Enter search terms to find related medical topics, multimedia and more.

Advanced Search:
- Use “ “ for exact phrases.
- For example: “pediatric abdominal pain”
- Use – to remove results with certain keywords.
- For example: abdominal pain -pediatric
- Use OR to account for alternate keywords.
- For example: teenager OR adolescent
Neonatal Pneumonia
, MD, University of Rochester School of Medicine and Dentistry
- Symptoms and Signs
Chlamydial Pneumonia
Neonatal pneumonia is lung infection in a neonate. Onset may be within hours of birth and part of a generalized sepsis syndrome or after 7 days and confined to the lungs. Signs may be limited to respiratory distress or progress to shock and death. Diagnosis is by clinical and laboratory evaluation for sepsis. Treatment is initial broad-spectrum antibiotics changed to organism-specific drugs as soon as possible.
(See also Overview of Pneumonia Overview of Pneumonia Pneumonia is acute inflammation of the lungs caused by infection. Initial diagnosis is usually based on chest x-ray and clinical findings. Causes, symptoms, treatment, preventive measures, and... read more in adults and Overview of Neonatal Infections Overview of Neonatal Infections Neonatal infection can be acquired In utero transplacentally or through ruptured membranes In the birth canal during delivery (intrapartum) From external sources after birth (postpartum) Common... read more .)
Pneumonia is the most common invasive bacterial infection after primary sepsis. Early-onset pneumonia is part of generalized sepsis that first manifests at or within hours of birth ( see Neonatal Sepsis Neonatal Sepsis Neonatal sepsis is invasive infection, usually bacterial, occurring during the neonatal period. Signs are multiple, nonspecific, and include diminished spontaneous activity, less vigorous sucking... read more ). Late-onset pneumonia usually occurs after 7 days of age, most commonly in neonatal intensive care units among infants who require prolonged endotracheal intubation because of lung disease (called ventilator-associated pneumonia).
Etiology of Neonatal Pneumonia
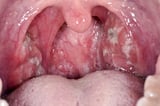
Symptoms and Signs of Neonatal Pneumonia
Late-onset hospital-acquired pneumonia manifests with unexplained worsening of the patient's respiratory status and increased quantities and a change in the quality of the respiratory secretions (eg, thick and brown). Infants may be acutely ill, with temperature instability and neutropenia.
Diagnosis of Neonatal Pneumonia
Chest x-ray
Evaluation includes chest x-ray, pulse oximetry, blood cultures, and Gram stain and culture of tracheal aspirate.
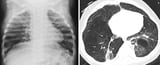
If Gram stain of tracheal aspirate shows a significant number of polymorphonuclear leukocytes and a single organism that is consistent with the one that grows from culture of the tracheal aspirate, the likelihood increases that this organism is the cause of the pneumonia. Because bacterial pneumonia in neonates may disseminate, a full evaluation for sepsis Diagnosis Neonatal sepsis is invasive infection, usually bacterial, occurring during the neonatal period. Signs are multiple, nonspecific, and include diminished spontaneous activity, less vigorous sucking... read more , including a lumbar puncture, should also be done. However, blood cultures are positive in only 2 to 5% of cases of hospital-acquired pneumonia.
Treatment of Neonatal Pneumonia
Usually vancomycin and a broad-spectrum beta-lactam drug
Treatment of Chlamydial Pneumonia
Erythromycin or azithromycin
The diagnosis of pneumonia secondary to Chlamydia trachomatis should prompt an evaluation of the mother and her partner because untreated maternal chlamydial infection may have complications such as pelvic inflammatory disease and sterility.

Was This Page Helpful?

Test your knowledge
Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada) — dedicated to using leading-edge science to save and improve lives around the world. Learn more about the MSD Manuals and our commitment to Global Medical Knowledge.
- Permissions
- Cookie Settings
- Terms of use
- Veterinary Manual
- IN THIS TOPIC
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
The epidemiology, microbiology, clinical manifestations, diagnosis, and treatment of neonatal pneumonia are reviewed here. Neonatal sepsis is discussed separately:
● (See "Clinical features, evaluation, and diagnosis of sepsis in term and late preterm neonates" .)
● (See "Management and outcome of sepsis in term and late preterm neonates" .)
● (See "Clinical features and diagnosis of bacterial sepsis in preterm infants <34 weeks gestation" .)
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 12 July 2023
Short-course antibiotic therapy for pneumonia in the neonatal intensive care unit
- Zachery S. Lewald ORCID: orcid.org/0009-0006-2711-7841 1 , 2 , 3 , 4 ,
- Pavel Prusakov ORCID: orcid.org/0000-0001-5787-5623 5 ,
- Jacqueline K. Magers ORCID: orcid.org/0000-0001-8422-8157 5 ,
- Matthew J. Kielt ORCID: orcid.org/0000-0002-4568-9070 2 , 3 ,
- Concepción de Alba Romero ORCID: orcid.org/0000-0003-0794-2466 2 , 3 , 6 ,
- Natalie O. White 7 ,
- Randy R. Miller ORCID: orcid.org/0000-0003-1524-6129 7 ,
- Richard Moraille 8 ,
- Anthony R. Theile 8 ,
- Pablo J. Sánchez ORCID: orcid.org/0000-0003-2437-1247 2 , 3 , 4 &
Nationwide Children’s Hospital Neonatal Antimicrobial Stewardship Program (NEO-ASP)
Journal of Perinatology volume 43 , pages 1145–1151 ( 2023 ) Cite this article
493 Accesses
2 Citations
14 Altmetric
Metrics details
- Outcomes research
- Respiratory tract diseases
To determine the adherence and safety outcomes of a 5-day antibiotic course with a “time-out” for treatment of “blood culture-negative” pneumonia in the NICU.
Study design
Prospective surveillance of all infants diagnosed with pneumonia at 7 NICUs from 8/2020-12/2021. Safety outcomes were defined a priori by re-initiation of antibiotic therapy within 14 days after discontinuation and overall and sepsis-related mortality.
128 infants were diagnosed with 136 episodes of pneumonia; 88% ( n = 119) were treated with 5 days of definitive antibiotic therapy. Antibiotics were restarted within 14 days in 22 (16%) of the 136 pneumonia episodes. However, only 3 (3%) of the 119 episodes of pneumonia treated for 5 days had antibiotics restarted for pneumonia. Mortality was 5% (7/128); 5 of the 7 deaths were assessed as sepsis-related.
Adherence to the 5-day definitive antibiotic treatment for “culture-negative” pneumonia was high and the intervention seemed safe.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Data availability
The de-identified dataset generated from the study is available from the corresponding author on reasonable request and following approval by the Institutional Review Board of Nationwide Children’s Hospital.
Prusakov P, Goff DA, Wozniak PS, Cassim A, Scipion CEA, Urzua S, et al. A global point prevalence survey of antimicrobial use in neonatal intensive care units: the no-more-antibiotics and resistance (NO-MAS-R) study. EClinicalMedicine. 2021;32:100727.
Article PubMed Central PubMed Google Scholar
Cantey JB, Wozniak PS, Sanchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatr Infect Dis J. 2015;34:267–72.
Article PubMed Google Scholar
Cantey JB, Pyle AK, Wozniak PS, Hynan LS, Sanchez PJ. Early antibiotic exposure and adverse outcomes in preterm, very low birth weight infants. J Pediatr. 2018;203:62–7.
Article CAS PubMed Google Scholar
Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66.
Ting JY, Roberts A, Sherlock R, Ojah C, Cieslak Z, Dunn M, et al. Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics. 2019;143:e20182286.
Ting JY, Synnes A, Roberts A, Deshpandey A, Dow K, Yoon EW, et al. Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr. 2016;170:1181–7.
Ting JY, Synnes A, Roberts A, Deshpandey AC, Dow K, Yang J, et al. Association of antibiotic utilization and neurodevelopmental outcomes among extremely low gestational age neonates without proven sepsis or necrotizing enterocolitis. Am J Perinatol. 2018;35:972–8.
Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–5.
Article CAS PubMed Central PubMed Google Scholar
Cantey JB, Huffman LW, Subramanian A, Marshall AS, Ballard AR, Lefevre C, et al. Antibiotic exposure and risk for death or bronchopulmonary dysplasia in very low birth weight infants. J Pediatr. 2017;181:289–93.e1.
Novitsky A, Tuttle D, Locke RG, Saiman L, Mackley A, Paul DA. Prolonged early antibiotic use and bronchopulmonary dysplasia in very low birth weight infants. Am J Perinatol. 2015;32:43–8.
Ong MS, Umetsu DT, Mandl KD. Consequences of antibiotics and infections in infancy: bugs, drugs, and wheezing. Ann Allergy Asthma Immunol. 2014;112:441–5.e1.
Nguyen LH, Ortqvist AK, Cao Y, Simon TG, Roelstraete B, Song M, et al. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol Hepatol. 2020;5:986–95.
Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063–9.
Mohamed TH, Abdi HH, Magers J, Prusakov P, Slaughter JL. Nephrotoxic medications and associated acute kidney injury in hospitalized neonates. J Nephrol. 2022;35:1679–87.
Bhattacharyya S, Darby RR, Raibagkar P, Gonzalez Castro LN, Berkowitz AL. Antibiotic-associated encephalopathy. Neurology. 2016;86:963–71.
Sutter R, Ruegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85:1332–41.
Cantey JB, Wozniak PS, Pruszynski JE, Sanchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis. 2016;16:1178–84.
Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67.
Kuitunen I, Jaaskelainen J, Korppi M, Renko M. Antibiotic treatment duration for community-acquired pneumonia in outpatient children in high-income countries-a systematic review and meta-analysis. Clin Infect Dis. 2023;76:e1123–e8.
American Academy of Pediatrics Committee on F. Newborn. Levels of neonatal care. Pediatrics. 2012;130:587–97.
Article Google Scholar
Magers J, Prusakov P, Speaks S, Conroy S, Sanchez PJ. Safety and efficacy of nafcillin for empiric therapy of late-onset sepsis in the NICU. Pediatrics. 2022;149:e2021052360.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. an evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9.
Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300–8.
Spellberg B. The new antibiotic mantra-"shorter is better". JAMA Intern Med. 2016;176:1254–5.
Pernica JM, Harman S, Kam AJ, Carciumaru R, Vanniyasingam T, Crawford T, et al. Short-course antimicrobial therapy for pediatric community-acquired pneumonia: the SAFER randomized clinical trial. JAMA Pediatr. 2021;175:475–82.
Uranga A, Espana PP, Bilbao A, Quintana JM, Arriaga I, Intxausti M, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176:1257–65.
Metersky ML, Klompas M, Kalil AC. Less is more: a 7-day course of antibiotics is the evidence-based treatment for pseudomonas aeruginosa ventilator-associated pneumonia. Clin Infect Dis. 2023;76:750–2.
Albin OR, Kaye KS, McCreary EK, Pogue JM. Less is more? Antibiotic treatment duration in pseudomonas aeruginosa ventilator-associated pneumonia. Clin Infect Dis. 2023;76:745–9.
Engle WD, Jackson GL, Sendelbach D, Ford D, Olesen B, Burton KM, et al. Neonatal pneumonia: comparison of 4 vs 7 days of antibiotic therapy in term and near-term infants. J Perinatol. 2000;20:421–6.
Engle WD, Jackson GL, Sendelbach DM, Stehel EK, Ford DM, McHugh KM, et al. Pneumonia in term neonates: laboratory studies and duration of antibiotic therapy. J Perinatol. 2003;23:372–7.
Goerens A, Lehnick D, Buttcher M, Daetwyler K, Fontana M, Genet P, et al. Neonatal ventilator associated pneumonia: a quality improvement initiative focusing on antimicrobial stewardship. Front Pediatr. 2018;6:262.
Araujo da Silva AR, Marques A, Di Biase C, Faitanin M, Murni I, Dramowski A, et al. Effectiveness of antimicrobial stewardship programmes in neonatology: a systematic review. Arch Dis Child. 2020;105:563–8.
Graus JM, Herbozo C, Hernandez R, Pantoja AF, Zegarra J. Managing antibiotics wisely in a neonatal intensive care unit in a low resource setting. J Perinatol. 2022;42:965–70.
Download references
Acknowledgements
The study was presented in part as poster presentations at the Pediatric Academic Societies Meeting, Denver, CO on 4/25/2022, Midwest Society for Pediatric Research, Chicago, IL on 9/29/2022, and St. Jude/PIDS Pediatric Infectious Diseases Research Conference, Memphis, TN, on 3/2/2023.
ZSL received a Pediatric Infectious Diseases Society Summer Research Scholar Award (SUMMERS) for his work on this study.
Author information
A full list of members and their affiliations appears in the Supplementary Information.
Authors and Affiliations
The Ohio State University, Columbus, OH, USA
Zachery S. Lewald
Department of Pediatrics, Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
Zachery S. Lewald, Matthew J. Kielt, Concepción de Alba Romero & Pablo J. Sánchez
Division of Neonatology, Nationwide Children’s Hospital, Center for Perinatal Research, Abigail Wexner Research Institute at Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
Division of Pediatric Infectious Diseases, Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
Zachery S. Lewald & Pablo J. Sánchez
Department of Pharmacy, Nationwide Children’s Hospital, Columbus, OH, USA
Pavel Prusakov, Jacqueline K. Magers, Caitlyn Schwirian, Wai-Yin Mandy Tam & Alexandra F. Burton
Division of Neonatology, Hospital 12 de Octubre, Madrid, Spain
Concepción de Alba Romero
Pediatrix Medical Group of Ohio, Columbus, OH, USA
Natalie O. White, Randy R. Miller & Maclain J. Magee
Central Ohio Newborn Medicine, Columbus, OH, USA
Richard Moraille & Anthony R. Theile
Department of Pediatrics, Division of Neonatology, Nationwide Children’s Hospital, Center for Perinatal Research, Abigail Wexner Research Institute at Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
Pablo J. Sánchez, Alexandra K. Medoro, Maria Jebbia & Roopali Bapat
Department of Pediatrics, Division of Pediatric Infectious Diseases, Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
Pablo J. Sánchez, Alexandra K. Medoro, Joshua R. Watson & Katia C. Halabi
Department of Neonatal Nurse Practitioners, Nationwide Children’s Hospital, The Ohio State Wexner Medical Center, Columbus, OH, USA
Melinda Albertson & Tommy Nathaniel Johnson-Roddenberry
Department of Clinical Outcomes, Nationwide Children’s Hospital, Columbus, OH, USA
Malak Abdel-Hadi
You can also search for this author in PubMed Google Scholar
- Pablo J. Sánchez
- , Jacqueline K. Magers
- , Pavel Prusakov
- , Natalie O. White
- , Randy R. Miller
- , Richard Moraille
- , Anthony R. Theile
- , Alexandra K. Medoro
- , Joshua R. Watson
- , Melinda Albertson
- , Caitlyn Schwirian
- , Wai-Yin Mandy Tam
- , Alexandra F. Burton
- , Tommy Nathaniel Johnson-Roddenberry
- , Maria Jebbia
- , Maclain J. Magee
- , Katia C. Halabi
- , Malak Abdel-Hadi
- & Roopali Bapat
Contributions
ZSL collected and analyzed the data, assisted with the analyses, wrote the first draft of the manuscript, and reviewed and revised the manuscript. PP conceptualized and designed the study, designed the data collection instruments, collected data, assisted with the analyses, and reviewed and revised the manuscript. JKM conceptualized and designed the study, collected data, and reviewed and revised the manuscript. MJK assisted with the analyses, reviewed and revised the manuscript. CdAR collected and helped to analyze the data, reviewed and revised the manuscript, and approved the final manuscript as submitted. NOW conceptualized and designed the study, reviewed and revised the manuscript. RRM conceptualized and designed the study, reviewed and revised the manuscript. RM conceptualized and designed the study, reviewed and revised the manuscript. ART conceptualized and designed the study, reviewed and revised the manuscript. PJS conceptualized and designed the study, coordinated and supervised data collection, analyzed the data, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Correspondence to Pablo J. Sánchez .
Ethics declarations
Competing interests.
PP received grant funding from Merck & Co. unrelated to this study. Other authors have no conflicts of interest relevant to this article to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Members of the nationwide children’s hospital neonatal antimicrobial stewardship program (neo-asp), rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Lewald, Z.S., Prusakov, P., Magers, J.K. et al. Short-course antibiotic therapy for pneumonia in the neonatal intensive care unit. J Perinatol 43 , 1145–1151 (2023). https://doi.org/10.1038/s41372-023-01720-6
Download citation
Received : 22 April 2023
Revised : 25 June 2023
Accepted : 04 July 2023
Published : 12 July 2023
Issue Date : September 2023
DOI : https://doi.org/10.1038/s41372-023-01720-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Volume 11 Supplement 3
Technical inputs, enhancements and applications of the Lives Saved Tool (LiST)
- Open access
- Published: 13 April 2011
Effect of case management on neonatal mortality due to sepsis and pneumonia
- Anita K M Zaidi 1 ,
- Hammad A Ganatra 1 ,
- Sana Syed 1 ,
- Simon Cousens 2 ,
- Anne CC Lee 3 ,
- Robert Black 3 ,
- Zulfiqar A Bhutta 1 &
- Joy E Lawn 4
BMC Public Health volume 11 , Article number: S13 ( 2011 ) Cite this article
15k Accesses
59 Citations
1 Altmetric
Metrics details
Each year almost one million newborns die from infections, mostly in low-income countries. Timely case management would save many lives but the relative mortality effect of varying strategies is unknown. We have estimated the effect of providing oral, or injectable antibiotics at home or in first-level facilities, and of in-patient hospital care on neonatal mortality from pneumonia and sepsis for use in the Lives Saved Tool (LiST).
We conducted systematic searches of multiple databases to identify relevant studies with mortality data. Standardized abstraction tables were used and study quality assessed by adapted GRADE criteria. Meta-analyses were undertaken where appropriate. For interventions with biological plausibility but low quality evidence, a Delphi process was undertaken to estimate effectiveness.
Searches of 2876 titles identified 7 studies. Among these, 4 evaluated oral antibiotics for neonatal pneumonia in non-randomised, concurrently controlled designs. Meta-analysis suggested reductions in all-cause neonatal mortality (RR 0.75 95% CI 0.64- 0.89; 4 studies) and neonatal pneumonia-specific mortality (RR 0.58 95% CI 0.41- 0.82; 3 studies). Two studies (1 RCT, 1 observational study), evaluated community-based neonatal care packages including injectable antibiotics and reported mortality reductions of 44% (RR= 0.56, 95% CI 0.41-0.77) and 34% (RR =0.66, 95% CI 0.47-0.93), but the interpretation of these results is complicated by co-interventions. A third, clinic-based, study reported a case-fatality ratio of 3.3% among neonates treated with injectable antibiotics as outpatients. No studies were identified evaluating injectable antibiotics alone for neonatal pneumonia. Delphi consensus (median from 20 respondents) effects on sepsis-specific mortality were 30% reduction for oral antibiotics, 65% for injectable antibiotics and 75% for injectable antibiotics on pneumonia-specific mortality. No trials were identified assessing effect of hospital management for neonatal infections and Delphi consensus suggested 80%, and 90% reductions for sepsis and pneumonia-specific mortality respectively.
Oral antibiotics administered in the community are effective for neonatal pneumonia mortality reduction based on a meta-analysis, but expert opinion suggests much higher impact from injectable antibiotics in the community or primary care level and even higher for facility-based care. Despite feasibility and low cost, these interventions are not widely available in many low income countries.
This work was supported by the Bill & Melinda Gates Foundation through a grant to the US Fund for UNICEF, and to Saving Newborn Lives Save the Children, through Save the Children US.
Deaths occurring in the neonatal period each year account for 41% (3.6 million) of all deaths in children under 5 years [ 1 ]. The majority of these deaths occur in low income countries and almost 1 million of these deaths are attributable to infectious causes including neonatal sepsis, meningitis and pneumonia [ 1 ]. These deaths occur because of lack of preventive care (clean birth care, breastfeeding) and appropriate case management [ 2 ]. Delays in treating neonatal infections of even a few hours may be fatal. Delays in illness recognition and care seeking, a dearth of primary health care providers, and limited access to facility care contribute to these deaths [ 3 ]. Recent trials have demonstrated the effect of community-based packages for prevention and treatment of neonatal bacterial infections, with the potential to save many lives [ 4 , 5 ].
Therapy with appropriate antibiotics and supportive management in neonatal nurseries is the cornerstone of management of neonatal sepsis and pneumonia, with strong biological plausibility that such therapy saves lives. Yet the quality of evidence is understandably affected by the ethical impossibility of undertaking randomized trials of antibiotic management compared with no antibiotic management. Nevertheless, given the limited access to care for sick neonates in low income countries, it is important to assess the potential mortality effect of oral antibiotics and injectable antibiotics delivered in domiciliary or primary care settings. Case management for hospitalized neonates is more expensive, but to guide policy and program investments we also need to know how much more effective it is compared to care delivered at home or in primary care settings.
The objective of this review is to provide estimates of the effectiveness of three interventions in preventing neonatal deaths from severe infection: (i) case management with oral antibiotic therapy alone for pneumonia and sepsis; (ii) case management with injectable antibiotics (± oral antibiotics) as an outpatient or at home for neonatal sepsis /meningitis and pneumonia; and (iii) hospital-based case management, including injectable antibiotics, intravenous fluids, oxygen therapy, second line injectable antibiotics if needed, and other supportive therapy (Table 1 ). These mortality effect estimates are used in the Lives Saved Tool (LiST) software, a user-friendly tool that estimates the number of lives saved by scaling up key interventions and helps in child survival planning in low income countries [ 6 , 7 ].
