Is Food Addictive? A Review of the Science
Affiliations.
- 1 Department of Psychology, University of Michigan, Ann Arbor, Michigan 48109, USA; email: [email protected].
- 2 Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA; email: [email protected].
- PMID: 34152831
- DOI: 10.1146/annurev-nutr-110420-111710
As ultraprocessed foods (i.e., foods composed of mostly cheap industrial sources of dietary energy and nutrients plus additives) have become more abundant in our food supply, rates of obesity and diet-related disease have increased simultaneously. Food addiction has emerged as a phenotype of significant empirical interest within the past decade, conceptualized most commonly as a substance-based addiction to ultraprocessed foods. We detail ( a ) how approaches used to understand substance-use disorders may be applicable for operationalizing food addiction, ( b ) evidence for the reinforcing potential of ingredients in ultraprocessed foods that may drive compulsive consumptions, ( c ) the utility of conceptualizing food addiction as a substance-use disorder versus a behavioral addiction, and ( d ) clinical and policy implications that may follow if ultraprocessed foods exhibit an addictive potential. Broadly, the existing literature suggests biological and behavioral parallels between food addiction and substance addictions, with ultraprocessed foods high in both added fat and refined carbohydrates being most implicated in addictive-like eating. Future research priorities are also discussed, including the need for longitudinal studies and the potential negative impact of addictive ultraprocessed foods on children.
Keywords: food addiction; food reward; obesity; overeating; substance-use disorders.

Publication types
- Behavior, Addictive*
- Feeding Behavior
- Food Addiction*
- Substance-Related Disorders*
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Circumspective
- Open access
- Published: 06 September 2018
Food addiction: a valid concept?
- Paul C. Fletcher 1 , 2 &
- Paul J. Kenny 3
Neuropsychopharmacology volume 43 , pages 2506–2513 ( 2018 ) Cite this article
31k Accesses
130 Citations
262 Altmetric
Metrics details
- Human behaviour
A Correction to this article was published on 07 December 2018
This article has been updated
Can food be addictive? What does it mean to be a food addict? Do common underlying neurobiological mechanisms contribute to drug and food addiction? These vexing questions have been the subject of considerable interest and debate in recent years, driven in large part by the major health concerns associated with dramatically increasing body weights and rates of obesity in the United States, Europe, and other regions with developed economies. No clear consensus has yet emerged on the validity of the concept of food addiction and whether some individuals who struggle to control their food intake can be considered food addicts. Some, including Fletcher, have argued that the concept of food addiction is unsupported, as many of the defining features of drug addiction are not seen in the context of feeding behaviors. Others, Kenny included, have argued that food and drug addiction share similar features that may reflect common underlying neural mechanisms. Here, Fletcher and Kenny argue the merits of these opposing positions on the concept of food addiction.
Similar content being viewed by others

Microdosing with psilocybin mushrooms: a double-blind placebo-controlled study
Federico Cavanna, Stephanie Muller, … Enzo Tagliazucchi

Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers
Joseph M. Rootman, Pamela Kryskow, … Zach Walsh

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls
Joseph M. Rootman, Maggie Kiraga, … Zach Walsh
Arguments against food addiction (PCF)
“I am asked by the learned doctor the cause and reason why opium causes sleep. To which I reply: because it has a dormitive property [virtus dormitiva], whose nature is to lull the senses to sleep.”
—Moliere, La malade imaginaire
Applying diagnoses to make sense of collections of symptoms and behaviors has advantages in sharpening communication and clarifying thought. It may carry benefits for the patient, too, offering a model that seems to make sense of what is otherwise baffling. But the hope, if we are simply to avoid the circularity alluded to in the quote above or, as Jaspers put, it “pseudo-insight through terminology” [ 1 ], is to go beyond simple naming and to reach an understanding of processes and mechanisms. The application of the term “food addiction” in humans is based on a set of features, held to resemble substance addictions. It carries the claim that this resemblance occurs because certain foods have effects on the brain comparable to those of addictive drugs. I suggest that there are problems with both of these claims. The first is questionable as the central features of substance addiction do not plausibly translate to food and consumption. The second because the assertion that foods have pharmacological effects on the brain demands strong and convincing evidence, which has not been found.
Food addiction, as an explanation for the often-distressing cravings, loss of control, and overconsumption experienced by many, particularly in relation to highly palatable foods, has been with us for many years [ 2 ] and, having more latterly becomes a focus for direct scientific study, the model has sought support in two broad sets of work. First, clinico-behavioral work has produced a descriptive framework based on the asserted resemblance between overconsumption of food and substance use. This has led to the development of a widely used scale [ 3 ], which measures the characteristics thought to be common to substance and, putatively, food addiction (i.e., craving, loss of control, excessive consumption, tolerance, withdrawal, and distress/dysfunction). Scores on this scale have subsequently been used as the basis for interpreting variance in functional neuroimaging measures, with such correlations being cited as evidence for the neurobiological reality of food addiction. Second, insights into the neurobiology of substance use and addiction have generated hypotheses in rodent and human work, particularly focusing on reward processing and the mesolimbic dopamine system. Such studies have established that particular regimens of palatable food availability may produce addiction-like brain changes, as well as binge-like eating and withdrawal symptoms. It is important to acknowledge that there are some differing positions held by supporters of the food addiction model, but the prevailing view seems reasonably characterized as follows: with exceptions [ 4 ], food addiction is considered as separable from obesity. While one study shows that 88% meeting criteria for food addiction are obese [ 5 ], it is important to note that food addiction is defined by behavioral patterns and experiences relating to eating rather than by weight status. Moreover, it is considered to be separate from those established clinical conditions with which it markedly overlaps, notably eating disorders marked by binge eating [ 2 ]. It is identified by some self-reported combination of the above features and held to resemble a substance addiction rather than a behavioral addiction (though see ref. [ 6 ]), such as gambling disorder [ 7 ]. Critically, the suggestion goes, it is associated with corresponding changes to the mesolimbic dopamine system, changes that underpin the transition from reward-driven to impulsive and compulsive eating.
There are several problems with this model. First, the addictive substance remains undiscovered—a problem that cannot be considered trivial. The model rests on a central assertion that either some category of foods, or some specific nutrients, exert a direct effect on the brain, enacting changes that ultimately hijack reward-related behaviors. Some have argued that sugar is the culprit though, as a whole, the evidence for sugar addiction remains deeply unconvincing [ 8 ]. Alternatively, others maintain that the refined and excessively palatable combinations of sugars and fats in Western diets increase addiction liability. But, as of now, no addictive substance has been identified. Second, there are crucial questions relating to how food addiction fits into the overall schema of problematic eating. Though initially applied to understanding obesity as a whole, the current view is that food addiction represents a specific construct that is strongly argued to be distinct even from the eating disorder that perhaps it most resembles: binge-eating disorder [ 2 ]. This clear distinction is challenged, however, by studies showing marked overlap between positive scores for food addiction (as measured on a dedicated scale—the Yale Food Addiction Scale (YFAS; see below)) and binge eating in the context of bulimia nervosa [ 9 , 10 , 11 , 12 ]. The overlap too between food addiction and binge-eating disorder is striking [ 13 ]. One conclusion from this might be that food addiction is not distinct from the symptom of binge eating, perhaps with food addiction (and its putative underlying neurobiological imbalance) providing a mechanistic explanation for the latter. However, it has been argued that these are distinct entities and should not be conflated [ 2 ]. There is a pressing need, therefore, to assess the discriminant validity of the scale. It is crucial, too, that we consider the validity and reliability of other characteristics held to be a feature of food addiction, most notably tolerance and withdrawal. For example, eating increased amounts of food and obtaining less pleasure from eating is taken as evidence of tolerance, while anxiety, dysphoria, and unspecified “physical symptoms” during abstinence from certain foods are viewed as withdrawal symptoms. It is inevitable that brief, non-interactive clinical scales will be imprecise. It is important, though, that scales are not taken to validate the phenomenon that they are seeking to measure [ 14 ]. Further considerations of the problems in applying the diagnosis of food addiction clinically have been discussed elsewhere [ 14 , 15 ].
Going beyond the phenotype, a critical aspect of the model, and one that moves it beyond a simple phenomenological description of a cluster of behaviors and symptoms, is the hypothesized set of neurobiological changes that underlie food addiction. The third concern therefore is especially important: there remains no convincing demonstration in humans that such neurobiological changes do indeed underlie these food addiction behaviors. We should see specifically altered structural and functional patterns in key nodes of the mesolimbic dopamine system in groups of individuals who exhibit food addiction behaviors. An early study suggested that this was indeed the case with reduced striatal dopamine receptor density in severely obese people [ 16 ]. But this finding, which is frequently cited in unqualified support for the food addiction model of obesity, has not been replicated and has been succeeded by several studies, which do not find such differences; e.g., refs. [ 17 , 18 , 19 ]. Reviewing the literature some 5 years ago, I and colleagues [ 20 , 21 ] suggested that the existing functional magnetic resonance imaging and positron-emission tomography studies should not be used as a basis for making claims about food addiction. Reviewing the field now, there is no more support for food addiction from human neuroimaging literature than there was then. Indeed, there is less.
