An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.


StatPearls [Internet].
Nephrotic syndrome.
Carolina Tapia ; Khalid Bashir .
Affiliations
Last Update: May 29, 2023 .
- Continuing Education Activity
Nephrotic syndrome (NS) is a clinical syndrome defined by massive proteinuria (greater than 40 mg/m^2 per hour) responsible for hypoalbuminemia (less than 30 g/L), with resulting hyperlipidemia, edema, and various complications. It is caused by increased permeability through the damaged basement membrane in the renal glomerulus. It results from an abnormality of glomerular permeability that may be primary with a disease-specific to the kidneys or secondary to congenital infections, diabetes, systemic lupus erythematosus, neoplasia, or certain drug use. This activity reviews the causes, pathophysiology, and presentation of nephrotic syndrome and highlights the role of the interprofessional team in its management.
- Identify the etiology of nephrotic syndrome.
- Review the presentation of patients with nephrotic syndrome.
- Summarize the treatment and management options available for nephrotic syndrome.
- Describe interprofessional team strategies for improving care and outcomes in patients with nephrotic syndrome.
- Introduction
Nephrotic syndrome (NS) is a clinical syndrome defined by massive proteinuria responsible for hypoalbuminemia, with resulting hyperlipidemia, edema, and various complications. It is caused by increased permeability through the damaged basement membrane in the renal glomerulus, especially infectious or thrombo-embolic. It results from an abnormality of glomerular permeability that may be primarily due to an intrinsic renal disease in the kidneys or secondary due to congenital infections, diabetes, systemic lupus erythematosus, neoplasia, or certain drug use. [1] [2] [3] Nephrotic-range proteinuria is defined as the urinary loss of 3 grams or more of proteins per 24 hours or, on a single spot urine sample, the presence of 2 g of protein per gram of urinary creatinine. This proteinuria can also result from other systemic diseases, such as amyloidosis. [4]
The disorder can affect people of all ages. In most children, the first sign of nephrotic syndrome is facial swelling. Adults usually present with dependent edema.
The nephrotic syndrome could affect adults and children of both genders and any race. Also, it could occur in a typical form or with nephritic syndrome. The latter denotes glomerular inflammation leading to hematuria and impaired renal function.
The first indication of nephrotic syndrome in children is the swelling of the face which then progresses to the entire body. Adults may present with dependent edema. Other common features are fatigue and loss of appetite.
Common primary causes of nephrotic syndrome are intrinsic kidney diseases, such as membranous nephropathy, minimal-change nephropathy, and focal glomerulosclerosis. Secondary causes may include systemic diseases, such as lupus erythematosus, diabetes mellitus, and amyloidosis. Congenital/hereditary focal glomerulosclerosis could occur because of genetic mutations in podocyte proteins, such as podocin, nephrin, or the cation channel 6 protein. [5] An episode of infectious diseases, particularly the upper respiratory tract, is a triggering factor in almost half of cases, an allergic reaction in a third of cases, and more rarely, an insect bite or vaccination. [6] Nephrotic syndrome can also result from drugs of abuse, including heroin. [7]
Secondary causes of nephrotic syndrome include the following:
- Diabetes mellitus
- Immune: lupus erythematosus, antibody vasculitis, Berger disease, glomeruli acute post-infectious nephritis, antineutrophil cytoplasmic neutrophils (ANCA), Goodpasture syndrome, extramembranous or membranoproliferative glomerulonephritis, thrombotic microangiopathy, alloantibodies from enzyme replacement therapy, or toxicity of nonsteroidal anti-inflammatory drugs (NSAIDs) or gold salts
- Infection: human immunodeficiency virus (HIV), hepatitis B virus, hepatitis C, cytomegalovirus, parvovirus B1, preeclampsia, toxoplasmosis, amyloidosis, and paraproteinemias
The most common cause in children is minimal change glomerulonephritis. In White adults, nephrotic syndrome is most frequently due to membranous nephropathy, whereas in populations of African ancestry, the most common cause of the nephrotic syndrome is focal segmental glomerulosclerosis.
One more scenario where nephrotic-range proteinuria can occur is in the third trimester of pregnancy, a classical preeclampsia finding. However, it may start de novo or be superimposed on chronic kidney disease from before. There would have been preexisting proteinuria in the latter, which worsened during pregnancy.
Medication may also cause nephrotic syndrome. This includes the following:
- The infrequent occurrence of minimal-change disease with nonsteroidal anti-inflammatory drugs (NSAIDs) [8]
- The occurrence of membranous glomerulonephritis with gold, bucillamine, and penicillamine use, which are used for rheumatic diseases [9]
- Focal glomerulosclerosis may occur due to bisphosphonates [10]
- Lithium and interferon therapy has been found to be associated with focal glomerulosclerosis [11]
- Epidemiology
Nephrotic syndrome is an important chronic disease in children. The estimated annual incidence of nephrotic syndrome in healthy children is two to seven new cases per 100,000 children less than 18 years of age. It is more common in boys than girls at younger ages, but once adolescence is reached, there is no significant difference between genders. Increased incidence and more severe diseases are seen in African American and Hispanic populations. [12]
We will look at the statistics from different regions of the world.
United States Statistics
Diabetic nephropathy associated with nephrotic syndrome is most common, with an estimated rate of around 50 cases per million population. In the pediatric population, nephrotic syndrome could occur at a rate of 20 cases per million. [13]
International Statistics
In India and Turkey, biopsy results in children with nephrotic syndrome have revealed similar histology types compared to what would be expected in Western countries. [14] [15] In Pakistani adult patients with nephrotic syndrome, the histological patterns of kidney biopsies are similar to those seen in western countries. [16]
In parts of the Middle East and Africa, glomerular diseases have also been linked with urogenital schistosomal infection. [17] However, tropical nephrotic syndrome due to parasitic diseases such as malaria or schistosomiasis may be non-existent.
Doe et al. reported causes of nephrotic syndrome in the African pediatric population where kidney biopsy most often revealed typical histologic findings, such as minimal change disease and focal and segmental glomerulosclerosis. [18] Nephrotic syndrome due to quartan malaria is not a very well-established phenomenon. In the Congo, Pakasa and Sumaili call attention to the fall of parasite-associated nephrotic syndrome. [19] [20]
Race-, sex-, and Age-related Demographics
Because diabetes mellitus is one of the major causes of nephrotic syndrome, American Indians, African Americans, and Hispanics have an increased incidence of nephrotic syndrome than White persons. HIV-associated nephropathy is a consequence of HIV infection that is uncommon in Whites; however, it is frequently seen in African Americans because of their greater prevalence of the ApoL1 alleles. [21] Focal glomerulosclerosis seems to be overrepresented as one of the causes of nephrotic syndrome in African-Americans as opposed to White children. [22] There is a male predominance in nephrotic syndrome, as seen in chronic kidney disease in general. This pattern is also observed in paraneoplastic membranous nephropathy. [23] However, lupus nephritis affects mostly women.
- Pathophysiology
The glomerular capillaries are lined by fenestrated endothelium, which sits on the glomerular basement membrane, covered by glomerular epithelium, or podocytes, which envelop the capillaries with the capillaries' cellular extensions called foot processes. These processes interdigitate with special cell-cell junctions called the slit diaphragm, which together form the glomerular filter. Normally, larger proteins (greater than 69 kD) are excluded from filtration. The destruction of podocytes above a critical mass also leads to irreversible glomerular damage. [24] [25] [26]
In a healthy person, the loss of plasma albumin through the glomerular filtration barrier is less than 0.1%. [27] Filtration of plasma water and solutes occurs extracellularly and through the filtration slits and endothelial fenestrae. The glomerular changes that may lead to proteinuria are damage to the glomerular basement membrane, the endothelial surface, or the podocytes. Albumin is the main constituent in proteinuria, accounting for 85%. Albumin carries a net negative charge. The loss of glomerular membrane negative charge plays an important role in causing albuminuria. A generalized defect in glomerular permeability is associated with nonselective proteinuria causing a glomerular leakage of various plasma proteins. This phenomenon does not allow a clear-cut separation of causes of proteinuria.
Pathogenesis of Edema
The following are the two hypotheses for the occurrence of edema in nephrotic syndrome:
Underfill Hypothesis
Increased glomerular permeability causes albuminuria, eventually leading to hypoalbuminemia. Consequently, hypoalbuminemia results in a decline in plasma colloid osmotic pressure, in turn causing increased transcapillary filtration of water in the body. Subsequently, this process leads to the development of edema. Capillary hydrostatic pressure and oncotic pressure control the fluid movement from the vascular compartment into the interstitium. Protein content mainly determines the oncotic pressure. For edema to occur, the amount of fluid filtered should exceed the maximal lymphatic flow, which happens secondary to a low enough intravascular oncotic pressure and a high enough capillary hydrostatic pressure. In nephrotic syndrome, this results in reduced plasma volume, with a secondary rise in sodium and water retention via the kidneys. [28]
Overfill Hypothesis
An alternative hypothesis states that an intrinsic defect in the renal tubules leads to a decline in sodium excretion. This might occur if the intraluminal protein directly causes renal epithelial sodium reabsorption. [29] The following points support this hypothesis:
- Sodium retention occurs even before the serum albumin level starts to fall
- Intravascular volume is normal or even raised in many patients with nephrotic syndrome
- There is an exaggerated peripheral capillary permeability to albumin, as reported in the radioisotopic technique in studies of 60 patients with nephrotic syndrome. [30] This would lead to increased interstitial oncotic pressure and fluid retention in the peripheral tissues.
- Histopathology
There are different types of glomerulonephritis causing nephrotic syndrome, and they all behave differently when it comes to histopathological features of the kidney biopsy.
Minimal change disease is the most common pathology found in childhood (77% to 85%). Usually idiopathic. Light microscopy of renal biopsy samples shows no change; on electron microscopy, effacement of the foot processes can be seen. [31] Immunofluorescent staining for immune complexes is negative.
Focal segmental glomerulosclerosis accounts for 10% to 15% of cases. Light microscopy of renal biopsy sample shows scarring, or sclerosis, of portions of selected glomeruli which can progress into global glomerular sclerosis and tubular atrophy. In most cases, negative immunofluorescence.
Membranoproliferative glomerulonephritis: More commonly presents as nephrotic syndrome. It involves immune complex deposition. Immunofluorescence staining shows a granular pattern. On light microscopy, one can see thickened basement membrane. [32]
Membranous glomerulonephritis: Just 2% to 4% of cases in children, but the most common type in adults. Thickened basement membrane and granular pattern on immunofluorescence. A characteristic “spike and dome” appearance is visible on electron microscopy, with membrane deposition growing around subepithelial immune complex deposition. [33]
- History and Physical
The first sign of nephrotic syndrome in the pediatric population is usually swelling on the face. This is followed by edema of the entire body. Adult patients can present with dependent edema. Frothy urine may be a presenting symptom. [34] Tiredness and lack of appetite are common features. A thrombotic consequence, such as deep venous thrombosis (DVT) of the calf veins or a pulmonary embolus, could be the first indication of nephrotic syndrome.
Additional features in a patient's history are related to the cause of the nephrotic syndrome. For instance, a recent commencement of NSAIDs suggests such drugs as the cause. Similarly, a more than 10-year history of diabetes mellitus with symptomatic neuropathy suggests diabetic nephropathy.
