health outcomes is prolific, including meta-analyses, cohort studies and case–control studies analyzing the risk of outcomes such as lung cancer 5 , 6 , 7 , chronic obstructive pulmonary disease (COPD) 8 , 9 , 10 and ischemic heart disease 11 , 12 , 13 , 14 due to smoking. There are few if any attempts, however, to systematically and comprehensively evaluate the landscape of evidence on smoking risk across a diverse range of health outcomes, with most current research focusing on risk or attributable burden of smoking for a specific condition 7 , 15 , thereby missing the opportunity to provide a comprehensive picture of the health risk experienced by smokers. Furthermore, although evidence surrounding specific health outcomes, such as lung cancer, has generated widespread consensus, findings about the attributable risk of other outcomes are much more heterogeneous and inconclusive 16 , 17 , 18 . These studies also vary in their risk definitions, with many comparing dichotomous exposure measures of ever smokers versus nonsmokers 19 , 20 . Others examine the distinct risks of current smokers and former smokers compared with never smokers 21 , 22 , 23 . Among the studies that do analyze dose–response relationships, there is large variation in the units and dose categories used in reporting their findings (for example, the use of pack-years or cigarettes per day) 24 , 25 , which complicates the comparability and consolidation of evidence. This, in turn, can obscure data that could inform personal health choices, public health practices and policy measures. Guidance on the health risks of smoking, such as the Surgeon General’s Reports on smoking 26 , 27 , is often based on experts’ evaluation of heterogenous evidence, which, although extremely useful and well suited to carefully consider nuances in the evidence, is fundamentally subjective.
The present study, as part of the Global Burden of Diseases, Risk Factors, and Injuries Study (GBD) 2020, re-estimated the continuous dose–response relationships (the mean risk functions and associated uncertainty estimates) between current smoking and 36 health outcomes (Supplementary Table 1 ) by identifying input studies using a systematic review approach and employing a meta-analytic method 28 . The 36 health outcomes that were selected based on existing evidence of a relationship included 16 cancers (lung cancer, esophageal cancer, stomach cancer, leukemia, liver cancer, laryngeal cancer, breast cancer, cervical cancer, colorectal cancer, lip and oral cavity cancer, nasopharyngeal cancer, other pharynx cancer (excluding nasopharynx cancer), pancreatic cancer, bladder cancer, kidney cancer and prostate cancer), 5 cardiovascular diseases (CVDs: ischemic heart disease, stroke, atrial fibrillation and flutter, aortic aneurysm and peripheral artery disease) and 15 other diseases (COPD, lower respiratory tract infections, tuberculosis, asthma, type 2 diabetes, Alzheimer’s disease and related dementias, Parkinson’s disease, multiple sclerosis, cataracts, gallbladder diseases, low back pain, peptic ulcer disease, rheumatoid arthritis, macular degeneration and fractures). Definitions of the outcomes are described in Supplementary Table 1 . We conducted a separate systematic review for each risk–outcome pair with the exception of cancers, which were done together in a single systematic review. This approach allowed us to systematically identify all relevant studies indexed in PubMed up to 31 May 2022, and we extracted relevant data on risk of smoking, including study characteristics, following a pre-specified template (Supplementary Table 2 ). The meta-analytic tool overcomes many of the limitations of traditional meta-analyses by incorporating between-study heterogeneity into the uncertainty of risk estimates, accounting for small numbers of studies, relaxing the assumption of log(linearity) applied to the risk functions, handling differences in exposure ranges between comparison groups, and systematically testing and adjusting for bias due to study designs and characteristics. We then estimated the burden-of-proof risk function (BPRF) for each risk–outcome pair, as proposed by Zheng et al. 29 ; the BPRF is a conservative risk function defined as the 5th quantile curve (for harmful risks) that reflects the smallest harmful effect at each level of exposure consistent with the available evidence. Given all available data for each outcome, the risk of smoking is at least as harmful as the BPRF indicates.
We used the BPRF for each risk–outcome pair to calculate risk–outcome scores (ROSs) and categorize the strength of evidence for the association between smoking and each health outcome using a star rating from 1 to 5. The interpretation of the star ratings is as follows: 1 star (*) indicates no evidence of association; 2 stars (**) correspond to a 0–15% increase in risk across average range of exposures for harmful risks; 3 stars (***) represent a 15–50% increase in risk; 4 stars (****) refer to >50–85% increase in risk; and 5 stars (*****) equal >85% increase in risk. The thresholds for each star rating were developed in consultation with collaborators and other stakeholders.
The increasing disease burden attributable to current smoking, particularly in low- and middle-income countries 4 , demonstrates the relevance of the present study, which quantifies the strength of the evidence using an objective, quantitative, comprehensive and comparative framework. Findings from the present study can be used to support policy makers in making informed smoking recommendations and regulations focusing on the associations for which the evidence is strongest (that is, the 4- and 5-star associations). However, associations with a lower star rating cannot be ignored, especially when the outcome has high prevalence or severity. A summary of the main findings, limitations and policy implications of the study is presented in Table 1 .
We evaluated the mean risk functions and the BPRFs for 36 health outcomes that are associated with current smoking 30 (Table 2 ). Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 31 for each of our systematic reviews, we identified studies reporting relative risk (RR) of incidence or mortality from each of the 36 selected outcomes for smokers compared with nonsmokers. We reviewed 21,108 records, which were identified to have been published between 1 May 2018 and 31 May 2022; this represents the most recent time period since the last systematic review of the available evidence for the GBD at the time of publication. The meta-analyses reported in the present study for each of the 36 health outcomes are based on evidence from a total of 793 studies published between 1970 and 2022 (Extended Data Fig. 1 – 5 and Supplementary Information 1.5 show the PRISMA diagrams for each outcome). Only prospective cohort and case–control studies were included for estimating dose–response risk curves, but cross-sectional studies were also included for estimating the age pattern of smoking risk on cardiovascular and circulatory disease (CVD) outcomes. Details on each, including the study’s design, data sources, number of participants, length of follow-up, confounders adjusted for in the input data and bias covariates included in the dose–response risk model, can be found in Supplementary Information 2 and 3 . The theoretical minimum risk exposure level used for current smoking was never smoking or zero 30 .
Five-star associations
When the most conservative interpretation of the evidence, that is, the BPRF, suggests that the average exposure (15th–85th percentiles of exposure) of smoking increases the risk of a health outcome by >85% (that is, ROS > 0.62), smoking and that outcome are categorized as a 5-star pair. Among the 36 outcomes, there are 5 that have a 5-star association with current smoking: laryngeal cancer (375% increase in risk based on the BPRF, 1.56 ROS), aortic aneurysm (150%, 0.92), peripheral artery disease (137%, 0.86), lung cancer (107%, 0.73) and other pharynx cancer (excluding nasopharynx cancer) (92%, 0.65).
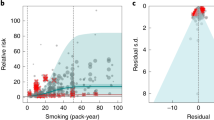
Results for all 5-star risk–outcome pairs are available in Table 2 and Supplementary Information 4.1 . In the present study, we provide detailed results for one example 5-star association: current smoking and lung cancer. We extracted 371 observations from 25 prospective cohort studies and 53 case–control studies across 25 locations (Supplementary Table 3 ) 5 , 6 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 . Exposure ranged from 1 pack-year to >112 pack-years, with the 85th percentile of exposure being 50.88 pack-years (Fig. 1a ).
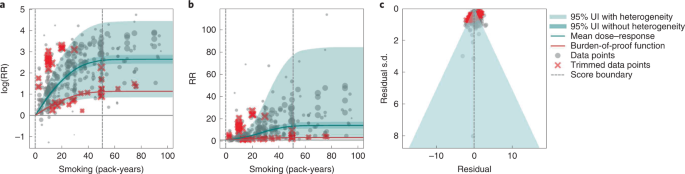
a , The log(RR) function. b , RR function. c , A modified funnel plot showing the residuals (relative to 0) on the x axis and the estimated s.d. that includes reported s.d. and between-study heterogeneity on the y axis.
We found a very strong and significant harmful relationship between pack-years of current smoking and the RR of lung cancer (Fig. 1b ). The mean RR of lung cancer at 20 pack-years of smoking was 5.11 (95% uncertainty interval (UI) inclusive of between-study heterogeneity = 1.84–14.99). At 50.88 pack-years (85th percentile of exposure), the mean RR of lung cancer was 13.42 (2.63–74.59). See Table 2 for mean RRs at other exposure levels. The BPRF, which represents the most conservative interpretation of the evidence (Fig. 1a ), suggests that smoking in the 15th–85th percentiles of exposure increases the risk of lung cancer by an average of 107%, yielding an ROS of 0.73.
The relationship between pack-years of current smoking and RR of lung cancer is nonlinear, with diminishing impact of further pack-years of smoking, particularly for middle-to-high exposure levels (Fig. 1b ). To reduce the effect of bias, we adjusted observations that did not account for more than five confounders, including age and sex, because they were the significant bias covariates identified by the bias covariate selection algorithm 29 (Supplementary Table 7 ). The reported RRs across studies were very heterogeneous. Our meta-analytic method, which accounts for the reported uncertainty in both the data and between-study heterogeneity, fit the data and covered the estimated residuals well (Fig. 1c ). After trimming 10% of outliers, we still detected publication bias in the results for lung cancer. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 5-star pairs.
Four-star associations
When the BPRF suggests that the average exposure of smoking increases the risk of a health outcome by 50–85% (that is, ROS > 0.41–0.62), smoking is categorized as having a 4-star association with that outcome. We identified three outcomes with a 4-star association with smoking: COPD (72% increase in risk based on the BPRF, 0.54 ROS), lower respiratory tract infection (54%, 0.43) and pancreatic cancer (52%, 0.42).
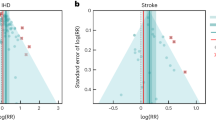
In the present study, we provide detailed results for one example 4-star association: current smoking and COPD. We extracted 51 observations from 11 prospective cohort studies and 4 case–control studies across 36 locations (Supplementary Table 3 ) 6 , 8 , 9 , 10 , 78 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 . Exposure ranged from 1 pack-year to 100 pack-years, with the 85th percentile of exposure in the exposed group being 49.75 pack-years.
We found a strong and significant harmful relationship between pack-years of current smoking and RR of COPD (Fig. 2b ). The mean RR of COPD at 20 pack-years was 3.17 (1.60–6.55; Table 2 reports RRs at other exposure levels). At the 85th percentile of exposure, the mean RR of COPD was 6.01 (2.08–18.58). The BPRF suggests that average smoking exposure raises the risk of COPD by an average of 72%, yielding an ROS of 0.54. The results for the other health outcomes that have an association with smoking rated as 4 stars are shown in Table 2 and Supplementary Information 4.2 .
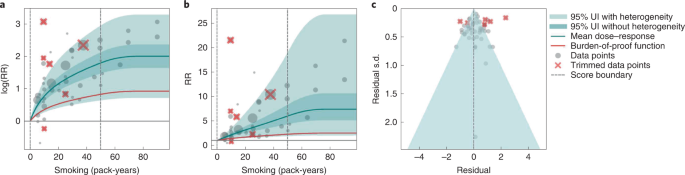
a , The log(RR) function. b , RR function. c , A modified funnel plot showing the residuals (relative to 0) on th e x axis and the estimated s.d. that includes the reported s.d. and between-study heterogeneity on the y axis.
The relationship between smoking and COPD is nonlinear, with diminishing impact of further pack-years of current smoking on risk of COPD, particularly for middle-to-high exposure levels (Fig. 2a ). To reduce the effect of bias, we adjusted observations that did not account for age and sex and/or were generated for individuals aged >65 years 116 , because they were the two significant bias covariates identified by the bias covariate selection algorithm (Supplementary Table 7 ). There was large heterogeneity in the reported RRs across studies, and our meta-analytic method fit the data and covered the estimated residuals well (Fig. 2b ). Although we trimmed 10% of outliers, publication bias was still detected in the results for COPD. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for reported RR data and alternative exposures across studies for the remaining health outcomes that have a 4-star association with smoking.
Three-star associations
When the BPRF suggests that the average exposure of smoking increases the risk of a health outcome by 15–50% (or, when protective, decreases the risk of an outcome by 13–34%; that is, ROS >0.14–0.41), the association between smoking and that outcome is categorized as having a 3-star rating. We identified 15 outcomes with a 3-star association: bladder cancer (40% increase in risk, 0.34 ROS); tuberculosis (31%, 0.27); esophageal cancer (29%, 0.26); cervical cancer, multiple sclerosis and rheumatoid arthritis (each 23–24%, 0.21); lower back pain (22%, 0.20); ischemic heart disease (20%, 0.19); peptic ulcer and macular degeneration (each 19–20%, 0.18); Parkinson's disease (protective risk, 15% decrease in risk, 0.16); and stomach cancer, stroke, type 2 diabetes and cataracts (each 15–17%, 0.14–0.16).
We present the findings on smoking and type 2 diabetes as an example of a 3-star risk association. We extracted 102 observations from 24 prospective cohort studies and 4 case–control studies across 15 locations (Supplementary Table 3 ) 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 . The exposure ranged from 1 cigarette to 60 cigarettes smoked per day, with the 85th percentile of exposure in the exposed group being 26.25 cigarettes smoked per day.
We found a moderate and significant harmful relationship between cigarettes smoked per day and the RR of type 2 diabetes (Fig. 3b ). The mean RR of type 2 diabetes at 20 cigarettes smoked per day was 1.49 (1.18–1.90; see Table 2 for other exposure levels). At the 85th percentile of exposure, the mean RR of type 2 diabetes was 1.54 (1.20–2.01). The BPRF suggests that average smoking exposure raises the risk of type 2 diabetes by an average of 16%, yielding an ROS of 0.15. See Table 2 and Supplementary Information 4.3 for results for the additional health outcomes with an association with smoking rated as 3 stars.
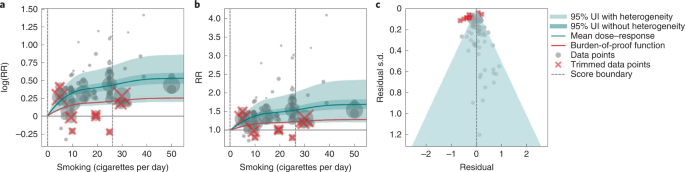
a , The log(RR) function. b , RR function. c , A modified funnel plot showing the residuals (relative to 0) on the x axis and the estimated s.d. that includes the reported s.d. and between-study heterogeneity on the y axis.
The relationship between smoking and type 2 diabetes is nonlinear, particularly for high exposure levels where the mean risk curve becomes flat (Fig. 3a ). We adjusted observations that were generated in subpopulations, because it was the only significant bias covariate identified by the bias covariate selection algorithm (Supplementary Table 7 ). There was moderate heterogeneity in the observed RR data across studies and our meta-analytic method fit the data and covered the estimated residuals extremely well (Fig. 3b,c ). After trimming 10% of outliers, we still detected publication bias in the results for type 2 diabetes. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 3-star pairs.
Two-star associations
When the BPRF suggests that the average exposure of smoking increases the risk of an outcome by 0–15% (that is, ROS 0.0–0.14), the association between smoking and that outcome is categorized as a 2-star rating. We identified six 2-star outcomes: nasopharyngeal cancer (14% increase in risk, 0.13 ROS); Alzheimer’s and other dementia (10%, 0.09); gallbladder diseases and atrial fibrillation and flutter (each 6%, 0.06); lip and oral cavity cancer (5%, 0.05); and breast cancer (4%, 0.04).
We present the findings on smoking and breast cancer as an example of a 2-star association. We extracted 93 observations from 14 prospective cohort studies and 9 case–control studies across 14 locations (Supplementary Table 3 ) 84 , 87 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 . The exposure ranged from 1 cigarette to >76 cigarettes smoked per day, with the 85th percentile of exposure in the exposed group being 34.10 cigarettes smoked per day.
We found a weak but significant relationship between pack-years of current smoking and RR of breast cancer (Extended Data Fig. 6 ). The mean RR of breast cancer at 20 pack-years was 1.17 (1.04–1.31; Table 2 reports other exposure levels). The BPRF suggests that average smoking exposure raises the risk of breast cancer by an average of 4%, yielding an ROS of 0.04. See Table 2 and Supplementary Information 4.4 for results on the additional health outcomes for which the association with smoking has been categorized as 2 stars.
The relationship between smoking and breast cancer is nonlinear, particularly for high exposure levels where the mean risk curve becomes flat (Extended Data Fig. 6a ). To reduce the effect of bias, we adjusted observations that were generated in subpopulations, because it was the only significant bias covariate identified by the bias covariate selection algorithm (Supplementary Table 7 ). There was heterogeneity in the reported RRs across studies, but our meta-analytic method fit the data and covered the estimated residuals (Extended Data Fig. 6b ). After trimming 10% of outliers, we did not detect publication bias in the results for breast cancer. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 2-star pairs.
One-star associations
When average exposure to smoking does not significantly increase (or decrease) the risk of an outcome, once between-study heterogeneity and other sources of uncertainty are accounted for (that is, ROS < 0), the association between smoking and that outcome is categorized as 1 star, indicating that there is not sufficient evidence for the effect of smoking on the outcome to reject the null (that is, there may be no association). There were seven outcomes with an association with smoking that rated as 1 star: colorectal and kidney cancer (each –0.01 ROS); leukemia (−0.04); fractures (−0.05); prostate cancer (−0.06); liver cancer (−0.32); and asthma (−0.64).
We use smoking and prostate cancer as examples of a 1-star association. We extracted 78 observations from 21 prospective cohort studies and 1 nested case–control study across 15 locations (Supplementary Table 3 ) 157 , 160 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 . The exposure among the exposed group ranged from 1 cigarette to 90 cigarettes smoked per day, with the 85th percentile of exposure in the exposed group being 29.73 cigarettes smoked per day.
Based on our conservative interpretation of the data, we did not find a significant relationship between cigarettes smoked per day and the RR of prostate cancer (Fig. 4B ). The exposure-averaged BPRF for prostate cancer was 0.94, which was opposite null from the full range of mean RRs, such as 1.16 (0.89–1.53) at 20 cigarettes smoked per day. The corresponding ROS was −0.06, which is consistent with no evidence of an association between smoking and increased risk of prostate cancer. See Table 2 and Supplementary Information 4.5 for results for the additional outcomes that have a 1-star association with smoking.