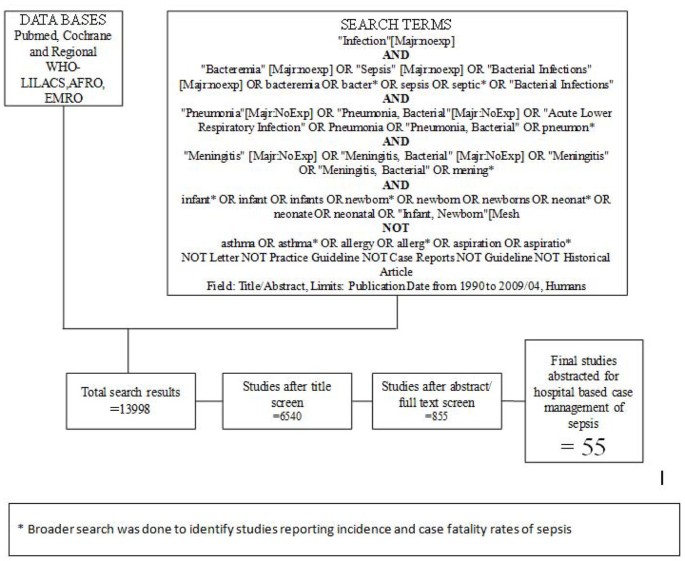
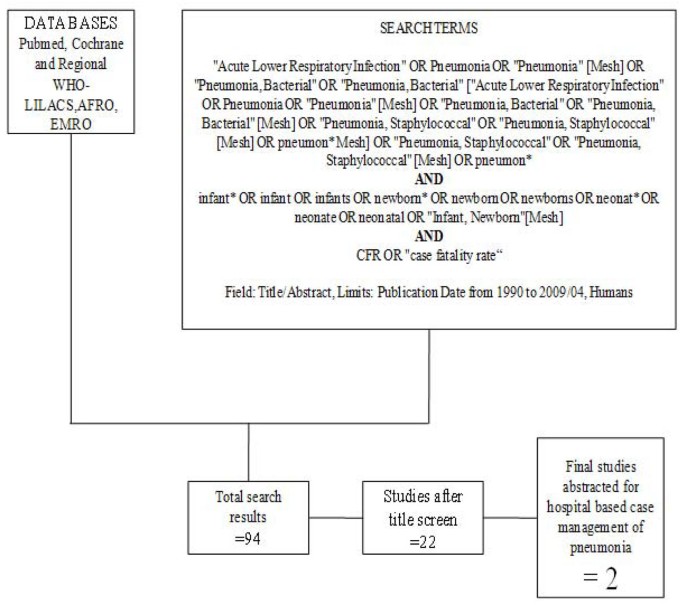
We searched all published literature as per CHERG systematic review guidelines[ 7 ]. Databases searched were PubMed, Cochrane Libraries and WHO regional databases from 1990 until April 2009 and included publications in any language (Figure 1 ). Search terms included various combinations of: sepsis, meningitis and pneumonia. For sepsis and pneumonia management at a hospital level we conducted two parallel searches (Figures 2 and 3 ). These were broader as we also wanted to identify studies reporting incidence and case fatality ratios (CFR) for a related study on global burden of neonatal sepsis. Titles and abstracts were reviewed and studies were included if data on one of the following outcomes was provided: all-cause mortality, sepsis/meningitis/pneumonia mortality and/or CFR. Furthermore, extensive efforts were made to contact investigators and program managers for unpublished data.
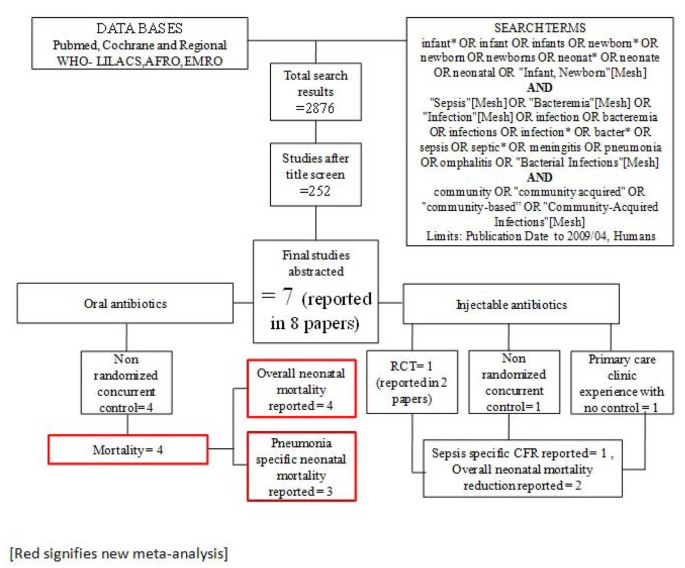
Searches and screening for community based management of sepsis and pneumonia.
Searches and screening for hospital management of sepsis
Searches and screening for hospital management of pneumonia.
Inclusion/exclusion criteria, abstraction
We reviewed all available observational studies, randomized controlled trials, systematic reviews, and meta-analyses, which included neonates and principally involved the management of serious neonatal infections. The search was limited to “humans”. We examined studies published from 1990 until April 2009.
We included randomized controlled trials, studies with concurrent controls, and observational studies with no control group if mortality outcomes were reported. All studies meeting final inclusion criteria were double data abstracted into a standardized form. We abstracted key variables with regard to the study identifiers and context, study design and limitations, intervention specifics, and mortality outcomes. We assessed the quality of each of these studies using a standard table employing an adapted version of GRADE[ 8 ] developed by the Child Health Epidemiology Reference Group (CHERG) [ 7 ]. For studies which reported mortality outcomes that were not neonatal specific, we contacted the authors to request the neonatal-specific data. All studies that were coded are included in Additional File 1 .
Case definition of sepsis
During our review of selected studies we were unable to find a standard definition for clinical neonatal sepsis or pneumonia (Table 2 ). Each study used different criteria although most are a variation on WHO IMCI approach. We therefore decided to accept authors’ definitions of sepsis and pneumonia, recognizing that these non-specific definitions lower mortality outcome estimates as many “non-sepsis” cases are included in an effort to maximize sensitivity.
Analyses and summary measures
All studies reporting mortality data for pneumonia and sepsis management, in community and hospital settings, were summarized according to the overall quality of evidence for each outcome and each data input type using an adapted version of the GRADE 21 protocol table [ 7 ]. When appropriate, we conducted meta-analyses to obtain pooled estimates of the risk ratios, using either the Mantel-Haenzsel or, when there was evidence of heterogeneity, the DerSimonian-Laird random effects estimator. 95% confidence intervals (CI) were also calculated. Statistical analyses were performed using STATA 10.0 ( http://www.stata.com ).
Delphi Process for Establishing Expert Consensus
For intervention-outcome combinations for which we did not identify moderate quality evidence, we sought expert consensus via the Delphi method. Individuals invited to participate were experts in newborn health and sepsis representing six WHO regions (South Asia, Africa, Western Europe, Eastern Europe, North America, Australia), and including multiple disciplines - international health, pediatric infectious diseases, clinical neonatology, and general pediatrics. Twenty (of twenty-three experts invited) agreed to participate in the Delphi process. The questionnaire was developed by JL, AZ, SC and SS, and refined after several rounds of pilot testing. The questionnaire was sent by email and included the background and aims of the Delphi and estimates of effect that were available from the literature for different scenarios. The median response and range were determined for each question. Consensus was defined a priori as an interquartile range in responses of not more than 30% for each question. For those estimates not reaching consensus, the plan was for results to be electronically distributed to the panel, virtual discussion allowed, and a second round of email questionnaires sent. However, consensus was achieved after one round of questionnaires and subsequent rounds were not necessary.
Studies identified
Our systematic searches for community management of sepsis and pneumonia identified 2876 titles (Figure 1 ) and after screening of titles, abstracts and relevant full texts, we located 7 studies of interest (reported in 8 papers) [ 9 – 16 ]. We identified 4 non randomised concurrently controlled studies, which evaluated oral antibiotics for pneumonia (Table 5 ) [ 10 , 14 – 16 ]. Three of these studies did not report disaggregated neonatal outcomes in the primary papers, but neonatal outcomes were available through abstracted forms from an earlier meta-analysis by Sazawal et al [ 17 ]. For management of neonatal sepsis using injectable antibiotics, we located 3 studies (reported in 4 papers) [ 9 , 11 – 13 ]. There was one observational primary clinic-based study without a control group [ 13 ], one RCT [ 12 ] and one non-randomised, concurrently controlled study [ 9 ]. The fourth paper reported observational data from individual infants evaluated during the RCT mentioned above and was not a separate study [ 11 ]. All the studies were from high neonatal mortality regions.
In our search for hospital-based studies of sepsis we found 55 studies from a total pool of 13998 studies which reported sepsis and/or meningitis mortality outcomes (Figure 2 ) [ 18 – 70 ]. For pneumonia, we found two studies from a total pool of 94 studies (Figure 3 ) [ 71 , 72 ].
The details of each study and quality assessment using GRADE are summarised in Tables 3 , 4 , 5 , and 6 .
Evidence for effectiveness of oral antibiotic therapy alone
Unpublished neonatal data were obtained from the principal investigators of the four studies identified and a new meta-analysis was done to update that of Sazawal et al[ 17 ]. We performed meta analyses for two outcomes: oral antibiotics were associated with reductions in both all-cause mortality (4 studies [ 10 , 14 – 16 ]: RR 0.75 95% CI 0.64- 0.89) (Figure 4 ) and pneumonia-specific mortality (3 studies [ 10 , 15 , 16 ]: RR 0.58 95% CI 0.41- 0.82) (Figure 5 ). Limitations included non-randomization, estimation of intervention coverage as precise coverage estimates were not available;and variability between studies of the intensity of co-interventions. We found no studies of the effect of oral antibiotics on sepsis-specific mortality. The Delphi consensus (median) was for a 28% reduction in sepsis-specific mortality with an interquartile range of 20% to 36.25% (Figure 6 ).
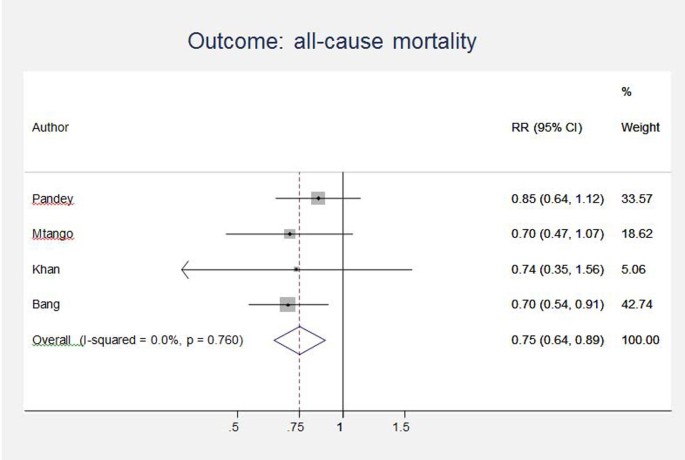
Meta-analysis of observational studies comparing oral antibiotics versus none in the community setting for babies: All cause mortality. Legend: Heterogeneity chi-squared = 1.17 (d.f. = 3) p = 0.760 I-squared (variation in RR attributable to heterogeneity) = 0.0% Test of RR=1 : z= 3.32 p = 0.001
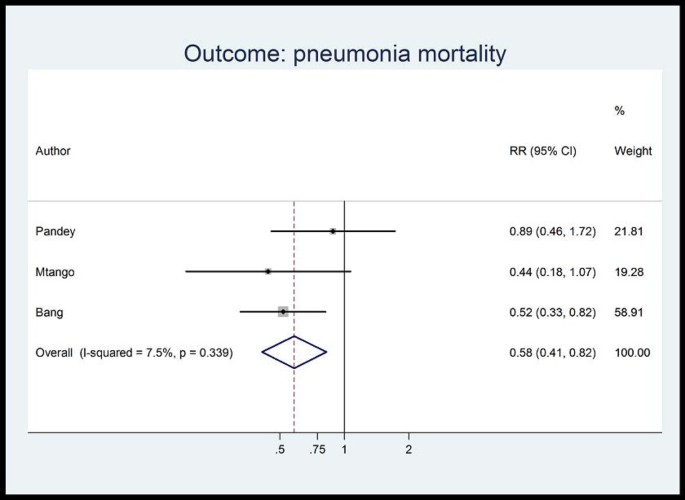
Meta-analysis of observational studies comparing oral antibiotics versus none in the community setting for babies: Pneumonia mortality. Legend: Heterogeneity chi-squared = 2.16 (d.f. = 2) p = 0.339 I-squared (variation in RR attributable to heterogeneity) = 7.5% Test of RR=1: z= 3.06 p = 0.002
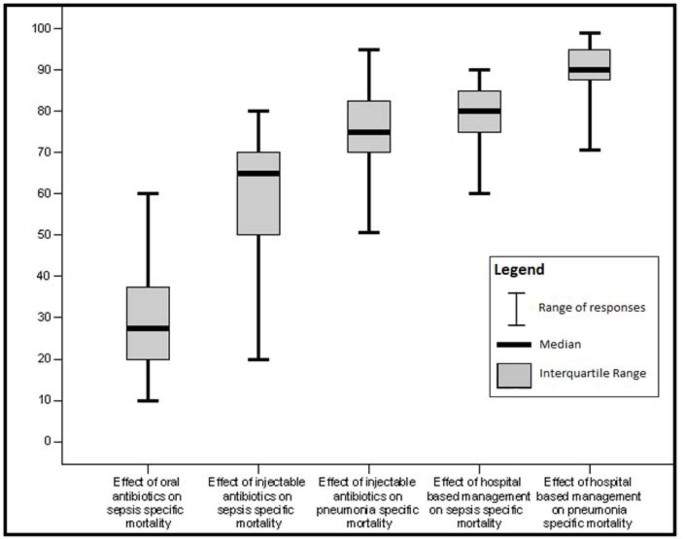
Box plot of Delphi expert opinion estimates of reduction in neonatal cause specific mortality due to pneumonia and sepsis/meningitis
Evidence for effectiveness of injectable antibiotic therapy (±oral antibiotics)
Three studies reported in four papers, were identified (Table 6 ) [ 9 , 11 – 13 ]. One, an RCT[ 12 ] evaluated the impact of a perinatal care package which included the administration of injectable antibiotics in domiciliary settings in situations where referral to hospital was not possible. This trial reported a reduction in all-cause neonatal mortality of 34% (RR=0.66, 95%CI 0.47-0.93). A second paper from the same study reported that the CFR for neonates who were evaluated and actually treated with injectable antibiotics was 4.4% [ 11 ]. A non-randomized, concurrently controlled study [ 9 ] also evaluated the impact of a home-based neonatal care package in which septic neonates were treated with injectable antibiotics when referral to hospital was not possible. The overall mortality reduction in the intervention arm of the trial was calculated to be 44% (RR=0.56 95% CI 0.41-0.77). A third, uncontrolled study [ 13 ] based in a primary care clinic reported a CFR of 3.3% among septic children treated with injectable antibiotics.
In both of the community-based studies [ 9 , 12 ] injectable antibiotics were only one component of comprehensive community-based newborn care packages, and therefore the effectiveness of injectable antibiotics alone in the community cannot be reliably estimated. The Delphi consensus for the effect of injectable antibiotics was for a 65% reduction (interquartile range of 50-70%) in sepsis-specific mortality and 75% reduction (interquartile range of 70-81.25%) in pneumonia-specific mortality in community-based settings (Figure 6 ).
Evidence for effectiveness of inpatient hospital case management
We found no trials assessing the impact of hospital-based case management and the observational studies of hospital management showed wide variation in effect. Searches conducted for studies reporting CFRs in neonates with pneumonia in health facilities revealed very few data. Two studies were identified with author-defined neonatal pneumonia; both were from low income, non-industrialised settings and reported CFRs of 14.4% [ 72 ] and 30.8% [ 71 ].
CFRs for neonatal sepsis, adjusted for the proportion of very low birth weight babies in the study, were plotted against national percentage skilled delivery, as a proxy for access to hospital-based case management of neonatal sepsis. In countries with a high proportion of births attended by skilled attendants, the predicted CFR for sepsis was 9.5%, whereas in countries with a low proportion (<30%) skilled birth attendance, the predicted CFR for sepsis with hospital care is 20-30% (Figure 7 ). A 68% reduction in the CFR for neonatal sepsis is predicted as one moves from 0% to100% skilled birth attendance. This reduction is likely to under estimate the effect of hospital-based case management since skilled birth attendance is likely to be a poor surrogate for effective facility case management of neonatal infections, but was used in the absence of coverage data for case management
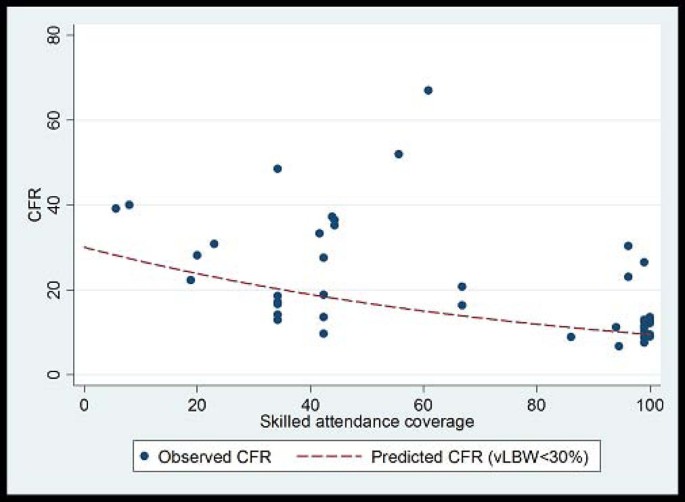
Plot of neonatal sepsis CFR versus percent skilled delivery as a marker of access to facility care. Model fitted: outcome = log(CFR) Covariates = Skilled attendant coverage and % babies vLBW Fitted line is predicted CFR for settings with % VLBW<30%. Predicted CFR at 0% skilled attendance is 30%. Predicted CFR at 100% skilled attendance is 9.5%. % reduction = 68.5% Coefficient skilled attendance is 0.12 on the log scale (95% CI -0.02 to -0.007); i.e. for each 1% increase in skilled attendance rate CFR is reduced by 1.1% (95% CI: 0.7% to 1.6%)
Although the quality of evidence is low according to GRADE criteria, the recommendation for case management of neonatal infections is strong, and this is standard practice globally. Table 7 provides a summary of the effect of case management on neonatal sepsis and pneumonia cause specific mortality, and GRADE of the estimate. Therefore the Delphi process was used to provide estimates for the effect of hospital care. The Delphi consensus was for a 80% reduction in sepsis-specific mortality (interquartile range 75% to 85%), and a 90% reduction in pneumonia-specific mortality (interquartile range 88.75% to 95%) (Figure 6 ).
Infections including sepsis, meningitis and pneumonia are responsible for almost a million neonatal deaths annually. Neonates are more susceptible to severe infections and the progression of disease is more rapid due to developmental immunodeficiency, resulting in high CFRs. Also, a significant proportion of infections may arise early, after vertical transmission from the mother [ 73 ]. Therefore, timely identification and appropriate management with antibiotics is an important strategy to reduce the burden of neonatal mortality due to infections. We have previously reported the evidence from observational and experimental studies in low income countries for community-based management of neonatal infections (pneumonia and sepsis) with oral and injectable antibiotics [ 74 – 76 ]. We have now undertaken a systematic review of available evidence, including from industrialized countries and facility settings, and where the quality of evidence is low we have undertaken a Delphi expert process to estimate the cause-specific mortality effect.
This review of effectiveness of the interventions is shaped in large part by the needs of the LiST model. In that model, increasing coverage of an intervention results in a reduction in deaths due to one or more specific causes or in reduction of a risk factor. Therefore the reviews and the GRADE process used were designed to develop estimates of the effect of an intervention in reducing death due to specific causes. For more details of the review methods, the adapted GRADE approach or the LiST model see related publications [ 6 , 7 ].
To our knowledge, this is the first review providing effectiveness estimates for case management options to reduce neonatal deaths due to neonatal sepsis/meningitis and pneumonia, in both community and facility settings. Theodoratou et al have previously estimated effectiveness of pneumonia case management in children under 5 years but they did not disaggregate neonatal mortality data from later child mortality [ 77 ]. The estimated effect of community case management on pneumonia mortality in children under 5 years of age in the analysis by Theodoratou et al is 70% (77). Oral antibiotics in community settings for neonatal pneumonia in our analysis were associated with a 42% reduction in pneumonia-specific mortality and a 25% reduction in all-cause neonatal mortality based on a meta-analysis of available trials. There is no evidence to estimate the effect of oral antibiotics on sepsis-specific mortality, but our Delphi process suggested a 28% reduction. Delphi-derived estimates for the effects of management using injectable antibiotics delivered in home or primary care settings came out at 65% for sepsis-specific mortality and 75% for pneumonia-specific mortality. These estimates are biologically plausible and consistent with published studies [ 9 , 12 ] which reported reductions in all-cause neonatal mortality (sepsis plus other causes) of 34% and 44% respectively with community-based packages including injectable antibiotics. CFRs reported from observational studies of hospital case management varied widely, from 6.7 to 67%. Our Delphi estimates suggested an 80% mortality reduction in sepsis deaths and a 90% reduction in pneumonia deaths with hospital case management.
There were 4 effectiveness trials assessing the impact of oral antibiotics on pneumonia-specific mortality in the community. Only one of these studies was randomized and the programmatic coverage of the intervention had to be estimated as coverage data were not routinely assessed or reported. The selection and intensity of co-interventions was not uniform between the studies. An additional limitation was the lack of clearly defined cause-of-death definitions by the authors. However, the effect sizes were remarkably consistent with each other, and therefore the evidence level was upgraded to moderate.
GRADE guidelines rank the evidence relating to the effect of injectable antibiotics on sepsis-specific mortality as low quality. The 3 studies identified were not uniform with respect to study designs; one was an effectiveness RCT, one was a non-randomized concurrent trial and the third was an observational study describing the experience from primary care clinic without a control group. Both the RCT [ 12 ] and the non-randomized concurrent trial[ 9 ], involved concurrent co-interventions alongside the administration of injectable antibiotics. This made it impossible to assess the impact of injectable antibiotics alone on sepsis mortality. Neither study reported the change in the sepsis-specific mortality rate in the intervention arm compared to control arm, and reported the impact on all-cause neonatal mortality only. The absence of randomization in one of the trials is a further limitation [ 9 ]. The main limitation to the observational study in a primary care clinic [ 13 ] was the absence of a control arm in the study.
We identified no controlled trials assessing the effect of hospital-based case management of neonatal infections. Such studies would be difficult or impossible to implement in an ethical fashion. Thus studies were limited to reporting CFRs for neonatal sepsis and meningitis. The studies were from varied settings, from both industrialized and low income countries, and reported widely varying CFRs. Only 2 of these observational studies reported CFRs for pneumonia. One of these studies reported a very high CFR for pneumonia due, we believe, to the high proportion of LBW babies in the sample (60%).