Fourth, there are several studies showing that controlled manipulation of food composition and availability provokes addiction-like patterns in rats. These studies convincingly demonstrate that experimentally constrained, intermittent availability of high-sugar, high-fat, or high-sugar plus high-fat diets provokes both compulsive patterns of eating in rats and a number of sequelae suggestive of addiction: notably, reduced dopamine receptor function, increased self-stimulation thresholds, and, in the case of sugar, anxious behaviors that may be relatable to withdrawal symptoms. Such studies constitute the most compelling evidence that highly palatable foods administered intermittently can produce changes in brain and behavior that replicate substance addiction. But, outstanding questions remain about whether and how these carefully controlled regimens translate to humans who, by and large, inhabit a very different food environment, one that is often characterized by constant and plentiful availability rather than constraint. To be clear, I do not deny the importance and value of these rodent models in developing our understanding of perturbations in food behaviors, but their emergence challenges us to translate them to humans, a challenge that has not been met. And it should be noted too that they raise important questions (see ref. [ 8 ] for fuller discussion): for example, it has been argued that it is the uncertainty of availability, rather than the emergence of addiction, that generates compulsive eating [ 22 ]. Moreover, while rats with intermittent access to a high-calorie food develop habit-like responding and corresponding activation in dorsolateral striatum, those with continued access to the same foods do not show this pattern [ 23 ].
When first expressing misgivings about the ready acceptance of food addiction as an explanation for obesity [ 20 ], our paper generated a brief correspondence [ 24 ] in which we all concluded with the cliched but invariably true suggestion that “more research is needed”. Reviewing the current literature, I suggest that little clarity has emerged: the case has not been further made that there is a convincing resemblance between problem food behaviors and substance addiction. Attempts to pinpoint the neurobiology of food addiction in humans have generated no consistent or replicated finding, overall failing to support the model and, in many areas, seeming quite strongly to contradict it. Furthermore, the most promising area of research, the modeling of addictive-like eating in rodents, has not progressed appreciably and, importantly, has not been followed-up in humans in a systematic way. And yet, there is a continued willingness to diagnose, measure, and apply food addiction as a validated concept in research.
The strong narrative around food addiction is, of course, understandable. A person prone to binges or overeating in general feels powerful cravings to consume; they often see little option but to succumb to these cravings, and this capitulation is a matter of shame and guilt. The language of addiction—“this is a biological drive that I alone cannot control”—fits well with this subjective experience, and it brings a means of communicating distress and helplessness. It may even be helpful in resisting cravings, though whether it is or not is an empirical question that has not been resolved. It is not for the scientist or clinician to police popular language. When a person asserts that they are addicted to sugar, they acknowledge and convey a distressing sense of helplessness and compulsion. The science narrative that is offered that food, like a drug, has affected a person’s brain such that their inhibitory control centers do not work properly, and their reward centers are malfunctioning, is simple and readily accepted. But, the scientist who offers this as the explanation had better be sure that the evidence exists to support it. My view is that it does not. Lacking this evidence, I suggest, severely constrains the explanatory power of the food addiction concept, leaving it perilously close to the sort of “virtus dormitiva” explanation sharply lampooned by Moliere.
Arguments in favor of food addiction (PJK)
“My drug of choice is food. I use food for the same reasons an addict uses drugs: to comfort, to soothe, to ease stress.”
—Oprah Winfrey
The term “addiction”, even when used in the context of drug abuse, is highly contentious. It means different things to different people; those suffering from the disorder or their loved ones, health-care professionals, researchers, self-help groups, religious organizations, and government agencies all have their own views on what it means to be an addict. For this reason, discrete behavioral characteristics in affected individuals that are considered core features of the disorder have been codified into checklists that are used in the formal diagnosis of substance use disorders (SUDs) [ 25 ]. It is little wonder then that the term addiction is so controversial when used in the context of food consumption. I hesitate to position myself as a defender of the concept of food addiction when it is such a nebulous term so open to misinterpretation. However, I consider it self-evident that at least some overweight individuals struggle to control their food intake even when their health and well-being depend on it in a manner that is analogous or even homologous to those affected by SUDs who struggle to control their drug use. After all, SUDs across different classes of drugs with different pharmacological actions are not defined by any shared physiological abnormality that can be objectively measured and used as a diagnostic, such as the elevated blood glucose in the case of diabetes, but rather by the manifestation of a behavioral abnormality that negatively impacts their life: specifically, the failure to control consummatory behavior despite repeated attempts to do so. Consequently, I am a willing defender of the notion that a history of overconsumption of energy-dense palatable food can remodel brain motivation circuits in a manner that renders some overweight individuals persistently vulnerable to the desirable properties of such food, which negatively impacts their health and well-being.
Food addiction, as currently diagnosed using the YFAS and related scales, is considered distinct from obesity. However, the negative consequences associated with a failure to control food intake are most obvious in overweight individuals, who often suffer social stigmatization and are at far greater risk of diabetes, cardiovascular disease, cancer, and other significant health concerns than lean individuals. For this reason, I have framed the arguments below in the context of overweight and obesity, but this should not disguise the fact that many lean individuals also suffer from patterns of dysregulated eating that share many features with SUDs.
Three of the most important clinical features of SUDs are feelings of deprivation when the substance is withheld, a propensity to relapse during periods of abstinence, and consumption that persists despite awareness of negative health, social, financial, or other consequences. Overweight individuals who experience real or perceived social, emotional, or health consequences because of their body weight will often express a desire to lose weight and will repeatedly attempt to do so [ 26 , 27 ], but limiting their food intake or the types of food that they consume over the prolonged time periods necessary to achieve and maintain a healthy body weight is notoriously difficult. Even those overweight individuals who turn to surgical interventions, highlighting their struggle to exert self-control over their diet, demonstrate remarkably high rates of recidivism, with many gradually increasing their consumption of energy-dense food items, and regaining previously lost body weight over time [ 28 ]. Hence, overweight individuals who are unable to exert control over their consummatory behavior, despite awareness of the negative consequences, demonstrate the same core failure to control consumption as those suffering from SUDs.
An issue often raised in the context of food addiction is whether the same brain systems are involved in relapse to weight gain in previously overweight individuals, through their failure to control the amount, frequency, or type of food consumed, as those systems involved in relapse to drug use in SUDs. The underlying assumption being that unless a core brain system involved in drug addiction is similarly impacted in those struggling to control their diet, then the construct of addiction should not be applied to food. There is now a preponderance of human functional imaging data showing that energy-dense palatable food can stimulate changes in the activity of many of the same brain structures and circuits known to be impacted by drugs of abuse. For example, palatable food stimulates reward-relevant activity in the striatum [ 29 , 30 , 31 ]. Moreover, weight gain is associated with altered striatal responses to palatable food or cues that predict the availability of such food [ 32 , 33 , 34 ], and allelic variation that influences vulnerability to weight gain and obesity can alter reward-relevant activity in the striatum [ 32 , 35 ]. In addition to the striatum, the activity of other brain areas thought to play an important role in drug addiction, such as prefrontal cortical regions and the amygdala, is similarly altered by consumption of palatable food and development of obesity [ 36 , 37 , 38 , 39 , 40 , 41 ]. The animal literature is also replete with evidence that palatable food and drugs of abuse can impact similar brain circuits, particularly the mesoaccumbens dopamine system, and that the development of obesity profoundly alters the function of these same circuits [ 42 , 43 , 44 , 45 , 46 ]. Hence, palatable food and weight gain profoundly impact activity and responsiveness of core components of the brain reward system. But which type of brain abnormality in drug users should be considered the true hallmark of addiction to which changes in the brains of overweight individuals should be compared? It seems unreasonable to assume that there is a specific addiction-relevant pattern of brain activity, at least those patterns that can be detected using current imaging modalities, which can be used to support or refute the existence of food addiction when the very same imaging approaches cannot be used as a diagnostic for drug addiction. Currently, all that can be concluded from these imaging studies is that palatable food and drugs of abuse can impact the function of similar regions of the brain. Ultimately, the value of identifying overlapping brain systems involved in regulating the motivational properties of palatable food and drugs of abuse rests on whether such knowledge facilitates the development of therapeutics that reduce problematic overeating and drug use. Available evidence is promising in this regard. For example, the cannabinoid receptor 1 antagonist rimonabant, developed as an antiobesity drug, facilitates smoking cessation in humans [ 47 ]. Similarly, the antiobesity drug lorcaserin, a serotonin 2C receptor antagonist, also facilitates smoking cessation [ 48 ]. These findings suggest that common underlying brain processes are likely involved in overeating and drug use.