Physical Examination
Edema is the most prominent feature of nephrotic syndrome, and in the beginning, it develops around the eyes and legs. Over time, the edema becomes generalized and leads to increasing weight and the development of ascites or pleural effusions. Hematuria and hypertension may be present less frequently, although these are more prominently seen in nephritic syndrome. [35]
Additional features on examination vary according to the cause of the nephrotic syndrome. Also, it depends on whether or not renal function impairment is present. For instance, in the case of longstanding diabetes mellitus, the patient could have diabetic retinopathy, which is closely associated with diabetic nephropathy. If the kidney function is impaired, the patient may have anemia, hypertension, or both.
Urine tests: Nephrotic-range proteinuria will be apparent by 3+ or 4+ readings on the dipstick or by semiquantitative testing by sulfosalicylic acid. A 3+ reading represents 300 mg/dL of urinary protein or more, which correlates with a daily loss of 3 g or more and thus is in the nephrotic range. Urine samples over 24 hours (for an accurate measure) and proteinuria (3 g protein) is diagnostic. [36] [37] [38]
Urinalysis may demonstrate casts (hyaline, granular, fatty, waxy, or epithelial cell). Lipiduria, the presence of free lipid or lipid within tubular cells, within casts, or as free globules, suggests a glomerular disorder.
Blood tests: The serum albumin level is classically low in nephrotic syndrome. Serum albumin often is less than the normal range of 3.5 to 4.5 g/dL. Creatinine concentrations vary by degree of renal impairment. Total cholesterol and triglyceride levels are typically increased.
- Serologic studies: The role of testing for secondary causes of nephrotic syndrome is controversial (because yield may be low). Tests are best done as indicated by clinical context. Consider: Serum glucose or glycosylated Hb (HbA), antinuclear antibodies, Hepatitis B and C serologic tests, serum or urine protein electrophoresis, cryoglobulins, rheumatoid factor, serologic test for syphilis (e.g., rapid plasma reagin), HIV antibody test, complement levels (CH50, C3, C4)
Test results may alter management and preclude the need for biopsy.
Ultrasonography: Individuals with a single kidney may be prone to developing focal glomerulosclerosis; having only one kidney is also a relative contraindication to kidney biopsy. Ultrasonography also demonstrates renal echogenicity. Increased renal echogenicity is consistent with intrarenal fibrosis.
Renal biopsy: This is indicated for the following: congenital nephrotic syndrome, children older than eight years at the onset, steroid resistance, frequent relapses or steroid dependency, significant nephritic manifestations. It is worth noting that in clinical practice, kidney biopsies frequently reveal glomerular diseases to be the cause of nephrotic-range proteinuria and not tubular diseases. This contradicts the idea that tubular function determines proteinuria. [39]
Phospholipase A Receptor (PLA R): it is a transmembrane receptor expressed on the surface of podocytes. 70% of cases with idiopathic membranous nephropathy have autoantibodies against PLA R. [40] There is a strong correlation between levels of this antibody and clinical disease activity. Therefore it helps in monitoring disease activity and treatment efficiency. [41] The absence of these autoantibodies could indicate secondary membranous nephropathy, such as that linked to cancers.
- Treatment / Management
A detailed assessment is necessary before starting corticosteroids. The patient's height, weight, and blood pressure should be monitored. Regular weight record helps in monitoring the decrease or increase of edema. Physical examination is carried out to detect infections and underlying systemic disorders. [42] [43] [44]
Specific treatment of nephrotic syndrome is dependent on its cause. Therefore, management varies between adult and pediatric populations. Kidney Disease Improving Global Outcomes (KDIGO) issued guidance in 2012 that included recommendations for treating nephrotic syndrome.
Specific Treatment in Children
Corticosteroids are mainly used for children with idiopathic nephrotic syndrome. Alternative immunosuppressive agents are often necessary for children with frequently relapsing or steroid-dependent nephrotic syndrome. Examples of these drugs include cyclophosphamide, mycophenolate mofetil (MMF), calcineurin inhibitors, and levamisole. In cases of steroid-resistant nephrotic syndrome, the first-line choice is calcineurin inhibitors, and if there is no response, then agents such as MMF or prolonged and/or intravenous pulse corticosteroids could be used. [45] [46] [47]
Rituximab, an anti-B cell antibody, has proved to be an effective steroid-sparing agent in the pediatric population. However, rituximab may fail to achieve drug-free remission in children dependent on both calcineurin inhibitors and steroids. Rituximab may also have a role in children with steroid-resistant disease. [45]
In children with complicated steroid-resistant nephrotic syndrome who respond to rituximab, Okutsu et al. observed that an additional rituximab treatment at B cell recovery may maintain prolonged remission. [48]
Specific Treatment in Adults
Treatment varies by etiology, as follows:
- Minimal change nephropathy in adults usually responds to prednisone.
- In lupus nephritis, prednisone combined with cyclophosphamide or mycophenolate mofetil induces remission.
- Secondary amyloidosis with nephrotic syndrome will improve with the anti-inflammatory management of the primary disease. [49]
Acute Nephrotic Syndrome in Childhood
Hospitalization is not usually necessary with close outpatient follow-up care and good parental and patient education. Hospitalization becomes helpful if any of the following are present:
- Generalized edema severe enough to result in respiratory distress
- Tense scrotal or labial edema
- Complications such as bacterial peritonitis, pneumonia, sepsis, or thromboembolism [50]
- Failure to thrive
- Uncertainty regarding the compliance of patient or family with treatment
Diuretics are usually needed. Furosemide (1 mg/kg/day) and spironolactone (2 mg/kg/day) help when fluid retention is severe enough, provided there are no signs of kidney failure or volume contraction. Achieving a satisfactory diuresis is hard when serum albumin level is less than 1.5 g/dL, so sometimes albumin has to be given.
To prevent infections, penicillin can be started in children with overt edema. Abdominal paracentesis is recommended in patients showing signs of peritonitis, and bacterial infections should be treated sooner. [51] Non-immune patients with varicella should receive immunoglobulin therapy if exposure to chickenpox occurs, and acyclovir should be started if the patient develops chickenpox.
Acute Nephrotic Syndrome in Adults
The principles of treatment in adults with acute nephrotic syndrome are not different from those for children. Diuretics, such as furosemide, spironolactone, and even metolazone, may be needed. Diuretic use may lead to volume depletion, which should be assessed by monitoring symptoms, weight, pulse, and blood pressure.
Anticoagulation has been suggested to prevent thromboembolic complications, but its role in primary prevention is not proven. Hypolipidemic agents could be used. [52]
In patients with secondary nephrotic syndrome, such as that secondary to diabetic nephropathy, some medications are widely used to reduce proteinuria, such as angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin 2 receptor blockers. [53] By reducing proteinuria, these drugs will lead to reduced intraglomerular pressure causing a reduction in systemic blood pressure.
Diet and Activity
The diet in patients with nephrotic syndrome is aimed to provide sufficient caloric and protein (1 g/kg/d) intake. Supplemental dietary proteins are of no proven value. A low-salt diet helps limit fluid retention and edema. [54]
Long-Term Monitoring
The patient's edema and proteinuria define the adjustment of diuretics and angiotensin antagonists. Follow-up in the nephrotic syndrome also involves immunizations and monitoring for steroid toxicity.
Routine immunizations should be deferred until there are no relapses and the patient has been off immunosuppressants for at least three months.
- Differential Diagnosis
The differential diagnoses for nephrotic syndrome include the following:
- Hepatic: insufficiency, hepatocellular cirrhosis, Budd-Chiari syndrome [55]
- Digestive: exudative enteropathy, lymphangiectasia, malnutrition
- Cardiac: hereditary angioneurotic edema
- Immune: anaphylaxis
- Renal: chronic glomerulonephritis, diabetic nephropathy, focal segmental glomerulosclerosis, HIV-associated nephropathy, IgA nephropathy, membranous glomerulonephritis, minimal change disease.
- Remission: Urine albumin nil or trace for three consecutive early morning specimens
- Relapse: Urine albumin 3+ or 4+ (or proteinuria greater than 40 mg/m^2/h) for three consecutive early morning specimens, having been in remission previously
- Frequent relapses: Two or more relapses in the initial six months or more than four relapses in any 12 months
- Steroid dependence: Two consecutive relapses when on alternate day steroids or within 14 days of its discontinuation
- Steroid resistance: Absence of remission despite therapy with daily prednisolone at a dose of 2 mg/kg per day for four weeks
- Congenital: presenting within the first three months of life, and in these children, there is usually a genetic mutation
The prognosis is excellent for patients with minimal change pathology, with most patients going into remission following corticosteroid treatment. [31] However, 85 to 90% of patients are steroid-responsive and may relapse, placing them at risk for steroid toxicity, systemic infections, and other complications.
For patients with focal-segmental glomerulosclerosis (FSGS), the prognosis is grave. [56] Generally will progress to an end-stage renal disease requiring dialysis and kidney transplant. Only around 20% of patients with focal glomerulosclerosis go into remission of proteinuria; another 10% improve but stay proteinuric. Between 25 and 30% of patients with FSGS develop end-stage renal disease (ESRD) within five years. There have been some studies to suggest a better 5-year renal outcome in Chinese adults with primary FSGS in comparison to the west. [57]
Of patients with membranous nephropathy, around 30% undergo spontaneous remission. However, for patients with persistent nephrotic syndrome, 40% to 50% develop ESRD over a period of ten years.
- Complications
Metabolic Consequences of Proteinuria
Following are the metabolic consequences of the nephrotic syndrome:
- Hypocalcemia and bone abnormalities
- Hyperlipidemia and atherosclerosis [58]
- Hypercoagulability
- Hypovolemia
Acute kidney injury may suggest underlying glomerulonephritis but is more commonly precipitated by hypovolemia or sepsis. Another proposition is that the edema of the kidneys causes a pressure-mediated reduction in the GFR. Additional consequences include the following:
- Hypertension due to reduced kidney function and fluid retention
- Edema of the gut could cause defective absorption resulting in malnutrition [59]
- Ascites and pleural effusions
- Generalized edema
- Respiratory distress
- Peritonitis
- Thromboembolism [60]
- Deterrence and Patient Education
Patients should be educated on taking a low-salt diet as it helps manage their symptoms. There are no restrictions on physical activity for patients with nephrotic syndrome, and staying active is preferred over bed rest as it reduces the risk of blood clots. Adverse effects of steroids, such as slowing growth, can be detected by monitoring patients every three months in the outpatient clinic. Patients should be given information that bone health is essential, and due to steroids, their bone health can be affected; therefore, supplemental calcium and vitamin D may be protective. [61] Patients should get a yearly checkup to look for cataracts. In the community, patients with nephrotic syndrome should have monitoring in terms of their vaccination.
- Enhancing Healthcare Team Outcomes
Because there are many causes of nephrotic syndrome, the condition is best managed by an interprofessional team. Once nephrotic syndrome is diagnosed, patient education is vital to prevent high morbidity.
Since most are outpatients, the pharmacist should encourage compliance with the medications. In addition, the doses of the drugs (diuretics and ACE inhibitors) may need continual reassessment depending on the patient's response. If the patient has been started on a corticosteroid, the pharmacist must assist the team by monitoring the patient for the adverse effects of these medications. The nurse should educate the patient on the importance of immunization and an appropriate diet.