We found some moderate quality evidence for intervention packages including antibiotics in community settings but ironically data are most lacking at facility level, and district hospital level is a critical gap [ 78 ]. Unlike the LiST review on neonatal resuscitation which identified several before-after studies of facility based neonatal resuscitation reporting mortality data, we were unable to find similar before-after studies on the effect of hospital-based case management of sepsis/meningitis/pneumonia. An understandable reason for this might be the ethical constraints precluding such studies. However, historical reviews from the pre-antibiotic era provide an insight into the CFR associated with untreated sepsis in facility settings. The best available evidence comes from the series of papers from Yale Medical Center reporting time trends for neonatal sepsis. These data show that in the 1920s and 1930s the CFR for blood culture confirmed sepsis stood at 90% [ 79 , 80 ]. With the introduction of antibiotics, the CFR decreased to 45% by 1965 [ 81 ], and with the subsequent introduction of intensive care units and advanced life support it came down to 16% by 1988[ 82 ], and 3% by 2003[ 83 ]. Such data highlight the effectiveness of hospital-based management in preventing neonatal mortality from sepsis.
As evident from our results, even oral or injectable antibiotics alone are highly effective in reducing deaths from neonatal sepsis or pneumonia. These interventions hold great potential to reduce the 1 million neonatal deaths each year. If substantial reduction in neonatal mortality is desired, both, community and facility-based interventions are required, linked by functioning referral systems, giving the potential to prevent hundreds of thousands of avoidable newborn deaths every year.
This work was supported in part by a grant to the US Fund for UNICEF for Child Health Epidemiology Reference Group from the Bill &Melinda Gates Foundation (grant 43386) to “Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries”, and by a grant to Save The Children USA from the Bill & Melinda Gates Foundation (Grant 50124) for "Saving Newborn Lives".
Abbreviations
Case Fatality Rate
Child Health Epidemiology Reference Group
Integrated Management of Childhood Illnesses
Lives Saved Tool
Randomized Controlled Trial.
Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, et al: Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010, 375 (9730): 1969-1987. 10.1016/S0140-6736(10)60549-1.
PubMed Google Scholar
Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L: Evidence-based, cost-effective interventions: how many newborn babies can we save?. Lancet. 2005, 365 (9463): 977-988. 10.1016/S0140-6736(05)71088-6.
Bhutta Z, Ali N, Hyder A, Wajid A: Perinatal and newborn care in Pakistan: seeing the unseen. 2004, Karachi: Oxford University Press
Google Scholar
Knippenberg R, Lawn JE, Darmstadt GL, Begkoyian G, Fogstad H, Walelign N, Paul VK: Systematic scaling up of neonatal care in countries. Lancet. 2005, 365 (9464): 1087-1098.
Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA: Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005, 115 (2 Suppl): 519-617.
Boschi-Pinto C, Young M, Black RE: The Child Health Epidemiology Reference Group reviews of the effectiveness of interventions to reduce maternal, neonatal and child mortality. International journal of epidemiology. 2010, 39 (Suppl 1): i3-6. 10.1093/ije/dyq018.
PubMed Central PubMed Google Scholar
Walker N, Fischer-Walker C, Bryce J, Bahl R, Cousens S: Standards for CHERG reviews of intervention effects on child survival. International journal of epidemiology. 2010, 39 (Suppl 1): i21-31. 10.1093/ije/dyq036.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, et al: Grading quality of evidence and strength of recommendations. BMJ. 2004, 328 (7454): 1490-10.1136/bmj.328.7454.1490.
Bang AT, Bang RA, Baitule SB, Reddy MH, Deshmukh MD: Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet. 1999, 354 (9194): 1955-1961. 10.1016/S0140-6736(99)03046-9.
CAS PubMed Google Scholar
Bang AT, Bang RA, Tale O, Sontakke P, Solanki J, Wargantiwar R, Kelzarkar P: Reduction in pneumonia mortality and total childhood mortality by means of community-based intervention trial in Gadchiroli, India. Lancet. 1990, 336 (8709): 201-206. 10.1016/0140-6736(90)91733-Q.
Baqui AH, Arifeen SE, Williams EK, Ahmed S, Mannan I, Rahman SM, Begum N, Seraji HR, Winch PJ, Santosham M, et al: Effectiveness of home-based management of newborn infections by community health workers in rural Bangladesh. The Pediatric infectious disease journal. 2009, 28 (4): 304-310. 10.1097/INF.0b013e31819069e8.
Baqui AH, El-Arifeen S, Darmstadt GL, Ahmed S, Williams EK, Seraji HR, Mannan I, Rahman SM, Shah R, Saha SK, et al: Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008, 371 (9628): 1936-1944. 10.1016/S0140-6736(08)60835-1.
Bhandari N, Bahl R, Bhatnagar V, Bhan MK: Treating sick young infants in urban slum setting. Lancet. 1996, 347 (9017): 1774-1775. 10.1016/S0140-6736(96)90856-9.
Khan AJ, Khan JA, Akbar M, Addiss DG: Acute respiratory infections in children: a case management intervention in Abbottabad District, Pakistan. Bulletin of the World Health Organization. 1990, 68 (5): 577-585.
PubMed Central CAS PubMed Google Scholar
Mtango FD, Neuvians D: Acute respiratory infections in children under five years. Control project in Bagamoyo District, Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1986, 80 (6): 851-858. 10.1016/0035-9203(86)90241-5.
Pandey MR, Daulaire NM, Starbuck ES, Houston RM, McPherson K: Reduction in total under-five mortality in western Nepal through community-based antimicrobial treatment of pneumonia. Lancet. 1991, 338 (8773): 993-997. 10.1016/0140-6736(91)91847-N.
Sazawal S, Black RE: Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. The Lancet infectious diseases. 2003, 3 (9): 547-556. 10.1016/S1473-3099(03)00737-0.
Adejuyigbe EA, Adeodu OO, ko-Nai KA, Taiwo O, Owa JA: Septicaemia in high risk neonates at a teaching hospital in Ile-Ife, Nigeria. East Afr Med J. 2001, 78 (10): 540-543.
Ahmed AS, Chowdhury MA, Hoque M, Darmstadt GL: Clinical and bacteriological profile of neonatal septicemia in a tertiary level pediatric hospital in Bangladesh. Indian Pediatr. 2002, 39 (11): 1034-1039.
Ali Z: Neonatal bacterial septicaemia at the Mount Hope Women's Hospital, Trinidad. Ann Trop Paediatr. 2004, 24 (1): 41-44. 10.1179/027249304225013367.
Anyebuno M, Newman M: Common causes of neonatal bacteraemia in Accra, Ghana. East Afr Med J. 1995, 72 (12): 805-808.
Basu S, Rathore P, Bhatia BD: Predictors of mortality in very low birth weight neonates in India. Singapore Med J. 2008, 49 (7): 556-560.
Bell Y, Barton M, Thame M, Nicholson A, Trotman H: Neonatal sepsis in Jamaican neonates. Ann Trop Paediatr. 2005, 25 (4): 293-296. 10.1179/146532805X72449.
Berger A, Salzer HR, Weninger M, Sageder B, Aspock C: Septicaemia in an Austrian neonatal intensive care unit: a 7-year analysis. Acta Paediatr. 1998, 87 (10): 1066-1069. 10.1080/080352598750031392.
Bhutta ZA, Yusuf K: Neonatal sepsis in Karachi: factors determining outcome and mortality. J Trop Pediatr. 1997, 43 (2): 65-70. 10.1093/tropej/43.2.65.
Boo NY, Chor CY: Six year trend of neonatal septicaemia in a large Malaysian maternity hospital. J Paediatr Child Health. 1994, 30 (1): 23-27. 10.1111/j.1440-1754.1994.tb00560.x.
Chang Chien HY, Chiu NC, Li WC, Huang FY: Characteristics of neonatal bacterial meningitis in a teaching hospital in Taiwan from 1984-1997. J Microbiol Immunol Infect. 2000, 33 (2): 100-104.
Chang CJ, Chang WN, Huang LT, Huang SC, Chang YC, Hung PL, Tasi CY, Lu CH, Cheng BC, Lee PY, et al: Neonatal bacterial meningitis in southern Taiwan. Pediatr Neurol. 2003, 29 (4): 288-294. 10.1016/S0887-8994(03)00273-X.
Cordero L, Rau R, Taylor D, Ayers LW: Enteric gram-negative bacilli bloodstream infections: 17 years' experience in a neonatal intensive care unit. Am J Infect Control. 2004, 32 (4): 189-195. 10.1016/j.ajic.2003.07.004.
Cordero L, Sananes M, Ayers LW: Bloodstream infections in a neonatal intensive-care unit: 12 years' experience with an antibiotic control program. Infect Control Hosp Epidemiol. 1999, 20 (4): 242-246. 10.1086/501619.
Daoud AS, al-Sheyyab M, bu-Ekteish F, Obeidat A, Ali AA, el-Shanti H: Neonatal meningitis in northern Jordan. J Trop Pediatr. 1996, 42 (5): 267-270. 10.1093/tropej/42.5.267.
Das PK, Basu K, Chakraborty P, Bhowmik PK: Clinical and bacteriological profile of neonatal infections in metropolitan city based medical college nursery. J Indian Med Assoc. 1999, 97 (1): 3-5.
Das PK, Basu K, Chakraborty S, Basak M, Bhowmik PK: Early neonatal morbidity and mortality in a city based medical college nursery. Indian J Public Health. 1998, 42 (1): 9-14.
Dawson KG, Emerson JC, Burns JL: Fifteen years of experience with bacterial meningitis. Pediatr Infect Dis J. 1999, 18 (9): 816-822. 10.1097/00006454-199909000-00014.
Galanakis E, Krallis N, Levidiotou S, Hotoura E, Andronikou S: Neonatal bacteraemia: a population-based study. Scand JI nfect Dis. 2002, 34 (8): 598-601. 10.1080/00365540110080809.
Gebremariam A: Neonatal meningitis in Addis Ababa: a 10-year review. Ann Trop Paediatr. 1998, 18 (4): 279-283.
Ghiorghis B: Neonatal sepsis in Addis Ababa, Ethiopia: a review of 151 bacteremic neonates. Ethiop Med J. 1997, 35 (3): 169-176.
Hervas JA, Alomar A, Salva F, Reina J, Benedi VJ: Neonatal sepsis and meningitis in Mallorca, Spain, 1977-1991. Clin Infect Dis. 1993, 16 (5): 719-724. 10.1093/clind/16.5.719.
Ishikawa T, Asano Y, Morishima T, Nagashima M, Sobue G, Watanabe K, Yamaguchi H: Epidemiology of bacterial meningitis in children: Aichi Prefecture, Japan, 1984-1993. Pediatr Neurol. 1996, 14 (3): 244-250. 10.1016/0887-8994(96)00024-0.
Jeena PM, Adhikari M, Carlin JB, Qazi S, Weber MW, Hamer DH: Clinical profile and predictors of severe illness in young South African infants (<60 days). S Afr Med J. 2008, 98 (11): 883-888.
Jiang JH, Chiu NC, Huang FY, Kao HA, Hsu CH, Hung HY, Chang JH, Peng CC: Neonatal sepsis in the neonatal intensive care unit: characteristics of early versus late onset. J Microbiol Immunol Infect. 2004, 37 (5): 301-306.
Kallman J, Kihlstrom E, Sjoberg L, Schollin J: Increase of staphylococci in neonatal septicaemia: a fourteen-year study. Acta Paediatr. 1997, 86 (5): 533-538. 10.1111/j.1651-2227.1997.tb08926.x.
Karthikeyan G, Premkumar K: Neonatal sepsis: Staphylococcus aureus as the predominant pathogen. Indian J Pediatr. 2001, 68 (8): 715-717. 10.1007/BF02752407.
Karunasekera KA, Pathirana D: A preliminary study on neonatal septicaemia in a tertiary referral hospital paediatric unit. Ceylon Med J. 1999, 44 (2): 81-86.
Kuruvilla KA, Pillai S, Jesudason M, Jana AK: Bacterial profile of sepsis in a neonatal unit in south India. Indian Pediatr. 1998, 35 (9): 851-858.
Laving AM, Musoke RN, Wasunna AO, Revathi G: Neonatal bacterial meningitis at the newborn unit of Kenyatta National Hospital. East Afr Med J. 2003, 80 (9): 456-462.
Lee NC, Chen SJ, Tang RB, Hwang BT: Neonatal bacteremia in a neonatal intensive care unit: analysis of causative organisms and antimicrobial susceptibility. J Chin Med Assoc. 2004, 67 (1): 15-20.
Lim NL, Wong YH, Boo NY, Kasim MS, Chor CY: Bacteraemic infections in a neonatal intensive care unit--a nine-month survey. Med J Malaysia. 1995, 50 (1): 59-63.
Lopez Sastre JB, Fernandez CB, Coto Cotallo GD, Ramos AA: Trends in the epidemiology of neonatal sepsis of vertical transmission in the era of group B streptococcal prevention. Acta Paediatr. 2005, 94 (4): 451-457. 10.1080/08035250410025302.
Mathur NB, Singh A, Sharma VK, Satyanarayana L: Evaluation of risk factors for fatal neonatal sepsis. Indian Pediatr. 1996, 33 (10): 817-822.
Milledge J, Calis JC, Graham SM, Phiri A, Wilson LK, Soko D, Mbvwinji M, Walsh AL, Rogerson SR, Molyneux ME, et al: Aetiology of neonatal sepsis in Blantyre, Malawi: 1996-2001. Ann Trop Paediatr. 2005, 25 (2): 101-110. 10.1179/146532805X45692.
Moore KL, Kainer MA, Badrawi N, Afifi S, Wasfy M, Bashir M, Jarvis WR, Graham TW, el-Kholy A, Gipson R, et al: Neonatal sepsis in Egypt associated with bacterial contamination of glucose-containing intravenous fluids. Pediatr Infect Dis J. 2005, 24 (7): 590-594. 10.1097/01.inf.0000168804.09875.95.
Moreno MT, Vargas S, Poveda R, Saez-Llorens X: Neonatal sepsis and meningitis in a developing Latin American country. Pediatr Infect Dis J. 1994, 13 (6): 516-520. 10.1097/00006454-199406000-00010.
Nnpd: Neonatal morbidity and mortality: report of the National Neonatal-Perinatal Database. Indian Pediatr. 1997, 34 (11): 1039-1042.
Palmer A, Weber M, Bojang K, McKay T, Adegbola R: Acute bacterial meningitis in The Gambia: a four-year review of paediatric hospital admissions. J Trop Pediatr. 1999, 45 (1): 51-53. 10.1093/tropej/45.1.51.
Park CH, Seo JH, Lim JY, Woo HO, Youn HS: Changing trend of neonatal infection: experience at a newly established regional medical center in Korea. Pediatr Int. 2007, 49 (1): 24-30. 10.1111/j.1442-200X.2007.02310.x.
Patel DM, Rhodes PG, LeBlanc MH, Graves GR, Glick C, Morrison J: Role of postnatal penicillin prophylaxis in prevention of neonatal group B streptococcus infection. Acta Paediatr. 1999, 88 (8): 874-879. 10.1111/j.1651-2227.1999.tb00064.x.
Persson E, Trollfors B, Brandberg LL, Tessin I: Septicaemia and meningitis in neonates and during early infancy in the Goteborg area of Sweden. Acta Paediatr. 2002, 91 (10): 1087-1092. 10.1080/080352502760311593.
Robillard PY, Perez JM, Hulsey TC, Perianin J, Gallais A, Janky E: Evaluation of neonatal sepsis screening in a tropical area. Part I: Major risk factors for bacterial carriage at birth in Guadeloupe. West Indian Med J. 2000, 49 (4): 312-315.
Simiyu DE: Morbidity and mortality of neonates admitted in general paediatric wards at Kenyatta National Hospital. East Afr Med J. 2003, 80 (12): 611-616.
Simiyu DE: Neonatal septicaemia in low birth weight infants at Kenyatta National Hospital, Nairobi. East Afr Med J. 2005, 82 (3): 148-152.
Smith PB, Cotten CM, Garges HP, Tiffany KF, Lenfestey RW, Moody MA, Li JS, Benjamin DK: A comparison of neonatal Gram-negative rod and Gram-positive cocci meningitis. J Perinatol. 2006, 26 (2): 111-114. 10.1038/sj.jp.7211438.
Sundaram V, Kumar P, Dutta S, Mukhopadhyay K, Ray P, Gautam V, Narang A: Blood culture confirmed bacterial sepsis in neonates in a North Indian tertiary care center: changes over the last decade. J Infect Dis. 2009, 62 (1): 46-50.
Tiskumara R, Fakharee SH, Liu CQ, Nuntnarumit P, Lui KM, Hammoud M, Lee JK, Chow CB, Shenoi A, Halliday R, et al: Neonatal infections in Asia. Arch Dis Child Fetal Neonatal Ed. 2009, 94 (2): F144-F148. 10.1136/adc.2008.139865.
Trotman H, Bell Y, Thame M, Nicholson AM, Barton M: Predictors of poor outcome in neonates with bacterial sepsis admitted to the University Hospital of the West Indies. West Indian Med J. 2006, 55 (2): 80-84.
Udo JJ, Anah MU, Ochigbo SO, Etuk IS, Ekanem AD: Neonatal morbidity and mortality in Calabar, Nigeria: a hospital-based study. Niger J Clin Pract. 2008, 11 (3): 285-289.
Waheed M, Laeeq A, Maqbool S: The etiology of neonatal sepsis and patterns of antibiotic resistance. J Coll Physicians Surg Pak. 2003, 13 (8): 449-452.
Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC: The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003, 167 (5): 695-701. 10.1164/rccm.200207-682OC.
Wolf H, Schaap AH, Smit BJ, Spanjaard L, Adriaanse AH: Liberal diagnosis and treatment of intrauterine infection reduces early-onset neonatal group B streptococcal infection but not sepsis by other pathogens. Infect Dis Obstet Gynecol. 2000, 8 (3-4): 143-150. 10.1002/1098-0997(2000)8:3/4<143::AID-IDOG8>3.0.CO;2-5.
Wolfler A, Silvani P, Musicco M, Antonelli M, Salvo I: Incidence of and mortality due to sepsis, severe sepsis and septic shock in Italian Pediatric Intensive Care Units: a prospective national survey. Intensive Care Med. 2008, 34 (9): 1690-1697. 10.1007/s00134-008-1148-y.
Kaushik SL, Parmar VR, Grover N, Grover PS, Kaushik R: Neonatal sepsis in hospital born babies. J Commun Dis. 1998, 30 (3): 147-152.
Parkash J, Das N: Pattern of admissions to neonatal unit. J Coll Physicians Surg Pak. 2005, 15 (6): 341-344.
Ganatra HA, Stoll BJ, Zaidi AK: International perspective on early-onset neonatal sepsis. Clin Perinatol. 2010, 37 (2): 501-523. 10.1016/j.clp.2010.02.004.
Bhutta ZA, Zaidi AK, Thaver D, Humayun Q, Ali S, Darmstadt GL: Management of newborn infections in primary care settings: a review of the evidence and implications for policy?. The Pediatric infectious disease journal. 2009, 28 (1 Suppl): S22-30. 10.1097/INF.0b013e31819588ac.
Darmstadt GL, Batra M, Zaidi AK: Parenteral antibiotics for the treatment of serious neonatal bacterial infections in developing country settings. The Pediatric infectious disease journal. 2009, 28 (1 Suppl): S37-42. 10.1097/INF.0b013e31819588c3.
Darmstadt GL, Batra M, Zaidi AK: Oral antibiotics in the management of serious neonatal bacterial infections in developing country communities. The Pediatric infectious disease journal. 2009, 28 (1 Suppl): S31-36. 10.1097/INF.0b013e3181958794.
Theodoratou E, Al-Jilaihawi S, Woodward F, Ferguson J, Jhass A, Balliet M, Kolcic I, Sadruddin S, Duke T, Rudan I, et al: The effect of case management on childhood pneumonia mortality in developing countries. International journal of epidemiology. 39 (Suppl 1): i155-171.
Bahl R, Martines J, Ali N, Bhan MK, Carlo W, Chan KY, Darmstadt GL, Hamer DH, Lawn JE, McMillan DD, et al: Research priorities to reduce global mortality from newborn infections by 2015. The Pediatric infectious disease journal. 2009, 28 (1 Suppl): S43-48. 10.1097/INF.0b013e31819588d7.
Dunham EC: Septicemia in the new-born. Archives of Pediatrics & Adolescent Medicine. 1933, 45 (2): 229-253. 10.1001/archpedi.1933.01950150002001.
Nyhan WL, Fousek MD: Septicemia of the newborn. Pediatrics. 1958, 22 (2): 268-278.
Gluck L, Wood HF, Fousek MD: Septicemia of the newborn. Pediatr Clin North Am. 1966, 13 (4): 1131-1148.
Gladstone IM, Ehrenkranz RA, Edberg SC, Baltimore RS: A ten-year review of neonatal sepsis and comparison with the previous fifty-year experience. The Pediatric infectious disease journal. 1990, 9 (11): 819-825. 10.1097/00006454-199011000-00009.
Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG: Seventy-five years of neonatal sepsis at Yale: 1928-2003. Pediatrics. 2005, 116 (3): 595-602. 10.1542/peds.2005-0552.
Young Infants Clinical Signs Study Group. Clinical signs that predict severe illness in children under age 2 months: a multicentre study. Lancet. 2008, 371 (9607): 135-142. 10.1016/S0140-6736(08)60106-3.
Download references
Acknowledgements
We are grateful to Rajiv Bahl for insightful review of an earlier draft of this paper. We are also grateful to the members of the Delphi Expert Panel including Rajiv Bahl, Abhay Bang, Abdullah Baqui, Zulfiqar Bhutta, Robert Black, Simon Cousens, Gary Darmstadt, Mike English, Luis Huicho, David Isaacs, Joy Lawn, Patrick Mark, Kim Mulholland, David Osrin, Vinod Paul, Igor Rudan, Cindy Stephen, Barbara Stoll, Steven Wall and Anita Zaidi.
This article has been published as part of BMC Public Health Volume 11 Supplement 3, 2011: Technical inputs, enhancements and applications of the Lives Saved Tool (LiST). The full contents of the supplement are available online at http://www.biomedcentral.com/1471-2458/11?issue=S3 .
Author information
Authors and affiliations.
