An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.12(8); 2020 Aug


Advantages and Disadvantages of the Ketogenic Diet: A Review Article
Jennifer t batch.
1 Internal Medicine, Orange Park Medical Center, Orange Park, USA
Sanjay P Lamsal
2 Radiology, University of Florida Health Jacksonville, Jacksonville, USA
Michelle Adkins
3 Pharmacy, University of Florida, Gainesville, USA
Senan Sultan
4 Endocrinology, Orange Park Medical Center, Orange Park, USA
Monica N Ramirez
5 Pharmacy, University of Florida Health Jacksonville, Jacksonville, USA
The ketogenic diet (KD) has gained immense popularity during the last decade, primarily because of its successful short-term effect on weight loss. In the United States, KD is utilized in a variety of patient populations for weight management, despite limited evidence regarding its efficacy and risks. This literature review provides an evaluation of data on the benefits and risks associated with the chronic use of KD, including its metabolic, endocrinological, and cardiovascular effects.
Introduction and background
Obesity is classified based on the body mass index (BMI) of the individual. A BMI of 18.5-24.9 kg/m 2 is considered to be the normal range, while a BMI of 25.0-29.9 is considered overweight and a BMI ≥30 is classified as obese (further classified as obesity class I if BMI is between 30.0-34.9, class II if BMI is between 35.0-39.9, and class III if BMI is ≥40.0). In 2016, the World Health Organization (WHO) reported that more than 1.9 billion (39%) adults were overweight globally and of these, over 650 million (13%) were obese [ 1 ]. Obesity is associated with multiple comorbidities including type 2 diabetes, hypertension, cardiovascular disease (CVD), cancer, sleep apnea, and obesity-hypoventilation syndrome (OHS). The effectiveness of different types of diets based on different macronutrient restrictions has been a topic of debate for the past few years. Some researchers support restriction in carbohydrate (CHO), while others endorse cutting down protein or fats [ 2 ].
This review article will focus on the ketogenic diet KD, which is defined as a low-carbohydrate diet (LCD) with a moderate amount of protein restriction to induce ketosis without restricting fat intake [ 3 ]. The concept of KD was initially developed in 1921 by Dr. Russel Wilder for the management of refractory seizures in pediatric patients [ 4 ]. Originally, the diet consisted of a 4:1 ratio of fat-to-CHO and protein. Fat provides upwards of 90% the caloric intake [ 5 ]. All variations of this diet, whether involving animal- or plant-based derivatives, are based on severely restricting overall intake of CHO with a goal of bringing it down to less than 50 g/day. A well-formulated KD limits protein intake moderately to less than 1 g/lb body weight, or 1.5 g/lb body weight for individuals performing heavy exercises. Additionally, the diet does not restrict fat intake while decreasing appetite and caloric intake, resulting in weight loss observed after the initiation of the diet [ 6 ].
Following CHO deprivation and depletion of glycogen stores, the body undergoes metabolic changes to provide an energy source for the body through gluconeogenesis and ketogenesis. Gluconeogenesis can be sustained for three days with adherence to an LCD, and subsequently, additional energy sources are necessary to meet the metabolic requirements of the body and brain. This is where the process of ketogenesis becomes indispensable, and the formation of ketone bodies is then used as the primary energy source by cells with mitochondria and, most importantly, the brain [ 6 ].
KD has been shown to effectively lead to weight loss, reduction in hyperinsulinemia, and improvement in insulin sensitivity. However, patients diagnosed with diabetes on insulin or oral hypoglycemic agents may suffer severe hypoglycemia if their medication regimen is not properly managed during the initiation of KD. Furthermore, the diet is limited and/or contraindicated in patients with liver failure, pancreatitis, inborn disorders of fat metabolism, primary carnitine deficiency, carnitine palmitoyltransferase deficiency, carnitine translocase deficiency, porphyria, and pyruvate kinase deficiency [ 6 ]. Common short-term side effects resulting from the initiation of KD have been referred to as “keto flu,” which encompasses symptoms including fatigue, headache, dizziness, nausea, vomiting, constipation, and low exercise tolerance [ 6 ]. Symptoms typically resolve after a few days to weeks as the body adjusts to the low CHO, ketogenic state. Long-term side effects include hepatic steatosis, kidney stones, hypoproteinemia, and vitamin deficiency. While the benefits of following KD have been extensively reported, long-term compliance with KD is a limiting factor. The sustainability of the diet has been called into question, and the prognosis of the diet’s effects after discontinuation must be examined.
1. Ketogenic diet and cardiovascular risk factors
Dyslipidemia
In a systematic review and meta-analysis of clinical trials performed by Santos et al., a total of 23 randomized controlled trials corresponding to 17 clinical investigations were analyzed. The authors concluded that LCD has positive effects on body weight, BMI, abdominal circumference, blood pressure, high-density lipoprotein cholesterol (HDL-C), triglycerides, glycemia, hemoglobin A1c (HbA1c), insulin, and C-reactive protein (CRP) [ 2 ]. However, despite the positive impact on cardiovascular risk factors, there is insufficient data to support KD in the long term as the studies were of relatively shorter duration, ranging from three to 36 months only.
In another meta-analysis performed by Bueno et al., a total of 13 randomized controlled trials were examined. The authors reported statistically significant results in the first six months of intervention, but at longer periods of 12-24 months, the statistical significance of outcomes decreased. In a majority of the studies analyzed, CHO intake was higher than the protocol allowed, which was less than 50 g of CHO per day. These similarities found during the follow-up period were likely a contributing factor to the observed decrease in statistical significance [ 7 ]. Also, participants following a very-low-carbohydrate ketogenic diet (VLCKD) had a significantly greater increase in low-density lipoprotein cholesterol (LDL-C) levels when compared to participants following a low-fat diet (LFD) (95% CI: 0.04 to 0.2; p=0.002). This increase in LDL-C may subsequently lead to the development of accelerated atherosclerosis and increases the risks associated with CVD.
The lack of evidence regarding long-term cardiovascular implications indicates that making recommendations against or in favor of KD should be a topic of further discussion. In a randomized controlled trial performed at the outpatient care of the Philadelphia Veterans Affairs Medical Center among adults of ≥18 years old with a BMI of ≥35 kg/m 2 , 64 participants were assigned to an LCD and 68 participants were assigned to a conventional diet. Analysis at one year of initiating the study found favorable metabolic effects on atherogenic dyslipidemia and glycemic control with participants on an LCD compared to participants on a conventional diet [ 8 ]. The mean weight change for participants assigned to the LCD group was -5.1 ±8.7 kg, while it was -3.1 ±8.4 kg for the conventional diet group. However, the differences in weight change were not significant [-1.9 kg (95% CI: -4.9 to 1.0 kg), p=0.20]. This reinforces the low sustainability of a long-term LCD and the high likelihood of regaining the weight lost.
Interestingly, multiple studies mention a “favorable” lipid profile associated with KD because it increases HDL-C and decreases triglyceride levels. Distinguishably, increases are observed with LDL-C and total cholesterol. A review article analyzing five randomized controlled trials concluded that, after six months, individuals assigned to an LCD lost more weight than individuals assigned to an LFD with a weight difference of -3.3 kg (95% CI: -5.3 to -1.4 kg). Nevertheless, this significant difference was not identified after one year of intervention (95% CI: -3.5 to 1.5 kg). Triglyceride levels had a significant decrease [-22.1 mg/dl (95% CI: -38.1 to -5.3 mg/dl)], and HDL-C levels underwent a significant increase [4.6 mg/dl (95% CI: 1.5 to -8.1 mg/dl)] in the LCD group when compared to the LFD group. Conversely, LDL-C and total cholesterol changed more favorably in the LFD group after six months of the intervention [ 9 ]. The mechanism postulated for this is mediated through lower CHO intake, inducing suppressed insulin production. Concomitantly, decreased insulin production inhibits 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase activation and stimulates HMG-CoA lyase involved in ketone production. Thus, following a low CHO diet inadvertently leads to increased production of LDL-C and hypothetically promotes atherosclerotic properties.
Differentiation in Fat Sources
As reported by Seidelmann et al. in a prospective cohort study and meta-analysis, it is not only a matter of CHO restriction but also the quality of food ingested. The study’s primary outcome measure was all-cause mortality. After multivariable adjustment and a median follow-up period of 25 years, a U-shape association was observed between the percentage of energy consumed from CHO and mortality [pooled hazard ratio (HR): 1.20 (95% CI: 1.09 to 1.32 for low CHO consumption); pooled HR: 1.23 (95% CI: 1.11 to 1.36 for high CHO consumption)] in the Atherosclerosis Risk in Communities (ARIC) cohort. The authors emphasized that both low (<40%) and high CHO consumption (>70%) conferred higher mortality when compared with moderate CHO intake. Further analysis of the results demonstrated that mortality was worse when fat and protein sources were animal-derived instead of plant-derived. A relationship may exist between decreasing mortality rates and long-term approach considerations in the replacement of CHO with plant-based fats and proteins such as vegetables, nuts, and whole grains [ 10 ].
Hypertension
A meta-analysis that included 13 studies aimed to examine whether participants assigned to a VLCKD (diet restriction to ≤50 g CHO/day) would achieve better long-term body weight and cardiovascular risk factor management when compared with participants assigned to a conventional LFD (diet restriction to <30% energy from fat). In the overall analysis, those assigned to the VLCKD group showed a statistically significant decrease in their primary outcome: body weight: -0.91 kg (95% CI: -2.49 to -0.37). Additionally, of the 13 studies, 11 were included in the systolic blood pressure (SBP) and diastolic blood pressure (DBP) subgroup analyses. A statistically greater reduction in DBP [-1.43 mmHg (95% CI: -2.49 to -0.37)] was reported in participants assigned to a VLCKD compared to participants assigned to an LFD. Furthermore, an increase was reported in secondary endpoints: HDL-C: 0.09 mmol/l (95% CI: 0.06 to 0.12), LDL-C: 0.12 mmol/l (95% CI: 0·04 to 0·2), and triacylglycerol (TAG): -0.18 mmol/l (95% CI: -0.27 to -0.08) [ 7 ].