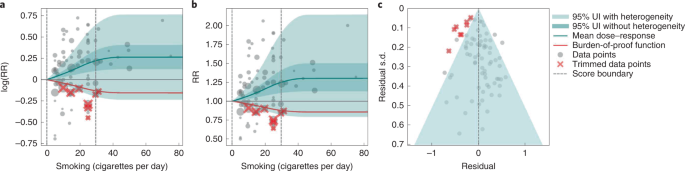
The relationship between smoking and prostate cancer is nonlinear, particularly for middle-to-high exposure levels where the mean risk curve becomes flat (Fig. 4a ). We did not adjust for any bias covariate because no significant bias covariates were selected by the algorithm (Supplementary Table 7 ). The RRs reported across studies were very heterogeneous, but our meta-analytic method fit the data and covered the estimated residuals well (Fig. 4b,c ). The ROS associated with the BPRF is −0.05, suggesting that the most conservative interpretation of all evidence, after accounting for between-study heterogeneity, indicates an inconclusive relationship between smoking exposure and the risk of prostate cancer. After trimming 10% of outliers, we still detected publication bias in the results for prostate cancer, which warrants further studies using sample populations. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 1-star pairs.
Age-specific dose–response risk for CVD outcomes
We produced age-specific dose–response risk curves for the five selected CVD outcomes ( Methods ). The ROS associated with each smoking–CVD pair was calculated based on the reference risk curve estimated using all risk data regardless of age information. Estimation of the BPRF, calculation of the associated ROS and star rating of the smoking–CVD pairs follow the same rules as the other non-CVD smoking–outcome pairs (Table 1 and Supplementary Figs. 2 – 4 ). Once we had estimated the reference dose–response risk curve for each CVD outcome, we determined the age group of the reference risk curve. The reference age group is 55–59 years for all CVD outcomes, except for peripheral artery disease, the reference age group for which is 60–64 years. We then estimated the age pattern of smoking on all CVD outcomes (Supplementary Fig. 2 ) and calculated age attenuation factors of the risk for each age group by comparing the risk of each age group with that of the reference age group, using the estimated age pattern (Supplementary Fig. 3 ). Last, we applied the draws of age attenuation factors of each age group to the dose–response risk curve for the reference age group to produce the age group-specific dose–response risk curves for each CVD outcome (Supplementary Fig. 4 ).
Using our burden-of-proof meta-analytic methods, we re-estimated the dose–response risk of smoking on 36 health outcomes that had previously been demonstrated to be associated with smoking 30 , 186 . Using these methods, which account for both the reported uncertainty of the data and the between-study heterogeneity, we found that 29 of the 36 smoking–outcome pairs are supported by evidence that suggests a significant dose–response relationship between smoking and the given outcome (28 with a harmful association and 1 with a protective association). Conversely, after accounting for between-study heterogeneity, the available evidence of smoking risk on seven outcomes (that is, colon and rectum cancer, kidney cancer, leukemia, prostate cancer, fractures, liver cancer and asthma) was insufficient to reject the null or draw definitive conclusions on their relationship to smoking. Among the 29 outcomes that have evidence supporting a significant relationship to smoking, 8 had strong-to-very-strong evidence of a relationship, meaning that, given all the available data on smoking risk, we estimate that average exposure to smoking increases the risk of those outcomes by >50% (4- and 5-star outcomes). The currently available evidence for the remaining 21 outcomes with a significant association with current smoking was weak to moderate, indicating that smoking increases the risk of those outcomes by at least >0–50% (2- and 3-star associations).
Even under our conservative interpretation of the data, smoking is irrefutably harmful to human health, with the greatest increases in risk occurring for laryngeal cancer, aortic aneurysm, peripheral artery disease, lung cancer and other pharynx cancer (excluding nasopharynx cancer), which collectively represent large causes of death and ill-health. The magnitude of and evidence for the associations between smoking and its leading health outcomes are among the highest currently analyzed in the burden-of-proof framework 29 . The star ratings assigned to each smoking–outcome pair offer policy makers a way of categorizing and comparing the evidence for a relationship between smoking and its potential health outcomes ( https://vizhub.healthdata.org/burden-of-proof ). We found that, for seven outcomes in our analysis, there was insufficient or inconsistent evidence to demonstrate a significant association with smoking. This is a key finding because it demonstrates the need for more high-quality data for these particular outcomes; availability of more data should improve the strength of evidence for whether or not there is an association between smoking and these health outcomes.
Our systematic review approach and meta-analytic methods have numerous benefits over existing systematic reviews and meta-analyses on the same topic that use traditional random effects models. First, our approach relaxes the log(linear) assumption, using a spline ensemble to estimate the risk 29 . Second, our approach allows variable reference groups and exposure ranges, allowing for more accurate estimates regardless of whether or not the underlying relative risk is log(linear). Furthermore, it can detect outliers in the data automatically. Finally, it quantifies uncertainty due to between-study heterogeneity while accounting for small numbers of studies, minimizing the risk that conclusions will be drawn based on spurious findings.
We believe that the results for the association between smoking and each of the 36 health outcomes generated by the present study, including the mean risk function, BPRF, ROS, average excess risk and star rating, could be useful to a range of stakeholders. Policy makers can formulate their decisions on smoking control priorities and resource allocation based on the magnitude of the effect and the consistency of the evidence relating smoking to each of the 36 outcomes, as represented by the ROS and star rating for each smoking–outcome association 187 . Physicians and public health practitioners can use the estimates of average increased risk and the star rating to educate patients and the general public about the risk of smoking and to promote smoking cessation 188 . Researchers can use the estimated mean risk function or BPRF to obtain the risk of an outcome at a given smoking exposure level, as well as uncertainty surrounding that estimate of risk. The results can also be used in the estimation of risk-attributable burden, that is, the deaths and disability-adjusted life-years due to each outcome that are attributable to smoking 30 , 186 . For the general public, these results could help them to better understand the risk of smoking and manage their health 189 .
Although our meta-analysis was comprehensive and carefully conducted, there are limitations to acknowledge. First, the bias covariates used, although carefully extracted and evaluated, were based on observable study characteristics and thus may not fully capture unobserved characteristics such as study quality or context, which might be major sources of bias. Second, if multiple risk estimates with different adjustment levels were reported in a given study, we included only the fully adjusted risk estimate and modeled the adjustment level according to the number of covariates adjusted for (rather than which covariates were adjusted for) and whether a standard adjustment for age and sex had been applied. This approach limited our ability to make full use of all available risk estimates in the literature. Third, although we evaluated the potential for publication bias in the data, we did not test for other forms of bias such as when studies are more consistent with each other than expected by chance 29 . Fourth, our analysis assumes that the relationships between smoking and health outcomes are similar across geographical regions and over time. We do not have sufficient evidence to quantify how the relationships may have evolved over time because the composition of smoking products has also changed over time. Perhaps some of the heterogeneity of the effect sizes in published studies reflects this; however, this cannot be discerned with the currently available information.
In the future, we plan to include crude and partially adjusted risk estimates in our analyses to fully incorporate all available risk estimates, to model the adjusted covariates in a more comprehensive way by mapping the adjusted covariates across all studies comprehensively and systematically, and to develop methods to evaluate additional forms of potential bias. We plan to update our results on a regular basis to provide timely and up-to-date evidence to stakeholders.
To conclude, we have re-estimated the dose–response risk of smoking on 36 health outcomes while synthesizing all the available evidence up to 31 May 2022. We found that, even after factoring in the heterogeneity between studies and other sources of uncertainty, smoking has a strong-to-very-strong association with a range of health outcomes and confirmed that smoking is irrefutably highly harmful to human health. We found that, due to small numbers of studies, inconsistency in the data, small effect sizes or a combination of these reasons, seven outcomes for which some previous research had found an association with smoking did not—under our meta-analytic framework and conservative approach to interpreting the data—have evidence of an association. Our estimates of the evidence for risk of smoking on 36 selected health outcomes have the potential to inform the many stakeholders of smoking control, including policy makers, researchers, public health professionals, physicians, smokers and the general public.
For the present study, we used a meta-analytic tool, MR-BRT (metaregression—Bayesian, regularized, trimmed), to estimate the dose–response risk curves of the risk of a health outcome across the range of current smoking levels along with uncertainty estimates 28 . Compared with traditional meta-analysis using linear mixed effect models, MR-BRT relaxes the assumption of a log(linear) relationship between exposure and risk, incorporates between-study heterogeneity into the uncertainty of risk estimates, handles estimates reported across different exposure categories, automatically identifies and trims outliers, and systematically tests and adjusts for bias due to study designs and characteristics. The meta-analytic methods employed by the present study followed the six main steps proposed by Zheng et al. 28 , 29 , namely: (1) enacting a systematic review approach and data extraction following a pre-specified and standardized protocol; (2) estimating the shape of the relationship between exposure and RR; (3) evaluating and adjusting for systematic bias as a function of study characteristics and risk estimation; (4) quantifying between-study heterogeneity while adjusting for within-study correlation and the number of studies; (5) evaluating potential publication or reporting biases; and (6) estimating the mean risk function and the BPRF, calculating the ROS and categorizing smoking–outcome pairs using a star-rating scheme from 1 to 5.
The estimates for our primary indicators of this work—mean RRs across a range of exposures, BRPFs, ROSs and star ratings for each risk–outcome pair—are not specific to or disaggregated by specific populations. We did not estimate RRs separately for different locations, sexes (although the RR of prostate cancer was estimated only for males and of cervical and breast cancer only for females) or age groups (although this analysis was applied to disease endpoints in adults aged ≥30 years only and, as detailed below, age-specific estimates were produced for the five CVD outcomes).
The present study complies with the PRISMA guidelines 190 (Supplementary Tables 9 and 10 and Supplementary Information 1.5 ) and Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) recommendations 191 (Supplementary Table 11 ). The study was approved by the University of Washington Institutional Review Board (study no. 9060). The systematic review approach was not registered.
Selecting health outcomes
In the present study, current smoking is defined as the current use of any smoked tobacco product on a daily or occasional basis. Health outcomes were initially selected using the World Cancer Research Fund criteria for convincing or probable evidence as described in Murray et al. 186 . The 36 health outcomes that were selected based on existing evidence of a relationship included 16 cancers (lung cancer, esophageal cancer, stomach cancer, leukemia, liver cancer, laryngeal cancer, breast cancer, cervical cancer, colorectal cancer, lip and oral cavity cancer, nasopharyngeal cancer, other pharynx cancer (excluding nasopharynx cancer), pancreatic cancer, bladder cancer, kidney cancer and prostate cancer), 5 CVDs (ischemic heart disease, stroke, atrial fibrillation and flutter, aortic aneurysm and peripheral artery disease) and 15 other diseases (COPD, lower respiratory tract infections, tuberculosis, asthma, type 2 diabetes, Alzheimer’s disease and related dementias, Parkinson’s disease, multiple sclerosis, cataracts, gallbladder diseases, low back pain, peptic ulcer disease, rheumatoid arthritis, macular degeneration and fracture). Definitions of the outcomes are described in Supplementary Table 1 .
Step 1: systematic review approach to literature search and data extraction
Informed by the systematic review approach we took for the GBD 2019 (ref. 30 ), for the present study we identified input studies in the literature using a systematic review approach for all 36 smoking–outcome pairs using updated search strings to identify all relevant studies indexed in PubMed up to 31 May 2022 and extracted data on smoking risk estimates. Briefly, the studies that were extracted represented several types of study design (for example, cohort and case–control studies), measured exposure in several different ways and varied in their choice of reference categories (where some compared current smokers with never smokers, whereas others compared current smokers with nonsmokers or former smokers). All these study characteristics were catalogued systematically and taken into consideration during the modeling part of the analysis.
In addition, for CVD outcomes, we also estimated the age pattern of risk associated with smoking. We applied a systematic review of literature approach for smoking risk for the five CVD outcomes. We developed a search string to search for studies reporting any association between binary smoking status (that is, current, former and ever smokers) and the five CVD outcomes from 1 January 1970 to 31 May 2022, and included only studies reporting age-specific risk (RR, odds ratio (OR), hazard ratio (HR)) of smoking status. The inclusion criteria and results of the systematic review approach are reported in accordance with PRISMA guidelines 31 . Details for each outcome on the search string used in the systematic review approach, refined inclusion and exclusion criteria, data extraction template and PRISMA diagram are given in Supplementary Information 1 . Title and/or abstract screening, full text screening and data extraction were conducted by 14 members of the research team and extracted data underwent manual quality assurance by the research team to verify accuracy.
Selecting exposure categories
Cumulative exposure in pack-years was the measure of exposure used for COPD and all cancer outcomes except for prostate cancer, to reflect the risk of both duration and intensity of current smoking on these outcomes. For prostate cancer, CVDs and all the other outcomes except for fractures, we used cigarette-equivalents smoked per day as the exposure for current smoking, because smoking intensity is generally thought to be more important than duration for these outcomes. For fractures, we used binary exposure, because there were few studies examining intensity or duration of smoking on fractures. The smoking–outcome pairs and the corresponding exposures are summarized in Supplementary Table 4 and are congruent with the GBD 2019 (refs. 30 , 186 ).
Steps 2–5: modeling dose–response RR of smoking on the selected health outcomes
Of the six steps proposed by Zheng et al. 29 , steps 2–5 cover the process of modeling dose–response risk curves. In step 2, we estimated the shape (or the ‘signal’) of the dose–response risk curves, integrating over different exposure ranges. To relax the log(linear) assumption usually applied to continuous dose–response risk and make the estimates robust to the placement of spline knots, we used an ensemble spline approach to fit the functional form of the dose–response relationship. The final ensemble model was a weighted combination of 50 models with random knot placement, with the weight of each model proportional to measures of model fit and total variation. To avoid the influence of extreme data and reduce publication bias, we trimmed 10% of data for each outcome as outliers. We also applied a monotonicity constraint to ensure that the mean risk curves were nondecreasing (or nonincreasing in the case of Parkinson’s disease).
In step 3, following the GRADE approach 192 , 193 , we quantified risk of bias across six domains, namely, representativeness of the study population, exposure, outcome, reverse causation, control for confounding and selection bias. Details about the bias covariates are provided in Supplementary Table 4 . We systematically tested for the effect of bias covariates using metaregression, selected significant bias covariates using the Lasso approach 194 , 195 and adjusted for the selected bias covariates in the final risk curve.
In step 4, we quantified between-study heterogeneity accounting for within-study correlation, uncertainty of the heterogeneity, as well as small number of studies. Specifically, we used a random intercept in the mixed-effects model to account for the within-study correlation and used a study-specific random slope with respect to the ‘signal’ to capture between-study heterogeneity. As between-study heterogeneity can be underestimated or even zero when the number of studies is small 196 , 197 , we used Fisher’s information matrix to estimate the uncertainty of the heterogeneity 198 and incorporated that uncertainty into the final results.
In step 5, in addition to generating funnel plots and visually inspecting for asymmetry (Figs. 1c , 2c , 3c and 4c and Extended Data Fig. 6c ) to identify potential publication bias, we also statistically tested for potential publication or reporting bias using Egger’s regression 199 . We flagged potential publication bias in the data but did not correct for it, which is in line with the general literature 10 , 200 , 201 . Full details about the modeling process have been published elsewhere 29 and model specifications for each outcome are in Supplementary Table 6 .
Step 6: estimating the mean risk function and the BPRF
In the final step, step 6, the metaregression model inclusive of the selected bias covariates from step 3 (for example, the highest adjustment level) was used to predict the mean risk function and its 95% UI, which incorporated the uncertainty of the mean effect, between-study heterogeneity and the uncertainty in the heterogeneity estimate accounting for small numbers of studies. Specifically, 1,000 draws were created for each 0.1 level of doses from 0 pack-years to 100 pack-years or cigarette-equivalents smoked per day using the Bayesian metaregression model. The mean of the 1,000 draws was used to estimate the mean risk at each exposure level, and the 25th and 95th draws were used to estimate the 95% UIs for the mean risk at each exposure level.
The BPRF 29 is a conservative estimate of risk function consistent with the available evidence, correcting for both between-study heterogeneity and systemic biases related to study characteristics. The BPRF is defined as either the 5th (if harmful) or 95th (if protective) quantile curve closest to the line of log(RR) of 0, which defines the null (Figs. 1a , 2b , 3a and 4a ). The BPRF represents the smallest harmful (or protective) effect of smoking on the corresponding outcome at each level of exposure that is consistent with the available evidence. A BPRF opposite null from the mean risk function indicates that insufficient evidence is available to reject null, that is, that there may not be an association between risk and outcome. Likewise, the further the BPRF is from null on the same side of null as the mean risk function, the higher the magnitude and evidence for the relationship. The BPRF can be interpreted as indicating that, even accounting for between-study heterogeneity and its uncertainty, the log(RR) across the studied smoking range is at least as high as the BPRF (or at least as low as the BPRF for a protective risk).
To quantify the strength of the evidence, we calculated the ROS for each smoking–outcome association as the signed value of the log(BPRF) averaged between the 15th and 85th percentiles of observed exposure levels for each outcome. The ROS is a single summary of the effect of smoking on the outcome, with higher positive ROSs corresponding to stronger and more consistent evidence and a higher average effect size of smoking and a negative ROS, suggesting that, based on the available evidence, there is no significant effect of smoking on the outcome after accounting for between-study heterogeneity.
For ease of communication, we further classified each smoking–outcome association into a star rating from 1 to 5. Briefly, 1-star associations have an ROS <0, indicating that there is insufficient evidence to find a significant association between smoking and the selected outcome. We divided the positive ROSs into ranges 0.0–0.14 (2-star), >0.14–0.41 (3-star), >0.41–0.62 (4-star) and >0.62 (5-star). These categories correspond to excess risk ranges for harmful risks of 0–15%, >15–50%, >50–85% and >85%. For protective risks, the ranges of exposure-averaged decreases in risk by star rating are 0–13% (2 stars), >13–34% (3 stars), >34–46% (4 stars) and >46% (5 stars).
Among the 36 smoking–outcome pairs analyzed, smoking fracture was the only binary risk–outcome pair, which was due to limited data on the dose–response risk of smoking on fracture 202 . The estimation of binary risk was simplified because the RR was merely a comparison between current smokers and nonsmokers or never smokers. The concept of ROS for continuous risk can naturally extend to binary risk because the BPRF is still defined as the 5th percentile of the effect size accounting for data uncertainty and between-study heterogeneity. However, binary ROSs must be divided by 2 to make them comparable with continuous ROSs, which were calculated by averaging the risk over the range between the 15th and the 85th percentiles of observed exposure levels. Full details about estimating mean risk functions, BPRFs and ROSs for both continuous and binary risk–outcome pairs can be found elsewhere 29 .
Estimating the age-specific risk function for CVD outcomes
For non-CVD outcomes, we assumed that the risk function was the same for all ages and all sexes, except for breast, cervical and prostate cancer, which were assumed to apply only to females or males, respectively. As the risk of smoking on CVD outcomes is known to attenuate with increasing age 203 , 204 , 205 , 206 , we adopted a four-step approach for GBD 2020 to produce age-specific dose–response risk curves for CVD outcomes.