Department of Paediatrics and Child Health, the Aga Khan University, Karachi, Pakistan
Anita K M Zaidi, Hammad A Ganatra, Sana Syed & Zulfiqar A Bhutta
London School of Tropical Medicine and Hygiene, London, UK
Simon Cousens
Johns Hopkins Bloomberg School of Public Health, International Health, Baltimore, MD, USA
Anne CC Lee & Robert Black
Saving Newborn Lives/Save the Children, Cape Town, South Africa
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Anita K M Zaidi .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
AZ and JL planned the review, SS and AZ undertook the searches and abstraction with input from JL and HG. SC undertook the meta-analyses. RB provided unpublished data from a previous investigator working group. JL, AZ and ACCL planned the Delphi. All authors contributed to the manuscript.
Electronic supplementary material
Additional file 1: study identifiers and context(xls 84 kb), rights and permissions.
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Zaidi, A.K.M., Ganatra, H.A., Syed, S. et al. Effect of case management on neonatal mortality due to sepsis and pneumonia. BMC Public Health 11 (Suppl 3), S13 (2011). https://doi.org/10.1186/1471-2458-11-S3-S13
Download citation
Published : 13 April 2011
DOI : https://doi.org/10.1186/1471-2458-11-S3-S13
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Case Management
- Neonatal Mortality
- Oral Antibiotic
- Neonatal Sepsis
- Neonatal Infection
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 27 July 2022
Clinical characteristics of hospitalized term and preterm infants with community-acquired viral pneumonia
- Xinxian Guan 1 na1 ,
- Shasha Gao 1 na1 ,
- He Zhao 2 ,
- Huiting Zhou 2 ,
- Yan Yang 1 ,
- Shenglin Yu 1 &
- Jian Wang 2
BMC Pediatrics volume 22 , Article number: 452 ( 2022 ) Cite this article
1822 Accesses
9 Altmetric
Metrics details
Pneumonia is a serious problem that threatens the health of newborns. This study aimed to investigate the clinical characteristics of hospitalized term and preterm infants with community-acquired viral pneumonia.
This was a retrospective analysis of cases of community-acquired viral pneumonia in the Neonatal Department. Nasopharyngeal aspirate (NPA) samples were collected for pathogen detection, and clinical data were collected. We analysed pathogenic species and clinical characteristics among these infants.
RSV is the main virus in term infants, and parainfluenza virus (PIV) 3 is the main virus in preterm infants. Patients infected with PIV3 were more susceptible to coinfection with bacteria than those with respiratory syncytial virus (RSV) infection ( p < 0.05). Preterm infants infected with PIV3 were more likely to be coinfected with bacteria than term infants ( p < 0.05), mainly gram-negative bacteria (especially Klebsiella pneumonia). Term infants with bacterial infection were more prone to fever, cyanosis, moist rales, three concave signs, elevated C-reactive protein (CRP) levels, respiratory failure and the need for higher level of oxygen support and mechanical ventilation than those with simple viral infection ( p < 0.05). The incidence of hyponatremia in neonatal community-acquired pneumonia (CAP) was high.
Conclusions
RSV and PIV3 were the leading causes of neonatal viral CAP. PIV3 infection is the main cause of viral CAP in preterm infants, and these individuals are more likely to be coinfected with bacteria than term infants, mainly gram-negative bacteria. Term infants with CAP coinfected with bacteria were more likely to have greater disease severity than those with single viral infections.
Peer Review reports
Introduction
Pneumonia is one of the most common infectious diseases in the neonatal period and accounts for 46% of all neonatal diseases [ 1 ]. Moreover, the mortality rate of pneumonia is 1.2%, which ranks highest among all neonatal infectious diseases; thus, pneumonia is a serious problem that threatens the health of newborns [ 2 ]. The main pathogens of neonatal pneumonia are bacteria, viruses, and fungi [ 3 ]. In recent years, many studies of bacterial pneumonia in neonates have been published [ 4 ], but information on viral pneumonia in neonates is limited. Many viruses can damage the airway epithelial layer, thus increasing the likelihood of both adherence to the respiratory tract and bacterial translocation, two of the critical first steps in causing infection [ 5 ]. Viruses can also lead to dysfunction of the immune system, thereby promoting bacterial infection [ 6 ]. We retrospectively analysed all preterm and term neonates with community-acquired viral pneumonia over a 5-year period to study the aetiology and clinical features of these infants.
Materials and methods
The present study was a retrospective analysis of newborn patients with community-acquired viral pneumonia. The general clinical data, clinical signs and symptoms, auxiliary examination results, complications and prognoses were collected and analysed from the hospital medical records system.
The inclusion criteria were as follows: the patients were hospitalized in the Neonatal Department of Children’s Hospital of Soochow University (Suzhou, China) between January 2017 and December 2021 and were diagnosed with community-acquired pneumonia (CAP); and the aetiology of these cases must be positive for respiratory syncytial virus (RSV), adenovirus, influenza virus (Inf) A, Inf B, or parainfluenza virus (PIV1, PIV2, and PIV3).
The exclusion criteria were as follows: patients with incomplete clinical data and severe basic diseases, such as congenital heart disease, congenital immunodeficiencies and incomplete medical data [ 7 , 8 ].
Diagnostic criteria and data collection
CAP refers to clinical signs and symptoms of pneumonia acquired outside a hospital setting [ 9 ] and is diagnosed based on clinical findings (fever, cough, and difficulty in breathing), physical examination findings (tachypnoea, chest retraction, and decreased breath sounds or rales), and radiological findings [ 10 ]. Chest radiographs were evaluated by a radiologist trained in reading and interpreting radiographs according to the World Health Organization’s (WHO) guidelines [ 11 ]. Respiratory failure is defined as the failure to maintain either normal delivery of oxygen to the tissues or normal removal of carbon dioxide from the tissues. Respiratory failure occurs when there is an imbalance between the respiratory workload and ventilatory strength and endurance. The suggested cutoffs for diagnosing respiratory failure include two or more of the following: PaCO2 > 60 mmHg, PaO2 < 50 mmHg or O2 saturation < 80% with an FiO2 of 1.0 and pH < 7.25 [ 12 ]. Heart failure (HF) is defined as the failure of the heart when it supplies blood to either systemic or pulmonary circulation at an appropriate rate of flow, or to receive venous return at an appropriate filling pressure, but produces adverse effects on the heart, the circulation, and the patient [ 13 ]. The diagnosis and treatment of HF are based on Canadian Cardiovascular Society Guidelines [ 13 ]. Pulmonary air leak syndrome (PALS) comprises several different clinical conditions, such as pulmonary interstitial emphysema (PIE), pneumomediastinum, pneumothorax and pneumopericardium, which all results from alveolar over distension and air leakage outside the lungs [ 14 ]. In this study, PALS is comprised of pneumothorax and pneumomediastinum. The treatment of mechanical ventilation (MV) in this research includes invasive MV and non-invasive ventilation (NIV), and invasive MV includes high frequency oscillatory ventilation (HFOV) and conventional mechanical ventilation (CMV).
Pathogen testing
Nasopharyngeal aspirates (NPA) were collected under strict aseptic operations within 24 h from all hospitalized patients ( n = 375) to identify the pathogen. The samples were divided into three subsamples for pathogen detection. One subsample was used to detect seven common respiratory viruses as previously described by direct immunofluorescence analysis, and the remaining two subsamples were used to detect and identify bacteria using bacterial culture and Mycoplasma by using PCR analysis. The study period overlapped with coronavirus disease 2019 (COVID-19) pandemic, COVID-19 real-time polymerase chain reaction (RT-PCR) test was performed by nasopharyngeal swab method for the infants from March 2020 to December 2021( n = 89).
Immunofluorescence analysis for respiratory virus pathogen detection
A total of 1–2 mL of NPA was mixed with PBS and centrifuged at (400–600) × g for 15 min. Then, the supernatant was discarded, and the remaining sample was washed three times with PBS (5 mL). PBS (0.5 to 1 mL) was added after centrifugation to make the cell suspension. Subsequently, seven wells (25 μL/well) were spotted on the slide, air-dried at room temperature, and fixed in cold acetone for 10 min. Then, 20 μL of immunofluorescent reagent (containing fluorescein-FITC-labelled monoclonal antibody) was added to each of the wells. After incubation at 37 °C for 30 min and rinsing 3 times with PBS, the glycerol buffer solution was air-dried and ready for further analysis. Seven common respiratory viruses (including RSV, adenovirus, InfA, Inf B, PIV1, PIV2, and PIV3) were detected by direct immunofluorescence analysis. A positive negative control is provided by the kit (D3 Ultra DFA respiratory virus screening and identification kit, Athens, Ohio, USA). Bright yellow–green fluorescence and/or fluorescent spots in the nucleus/cytoplasm were considered positive.
RT-PCR for COVID-19
Laboratory confirmation of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) RNA was performed by RT-PCR. Briefly, according to the manufacturer’s instruction, we extracted the nucleic acid from nasopharyngeal swab samples by using the Viral Nucleic Acid Kit (Health, Ningbo, China). We also used COVID-19 detection kits (Bioperfectus, Taizhou, China) in detecting the ORF1ab and the N genes. At the same time, we adopted the procedure of RT-PCR assay from the WHO protocol (2020b). A positive test was defined as a cycle threshold value (Ct value) less than 35 and laboratory confirmation of COVID-19 was based on the positive results for both ORF1ab and the N genes.
Bacterial culture for bacterial detection
Bacteria were tested by inoculating NPA samples on blood plates that were read after incubating for 18–20 h. If bacterial growth was > 10 4 colony forming units/mL, it was considered significant. Morphology selection depended on experienced clinical laboratory physicians.
PCRs for Mycoplasma
Mycoplasma pneumoniae were detected by PCR. NPA samples were centrifuged at 12,000 × g for 5 min. DNA was obtained from the NPA samples (200 μL) using DNA-EZ Reagents (Sangon Biotech, Shanghai, China) in accordance with the manufacturer’s instructions. A final volume of 100 μL containing DNA was eluted for detection of Mycoplasma pneumoniae gene amplification via real-time PCR.
Statistical analysis
Statistical analysis was performed using SPSS v.17.0 for Windows (SPSS Inc., Chicago, IL). Normally distributed data are expressed as the mean ± standard deviation, and nonnormally distributed data are expressed as the median and interquartile range. Normally distributed data were compared using the independent samples t test, and nonnormally distributed data were compared using the Kruskal–Wallis test. Categorical data are presented as numbers and percentages. The chi-square and Fisher exact tests were used to compare categorical data. All tests were two-tailed, and P < 0.05 was considered statistically significant.
Three hundred seventy-five newborns were enrolled and retrospectively analysed in this study. No deaths were reported. There were 248 males and 127 females. Of the 375 patients, 344 were term infants, and 31 were preterm infants. All term infants were younger than 28 days old at admission. The postmenstrual age (PMA) of all preterm infants was less than 44 weeks after birth.

Comparison of pathogens in preterm and term infants
Of the 375 community-acquired viral pneumonia cases, 140 patients were coinfected with bacteria, 3 patients were coinfected with mycoplasma. The types of bacteria included Staphylococcus aureus ( n = 42), Escherichia coli ( n = 32), Klebsiella pneumoniae ( n = 22), Streptococcus viridans ( n = 20), Moraxella catarrhalis ( n = 13), Aerobacter cloacae ( n = 5), Enterobacter aerogenes ( n = 3) , Haemophilus influenzae ( n = 2) and Proteus mirabilis ( n = 1). All admitted infants through the pandemic were tested negative for COVID-19.
Full-term infants were more likely to be infected with RSV than preterm infants ( p < 0.001). Preterm infants were more likely to be infected with PIV3 than term infants ( p < 0.001). In addition, preterm infants were more likely to be coinfected with bacteria than term infants ( p < 0.001), especially Gram-negative bacteria ( p < 0.001), such as Klebsiella pneumoniae ( p < 0.001) (Table 1 ).
In all cases of community-acquired viral pneumonia, regardless of the viral infection, newborns with coinfection, especially bacterial infection, were more prone to respiratory failure (43/140). The probability of respiratory failure in children with simple virus infection (30/232) was lower (χ2 = 17.51, p < 0.001) than in those coinfected with bacteria (Table 2 ).
The distribution of virus species and monthly distribution of respiratory virus detection
Among the 375 enrolled patients, 322 were infected with RSV alone (85.9%), 2 were infected with both RSV and Inf A (0.5%), 1 was infected with both RSV and PIV3 (0.3%), 35 were infected with PIV3 alone (9.3%), 10 were infected with Inf A alone (2.7%) and 5 were infected with Inf B alone (1.3%). No patient was infected with adenovirus, PIV1 or PIV2. RSV infection mainly occurred in January, February, November and December, which showed obvious seasonal prevalence. However, PIV3 infection did not show significant seasonal prevalence (Fig. 1 ).
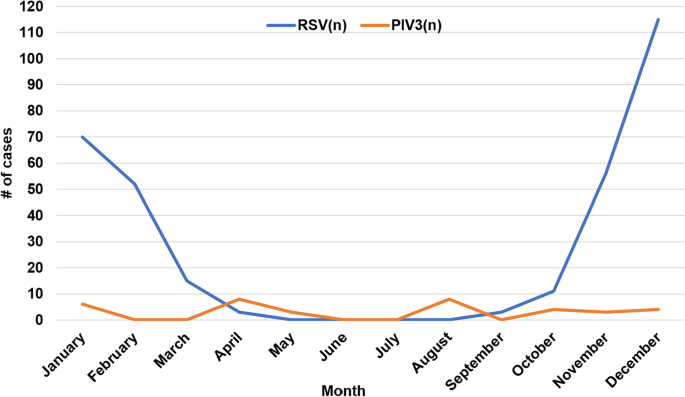
Monthly distribution of CAP in neonates with RSV and PIV3 infection
Patients infected with PIV3 were more prone to co-infection with bacteria than those with RSV infection. Preterm infants were more susceptible to co-infection with bacteria than term infants
In all children with community-acquired viral pneumonia, their combined bacterial infection was compared. One child with mixed infection of RSV and PIV3 was excluded because he had no bacterial infection. The prevalence of RSV pneumonia complicated with bacterial infection was 33.0% (107/324), and the prevalence of PIV3 pneumonia complicated with bacterial infection was 60% (21/35). Because the total number of children with other types of viral (Inf A and Inf B) pneumonia was small, statistical analysis was carried out together, and the prevalence of combined bacterial infection was 80% (12/15). Statistical analysis showed that patients infected with PIV3 and other viruses were more likely to be coinfected with bacteria than patients infected with RSV ( p < 0.05). Among RSV-infected patients, preterm infants were more likely to be complicated ( p < 0.01) with bacterial infection than term infants. The same trend was found in PIV 3-infected infants ( p < 0.05) (Fig. 2 ) .
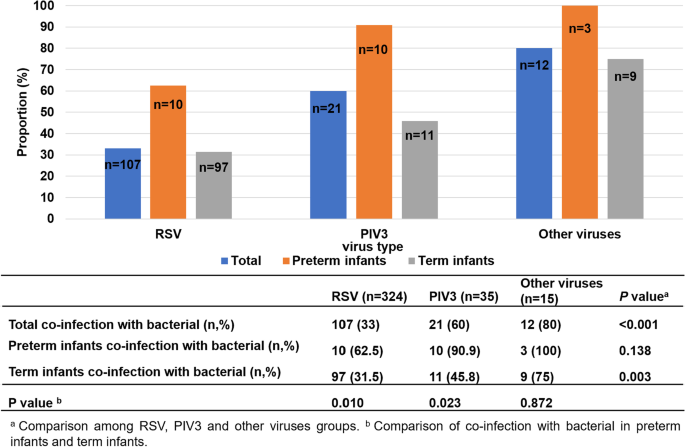
The proportion of coinfection with bacteria of different viruses in preterm and term infants
Clinical characteristics of coinfected with bacteria in term infants and premature infants with viral pneumonia
Of the 375 patients with community-acquired viral pneumonia, two cases of coinfection with mycoplasma in term infants and one case of coinfection with mycoplasma in preterm infants were excluded from the analysis. Therefore, the clinical features of coinfection with bacteria among term infants (342 cases) and premature infants (30 cases) with viral pneumonia are summarized in Table 2 . The percentage of coinfection with bacteria was different between term and preterm infants ( p < 0.05). There were 117 term infants coinfected with bacteria (117/342), and there were 23 preterm infants coinfected with bacteria (23/30). Our results suggested that premature infants are more likely to be complicated with bacterial infection.
For laboratory tests, the white blood cell (WBC) count was not affected by coinfection with bacteria in term or preterm infants. The proportion of neutrophils to WBCs was not different between term infants with coinfection with bacteria and those with simple virus infection. However, in preterm infants, the prevalence of neutrophils to WBCs was different between those with coinfection with bacteria and those with simple virus infection. The level of C-reactive protein (CRP) in term infants was different between those with coinfection with bacteria and those with simple virus infection. The level of CRP was not affected in preterm infants with co-infection with bacteria and those with simple virus infection.
In the term infant group, children with bacterial infection are more likely to have fever and cyanosis symptoms than children with simple virus infection. The three concave signs are more obvious among term infants with bacterial infection, their CRP values are higher ( p < 0.01), they are more likely to be complicated with respiratory failure, and they are more likely to need oxygen support and mechanical ventilation treatment, including invasive and non-invasive mechanical ventilation. The signs of pulmonary moist rales in children with simple virus infection were significantly more common than those in children with bacterial infection.
In the preterm infant group, the hospitalization time of infants with bacterial infection was longer than that of infants with simple virus infection, and the prevalence of neutrophils to WBC was lower ( p < 0.014) than that of infants with simple virus infection.
Neonatal immune dysfunction, as well as neonatal lung development, is not fully developed and vulnerable to the invasion of external pathogens [ 15 ]. Pathogens can be transmitted to newborns through droplets and contact [ 16 ]. Because of the complexity of the community environment and the variation in regions and seasons, the distribution of pathogens also differs. In addition, the incidence of disease increases rapidly, which is the focus of current research [ 17 ].
In our study, the peak incidence of RSV pneumonia occurred during the winter and early spring. Similar results were reported in our previous study with a subtropical climate [ 18 , 19 ], and the peak of RSV activity was in the winter and spring seasons [ 19 ].
In Suzhou, China, the infection rate of COVID-19 was low, epidemic data is obtained from the Sina Real-Time Epidemic website ( https://news.sina.cn/zt_d/yiqing0121 ) [ 20 ]. We had tested hospitalized children for SARS-CoV-2 and there were no positive cases during study period. The current research showed that RSV and PIV3 were the major viral pathogens in neonatal community-acquired viral pneumonia, especially RSV. Few patients were infected with InfA or InfB. RSV was the main virus in term newborns. PIV3 was the main virus in preterm infants. Unfortunately, there are currently no vaccines available against pathogens (RSV, PIVs, influenza virus) for infants under 6 months of age [ 21 ]. In newborns, especially in preterm infants, palivizumab is the only licenced treatment to help reduce the burden of RSV [ 22 ]. Unfortunately, palivizumab use is limited in the Suzhou area. Additionally, Preterm infants are more likely to co-infect with bacteria than term infants, especially gram-negative bacteria, such as Klebsiella pneumoniae . Therefore, in clinical work, we can preliminarily distinguish potential pathogens for newborns with CAP based on whether they are preterm or term infants and thus select the targeted treatment schemes.
In the present study, patients with PIV3 infection were more likely to be infected with bacteria than patients with RSV infection (60.0% vs. 33.0%). Additionally, patients with other virus (InfA, InfB) infections were more likely to be infected with bacteria than patients with RSV infections (80.0% vs. 33.0%). Among RSV-infected patients, preterm infants were more likely to be complicated with bacterial infection than term infants, and the same trend was found in PIV3-infected infants. This result suggested that preterm infants are more susceptible to coinfection with bacteria than term infants. Therefore, in the clinic, when treating patients with PIV3 and other virus (InfA, InfB) infections or preterm infants, we should be alert to the possibility of combined bacterial infection and relax the indications for the use of antibiotics on the basis of support and symptomatic treatment. For patients with RSV infection, antibiotics should be used as soon as a concurrent bacterial infection is detected.
Not everyone with neonatal viral pneumonia will have prodromal symptoms, as the incidence of these symptoms usually varies between 30 and 65% depending on the pathogen [ 23 ]. In this study, patients with mild infections only showed symptoms of mild cough and low fever, while patients with severe infections had serious cough, high fever, apnoea, cyanosis, tachypnoea, refusal to feed, vomit or diarrhoea, three concave signs, increased moist rales, wheezing in the lungs, and complications with respiratory failure, HF and PALS.
This study also showed that preterm infants are more susceptible to coinfection, especially for bacteria, which is related to the lower immune function of preterm infants than that of term infants [ 24 , 25 ]. The trachea of premature infants is narrow, and the wall of the trachea easily collapses. The abundant capillaries and weak ciliary movement function provide a good environment for the attachment and reproduction of pathogenic bacteria [ 26 ]. Because the immune system of premature infants is not fully developed, their immune function is low [ 27 , 28 ]. Neonatal immune function, especially that of the local airway, is also underdeveloped with lower levels of secretory IgA, which serves an anti-infectious role [ 29 ]. Foetal immunoglobulins are mostly transmitted from the mother, but this physiological process mainly occurs in the middle and late stages of pregnancy. The IgG level of full-term newborns can reach the maternal level [ 30 ]. Respiratory virus infection is often accompanied by bacterial infection [ 31 ]. Because the humoral and cellular immunity of premature infants at small gestational age are at a low level [ 24 ], the damaged respiratory mucosa and inhibited immune function by respiratory viruses induce the risk of bacterial infection. Therefore, newborns are more easily infected by a variety of pathogens. Therefore, targeted measures, such as perinatal detection and health care, should be taken to reduce the birth of premature infants. When pneumonia occurs in premature infants, corresponding treatment measures, including respiratory support, immune support, enteral nutrition, parenteral nutrition support and sodium supplements, should be actively adopted.
Hyponatremia is relatively common in pneumonia, with one large Italian series reporting a rate of 45% [ 32 ]. In our study, the overall incidence of hyponatremia was 30.9% (116/375); however, hyponatremia was mild in the majority of cases, from 130 mmol/l to 135 mmol/l. Studies in developing countries have shown this to be associated with increasing severity of pneumonia and risk of death. Factors increasing the risk of dehydration and potentially hyponatremia include reduced nutrient/water intake and increased evaporative losses as a result of both increased respiratory rate and increased core temperature. Sometimes it may be a result of additional losses from vomiting and diarrhoea. Pneumonia is widely cited as a potential cause of syndrome of inappropriate antidiuretic hormone secretion (SIADH) [ 33 ], but no studies have examined the biochemistry and pathophysiology of hyponatremia in children with pneumonia with sufficient rigor to be able to differentiate adequately between SIADH and salt depletion; therefore, hyponatremia has frequently been ascribed to SIADH. Some scholars suspect that hyponatremia principally occurs secondarily to dehydration in most children considered to have SIADH. This has major implications for acute patient management, as SIADH is managed with fluid restriction, and dehydration clearly requires rapid volume replacement. Further studies are urgently required to address this question in the future.
