Ensuring the validity of clinical outcomes assessment data
The value of rater training

Advancements in Artificial Intelligence for site selection
Using human-enabled AI to enhance decision-making and minimise risk
A multifaceted risk factor: Addressing obesity's impact across the disease spectrum
Optimising biotech funding whitepaper series.
2023 biotech sector survey
Navigating biotech's challenges and embracing a promising tomorrow.
EMA guideline on computerised systems and electronic data in clinical trials
Key considerations on the impact of the new framework of globally applicable standards.
Navigating the regulatory labyrinth of technology in decentralised clinical trials
Keeping up to date and implementing the latest guidelines.
Considerations for DTx clinical trials in CNS indications
Successful digital therapeutic clinical trials.
Featured Solutions
Digital Health Technologies
ICON acquires HumanFirst, a cloud-based technology company for life sciences supporting precision measurement in patient centred clinical research.
ICON provides full service outsourcing and flexible support for biotech specific needs such as due diligence and asset valuation.
Cardiac Safety Solutions
End-to-end cardiac safety solutions, including ECG, event monitoring, BPM, long-term Holter monitoring, ECHO and MUGA studies.
Early Clinical and Bioanalytical Solutions
Innovative early clinical solutions that will advance your drug development strategy.
Site & Patient Solutions
Transforming recruitment through patient-centric trials and real-world, real-time data.
Market Access
Expertise in mission-critical pricing, market access, and reimbursement.
Navigating the complexity of real-world healthcare data: choosing the right tools at the right time
Watch the webinar.
The future of pharmacovigilance: Exploring automation and AI in literature surveillance
Streamlining IRT in clinical trials: Simplify, optimise, succeed
Icon insights.
- 01 Apr 2024
SDoH data for efficient, patient-centred clinical trials
Insights from PHUSE US Connect 2024
- 27 Mar 2024
Diabesity: Overlapping pathophysiology informs multi-indication treatment
- 25 Mar 2024
Site Training for Clinical Trial Success
- 28 Feb 2024
Beyond awareness: Driving progress on the Rare Disease journey
- 21 Feb 2024
Rare disease trials require effective participation support strategies to succeed
Strategies for de-risking rare disease programmes during research and development
- 13 Feb 2024
Insights from the 2024 ISCR Annual Conference: India’s evolving clinical research arena
- 26 Jan 2024
Ensuring safety and compliance: The essentials of outsourcing pharmacovigilance
What’s happening in icon, how can we help.
- Cell and Gene Therapies
- Decentralised Clinical Trials
- Early Clinical
- Medical Device
- Rare & Orphan Diseases
- Real World Evidence
- Site & Patient Recruitment
- Strategic Solutions

- Central Middlesex Hospital
- testimonials
- trial design
- subject recruitment
- clinical research
- project management
- data management
- medical writing
- computer systems validation (CSV) and IT
- quality services
- adaptive studies
- bioavailability and bioequivalence
- bridging trials
- drug-drug interactions
- first-in-human healthy volunteer trials – SAD & MAD
- first-in-human patient trials
- food effect
- phase 2 patient studies
- thorough QT trials
- analysis of drug concentrations
- microdose trials
- polysomnography
- precision ECG
- cardiovascular
- central nervous system
- dermatological
- gastrointestinal
- haematological
- respiratory
- biologicals and biosimilars
- radiolabelled
- small molecules
- publications
Hammersmith Medicines Research Cumberland Avenue London NW10 7EW
For enquiry 020 8961 4130
Specialists in clinical pharmacology
Phase 1 and early phase 2 trials
Over 900 clinical trials completed since 1993 complete an online enquiry form
For pharmaceutical or biotechnology companies worldwide complete an online enquiry form
Fully integrated services from study design to final report complete an online enquiry form
- Specialist services read more
- Want to volunteer at HMR? find out more
- Careers working at HMR
- Corporate view more

Facilities and services
- We specialise in early clinical trials of study drugs for pharmaceutical and biotechnology companies worldwide.
- Founded in 1993, HMR is now the biggest CRO of its kind in the UK and one of the biggest in Europe.
- We have 270 staff, including a resident 24/7 resuscitation team, extensive purpose-built facilities, 145 beds, and on-site pharmacy, radiopharmacy, and laboratory.
- We can provide a full service from design to clinical study report, all in one building in London, UK.
- Our level of service and commitment is the same for all companies, regardless of size.
- HMR has: MHRA Phase 1 Accreditation for FIH trials; Manufacturing and Import Licence for study drugs [MIA(IMP)]; Home Office licence for all controlled drug substances; and Environment Agency licence for trials of radiolabelled study drugs.
- Our Quality System is compliant with ISO 9001, our Laboratory with ISO 17025, and our Information Security Management System with ISO 27001.
- We’ve won three Queen’s Awards for Exports or Enterprise, the most prestigious of UK business awards.

Our experience
- We have completed >900 trials for pharmaceutical and biotechnology companies from 23 countries worldwide, including all 15 major companies by revenue, and >200 small-to-medium sized or virtual companies.
- We can do almost all types of trial, from simple bioequivalent to complex trials, such as endoscopy, sleep, radiopharmacy, bridging, QTc, and PET, fMRI or CT imaging.
- Many of our trials are first-in-human (FIH) and proof-of-concept in design and range from small to large and complex trials with adaptive protocol and several parts.
- Most of the study drugs are small molecules, but we also do many trials of biological products, such as recombinant proteins, monoclonal antibodies, vaccines, and small interfering RNA molecules.
- Most of our trials are in healthy subjects, but many include patients with the target disease; some trials involve only patients.
- We have a large database of healthy subjects of all ages and ethnicities, and many types of patient.
- We collaborate with London universities, hospitals, and general practices for trials requiring patients.

Why choose HMR?
- We don’t have a business development team. We rely solely on reputation, recommendations, record, and repeat business, a unique testament to the quality of our services.
- The UK is a good place to do clinical trials: the regulatory system is rigorous, efficient, and supportive of clinical pharmacology and sponsors and can help sponsors to plan their FIH trials.
- The MHRA and FDA inspectors have never reported any important GCP or GMP findings.
- Our pharmacy prepares and QP releases all types of dose. Our laboratory provides rapid turnaround of safety samples, and a wide range of biomarkers, such as flow cytometry, cytokine, coagulation, platelet aggregation, and PCR tests.
- We developed TOPS, a database to register participants in UK phase 1 trials; use is now mandatory for regulatory approval of phase 1 trials.
- We PCR test all subjects for SARS-Cov-2, and admit only negative subjects. We PCR test all front-line staff regularly. So far we’ve kept the virus out of our trials.
- Together with sponsors, we publish results of our trials in peer-reviewed journals.
HMR offers Biomedical Scientist Apprenticeships
HMR offers Biomedical Scientist apprenticeships with a BSc (Hons) in Applied Bioscience from the University of...
HMR achieves ISO 27001 information security certification
HMR attained ISO 27001 information security certification in November 2021. This further builds on our Cyber Essentials...
HMR data expertise contributes to improved NHS bowel cancer screening
Our comprehensive Statistics and Data Management service has contributed to a pioneering, improved, bowel cancer...
HMR offers Clinical Trials Specialist apprenticeships
HMR offers Clinical Trials Specialist apprenticeships with a BSc in Applied Bioscience from the University of Kent. We...
HMR one of the first 5 labs in UK to be accredited for COVID-19 testing
The United Kingdom Accreditation Service (UKAS) has announced that HMR’s central laboratory is one of the first 5...
HMR provides Covid-19 testing for NHS
HMR is volunteering laboratory services, free of charge, to the NHS, as part of the effort to test front-line staff for...
Privacy Overview
+44 (0) 1753 578 080
Scientific Advisory Board
Our Services
Clinical Trial Management Services
Central laboratory services, clinical pharmacology.
Scintigraphy
Pharmacovigilance
Regulatory Affairs
Clinical & Medical Writing
Site Management & Monitoring
IMP Management
Biometric Services
Bioanalytical Laboratory Services
Routine Safety Testing
Immunoassays
Our Expertise
AI : Causal Modeling
CNS Indications
Oncology, including Rare Oncology
Rare & Ultra-Rare Disease Trials
White papers
Case Studies
Clinical Trial Publications
- Latest News
- Join Our Team
View About Us
View Our Services
View Clinical Trial Management Services
View Central Laboratory Services
View Our Expertise
View Resources
A Global CRO Delivering early to late phase clinical trials
Say hello to a responsive, specialist, flexible approach
We are Simbec-Orion, who for almost five decades, have been delivering clinical trial management services across a wide range of therapeutic indications and phases. The clinical research organisation with a flexible, specialist approach, we strive to become a trusted partner for our clients. Our passion as a CRO is rare diseases and oncology.

Our latest on-demand Webinar
The value of Early Regulatory Engagement: How Scientific Advice Can Support your Clinical Development
In our latest webinar, Dr Kirsty Wydenbach, Head of Regulatory Strategy, Weatherden and valued member of our independent Drug Development Advisory Board, shares her insights on the benefits of early, proactive engagement with regulators through Regulatory Scientific Advice.

Our experience defines our expertise
Responding to the evolving needs of our clients has made us the contract research organisation we are today. Offering a full-service clinical development portfolio, but with the size, agility, and structure to respond rapidly when needed. With a team of experienced management, clinical research and scientific advisory specialists, we deliver precise clinical development solutions with expertise.
From First-in-Human phase I, including a full range of late-phase clinical pharmacology studies, through to phase III studies, central laboratory services, and post-marketing, we are the CRO ready to take on the challenge. Whatever the indication or compound you are passionate about, wherever you are in your clinical development journey, we will act as an extension of your team to manage every element of your clinical trial.
Integrated, full-service support
Fabrice Chartier, CEO shares insights into what makes Simbec-Orion a unique Contract Research Organisation.
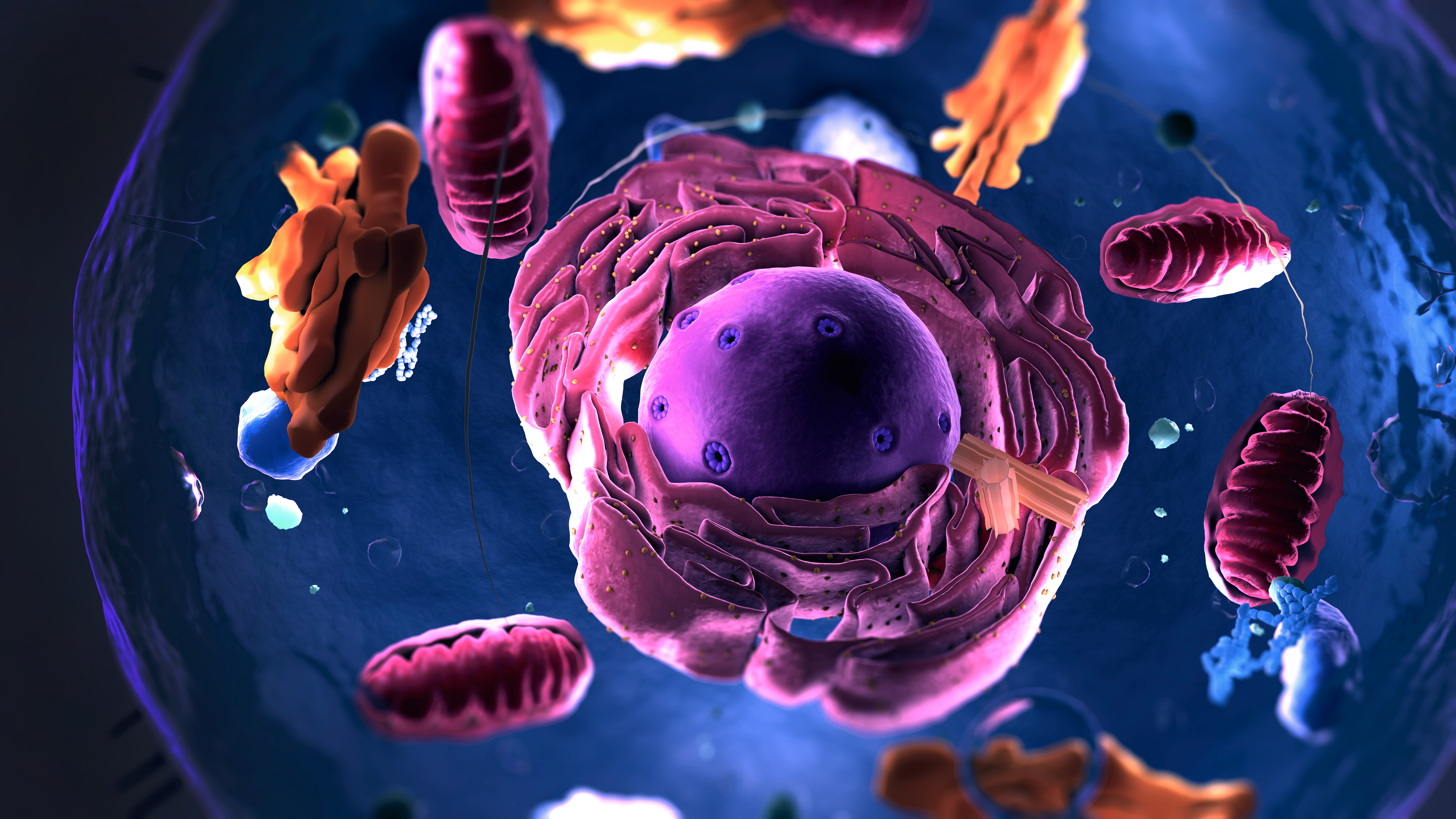
Our clinical development solutions
We support clients with a range of functional and full-service clinical development solutions. Our wide range of clinical services include clinical trial management, clinical pharmacology, central laboratory support, and more – all customised to your project’s specific needs. Find out more about our clinical development services.

As a full-service facility, we carry out all types of studies at our purpose-built site. 90% of our work is with Investigational Medicinal Products, in all drug delivery routes.
Clinical pharmacology is a key part of our CRO services, so if you need support managing a clinical trial, there are many ways our expertise can help.
Our expert biometrics team delivers accurate and adaptive clinical data management services that support the success of your drug development programme.
Conducting statistical analysis, planning, and reporting across all clinical phases, they will create a quality biostatistical framework that presents clinical trial data clearly and correctly for regulatory agencies.

We are a CRO with in-depth experience in the most challenging therapeutic areas, including oncology, rare and orphan diseases, respiratory, dermatology, vaccines and anti-infectives.
With more than 25 years’ experience in the clinical research sector, we offer a comprehensive service you can trust – with an international reach. All with the highest level of quality, agility, and responsive customer service.
Therapy areas in detail
The clinical research organisation with wide therapeutic experience, specialising in rare disease and oncology clinical development solutions.
Rare Diseases
Therapeutic Areas
Pinpointing our clinical trials around the world
Headquartered in South Wales, UK, we work on an international scale and are currently supporting Phase I-IV clinical trials across:
Employees with international reach

What our clients say about us
The people working at Simbec Orion are dedicated, very professional and they cover all aspects of clinical research. A team will be set up for your project, who will have a strong focus on your study in a very open minded and friendly atmosphere. The studies I have outsourced to Simbec Orion, have been successfully managed and performed. I will use Simbec Orion in the future and I can strongly recommend them to other pharmaceutical companies.
Tina Lidén Mascher, Head of Clinical Operations
Got a question? Get in Touch or Submit an RFI/RFP
Our team is on hand to answer any questions you might have relating to the work we do at Simbec-Orion. Just fill in the form below, making sure to tick the boxes that apply, and we’ll respond as quickly as we can.
Looking to volunteer for a clinical trial?
Visit our dedicated volunteer website here , or email [email protected]
Follow us on Social Media
+44 (0) 2036426654 [email protected]
Specialists in adaptive approaches for successful clinical trial delivery
“Together we make it happen”
Trusted CRO partner to world leading organisations
PHARMExcel is an award-winning, full-service Contract Research Organisation (CRO) providing a flexible and innovative approach to clinical trial delivery.
The company offers in-depth knowledge and experience of the clinical research environment, particularly in the UK, and has a network of regulatory, and other industry associates, allowing us to provide a global reach.
Delivering excellence at all times

Forging Paths: A Closer Look at Rare Disease Clinical Trials in the UK

PHARMExcel Secures Top 5 Spot Among Clinical Management Solutions Providers in the UK

A CEO Reflection: Raising the bar in clinical research outsourcing

How PHARMExcel Collaborated with ECMC to Deliver a New Revolutionary Radiation Therapy
Contact us today
We know that successful partnerships can only be advanced where there is a true understanding of requirements and expectations. PHARMExcel welcome the opportunity to learn more about your specific needs so we can best determine the right delivery approach for you.
|
© Copyright 2024 PHARMExcel | Website by Union 10 Design

Driving innovation
In clinical research, in clinical research.

At the forefront of drug evolution
-min.jpg)
We are Richmond Pharmacology.
Centre of excellence., work with us.