Another argument often used against the notion of a food-directed use disorder is the question of which ingredient in food is the responsible agent. The underlying assumption here is that only those food items that contain this agent will support addiction-relevant behaviors. Recently, arguments have been made for the involvement of refined sugars [ 49 , 50 ]. My own view is that it is not necessarily a single macronutrient that is responsible for maladaptive eating but rather the combinations of macronutrients in palatable high-calorie food items that do not occur naturally, but that when combined can pack a supraphysiological punch to brain motivation circuits that is sufficient to modify subsequent consummatory behaviors. Emerging evidence suggests that this may indeed be the case. For example, a recent study in humans found that blended food items high in both fat and carbohydrate were more valued than palatable food items high in fat or carbohydrate alone, and that the blended food had a greater impact on the activity on brain areas involved in reward than the single-nutrient food items [ 51 ]. Hence, diets consisting of palatable food items that are rich in energy-dense macronutrients may render less-palatable but healthier food options less attractive and shift dietary preferences toward these highly rewarding, calorie-laden options.
Addiction should not be viewed as a single unitary disorder, related to core deficits in one or more of the same brain system and distinguished only by the users’ drug of choice. Instead, use disorders should be viewed as a constellation of related syndromes that share similar but not entirely overlapping brain and behavior abnormalities, the most conspicuous of which is a failure to control consumption. Indeed, core features of SUDs can differ dramatically depending on which substance is being used, reflecting different underlying neurobiological processes at play. For example, cocaine use disorder is characterized by cycles of binge consumption interspersed by periods of abstinence, with the amounts of cocaine used sufficiently to induce intoxication and often exceeding the amounts that the user wished to limit themselves to, resulting in overt signs of overdosing [ 52 ]. By contrast, tobacco use disorder is characterized by remarkably stable and highly regular patterns of daily use, with no overt signs of intoxication, and binge-like consumption not a general feature of the habit [ 53 ]. Yet, considering the well-known detrimental health consequences of tobacco smoking, and the struggle that habitual smokers experience when trying to quit, it is difficult to argue that a tobacco smoker is any less “addicted” than someone who binges on cocaine. It is worth pointing out that until recently, tobacco smoking was also the subject of much heated debate about whether it constituted a simple habit or warranted the moniker of full-blown addiction [ 54 , 55 ]. Before that, the same type of debate centered on cocaine [ 56 ]. When viewed from this perspective, it should come as no surprise that overweight individuals who struggle to control their food intake will show brain and behavior abnormalities that are similar in some respects to the prototypic features of psychomotor stimulant, alcohol, tobacco, or opioid addiction, yet differ from these disorders in much the same way that these drug addictions differ from each other. Ultimately, the questions we must ask when considering whether food consumption can, in some circumstances, be considered a use disorder are: does the affected individual fail to exert control over consummation of a substance from which they derive pleasure despite knowledge of potentially severe consequences? Does the affected individual experience feelings of deprivation when the substance is not available or they try to abstain? Does the affected individual show vulnerability to relapse during periods of abstinence? For some overweight individuals at least, the answer to each of these questions is a resounding yes. Precisely, how these features can be codified into checklists of symptoms to identify individuals who are most affected, and the utility of such checklists in a clinical or research setting, should not distract from struggle that some individuals face when trying to exert control over their diet.
Ultimately, I agree with those who express concern about the concept of food addiction. This concept is too nebulous and loaded to convey proper meaning. However, some individuals clearly demonstrate a failure to exert control over their food choices, despite a desire to do so, and as a result experience significant negative consequences. Our ever-increasing understanding of how drugs of abuse remodel motivation circuits in the brain to precipitate compulsive drug use can and should serve as a heuristic to better understand the brain mechanisms of overeating in overweight individuals.
How to move the field forward
We both agree that questions on the concept of food addiction are heavily confounded by terminology. As noted above, the term addiction is simply too imprecise to be meaningful from a clinical perspective. We also agree that there are patterns of behavior and subjective experiences related to food consumption that bear a resemblance to SUDs, most notably the strong urge to consume, which can become more powerful with abstinence and override personal desires to limit consumption. Where we differ is on the issue of the biological equivalence between those struggling to control their food or drug intake and whether this signifies a deeper resemblance between overeating and drug addiction. More specifically, do strong urges to consume food simply reflect innate biological drives, which can differ between individuals, and which are undoubtedly further influenced by patterns of overconsumption and ensuing weight gain but which should not be conflated with the notion of an addict seeking drugs (Fletcher)? Or, instead, are such urges and associated overeating reflective of a breakdown of behavioral control systems related to neurobiological abnormalities that can be considered analogous or even homologous to those that contribute to SUDs (Kenny)? These differences in opinion speak directly to whether the diagnosis of “food addiction”, as defined by existing instruments such as the YFAS or other instruments that will likely emerge in coming years, captures a legitimate clinical entity that is distinct from other patterns of disordered eating.
A critical evaluation of the concept of food addiction should not seek to undermine the personal and clinical reality of those experiencing strong urges to consume. Instead, the focus should be placed firmly on generating empirical evidence that supports or refutes this concept. Perhaps the most practical scientific strategy to explore the case for food addiction is to begin with our current understanding of how drugs of abuse remodel brain motivation circuits to precipitate compulsive drug seeking, and factors that influence this process. Critically, this approach might adopt two core principles. First, it begins not by assuming that overeating is a form of addiction, although it allows for the possibility that ultimately there may emerge evidence to suggest that at least certain forms of such overconsumption may show high levels of overlap. Rather, the initial aim would be a systematic approach, using insights from the drug addiction literature to focus and sharpen scientific questions relating to obesity. Second, the emphasis would move away from focusing exclusively upon similarities between excessive food and substance use and would acknowledge too the importance of differences in their patterns of use. The functional significance of both the commonalities and the differences in the overuse of food and drugs should be explored together in order to generate a more complete picture of where the food addiction model succeeds and fails. Indeed, as with all useful models, one can learn as much from its limitations as from its success. This is important because we both feel that current characterizations of the food addiction model lack the specificity that would make the model breakable: a sine qua non for a useful model [ 57 ]. Overall, we suggest, in demanding less dogmatic or polarized positions, this reframes the question to a much more scientifically compelling and tractable one.
Practical difficulties present themselves when trying to apply drug addiction approaches to the question of food addiction. Even in the case of SUDs, where there is a known agent with an established pharmacology and the picture is not complicated by a preexisting innate biological need for the substance, the brain mechanisms are highly complex, with pronounced variability across individuals and drugs. Despite this complexity, robust molecular, epigenetic, synaptic, cellular, and circuit-based adaptive responses to drugs of abuse, thought to be relevant to the emergence of compulsive drug use in humans, have been detected in laboratory animals [ 58 ]. In addition, genetic vulnerabilities that influence these processes have been identified in recent years. Of course, the complexities are magnified significantly in the context of food consumption, which is shaped and regulated by a host of parallel systems with built-in redundancies as well as by complex economic and sociocultural factors. We must consider, therefore, the appreciable challenges when applying concepts in drug addiction to understand food consumption. Nonetheless, some of the important (but by no means the only) concepts from the addiction literature that should be considered in the context of food consumption are explained below.
First, most major drugs of abuse profoundly dysregulate striatal glutamate homeostasis [ 59 ], such that environmental stimuli associated with their delivery can evoke “supraphysiological” increases in glutamate spillover, particularly in the nucleus accumbens core [ 60 ]. Such cue-evoked increases in glutamate transmission trigger synaptic and structural plasticity in the striatum, reflected by alterations in AMPA/NMDA receptor ratios and new dendritic spine formation [ 60 ]. This action, in turn, is thought to contribute to vigorous drug seeking during periods of abstinence. Hence, it will be important to determine whether excessive consumption of palatable foods, and associated weight gain, also induces disruption in striatal glutamate homeostasis, a disruption that may perhaps offer important targets for therapeutic interventions aimed at curbing consumption or preventing relapse. The complexity of applying this aspect of the model to foods should not be underestimated: for example, excessive consumption of palatable foods may not lead to weight gain (e.g., in bulimia nervosa). Moreover, in many cases, particularly of early-onset obesity, primary disturbances lie outside the striatum, for example, within the leptin–melanocortin circuitry of the hypothalamus [ 61 ]. Thus, putative striatal alterations will need to be considered within a context of more extensive, albeit subtle, brain changes.
Second, a history of extended access to psychomotor stimulants, alcohol, or opioids in laboratory rodents and in human subjects is associated with profound deficits in brain reward function [ 62 ]. It has been hypothesized that drug-induced reward deficits precipitate the emergence of compulsive drug-seeking behaviors [ 63 ], and are related to the recruitment of brain aversion and stress systems such as the κ opioid receptor-mediated transmission [ 64 ]. Recently, it was shown that overconsumption of palatable energy-dense food and the development of obesity in laboratory rats similarly disrupts brain reward function [ 65 ]. While consistent evidence has yet to emerge that this translates to human obesity, this finding suggests that homeostatic adaptive responses occur in brain reward circuits in response to overconsumption of rewarding food or drugs. It will be important to determine if common underlying neuromolecular mechanisms are involved in drug- and food-induced alterations in hedonic responsiveness. Moreover, will amelioration of these adaptive responses in brain reward systems reduce the desire to use food and drugs in humans?