In contrast, a meta-analysis of 11 randomized controlled trials proposed to assess the effects of LCD vs LFD on weight loss and risk factors of CVD reported that cardiovascular risk factors such as SBP and DBP were not found to be statistically significant among both groups. A dietary intervention of six months or longer was implemented, and participants assigned to the LCD group had greater reductions in body weight [-2.17 kg (95% CI: -3.36 to -0.99)] and TAG [-0.26 mmol/l, (95% CI: -0.37 to -0.15)]. On the other hand, increases in LDL-C [0.16 mmol/l (95% CI: 0.003 to 0.33)] and HDL-C [0.14 mmol/l, (95% CI: 0.09 to 0.19)] were observed [ 11 ].
2. Effects of the ketogenic diet on endocrinology and metabolism
Polycystic Ovarian Syndrome
KD has been postulated to positively impact women diagnosed with polycystic ovarian syndrome (PCOS). Women with PCOS experience symptoms of irregular/absent menses, infertility, obesity, and other phenotypical effects of hyperandrogenism such as hirsutism. PCOS is closely associated with other metabolic and endocrinological irregularities, which include insulin resistance, hyperinsulinemia, type 2 diabetes mellitus, dyslipidemia, and hyperandrogenism [ 12 ]. PCOS is accompanied by key features such as insulin resistance, androgen excess, and abnormal gonadotropin dynamics. In turn, treatment is targeted towards improving insulin resistance, weight loss, decreasing luteinizing hormone (LH) and follicular stimulating hormone (FSH) ratios, and excess androgens. A study by Mavropoulos et al. implemented KD for women between the ages of 18-45 years diagnosed with PCOS, with a BMI greater than 27 kg/m2, and no other serious medical conditions. Participants adhered to a six-month period of strict KD consisting of less than 20 g of CHO per day with unlimited consumption of animal-based foods. After 24 weeks, the results of the study (pre- and post-design) showed a statistically significant decrease in fasting serum insulin (23.5 to 8.2, p=0.002), LH-to-FSH ratio (2.23 to 1.21, p≤0.05), and free testosterone (2.19 to 1.70, p≤0.05). Furthermore, the study subjects had an overall mean body weight change from baseline of -12.1% and a mean decrease in BMI of 4.0 kg/m2 (p=0.0006) [ 12 ]. Despite the results of the pilot study demonstrating a positive impact, there are limitations in generalization due to the small sample sizes of the study.
A crossover study by Gower et al. included participants with PCOS who were randomly assigned to either a standard diet or an LCD. The results demonstrated that LCD can lead to decreases in glycemia, fasting insulin, testosterone, and insulin sensitivity. However, the study had limitations due to the broad age range of participants and the small sample size, thereby rendering it inadequate in terms of generalizability [ 13 ]. Similar results were reported by Paoli et al., with significant reductions in BMI, glycemia, insulin, LDL-C, HDL-C, triglycerides, LH, testosterone, and dehydroepiandrosterone sulfate (DHEAS). Even though a reversal of the LH-to-FSH ratio was observed initially, it was not reported after 12 weeks. Limitations of this study include small sample size, single-arm design, lack of infertility measurements, and a short intervention time interval [ 14 ].
Diabetes Mellitus and Insulin
The term “Ketogenic Diet” may lead to apprehension in diabetic patients given its association with the well-known, life-threatening condition of ketoacidosis. It is important to note that during nutritional ketosis, the concentrations of beta-hydroxybutyrate and acetone are of low levels and do not cause any alterations in the pH of blood [ 6 ]. Management of type 1 and type 2 diabetes generally consists of medication adjustments targeted toward glycemic control and an HbA1c level of <7%. As mentioned previously with regard to patients with PCOS, the same benefits of following a VLCKD apply to patients with diabetes as well. They include decreased glycemia, lower levels of fasting insulin, decreased insulin resistance, and potentiating decreased requirements of insulin and/or oral glycemic medications. Thus, VLCKD has become popular among patients suffering from diabetes and obesity; nevertheless, the appropriateness of this diet is still debated.
In an outpatient clinic study by Yancy et al., overweight patients diagnosed with type 2 diabetes were made to follow a VLCKD throughout 16 weeks with the primary outcome measure of monitoring blood glucose control through HbA1c levels. The 28 participants enrolled were restricted to less than 20 g of CHO per day. At the end of the 16-week timeframe, HbA1c decreased from 7.5 ±1.4% to 6.3 ±1.0% (p<0.001). The absolute decrease in HgA1c was approximately 1.0% in 11 participants (52%). The relative decrease in HgA1c from baseline was >10% in 14 participants, and >20% in six participants. Furthermore, seven participants had their baseline diabetic medications discontinued, 10 participants had their baseline medications decreased, while four participants had unchanged requirements regarding baseline medications [ 15 ].
A similar observational study was performed by Leow et al. to evaluate the glycemic benefits of a VLCKD in patients diagnosed with type 1 diabetes. The study had a total of 11 eligible participants based on the study inclusion criteria, which included type 1 diabetes of ≥2 years, not taking any medications other than insulin, fasting blood beta-hydroxybutyrate levels of ≥0.4 mmol/l, and C-peptide levels of <0.05. Participants were required to follow a VLCKD with ingestion of less than 55 g of CHO a day for more than six months. The median duration of intervention was 1.5 years (range: 0.6-3 years). All participants had their HbA1c, C-peptide, beta-hydroxybutyrate levels, lipoprotein profile, markers of liver and kidney function, height, body mass, and blood pressure measurements taken after overnight fasting. The participants were found to have a mean HbA1c of 5.3% ±0.4%; mean and median blood glucose levels determined from continuous glucose monitoring were 5.8 ±1.2 and 5.5 (3.1-8.4) mmol/l, respectively. Daily blood glucose variability, expressed as standard deviation (SD) and coefficient of variation, was 1.5 ±0.7 mmol/l and 26.4% ±8.0%, respectively. The mean and median magnitude of postprandial blood glucose excursions were 0.8 ±1.5 and 0.5 (0-2.2) mmol/l, respectively. Hypoglycemic events were detected with continuous glucose monitoring; patients experienced 0.9 (0.0-2.0) episodes of hypoglycemia per day, defined as blood glucose levels of <3.0 mmol/l [ 5 ]. The results of this study indicate that adherence to KD in type 1 diabetics is associated with well-controlled HbA1c levels and minimal glycemic variability. Although this shows some evidence regarding the normalization of HbA1c, the diet comes with increased risks of hypoglycemic episodes. Hence, it is important to emphasize that insulin regimens, as well as oral hypoglycemic agents, must be closely monitored and adjusted in any diabetic patient following a VLCKD regimen.
Long-term adherence to KD is a major challenge and that is why this type of diet is considered non-sustainable. A comparison of different meta-analyses, review articles, and interventional studies revealed that no uniformity was established in the reported results. The limitations of most studies are attributed to small sample sizes, short duration of interventions, and high participant dropout rates. Due to the above-mentioned issues, even though some studies show positive results, we cannot consider them applicable to the general population; especially given that patients with diabetes or obesity often have other comorbid conditions such as dyslipidemia and CVD.
In a controlled study involving 20 participants receiving a nutritional intervention with VLCKD, a significant improvement in anthropometric and biochemical parameters was observed after eight weeks of therapy. This included a reduction in BMI, LDL-C, triglycerides, insulinemia, and liver transaminases. Additionally, it was reported that VLCKD also reduced inflammation [ 16 ]. Limitations once again include small sample size and a short period of intervention. In a meta-analysis of 11 studies, significant weight reductions were reported in the LCD groups when compared to LFD groups. Interestingly, the authors mentioned that this was attributed to lower energy intake rather than the macronutrient composition [ 11 ].
The aim of The Diet Intervention Examining the Factors Interacting with Treatment Success (DIETFITS) randomized clinical trial was to determine the effect of healthy low-fat (HLF) diet vs a healthy low-carbohydrate (HLC) diet on weight change and if genotype pattern or insulin secretion were related to the dietary effects on weight loss. The clinical trial, involving 609 overweight adults, did not demonstrate any results of statistical significance regarding its primary outcome measure, which was weight change with an HLF diet or HLC diet over 12 months. Similarly, neither type of diet showed outcomes of statistical significance in genotype pattern interaction or baseline insulin secretion interaction with 12-month weight loss. It can be assumed that it would be difficult to identify which type of diet is better for any individual. Thus, this leads us to conclude that dietary modifications remain key to successful weight loss [ 17 ].
Lastly, a multicenter randomized controlled trial by Ebbeling et al. randomly assigned participants who achieved target weight loss to either a low CHO, moderate CHO, or high CHO diet. This 20-week interventional study found that independent of body weight, total energy expenditure was significantly greater in participants assigned to low CHO diets compared to high CHO diets of similar protein content. Moreover, a significant decrease in metabolic hormonal response with both ghrelin and leptin was reported in the participants assigned to the low CHO diet compared with those assigned to the high CHO diet. These results led the authors to conclude that these metabolic effects and the correlation of dietary quality with energy expenditure may be helpful in the treatment of obesity [ 18 ].
Conclusions
Based on our review, within the first 6-12 months of initiating KD, transient decreases in blood pressure, triglycerides, and glycosylated hemoglobin, as well as increases in HDL and weight loss may be observed. However, the aforementioned effects are generally not seen after 12 months of therapy, as the changes reported in the studies we reviewed are not statistically significant. Further research is warranted to evaluate the long-term implications of KD. Despite the diet's favorable effect on HDL-C, the concomitant increases in LDL-C and very-low-density lipoproteins (VLDL) may lead to increased cardiovascular risks. Additionally, the dietary restrictions required to sustain ketosis may actually lead to its low sustainability. Unfortunately, most available studies lack generalizability and validity due to their small sample sizes and short study durations. Due to the limited amount of robust studies and lack of strong evidence evaluating the diet’s potential risks, recommendations supporting VLCKD in patients with no comorbidities, or cardiometabolic and endocrinologic diseases should be made at the provider’s discretion.
Funding Statement
This research was supported (in whole or in part) by HCA and/or an HCA-affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA or any of its affiliated entities.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Ketogenic Diet: Overview Presentation
Diet as the way to lose weight.
- Many people want to lose weight.
- Every person has their reasons.
- The issue of weight loss is relevant, but requires careful monitoring.
Often, many people think about losing extra weight. Every person has their reasons for doing so – one wants to lead a healthier life, while others want to attract people. One way or another, the questions of weight loss are acute for humanity; for this reason, quite often among the already known classic methods of weight loss new and radical options that can aggravate human health appear. In order to prevent unnecessary health issues and achieve the desired effect, nutritionists offer patients to use the rules of the popular ketogenic diet.
