Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State

Pathophysiology and Clinical Presentation
Pathophysiology:
Type 1 Diabetes Mellitus is a syndrome characterized by hyperglycemia and insulin deficiency resulting from the loss of beta cells in pancreatic islets (Mapes & Faulds, 2014). Nonimmune (type 1B diabetes), occurs secondary to other diseases and is much less common than autoimmune (type 1A). The destruction of beta cells in Type 1A diabetes results from the interaction of both genetic and environmental factors. Although the genetic susceptibility is not well understood, type 1 diabetes is most strongly associated with major histocompatibility complex (MHC), specifically histocompatibility leukocyte antigen (HLA) class II alleles (HLA-DQ and HLA-DR) (McCance & Heuther, 2014). Type 1 diabetes is less hereditary than type 2 but 7-13% of patients also have a first degree relative with type 1 diabetes (Mapes & Faulds, 2014). Environmental factors include viral infections (especially enteroviruses), exposure to infectious microorganisms (such as Helicobacter pylori ), exposure to cow’s milk proteins and a lack of vitamin D (McCance & Heuther, 2014).
The destruction of insulin-producing beta cells in the pancreas starts with the formation of autoantigens. These autoantigens are ingested by antigen-presenting cells which activate T helper 1 (Th1) and T helper 2 (Th2) lmphocytes. Activated Th1 lymphocytes secrete interluekin-2 (IL-2) and interferon. IL-2 activates autoantigen-specific T cytotoxic lymphocytes which destroy islet cells through the secretion of toxic perforins and granzymes. Interferon activates macrophages and stimulates the release of inflammatory cytokines (including IL-1 and tumor necrosis factor [TNF]) which further destroy beta cells (McCance & Heuther, 2014). Activated Th2 lymphocytes produce IL-4 which stimulates B lymphocytes to proliferate and produce islet cell autoantibodies (ICAs) and anti-glutamic acid decarboxylase (antiGAD65) antibodies. AntiGAD65 is an enzyme that helps control the release of insulin from beta cells and can be used to determine the cause of diabetes (McCance & Heuther, 2014). Insulin autoantibodies [IAAs]) and zinc transporter 8 (Znt8) protein are also associated with type 1 diabetes mellitus. Despite it’s complicated pathophysiology, it is important to understand the destruction of beta cells in type 1 diabetes because it leads to a lack of insulin and amylin. Without insulin or amylin the body cannot promote glucose disappearance or limit glucose appearance from the bloodstream, respectively, resulting in hyperglycemia (Mapes & Faulds, 2014).
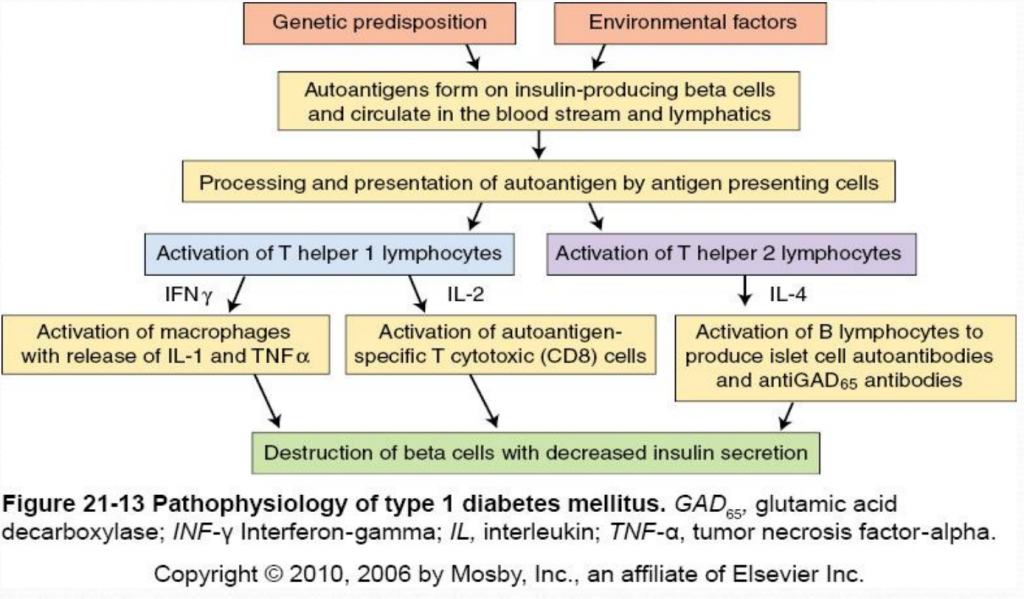
Clinical Presentation:
Type 1 diabetes does not present clinically until 80-90% of the beta cells have been destroyed (McCance & Heuther, 2014). Because insulin stimulates glucose uptake into tissues, stores glycose as glycogen, inhibits glucagon secretion and inhibits glucose production from the liver, the destruction of insulin-producing beta cells causes hyperglycemia (Mapes & Faulds, 2014). Type 1 diabetics may present with abrupt onset of diabetic ketoacidosis, polyuria, polyphagia, polydipsia, or rapid weight loss with marked hyperglycemia (Mapes & Faulds, 2014). To diagnose diabetes, patients must have an A1C level greater than 6.5% percent on two separate tests; the presence of ketones in the urine and/or autoantibodies in the blood can distinguish type 1 from type 2 diabetes (Mayo Clinic, 2014).

2 thoughts on “ Pathophysiology and Clinical Presentation ”
none for now
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Patient Care & Health Information
- Diseases & Conditions
- Type 1 diabetes
What is type 1 diabetes? A Mayo Clinic expert explains
Learn more about type 1 diabetes from endocrinologist Yogish Kudva, M.B.B.S.
I'm Dr. Yogish C. Kudva an endocrinologist at Mayo Clinic. In this video, we'll cover the basics of type 1 diabetes. What is it? Who gets it? The symptoms, diagnosis, and treatment. Whether you're looking for answers for yourself or someone you love. We are here to give you the best information available. Type 1 diabetes is a chronic condition that affects the insulin making cells of the pancreas. It's estimated that about 1.25 million Americans live with it. People with type 1 diabetes don't make enough insulin. An important hormone produced by the pancreas. Insulin allows your cells to store sugar or glucose and fat and produce energy. Unfortunately, there is no known cure. But treatment can prevent complications and also improve everyday life for patients with type 1 diabetes. Lots of people with type 1 diabetes live a full life. And the more we learn and develop treatment for the disorder, the better the outcome.
We don't know what exactly causes type 1 diabetes. We believe that it is an auto-immune disorder where the body mistakenly destroys insulin producing cells in the pancreas. Typically, the pancreas secretes insulin into the bloodstream. The insulin circulates, letting sugar enter your cells. This sugar or glucose, is the main source of energy for cells in the brain, muscle cells, and other tissues. However, once most insulin producing cells are destroyed, the pancreas can't produce enough insulin, meaning the glucose can't enter the cells, resulting in an excess of blood sugar floating in the bloodstream. This can cause life-threatening complications. And this condition is called diabetic ketoacidosis. Although we don't know what causes it, we do know certain factors can contribute to the onset of type 1 diabetes. Family history. Anyone with a parent or sibling with type 1 diabetes has a slightly increased risk of developing it. Genetics. The presence of certain genes can also indicate an increased risk. Geography. Type 1 diabetes becomes more common as you travel away from the equator. Age, although it can occur at any age there are two noticeable peaks. The first occurs in children between four and seven years of age and the second is between 10 and 14 years old.
Signs and symptoms of type 1 diabetes can appear rather suddenly, especially in children. They may include increased thirst, frequent urination, bed wetting in children who previously didn't wet the bed. Extreme hunger, unintended weight loss, fatigue and weakness, blurred vision, irritability, and other mood changes. If you or your child are experiencing any of these symptoms, you should talk to your doctor.
The best way to determine if you have type 1 diabetes is a blood test. There are different methods such as an A1C test, a random blood sugar test, or a fasting blood sugar test. They are all effective and your doctor can help determine what's appropriate for you. If you are diagnosed with diabetes, your doctor may order additional tests to check for antibodies that are common in type 1 diabetes in the test called C-peptide, which measures the amount of insulin produced when checked simultaneously with a fasting glucose. These tests can help distinguish between type 1 and type 2 diabetes when a diagnosis is uncertain.
If you have been diagnosed with type 1 diabetes, you may be wondering what treatment looks like. It could mean taking insulin, counting carbohydrates, fat protein, and monitoring your glucose frequently, eating healthy foods, and exercising regularly to maintain a healthy weight. Generally, those with type 1 diabetes will need lifelong insulin therapy. There are many different types of insulin and more are being developed that are more efficient. And what you may take may change. Again, your doctor will help you navigate what's right for you. A significant advance in treatment from the last several years has been the development and availability of continuous glucose monitoring and insulin pumps that automatically adjust insulin working with the continuous glucose monitor. This type of treatment is the best treatment at this time for type 1 diabetes. This is an exciting time for patients and for physicians that are keen to develop, prescribe such therapies. Surgery is another option. A successful pancreas transplant can erase the need for additional insulin. However, transplants aren't always available, not successful and the procedure can pose serious risks. Sometimes it may outweigh the dangers of diabetes itself. So transplants are often reserved for those with very difficult to manage conditions. A successful transplant can bring life transforming results. However, surgery is always a serious endeavor and requires ample research and concentration from you, your family, and your medical team.
The fact that we don't know what causes type 1 diabetes can be alarming. The fact that we don't have a cure for it even more so. But with the right doctor, medical team and treatment, type 1 diabetes can be managed. So those who live with it can get on living. If you would like to learn even more about type 1 diabetes, watch our other related videos or visit mayoclinic.org. We wish you well.
Type 1 diabetes, once known as juvenile diabetes or insulin-dependent diabetes, is a chronic condition. In this condition, the pancreas makes little or no insulin. Insulin is a hormone the body uses to allow sugar (glucose) to enter cells to produce energy.
Different factors, such as genetics and some viruses, may cause type 1 diabetes. Although type 1 diabetes usually appears during childhood or adolescence, it can develop in adults.
Even after a lot of research, type 1 diabetes has no cure. Treatment is directed toward managing the amount of sugar in the blood using insulin, diet and lifestyle to prevent complications.
Products & Services
- A Book: The Essential Diabetes Book
Type 1 diabetes symptoms can appear suddenly and may include:
- Feeling more thirsty than usual
- Urinating a lot
- Bed-wetting in children who have never wet the bed during the night
- Feeling very hungry
- Losing weight without trying
- Feeling irritable or having other mood changes
- Feeling tired and weak
- Having blurry vision
When to see a doctor
Talk to your health care provider if you notice any of the above symptoms in you or your child.
The exact cause of type 1 diabetes is unknown. Usually, the body's own immune system — which normally fights harmful bacteria and viruses — destroys the insulin-producing (islet) cells in the pancreas. Other possible causes include:
- Exposure to viruses and other environmental factors
The role of insulin
Once a large number of islet cells are destroyed, the body will produce little or no insulin. Insulin is a hormone that comes from a gland behind and below the stomach (pancreas).
- The pancreas puts insulin into the bloodstream.
- Insulin travels through the body, allowing sugar to enter the cells.
- Insulin lowers the amount of sugar in the bloodstream.
- As the blood sugar level drops, the pancreas puts less insulin into the bloodstream.
The role of glucose
Glucose — a sugar — is a main source of energy for the cells that make up muscles and other tissues.
- Glucose comes from two major sources: food and the liver.
- Sugar is absorbed into the bloodstream, where it enters cells with the help of insulin.
- The liver stores glucose in the form of glycogen.
- When glucose levels are low, such as when you haven't eaten in a while, the liver breaks down the stored glycogen into glucose. This keeps glucose levels within a typical range.
In type 1 diabetes, there's no insulin to let glucose into the cells. Because of this, sugar builds up in the bloodstream. This can cause life-threatening complications.
Risk factors
Some factors that can raise your risk for type 1 diabetes include:
- Family history. Anyone with a parent or sibling with type 1 diabetes has a slightly higher risk of developing the condition.
- Genetics. Having certain genes increases the risk of developing type 1 diabetes.
- Geography. The number of people who have type 1 diabetes tends to be higher as you travel away from the equator.
- Age. Type 1 diabetes can appear at any age, but it appears at two noticeable peaks. The first peak occurs in children between 4 and 7 years old. The second is in children between 10 and 14 years old.
Complications
Over time, type 1 diabetes complications can affect major organs in the body. These organs include the heart, blood vessels, nerves, eyes and kidneys. Having a normal blood sugar level can lower the risk of many complications.
Diabetes complications can lead to disabilities or even threaten your life.
- Heart and blood vessel disease. Diabetes increases the risk of some problems with the heart and blood vessels. These include coronary artery disease with chest pain (angina), heart attack, stroke, narrowing of the arteries (atherosclerosis) and high blood pressure.
Nerve damage (neuropathy). Too much sugar in the blood can injure the walls of the tiny blood vessels (capillaries) that feed the nerves. This is especially true in the legs. This can cause tingling, numbness, burning or pain. This usually begins at the tips of the toes or fingers and spreads upward. Poorly controlled blood sugar could cause you to lose all sense of feeling in the affected limbs over time.
Damage to the nerves that affect the digestive system can cause problems with nausea, vomiting, diarrhea or constipation. For men, erectile dysfunction may be an issue.
- Kidney damage (nephropathy). The kidneys have millions of tiny blood vessels that keep waste from entering the blood. Diabetes can damage this system. Severe damage can lead to kidney failure or end-stage kidney disease that can't be reversed. End-stage kidney disease needs to be treated with mechanical filtering of the kidneys (dialysis) or a kidney transplant.
- Eye damage. Diabetes can damage the blood vessels in the retina (part of the eye that senses light) (diabetic retinopathy). This could cause blindness. Diabetes also increases the risk of other serious vision conditions, such as cataracts and glaucoma.
- Foot damage. Nerve damage in the feet or poor blood flow to the feet increases the risk of some foot complications. Left untreated, cuts and blisters can become serious infections. These infections may need to be treated with toe, foot or leg removal (amputation).
- Skin and mouth conditions. Diabetes may leave you more prone to infections of the skin and mouth. These include bacterial and fungal infections. Gum disease and dry mouth also are more likely.
- Pregnancy complications. High blood sugar levels can be dangerous for both the parent and the baby. The risk of miscarriage, stillbirth and birth defects increases when diabetes isn't well-controlled. For the parent, diabetes increases the risk of diabetic ketoacidosis, diabetic eye problems (retinopathy), pregnancy-induced high blood pressure and preeclampsia.
There's no known way to prevent type 1 diabetes. But researchers are working on preventing the disease or further damage of the islet cells in people who are newly diagnosed.
Ask your provider if you might be eligible for one of these clinical trials. It is important to carefully weigh the risks and benefits of any treatment available in a trial.
- Summary of revisions: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-Srev.
- Papadakis MA, et al., eds. Diabetes mellitus. In: Current Medical Diagnosis & Treatment 2022. 61st ed. McGraw Hill; 2022. https://accessmedicine.mhmedical.com. Accessed May 4, 2022.
- What is diabetes? National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes. Accessed May 4, 2022.
- Levitsky LL, et al. Epidemiology, presentation, and diagnosis of type 1 diabetes mellitus in children and adolescents. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Diabetes mellitus (DM). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Accessed May 4, 2022.
- AskMayoExpert. Type 1 diabetes mellitus. Mayo Clinic; 2021.
- Robertson RP. Pancreas and islet transplantation in diabetes mellitus. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Levitsky LL, et al. Management of type 1 diabetes mellitus in children during illness, procedures, school, or travel. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Hyperglycemia (high blood glucose). American Diabetes Association. https://www.diabetes.org/healthy-living/medication-treatments/blood-glucose-testing-and-control/hyperglycemia. Accessed May 4, 2022.
- Diabetes and DKA (ketoacidosis). American Diabetes Association. https://www.diabetes.org/diabetes/dka-ketoacidosis-ketones. Accessed May 4, 2022.
- Insulin resistance & prediabetes. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/prediabetes-insulin-resistance. Accessed May 4, 2022.
- Blood sugar and insulin at work. American Diabetes Association. https://www.diabetes.org/tools-support/diabetes-prevention/high-blood-sugar. Accessed May 4, 2022.
- Inzucchi SE, et al. Glycemic control and vascular complications in type 1 diabetes. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Diabetes and oral health. American Diabetes Association. https://www.diabetes.org/diabetes/keeping-your-mouth-healthy. Accessed May 4, 2022.
- Drug treatment of diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/drug-treatment-of-diabetes-mellitus. Accessed May 4, 2022.
- Weinstock DK, et al. Management of blood glucose in adults with type 1 diabetes mellitus. https://www.uptodate.com/contents/search. Accessed May 7, 2022.
- FDA proves first automated insulin delivery device for type 1 diabetes. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-automated-insulin-delivery-device-type-1-diabetes. Accessed May 4, 2022.
- Boughton CK, et al. Advances in artificial pancreas systems. Science Translational Medicine. 2019; doi:10.1126/scitranslmed.aaw4949.
- Hypoglycemia (low blood sugar). American Diabetes Association. https://www.diabetes.org/healthy-living/medication-treatments/blood-glucose-testing-and-control/hypoglycemia. Accessed May 4, 2022.
- Diabetes in the workplace and the ADA. U.S. Equal Opportunity Employment Commission. https://www.eeoc.gov/laws/guidance/diabetes-workplace-and-ada. Accessed May 4, 2022.
- Cardiovascular disease and risk management: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S010.
- Diabetes technology. Standards of Medical Care in Diabetes — 2022. 2022; doi:10.2337/dc22-S007.
- FDA authorizes a second artificial pancreas system. JDRF. https://www.jdrf.org/blog/2019/12/13/jdrf-reports-fda-authorizes-second-artificial-pancreas-system/. Accessed May 4, 2022.
- Classification and diagnosis of diabetes: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S002.
- Retinopathy, neuropathy, and foot care: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S012.
- Glycemic targets: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S012.
- Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S009.
- Facilitating behavior change and well-being to improve health outcomes: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S005.
- Centers for Disease Control and Prevention. Use of hepatitis B vaccination for adults with diabetes mellitus: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 2011;60:1709.
- Management of diabetes in pregnancy: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S015.
- Older adults: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S013.
- FDA approves first-of-its-kind automated insulin delivery and monitoring system for use in young pediatric patients. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-automated-insulin-delivery-and-monitoring-system-use-young-pediatric#:~:text=Today, the U.S. Food and,by individuals aged 2 to. Accessed May 8, 2022.
- What you need to know: Getting a COVID-19 vaccine. American Diabetes Association. https://www.diabetes.org/coronavirus-covid-19/vaccination-guide. Accessed June 1, 2022.
News from Mayo Clinic
- Driven by family, fueled by hope: Mayo Clinic researcher fights against Type 1 diabetes Aug. 06, 2023, 11:00 a.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
There are unique challenges in caring for children and adolescents with T1DM that differentiate pediatric from adult care. These include the obvious differences in the size of the patients, developmental issues such as the unpredictability of a toddler's dietary intake and activity level and inability to communicate symptoms of hypoglycemia, and medical issues such as the increased risk of hypoglycemia and diabetic ketoacidosis (DKA). Because of these considerations, the management of a child with T1DM must take into account the age and developmental maturity of the child.
Most children with T1DM present relatively early with the classic signs and symptoms of hyperglycemia and deficient insulin release, including polyuria and polydipsia, sometimes associated with polyphagia and weight loss. Approximately 30 percent of children present with severe insulin deficiency and DKA [ 1,2 ]. The topic below provides an overview of the management of T1DM in children who are not in DKA. Details of insulin therapy, including regimens, pumps, and blood glucose monitoring, are presented separately. (See "Insulin therapy for children and adolescents with type 1 diabetes mellitus" .)
Other aspects of childhood-onset T1DM are discussed separately:
● Routine management:
What Is Type 1 Diabetes?

