- Case Report
- Open access
- Published: 11 April 2022

Clinical features and outcomes of four atypical hemolytic uremic syndrome cases at a single institution in Miyazaki Prefecture from 2015 to 2019
- Noriaki Kawano 1 na1 ,
- Tomohiro Abe 2 na1 ,
- Naoko Ikeda 1 na1 ,
- Yuri Nagahiro 3 na1 ,
- Sayaka Kawano 1 ,
- Taro Tochigi 1 ,
- Takashi Nakaike 1 ,
- Kiyoshi Yamashita 1 ,
- Keisuke Kubo 2 ,
- Atsushi Yamanaka 1 ,
- Sohshi Terasaka 4 ,
- Kousuke Marutsuka 5 ,
- Koichi Mashiba 1 ,
- Ikuo Kikuchi 1 ,
- Kazuya Shimoda 6 ,
- Masanori Matsumoto 7 &
- Hidenobu Ochiai 2
Renal Replacement Therapy volume 8 , Article number: 15 ( 2022 ) Cite this article
2456 Accesses
1 Citations
Metrics details
Although atypical hemolytic uremic syndrome (aHUS) is a life-threatening clinical entity that was characterized by thrombotic microangiopathy (TMA) with the activation of the complement system and the efficient treatment of eculizumab, the clinical features of aHUS have been unclear because of the rare incidence.
Case presentation
We retrospectively analyzed 4 aHUS cases at a single institution during 2015–2019. Here, we presented 4 aHUS cases with renal transplantation (one case), influenza/acute interstitial pneumonia/disseminated intravascular coagulation (two cases), and severe fever with thrombocytopenia syndrome (one case), respectively. Initial clinical symptoms were microangiopathic hemolytic anemia (four cases), renal dysfunction (four cases), thrombocytopenia (four cases), and pulmonary hemorrhage (three cases) consisted with TMA features. Subsequent further examinations ruled out thrombotic thrombocytopenic purpura, Shiga toxin-producing E.coli-induced hemolytic uremic syndrome, and secondary TMA. Taken these findings together, we made the clinical diagnosis of aHUS. Furthermore, all cases also presented the high levels of plasma soluble C5b-9 (871.1 ng/ml, 1144.3 ng/ml, 929.2 ng/ml, and 337.5 ng/ml), suggesting persistent activation of complementary system. Regarding the treatment, plasma exchange (PE) (four cases) and eculizumab (two cases) therapy were administered for aHUS cases. Consequently, case 2 and case 4 were still alive with 768 days and 235 days, respectively. The other two cases were dead at 34 days and 13 days, respectively. Finally, although the previous reported genetic pathogenetic mutations were not detected in our cases, multiple genetic variants of complement factors were detected as CFH (H402Y, E936D), and THBD (A473V) in case 1, CFH (V62I, H402Y, V837I) in case 2, and CFH (H402Y, E 936D) and THBD (A473V) in case 3, CFH (V62I, H402Y, E936D) and THBD (473V) in case 4, respectively.
Conclusions
Because of still high mortality in our study, an urgent diagnosis of aHUS and subsequent immediate treatment including PE and eculizumab should be essential in clinical practice. Furthermore, the multiple genetic variants and the triggers may be related to one of the pathogenesis of aHUS. Thus, we assume that such a case-oriented study would be highly useful to the physicians who directly care for aHUS cases in clinical practice.
Introduction
Thrombotic microangiopathy (TMA) is a rare and life-threatening systemic disease that is characterized by the triad of microangiopathic hemolytic anemia, destructive thrombocytopenia, and acute renal failure due to endothelial damage [ 1 , 2 ]. Among TMA, thrombotic thrombocytopenic purpura (TTP), Shiga toxin-producing E.coli-induced hemolytic uremic syndrome (STEC-HUS), atypical hemolytic uremic syndrome (aHUS), and secondary TMA are classified by current guidelines [ 1 , 2 ].
Regarding the clinical diagnosis for aHUS, the guideline was published to help the clinical diagnosis of aHUS in clinical practice [ 3 , 4 , 5 , 6 ]. Especially, aHUS is characterized by the persistent activation of the complementary system. Furthermore, aHUS is essential to rule out TTP, STEC-HUS, and secondary TMA [ 3 , 4 , 5 , 6 ]. Thus, the further examination of the ADAMTS 13 activity/the inhibitor of AMDAMTS 13, anti-E. coli O157 lipopolysaccharide (LPS) IgG, and the treatment response by the treatment for the underlying diseases are also essential to rule out TTP, STEC-HUS, and secondary TMA, respectively [ 3 , 4 , 5 , 6 ].
Regarding the treatment for aHUS [ 7 , 8 , 9 , 10 ], eculizumab (a recombinant humanized monoclonal anti-C5 antibody) was recently reported to be efficiently administered for aHUS by the mechanism of the binding to the epitope of C5 and subsequently inhibit the formation of C5a and the terminal complement complex (C5b-9) by the suppression of excessive complement activation in aHUS [ 7 , 8 , 9 , 10 ].
Therefore, the initial clinical manifestation of and clinical findings of aHUS presented still different, diverse, and heterogeneous features, the precise clinical diagnosis and subsequent appropriate treatment were still difficult for the physicians to perform in clinical practice. Furthermore, the pathogenesis and treatment outcomes of aHUS have been still unclear due to the rare incidences of aHUS patients and the presence of aHUS patients without genetic abnormalities [ 1 , 2 ].
Thus, it should be crucial to describe the detailed clinical features and treatment outcome of aHUS in clinical practice. Herein, we described and analyzed the detailed clinical course and treatment outcomes of four aHUS cases at Miyazaki Prefectural Miyazaki Hospital during 2015–2019.
Patients and methods
From January 2015 to September 2019, according to the guideline of aHUS [ 3 , 4 , 5 , 6 ], we made a definitive diagnosis of aHUS by the presence of features of TMA (the presence of all 3 classical symptoms including thrombocytopenia, microangiopathic hemolytic anemia (MAHA), and renal insufficiency) with the normal value of a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13 (ADAMTS 13) activity (> 10%), the negative findings of STEC and the exclusion of secondary TMA by judging no treatment response in the treatment for the underlying diseases. ADAMTS13 activity was determined by a chromogenic ADAMTS13-act-ELISA (Chr-act-ELISA) with a detection limit of 0.5% of the normal level [ 11 ]. In case 1, to administer eculizumab for aHUS, we confronted the necessity of the objective markers, suggesting the persistent activation of the complementary system. To elucidate the activation of the complement system, we measured plasma soluble C5b-9 (sC5b-9) in aHUS patients [ 12 ]. Plasma sC5b-9 (normal range: 37.0–260.6) was measured by the ELISA method in Miyazaki University [ 12 ] (Micro Vue sC5b-9 Plus Enzyme Immunoassay; Quidel, San Diego, USA). Thus, in case 1 of postmortem analysis, we measured and obtained the objective markers of plasma sC5b-9, suggesting the persistent activation of the complementary system. Thus, the other 3 cases including cases 2, 3, and 4 were prospectively measured plasma sC5b-9 to evaluate the activation of the complementary system in aHUS patients. Additionally, the genetic analysis of the exome sequencing was performed by the next-generation sequencing (NGS) for factors such as complement factor (CFH), C3, complement factor I (CFI), complement factor B (CFB), membrane cofactor protein (MCP), and thrombomodulin (THBD) in TMA registry systems [ 12 , 13 ]. We obtained the consent from patients or families.
As for treatment for aHUS, we performed plasma exchange (PE) therapy with fresh frozen plasma (FFP) at 60 mL/kg body weight until we observed the recovery of the following variables: platelet count (> 150 × 10 9 /L), lactate dehydrogenase (LDH), and acute renal insufficiency [ 7 , 8 , 9 , 10 ]. As for eculizumab therapy for aHUS, eculizumab was immediately administered after the clinical diagnosis of aHUS with confirmation of sC5b-9 elevation according to the clinical guide of aHUS in 2015 and phase 2/3 trial and Post marketing-surveillance in Japan [ 4 , 9 , 10 ]. Basically, eculizumab was administered at a dose of 900 mg/sqm, weekly over 4 courses, and subsequently administered at a dose of 1200 mg/sqm, biweekly until no response or incapability of administration because of adverse effects.
Response criteria were as follows. Complete remission (CR) of TMA was evaluated and defined by the hematologic normalization (platelet count (> 150 × 10 9 /L), LDH < upper limit of normal range), and preservation of kidney function (< 25% serum creatinine increase from baseline) after 4 weeks later at treatment.
Regarding the problems in eculizumab therapy for aHUS, the risk of meningeal infection was reported to increase [ 4 , 9 , 10 ]. To reduce the risk of meningeal infection, meningeal vaccination was recommended by the clinical guide of aHUS in 2015 at two weeks before administration of eculizumab [ 4 , 9 , 10 ]. Thus, 2 cases (case 3 and case 4) were administered the vaccination of meningeal infection before administration of eculizumab.
The summary of clinical and laboratory findings, treatment regimen, and therapeutic outcomes in case series
Clinical and laboratory findings on the admission of 4 aHUS cases as well as their treatment regimens and therapeutic outcomes are summarized in Table 1 . Here, we summarized the detailed clinical course and treatment outcome of 4 aHUS cases as follows. Thus, we presented 4 aHUS cases (59, 57, 74, and 67 years) with renal transplantation (one case), influenza/acute interstitial pneumonia (AIP) (two cases), and severe fever with thrombocytopenia syndrome (SFTS) (one case), respectively. Initial symptoms were microangiopathic hemolytic anemia (four cases), renal dysfunction (four cases), thrombocytopenia (four cases), and pulmonary hemorrhage (three cases) consistent with the triad of TMA. In peripheral blood analysis, two, three, four, and three percentage of schistocyte was detected in case 1, case 2, case 3, and case 4, respectively. In all cases, haptoglobin was not detected. Further examination revealed that TTP, STEC-HUS, and secondary TMA are excluded by the laboratory findings of the normal ADAMTS 13 activity (> 10%)/no inhibitor of ADAMTS 13, the negative findings of STEC, and no treatment response for the underlying diseases, respectively. Taken these findings together, we finally made the diagnosis of aHUS based on the clinical guideline [ 3 , 4 , 5 , 6 ]. Furthermore, in all cases, high levels of plasma sC5b-9 (871.1 ng/ml, 1144.3 ng/ml, 929.2 ng/ml, and 337.5 ng/ml) remarkably supported the persistent activation of the complementary system.
Regarding the treatment, PE therapy (four cases) and eculizumab therapy (two cases) were administered for aHUS cases. Two cases (case 2 and case 4) out of four cases achieved a CR of TMA. Consequently, case 2 and case 4 were still alive with 768 days and 235 days, respectively. The other two cases (case 1 and case 3) were dead at 34 days and 13 days, respectively. Finally, the genetic variants of complement factors were detected as CFH (H402Y, E936D) and THBD (A473V) in case 1, CFH (V62I, H402Y, V837I) in case 2, and CFH (H402Y, E 936D) and THBD (A473V) in case 3, CFH (V62I, H402Y, E936D) and THBD (473V) in case 4, respectively (Table 3 ).
Herein, we described the detailed clinical course and treatment outcomes of four aHUS cases at our hospital during 2015–2019.
In March 2017, a 59-year-old female case was admitted to our hospital for living donor renal transplantation from her sister. Regarding the history of kidney dysfunction, in 2005, the case developed chronic renal failure (CRF) (CKD: stage5) due to chronic glomerulonephritis (etiology unknown due to no renal biopsy) and was treated by hemodialysis. Thus, the case enrolled the cadaveric renal transplantation in Japan. Furthermore, additional past history was cholecystectomy for cholelithiasis and gastric cancer (stage 1) for surgery at 53 years. The family history of the father was expired by uremia (etiology unknown). The clinical course and treatment outcome is shown in Fig. 1 . Physical examination was not particular. On March 15, 2017, living-donor renal transplantation from sister was administered under preoperative rituximab and double filtration plasmapheresis (DFFP) administration according to the incompatibility of ABO mismatched donor protocol. After renal transplantation, the transplanted kidney presented rapid recovery of renal function in urine output. However, on day 7, the patient showed the acute respiratory failure due to pulmonary hemorrhage, acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS), and the sudden decrease of urine output. Furthermore, the case was critically cared by mechanical intubation and continuous hemodiafiltration (CHDF) (day 7–day 34). However, on day 16, the case showed the clinical findings of schistocytes, microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and renal dysfunction. The case was consulted to our hematologists for further examination for TMA including TTP and aHUS. Further examination excluded TTP, STEC-HUS, and secondary TMA by the laboratory findings of the normal ADAMTS 13 activity (48.8% > 10%)/no inhibitor of ADAMTS 13, the negative findings of STEC, and no treatment response for the underlying diseases, respectively. In case 1, to rule out the possibilities of tacrolimus-induced TMA, we ceased tacrolimus after the clinical diagnosis of TMA. Therefore, the differential diagnosis of aHUS is very difficult, we cautiously ruled out the differential diagnosis according to the guideline of aHUS [ 3 , 4 , 5 , 6 ]. Taken these findings together, we made clinically diagnosed with aHUS according to the clinical guideline of aHUS [ 3 , 4 , 5 , 6 ]. After the clinical diagnosis of aHUS, immediate PE therapy and mPSL pulse therapy were initiated on day 16. Three times of PE therapy transiently resolved the amount of blood transfusion (red cell concentrate and platelet concentrate) and the amount of urine output. Furthermore, in renal biopsy on day 19, H.E. staining specimen was consistent with endothelial damage findings of aHUS (Fig. 2 A). However, the combination of mPSL pulse therapy and PE therapy did not resolve the intubation of mechanical ventilation and hemodialysis. Although we urgently examined the administration of eculizumab treatment, the case unfortunately developed the severe septic shock due to the pseudomonas aeruginosa. Finally, we did not administer eculizumab treatment due to the presence of active infection. Consequently, despite of these intensive cares, the case expired by the rapid progression of aHUS, ARDS, and multiple organ failure at day 34.
