Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations
Affiliation.
- 1 WHO, Immunization, Vaccines and Biologicals, 20 Ave Appia, CH-1211 Geneva 27, Switzerland.
- PMID: 28596091
- DOI: 10.1016/j.vaccine.2017.05.069
This article presents the World Health Organization's (WHO) recommendations on the use of human papillomavirus (HPV) vaccines excerpted from the WHO position paper on Human papillomavirus vaccines: WHO position paper, May 2017, published in the Weekly Epidemiological Record [1]. This position paper replaces the 2014 WHO position paper on HPV vaccines [2]. The position paper focuses primarily on the prevention of cervical cancer, but also considers the broader spectrum of cancers and other diseases preventable by HPV vaccination. It incorporates recent developments concerning HPV vaccines, including the licensure of a nonavalent (9-valent) vaccine and recent data on vaccine effectiveness, and provides guidance on the choice of vaccine. New recommendations are proposed regarding vaccination strategies targeting girls only or both girls and boys, and vaccination of multiple birth cohorts [3]. Footnotes to this paper provide a number of core references including references to grading tables that assess the quality of the scientific evidence, and to the evidence-to-recommendation table. In accordance with its mandate to provide guidance to Member States on health policy matters, WHO issues a series of regularly updated position papers on vaccines and combinations of vaccines against diseases that have an international public health impact. These papers are concerned primarily with the use of vaccines in large-scale immunization programmes; they summarize essential background information on diseases and vaccines, and conclude with WHO's current position on the use of vaccines in the global context. Recommendations on the use of HPV vaccines were discussed by SAGE in October 2016; evidence presented at these meetings can be accessed at: www.who.int/immunization/sage/meetings/2016/october/presentations_background_docs/en/.
Keywords: Cervical cancer; HPV; Human papillomavirus; Licensurenonavalent(9-valent) vaccine; Vaccination strategies; WHO position paper; World Health Organization.
Copyright © 2017. Published by Elsevier Ltd.
- Health Policy
- Immunization Programs / standards
- Immunization Schedule
- Papillomavirus Infections / immunology*
- Papillomavirus Infections / prevention & control*
- Papillomavirus Vaccines / immunology*
- Papillomavirus Vaccines / standards*
- Public Health / standards
- Vaccination / standards
- World Health Organization
- Papillomavirus Vaccines

Grants and funding
- 001/WHO_/World Health Organization/International
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Human papillomavirus...
Human papillomavirus vaccination and cervical cancer risk
- Related content
- Peer review
This article has a correction. Please see:
- Human papillomavirus vaccination and cervical cancer risk - December 07, 2023
- Lisa Rahangdale , professor 1 2 3 ,
- Chemtai Mungo , assistant professor 1 2 3 ,
- Siobhan O’Connor , associate professor 4 ,
- Carla J Chibwesha , associate professor 1 5 ,
- Noel T Brewer , Gillings distinguished professor 2 6
- 1 Department of Obstetrics and Gynecology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
- 2 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
- 3 Center for AIDS Research, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
- 4 Department of Pathology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
- 5 Clinical HIV Research Unit, University of the Witwatersrand, Johannesburg, South Africa
- 6 Department of Health Behavior, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
- Correspondence to: L Rahangdale lisa_rahangdale{at}med.unc.edu
Persistent human papillomavirus infection is the central cause of cervical cancer, the leading cause of cancer death among women worldwide. Clear evidence from both randomized trials and population based studies shows that vaccination against human papillomavirus reduces the incidence of cervical pre-cancer. These data suggest that the vaccine reduces the incidence of cervical cancer. However, human papillomavirus vaccine coverage is inadequate in all countries, especially in low and middle income countries where disease burden is highest. Supply side strategies to improve coverage include increasing the availability of low cost vaccines, school located delivery, single dose vaccine schedules, and development of vaccines that do not need refrigeration. Demand side strategies include enhancing provider recommendations, correcting misinformation, and public awareness campaigns. The near elimination of cervical cancer is achievable through increased uptake of human papillomavirus vaccination and efforts to increase screening for cervical cancer, especially when enacted to reduce disparities in across the world.
Introduction
The progression of cervical cancer is well characterized ( fig 1 ). 1 2 High risk human papillomavirus (hrHPV) infects metaplastic cells at the cervical transformation zone and integrates into the host genome, 3 leading to inactivation of the tumor suppressor genes p53 and Rb , cell proliferation, and accumulation of mutations. 4 Genetic predisposition, hormonal factors, host immune response, and cigarette smoking increase susceptibility to hrHPV infection. .3 As persistent human papillomavirus infection is the central cause of invasive cervical squamous cell carcinoma (ICC), 5 and pre-cancerous lesions are generally detectable, prevention of cervical cancer relies primarily on preventing infection through human papillomavirus vaccination (primary prevention) and detecting and treating pre-cancerous lesions (also known as high grade cervical intraepithelial neoplasia (CIN2/3) or adenocarcinoma in situ (ACIS)) before they progress to cancer (secondary prevention).

Progression of cervical disease after human papillomavirus infection. CIN=cervical intraepithelial neoplasia. Adapted from references 1 and 2
- Download figure
- Open in new tab
- Download powerpoint
The first human papillomavirus vaccine was introduced into clinical care in 2006. 6 Bivalent (human papillomavirus 16/18), quadrivalent (human papillomavirus 6/11/16/18), and nonavalent (human papillomavirus 6/11/16/18/31/33/45/52/58) vaccines are prequalified by the World Health Organization and widely licensed. 7 All available vaccines provide protection against human papillomavirus 16 and 18, as approximately 70% of cervical cancers worldwide are attributable to these two virus types 5 ; the nonavalent vaccine prevents subtypes that account for an additional 19%. 8
WHO set a target for 194 countries to adopt human papillomavirus vaccination by 2030 in its Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem . 9 By 2020, however, only 114 countries had introduced human papillomavirus vaccines; most of these are high income countries. 10 Less than 25% of low income countries have human papillomavirus vaccination as part of their national immunization schedules. Most gaps in the introduction and coverage of human papillomavirus vaccine are in regions of Africa and Asia where the burden of cervical cancer is also high. 10
Global efforts to nearly eliminate cervical cancer focus on expanding access to human papillomavirus vaccination and cervical cancer screening. 9 In this article, we summarize clinical data on the efficacy and effectiveness of human papillomavirus vaccination, its potential impact on incidence of ICC, and strategies to increase access to and uptake of vaccination for general practitioners and specialists in positions to offer human papillomavirus vaccination to individual patients and/or affect policy.
Sources and selection criteria
We developed the tables to illustrate the evidence on the impact of human papillomavirus vaccination on cervical pre-cancer and, potentially, cervical cancer. We searched PubMed, Embase, and CINHAL by using the following predefined terms: (HPV OR human papillomavirus OR human papilloma virus) AND (vaccine OR vaccines OR immunization OR immunisation OR shot) AND (CIN OR cervical intraepithelial neoplasia) AND (impact OR effect OR effectiveness). After the initial search, we selected the “randomized controlled trial” filter under “article type” on PubMed. We also searched lists of references and lists of studies that had cited the specific study, linking to studies published on PubMed or Embase. The literature search for the tables was completed on 16 May 2022 and included studies up to this date. Quality criteria used to select papers for the tables included randomized control trials and population based cohort (registries or databases) studies in which both human papillomavirus vaccination and pathologic diagnosis were documented in the dataset. We did not include studies based on self-report of vaccination or having hrHPV infection as the only endpoint. Additionally, we prioritized the most recent national and international recommendations on human papillomavirus vaccination for inclusion in the human papillomavirus vaccination guidelines and recommendations section.
Epidemiology
Cervical cancer is the fourth most common cancer among women and other people with a cervix worldwide, with a global incidence of 13.3 per 100 000 in 2020. 11 Eight of every 10 cases of ICC occur in low and middle income countries (LMICs)—a disparity driven by inequity in access to prevention and treatment of cancer. 12 13 Africa has an estimated 20% of the world’s ICC, about 120 000 cases per year. More specifically, ICC is the leading cause of death from cancer among women in sub-Saharan Africa, 14 15 which is also the global epicenter of the HIV pandemic. 16 More than 11 million women living with HIV are in sub-Saharan Africa. In addition to many barriers in access to human papillomavirus vaccination and cervical screening, these women face a substantially higher risk of persistent hrHPV infection. 17 18 19 Women living with HIV are also more likely to be diagnosed as having cervical cancer or cervical pre-cancer. 20
Incidence of ICC in high income countries has decreased over the past three decades but remains an important public health concern. 21 Europe is a high income region where an estimated 58 169 women annually are found to have ICC (10.7 per 100 000 women) and 25 989 women die from ICC. 22 By contrast, Australia will have an estimated 942 new diagnoses of ICC (7.1 per 100 000 women) and 222 deaths in 2022. 23 Figure 2 illustrates risks worldwide in relation to a high income country such as the United States. 24 25 The US had 12 795 new cases of ICC (7.5 per 100 000 women) 26 and 4152 deaths in 2019. The numbers have been falling in the past several decades, largely owing to screening for cervical cancer. 27 However, serial national cross sectional household surveys indicate that guideline concordant rates of screening went down between 2005 and 2019 in the US. 28 This drop is likely more significant than reported owing to the tendency to over-report screening in self-report studies, 29 as well as the subsequent covid-19 pandemic.