For those children who have a failure to thrive, a dietary consult should be sought. Many of these children may require vitamin D or calcium supplements to prevent bone loss. The nurse should also educate the family on how to measure urine output daily and record the amount, as this will provide an indication of how the disease is progressing. Finally, a dietary consult should be obtained to educate the patient on a low-salt diet to prevent an aggravation of the edema. Only through such an approach can the morbidity of nephrotic syndrome be lowered. [62] [63] [Level 5]
Due to the rarity and complexity of this disease, an interprofessional approach to evaluation, treatment, and education of the patient and family will lead to the best outcomes. [Level 5]
Prior to the era of antibiotics, survival was rare for patients with nephrotic syndrome. Today, most patients with nephrotic syndrome survive, and the prognosis usually depends on the cause of kidney dysfunction. However, the prognosis in infants with nephrotic syndrome is still poor, and only those who can undergo dialysis or kidney transplantation have good survival. In patients who develop focal glomerulosclerosis, remission from proteinuria is only seen in one-third of patients. Because of frequent relapses, many of these patients require long-term corticosteroids and consequently also develop many adverse effects from these medications. About a third of these patients will require dialysis within five years.
The best prognosis is for patients with minimal change nephropathy, with few relapses, and less than 5% require long-term corticosteroids. The long-term risk of renal failure in these patients is low. Patients who show a poor response to steroids usually have poor outcomes. For those who develop nephrotic syndrome due to a secondary cause, the morbidity is primarily related to the cause. Diabetic patients who respond to ACE inhibitors may develop a slowing down of proteinuria and stabilize renal function. Those who develop amyloidosis will usually have a guarded prognosis. [64] [65] [66] [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Carolina Tapia declares no relevant financial relationships with ineligible companies.
Disclosure: Khalid Bashir declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Tapia C, Bashir K. Nephrotic Syndrome. [Updated 2023 May 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Diagnosis and management of nephrotic syndrome. [Practitioner. 2017] Diagnosis and management of nephrotic syndrome. McCloskey O, Maxwell AP. Practitioner. 2017 Feb; 261(1801):11-5.
- Review Clinical presentation & management of glomerular diseases: hematuria, nephritic & nephrotic syndrome. [Mo Med. 2011] Review Clinical presentation & management of glomerular diseases: hematuria, nephritic & nephrotic syndrome. Khanna R. Mo Med. 2011 Jan-Feb; 108(1):33-6.
- Thin basement membrane disease with heavy proteinuria or nephrotic syndrome at presentation. [Am J Kidney Dis. 2000] Thin basement membrane disease with heavy proteinuria or nephrotic syndrome at presentation. Nogueira M, Cartwright J Jr, Horn K, Doe N, Shappell S, Barrios R, Coroneos E, Truong LD. Am J Kidney Dis. 2000 Apr; 35(4):E15.
- Review Nephrotic Syndrome for the Internist. [Med Clin North Am. 2023] Review Nephrotic Syndrome for the Internist. Zabala Ramirez MJ, Stein EJ, Jain K. Med Clin North Am. 2023 Jul; 107(4):727-737. Epub 2023 Apr 8.
- Review Clinicopathologic correlations in the nephrotic syndrome. [Paediatrician. 1979] Review Clinicopathologic correlations in the nephrotic syndrome. Habib R, Lévy M, Gubler MC. Paediatrician. 1979; 8(5-6):325-48.
Recent Activity
- Nephrotic Syndrome - StatPearls Nephrotic Syndrome - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

CHARLES KODNER, MD
Am Fam Physician. 2016;93(6):479-485
Patient information : See related handout on nephrotic syndrome , written by the author of this article.
Author disclosure: No relevant financial affiliations.
Nephrotic syndrome (NS) consists of peripheral edema, heavy proteinuria, and hypoalbuminemia, often with hyperlipidemia. Patients typically present with edema and fatigue, without evidence of heart failure or severe liver disease. The diagnosis of NS is based on typical clinical features with confirmation of heavy proteinuria and hypoalbuminemia. The patient history and selected diagnostic studies rule out important secondary causes, including diabetes mellitus, systemic lupus erythematosus, and medication adverse effects. Most cases of NS are considered idiopathic or primary; membranous nephropathy and focal segmental glomerulosclerosis are the most common histologic subtypes of primary NS in adults. Important complications of NS include venous thrombosis and hyperlipidemia; other potential complications include infection and acute kidney injury. Spontaneous acute kidney injury from NS is rare but can occur as a result of the underlying medical problem. Despite a lack of evidence-based guidelines, treatment consisting of sodium restriction, fluid restriction, loop diuretics, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy, and careful assessment for possible disease complications is appropriate for most patients. Renal biopsy is often recommended, although it may be most useful in patients with suspected underlying systemic lupus erythematosus or other renal disorders, in whom biopsy can guide management and prognosis. Immunosuppressive treatment, including corticosteroids, is often used for NS, although evidence is lacking. Routine prophylactic treatment to prevent infection or thrombosis is not recommended. A nephrologist should be consulted about use of anticoagulation and immunosuppressants, need for renal biopsy, and for other areas of uncertainty.
Nephrotic syndrome (NS) consists of peripheral edema, heavy proteinuria, and hypoalbuminemia, often with hyperlipidemia. Patients typically present with edema and fatigue, without heart failure or severe liver disease. Although there is limited evidence to guide management decisions, recent expert consensus guidelines and systematic reviews provide updated recommendations. This article focuses on diagnosis and management of NS in adults, which is different from that in children.
Epidemiology
The annual incidence of NS in adults is three per 100,000 persons. Approximately 80% to 90% of NS cases in adults are idiopathic. Membranous nephropathy is the most common cause in whites, and focal segmental glomerulosclerosis is most common in blacks; each of these disorders accounts for approximately 30% to 35% of NS cases in adults. Minimal change disease and immunoglobulin A nephropathy each account for approximately 15% of cases. The remaining 10% of cases are secondary to an underlying medical condition. 1
Assessing the cause of NS is important in guiding management decisions. Many underlying systemic conditions can cause NS, although type 2 diabetes mellitus and systemic lupus erythematosus are most common. NS may not present as a primary diagnosis, but instead as one of multiple disease manifestations, particularly in systemic lupus erythematosus. A published case report of a 29-year-old pregnant woman with lupus nephritis, preeclampsia, NS, and hemolytic anemia illustrates this scenario. 2 Secondary causes of NS are listed in Table 1 . 1 , 3
Pathophysiology
The mechanism of edema formation in NS is unclear. The primary defect seems to be increased glomerular permeability to albumin and other plasma proteins. Primary renal sodium retention and decreased oncotic pressure from hypoalbuminemia lead to increased extravasation of fluid from the intravascular space into the interstitial space, resulting in edema. 4
The pathophysiology of thrombogenesis in NS is also not completely understood but seems to be multifactorial, involving loss of coagulation regulatory proteins and a shift in the hemostatic balance toward a prothrombotic milieu. 5 Patients with NS and prothrombotic genetic mutations have a further increased risk of thrombosis.
Diagnostic Evaluation
New-onset edema, particularly in the lower extremities, is the most common presenting symptom of NS. Depending on disease severity, patients may have edema extending to the proximal lower extremities, lower abdomen, or genitalia. Ascites, periorbital edema, hypertension, and pleural effusion are also possible presenting features. Patients may report foamy urine, exertional dyspnea or fatigue, and significant fluid-associated weight gain. 1 , 3
The diagnostic criteria for NS are listed in Table 2 . 1 Confirmation of proteinuria via 24-hour urine collection is cumbersome for patients, and the specimen can be collected incorrectly. The protein-to-creatinine ratio from a single urine sample is commonly used to diagnose nephrotic-range proteinuria. Although this spot test has limited accuracy in patients who exercise heavily, are gaining or losing muscle mass, or have similar factors, in general, it is sufficient for diagnosing heavy proteinuria. 1
Further diagnostic assessment of patients with NS has three goals: to assess for complications, identify underlying disease, and potentially determine the histologic type of idiopathic NS. The role of renal biopsy in patients with NS is controversial, and there are no evidence-based guidelines regarding indications for biopsy. Whether biopsy is performed often depends on the preferences of consulting nephrologists. In patients with NS from a known secondary cause and who are responding to treatment appropriately, biopsy will likely add little to treatment. Biopsy may be more useful for treatment and prognosis in patients with idiopathic NS of an unknown histologic disease type or with suspected underlying systemic lupus erythematosus or other renal disorders.
Complications
Various systemic complications are commonly associated with NS. These are thought to result from overproduction of hepatic proteins and loss of low-molecular-weight proteins in the urine, although the specific mechanisms have not been fully described. 5 It is generally not necessary to screen otherwise asymptomatic patients for these complications. Figure 1 is an algorithm for the diagnosis and management of NS. 1
VENOUS THROMBOSIS
Venous thrombosis is one of the most important complications of NS, but the true incidence and risk are difficult to determine because of the heterogeneity of the clinical manifestations and causes of NS. The most common sites of venous thrombosis in adults are in the deep veins of the lower limbs, although thrombosis can also occur in the renal veins and can cause pulmonary embolism. Arterial thrombosis is rare in patients with NS. 1
In a historical case series of patients with NS, venous thrombosis of the lower limb occurred in 8% of patients, and renal venous thrombosis occurred in up to 25% of patients. However, more recent data suggest a much lower risk of venous thrombosis in patients with NS. 6 In a retrospective study, deep venous thrombosis occurred in 1.5% of adults with NS, and renal venous thrombosis occurred in 0.5% of adults with NS. 6 Venous thrombosis is much more common in adults than in children and is more common in adults with membranous nephropathy than other histologies, 5 with an incidence of up to 7%. 7 Unless the patient's history suggests a thromboembolic complication, screening otherwise asymptomatic patients for thromboembolic events is not indicated.
Bacterial infections, especially cellulitis, are a potential complication of NS. A Cochrane review found no relevant studies of infections in adults with NS. 8 There are no reliable data on the incidence of infection as a complication of NS and no current guidelines for the use of prophylactic antibiotics in adults with NS.
RENAL FAILURE
Acute kidney injury is considered a rare spontaneous complication of NS. It can coexist with NS when it is caused by the same factors that lead to edema and proteinuria, such as lupus nephritis and drug-induced interstitial nephritis. 1 , 9 Although acute kidney injury is uncommon in NS, tests for renal function, quantification of proteinuria, serum chemistry, and lipid profile are appropriate to assess renal function and determine the degree of hyperlipidemia. Table 3 shows the differential diagnosis of acute kidney injury in patients with NS. 3
HYPERLIPIDEMIA
Elevated lipid levels (potentially markedly elevated) are a common feature of NS. Any subtype of lipoprotein concentrations can be elevated. There are no recent epidemiologic data to indicate how common or severe this complication is, and no recent data regarding the impact of treatment for dyslipidemia associated with NS. However, resolving proteinuria and any underlying disease process is believed to improve or resolve the dyslipidemia. 1 , 10
Management of NS is limited by a lack of clear evidence-based guidelines, although recent expert consensus guidelines provide useful recommendations. 11 In addition to correction of treatable causes, management includes general measures to treat symptoms such as edema and, in some cases, immunosuppressant treatment of the renal pathology.