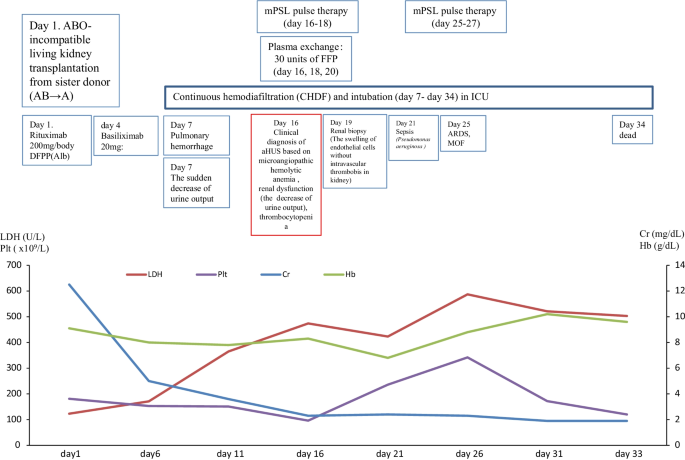
The clinical course of case 1
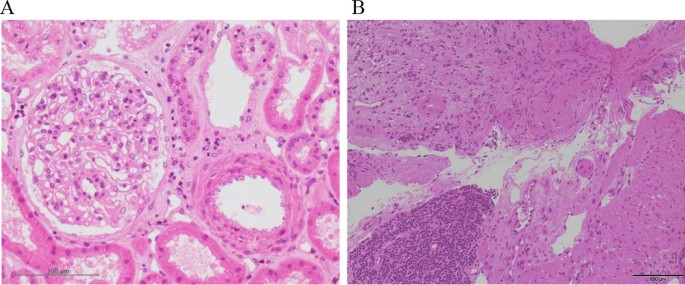
Pathological findings of case 1 and case 2. A In renal biopsy of case 1 on day 19, kidney specimen with H.E. staining showed the findings of the swelling of endothelial cells without intravascular thrombosis in kidney. These findings were consistent with endothelial damage findings of aHUS. B In colon biopsy of case 2 on day 11, colon specimen with H.E. staining also revealed the findings of colon ulcer and the presence of thrombi in small vessels. These findings were consistent with aHUS
Retrospectively, we strongly realized the necessity of the objective confirmation of the persistent activation of the complementary system to appropriately administer eculizumab for case 1. Thus, we retrospectively performed the postmortem examination of the complementary system by the specimen that was preserved at a clinical diagnosis of aHUS. First, the laboratory findings of the complementary system in aHUS patients are shown in Table 2 . Notably, the high levels of plasma sC5b-9 levels (871.12 ng/ml) at day 16 supported the activation of the complement system in aHUS (Table 2 ). Second, the genetic analysis is shown in Table 3 . The genetic analysis of the exome sequencing by the NGS for 6 factor-related complement system (CFH, C3, CFI, CFB, MCP, and THBD) revealed the no previous pathogenetic mutations. However, the genetic variants of complement factors were detected as CFH (H402Y, E936D) and THBD (A473V) in case 1.
In December 2017, a 57-year-old female was referred to our hospital to treat developed influenza, AIP, and DIC. The clinical course and treatment outcome is shown in Fig. 3 . We administered peramivir, mPSL pulse therapy, and recombinant human soluble thrombomodulin (rhTM) for influenza, AIP, and DIC, respectively. Although these treatments were performed, the case developed the clinical findings of schistocytes, MAHA, renal dysfunction, and pulmonary hemorrhage. On January 4, 2018, based on the guideline of aHUS, we clinically made the diagnosis of TMA including TTP and aHUS. In case 2 with poor PS and life-threatening multiple organ failure, we strongly suspected clinical TTP and immediately administered PE therapy. Immediately, the case was intensively treated with the intubation and CHDF in ICU (day 7–day 21). Further examination excluded TTP, STEC-HUS, and secondary TMA by the laboratory findings of the normal ADAMTS 13 activity (65.4% > 10%)/no inhibitor of ADAMTS 13, the negative findings of STEC, and no treatment response for the underlying diseases, respectively. Thus, taken these findings together, we finally made the diagnosis with aHUS according to the clinical guideline of aHUS [ 3 , 4 , 5 , 6 ]. Moreover, colon biopsy findings also revealed the findings of colon ulcer and the presence of thrombi in small vessels consistent with aHUS (Fig. 2 B). Consequently, the subsequent and immediate 4 courses of PE therapy led to the rapid improvement in the schistocyte, findings of MAHA, AIP, renal dysfunction, pulmonary hemorrhage, and colon ulcer. Thus, the case attained CR and disease-free at 768 days with PSL 5 mg/day. Retrospectively, we revealed the high levels of plasma sC5b-9 (1144.3 ng/ml) in preserved specimen on day 7 and confirmed the persistent activation of the complementary system in case 2. These findings may support the persistent activation of the complement system in case 2 (Table 2 ).
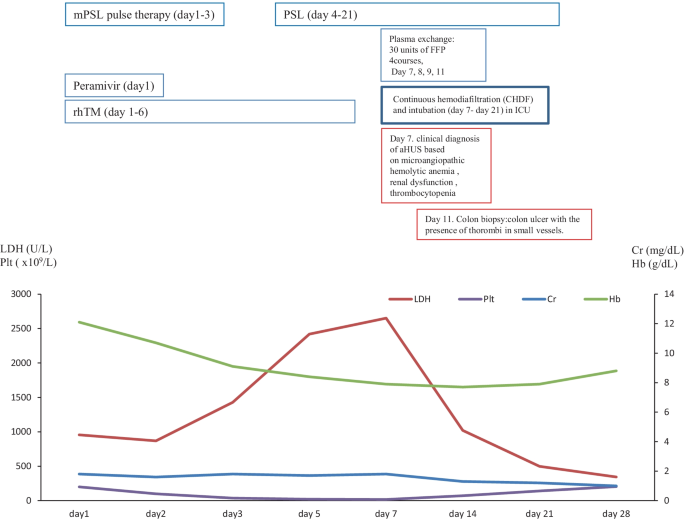
The clinical course of case 2
Three months after clinical diagnosis of aHUS, the laboratory findings of the complementary system and the genetic analysis was realized and shown in Tables 2 and 3 , respectively. The laboratory findings of the complementary system of the elevation of C5b-9 and Ba were consistent with the activation of the complementary system. The genetic analysis of the exome sequencing by the NGS for 6 factor-related complement system (CFH, C3, CFI, CFB, MCP, and THBD) revealed the no previous pathogenetic mutations. However, the genetic variants of complement factors were detected as CFH (V62I, H402Y, V837I) in case 2.
In January 2018, a 74-year-old male was referred to our hospital to treat developed influenza-mimicking symptoms, AIP and DIC. The clinical course and treatment outcome is shown in Fig. 4 . We administered peramivir, mPSL pulse therapy, and rhTM for influenza, AIP, and DIC, respectively. Although these treatments were performed, the case developed the clinical findings of schistocytes, MAHA, renal dysfunction, and pulmonary hemorrhage. On January 31, 2018 (day 4), based on the guideline of aHUS, we clinically made the diagnosis of TMA including TTP and aHUS.
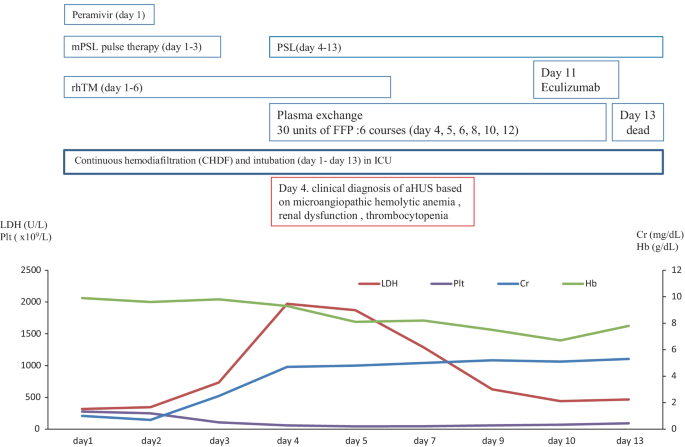
The clinical course of case 3
Due to the poor PS and the life-threatening multiple organ failure in case 3, we strongly suspected clinical TTP and immediately administered PE therapy. Immediately, the case was intensively treated with the intubation and CHDF in ICU (day 1–13). Further examination excluded TTP, STEC-HUS, and secondary TMA by the laboratory findings of the normal ADAMTS 13 activity (59.8% > 10%)/no inhibitor of ADAMTS 13, the negative findings of STEC, and no treatment response for the underlying diseases, respectively. Thus, taken these findings together, we finally made the diagnosis of aHUS according to the clinical guideline of aHUS [ 3 , 4 , 5 , 6 ]. Notably, we prospectively confirmed the persistent activation of the complementary system by the high levels of plasma sC5b-9 (929.2 ng/ml), supporting the persistent activation of the complement system on day 11 after admission (Table 2 ). Renal biopsy was not performed because of the thrombocytopenia, abnormal coagulopathy, and bleeding tendency. Therefore, subsequent and immediate 6 courses of PE therapy did not recover the improvement in the clinical and laboratory findings of aHUS regarding the renal function and the lung lesions, we administered one course of eculizumab. However, the progression of renal dysfunction and AIP/ARDS led to the death (day 13).
Three months after clinical diagnosis of aHUS, the laboratory findings of the complementary system and the genetic analysis was realized and shown in Tables 2 and 3 , respectively. The laboratory findings of the complementary system of the elevation of C5b-9 and Ba were consistent with the activation of the complementary system. The genetic analysis of the exome sequencing by the NGS for 6 factor-related complement system (CFH, C3, CFI, CFB, MCP, and THBD) revealed the no previous pathogenetic mutations. However, the genetic variants of complement factors were detected as CFH (H402Y, E 936D) and THBD (A473V) in case 3.
In June 2018, a 67-year-old female was referred to our hospital to treat the severe fever with thrombocytopenia syndrome (SFTS). The clinical course and treatment outcome is shown in Fig. 5 . On admission, the case also showed the clinical findings of schistocytes, MAHA, renal dysfunction, and liver dysfunction. On July 7, 2018, based on the guideline of aHUS, we made the clinical diagnosis of TMA including TTP and aHUS. Subsequently, we immediately and intensively treated with PE therapy under the intubation and CHDF in ICU (day 1–day 24). Further examination excluded TTP, STEC-HUS, and secondary TMA by the laboratory findings of the normal ADAMTS 13 activity (67.2% > 10%)/no inhibitor of ADAMTS 13, the negative findings of STEC, and no treatment response for the underlying diseases, respectively. Thus, taken these findings together we finally made the diagnosis of aHUS according to the clinical guideline of aHUS [ 3 , 4 , 5 , 6 ]. Notably, we also prospectively confirmed the persistent activation of the complementary system by the high levels of plasma sC5b-9 (337.5 ng/ml), supporting the persistent activation of the complement system on day 10 after admission (Table 2 ). Renal biopsy was not performed because of the thrombocytopenia, abnormal coagulopathy, and bleeding tendency. Although subsequent and immediate four courses of PE therapy led to the improvement in the value of platelet and LDH, the renal function still progressed. Thus, we immediately administered eculizumab on day 10 after the diagnosis of aHUS. Thus, the case attained CR and disease-free at 235 days with PSL 5 mg/day.
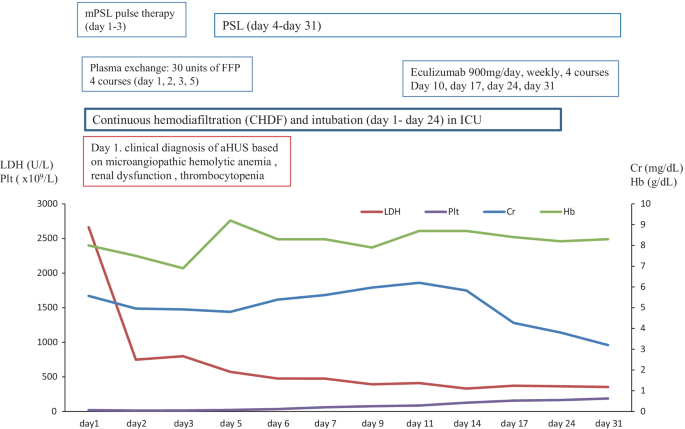
The clinical course of case 4
Three months after clinical diagnosis of aHUS, the laboratory findings of the complementary system and the genetic analysis was realized and shown in Tables 2 and 3 , respectively. The laboratory findings of the complementary system of the elevation of C5b-9 and Ba were consistent with the activation of the complementary system. The genetic analysis of the exome sequencing by the NGS for 6 factor-related complement system (CFH, C3, CFI, CFB, MCP, and THBD) revealed the no previous pathogenetic mutations. However, the genetic variants of complement factors were detected as CFH (V62I, H402Y, E936D) and THBD (A473V) in case 4.