First, we estimated the reference dose–response risk of smoking for each CVD outcome using dose-specific RR data for each outcome regardless of the age group information. This step was identical to that implemented for the other non-CVD outcomes. Once we had generated the reference curve, we determined the age group associated with it by calculating the weighted mean age across all dose-specific RR data (weighted by the reciprocal of the s.e.m. of each datum). For example, if the weighted mean age of all dose-specific RR data was 56.5, we estimated the age group associated with the reference risk curve to be aged 55–59 years. For cohort studies, the age range associated with the RR estimate was calculated as a mean age at baseline plus the mean/median years of follow-up (if only the maximum years of follow-up were reported, we would halve this value and add it to the mean age at baseline). For case–control studies, the age range associated with the OR estimate was simply the reported mean age at baseline (if mean age was not reported, we used the midpoint of the age range instead).
In the third step, we extracted age group-specific RR data and relevant bias covariates from the studies identified in our systematic review approach of age-specific smoking risk on CVD outcomes, and used MR-BRT to model the age pattern of excess risk (that is, RR-1) of smoking on CVD outcomes with age group-specific excess RR data for all CVD outcomes. We modeled the age pattern of smoking risk on CVDs following the same steps we implemented for modeling dose–response risk curves. In the final model, we included a spline on age, random slope on age by study and the bias covariate encoding exposure definition (that is, current, former and ever smokers), which was picked by the variable selection algorithm 28 , 29 . When predicting the age pattern of the excess risk of smoking on CVD outcomes using the fitted model, we did not include between-study heterogeneity to reduce uncertainty in the prediction.
In the fourth step, we calculated the age attenuation factors of excess risk compared with the reference age group for each CVD outcome as the ratio of the estimated excess risk for each age group to the excess risk for the reference age group. We performed the calculation at the draw level to obtain 1,000 draws of the age attenuation factors for each age group. Once we had estimated the age attenuation factors, we carried out the last step, which consisted of adjusting the risk curve for the reference age group from step 1 using equation (1) to produce the age group-specific risk curves for each CVD outcome:
We implemented the age adjustment at the draw level so that the uncertainty of the age attenuation factors could be naturally incorporated into the final adjusted age-specific RR curves. A PRISMA diagram detailing the systematic review approach, a description of the studies included and the full details about the methods are in Supplementary Information 1.5 and 5.2 .
Estimating the theoretical minimum risk exposure level
The theoretical minimum risk exposure level for smoking was 0, that is, no individuals in the population are current or former smokers.
Model validation
The validity of the meta-analytic tool has been extensively evaluated by Zheng and colleagues using simulation experiments 28 , 29 . For the present study, we conducted two additional sensitivity analyses to examine how the shape of the risk curves was impacted by applying a monotonicity constraint and trimming 10% of data. We present the results of these sensitivity analyses in Supplementary Information 6 . In addition to the sensitivity analyses, the dose–response risk estimates were also validated by plotting the mean risk function along with its 95% UI against both the extracted dose-specific RR data from the studies included and our previous dose–response risk estimates from the GBD 2019 (ref. 30 ). The mean risk functions along with the 95% UIs were validated based on data fit and the level, shape and plausibility of the dose–response risk curves. All curves were validated by all authors and reviewed by an external expert panel, comprising professors with relevant experience from universities including Johns Hopkins University, Karolinska Institute and University of Barcelona; senior scientists working in relevant departments at the WHO and the Center for Disease Control and Prevention (CDC) and directors of nongovernmental organizations such as the Campaign for Tobacco-Free Kids.
Statistical analysis
Analyses were carried out using R v.3.6.3, Python v.3.8 and Stata v.16.
Statistics and reproducibility
The study was a secondary analysis of existing data involving systematic reviews and meta-analyses. No statistical method was used to predetermine sample size. As the study did not involve primary data collection, randomization and blinding, data exclusions were not relevant to the present study, and, as such, no data were excluded and we performed no randomization or blinding. We have made our data and code available to foster reproducibility.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The findings from the present study are supported by data available in the published literature. Data sources and citations for each risk–outcome pair can be downloaded using the ‘download’ button on each risk curve page currently available at https://vizhub.healthdata.org/burden-of-proof . Study characteristics and citations for all input data used in the analyses are also provided in Supplementary Table 3 , and Supplementary Table 2 provides a template of the data collection form.

Code availability
All code used for these analyses is publicly available online ( https://github.com/ihmeuw-msca/burden-of-proof ).
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung. Br. Med. J. 2 , 739–748 (1950).
Article CAS PubMed PubMed Central Google Scholar
Di Cicco, M. E., Ragazzo, V. & Jacinto, T. Mortality in relation to smoking: the British Doctors Study. Breathe 12 , 275–276 (2016).
Article PubMed PubMed Central Google Scholar
World Health Organization. WHO Framework Convention on Tobacco Control 36 (WHO, 2003).
Dai, X., Gakidou, E. & Lopez, A. D. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob. Control 31 , 129–137 (2022).
Article PubMed Google Scholar
Dikshit, R. P. & Kanhere, S. Tobacco habits and risk of lung, oropharyngeal and oral cavity cancer: a population-based case-control study in Bhopal, India. Int. J. Epidemiol. 29 , 609–614 (2000).
Article CAS PubMed Google Scholar
Liaw, K. M. & Chen, C. J. Mortality attributable to cigarette smoking in Taiwan: a 12-year follow-up study. Tob. Control 7 , 141–148 (1998).
Gandini, S. et al. Tobacco smoking and cancer: a meta-analysis. Int. J. Cancer 122 , 155–164 (2008).
Deng, X., Yuan, C. & Chang, D. Interactions between single nucleotide polymorphism of SERPINA1 gene and smoking in association with COPD: a case–control study. Int. J. Chron. Obstruct. Pulmon. Dis. 12 , 259–265 (2017).
Leem, A. Y., Park, B., Kim, Y. S., Jung, J. Y. & Won, S. Incidence and risk of chronic obstructive pulmonary disease in a Korean community-based cohort. Int. J. Chron. Obstruct. Pulmon. Dis. 13 , 509–517 (2018).
Forey, B. A., Thornton, A. J. & Lee, P. N. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulmon. Med. 11 , 36 (2011).
Article Google Scholar
Tan, J. et al. Smoking, blood pressure, and cardiovascular disease mortality in a large cohort of Chinese men with 15 years follow-up. Int. J. Environ. Res. Public Health 15 , E1026 (2018).
Doll, R., Peto, R., Boreham, J. & Sutherland, I. Mortality in relation to smoking: 50 years’ observations on male British doctors. Br. Med. J. 328 , 1519 (2004).
Huxley, R. R. & Woodward, M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet 378 , 1297–1305 (2011).
Hbejan, K. Smoking effect on ischemic heart disease in young patients. Heart Views 12 , 1–6 (2011).
Chao, H. et al. A meta-analysis of active smoking and risk of meningioma. Tob. Induc. Dis. 19 , 34 (2021).
Shi, H., Shao, X. & Hong, Y. Association between cigarette smoking and the susceptibility of acute myeloid leukemia: a systematic review and meta-analysis. Eur. Rev. Med Pharm. Sci. 23 , 10049–10057 (2019).
CAS Google Scholar
Macacu, A., Autier, P., Boniol, M. & Boyle, P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res. Treat. 154 , 213–224 (2015).
Pujades-Rodriguez, M. et al. Heterogeneous associations between smoking and a wide range of initial presentations of cardiovascular disease in 1 937 360 people in England: lifetime risks and implications for risk prediction. Int. J. Epidemiol. 44 , 129–141 (2015).
Kanazir, M. et al. Risk factors for hepatocellular carcinoma: a case-control study in Belgrade (Serbia). Tumori 96 , 911–917 (2010).
Pytynia, K. B. et al. Matched-pair analysis of survival of never smokers and ever smokers with squamous cell carcinoma of the head and neck. J. Clin. Oncol. 22 , 3981–3988 (2004).
Barengo, N. C., Antikainen, R., Harald, K. & Jousilahti, P. Smoking and cancer, cardiovascular and total mortality among older adults: the Finrisk Study. Prev. Med. Rep. 14 , 100875 (2019).
Guo, Y. et al. Modifiable risk factors for cognitive impairment in Parkinson’s disease: a systematic review and meta-analysis of prospective cohort studies. Mov. Disord. 34 , 876–883 (2019).
Aune, D., Vatten, L. J. & Boffetta, P. Tobacco smoking and the risk of gallbladder disease. Eur. J. Epidemiol. 31 , 643–653 (2016).
Qin, L., Deng, H.-Y., Chen, S.-J. & Wei, W. Relationship between cigarette smoking and risk of chronic myeloid leukaemia: a meta-analysis of epidemiological studies. Hematology 22 , 193–200 (2017).
Petrick, J. L. et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Br. J. Cancer 118 , 1005–1012 (2018).
United States Department of Health, Education and Welfare. Smoking and Health. Report of the Advisory Committee on Smoking and Health to the Surgeon General of the United States Public Health Service https://www.cdc.gov/tobacco/data_statistics/sgr/index.htm (US DHEW, 1964).
United States Public Health Service Office of the Surgeon General & National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking Cessation: A Report of the Surgeon General . (US Department of Health and Human Services, 2020).
Zheng, P., Barber, R., Sorensen, R. J. D., Murray, C. J. L. & Aravkin, A. Y. Trimmed constrained mixed effects models: formulations and algorithms. J. Comput. Graph Stat. 30 , 544–556 (2021).
Zheng, P. et al. The Burden of Proof studies: assessing the evidence of risk. Nat. Med. in press (2022).
Reitsma, M. B. et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397 , 2337–2360 (2021).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br. Med. J. 339 , b2535 (2009).
Liu, Z. Y., He, X. Z. & Chapman, R. S. Smoking and other risk factors for lung cancer in Xuanwei, China. Int. J. Epidemiol. 20 , 26–31 (1991).
Brownson, R. C., Reif, J. S., Keefe, T. J., Ferguson, S. W. & Pritzl, J. A. Risk factors for adenocarcinoma of the lung. Am. J. Epidemiol. 125 , 25–34 (1987).
Marugame, T. et al. Lung cancer death rates by smoking status: comparison of the Three-Prefecture Cohort study in Japan to the Cancer Prevention Study II in the USA. Cancer Sci. 96 , 120–126 (2005).
Dosemeci, M., Gokmen, I., Unsal, M., Hayes, R. B. & Blair, A. Tobacco, alcohol use, and risks of laryngeal and lung cancer by subsite and histologic type in Turkey. Cancer Causes Control 8 , 729–737 (1997).
Freedman, N. D. et al. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int. J. Epidemiol. 45 , 846–856 (2016).
Bae, J.-M. et al. Lung cancer incidence by smoking status in Korean men: 16 years of observations in the Seoul Male Cancer Cohort study. J. Korean Med. Sci. 28 , 636–637 (2013).
Everatt, R., Kuzmickienė, I., Virvičiūtė, D. & Tamošiūnas, A. Cigarette smoking, educational level and total and site-specific cancer: a cohort study in men in Lithuania. Eur. J. Cancer Prev. 23 , 579–586 (2014).
Nordlund, L. A., Carstensen, J. M. & Pershagen, G. Are male and female smokers at equal risk of smoking-related cancer: evidence from a Swedish prospective study. Scand. J. Public Health 27 , 56–62 (1999).
Siemiatycki, J., Krewski, D., Franco, E. & Kaiserman, M. Associations between cigarette smoking and each of 21 types of cancer: a multi-site case–control study. Int. J. Epidemiol. 24 , 504–514 (1995).
Chyou, P. H., Nomura, A. M. & Stemmermann, G. N. A prospective study of the attributable risk of cancer due to cigarette smoking. Am. J. Public Health 82 , 37–40 (1992).
Potter, J. D., Sellers, T. A., Folsom, A. R. & McGovern, P. G. Alcohol, beer, and lung cancer in postmenopausal women. The Iowa Women’s Health Study. Ann. Epidemiol. 2 , 587–595 (1992).
Chyou, P. H., Nomura, A. M., Stemmermann, G. N. & Kato, I. Lung cancer: a prospective study of smoking, occupation, and nutrient intake. Arch. Environ. Health 48 , 69–72 (1993).
Pesch, B. et al. Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case–control studies. Int. J. Cancer 131 , 1210–1219 (2012).
Jöckel, K. H. et al. Occupational and environmental hazards associated with lung cancer. Int. J. Epidemiol. 21 , 202–213 (1992).
Jöckel, K. H., Ahrens, W., Jahn, I., Pohlabeln, H. & Bolm-Audorff, U. Occupational risk factors for lung cancer: a case-control study in West Germany. Int. J. Epidemiol. 27 , 549–560 (1998).
Lei, Y. X., Cai, W. C., Chen, Y. Z. & Du, Y. X. Some lifestyle factors in human lung cancer: a case-control study of 792 lung cancer cases. Lung Cancer 14 , S121–S136 (1996).
Pawlega, J., Rachtan, J. & Dyba, T. Evaluation of certain risk factors for lung cancer in Cracow (Poland)—a case–control study. Acta Oncol. 36 , 471–476 (1997).
Mao, Y. et al. Socioeconomic status and lung cancer risk in Canada. Int. J. Epidemiol. 30 , 809–817 (2001).
Barbone, F., Bovenzi, M., Cavallieri, F. & Stanta, G. Cigarette smoking and histologic type of lung cancer in men. Chest 112 , 1474–1479 (1997).
Matos, E., Vilensky, M., Boffetta, P. & Kogevinas, M. Lung cancer and smoking: a case–control study in Buenos Aires, Argentina. Lung Cancer 21 , 155–163 (1998).
Simonato, L. et al. Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int. J. Cancer 91 , 876–887 (2001).
Risch, H. A. et al. Are female smokers at higher risk for lung cancer than male smokers? A case–control analysis by histologic type. Am. J. Epidemiol. 138 , 281–293 (1993).
Sankaranarayanan, R. et al. A case–control study of diet and lung cancer in Kerala, south India. Int. J. Cancer 58 , 644–649 (1994).
Band, P. R. et al. Identification of occupational cancer risks in British Columbia. Part I: methodology, descriptive results, and analysis of cancer risks, by cigarette smoking categories of 15,463 incident cancer cases. J. Occup. Environ. Med. 41 , 224–232 (1999).
Becher, H., Jöckel, K. H., Timm, J., Wichmann, H. E. & Drescher, K. Smoking cessation and nonsmoking intervals: effect of different smoking patterns on lung cancer risk. Cancer Causes Control 2 , 381–387 (1991).
Brockmöller, J., Kerb, R., Drakoulis, N., Nitz, M. & Roots, I. Genotype and phenotype of glutathione S-transferase class mu isoenzymes mu and psi in lung cancer patients and controls. Cancer Res. 53 , 1004–1011 (1993).
PubMed Google Scholar
Vena, J. E., Byers, T. E., Cookfair, D. & Swanson, M. Occupation and lung cancer risk. An analysis by histologic subtypes. Cancer 56 , 910–917 (1985).
Cascorbi, I. et al. Homozygous rapid arylamine N -acetyltransferase (NAT2) genotype as a susceptibility factor for lung cancer. Cancer Res. 56 , 3961–3966 (1996).
CAS PubMed Google Scholar
Chiazze, L., Watkins, D. K. & Fryar, C. A case–control study of malignant and non-malignant respiratory disease among employees of a fiberglass manufacturing facility. Br. J. Ind. Med 49 , 326–331 (1992).
CAS PubMed PubMed Central Google Scholar
Ando, M. et al. Attributable and absolute risk of lung cancer death by smoking status: findings from the Japan Collaborative Cohort Study. Int. J. Cancer 105 , 249–254 (2003).
De Matteis, S. et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am. J. Epidemiol. 177 , 601–612 (2013).
He, Y. et al. Changes in smoking behavior and subsequent mortality risk during a 35-year follow-up of a cohort in Xi’an, China. Am. J. Epidemiol. 179 , 1060–1070 (2014).
Nishino, Y. et al. Cancer incidence profiles in the Miyagi Cohort Study. J. Epidemiol. 14 , S7–S11 (2004).
Papadopoulos, A. et al. Cigarette smoking and lung cancer in women: results of the French ICARE case–control study. Lung Cancer 74 , 369–377 (2011).
Shimazu, T. et al. Alcohol and risk of lung cancer among Japanese men: data from a large-scale population-based cohort study, the JPHC study. Cancer Causes Control 19 , 1095–1102 (2008).
Tindle, H. A. et al. Lifetime smoking history and risk of lung cancer: results from the Framingham Heart Study. J. Natl Cancer Inst. 110 , 1201–1207 (2018).
PubMed PubMed Central Google Scholar
Yong, L. C. et al. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am. J. Epidemiol. 146 , 231–243 (1997).
Hansen, M. S. et al. Sex differences in risk of smoking-associated lung cancer: results from a cohort of 600,000 Norwegians. Am. J. Epidemiol. 187 , 971–981 (2018).
Boffetta, P. et al. Tobacco smoking as a risk factor of bronchioloalveolar carcinoma of the lung: pooled analysis of seven case-control studies in the International Lung Cancer Consortium (ILCCO). Cancer Causes Control 22 , 73–79 (2011).
Yun, Y. D. et al. Hazard ratio of smoking on lung cancer in Korea according to histological type and gender. Lung 194 , 281–289 (2016).
Suzuki, I. et al. Risk factors for lung cancer in Rio de Janeiro, Brazil: a case–control study. Lung Cancer 11 , 179–190 (1994).
De Stefani, E., Deneo-Pellegrini, H., Carzoglio, J. C., Ronco, A. & Mendilaharsu, M. Dietary nitrosodimethylamine and the risk of lung cancer: a case–control study from Uruguay. Cancer Epidemiol. Biomark. Prev. 5 , 679–682 (1996).
Google Scholar
Kreuzer, M. et al. Risk factors for lung cancer in young adults. Am. J. Epidemiol. 147 , 1028–1037 (1998).
Armadans-Gil, L., Vaqué-Rafart, J., Rosselló, J., Olona, M. & Alseda, M. Cigarette smoking and male lung cancer risk with special regard to type of tobacco. Int. J. Epidemiol. 28 , 614–619 (1999).
Kubík, A. K., Zatloukal, P., Tomásek, L. & Petruzelka, L. Lung cancer risk among Czech women: a case–control study. Prev. Med. 34 , 436–444 (2002).
Rachtan, J. Smoking, passive smoking and lung cancer cell types among women in Poland. Lung Cancer 35 , 129–136 (2002).
Thun, M. J. et al. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 368 , 351–364 (2013).
Zatloukal, P., Kubík, A., Pauk, N., Tomásek, L. & Petruzelka, L. Adenocarcinoma of the lung among women: risk associated with smoking, prior lung disease, diet and menstrual and pregnancy history. Lung Cancer 41 , 283–293 (2003).
Hansen, M. S., Licaj, I., Braaten, T., Lund, E. & Gram, I. T. The fraction of lung cancer attributable to smoking in the Norwegian Women and Cancer (NOWAC) Study. Br. J. Cancer 124 , 658–662 (2021).