Numbers of human papillomavirus associated cancers. Adapted from references 21 and 22
The incidence of ICC in Asia (12.7 per 100 000) 30 was lower than in Africa (24.6 per 100 000) 31 but nearly twice that in Europe and the US in 2020. An upward trend in cases is noted in both sub-Saharan Africa and east Asia. 21 The incidence in Latin America and the Caribbean was estimated at 14.9 per 100 000 women in 2020. 32 Disproportionately higher incidence and mortality occurs in Latin America and the Caribbean, with mortality rates three times higher than in North America and accounting for 89% of ICC deaths in the Americas. 33
Impact of human papillomavirus on risk of cervical cancer
Extensive evidence supports the safety and efficacy of human papillomavirus vaccination for prevention of precursors of ICC. A Cochrane review included 26 trials (73 428 participants), of which 10 concluded that human papillomavirus vaccination leads to prevention of cervical pre-cancer, particularly in adolescent girls and women who were negative for human papillomavirus before vaccination. 34 A systematic review and meta-analysis with data for more than 60 million people found that the risk of CIN2+ decreased by 51% (relative risk 0.49, 95% confidence interval 0.42 to 0.58) among 15-19 year old female patients vaccinated against human papillomavirus and by 31% (0.69, 0.57 to 0.84) among vaccinated 20-24 year olds. 35 Table 1 includes multiple randomized clinical trials that were designed to show safety and hrHPV 16/18 genotype specific efficacy for reduction of cervical pre-cancer (CIN2+) after bivalent, quadrivalent, and nonavalent human papillomavirus vaccination. 36 37 38 39 40 Table 2 shows data from multiple population based observational studies using national registries or databases. Vaccine effectiveness for CIN2+ has been demonstrated with one, two, and three doses of vaccine. 41 42 43 44 45 46 47 48 49
Double blinded randomized controlled trials of human papillomavirus (HPV) vaccination (three doses) and efficacy
- View inline
Population based observational studies of human papillomavirus vaccination (one, two, or three doses) and vaccine effectiveness
Given these positive results, interest is increasing in how vaccination programs may reduce the incidence of ICC through herd immunity and achieve the near elimination of cervical cancer. Australia’s national human papillomavirus vaccination program started in 2007 for girls aged 12-13 and in 2013 for boys the same age, with catch-up to age 26. A review of epidemiologic studies in Australia found a decline in the national incidence of CIN2+/ACIS in women up to age 29. 50 The authors estimated that 72% of cervical cancers would be prevented by quadrivalent human papillomavirus vaccine and an additional 15% prevented by the introduction of the nonavalent vaccine. Australia’s prevention program of vaccination and screening is on track to lower ICC incidence to below four per 100 000 women by 2035, making the country the first to nearly eliminate cervical cancer. 51
Data from 2006 to 2017 from Sweden’s national human papillomavirus vaccination program in girls and women aged 10-30 years were used to assess for risk of ICC, specifically. In a female population of more than 1.6 million, Sweden had 538 cases of ICC in the unvaccinated population compared with 19 cases in the vaccinated (at least one dose) population. The adjusted incidence rate ratio was 0.12 (95% confidence interval 0.00 to 0.34) and 0.47 (0.27 to 0.75) for those vaccinated before age 17 or between 17 and 30 years, respectively. 52
A modeling study examined the effect of England’s national human papillomavirus vaccination program that started in 2008 in 12-13 year old girls with catch-up to 18 years. It showed a risk reduction of 97% (95% confidence interval 96% to 98%) for CIN3 and 34% (25% to 41%) for cancer in the cohort vaccinated at ages 12-13 years. A reduction in risk was also seen in those vaccinated in the catch-up group of up to 18 years. 53 Outcomes were based on data from 20-30 year old women, a population with low rates of cervical cancer.
Japan’s initial human papillomavirus vaccination program for 12-16 year old girls started in 2010 and showed a decline in human papillomavirus 16/18 CIN2-3/ACIS from 48% to 33%, especially in women first vaccinated before age 20. 54 Japan suspended its human papillomavirus vaccination program in 2013 (see “Barriers to vaccination” below) and restarted it in 2022. The suspension is estimated to have led to an additional 24 600 to 27 300 diagnoses of cervical cancer and 5000-5700 deaths from cervical cancer. 22 55
Guidelines and recommendations
Several national and international vaccination schedules include human papillomavirus vaccination for primary prevention of cervical cancer, other human papillomavirus related cancers, and genital warts. 56 57 58 59 As of December 2021, all European Union/European Economic Area countries had human papillomavirus vaccination in their national vaccination schedules. Rwanda was the first African nation to implement a comprehensive human papillomavirus vaccination program in 2011 and is the only African country to meet the WHO target of a 90% vaccination rate for girls by age 15. 60 Whereas less than a decade was needed for 80% of high income countries to adopt human papillomavirus vaccination, only 41% of LMICs have been able to do so. 61
All recommendations call for a two dose human papillomavirus vaccination schedule for girls aged 9-14 years, although the starting age varies across countries. If vaccination starts after age 15 or if the individual is immunocompromised, most recommendations are for three doses of vaccine. 56 57 58 62 63 These parameters are similar across countries with national programs, whether high income countries or LMICs. Catch-up vaccination of adults up through 26 years of age for those who have not previously been vaccinated or completed their vaccination series is recommended in most countries, but it is not included in all national programs.
The focus for most national programs is the younger adolescent group, although the nonavalent vaccine is licensed for use up to age 45 years in many countries. The US Centers for Disease Control and Prevention recommends shared decision making between patients and healthcare providers regarding vaccination for adults aged 27 through 45 years, as the public health benefit of human papillomavirus vaccination in this age range is minimal. 58 By contrast, the American Cancer Society does not recommend vaccination in the 27-45 age group, citing lower effectiveness and low potential for prevention of cancer for this group. 63 Ethical dilemmas exist regarding vaccination of older women and boys when access to vaccination is limited in the target adolescent female populations in LMICs, where the burden of cervical cancer is highest, vaccination rates are lowest, and reduction of cervical cancer is the main goal.
Assuming optimal supply and demand for human papillomavirus vaccination, the HPV-FASTER proposal describes an approach to offer catch-up vaccination to 26-45 year olds in combination with targeted cervical cancer screening to accelerate the near elimination of cervical cancer in central and eastern Europe, Latin America, Asia, and some parts of Africa. 64 The proposal indicates that systematic vaccination of women up to age 30, continued opportunity to vaccinate women up to 45-50 years, and an abridged number of visits for human papillomavirus based cervical cancer screening may be a cost effective strategy. 64 Cost effectiveness data from France indicate that 34% of ICC could be averted with vaccination up to age 40 years. 65
A one dose human papillomavirus schedule can reduce infection and early stage disease according to nested observational and pilot studies ( table 2 ). 66 In April 2022 WHO’s Strategic Advisory Group of Experts on Immunization updated its human papillomavirus vaccination dosing recommendations as follows: one or two doses for the primary target of girls aged 9-14 years; one or two doses for young women aged 15-20 years; two doses separated by six months for women older than 21 years. 67 A three dose schedule, if feasible, is still recommended for immunocompromised individuals, including women living with HIV. 67 If countries adopt this new single dose recommendation, barriers related to cost and access to vaccination could be overcome, especially in LMICs with low vaccination coverage.
Gender neutral vaccination programs
WHO’s recommendation also allows for boys and men to get human papillomavirus vaccine on the same schedule as girls and women. Gender neutral human papillomavirus vaccination programs, which include both boys and girls, provide several benefits including more rapid population impact through herd immunity, indirect protection of unvaccinated women, and direct protection of boys and men, including men who have sex with men. 68 Although many high income countries have gender neutral human papillomavirus vaccination programs, around two thirds of the countries that provide human papillomavirus vaccine to adolescents do so only for girls. 69
The human papillomavirus vaccination guidelines of the American Society of Clinical Oncology (ASCO), which stratify recommendations on the basis of resource settings, support extension of vaccination to boys in high resource settings if vaccine coverage is low (<50%) in the priority 9-14 year old female population. This is because the cost effectiveness of vaccinating boys for the purpose of cervical cancer prevention is low, unless vaccine coverage in the target population (girls aged 9-14 years) is also low. 57 Similarly, in resource limited settings, if human papillomavirus vaccination of girls is above 50%, ASCO recommends against vaccination of boys as this strategy is not thought to be cost effective for cervical cancer prevention, specifically. However, if resources allow, human papillomavirus vaccination can be extended to boys to prevent other human papillomavirus related cancers, and modeling shows that a gender neutral approach is cost effective when considering these cancers. 70
Several studies have confirmed the cost effectiveness of a gender neutral strategy when considering the impact on all human papillomavirus related diseases, including penile and oropharyngeal cancer. 68 70 The US, Australia, and about half of European countries currently include boys in vaccination programs. 71 No country in Africa offers human papillomavirus vaccination to boys as part of its national program. 72
Barriers to vaccination
Despite robust safety and clinical efficacy data, global human papillomavirus vaccination coverage for girls is approximately 18% for a first dose and 13% for series completion. 73 The WHO goal for human papillomavirus vaccination is 90% of girls fully vaccinated by their 15th birthday, but few countries have met or are anywhere close to this goal. 9 Whereas countries such as Australia and the UK have high human papillomavirus vaccination coverage, the US is far from its goal of 80% coverage for boys and girl aged 13-15 years. 74 Human papillomavirus vaccination is marked by disparities, with much lower uptake in LMICs where the burden of ICC is highest; few of these countries even offer human papillomavirus vaccine as part of their national immunization schedules. 9 Figure 2 illustrates global differences in human papillomavirus associated cancers.
Global human papillomavirus vaccination coverage dropped for the first time in 2020. 46 Most affected were the Americas and Africa; by contrast, other areas had small increases. Owing to a drop in well child visits, which persists to date, the US provided several million fewer doses of human papillomavirus vaccine in 2020-22 than would be expected on the basis of 2019 levels. 75 Several factors related to supply and demand account for low uptake of human papillomavirus vaccination, even before the pandemic. On the supply side, the cost of human papillomavirus vaccines makes them unaffordable for many LMICs and disproportionately accessible to high income countries. A previous worldwide shortage of the vaccines limiting availability for LMICs has abated, and efforts by Gavi, the Vaccine Alliance, WHO, and other organizations to provide the vaccines at dramatically lower costs have increased access in LMICs. Finally, a continued logistic barrier to administration of vaccine is that currently licensed human papillomavirus vaccines all require refrigeration.
On the demand side, misinformation, cultural views on sex, and mistrust of the medical system have contributed to low confidence in the vaccine by parents. Programmatic problems have further limited demand in some areas. Some countries have experienced unsubstantiated safety scares around the vaccine, which led to drops in coverage. In 2013 the national immunization program in Japan was suspended owing to reports of adverse events in girls, leading to vaccination rates going from 70% to less than 1% of eligible girls. 76 77 After further safety data, reassurance from WHO, insistence from Japanese academic societies, and Japanese data indicating that similar symptoms occurred in unvaccinated girls, the program was restarted in 2022. 76 The importance of the voices of political and public health leaders was also shown when a national information campaign helped Denmark’s human papillomavirus vaccination rates to rebound four years after negative media coverage started in 2013. 78 Pan American Health Organization countries are using monitoring of social media and proactive social media campaigns to manage negative information and rumors that have been associated with low vaccination uptake in the region. 61 79
In settings where limited supply is not a problem, demand plays a role in how individuals access a vaccine. Delegation of promotion of vaccine to individual providers or even the manufacturers has been less effective than centralized and integrated efforts. In particular, promotion by industry seems to have unsettled some parents. 80 School located provision reliably yields the highest coverage, 81 but many countries continue to rely on provision in primary care settings or pharmacies.
Opportunities to reduce the barriers discussed above include securing sufficient and affordable vaccine doses, school located delivery of vaccination, registries, innovations in communication to combat misinformation about vaccines, and implementation of supportive national recommendations and policies. 9 Community based strategies should also be present for girls who do not attend school. New recommendations for one dose vaccination schedules in adolescent girls will help to mitigate problems with high cost and potential shortages if adopted widely and may increase demand if only a single dose is needed. Changes in the formulation of vaccines to limit need for refrigeration and co-formulation with other vaccines will also increase access. 12
Owing to controversies about human papillomavirus vaccination, proactive television, radio, or social media communication strategies to quickly combat rumors and misconceptions are needed. 61 79 Improving provider recommendations will build confidence about and demand for the vaccine. Increasing public understanding of the impact of human papillomavirus vaccination on the risk of cervical cancer and other anogenital and head and neck cancers may provide an additional impetus for vaccination. 82 Traditional venues for awareness campaigns must be adjusted by understanding how women and parents obtain information online and working within these platforms. Strategies that benefit LMICs may also work in high income countries to reach historically marginalized girls and women with less access to vaccination and screening and higher risk of cervical cancer.
Combined human papillomavirus vaccination and cervical cancer screening
WHO and US, European, and Australian organizations have called for the near elimination of cervical cancer. A statistical modeling study based on high quality cancer registry data of worldwide trends in ICC developed from the International Agency for Research on Cancer’s Cancer Incidence in Five Continents series shows a way forward. According to data from 37 registries in 20 countries, with no changes in rates of human papillomavirus vaccination or cervical cancer screening, the annual number of ICC cases globally will increase from an estimated 600 000 in 2020 to 1.3 million in 2069 as a result of increases in population, aging, and underlying risk factors for exposure to hrHPV. 83 An estimated 44.4 million women will be diagnosed as having ICC during this time period, with two thirds of these cases being in LMICs. However, assuming a rapid scale up of human papillomavirus vaccine coverage to more than 80% of adolescent girls along with continued or increased screening for cervical cancer in adult women, an estimated 13 million cases of ICC could be averted in LMICs. This type of global strategy could lead to an incidence of ICC of less than four per 100 000 women across all countries worldwide—similar to the proposed WHO goal for the near elimination of cervical cancer. 9 83 Secondary prevention through screening for cervical cancer remains essential to prevention of ICC, especially in older women and women living with HIV because these women either are not eligible for vaccination or have a higher risk of cervical pre-cancer and cancer.
WHO’s near elimination plan’s foundation is based on attainment of several of the sustainable development goals such as ending poverty, ensuring access to sexual and reproductive healthcare, gender empowerment, and reduction of inequality among countries. 9 Interventions specific to cervical cancer include primary prevention through human papillomavirus vaccination in 9-14 year olds and secondary prevention in women over 30 years old through screening and treatment of cervical pre-cancer.
The need for innovation in cervical cancer screening will remain relevant even in countries that reach high vaccination rates, as high vaccination coverage may eventually reduce the accuracy of current cervical cancer screening methods. As cervical cancer becomes rarer, the ability of our screening tests to detect cases will drop and the rate of false positive screening results and associated over-testing, over-treatment, and avoidable harms will increase. Although data on the beneficial effect of widespread human papillomavirus vaccination are compelling, no country is at the stage to consider reducing screening for cervical cancer as a result of human papillomavirus vaccination, particularly with lapses in preventive healthcare with the covid-19 pandemic.
Emerging vaccine options
In 2022 the Serum Institute of India announced the development of a quadrivalent prophylactic human papillomavirus vaccine (estimated cost of $2.50 to $5.00 per dose) which will become available in India in 2023 and for export in 2024. 84 85 Given that cost has been a major barrier to expansion of human papillomavirus vaccination coverage, the availability and efficacy of this new vaccine will be a major game changer in efforts to prevent cervical cancer. A phase 3 clinical trial for an 11 valent prophylactic vaccine is ongoing in China (ClinicalTrials.gov NCT05262010 ). Several trials of next generation vaccines are planned or ongoing using L2 rather than the currently licensed vaccines with L1 virus-like particles to develop an antigen response, meaning that the vaccine will probably not require refrigeration and allowing cross protection over multiple genotypes.
Additional clinical trials of currently licensed human papillomavirus vaccines are being conducted with focus on populations at risk such as transplant recipients ( NCT03036930 ) and people with HIV ( NCT04982614 , NCT05495906 ), as well as dose reduction studies in adolescents, people with HIV, and boys and men ( NCT03728881 , NCT04688476 , NCT03943875 , NCT03832049 , NCT05495906 , NCT05173324 , NCT04953130 ). Further research into use of the nonovalent vaccine as an adjuvant to excision for cervical pre-cancer is ongoing ( NCT03848039 ).
Therapeutic vaccines studies using cell mediated immunity against existing infection have shown little success to date. 86 Current trials are studying novel vaccines for adjuvant treatment of cervical cancer and other diseases caused by human papillomavirus ( NCT04800978 , NCT04084951 , NCT02405221 , NCT04432597 , NCT00788164 , NCT0341848, NCT03947775 ).
Human papillomavirus vaccination is highly effective in preventing infection with the virus, 87 cervical pre-cancers, 52 ICC, 53 and several other diseases. 88 The near elimination of cervical cancer is possible in countries with robust uptake of the vaccine. Opportunities to increase the uptake of human papillomavirus vaccine exist in several domains. However, vaccine distribution continues to be inequitable among and within countries according to income, race/ethnicity, and gender. Given global disparities, most LMICs will be delayed by decades in achieving, or will never achieve, near elimination of cervical cancer. The covid-19 pandemic has further set some countries back. Deliberate action will be needed to unwind these deeply rooted inequities. Inaction will only perpetuate them, further widening the gap between countries and people of wealth and those who are less privileged.
ICC is a preventable cancer affecting millions of women worldwide. Near elimination of ICC will require new partnerships between leaders in public health, governments, non-governmental organizations, communities, and patient advocates along with the healthcare teams who see patients. Advancement of the single dose strategy, development of low cost vaccines, co-formulation of human papillomavirus vaccine with other routine vaccines that do not need refrigeration, and combating vaccine misinformation are areas of necessary research and policy change. 12 Action in these areas and other initiatives to increase the supply of and demand for human papillomavirus vaccination, particularly in marginalized or remote populations and LMICs, are essential.
Questions for future research
How can a human papillomavirus vaccine be developed that does not need to be refrigerated, to support low and middle income country settings where refrigeration is not reliably available?
How can health workers in low and middle income countries most effectively recommend human papillomavirus vaccination?
What level of human papillomavirus vaccination coverage could affect cervical cancer screening guidelines?
How will herd immunity for human papillomavirus affect the risk for or incidence of other human papillomavirus related cancers or non-cancer related outcomes?
Patient involvement
A draft of the article was reviewed by a group of cervical cancer survivors and members of the non-profit cervical cancer and awareness group, Cervivor, located in the United States. After giving informed consent, another cervical cancer survivor affiliated with the cancer support network SHARE gave the following perspective: “After many trips to my local health department and misdiagnosis of symptoms which I was told were related to a bacterial infection, I was diagnosed with cervical cancer, which later metastasized. Had the human papillomavirus vaccine been available when I was a teenager, my life would have taken a very different direction. I was not only infected with human papillomavirus once, but with two different types, one of which ultimately led to cancer. I feel as though my socioeconomic situation played a huge role in my largely flying under the radar. Having the option to vaccinate can help people around the world who struggle with access to care not have to navigate treatment of a cancer diagnosis.”
Acknowledgments
We acknowledge the contribution of the cervical cancer survivors who reviewed and commented on this article as listed under “Patient involvement.” We also acknowledge Erin McCallum for her organization of author contributions, support of LR with the literature search, and compilation of references.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: LR had the idea for the article, did the literature search, and led the writing of the manuscript. CM, SO, CJC, and NTB contributed to the writing of the manuscript. LR is the guarantor.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following interests: LR has received research funding on antiretroviral therapy from Merck and Co, Inc; CC has been awarded the Merck HPV Investigator Studies Program (MISP) for cervical cancer prevention research; NB has served as a paid adviser on vaccine behavior research to the Centers for Disease Control and Prevention, World Health Organization, Merck and Co, Inc, Novartis, and Sanofi.
Provenance and peer review: Commissioned; externally peer reviewed.
- Solomon D ,
- Forum Group Members ,
- Bethesda 2001 Workshop
- Syrjänen SM ,
- Syrjänen KJ
- Castellsagué X
- Markowitz LE ,
- Saraiya M ,
- Lawson HW ,
- Chesson H ,
- Centers for Disease Control and Prevention (CDC) ,
- Advisory Committee on Immunization Practices (ACIP)
- ↵ World Health Organization. Prequalified vaccines. https://extranet.who.int/pqweb/vaccines/prequalified-vaccines?page=8 .
- Serrano B ,
- de Sanjosé S ,
- ↵ World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. 2020. https://www.who.int/publications/i/item/9789240014107 .
- ↵ World Health Organization. Introduction of HPV (Human Papilloma Virus) vaccine. https://immunizationdata.who.int/pages/vaccine-intro-by-antigen/hpv.html?ISO_3_CODE=&YEAR= .
- Siegel RL ,
- Oberlin AM ,
- Rahangdale L ,
- Chinula L ,
- Fuseini NM ,
- Chibwesha CJ
- Masuyer E ,
- ↵ Joint United Nations Programme on HIV and AIDS (UNAIDS). AIDSinfo. https://aidsinfo.unaids.org/ .
- Clifford GM ,
- Gonçalves MAG ,
- Franceschi S ,
- HPV and HIV Study Group
- Williamson A-L ,
- Anastos K ,
- Hoover DR ,
- Einstein MH ,
- ↵ Bruni L, Albero G, Serrano B, et al; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Europe: Summary Report 22. 2021. https://hpvcentre.net/statistics/reports/XEX.pdf .
- ↵ Australia C. Cervical cancer in Australia statistics. https://www.canceraustralia.gov.au/cancer-types/cervical-cancer/statistics .
- de Martel C ,
- ↵ The President’s Cancer Panel. HPV Vaccination For Cancer Prevention: Progress, Opportunities, and a Renewed Call to Action. 2018. https://prescancerpanel.cancer.gov/report/hpvupdate/ .
- ↵ Centers for Disease Control and Prevention. United States Cancer Statistics: Data Visualizations. https://gis.cdc.gov/Cancer/USCS/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcancer%2Fdataviz%2Findex.htm#/AtAGlance/ .
- ↵ National Cancer Institute. Cancer Stat Facts: Cervical Cancer. https://seer.cancer.gov/statfacts/html/cervix.html .
- Rauscher GH ,
- Johnson TP ,
- ↵ International Agency for Research on Cancer. Population fact sheets. https://gco.iarc.fr/today/fact-sheets-populations .
- ↵ International Agency for Research on Cancer. Africa. https://gco.iarc.fr/today/data/factsheets/populations/903-africa-fact-sheets.pdf .
- ↵ International Agency for Research on Cancer. Latin America and the Caribbean. https://gco.iarc.fr/today/data/factsheets/populations/904-latin-america-and-the-caribbean-fact-sheets.pdf .
- ↵ Pan American Health Organization. Cervical Cancer. https://www.paho.org/en/topics/cervical-cancer .
- Simoens C ,
- Martin-Hirsch PP
- Brisson M ,
- HPV Vaccination Impact Study Group
- FUTURE II Study Group
- Wheeler CM ,
- Skinner SR ,
- Del Rosario-Raymundo MR ,
- VIVIANE Study Group
- Paavonen J ,
- Salmerón J ,
- HPV PATRICIA Study Group
- Giuliano AR ,
- Herrero R ,
- Costa Rica Vaccine Trial Group
- Pollock KGJ ,
- Kavanagh K ,
- Herweijer E ,
- Sundström K ,
- Arnheim-Dahlström L
- Dillner J ,
- Brotherton JM ,
- Rompotis C ,
- Rodriguez AM ,
- Konishi H ,
- Sauvaget C ,
- Verdoodt F ,
- Dehlendorff C ,
- Hortlund M ,
- Pillsbury A ,
- ↵ Cancer Council NSW. Eliminating cervical cancer in Australia by 2035. https://www.cancercouncil.com.au/research-pt/eliminating-cervical-cancer-in-australia-by-2035/ .
- Elfström KM ,
- Falcaro M ,
- Castañon A ,
- Matsumoto K ,
- Yaegashi N ,
- MINT Study Group
- Kyrgiou M ,
- Arrossi S ,
- Garland S ,
- Szilagyi PG ,
- Chesson HW ,
- Romero JR ,
- Markowitz LE
- ↵ South Africa National Department of Health. Cervical Cancer Prevention and Control Policy. 2017. https://www.health.gov.za/wp-content/uploads/2021/07/cervical-cancer-policy.pdf .
- Sayinzoga F ,
- Umulisa MC ,
- Sibomana H ,
- Baussano I ,
- Clifford GM
- LaMontagne DS ,
- Atuhebwe P ,
- World Health Organization
- Andrews KS ,
- Manassaram-Baptiste D ,
- Fontham ETH ,
- American Cancer Society Guideline Development Group
- Demarteau N ,
- Detournay B ,
- El Hasnaoui A ,
- Standaert B
- Whitworth HS ,
- Gallagher KE ,
- ↵ World Health Organization. One-dose Human Papillomavirus (HPV) vaccine offers solid protection against cervical cancer. 2022. https://www.who.int/news/item/11-04-2022-one-dose-human-papillomavirus-(hpv)-vaccine-offers-solid-protection-against-cervical-cancer .
- Hutubessy R ,
- Chaiyakunapruk N
- Saura-Lázaro A ,
- Montoliu A ,
- Linertová R ,
- Guirado-Fuentes C ,
- Mar Medina J ,
- Imaz-Iglesia I ,
- Rodríguez-Rodríguez L ,
- Carmona-Rodríguez M
- ↵ European Cancer Organisation. Action Area 1: HPV Prevention Via Gender Neutral Vaccination Programmes. https://www.europeancancer.org/2-standard/107-hpv-action-area-1-hpv-prevention-via-gender-neutral-vaccination-programmes .
- Chido-Amajuoyi OG ,
- Fokom Domgue J ,
- Obi-Jeff C ,
- Schmeler K ,
- ↵ World Health Organization. Strategic Advisory Group of Experts on Immunization 4-7 April 2022. https://terrance.who.int/mediacentre/data/sage/SAGE_Slidedeck_Apr2022.pdf
- Elam-Evans LD ,
- Singleton JA ,
- ↵ National C enter for Immunization & Respiratory Diseases. National and State-Level HPV Vaccination Coverage. 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-HPV-Stokley-508.pdf .
- ↵ Normile D. Japan relaunches its HPV vaccination drive. For thousands of women, it may be too late. 2022. https://www.science.org/content/article/japan-relaunches-its-hpv-vaccination-drive-thousands-women-it-may-be-too-late .
- Kadooka Y ,
- Hansen PR ,
- Schmidtblaicher M ,
- ↵ Pan American Health Organization. HPV Vaccine: Lessons Learned in the Region of the Americas. Immunization Newsletter. 2018 https://www.paho.org/hq/index.php?option=com_docman&view=download&alias=46153-immunization-newsletter-june-2018&category_slug=immunization-newsletter-9&Itemid=270&lang=en .
- Colgrove J ,
- Niccolai LM ,
- Kamolratanakul S ,
- Pitisuttithum P
- Steinberg J ,
- Caruana M ,
- ↵ Reuters. India develops its first cervical cancer vaccine. 2022. https://www.reuters.com/business/healthcare-pharmaceuticals/india-develops-its-first-cervical-cancer-vaccine-2022-09-01 .
- ↵ The Hindu. HPV vaccine production delayed due to COVID focus, supply in India from early 2023: Serum Institute. 2022. https://www.thehindu.com/sci-tech/health/hpv-vaccine-production-delayed-due-to-covid-focus-supply-in-india-to-begin-in-early-2023-serum-institute/article66039271.ece .
- Rosenblum HG ,
- Gargano JW ,
- Querec TD ,
- Open access
- Published: 29 June 2023
A systematic review of interventions to promote HPV vaccination globally
- Cam Escoffery 1 ,
- Courtney Petagna 1 ,
- Christine Agnone 1 ,
- Stephen Perez 1 ,
- Lindsay B. Saber 1 ,
- Grace Ryan 2 ,
- Meena Dhir 1 ,
- Swathi Sekar 1 ,
- Katherine A. Yeager 3 ,
- Caitlin B. Biddell 4 ,
- Purnima Madhivanan 5 ,
- Stephanie Lee 3 ,
- Amanda S. English 6 ,
- Lara Savas 7 ,
- Eliza Daly 8 ,
- Thuy Vu 9 &
- Maria E. Fernandez 7
BMC Public Health volume 23 , Article number: 1262 ( 2023 ) Cite this article
4923 Accesses
3 Citations
3 Altmetric
Metrics details
Despite the human papillomavirus (HPV) vaccine being a safe, effective cancer prevention method, its uptake is suboptimal in the United States (U.S.). Previous research has found a variety of intervention strategies (environmental and behavioral) to increase its uptake. The purpose of the study is to systematically review the literature on interventions that promote HPV vaccination from 2015 to 2020.
We updated a systematic review of interventions to promote HPV vaccine uptake globally. We ran keyword searches in six bibliographic databases. Target audience, design, level of intervention, components and outcomes were abstracted from the full-text articles in Excel databases.
Of the 79 articles, most were conducted in the U.S. (72.2%) and in clinical (40.5%) or school settings (32.9%), and were directed at a single level (76.3%) of the socio-ecological model. Related to the intervention type, most were informational ( n = 25, 31.6%) or patient-targeted decision support ( n = 23, 29.1%). About 24% were multi-level interventions, with 16 (88.9%) combining two levels. Twenty-seven (33.8%) reported using theory in intervention development. Of those reporting HPV vaccine outcomes, post-intervention vaccine initiation ranged from 5% to 99.2%, while series completion ranged from 6.8% to 93.0%. Facilitators to implementation were the use of patient navigators and user-friendly resources, while barriers included costs, time to implement and difficulties of integrating interventions into the organizational workflow.
Conclusions
There is a strong need to expand the implementation of HPV-vaccine promotion interventions beyond education alone and at a single level of intervention. Development and evaluation of effective strategies and multi-level interventions may increase the uptake of the HPV vaccine among adolescents and young adults.
Peer Review reports
The human papillomavirus (HPV) is the most common infection that can lead to cancer later in life. There are 570,000 incident cancer cases per year in females and 60,000 incident cancer cases in males attributable to HPV globally [ 1 ].HPV can lead to cancers of the cervix, vagina, and vulva for females, penis cancer for males, and anus and oropharyngeal cancers for both [ 1 ]. The World Health Organization has a vision to eliminate HPV-related cancers, particularly cervical cancer, worldwide by 2030 [ 2 ]. Similarly, in the U.S., Healthy People 2030 has an objective to increase the proportion of adolescents who receive recommended doses of the HPV vaccine from a baseline of 48.0% to 80.0% [ 3 ].
HPV vaccination can prevent more than 90% of cancers due to HPV infections [ 4 , 5 ]. Vaccination starts at age 9 and the catch up is recommended through age 26. If not adequately vaccinated, persons up to the age of 45 can be considered for vaccination but with shared decision-making between the patient and provider [ 6 ]. Primary prevention is from ages 9–14 globally [ 7 ]. The HPV vaccine is commonly recommended during routine vaccinations to children ages 11–12 and there is a push from public health professionals and providers to start as early as 9 in the U.S [ 8 ]. Globally, an estimated 15% of girls are fully vaccinated against HPV [ 9 ]. In the U.S., about 58.5% of adolescents were up-to-date on HPV vaccination in 2020, with 61% of females being fully vaccinated versus 56% of males [ 10 ]. Public health efforts are needed to increase the global rates of HPV vaccination.
Worldwide, there have been a few reviews of interventions focused on improving HPV vaccination rates [ 11 , 12 , 13 , 14 , 15 ]. Interventions to promote HPV vaccination have typically targeted parents, adolescents, young adults, and providers.. HPV vaccination interventions have targeted various socio-ecological levels that influence HPV vaccination to ultimately effect change. Some focus only on the individual level (e.g., via education such as informational text included with reminders), whereas others may include changes to policy (e.g., via formalized requirements, such as school mandates). Multi-level and multi-component interventions are increasingly used [ 12 , 13 , 15 ] and address health disparities [ 16 , 17 ]. Multi-level interventions target two or more levels of influence at or around the same time; the approaches implemented at each level typically may vary in type (e.g., behavioral, health systems, or policy) [ 16 , 18 ]. It is important to understand the wide range of levels that can be utilized in interventions from single-level to multi-level and how those levels can impact the desired outcome of vaccination.
This study aimed to conduct a systematic review of HPV interventions by synthesizing literature published from May 2015 to March 2020, related to promoting HPV vaccine uptake and/or completion in the U.S and internationally. A previous systematic review and meta-analysis in the United States found a combination of provider- and community-level interventions were effective [ 11 ]. Our review was intended to update this review of interventions for HPV vaccine promotion with more rigorous methodology, including exploration of sources of heterogeneity and quality assessment. Another purpose of the study was to improve the understanding of multi-level interventions for HPV vaccine promotion. The review questions included: 1) What are the targeted audiences and levels of intervention for HPV vaccination interventions?, 2) What are common components of the interventions?, 3) What were facilitators and barriers to implementation of the vaccination interventions?, and 4) What are the study outcomes measured including the rates of HPV vaccination initiation and completion and their effectiveness? Our resulting study provides a strong contribution to the literature that can be used to inform future promotion efforts that aim to increase HPV vaccine uptake.
We conducted a systematic review of the peer-reviewed published literature, using methods following the PRISMA guidelines [ 19 ]. The team included cancer control researchers and master’s and doctoral students in public health and nursing fields.
Search strategy
The lead author, in collaboration with a health sciences librarian, created a search strategy using text and MeSH terms (Supplemental Table 1 ). We searched for relevant articles in six bibliographic databases, including Medline, CINAHL, Embase, Web of Science, Cochrane Reviews, and SCOPUS. Some of the keywords searched alone or in combination were children, pediatric, young adult, parent, behavioral therapy, prevention, and human papilloma virus. An additional manual search was performed of the bibliographies of relevant studies identified from the database search. The team reviewed the articles found in the search and removed duplicates.
Inclusion criteria
To be included in the review, an article had to: a) aim to increase HPV vaccination through at least one intervention; b) report an outcome based on the intervention (e.g., increase knowledge of HPV, report on HPV vaccine outcomes determined either by self-report or medical records; c) be published between May 2015 through March 2020; and d) be published in English. Studies that tested single or multi-level interventions were included. Screening was conducted in two stages with the initial stage evaluating titles and abstracts reviewed by 3 authors (CE, CA, and MD), and a second stage screening full text articles independently reviewed by the same 3 authors. Discrepancies were resolved through discussion at team meetings. Studies were excluded if they did not describe a primary intervention aimed at increasing HPV vaccination, were systematic reviews or articles with just a program description, or had no study outcomes. Those that met eligibility through abstract review were included in the full-text review. After the full article review, the articles were examined further to see if they met the eligibility criteria, and 33 were excluded.
Data extraction
We retrieved the full text of eligible studies for review and abstraction. We then created a detailed codebook for data collection. Data extraction tables for the article and quality assessment were developed and maintained in an Excel database. They were modified following discussions between three reviewers before data extraction. Data extracted included study location, target population, sample description, and setting; intervention details consisted of study design, description of the intervention (e.g., control group components, if applicable), level(s) of intervention, delivery and barriers to implementation and vaccination, and outcomes of the study. We piloted the forms with five studies and made refinements to the codebook and Excel database. We invited cancer and implementation science researchers from the Cancer Prevention and Control Research Network [ 20 ] and doctoral and MPH students from the participating institutions to be trained as data abstractors and abstract data from the final included articles. There were a total of 15 reviewers (CA, CP, CE, MD, SS, CB, MF, AE, LS, ED, GR, KY, SL, TV, and PM). For quality control, we had 2 abstractors for each study, and we merged the data when consensus was reached for each article. The abstractors also performed study quality assessment for the articles they abstracted. The pair of abstractors came to an agreement if there were discrepancies. If there was a disagreement or question about a study quality answer, then the core team (CA, CP, and CE) had a discussion and came to an agreement on the study quality question.
Quality assessment
For this assessment, we employed the NCI Quality Rating assessment for Pre and Posttest Designs to conduct quality assessment of the included articles [ 21 ]. This assessment included 12 items which included: whether the objectives, intervention, and eligibility requirements were clearly stated, had a sample adequate for confidence in the data, had a loss to follow-up of 20% or less, and measured changes in outcomes of interest before and after the intervention.
Synthesis of the results
We compiled all article abstractions into one database. We ran descriptive statistics and created summary scores for study setting and program component descriptions. The Community Guide categories (education, technology, vaccine access, incentive, provider education, health system change, community wide campaign, and policy) were used to organize the interventions into informational; behavioral change for participants, providers or both; or environmental (small-no government involvement such as organizational policy change or large policy-formal laws, rules or regulations, national or local government involvement). These categories also were applied in the Walling et al. systematic review [ 12 ]. We also created summary tables for study characteristics, outcomes, and quality ratings. The primary outcome was HPV vaccine initiation and/or completion, although we reported on other outcomes related to HPV vaccination determinants, or factors to increase vaccination (i.e., parental knowledge, awareness, self-efficacy, acceptability, attitudes and beliefs, and vaccine intention). We examined the range of HPV vaccine initiation and completion for adolescents and/or young adults.
The search identified 1,201 studies after removing duplicates. As a result of the title and abstract screen, 1,045 studies were excluded due to not being an intervention study or not reporting outcomes. The full-text of the remaining 152 articles were reviewed, leading to the exclusion of an additional 72 articles that did not have descriptions of the intervention or outcome data. This resulted in 79 articles included in the review for data extraction (Fig. 1 ). Table 1 shows the main characteristics of the included studies published between 2015 and 2020.
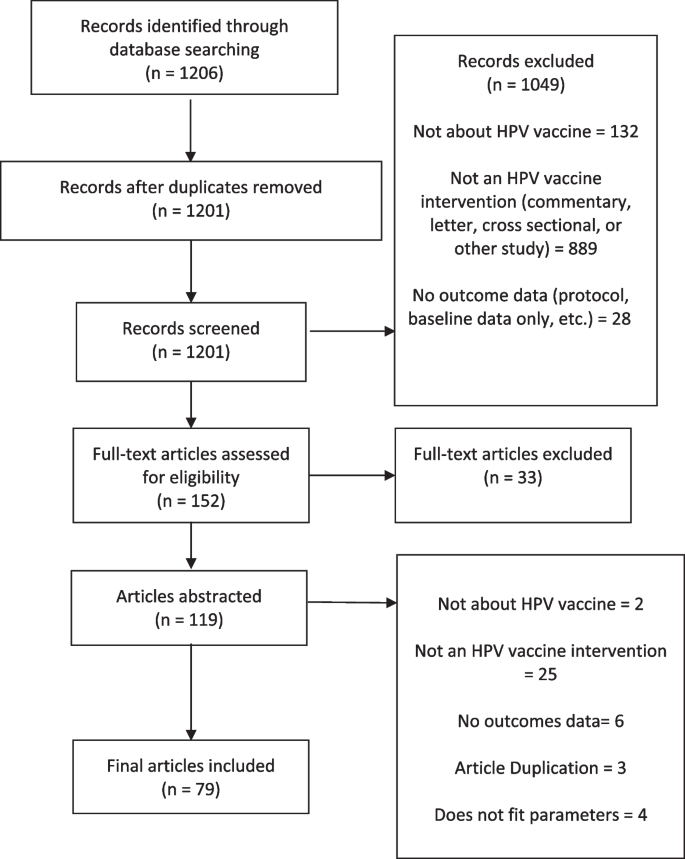
HPV Vaccination intervention systematic review flowchart
Study setting and design
Of the 79 intervention articles, 57 (72.2%) were conducted in the U.S. Other studies were conducted in Europe ( n = 10, 12.7%), Africa ( n = 4, 5.1%), Asia (3, 3.80%), Australia (3, 3.80%), Central/South America (1, 1.27%), and Canada (1, 1.27%). Forty-five studies (57.0%) employed an experimental design, 18 (22.8%) used a quasi-experimental design, and 16 (20.3%) employed a non-experimental design.
Setting and population focus
Intervention settings included clinics (32, 40.5%), schools (26, 32.9%), communities (10, 12.7%), an organization (1, 1.3%), a health insurance system, and online (10, 11.4%). Study samples ranged from 36 to 8,062.
Of the 79 studies, most interventions targeted adolescents only (39 studies, 49%) [ 22 , 25 , 27 , 29 , 31 , 32 , 34 , 35 , 36 , 40 , 43 , 44 , 46 , 48 , 50 , 51 , 52 , 53 , 54 , 55 , 60 , 61 , 62 , 63 , 64 , 65 , 68 , 69 , 72 , 73 , 74 , 75 , 85 , 90 , 91 , 92 , 94 , 98 , 100 ], of which 15 (38%) included girls only, 17 (44%) included both boys and girls, 3 (8%) included boys only, and 4 (10%) did not report. Other interventions focused on young adults ages 18–34 years (20 studies, 25%) [ 22 , 23 , 24 , 25 , 26 , 28 , 34 , 38 , 47 , 49 , 57 , 58 , 69 , 73 , 78 , 83 , 89 , 93 , 97 , 99 ], parents (27 studies, 34%) [ 25 , 33 , 41 , 43 , 45 , 50 , 51 , 52 , 56 , 61 , 63 , 66 , 70 , 75 , 76 , 78 , 79 , 81 , 82 , 84 , 86 , 90 , 91 , 92 , 93 , 96 , 100 ], healthcare providers (13 studies, 17%) [ 30 , 37 , 39 , 47 , 59 , 66 , 67 , 69 , 71 , 80 , 87 , 88 , 95 ], or did not report (1 study, 1%) [ 77 ].
Twenty-one interventions included multiple target populations as participants. Common combinations of participants included parents and adolescents (11 studies) [ 43 , 50 , 51 , 52 , 61 , 63 , 75 , 90 , 91 , 92 , 100 ], adolescents and young adults (4 studies) [ 22 , 26 , 34 , 73 ], clinicians and young adults (1 study) [ 47 ], parents and young adults (3 studies) [ 25 , 78 , 93 ], parents and clinicians (1 study) [ 66 ], and clinicians, adolescents, and young adults (1 study) [ 69 ]. Only three studies included only male adolescents or young adult study populations (2 were adolescents only, and the last one was both adolescents and young adults).
Eight of the 79 studies (10.1%) included a large proportion of parents from diverse racial and ethnic identities (defined as ≥ 50% other races than White) [ 33 , 45 , 56 , 70 , 76 , 79 , 81 , 100 ], 6 (7.6%) included adolescents from diverse groups [ 27 , 40 , 64 , 65 , 74 , 98 ], 8 (10.1%) included both parents and children from diverse groups [ 38 , 51 , 52 , 61 , 63 , 75 , 90 , 91 ], 6 (7.6%) included young adults from diverse groups [ 26 , 35 , 57 , 60 , 80 , 97 ], and 1 included both young adults and children from diverse groups (1.3%) [ 34 ].
Socio-ecological levels
Based on a review of the reported intervention components, the audiences they targeted, and the socio-ecological model, most studies were conducted at the individual level (44, 55.7%), followed by interpersonal level (10, 12.7%), community level (3, 3.8%), and clinic level (4, 5.0%).
Multi-level interventions
Although most interventions were directed at a single level of the socio-ecologicl level ( n = 61, 76.3%), 23.7% ( n = 18) were multi-level. Sixteen (88.9%) combined two levels [ 27 , 32 , 39 , 42 , 43 , 55 , 63 , 66 , 69 , 71 , 73 , 75 , 77 , 82 , 98 , 100 ], and 2 (9.1%) combined three levels (Fig. 2 ) [ 47 , 67 ]. Common combinations of the levels included provider and clinical (5 studies) [ 66 , 69 , 71 , 73 , 82 ], interpersonal and clinical (4 studies) [ 27 , 39 , 43 , 77 ], individual and interpersonal (2 studies) [ 32 , 100 ], individual and clinical (2 studies) [ 42 , 98 ], and individual and community (2 studies) [ 55 , 75 ]. Meyer et. al aimed to use an electronic point-of-care prompt and 2-h lecture for providers to increase HPV vaccine uptake in retail clinics (provider and clinical interventions) [ 73 ]. Staras et al. sought to increase HPV vaccine initiation among publicly insured Florida adolescents ages 11–17 using a quasi-experimental factorial design with four study arms: 1) postcard campaign, 2) in-clinic Health Information Technology (HIT) system, 3) postcard campaign and in-clinic HIT system, and 4) usual care (individual and clinical interventions) [ 98 ]. Paskett et al. developed a program focused on HPV vaccine uptake among parents who have adolescent girls ages 9–17 who have not received the HPV vaccine, which would include vaccinations (individual and provider interventions) [ 82 ]. The 3-level combinations included: 1 study with individual, interpersonal, and clinical interventions [ 67 ], and 1 study with individual, clinical, and community interventions [ 47 ]. For example, Malo et al. created a 3-level intervention for parents to analyze which messages were most motivating to persuade them to administer the HPV vaccine to their child, for educating and training physicians, physician assistants, nurse practitioners and nurses who serve at primary clinics specialized in pediatrics or family medicine about the most persuasive messages in speaking to parents about the HPV vaccine for their children (individual, interpersonal, and clinical interventions) [ 67 ].
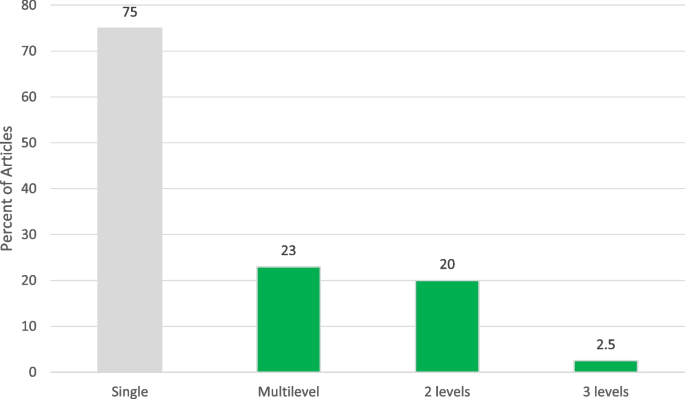
Levels of Interventions among Included Studies
Intervention components
The duration of interventions ranged from 10 min to 18 months among the studies reporting intervention time frames. Twenty-seven interventions (33.8%) reported using theory in intervention development [ 23 , 24 , 31 , 35 , 36 , 40 , 45 , 47 , 48 , 50 , 53 , 54 , 58 , 61 , 63 , 67 , 70 , 72 , 74 , 75 , 76 , 81 , 82 , 83 , 86 , 100 ]. Theories or frameworks referenced included the Elaboration Likelihood Model, Culture-centric narrative theory, Health Belief Model, Theory of Reasoned Action/Planned Behavior, Moral Norm and Social Cognitive Theory.
Intervention components varied from education to offering vaccination (vaccine access). The most common intervention components were individual education of parents and/or adolescents (60, 76.0%); use of technology such as websites, PowerPoints, and text messages (21, 26.6%); and provider education (16, 20.3%). Examples of educational messaging were: expressing the benefit of the HPV vaccine, providing cervical and breast cancer prevention education, supplying educational handouts at an eighth-grade reading level, and displaying facts on posters about HPV and the HPV vaccine (i.e. both genders can receive the vaccine). The websites provided factual information on HPV and the HPV vaccine including statistics on the incidence of HPV infection and cervical cancer, risks associated with HPV infection, costs of vaccination, safety and efficacy of the HPV vaccine, and suggestions for how to talk to a doctor about the vaccine. Other components included patient reminders (13, 16.5%) [ 27 , 50 , 51 , 62 , 63 , 70 , 71 , 74 , 77 , 83 , 89 , 90 , 99 ], improving access to the HPV vaccine (6, 7.6%) [ 29 , 55 , 64 , 75 , 85 , 89 ], health systems change (6, 7.6%) [ 43 , 69 , 75 , 77 , 81 , 98 ], incentives (4, 5.1%) [ 46 , 62 , 68 , 92 ], and community-wide campaigns or outreach (3, 3.8%) [ 32 , 45 , 75 ]. Patient reminders included phone calls, text messages, mailing reminders, and reminder-recall letters prompting adolescents to sign up for an appointment via a website. Several ways to improve access to the HPV vaccine consisted of utilizing school-based programs and expanding HPV vaccination programs in countries where there were no existing HPV vaccine programs. For incentives, gift cards (e.g., general merchandise and department stores, fashion and footwear retailers, bookstores, jewelry shops, motoring stores, and home improvement stores) and vaccine vouchers were used. Some studies combined two components (29, 36.7%) [ 24 , 27 , 28 , 29 , 31 , 32 , 33 , 34 , 35 , 39 , 40 , 44 , 45 , 47 , 50 , 55 , 65 , 66 , 71 , 72 , 81 , 83 , 85 , 86 , 87 , 88 , 90 , 97 , 98 ], three components (6, 7.6%) [ 51 , 62 , 63 , 69 , 74 , 82 ] or four components (3, 3.8%) [ 75 , 77 , 89 ]. Common intervention combinations included education and technology (18 studies, 23%) [ 24 , 28 , 31 , 33 , 34 , 35 , 40 , 44 , 51 , 63 , 65 , 72 , 74 , 82 , 86 , 88 , 89 , 97 ], education and reminders (9 studies, 11%) [ 50 , 51 , 62 , 63 , 74 , 77 , 83 , 89 , 90 ], education and vaccine access (5 studies) [ 29 , 55 , 75 , 85 , 89 ], and provider education and technology (4 studies, 5%) [ 39 , 69 , 82 , 87 ].
Community guide intervention categorization
We reported on the categorization of the interventions based on the Community Guide’s categorization framework to assess the design and execution of health-related evidence-based interventions [ 12 ]. The most common type of HPV vaccination interventions were informational interventions (25, 31.7%). Of the behavioral interventions, 23 (29.1%) [ 24 , 26 , 32 , 34 , 35 , 36 , 38 , 40 , 44 , 46 , 48 , 51 , 52 , 55 , 61 , 68 , 76 , 79 , 81 , 91 , 92 , 97 , 100 ] were patient-targeted decision support, 9 (11.4%) [ 50 , 62 , 63 , 70 , 74 , 83 , 89 , 90 , 99 ] were patient-targeted reminders, 12 (15.2%) [ 22 , 30 , 37 , 39 , 43 , 59 , 67 , 69 , 71 , 73 , 87 , 95 ] were provider-targeted, 8 (10.1%) [ 27 , 47 , 66 , 75 , 77 , 82 , 85 , 98 ] were both patient and provider targeted interventions. Only 2 (2.5%) [ 29 , 64 ] were related to environmental interventions related to small policies (i.e., organizational guidelines, no government involvement) (Fig. 3 ).
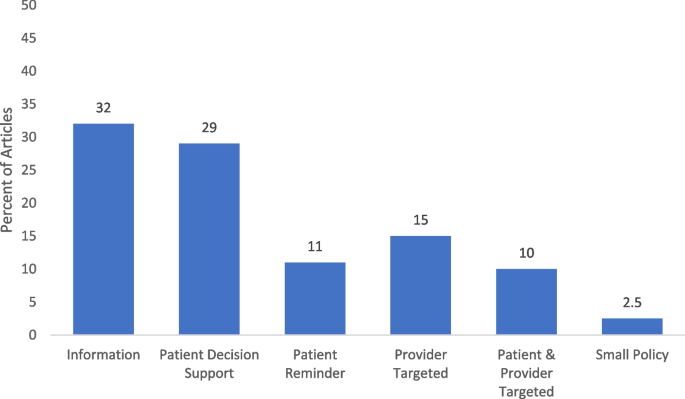
Intervention component categorizations based on community guide among included studies. Note. Interventions may have more than one intervention components
Facilitators and barriers to intervention implementation
Several studies reported facilitators (13 studies, 16.5%) [ 26 , 27 , 29 , 30 , 32 , 36 , 37 , 40 , 45 , 47 , 48 , 63 , 65 ] and barriers (22 studies, 27.58%) [ 24 , 26 , 27 , 30 , 36 , 37 , 40 , 43 , 44 , 50 , 61 , 67 , 68 , 73 , 74 , 77 , 79 , 87 , 89 , 93 , 98 , 99 ] to intervention implementation. Facilitators included use of patient navigators and user-friendly resources [ 26 , 27 , 36 ], interactive information sessions [ 29 , 30 , 37 ], low-cost interventions [ 30 , 40 , 47 ], and quality improvement initiatives [ 30 , 37 ]. Barriers to implementation were related to cost [ 24 , 27 , 93 , 99 ], time constraints with the given intervention [ 30 , 36 , 43 , 67 , 87 ] and integrating the intervention into clinical workflow [ 37 , 73 , 87 , 98 ]. Other barriers included mobility of parents and technology challenges.
HPV Intervention outcomes
Forty-two studies (53.2%) [ 22 , 24 , 26 , 27 , 29 , 32 , 34 , 37 , 38 , 39 , 40 , 44 , 47 , 48 , 50 , 51 , 52 , 53 , 55 , 61 , 62 , 63 , 68 , 69 , 71 , 73 , 74 , 75 , 76 , 77 , 79 , 81 , 82 , 83 , 85 , 89 , 90 , 91 , 97 , 98 , 99 , 100 ] reported on HPV vaccination outcomes, with 38 (48.1%) [ 22 , 24 , 26 , 27 , 29 , 32 , 34 , 37 , 38 , 39 , 40 , 44 , 47 , 50 , 51 , 52 , 53 , 55 , 61 , 62 , 68 , 69 , 71 , 73 , 74 , 75 , 76 , 77 , 79 , 81 , 82 , 83 , 85 , 91 , 97 , 98 , 99 , 100 ] reporting HPV vaccine initiation and 26 (32.9%) [ 22 , 26 , 27 , 29 , 34 , 37 , 38 , 39 , 40 , 48 , 50 , 52 , 55 , 63 , 68 , 71 , 74 , 75 , 76 , 77 , 81 , 89 , 90 , 91 , 97 , 99 , 100 ] reporting vaccine series completion. Post-intervention vaccine initiation ranged from 5% to 99.2%, while series completion ranged from 6.8% to 93%. For the experimental studies ( n = 47), 11 (23.4%) measured vaccine initiation [ 24 , 34 , 38 , 48 , 51 , 54 , 61 , 82 , 83 , 85 , 86 ], and 3 (6.4%) measured completion [ 89 , 90 , 99 ]. Eleven (23.4%) assessed initiation and completion as outcomes (Table 2 ) [ 39 , 40 , 44 , 50 , 52 , 53 , 68 , 71 , 81 , 91 , 100 ]. Of the interventions that only measured vaccine initiation, 3 out of 11 (27%) found a significant increase in vaccine initiation [ 48 , 82 , 85 ]. For the interventions that measured both as an outcome, 3 out of the 11 (27%) found a significant increase in vaccine initiation [ 50 , 71 , 100 ]. Therefore, a total of 6 (12.8%) interventions demonstrated a significant increase in vaccine initiation [ 48 , 50 , 71 , 82 , 85 , 100 ]. For the interventions that measured both vaccine initiation and completion, 1 (9.1%) reported a significant increase in completion only [ 81 ] and 2 (18.2%) in both vaccine initiation and completion [ 39 , 68 ]. Of the interventions with quasi-experimental studies ( n = 16), 5 (31.3%) were studies with comparison groups [ 30 , 55 , 62 , 69 , 98 ] and 11 (68.8%) were studies with pre and post intervention data collection (Table 3 ) [ 22 , 25 , 47 , 59 , 63 , 73 , 76 , 79 , 93 , 95 , 97 ]. Out of the quasi-experimental interventions with comparison groups ( n = 5), 3 (60%) measured vaccine initiation [ 62 , 69 , 98 ], and 1 (20%) assessed both initiation and completion [ 55 ]. Of those, 2 (40%) demonstrated significant increase in vaccine initiation [ 62 , 98 ], 0 in completion, and 1 (20%) in both as an outcome [ 55 ]. Out of the quasi-experimental interventions with pre and post-intervention designs ( n = 11), 2 (18.2%) measured initiation [ 47 , 79 ], 1 (9.1%) measured completion [ 63 ], and 4 (36.4%) assessed both as outcomes [ 22 , 73 , 76 , 97 ]. One (9.1%) reported a significant increase in vaccine initiation [ 47 ] 1 (9.1%) in completion [ 63 ]; and 2 (18.2%) in both [ 73 , 76 ].
Other common intervention outcomes included measures of parental knowledge (18, 32.1%), self-efficacy (7, 12.5%), acceptability (7, 12.5%), and attitudes and beliefs (6, 10.7%). For adolescents, other outcome measures were knowledge (8, 34.5%), awareness (3, 13.0%), and attitudes and beliefs (3, 13.0%). For young adults, these measures included knowledge (14, 35.9%), attitudes and beliefs (7, 17.9%), and self-efficacy (4, 10.3%). Out of 79 studies, 15 (19%) measured vaccine intention.
The study quality (SQ) assessment included 12 criteria items with response options as 0 = no or 1 = yes. The results showed that SQ1 (the study had a clear objective) was the most common criterion met, with 79 (98.8%) studies meeting this criterion. This was followed by SQ3 (participants in the study are representative of those who would be eligible), which was met by 68 (85%) studies. SQ2 (eligibility criteria clearly described) and SQ6 (delivered consistently across the study population) were tied for third place, with 67 (83.75%) studies meeting these criteria. On the other hand, SQ8 (people assessing the outcomes blinded to participants' exposures/interventions) was the least met criterion, with only 9 (11.25%) studies meeting this criterion. SQ12 (the study took into account the use of individual-level data to determine effects at the group level) was met by 15 (18.75%) studies. SQ11 (outcome measured multiple times) was met by 19 (23.75%) studies, while SQ9 (loss to follow-up after baseline 20% or less) was met by 30 (37.50%) studies. Overall, 60% ( n = 48) and 32.5% ( n = 26)were rated as Good or Fair in quality, respectively. Six (0.75%) studies were rated as Poor. For a detailed presentation of the quality elements and overall quality scores, please refer to Supplementary Table 2 .
We conducted a systematic review to assess interventions for HPV vaccine promotion. Our goal was to better describe common target populations of HPV vaccine interventions, common intervention levels and components, barriers and facilitators to intervention implementation, and the relationship between types of interventions and HPV-vaccine related outcomes. Previous systematic reviews have identified the breadth of intervention designs and contributed to our understanding of relative effectiveness of different intervention types [ 12 , 14 , 101 ]; however, given the advances in HPV vaccination research over the last several years, an update to these reviews was warranted. Moreover, previous systematic reviews have had a limited scope in terms of study settings, study designs, or topics and our goal here was to conduct a global and comprehensive review of interventions [ 14 , 15 , 102 , 103 ]. We reported on the level of socio-ecological that each intervention targets, barriers and facilitators to the implementation of these interventions, and intervention with outcomes such as initiation and completion rates from the U.S. and other countries. In our update to these reviews, we found that while intervention components were described thoroughly to contribute to our knowledge of types of interventions being implemented, fewer details about barriers and facilitators and HPV vaccine-related outcomes (particularly vaccination rates) were included. There were few patterns to be discerned in which types of interventions were found to be most effective, and in fact, among those that did report, only 20.3% reported significant increases in either initiation or completion or both. Despite this, our findings offer six key insights into the types of interventions being implemented that make effective interventions.
From intervention research, we know that there are certain “components” that can help to promote successful intervention implementation and outcomes. For HPV vaccination specifically, we know that working with healthcare providers is an effective strategy [ 11 ]. More broadly, literature suggests that interventions are more effective when they focus on implementation at multiple levels [ 82 ] and use theory in intervention development [ 104 ]. However, in our review, we found that overall, many of the interventions identified did not adhere to these best practices; only 23% of the interventions were multi-level (18 total) and 34% employed theory (27 total).
We used the Community Guide and the Walling et al. systematic review classification of interventions such as informational, behavioral, and environmental to categorize and rank interventions [ 11 ]. Firstly, our review revealed the most commonly implemented interventions were not the types of interventions that had previously been shown to have the greatest impact. For example, while the success of behavioral provider and clinic-focused interventions (particularly ones that promote changes to systems like utilizing reminder-recall and encouraging strong recommendations) is well-documented [ 11 ], in our study we found other types of interventions were more often used. For example, information-providing interventions (used to increase knowledge of HPV, HPV-associated cancers and the HPV vaccine [ 11 ]) were most common (31.7%) followed by patient decision support interventions (29.1%). Among these intervention categories, the intensity of the activities ranged widely. For example, in our study among information-providing interventions some studies employed a passive approach by offering pamphlets and educational materials [ 60 ] whereas others were more active and included live presentations [ 57 , 65 ]. Yet, educational, or information-giving interventions have been found to be less effective in increasing uptake or completion [ 103 ]. The interventions being implemented are not the types that have been shown to be most effective, which is consistent with other research that has identified a discrepancy between the implementation of interventions or strategies that are most effective compared to interventions that may be deemed “easiest” to implement [ 105 , 106 ].