In all patients involved in this study, the main pathogens that were co-infected were Staphylococcus aureus , Escherichia coli , Klebsiella pneumoniae and Streptococcus viridans , which was similar to other reports [ 34 ]. In addition, this article also found that in term infants with concurrent bacterial infections, the symptoms are more severe and more likely to have respiratory failure. Some of these patients require not only oxygen support but also NIV or invasive MV. Therefore, term infants with viral and bacterial infections are more severe than those with pure viral infections [ 35 , 36 ], and more attention should be devoted to respiratory management [ 37 , 38 ] and supportive care. Once the results of NPA culture are confirmed (before those of drug sensitivity testing are clear), appropriate antibiotics for common bacteria can be empirically selected. After the report of bacterial drug sensitivity tests, the type of antibiotics should be adjusted according to the treatment effect.
Neutrophils play an important and active role in the body's nonspecific immunity. In the present study, the total number of leukocytes in preterm infants combined with bacterial infection was numerically higher than that in preterm patients with simple virus infection; although the difference was not statistically significant, the prevalence of neutrophils to WBC was lower than that in patients with simple virus infection ( p < 0.05). The hospital stay in preterm infants with bacterial infection is also longer than that in preterm patients with simple virus infection. When bacteria and other microbial pathogens invade and inflammatory reactions occur, they can reach the inflammatory site under the influence of chemokines, devour bacteria and tissue fragments, and prevent the diffusion of pathogenic microorganisms in the body [ 39 ]. When the inflammatory reaction is strong, a large number of neutrophils stored in bone marrow are released into the blood, and the level of neutrophils increases. However, neutrophils were depleted in bone marrow when the infection was very serious, resulting in a decrease in neutrophil expression levels [ 40 ]. Therefore, when the prevalence of neutrophils to WBC is reduced in preterm infants, the risk of bacterial infection may increase, resulting in a prolonged hospital stay.
RSV and PIV3 are the main pathogens of neonatal viral pneumonia. It is necessary to consider the possibility of RSV infection in neonates with viral CAP in winter and early spring. In PIV3-infected newborns and premature infants, we should be alert to the possibility of combined bacterial infection. Preterm infants are more prone to PIV3 infection and coinfection with bacteria. Term infants are more prone to be infected with RSV. Term infants with bacterial infection are more prone to respiratory failure than simple virus infection. Patients with respiratory failure should be closely monitored and supported in a timely manner by inhaling oxygen and ventilation. When the prevalence of neutrophils to WBCs in preterm infants is reduced, it is easily complicated by bacterial infection. Additionally, hyponatremia cannot be ignored in children with pneumonia.
However, this study also has some shortcomings: the number of patients involved, especially preterm infants, was small. In the future, we will conduct more joint research with hospitals and communities in multiple regions. We will expand the inclusion criteria and include some children with insignificant performance in the study.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the datasets need to be kept confidential, but they are available from the corresponding author upon reasonable request.
Abbreviations
Nasopharyngeal aspirates
Community-acquired pneumonia
Respiratory syncytial virus
Parainfluenza virus
Influenza virus
C-reactive protein
World Health Organization
Postmenstrual age
Syndrome of inappropriate antidiuretic hormone secretion
Real-time polymerase chain reaction
Severe Acute Respiratory Syndrome Coronavirus 2
Coronavirus disease 2019
Heart failure; PALS: pulmonary air leak syndrome
Pulmonary interstitial emphysema
Mechanical ventilation
High frequency oscillatory ventilation
Conventional mechanical ventilation
Non-invasive ventilation
Malarbi S, Gunn-Charlton JK, Burnett AC, Prentice TM, Williams A, Mitchell P, Wray A, Hunt RW. Outcome of vein of Galen malformation presenting in the neonatal period. Arch Dis Child. 2019;104(11):1064–9.
Article PubMed Google Scholar
Backhaus E, Berg S, Andersson R, Ockborn G, Malmstrom P, Dahl M, Nasic S, Trollfors B. Epidemiology of invasive pneumococcal infections: manifestations, incidence and case fatality rate correlated to age, gender and risk factors. BMC Infect Dis. 2016;16:367.
Article PubMed PubMed Central Google Scholar
Zhang DS, Chen C, Zhou W, Chen J, Mu DZ. Pathogens and risk factors for ventilator-associated pneumonia in neonats. Chin J Contemp Pediatr. 2013;15(1):14–8.
Google Scholar
Viasus D, Ramos O, Ramos L, Simonetti AF, Carratala J. Solithromycin for the treatment of community-acquired bacterial pneumonia. Expert Rev Respir Med. 2017;11(1):5–12.
Article CAS PubMed Google Scholar
Cawcutt K, Kalil AC. Pneumonia with bacterial and viral coinfection. Curr Opin Crit Care. 2017;23(5):385–90.
Deng JC. Viral-bacterial interactions-therapeutic implications. Influenza Other Respir Viruses. 2013;7(Suppl 3):24–35.
Breese C, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, et al. Respiratory Syncytial Virus-Associated Hospitalizations Among Children Less Than 24 Months of Age. Pediatrics. 2013;132(2):E341–8.
Article Google Scholar
Mori M, Morio T, Ito S, Morimoto A, Ota S, Mizuta K, Iwata T, Hara T, Saji T. Risks and prevention of severe RS virus infection among children with immunodeficiency and Down’s syndrome. J Infect Chemother. 2014;20(7–8):455–9.
Temel MT, Demiryurek S, Temel L, Saracaloglu A, Eke N, Baysalman E, Mammadov A, Coskun ME, Demiryurek AT. Dynamic thiol/disulfide homeostasis in children with community-acquired pneumonia. Pediatr Int. 2019;61(3):252–7.
Neuman MI, Monuteaux MC, Scully KJ, Bachur RG. Prediction of pneumonia in a pediatric emergency department. Pediatrics. 2011;128(2):246–53.
Azab SF, Sherief LM, Saleh SH, Elsaeed WF, Elshafie MA, Abdelsalam SM. Impact of the socioeconomic status on the severity and outcome of community-acquired pneumonia among Egyptian children: a cohort study. Infect Dis Poverty. 2014;3:14.
Wen SW, Smith G, Yang Q, Walker M. Epidemiology of preterm birth and neonatal outcome. Semin Fetal Neonatal Med. 2004;9(6):429–35.
Kantor PF, Lougheed J, Dancea A, McGillion M, Barbosa N, Chan C, Dillenburg R, Atallah J, Buchholz H, Chant-Gambacort C, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013;29(12):1535–52.
Markovic-Sovtic G, Nikolic T, Sovtic A, Martic JM, Rakonjac Z. Pulmonary air leak syndrome in term and late preterm neonates. Srp Ark Celok Lek. 2019;147(9–10):578–82.
Sow FB, Gallup JM, Meyerholz DK, Ackermann MR. Gene profiling studies in the neonatal ovine lung show enhancing effects of VEGF on the immune response. Dev Comp Immunol. 2009;33(6):761–71.
Article CAS PubMed PubMed Central Google Scholar
Mawdsley JL, Bardgett RD, Merry RJ, Pain BF, Theodorou MK. Pathogens in livestock waste, their potential for movement through soil and environmental pollution. Appl Soil Ecol. 1995;2(1):1–15.
George EK, Mearin ML, Franken HC, Houwen RH, Hirasing RA, Vandenbroucke JP. Twenty years of childhood coeliac disease in The Netherlands: a rapidly increasing incidence? Gut. 1997;40(1):61–6.
Chen Z, Zhu Y, Wang Y, Zhou W, Yan Y, Zhu C, Zhang X, Sun H, Ji W. Association of meteorological factors with childhood viral acute respiratory infections in subtropical China: an analysis over 11 years. Arch Virol. 2014;159(4):631–9.
Lu L, Yan Y, Yang B, Xiao Z, Feng X, Wang Y, Ji W, Mize M, Hao C, Chen Z. Epidemiological and clinical profiles of respiratory syncytial virus infection in hospitalized neonates in Suzhou. China BMC Infect Dis. 2015;15:431.
Zhao Z, Zhou Y, Li W, Fan X, Huang Q, Tang Z, Li H, Wang J, Li J, Wu J. Discussion on China’s anti-epidemic response based on the Protocol on Prevention and Control of Coronavirus Disease 2019 from Chinese Authority. Int J Health Plann Manage. 2022;37(3):1205–20.
Crofts KF, Alexander-Miller MA. Challenges for the Newborn Immune Response to Respiratory Virus Infection and Vaccination. Vaccines (Basel). 2020;8(4):558.
CAS Google Scholar
Luna MS, Manzoni P, Paes B, Baraldi E, Cossey V, Kugelman A, Chawla R, Dotta A, Fernandez RR, Resch B, et al. Expert consensus on palivizumab use for respiratory syncytial virus in developed countries. Paediatr Respir Rev. 2020;33:35–44.
PubMed Google Scholar
Sanz F, Restrepo MI, Fernandez-Fabrellas E, Cervera A, Briones ML, Novella L, Aguar MC, Chiner E, Fernandez JF, Blanquer J. Does prolonged onset of symptoms have a prognostic significance in community-acquired pneumonia? Respirology. 2014;19(7):1073–9.
Ricci D, Romeo DM, Serrao F, Gallini F, Leone D, Longo M, Albamonte E, Romeo MG, Mazzone D, Romagnoli C, et al. Early assessment of visual function in preterm infants: How early is early? Early Human Dev. 2010;86(1):29–33.
Hentati N, Thabet AB, Bouraoui A, Ammous D, Gargouri A. PO-0689 Assessment Of Visual Function In Preterm Infants: Comparative Study About 68 Cases. Arch Dis Child. 2014;99(Suppl 2):A479–A479.
Collins A, Weitkamp JH, Wynn JL. Why are preterm newborns at increased risk of infection? Arch Dis Child Fetal Neonatal Ed. 2018;103(4):F391–4.
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Vento M, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology. 2017;111(2):107–25.
Rincon JC, Cuenca AL, Raymond SL, Mathias B, Nacionales DC, Ungaro R, Efron PA, Wynn JL, Moldawer LL, Larson SD. Adjuvant pretreatment with alum protects neonatal mice in sepsis through myeloid cell activation. Clin Exp Immunol. 2018;191(3):268–78.
He L, Yang L, Zhang H, Luo Q. Efficacy and safety of interferon on neonates with respiratory syncytial virus pneumonia. Exp Ther Med. 2020;20(6):220.
Pou C, Nkulikiyimfura D, Henckel E, Olin A, Lakshmikanth T, Mikes J, Wang J, Chen Y, Bernhardsson AK, Gustafsson A, et al. The repertoire of maternal anti-viral antibodies in human newborns. Nat Med. 2019;25(4):591–6.
Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004;23(1 Suppl):S87-97.
Don M, Valerio G, Canciani M, Korppi M. Hyponatremia in radiologically confirmed pediatric community-acquired pneumonia. Pediatr Emerg Care. 2014;30(1):76.
Don M, Valerio G, Korppi M, Canciani M. Hyponatremia in pediatric community-acquired pneumonia. Pediatr Nephrol. 2008;23(12):2247–53.
Stanley IJ, Kajumbula H, Bazira J, Kansiime C, Rwego IB, Asiimwe BB. Multidrug resistance among Escherichia coli and Klebsiella pneumoniae carried in the gut of out-patients from pastoralist communities of Kasese district, Uganda. PLoS ONE. 2018;13(7): e0200093.
Volakli E, Spies C, Michalopoulos A, Groeneveld AB, Sakr Y, Vincent JL. Infections of respiratory or abdominal origin in ICU patients: what are the differences? Crit Care. 2010;14(2):R32.
Koga M, Takahashi T, Kawai M, Fujihara K, Kanda T. A serological analysis of viral and bacterial infections associated with neuromyelitis optica. J Neurol Sci. 2011;300(1–2):19–22.
Orimoloye OA, Kambhampati S, Hicks AJ 3rd, Al Rifai M, Silverman MG, Whelton S, Qureshi W, Ehrman JK, Keteyian SJ, Brawner CA, et al. Higher cardiorespiratory fitness predicts long-term survival in patients with heart failure and preserved ejection fraction: the Henry Ford Exercise Testing (FIT) Project. Arch Med Sci. 2019;15(2):350–8.
Shook BC, Lin K. Recent Advances in Developing Antiviral Therapies for Respiratory Syncytial Virus. Top Curr Chem (Cham). 2017;375(2):40.
Stella SL, Velasco-Acosta DA, Skenandore C, Zhou Z, Steelman A, Luchini D, Cardoso FC. Improved uterine immune mediators in Holstein cows supplemented with rumen-protected methionine and discovery of neutrophil extracellular traps (NET). Theriogenology. 2018;114:116–25.
Qiao Y, Liang X, Yan Y, Lu Y, Zhang D, Yao W, Wu W, Yan Z. Identification of Exosomal miRNAs in Rats With Pulmonary Neutrophilic Inflammation Induced by Zinc Oxide Nanoparticles. Front Physiol. 2018;9:217.
Download references
Acknowledgements
This work was supported by Jiangsu maternal and child health research project [Shenglin Yu, grant number F202021].
Financial support for this study was provided by the Foundation of Jiangsu Province Health Committee (F202021).
Author information
Xinxian Guan and Shasha Gao contributed equally to this work.
Authors and Affiliations
Department of Neonatology, Children’s Hospital of Soochow University, Suzhou, China
Xinxian Guan, Shasha Gao, Yan Yang & Shenglin Yu
Institute of Pediatric Research, Children’s Hospital of Soochow University, Suzhou, China
He Zhao, Huiting Zhou & Jian Wang
You can also search for this author in PubMed Google Scholar
Contributions
Xinxian Guan and Shasha Gao wrote the main manuscript text and analysed the data. He Zhao and Huiting Zhou collected laboratory data and prepared Figs. 1 and 2 . Yan Yang collected clinical data and prepared Tables 1 and 2 . Shenglin Yu and Jian Wang have made substantial contributions to the conception and design. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Shenglin Yu or Jian Wang .
Ethics declarations
Ethics approval and consent to participate.
All procedures performed in studies involving human participants were carried out in accordance with the Committee on Publication Ethics and the International Committee of Medical Journal Editors. This study was approved by the Ethics Committee of Children’s Hospital of Soochow University (under number 2020CS004). The study was retrospective, and the data were anonymous, so the requirement for informed consent was waived by the Ethics Committee of Children’s Hospital of Soochow University.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Guan, X., Gao, S., Zhao, H. et al. Clinical characteristics of hospitalized term and preterm infants with community-acquired viral pneumonia. BMC Pediatr 22 , 452 (2022). https://doi.org/10.1186/s12887-022-03508-7
Download citation
Received : 31 March 2022
Accepted : 18 July 2022
Published : 27 July 2022
DOI : https://doi.org/10.1186/s12887-022-03508-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coinfection
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Effect of case management on neonatal mortality due to sepsis and pneumonia
Affiliation.
- 1 Department of Paediatrics and Child Health, the Aga Khan University, Karachi, Pakistan. [email protected]
- PMID: 21501430
- PMCID: PMC3231886
- DOI: 10.1186/1471-2458-11-S3-S13
Background: Each year almost one million newborns die from infections, mostly in low-income countries. Timely case management would save many lives but the relative mortality effect of varying strategies is unknown. We have estimated the effect of providing oral, or injectable antibiotics at home or in first-level facilities, and of in-patient hospital care on neonatal mortality from pneumonia and sepsis for use in the Lives Saved Tool (LiST).
Methods: We conducted systematic searches of multiple databases to identify relevant studies with mortality data. Standardized abstraction tables were used and study quality assessed by adapted GRADE criteria. Meta-analyses were undertaken where appropriate. For interventions with biological plausibility but low quality evidence, a Delphi process was undertaken to estimate effectiveness.
Results: Searches of 2876 titles identified 7 studies. Among these, 4 evaluated oral antibiotics for neonatal pneumonia in non-randomised, concurrently controlled designs. Meta-analysis suggested reductions in all-cause neonatal mortality (RR 0.75 95% CI 0.64- 0.89; 4 studies) and neonatal pneumonia-specific mortality (RR 0.58 95% CI 0.41- 0.82; 3 studies). Two studies (1 RCT, 1 observational study), evaluated community-based neonatal care packages including injectable antibiotics and reported mortality reductions of 44% (RR = 0.56, 95% CI 0.41-0.77) and 34% (RR = 0.66, 95% CI 0.47-0.93), but the interpretation of these results is complicated by co-interventions. A third, clinic-based, study reported a case-fatality ratio of 3.3% among neonates treated with injectable antibiotics as outpatients. No studies were identified evaluating injectable antibiotics alone for neonatal pneumonia. Delphi consensus (median from 20 respondents) effects on sepsis-specific mortality were 30% reduction for oral antibiotics, 65% for injectable antibiotics and 75% for injectable antibiotics on pneumonia-specific mortality. No trials were identified assessing effect of hospital management for neonatal infections and Delphi consensus suggested 80%, and 90% reductions for sepsis and pneumonia-specific mortality respectively.
Conclusion: Oral antibiotics administered in the community are effective for neonatal pneumonia mortality reduction based on a meta-analysis, but expert opinion suggests much higher impact from injectable antibiotics in the community or primary care level and even higher for facility-based care. Despite feasibility and low cost, these interventions are not widely available in many low income countries.
Funding: This work was supported by the Bill & Melinda Gates Foundation through a grant to the US Fund for UNICEF, and to Saving Newborn Lives Save the Children, through Save the Children US.
Publication types
- Meta-Analysis
- Research Support, Non-U.S. Gov't
- Anti-Bacterial Agents / therapeutic use*
- Case Management
- Controlled Clinical Trials as Topic
- Infant Mortality*
- Infant, Newborn
- Pneumonia / drug therapy*
- Pneumonia / mortality
- Sepsis / drug therapy*
- Sepsis / mortality
- Treatment Outcome
- Anti-Bacterial Agents
- Case report
- Open access
- Published: 25 March 2024
Stenotrophomonas maltophilia neonatal sepsis: a case report
- Williams Oluwatosin Adefila ORCID: orcid.org/0000-0002-1928-4862 1 ,
- Isaac Osie 1 , 2 ,
- Modou Lamin Keita 1 ,
- Baleng Mahama Wutor 1 ,
- Abdulsalam Olawale Yusuf 1 ,
- Ilias Hossain 1 ,
- Minteh Molfa 1 ,
- Ousman Barjo 1 ,
- Rasheed Salaudeen 1 &
- Grant Mackenzie 1 , 2 , 3 , 4
Journal of Medical Case Reports volume 18 , Article number: 180 ( 2024 ) Cite this article
177 Accesses
1 Altmetric
Metrics details
Stenotrophomonas maltophilia is a gram-negative bacteria known for causing opportunistic and nosocomial infections in humans. S. maltophilia is an emerging pathogen of concern due to it’s increasing prevalence, diverse disease spectrum, intrinsic multi-drug resistance and high mortality rates in immunocompromised individuals. S. maltophilia is a rare cause of neonatal sepsis associated with significant morbidity and mortality. The bacterium’s multi-drug resistance poses a considerable challenge for treatment, with various mechanisms contributing to its resistance.
Case presentation
We report a case involving a 40-h-old male African neonate who exhibited symptoms of neonatal sepsis. The blood culture revealed Stenotrophomonas maltophilia , which was sensitive to ciprofloxacin and gentamicin but resistant to other antibiotics. Lumbar puncture for CSF could not be done because the father declined.
We treated the newborn with the empirical first-line antibiotics as per the national guideline intravenous ampicillin and gentamicin for six days, and the child recovered fully with a repeated negative blood culture.
Conclusions
This report describes a neonatal sepsis case caused by S. maltophilia, a multi-drug resistant bacteria and a rare cause of neonatal sepsis. We report that early detection of the bacterial and antimicrobial management based on local antibiogram data may be essential for successful patient’s management.
Peer Review reports
Stenotrophomonas maltophilia causes human infections ranging from bacteremia, sepsis, endocarditis, pneumonia, and meningitis. It is a gram-negative, glucose non-fermentative aerobic rod with low virulence. The bacteria was described in 1961 by Hugh and Ryschenkow [ 1 ].
S. maltophilia is often pathogenic in immunocompromised and hospitalized patients, especially in the intensive care unit (ICU) [ 2 ]. It can also produce biofilms on prosthetic devices, such as catheters, mechanical ventilators, and feeding tubes [ 3 ]. The mortality rate of an S. maltophilia infection is high, primarily when associated with pneumonia (or bacteremia) and antibiotic resistance [ 4 ]. The resistance can be intrinsic, or the bacteria can develop resistance to antibiotics over time. The increasing use of antibiotics, especially broad-spectrum antibiotics, has contributed to the rise of multidrug-resistant (MDR) strains of S. maltophilia . MDR strains are resistant to a wide range of antibiotics, making them difficult to treat [ 5 ]. The outbreak of SARS-CoV-2 may have contributed to the increase in the prevalence of MDR bacteria, including S. maltophilia , because of the increased irrational use of antibiotics to treat COVID-19 [ 6 ].
A 40-h-old male African neonate was brought by the mother to our health facility, Bansang Hospital, at 7:30 am. Bansang Hospital (a secondary care facility) is in rural Gambia. It is the main referral centre for the country’s two largest regions (Central and Upper River Region). The mother complained of a persistently high fever that started about a day after delivery. She also complained of the child’s refusal to breastfeed with poor suckling ability, and was highly irritable. The mother also complained that the child had multiple episodic jerky movements, clenching the fist, lip-smacking, upward eye rolling, and limb stiffness during each episode. She noticed that the child had abnormal rapid breathing and a gradual decline in the child’s alertness. There was no history of vomiting or passage of loose, watery stool. There was no history of yellowish discolouration of the eye or the skin.
The mother was a primigravid, carrying the pregnancy to term with only two antenatal visits. She was not a known diabetic or hypertensive. She was not on any long-term medication. She was HIV-negative and received the required prenatal tetanus toxoid vaccination.