GENERAL TREATMENT MEASURES
Because of the possible pathophysiologic role of sodium retention, some experts recommend that routine treatment of patients with NS include restricting dietary sodium to less than 3 g per day and restricting fluid to less than 1,500 mL per day. 1
TREATING EDEMA
Patients with nephrosis are resistant to diuretics, even if the glomerular filtration rate is normal. Loop diuretics act in the renal tubule and must be protein-bound to be effective. Serum proteins are reduced in NS, limiting the effectiveness of loop diuretics, and patients may require higher-than-normal doses. 3 Other mechanisms for diuretic resistance are also possible. Oral loop diuretics with twice-daily administration are usually preferred because of the longer duration of action. However, with severe NS and edema, gastrointestinal absorption of the diuretic may be uncertain because of intestinal wall edema, and intravenous diuretics may be necessary. Diuresis should be relatively gradual and guided by daily weight assessment, with a target of 2 to 4 lb (1 to 2 kg) per day. 3
Furosemide (Lasix) at 40 mg orally twice daily or bumetanide at 1 mg twice daily is a reasonable starting dosage, with approximate doubling of the dose every one to three days if there is inadequate improvement in edema or other evidence of fluid overload. 3 An approximate upper limit for furosemide is 240 mg per dose or 600 mg total per day, 12 but there is no clear evidence or rationale for this limit. If there is still an inadequate clinical response, patients may be treated by changing to intravenous loop diuretics, adding oral thiazide diuretics, or giving an intravenous bolus of 20% human albumin prior to an intravenous diuretic bolus. 3
ANTICOAGULATION FOR VENOUS THROMBOSIS
Despite the known risk of venous thrombosis in patients with NS, there are no randomized controlled trials to guide whether prophylactic anticoagulation should be used and for how long. 1
Adult patients with NS should be assessed individually for underlying disease. Additional considerations are the severity of NS (i.e., serum albumin less than 2.0 to 2.5 g per dL [20 to 25 g per L] may be more likely to prompt anticoagulation prophylaxis 7 ), preexisting thrombophilic states, and the overall likelihood of serious bleeding events from the use of oral anticoagulation. The decision to treat with anticoagulants should be made individually. 13 Although the benefits of anticoagulation may outweigh the risks in selected patients at high risk of venous thrombosis (e.g., those with known prothrombotic tendency or a history of venous thrombosis), anticoagulation is not routinely used for primary prevention of thrombotic events in patients with NS. 6
TREATING AND PREVENTING INFECTION
Infection has been reported in up to 20% of adults with NS, although it is unclear if NS is causative or if the infection is a result of hospitalization, corticosteroid use, or other factors. 1 A Cochrane review found no strong evidence to recommend a specific intervention to prevent infection in adults with NS. 8
TREATING DYSLIPIDEMIA
A recent Cochrane review found insufficient evidence to determine if lipid-lowering agents are helpful in managing dyslipidemia in adults with NS and no other indications for treatment based on previously obtained lipid levels. 10
ANTIPROTEINURIC TREATMENT
Treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers appears to reduce the risk of venous thrombosis, although this has not been confirmed. 14 Treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers is often recommended for patients with NS because of their known antiproteinuric effects. However the degree of benefit for specific outcomes, such as renal failure or recovery, improvement in edema, or need for dialysis, is unproven, and the evidence supporting the routine use of these medications is conflicting.
IMMUNOSUPPRESSIVE THERAPY
Corticosteroids are often used in the treatment of NS despite an absence of supporting data. In recent years, corticosteroids and other immunosuppressive treatments have been investigated for use in NS ( Table 4 ) . 15 A Cochrane review showed that combining an alkylating agent with a corticosteroid has short- and long-term benefits for membranous nephropathy in adults with NS. 15 In general, immunosuppressive treatment has no proven benefit for most adults with idiopathic NS, and the potential risks may outweigh any benefits. The role of such treatment and specific treatment decisions, such as type and duration of therapy, depend on clinical factors and potentially on the histologic diagnosis identified on biopsy. If NS is steroid-resistant or does not improve, other immunosuppressive treatments should be considered in cooperation with a nephrologist. Immunosuppressive therapy for NS secondary to systemic lupus erythematosus is highly effective and supported by multiple studies, and may lead to partial or complete remission in patients with minimal change disease or primary focal segmental glomerulosclerosis.
The prognosis for NS is highly dependent on the underlying cause, the disease histology, and patient clinical factors. Although many patients improve with appropriate supportive care and do not require any specific therapy, others worsen despite aggressive, specific therapy and may require dialysis. In one study, routine treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, plus selective use of corticosteroids or other immunosuppressants, led to a remission rate of 76%, with 12% of patients requiring hemodialysis. 16
Idiopathic membranous nephropathy is one of the most common forms of primary NS in adults, and has a generally favorable prognosis. 15 The prognosis for this illness roughly follows a “rule of thirds”: about one-third of patients have a benign course with a high rate of remission; one-third have ongoing evidence of proteinuria or edema but maintain normal renal function; and somewhat less than one-third of patients progress toward end-stage renal disease within 10 years. 15
Adults with primary focal segmental glomerulosclerosis, however, tend to have a poorer prognosis, and the degree of proteinuria is a significant prognostic factor. Although about one-half of patients with nephrotic-range proteinuria progress to end-stage renal disease over five to 10 years, patients with very heavy proteinuria (10 to 14 g per day) will develop end-stage renal disease on average within two to three years. 17
Subspecialist Consultation
Consultation with nephrologists should guide decisions about use of anticoagulation and immunosuppressants, need for renal biopsy, and for other areas of uncertainty.
Data Sources : A Medline literature search was conducted using the key term nephrotic syndrome. The search was limited to English, human, core clinical journals ( Abridged Index Medicus ), and publication years between 2005 and 2015. Additional searches were conducted combining the baseline nephrotic syndrome search with other relevant key words, such as venous thrombosis, hyperlipidemia, infection, and acute kidney injury. Relevant original articles cited in reviews were used as the sources for cited data. Search dates: January 25, 2015, and December 10, 2015.
This review updates a previous article on this topic by the author. 18
Hull RP, Goldsmith DJ. Nephrotic syndrome in adults. BMJ. 2008;336(7654):1185-1189.
Williams WW, Ecker JL, Thadhani RI, Rahemtullah A. Case records of the Massachusetts General Hospital. Case 38-2005. A 29-year-old pregnant woman with the nephrotic syndrome and hypertension. N Engl J Med. 2005;353(24):2590-2600.
Floege J. Introduction to glomerular disease: clinical presentations. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology . 5th ed. Philadelphia, Pa.: Elsevier Saunders; 2015.
Siddall EC, Radhakrishnan J. The pathophysiology of edema formation in the nephrotic syndrome. Kidney Int. 2012;82(6):635-642.
Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7(3):513-520.
Kayali F, Najjar R, Aswad F, Matta F, Stein PD. Venous thromboembolism in patients hospitalized with nephrotic syndrome. Am J Med. 2008;121(3):226-230.
Pincus KJ, Hynicka LM. Prophylaxis of thromboembolic events in patients with nephrotic syndrome. Ann Pharmacother. 2013;47(5):725-734.
Wu HM, Tang JL, Cao L, Sha ZH, Li Y. Interventions for preventing infection in nephrotic syndrome. Cochrane Database Syst Rev. 2012;4:CD003964.
Koomans HA. Pathophysiology of acute renal failure in idiopatic nephrotic syndrome. Nephrol Dial Transplant. 2001;16(2):221-224.
Kong X, Yuan H, Fan J, Li Z, Wu T, Jiang L. Lipid-lowering agents for nephrotic syndrome. Cochrane Database Syst Rev. 2013;12:CD005425.
Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—application to the individual patient. Kidney Int. 2012;82(8):840-856.
Furosemide dosage. Drugs.com. http://www.drugs.com/dosage/furosemide.html . Accessed January 13, 2016.
Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome. J Am Soc Nephrol. 2007;18(8):2221-2225.
Mahmoodi BK, Mulder AB, Waanders F, et al. The impact of antiproteinuric therapy on the prothrombotic state in patients with overt proteinuria. J Thromb Haemost. 2011;9(12):2416-2423.
Chen Y, Schieppati A, Chen X, et al. Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2014;10:CD004293.
McQuarrie EP, Stirling CM, Geddes CC. Idiopathic membranous nephropathy and nephrotic syndrome. Nephrol Dial Transplant. 2012;27(1):235-242.
Korbet SM. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23(11):1769-1776.
Kodner C. Nephrotic syndrome in adults: diagnosis and management. Am Fam Physician. 2009;80(10):1129-1134.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2016 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Nephrotic syndrome
On this page, preparing for your appointment.
Tests and procedures used to diagnose nephrotic syndrome include:
- Urine tests. A urinalysis can reveal abnormalities in your urine, such as large amounts of protein. You might be asked to collect urine samples over 24 hours.
- Blood tests. A blood test can show low levels of the protein albumin and often decreased levels of blood protein overall. Loss of albumin is often associated with an increase in blood cholesterol and blood triglycerides. The creatinine and urea nitrogen levels in your blood also might be measured to assess your overall kidney function.
- Kidney biopsy. Your doctor might recommend removing a small sample of kidney tissue for testing. During a kidney biopsy, a needle is inserted through your skin and into your kidney. Kidney tissue is collected and sent to a lab for testing.
More Information
- Kidney biopsy
Treatment for nephrotic syndrome involves treating any medical condition that might be causing your nephrotic syndrome. Your doctor might also recommend medications and changes in your diet to help control your signs and symptoms or treat complications of nephrotic syndrome.
Medications might include:
Blood pressure medications. Drugs called angiotensin-converting enzyme (ACE) inhibitors reduce blood pressure and the amount of protein released in urine. Medications in this category include lisinopril (Prinivil, Qbrelis, Zestril), benazepril (Lotensin), captopril and enalapril (Vasotec).
Another group of drugs that works similarly is called angiotensin II receptor blockers (ARBs) and includes losartan (Cozaar) and valsartan (Diovan). Other medications, such as renin inhibitors, also might be used, though angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are generally used first.
- Water pills (diuretics). These help control swelling by increasing your kidneys' fluid output. Diuretic medications typically include furosemide (Lasix). Others include spironolactone (Aldactone, Carospir) and thiazides, such as hydrochlorothiazide or metolazone (Zaroxolyn).
Cholesterol-reducing medications. Statins can help lower cholesterol levels. However, it's not clear whether cholesterol-lowering medications can improve the outcomes for people with nephrotic syndrome, such as avoiding heart attacks or decreasing the risk of early death.
Statins include atorvastatin (Lipitor), fluvastatin (Lescol XL), lovastatin (Altoprev), pravastatin (Pravachol), rosuvastatin (Crestor, Ezallor) and simvastatin (Zocor).
- Blood thinners (anticoagulants). These might be prescribed to decrease your blood's ability to clot, especially if you've had a blood clot. Anticoagulants include heparin, warfarin (Coumadin, Jantoven), dabigatran (Pradaxa), apixaban (Eliquis) and rivaroxaban (Xarelto).
- Immune system-suppressing medications. Medications to control the immune system, such as corticosteroids, can decrease the inflammation that accompanies some of the conditions that can cause nephrotic syndrome. Medications include rituximab (Rituxan), cyclosporine and cyclophosphamide.
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Changes to your diet might help with nephrotic syndrome. Your doctor might refer you to a dietitian, who might recommend that you do the following:
- Choose lean sources of protein. Plant-based protein is helpful in kidney disease.
- Reduce the amount of fat and cholesterol in your diet to help control your blood cholesterol levels.
- Eat a low-salt diet to help control swelling.
- Reduce the amount of liquid in your diet.
Start by seeing your primary care doctor. If your doctor suspects you or your child has a kidney problem, such as nephrotic syndrome, you might be referred to a doctor who specializes in the kidneys (nephrologist).
Here's some information to help you get ready for your appointment.