People of all ages can develop type 1 diabetes.
If you have type 1 diabetes, your pancreas doesn’t make insulin or makes very little insulin. Insulin helps blood sugar enter the cells in your body for use as energy. Without insulin, blood sugar can’t get into cells and builds up in the bloodstream. High blood sugar is damaging to the body and causes many of the symptoms and complications of diabetes.
Type 1 diabetes was once called insulin-dependent or juvenile diabetes, but it can develop at any age.
Type 1 diabetes is less common than type 2 —about 5-10% of people with diabetes have type 1. Currently, no one knows how to prevent type 1 diabetes, but it can be treated successfully by:
- Following your doctor’s recommendations for living a healthy lifestyle.
- Managing your blood sugar.
- Getting regular health checkups.
- Getting diabetes self-management education and support .
For Parents
If your child has type 1 diabetes—especially a young child—you’ll handle diabetes care on a day-to-day basis. Daily care will include serving healthy foods, giving insulin injections, and watching for and treating hypoglycemia (low blood sugar). You’ll also need to stay in close contact with your child’s health care team. They will help you understand the treatment plan and how to help your child stay healthy.
Much of the information that follows applies to children as well as adults. You can also visit JDRF’s T1D Resources for more information on managing your child’s type 1 diabetes.
What Causes Type 1 Diabetes?
Type 1 diabetes is thought to be caused by an autoimmune reaction (the body attacks itself by mistake). This reaction destroys the cells in the pancreas that make insulin, called beta cells. This process can go on for months or years before any symptoms appear.
Some people have certain genes (traits passed on from parent to child) that make them more likely to develop type 1 diabetes. However, many of them won’t go on to have type 1 diabetes even if they have the genes. A trigger in the environment, such as a virus, may also play a part in developing type 1 diabetes. Diet and lifestyle habits don’t cause type 1 diabetes.
Symptoms and Risk Factors
It can take months or years before symptoms of type 1 diabetes are noticed. Type 1 diabetes symptoms can develop in just a few weeks or months. Once symptoms appear, they can be severe.
Some type 1 diabetes symptoms are similar to symptoms of other health conditions. Don’t guess! If you think you could have type 1 diabetes, see your doctor to get your blood sugar tested. Untreated diabetes can lead to very serious—even fatal—health problems.
Risk factors for type 1 diabetes are not as clear as for prediabetes and type 2 diabetes. However, studies show that family history plays a part.
Testing for Type 1 Diabetes
A simple blood test will let you know if you have diabetes. If you were tested at a health fair or pharmacy, follow up at a clinic or doctor’s office. That way you’ll be sure the results are accurate.
If your doctor thinks you have type 1 diabetes, your blood may also be tested for autoantibodies. These substances indicate your body is attacking itself and are often found with type 1 diabetes but not with type 2. You may have your urine tested for ketones too. Ketones are produced when your body burns fat for energy. Having ketones in your urine indicates you have type 1 diabetes instead of type 2.
Managing Diabetes
Unlike many health conditions, diabetes is managed mostly by you, with support from your health care team:
- Primary care doctor
- Foot doctor
- Registered dietitian nutritionist
- Diabetes educator
Also ask your family, teachers, and other important people in your life for help and support. Managing diabetes can be challenging, but everything you do to improve your health is worth it!
If you have type 1 diabetes, you’ll need to take insulin shots (or wear an insulin pump) every day. Insulin is needed to manage your blood sugar levels and give your body energy. You can’t take insulin as a pill. That’s because the acid in your stomach would destroy it before it could get into your bloodstream. Your doctor will work with you to figure out the most effective type and dosage of insulin for you.
You’ll also need to do regular blood sugar checks . Ask your doctor how often you should check it and what your target blood sugar levels should be. Keeping your blood sugar levels as close to target as possible will help you prevent or delay diabetes-related complications .
Stress is a part of life, but it can make managing diabetes harder. Both managing your blood sugar levels and dealing with daily diabetes care can be tougher to do. Regular physical activity, getting enough sleep, and exercises to relax can help. Talk to your doctor and diabetes educator about these and other ways you can manage stress.
Healthy lifestyle habits are really important too:
- Making healthy food choices
- Being physically active
- Controlling your blood pressure
- Controlling your cholesterol
Make regular appointments with your health care team. They’ll help you stay on track with your treatment plan and offer new ideas and strategies if needed.
Hypoglycemia and Diabetic Ketoacidosis
These 2 conditions are common complications of diabetes, and you’ll need to know how to handle them. Meet with your doctor for step-by-step instructions. You may want to bring a family member with you to the appointment so they learn the steps too.
Hypoglycemia (low blood sugar) can happen quickly and needs to be treated quickly. It’s most often caused by:
- Too much insulin.
- Waiting too long for a meal or snack.
- Not eating enough.
- Getting extra physical activity.
Talk to your doctor if you have low blood sugar several times a week. Your treatment plan may need to be changed.
Diabetic ketoacidosis (DKA) is a serious complication of diabetes that can be life-threatening. DKA develops when you don’t have enough insulin to let blood sugar into your cells. Very high blood sugar and low insulin levels lead to DKA. The two most common causes are illness and missing insulin shots. Talk with your doctor and make sure you understand how you can prevent and treat DKA.
Get Diabetes Education
Meeting with a diabetes educator is a great way to get support and guidance, including how to:
- Develop and stick to a healthy eating and activity plan
- Test your blood sugar and keep a record of the results
- Recognize the signs of high or low blood sugar and what to do about it
- Give yourself insulin by syringe, pen, or pump
- Monitor your feet, skin, and eyes to catch problems early
- Buy diabetes supplies and store them properly
- Manage stress and deal with daily diabetes care
Ask your doctor about diabetes self-management education and support services and to recommend a diabetes educator. You can also search this nationwide directory for a list of programs in your community.
Get Support
Tap into online diabetes communities for encouragement, insights, and support. Check out the American Diabetes Association’s Community page and JDRF’s TypeOneNation . Both are great ways to connect with others who share your experience.
- Type 1 Diabetes Resources and Support from JDRF
- Living With Diabetes
- Just Diagnosed With Type 1 Diabetes
- Learn About Diabetic Ketoacidosis
- 4 Ways To Take Insulin
- Making the Leap From Type 1 Teen to Adult
To receive updates about diabetes topics, enter your email address:
- Diabetes Home
- State, Local, and National Partner Diabetes Programs
- National Diabetes Prevention Program
- Native Diabetes Wellness Program
- Chronic Kidney Disease
- Vision Health Initiative
- Heart Disease and Stroke
- Overweight & Obesity
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- UBC Directories
- UBC Quick Links
- The University of British Columbia
- a place of mind
- Learn Pediatrics
- Diabetes: Approach to First Presentation
Click for pdf: Diabetes
General presentation
Diabetes mellitus (DM) is an important endocrine disorder that presents commonly in children and adolescents. There are two types of diabetes mellitus: type 1 and type 2. Type 1 DM is one of the most common chronic diseases in children and is characterized by insulin deficiency as a result of autoimmune destruction of pancreatic beta islet cells; whereas type 2 DM is the presence of high blood glucose with insulin resistance and relative insulin deficiency. Diabetes mellitus is a chronic condition that requires long-term follow-up and adequate patient (and parent) education to maintain good glycemic control to prevent long-term complications.
Epidemiology
Approximately 2/3 of all new diabetes diagnoses in patients less than 19 years of age in the United States are type 1 DM. Over 300,000 Canadians have type 1 DM, with a 3-5% increase each year; especially in children aged 5-9. Typically, the age of onset has a bimodal distribution, with the first peak in children 4-6 years old, and the second peak in children 10-14 years old (early puberty). Unlike other autoimmune diseases, the overall incidence appears to be equal in both genders. There is a higher risk of developing this condition in children with close relatives who have type 1 DM.
The incidence of type 2 DM has increased 10 fold in the last decade. There is an estimated 3600/100,000 cases of type 2 DM in Canadian adolescents and 1100/100,000 cases in Canadian children. This value may be as high as 1% in Canadian aboriginal youths and children. There is a strong association between increasing rates of obesity in the pediatric population and the development of type 2 DM.
Basic Physiology
In type 1 DM, there is autoimmune-mediated destruction of insulin-producing pancreatic beta cells that results in insulin deficiency. It is a progressive condition that occurs in genetically susceptible individuals. The autoimmune destruction can be triggered by various environmental agents. Some proposed environmental factors include pregnancy-related and perinatal influences, viruses, cow’s milk and cereals. There is a long latency period (where the patient is asymptomatic and euglycemic) between the onset of beta cell destruction and clinical presentation of diabetes mellitus. A large number of functional beta cells must be lost before clinical symptoms like hyperglycemia occurs.
Genetic polymorphisms in six genes have been shown to be associated with type 1 DM. Major Histocompatibility Complex genes and elsewhere in the genome all contribute to the risk, but only the HLA alleles seem to have a large effect.
The natural history has four stages:
- Preclinical autoimmune destruction of pancreatic beta cells
- Onset of clinical symptoms
- Transient remission
- Established diabetes with acute and chronic complications
Type 2 DM is a complex, multifactorial disease characterized by both relative insulin deficiency and insulin resistance with various environmental and behavioural risk factors. Increased hepatic glucose production, insulin resistance and progressive loss of glucose-stimulated insulin release all contribute to the development of hyperglycemia. In Type 2 DM, pancreatic beta cells retain the ability to produce insulin, but levels are not adequate to counteract the developing insulin resistance. The current theory is that insulin resistance develops first, followed and complicated by gradual destruction of beta cells. Insulin resistance worsens with obesity and physical inactivity; and improves with weight loss and increased physical exercise.
Puberty also plays a role in the development of type 2 DM in adolescents. During this period, insulin sensitivity is approximately 30% lower than that of preadolescents or adults, which results in hyperinsulinemia as a compensatory mechanism. In adolescents with both genetic predisposition and negative environmental contributors, this period of relative insulin resistance may result in a decompensated state (inadequate insulin secretion and glucose intolerance). The resulting hyperglycemic state may cause worsening abnormalities of insulin secretion and action, starting a vicious cycle that progress beyond the adolescent years.
Clinical Presentation
Childhood type 1 DM can present in the following ways:
Classic new onset:
Hyperglycemia without acidosis
Symptoms include:
- Can present as nocturia, bedwetting, daytime incontinence in a previously continent child
- Polydipsia – due to increased serum osmolality and hypovolemia
- Impaired glucose utilization in skeletal muscle and increased fat and muscle breakdown
Diabetic ketoacidosis
Similar symptoms but are usually more severe
- Clinical: polydipsia, polyuria, dehydration, hypotension, ketosis, etc.
- Metabolic: hyperglycemia, glycosuria, metabolic acidosis, ketonemia, etc.
Reported frequency varies between 15-67%
- Young children (<6) from low socioeconomic backgrounds are more likely to present with diabetic ketoacidosis
Silent Presentation
Diagnosis before onset of clinical symptoms
Typically occurs in children with a family member with type 1 DM (close monitoring)
Childhood type 2 DM can present in the following ways:
- Hyperglycemia, ketonuria, acidosis
- Frequency varies between 5-25%
Hyperosmolar hyperglycemic state
- Marked hyperglycemia (>33.3 mmol/L) and severe dehydration but no ketonuria
- Less common in adolescents
Symptomatic
- Due to hyperglycemia and include: polyuria, polydipsia, and nocturia
- Recent weight loss is less frequent
- Adolescent girls: vaginal discharge due to candida infection may be initial presentation
Asymptomatic
- Identified based on screening (for type 2 DM or urinalysis as part of a regular physical exam)
Questions to ask
Historical Investigation
Presenting condition:
- Have you been very thirsty? Do you drink a lot?
- Have you been urinating more than usual?
- Has the child had any bedwetting episodes?
- Has there been any recent weight loss?
- Have you been feeling tired lately?
- Have you noticed an increased appetite lately?
- Has the child had more frequent minor skin infections?
Predisposing factors:
- Have you had any viral infections recently?
- What kinds of exercise do you participate in on a regular basis? How frequent do you exercise? How long do you exercise each time?
- How many hours a day do you spend watching TV, using the computer, and play video games?
- What do you normally eat? What is the portion size? How many meals do you have per day? Do you normally eat out or home cooked meals? Do you eat as a family? Do you eat at the table or in front of the tv?
Family history:
- Are there any family members with insulin-dependent diabetes mellitus?
- Are there any family members with autoimmune conditions?
- Does your mother or father have diabetes?
- Are there any other family members with diabetes? (grandparents, aunts, uncles, brothers, sisters, etc.)
Physical Examination
Do a complete physical exam with particular attention to the following:
- Assess hydration status
- Assess circulation: HR, BP, capillary refill
- Temperature: coexisting infection
- Use growth chart to check for weight loss
- Neck examination: look for thyroid abnormalities
- Respiratory: respiratory rate (hyperventilation – DKA), auscultation (respiratory infection), ketones on breath (DKA)
- Measure body weight and height, calculate BMI
- Measure lying and standing BP
- Inspect skin for acanthosisnigricans
- Examine feet to look for decreased sensation and circulation (pulses)
- Measure visual acuity
Differential diagnosis
- DM types 1 and 2
- Diabetes insipidus
- Urinary tract infection
- Malabsorption (e.g. Celiac disease)
- Secondary diabetes
- Maturity-onset diabetes of the young
Procedures for investigation
Diagnostic Criteria
- Fasting plasma glucose >7 mmol/L (no caloric intake for at least 8 hours)
- Symptoms of hyperglycemia, random venous plasma glucose >11.1 mmol/L
- Abnormal oral glucose tolerance test – plasma glucose >11.1 mmol/L measured 2 hours after a glucose load of 1.75 g/kg (max 75g)
- Glycated hemoglobin (A1C) ≥ 6.5%
Other Investigations
- Urinalysis for glucosuria and ketonuria
- Urinalysis for microalbuminemia
- Alemzadeh R, Wyatt DT. Section 6 – Diabetes mellitus in children. In: Kliegman: Nelson Textbook of Pediatrics. 18 th ed. Saunders, Pennsylvania. 2007
- Eisenbarth GS, McCulloch DK. Pathogenesis of type 1 diabetes mellitus. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2011
- Laffel L, Svoren B. Epidemiology, presentation, and diagnosis of type 2 diabetes mellitus in children and adolescents. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2011
- Levinson P, Nelson BA, Scherger JE. Diabetes mellitus type 1 in children. [Online]. 2007. Availabe from: FirstConsult, MDConsult. [cited 2011 Jan 15]
- Levitsky LL, Misra M. Epidemiology, presentation, and diagnosis of type 1 diabetes mellitus in children and adolescents. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2011
- McCulloch DK, Robertson RP. Pathogenesis of type 2 diabetes mellitus. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2011
- Panagiotopoulos C. Type 2 diabetes in children and adolescents. BCMJ. 2004;46(9): 461-466
- Scherger JE, McIntire SDC, Escobar O, Heinzman DM. Diabetes mellitus type 2 in children. [Online]. 2007. Availabe from: FirstConsult, MDConsult. [cited 2011 Jan 15]
Acknowledgements
Written by: Ying Yao
Edited by: Dianna Louie
Last updated on November 10, 2011 @5:01 pm
Feedback: How useful was the above information?
Post comment click here to cancel reply..
You must be logged in to post a comment.
- Approach to the Child with a fever and rash
- Approach to Bradycardia
- Basics of cardiac pharmacology
- Approach to cardiac history taking
- Congestive heart failure in children
- Approach to Pediatric Hypertension
- Approach to Cyanotic Congenital Heart Disease in the Newborn
- Approach to Syncope: Is it Cardiac or Not?
- Approach to Pediatric Tachycardia
- Approach to Pediatric Chest Pain
- Approach to Cardiac Murmurs
- Normal Cardiac Physiology – Transition From Fetal to Neonatal
- Basic Physiology and Approach to Heart Sounds
- Approach to Pediatric ECGs
- Care of a Child with Turner Syndrome
- Approach to the Underweight Child
- Normal sexual maturity rating
- Hypothyroidism
- Approach to the Short Child
- Approach to Vomiting
- Suspected Foreign Body Ingestion
- Approach to a Mediastinal Mass
- Toxic Ingestion
- Nutritional Deficiencies
- Pharmacology of Common Agents Used in Gastrointestinal Conditions
- Approach to Pediatric Abdominal Pain
- Pediatric Gastrointestinal History Taking
- Approach to diarrhea
- Approach to a picky eater
- Constipation
- Hepatomegaly
- Splenomegaly
- Approach to Abdominal Mass
- Failure to Thrive
- Approach to Skin Lesions
- Common Paediatric Skin Conditions & Birthmarks
- Approach to the child with mental health concerns
- Child with a sore ear (Otalgia)
- To vaccinate or not to vaccinate
- Approach to a the Child with a Fever and Rash
- Approach to a Routine Adolescent Interview
- Sore Throat in Children – Clinical Considerations and Evaluation
- Approach to Strabismus
- Approach to Adolescent Substance Use
- Approach to the Child Who is Dry
- Conjunctivitis: Approach to the Child with a Red Eye
- Acne In Teens
- Pediatric Neck Mass
- Fetal Alcohol Spectrum Disorder
- To Circumcise or Not to Circumcise
- Approach to inborn errors of metabolism
- Infants of Diabetic Mothers
- Fever in the Newborn Period
- Neonatal Thrombocytopenia
- Consequences of Prematurity
- Respiratory Distress in the Newborn
- Approach to Neonatal Cyanosis
- Approach to the Child with IUGR/SGA
- Neonatal Jaundice
- Breastfeeding Problems
- Diaper Rash: Clinical Considerations and Evaluation
- The Pediatric Neurological History
- Evaluation of Pediatric Development (Normal)
- Closed Head Injury in Pediatrics
- Approach to the Ataxic Child
- Approach to the Child with a Seizure
- Basics to the Approach of Developmental Delay
- Lumbar Puncture in Pediatrics
- The Basics of Cerebral Palsy
- Approach to a Child with a Headache
- Febrile Seizures
- Approach to the Comatose Child
- Basics of Spina Bifida
- Principles of Pharmacotherapy in Neurology
- Easy Bleeding
- Non-Neonatal Jaundice
- Approach to Lymphadenopathy
- Tumor Lysis Syndrome
- Febrile Neutropenia
- Pediatric Neutropenia
- Blood Transfusion Reactions
- Approach to Sickle Cell Disease
- Iron-deficiency and Health Consequences in Children
- Easy Clotting
- Approach to Thrombocytopenia
- Approach to Pediatric Leukemias and Lymphomas
- Approach to Thalassemia
- Approach to Non-Accidental Injuries
- Common Pediatric Bone Diseases-Approach to Pathological Fractures
- Infant with an Abnormal Hip Exam
- Knock Kneed Children
- Elbow Injuries
- Approach to the Child with a Limp
- Pediatric Fractures
- Clearing the C - Spine
- Approach to a Child With a Cough
- Approach to Pediatric Hemoptysis
- Approach to Pediatric Dyspnea
- Introduction
- Systemic Exam
- Kidneys and Bladder
- Male Infants
- Female Infants
- Acute Assessment
- General exam
- Peripheral palpation
- Precordium palpation
- Auscultation
- General Inspection
- Genito-Urinary
- Musculoskeletal
- Neurological
- Mental Status Exam
- Cranial Nerve Exam
- Sensory Exam
- Cerebellar Exam
- Other Resources
- Physical Exam Setup
- Screening MSK Exam
- Focused MSK Exams
Emergency Procedures | Accessibility | Contact UBC | © Copyright The University of British Columbia

Type 1 Diabetes Mellitus
Mar 27, 2019
860 likes | 1.27k Views
Type 1 Diabetes Mellitus. Treatment. Goals of T1DM Management. Utilize intensive therapy aimed at near-normal BG and A1C levels Prevent diabetic ketoacidosis and severe hypoglycemia Achieve the highest quality of life compatible with the daily demands of diabetes management
Share Presentation
- juvenile diabetes research foundation
- diabetes technol ther
- swedish ndr jdrf
- diabetes duration
- caloric intake