Everything New is Actually Well-forgotten Old
- In the early twentieth century, ketogenic diet was used to treat epilepsy.
- Carbohydrate deficiency in the diet caused insulin reduction.
- With the advent of drugs, the diet has been relegated to the background.
- It was only thirty years ago that nutritionists rediscovered the ketogenic diet.
Recently, ketogenic diets have been used by a large number of young and adult people, and this method of starvation has become incredibly popular. However, if one looks at the historical context of ketogenic diets, it is clear that the diet has a rather long and mixed history. This diet was first introduced in the early 20th century to treat diseases of the nervous system, particularly epilepsy (Rho 5). A prerequisite for the appearance of the diet was the use of fasting: when food ceased to arrive, insulin stopped being produced in the body, significantly affecting the central nervous system (Rho 5). Such fasting was indeed valid, but it was impossible to use it for a long time. The situation was particularly difficult for children, as deprivation of food posed a serious health hazard (Rho 6). At that time, a diet similar to healing fasting was developed, but it gave the body energy from fats. It showed excellent results – people with epilepsy practically stopped having seizures. Indeed, the success of the ketogenic diet was not celebrated for long: soon, there were specially developed drugs, as a result of which the diet was forgotten for some time. It was only in the 1990s, when the problem of non-universal antiepileptic drugs for patients matured, that such a diet became discussed again. Information about ketogenic diets started to spread in the media, and the “new” weight-loss remedy began to be popularized.

The Mechanism of the Diet
The rule is simple: lack of carbohydrates leads to burning of fats. The body draws energy from burning its own deposits or with food – as a result, the body weight is reduced.
The meaning of the ketogenic diet is that when carbohydrates are stopped when glucose concentration in blood decreases, the body searches for other sources of energy and burns fat. This process involves both fats from food and the body’s supplies. In the reorganization of diet, the liver produces ketone bodies (acetoacetate), which participate in the oxidation of fatty acids and are used as energy by many organs, including the brain. This condition is called ketosis (Yancy, Mitchell, & Westman 1734). As glucose disappears from the blood, the production of insulin, a hormone that prevents fat burning, is reduced.

Scientific Evidence
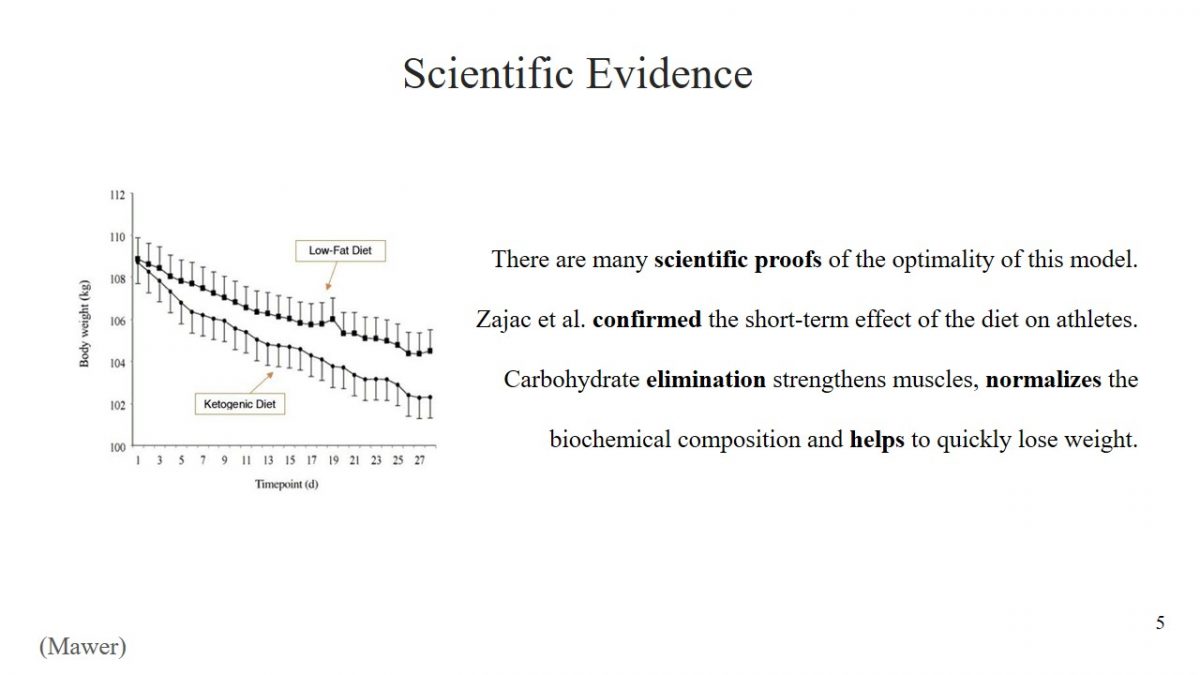
- There are many scientific proofs of the optimality of this model.
- Zajac et al. confirmed the short-term effect of the diet on athletes.
- Carbohydrate elimination strengthens muscles, normalizes the biochemical composition and helps to quickly lose weight.
The decision to use any diet must be meaningful, and the effectiveness of starvation has been scientifically proven. For example, Zajac et al. found that a ketogenic diet has a positive effect on the performance of athletes (2496). The study determined what changes would result from changing diets to predominantly fatty young cyclists. Scientists concluded that the short-term effect of a ketogenic diet could preserve muscle structures in athletes after exercise, reduce body weight, and improve biochemical composition. An incredible achievement of Zajac et al. is the discovery that a ketogenic diet has a positive effect on a person’s breathing ability (2498).
Who is Ketogenic Diet Good for
- Meat lovers;
- Those who want to save muscle mass;
- Patients with diabetes mellitus.
Despite the complexity of the first days and strict diet restrictions, for some people’s ketogenic diets are ideal. It is worth trying out for the following categories of people who want to lose weight:
- Meat lovers. Many diets offer a drastic reduction in meat-eating or complete rejection, but a ketogenic diet does not restrict it.
- Those who want to save muscle mass. Normally diets can reduce muscle mass, but a ketogenic diet does not destroy muscle fiber structures, which is more suitable for professional athletes.
- Patients with diabetes mellitus. Ketosis can control blood sugar levels (Yancy, Mitchell, & Westman 1734). It is a type of professional diet, so a doctor’s consultation is necessary.

Mistakes that are Made
Lack of education can lead to:
- Carbohydrate consumption.
- Consumption of large quantities of proteins.
Due to their lack of education, beginners often make typical mistakes when using a ketogenic diet. Most of these errors will neutralize the positive effects of the diet, but others can have adverse effects on health. The most common mistake is when a person neglects the basic rule of diet and eats small amounts of carbohydrates. However, it is essential to understand that even a slice of eaten bread delays the start of ketosis. The second error is the opposite: people often completely give up carbohydrates, but in addition, they increase the amount of protein they consume. Protein poisoning leads to the destruction of the liver, kidneys, and digestive system problems.
The most challenging part of the keto diet is getting used to an entirely new way of eating, especially the rejection of most foods with high carbohydrate content and the addition of fat. Large amounts of natural fat can be found in vegetable and animal oils. Low-carbohydrate meat products may include beef, poultry and eggs, and fish. Non-starchy foods such as avocados, tomatoes, cabbage, and broccoli are allowed types of vegetables. On a ketogenic diet, it is possible to eat fruits and berries such as raspberries, kiwi, lemons, and blueberries.
What Does a Person Get: Arguments “For”
- The scientific validity of diet.
- Rapidity of fat burning.
- Comfortable process.
- Long-term result.
- Cholesterol Control.
- Reducing epileptic seizures.
- Improving skin condition.
Those who decide to use ketogenic diet rules for weight loss should understand the benefits that await them. First, the effectiveness of such a diet has been scientifically proven (Zajac et al. 2493). Secondly, with such a diet, a person burns excess fat very quickly. Also, unlike other diets, the patient has no sense of hunger, so fasting is comfortable. Also, it should be noted that the result obtained is quite long term as the person is not hungry, and the body is not stressed. Several scientific studies demonstrate that a ketogenic diet improves cholesterol levels (“Pros and Cons” 1). This reduces the likelihood of cardiovascular disease. In addition, it should be remembered that a diet is right for people with epilepsy – the number of seizures and convulsions decreases (Feldman 36). In the world of cosmetology, there is a belief that a ketogenic diet has a positive effect on skin health by inhibiting the growth of acne and pimples (Feldman 36).
What a Person will Face: Arguments “Against”
- This diet is unbalanced
- Like any diet, it requires willpower.
- It is quite long, can be used for years.
- Side effects are possible.
It is essential to understand that there are no ideal weight loss models. A ketogenic diet is rich in deficiencies that can significantly worsen a person’s condition (Pros and Cons” 2). For this reason, it is necessary to consult a nutritionist before using such a diet.
- First, it should be noted that the ketogenic diet is unbalanced – it excludes the intake of several nutrients, vitamins, and trace elements.
- Secondly, it requires motivation and responsibility – one has to give up favorite dishes and regularly calculate the number of calories.
- The third disadvantage of the diet is duration: a ketogenic diet can last from 3-4 weeks to a year. There is no point in staying on a diet for less than three weeks because, during this time, the body will go through ketogenic adaptation and only begin to get all the benefits of this diet.
- It also has many side effects: constipation, nausea and vomiting, growth disorders, kidney stones, and changes in blood lipids.
What Else Should be Mentioned
What must be said is :
- This diet is not suitable for pregnant and sick patients.
- Long-term effects require careful consideration.
- Each diet is selected individually and requires analysis by a specialist.
The ketogenic diet has another disadvantage, which, however, cannot be unequivocally attributed to the general disadvantages. The fact is that this type of diet is not suitable for all population groups, and is contraindicated for medical reasons to pregnant women and people with kidney and liver diseases (Feldman 34). The effects of a long-term diet on healthy people have not yet been well understood. Dieticians are advised to choose a diet that can be sustained throughout life. Short-term effects may be successful, but the method is not suitable for everyone. Dietary recommendations are tailored to each individual and following the next trend without consulting a doctor is not recommended.
Works Cited
Feldman, Ellen. “Ketogenic Diet for Refractory Pediatric Seizures.” Integrative Medicine Alert , vol. 22, no. 9, 2019, pp. 32-37.
Mawer, Rudy. “ 10 Graphs That Show the Power of a Ketogenic Diet. ” heathline . 2018. Web.