The pregnancy was carried to term. There was a history of premature membrane rupture and liquor drainage for more than two days before the onset of labour. The draining amniotic fluid was not blood-stained but had a foul odour. The mother also complained of a foul-smelling whitish-brown vaginal discharge with no associated itching two weeks before the delivery. The mother did not receive any antibiotics before delivery. She delivered in the hospital through spontaneous vaginal delivery after more than 18 h of prolonged labour. The child’s birth weight was 3kg, and the newborn did not cry immediately after birth, necessitating further resuscitation. Apgar score in the first minute was three, and at ten minutes, it was seven. After a successful resuscitation, the child was allowed to breastfeed, and the mother described the suckling reflex as weak. They were discharged home less than 24 h after delivery.
The parents noticed the newborn having a persistent high-grade fever that started the following day after birth, not feeding, and becoming weak at home. The grandmother gave the newborn some undetermined syrups to reduce the fever. The newborn was not improving; hence, the mother brought him to the hospital.
Physical examination revealed an acutely ill-looking newborn with hyperpyrexia (temperature of 39.1 degrees Celsius), not pale, anicteric, not cyanosed, responsive to pain only and having seizures. The child weighed 3kg.
The central nervous system examination showed a newborn with poor suckling reflex, unresponsive except to pain, poor Moro reflex, normal fontanelles, normal tones globally and lethargic.
The respiratory rate was 65 breaths per minute, hypoxia with oxygen saturation of 80%, absent subcostal retraction, nil dullness to chest percussion, and broncho-vesicular breath sounds.
His heart rate was 153 beats per minute, with no abnormal heart sounds on auscultation.
The rest of the physical examination was unremarkable.
The random blood sugar was 5.1 mmol/l (91.8 mg/dl). We requested a blood culture, microscopy, and sensitivity, and a chest x-ray.
We counselled the parents for lumbar puncture for cerebrospinal fluid analysis, but the father declined.
A diagnosis of early-onset neonatal sepsis with a differential diagnosis of neonatal meningitis and perinatal asphyxia was made.
The newborn was started on empirical first-line antibiotics as per the national guideline: IV ampicillin 50mg/kg every 6 h and IV gentamicin 5mg/kg daily. Also, we put the child on nil per oral, intravenous maintenance fluid 34mls every three hours and a stat dose of IV phenobarbitone. Moreover, we placed the child on humidified oxygen, one litre per minute, through a nasal prong and monitored the vital signs, including random blood sugar.
Results of investigation
The chest x-ray findings were essentially normal. The blood culture yielded glucose non-fermenting colonies, and the Gram stain showed gram-negative rods. We processed the bacterium for identification using the Analytical Profile Index (API) 20E. The identification number 1202000 on the API is consistent with Stenotrophomonas maltophilia. Antibiotic susceptibility testing of the pathogen using the disc diffusion method showed sensitivity to ciprofloxacin and gentamicin. The organism was resistant to ceftriaxone, tetracycline, chloramphenicol, and ampicillin.
Progress report
After 48 h of admission, the fever subsided, and the child had one episode of convulsion. He was alert but had a poor suckling reflex. After 72 h of hospital admission, he had no seizures in the previous 36 h, had started breastfeeding, and no complaints or abnormalities were noted. After four days of hospital admission, he had a normal suckling reflex, and the mother had adequate lactation. No anomaly was detected, and we stopped the intravenous maintenance fluid. We did not change the antibiotic regimen because the child improved well clinically on the initial antibiotics administered. There were no observed adverse effects or complications during the treatment. After six days on admission, the newborn was discharged home for a follow-up in one week. The mother had no complaints at follow-up, and the child was feeding well with no abnormal signs.
Discussion and conclusions
The prevalence of Stenotrophomonas maltophilia isolation described from different hospitals ranged from 7.1 to 37.7 cases per 10,000 discharges or 18.3 cases per 1,000 patient days. The majority of these reports were investigated after a perceived increase in the incidence and prevalence of the organism[ 7 ].
Neonatal sepsis is a leading cause of death in newborns in sub-Saharan Africa. It is estimated that 380,000 to 2 million cases of neonatal sepsis occur in sub-Saharan Africa each year and that 270,000 are fatal [ 7 ]. Neonatal resuscitation, low birth weight, and prematurity are risk factors for neonatal sepsis. Prolonged and premature membrane rupture, multiple vaginal examinations, meconium-stained amniotic fluid, and intrapartum fever are maternal risk factors for neonatal sepsis [ 8 ]. Gram-positive bacteria (Staphylococcus aureus , Streptococcus pyogenes, group B streptococcus, and group D streptococcus ) and gram-negative bacteria ( Klebsiella, Escherichia coli, Pseudomonas, Enterobacter spp, and Salmonella spp.) are the most common causes of neonatal sepsis in sub-Saharan Africa [ 9 , 10 , 11 ].
S . maltophilia is a rare cause of neonatal sepsis globally and in sub-Saharan Africa, but its emergence has significant implications for patient management due to antimicrobial resistance [ 12 ]. S. maltophilia is categorized by the World Health Organization (WHO) categorized as a primary multi-drug-resistant bacteria in hospital settings [ 13 ]. The prevalence of S. maltophilia infections increased in the general population, with more cases in the Western Pacific regions (from 6% to 18.6%) and lower prevalence in the American regions (from 3.2% to 5.7%) in 1991 and 2019 [ 13 ]. S. maltophilia has exhibited an increasing tendency towards drug resistance through multiple mechanisms. These mechanisms include the expression of resistance genes acquired from the environment, reduced outer membrane permeability, and transposons encoded within the chromosome and plasmids [ 14 ]. Furthermore, other mechanisms of antimicrobial resistance include the production of β-lactamase enzymes, integrons, biofilms, and multi-drug efflux [ 15 , 16 , 17 ].
Our patient’s mother had prolonged prelabour rupture of membranes and prolonged labour. These risk factors predisposed the neonate to acquire sepsis with S. maltophilia. Also, the neonate had perinatal asphyxia and needed to be resuscitated. This was another avenue through which the neonate may have acquired the infection.
Even though prematurity and low birth weight have been reported as risk factors for S. maltophilia infection in the newborn , our case was term and had a normal birth weight but with significant other risk factors for sepsis [ 17 , 18 ]. Since 1977, the first case of S. maltophilia was documented, and only very few cases of meningitis associated with S. maltophilia have been documented [ 19 , 20 ]. The management of S. maltophilia infection is challenging due to the bacterium’s resistance to multiple antimicrobials used to treat Gram-negative infections [ 10 ]. Trimethoprim-sulfamethoxazole and flouroquininolones are typically effective against S. maltophilia strains, but due to its unfavourable side effects, it is not recommended for use in newborns [ 21 ]. In our patient, we found that the pathogen was susceptible to ciprofloxacin and gentamicin and resistant to ceftriaxone, tetracycline, chloramphenicol, and ampicillin. The organism’s resistance to commonly available antibiotics such as ampicillin and ceftriaxone, poses a challenge to effective clinical management. It increases the chances of neonatal mortality and morbidity, especially in resource-limited settings.
Neonatal sepsis can be devastating due to its high mortality and potential long-term consequences and the risk is further amplified when S. maltophilia is indicated [ 22 ]. However, this can be prevented through early detection. Early detection of S. maltophilia and management is critical because of its aggressive nature as an opportunistic pathogen, non-specific neonatal symptoms, rapid progression, and improved treatment outcomes [ 23 ].
The index newborn responded well to the first-line empirical treatment combination of ampicillin and gentamicin, and the child recovered fully. Hence, it is essential to note that the chosen antibiotic combination had a high success rate despite the organism’s inherent multi-drug resistance. Furthermore, this case has highlighted the role of S. maltophilia as an emerging cause of nosocomial infections in vulnerable patients such as neonates in resource limited settings. Clinicians should have a high suspicion to identify patients at high risk for S. maltophilia infections. Early detection and prompt management using clinical and microbiological evidence are paramount to successfully managing S. maltophilia neonatal sepsis.
Due to the parents’ refusal to perform a lumbar puncture to confirm our suspicion of meningitis, a blood culture was essential for diagnosing S. maltophilia infection in our patient. We had other limitations in conducting further crucial diagnostic investigations like complete blood count and C-reactive protein because our laboratory is under-equipped.
This report shows that although S. maltophilia is a rare cause of neonatal sepsis, early detection and management based on local antibiogram data is essential for excellent patient outcomes. The lack of microbiology services in many settings in sub-Saharan Africa will mean that infections with S. maltophilia are under-detected and often inadequately treated.
Availability of data
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
Intravenous
Intensive care unit
Multi-drug resistant
Analytical Profile Index
World Health Organization
Ibrahim J, Hamwi N, Rabei H, Abdelghafar M, Al-Dulaimi Z, Al TH. Stenotrophomonas maltophilia meningitis in a term healthy neonate: a case report and literature review. Case Rep Pediatr. 2018;13(2018):1543934.
Google Scholar
Dimopoulos G, Garnacho-Montero J, Paramythiotou E, Gutierrez-Pizarraya A, Gogos C, Adriansen-Pérez M, et al . Upraising Stenotrophomonas maltophilia in critically ill patients: a new enemy? Diagnostics. 2023;13(6):1106.
Article CAS PubMed PubMed Central Google Scholar
Pompilio A, Ranalli M, Piccirilli A, Perilli M, Vukovic D, Savic B, et al . Biofilm formation among Stenotrophomonas maltophilia Isolates has clinical relevance: the ANSELM prospective multicenter study. Microorganisms. 2020;9(1):49.
Article PubMed PubMed Central Google Scholar
Zöllner SK, Kampmeier S, Froböse NJ, Herbrüggen H, Masjosthusmann K, van den Heuvel A, et al . Stenotrophomonas maltophilia infections in pediatric patients—experience at a European center for pediatric hematology and oncology. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.752037 .
Hafiz TA, Aldawood E, Albloshi A, Alghamdi SS, Mubaraki MA, Alyami AS, et al . Stenotrophomonas maltophilia epidemiology, resistance characteristics, and clinical outcomes: understanding of the recent three years’ trends. Microorganisms. 2022;10(12):2506.
Petrakis V, Panopoulou M, Rafailidis P, Lemonakis N, Lazaridis G, Terzi I, et al . The impact of the COVID-19 pandemic on antimicrobial resistance and management of bloodstream infections. Pathogens. 2023;12(6):780.
Toro MDD, Rodríguez-Baño J, Herrero M, Rivero A, García-Ordoñez MA, Corzo J, et al . Clinical epidemiology of Stenotrophomonas maltophilia colonization and infection: a multicenter study. Medicine. 2002;81(3):228.
Article PubMed Google Scholar
Ranjeva SL, Warf BC, Schiff SJ. Economic burden of neonatal sepsis in sub-Saharan Africa. BMJ Glob Health. 2018;3(1): e000347.
Bech CM, Stensgaard CN, Lund S, Holm-Hansen C, Brok JS, Nygaard U, et al . Risk factors for neonatal sepsis in Sub-Saharan Africa: a systematic review with meta-analysis. BMJ Open. 2022;12(9): e054491.
Okomo U, Akpalu ENK, Doare KL, Roca A, Cousens S, Jarde A, et al . Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect Dis. 2019;19(11):1219–34.
Article CAS PubMed Google Scholar
Okomo UA, Darboe S, Bah SY, Ayorinde A, Jarju S, Sesay AK, et al . Maternal colonization and early-onset neonatal bacterial sepsis in the Gambia, West Africa: a genomic analysis of vertical transmission. Clin Microbiol Infect. 2023;29(3):386.e1-386.e9.
Blumenröder S, Wilson D, Ndaboine E, Mirambo MM, Mushi MF, Bader O, et al . Neonatal infection in Sub-Saharan Africa: a cross-sectional pilot study on bacterial pathogens and maternal risk factors. Front Microbiol. 2023. https://doi.org/10.3389/2Ffmicb.2023.1171651 .
Soren C, Jagtap S, Malathi V. Stenotrophomonas maltophilia : a rare cause of early onset neonatal sepsis. Int J Contemp Pediatr. 2018;5(5):2006–7.
Article Google Scholar
Geller M, Nunes CP, Oliveira L, Nigri RS. maltophilia pneumonia: a case report. Respir Med Case Rep. 2018;9(24):44–5.
Banar M, Sattari-Maraji A, Bayatinejad G, Ebrahimi E, Jabalameli L, Beigverdi R, et al . Global prevalence and antibiotic resistance in clinical isolates of Stenotrophomonas maltophilia : a systematic review and meta-analysis. Front Med. 2023. https://doi.org/10.3389/fmed.2023.1163439 .
Gil-Gil T, Martínez JL, Blanco P. Mechanisms of antimicrobial resistance in Stenotrophomonas maltophilia : a review of current knowledge. Expert Rev Anti Infect Ther. 2020;18(4):335–47.
Behera B. Stenotrophomonas maltophilia , an emerging pathogen in newborns: three case reports and a review of the literature. WJCID. 2021;11(1):11–8.
Mutlu M, Yılmaz G, Aslan Y, Bayramoğlu G. Risk factors and clinical characteristics of Stenotrophomonas maltophilia infections in neonates. J Microbiol Immunol Infect. 2011;44(6):467–72.
Yemisen M, Mete B, Tunali Y, Yentur E, Ozturk R. A meningitis case due to Stenotrophomonas maltophilia and review of the literature. Int J Infect Dis. 2008;12(6):e125-127.
Libanore M, Bicocchi R, Pantaleoni M, Ghinelli F. Community-acquired infection due to Stenotrophomonas maltophilia : a rare cause of meningitis. Int J Infect Dis. 2004;8(5):317–9.
Jia W, Wang J, Xu H, Li G. Resistance of Stenotrophomonas maltophilia to fluoroquinolones: prevalence in a university hospital and possible mechanisms. Int J Environ Res Public Health. 2015;12(5):5177.
Verma A, Patnaik SK, Suryawanshi P. Stenotrophomonas maltophilia sepsis in preterm neonates. Medical Journal Armed Forces India [Internet]. 2023. https://www.sciencedirect.com/science/article/pii/S0377123723000102 . Accessed 9 Jan 2024.
Alsuhaibani M, Aljarbou A, Althawadi S, Alsweed A, Al-Hajjar S. Stenotrophomonas maltophilia bacteremia in children: risk factors and mortality rate. Antimicrob Resist Infect Control. 2021;10(1):19.
Download references
Acknowledgements
We appreciate the paediatric team of the Bansang Hospital, the microbiology team and the haematology department for the excellent communication and management of this patient.
No funds were used to produce this manuscript.
Author information
Authors and affiliations.
Medical Research Council Unit The Gambia at London, School of Hygiene and Tropical Medicine, West Africa, PO Box 273, Fajara, The Gambia
Williams Oluwatosin Adefila, Isaac Osie, Modou Lamin Keita, Baleng Mahama Wutor, Abdulsalam Olawale Yusuf, Ilias Hossain, Minteh Molfa, Ousman Barjo, Rasheed Salaudeen & Grant Mackenzie
Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK
Isaac Osie & Grant Mackenzie
Murdoch Children’s Research Institute, Melbourne, Australia
Grant Mackenzie
Department of Paediatrics, University of Melbourne, Melbourne, Australia
You can also search for this author in PubMed Google Scholar
Contributions
OB, MM, and RS provided the microbiology laboratory work results and assisted in drafting the case report. BMW and AOY assisted in the drafting of the case report. MLK assisted in the patient’s management and also in writing the manuscript. IO and GM provided clinical work support, supervision, and writing of the case report manuscript.
Corresponding author
Correspondence to Williams Oluwatosin Adefila .
Ethics declarations
Ethics approval and consent to participate.
The clinical care provided was in a clinical trial approved by the Gambian Government Ministry of Health and Medical Research Council Gambia Joint Ethics Committee.
Consent for publication
We obtained informed consent from the patient’s father to publish this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests" in this section.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Adefila, W.O., Osie, I., Keita, M.L. et al. Stenotrophomonas maltophilia neonatal sepsis: a case report. J Med Case Reports 18 , 180 (2024). https://doi.org/10.1186/s13256-024-04479-2
Download citation
Received : 29 November 2023
Accepted : 28 February 2024
Published : 25 March 2024
DOI : https://doi.org/10.1186/s13256-024-04479-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neonatal sepsis
- Stenotrophomonas maltophilia
- Antibiotic treatment
- Multidrug-resistant
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Public Health

Effect of case management on neonatal mortality due to sepsis and pneumonia
Anita k m zaidi.
1 Department of Paediatrics and Child Health, the Aga Khan University, Karachi, Pakistan
Hammad A Ganatra
Simon cousens.
2 London School of Tropical Medicine and Hygiene, London, UK
Anne CC Lee
3 Johns Hopkins Bloomberg School of Public Health, International Health, Baltimore MD, USA
Robert Black
Zulfiqar a bhutta.
4 Saving Newborn Lives/Save the Children, Cape Town, South Africa
Associated Data
Each year almost one million newborns die from infections, mostly in low-income countries. Timely case management would save many lives but the relative mortality effect of varying strategies is unknown. We have estimated the effect of providing oral, or injectable antibiotics at home or in first-level facilities, and of in-patient hospital care on neonatal mortality from pneumonia and sepsis for use in the Lives Saved Tool (LiST).
We conducted systematic searches of multiple databases to identify relevant studies with mortality data. Standardized abstraction tables were used and study quality assessed by adapted GRADE criteria. Meta-analyses were undertaken where appropriate. For interventions with biological plausibility but low quality evidence, a Delphi process was undertaken to estimate effectiveness.
Searches of 2876 titles identified 7 studies. Among these, 4 evaluated oral antibiotics for neonatal pneumonia in non-randomised, concurrently controlled designs. Meta-analysis suggested reductions in all-cause neonatal mortality (RR 0.75 95% CI 0.64- 0.89; 4 studies) and neonatal pneumonia-specific mortality (RR 0.58 95% CI 0.41- 0.82; 3 studies). Two studies (1 RCT, 1 observational study), evaluated community-based neonatal care packages including injectable antibiotics and reported mortality reductions of 44% (RR= 0.56, 95% CI 0.41-0.77) and 34% (RR =0.66, 95% CI 0.47-0.93), but the interpretation of these results is complicated by co-interventions. A third, clinic-based, study reported a case-fatality ratio of 3.3% among neonates treated with injectable antibiotics as outpatients. No studies were identified evaluating injectable antibiotics alone for neonatal pneumonia. Delphi consensus (median from 20 respondents) effects on sepsis-specific mortality were 30% reduction for oral antibiotics, 65% for injectable antibiotics and 75% for injectable antibiotics on pneumonia-specific mortality. No trials were identified assessing effect of hospital management for neonatal infections and Delphi consensus suggested 80%, and 90% reductions for sepsis and pneumonia-specific mortality respectively.
Oral antibiotics administered in the community are effective for neonatal pneumonia mortality reduction based on a meta-analysis, but expert opinion suggests much higher impact from injectable antibiotics in the community or primary care level and even higher for facility-based care. Despite feasibility and low cost, these interventions are not widely available in many low income countries.
This work was supported by the Bill & Melinda Gates Foundation through a grant to the US Fund for UNICEF, and to Saving Newborn Lives Save the Children, through Save the Children US.
Deaths occurring in the neonatal period each year account for 41% (3.6 million) of all deaths in children under 5 years [ 1 ]. The majority of these deaths occur in low income countries and almost 1 million of these deaths are attributable to infectious causes including neonatal sepsis, meningitis and pneumonia [ 1 ]. These deaths occur because of lack of preventive care (clean birth care, breastfeeding) and appropriate case management [ 2 ]. Delays in treating neonatal infections of even a few hours may be fatal. Delays in illness recognition and care seeking, a dearth of primary health care providers, and limited access to facility care contribute to these deaths [ 3 ]. Recent trials have demonstrated the effect of community-based packages for prevention and treatment of neonatal bacterial infections, with the potential to save many lives [ 4 , 5 ].
Therapy with appropriate antibiotics and supportive management in neonatal nurseries is the cornerstone of management of neonatal sepsis and pneumonia, with strong biological plausibility that such therapy saves lives. Yet the quality of evidence is understandably affected by the ethical impossibility of undertaking randomized trials of antibiotic management compared with no antibiotic management. Nevertheless, given the limited access to care for sick neonates in low income countries, it is important to assess the potential mortality effect of oral antibiotics and injectable antibiotics delivered in domiciliary or primary care settings. Case management for hospitalized neonates is more expensive, but to guide policy and program investments we also need to know how much more effective it is compared to care delivered at home or in primary care settings.
The objective of this review is to provide estimates of the effectiveness of three interventions in preventing neonatal deaths from severe infection: (i) case management with oral antibiotic therapy alone for pneumonia and sepsis; (ii) case management with injectable antibiotics (± oral antibiotics) as an outpatient or at home for neonatal sepsis /meningitis and pneumonia; and (iii) hospital-based case management, including injectable antibiotics, intravenous fluids, oxygen therapy, second line injectable antibiotics if needed, and other supportive therapy (Table (Table1). 1 ). These mortality effect estimates are used in the Lives Saved Tool (LiST) software, a user-friendly tool that estimates the number of lives saved by scaling up key interventions and helps in child survival planning in low income countries [ 6 , 7 ].
Definitions of interventions reviewed
We searched all published literature as per CHERG systematic review guidelines[ 7 ]. Databases searched were PubMed, Cochrane Libraries and WHO regional databases from 1990 until April 2009 and included publications in any language (Figure (Figure1). 1 ). Search terms included various combinations of: sepsis, meningitis and pneumonia. For sepsis and pneumonia management at a hospital level we conducted two parallel searches (Figures (Figures2 2 and and3). 3 ). These were broader as we also wanted to identify studies reporting incidence and case fatality ratios (CFR) for a related study on global burden of neonatal sepsis. Titles and abstracts were reviewed and studies were included if data on one of the following outcomes was provided: all-cause mortality, sepsis/meningitis/pneumonia mortality and/or CFR. Furthermore, extensive efforts were made to contact investigators and program managers for unpublished data.

Searches and screening for community based management of sepsis and pneumonia.

Searches and screening for hospital management of sepsis

Searches and screening for hospital management of pneumonia.
Inclusion/exclusion criteria, abstraction
We reviewed all available observational studies, randomized controlled trials, systematic reviews, and meta-analyses, which included neonates and principally involved the management of serious neonatal infections. The search was limited to “humans”. We examined studies published from 1990 until April 2009.