Presentation Transcript
Type 1 Diabetes Mellitus Treatment
Goals of T1DM Management Utilize intensive therapy aimed at near-normal BG and A1C levels Prevent diabetic ketoacidosis and severe hypoglycemia Achieve the highest quality of life compatible with the daily demands of diabetes management In children, achieve normal growth and physical development and psychological maturation Establish realistic goals adapted to each individual’s circumstances
Routine Care Recommendations for Patients With T1DM A/C, albumin/creatinine ratio. American Diabetes Association. Diabetes Care 2005;28:186-212..
AACE Comprehensive Diabetes Care: Glucose Goals Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53.
Glycemic Control in T1DM
Poor Glycemic Control Among Youth With Diabetes SEARCH for Diabetes in Youth Cross-sectional analysis of data from a 6-center US study of diabetes in youth, N=3947 individuals with T1DM Petitti DB, et al. J Pediatr. 2009;155:668-72.e1-3
Suboptimal Glycemic Control in Adults With T1DM STAR 3 (MDI) JDRF (CGM) Swedish NDR JDRF (no CGM) STAR 3 (CGM/Pump) Pittsburgh EDC EDIC (DCCT Intensive Cohort) EDIC, Epidemiology of Diabetes Interventions and Complications; Pittsburgh EDC, Pittsburgh Epidemiology of Diabetes Complications; Swedish NDR, Swedish National Diabetes Register; Star 3, Sensor Augmented Pump Therapy for A1C Reduction; JDRF, Juvenile Diabetes Research Foundation; CGM, continuous glucose monitor. Nathan DM, et al. Arch Intern Med . 2009;169:1307-1316; Eeg-Olofsson K, et al. Diabetes Care. 2007;30:496-502; Bergenstal RM, et al. N Engl J Med. 2010;363:311-320; JDRF CGM Study Group. N Engl J Med. 2008;359:1446-1476.
Predictors of Poor Glycemic Control Younger age Longer diabetes duration Weight <85th percentile Not living in a 2-parent household Type of diabetes care provider Nonwhite race/ethnicity Female gender Lower parental education Poor early glycemic control (2nd year after diagnosis; predictive of poor glycemic control later) Petitti DB, et al. J Pediatr. 2009;155:668-672.e1-3; Chemtob CM, et al. J Diabetes. 2011;3:153-157.
Glucose Variability and Health Outcomes: Direct and Indirect Pathways Glucose variability Fear of hypoglycemia Reluctance to intensify therapy Quality of life High A1C Severe hypoglycemia Complications Morbidity Mortality Controversial Irvine AA, et al. Health Psychol. 1992;11:135-138; Thompson CJ, et al. Diabetes Care. 1996;19:876-879;Reach G. Diabetes Technol Ther. 2008;10:69-80.
DCCT and EDIC Findings Intensive treatment should be started as soon as is safely possible after the onset of T1DM and maintained thereafter Intensive treatment reduced the risks of retinopathy, nephropathy, and neuropathy by 35% to 90% compared with conventional treatment Absolute risks of retinopathy and nephropathy were proportional to the A1C Intensive treatment was most effective when begun early, before complications were detectable Risk reductions achieved at a median A1C 7.3% for intensive treatment (vs 9.1% for conventional) Benefits of 6.5 years of intensive treatment extended well beyond the period of most intensive implementation(“metabolic memory”) DCCT/EDIC Research Group. JAMA. 2002;15;287:2563-2569.
DCCT/EDIC: Long-Term Benefits of Early Intensive Glycemic Control Intensive glycemic control over a mean of 6.5 years reduced CVD complications by 57% after a mean of 17 years of follow-up Nathan DM, et al. N Engl J Med. 2005;353:2643-2653.
Sustained Effect of Intensive Treatment on Development and Progression of Nephropathy in T1DM Annual Prevalence Cumulative Incidence DCCT/EDIC. JAMA. 2003;290:2159-2167.
Effect of Intensive Treatment on Development and Progression of Retinopathy in T1DM DCCT. N Engl J Med. 1993;329:977-986.
STAR 3 SAP (all ages): 13.3 per 100 pt-yrs; A1C (1 yr): 8.3% 7.5% Severe Hypoglycemia and A1C: DCCT (1993), JDRF (2008), and STAR 3 (2010) Studies DCCT (intensive therapy): 62 per 100 pt-yrs, A1C(6.5 yr): 9.0% 7.2% JDRF CGM (adults, 1 subject excluded): 20.0 per 100 pt-yrs; A1C (6 mo): 7.5% 7.1% DCCT. N Engl J Med. 1993;329:977-986. JDRF CGM Study Group. N Engl J Med. 2008;359:1465-1476. Bergenstal RM, et al. N Engl J Med. 2010;363:311-20.
Treatment of Hyperglycemia in T1DM
Therapeutic Options for Persons With T1DM • Multiple daily injections of rapid acting insulin with meals combined with a daily basal insulin • Other regimens such as premixed insulin are also used in certain clinical situations • Continuous subcutaneous insulin infusion via an insulin pump • Adjunctive therapy with pramlintide Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53.
Recent Advances in the Care of Persons With T1DM Development of insulin analogues Insulin pump therapy Home glucose monitoring Advent of continuous glucose monitoring (CGM)
Treatment of Hyperglycemia in T1DM Insulin Options
Physiologic Multiple Injection Regimens: The Basal-Bolus Insulin Concept • Basal insulin • Controls glucose production between meals and overnight • Near-constant levels • Usually ~50% of daily needs • Bolus insulin (mealtime or prandial) • Limits hyperglycemia after meals • Immediate rise and sharp peak at 1 hour post-meal • 10% to 20% of total daily insulin requirement at each meal • For ideal insulin replacement therapy, each component should come from a different insulin with a specific profile or via an insulin pump (with 1 insulin) Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53.
Pharmacokinetics of Insulin Products Rapid (lispro, aspart, glulisine) Insulin Level Short (regular) Intermediate (NPH) Long (glargine) Long (detemir) 0 2 4 6 8 10 12 14 16 18 20 22 24 Hours Adapted from Hirsch I. N Engl J Med. 2005;352:174-183.
Basal/Bolus Treatment Program With Rapid-Acting and Long-Acting Analogs Rapid (lispro, aspart, glulisine) Rapid (lispro, aspart, glulisine) Rapid (lispro, aspart, glulisine) Plasma insulin Glargine or detemir 4:00 8:00 12:00 16:00 20:00 24:00 4:00 8:00 Breakfast Lunch Dinner Bed
Treatment of Hyperglycemia in T1DM Pramlintide
Aronoff SL, et al. Diabetes Spectrum. 2004;17:183-190;Brown L, et al. Sci Transl Med. 2010;2:27ps18; Lebovitz HE. Nat Rev Endocrinol. 2010;6:326-334. Insulin Replacement Not Always Sufficient for Glucose Control in T1DM • Normal glucose regulation involves multiple hormones (eg, insulin, glucagon, amylin, incretins) and multiple organ systems (eg, pancreas, liver, stomach, brain) • Insulin replacement therapy does not fully mimic the actions of insulin secreted by the pancreas in a healthy individual • Insulin exposure in the liver is lower with replacement therapy than with natural production, resulting in inadequate suppression of endogenous glucose production • Higher doses of insulin are required to achieve sufficient suppression of endogenous glucose production, but these are associated with hypoglycemia and weight gain
Kruger D, et al. Diabetes Educ. 1999;25:389-398. Amylin Is Deficient in Patients with T1DM Normal Diurnal Insulin and Amylin Secretion in Healthy Adults (N=6) Meal Meal Meal 30 Insulin Amylin 600 25 Amylin Secretion in Individuals With and Without T1DM Meal 20 400 Plasma Insulin (pM) Plasma Amylin (pM) 20 15 200 10 No T1DM (n = 27) 15 5 0 7:00 24:00 12:00 17:00 Time (24 h) Plasma Amylin (pM) 10 T1DM (n = 190) 5 0 -30 0 30 60 90 120 150 180 Time After Meal (min)
Pramlintide • Human amylin analog with pharmacokinetic and pharmacodynamic properties similar to endogenous hormone • Mechanism of action • Promotes satiety and reduces caloric intake • Slows gastric emptying • Inhibits inappropriately high postprandial glucagon secretion Inzucchi SE, et al. Diabetes Care. 2012;35:1364-1379.
Continuous Subcutaneous Insulin Infusion
Meal Meal Meal Bolus (meal) insulin needs Normal Insulin Secretion 60 50 40 Serum insulin(µU/mL) 30 20 Basal (background) insulin needs 10 0 0 2 4 6 8 10 12 14 16 18 20 22 24 Time
CSII With Rapid-Acting Analog Morning Afternoon Evening Night Bolus Bolus Bolus Insulin effect Basal Infusion Bedtime Breakfast Lunch Dinner
Features of Modern Insulin Pumps Not Shared by MDI • Variable basal and prandial infusion rates • Meal profiles (eg, square/extended/dual wave), preset basal rate changes, etc • Onboard calculators for meal insulin boluses • Alarms/reminders (eg, missed bolus) • Ability to download pump data to computer • Integration with CGM for automatic feedback control (“semi-closed loop”) CGM, continuous glucose monitoring; MDI, multiple daily injections.
Technological Features of Insulin Pumps* * Will vary by insulin pump make and model. BG, blood glucose.
Improved Control With CSII 8.5 8.0 Before 12 months >24 months 7.5 7.0 A1C 6.5 6.0 5.5 <7 years 7-11 years 12-18 years Age Ahern JA, et al. Pediatr Diabetes. 2002;3:10-15.
Reduced Risk of Severe Hypoglycemia (Seizure/Coma) 40 35 30 25 Patients with seizure or coma (%) 20 15 10 5 0 12 Months Pre-Pump 12 Months Pump Rx Ahern JA, et al. Pediatr Diabetes. 2002;3:10-15.
Other Nonrandomized Pediatric Studies (N>1000) • Switching to CSII results in • Lower A1C (by ~0.5%-0.6%) • Mean A1C ~7.5%-7.6% • Less hypoglycemia • Less glucose variability • No excessive weight gain • Greater patient satisfaction and quality of life Tamborlane WV, et al. Rev Endo Metab Disorders. 2006;7:205-213.
MDI vs CSII: 2008 Meta-analysis • Rate of severe hypoglycemia T1DM was markedly lower during CSII than MDI, with greatest reductions in • Patients with most severe hypoglycemia on MDI • Patients with longest duration of diabetes • Greatest improvement in A1C occurred in patients with the highest A1C on MDI Pickup JC, Sutton AJ. Diabet Med. 2008;25:765-774.
Severe Hypoglycemia With MDI vs CSII: 2008 Meta-analysis Severe hypoglycemia reduced by ~75% by switching to pump therapy No difference between randomized, controlled trials and before/after studies Rate ratio 4.19 (95% CI 2.86-6.13) Pickup JC, Sutton AJ. Diabet Med. 2008;25:765-774.
CSII vs MDI: 2010 Meta-Analysis • 23 studies randomized 976 participants with T1DM to either intervention • Statistically significant difference in A1C favoring CSII • Weighted mean difference: -0.3%(95% confidence interval -0.1 to -0.4) • Severe hypoglycemia appeared to be reduced in those using CSII • Quality of life measures favored CSII Misso ML, et al. Cochrane Database Syst Rev. 2010:CD005103.
CSII vs MDI: 2012 Meta-Analysis Children/adolescents with T1DM Adults with T1DM Adults with T2DM Yeh HC, et al. Ann Intern Med. 2012;157:336-347.
2006 Berlin Consensus Conference on Pumps in Pediatrics • CSII strongly recommended for children with • Recurrent severe hypoglycemia • A1C above target range for age • Unacceptable fluctuations in blood glucose • Microvascular complications • Lifestyle compromised by insulin regimen • CSII may also be beneficial in • Very young children • Dawn phenomenon • Competitive athletes Almost all pediatric patients with T1DM are candidates for CSII Phillip M, et al. Diabetes Care. 2007;30:1653-1662.
Insulin Pump Use in Children Advantages Disadvantages Remembering to give insulin boluses with food intake Ketonuria or ketoacidosis Psychological factors Expense Weight gain Skin infections Insulin unavailability and instability Infusion site locations and set changes Physical/logistical considerations • Improved blood sugar control • Insulin availability and convenience • Use of multiple basal rates, temporary basal rates • Ease of administering multiple boluses • Reduction of hypoglycemia • Flexibility and freedom • Control of post-meal blood sugar/CGM values • Ease of adjusting insulin doses with exercise and travel Maahs DM, et al. Diabetes Technol Ther. 2010; 12(S1):S-59-S-65.
Characteristics of SuccessfulCSII Patients • Access to diabetes team knowledgeable in CSII, with 24/7 HCP access (physician or RN/CDE) • Insurance • Adequate intellectual ability to • Understand glycemic trending, even without CGM • Master carbohydrate counting or similar system for estimation of prandial insulin dosing (frequent SMBG can make up for poor carb estimation) • Understand basics of insulin therapy, including how to correct hyperglycemia before and after meals
Characteristics of SuccessfulCSII Physicians Time to spend with the patient Consistent philosophy of insulin use among all members of diabetes healthcare team Electronic infrastructure in the office or clinic to faciliate downloads and utilize the technology most effectively Basic understanding of principles of insulin use (MDI or CSII)
Definitions in the Context of Insulin Pumps • Pharmacodynamics vs pharmacokinetics • Insulin-on-board (IOB) • Amount of insulin from the last bolus that has not yet been absorbed based on pharmacodynamic (not pharmacokinetic) data • Insulin stacking • Correction dose of insulin, used to treat before-meal or between-meal hyperglycemia in a situation when there is still significant IOB • Insulin sensitivity factor • Correction factor based on amount of glucose reduction (mg/dL) expected from 1 unit of insulin for the individual patient
CSII: “Smart Pump” Limitations • All modern pumps include a “bolus calculator” with goal of preventing insulin stacking, but patient must still • Check blood glucose • Understand “glycemic trends” • Estimate carbohydrate content with reasonable accuracy • Account for lag time • Assume no variability of food or insulin absorption • Use appropriate IOB
Glycemic Control and CSII Patients with T1DM switched from MDI to pump therapy (N=104) • A1C on CSII significantly correlated with prior A1C on MDI (r=0.66; P<0.001). Nixon R, Pickup JC. Diabetes Technol Ther. 2011;13:93-98.
Continuous Glucose Monitoring
Definitions • Professional CGM • Equipment owned by the provider • Patient “masked” (not blinded) to CGM data • Personal CGM • Device owned by patient • Blood glucose data visible, able to be seen continuously
CGM in T1DM:JDRF Sensor Trial • Patients • Baseline A1C >7.0% • Age cohorts • 8-14 years (n=114) • 15-24 years (n=110) • ≥25 years (n=98) • Improvement sustained for 12 months in patients aged ≥25 years • No significant difference between CGM and control group among patients <25 years of age Patients ≥25 Years of Age P<0.001 JDRF CGM Study Group. New Engl J Med. 2008;359:1464-1476.
Change in A1C Over Time: JDRF Sensor Trial Patients ≥25 Years of Age JDRF CGM Study Group. N Engl J Med. 2008;359:1464-1476.
Relationship Between Frequency of CGM Use and Change in A1C:JDRF Sensor Trial JDRF CGM Study. Diabetes Care. 2009;32:1947-1953.
A1C Goal Attainment:JDRF Sensor Trial P<0.001 JDRF CGM Study. Diabetes Care. 2009;32:1947-1953.
- More by User

Diabetes Mellitus Type 1 Diabetes and Its Current Treatments
Diabetes Mellitus Type 1 Diabetes and Its Current Treatments. Michelle Adams CHEM 5389 April 3, 2007. Presentation Outline. Diabetes What is Diabetes? How do people get diabetes? What are the signs and symptoms? Importance of Control – Complications of Diabetes Control Being In Control
925 views • 26 slides

type 1 diabetes mellitus
http://www.our-diabetic-life.com Here are information on Type 1 Diabetes Mellitus
684 views • 5 slides

Type - 1 Diabetes Mellitus :Indian and Global Scene
694 views • 43 slides

Pediatric Type 1 Diabetes Mellitus
Pediatric Type 1 Diabetes Mellitus. Chelsea Stegman and Kelly Davis. Test your Knowledge. Type 1 Diabetes Mellitus is a/an _____ disease. a. Thyroid b. Bacterial c. Viral d. Autoimmune. Test your Knowledge. Type 1 Diabetes is most commonly diagnosed amongst: A. Overweight adults
893 views • 21 slides

Type 2 Diabetes Mellitus
Type 2 Diabetes Mellitus. Aetiology, Pathogenesis, History, and Treatment. The Diabetes Mellitus epidemic. Estimated 180 million people in the world have DM. That’s roughly 6% of the world population. These numbers are estimated to double by 2030.
734 views • 19 slides

Diabetes Mellitus Type 1
Objectives. Recognize the difference between Type 1 and Type 2 DMIdentify the etoiology, epidemiology, pathophysiology, and common clinical manifestations of DM1.Know the pathophysiology, common presentation, labs, complications and proper treatment of DKABe familiar with different types of insul
753 views • 38 slides

Diabetes Mellitus Type 2
Diabetes Mellitus Type 2. Dr. Vinod Sanghi MD. Overview of Diagnosed and Undiagnosed Diabetes in the United States—2000. Diabetes in India. People with Diabetes : 40 million In 2025 : About 70 million people with diabetes DIABETES CAPITAL OF THE WORLD.
750 views • 37 slides
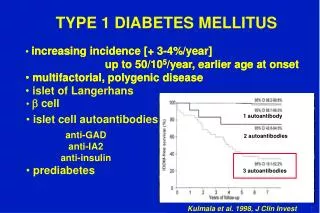
TYPE 1 DIABETES MELLITUS
increasing incidence [ + 3-4%/year ] up to 50/10 5 /year, earlier age at onset multifactorial, polygenic disease islet of Langerhans. 1 autoantibody. 2 autoantibodies. b cell. 3 autoantibodies. islet cell autoantibodies. prediabetes.
506 views • 18 slides

Type 1 Diabetes Mellitus – insulins Key slides
Type 1 Diabetes Mellitus – insulins Key slides. Type 1 vs. type 2 diabetes Lambert P, et al. Medicine 2006; 34(2): 47-51 Nolan JJ. Medicine 2006; 34(2): 52-56. Features of type 2 diabetes Usually presents in over-30s (but also seen increasingly in younger people)
309 views • 9 slides

Type I Diabetes Mellitus
Type I Diabetes Mellitus. MR 8/14/09 J.Chen. Management. Insulin Monitoring Nutrition Exercise Education. Insulin. Insulin Requirements. Preadolescent-0.5-1 unit/kg/day Adolescents-0.8-1.2 units/kg/day During honeymoon period-<0.5 units/kg/day. Starting an Insulin Regimen.
313 views • 15 slides

Diabetes mellitus type 1 (Type 1 diabetes, T1D, T1DM , IDDM, juvenile diabetes):
Diabetes mellitus type 1 (Type 1 diabetes, T1D, T1DM , IDDM, juvenile diabetes):. is a form of diabetes mellitus . Type 1 diabetes is an autoimmune disease [1] that results in destruction of insulin -producing beta cells of the pancreas .
1.31k views • 54 slides

Pediatric Type 1 Diabetes Mellitus. Chelsea Stegman and Kelly Davis. Test your Knowledge . Type 1 Diabetes Mellitus is a/an _____ disease. a. Thyroid b. Bacterial c. Viral d. Autoimmune. Test your Knowledge. Type 1 Diabetes is most commonly diagnosed amongst: A. Overweight adults
669 views • 21 slides

Diabetes Mellitus type 1 & Cerebrovascular disease
Evaluation of Vascular Resistance in the Intracerebral and Extracerebral Arteries in Patients with Diabetes Mellitus type 1 - preliminary results.
382 views • 14 slides

Diabetes Mellitus (Type 2)
Diabetes Mellitus (Type 2). By Madison and Jemma. What is it? Description. Diabetes Mellitus is a chronic condition in which the sufferer is unable to utilise blood glucose correctly. There are three types of this NHPA: type 1 diabetes, type 2 diabetes, gestational diabetes. Different types:.
518 views • 10 slides

TYPE 2 DIABETES MELLITUS
TYPE 2 DIABETES MELLITUS. Cynthia Brown, MN, ANP, CDE. Type 2 Diabetes Mellitus. Epidemiology: 25 million Americans or 8.3% 7 million undiagnosed 1.9 million older than 20 diagnosed in 2010 7 th leading cause of death In 2007, cost of treating $174 billion
455 views • 27 slides

Diabetes Mellitus Type 2. Diabetes Mellitus. Diabetes mellitus is “a group of common metabolic disorders that share the phenotype of hyperglycemia.” (HPIM 17 th ed.)
532 views • 13 slides


Diabetes Mellitus, Type 2
Diabetes Mellitus, Type 2. Presentation By Heather Hawley. Epidemiology.
822 views • 20 slides

Autoimmune Insulin-dependent diabetes mellitus (Type 1): (IDDM-type 1)
Autoimmune Insulin-dependent diabetes mellitus (Type 1): (IDDM-type 1). -IDDM is a type of diabetes that results from autoimmune destruction of insulin-producing pancreatic beta cells of the islets of Langerhans . - Incidence varies from ( 8 to 17 ) per 100,000 in Northern
377 views • 17 slides

Facts about Type I Diabetes Mellitus
Facts about Type I Diabetes Mellitus. “ Diabetes was long thought to be a kidney disease (Greek & Arabic Methodology). “ Thomas Willis (1621 - 1679), discovered the sweetness of urine, hence, the name Diabetes Mellitus arised”. “Mathew Dobson (1776), identified glycosuria.
535 views • 36 slides

DIABETES MELLITUS Type 1
DIABETES MELLITUS Type 1. By Dana Beaver, RN. Diabetes Mellitus. Is one of the oldest conditions known to man, having been identified in 1500 B.C. (Selekman, J., 2006). What is Diabetes?. Diabetes is a disease in which the body does not produce or properly use insulin
1.19k views • 28 slides

Diabetes Mellitus Type II
Diabetes Mellitus Type II. Beta Cell Failure in DM T2. signaling pathways implicated in β -cell failure. Controls organismal growth and differentiation. Wnt Signaling Pathway. Wnt signalling Pathway and DM T2.
614 views • 40 slides