Secondly, despite extensive research showing the increased effectiveness of multi-level interventions [ 82 ], there were limited interventions included in this review that were multi-level (23%). For example, The Community Guide has found insufficient evidence for provider or patient education alone to increase vaccination, but it has found that using education in combination with provider-focused interventions (i.e. provider reminders; assessment and feedback) has been successful [ 107 ]. In this review, 75% of the interventions reported intervening on only a single level, most commonly in clinical or school-based settings focused on individuals or providers. Future interventions to promote HPV vaccination should prioritize intervening at multiple levels to more effectively improve vaccine outcomes and discern which combination of levels results in higher vaccination.
Thirdly, using theory is well-documented as a best practice in intervention development and implementation [ 104 , 108 ]; however only one-third of the interventions in this review used theory in the design of their program strategies. It is highly possible that some of these interventions did in fact use theory or theoretical constructs to guide their research, but did not report it explicitly. The Health Belief Model, Theory of Planned Behavior, Social Cognitive Theory and the Elaboration Likelihood Model were the most commonly utilized; this is consistent with a recent systematic review exploring the use of theory in HPV vaccine interventions [ 102 ]. Using theory allows for understanding why specific interventions may be effective (or not effective) and for comparison across multiple studies. Thus, future HPV vaccine interventions should report more broadly on the use of theory in their intervention development and how constructs are employed in their design of intervention components or assessed in evaluation.
Fourthly, the effectiveness of these interventions was difficult to discern due to heterogeneity in measurement, outcomes, and study designs. Unfortunately, it is difficult to speak to what types of interventions were most effective as only about half reported on vaccine initiation (48%) and less than a third (32%) reported on vaccine series completion. Other commonly assessed outcomes included parental knowledge [ 33 , 90 , 91 , 100 ], self-efficacy [ 35 , 48 , 54 , 70 , 72 , 75 , 76 , 82 , 86 , 96 ], attitudes/beliefs [ 23 , 31 , 48 , 49 , 51 , 54 , 58 , 65 , 72 , 75 , 80 , 82 , 86 , 97 , 100 ], and acceptability [ 28 , 34 , 41 , 43 , 50 , 72 , 78 , 79 , 92 ]. There is mixed evidence on whether these outcomes are associated with uptake. For example, one meta-analysis found that parents’ beliefs, attitudes and intentions were positively associated with HPV vaccine uptake [ 109 ], while other studies have found intention to be unrelated to uptake, particularly in multivariable models, other factors seem to attenuate the effect of intention [ 110 ]. Moreover, many of the studies included in this review were quasi- or non-experimental, making it difficult to draw inferences about the effectiveness of any of the outcomes reported. Only about half focused on vaccine series initiation and completion. There are promising findings that a proportion of the interventions that reported significant changes in vaccination uptake or completion are multi-level and multi-component. Future intervention studies should focus on using rigorous methods to assess the effectiveness of different types of interventions, including investigating vaccination outcomes of series initiation or completion, and having longer-term follow-up to be able to assess longer-term outcomes. In addition, evaluation of multi-level interventions for the promotion of HPV vaccination should be conducted to contribute to their evidence of effectiveness.
Fifthly, related to the lack of reporting on intervention outcomes was a lack of reporting on implementation barriers and facilitators. Less than 20% of studies reported on facilitators and less than 30% reported on barriers. This is a similar finding to the review conducted by Smulian et al. (2016), who also reported a lack of reporting on barriers and facilitators [ 11 ]. This kind of information is critical in understanding program implementation, adaptation, and tailoring for different settings [ 24 , 68 , 93 ]. Recently, the use of hybrid trials, which can be used to assess both effectiveness and implementation outcomes, is emerging among implementation research [ 111 , 112 ]. In the future, researchers could prioritize conducting these hybrid trials so that we can not only identify those interventions that are most effective, but also important implementation determinants that can inform sustainability and scalability in multiple types of healthcare settings.
Finally, it is important to note that it is a critical time, in the era of the COVID-19 pandemic to disseminate effective cancer prevention interventions. HPV vaccination rates have fallen during the pandemic [ 113 , 114 ] and competing priorities have led to less time for clinics to devote to vaccine promotion [ 115 ]. Coupled with recent data suggesting that concerns about HPV vaccine safety are rising [ 116 ], this is indicative of a need to identify what works and how to implement it to prevent future generations from being susceptible to HPV-associated cancers. Overall, increased reporting of both vaccine outcomes as well as barriers and facilitators to vaccination will move the field forward and provide data to help researchers determine which types of interventions to prioritize.
Strengths and limitations
Our study was strengthened by the inclusion of interventions globally and our focus on understanding multi-level intervention strategies. By categorizing interventions at different levels (e.g., individual, interpersonal, clinical) we have added to the growing literature on multi-level interventions. Additionally, almost 30% of the studies included were conducted outside of the United States. This finding helps to add to the growing global literature on HPV vaccine interventions and allows for comparability between the U.S. and other countries that continue to struggle with low HPV vaccination rates [ 2 ]. However, this should simultaneously be recognized as a potential limitation, as results may not be generalizable across all global geographies. While studies from North and South America, Europe, Africa, Asia, and Australia were included, there were only several from each continent (other than North America) which limits the generalizability of results. Similarly, less than 15% of studies included parents or children from diverse racial and ethnic identities (defined as ≥ 50% other races than White). This makes it hard to assess the impact of interventions for HPV vaccination on racially and ethnically diverse populations. Future HPV vaccination research should focus on these populations to test intervention effectiveness. We also were limited by only reporting on articles written in English and may be missing HPV vaccination interventions written in other languages.
Another key limitation is the lack of reporting vaccine-related outcomes in studies. Just over 50% reported either initiation and/or completion outcomes. This fact with varying study designs makes it difficult to collectively assess intervention effectiveness through data synthesis. Moreover, 40% of the studies were rated as “fair” or “poor” quality in our quality assessment, primarily due to studies not including multiple time points for outcome measures, not blinding participants in intervention studies, and for group-level studies not reporting on individual-level data to determine group-level effects. These limitations identify key gaps in the literature and that future research should focus on including more diverse populations in interventions, employing more rigorous study designs, and including vaccine initiation and completion rates.
In 2020, the World Health Organization adopted a Global Strategy to eliminate cervical cancer, aiming for 90% of girls to be fully vaccinated by age 15 [ 2 ]. Given that males can suffer from HPV-associated cancers as well, many countries have expanded their vaccination programs to include males. However, worldwide, most countries fall far short of this 90% goal. Therefore, there is a strong need to expand implementation of HPV-vaccine promotion interventions beyond education alone and at a single level and use rigorous intervention designs. Inclusion of longer-term interventions and more evaluations focusing on vaccine initiation and/or completion would be helpful to truly understand what is most effective in improving HPV vaccination rates. Many of the interventions included in this review did not report vaccine uptake data; relied on strategies found to be less effective (e.g., education alone); did not use or not report on use of theory; did not report on barriers and facilitators to implementation; or addressed a single level for intervention. Improving on the design and evaluation of HPV vaccination interventions is particularly critical at this moment as many adolescents missed vaccinations during the COVID-19 pandemic and vaccine hesitancy is growing. Improving our understanding of which interventions to prioritize for implementation will be important to ensure future generations of adolescents are protected against HPV-associated cancers.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Coronavirus disease of 2019
Health Information Technology
Human papillomavirus
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70. https://doi.org/10.1002/ijc.30716 .
Article CAS PubMed PubMed Central Google Scholar
WHO. Global strategy to accelerate the elimination of cervical cancer as a public health problem. World Health Organization. ( https://www.who.int/publications/i/item/9789240014107 ).
USDHHS. Healthy People 2030. U.S. Department of health and human services. ( https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08 ).
CDC. Cancers caused by HPV are preventable. Centers for disease control and prevention. ( https://www.cdc.gov/hpv/hcp/protecting-patients.html ).
Rosenblum HG, Lewis RM, Gargano JW, Querec TD, Unger ER, Markowitz LE. Human Papillomavirus Vaccine Impact and Effectiveness Through 12 Years After Vaccine Introduction in the United States, 2003 to 2018. Ann Intern Med 2022. https://doi.org/10.7326/M21-3798
Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. https://doi.org/10.15585/mmwr.mm6832a3 .
Article PubMed PubMed Central Google Scholar
World Health Organization. Cervical cancer. www.who.int . Published February 22, 2022. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
Petrosky E, Bocchini JA, Jr., Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015;64(11):300–4. ( https://www.ncbi.nlm.nih.gov/pubmed/25811679 ).
Bruni L, Saura-Lazaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144.
Article Google Scholar
Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(35):1183–90. https://doi.org/10.15585/mmwr.mm7035a1 .
Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: A systematic review. Hum Vaccin Immunother. 2016;12(6):1566–88. https://doi.org/10.1080/21645515.2015.1125055 .
Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1):e20153863. https://doi.org/10.1542/peds.2015-3863 .
Article PubMed Google Scholar
Mavundza EJ, Iwu-Jaja CJ, Wiyeh AB, et al. A systematic review of interventions to improve HPV vaccination coverage. Vaccines (Basel). 2021;9(7):687. https://doi.org/10.3390/vaccines9070687 .
Niccolai LM, Hansen CE. Practice- and community-based interventions to increase human papillomavirus vaccine coverage: a systematic review. JAMA Pediatr. 2015;169(7):686–92. https://doi.org/10.1001/jamapediatrics.2015.0310 .
Acampora A, Grossi A, Barbara A, et al. Increasing HPV vaccination uptake among adolescents: a systematic review. Int J Environ Res Public Health. 2020;17(21):7997. https://doi.org/10.3390/ijerph17217997 .
Agurs-Collins T, Persky S, Paskett ED, et al. Designing and assessing multilevel interventions to improve minority health and reduce health disparities. Am J Public Health. 2019;109(S1):S86–93. https://doi.org/10.2105/AJPH.2018.304730 .
Fisher H, Trotter CL, Audrey S, MacDonald-Wallis K, Hickman M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int J Epidemiol. 2013;42(3):896–908. https://doi.org/10.1093/ije/dyt049 .
Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the national institutes of health centers for population health and health disparities. Am J Public Health. 2008;98(9):1608–15. https://doi.org/10.2105/AJPH.2006.102525 .
PRISMA. Preferred reporting items for systematic reviews and meta-analyses (PRISMA). ( http://www.prisma-statement.org/ ).
Ribisl KM, Fernandez ME, Friedman DB, et al. Impact of the cancer prevention and control research network: accelerating the translation of research into practice. Am J Prev Med. 2017;52(3 Suppl 3):S233–40. https://doi.org/10.1016/j.amepre.2016.08.026 .
National Heart, Lung, and Blood Institute (NHLBI). Study quality assessment tools. ( https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools ).
Austin B, Morgan H. Improving human papillomavirus vaccine uptake in the family practice setting. J Nurse Pract. 2019;15(6):e123–5. https://doi.org/10.1016/j.nurpra.2019.01.022 .
Baxter CE, Barata PC. The paradox of HPV vaccines: how to reach sexually inexperienced women for protection against a sexually transmitted infection. Women’s health issues. 2011;21(3):239–45. https://doi.org/10.1016/j.whi.2010.11.007 . (In eng).
Bennett AT, Patel DA, Carlos RC, et al. Human papillomavirus vaccine uptake after a tailored, online educational intervention for female university students: a randomized controlled trial. J Womens Health (Larchmt). 2015;24(11):950–7. https://doi.org/10.1089/jwh.2015.5251 . (In eng).
Berenson AB, Rahman M, Hirth JM, Rupp RE, Sarpong KO. A brief educational intervention increases providers’ human papillomavirus vaccine knowledge. Hum Vaccin Immunother. 2015;11(6):1331–6. https://doi.org/10.1080/21645515.2015.1022691 . In eng.
Berenson AB, Rahman M, Hirth JM, Rupp RE, Sarpong KO. A human papillomavirus vaccination program for low-income postpartum women. Am J Obstet Gynecol. 2016;215(3):318.e1-9. https://doi.org/10.1016/j.ajog.2016.02.032 . (In eng).
Berenson AB, Rupp R, Dinehart EE, Cofie LE, Kuo YF, Hirth JM. Achieving high HPV vaccine completion rates in a pediatric clinic population. Hum Vaccines Immunother. 2019;15(7–8):1562–9. https://doi.org/10.1080/21645515.2018.1533778 . Article In English.
Bonafide KE, Vanable PA. Male human papillomavirus vaccine acceptance is enhanced by a brief intervention that emphasizes both male-specific vaccine benefits and altruistic motives. Sex Transm Dis. 2015;42(2):76–80. https://doi.org/10.1097/olq.0000000000000226 . (In eng).
Botha MH, van der Merwe FH, Snyman LC, Dreyer G. The vaccine and cervical cancer screen (VACCS) project: acceptance of human papillomavirus vaccination in a school-based programme in two provinces of South Africa. S Afr Med J. 2015;105(1):40–3. https://doi.org/10.7196/samj.8419 . In eng.
Article CAS PubMed Google Scholar
Calo WA, Gilkey MB, Leeman J, et al. Coaching primary care clinics for HPV vaccination quality improvement: comparing in-person and webinar implementation. Transl Behav Med. 2019;9(1):23–31. https://doi.org/10.1093/tbm/iby008 . (Journal: Article).
Carolan K, Verran J, Crossley M, Redfern J, Whitton N, Amos M. Impact of educational interventions on adolescent attitudes and knowledge regarding vaccination: a pilot study. Plos One. 2018;13(1):14. https://doi.org/10.1371/journal.pone.0190984 . Article In English.
Article CAS Google Scholar
Chigbu CO, Onyebuchi AK, Onyeka TC, Odugu BU, Dim CC. The impact of community health educators on uptake of cervical and breast cancer prevention services in Nigeria. Int J Gynecol Obstetr. 2017;137(3):319–24. https://doi.org/10.1002/ijgo.12150 . (Article) (In English).
Cipriano JJ, Scoloveno R, Kelly A. Increasing parental knowledge related to the Human Papillomavirus (HPV) Vaccine. J Pediatr Health Care. 2018;32(1):29–35. https://doi.org/10.1016/j.pedhc.2017.06.006 . (Article) (In English).
Cory L, Cha B, Ellenberg S, et al. Effects of educational interventions on human papillomavirus vaccine acceptability: a randomized controlled trial. Obstet Gynecol. 2019;134(2):376–84. https://doi.org/10.1097/aog.0000000000003379 . (In eng).
Darville G, Anderson-Lewis C, Stellefson M, et al. Customization of avatars in a HPV digital gaming intervention for college-age males: an experimental study. Simul Gaming. 2018;49(5):515–37. https://doi.org/10.1177/1046878118799472 . (Article).
Davies C, Skinner SR, Stoney T, et al. “Is it like one of those infectious kind of things?” The importance of educating young people about HPV and HPV vaccination at school. Sex Educ-Sex Soc Learn. 2017;17(3):256–75. https://doi.org/10.1080/14681811.2017.1300770 (Article) (In English).
Dawson R, Lemmon K, Trivedi NJ, Hansen S. Improving human papilloma virus vaccination rates throughout military treatment facilities. Vaccine. 2018;36(11):1361–7. https://doi.org/10.1016/j.vaccine.2018.02.007 . (In eng).
Dempsey AF, Maertens J, Sevick C, Jimenez-Zambrano A, Juarez-Colunga E. A randomized, controlled, pragmatic trial of an iPad-based, tailored messaging intervention to increase human papillomavirus vaccination among Latinos. Human Vaccines Immunother. 2019;15(7–8):1577–84. https://doi.org/10.1080/21645515.2018.1559685 . (Article) (In English).
Dempsey AF, Pyrznawoski J, Lockhart S, et al. Effect of a health care professional communication training intervention on adolescent human papillomavirus vaccination: a cluster randomized clinical trial. JAMA Pediatr. 2018;172(5):e180016. https://doi.org/10.1001/jamapediatrics.2018.0016 . (In eng).
DiClemente RJ, Murray CC, Graham T, Still J. Overcoming barriers to HPV vaccination: A randomized clinical trial of a culturally-tailored, media intervention among African American girls. Hum Vaccin Immunother. 2015;11(12):2883–94. https://doi.org/10.1080/21645515.2015.1070996 (In eng).
Donahue K, Hendrix K, Sturm L, Zimet G. Provider communication and mothers’ willingness to vaccinate against human papillomavirus and influenza: a randomized health messaging trial. Acad Pediatr. 2018;18(2):145–53. https://doi.org/10.1016/j.acap.2017.07.007 . (In eng).
Dreyer G, van der Merwe FH, Botha MH, et al. School-based human papillomavirus vaccination: An opportunity to increase knowledge about cervical cancer and improve uptake of screening. S Afr Med J. 2015;105(11):912–6. https://doi.org/10.7196/SAMJ.2015.v105i11.9814 .
Edwards T, Hooper GL. A school-based intervention to increase HPV vaccination rates. J Doct Nurs Pract. 2019;12(2):196–201. https://doi.org/10.1891/2380-9418.12.2.196
Esposito S, Bianchini S, Tagliabue C, et al. Impact of a website based educational program for increasing vaccination coverage among adolescents. Human Vaccines Immunother. 2018;14(4):961–8. https://doi.org/10.1080/21645515.2017.1359453 (Article) (In English).
Ford ME, Cannady K, Nahhas GJ, et al. Assessing an intervention to increase knowledge related to cervical cancer and the HPV vaccine. Adv Cancer Res. 2020;146:115–37.
Forster AS, Cornelius V, Rockliffe L, Marlow LA, Bedford H, Waller J. A cluster randomised feasibility study of an adolescent incentive intervention to increase uptake of HPV vaccination. Br J Cancer. 2017;117(8):1121–7. https://doi.org/10.1038/bjc.2017.284 (In eng).
Gerend MA, Murdock C, Grove K. An intervention for increasing HPV vaccination on a university campus. Vaccine. 2020;38(4):725–9. https://doi.org/10.1016/j.vaccine.2019.11.028 . (Article).
Grandahl M, Rosenblad A, Stenhammar C, et al. School-based intervention for the prevention of HPV among adolescents: a cluster randomised controlled study. BMJ open. 2016;6(1):e009875 (Journal Article; Randomized Controlled Trial; Research Support, Non‐U.S. Gov't).
Gualano MR, Thomas R, Stillo M, et al. What is the most useful tool in HPV vaccine promotion? Results from an experimental study. Hum Vaccin Immunother. 2019;15(7–8):1607–14. https://doi.org/10.1080/21645515.2018.1526552 . (In eng).
Henrikson NB, Zhu W, Baba L, et al. Outreach and reminders to improve human papillomavirus vaccination in an Integrated primary care system. Clin Pediatr (Phila). 2018;57(13):1523–31. https://doi.org/10.1177/0009922818787868 (In eng).
Hofstetter AM, Barrett A, Camargo S, Rosenthal SL, Stockwell MS. Text message reminders for vaccination of adolescents with chronic medical conditions: A randomized clinical trial. Vaccine. 2017;35(35):4554–60. https://doi.org/10.1016/j.vaccine.2017.07.022 (Article).
Joseph NP, Bernstein J, Pelton S, et al. Brief client-centered motivational and behavioral intervention to promote HPV vaccination in a hard-to-reach population: a pilot randomized controlled trial. Clin Pediatr (Phila). 2016;55(9):851–9. https://doi.org/10.1177/0009922815616244 (In eng).
Juraskova I, Bari RA, O’Brien MT, McCaffery KJ. HPV vaccine promotion: does referring to both cervical cancer and genital warts affect Intended and Actual Vaccination Behavior? Women’s Health Issues. 2011;21(1):71–9. https://doi.org/10.1016/j.whi.2010.08.004 (Journal: Article).
Juraskova I, O’Brien M, Mullan B, Bari R, Laidsaar-Powell R, McCaffery K. HPV vaccination and the effect of information framing on intentions and behaviour: an application of the theory of planned behaviour and moral norm. International journal of behavioral medicine. 2012;19(4):518–25. https://doi.org/10.1007/s12529-011-9182-5 (Journal Article; Randomized Controlled Trial).
Kaul S, Do TQN, Hsu E, Schmeler KM, Montealegre JR, Rodriguez AM. School-based human papillomavirus vaccination program for increasing vaccine uptake in an underserved area in Texas. Papillomavirus Res. 2019;8:8. https://doi.org/10.1016/j.pvr.2019.100189 (Article).
Kepka D, Coronado GD, Rodriguez HP, Thompson B. Evaluation of a radionovela to promote HPV vaccine awareness and knowledge among Hispanic parents. J Commun Health. 2011;36(6):957–65. https://doi.org/10.1007/s10900-011-9395-1 . (Journal Article; Randomized Controlled Trial; Research Support, N.I.H., Extramural; Research Support, U.S. Gov't, P.H.S.).
Kester LM, Shedd-Steele RB, Dotson-Roberts CA, Smith J, Zimet GD. The effects of a brief educational intervention on human papillomavirus knowledge and intention to initiate HPV vaccination in 18–26 year old young adults. Gynecol Oncol. 2014;132(SUPPL1):S9–12. https://doi.org/10.1016/j.ygyno.2013.12.033 . (Journal: Article).
Kim J, Nan X. Effects of consideration of future consequences and temporal framing on acceptance of the HPV vaccine among young adults. Health Commun. 2016;31(9):1089–96. https://doi.org/10.1080/10410236.2015.1038774 .
Kumar MM, Boies EG, Sawyer MH, Kennedy M, Williams C, Rhee KE. A brief provider training video improves comfort with recommending the human papillomavirus vaccine. Clin Pediatr (Phila). 2019;58(1):17–23. https://doi.org/10.1177/0009922818805217 . (In eng).
Kwang NB, Mahayudin T, Yien HL, Abdul Karim AK, Teik CK, Shan LP. Effect of an Educational intervention on knowledge of human papillomavirus vaccination among Pre-University Students in Malaysia. Asian pacific journal of cancer prevention. 2016;17(1):267–74. https://doi.org/10.1002/central/CN-01474250/full . (Article).
Lee H, Kim M, Cooley ME, et al. Using narrative intervention for HPV vaccine behavior change among Khmer mothers and daughters: A pilot RCT to examine feasibility, acceptability, and preliminary effectiveness. Appl Nurs Res. 2018;40:51–60. https://doi.org/10.1016/j.apnr.2017.12.008 (In eng).
Lefevere E, Hens N, De Smet F, Beutels P. The impact of non-financial and financial encouragements on participation in non school-based human papillomavirus vaccination: a retrospective cohort study. Eur J Health Econ. 2016;17(3):305–15. https://doi.org/10.1007/s10198-015-0680-2 (In eng).
Lennon T, Gundacker C, Nugent M, et al. Ancillary benefit of increased HPV immunization rates following a CBPR approach to address immunization disparities in younger siblings. Journal of Community Health. 2019;44(3):544–51. https://doi.org/10.1007/s10900-018-00610-9 Article) (In English).
Lin L, Macias Parra M, Sierra VY, et al. Long-term immunogenicity and safety of the AS04-adjuvanted human papillomavirus-16/18 vaccine in four- to six-year-old girls: three-year follow-up of a randomized phase III trial. Pediatr Infect Dis J. 2019;38(10):1061–7. https://doi.org/10.1097/INF.0000000000002437 . (Journal: Article).
Liu CR, Liang H, Zhang X, et al. Effect of an educational intervention on HPV knowledge and attitudes towards HPV and its vaccines among junior middle school students in Chengdu, China. BMC Public Health. 2019;19(1):488. https://doi.org/10.1002/central/CN-01953799/full . (Journal Article).
Malo TL, Gilkey MB, Hall ME, Shah PD, Brewer NT. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25(10):1383–91. https://doi.org/10.1158/1055-9965.Epi-16-0224 . (In eng).
Malo TL, Hall ME, Brewer NT, Lathren CR, Gilkey MB. Why is announcement training more effective than conversation training for introducing HPV vaccination? A theory-based investigation. Implement Sci. 2018;13(1):57. https://doi.org/10.1186/s13012-018-0743-8 . (In eng).
Mantzari E, Vogt F, Marteau TM. Financial incentives for increasing uptake of HPV vaccinations: a randomized controlled trial. Health Psychol. 2015;34(2):160–71. https://doi.org/10.1037/hea0000088 . (In eng).
Marchand-Ciriello L, Foustoukos A, Fantasia HC. Intervention to Increase human papillomavirus vaccine initiation rates in adolescent males. JNP-J Nurse Pract. 2020;16(1):79–82. https://doi.org/10.1016/j.nurpra.2019.06.018 . (Editorial Material) (In English).
McGlone MS, Stephens KK, Rodriguez SA, Fernandez ME. Persuasive texts for prompting action: agency assignment in HPV vaccination reminders. Vaccine. 2017;35(34):4295–7. https://doi.org/10.1016/j.vaccine.2017.06.080 . (Article).
McLean HQ, VanWormer JJ, Chow BDW, et al. Improving human papillomavirus vaccine use in an integrated health system: impact of a provider and staff intervention. J Adolesc Health. 2017;61(2):252–8. https://doi.org/10.1016/j.jadohealth.2017.02.019 . (In eng).
McRee AL, Shoben A, Bauermeister JA, Katz ML, Paskett ED, Reiter PL. Outsmart HPV: Acceptability and short-term effects of a web-based HPV vaccination intervention for young adult gay and bisexual men. Vaccine. 2018;36(52):8158–64. https://doi.org/10.1016/j.vaccine.2018.01.009 . (In eng).
Meyer AF, Borkovskiy NL, Brickley JL, et al. Impact of electronic point-of-care prompts on human papillomavirus vaccine uptake in retail clinics. Am J Prev Med. 2018;55(6):822–9. https://doi.org/10.1016/j.amepre.2018.06.027 (In eng).
Mohanty S, Leader AE, Gibeau E, Johnson C. Using facebook to reach adolescents for human papillomavirus (HPV) vaccination. Vaccine. 2018;36(40):5955–61. https://doi.org/10.1016/j.vaccine.2018.08.060 (In eng).
Molokwu J, Dwivedi A, Mallawaarachchi I, Hernandez A, Shokar N. Tiempo de Vacunarte (time to get vaccinated): Outcomes of an intervention to improve HPV vaccination rates in a predominantly Hispanic community. Prev Med. 2019;121:115–20. https://doi.org/10.1016/j.ypmed.2019.02.004 . (In eng).
Morales-Campos DY, Parra-Medina DA. Predictors of HPV vaccine initiation and completion among hispanic mothers of 11–17 year old daughters living along the Texas-Mexico border. Cancer Epidemiology Biomarkers and Prevention 2016;25(3):B90. (Conference Abstract) (In English). https://doi.org/10.1158/1538-7755.DISP15-B90 .
Nissen M, Kerkvliet JL, Polkinghorn A, Pugsley L. Increasing rates of human pipillomavirus vaccination in family practice: a quality improvement project. S D Med. 2019;72(8):354–60 (In eng).
PubMed Google Scholar
Nwanodi O, Salisbury H, Bay C. Multimodal counseling interventions: effect on human papilloma virus vaccination acceptance. Healthcare (Basel). 2017;5(4):86. https://doi.org/10.3390/healthcare5040086 . (In eng).
Obulaney PA, Gilliland I, Cassells H. Increasing cervical cancer and human papillomavirus prevention knowledge and HPV vaccine uptake through mother/daughter education. J Community Health Nurs. 2016;33(1):54 66-quiz 66-7. https://doi.org/10.1080/07370016.2016.1120595 . (In eng).
Padmanabha N, Kini JR, Alwani AA, Sardesai A. Acceptability of human papillomavirus vaccination among medical students in Mangalore. India Vaccine. 2019;37(9):1174–81. https://doi.org/10.1016/j.vaccine.2019.01.032 . (Article) (In English).
Parra-Medina D, Morales-Campos DY, Mojica C, Ramirez AG. Promotora outreach, education and navigation support for HPV vaccination to Hispanic women with unvaccinated daughters. J Cancer Educ. 2015;30(2):353–9. https://doi.org/10.1007/s13187-014-0680-4 . (In eng).
Paskett ED, Krok-Schoen JL, Pennell ML, et al. Results of a multilevel intervention trial to increase human papillomavirus (HPV) vaccine uptake among adolescent girls. Cancer Epidemiol Biomarkers Prev. 2016;25(4):593–602. https://doi.org/10.1158/1055-9965.Epi-15-1243 . (In eng).
Patel DA, Zochowski M, Peterman S, Dempsey AF, Ernst S, Dalton VK. Human papillomavirus vaccine intent and uptake among female college students. J Am College Health. 2012;60(2):151–61. https://doi.org/10.1080/07448481.2011.580028 . (Journal Article; Randomized Controlled Trial; Research Support, N.I.H., Extramural; Research Support, U.S. Gov't, P.H.S.).
Porter RM, Amin AB, Bednarczyk RA, Omer SB. Cancer-salient messaging for human papillomavirus vaccine uptake: a randomized controlled trial. Vaccine. 2018;36(18):2494–500. https://doi.org/10.1016/j.vaccine.2018.01.040 . (In eng).
Poscia A, Pastorino R, Boccia S, Ricciardi W, Spadea A. The impact of a school-based multicomponent intervention for promoting vaccine uptake in Italian adolescents: a retrospective cohort study. Ann Ist Super Sanita. 2019;55(2):124–30. https://doi.org/10.4415/ann_19_02_04 (In eng).
Pot M, Paulussen TG, Ruiter RA, et al. Effectiveness of a web-based tailored intervention with virtual assistants promoting the acceptability of HPV vaccination among mothers of invited girls: randomized controlled trial. J Med Internet Res. 2017;19(9):e312. https://doi.org/10.2196/jmir.7449 . (In eng).
Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313–20. https://doi.org/10.1080/10810730.2018.1442530 . (In eng).
Rhodes D, Visker JD, Cox C, Sas A, Banez JC. Effects of an online educational module on school nurses’ knowledge of HPV vaccination. J Cont Educ Nurs. 2017;48(9):431–6. https://doi.org/10.3928/00220124-20170816-10 . (Article) (In English).
Richman AR, Maddy L, Torres E, Goldberg EJ. A randomized intervention study to evaluate whether electronic messaging can increase human papillomavirus vaccine completion and knowledge among college students. J Am Coll Health. 2016;64(4):269–78. https://doi.org/10.1080/07448481.2015.1117466 . (In eng).
Richman AR, Torres E, Wu Q, et al. Text and Email Messaging for Increasing Human Papillomavirus Vaccine Completion among Uninsured or Medicaid-insured Adolescents in Rural Eastern North Carolina. J Health Care Poor Underserved. 2019;30(4):1499–517. https://doi.org/10.1353/hpu.2019.0090 .
Rickert VI, Auslander BA, Cox DS, Rosenthal SL, Rupp RE, Zimet GD. School-based HPV immunization of young adolescents: effects of two brief health interventions. Hum Vaccin Immunother. 2015;11(2):315–21. https://doi.org/10.1080/21645515.2014.1004022 . (In eng).
Rockliffe L, Chorley AJ, McBride E, Waller J, Forster AS. Assessing the acceptability of incentivising HPV vaccination consent form return as a means of increasing uptake. BMC Public Health. 2018;18(1):382. https://doi.org/10.1186/s12889-018-5278-z . (In eng).
Roussos-Ross K, Foster L, Peterson HV, Decesare J. Do educational seminars for the human papillomavirus vaccine improve attitudes toward the value of vaccination? J Pediatr Adolesc Gynecol. 2017;30(4):456–9. https://doi.org/10.1016/j.jpag.2016.12.003 . (Article) (In English).
Sadoh AE, Okonkwobo C, Nwaneri DU, et al. Effect of peer education on knowledge of human papilloma virus and cervical cancer among female adolescent students in Benin City. Nigeria Ann Glob Health. 2018;84(1):121–8. https://doi.org/10.29024/aogh.24 . (In eng).
Schnaith AM, Evans EM, Vogt C, et al. An innovative medical school curriculum to address human papillomavirus vaccine hesitancy. Vaccine. 2018;36(26):3830–5. https://doi.org/10.1016/j.vaccine.2018.05.014 . (In eng).
Shah PD, Calo WA, Gilkey MB, et al. Questions and concerns about HPV vaccine: a communication experiment. Pediatrics. 2019;143(2):10. https://doi.org/10.1542/peds.2018-1872 . (Article) (In English).
Staples JN, Wong MS, Rimel BJ. An educational intervention to improve human papilloma virus (HPV) and cervical cancer knowledge among African American college students. Gynecol Oncol. 2018;149(1):101–5. https://doi.org/10.1016/j.ygyno.2017.10.015 . (In eng).
Staras SAS, Vadaparampil ST, Livingston MD, Thompson LA, Sanders AH, Shenkman EA. Increasing human papillomavirus vaccine initiation among publicly insured florida adolescents. J Adolesc Health. 2015;56(5):S40–6. https://doi.org/10.1016/j.jadohealth.2014.11.024 . (Article) (In English).
Stern L, Unger Z, Debevec E, Ginde S, Morfesis J, Patel A. Staying on track: a cluster randomized controlled trial of automated reminders for HPV vaccine series completion. Contraception. 2013;88(3):438–9. https://doi.org/10.1016/j.contraception.2013.05.036 . (Journal: Conference Abstract).
Underwood NL, Weiss P, Gargano LM, et al. Human papillomavirus vaccination among adolescents in Georgia. Hum Vaccin Immunother. 2015;11(7):1703–8. https://doi.org/10.1080/21645515.2015.1035848 . (In eng).
Rodriguez AM, Do TQN, Goodman M, Schmeler KM, Kaul S, Kuo YF. Human papillomavirus vaccine interventions in the U.S.: a systematic review and meta-analysis. Am J Prev Med. 2019;56(4):591–602. https://doi.org/10.1016/j.amepre.2018.10.033 .
Xiao X, Lee DKL, Wong RM, Borah P. The impact of theory in HPV vaccination promotion research: a systematic review and meta-analysis. Am J Health Promot. 2021;35(7):1002–14. https://doi.org/10.1177/08901171211012524 .
Fu LY, Bonhomme LA, Cooper SC, Joseph JG, Zimet GD. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. 2014;32(17):1901–20. https://doi.org/10.1016/j.vaccine.2014.01.091 .
Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31:399–418. https://doi.org/10.1146/annurev.publhealth.012809.103604 .
Askelson N, Ryan G, McRee AL, et al. Using concept mapping to identify opportunities for HPV vaccination efforts: Perspectives from the Midwest and West Coast. Eval Program Plann. 2021;89:102010. https://doi.org/10.1016/j.evalprogplan.2021.102010 .
Askelson NM, Ryan G, Seegmiller L, Pieper F, Kintigh B, Callaghan D. Implementation challenges and opportunities related to HPV vaccination quality improvement in primary care clinics in a rural state. J Community Health. 2019;44(4):790–5. https://doi.org/10.1007/s10900-019-00676-z .
Vaccination Programs: Health Care System-Based Interventions Implemented in Combination. The Community Guide. 2014 ( https://www.thecommunityguide.org/findings/vaccination-programs-health-care-system-based-interventions-implemented-combination ).
Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N. Changing the behavior of healthcare professionals: the use of theory in promoting the uptake of research findings. J Clin Epidemiol. 2005;58(2):107–12. https://doi.org/10.1016/j.jclinepi.2004.09.002 .
Newman PA, Logie CH, Lacombe-Duncan A, et al. Parents’ uptake of human papillomavirus vaccines for their children: a systematic review and meta-analysis of observational studies. BMJ Open. 2018;8(4):e019206. https://doi.org/10.1136/bmjopen-2017-019206 .
Bowyer HL, Forster AS, Marlow LA, Waller J. Predicting human papillomavirus vaccination behaviour among adolescent girls in England: results from a prospective survey. J Fam Plann Reprod Health Care. 2014;40(1):14–22. https://doi.org/10.1136/jfprhc-2013-100583 .
Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26. https://doi.org/10.1097/MLR.0b013e3182408812 .
Landes SJ, McBain SA, Curran GM. An introduction to effectiveness-implementation hybrid designs. Psychiatry Res. 2019;280:112513. https://doi.org/10.1016/j.psychres.2019.112513 .
Daniels V, Saxena K, Roberts C, et al. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: a model based analysis. Vaccine. 2021;39(20):2731–5. https://doi.org/10.1016/j.vaccine.2021.04.003 .
Patel Murthy B, Zell E, Kirtland K, et al. Impact of the COVID-19 pandemic on administration of selected routine childhood and adolescent vaccinations -10 U.S.Jurisdictions, March-September 2020. MMWR Morb Mortal Wkly Rep. 2021;70(23):840–5. https://doi.org/10.15585/mmwr.mm7023a2 .
Ryan G, Gilbert PA, Ashida S, Charlton ME, Scherer A, Askelson NM. Challenges to adolescent HPV vaccination and implementation of evidence-based interventions to promote vaccine uptake during the COVID-19 pandemic: “HPV is probably not at the top of our list.” Prev Chronic Dis. 2022;19:E15. https://doi.org/10.5888/pcd19.210378 .
Sonawane K, Lin YY, Damgacioglu H, et al. Trends in human papillomavirus vaccine safety concerns and adverse event reporting in the United States. JAMA Netw Open. 2021;4(9):2124502. https://doi.org/10.1001/jamanetworkopen.2021.24502 .
Download references
Acknowledgements
Not applicable.
This study was supported by Centers for Disease Control and Prevention, SIP 19–005 Cancer Prevention and Control Research Network, U48DP006377, U48DP006389, U48DP006400, U48DP006413, U48DP006408 and U48DP006398. CBB was supported by a NIH Cancer Care Quality Training Program grant, UNC-CH, Grant No. T32-CA-116339. GR was supported by the National Cancer Institute Grant No. T32-CA-172009. PM was supported by P30CA023074-41. The funders had no role in the study design, data collection, analysis, and interpretation of data and in writing the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDC or NIH.
Author information
Authors and affiliations.
Rollins School of Public Health, Emory University, 1518 Clifton Road, NE, Atlanta, GA, 30322, 404-727-4701, USA
Cam Escoffery, Courtney Petagna, Christine Agnone, Stephen Perez, Lindsay B. Saber, Meena Dhir & Swathi Sekar
Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA
Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA, USA
Katherine A. Yeager & Stephanie Lee
Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, Durham, NC, USA
Caitlin B. Biddell
Mel & Enid Zuckerman College of Public Health, University of Arizona, Tucson, USA
Purnima Madhivanan
Institute for Health Disparities, University of North Texas Health Science Center, Fort Worth, TX, USA
Amanda S. English
Center for Health Promotion and Prevention Research, School of Public Health, University of Texas Health Science Center at Houston, Houston, TX, USA
Lara Savas & Maria E. Fernandez
Prevention Research Center, College of Public Health, University of Iowa, Iowa City, IA, USA
Health Promotion Research Center, School of Public Health, University of Washington, Seattle, WA, USA
You can also search for this author in PubMed Google Scholar
Contributions
CE is responsible for the study design and oversight of the study. CE, CP, CA, MD, GR, SS, KY, CB, PM, SL, AE, LS, ED, TH and MF contributed to data abstraction and review of the manuscript. CE, CP, LS and SP contributed the analysis plan of the manuscript and they and MD, GR wrote the manuscript. All authors read, edited and approved the final manuscript.
Corresponding author
Correspondence to Cam Escoffery .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional files 1: supplemental table 1..
Systematic Review of HPV Vaccination Intervention Search Terms. Supplemental Table 2. Quality Assessment of Included Articles* .
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Escoffery, C., Petagna, C., Agnone, C. et al. A systematic review of interventions to promote HPV vaccination globally. BMC Public Health 23 , 1262 (2023). https://doi.org/10.1186/s12889-023-15876-5
Download citation
Received : 22 September 2022
Accepted : 11 May 2023
Published : 29 June 2023
DOI : https://doi.org/10.1186/s12889-023-15876-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Young adults
- Health promotion
- HPV vaccination
- Vaccination
- Interventions
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Research Article
- Open access
- Published: 17 November 2022
HPV knowledge and vaccine acceptability: a survey-based study among parents of adolescents (KAPPAS study)
- Noelia López ORCID: orcid.org/0000-0001-8568-4752 1 ,
- Ignacio Salamanca de la Cueva 2 ,
- Elena Taborga 3 ,
- Auxiliadora Fernández de Alba 2 ,
- Inés Cabeza 4 ,
- Reyes Mazas Raba 5 ,
- Josep Marès 6 ,
- Patricia Company 7 ,
- Bruno Herrera 1 &
- Manuel Cotarelo 1
Infectious Agents and Cancer volume 17 , Article number: 55 ( 2022 ) Cite this article
6015 Accesses
2 Citations
1 Altmetric
Metrics details
Human papillomavirus (HPV) infection is recognized as one of the major causes of infection-related cancer worldwide. In Spain, the HPV vaccination program started in 2007 and until 2022, it targeted 12-year-old girls.
This was a cross-sectional, multicenter survey-based research carried out at 24 pediatric offices to describe HPV knowledge and vaccine acceptability in parents of children aged between 9 and 14 years-old in Spain. Parents were randomly selected from the medical records following specific quotas to ensure representativeness. The survey included five sections that aim to collect information about sociodemographic characteristics, knowledge of HPV, knowledge and acceptability of vaccines in general, HPV vaccination knowledge and HPV vaccine acceptability. Each section was constituted by a number of close questions with different answer options. Specific scores were assigned to each possible answer to these questions. Based on these scores, four composite variables were created to assess HPV knowledge, HPV vaccine knowledge, HPV vaccine acceptability and vaccines knowledge and acceptability in general. A latent class analysis was performed to identify different group of respondents according to their HPV vaccine acceptability.
A total of 1405 valid surveys were included, with 86.19% of the respondents being mothers. The mean score of HPV knowledge was 28.92 out of 40 (maximum value) (95% CI 28.70–29.20) and the mean score of HPV vaccine acceptability was 3.37 out of 5 (maximum value). One third of parents still need more information to take a final decision about HPV vaccination in their children. Parents perceived that females were more likely to become infected than males and tended to associate HPV infection mainly with cervical cancer, showing a. a lack of information about other HPV-related diseases affecting males.
Conclusions
This study results highlight the need for future actions and educational initiatives to raise awareness of HPV consequences in both genders and to contribute to achieving the elimination of HPV-related diseases beyond cervical cancer.
Human papillomavirus (HPV) infection is recognized as one of the major causes of infection-related cancer worldwide, as well as a causal factor of other diseases such as genital warts or recurrent respiratory papillomatosis [ 1 ].
Nowadays, HPV infections are regarded as the most common sexually transmitted infections in the world. Indeed, 80% of sexually active people will become infected at some point in their lifetime. However, most of these infections are typically controlled immunologically within 1–2 years, although if they persist, they can cause different types of cancer, such as cervical cancer or oropharyngeal cancer [ 1 , 2 , 3 ]. Although the HPV-related burden of disease used to be higher in females than in males in most countries, the latest epidemiological studies point to an increasing trend in the incidence of anal and oropharyngeal cancer in men [ 4 ]. Immunization against HPV infection is the most promising strategy for the prevention of one of the most common sexually transmitted infections worldwide [ 1 ].
In 2020, the World Health Organization/The United Nations Children's Emergency Fund (WHO/UNICEF) published the Estimates of National HPV Immunization Coverage from 2010 to 2019. According to this publication, 107 (55%) of the 194 WHO Member States had introduced HPV vaccination [ 5 ]. America and Europe are by far the WHO regions with the most introductions, and 85% and 77% of their countries, respectively, have already introduced the HPV vaccination, with almost one third of the programs (33 out of 107) being gender-neutral [ 5 ]. Many countries have recognized the value of HPV gender-neutral vaccination programs for the purpose of achieving the goal of eliminating not only cervical cancer but all HPV-related diseases [ 6 ]. According to the latest data published by the European Cancer Organization in 2020, 26 countries in the European region of the WHO are or will be including boys in their national HPV vaccination programs [ 6 ]. This represents almost half (48%) of all the countries in this region [ 6 ]. In Spain, HPV vaccination was introduced into the national immunization program in 2007–2008. Until 2022, it targeted 12-year-old girls [ 7 ], with a mean vaccination coverage rate (VCR) of 81.8% in 2020 (latest data available) [ 8 ].
The latest vaccination calendar published by the Spanish Association of Pediatricians (AEP), dated January 2022, recommends HPV vaccination for girls and boys at the age of 12 years [ 9 ]. However, although this Society [ 9 ] recommends including boys in HPV vaccination programs since 2018, the funded Spanish Immunization Program continues to target only female adolescents and specific high-risk groups. Regardless of gender, it is important to stress that the parents of adolescents play a key role in vaccination decision-making, and the success of the vaccination program relies largely on parental decision-making [ 10 ].
Our group published a systematic literature review in 2020 about HPV knowledge and vaccine acceptability among European adolescents and their parents. This review concluded that since HPV knowledge and vaccine acceptability were still modest and varied widely between studies across EU countries, coordinated efforts should be made to provide the relevant population with information to allow informed decision-making on HPV vaccination [ 10 ]. If the information received by the parents is not properly balanced there might be a negative impact on HPV vaccine acceptability and therefore the HPV-related disease elimination goal would not be achievable [ 6 ].
To our knowledge, as yet no studies to assess knowledge of HPV and vaccine acceptability among parents of children (girls and boys) have been performed at national level in Spain. Spanish studies already published are mainly regional and have focused exclusively on describing acceptability in the female or adult populations at regional level [ 11 , 12 , 13 ]. As the VCR differs substantially between regions, a national study to evaluate knowledge of HPV and vaccine acceptability for girls and boys is called for.
The objective of this study, KAPPAS study (Knowledge and Acceptability of Papillomavirus Vaccines in Parents of Adolescents in Spain ), was to describe HPV knowledge and HPV vaccination acceptability among parents of girls and boys aged between 9 and 14 years living in Spain. Moreover, the study assessed the correlation between HPV knowledge and HPV vaccine acceptability and the influence of different sociodemographic variables.
This paper focuses on the results of the level of HPV knowledge and vaccine acceptability in parents of adolescents in Spain. The analysis of the factors involved in HPV knowledge and vaccine acceptability has been addressed in a separate publication [ 14 ].
Study design and setting
This was a cross-sectional, multicenter survey-based research carried out at twenty-four (public and private) pediatric offices in Spain between May 2019 and April 2020. Sample size and sample distribution was estimated to assess primary and secondary endpoints with appropriate precision (≤ 5%) per each stratum of interest.
Due to the COVID-19 pandemic, only surveys completed before 16 April were considered valid for the analysis to ensure external validity of our results; as COVID-19 pandemic could interfere in vaccine perceptions in the overall population. The study recruited the fathers, mothers or legal guardians of children (girls and/or boys) aged between 9 and 14 years who had been living in Spain for at least the last 12 months.
The study obtained the favorable approval of the reference Investigational Ethical Committee (IEC). The rest of IECs requested the evaluation or only the registration of the protocol as necessary per their local guidance.
Survey development
A structured survey, in Spanish language, was developed to collect epidemiological variables as well as knowledge- and acceptance-related measurements. It was designed based on a previous systematic literature review [ 11 ] carried out by our group to identify published studies and items used to evaluate parental and/or adolescent HPV knowledge and/or HPV vaccination acceptability. Based on the inputs found in the systematic review, a draft questionnaire was developed and then validated by an Expert Committee comprised of 4 pediatricians who were experts in adolescents and HPV. The draft questionnaire was afterwards tested through cognitive debriefing methodology on a representative sample of 12 parents of children between 9 and 14 years old following a fine-tuning of the wording and comprehensiveness of the questionnaire according to participants’ perceptions and suggestions. The Experts Committee validated the final version of the questionnaire.
The final survey (Additional file 1 : Material S1) included five sections: (1) sociodemographic characteristics (15 items); (2) knowledge of HPV (9 items); (3) knowledge of vaccines and their acceptability in general (5 items); (4) HPV vaccination knowledge (8 items); (5) HPV vaccine acceptability (7 items). All questions were closed with multiple choice of answers, using the appropriate scale of response according to the specific type of question (yes/no, yes/no/not sure, ordinal scale of level of agreement or specific response options, when needed) (Additional file 1 : Material S2).
Data collection
Representative centers across different Spanish regions were invited to participate. A preliminary selection of sites was performed by the Committee of Experts of the study-a stratification based on HPV VCR was performed to ensure adequate representativeness. To those centers preliminary selected, a feasibility questionnaire was sent, to ensure that their willingness and availability to participate in this study, and to ensure that they had enough patients between 9 and 14 y.o in order to be able to send the questionnaire to the parents of those patients.. Active recall recruitment process was designed to prevent any selection bias such as chronically ill patients who might attend pediatricians’ offices more frequently (Additional file 1 : Figure S1). All children aged between 9 and 14 years were identified from investigator databases or medical records and were divided into 4 stratification quotas based on gender and age (males 9–11 y.o; females 9–11 y.o,; males 12–14 y.o and females 12–14 y.o). Spanish regions were classified into 2 categories according to their HPV VCR: low VCR: < 77.8% / high VCR: ≥ 77.8%). In 2107, when the protocol was drafted, the mean national VCR was 77.8%.
The investigators actively invited parents following the order generated by a randomization tool by telephone and according to the stratification quotas to ensure representativeness. The survey could be completed either online or in a paper format in an autonomous manner. The investigator sent the participant an e-mail/mobile text message with a link to the survey and a code. Each participant could access the link, introduce the code and confirm his/her acceptance to participate and afterwards fill the online survey (Additional file 1 : Figure S1). For the paper-pen option, the participant could either collect the questionnaire in the office or print it directly from the same link. After completion, the questionnaire could be sent through pre-paid envelopes or be delivered at the doctor’s office. Per protocol, the survey should not be completed in the presence of the pediatrician, nor should anyone from his team interfere with the participant’s answers.
Statistical analysis
A descriptive analysis of the qualitative and quantitative variables was performed. The qualitative variables were described by means of frequencies and percentages. Normality data test were performed to choose the appropriate statistical tests accordingly. The quantitative variables were described by n, mean, standard deviation, 95% confidence interval (CI), median, interquartile interval (25 th and 75 th percentiles) and minimum and maximum according to the distribution. The CI was calculated with the Clopper-Pearson method for binomial proportions and the Sison and Glaz (1995) method for multinomial proportions. The Pearson’s correlation coefficient was calculated to study the correlation between quantitative variables. 95% CI were built using a bootstrapping method (N = 1000 iterations).
Four composite variables on knowledge and acceptability were created based on the responses to the items in sections 2–5 of the questionnaire. The points were summated to create a total score and the results were described with the mean and 95% CI. Details on score assignment can be found in Additional file 1 : Material S2.
Degree of HPV knowledge total score ranged from 0 to 40, Degree of HPV vaccine acceptability ranged from 0 to 5, Degree of HPV vaccination knowledge ranged from 0 to 21 and Degree of knowledge of vaccines and their acceptability in general ranged from minus 10 to 10.
In order to identify the different profiles of parents who answered the survey, a latent class analysis (LCA) was conducted. The different response patterns were used to classify parents into groups (classes) according to answer similarities. LCA uses categorical data to create the groups, and the results provide the probability of a respondent belonging to each class. The questions included in the LCA were selected based on the previous assessment on their relevance in HPV vaccine acceptability: 2.4, 2.5, 3.1–3.5, 4.6, 4.7 and 5.1–5.4.
A total of 3110 participants were selected and contacted. 1071 did not answer and 555 refused participation, which translates into a response rate of 47.7%. After exclusion of unanswered and the invalid surveys, (n = 79) 1405 surveys were considered valid for the analysis (1116 online and 289 paper-based) (see reasons of invalid surveys in Additional file 1 : Figure S2).
Sociodemographic characteristics
Most of the recruitment sites were public (68.0%) and were located in a region considered as low VCR (55.9%) (Table 1 ). Distribution according to gender and age was similar in each stratification group.
Most respondents were mothers (86.2%), between 40 and 49 years (69.1%), with a university degree (35.6%), in full-time employment (61.8%), living in a place with more than 50,000 inhabitants (37.0%), of Spanish nationality (97.4%), married (81.7%), with 2 children (63.1%) and not vaccinated against HPV (76.6%). Only 7.6% of the parents stated that they had been vaccinated against HPV, and 15.8% of them were unsure of their vaccination status (Table 1 ). Of the children about whom the survey was completed, 736 (52.4%) were girls with a mean age of 11.5 (SD: 1.6) years. Eight hundred and ninety-five (63.7%) of the children had not been vaccinated against HPV and 391 (27.8%) had. For the rest, the vaccination status was reported as “unknown”.
HPV knowledge
The majority of the respondents (90.7%) had heard of HPV infection. The pediatrician (44.8%) was the most common source of information, followed by family and friends (40.4%) and the Internet (39.3%).
The participants had a medium-to-high degree of HPV knowledge, with a mean score of 28.9 out of 40 (95% CI 28.7–29.2). Additional data are provided in the Additional file 1 : Figure S3 and S4. In general, the parents agreed that HPV was a serious health problem (39.9% strongly agree, 53.1% agree) and that it was one of the most common sexually transmitted diseases (17.9% strongly agree, 52.2% agree).
The respondents correctly answered that HPV is a sexually transmitted disease (89.2%), although 10.8% were not sure how it is transmitted (Additional file 1 : Figure S5). Most of the parents considered than women (89.2%) or girls (69.1%) could get infected by HPV. In contrast, only 50.2% and 67.2% considered that boys and men could be infected, respectively (data not shown). Regarding possible diseases related to HPV infection, the majority of the respondents considered HPV infection to be related to cervical cancer (73.7%). However, in Fig. 1 it is shown that parents were less aware of the role of HPV in other diseases.
Diseases related to HPV
In terms of HPV prevention; most of the parents answered that it could be prevented by the HPV vaccine (87.2%) and condom use (80.9%). A lower proportion stated that delayed sexual debut (10.2%), personal hygiene (13.9%) and monogamy (14.2%) were also reported mechanisms for preventing HPV infections. Seven percent were not sure how to prevent this infection (data not shown). Additional data are provided in the Additional file 1 : Figure S5.
In order to obtain more information about HPV infection, most parents would rather consult healthcare professionals such as pediatricians (80.1%), family doctors (68.3%) and gynecologists (78.1%). Only 19.8% of the respondents would consult the Internet or the social media (Data not shown).
HPV vaccine acceptability
The respondents had a medium-to-high degree of HPV vaccine acceptability, translating into a mean score of 3.37 out of 5 (95% CI 3.30–3.44) (Additional file 1 : Figure S3).
In general, the participants presented a high level of agreement (strongly agree + agree) in considering that HPV vaccination is necessary in girls and boys. However, the results revealed that a higher proportion of parents considered it necessary in girls compared to boys (Fig. 2 and Additional file 1 : S8).
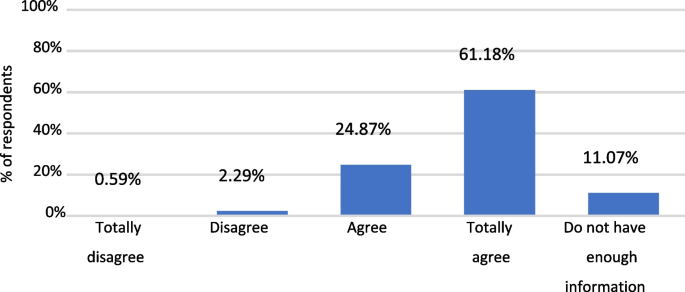
Answers to question: “I would vaccinate my son/daugther against HPV”
The main reasons for having the child vaccinated were to protect them against sexually transmitted diseases (67.4%) or against cancer and/or genital warts (77.4%), whereas the reasons for not having them vaccinated included lack of information (27.9%), fear of possible adverse events (20.9%) and other unspecified reasons (29.3%) (Additional file 1 : Figure S7).
With regard to the type of information needed for the HPV vaccination to be acceptable for parents who initially disagreed to vaccinate their daughter/son: 55.7% would request information about vaccine safety; 54.5% would need a doctor’s recommendation, 51.8% about HPV vaccine efficacy, 49% about the HPV vaccine in general and 46.1% about HPV infection (Data not shown).
The proportion of parents that would consult a pediatrician to obtain further information about the HPV vaccine represented 93.6% (Fig. 3 ).
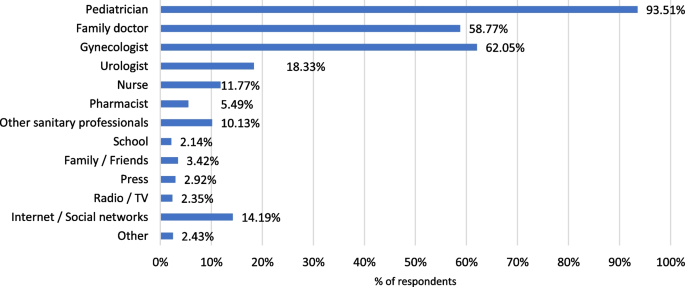
Main sources to be consulted for obtaining more information about HPV vaccine, according to participant’s opinion
Knowledge of HPV vaccination
The parents evinced an intermediate-to-high degree of knowledge of HPV vaccination, with a mean score of 15.5 out of 21 (95% CI 15.3–15.6) (Additional file 1 : Figure S3 and S4).
As it was observed with HPV infection, 92.1% of the respondents had heard of the HPV vaccine, the main source of information being the pediatrician (62.3%). Family and friends (34.5%), the gynecologist (27.8%) and the Internet (25.1%) were also mentioned as sources of information about the HPV vaccination.
Only 57.5% of the participants provided a correct answer indicating that the HPV vaccine was funded only for girls, whereas up to 25.1% of the parents did not know if the HPV vaccine was funded as part of the Spanish vaccination program (data not shown).
When the parents were questioned about the recommended age for vaccination, most of them answered correctly, with a mean (SD) reported age of 12.10 (1.21) years.
As occurred with HPV infection, 90.8% and 55.2% of the parents knew that girls and women, respectively, can be vaccinated against HPV. In contrast, the proportion of participants that considered that male populations could be eligible for the HPV vaccine was lower: only 60.1% and 37.9% of the parents considered that boys and men, respectively, could be vaccinated (Data not shown).
Up to 75.9% of the parents concurred (strongly agree + agree) in considering the HPV vaccine as effective, and 76.8% agreed that its benefits outweigh the risks. Nevertheless, it is important to point out that nearly 20% of the parents opined that they lacked sufficient information to answer (data not shown).
The HPV vaccine results tallied with the answers in the HPV knowledge section, HPV-related diseases. Thus, 80.0% of the participants considered that cervical cancer could be prevented with the vaccine, although the percentage was much lower for other diseases, such as genitals warts or anal cancer, that also affect males (Fig. 4 ). Additional data are shown in Additional file 1 : Figure S6.