Mawer, Rudy. “ The Ketogenic Diet: A Detailed Beginner’s Guide to Keto. ” heathline . 2018. Web.
“Pros and Cons of Low-Carb/Ketogenic Diets.” Nutrition Letter , vol. 37, no. 10, 2019, pp. 1-2.
Rho, Jong M. “How Does the Ketogenic Diet Induce Anti-Seizure Effects?” Neuroscience letters , vol. 637, no. 1, 2017, pp. 4-10.
Yancy, William S., Nia S. Mitchell, and Eric C. Westman. “Ketogenic Diet for Obesity and Diabetes.” JAMA Internal Medicine , vol. 179, no. 12, 2019, pp. 1734-1735.
Zajac, Adam, et al. “The Effects of a Ketogenic Diet on Exercise Metabolism and Physical Performance in Off-Road Cyclists.” Nutrients , vol. 6, no. 7, 2014, pp. 2493-2508.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, July 15). Ketogenic Diet: Overview. https://ivypanda.com/essays/ketogenic-diet-overview/
"Ketogenic Diet: Overview." IvyPanda , 15 July 2022, ivypanda.com/essays/ketogenic-diet-overview/.
IvyPanda . (2022) 'Ketogenic Diet: Overview'. 15 July.
IvyPanda . 2022. "Ketogenic Diet: Overview." July 15, 2022. https://ivypanda.com/essays/ketogenic-diet-overview/.
1. IvyPanda . "Ketogenic Diet: Overview." July 15, 2022. https://ivypanda.com/essays/ketogenic-diet-overview/.
Bibliography
IvyPanda . "Ketogenic Diet: Overview." July 15, 2022. https://ivypanda.com/essays/ketogenic-diet-overview/.
- Ketogenic Diets: Carbohydrate Count
- Ketogenic Diets and It Uses for Epilepsy Management in Children
- Ketogenic Diet: Potential Health Benefits and Risks
- The Aspects of the Ketogenic Diet
- Popular Diets: The Ketogenic Diet
- Aspects of the Ketogenic Diet: Pros and Cons
- Pros and Cons of Different Diets
- Going Keto: Is It Worth It?
- Very-Low-Calorie Ketogenic Diet and Kidney Failure
- Analysis of Low Carb and High-Fat Diets
- Canada Food Guide Overview
- Dietary Specifications: Medicinal Meals in China
- Steps in Ensuring a Healthy Diet in College
- The Process of Cheese Production
- Unhealthy Fad Diets: Fact or Hoax
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 30 November 2020
Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis
- Xiaojie Yuan 1 na1 ,
- Jiping Wang 1 na1 ,
- Shuo Yang 2 na1 ,
- Mei Gao 2 ,
- Lingxia Cao 2 ,
- Xumei Li 1 ,
- Dongxu Hong 1 ,
- Suyan Tian 3 &
- Chenglin Sun ORCID: orcid.org/0000-0003-3570-1918 1 , 2
Nutrition & Diabetes volume 10 , Article number: 38 ( 2020 ) Cite this article
35k Accesses
90 Citations
481 Altmetric
Metrics details
- Type 2 diabetes
At present, the beneficial effect of the ketogenic diet (KD) on weight loss in obese patients is generally recognized. However, a systematic research on the role of KD in the improvement of glycemic and lipid metabolism of patients with diabetes is still found scarce.
This meta-study employed the meta-analysis model of random effects or of fixed effects to analyze the average difference before and after KD and the corresponding 95% CI, thereby evaluating the effect of KD on T2DM.
After KD intervention, in terms of glycemic control, the level of fasting blood glucose decreased by 1.29 mmol/L (95% CI: −1.78 to −0.79) on average, and glycated hemoglobin A1c by 1.07 (95% CI: −1.37 to −0.78). As for lipid metabolism, triglyceride was decreased by 0.72 (95% CI: −1.01 to −0.43) on average, total cholesterol by 0.33 (95% CI: −0.66 to −0.01), and low-density lipoprotein by 0.05 (95% CI: −0.25 to −0.15); yet, high-density lipoprotein increased by 0.14 (95% CI: 0.03−0.25). In addition, patients’ weight decreased by 8.66 (95% CI: −11.40 to −5.92), waist circumference by 9.17 (95% CI: −10.67 to −7.66), and BMI by 3.13 (95% CI: −3.31 to −2.95).
KD not only has a therapeutic effect on glycemic and lipid control among patients with T2DM but also significantly contributes to their weight loss.
Similar content being viewed by others
The effects of vegetarian diets on glycemia and lipid parameters in adult patients with overweight and obesity: a systematic review and meta-analysis
Yang Xu, Guli Mo, … Chuan Li
The effects of low-fat, high-carbohydrate diets vs. low-carbohydrate, high-fat diets on weight, blood pressure, serum liquids and blood glucose: a systematic review and meta-analysis
Qing Yang, Xinyue Lang, … Yan Liang
Adherence to ketogenic diet in lifestyle interventions in adults with overweight or obesity and type 2 diabetes: a scoping review
Shiyu Li, Yan Du, … Jing Wang
Introduction
Diabetes mellitus (DM) is the world’s leading cause for motility and morbidity, and the disease has become a major public health burden worldwide. It is estimated that the prevalence of diabetes in adults worldwide is over 300 million, and it will increase by 55% by 2035 1 . Obesity or overweight is one of the essential risk factors for diabetes and contributes to a twice-higher risk to develop DM 2 , 3 . Thus, dietary therapy aiming at weight loss is typically recommended in clinical practice 4 . Due to the fact that diabetes and its complications affect many aspects of physiology, the benefits of weight reduction are not limited to glycemic control but are also related to many cardiovascular risk factors such as blood pressure, high-density lipoprotein (HDL), total cholesterol (TC) and triglyceride (TG) 2 .
Medical nutrition, as part of the comprehensive treatment of DM with obesity with a primary goal of weight reduction, is the most simple, effective and economical choice of intervention. The dietary approach for body weight reduction can be obtained from many strategies, including a low-calorie diet, a very low-calorie diet, high-protein diet, and so on. Ketogenic diet (KD), which contains a very low level of carbohydrates (<55 g/d) with the main energy sources of lipid and protein, and which causes ketosis and simulates the physiological state of fasting, has been well reported to be effective for weight loss and glycemic control 4 , 5 , 6 , 7 , 8 , 9 . Previous meta-analyses have proved the efficacy of KD in body weight reduction 2 , 10 , 11 ; however, systemic reviews on the effect of KD on weight reduction and glycolipid metabolism in patients with DM are still limited. Westman et al. 12 and Partsalaki et al. 13 demonstrated that KD improved type 2 diabetes mellitus (T2DM) by reducing the glycemic response caused by carbohydrate and improving potential insulin resistance. Leonetti et al. 14 and Walton et al. 15 reported reduced TG and TC with increased HDL levels after KD consumption for a lipid profile. However, controversies are still existing; studies revealed that a low-carbohydrate, high-fat diet may exacerbate the lipid profile in patients with diabetes, although glycemic control improved with hypoglycemic medications 16 , 17 , 18 . Therefore, the purpose of the current review was to conduct a meta-analysis on the effects of a KD in patients with diabetes.
Considering the potential benefits of KD in diabetes management and weight reduction, and considering fasting blood glucose and glycated hemoglobin A1c (HbA1c) as common biomarkers for long-term glycemic control, HDL, LDL, TC, and TG levels are included in the current analysis to determine the changes of metabolic disorders in glucose and lipid metabolism. In addition, the homeostatic model assessment of insulin resistance (HOMA-IR) is considered as a reflection of insulin resistance reversal.
Materials and methods
Literature search.
In this meta-analysis, only studies published in English were considered, which were identified by searching the PubMed and MEDLINE databases. The keywords used for this literature search are T2DM or diabetes mellitus, ketogenic diet, obesity, and human. The search was finished on September 20, 2019. This meta-analysis was planned and performed according to the Preferred Reporting Items for Systemic Reviews (PRISM) guideline (Fig. 1 ).
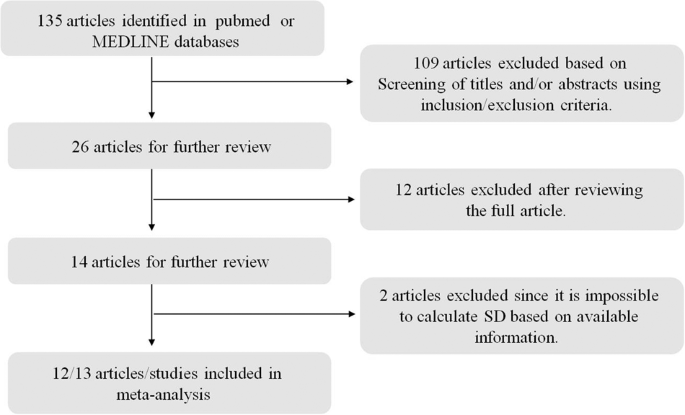
Only studies published in English were considered, which were identified by searching the PubMed and MEDLINE databases. The keywords used for this literature search are T2DM or diabetes mellitus, ketogenic diet, obesity, and human. The search was finished on September 20, 2019.
Inclusive/exclusive criteria
Studies that met the following inclusive criteria were included: (1) the disease of interest is type II diabetes; (2) the therapeutic diet under consideration is KD; (3) the study was carried out on humans; animal experiments are not included; and (4) the summary statistics of the mean difference between before and after KD (if both means for before and after measurements are available, then we took the difference of these two statistics to obtain the desired mean difference), their corresponding standard error or 95% CI (according to this, the standard error was calculated) or p values (according to this, the corresponding t statistics and subsequently the standard error were calculated) are available.
Exclusive criteria: (1) case report studies were excluded; (2) meta-analysis or review studies were excluded; (3) studies on other diseases rather than type II diabetes were excluded; and (4) if only the respective mean and standard errors were available, such studies were excluded given it is hard to get an accurate estimation for the standard error of mean difference (since both measurements were on the same patient, they should be correlated to each other, and hence it is impossible to estimate this correlation).

