

Ethics & Governance Contacts
For enquiries or advice about applications for ethical and scientific review , please contact the Executive Officer of the relevant Human Research Ethics Committee .
You may also contact the relevant Human Research Ethics Committee for more information about post-approval reporting and study management. This includes advice relating to eligibility for expedited review; meeting and application submission dates; completion of the application form(s); and submission of the application form(s) and supporting documents.
A Certified Human Research Ethics Committee is one that is hosted by an institution that has been certified by the National Health and Medical Research Council to participate in the national approach to single ethical review. Certification details can be accessed through the National Health and Medical Research Council Human Research Ethics Portal .
For enquiries or advice about applications for site authorisation (i.e. site specific assessments and access requests) please contact the relevant Research Governance Officer .
NSW Health Early Phase Clinical Trials HRECs
Use the + to view more information on each organisation.
- Organisation
- Bellberry Limited Bellberry Limited "> Bellberry Limited ">
- 08 8361 3222
Chief Executive: Kylie Sproston Early Phase Clinical Trials Manager: Jerneen Williams Email: [email protected] Phone: 08 8122 4575 Address: 123 Glen Osmond Road Eastwood, Adelaide South Australia, 5063
- Sydney Children's Hospitals Network Sydney Children's Hospitals Network "> Sydney Children's Hospitals Network ">
- Paediatrics
- (02) 9845 3105
Executive Officer: Ms Asra Gholami Chair: A/Prof Sarah Garnett Early Phase Clinical Trials Research Ethics Officer: Melinda Giles Phone: (02) 9845 3105 Email: [email protected] Certified HREC: Yes National Mutual Acceptance: Yes Address: Kids Research Institute Children's Hospital at Westmead Locked Bag 4001 Westmead NSW 2145
NSW Human Research Ethics Committees
Use the + to view more information on each organisation’s Human Research Ethics Committee.
- Central Coast Local Health District
- There is no local HREC for Central Coast Local Health District
- 02 4320 2085
Executive Officer : Dr Katherine Bolton, Research Manager Governance Officer: Brianna Attard and Yin Wang Certified HREC: No National Mutual Acceptance: Yes Address: Research Office Level 10 Central Coast Research Institute 77A Holden Street Gosford NSW 2065 Address for Agreements: CCLHD, Holden Street Gosford NSW 2250
- Illawarra Shoalhaven Local Health District
- University of Wollongong and Illawarra Shoalhaven LHD Health and Medical HREC [EC00150]
- 02 4221 5041
Executive Officer : Ms Zoe Wulff, Research Ethics Manager Chair: Dr Susan Thomas Certified HREC: Yes National Mutual Acceptance: Yes Address: Research Services Office, University of Wollongong Northfields Avenue Wollongong NSW 2522
- Nepean Blue Mountains Local Health District
- Nepean Blue Mountains Local Health District HREC [EC00151]
- 02 4734 1998
Executive Officer : Ms Tanya Harley Chair: Prof Ian Seppelt Certified HREC: Yes National Mutual Acceptance: Yes Address: Research Office Level 5 South Block, Nepean Hospital PO Box 63 Penrith NSW 2751
- Northern Sydney Local Health District
- Northern Sydney Local Health District HREC [EC00112]
- 02 9926 4590
Executive Officer : Olivia Hu Chair: Dr Greta Ridley Certified HREC: Yes National Mutual Acceptance: Yes Address: Research Office Level 13, Kolling Building, Royal North Shore Hospital Reserve Road St Leonards NSW 2065
- Royal Rehab
- Royal Rehab HREC [EC00133]
- 02 9808 9285
Executive Officer : Ms Elizabeth Drolz Additional information: Chair: TBC Certified HREC: No National Mutual Acceptance: No The HREC will not be accepting any new proposals for review, however it will continue to monitor existing approved research proposals. Address: Royal Rehab PO Box 6, Ryde NSW 1680
- South Eastern Sydney Local Health District
- South Eastern Sydney Local Health District HREC [EC00134]
- 02 8797 7605
Executive Officer : Marie Le Bechennec, A/Research Ethics and Governance Manager Chair: Professor Georgina Hold Certified HREC: Yes National Mutual Acceptance: Yes Address: G71, East Wing, Edmund Blackett Building Prince of Wales Hospital Cnr Avoca and High Streets Randwick NSW 2031
- South Western Sydney Local Health District
- South Western Sydney Local Health District HREC [EC00136]
- 02 8738 8304
Executive Officer : Dr Shakti Shukla Chair: Professor Murray Killingsworth Certified HREC: Yes National Mutual Acceptance: Yes Address: Research Directorate Locked Bag 7279 Eastern Campus Liverpool BC NSW 1871 Delivery Address: South Western Sydney Local Health District Executive Office Liverpool Hospital Eastern Campus Scrivener Street Liverpool NSW 2170
- St Vincent's Hospital Sydney
- St Vincent’s Hospital HREC [EC00140]
- 02 8382 4960
Executive Officer : Dr Pamela Blaikie, Research Office Manager Chair: Dr Jane Carland Certified HREC: Yes National Mutual Acceptance: Yes Address: St Vincent's Hospital Sydney Research Office Translational Research Centre 97-105 Boundary Street Darlinghurst NSW 2010
- Sydney Children's Hospital Network
- Sydney Children’s Hospitals Network HREC [EC00130]
- Research Office: 02 9845 3066 Early Phase Clinical Trials Ethics Officer: 02 9845 3105
Executive Officer : Dr Clare Bayram Early Phase Clinical Trials Ethics Officer: Ms Melinda Giles Chair: A/Prof Sarah Garnett Certified HREC: Yes National Mutual Acceptance: Yes Address: Kids Research The Children's Hospital at Westmead Locked Bag 4001 Westmead NSW 2145
- Sydney Local Health District
- Sydney Local Health District HREC (CRGH) [EC00118]
- 02 9767 5622
Executive Officer : Ms Kate Flinders, Research Office Manager Chair: David Le Couteur Certified HREC: Yes National Mutual Acceptance: Yes Address: Research Office Ground Floor, Building 20 Concord Repatriation General Hospital Concord NSW 2139
- Sydney Local Health District Ethics Review Committee (RPAH Zone) [EC00113]