Autoimmune Insulin Dependent Diabetes Mellitus (Type 1 Diabetes Mellitus) :
Autoimmune Insulin Dependent Diabetes Mellitus (Type 1 Diabetes Mellitus) :. Major immunologic Features: HLA-DR3 and DR4 haplotype expression on the beta cells of the islets of Langerhans. Presence of reactive Autoantibodies directed against multiple antigens of islets beta cells.
798 views • 27 slides
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Correspondence
- Published: 21 March 2024
Slowly progressive insulin-dependent diabetes mellitus in type 1 diabetes endotype 2
- Tetsuro Kobayashi ORCID: orcid.org/0000-0002-4364-2527 1 , 2 &
- Takashi Kadowaki 1 , 3
Nature Reviews Endocrinology ( 2024 ) Cite this article
Metrics details
- Type 1 diabetes
- Type 2 diabetes
You have full access to this article via your institution.
We have read with great interest the Review by Maria J. Redondo and Noel G. Morgan (Redondo, M.J., Morgan, N.G. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat. Rev. Endocrinol . 19 , 542–554 (2023)) 1 . The authors propose a new concept to clarify the intrinsically unique pathological processes in the heterogenous atypical endotype of type 1 diabetes mellitus (T1DE2) and to explore specific approaches for prediction, prevention and treatment. T1DE2 is sometimes assumed to be a mix of type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) because a proportion of people with insulin-independent diabetes mellitus have islet autoantibodies, a marker of T1DM, as well as obesity and insulin resistance, markers of T2DM 1 . However, systematic data on pathobiological findings in pancreas tissue from people with T1DE2 are rarely reported, but there are some systematic studies on the endotype of typical type 1 diabetes mellitus (T1DE1) 2 , 3 .
To make the concept of T1DE2 clearer, we present the distinct pathobiological findings of an atypical form of T1DM, slowly progressive insulin-dependent diabetes mellitus (SPIDDM) 4 , 5 , 6 , 7 , as cited in the article 1 . SPIDDM onset predominantly occurs during adolescence or adulthood, and β-cell function usually decreases gradually until reaching the insulin-dependent stage 4 , 5 . In our study, people with SPIDDM had no history of obesity (defined as BMI >30.0 kg m −2 ) (refs. 6 , 7 ). Most people with SPIDDM had T1DM-susceptible HLA-DR and HLA-DQ haplotypes 4 , 6 .
The most predominant features of SPIDDM examined by in situ hybridization and immuno-histochemical methods indicate persistent enterovirus infection in the islet cells as well as in exocrine acinar cells 7 . Persistent enterovirus infection over decades in typical T1DM is not reported 2 , 3 . In addition, innate immune responses including melanoma associated protein 5 (MDA5), innate immune receptor and IFNβ1 expression gradually decreased with the duration of SPIDDM 7 . The suppressed innate immunity in SPIDDM was histologically related to the cleavage of MDA5 and IFNβ1 in islet cells by protease 2 (2A pro ) (ref. 7 ). 2A pro is encoded by enteroviruses to cleave the enterovirus-preprotein to enable the assembly of the virus envelope protein 8 . 2A pro potentially has proteolytic activity and could therefore damage neighbouring β-cells 7 . 2A pro activity in coxsackie virus B3-induced chronic cardiomyopathy was reported to have a causative role on the cleavage and/or damage of cardiomyocyte dystrophin-glycoprotein complex 9 .
The inflammation of islets in SPIDDM is less aggressive than in typical T1DM, probably due to a weakened innate immune response. This weakened innate immunity can be seen in the low numbers of infiltrating CD8 + T cells in the pancreatic islets and the weak chemokine expression and MHC class I hyperexpression on β-cells in SPIDDM 6 , 7 , sharply contrasting with the aggressive attack of CD8 + T cells and cytopathic effects on β-cells observed in fulminant T1DM 10 . We could not find islet amylin polypeptide (IAPP)-positive amyloid deposition in the residual islet β-cells in SPIDDM 6 , a marker of T2DM.
In summary, SPIDDM is strongly associated with persistent enterovirus infection that disables innate immunity through the MDA5–IFNβ1 axis and is associated with autoimmunity and T1DM-asssociated HLA haplotypes 4 , 6 . Association was not found with T2DM in our study; people with SPIDDM had no islet IAPP-amyloid deposition 6 . Our findings will contribute to the clarification of the T1DE2 endotype proposed by Redondo and Morgan 1 .
There is a reply to this letter by Redondo, M. and Morgan, N. G. Nat. Rev. Endocrinol . https://doi.org/10.1038/s41574-024-00977-x (2024).
Redondo, M. J. & Morgan, N. G. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 19 , 542–555 (2023).
Article PubMed Google Scholar
Foulis, A. K. et al. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 29 , 267–274 (1986).
Article CAS PubMed Google Scholar
Morgan, N. G. & Richardson, S. J. Fifty years of pancreatic islet pathology in human type 1 diabetes: insights gained and progress made. Diabetologia 61 , 2499–2506 (2018).
Article PubMed PubMed Central Google Scholar
Kobayashi, T. et al. Immunogenetic and clinical characterization of slowly progressive IDDM. Diabetes Care 16 , 780–788 (1993).
Kobayashi, T. Subtype of insulin-dependent diabetes mellitus (IDDM) in Japan: slowly progressive IDDM – the clinical characteristics and pathogenesis of the syndrome. Diabetes Res. Clin. Pract. 24 , S95–S99 (1994).
Aida, K. et al. Distinct inflammatory changes of the pancreas of slowly progressive insulin-dependent (type 1) diabetes. Pancreas 47 , 1101–1109 (2018).
Article CAS PubMed PubMed Central Google Scholar
Fukui, T. et al. Bi-glandular and persistent enterovirus infection and distinct changes of the pancreas in slowly progressive type 1 diabetes mellitus. Sci. Rep. 13 , 6977–6993 (2023).
Laitinen, O. H. et al. Enteroviral proteases: structure, host interactions and pathogenicity. Rev. Med. Virol. 26 , 251–267 (2016).
Bouin, A. et al. Enterovirus persistence in cardiac cells of patients with idiopathic dilated cardiomyopathy is linked to 5’ terminal genomic RNA-deleted viral populations with viral-encoded proteinase activities. Circulation 139 , 2326–2338 (2019).
Aida, K. et al. RIG-I- and MDA5-initiated innate immunity linked with adaptive immunity accelerates beta-cell death in fulminant type 1 diabetes. Diabetes 60 , 884–889 (2011).
Download references
Acknowledgements
T. Kobayashi acknowledges the support of research grants from the Japan Society for Promotion of Science KAKENHI (grant nos.15K09406 and 21K08541) and the support of funding from Yasuyuki Yokoyama, CEO of Yokoyamasangyo.
Author information
Authors and affiliations.
Department of Endocrinology and Metabolism, Toranomon Hospital, Tokyo, Japan
Tetsuro Kobayashi & Takashi Kadowaki
Division of Immunology and Molecular Medicine, Okinaka Memorial Institute for Medical Research, Tokyo, Japan
Tetsuro Kobayashi
Division of Endocrinology and Metabolism, Okinaka Memorial Institute for Medical Research, Tokyo, Japan
Takashi Kadowaki
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Tetsuro Kobayashi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Kobayashi, T., Kadowaki, T. Slowly progressive insulin-dependent diabetes mellitus in type 1 diabetes endotype 2. Nat Rev Endocrinol (2024). https://doi.org/10.1038/s41574-024-00975-z
Download citation
Published : 21 March 2024
DOI : https://doi.org/10.1038/s41574-024-00975-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

How does type 1 diabetes alter muscle structure and blood supply?
I n a recent study conducted by the Institute of Anatomy, Faculty of Medicine, University of Ljubljana, researchers have provided new insights into the detrimental effects of type 1 diabetes mellitus (T1DM) on skeletal muscle structure and capillary networks. Utilizing state-of-the-art 3D imaging technology, this comprehensive study marks a significant leap in understanding the multifaceted impact of T1DM on the body's muscular system.
Diabetes mellitus disrupts the regulation of glucose levels, leading to high blood sugar and a myriad of related health issues. T1DM, characterized by the immune-mediated destruction of insulin-producing pancreatic β cells, has profound effects on various organs, especially skeletal muscles, which play a crucial role in glucose uptake and regulation.
This study , published in the journal Biomolecules and Biomedicine , aimed to explore the structural and functional adaptations of skeletal muscles to the metabolic disturbances caused by T1DM.
The hidden changes in muscle and blood vessels
Conducted on female C57BL/6J-OlaHsd mice using a streptozotocin (STZ)-induced model to simulate T1DM, the research focused on critical muscles like the soleus, gluteus maximus, and gastrocnemius. Researchers meticulously analyzed the expression of myosin heavy chain (MyHC) isoforms and the intricacies of the 3D capillary network.
"Our study provides a deeper understanding of how type 1 diabetes not only affects muscle fiber composition but also significantly alters the capillary networks that are essential for muscle health," explained Nejc Umek, the study's lead author.
The research revealed that, despite the composition of fast-twitch type 2b fibers remaining consistent, notable differences were observed in the diabetic mice's soleus muscle, which showed a reduced proportion of type 2a fibers and diminished fiber diameters across all muscles analyzed.
Additionally, an intriguing increase in capillary length per muscle volume was discovered in the gluteus maximus of diabetic mice, suggesting an adaptive mechanism to counterbalance muscle fiber atrophy induced by diabetes.
Methodological advances and key discoveries
The study utilized female mice, addressing a gap in diabetes research that often overlooks gender differences in disease progression and response to treatment. Through a single intraperitoneal administration of STZ, researchers successfully induced T1DM, confirmed by significantly elevated fasting glucose levels. This model allowed for an in-depth examination of diabetes-induced changes in a controlled environment.
By employing antibodies specific to different MyHC isoforms and cutting-edge 3D imaging, the team could precisely quantify changes in muscle fiber types and the capillary network. "The advanced 3D imaging techniques we used represent a significant improvement over traditional 2D analyses, offering a more detailed and accurate depiction of the capillary network changes in diabetic muscle tissue," stated Erika Cvetko, the study's senior author.
Implications for diabetes management and future directions
The findings from this collaborative research effort highlight the necessity for comprehensive diabetes management plans that encompass not only glucose regulation but also the preservation of muscle structure and function. "Understanding the specific alterations in muscle tissue due to type 1 diabetes paves the way for developing targeted therapies that could significantly improve patient outcomes," Cvetko added.
The study's revelations about the increased capillary length per muscle volume in diabetic mice underscore the body's potential compensatory responses to the structural changes induced by diabetes. These insights are crucial for designing interventions that aim to mitigate muscle deterioration and enhance overall diabetes care.
This novel study contributes significantly to the body of knowledge on diabetes and its systemic effects, particularly on skeletal muscle health. By highlighting the critical role of maintaining muscle integrity and vascular supply in the management of T1DM, the research opens new avenues for therapeutic strategies and underscores the importance of multidisciplinary approaches in tackling this complex disease.
More information: Nejc Umek et al, Skeletal muscle myosin heavy chain expression and 3D capillary network changes in streptozotocin-induced diabetic female mice, Biomolecules and Biomedicine (2023). DOI: 10.17305/bb.2023.9843
Provided by Association of Basic Medical Sciences of FBIH
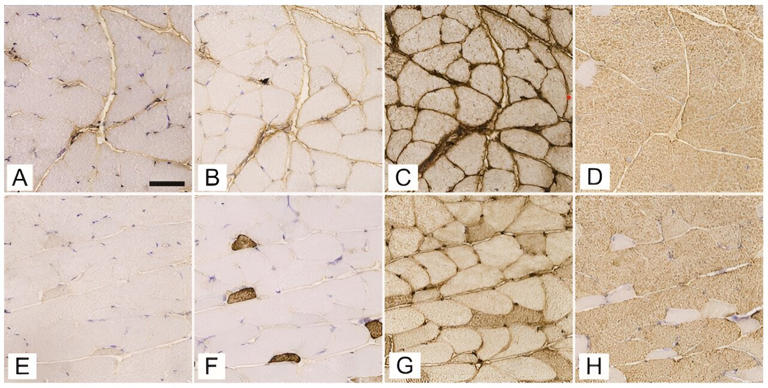
The Impact of Parental Electronic Health Literacy on Disease Management and Outcomes in Pediatric Type 1 Diabetes Mellitus: Cross-Sectional Clinical Study
Affiliations.
- 1 Doctoral School, Semmelweis University, Budapest, Hungary.
- 2 Health Economics Research Center, University Research and Innovation Center, Óbuda University, Budapest, Hungary.
- 3 Pediatric Center, Semmelweis University, Budapest, Hungary.
- 4 Musculoskeletal Research Unit, University of Bristol, Bristol, United Kingdom.
- 5 Physiological Controls Research Center, University Research and Innovation Center, Óbuda University, Budapest, Hungary.
- PMID: 38506893
- DOI: 10.2196/54807
Background: Despite the growing uptake of smart technologies in pediatric type 1 diabetes mellitus (T1DM) care, little is known about caregiving parents' skills to deal with electronic health information sources.
Objective: We aimed to assess the electronic health literacy of parents caring for children with T1DM and investigate its associations with disease management and children's outcomes.
Methods: A cross-sectional survey was performed involving 150 parent-child (8-14 years old with T1DM) dyads in a university pediatric diabetology center. Parents' electronic health literacy (eHealth Literacy Scale [eHEALS]), general health literacy (Chew questionnaire and Newest Vital Sign [NVS]), and attitudes toward T1DM care (Parental Self-Efficacy Scale for Diabetes Management [PSESDM] and Hypoglycemia Fear Survey [HFS]) were investigated. Children's treatment, HbA 1c level, and quality of life (Pediatric Quality of Life Inventory Diabetes Module [PedsQL Diab] and EQ-5D-Y-3L) were assessed. Multiple linear regression analysis was performed to investigate the determining factors of 6-month average HbA 1c .
Results: Of the 150 children, 38 (25.3%) used a pen, 55 (36.7%) used a pen plus a sensor, 6 (4.0%) used an insulin pump, and 51 (34.0%) used an insulin pump plus a sensor. Parents' average eHEALS score (mean 31.2, SD 4.9) differed significantly by educational level (P=.04) and the children's treatment (P=.005), being the highest in the pump + sensor subgroup. The eHEALS score showed significant Pearson correlations with the Chew score (r=-0.45; P<.001), NVS score (r=0.25; P=.002), and PSESDM score (r=0.35; P<.001) but not with the children's HbA 1c (r=-0.143; P=.08), PedsQL Diab (r=-0.0002; P>.99), and EQ-5D-Y-3L outcomes (r=-0.13; P=.12). Regression analysis revealed significant associations of the child's HbA 1c level with sex (β=0.58; P=.008), treatment modality (pen + sensor: β=-0.66; P=.03; pump + sensor: β=-0.93; P=.007), and parents' self-efficacy (PSESDM; β=-0.08; P=.001).
Conclusions: Significantly higher parental electronic health literacy was found in T1DM children using a glucose sensor. The electronic health literacy level was associated with parents' diabetes management attitude but not with the child's glycemic control. Studies further investigating the role of parental electronic health literacy in T1DM children managed at different levels of care and the local context are encouraged.
Keywords: caregivers; child; diabetes mellitus; electronic health literacy; parents.
©Áron Hölgyesi, Andrea Luczay, Péter Tóth-Heyn, Eszter Muzslay, Eszter Világos, Attila J Szabó, Petra Baji, Levente Kovács, László Gulácsi, Zsombor Zrubka, Márta Péntek. Originally published in JMIR Pediatrics and Parenting (https://pediatrics.jmir.org), 20.03.2024.
Early onset type 2 diabetes mellitus: an update
- Open access
- Published: 12 March 2024
Cite this article
You have full access to this open access article
- Myrsini Strati ORCID: orcid.org/0009-0006-2060-6415 1 ,
- Melpomeni Moustaki ORCID: orcid.org/0009-0007-2918-7461 2 ,
- Theodora Psaltopoulou ORCID: orcid.org/0000-0002-1404-9716 3 ,
- Andromachi Vryonidou 2 &
- Stavroula A. Paschou ORCID: orcid.org/0000-0002-0651-1376 3
549 Accesses
1 Altmetric
Explore all metrics
The incidence and prevalence of type 2 diabetes mellitus (T2DM) in young individuals (aged <40 years) have significantly increased in recent years, approximating two to threefold increase in the respective rates. Numerous risk factors including severe obesity, family history, ethnicity, maternal diabetes or gestational diabetes, and female sex contribute to a younger age of onset. In terms of pathogenesis, impaired insulin secretion is the key operating mechanism, alongside with ectopic adiposity-related insulin resistance. T2DM diagnosis in a young adult requires the exclusion of type 1 diabetes mellitus (T1DM), latent autoimmune diabetes of adults (LADA) and maturity-onset diabetes of the young (MODY). The establishment of such diagnosis is critical for prognosis, because early-onset T2DM is associated with rapid deterioration in pancreatic β-cell secretory function leading to earlier initiation of insulin therapy. Furthermore, mortality and lifetime risk of developing complications, especially microvascular, is increased in these patients compared to both later-onset T2DM and T1DM patients; also, the latter are often developed earlier in the course of disease. The management of early-onset T2DM follows the same guidelines as in later-onset T2DM; yet patients aged 18–39 years are underrepresented in the big clinical trials on which the development of guidelines is based. Finally, young people with T2DM face significant challenges associated with social determinants, which compromise their adherence to therapy and induce diabetes distress. Future research focusing on the pathogenesis of β-cell decline and complications, as well as on specific treatment shall lead to better understanding and management of early-onset T2DM.
Similar content being viewed by others
Young-onset type 2 diabetes mellitus — implications for morbidity and mortality
Dianna J. Magliano, Julian W. Sacre, … Jonathan E. Shaw

