- Case report
- Open access
- Published: 01 December 2021

Rabies is still a fatal but neglected disease: a case report
- Y. A. Amoako ORCID: orcid.org/0000-0002-4642-789X 1 , 2 ,
- P. El-Duah 1 , 3 ,
- A. A. Sylverken 1 , 4 ,
- M. Owusu 1 , 5 ,
- R. Yeboah 1 ,
- R. Gorman 1 ,
- T. Adade 1 ,
- J. Bonney 1 ,
- W. Tasiame 1 , 3 ,
- K. Nyarko-Jectey 6 ,
- T. Binger 1 ,
- V. M. Corman 3 ,
- C. Drosten 3 &
- R. O. Phillips 1 , 2
Journal of Medical Case Reports volume 15 , Article number: 575 ( 2021 ) Cite this article
12k Accesses
7 Citations
19 Altmetric
Metrics details
Rabies, caused by a lyssavirus, is a viral zoonosis that affects people in many parts of the world, especially those in low income countries. Contact with domestic animals, especially dogs, is the main source of human infections. Humans may present with the disease only after a long period of exposure. Nearly half of rabies cases occur in children <15 years old. We report on a fatal case of rabies in a Ghanaian school child 5 years after the exposure incident, and the vital role of molecular tools in the confirmation of the diagnosis.
Case presentation
The patient, an 11-year-old junior high school Ghanaian student from the Obuasi Municipality in Ghana, presented with aggressive behavior, which rapidly progressed to confusion and loss of consciousness within a day of onset. Her parents reported that the patient had experienced a bite from a stray dog on her right leg 5 years prior to presentation, for which no antirabies prophylaxis was given. The patient died within minutes of arrival in hospital (within 24 hours of symptom onset). Real-time polymerase chain reaction testing of cerebrospinal fluid obtained after her death confirmed the diagnosis of rabies. Subsequent phylogenetic analysis showed the virus to belong to the Africa 2 lineage of rabies viruses, which is one of the predominant circulating lineages in Ghana.
The incubation period of rabies is highly variable so patients may only present with symptoms long after the exposure incident. Appropriate molecular testing tools, when available as part of rabies control programmes, are vital in confirming cases of rabies.
Peer Review reports
Rabies, a viral zoonosis caused by a lyssavirus, is a vaccine-preventable, neglected tropical disease (NTD) that occurs in more than 150 countries and territories [ 1 ]. In the USA, the majority of rabies cases reported to the Centers for Disease Control and Prevention (CDC) each year occur in wild animals such as bats, raccoons, skunks, and foxes [ 2 ]. However, rabies can affect any mammal, including humans. Dogs are the main source of human rabies deaths, contributing up to 99% of all rabies transmissions to humans. The rabies virus infects the central nervous system of mammals, ultimately causing disease in the brain and death. Infection causes tens of thousands of deaths every year, mainly in Asia and Africa. Approximately 40% of people bitten by suspect rabid animals are children under 15 years of age. Rabies elimination is feasible through vaccination of dogs and prevention of dog bites [ 1 ]. In Ghana, rabies remains an important public health threat, with case fatality rate of 100% [ 3 , 4 , 5 , 6 ].
We report on a case of rabies in an 11-year-old Ghanaian student and discuss the essential role of accurate diagnosis in rabies control.
The patient was an 11-year-old junior high school Ghanaian student from the Obuasi Municipality in Ghana, with no known previous illnesses. She was the third child from a family of five children. She presented with a day’s history of aggressive behavior, which rapidly progressed to confusion and loss of consciousness. Her parents reported that the patient had experienced a bite from a stray dog on her right leg 5 years prior to presentation. Additionally, her parents described episodes of hydrophobia within the preceding year; however, they did not make much of it as they considered it to be mild and due to the ‘playful nature of children’. Other prodromal symptoms such as fever, general malaise, sore throat, anorexia, and muscle weakness were absent. There was no history of dysphagia. The patient was brought to the hospital within a day of onset of her symptoms and died from cardiorespiratory failure within minutes of arrival in hospital (within 24 hours of symptom onset). Initial assessment revealed an ill-looking, unconscious patient, who was afebrile with a respiratory rate of 14 cycles per minute. The neck was supple and Kernig’s sign was negative. The pupils were dilated and sluggishly reactive to light. Real-time polymerase chain reaction (RT-PCR) testing of cerebrospinal fluid obtained after her death confirmed the diagnosis of rabies. The corpse was handled and buried/disposed in accordance with standard local protocols in Ghana [ 7 ]. The contacts of the patient were subsequently counseled and offered rabies vaccination. All the contacts of the patient were free of rabies symptoms after 12 months of follow-up.
Virus characteristics
Given the reported long incubation period of the case, sequencing and phylogenetic analysis of the detected virus was performed to assess the possibility of another origin of the virus other than the reported dog bite, such as from bats, which may not have been recognized. Viral Ribonucleic acid (RNA) was extracted from the patient’s cerebrospinal fluid (CSF) using the Qiagen Viral RNA mini spin kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Presence of rabies RNA was confirmed by RT-PCR testing using a Lyssa-Virus RT-PCR kit (Tib Molbiol, Berlin, Germany) and a LightCycler Multiplex RNA Virus Master (Roche, Penzberg, Germany). We applied a high-throughput sequencing (HTS) approach for whole genome sequencing using the KAPA RNA Hyper Prep Kit (Roche Molecular Diagnostics, Basel, Switzerland) for library preparation and the 150-cycle NextSeq reagent v3 cartridge (Illumina, San Diego, California, US), according to manufacturer’s instructions.
Bidirectional reads from the HTS run were assembled against a reference rabies sequence from GenBank and annotated using Geneious prime 2019 ( https://www.geneious.com ). Phylogenetic analysis was done by maximum likelihood reconstruction using the PHYML [ 8 ] plugin in Geneious prime with 500 bootstrap replicates.
The sequence obtained was found to be most closely related to a Rabies virus (Accession number: NC_001542), sharing an 85.4% pairwise sequence identity and forming a monophyletic pairing with this virus when compared with other reference Lyssaviruses from GenBank (Fig. 1 ). Comparison of the full nucleoprotein coding region to those of a representative subset of African rabies viruses of various lineages [ 9 ] showed the virus to belong to the Africa 2 lineage of rabies viruses, which is one of the predominant circulating lineages in Ghana [ 10 ] (Fig. 2 ). The full genome obtained in this study was submitted to GenBank and assigned accession number MT107888.
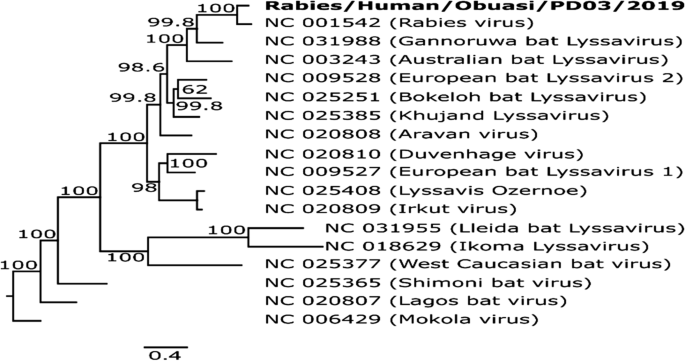
Phylogenetic tree comparing Lyssavirus genotypes. Tree was generated using maximum likelihood reconstruction by the general time reversible model with a gamma distribution and proportion of invariable sites (GTR+I+G). The tree is based on whole genome sequences and was rooted with a Mokola virus (Genotype 3). Tips were labeled with accession numbers and virus names in brackets. The sequence obtained in this study is shown by bold type font
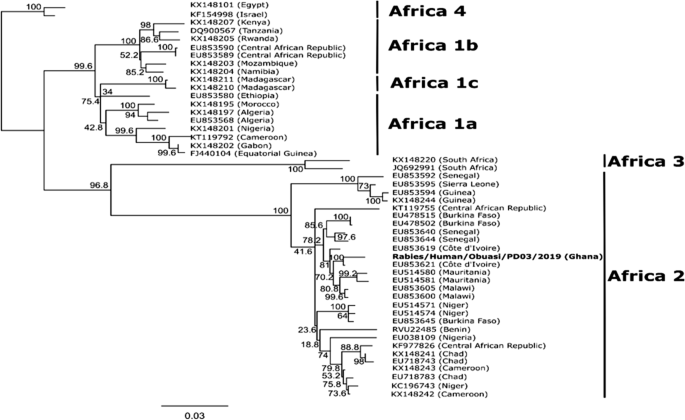
Phylogenetic tree comparing rabies viruses from Africa. Tree was generated using maximum likelihood reconstruction by a transition model with a gamma distribution and proportion of invariable sites (TIM1+G+I). The tree is based on complete nucleoprotein sequences and rooted with the Africa 4 lineage branch. Tips are labeled with accession numbers and country of origin in brackets. The sequence obtained in this study is shown by a bold type font
Ethical considerations
Ethical approval for this study was obtained from the Scientific and Ethical review Committee of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology (KNUST) (CHPRE/AP/462/19). Written informed consent was also obtained from parent of the patient for publication of this case report.
Rabies is a neglected tropical disease of poor and vulnerable populations, with deaths due to rabies often not reported. Rabies is nearly always fatal once symptoms appear. Although 100% preventable, over 59,000 people, mostly in under-served areas, in over 150 countries, die of rabies every year as human vaccines and immunoglobulin are not readily available or accessible [ 1 , 5 ]. Operationally in Ghana [ 7 , 11 ], all cases of dog bites are considered suspected cases of rabies, and a confirmed case is defined as a suspected case with clinical and or laboratory confirmation. The clinical confirmation of rabies is based on a history of dog bite that is followed by classical symptoms such as anxiety, agitation, paralysis, excessive salivation, and hydrophobia. The patient presented in this report met the case definition and tested positive by PCR and subsequent phylogenetic analysis as described.
The incubation period for rabies is typically 2–3 months but may vary from 1 week to 1 year. This period may vary based on the location of the exposure site (how far away it is from the brain), the type of rabies virus, viral load, and any existing immunity [ 1 ]. Rabies causes an acute progressive viral encephalomyelitis. The first symptoms of rabies may be very similar to those of flu including general weakness or discomfort, fever, or headache. The disease may present as furious or paralytic rabies. Furious rabies presents with signs of hyperactivity, excitable behavior, hydrophobia, and sometimes aerophobia. Death occurs after a few days due to cardio-respiratory arrest. Paralytic rabies accounts for about 20% of the total number of human cases and runs a less dramatic and usually longer course than the furious form. Muscles gradually become paralyzed, starting at the site of the bite or scratch. A coma slowly develops, and eventually death occurs. The paralytic form of rabies is often misdiagnosed, contributing to the under-reporting of the disease.
In a previous study from Ghana [ 3 ], the time between exposure and the onset of symptoms ranged between 3 weeks and 4 months, with 52.4% of cases reporting the onset of symptoms approximately 2 months after exposure. There was a history of a dog bite about 5 years prior to the onset of symptom in the patient presented in this report; this represents a rather long incubation period and was possibly influenced by recall bias from the parents who gave the clinical history. A more reasonable scenario will be that the patient had some further exposure to the rabies virus. The lineage of the detected virus implicates a common circulating rabies virus, most likely from dogs, and suggests she could have been innocuously exposed to the saliva or been scratched by an infected stray dog in the immediate period preceding her demise; but we have no way of confirming this. This assessment is in line with that of another study in Bangladesh that also found three patients with reported incubation period in excess of 1000 days, which was attributed to recall bias and likely recurrent exposures following the first bite incident [ 12 ]. A further possibility is that the virus was replicating slowly with the establishment of a latent infection following the initial exposure 5 years earlier, with subsequent reactivation of the neurotropic virus infection in later years. Although rare, long incubation period for rabies have been reported. Shankar and colleagues [ 13 ] reported a case of rabies encephalitis with a possible 25 year incubation period and suggested that reactivation of a latent infection may have played a role in the pathogenesis of the disease. In that study, the diagnosis of rabies was established by histopathology. An incubation period longer than > 6.5 years was reported in a 10-year-old girl of Vietnamese origin in whom rabies developed after she had lived continuously in Australia for almost 5 years [ 14 ]. Viral molecular epidemiological tools as used in our study provide insight into the migratory pattern of the virus-carrying animal and human vectors, but not the mechanism of viral latency [ 14 , 15 ].
In Ghana, rabies is endemic and cases of human rabies are under reported, as in other developing countries [ 1 , 6 ]. Twenty-one cases of rabies were seen at a tertiary facility in Ghana over a 25 month period, with more than half of cases aged >18 years [ 3 ]. Among that population, hydrophobia and agitation were the most common symptoms, and the case fatality rate was 100% with about 60% of cases dying within 24 hours of admission. The longest duration of stay recorded in that study was 5 days. Our patient had similar symptoms and died within 24 hours of hospitalization, in keeping with the aggressive course of the illness.
The veterinary services in Ghana are often limited in the diagnosis of rabies, as Sellers’ stain and fluorescent antibody test, commonly used techniques in diagnosing rabies, are mostly unavailable. The clinical diagnosis of human rabies is partly based on a positive rabies test result of the offending animal from the veterinary services. Preventive and control measures in Ghana to reduce the incidence of human rabies have been targeted at improving the vaccination of dogs against rabies, stray dog removal, and providing pre-/ post-exposure vaccinations of humans; however, these measures have been irregular and not sustained [ 6 , 16 ].
The differential diagnosis for this case includes other etiologies of central nervous infections (such as bacterial, fungal, and other viruses and abscess) and intracranial tumors. Although these other etiologies were not sought for, the classic clinical presentation, together with the PCR and phylogenetic characterization of the rabies virus in the patient’s CSF make these other differential etiologies less likely.
Laboratory diagnosis of rabies infection in humans is difficult after exposure to the virus before the onset of clinical symptoms. Clinical diagnosis of rabies is often made when rabies-specific signs, such as hydrophobia or aerophobia, are present. Human rabies can be confirmed during clinical disease stage and postmortem by detecting viral antigens, whole virus, or nucleic acids in infected tissues (brain, skin, urine, or saliva) using various diagnostic techniques[ 1 ]. Accurate diagnosis of rabies in exposed persons will enable the institution of appropriate care. In the incident case, rabies confirmation by PCR testing of cerebrospinal fluid only occurred after death of the patient. In under-served populations where the threat of rabies is highest, diagnostic facilities are largely absent, thus hampering early and accurate diagnosis. Indeed, in most cases, the diagnosis is only presumptively made on clinical grounds [ 3 , 17 ]. Over the past several years, the Kumasi Centre for Collaborative Research in Tropical Medicine (KCCR) has provided support services in the area of rabies diagnosis to the Ashanti Regional Directorate of Health Services (RDHS) in Ghana. The KCCR performs PCR testing on samples received from the RDHS. Such collaborations are essential in facilitating diagnosis and guiding treatment decisions and rabies control efforts within the country.
The rabies strain reported in this case is presumably from a domestic animal (dog) contact. This is in keeping with previous accounts from Ghana [ 3 , 6 , 16 ] and globally [ 1 ] that implicate domestic dogs as being responsible for most rabies virus transmission to humans. A recent study in the Ashanti region of Ghana showed a high dog to household ratio, and that 80.3% of the dogs were not restricted and 49.9% were allowed to enter neighbors' households [ 18 ]. In that same study, dog rabies vaccination coverage was low, ranging from 28.1% to 64.9%. This calls for improved efforts to target the vaccination of all dogs in Ghana to prevent spread from stray animals.
While the knowledge of rabies transmission is high in Ghana, about 65% of people studied in a peri-urban setting believed in traditional ways of treatment such as concoctions, herbs, and consumption of the offending dogs’ organs [ 18 ]. This practice has the tendency to delay access to care for people exposed to rabies and contribute to rabies mortality within the Ghanaian population. Even after exposure, the tragic loss of lives from rabies is preventable, since effective post-exposure prophylaxis (PEP) is available in the form of wound care, rabies immunoglobulin, and rabies vaccine. Rabies immunoglobulin in addition to rabies vaccine is indicated for category III exposures, which include bites or scratches that penetrate the skin (as occurred in this case), licking of mucous membranes or broken skin, and direct contact with bats. Laryea and colleagues [ 3 ] reported that most patients do not seek care after exposure to rabies. Even for the third of people who seek care post exposure, they do not get access to the recommended PEP. In an Ethiopian study, 77% of suspected rabid dog bite victims visited a health center, and 57% received sufficient doses of PEP. The likelihood of seeking medical services following rabies exposure was higher for high-income earners, people bitten by dogs of unknown ownership, where the bite was severe especially on the leg, and where the victim lived close to the nearest health center [ 19 ]. Increasing access to health facilities delivering post-exposure services can lead to improved health-seeking behavior in patients following rabies exposure and reduce the mortality associated with rabies.
Rabies control efforts requires concerted collaboration between agencies in multiple sectors. In Ghana, a parallel and uncoordinated system of rabies surveillance is maintained by the health and veterinary services, with gross disparities in the number of reported events and an overall impression of under-reporting [ 11 ]. Tackling the scourge of a zoonosis such as rabies using the ‘One Health Approach’ requires a collaborative and multi-disciplinary effort that cuts across the boundaries of animal, human, and environmental health to undertake risk assessments, and to develop plans for response and control [ 20 , 21 ]. The WHO, the World Organisation for Animal Health (OIE), the Food and Agriculture Organization of the United Nations (FAO), and the Global Alliance for Rabies Control (GARC) have established a global multi-sectoral “United Against Rabies” collaboration to provide a common strategy to achieve "Zero human rabies deaths by 2030" [ 22 ]. In Ghana, such multi-sectoral collaboration will involve the Ministry of Health and public health authorities, veterinary services, Ministry of Local Government and Rural Development, and Municipal and district assemblies among others, as important stakeholders.
The median age of rabies victims in a previous study [ 11 ] from Ghana was 9 years (range 3–72 years) and the patient presented in this report was aged 11 years. Since the at-risk population includes a large proportion of children of school going age, an important control measure might be increasing awareness of rabies in this age group. In a Malawian study, knowledge of rabies and how to be safe around dogs was greater among school children who had received a school lesson on rabies compared with those who had not received the lesson, but had been exposed to a rabies vaccination campaign in their community (both p < 0.001), indicating that the lesson itself was critical in improving knowledge [ 23 ]. The primary school education curriculum should include basic content to educate young children on the dangers of an animal bite and encourage them to seek help. Education in rural and urban communities targeting community leaders, chiefs, farmers, pet owners, and schools on rabies prevention will create awareness among the public and aid rabies control efforts.
The incubation period of rabies is highly variable, so patients may only present with symptoms long after the incident exposure. Rabies should be considered in the differential diagnosis of patients who present with encephalopathy. Appropriate molecular testing tools are vital in confirming and documenting cases of rabies in people who meet the case definition. There is a need to increase knowledge and awareness of rabies and provide appropriate post-exposure prophylaxis to reduce the incidence of human rabies and the associated fatalities.
Availability of data and materials
The datasets obtained and analysed during the current study are available from the corresponding author on reasonable request. The full genome obtained in this study was submitted to GenBank and assigned accession number MT107888.
Abbreviations
Neglected tropical disease
Centers for Disease Control
Polymerase chain reaction
Real-time polymerase chain reaction
Ribonucleic acid
High-throughput sequencing
Cerebrospinal fluid
Post-exposure prophylaxis
World Organisation for Animal Health
Food and Agriculture Organization
Global Alliance for Rabies Control
WHO. Rabies. https://www.who.int/news-room/fact-sheets/detail/rabies . Accessed 7 Aug 2020.
CDC. Rabies. https://www.cdc.gov/rabies/about.html . Accessed 7 Aug 2020.
Laryea D, Owusu R, Arthur J, Agyemang E, Spangenberg K. Human rabies in Kumasi: a growing public health concern. Afr J Curr Med Res. 2017;1:1.
Google Scholar
Punguyire DT, Osei-Tutu A, Aleser EV, Letsa T. Level and pattern of human rabies and dog bites in Techiman Municipality in the Middle Belt of Ghana: a six year retrospective records review. Pan Afr Med J. 2017;28:281.
Article Google Scholar
Kenu E, Ganu V, Noora CL, Adanu R, Lartey M. Management of dog bites by frontline service providers in primary healthcare facilities in the Greater Accra Region of Ghana, 2014–2015. Infect Dis Poverty. 2018;7(1):18.
Belcher DW, Wurapa FK, Atuora DO. Endemic rabies in Ghana. Epidemiology and control measures. Am J Trop Med Hyg. 1976;25(5):724–9.
Article CAS Google Scholar
Ghana TgfIDSaRi. https://www.moh.gov.gh/wp-content/uploads/2016/02/Integrated-Disease-Surveillance-and-Response-Ghana-Guidelines.pdf . Accessed 7 Aug 2020.
Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–21.
Sadeuh-Mba SA, Momo JB, Besong L, Loul S, Njouom R. Molecular characterization and phylogenetic relatedness of dog-derived rabies viruses circulating in Cameroon between 2010 and 2016. PLoS Negl Trop Dis. 2017;11(10):e0006041.
Hayman DT, Johnson N, Horton DL, Hedge J, Wakeley PR, Banyard AC, et al. Evolutionary history of rabies in Ghana. PLoS Negl Trop Dis. 2011;5(4):e1001.
Adomako B-Y, Baiden F, Sackey S, Ameme DK, Wurapa F, Nyarko KM, et al. Dog bites and rabies in the eastern region of Ghana in 2013–2015: a call for a one-health approach. J Trop Med. 2018;2018:6139013.
Ghosh S, Rana MS, Islam MK, Chowdhury S, Haider N, Kafi MAH, et al. Trends and clinico-epidemiological features of human rabies cases in Bangladesh 2006–2018. Sci Rep. 2020;10(1):2410.
Shankar SK, Mahadevan A, Sapico SD, Ghodkirekar MSG, Pinto RGW, Madhusudana SN. Rabies viral encephalitis with probable 25 year incubation period! Ann Indian Acad Neurol. 2012;15(3):221–3.
Johnson N, Fooks A, McColl K. Human rabies case with long incubation, Australia. Emerg Infect Dis. 2008;14(12):1950–1.
McColl KA, Gould AR, Selleck PW, Hooper PT, Westbury HA, Smith JS. Polymerase chain reaction and other laboratory techniques in the diagnosis of long incubation rabies in Australia. Aust Vet J. 1993;70(3):84–9.
Alonge DO, Abu SA. Rabies in Ghana West Africa. Int J Zoon. 1984;11(1):53–8.
CAS Google Scholar
Apanga PA, Awoonor-Williams JK, Acheampong M, Adam MA. A presumptive case of human rabies: a rare survived case in rural Ghana. Front Public Health. 2016;4:256.
Tasiame W, Johnson S, Burimuah V, Akyereko E, Amemor E. Dog population structure in Kumasi, Ghana: a missing link towards rabies control. Pan Afr Med J. 2019;33:13.
Beyene TJ, Mourits MCM, Revie CW, Hogeveen H. Determinants of health seeking behaviour following rabies exposure in Ethiopia. Zoonoses Public Health. 2018;65(4):443–53.
Mackenzie JS, Jeggo M. The one health approach-why is it so important? Trop Med Infect Dis. 2019;4:2.
Cunningham AA, Daszak P, Wood JLN. One Health, emerging infectious diseases and wildlife: two decades of progress? Phil R Soc London B. 2017;372:1725.
Zero by 30: The Global Strategic Plan to Prevent Human Deaths from Dog-Transmitted Rabies by 2030. https://rabiesalliance.org/resource/united-against-rabies-plan-executive-summary . Accessed 7 Aug 2020.
Burdon Bailey JL, Gamble L, Gibson AD, Bronsvoort BMD, Handel IG, Mellanby RJ, et al. A rabies lesson improves rabies knowledge amongst primary school children in Zomba, Malawi. PLoS Negl Trop Dis. 2018;12(3):e0006293.
Download references
Acknowledgements
Not applicable.
This publication is part of the PANDORA-ID-NET (EDCTP Reg/Grant RIA2016E-1609), funded by the European and Developing Countries Clinical Trials Partnership (EDCTP2) programme, which is supported under Horizon 2020, the European Union’s Framework Programme for Research and Innovation. The views and opinions of authors expressed herein do not necessarily state or reflect those of EDCTP. The EDCTP had no role in the design of the study and collection, analysis, and interpretation of data, and in writing the manuscript.
Author information
Authors and affiliations.
Kumasi Centre for Collaborative Research, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Y. A. Amoako, P. El-Duah, A. A. Sylverken, M. Owusu, R. Yeboah, R. Gorman, T. Adade, J. Bonney, W. Tasiame, T. Binger & R. O. Phillips
Department of Medicine, School of Medicine and Dentistry, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Y. A. Amoako & R. O. Phillips
Institute of Virology, Charite Universitatsmedizin Berlin, Berlin, Germany
P. El-Duah, W. Tasiame, V. M. Corman & C. Drosten
Department of Theoretical and Applied Biology, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
A. A. Sylverken
Department of Medical Laboratory Technology, College of Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Obuasi Municipal Health Directorate, Obuasi, Ghana
K. Nyarko-Jectey
You can also search for this author in PubMed Google Scholar
Contributions
YAA, PED, CD, and ROP conceived the case report and its design. AAS, MO, RY, RG, TA, JB, WT, KNJ, TB, and VMC participated in data collection and analysis. YAA and PED wrote the manuscript and reviewed it for important intellectual content. All authors reviewed and approved the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Y. A. Amoako .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was obtained from the Committee on Human Research, Publication and Ethics of the School of Medicine and Dentistry of the Kwame Nkrumah University of Science and Technology, Kumasi, Ghana (CHPRE/AP/462/19).
Consent for publication
Written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Amoako, Y.A., El-Duah, P., Sylverken, A.A. et al. Rabies is still a fatal but neglected disease: a case report. J Med Case Reports 15 , 575 (2021). https://doi.org/10.1186/s13256-021-03164-y
Download citation
Received : 24 August 2020
Accepted : 20 October 2021
Published : 01 December 2021
DOI : https://doi.org/10.1186/s13256-021-03164-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Incubation period
- Diagnostic testing
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
October 8, 2008
Medical Mystery: Only One Person Has Survived Rabies without Vaccine--But How?
ScientificAmerican.com talks with the first known survivor of rabies four years later
By Jordan Lite
Four years after she nearly died from rabies, Jeanna Giese is being heralded as the first person known to have survived the virus without receiving a preventative vaccine. But Giese (pronounced Gee-See) says she would gladly share that honor with others if only doctors could show that the treatment used to save her could spare other victims as well. "They shouldn't stop 'till it's perfected," said Giese, now 19, during a recent interview about physicians' quest to refine the technique that may have kept her alive. Giese's wish may come true. Another young girl infected with rabies is still alive more than a month after doctors induced a coma to put her symptoms on hold, just as they did with Giese. Yolanda Caicedo, an infectious disease specialist at Hospital Universitario del Valle in Cali, Colombia, who is treating the latest survivor, confirmed reports in the Colombian newspaper El País that the victim is an eight-year-old girl who came down with symptoms in August, about a month after she was bitten by an apparently rabid cat. Caicedo said that the family had sought treatment for the bite in Bolivar, at a hospital about three hours by foot from their rural home, but that the child, Nelsy Gomez, did not receive the series of vaccines that can prevent the virus from turning into full-blown rabies. The five shots contain minute amounts of the dead rabies virus and are designed to nudge the body into developing antibodies to fight it. Patients are also given a shot of immunoglobulin (in this case a synthesized rabies antibody) to protect them while their immune systems produce antibodies to the vaccine virus. But the combination is only effective within six days of infection, before symptoms show up; when Gomez developed signs of the disease, it was too late for the shots. With no other options available, doctors induced a coma. Caicedo is hopeful, but indicated that Gomez will face a long, slow recovery. She would not say how long Gomez was comatose but told ScientificAmerican.com that she had been awake for "a few days" and is stable. The child can move her fingers but cannot walk or eat on her own, and her eyes are open but she cannot speak yet and physicians are not sure if she can see, Caicedo says. Giese, informed of the case, says that she "hopes and prays" that Gomez will survive. Giese was the keynote speaker at a conference last week in Atlanta, where scientists gathered to discuss the latest research being conducted on ways to battle the deadly disease. During her talk, she urged physicians to continue efforts to pin down treatments that work. Giese was 15 when she was infected after being bitten by a rabid bat she had picked up outside her church in her hometown of Fond du Lac, Wisc.
Her parents cleaned the superficial wound and she says they did not believe it was necessary to seek further medical treatment. "We never thought of rabies," she says. By the time Giese began displaying signs of rabies three weeks later—fatigue, double vision, vomiting and tingling in her left arm—it was too late for the antirabies vaccine cocktail. Instead of giving her up for dead, the doctors decided to "shut the brain down and wait for the cavalry to come" by inducing a coma to give her own immune system time to build up antibodies against the virus, says Rodney Willoughby , an infectious disease specialist who treated Giese at the Children's Hospital of Wisconsin in Milwaukee. Willoughby devised the treatment credited with saving Giese there, which has since become known as the Milwaukee protocol. Rabies kills by compromising the brain's ability to regulate breathing, salivation and heartbeat; ultimately, victims drown in their own spit or blood, or cannot breathe because of muscle spasms in their diaphragms. One fifth die from fatal heart arrhythmia. Doctors believed that Giese might survive if they suppressed her brain function by sedating her while her immune system attacked the rabies virus. This was the first time the therapy was attempted, and doctors had no clue if it would work or, if it did, whether it would leave her brain damaged. But Willoughby says it was the only chance doctors had of saving her. When she arrived at the hospital, Giese couldn't talk, sit or stand and fell in and out of consciousness—she also needed to be intubated to help her breathe. "She was critically ill," Willoughby recalls, "and looked as if she might die within the day." In addition to inducing the coma, doctors also gave her the antivirals ribavarin and amantadine. They tapered off the anesthetics after about a week, when tests showed that Giese's immune system was battling the virus. For about six months after awakening from the coma, physicians also gave her a compound called tetrahydrobiopterin that is chemically similar to the B-complex vitamin folic acid, which may have improved her speech and ability to eat, Willoughby says. He notes that physicians gave her the supplement after tests showed that she had a deficiency of the compound, which is known to boost production of serotonin and dopamine neurotransmitters needed to perform motor, speech and other routine bodily functions.
Remarkably, Giese survived. She recovered most of her cognitive functions within a few months, and other skills within a year, Willoughby says. She got her driver's license and is now a sophomore at Marian University in Fond du Lac, where she is majoring in biology. There are lingering signs of her illness: Giese, once an avid athlete, says she now lists to one side when she runs and walks and no longer plays volleyball, basketball and softball as she once did. She also speaks more slowly and sometimes not as clearly as before her illness, but Willoughby says these effects may fade over time. Giese is " pretty much normal ," says Willoughby, an associate professor of pediatrics at the Medical College of Wisconsin in Milwaukee. "She continues to get better, counter to conventional medical thinking." Rabies has an incubation period of two weeks to three months and kills within a week of the symptoms showing up. The vaccine series and other immune therapies are useless at this point and may even speed up and increase the severity of the symptoms. Usually, patients are made as comfortable as possible in the hospital or, in countries without sophisticated health care, sent home to die an agonizing death. Antiviral drugs and immune therapies including steroids, disease-fighting interferon-alpha and poly IC (which stimulates the body's own production of interferon-alpha) have been tried, but none have been shown to be lifesaving on their own, Willoughby says. Over the past four years, the Milwaukee protocol to differing degrees has been used a dozen times, but until now Giese was the sole survivor. Exactly why she lived—and the others died—is still a mystery. In a 2005 report on her case in The New England Journal of Medicine , Willoughby speculated that she may have been infected with a rare, weakened version of the virus. Today, he chalks Giese's survival up to aggressive intensive care, the decision to sedate her "and 10 percent sheer luck." Which element of that combination made the difference, and whether the antivirals she was given helped save her is unknown. "In all honesty, we were probably just pretty lucky," he says. Only another survivor, and then animal and clinical trials, will show if the therapy works, and why, he says. The U.S. Centers for Disease Control and Prevention (CDC) plans to test the protocol on rabies-infected ferrets; Thai and Canadian doctors, who unsuccessfully treated a 33-year-old man with rabies with the Milwaukee protocol, recommended in the Journal of NeuroVirology two years ago that physicians exercise "caution" in using the treatment, because it is too expensive and lacks " a clear scientific rationale." Willoughby says it cost about $800,000 to treat Giese.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Rabies is 100 percent preventable with vaccinations if patients receive them before the onset of symptoms, including hallucinations, delirium, muscle spasms, paralysis and hydrophobia. Yet an estimated 55,000 people, mostly in Asia and Africa, die from it annually because of misdiagnosis or because the illness is not recognized until it has taken hold, according to the journal Neurologic Clinics . Often, patients dismiss the potential seriousness of bites, cannot afford follow-up medical treatment or, in some situations, are unaware they've been bitten, as was the case of a 13-year-old Connecticut girl who died of rabies in 1995 . Vaccine shortages as one manufacturer, Bridgewater, N.J.–based sanofi–aventis, upgrades its factory to meet U.S. Food and Drug Administration requirements , and chronic shortfalls of immunoglobulin also play a role in the fatalities. The vaccine-immunoglobulin regimen costs $1,200 to $2,000 in industrialized nations and $100 to $300 in developing countries—an out-of-reach sum for many people, Willoughby says. Though it's promising that Gomez is still alive, "The hope that the outcome will necessarily be the same as with Jeanna, particularly in a developing country, is expecting a bit much," laments Charles Rupprecht, chief of the CDC's Rabies Program Willoughby acknowledges that even if Giese's success is reproducible—and the Milwaukee protocol perfected—it likely will only be available for use in 10 percent of cases, because of limited medical facilities in developing countries. "Re-creating that in a place stricken with poverty, you get into ethical issues of whether we should do this when we should be about prevention; and does that society have the ability to rehabilitate a patient who may survive but with severe [side effects]?" Rupprecht says. "Jeanna created several ethical issues for all of us to deal with this bug." Giese says that the fourth-year anniversary of her illness has brought up some bitter memories that she'll probably never shake, but she's glad to be alive—and doing as well as she is. "It takes some getting used to, but I've kind of come to terms with the fact that I'm the only…[survivor]," she says. "At 15, I never would have thought that anything like this would ever happen, and that I lived is just amazing." An animal lover who owns a dog, two rabbits and six birds, she hopes to one day open a sanctuary in Fond du Lac for endangered animals, including "big predators like lions and tigers and wolves," and maybe even bats , too. "I'm not scared of them at all," Giese says of bats. "I'm more passionate about animals than I was before. Animals are my happiness and reason for living." Additional reporting by Barbara Juncosa
- Skip to main content
- Keyboard shortcuts for audio player
A Man Died From Rabies In Illinois. Here's Why That's So Unusual In The U.S.