Meet the experts
-min.jpg)
Richmond Pharmacology has a team of experts with extensive knowledge of clinical research trials.
Careers at Richmond

We are looking for people who love what they do, strive for excellence and who want to make a difference.
.jpg)
Learn more about Richmond Pharmacology by viewing some of our videos.
Latest news
Single-dose gene silencing cure for fatal heart disease comes one step closer, how does social time contribute to a positive workplace culture, isa symposium 2024.

Knowledge Base
Connect with us.


From start to finish Ixia Clinical will expertly manage all your clinical project requirements
Clinical Research Organisation – Ixia Clinical
IXIA CLINICAL is an adaptable Clinical Research Organisation (CRO) based just outside London, UK, with a team of experienced clinical professionals operating globally in the pharmaceutical and biotechnology industries. Our expert networks of knowledgeable clinical research consultants can provide comprehensive assistance to support your clinical trials in the UK, Europe, the USA and the rest of the world.
Our staff are experienced in managing clinical projects right from conception to completion. We are experts in gaining regulatory and ethics approvals. Having supported numerous clients both in the preparation for and throughout regulatory audits and inspections, we are the perfect partner to advise and assist you.
We will work closely with your team to achieve the results desired and meet the challenges your project presents.
At Ixia Clinical we are experts in the planning, management and delivery of phase II, phase III and phase IV clinical trials, registry studies and pivotal or post-approval medical device studies across a wide variety of therapeutic and specialist areas.
Our experience allows us to provide highly customised solutions to meet your detailed objectives. These solutions can be delivered as a full service project solution, or if you prefer we are also used to working as part of a wider network to deliver solutions as part of a larger team.
Clinical Study Project Management Services
Our business is founded on providing a highly organised approach to clinical study project management. Our approach is defined by our unique processes which are highly structured and documented in straightforward, user-friendly standard operating procedures which enable us to deliver a comprehensive and successful solution on every project.
We regularly monitor and report on progress throughout the project life-cycle ensuring each project is kept within agreed timelines and our project costs adhere to agreed project budgets at all times.
Regulatory & Ethics Submissions
At Ixia Clinical we are highly experienced at dealing with regulatory bodies, ethics committees and other institutional bodies. We are experts in skilfully navigating the requirements for the preparation and submission of trials for ethical and regulatory and consideration in the shortest possible timeframes.
Our team provide regulatory support at all phases of the product life-cycle. We are flexible to meet the needs of your project and can advise on the most effective solutions and strategies to achieve your project objectives.
Clinical Trial Set Up and Monitoring
The set up process and ongoing continued monitoring of clinical trials and medical device studies is the bedrock of our business. Covering the process from study conception right through to project completion, our team are highly skilled at all facets of the clinical trials and medical device studies process.
From evaluating the suitability of investigators and their sites, through site set up and patient recruitment, to the clinical monitoring process and finally the completion and closure of the trial on site. Our team are highly experienced at managing the clinical trials and medical device study process at all phases of the development life-cycle.
Clinical Study Feasibility
Ensuring the viability of studies is critical during the funding phase and our team are experts at helping to identify whether a trial can be delivered effectively. Our excellent working relationships with some of the most influential and high profile consultants, not just in the UK but worldwide, enables us to deliver comprehensive feasibility studies for projects across multi disciplines and requirements.
Site Assessment & Selection
For the successful completion of trials, it is critical that the investigator sites chosen are able to manage the trial process on time and within budget to ensure the high quality production of data.
We work with teams across the UK and beyond to help select sites and investigators that are highly competent and deliver excellent levels of data for every trial we manage.
Patient Recruitment
Ensuring successful patient recruitment is key to the success of any trial and our record of achieving agreed patient recruitment targets is excellent.
With careful project planning at the outset, followed by a comprehensive ongoing problem identification and resolution process, we are able to resolve recruitment challenges quickly and effectively resulting in a successful rate of patient recruitment and retention.
For more information about our clinical study and medical device study procedures and how we can help you achieve your study objectives, please contact our team .
Latest news
Health research funders provide £4.8bn a year to improvement of human health, shows ukcrc report.
A new report commissioned by the UK Clinical Research Collaboration has found that UK research funders provided £4.8bn of funding

Welcome to the UK Clinical Research Collaboration
Strengthening experimental medicine.
The major funding bodies within the UKCRC have undertaken several joint initiatives to strengthen research in experimental medicine.

UKCRC Registered Clinical Trials Units
A Registration Process has been established to recognise CTUs that have the expertise to centrally coordinate high quality multi-centre clinical trials.

Health Research Analysis
The Health Research Classification System was used to analyse the health research portfolios of the UK’s government and charity funders in two major reports.

Joint Funding Initiatives
The UKCRC provides a forum to facilitate coordination between funding bodies in order to address barriers and maximise opportunities in health research in the UK.

Regulation and Governance
The UKCRC Partners have established initiatives to streamline the regulatory and governance environment in the UK.

Clinical Research Networks
Clinical research networks have been established across the UK to provide the infrastructure to support high quality clinical research studies.

The UK Clinical Research Collaboration (UKCRC) Partners' goal is to establish the UK as a world leader in clinical research. The UKCRC provides a forum that enables all Partners to work together to transform the clinical research environment in the UK.
The forum promotes a strategic approach to the identification of opportunities and obstacles to clinical research and their resolution. In so doing the UKCRC aims to benefit the public and patients by improving national health and increasing national wealth.