Zhang, P. et al. Association of smoking and polygenic risk with the incidence of lung cancer: a prospective cohort study. Br. J. Cancer 126 , 1637–1646 (2022).
Weber, M. F. et al. Cancer incidence and cancer death in relation to tobacco smoking in a population-based Australian cohort study. Int. J. Cancer 149 , 1076–1088 (2021).
Guo, L.-W. et al. A risk prediction model for selecting high-risk population for computed tomography lung cancer screening in China. Lung Cancer 163 , 27–34 (2022).
Mezzoiuso, A. G., Odone, A., Signorelli, C. & Russo, A. G. Association between smoking and cancers among women: results from the FRiCaM multisite cohort study. J. Cancer 12 , 3136–3144 (2021).
Hawrysz, I., Wadolowska, L., Slowinska, M. A., Czerwinska, A. & Golota, J. J. Adherence to prudent and mediterranean dietary patterns is inversely associated with lung cancer in moderate but not heavy male Polish smokers: a case–control study. Nutrients 12 , E3788 (2020).
Huang, C.-C., Lai, C.-Y., Tsai, C.-H., Wang, J.-Y. & Wong, R.-H. Combined effects of cigarette smoking, DNA methyltransferase 3B genetic polymorphism, and DNA damage on lung cancer. BMC Cancer 21 , 1066 (2021).
Viner, B., Barberio, A. M., Haig, T. R., Friedenreich, C. M. & Brenner, D. R. The individual and combined effects of alcohol consumption and cigarette smoking on site-specific cancer risk in a prospective cohort of 26,607 adults: results from Alberta’s Tomorrow Project. Cancer Causes Control 30 , 1313–1326 (2019).
Park, E. Y., Lim, M. K., Park, E., Oh, J.-K. & Lee, D.-H. Relationship between urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and lung cancer risk in the general population: a community-based prospective cohort study. Front. Oncol. 11 , 611674 (2021).
De Stefani, E., Deneo-Pellegrini, H., Mendilaharsu, M., Carzoglio, J. C. & Ronco, A. Dietary fat and lung cancer: a case–control study in Uruguay. Cancer Causes Control 8 , 913–921 (1997).
Wünsch-Filho, V., Moncau, J. E., Mirabelli, D. & Boffetta, P. Occupational risk factors of lung cancer in São Paulo, Brazil. Scand. J. Work Environ. Health 24 , 118–124 (1998).
Hu, J. et al. A case-control study of diet and lung cancer in northeast China. Int. J. Cancer 71 , 924–931 (1997).
Jia, G., Wen, W., Massion, P. P., Shu, X.-O. & Zheng, W. Incorporating both genetic and tobacco smoking data to identify high-risk smokers for lung cancer screening. Carcinogenesis 42 , 874–879 (2021).
Rusmaully, J. et al. Risk of lung cancer among women in relation to lifetime history of tobacco smoking: a population-based case–control study in France (the WELCA study). BMC Cancer 21 , 711 (2021).
Jin, K. et al. Tobacco smoking modifies the association between hormonal factors and lung cancer occurrence among post-menopausal Chinese women. Transl. Oncol. 12 , 819–827 (2019).
Tse, L. A., Wang, F., Wong, M. C.-S., Au, J. S.-K. & Yu, I. T.-S. Risk assessment and prediction for lung cancer among Hong Kong Chinese men. BMC Cancer 22 , 585 (2022).
Huang, C.-C. et al. Joint effects of cigarette smoking and green tea consumption with miR-29b and DNMT3b mRNA expression in the development of lung cancer. Genes 13 , 836 (2022).
Hosseini, M. et al. Environmental risk factors for lung cancer in Iran: a case–control study. Int. J. Epidemiol. 38 , 989–996 (2009).
Naghibzadeh-Tahami, A. et al. Is opium use associated with an increased risk of lung cancer? A case–control study. BMC Cancer 20 , 807 (2020).
Shimatani, K., Ito, H., Matsuo, K., Tajima, K. & Takezaki, T. Cumulative cigarette tar exposure and lung cancer risk among Japanese smokers. Jpn J. Clin. Oncol. 50 , 1009–1017 (2020).
Lai, C.-Y. et al. Genetic polymorphism of catechol- O -methyltransferase modulates the association of green tea consumption and lung cancer. Eur. J. Cancer Prev. 28 , 316–322 (2019).
Schwartz, A. G. et al. Hormone use, reproductive history, and risk of lung cancer: the Women’s Health Initiative studies. J. Thorac. Oncol. 10 , 1004–1013 (2015).
Kreuzer, M., Gerken, M., Heinrich, J., Kreienbrock, L. & Wichmann, H.-E. Hormonal factors and risk of lung cancer among women? Int. J. Epidemiol. 32 , 263–271 (2003).
Sreeja, L. et al. Possible risk modification by CYP1A1, GSTM1 and GSTT1 gene polymorphisms in lung cancer susceptibility in a South Indian population. J. Hum. Genet. 50 , 618–627 (2005).
Siemiatycki, J. et al. Are the apparent effects of cigarette smoking on lung and bladder cancers due to uncontrolled confounding by occupational exposures? Epidemiology 5 , 57–65 (1994).
Chan-Yeung, M. et al. Risk factors associated with lung cancer in Hong Kong. Lung Cancer 40 , 131–140 (2003).
Lawania, S., Singh, N., Behera, D. & Sharma, S. Xeroderma pigmentosum complementation group D polymorphism toward lung cancer susceptibility survival and response in patients treated with platinum chemotherapy. Future Oncol. 13 , 2645–2665 (2017).
De Stefani, E. et al. Mate drinking and risk of lung cancer in males: a case-control study from Uruguay. Cancer Epidemiol. Biomark. Prev. 5 , 515–519 (1996).
Pérez-Padilla, R. et al. Exposure to biomass smoke and chronic airway disease in Mexican women. A case-control study. Am. J. Respir. Crit. Care Med. 154 , 701–706 (1996).
Zhang, X.-R. et al. Glucosamine use, smoking and risk of incident chronic obstructive pulmonary disease: a large prospective cohort study. Br. J. Nutr . https://doi.org/10.1017/S000711452100372X (2021).
Johannessen, A., Omenaas, E., Bakke, P. & Gulsvik, A. Incidence of GOLD-defined chronic obstructive pulmonary disease in a general adult population. Int. J. Tuberc. Lung Dis. 9 , 926–932 (2005).
Fox, J. Life-style and mortality: a large-scale census-based cohort study in Japan. J. Epidemiol. Community Health 45 , 173 (1991).
Article PubMed Central Google Scholar
Thomson, B. et al. Low-intensity daily smoking and cause-specific mortality in Mexico: prospective study of 150 000 adults. Int. J. Epidemiol. 50 , 955–964 (2021).
van Durme, Y. M. T. A. et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest 135 , 368–377 (2009).
Li, L. et al. SERPINE2 rs16865421 polymorphism is associated with a lower risk of chronic obstructive pulmonary disease in the Uygur population: a case–control study. J. Gene Med. 21 , e3106 (2019).
Ganbold, C. et al. The cumulative effect of gene-gene interactions between GSTM1 , CHRNA3 , CHRNA5 and SOD3 gene polymorphisms combined with smoking on COPD risk. Int. J. Chron. Obstruct. Pulmon. Dis. 16 , 2857–2868 (2021).
Omori, H. et al. Twelve-year cumulative incidence of airflow obstruction among Japanese males. Intern. Med. 50 , 1537–1544 (2011).
Manson, J. E., Ajani, U. A., Liu, S., Nathan, D. M. & Hennekens, C. H. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am. J. Med. 109 , 538–542 (2000).
Lv, J. et al. Adherence to a healthy lifestyle and the risk of type 2 diabetes in Chinese adults. Int. J. Epidemiol. 46 , 1410–1420 (2017).
Waki, K. et al. Alcohol consumption and other risk factors for self-reported diabetes among middle-aged Japanese: a population-based prospective study in the JPHC study cohort I. Diabet. Med. 22 , 323–331 (2005).
Meisinger, C., Döring, A., Thorand, B. & Löwel, H. Association of cigarette smoking and tar and nicotine intake with development of type 2 diabetes mellitus in men and women from the general population: the MONICA/KORA Augsburg Cohort Study. Diabetologia 49 , 1770–1776 (2006).
Huh, Y. et al. Association of smoking status with the risk of type 2 diabetes among young adults: a nationwide cohort study in South Korea. Nicotine Tob. Res. 24 , 1234–1240 (2022).
Sawada, S. S., Lee, I.-M., Muto, T., Matuszaki, K. & Blair, S. N. Cardiorespiratory fitness and the incidence of type 2 diabetes: prospective study of Japanese men. Diabetes Care 26 , 2918–2922 (2003).
Will, J. C., Galuska, D. A., Ford, E. S., Mokdad, A. & Calle, E. E. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int. J. Epidemiol. 30 , 540–546 (2001).
Nakanishi, N., Nakamura, K., Matsuo, Y., Suzuki, K. & Tatara, K. Cigarette smoking and risk for impaired fasting glucose and type 2 diabetes in middle-aged Japanese men. Ann. Intern. Med. 133 , 183–191 (2000).
Sairenchi, T. et al. Cigarette smoking and risk of type 2 diabetes mellitus among middle-aged and elderly Japanese men and women. Am. J. Epidemiol. 160 , 158–162 (2004).
Hou, X. et al. Cigarette smoking is associated with a lower prevalence of newly diagnosed diabetes screened by OGTT than non-smoking in Chinese men with normal weight. PLoS ONE 11 , e0149234 (2016).
Hu, F. B. et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 345 , 790–797 (2001).
Teratani, T. et al. Dose-response relationship between tobacco or alcohol consumption and the development of diabetes mellitus in Japanese male workers. Drug Alcohol Depend. 125 , 276–282 (2012).
Kawakami, N., Takatsuka, N., Shimizu, H. & Ishibashi, H. Effects of smoking on the incidence of non-insulin-dependent diabetes mellitus. Replication and extension in a Japanese cohort of male employees. Am. J. Epidemiol. 145 , 103–109 (1997).
Patja, K. et al. Effects of smoking, obesity and physical activity on the risk of type 2 diabetes in middle-aged Finnish men and women. J. Intern. Med. 258 , 356–362 (2005).
White, W. B. et al. High-intensity cigarette smoking is associated with incident diabetes mellitus in Black adults: the Jackson Heart Study. J. Am. Heart Assoc. 7 , e007413 (2018).
Uchimoto, S. et al. Impact of cigarette smoking on the incidence of Type 2 diabetes mellitus in middle-aged Japanese men: the Osaka Health Survey. Diabet. Med . 16 , 951–955 (1999).
Rimm, E. B., Chan, J., Stampfer, M. J., Colditz, G. A. & Willett, W. C. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. Br. Med. J. 310 , 555–559 (1995).
Article CAS Google Scholar
Hilawe, E. H. et al. Smoking and diabetes: is the association mediated by adiponectin, leptin, or C-reactive protein? J. Epidemiol. 25 , 99–109 (2015).
InterAct, Consortium et al. Smoking and long-term risk of type 2 diabetes: the EPIC-InterAct study in European populations. Diabetes Care 37 , 3164–3171 (2014).
Jee, S. H., Foong, A. W., Hur, N. W. & Samet, J. M. Smoking and risk for diabetes incidence and mortality in Korean men and women. Diabetes Care 33 , 2567–2572 (2010).
Rasouli, B. et al. Smoking and the risk of LADA: results from a Swedish population-based case-control study. Diabetes Care 39 , 794–800 (2016).
Wannamethee, S. G., Shaper, A. G. & Perry, I. J., British Regional Heart Study. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care 24 , 1590–1595 (2001).
Radzeviciene, L. & Ostrauskas, R. Smoking habits and type 2 diabetes mellitus in women. Women Health 58 , 884–897 (2018).
Carlsson, S., Midthjell, K. & Grill, V., Nord-Trøndelag Study. Smoking is associated with an increased risk of type 2 diabetes but a decreased risk of autoimmune diabetes in adults: an 11-year follow-up of incidence of diabetes in the Nord-Trøndelag study. Diabetologia 47 , 1953–1956 (2004).
Akter, S. et al. Smoking, smoking cessation, and the risk of type 2 diabetes among Japanese adults: Japan Epidemiology Collaboration on Occupational Health Study. PLoS ONE 10 , e0132166 (2015).
Pirie, K. et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 381 , 133–141 (2013).
Park, C.-H. et al. [The effect of smoking status upon occurrence of impaired fasting glucose or type 2 diabetes in Korean men]. J. Prev. Med. Public Health 41 , 249–254 (2008).
Doi, Y. et al. Two risk score models for predicting incident Type 2 diabetes in Japan. Diabet. Med. 29 , 107–114 (2012).
van den Brandt, P. A. A possible dual effect of cigarette smoking on the risk of postmenopausal breast cancer. Eur. J. Epidemiol. 32 , 683–690 (2017).
Dossus, L. et al. Active and passive cigarette smoking and breast cancer risk: results from the EPIC cohort. Int. J. Cancer 134 , 1871–1888 (2014).
Kawai, M., Malone, K. E., Tang, M.-T. C. & Li, C. I. Active smoking and the risk of estrogen receptor-positive and triple-negative breast cancer among women ages 20 to 44 years. Cancer 120 , 1026–1034 (2014).
Reynolds, P. et al. Active smoking, household passive smoking, and breast cancer: evidence from the California Teachers Study. J. Natl Cancer Inst. 96 , 29–37 (2004).
Ellingjord-Dale, M. et al. Alcohol, physical activity, smoking, and breast cancer subtypes in a large, nested case-control study from the Norwegian Breast Cancer Screening Program. Cancer Epidemiol. Biomark. Prev. 26 , 1736–1744 (2017).
Arthur, R. et al. Association between lifestyle, menstrual/reproductive history, and histological factors and risk of breast cancer in women biopsied for benign breast disease. Breast Cancer Res. Treat. 165 , 623–631 (2017).
Luo, J. et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. Br. Med. J. 342 , d1016 (2011).
White, A. J., D’Aloisio, A. A., Nichols, H. B., DeRoo, L. A. & Sandler, D. P. Breast cancer and exposure to tobacco smoke during potential windows of susceptibility. Cancer Causes Control 28 , 667–675 (2017).
Gram, I. T. et al. Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol. Biomark. Prev. 14 , 61–66 (2005).
Gammon, M. D. et al. Cigarette smoking and breast cancer risk among young women (United States). Cancer Causes Control 9 , 583–590 (1998).
Magnusson, C., Wedrén, S. & Rosenberg, L. U. Cigarette smoking and breast cancer risk: a population-based study in Sweden. Br. J. Cancer 97 , 1287–1290 (2007).
Chu, S. Y. et al. Cigarette smoking and the risk of breast cancer. Am. J. Epidemiol. 131 , 244–253 (1990).
Lemogne, C. et al. Depression and the risk of cancer: a 15-year follow-up study of the GAZEL cohort. Am. J. Epidemiol. 178 , 1712–1720 (2013).
Morabia, A., Bernstein, M., Héritier, S. & Khatchatrian, N. Relation of breast cancer with passive and active exposure to tobacco smoke. Am. J. Epidemiol. 143 , 918–928 (1996).
Conlon, M. S. C., Johnson, K. C., Bewick, M. A., Lafrenie, R. M. & Donner, A. Smoking (active and passive), N -acetyltransferase 2, and risk of breast cancer. Cancer Epidemiol. 34 , 142–149 (2010).
Ozasa, K., Japan Collaborative Cohort Study for Evaluation of Cancer. Smoking and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac. J. Cancer Prev. 8 , 89–96 (2007).
Jones, M. E., Schoemaker, M. J., Wright, L. B., Ashworth, A. & Swerdlow, A. J. Smoking and risk of breast cancer in the Generations Study cohort. Breast Cancer Res. 19 , 118 (2017).
Bjerkaas, E. et al. Smoking duration before first childbirth: an emerging risk factor for breast cancer? Results from 302,865 Norwegian women. Cancer Causes Control 24 , 1347–1356 (2013).
Gram, I. T., Little, M. A., Lund, E. & Braaten, T. The fraction of breast cancer attributable to smoking: the Norwegian women and cancer study 1991–2012. Br. J. Cancer 115 , 616–623 (2016).
Li, C. I., Malone, K. E. & Daling, J. R. The relationship between various measures of cigarette smoking and risk of breast cancer among older women 65–79 years of age (United States). Cancer Causes Control 16 , 975–985 (2005).
Xue, F., Willett, W. C., Rosner, B. A., Hankinson, S. E. & Michels, K. B. Cigarette smoking and the incidence of breast cancer. Arch. Intern. Med. 171 , 125–133 (2011).
Parker, A. S., Cerhan, J. R., Putnam, S. D., Cantor, K. P. & Lynch, C. F. A cohort study of farming and risk of prostate cancer in Iowa. Epidemiology 10 , 452–455 (1999).
Sawada, N. et al. Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: the Japan Public Health Center-based prospective study. Int. J. Cancer 134 , 971–978 (2014).
Hiatt, R. A., Armstrong, M. A., Klatsky, A. L. & Sidney, S. Alcohol consumption, smoking, and other risk factors and prostate cancer in a large health plan cohort in California (United States). Cancer Causes Control 5 , 66–72 (1994).
Cerhan, J. R. et al. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control 8 , 229–238 (1997).
Watters, J. L., Park, Y., Hollenbeck, A., Schatzkin, A. & Albanes, D. Cigarette smoking and prostate cancer in a prospective US cohort study. Cancer Epidemiol. Biomark. Prev. 18 , 2427–2435 (2009).
Butler, L. M., Wang, R., Wong, A. S., Koh, W.-P. & Yu, M. C. Cigarette smoking and risk of prostate cancer among Singapore Chinese. Cancer Causes Control 20 , 1967–1974 (2009).
Lotufo, P. A., Lee, I. M., Ajani, U. A., Hennekens, C. H. & Manson, J. E. Cigarette smoking and risk of prostate cancer in the physicians’ health study (United States). Int. J. Cancer 87 , 141–144 (2000).
Hsing, A. W. et al. Diet, tobacco use, and fatal prostate cancer: results from the Lutheran Brotherhood Cohort Study. Cancer Res. 50 , 6836–6840 (1990).
Veierød, M. B., Laake, P. & Thelle, D. S. Dietary fat intake and risk of prostate cancer: a prospective study of 25,708 Norwegian men. Int. J. Cancer 73 , 634–638 (1997).
Meyer, J., Rohrmann, S., Bopp, M. & Faeh, D. & Swiss National Cohort Study Group. Impact of smoking and excess body weight on overall and site-specific cancer mortality risk. Cancer Epidemiol. Biomark. Prev . 24 , 1516–1522 (2015).