We included randomized controlled trials, studies with concurrent controls, and observational studies with no control group if mortality outcomes were reported. All studies meeting final inclusion criteria were double data abstracted into a standardized form. We abstracted key variables with regard to the study identifiers and context, study design and limitations, intervention specifics, and mortality outcomes. We assessed the quality of each of these studies using a standard table employing an adapted version of GRADE[ 8 ] developed by the Child Health Epidemiology Reference Group (CHERG) [ 7 ]. For studies which reported mortality outcomes that were not neonatal specific, we contacted the authors to request the neonatal-specific data. All studies that were coded are included in Additional File 1 .
Case definition of sepsis
During our review of selected studies we were unable to find a standard definition for clinical neonatal sepsis or pneumonia (Table (Table2). 2 ). Each study used different criteria although most are a variation on WHO IMCI approach. We therefore decided to accept authors’ definitions of sepsis and pneumonia, recognizing that these non-specific definitions lower mortality outcome estimates as many “non-sepsis” cases are included in an effort to maximize sensitivity.
Varying definitions of neonatal sepsis used by investigators and clinicians
Analyses and summary measures
All studies reporting mortality data for pneumonia and sepsis management, in community and hospital settings, were summarized according to the overall quality of evidence for each outcome and each data input type using an adapted version of the GRADE 21 protocol table [ 7 ]. When appropriate, we conducted meta-analyses to obtain pooled estimates of the risk ratios, using either the Mantel-Haenzsel or, when there was evidence of heterogeneity, the DerSimonian-Laird random effects estimator. 95% confidence intervals (CI) were also calculated. Statistical analyses were performed using STATA 10.0 ( http://www.stata.com ).
Delphi Process for Establishing Expert Consensus
For intervention-outcome combinations for which we did not identify moderate quality evidence, we sought expert consensus via the Delphi method. Individuals invited to participate were experts in newborn health and sepsis representing six WHO regions (South Asia, Africa, Western Europe, Eastern Europe, North America, Australia), and including multiple disciplines - international health, pediatric infectious diseases, clinical neonatology, and general pediatrics. Twenty (of twenty-three experts invited) agreed to participate in the Delphi process. The questionnaire was developed by JL, AZ, SC and SS, and refined after several rounds of pilot testing. The questionnaire was sent by email and included the background and aims of the Delphi and estimates of effect that were available from the literature for different scenarios. The median response and range were determined for each question. Consensus was defined a priori as an interquartile range in responses of not more than 30% for each question. For those estimates not reaching consensus, the plan was for results to be electronically distributed to the panel, virtual discussion allowed, and a second round of email questionnaires sent. However, consensus was achieved after one round of questionnaires and subsequent rounds were not necessary.
Studies identified
Our systematic searches for community management of sepsis and pneumonia identified 2876 titles (Figure (Figure1) 1 ) and after screening of titles, abstracts and relevant full texts, we located 7 studies of interest (reported in 8 papers) [ 9 - 16 ]. We identified 4 non randomised concurrently controlled studies, which evaluated oral antibiotics for pneumonia (Table (Table5) 5 ) [ 10 , 14 - 16 ]. Three of these studies did not report disaggregated neonatal outcomes in the primary papers, but neonatal outcomes were available through abstracted forms from an earlier meta-analysis by Sazawal et al [ 17 ]. For management of neonatal sepsis using injectable antibiotics, we located 3 studies (reported in 4 papers) [ 9 , 11 - 13 ]. There was one observational primary clinic-based study without a control group [ 13 ], one RCT [ 12 ] and one non-randomised, concurrently controlled study [ 9 ]. The fourth paper reported observational data from individual infants evaluated during the RCT mentioned above and was not a separate study [ 11 ]. All the studies were from high neonatal mortality regions.
Summary of community-based studies for case management with oral antibiotics for and effect on cause specific neonatal mortality due to pneumonia
In our search for hospital-based studies of sepsis we found 55 studies from a total pool of 13998 studies which reported sepsis and/or meningitis mortality outcomes (Figure (Figure2) 2 ) [ 18 - 70 ]. For pneumonia, we found two studies from a total pool of 94 studies (Figure (Figure3) 3 ) [ 71 , 72 ].
The details of each study and quality assessment using GRADE are summarised in Tables Tables3, 3 , ,4, 4 , ,5, 5 , and and6 6 .
GRADE assessment of studies of effect of case management on cause specific neonatal mortality due to pneumonia
GRADE assessment of studies of case management on cause specific neonatal mortality due to neonatal sepsis
*NMR LEVELs (1=NMR <5 per 1000 live births, 2=NMR 6 to 15 per 100 live births, 3= NMR 15 to 30 per 100 live births, 4=NMR 31-45 per 100 live births 5=NMR >45 per 100 live births
Summary of community-based studies including injectable antibiotics for case management of neonatal sepsis (observational, quasi experimental, and RCT)
* Combined data from years 2 and 3 of trial i.e. 1996-1997 and 1997-1998.
**Observational data on individual infants evaluated during the cluster randomized trial by Baqui et al. Control group is families unable to comply with referral and were not offered treatment with injectable antibiotics at home.
Evidence for effectiveness of oral antibiotic therapy alone
Unpublished neonatal data were obtained from the principal investigators of the four studies identified and a new meta-analysis was done to update that of Sazawal et al[ 17 ]. We performed meta analyses for two outcomes: oral antibiotics were associated with reductions in both all-cause mortality (4 studies [ 10 , 14 - 16 ]: RR 0.75 95% CI 0.64- 0.89) (Figure (Figure4) 4 ) and pneumonia-specific mortality (3 studies [ 10 , 15 , 16 ]: RR 0.58 95% CI 0.41- 0.82) (Figure (Figure5). 5 ). Limitations included non-randomization, estimation of intervention coverage as precise coverage estimates were not available;and variability between studies of the intensity of co-interventions. We found no studies of the effect of oral antibiotics on sepsis-specific mortality. The Delphi consensus (median) was for a 28% reduction in sepsis-specific mortality with an interquartile range of 20% to 36.25% (Figure (Figure6 6 ).

Meta-analysis of observational studies comparing oral antibiotics versus none in the community setting for babies: All cause mortality. Legend: Heterogeneity chi-squared = 1.17 (d.f. = 3) p = 0.760 I-squared (variation in RR attributable to heterogeneity) = 0.0% Test of RR=1 : z= 3.32 p = 0.001

Meta-analysis of observational studies comparing oral antibiotics versus none in the community setting for babies: Pneumonia mortality. Legend: Heterogeneity chi-squared = 2.16 (d.f. = 2) p = 0.339 I-squared (variation in RR attributable to heterogeneity) = 7.5% Test of RR=1: z= 3.06 p = 0.002

Box plot of Delphi expert opinion estimates of reduction in neonatal cause specific mortality due to pneumonia and sepsis/meningitis
Evidence for effectiveness of injectable antibiotic therapy (±oral antibiotics)
Three studies reported in four papers, were identified (Table (Table6) 6 ) [ 9 , 11 - 13 ]. One, an RCT[ 12 ] evaluated the impact of a perinatal care package which included the administration of injectable antibiotics in domiciliary settings in situations where referral to hospital was not possible. This trial reported a reduction in all-cause neonatal mortality of 34% (RR=0.66, 95%CI 0.47-0.93). A second paper from the same study reported that the CFR for neonates who were evaluated and actually treated with injectable antibiotics was 4.4% [ 11 ]. A non-randomized, concurrently controlled study [ 9 ] also evaluated the impact of a home-based neonatal care package in which septic neonates were treated with injectable antibiotics when referral to hospital was not possible. The overall mortality reduction in the intervention arm of the trial was calculated to be 44% (RR=0.56 95% CI 0.41-0.77). A third, uncontrolled study [ 13 ] based in a primary care clinic reported a CFR of 3.3% among septic children treated with injectable antibiotics.
In both of the community-based studies [ 9 , 12 ] injectable antibiotics were only one component of comprehensive community-based newborn care packages, and therefore the effectiveness of injectable antibiotics alone in the community cannot be reliably estimated. The Delphi consensus for the effect of injectable antibiotics was for a 65% reduction (interquartile range of 50-70%) in sepsis-specific mortality and 75% reduction (interquartile range of 70-81.25%) in pneumonia-specific mortality in community-based settings (Figure (Figure6 6 ).
Evidence for effectiveness of inpatient hospital case management
We found no trials assessing the impact of hospital-based case management and the observational studies of hospital management showed wide variation in effect. Searches conducted for studies reporting CFRs in neonates with pneumonia in health facilities revealed very few data. Two studies were identified with author-defined neonatal pneumonia; both were from low income, non-industrialised settings and reported CFRs of 14.4% [ 72 ] and 30.8% [ 71 ].
CFRs for neonatal sepsis, adjusted for the proportion of very low birth weight babies in the study, were plotted against national percentage skilled delivery, as a proxy for access to hospital-based case management of neonatal sepsis. In countries with a high proportion of births attended by skilled attendants, the predicted CFR for sepsis was 9.5%, whereas in countries with a low proportion (<30%) skilled birth attendance, the predicted CFR for sepsis with hospital care is 20-30% (Figure (Figure7). 7 ). A 68% reduction in the CFR for neonatal sepsis is predicted as one moves from 0% to100% skilled birth attendance. This reduction is likely to under estimate the effect of hospital-based case management since skilled birth attendance is likely to be a poor surrogate for effective facility case management of neonatal infections, but was used in the absence of coverage data for case management

Plot of neonatal sepsis CFR versus percent skilled delivery as a marker of access to facility care. Model fitted: outcome = log(CFR) Covariates = Skilled attendant coverage and % babies vLBW Fitted line is predicted CFR for settings with % VLBW<30%. Predicted CFR at 0% skilled attendance is 30%. Predicted CFR at 100% skilled attendance is 9.5%. % reduction = 68.5% Coefficient skilled attendance is 0.12 on the log scale (95% CI -0.02 to -0.007); i.e. for each 1% increase in skilled attendance rate CFR is reduced by 1.1% (95% CI: 0.7% to 1.6%)
Although the quality of evidence is low according to GRADE criteria, the recommendation for case management of neonatal infections is strong, and this is standard practice globally. Table Table7 7 provides a summary of the effect of case management on neonatal sepsis and pneumonia cause specific mortality, and GRADE of the estimate. Therefore the Delphi process was used to provide estimates for the effect of hospital care. The Delphi consensus was for a 80% reduction in sepsis-specific mortality (interquartile range 75% to 85%), and a 90% reduction in pneumonia-specific mortality (interquartile range 88.75% to 95%) (Figure (Figure6 6 ).
Effect of case management on neonatal sepsis and pneumonia cause specific mortality, and GRADE of the estimate
Infections including sepsis, meningitis and pneumonia are responsible for almost a million neonatal deaths annually. Neonates are more susceptible to severe infections and the progression of disease is more rapid due to developmental immunodeficiency, resulting in high CFRs. Also, a significant proportion of infections may arise early, after vertical transmission from the mother [ 73 ]. Therefore, timely identification and appropriate management with antibiotics is an important strategy to reduce the burden of neonatal mortality due to infections. We have previously reported the evidence from observational and experimental studies in low income countries for community-based management of neonatal infections (pneumonia and sepsis) with oral and injectable antibiotics [ 74 - 76 ]. We have now undertaken a systematic review of available evidence, including from industrialized countries and facility settings, and where the quality of evidence is low we have undertaken a Delphi expert process to estimate the cause-specific mortality effect.
This review of effectiveness of the interventions is shaped in large part by the needs of the LiST model. In that model, increasing coverage of an intervention results in a reduction in deaths due to one or more specific causes or in reduction of a risk factor. Therefore the reviews and the GRADE process used were designed to develop estimates of the effect of an intervention in reducing death due to specific causes. For more details of the review methods, the adapted GRADE approach or the LiST model see related publications [ 6 , 7 ].
To our knowledge, this is the first review providing effectiveness estimates for case management options to reduce neonatal deaths due to neonatal sepsis/meningitis and pneumonia, in both community and facility settings. Theodoratou et al have previously estimated effectiveness of pneumonia case management in children under 5 years but they did not disaggregate neonatal mortality data from later child mortality [ 77 ]. The estimated effect of community case management on pneumonia mortality in children under 5 years of age in the analysis by Theodoratou et al is 70% (77). Oral antibiotics in community settings for neonatal pneumonia in our analysis were associated with a 42% reduction in pneumonia-specific mortality and a 25% reduction in all-cause neonatal mortality based on a meta-analysis of available trials. There is no evidence to estimate the effect of oral antibiotics on sepsis-specific mortality, but our Delphi process suggested a 28% reduction. Delphi-derived estimates for the effects of management using injectable antibiotics delivered in home or primary care settings came out at 65% for sepsis-specific mortality and 75% for pneumonia-specific mortality. These estimates are biologically plausible and consistent with published studies [ 9 , 12 ] which reported reductions in all-cause neonatal mortality (sepsis plus other causes) of 34% and 44% respectively with community-based packages including injectable antibiotics. CFRs reported from observational studies of hospital case management varied widely, from 6.7 to 67%. Our Delphi estimates suggested an 80% mortality reduction in sepsis deaths and a 90% reduction in pneumonia deaths with hospital case management.
There were 4 effectiveness trials assessing the impact of oral antibiotics on pneumonia-specific mortality in the community. Only one of these studies was randomized and the programmatic coverage of the intervention had to be estimated as coverage data were not routinely assessed or reported. The selection and intensity of co-interventions was not uniform between the studies. An additional limitation was the lack of clearly defined cause-of-death definitions by the authors. However, the effect sizes were remarkably consistent with each other, and therefore the evidence level was upgraded to moderate.
GRADE guidelines rank the evidence relating to the effect of injectable antibiotics on sepsis-specific mortality as low quality. The 3 studies identified were not uniform with respect to study designs; one was an effectiveness RCT, one was a non-randomized concurrent trial and the third was an observational study describing the experience from primary care clinic without a control group. Both the RCT [ 12 ] and the non-randomized concurrent trial[ 9 ], involved concurrent co-interventions alongside the administration of injectable antibiotics. This made it impossible to assess the impact of injectable antibiotics alone on sepsis mortality. Neither study reported the change in the sepsis-specific mortality rate in the intervention arm compared to control arm, and reported the impact on all-cause neonatal mortality only. The absence of randomization in one of the trials is a further limitation [ 9 ]. The main limitation to the observational study in a primary care clinic [ 13 ] was the absence of a control arm in the study.
We identified no controlled trials assessing the effect of hospital-based case management of neonatal infections. Such studies would be difficult or impossible to implement in an ethical fashion. Thus studies were limited to reporting CFRs for neonatal sepsis and meningitis. The studies were from varied settings, from both industrialized and low income countries, and reported widely varying CFRs. Only 2 of these observational studies reported CFRs for pneumonia. One of these studies reported a very high CFR for pneumonia due, we believe, to the high proportion of LBW babies in the sample (60%).
We found some moderate quality evidence for intervention packages including antibiotics in community settings but ironically data are most lacking at facility level, and district hospital level is a critical gap [ 78 ]. Unlike the LiST review on neonatal resuscitation which identified several before-after studies of facility based neonatal resuscitation reporting mortality data, we were unable to find similar before-after studies on the effect of hospital-based case management of sepsis/meningitis/pneumonia. An understandable reason for this might be the ethical constraints precluding such studies. However, historical reviews from the pre-antibiotic era provide an insight into the CFR associated with untreated sepsis in facility settings. The best available evidence comes from the series of papers from Yale Medical Center reporting time trends for neonatal sepsis. These data show that in the 1920s and 1930s the CFR for blood culture confirmed sepsis stood at 90% [ 79 , 80 ]. With the introduction of antibiotics, the CFR decreased to 45% by 1965 [ 81 ], and with the subsequent introduction of intensive care units and advanced life support it came down to 16% by 1988[ 82 ], and 3% by 2003[ 83 ]. Such data highlight the effectiveness of hospital-based management in preventing neonatal mortality from sepsis.
As evident from our results, even oral or injectable antibiotics alone are highly effective in reducing deaths from neonatal sepsis or pneumonia. These interventions hold great potential to reduce the 1 million neonatal deaths each year. If substantial reduction in neonatal mortality is desired, both, community and facility-based interventions are required, linked by functioning referral systems, giving the potential to prevent hundreds of thousands of avoidable newborn deaths every year.
List of abbreviations used
CFR: Case Fatality Rate; CHERG: Child Health Epidemiology Reference Group; IMCI: Integrated Management of Childhood Illnesses; LiST: Lives Saved Tool; RCT: Randomized Controlled Trial.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AZ and JL planned the review, SS and AZ undertook the searches and abstraction with input from JL and HG. SC undertook the meta-analyses. RB provided unpublished data from a previous investigator working group. JL, AZ and ACCL planned the Delphi. All authors contributed to the manuscript.
This work was supported in part by a grant to the US Fund for UNICEF for Child Health Epidemiology Reference Group from the Bill &Melinda Gates Foundation (grant 43386) to “Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries”, and by a grant to Save The Children USA from the Bill & Melinda Gates Foundation (Grant 50124) for "Saving Newborn Lives".
Supplementary Material
Study identifiers and context
Acknowledgements
We are grateful to Rajiv Bahl for insightful review of an earlier draft of this paper. We are also grateful to the members of the Delphi Expert Panel including Rajiv Bahl, Abhay Bang, Abdullah Baqui, Zulfiqar Bhutta, Robert Black, Simon Cousens, Gary Darmstadt, Mike English, Luis Huicho, David Isaacs, Joy Lawn, Patrick Mark, Kim Mulholland, David Osrin, Vinod Paul, Igor Rudan, Cindy Stephen, Barbara Stoll, Steven Wall and Anita Zaidi.
This article has been published as part of BMC Public Health Volume 11 Supplement 3, 2011: Technical inputs, enhancements and applications of the Lives Saved Tool (LiST). The full contents of the supplement are available online at http://www.biomedcentral.com/1471-2458/11?issue=S3 .