Top 17 Clinical Research Organizations (CRO) in 2023
In clinical research and treatment development, clinical research organizations (CROs) are frequently a sponsor’s most important partner and ally.
Depending on the nature of the clinical trial, and your existing capabilities as a sponsor to run the trial, the CRO company of your choice will typically be responsible for facilitating most of the micro and macro processes that go into designing and running a successful clinical trial.
When contracting a CRO to help you with your trial, you are transferring over a large portion of responsibility into the hands of your clinical research partner. The CRO of your choice will have the responsibility to control a variety of factors and processes of a clinical trial, and depending on their expertise, team structures, service offerings, internal resources and many other capabilities.
Your ability to find and contract a top CRO company that is the right fit for your unique trial will be a determinant of whether or not you will be able to operate a high-quality clinical trial that meets your expected timelines, budget and delivers a top-notch patient experience.
At ClaraHealth (a patient-centric recruitment acceleration platform) , we have put together an extensive list of the top CRO companies in the US and around the world.
This is not a cro rankings list, but rather a compiled list of some of the top clinical research organizations around the world. We have highlighted their strengths and core service offerings to make it easier for you to find the right fit clinical research partner.
In addition, we’ve put together a list of 9 fundamental questions to ask the prospective clinical research organization , which will help you to save time and ensure a right fit in picking the CRO.
Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research.
The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
Some clinical trial solutions offered by IQVIA include:
- Assistance with protocol design
- Design of phase 1 clinical trials
- Assessment and improvement of phase 2 and 3 clinical trials
- Site identification & selection
- Patient recruitment
- Access to global laboratories via their wholly owned subsidiary Q2 Solutions
Parexel is a global clinical research organization that was founded in 1982, and specializes in conducting clinical studies on behalf of its pharmaceutical partners in order to accelerate and ensure the drug approval process of up-and-coming potential treatments. It currently operates in more than 50 countries, and is run by more than 18,000 employees around the world.
The company has a wide range of service offerings, covering nearly every type of clinical trial service to assist sponsors in running successful clinical studies.
Some clinical trial solutions offered by Parexel include:
- Clinical trial design and development for early phase, phase 2 & 3, and late phase clinical trials
- Clinical data management
- Decentralized clinical trials
- Clinical supply chain management
- Medical writing
- Regulatory affairs consulting
- Pharmacovigilance
3. PRA Health Sciences
PRA Health Sciences is one of the largest contract research organizations in the world. Founded in 1976 under the name “Anti-Inflammatory Drug Study Group”, the company was renamed to PRA in 1982. PRA Health Sciences employees more than 17,000 people, and provides coverage to more than 90 countries.
In 2021, PRA Health Sciences was acquired by the Ireland-headquartered global CRO leader ICON, which is also reviewed in this list.
Some clinical trial solutions offered by PRA Health Sciences include:
- Decentralized Clinical Trials Platform
- Protocol Consultation & Study Design
- Onsite Support services
- Customized Solutions for Biotech (such as asset valuation, regulatory strategy, engagement and support, drug development strategy and funding solutions)
- Clinical Diagnostics
- Site Commercial Solutions
- PRA’s Laboratories for Drug Development
Headquartered in Ireland, ICON was founded in 1990 in Dublin by co-founders John Climax and Ronan Lambre. The company has since grown to be one of the largest CROs in the world. As of September 2020, the company employs more than 15,000 people in 94 locations and across 40 countries.
ICON offers clinical research services which include consulting, clinical development and commercialization across a wide range of therapeutic areas.
In 2021, ICON acquired PRA Health Sciences, which is another CRO and global leader in clinical research services.
Some clinical trial solutions offered by ICON:
- Commercial Positioning
- Early Phase
- Functional Services Provision
- Laboratories
- Language Services
- Medical Imaging
- Real World Intelligence
- Site & Patient Solutions
- COVID-19 Clinical Operations
5. Syneos Health
Formerly known as InVentiv Health Incorporated and INC Research, Syneos Health is a publicly listed and global contract research organization. The company is based in Morrisville, North Carolina, and specializes in assisting companies with late-stage clinical trials. Syneos Health currently employs more than 25,000 people, and has offices across 91 locations.
In early 2018, INC Research was acquired inVentiv Health, and the merged company was named Syneos Health.
Some clinical trial solutions offered by Syneos Health include:
- Decentralized Clinical Trials Solutions
- Bioanalytical Solutions
- Phase II-III/Phase IIIb-IIIV
- Medical Device Diagnostics
- Clinical Data Management
- Clinical Project Management
- Clinical Monitoring
- Drug Safety & Pharmacovigilance
- Site and Patient Access
6. Labcorp Drug Development (Formerly Covance)
Formerly known as Covance and renamed to Labcorp Drug Development in early 2021, this CRO is one of the largest contract research organizations in the world. The company claims to provide the world’s largest central laboratory network, and has been rated as one of the best places to work for LGBTQ+ equality by the Human Rights Campaign organization in 2018 to 2021. Currently, Labcorp employs over 70,000 people and is able to support clinical research efforts in almost 100 countries around the world.
Some clinical trial solutions offered by Labcorp Drug Development include:
- Preclinical Services
- Clinical Trials
- Clinical Trial Laboratory Services
- Post-Marketing Solutions
- Medical Devices
- Data & Technology
Also known as Pharmaceutical Product Development, PPD is a large global contract research organization headquartered in Wilmington, North Carolina. Started as a one-person consulting firm in 1985, PPD has grown to over 27,000 employees worldwide, and provides a wide range of clinical research services to pharmaceutical and biotech companies.
Some clinical trial solutions offered by PPD include:
- Clinical Development
- Early Development
- Peri- and Post-Approval
- PPD Biotech
- PPD Laboratories
- Product Development and Consulting
- Site and Patient Centric Solutions
8. Fisher Clinical Services
Part of Thermo Fisher Scientific, Fisher Clinical Services is a global clinical research organization with headquarters in Center Valley, Philadelphia.
The company has been in the business of clinical supply chain management for over 20 years, and is focused exclusively on working with the packaging and distribution requirements of clinical trials across the globe.
Some clinical trial solutions offered by Fisher Clinical Services include:
- Biologistics Management
- Cell & Gene Therapy
- Clinical Ancillary Management
- Clinical Label Services
- Clinical Trial Packaging & Storage
- Clinical Supply Optimization Services
- Cold Chain Management & Expertise
- Direct-to-Patient
- Distribution & Logistics
- Strategic Comparator Sourcing
- Public Health Research
Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
KCR operates globally, and has offices in North America, Western Europe, Central Europe and Eartern Europe. The company currently employs more than 700 staff.
Some clinical trial solutions offered by KCR include:
- Trial Execution
10. Medpace
Founded in 1992 and based in Cincinnati, Ohio, Medpace is a midsize clinical contract research organization. The company has operations in over 45 countries, and employs over 2,800 people. Medpace provides support services for Phase I-IV clinical trials for pharmaceutical and biotechnology companies, which include central laboratory services and regulatory services.
Some clinical trial solutions offered by Medpace include:
- Biostatistics and Data Sciences
- Clinical Trial Management
- Drug Safety and Pharmacovigilance
- Medical Writing
- Quality Assurance
- Regulatory Affairs
- Risk-Based Monitoring
- Medpace Laboratories
11. Clintec
Now in business for over 22 years, Clintec is a medium-sized global contract research organization for pharmaceutical, biotech and medical device industries, with large expertise in oncology and rare diseases.
The company provides the flexibility and agility of a smaller-sized CRO, while also having a wide global coverage that large CRO companies are known for. Clintec is based in more than 50 countries, and was acquired by the leading global CRO IQVIA in late 2018.
Some clinical trial solutions offered by Clintec include:
- Project Management
- Data Management
- Biostatistics
- Global Feasibilities
- Patient Recruitment & Retention
12. Worldwide Clinical Trials
Bringing over 30 years of experience to the clinical research market, Worldwide Clinical Trials is a leading medium-sized global contract research organization. Founded by physicians with a dedication and commitment to advancing medical research, Worldwide Clinical Trials was the first customer-centric CRO.
Currently the company has coverage in more than 60 countries, and has extensive experience in a wide range of therapeutic areas, including central nervous system, metabolic, cardiovascular, oncology, rare diseases and general medicine.
Some clinical trial solutions offered by Worldwide Clinical Trials include:
- Bioanalytical Lab
- Early Phase Development
- Clinical Phase IIB-II Clinical Trials
- Phase IIIB-IV Clinical Trials
- Trial Management Technologies
Named #1 CRO in the world for operational excellence at the 2021 CRO Leadership Awards, CTI Clinical Trial And Consulting Services is a medium-sized global contract research organization that has been serving pharmaceutical companies since 1999.
Based in Covington, Kentucky, CTI has offices around the world in more than 60 countries, with coverage in North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific regions.
Some clinical trial solutions offered by CTI include:
- Feasibility
- Regulatory Affairs Study Start-Up
- Medical Monitoring
- Safety & Pharmacovigilance
- Clinical Services
14. Wuxi AppTec
Founded in 2000 as WuXi PharmaTech in the city of Wuxi, China, Wuxi AppTec has grown from a single laboratory into a leading global contract research organization with more than 28,000 employees, including 23,000 scientists and more than 30 research & development and manufacturing sites around the world.
With offices in Asia, U.S, Europe and the Middle East, the company is able to provide coverage to more than 30 countries around the world.
Some clinical trial solutions offered by Wuxi AppTec include:
- Small Molecule Drug R&D and Manufacturing
- Cell Therapy and Gene Therapy
- Drug R&D and Medical Device Testing
- Clinical Services (Phase I-IV)
15. Advanced Clinical
Founded in 1994 and based out of Deerfield, Illinois, Advanced Clinical is a midsize and full-service CRO that helps sponsors with running clinical trials. The company employs more than 700 staff, and offers a wide variety of services across many therapeutic areas. Advanced Clinical has global representation in over 50 countries around the world.
Some clinical trial solutions offered by Advanced Clinical include:
- eTMF & Document Management
- Global Medical Services
- Quality & Validation
16. Pharm-Olam
Pharm-Olam is a leading midsize CRO with global headquarters located in Houston, Texas and its European headquarters in Bracknell, United Kingdom. The company employs more than 800 staff, and has 25 offices around the world, with a global coverage in more than 60 countries.
The company has therapeutic expertise in 5 areas, including Rare & Orphan Disease, Infectious Disease & Vaccine, Oncology-Hematology, Allergy and Autoimmune.
Some clinical trial solutions offered by Pharm-Olam include:
- Study Feasibility
- Site Activation
- Patient Recruitment
- Medical Affairs
- Compliance & Training
- Clinical Monitoring & Operations
17. Clinipace
Founded in 2003 and based out of Morrisville, North Carolina, Clinipace is a global midsize full-service CRO with a focus on solution customization for clinical trials. The company has a large global coverage in more than 50 countries, and has offices in North America, South America, Europe and Asia-Pacific regions.
Clinipace’s therapeutic focus areas include Oncology, Nephrology and Urology, Rare Disease, Gastroenterology and Women’s Health. The company also has complete therapeutic expertise in Infectious Disease & Vaccines, Cardiology, CNS, Immunology, and Respiratory.
Some clinical trial solutions offered by Clinipace include:
- Clinical Analytics
- Clinical Technology and Ecosystem
- Functional Service Partnership (FSP)
- Regulatory & Strategic Product Development
9 Fundamental Questions To Ask A Top CRO Company Before Signing The Contract
1. which services does the cro provide.
CROs offload a lot of operational tasks from trial sponsors, which can touch any component of clinical trial operations. From formulating an overall study strategy and implementing technologies to support the operational processes of the trial, to picking and identifying sites, and supporting patients during the trial, the range of clinical services offered by a CRO tends to be vast and inclusive of all the typical services and support you will require for running a successful clinical trial.
However, not all CROs are the same in their service offerings, or are able to offer the same depth of capability within a seemingly same clinical trial support process. For this reason it is important to understand exactly which kind of clinical services and support you are looking to receive from the prospective CRO when running your clinical trial.
While services such as clinical monitoring and clinical trial management are offered by the majority of CROs, the specific needs of each trial are unique, and for this reason it is important to first identify what will be the unique services your trial requires. Completing this internal analysis first will help you to understand the extent to which a potential CRO partner will be able to provide all of these services.
Some CROs specialize in specific clinical trial functions which the company may label as a “core services”, in which case this is a sign the company will have more expertise, experience, and will be set up in a way to maximize their capabilities in providing support for these services compared to other services that the CRO offers.
For example, a CRO may include patient recruitment as part of its “core services”, which implies that they are highly skilled in and have the necessary infrastructure to design and implement a high-quality patient recruitment strategy.
Clara Health CRO Support Services: At Clara Health our specialty services include technology-augmented digital and patient advocacy recruitment, as well as patient support via our signature patient recruitment platform, which we use to upgrade clinical trials and deliver results sponsors look for in their recruitment and retention campaigns.
At Clara, we work alongside CROs to supplement and support clinical trials with modern and personalized capabilities that CROs do not typically have the bandwidth, corporate structure or infrastructure to support.
If you would like to learn more about exactly how our platform can upgrade your unique trial, feel free to book a Free 30 Minute Consultation Session Here with one of our in-house experts.
2. What Related Experience Does The CRO Have?
It is helpful to ask the prospective CRO company if they have any relevant experience in running clinical trials that would be an asset in designing and running your study. Previous experience in a related therapeutic area or in running a trial with a similar design allows CROs to have a deeper understanding into potential opportunities and challenges, increasing the likelihood of your clinical study being successful.
For example, if a sponsor is planning to run a trial in oncology, for the purpose of site identification and selection it would be valuable to partner with a CRO vendor that has expertise in this area, as they likely already have a good understanding of which sites will lead to optimal results.
However, it is also important to consider all factors when selecting a CRO vendor and not to rely on therapeutic experience as the sole qualifier for whether or not a potential CRO is a fit for your trial. While previous experience is beneficial, some sponsors close themselves off from working with vendors that have not worked in their therapeutic area, which significantly limits options when choosing a CRO partner that is truly a good fit for their clinical study.
This can impact the end result of your clinical study, as sponsors that are not successful in choosing a CRO vendor that is the right overall fit may face difficulties if the needs of their clinical study aren’t being properly met.
Clara Health: We have worked to provide support for clinical trials across a wide range of therapeutic areas and trial designs. Our specialty is filling in the gaps that CROs traditionally did not have to think about, which include digital patient recruitment, patient advocacy recruitment, and technology-augmented patient support.
Additionally, we are constantly building our proprietary data and running tests in a variety of therapeutic areas. These research efforts allow us to have a detailed understanding of the expected level of difficulty when recruiting particular patient populations, as well as allow us to predict with accuracy which segments of the targeted population will be likely to qualify in a particular study.
3. What Are The Communication Workflows & Expectations For Performing And Delivering Contracted Services?
It is important that you clarify what the expectations for communication will be between your prospective CRO vendor and your internal teams, as you will most likely be working with the CRO of your choice for the entire duration of your clinical trial.
There are a vast variety of factors and success determinants for a clinical trial, which are continuously undergoing change as the study unfolds. For this reason, it is recommended that you work with a CRO that is proactive in their communication, so that you are kept up to date with information about important changes as your clinical trial progresses.
A vendor that is proactive rather than reactive in their communication and approach to dealing with arising issues is one of the most important qualities in CRO. Challenging situations will naturally arise, and the promptness with which they are taken care of will significantly impact your clinical trial’s degree of success. Therefore, seeking a vendor that is able to match the standard of communication that you as a sponsor would like to experience throughout the duration of your partnership is one of the most critical steps in determining which CRO is the right fit for your clinical trial.
We’ve included a few additional questions pertaining to the communication structure and reporting expectations that you can ask a prospective CRO vendor to determine the degree of fit in this particular category:
Communication Expectations:
- If we were to move forward with you, which of your team members will be our main point of contact?
- How available will you be outside of the scheduled meetings to address any of our concerns or additional requests?
- What will be the frequency at which update meetings will be conducted, and who will be present at those meetings?
- Which clinical study processes will be reported on, and what will be the workflow for how we will receive this information?
- What will be the cadence at which we will receive progress reports?
- Would we be able to access metrics electronically via an interactive dashboard, or will you send us formal reports?
Clara Health: At Clara Health, we directly interact and actively work with several key stakeholders involved in running a clinical trial, which includes sponsors, CROs, sites, and patients. This unique position allows us to have a centralized perspective which helps us to see all the moving parts of a clinical trial at the same time, which helps to identify issues and relay this vital information and insight back to the sponsor (or other appropriate stakeholders) in the shortest time possible.
The ability to access this perspective allows us to gather the most accurate, complete, and up-to-date information about how the clinical trial is unfolding, and quickly becomes very valuable to sponsors for their clinical trial.
As an example, we may receive feedback from patients about having an unsatisfactory experience with a particular study site. We are able to aggregate and analyze this information, and relay our findings back to the sponsor and the study site to improve the experience for other patients.
4. What Is The CRO’s Client Satisfaction Record?
It is a good practice to request information or metrics from the prospective CRO vendor that can point to the degree of satisfaction of their past clients. Prior to signing the contract, vendors will naturally do their best to uplift their image and future value to you during their sales conversations with you and your team. It can be tricky to get an objective understanding of what the partnership experience will actually entail, especially when there are multiple vendors fighting for your commitment.
We recommend that you ask the prospective vendor to provide success metrics regarding areas of clinical trial operations that are going to be important for your trial.
For example, you may be interested in learning about the vendor’s relationship to finances, in which case it will be useful to ask them about situations in which they went over the planned budget, and investigate into the reasons behind that. Alternatively you may be concerned about potential delays in timelines, in which case it would be helpful to learn about metrics regarding the CRO’s ability to meet timeline expectations.
You may also request to talk to the prospective CRO’s past clients, which will help you to gain insight into what the relationship was like and give you the opportunity to examine if the way in which the particular CRO manages its relationships and performs its services meets the expectations that you would have for your potential relationship and for your clinical trial.
Clara Health: At Clara Health, our relationships with our partners and with our patients are most important to us. In the unique position where we fit in the clinical trial process, we have the opportunity to directly co-create the clinical trial patient experience with a variety of stakeholders, including sponsors, sites, CROs, and patients.
Our company’s values and culture have been directed and developed to be such that the client and patient experience is at the top of priority for all of our internal teams, and we work to provide the best quality of care to all stakeholders.
We have many testimonials from every type of partner we’ve worked with which we can happily share with you.
5. How Do You Adapt When Encountering Challenges With Running A Clinical Trial?
It is inevitable that challenges and unforeseen changes will arise throughout the operational clinical trial process, and for this reason it is important to work with a CRO vendor that can provide you with evidence of their flexibility and ability to adapt to sudden changes.
The ideal CRO partner is one that is highly consultative throughout the entire process, and has an ability and the initiative to deal with challenges at their seed stage, prior to them turning into major obstacles for the success of your trial.
CROs naturally have a large reach, and there are a lot of different clinical trial mechanisms and processes that are under their control. They are able to monitor and respond to what is going on in every key link in the chain of the clinical trial operation.
It is reasonable to expect this level of oversight from a CRO, and additional questions that can help you gain insight into this include:
- What are some examples where the CRO was effective at monitoring the health of clinical trials they’ve helped operate in the past?
- How quickly does the CRO respond to challenges or opportunities for improving the clinical trial experience?
- How well does the CRO gather & process information from study sites, study teams, patients & the sponsor, and what are their typical data analysis workflows?
It is also recommended to speak to the prospective CROs past clients to help you gain insight into how well they respond and adapt to the naturally arising challenges in clinical trials.
Clara Health: While CROs do have a large reach within the clinical trial, no CRO has complete visibility into every clinical process. They are not typically set up to support full visibility, which can manifest as a potential threat to your clinical trial as it unfolds. This is especially true for parts of the clinical trial processes that CROs naturally do not specialize and often subcontract, such as clinical trial recruitment.
At Clara, we are in a unique position in relation to other key partners involved in operating the clinical trial. We are in direct and frequent contact with patients, CROs, study sites, study teams, and the sponsor, and have a very deep understanding of the patient pipeline. This allows us the unique ability to go very deep into specific parts of the recruitment chain and investigate what is working and what is not working.
In addition, Clara functions as a resource for all partners in the clinical trial. For example, we work directly with site teams to ensure that they have access to a 3rd party that they can relay their needs to and receive fast support in case there is anything they require that can improve the patient recruitment process.
6. Which Parts Of Operating The Clinical Trial Will You Be Outsourcing?
Since there are so many processes and mechanisms that go into operating a clinical trial, CROs will always outsource some parts of running and managing the study. While you can expect that the prospective CRO will subcontract some of the work, it is important to find out which exact parts the clinical study will be outsourced.
There are certain basic and key clinical processes (such as site selection) that CROs almost always help with, and if you find that these parts of your trial are going to be subcontracted to another company, it is recommended to find out why the CROs operations are set up this way and how this would impact the service you will receive.
Ultimately what matters to you as a partner and client is that the quality of service and care that you will receive will be up to standard, and meet what was promised and what you are expecting. While this trust is important after you have signed the contract, it is recommended that prior to entering into such a significant commitment that you have evidence and the conviction that the CRO of your choice is truly the right fit and will deliver the quality of service that was being discussed.
Since it is impossible to predict exactly what the quality of this relationship and services performed will actually be like in practice, it is recommended that you understand the details of what will be done for your trial and how. Investigating how the CRO outsources and subcontracts services for a clinical trial will help you to gain necessary insight that you would need to make the correct vendor selection decision.
Clara Health: At Clara, we maximize the effectiveness of the digital component across the entire digital & recruitment spectrum, which is added on top of the existing capabilities of the CROs and other vendors involved in operating your clinical trial. In addition, we offer services that augment the CROs efforts, which has the potential to significantly improve the patient experience, operations flows, recruitment and retention performance, which is so important in ensuring the success of a clinical trial.
For example, if a CRO wants to have a great site relationship, we are able to come in as a third party on behalf of the sponsor and CRO and act as a resource and additional support for sites.
In another example, If a sponsor wants to have great relationships with the patient community, Clara is able to come in on behalf of the sponsor and develop these relationships while being perceived more neutrally by the patient community.
7. Do You Have Experience Running International Trials?
If you are planning on operating an international clinical trial, it is recommended to work with a CRO that has extensive experience in this area. While many CROs will offer near-global coverage, the level of experience with specific geographic locations can significantly vary from one vendor to another.
It is important to work with a CRO that has experience running clinical trials in the specific countries and regions you are planning to conduct your research in. Being compliant with the local rules and regulations for clinical testing is a very complex process that requires existing understanding and familiarity in order to ensure logistical smoothness and to mitigate legal risks. In operating a clinical trial, there are a multitude of clinical services and processes, which can greatly vary across the many regions in which you can conduct clinical testing.
A CRO that is lacking experience in operating international trials or operating in particular regions where you plan on conducting research may not be able to meet your desired quality and agility expectations, and therefore may not be the right fit for your international clinical trial.
Clara Health: In the past, we have provided international patient recruitment and digitally-augmented trial support services for clinical trials in the EU, Canada, UK, Australia and South America.
Clara Health is fully compliant to operate international studies everywhere in the world, with the exception of Russia and China.
8. What Is Your Relationship With Patients?
Patient-centric approach to designing and operating a clinical trial is becoming more and more crucial in the clinical research space. The ability of a sponsor and their CRO partner to understand the needs and characteristics of their target patient community is a significant determinant of whether or not the study will be a success.
A sponsor that has close and authentic relationships with the patient community tends to have a deeper understanding of how to create the best clinical trial experience that will attract patients and keep their interest throughout the clinical trial.
In addition, strong relationships with patients allow sponsors and CROs to forecast recruitment and patient retention pipeline with much higher accuracy. This ability is critical for ensuring the success of the trial and mitigating the risk of low enrollment. After an understanding of the patient population is acquired, sponsors gain the necessary insight to design a clinical trial that is not only favorable to their research results, but is also practical and will result in the enrollment numbers they are looking for.
While many CROs have already recognized the importance of patient-centricity and evolved the ways in which they design and operate clinical trials, other CROs have not yet made such a pivot in their values. It is important to understand the degree of importance the prospective CRO places on creating a favorable patient experience, and what kind of infrastructure the company has to support it.
At Clara, we recommend choosing a CRO partner that is adapting to the patient-centric model which is becoming more and more important for running a successful clinical trial.
Clara Health: Since early stages of our development, we’ve had a dedicated patient advocacy team that has been integral in shaping our company’s vision and operations. We have built our entire platform and recruitment infrastructure around creating the best experience for patients. Our teams, corporate values, service offerings and company infrastructure all work in the service of the patient.
In addition, over the many years of being in business we have heavily invested in building authentic patient community relationships that span across a variety of therapeutic areas. This has given us a unique ability to receive feedback directly from patients that is genuine and authentic around marketing materials, strategy for patient recruitment, and other services that we build for specific trials.
This ability to build partnerships with the patient community in an authentic way gives us a very unique ability to engage with the patient community on behalf of a pharmaceutical company, allowing our sponsor & CRO partners the opportunity to start conversations with patients through our in-house patient advocacy team.
If you would like to learn how Clara can help you to build a strong & authentic relationship with your target patient community, get in touch with us and we’d be happy to share our capabilities and previous results with you as they relate to your current or upcoming clinical trial.
9. How Is The CRO Going To Utilize Patient Input For Developing The Trial?
In the initial stages of clinical trial design, sponsors often determine the ideal patient profiles that would help them to drive the most favorable research outcomes for their study. While it is important for the success of your trial to determine who your ideal patients are, very often these projections do not match up with what is viable in practice.
At Clara, we often encounter study protocols that are not set up realistically for successful recruitment to be possible.
Common mistakes that are made when determining trial eligibility criteria and trial design include:
- Overestimating the interest in the clinical trial from the target patient population
- A lack of patient focus in the trial design
- A lack of convenience for patients in their participation
- Complicated and/or inefficient study experience flows
- Crafting the eligibility criteria around the patient population that is most likely to lead to favorable study outcomes, without conducting sufficient research to more accurately estimate the recruitment and retention difficulty of the group for a particular study
It is natural for there to be a “push & pull” between the research ideal and the real world practicality. It is important to determine the correct balance between these two sides for your trial, as going too far in either direction will decrease the chance of your clinical study’s success.
The nature of the industry as it is right now is such that there is excess research idealization and not enough emphasis on patient centricity. This distorted orientation has resulted in many clinical trials being unsuccessful, negatively impacting sponsors, patients and the entire clinical trials industry.
The ideal CRO partner should help you make sure that your protocol design sets your study up for success. The CRO should be able to help you determine the proper balance between the research ideal and the real world practicality, and back up their findings with sufficient research and patient data that can project your trial being a success.
Clara Health: When formulating a recruitment and retention plan for our clients, we begin with conducting thorough research into the target trial patient population. This allows us to get a clear understanding of which recruitment channels will yield the best results and what kind of marketing materials will resonate with the prospective study participants.
To ensure accuracy and real-world applicability of our research, we consult and collaborate with our internal patient advocacy and patient support teams, as well as with our clients and patients representing the target trial patient profiles. We then tie our findings back with any existing proprietary data that we have in connection with the therapeutic area or the prospective target patient group.
Our unique position within the clinical recruitment chain gives us the presence and deep-rooted access needed to effectively tap into any of the three patient traffic sources: digital recruitment, offline recruitment, or patient advocacy recruitment.
Once a recruitment campaign has gone live, we constantly monitor, analyze and optimize our performance to make sure that the processes we have in place are as efficient as possible and drive the greatest results. In addition, we have the capability to layer in any traditional advertising (such as billboard ads) if requested by the study sponsor.
Clinical Research Organization (CRO) for Clinical Trials in the United Kingdom
Patricio ledesma, contract research organization, 7 june, 2021.
If you need a CRO to manage a clinical trial in the UK, please contact us here
Are you a biotech or pharmaceutical company planning a clinical trial in the United Kingdom?
Sofpromed is a clinical research organization (CRO) with clinical trial management capabilities in the UK.
We help biotechnology and pharmaceutical companies worldwide conduct phase I-IV clinical trials with drugs and medical devices in the United Kingdom.
Clinical Trial Management Services in the UK
Sofpromed offers the full range of CRO services needed to carry out a clinical trial in the United Kingdom.
Our service portfolio includes:
- Regulatory affairs
- Clinical trial submissions to MHRA
- Clinical trial submissions to ethics committees
- Site selection and activation in the UK
- Clinical site management
- Onsite monitoring
- Clinical data management
- Biostatistics and statistical programming
- Pharmacovigilance
- Drug logistics
- Biological sample management
- Medical writing
- Project management
- Clinical trial auditing
We help US, EU, and Asian sponsors identify the best sites in the United Kingdom to execute their clinical studies.
We provide local regulatory and clinical operations staff (e.g. regulatory specialists, clinical research associates, clinical trial assistants, clinical project managers) to take care of clinical trial start-up, reporting, monitoring, and site management tasks in hospitals located across the United Kingdom.
We also provide specialized data management and statistical programming services to ensure the best quality of data in your clinical trial.
Clinical Trial Services in the United Kingdom in Multiple Therapeutic Areas
Sofpromed is able to manage clinical trials in the UK in multiple therapeutic areas, including the following, among others:
- Cardiovascular
- Central nervous system (CNS)
- Dermatology
- Infectious diseases
- Respiratory diseases
Although Sofpromed can handle trials in multiple diseases, we have particular expertise in managing clinical trials in cancer.
Therefore, Sofpromed is the perfect CRO partner to conduct oncology clinical trials in the United Kingdom.
We manage clinical trials in some of the UK’s top clinical sites like the Institute of Cancer Research (ICR) and The Royal Marsden NHS Foundation Trust, in London.
Why Conducting a Clinical Trial in the UK?
The United Kingdom is one of the top countries in the world to carry out clinical trials.
As of June of 2021, the American clinical trial database ( clinicaltrials.gov ) displays a total of 20,182 registered clinical studies for the United Kingdom, which shows the leadership of the UK in the global clinical research market.
Together with world-class research funders, the NHS, research charities and global pharmaceutical companies, the UK strives to continue to be a leader in clinical research.
The pharmaceutical industry invested £4.5 billion in UK R&D in 2018, with the global industry predicted to spend $232.5 billion a year on R&D by 2026 [1] .
Since 2017, Phase I clinical trial activity has declined in the UK, with 95 trials initiated in 2018, compared with 117 in 2017. This is a trend seen across European comparators, with the UK maintaining its lead in Europe for Phase I trials. For Phase II, the UK ranks second globally, with 268 trials initiated in 2018, compared with the USA, ranking first with 1,021 trials initiated [2].
The UK’s strong oncology research environment ranks it second in Europe, behind Spain, and fourth globally, with 226 trials initiated in 2018. For research in diseases of the nervous system and infectious diseases, the UK leads in Europe, with 66 and 46 clinical trials initiated in 2018, respectively [2].
Get CRO Support to Run Your Clinical Trial in the United Kingdom
Sofpromed provides full clinical operations support to sponsors planning clinical trials in the UK. We can manage your study from beginning to end.
We help biotechnology and pharmaceutical companies around the world achieve quick clinical trial start-up in the United Kingdom, providing high-quality services in regulatory affairs, clinical operations, pharmacovigilance, data management, biostatistics, and drug logistics, among others.
Do not hesitate to contact us if you need support to run your next clinical study in the UK.
For more information about conducting clinical trials in the United Kingdom, please contact us at: [email protected]
References:
[1] EvaluatePharma. EvaluatePharma World Preview 2020, Outlook to 2026 [Internet]. Evaluate.com. 2020 [cited 2020 Aug 4]. Available from: https://www.evaluate.com/thought-leadership/pharma/evaluatepharma-world-preview-2020-outlook-2026
[2] Clinical trials – How the UK can transform the clinical research environment