What you can do
When you make the appointment, ask if there's anything you need to do in advance, such as restrict your diet. Take a family member or friend along, if possible, to help you remember the information you'll be given.
Make a list of:
- Your or your child's symptoms and when they began
- Key personal information, including major stresses or recent life changes
- All medications, vitamins or other supplements you or your child takes, including doses
- Questions to ask your doctor
For nephrotic syndrome, some questions to ask include:
- What's the most likely cause of my or my child's nephrotic syndrome?
- What tests do I or my child need?
- Is this condition likely temporary?
- What are the treatment options? And which do you recommend?
- Are there changes I can make to my or my child's diet? Could consulting a dietitian help?
- How can I best manage this condition with my or my child's other medical conditions?
- Are there brochures or other printed material that I can have? What websites do you recommend?
What to expect from your doctor
Your doctor is likely to ask you questions, such as:
- Do symptoms come and go, or do you have them all the time?
- How severe are the symptoms?
- Does anything seem to improve the symptoms?
- What, if anything, appears to worsen the symptoms?
Feb 23, 2022
- Ferri FF. Nephrotic syndrome. In: Ferri's Clinical Advisor 2020. Elsevier; 2020. https://www.clinicalkey.com. Accessed Nov. 22, 2019.
- Nephrotic syndrome in adults. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/kidney-disease/nephrotic-syndrome-adults. Accessed Nov. 22, 2019.
- Kelepouris E, et al. Overview of heavy proteinuria and the nephrotic syndrome. https://www.uptodate.com/contents/search. Accessed Nov. 24, 2019.
- A to Z health guide: Nephrotic syndrome. National Kidney Foundation. https://www.kidney.org/atoz/content/nephrotic. Accessed Nov. 22, 2019.
- Childhood nephrotic syndrome. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/kidney-disease/children/childhood-nephrotic-syndrome. Accessed Nov. 22, 2019.
- Symptoms & causes
- Doctors & departments
- Diseases & Conditions
- Nephrotic syndrome diagnosis & treatment
Associated Procedures
Products & services.
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Assortment of Compression Products at Mayo Clinic Store
- Newsletter: Mayo Clinic Health Letter — Digital Edition
CON-XXXXXXXX
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- Register / Log in
Nephritic syndrome
An acute presentation in patients with glomerular inflammation (e.g., hematuria with red blood cell casts, pyuria, and mild proteinuria) that results in hypertension, oliguria, and fluid retention with edema. Can be caused by various autoimmune disorders, hereditary disorders, and infectious diseases.

Nephrotic Syndrome: Understanding Kidney Disorder
A kidney illness known as nephrotic syndrome is characterized by high cholesterol levels, swelling (edema), low blood protein levels, and excess protein in the urine.
Recognizing the signs of this condition is essential for correct diagnosis and efficient care as it can be brought on by a number of different kidney diseases. In this post, we examine the typical Nephrotic Syndrome symptoms, its underlying causes, and potential cures.
Understanding Nephrotic Syndrome
A collection of symptoms known as nephrotic syndrome are brought on by damage to the glomeruli, which are the kidneys' microscopic filtering cells. Proteinuria (excess protein in the urine) is caused when these filtration systems are impaired and allow the protein to escape into the urine.
Edema (swelling) in several areas of the body, including the legs, ankles, and face, can result from this protein loss. Nephrotic Syndrome can also increase cholesterol levels, which can cause issues with blood flow and general health.

Symptoms of Nephrotic Syndrome
1. proteinuria:.
Proteinuria, a condition in which the kidneys produce excessive amounts of protein in the urine, is one of the main signs of nephrotic syndrome. Because of the increased protein concentration, the urine may seem frothy or bubbly. A low level of albumin, a necessary blood protein, can result from protein loss , which is known as hypoalbuminemia.
2. Edema (Swelling):
Edema, a frequent sign of Nephrosis, is brought on by protein loss in the blood. Protein levels drop, which lowers the blood vessel's oncotic pressure, allowing fluid to flow out and build up in the tissues, causing swelling. Although it can affect other parts of the body as well, edema is most visible in the legs, ankles, and face.
3. Hyperlipidemia:
Hyperlipidemia, also known as an increase in blood cholesterol levels, can result from Nephrosis. Increased blood lipid and cholesterol levels can increase the risk of cardiovascular issues like heart disease and stroke.
4. Fatigue and Weakness:
It is possible to experience a lack of energy, exhaustion, and weakness as a result of the loss of vital proteins from the blood, especially albumin. Lethargy is caused by the body's failure to maintain normal protein levels, which affects a number of physiological functions.
5. Decreased Appetite:
Due to how protein loss affects the body's metabolism, people with Nephrosis may find they have less hunger. This diminished appetite may be a factor in undernutrition and weight loss.
6. Foamy Urine:
When urinating, too much protein can make the urine appear frothy. This foamy or bubbly urine is an obvious indicator of proteinuria and needs to be evaluated by a doctor.
7. Elevated Blood Pressure:
Nephrosis may occasionally result in a rise in blood pressure. If unchecked, high blood pressure can exacerbate renal damage already present and advance the disease.
8. Susceptibility to Infections:
Immune system deterioration brought on by the loss of immunoglobulins and other vital blood proteins can increase infection susceptibility in people with Nephrosis. Infections that are severe or recurrent may result from this sensitivity.
Proteinuria, edema, hyperlipidemia, tiredness, and other kidney-related symptoms are all signs of nephrotic syndrome. Early detection of these symptoms is essential for accurate diagnosis and effective care. Timely intervention can assist maintain kidney function and enhance the quality of life for those with Nephrosis. This is in addition to comprehensive medical care.

Unknown Error
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

solar eclipse
25 templates

education technology
180 templates

32 templates

28 templates

thanksgiving
38 templates

Nephrotic Syndrome
Nephrotic syndrome presentation, free google slides theme and powerpoint template.
Download the "Nephrotic Syndrome" presentation for PowerPoint or Google Slides. Taking care of yourself and of those around you is key! By learning about various illnesses and how they are spread, people can get a better understanding of them and make informed decisions about eating, exercise, and seeking medical attention. This Google Slides theme and PowerPoint template is editable and can be used to teach the general public or medical students about a certain disease.
Features of this template
- 100% editable and easy to modify
- Different slides to impress your audience
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides and Microsoft PowerPoint
- Includes information about fonts, colors, and credits of the resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access

Register for free and start editing online
- Open supplemental data
- Reference Manager
- Simple TEXT file
People also looked at
Case report article, case report: novel compound heterozygous tprkb variants cause galloway-mowat syndrome.

- 1 Department of Pediatrics, Hamamatsu University School of Medicine, Hamamatsu, Japan
- 2 Department of Biochemistry, Hamamatsu University School of Medicine, Hamamatsu, Japan
- 3 Department of Pediatrics, Hamamatsu Medical Center, Hamamatsu, Japan
- 4 Department of Hamamatsu Child Health and Development, Hamamatsu University School of Medicine, Hamamatsu, Japan
Background: Galloway-Mowat syndrome (GAMOS) is a rare genetic disease characterized by early-onset nephrotic syndrome and microcephaly with central nervous system abnormalities. Pathogenic variants in genes encoding kinase, endopeptidase, and other proteins of small size (KEOPS) complex subunits cause GAMOS. The subunit TPRKB (TP53RK binding protein) has been reported in only two patients with GAMOS with homozygous missense variants.
Clinical report: Herein, we described a three-year-old male with GAMOS. He exhibited developmental delay, developmental regression, microcephaly, distinctive facial features, skeletal abnormalities, and epilepsy. Brain magnetic resonance imaging revealed progressive brain atrophy, delayed myelination, T2-hypointense signals in the thalamus, and multiple intracranial abnormal signals on diffusion-weighted imaging. He presented with relapsing nephrotic proteinuria exacerbated by upper respiratory tract infections and progressive renal function decline. Exome sequencing identified compound heterozygous missense and frameshift variants in TPRKB : c.224dup, p.(Ser76IlefsTer3) and c.247C>T, p.(Leu83Phe).
Conclusions: Our study supports that pathogenic TPRKB variants cause KEOPS complex-related GAMOS.
Introduction
Galloway-Mowat syndrome (GAMOS; MIM#251300) is a rare genetic disease characterized by early-onset nephrotic syndrome (NS), microcephaly, and brain anomalies that result in severely delayed psychomotor development ( 1 ). The renal prognosis of GAMOS is poor, and renal replacement therapy or renal transplantation is necessary for survival ( 2 ). GAMOS is clinically and genetically heterogeneous. Other clinical features include dysmorphic facial features, skeletal anomalies, and esophageal hiatal hernias. Several genes have been reported to be associated with GAMOS, including WDR73 , LAGE3 , OSGEP , TP53RK , TPRKB , WDR4 , NUP107 , NUP133 , GON7, YRDC , and PRDM15 ( 2 – 8 ).
TPRKB (TP53RK binding protein) (MIM*608680) encodes a subunit of the highly conserved kinase, endopeptidase, and other proteins of small size (KEOPS) complex. The human KEOPS complex comprises OSGEP, TP53RK, TPRKB, LAGE3, and GON7 ( 9 ). It regulates the universal chemical modification of tRNA, N 6 -threonylcarbamoyl adenosine (t 6 A), which is essential for normal cell growth and accurate translation by ribosomes ( 10 ). The KEOPS complex is involved in human podocyte migration through impaired cell proliferation, increased apoptosis, genomic instability, and defects in actin regulation ( 4 ). Biallelic pathogenic variants in genes of the KEOPS complex are responsible for GAMOS in many patients ( 3 , 4 ). Renal biopsies of patients with KEOPS complex-related GAMOS reveals mainly focal segmental glomerulosclerosis or diffuse mesangial sclerosis, with partial podocyte foot process effacement on electron microscopy ( 4 ). In contrast, GAMOS 5 (MIM#617731) caused by TPRKB has only been reported in two patients with homozygous missense variants ( 4 ). Herein, we report the case of a patient with GAMOS with compound heterozygous missense and frameshift variants in TPRKB .
Clinical report
After 40 weeks of gestation without asphyxia, a Japanese boy was born to nonconsanguineous, healthy parents as their first child. His birth weight was 3,450 g [+1.04 standard deviation (SD)], his length was 51.0 cm (+0.90 SD), and his occipitofrontal circumference was 35.0 cm (+1.25 SD). Distinctive facial features such as widely spaced eyes and pointed chin, pectus excavatum, tapered fingers, esotropia, and muscle hypotonia, were noted ( Figures 1A–D ). He could hold his head up at five months of age and sit with support at eight months of age. At nine months of age, he babbled but could not maintain a sitting position, even with support. At one year and one month of age, antiepileptic drugs were initiated because of the onset of epileptic spasms and focal to bilateral tonic-clonic seizures. His development regressed, losing interest in toys at one year and three months of age and becoming unable to hold his head up at one year and eight months of age. Tube feeding was initiated because of dysphagia and weight loss at two years and one month of age, followed by gastrostomy at three years of age.