Emerging Type 2 Diabetes in Young Adults
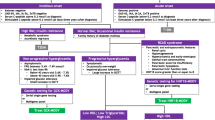
The epidemiology, molecular pathogenesis, diagnosis, and treatment of maturity-onset diabetes of the young (MODY)
Ken Munene Nkonge, Dennis Karani Nkonge & Teresa Njeri Nkonge
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a global pandemic, currently affecting 1 in 10 individuals aged 20–79 years and ranking among the leading causes of premature mortality. Its global incidence and prevalence are continuously increasing, with the latter being anticipated to reach 784 million by 2045 [ 1 ]. Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and insufficient insulin secretion [ 2 ] and accounts for more than 90% of all types of DM worldwide [ 1 ]. Although previously known as a metabolic disorder occurring in middle and late adulthood, the increasing rates of T2DM in children, adolescents, and young adults in recent years and especially after the 2000s, as reflected by almost tripling of the respective standardized incidence ratio in individuals up to the age of 40 years, raises serious public health concerns [ 1 , 2 , 3 , 4 , 5 , 6 , 7 ]. Accumulating evidence indicates that early-onset T2DM is associated with a more severe disease phenotype, characterized by faster decline in β-cell secretory function, leading to necessitation of insulin therapy earlier in the course of disease, alongside with increased lifetime risk of developing unfavorable long-term outcomes [ 2 ]. Despite the increased prevalence and severity of this clinical entity, several pathogenetic and clinical aspects are not entirely understood. This review aims to explore the risk factors, pathophysiology, clinical presentation, differential diagnosis, complications, and management of early-onset T2DM, based on evidence acquired from clinical studies over the last 2 decades.
Risk factors
The upward trend of early-onset T2DM is associated with numerous different risk factors, which demonstrate the disease’s multifactorial character. Worryingly, obesity, showing escalating rates especially among young populations and being mainly driven by unhealthy lifestyle habits, appears to be the major contributor [ 2 ]. In a study aiming to assess the phenotypic characteristics and risk factors leading to early-onset T2DM (<40 years of age) conducted by Lascar et al. 95% of the participants were found to be either overweight or obese [ 8 ]. Similarly, in a nationwide-based study in Israel of ~1.5 million adolescents, Twig et al. described that severe obesity (body mass index, BMI > 35 kg/m 2 ) increases the risk for T2DM in early adulthood in both sexes [ 9 ].
Furthermore, genetic predisposition and family history of T2DM seem to be strong predictors of early presentation of the disease, with 60% of patients having one parent affected by T2DM [ 10 ]. Unlike type 1 diabetes mellitus (T1DM), where ∼90% of patients have a negative family history [ 11 ], 74–100% of children presenting with T2DM are estimated to have a 1st or 2nd-degree relative with T2DM [ 12 ].
Notably, early-onset T2DM is highly represented in people originating from specific ethnic groups, such as Indigenous Australians, Pima Indians, Native Americans, and Canada’s First Nation people, a fact that might be linked either to genetic predisposition or to lower socioeconomic status [ 10 ]. Τhe Progress in Diabetes Genetics in Youth (ProDiGY) Consortium collected data from three studies (TODAY Study, SEARCH Study, and T2D-GENES) in order to explore the effect of various genetic variants predisposing specifically to youth-onset T2DM and discovered seven genome wide associations. [ 13 ].
Moreover, Pettitt et al. in the SEARCH for diabetes study found that early life determinants affecting the intrauterine environment, such as maternal gestational DM and obesity, are associated with increased risk of developing T2DM earlier in life [ 14 ]. In addition, a systematic review and meta-analysis demonstrated that newborns small for gestational age (SGA), compared to the ones with adequate size for gestational age, have an increased risk (2.33-fold higher) of developing T2DM in childhood or adolescence years [ 15 ].
Finally, female sex, especially if polycystic ovarian syndrome (PCOS) is present, predisposes to earlier presentation of T2DM [ 8 ]. Last but not least, other, well recognized risk factors for late-onset T2DM such as non-alcoholic fatty liver disease (NAFLD), hypertension [ 16 ], dyslipidemia, and albuminuria are frequently encountered among young patients with T2DM as well (Table 1 ) [ 10 ].
Pathophysiology
Compared to T1DM in the same age and T2DM in middle and late adulthood, early-onset T2DM, including both children and young adults, is shown to have higher rate of complications and more rapid deterioration of β-cell function respectively [ 1 , 10 , 17 ]. Although the pathophysiology and precise pathways through which T2DM develops in young people are not fully described yet, the overall etiology is similar to that of later-onset T2DM, including insulin resistance and β-cell dysfunction [ 10 ].
Insulin resistance
Insulin resistance is a metabolic state characterized by great complexity, induced by multiple suggested pathways.
In the first place, obesity is more common in early-onset T2DM compared to later-onset T2DM (95% vs. 50%) and it is considered to be one of the key drivers in developing the early-onset phenotype of the disease [ 10 ]. Indeed, BMI and age of T2DM onset are shown to have a strong inverse correlation [ 18 ].
More important than obesity per se seems to be the unfavorable distribution of adipose tissue, characterized by decreased subcutaneous adipose tissue and increased intramyocellular and intrahepatic lipid content, which is also more prominent in patients with early-onset T2DM. In fact, intrahepatic fat, which is emerging as the most important marker of insulin resistance, is threefold higher compared to later-onset T2DM and BMI-matched peers without diabetes [ 2 , 19 ]. The ectopic, intracellular accumulation of excess lipid metabolites in skeletal muscle and liver leads to post-receptor impairment of insulin signaling. In skeletal muscle, there is inhibition of insulin-receptor substrate tyrosine phosphorylation and hence of the following phosphatidylinositol 3 kinase activity and glucose transporter 4 transportation to the cell surface, resulting into decreased glucose uptake and glycogenesis, which is actually considered as the primary metabolic defect in T2DM pathogenesis [ 20 ]. Similarly, in liver, there is inhibition of glycogen synthase activity and stimulation of gluconeogenic enzymes expression, leading to attenuated glycogenesis and enhanced gluconeogenesis [ 21 ].
Furthermore, youth populations with T2DM show evidence of systemic inflammation, which appears to be interrelated with hepatokines’ and adipokines’ profile. According to two cross-sectional studies comparing obese adolescents with and without T2DM matched for BMI, sex, and age, the former demonstrate higher levels of the hepatokines fibroblast growth factor 21 (FGF21) and fetuin-A, as well as of high-sensitivity C-reactive protein (hsCRP), tumor necrosis factor alpha (TNF-α) and interleukin 1 beta [ 22 , 23 ]. Interestingly, there is a positive correlation between FGF21 and hsCRP and a negative correlation between leptin and TNF-α [ 23 ]. Given that FGF21 is known to have an insulin-sensitizing effect, these findings suggest an inflammation-induced FGF-resistant state, in the absence of leptin resistance in this setting [ 23 ]. In turn, fetuin-A is associated with intrahepatic fat and suggested to induce insulin resistance in parallel with promoting low-grade inflammation, leading to a mutually amplifying loop between them [ 22 ].
Additionally, intrauterine environment emerges as a key player of insulin resistance in this setting. According to the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS), there is linear association between maternal and child glycemia across the spectrum of glucose levels, alongside with inverse association between maternal glucose levels at all time points of 2-h oral glucose tolerance test and child insulin sensitivity, assessed by Matsuda index, independently of mother or child BMI and family history. These results highlight the contribution of exposure to higher levels of glucose in utero to development of insulin resistance earlier in life [ 24 ]. Moreover, low birth weight (LBW) and SGA were also associated with 0.20 increased mean levels of homeostasis model assessment of insulin resistance in a systematic review and meta-analysis. The exact mechanisms underlying this phenomenon remain unexplained, but several hypotheses have been developed including overfeeding tendency during perinatal period of LBW newborns (“the early catch-up theory”) and metabolic stress events in utero causing aberrant vasculature and endocrine dysregulation (“the fetal programming theory”). [ 15 ].
Moreover, hormonal changes throughout puberty are also considered to amplify insulin resistance. The expected surge in growth hormone (GH) followed by extensive lipid breakdown and increase in free fatty acids concentration in the bloodstream comprises the main mechanism of short-term increase in insulin resistance during this period [ 25 ].
Finally, several studies describe a positive association between metabolic disease and sleep deprivation, with the latter being prominent among adolescents in recent years. Chronic sleep deprivation is associated with higher cortisol levels, inflammatory markers, and decreased testosterone levels. Additionally, by altering the expression of several appetite-related hormones (e.g., orexin, ghrelin) sleep deprivation is described to cause an imbalance in central appetite regulation controlled by the hypothalamus, and therefore to increase the risk of obesity [ 26 ].
β-cell dysfunction
In order to compensate for the increased insulin resistance, β-cells initially increase insulin secretion. However, over time, the number and secretory response of healthy β-cells decreases due to glucolipotoxicity, endoplasmic reticulum stress, mitochondrial dysfunction and inflammation, which crosstalk with genetic and epigenetic factors [ 27 ]. The substantial difference between early- and later-onset T2DM is that the impairment of β-cell function progresses more rapidly, highlighting that impaired insulin secretion is the key operating pathophysiologic mechanism. In particular, the yearly β-cell function deterioration in early-onset T2DM was reported to be 20–35% by The Multi-Ethnic Treatment Options for T2DM in Adolescents and Youth (TODAY) study of 699 youth and adolescents with T2DM (mean age 14 years, USA), much higher compared to 7% per year in later-onset T2DM [ 28 ].
The exact mechanisms underpinning the accelerated loss of β-cell function in early-onset T2DM are not entirely understood. A widely accepted hypothesis in explanation of this phenomenon is that insulin hypersecretion, assessed by insulin and C -peptide levels, at the initial stages of impaired glucose tolerance (IGT) or newly diagnosed T2DM is more pronounced in children and adolescents compared to older adults, and thus, might lead to faster β-cell exhaustion [ 29 ]. In this regard, increases in hormones related to growth and puberty, such as GH and sex steroids have been shown to overstimulate β-cells, which express GH, estrogen, and androgen receptors [ 25 , 30 ]. Considering that insulin sensitivity decreases by 30–50% during puberty even in healthy, normal weight children, the above hormonal stimulation most likely comprises a compensatory mechanism, which, however, may not be adequate in individuals prone to β-cell dysfunction, who may present with insulin deficiency during this period [ 27 ].
Additionally, intrauterine environment and birth weight appear to affect substantially β-cell function besides insulin sensitivity. According to HAPO FUS, maternal glycemia is inversely associated with child’s disposition index (DI), a measure of pancreatic β-cell function which is shown to predict the progression to T2DM [ 24 ]. Similarly, obese children born SGA demonstrate deficit in early insulin response and DI [ 31 ]. Apart from stressing β-cell via increasing insulin resistance, pathological intrauterine environment appears to affect β-cell function per se. Data from a large study comparing 568 non-diabetic offspring of mothers and fathers with early-onset T2DM, for the same degree of insulin resistance, the former had lower early insulin response, highlighting the importance of intrauterine hyperglycemia in fetal β-cell programming even among patients with equal genetic predisposition to early-onset T2DM [ 32 ]. Similarly, a previous small study dated in 1970s, which compared pancreatic biopsies of SGA and normal weight babies of similar gestational age that died within the first 48 h after birth, indicated that the former had decreased fetal endocrine pancreatic tissue and percentage of β-cells [ 33 ].
Furthermore, impaired proinsulin processing, reflected at increased proinsulin levels and proinsulin-to-insulin ratio, has been demonstrated in two studies in young subjects with T2DM, including the large-scale TODAY study [ 34 ]. According to the latter, impaired proinsulin processing comprises an early predictor of glycemic control deterioration [ 35 ]. The underlying mechanisms have not been elucidated. However, considering that hyperproinsulinemia does not precede the onset of T2DM points away from the possibility of being compensatory in young patients [ 34 ].
Genetically-wise, there are few data regarding specific genotypes linked to early-onset T2DM. Particularly, rs3738435 variant of muscarinic acetylcholine receptor subtype 3 (CHRM3) gene is associated with decreased acute insulin response and increased risk of early-onset T2DM in Pima Indians [ 36 ], and rs7903146 variant of transcription factor 7-like protein 2 (TCF7L2) is linked to decreased β-cell responsivity and DI, impaired proinsulin processing, and increased risk of IGT and T2DM in obese adolescents of Caucasian and African American origin [ 37 ]. Regarding the contribution of epigenetic factors, rodent data indicate that depletion of m6 mRNA methylation leads to islet phenotype of early-onset T2DM, via inducing cell-cycle arrest and impairing insulin secretion via inhibition of protein kinase B (AKT) phosphorylation and pancreatic duodenal homeobox 1 (PDX1) protein levels [ 38 ].
In terms of environmental factors, as recently reviewed, adequate intake of specific micronutrients, such as vitamin D, calcium, vitamin A, zinc, and iron is important for the preservation of β-cell function. Interestingly, some of these factors, such as vitamins A and D appear to be essential for the development of fetal pancreatic islets. Nevertheless, the role of nutrients in pathogenesis of early-onset T2DM is scarcely studied [ 27 ]. Finally, recently published data reveal that the second phase of insulin secretion is inversely correlated with bedtime, which is later in patients with early-onset T2DM than in those with later-onset T2DM [ 39 ].
Compared to T1DM
Early-onset T2DM and T1DM are both a result of the loss of function and mass of β-cells in pancreatic islets, but their underlying pathogenic mechanisms are distinguished by fundamental differences. In early-onset T2DM, β-cell failure is the end pathophysiological stage, preceded by insulin hypersecretion which initially compensates for the increased insulin resistance mainly on the grounds of obesity, ectopic adiposity, puberty, and inflammation. The faster exhaustion of β-cell reserve in this setting is multifactorial, being related to higher insulin resistance, fetal programming in utero, genetic, epigenetic, and environmental parameters [ 27 ]. On the contrary, in T1DM, β-cell failure due to autoimmune-mediated β-cell apoptosis is the initial event in pathogenesis. More specifically, the key mechanism underlying T1DM is driven by the presence of autoreactive T-lymphocytes and macrophages which target β-cell surface antigens and release cytokines in the microenvironment of the pancreatic islets resulting in an inflammatory reaction, called “insulitis”, and progressive β-cell death. In most cases, these events cause a total deficiency of insulin secretion and therefore lifelong exogenous insulin replacement therapy is required [ 40 , 41 ]. Despite the increased evidence of insulin resistance in young patients with T1DM leading to overlap of clinical phenotype between the two types of DM, this event is not intrinsic to pathophysiology; rather, it is secondary to obesity, inflammation, exogenous insulin treatment in parallel with decreased insulin delivery to portal circulation, and, to certain degree, determined by genetics and ethnicity [ 42 ].
Clinical presentation and differential diagnosis
Early-onset T2DM is strongly associated with obesity, metabolic syndrome features, insulin resistance, family history, and necessitates faster progression to insulin therapy, i.e., in 2–5 years after diagnosis in >50% of patients [ 2 ]. Recently, a study led by Baek et al. demonstrated that patients with early-onset T2DM present with higher levels of fasting glucose and HbA1 at the time of diagnosis and have poorer glycemic control and higher glycemic variability compared to middle- and older- onset age groups [ 43 ].
The classification of different forms of diabetes in young populations presents significant challenges, as the differential diagnosis spectrum is wider than in older populations. Early-onset T2DM overlaps with clinical patterns commonly seen both in T1DM, latent autoimmune diabetes of adults (LADA), maturity-onset diabetes of the young (MODY). For instance, the more aggressive phenotype of the disease, accompanied by early insulinopenia and initiation of insulin therapy, overlaps with clinical characteristics of T1DM, LADA, and MODY types 1 and 3.
Unlike T2DM, immune-mediated diabetes (T1DM, LADA) is identified by the presence of autoimmune markers. The presence of two or more autoantibodies that target antigens of the β-cell secretory granules is indicative of either T1DM or LADA. These include islet cell autoantibodies, glutamic acid decarboxylase autoantibodies, insulin autoantibodies, tyrosine phosphatases islet antigen 2 autoantibodies (IA-2A and IA-2β), and also zinc transporter 8 autoantibodies [ 44 ]. Although β-cell destruction and corresponding insulin reserve vary among individuals with autoimmune diabetes, by the time that full-blown picture of insulinopenia is established, plasma C-peptide level is usually undetectable and may be used to differentiate immune-mediated diabetes from other DM types [ 44 , 45 ], except from the honeymoon phase of LADA. Importantly, considering the continuously increasing prevalence of obesity in the general population, BMI is not considered a reliable feature to differentiate between T2DM from T1DM and should not preclude autoimmune diabetes testing [ 46 ]. Additionally, due to overlapping genetic susceptibility, autoimmune diabetes is often associated and can co-exist with additional autoimmune disorders such as autoimmune thyroid disease, celiac disease, autoimmune hepatitis, and others; thus, should be suspected in patients with an autoimmune background [ 47 ] .
MODY, the most common type of monogenic diabetes, is caused by a single-gene mutation resulting in impaired β-cell function and decreased insulin secretion. Similarly to immune-mediated diabetes and early-onset T2DM, MODY also typically presents during adolescence or early adulthood years. At least 14 subtypes have been identified to date, based on the specific mutated gene with the majority of cases being classified into three main subtypes (MODY 1–3). Clinical features, age of onset, and management differ depending on specific gene affected. Notably, MODY 1 and MODY 3 present with phenotype of insulin deficiency, including polyuria, polydipsia, and weight loss. Their substantial difference with both early-onset T2DM and T1DM is that insulin secretion is restored by treatment with sulfonylureas. In addition, patients with MODY 1 usually have history of macrosomia and neonatal hyperglycemia, which is also common in patients with early-onset T2DM exposed to hyperglycemia in utero; however, in MODY 1 cases such manifestations may occur even in the absence of maternal or gestational DM. Contrary to the above subtypes, MODY 2 presents with mild fasting hyperglycemia, neither requiring treatment nor being associated with chronic complications; therefore, it can be easily distinguished from early-onset T2DM based one the absence of progression. Importantly, the autosomal dominant transmission in most forms of MODY overlaps with the strong association between early-onset T2DM and positive family history. In addition, similarly to immune-mediated diabetes, overweight or obesity may also be present in patients with MODY and should not preclude genetic testing for MODY panel, especially if no other markers of metabolic syndrome are present, not all affected relatives are obese and autosomal dominant mode of inheritance is present [ 44 , 48 ]. Considering these overlapping features as well as the possibility of inadequate genetic testing, MODY patients, although representing a small fraction of all diabetes types, may remain underrecognized. Indeed, 50–90% of MODY cases are reported to be misdiagnosed as T1DM or T2DM [ 49 ].
Complications and mortality
Given the rising incidence and the faster progression of T2DM in young populations, several studies during the past few years aimed to investigate the association between the early age at diagnosis and the risk of developing several complications, compared to later-onset T2DM or T1DM. Several questions have also been stated about whether the possible higher risk of morbidity and mortality is attributed to a more aggressive phenotype of the disease, a more prolonged duration of diabetes, or both. The contribution of various risk factors to the development of some complications was also examined in several studies (Table 2 ).
In the prospective, follow-up study conducted after the completion of TODAY study, aiming to assess the occurrence of T2DM-related complications among youth (mean age 26.4 ± 2.8 and mean time since diagnosis 13.3 years), at least one microvascular complication developed in 60.1% and at least two in 28.4% of the participants. Complications examined included hypertension, dyslipidemia, and microvascular complications such as neuropathy, nephropathy, and retinopathy [ 50 ]. Moreover, according to the SEARCH for Diabetes in Youth Study hyperglycemia was positively associated with dyslipidemia in adolescents with T2DM; in fact, there was a 20% and 13% higher risk of either deterioration or maintaining abnormal LDL-C and triglycerides levels respectively for each 1% increase in glycated hemoglobin (HbA1c) in young adolescents with T2DM [ 51 ]. However, this correlation does not apply exclusively in early-onset T2DM populations. In a study including 1747 T2DM patients of all age ranges (average 58.6 years), a positive association between blood glucose levels and TG, LDL-C, TG/HDL-C, and LDL/HDL-C was also established [ 52 ]. Additionally, in a meta-analysis integrating data from 26 observational studies comprising, in total, over a million of participants worldwide, Nanayakkara et al. established an inverse association between age at T2DM diagnosis and all-cause mortality, macrovascular, and microvascular disease [ 53 ].
Furthermore, a study by Sattar et al. collecting data from the Swedish National Diabetes Registry from 1998 to 2013 aimed to examine the association between age at T2DM diagnosis and risk of cardiovascular disease (CVD) and mortality and reported higher risk of all analyzed outcomes in patients with younger age at T2DM diagnosis [ 54 ]. Similarly, in a population-based cohort in Australia by Huo et al. younger age of onset is associated with all-cause mortality as well as with higher rates of cardiovascular (CV) mortality, emphasizing the importance of effective CVD risk factor management [ 55 ].
Apart from the traditional complications of diabetes, additional complications such as subclinical hearing impairment and infertility, are also described in young patients with T2DM [ 2 , 56 , 57 ]. Such complications might remain underrecognized but can substantially affect the quality of life of these patients. In addition, early-onset T2DM patients may be more prone to mental health disorders. In a study of 1114 patients, Riaz et. al observed that the diagnosis of T2DM in patients <40 years of age is associated with an increased risk of developing depression [ 58 ].
Compared to later-onset T2DM
In an observational study of 10,447 patients with T2DM, Cho et al. aimed to compare the prevalence of diabetic retinopathy, neuropathy, nephropathy, and carotid artery plaque formation between patients with early (diagnosed <40 years) and late (diagnosed >40 years)-onset T2DM and found higher prevalence of these complications in the early-onset group. However, after adjustment for the duration of the disease, only the prevalence of neuropathy remained significantly higher [ 59 ].
To minimize the effect of disease duration, Al-Saeed et al. compared the prevalence of complications and mortality in patients with early-onset T2DM (diagnosed between 15 and 30 years) to patients with later-onset T2DM (diagnosed between 40 and 50 years), matched for disease duration, glycemic control, and male to female ratio. As demonstrated, albuminuria and neuropathy were more prominent in the young-onset group, without differences in retinopathy among the two groups. This outcome is attributed to the “inherent” morbidity of having T2DM at a younger age by the authors. Interestingly though, the younger-onset patients were less frequently treated for hypertension and dyslipidemia in this study, possibly reflecting another possible cause. In addition, the standardized mortality ratio (SMR) was shown to be markedly higher in the young-onset group, reaching sixfold increase compared to that of the background population by the age of 40 years. This observation indicates that morbidity and mortality are not only higher in the young-onset group, but also occur early [ 60 ].
Furthermore, Unnikrishnan et al. also compared the risk factors and complications between T2DM patients diagnosed ≤25 years and ≥50 years and described poorer glycemic and lipid control in the younger-aged group. After adjusting for various risk factors, the early-onset group was shown to have higher risk of developing retinopathy [ 61 ]. Finally, according to a retrospective study in China led by Huang et al. early age of T2DM onset increases the risk of developing long-term microvascular, but not macrovascular complications [ 62 ].
In the observational SEARCH for Diabetes in Youth Study, conducted from 2002 to 2015 and comprising 2018 participants with type 1 and 2 diabetes with age of diagnosis <20 years, Dabelea et al. demonstrated that the prevalence of diabetic kidney disease (CKD), retinopathy and peripheral neuropathy was higher among the early-onset type 2 group, after adjustment for differences in various risk factors. No significant differences in arterial stiffness and hypertension were reported compared to the T1DM group [ 63 ].
In a more recent longitudinal study conducted also in the context of the SEARCH Study group, Shah et al. compared the arterial stiffness and heart rate variability (HRV) in participants with young-onset type 1 vs. type 2 diabetes and demonstrated that both factors worsened moreover time in patients with T2DM, and especially in those with signs of metabolic syndrome [ 64 ].
Eppens et al. compared the prevalence of various complications among patients with T1DM and T2DM diagnosed before 18 years and reported higher rates of microalbuminuria and hypertension in T2DM patients, higher rates of retinopathy in T1DM patients and no differences in neuropathy rates. The only risk factor reported to have an association with higher albuminuria rates in the T2DM group was higher HbA1c. [ 65 ].
Constantino et al. also aimed to compare long-term outcomes and survival rates between patients with T1DM and T2DM with age of onset ranging from 15 to 30 years. Similarly, twofold higher mortality rates were demonstrated in T2DM patients compared to T1DM peers, with similar age and duration. A significantly higher prevalence of albuminuria as well as macrovascular risk factors and complications were described in the T2DM cohort, but no differences in retinopathy and renal disease [ 66 ].
In a cross-sectional study in the UK, Song et al. also compared the risk of complications between the groups of young-onset T2DM and T1DM. After adjusting for various risk factors, the T2DM group was shown to be more predisposed to cardiovascular disease and neuropathy, and to have similar risk for developing retinopathy compared to the T1DM group [ 67 ].
Little evidence exists to specify the therapeutic management for early-onset T2DM. Thus, treatment planning is mainly based on existing T2DM evidence-based protocols [ 2 ]. Importantly, a recent study by Sargeant et al. reported that adult patients with early-onset T2DM (aged 18–39 years) are severely underrepresented in prominent clinical trials on which diabetes management guidelines are based [ 68 ]. A multidisciplinary care team, including pediatric and adult endocrinologists, educators, dietitians, psychologists, and other specialists is essential in order to achieve the best possible outcomes [ 69 ].
With the aim of decreasing the risk of the severe and rapidly developing complications of the disease, achieving adequate glycemic control is fundamental [ 2 ]. Lifestyle interventions, including achieving/ maintaining optimal weight, healthy dietary habits, regular exercise, smoking cessation, and limiting alcohol consumption are key pillars of treatment and together with diabetes self-management education should be prioritized and considered as the first line and essential component of the patient’s management plan [ 70 ].
Whether remission of early-onset T2DM is feasible if proper management of lifestyle risk factors is achieved is a matter of great importance. The Diabetes Remission Clinical Trial (DiRECT study) aimed to examine whether an intensive weight management program (total diet replacement) delivered in routine care, along with discontinuation of all diabetes medication, could attain remission of T2DM, defined as HbA 1c < 6.5%. Participants included in this study aged 20–65 years, were diagnosed within 6 years, had BMI of 27–45 kg/m 2 and were not treated with insulin. Primary results at 12 months were weight loss of ≥15 kg in 24% and T2DM remission in 46% of participants in the intervention group (vs. 0% and 4% in control group who was treated according to guidelines), suggesting that T2DM can be reversed if appropriate dietary and lifestyle interventions are applied shortly after diagnosis [ 71 ]. In a 48-month analysis of the same study weight loss of ≥15 kg was maintained by 11% and T2DM remission by 36% of participants of the intervention group. Importantly, 70% of those who maintained weight loss of ≥15 kg managed to achieve a durable remission, emphasizing the strong association between the two [ 72 ]. Similarly, the DIADEM-I randomized control trial, was designed to investigate the impact of intensive lifestyle intervention (total diet replacement and physical activity) on weight loss and T2DM remission but compared to DiRECT study it included younger patients (aged 18–50 years) with a shorter disease duration (≤3 years). Patients had a BMI of ≥27 kg/m 2 . After 12 months, the mean weight loss in the intervention group was 11.98 kg and remission of T2DM was achieved in 61% of the participants (compared to 3.98 kg and 12% in the control group who was treated with usual diabetes care) [ 73 ]. Βoth studies included patients not receiving insulin treatment at baseline, therefore without profound β-cell dysfunction, highlighting the importance of early interventions targeting obesity-driven insulin resistance.
In addition, whether restoration of β-cell function is possible if risk factors are controlled was investigated by several studies that included T2DM patients of all age ranges. Further metabolic investigations, including examinations for β-cell function, were performed on a subgroup of participants of the DiRECT study. On this study, Taylor et al. described a recovery of first-phase insulin secretion among the responder’s group, that was also sustained during the maintenance period, compared to no-change in the non-responders group. Responders vs. non-responders were defined by a shorter disease duration, highlighting that T2DM could be considered a reversible condition, if appropriate weight management interventions are applied early in the course of disease. Importantly, in the same study, ectopic adipose tissue content in liver and pancreas was decreased in both groups after weight loss [ 74 ]. However, further evidence is required regarding the extent of β-cell function reversibility, specifically in early-onset T2DM populations.
Importantly, in a considerable number of cases of adolescents with T2DM, adequate glycemic control cannot be achieved or maintained on a long-term basis if lifestyle interventions are applied alone [ 2 , 75 , 76 ]. Indeed, according to a study by Herbst et al. comprising 578 patients (mean age 15.5 ± 2.1 years), while regular physical activity is associated with lower HbA1c, higher high-density lipoprotein (HDL) and lower standardized BMI, it does not appear to de-escalate the required treatment regime [ 77 ].