Patricio Ledesma ( B.Eng Concordia University, Montreal, Canada and Master’s Degree in Clinical Trials, University of Seville, Spain) is the Head of Clinical Operations and Founder at Sofpromed CRO. Patricio is a professional consultant providing comprehensive, one-stop expert advice and guidance to biotechnology and pharmaceutical companies worldwide in the field of clinical trials and drug development. He is personally and enthusiastically devoted to helping biotech Chief Executive, Operations, Scientific, Medical, and Regulatory Officers in the planning and execution of phase I-IV clinical trials across North America, Europe, Asia-Pacific, Latin America, and Middle East regions. You can contact Patricio at: +34 607 939 266 [email protected]
To our articles and updates
- Follow Follow
You may also be interested…


What Are Community-Based Clinical Trials?
Community-Based Clinical Trials
If you are a Sponsor seeking to run a community-based clinical trial in underserved populations, please contact us at [email protected] Clinical trials are fundamental to advance medical knowledge and improve patient care. However, one significant challenge in...

Clinical Research Site Networks: Accelerating Clinical Trials
Site Networks
If you are a Sponsor seeking to run a clinical trial through a clinical research site network, please contact us at [email protected] Clinical research plays a central role in advancing medical treatments and improving healthcare outcomes. To ensure the smooth...

How Community-Based Clinical Trials Are Advancing Healthcare in Underserved Populations
If you are a Sponsor interested in running a community-based clinical trial in underserved populations, please contact us at [email protected] Clinical trials are instrumental in advancing healthcare by evaluating the safety and effectiveness of new treatments and...

Advantages of Clinical Research Site Networks in North Carolina
If you are a Sponsor interested in running a clinical trial through a clinical research site network in North Carolina, please contact us at [email protected] Clinical research plays a pivotal role in advancing medical knowledge, improving patient care, and driving...

Benefits of Clinical Research Site Networks in Illinois
If you are a Sponsor interested in running a clinical trial through a clinical research site network in Illinois, please contact us at [email protected] Clinical site networks play a central role in advancing medical research and improving patient care. In this...

Advantages of Clinical Research Site Networks in Pennsylvania
If you are a Sponsor interested in running a clinical trial through a clinical research site network, please contact us at [email protected] Pennsylvania is a hub for clinical research, with numerous reputable clinical site networks offering a wide range of trials to...

Benefits of Clinical Research Site Networks in New York
If you are a Sponsor interested in running a clinical trial through a clinical research site network in New York, please contact us at [email protected] New York, with its vibrant healthcare landscape, is home to several prominent clinical research site networks.In...

Enhancing Clinical Trial Diversity Through Community-Based Site Networks
If you are a Sponsor interested in running a clinical trial through a community-based clinical research site network, please contact us at [email protected] One significant challenge in clinical trials is the lack of diversity among participants, particularly from...
Patricio Ledesma ( B.Eng Concordia University, Montreal, Canada and Master’s Degree in Clinical Trials, University of Seville, Spain) is the Head of Clinical Operations and Founder at Sofpromed CRO. Patricio is a professional consultant providing comprehensive, one-stop expert advice and guidance to biotechnology and pharmaceutical companies worldwide in the field of clinical trials and drug development. He is personally and enthusiastically devoted to helping biotech Chief Executive, Operations, Scientific, Medical, and Regulatory Officers in the planning and execution of phase I-IV clinical trials across North America, Europe, Asia-Pacific, Latin America, and Middle East regions. You can contact Patricio at: +34 607 939 266 [email protected]
Internet Explorer is no longer supported by Microsoft. To browse the NIHR site please use a modern, secure browser like Google Chrome, Mozilla Firefox, or Microsoft Edge.

Clinical Research Network

The NIHR Clinical Research Network (CRN) supports patients, the public and health and care organisations across England to participate in high-quality research, thereby advancing knowledge and improving care. The CRN supports research being delivered through 30 specialty therapy areas and 15 Local Clinical Research Networks . These provide a network of research expertise and clinical leadership to deliver research studies on the NIHR CRN Portfolio of studies . Find out more about our performance and key statistics relating to our activity.
From April 2024, the CRN will transition to a new organisation, the NIHR Research Delivery Network (RDN).
Research Delivery Network
The NIHR RDN is being established to support the delivery of high quality research that enables the best care for our population. The new organisation will build on the success of the CRN to support the country's world-class research system to deliver for the future.
The RDN will operate as one organisation across England and will play a critical and active role in implementing government policy, including:
- the Life Sciences Vision
- the Future of UK Clinical Research Delivery vision
- policy for life sciences research and development
- responding to the findings of the Lord O'Shaughnessy review
The RDN will support:
- clinical trials and other well-designed health and social care research studies (including studies that are delivered outside of an NHS setting)
- public health studies that require the recruitment of individuals within an NHS setting - this may include acute, ambulance, mental health, community or primary care
Growing the amount of commercial clinical research will be a key strategic ambition for the new network. The RDN will play a critical role in supporting the implementation of the Government's response to the Lord O'Shaughnessy report, which set out a clear blueprint for how the UK can return to its global leadership role in the support of commercial clinical trials and world-class research.
How will the RDN work in a different way to the CRN?
- The RDN will operate as one organisation across England. The RDN will have a shared vision and purpose, delivering a consistent experience for the research and healthcare communities. Innovations in one region will be shared and replicated across the country. It will be rooted in the local experience and needs of the research system and the populations it serves.
- The 12 Regional Research Delivery Networks will work as one with the Coordinating Centre and the Department of Health and Social Care to deliver a consistent, high quality and responsive service. The RDN will draw strength from its collaborative leadership model, with joint leadership composed from regional leaders, Coordinating Centre and DHSC colleagues. Regional context, expertise and relationships will enhance the quality of national decision making, leadership and coordination.
- The RDN will provide a range of services and support for research delivery, listening and addressing the needs of customers and working in collaboration with R&D teams. It will also support research sites with recruitment resources, tools to support research delivery, analysis and advice, and workforce development.
- The RDN will develop the strategic capacity and capability of the research system. The work of the RDN will build capacity for delivery partners to conduct research, with more researchers, sites, studies and participants involved in research. It will continue to bring research to under-served regions and communities with major health and care needs, considering the need for research inclusion and for the benefits of research to be spread across the whole country.
- The RDN will continuously learn and adapt to the changing domestic and global environment for health and care, life sciences and health research. It will work in collaboration with partners across UK health and care research to help drive improved efficiency across the system.
Regional Research Delivery Networks
NIHR Regional Research Delivery Networks (RRDNs) will develop excellent relationships with organisations commissioning and providing health and social care across their regions. They will help support research and promote the value of research in meeting the needs of local populations.
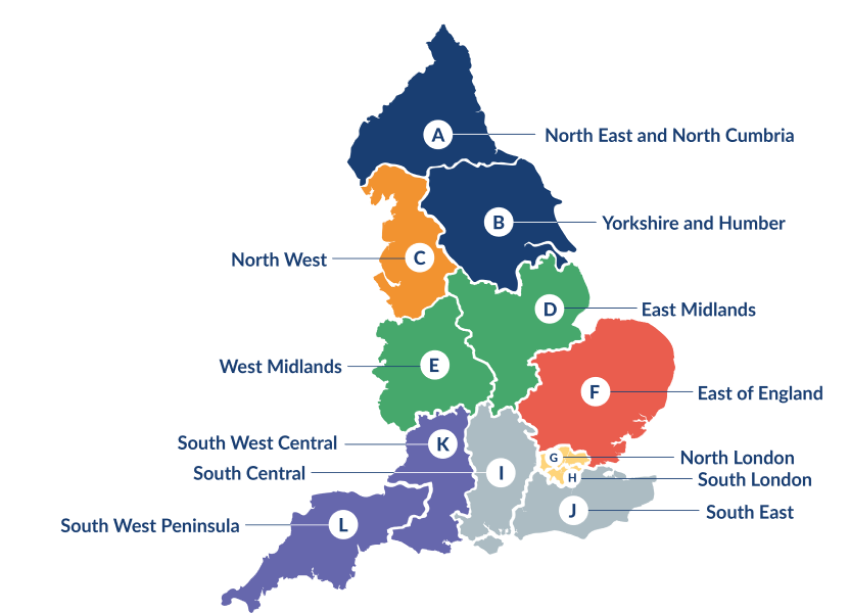
The NIHR RRDN map (pictured above) shows the geography of the 12 RRDNs that will make up the NIHR RDN from October 2024. These 12 RRDNs and their host organisations are listed below:
- North East and North Cumbria - hosted by The Newcastle upon Tyne Hospitals NHS Foundation Trust- Morag Burton, Director
- Yorkshire and Humber - hosted by Leeds Teaching Hospitals NHS Trust- Amber O'Malley, Director
- North West- hosted by Manchester University NHS Foundation Trust- Andy Ustianowski, Director
- East Midlands - hosted by University Hospitals of Leicester NHS Trust- Elizabeth Moss, Director
- West Midlands - hosted by The Royal Wolverhampton NHS Trust- Matt Brookes, Director
- East of England - hosted by Norfolk and Norwich University NHS Foundation Trust- Helen Macdonald, Director
- North London - hosted by Barts Health NHS Trust- Sharon Barrett, Director
- South London - hosted by Guy's & St Thomas' NHS Foundation Trust- James Lyddiard, Director
- South Central - hosted by University Hospital Southampton NHS Foundation Trust- Clare Rook, Director
- South East - hosted by Royal Surrey NHS Foundation Trust- Stephen Barnett, Director
- South West Central - hosted by University Hospitals Bristol and Weston NHS Foundation Trust- Kyla Thomas, Director
- South West Peninsula - hosted by Royal Devon University Healthcare NHS Foundation Trust- To be announced
The RDN Coordinating Centre will be hosted by the University of Leeds.
Together we are beating cancer
About cancer
Cancer types
Breast cancer
- Bowel cancer
Lung cancer
Prostate cancer.
Cancers in general
- Clinical trials
- Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