Putnam, S. D. et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann. Epidemiol. 10 , 361–369 (2000).
Taghizadeh, N., Vonk, J. M. & Boezen, H. M. Lifetime smoking history and cause-specific mortality in a cohort study with 43 years of follow-up. PLoS ONE 11 , e0153310 (2016).
Park, S.-Y. et al. Racial/ethnic differences in lifestyle-related factors and prostate cancer risk: the Multiethnic Cohort Study. Cancer Causes Control 26 , 1507–1515 (2015).
Nomura, A. M., Lee, J., Stemmermann, G. N. & Combs, G. F. Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 9 , 883–887 (2000).
Rodriguez, C., Tatham, L. M., Thun, M. J., Calle, E. E. & Heath, C. W. Smoking and fatal prostate cancer in a large cohort of adult men. Am. J. Epidemiol. 145 , 466–475 (1997).
Rohrmann, S. et al. Smoking and risk of fatal prostate cancer in a prospective U.S. study. Urology 69 , 721–725 (2007).
Giovannucci, E. et al. Smoking and risk of total and fatal prostate cancer in United States health professionals. Cancer Epidemiol. Biomark. Prev. 8 , 277–282 (1999).
Rohrmann, S. et al. Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 108 , 708–714 (2013).
Lund Nilsen, T. I., Johnsen, R. & Vatten, L. J. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br. J. Cancer 82 , 1358–1363 (2000).
Hsing, A. W., McLaughlin, J. K., Hrubec, Z., Blot, W. J. & Fraumeni, J. F. Tobacco use and prostate cancer: 26-year follow-up of US veterans. Am. J. Epidemiol. 133 , 437–441 (1991).
Murray, C. J. L. et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1223–1249 (2020).
Bero, L. A. & Jadad, A. R. How consumers and policymakers can use systematic reviews for decision making. Ann. Intern. Med. 127 , 37–42 (1997).
Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb. Mortal. Wkly Rep. 58 , 1227–1232 (2009).
Prochaska, J. O. & Goldstein, M. G. Process of smoking cessation: implications for clinicians. Clin. Chest Med. 12 , 727–735 (1991).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br. Med. J. 372 , n71 (2021).
Stevens, G. A. et al. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet 388 , e19–e23 (2016).
BMJ Best Practice. What is GRADE? https://bestpractice.bmj.com/info/us/toolkit/learn-ebm/what-is-grade (BMJ, 2021).
The GRADE Working Group. GRADE handbook . https://gdt.gradepro.org/app/handbook/handbook.html (The GRADE Working Group, 2013).
Efron, B., Hastie, T., Johnstone, I. & Tibshirani, R. Least angle regression. Ann. Stat. 32 , 407–499 (2004).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 58 , 267–288 (1996).
von Hippel, P. T. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 15 , 35 (2015).
Kontopantelis, E., Springate, D. A. & Reeves, D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS ONE 8 , e69930 (2013).
Biggerstaff, B. J. & Tweedie, R. L. Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat. Med. 16 , 753–768 (1997).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315 , 629–634 (1997).
Lee, P. N., Forey, B. A. & Coombs, K. J. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 12 , 385 (2012).
Rücker, G., Carpenter, J. R. & Schwarzer, G. Detecting and adjusting for small-study effects in meta-analysis. Biometr. J. 53 , 351–368 (2011).
Wu, Z.-J., Zhao, P., Liu, B. & Yuan, Z.-C. Effect of cigarette smoking on risk of hip fracture in men: a meta-analysis of 14 prospective cohort studies. PLoS ONE 11 , e0168990 (2016).
Thun, M. J. et al. in Cigarette Smoking Behaviour in the United States: changes in cigarette-related disease risks and their implication for prevention and control (eds Burns, D.M. et al.) Tobacco Control Monograph No. 8 Ch. 4 (National Cancer Institute, 1997).
Tolstrup, J. S. et al. Smoking and risk of coronary heart disease in younger, middle-aged, and older adults. Am. J. Public Health 104 , 96–102 (2014).
Jonas, M. A., Oates, J. A., Ockene, J. K. & Hennekens, C. H. Statement on smoking and cardiovascular disease for health care professionals. American Heart Association. Circulation 86 , 1664–1669 (1992).
Khan, S. S. et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J. Am. Heart Assoc. 10 , e021751 (2021).
Download references
Acknowledgements
Research reported in this publication was supported by the Bill & Melinda Gates Foundation and Bloomberg Philanthropies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The study funders had no role in study design, data collection, data analysis, data interpretation, writing of the final report or the decision to publish.
We thank the Tobacco Metrics Team Advisory Group for their valuable input and review of the work. The members of the Advisory Group are: P. Allebeck, R. Chandora, J. Drope, M. Eriksen, E. Fernández, H. Gouda, R. Kennedy, D. McGoldrick, L. Pan, K. Schotte, E. Sebrie, J. Soriano, M. Tynan and K. Welding.
Author information
Authors and affiliations.
Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA
Xiaochen Dai, Gabriela F. Gil, Marissa B. Reitsma, Noah S. Ahmad, Jason A. Anderson, Catherine Bisignano, Sinclair Carr, Rachel Feldman, Simon I. Hay, Jiawei He, Vincent Iannucci, Hilary R. Lawlor, Matthew J. Malloy, Laurie B. Marczak, Susan A. McLaughlin, Larissa Morikawa, Erin C. Mullany, Sneha I. Nicholson, Erin M. O’Connell, Chukwuma Okereke, Reed J. D. Sorensen, Joanna Whisnant, Aleksandr Y. Aravkin, Peng Zheng, Christopher J. L. Murray & Emmanuela Gakidou
Department of Health Metrics Sciences, School of Medicine, University of Washington, Seattle, WA, USA
Xiaochen Dai, Simon I. Hay, Jiawei He, Peng Zheng, Christopher J. L. Murray & Emmanuela Gakidou
Department of Applied Mathematics, University of Washington, Seattle, WA, USA
- Aleksandr Y. Aravkin
You can also search for this author in PubMed Google Scholar
Contributions
X.D., S.I.H., S.A.M., E.C.M., E.M.O., C.J.L.M. and E.G. managed the estimation or publications process. X.D. and G.F.G. wrote the first draft of the manuscript. X.D. and P.Z. had primary responsibility for applying analytical methods to produce estimates. X.D., G.F.G., N.S.A., J.A.A., S.C., R.F., V.I., M.J.M., L.M., S.I.N., C.O., M.B.R. and J.W. had primary responsibility for seeking, cataloguing, extracting or cleaning data, and for designing or coding figures and tables. X.D., G.F.G., M.B.R., N.S.A., H.R.L., C.O. and J.W. provided data or critical feedback on data sources. X.D., J.H., R.J.D.S., A.Y.A., P.Z., C.J.L.M. and E.G. developed methods or computational machinery. X.D., G.F.G., M.B.R., S.I.H., J.H., R.J.D.S., A.Y.A., P.Z., C.J.L.M. and E.G. provided critical feedback on methods or results. X.D., G.F.G., M.B.R., C.B., S.I.H., L.B.M., S.A.M., A.Y.A. and E.G. drafted the work or revised it critically for important intellectual content. X.D., S.I.H., L.B.M., E.C.M., E.M.O. and E.G. managed the overall research enterprise.
Corresponding author
Correspondence to Xiaochen Dai .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Medicine thanks Frederic Sitas and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jennifer Sargent and Ming Yang, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 prisma 2020 flow diagram for an updated systematic review of the smoking and tracheal, bronchus, and lung cancer risk-outcome pair..
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and lung cancer conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 2 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Chronic obstructive pulmonary disease risk-outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and chronic obstructive pulmonary disease conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 3 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Diabetes mellitus type 2 risk- outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and type 2 diabetes conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 4 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Breast cancer risk-outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and breast cancer conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 5 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Prostate cancer risk-outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and prostate cancer conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 6 Smoking and Breast Cancer.
a , log-relative risk function. b , relative risk function. c , A modified funnel plot showing the residuals (relative to 0) on the x-axis and the estimated standard deviation (SD) that includes reported SD and between-study heterogeneity on the y-axis.
Supplementary information
Supplementary information.
Supplementary Information 1: Data source identification and assessment. Supplementary Information 2: Data inputs. Supplementary Information 3: Study quality and bias assessment. Supplementary Information 4: The dose–response RR curves and their 95% UIs for all smoking–outcome pairs. Supplementary Information 5: Supplementary methods. Supplementary Information 6: Sensitivity analysis. Supplementary Information 7: Binary smoking–outcome pair. Supplementary Information 8: Risk curve details. Supplementary Information 9: GATHER and PRISMA checklists.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Dai, X., Gil, G.F., Reitsma, M.B. et al. Health effects associated with smoking: a Burden of Proof study. Nat Med 28 , 2045–2055 (2022). https://doi.org/10.1038/s41591-022-01978-x
Download citation
Received : 11 April 2022
Accepted : 28 July 2022
Published : 10 October 2022
Issue Date : October 2022
DOI : https://doi.org/10.1038/s41591-022-01978-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Gabriela F. Gil
- Jason A. Anderson
- Emmanuela Gakidou
Nature Communications (2024)
- Luisa S. Flor
Nature Medicine (2024)
Metabolic profiling of smoking, associations with type 2 diabetes and interaction with genetic susceptibility
- Sofia Carlsson
European Journal of Epidemiology (2024)
Global burden of prostate cancer attributable to smoking among males in 204 countries and territories, 1990–2019
- Hanfei Zhang
- Dingping Huang
- Daqing Hong
BMC Cancer (2023)
Reply to: Concerns about the Burden of Proof studies
- Susan A. McLaughlin
- Christopher J. L. Murray
Nature Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Health Effects of Cigarette Smoking
Smoking and death, smoking and increased health risks, smoking and cardiovascular disease, smoking and respiratory disease, smoking and cancer, smoking and other health risks, quitting and reduced risks.
Cigarette smoking harms nearly every organ of the body, causes many diseases, and reduces the health of smokers in general. 1,2
Quitting smoking lowers your risk for smoking-related diseases and can add years to your life. 1,2
Cigarette smoking is the leading cause of preventable death in the United States. 1
- Cigarette smoking causes more than 480,000 deaths each year in the United States. This is nearly one in five deaths. 1,2,3
- Human immunodeficiency virus (HIV)
- Illegal drug use
- Alcohol use
- Motor vehicle injuries
- Firearm-related incidents
- More than 10 times as many U.S. citizens have died prematurely from cigarette smoking than have died in all the wars fought by the United States. 1
- Smoking causes about 90% (or 9 out of 10) of all lung cancer deaths. 1,2 More women die from lung cancer each year than from breast cancer. 5
- Smoking causes about 80% (or 8 out of 10) of all deaths from chronic obstructive pulmonary disease (COPD). 1
- Cigarette smoking increases risk for death from all causes in men and women. 1
- The risk of dying from cigarette smoking has increased over the last 50 years in the U.S. 1
Smokers are more likely than nonsmokers to develop heart disease, stroke, and lung cancer. 1
- For coronary heart disease by 2 to 4 times 1,6
- For stroke by 2 to 4 times 1
- Of men developing lung cancer by 25 times 1
- Of women developing lung cancer by 25.7 times 1
- Smoking causes diminished overall health, increased absenteeism from work, and increased health care utilization and cost. 1
Smokers are at greater risk for diseases that affect the heart and blood vessels (cardiovascular disease). 1,2
- Smoking causes stroke and coronary heart disease, which are among the leading causes of death in the United States. 1,3
- Even people who smoke fewer than five cigarettes a day can have early signs of cardiovascular disease. 1
- Smoking damages blood vessels and can make them thicken and grow narrower. This makes your heart beat faster and your blood pressure go up. Clots can also form. 1,2
- A clot blocks the blood flow to part of your brain;
- A blood vessel in or around your brain bursts. 1,2
- Blockages caused by smoking can also reduce blood flow to your legs and skin. 1,2
Smoking can cause lung disease by damaging your airways and the small air sacs (alveoli) found in your lungs. 1,2
- Lung diseases caused by smoking include COPD, which includes emphysema and chronic bronchitis. 1,2
- Cigarette smoking causes most cases of lung cancer. 1,2
- If you have asthma, tobacco smoke can trigger an attack or make an attack worse. 1,2
- Smokers are 12 to 13 times more likely to die from COPD than nonsmokers. 1
Smoking can cause cancer almost anywhere in your body: 1,2
- Blood (acute myeloid leukemia)
- Colon and rectum (colorectal)
- Kidney and ureter
- Oropharynx (includes parts of the throat, tongue, soft palate, and the tonsils)
- Trachea, bronchus, and lung
Smoking also increases the risk of dying from cancer and other diseases in cancer patients and survivors. 1
If nobody smoked, one of every three cancer deaths in the United States would not happen. 1,2
Smoking harms nearly every organ of the body and affects a person’s overall health. 1,2
- Preterm (early) delivery
- Stillbirth (death of the baby before birth)
- Low birth weight
- Sudden infant death syndrome (known as SIDS or crib death)
- Ectopic pregnancy
- Orofacial clefts in infants
- Smoking can also affect men’s sperm, which can reduce fertility and also increase risks for birth defects and miscarriage. 2
- Women past childbearing years who smoke have weaker bones than women who never smoked. They are also at greater risk for broken bones.
- Smoking affects the health of your teeth and gums and can cause tooth loss. 1
- Smoking can increase your risk for cataracts (clouding of the eye’s lens that makes it hard for you to see). It can also cause age-related macular degeneration (AMD). AMD is damage to a small spot near the center of the retina, the part of the eye needed for central vision. 1
- Smoking is a cause of type 2 diabetes mellitus and can make it harder to control. The risk of developing diabetes is 30–40% higher for active smokers than nonsmokers. 1,2
- Smoking causes general adverse effects on the body, including inflammation and decreased immune function. 1
- Smoking is a cause of rheumatoid arthritis. 1
- Quitting smoking is one of the most important actions people can take to improve their health. This is true regardless of their age or how long they have been smoking. Visit the Benefits of Quitting page for more information about how quitting smoking can improve your health.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General . Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014 [accessed 2017 Apr 20].
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: What It Means to You . Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010 [accessed 2017 Apr 20].
- Centers for Disease Control and Prevention. QuickStats: Number of Deaths from 10 Leading Causes—National Vital Statistics System, United States, 2010 . Morbidity and Mortality Weekly Report 2013:62(08);155. [accessed 2017 Apr 20].
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual Causes of Death in the United States . JAMA: Journal of the American Medical Association 2004;291(10):1238–45 [cited 2017 Apr 20].
- U.S. Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General . Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General, 2001 [accessed 2017 Apr 20].
- U.S. Department of Health and Human Services. Reducing the Health Consequences of Smoking: 25 Years of Progress. A Report of the Surgeon General . Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 1989 [accessed 2017 Apr 20].
To receive email updates about Smoking & Tobacco Use, enter your email address:
- Tips From Former Smokers ®
- Division of Cancer Prevention and Control
- Lung Cancer
- National Comprehensive Cancer Control Program
- Division of Reproductive Health

Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2012.

Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General.
1 introduction, summary, and conclusions.
- Introduction
Tobacco use is a global epidemic among young people. As with adults, it poses a serious health threat to youth and young adults in the United States and has significant implications for this nation’s public and economic health in the future ( Perry et al. 1994 ; Kessler 1995 ). The impact of cigarette smoking and other tobacco use on chronic disease, which accounts for 75% of American spending on health care ( Anderson 2010 ), is well-documented and undeniable. Although progress has been made since the first Surgeon General’s report on smoking and health in 1964 ( U.S. Department of Health, Education, and Welfare [USDHEW] 1964 ), nearly one in four high school seniors is a current smoker. Most young smokers become adult smokers. One-half of adult smokers die prematurely from tobacco-related diseases ( Fagerström 2002 ; Doll et al. 2004 ). Despite thousands of programs to reduce youth smoking and hundreds of thousands of media stories on the dangers of tobacco use, generation after generation continues to use these deadly products, and family after family continues to suffer the devastating consequences. Yet a robust science base exists on social, biological, and environmental factors that influence young people to use tobacco, the physiology of progression from experimentation to addiction, other health effects of tobacco use, the epidemiology of youth and young adult tobacco use, and evidence-based interventions that have proven effective at reducing both initiation and prevalence of tobacco use among young people. Those are precisely the issues examined in this report, which aims to support the application of this robust science base.
Nearly all tobacco use begins in childhood and adolescence ( U.S. Department of Health and Human Services [USDHHS] 1994 ). In all, 88% of adult smokers who smoke daily report that they started smoking by the age of 18 years (see Chapter 3 , “The Epidemiology of Tobacco Use Among Young People in the United States and Worldwide”). This is a time in life of great vulnerability to social influences ( Steinberg 2004 ), such as those offered through the marketing of tobacco products and the modeling of smoking by attractive role models, as in movies ( Dalton et al. 2009 ), which have especially strong effects on the young. This is also a time in life of heightened sensitivity to normative influences: as tobacco use is less tolerated in public areas and there are fewer social or regular users of tobacco, use decreases among youth ( Alesci et al. 2003 ). And so, as we adults quit, we help protect our children.
Cigarettes are the only legal consumer products in the world that cause one-half of their long-term users to die prematurely ( Fagerström 2002 ; Doll et al. 2004 ). As this epidemic continues to take its toll in the United States, it is also increasing in low- and middle-income countries that are least able to afford the resulting health and economic consequences ( Peto and Lopez 2001 ; Reddy et al. 2006 ). It is past time to end this epidemic. To do so, primary prevention is required, for which our focus must be on youth and young adults. As noted in this report, we now have a set of proven tools and policies that can drastically lower youth initiation and use of tobacco products. Fully committing to using these tools and executing these policies consistently and aggressively is the most straight forward and effective to making future generations tobacco-free.
The 1994 Surgeon General’s Report
This Surgeon General’s report on tobacco is the second to focus solely on young people since these reports began in 1964. Its main purpose is to update the science of smoking among youth since the first comprehensive Surgeon General’s report on tobacco use by youth, Preventing Tobacco Use Among Young People , was published in 1994 ( USDHHS 1994 ). That report concluded that if young people can remain free of tobacco until 18 years of age, most will never start to smoke. The report documented the addiction process for young people and how the symptoms of addiction in youth are similar to those in adults. Tobacco was also presented as a gateway drug among young people, because its use generally precedes and increases the risk of using illicit drugs. Cigarette advertising and promotional activities were seen as a potent way to increase the risk of cigarette smoking among young people, while community-wide efforts were shown to have been successful in reducing tobacco use among youth. All of these conclusions remain important, relevant, and accurate, as documented in the current report, but there has been considerable research since 1994 that greatly expands our knowledge about tobacco use among youth, its prevention, and the dynamics of cessation among young people. Thus, there is a compelling need for the current report.