Despite the rising incidence and faster progression of T2DM in younger ages, there are currently limited studies regarding oral hypoglycemic drugs in children and young adults. A Scandinavian population-based study from 2010 to 2019 aimed to examine the use of non-insulin antidiabetic drugs in patients <24 years and found that metformin was by far the most commonly prescribed medication in this age group, followed by glucagon-like peptide 1 (GLP-1) analogs, the use of which had an eightfold increase during the period that this study was conducted [ 78 ].
Pharmacotherapy options for patients <18 years are limited due to little existing evidence in regard to their safety and efficacy. Since 2000, metformin has been the only approved oral medication available for this age group. Empagliflozin, a sodium-glucose co-transporter-2 inhibitor that has revolutionized adult T2DM care in the past few years, was very recently approved by the US Food and Drug Administration for use in pediatric populations 10 years and older with T2DM [ 79 ]. Injectable GLP-1 agonists, liraglutide (daily) and exenatide extended release (once-weekly) have also been approved for T2DM treatment in the youth since 2019 and 2021 respectively [ 80 , 81 ]. The rapid deterioration of β-cell function observed in early-onset phenotype usually leads to necessitation of insulin therapy at earlier stages in those patients compared to the later-onset T2DM (in >50% of patients by 2–5 years after diagnosis) [ 2 , 60 ].
Results from the TODAY study, a multicentered randomized clinical trial combining lifestyle and drug treatment choices in 699 obese young patients (aged 10–17 years) with T2DM reported that metformin monotherapy resulted in durable glycemic control (HbA1c < 8% without requirement of insulin therapy) in only 50% of the participants, metformin plus rosiglitazone had superior results to metformin monotherapy and metformin combined to lifestyle intervention program had no difference to metformin alone. Further analysis of this study reported that treatment response differs in relation to ethnic origin and sex, with non-Hispanic Blacks having the poorest response and females having the best response to the combination treatment (metformin plus rosiglitazone) [ 82 ].
Moreover, with the rise in the prevalence of T2DM in young populations, including females of childbearing age, a rise in pregnancies complicated by type 2 diabetes is also anticipated. For instance, despite strong birth control recommendations on participants, 10.2% of female patients in the TODAY study experienced a pregnancy, with a considerable percentage resulting in unfavorable outcomes, such as pregnancy endings and major congenital abnormalities [ 83 ].
Another randomized clinical trial, The Restoring Insulin Secretion (RISE) Pediatric Medication Study, including 91 overweight or obese patients (aged 10–19 years) with IGT or newly diagnosed T2DM (<6 months), compared two different treatment approaches, 3 months of insulin glargine followed by 9 months of metformin or 12 months metformin alone, in order to evaluate the β-cell function during and after treatment. Neither of the two treatment alternatives succeeded in preventing β-cell function deterioration, emphasizing the need for new approaches aiming to maintain β-cell function [ 84 ].
Metabolic bariatric surgery is nowadays a common and effective treatment option for obese adults with T2DM. As for its role in management of youth obese populations with early-onset T2DM, there are currently limited, yet encouraging data available in regard to maintenance of glycemic control compared to non-surgical treatment [ 2 , 85 , 86 ]. Therefore, additional research in this field, including safety and long-term outcomes in this high-risk population is of utmost importance.
Societal effects and challenges
Early-onset T2DM poses a number of challenges to public health care, distinctly different than if it presented later in life. Providing health care to those with an earlier diagnosis of T2DM can be challenging in terms of engaging with full-time working or studying populations, including women of reproductive age, in order to achieve appropriate glycemic targets. Additionally, Agarwal et al. in the context of the SEARCH for Diabetes Youth Study demonstrated that transferring from pediatric to adult care (or even no care, in 15%) resulted in worsening glycemic control due to loss of follow-up [ 87 ].
Moreover, a cross-sectional survey conducted in China showed that young adults with T2DM suffer much higher diabetes distress levels (90.82%) than elderly patients, which is amplified by social and economic-related responsibilities [ 88 ]. Recent data from the TODAY2 cohort study showed that low medication adherence is common in youth-onset T2DM patients (65.4%) and associated with certain need insecurities (e.g., housing) and interfering beliefs, such as that medications might be overused or harmful [ 89 ]. Recently, data from a study conducted by the Canadian Diabetes Association revealed that Covid-19 pandemic had great impact on the lives of adolescents and young adults living with T2DM, with only 70% of them having the availability of telephone or virtual clinic appointments with their healthcare provider, and most of them reporting worsening of their eating and exercising habits [ 90 ].
Conclusions
DM is one of the major global public health challenges of the 21st century with a continuously rising prevalence and incidence. This review aimed to provide an overview of the up-to date knowledge with regard to early-onset T2DM.
Numerous modifiable and non-modifiable risk factors are reported to induce the early-phenotype disease presentation, among which, the escalating rates of overweight and obesity in pediatric and adolescent populations appear to be the most prominent. Furthermore, ectopic adiposity, especially in liver, is substantially higher in younger than older patients with T2DM and appears to be a key player of insulin resistance in this population. However, the striking difference with later-onset T2DM, is that the ability of pancreatic β-cells to compensate for the increased insulin resistance is early lost, highlighting the impairment of insulin secretion as the principal pathophysiologic mechanism. The exact pathophysiology pathways underpinning rapid β-cell decline are not clear; future research focusing on genetic, epigenetic, hormonal, and environmental parameters could shed more light on this phenomenon.
Apart from progressing faster, this clinical entity, compared to later-onset T2DM and T1DM, results in higher and earlier development of complications, especially microvascular, as well as increased relative morbidity and mortality. Importantly, these outcomes still remain not fully explained in terms of pathophysiology. Possibly, the increased prevalence of complications could be attributed to a more aggressive disease phenotype, given that it is also observed in studies controlled for diabetes duration and glycemic control. Nevertheless, long-term effects have only recently started to become apparent, and further research is required in order to specify the associated risk factors and develop appropriate prevention, screening, and management strategies.
For the time being, the therapeutic management of early-onset T2DM follows the same principles as in later-onset T2DM. Nevertheless, if a different pathogenesis is suspected, a different therapeutic management would seem plausible. Large, prospective, interventional controlled studies are required in order to develop treatment guidelines tailored to early-onset T2DM.
Last but not least, the challenges associated with social determinants in young populations have substantial impact on diabetes distress, low adherence to therapy and follow-up, prompting us to encompass structured psychosocial support and education in management of young patients with T2DM, following the paradigm of relevant strategies for T1DM patients.
In conclusion, early-onset T2DM is an emerging public health problem, with aggressive phenotype and great overall impact in life of the affected individuals. In order to optimize prevention and treatment strategies, it might be essential to reconceptualize it as a different form of DM; to this direction though, more pathogenetic and clinical aspects need to be elucidated.
International Diabetes Federation. IDF Diabetes Atlas 10th Edition, 2021. https://diabetesatlas.org/
N. Lascar, J. Brown, H. Pattison, A.H. Barnett, C.J. Bailey, S. Bellary, Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 6 , 69–80 (2018). https://doi.org/10.1016/S2213-8587(17)30186-9
Article PubMed Google Scholar
W. Perng, R. Conway, E. Mayer-Davis, D. Dabelea, Youth-onset type 2 diabetes: the epidemiology of an awakening epidemic. Diabetes Care 46 , 490–499 (2023). https://doi.org/10.2337/dci22-0046
Article CAS PubMed PubMed Central Google Scholar
E.J. Mayer-Davis, J.M. Lawrence, D. Dabelea, J. Divers, S. Isom, L. Dolan, G. Imperatore, B. Linder, S. Marcovina, D.J. Pettitt, C. Pihoker, S. Saydah, L. Wagenknecht, Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N. Engl. J. Med. 376 , 1419–1429 (2017). https://doi.org/10.1056/NEJMoa1610187
Article PubMed PubMed Central Google Scholar
D. Dabelea, E.J. Mayer-Davis, S. Saydah, G. Imperatore, B. Linder, J. Divers, R. Bell, A. Badaru, J.W. Talton, T. Crume, A.D. Liese, A.T. Merchant, J.M. Lawrence, K. Reynolds, L. Dolan, L.L. Liu, R.F. Hamman, Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 311 , 1778 (2014). https://doi.org/10.1001/jama.2014.3201
S.E. Holden, A.H. Barnett, J.R. Peters, S. Jenkins-Jones, C.D. Poole, C.L. Morgan, C.J. Currie, The incidence of type 2 diabetes in the United Kingdom from 1991 to 2010. Diabetes Obes. Metab. 15 , 844–852 (2013). https://doi.org/10.1111/dom.12123
Article CAS PubMed Google Scholar
O. Pinhas-Hamiel, P. Zeitler, The global spread of type 2 diabetes mellitus in children and adolescents. J. Pediatr. 146 , 693–700 (2005). https://doi.org/10.1016/j.jpeds.2004.12.042
N. Lascar, Q.-A. Altaf, N.T. Raymond, J. E. P. Brown, H. Pattison, A. Barnett, C.J. Bailey, S. Bellary, Phenotypic characteristics and risk factors in a multi-ethnic cohort of young adults with type 2 diabetes. Curr. Med. Res. Opin. 35 , 1893–1900 (2019). https://doi.org/10.1080/03007995.2019.1638239
G. Twig, I. Zucker, A. Afek, T. Cukierman-Yaffe, C.D. Bendor, E. Derazne, M. Lutski, T. Shohat, O. Mosenzon, D. Tzur, O. Pinhas-Hamiel, S. Tiosano, I. Raz, H.C. Gerstein, A. Tirosh, Adolescent obesity and early-onset type 2 diabetes. Diabetes Care 43 , 1487–1495 (2020). https://doi.org/10.2337/dc19-1988
D.J. Magliano, J.W. Sacre, J.L. Harding, E.W. Gregg, P.Z. Zimmet, J.E. Shaw, Young-onset type 2 diabetes mellitus—implications for morbidity and mortality. Nat. Rev. Endocrinol. 16 , 321–331 (2020). https://doi.org/10.1038/s41574-020-0334-z
E.K. Sims, R.E.J. Besser, C. Dayan, C. Geno Rasmussen, C. Greenbaum, K.J. Griffin, W. Hagopian, M. Knip, A.E. Long, F. Martin, C. Mathieu, M. Rewers, A.K. Steck, J.M. Wentworth, S.S. Rich, O. Kordonouri, A.-G. Ziegler, K.C. Herold, Screening for type 1 diabetes in the general population: a status report and perspective. Diabetes 71 , 610–623 (2022). https://doi.org/10.2337/dbi20-0054
American Diabetes Association, Type 2 diabetes in children and adolescents. Pediatrics 105 , 671–680 (2000). https://doi.org/10.1542/peds.105.3.671
Article Google Scholar
S. Srinivasan, L. Chen, J. Todd, J. Divers, S. Gidding, S. Chernausek, R.A. Gubitosi-Klug, M.M. Kelsey, R. Shah, M.H. Black, L.E. Wagenknecht, A. Manning, J. Flannick, G. Imperatore, J.M. Mercader, D. Dabelea, J.C. Florez, The first genome-wide association study for type 2 diabetes in youth: The Progress in Diabetes Genetics in Youth (ProDiGY) Consortium. Diabetes 70 , 996–1005 (2021). https://doi.org/10.2337/db20-0443
D.J. Pettitt, J.M. Lawrence, J. Beyer, T.A. Hillier, A.D. Liese, B. Mayer-Davis, B. Loots, G. Imperatore, L. Liu, L.M. Dolan, B. Linder, D. Dabelea, Association between maternal diabetes in utero and age at offspring’s diagnosis of type 2 diabetes. Diabetes Care 31 , 2126–2130 (2008). https://doi.org/10.2337/dc08-0769
N. Martín‐Calvo, L. Goni, J.A. Tur, J.A. Martínez, Low birth weight and small for gestational age are associated with complications of childhood and adolescence obesity: systematic review and meta‐analysis. Obes. Rev. 23 , e13380 (2022). https://doi.org/10.1111/obr.13380
B. Fishman, E. Grossman, I. Zucker, O. Orr, M. Lutski, A. Bardugo, C.D. Bendor, Y. Leiba, T. Cukierman-Yaffe, E. Derazne, O. Mosenzon, D. Tzur, Z. Beer, O. Pinhas-Hamiel, T. Fishman, A. Afek, A. Tirosh, I. Raz, H.C. Gerstein, G. Twig, Adolescent hypertension and risk for early-onset type 2 diabetes: a nationwide study of 1.9 million Israeli adolescents. Diabetes Care 44 , e6–e8 (2021). https://doi.org/10.2337/dc20-1752
J. Pan, W. Jia, Early-onset diabetes: an epidemic in China. Front. Med. 12 , 624–633 (2018). https://doi.org/10.1007/s11684-018-0669-1
T.A. Hillier, K.L. Pedula, Characteristics of an adult population with newly diagnosed type 2 diabetes. Diabetes Care 24 , 1522–1527 (2001). https://doi.org/10.2337/diacare.24.9.1522
E. D’Adamo, S. Caprio, Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care 34 , S161–S165 (2011). https://doi.org/10.2337/dc11-s212
K.F. Petersen, G.I. Shulman, Etiology of insulin resistance. Am. J. Med. 119 , S10–S16 (2006). https://doi.org/10.1016/j.amjmed.2006.01.009
G.I. Shulman, Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 371 , 1131–1141 (2014). https://doi.org/10.1056/nejmra1011035
T. Reinehr, B. Karges, T. Meissner, S. Wiegand, M. Fritsch, R.W. Holl, J. Woelfle, Fibroblast growth factor 21 and fetuin-a in obese adolescents with and without type 2 diabetes. J. Clin. Endocrinol. Metab. 100 , 3004–3010 (2015). https://doi.org/10.1210/jc.2015-2192
T. Reinehr, B. Karges, T. Meissner, S. Wiegand, B. Stoffel-Wagner, R.W. Holl, J. Woelfle, Inflammatory markers in obese adolescents with type 2 diabetes and their relationship to hepatokines and adipokines. J. Pediatr. 173 , 131–135 (2016). https://doi.org/10.1016/j.jpeds.2016.02.055
D.M. Scholtens, A. Kuang, L.P. Lowe, J. Hamilton, J.M. Lawrence, Y. Lebenthal, W.J. Brickman, P. Clayton, R.C. Ma, D. McCance, W.H. Tam, P.M. Catalano, B. Linder, A.R. Dyer, W.L. Lowe, B.E. Metzger, C. Deerochanawong, T. Tanaphonpoonsuk, S.B.U. Chotigeat, W. Manyam, M. Forde, A. Greenidge, K. Neblett, P.M. Lashley, D. Walcott, K. Corry, L. Francis, J. Irwin, A. Langan, D.R. McCance, M. Mousavi, I. Young, J. Gutierrez, J. Jimenez, J.M. Lawrence, D.A. Sacks, H.S. Takhar, E. Tanton, W.J. Brickman, J. Howard, J.L. Josefson, L. Miller, J. Bjaloncik, P.M. Catalano, A. Davis, M. Koontz, L. Presley, S. Smith, A. Tyhulski, A.M. Li, R.C. Ma, R. Ozaki, W.H. Tam, M. Wong, C.S.M. Yuen, P.E. Clayton, A. Khan, A. Vyas, M. Maresh, H. Benzaquen, N. Glickman, A. Hamou, O. Hermon, O. Horesh, Y. Keren, Y. Lebenthal, S. Shalitin, K. Cordeiro, J. Hamilton, H.Y. Nguyen, S. Steele, F. Chen, A.R. Dyer, W. Huang, A. Kuang, M. Jimenez, L.P. Lowe, W.L. Lowe, B.E. Metzger, M. Nodzenski, A. Reisetter, D. Scholtens, O. Talbot, P. Yim, D. Dunger, A. Thomas, M. Horlick, B. Linder, A. Unalp-Arida, G. Grave, Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care 42 , 381–392 (2019). https://doi.org/10.2337/dc18-2021
B. Valaiyapathi, B. Gower, A.P. Ashraf, Pathophysiology of type 2 diabetes in children and adolescents. Curr. Diabetes Rev. 16 , 220–229 (2020). https://doi.org/10.2174/1573399814666180608074510
S. Liu, X. Wang, Q. Zheng, L. Gao, Q. Sun, Sleep deprivation and central appetite regulation. Nutrients 14 , 5196 (2022). https://doi.org/10.3390/nu14245196
A. Serbis, V. Giapros, K. Tsamis, F. Balomenou, A. Galli-Tsinopoulou, E. Siomou, Beta cell dysfunction in youth- and adult-onset type 2 diabetes: an extensive narrative review with a special focus on the role of nutrients. Nutrients 15 , 2217 (2023). https://doi.org/10.3390/nu15092217
TODAY Study Group, Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 36 , 1749–1757 (2013). https://doi.org/10.2337/dc12-2393
Article CAS PubMed Central Google Scholar
T. Barrett, M.Y. Jalaludin, S. Turan, M. Hafez, N. Shehadeh, Rapid progression of type 2 diabetes and related complications in children and young people—A literature review. Pediatr. Diabetes 21 , 158–172 (2020). https://doi.org/10.1111/pedi.12953
Y. Wu, C. Liu, H. Sun, A. Vijayakumar, P.R. Giglou, R. Qiao, J. Oppenheimer, S. Yakar, D. Leroith, Growth hormone receptor regulates β cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J. Clin. Investig. 121 , 2422–2426 (2011). https://doi.org/10.1172/JCI45027DS1
C. Brufani, A. Grossi, D. Fintini, A. Tozzi, V. Nocerino, P.I. Patera, G. Ubertini, O. Porzio, F. Barbetti, M. Cappa, Obese children with low birth weight demonstrate impaired β-cell function during oral glucose tolerance test. J. Clin. Endocrinol. Metab. 94 , 4448–4452 (2009). https://doi.org/10.1210/jc.2009-1079
R. Singh, E. Pearson, P.J. Avery, M.I. McCarthy, J.C. Levy, G.A. Hitman, M. Sampson, M. Walker, A.T. Hattersley, Reduced beta cell function in offspring of mothers with young-onset type 2 diabetes. Diabetologia 49 , 1876–1880 (2006). https://doi.org/10.1007/s00125-006-0285-5
F.A. Van Assche, F. De Prins, L. Aerts, M. Verjans, The endocrine pancreas in small-for-dates infants. Br. J. Obstet. Gynaecol. 84 , 751–753 (1977). https://doi.org/10.1111/j.1471-0528.1977.tb12486.x
R. Weiss, S. Caprio, M. Trombetta, S.E. Taksali, W.V. Tamborlane, R. Bonadonna, β-cell function across the spectrum of glucose tolerance in obese youth. Diabetes 54 , 1735–1743 (2005). https://doi.org/10.2337/diabetes.54.6.1735
P. Zeitler, L. El Ghormli, S. Arslanian, S. Caprio, E. Isganaitis, M.K. Kelsey, R.S. Weinstock, N.H. White, K. Drews, Deterioration of glycemic control in youth-onset type 2 diabetes: what are the early and late predictors? J. Clin. Endocrinol. Metab. 107 , E3384–E3394 (2022). https://doi.org/10.1210/clinem/dgac254
Y. Guo, M. Traurig, L. Ma, S. Kobes, I. Harper, A.M. Infante, C. Bogardus, L.J. Baier, M. Prochazka, CHRM3 gene variation is associated with decreased acute insulin secretion and increased risk for early-onset type 2 diabetes in Pima Indians. Diabetes 55 , 3625–3629 (2006). https://doi.org/10.2337/db06-0379
C. Cropano, N. Santoro, L. Groop, C.D. Man, C. Cobelli, A. Galderisi, R. Kursawe, B. Pierpont, M. Goffredo, S. Caprio, The rs7903146 variant in the tcf7l2 gene increases the risk of prediabetes/type 2 diabetes in obese adolescents by impairing b-cell function and hepatic insulin sensitivity. Diabetes Care 40 , 1082–1089 (2017). https://doi.org/10.2337/dc17-0290
D.F. De Jesus, Z. Zhang, S. Kahraman, N.K. Brown, M. Chen, J. Hu, M.K. Gupta, C. He, R.N. Kulkarni, m6A mRNA methylation regulates human β-cell biology in physiological states and in type 2 diabetes. Nat. Metab. 1 , 765–774 (2019). https://doi.org/10.1038/s42255-019-0089-9
M. Ma, T. Jiang, D. Zhang, X. Yao, Z. Wen, L. Xiu, Association of bedtime with early-onset diabetes and islet beta cell function in patients with newly diagnosed type 2 diabetes mellitus. Nat. Sci. Sleep 15 , 653–662 (2023). https://doi.org/10.2147/NSS.S413992
D.L. Eizirik, L. Pasquali, M. Cnop, Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat. Rev. Endocrinol. 16 , 349–362 (2020). https://doi.org/10.1038/s41574-020-0355-7
A. Zajec, K. Trebušak Podkrajšek, T. Tesovnik, R. Šket, B. Čugalj Kern, B. Jenko Bizjan, D. Šmigoc Schweiger, T. Battelino, J. Kovač, Pathogenesis of type 1 diabetes: established facts and new insights. Genes 13 , 706 (2022). https://doi.org/10.3390/genes13040706
A. Khadilkar, C. Oza, S.A. Mondkar, Insulin resistance in adolescents and youth with type 1 diabetes: a review of problems and solutions. Clin. Med. Insights Endocrinol. Diabetes 16 , 11795514231206730 (2023). https://doi.org/10.1177/11795514231206730
H. Baek, J.-Y. Park, J. Yu, J. Lee, Y. Yang, J. Ha, S.H. Lee, J.H. Cho, D.-J. Lim, H.-S. Kim, Characteristics of glycemic control and long-term complications in patients with young-onset type 2 diabetes. Endocrinol. Metab. 37 , 641–651 (2022). https://doi.org/10.3803/EnM.2022.1501
Article CAS Google Scholar
N.A. ElSayed, G. Aleppo, V.R. Aroda, R.R. Bannuru, F.M. Brown, D. Bruemmer, B.S. Collins, J.L. Gaglia, M.E. Hilliard, D. Isaacs, E.L. Johnson, S. Kahan, K. Khunti, J. Leon, S.K. Lyons, M.Lou Perry, P. Prahalad, R.E. Pratley, J.J. Seley, R.C. Stanton, R.A. Gabbay, 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care 46 , S19–S40 (2023). https://doi.org/10.2337/dc23-S002
E. Leighton, C.A. Sainsbury, G.C. Jones, A practical review of C-peptide testing in diabetes. Diabetes Ther. 8 , 475–487 (2017). https://doi.org/10.1007/s13300-017-0265-4
R. Unnikrishnan, V.N. Shah, V. Mohan, Challenges in diagnosis and management of diabetes in the young. Clin. Diabetes Endocrinol. 2 , 18 (2016). https://doi.org/10.1186/s40842-016-0036-6
J.M. Barker, Type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J. Clin. Endocrinol. Metab. 91 , 1210–1217 (2006). https://doi.org/10.1210/jc.2005-1679
S.A.W. Greeley, M. Polak, P.R. Njølstad, F. Barbetti, R. Williams, L. Castano, K. Raile, D.V. Chi, A. Habeb, A.T. Hattersley, E. Codner, ISPAD Clinical Practice Consensus Guidelines 2022: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr. Diabetes 23 , 1188–1211 (2022). https://doi.org/10.1111/pedi.13426
M. Tosur, L.H. Philipson, Precision diabetes: lessons learned from maturity‐onset diabetes of the young (MODY). J. Diabetes Investig. 13 , 1465–1471 (2022). https://doi.org/10.1111/jdi.13860
TODAY Study Group, Long-term complications in youth-onset type 2 diabetes. N. Engl. J. Med. 385 , 416–426 (2021). https://doi.org/10.1056/NEJMoa2100165
Article PubMed Central Google Scholar
R.P. Brady, A.S. Shah, E.T. Jensen, J.M. Stafford, R.B. D’Agostino, L.M. Dolan, L. Knight, G. Imperatore, C.B. Turley, A.D. Liese, E.M. Urbina, J.M. Lawrence, C. Pihoker, S. Marcovina, D. Dabelea, Glycemic control is associated with dyslipidemia over time in youth with type 2 diabetes: The SEARCH for Diabetes in Youth Study. Pediatr. Diabetes 22 , 951–959 (2021). https://doi.org/10.1111/pedi.13253
L. Wang, N. Yan, M. Zhang, R. Pan, Y. Dang, Y. Niu, The association between blood glucose levels and lipids or lipid ratios in type 2 diabetes patients: a cross-sectional study. Front. Endocrinol. 13 , 969080 (2022). https://doi.org/10.3389/fendo.2022.969080
N. Nanayakkara, A.J. Curtis, S. Heritier, A.M. Gadowski, M.E. Pavkov, T. Kenealy, D.R. Owens, R.L. Thomas, S. Song, J. Wong, J.C.-N. Chan, A.O.-Y. Luk, G. Penno, L. Ji, V. Mohan, A. Amutha, P. Romero-Aroca, D. Gasevic, D.J. Magliano, H.J. Teede, J. Chalmers, S. Zoungas, Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses. Diabetologia 64 , 275–287 (2021). https://doi.org/10.1007/s00125-020-05319-w
N. Sattar, A. Rawshani, S. Franzén, A. Rawshani, A.-M. Svensson, A. Rosengren, D.K. McGuire, B. Eliasson, S. Gudbjörnsdottir, Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 139 , 2228–2237 (2019). https://doi.org/10.1161/CIRCULATIONAHA.118.037885
L. Huo, D.J. Magliano, F. Rancière, J.L. Harding, N. Nanayakkara, J.E. Shaw, B. Carstensen, Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997–2011. Diabetologia 61 , 1055–1063 (2018). https://doi.org/10.1007/s00125-018-4544-z
I. Lerman-Garber, D. Cuevas-Ramos, S. Valdés, L. Enríquez, M. Lobato, M. Osornio, A.R. Escobedo, V. Pascual-Ramos, R. Mehta, J. Ramírez-Anguiano, F.J. Gómez-Pérez, Sensorineural hearing loss-a common finding in early-onset type 2 diabetes me llitus. Endocr. Pract. 18 , 549–557 (2012). https://doi.org/10.4158/EP11389.OR
A. Bener, A.A. Al-Ansari, M. Zirie, A.O.A.A. Al-Hamaq, Is male fertility associated with type 2 diabetes mellitus? Int. Urol. Nephrol. 41 , 777–784 (2009). https://doi.org/10.1007/s11255-009-9565-6
B.K. Riaz, S. Selim, M. Neo, M.N. Karim, M.M. Zaman, Risk of depression among early onset type 2 diabetes mellitus patients. Dubai Diabetes Endocrinol. J. 27 , 55–65 (2021). https://doi.org/10.1159/000515683
Y. Cho, H.-S. Park, B.W. Huh, S.H. Seo, D.H. Seo, S.H. Ahn, S. Hong, Y.J. Suh, S.H. Kim, Prevalence and risk of diabetic complications in young-onset versus late-onset type 2 diabetes mellitus. Diabetes Metab. 48 , 101389 (2022). https://doi.org/10.1016/j.diabet.2022.101389
A.H. Al-Saeed, M.I. Constantino, L. Molyneaux, M. D’Souza, F. Limacher-Gisler, C. Luo, T. Wu, S.M. Twigg, D.K. Yue, J. Wong, An Inverse relationship between age of type 2 diabetes onset and complication risk and mortality: the impact of youth-onset type 2 diabetes. Diabetes Care 39 , 823–829 (2016). https://doi.org/10.2337/dc15-0991
R. Unnikrishnan, R.M. Anjana, A. Amutha, H. Ranjani, S. Jebarani, M.K. Ali, K. Narayan, V. Mohan, Younger-onset versus older-onset type 2 diabetes: clinical profile and complications. J. Diabetes Complications 31 , 971–975 (2017). https://doi.org/10.1016/j.jdiacomp.2017.03.007
L. Huang, P. Wu, Y. Zhang, Y. Lin, X. Shen, F. Zhao, S. Yan, Relationship between onset age of type 2 diabetes mellitus and vascular complications based on propensity score matching analysis. J. Diabetes Investig. 13 , 1062–1072 (2022). https://doi.org/10.1111/jdi.13763
D. Dabelea, J.M. Stafford, E.J. Mayer-Davis, R. D’Agostino, L. Dolan, G. Imperatore, B. Linder, J.M. Lawrence, S.M. Marcovina, A.K. Mottl, M.H. Black, R. Pop-Busui, S. Saydah, R.F. Hamman, C. Pihoker, Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 317 , 825 (2017). https://doi.org/10.1001/jama.2017.0686
A.S. Shah, S. Isom, R. D’Agostino, L.M. Dolan, D. Dabelea, G. Imperatore, A. Mottl, E. Lustigova, C. Pihoker, S. Marcovina, E.M. Urbina, Longitudinal changes in arterial stiffness and heart rate variability in youth-onset type 1 versus type 2 diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care 45 , 1647–1656 (2022). https://doi.org/10.2337/dc21-2426
M.C. Eppens, M.E. Craig, J. Cusumano, S. Hing, A.K.F. Chan, N.J. Howard, M. Silink, K.C. Donaghue, Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 29 , 1300–1306 (2006). https://doi.org/10.2337/dc05-2470
M.I. Constantino, L. Molyneaux, F. Limacher-Gisler, A. Al-Saeed, C. Luo, T. Wu, S.M. Twigg, D.K. Yue, J. Wong, Long-term complications and mortality in young-onset diabetes. Diabetes Care 36 , 3863–3869 (2013). https://doi.org/10.2337/dc12-2455
S.H. Song, Complication characteristics between young-onset type 2 versus type 1 diabetes in a UK population. BMJ Open Diabetes Res. Care 3 , e000044 (2015). https://doi.org/10.1136/bmjdrc-2014-000044
J.A. Sargeant, E.M. Brady, F. Zaccardi, F. Tippins, D.R. Webb, V.R. Aroda, E.W. Gregg, K. Khunti, M.J. Davies, Adults with early-onset type 2 diabetes (aged 18–39 years) are severely underrepresented in diabetes clinical research trials. Diabetologia 63 , 1516–1520 (2020). https://doi.org/10.1007/s00125-020-05174-9
K.-T. Kao, M.A. Sabin, Type 2 diabetes mellitus in children and adolescents. Aust. Fam. Physician 45 , 401–406 (2016)
PubMed Google Scholar
S.L. Samson, P. Vellanki, L. Blonde, E.A. Christofides, R.J. Galindo, I.B. Hirsch, S.D. Isaacs, K.E. Izuora, C.C. Low Wang, C.L. Twining, G.E. Umpierrez, W.M. Valencia, American Association of Clinical Endocrinology Consensus Statement: comprehensive type 2 diabetes management algorithm – 2023 Update. Endocr. Pract. 29 , 305–340 (2023). https://doi.org/10.1016/j.eprac.2023.02.001
M.E. Lean, W.S. Leslie, A.C. Barnes, N. Brosnahan, G. Thom, L. McCombie, C. Peters, S. Zhyzhneuskaya, A. Al-Mrabeh, K.G. Hollingsworth, A.M. Rodrigues, L. Rehackova, A.J. Adamson, F.F. Sniehotta, J.C. Mathers, H.M. Ross, Y. McIlvenna, R. Stefanetti, M. Trenell, P. Welsh, S. Kean, I. Ford, A. McConnachie, N. Sattar, R. Taylor, Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 391 , 541–551 (2018). https://doi.org/10.1016/S0140-6736(17)33102-1
M.E.J. Lean, W.S. Leslie, A.C. Barnes, N. Brosnahan, G. Thom, L. McCombie, C. Peters, S. Zhyzhneuskaya, A. Al-Mrabeh, K.G. Hollingsworth, A.M. Rodrigues, L. Rehackova, A.J. Adamson, F.F. Sniehotta, J.C. Mathers, H.M. Ross, Y. McIlvenna, P. Welsh, S. Kean, I. Ford, A. McConnachie, C.-M. Messow, N. Sattar, R. Taylor, Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 7 , 344–355 (2019). https://doi.org/10.1016/S2213-8587(19)30068-3
S. Taheri, H. Zaghloul, O. Chagoury, S. Elhadad, S.H. Ahmed, N. El Khatib, R.A. Amona, K. El Nahas, N. Suleiman, A. Alnaama, A. Al-Hamaq, M. Charlson, M.T. Wells, S. Al-Abdulla, A.B. Abou-Samra, Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): an open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 8 , 477–489 (2020). https://doi.org/10.1016/S2213-8587(20)30117-0
R. Taylor, A. Al-Mrabeh, S. Zhyzhneuskaya, C. Peters, A.C. Barnes, B.S. Aribisala, K.G. Hollingsworth, J.C. Mathers, N. Sattar, M.E.J. Lean, Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab. 28 , 547–556.e3 (2018). https://doi.org/10.1016/j.cmet.2018.07.003
G. Rao, E.T. Jensen, Type 2 diabetes in youth. Glob. Pediatr. Health 7 , 2333794X2098134 (2020). https://doi.org/10.1177/2333794X20981343
J.L. Miller, J.H. Silverstein, The management of type 2 diabetes mellitus in children and adolescents. J. Pediatr. Endocrinol. Metab. 18 , 111–123 (2005). https://doi.org/10.1515/JPEM.2005.18.2.111
A. Herbst, T. Kapellen, E. Schober, C. Graf, T. Meissner, R. Holl, Impact of regular physical activity on blood glucose control and cardiovascular risk factors in adolescents with type 2 diabetes mellitus - a multicenter study of 578 patients from 225 centres. Pediatr. Diabetes 16 , 204–210 (2015). https://doi.org/10.1111/pedi.12144
H.K. Jensen, L. Rasmussen, K. Furu, Ø. Karlstad, M. Linder, C.E. Cesta, A. Pottegård, Use of non‐insulin antidiabetic drugs in children and young adults—A Scandinavian drug utilization study from 2010–2019. Br. J. Clin. Pharmacol. 87 , 4470–4475 (2021). https://doi.org/10.1111/bcp.14867
FDA approves new class of medicines to treat pediatric type 2 diabetes (2023). https://www.fda.gov/news-events/press-announcements/fda-approves-new-class-medicines-treat-pediatric-type-2-diabetes
FDA approves treatment for pediatric patients with type 2 diabetes—drug information update (2019). https://content.govdelivery.com/accounts/USFDA/bulletins/2e98d66
FDA approves new treatment for pediatric patients with type 2 diabetes (2021). https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-pediatric-patients-type-2-diabetes
TODAY Study Group, A Clinical Trial to Maintain Glycemic Control in Youth with Type 2 Diabetes. N. Engl. J. Med. 366 , 2247–2256 (2012). https://doi.org/10.1056/NEJMoa1109333
G.J. Klingensmith, L. Pyle, K.J. Nadeau, L.A. Barbour, R.S. Goland, S.M. Willi, B. Linder, N.H. White, Pregnancy outcomes in youth with type 2 diabetes: the TODAY study experience. Diabetes Care 39 , 122–129 (2016). https://doi.org/10.2337/dc15-1206
K.J. Nadeau, T.S. Hannon, S.L. Edelstein, S.A. Arslanian, S. Caprio, E.W. Leschek, P.S. Zeitler, T.A. Buchanan, D.A. Ehrmann, K.J. Mather, S.E. Kahn, S. Gross, J. Williams, M. Cree-Green, Y.G. Reyes, K. Vissat, K. Brown, N. Guerra, K. Porter, M. Savoye, B. Pierpont, T. Garrett, A. Lteif, A. Patel, R. Chisholm, K. Moore, V. Pirics, L. Pratt, K.A. Temple, A. Rue, E. Barengolts, B. Mokhlesi, E. Van Cauter, S. Sam, M.A. Miller, K.M. Atkinson, J.P. Palmer, K.M. Utzschneider, T. Gebremedhin, A. Kernan-Schloss, A. Kozedub, B.K. Montgomery, E.J. Morse, A.H. Xiang, E. Trigo, E. Beale, F.N. Hendee, N. Katkhouda, K. Nayak, M. Martinez, C. Montgomery, X. Wang, J.M. Lachin, A.N. Hogan, S. Marcovina, J. Harting, J. Albers, D. Hill, P.J. Savage, Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 41 , 1717–1725 (2018). https://doi.org/10.2337/dc18-0787
T.H. Inge, L.M. Laffel, T.M. Jenkins, M.D. Marcus, N.I. Leibel, M.L. Brandt, M. Haymond, E.M. Urbina, L.M. Dolan, P.S. Zeitler, Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr. 172 , 452 (2018). https://doi.org/10.1001/jamapediatrics.2017.5763
G. Mingrone, Pros and cons of bariatric surgery in adolescents. Lancet Diabetes Endocrinol. 5 , 152–154 (2017). https://doi.org/10.1016/S2213-8587(16)30425-9
S. Agarwal, J.K. Raymond, S. Isom, J.M. Lawrence, G. Klingensmith, C. Pihoker, S. Corathers, S. Saydah, R.B. D’Agostino, D. Dabelea, Transfer from paediatric to adult care for young adults with type 2 diabetes: the SEARCH for Diabetes in Youth Study. Diabet. Med. 35 , 504–512 (2018). https://doi.org/10.1111/dme.13589
Y. Hu, L. Li, J. Zhang, Diabetes distress in young adults with type 2 diabetes: a cross-sectional survey in China. J. Diabetes Res. 2020 , 1–6 (2020). https://doi.org/10.1155/2020/4814378
P.M. Trief, D. Uschner, S. Kalichman, B.J. Anderson, L.M. Fette, H. Wen, J.D. Bulger, R.S. Weinstock, Psychosocial factors predict medication adherence in young adults with youth‐onset type 2 diabetes: longitudinal results from the TODAY2 iCount study. Diabet. Med. 40 , e15062 (2023). https://doi.org/10.1111/dme.15062
M. Carino, Z. Quill, M. Gabbs, E. Sellers, J. Hamilton, T. Pinto, M. Jetha, J. Ho, O.G. Alecio, A. Dart, B. Wicklow, Impact of COVID-19 pandemic on adolescents and young adults living with type 2 diabetes. Can. J. Diabetes 46 , 404–410 (2022). https://doi.org/10.1016/j.jcjd.2022.01.002
Download references
Open access funding provided by HEAL-Link Greece.
Author information
Authors and affiliations.
School of Medicine, University of Patras, Patras, Greece
Myrsini Strati
Department of Endocrinology and Diabetes Center, Hellenic Red Cross Hospital, Athens, Greece
Melpomeni Moustaki & Andromachi Vryonidou
Endocrine Unit and Diabetes Center, Department of Clinical Therapeutics, Alexandra Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
Theodora Psaltopoulou & Stavroula A. Paschou
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stavroula A. Paschou .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Strati, M., Moustaki, M., Psaltopoulou, T. et al. Early onset type 2 diabetes mellitus: an update. Endocrine (2024). https://doi.org/10.1007/s12020-024-03772-w
Download citation
Received : 16 November 2023
Accepted : 02 March 2024
Published : 12 March 2024
DOI : https://doi.org/10.1007/s12020-024-03772-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetes mellitus
- Type 2 diabetes mellitus
- Early-onset
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ann Saudi Med
- v.31(3); May-Jun 2011