Diseases that can be prevented by HPV vaccination, according to participant’s opinion
Knowledge and acceptability of vaccines in general
Knowledge and acceptability of vaccines was high, 6.6 out of 10 (95% CI 6.4–6.8). In fact, 25.8% of the participants obtained the maximum score (10 points).
More details are included in Additional file 1 : Figure S9.
Correlations
There were significant and positive correlations between all variables (vaccine knowledge, HPV vaccine knowledge and HPV vaccine acceptability), and parents who scored high in one variable tended to score high in the other variables (Fig. 5 ).
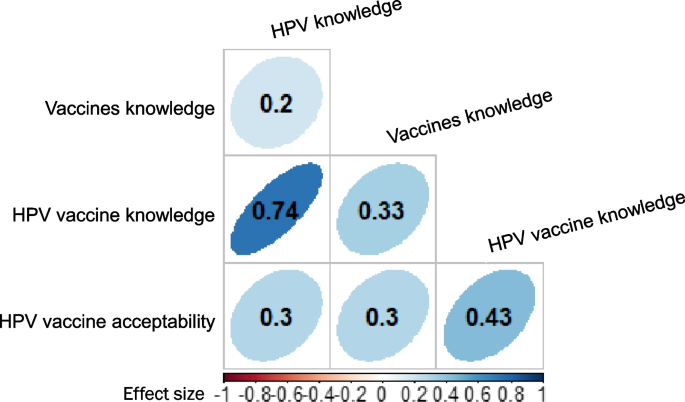
Correlations between knowledge and vaccine acceptability. p < 0.001 for all correlations. The colour intensity and shape indicate the strength of the correlation
The highest correlation was observed between HPV knowledge and HPV vaccine knowledge (0.7), followed by the correlation between HPV vaccination knowledge and HPV vaccine acceptability (0.4). Lower correlations were observed between other pairs of variables, although they were statistically significant (Fig. 5 ).
Typologies of respondents
The results of the LCA analysis allowed us to conclude that there were 4 groups of parents, according to different response patterns:
Class 1 (the probability of belonging to this group was 0.47): they consider that vaccines are useful, effective, beneficial and also that parents who do not have their children vaccinated put other people at risk. On the other hand, they think that both girls and boys should be vaccinated and that their doctor has recommended such vaccination. This group of parents showed a high agreement in considering HPV as a sexually transmitted disease and a serious health problem.
Class 2 (probability: 0.34): they consider that vaccines may be useful, effective, beneficial and also that parents who do not have their children vaccinated put other people at risk but that they may need more information. They think that it might be necessary to have both girls and boys vaccinated and that their doctor has recommended such vaccination. This group of parents agreed in considering HPV as a sexually transmitted disease and a serious health problem. Although these parents may have their sons vaccinated, they may need further information about HPV or its vaccine.
Class 3 (probability: 0.15): this group of parents are very unsure or lack sufficient information about HPV and its vaccine. While they are not afraid of having their child vaccinated, this lack of information could increase their indecision.
Class 4 (probability: 0.04): this group of parents have a higher probability of not accepting the vaccine as they believed that vaccines are not useful, are not safe and are ineffective. In addition, they did not regard HPV as a common sexually transmitted disease and a serious health problem. Furthermore, they tended to think that there is no need to have boys and girls vaccinated. In this group of parents, a high proportion stated that their doctor did not recommend the HPV vaccine.
According to our results, thirteen years after the beginning of the HPV vaccination program in Spain, the degree of knowledge of HPV among parents of adolescents is still modest, although HPV vaccine acceptability is medium–high. There was still a clear tendency to relate HPV to girls and females [ 6 ].
Parents perceived that females were more likely to become infected than males and tended to associate HPV infection mainly with cervical cancer. In addition, there was a lack of information about other HPV-related diseases, and even more about those affecting males. These results contrast with the real burden of HPV-related diseases, which is substantial in both genders, causing not only cervical cancer but also anal, penile, vaginal, vulval and oropharyngeal cancers, in addition to genital warts and recurrent respiratory papillomatosis (RRP) [ 6 ]. In this context, it is important to highlight that the current objective of HPV vaccination programs in high-income countries is the elimination HPV-related diseases, not only cervical cancer [ 15 ]. To this aim establishing gender-neutral vaccination program would therefore seem to be crucial.
Our findings are similar to those of other HPV vaccine awareness studies performed in other European countries, such as the outcomes of our own systematic review published in 2020 [ 11 ]. This review found that HPV knowledge and acceptability of the HPV vaccine continued to be modest and varied widely across EU countries, with insufficient information and safety concerns being the main barriers to vaccination acceptability [ 11 ]. A more recent review [ 16 ] also identified a modest degree of HPV and HPV vaccine knowledge among the male population. In line with the results of previous studies [ 11 , 16 , 17 , 18 ], the KAPPAS study emphasizes the need to implement actions to provide the relevant population with information, including the possible impact of HPV in males, and therefore empower them to make informed decisions. The significant and positive correlations between HPV knowledge and HPV vaccine knowledge as well as between HPV vaccination knowledge and HPV vaccine acceptability found in our study also underscore the importance of awareness-raising campaigns.
In our study, the LCA revealed that approximately more than one third of the parents may still have insufficient information about HPV and its vaccine and evince a certain indecision in vaccinating their children, particularly boys. This result reinforces the need for educational activities targeting parents that are still hesitant to HPV vaccination to ensure the success of HPV immunization programs. On the other hand, in our study, the percentage of parents who present a higher probability of not accepting the HPV vaccine or vaccines in general is very small (3.80%). This correlated with the high VCR in our country of HPV and the rest of the vaccines, one of the highest in Europe. Our LCA analysis may also help healthcare professionals to identify different types of parents and provide them with balanced information targeting their needs in order to enable them to take informed decisions about their children’s HPV vaccination.
Similar results were published in 2015 in an pan-European study that examined A study published in 2015 examining parental views of HPV vaccination of sons in France, Germany, Italy and the UK [ 19 ] found that approximately three quarters of the parents in the UK, Germany and Italy were in favor of the HPV vaccination for their sons. The favorable parents sought to protect their sons from the disease and regarded gender equality as important. Parents in doubt about male HPV vaccination needed more information about HPV diseases and HPV vaccination among males. The rejecting parents were generally skeptical of vaccines and feared the side effects of vaccination. Although all these countries now include boys in their HPV immunization programs, this study was conducted before this inclusion and examined countries with significant differences in vaccination coverage [ 20 , 21 ]. Parents in countries with high HPV vaccination coverage rates (UK and Italy) tended to recognize the importance of national vaccination programs. Parents in countries with limited HPV vaccination coverage rate (Germany and France) felt a greater need for information from healthcare professionals (HCP) and the public health authorities. The authors concluded that by providing brief information about HPV in both genders, parental acceptance of HPV vaccination for sons could be as high as for girls [ 19 ]. This was confirmed by the recent WHO/UNICEF estimations, published in 2020, Bruni et al. [ 5 ] which have shown that the VCR in boys and girls is similar in countries with gender-neutral HPV vaccination programs.
Our study is the first of its kind conducted in Spain at a national level that sheds some light on the knowledge and acceptability of HPV and its vaccine.
Some limitations derived from the nature of this study should be borne in mind. Firstly, the potential sources of bias in this study comprised a selection bias due to the parents’ acceptance to participate in the survey and the inclusion of the population entered in medical records. In addition, parent-reported information is subjective and may be affected by social desirability, imprecision or mistakes in interpreting the questions.
More than 12 years after the implementation of the HPV vaccination program in our country, parental HPV knowledge and vaccine acceptability is medium-to-high. However, HPV is still associated with the female gender, with important lack of knowledge of HPV consequences in males. Moreover, our results also point out that more than one third of parents still need more information to vaccinate their children against HPV. Providing parents with adequate and well-balanced information is crucial to ensure the success of HPV vaccination programs.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due participants privacy protection but are available from the corresponding author on reasonable request.
Abbreviations
Spanish Association of Pediatricians
Confidence interval
Coronavirus disease
European Union
Healthcare professionals
Human papillomavirus
Investigational ethical committee
Latent class analysis
Recurrent respiratory papillomatosis
United Kingdom
United Nations International Children's Emergency Fund
Vaccine coverage rate
World Health Organization
Bosch FX, Broker TR, Forman D, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl 7):H1–31.
Article PubMed PubMed Central Google Scholar
Schiffman M, Doorbar J, Wentzensen N, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086.
Article PubMed Google Scholar
Weaver BA. Epidemiology and natural history of genital human papillomavirus infection. J Am Osteopath Assoc. 2006;106:S2–8.
PubMed Google Scholar
Hartwig S, St Guily JL, Dominiak-Felden G, et al. Estimation of the overall burden of cancers, precancerous lesions, and genital warts attributable to 9-valent HPV vaccine types in women and men in Europe. Infect Agent Cancer. 2017;12:19.
Bruni L, Saura-Lazaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144:106399.
European Cancer Organization. Viral Protection: Achieving the Possible. A Four Step Plan for Eliminating HPV Cancers in Europe. https://www.europeancancer.org/resources/159:viral-protection-achievingthe-possible-a-four-step-plan-for-eliminating-hpv-cancers-in-europe.html . Accessed 25 March 2021.
Ministerio de Sanidad Servicios Sociales e Igualdad. Spanish Vaccination Calendar 2021. https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/calendario-y-coberturas/home.htm . Accessed 22 March 2021.
Ministerio de Sanidad. Servicios Sociales e Igualdad - Profesionales - Vacunas Coberturas de Vacunación. 2022. https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/calendario-y-coberturas/coberturas/docs/Tabla11.pdf . Accessed 03 March 2022.
García FJA, Ortega MJC, Aldeán JÁ, Garcés-Sánchez M, Llanos EG, et al. en representación del Comité Asesor de Vacunas de la Asociación Española de Pediatría (CAV-AEP). Immunisation schedule of the Pediatric Spanish Association: 2022 recommendations. Anales de Pediatría (English Edition). 2022;96(1):59.e1–59.e10.
Lopez N, Garces-Sanchez M, Panizo MB, et al. HPV knowledge and vaccine acceptance among European adolescents and their parents: a systematic literature review. Public Health Rev. 2020;41:10.
Navarro-Illana P, Caballero P, Tuells J, et al. Acceptability of human papillomavirus vaccine in mothers from Valencia (Spain). An Pediatr (Barc). 2015;83:318–27.
Article CAS Google Scholar
Caballero-Perez P, Tuells J, Rementeria J, et al. Acceptability of the HPV vaccine among Spanish university students in the pre-vaccine era: a cross-sectional study. Rev Esp Quimioter. 2015;28:21–8.
Navarro-Illana P, Navarro-Illana E, Vila-Candel R, Diez-Domingo J. Drivers for human papillomavirus vaccination in Valencia (Spain). Gac Sanit. 2018;32:454–8.
López N, Salamanca de la Cueva I, Vergés E, Suárez Vicent E, Sánchez A, López AB, Panizo-Santos MB, Garcés-Sánchez M, Montesdeoca A, Rivera AJ, Cotarelo MS. Factors influencing HPV knowledge and vaccine acceptability in parents of adolescent children: results from a survey-based study (KAPPAS study). Hum Vaccines Immunother. 2022;18(1):2024065.
Article Google Scholar
European. Cancer Organisation. Eliminating HPV-Caused Cancers and Diseases in Europe. 2019. Report available in: https://www.europeancancer.org/resources/51:eliminating-hpv-caused-cancers-and-diseases-in-europe-case-for-action.html . Accessed 03 March 2022.
Radisic G, Chapman J, Flight I, Wilson C. Factors associated with parents’ attitudes to the HPV vaccination of their adolescent sons: a systematic review. Prev Med. 2017;95:26–37.
Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138:e20153863.
Rodriguez AM, Do TQN, Goodman M, et al. Human papillomavirus vaccine interventions in the U.S.: a systematic review and meta-analysis. Am J Prev Med. 2019;56:591–602.
Lee Mortensen GAM, Idtaleb L. Parental attitudes towards male human papillomavirus vaccination: a pan-European cross-sectional survey Infectious Disease epidemiology. BMC Public Health. 2015;15:1–10. https://doi.org/10.1186/s12889-015-1863-6 .
Ministerio della salute. Piano nazionale prevenzione vaccinale. http://www.salute.gov.it/portale/vaccinazioni/dettaglioContenutiVaccinazioni.jsp?lingua=italiano&id=4828&area=vaccinazioni&menu=vuoto . Accessed 1 Sept 2021.
Public Health England. NHS Public Health functions agreement 2019–2020. HPV programme. 2019. https://www.england.nhs.uk/wp-content/uploads/2017/04/Service-Specification-No.11-Human-Papillomavirus-Virus-v2-080819.pdf . Accessed 7 Sept 2021.
Download references
Acknowledgements
Investigators of the KAPPAS study: Magdalena Aga (CS Repélega); Isabel Cañabate (CS Churriana); Cynthia Crespo (CAP Montclar); Mara Garcés (CS Nazaret); Ana Belén López (IHP Córdoba); Gema Belén López (IHP Córdoba); María Martín (CS La Cala de Mijas); Abián Montesdeoca (CS Guanarteme); Ana María Nocea (CS Condes de Barcelona); Mª Belén Panizo (CS Illescas); Lizeth Peña (CS Pla Vinalopó); Victoria Planelles (CS Paiporta); Almudena Sánchez (CAP Les Hortes); Eva Suarez (CS Burriana II); Isabel Úbeda (CS L'Eliana); Edelmiro Verges (CS Binissalem, CS Alaro). Editorial assistance and medical writing support were provided by Esther Tapia, PhD and Adelphi Targis, SL.
This study has been funded and sponsored by MSD Spain.
Author information
Authors and affiliations.
Medical Affairs Department, MSD Spain, C/Josefa Valcarcel, 38, 28027, Madrid, Spain
Noelia López, Bruno Herrera & Manuel Cotarelo
Instituto Hispalense de Pediatría, Seville, Spain
Ignacio Salamanca de la Cueva & Auxiliadora Fernández de Alba
Centro de Salud de Villalegre - La Luz, Avilés, Asturias, Spain
Elena Taborga
Healthcare Centre Galdakao, Vizcaya, Spain
Inés Cabeza
Healthcare Centre Gama, Cantabria, Spain
Reyes Mazas Raba
Institut Pediàtric MARES-RIERA, Girona, Spain
Josep Marès
Healthcare Centre Pla, Elche, Alicante, Spain
Patricia Company
You can also search for this author in PubMed Google Scholar
Contributions
NL was responsible for original idea and design of the study, reviewed the results, and actively prepared and reviewed the manuscript. MC and BH reviewed results and manuscript. ISdlC participated in the design of the study and recruitment, reviewed results and reviewed the manuscript. The other authors participated in the recruitment, reviewed results and manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Noelia López .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Comité de Ética de la investigación con medicamentos de Euskadi (CEIm-E) reference ethics committee on 08th January, 2019.
Acceptance to participate was obtained from all subjects involved in the study.
Consent for publication
Not applicable.