- Clinical trial organisations
This page has information about
Organisations in the UK
Cancer research uk.
Cancer Research UK is the largest cancer research organisation in the world outside the USA. We fund research on all aspects of cancer from its causes to prevention and treatment.
The Centre for Drug Development (CDD) also develops and assesses new treatments for patients with cancer.
The Cancer Research UK website are committed to producing high quality information for people affected by cancer. This includes lots of easy to understand information about cancer. There is also a database of clinical trials.
As well as looking at the information on this website you can call our nurse freephone helpline on 0808 800 4040. They are available from Monday to Friday, 9am to 5pm. Or you can send us a question online.
- Contact our cancer information nurses
Cancer Chat is our online forum where you can share experiences.
- Cancer Chat discussion forum
Experimental Cancer Medicines Centre (ECMC) Network
The ECMC network aims to bring together cancer doctors, research nurses and scientists to make clinical trials of new treatments quicker, easier and safer. The ECMC network is made up of 18 adult centres and 11 paediatric locations across the UK. They are all involved with clinical trials of experimental treatments and tests.
The ECMC initiative was set up in 2007 and is funded by Cancer Research UK and the Departments of Health of England, Scotland, Wales and Northern Ireland.
The centres use the money to run trials of new and experimental treatments. ECMC trials are mostly small phase 1 and phase 2 trials looking at new treatments. They also analyse thousands of blood and tissue samples (biopsies) to help find out more about how treatments work and what happens to cancer cells.
- Visit the ECMC website
Medical Research Council (MRC)
The Medical Research Council is a national organisation funded by the UK Government. They work closely with the Department of Health and Social Care and other organisations. They fund research in all areas of health care, including cancer. Their research ranges from laboratory work to clinical trials with patients.
Phone: 01793 416200
- Visit the MRC website
National Institute for Health Research (NIHR)
The National Institute for Health Research (NIHR) is mainly funded by the Government through the Department of Health and Social Care.
They provide the staff, facilities and technology to help the research process. They work in partnership with the NHS, universities, local government, other research funders, patients and the public. Their aim is to improve the health and wealth of the nation through research.
Be Part of Research is run by the NIHR. The website has information about clinical trials and other research running in the UK.
Email : [email protected]
- Visit the NIHR website
National Cancer Research Institute (NCRI)
The National Cancer Research Institute was set up in 2001. It is a partnership between the UK government, charities and industry. The aim of the NCRI is to oversee and coordinate cancer research in the UK. And to continue to improve the quality and relevance of cancer research.
Email: [email protected] Phone : 020 3469 8798
- Visit the NCRI website
NIHR Health Technology Assessment (HTA) programme
The Health Technology Assessment (HTA) Programme is funded by the NIHR. It is a national research programme that helps assess tests and treatments to see how good and how cost effective they are. The HTA funds research and clinical trials in all areas of healthcare including prevention, treatment and rehabilitation.
Email: [email protected]
- Visit the NIHR Health Technology Assessment (HTA) programme website
International organisations
European organisation for research and treatment of cancer (eortc).
The EORTC was founded in 1962 to improve the standard of cancer treatment in Europe. They fund research into cancer, including the development of new drugs. They also fund and co-ordinate a large number of clinical trials across Europe.
Contact the EORTC
- Visit the EORTC website
The European Society for Blood and Marrow Transplantation (EBMT)
The EBMT aims to bring together doctors treating patients with bone marrow transplants, to share experiences and work together. They also fund research. They have offices in the UK, Spain, the Netherlands and France.
Email: [email protected] Phone: 0207 188 8402
- Visit the EBMT website
International Cancer Research Partnership (ICRP)
The International Cancer Research Partnership (ICRP) is a database of information about cancer research that is funded by organisations in the UK and other countries. It was set up in 2003 to help researchers find information about current cancer research projects. Members of the public can also look at the information on the ICRP website, but it tends to be written for medical professionals.
- Visit the ICRP website
International Standard Randomised Controlled Trial Number (ISCTRN)
The ISCTRN is a database of trials that are running worldwide. It was set up in 2000 and gives each trial a unique identifying number. It includes trials for cancer as well as other diseases. The aim is to ensure that trials and the results of trials are easy and available to access.
- Visit the ISCTRN website
National Cancer Institute (NCI)
The National Cancer Institute (NCI) is an American organisation that carries out cancer research. They fund laboratory research and clinical trials for cancer patients in the US and around the world. They provide information about cancer and its treatments in English and Spanish.
- Visit the NCI website
Trials organisations working with specific cancers
We have listed some organisations below that are involved in research for particular types of cancer.
Bowel (colorectal) cancer
Guts UK (used to be called CORE) is a charity that supports research into a large range of digestive disorders, including cancer. They also produce information for patients.
Email: [email protected] Phone: 020 7486 0341
- Visit the Guts UK website
The Bobby Moore Fund
The Bobby Moore Fund raises money for research into bowel cancer. They aim to raise awareness of symptoms so that people are diagnosed earlier.
Email: [email protected] Phone: 0870 609 1966
- Visit The Bobby Moore fund website
Brain Tumours
The brain tumour charity.
The Brain Tumour Charity funds research into brain tumours and offers support and information to those affected. It also raises awareness and influences policy.
Email: [email protected] Helpline: 0808 800 0004
- Visit The Brain Tumour Charity Website
Breast Cancer Now
A UK wide organisation that funds research into breast cancer and campaigns for better treatments and services. They have their own research centre within the Institute of Cancer Research in Sutton, Surrey. They also offer support and information for people affected by breast cancer.
Email: [email protected] Phone: 0808 800 6000
- Visit the Breast Cancer Now website
Breast International Group (BIG)
Breast International Group (BIG) was founded in 1996 by a group of breast cancer specialists and is based in Belgium. They share knowledge and information about research and clinical trials and aim to speed up recruitment onto clinical trials. They fund and co-ordinate breast cancer trials to improve treatments and quality of life.
Email: [email protected] Phone: +32 2 486 16 00
- Visit the BIG website
Blood cancer
Blood cancer uk.
Blood Cancer UK raises money for research into the causes and treatment of leukaemia, myeloma and lymphoma. They also provide information and support and produce information booklets
Blood Cancer UK have a trials support service. You can contact one of their nurse advisors to find out about trials that are running in the UK. The nurses are experienced in blood cancers and clinical trials. They can talk through the available trials with you and see if there are any suitable trials for you to join.
To arrange a referral for this service, visit the Blood Cancer UK trial referral page
Email: [email protected] Phone: 0808 2080 888
- Visit the Blood Cancer UK website
Myeloma UK fund research looking at patient care and treatments for myeloma. They provide information and support for patients and their families. They also produce booklets and have details of myeloma support groups in the UK
Tel: 0800 980 3332 Email: [email protected]
- Visit the Myeloma UK website
UK Myeloma Forum
The UK Myeloma Forum (UKMF) develops and promotes clinical trials for myeloma. They also develop guidelines for treatment and provide information for patients and health care professionals.
- Visit the UK Myeloma Forum website
Roy Castle Lung Cancer Foundation
This organisation, founded in Roy Castle's memory, funds research into lung cancer and provides support for those affected. Their two main areas of research are early detection of lung cancer and improving the patient experience
Email: [email protected] Phone: 0333 323 720
- Visit the Royal Castle Lung Cancer Foundation
Melanoma skin cancer
Melanoma focus .
Melanoma Focus fund research into melanoma. They also provide support and information for patients, carers and healthcare professionals.
Helpline: 0808 801 0777
- Visit the Melanoma Focus website
Prostate Cancer UK
Prostate Cancer UK fund research focussing on prevention, diagnosis and treatments. They provide information and support for patients with prostate cancer. They have a helpline and information about support groups, and produce leaflets for patients.
Helpline: 0800 074 8383
Get support from specialist nurses
- Visit the Prostate Cancer UK website
Related information
What are clinical trials?
How to find a clinical trial
Last reviewed
Find a trial, about cancer.
- Spot cancer early
- Talking to your doctor
- What is cancer?
- What clinical trials are
- How to find a clinical trial
- How to join a clinical trial
- What you should be told about a clinical trial
- How clinical trials are planned and organised
- Clinical trial results
- What to ask your doctor about clinical trials
- Children's Cancers
- Sign up for relevant cancer information and support
- How do I check for cancer?
- Welcome to Cancer Chat

Rate this page:


Research For You
Improving quality of life.
MAC Clinical Research is the UK’s leading clinical trials organisation. As an award winning healthcare organisation, we are dedicated to developing new and improved treatments for a range of medical conditions.
Every year across the UK thousands of people take part in medical trials. Volunteers, just like you, play a significant role in helping to find tomorrow’s breakthrough medications. Without these participants there would be no advances in treatment options.
By taking part in a clinical trial with MAC you can make a real difference, and you can benefit in many different ways.
LATEST POSTS

National DNA Day: Is Osteoarthritis Genetic?
As we celebrate National DNA Day, a day dedicated to commemorating the discovery of the DNA double helix in 1953

The Crucial Role of Vaccines and Collective Action
With World Immunisation Week upon us, it serves as a poignant reminder of the critical role vaccines play in safeguarding

Allergy Awareness Week: The Body’s Immune Response to Allergies
Millions of people around the globe live with allergies, symptoms of which can range from mildly irritating to potentially life-threatening.
Patient Testimonials

Find out about our current trials near you...

The UK's leading Clinical Trials Sites
- 0800 633 5507
- [email protected]
quick links
- Join A Trial
- MAC Clinical Research
- Privacy Policy
- Cookie Policy

Download The Research For You App

Copyright © 2024 MAC Research, All rights reserved.
Privacy Overview
Top 12 Clinical Research Organisations (CROs) in 2023

- Author Company: PharmiWeb.Jobs
- Author Name: Lucy Walters
- Author Email: [email protected]
- Author Website: https://www.pharmiweb.jobs/
The global clinical trials market was estimated to be worth $38.7 billion in 2021 and is expected to reach £52 billion by 2026. In this article, we look at the top 12 CROs in the world , highlighting the companies driving this considerable growth and accelerating research and development across the globe.
Recognised as the world’s largest and most comprehensive CRO powered by Healthcare Intelligence, ICON provides outsourced development and commercialisation services to pharmaceutical, biotechnology, medical device and government and public health organisations. ICON has a global network of offices in 46 countries, working on specialities including Medical Devices, Therapeutics, Government and Public Health Solutions, Clinical Research Services, Commercialisation and Outcomes, and more.
With approximately 70,000 employees, IQVIA is a leading provider of advanced analytics, technology solutions and clinical research services to the life sciences industry. Operating in more than 100 countries, IQVIA’s Clinical Research solutions include Protocol Design, Phase I Trials, Phase IIb / III Trials, Site Identification, Patient Recruitment and Global Laboratories.
With more than 20,000 employees globally, Parexel is one of the world’s largest CROs and provides contract research, consulting, and medical communications services to the worldwide pharmaceutical, biotechnology, and medical device industries. Parexel provides the full range of Phase I to IV clinical development services, with global study locations in Baltimore, US, London, UK, Los Angeles, US, and Berlin, Germany.
Syneos Health
Syneos Health is the only fully integrated biopharmaceutical solutions organisation built to accelerate customer success, with 29,000+ employees working across more than 110 countries. The company’s Clinical Development services include Decentralized Solutions, Bioanalytical Solutions, Early Phase, Phase II–III, Phase IIIb – IV, Real World and Late Phase, and Medical Device and Diagnostics. In 2022, Syneos Health was the winner of the Life Sciences Award for Most Innovative CRO.
Labcorp Drug Development
With more than 70,000 employees, Labcorp is a leading global life sciences company with unparalleled diagnostics and drug development capabilities. The company is driven to help medical, biotech, and pharmaceutical companies transform ideas into innovations, and supports clinical trial activities in approximately 100 countries. Labcorp plans to spin-off its clinical development business, to be named Fortrea, in mid-2023.
Part of Thermo Fisher Scientific, PPD is a leading global CRO with 130,000+ employees, working to make the world healthier, cleaner, and safer. The company offers a broad range of clinical development and analytical services, helping to accelerate medicines from early development through regulatory approval and market access. PPD works with a broad range of therapeutic areas, including Biosimilar Development, Cell & Gene Therapy, Immunology and Rheumatology, Infectious Diseases, Women’s Health, and more.
In 2022, Medpace celebrated 30 years of accelerating the development of medical therapeutics, with approximately 5,000 employees working across 41 countries. The company provides medical and regulatory leadership and guidance throughout the clinical development life cycle, along with efficient execution of studies around the world, from Early Phase to Late Phase. Medpace also earned the 2022 CRO Leadership Awards for Capability, Compatibility, Expertise, Quality, and Reliability.
Charles River Laboratories
With 20,000+ employees worldwide, Charles River Laboratories provides essential products and services that help pharmaceutical and biotechnology companies, government agencies and leading academic institutions across the globe to accelerate their research and drug development efforts. The company currently operates more than 110 facilities across 20+ countries, working on therapeutic areas including COVID-19, Cardiovascular Disease, Immunology, Infectious Diseases, and Rare Diseases.
WuXi AppTec
WuXi AppTec is a global company providing a broad portfolio of R&D and manufacturing services to help pharmaceutical and healthcare companies across the globe advance discoveries and deliver ground-breaking treatments. The company currently has more than 45,000 employees globally, including 42,000 scientists. WuXi Clinical, a wholly owned subsidiary of WuXi AppTec, is a global CRO providing a comprehensive range of clinical development services for the development of pharmaceuticals, biologics, and medical devices.
CTI is one of the 20 largest CROs in the world, with associates in 60+ countries across six continents, including 12 locations in Europe. The company is the highest-ranked CRO in the world after being named a winner in all 5 core categories of the 2022 CRO Leadership Award. CTI provides its partners with a full range of clinical services, across therapeutic areas including Rare / Orphan Diseases, Regenerative Medicine / Gene Therapy, Immunology, Transplantation, and Hematology / Oncology.
Worldwide Clinical Trials
With approximately 3,000 employees and operating in more than 60 countries, Worldwide Clinical Trials is a global, midsize CRO providing top-performing bioanalytical and Phase I-IV clinical development services to biotechnology and pharmaceutical companies. The company’s major therapeutic focus areas include Cardiovascular, Metabolic, Neuroscience, Oncology, and Rare Diseases. In 2022, they were recognised for excellence and honoured in 5 categories in the CRO Leadership Awards.
PSI CRO is a privately-owned, full-service CRO operating globally, with 27,000+ employees in more than 60 countries. Committed to being the best CRO in the world, the company specialises in the planning and execution of global pivotal registration clinical trials, supporting trials across multiple countries and continents. PSI was named a 2022 CRO Leadership Award Champion in the categories of Compatibility, Quality, and Reliability.
You are leaving PharmiWeb.com

Disclaimer: You are now leaving PharmiWeb.com website and are going to a website that is not operated by us. We are not responsible for the content or availability of linked sites.
ABOUT THIRD PARTY LINKS ON OUR SITE
PharmiWeb.com offers links to other third party websites that may be of interest to our website visitors. The links provided in our website are provided solely for your convenience and may assist you in locating other useful information on the Internet. When you click on these links you will leave the PharmiWeb.com website and will be redirected to another site. These sites are not under the control of PharmiWeb.com.
PharmiWeb.com is not responsible for the content of linked third party websites. We are not an agent for these third parties nor do we endorse or guarantee their products. We make no representation or warranty regarding the accuracy of the information contained in the linked sites. We suggest that you always verify the information obtained from linked websites before acting upon this information.
Also, please be aware that the security and privacy policies on these sites may be different than PharmiWeb.com policies, so please read third party privacy and security policies closely.
If you have any questions or concerns about the products and services offered on linked third party websites, please contact the third party directly.

Bespoke Clinical Research Organisation (CRO)
A results-driven, bespoke clinical research organisation.
Clinical trials are changing. Through new processes, improved guidance, and a growing trend for the inclusion of underrepresented demographics and cultures, these changes are making waves across the industry and are facilitating the development of more effective vaccines, safer treatments, and new, innovative therapies that are transforming people’s lives and contributing towards improved life expectancies.
However, at Clinicology we understand that these changes to operational processes haven’t fully eradicated issues within the industry; they’ve simply created a new set of challenges that research teams need to tackle. Challenges such as finding teams with knowledge of local legislation, identifying suitable sites in unfamiliar territory, and monitoring findings and results not just on a national scale, but on a global scale.
At a time when clinical research is becoming increasingly complex, teams are beginning to realise the stark realities of running their own trials. Trials are taking longer to complete than originally anticipated, workloads are increasing, and it’s been reported that almost 50% of R&D budgets are now being spent on clinical trials alone. There is an urgent need to address these problems now to ensure success in the future.
Your clinical trial, in safe hands
Our core ethos is to deliver cost-effective, smart, strategic, and innovative results-driven solutions to our clients, no matter what they need. Specializing in Phase I-IV CTIMP and medical device studies, site startup, monitoring, feasibility, and patient recruitment, our professional full service network covers a broad range of niche study and research areas including rare diseases, oncology, cardiology, ophthalmology, dermatology, diabetes, vaccines and more.
Why waste valuable time, money, and skills on site setup and operational management? These important resources are best applied to core research tasks so you can focus on aspects of research that really make a difference. By partnering with our dedicated clinical research organisation, you can take the pressure off your key talent while having peace of mind that your trial is in safe hands.
Contact us to discuss your challenges and how we can help today.

About our founder
Clinicology was founded by Mark Thomas, previously founding Director and Board Member of a medium sized global CRO, who has gained exceptional industry knowledge since 1995.
Looking for help?
Description
TRUSTED BY PHARMA & BIOTECH FOR STATISTICAL & DATA EXPERTISE
Bring your drugs to market with fast and reliable access to experts from one of the world's largest global biometric Clinical Research Organizations.
Statistical Analysis, Data Capture and Clinical Trial Reporting

BIOSTATISTICS

STATISTICAL PROGRAMMING
CLINICAL DATA MANAGEMENT

BIOSTATISTICAL CONSULTANCY
The three steps to a faster drug approval, schedule a meeting and speak with a clinical data expert, a bespoke plan will be created in line with your needs, our clinical data experts become a part of your team, our areas of expertise:, protocol design and power calculations.
Get the right protocol design for the right scientific questions of your trial and boost the chance of success with sound sample size calculation.
CDISC/ADaM Mapping
Convert all your trial research data into one globally regulatory recognized data standard for clinical research.
EDC & Data Capture Options
Utilize the latest data capture technologies such as wearable and ePRO devices and take a flexible approach to selecting the right electronic data capture (EDC) system for your trial.
ISS/ISE Support
Overcome the challenges of combining multiple studies to review the safety and efficacy of an IND/NDA.
Centralized Statistical Monitoring & Data Quality Oversight
Help assure that your regulatory submission isn’t needlessly delayed through a lack of data quality or integrity as you discover data anomalies, outliers and other unnoticeable trends to improve the chance of your regulatory approval.
Clinical Study Designs/Adaptive Trials
An optimally designed trial can reduce costs and save time, either by requiring a smaller sample size or by stopping a trial earlier in time without continued wasted efforts.
PK/PD Services
Get the specialist software and expertise you need to discover what the body does to your investigational drug and where it goes with Pharmacokinetics (PK) and Pharmacodynamics (PD) analysis.
Clinical Data Visualizations
Gain early insights into your pre-database lock data with free Tables, Listings and Figures visualizations to give you an idea of the potential trial reporting results that lay ahead.
Oncology trials face many challenges in the collection, analysis and reporting of study data, and selecting a partner that is specializes in data instead of a traditional full-service CRO can make all the difference in your studies success.
Select your preferred resourcing model
Functional Service Provision
Gain rapid access to resource of functional biometric teams and become flexible to any peaks and troughs in your pipeline.
Fixed Cost Projects
Gain resource on a single project by project basis for the biometric support you need, when needed.
Utilize Contractors
Handle a single or multiple contracts and the convenience of adding additional resources on a smaller scale.
What Our Clients Say
“Quanticate is a clinical data expert and as a consultant I find they are easy to deal with, making my life much easier. Good communication and expectations management mean that we have built a good relationship over time which enables me to provide an excellent service to my clients in a timely and effective manner.”
“Quanticate is one of the best CROs I have ever worked with in the past and they are really outstanding at biometrics. Quanticate supported us with the analysis of a large phase 3 study, an integrated summary of efficacy and interactions with regulatory authorities. We have a great relationship with Quanticate at Fertility Biotech; they’re helpful and take the time to understand our needs. I would recommend them to anyone looking for any biometrics support. ”
FREE WHITEPAPERS & WEBINARS
Resources to a successful drug approval
Contact details.
Address - UK HQ:
Address - US HQ:
BLOG TOPICS
- SAS Programming (29)
- Clinical Trials (26)
- Statistical Programming (26)
- Regulatory Requirements (23)
FOLLOW QUANTICATE ON:

© 2024 Quanticate
Therapeutic Expertise
Participate

Explore end-to-end solutions throughout development — from portfolio optimization and regulatory strategy, to Phase I-IV clinical trials, market access planning, and more.
- Portfolio management and asset valuation
- Early development and innovation
- Integrated clinical development
- Approval and access
- Value substantiation lifecycle management
HOW WE DO IT
- Operational excellence
- Delivery models
- Building patient insights into assets, profile and claims
- Portfolio Optimization
- Asset Valuation and Indication Prioritization
- Early Evidence Review
- Model-Based Drug Development
- Integrated Development Strategy and Planning
- Phase I Clinical Trials
- Proof of Concept Studies: Phase IB-IIA
- Patient Engagement Strategy and Enrollment Solutions
- Patient Inclusion
- Site Alliance Network and KOL Engagement
- Protocol Optimization
- Regulatory Strategy
- Market Access Strategy and Delivery
- Biomarker and Genomic Medicine Strategy
- Clinical Trial Supply & Logistics
- Medical Communications
- Phase IIB-IV Clinical Trials
- Real World Evidence
- Protocol-Driven, Customized Site Solution Strategy
- Regulatory Strategy, Submissions, Compliance, and Outsourcing
- Clinical Development Technology Optimization
- Global Regulatory Submissions and Outsourcing
- Compliance and Risk Management
- Real-World Evidence, Market Access Strategy and Planning
- Regulatory Compliance, Drug Safety and Pharmacovigilance
- Lifecycle Optimization
- Leveraging AI and digital in clinical development
- Biotech Clinical Trial Solutions
- Gain an advantage through FSP

Parexel Biotech provides the end-to-end capabilities you’ll need to succeed. We're fast, flexible, and dedicated to impacting patient lives.