- 02 9515 6766
Executive Officer : Ms Rosemary Carney Chair: Dr Robert Loblay Certified HREC: Yes National Mutual Acceptance: Yes Address: Research Development Office Suite 210A, RPAH Medical Centre 100 Carillon Avenue Newtown NSW 2042
- Western Sydney Local Health District
- Western Sydney Local Health District HREC [EC00152]
- 02 8890 8183
Executive Officer : Ms Kellie Hansen Chair: A/Prof Clement Loy Certified HREC: Yes National Mutual Acceptance: Yes Address: Research Office Westmead Hospital, Rm 2050, Level 2 Research and Education Network Building Westmead NSW 2145
- Far West Local Health Districts
- Greater Western Area Health Service HREC [EC00399]
- 02 6330 5948
Executive Officer : Mr Phil Sanders Chair: A/Professor Tony Brown Certified HREC: No National Mutual Acceptance: No This HREC is permitted to conduct single ethical review for multi-centre research in NSW only. Address: 39 Hampden Park Road PO Box 143 Bathurst NSW 2795
- Western NSW Local Health Districts
- Hunter New England Local Health District
- Hunter New England HREC [EC00403]
- 02 4921 4140
Executive Officer : Dr Sarah Moberley, Manager Research Ethics and Governance Chair: Ms Mandy Hunter Certified HREC: Yes National Mutual Acceptance: Yes Address: Awabakal Country Level 3, POD, HMRI Lot 1 Kookaburra Circuit New Lambton Heights NSW 2305 Mailing Address: HNELHD Research Office John Hunter Hospital Lookout Road New Lambton Heights NSW 2305 ABN : 63 598 010 203
- Mid North Coast Local Health Districts
- North Coast NSW HREC [EC00415]
- 0421 028 924
Executive Officer : Ms Rebecca Lavery, A/Executive Officer Chair: (TBA) Population Health and Health Services Studies: Mr Paul Corben Certified HREC: No National Mutual Acceptance: No Address: PO Box 821 Murwillumbah NSW 2484
- Northern NSW Local Health Districts
- Murrumbidgee Local Health District
- 0436 679 115
There is no local HREC for Murrumbidgee Local Health District (MLHD). However, via an agreement with Western NSW Local Health District, the Greater Western Human Research Ethics Committee (HREC) conducts ethical review for all new single centre applications for research to be conducted in MLHD. Please contact the Greater Western HREC for further information about ethical review at phone (02) 6330 5948 or e-mail [email protected] . After ethical approval has been granted by the Greater Western HREC, MLHD conducts its own research governance site review to determine whether research is approved to proceed at sites in MLHD. If you have questions about the research governance site review process then please contact the MLHD Research Governance Officer, Dr Janelle Thomas, at [email protected] or on (02) 5943 2014 .
- Southern NSW Local Health District
- 02 6492 9682
Executive Officer : Ms Sally Josh, Research Governance Officer Certified HREC: NA National Mutual Acceptance: NA There is no local HREC for Southern NSW Local Health District. However, via an agreement with Western NSW Local Health District, the Greater Western HREC conducts review for: · all new single centre applications that are to be conducted in Southern NSW Local Health District · projects that are currently running in Southern NSW Local Health District (i.e. those reviewed by the former GSAHS HREC) that require review or acknowledgement of documentation (e.g. amendments and reports). Contact the Greater Western HREC for further information. Southern NSW Local Health District conducts its own research governance review. Address: Research Governance Office Southern NSW Local Health District Community Health Building, Bega District Hospital PO Box 173 Bega NSW 2550
- Aboriginal Health and Medical Research Council of NSW
- AH&MRC Ethics Committee [EC00342]
- 02 9212 4777
Ethics Manager : Sonny Green Chair: Aunty Val Keed Certified HREC: No National Mutual Acceptance: No Note: All human research projects affecting the health and wellbeing of Aboriginal people and communities in NSW must be reviewed by the Ethics Committee of the Aboriginal Health and Medical Research Council of NSW (AH&MRC). This is the case even if the project has been reviewed by a NSW Health HREC. Address: AH&MRC Ethics Committee PO Box 1565 Strawberry Hills NSW 2012
- Justice Health & Forensic Mental Health
- Justice Health HREC [EC00119]
- 02 9700 3443
Executive Officer : Ms Josie Cullen Chair: Dr Megan Williams Certified HREC: No National Mutual Acceptance: No Note: All human research projects undertaken with people in the correctional environment in NSW must be reviewed by this HREC. Address: Justice Health and Forensic Mental Health Network PO Box 150 Matraville NSW 2036
- NSW Ministry of Health & Cancer Institute NSW
- NSW Population and Health Services Research Ethics Committee [EC00410
- 02 8374 5689 or 8374 3610
Executive Officer : Ms Chehani Alles Project Support Officer, Research Ethics: Mr Patrick Borzycki Chair: Prof David Roder Certified HREC: Yes National Mutual Acceptance: No Note: this is a Cancer Institute NSW and NSW Ministry of Health joint committee - all research involving access (including linkage) to statewide data collections owned or managed by NSW Health or the Cancer Institute NSW must be reviewed by this HREC. Address: Cancer Institute NSW PO Box 41 Alexandria NSW 1435
- Albury Wodonga Health
- Albury Wodonga Human Research Ethics Committee [EC00117]
- 02 6058 4356
Executive Officer : Ms Judy Rumler, Administration Officer Chair: Mr Greg Pearl Certified HREC: No National Mutual Acceptance: No In 2009 the former Albury Base Hospital merged with Wodonga Hospital to form Albury Wodonga Health. All human research projects undertaken at Albury Wodonga Health must be reviewed by the Albury Wodonga Human Research Ethics Committee, which operates under the Victorian System. Address: Albury Wodonga Human Research Ethics Committee Albury Wodonga Health PO Box 156 Wodonga VIC 3689
NSW Research Governance Officers
Use the + to view more information on each organisation’s Research Office.