Statistical analysis
The effects of KD on type II diabetes were estimated by the mean difference after KD versus before KD and their corresponding 95% CIs in random-effects meta-analysis models or fixed-effect meta-analysis models. To determine which model should be used, heterogeneity among studies was evaluated by the Cochrane’s Q statistic corresponding p values and the I 2 statistics. If the p value was <0.05 and I 2 > 0.5, a random-effect meta-analysis model was used. Otherwise, a fixed-effect meta-analysis model was chosen. Additionally, potential bias was assessed by using funnel plots, in which effect sizes versus standard errors were diagrammed. All statistical analysis was carried out in the R software, version 3.5 ( www.r-project.org ) 19 , 20 , 21 .
There are 13 studies included in this meta-analysis; the details of these 13 studies are presented in Table 1 . In total, 567 subjects were included in the final meta-analysis. From the perspective of glucose metabolism, lipid metabolism, and weight control, the effects of KD on T2DM were systemically reviewed by comparing the after-intervention measures with before-intervention measures of several biomarkers for the same patient. The variables used to surrogate for carbohydrate metabolism are included fasting glucose level and HbA1c; for lipid metabolism TC, TG, HDL and LDL; and for weight loss body weight, BMI and waist circumference. For all variables except BMI and waist, random-effect models were adopted according to the Q statistic p value and I 2 statistics.
Using the meta-analysis method, we found that the fasting blood glucose level was decreased 1.29 mmol/l (95% CI: −1.78 to −0.79) after the intervention of KD, compared to before such an intervention (based on ten articles that have the summary statistics for the difference between after- and before-intervention measures). As far as HbA1c is concerned, we found that the reduced proportion of HbA1c is more significant after the KD implementation, with a difference of −1.07% (95% CI: −1.37 to −0.78), which is regarded as the ideal therapeutic effect of drugs that is possible to be achieved on HbA1c. The forest plots for these two carbohydrates metabolism indices are given in Fig. 2 .
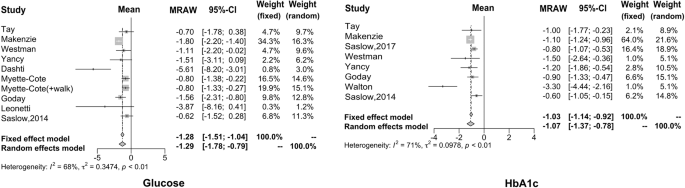
The reduced proportion of HbA1c is more significant after the KD implementation, which is regarded as the ideal therapeutic effect of drugs that is possible to be achieved on HbA1c.
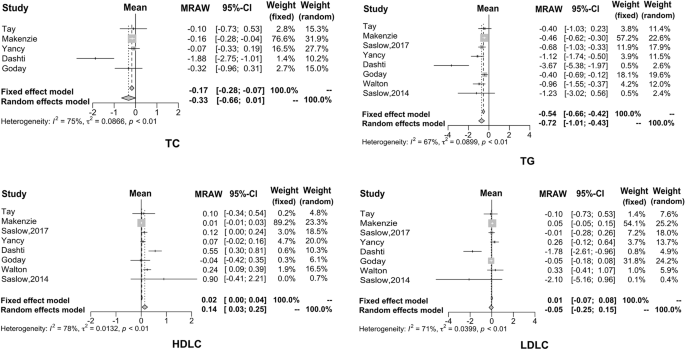
In this study, eight articles investigated the effect of KD on the lipid metabolism of diabetic patients, but only five papers analyzed total cholesterol. It can be seen that after KD consumption, TG decreased by 0.72 mmol/L (95% CI: −1.01 to −0.43), TC decreased by 0.33 mmol/L (95% CI: −0.66 to −0.01), and LDL decreased by 0.05 mmol/L (95% CI: −0.25 to −0.15). On the other hand, HDL increased by 0.14 mmol/L (95% CI: 0.03−0.25). The forest plots for these four biomarkers are shown in Fig. 3 .
It can be seen that after KD consumption, TG, TC, and LDL decreased. On the other hand, HDL increased.
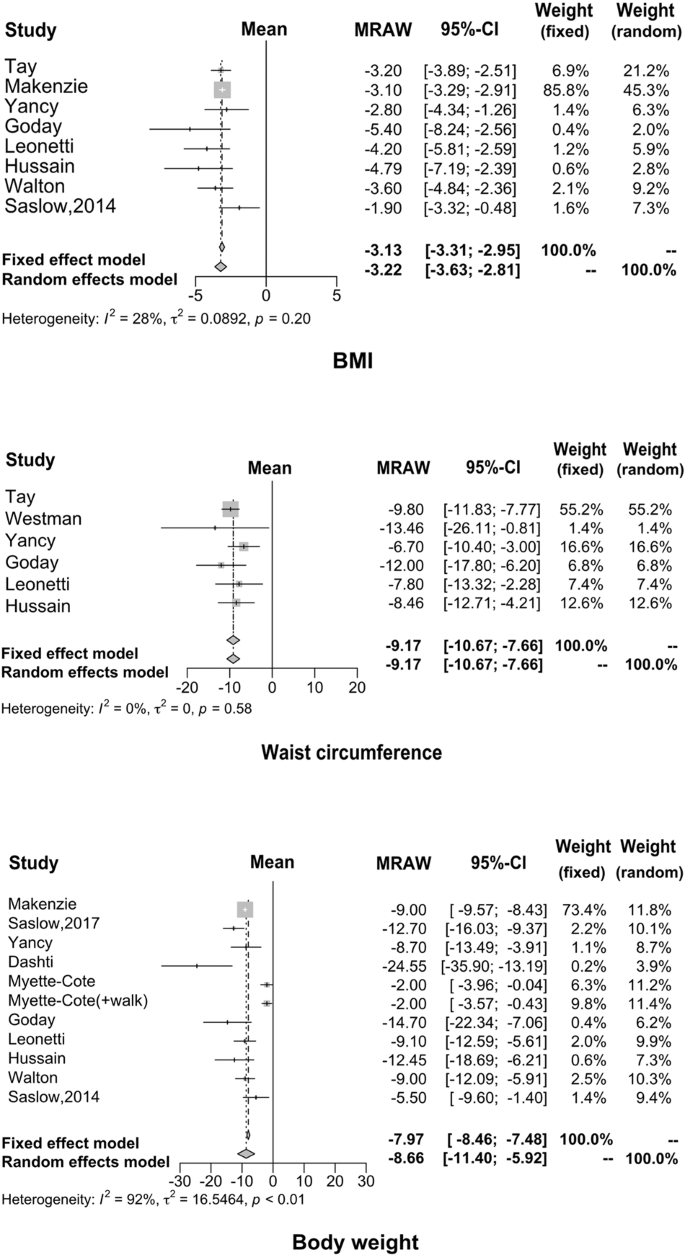
Regarding weight loss, many studies have demonstrated that KD has a positive effect by providing effective control over obesity. The results of our meta-analysis are consistent with previous results. Specifically, the average weight decreased by 8.66 kg (95% CI: −11.40 to −5.92), waist circumference reduced by 9.17 cm (95% CI: −10.67 to −7.66) and BMI reduced by 3.13 kg/m 2 (95% CI: −3.31 to −2.95), as shown in Fig. 4 .
Many studies have demonstrated that KD has a positive effect by providing effective control over obesity; our findings were consistent with the previous reports.
The American Diabetes Society (ADA) recommended physical activity, dietary management, and medical intake and other approaches should be applied simultaneously to manage blood glucose levels, and other abnormal metabolic factors. KD showed numerous health benefits to patients with T2DM 22 , 23 . KD provides energy through fat oxidation. When the human body experienced extreme hunger or very limited carbohydrate, the ketone body was produced and released to circulation by hepatic transformation of fatty acids 24 , 25 . Nutritional ketosis is different from severe pathological diabetic ketosis; the blood ketone body remained at 0.5−3.0 mmol/L with reduced blood glucose and normal blood pH, with no symptoms in nutritional ketosis 26 .
The possible mechanism for the health benefit of KD on patients with T2DM is that the extreme restriction of carbohydrate reduces the intestinal absorption of mono-saccharide, which leads to lower blood glucose level and reduces the fluctuation of blood glucose, and its effectiveness on regulating glucose metabolism was confirmed by a large body of evidence 27 , 28 . The current study analyzed 13 studies from literature focusing on diabetic patients; the results showed that the reduction of blood glucose ranges from 0.62 to 5.61 mmol/L. Higher reduction amplitudes were reported by Dashti 29 and Leonetti et al. 14 of 5.61 mmol/L (weight random 3.0%) and 3.87 mmol/L (weight random 1.2%), respectively; other reductions in blood glucose were all lower than 1.8 mmol/L. The possible reason for the higher reduction found in these two studies could be the higher blood glucose level included in the studies, and also that the average blood glucose concentration was above 10.0 mmol/L, leading to the possibility of a larger reduction; however, their contribution to the overall effect estimations in the meta-analysis was low. The average changes in fasting blood glucose after the KD consumption among the selected studies were −1.29 mmol/L, indicating the effectiveness of the KD in lowering fasting blood glucose.
No studies included in this meta-analysis evaluated the effect of KD on postprandial glucose level; unlike medications, dietetic therapy showed a long-term effect on glucose regulation 4 , 16 , and HbA1c was analyzed to evaluate the long-term effect of KD. HbA1c effectively reflects the blood glucose control in the past 2−3 months in patients with diabetes. It is reported that the risk of cardiac infarction and micro-vascular complications reduced by 14% and 37%, respectively, when HbA1c reduced by 1%. Therefore, the HbA1c level showed essential clinical significance in evaluating the blood glucose control, revealing the potential problems in the treatment and thereby guiding the therapeutic schedule 30 , 31 . Eight of the selected studies showed a reduction of HbA1c after KD consumption, the changes ranging from −0.6% to −3.3%; HbA1c reduced <1.5% in the majority of the studies included in the current analysis besides the study conducted by Walton (−3.3%; weight random 5.1%) 15 . The possible explanation for such strong improvement of HbA1c could be that Walton’s study had enrolled a limited number of patients and thus the compliance of patients to KD therapy can be guaranteed. Moreover, the studied subjects were newly diagnosed diabetic patients who were under dietary management without taking glucose-lowering medications; newly diagnosed subjects persist well in the study. Considering the causal factors comprehensively, the above study showed an ideal reduction in HbA1c. The average reduction of HbA1c was 1.07 in the current analysis of the selected eight studies, indicating that dietary management may also achieve the ideal therapeutic effects of medication.