Pattern of presentation in type 1 diabetic patients at the diabetes center of a university hospital
Abdulaziz m. al rashed.
From the Department of Pediatrics, King Abdulaziz University Hospital, College of Medicine, King Saud University, Riyadh, Saudi Arabia
BACKGROUND AND OBJECTIVES:
Diabetes mellitus (DM) is a major health problem worldwide. This study aimed to investigate the pattern of presentation and complications of pediatric diabetes.
DESIGN AND SETTING:
Retrospective study of children treated at a diabetes clinic at a university hospitalfor diabetes over 12-year period.
PATIENTS AND METHODS:
We collected data on the age at onset, sex, clinical presentation, duration of symptoms before diagnosis, and partial remission rate that were obtained from the hospital medical records, the National Diabetes Registry, and the statistics department.
Of 369 diabetic children, most (n=321) children had polyuria (92%) 321/369=87% as the presenting symptom; other symptoms included polydipsia (310 patients, 88.8% 310/369=84%), weight loss (292 patients, 83.9%), nocturia (240 patients, 68.8% 240/369=65%), diabetic ketoacidosis (DKA) (174 patients, 49.9% 174/369=47.20%), and abdominal pain (172 patients, 49.3% 174/369=46.6%). Presenting symptoms were missing in 20 files, so the percentages were calculated among 349 patients. Most patients had acute diabetic complications such as hypoglycemia (222 patients, 62%) and DKA (88 patients, 38.1%, but none had severe complications such as coma and cerebral edema. Chronic complications included retinopathy (4 patients, 1.3%), neuropathy (2 patients, 0.6%), coronary heart disease (2 patients, 0.6%), and nephropathy (1 patient, 0.4%).
CONCLUSION:
The pattern of presentation of type 1 diabetes has changed as the incidence of DKA has decreased; unlike in previous studies, DKA was not the most common presenting symptom in this study. Chronic complications of diabetes, such as retinopathy, neuropathy, coronary heart disease, and nephropathy are mostly rare but still present. These complications might be prevented by achieving better awareness of the need for glycemic control.
Diabetes mellitus (DM) is a major health problem worldwide. Current studies have revealed a definite global increase in the incidence and prevalence of diabetes, with the World Health Organization (WHO) projecting that there will be almost 221 million cases in the year 2010 and up to 285 million cases in the year 2025. 1 It is the fourth or fifth leading cause of death in most developed countries. 1 , 2 Although this increase is mainly expected in type 2 diabetes, a parallel increase in childhood diabetes, including type 1 and 2 diabetes, has been reported. 3 DM in children has previously been considered rare in African and Asian populations. 4 – 8 The WHO Diabetes Mondiale (WHO DIAMOND) project group has reported a worldwide increase in the incidence and variation (over 400-fold) of type 1 diabetes, with the highest occurring in Finland (over 45 per 100 000 children under the age of 15 years) and the lowest in parts of China and Fiji. 9
DM in children in Saudi Arabia has not been studied well and further studies are needed. 10 Little local information on the disease is available, and most cases reported have been of type 2 DM. 11 The epidemiology and characteristics of DM, particularly insulin-dependent DM, are not known in the Saudi community, and only a small amount of data is available. 11 Moreover, the data confirm the need to develop a national registry and the need for further epidemiological research. 12 Furthermore, adolescents are not examined in pediatric clinics, and they do not receive adequate attention in adult clinics. 13
Saudi Arabia is a unique country among developing nations in view of its excellent economic status and relatively low literacy rate, particularly among mothers, in addition to the cultural and religious background, which might influence the management of diabetes. 14 The presentation of type 1 diabetes in Saudi children seems to differ from that in children from Western countries. 15 The most common clinical sign is diabetic ketoacidosis (DKA), which is observed in 67.2% of the patients. 11 DKA is the most serious presenting symptom of type 1 DM. The frequency and severity of DKA at presentation vary significantly worldwide. 16 In Saudi Arabia, studies have revealed that DKA is present in 55% to 77% of the DM cases. 15 , 17 Ketoacidosis is the most common presenting symptom of childhood DM in this region. 18
This study presents some of the epidemiological and clinical features and complications of childhood DM as recorded in the Diabetes Center at King Abdulaziz University Hospital, Riyadh, Saudi Arabia. The Diabetes Center receives patients from Riyadh District and suburban areas; in addition, it is a tertiary care center that receives referred patients from different cities in the country. The objective of this study was to investigate the pattern of presentation of pediatric diabetes in patients enrolled in the diabetes center of a university hospital and to review the complications of diabetes in the study group.
PATIENTS AND METHODS
All diabetic children who were enrolled in the study from among those treated at the King Abdulaziz University Hospital over a 12-year period from 1993 to 2005. Vital data for the study were extracted from several sources, including hospital medical records, the National Diabetes Registry, and the statistics department. The data were extracted by an experienced physician under the strict supervision of the author, who also checked for the consistency and completeness of the extracted data. The recorded information included the age at onset, sex, nationality, consanguinity, clinical presentation, duration of symptoms before diagnosis, and partial remission rate (which was defined according to the criteria of the International Study Group of Diabetes in Children and Adolescents as a period of freedom from clinical symptoms of diabetes with insulin requirements of <0.5 units/kg/day and absent or minimal glycosuria for more than 4 weeks). During this study, type 1 diabetes was predominantly diagnosed on the basis of the clinical and biological features. Polyuria, polydipsia, weight loss and fatigability were the principal clinical features for diagnosis. Significant hyperglycemia was taken into account as a biological feature according to the National Diabetes Data Group criteria of fasting blood glucose of >140 mg/dL (>7.7 mmol/L), 2-hour postprandial blood glucose level of >200 mg/dL (>11.1 mmol/L), and glycosuria with or without ketonuria.
Both clinical and biological features were included in the diagnosis of DKA. Clinical features such as vomiting, abdominal pain, moderate-to-severe dehydration, and stupor, in addition to hyperglycemia with blood glucose levels exceeding 15 mmol/L, ketonuria and metabolic acidosis with a bicarbonate level of <15 mmol/L, played significant roles in determining DKA. The chronic complications such as retinopathy, nephropathy and neuropathy were identified by ophthalmic findings indicative of retinopathy, persistent microalbuminuria, and abnormal nerve conductions, respectively. Data analyses (chi square tests, Fischer exact test) were performed using the statistical packages STATA, R, and Minitab.
Of the 369 diabetic patients, 159 (43.1%) patients were between 11 and 15 years of age. The age groups 6-10 years and >15 years consisted of a similar number of patients—100 (27.1%) and 97 (26.3%) patients, respectively. Only 13 (3.5%) patients were less than 5 years old. The mean (standard deviation) age was 12.3 (4.0) years with a range of 2-18 years ( Table 1 ). Of the enrolled patients, 175 (47.4%) were male and 194 (52.6%) were female. The study group included 324 (87.8%) Saudi patients and 45 (12.2%) patients of different Arab nationalities. A positive family history of DM was recorded in 260 (73.7%) patients, including both type 1 and type 2 diabetes patients. The overall mean (SD) duration of diabetes was 4.6 (3.7) years. There were two major peaks of age at diagnosis, one at the age of 7 years and the other at 11 years, with a sharp drop after the age of 11 years; the curve almost reached a plateau at the age of 18 years ( Figure 1 ). Most patients (134 patients, 58.5%) had a less than 15 days duration of symptoms before diagnosis. The duration of symptoms before diagnosis ranged from 1 to 365 days, with a median of 14 days ( Table 1 ). The mean total insulin intake was 36.0 units/d, with a range of 2-106 units/d and a median of 37 units/d. Partial remission was observed in 21 (9.1%) patients ( Table 1 ). Numbers of patients by age group, duration of diabetes, family history, diabetic complications, and were above 17 years of age at the time of diagnosis, and these were the oldest patients in this study. Two peaks [peaks of age at time of diagnosis?] were observed, one as early as at 12 days of age in a case that was diagnosed in another hospital and referred to the Diabetes Center of King Abdulaziz University Hospital. Three patients duration of partial remission by the other variables are presented in Tables Tables2a, 2a , ,2b, 2b , and and2c 2c .
Characteristics of pediatric diabetic patients attending the diabetes center at a university hospital (1993-2005) (n=369).