- Albury Wodonga Health Albury Wodonga Health "> Albury Wodonga Health ">
- Dr Glenn Davies, Executive Director Medical Services & Clinical Governance
- 02 6051 7649
Address: Albury Wodonga Joint Hospitals' Ethics Committee Albury Wodonga Health Wodonga Campus Vermont St (PO Box 156) Wodonga VIC 3689
- Calvary Mater Hospital Newcastle
- Melissa Gavenlock, Research Governance Officer
- 02 4014 4784
- Cancer Council NSW Cancer Council NSW "> Cancer Council NSW ">
- Dr John Williams, Research Strategy Unit
- 02 9334 1993
Facilities covered include the Cancer Council NSW offices and Information Centres or Hubs in NSW and the Centre for Health Research and Psycho-oncology (University of Newcastle) for some research studies. Address: Cancer Council NSW PO Box 572 Kings Cross NSW 1340
- Central Coast Local Health District Central Coast Local Health District "> Central Coast Local Health District ">
- Dr Katherine Bolton, Research Manager, Brianna Attard and Yin Wang, Research Governance Officers
Facilities include: Gosford Hospital, Long Jetty Hospital, Woy Woy Hospital, Wyong Hospital Address: Research Office, Level 10 Central Coast Research Institute 77A Holden Street Gosford NSW 2250 Address for Agreements: CCLHD, Holden Street Gosford NSW 2250 Australia ABN: 88 523 389 096
- Western NSW and Far West Local Health Districts Western NSW and Far West Local Health Districts "> Western NSW and Far West Local Health Districts ">
- Mr Phil Sanders, Research Governance Officer
Address: Level 1, 230 Howick Street PO Box 143 Bathurst NSW 2795 Address for Agreements: 29 Hawthorn Street Dubbo NSW 2830 ABN: 50 629 556 404
- HammondCare HammondCare "> HammondCare ">
- Dr Najwa Reynolds, Research and Governance
- 02 8788 3955
Facilities covered include: Greenwich Hospital, Neringah Hospital, Braeside Hospital, Northern Beaches Palliative Care Service Address: Pallister House, 97-115 River Road Greenwich NSW 2065
- Hunter New England Local Health District Hunter New England Local Health District "> Hunter New England Local Health District ">
- Kristy Morris, Manager Research Governance
Address: Hunter New England Local Health District Lookout Road New Lambton NSW 2305
- Illawarra Shoalhaven Local Health District Illawarra Shoalhaven Local Health District "> Illawarra Shoalhaven Local Health District ">
- Research Support Office
- 02 4253 4800
Facilities covered include: Bulli Hospital, Coledale Hospital, David Berry Hospital, Kiama Hospital and Community Health Service, Milton-Ulladulla Hospital, Port Kembla Hospital, Shellharbour Hospital, Shoalhaven District Memorial Hospital, Wollongong Hospital Address: Wollongong Hospital, Block C, Level 8 LMB 8808 SCMC NSW 2521
- Justice Health & Forensic Mental Health Network Justice Health & Forensic Mental Health Network "> Justice Health & Forensic Mental Health Network ">
- Victoria Bell
Address: Justice Health & Forensic Mental Health Network PO Box 150 Matraville NSW 2036
- Mid North Coast Local Health District-Coffs Clinical Network Mid North Coast Local Health District-Coffs Clinical Network "> Mid North Coast Local Health District-Coffs Clinical Network ">
- Colleen Nosworthy, Research Governance Officer
- 0428 882 170
Facilities covered include: Bellinger River District Hospital, Coffs Harbour Health Campus, Dorrigo Multi Purpose Service, Macksville District Hospital Address: Port Macquarie Base Hospital Wrights Road PO Box 126 Port Macquarie NSW 2444 ABN: 57 946 356 658 Mobile: 0428 882 170
- Diana Stephens, Research Governance Officer
- 0473 853 782
Facilities covered include: Kempsey District Hospital, Port Macquarie Base Hospital, Wauchope District Memorial Hospital Address: Executive Administration Offices Kempsey District Hospital River St Kempsey NSW 2440 Mobile: 0428 882 170
- Murrumbidgee Local Health District Murrumbidgee Local Health District "> Murrumbidgee Local Health District ">
- Dr Janelle Thomas, Research Governance Officer
- 02 5943 2014
Address: 193-195 Morgan Street Wagga Wagga NSW 2650
- Nepean Blue Mountains Local Health District Nepean Blue Mountains Local Health District "> Nepean Blue Mountains Local Health District ">
- Medhia Survery, Research Governance Officer
- [email protected]
Facilities covered include: Blue Mountains Hospital, Community Health Centres within Nepean Blue Mountains Local Health District, Lithgow Hospital, Mental Health Centres within Nepean Blue Mountains Local Health District, Nepean Hospital, Portland Tabulam Health Centres within Nepean Blue Mountains Local Health District, Springwood Hospital Address: Nepean Blue Mountains Local Health District 2-6 Station Street Penrith NSW 2750 ABN: 31 910 677 424
- Northern NSW Local Health District Northern NSW Local Health District "> Northern NSW Local Health District ">
- Rebecca Lavery
- 02 6672 0269
Facilities covered include: Ballina District Hospital, Bonalbo Hospital, Byron Central Hospital, Casino and District Memorial Hospital, Kyogle Memorial Hospital, Lismore Base Hospital, Murwillumbah District Hospital, Nimbin Multi Purpose Centre, The Tweed Hospital, Urbenville Rural Hospital and Health Service, Grafton Base Hospital, McLean District Hospital Address: Crawford House 70-72 Hunter Street Lismore NSW 2480 ABN: 67 284 856 520
- Northern Sydney Local Health District Northern Sydney Local Health District "> Northern Sydney Local Health District ">