Utilize our expertise across therapeutic areas, combining innovative trial designs, leading clinical and regulatory expertise, global reach, and a passion for changing patient lives.
- Neuroscience
- General medicine
- Infectious disease & vaccines
- Inflammation & immunology
Cross-Therapeutic Expertise
- Cell & gene therapies
- Rare diseases

Our experts help you stay at the forefront of the industry - and ahead of change.
New Medicines, Novel Insights
- Advancing rare disease drug development
- Accelerating development of cell and gene therapies
- Achieving patient-guided drug development
Discussions on Diversity
- Chapter 1 Bridging the Gap
- Chapter 2 Beyond the Binary
The Regulatory Navigator
- BIOSECURE Act: Implications for US-based drug developers
- Exploring ICH Q5A revision 2
- Potency assurance for CGT products: FDA's new draft guidance
Latest Report

New Medicines, Novel Insights: Achieving patient-guided drug development

Thinking about joining a clinical trial? Learn the drug development process, what it’s like to participate, how to find a trial, and answers to frequently asked questions.
INTERESTED IN PARTICIPATING?
- Healthy Volunteers
- Patient Volunteers
- Patient Advocacy Groups
HEAR FROM REAL PATIENTS
- Patient Stories
TRIAL SITES

Want to collaborate with us to offer clinical trials at your site? We would welcome the opportunity to discuss.
We are one of the largest CROs in the world, speeding life-changing medicine to market by engaging patients With Heart ™. Learn about who we are, what we do, and what we believe.
- Management team
- Global reach
- Our DE&I strategy
- Compliance, Tax & Privacy
- Meet us at an event
- Jamie Macdonald
- Peyton Howell
- Michael Crowley
- Jonathan Shough
- Sheri McCoy
- Michael Bruun
- John Groetelaars
- Susan Salka
- Global Reach
- Our DE&I Strategy
- Our ESG Strategy
- Compliance & Ethics
- Tax Strategy
- Privacy Policy
- Supplier Data Privacy Requirements
- Terms of Use
- French Gender Pay Data
- UK Gender Pay Data
- UK Modern Slavery Act Statement
- EU-U.S. Data Privacy Framework (EU-U.S. DPF)
- Supplier Diversity Policy
- World Vaccine Congress
- Patients as Partners U.S.
- MAPS Americas
- ASCPT 2024 Annual Meeting
- Managing Complexity in Cell and Gene Trials: Establishing an RBQM Strategy that Drives Better Performance
- EDI in clinical research: The case for adaptive strategies at every step
- 17th ISCR ANNUAL CONFERENCE 2024
- IncluDE Site Solutions Summit
- AAN Annual Meeting
- ASGCT Annual Meeting
- Dermatology Drug Development Summit EU
- World Orphan Drug Congress US
- AACR Annual Meeting
- BIO International Convention
- DIA Global Annual Meeting
- International Society for Cell & Gene Therapy
- Final countdown: How to transition your trials under EU-CTR
- SCRS: Oncology Site Solutions Summit
- Accelerating Vaccine Development Through Operational Excellence
- Alzheimer’s Disease vs Multiple Sclerosis Drug Development: Similarities, Differences & New Perspectives
- Patients as Partners EU
- Best of ASCO
- World Drug Safety Congress US
- World Drug Safety Congress EU
What can we help you find today?
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
Patient Story
- Cell & Gene Therapy
- Rare Diseases
- Inflammation & Immunology
- Neurosciences
One night, while watching TV with her husband, Robyn felt a lump in her neck.
She had large B-cell lymphoma — an aggressive cancer diagnosed in 150,000 patients each year globally.
As a doctor, she knew the risk. She got a CAT scan, looked at the images, and her world stopped.
Four years later the lymphoma returned. Robyn’s care team prescribed a further six cycles of a brutal chemotherapy, followed by an autologous stem cell transplant (ASCT) and external beam radiation. Robyn’s ASCT treatment was further complicated by septic shock requiring a stay in intensive care.
Robyn entered remission again, but it was short-lived, with the lymphoma returning nine months later. Treatment options for Robyn were now bleak and limited.
Robyn immediately sought treatment. With her husband by her side, she underwent many rounds of chemotherapy and went into remission.
Feeling defeated, she found a clinical trial for car t-cell therapy, a new treatment that reengineers white blood cells to target and eradicate cancer., she thought this was her last chance., a week after receiving treatment, her lymph nodes shrunk. within three months, there was no evidence of the disease., today, she’s cancer free — and sharing her experiences to help us better meet the needs of patients like her in our cell and gene therapy trials., lives can change when you design cell and gene therapy trials with speed and precision..
- Utilize the right experts, focused on the right indications
- Access even hard-to-find patient populations
- Pinpoint the perfect sites for your trial
- Satisfy global regulations, to get your treatment to market safely and quickly
What we do, we do
Our Experts
Our cell and gene therapy specialists collaborate to help get your innovative treatments to patients like Robyn faster.
MORE EXPERTS
Steve Winitsky
Vice President, Tech...
Stefan Zietze
Executive Director, ...
Hicham El Bariqi
Director, Cell & Gen...
Jingke Yang, M.D., Ph.D.
Global TA Section He...
Chris Learn, Ph.D., PMP

Vice President, Cell and Gene Therapy, Center of Excellence
With 20+ years of trial execution and team management experience, Chris leads development for our cell and gene therapeutic area. He reinforces our patient-first focus to help ensure your trials are designed to meet patient needs.
"Being able to capture and understand the patient’s perspective — not just as a patient, but as a person — and use their insight to guide my decisions is what it means to work With Heart™."
Our diverse experiences ensure you get the expertise you need, no matter the indication.
Our experience with CAGT clinical trial sites around the world allows us to accelerate study start-ups.
- North America
- South America
- Middle East & Africa
Access to global EMR data enables us to pinpoint the perfect sites for your trial.

Our cross-functional team overcomes any technical, logistical, and strategic challenges.
Our highly tailored recruitment and retention strategies drive access to even hard-to-find patient populations.
What can we do to help you change patient lives?
See CAGT capabilities Visit all therapeutic areas Explore CAGT careers
Want our latest insights for cell and gene therapy?
Ready to speak to someone on our team?
Are you a patient interested in a clinical trial?
We focus on patients, because they inspire us to deliver better trials, faster than ever . So we can make a difference for more patients like Robyn.
Who we are,, parexel is proudly among the world’s largest clinical research organizations.
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise , our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind , to make clinical research a care option for anyone, anywhere.
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Health and social care
- Research and innovation in health and social care
- The Future of UK Clinical Research Delivery: 2022 to 2025 implementation plan
- Department of Health & Social Care
The Future of Clinical Research Delivery: 2022 to 2025 implementation plan
Published 30 June 2022