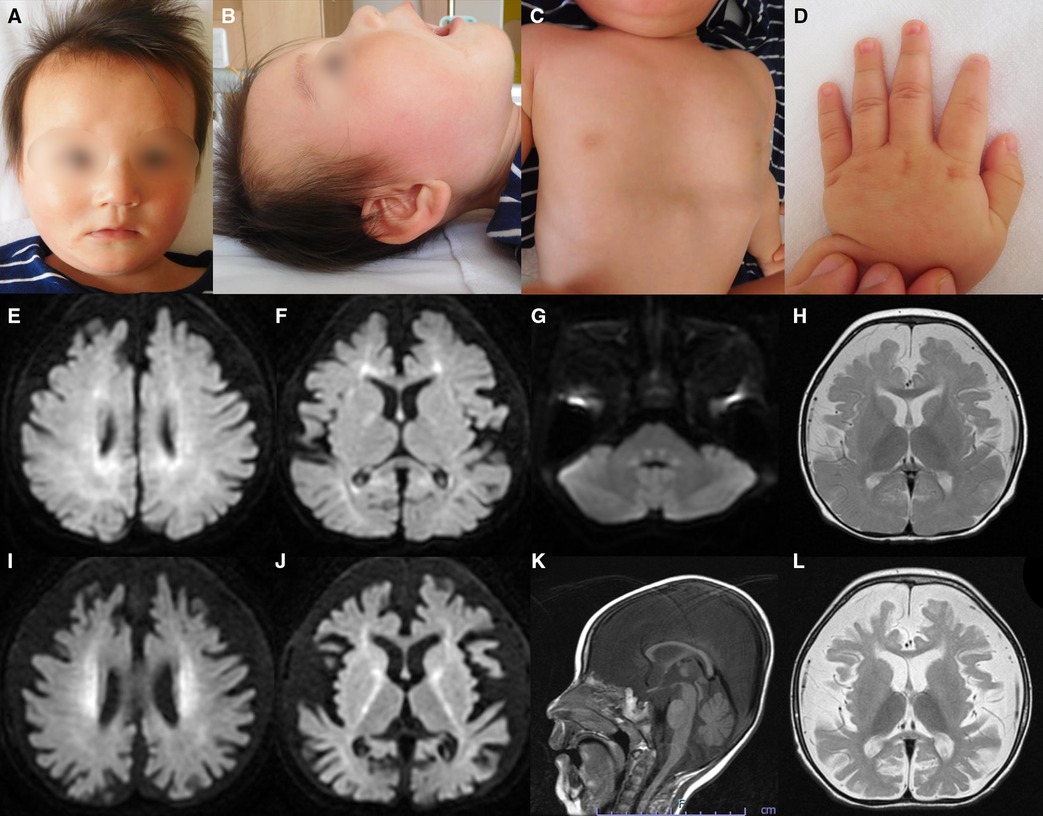
Figure 1 . Representative clinical findings. ( A – D ) Photographs of the patient at ten months of age. Widely spaced eyes, epicanthus, left esotropia, medial sparse eyebrow, pointed chin, and thin upper lip vermilion are observed ( A , B ). Pectus excavatum ( C ) and tapered fingers ( D ) are recognized. ( E – L ) Brain magnetic resonance imaging (MRI) at ten months of age ( E – H ) and one year and eight months of age ( I – L ). Axial diffusion-weighted imaging (DWI) demonstrated elevated signal in the bilateral periventricular white matter, deep and subcortical white matter of the bilateral posterior lobe, posterior limb of the internal capsule, and dorsal part of the pons ( E – G ). An axial T2-weighted image shows brain atrophy with frontal white matter dominance and diffuse hypointense signal of the thalamus ( H ) DWI hyperintensities of the bilateral corona radiata and internal capsule are more distinct ( I , J ). A sagittal T1-weighted image shows atrophy of cerebellar vermis and thinning of the corpus callosum ( K ). An axial T2-weighted image reveals progressive brain atrophy and no hypointensity in the subcortical white matter. Hypointensity in the anterior limb of the internal capsule is obscured ( L ).
He was diagnosed with proteinuria at six months of age. The spot urine protein/creatinine ratio (UPCR) was 3.33 g/g at one year and one month of age. At 2 years and eight months of age, he was diagnosed with NS due to increased proteinuria (UPCR 17.23 g/g) and hypoalbuminemia (Alb 2.9 g/dl) induced by an acute upper respiratory tract infection. As the infection improved, the proteinuria decreased (UPCR 0.97 g/g). He initiated treatment with valsartan. Subsequently, NS relapsed during respiratory infections, with serum albumin ranging from 2.2 to 3.9 g/dl and UPCR ranging from 0.73 to 17.23 g/g. Serum creatine was low (0.15 mg/dl), while the cystatin C value, which was 0.46 mg/L at 2 years of age, increased to 0.66 mg/L at 3 years of age, suggesting a progressive decline in renal function. Renal biopsy was not performed because of respiratory depression during anesthesia.
Metabolic screenings of blood and cerebrospinal fluid, including amino acids, lactic acid, and pyruvic acid, were unremarkable. Interictal electroencephalogram at one year and eight months of age revealed multifocal spikes with slow background activity. Bilateral visual evoked potentials were absent. Electrocardiography, auditory brainstem response, nerve conduction studies, electroretinography, and echocardiography findings were normal. Radiography revealed no bone abnormalities other than scoliosis and pectus excavatum. Abdominal computed tomography scans of the kidneys, bladder, liver, and spleen revealed no abnormalities. Brain magnetic resonance imaging (MRI) showed a diffuse T2-hypointense signal in the thalamus and numerous characteristic aberrant DWI signals in the white matter, internal capsule, and dorsal part of the pons ( Figures 1E–J, L ). At one year and eight months of age, there was no T2-hypointensity in the subcortical white matter, suggesting hypomyelination ( Figure 1L ). The T2-hypointensity in the anterior limb of the internal capsule observed at 10 months of age was indistinct at one year and eight months of age, suggesting demyelination ( Figures 1H, L ). Progressive atrophy of the cerebral hemispheres and cerebellum was observed ( Figures 1K, L ).
The last physical examination at three years of age showed a body weight of 12.0 kg (−1.1 SD), height of 97.0 cm (+1.1 SD), and head circumference of 42.5 cm (−4.4 SD). He had a social smile, but his visual tracking was poor. He had constant stridor. Hypotonia of the upper limbs and trunk and spasticity of the lower limbs were noted. His deep tendon reflexes were hyperactive with lower-extremity dominance, and the Babinski reflex and ankle clonus were observed. These findings were suggestive of GAMOS. Progression of neuronal symptoms and repeated worsening of urinary proteinuria due to infectious disease are shown along the timeline in Figure 2 .
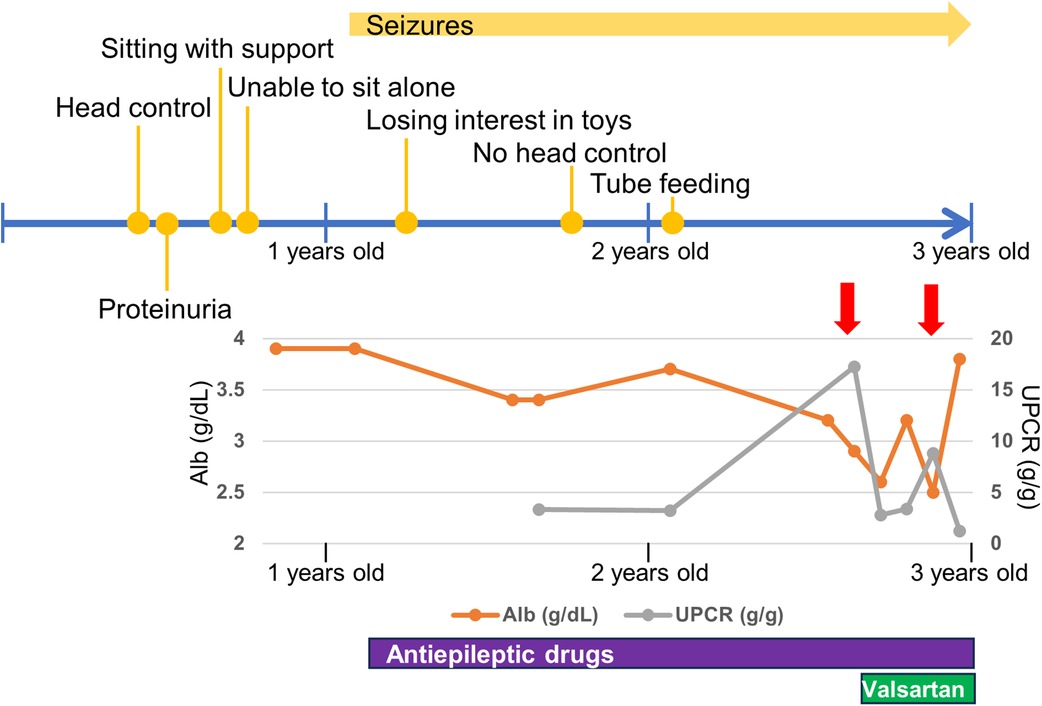
Figure 2 . Timeline of neurological and renal symptoms. Red allows indicate respiratory infections. Alb, serum albumin; UPCR, spot urine protein/creatinine ratio.
Ethical compliance
This study was approved by the Institutional Review Board Committee of Hamamatsu University School of Medicine (16-076) and was performed after obtaining written informed consent. Consent to publish identifiable images was obtained from the patient's parents.
Variant sequencing
Genomic DNA was extracted from the blood leukocytes of this family. The patient's DNA for exome sequencing was captured using an xGen Exome Research Panel v2 kit (IDT, Coralville, IA, USA) and sequenced on a NextSeq500 (Illumina, San Diego, CA, USA) with 75-bp paired-end reads. Data processing, variant calling, annotation, and filtering were performed as previously described ( 11 ). We identified compound heterozygous variants of TPRKB (NM_016058.5), c.224dup, p.(Ser76IlefsTer3) and c.247C > T, p.(Leu83Phe) ( Figure 3A ). The allele frequencies of the c.224dup and c.247C>T variants were 0.0055% (6/108,604 alleles) and 0.0064% (7/108,604 alleles), respectively, in the ToMMo 54KJPN Allele Frequency Panel (v20230626) ( https://jmorp.megabank.tohoku.ac.jp/ ). The allele frequencies in an East Asian population in the gnomAD v4.0.0 ( http://gnomad.broadinstitute.org/ ) were 0.005039% (2/39,694 alleles) and 0.002520% (1/39,684 alleles), respectively. The c.247C>T, p.(Leu83Phe) variant was predicted to be deleterious using in silico pathogenicity prediction tools ( Supplementary Table S1 ), and the Leu83 residue is highly evolutionarily conserved ( Figure 3B ). The Leu83Phe substitution, like the previously reported TPRKB variants Leu136Pro (α6) and Tyr149Cys (α7), is located at the α helix (α3), a deeply buried position that may affect the structural integrity of the protein ( 4 , 12 ) ( Figure 2B ; Supplementary Figure S1 ). No other likely pathogenic variants were identified among the candidate variants ( Supplementary Tables S1, S2 ). No candidate pathogenic copy number variants were detected using the eXome-hidden Markov model or jNord methods ( 13 , 14 ). According to the American College of Medical Genetics and Genomics guideline 2015, the c.224dup variant was classified as likely pathogenic and the c.247C>T variant uncertain significance ( Supplementary Tables S1, S2 ). However, since our case had characteristic GAMOS symptoms such as nephrotic proteinuria and central nervous system abnormalities, we concluded that the TPRKB variants were likely responsible in this case.