Tobacco Control Developments
Since 1994, multiple legal and scientific developments have altered the tobacco control environment and thus have affected smoking among youth. The states and the U.S. Department of Justice brought lawsuits against cigarette companies, with the result that many internal documents of the tobacco industry have been made public and have been analyzed and introduced into the science of tobacco control. Also, the 1998 Master Settlement Agreement with the tobacco companies resulted in the elimination of billboard and transit advertising as well as print advertising that directly targeted underage youth and limitations on the use of brand sponsorships ( National Association of Attorneys General [NAAG] 1998 ). This settlement also created the American Legacy Foundation, which implemented a nationwide antismoking campaign targeting youth. In 2009, the U.S. Congress passed a law that gave the U.S. Food and Drug Administration authority to regulate tobacco products in order to promote the public’s health ( Family Smoking Prevention and Tobacco Control Act 2009 ). Certain tobacco companies are now subject to regulations limiting their ability to market to young people. In addition, they have had to reimburse state governments (through agreements made with some states and the Master Settlement Agreement) for some health care costs. Due in part to these changes, there was a decrease in tobacco use among adults and among youth following the Master Settlement Agreement, which is documented in this current report.
Recent Surgeon General Reports Addressing Youth Issues
Other reports of the Surgeon General since 1994 have also included major conclusions that relate to tobacco use among youth ( Office of the Surgeon General 2010 ). In 1998, the report focused on tobacco use among U.S. racial/ethnic minority groups ( USDHHS 1998 ) and noted that cigarette smoking among Black and Hispanic youth increased in the 1990s following declines among all racial/ethnic groups in the 1980s; this was particularly notable among Black youth, and culturally appropriate interventions were suggested. In 2000, the report focused on reducing tobacco use ( USDHHS 2000b ). A major conclusion of that report was that school-based interventions, when implemented with community- and media-based activities, could reduce or postpone the onset of smoking among adolescents by 20–40%. That report also noted that effective regulation of tobacco advertising and promotional activities directed at young people would very likely reduce the prevalence and onset of smoking. In 2001, the Surgeon General’s report focused on women and smoking ( USDHHS 2001 ). Besides reinforcing much of what was discussed in earlier reports, this report documented that girls were more affected than boys by the desire to smoke for the purpose of weight control. Given the ongoing obesity epidemic ( Bonnie et al. 2007 ), the current report includes a more extensive review of research in this area.
The 2004 Surgeon General’s report on the health consequences of smoking ( USDHHS 2004 ) concluded that there is sufficient evidence to infer that a causal relationship exists between active smoking and (a) impaired lung growth during childhood and adolescence; (b) early onset of decline in lung function during late adolescence and early adulthood; (c) respiratory signs and symptoms in children and adolescents, including coughing, phlegm, wheezing, and dyspnea; and (d) asthma-related symptoms (e.g., wheezing) in childhood and adolescence. The 2004 Surgeon General’s report further provided evidence that cigarette smoking in young people is associated with the development of atherosclerosis.
The 2010 Surgeon General’s report on the biology of tobacco focused on the understanding of biological and behavioral mechanisms that might underlie the pathogenicity of tobacco smoke ( USDHHS 2010 ). Although there are no specific conclusions in that report regarding adolescent addiction, it does describe evidence indicating that adolescents can become dependent at even low levels of consumption. Two studies ( Adriani et al. 2003 ; Schochet et al. 2005 ) referenced in that report suggest that because the adolescent brain is still developing, it may be more susceptible and receptive to nicotine than the adult brain.
Scientific Reviews
Since 1994, several scientific reviews related to one or more aspects of tobacco use among youth have been undertaken that also serve as a foundation for the current report. The Institute of Medicine (IOM) ( Lynch and Bonnie 1994 ) released Growing Up Tobacco Free: Preventing Nicotine Addiction in Children and Youths, a report that provided policy recommendations based on research to that date. In 1998, IOM provided a white paper, Taking Action to Reduce Tobacco Use, on strategies to reduce the increasing prevalence (at that time) of smoking among young people and adults. More recently, IOM ( Bonnie et al. 2007 ) released a comprehensive report entitled Ending the Tobacco Problem: A Blueprint for the Nation . Although that report covered multiple potential approaches to tobacco control, not just those focused on youth, it characterized the overarching goal of reducing smoking as involving three distinct steps: “reducing the rate of initiation of smoking among youth (IOM [ Lynch and Bonnie] 1994 ), reducing involuntary tobacco smoke exposure ( National Research Council 1986 ), and helping people quit smoking” (p. 3). Thus, reducing onset was seen as one of the primary goals of tobacco control.
As part of USDHHS continuing efforts to assess the health of the nation, prevent disease, and promote health, the department released, in 2000, Healthy People 2010 and, in 2010, Healthy People 2020 ( USDHHS 2000a , 2011 ). Healthy People provides science-based, 10-year national objectives for improving the health of all Americans. For 3 decades, Healthy People has established benchmarks and monitored progress over time in order to encourage collaborations across sectors, guide individuals toward making informed health decisions, and measure the impact of prevention activities. Each iteration of Healthy People serves as the nation’s disease prevention and health promotion roadmap for the decade. Both Healthy People 2010 and Healthy People 2020 highlight “Tobacco Use” as one of the nation’s “Leading Health Indicators,” feature “Tobacco Use” as one of its topic areas, and identify specific measurable tobacco-related objectives and targets for the nation to strive for. Healthy People 2010 and Healthy People 2020 provide tobacco objectives based on the most current science and detailed population-based data to drive action, assess tobacco use among young people, and identify racial and ethnic disparities. Additionally, many of the Healthy People 2010 and 2020 tobacco objectives address reductions of tobacco use among youth and target decreases in tobacco advertising in venues most often influencing young people. A complete list of the healthy people 2020 objectives can be found on their Web site ( USDHHS 2011 ).
In addition, the National Cancer Institute (NCI) of the National Institutes of Health has published monographs pertinent to the topic of tobacco use among youth. In 2001, NCI published Monograph 14, Changing Adolescent Smoking Prevalence , which reviewed data on smoking among youth in the 1990s, highlighted important statewide intervention programs, presented data on the influence of marketing by the tobacco industry and the pricing of cigarettes, and examined differences in smoking by racial/ethnic subgroup ( NCI 2001 ). In 2008, NCI published Monograph 19, The Role of the Media in Promoting and Reducing Tobacco Use ( NCI 2008 ). Although young people were not the sole focus of this Monograph, the causal relationship between tobacco advertising and promotion and increased tobacco use, the impact on youth of depictions of smoking in movies, and the success of media campaigns in reducing youth tobacco use were highlighted as major conclusions of the report.
The Community Preventive Services Task Force (2011) provides evidence-based recommendations about community preventive services, programs, and policies on a range of topics including tobacco use prevention and cessation ( Task Force on Community Preventive Services 2001 , 2005 ). Evidence reviews addressing interventions to reduce tobacco use initiation and restricting minors’ access to tobacco products were cited and used to inform the reviews in the current report. The Cochrane Collaboration (2010) has also substantially contributed to the review literature on youth and tobacco use by producing relevant systematic assessments of health-related programs and interventions. Relevant to this Surgeon General’s report are Cochrane reviews on interventions using mass media ( Sowden 1998 ), community interventions to prevent smoking ( Sowden and Stead 2003 ), the effects of advertising and promotional activities on smoking among youth ( Lovato et al. 2003 , 2011 ), preventing tobacco sales to minors ( Stead and Lancaster 2005 ), school-based programs ( Thomas and Perara 2006 ), programs for young people to quit using tobacco ( Grimshaw and Stanton 2006 ), and family programs for preventing smoking by youth ( Thomas et al. 2007 ). These reviews have been cited throughout the current report when appropriate.
In summary, substantial new research has added to our knowledge and understanding of tobacco use and control as it relates to youth since the 1994 Surgeon General’s report, including updates and new data in subsequent Surgeon General’s reports, in IOM reports, in NCI Monographs, and in Cochrane Collaboration reviews, in addition to hundreds of peer-reviewed publications, book chapters, policy reports, and systematic reviews. Although this report is a follow-up to the 1994 report, other important reviews have been undertaken in the past 18 years and have served to fill the gap during an especially active and important time in research on tobacco control among youth.
- Focus of the Report
Young People
This report focuses on “young people.” In general, work was reviewed on the health consequences, epidemiology, etiology, reduction, and prevention of tobacco use for those in the young adolescent (11–14 years of age), adolescent (15–17 years of age), and young adult (18–25 years of age) age groups. When possible, an effort was made to be specific about the age group to which a particular analysis, study, or conclusion applies. Because hundreds of articles, books, and reports were reviewed, however, there are, unavoidably, inconsistencies in the terminology used. “Adolescents,” “children,” and “youth” are used mostly interchangeably throughout this report. In general, this group encompasses those 11–17 years of age, although “children” is a more general term that will include those younger than 11 years of age. Generally, those who are 18–25 years old are considered young adults (even though, developmentally, the period between 18–20 years of age is often labeled late adolescence), and those 26 years of age or older are considered adults.
In addition, it is important to note that the report is concerned with active smoking or use of smokeless tobacco on the part of the young person. The report does not consider young people’s exposure to secondhand smoke, also referred to as involuntary or passive smoking, which was discussed in the 2006 report of the Surgeon General ( USDHHS 2006 ). Additionally, the report does not discuss research on children younger than 11 years old; there is very little evidence of tobacco use in the United States by children younger than 11 years of age, and although there may be some predictors of later tobacco use in those younger years, the research on active tobacco use among youth has been focused on those 11 years of age and older.
Tobacco Use
Although cigarette smoking is the most common form of tobacco use in the United States, this report focuses on other forms as well, such as using smokeless tobacco (including chew and snuff) and smoking a product other than a cigarette, such as a pipe, cigar, or bidi (tobacco wrapped in tendu leaves). Because for young people the use of one form of tobacco has been associated with use of other tobacco products, it is particularly important to monitor all forms of tobacco use in this age group. The term “tobacco use” in this report indicates use of any tobacco product. When the word “smoking” is used alone, it refers to cigarette smoking.
- Organization of the Report
This chapter begins by providing a short synopsis of other reports that have addressed smoking among youth and, after listing the major conclusions of this report, will end by presenting conclusions specific to each chapter. Chapter 2 of this report (“The Health Consequences of Tobacco Use Among Young People”) focuses on the diseases caused by early tobacco use, the addiction process, the relation of body weight to smoking, respiratory and pulmonary problems associated with tobacco use, and cardiovascular effects. Chapter 3 (“The Epidemiology of Tobacco Use Among Young People in the United States and Worldwide”) provides recent and long-term cross-sectional and longitudinal data on cigarette smoking, use of smokeless tobacco, and the use of other tobacco products by young people, by racial/ethnic group and gender, primarily in the United States, but including some worldwide data as well. Chapter 4 (“Social, Environmental, Cognitive, and Genetic Influences on the Use of Tobacco Among Youth”) identifies the primary risk factors associated with tobacco use among youth at four levels, including the larger social and physical environments, smaller social groups, cognitive factors, and genetics and neurobiology. Chapter 5 (“The Tobacco Industry’s Influences on the Use of Tobacco Among Youth”) includes data on marketing expenditures for the tobacco industry over time and by category, the effects of cigarette advertising and promotional activities on young people’s smoking, the effects of price and packaging on use, the use of the Internet and movies to market tobacco products, and an evaluation of efforts by the tobacco industry to prevent tobacco use among young people. Chapter 6 (“Efforts to Prevent and Reduce Tobacco Use Among Young People”) provides evidence on the effectiveness of family-based, clinic-based, and school-based programs, mass media campaigns, regulatory and legislative approaches, increased cigarette prices, and community and statewide efforts in the fight against tobacco use among youth. Chapter 7 (“A Vision for Ending the Tobacco Epidemic”) points to next steps in preventing and reducing tobacco use among young people.
- Preparation of the Report
This report of the Surgeon General was prepared by the Office on Smoking and Health (OSH), National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC), USDHHS. In 2008, 18 external independent scientists reviewed the 1994 report and suggested areas to be added and updated. These scientists also suggested chapter editors and a senior scientific editor, who were contacted by OSH. Each chapter editor named external scientists who could contribute, and 33 content experts prepared draft sections. The draft sections were consolidated into chapters by the chapter editors and then reviewed by the senior scientific editor, with technical editing performed by CDC. The chapters were sent individually to 34 peer reviewers who are experts in the areas covered and who reviewed the chapters for scientific accuracy and comprehensiveness. The entire manuscript was then sent to more than 25 external senior scientists who reviewed the science of the entire document. After each review cycle, the drafts were revised by the chapter and senior scientific editor on the basis of the experts’ comments. Subsequently, the report was reviewed by various agencies within USDHHS. Publication lags prevent up-to-the-minute inclusion of all recently published articles and data, and so some more recent publications may not be cited in this report.
- Evaluation of the Evidence
Since the first Surgeon General’s report in 1964 on smoking and health ( USDHEW 1964 ), major conclusions concerning the conditions and diseases caused by cigarette smoking and the use of smokeless tobacco have been based on explicit criteria for causal inference ( USDHHS 2004 ). Although a number of different criteria have been proposed for causal inference since the 1960s, this report focuses on the five commonly accepted criteria that were used in the original 1964 report and that are discussed in greater detail in the 2004 report on the health consequences of smoking ( USDHHS 2004 ). The five criteria refer to the examination of the association between two variables, such as a risk factor (e.g., smoking) and an outcome (e.g., lung cancer). Causal inference between these variables is based on (1) the consistency of the association across multiple studies; this is the persistent finding of an association in different persons, places, circumstances, and times; (2) the degree of the strength of association, that is, the magnitude and statistical significance of the association in multiple studies; (3) the specificity of the association to clearly demonstrate that tobacco use is robustly associated with the condition, even if tobacco use has multiple effects and multiple causes exist for the condition; (4) the temporal relationship of the association so that tobacco use precedes disease onset; and (5) the coherence of the association, that is, the argument that the association makes scientific sense, given data from other sources and understanding of biological and psychosocial mechanisms ( USDHHS 2004 ). Since the 2004 Surgeon General’s report, The Health Consequences of Smoking , a four-level hierarchy ( Table 1.1 ) has been used to assess the research data on associations discussed in these reports ( USDHHS 2004 ). In general, this assessment was done by the chapter editors and then reviewed as appropriate by peer reviewers, senior scientists, and the scientific editors. For a relationship to be considered sufficient to be characterized as causal, multiple studies over time provided evidence in support of each criteria.
Four-level hierarchy for classifying the strength of causal inferences based on available evidence.
When a causal association is presented in the chapter conclusions in this report, these four levels are used to describe the strength of the evidence of the association, from causal (1) to not causal (4). Within the report, other terms are used to discuss the evidence to date (i.e., mixed, limited, and equivocal evidence), which generally represent an inadequacy of data to inform a conclusion.
However, an assessment of a casual relationship is not utilized in presenting all of the report’s conclusions. The major conclusions are written to be important summary statements that are easily understood by those reading the report. Some conclusions, particularly those found in Chapter 3 (epidemiology), provide observations and data related to tobacco use among young people, and are generally not examinations of causal relationships. For those conclusions that are written using the hierarchy above, a careful and extensive review of the literature has been undertaken for this report, based on the accepted causal criteria ( USDHHS 2004 ). Evidence that was characterized as Level 1 or Level 2 was prioritized for inclusion as chapter conclusions.
In additional to causal inferences, statistical estimation and hypothesis testing of associations are presented. For example, confidence intervals have been added to the tables in the chapter on the epidemiology of youth tobacco use (see Chapter 3 ), and statistical testing has been conducted for that chapter when appropriate. The chapter on efforts to prevent tobacco use discusses the relative improvement in tobacco use rates when implementing one type of program (or policy) versus a control program. Statistical methods, including meta-analytic methods and longitudinal trajectory analyses, are also presented to ensure that the methods of evaluating data are up to date with the current cutting-edge research that has been reviewed. Regardless of the methods used to assess significance, the five causal criteria discussed above were applied in developing the conclusions of each chapter and the report.
- Major Conclusions
- Cigarette smoking by youth and young adults has immediate adverse health consequences, including addiction, and accelerates the development of chronic diseases across the full life course.
- Prevention efforts must focus on both adolescents and young adults because among adults who become daily smokers, nearly all first use of cigarettes occurs by 18 years of age (88%), with 99% of first use by 26 years of age.
- Advertising and promotional activities by tobacco companies have been shown to cause the onset and continuation of smoking among adolescents and young adults.
- After years of steady progress, declines in the use of tobacco by youth and young adults have slowed for cigarette smoking and stalled for smokeless tobacco use.
- Coordinated, multicomponent interventions that combine mass media campaigns, price increases including those that result from tax increases, school-based policies and programs, and statewide or community-wide changes in smoke-free policies and norms are effective in reducing the initiation, prevalence, and intensity of smoking among youth and young adults.
- Chapter Conclusions
The following are the conclusions presented in the substantive chapters of this report.
Chapter 2. The Health Consequences of Tobacco Use Among Young People
- The evidence is sufficient to conclude that there is a causal relationship between smoking and addiction to nicotine, beginning in adolescence and young adulthood.
- The evidence is suggestive but not sufficient to conclude that smoking contributes to future use of marijuana and other illicit drugs.
- The evidence is suggestive but not sufficient to conclude that smoking by adolescents and young adults is not associated with significant weight loss, contrary to young people’s beliefs.
- The evidence is sufficient to conclude that there is a causal relationship between active smoking and both reduced lung function and impaired lung growth during childhood and adolescence.
- The evidence is sufficient to conclude that there is a causal relationship between active smoking and wheezing severe enough to be diagnosed as asthma in susceptible child and adolescent populations.
- The evidence is sufficient to conclude that there is a causal relationship between smoking in adolescence and young adulthood and early abdominal aortic atherosclerosis in young adults.
- The evidence is suggestive but not sufficient to conclude that there is a causal relationship between smoking in adolescence and young adulthood and coronary artery atherosclerosis in adulthood.
Chapter 3. The Epidemiology of Tobacco Use Among Young People in the United States and Worldwide
- Among adults who become daily smokers, nearly all first use of cigarettes occurs by 18 years of age (88%), with 99% of first use by 26 years of age.
- Almost one in four high school seniors is a current (in the past 30 days) cigarette smoker, compared with one in three young adults and one in five adults. About 1 in 10 high school senior males is a current smokeless tobacco user, and about 1 in 5 high school senior males is a current cigar smoker.
- Among adolescents and young adults, cigarette smoking declined from the late 1990s, particularly after the Master Settlement Agreement in 1998. This decline has slowed in recent years, however.
- Significant disparities in tobacco use remain among young people nationwide. The prevalence of cigarette smoking is highest among American Indians and Alaska Natives, followed by Whites and Hispanics, and then Asians and Blacks. The prevalence of cigarette smoking is also highest among lower socioeconomic status youth.