Third, overconsumption of drugs of abuse can facilitate the emergence of habitual-like consummatory behaviors, characterized by their relative insensitivity to the current value of a reinforcer that the animal is responding for [ 66 , 67 ]. The ability of drugs of abuse to enhance habitual-like patterns of responding may play a role in the persistence of the drug-taking habit. In addition, a history of extended drug access can precipitate drug-taking behaviors that become progressively less sensitive to negative consequences [ 68 , 69 ]. Emerging evidence suggests that overconsumption of palatable food can similarly precipitate habitual- and compulsive-like patterns of food intake in rats [ 65 , 70 ] Hence, it will be important to determine if common underlying neurobiological mechanisms contribute to these addiction-relevant patterns of consumption and, again, if manipulating these processes may help to ameliorate overconsumption. It will, however, be profoundly challenging to determine whether rodent findings extend to humans, given the challenges posed by precise but unobtrusive characterization of naturalistic eating behaviors and by difficulties in replicating the conditions under which habit-like responses can be generated, and thereby investigated, in humans ( https://osf.io/5pbmz/ ).
Fourth, drug addiction is often hypothesized to reflect a state of “hypofrontality” in which excessive drug use induces deficits in the function of higher-order cortical centers in the brain, resulting in a progressive loss of executive control over drug-seeking behaviors [ 71 ]. Such conceptualizations often consider addiction as a circuit-based disorder, in which top-down signals from the cortex to limbic, basal ganglia, and midbrain regions to inhibit consummatory behavior progressively weaken, whereas bottom-up urges to seek and consume drugs of abuse persist or even strengthen [ 72 ]. Recent animal and human findings are consistent with a circuit-based view of drug addiction that is controlled by executive centers in the cortex [ 71 , 73 ]. Hence, it will be important to thoroughly explore the consequences of overconsumption of palatable energy-dense food on cortical control centers in laboratory animals and humans. Emerging data suggest that higher-order cortical systems indeed undergo robust remodeling in response to such diets [ 74 ], but the functional consequences of such cortical remodeling remain poorly understood. Moreover, though ventromedial prefrontal cortex in humans is emerging as a consistent site of structural change in relation to elevated body weight [ 75 ], the functional significance of this is by no means clear and functional neuroimaging as a whole does not support any simple notions of hypofrontality as a causative or maintaining factor in obesity.
Fifth, we both agree that using “overeating” as a metric for addiction is problematic, as it is not necessarily the amount of food that is consumed but rather the type and pattern of consumption that is the issue. Drug addiction is often conceptualized as a disorder of decision-making, whereby the value of the drug increases to the point that the user will choose to consume the drug at the expense of competing natural reinforcers or behaviors. In recent years, Ahmed and colleagues have shown that the majority of rodents, when presented with a mutually exclusive choice between a natural reward such as sucrose or a drug reward, such as a cocaine infusion or delivery of alcohol, will select the natural reward over the drug reward [ 76 , 77 , 78 , 79 , 80 ]. Only a small minority of animals will select the drug reward, with this population considered the “addiction vulnerable” animals. Most recently, it was shown that constitutively enhanced GABAergic transmission in the central nucleus of the amygdala may explain the increased value of alcohol in vulnerable animals, as reflected by their choice to consume alcohol at the expense of competing saccharin rewards [ 81 ]. Currently, very little is known about the neurobiological mechanisms that regulate the choice to consume palatable energy-dense food at the expense of healthier but less-palatable options and the role that intermittency of access to palatable food can play in influencing such choices [ 22 ]. The use of choice procedures similar to those championed by Ahmed to understand maladaptive choice behaviors in the context of drug addiction may facilitate greater understanding of how long-lasting shifts in dietary preferences toward highly rewarding energy-dense food items can occur.
Sixth, large-scale genome-wide association studies (GWAS) in humans are beginning to identify genetic variants that are robustly associated with complex traits or phenotypes, such as cannabis use ( https://www.biorxiv.org/content/early/2018/01/08/234294 ), problematic alcohol use (see https://www.biorxiv.org/content/biorxiv/early/2018/03/08/275917.full.pdf ), levels of tobacco smoking [ 82 ], and measures of adiposity [ 83 ]. If substance use and obesity do share a common etiology, these studies are well suited to identify and characterize it. While early candidate gene studies have suggested such overlap, these have proved to be inconsistent; e.g., ref. [ 84 ]. Moreover, initial results from these GWAS suggest that the genetic variants that confer risk for substance use phenotypes do not substantially increase the risk for obesity—if at all. In fact, the results from these GWAS suggest that the common variant genetic architectures of substance use and obesity are largely distinct from one another despite both having high expression levels in brain tissue [ 83 , 85 ]. It should be borne in mind that this is a field that is developing rapidly, and changing techniques and emerging findings will require careful monitoring with respect to the question at hand.
It is important to bear in mind that while we support the view of drug use as a potential, albeit simplified, model for aberrant food intake, many of the gains that have been made in our understanding of drug use have been based on the reverse modeling process—specifically, by considering it in terms of a hijacking of the systems underlying natural rewards, including foods. There is no contradiction here. While it would certainly be circular to argue, as some have done, that, because foods activate reward circuitry, this suggests that foods are as perilous as drugs, it is nonetheless useful to take an iterative approach in which observations of perturbations associated with substance use can contribute to guiding and interpreting observations related to health-harming overconsumption, and vice versa. But such an approach must acknowledge notable differences between obesity and drug addiction. Reward circuitry, which is the core focus of both, does not function in isolation from other homeostatic and higher-order functions of the brain or from the powerful effects of neural, hormonal, and metabolic signals from the rest of the body. As well as modulating reward-related circuitry [ 86 ], these signals have the capacity to shift and shape higher-order cognition and perception [ 87 , 88 ]. With this in mind, it is important to consider whether mechanisms of appetitive regulation can provide a conceptual framework to better understand drug addiction. All vertebrates have whole-organ systems adapted to sense, consume, digest, and eliminate food, and each level of this process involves exquisitely controlled bidirectional communication between these organ systems and those brain systems involved in appetite control. Consequently, those interested in obesity, binge eating, and other forms of disordered eating do not view the brain in isolation but rather take a holistic view of the body and how it is perturbed in affected individuals. Indeed, slower hormonal and faster vagally transmitted signals from organ systems involved in energy homeostasis, such as the pancreas, liver, and gut, are central to any conceptualization of disordered feeding behaviors and associated diseases. With the exception of alcohol use [ 89 ], research conducted into the role of organ systems other than the brain in drug addiction has been sparse. Do peripheral organ systems transmit information to brain motivation circuits in response to drug consumption, and is such peripherally derived information relevant to addiction? In addition to hunger-related signals derived from peripheral organs that act on brain circuits to promote food intake, there are complex systems that serve to inhibit appetite, and failure of such appetite-suppressing systems is often incorporated into conceptualizations of overeating and obesity. Do circuits that inhibit food consumption also play a role in controlling drug intake, and does their dysregulation contribute to the emergence of drug addiction? These are important questions that can help bridge the fields of maladaptive food and drug consumption.
We believe that even those who feel skepticism over the validity and current evidence base of the food addiction model would recognize the potential value and synergy in drawing these fields together. There are many ways in which they may prove mutually informative. But information will be lost if we begin with the assumption that drug addiction processes explain food overconsumption and schedule our empirical endeavors exclusively toward a survey of similarities, some of which are superficial and imprecise. Ultimately, drug addiction could provide a useful model for aspects of food overconsumption, just as consumption of foods and other natural rewards serves a useful purpose to better understand drug addiction. Of course, such synergy may not necessarily translate directly to the clinic [ 90 ], but it may well help to guide future targeted therapeutic efforts and contribute to our understanding of whether superficial clinical similarities are underpinned by a deeper resemblance.
Change history
07 december 2018.
This article was originally published under standard licence, but has now been made available under a [CC BY 4.0] license. The PDF and HTML versions of the paper have been modified accordingly.
Jaspers K. General psychopathology. Manchester: Manchester University Press; 1963.
Google Scholar
Davis C. Evolutionary and neuropsychological perspectives on addictive behaviors and addictive substances: relevance to the “food addiction” construct. Subst Abus Rehabil. 2014;5:129–37.
Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52:430–6.
PubMed Google Scholar
Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18.
CAS PubMed Google Scholar
Pedram P, Wadden D, Amini P, Gulliver W, Randell E, Cahill F, et al. Food addiction: its prevalence and significant association with obesity in the general population. PLoS ONE. 2013;8:e74832.
CAS PubMed PubMed Central Google Scholar
Hebebrand J, Albayrak O, Adan R, Antel J, Dieguez C, de Jong J, et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci Biobehav Rev. 2014;47:295–306.