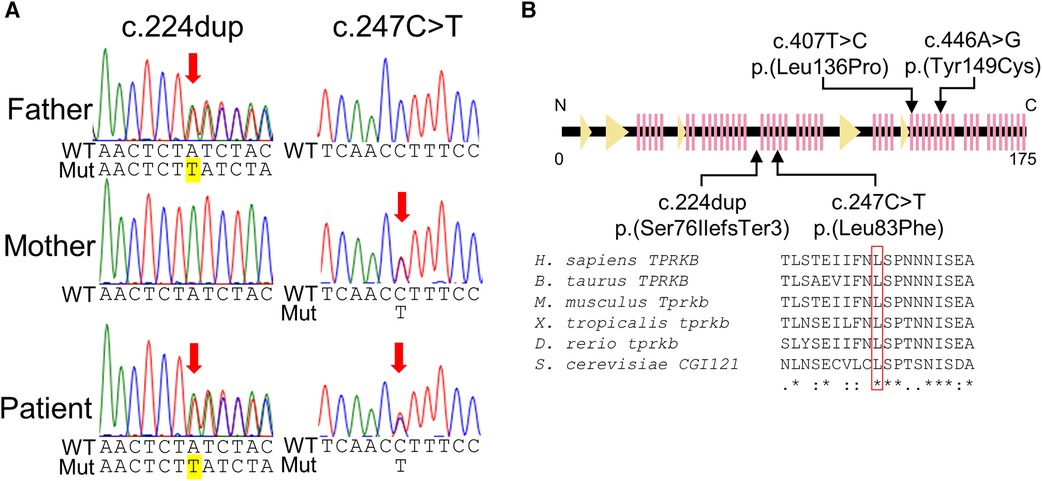
Figure 3 . TPRKB variants in the patients with Galloway-Mowat syndrome. ( A ) Compound heterozygous variants in TPRKB . Sanger sequencing shows a paternal T duplication (yellow) on the left and a maternal missense variant on the right. ( B ) Schematic presentation of the TPRKB protein and location of altered residues. Previously reported TPRKB variants are depicted above, and the variants identified in our case are shown below. The predicted secondary structure modified from the PDB (code 6WQX; https://www.rcsb.org/ ) is indicated for β-strand and α-helix conformation as triangle and zigzag lines, respectively. Multiple amino acid sequences of TPRKB were aligned using the ClustalW tool ( http://www.genome.jp/tools/clustalw ).
In this study, we describe the case of a patient with novel compound heterozygous TPRKB variants. Two individuals with GAMOS with TPRKB variants have been previously described, and the clinical manifestations of the three cases of TPRKB variants, including our patient, are summarized in Table 1 ( 4 ). Microcephaly, global developmental delay, spasticity, and distinctive facial features were common to all three cases. Most patients with GAMOS associated with the KEOPS complex had microcephaly, developmental delay, and distinctive facial features, whereas spasticity was not commonly reported (9/33) ( 4 ). This may be because patients with GAMOS with TPRKB variants were relatively older children in whom spasticity was evident due to progression of brain atrophy. In our case, proteinuria was diagnosed earlier than in other patients with TPRKB variants, possibly because of the loss-of-function variant. The age of onset of proteinuria in patients with TPRKB-related GAMOS was later than that in patients with KEOPS complex-related GAMOS other than TPRKB-related GAMOS (median, three months) ( 4 ). Furthermore, the median age of death for GAMOS cases with variants of KEOPS complex-encoding genes other than TPRKB was reported to be six months of age. In contrast, one individual with TPRKB variant died at 6.8 years of age, and two cases survived at three and six years. TPRKB-related GAMOS may be milder than GAMOS caused by other KEOPS complex-related genes.
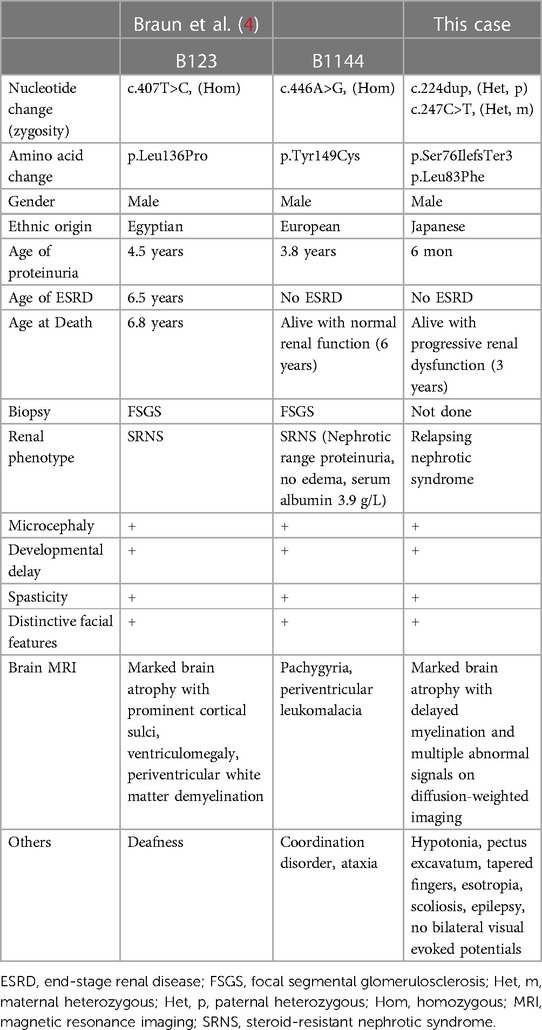
Table 1 . Clinical features of TPRKB -associated Galloway-Mowat syndrome .
The most frequently observed brain MRI anomalies in the GAMOS associated with the KEOPS complex include cortical and cerebellar atrophy, gyration abnormalities, and myelination defects ( 4 ). Abnormal DWI signals, as in our case, have been reported in patients with GAMOS with pathogenic variants of TP53RK and OSGEP , which belong to the KEOPS complex ( 15 , 16 ). MRI of another case of GAMOS attributed to TP53RK showed diffuse T2-hypointense signals in the thalamus ( 17 ). In animal studies, knockout of tprkb in zebrafish larvae recapitulated microcephaly, and Tprkb knockout mouse embryos showed a significant reduction in brain size, cortex length, cortex-midbrain midline length, and cortex width ( 4 ). Proteomic profiling of fibroblasts from patients with GAMOS caused by OSGEP variants revealed upregulation of the P2X7 receptor signaling complex pathway ( 15 ). In multiple sclerosis, the sustained activation of P2X7 receptors induces oligodendrocyte death, demyelination, neuroinflammatory processes, and neurodegeneration ( 18 ). The activation of P2X7 receptors may be involved in the brain phenotype of KEOPS complex-related GAMOS.
Our patient had NS with repeated relapses concurrently with respiratory infections. Increased edema in patients with congenital NS is especially common during infection ( 19 ). Relapsing NS with mild persistent proteinuria and relapses of the nephrotic range associated with upper respiratory tract infections have been described in familial cases of the NPHS1 variants encoding nephrin, an essential component of the interpodocyte-spanning slit diaphragm ( 20 ). The molecular mechanism of NS relapse caused by genetic defects remains to be elucidated and requires further investigation.
The patients with KEOPS complex-related GAMOS have severe renal and neurological symptoms and die in early childhood ( 4 ). Kidney complications are the direct cause of most GAMOS deaths, and the patients with GAMOS should be screened regularly for proteinuria and renal function and evaluated closely ( 21 ). The genetic diagnosis of GAMOS is useful for family planning, and prenatal and preimplantation testing is available after genetic counseling.
In conclusion, we identified a patient with GAMOS and compound heterozygous TPRKB variants who had nephrotic proteinuria, developmental delay, developmental regression, microcephaly, distinctive facial features, skeletal abnormalities, epilepsy, progressive brain atrophy, delayed myelination, T2-hypointense signals in the thalamus, and multiple intracranial abnormal signals. This is the first case of a patient with GAMOS associated with a frameshift variant in TPRKB . Further accumulation of cases is necessary to establish the common clinical features in patients with pathogenic TPRKB variants.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board Committee of Hamamatsu University School of Medicine (16-076). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
THi: Data curation, Formal Analysis, Funding acquisition, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing. THa: Data curation, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing. YI: Resources, Writing – original draft, Writing – review & editing. RU: Data curation, Resources, Writing – original draft, Writing – review & editing. HU: Data curation, Investigation, Resources, Writing – original draft, Writing – review & editing. RK: Data curation, Investigation, Writing – original draft, Writing – review & editing. HI: Data curation, Resources, Writing – original draft, Writing – review & editing. TO: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. HS: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. TF: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research is supported by a donation from Hamamatsu City to the Endowed chair of Hamamatsu Child Health and Development. This work was supported by the Japan Agency for Medical Research and Development (AMED) (JP23ek0109549 to TO, JP23ek0109674 and JP23ek0109637 to HS), Grants-in-Aid for Scientific Research (B) (JP20H03641 and JP23H02875 to HS), Grant-in-Aid for Early-Career Scientists (23K14944 to TH), Grant-in-Aid for Research Activity Start-up (22K20852 to TH) from the Japan Society for the Promotion of Science (JSPS) KAKENHI, Japan Intractable Diseases (Nanbyo) Research Foundation (2020A02), and the Takeda Science Foundation.