- Use of smokeless tobacco and cigars declined in the late 1990s, but the declines appear to have stalled in the last 5 years. The latest data show the use of smokeless tobacco is increasing among White high school males, and cigar smoking may be increasing among Black high school females.
- Concurrent use of multiple tobacco products is prevalent among youth. Among those who use tobacco, nearly one-third of high school females and more than one-half of high school males report using more than one tobacco product in the last 30 days.
- Rates of tobacco use remain low among girls relative to boys in many developing countries, however, the gender gap between adolescent females and males is narrow in many countries around the globe.
Chapter 4. Social, Environmental, Cognitive, and Genetic Influences on the Use of Tobacco Among Youth
- Given their developmental stage, adolescents and young adults are uniquely susceptible to social and environmental influences to use tobacco.
- Socioeconomic factors and educational attainment influence the development of youth smoking behavior. The adolescents most likely to begin to use tobacco and progress to regular use are those who have lower academic achievement.
- The evidence is sufficient to conclude that there is a causal relationship between peer group social influences and the initiation and maintenance of smoking behaviors during adolescence.
- Affective processes play an important role in youth smoking behavior, with a strong association between youth smoking and negative affect.
- The evidence is suggestive that tobacco use is a heritable trait, more so for regular use than for onset. The expression of genetic risk for smoking among young people may be moderated by small-group and larger social-environmental factors.
Chapter 5. The Tobacco Industry’s Influences on the Use of Tobacco Among Youth
- In 2008, tobacco companies spent $9.94 billion on the marketing of cigarettes and $547 million on the marketing of smokeless tobacco. Spending on cigarette marketing is 48% higher than in 1998, the year of the Master Settlement Agreement. Expenditures for marketing smokeless tobacco are 277% higher than in 1998.
- Tobacco company expenditures have become increasingly concentrated on marketing efforts that reduce the prices of targeted tobacco products. Such expenditures accounted for approximately 84% of cigarette marketing and more than 77% of the marketing of smokeless tobacco products in 2008.
- The evidence is sufficient to conclude that there is a causal relationship between advertising and promotional efforts of the tobacco companies and the initiation and progression of tobacco use among young people.
- The evidence is suggestive but not sufficient to conclude that tobacco companies have changed the packaging and design of their products in ways that have increased these products’ appeal to adolescents and young adults.
- The tobacco companies’ activities and programs for the prevention of youth smoking have not demonstrated an impact on the initiation or prevalence of smoking among young people.
- The evidence is sufficient to conclude that there is a causal relationship between depictions of smoking in the movies and the initiation of smoking among young people.
Chapter 6. Efforts to Prevent and Reduce Tobacco Use Among Young People
- The evidence is sufficient to conclude that mass media campaigns, comprehensive community programs, and comprehensive statewide tobacco control programs can prevent the initiation of tobacco use and reduce its prevalence among youth.
- The evidence is sufficient to conclude that increases in cigarette prices reduce the initiation, prevalence, and intensity of smoking among youth and young adults.
- The evidence is sufficient to conclude that school-based programs with evidence of effectiveness, containing specific components, can produce at least short-term effects and reduce the prevalence of tobacco use among school-aged youth.
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during peri-adolescence in rats. Journal of Neuroscience. 2003; 23 (11):4712–6. [ PMC free article : PMC6740776 ] [ PubMed : 12805310 ]
- Alesci NL, Forster JL, Blaine T. Smoking visibility, perceived acceptability, and frequency in various locations among youth and adults. Preventive Medicine. 2003; 36 (3):272–81. [ PubMed : 12634018 ]
- Anderson G. Chronic Care: Making the Case for Ongoing Care. Princeton (NJ): Robert Wood Johnson Foundation; 2010. [accessed: November 30, 2011]. < http://www .rwjf.org/files /research/50968chronic .care.chartbook.pdf >.
- Bonnie RJ, Stratton K, Wallace RB, editors. Ending the Tobacco Problem: A Blueprint for the Nation. Washington: National Academies Press; 2007.
- Cochrane Collaboration. Home page. 2010. [accessed: November 30, 2010]. < http://www .cochrane.org/ >.
- Community Preventive Services Task Force. First Annual Report to Congress and to Agencies Related to the Work of the Task Force. Community Preventive Services Task Force. 2011. [accessed: January 9, 2012]. < http://www .thecommunityguide .org/library /ARC2011/congress-report-full.pdf >.
- Dalton MA, Beach ML, Adachi-Mejia AM, Longacre MR, Matzkin AL, Sargent JD, Heatherton TF, Titus-Ernstoff L. Early exposure to movie smoking predicts established smoking by older teens and young adults. Pediatrics. 2009; 123 (4):e551–e558. [ PMC free article : PMC2758519 ] [ PubMed : 19336346 ]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ (British Medical Journal). 2004; 32 :1519. [ PMC free article : PMC437139 ] [ PubMed : 15213107 ] [ CrossRef ]
- Fagerström K. The epidemiology of smoking: health consequences and benefits of cessation. Drugs. 2002; 62 (Suppl 2):1–9. [ PubMed : 12109931 ]
- Family Smoking Prevention and Tobacco Control Act, Public Law 111-31, 123 U.S. Statutes at Large 1776 (2009)
- Grimshaw G, Stanton A. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews. 2006;(4):CD003289. [ PubMed : 17054164 ] [ CrossRef ]
- Kessler DA. Nicotine addiction in young people. New England Journal of Medicine. 1995; 333 (3):186–9. [ PubMed : 7791824 ]
- Lovato C, Linn G, Stead LF, Best A. Impact of tobacco advertising and promotion on increasing adolescent smoking behaviours. Cochrane Database of Systematic Reviews. 2003;(4):CD003439. [ PubMed : 14583977 ] [ CrossRef ]
- Lovato C, Watts A, Stead LF. Impact of tobacco advertising and promotion on increasing adolescent smoking behaviours. Cochrane Database of Systematic Reviews. 2011;(10):CD003439. [ PMC free article : PMC7173757 ] [ PubMed : 21975739 ] [ CrossRef ]
- Lynch BS, Bonnie RJ, editors. Growing Up Tobacco Free: Preventing Nicotine Addiction in Children and Youths. Washington: National Academies Press; 1994. [ PubMed : 25144107 ]
- National Association of Attorneys General. Master Settlement Agreement. 1998. [accessed: June 9, 2011]. < http://www .naag.org/back-pages /naag/tobacco /msa/msa-pdf/MSA%20with %20Sig%20Pages%20and%20Exhibits .pdf/file_view >.
- National Cancer Institute. Changing Adolescent Smoking Prevalence. Bethesda (MD): U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 2001. Smoking and Tobacco Control Monograph No. 14. NIH Publication. No. 02-5086.
- National Cancer Institute. The Role of the Media in Promoting and Reducing Tobacco Use. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2008. Tobacco Control Monograph No. 19. NIH Publication No. 07-6242.
- National Research Council. Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects. Washington: National Academy Press; 1986. [ PubMed : 25032469 ]
- Office of the Surgeon General Reports of the Surgeon General, U.S. Public Health Service. 2010. [accessed: November 30, 2010]. < http://www .surgeongeneral .gov/library/reports/index.html >.
- Perry CL, Eriksen M, Giovino G. Tobacco use: a pediatric epidemic [editorial] Tobacco Control. 1994; 3 (2):97–8.
- Peto R, Lopez AD. Future worldwide health effects of current smoking patterns. In: Koop CE, Pearson CE, Schwarz MR, editors. Critical Issues in Global Health. San Francisco: Wiley (Jossey-Bass); 2001. pp. 154–61.
- Reddy KS, Perry CL, Stigler MH, Arora M. Differences in tobacco use among young people in urban India by sex, socioeconomic status, age, and school grade: assessment of baseline survey data. Lancet. 2006; 367 (9510):589–94. [ PubMed : 16488802 ]
- Schochet TL, Kelley AE, Landry CF. Differential expression of arc mRNA and other plasticity-related genes induced by nicotine in adolescent rat forebrain. Neuroscience. 2005; 135 (1):285–97. [ PMC free article : PMC1599838 ] [ PubMed : 16084664 ]
- Sowden AJ. Mass media interventions for preventing smoking in young people. Cochrane Database of Systematic Reviews. 1998;(4):CD001006. [ PubMed : 10796581 ] [ CrossRef ]
- Sowden AJ, Stead LF. Community interventions for preventing smoking in young people. Cochrane Database of Systematic Reviews. 2003;(1):CD001291. [ PubMed : 12535406 ] [ CrossRef ]
- Stead LF, Lancaster T. Interventions for preventing tobacco sales to minors. Cochrane Database of Systematic Reviews. 2005;(1):CD001497. [ PubMed : 15674880 ] [ CrossRef ]
- Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004; 1021 :51–8. [ PubMed : 15251873 ]
- Task Force on Community Preventive Services. Recommendations regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. American Journal of Preventive Medicine. 2001; 20 (2 Suppl):S10–S15. [ PubMed : 11173214 ]
- Task Force on Community Preventive Services. Tobacco. In: Zaza S, Briss PA, Harris KW, editors. The Guide to Preventive Services: What Works to Promote Health? New York: Oxford University Press; 2005. pp. 3–79. < http://www .thecommunityguide .org/tobacco/Tobacco.pdf >.
- Thomas RE, Baker PRA, Lorenzetti D. Family-based programmes for preventing smoking by children and adolescents. Cochrane Database of Systematic Reviews. 2007;(1):CD004493. [ PubMed : 17253511 ] [ CrossRef ]
- Thomas RE, Perera R. School-based programmes for preventing smoking. Cochrane Database of Systematic Reviews. 2006;(3):CD001293. [ PubMed : 16855966 ] [ CrossRef ]
- US Department of Health and Human Services. Preventing Tobacco Use Among Young People A Report of the Surgeon General. Atlanta (GA): US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1994.
- US Department of Health and Human Services. Tobacco Use Among US Racial/Ethnic Minority Groups—African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1998.
- U.S. Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2nd ed. Washington: U.S. Government Printing Office; 2000.
- US Department of Health and Human Services. Reducing Tobacco Use: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2000.
- US Department of Health and Human Services. Women and Smoking A Report of the Surgeon General. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001.
- US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
- US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [ PubMed : 20669524 ]
- US Department of Health and Human Services. How Tobacco Smoke Causes Disease—The Biology and Behavioral Basis for Tobacco-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [ PubMed : 21452462 ]
- U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. 2011. [accessed: November 1, 2011]. < http://www .healthypeople .gov/2020/default.aspx >.
- US Department of Health, Education, and Welfare. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control; 1964. PHS Publication No. 1103.
- Cite this Page National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2012. 1, Introduction, Summary, and Conclusions.
- PDF version of this title (18M)
In this Page
Other titles in these collections.
- Reports of the Surgeon General
- Health Services/Technology Assessment Text (HSTAT)
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Introduction, Summary, and Conclusions - Preventing Tobacco Use Among Youth and ... Introduction, Summary, and Conclusions - Preventing Tobacco Use Among Youth and Young Adults
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Home — Essay Samples — Nursing & Health — Smoking — The Effects Of Smoking On Health
The Effects of Smoking on Health
- Categories: Smoking Tobacco
About this sample

Words: 491 |
Published: Mar 1, 2019
Words: 491 | Page: 1 | 3 min read

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr Jacklynne
Verified writer
- Expert in: Nursing & Health

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
2 pages / 712 words
6 pages / 2528 words
3 pages / 1160 words
3 pages / 1162 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Smoking
Smoking is a prevalent practice in many societies, with approximately 1 billion people engaging in this habit. The act of smoking involves burning substances, such as tobacco or cannabis, and inhaling the resulting smoke into [...]
Smoking has numerous health effects, both short-term and long-term. Some of the short-term effects include bad breath, yellow teeth, and decreased sense of taste and smell. The long-term effects, however, are much more severe. [...]
Understanding the Effects of Smoking Smoking is a habit that has been linked to a myriad of health risks, including lung cancer, heart disease, and respiratory illnesses. In addition to the physical health risks, smoking also [...]
Initial impressions of a person smoking Association of smoking with wealth and maturity Irony of anti-smoking education Relaxation and stress reduction Taking breaks and social interactions Life skills [...]
For years there has been conflicting research whether smoking should be banned or not and it is a significant issue today. Many people have given up smoking while others still continue to smoke. Smoking is the inhalation and [...]
Stop smoking it can cost you your life! What is smoking? How can something small cause so much harm to the world? Smoking is an addictive drug that can cause death or cancer it has caused, More than 10 times as many U.S. [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
High-potency marijuana highlights the risk of cannabis-induced psychiatric disorders
Anders Gilliand was just 17 years old when he started to lose contact with reality.
“He thought that there were higher beings that were communicating with him to tell him what to do or who he was,” said his mother, Kristin Gilliand, who lives in Nashville.
Her son, who had been using marijuana since he was 14 years old, was diagnosed with schizophrenia, a chronic psychiatric disorder that can include symptoms like delusions, hallucinations and disorganized speech.
He started taking anti-psychotic medication but ultimately stopped because of the side effects he experienced. To try and quell the voices in his head, he began using heroin and died of an accidental drug overdose in 2019 when he was 22.
“If he had never started using cannabis, he might still be here,” said Gilliand, a neuroscientist at Vanderbilt University. While there is a family history of schizophrenia, she believes that her son’s marijuana use led to psychotic episodes and triggered the condition.
Anders was among a growing number of young adults, particularly men, who are at increased risk of developing psychosis from marijuana use. Evidence from separate Danish and British studies , among others, suggest a link between heavy marijuana use and psychiatric disorders such as depression, bipolar disorder and schizophrenia. Researchers believe that high levels of THC — the psychoactive component in the cannabis plant that causes the high — could set off these conditions in people who have a genetic risk. THC levels in marijuana have been getting stronger for decades.

“We’re definitely seeing a rise in cannabis-induced psychosis among teenagers,” said child psychiatrist Dr. Christian Thurstone, an addiction specialist at the University of Colorado School of Medicine in Denver.
Is higher potency marijuana more dangerous?
The more potent the cannabis products, the more likely users are to have adverse effects, said Nora Volkow, director of the National Institute on Drug Abuse.
“Those who consume the highest doses are the ones that are going to have the highest risk of becoming psychotic,” she said.
There’s limited research on adverse effects of high levels of THC, although a European study published in 2020 found that high-potency cannabis products carried a greater risk of hallucinations and delusions compared with lower potency types.
“It seems like there’s an association between cannabis strength and increased risk for psychosis but more work needs to be done there,” said Ziva Cooper, director of the center for cannabis and cannabinoids at UCLA.
As many as half of people with cannabis-induced psychosis may go on to develop either schizophrenia or bipolar disorder, research suggests.
Young adults and teenagers should be especially concerned, Thurstone said
“The studies that we have so far clearly indicate that the risk for psychosis is dose dependent, meaning that the more marijuana somebody’s exposed to especially in adolescence, the greater the risk of developing psychosis, schizophrenia and severe mental illness,” he said.
More news on marijuana and health
- Risks of marijuana and THC on the heart: What you need to know.
- Marijuana use as little as once a month linked to heart risks.
- Marijuana use sent more kids to the ER during the pandemic.
Another issue with higher strength products is the potential for developing cannabis use disorder, or an addiction to marijuana. When people are exposed frequently to higher strengths of cannabis, they may be more likely to develop cannabis use disorder, although further research is still needed to say definitively.
“There’s no longer any scientific debate that marijuana can not just be psychologically addictive or habit forming, but also physically habit forming,” Thurstone said. “It’s a substance that produces tolerance so people have to use more and more to have the same effect.”
About 1 in 10 people who begin using cannabis will become addicted, according to the Centers for Disease Control .
How cannabis potency may be linked to psychosis
Marijuana overactivates molecules in our brain known as cannabinoid receptors, which cause the high. When these brain receptors are stimulated, it can cause difficulty with thinking and problem-solving, as well as impaired memory.
How marijuana use may trigger psychosis isn’t fully understood, although scientists believe it’s interfering with our brains ability to distinguish between what’s going on in our heads versus the real world.
“Marijuana in the 60s, 70s, 80s and early 90s was about 2% to 3% THC,” said Thurstone, who has tracked the rise of high-potency THC products in smoke shops and dispensaries. “Nowadays, with the commercialized products, they are routinely 20 plus percent — so about 10 times more potent.”
Patrick Johnson, assistant store manager at Frost Exotic dispensary in Colorado, has been in the cannabis industry since 2009 and has seen the potency rise firsthand. Johnson said THC levels really started to take off after marijuana became legal for recreational use in Colorado in 2014.
Since then, 24 states, two territories and Washington, D.C. have legalized marijuana for medical and recreational use.
With more people around the country using weed legally, there’s been greater consumer demand for more powerful weed, experts say.
“After recreational [legalization] is when I have personally seen it go from like 19 or 20% up to like 30 or 35%,” Johnson said.
Currently in his shop they carry strains as low as 14% up to 30%. Most customers have a preference for the strong stuff, Johnson said.
One reason that potency has been getting stronger over the years in cannabis products is because customers may build a tolerance to the drug, said Mahmoud ElSohly, professor of pharmaceutics and cannabis researcher at the University of Mississippi. He has been studying this issue in collaboration with the National Institute of Drug Abuse, finding that the average potency has risen from 3% to 15% from 1995 to 2021.
“People keep needing higher and higher potency products to get the degree of high they’re looking for,” he said.
In the past, a joint with 2% THC may have been enough to get most people high, ElSohly said. With heavier usage, many people now have built up a tolerance and may need to smoke multiple joints with 2% THC or buy a single joint with 6% to get a similar feeling.
Is one form of marijuana safer?
Cannabis strength primarily refers to the THC content in the marijuana flower or bud, which is the smokable part of the plant.
While THC levels can be close to 40% in the flower, other products, such as concentrates or oils may contain amounts as high as 95%.
The problem, said UCLA’s Cooper, is that there isn’t yet a widely accepted standard dose like there is with alcohol, so predicting how someone will react to different cannabis products can be difficult.
It’s also challenging to develop a unit dose for inhaled combusted products. While a typical joint may contain between 100 to 200 milligrams of THC, that doesn’t tell the whole story, Cooper said.
How much THC a marijuana smoker is exposed to can vary. How long and how deep are they inhaling? Or how long do they wait in between puffs as a lot of THC is lost to "sidestream" smoke, which comes from the burning end of the joint in between hits.
In comparison, marijuana edibles such as gummies, cookies and brownies are typically 5 to 10 milligrams per dose. There is a movement toward establishing a unit dose for edibles and limiting how much THC can be consumed at once. In New York state, for example, that number is 10 mg per serving.
How high can the THC go?
“I don’t necessarily think it may raise much more,” said Volkow, from the National Institute on Drug Abuse. “There may be a level at which sometimes too much can become aversive so people smoke and get very agitated or paranoid.”