Research Governance Officer: Dylan Chin Research Governance Officer: Vidura Perera Facilities covered include: Hornsby Hospital, Macquarie Hospital, Mona Vale Hospital, Royal North Shore Hospital, Ryde Hospital Address: Executive Unit Level 14, Kolling Building Royal North Shore Hospital Reserve Road St Leonards NSW 2065
- NSW Ambulance NSW Ambulance "> NSW Ambulance ">
- Sandra Ware
- 0418 671 573
Facilities covered include all operational units and corporate services within the Ambulance Service of NSW except Health Emergency and Aeromedical Services. Address: NSW Ambulance Church St Locked Bag 105 Rozelle NSW 2039 ABN: 69 291 930 156
- Ms Sandra Ware, Research Manager
- 02 9707 8800
Address: 670 Drover Road Bankstown Airport PO Box 58 Georges Hall NSW 2198
- NSW Health Pathology
- Andrew Harre, Research Governance Officer
- 02 9464 4766 or 0436 818 727
- Royal Rehabilitation Centre Sydney Royal Rehabilitation Centre Sydney "> Royal Rehabilitation Centre Sydney ">
- Dr Julie Pryor
- 0427 772 074
- [email protected]
Address: Royal Rehabilitation Centre Sydney PO Box 6 Ryde NSW 1680
- South Eastern Sydney Local Health District South Eastern Sydney Local Health District "> South Eastern Sydney Local Health District ">
- Marie Le Bechennec, Operations & Governance Manager
Facilities covered include: Prince of Wales Hospital, St George Hospital, Royal Hospital for Women, Sutherland Hospital, Sydney and Sydney Eye Hospital, War Memorial Hospital, Albion Street Centre, Kirketon Road Centre and K2, Langton Centre, The Garrawarra Centre, Gower Wilson Memorial Hospital (Lord Howe Island) Address: Prince of Wales Hospital G71, East Wing, Edmund Blackett Building Cnr High & Avoca Streets Randwick NSW 2031
- South Western Sydney Local Health District South Western Sydney Local Health District "> South Western Sydney Local Health District ">
- Dr Shakti Shukla, Research Governance Officer
Facilities covered include: Bankstown-Lidcombe Hospital, Bowral Hospital, Camden Hospital, Campbelltown Hospital, Fairfield Hospital, Liverpool Hospital Address: Research Directorate Locked Bag 7279 Eastern Campus Liverpool BC NSW 1871 Delivery Address: South Western Sydney Local Health District Executive Office Liverpool Hospital Eastern Campus Scrivener Street Liverpool NSW 2170 ABN: 46 738 965 845
- Southern NSW Local Health District Southern NSW Local Health District "> Southern NSW Local Health District ">
- Nicole Yates, Research Office Contact
- 0476 802 843
Address: Southern NSW Local Health District Clinical Governance Unit Community Health Building PO Box 173 Bega NSW 2550
- St Vincent's Hospital Sydney St Vincent's Hospital Sydney "> St Vincent's Hospital Sydney ">
- Dr Sabine Giesebrecht, Research Governance Officer
Facilities covered include: Sacred Heart Hospice, St Joseph's Hospital, St Vincent 's Hospital, St Vincent's Private Hospital*, The Mater*, Victor Chang Cardiac Research Institute* *These facilities are private sites. Please contact the RGO for research to be undertaken at these sites. Address: St Vincent's Hospital Sydney Limited 390 Victoria Street Darlinghurst NSW 2010 ABN: 77 054 838 87
- Sydney Children's Hospitals Speciality Network - Randwick and Westmead Sydney Children's Hospitals Speciality Network - Randwick and Westmead "> Sydney Children's Hospitals Speciality Network - Randwick and Westmead ">
- Dr Lucia Smith, Research Governance Manager
- 02 9845 3011
Facilities covered include: Sydney Children's Hospital Randwick, The Children's Hospital at Westmead (also known as the Royal Alexandra Hospital for Children), Newborn & Paediatric Emergency Transport Service (NETS), New South Wales Pregnancy and Newborn Services Network (PSN), Children's Court Clinic Address: Kids Research The Children's Hospital at Westmead Locked Bag 4001 Westmead NSW 2145 ABN: 53 188 579 090
- Sydney Local Health District - Royal Prince Alfred Hospital Sydney Local Health District - Royal Prince Alfred Hospital "> Sydney Local Health District - Royal Prince Alfred Hospital ">
- Estelle Ali
- 02 9515 7899
Facilities covered include: Balmain Hospital, Canterbury Hospital, Royal Prince Alfred Hospital, Sydney Dental Hospital Address: Research Development Office Suite 210A, RPAH Medical Centre 100 Carillon Avenue Newtown NSW 2042
- Sydney Local Health District - Concord Repatriation General Hospital Sydney Local Health District - Concord Repatriation General Hospital "> Sydney Local Health District - Concord Repatriation General Hospital ">
- Sarah Truong
Address: Research Office Ground Floor, Building 20 Concord Repatriation General Hospital Concord NSW 2139
- Western Sydney Local Health District Western Sydney Local Health District "> Western Sydney Local Health District ">
- Pauline Geale and Tiffany Jessop
- 02 8890 9007
Facilities covered include: Auburn Hospital, Blacktown Hospital, Cumberland Hospital, Mt Druitt Hospital, Westmead Hospital Community Health Centres within Western Sydney Local Health District Address: Westmead Hospital Rm 2050, Level 2, Research and Education Network Building Westmead NSW 2145
Updated 1 year ago
Popular Searches:
- clinical trial expertise
- clinical trial support
- clinical trials
- collaboration opportunities
- commercialisation
- early phase clinical trials
- establish a clinical trial
- ethics and governance
- facilitate resolution
- good clinical practice
- human research ethics committee
- key contacts
- motor neurone disease
- news articles
- problem solving
- publications
- solution service
- standard operating procedures
- start a clinical trial
- translational research

Human research ethics
Research conducted at another organisation, external organisations.