Deepa Shivaram

Rabies is a preventable viral disease. Human fatalities are rare and typically occur in people who don't get treatment quickly. Here, a vial and box of rabies vaccine. Adriana Adie/NurPhoto via Getty Images hide caption
Rabies is a preventable viral disease. Human fatalities are rare and typically occur in people who don't get treatment quickly. Here, a vial and box of rabies vaccine.
Be aware if you've got bats in your home. That's the message from the Illinois Department of Health as it announced that an 80-year-old man died of rabies after waking up to find a bat on his neck. It is the first human case of rabies in the state since 1954.
The man refused rabies treatment at the time of the incident in mid-August, health officials said in a press release . A month later, he started experiencing rabies symptoms such as neck pain, headache, difficulty controlling his arms, finger numbness and difficulty speaking.

Shots - Health News
Bats in the bedroom can spread rabies without an obvious bite.
Rabies infections in humans are extremely rare in the United States, since the disease is preventable and treatable. Typically one to three cases are reported each year, and there were no cases reported in 2019 , according to the most recent data available from the CDC.
But rabies exposure is far more common; 60,000 Americans receive the post-exposure treatment every year. Without prompt treatment, though, the virus infects the nervous system and is typically fatal.

The U.S. Bans Importing Dogs From 113 Countries After Rise In False Rabies Records
Lake County Health Department Executive Director Mark Pfister said the case of the man who died this week emphasizes the need for more public health awareness of the risks of rabies.
"Rabies infections in people are rare in the United States; however, once symptoms begin, rabies is almost always fatal, making it vital that an exposed person receive appropriate treatment to prevent the onset of rabies as soon as possible," Pfister said.
Illinois health officials say bats are the most common animal found with rabies in the state. The man who died had a colony of bats living in his home.
- public health
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 19 May 2022
Elimination of human rabies in Goa, India through an integrated One Health approach
- A. D. Gibson ORCID: orcid.org/0000-0002-4641-2583 1 , 2 na1 ,
- G. Yale 3 na1 ,
- J. Corfmat 3 na1 ,
- M. Appupillai 3 na1 ,
- C. M. Gigante 4 ,
- M. Lopes 5 ,
- U. Betodkar 6 ,
- N. C. Costa 5 ,
- K. A. Fernandes 7 ,
- P. Mathapati 3 ,
- P. M. Suryawanshi 6 ,
- N. Otter 3 , 7 ,
- G. Thomas 1 ,
- P. Ohal 3 ,
- I. Airikkala-Otter 7 ,
- F. Lohr ORCID: orcid.org/0000-0002-7158-3122 1 ,
- C. E. Rupprecht 8 ,
- A. King 9 ,
- D. Sutton 10 ,
- I. Deuzeman 9 ,
- Y. Li ORCID: orcid.org/0000-0001-8815-6816 4 ,
- R. M. Wallace 4 ,
- R. S. Mani 11 ,
- G. Gongal 12 ,
- I. G. Handel 2 ,
- M. Bronsvoort ORCID: orcid.org/0000-0002-3271-8485 2 ,
- V. Naik 5 na2 ,
- S. Desai 5 na2 ,
- S. Mazeri 1 , 2 na2 ,
- L. Gamble 1 na2 &
- R. J. Mellanby 2 na2
Nature Communications volume 13 , Article number: 2788 ( 2022 ) Cite this article
12k Accesses
23 Citations
89 Altmetric
Metrics details
- Developing world
- Epidemiology
- Viral epidemiology
- Viral genetics
Dog-mediated rabies kills tens of thousands of people each year in India, representing one third of the estimated global rabies burden. Whilst the World Health Organization (WHO), World Organization for Animal Health (OIE) and the Food and Agriculture Organization of the United Nations (FAO) have set a target for global dog-mediated human rabies elimination by 2030, examples of large-scale dog vaccination programs demonstrating elimination remain limited in Africa and Asia. We describe the development of a data-driven rabies elimination program from 2013 to 2019 in Goa State, India, culminating in human rabies elimination and a 92% reduction in monthly canine rabies cases. Smartphone technology enabled systematic spatial direction of remote teams to vaccinate over 95,000 dogs at 70% vaccination coverage, and rabies education teams to reach 150,000 children annually. An estimated 2249 disability-adjusted life years (DALYs) were averted over the program period at 526 USD per DALY, making the intervention ‘very cost-effective’ by WHO definitions. This One Health program demonstrates that human rabies elimination is achievable at the state level in India.
Similar content being viewed by others
Ecological countermeasures to prevent pathogen spillover and subsequent pandemics
Raina K. Plowright, Aliyu N. Ahmed, … Annika T. H. Keeley

A guide to vaccinology: from basic principles to new developments
Andrew J. Pollard & Else M. Bijker

Infectious disease in an era of global change
Rachel E. Baker, Ayesha S. Mahmud, … C. Jessica E. Metcalf
Introduction
Rabies is a devastating and societally important zoonotic disease, which is transmitted principally to humans through the bite of infected dogs. This acute, progressive viral encephalitis has the highest case fatality of any infectious disease and kills tens of thousands of people annually, with children and impoverished communities being affected disproportionately 1 , 2 .
India is estimated to suffer the greatest rabies burden of any country, both in terms of annual human deaths and disability-adjusted life years (DALYs) 1 . Although the timely delivery of human post-exposure prophylaxis (PEP) prevents death from rabies, focusing on the post-bite treatment of people (a dead-end host) has no impact on the incidence of rabies in the canine reservoir population, leaving other members of the community vulnerable to acquiring the disease 3 . The effectiveness of mass dog vaccination in eliminating rabies from the reservoir animal population, and thereby preventing viral transmission to humans, has been known for over a century 4 , enabling dog-mediated rabies to be eliminated in numerous countries 5 , 6 , 7 . Modern rabies management highlights the importance of achieving zoonotic disease prevention and control through consideration of human, animal, and environmental components in a One Health approach 8 .
In most endemic settings without vaccination, the reproductive number of rabies in dogs is below two under natural conditions, falling below one where over 40% of the dog population is vaccinated 9 . To account for population turnover, annual vaccination of over 70% of dogs has been shown to successfully eliminate viral perpetuation, making canine rabies an ideal candidate for worldwide elimination 9 , 10 . Due to the particular ecology of dogs in India, where millions of dogs are free-roaming and hard-to-reach 11 , rabies is considered very challenging to eliminate, as is reflected in the complete paucity of examples of rabies elimination in any Indian state 12 . The reasons for this failure are invariably multifactorial, but achieving a step-change in the political prioritization of rabies control and surmounting the logistical challenges in reaching vaccination coverages sufficient to control the disease is critical to the quest for canine rabies elimination at the state level 11 , 12 , 13 .
Here, we report how these challenges were overcome in Goa, India through a collaboration between local government, non-governmental organizations, and academic partners, culminating in the elimination of human rabies, for the first time, at the state level in India. The One Health program consisted of three core areas of activity: dog vaccination; rabies education; and intensified human and animal rabies surveillance.
Dog vaccination
The central goal of this One Health program was to eliminate human rabies deaths by reducing rabies incidence in the canine reservoir through mass dog vaccination. This was achieved with mobile dog vaccination teams aiming for high coverage in both the free-roaming and owned confined dog populations throughout the state. Remote vaccination teams were spatially directed through assigned polygons displayed on a smartphone app, enabling managers to deliver vaccination resources to a specific geographic area at the sub-village scale 14 , 15 . The GPS and details of each dog vaccination were recorded offline in the app and subsequently shared with project managers through an administrator website. Vaccination teams rotated through the administrative regions of Goa (talukas) (Fig. 1 ), re-starting the state campaign cycle on an approximately annual basis (Supplementary Figs. 2 – 4 ). A combination of door-to-door (DD) and capture-vaccinate-release (CVR) methods were used to access dogs for parenteral vaccination. DD vaccination involved teams walking house-to-house offering owners an opportunity to have their dog vaccinated, whilst CVR consisted of teams using nets to catch and vaccinate dogs that could not be restrained manually.
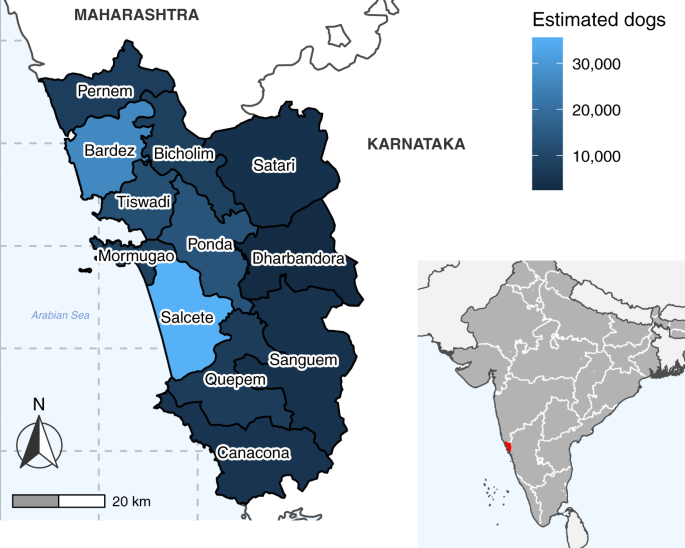
Choropleth map of Goa state showing taluka boundaries and colored by the estimated dog population. Dog population estimates were calculated from mean vaccination coverage and mean number of dogs vaccinated per taluka during vaccination cycles in which comprehensive post-vaccination surveys took place (Supplementary Methods, Supplementary Fig. 7 ). Inset map shows the state borders of India (white lines) and the location of Goa state (red). India state and Goa taluka boundaries were sourced from https://gadm.org .
The annual vaccination output increased, both in terms of geographic extent and a total number of dog vaccinations, through program refinement from 2013 to 2017 (Fig. 2 ). Intensive state-wide vaccination was achieved for the first time in 2017, vaccinating 97,277 dogs in an estimated total population of 137,353 dogs. This output was sustained through 2018 and 2019 (Fig. 2 ). A total of 426,119 rabies vaccine doses were administered to dogs between 2013 and 2019 (Fig. 2 , Supplementary Fig. 3 , Supplementary Table 1 ).
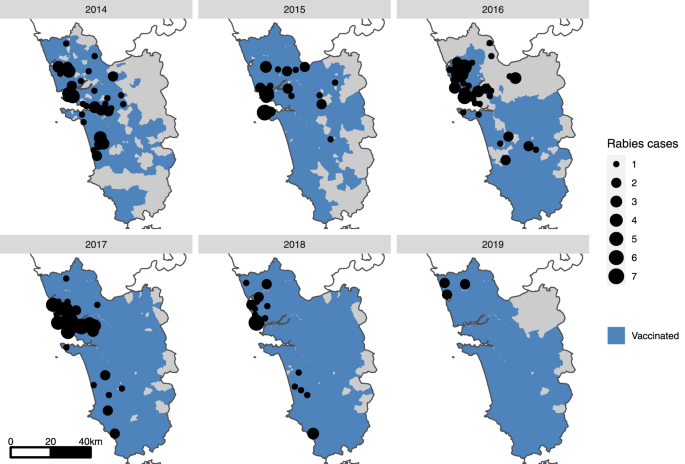
Maps of Goa state (gray shading) showing the villages/municipalities in which vaccination took place each year (blue shading) and positive canine rabies cases by village (black circles). A small-scale pilot dog vaccination campaign was conducted in 2013 (Supplementary Fig. 3 ), however, no location-specific canine rabies surveillance data were available at this time.
Vaccination methods were evaluated using post-vaccination dog-sight surveys to estimate coverage. A total of 3188 post-vaccination surveys were conducted during the period of study, recording 280,859 dog sightings. Final vaccination coverage was estimated from the last survey of each area, resulting in the omission of 793 surveys from the final analysis. The mean vaccination coverage in the 2016 campaign was 71.8% in all sighted dogs and 60.1% in roaming dogs, however, some areas of the state were not vaccinated. In 2017 intensive methods were applied state-wide achieving an estimated coverage of 71.7% in all dogs sighted and 53.1% in the roaming population (Supplementary Fig. 5 ).
Both ownership and confinement data were available for over 90% of dog vaccination records ( n = 384,149), of which 52% ( n = 199,887) were owned dogs and 48% ( n = 184,262) were unowned dogs. Unowned dogs were inherently always roaming, while owned dogs were either always roaming ( n = 35,823, 17.9%), allowed to roam for some of the time ( n = 100,618, 50.3%) or always confined (never roaming) ( n = 63,446, 31.7%). Consequently, most dogs vaccinated (83.5%) were among the roaming dog population for some or all of the time (Supplementary Fig. 4 ). The proportion of dogs vaccinated that were owned differed significantly between urban and rural settings. Of 213,467 dogs vaccinated in urban areas, 45.1% (CI: 44.9–45.4) were recorded as owned, as compared to 60.6% (CI: 60.4– 60.9) of 170,682 dogs vaccinated in rural settings (test of equal proportions p < 0.001).
Operational efficiency was improved through iterative refinement of vaccination methods. The initial approach, focusing on net-catching of unowned dogs in 2013, was adjusted to include the vaccination of owned dogs from October 2015. Smaller two-person DD vaccination teams were introduced in 2018 to more efficiently vaccinate dogs that could be restrained by hand, which was the case for 64.2% of all dogs vaccinated (restraint data available from 2018). Nevertheless, specialist equipment was still needed to access a considerable proportion of dogs, with 16.3% of owned dogs and 56.5% of unowned dogs being restrained by a net. The mean number of active vaccination teams per day increased from 2.5 (95% CI: 2.4–2.7) CVR teams in 2016 to a total of 7.7 (CI 7.4–8.0) (4.4 CVR teams and 3.3 DD teams) in 2019. Dog vaccination program-specific costs were available for 2018 and 2019, producing a mean cost per dog vaccinated of 3.45 USD (Supplementary Table 5 ).
Rabies education
The second pillar of the program was an education initiative whose primary focus was on teaching school children about rabies, how to avoid dog bites and what to do if bitten. The program also emphasized the importance and social value of ensuring as many dogs as possible were vaccinated against rabies each year. In total, school-based rabies education classes were delivered to 694,271 school children and 31,251 teachers between 2014 and 2019 (Supplementary Table 1 ). The scale of the school education program increased from 2014, plateauing from 2017 onwards with the delivery of educational lessons to ~170,000 children per year across 1400 schools in Goa. Activities to distribute rabies educational messages throughout communities intensified with a similar timeframe which resulted in the delivery of rabies lessons to 155,079 people through community groups, local authorities, and public events (Supplementary Table 1 ).
Dog rabies surveillance
The third pillar of the program was rabies surveillance within the dog population. Enhanced canine rabies surveillance was driven by improving the reporting of suspected rabid dogs from across public and private sectors. This was centrally managed through a widely publicized Rabies Hotline phone service launched in 2014.
Details of phone calls to the Rabies Hotline were available from October 2017, totaling 7372 and increasing from an average of 50.2 calls per week in 2018 (95% CI: 42.9–57.5) to 78.9 calls per week in 2019 (CI: 69.9–87.9) (Supplementary Fig. 8 , Supplementary Table 2 ). Mapping the origin of calls showed widespread engagement with the Rabies Hotline throughout the state (Supplementary Fig. 9 ). The most common reasons for contacting the Rabies Hotline were requests for vaccination of dogs (44.3%), reporting sick or injured dogs (without typical signs of rabies) (32.4%), and dog nuisance (6.7%). Despite increasing total calls, the rate of calls reporting suspect rabid animals reduced from a mean of 6.7 per month in 2017 to 4.8 in 2018 and 4.5 in 2019 (Supplementary Fig. 8 ).
Reports of suspect rabid animals initiated a veterinary field investigation to examine the animal and interview people involved or exposed. Systems for the management and testing of suspect rabid animals were established in 2014. Samples were initially shipped to the WHO reference laboratory for rabies in Bangalore (NIMHANS) for direct fluorescent antibody (DFA) test diagnosis. A rapid lateral flow assay (LFA) was used as a field-side tool in screening cases 16 , however, LFA results did not inform human PEP decisions. In 2016, the capacity to perform the DFA was established at the Goa Disease Investigation Unit (Department of Animal Husbandry & Veterinary Services) laboratory to provide routine timely rabies diagnosis ( Supplementary Methods ).
Seventy-three canine rabies cases were confirmed in the first 6 months of surveillance in 2014, with the highest monthly report of the study period occurring in July 2014 at 20 cases (Supplementary Fig. 10 ). The mean state-wide occurrence of canine rabies cases decreased from 10.6 cases per month in 2014 to 0.8 in 2019, a decrease of 92% (Supplementary Fig. 10 , Supplementary Table 2 ). The regional distribution of cases also changed significantly across the study period. No canine rabies cases were detected in 11 of Goa’s 12 taluka regions for over a year, from November 2018 until December 2019 (Figs. 2 , 3 , Supplementary Fig. 11 ). The continued occurrence of cases in the later stages of the program was confined to the northern region of Goa, bordering unvaccinated dog populations in the neighboring state (Figs. 2 , 3 ).