Distribution curve of age of diagnosis pediatric patients attending the diabetes center at a university hospital (1993-2005).
Sex of pediatric diabetic patients by age group

Sex and and age group by duration of diabetes

Sex, age group and duration by family history of diabetes, diabetic complications and duration of partial remission

The most frequent presenting symptoms were polyuria, polydipsia, weight loss, nocturia ( Table 3 ) while DKA was present in about half ( Table 3 ). Because of missing data, not all information on all parients was available. The data on fatigability was available for 231 patients; fatigability was observed in 179 of these 231 patients. The less frequent symptoms included fever, obesity, delayed wound healing, vomiting, loss of consciousness, and diarrhea; a history of preceding illness was also less frequent. Ten (4.3%) patients of the studied cohort were asymptomatic. Most patients had acute diabetic complications such as hypoglycemia, and DKA ( Table 4 ). None of the patients had severe complications such as coma and cerebral edema. Chronic complications included retinopathy, neuropathy, coronary heart disease and nephropathy.
Symptoms of pediatric diabetic patients on presentation at the diabetes center according sex, age group, and duration of symptoms

Diabetic complications (acute and chronic)

Among the patients in the study, diabetes was diagnosed as early as at 12 days of age in a case that was diagnosed in another hospital and referred to the Diabetes Center of King Abdulaziz University Hospital. Three patients were above 17 years of age at the time of diagnosis, and these were the oldest patients in this study. Two peaks in age at time of diagnosis were observed, one at 7 and the other at 11 years of age. In a study by Salman et al, the age at onset ranged from 7.5 months to 12 years, with a peak at around 5-7 years and 11-14 years, respectively. The second peak in this study was observed to occur in the age range similar to that reported by Abdullah (10-13 years), while the first peak was observed to occur slightly earlier (4-6 years). 19 , 20 In the study by Abdullah, the youngest patient was 6 months old at diagnosis.
The present study showed a female preponderance, with 194 (52.6%) females versus 175 (47.4%) males; such a female preponderance was also observed in the series conducted by Salman et al, wherein 53.6% of patients were female. On the contrary, the series conducted by Abdullah showed a male preponderance, with a male-to-female ratio of 1.3:1; this ratio is similar to the ratios observed in the UK, Denmark and India. 19 , 20 In this study, the duration of symptoms before diagnosis was 1-35 days with a median of 14 days as compared to a duration of 2-60 days with a mean of 18.2 days in the series conducted by Salman et al.
The most common clinical presentations in the present study were polyuria (92%) and polydipsia (88.8%). In the study by Salman et al, DKA was the most common clinical presentation and was observed in 74 (67.3%) patients; while in the present study, DKA was observed in 49.9% of the patients. In the study by Abdullah, 55% of the patients presented with DKA. Studies in Malaysia revealed a figure (48%) similar to that in the present study, while studies in Philippines and India revealed figures of 63% and 20%-40%, respectively. 6 , 21 , 22 DKA is considered uncommon in Japan and Indonesia. 23 , 24 DKA was observed in 49.9% of the patients in this series; thus DKA was less common in this study than in other local studies, such as those by Salman et al (DKA was observed in 67.2% of the patients) and Abdullah (DKA observed in 55% of the patients). This difference may be explained by a higher level of awareness among parents and improvement in health services with early diagnosis.
The partial remission rate in this study was only 9.1%, which is lower than the rates observed in the studies by Abdullah (32%) and Salman et al. (30.9%). It correlates to those studies in relation to age group; none of the patients below 5 years of age had any episode. Partial remission is considered more common when diabetes is diagnosed in older children and teenagers, and most patients in the present study were diagnosed when they were less than 11 years of age ( Figure 1 ); this might explain the low rate of partial remission observed in this study. The lower incidence of DKA may further explain the low rate of partial remission.
A positive family history of both types (1 and 2) of diabetes was observed in 73.7% of the patients in this study; this figure is higher than that reported in the study by Abdullah (56.7%). DM occurs significantly more frequently in the parents and siblings of diabetics than in those of the control population. 25 , 26 In the study by Salman et al,, both the consanguinity rate and family history of type 1 and 2 diabetes were higher than those reported in the literature and also in a similar local study. 25 – 29
The treatment of DM in children requires the provision of a comprehensive, well-coordinated and continuous service. This is best achieved by teamwork. Adolescents or “young adults” in Saudi Arabia and in some other non-Western countries are not examined at pediatric clinics and do not receive adequate attention at adult clinics. Studies of the microvascular complications in non-insulin-dependent DM patients suggest that the onset of these complications occurs at least 4-6 years before clinical diagnosis. Evidence shows that strict glycemic control prevents microvascular complications. 30
In summary, the incidence of DKA was lower than that reported in previous studies; in addition, unlike in previous studies, DKA was not the most common clinical presentation. This difference is due to better awareness and early diagnosis. Additionally, the partial remission rate was lower, which indicates early diagnosis. Although chronic complications are uncommon in children, retinopathy, neuropathy, coronary heart disease and nephropathy have been observed; this necessitates an awareness among physicians, caretakers and patients about the importance of early diagnosis and strict control of DM. The incidence of family history was higher than that reported previously, which can be explained by the higher rate of consanguinity in the Saudi community. This observation indicates the need for further genetic studies of DM in the Saudi population.

IMAGES
VIDEO
COMMENTS
The most common symptoms of type 1 diabetes mellitus (DM) are polyuria, polydipsia, and polyphagia, along with lassitude, nausea, and blurred vision, all of which result from the hyperglycemia itself. ... Symptoms at the time of the first clinical presentation can usually be traced back several days to several weeks. However, beta-cell ...
Type 1 Diabetes Mellitus is a syndrome characterized by hyperglycemia and insulin deficiency resulting from the loss of beta cells in pancreatic islets (Mapes & Faulds, 2014). ... Clinical Presentation: Type 1 diabetes does not present clinically until 80-90% of the beta cells have been destroyed (McCance & Heuther, 2014). Because insulin ...
This topic will review the clinical presentation, diagnosis, and initial evaluation of diabetes in nonpregnant adults. Screening for and prevention of diabetes, the etiologic classification of diabetes mellitus, the treatment of diabetes, as well as diabetes during pregnancy are discussed separately. (See "Screening for type 2 diabetes mellitus" .)
Type 1 diabetes, once known as juvenile diabetes or insulin-dependent diabetes, is a chronic condition. In this condition, the pancreas makes little or no insulin. Insulin is a hormone the body uses to allow sugar (glucose) to enter cells to produce energy. Different factors, such as genetics and some viruses, may cause type 1 diabetes.
Type 1 diabetes mellitus (T1D) is an autoimmune disease that leads to the destruction of insulin-producing pancreatic beta cells. There is heterogeneity in the metabolic, genetic, and immunogenetic characteristics of T1D and age-related differences, requiring a personalized approach for each individual. Loss of insulin secretion can occur quickly or gradually.
Type 1 diabetes mellitus is a chronic medical condition that occurs when the pancreas, an organ in the abdomen, produces very little or no insulin ( figure 1 ). Insulin is a hormone that helps the body to use glucose for energy. Glucose is a sugar that comes, in large part, from foods we eat.
What is the difference between Type 1 diabetes and Type 2 diabetes? While Type 1 diabetes and Type 2 diabetes are both forms of diabetes mellitus (as opposed to diabetes insipidus) that lead to hyperglycemia (high blood sugar), they are distinct from each other.. In Type 2 diabetes (T2D), your pancreas doesn't make enough insulin and/or your body doesn't always use that insulin as it ...
Type 1 diabetes mellitus (DM) is a multisystem disease with both biochemical and anatomic/structural consequences. ... (See Presentation.) Treatment of type 1 DM requires lifelong insulin therapy. A multidisciplinary approach by the physician, nurse, and dietitian, with regular specialist consultation, is needed to control glycemia, as well as ...
Abstract. This article reviews our current understanding of the etiology, presentation, and management of type 1 diabetes. The discussion includes a review of the natural history of diabetes, the complex relationship between genetic and environmental risk for type 1 diabetes, and current methods for prediction of type 1 diabetes.
Type 1 diabetes mellitus (T1DM), one of the most common chronic diseases in childhood, is caused by insulin deficiency resulting from the destruction of insulin-producing pancreatic beta cells. (See "Pathogenesis of type 1 diabetes mellitus" .) There are unique challenges in caring for children and adolescents with T1DM that differentiate ...
Type 1 diabetes is thought to be caused by an autoimmune reaction (the body attacks itself by mistake). This reaction destroys the cells in the pancreas that make insulin, called beta cells. This process can go on for months or years before any symptoms appear. Some people have certain genes (traits passed on from parent to child) that make ...
Diabetes mellitus is a chronic heterogeneous metabolic disorder with complex pathogenesis. It is characterized by elevated blood glucose levels or hyperglycemia, which results from abnormalities in either insulin secretion or insulin action or both. Hyperglycemia manifests in various forms with a varied presentation and results in carbohydrate ...
This activity reviews the evaluation and management of type 1 diabetes mellitus in children and highlights the role of interprofessional team members in collaborating to provide well-coordinated care and enhance outcomes for affected patients. ... At presentation, children usually have a history of polyuria, polydipsia and weight loss for days ...
General presentation. Diabetes mellitus (DM) is an important endocrine disorder that presents commonly in children and adolescents. There are two types of diabetes mellitus: type 1 and type 2. Type 1 DM is one of the most common chronic diseases in children and is characterized by insulin deficiency as a result of autoimmune destruction of ...
Type 1 diabetes mellitus (T1D) is a heterogeneous disorder characterized by autoimmune-mediated destruction of pancreatic beta cells that culminates in absolute insulin deficiency. T1D is most commonly diagnosed in children and adolescents, usually presents with symptomatic hyperglycemia, and imparts the immediate need for exogenous insulin ...
Type 2 Diabetes Warning Signs. Warning Signs and Symptoms - Can occur slowly over time. Blurred vision. Tingling or numbness in legs, feet or fingers. Recurring skin, gum or urinary tract infections. Drowsiness. Slow healing of cuts and bruises. Any symptoms that occur with Type 1 diabetes.
Candidiasis may develop, especially in the groin and in flexural areas. Most pediatric patients with diabetes have type 1 diabetes mellitus (T1DM) and a lifetime dependence on exogenous insulin. Diabetes mellitus (DM) is a chronic metabolic disorder caused by an absolute or relative deficiency of insulin, an anabolic hormone.
Presentation Transcript. Type 1 Diabetes Mellitus Treatment. Goals of T1DM Management Utilize intensive therapy aimed at near-normal BG and A1C levels Prevent diabetic ketoacidosis and severe hypoglycemia Achieve the highest quality of life compatible with the daily demands of diabetes management In children, achieve normal growth and physical ...
The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 29 , 267-274 ...
Diabetes mellitus—more commonly known as diabetes—is a chronic disease that occurs when you have higher than normal levels of blood glucose (or, blood sugar). Glucose is the body's main ...
Comparison between type 1 diabetes mellitus mice (black columns; n=12) and non-diabetic mice (gray columns; n=12). Data are presented as mean±standard deviation. *P 0.05.
Aim: Determination of blood flow parameters in the ophthalmic artery and central retinal artery using Doppler ultrasound in patients with type 1 diabetes mellitus without fundus signs of diabetic retinopathy and with mild non-proliferative retinopathy. Material and methods: To eliminate the impact of other systemic factors on vascular flow, the study enrolled a total of 80 patients with type 1 ...
Diabetes mellitus is a metabolic disease characterized by insulin deficiency and/or insulin resistance. Type 1 diabetes results from the autoimmune destruction of pancreatic β cells and is typically found in younger patients while type 2 diabetes occurs due to insulin resistance and is most often found in middle aged to older adults. Each type ...
Type 1 Diabetes Mellitus: Etiology, Presentation, and Management Michael J. Haller, MDa, Mark A. Atkinson, PhDb, Desmond Schatz, MDc,T aDivision of Pediatric Endocrinology, University of Florida College of Medicine, PO Box 100296, Gainesville, FL 32610, USA bThe Center for Immunology and Transplantation, University of Florida College of Medicine, Room R3-128, ARB, Gainesville, FL 32610-0275, USA
Background: Despite the growing uptake of smart technologies in pediatric type 1 diabetes mellitus (T1DM) care, little is known about caregiving parents' skills to deal with electronic health information sources. Objective: We aimed to assess the electronic health literacy of parents caring for children with T1DM and investigate its associations with disease management and children's outcomes.
The objective of the study was to assess the expression of proteins responsible for placental lipid transport in term pregnancies complicated by well-controlled gestational (GDM) and type 1 diabetes mellitus (PGDM). A total of 80 placental samples were obtained from patients diagnosed with PGDM (n = 20), GDM treated with diet (GDMG1, n = 20), GDM treated with diet and insulin (GDMG2, n = 20 ...
One presentation is regarding the objectives and design of the ongoing Phase 2 clinical trial enrolling patients with Type 2 diabetes, dyslipidemia, and NAFLD. The other presentation is regarding the mechanism of action of MN-001/MN-002 in lipid metabolism, particularly the effects on cholesterol efflux capacity.
The incidence and prevalence of type 2 diabetes mellitus (T2DM) in young individuals (aged <40 years) have significantly increased in recent years, approximating two to threefold increase in the respective rates. Numerous risk factors including severe obesity, family history, ethnicity, maternal diabetes or gestational diabetes, and female sex contribute to a younger age of onset. In terms of ...
GIP/GLP-1 RA) for the treatment of type 2 diabetes mellitus in adults. NIE TA 924 has positioned Mounjaro® the same as GLP-1 RAs, and the same criteria for prescribing and continuing treatment apply. ommon Side effects Dual GIP/GLP-1 receptor agonist drugs lower glucose levels by reducing appetite (cerebral effect and by
Diabetes mellitus (DM) is a major health problem worldwide. Current studies have revealed a definite global increase in the incidence and prevalence of diabetes, with the World Health Organization (WHO) projecting that there will be almost 221 million cases in the year 2010 and up to 285 million cases in the year 2025. 1 It is the fourth or fifth leading cause of death in most developed ...