Competing interests
NL, BH, and MC are full-time employee of MSD Spain. ISdlC has received grants and/or honoraria as a consultant/advisor or attending conferences and practical courses from GlaxoSmithKline, Sanofi Pasteur, MSD and Pfizer. ET has received honoraria from MSD as an investigator for this study. AFdA has received honoraria from MSD as an investigator for this study. IC has received honoraria from MSD as an investigator for this study and grants for medical education activities. She has also participated as speaker for other pharmaceutical companies. RMR has received honoraria from MSD as investigator for this study. JM has received grants from MSD as an investigator for this study and payments for lectures including service on speaker bureau and as a board membership from GlaxoSmithKline, Sanofi Pasteur, MSD and Pfizer. PC has received honoraria from MSD as investigator for this study.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: material s1..
Questionnaire used in the KAPPAS study. Material S2 . Scores Keys assigned to each item to compute global scores. Material S3 . Additional information of methodology and results. Figure S1 . Recruitment and data collection process. Figure S2 . Flowchart of participants. Figure S3 . Total scores and distribution of respondents with regard to A) HPV knowledge, B) HPV vaccine knowledge, C) HPV vaccine acceptability, D) knowledge and acceptability of vaccines in general. Figure S4 . Box representation of total scores. Figure S5 . Wrong/Right answers for HPV knowledge. Figure S6 . Wrong/Right answers for HPV vaccine knowledge. Figure S7 . Reasons to vaccinated and to not vaccinate the child. Figure S8 : Responses to questions related to HPV vaccine acceptability. Figure S9 : Responses to questions related to Knowledge and acceptability of vaccines in general.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
López, N., de la Cueva, I.S., Taborga, E. et al. HPV knowledge and vaccine acceptability: a survey-based study among parents of adolescents (KAPPAS study). Infect Agents Cancer 17 , 55 (2022). https://doi.org/10.1186/s13027-022-00467-7
Download citation
Received : 07 October 2021
Accepted : 31 October 2022
Published : 17 November 2022
DOI : https://doi.org/10.1186/s13027-022-00467-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- HPV vaccine
- Vaccination
- Acceptability
- HPV-related diseases
Infectious Agents and Cancer
ISSN: 1750-9378
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Human papilloma virus articles from across Nature Portfolio
Human papilloma virus (HPV) is an infectious agent belonging to the virus family Papillomaviridae, members of which have tropism for cutaneous epithelium and mucosal epithelium. There are more than 120 different HPV types, with HPV-16 and HPV-18 having a strong association with cervical cancer.
Latest Research and Reviews
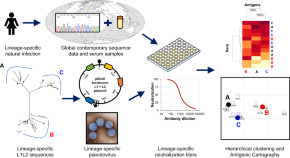
Global evaluation of lineage-specific human papillomavirus capsid antigenicity using antibodies elicited by natural infection
Human Papillomaviruses (HPV) are classified in lineages based on their sequence. Here, the authors test neutralizing activity of sera from naturally infected women against vaccine-preventable HPV variants, delineating lineage-specific antibody responses.
- Gathoni Kamuyu
- Filomeno Coelho da Silva
- Simon Beddows
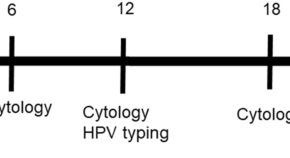
A prospective clinical trial of diathermy ablation for patients with high-grade cervical intraepithelial neoplasia from a single institution in Japan
- Takeji Mitani
- Iwao Kukimoto
- Takuma Fujii
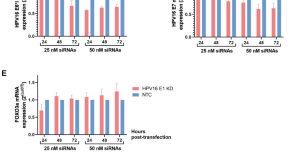
HPV16E1 downregulation altered the cell characteristics involved in cervical cancer development
- Thanayod Sasivimolrattana
- Arkom Chaiwongkot
- Parvapan Bhattarakosol