Asterisk denotes talukas that immediately border areas of high dog density in other states in which rabies remains endemic. Month-wise estimated vaccination coverage was calculated from the number of vaccinations delivered by campaign cycle and total estimated dog population by region, with a month-wise estimate of population turnover.
Analysis of the distribution of confirmed canine rabies cases by taluka over time revealed that cases were not homogeneously distributed. Cases predominated in areas of high dog density with a multivariable mixed-effects logistic regression model estimating that the odds of a taluka having at least one confirmed rabies case in a month increased as the taluka’s free-roaming dog population density increased (Fig. 4 , Supplementary Table 6 ). The model also showed that the odds of at least one rabies case occurring decreased as rolling mean 12-month vaccination coverage increased. Two talukas, Pernem and Canacona, neighboring unvaccinated dog populations at the north and south borders of the state did not follow this pattern as canine rabies cases continued to occur in the face of sustained high vaccination coverage (Fig. 3 ).
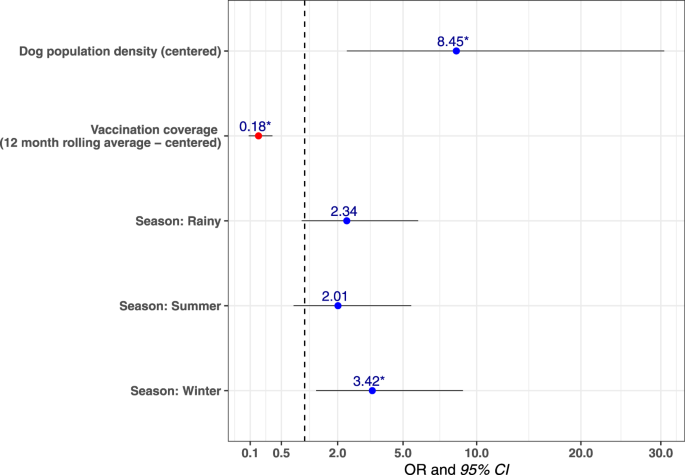
Multivariable mixed-effects logistic regression model predicting a taluka having at least one confirmed dog rabies case in a particular month ( n = 624). Figure shows the Odds Ratio (points) and 95% confidence intervals (horizontal lines). Asterisks indicate a p value < 0.05. The model shows that the odds of a taluka having at least one positive dog rabies case in a particular month increased as roaming dog population density increased. Similarly, as the rolling mean 12-month vaccination coverage increased, the odds of a positive rabies case were reduced. The odds of identifying at least one rabies case during the monsoon season (the reference season in the model) were lower, especially compared to the winter season, which had significantly higher odds. The AUC was calculated as 0.73, indicating that the model was very good at predicting the outcome.
Rabies virus sequencing was conducted to explore the molecular epidemiology of canine rabies in Goa 17 . Ninety-seven glycoprotein and 80 nucleoprotein gene sequences were generated from samples randomly selected from a bank of rabid dog brain samples spanning 2016 to 2018 (Supplementary Data 2 ). The sequences formed three major groups based on phylogenetic analysis, average nucleotide identity, and haploid network analysis of the glycoprotein gene: seventy sequences fell into Group 1; sixteen into Group 2; and eight into Group 3 (Fig. 5 , Supplementary Fig. 15 – 17 ). The high degree of sequence conservation within these groups indicated that they represented co-circulating lineages predominating in discrete geographic regions of Goa state: Group 1 in North Goa; Group 2 in South Goa; and Group 3 in the north border region, with the exception of Goa_A_04-03-2018 (Fig. 5 ). One sequence, from a rabid dog brought from Karnataka to Goa post-mortem (Goa_A_10-03-2017), was identified as a recently imported case into the region from north India, displaying <97% identity to all other samples (Supplementary Table 7 , Supplementary Fig. 16 ).
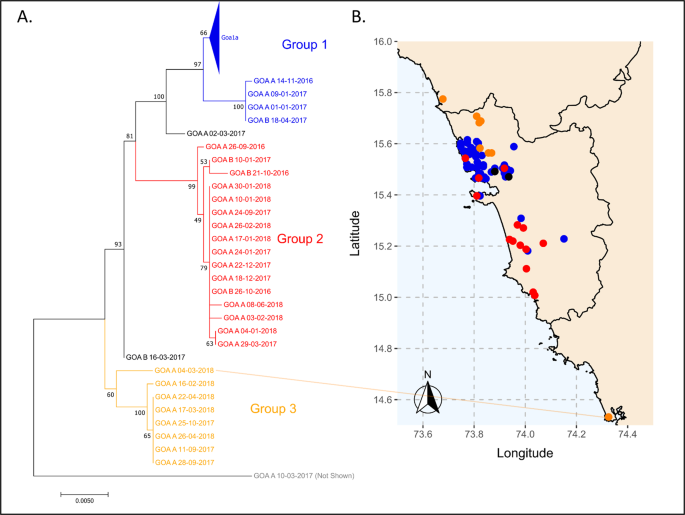
Phylogenetic analysis of 97 rabies virus glycoprotein gene sequences from Goa, India 2016–2018. The phylogenetic tree was calculated by maximum likelihood (GTR + G + I). Numbers on branch points represent bootstrap support based on 1000 replicates. The colors on the tree correspond to colored points on the Goa map. Sequences in Goa1a are collapsed for viewing convenience; a list of samples in each group is in Supplementary Data 2 . Positions on the map are approximate. Some points deviate slightly from their true location to allow for visualization of multiple samples from the same location. The location of sample Goa A 04-03-2018 (Karnataka) is highlighted by a line. The scale bar indicates the number of changes per site.
Time-scaled phylogenetic analysis of the glycoprotein gene estimated that Group 1 originated in 2009 (95% CI: 2006.6–2011.7), Group 2 in 2012 (95% CI: 2010.2–2014.6), and Group 3 in 2009 (95% CI: 2005.5–2013.3), with the most recent common ancestor between the groups estimated to be in 2003 (1999.4–2007.3) (Figs. 5 , 6 ). A sub-group of Group 3, excluding sample GOA_A_04-03-18 from Karnataka, was estimated to arise around 2014. Similar estimates (within 2.5 years) were obtained using the complete nucleoprotein gene (Supplemental Fig. 19 ).
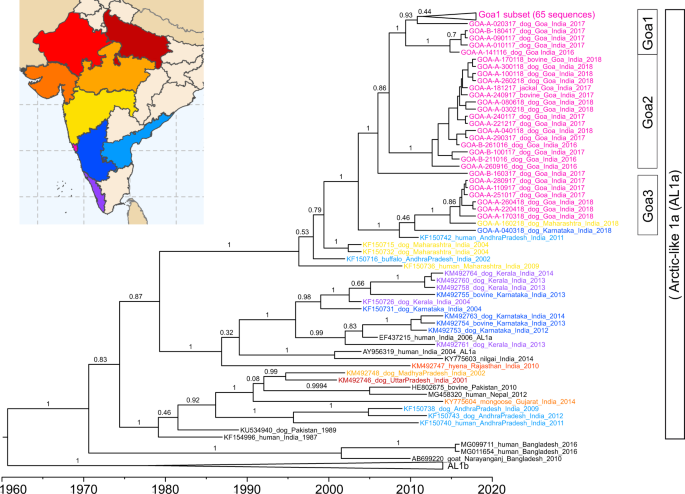
Time-scaled phylogeny of partial glycoprotein gene sequences (1317 nt) of Goa samples, Karnataka samples, and Maharashtra samples generated in this study with representative reference sequences from India belonging to the Arctic-like 1a rabies virus lineage. Representative samples from the Arctic-like 1b lineages were included as an outgroup. Scale at the bottom indicates year. Sample IDs are colored based on the state of sample collection, according to the coloration on the map. Bars to the right indicate members of Goa1, Goa2, and Goa3 groups. Numbers at the branches indicate posterior support values. AL1b: Arctic-like 1b.
All Goa sequences generated in this study clustered within the Arctic-like 1a rabies virus variant lineage and were most similar to sequences from the neighboring states of Karnataka, Maharashtra, and Andhra Pradesh (Fig. 6 ). Samples in Groups 1 and 2 clustered separately from sequences generated in other studies. However, Group 3 samples clustered with a rabies virus reference sequence from a human case in Andhra Pradesh, India in 2011 (Fig. 6 ).
Additional statistical cluster analysis of the spatio-temporal distribution of rabies cases identified one statistically significant cluster centered in North Goa from 2016 to 2017 (Supplementary Figs. 20 and 21 ). This statistically significant rise in canine rabies, above what would be expected if cases were distributed equally according to the population at risk, occurred where the Group 1 and Group 3 rabies virus lineages coincided. This region was close to the northern border where the Goan dog population interacts with unvaccinated dogs in neighboring districts of Maharashtra (Supplementary Fig. 21 ).
Public health impact
To assess the human impact of the One Health program, information on dog bites and human rabies deaths was acquired from the Directorate of Health, Government of Goa. Human rabies deaths reduced from 17 in 2014 to zero in 2018 and 2019 (Fig. 7 , Supplementary Table 2 ). This decline in human deaths occurred during a period of increased human rabies surveillance resulting from numerous rabies-focused activities engaging the human medical profession in Goa ( Supplementary Methods ). The number of reported dog bites increased from 785 per 100,000 people in 2012 to 1430 per 100,000 people in 2019 (Supplementary Fig. 22 , Supplementary Table 2 ).
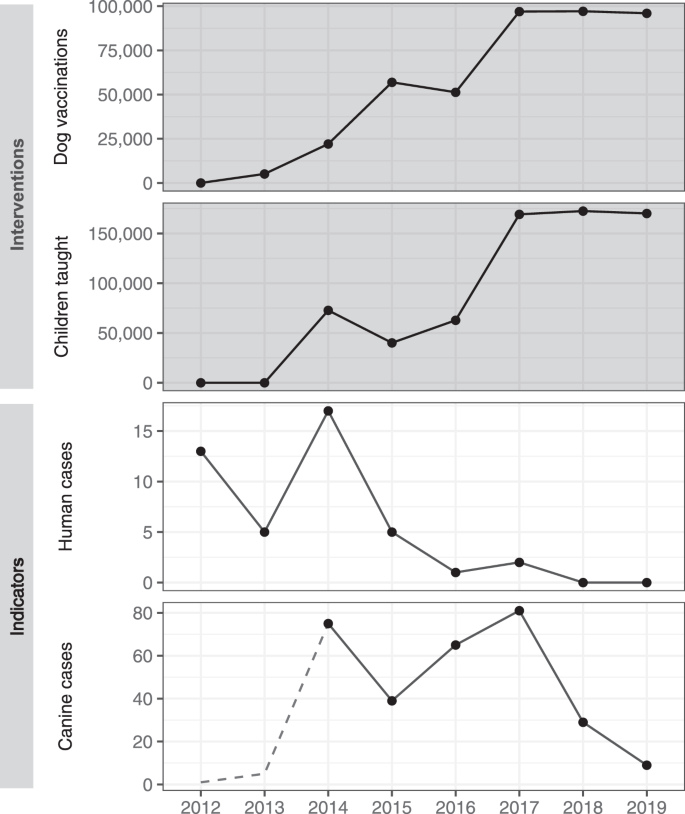
Graphs show intervention outputs (dark background) of annual total dog vaccinations and children taught about rabies and indicators of rabies control (light background) of annual human rabies deaths and confirmed canine rabies cases. The dotted line in canine cases indicates a period prior to the enhancement of animal rabies surveillance activities. The decrease in canine rabies cases in 2015 is due to the cessation of canine rabies surveillance activities between October 2014 and September 2015.
A previously published model (RabiesEcon 18 , 19 ) was used to estimate the cost-effectiveness of the intervention. Goa-specific programmatic data were used to populate the model, including human and dog population data; annual dog vaccination coverage; program costs; PEP costs; and estimated rates of access to PEP (Supplementary Data 3 , Supplementary Table 5 ). The estimated mean cost per death averted and cost per DALY averted from 2013 to 2019 were 14,866 USD and 526 USD respectively. During this period the program was estimated to result in a total of 2,249 DALYS averted, and 80 deaths averted as compared to no intervention. Over a 10-year projection (2013–2023), the intervention was estimated to prevent 121 human rabies deaths and 3427 DALYS at a mean cost of 567 USD per DALY averted. The model predictions of human rabies deaths, human rabies exposures, and total estimated intervention cost were concordant with empirical values from the program area.
This collaborative One Health program demonstrates the feasibility and cost-effectiveness of human rabies elimination at the state level in India, offering a tangible example of success in the quest to eliminate canine-mediated human rabies deaths by 2030. Despite the terrible toll rabies exerts in India, the inaccessible nature of the huge free-roaming dog population resulted in the modern discourse that, while local elimination of rabies within restricted communities is feasible, extending these approaches to a state-level still presents a near-insurmountable practical and logistical challenge 8 , 11 , 20 , 21 . Such failings resulted in calls to the scientific community to refocus rabies research towards programs that advanced the field implementation and evaluation of rabies elimination strategies 22 , 23 . The current initiative integrating mass dog vaccination, education, and rabies surveillance, underpinned by innovations in mHealth remote team direction, data capture, and real-time analysis, specifically addressed this challenge and has shown robustly that freedom from dog-mediated human rabies deaths is feasible and within reach.
Dog accessibility is a major barrier to the efficient application of high-coverage state-wide dog vaccination campaigns in India. Whilst central point dog vaccination approaches have achieved high-number, high-coverage outputs in some African settings 10 , 24 , the high proportion of stray dogs in India limits the likely success of such methods in achieving herd immunity against rabies 11 . Similarly, DD campaigns centered on catching dogs by hand had limited success 25 . The majority of dogs vaccinated in the Goa program were caught by hand, however, achieving adequate vaccination coverage was not possible without specialist equipment and expertise through net-catching. Advanced vaccination techniques are therefore necessary to interrupt rabies virus transmission in such settings. Regular assessment of post-vaccination coverage during the early stages of program development proved to be critical in identifying where the vaccination approach was successful and where improvement was needed 26 . The mean cost of 3.45 USD per dog vaccinated was higher than reported global averages (2.18 USD), but within the range reported by other mass dog vaccination interventions (1.13–15.62 USD) 27 , 28 . This appears reasonable given the large free-roaming dog population in Goa and reliance on net capture to reach adequate vaccination coverages. The use of oral rabies vaccination (ORV) of dogs in combination with parenteral methods offers the potential to further optimize the current approach to intensively vaccinate otherwise inaccessible dog populations across large states in a short period of time 20 , 29 , 30 . An economic study of ORV in dogs found that communities similar to Goa could likely implement this approach at a similar cost per dog vaccinated 31 .
According to the WHO criteria of cost-effectiveness 32 , the intervention can be considered “very cost-effective”. The cost of each year of healthy life (i.e., DALY averted) of 526 USD was a quarter of the gross domestic product per capita for India (2100 USD) 33 , and 13 times lower than that of Goa state (7029 USD) 34 in 2019. The estimated cost per DALY averted from the Goa program was lower than statistical estimations of rabies interventional cost-effectiveness in India and Sri Lanka at 1064 USD and 1401 USD per DALYs respectively 35 , 36 , but higher than a recent report in Rajasthan at 40 USD per DALY averted 37 . This variability is likely a reflection of the non-standardization of economic modeling methodologies. However, unlike many assessments based on estimated values, state-wide implementation of the current program through a single government collaborator enabled comprehensive inputs to be based on programmatic operational and surveillance data. In the current analysis, we assumed that rates of PEP administration would not change until policies were updated to reflect the reduced risk and limited need for PEP. Assuming these changes are made in the lifetime of this program, the interventions would be even more cost-effective. The collaborative structure of the Goa program drove rapid innovation and expansion of activities, leveraging external funding to support the refinement of methodology and development of tools to increase efficiency, whilst the local government retained control and leadership of the intervention. Similar collaborative approaches were central to the global effort to eradicate polio 38 and have been beneficial in rabies control initiatives elsewhere 39 , 40 . Proposed innovative funding structures, such as development impact bonds, may offer novel investment sources to stimulate the expansion of rabies control activities 41 .
The use of smartphone technology revolutionized the systematic, spatial management of the vaccination workforce in Goa 15 . A study in Haiti found that the same smartphone application used to spatially direct vaccination teams at the sub-village level, significantly increased vaccination coverage compared with non-technology-aided methods 14 . Such technology-aided vaccination strategies have the potential to minimize unvaccinated pockets in the population and therefore hasten viral elimination 42 . Similar strategies to spatially dissect national public health programs for delivery at the community level, described as “microplans”, have been reported as significant to the successful delivery of polio and other disease control interventions 43 , 44 , 45 . The availability of GPS location and dog-specific data for every animal vaccinated provided unprecedented transparency in reporting to government stakeholders in addition to a wellspring of insights into dog population ecology from which to optimize vaccination strategy.
The state-wide education initiative in schools directed rabies awareness towards children, the demographic at disproportionate risk of death from rabies 46 . Similar class structures, combining presentation, theater, and question-answer methods have been demonstrated to be effective in other settings 47 . Rabies educational content was integrated into the Government of Goa school science curriculum for children aged 11–12 years in 2020, helping to ensure sustained awareness of the disease whilst regional control efforts grow. The increase in dog-bite presentations at bite clinics during the project period may reflect the widespread community awareness of rabies brought about by the community education program and counters the traditional view that bite cases fall following rabies control 48 . Concurrent development of integrated bite case management (IBCM) systems to reduce unnecessary PEP in people bitten by healthy dogs would maintain cost-effective use of PEP in the face of this increase 49 , 50 .
Enhancing state capacity for the detection and diagnosis of rabies in dogs was critical to gaining insight into the scale of the problem; monitoring the impact of dog vaccination activities; and providing incentives for continued support from all stakeholders. In addition to these benefits, the removal of rabid dogs may also have hastened elimination by preventing ongoing rabies virus transmission 51 , 52 . Canine rabies surveillance focused on three main areas of enhancement: reporting, detection, and diagnosis. The Rabies Hotline proved to be highly effective in soliciting reports of suspect rabies cases from the public, police, animal welfare, human health, and veterinary sectors. Full-time Rabies Surveillance Officers capable of deploying to anywhere in the state at short notice ensured the timely veterinary investigation and testing of suspect rabid animals.
Prior to the local availability of OIE-approved rabies diagnostic tests, the use of rapid diagnostic tests motivated veterinarians to retrieve samples from suspect rabid animals under difficult field conditions 16 and demonstrated the urgent need for in-state laboratory rabies diagnostic capabilities. Reports of poor quality control and low sensitivity of these tests, meant that negative results could not be considered valid and guidelines for their use should be a point of consideration in the development of national programs 53 . The establishment of DFA testing capacity in the government veterinary laboratory in 2016 was essential for robust state-level rabies surveillance and to ultimately demonstrate canine rabies freedom as recognized by OIE.
The use of portable MinION technology in Goa state revealed the potential for sequencing at regional veterinary laboratories to enhance state-level control strategies through a greater understanding of rabies virus transmission dynamics 17 , 54 . Time-scaled phylogenetic analysis of Goa sequences revealed a recent common ancestor around 2003–2005, with subsequent diversification into the three lineages, identified in this study as Goa1, Goa2, and Goa3. Further diversification of the Goa3 samples from the northern Goa border region around 2014, coincided with an increase in reported rabies cases in the area between 2014 and 2018. This finding supported the conclusion of the spatio-temporal cluster analysis indicating a potential site of the continued reintroduction of canine rabies. Expansion of dog vaccination beyond Goa’s borders is underway to reduce the risk of direct rabies virus reintroduction through dog movement at the border. The identification of a rabid dog importation into the greater region from northern India highlighted the risk of inter-state spread of rabies virus via human-mediated transport of dogs. National and regional rabies control will invariably require widespread coordination of vaccination efforts as was found to be critical to the success of programs in Europe and Latin America 55 , 56 . The continued monitoring of rabies virus sequences in Goa will provide a detailed picture of rabies virus transmission to further optimize control strategies on a larger scale.
The predominance of canine rabies in regions of high human and dog population density in Goa addressed the question of where efforts should be prioritized during the early phases of mass dog vaccination scale-up, when resources may be insufficient to vaccinate an entire state or district. The findings of the current study support an approach to accelerate the development of dog vaccination campaigns in Indian metropolis settings and the subsequent propagation of efforts into peri-urban and rural settings. This strategy was effective in regional rabies elimination efforts in Latin America, where nascent dog vaccination programs focused on large urban centers. These activities enabled the development of expertize, capacity, and political momentum to progress towards widespread initiatives ultimately resulting in the inter-state success of a magnitude needing to be replicated in India 20 , 56 . However, it is important to note that whilst urban centers may present the largest canine rabies burden, dog populations in peri-urban and rural regions can sustain rabies virus transmission 57 and the limited access to PEP in rural communities often results in a disproportionate human rabies burden in this areas 3 . Further research is required to explore opportunities to spatially prioritize dog vaccination for maximum cost-efficacy of elimination programs.
National implementation of effective canine rabies control in India would represent the greatest achievement by a single country in the endeavor to eliminate dog-mediated human rabies by 2030. Clearly, this would require enormous mobilization of resources and sustained political commitment. Although the outputs of the current study would need to be amplified several hundred times over to be replicated at the national scale, it showcases the feasibility, cost-effectiveness, and considerable public health impacts of One Health interventions for rabies control at the state level in India. Enduring political support, modern technologies in program management, and intersectoral, transdisciplinary collaboration was pivotal to the success of this multi-year effort and provide a clear rationale for other states to follow. The growth trajectory of the Goa rabies control program aligned with that outlined previously by Wallace et al. 58 , with phases of preparation, scale-up, and sustained vaccination, resulting in rapid impacts on human and canine rabies incidence within three years of scale-up. Whilst dog accessibility presents a major challenge to achieving herd immunity in a predominantly unowned dog population, this study demonstrates that effective mass dog vaccination campaigns are feasible in India and can achieve canine rabies virus elimination across large geographic areas. In 2021, Goa became the first Indian state to be declared a Rabies Controlled Area 59 under the Prevention and Control of Infectious and Contagious Diseases in Animals Act, 2009, ensuring legislation to maintain rabies control activities and setting a precedent for other states. The methods and technology developed through the current study can be leveraged to support the planning of national rabies control efforts in South Asia and accelerate similar examples of success in driving towards the 2030 goal of global dog-mediated human rabies elimination.
The period of study was from 10/09/2013 to 31/12/2019, which coincides with the launch of the first pilot dog vaccination and education initiative and the end of the fifth year of large-scale vaccination activities. The Government of Goa Department for Animal Husbandry oversaw the project protocols and methods for mass dog vaccination and animal rabies surveillance, with input from the Goa Veterinary Association to adhere to all relevant ethical regulations. A veterinary ethical review was provided by the University of Edinburgh Veterinary Ethical Review Committee.
Goa covers an area of 3700 square kilometers and has an estimated human population of 1.5 million, with 62% of people residing in areas defined as “urban” 60 . The state is bordered to the west by the Arabian Sea and to the east by the Western Ghats mountain range (Fig. 1 ). Tourism is a major industry. Goa is one of India’s more developed states, as measured by the United Nation’s human development index (HDI), with an HDI of 0.76 in 2017 as compared to India's national HDI of 0.64 61 , 62 . The state is divided administratively into two Districts, North Goa and South Goa, which are further divided into a total of 12 talukas. These talukas are made up of local administrative units of village panchayats (villages) and municipalities (towns and cities), of which there are a total of 420 in Goa.
The non-governmental organization Mission Rabies ( www.missionrabies.com ) began investigation of mass dog vaccination methods in September 2013 with a two-week pilot vaccination campaign, followed by a dog vaccination and sterilization campaign in densely populated regions from March to September 2014. In September 2015, the Government of Goa established a formal collaboration through a Memorandum of Understanding with Mission Rabies to support the implementation of a state-wide dog vaccination initiative, rabies surveillance, and education program. The aim was to systematically vaccinate at least 70% of the dog population on an annual basis using a combination of DD and CVR methods (Supplementary Fig. 1 ) 2 , 9 . Vaccinations were provided free of charge and each dog was administered with a 1 ml dose of rabies vaccine (Nobivac® Rabies–MSD Animal Health) either subcutaneously or intramuscularly, depending on animal position and restraint method. Each dog was marked with non-toxic paint on the top of the head, lasting for several days to enable identification of vaccination status on post-vaccination surveys. Consent was obtained from an owner prior to vaccination of dogs that were identifiably owned.
Vaccination team direction and program monitoring were performed using the WVS App, a purpose-built mHealth technology described previously 15 , 26 . The information recorded offline for each dog at the time of vaccination included: vaccination team ID; time; date; GPS; sex; age; ownership; neuter status; confinement; and health status. A web-based interface enabled project managers to review daily the geographic extent of vaccination work and to spatially direct vaccination and survey teams to sub-village ‘Working Zones’ according to vaccination output 26 .
Between 2013 and 2017 dog vaccination was conducted entirely by CVR teams typically consisting of seven or more people traveling by truck: one vaccinator, one assistant, one driver, and four dog catchers using nets. Dogs that could be held by hand, either by an owner or the team, were manually restrained for vaccination. Dogs that could not be manually restrained were caught using nets (Supplementary Fig. 1 ). Two-person DD vaccination teams, consisting of a vaccinator and assistant traveling by scooter were introduced in 2018 to improve operational efficiency. DD teams vaccinated in Working Zones first, targeting dogs that could be restrained by hand and therefore did not require a large team of net-catchers. CVR teams subsequently followed in these regions to catch and vaccinate difficult to handle dogs that had not been vaccinated by DD teams, as indicated by the absence of a vaccination paint mark.
Post-vaccination dog-sight survey methods have been described previously and enabled immediate re-deployment of vaccination teams to Working Zones with low vaccination coverage 26 . In 2013 and 2014 only free-roaming dogs sighted were recorded, however, from 2015, dogs confined to private property at the time of sighting were also recorded. These surveys were performed following the completion of dog vaccination in each Working Zone to evaluate vaccination methodologies until 2017. In 2018 and 2019, post-vaccination surveys were used to spot-check coverage, and whilst vaccination teams were re-deployed to boost areas of low coverage, repeat surveys were not conducted as had been done in previous years. Therefore, surveys from 2018 and 2019 were not included in the analysis of vaccination coverage assessment.
Alongside the state-wide systematic mass dog vaccination program, Mission Rabies implemented a concurrent education initiative focused on the delivery of structured lessons to children in schools and educational sessions to community groups. Typically, the education program was implemented through three rabies education officers who moved systematically across the state ahead of the vaccination schedule, delivering rabies lessons in schools and sessions to the community ( Supplementary Materials ) 15 . Rabies lessons were 15–30 minutes in duration, were adjusted to the age group, and fell under the following headings: Rabies is serious; Stopping dog bites; Rabies first aid; and Rabies is preventable 47 . In the later stages of the project, events to train schoolteachers in rabies lesson delivery were also conducted.
Rabies surveillance