In our study regarding case series analysis of four atypical HUS patients with various clinical courses, we showed that plasma sC5b-9 may be a useful diagnostic marker of aHUS although it is still controversial whether plasma sC5b-9 is a diagnostic marker of aHUS or not. Second, regarding the early appropriate treatment for aHUS among TMA, all four cases were immediately administered PE therapy and/or eculizumab. Notably, the multiple genetic variants of various complement-related genes in our study may be one of pathogenesis associated with the development of aHUS because 50% of aHUS had no pathogenetic mutations that was previously reported in the background. Furthermore, the severe infections may be the trigger of the development of aHUS in all cases. Our study may indicate important suggestions for early diagnosis and subsequent early treatment and understanding of the pathological mechanism of aHUS. Therefore, our reports were only four case experiences and aHUS is a rare disease, further accumulation of many cases is also necessary in the future.
Thus, we focused on (i) the importance of the precise clinical diagnosis of aHUS among TMA, (ii) the early appropriate treatment for aHUS among TMA, and (iii) the pathogenesis of aHUS.
First, regarding the importance of the precise clinical diagnosis of aHUS among TMA, we revealed that the elevation of plasma sC5b-9 may be useful and helpful to support the persistent activation of the complementary system in aHUS. Fujita Y et al. reported a case treated with successful eculizumab for severe TMA secondary to surgical invasive stress with the activation of the complementary system [ 14 ]. Thus, Fujita Y et al. suggested that the strategy of clinical diagnosis based on the limited laboratory findings and immediate treatment in aHUS should be important to save the lives of aHUS patients [ 14 ]. Furthermore, in the clinical setting, the results from a genetic test often arrive after a long time. Thus, Fujita et al. strongly indicated that the early use of eculizumab could save the patients' life in the case of complement-mediated TMA according to the proposal of various clinical diagnostic algorithms for TMA that permit eculizumab administration for possible aHUS at the very early stage when PE therapy is not effective or TTP and HUS have been excluded [ 14 ].
In our case study, according to the guideline of aHUS [ 3 , 4 , 5 , 6 ], we made an immediate clinical diagnosis of TMA including TTP and aHUS with the presence of features of TMA (the presence of all 3 classical symptoms including thrombocytopenia, MAHA, and renal insufficiency). Subsequently, we immediately administered PE therapy because of the aggressive and life-threatening clinical course of aHUS. Further examinations of the normal ADAMTS 13 activity (> 10%), the negative findings of STEC, and the exclusion of secondary TMA by judging no treatment response in the treatment for the underlying diseases made us to rule out TTP, STEC-HUS, and secondary TMA. As for the objective markers, suggesting the persistent activation of the complementary system, we utilized the plasma sC5b-9 value for evaluating the persistent activation of the complementary system in aHUS cases. However, as for a marker of aHUS, plasma sC5b-9 has been controversial. Regarding the positive previous reports, Abe et al. reported that plasma sC5b-9 was useful to evaluate the persistent activation of complementary system in the case with the complement-mediated TMA secondary to sepsis-induced DIC treated with eculizumab [ 12 ]. Furthermore, several groups also reported that the elevated plasma sC5b-9 may be useful to support the persistent activation of the complementary system in aHUS. [ 15 , 16 , 17 , 18 ]. Cataland SR et al., reported that plasma sC5b-9 in aHUS ( N = 19) were median 1098 (422–4840) in aHUS patients [ 15 ]. Belgian guidelines recommend the diagnostic test of aHUS as plasma sC5b-9 [ 16 ]. Furthermore, in Australian and New Zealand's consensus report [ 17 ], plasma C5b-9 is recommended for Complement protein assays in suspected aHUS patients. Regarding the negative previous reports, in Kidney International 2017, there is a paucity of data to support the reliability of this assay as a true marker of disease pathology [ 5 ]. Furthermore, Noris et al. reported that endothelial C5b-9 deposits instead of plasma sC5b-9 are suitable markers of complement activation in this disease [ 19 ].
Notably, in all four aHUS case series study, the high levels of plasma sC5b-9 (871.1 ng/ml, 1144.3 ng/ml, 929.2 ng/ml, and 337.5 ng/ml) supported the activation of the complement system in aHUS patients at the clinical diagnosis of aHUS, respectively (Table 2 ). Thus, in our study, the plasma sC5b-9 may be a useful diagnostic marker of a HUS in all four cases. Thus, plasma sC5b-9 elevation may support the early diagnosis of aHUS and provide the prompt treatment by eculizumab on the evidence of the complementary activation. In the future, to clarify the significance of plasma sC5b-9 for the diagnosis and the treatment, the further accumulation of cases should be essential to elucidate the impact of plasma sC5b-9 in the diagnosis of aHUS.
Second, regarding the early appropriate treatment for aHUS among TMA, all four cases were immediately administered PE therapy and/or eculizumab. During PE therapy for TMA, we differentiated the clinical diagnosis of TMA including TTP, STEC-HUS, and secondary TMA. Thus, only 2 cases were administered eculizumab for aHUS in our study. In only case 4, eculizumab therapy led to the improvement in aHUS and complete remission. In case 3, the case aggressively progressed AIP with pulmonary hemorrhage although we administered eculizumab for aHUS. In case 1, eculizumab was not administered because of severe sepsis by pseudomonas aeruginosa. In case 2, PE and rituximab therapy led to CR before the administration of eculizumab for aHUS. Thus, in confront with TMA, we should consider the diagnosis of aHUS for differential diagnosis. Furthermore, based on the diagnostic algorithm from KDIGO in 2017 and the clinical Japanese guide of aHUS in 2015, we immediately should tackle the treatment of aHUS by using PE therapy and/or eculizumab.
Finally, the pathogenesis of aHUS, our cases had the multiple (three or four) genetic variants as the genetic predisposition in all four cases. Furthermore, the severe infections (renal transplantation, influenza/AIP/DIC, influenza-mimicking symptoms/AIP/DIC, SFTS) may trigger the development of aHUS in all four cases.
Riedl et al. proposed the multiple hit hypothesis of aHUS consisting of the genetic predispositions in the background and the strong triggers may overcome the threshold of the development of aHUS [ 20 ]. Although the previous reports regarding genetic analysis for aHUS were limited, only 50% of aHUS was reported to have the pathogenetic genetic mutations [ 1 , 2 , 3 , 4 , 5 , 6 ]. Thus, in another 50% of aHUS had no pathogenetic genetic mutations that was previously reported. Thus, the genetic pathogenesis of aHUS in the background has been still unclear. However, recently, specific genetic variants, numbers of genetic variants, and combinations of genetic variants were reported to be one of the mechanisms in the development of aHUS [ 10 , 12 , 21 , 22 , 23 , 36 , 37 , 38 ]. At present, it has been unclear and there is no consensus whether which kind of genetic variants or which the number of genetic variants, or which combination of the genetic variant may be related to the pathogenesis of the onset of aHUS.
As for the kind of genetic variants, previously, Caprioli et al. reported that CFH-253T and/or E936D variations have been associated with aHUS [ 37 , 38 ]. Furthermore, Abe et al. reported that genetic variants of CFH-H402Y and V62I were also related to aHUS [ 12 ]. In short, we summarized that the genetic variants in previous reported aHUS patients in Japan (Table 4 ). In our study, our four aHUS cases showed the genetic variants of CFH-H402Y [ 10 , 12 , 36 ], E936D [ 10 , 36 ], V62I [ 12 , 23 ], V837I [ 23 ], and THBD-A473V [ 23 , 36 ].
As for the number of genetic variants, Recently, Jodele reported that among 77 TMA patients after allogeneic hematopoietic stem cell transplantation (allo-HSCT) undergoing genetic testing, allo-HSCT recipients (9%) with multiple (≥ 3) complement gene variants including the common variants (> 1% of allele incidence) are at high risk for severe TMA [ 21 ]. Furthermore, Bresin et al. revealed that among 60 aHUS cases, multiple genetic variants also showed the penetration of aHUS [ 22 ]. Thus, multiple genetic variants of complement factors (≥ 3) may be the genetic predisposition of aHUS in the background. In previous reports from Japan, 18 out of 37 cases had multiple genetic variants of complement factors (≥ 3) (Table 4 ). In our study, the genetic variants of complement factors were detected as CFH (H402Y, E936D) and THBD (A473V) in case 1, CFH (V62I, H402Y, V837I) in case 2, and CFH (H402Y, E 936D) and THBD (A473V) in case 3, and CFH (V62I, H402Y, E936D) and THBD (A473V) in case 4, respectively.
As for the combination of genetic variants, in our study, four cases showed a new combination of genetic variants which was not previously reported. Thus, our study strongly supports that the multiple genetic variants that was not the pathogenic genetic change of complementary system may elevate the risk of the onset of aHUS.
Furthermore, our four cases previously experienced the severe trigger such as severe infection such as renal transplantation, influenza/AIP complicated with DIC, SFTS, and others before the development of aHUS. Previously, Abe et al. reported that the case presented the complement-mediated TMA secondary to sepsis-induced DIC successfully treated with eculizumab [ 12 ]. Based on the TMA development after DIC in Sakamakis' case report and the Oklahoma TTP/HUS registry (10 out of 31 TMA cases) [ 12 , 39 , 40 ], Abe et al. discussed that DIC may trigger the TMA development and accelerate according to the findings that the coagulation and complement systems mutually induce positive feedback [ 12 ]. In our cases, among 4 aHUS patients, two patients severely complicated DIC before the development of aHUS. Thus, the coagulation and complement systems may also co-activated in aHUS patients. In the future, further accumulation of the cases was needed to clarify the relationship between the genetic variants and the triggers for the development of aHUS in clinical practice.
In conclusion, in our case series report regarding 4 aHUS cases, we described different, diverse, and heterogeneous features of initial clinical manifestation, clinical symptoms, and clinical findings of aHUS in clinical practice. Notably, plasma sC5b-9 examination may be useful to make the diagnosis of aHUS. Subsequent treatment such as PE and eculizumab should be essential to improve the high mortality. Furthermore, multiple genetic variants and the trigger may be related to the pathogenesis of aHUS. Thus, we assume that such a case-oriented study would be highly useful to the physicians who directly care for aHUS cases in clinical practice.
Availability of data and materials
Please contact the author for data requests.
Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–87.
Article CAS Google Scholar
Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390:681–96.
Article Google Scholar
Loirat C, Fakhouri F, Ariceta G, et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39.
Kato H, Nangaku M, Hataya H, et al. Joint Committee for the Revision of Clinical Guides of Atypical Hemolytic Uremic Syndrome in Japan. Clinical guides for atypical hemolytic uremic syndrome in Japan. Clin Exp Nephrol. 2016;20:536–43.
Goodship TH, Cook HT, Fakhouri F, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91:539–51.
Kato H, Nangaku M, Okada H, Kagami S. Controversies of the classification of TMA and the terminology of aHUS. Clin Exp Nephrol. 2018;22:979–80.
Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–81.
Licht C, Greenbaum LA, Muus P, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–73.
Kato H, Miyakawa Y, Hidaka Y, et al. Safety and effectiveness of eculizumab for adult patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol. 2019;23:65–75.
Ito S, Hidaka Y, Inoue N, et al. Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol. 2019;23:112–21.
Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fu-jimura Y. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Trans-fusion. 2006;46:1444–52.
CAS Google Scholar
Abe T, Sasaki A, Ueda T, Miyakawa Y, Ochiai H. Complement-mediated thrombotic microangiopathy secondary to sepsis-induced disseminated intravascular coagulation successfully treated with eculizumab: a case report. Medicine. 2017;96: e6056.
Mukai S, Hidaka Y, Hirota-Kawadobora M, et al. Factor H gene variants in Japanese: its relation to atypical hemolytic uremic syndrome. Mol Immunol. 2011;49:48–55.
Fujita Y, Terashita M, Yazawa M, et al. Eculizumab for severe thrombotic microangiopathy secondary to surgical invasive stress and bleeding. Intern Med. 2020;59:93–9.
Cataland SR, Holers VM, Geyer S, Yang S, Wu HM. Biomarkers of terminal complement activation confirm the diagnosis of aHUS and differentiate aHUS from TTP. Blood. 2014;123:3733–8.
Claes KJ, Massart A, Collard L, et al. Belgian consensus statement on the diagnosis and management of patients with atypical hemolytic uremic syndrome. Acta Clin Belg. 2018;73:80–9.