HOMA-IR is considered as an indicator to evaluate the status of insulin resistance. Insulin resistance as a clinical characteristic of T2DM is closely related to obesity. Improving insulin resistance is one of the major targets in diabetes treatment 32 , 33 , 34 . However, studies focusing on the role of KD in the improvement of insulin resistance in patients with diabetes are very limited; most of the studies focused on the effect in obese subjects 35 , 36 . For instance, a controlled clinical trial aiming at the effects of KD consumption in obese people without diabetes revealed that HOMA-IR decreased by about 2.0 after KD consumption for 6 weeks 37 . The current analysis showed consistent changes in the studies that included HOMA-IR evaluation, with reduction ranging from −0.4 to −3.4; the reason for the significant reduction of 3.4 in the study by Tay et al. 38 is that the population included was obese diabetic patients with BMI higher than 30 kg/m 2 . Obesity is closely related to insulin resistance; KD consumption is confirmed to be effective in reducing body weight, and it is expected that KD may improve insulin resistance in obese diabetic patients 39 . During the ketogenesis, the sensitivity of the insulin receptor is promoted; therefore, KD not only ensures the supply of basic nutrients but also maintains a negative balance of energy, and reduces the fluctuation and reduction of insulin secretion caused by reduced carbohydrate intake as well, which eventually leads to improved insulin sensitivity 40 , 41 , 42 , 43 .
Consumption of KD not only improved glucose metabolism, but a large body of evidence has reported that KD improved lipid metabolism as well. Hussain et al. 4 reported that KD reduced TG and TC, and increased HDL level, thus ameliorating the status of dyslipidemia. In the present study, eight studies included showed results of lipid metabolism in diabetic patients after KD consumption; however, only five analyzed the TC levels. The current results showed the mean reduction of TG was 0.72 mmol/L, TC was 0.33 mmol/L, and LDL was 0.05 mmol/L, while the increase of HDL was 0.14 mmol/L. The higher amplitude of variation occurred in the Dashti et al. study 29 . This study reported that TG reduced by 3.67 mmol/L, TC reduced by 1.88 mmol/L, and LDL reduced by 1.78 mmol/L, while HDL increased by 0.14 mmol/L. Changes in the amplitude of the lipid biomarkers were all at the higher end in the above study. Both glucose and lipid metabolism showed great improvement after KD consumption in such a study; the characteristics of subjects recruited were closely correlated. The study recruited 31 obese subjects with hyperglycemia, dyslipidemia, and BMI over 30 kg/m 2 . The baseline TG, TC, and LDL were higher than those of typical patients with T2DM, which may contribute to the significant changes after the intervention. Consumption of KD showed a significant therapeutic effect in common patients with T2DM, including the Dashti 29 study. Disorders of lipid metabolism are particularly strong among patients with insulin resistance in T2DM. Dyslipidemia is lipotoxic to cells, leading to and/or aggravating insulin resistance. Its typical manifestation is the increase of TG and free fatty acid (FFA) 44 , 45 , 46 , 47 . Increased FFA is an independent pathogenic factor for insulin resistance and can possibly increase the risk for cardiovascular diseases 48 , 49 . Therefore, the improvement of dyslipidemia is beneficial for not only regulating insulin sensitivity but also controlling the occurrence and progression of diabetic complications 50 , 51 .
Numerous studies have confirmed the role of KD consumption in weight reduction in obese patients 35 , 36 , 37 , 40 , 41 , 42 , 43 , 52 ; the current meta-analysis focused on the effect of KD on weight reduction in obese diabetic patients. The results showed the average reduction of body weight was 8.66 kg, waist circumference was 9.17 cm, and BMI was 3.22 kg/m 2 , which were consistent with previous studies in nondiabetic patients. We also found that KD reduced systolic blood pressure by 4.30 (95% CI: −7.02 to 1.58) and diastolic blood pressure by 5.14 (95% CI: −10.18 to 0.10) in patients with T2DM, which possibly benefit from the improvement of body weight 51 .
Besides the mediation of glucose and lipid metabolism, KD may also benefit other clinical symptoms in diabetic patients, including insomnia, chills, constipation, pruritus, numbness of limbs, hypopsia, fatty liver, hypertension, and reduced cardiac function.
The potential side effects of KD were only mentioned in two of the studies 14 , 41 included in the meta-analysis; thus it is impossible to perform a systematic review in terms of the risks associated with KD consumption. Specifically, Goday and Leonetti’s 14 , 41 study investigated the adverse reactions of KD. Goday et al. 41 mentioned that fatigue, headache, nausea and vomiting were more common in the KD diet group after a 2-week intervention, while constipation and orthostatic hypotension were more common after 10 weeks. It was revealed by Leonetti et al. 14 that in the early stages of applying the KD, patients reported a sense of hunger, but it could be significantly alleviated with the progress of the intervention. Even though headache, nausea, vomiting, constipation, diarrhea, and other symptoms were reported during the study, the symptoms were mild and lasted for a short time, not relating to clinical practice.
Limitations
Only 13 studies were included in the current analysis, with limited studies focusing on the effect of KD in patients with T2DM worldwide. For instance, no analysis was conducted on HOMA-IR even though there was a trend of improvement; also, very limited literature was available. All studies included in this meta-analysis were carried out among Caucasian diabetic patients (no East Asians included); however, the majority of East Asian diabetic patients showed insulin resistance with central obesity and defect in insulin secretion. Therefore, clinical trials conducted among East Asians are highly desirable to confirm whether there is an improvement in the secretion function of islet cells other than improved regulation of glucose and lipid metabolism. Moreover, the current study analyzed the data without assigning studies into time duration due to the limited number of studies and the missing data of insulin and lipid biomarkers; in addition, the duration of the follow-up was decentralized into days, months, and years. The available studies concerning the effects of ketogenic diet in patients with diabetes are very limited; it is impossible to summarize a similar follow-up interval for statistical analysis of time points. However, the current results suggested that ketogenic diet consumption contributed to therapeutic effects despite the length of the term of intervention. The analysis of the difference before and after the intervention may also give credit to the clinical efficacy of the diet therapy. In current clinical practice, a majority of the patients have to use a combination of multiple drugs to improve their glycolipid metabolism. Drug therapy is a heavy mental and economical burden to patients. Given the fact that most of the patients are confused regarding a proper dietary therapy plan, it is essential to recommend a feasible dietary therapy plan to transmit a positive message to both patients with diabetes and physicians majored in the area of diabetes.
Based on a meta-analysis that systematically reviewed 13 relevant studies, we found that ketogenic diet can not only control fasting blood glucose and reduce glycosylated hemoglobin, but also improve lipid metabolism. Additionally, ketogenic diet can reduce BMI and body weight. Therefore, ketogenic diet may be used as part of the integrated management of type 2 diabetes.
Guariguata, L. et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pr. 103 , 139–149 (2014).
Google Scholar
Yu, Z. et al. Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in T2DM: a systematic review and metaanalysis of randomized controlled trials. Clin. Nutr. 39 , 1724–1734 (2020).
Article CAS Google Scholar
Guh, D. P. et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9 , 88 (2009).
Article Google Scholar
Hussain, T. A. et al. Effect of low-calorie versus low-carbohydrate ketogenic diet in T2DM. Nutrition 28 , 1061–1021 (2012).
Hall, K. D. et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 104 , 324–333 (2016).
Hamdy, O. et al. Fat versus carbohydrate-based energy-restricted diets for weight loss in patients with T2DM. Curr. Diab. Rep. 18 , 128 (2018).
Joshi, S., Ostfeld, R. J. & McMacken, M. The ketogenic diet for obesity and diabetes-enthusiasm outpaces evidence. JAMA Intern. Med. 179 , 1163–1164 (2019).
Saslow, L. R. et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with T2DM: a randomized controlled trial. J. Med. Internet Res. 19 , e36 (2017).
Saslow, L. R. et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with T2DM mellitus or prediabetes. Nutr. Diabetes 7 , 304 (2017).
Meng, Y. et al. Efficacy of low carbohydrate diet for T2DM mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pr. 131 , 124–131 (2017).
Castellana, M. et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 21 , 5–16 (2019).
Westman, E. C., Yancy, W. S. Jr., Mavropoulos, J. C., Marquart, M. & McDuffie, J. R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in T2DM mellitus. Nutr. Metab. 5 , 36 (2008).
Partsalaki, I., Karvela, A. & Spiliotis, B. E. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 25 , 697–704 (2012).
Leonetti, F. et al. Very low-carbohydrate ketogenic diet before bariatric surgery: prospective evaluation of a sequential diet. Obes. Surg. 25 , 64–71 (2015).
Walton, C. M., Perry, K., Hart, R. H., Berry, S. L. & Bikman, B. T. Improvement in glycemic and lipid profiles in type 2 diabetics with a 90-day ketogenic diet. J. Diabetes Res. 14 , 8681959 (2019).
Leow, Z. Z. X., Guelfi, K. J., Davis, E. A., Jones, T. W. & Fournier, P. A. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with Type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet. Med. 35 , 9 (2018).
Azevedo, D. L. P. et al. Effect of classic ketogenic diet treatment on lipoprotein subfractions in children and adolescents with refractory epilepsy. Nutrition 33 , 271–277 (2017).
Krebs, J. D. et al. Improvements in glucose metabolism and insulin sensitivity with a low-carbohydrate diet in obese patients with T2DM. J. Am. Coll. Nutr. 32 , 11–17 (2013).
Lau, J., Ioannidis, J. P. & Schmid, C. H. Quantitative synthesis in systematic reviews. Ann. Intern. Med. 127 , 820–826 (1997).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 , 1539–1558 (2002).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 7 , 177–188 (1986).
McKenzie, A. L. et al. A Novel Intervention Including Individualized Nutritional Recommendations Reduces Hemoglobin A1c Level, Medication Use, and Weight in Type 2 Diabetes. JMIR Diabetes 2 , e5 (2017).
Yancy, W. S. et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. (Lond) 2 , 34 (2005).
Athinarayanan, S. J. et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of T2DM: a 2-year non-randomized clinical trial. Front. Endocrinol. 10 , 348 (2019).
Laffel, L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 15 , 412–426 (1999).
Gershuni, V. M., Yan, S. L. & Medici, V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr. Nutr. Rep. 7 , 97–106 (2018).
Yancy, W. S., Vernon, M. C. & Westman, E. C. A pilot trial of a low-carbohydrate, ketogenic diet in patients with T2DM. Metab. Syndr. Relat. Disord. 1 , 239–243 (2003).