Acknowledgments
We would like to thank the patients for participating in this work. We thank Editage ( www.editage.jp ) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1360867/full#supplementary-material
1. Galloway WH, Mowat AP. Congenital microcephaly with hiatus hernia and nephrotic syndrome in two sibs. J Med Genet . (1968) 5(4):319–21. doi: 10.1136/jmg.5.4.319
PubMed Abstract | Crossref Full Text | Google Scholar
2. Braun DA, Shril S, Sinha A, Schneider R, Tan W, Ashraf S, et al. Mutations in Wdr4 as a new cause of Galloway-Mowat syndrome. Am J Med Genet A . (2018) 176(11):2460–5. doi: 10.1002/ajmg.a.40489
3. Arrondel C, Missoury S, Snoek R, Patat J, Menara G, Collinet B, et al. Defects in T(6)a trna modification due to Gon7 and yrdc mutations lead to Galloway-Mowat syndrome. Nat Commun . (2019) 10(1):3967. doi: 10.1038/s41467-019-11951-x
4. Braun DA, Rao J, Mollet G, Schapiro D, Daugeron MC, Tan W, et al. Mutations in keops-complex genes cause nephrotic syndrome with primary microcephaly. Nat Genet . (2017) 49(10):1529–38. doi: 10.1038/ng.3933
5. Colin E, Huynh Cong E, Mollet G, Guichet A, Gribouval O, Arrondel C, et al. Loss-of-function mutations in Wdr73 are responsible for microcephaly and steroid-resistant nephrotic syndrome: Galloway-Mowat syndrome. Am J Hum Genet . (2014) 95(6):637–48. doi: 10.1016/j.ajhg.2014.10.011
6. Fujita A, Tsukaguchi H, Koshimizu E, Nakazato H, Itoh K, Kuraoka S, et al. Homozygous splicing mutation in Nup133 causes Galloway-Mowat syndrome. Ann Neurol . (2018) 84(6):814–28. doi: 10.1002/ana.25370
7. Mann N, Mzoughi S, Schneider R, Kühl SJ, Schanze D, Klämbt V, et al. Mutations in Prdm15 are a novel cause of Galloway-Mowat syndrome. J Am Soc Nephrol . (2021) 32(3):580–96. doi: 10.1681/asn.2020040490
8. Rosti RO, Sotak BN, Bielas SL, Bhat G, Silhavy JL, Aslanger AD, et al. Homozygous mutation in Nup107 leads to microcephaly with steroid-resistant nephrotic condition similar to Galloway-Mowat syndrome. J Med Genet . (2017) 54(6):399–403. doi: 10.1136/jmedgenet-2016-104237
9. Wan LC, Maisonneuve P, Szilard RK, Lambert JP, Ng TF, Manczyk N, et al. Proteomic analysis of the human keops complex identifies C14orf142 as a core subunit homologous to yeast Gon7. Nucleic Acids Res . (2017) 45(2):805–17. doi: 10.1093/nar/gkw1181
10. Perrochia L, Guetta D, Hecker A, Forterre P, Basta T. Functional assignment of keops/ekc complex subunits in the biosynthesis of the universal T6a trna modification. Nucleic Acids Res . (2013) 41(20):9484–99. doi: 10.1093/nar/gkt720
11. Komatsu K, Fukumura S, Minagawa K, Nakashima M, Saitsu H. A new case of concurrent existence of Prrt2-associated paroxysmal movement disorders with C.649dup variant and 16p11.2 microdeletion syndrome. Brain Dev . (2022) 44(7):474–9. doi: 10.1016/j.braindev.2022.03.008
12. Li J, Ma X, Banerjee S, Chen H, Ma W, Bode AM, et al. Crystal structure of the human prpk-tprkb complex. Commun Biol . (2021) 4(1):167. doi: 10.1038/s42003-021-01683-4
13. Fromer M, Moran JL, Chambert K, Banks E, Bergen SE, Ruderfer DM, et al. Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am J Hum Genet . (2012) 91(4):597–607. doi: 10.1016/j.ajhg.2012.08.005
14. Uchiyama Y, Yamaguchi D, Iwama K, Miyatake S, Hamanaka K, Tsuchida N, et al. Efficient detection of copy-number variations using exome data: batch- and sex-based analyses. Hum Mutat . (2021) 42(1):50–65. doi: 10.1002/humu.24129
15. Alghamdi M A, Benabdelkamel H, Masood A, Saheb Sharif-Askari N, Hachim MY, Alsheikh H, et al. Genomic, proteomic, and phenotypic spectrum of novel O-sialoglycoprotein endopeptidase variant in four affected individuals with Galloway-Mowat syndrome. Front Genet . (2022) 13:806190. doi: 10.3389/fgene.2022.806190
16. Chen J, Ye GB, Huang JR, Peng M, Gu WY, Xiong P, et al. Novel Tp53rk variants cause varied clinical features of Galloway-Mowat syndrome without nephrotic syndrome in three unrelated Chinese patients. Front Mol Neurosci . (2023) 16:1116949. doi: 10.3389/fnmol.2023.1116949
17. Treimer E, Kalayci T, Schumann S, Suer I, Greco S, Schanze D, et al. Functional characterization of a novel Tp53rk mutation identified in a family with Galloway-Mowat syndrome. Hum Mutat . (2022) 43(12):1866–71. doi: 10.1002/humu.24472
18. Andrejew R, Oliveira-Giacomelli Á, Ribeiro DE, Glaser T, Arnaud-Sampaio VF, Lameu C, et al. The P2x7 receptor: central hub of brain diseases. Front Mol Neurosci . (2020) 13:124. doi: 10.3389/fnmol.2020.00124
19. Hölttä T, Jalanko H. Congenital nephrotic syndrome: is early aggressive treatment needed? Yes. Pediatr Nephrol . (2020) 35(10):1985–90. doi: 10.1007/s00467-020-04578-4
Crossref Full Text | Google Scholar
20. Kitamura A, Tsukaguchi H, Hiramoto R, Shono A, Doi T, Kagami S, et al. A familial childhood-onset relapsing nephrotic syndrome. Kidney Int . (2007) 71(9):946–51. doi: 10.1038/sj.ki.5002110
21. Chen Y, Yang Y, Yang Y, Rao J, Bai H. Diagnosis delay a family of Galloway-Mowat syndrome caused by a classical splicing mutation of Lage3. BMC Nephrol . (2023) 24(1):29. doi: 10.1186/s12882-022-03000-5
Keywords: TPRKB , Galloway-Mowat syndrome, exome sequencing, KEOPS complex, nephrotic proteinuria
Citation: Hiraide T, Hayashi T, Ito Y, Urushibata R, Uchida H, Kitagata R, Ishigaki H, Ogata T, Saitsu H and Fukuda T (2024) Case Report: Novel compound heterozygous TPRKB variants cause Galloway-Mowat syndrome. Front. Pediatr. 12:1360867. doi: 10.3389/fped.2024.1360867
Received: 24 December 2023; Accepted: 20 March 2024; Published: 3 April 2024.
Reviewed by:
© 2024 Hiraide, Hayashi, Ito, Urushibata, Uchida, Kitagata, Ishigaki, Ogata, Saitsu and Fukuda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tokiko Fukuda [email protected]

IMAGES
VIDEO
COMMENTS
Nephrotic syndrome (NS) is a clinical syndrome defined by massive proteinuria responsible for hypoalbuminemia, with resulting hyperlipidemia, edema, and various complications. It is caused by increased permeability through the damaged basement membrane in the renal glomerulus, especially infectious or thrombo-embolic. It results from an abnormality of glomerular permeability that may be ...
History. The first sign of nephrotic syndrome in children is usually swelling of the face; this is followed by swelling of the entire body. Adults can present with dependent edema. Foamy urine may be a presenting feature. Fatigue and loss of appetite are common symptoms. A thrombotic complication, such as deep venous thrombosis of the calf ...
Nephrotic syndrome (NS) consists of peripheral edema, heavy proteinuria, and hypoalbuminemia, often with hyperlipidemia. Patients typically present with edema and fatigue, without evidence of ...
The term "nephrotic syndrome" refers to a distinct constellation of clinical and laboratory features of kidney disease. It is specifically defined by the presence of heavy proteinuria (protein excretion greater than 3.5 g/24 hours), hypoalbuminemia (less than 3.5 g/dL), and peripheral edema. Hyperlipidemia and thrombotic disease are also ...
Nephrotic syndrome has many causes, ... (See Presentation.) Classification. Nephrotic syndrome can be primary, being a disease specific to the kidneys, or it can be secondary, being a renal manifestation of a systemic general illness. In all cases, injury to glomeruli is an essential feature. Kidney diseases that affect tubules and interstitium ...
Nephrotic syndrome is a kidney disorder that causes your body to pass too much protein in your urine. Nephrotic syndrome is usually caused by damage to the clusters of small blood vessels in your kidneys that filter waste and excess water from your blood. The condition causes swelling, particularly in your feet and ankles, and increases the ...
Nephrotic syndrome occurs in about 1 in every 50,000 children each year. Most children receive a nephrotic syndrome diagnosis between the ages of 2 and 5. Boys and children assigned male at birth (AMAB) are about twice as likely to have nephrotic syndrome as girls or children assigned female at birth (AFAB).
Tests and procedures used to diagnose nephrotic syndrome include: Urine tests. A urinalysis can reveal abnormalities in your urine, such as large amounts of protein. You might be asked to collect urine samples over 24 hours. Blood tests. A blood test can show low levels of the protein albumin and often decreased levels of blood protein overall.
Nephrotic syndrome is one cause of end-stage kidney disease. Because edema is a common presenting feature and hypertension and dyslipidemia are often present in nephrotic syndrome, it is important for the primary care physician to suspect this entity. Common causes in adults include diabetic nephropathy, focal segmental glomerulosclerosis, and membranous nephropathy.
Nephrotic Syndrome what you should know • Depending on the disease and person's overall health, dietary changes and medicines are used to: - Lower excess salt and fluids in the body - Lower loss of protein in the urine - Lower cholesterol in the blood • Certain medicines that suppress or "calm" the immune system can be used.
Introduction. Nephrotic syndrome (NS) is the most common glomerular disease in children, but its occurrence in the first year of life is rare. It is defined as congenital nephrotic syndrome (CNS) if the disease onset is in the first 3 months of life, or infantile nephrotic syndrome (INS) if it occurs between 3 and 12 months of life.
Nephrotic syndrome is a collection of symptoms due to kidney damage. This includes protein in the urine, low blood albumin levels, high blood lipids, and significant swelling. Other symptoms may include weight gain, feeling tired, and foamy urine. Complications may include blood clots, infections, and high blood pressure.. Causes include a number of kidney diseases such as focal segmental ...
Nephritic syndrome. An acute presentation in patients with glomerular inflammation (e.g., hematuria with red blood cell casts, pyuria, and mild proteinuria) that results in hypertension, oliguria, and fluid retention with edema. Can be caused by various autoimmune disorders, hereditary disorders, and infectious diseases.
Nephrotic syndrome is one of the principal presentations of kidney disease, reflecting the pathophysiological effects of urinary losses of large quantities of protein. It is defined as urine total protein excretion of 3.5 g/d or greater, low serum albumin level, high serum cholesterol level, and peripheral edema.
Children are typically healthy prior to the onset of INS and, except for the history of allergy and atopy noted above, do not usually have a significant past medical history related to INS. Pediatric nephrotic syndrome, also known as nephrosis, is defined by the presence of nephrotic-range proteinuria, edema, hyperlipidemia, and hypoalbuminemia.
Nephrotic syndrome (NS) is a condition characterized by increased permeability of the glomerular filtration barrier (GFB) leading to proteinuria with consequent hypoalbumine-mia, edema, and hyperlipidemia. Nephrotic-range protein-uria in children is de fined as a urine protein-to-creatinine ratio of 200 mg/mmol or greater ($2 mg/mg) or 24-hour ...
This Osmosis High-Yield Note provides an overview of Nephrotic syndrome essentials. All Osmosis Notes are clearly laid-out and contain striking images, tables, and diagrams to help visual learners understand complex topics quickly and efficiently. Find more information about Nephrotic syndrome: Osmosis Nephrotic syndrome high-yield notes offers ...
A kidney illness known as nephrotic syndrome is characterized by high cholesterol levels, swelling (edema), low blood protein levels, and excess protein in the urine. Recognizing the signs of this ...
International Phone Number: +1-781-392-2000. Email: [email protected]. Support Center Hours: Monday - Friday, 7 AM - 9 PM ET (-5 GMT) Current Support Center Time & Date: Monday, September 12, 2023 11:36 AM ET (-5 GMT)
Introduction. Idiopathic nephrotic syndrome (INS) is the most frequent glomerular disease in childhood, characterized by a damage of the glomerular filtration barrier leading to loss of protein in the urine, hypoalbuminemia and oedema ().At first presentation, patients receive a standard course of oral prednisone, to which the majority of children respond within 4 weeks and are therefore ...
Download the "Nephrotic Syndrome" presentation for PowerPoint or Google Slides. Taking care of yourself and of those around you is key! By learning about various illnesses and how they are spread, people can get a better understanding of them and make informed decisions about eating, exercise, and seeking medical attention. This Google Slides ...
BackgroundGalloway-Mowat syndrome (GAMOS) is a rare genetic disease characterized by early-onset nephrotic syndrome and microcephaly with central nervous system abnormalities. Pathogenic variants in genes encoding kinase, endopeptidase, and other proteins of small size (KEOPS) complex subunits cause GAMOS. The subunit TPRKB (TP53RK binding protein) has been reported in only two patients with ...
Introduction. Pierson syndrome is a rare genetic disorder characterized by a complex array of renal and ocular manifestations, presenting significant challenges in diagnosis and management. Its hallmark features include congenital nephrotic syndrome and ocular anomalies such as microcoria and cataracts. Despite its rarity, understanding the ...