Fox LC, Cohney SJ, Kausman JY, et al. Consensus opinion on diagnosis and management of thrombotic microangiopathy in Australia and New Zealand. Intern Med J. 2018;48(6):624–36.
Wehling C, Amon O, Bommer M, et al. Monitoring of complement activation biomarkers and eculizumab in complement-mediated renal disorders. Clin Exp Immunol. 2017;187:304–15.
Noris M, Galbusera M, Gastoldi S, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124:1715–26.
Riedl M, Fakhouri F, Le Quintrec M, et al. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin Thromb Hemost. 2014;40:444–64.
Jodele S, Zhang K, Zou F, et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood. 2016;127:989–96.
Bresin E, Rurali E, Caprioli J, et al. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013;24:475–86.
Matsumoto T, Fan X, Ishikawa E, et al. Analysis of patients with atypical hemolytic uremic syndrome treated at the Mie University Hospital: concentration of C3 p.I1157T mutation. Int J Hematol. 2014;100:437–42.
Toyoda H, Wada H, Miyata T, et al. Disease recurrence after early discontinuation of eculizumab in a patient with atypical hemolytic uremic syndrome with complement C3 I1157T mutation. Pediatr Hematol Oncol. 2016;38:e137-139.
Okumi M, Omoto K, Unagami K, et al. Eculizumab for the treatment of atypical hemolytic uremic syndrome recurrence after kidney transplantation associated with complement factor H mutations: a case report with a 5-year follow-up. Int Urol Nephrol. 2016;48:817–8.
Omura T, Watanabe E, Otsuka Y, et al. Complete remission of thrombotic microangiopathy after treatment with eculizumab in a patient with non-Shiga toxin-associated bacterial enteritis: a case report. Medicine. 2016;95: e4104.
Matsukuma E, Imamura A, Iwata Y, et al. Postoperative atypical hemolytic uremic syndrome associated with complement c3 mutation. Case Rep Nephrol. 2014;2014: 784943.
PubMed PubMed Central Google Scholar
Yamaguchi M, Hori M, Hiroshi N, et al. Postpartum atypical hemolytic uremic syndrome with complement factor H mutation complicated by reversible cerebrovascular constriction syndrome successfully treated with eculizumab. Thromb Res. 2017;151:79–81.
Terano C, Ishikura K, Hamada R, et al. Practical issues in using eculizumab for children with atypical haemolytic uraemic syndrome in the acute phase: a review of four patients. Nephrology. 2018;23:539–45.
Hasegawa D, Saito A, Nino N, et al. Practical issues in using eculizumab for children with atypical haemolytic uraemic syndrome in the acute phase: a review of four patients. J Pediatr Hematol Oncol. 2018;40:41–4.
Nagata A, Ohara A, Wakasugi D, et al. A case of atypical hemolytic uremic syndrome successfully weaned from plasma exchange by treatment with eculizumab. Nihon Jinzo Gakkai Shi. 2014;56:606–11.
PubMed Google Scholar
Sakaguchi H, Iwamura H, Orita M, et al. The successful treatment of eculizumab for a case of atypical hemolytic uremic syndrome with H factor. J Jpn Pediatr Soc. 2017;121:1196–202.
Google Scholar
Okano M, Matsumoto T, Nakamori Y, et al. Atypical hemolytic uremic syndrome with C3 p.I1157T missense mutation successfully treated with eculizumab. Rinsho Ketsueki. 2018;59:178–81.
Nakamura H, Anayama M, Makino M, et al. Atypical hemolytic uremic syndrome associated with complement factor H mutation and IgA nephropathy: a case report successfully treated with Eculizumab. Nephron. 2018;138:324–7.
Nozawa A, Ozeki M, Hori T, et al. A heterozygous CFHR3-CFHR1 gene deletion in a pediatric patient with transplant-associated thrombotic microangiopathy who was treated with Eculizumab. J Pediatr Hematol Oncol. 2018;40:544–6.
Kato H, Miyakawa Y, Hidaka Y, et al. Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol. 2019;23:65–75.
Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–59.
Caprioli J, Castelletti F, Bucchioni S, et al. International Registry of Recurrent and Familial HUS/TTP. Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: the C-257T, the A2089G, and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet. 2003;12:3385–95.
Sakamaki Y, Konishi K, Hayashi K, et al. Renal thrombotic microangiopathy in a patient with septic disseminated intravascular coagulation. BMC Nephrol. 2013;14:260. https://doi.org/10.1186/1471-2369-14-260 .
Article PubMed PubMed Central Google Scholar
Booth KK, Terrell DR, Vesely SK, George JN. Systemic infections mimicking thrombotic thrombocytopenic purpura. Am J Hematol. 2011;86:743–51.
Download references
Acknowledgements
We thank the TMA registry in Japan for analyzing the genetic findings of the complementary system.
Author information
Noriaki Kawano, Tomohiro Abe, Naoko Ikeda, and Yuri Nagahiro are co-first authors and contributed equally to this work
Authors and Affiliations
Department of Internal Medicine, Miyazaki Prefectural Hospital, Takamatsu, Miyazaki, 5-30, Japan
Noriaki Kawano, Naoko Ikeda, Sayaka Kawano, Taro Tochigi, Takashi Nakaike, Kiyoshi Yamashita, Atsushi Yamanaka, Koichi Mashiba & Ikuo Kikuchi
Trauma and Critical Care Center, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan
Tomohiro Abe, Keisuke Kubo & Hidenobu Ochiai
Department of Psychiatry, Jozan Hospital, Kumamoto, Japan
Yuri Nagahiro
Department of Surgery, Miyazaki Prefectural Hospital, Miyazaki, 5-30 Takamatsu, Japan
Sohshi Terasaka
Department of Pathology, Miyazaki Prefectural Hospital, Miyazaki, 5-30 Takamatsu, Japan
Kousuke Marutsuka
Division of Gastroenterology and Hematology, Department of Internal Medicine, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan
Kazuya Shimoda
Department of Blood Transfusion Medicine, Nara Medical University, Kashihara, Nara, Japan
Masanori Matsumoto
You can also search for this author in PubMed Google Scholar
Contributions
NK cared for the 4 aHUS cases, retrospectively analyzed aHUS cases, and wrote the papers. TA cared for the 4 aHUS cases, analyzed aHUS cases, and measured sC5b-9. NI retrospectively analyzed aHUS cases and cared for the 4 aHUS cases. YN cared for the 4 aHUS cases. SK cared for the 4 aHUS cases. TT cared for the 4 aHUS cases. TN cared for the 4 aHUS cases. KY cared for the 4 aHUS cases. KK cared for the 4 aHUS cases. AY cared for the 4 aHUS cases. ST cared about the one aHUS case. KM analyzed the pathological findings. KM cared for the 4 aHUS cases. IK cared for the 4 aHUS cases. KS retrospectively analyzed aHUS cases and advised the diagnosis and treatment of aHUS. MM retrospectively analyzed aHUS cases and advised the diagnosis and treatment of aHUS. HO retrospectively analyzed aHUS cases and advised the diagnosis and treatment of aHUS. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Noriaki Kawano .
Ethics declarations
Ethics approval and consent to participate.
This retrospective study was conducted in compliance with good clinical practices and the ethical principles of the Declaration of Helsinki. We received approval for this study from the ethics committees in Miyazaki Prefectural Miyazaki Hospital.
Consent for publication
We received the consent from the patients and families regarding using the person's data including the individual details and the images. Furthermore, we received the consent for publication from the cases and families by informed consent. Case 2 was reported in Am J Case Rep, 2021 ( https://doi.org/10.12659/AJCR.932251 ).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kawano, N., Abe, T., Ikeda, N. et al. Clinical features and outcomes of four atypical hemolytic uremic syndrome cases at a single institution in Miyazaki Prefecture from 2015 to 2019. Ren Replace Ther 8 , 15 (2022). https://doi.org/10.1186/s41100-022-00396-6
Download citation
Received : 29 June 2020
Accepted : 06 March 2022
Published : 11 April 2022
DOI : https://doi.org/10.1186/s41100-022-00396-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Atypical hemolytic uremic syndrome
- Excessive complement activation
- Genetic variants in the complementary system
Renal Replacement Therapy
ISSN: 2059-1381
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
This topic will provide an overview of the different causes, evaluation, and initial management of HUS in children. HUS in adults is discussed separately. (See "Thrombotic microangiopathies (TMAs) with acute kidney injury (AKI) in adults: CM-TMA and ST-HUS" .)
DEFINITIONS
Thrombotic microangiopathy describes a specific pathologic lesion in which abnormalities in the vessel wall of arterioles and capillaries lead to microvascular thrombosis. It includes several primary disorders including thrombotic thrombocytopenic purpura, Shiga toxin-mediated HUS and complement-mediated HUS or TMA. (See "Diagnostic approach to suspected TTP, HUS, or other thrombotic microangiopathy (TMA)" .)
Classification — In the past, HUS had been divided into diarrhea-positive and diarrhea-negative HUS. The former, also referred to as typical HUS, primarily resulted from Shiga toxin-producing Escherichia coli (STEC) infections, and less frequently from Shigella dysenteriae type 1 infection. All other causes of HUS were referred to as atypical HUS or assigned to the diarrhea-negative HUS, even though some patients with non-STEC-associated HUS also presented with diarrhea.
However, ongoing research has provided a better understanding of the underlying causes of HUS, especially those due to genetic mutations in the alternative pathway of complement [ 2,3 ]. As a result, the following classification has been developed based on pathophysiological considerations and triggering factors [ 4 ]:
Virginia Henderson's Writings on the Nature of Nursing: An Exemplar of Nursing Practice
Affiliations.
- 1 Department of Nursing, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, IR Iran.
- 2 Department of Midwifery, Zeynab (P.B.U.H) School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran.
- 3 Ann & Robert H. Lurie Children's Hospital of Chicago, USA.
- PMID: 36994952
- DOI: 10.1177/08943184221150255
Virginia Henderson's views on the nature of nursing continues to serve patients. Henderson emphasized that with the increasing complexity and technology in healthcare, nursing has never had a more important opportunity to contribute to the placement of the patient in the best conditions to achieve health. The aim of this article is to highlight a case study that employed Henderson's principles and plan of care to assist a child with a diagnosis of hemolytic uremic syndrome (HUS) through the performance of activities centering on achieving health and recovery.
Keywords: Virginia Henderson; hemolytic uremic syndrome; nursing care plans.
- Nursing Care*

- Physician Physician Board Reviews Physician Associate Board Reviews CME Lifetime CME Free CME
- Student USMLE Step 1 USMLE Step 2 USMLE Step 3 COMLEX Level 1 COMLEX Level 2 COMLEX Level 3 96 Medical School Exams Student Resource Center NCLEX - RN NCLEX - LPN/LVN/PN 24 Nursing Exams
- Nurse Practitioner APRN/NP Board Reviews CNS Certification Reviews CE - Nurse Practitioner FREE CE
- Nurse RN Certification Reviews CE - Nurse FREE CE
- Pharmacist Pharmacy Board Exam Prep CE - Pharmacist
- Allied Allied Health Exam Prep Dentist Exams CE - Social Worker CE - Dentist
- Point of Care
- Free CME/CE
Hemolytic Uremic Syndrome
Introduction.
Hemolytic uremic syndrome (HUS) is a thrombotic microangiopathy (TMA) characterized by thrombocytopenia, microangiopathic hemolytic anemia, and acute kidney injury. HUS is most commonly caused by Shiga toxin (typical HUS) or, less commonly, infections or genetic abnormalities activating the alternative complement pathway (atypical HUS). Additional causes can be secondary to malignancy, autoimmune disorders, genetic mutations, and medication use. [1] Extrarenal manifestations are common in HUS, particularly neurological symptoms. [1] Prompt recognition of HUS's varied etiologies and manifestations is essential for timely diagnosis and intervention, optimizing patient outcomes.