The National Statement on Ethical Conduct in Human Research recommends that institutions adopt processes to eliminate the unnecessary duplication of ethical review.
To meet this, the University of Newcastle’s Human Research Ethics Committee (HREC) may recognise an ethics approval from an external HREC, as long as that HREC is registered with the National Health & Medical Research Council.
It may also define the circumstances in which ethical clearance from the University HREC is not required, such as approval from an external HREC where a formal agreement is in place with that institution.
Research projects that have obtained approval from an external human research ethics committee recognised by the University of Newcastle must register that approval with the University’s HREC and provide all associated documentation to support that approval prior to the commencement of the approved research activity.
Examples of external registered HRECs include the Human Research Ethics Committees at Local Health Districts in NSW, other state-based health departments and hospitals, research institutes, national medical councils, etc.
Hunter New England and Central Coast Local Health Districts
If research involves patients, facilities or records of Hunter New England Health, an application for ethics approval must be made to the Hunter New England Health Research Ethics Committee .
In most cases, if the research is to be conducted by staff or students of the University of Newcastle (and provided ethics approval is granted by the Hunter New England Health HREC) it will not be necessary to make a full application to the University HREC.
However, this approval must be registered with the University of Newcastle’s HREC of the research activity, and researchers will not be covered by the University’s insurance provisions if the project is not registered.
For further information about Hunter New England Health ethics processes, contact:
HNE Research Office Hunter New England Local Health District E: [email protected] P: (02) 4921-4140 L: Research Ethics | Research Governance | Research Development
The Central Coast Local Health District does not currently have a human research ethics committee. Instead, it has a standing agreement with HNE Health that ‘more than low risk’ applications will be reviewed by the Hunter New England Health HREC. For ‘low risk’ and ‘negligible risk’ applications, the Central Coast LHD uses local review processes.
Schools and pre-schools
In addition to being approved by a registered HREC, research projects that involve NSW Government pre-schools or schools must also be approved by the NSW Department of Education via its State Education Research Applications Process (SERAP).
The study information/invitation for research participants that is submitted to the NSW Department of Education as part of an application for approval to conduct the research in government schools must be the same version as that submitted to and/or approved by the approving HREC.
It will be necessary to prepare a human ethics application and a SERAP application that take into account both the SERAP Guidelines and the recommended content outlined in the University's Participant Information Sheet and Consent Form, which University staff and HDR candidates can access via our internal ReSearchHub .
For details about NSW Department of Education requirements, contact:
Robert Stevens Manager, Quality Assurance/Research Policy, Planning and Reporting NSW Department of Education [email protected] (02) 9244 5060
For additional advice
Visit the 'Research with external ethics approval' page.
General enquiries
Contact a team member
The University of Newcastle acknowledges the traditional custodians of the lands within our footprint areas: Awabakal, Darkinjung, Biripai, Worimi, Wonnarua, and Eora Nations. We also pay respect to the wisdom of our Elders past and present.
Are you visiting our site from South Asia ? Head to our dedicated page with all the information you need to study at the University of Newcastle. Close
您是否在中国访问我们的网址? 前往 专属页面 ,查询你在纽卡斯尔大学学习所需的所有信息。 Close

Leading research for life changing results
Medical Research
Leading Research for Life Changing Results

- MEDICAL RESEARCH
- RESEARCHERS
- Carlos Garcia-Esperon
Dr Carlos Garcia-Esperon
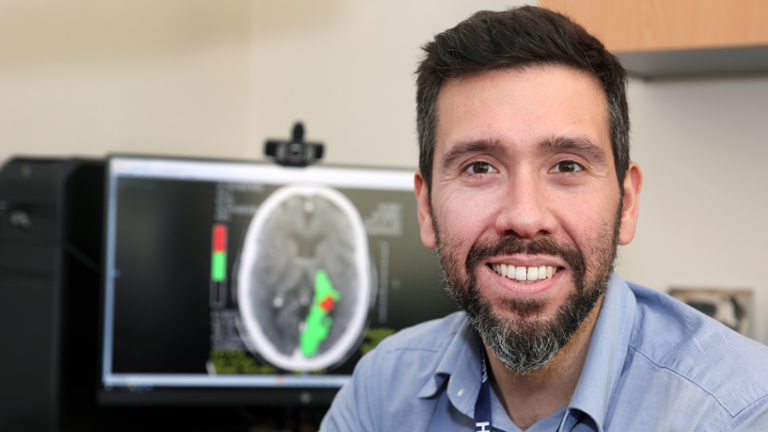
Dr Carlos Garcia-Esperon is a stroke neurologist and researcher at John Hunter Hospital where he is Director of Hunter Stroke Services. Dr Garcia-Esperon undertook a Bachelor of Medicine at the University of Santiago de Compostela in Spain. He then completed a dissertation in ischaemic stroke at the University of Bern while undertaking further clinical training in Stroke at the Kantonsspital Aarau. He subsequently undertook a Stroke Fellowship at John Hunter Hospital, and in 2020 completed a PhD program in brain imaging and thrombolytic therapy in acute stroke at the University of Newcastle.