SIRT1 is an actionable target to restore p53 function in HPV-associated cancer therapy
- Irene Lo Cigno
- Federica Calati
- Marisa Gariglio
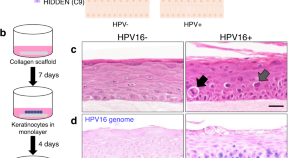
Single cell transcriptomic analysis of HPV16-infected epithelium identifies a keratinocyte subpopulation implicated in cancer
The role of keratinocyte subpopulations in the different phases of the viral cycle during HPV16 infection remains to be characterised. Here, single cell RNA sequencing of HPV16 infected and uninfected organoids identifies 12 distinct keratinocyte populations including an HPV-reprogrammed keratinocyte subpopulation that is linked to cancer.
- Mary C. Bedard
- Tafadzwa Chihanga
- Susanne I. Wells
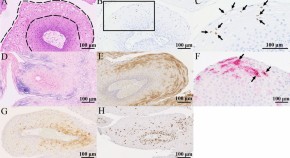
Association between human papillomavirus particle production and the severity of recurrent respiratory papillomatosis
- Satoshi Yamada
- Toshiya Itoh
- Hideya Kawasaki
News and Comment

A refined view of tumour-associated B cells
Wieland et al. report that the tumour microenvironment of human papillomavirus (HPV)-positive head and neck squamous cell carcinomas contains HPV-specific B cells that actively secrete HPV-specific antibodies.
- Sarah Seton-Rogers

Toll of vaccine hesitancy
- Jamie Horder

Benevolent viruses in skin cancer
Strickley, Messerschmidt et al. show that beta human papilloma virus (β-HPV) infection itself is not causal in cutaneous squamous cell carcinoma (SCC) development in the context of immunosuppression — instead, the loss of β-HPV-mediated T cell immunity promotes SCC.
- Ulrike Harjes
TIL infusions effective in HPV-associated cancers
- Peter Sidaway