Thrombotic microangiopathy encompasses various systemic diseases in which endothelial damage causes thrombosis in the microvasculature, including capillaries, arterioles, and venules, resulting in consumptive platelet aggregations. This leads to mechanical shearing of the red blood cells (RBCs), Coombs-negative hemolytic anemia, and end-organ damage. The triad of thrombocytopenia, hemolytic anemia, and ischemic end-organ damage defines thrombotic microangiopathy. [2] [3] Some of the more common TMAs from which HUS must be differentiated are thrombotic thrombocytopenic purpura (TTP); syndrome of hemolysis, elevated liver enzymes and low platelets (HELLP); and disseminated intravascular coagulation (DIC). Despite similar pathogeneses, the treatments of these entities differ significantly. [2] [4]
Prior classifications of HUS often depended on the presence or absence of bloody diarrhea, with its presence used to diagnose typical HUS associated with Shiga toxin. However, atypical HUS can also present with bloody diarrhea in up to 30% of cases, so an etiology-based classification system is preferred. [3]
Typical HUS
Shiga toxin–producing Escherichia coli (STEC), called typical HUS, is the most common cause of HUS. Typical HUS comprises 90% to 95% of HUS cases and commonly arises from consuming contaminated food or drink and through person-to-person contact. The incidence of HUS among individuals infected with STEC ranges from 5% to 15%, predominantly affecting children younger than 5. [3] Typically, a presentation of bloody diarrhea occurs around day 2 or 3 following exposure, with HUS onset developing 3 to 10 days after the start of diarrhea. [3] Other common symptoms are vomiting (67%), fever (37%), and abdominal pain (29%). [4] [5]
Atypical HUS
Atypical HUS (aHUS) constitutes 5% to 10% of HUS cases and is linked to genetic mutations affecting the alternative complement pathway. Under physiological conditions, the alternative complement system is continuously active at low levels. However, inflammatory conditions such as infections can induce endothelial damage, triggering the activation of the coagulation cascade and causing a TMA presentation. [6] [7]
The initial clinical manifestation of aHUS typically involves nonspecific symptoms like fatigue, pallor, or somnolence. These symptoms can progress to signs of acute kidney injury (AKI), including oliguria, uremia, and fluid overload. The risk of progression to stage 3 or 4 chronic kidney disease (CKD) and end-stage renal disease (ESRD) in aHUS is high. In contrast to typical HUS, patients with aHUS often fail to regain kidney function without treatment. Untreated, approximately 50% of aHUS cases progress to dialysis dependency, with a mortality rate of 25%. [7] [6] [8]
Similar to typical HUS, aHUS may exhibit extrarenal manifestations, notably cardiac and neurological, including heart failure, pulmonary hypertension, seizures, coma, and blindness. These manifestations significantly contribute to the morbidity and mortality associated with aHUS. [6] [8] In contrast to typical HUS, patients with aHUS commonly relapse; patients must be monitored closely for relapse after treatment is discontinued. [7] [8]
Secondary HUS
The last category of HUS involves patients with HUS secondary to underlying conditions or infections, commonly presenting as aHUS with abnormal complement system activation. The most significant component of this category is HUS caused by S treptococcus pneumoniae, accounting for 5% to 15% of all cases of HUS in children. [5] S pneumoniae releases neuraminidase, exposing cellular antigens and activating the alternative complement system. This is the only cause of Coombs-positive HUS, and early antibiotic administration is indicated. [8]
Other causes of secondary aHUS are as follows:
- inherited vitamin B12 (cobalamin) metabolism disorders;
- diacydiacylglycerol kinase ε (DGKE) mutations;
- influenza virus;
- autoimmune disease, eg, systemic lupus erythematosus, antiphospholipid antibody syndrome;
- drugs, eg, quinine, calcineurin inhibitors, chemotherapeutic agents; and
- malignant hypertension. [7]
Recent studies have shown that COVID-19 can trigger aHUS in adults and children. [8] Pregnancy triggers aHUS activation, leading to increased pregnancy complications in women with established aHUS compared to those without the condition. [9] [10]
Register For Free And Read The Full Article
Learn more about a subscription to statpearls point-of-care.
Shiga toxin causing typical HUS can be divided into 2 main subtypes— Stx1 and Stx2. Stx2 is associated with more severe disease and a greater need for renal replacement therapy. [3] Traditionally, Escherichia coli 0157:H7 (E coli 0157:H7) has been linked to typical HUS; however, in recent years, non-0157 serotypes have become dominant. The most common cause of typical HUS in North America and Europe is E coli 026:H11 . Shiga toxin from Shigella dysenteriae produces a similar disease pattern to STEC, except symptoms are much more severe, and the fatality of HUS with Shigella dysenteriae is estimated at 36%. [11] Rarely, Salmonella spp are also associated with HUS caused by STEC.(doi: 10.1007/978-3-662-52972-0_26 )
The cause of aHUS is the abnormal activation of the alternative complement pathway. The complement system is part of the innate immune system and is composed of 3 pathways of activation: classical, alternative, and lectin-binding. The initial steps of each separate pathway converge at the step of forming C3 convertase, leading to the formation of the membrane-attack complex (MAC), which then lyses target cells.
In aHUS, the complement pathway becomes uncontrolled, most often due to mutations in genes for alternative path initiators, regulatory proteins, or autoantibodies-to-regulatory proteins. The most common identifiable mutation in aHUS is a pathogenic variant of the CFH gene, which encodes Factor H—the main regulatory protein of the alternative complement pathway at the C3b level. [6] [7] Because of incomplete penetrance, a second inciting agent, such as infection, is often required for aHUS to develop, even if genetic abnormalities are present. [8]
Epidemiology
HUS and aHUS are most often associated with children younger than 10, with most cases in those younger than 5. [4] Globally, STEC causes 43 acute illnesses per 100,000 person-years and 3890 cases of HUS. [12] STEC-HUS is one of the most common causes of pediatric renal replacement therapy. A large retrospective study showed that 15% of children (younger than 18) who presented to the emergency department with bloody diarrhea developed STEC-HUS. [13]
STEC-HUS incidence is estimated at 0.57 cases per 100,000 children, and in the highest risk group—children aged 6 months to 2 years—the incidence is as high as 3 per 100,000 children. [3] Incidence directly coordinates with environmental exposure and agricultural practices, such as cattle raising, and most patients are diagnosed between April and September when cattle show higher colonization of STEC. [3]
In contrast to HUS, aHUS is notably less frequent; nevertheless, aHUS exhibits substantially elevated morbidity and mortality rates. Like typical HUS, atypical HUS affects young children, predominantly those younger than 5, and prevalence is estimated at 2 to 9 cases per million people aged 20 years or younger. [6] Cases due to S Pneumonia usually occur in the winter during cold season. (doi: 10.1007/978-3-662-52972-0_26 )
Pathophysiology
HUS is typically associated with bacterial infection resulting from the consumption of undercooked beef, unpasteurized milk, or other food or drink contaminated by cattle manure; cattle are asymptomatic carriers of STEC. [3] Once ingested, STEC penetrates the mucous layer of the intestine and secretes Shiga toxin, which binds to the receptor Gb3. The Shiga toxin/Gb3 complex binds to cell ribosomes, inhibiting protein synthesis and causing apoptosis; inflammatory cytokines are also produced. [3]
In addition to cytotoxic effects, Shiga toxin is capable of activating the complement system by inhibiting complement factor H. Upon entering the bloodstream, Shiga toxin persists in binding to cells via the Gb3 receptor, with the highest prevalence found in the glomerular microvasculature. Endothelial damage is caused by 1) direct cytotoxicity of Shiga toxin, 2) disturbance of the hemostatic pathway, 3) increased cytokine release, and 4) alternative pathway activation. [3] This endothelial damage then initiates the pathology associated with TMA.
In aHUS, the alternative complement pathway is activated as described above, with particular emphasis on regulatory Factor H that stabilizes C3 and inactivates C3b. [3] aHUS is usually associated with a genetic abnormality affecting the regulation in the alternative complement system coupled with an inciting stress such as infection. Secondary HUS generally follows the same pathophysiology pattern as aHUS.

Histopathology
Most biopsies performed on patients with HUS occurred prior to 1990 because patients with suspected HUS are not routinely biopsied due to the presence of thrombocytopenia and general instability. In biopsies that were performed, light microscopy revealed fragmented RBC in glomerular capillary loops and variable fibrin staining in glomerular capillaries and renal arterioles. Electron microscopy shows endothelial and mesangial cell deposits of fibrin and proteinaceous material pushing into the capillary lumen giving the appearance of a glomerular basement membrane with a double contour. In typical STEC-associated HUS, staining for C1q, C3, or C4 is not observed.(doi: 10.1007/978-3-662-52972-0_26 )
Despite extra-renal manifestations, other organs are generally not biopsied with the exception of the gastrointestinal tract which can show extensive vascular thrombosis histologically.(doi: 10.1007/978-3-662-52972-0_26 )
History and Physical
The typical patient is a child younger than 5 with painful diarrhea and abdominal cramping between 1 and 10 days (median 4 d) after exposure to STEC; fever and vomiting may also be present. HUS generally begins 5 to 13 days after the start of diarrhea (median 6.5-7 d). [3] HUS symptoms include renal symptoms, such as anuria, oliguria, and fluid overload, and symptoms related to anemia, such as syncope, fatigue, and pallor. Petechiae and easy bleeding may be notable due to thrombocytopenia.
Although less common in adults, when HUS emerges, particularly during food-related outbreaks, its clinical trajectory displays greater variability and an unfavorable prognosis. Notably, most HUS-related deaths occur in adults older than 60. In adult patients, neurological symptoms such as confusion, seizures, and coma are markedly more prevalent than in children. Older patients often also have neuropsychiatric symptoms. [12]
The initial clinical presentation of aHUS is usually nonspecific symptoms such as fatigue, pallor, or somnolence, which can progress to signs of acute kidney injury (AKI), such as oliguria, uremia, and fluid overload. If the inciting factor is S Pneumoniae , patients may have underlying pneumonia, empyema, or meningitis. Diarrhea, including bloody diarrhea, can also be prominent in atypical HUS. [3]
Extrarenal manifestations are common with both typical and atypical HUS. Neurologic and cardiac symptoms are often responsible for much of the morbidity and mortality of HUS. [3]
The diagnosis of HUS requires a high index of suspicion based on symptoms, travel history, laboratory data, and dietary history. [14] [15] [16] Initial tests should include a complete blood count with differential and comprehensive blood metabolic panel. Elevated LDH and indirect bilirubin, as well as low haptoglobin and elevated plasma hemoglobin, are diagnostic of hemolytic anemia, as are schistocytes on peripheral smear. A Coombs test should be negative, with the exception of HUS caused by S pneumonia .
Up to 20% of patients have elevated amylase and lipase due to pancreatic damage, which may be accompanied by hyperglycemia.(doi: 10.1007/978-3-662-52972-0_26 ) A stool sample should be collected to test for Shiga toxin whenever diarrhea is present. The results may not be positive for the Shiga toxin if it has been cleared or is avidly bound to the endothelium. If clinically indicated, testing for the presence of S dysenteriae and S pneumonia should also be performed.
Low complement levels are suggestive of, but not specific to aHUS, as typical HUS also causes immune system abnormalities. [5] Patients may have hyponatremia, hyperkalemia, and other electrolyte abnormalities from acute kidney injury as the disease progresses. Genetic testing can also be sent to evaluate for genetic causes of aHUS.
An ADAMTS13 level should be sent to rule out TTP. Abnormal coagulation studies, such as prolonged prothrombin time (PTT), activated partial thromboplastin time (aPTT), elevated D dimer, and elevated fibrin degradation products, are suggestive of DIC.
All of the above tests have therapeutic value, but if clinical suspicion is high, treatment should not be delayed while waiting for all testing results, as early treatment initiation is associated with improved outcomes.
Treatment / Management
The management of typical HUS caused by STEC is generally supportive. Patients are often volume depleted, and less volume resuscitation has been linked to an increased need for renal replacement therapy, which half of all patients require. [3] [13] Blood transfusions are provided as clinically needed, and platelet transfusions should be given sparingly to avoid thrombotic complications. (B3)
Antimotility agents and antibiotics are avoided in typical HUS caused by STEC, as they correlate with poorer outcomes, likely due to heightened Shiga toxin exposure. On the other hand, if S dysenteriae or S pneumonia is present, early antibiotics are associated with improved outcomes.(doi: 10.1007/978-3-662-52972-0_26 )
For aHUS, early treatment is crucial to avoid end-stage renal disease (ESRD) and mortality. The cornerstones of treatment for aHUS are first-line treatment with eculizumab and second-line with plasma exchange. [17] Eculizumab is a recombinant monoclonal antibody that targets the C5 component of complement activation by preventing its cleavage; early eculizumab administration improves its effectiveness. [7] [17] The advent of eculizumab treatment has decreased progression to ESRD or death in children from 30% to 50% down to 9% and in adults from 60% down to 6% to 15%. [5] (B2)
Ravulizumab is a new C5 inhibitor approved for use in the USA in 2019 and the EU in 2020. Ravulizumab also binds to C5, preventing cleavage, and it has similar efficacy to eculizumab but with 4 times as long of a half-life. [8] Prior to eculizumab, plasma exchange was the standard of care for HUS and is still used if eculizumab is not available or in addition if clinically indicated. (B3)
One ongoing question is how to treat patients who develop ESRD due to aHUS because patients with aHUS often develop renal failure necessitating kidney transplant, and there is a high recurrence rate of aHUS in the transplanted kidney. Studies suggest that prophylactic administration of eculizumab in patients with a high risk of aHUS recurrence prolongs graft survival and may also be cost-effective in the high-risk groups despite the drug's high cost. [7] [17] Adverse effects of eculizumab are infection by encapsulated organisms such as S pneumoniae and Haemophilus influenzae, and all patients should be given appropriate vaccines and monitored. [7] (B2)
The treatment for secondary HUS is largely dependent on the treatment of the underlying condition. Small studies have shown eculizumab to have some efficacy in treating pregnancy-related secondary HUS; however, many of these patients are shown to have an underlying genetic component predisposing them to aHUS as well. [10] [18] In another small study, patients with secondary HUS who had worsening renal function despite treatment of underlying disease were administered eculizumab with improvement in symptoms within the kidneys and extrarenal symptoms. [18]
Differential Diagnosis
Initially HUS may present similarly to other TMAs such as TTP, DIC, HELLP, and systemic vasculitis. Often clinical presentations and laboratory testing will rule out other causes.