A central public rabies reporting and response service was established in March 2014, prior to which there was no structured process for the reporting of suspect rabid animals. A Rabies Hotline phone number was widely publicized to the public, government, and private sectors for the reporting of suspect rabid animals 24 hours a day, 7 days a week ( Supplementary Methods , Supplementary Figure 1 ). Notification of a suspect rabies case to the Rabies Hotline triggered an immediate field investigation to examine the animal, and if necessary, submit samples for testing at the Goa Disease Investigation Unit ( Supplementary Methods ). The Rabies Hotline and rabies response activities were not active from October 2014 to September 2015. Canine rabies surveillance intensity increased in April 2018 through the incorporation of IBCM methods described elsewhere 50 . Suspected human rabies cases were managed and diagnosed at the Goa Medical College, with numerous events and initiatives taking place during the study period to raise awareness of the importance of human rabies surveillance in the medical profession ( Supplementary Methods ). PEP was available free of charge to those presenting for treatment of dog bites at government medical facilities throughout the state.
Data analysis
Data for dog vaccinations, educational events, post-vaccination dog surveys, notifications of suspect animal rabies cases and suspect animal rabies case investigations were exported from the WVS App database in CSV format. Analysis was performed using R version 3.6.2. Manual reports and field records were used to verify App data. Post-vaccination surveys and dog vaccination data were used to calculate mean dog vaccination coverage and month-wise vaccination coverage by taluka ( Supplementary Methods ).
Logistic regression model
A mixed-effects multivariable logistic regression model was used to identify factors associated with a taluka having at least one confirmed dog rabies case each month (Supplementary Software 1 ). The number of confirmed dog rabies cases for each taluka each month was used to create the binary outcome variable in the model. If a taluka had at least one case in a given month it was considered positive, and if no cases were recorded that month the taluka was considered negative. Explanatory variables investigated included free-roaming dog population, free-roaming dog population density, estimated monthly vaccination coverage, estimated 12-month rolling mean coverage, season, and whether the taluka borders unvaccinated dog regions (Supplementary Data 1 ). The seasonal timeframes used have been described previously 63 . The data were randomly split into a training and testing dataset using a 70:30 ratio using R package caret 64 . Univariable analysis was used and any variable with a p value of <0.15 was considered for the final model. To investigate whether numerical variables had a linear relationship with the log-odds of the outcome, these were split into quartiles and univariable models were visualized to assess the relationship. Manual forward variable selection was conducted and the final model was chosen based on the lowest Akaike information criterion (AIC). The final model was validated, testing its ability to predict the outcome in the test dataset by estimating the area under the curve using R package ROCR 65 .
Phylogenetic analysis
Rabies viral sequencing of glycoprotein and nucleoprotein genes was performed on an archive of positive canine rabies brain samples spanning 2016 to 2018 using a MinION sequencer (Oxford Nanopore Technologies) at the Goa Disease Investigation Unit ( Supplementary Methods ). Phylogenetic analysis was performed to evaluate the similarity between samples and to compare them with historic references 17 ( Supplementary Methods ).
Time-scaled phylogenies were generated from complete nucleoprotein and partial glycoprotein gene (1317 nt) data using BEAST v1.10.4 and GTR + G + I substitution model with two partitions (1 + 2 and 3) and an uncorrelated relaxed lognormal molecular clock. Years of sample collection were used as tip dates, with an uncertainty of 1. The mean of the clock rate prior was set to 10 -4 (normal distribution, SD = 1). Both analyses lacked calibration data points and estimated most recent common ancestor ages should be considered relative and are highly influenced by the samples included, which was biased toward recent samples due to improved access to sequencing. Maximum clade credibility trees were generated using TreeAnnotator and visualized in FigTree v1.4.4. Map of India was generated from shapefile data at GADM.org using raster, ggspatial, and ggplot2 packages in RStudio (R v4.0.2). Figures were finished in InkScape. Reference sequences included in the time-scaled phylogenetic analyses were chosen based on sequence length (full-length nucleoprotein gene and at least 1317 nt of glycoprotein gene), isolation in India or neighboring countries, inclusion in Arctic-like 1a lineage, year of isolation available, and sequence quality. Sequences published by Deventhiran et al. (2018) 66 were not included in these analyses as several sequencing errors were observed and phylogenetic clustering of nucleoprotein and glycoprotein gene sequences from that study was inconsistent. Arctic rabies virus variant sequences KX148105 and JQ148105 , Arctic-like 3 sequence KX148228 , and Arctic-like 1b sequences KX148225 , KX148226 , HE802676 , KX148227 , KF150745 , KF150744 , and MK760761 were used as outgroups 67 .
Spatio-temporal cluster analysis
A space-time scan statistic was used to detect statistically significant spatio-temporal clusters of canine rabies using a discrete Poisson retrospective space-time probability model 68 as used in a number of other studies of rabies distribution 69 , 70 (Supplementary Software 2 ). For the purposes of this analysis, the village was considered the study unit, and canine rabies cases recorded each month were aggregated at the centroid of each village for use in the model. The human population of each village was used as a proxy for the population at risk. Cluster analyses were performed using SaTScan™ v9.6 software 71 through R package rsatscan 72 . Satscan uses Monte Carlo hypothesis testing to obtain the p values. For this analysis, we used 9999 Monte Carlo replications, and a cluster was considered statistically significant if the p value was <0.05. The maximum size for the mobile window of the scan was set as 20% of the population at risk with a circular shape.
Cost per dog vaccinated
In-country annual program expenditures were used alongside annual dog vaccination output to estimate the cost per dog vaccinated. This expenditure was determined from account records reporting on Goa expenditure on all sources of income, including government and charitable grants, and the estimated value of donated vaccine (Supplementary Tables 3 – 5 ). A breakdown of dog vaccination program expenditures, within total program expenditures, was only available for 2018 and 2019 (Supplementary Table 5 ).
Cost-effectiveness model
The RabiesEcon model, developed by the US Centers for Disease Control and Prevention, was used to estimate the impact and cost-effectiveness of the intervention (Supplementary Data 3 ) 18 , 19 , 73 . Goa values were inputted into the RabiesEcon version used by Kunkel et al. 18 to assess the additional costs and benefits of the Goa rabies control program as compared to no intervention. Input values from Goa, including human and dog population data, cost of dog vaccination and PEP, annual dog vaccination output, and estimated rates of access to PEP during and after the program (Supplementary Data 3 ). Predicted outcomes such as estimated human exposures and human rabies deaths were cross-checked with real-world data. The RabiesEcon model was published by Kunkel et al. (2021) was modified to account for costs associated with PEP administration resulting from exposures to animals that were unavailable for diagnostic testing. This adjustment was made by including a feature to allow the user to estimate the reduction in PEP after the implementation of the rabies control program. The equation in the tab “Data”, row 27 was modified to reflect the larger of either the pre-program costs multiplied by the user-defined PEP reduction rate or the number of true rabies virus exposures estimated by the model. As part of the data inputs to the model, it was assumed that there was no reduction in PEP administration during or after the intervention. All annual costs were discounted at 3%. The costs associated with surgical sterilization of dogs by non-governmental organizations were not considered in the model.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. All sequences generated as part of this study are deposited in GenBank (accession codes: MW054945 – MW055041 ). Source data are provided in this paper.
Code availability
The R code used in the logistical regression and spatio-temporal analysis is included in the Supplementary Files .
Hampson, K. et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis . 9 , e0003709 (2015).
World Health Organization & WHO. WHO Expert Consultation on rabies. Third report. World Health Organization technical report series, 1012 (2018).
Sudarshan, M. K. et al. An epidemiological study of animal bites in India: results of a WHO sponsored national multi-centric rabies survey. J. Commun. Dis. 38 , 32–39 (2006).
CAS PubMed Google Scholar
Umeno, S. & Doi, Y. A study on the antirabic inoculation of dogs, and the results of its practical application. Kitasato Arch. Exp. Med. 4 , 89–108 (1921).
Google Scholar
Kurosawa, A. et al. The rise and fall of rabies in Japan: a quantitative history of rabies epidemics in Osaka Prefecture, 1914–1933. PLoS Negl. Trop. Dis. 11 , 1–19 (2017).
Article Google Scholar
Schneider, M. C. et al. Current status of human rabies transmitted by dogs in Latin America. Cad. Saude Publica 23 , 2049–2063 (2007).
Article PubMed Google Scholar
Blanton, J. D., Hanlon, C. A. & Rupprecht, C. E. Rabies surveillance in the United States during 2006. J. Am. Vet. Med. Assoc. 231 , 540–556 (2007).
Rupprecht, C. E. et al. A history of rabies—The foundation for global canine rabies elimination. Rabies (Elsevier Inc., 2020). https://doi.org/10.1016/b978-0-12-818705-0.00001-7 (Elsevier Inc., 2020).
Hampson, K. et al. Transmission dynamics and prospects for the elimination of canine Rabies. PLoS Biol. 7 , 0462–0471 (2009).
Article CAS Google Scholar
Zinsstag, J. et al. Vaccination of dogs in an African city interrupts rabies transmission and reduces human exposure. Sci. Transl. Med . 9 , eaaf6984 (2017).
Tiwari, H. K., Robertson, I. D., O’Dea, M. & Vanak, A. T. Demographic characteristics of free-roaming dogs (FRD) in rural and urban India following a photographic sight-resight survey. Sci. Rep. 9 , 1–10 (2019).
Kakkar, M., Venkataramanan, V., Krishnan, S., Chauhan, R. S. & Abbas, S. S. Moving from rabies research to rabies control: lessons from India. PLoS Negl. Trop. Dis. 6 , e1748 (2012).
Article PubMed PubMed Central Google Scholar
Radhakrishnan, S., Vanak, A. T., Nouvellet, P. & Donnelly, C. A. Rabies as a public health concern in India–a historical perspective. 1–21 https://doi.org/10.3390/tropicalmed5040162 (2020).
Monroe, B. et al. Every dog has its data: evaluation of a technology-aided canine rabies vaccination campaign to implement a microplanning approach. Front. Public Heal . 9 , 757668 (2021).
Gibson, A. D. et al. One million dog vaccinations recorded on mHealth innovation used to direct teams in numerous rabies control campaigns. PLoS One 13 , e0200942 (2018).
Article PubMed PubMed Central CAS Google Scholar
Yale, G. et al. Evaluation of an immunochromatographic assay as a canine rabies surveillance tool in Goa, India. Viruses 11 , 1–10 (2019).
Gigante, C. M. et al. Portable rabies virus sequencing in canine rabies endemic countries using the oxford nanopore MinION. Viruses 12 , 1255 (2020).
Article CAS PubMed Central Google Scholar
Kunkel, A. et al. The urgency of resuming disrupted dog rabies vaccination campaigns: a modeling and cost-effectiveness analysis. Sci. Rep. 11 , 1–8 (2021).
Borse, R. H. et al. Cost-effectiveness of dog rabies vaccination programs in East Africa. PLoS Negl. Trop. Dis. 12 , e0006490 (2018).
Gibson, A. D. et al. Reviewing solutions of scale for canine rabies elimination in India. Trop. Med. Infect. Dis. 5 , 1–22 (2020).
Shahid, S. & Kakkar, M. Rabies control in India: a need to close the gap between research and policy. Bull. World Heal. Organ 93 , 131–132 (2015).
Cleaveland, S., Lankester, F., Townsend, S., Lembo, T. & Hampson, K. Rabies control and elimination: a test case for One Health. Vet. Rec. 175 , 188–193 (2014).
Zinsstag, J. Towards a science of rabies elimination. Infect. Dis . Poverty 2 , 22 (2013).
Mazeri, S. et al. Using data-driven approaches to improve delivery of animal health care interventions for public health. Proc. Natl Acad. Sci. USA 118 , e2003722118 (2021).
Article CAS PubMed PubMed Central Google Scholar
Belsare, A. V. & Gompper, M. E. Assessing demographic and epidemiologic parameters of rural dog populations in India during mass vaccination campaigns. Prev. Vet. Med. 111 , 139–146 (2013).
Gibson, A. D. et al. Vaccinate-assess-move method of mass canine rabies vaccination utilising mobile technology data collection in Ranchi, India. BMC Infect. Dis. 15 , 589 (2015).
Undurraga, E. A. et al. Costs and effectiveness of alternative dog vaccination strategies to improve dog population coverage in rural and urban settings during a rabies outbreak. Vaccine 38 , 6162–6173 (2020).
Elser, J. L., Hatch, B. G., Taylor, L. H., Nel, L. H. & Shwiff, S. A. Towards canine rabies elimination: economic comparisons of three project sites. Transbound. Emerg. Dis. 65 , 135–145 (2018).
Article CAS PubMed Google Scholar
Gibson, A. D. et al. Oral bait handout as a method to access roaming dogs for rabies vaccination in Goa, India: a proof of principle study. Vaccin. X 1 , 100015 (2019).
Chanachai, K. et al. Feasibility and effectiveness studies with oral vaccination of free ‐ roaming dogs against rabies in Thailand. Viruses 13 , 1–13 (2021).
Wallace, R. M. et al. Estimating the effectiveness of vaccine programs in dog populations. Epidemiol. Infect. 147 , e247 (2019).
World Health Organisation. Making choices in health: WHO guide to cost-effectiveness analysis. vol. 1 (World Health Organisation, 2003).
The World Bank. GDP per capita - India. World Bank national accounts data, and OECD National Accounts data files https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?end=2020&locations=IN&start=1960&view=chart (2019).
Statistics Times. Indian states by GDP per capita. https://statisticstimes.com/economy/india/indian-states-gdp-per-capita.php (2021).
Fitzpatrick, M. C. et al. One Health approach to cost-effective rabies control in India. Proc. Natl Acad. Sci. 113 , 14574–14581 (2016).
Häsler, B. et al. A one health framework for the evaluation of rabies control programmes: a case study from Colombo City, Sri Lanka. PLoS Negl. Trop. Dis. 8 , e3270 (2014).
Larkins, A. J., Reece, J. F., Shaw, A. P. M. & Thrusfield, M. V. An economic case study of the control of dog-mediated rabies by an animal welfare organisation in Jaipur, India. Prev. Vet. Med. 183 , 105120 (2020).
Aylward, B. & Tangermann, R. The global polio eradication initiative: lessons learned and prospects for success. Vaccine 29 , D80–D85 (2011).
Taylor, L. Eliminating canine rabies: the role of public-private partnerships. Antivir. Res 98 , 314–318 (2013).
Léchenne, M. et al. Increasing rabies data availability: the example of a One Health research project in Chad, Côte d’Ivoire and Mali. Acta Trop. 215 , 105808 (2021).
Anyiam, F. et al. Cost-estimate and proposal for a development impact bond for canine rabies elimination by mass vaccination in Chad. Acta Trop. 175 , 112–120 (2017).
Ferguson, E. A. et al. Heterogeneity in the spread and control of infectious disease: consequences for the elimination of canine rabies. Sci. Rep. 5 , 18232 (2015).
Article ADS CAS PubMed PubMed Central Google Scholar
Teshome, S., Desai, S., Kim, J. H., Belay, D. & Mogasale, V. Feasibility and costs of a targeted cholera vaccination campaign in Ethiopia. Hum. Vaccin Immunother. 14 , 2427–2433 (2018).
WHO & UNICEF. Microplanning for Immunization Service Delivery Using the Reaching Every District (RED) Strategy. Expanded Programme on Immunization of the Department of Immunization, Vaccines and Biologicals https://www.who.int/immunization/sage/9_Final_RED_280909.pdf (2009).
Aylward, R. B. Eradicating polio: today’s challenges and tomorrow’s legacy. Ann. Trop. Med. Parasitol. 100 , 401–413 (2006).
Knobel, D. L. et al. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ 83 , 360–368 (2005).
PubMed PubMed Central Google Scholar
Burdon Bailey, J. L. et al. A rabies lesson improves rabies knowledge amongst primary school children in Zomba, Malawi. PLoS Negl. Trop. Dis. 12 , e0006293 (2018).
Cleaveland, S., Kaare, M., Tiringa, P., Mlengeya, T. & Barrat, J. A dog rabies vaccination campaign in rural Africa: impact on the incidence of dog rabies and human dog-bite injuries. Vaccine 21 , 1965–1973 (2003).
Undurraga, E. A. et al. Cost-effectiveness evaluation of a novel integrated bite case management program for the control of human rabies, Haiti 2014–2015. Am. J. Trop. Med. Hyg. 96 , 1307–1317 (2017).
Etheart, M. D. et al. Effect of counselling on health-care-seeking behaviours and rabies vaccination adherence after dog bites in Haiti, 2014–15: a retrospective follow-up survey. Lancet Glob. Heal. 5 , e1017–e1025 (2017).
Laager, M. et al. A metapopulation model of dog rabies transmission in N’Djamena, Chad. J. Theor. Biol. 462 , 408–417 (2019).
Article ADS MathSciNet PubMed MATH Google Scholar
Wallace, R. M. et al. Establishment of a canine rabies burden in Haiti through the implementation of a novel surveillance program. PLoS Negl. Trop. Dis. 9 , e0004245 (2015).
Eggerbauer, E. et al. Evaluation of six commercially available rapid immunochromatographic tests for the diagnosis of rabies in brain material. PLoS Negl. Trop. Dis. 10 , e0004776 (2016).
Brunker, K. et al. Rapid in-country sequencing of whole virus genomes to inform rabies elimination programmes. Wellcome Open Res 5 , 1–30 (2020).
Müller, T. F., Schröder, R., Wysocki, P., Mettenleiter, T. C. & Freuling, C. M. Spatio-temporal use of oral rabies vaccines in fox rabies elimination programmes in Europe. PLoS Negl. Trop. Dis. 9 , e0003953 (2015).
Belotto, A. J. The pan american health organization role in the control of rabies in latin america. Dev. Biol. 119 , 213–216 (2004).
CAS Google Scholar
Bourhy, H. et al. Revealing the micro-scale signature of endemic zoonotic disease transmission in an African urban setting. PLoS Pathog. 12 , e1005525 (2016).
Wallace, R. M., Undurraga, E. A., Blanton, J. D., Cleaton, J. & Franka, R. Elimination of dog-mediated human rabies deaths by 2030: Needs assessment and alternatives for progress based on dog vaccination. Front. Vet. Sci. 4 , 1–14 (2017).
Department of Animal Husbandry & Veterinary services - Government of Goa. 1 4-9-AH/Rabies control/2021-22 Rabies Dis. control State Goa . Off. Gaz. 1 , 193 (2021).
Government of India, Ministry of Home Affairs & Office of the Registrar General & Census Commissioner. Census of India . https://www.census2011.co.in/ (2011).
Institute for Management Research Radbound University. Subnational Human Development Index. Global Data Lab https://globaldatalab.org/shdi/ (2019).
United Nations Development Programme. Human Development Index. Human Development Reports http://hdr.undp.org/en/content/human-development-index-hdi (2019).
Fielding, H. R. et al. Timing of reproduction and association with environmental factors in female free-roaming dogs in southern India. Prev. Vet. Med. 187 , 105249 (2020).
Kuhn, M. caret: Classification and Regression Training. R package. https://cran.r-project.org/web/packages/caret/caret.pdf (2021).
Sing, T., Sander, O., Beerenwinkel, N. & Lengauer, T. ROCR: Visualizing classifier performance in R. Bioinformatics 21 , 3940–3941 (2005).
Deventhiran, J. et al . Continued circulation, recombination and evolution of the ancient subcontinent lineage despite predominance of the recent arctic-like lineage rabies viruses (RABV) in India. bioRxiv (preprint) 398180 (2018) https://doi.org/10.1101/398180 .
Troupin, C. et al. Large-scale phylogenomic analysis reveals the complex evolutionary history of rabies virus in multiple carnivore hosts. PLoS Pathog. 12 , e1006041 (2016).
Kulldorff, M., Athas, W. F., Feuer, E. J., Miller, B. A. & Key, C. R. Evaluating cluster alarms: a space-time scan statistic and brain cancer in Los Alamos, New Mexico. Am. J. Public Health 88 , 1377–1380 (1998).
Arias-Orozco, P. et al. Spatiotemporal analysis of canine rabies in El Salvador: violence and poverty as social factors of canine rabies. PLoS One 13 , e0201305 (2018).
Bouslama, Z., Belkhiria, J. A., Turki, I. & Kharmachi, H. Spatio-temporal evolution of canine rabies in Tunisia, 2011–2016. Prev. Vet. Med. 185 , 105195 (2020).
Kulldorff, M. & Information Management Services ISv. Software for the spatial and space-time scan statistics. https://www.satscan.org/ (2009).
Kleinman, K. rsatscan: Tools, Classes, and Methods for Interfacing with SaTScan Stand-Alone Software. https://rdrr.io/cran/rsatscan/ (2015).
Jeon, S. et al. Determining the post-elimination level of vaccination needed to prevent re-establishment of dog rabies. PLoS Negl. Trop. Dis. 13 , e0007869 (2019).
Download references
Acknowledgements
The Goa rabies control program was funded by the Government of Goa and Dogs Trust Worldwide. MSD Animal Health donated all Nobivac® Rabies vaccines used in the program. Rotary International (Rotary Clubs of Gainesville USA, Mapusa, Panjim, Riveira, Miramar, Panjim Midtown, Gainesville Sunrise, and Downtown Gainesville) donated vehicles used in dog vaccination and post-vaccination surveys. The World Health Organization contributed to Lateral Flow Assays (Bionote). B.M.d.B. was supported by Biotechnology and Biological Sciences Research Council through the Institute Strategic Program funding (BB/ J004235/1 and BB/P013740/1). Phylogenetic analysis was funded in part by the Office of Advanced Molecular Detection and Global Health Security (United States Centers for Disease Control and Prevention). C.M.G. was supported in part by an appointment to the Research Participation Program at CDC, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC. We thank all the staff of Mission Rabies and Worldwide Veterinary Service, volunteers, local government veterinarians, and NGOs who have supported and contributed to the success of the campaign. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the United States government or the Centers for Disease Control and Prevention. Use of trade names, product names, or commercial sources is for identification only and does not imply endorsement by the United States government.
Author information
These authors contributed equally: A. D. Gibson, G. Yale, J. Corfmat, M. Appupillai
These authors jointly supervised this work: V. Naik, S. Desai, S. Mazeri, L. Gamble, R. J Mellanby.
Authors and Affiliations
Mission Rabies, Cranborne, Dorset, United Kingdom
A. D. Gibson, G. Thomas, F. Lohr, S. Mazeri & L. Gamble
The Roslin Institute and The Royal (Dick) School of Veterinary Studies, The University of Edinburgh, Easter Bush Veterinary Centre, Roslin, Midlothian, United Kingdom
A. D. Gibson, I. G. Handel, M. Bronsvoort, S. Mazeri & R. J. Mellanby
Mission Rabies, Tonca, Panjim, Goa, India
G. Yale, J. Corfmat, M. Appupillai, P. Mathapati, N. Otter & P. Ohal
Poxvirus and Rabies Branch, Centers for Disease Control and Prevention, Atlanta, GA, USA
C. M. Gigante, Y. Li & R. M. Wallace
Department of Animal Husbandry & Veterinary Services, Government of Goa, Panaji, India
M. Lopes, N. C. Costa, V. Naik & S. Desai
Directorate of Health Services, Government of Goa, Panaji, India
U. Betodkar & P. M. Suryawanshi
Worldwide Veterinary Service India, Ooty, Tamil Nadu, India
K. A. Fernandes, N. Otter & I. Airikkala-Otter
LYSSA LLC, Atlanta, Georgia, United States
C. E. Rupprecht
Merck Animal Health, Madison, NJ, USA
A. King & I. Deuzeman
MSD Animal Health, Walton Manor, Walton, Milton Keynes, MK7 7AJ, United Kingdom
Department of Neurovirology, WHO Collaborating Centre for Reference and Research in Rabies, National Institute of Mental Health and Neurosciences, Bengaluru, India
WHO Regional Office for South East Asia, New Delhi, India
You can also search for this author in PubMed Google Scholar
Contributions
A.D.G., S.M., L.G., and R.J.M. conceived the study. A.D.G., G.Y., J.C., M.A., and S.M. were responsible for data curation. L.G. led the acquisition of funds for the project, with M.A. responsible for the acquisition of in-country funding. J.C. oversaw management of the rabies mass dog vaccination program, supervised by A.D.G., V.N., and SD. G.Y. oversaw the management of rabies surveillance activities, supervised by A.D.G., V.N., and S.D. M.A. oversaw management of the rabies education program, supervised by GT, V.N., and S.D. A.D.G., G.Y., N.C., P.M., and R.S.M. contributed to rabies diagnostic work. A.D.G., G.Y., J.C., M.A., S.D., U.B., N.C., V.N., K.A.F., M.L., P.M., N.O., G.T., P.O., I.A.O., F.L., and L.G. contributed to program implementation, data collection, and project administration and reporting. U.B. and P.S. were responsible for the collation and reporting of human medical data. A.D.G. performed the formal analysis with supervision from S.M., I.G.H., B.M.B., and R.J.M. S.M. performed the statistical modeling, supervised by I.G.H. and B.M.B. C.G. and G.Y. performed the laboratory sequencing of rabies viruses and CG performed the phylogenetic analysis of molecular genetic data, supervised by Y.L. A.D.G. and R.M.W. performed the cost-effectiveness analysis. A.D.G., G.Y., J.C., M.A., S.D., U.B., C.G., V.N., K.A.F., M.L., P.S., F.L., C.E.R., A.K., D.S., I.D., Y.L., R.M.W., R.S.M., G.G., I.G.H., B.M.B., S.M., L.G., and R.J.M. provided a substantial intellectual contribution. S.D., U.B., V.N., M.L., P.S., N.O., G.T., I.A.O., F.L., C.E.R., A.K., D.S., I.D., and G.G. provided administrative, technical, or supervisory support. A.D.G., CG, S.M., and R.J.M. wrote the original manuscript. G.Y., J.C., M.A., S.D., U.B., C.G., N.C., V.N., K.A.F., M.L., P.S., G.T., F.L., C.E.R., A.K., D.S., I.D., Y.L., R.M.W., R.S.M., G.G., I.G.H., M.D.C., and L.G. contributed to review and editing of the manuscript.
Corresponding author
Correspondence to A. D. Gibson .
Ethics declarations
Competing interests.
D.S., A.K., and I.D. are employees of MSD Animal Health/Merck Animal Health. Other authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Darryn Knobel, Jakob Zinsstag, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, description of additional supplementary files, supplementary data 1, supplementary data 2, supplementary data 3, supplementary software 1, supplementary software 2, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Gibson, A.D., Yale, G., Corfmat, J. et al. Elimination of human rabies in Goa, India through an integrated One Health approach. Nat Commun 13 , 2788 (2022). https://doi.org/10.1038/s41467-022-30371-y
Download citation
Received : 18 July 2021
Accepted : 27 April 2022
Published : 19 May 2022
DOI : https://doi.org/10.1038/s41467-022-30371-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Rabies in a postpandemic world: resilient reservoirs, redoubtable riposte, recurrent roadblocks, and resolute recidivism.
- Charles E. Rupprecht
- Philip P. Mshelbwala
- Ivan V. Kuzmin
Animal Diseases (2023)
Integrating full and partial genome sequences to decipher the global spread of canine rabies virus
- Andrew Holtz
- Anna Zhukova
Nature Communications (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Sign up for News & Events
- Join Our Campaigns
- Become a Member
- A Significant Public Health Problem
- A Neglected Tropical Disease
- The Case for Investment
- The Rabies Roadmap
- Events and Courses
News and Case Studies
- Become a United Against Rabies Forum Member
- Frequently Asked Questions