JCVI defers decision on HPV
Quick links.
- Explore articles by subject
- Guide to authors
- Editorial policies
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

The HPV vaccine
- HPV vaccination helps to prevent cancer by protecting against HPV. HPV increases the risk of some types of cancer and causes almost all cases of cervical cancer.
- The HPV vaccine is offered to all children between the ages of 11-13 in the UK.
- It is also available to people up to the age of 25 who missed their vaccination when offered it, men who have sex with men, and some transgender people.
The human papillomavirus (HPV) is a very common virus. It usually doesn’t cause any problems. For most people, HPV will be cleared from the body and they will never know they’ve had it.
But some types of HPV can cause problems such as genital warts, and ‘high-risk’ types of HPV increase the risk of some cancers. High-risk HPV can cause changes to the DNA in our cells and make them more likely to turn cancerous.
Find out more about what HPV is and how it can cause cancer.
Content not working due to cookie settings.
What is the HPV vaccine?
The HPV vaccine protects against HPV infection. The HPV vaccine used in the NHS vaccination programme is called Gardasil 9. It protects against nine types of HPV - HPV 6, 11, 16, 18, 31, 33, 45, 52 and 58.
Most people under the age of 25 will need just one dose of the vaccine.
Sometimes this vaccination is called the ‘cervical cancer vaccine’ because it has been proven to reduce rates of cervical cancer in England.
But the vaccine also protects against types of HPV that can cause cancers of the mouth, throat, vulva, vagina, penis and anus. It also protects against genital warts.
The NHS has further information on the HPV vaccine, including dosing and potential side effects.
Why get the HPV vaccine?
The HPV vaccine has been proven to be safe and effective at protecting against HPV and reducing HPV infections. This reduces the risk of cancers caused by HPV.
The HPV vaccine plays an important role in preventing cervical cancer, as almost all cases of cervical cancer are caused by HPV. HPV 16 and 18 cause about 7 in 10 cases of cervical cancer, and the vaccine protects against these HPV types.
As HPV is linked to cancers of the vulva, vagina, penis, anus and some types of mouth and throat cancer, the vaccine may also help lower the risk of these cancer types.
The vaccine also provides protection against genital warts, by protecting against HPV 6 and 11 which cause the majority of cases.
How does the HPV vaccine work?
The HPV vaccine tricks the body into thinking it has been exposed to the HPV virus. This triggers the body’s immune system to produce antibodies to fight against HPV. Antibodies are usually how our body clears an infection.
Because our immune system has a memory, the body will now be able to quickly recognise HPV and produce antibodies if the body is exposed to HPV in the future. This means it will be quicker and easier for the body to clear HPV.
Studies have already shown that protection by the HPV vaccine lasts for around 10 years. As the vaccine continues to be used, we expect studies will show that it lasts for much longer.
The NHS has more information on how vaccines work.
dfs_0060_how_does_the_hpv_vaccine_work_r3.png
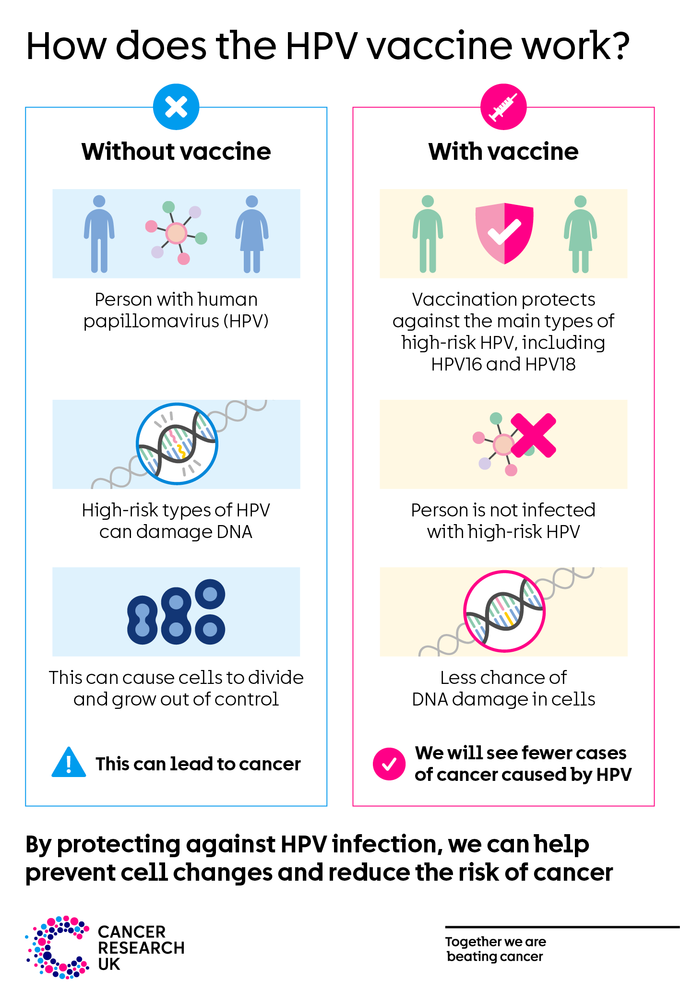
Who can get the HPV vaccine?
HPV vaccination is most effective in people who have not been exposed to HPV. This is why it’s recommended to vaccinate children at the age of 11-13. By adulthood most people will have been exposed to HPV, so vaccination won’t be as effective.
The groups listed below can get the HPV vaccine for free. If you aren’t in one of these groups, you can pay to have the vaccine privately if you want it.
Other people may be offered the vaccine for free on a case-by-case basis if they are at higher risk of HPV. If you’re not sure if you are eligible for the HPV vaccine or have questions about it, speak to your doctor.
All children aged 11-13
Since September 2019, all children aged 11-13 in the UK are offered the HPV vaccine. That’s all children in year 8 in England, Northern Ireland and Wales, or S1 in Scotland. The vaccine is usually given in schools. Children who are home-schooled can get it via their GP.
People younger than 25 who missed their HPV vaccination
People who were offered the HPV vaccination but did not have it can still get the vaccine for free, up to the age of 25.
Talk to your GP, school nurse, or school immunisation team about this.
Men who have sex with men
Men who have sex with men can request the vaccine for free through sexual health and HIV clinics in the UK, up to the age of 45.
Studies show men who have sex with men may be at increased risk of anal cancer. Having the HPV vaccination may help to prevent anal cancer by protecting against HPV.
Transgender people
Some transgender (trans) people can get the vaccine for free through sexual health and HIV clinics in the UK:
- Trans men (men assigned female at birth) younger than 45 who have sex with other men
- Trans women (women assigned male at birth) younger than 45, on a case-by-case basis
But if you have already had the vaccine as part of the vaccination programme for school children, there is no need to have it again.
Do I need to go to cervical screening if I’ve had my HPV vaccination?
Yes, you should still consider taking part in cervical screening if you’ve had the HPV vaccine. The HPV vaccine doesn’t protect against all types of HPV that cause cervical cancer. And cervical screening is another effective way to prevent cervical cancer.
Cervical screening in the UK is for most women, some transgender men and some non-binary people who are aged between 25 and 64.
Cervical screening aims to detect HPV and spot early cell changes in the cervix caused by HPV. This means any abnormal cells can be monitored or treated before they have a chance to become cancer.
Cervical screening is for people without symptoms. Don’t wait for your next screening appointment if you’ve noticed anything that’s not normal for you, speak to your doctor.
Find out more about cervical screening
Find out more about cancer screening for people who are trans or non-binary
Key references
The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02178-4/fulltext
Lehtinen M, Lagheden C, Luostarinen T, et al. Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point—registry-based follow-up of three cohorts from randomized trials. BMJ Open. 2017;7(8):e015867. doi:10.1136/bmjopen-2017-015867
National Health Service (NHS). HPV vaccine overview. NHS Website. 2019; (accessed March 2021). https://www.nhs.uk/conditions/vaccinations/hpv-human-papillomavirus-vaccine/
Last reviewed
Rate this page:.

We want to make working with Washington state government easier The state of Washington wants to learn more about your interactions with its government services to better support your needs. Please take this 15-minute survey for a chance to win a $200 gift card (your choice of 1,000+ options)!
About Us | Contact Us | Newsroom
Human Papillomavirus (HPV) Vaccine at Age Nine
Hpv vaccine is most effective between ages 9 and 12 years.

- Doctors and nurses recommend the HPV vaccine for children starting at age 9 years.
- The HPV vaccine is most effective at this age, it produces the most infection-fighting cells, or antibodies. This also ensures immunity is already in place before any exposure to the virus.
- If your teen hasn't received the vaccine, it is not too late! Talk to their doctor or nurse about getting them immunized as soon as possible. This is a recommended vaccine up through age 26.
- If you are an adult age 27 through age 45 , talk with your health care provider to see if you should get the HPV vaccine.
Efforts to Increase HPV Vaccination Coverage Rates
Since 2017, the Washington State Department of Health (DOH) has worked with providers and advocates around the state to improve HPV vaccination coverage rates. In 2022, the Washington State Vaccine Advisory Committee passed a motion to routinely start HPV vaccination at age nine and to track and publish state and county data on HPV vaccination coverage rates for children ages 9-10 years.
As a result, in January 2023, DOH updated the software in the state's immunization information system that determines vaccine eligibility to recommend HPV vaccination at age nine instead of age 11. With this update, providers received a reminder to start the HPV vaccine series once a child turns nine years old. This provides more time for the child to complete the two-dose series before they turn 13.
IIS Update Notification to Providers
HPV At Nine Provider Letter (PDF)
HPV at Nine Research
Research over the years shows that the HPV vaccine is most effective when given between ages 9 and 12. Below are some of the research papers that support this:
- For a more depth look at Washington state’s HPV at nine initiative: Effect of immunization registry-based provider reminder to initiate HPV vaccination at age 9, Washington state (tandfonline.com)
- Why the American Academy of Pediatrics recommends initiating HPV vaccine at age 9 Pub Med (nih.gov)
- Article collection: HPV Vaccination Starting at Age 9 (tandfonline.com)
HPV At Nine Resources
- Spanish (PDF)
- Marshallese (PDF)
- Russian (PDF)
- Ukrainian (PDF)
- Vietnamese (PDF)
- Talking to Parents About HPV Vaccines (PDF) (CDC)
- HPV Vaccination Algorithm (PDF)
HPV Dose Reminder Cards:
Age 9-14, English (PDF) / Age 9-14, Spanish (PDF)
Age 15+ English (PDF) / Age 15+ Spanish (PDF)
Adolescent Immunization Schedule:
- Korean (PDF)
- Punjabi (PDF)
Additional Places to Find HPV at Nine Resources:
- HPV Information for Parents (CDC)
- Resource Center (National HPV Roundtable)
- Vaccines – WCAAP - Washington Chapter of the American Academy of Pediatrics
HPV at Nine Webinars and Trainings
Past and upcoming trainings for improving HPV vaccination at age nine rates. Continuing Education (CE) credits available.
- HPV Vaccine Starts at 9: Why? How? Now! Cancer Prevention Made Easy
- Strategies for Improving HPV Vaccination Rates (nfid.org)
- Cancer Prevention through Human Papillomavirus (HPV) Vaccination (nfid.org)
- You Are the Key to HPV Cancer Prevention – 2022 (CDC)
Quality Improvement for HPV Vaccination
Several resources exist to help providers and clinics with improving immunization rates.
- Immunization Quality Improvement for Providers (IQIP)
- Training Tools (HPV IQ)
- Washington Child Health Improvement Partnership (wa-chip.org)
- Scheduling Future Coverage Rate Reports: Example provided for HPV 2 dose series for age 9-10 years (youtube.com)
Washington State HPV Free Taskforce
Are you interested in getting more involved with HPV vaccination efforts? If so, the Washington State HPV Free Taskforce is for you! Their mission is to increase human papillomavirus (HPV) vaccination rates in Washington state and reduce the amount of HPV associated disease by engaging and supporting diverse partners and increasing knowledge about HPV and cancer prevention.
The taskforce includes representatives from various sectors including Federally Qualified Health Centers, managed care organizations/payers, hospitals, primary care providers, government, tribal entities, HPV cancer survivors, and others involved in HPV vaccination and prevention. For more information visit Washington State HPV Free Task Force (immunitycommunitywa.org).
Please familiarize yourself with the Taskforce’s Group Agreements (PDF) prior to attending our meetings.
- Care-a-Van Mobile Health Services
- Disability Organizations
- Drug User Health
- Food Safety
- Healthy Aging
- Healthy Home
- Illness and Disease
- Immunization
- Infants and Children
- Injury and Violence Prevention
- Men's Health
- Nutrition and Physical Activity
- Oral Health
- Poisoning and Drug Overdose
- Sexual and Reproductive Health
- Adolescents & Young Adults
- Women's Health
- Air Quality
- Climate and Health
- Contaminants
- Drinking Water
- Essentials for Childhood Initiative
- Health Equity
- Healthy Communities Washington
- Wastewater Management
- Water Recreation
- Worksite Wellness
- Facilities - New, Renew or Update
- File Complaint About Provider or Facility
- Healthcare Enforcement and Licensing Modernization Solution
- Healthcare Professional Credentialing Requirements
- Medical Commission
- Board of Nursing
- Professions - New, Renew or Update
- Provider Credential Search
- Veterans, Service Members and their Families
- Vital Records
- Data Guidelines
- Data Systems
- Data Topics A-Z
- Diseases and Chronic Conditions
- Environmental Health
- Health Behaviors
- Health Statistics
- Healthcare in Washington
- Injury Violence and Poisoning
- State Health Assessment
- Washington Tracking Network (WTN)
- Bioterrorism and Terrorism
- Emergency Information for Specific Groups
- Get Ready for an Emergency
- Severe Weather and Natural Disasters
- Emergency Contacts and Numbers
- Clinical Laboratory Reporting
- Emergency Medical Services (EMS) Systems
- Emergency Preparedness
- Healthcare Professions and Facilities
- Lactation and Infant Feeding-Friendly Environments
- Notifiable Conditions
- Public Health Laboratories
- Public Health System Resources and Services
- Rural Health
- Tribal Public Health and Relations
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Med J Islam Repub Iran
Human papilloma virus: A review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers
Maryam soheili.
1 School of Kinesiology and Health Science, York University, Toronto, Canada
Hossein Keyvani
2 Department of Medical Virology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
3 Gastrointestinal and Liver Disease Research Center, Iran University of Medical Sciences, Tehran, Iran
Marzieh Soheili
4 Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
5 Human Revivification Society of Congress 60, Tehran, Iran
Sherko Nasseri
6 Cellular and Molecular Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran
7 Department of Molecular Medicine and Medical Genetics, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran
Background: Human papillomavirus (HPV) infection is considered as the most common viral sexually transmitted infection worldwide. This poses an increasingly interdisciplinary medical challenge. Since there is vast scattered information in databases about HPV and the correlated diseases, we decided to collect useful data so that the experts can get a more comprehensive view of HPV.
Methods: In this article, HPV-associated diseases, prevalence, prevention, and new treatments are discussed. The retrieved articles reporting the latest data about the required information for our review were selected through searching in Web of Science, Scopus, Medline (PubMed), EMBASE, Cochrane Library, Ovid, and CINHAL with language limitations of English and German.
Results: There are 2 groups of HPVs: (1) low-risk HPV types that can lead to genital warts, and (2) high-risk HPV types that are involved in HPV-associated oncogenesis. About 70% of all sexually active women are infected and most of these infections heal within many weeks or months. In the case of HPV-persistence, a risk of preneoplasia or carcinoma exists. These types of viruses are responsible for the existence of genitoanal, gastrointestinal, urinary tract, and head and neck tumors. There is still no definite successful treatment. The detection of HPV-related condylomata occurs macroscopically in women and men, and the diagnosis of the precursors of cervical carcinoma in women is possible by Pap smear.
Conclusion: For extragenital manifestations, there is no structured early detection program. Meanwhile, studies on HPV vaccines confirm that they should be used for the primary prevention of HPV-dependent diseases. However, we need more research to find out the real advantages and disadvantages of vaccines.
↑ What is “already known” in this topic:
There are numerous papers in the field of sexual transmitted diseases (STDs), especially HPV. However, most of them do not have comprehensive data involving the main facts. Some of them have only evaluated the epidemiology or carcinogenesis or screening methods. And some have only assessed one type of cancer correlated with HPV. Thus, there is no distinct information among concepts.
→ What this article adds:
This study presents comprehensive updated data about HPV, the laboratory methods, and all its correlated cancers. These combined data are highly useful for the clinicians and laboratory specialists. This is the main reason that we decided not to divide this article into several papers. The readers do not need to look for scattered information to find their required data. A vast part of the information is available in tables and figures that are easily reachable by the readers.
Introduction
Sexually transmitted infections (STIs) are initially developed by sexual contact and have a high rate of morbidity and mortality worldwide, affecting 50% to 70% of sexually active individuals ( 1 , 2 ). Human papillomaviruses (HPVs) are a large and diverse group of epitheliotropic double-stranded DNA viruses ( 3 ). There are up to 225 types of HPVs divi ded into 5 groups (α, ß, γ, µ, and ν) ( 4 ). The exact classification of each group is shown in Figure 1 . A subgroup of about 15 of the α-types (high-risk (HR)-HPV types) can lead to invasive carcinomas ( 5 , 6 ).

HPV classification: Red color: High-risk HPVs, Green color: Low-risk HPVs, Yellow colors: probably high-risk HPVs, Blue color: Unknown
Persistent HPV infection is one of the important sexual transmitted diseases (STDs) associated with more than 5% of all cancers in the world ( 7 ). In other words, globally more than half of all malignancies related to infection are caused by HPV ( 8 ). Approximately 90% of the HPV viruses clear or become dormant in 1 to 2 years after infection. Statistics show that the majority of women who had a positive test for a high-risk HPV serotype developed cervical cancer after 3 to 5 years ( 9 ). HPVs mostly cause nonpersistent acute infections. Hence, like other oncoviruses, there is a striking gap between the times of diagnosis of the chronic infection and its early stages ( 10 ). Some studies report that infection with at least 1 high-risk HPV will occur during the lifetime of 60% of sexually active individuals ( 11 ). They are often eliminated by the immune system within 1 or 2 years after exposure ( 12 ). The virus in the remaining cases that persists for a long time affords lesions that can bring malignancies ( 13 ). Therefore, early diagnosis of HPV infection and HPV induced lesions is highly important to prevent cancer development ( 14 ). In this review, we tried to collect all data about the prevalence, mechanism of action and carcinogenesis, the correlated disases as well as a brief overview about laboratory studies, screening, diagnosis, and therapies of each disease. This collection has been prepared by the newest data of valid databases and websites, such as Centers for Disease Control and Prevention (CDC) and human papillomavirus (HPV) centers. This information can help us to be aware of several factors when a patient with HPV comes to us for screening or treatment.
Search Syntax and Search Strategy
All original published articles were searched in international databases, including Web of Science, Scopus, Medline (PubMed), EMBASE, Cochrane Library, Ovid, and CINHAL to retrieve all articles reporting the latest data about epidemiology, diagnostic methods, and treatment of diseases related to HPV that were not discussed comprehensively in previous studies. A search of these databases was performed by the researchers with hand searching through the reference lists and gray literature. We searched from January 1985 to March 2020, with language limitation of English and German. All related components were related to the following keywords: “Human Papilloma Virus”, “HPV”, “Epodemiology”, “Carcinogenesis”, “Diagnostic methods”, “Screening methods”, “Treatment”, “Cervical cancer”, “Cervical neoplasm”, “Cervical malignancy”, “Vulvar and vaginal cancer”, “Vulvar and vaginal neoplasm”, “Vulvar and vaginal malignancy”, “Anal cancer”, “Anal neoplasm”, “Anal malignancy”,”Esophageal cancer”, “Esophageal neoplasm”, “Esophageal malignancy”, “Colorectal cancer”, “Colorectal neoplasm”, “Colorectal maliganany”, “Prostate cancer”, “Prostate neoplasm”, “Prostate malignancy”, “Urothelial cancer”, “Urothelial eoplasm”, “Urothelial malignancy”, “Testicular cancer”, “Testicular neoplasm”, “Testicular malignancy”, “Renal cancer”, “Renal neoplasm”, “Renal malignany”, “Penile cancer”, “Penile neoplasm”, “Penile malignancy”, “Head and neck squamous cell carcinoma”, “Head and neck cancer”, “Head and neck neoplasm”, “Head and neck malignancy”, “cutaneous squamous cell carcinoma”, “Warts”, “HPV Vaccines”, and “HPV DNA Tests.
Screening and Selection
TheReference Manager bibliographic software was applied to manage the searched citations. Duplicate entries were checked by considering the title of the published papers, authors, year of publication, and specifications of the sources types. In case of questionable records, the texts were compared. We reviewed the initial search results, and after reviewing each article by title and available abstract, some of the articles were excluded. Evaluating the papers under consideration was based on the inclusion and exclusion criteria by 2 researchers separately (Sh.N. and Maryam S.). About 546 papers were retrieved; 353 of which were selected and evaluated to extract the required information. In case of finding no data in the studies available in databases, we tried to apply valid websites such as HPV center or CDC that have been cited in references.
Eligibility Criteria
All observational studies that assessed the prevalence of HPV and related cancers, screening and diagnostic methods, and total therapeutic managements were included. We excluded duplicate citations, oral presentation, posters, and articles where their abstract and full-text were not available. The original articles and reviews were included.
Data Extraction
Three steps of assessment for titles, abstracts, and full-texts were done. The full-text of each selected article was retrieved for detailed evaluation. Data were extracted using a checklist involving publication year, authors, type of cancer, laboratory tests, screening methods, diagnostic methods, and treatment. All steps from search to final data extraction were followed independently by 2 research experts (Sh. N., Marzieh S).
Epidemiology and Transmission
About 50% to 80% of sexually active females will be infected with HPV during their lifetime ( 15 ). The estimated global HPV prevalence is 11.7%. South Africa (17.4.0%), Eastern Africa (33.6%), Eastern Europe (21.4%), Western Europe (9.0%), Eastern Europe (21.4%), and Caribbean (35.4%) showed the highest HPV prevalences ( 16 , 17 ). Female sex workers (FSWs) are among the most susceptible groups to acquire HPV infection and develop cervical intraepithelial neoplasia and cervical cancer. In a meta-analysis done by Farhmand et al, it is demonstrated that the pooled HPV prevalence was 42.6%. HPV-16, HPV-52, and HPV-53 were the most common high-risk HPV types found among FSWs ( 18 ). Since there are no definite antiviral treatments for HPV, the high prevalence of genital HPV has been a great concern in the world ( 19 ). Another research evaluating 645 sexually active innercity young females reported a cervical HPV prevalence of 54% ( 20 ). Assessments of 12.7 million cancers occurring globally in 2008 showed that HPV infection is related to almost 100% of cervical cancers, 90% to 93% of anal canal cancers, 12% to 63% of oropharyngeal cancers, 40% to 64% of vaginal cancers, 40% to 51% of vulvar cancers, and 36% to 40% of penile cancers. Virtually 5% (610 000) of the cases had HPV associated with anogenital or oral cancers. Nonmelanoma skin cancers are the increased risk of cutaneous HPV types. The initial risk factor for occurring the anogenital and oral HPV infection among males and females is sexual behavior ( 21 ).
HPV Infection and Carcinogenesis
HPV infection can be persistent and carcinogenic through integrating the viral DNA into the host genome. Then, it omits the early and late HPV genes known as E2, E4, E5, L1, and L2. Two early viral proteins have oncogenic potentials: E6 and E7 ( 22 , 23 ). The high-risk HPVs (HR-HPVs) that are also carcinogens have a great ability to remain in human keratinocytes in vitro through a chronic status. These viruses penetrate the cervical epithelial cells and express the oncogenic protein E6 and E7, leading to the inactivation of host regulatory proteins p53 and then retinoblastoma protein (RB). The viral antigen is a very specific type of antigen marking tumor cells. It is presented in viral-mediated carcinogenesis. Thus, the point that should be considered is that the best target to have an antitumor therapeutic goal is this antigen that is expressed just by the infected cells. This is somehow a way to block the autoimmunity cascade. Viral antigens can also act along with tumor-associated antigens in case of the dependence of oncogenes on expressing viral oncoproteins in infected cells. Scientists try to develop anticancer gene therapy because of the relationship between HPV infection and cervical cancer ( 24 ). When HPV starts to integrate into the host genome, the malignant transformation occurs. Hereby, E6 and E7 are expressed. Therefore, they have been targeted by several types of vaccines. It is proven that vaccines have a satisfactory result against these antigens in HPV-induced cervical dysplasia ( 25 - 27 ). They are applied in the clinical trial phase to treat cervical and head and neck cancers ( 28 ). The integration of the genome to the cell is necessary to induce cervical cancer. So persistent HPV infection is not the only or sufficient factor to this point ( 29 ). HPV 18 has a great power to integrate into the host genome leading to malignancy ( 30 - 32 ). The fragile sites of chromosome have this integration that causes disruption to the open-reading frame of E2 and less commonly E1, E4 or E5 ( 29 , 30 ). Tables 1 and and2 2 present complete data of the statistics as well as present screening methods, diagnostic methods, and available treatments of different types of HPV-related diseases.
Molecular Diagnosis of HPV
The molecular techniques are the main instrument to detect HPV DNA because of hard cultivation of HPV in culture systems. Although the E1 gene is used, the L1 and L2 late genes encode viral capsid proteins are used for HPV genotypes detection. The diagnostic techniques for detection and genotyping of HPV were classified as shown in Figure 2 . All the commercially available tests are listed in Table 3 .