Thrombotic Thrombocytopenic Purpura (TTP)
TTP is thrombotic microangiopathy characterized by a pentad of hemolytic anemia, thrombocytopenia, renal dysfunction, fever, and neurological dysfunction. TTP is due to a deficiency or mutation in "a disintegrin and metalloproteinase with a thrombospondin type 1 motif member 13" (ADAMTS13) and usually has adult-onset symptoms.
Disseminated Intravascular Coagulation (DIC)
DIC is the systemic activation of the coagulation cascade and is characterized by abnormal coagulation studies, including prolonged prothrombin time and activated partial thromboplastin time, elevated D dimer, and elevated fibrin degradation products, which are usually normal in HUS. Patients with DIC usually have serious underlying illnesses such as septic shock, trauma, or malignancy.
HELLP Syndrome
HELLP syndrome is observed in women pregnant in the third trimester or immediately postpartum, and it is characterized by hemolysis of red blood cells, elevated liver enzymes, and a low platelet count usually occurring with preeclampsia.
Systemic Vasculitis
Patients with systemic vasculitis typically present with inflammatory signs like fever, rash, and arthralgia, and lack prodromal diarrhea. Patients generally have a markedly elevated erythrocyte sedimentation rate.
The prognosis of typical HUS is generally good with mortality estimated at 5% overall. [19] [20] [21] However, up to 25% of patients with HUS develop long-term renal insufficiency with a glomerular filtration rate <80 mL/min/1.73 m 2 , hypertension, or proteinuria, which could predispose patients to increased renal insufficiency as they get older. The most significant prognostic indicator of ongoing renal dysfunction is the length of time on dialysis, with long-term complications evident after 2 to 3 weeks of dialysis dependency. [3] One exception to the low mortality rate of typical HUS is in adults older than 60 who comprise most fatalities. [12]
The course of aHUS has traditionally been much less benign than typical HUS; however, the advent of eculizumab treatment has decreased progression to ESRD or death in children from 30% to 50% down to 9% and in adults from 60% to 6% down to 15%. [5]
Enhancing Healthcare Team Outcomes
Although HUS has less than 5% mortality, it can lead to long-term renal complications, especially in children. Timely diagnosis and appropriate management are crucial. A high index of suspicion in children presenting with symptoms related to HUS and appropriate investigations can lead to better patient outcomes. Clinicians must monitor for reduced hemoglobin and platelet counts and signs related to anemia and thrombocytopenia.
Including a nephrologist in the care team is crucial for patients who develop acute renal failure and need dialysis. Thus, proper coordination among interprofessional team members, consisting of physicians, advanced practice practitioners, nurses, pharmacists, and nephrologists, is optimal. Prompt recognition of HUS's varied etiologies and manifestations is essential for timely diagnosis and intervention, optimizing patient outcomes.
Bayer G, von Tokarski F, Thoreau B, Bauvois A, Barbet C, Cloarec S, Mérieau E, Lachot S, Garot D, Bernard L, Gyan E, Perrotin F, Pouplard C, Maillot F, Gatault P, Sautenet B, Rusch E, Buchler M, Vigneau C, Fakhouri F, Halimi JM. Etiology and Outcomes of Thrombotic Microangiopathies. Clinical journal of the American Society of Nephrology : CJASN. 2019 Apr 5:14(4):557-566. doi: 10.2215/CJN.11470918. Epub 2019 Mar 12 [PubMed PMID: 30862697]
Bommer M, Wölfle-Guter M, Bohl S, Kuchenbauer F. The Differential Diagnosis and Treatment of Thrombotic Microangiopathies. Deutsches Arzteblatt international. 2018 May 11:115(19):327-334. doi: 10.3238/arztebl.2018.0327. Epub [PubMed PMID: 29875054]
Joseph A, Cointe A, Mariani Kurkdjian P, Rafat C, Hertig A. Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review. Toxins. 2020 Jan 21:12(2):. doi: 10.3390/toxins12020067. Epub 2020 Jan 21 [PubMed PMID: 31973203]
Ylinen E, Salmenlinna S, Halkilahti J, Jahnukainen T, Korhonen L, Virkkala T, Rimhanen-Finne R, Nuutinen M, Kataja J, Arikoski P, Linkosalo L, Bai X, Matussek A, Jalanko H, Saxén H. Hemolytic uremic syndrome caused by Shiga toxin-producing Escherichia coli in children: incidence, risk factors, and clinical outcome. Pediatric nephrology (Berlin, Germany). 2020 Sep:35(9):1749-1759. doi: 10.1007/s00467-020-04560-0. Epub 2020 Apr 22 [PubMed PMID: 32323005]
Palma LMP, Vaisbich-Guimarães MH, Sridharan M, Tran CL, Sethi S. Thrombotic microangiopathy in children. Pediatric nephrology (Berlin, Germany). 2022 Sep:37(9):1967-1980. doi: 10.1007/s00467-021-05370-8. Epub 2022 Jan 18 [PubMed PMID: 35041041]
Yerigeri K, Kadatane S, Mongan K, Boyer O, Burke LLG, Sethi SK, Licht C, Raina R. Atypical Hemolytic-Uremic Syndrome: Genetic Basis, Clinical Manifestations, and a Multidisciplinary Approach to Management. Journal of multidisciplinary healthcare. 2023:16():2233-2249. doi: 10.2147/JMDH.S245620. Epub 2023 Aug 4 [PubMed PMID: 37560408]
Raina R, Grewal MK, Radhakrishnan Y, Tatineni V, DeCoy M, Burke LL, Bagga A. Optimal management of atypical hemolytic uremic disease: challenges and solutions. International journal of nephrology and renovascular disease. 2019:12():183-204. doi: 10.2147/IJNRD.S215370. Epub 2019 Sep 4 [PubMed PMID: 31564951]
Raina R, Vijayvargiya N, Khooblall A, Melachuri M, Deshpande S, Sharma D, Mathur K, Arora M, Sethi SK, Sandhu S. Pediatric Atypical Hemolytic Uremic Syndrome Advances. Cells. 2021 Dec 18:10(12):. doi: 10.3390/cells10123580. Epub 2021 Dec 18 [PubMed PMID: 34944087]
Gupta M, Govindappagari S, Burwick RM. Pregnancy-Associated Atypical Hemolytic Uremic Syndrome: A Systematic Review. Obstetrics and gynecology. 2020 Jan:135(1):46-58. doi: 10.1097/AOG.0000000000003554. Epub [PubMed PMID: 31809447]
Bruel A, Kavanagh D, Noris M, Delmas Y, Wong EKS, Bresin E, Provôt F, Brocklebank V, Mele C, Remuzzi G, Loirat C, Frémeaux-Bacchi V, Fakhouri F. Hemolytic Uremic Syndrome in Pregnancy and Postpartum. Clinical journal of the American Society of Nephrology : CJASN. 2017 Aug 7:12(8):1237-1247. doi: 10.2215/CJN.00280117. Epub 2017 Jun 8 [PubMed PMID: 28596415]
Mattock E, Blocker AJ. How Do the Virulence Factors of Shigella Work Together to Cause Disease? Frontiers in cellular and infection microbiology. 2017:7():64. doi: 10.3389/fcimb.2017.00064. Epub 2017 Mar 24 [PubMed PMID: 28393050]
Travert B, Rafat C, Mariani P, Cointe A, Dossier A, Coppo P, Joseph A. Shiga Toxin-Associated Hemolytic Uremic Syndrome: Specificities of Adult Patients and Implications for Critical Care Management. Toxins. 2021 Apr 26:13(5):. doi: 10.3390/toxins13050306. Epub 2021 Apr 26 [PubMed PMID: 33925836]
McKee RS, Schnadower D, Tarr PI, Xie J, Finkelstein Y, Desai N, Lane RD, Bergmann KR, Kaplan RL, Hariharan S, Cruz AT, Cohen DM, Dixon A, Ramgopal S, Rominger A, Powell EC, Kilgar J, Michelson KA, Beer D, Bitzan M, Pruitt CM, Yen K, Meckler GD, Plint AC, Bradin S, Abramo TJ, Gouin S, Kam AJ, Schuh A, Balamuth F, Hunley TE, Kanegaye JT, Jones NE, Avva U, Porter R, Fein DM, Louie JP, Freedman SB, Pediatric Emergency Medicine Collaborative Research Committee and Pediatric Emergency Research Canada. Predicting Hemolytic Uremic Syndrome and Renal Replacement Therapy in Shiga Toxin-producing Escherichia coli-infected Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 Apr 10:70(8):1643-1651. doi: 10.1093/cid/ciz432. Epub [PubMed PMID: 31125419]
Bouwmeester RN, Bormans EMG, Duineveld C, van Zuilen AD, van de Logt AE, Wetzels JFM, van de Kar NCAJ. COVID-19 vaccination and Atypical hemolytic uremic syndrome. Frontiers in immunology. 2022:13():1056153. doi: 10.3389/fimmu.2022.1056153. Epub 2022 Dec 1 [PubMed PMID: 36531998]
Boldig K, Batra R, Villegas A. COVID-19: A Rare Cause of Hemolytic Uremic Syndrome. Cureus. 2022 Aug:14(8):e27962. doi: 10.7759/cureus.27962. Epub 2022 Aug 13 [PubMed PMID: 36120203]
Netti GS, Santangelo L, Paulucci L, Piscopo G, Torres DD, Carbone V, Giordano P, Spadaccino F, Castellano G, Stallone G, Gesualdo L, Chironna M, Ranieri E, Giordano M. Low C3 Serum Levels Predict Severe Forms of STEC-HUS With Neurologic Involvement. Frontiers in medicine. 2020:7():357. doi: 10.3389/fmed.2020.00357. Epub 2020 Jun 26 [PubMed PMID: 32671083]
Zuber J, Frimat M, Caillard S, Kamar N, Gatault P, Petitprez F, Couzi L, Jourde-Chiche N, Chatelet V, Gaisne R, Bertrand D, Bamoulid J, Louis M, Sberro Soussan R, Navarro D, Westeel PF, Frimat L, Colosio C, Thierry A, Rivalan J, Albano L, Arzouk N, Cornec-Le Gall E, Claisse G, Elias M, El Karoui K, Chauvet S, Coindre JP, Rerolle JP, Tricot L, Sayegh J, Garrouste C, Charasse C, Delmas Y, Massy Z, Hourmant M, Servais A, Loirat C, Fakhouri F, Pouteil-Noble C, Peraldi MN, Legendre C, Rondeau E, Le Quintrec M, Frémeaux-Bacchi V. Use of Highly Individualized Complement Blockade Has Revolutionized Clinical Outcomes after Kidney Transplantation and Renal Epidemiology of Atypical Hemolytic Uremic Syndrome. Journal of the American Society of Nephrology : JASN. 2019 Dec:30(12):2449-2463. doi: 10.1681/ASN.2019040331. Epub 2019 Oct 1 [PubMed PMID: 31575699]
Cavero T, Rabasco C, López A, Román E, Ávila A, Sevillano Á, Huerta A, Rojas-Rivera J, Fuentes C, Blasco M, Jarque A, García A, Mendizabal S, Gavela E, Macía M, Quintana LF, María Romera A, Borrego J, Arjona E, Espinosa M, Portolés J, Gracia-Iguacel C, González-Parra E, Aljama P, Morales E, Cao M, Rodríguez de Córdoba S, Praga M. Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2017 Mar 1:32(3):466-474. doi: 10.1093/ndt/gfw453. Epub [PubMed PMID: 28339660]
Siegler RL, Pavia AT, Christofferson RD, Milligan MK. A 20-year population-based study of postdiarrheal hemolytic uremic syndrome in Utah. Pediatrics. 1994 Jul:94(1):35-40 [PubMed PMID: 8008534]
Siegler RL, Milligan MK, Burningham TH, Christofferson RD, Chang SY, Jorde LB. Long-term outcome and prognostic indicators in the hemolytic-uremic syndrome. The Journal of pediatrics. 1991 Feb:118(2):195-200 [PubMed PMID: 1993944]
Fitzpatrick MM, Shah V, Trompeter RS, Dillon MJ, Barratt TM. Long term renal outcome of childhood haemolytic uraemic syndrome. BMJ (Clinical research ed.). 1991 Aug 31:303(6801):489-92 [PubMed PMID: 1912857]
Use the mouse wheel to zoom in and out, click and drag to pan the image
Breastfeeding and hemolytic uremic syndrome
- Research Letter
- Published: 27 April 2024
Cite this article

- Mario Giordano ORCID: orcid.org/0000-0003-4224-636X 1 ,
- Manuela Gallo 1 &
- Maria Paola Ferrante 1
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Myojin S, Pak K, Sako M et al (2022) Interventions for Shiga toxin-producing Escherichia coli gastroenteritis and risk of hemolytic uremic syndrome: A population-based matched case control study. PLoS One 17:e0263349. https://doi.org/10.1371/journal.pone.0263349
Article CAS PubMed PubMed Central Google Scholar
Giordano M, Baldassarre ME, Palmieri V et al (2019) Management of STEC Gastroenteritis: Is There a Role for Probiotics? Int J Environ Res Public Health 16:1649. https://doi.org/10.3390/ijerph16091649
Victora CG, Bahl R, Barros AJ, França GV et al (2016) Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387:475–490. https://doi.org/10.1016/S0140-6736(15)01024-7
Article PubMed Google Scholar
Loconsole D, Giordano M, Centrone F et al (2020) Epidemiology of Shiga Toxin-Producing Escherichia coli Infections in Southern Italy after Implementation of Symptom-Based Surveillance of Bloody Diarrhea in the Pediatric Population. Int J Environ Res Public Health 17:5137. https://doi.org/10.3390/ijerph17145137
Article PubMed PubMed Central Google Scholar
Download references
Author information
Authors and affiliations.