Reperfusion therapies for ischaemic stroke are amongst the most beneficial and cost-effective therapies in medicine but are highly time critical. With a focus on an equity of access to acute stroke therapies in rural and regional centres, Dr Garcia Esperon’s research aims to integrate advanced brain imaging, targeted acute stroke therapies and enhanced clinician education to optimise outcomes for acute stroke patients in these areas. Together with Prof Spratt, Levi and Parsons, he pioneered Telestroke in NSW, to deliver imaging and neurologist supported acute stroke therapies in rural hospitals in NSW. This was expanded from a proof of concept to a state-wide network. He was the lead author on the analysis of the pilot project, which was instrumental in obtaining funding from Ministry of Health.
A project based on this work won the Health and Research Innovation category of the 2020 Hunter New England Health Excellence Awards. He has been awarded a Hunter New England Clinical and Health Service Research Fellowship and a Viertel Foundation Clinical Investigator Award to build on this research.
Dr Garcia Esperon has published his work in high impact journals and has presented at national and international conferences. He has also spoken on several occasions at the invitation-only International Symposium on Thrombolysis, Thrombectomy and Acute Stroke Therapy and the European and American annual stroke conferences. Locally, he has presented widely in regional and rural centres in NSW on the role of telethrombolysis in stroke management and educating healthcare practitioners in its role in acute stroke treatment.
Research Interests
- Pre-hospital stroke triage and use of pre-hospital stroke scales to enhance the timely delivery of reperfusion therapies for ischaemic stroke.
- Telestroke to boost access to stroke specialists, advanced imaging techniques and reperfusion therapies in rural areas.
- Brain perfusion imaging, particularly in large ischaemic core strokes and the potential benefit of endovascular thrombectomy.
- Use of cardiac CT in the hyperacute stroke phase
- Delivery of new models of remote stroke education using virtual reality.
Why did you get into research?
I am originally from rural Spain, where my grandparents were farmers, and I have experienced the imbalance in access to healthcare services due to geographic location. So, I steered the focus of my career towards enhancing access to reperfusion therapies for ischaemic stroke, with a special focus on rural areas.
What would be the ultimate goal for your research?
My research has always been driven by a desire to improve outcomes for stroke patients. I have clinical research expertise and I have sought to use this to advance knowledge and knowledge translation in stroke research.
Ischaemic stroke treatment has been transformed by the availability of reperfusion therapies. These are among the most effective therapies in recent medicine but to ensure that the benefits are realised for patients, we need to ensure that patients get this care quickly. I have been fortunate to work with colleagues who share my vision, and we have pioneered approaches to pre-hospital triage and telestroke.

Future Focus
My future focus includes the growth of research productivity in established projects with rural hospitals, and the development of new projects to build on research efforts in the treatment of acute stroke. I want to develop other initiatives to improve stroke outcome in rural Australia and close the rural-metropolitan gap for stroke patients.
Specialised/Technical Skills
- Cerebrovascular disease
- Ischaemic stroke
- Acute stroke therapies
- Multimodal CT
- Neuroimaging
- Reperfusion
Affiliations
- Heart and Stroke Research Program
- Staff Specialist Neurologist
- Director, Hunter Stroke Services
- Clinical and Health Service Research Fellow
- Member, Hunter New England Human Research Ethics Committee
- Acute Stroke Team
- Conjoint Lecturer, School of Medicine and Public Health
- Executive Committee Member, Priority Research Centre Stroke and Brain Injury
- Treasurer
- Clinical Council Member
- Member of the Hunter New England Research Ethics Committee
- Committee Member
- View Carlos Garcia-Esperon's University of Newcastle profile
- Main Navigation
- Main Content

- Current Students
- Give to UNE
- Human Research Ethics
- Research Integrity & Ethics
Human Research Ethics Committee
- Research Performance
- Applying for ethics approval
- Researcher resources
- HREC EOI Form.docx
- Useful links
- Animal Ethics
- Research Integrity
- Research Integrity Advisors
- Contact Research Integrity & Ethics
- Centres, Institutes, CRCs
- Graduate Research School
- Research Themes and Clusters
- Office of the Deputy Vice-Chancellor (Research)
The Human Research Ethics Committee (HREC) meets monthly to discuss Greater than Low Risk applications (HREA) applications and weekly for Negligible or Low Risk applications for ethics approval.
Negligible and Low risk meetings for 2024 will be held each Thursday . The application submission date for all expedited applications is 12 noon Tuesday, the week prior to the meeting. The first meeting date for Negligible and Low risk applications for 2024 will be Tuesday 1st February with a closing date of 23rd January. The final Negligible and Low risk meeting date for 2024 will be held on the 5th December.
The 2024 Meeting Dates can be downloaded here. This also include the first and last E2 meeting dates.
Weekly Zoom Drop-In with the Chair & Human Research Ethics Officer - Tuesday 2pm
If you require another meeting time, please contact [email protected] and this will be arranged., committee membership.
Expression of Interest in joining the HREC can be submitted any time and will be considered for any vacancies as they become available.
Please complete the Expression of Interest form. Submission details are included on the form.