The schematic classification of diagnostic techniques for detection and genotyping of HPV
1. Nucleic Acid Detection-based Methods
1.1. nucleic acid amplification-based methods.
These methods are generally based on the polymerase chain reaction that is used for amplifying, detecting, and typing the HPV DNA by the use of degenerate primers MY09/MY11 or PGMY09/11, GP5+/6+ and SPF10 to amplify the viral capsid L1 gene ( 33 - 35 ).
1. The Conventional PCR-based Methods: The conventional PCR based methods are single or double nested-PCR ( 36 , 37 ) multiplex-PCR ( 38 ) and nested-PCR-RFLP assay ( 34 ). Then, the PCR product is amplified by targeting a type-specific DNA sequence or treatment with restriction enzyme to determine the specific sequence existing in the sample related to a specific type of HPV ( 39 , 40 )
2. The PCR Following by Hybridization-based Methods: The methods-based amplification of target DNA following hybridization include traditional PCR in situ hybridization (PISH), microplate colorimetric hybridization assay (MCHA), the linear array for HPV genotyping, and the reverse line hybridization. The PISH technique is the typical PCR performed on the slide of intact paraffin-embedded tissue, and then hybridized with specific DNA probes ( 41 , 42 ). The MCHA is a method based on PCR followed by colorimetric hybridization to type-specific probes on microplates ( 43 ). In the reverse line blot assays and the linear array HPV genotyping, after the amplifying step, the sequences are fixed on a membrane strip and detected by type-specific probe ( 39 , 44 ).
3. PCR-based Fluorescent-based Array: Microarray-based HPV genotyping assays employ the PCR to amplificate the viral genome fragment and then hybridize with several HPV-specific oligonucleotide probes attached on the surface of an insoluble supporter like bead or DNA chip ( 45 - 53 ). The suspension array genotyping assays use bead-based technology, which is based on the use of polystyrene beads dyed with 2 spectrally distinct fluorophores (red and infrared), and each bead set is coupled with a specific oligonucleotide probe for 1 HPV type. The HPV sequences are amplified, denatured, and hybridized with the bead-bound probes. Then, hybridized biotinylated amplicons that are labeled by using phycoerythrin and streptavidin are served as a reporter fluorophore. The bead sets are then read and analyzed on a Luminex analyzer ( 54 - 60 ).
4. The Real-time PCR-based HPV Genotyping-based Methods: The real-time PCR is reliable and sensitive, with high accuracy and validity in HPV-DNA detection and genotyping. Also, the viral load quantification and the capability of multisample qualification with different fluorochromes is the advantage. In this method the fluorescent probes in cooperation with PCR primers allow for quantification of the viral genome and are presented in a sample as the name “viral load”( 61 , 62 ) ( 63 - 65 ). The cobas 4800 HPV test uses multiplex real-time PCR and nucleic acid hybridization with 4 different fluorescent reporter probes that concurrently detects the L1 gene ( 64 , 66 ).
5. HPV E6/E7 mRNA-based Screening Assays: The detection of viral mRNA is based on transcription-mediated amplification of full-length E6/E7 transcripts accomplished by target capture. Reverse-transcriptase-PCR incorporates is a RT step following real-time quantitative PCR. The oncoproteins E6 and E7 are the most relevant transcripts for diagnostic and carcinogenesis follow-up. ( 67 , 68 ). The main techniques used to detect mRNA for E6/E7 oncogenes are 3 commercial assays shown in Table 3 ( 45 , 68 - 71 ).
1.2 Signal Amplification
Signal amplification describes methods-based probe molecule, which are hybridized to the target nucleic acid sequence and generate the signal related to amplification rate ( 72 ). Signal amplification technologies include branched DNA (bDNA) and hybrid capture (HC) assays ( 73 , 74 ). The hybrid capture method is the most widely-used signal amplification method that briefly samples DNA hybridized with the cocktail of RNA probes. The RNA-DNA hybrids indicate the presence of HPV DNA and subsequently it is revealed by the nonradioactive signal-amplification method to aid detection ( 73 - 76 ). The Digene HCII technology served as the second version of hybrid capture ( 77 ).The bDNA assay directly measures nucleic acid molecules and is based on binding the subset of “target probes” bound to specific nucleotide sequences (exist in the sample) as in situ hybridization (bDNA ISH) ( 72 , 74 ). The Cervista HPV test is a signal amplification method that uses 2 types of isothermal reactions, which is briefly based on the enzymatic cleaves the FRET oligonucleotides between the fluorophore and quencher molecule, resulting in the production of a fluorescence signal ( 39 , 42 , 74 ).
1.3 Noucleotid Hybridization-based Methods
The nonamplified HPV techniques include in southern/dot blot hybridization, and in situ hybridization (ISH). This method is generally time-consuming, requires more skill, necessary equipments, and is not as sensitive and reliable as the molecular methods. briefly, in HPV DNA detection by southern blot, the sample extracted DNA is digested by restriction enzymes and then runs in agarose gel electrophoresis to separate the digested DNA based on the size, and then is transferred to a nitrocellulose or nylon membrane and finally hybridized with cloned HPV genomic probes labeled with isotopic (P 32 ) or nonisotopic (digoxigenin) techniques. The detection procedure in ISH occurs right on the fixed nuclei of infected cells (in situ) and hybridization reaction is evaluated microscopically ( 78 , 79 ) In comparison with PCR, the blot hybridization-based method has higher specificity but is less sensitive ( 42 , 80 , 81 ).
2. Immune-biochemical-based Methods
1. HPV serology — ELISA assay: The serological tests to determine the HPV virus are performed based on VLPs (virus-like particles)( 82 - 84 ), and the sensitivity is about 50%( 85 ). Three forms of Elisa method include ( 1 ) Direct assays binding to HPV VLPs on the microplate; ( 2 ) indirect assays binding to HPV VLPs to the microplate via anti-VLP antibodies; and ( 3 ) Competitive assay by which the antigen is coated on fluorescent beads (Luminex-based assays) exposed to the sample ( 54 , 58 , 86 ).
2. HPV Neutralization Assay: High-throughput pseudovirion-based neutralization assay (HT-PBNA) with excellent repeatability and run-to-run reproducibility was developed for HPVs ( 86 ). HPV neutralization assays rely on neutralization of one of the following items: authentic virions, pseudotyped virions that are capsids carrying a reporter gene on their surface, and pseudovirions (PsVs) that have encapsidated reporter genes to assess anti-VLP conformational antibodies neutralizing activity. The neutralization assays are the gold standard to assess the protective potential of antibodies induced by HPVs vaccines in experimental systems ( 87 - 89 ).
The Potential Biomarkers for HPV Detection
The biomarkers related to high-risk HPV infection can help to enhance the sensitivity of cervical cytology screening, reduce false-negative diagnoses, monitoring and prognosis of related diseases.
The potential biomarker in cervical cancer includes in p16INK4a, Ki-67 that are the target of E6 and E7 oncoprotein ( 90 - 93 ). There are many molecular targets of E6 and E7, such as Wnt/β-catenin/Notch ( 94 , 95 ), PI3K/AKT/mTOR pathway ( 96 ), P53, and PRb( 97 , 98 ). The molecular targets of HPV E5 oncoprotein are the cell surface receptors like EGFR ( 99 , 100 ), p21Wafl/Sdil/Cip( 101 ), p27KIP1( 102 ), COX-2, VEGF, and Cav-1( 102 ). Besides, the host microRNAs have been affected by HPV proteins E5, E6, and E7. Increased expression of miR-16, miR-25, miR-92a, and miR-378, and decreased expression of miR-22, miR-15a, miR-15b, miR-21and miR29a miR-16, miR-27a, miR-29a, and miR-100 are attributed to viral oncoprotein E6 or E7 ( 103 - 108 ). The miRNA-944( 109 ) and miRNA-155( 110 ) overexpression as well as downregulation of miRNA-375 ( 111 ) can potentially be served as a biomarker for cervical cancer follow-up. Dysregulation of miR-375/AEG-1 Axis by HPV high-risk 16 and 18 E6/E7 promotes cellular proliferation, migration, and invasion in cervical cancer ( 112 ).
Condyloma acuminate (CA) or wart is one of the most common sexually transmitted diseases in the world ( 15 ). The incubation period is 3 weeks to 8 months, and the clinical manifestation takes 2 to 3 months ( 113 ). The viruses producing genital warts are usually the low-risk types of HPV: 6, 11, 42, 43, and 44. types 6 and 11 are detected in 90% of cases. A study in 2009 has reported that 31% of patients with CA have a high-risk HPV as coinfection ( 114 ). A cohort study in 2019 reprted that the high-risk HPV genotypes could also be associated with warts. Particularly, a large number of patients with CIN2-3 and milder lesions with CIN1 were carriers of a virus of a HPV-HR genotype ( 115 ). Typically, CA is mostly discrete, skin-colored, brown or whitish, pedunculated,or broad-based with a variation in size from 1 millimeter to several centimeters ( 116 ). They are usually found on the external genitalia, perineum, perianal area, and oral cavity ( 117 ). Warts are most often diagnosed through their clinical appearance. Laboratory tests for the presence of HPV are not recommended for the diagnosis of CA. Histologic examination of biopsy specimens can be performed to rule out intraepithelial or invasive squamous cell carcinomas SCCs, which can coexist with or appear similar to anogenital warts ( 118 ). Their feature in histologic evaluation involves epidermal hyperplasia, parakeratosis, koilocytosis, and papillomatosis ( 119 ). Unfortunately, there is not any definite antiviral therapy to treat anogenital warts. Recurrence rate is high (25%-65%), which is may be due to the widespread infection and subclinical lesions ( 120 ). Also, most of the treatments are time-consuming and uncomfortable ( 121 ). Since there are no specific therapy options, the therapist should consider many points for the treatment such as the number, size, morphology, anatomic location, patient preference and side effects ( 122 ). The available therapies are effective for 60% to 90% of nonimmunosuppressed patients ( 121 , 123 ). About trichloroacetic acid (TCA), it is investigated that 71% to 79% of cases regressed from high grade squamous intraepithelial lesion (HSIL) to low-grade squamous intraepithelial lesion (LSIL) or complete resolution when using TCA ( 124 ). The current treatments used for warts are as below:
1. Topical, destructive, surgical: podophyllotoxin and podophyllin, imiquimod, sinecatechins, intralesional immunotherapy with skin test antigens, cidofovir, 5-fluorouracil (5-FU), TCA, cryotherapy, potassium hydroxide, surgical excision, laser therapy, and photodynamic therapy
2. Systemic: interferon, isotretinoin ( 125 ).
The Risk of Infertility
Infertility means the inability of a couple to have a gestational process after 1 year of unprotected sex. Approximately 10% to 20% of couples at the reproductive age are suffering from infertility. Also, 50% of the reasons for infertility among couples relate to male factors, such as sexual dysfunction, congenital dysplasia, endocrine disorders, varicocele, immune factors, and sexually transmitted infections ( 126 - 128 ). It is established that there is a correlation between cervical disease and pregnancy complications ( 129 ). Nonetheless, we do not have any definite evidence recognizing the influence of HPV infection on pregnancy, such as preeclampsia, preterm labor, or fetal growth restriction ( 130 ). In men, the story is as in women. The literature supporting a link between HPV and infertility is still controversial ( 131 ). Seminal HPV infection is common worldwide, which may be correlated with the risk of male infertility through affecting sperm abnormalities, such as low sperm count and motility. Some studies have reported that HPV16 and 52 in men and HPV 58 in women are the most common types of HPVs leading to infertility ( 24 , 132 ).
Vaccination
There is no definite therapy for HPV infection. Sometimes it is eliminated on its own. Some scientists believe that it cannot be cleared completely and may only switch to undetectable levels ( 133 ). We have several types of therapeutic vaccines evaluated in preclinical and clinical trials: live vector, protein or peptide, nucleic acid, and cell-based vaccines ( 134 ). However, 3 HPV vaccines are currently available in the vaccination program: cervarix (bivalent vaccine for HPV 16, 18), gardasil (quadrivalent vaccine for HPV 6, 11, 16, 18), and nonavalent vaccine (for HPV 6, 11, 16, 18, 31, 33, 45, 52, 58). These vaccines can target between 2 and 7 oncogenic HPV serotypes ( 135 ). They promise, in the long-term (30-50 years), to reduce the incidence of disease associated with HPV vaccine types ( 136 ). Some studies have proved that HPV vaccination is a secure and efficient method to prevent cancer ( 137 , 138 ). Steben et al in 2018 evaluated 10 years of clinical experience in Canada. The results indicated that the prevalence of HPV types 16, 18, 6, and 11 was lower in qHPV-vaccinated than unvaccinated individuals (1.5% vs 11%). The risk of anogenital warts incidence had a decrease by up to about 45% in vaccinated population in cohort studies and the incidence of cervical intraepithelial neoplastic had a significant decrease by up to 86%. These researchers concluded that the programs of HPV vaccination constitute an effective and useful public health initiative ( 139 ). Unfortunately, there is limited vaccination coverage of large populations in the world. Therefore, a gross unvaccinated population remains at a high risk of HPV-associated disease ( 14 ). Additionally, some researches have demonstrated that current vaccines will not be useful for preventing all types of HPV-related cancers ( 140 ). Also, the HPV subtype distribution in cervical cancer varies throughout the world ( 141 , 142 ). Japanese scientists in 2019 reported a significant number of adolescent girls complaining of unusual symptoms after HPV vaccination. The vast majority of them had psychiatric illness in the absence of any pathologic findings in radiological images or laboratory tests. So the recommendation of vaccination was withdrawn by the Japanese Ministry of Public Health ( 143 ). At present, the vaccination program is implemented in Argentina, Australia, Austria, Belgium, Bhutan, Brazil, Brunei, Darussalam, Canada, Colombia, Cook Islands, Czech Republic (the), Denmark, Fiji, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Japan, Kiribati, Latvia, Lesotho, Luxembourg, Malaysia, Marshall Islands (the), Mexico, Micronesia, Netherlands, New Zealand, Norway, Palau, Panama, Paraguay, Portugal, Rwanda, San Marino, Singapore, Slovenia, Spain, Sweden, Switzerland, United Kingdom of Great Britain, the former Yugoslav Republic of Macedonia, Uganda, and Northern Ireland, Trinidad and Tobago, United States of America (the), and Uruguay ( 144 ).
HPV transmission risk is increased among multipartners. It can originate from a genital area and extend to other parts like the anus region. Neonates can be infected by their mothers during pregnancy or the delivery period through vertical and perinatal transmission ( 145 ). The transmitted couples are sexually more active with a history of not using condoms regularly. It shows that condoms can be effective, along with the prophylactic vaccines. Despite the 3% of transmitting couples, more than 50% of nontransmitting couples presented that during the previous 4 months they used condoms in 100% of intercourses ( 146 ). So condoms can be used to protect against HPV but since they do not cover every possible HPV-infected area of the body, they cannot offer full protection ( 147 ). According to a cohort study done by Hernandez et al, genital transmission from women to men occurs more frequently than from men to women. The primary source of transmission to the cervix is the penis. The cervix and urethra are the primary sources of infection to male genitals. Sexual transmission implicates also the scrotum, the anus of women, and the hands of both genders. In heterosexual transmission the anus of women plays an important role as a major source and target. It is found that HPV transmission does not definitely influence the target organs because it depends on the tissue or genotype differences or both ( 146 ).
Epidemiologist research finds that HR-HPVs play an important role in cervical cancer and bacterial vaginosis ( 148 , 149 ). After the integration of the genome, E6 oncogene is expressed. Many cells have changed by the E6 protein, leading to inhibition of apoptosis and increased telomerase function. This is why E6 protein can prolong cellular lifespan. It functions as a transcriptional activator accompanied by this prolonged lifespan and transforms cells ( 150 ). HR-HPV E7 allows the cell to increase its transforming activities. Therefore, human keratinocytes are immortalized because they interact with factors that regulate cell growth ( 151 ). Based on the our results, the expression of E6 and E7 can be blocked by E2. After disrupting, it enables uninhibited E6 and E7 oncoprotein activity ( 31 ). The differences between low-risk and high-risk HPVs in producing warts or cancers is mainly in E6 and E7 function. In low-risk HPVs, E6/E7 expression stimulates cell cycle entry in the upper epithelial layers (differentiating cells), allowing genome amplification in both low and high-risk HPVs. Also, basal cell proliferation may still be stimulated by growth factors, but not E6/E7. There is little or no expression of E6/E7. About high-risk HPVs, E6/E7 expression stimulates additional cell cycle entry and cell proliferation in the basal and parabasal epithelial layers leading to neoplasia. E6/E7 inhibit immune response to tolerate viral gene expression ( 152 ). HPV vaccines provide a promising primary approach to prevent malignancies. Because HPV acquisition generally occurs soon after first sexual activity, vaccine effectiveness will be lower in older age groups because of prior infections. Evidence suggests that although HPV vaccination is safe for adults aged 27 to 45 years, population benefit would be minimal; nevertheless, some adults who are not adequately vaccinated might be at risk for new HPV infection and might benefit from vaccination in this age range. Vaccination is routinely recommended at age 11 or 12 years; it can be given starting at age 9 years. Although catch-up HPV vaccination is recommended for all persons through age 26 years who are not adequately vaccinated, it is not recommended for all adults aged >26 years. Instead, shared clinical decision-making regarding HPV vaccination is recommended for some adults aged 27 to 45 years who are not adequately vaccinated. HPV vaccines are not licensed to be used in adults aged >45 years ( 153 ).
This was a comprehensive review study involving 353 valid publications evaluating all important aspects of HPVs and their related cancers. Most of the studies have evaluated only 1 or 2 types of cancers correlated with HPVs. The need for easily accessible comprehensive data in this field seemed to be necessary. Thus, we tried to provide such data for clinicians and laboratory specialists working on HPV infection.
Limitations
The major limitations of this review were lack of data in some countries and nonvalid data in developing countries. Some papers had poor or unclear information or evaluation. In some cases, it was not possible to find valuable data. Thus, we applied the phrase “not found” for such cases.
About 50% to 80% of sexually active females will be infected with HPV during their lifetime. The global HPV prevalence has been estimated to beabout 11.7%. The major burden of HPV infection is the carcinogenic effect of high-risk HPVs. Since there is no definite treatment for HPV, the high prevalence of genital HPV has been a great concern in the world. At present, vaccination has been introduced as the best prevention and treatment method for HPV infection. If complete effectiveness of vaccination is expected, future vaccines should be multivalent for all described oncogenic HPV types. Nonetheless, these vaccines will be much more expensive than current formulations ( 14 ). Although some researchers have reported positive clinical effectiveness of vaccines in reducing malignancy, large population-based clinical studies of these vaccines are necessary to assess the true impact of vaccination ( 135 , 154 , 155 ). We believe that routine use of HPV vaccines needs much more care and assessment because there are many doubts and questions about these vaccines.
Conflict of Interests
The authors declare that they have no competing interests.
Cite this article as: Soheili M, Keyvani H, Soheili M, Nasseri Sh. Human papilloma virus: A review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers. Med J Islam Repub Iran. 2021 (22 May);35:65. https://doi.org/10.47176/mjiri.35.65
Conflicts of Interest: None declared
Funding: None

IMAGES
COMMENTS
3. Mechanisms of Vaccinations. Currently, the licensed HPV vaccines are developed based on a virus-like particle (VLP) of the major papillomavirus capsid protein L1 [].Since VLPs are merely protein and do not contain viral genome, these are considered non-infectious and non-oncogenic, and thus are safer than HPV-attenuated vaccines [].VLPs can be produced in bacteria, yeast, or insect cells.
Results. During the study period, we evaluated girls and women for cervical cancer until their 31st birthday. Cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV ...
Vaccination of young women with qHPV vaccine offers durable protection against HPV16/18-related high-grade cervical dysplasia for ≥12 years, with a trend toward continued protection through 14 years post-vaccination, and induces sustained HPV6/11/16/18 antibody responses for up to 14 years post-vaccination. There was no evidence of waning immunity, suggesting no need for a booster dose ...
Over the first 18 months of this ongoing trial, the efficacy of single-dose bivalent or nonavalent HPV vaccine was very high among Kenyan adolescent girls and young women, demonstrating high levels of protection against vaccine-specific oncogenic HPV infection. Protection against HPV 16/18 infection was 97.5% for both vaccines.
The cancer registry data used in this paper are held by the National Cancer Registration and Analysis Service, PHE. ... Evidence for single-dose protection by the bivalent HPV vaccine—review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. 2018; 36: 4774-4782. View in Article Scopus (92) PubMed;
This position paper is concerned with vaccines and vaccination against diseases caused by human papillomaviruses (HPVs). Its primary focus is the prevention of cervical cancer, given the role of prophylactic HPV vaccination as a foundational pillar of the WHO Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem.
This position paper replaces the 2014 WHO position paper on HPV vaccines [2]. The position paper focuses primarily on the prevention of cervical cancer, but also considers the broader spectrum of cancers and other diseases preventable by HPV vaccination. It incorporates recent developments concerning HPV vaccines, including the licensure of a ...
Objective To explore the efficacy of human papillomavirus (HPV) vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment. Design Systematic review and meta-analysis Data sources PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov were screened from inception to 31 March 2021. Review ...
Since 2006, routine HPV vaccination has been recommended for girls 11 or 12 years of age; vaccination can be started at 9 years of age. Boys were included in the vaccination program in 2011 ...
Persistent human papillomavirus infection is the central cause of cervical cancer, the leading cause of cancer death among women worldwide. Clear evidence from both randomized trials and population based studies shows that vaccination against human papillomavirus reduces the incidence of cervical pre-cancer. These data suggest that the vaccine reduces the incidence of cervical cancer. However ...
Despite the human papillomavirus (HPV) vaccine being a safe, effective cancer prevention method, its uptake is suboptimal in the United States (U.S.). Previous research has found a variety of intervention strategies (environmental and behavioral) to increase its uptake. The purpose of the study is to systematically review the literature on interventions that promote HPV vaccination from 2015 ...
The virus types currently in the nonavalent HPV vaccine are indicated with a black dot ... This paper explains why the HPV vaccine is so ... International Agency for Research on Cancer ...
In this systematic review, we synthesized research using the socioecological framework model to examine individual-level, relationship-level, community-level, and societal-level factors that influence HPV vaccine initiation and completion among U.S. adolescents. ... The odds of HPV vaccine initiation and completion was 1.46 to 7.89 times higher ...
Human papillomavirus (HPV) infection is recognized as one of the major causes of infection-related cancer worldwide. In Spain, the HPV vaccination program started in 2007 and until 2022, it targeted 12-year-old girls. This was a cross-sectional, multicenter survey-based research carried out at 24 pediatric offices to describe HPV knowledge and vaccine acceptability in parents of children aged ...
Human Papillomaviruses (HPV) are classified in lineages based on their sequence. Here, the authors test neutralizing activity of sera from naturally infected women against vaccine-preventable HPV ...
In this article, ACROBiosystems discusses how the HPV vaccine and research into it, could help combat cervical cancer. ... paper or report: APA. ViruStop - An ACROBiosystems Brand. (2024, January 29).
The HPV vaccine plays an important role in preventing cervical cancer, as almost all cases of cervical cancer are caused by HPV. HPV 16 and 18 cause about 7 in 10 cases of cervical cancer, and the vaccine protects against these HPV types. A 2021 study found that cervical cancer rates were reduced by almost 90% in women in their 20s in England ...
Research over the years shows that the HPV vaccine is most effective when given between ages 9 and 12. Below are some of the research papers that support this: For a more depth look at Washington state's HPV at nine initiative: Effect of immunization registry-based provider reminder to initiate HPV vaccination at age 9, Washington state ...
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States with approximately 80% of women having acquired an infection by the age of 50. 1 Although most HPV infections clear, persistent HPV infection is strongly associated with risk of cervical cancer and genital warts. The recently approved quadrivalent (types 6, 11, 16, and 18) HPV vaccine targets the ...
Meanwhile, studies on HPV vaccines confirm that they should be used for the primary prevention of HPV-dependent diseases. However, we need more research to find out the real advantages and disadvantages of vaccines. ... There are numerous papers in the field of sexual transmitted diseases (STDs), especially HPV. ... Another research evaluating ...