Schulte EM, Potenza MN, Gearhardt AN. A commentary on the “eating addiction” versus “food addiction” perspectives on addictive-like food consumption. Appetite. 2017;115:9–15.
Westwater ML, Fletcher PC, Ziauddeen H. Sugar addiction: the state of the science. Eur J Nutr. 2016;55(Suppl 2):55–69.
de Vries SK, Meule A. Food addiction and bulimia nervosa: new data based on the Yale Food Addiction Scale 2.0. Eur Eat Disord Rev. 2016;24:518–22.
Hilker I, Sanchez I, Steward T, Jimenez-Murcia S, Granero R, Gearhardt AN, et al. Food addiction in bulimia nervosa: clinical correlates and association with response to a brief psychoeducational intervention. Eur Eat Disord Rev. 2016;24:482–8.
Meule A, Heckel D, Kubler A. Factor structure and item analysis of the Yale Food Addiction Scale in obese candidates for bariatric surgery. Eur Eat Disord Rev. 2012;20:419–22.
Meule A, von Rezori V, Blechert J. Food addiction and bulimia nervosa. Eur Eat Disord Rev. 2014;22:331–7.
Gearhardt AN, White MA, Masheb RM, Morgan PT, Crosby RD, Grilo CM. An examination of the food addiction construct in obese patients with binge eating disorder. Int J Eat Disord. 2012;45:657–63.
Finlayson G. Food addiction and obesity: unnecessary medicalization of hedonic overeating. Nat Rev Endocrinol. 2017;13:493–8.
Long CG, Blundell JE, Finlayson G. A systematic review of the application and correlates of YFAS-diagnosed ‘food addiction’ in humans: are eating-related ‘addictions’ a cause for concern or empty concepts? Obes Facts. 2015;8:386–401.
PubMed PubMed Central Google Scholar
Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7.
Dang LC, Samanez-Larkin GR, Castrellon JJ, Perkins SF, Cowan RL, Zald DH. Associations between dopamine D2 receptor availability and BMI depend on age. Neuroimage. 2016;138:176–83.
Eisenstein SA, Bischoff AN, Gredysa DM, Antenor-Dorsey JA, Koller JM, Al-Lozi A, et al. Emotional eating phenotype is associated with central dopamine D2 receptor binding independent of body mass index. Sci Rep. 2015;5:11283.
Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased mu-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35:3959–65.
Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. 2012;13:279–86.
Ziauddeen H, Fletcher PC. Is food addiction a valid and useful concept? Obes Rev. 2013;14:19–28.
Corwin RL. The face of uncertainty eats. Curr Drug Abus Rev. 2011;4:174–81.
Furlong TM, Jayaweera HK, Balleine BW, Corbit LH. Binge-like consumption of a palatable food accelerates habitual control of behavior and is dependent on activation of the dorsolateral striatum. J Neurosci. 2014;34:5012–22.
Avena NM, Gearhardt AN, Gold MS, Wang GJ, Potenza MN. Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data. Nat Rev Neurosci. 2012;13:514. author reply 514
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Arlington, VA: American Psychiatric Publishing; 2013.
Puhl RM, Moss-Racusin CA, Schwartz MB, Brownell KD. Weight stigmatization and bias reduction: perspectives of overweight and obese adults. Health Educ Res. 2008;23:347–58.
Booth ML, Wilkenfeld RL, Pagnini DL, Booth SL, King LA. Perceptions of adolescents on overweight and obesity: the weight of opinion study. J Paediatr Child Health. 2008;44:248–52.
Saunders R. Compulsive eating and gastric bypass surgery: what does hunger have to do with it? Obes Surg. 2001;11:757–61.
Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(Pt 9):1720–33.
Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31:4360–6.
Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring). 2014;22:2544–51.
Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–52.
Stice E, Yokum S. Gain in body fat is associated with increased striatal response to palatable food cues, whereas body fat stability is associated with decreased striatal response. J Neurosci. 2016;36:6949–56.
Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–9.
van der Klaauw AA, von dem Hagen EA, Keogh JM, Henning E, O’Rahilly S, Lawrence AD, et al. Obesity-associated melanocortin-4 receptor mutations are associated with changes in the brain response to food cues. J Clin Endocrinol Metab. 2014;99:E2101–6.
Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, et al. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27:669–76.
Killgore WD, Weber M, Schwab ZJ, Kipman M, DelDonno SR, Webb CA, et al. Cortico-limbic responsiveness to high-calorie food images predicts weight status among women. Int J Obes (Lond). 2013;37:1435–42.
CAS Google Scholar
Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–94.
Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63:891–902.
Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–58.
van Bloemendaal L, RGI J, Ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186–96.
Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–9.
Martel P, Fantino M. Influence of the amount of food ingested on mesolimbic dopaminergic system activity: a microdialysis study. Pharmacol Biochem Behav. 1996;55:297–302.
Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999;11:4389–97.
Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Increased dopamine metabolism in the nucleus accumbens and striatum following consumption of a nutritive meal but not a palatable non-nutritive saccharin solution. Pharmacol Biochem Behav. 1986;25:1095–100.
Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15(7 Pt 2):5169–78.
Cahill K, Ussher M. Cannabinoid type 1 receptor antagonists (rimonabant) for smoking cessation. Cochrane Database Syst Rev: 2007;CD005353.
Shanahan WR, Rose JE, Glicklich A, Stubbe S, Sanchez-Kam M. Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine Tob Res. 2017;19:944–51.
Ahmed SH, Guillem K, Vandaele Y. Sugar addiction: pushing the drug-sugar analogy to the limit. Curr Opin Clin Nutr Metab Care. 2013;16:434–9.
Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–88.
DiFeliceantonio AG, Coppin G, Rigoux L, Thanarajah SE, Dagher A, Tittgemeyer M, et al. Supra-additive effects of combining fat and carbohydrate on food reward. Cell Metab. 2018;28:33–44.e3.
Decorte T. Quality control by cocaine users: underdeveloped harm reduction strategies. Eur Addict Res. 2001;7:161–75.
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Patterns of tobacco use among U.S. youth, young adults, and adults. In: (US)CfDCaP, editor. The health consequences of smoking-50 years of progress: a report of the surgeon general. US Department of Health and Human Services, Atlanta, GA; 2014.
Russell MA. The smoking habit and its classification. Practitioner. 1974;212:791–800.
Warburton DM. Is nicotine use an addiction? Psychologist. 1989;4:166–70.
Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–6.
Teufel C, Fletcher PC. The promises and pitfalls of applying computational models to neurological and psychiatric disorders. Brain. 2016;139(Pt 10):2600–8.
Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–76.
Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–9.
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev. 2016;68:816–71.
O’Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57:2905–10.
Kenny PJ, Hoyer D, Koob GF. Animal models of addiction and neuropsychiatric disorders and their role in drug discovery: honoring the legacy of Athina Markou. Biol Psychiatry. 2018;83:940–6.
Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53.
Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl). 2010;210:121–35.
Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–41.
Ersche KD, Gillan CM, Jones PS, Williams GB, Ward LH, Luijten M, et al. Carrots and sticks fail to change behavior in cocaine addiction. Science. 2016;352:1468–71.
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9.
Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7.
Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–9.
Tantot F, Parkes SL, Marchand AR, Boitard C, Naneix F, Laye S, et al. The effect of high-fat diet consumption on appetitive instrumental behavior in rats. Appetite. 2017;108:203–11.
Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–62.
Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci. 2017;18:685–93.
Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: a pilot study. Eur Neuropsychopharmacol. 2016;26:37–44.
Thompson JL, Drysdale M, Baimel C, Kaur M, MacGowan T, Pitman KA, et al. Obesity-induced structural and neuronal plasticity in the lateral orbitofrontal cortex. Neuropsychopharmacology. 2017;42:1480–90.
Medic N, Ziauddeen H, Ersche KD, Farooqi IS, Bullmore ET, Nathan PJ, et al. Increased body mass index is associated with specific regional alterations in brain structure. Int J Obes (Lond). 2016;40:1177–82.
Freese L, Durand A, Guillem K, Ahmed SH. Pre-trial cocaine biases choice toward cocaine through suppression of the nondrug option. Pharmacol Biochem Behav. 2018;173:65–73.
Huynh C, Fam J, Ahmed SH, Clemens KJ. Rats quit nicotine for a sweet reward following an extensive history of nicotine use. Addict Biol. 2017;22:142–51.
Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20.
Madsen HB, Ahmed SH. Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol. 2015;20:433–44.
Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, et al. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS ONE. 2010;5:e11592.
Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, et al. A molecular mechanism for choosing alcohol over an alternative reward. Science. 2018;360:1321–6.
Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–42.
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206.
Munafo MR, Matheson IJ, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case-control studies and evidence of publication bias. Mol Psychiatry. 2007;12:454–61.
Sanchez-Roige S, Gray JC, MacKillop J, Chen CH, Palmer AA. The genetics of human personality. Genes Brain Behav. 2018;17:e12439.
Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355.
Owens AP, Allen M, Ondobaka S, Friston KJ. Interoceptive inference: from computational neuroscience to clinic. Neurosci Biobehav Rev. 2018;90:174–83.