Pediatric Nephrology and Dialysis Unit, Pediatric Hospital “Giovanni XXIII”, Bari, Italy
Mario Giordano, Manuela Gallo & Maria Paola Ferrante
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mario Giordano .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Giordano, M., Gallo, M. & Ferrante, M.P. Breastfeeding and hemolytic uremic syndrome. Pediatr Nephrol (2024). https://doi.org/10.1007/s00467-024-06359-9
Download citation
Received : 18 February 2024
Revised : 18 March 2024
Accepted : 18 March 2024
Published : 27 April 2024
DOI : https://doi.org/10.1007/s00467-024-06359-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Med Case Rep

Atypical hemolytic uremic syndrome: a case report
B. m. d. b. basnayake.
1 Department of Nephrology and Renal Transplant, Teaching Hospital Kandy, Kandy, Sri Lanka
A. W. M. Wazil
N. nanayakkara, s. m. d. k. samarakoon.
2 Department of Transfusion Medicine, Teaching Hospital Kandy, Kandy, Sri Lanka
E. M. S. K. Senavirathne
B. u. e. w. d. r. thangarajah, n. karunasena, r. m. b. s. s. mahanama.
Thrombotic microangiopathy is a pathological condition comprised of microvascular thrombosis involving any organ of the body leading to thrombocytopenia, Coombs-negative hemolytic anemia, and end-organ damage. The most common forms of thrombotic microangiopathies are Shiga toxin-producing Escherichia coli -mediated hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, and atypical hemolytic uremic syndrome. The atypical hemolytic uremic syndrome occurs due to genetic and acquired mutations in complement regulatory factors and to complement activation factors in the immune system, mainly the alternative pathway. Clinical manifestations and outcomes differ with the prevalent mutations of the patient. Currently, available treatment modalities are therapeutic plasma exchange and a monoclonal antibody against C5, eculizumab. We report a case of a Sri Lankan girl diagnosed with atypical hemolytic uremic syndrome complicated with septicemia, hemolytic anemia, acute kidney injury, pulmonary hemorrhage with respiratory failure, and hypertension who had a complete remission following long-term (30 months) therapeutic plasma exchange.
Case presentation
A 15-year-old Sri Lankan girl was transferred from a local hospital with the features of septicemia and acute kidney injury for specialized management. She had high blood pressure (180/100 mmHg) on admission. She underwent appendicectomy based on suspicion of acute appendicitis as the cause of sepsis. Following surgery, her condition deteriorated, and intensive care unit management was warranted because she developed pulmonary hemorrhages and respiratory failure requiring mechanical ventilation and renal replacement therapy in the form of hemodialysis. Her blood investigations showed microangiopathic hemolytic anemia, thrombocytopenia, elevated lactate dehydrogenase, and reduced human complement C3 levels, together with a normal coagulation profile. She was diagnosed with atypical hemolytic uremic syndrome and was initiated on therapeutic plasma exchange and other supportive therapy, including corticosteroids. Following a lengthy course of plasma exchange, complete recovery was achieved.
The atypical hemolytic uremic syndrome is a rare disease entity requiring a high index of suspicion to diagnose. It is a diagnosis of exclusion. Early diagnosis with prompt treatment will render a better outcome. The atypical hemolytic uremic syndrome needs to be considered in all patients with thrombotic microangiopathy.
Thrombotic microangiopathy (TMA) is characterized by thrombocytopenia and Coombs test-negative hemolytic anemia with end-organ damage. Any organ of the body can be involved, commonly the kidney, central nervous system, gastrointestinal tract, lungs, and heart [ 1 ]. TMA is a pathological condition resulting in thrombosis of capillaries and arterioles. It is due to endothelial injury that leads to the activation of platelets causing platelet aggregation and thrombus formation. Due to the consumption of platelets, thrombocytopenia ensues. Small vessel occlusion leads to organ hypoxia and ischemic organ damage [ 1 , 2 ]. According to recent updates, TMA is classified into three major categories. These include Shiga toxin-producing Escherichia coli (STEC) hemolytic uremic syndrome (HUS), thrombotic thrombocytopenic purpura (TTP), and atypical hemolytic uremic syndrome (aHUS), which is further classified according to the etiology. Etiology-based nomenclature, such as pregnancy-induced aHUS, post-transplant aHUS, drug-induced, infection-induced aHUS, and autoimmune disease-associated aHUS, is also widely used currently [ 3 ].
Even though the clinical manifestations of TMA are similar, pathophysiologies differ from each other. TTP occurs due to severe deficiency of ADAMTS-13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13). ADAMTS-13 is a specific cleaving protease of von Willebrand factor (vWF). Absent cleavage of ultralarge vWF multimers due to lack of ADAMTS-13 activity will promote thrombus formation in small vessels [ 4 , 5 ]. In STEC-HUS, Shiga toxin exerts direct toxicity to cells by binding to the globotriaosylceramide receptors on the target cell surface and escorting to cytotoxicity. Cytotoxicity includes protein synthesis and apoptosis. Shiga toxin also increases the secretion of abnormally sized vWF multimers from endothelial cells [ 4 , 6 ]. aHUS is due to the uncontrolled complement activation [ 7 ]. We report a case of a Sri Lankan patient diagnosed with aHUS who was treated successfully with plasma exchange therapy. To our knowledge, there is only a handful of case reports on aHUS in Sri Lanka, and this is the only reported case with complete recovery following a lengthy course of plasma exchange in Sri Lanka.
A 15-year-old, previously healthy Sri Lankan girl with no significant medical history or family history of hypertension or diabetes mellitus was admitted to a peripheral hospital with fever and skin rash of 2 weeks’ duration that was treated as chickenpox. The diagnosis of chickenpox was confirmed by a dermatologist. She developed severe lower abdominal pain. She was oliguric and tachycardic (pulse rate of 102 beats per minute), and her blood pressure was elevated to 180/100 mm Hg. Her temperature was 38.1 °C. The result of her neurological examination was normal. Her serum creatinine was 210 μmol/L (normal range 80–130 μmol/L), and she had active urinary sediments (urine full report, red cells 100–150 with 50% dysmorphic red cells). She was clinically diagnosed with acute appendicitis, confirmed with ultrasound findings and complicated with sepsis-induced acute kidney injury. She was initiated on intravenous cefuroxime 750 mg 8-hourly and intravenous metronidazole 500 mg 8-hourly and was transferred to the nephrology unit of Teaching Hospital Kandy for specialized care.
She was referred to the surgical unit, where an appendectomy was performed while she was under general anesthesia. Her appendix was inflamed, but it was not perforated. Both intravenous cefuroxime 750 mg 8-hourly and intravenous metronidazole 500 mg 8-hourly were continued. In preoperative assessment, her blood pressure was 150/94 mmHg, and her serum creatinine level was high (226 μmol/L) with hyperkalemia (5.8 mmol/L), which was corrected with a potassium-lowering regimen prior to induction of anesthesia. During the surgery, her blood pressure was under control, and her recovery was also uneventful. Later, the diagnosis of appendicitis was confirmed with the histological findings.
On postsurgery day 1, she was anuric and severely acidotic, and her creatinine level further inclined. Urgent hemodialysis was offered, and input-output was strictly monitored. A renal biopsy was performed.
On postsurgery day 3, she developed high-grade fever again, and therefore surgical site infection or femoral vascular catheter infection was suspected. Intravenous antibiotics were changed; intravenous flucloxacillin 500 mg 6-hourly was added according to the microbiology team’s opinion. The vascular catheter was removed, and catheter tip and blood samples, which were taken with aseptic nontouch technique, were sent for cultures. The culture results were negative for aerobic and anaerobic bacteria and fungi, and surgical site infection was also excluded. Findings of an echocardiogram ruled out infective endocarditis.
The patient’s full blood count showed hemoglobin of 7.6 g/dl, platelet count of 68 × 10 9 /L, and white cell count of 19.5 × 10 9 /L. Blood film revealed features of microangiopathic hemolytic anemia. The patient’s serum creatinine was 312 μmol/L. Her liver enzymes were within the normal range. Her coagulation profile was normal, including the thromboelastogram. Her lactate dehydrogenase (LDH) level was 3124 U/L. Her reticulocyte count was 7.27%. Her D-dimer was negative at 0.78 mg/L (< 1). Her Coombs test result was negative. With the blood film evidence and other test result findings, together with unexplained fever, TTP was suspected. She was admitted to the intensive care unit (ICU) and was initiated on therapeutic plasma exchange (TPE) together with cryo-poor plasma as the replacement fluid.
Her ICU stay was complicated with pulmonary hemorrhage with lower respiratory tract infection followed by respiratory failure requiring mechanical ventilation, and intravenous antibiotics were upgraded to meropenem 1 g 12-hourly and levofloxacin 500 mg once per day. Initially, plasma exchange was carried out daily (for 14 days) and then every other day (for 28 days). She was offered hemodialysis every other day for 14 days, every third day for 21 days, and every fourth day for 24 days. Hemodialysis was stopped following the improvement of urine output.
Histological examination of renal biopsy revealed fibrinoid necrosis of small arteries. The patient’s complement C3 was 57.6 mg/dl (normal range 90–180), and her complement C4 was 17.4 mg/dl (10–40). Results of tests for antineutrophil cytoplasmic antibodies, antinuclear antibody, and double-stranded deoxyribonucleic acid (DNA) were negative. Test results for hepatitis B surface antigen, hepatitis C antibody, human immunodeficiency virus, and VDRL (Venereal Disease Research Laboratory) were negative. Blood culture results were negative for E. coli . Investigation findings together with hypertension, blood picture features of microangiopathic hemolytic anemia, kidney injury, and pulmonary hemorrhage associated with respiratory failure prompted the diagnosis of atypical hemolytic uremic syndrome. ADAMTS-13 level and genetic studies were not done, owing to the unavailability of resources.
As the long-term management plan, the patient was offered long-term plasma exchange in a tapering regimen. Initially, two cycles per week were arranged. She gradually responded to treatment, and investigation results were improving. She stayed in the ICU for 14 days and was discharged from the hospital after a total of 69 days with two antihypertensive agents and a tapering regimen of oral prednisolone.
She was regularly followed up in the nephrology clinic, and TPE was offered once per week for 6 months and then tapered to once in 2 weeks, 4 weeks, 6 weeks, and 8 weeks. Following a total TPE course of 30 months, plasma exchange therapy was omitted. Her hemoglobin, platelet count, serum creatinine, LDH, reticulocyte count, and blood film were all within normal ranges.
Our patient represents a typical case of aHUS with a severe form of this disease. She developed a number of complications, such as septicemia, refractory hypertension, and pulmonary hemorrhage with respiratory failure. She was also in an oligoanuric state for almost 2 months. This case proves that early diagnosis and prompt treatment with TPE and renal replacement therapy will lead to a complete recovery from aHUS.
aHUS occurs due to complement-related abnormalities. The complement system is a part of the immune system that destroys microorganisms and damaged cells by enhancing the ability of antibodies and phagocytic cells via complement activation. It also promotes inflammation and attacks the pathogen’s cell membrane. It is activated via three pathways: the classical pathway, the alternative pathway (AP), and the lectin pathway. Uncontrolled activation of complements in AP is strongly associated with aHUS [ 4 , 8 , 9 ].
AP is continuously activated at a low level due to spontaneous C3 hydrolysis because it does not need specific initiators. C3 internal thioester bond is hydrolyzed by H 2 O easily and forms C3(H 2 O). Then it interacts with complement factor B (CFB) followed by cleavage by complement factor D (CFD) and generates C3(H 2 O)Bb. For the cleavage of C3 into C3a and C3b by C3 convertase enzyme complex, the C3(H 2 O)Bb molecule works as an initiator. However, complement factor I (CFI), complement factor H (CFH), and membrane cofactor protein (MCP) rapidly inactivate C3b. C3b may bind with CFB to form C3bB. This complex then is cleaved by CFD into Ba and Bb. Bb remains bound to C3b to produce C3bBb, which is the C3 convertase in AP. C3 convertase may recruit another C3b and produce C3bBbC3b, which is the C5 convertase. C5 convertase cleaves C5 into C5a and C5b molecules. The C5b molecule then binds to C6, C7, C8, and C9 to generate the membrane attack complex (MAC). MAC destroys the target directly by the formation of membrane pores [ 4 , 10 , 11 ].
Abnormalities of complement activation factors, such as CFB and CFD, and complement regulatory factors, such as CFI, CFH, and MCP, are associated with the development of aHUS. These abnormalities are due to uncontrolled complement activation by the loss-of-function variants in complement-regulatory proteins or gain-of-function variants in complement activation factors [ 4 , 12 ]. Multiple genetic and acquired complement abnormalities have been identified.