Case Study: Goa
Working together to achieve ‘rabies-controlled’ status.

Case Study: Namibia
Fighting rabies with a multisectoral One Health approach.

Case Study: Mexico
The Health Ministry is responsible for canine rabies control
Select category
United against rabies forum steering group announced, creation date: 4 march 2024, produced by: united against rabies.
We are delighted to share the members of the United Against Rabies Forum Steering Group, designated for the next two years (2024 – 2025).

WHO poster supports confident PEP administration
Creation date: 5 march 2024.
The World Health Organization has published a new visual tool for health workers to use in order to make informed decisions about administration of Rabies PEP (Post Exposure Prophylaxis). Set out as a decision-making tree, the poster is meant to be a quick reference guide, and can be downloaded and printed from the WHO website.
Tags: One Health , PEP Post-Exposure Prophylaxis
United Against Rabies Photo Competition
Creation date: 6 november 2023.
We invite you to participate in the “Rabies: All for 1, One Health for all” United Against Rabies Photo Competition.
Tags: Advocacy , United Against Rabies Forum , World Rabies Day
Rabies Today Podcast
Creation date: 3 november 2023.
Tags: Podcast , United Against Rabies Forum
Cambodia puts One Health into practice to tackle rabies
Creation date: 21 july 2023, united against rabies thanks gavi for decision to roll out support for human rabies vaccines, creation date: 12 july 2023.
Tags: One Health , PEP Post-Exposure Prophylaxis , United Against Rabies Forum
One Health for Dog-mediated Rabies Elimination in Asia
Creation date: 29 june 2023, the lancet: a call to accelerate an end to human rabies deaths, creation date: 30 january 2023, rabies experts urge gavi to implement pep investment, rabies prevention and control – lessons from chongqing, china, creation date: 20 october 2022.
Tags: Dog Vaccination Campaign , Education
- Open access
- Published: 05 July 2017
Bites from the same dog, different outcomes for two patients: a case report
- Xue-Yong Huang 1 , 2 , 3 ,
- Xing-Le Li 1 , 2 ,
- Shu-Yu Wu 4 , 5 ,
- Yu-Lei Gu 6 ,
- Xin-Jun Lv 7 ,
- John David Klena 4 , 5 &
- Bian-Li Xu ORCID: orcid.org/0000-0003-4966-3457 1 , 2
Infectious Diseases of Poverty volume 6 , Article number: 107 ( 2017 ) Cite this article
6965 Accesses
2 Citations
1 Altmetric
Metrics details
Rabies is a serious reemerging zoonosis in China. At present human rabies cases are primarily diagnosed based on clinical presentation.
Case presentation
In August 2012, a woman and her son were attacked by a stray dog in Henan, China. The son received rabies postexposure prophylaxis (wound treatment followed by vaccine, no immunoglobulin), however, the mother did not. Rabies infection was subsequently laboratory confirmed in the mother and she died in December; her son is alive and healthy after 2 years of follow-up.
This report documents that the timely utilization of postexposure prophylaxis is a required measure in preventing rabies after exposure to an animal bite.
Multilingual abstract
Please see Additional file 1 for translations of the abstract into the five official working languages of United Nations.
Rabies is a zoonotic infectious disease caused by lyssaviruses. The rabies virus (RV) is the most important lyssavirus and is widely distributed across the globe, with a human mortality rate of almost 100% after the onset of symptoms [ 1 ]. China reports the second highest number of human rabies deaths, only after India [ 2 ]. Although the number of cases of human rabies has been decreasing since 2007, the disease remains an important public health threat in mainland China [ 3 ]. Dogs are the main source of infection and are the primary vector for human rabies in rural China [ 4 ].
The purpose of this report is to present the different outcomes in two patients (a mother and a son) bitten by the same dog. The mother did not receive rabies postexposure prophylaxis (PEP) and died of rabies, while her son had no illness or symptoms after receiving the rabies PEP. The report also summarizes how to systematically diagnose a suspected rabies case and a laboratory confirmed rabies case, as well as how to carry out effective prevention and control measures for the disease.
On 24 November 2012, a 31-year-old woman was diagnosed as a suspected rabies case by the First Affiliated Hospital of Zhengzhou University, which reported the case to the Henan Center for Disease Control and Prevention (CDC). A detailed epidemiological investigation of the suspected rabies case was performed.
The woman was a farmer living in a rural area of Xuchang, Henan Province, China. She lived with her husband and two sons, and said she had not travelled anywhere recently. At about 17:00 on 25 August 2012, the woman and her 7-year-old son were attacked by a stray dog while walking nearby to their village; the woman sustained bites on her right thigh while her son had bites on his left calf. Villagers caught and killed the dog, and it was buried on the village outskirts without laboratory investigation for RV. Believing the wound would result in a severe disability for her child, the mother washed the son’s bites with municipal tap water (without soap) shortly after the incident. On 26 August, she took her son to the healthcare unit in their village, where the boy received a rabies vaccine and completed a full course of standard vaccines (freeze-dried rabies vaccine for human use [Vero cells], Liaoning Chengda Biotechnology Co., Ltd. Dalian City, Liaoning Province, China; a five-dose vaccination regimen on days 0, 3, 7, 14, and 28). In contrast, the mother did not recognize the risk posed to her by the dog bite, and only washed her own wounds the following day, following the doctor’s advice. However, she declined the rabies vaccine for economic reasons. Both the mother’s and son’s wounds were determined by the doctor to constitute category III exposure bites (single or multiple transdermal bites or scratches; contamination of mucous membrane with saliva, i.e. licks), according to the classification criteria of the World Health Organization (WHO) [ 1 ]. However, neither the mother nor her son were treated as recommended by the WHO for category III rabies exposure, which requires wound cleaning, rabies vaccination, and direct wound infiltration with rabies immunoglobulin (RIG) [ 1 ]. The rabies vaccine is not in the Chinese National Immunization Scheme, so rabies vaccine and RIG are currently provided for a fee in China.
On 20 November 2012, the woman presented with a persistent fever (39 °C), nausea, vomiting, chest tightness, and agitation to the Xiangcheng County People’s Hospital. She received a diagnosis of encephalitis and was treated for 2 days with cefoperazone, sulbactam, levofloxacin, and supportive treatment, including oxygen therapy, intravenous rehydration, and maintenance of adequate electrolyte balance. On 22 November, her symptoms worsened and she developed a mild coma, drooling and melena; she was then transferred to the Xuchang City Central Hospital. On 24 November, she was transferred to the First Affiliated Hospital of Zhengzhou University, where she received a diagnosis of suspected rabies and was treated with symptomatic and supportive therapies. Her condition continued to deteriorate and she died on 6 December 2012.
On 4 December 2012, saliva, serum, and cerebrospinal fluid (CSF) from the patient were collected. On 15 December 2012, a serum sample was collected from her son, who was in good health when sampled. Both sets of specimens were transported under refrigeration to the Henan CDC for testing.
Total ribonucleic acid (RNA) was extracted from the CSF and saliva, and reverse transcribed to cDNA. RV N and G genes were amplified using nested polymerase chain reaction (PCR), and negative controls (RNAse-free water) and positive controls (positive CSF specimens were preserved in our laboratory) were included in each set of reactions [ 5 ]. Amplification products were detected after electrophoresis using 2% agarose gel. The N and G genes were amplified from the woman patient’s saliva, but not from her CSF (see Figure 1 ). Amplification products were purified and sequenced using an automated ABI 3730 DNA Sequencer (Applied Biosystems™, Foster City, CA, USA) from Sangon Biotech Co., Ltd. (Shanghai, China). Molecular phylogenetic analysis was conducted using the maximum likelihood method based on the Kimura’s two parameter model with MEGA 5 software (available at: http://mega.software.informer.com/5.0/ ) [ 6 ]. Nucleotide sequence from the female (Henan) patient (Henan JSS, GenBank accession number KP221203) G protein was compared against nucleotide sequences of G protein genes from RV identified in GenBank (Table 1 ). Henan JSS, along with previous Henan RV strains, the Chinese vaccine strain, and 8743THA (representing strains of RV genotype one) were grouped into GT1 (see Figure 2 ).
Amplification products of the N and G genes of RV. Lane M, DL2000 DNA Marker; lane 1, N gene amplification product using RNA obtained from patient’s CSF; lane 2, N gene amplification product using RNA obtained from patient’s saliva; lane 3, positive control; lane 4, negative control; lane 5, G gene amplification product using RNA obtained from patient’s CSF; lane 6, G protein gene amplification product using RNA obtained from patient’s saliva; lane 7, positive control; lane 8, negative control
Phylogenetic analysis based on the G gene nucleotide sequences of RV from Henan. With the exception of Henan JSS, the G gene sequences were collected from GenBank. The black spot indicates Henan JSS, the G gene that was amplified by PCR and subsequently sequenced in the present study
The rabies virus neutralizing antibody (RVNA) titers in the sera were assayed by a standard rapid fluorescent focus inhibition test with some modifications [ 7 ]. A serum specimen from the patient was collected on day 12 of her illness; her son’s serum was collected 3 months after the bites. Serum RVNA titers of the mother and her son were 0.68 IU/ml and 2.29 IU/ml, respectively. The mother and son were considered positive according to the diagnosis criteria for RVNA reactions (RVNA titers ≥0.05 IU/ml, the WHO recommended protective level) [ 8 ].
Because the son did not receive immediate RIG treatment, he remained at possible risk for RV infection [ 9 , 10 ]. RVNA of the son has been actively monitored; his health condition has been assessed every 6 months post his initial result. Fortunately, the son is alive and healthy after 2 years of follow-up.
Rabies is a fatal disease, yet it is preventable using proven, effective measures including immediate wound washing with soap and water or other detergents that can kill the virus, vaccine in cases of category III exposures, and wound infiltration with RIG [ 11 , 12 , 13 ].
This RV case highlights several important issues in the recognition and treatment of rabies. Prior to transfer to the First Affiliated Hospital of Zhengzhou University, the female patient was hospitalized in a local county and then a municipal hospital, where a clinical diagnosis could not be made with certainty. In these settings, medical personnel have limited experience with rabies recognition and diagnosis.
Secondly, the patient had almost no typical clinical manifestations of rabies, making it more difficult to diagnose her illness. After transfer to the First Affiliated Hospital of Zhengzhou University, the patient was suspected to have a RV infection based on the history of being attacked by a dog and after ruling out other probable causes of craniocerebral injury. Epidemiological history plays an important role in the clinical diagnosis of rabies [ 14 , 15 , 16 ]. However, the first two hospitals did not ask about the dog bite history of the patient.
The case reported here highlights the challenge of diagnosing and treating rabies patients in rural areas of China. Public health agencies should increase public awareness around the risk associated with dog bites and improve the application and availability of high-quality anti-rabies vaccines and RIG, in order to prevent rabies infection in China. Controlling rabies through pet vaccination schemes, particularly for dogs, is also an important strategy for reducing the rate of human exposure to rabies.
This report documents the outcomes of bites from the same dog based on different treatment of two cases. Rabies is preventable using effective measures including immediate wound washing, vaccine therapy, and wound infiltration with RIG.
Abbreviations
Center for Disease Control and Prevention
Cerebrospinal fluid
Polymerase chain reaction
Postexposure prophylaxis
Rabies immunoglobulin
Ribonucleic acid
Rabies virus
Rabies virus neutralizing antibody
World Health Organization
Quiambao BP, Dytioco HZ, Dizon RM, Crisostomo ME, Laot TM, Teuwen DE. Rabies post-exposure prophylaxis in the Philippines: health status of patients having received purified equine F(ab')(2) fragment rabies immunoglobulin (Favirab). PLoS Negl Trop Dis. 2008;2(5):e243.
Article PubMed PubMed Central Google Scholar
Yin W, Dong J, Tu C, Edwards J, Guo F, Zhou H, et al. Challenges and needs for China to eliminate rabies. Infect Dis Poverty. 2013;2(1):23.
Zhu WY, Liang GD. Current status of canine rabies in China. Biomed Environ Sci. 2012;25(5):602–5.
PubMed Google Scholar
Rudd RJ, Appler KA, Wong SJ. Presence of cross-reactions with other viral encephalitides in the indirect fluorescent-antibody test for diagnosis of rabies. J Clin Microbiol. 2013;51(12):4079–82.
Dacheux L, Wacharapluesadee S, Hemachudha T, Meslin FX, Buchy P, Reynes JM, et al. More accurate insight into the incidence of human rabies in developing countries through validated laboratory techniques. PLoS Negl Trop Dis. 2010;4(11):e765.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–9.
Article CAS PubMed PubMed Central Google Scholar
Madhusudana SN, Malavalli BV, Thankappan UP, Sundramoorthy S, Belludi AY, Pulagumbaly SB, et al. Development and evaluation of a new immunohistochemistry-based test for the detection of rabies virus neutralizing antibodies. Hum Vaccin Immunother. 2014;10(5):1359–65.
Fang Y, Chen L, Liu MQ, Zhu ZG, Zhu ZR, Hu Q. Comparison of safety and immunogenicity of PVRV and PCECV immunized in patients with WHO category II animal exposure: a study based on different age groups. PLoS Negl Trop Dis. 2014;8(12):e3412.
Si H, Guo ZM, Hao YT, Liu YG, Zhang DM, Rao SQ, et al. Rabies trend in China (1990-2007) and post-exposure prophylaxis in the Guangdong province. BMC Infect Dis. 2008;8:113.
Gowda VK, Basavaraja GV, Reddy H, Ramaswamy P. Paralytic rabies following cat scratch and intra-dermal anti-rabies vaccination. J Pediatr Neurosci. 2014;9(2):154–5.
Shantavasinkul P, Wilde H. Postexposure prophylaxis for rabies in resource-limited/poor countries. Adv Virus Res. 2011;79:291–307.
Article CAS PubMed Google Scholar
Abubakar SA, Bakari AG. Incidence of dog bite injuries and clinical rabies in a tertiary health care institution: a 10-year retrospective study. Ann Afr Med. 2012;11(2):108–11.
Stahl JP, Gautret P, Ribadeau-Dumas F, Strady C, Le Moal G, Souala F, et al. Update on human rabies in a dog- and fox-rabies-free country. Med Mal Infect. 2014;44(7):292–301.
Article PubMed Google Scholar
Susilawathi NM, Darwinata AE, Dwija IB, Budayanti NS, Wirasandhi GA, Subrata K, et al. Epidemiological and clinical features of human rabies cases in Bali 2008-2010. BMC Infect Dis. 2012;12:81.
Yu J, Li H, Tang Q, Rayner S, Han N, Guo Z, et al. The spatial and temporal dynamics of rabies in China. PLoS Negl Trop Dis. 2012;6(5):e1640.
Udow SJ, Marrie RA, Jackson AC. Clinical features of dog- and bat-acquired rabies in humans. Clin Infect Dis. 2013;57(5):689–96.
Download references
Acknowledgements
Not applicable.
This work was sponsored by the Science and Technology Bureau of Henan Province (152,102,310,133, 164,100,510,008) and the National Natural Science Foundation of China (81573204).
Availability of data and materials
Authors’ contributions.
X-YH and B-LX contributed to the study design and drafting of the paper. Y-LG contributed to the data collection and analysis. X-LL and X-JL contributed to the laboratory testing. S-YW and JDK made critical revision of the paper for important intellectual content. All authors read and approved the final paper for publication.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patients’ families (the legal guardians of the patient and her boy) for publication of this case. A copy of the written consent is available for review by the editor of this journal.
Ethics approval and consent to participate
This research was approved by the Ethical Committee of the Henan CDC, and the committee’s reference number is 2016-KY-003-03. The patients’ families gave written informed consent for the use of the patients’ clinical samples.
Disclaimers of the US CDCs
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US CDCs.
Author information
Authors and affiliations.
Henan Center for Disease Control and Prevention, Zhengzhou, China
Xue-Yong Huang, Xing-Le Li & Bian-Li Xu
Henan Key Laboratory of Pathogenic Microorganisms, Zhengzhou, China
Henan Collaborative Innovation Center of Molecular Diagnosis and Laboratory Medicine, Xinxiang, China
Xue-Yong Huang
International Emerging Infections Program, United States Centers for Disease Control and Prevention, Beijing, China
Shu-Yu Wu & John David Klena
Global Disease Detection Branch, Division of Global Health Protection, Center for Global Health, United States Centers for Disease Control and Prevention, Atlanta, GA, USA
The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Chinese Center for Disease Control and Prevention, Beijing, China
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Bian-Li Xu .
Additional file
Additional file 1:.
Multilingual abstract in the five official working languages of the United Nations. (PDF 673 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Huang, XY., Li, XL., Wu, SY. et al. Bites from the same dog, different outcomes for two patients: a case report. Infect Dis Poverty 6 , 107 (2017). https://doi.org/10.1186/s40249-017-0321-3
Download citation
Received : 14 December 2016
Accepted : 31 May 2017
Published : 05 July 2017
DOI : https://doi.org/10.1186/s40249-017-0321-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Encephalitis
- Neglected diseases
- Rabies atypical manifestations
- Rabies laboratory diagnosis
- Rabies postexposure prophylaxis
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Loading metrics
Open Access
Peer-reviewed
Research Article
A One Health Framework for the Evaluation of Rabies Control Programmes: A Case Study from Colombo City, Sri Lanka
* E-mail: [email protected]
Affiliations Veterinary Epidemiology Economics and Public Health Group, Royal Veterinary College, North Mymms, Hatfield, United Kingdom, Leverhulme Centre for Integrative Research on Agriculture and Health, Royal Veterinary College, North Mymms, Hatfield, United Kingdom
Affiliation Conservation Research Ltd, Great Shelford, Cambridge, United Kingdom
Affiliation Veterinary Epidemiology Economics and Public Health Group, Royal Veterinary College, North Mymms, Hatfield, United Kingdom
Affiliation Blue Paw Trust, Colombo, Sri Lanka
- Barbara Häsler,
- Elly Hiby,
- Will Gilbert,
- Nalinika Obeyesekere,
- Houda Bennani,
- Jonathan Rushton