She is optimistic that the THC levels of available marijuana flower will not rise as high as 50%.
There is a limit to how much THC the plant can produce, although manufacturers are finding clever ways to boost the chemical, Cooper said.
“The industry is adding more THC to the plant products,” including infusing pre-rolled cannabis cigarettes with additional THC, she said. “We’re starting to see that people are being exposed to levels of THC we just haven’t seen in the past.
Akshay Syal, M.D., is a medical fellow with the NBC News Health and Medical Unit.
Kate Snow is a senior national correspondent for NBC News and an anchor for NBC Nightly News.
Patrick Martin is a producer in the NBC News Health & Medical Unit.
Is it dangerous to smoke weed? What you need to know about using marijuana.
Using marijuana at a young age can have lifelong consequences. the drug interferes with the development of the brain..
The push to legalize marijuana at the federal level has gained ground ever since California legalized it for medical use nearly 30 years ago. Recreational marijuana is now permissible in 24 states and Washington, D.C. Only four states still outlaw marijuana with no medical exceptions.
In 2022, President Joe Biden ordered a review of the drug's status as a Schedule I substance, which denotes that a drug has no accepted medical use and has high potential for abuse. If marijuana is reclassified, a renewed push for national legalization will surely follow.
We aren't here to tell you whether to vote for or against legalizing marijuana. But as a doctor and mental health professional, we're concerned that many Americans may come away from this legalization push with a belief that marijuana is harmless − if not healthful.
That's not quite right, as a growing body of scientific literature shows, including a new landmark study in the Journal of the American Heart Association .
Marijuana can help relieve symptoms of illness
Let's be fair: Marijuana has been shown to alleviate the symptoms and side effects of certain conditions.
Scientific research suggests that the drug can reduce vomiting and nausea in patients undergoing chemotherapy, reduce muscle spasms in patients with multiple sclerosis and provide short-term relief for adults with chronic pain.
Your quality of life: Do I have to get chemo to treat my cancer? That answer is changing as treatments evolve.
There's some evidence that marijuana might reduce tics in people with Tourette syndrome , increase appetite and reduce weight loss in people with HIV and improve sleep quality for people with sleep apnea.
But because medical marijuana is federally illegal, there have been relatively few comprehensive and scientifically rigorous studies on its potential benefits. All these areas require further research, including whether benefits actually outweigh risks.
And let's not get suckered into the cannabis industry's focus on medical utility. For the majority of users, marijuana is a recreational drug that brings pleasure, pure and simple.
Risks of marijuana use include heart attacks and strokes
Unfortunately, that's not all it brings. The risks are real.
An American Heart Association study analyzed data from more than 430,000 adults collected over four years. Researchers found that marijuana use is linked to a significantly higher risk of heart attack and stroke, with the risk increasing with frequency of use. Daily users had a 25% higher chance of heart attack and a 42% higher chance of stroke than non-users. And the increased danger exists whether users smoke, vape or eat their cannabis products.
It's also important to note that thanks to advances in agricultural technology, the potency of marijuana's psychoactive ingredient – tetrahydrocannabinol, or THC − has dramatically increased.
Today's marijuana is nothing like the flowers and leaves that filled the joints smoked at Woodstock in 1969. At that time, marijuana contained less than 2% THC. By the '90s, that had doubled to about 4%. Today, THC content in the most popular strains of weed falls between 17% and 28%. Concentrated oils or "dabs," meanwhile, can contain upwards of 95% concentration.
The higher the potency, the greater the risk of addiction − despite the common misconception that marijuana is not addictive.
Not your grandma's weed: Why potency limits must be part of any push to legalize cannabis
A 2014 study from the New England Journal of Medicine showed that nearly 10% of people who try out marijuana get hooked . That figure increases to 17% among those who first try weed in adolescence and to 25% among those who get high every day.
Marijuana is not a harmless substance, especially for adolescents whose brains have yet to fully develop. Yet teenage marijuana use is also at its highest level this century.
Nearly 80% of cannabis users try the drug for the first time as a teenager . In the years following its legalization in Colorado via a 2012 referendum, marijuana use among 12- to 17-year-olds increased 65%.
Using marijuana at a young age can have lifelong consequences. The drug interferes with the development of the brain . Impaired attention, problems with memory and difficulty learning are all potential side effects of early exposure to marijuana.
Studies have shown that frequent marijuana use can fundamentally alter the brain's prefrontal cortex (our brain's "personality center"), the cerebellum (which controls movement and balance) and the amygdala (which processes emotions and memories).
Recognition of the mental health risks of marijuana use is also growing. A six-year study found that teenage girls are five times more likely to develop depression or anxiety if they smoke weed every day. Because many use marijuana as a coping mechanism for anxiety and depression, they can get themselves into a vicious cycle of dependency and worsening mental health.
Recent research from the National Institutes of Health has linked cannabis use disorder − which afflicts more than 1 in 5 users − to an increased risk of developing schizophrenia.Among men in their 20s, as many as 30% of schizophrenia cases would have been prevented but for marijuana use.
Once people get hooked, marijuana can be incredibly difficult to quit. Withdrawal symptoms include depression, insomnia, anger, irritability and, of course, intense cravings to get high again.
Against all this data, it's a dangerous folly to think that getting high poses no health risks.
At a time when social acceptance of marijuana and access to the drug have skyrocketed, even as youth mental health indicators are plummeting, it's more important than ever to reexamine the notion that weed is harmless.
Phil McGraw, Ph.D., of daytime TV's "Dr. Phil," is one of the most well-known mental health professionals in the world and founder of Merit Street Media cable network, where he hosts " Dr. Phil Primetime ." Dr. John Whyte is chief medical officer of WebMD .
Smoking Habit, Its Causes and Effects Essay
Smoking is one of the factors that are considered the leading causes of several health problems in the current society. As Fritz (2008) says, there is a need to encourage people to adopt a lifestyle that would help in protecting the health of an individual. The above scholar is of the view that many professionals, including doctors, support the need to maintain a healthy lifestyle. Other professionals, such as psychologists and teachers, have firsthand experience of how some health complications would negatively affect some users. The cost of living is on the rise, while employment opportunities are shrinking. The health facilities are under pressure to increase their capacity, but due to the limited resources, the government has not been possible to meet the demands needed in the hospitals. This has seen the cost of accessing proper medical attention skyrocket within the past decade. This has been the main reason why people are constantly advised to adopt healthy lifestyles that would ensure that they keep doctors away.
Most smokers would develop the smoking habit out of the fun. They consider this behavior a form of lifestyle that would help them be categorized in a specific group of people. Some start smoking as a way of gaining acceptance to a certain group of people. As Wong (2000) says, no one is born a smoker. Similarly, smoking is not medicinal, and as such, it is not possible that one could have been addicted because of a medical condition. Getting into addiction as a chain smoker always starts of one’s own willingness to be a smoker. However, after a successful introduction into the habit, the behavior of an individual would change forever. Smoking will cease being an activity done to generate fun. It would be a necessity without which the body might not function properly. Indeed, there has always been a massive global campaign against smoking. A section of the society may be wondering why this vice has attracted the attention of various professionals and the public in general. Smoking has several severe health conditions that make it unfit for people. It affects the health of an individual to the extent that it might lead to amputation. Several individuals have lost some parts of their bodies simply because of smoking.
Liver cirrhosis is one of the main health complications that result from smoking. Have you ever wondered where all smoke that one inhale goes? The best physical test would be to study the chimneys or the car exhaust for a while. When the car is newly taken from the showroom, the exhaust is sparkling clean. After driving the car for a while, the exhaust would develop black soot. The same would be the case with the chimney. As time goes by and as the chimney or the exhaust is continually put into use, the soot gets bigger and uglier. It reaches a time when the soot has to be scraped off to increase the efficiency and make the exhaust or the chimney more effective.
Similarly, this is what happens to the lungs and the entire respiratory organs as one persists with the smoking habit (Hawkins, Mothersbaugh, & Best, 2010). The soot would start developing in the lungs as one continues to smoke. When this habit is not changed as soon as possible, the individual would have an infection of the lungs as the smoke accumulates. The soot would settle in the chambers of the lungs, blocking them from functioning completely. This would render the lungs ineffective. The liver would face difficulties in ensuring that the lung is cleaned to allow it to perform its functions. This means that the soot will be transferred into the liver. The liver will try to eliminate this contamination for a while. However, as their volume becomes unbearable, it would affect the liver to the extent that it would not be functioning. This contaminant will negatively affect the liver leading to what is always referred to as liver cirrhosis. This health complication can lead to death if it is not addressed appropriately.
Smoking is also known to contribute to other health conditions. According to Graham (2010), smoking has been confirmed to be the leading cause of some forms of cancer. The above scholar says that smoking always increases the chances of one developing such cancers as cancer of the throat and mouth. Cancer is a medical condition that has been considered the leading cause of death in the world today. A case in point is the death of Apple Inc’s founder and former chief executive, Steve Jobs. Steve Jobs was a chain smoker who heavily relied on smoking to make the body system function properly. Since this habit is welcome in society, the famous CEO did not consider it a factor that could complicate his health. When this realization dawned on him, it was too late. The smoke had massively affected him, and he was diagnosed with cancer. He ignored the advice of the medics to quit smoking and adopt a different behavior that would save his life, but he was reluctant to do so.
Consequently, he was brought to his humbling knees by this complication. The demise of Steve Jobs should be a wakeup call to all smokers and those planning to join smoking. These people should know that smoking is dangerous. There are other consequences of smoking, such as changing the coloration of the teeth. The smoke makes the teeth to develop a brown coloration that may not be pleasant, especially among the youth and the middle-aged individuals who would always want to be presentable to others. Smoking may also make one be alienated from friends. Some people hate smoking with a passion. This may force one to drop trusted friends because of this habit.
Smoking is a habit that may be easy to start, but getting out of this vice might be one of the biggest challenges in one’s lifetime. Psychologists have always stated that quitting smoking is not as easy as quitting other addictions, such as alcoholism. Although it is not an impossibility, the process of quitting this habit is always complex and may be accompanied by some pain, especially when an individual reaches an advanced stage. As such, it is always important that this habit should not be started in the first place. One gains no advantage by being a smoker. However, the health and social complications that are accompanied by this are always devastating. One faces a possibility of rejection from trusted friends, besides developing the dreaded cancer disease. This may change the lifestyle of an individual permanently.
Fritz, R. (2008). The power of a positive attitude: Discovering the key to success . New York: American Management Association.
Graham, J. (2010). Critical thinking in consumer behavior: Cases and experiential exercises . Boston: Prentice Hall.
Hawkins, D., Mothersbaugh, D., & Best, R. (2010). Consumer behavior: Building marketing strategy . Boston: McGraw Hill.
Wong, R. (2000). Motivation: A bio-behavioral approach . Cambridge: Cambridge University Press.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2020, July 29). Smoking Habit, Its Causes and Effects. https://ivypanda.com/essays/smoking-habit-its-causes-and-effects/
"Smoking Habit, Its Causes and Effects." IvyPanda , 29 July 2020, ivypanda.com/essays/smoking-habit-its-causes-and-effects/.
IvyPanda . (2020) 'Smoking Habit, Its Causes and Effects'. 29 July.
IvyPanda . 2020. "Smoking Habit, Its Causes and Effects." July 29, 2020. https://ivypanda.com/essays/smoking-habit-its-causes-and-effects/.
1. IvyPanda . "Smoking Habit, Its Causes and Effects." July 29, 2020. https://ivypanda.com/essays/smoking-habit-its-causes-and-effects/.
Bibliography
IvyPanda . "Smoking Habit, Its Causes and Effects." July 29, 2020. https://ivypanda.com/essays/smoking-habit-its-causes-and-effects/.
- Literature Studies: “The Chimney Sweeper” by W. Blake
- Comparison: "The Chimney Sweeper" and "The Love Song of J. Alfred Prufrock"
- “The Chimney Sweeper” by William Blake: Social Impact of the Industrial Revolution
- Child Innocence against Exploitative Nature in “The Chimney Sweeper”
- How Aviation Impacts Climate Change
- Quitting Smoking: Strategies and Consequences
- Importance of Quitting Smoking
- Environmental Factors and Health Promotion: Indoor and Outdoor Air Pollution
- On Why Global Warming Is a Reality
- How to Light a Fire in a Fireplace
- Anthropology of Culture and Medicine
- Nonurgent Emergency Room Visit' Effects
- Why Healthcare Should Be Free?
- Obesity Risk Factors in Dallas: Windshield Surveys
- Arlington Community and Healthy Living Conditions

IMAGES
VIDEO
COMMENTS
500 Words Essay On Smoking. One of the most common problems we are facing in today's world which is killing people is smoking. A lot of people pick up this habit because of stress, personal issues and more. In fact, some even begin showing it off. When someone smokes a cigarette, they not only hurt themselves but everyone around them.
Introduction. Tobacco use, including smoking, has become a universally recognized issue that endangers the health of the population of our entire planet through both active and second-hand smoking. Pro-tobacco arguments are next to non-existent, while its harm is well-documented and proven through past and contemporary studies (Jha et al., 2013).
Smoking is one of the most common negative habits that people indulge in. Many health experts have warned that smoking is unhealthy and dangerous to the human health. This essay will discuss the negative effects of smoking on human beings.
Smoking: Causes and Effects Essay. Among numerous bad habits of modern society smoking seems to be of the greatest importance. Not only does it affect the person who smokes, but also those who are around him. Many people argue about the appropriate definition of smoking, whether it is a disease or just a bad habit.
500 Words Essay on Harmful Effects of Smoking Introduction. Smoking is a prevalent habit, often started out of curiosity, peer pressure, or stress management. However, its harmful effects are well-documented, impacting nearly every organ in the human body. Despite the widespread knowledge of its adverse effects, smoking continues to be a ...
500 Words Essay on Negative Effects Of Smoking Introduction. Smoking is a habit that many people pick up due to various reasons, such as stress, peer pressure, or even out of curiosity. Despite its popularity, smoking has many negative effects on our health and the environment. This essay will discuss these harmful effects in simple terms.
Smoking. Smoking is the practice of inhaling smoke from burning plant material. Nicotine works on your brain to create a relaxing, pleasurable feeling that makes it tough to quit. But smoking tobacco puts you at risk for cancer, stroke, heart attack, lung disease and other health issues. Nicotine replacements and lifestyle changes may help you ...
This article discusses why smoking is bad for health and reasons to quit. Smoking can cause harm throughout the body, including the heart, brain, and lungs. ... (2016). Smoking and its effects on ...
Persuasive Essay Examples About Smoking. Smoking is one of the leading causes of preventable death in the world. It leads to adverse health effects, including lung cancer, heart disease, and damage to the respiratory tract. However, the number of people who smoke cigarettes has been on the rise globally. A lot has been written on topics related ...
We identified three outcomes with a 4-star association with smoking: COPD (72% increase in risk based on the BPRF, 0.54 ROS), lower respiratory tract infection (54%, 0.43) and pancreatic cancer ...
Health impact of smoking. Table Table1 1 lists the main causes of death from smoking. Tobacco smoking is estimated to lead to the premature death of approximately 6 million people worldwide and 96,000 in the UK each year (Action on Smoking and Health, 2016b; World Health Organization, 2013).A 'premature death from smoking' is defined as a death from a smoking-related disease in an ...
Smoking affects the lungs and respiratory organs causing such terrible diseases as cancer. Among the most wider spread diseases are peptic ulcers, cancer of the larynx, kidney, pancreas, and other major organs. The resins from the smoke enter the blood and ruin cells. This process is inevitable if a person smokes for years.
Smoking causes stroke and coronary heart disease, which are among the leading causes of death in the United States. 1,3. Even people who smoke fewer than five cigarettes a day can have early signs of cardiovascular disease. 1. Smoking damages blood vessels and can make them thicken and grow narrower.
Smoking has a number of negative physiological, social, and psychological impacts that can seriously affect a person's life.This is just a smoking essay introduction. Reading the essay on smoking will discuss the various negative effects of smoking as well as preventative measures. Read and download this smoking in public places essay pdf here.
Smoking Essay Smoking is a widespread habit that involves inhaling smoke from the burning of tobacco. It is a highly addictive habit that has numerous negative effects on the body, including lung cancer, heart disease, and respiratory issues. Writing an essay on smoking can be a challenging task, but it is an important topic to discuss.
Health effects of smoking. The decision to smoke cigarettes is often framed as a personal choice, but it is important to consider the broader implications of this habit. The negative health effects of smoking are well-established, with numerous studies linking it to lung cancer, heart disease, and a range of other serious conditions.
Tobacco use is a global epidemic among young people. As with adults, it poses a serious health threat to youth and young adults in the United States and has significant implications for this nation's public and economic health in the future (Perry et al. 1994; Kessler 1995). The impact of cigarette smoking and other tobacco use on chronic disease, which accounts for 75% of American spending ...
I think smoking is bad for you because it can kill your cat, dog or pets. It can also kill your family. You can die from cancer by smoking. Every time you smoke you are exposing toxic chemicals to the air which somebody else is going to breath and possibly die from. For example, you are putting people at risk every time you smoke.
This damages the blood vessels. Smoking can result in stroke and heart attacks since it hinders blood flow, interrupting oxygen to various parts of the body, such as feet and hands. Introduction of cigarettes with low tar does not reduce these effects since smokers often prefer deeper puffs and hold the smoke in lungs for a long period.
Smoking as a social and psychological problem. Smoking in an economical way causes costs to increase. Social habits, for example, deprivation of senses, dullness, anxiety, stress, and smoking play a major role in causing all these things. Smoking causes stress, so it is bad for both types of people smoking and non-smoking.
Human body is very vulnerable to harmful effects of smoking, and it can harm our heart, lungs, blood circulation, bones, stomach, mouth, eyes, skin, reproduction and fertility. Smoking effect on heart and lung in very serious manner, in case of heart nicotine raises blood pressure and blood gets clot easily. Carbon monoxide raids the blood of ...
Smoking and Its Negative Effects on Human Beings. Therefore, people need to be made aware of dental and other health problems they are likely to experience as a result of smoking. Hookah Smoking and Its Risks. The third component of a hookah is the hose. This is located at the bottom of the hookah and acts as a base.
There's limited research on adverse effects of high levels of THC, although a European study published in 2020 found that high-potency cannabis products carried a greater risk of hallucinations ...
Researchers found that marijuana use is linked to a significantly higher risk of heart attack and stroke, with the risk increasing with frequency of use. Daily users had a 25% higher chance of ...
Smoking is also known to contribute to other health conditions. According to Graham (2010), smoking has been confirmed to be the leading cause of some forms of cancer. The above scholar says that smoking always increases the chances of one developing such cancers as cancer of the throat and mouth. Cancer is a medical condition that has been ...