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R. et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010; 375 (9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005; 365 (9463):977–988. doi: 10.1016/S0140-6736(05)71088-6. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhutta Z, Ali N, Hyder A, Wajid A. Perinatal and newborn care in Pakistan: seeing the unseen. Karachi: Oxford University Press; 2004. [ Google Scholar ]
- Knippenberg R, Lawn JE, Darmstadt GL, Begkoyian G, Fogstad H, Walelign N, Paul VK. Systematic scaling up of neonatal care in countries. Lancet. 2005; 365 (9464):1087–1098. [ PubMed ] [ Google Scholar ]
- Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005; 115 (2 Suppl):519–617. [ PubMed ] [ Google Scholar ]
- Boschi-Pinto C, Young M, Black RE. The Child Health Epidemiology Reference Group reviews of the effectiveness of interventions to reduce maternal, neonatal and child mortality. International journal of epidemiology. 2010; 39 (Suppl 1):i3–6. doi: 10.1093/ije/dyq018. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Walker N, Fischer-Walker C, Bryce J, Bahl R, Cousens S. Standards for CHERG reviews of intervention effects on child survival. International journal of epidemiology. 2010; 39 (Suppl 1):i21–31. doi: 10.1093/ije/dyq036. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D. et al. Grading quality of evidence and strength of recommendations. BMJ. 2004; 328 (7454):1490. doi: 10.1136/bmj.328.7454.1490. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bang AT, Bang RA, Baitule SB, Reddy MH, Deshmukh MD. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet. 1999; 354 (9194):1955–1961. doi: 10.1016/S0140-6736(99)03046-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bang AT, Bang RA, Tale O, Sontakke P, Solanki J, Wargantiwar R, Kelzarkar P. Reduction in pneumonia mortality and total childhood mortality by means of community-based intervention trial in Gadchiroli, India. Lancet. 1990; 336 (8709):201–206. doi: 10.1016/0140-6736(90)91733-Q. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baqui AH, Arifeen SE, Williams EK, Ahmed S, Mannan I, Rahman SM, Begum N, Seraji HR, Winch PJ, Santosham M. et al. Effectiveness of home-based management of newborn infections by community health workers in rural Bangladesh. The Pediatric infectious disease journal. 2009; 28 (4):304–310. doi: 10.1097/INF.0b013e31819069e8. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baqui AH, El-Arifeen S, Darmstadt GL, Ahmed S, Williams EK, Seraji HR, Mannan I, Rahman SM, Shah R, Saha SK. et al. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008; 371 (9628):1936–1944. doi: 10.1016/S0140-6736(08)60835-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhandari N, Bahl R, Bhatnagar V, Bhan MK. Treating sick young infants in urban slum setting. Lancet. 1996; 347 (9017):1774–1775. doi: 10.1016/S0140-6736(96)90856-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khan AJ, Khan JA, Akbar M, Addiss DG. Acute respiratory infections in children: a case management intervention in Abbottabad District, Pakistan. Bulletin of the World Health Organization. 1990; 68 (5):577–585. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mtango FD, Neuvians D. Acute respiratory infections in children under five years. Control project in Bagamoyo District, Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1986; 80 (6):851–858. doi: 10.1016/0035-9203(86)90241-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pandey MR, Daulaire NM, Starbuck ES, Houston RM, McPherson K. Reduction in total under-five mortality in western Nepal through community-based antimicrobial treatment of pneumonia. Lancet. 1991; 338 (8773):993–997. doi: 10.1016/0140-6736(91)91847-N. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sazawal S, Black RE. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. The Lancet infectious diseases. 2003; 3 (9):547–556. doi: 10.1016/S1473-3099(03)00737-0. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Adejuyigbe EA, Adeodu OO, ko-Nai KA, Taiwo O, Owa JA. Septicaemia in high risk neonates at a teaching hospital in Ile-Ife, Nigeria. East Afr Med J. 2001; 78 (10):540–543. [ PubMed ] [ Google Scholar ]
- Ahmed AS, Chowdhury MA, Hoque M, Darmstadt GL. Clinical and bacteriological profile of neonatal septicemia in a tertiary level pediatric hospital in Bangladesh. Indian Pediatr. 2002; 39 (11):1034–1039. [ PubMed ] [ Google Scholar ]
- Ali Z. Neonatal bacterial septicaemia at the Mount Hope Women's Hospital, Trinidad. Ann Trop Paediatr. 2004; 24 (1):41–44. doi: 10.1179/027249304225013367. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anyebuno M, Newman M. Common causes of neonatal bacteraemia in Accra, Ghana. East Afr Med J. 1995; 72 (12):805–808. [ PubMed ] [ Google Scholar ]
- Basu S, Rathore P, Bhatia BD. Predictors of mortality in very low birth weight neonates in India. Singapore Med J. 2008; 49 (7):556–560. [ PubMed ] [ Google Scholar ]
- Bell Y, Barton M, Thame M, Nicholson A, Trotman H. Neonatal sepsis in Jamaican neonates. Ann Trop Paediatr. 2005; 25 (4):293–296. doi: 10.1179/146532805X72449. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Berger A, Salzer HR, Weninger M, Sageder B, Aspock C. Septicaemia in an Austrian neonatal intensive care unit: a 7-year analysis. Acta Paediatr. 1998; 87 (10):1066–1069. doi: 10.1080/080352598750031392. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhutta ZA, Yusuf K. Neonatal sepsis in Karachi: factors determining outcome and mortality. J Trop Pediatr. 1997; 43 (2):65–70. doi: 10.1093/tropej/43.2.65. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Boo NY, Chor CY. Six year trend of neonatal septicaemia in a large Malaysian maternity hospital. J Paediatr Child Health. 1994; 30 (1):23–27. doi: 10.1111/j.1440-1754.1994.tb00560.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chang Chien HY, Chiu NC, Li WC, Huang FY. Characteristics of neonatal bacterial meningitis in a teaching hospital in Taiwan from 1984-1997. J Microbiol Immunol Infect. 2000; 33 (2):100–104. [ PubMed ] [ Google Scholar ]
- Chang CJ, Chang WN, Huang LT, Huang SC, Chang YC, Hung PL, Tasi CY, Lu CH, Cheng BC, Lee PY. et al. Neonatal bacterial meningitis in southern Taiwan. Pediatr Neurol. 2003; 29 (4):288–294. doi: 10.1016/S0887-8994(03)00273-X. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cordero L, Rau R, Taylor D, Ayers LW. Enteric gram-negative bacilli bloodstream infections: 17 years' experience in a neonatal intensive care unit. Am J Infect Control. 2004; 32 (4):189–195. doi: 10.1016/j.ajic.2003.07.004. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cordero L, Sananes M, Ayers LW. Bloodstream infections in a neonatal intensive-care unit: 12 years' experience with an antibiotic control program. Infect Control Hosp Epidemiol. 1999; 20 (4):242–246. doi: 10.1086/501619. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Daoud AS, al-Sheyyab M, bu-Ekteish F, Obeidat A, Ali AA, el-Shanti H. Neonatal meningitis in northern Jordan. J Trop Pediatr. 1996; 42 (5):267–270. doi: 10.1093/tropej/42.5.267. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Das PK, Basu K, Chakraborty P, Bhowmik PK. Clinical and bacteriological profile of neonatal infections in metropolitan city based medical college nursery. J Indian Med Assoc. 1999; 97 (1):3–5. [ PubMed ] [ Google Scholar ]
- Das PK, Basu K, Chakraborty S, Basak M, Bhowmik PK. Early neonatal morbidity and mortality in a city based medical college nursery. Indian J Public Health. 1998; 42 (1):9–14. [ PubMed ] [ Google Scholar ]
- Dawson KG, Emerson JC, Burns JL. Fifteen years of experience with bacterial meningitis. Pediatr Infect Dis J. 1999; 18 (9):816–822. doi: 10.1097/00006454-199909000-00014. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Galanakis E, Krallis N, Levidiotou S, Hotoura E, Andronikou S. Neonatal bacteraemia: a population-based study. Scand JI nfect Dis. 2002; 34 (8):598–601. doi: 10.1080/00365540110080809. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gebremariam A. Neonatal meningitis in Addis Ababa: a 10-year review. Ann Trop Paediatr. 1998; 18 (4):279–283. [ PubMed ] [ Google Scholar ]
- Ghiorghis B. Neonatal sepsis in Addis Ababa, Ethiopia: a review of 151 bacteremic neonates. Ethiop Med J. 1997; 35 (3):169–176. [ PubMed ] [ Google Scholar ]
- Hervas JA, Alomar A, Salva F, Reina J, Benedi VJ. Neonatal sepsis and meningitis in Mallorca, Spain, 1977-1991. Clin Infect Dis. 1993; 16 (5):719–724. doi: 10.1093/clind/16.5.719. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ishikawa T, Asano Y, Morishima T, Nagashima M, Sobue G, Watanabe K, Yamaguchi H. Epidemiology of bacterial meningitis in children: Aichi Prefecture, Japan, 1984-1993. Pediatr Neurol. 1996; 14 (3):244–250. doi: 10.1016/0887-8994(96)00024-0. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jeena PM, Adhikari M, Carlin JB, Qazi S, Weber MW, Hamer DH. Clinical profile and predictors of severe illness in young South African infants (<60 days) S Afr Med J. 2008; 98 (11):883–888. [ PubMed ] [ Google Scholar ]
- Jiang JH, Chiu NC, Huang FY, Kao HA, Hsu CH, Hung HY, Chang JH, Peng CC. Neonatal sepsis in the neonatal intensive care unit: characteristics of early versus late onset. J Microbiol Immunol Infect. 2004; 37 (5):301–306. [ PubMed ] [ Google Scholar ]
- Kallman J, Kihlstrom E, Sjoberg L, Schollin J. Increase of staphylococci in neonatal septicaemia: a fourteen-year study. Acta Paediatr. 1997; 86 (5):533–538. doi: 10.1111/j.1651-2227.1997.tb08926.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karthikeyan G, Premkumar K. Neonatal sepsis: Staphylococcus aureus as the predominant pathogen. Indian J Pediatr. 2001; 68 (8):715–717. doi: 10.1007/BF02752407. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karunasekera KA, Pathirana D. A preliminary study on neonatal septicaemia in a tertiary referral hospital paediatric unit. Ceylon Med J. 1999; 44 (2):81–86. [ PubMed ] [ Google Scholar ]
- Kuruvilla KA, Pillai S, Jesudason M, Jana AK. Bacterial profile of sepsis in a neonatal unit in south India. Indian Pediatr. 1998; 35 (9):851–858. [ PubMed ] [ Google Scholar ]
- Laving AM, Musoke RN, Wasunna AO, Revathi G. Neonatal bacterial meningitis at the newborn unit of Kenyatta National Hospital. East Afr Med J. 2003; 80 (9):456–462. [ PubMed ] [ Google Scholar ]
- Lee NC, Chen SJ, Tang RB, Hwang BT. Neonatal bacteremia in a neonatal intensive care unit: analysis of causative organisms and antimicrobial susceptibility. J Chin Med Assoc. 2004; 67 (1):15–20. [ PubMed ] [ Google Scholar ]
- Lim NL, Wong YH, Boo NY, Kasim MS, Chor CY. Bacteraemic infections in a neonatal intensive care unit--a nine-month survey. Med J Malaysia. 1995; 50 (1):59–63. [ PubMed ] [ Google Scholar ]
- Lopez Sastre JB, Fernandez CB, Coto Cotallo GD, Ramos AA. Trends in the epidemiology of neonatal sepsis of vertical transmission in the era of group B streptococcal prevention. Acta Paediatr. 2005; 94 (4):451–457. doi: 10.1080/08035250410025302. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mathur NB, Singh A, Sharma VK, Satyanarayana L. Evaluation of risk factors for fatal neonatal sepsis. Indian Pediatr. 1996; 33 (10):817–822. [ PubMed ] [ Google Scholar ]
- Milledge J, Calis JC, Graham SM, Phiri A, Wilson LK, Soko D, Mbvwinji M, Walsh AL, Rogerson SR, Molyneux ME. et al. Aetiology of neonatal sepsis in Blantyre, Malawi: 1996-2001. Ann Trop Paediatr. 2005; 25 (2):101–110. doi: 10.1179/146532805X45692. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moore KL, Kainer MA, Badrawi N, Afifi S, Wasfy M, Bashir M, Jarvis WR, Graham TW, el-Kholy A, Gipson R. et al. Neonatal sepsis in Egypt associated with bacterial contamination of glucose-containing intravenous fluids. Pediatr Infect Dis J. 2005; 24 (7):590–594. doi: 10.1097/01.inf.0000168804.09875.95. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moreno MT, Vargas S, Poveda R, Saez-Llorens X. Neonatal sepsis and meningitis in a developing Latin American country. Pediatr Infect Dis J. 1994; 13 (6):516–520. doi: 10.1097/00006454-199406000-00010. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nnpd. Neonatal morbidity and mortality: report of the National Neonatal-Perinatal Database. Indian Pediatr. 1997; 34 (11):1039–1042. [ PubMed ] [ Google Scholar ]
- Palmer A, Weber M, Bojang K, McKay T, Adegbola R. Acute bacterial meningitis in The Gambia: a four-year review of paediatric hospital admissions. J Trop Pediatr. 1999; 45 (1):51–53. doi: 10.1093/tropej/45.1.51. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Park CH, Seo JH, Lim JY, Woo HO, Youn HS. Changing trend of neonatal infection: experience at a newly established regional medical center in Korea. Pediatr Int. 2007; 49 (1):24–30. doi: 10.1111/j.1442-200X.2007.02310.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patel DM, Rhodes PG, LeBlanc MH, Graves GR, Glick C, Morrison J. Role of postnatal penicillin prophylaxis in prevention of neonatal group B streptococcus infection. Acta Paediatr. 1999; 88 (8):874–879. doi: 10.1111/j.1651-2227.1999.tb00064.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Persson E, Trollfors B, Brandberg LL, Tessin I. Septicaemia and meningitis in neonates and during early infancy in the Goteborg area of Sweden. Acta Paediatr. 2002; 91 (10):1087–1092. doi: 10.1080/080352502760311593. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Robillard PY, Perez JM, Hulsey TC, Perianin J, Gallais A, Janky E. Evaluation of neonatal sepsis screening in a tropical area. Part I: Major risk factors for bacterial carriage at birth in Guadeloupe. West Indian Med J. 2000; 49 (4):312–315. [ PubMed ] [ Google Scholar ]
- Simiyu DE. Morbidity and mortality of neonates admitted in general paediatric wards at Kenyatta National Hospital. East Afr Med J. 2003; 80 (12):611–616. [ PubMed ] [ Google Scholar ]
- Simiyu DE. Neonatal septicaemia in low birth weight infants at Kenyatta National Hospital, Nairobi. East Afr Med J. 2005; 82 (3):148–152. [ PubMed ] [ Google Scholar ]
- Smith PB, Cotten CM, Garges HP, Tiffany KF, Lenfestey RW, Moody MA, Li JS, Benjamin DK Jr.. A comparison of neonatal Gram-negative rod and Gram-positive cocci meningitis. J Perinatol. 2006; 26 (2):111–114. doi: 10.1038/sj.jp.7211438. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sundaram V, Kumar P, Dutta S, Mukhopadhyay K, Ray P, Gautam V, Narang A. Blood culture confirmed bacterial sepsis in neonates in a North Indian tertiary care center: changes over the last decade. J Infect Dis. 2009; 62 (1):46–50. [ PubMed ] [ Google Scholar ]
- Tiskumara R, Fakharee SH, Liu CQ, Nuntnarumit P, Lui KM, Hammoud M, Lee JK, Chow CB, Shenoi A, Halliday R. et al. Neonatal infections in Asia. Arch Dis Child Fetal Neonatal Ed. 2009; 94 (2):F144–F148. doi: 10.1136/adc.2008.139865. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Trotman H, Bell Y, Thame M, Nicholson AM, Barton M. Predictors of poor outcome in neonates with bacterial sepsis admitted to the University Hospital of the West Indies. West Indian Med J. 2006; 55 (2):80–84. [ PubMed ] [ Google Scholar ]
- Udo JJ, Anah MU, Ochigbo SO, Etuk IS, Ekanem AD. Neonatal morbidity and mortality in Calabar, Nigeria: a hospital-based study. Niger J Clin Pract. 2008; 11 (3):285–289. [ PubMed ] [ Google Scholar ]
- Waheed M, Laeeq A, Maqbool S. The etiology of neonatal sepsis and patterns of antibiotic resistance. J Coll Physicians Surg Pak. 2003; 13 (8):449–452. [ PubMed ] [ Google Scholar ]
- Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003; 167 (5):695–701. doi: 10.1164/rccm.200207-682OC. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wolf H, Schaap AH, Smit BJ, Spanjaard L, Adriaanse AH. Liberal diagnosis and treatment of intrauterine infection reduces early-onset neonatal group B streptococcal infection but not sepsis by other pathogens. Infect Dis Obstet Gynecol. 2000; 8 (3-4):143–150. doi: 10.1002/1098-0997(2000)8:3/4<143::AID-IDOG8>3.0.CO;2-5. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wolfler A, Silvani P, Musicco M, Antonelli M, Salvo I. Incidence of and mortality due to sepsis, severe sepsis and septic shock in Italian Pediatric Intensive Care Units: a prospective national survey. Intensive Care Med. 2008; 34 (9):1690–1697. doi: 10.1007/s00134-008-1148-y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kaushik SL, Parmar VR, Grover N, Grover PS, Kaushik R. Neonatal sepsis in hospital born babies. J Commun Dis. 1998; 30 (3):147–152. [ PubMed ] [ Google Scholar ]
- Parkash J, Das N. Pattern of admissions to neonatal unit. J Coll Physicians Surg Pak. 2005; 15 (6):341–344. [ PubMed ] [ Google Scholar ]
- Ganatra HA, Stoll BJ, Zaidi AK. International perspective on early-onset neonatal sepsis. Clin Perinatol. 2010; 37 (2):501–523. doi: 10.1016/j.clp.2010.02.004. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhutta ZA, Zaidi AK, Thaver D, Humayun Q, Ali S, Darmstadt GL. Management of newborn infections in primary care settings: a review of the evidence and implications for policy? The Pediatric infectious disease journal. 2009; 28 (1 Suppl):S22–30. doi: 10.1097/INF.0b013e31819588ac. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Darmstadt GL, Batra M, Zaidi AK. Parenteral antibiotics for the treatment of serious neonatal bacterial infections in developing country settings. The Pediatric infectious disease journal. 2009; 28 (1 Suppl):S37–42. doi: 10.1097/INF.0b013e31819588c3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Darmstadt GL, Batra M, Zaidi AK. Oral antibiotics in the management of serious neonatal bacterial infections in developing country communities. The Pediatric infectious disease journal. 2009; 28 (1 Suppl):S31–36. doi: 10.1097/INF.0b013e3181958794. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Theodoratou E, Al-Jilaihawi S, Woodward F, Ferguson J, Jhass A, Balliet M, Kolcic I, Sadruddin S, Duke T, Rudan I, The effect of case management on childhood pneumonia mortality in developing countries. International journal of epidemiology. pp. i155–171. [ PMC free article ] [ PubMed ]
- Bahl R, Martines J, Ali N, Bhan MK, Carlo W, Chan KY, Darmstadt GL, Hamer DH, Lawn JE, McMillan DD. et al. Research priorities to reduce global mortality from newborn infections by 2015. The Pediatric infectious disease journal. 2009; 28 (1 Suppl):S43–48. doi: 10.1097/INF.0b013e31819588d7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dunham EC. Septicemia in the new-born. Archives of Pediatrics & Adolescent Medicine. 1933; 45 (2):229–253. [ Google Scholar ]
- Nyhan WL, Fousek MD. Septicemia of the newborn. Pediatrics. 1958; 22 (2):268–278. [ PubMed ] [ Google Scholar ]
- Gluck L, Wood HF, Fousek MD. Septicemia of the newborn. Pediatr Clin North Am. 1966; 13 (4):1131–1148. [ PubMed ] [ Google Scholar ]
- Gladstone IM, Ehrenkranz RA, Edberg SC, Baltimore RS. A ten-year review of neonatal sepsis and comparison with the previous fifty-year experience. The Pediatric infectious disease journal. 1990; 9 (11):819–825. doi: 10.1097/00006454-199011000-00009. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG. Seventy-five years of neonatal sepsis at Yale: 1928-2003. Pediatrics. 2005; 116 (3):595–602. doi: 10.1542/peds.2005-0552. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Young Infants Clinical Signs Study Group. Clinical signs that predict severe illness in children under age 2 months: a multicentre study. Lancet. 2008; 371 (9607):135–142. doi: 10.1016/S0140-6736(08)60106-3. [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
COMMENTS
Neonatal pneumonia is one of the most common infection in neonates which can causes death. Neonatal pneumonia occurs in babies since birth until the age of 28 days. Several risk factors are known ...
Presentation of Case. Dr. Sarita U. Patil (Allergy and Immunology): A 9-year-old boy was admitted to this hospital because of fever, cough, respiratory distress, and chest pain. The patient had ...
Neonatal pneumonia is lung infection in a neonate. Onset may be within hours of birth and part of a generalized sepsis syndrome or after 7 days and confined to the lungs. Signs may be limited to respiratory distress or progress to shock and death. Diagnosis is by clinical and laboratory evaluation for sepsis. Treatment is initial broad-spectrum ...
This case study aimed to represent the actual scenario of severe childhood pneumonia case management at community clinic. Considering that circumstances, International Centre for Diarrheal Disease Research, Bangladesh (icddr,b) developed an innovative day care management approach as safe, effective and less expensive alternative to hospital ...
Pneumonia is an important cause of neonatal infection and accounts for significant morbidity and mortality, especially in developing countries [ 1,2 ]. The epidemiology, microbiology, clinical manifestations, diagnosis, and treatment of neonatal pneumonia are reviewed here. Neonatal sepsis is discussed separately:
The clinical signs in neonatal pneumonia mimic other conditions like TTN, RDS or MAS, making it difficult to distinguish them , , . Assessment of respiratory distress in the neonate ... in order to identify studies using case definitions or, in their absence, providing clinical descriptions of the case material. This review resulted in a ...
Presentation of Case. A newborn boy was admitted to the neonatal intensive care unit (NICU) of this hospital because of respiratory distress. The patient was born at a gestational age of 29 weeks ...
A 41-month longitudinal study of neonatal nosocomial infections identified a significantly increased risk of pneumonia among patients with birth weight <1500 g . However, a prospective study of VAP in almost 200 neonates intubated for at least 48 h identified duration of mechanical ventilation as the sole independent risk factor for pneumonia ...
As treatment duration for neonatal pneumonia is based on sparse evidence , ... a national case-control study in Sweden. Lancet Gastroenterol Hepatol. 2020;5:986-95. ...
Scoping review. 1. Introduction. The first month of life, also called the neonatal period, is the most crucial time for survival of a child. Globally, around 2.5 million neonates died in 2018 (nearly 7000 deaths per day), of which one-third occurred on the first day of life. 1 With a neonatal mortality rate of 22.7 per 1000 live births, 1 India ...
The pneumonia case-management intervention was associated with a greater difference in neonatal pneumonia mortality between intervention and control areas than in all neonatal mortality . All studies showed a reduction in the point estimate of between 8% and 56%, but only two studies had an upper confidence limit less than one.
Background Each year almost one million newborns die from infections, mostly in low-income countries. Timely case management would save many lives but the relative mortality effect of varying strategies is unknown. We have estimated the effect of providing oral, or injectable antibiotics at home or in first-level facilities, and of in-patient hospital care on neonatal mortality from pneumonia ...
Cathy's membranes were ruptured for 20 hours. High vaginal swab at 36 weeks was +ve for group B strep. Cathy was given prophylactic antibiotics when her membranes ruptured. Nathan is Cathy's second baby. Cathy is breastfeeding. 1. Assign roles to each player. 2. Set up the room with baby in open cot.
Pneumonia is a serious problem that threatens the health of newborns. This study aimed to investigate the clinical characteristics of hospitalized term and preterm infants with community-acquired viral pneumonia. This was a retrospective analysis of cases of community-acquired viral pneumonia in the Neonatal Department. Nasopharyngeal aspirate (NPA) samples were collected for pathogen ...
Pneumonia is a disease of the lower airway that occurs when viruses, bacteria, fungi, or a combination of these, cause inflammation and fluid accumulation in the pulmonary parenchyma.[1] Globally, pneumonia is a leading cause of morbidity and mortality in children younger than the age of 5 years.[2] Although the majority of deaths attributed to pneumonia in children are mostly in the ...
To improve the case management of pneumonia in low- and middle-income countries, the World Health Or-ganization (WHO) developed the Integrated Management of Childhood Illness guidelines in 1995. In 2005, these guidelines4 defined severe pneumonia as the presence of cough or dificulty in breathing and tachypnoea (> 50 breaths per minute for ...
Neonatal Pneumonia Case Study - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. neonatal pneumonia
As such, neonatal pneumonia demonstrates an important, high-stakes clinical scenario that highlights the necessity of cross-speciality cohesion. Group B streptococcal infection screening and management, in conjunction with ease in assessing appropriate antibiotic therapy of neonates and infants in the case of pneumonia, is as pivotal as ...
The pneumonia case-management intervention was associated with a greater difference in neonatal pneumonia mortality between intervention and control areas than in all neonatal mortality (figure 1). All studies showed a reduction in the point estimate of between 8% and 56%, but only two studies had an upper confidence limit less than one.
A third, clinic-based, study reported a case-fatality ratio of 3.3% among neonates treated with injectable antibiotics as outpatients. No studies were identified evaluating injectable antibiotics alone for neonatal pneumonia. Delphi consensus (median from 20 respondents) effects on sepsis-specific mortality were 30% reduction for oral ...
Thus, in any case of pneumonia unresponsive to antibiotics, Legionnaires' disease should be considered and specific tests to verify the diagnosis should be performed . Legionella spp. is a rare cause of neonatal pneumonia, but the high mortality rate of 50% in children younger than one year is still being discussed in the literature [ 29 ].
Stenotrophomonas maltophilia causes human infections ranging from bacteremia, sepsis, endocarditis, pneumonia, and meningitis.It is a gram-negative, glucose non-fermentative aerobic rod with low virulence. The bacteria was described in 1961 by Hugh and Ryschenkow [].S. maltophilia is often pathogenic in immunocompromised and hospitalized patients, especially in the intensive care unit (ICU) [].
Case-study-www - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. The patient manifested cough which is consistent with pneumonia caused by an infection in the respiratory tract. Fever is also present due to the inflammatory process. Apnea was not present which is a good sign as it indicates the infection did not ...
Background. Deaths occurring in the neonatal period each year account for 41% (3.6 million) of all deaths in children under 5 years [].The majority of these deaths occur in low income countries and almost 1 million of these deaths are attributable to infectious causes including neonatal sepsis, meningitis and pneumonia [].These deaths occur because of lack of preventive care (clean birth care ...