Bolla, A. M., Caretto, A., Laurenzi, A., Scavini, M. & Piemonti, L. Low-carb and ketogenic diets in type 1 and type 2 diabetes. Nutrients 26 , 962 (2019).
Dashti, H. M. et al . Beneficial effects of ketogenic diet in obese diabetic subjects. Mol. Cell Biochem . 302 , 249–256 (2007).
Yancy, W. S. Jr. et al. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch. Intern. Med . 170 , 136–145 (2010).
Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of T2DM (UKPDS 35): prospective observational study. BMJ 321 , 405–412 (2000).
Nakanishi, S. et al. Comparison of HbA1c levels and body mass index for prevention of diabetic kidney disease: a retrospective longitudinal study using outpatient clinical data in Japanese patients with T2DM mellitus. Diabetes Res. Clin. Pr . 155 , 107807 (2019).
Dehghan, P. & Abbasalizad, F. M. Dietary acid load, blood pressure, fasting blood sugar and biomarkers of insulin resistance among adults: findings from an updated systematic review and meta-analysis. Int. J. Clin. Pr. 74 , 4 (2019).
Avtanski, D., Pavlov, V. A., Tracey, K. J. & Poretsky, L. Characterization of inflammation and insulin resistance in high-fat diet-induced male C57BL/6J mouse model of obesity. Anim. Model Exp. Med . 2 , 252–258 (2019).
Saslow, L. R. et al. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS One 9 , e91027 (2014).
Stocker, R. K., Reber, A. E., Bally, L., Nuoffer, J. M. & Stanga, Z. Ketogenic diet and its evidence-based therapeutic implementation in endocrine diseases. Praxis 108 , 541–553 (2019).
Handley, R. T., Bentley, R. E., Brown, T. L. & Annan, A. A. Successful treatment of obesity and insulin resistance via ketogenic diet status post Roux-en-Y. BMJ Case Rep. 2018 , bcr2018225643 (2018).
Tay, J. et al. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. Am. J. Clin. Nutr . 102 , 780–790 (2015).
Kinzig, K. P., Honors, M. A. & Hargrave, S. L. Insulin sensitivity and glucose tolerance are altered by maintenance on a ketogenic diet. Endocrinology 151 , 3105–3114 (2010).
Cox, N., Gibas, S., Salisbury, M., Gomer, J. & Gibas, K. Ketogenic diets potentially reverse Type II diabetes and ameliorate clinical depression: a case study. Diabetes Metab. Syndr . 13 , 1475–1479 (2019).
Goday, A. et al. Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr Diabetes 6 , e230 (2016).
Cohen, C. W. et al. A ketogenic diet reduces central obesity and serum Insulin in women with ovarian or endometrial cancer. J. Nutr . 148 , 1253–1260 (2018).
Myette-Cote, E. et al. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes: a randomized trial. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315 , R1210–R1219 (2018).
Zhu, B. et al. Lipid oversupply induces CD36 sarcolemmal translocation via dual modulation of PKCzeta and TBC1D1: an early event prior to insulin resistance. Theranostics 10 , 1332–1354 (2020).
Akhtar, D. H., Iqbal, U., Vazquez-Montesino, L. M., Dennis, B. B. & Ahmed, A. Pathogenesis of insulin resistance and atherogenic dyslipidemia in nonalcoholic fatty liver disease. J. Clin. Transl. Hepatol. 7 , 362–370 (2019).
Iqbal, J., Al Qarni, A., Hawwari, A., Alghanem, A. F. & Ahmed, G. Metabolic syndrome, dyslipidemia and regulation of lipoprotein metabolism. Curr. Diabetes Rev . 14 , 427–433 (2018).
Laakso, M. & Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol . 10 , 293–302 (2014).
Han, L. et al. Free fatty acid can induce cardiac dysfunction and alter insulin signaling pathways in the heart. Lipids Health Dis. 17 , 185 (2018).
Yu, S. et al. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res. Ther . 10 , 333 (2019).
Ponce, A. J. et al. Low prolactin levels are associated with visceral adipocyte hypertrophy and insulin resistance in humans. Endocrine 67 , 331–343 (2020).
Karasek, D. & Vaverkova, H. Diabetic dyslipidemia and microvascular complications of diabetes. Vnitr. Lek. 64 , 17–24 (2018).
Sajoux, I. et al. Effect of a very-low-calorie ketogenic diet on circulating myokine levels compared with the effect of bariatric surgery or a low-calorie diet in patients with obesity. Nutrients 11 , 2368 (2019).
Download references
Acknowledgements
This study was supported by the Science Technology Department of Jilin Province (20180623006TC and 20200404213YY) and the Interdisciplinary Project of First Hospital of Jilin University (JDYYJC010) and Transformation Project of First Hospital of Jilin University (JDYYZH-1902019) and Education Department of Jilin Province (JJKH20190032KJ and JJKH20201081KJ).
Author information
These authors contributed equally: Xiaojie Yuan, Jiping Wang, Shuo Yang
Authors and Affiliations
Department of Clinical Nutrition, First Hospital of Jilin University, 1 Xinmin Street, 130021, Changchun, Jilin, China
Xiaojie Yuan, Jiping Wang, Xumei Li, Dongxu Hong & Chenglin Sun
Department of Endocrinology and Metabolism, First Hospital of Jilin University, 1 Xinmin Street, 130021, Changchun, Jilin, China
Shuo Yang, Mei Gao, Lingxia Cao & Chenglin Sun
Division of Clinical Research, First Hospital of Jilin University, 1 Xinmin Street, 130021, Changchun, Jilin, China
You can also search for this author in PubMed Google Scholar
Contributions
X.Y., J.W., S.Y., S.T. and C.S. were responsible for writing, M.G., L.C., X.L. and S.Y. were responsible for the literature collection and data management, D.H. and S.T. for statistical analysis, C.S. and S.T. are in charge of the overall research design and supervision.
Corresponding authors
Correspondence to Suyan Tian or Chenglin Sun .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yuan, X., Wang, J., Yang, S. et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr. Diabetes 10 , 38 (2020). https://doi.org/10.1038/s41387-020-00142-z
Download citation
Received : 06 July 2020
Revised : 19 October 2020
Accepted : 06 November 2020
Published : 30 November 2020
DOI : https://doi.org/10.1038/s41387-020-00142-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential.
- Yu-Ling Xiao
- Yi-Zhou Jiang
Signal Transduction and Targeted Therapy (2024)
Effects of ketogenic diet on health outcomes: an umbrella review of meta-analyses of randomized clinical trials
- Chanthawat Patikorn
- Pantakarn Saidoung
- Nathorn Chaiyakunapruk
BMC Medicine (2023)
Nutrition & Diabetes (2023)
Regional differences in the reduction in cerebral FDG uptake induced by the ketogenic diet
- O. A. Bennett
- S. C. Ramsay
- D. A. Pattison
European Journal of Hybrid Imaging (2022)
VLCKD: a real time safety study in obesity
- Luigi Barrea
- Ludovica Verde
- Giovanna Muscogiuri
Journal of Translational Medicine (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Advances in Diabetes Research and Management pp 247–256 Cite as
Influence of Ketogenic Diet on Diabetes
- Natesan Sella Raja 2 ,
- Varsha Singh 2 &
- Subhashree Sivakumar 2
- First Online: 10 March 2023
566 Accesses
The effectiveness of limiting the intake of carbohydrates in obese individuals and people with diabetes is still subject to debate, even though ketogenic diets are widespread among clinicians and patients. Studies that have been published are contentious, probably due to the overall vagueness of these diets. In addition to the inherent complexity of dietary treatments, it is challenging to compare the findings of various studies. Despite the research showing that consuming fewer carbohydrates reduces body weight and improves glucose control in people with type 2 diabetes, little is known regarding this strategy’s sustainability, safety, and long-term efficacy. Ketogenic diet results in rapid and healthy weight loss and positive biomarker improvements, such as a decrease in serum hemoglobin A1c (HbA1c) in those with type 2 diabetes. Many doctors are reluctant to recommend it because it substantially raises low lipoprotein cholesterol levels. There is a significant concern about the possible long-term effects of the widespread adoption of this diet by broad parts of the public, given the popularity of the ketogenic diet, especially among persons who do not need to lose weight. In this chapter, we summarize about the ketogenic diet, the potential impact of ketogenic diets on diabetic patients, and the physiological changes experienced by an individual, followed by observational studies in diabetic patients.