Tallon-Baudry C, Campana F, Park HD, Babo-Rebelo M. The neural monitoring of visceral inputs, rather than attention, accounts for first-person perspective in conscious vision. Cortex. 2018;102:139–49.
Morris LS, Voon V, Leggio L. Stress, motivation, and the gut-brain axis: a focus on the ghrelin system and alcohol use disorder. Alcohol Clin Exp Res. 2018.
Wilson GT. Eating disorders, obesity and addiction. Eur Eat Disord Rev. 2010;18:341–51.
Download references
Acknowledgements
PCF is supported by the Wellcome Trust and the Bernard Wolfe Health Neuroscience Fund. He is grateful to Hisham Ziauddeen and Margaret Westwater for their erudite discussion and suggestions around this topic. PJK was supported by the National Institutes of Health and is grateful to Drs. Alexandra DiFeliceantonio and Richard O’Connor for helpful discussions.
Author information
Authors and affiliations.
Department of Psychiatry, University of Cambridge, Cambridge, CB2 8AH, UK
Paul C. Fletcher
Cambridgeshire and Peterborough NHS Foundation Trust, Cambrdge, CB21 5EF, UK
Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, 10029, USA
Paul J. Kenny
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Paul C. Fletcher .
Ethics declarations
Competing interests.
PJK is a co-founder and shareholder in Eolas Therapeutics Inc., has a patent license agreement with AstraZeneca, receives research support from Eli Lilly, and has received consulting fees from Takenda Pharmaceuticals, USA. PCF has received consulting fees from GlaxoSmithKline.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Fletcher, P.C., Kenny, P.J. Food addiction: a valid concept?. Neuropsychopharmacol 43 , 2506–2513 (2018). https://doi.org/10.1038/s41386-018-0203-9
Download citation
Received : 22 June 2018
Revised : 12 August 2018
Accepted : 15 August 2018
Published : 06 September 2018
Issue Date : December 2018
DOI : https://doi.org/10.1038/s41386-018-0203-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Food addiction and its relationship with other eating behaviours among spanish university students.
- Tamara Escrivá-Martínez
- Laura Galiana
- Rosa M. Baños
Journal of Eating Disorders (2023)
Disentangling the role of NAc D1 and D2 cells in hedonic eating
- Mathilde C. C. Guillaumin
- Paulius Viskaitis
- Daria Peleg-Raibstein
Molecular Psychiatry (2023)
Towards precision medicine in bariatric surgery prescription
- Sofia S. Pereira
- Marta Guimarães
- Mariana P. Monteiro
Reviews in Endocrine and Metabolic Disorders (2023)
The habit, choice, intention, and perception of raw beef consumers on raw beef-eating: the health risk management perspective
- Daniel Teshome Gebeyehu
- Biruk Alemu
- Gemechu Belete
BMC Nutrition (2022)
Validation of the Arabic version of the modified Yale Food Addiction Scale in the general population in Lebanon
- Souheil Hallit
- Anna Brytek-Matera
- Sahar Obeid
Journal of Eating Disorders (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
September 11, 2023
Food Can Be Literally Addictive, New Evidence Suggests
Highly processed foods resemble drugs of misuse in a number of disturbing ways
By Marta Zaraska

Julia Garan/Alamy Stock Photo
Given the option, most rats will choose sugar instead of cocaine . Their lust for the carbohydrate is so intense that they will go as far as to self-administer electric shocks in their desperation to consume sugar. Rats aren’t alone in this drive. Humans, it seems, do something similar. People who’ve had bariatric surgery sometimes continue to overindulge in highly processed foods , those made from white flour, sugar, butter, and the like, even if it means later enduring vomiting and diarrhea. Daily snacking on processed foods, recent studies show, rewires the brain’s reward circuits . Cravings for tasty meals light up the brain just like cravings for cocaine do, prompting some researchers to ask whether products such as fries or cookies can trigger addiction akin to that associated with drugs or alcohol.
Yet the issue is by no means settled. An ongoing debate persists over whether these foods are truly addictive . Processed foods might provoke compulsive behaviors that reinforce the need to consume more, but do they really have mood-altering effects, another criterion used to define an addiction?
Answers to these questions are complicated by the enormous variety of foods we consume. There is no single opiatelike substance that can be identified as leading someone to become a food addict. Arguments in favor of food addiction suggest that if carbohydrates and fats are mixed together in unnaturally large doses, this creates a rapid “delivery system” for nutrients that results in physiological effects on the brain’s reward system that resemble those produced by cocaine or nicotine.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
To examine how this affects actual behaviors, researchers developed a measurement to examine the strong pull that highly processed food exerts on humans. In 2009 the Yale Food Addiction Scale emerged. It is used to assess whether a person displays behavioral patterns that would merit fries, shakes and other palatable foods being classified as addictive substances.
Using this measurement technique, a 2022 meta-analysis suggested that 20 percent of adults are addicted to food. People in this group go out of their way to obtain their favorite foods and often eat to the point of feeling physically ill. They experience withdrawal, fail to quit eating certain foods and continue their consumption pattern despite adverse consequences, such as disruptions to their daily routines and social activities. These are all criteria set out by the Yale Food Addiction Scale , which is adapted from measures used to gauge substance use disorders. The definition of food addiction is separable from obesity. Surprisingly, many people who tick the boxes for food addiction maintain a typical weight. If anything, food addiction is the closest cousin to binge eating disorder, says Alexandra DiFeliceantonio , a neuroscientist at the Fralin Biomedical Research Institute at Virginia Tech Carilion. Both cause people to exhibit a lack of control in the way food is consumed, but the definition for a substance use disorder also includes cravings, withdrawal symptoms and continued use despite negative consequences.
Critics of this research suggest that you can’t get addicted to something that’s essential to life. What’s more, while science has pinpointed nicotine in cigarettes and ethanol in wine or beer as the substances responsible for keeping people hooked, no such clear-cut equivalent exists for food . “It’s very difficult to prove that there are these nutrients in food that directly cause addiction,” says Johannes Hebebrand , a psychiatrist at the University of Duisburg-Essen in Germany.
Yet Ashley Gearhardt , a clinical psychologist at the University of Michigan, argues that highly processed foods are vastly different from what our ancestors used to consume. “Foods that are very high in fat and carbohydrate in a kind of an equal ratio—they don’t exist naturally,” she says. “It’s something that’s designed by food scientists in a laboratory to look a certain way, feel a certain way in your mouth, smell a certain way when you open the package.” A 2021 study showed, for example, that people with binge eating disorder exclusively overeat ultraprocessed foods . “People aren’t losing control over beans,” Gearhardt says.
Early research on rats suggested that sucrose keeps animals hooked. “They want more and more and more. Each day, they’ll show signs of craving,” says Nicole Avena, a neuroscientist at the Icahn School of Medicine at Mount Sinai . Sugars are present in many natural foods, from bananas to beets. Yet, as Avena points out, it’s all about packaging. A piece of fruit, she says, “has the appropriate amount of sugar in it, based on how much fiber it contains. Also, it has other nutrients that are going to minimize or mitigate the effects that that sugar might have on our brain.”
What matters, the scientists argue, is the dosage and the speed of absorption of a substance. Most people don’t consume pure ethanol, for example. Instead they opt for wine or beer, which contain a small amount of the addictive substance. (Most beer is more than 90 percent water .) Similarly, few of us indulge in sucrose by the spoonful. Nicotine also mixes with other ingredients and is carefully dosed. It’s naturally present in eggplants and tomatoes , but you won’t become an addict by indulging in vegetables.
When it comes to ultraprocessed snacks, sugar often goes together with fat—a combination that could make such foods even more addictive. A 2018 study by DiFeliceantonio and her colleagues showed that, compared with equally caloric foods containing only fat or only a carbohydrate, those made with both ingredients are far more efficient at activating the striatum, a part of the brain’s reward center that is implicated in addictions.
For a 2023 study, DiFeliceantonio and her colleagues randomly assigned 82 people to snack on either high-fat, high-sugar yogurts or low-sugar, low-fat ones for eight weeks. The scientists discovered not only that the first group’s preference for the healthier yogurts decreased after the trial but that their brain activation patterns changed, too . When they tasted fatty, sugary milkshakes, those who had been indulging in high-fat, high-sugar snacks had an increased response in their reward circuits, including the striatum. “Ultraprocessed foods are hijacking the brain in a way you’d see with addiction to drugs,” Avena says.
One of the hallmarks of drug addiction, she says, is the release of dopamine in the brain’s reward regions. The potency of a fatty, sugary treat in triggering this release was highlighted by a 2023 study in which scientists used positron-emission tomography on a small sample of volunteers. The results showed that indulging in a milkshake leads to a significant release of dopamine in healthy people that can be about one third of what is usually seen with amphetamines—a group of highly addictive stimulant drugs, such as “speed.”