© Crown copyright 2022
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/the-future-of-uk-clinical-research-delivery-2022-to-2025-implementation-plan/the-future-of-clinical-research-delivery-2022-to-2025-implementation-plan
Ministerial foreword
In 2021 we, the UK and devolved governments, set out our vision for the future of clinical research delivery. Saving and Improving Lives: The Future of UK Clinical Research Delivery lays out our ambition to create a world-leading UK clinical research environment that is more efficient, more effective and more resilient, with research delivery embedded across the NHS. We also set out our plans for 2021 to 2022 , as the first steps in delivering on the vision.
A digitally enabled, pro-innovation and people-centred clinical research environment is key to realising our ambitions to make the UK a world-leading hub for life sciences that delivers improved health outcomes for our citizens and attracts investment from all over the world. We will harness the explosion in innovative technologies to benefit patient outcomes and make a tangible difference to people’s lives across the UK. Clinical research is crucial to these efforts, as the lynchpin to driving improvements in healthcare.
As we emerge from the shadow of the pandemic and look to the future, we will work together to ensure that the UK is seen to be one of the best places in the world to deliver cutting-edge clinical research. We are working hard both to recover research delivery in the NHS and to use this moment as a catalyst for transformation, building increased resilience and embedding innovative practice as we go. The cross-sector partnerships built through the UK Clinical Research Recovery, Resilience and Growth ( RRG ) programme provide the strong foundations we need to succeed, drawing on expertise and support from industry, academia, charities, patients and the public, regulators, funders and the NHS.
Our vision was clear on the importance of unleashing the true potential of clinical research across the UK, addressing long-standing health inequalities and improving the lives of us all. We, the UK and devolved governments, are excited to set out the next stages as we look to turn our vision into a reality and build a clinical research system of the future.
Lord Kamall of Edmonton in the London Borough of Enfield Parliamentary Under Secretary of State for Technology, Innovation and Life Sciences Department of Health and Social Care
Robin Swann Minister for Health Northern Ireland Executive
Eluned Morgan Minister for Health and Social Services Welsh Government
Humza Yousaf Cabinet Secretary for Health and Social Care Scottish Government
Executive summary
In March 2021, we published our bold and ambitious 10 year vision: Saving and Improving Lives: The Future of UK Clinical Research Delivery . This was followed in June 2021 by The Future of UK Clinical Research Delivery: 2021 to 2022 implementation plan setting out the steps we would take to progress the vision in 2021 to 2022.
This phase 2 plan summarises the progress that we have made so far and the actions that we will take over the next 3 years, from 2022 to 2025, ensuring we make the progress necessary to achieve our vision in full by 2031.
This plan has been developed by the cross-sector UK Clinical Research RRG Programme in consultation with stakeholders from across the clinical research ecosystem. Our plan is centred around the 5 overarching themes identified in the vision:
- a sustainable and supported research workforce to ensure that healthcare staff of all backgrounds and roles are given the right support to deliver clinical research as an essential part of care
- clinical research embedded in the NHS so that research is increasingly seen as an essential part of healthcare to generate evidence about effective diagnosis, treatment and prevention
- people-centred research to make it easier for patients, service users and members of the public across the UK to access research and be involved in the design of research, and to have the opportunity to participate
- streamlined, efficient and innovative research so that the UK is seen as one of the best places in the world to conduct cutting-edge clinical research, driving innovation in healthcare
- research enabled by data and digital tools to ensure the best use of resources, leveraging the strength of UK health data assets to allow for more high-quality research to be delivered
We have made significant progress over the past year – a new combined review process has led to halving of approval times for new Clinical Trials of Investigational Medicinal Products (CTIMPs) since January 2022 compared to previous separate applications, streamlining the route through the regulatory journey for researchers, the world-leading £200 million data for research and development programme has been announced to invest in health data infrastructure in England with devolved administrations aligning and strengthening their infrastructure; and a new UK-wide professional accreditation scheme for Clinical Research Practitioners ( CRP ) has been launched to help double the size of this important workforce in the future.
However, the recovery of research delivery following the pandemic remains challenging. The Department for Health and Social Care (DHSC) and NHS England are taking firm action to address this, with the support of the devolved administrations, through the ‘Research Reset’ programme. We are committed not only to returning to pre-pandemic levels of performance, but to using this as an opportunity to reform and catalyse the transformation needed to create the flourishing, responsive and resilient system set out in our vision.
The phase 2 plan is aligned with funding confirmed through the government spending review for April 2022 to March 2025 and includes up to £150 million of additional funding from the National Institute for Health and Care Research ( NIHR ) and £25 million additional funding from RRG partners across the UK, complementing up to £200 million in England for the data for research and development programme announced in March 2022 and demonstrating the government’s ongoing commitment to delivering on the UK’s potential as a global life sciences superpower. This funding will enable RRG partners to:
- recover the UK’s capacity to deliver research through DHSC and NHS England’s Research Reset programme, and aligned work in the devolved administrations, aiming for 80% of all open studies on the NIHR Clinical Research Network (CRN) portfolio to be delivering to time and target by June 2023
- ensure we can recognise and support our expert workforce, and develop robust workforce plans, providing the basis for strategic investment in capacity development to support achievement of our vision in full
- broaden responsibility and accountability for research across the NHS, and improve measurement, visibility and recognition of those supporting the delivery of clinical research studies
- achieve a sector-wide, sustained shift in how studies are designed and delivered so that inclusive, practicable and accessible research is delivered with and for the people with the greatest need and in ways that enable us to tackle the greatest challenges facing the NHS
- streamline processes, strengthen our regulatory environment and ensure faster approval, set-up and delivery of studies with more predictability and less variation, as well as make it easier to understand and access the UK’s clinical research offer, thereby utilising the unique opportunity to develop a more flexible and improved regulatory model for clinical research outside the EU and improving our attractiveness as a leading destination to conduct cutting edge and global multi-centre clinical studies
- invest in the infrastructure and tools needed to implement people-centred, innovative data and digitally-enabled methods and increase partnership working across the health data ecosystem to ensure people across the whole of the UK can benefit from these approaches
The RRG programme will oversee the delivery of this plan, continuing to work in partnership with stakeholders across the sector and regularly revisiting the original vision to consider any further actions that will be needed to deliver it in full. In doing so, we will ensure that the NHS is able to tackle the healthcare challenges of the future and people across the UK and around the world will benefit from better health outcomes.
Further information about the RRG programme, including our delivery partners and governance , are available on the dedicated Recovery Resilience and Growth website . Detailed summaries of our progress to date and our future plans will be published on the site on an ongoing basis, providing a central point of information and updates about the programme and our progress towards achievement of the vision. You can also sign up to receive regular email updates on our progress.
UK-wide approach
Health policy is a devolved responsibility, where each of the UK administrations has distinct ownership over implementation. However, we are committed to delivering on a vision with a UK-wide reach and in pursuit of a common goal: to create a seamless and interoperable service across the UK to support clinical research delivery, shaping the future of healthcare and improving people’s lives.
We are therefore further strengthening a joined-up system, where sponsors of both commercial and non-commercial research can easily deliver studies across the UK and people can more easily participate. To ensure compatible and consistent ways of working across England, Scotland, Wales and Northern Ireland many commitments in this plan are focused on UK-wide implementation. Organisations such as MHRA ( Medicines and Healthcare products Regulatory Agency ) and systems such as IRAS ( Integrated Research Application System ) have a UK-wide reach and their actions will have impacts across the country. In some instances, actions are being led by a specific organisation on behalf of the UK, while others will be delivered through UK partnerships – recognising the different legislative and delivery contexts across the UK government and devolved administrations.
The needs of UK citizens and our health research system are broad and diverse. We are committed to maintaining a rich and balanced portfolio of studies in rare and common diseases, ranging from complex, intensive studies in small, highly targeted populations to pragmatic population health research in large cohorts, using a range of methodologies and methods as appropriate to the research questions.
Our vision focuses specifically on the future of UK clinical research delivery. Other types of research, including social care and public health research, are vitally important to provide the evidence necessary to support policy making and service delivery in these areas. Many partners involved in the RRG programme support this broader programme of research activity and other work programmes are underway to enable their development. We expect that many of the improvements we make in the clinical research environment will have benefits for other kinds of research and will work across our organisations and with wider groups of stakeholders to ensure the lessons are shared.
Research reset
As we recover from the pandemic, clinical research delivery is facing unprecedented challenges and there is an urgent need to reset the UK’s research portfolio so we can build for a stronger future.
The number of studies in the NHS is now higher than ever before. This is accounted for by the additional COVID-19 studies, other research that has remained on the portfolio from before the pandemic and has been paused or delayed, together with new studies being funded and coming into the system. In addition, the number of studies in set up is now much higher than pre-pandemic, further increasing the workload for NHS R&D offices and research delivery teams. This is taking place in the context of the recovery of wider NHS services and resourcing the high number of studies is challenging. Throughout this, the resilience of the workforce has been remarkable.
Recovery of the UK’s capacity to deliver clinical research is essential if we are to deliver the ambitions set out in this phase 2 plan. Indeed, many of the challenges the vision seeks to address have been exacerbated by the pandemic, so Research Reset and reform go hand in hand.
Since summer 2020, all delivery partners across the sector have been working to restore the diverse and balanced portfolio of studies which were impacted due to the COVID-19 pandemic. While this has had some positive impact, it has not resulted in the restoration of activity across all studies that were underway before the pandemic. We are taking further action through the Research Reset programme to build back a thriving, sustainable and diverse research and development portfolio within the NHS.
Our objective in implementing Research Reset is to give as many studies as possible the chance of completing and yielding results, generating the evidence needed to improve care and sustain our health system. However, this will require closure of studies that are not viable in the current context to free delivery resources in the system for those studies that can deliver. Lessons must also be learned to reform and increase the resilience of our research system. As part of this we have asked funders and research sponsors to review their active studies to assess the viability of delivering these within the capacity available.
Our aim is for 80% of all open studies on the NIHR CRN portfolio to be delivering to time and target by June 2023. We will take an agile approach to the Research Reset programme, continuously assessing whether further action is required with the input of stakeholders across the sector including patients and the public.
The devolved governments support this approach and we are working together across the UK to ensure synergistic arrangements are in place to promote the smooth delivery of cross-border studies. Each devolved administration will also review possible new eligibility criteria for national delivery support to ensure deliverability within available resources is feasible.
A sustainable and supported research workforce
The UK clinical research workforce has been fundamental to our collective success to date and will be critical to the achievement of our vision in the future. Healthcare and research staff of all backgrounds must be offered rewarding, challenging and exciting careers within clinical research, so that the most talented people can be brought into clinical research, including research delivery and R&D management, as a life-long career. This will help to bolster the capacity of the clinical research system and support a motivated and sustainable workforce. Collectively, we can realise the potential of UK clinical research to improve outcomes for people across the country, sustain our NHS and improve the economy.
Progress over the past 12 months:
- in England, to support the drive to recover the portfolio, DHSC provided over £30 million of additional funding via the NIHR Clinical Research Network (CRN) in the 2021 to 2022 financial year to increase research delivery capacity, especially in community settings and with a key focus on achieving flexibility and agility in the workforce. The Welsh Government provided £1.7 million to support additional capacity in order to achieve the recovery of non-COVID-19 research, including development of research capacity outside of hospital settings. £3 million of funding from the Department of Health in Northern Ireland has been provided to support the work of a Taskforce established to address clinical research recovery in Northern Ireland
- the NIHR , working with the devolved administrations, launched a UK census for nurses and midwives working in clinical research in order to understand the true size of this workforce. Data was also sought on location, speciality and banding or grade. It was able to identify that there are at least 7,469 research nurses and midwives across the UK and Ireland working at every level and within all areas of healthcare. This census demonstrates the breadth and depth of nurse and midwife involvement in research across the healthcare sector
- in June 2021, NIHR on behalf of the UK launched a new UK-wide professional accreditation scheme for Clinical Research Practitioners ( CRP ) as part of efforts to double the number of this important workforce over the next few years. Over 1,000 members have already signed up to the CRP directory
- NIHR also launched the UK wide Associate Principal Investigator Scheme, which aims to make research a routine part of clinical training so doctors, nurses and allied health professions can become the principle investigators of the future. Over 1,000 health and care professionals had registered for the scheme by April 2022
- in February 2022, Wales published a vision for research career pathways that outlines recommendations to improve support and encourage more health and social care professionals to embark on research careers
Phase 2 commitments
To continue this progress and build towards a sustainable and supported research workforce, we will ensure we can retain and recognise our expert staff and develop robust workforce plans to provide the basis for strategic investment in capacity development:
- the RRG Programme will lead the development of a cross-sector research workforce plan to support implementation of our vision in full. Developed over 2022 to 2023, this plan will guide additional investment in our workforce from 2024
- RRG partners will ensure workforce plans developed by key healthcare organisations include research requirements, particularly noting the knowledge and skills needed across the wider workforce to deliver research as an essential part of high-quality care. This will include the NHS People Plan , coordinating with Health Education England and DHSC, and equivalent plans in the devolved administrations
- NHS England, working with its partners is developing a comprehensive, long-term NHS workforce plan. This will include consideration of research requirements to support the delivery of high-quality care
- Health Education and Improvement Wales, working closely with Welsh Government and the NHS, will develop plans to support and facilitate the nursing, midwifery, allied healthcare professionals and health sciences professions in embracing research as part of their roles and career pathways. Through the development of competency and skills frameworks, Health and Care Research Wales is working to support the inclusion of research delivery roles
- NIHR will provide investment to support NHS R&D transformation, increase research capacity including nurses, midwives and allied healthcare professionals, and provide more opportunities for rewarding careers in research
- the RRG partners will expand the package of training programmes for the research workforce including through the RCP- NIHR Credentialing Scheme, the NIHR Associate PI scheme, the NIHR Nurse and Midwife Leaders Programme, an NHS England programme for executive nurses in Trusts and Integrated Care Systems (ICSs), and a research matron’s toolkit
- NIHR and the devolved administrations will invest in learning and support for researchers, so that they are equipped with the expertise and cultural competency to design and deliver people-centred studies to meet the needs of patients, service users and the public, including those from underserved communities and groups not traditionally served by research
- in support for NHS R&D transformation, Wales will invest in a new Health and Care Research Wales Faculty, which will include increased investment in the NHS Research Time scheme to help develop the next generation of principal and chief investigators in the NHS alongside enhanced mentorship schemes
Clinical research delivery embedded in the NHS
Our aim is to create a step change in the delivery of clinical research in the NHS, so that research is increasingly seen as an essential part of healthcare. Making research an intrinsic part of clinical care means that patients and service users can expect to have access to the most cutting-edge treatments and technologies. We want the NHS to actively participate in generating evidence about effective diagnosis, prevention and treatment through research. By acknowledging the important role of the whole of the healthcare workforce in clinical research delivery, we can ensure everyone is empowered to get involved in research and further boost overall capacity for research in the NHS and wider health system. Measuring clinical research will also support NHS leaders to drive behaviour change and incentivise more engagement in research activity. Finally, ensuring clinical research is embedded within the NHS will be essential in giving the UK the capacity to grow in an increasingly competitive global market.
Progress in Phase 1:
the UK Research and Development (UKRD) and NHS R&D Forum, with NIHR , developed the ‘Best Patient Care, Clinical Research and You’ online guide that aims to help busy non-research staff become more aware of the impact of research in their trust
the General Medical Council (GMC) published its position statement Normalising Research - Promoting Research for all Doctors
the Allied Health Professions’ Research and Innovation Strategy was published, addressing the key areas which impact research and innovation across all health professions in England
the NHS Chief Nursing Officer (CNO) for England published the strategic plan for research for nurses. The plan aims to create a people-centred research environment that empowers nurses to lead, participate in and deliver clinical research that is fully embedded in practice and professional decision making
together with existing strategies in the devolved administrations, we are continuing the development of UK-wide support for the key professional groups
In order to more deeply embed clinical research in the NHS, we will take action to broaden responsibility and accountability for research across the NHS, and improve measurement, visibility and recognition of those supporting the delivery of clinical research studies. The role of healthcare leaders and professionals will be vital in this:
NHS England and the devolved administrations will each develop clear and tangible plans to work towards embedding responsibility and accountability for research in healthcare delivery
- NHS England and the devolved administrations will use existing legal duties and planning frameworks to promote and facilitate research. Each administration will develop assurance frameworks and use existing channels such as annual reports and joint forward plans to help cement the importance of research as a core duty. In England this will include the implementation of the Health and Care Act . Integrated Care Boards (ICBs), NHS England and the Secretary of State for Health and Social Care will all have enhanced duties to report on how they are promoting and facilitating research. NHS England will also lead development of a research framework for ICBs to help them understand and fulfil the minimum expectations around research that the Health and Care Act sets. This will herald a significant shift in how research is considered within the NHS and drive a greater responsibility for more research activity across all sites. In Wales, we will explore opportunities provided through the development of the NHS Executive in Wales to strengthen the national oversight of NHS research
- we will work across the UK administrations to introduce new metrics and measures to increase the visibility and recognition for undertaking and supporting clinical research across NHS organisations
- NIHR , working in partnership with NHS England and the devolved governments, like the Scottish Health Research Register (SHARE), will continue to enhance the UK Be Part of Research platform through collaboration with other existing registries. National digital channels (for example the NHS App or NHS website) will feed into the Be Part of Research platform
The RRG programme will ensure strategic co-ordination of this work across the UK clinical research ecosystem, supporting progress and ensuring alignment of initiatives, as well as identifying key areas where we can go further in the next 3 years.
People-centred research
The vision set out our ambition for more people-centred research, designed to make it easier for patients, service users and members of the public to access research of relevance to them and be involved in its design. To achieve this, delivery of research in community, primary care and virtual settings needs to increase, with delivery designed around the needs of the people participating in it. Alongside this, we will ensure we maintain our world-leading specialist research infrastructure, which provides opportunities for people to access early-phase studies, complex therapies and devices.
- delivering studies such as PANORAMIC and IBS-RELIEVE has demonstrated the UK’s growing ability to harness technology and conduct studies virtually and in the community
- HRA and MHRA, in collaboration with NHS Research Scotland, Health and Social Care Northern Ireland (the equivalent to the NHS in Northern Ireland), and Health and Social Care Research Wales, have published UK-wide guidance on the set up of interventional research to enable research to be delivered across organisational boundaries and to help take research to where people might find it easier to take part, for example using hub and spoke models
- the NIHR led UK Working Group on Remote Trial Delivery published a report in June, which discussed the challenges and opportunities in remote trial delivery and provided guidance for researchers
- the NIHR Race Equality Framework was piloted by industry. This self-assessment tool helps organisations to improve racial equality in health and care research
- partners across the UK are working together to ensure patient and public involvement in research in a variety of ways including through regulation, ethics, payment for public contributors and development of new public engagement strategies. This includes the publication of a shared commitment to public involvement in research to ensure involvement is built into study design, delivery, and dissemination
- in Northern Ireland the Clinical Research Recovery Resilience and Growth Taskforce implementation plan includes a patient and public engagement and involvement sub-group, which is focused on the development of patient and public centred priorities, and an innovation sub-group planning approaches to innovative and people-centred trial design
- in Wales, the ‘Discover your Role’ programme is underway, with a co-created action plan to ensure that people are at the heart of new developments in research
- the NHS Research Scotland patient and public involvement workshop series completed and reported in September 2021. Findings from the workshops and the Scottish Patient Public Involvement Survey are informing work to support greater visibility and connectivity, increased diversity and representation, and a review of the current mechanisms for pre-award funding
- RRG partners have partnered with the International Standard Randomised Controlled Trial Number (ISRCTN) registry to make it easy for researchers to fulfil their transparency responsibilities. Trial registration is the first step to ensuring research transparency from the outset, and from 2022 the HRA began automatic registration of clinical research with ISRCTN , taking the burden away from research sponsors and researchers
Our aim will be to achieve a sector-wide, sustained shift in how studies are designed and delivered so that inclusive, practicable and accessible research is delivered with and for the people with the greatest need and in ways that enable us to tackle the greatest challenges facing the NHS. The UK’s ability to deliver diverse trials and studies will also give us a competitive advantage on the global stage, attracting researchers from around the world to base their studies here:
- the HRA is leading a cross-sector project , co-produced with public contributors, to collect evidence about how high quality, people-centred clinical research is done well: finding out what matters most, what ‘good’ looks like and what might be making it difficult. It will make recommendations to help improve the way clinical research happens in the UK and disseminate information about actions and resources developed by partners
- NIHR will invest in the development of skills and tools for innovative trial delivery, increasing the confidence and ability of our researchers to design and deliver studies in people-centred ways
- NHS England will launch a toolkit that could be used by researchers across the UK to help them engage more effectively with selected underserved communities. NIHR will also promote increased use of the resources developed by the NIHR INCLUDE project project which enable researchers to increase inclusion of underserved communities in their research
- NIHR and NHS Digital will develop mechanisms to monitor the diversity of people participating in NIHR Clinical Research Network portfolio studies in England in order that we can understand where improvement is needed and what action will be most effective.
- in England, the NHS Accelerated Access Collaborative will invest in demand signalling (the process of identifying, prioritising and articulating the most important research questions) and horizon scanning (the process of identifying and better understanding emerging transformational technologies of potential benefit to the NHS and our communities) to improve identification of the most needed treatments and technologies and rapidly bring these into clinical use
- in Scotland, SHIP is leading the new Scottish Health and Industry Partnership Demand Signalling Plan. This new framework will support identification and decision making around key strategic challenges and operational pressures to accelerate NHS Scotland Re-mobilisation, Recovery and Re-design, aligning with delivery of the NHS Recovery Plan 2021 to 2026, and the Life Science Vision healthcare missions
- medical research charities play an important role in supporting people-centred research, utilising their contacts with patients and communities, and prioritising their needs when setting a research agenda. The Association of Medical Research Charities (AMRC) will be working with NIHR and NHS England to formalise this work – and will share findings once developed across the UK
The RRG programme will ensure strategic co-ordination of this work across the UK clinical research ecosystem, supporting progress and ensuring alignment of initiatives, as well as identifying key areas where we can go further within the next 3 years.
Work is also underway to improve access to research through digitised recruitment as detailed in the section on research delivery enabled by data and digital tools.
Streamlined, efficient and innovative research
Facilitating research to happen quickly and predictably will not only bolster our economy and status as a life sciences superpower, but will also drive innovation, which translates into improved care. We have the opportunity to develop a more flexible and improved regulatory model for clinical research outside the EU in the best interests of patients and the public, and since the publication of the vision we have been building towards our aims of supporting a more streamlined, efficient, and effective clinical research environment.
Progress in phase 1:
- in a new approach to licensing and regulation implemented by the MHRA, NICE, the All Wales Therapeutics and Toxicology Centre (AWTTC) and the Scottish Medicines Consortium (SMC), over 100 innovation passports have been issued through the Innovative Licensing and Access Pathway (ILAP), to robustly and safely support the path to market of the most innovative, transformative treatments
- the combined review from the MHRA and the UK Research Ethics Service, in collaboration with the HRA facilitates speedier set up for clinical research trials by requiring applicants to only make a single application for both Clinical Trial Authorisation (CTA) and Research Ethics Committee (REC) approval. Since January 2022, all new Clinical Trials of Investigational Medicinal Products (CTIMPS) in the UK have been benefiting from the combined review, halving the approval time compared with separate applications over the period 2018 to 2021
- the range of model UK contracts agreed with industry and the NHS has been expanded including the first UK-wide model Clinical Investigations Agreement (UK mCIA) for research in medical devices, and the first Model Confidentiality Disclosure Agreement (mCDA) for use by companies with potential NHS sites has also been launched
- the MHRA ran a public consultation on proposals for legislative changes for clinical research. The proposals aim to promote patient and public involvement in clinical research, increase the diversity of participants, streamline clinical research approvals, enable innovation, and enhance clinical research transparency. The consultation sought the views of the wider public, clinical research participants, researchers, developers, manufacturers, sponsors, investigators, and healthcare professionals to help shape this important future legislation and over 2,000 responses were received
- NHS England published refreshed guidance on Excess Treatment Costs (ETCs), expanding the framework to include studies where Clinical Commissioning Groups are the commissioner for the service where the study takes place and setting out the provider types which can utilise the national payment system in England. From April 2022 the provider thresholds for ETCs has been reduced, meaning that the number of providers who receive ETCs will increase
In our next phase of work, we will streamline processes, further strengthen our regulatory environment and ensure faster approval, set-up and delivery of studies with more predictability and less variation. Significant emphasis will be placed on reducing unwarranted variation in ways of working across sites and other research infrastructure, so that conducting clinical research in the UK is high quality, predictable and reliable. This will be particularly important for commercial contract research as speed and predictability is key to the UK’s competitiveness and our ability to attract global multi-centre research studies into the NHS.
The UK is globally recognised for its scientific expertise and dedicated research infrastructure. However, the devolved healthcare systems and competition between organisations has created a complex landscape which is difficult to navigate and creates barriers for researchers and companies. We will work across the UK clinical research system to ensure it is easier to understand and is attractive as a leading destination to conduct cutting edge clinical studies.
To improve research approvals and strengthen our regulatory frameworks:
- a single UK approval service will replace HRA and HCRW Approval and equivalent process in Northern Ireland and Scotland, and site permission and confirmation processes across the UK
- MHRA will work with HRA in continuing the development of IRAS to streamline health technology and medicines research, and HRA will explore whether it is viable to embed a fast-track ethics review as part of combined review
- HRA will lead UK-wide work to further expand the suite of model agreements, including decentralised and other innovative delivery models as well as particular fields of innovative products such as Advanced Therapy Medicinal Products
- following public consultation on proposals for legislative changes for clinical research, the MHRA is now carefully analysing the responses received, preparing a Government response and developing secondary legislation to improve and strengthen our clinical research legislation
- MHRA will support risk-proportionate trial conduct and monitoring, including through Good Clinical Practice (GCP) guidance and pragmatic investigator guidance, and will work with HRA to develop guidance on use of in vitro diagnostics (IVDs) in clinical research
- MHRA and HRA will also establish a comprehensive stakeholder reference group to assist with guidance generation on new legislation and ensure there is a common understanding of regulatory requirements that will enhance the UK’s international attractiveness as a place to conduct multinational trials
To improve study set-up:
- learning lessons from delivering COVID-19 research, we will enhance our early feedback service offer via the NIHR CRN to support study design that is optimised for delivery and explore how we can further match research delivery demand to capacity across the UK
- we will implement the UK-wide National Contract Value Review (NCVR), with the aim of expediting the costing elements of the contracting process across NHS Trusts to ensure costing does not delay study set-up. From 1 April 2022, the NCVR will begin to replace the current time-consuming process whereby each NHS organisation negotiates with each commercial sponsor for every study in order to agree bespoke contract value. The programme will be monitored throughout implementation to ensure lessons can be learnt and the process improved to ensure it achieves its aims. The existing single cost and contract review model in Scotland and across the NIHR Patient Recruitment Centres in England will integrate with NCVR as it develops, supporting more effective UK alignment and efficiency
- the Experimental Cancer Medicine Centre (ECMC) Network, with support from MHRA and HRA, will complete their pilot to set up Phase I oncology trials within 80 days of IRAS submission. Learning from this programme will be shared to enable improved set-up performance in other specialities
- RRG programme partners will identify and establish mechanisms to achieve efficient costing and contracting across other parts of the health system, supporting and enabling an increase in decentralised study designs and research taking place in primary care and community settings.
- DHSC and NHS England will lead a review of their current Excess Treatment Costs (ETC) process in England to review experiences of the policy and t explore how best we can support non-commercial research in the NHS
To make the UK offer easier to navigate:
- understand UK capabilities to deliver their study at all stages of the protocol development and delivery pathway
- connect with the right part of the system to help them at the right time
- access the network of expertise and resources available to create a package of support to deliver studies efficiently
- MHRA, NICE, AWTTC and SMC will work with partners across the UK to develop ILAP as an effective route into the UK research system, particularly through the development of a support toolkit
- the further development of IRAS will also provide navigation and signposting through the research journey, directing applicants to relevant guidance and advice. Through interfaces with other systems it will reduce burden and duplication
Research delivery enabled by data and digital tools
The UK’s health data offering is one of our global strengths due to our national health systems and cradle-to-grave healthcare records. Investing in data and digital tools, and making ethical use of them to support clinical research, for example by making it easier to recruit and follow-up participants, increases the efficiency and effectiveness of the clinical research process. These tools also increase the resilience and sustainability of the healthcare system and reduce the burden on the NHS workforce.
- the data strategy for health and social care in England was published in June 2022
- up to £200 million committed to support NHS-led health research (subject to business case) was announced on 2 March 2022 to invest in health data infrastructure to support research and development in England, with parallel activity in the devolved governments
the NHS-Galleri trial demonstrated the potential for the use of healthcare data to support rapid, large scale recruitment to and delivery of clinical studies in the NHS. The Accelerated Access Collaborative (AAC), led by NHS England, coordinated the design and set up of a 2 part, real-world demonstration project involving clinical data capture from NHS Digital and NIHR , and was a demonstrator for the ‘Find, Recruit and Follow-up’ service and NHS DigiTrials. The trial has already passed the halfway point in their recruitment of participants, with over 100,000 enrolled following the launch in autumn 2021
- each delivery partner funded as part of year one of the ‘Find Recruit and Follow-up’ service launched Minimum Viable Products (MVPs) of their services including: NHS DigiTrials, which has successfully facilitated 28 active trials through its service with a further 8 in application and 12 in pre-application; NIHR CRN launched its early stage ‘concierge’ service, with 2 companies and 4 data service providers as early users; and HRA, which agreed an approach to review by the Confidentiality Advisory Group which will enable more efficient study set up in future. In addition, the MHRA Clinical Practice Research Datalink (CPRD) has launched SPRINT (Speedy Recruitment into Trials ), a data-enabled research service that facilitates rapid feasibility and patient recruitment into industry sponsored phase 2 to 4 trials across the UK
- making use of real-world data (RWD) in and for clinical research is now a reality, supported by MHRA’s published guidance . This is the start of a series of guidelines to provide general points to consider for sponsors planning to conduct clinical research using RWD to support regulatory decision making
The next 3 years will see a revolution in how we use data across the health system. We will go further in utilising innovative data-driven methods and digital tools to transform the way we design, manage and deliver people-centred clinical research studies across the whole of the UK. We will achieve this by increasing the use of data and digital tools in recruitment and follow up, and by improving access to data via Trusted Research Environments (TREs: a type of Secure Data Environment, secure spaces where approved researchers can access rich, linked datasets) and through increased partnership working across the UK health data ecosystem.
We are very clear that the opportunity to use health data must be done in a way which is secure and trusted by members of the public, so governance and oversight processes must be both as efficient as possible and transparent, robust and trustworthy. Public trust and understanding of how data is being used to support research continues to be critical in developing appropriate activities. We will be working together to consider how to implement recommendations from the Goldacre Review , and ensuring that all work is supported by comprehensive public involvement and engagement activity.
To improve study planning, recruitment and follow-up:
- the Find, Recruit and Follow-up service will work across the 4 administrations to consider how activity can be expanded to include SAIL, Scottish Health Research Register, data infrastructure in Northern Ireland, NIHR BioResource and other key national data infrastructure, increasing opportunities for people to quickly and easily access research of relevance to them
- NHS DigiTrials and CPRD (via MHRA) will enable a significant increase in the scale of identification of people who match the eligibility criteria for specific studies in order that they can be given the opportunity to participate in research. They will also support increased use of routine healthcare data to streamline reporting of follow-up data, increasing predictability and releasing delivery capacity in the NHS
- in England, the Data for R&D Programme will invest in health data infrastructure for research and development, supported by comprehensive PPI and engagement throughout the programme, including embedded within its governance
- NIHR will invest in data and digital platforms such as Be Part of Research and NIHR BioResource, and provide the tools and support necessary to deliver virtual and decentralised studies. Increased interoperability between regulatory, NHS and NIHR platforms will enable further streamlining of processes for researchers
- in Wales, a digital recruitment programme will be developed through partnership between Health and Care Research Wales, SAIL Databank and the NHS Wales National Data Resource programme, to develop services that utilise data resources to drive research delivery. An Expert Working Group has been established to guide on the development of this ‘data for research’ programme. A pilot service has been funded to use SAIL data to provide rapid intelligence to aid placement of research trials in Wales to support most effective recruitment
- in Scotland, scoping work and stakeholder engagement is informing plans for developments to support increased use of NHS data and digital technology to accelerate clinical trial delivery, and for further development of the Scottish Health Research Register (SHARE) to support recruitment to health research studies. We will continue to support the already established regional NHS Scotland controlled data safe havens (Trusted Research Environments) and their collaboration with the newly established Research Data Scotland to support use of data in research. We will also look for opportunities to support research and innovation as part of the forthcoming Scottish Government Data Strategy for Health and Social Care
- in Northern Ireland, the RRG Taskforce data and digital sub-group will lead work to prepare the NI data infrastructure to support digitally-enabled trials and participate in UK-wide initiatives such as the ‘Find, Recruit and Follow-up’ service.
To improve access to data and TREs:
- over the next 3 years NHS England will build upon foundational investments made in 2021 and 2022 in an interoperable network of TREs. At a national level, we will expand the scale, scope and capacity of the NHS Digital TRE to enable more users to have timely and secure access to a range of national datasets. At a regional level, we will develop a small network of regional ‘Sub National TREs’ in England, each covering a population of more than 5 million citizens and enabling access to near real time, multimodal data particularly amenable to the development of AI algorithms
- the Data for R&D Programme within NHS England will expand the ability for researchers to access a range of rich linked genomic datasets, creating linkages across the various health data systems so that genomic data can be used to support innovation and patients and service users can benefit from the provision of innovative genomic healthcare. The Genome UK Implementation Coordination Group Data Working Group will lead work looking to link genomic datasets from across the UK, and federate these where appropriate, as set out in the Genome UK: shared commitments for UK-wide implementation 2022 to 2025
- in Scotland, we will continue to support the already established regional NHS controlled TREs and their collaboration with the newly established Research Data Scotland to support use of data in research
- in Wales, we will continue to invest and grow the internationally recognised expertise and TRE available via the SAIL Databank, offering national population coverage and secure access to billions of person-based records
- in Northern Ireland, the Honest Broker Service and the more recently established Northern Ireland TRE will be supported to further develop secure access to data for research. This will sit alongside a sustained public dialogue and progression of the enactment of secondary uses legislation to facilitate data access for research in Northern Ireland.
Connecting these developments into a coherent UK offer will bring added benefit, therefore to unite plans:
- the RRG programme will ensure strategic co-ordination of this work across the UK clinical research ecosystem, supporting progress and ensuring alignment of initiatives, as well as identifying key areas where we can go further within the next 3 years to take steps towards fully realising our overarching vision
- an RRG data and digital subgroup will be established to enhance collaboration across the sector and ensure people across the whole of the UK benefit from research delivered using data and/or digitally-enabled approaches
Governance, detailed plans and ongoing updates
The UK Clinical Research RRG programme will oversee the delivery of this plan, continuing to work in partnership with stakeholders across the sector and regularly revisit the original vision to consider any further actions needed to deliver on the 10 year vision. In doing so, we will ensure that the NHS is able to tackle the healthcare challenges of the future enabling people across the UK and around the world to benefit from better health outcomes.
Given the scope of the work and the fast pace of change in clinical research, we will keep the specifics of this plan under review via the RRG programme and adapt delivery as needed. This flexibility will allow us to meet emerging challenges and ensure that the outcomes are aligned to the most pressing issues to realise our shared ambitions.
Progress will be measured by the RRG Programme Board and the Ministerially-chaired Oversight Group, ensuring we are delivering on the commitments set out in this plan and that they are having the intended impact on the UK clinical research system. Specific measures for success will be published on the RRG website later in 2022.
We will publish a Phase 3 plan in 2025 to 2026 to align with the next government spending review period. The Phase 3 plan will showcase our progress and lay out the next steps needed to ensure the vision is delivered.
Achievement of our plan will require action across the whole sector, but by building on the foundations of collaboration and partnership that we have created through RRG programme we can collectively work through current challenges and see this vision become a reality.
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. We’ll send you a link to a feedback form. It will take only 2 minutes to fill in. Don’t worry we won’t send you spam or share your email address with anyone.