- Terms of Reference
- Application information
- Study options
- How to apply
- Scholarships
- Study online
- Study on campus
- Regional Study Centres
- International
- Fees and costs
- English Language Requirements
- UNE Armidale
- UNE Accommodation
- UNE Tamworth
- Events Calendar
- Safe Communities
- High Schools
- Businesses and Community
- Aboriginal and Torres Strait Islander Community
- Alumni Community
- Honorary Appointments
- Teachers & Education Students
- Our Values and Culture
- University Structure
- Faculty of Humanities, Arts, Social Sciences and Education
- Faculty of Medicine and Health
- Faculty of Science, Agriculture, Business and Law
- Principal Dates 2024
- Rankings and Ratings
- Annual reports
- Right to Information
- Accessibility
- CRICOS Provider Number 00003G
- TEQSA Provider Code: PRV12054 Australian University
- ABN: 75 792 454 315
- UNE is a member of the Regional Universities Network
© University of New England, 2024

The University of New England respects and acknowledges that its people, courses and facilities are built on land, and surrounded by a sense of belonging, both ancient and contemporary, of the world's oldest living culture. In doing so, UNE values and respects Indigenous knowledge systems as a vital part of the knowledge capital of Australia. We recognise the strength, resilience and capacity of the Aboriginal community and pay our respects to the Elders past, present and future.
- No category
Variation Application Form - Hunter New England Health

Related documents

Add this document to collection(s)
You can add this document to your study collection(s)
Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer
A NSW Government website

HREC Meeting Dates
Hunter new england human research ethics committee (hnehrec) & clinical trials subcommittee (ctsc) meeting dates.
Applications are due on the last working day of each month
(except December as the Committee and its subcommittee do not meet in January).
Late applications will not be accepted
Please Note: It is mandatory for all NEW Single and Multi-Site Applications to be completed and submitted in REGIS.
Mechanism evaluation of a lifestyle intervention for patients with musculoskeletal pain who are overweight or obese: protocol for a causal mediation analysis
Affiliations.
- 1 School of Medicine and Public Health, Hunter Medical Research Institute, University of Newcastle, Newcastle, Australia.
- 2 Centre for Rehabilitation Research, Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, United Kingdom.
- 3 Centre for Pain, Health and Lifestyle.
- 4 Neuroscience Research Australia, University of New South Wales, Sydney, Australia.
- 5 Hunter New England Population Health, Wallsend, Australia.
- 6 Musculoskeletal Division, The George Institute for Global Health, University of Sydney, Sydney, Australia.
- 7 Ambulatory Care Centre, John Hunter Hospital, Newcastle, Australia.
- PMID: 28674135
- PMCID: PMC5734414
- DOI: 10.1136/bmjopen-2016-014652
Introduction: Low back pain (LBP) and knee osteoarthritis (OA) are highly prevalent and disabling conditions that cause societal and economic impact worldwide. Two randomised controlled trials (RCTs) will evaluate the effectiveness of a multicomponent lifestyle intervention for patients with LBP and knee OA who are overweight or obese. The key targets of this intervention are to improve physical activity, modify diet and correct pain beliefs. These factors may explain how a lifestyle intervention exerts its effects on key patient-relevant outcomes: pain, disability and quality of life. The aim of this protocol is to describe a planned analysis of a mechanism evaluation for a lifestyle intervention for overweight or obese patients with LBP and knee OA.
Methods and analysis: Causal mediation analyses of 2 two-armed RCTs. Both trials are part of a cohort-multiple RCT, embedded in routine health service delivery. In each respective trial, 160 patients with LBP and 120 patients with knee OA waiting for orthopaedic consultation will be randomised to a lifestyle intervention, or to remain part of the original cohort. The intervention consists of education and advice about the benefits of weight loss and physical activity, and the Australian New South Wales Get Healthy Service. All outcome measures including patient characteristics, primary and alternative mediators, outcomes, and potential confounders will be measured at baseline (T0). The primary mediator, weight, will be measured at 6 months post randomisation; alternative mediators including diet, physical activity and pain beliefs will be measured at 6 weeks post randomisation. All outcomes (pain, disability and quality of life) will be measured at 6 months post randomisation. Data will be analysed using causal mediation analysis with sensitivity analyses for sequential ignorability. All mediation models were specified a priori before completing data collection and without prior knowledge about the effectiveness of the intervention.
Ethics and dissemination: The study is approved by the Hunter New England Health Human Research Ethics Committee (13/12/11/5.18) and the University of Newcastle Human Research Ethics Committee (H-2015-0043). The results will be disseminated in peer-reviewed journals and at scientific conferences.
Trial registration number: ACTRN12615000490572 and ACTRN12615000478516; Pre-results.
Keywords: Back pain; Lifestyle; Mechanism evaluation; Mediation Analysis; Osteoarthritis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Publication types
- Randomized Controlled Trial
- Research Support, Non-U.S. Gov't
- Clinical Protocols
- Cohort Studies
- Exercise Therapy
- Life Style*
- Low Back Pain / rehabilitation*
- Musculoskeletal Pain / rehabilitation
- New South Wales
- Obesity / complications
- Obesity / rehabilitation*
- Osteoarthritis, Knee / rehabilitation*
- Overweight / complications
- Overweight / rehabilitation*
- Quality of Life
- Referral and Consultation
- Risk Reduction Behavior*
Associated data
- ANZCTR/ACTRN12615000490572
- ANZCTR/ACTRN12615000478516

IMAGES
COMMENTS
The Hunter New England Human Research Ethics Committee is constituted according to the National Statement on Ethical Conduct in Human Research 2023, and its membership is maintained in excess of the minimum requirements of Section 5.1.30, which are as follows:. A chairperson; Laywoman with no affiliation with the institution and drawn from the local community
Hunter New England Human Research Ethics Committee Level 3, Pod, HMRI, Lot 1, Kookaburra Circuit, New Lambton Heights NSW 2305. Telephone: 02 4921 4140. Email: [email protected]. Should the Complaint relate to the review of an Application or any other of the Hunter New England Human Research Ethics Committee's activities ...
Hunter New England Training in Psychiatry (HNET) ... Research Ethics . HNE Human Research Ethics Committee. HREC Meeting Dates. Submitting an Ethics Application . Forms and Templates. Ethics and Governance Review Fees for Clinical Trials. Reporting Requirements. Policies and Guidelines.