- Published: October 23, 2014
- https://doi.org/10.1371/journal.pntd.0003270
- Reader Comments
One Health addresses complex challenges to promote the health of all species and the environment by integrating relevant sciences at systems level. Its application to zoonotic diseases is recommended, but few coherent frameworks exist that combine approaches from multiple disciplines. Rabies requires an interdisciplinary approach for effective and efficient management.
Methodology/Principal Findings
A framework is proposed to assess the value of rabies interventions holistically. The economic assessment compares additional monetary and non-monetary costs and benefits of an intervention taking into account epidemiological, animal welfare, societal impact and cost data. It is complemented by an ethical assessment. The framework is applied to Colombo City, Sri Lanka, where modified dog rabies intervention measures were implemented in 2007. The two options included for analysis were the control measures in place until 2006 (“baseline scenario”) and the new comprehensive intervention measures (“intervention”) for a four-year duration. Differences in control cost; monetary human health costs after exposure; Disability-Adjusted Life Years (DALYs) lost due to human rabies deaths and the psychological burden following a bite; negative impact on animal welfare; epidemiological indicators; social acceptance of dogs; and ethical considerations were estimated using a mixed method approach including primary and secondary data. Over the four years analysed, the intervention cost US $1.03 million more than the baseline scenario in 2011 prices (adjusted for inflation) and caused a reduction in dog rabies cases; 738 DALYs averted; an increase in acceptability among non-dog owners; a perception of positive changes in society including a decrease in the number of roaming dogs; and a net reduction in the impact on animal welfare from intermediate-high to low-intermediate.
Conclusions
The findings illustrate the multiple outcomes relevant to stakeholders and allow greater understanding of the value of the implemented rabies control measures, thereby providing a solid foundation for informed decision-making and sustainable control.
Author Summary
Successful rabies control generates benefits in terms of improved human and animal health and well-being and safer environments. A key requirement of successful and sustainable rabies control is empowering policy makers to make decisions in an efficient manner; essential to this is the availability of evidence supporting the design and implementation of the most cost-effective strategies. Because there are many, at times differing, stakeholder interests and priorities in the control of zoonotic diseases, it is important to assess intervention strategies in a holistic way. This paper describes how different methods and data from multiple disciplines can be integrated in a One Health framework to provide decision-makers with relevant information, and applies it to a case study of rabies control in Colombo City, Sri Lanka. In Colombo City, a new comprehensive intervention was initiated in 2007 based on vaccination, sterilisation, education, and dog managed zones. Results showed that for the four year time period considered, the new measures overall cost approximately US $ 1 million more than the previous programme, but achieved a reduction in dog rabies cases and human distress due to dog bites, reduced animal suffering and stimulated a perception of positive changes in society. All these achievements have a value that can be compared against the monetary cost of the programme to judge its overall worth.
Citation: Häsler B, Hiby E, Gilbert W, Obeyesekere N, Bennani H, Rushton J (2014) A One Health Framework for the Evaluation of Rabies Control Programmes: A Case Study from Colombo City, Sri Lanka. PLoS Negl Trop Dis 8(10): e3270. https://doi.org/10.1371/journal.pntd.0003270
Editor: Joseph Raymond Zunt, University of Washington, United States of America
Received: March 21, 2014; Accepted: September 14, 2014; Published: October 23, 2014
Copyright: © 2014 Häesler et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding: This study received financial support from the World Society for the Protection of Animals (WSPA). BH acknowledges financial support from the Leverhulme Centre for Integrative Research on Agriculture and Health (LCIRAH). A former staff member of WSPA (EH) provided information and data on the rabies situation and the ongoing rabies control programme in Colombo City which formed the basis for the development of the study design. LCIRAH had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The One Health paradigm aims to effectively manage complex risks affecting human, animal, and environmental health by forging new interdisciplinary partnerships and collaborations. Rabies, an acute progressive encephalomyelitis with almost 100% case fatality rate caused by viruses in the genus Lyssavirus , is a zoonotic disease that is responsible for an estimated 55,000 human deaths, tens of millions of human exposures, and substantial animal losses annually [1] . It requires a generalised approach if it is to be managed effectively and efficiently [2] .
While One Health thinking has come into vogue, systematic integration of various disciplines such as biological, environmental, social, and health sciences to manage health more holistically is often complicated by interdisciplinary and intersectoral barriers to effective collaboration [3] . One major challenge is the paradigm debate caused by the philosophical assumptions that guide the collection and analysis of quantitative (post-positivist) and qualitative (constructivist) data which may be viewed differently by disciplines. It has been suggested that using both approaches in the same study provides, in combination, a superior understanding of research problems than either approach alone [4] . Another important barrier is the current institutional architecture in which public funds are allocated to specific ministries thereby hindering development of joint public health programmes, which in the case of zoonotic diseases can result in a fragmented approach to control.
The most important vector for maintenance of rabies virus and transmission to humans is the domestic dog, with over 90% of human cases attributable to dog bites. The tools to eliminate rabies from animal populations exist, yet relatively few countries are currently rabies-free placing a major strain on public health budgets. Nearly all human rabies deaths occur in developing countries because they are lacking the resources and capacity to provide both adequate pre-exposure prophylaxis and post-exposure prophylaxis (PEP) in humans and effective management of the virus in animal populations. The World Health Organisation estimates that the annual cost of rabies may be in excess of US $6 billion per year including an estimated US $1.6 billion for PEP [5] . Where rabies control has been successful, efforts have been based on quarantine in an advantageous geographical location (e.g. United Kingdom) or the systematic mass vaccination of domestic and wild host populations (e.g. mainland Europe). In the long term, controlling rabies in the dog population through mass dog vaccination has been shown to be more cost-effective than human PEP alone [6] . The World Health Organisation, the World Organisation for Animal Health, and the Food and Agriculture Organisation of the United Nations acknowledge the need for intersectoral collaboration to manage rabies [5] . However, the systematic control of rabies in animal populations requires financial resources, and the technical capacity to plan, implement and evaluate the vaccination campaign; aspects that are often lacking in affected countries.
Sustaining control demands political, societal and financial backing to maintain the campaign as well as the logistic and human resource capacity to deliver vaccine, and knowledge of, and access to, target populations. On-going collection of data through surveillance systems to monitor and evaluate the economic and technical efficiency of campaigns is necessary to ensure objectives are being achieved, and surveillance must be continuous following eradication to detect re-emergence of the virus promptly. Many of these components need the active support of the public in affected areas. In many countries where rabies is endemic these requisite criteria are not met, and interventions against other diseases are given a higher priority. As a result rabies is considered a neglected disease.
Modern science tends to abstract phenomena and reduce reality into smaller portions that can be easily understood and, as much as possible, be expressed in mathematical terms. While these mathematical abstractions are critical in modelling the dynamics of disease in a population and to assess the effectiveness of interventions, they do not provide an understanding of the support for rabies control measures in society nor do they shed any light on wider-reaching issues such as ethical concerns or animal welfare, in short, they oversimplify reality. For example, anecdotal evidence suggests that some people are not supportive of rabies control measures such as dog culling and actually jeopardise the process by hiding or moving their dogs. Thus, both reductionist in-depth studies, as well as collaboration with other disciplines are needed to understand and plan sustainable and publicly acceptable control programmes.
Many projects have focused on individual components of rabies impact, for example the use of pre-exposure prophylaxis and PEP in humans [7] – [10] , the effectiveness of different strategies for dog vaccination [11] , [12] , willingness-to-pay for dog vaccination [13] and the indirect costs of rabies exposure [14] . However, they have all been assessed independently. Assessed in conjunction, they provide important insights into the positive and negative consequences of rabies management and build a robust basis for informed decision-making.
This paper proposes a generic framework for the assessment of rabies interventions encompassing a wide range of positive and negative consequences and local conditions in order to assess economic efficiency and illustrates its use by applying it to the rabies control programme in Colombo City, Sri Lanka.
The framework
The heart of the framework is the economic assessment that compares the additional costs and benefits of an intervention in monetary and non-monetary terms taking into account epidemiological, animal welfare, societal impact and cost data ( Figure 1 ). The economic assessment is complemented by an ethical assessment that provides an additional perspective. These components are connected as described below, establishing a framework for the assessment of rabies control. While the underlying principles and concepts are generic, the focus and the resulting data needs are presented here for rabies.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pntd.0003270.g001
Economic assessment.
All rational decision-making involves an evaluation of relevant pros and cons; the logic of assessing the positive and negative consequences of a decision is unarguable and intuitively appealing [15] . Any investment in rabies control can be considered worthwhile if the additional outcome outweighs the additional costs. Two popular formalised techniques for decision-making based on the fundamental economic principle of marginality are cost-benefit analysis (CBA), where positive and negative aspects of a decision are expressed in monetary terms; and cost-effectiveness analysis (CEA), where the outcomes are expressed in terms of monetary costs per unit of effect (e.g. cost per life year gained) [16] .
The societal impact of rabies expressed in monetary terms includes PEP and treatment costs for humans and animals following exposure (e.g. wound treatment, application of immunoglobulin and vaccines), production losses (e.g. mortality of livestock or companion animals), expenditures for surveillance in animals and humans (e.g. recording of the number of dog bites or dog rabies cases), expenses for intervention measures (e.g. mass vaccination campaigns in dogs, educational programmes to avoid exposure), epidemiological investigations (e.g. disease outbreak investigation), and indirect loss of income due to absence from work (e.g. caring for diseased family members).
Expressing effectiveness in non-monetary terms is particularly appealing for disease control objectives where outcomes have a value to society, but are difficult to measure in money units. The interpretation or value of the effectiveness measure depends on the importance, worth, or usefulness society attaches to something, reflecting peoples' judgement of what is relevant in life. Consequently, decision thresholds related to such effectiveness measures may vary according to the evaluation context [17] . Such measures include human rabies deaths and psychological distress due to fear, anxiety or other feelings (commonly expressed in disability-adjusted-life-years - DALYs), and animal welfare.
The value of animal welfare is a “reflection of a natural human reaction, the satisfaction, assurance and comfort derived from the knowledge that a sentient being is being treated in an appropriate manner” and is based on ethical or cultural values, individual preferences or sensitivities [18] . If animal disease causes a sense of discomfort and unease in people by, for example, evoking fear of rabies infection or disgust because of animals in the population being ill, this is expressive of a disutility or loss of benefit, which affects peoples' quality of life or happiness. Implementation of a disease control programme in animals that improves the environmental and social wellness of people causes positive externalities which can be assessed by using happiness or quality of life metrics available, such as self-perceived quality of life or gross national happiness [19] , [20] , or by defining a suitable effectiveness measure that allows quantifying the positive externality taking into account for example lifestyle stress, living environment, or life-satisfaction.
Epidemiological assessment.
Veterinary epidemiology describes the “frequency of disease occurrence and how disease, productivity, welfare and well-being are affected by the interaction of different factors or determinants” [21] . These determinants can then be manipulated to reduce the frequency of disease occurrence by creating effective risk mitigation programmes to improve the health of populations. Essentially, in epidemiological analysis, data are gathered which are then analysed using qualitative or quantitative approaches or hypotheses. Epidemiological studies therefore provide information about the technical efficiency of disease control measures, a pre-requisite for any economic analysis of animal disease control. For ex post analyses, empirical data may be collected on the technical impact control activities had on disease in the population (e.g. changes in prevalence or incidence), while epidemiological models provide critical inputs for ex ante economic assessments by predicting patterns of disease occurrence and studying the effect of mitigation strategies on the disease dynamics in a population.
Animal welfare assessment.
Animal welfare science identifies the various factors that affect the welfare state of the animal (e.g. nutrition, health, pain and discomfort, anxiety or frustration, vitality, behavioural freedom) with the inference that improvement in any of these variables leads to better welfare. The methods used for animal welfare assessment can be broadly divided into two groups depending on the parameters they take into account, namely animal-based and environment-based assessments [22] . The first group assesses a change in physiological and behaviour responses indicative of a change in animal welfare through direct behavioural observations (e.g. flight distance, lethargy, vocalization) and stress measurements (e.g. glucocorticoid, heart rate, opioids) that reflect the underlying physical and psychological states of the animals. The second group includes indirect methods that focus on the environmental aspects thought to be relevant to animal welfare, such as space allowance, or social contact [23] , and is less demanding in terms of ease of recording, necessary experience and time.
There is no single, reliable measure of an animal's welfare [24] . The best indicators of an animal's welfare depend on the species of animal involved, and the context in which it is being assessed. From the animal's viewpoint, a reaction to a control measure such as poisoning is independent of the context, but the selection of animal welfare measures for an economic analysis needs to reflect the context and value system of the society in question. Positive and negative consequences of a programme on animal welfare can, for example, take into account parameters on health (unhealthy animals may experience pain or discomfort), productivity (potentially valuable for measuring progress in animal welfare in environments that systematically monitor animal welfare, such as laboratories), behaviour (provides an immediate reflection of the animal's emotional state) and physiology (quantitative approach useful for before-and-after assessments).
Social assessment.
With respect to impact, animal disease and its control produces externalities; for example emotional distress experienced when performing or witnessing the culling of animals, frustration, anger, feelings of loss of control, fear and uncertainty, and the loss of social (support) structures due to movement bans as experienced by the farming community during the foot-and-mouth disease outbreak in the United Kingdom in 2001 [25] . If disease control leads to an improved quality of life, the use of one of the many approaches available to measure this change may be indicated, which evolve around three principal concepts: 1) the availability of resources and commodities, 2) the notion of subjective well-being, and 3) the fulfilment of individual capabilities [26] .
The second principal aspect of a social assessment in relation to disease control revolves around peoples' attitudes, judgments, beliefs and behaviour related to disease control. Social-cognitive models, such as the theory of planned behaviour have been widely applied within the health and disease control fields [27] – [29] . These theories devise a model linking people's attitudes to intent to perform particular behaviours. They have been proven effective in predicting and explaining behaviours and are considered useful tools in disease management [28] , [29] . A social assessment, including a survey of attitudes toward disease control, provides a degree of insight into how people are likely to respond to control measures. Public support or antipathy for disease control may drastically influence the effectiveness of intervention programmes.
Ethical assessment.
Five standard ethical approaches are recommended to be used to assess the ethical dimension of rabies and its control: 1) the common good approach argues that relationships in society are the basis of ethical reasoning and calls attention to the welfare of everyone (hence, options which best serve the community as whole and not just some members are preferable); 2) the utilitarian approach emphasizes that the ethical action is the one that produces the greatest balance of good over harm; 3) the rights approach assesses which option best respects the rights of all who have a stake; 4) the fairness approach assesses which option treats individuals equally or proportionately; and 5) the virtue approach assesses which option allows people to act as the sort of person they want to be.
Application of the framework to a case study in Colombo City, Sri Lanka
In Colombo City, canine rabies has been endemic for several decades. The national anti-rabies strategy aims to protect people who are exposed and those at risk of contracting the disease, establish dog population immunity and to control the dog population. A well regulated system of PEP is in place, limiting the average number of human rabies cases between 1995 and 2011 to 0.65 per year in a city of 650,000 (unpublished data, Veterinary Department of Colombo Municipal Council). The Veterinary Department of Colombo Municipal Council used to combat rabies through culling of roaming dogs via carbon monoxide and carbon dioxide poisoning in a gas chamber and vaccination of owned dogs, but canine rabies cases continued to persist in the city. From 2007 to 2012, following cessation of culling by Presidential decree in 2006, a modified comprehensive intervention to control rabies was implemented, which included mass vaccination of dogs, targeted sterilisation of both owned and unowned dogs, education of children and adults in bite prevention and rabies awareness, and development of dog managed zones in public areas. The stakeholders involved in the intervention hypothesised that the new measures would lead to a decrease in the number of dog rabies cases, an associated reduction in the administration of PEP to people, an increased acceptance of dogs in society, and overall a positive net value of the intervention in Colombo City. The aim of this case study was to assess the economic value of the intervention explicitly taking into account monetary and non-monetary consequences resulting from the change in rabies prevalence, animal welfare and social acceptance.
Study site and data collection.
The case study focused on Colombo City, which is composed of 47 wards or sub-districts. An ex post assessment was conducted for a four year duration of implementation of the intervention from its start in June 2007 up to June 2011. To inform the economic assessment, primary and secondary data were collected and collated between May and September 2011 taking into account the components described in the framework outlined above.
Ethics statement.
For the primary data collection, namely the focus group discussions for the social acceptance assessment, ethical approval was received from the Royal Veterinary College's Ethics and Welfare Committee (approval number URN 2014 0108H-R). Focus groups participants were informed about the purpose and procedures of the study. Oral informed consent was obtained and recorded, as not all participants were literate. The use of oral consent was approved by the Royal Veterinary College's Ethics and Welfare Committee. Participation was completely voluntary and participants could withdraw from the focus group discussion at any time. All results were coded and treated confidentially.
General overview, software, and sensitivity analysis.
The study comprised four main steps, namely 1) identification of intervention and baseline options to be assessed; 2) identification of their monetary and non-monetary costs and benefits including epidemiological, social and animal welfare consequences; 3) measurement and valuation of the monetary and non-monetary costs and benefits; and 4) comparison of costs and benefits of the options identified. The assessment was complemented by a discussion on ethical considerations.
An overview of the intervention activities and relevant data were obtained by reading reports, articles and guidelines referring to the intervention and by consulting staff members involved in the planning and implementation. The two options included for analysis were the rabies control activities in place from 2002 to 2006 (“the baseline scenario”) and the new intervention with the activities summarised in Table 1 .
https://doi.org/10.1371/journal.pntd.0003270.t001
The following effects were estimated both for the intervention and the baseline scenario: 1) Monetary expenditures (in 2011 US $) for the implementation of the rabies control activities in the human and dog populations; 2) DALYs lost due to human rabies deaths and psychological distress following a bite from a suspect rabid dog; 3) Impact of the rabies control activities in the dog population on animal welfare expressed in animal welfare scores; and 4) People's acceptance of dogs in society expressed in acceptance scores and qualitative descriptions. Next, the net values were estimated by calculating the difference of these effects between the baseline scenario and the intervention (described in detail in subsequent sections). Livestock losses due to rabies in Colombo City were not reported and therefore not considered in the analysis.
The economic assessment.
https://doi.org/10.1371/journal.pntd.0003270.t002
The average DALYs lost per human rabies death for Asia were calculated based on published estimates from Knobel et al. [1] by dividing the estimated 994,607 DALYs lost due to human rabies death in Asia (composite score of the years of life lost due to premature mortality and the years of life lived with a disability) by the estimated 31,539 human rabies deaths in Asia. This resulted in a loss of 27.99 DALYs per human rabies death. The total DALYs lost due to human rabies deaths for the baseline scenario and the intervention, respectively, were calculated by multiplying the 27.99 DALYs lost per human rabies death by the number of recorded human rabies deaths in Colombo City (see “epidemiological input parameters” below).
Human wellbeing was expected to be affected by the psychological burden of fear and trauma induced by bites from dogs that may be rabies infected. To estimate the DALYs lost per dog bite, data from the literature concerning the psychological burden of rabies and the number of dog bites in Asia was used. The psychological burden of rabies of 139,893 DALYs lost each year in Asia derived from the World Health Organisations's expert consultation on rabies [30] were divided by 3,529,300, the estimated number of bites from suspected rabid dogs in Asia [1] , to estimate the DALYs lost per dog bite. This resulted in 0.040 DALYs lost per dog bite. The total loss of DALYs related to the distress experienced following a dog bite for the baseline scenario and the intervention, respectively, were calculated by multiplying the 0.040 DALYs lost per dog bite by the number of dog bites occurring in Colombo City (see “epidemiological input parameters” below).
Finally, the loss of DALYs resulting from human rabies deaths and psychological distress were summed to estimate the total DALYs lost for the baseline scenario and the intervention, respectively.
The animal welfare assessment.
A qualitative scoring system was developed to assess the impact of rabies and its control on dog welfare. The situations identified where rabies and its control have a potential impact on dog welfare are listed in Table 3 . For each situation, a set of conditions potentially affecting animal welfare was identified, e.g. pain, physical injuries, and dyspnoea ( Table 3 ). An impact scale was used for assigning a grade to reflect the level of impact of each condition listed. Scores were attributed to the frequency (proportion of animals in the situation that are affected), severity and duration of the condition according to the following scheme: 0 = no impact, 1 = mild impact, 2 = moderate impact, 3 = severe impact and 4 = extreme impact.
https://doi.org/10.1371/journal.pntd.0003270.t003
The attribution of scores was based on data collected during field visits, viewing of videos taken and a literature review of the physiological and clinical signs that can occur with each situation. It was assumed that the conditions causing pain and discomfort in humans would also do so in animals. The scores were allocated by assessing the information available related to a particular situation such as distress, pain and suffering of animals (based on different symptoms). The scores were allocated relative to each other by first identifying the least stressful and painful method and then assigning scores to other scenarios in comparison to this reference point. First, scores were allocated to the different conditions and then combined to an overall score for the situation. In a next step, the number of dogs in the situations for the baseline scenario and intervention was taken into account and the final score per situation assigned judging whether the score would change if few or very many dogs would be in the situation. Finally, an overall animal suffering score for the baseline scenario and the intervention was assigned.
The scores were attributed by a group of three animal health scientists, namely a professor in animal welfare physiology, a veterinary scientist with expertise in economics and epidemiology; and a veterinary public health specialist. First, the scores were attributed by each scientist individually using the information provided as listed in the Text S1 . Next, the three scientists met to discuss the attributed scores and agree on a common score. All three group members respected the opinions of the others and contributed to an objective and professional discussion.
Epidemiological input parameters.
Epidemiological data needed for inclusion in the economic assessment were the number of dog bites, the number of people presenting with dog bites at health centres, the number of human rabies deaths, the number of dog rabies cases, the number of dogs vaccinated, the number of dogs sterilised, and the number of dogs culled by different means. Various secondary data sources were used to gather these data; there was no primary data collection.
The number of dog bites in Colombo City was estimated based on two independent surveys (not related to this study) conducted by members of the non-government organisation Blue Paw Trust and supported by the World Society for the Protection of Animals (unpublished data). Wards were chosen using random selection from 47 wards in the Colombo Municipal Council, one ward initially selected was removed due to the largely inaccessible military area it contained and replaced with another ward. This resulted in a sample of seven wards; namely Wards 1, 7, 15, 31, 39, 41, 47 and a sampling fraction of 0.15. The first survey which represented the baseline scenario was conducted in June and July 2007 on a representative sample of 277 households. The second survey which represented the intervention was conducted in September 2010 on a representative sample of 117 households in four wards (Wards 1, 7, 15, 41; Wards 31, 39 and 47 were excluded in the second survey, because of low participation rates in the first survey); a sampling fraction of 0.09. Every 10th household encountered was included, starting from a convenient central point within the ward. A questionnaire was administered by one person of a team of trained interviewers to every eligible dog-owning household, and to every 10th non-dog-owning household. A household was considered eligible for interview if at least one adult occupant (≥16 years) was present and from whom consent was obtained for the interview. The questionnaire contained sections on household demographics, dog ownership, care provision and welfare status of any dogs present, and attitudes towards dogs.
The number of human deaths in Colombo City for the duration of the intervention was derived from data provided by the Colombo City Municipal Council based on official public health statistics. The average number of residents presenting with dog bites was provided by the national hospital based on their hospital records. The rate of reporting was defined as the number of residents presenting with dog bites divided by the estimated number of dog bites based on the survey data for the intervention and the baseline scenario, respectively.
The numbers of dogs per situation as described in the animal welfare assessment were derived from data provided by the Veterinary Department of Colombo Municipal Council and the Blue Paw Trust based on their own statistics. Figures for the intervention were directly taken from these statistics, apart from inputs for the situations ‘number of dogs caught in a net and vaccinated’ and ‘number of dogs held by owners’, where the BPT vaccination teams were asked to record the proportion of each category during five weeks in summer 2011 while vaccinating dogs. This proportion was multiplied by the total number of dogs vaccinated by the BPT to get an approximation of the number of dogs in these two situations. The dogs vaccinated by the staff from the animal control facility during the intervention were either vaccinated at peoples' homes or brought for vaccination to the animal control facility by their owners. Hence, they were not caught by net, but handled by their owners, i.e. all fell under the second situation. Assumptions were made for the baseline scenario as follows: It was assumed that the number of dog rabies cases would be comparable to the situation before implementation of the presidential decree and under guidance of the same veterinary officer in charge of the animal control facility (i.e. the period 2001 to 2005). The number of dogs culled in a gas chamber using carbon monoxide and carbon dioxide from a combustion engine was approximated using the annual average of dogs culled in Colombo City from 1999 to 2005. The government veterinary service in the past did not catch dogs using a net for vaccination and it was assumed that they would not have changed their practices. The number of dogs caught in a net and vaccinated for the baseline scenario was therefore set to zero. For the number of dogs held by owners and vaccinated it was assumed that the frequency of vaccination by staff from the animal control facility would have stayed at the same level as in previous years under the guidance of the same veterinary office in charge of the animal control facility (i.e. the period 2001 to 2005).
The social acceptance assessment.
Eleven attitude statements from the two surveys conducted as described above were used as an indicator of the level of acceptance of dogs in the population. They all used a seven level Likert scale (strongly disagree, moderately disagree, slightly disagree, unsure, slightly agree, moderately agree, strongly agree) and were as follows:
- Street dogs pose a danger to people
- I like having dogs around on my street
- The welfare of street dogs is important to me
- Street dogs should be looked after by the community
- I like dogs very much
- People should not feed street dogs
- I don't like being close to dogs
- Street dogs should not be allowed to breed
- If a dog of mine got a skin disease, I would not want it around the house
- It is not acceptable to kill dogs
- Dogs add happiness to people's lives
A summative score per respondent was generated to reflect individuals' acceptance of dogs. Scores of 1 to 7 were attributed with 1 meaning ‘strongly disagree’ with the statement and 7 ‘strongly agree’. The scores of negative statements were reversed (i.e. statements 1, 6, 7, 8 and 9) so that all of the individual item scores had the same direction, which allowed obtaining an overall score indicating acceptance. With this scoring system a minimum score of 11 meant total non-acceptance and a maximum score of 77 total acceptance. Descriptive statistics, Kruskal-Wallis and Wilcoxon rank-sum tests were used to compare the acceptance scores between dog owners and non-dog owners in 2007 and 2010. Finally, Wilcoxon rank-sum tests were used to compare the overall total score from 2007 with the overall total score from 2010. The significance level was set at 5%.
The two surveys were complemented by nine focus groups held with 61 participants. The participants were asked to consider the current situation and think back to five years previously, before the intervention started, in order to establish a public perception of what had changed. They were specifically asked to express any concerns regarding roaming dogs and indicate what size roaming dog population would be acceptable to them. It was assumed that people not expressing any concerns regarding roaming dogs would have a high acceptance. Further, the support of rabies control and dog population management measures as well as peoples' behaviour in case of dog bites was assessed. From August to September 2011, nine focus group discussions were organised, facilitated and summarised by staff members from the Blue Paw Trust. The community liaison officers in Wards 30, 31, 33, 34, and 43 were contacted and asked to invite mixed groups of people (mixed gender, age, professions, non-dog owners and dog owners) from two income strata; high income and low income. The community liaison officers identified the main community leader in each of the sample wards who was familiar with the project. This person then got in touch with people from the community to organise two groups of 10 people each from high and low socio-economic backgrounds. The selection of wards and participants was based on convenience. No payments were offered for participation, but refreshments were provided. In each focus group, participants were:
- 1- Provided with a map of the ward and asked to indicate the locations of roaming dogs
- 2- Encouraged to list and rank the concerns regarding roaming dogs in the past (five years ago) and at present.
- 3- Asked to discuss what an acceptable dog population was and to indicate the following figures: Number of houses in their ward, estimated number of roaming dogs before 2007, estimated number of roaming dogs now, acceptable number of ownerless roaming dogs, and acceptable total roaming dogs.
- 4- Invited to describe how the present situation was compared to 5 years ago
- 5- Asked to discuss what interventions should be implemented if the number of dogs increased substantially
- 6- Posed the question: “What do you do/would you do when bitten? Have you ever been bitten? Would you react differently now than a couple of years ago and if yes, why?”
One enumerator facilitated the discussion, while another one took notes. The facilitator made sure to create a comfortable atmosphere and to encourage people to openly share their thoughts and concerns. Participants were assured that the data would be handled anonymously and that their answers did not have any negative consequences for them. The notes were summarised afterwards and translated into English by the enumerators. Descriptive statistics were presented and the number of dog related problems compared in the past and present compared using Wilcoxon test and McNemar's test. The significance level was set at 5%.
Epidemiological data
The survey in 2007 found 23 dog bites in 1,063 household members or an annual incidence rate of 0.0216. The survey in 2010 found 8 dog bites in 559 household members or an annual incidence rate of 0.0143. The difference in incidence rate in 2007 and 2010 was not significant (p = 0.3105, significance level set at 5%). Extrapolating these dog bite incidence rates to the total population of Colombo City of 642,163 in 2007 and 644,450 in 2010, respectively, resulted in the following inputs for the economic assessment: 13,871 annual dog bites for the baseline scenario and 9,216 annual dog bites for the intervention. These figures were multiplied by four to estimate the total number of dog bites for a four year period, which resulted in 55,484 and 36,864 dog bites for the baseline scenario and the intervention, respectively. The average number of human deaths for the four year duration of the intervention and the baseline scenario, respectively, was three human deaths each for the four year period. The national hospital reported that in May 2006, 131 people sought care following dog bites and in May 2011, 160 people were recorded. These monthly figures were multiplied by 48 to estimate proxies for the number of people seeking medical attention for dog bites in Colombo City for the baseline scenario (n = 6,288) and the intervention (n = 7,680), respectively. The estimated rate of reporting was 0.11 for the baseline scenario and 0.21 for the intervention, respectively.
The number of dog rabies cases was 19 for 2007 (proportionally estimated from annual figure for the period June to December), 17 in 2008, 20 in 2009, 10 in 2010, and 2 in 2011 (until June). For the baseline scenario, the estimated average number of dog rabies cases per year was 43, i.e. 172 for the four year duration. The number of dogs culled with a mixture of carbon monoxide and dioxide in the exhaust fumes produced by a freestanding combustion engine was zero in the intervention due to the presidential decree in 2006 that stopped the elimination of dogs and an estimated 9,384 in the baseline scenario for the four years. Field data from Colombo City collected by the BPT from 5 July to 13 August 2011 during 24 vaccination sessions in 12 different wards (total dogs vaccinated = 658) showed that a mean 28% (SD = 21.9%) of the total dogs vaccinated were held by people from the community (owner or other people) and the remaining dogs were caught in a net for vaccination. Using this proportion to estimate the number of dogs in the situation ‘dogs held by owner and vaccinated’ resulted in 36,300 dogs for the intervention and 25,013 dogs for the baseline scenario for the four years. The number of dogs in the situation ‘catch in net and vaccinate’ was estimated at 10,740 for the four years of intervention. The number of dogs sterilised in the intervention during the four years was 5,323 in total based on records from the Blue Paw Trust.
Comparison of non-monetary and monetary costs and benefits
Table 4 summarises the additional investment and the additional outcomes in monetary and non-monetary terms resulting from the intervention when compared with the baseline scenario over a time period of four years. The overall costs of the intervention were US $1.03 million, which was the sum of the additional investment of US $818,851 for the control measures in the animal health sector and the additional US $215,064 spent on monetary human health costs. The net benefits from the intervention were 738 DALYs averted resulting from the reduction in dog bites, increased acceptance of roaming dogs in society and improved animal welfare. The detailed findings are presented below.
https://doi.org/10.1371/journal.pntd.0003270.t004
Costs of dog rabies control activities
Table 5 illustrates the total costs incurred for dog rabies control activities for the intervention from different organisations involved (Sri Lankan government, Blue Paw Trust). Table 6 lists the total costs incurred by the Sri Lankan government for dog rabies control in the years 2002 to 2006 which reflect the control costs in the baseline scenario. In the intervention, the largest proportion of the total costs was staff costs (33%), followed by implementation costs (21%), other costs (19%), and planning and preparation costs (11%). In the baseline scenario, the costs for implementation activities contributed most (about 92%) to the total annual costs in all years. The difference in costs between the baseline scenario and the intervention over a time period of four years was US $818,851.
https://doi.org/10.1371/journal.pntd.0003270.t005
https://doi.org/10.1371/journal.pntd.0003270.t006
Monetary and non-monetary human health costs
The total human health cost per dog bite was estimated at US $159 without using immunoglobulin, US $163 with equine immunoglobulin and US $39 for the people who only needed medical care, but not vaccination. The total human health costs for the four years of intervention and the baseline scenario were US $1,179,925 and US $964,861, respectively ( Table 4 ). The difference between the two was US $215,064.
The total DALYs lost for the four years related to psychological distress were 1,461 for a total 36,864 dog bites in the intervention and 2,199 for a total 55,484 dog bites in the baseline scenario, respectively. The total DALYs lost for a four year period related to human deaths were 83.97 for both the intervention and the baseline scenario with three human deaths each. The total number of DALYs averted in the intervention period as compared to the baseline scenario for the four year period was 738.
The sensitivity analyses on the input variables that determined the outcomes “difference in monetary human health costs” and “DALYs averted” over the four years are illustrated in Figures 2 and 3 . For the outcome “difference in monetary human health costs” the most influential variables were the number of people bitten and seeking treatment in the intervention (outcome changed by 82%) and the baseline scenario (outcome changed by 67%), respectively, followed by the overhead cost per hospital visit (outcome changed by 13%) and the proportion of people presented with dog bites receiving PEP (outcome changed by 11%). All other input variables caused changes in outcome of 1% or less ( Figure 2 ). The difference in monetary human health costs when varying the two most influential inputs number of people bitten and seeking treatment in the intervention and baseline scenario, respectively, between −30% and +30% from the base is shown in Table 7 . The results demonstrate by how much the inputs need to change for the intervention to create a benefit in terms of monetary human health costs. When keeping the base value for the baseline scenario constant, a reduction of the intervention input by at least 20% would lead to a monetary benefit in the human health sector. The additional expenditures for the intervention spent by the animal health sector could be recovered by monetary human health benefits if, ceteris paribus , the input people seeking treatment in the intervention was 950 (12% of the base value) or the input people seeking treatment in the baseline scenario was 13,026 (207% of the base value).
Sensitivity analysis results where distinct input variables were varied by ±15% and the impact measured on the difference in monetary human health costs (in 2011 US $) between the intervention and the baseline scenario (BS). ◊ = base value = US $ -215,064.
https://doi.org/10.1371/journal.pntd.0003270.g002
Sensitivity analysis results where distinct input variables were varied by ±15% and the impact measured on the Disability Adjusted Life Years (DALYs) averted. ◊ = base value = 738 DALYs averted.
https://doi.org/10.1371/journal.pntd.0003270.g003
https://doi.org/10.1371/journal.pntd.0003270.t007
For the outcome “DALYs averted” the most influential variables were the number of dog bites in the baseline scenario (outcome changed by 45%) and in the intervention (outcome changed by 30%), respectively, followed by the DALYs lost per dog bite due to psychological distress (outcome changed by 15%). The DALYs lost per human rabies death did not influence the outcome ( Figure 3 ).
Animal welfare assessment
Table 8 and Table 9 illustrate the score per situation without taking into account dog numbers and the score per situation taking into account dog numbers. For the intervention, the qualitative estimates ranged between very low and high. For the baseline scenario, the estimates ranged between very low and very high. The overall score was estimated as low-intermediate for the intervention and intermediate-high for the baseline scenario.
https://doi.org/10.1371/journal.pntd.0003270.t008
https://doi.org/10.1371/journal.pntd.0003270.t009
Social acceptance assessment
Table 10 summarises the overall acceptance scores for the baseline scenario and the intervention among dog owners and non-dog owners derived from the two surveys. The Kruskal-Wallis rank test to compare different groups showed that the differences between the four groups of dog owners and non-dog owners were statistically significant (p = 0.001). The post-hoc Wilcoxon rank-sum tests yielded a significant difference between dog owners and non-dog owners in 2007 (z = 8.22, p<0.0001), dog owners and non-dog owners in 2010 (z = 3.836, p = 0.0001), and non-dog owners in 2007 and 2010 (z = −2.71, p = 0.0068). There was no significant difference between all participants in the baseline scenario and the intervention (z = −0.938, p = 0.35).
https://doi.org/10.1371/journal.pntd.0003270.t010
Of the 61 focus group participants, 53 were women and 8 were men. There were 17 housewives and 28 who did not indicate their professions. The rest of the occupations included salesmen, students, nursery teachers, garment makers, an architect and business people. When asked about dog-related issues in the past, the groups described significantly more problems for the past than the present, specifically past problems 7.8±1.5 and present problems 3.3±1.2 (Wilcoxon test, p<0.01). Figure 4 illustrates the number of dog related problems reported by the nine focus groups. Significantly fewer groups mentioned rabies and breeding or puppies as problems at present than in the past (Mc Nemar's test, p<0.05). The stark decrease in the perception of rabies as a problem was explained by workshop participants as being due to possession of knowledge about the disease and knowing what to do when bitten by a dog.
The number of focus groups (1 to 9) that listed specific dog related problems perceived for the years 2006 (blue line) and 2011 (red line) in Colombo City, Sri Lanka.
https://doi.org/10.1371/journal.pntd.0003270.g004
The population control measures mentioned by participants were sterilisation, vaccination, shelter, re-homing, treatments, birth control injection, dumping, education, and awareness campaigns. The highest preference across all groups was given to sterilisation, vaccination and education. None of the groups mentioned culling as a means of population control.
All focus groups indicated that their behaviour following a dog bite had changed. Many groups reported the application of Murunga (a local plant) in the past, but would nowadays wash the wound with soap and running water and go to a hospital to seek treatment.
The mean acceptable total number of roaming dogs reported in the vicinity (i.e. street) was 2 (SD 2, range 0 to 10). There was a significant difference in levels of roaming dogs reported for the past and the present across all focus groups (p<0.001) ( Table 11 ). There was no significant difference in the total number of roaming dogs reported by income levels (p = 0.184), whether the household reared dogs (p = 0.708), gender (p = 0.535), and occupation of participants (p = 0.696).
https://doi.org/10.1371/journal.pntd.0003270.t011
Ethical considerations
The economic analysis showed that the use of an additional US $818,851 in the animal health sector to combat rabies and manage the dog population in Colombo City had both negative and positive consequences in society when contrasting the intervention and the baseline scenario. Non-monetary benefits included an increase in the acceptance of roaming dogs among non-dog owners and dog owners, a reduction in animal suffering, and 738 DALYs averted. The increased acceptance of roaming dogs and the DALYs averted increased well-being of society. While reducing animal suffering overall, the intervention strategy at the same time compromised animal welfare (e.g. due to sterilisation or catching in a net). Negative consequences included an increase of US $215,064 in human health costs related to seeking health care following dog bites. Hence, there was a net cost to society in monetary terms of US $1.03 m and a net benefit in non-monetary terms. The lower number of estimated dog bites and the improvement in reporting of bites and treatment of people indicated that the risk to people of contracting rabies was decreasing. The intervention was shown to be effective, as the official number of dog rabies cases decreased from an average of 43 cases per year (2001 to 2005) to just two cases in the first six months of 2011.
Ethical aspects relating to the rights and fairness approach in dogs and humans as well as the virtue approach in people included the following:
- Rights: The right of people not to be injured was promoted in the intervention by an estimated decrease in the number of dog bites. The culling of dogs in the baseline scenario violated the right to follow religious beliefs, because it was against the norms of the mainly Buddhist population in Colombo City ( http://www.statistics.gov.lk ).
- Fairness: In both scenarios all dog owners were treated equally, because they all had the same possibilities to get their dogs vaccinated. In the intervention, non-dog owners were also targeted as part of the education activity, which was not the case in the baseline scenario.
- Virtue: By not taking life or taking life without suffering, veterinarians implementing the rabies control measures were given the possibility to be good practitioners (intervention). By treating all dogs and their owners equally, policy and decision-makers planning and implementing the rabies control measures showed fairness and generosity (both scenarios). People valuing dogs as companions were reinforced in their feelings of love and fidelity by observing the Blue Paw Trust team working in the field (intervention). Not having to hide dogs to avoid their culling indirectly promoted virtue (intervention).
- Rights: The baseline scenario violated the right to life because dogs were culled on a large scale for the purpose of population and rabies control. The intervention respected the right to life by pursuing a strategy without culling. The dogs' right to live their lives without molestation was violated by sterilisation and catching, but prevented more suffering and harm than it imposed on them.
- Fairness: In the intervention, all dogs were included in the vaccination campaign, while in the baseline scenario only owned dogs were vaccinated. Also, the culling activities in the baseline scenario were unfair, because they only targeted roaming dogs.
The judgement if the good of the intervention outweighed the harm (the utilitarian approach) and if it best served the community as whole and not just some members (the common good approach) depends on how decision-makers prioritise ethical issues. It might be argued that the avoidance of animal suffering and the increased well-being of people justified the net monetary cost of the strategy. Others might attribute more weight to monetary values resulting from the control activities.
The article proposes a comprehensive framework for assessing multiple aspects of rabies control and combining them in an economic analysis. It is composed of five components (epidemiological, economic, social, animal welfare and ethical assessments) that are all interlinked to guide decision-making and the allocation of resources. While almost all parts were covered individually in previous studies, to the authors' knowledge there are no publications on rabies control that cover all these aspects in the spirit of One Health and link them in an economic analysis. The advantage of the framework is its comprehensive nature that provides decision-makers with a wide array of information that they need to be able to take informed decisions on disease management. However, it requires capacity in multiple disciplines, extensive data collection and an acknowledgment of the multi-factorial processes of decision-making. Similar elements essential for One Health decision making have also been identified by others. For example, a framework published after this study was conducted for the estimation of the economic costs of zoonoses [31] conceptually linked epidemiological and economic models and placed them in the context of wider risk management strategies including assessment of the context, hazard identification, risk assessment, capacity building and communication. The approach proposed here can be considered as an expansion of the risk assessment and risk management steps described in the other framework, whilst providing more detail on a specific disease (i.e. rabies) and the associated effects.
The comparison of additional costs with both monetary and non-monetary outcomes required presenting the results in an unconventional way. On the one hand, this presentation allowed reflecting the complexity of the real world and the various economic consequences related to a decision. On the other hand, the combination of negative monetary and positive non-monetary outcomes made the interpretation more challenging than a conventional net present value or cost-benefit ratio. Cost-benefit analysis is an approach that is intuitively appealing, because it assesses the positive and negative consequences of a strategy in a common unit, generally money. Cost-effectiveness analysis uses the same basic approach, but presents the outcome of a strategy in non-monetary units. The selection of an appropriate measure of effectiveness is critical, and must be in accordance with the control objective. A “CEA is only as valid as its underlying measures of effectiveness and cost” [32] , but unlike in health economics, where attempts have been made to harmonise CEA methodologies and encourage comparability of studies [33] , there are no specific guidelines available yet for its application in animal health. Currently, due to variability of interests, approaches, designs, capacity and resource availability of organisations involved in rabies control, any incremental cost-effectiveness analyses going beyond human health will vary depending on the outcome measures defined. If the scientific community was to find an agreement on a standardised approach to measure outcomes of rabies control in an integrated way, the economic efficiency of such control measures could be compared internationally and the best approach chosen. As long as there is no standardisation of effectiveness measures for rabies or disease control in general, the variety in outcomes will make a meta-analysis difficult or even impossible. The presented framework is a starting point that may help to create awareness and stimulate discussion.
A range of approaches were used in the case study to cover the multifaceted control measures implemented which were expected to decrease the number of dog rabies cases, to reduce the number of PEP applied to people, to increase acceptance of dogs in society, and to generate a positive net value overall. The case study illustrates the various components of the proposed framework in a developing country context. Because of the limited availability of resources for the case study, secondary data were used whenever possible and where primary data collection was necessary, low-cost approaches were considered for data collection. While the case study is subject to various limitations as described below, it provides information for Sri Lankan stakeholders involved in rabies control on the profitability and cost-effectiveness of the implemented intervention and demonstrates the advantages and challenges of the proposed framework.
Importantly, the number of dog rabies cases was drastically reduced during the time of the intervention to only two in the last six months of the study period compared to a previous high number of dog rabies cases (an average of 43 per year in the period of 2001 to 2005). This indicated that high enough vaccination coverage was achieved and that good progress was being made towards the elimination of rabies in the years 2014–2015, the specified long term target. Given that rabies is still prevalent in other parts of the island, it is important to continue intervention and surveillance efforts in Colombo City to maintain the favourable situation until rabies can be eliminated island-wide.
One critical variable in the estimation of monetary and non-monetary human health consequences was the number of dog bites. While the number of people seeking health care following a dog bite derived from data from the national hospital showed an increase from 2006 to 2011, the numbers derived from the two surveys in 2007 and 2010 showed a decrease in the number of dog bites. There are four possible explanations for this increase: 1) people were more aware of rabies prophylaxis and went to the hospitals more often, 2) there was a better system in place to record dog bites in hospitals, 3) there were effectively more dog bites, and 4) unknown factors related to the two months of data provided caused a fluctuation in numbers (a comprehensive data set for the entire period of 2006 to 2011 was not available). Given the fact that the intervention substantially decreased the number of dog rabies cases in the population, an increase in the number of dog bites seems highly unlikely. This hypothesis is corroborated by the survey and focus group data. Because the survey data showed a decrease in the number of dog bites and the focus groups an increase in disease awareness, it is most likely that the increase in the number of registered dog bites was due to a higher number of people seeking medical advice in case of dog bites. The analysis of the focus groups demonstrated that people's reaction following a dog bite had changed. All focus groups reported that they would now wash the wound with soap and water and go to the hospital to receive PEP. Also, the development of a better system to record bites in hospitals in recent years was expected to have had a positive impact on the number of registered cases (personal communication Dr Obeyesekere).
The difference between the number of dog bites collected from the national hospital and the number estimated from the surveys provided an indication of the rate of under-reporting. The estimated reporting rates indicated an improvement in dog bite reporting in the intervention compared to the baseline scenario. This observation further confirmed the increased rabies awareness of people in the community. However, it also showed that a considerable part of the population did not seek medical attention after being bitten by a dog. As long as rabies is not eradicated from the dog population, people should constantly be informed about the appropriate behaviour in case of a dog bite.
The increase of registered dog bite cases in health centres caused an increase in human health costs. For the savings in monetary human health costs to cover the additional investment made in the animal health sector, the number of people seeking treatment following dog bites would have to be reduced drastically as shown in the sensitivity analysis. It is expected that the number of people seeking medical advice will remain high or increase despite a reduction in dog bites, because the on-going intervention activities constantly promote disease awareness. Only elimination of rabies from the dog population will allow reducing the provision of PEP after dog bites. As long as rabies is endemic in the dog population, people bitten by rabies-suspect animals should get a thorough assessment by health professionals and PEP, as recommended by World Health Organisation guidelines. The only way to reduce public health costs in a rabies endemic situation is to find cheaper and equally effective methods of PEP. The public health sector has already initiated such cost savings by using intradermal vaccines and only administering immunoglobulin in priority cases following a sound history taking and assessment.
Remarkably, there was a considerable reduction in the number of problems listed in all focus groups. Nearly all groups reported that there had been a reduction in rabies, barking, puppies and breeding behaviour and dog fights since the implementation of the intervention. Thus, dogs were perceived more favourably by people, because they looked healthier and showed reduced breeding and nuisance behaviour. Moreover, some focus group participants indicated that their fear of rabies had decreased drastically, because of their improved knowledge of the disease. The selection of participants was performed independently by the community liaison officers in collaboration with community leaders and therefore not influenced by the staff of the BPT. Because the community liaison officers did not receive fixed criteria about socio-economic status of participants, it is likely that ‘high’ socioeconomic groups represented more the middle level, as those at the truly high end did not have the time or interest to participate and were not known well to the community leaders. To promote open sharing of thoughts and concerns, the facilitator made sure to create a comfortable atmosphere and assured participants that the data would be handled anonymously and that their answers did not have any negative consequences for them. However, it is still possible that a few participants may have felt that a less than positive evaluation would result in discontinuation of the project. While such behaviour introduces bias into the results, it also reflects the social desirability of the project, i.e. a community wanting the project to continue is in itself an indication of the degree of perceived success. A source of bias that could not be controlled was the imbalance in gender representation in the focus groups. Only a few men were able to join the focus groups, which was due to the fact that all groups met during the day when the men were at work.
While a variety of approaches are available to assess animal welfare (e.g. welfare assessment protocols for commercial livestock), there are no guidelines in place for the systematic assessment of the impact of rabies and its control on animal welfare. Therefore, we developed a qualitative approach to assess defined situations related to rabies and its control that may negatively affect animal welfare. The assessment was a combination of field data, scientific literature, logical reasoning and professional judgment. Importantly, the scores attributed to the different situations were relative and not absolute. The development of an absolute scoring system would require systematic measurement of physiological and behavioural parameters, which was not within the scope of this project. Taking into account the numbers of dogs in the situation, the highest score (‘very high’) was attributed to the situation culling dogs via carbon monoxide and carbon dioxide poisoning using the exhaust fumes of a combustion engine, and the lowest scores to the situation of holding dogs by the owner or people from the community, and vaccination. Thus, replacing the culling of dogs by other intervention strategies reduced animal suffering. Because none of the focus groups mentioned culling of dogs as an intervention strategy for rabies or population control, it is most likely that the avoidance of culling dogs not only promotes animal welfare, but also the well-being of people in society who care for the dogs.
The ethical assessment helped guide the interpretation of the results. However, it did not attribute weights to the different criteria analysed. Such weights were expected to differ among decision-makers depending on the political agenda, local norms and customs, available resources, experience and personal preferences.
Further benefits that were not quantified in the analysis and remain open to further research include a potential reduction of rabies cases in other animals, promotion of responsible dog ownership and thus better animal welfare, and the decrease of fear in the human population.
This case study explicitly took into account a range of factors that impact on the value of rabies control measures. By combining different monetary and non-monetary aspects, it not only provided information about the impact of rabies control on monetary public health costs, but also important insights about non-monetary effects, particularly animal welfare and social acceptability that were not only valuable outcomes in themselves, but also helped to explain and support some of the other findings. For example, the epidemiological data on the number of dog rabies cases as well as the information from the surveys on dog bites and the focus groups on disease awareness provide an explanation for the increase in human health costs. Linkages between the individual components could be more formalised by for example making social assessments an integral part of epidemiological analysis.
The proposed framework provides a first proposal for looking at rabies control in a holistic way and covers multiple facets that inform decision-making. The framework is expected to help planning impact evaluations of rabies control so that future data collection protocols can take into account not only the health costs, but also consider factors like social acceptance and animal welfare. It thereby helps to conduct integrated assessments for zoonotic disease control and can be further developed to address more complex One Health challenges.
Supporting Information
Information relevant to the animal welfare assessment.
https://doi.org/10.1371/journal.pntd.0003270.s001
Acknowledgments
We thank all study participants of Colombo City for their cooperation and willingness to participate. We are grateful to the staff members of the Blue Paw Trust for their support with data collection. The authors thank Prof. Neville Gregory (staff member Royal Veterinary College at the time of analysis) for his guidance in developing the animal welfare assessment and the various data providers for their assistance and sharing of data.
Author Contributions
Analyzed the data: BH HB WG. Contributed reagents/materials/analysis tools: BH EH NO JR HB WG. Wrote the paper: BH EH NO JR HB WG. Developed the One Health evaluation framework and the study design: BH JR HB WG. Conceived the data collection protocols: BH NO HB JR. Conducted data collection: BH NO HB JR.
- View Article
- Google Scholar
- 16. Drummond MF (1997) Methods for the economic evaluation of health care programmes. 2nd ed. Oxford: Oxford University Press.
- 21. Pfeiffer DU (2010) Veterinary Epidemiology: An Introduction. Oxford: Wiley-Blackwell.
- 26. Vesan P. and Bizzotto G. (2011) ―Quality of life Europe: conceptual approaches and empirical definitions. Walqing working paper 2011.5. http://www.walqing.eu/fileadmin/download/external_website/Newsletters___policy_briefs/WALQING_244597_WPaper2011.5_WP4LitReview.pdf (accessed October 2011)
By providing an email address. I agree to the Terms of Use and acknowledge that I have read the Privacy Policy .
Slaughtered dog Killua in Cam Sur tested positive for rabies, says PAWS