CFH abnormalities have the highest frequency, accounting for 20–30% of cases. The main effects are impaired CFH binding to C3b and/or glycosaminoglycans on host cell surface and decreased cofactor activity [ 13 – 15 ]. Effects of CFI abnormalities are impaired CFI secretion and reduced proteolytic activity. MCP abnormalities have a frequency of 8–10% with reduction in MCP expression and decreased C3b binding and cofactor activity [ 16 , 17 ]. Anti-CFH antibody inhibits the complement-regulatory function of CFH [ 10 , 18 ].
C3 defects result in resistance to CFI-mediated inactivation of C3b and also generation of hyperactive C3 convertase [ 19 ]. Abnormalities in CFB lead to the resistance of convertase to decay by CFH and formation of hyperactive C3 convertase [ 20 ]. Several coagulation-related factor abnormalities are also recognized; thrombomodulin, diacylglycerol kinase epsilon, and plasminogen abnormalities are related to the development of aHUS [ 10 , 21 – 23 ].
Clinical manifestations and outcome differ with underlying genetic or acquired defects in the complement system. Patients with aHUS, who have genetic mutations, are commonly affected during childhood (67%) [ 24 ]. Acute episodes of aHUS present with severe Coombs-negative hemolytic anemia, thrombocytopenia, and acute kidney injury. Extrarenal manifestations occur in 20% of cases [ 10 , 25 ]. Among patients with CFH, CFI, and C3 mutations, 60–70% will progress to permanent renal damage or die during the initial episode or progress to end-stage renal failure (ESRF) following relapses. But in children with CFH antibodies, only 30% of patients end up with ESRF [ 24 ]. CFB abnormalities are related to worse renal prognosis, whereas CFH mutations are associated with more cardiovascular complications and a higher mortality [ 20 , 25 ]. Ten-year survival in patients with CFH abnormalities is 50%, but patients with CFI or C3 mutations or anti-CFH antibodies have higher survival rates of 80–90% at 10 years [ 24 ]. The best prognosis is seen in patients with MCP mutations because they have complete remission at a rate of 80–90%. Even though recurrence is frequent, 80% of patients will not require hemodialysis [ 24 , 25 ].
Current therapeutic options for aHUS are plasma exchange and eculizumab. Empirically, TPE is considered as the first-line treatment. It assists in clearing abnormal complement-related factors such as anti-CFH autoantibodies and provides complement-regulatory factors to the circulation [ 26 ]. Although TPE is associated with a reduction of mortality from 50% to 25%, in a 3-year follow-up, 48% of pediatric patients and 67% of adult patients died or progressed to ESRF [ 15 , 26 , 27 ]. TPE combined with immunosuppressive drugs such as corticosteroids and azathioprine or mycophenolate mofetil and an anti-CD20 antibody (rituximab) has shown long-term dialysis-free survival in 60–70% of patients [ 10 , 28 ].
Eculizumab has become the gold standard treatment for aHUS with a good efficacy and safety profile. It is a monoclonal antibody against C5. It blocks the cleavage of C5 to C5a and C5b. C3b binding to C6, C7, C8, and C9 is prevented, and formation of MAC is blocked [ 29 ]. Eculizumab is recommended as the first-line therapy for children because of their increased risk of developing catheter-related complications such as infections with regard to TPE [ 30 ]. The duration of treatment with eculizumab in aHUS is unknown. Original approval was for lifelong treatment, whereas emerging evidence suggests that aHUS may not require indefinite eculizumab treatment and cessation may help mitigate sequelae of therapy [ 31 ]. However, when eculizumab is unavailable in resource-poor settings such as Sri Lanka, TPE may be the first treatment of choice. In our patient, TPE was a successful treatment, because she completely recovered following long-term therapy.
Diagnosing aHUS is a challenge for a variety of reasons. It is a rare disease entity; complement diagnostics are not reliable; 30–50% of patients do not have genetic or acquired mutations in the complement system; TMA occur in the context of complement-activating conditions; no specific test is available to confirm the diagnosis of aHUS; and aHUS is a diagnosis of exclusion. Early diagnosis and therapy improves outcome. aHUS should be considered in all patients with TMA.
Acknowledgments
We appreciate Dr. P. N. S. Premathilake, who provided assistance with medical writing.
Abbreviations
Authors’ contributions.
AWMW, NN, and SMDKS made the clinical diagnosis and supervised manuscript drafting. BMDBB drafted the first manuscript and reviewed the literature. AWMW, NN, SMDKS, EMSKS, BUEWDRT, BMDBB, NK, and RMBSSM were involved in the management of the patient. All authors read and approved the final manuscript.
Authors’ information
AWMW [MBBS, MD, MRCP(UK)] and NN (MBBS, MD), consultant nephrologists, are working at the Teaching Hospital Kandy, Sri Lanka. SMDKS (MBBS, MD, DTM), consultant transfusion physician, is working at Teaching Hospital Kandy. BMDBB, BUEWDRT, NK, and RMBSSM are senior registrars in nephrology working at the Teaching Hospital Kandy. EMSKS is a senior registrar in transfusion medicine working at Teaching Hospital Kandy.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
B. M. D. B. Basnayake, Email: moc.oohay@ekayansabbdmb .
A. W. M. Wazil, Email: moc.liamtoh@lizawmwa .
N. Nanayakkara, Email: moc.liamg@3134ahtnahsiN .
S. M. D. K. Samarakoon, Email: moc.oohay@nookaramas_d .
E. M. S. K. Senavirathne, Email: [email protected] .
B. U. E. W. D. R. Thangarajah, Email: moc.liamg@nihammarb .
N. Karunasena, Email: moc.oohay@einamahsin .
R. M. B. S. S. Mahanama, Email: moc.liamg@ilamirsahsihddub .

IMAGES
VIDEO
COMMENTS
Typical hemolytic uremic syndrome (HUS) is a leading cause of community acquired acute kidney injury in infants and young children. It is defined as a triad of microangiopathic hemolytic anemia, thrombocytopenia and renal insufficiency associated with Shiga toxin-producing Escherichia coli (1). Between February and November 2016, there was an ...
Abstract. Thrombotic microangiopathies (TMA) are microvascular occlusive disorders characterized by platelet aggregation and mechanical damage to erythrocytes, clinically characterized by microangiopatic haemolytic anemia, thrombocytopenia and organ injury. We are reporting a case of a woman patient with severe hemolytic uremic syndrome ...
Hemolytic uremic syndrome (HUS) is a thrombotic microangiopathy (TMA) characterized by thrombocytopenia, microangiopathic hemolytic anemia, and acute kidney injury. HUS is most commonly caused by Shiga toxin (typical HUS) or, less commonly, infections or genetic abnormalities activating the alternative complement pathway (atypical HUS). Additional causes can be secondary to malignancy ...
Contributors. Hemolytic uremic syndrome, or HUS for short, is a condition characterized by the triad of microangiopathic hemolytic anemia, or breakdown of red blood cells inside small blood vessels, thrombocytopenia, or low platelets, and acute kidney injury. Alright, now first, let's quickly review the physiology behind hemostasis or clotting.
Introduction: Typical hemolytic uremic syndrome (HUS) is a leading cause of community acquired acute kidney injury in infants and young children. It is defined as a triad of microangiopathic hemolytic anemia, thrombocytopenia and renal insufficiency associated with Shiga toxin-producing Escherichia coli. In this case of HUS that we are going to ...
Hemolytic uremic syndrome is a complex disease that impacts multiple body systems. Knowledge gained from cases has increased understanding of etiologic factors, presenting symptoms, diagnostic laboratory findings, and the disease process. In rare cases, severe neurological symptoms are evident. This 20-year-old woman presented with bloody ...
The aim of this article is to highlight a case study that employed Henderson's principles and plan of care to assist a child with a diagnosis of hemolytic uremic syndrome (HUS) through the performance of activities centering on achieving health and recovery. ... hemolytic uremic syndrome; nursing care plans; Virginia Henderson; Rights and ...
The remaining 10%, without any preceding diarrhea, are diagnosed with atypical HUS.Aim The objective of the study was the presentation the case of two patient with hemolytic uremic syndrome ...
Abstract. Hemolytic uremic syndrome is a complex disease that impacts multiple body systems. Knowledge gained from cases has increased understanding of etiologic factors, presenting symptoms ...
Hemolytic Uremic Syndrome ... solutions to challenging patient care issues. Case studies or descriptions may be submitted with or without discussion or solutions. References, tables, gures, and illustrations can be included. Materials or inq uiries should be directed to Oncology Nursing Forum Associate Editor Susan Moore, RN, MSN, ANP, ...
Diagnosing Hemolytic Uremic Syndrome (HUS) is done clinically, based on the classical triad of microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury. HUS can be established by laboratory examination, such as a complete blood count, with differential, and peripheral blood smear, renal functions studies, and urinalysis.
Abstract. Hemolytic uremic syndrome (HUS) is a triad of thrombocytopenia, microangiopathic hemolysis, and acute kidney injury, within the broader category of thrombotic microangiopathy (TMA). The most common form of HUS in children is due to Shigatoxin-producing E. coli infection, although other forms due to invasive pneumococcal disease ...
Background Although atypical hemolytic uremic syndrome (aHUS) is a life-threatening clinical entity that was characterized by thrombotic microangiopathy (TMA) with the activation of the complement system and the efficient treatment of eculizumab, the clinical features of aHUS have been unclear because of the rare incidence. Case presentation We retrospectively analyzed 4 aHUS cases at a single ...
Hemolytic-Uremic Syndrome / therapy. Humans. Patient Care Planning*. Renal Dialysis. The nursing interventions necessary to care for this critically ill child were professionally challenging. Meeting the psychosocial, educational, and supportive needs of the family during this period of uncertainty, as well as, providing the dialysis therapy ...
The hemolytic uremic syndrome (HUS) is defined by the simultaneous occurrence of microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury [ 1 ]. It is one of the main causes of acute kidney injury in children. Although all pediatric cases exhibit the classic triad of findings that define HUS, there are a number of various ...
Abstract. Typical hemolytic uremic syndrome (HUS) in adults is an uncommon clinical occurrence and has been rarely reported in the literature. Typical HUS is mainly caused by Shiga toxin-producing Escherichia coli (STEC) and is typically a pediatric disease. Worldwide outbreaks have been reported, one of the largest and most recent being in ...
Cathy discusses hemolytic uremic syndrome. She explains the pathophysiology, risk factors, signs/symptoms, expected labs, treatment, and prevention of hemoly...
Atypical hemolytic uremic syndrome (aHUS) is an ultrarare disease affecting approximately 2 cases per 100,000 per year, of which children comprise 0.26-0.75 cases per 100,000 [].It is a rapidly progressive illness, frequently causing severe acute kidney injury requiring dialytic support and multisystem complications [].Dysregulation of the alternative complement pathway is key to its ...
Hemolytic uremic syndrome (HUS) is a condition that affects blood vessels in your kidneys. It damages your kidneys and may cause blood clots. E.coli is the most common cause of HUS. In most cases, symptoms include diarrhea, abdominal pain, high blood pressure and low urine output. Treatment includes IV fluids, IV blood plasma and medications.
The aim of this article is to highlight a case study that employed Henderson's principles and plan of care to assist a child with a diagnosis of hemolytic uremic syndrome (HUS) through the performance of activities centering on achieving health and recovery. ... Keywords: Virginia Henderson; hemolytic uremic syndrome; nursing care plans. MeSH ...
Introduction. Hemolytic uremic syndrome (HUS) is a thrombotic microangiopathy (TMA) characterized by thrombocytopenia, microangiopathic hemolytic anemia, and acute kidney injury. HUS is most commonly caused by Shiga toxin (typical HUS) or, less commonly, infections or genetic abnormalities activating the alternative complement pathway (atypical ...
Hemolytic-uremic syndrome (HUS) is one of the most common etiologies for acute kidney injury and an important cause of acquired chronic kidney disease in children. 1 HUS is generally classified into two main types: typical or diarrhea-associated (D+HUS) and atypical (aHUS) or diarrhea-negative HUS. In children, 90% of HUS cases are associated ...
In conclusion, our study, although limited by the small size of the sample examined, excludes a protective role by BF on STEC-HUS course. However, in our territory, a surveillance protocol for bloody diarrhea has been activated [ 4 ], which recognized, in the period 06/2018 to 30/11/2022, out of a total of 2409 cases, 201 STEC infections (8.34%).
Conclusion. The atypical hemolytic uremic syndrome is a rare disease entity requiring a high index of suspicion to diagnose. It is a diagnosis of exclusion. Early diagnosis with prompt treatment will render a better outcome. The atypical hemolytic uremic syndrome needs to be considered in all patients with thrombotic microangiopathy.
Case study hemolytic uremic syndrome scenario mark is who lives at home with his parents and sister. the family has city water and black lab, which is in good ... Concepts Of Nursing: The Childbearing/Child Caring Family (NUR 230) ... Hemolytic Uremic Syndrome Scenario Mark is a 4-year-old who lives at home with his parents and two-year-old ...