"The CCRA greatly welcomes the review, announced last week, into commercial clinical trials in the UK. The review will be led by Lord James O’Shaughnessy and will address the worrying challenges that currently confront the industry. It is intended that the review will put forward recommendations that will restore the global competitiveness of the UK industry and support for NHS patients. We look forward to working with Government and our colleagues, from all organisations in the field, to find solutions to the issues that are being faced, particularly by SME’s in the CRO industry."
- Becoming a participant in clinical trials
- Frequently Asked Questions (FAQs)


IMAGES
COMMENTS
Global Contract Research Organizations in United Kingdom. 54gene's Clinical Programs Group (CPG) Website: www.54gene.com Email: [email protected] Phone: +971 58 594 9926; +1 310-266-9926. 54gene's Clinical Programs Group (CPG) is a contract research organization that provides innovative, customizable, and cost-effective clinical trials solutions...
Ensuring safety and compliance: The essentials of outsourcing pharmacovigilance. ICON is the world's leading clinical research organisation, providing outsourced clincal development and commercialisation services to the pharmaceutical, biotechnology and medical device industries.
London's Leading Clinical Research Organisation (CRO). We have carried out over 650 successful trials since 1993. Clinical trials of study drugs for pharmaceutical and biotechnology companies worldwide. ... HMR is now the biggest CRO of its kind in the UK and one of the biggest in Europe. We have 270 staff, including a resident 24/7 ...
Klaria AB. Simbec-Orion is an experienced, full-service Contract Research Organisation (CRO), with offices across the UK, Europe, and the United States. Established for over 45 years, we provide clinical trial expertise to a wide range of small to mid-sized biotech and pharmaceutical partners across Europe, North America and beyond.
PHARMExcel is an award-winning, full-service Contract Research Organisation (CRO) providing a flexible and innovative approach to clinical trial delivery.. The company offers in-depth knowledge and experience of the clinical research environment, particularly in the UK, and has a network of regulatory, and other industry associates, allowing us to provide a global reach.
We are the UK's leading clinical research organisation, successfully conducting over 300 early phase studies since 2001. Speak to our team of experts today. By clicking "Accept All Cookies" , you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Clinical Research Organisation - Ixia Clinical. IXIA CLINICAL is an adaptable Clinical Research Organisation (CRO) based just outside London, UK, with a team of experienced clinical professionals operating globally in the pharmaceutical and biotechnology industries. Our expert networks of knowledgeable clinical research consultants can provide comprehensive assistance to support your ...
Established in the UK in the year 2012, ASSAY Clinical Research started with a group of clinical research professionals who have been providing a variety of services for the pharmaceutical industry and other clinical research organizations. Gradually, ASSAY has gained recognition for its quality, timely services with affordable costs.
The UK Clinical Research Collaboration (UKCRC) Partners' goal is to establish the UK as a world leader in clinical research. The UKCRC provides a forum that enables all Partners to work together to transform the clinical research environment in the UK. The forum promotes a strategic approach to the identification of opportunities and obstacles ...
Model Clinical Trial Agreement (mCTA) and Clinical Research Organisation Model Clinical Trial Agreement (CRO-mCTA) (January 2021) - Guidance (January 2021) 3 | P a g e The first CRO-mCTA was published in 2007. Based on mCTA 2006, it allowed unmodified use of a template agreement in circumstances where a CRO undertook site
9. KCR. Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
As of June of 2021, the American clinical trial database (clinicaltrials.gov) displays a total of 20,182 registered clinical studies for the United Kingdom, which shows the leadership of the UK in the global clinical research market. Together with world-class research funders, the NHS, research charities and global pharmaceutical companies, the ...
These provide a network of research expertise and clinical leadership to deliver research studies on the NIHR CRN Portfolio of studies. Find out more about our performance and key statistics relating to our activity. From April 2024, the CRN will transition to a new organisation, the NIHR Research Delivery Network (RDN). Research Delivery Network
To promote the benefits of clinical contract research to the pharmaceutical and biotechnology industries. To work to safeguard the health, welfare and interests of all participants in clinical trials. To provide networking opportunities for our members both in the UK and overseas. To provide training courses tailored to the needs of members.
It is a partnership between the UK government, charities and industry. The aim of the NCRI is to oversee and coordinate cancer research in the UK. And to continue to improve the quality and relevance of cancer research. Email: [email protected]. Phone: 020 3469 8798. Visit the NCRI website.
MAC Clinical Research is the UK's leading clinical trials organisation. As an award winning healthcare organisation, we are dedicated to developing new and improved treatments for a range of medical conditions. Every year across the UK thousands of people take part in medical trials. Volunteers, just like you, play a significant role in ...
The global clinical trials market was estimated to be worth $38.7 billion in 2021 and is expected to reach £52 billion by 2026. In this article, we look at the top 12 CROs in the world, highlighting the companies driving this considerable growth and accelerating research and development across the globe.. ICON. Recognised as the world's largest and most comprehensive CRO powered by ...
A results driven, bespoke Clinical Research Organisation (CRO) with an agile and expert team to meet your clinical research needs. Skip to content. Our Company. ... Surrey Research Park Guildford GU2 7YG UK Tel: +44 (0)1483 977 777. Level 21, Al Habtoor Business Tower, Dubai Marina, UAE Tel: +971 4 453 2803. CRO Services. Project management;
A leading global Biometric Clinical Research Organization (CRO) dedicated to providing expertise in statistical analysis, data capture and clinical trial reporting ... Address - UK HQ: Quanticate. Bevan House, 9-11 Bancroft Court Hitchin, Hertfordshire SG5 1LH United Kingdom Tel: +44 (0)1462 440 084 Fax: +44 (0)1462 440 086
Parexel is among the world's largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals works in ...
Parexel is proudly among the world's largestclinical research organizations. A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical ...
Executive summary. In March 2021, we published our bold and ambitious 10 year vision: Saving and Improving Lives: The Future of UK Clinical Research Delivery. This was followed in June 2021 by The ...
The CCRA 2024 Summer Reception will be held on Thursday, 11th July 2024 from 18.30 - 21.00 hrs, for further details contact [email protected].