The addictive potential of ultraprocessed foods may not relate just to dopamine, however. A 2023 study revealed the importance of the cannabinoid receptor 2 (CB2) in getting hooked on certain foods (in this particular case, chocolate-flavored pellets , because the subjects were mice). Rodents lacking these receptors in the brain are not only less likely to become addicted to cocaine or alcohol, the research showed, but also less prone to food addiction—a finding that may open new paths for treatment of binge eating.
Research on weight-loss drugs provides further evidence that overeating and substance misuse may share common brain processes. Semaglutide (sold under the brand names Ozempic and Wegovy) induces weight loss by mimicking the insulin-increasing gut hormone glucagonlike peptide-1 (GLP-1) , and it could potentially aid those struggling with various addictions, too. Animal experiments suggest, for example, that it can reduce dependence on cocaine and opioids. “That supports the argument that foods and drugs, in many ways, can act on the same brain systems,” Avena says.
What’s more, both illegal drugs and processed foods can induce cravings in the same reward areas of the brain—as demonstrated by a 2023 functional magnetic resonance imaging (fMRI) study. When researchers showed pictures of cocaine to drug addicts or photographs of donuts to healthy people, the same brain regions—ranging from the ventral striatum and amygdala to the cerebellum— lit up in both groups . And the stronger the volunteers’ reported craving was, the more intense their neural response was as well.
Withdrawal symptoms, another classic feature of addiction, also seem to be present in connection with ultraprocessed foods. While it’s unlikely that anyone experiences physical shakes from quitting cookies, parents who attempt to restrict their children’s intake of sugar-sweetened drinks have reported symptoms such as headaches, irritability and social withdrawal in their kids. Similarly, adolescents instructed to abstain from their high intake of sodas for three days complained of decreased motivation and ability to concentrate—along with increased headaches.
Critics of the idea that certain foods may be addictive point out that treats such as burgers don’t induce the same kind of “high” that one might experience with opioids or alcohol. “This is totally missing in all the food addiction stories,” Hebebrand says. For her part, Gearhardt is not convinced. “By that principle, cigarettes are not addictive, right? You can drive your car while you smoke cigarettes. You can watch your children while you smoke cigarettes,” she says. She points to studies indicating that chocolate does have a psychoactive effect and can induce feelings of euphoria at least as much as intravenous nicotine given to smokers can .
In 2022 Gearhardt and DiFeliceantonio published an opinion piece in the journal Addiction arguing that highly processed foods should be classified as addictive based on a 1988 Surgeon General report on tobacco products. That document outlined scientific evidence behind cigarettes’ addictive nature, including their psychoactive effects and potential to trigger compulsive use. Similar evidence, the scientists argue, already exists for ultraprocessed foods. “If we apply that same criteria to this specific class of foods, it meets every single checkbox,” Gearhardt says.
Hebebrand worries, however, that rushing to classify certain foods as addictive could let the sugar industry off the hook too easily. “They can always say, ‘Well, this is a matter of debate; we don’t know if it really exists,’” he says. The industry has already sponsored research that argues against the existence of sugar addiction, which, for Gearhardt, suggests that it may be following “ the playbook of the tobacco industry .” After all, nicotine wasn’t a clear-cut candidate for an addictive substance, either: it lacks significant mind-altering effects and is not found in large amounts in foods, and researchers don’t know the dosage at which it becomes addictive. As a result, and with the help of the tobacco industry, the addictive nature of tobacco was denied for decades. Considering ultraprocessed foods’ detrimental health effects—a 2021 meta-analysis showed such products raise mortality risk by 25 percent —Gearhardt argues that it’s better to chance misclassifying ultraprocessed foods as addictive than to fail to label them as such when warranted. “It’s cigarettes all over again,” she says.
ORIGINAL RESEARCH article
Food addiction and its relationship to weight- and addiction-related psychological parameters in individuals with overweight and obesity.

- 1 Department of Psychosomatic Medicine and Psychotherapy, LWL-University Hospital, Ruhr University Bochum, Bochum, Germany
- 2 Department of Clinical Psychology and Psychotherapy, University of Bamberg, Bamberg, Germany
- 3 Department of Pathopsychology, University of Bamberg, Bamberg, Germany
Background and Aims: It is assumed that a relevant subgroup of individuals experiences an addiction-like eating behaviour (Food Addiction), characterized by an impaired control over eating behaviour, emotional eating and food craving. Individuals experiencing Food Addiction partially share common symptomatology with Binge-Eating-Disorder and Bulimia Nervosa. The aim of this study was to investigate the prevalence of Food Addiction, general psychopathology, and associations with weight- and addiction-related constructs in individuals with overweight and obesity, who did not suffer from Binge-Eating-Disorder or Bulimia Nervosa.
Methods: N =213 (67.1% female; M BMI =33.35kg/m 2 , SD BMI =3.79kg/m 2 ) participants who were included in a weight loss program (I-GENDO project) reported BMI and completed questionnaires before the start of the treatment. Food Addiction severity, depressive symptoms, alcohol use disorder, internet use disorder, psychological distress, impulsivity personality trait, impulsive and emotional eating behaviour, food related inhibitory control, weight bias internalization, and self-efficacy were assessed.
Results: The prevalence of Food Addiction was 15% with higher, although not statistically significant, prevalence in female (18.2%) compared to male (8.6%) participants. Food Addiction was associated with higher BMI at baseline assessment, low self-esteem, impulsive and emotional eating behaviour, weight bias internalization, and deficits in food-related inhibitory control. In addition, correlations were found between Food Addiction and severity of depressive symptoms, internet use disorder, and psychological distress.
Conclusion: A relevant subgroup of participants experiences Food Addiction even when controlling for Binge-Eating-Disorder and Bulimia Nervosa. Future studies are warranted that investigate whether Food Addiction affects treatment success.
Introduction
In the last decades, the prevalence of overweight and obesity has increased dramatically worldwide, causing not only physical, but also mental health problems, including depression and anxiety disorders ( Chu et al., 2019 ). Various weight loss programs (WLPs) have been developed mainly based on physical activity, diets, and change in eating habits ( Jensen and Ryan, 2014 ). These lifestyle interventions often lack long-term effectiveness ( Jeffery et al., 2000 ; Ross Middleton et al., 2012 ) due to an “obesogenic environment” ( Swinburn et al., 1999 ) that promotes gaining weight, and is not conducive to weight loss within the home or workplace (hypercaloric diet, physical inactivity). Furthermore, relevant individual psychological aspects associated with eating behaviour are not sufficiently considered ( Chalk, 2004 ).
A subgroup of individuals with overweight and obesity describes themselves as being “addicted” to food, characterized by an impaired control over their eating behaviour, emotional eating and food craving ( Davis et al., 2013 ; Meule, 2015 ). Firstly, the addiction model of overeating was introduced by self-help groups and is even today controversially discussed in the scientific community ( Naish et al., 2018 ; Hebebrand and Gearhardt, 2021 ). In 2009, the Yale Food Addiction Scale (YFAS), the first formal measure of Food Addiction (FA), was introduced. Originally based on the DSM-IV criteria for diagnosing substance use disorders (SUD) the YFAS was adapted to addictive overeating ( Gearhardt et al., 2009 ). With the updated criteria for addictive disorders in the DSM-5, the YFAS 2.0 was developed in 2016 ( Gearhardt et al., 2016 ). Until now, FA is not formally included as a distinct category in DSM-5 or ICD-11, but is based on criteria for addictive disorders as defined in these diagnostic manuals.
The concept of FA gains support from neurobiological findings, reporting food related brain activities, i.e., in the dopaminergic reward system, similar to those of drug intake ( Gearhardt et al., 2011 ; Grosshans et al., 2012 ; Beitscher-Campbell et al., 2016 ; Novelle and Diéguez, 2018 ). Consistent with neurobiological similarities, individuals experiencing FA share specific behavioural deficits and personality traits with individuals suffering from SUDs, such as increased impulsivity ( VanderBroek-Stice et al., 2017 ). Impulsivity is a multidimensional construct characterized by deficient inhibitory processes and low self-control. There are mainly three components of impulsivity to be distinguished: impulsive personality trait , impulsive actions , i.e., deficits in behavioural inhibition (action withdrawal, action cancellation), and impulsive choices , i.e., the tendency towards immediate rewards instead of long-term goals (delay discounting; Hofmann et al., 2009 ; Bari and Robbins, 2013 ; Mole et al., 2015 ). Individuals experiencing FA report a tendency to consume immediate disposable high-caloric food, as well as problems in resisting eating impulses ( Meule, 2017 ; VanderBroek-Stice et al., 2017 ; Preuss et al., 2019 ). In general, individuals experiencing FA are characterized by high impulsivity traits ( Mobbs et al., 2010 ; Churchill and Jessop, 2011 ; Murphy et al., 2014 ). Individuals with overweight and obesity experiencing weight stigmatization are likely to internalize negative stereotypes and to attribute them to their own weight (weight bias internalization, WBI; Puhl and Heuer, 2009 ). WBI is assumed to be linked to emotional eating and FA, resulting in maladaptive eating patterns ( Baldofski et al., 2016 ). FA is associated with low self-esteem, high psychological distress, lower quality of life and depressive symptoms ( Gearhardt et al., 2012 ; Burmeister et al., 2013 ; Minhas et al., 2021 ; Vidmar et al., 2021 ). From a psychobiological perspective, there is the assumption that similar processes in FA and other addiction disorders may be operating, which might explain the similarities and the high comorbidity of FA with substance-related disorders and behavioural addictions ( Davis and Carter, 2009 ; García-García et al., 2014 ; Marmet et al., 2019 ).