Golden Retriever Killua was killed and found by his keepers in a sack. (File photo courtesy of Vina Rachelle / Facebook)
MANILA, Philippines — The slaughtered golden retriever in Camarines Sur tested positive for rabies, according to the Philippine Animal Welfare Society (PAWS).
PAWS Director Anna Cabrera told INQUIRER.net in a phone interview on Monday that this was based on the test conducted by the Bureau of Animal Industry (BAI) over the weekend.
However, Cabrera noted that several factors may affect the test result.
“While the result of the testing may not be accurate due to the fact that the body had already been buried for five days prior to testing and may have been contaminated from being in an area where many stray dogs have already been slaughtered, PAWS is making this announcement to ensure that any bites or scratches will be reported promptly in the interest of public health and safety,” Cabrera said in a statement.
Cabrera said they also immediately informed the owner, who is now considered at risk for the virus after hugging “the bloodied body of her beloved dog when she found him at a known dog slaughter area.”
The dog named Killua was killed in Barangay Sta Cruz in Bato town on March 17.
A certain Anthony Solares claimed that Killua had supposedly chased his child.
But in CCTV footage posted by Vina Rachelle Arazas – Killua’s owner – on Facebook, Solares was seen hitting Killua as the dog was running around, trying to escape.
The standard protocol for a suspected rabid animal is for it to “be observed for fourteen (14) days or, in case of highly suspected rabies cases, be humanely euthanized with no damage to the head,” according to the BAI’s Manual of Procedure for Rabies.
Cabrera also learned that Solares owned a carinderia, but she clarified to INQUIRER.net that it could not be determined if they were selling dog meat.
But she noted that “Solares owns a carinderia business which sells meat viands near the dog slaughter area.”
Arazas, as well as consumers of stray dogs in the area, should get post-exposure vaccines, said PAWS.
READ: PAWS finds Killua killer not acting on self defense
Rabies is estimated to kill 59,000 people every year, according to the World Health Organization (WHO).
Subscribe to our daily newsletter
As of March 2024, the Philippines already has 84 cases of rabies, which is two cases higher than the same period last year, as per the Department of Health (DOH).
The DOH did not mention how many of the rabies victims died, but the WHO noted that the virus is virtually 100 percent fatal.
Disclaimer: Comments do not represent the views of INQUIRER.net. We reserve the right to exclude comments which are inconsistent with our editorial standards. FULL DISCLAIMER
© copyright 1997-2024 inquirer.net | all rights reserved.
We use cookies to ensure you get the best experience on our website. By continuing, you are agreeing to our use of cookies. To find out more, please click this link.
Bangor Daily News
Maine news, sports, politics, election results, and obituaries
More than half of pet owners don’t believe in vaccinating their animals

Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to print (Opens in new window)
- Click to email a link to a friend (Opens in new window)

A study released last fall indicates more than 37 percent of dog owners believe that canine vaccinations could cause their pets to develop autism — a belief that animal health experts say is wrong on several levels.
Published in the online journal Vaccine , the study “Sick as a dog? The prevalence, politicization and health policy consequences of canine vaccine hesitancy,” found that more than half of the country’s dog owners have some level of anti-vax feelings when it comes to their pets.
It’s leaving veterinarians in Maine frustrated by clients who refuse the important medical care for their pets.
The link between human vaccines and the onset of autism has been disproven by peer-reviewed scientific studies , and there is no scientific evidence that domestic pets can have autism, vets said.
“I have never heard of autism in dogs,” said Dr. Kate Domenico, president of the Maine Veterinary Medical Association. “How would you assess your dog? I would love to know exactly how you would classify your dog as autistic.”
Vaccine opposition is nothing new, although it’s better known when it comes to humans. But it’s picking up steam, according to a 2023 study on human attitudes toward vaccines published in The Lancet , and it’s bringing pets along for the ride.
The study was presented to The Lancet Commission on Vaccine Refusal, Acceptance, and Demand in the USA, co-hosted by the Yale Institute for Global Health and the Baylor College of Medicine. The authors, who are all members of the commission, said that over the last 20 years, the human anti-vaccine movement has evolved from what it termed a “fringe subculture” into an increasingly organized network.
The study’s authors said the anti-vax movement has grown to the point it is having negative impacts on public health, such as the return of whooping cough and measles — both illnesses that were essentially eradicated by vaccines until recent years.
The same anti-vaccination attitudes that brought back measles are spilling over into domestic pet care, according to animal health experts.
The dog study pointed to the explosion of human vaccine mistrust during the pandemic. That mistrust created what researchers call a “spillover effect” to domestic pet vaccinations, according to the study.
Hesitancy or outright refusal to have pets vaccinated is nothing new, but the growing number of people opposing dog and cat vaccines nationwide is cause for concern, according to animal health experts in Maine. Besides animal owners’ concerns about autism, other factors that play into their decisions to not vaccinate pets include access to veterinary care and financial constraints, Domenico said.
In the “Vaccine” study, researchers found that 45 percent of U.S. households own dogs. Of those, close to 40 percent believe that canine vaccines are unsafe, more than 20 percent believe these vaccines are ineffective and 30 percent consider them to be medically unnecessary.
It’s a trend veterinarians in Maine would like to see reversed.
“Vaccines do indeed strengthen immunity against potentially serious or even fatal diseases, especially when exposure to other animals occurs, such as in boarding kennels, pet shops, animal shelters or shows,” said Robert Causey, DVM, associate professor of animal and veterinary sciences at the University of Maine. “Vaccines also provide protection against wildlife-borne diseases, such as rabies, and environmental disease such as tetanus.”
It’s not unheard of, but it is extremely rare for a vaccinated dog or cat to contract rabies, according to a published study by the American Veterinary Medical Association.
The research showed that out of 1,100 reported cases of canine and feline rabies in 21 states, the majority of the animals — 97 percent — had not been vaccinated. The remaining 3 percent had a history of rabies vaccinations.
In its most recent numbers, the Maine Centers for Disease Control reported 30 cases of rabies in the state in the first half of 2023. All cases were found in small, wild mammals.
Because domestic animals can serve as a bridge between wildlife rabies reservoirs and humans, vaccination of pets is one of the most effective public health tools available to protect human health, according to the World Health Organization .
Getting pets vaccinated against rabies is a tough sell when owners come in armed with erroneous information they heard from well-meaning people who regularly work with pets like groomers, breeders and pet shop employees, Domenico said.
She has had clients come in repeating wrong information gleaned from the pet industry, such as their particular breed of animal does not require any vaccines or does not need them until they are 6 months old.
Neither is ever the case, she said.
Veterinarians agree some pets may react to vaccines, much like humans react to their annual flu shots. In both cases, there’s no reason not to be vaccinated.
“All vaccines can pose some degree of risk, but this is where a conversation with one’s local veterinarian can be very helpful,” Causey said. “One can have a discussion, based on the pet’s age [and] lifestyle, which vaccines are necessary to protect against severe disease, and which may be considered elective.”
Those electives, also called non-core vaccines, include bordetella, leptospirosis, Lyme disease and influenza for dogs and feline leukemia for cats.
“I have seen dogs who have died of kidney disease because their owners refused to get them vaccinated for leptospirosis,” Domenico said. “It did not have to happen.”
Leptospirosis is a deadly bacterial disease most commonly spread through the urine of wild animals. So if a pet drinks puddle water out in the Maine woods, it could easily be exposed to it.
Non-elective or core vaccines are rabies and distemper, and are required by law in Maine.
To further reduce risk of a reaction, Domenico said pet owners can opt to spread individual vaccines out over a period of time.
“Every veterinarian should evaluate every patient to determine whether or not that pet needs those non-core vaccines,” Domenico said. “It should be based on risk of exposure.”
More articles from the BDN
Julia bayly.
Julia Bayly is a reporter at the Bangor Daily News with a regular bi-weekly column. Julia has been a freelance travel writer/photographer since 2000. More by Julia Bayly
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Ron Koury ; Steven J. Warrington .
Affiliations
Last Update: October 31, 2022 .
- Continuing Education Activity
Rabies causes viral encephalitis which kills up to 70000 people/year worldwide. Infected animal saliva transmits viral encephalitis to humans. Rabies is one of the oldest known diseases in history with cases dating back to 4000 years ago. For most of human history, a bite from a rabid animal was uniformly fatal. In the past, people were so scared of rabies that after being bitten by a potentially rabid animal, many would commit suicide. This activity describes the pathophysiology of rabies and stresses the importance of an interprofessional team in its management.
- Identify the etiology of rabies.
- Review the pathophysiology of rabies.
- Outline the treatment and management options available for rabies.
- Describe interprofessional team strategies for improving care and outcomes in patients with rabies.
- Introduction
Rabies causes viral encephalitis which kills up to 70,000 people/year worldwide. Infected animal saliva transmits viral encephalitis to humans. Rabies is one of the oldest known diseases in history with cases dating back to 4000 years ago. For most of human history, a bite from a rabid animal was uniformly fatal. In the past, people were so scared of rabies that after being bitten by a potentially rabid animal, many would commit suicide. Pasteur's rabies vaccine from 1885 has led to such intense prophylaxis in developed countries, that in the United States, for example, there have only been about two rabies deaths per year for the past two decades; less developed countries are not so lucky. [1] [2] [3]
The Rhabdoviridae family of viruses that are bullet-shaped and composed of two parts cause rabies. The first part is considered more structural and is a viral envelope while the second part is more functional and contains the ribonucleocapsid core. The virus is most commonly spread through the bite of an infected mammal, including domestic and wild ones, but transmission can occur from saliva through broken skin or mucous membranes. Other routes of infection include inhalation of the virus in an aerosolized form, ingestion, transplacentally, and even through organ transplants. [4] [5]
- Epidemiology
Researchers estimate that 30,000 to 70,000 deaths are attributable to rabies each year, with less developed countries affected more. In the United States, there are few human cases reported, though that may be due to the widespread use of post-exposure prophylaxis and the prevention programs in place. In developed countries, domesticated animals have only been responsible for about 10% of cases of rabies transmission, while wild animals such as skunks, raccoons, foxes, and especially bats are responsible for the rest of the cases. Any mammal may carry rabies, and so while small rodents and the rabbit family usually are considered safe as they are not expected to survive an inoculating wound from a rabid animal, there have been anecdotal reports of rabies caused by transmission from rats. As animal carriers vary by region, it is important to know your region’s carriers to help determine who may need prophylaxis. [6] [7]
- Pathophysiology
Following viral transmission, the rhabdovirus travels through the peripheral nervous system targeting the central nerves, which then leads to encephalomyelitis. In humans, the first symptoms seem like any other nonspecific viral syndrome (fever, malaise, headache). These benign symptoms may then progress to anxiety, then to agitation, and then to frank delirium. One very consistent symptom after a rabid bite is tingling at the bite site within the first few days. Interestingly enough, after the virus has spread from peripheral nerves to the central nervous system (CNS), it then travels back to the peripheral nervous system, particularly affecting highly innervated areas (e.g., salivary glands). The "frothing" as portrayed in the movies Cujo and Old Yeller, is due to hypersalivation, and victims can suffer from intense pharyngeal muscle spasm at the mere sight, taste, or sound of water. This is called "hydrophobia." Eventually, the virus progresses to complete failure of the entire nervous system which causes a quick death. While animals tend to die within ten days, the incubation period following inoculation can last two weeks to six years, averaging a few months. Determining factors for the time of onset include the viral load, location of exposure, and severity of the wound. The virus ultimately affects the central nervous system, usually affecting the brainstem more severely. The toxic effects occur through an inflammatory response, with functional changes not completely understood. Ultimately the virus is suspected to affect neurotransmission, and apoptosis may occur through virus-dependent and cell-dependent routes. Once clinical features are seen, rabies is universally fatal. [8]
- Histopathology
Autopsy studies have revealed that the brain usually is swollen, congested, and has an acute inflammatory process. In most cases, the presence of neuronal death is rare. Immunochemical staining will reveal deposits of the virion in the nerve cytoplasm. Negri bodies are often seen on light microscopy but only in about two-thirds of cases.
- History and Physical
The history of a rabies-infected patient may be simple and straightforward with a known bite from a rabid animal. Unfortunately, it may be challenging to obtain a history pointing towards rabies due to the potential for a long incubation period and multiple potential transmission methods.
There are five stages of rabies following inoculation: incubation; prodrome; acute neurologic illness; coma; and death.
Incubation is the period defined as an inoculation to the first onset of symptoms and can range from days to years.
The prodrome phase includes nonspecific symptoms similar to flu-like illnesses with gastrointestinal symptoms, myalgias, and fevers being some of the possible symptoms.
The third stage of rabies is when neurologic symptoms occur. These are classified into one of three categories: encephalitic (also considered "furious"), paralytic (also considered "dumb"), and a rare non-classic form.
- The encephalitic form is most common and presents in approximately 85% of cases. These patients may exhibit hydrophobia or aerophobia, which is when spasms develop as a result of stimuli such as swallowing liquids (hydrophobia). Agitation and changes in mentation can occur during the encephalitic form, with the potential for autonomic dysfunction, increased deep tendon reflexes, nuchal rigidity, and finding positive Babinski sign. Other examination findings outside the nervous system can include tachycardia, tachypnea, and fever. This progresses rapidly to hyperactivity.
- The paralytic form of rabies is less common and noted to occur less than 20% of the time. These patients may be confused with Guillain-Barre syndrome as the classically associated hydrophobia, and irritability is not seen. Weakness is a hallmark, though patients may also have altered mentation, ongoing fevers, and bladder dysfunction.
- The final form of rabies is considered non-classic and is rare, generally associated with seizures and more profound motor and sensory symptoms.
Stage 4 of rabies is the coma stage and usually begins within ten days of stage 3. Patients may have ongoing hydrophobia, develop prolonged apnea periods, and have flaccid paralysis.
Following the onset of stage 4, without supportive care due to cardiopulmonary failure, most patients experience death within two to three days. Even with supportive therapy, virtually zero patients survive rabies.
Without a clear-cut rabid bite history, rabies is often a diagnosis of exclusion. In early stages, it may manifest similar to influenza, Coxsackie, enterovirus, and herpes. In later stages, rabies may present similarly to delirium tremens, tetanus, botulism, diphtheria, tick-borne diseases, and Guillain Barre. It is common for physicians to check CBC, electrolytes, cultures, CT, chest x-ray, and MRI and still, have no idea that rabies is the culprit. Unless isolated in a rabies-specific viral culture, detected by polymerase chain reaction (PCR) in saliva, found to have positive antibody titer, or isolated in cerebrospinal fluid (CSF), the diagnosis may continue to be elusive until too late.
Rabies can be diagnosed through multiple routes using CSF, blood, saliva, tears, and tissue biopsies (neck, immunofluorescent stain). CSF analysis can show a pleocytosis and may allow isolation of the virus. The Centers for Disease Control and Prevention notes that no single test is enough to rule in or out rabies. Ultimately, a high level of suspicion is required in developed countries due to the rarity of the disease. [9] [10] [11]
If the biting animal can be euthanized and tested then that may prevent the need to administer post-exposure prophylaxis. Public health may be able to facilitate the testing of the animal.
- Treatment / Management
There is no effective treatment for rabies. Prevention is the mainstay of treatment including programs involving domestic animal vaccination, education, and monitoring. [12] [13] [14]
Wound care is the first step in the treatment of any individual with a feared rabies exposure. Appropriate wound care alone has been noted to be almost 100% effective if initiated within three hours of inoculation. Recommendations include scrubbing the wound and surrounding area with soap and water (solutions include 20% soap solution, povidone, and alcohol solutions), and swabbing deeply for puncture wounds, with irrigation. After cleaning the wound thoroughly the application of a virucidal agent such as benzalkonium chloride or povidone-iodine is recommended.
In the United States, when a bite is known to be from a bat, skunk, raccoon, or fox, treat immediately with rabies vaccine and rabies immune globulin. For all other bites, consult the public health department. Outside of the United States, a dog bite should be treated immediately with vaccine and rabies immune globulin.
Treatment is then initiated based on if the patient was previously immunized or not. For patients with previous immunization, a typical treatment may be with a human diploid cell vaccine or purified chick embryo cell vaccine at a dose of 1 mL injected intramuscularly on the day it occurs (day 0) and on day 3.
If the patient has not been previously immunized, the treatment still involves dosing with one of the two vaccines listed above with 1 mL given intramuscularly on days 0, 3, 7, and 14 (and on day 28 if the individual is immunosuppressed). The dose of the vaccine should be given at a site distant from where the second part of treatment (human rabies immune globulin or HRIG) is given. These unimmunized patients are treated with human rabies immune globulin as well at a dose of 20 IU/kg, with a preference to infiltrate as much of that dose around the wound as possible. Any remaining dose of human rabies immune globulin not infiltrated into the wound is then given intramuscularly, and as mentioned above is given at a site distant from the vaccine.
Recently, recommendations have been updated in the United States, and since bats are by far the major source of rabies here, any person who awakens from sleep and finds a bat in the room should be urgently immunized.
- Differential Diagnosis
- Poisoning with belladonna alkaloids
- Jacob Creutzfeldt disease
- Brain tumor
- Encephalitis
- Complications
- Fasciculations
- Autonomic instability
- Consultations
- Neurologist
- Infectious disease
- Neurosurgeon
- Public health
- Enhancing Healthcare Team Outcomes
When a diagnosis of rabies is made, an interprofessional team is necessary as the repercussions of this infection go way beyond the acute infection.
Blood transfusions have not been documented to transmit rabies, though it has been suggested to hold donation for one year following exposure prophylaxis. If no exposure was noted, but vaccination occurred there is a recommendation to wait four weeks.
Since rabies is entrenched within the native animal population in the United States, there will continue to be human exposure to this fatal disease. Public health officials take a very active role in preventing rabies, both before exposures as well as after possible exposures.
Animal control has to be notified to determine if the domestic animals require vaccination. In addition, vaccination of workers who may be exposed to rabies may also be necessary.
Patients need to be educated on avoiding contact with wildlife, and if ever bitten, the area should be thoroughly washed to lower the risk of rabies transmission. All dead and sick animals should be handled with heavy gloves. If bitten by a wild animal, one should seek immediate medical assistance. [15] [16] [17] (Level V)
For those who develop symptoms of rabies, survival is rare. Only a handful of survivors exist in the USA after acquiring rabies. For those without symptoms but with rabies vaccine prophylaxis, survival is assured. Individuals who are bitten by a rabid animal need the rabies vaccine and immunoglobulin ASAP for survival- once the symptoms appear, death is inevitable. [18] [19] [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Ron Koury declares no relevant financial relationships with ineligible companies.
Disclosure: Steven Warrington declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Koury R, Warrington SJ. Rabies. [Updated 2022 Oct 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Overview, prevention, and treatment of rabies. [Mayo Clin Proc. 2004] Review Overview, prevention, and treatment of rabies. Hankins DG, Rosekrans JA. Mayo Clin Proc. 2004 May; 79(5):671-6.
- Retrospective Cohort Study to Assess the Risk of Rabies in Biting Dogs, 2013⁻2015, Republic of Haiti. [Trop Med Infect Dis. 2017] Retrospective Cohort Study to Assess the Risk of Rabies in Biting Dogs, 2013⁻2015, Republic of Haiti. Medley AM, Millien MF, Blanton JD, Ma X, Augustin P, Crowdis K, Wallace RM. Trop Med Infect Dis. 2017 Jun 12; 2(2). Epub 2017 Jun 12.
- [Old and new prescriptions for infectious diseases and the newest recipes for biomedical products in plants]. [Arch Immunol Ther Exp (Warsz)....] [Old and new prescriptions for infectious diseases and the newest recipes for biomedical products in plants]. Koprowski H. Arch Immunol Ther Exp (Warsz). 2002; 50(6):365-9.
- Review Rabies: epidemiology, pathogenesis, and prophylaxis. [Adv Ther. 2007] Review Rabies: epidemiology, pathogenesis, and prophylaxis. Leung AK, Davies HD, Hon KL. Adv Ther. 2007 Nov-Dec; 24(6):1340-7.
- Review Epidemiology of human rabies in the United States, 1980 to 1996. [Ann Intern Med. 1998] Review Epidemiology of human rabies in the United States, 1980 to 1996. Noah DL, Drenzek CL, Smith JS, Krebs JW, Orciari L, Shaddock J, Sanderlin D, Whitfield S, Fekadu M, Olson JG, et al. Ann Intern Med. 1998 Jun 1; 128(11):922-30.
Recent Activity
- Rabies - StatPearls Rabies - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
COMMENTS
Background Rabies, caused by a lyssavirus, is a viral zoonosis that affects people in many parts of the world, especially those in low income countries. Contact with domestic animals, especially dogs, is the main source of human infections. Humans may present with the disease only after a long period of exposure. Nearly half of rabies cases occur in children <15 years old. We report on a fatal ...
The U.S. Centers for Disease Control and Prevention (CDC) plans to test the protocol on rabies-infected ferrets; Thai and Canadian doctors, who unsuccessfully treated a 33-year-old man with rabies ...
Introduction. Rabies, an acute progressive encephalomyelitis caused by infection with viruses of the Lyssavirus genus, continues to kill about 20,000 people every year in India, accounting for almost a third of the 61,000 global human deaths due to rabies. 1, 2 Usually transmitted through the saliva of an infected animal, rabies encephalitis has the highest fatality rate among infectious ...
A U.S. citizen was bitten by a puppy while in India; rabies postexposure prophylaxis was not sought. The traveler developed rabies upon return to the United States and died during hospitalization. Seventy-two health care providers were exposed to infectious materials. Treatment for exposures cost approximately $235,000.
This study provides insight on a presumptive case of human rabies that survived despite non-administration of rabies vaccine after exposure. It also exposes the weaknesses in the health and veterinary systems in rural Ghana regarding rabies surveillance and case management. Keywords: rabies, human rabies, dog bite, vaccination, surveillance.
Studies conducted in sub-Saharan Africa show that most of the rabies cases in animals and humans are caused by canine rabies virus, mostly transmitted by domestic dogs and thus comprehensive and ...
CLINICAL CASE STUDY The following clinical case study serves as a timely reminder that rabies is still with us. As illustrated in this case, the tools for making an antemortem diagnosis are getting better. However, in low-prevalence areas, the possibility of rabies is often not entertained until late in the course or after death.The delay in ...
That's the message from the Illinois Department of Health as it announced that an 80-year-old man died of rabies after waking up to find a bat on his neck. It is the first human case of rabies in ...
Of these, 85 percent were bites by stray dogs and 60 percent of human cases were in children and youth aged five to 18 years. A comparison with pre-2011 dog bite incidence data from the Idlib Medical Directorate was underway. Anecdotal information has indicated an increase of rabies cases among grazing animals.
† During 1960-2018, approximately 70% of 89 human rabies cases acquired in the United States were caused by exposures to bats (1). Although human rabies deaths in the United States are rare, rabid animals and rabies exposures are relatively common (2). Since 2014, all states except Hawaii have reported rabid bats.
A presumptive diagnosis of rabies was made, and they passed away with in 48 hours of hospitalization. Our case initially presented with agitation, high-grade fever, photophobia, and hydrophobia after a bite of a honey badger before four weeks. After 48 hours of hospitalization in the ICU, he passed away. No further tests were conducted.
Post-exposure prophylaxis (PEP) for rabies is widely administered and highly effective. Nevertheless, sporadic breakthrough infections (ie, rabies in people who have started PEP) have been reported. We conducted a systematic review of articles published between Jan 1, 1980 and June 1, 2022 to characterise breakthrough infections. After reviewing 3380 articles from across all continents, we ...
Notification of a suspect rabies case to the Rabies Hotline triggered an ... A. J., Reece, J. F., Shaw, A. P. M. & Thrusfield, M. V. An economic case study of the control of dog-mediated rabies by ...
This study was a retrospective hospital-based case record review of all patients admitted to SLH with a clinical diagnosis of rabies between January 1, 2006, and December 31, 2015. Data collection At SLH, physicians diagnose human rabies clinically based on a history of animal bite or non-bite exposure with hydrophobia and/or aerophobia, or ...
Creation Date: 20 October 2022. Categories. News Category. Case Study. Tags: Dog Vaccination Campaign, Education. The United Against Rabies Forum brings together governments, vaccine producers, researchers, NGOs and development partners to end human deaths from dog-mediated rabies.
The review reported only quantitative studies on rabies surveillance, prevention and control. ... Integrated bites case management/rabies disease surveillance, prevention and control: 31 32 37 44 98 99: Studies have shown the importance of coordinated surveillance, prevention and control in the eradication of rabies. ...
Background Rabies is a serious reemerging zoonosis in China. At present human rabies cases are primarily diagnosed based on clinical presentation. Case presentation In August 2012, a woman and her son were attacked by a stray dog in Henan, China. The son received rabies postexposure prophylaxis (wound treatment followed by vaccine, no immunoglobulin), however, the mother did not. Rabies ...
However, we have recently encountered a case of human rabies which arose through a rare transmission method, and we believe that lessons can and should be learnt from this incident. On June 22, 2014, a middle-aged male worker suffered a laceration to his right thumb from a cutter knife. The wound was 1.5 cm in length, and was accompanied by ...
Human Rabies. Cases of human rabies cases in the United States are rare, with only 1 to 3 cases reported annually. Twenty-five cases of human rabies have been reported in the United States in the past decade (2009-2018). Seven of these infections were acquired outside of the U.S. and its territories.
Author Summary Successful rabies control generates benefits in terms of improved human and animal health and well-being and safer environments. A key requirement of successful and sustainable rabies control is empowering policy makers to make decisions in an efficient manner; essential to this is the availability of evidence supporting the design and implementation of the most cost-effective ...
The standard protocol for a suspected rabid animal is for it to "be observed for fourteen (14) days or, in case of highly suspected rabies cases, be humanely euthanized with no damage to the ...
A PubMed search using keywords "novel surveillance" OR "integrated bite case management" AND "rabies" identified only eleven studies from four countries: Chad (10, 17), Haiti (4, 14, 16, 18-20), the Philippines (12, 21), and Tanzania . Although several IBCM programs have been implemented within the last decade, the approaches ...
The remaining 3 percent had a history of rabies vaccinations. In its most recent numbers, the Maine Centers for Disease Control reported 30 cases of rabies in the state in the first half of 2023 ...
Pretest: Rabies ― The Global Connection, Step 1. Rabies Case Studies/Fact or Fiction, Step 2. Rabies Webquest: An Internet Scavenger Hunt, Step 3. Rabies Educational Pamphlet, Step 5. Rabies Educational Pamphlet Rubric Step 5. The teacher should also make copies of Public Health Problem: Rabies in India assignment sheets, Step 4 for each ...
A major new study released by The Lancet Neurology shows that, in 2021, more than 3 billion people worldwide were living with a neurological condition. The World Health Organization (WHO) contributed to the analysis of the Global Burden of Disease, Injuries, and Risk Factor Study (GBD) 2021 data. Neurological conditions are now the leading cause of ill health and disability worldwide.
Rabies causes viral encephalitis which kills up to 70,000 people/year worldwide. Infected animal saliva transmits viral encephalitis to humans. Rabies is one of the oldest known diseases in history with cases dating back to 4000 years ago. For most of human history, a bite from a rabid animal was uniformly fatal. In the past, people were so scared of rabies that after being bitten by a ...