- Ketogenic diet
- Physiological changes
- Observational studies
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Aguirre Castaneda, R. L., Mack, K. J., & Lteif, A. (2012). Successful treatment of type 1 diabetes and seizures with combined ketogenic diet and insulin. Pediatrics, 129 (2), e511. https://doi.org/10.1542/PEDS.2011-0741
Article PubMed Google Scholar
American Diabetes Association. (2017). 5. Lifestyle management: Standards of medical care in diabetes-2019. Diabetes Care, 40 , S33–S43. https://doi.org/10.2337/dc17-S007
Article Google Scholar
American Diabetes Association. (2018). 5. Lifestyle management: Standards of medical care in diabetes-2019. Diabetes Care, 42 , S46–S60. https://doi.org/10.2337/dc19-S005
American Diabetes Association. (2019). 6. Glycemic targets: Standards of medical care in diabetes-2019. Diabetes Care, 42 (Suppl 1), S61–S70. https://doi.org/10.2337/DC19-S006
Arora, S. K., & McFarlane, S. I. (2005). The case for low carbohydrate diets in diabetes management. Nutrition & Metabolism, 2 , 16. https://doi.org/10.1186/1743-7075-2-16
Article CAS Google Scholar
Bach, A. C., & Babayan, V. K. (1982). Medium-chain triglycerides: An update . Retrieved from https://academic.oup.com/ajcn/article-abstract/36/5/950/4693611
Barzegar, M., Afghan, M., Tarmahi, V., Behtari, M., Rahimi Khamaneh, S., & Raeisi, S. (2021). Ketogenic diet: Overview, types, and possible anti-seizure mechanisms. Nutritional Neuroscience, 24 (4), 307–316. https://doi.org/10.1080/1028415X.2019.1627769
Article CAS PubMed Google Scholar
Boden, G., Sargrad, K., Homko, C., Mozzoli, M., & Stein, T. P. (2005). Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Annals of Internal Medicine, 142 (6), 403. https://doi.org/10.7326/0003-4819-142-6-200503150-00006
Chandel, N. S. (2021). Glycolysis. Cold Spring Harbor Perspectives in Biology, 13 (5), a040535. https://doi.org/10.1101/CSHPERSPECT.A040535
Dhamija, R., Eckert, S., & Wirrell, E. (2013). Ketogenic diet. The Canadian Journal of Neurological Sciences. Le Journal Canadien Des Sciences Neurologiques, 40 (2), 158–167. https://doi.org/10.1017/S0317167100013676
George, F. C. (2006). Fuel metabolism in starvation. Annual Review of Nutrition, 26 , 1–22. https://doi.org/10.1146/ANNUREV.NUTR.26.061505.111258
Gershuni, V. M., Yan, S. L., & Medici, V. (2018). Nutritional ketosis for weight management and reversal of metabolic syndrome. Current Nutrition Reports, 7 (3), 97–106. https://doi.org/10.1007/S13668-018-0235-0
Article CAS PubMed PubMed Central Google Scholar
Halton, T. L., & Hu, F. B. (2013). The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. Journal of the American Nutrition Association., 23 (5), 373–385. https://doi.org/10.1080/07315724.2004.10719381
Hammami, M. M., Bouchama, A., Al-Sedairy, S., Shail, E., AlOhaly, Y., & Mohamed, G. E. D. (1997). Concentrations of soluble tumor necrosis factor and interleukin-6 receptors in heatstroke and heatstress. Critical Care Medicine, 25 (8), 1314–1319. https://doi.org/10.1097/00003246-199708000-00017
Hu, T., Mills, K. T., Yao, L., Demanelis, K., Eloustaz, M., Yancy, W. S., Kelly, T. N., He, J., & Bazzano, L. A. (2012). Systematic reviews and meta-and pooled analyses effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: A meta-analysis of randomized controlled clinical trials. American Journal of Epidemiology, 176 (7), 44–54. https://doi.org/10.1093/aje/kws264
Hussain, T. A., Mathew, T. C., Dashti, A. A., Asfar, S. M., Al-Zaid, N., & Dashti, H. M. (2012). Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition, 28 (10), 1016–1021. https://doi.org/10.1016/j.nut.2012.01.016
Ioannidis, J. P. A. (2018). The challenge of reforming nutritional epidemiologic research. JAMA - Journal of the American Medical Association, 320 (10), 969–970. https://doi.org/10.1001/JAMA.2018.11025
Jensen, N. J., Wodschow, H. Z., Nilsson, M., & Rungby, J. (2020). Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. International Journal of Molecular Sciences, 21 (22), 8767. https://doi.org/10.3390/IJMS21228767
Johnstone, A. M., Horgan, G. W., Murison, S. D., Bremner, D. M., & Lobley, G. E. (2008). Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. The American Journal of Clinical Nutrition, 87 (1), 44–55. https://doi.org/10.1093/AJCN/87.1.44
Jornayvaz, F. R., Samuel, V. T., & Shulman, G. I. (2010). The role of muscle insulin resistance in the pathogenesis of Atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annual Review of Nutrition, 30 , 273–290. https://doi.org/10.1146/ANNUREV.NUTR.012809.104726
Kakodkar, P. (2020). Ketogenic diet: Biochemistry, weight loss and clinical applications. Nutrition and Food Science International Journal, 10 (2), 10.19080/nfsij.2020.10.555782.
Klein, S., Sheard, N. F., Pi-Sunyer, X., Daly, A., Wylie-Rosett, J., Kulkarni, K., & Clark, N. G. (2004). Weight management through lifestyle modification for the prevention and management of type 2 diabetes: Rationale and strategies. A statement from the American Diabetes Association, the north American Association for the Study of obesity, and the American Society for Clinical Nutrition. The American Journal of Clinical Nutrition, 80 (2), 257–263. https://doi.org/10.1093/AJCN/80.2.257
Kossoff, E. H., & Dorward, J. L. (2008). The modified Atkins diet. Epilepsia, 49 (Suppl. 8), 37–41. https://doi.org/10.1111/j.1528-1167.2008.01831.x
Li, S., Lin, G., Chen, J., Chen, Z., Xu, F., Zhu, F., Zhang, J., & Yuan, S. (2022). The effect of the regular ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocrine Disorders, 22 (1), 1–6. https://doi.org/10.1186/S12902-022-00947-2/TABLES/3
Maalouf, M., Sullivan, P. G., Davis, L., Kim, D. Y., & Rho, J. M. (2007). Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience, 145 (1), 256. https://doi.org/10.1016/J.NEUROSCIENCE.2006.11.065
Masino, S. A., & Rho, J. M. (2012). Mechanisms of Ketogenic diet action - Jasper’s basic mechanisms of the epilepsies - NCBI bookshelf . Retrieved August 13, 2022, from https://www.ncbi.nlm.nih.gov/books/NBK98219/#!po=3.52113
Paoli, A., Grimaldi, K., Bianco, A., Lodi, A., Cenci, L., & Parmagnani, A. (2012). Medium-term effects of a ketogenic and Mediterranean diet on resting energy expenditure and respiratory ratio. BMC Proceedings, 6 (Suppl 3), P37. https://doi.org/10.1186/1753-6561-6-S3-P37
Article PubMed Central Google Scholar
Paoli, A., Rubini, A., Volek, J. S., & Grimaldi, K. A. (2013). Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. European Journal of Clinical Nutrition, 67 (8), 789–796. https://doi.org/10.1038/ejcn.2013.116
Paoli, A., Parmagnani, A., & Bianco, A. (2014). Ketogenic diet and phytoextracts. Comparison of the efficacy of Mediterranean, Zone, and Tisanoreica diet on some health risk factors. Agro Food Industry Hi Tech, 21 (4), 24–29. https://www.researchgate.net/publication/230577564
Google Scholar
Partsalaki, I., Karvela, A., & Spiliotis, B. E. (2012). Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. Journal of Pediatric Endocrinology and Metabolism, 25 (7–8), 697–704. https://doi.org/10.1515/JPEM-2012-0131/MACHINEREADABLECITATION/RIS
Wang, M. L., Gellar, L., Nathanson, B. H., Pbert, L., Ma, Y., Ockene, I., & Rosal, M. C. (2015). Decrease in glycemic index associated with improved glycemic control among Latinos with type 2 diabetes. Journal of the Academy of Nutrition and Dietetics, 115 (6), 898–906. https://doi.org/10.1016/J.JAND.2014.10.012
Webster, C., Murphy, T. E., Larmuth, K. M., Noakes, T. D., & Smith, J. A. (2019). Diet, diabetes status, and personal experiences of individuals with type 2 diabetes who self-selected and followed a low carbohydrate, high-fat diet. Diabetes, Metabolic Syndrome and Obesity . Retrieved August 12, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6901382/
Wong, K., Raffray, M., Roy-Fleming, A., Blunden, R. D., & Brazeau, A.-S. (2021). The ketogenic diet as a standard way of eating in adults with type 1 and type 2 diabetes: A qualitative study. Canadian Journal of Diabetes . Retrieved August 12, 2022, from https://www.sciencedirect.com/science/article/pii/S1499267120302008
Download references
Author information
Authors and affiliations.
Membrane-Protein Interaction Lab, Department of Genetic Engineering, School of Bio-engineering, SRM Institute of Science and Technology, Kattankulathur, Chennai, Tamil Nadu, India
Natesan Sella Raja, Varsha Singh & Subhashree Sivakumar
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Faculty of Dentistry Department of Biochemistry, Jamia Millia Islamia, New Delhi, India
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter.
Raja, N.S., Singh, V., Sivakumar, S. (2023). Influence of Ketogenic Diet on Diabetes. In: Noor, R. (eds) Advances in Diabetes Research and Management. Springer, Singapore. https://doi.org/10.1007/978-981-19-0027-3_11
Download citation
DOI : https://doi.org/10.1007/978-981-19-0027-3_11
Published : 10 March 2023
Publisher Name : Springer, Singapore
Print ISBN : 978-981-19-0026-6
Online ISBN : 978-981-19-0027-3
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
VIDEO
COMMENTS
A ketogenic diet primarily consists of high fat intake, moderate protein consumption, and low carbohydrate intake. The macronutrient distribution typically ranges from approximately 55% to 60% fat, 30% to 35% protein, and 5% to 10% carbohydrates. For instance, in a 2000 kcal per day diet, the carbohydrate allowance would amount to approximately ...
The ketogenic diet (KD) is a high-fat, adequate-protein, and very-lowcarbohydrate diet regiment that mimics the metabolism of the fasting state (1), for the purpose of harnessing its high fat ...
Introduction and background. Obesity is classified based on the body mass index (BMI) of the individual. A BMI of 18.5-24.9 kg/m 2 is considered to be the normal range, while a BMI of 25.0-29.9 is considered overweight and a BMI ≥30 is classified as obese (further classified as obesity class I if BMI is between 30.0-34.9, class II if BMI is between 35.0-39.9, and class III if BMI is ≥40.0).
The consumption of a high-fat, low-carbohydrate diet (ketogenic diet) has diverse effects on health and is expected to have therapeutic value in neurological disorders, metabolic syndrome, and cancer.
3.1. Anti-Inflammatory, Cardioprotective Potential of the State of Ketosis (Ketone Bodies) The ketogenic diet initiates the increased production of ketone bodies (i.e., β-hy-droxybutyrate (the main ketone body), acetone and acetoacetate) in the body. Thus, it places the body into a state of nutritional ketosis.
A ketogenic diet is rich in deficiencies that can significantly worsen a person’s condition (Pros and Cons” 2). For this reason, it is necessary to consult a nutritionist before using such a diet. First, it should be noted that the ketogenic diet is unbalanced – it excludes the intake of several nutrients, vitamins, and trace elements.
the effects of the ketogenic diet (kd) on inflammatory pain by livia s. wyss . a thesis submitted to the faculty of the neuroscience program in candidacy for the baccalaureate degree with honors in neuroscience neuroscience program hartford, connecticut may 16, 2016
The effects of low-fat, high-carbohydrate diets vs. low-carbohydrate, high-fat diets on weight, blood pressure, serum liquids and blood glucose: a systematic review and meta-analysis
The basic idea behind the ketogenic diet is to instruct the body to metabolize fat for energy rather than glucose, which is the primary source of adenosine triphosphates (ATPs) under normal conditions. Type 2 diabetes is characterized by resistance to insulin, which results in poor glucose uptake by cells.