A Certified Human Research Ethics Committee is one that is hosted by an institution that has been certified by the National Health and Medical Research Council to participate in the national approach to single ethical review. ... Hunter New England Local Health District. Hunter New England HREC [EC00403] 02 4921 4140;
When registering a human research ethics approval from the University to the Hunter New England Heath HREC, submit the following documentation to the HNE Health Manager of Research Ethics and Governance prior to commencement of an approved activity:. copy of the University HREC approval letter
Ethical approval has been granted by Macquarie University (Project ID 23816) and Hunter New England (Project ID 2020/ETH02186) Human Research Ethics Committees. The findings will be published in peer-reviewed journals. Results will be fed back to partner organisations and roundtable discussions with …
Hunter New England and Central Coast Local Health Districts. If research involves patients, facilities or records of Hunter New England Health, an application for ethics approval must be made to the Hunter New England Health Research Ethics Committee.. In most cases, if the research is to be conducted by staff or students of the University of Newcastle (and provided ethics approval is granted ...
Director, Hunter Stroke Services; Clinical and Health Service Research Fellow; Member, Hunter New England Human Research Ethics Committee; Acute Stroke Team University of Newcastle. Conjoint Lecturer, School of Medicine and Public Health; Executive Committee Member, Priority Research Centre Stroke and Brain Injury Australasian Stroke Academy ...
Results: This study was approved by the Hunter New England Human Research Ethics Committee (HNEHREC Reference No. 18/10/17/4.02). Preimplementation data collection (T 0 ) was completed in March 2020. As of November 2020, 87% (13/15) wards have completed implementation and are undertaking postimplementation data collection (T 1 ).
The Human Research Ethics Committee (HREC) meets monthly to discuss Greater than Low Risk applications (HREA) applications and weekly for Negligible or Low Risk applications for ethics approval. Negligible and Low risk meetings for 2024 will be held each Thursday.The application submission date for all expedited applications is 12 noon Tuesday, the week prior to the meeting.
Research Projects that will require full review by the Hunter New England Human Research Ethics Committee (HNEHREC): Interventions and therapies, including clinical and non-clinical trials and innovations or new treatment modalities; Active concealment or planned deception of participants; Exposure of illegal activities;
The study will generate new evidence on the challenges, strategies and benefits of normalising ACP into practice in acute and community settings. Trial registration: This project has been approved by the Hunter New England Human Research Ethics Committee (Approval No. 17/12/13/4.16). It has also been retrospectively registered on 3 October 2018 ...
Australian College of Applied Professions Human Research Ethics Committee. EC00447. Registered. Nepean Blue Mountains Local Health District. Nepean Blue Mountains Local Health District. Nepean Blue Mountains Local Health District Research Office. EC00151. Registered and Certified. NSW Population and Health Service Research Ethics Committee.
The NEW1000 study has been approved and will be monitored by the Hunter New England Human Research Ethics Committee. ... Hunter Medical Research Institute and Hunter New England LHD. The NEW1000 Study Hunter Medical Research Institute Kookaburra Circuit, New Lambton Heights, NSW 2305 Ph. (02) 4042 0329
Hunter New England Human Research Ethics Committee Terms of Reference Version 4 dated September 2020 3.5 The HNEHREC will undertake its review of applications in a timely and efficient manner in line with the metrics program detailed in the NSW Health Chief Executive's Service Agreement and any other agreed Key Performance Indicators. 4.
Ethics and dissemination: This study has been approved by the Hunter New England Human Research Ethics Committee (2019/ETH13349). Study findings will be presented at local and national conferences and within scientific research journals. Trial registration number: ACTRN12619001543178.
This Application was reviewed as a Greater than low risk review pathway and was initially considered by the Hunter New England Human Research Ethics Committee at its meeting held on 14 December 2022. The project was determined to meet the requirements of the National Statement on Ethical Conduct in Human Research (2007) and was APPROVED.
HUNTER NEW ENGLAND HUMAN RESEARCH ETHICS COMMITTEE APPLICATION FOR VARIATION OF ETHICS APPROVAL FOR RESEARCH INVOLVING HUMANS Version: August 2015 NOTES: This form is to be used for requesting approval for proposed variations to research projects involving humans which have already been approved by Hunter New England Human Research Ethics Committee of Hunter New England Health. Variations ...
Ethics and dissemination: The study is approved by the Hunter New England Health Human Research Ethics Committee (13/12/11/5.18) and the University of Newcastle Human Research Ethics Committee (H-2015-0043). The results will be disseminated in peer-reviewed journals and at scientific conferences.
Research Ethics & Governance, the Hunter New England Human Research Ethics Committee in consultation with a member of the Clinical Trials Sub-Committee, has granted ethical approval of the above project. The following documentation has been reviewed and approved by the Hunter New England Human Research Ethics Committee:
HNELHD Research Office, John Hunter Hospital Mail Room, Lookout Rd, New Lambton Heights NSW 2305. Phone: (02) 4921 4140. Email: [email protected]. Office location: Awabakal Country: Level 3 POD, Hunter Medical Research Institute, Lot 1 Kookaburra Circuit, New Lambton. Feedback Assist Widget.
Hunter New England Human Research Ethics Committee (HNEHREC) & Clinical Trials Subcommittee (CTSC) Meeting Dates. Ethics Application Submission Close Date. CTSC & HNEHREC Meeting Dates. 31 January 2024. 14 February 2024.
Ethics and dissemination: The study is approved by the Hunter New England Health Human Research Ethics Committee (13/12/11/5.18) and the University of Newcastle Human Research Ethics Committee (H-2015-0043). The results will be disseminated in peer-reviewed journals and at scientific conferences.