In Germany, about 7.9% of the general population suffer from FA, with increasing prevalence rates in individuals with overweight (17.2%; Hauck et al., 2017 ). According to a meta-analysis of N =25 studies, the prevalence of FA is higher in individuals with overweight and obesity (weighted mean prevalence, WMP: 25%), females (WMP: 12%) and individuals aged over 35years (WMP: 22%; Pursey et al., 2014 ). To date, numerous heterogeneous results about the effect of FA on body weight and the influence on the success of WLPs have been published. In general, FA symptom severity is associated with higher BMI ( Pursey et al., 2014 ). Some studies report higher study attrition and lower effectiveness of WLPs in individuals experiencing FA, while other studies did not find differences to individuals who are not experiencing FA ( Burmeister et al., 2013 ; Clark and Saules, 2013 ; Meule et al., 2015 ; Fielding-Singh et al., 2019 ). Yet, the prevalence of FA in WLP samples is high (up to 34,5–38%; Meule et al., 2015 ; Vidmar et al., 2021 ). At least partially, some authors explain the relationship between FA and weight history as a result of comorbid mental disorders, particularly eating disorders ( Eichen et al., 2013 ; Lent et al., 2014 ; Chao et al., 2019 ). Prevalence rates of FA in adults with Binge-Eating-Disorder (BED) range from 40 to 50%, while in individuals with Bulimia Nervosa (BN) the prevalence rises over 80% ( Gearhardt et al., 2014 ; De Vries and Meule, 2016 ). Additionally, the prevalence of eating disorders, i.e., BED (up to 30%), are also higher in participants undergoing weight loss interventions ( Herpertz et al., 2006 ; Blaine and Rodman, 2007 ; Davis, 2015 ).
The high comorbidity may be due to the fact that BED, BN and FA share common mechanisms such as binge-eating and reward dysfunction ( Burrows et al., 2018 ). Especially the emotional component (e.g., fear of not being able to stop and guilt surrounding binge eating episodes) of BED seems to be associated with increased FA severity ( Burrows et al., 2017 ). Thus, FA overlaps with BN and BED ( Gearhardt et al., 2013 ; Meule et al., 2014 ; Meule, 2019b ). Yet, there are also dissimilarities, which distinguish FA from BED and BN ( Schulte et al., 2020 ). When conceptualizing FA as a specific form of SUDs, highly palatable and processed foods act as drugs. Yet, individuals experiencing FA need not necessarily binge these drugs, but could also graze them throughout the day, similar to other SUDs. Additionally, some studies report specific food-related withdrawal and tolerance symptoms ( Avena et al., 2008 ; Burger and Stice, 2012 ).
Taken together, despite its similarities to other SUDs FA is currently not an officially recognized diagnosis. One contentious issue in the recent debate about the need of an FA diagnosis is the mentioned overlap of FA symptoms with those of other eating disorders ( Gearhardt and Hebebrand, 2021 ; Hebebrand and Gearhardt, 2021 ). In order to contribute to this question, we analysed FA in a sample of individuals with overweight and obesity participating at a 12-week long WLP, who did not suffer from BED or BN.
The aims of our study were:
1. To analyse the prevalence of FA in a sample of individuals with overweight and obesity seeking treatment, who did not suffer from BED or BN.
2. To analyse relationships between FA severity, eating behaviours, general psychopathology and weight- and addiction-related factors, such as impulsivity, food related inhibitory control, self-efficacy and weight bias internalization.
3. To further characterize the subgroup of FA in contrast to non-food-addicted (NFA) participants.
Materials and Methods
The dataset of this manuscript is retrieved from the I-GENDO project ( Gender-sensitive Enhancement of Common Weight Loss Strategies for Overweight and Obesity , ClinicalTrials.gov Identifier: NCT04080193) a multicentre randomized controlled trial to assess the effectivity of a gender sensitive individualized smartphone-based intervention to reduce weight. Interested individuals were recruited from August 2019 until August 2020 via newspaper articles, radio features and oral presentations at rehab centres. At the baseline assessment the participants completed questionnaires and reported their current weight (kg) and height (m), of which the individual BMI (kg/m 2 ) was calculated.
Participants
Table 1 displays the eligibility criteria of the I-GENDO project. Following the guidelines of the German Society for General and Visceral Surgery (DGAV) and the German Association for the Study of Obesity (DAG) individuals with obesity class III (BMI>39.9) suffer from a complex multifactorial framework of psychological, social and physical problems and are therefore recommended to undergo a bariatric surgery. In order to avoid potentially confounding effects, we excluded individuals with obesity class III from participation, but provided further support. During the recruitment process we screened for major depression with the PHQ-9 ( Löwe et al., 2002 ). Interested subjects who scored above the cut-off for major depression (≥ 20) were excluded from participation ( Kroenke et al., 2001 ). Furthermore, if suicidal ideation was reported, people were contacted and subsequently diagnosed via telephone with a structured interview to clarify suicidal intentions by experienced psychologists. When suicidal ideation could not be convincingly negated by clinical judgement, subjects were referred further and excluded from this study. In prior studies heavy drinking was consistently associated with gaining weight, which we expected could influence the effectiveness of the WLP ( Traversy and Chaput, 2015 ). Using the Alcohol Use Disorders Identification Test (AUDIT, Saunders et al., 1993 ) subjects were excluded when the sum score of the AUDIT was 15 or more, suggesting hazardous alcohol consumption ( Conigrave et al., 1995 ). In case of suspected eating disorders, as assessed by the Munich ED-Quest ( Fichter et al., 2015 ), subjects were contacted and subsequently diagnosed by experienced psychologists using the German version of the Eating Disorder Examination (EDE), a clinical interview for the assessment of eating disorder specific psychopathology ( Hilbert and Tuschen-Caffier, 2016 ). Individuals who were diagnosed with BN or BED were excluded from participation.

Table 1 . Eligibility criteria of the I-GENDO project.
The final sample consisted of N =213 (67.1% female) individuals with obesity or overweight (M BMI =33.35kg/m 2 , SD BMI =3.79kg/m 2 ). Age ranged between 19 and 71years (M age =46.45years, SD age =12.13years).
Food Addiction (YFAS 2.0)
Addiction-like eating (during the past 12months) was measured using the YFAS Scale 2.0 ( Gearhardt et al., 2016 ). The YFAS 2.0 consists of 35 items, which are scored on an 8-point scale ranging from never to every day . Based on the 11 diagnostic criteria for SUDs in the DSM-5 (e.g., craving, tolerance or withdrawal) a symptom score ranging between 0 and 11 is calculated, reflecting the FA symptom severity. Additionally, clinically significant impairments or distress due to the eating behaviour are assessed. Food addiction (FA) is diagnosed when two or more symptoms are met (=symptom score≥2) together with clinically significant impairments or distress due to the eating behaviour. The German version of the YFAS 2.0 was used ( Meule et al., 2017 ) and showed excellent internal consistency, α=0.91, in the present sample.
Eating Disorders (Munich ED-Quest)
The Munich Eating and Feeding Disorder Questionnaire (Munich ED-Quest, Fichter et al., 2015 ) was used to screen for BN and BED. The whole questionnaire consists of 65 items assessing core symptoms of BN, BED, Anorexia Nervosa, Purging Disorder and Night Eating Syndrome within the last 3months. In our study, only 32 items were assessed screening for core symptoms of BED and BN based on the DSM-5 criterions (e.g., binge eating episodes, inappropriate compensatory behaviour, significant distress caused by binge eating episodes). Participants were instructed to indicate the severity of their symptomatology by either rating the corresponding items on a 5-Point Likert scale ranging from 0 to 4, or by estimating average frequencies (e.g., of binge eating episodes). Reliability of Munich ED-Quest (internal consistency of α=0.94) and validity are determined in a clinical sample of eating disorder patients in Germany ( Fichter et al., 2015 ).
Depression (PHQ-9)
Depressive symptoms in the last 2weeks were measured using the Patients-Health-Questionnaire-9 (PHQ-9, Löwe et al., 2002 ). The screening instrument consists of nine items, corresponding to the DSM-IV major depression criteria, which are scored on a 4-point scale ranging from not at all (0) to nearly every day (3). The symptom score ranges between 0 and 27, with scores above 20 indicating major depression ( Kroenke et al., 2001 ).
Alcohol Use Disorder (AUDIT)
The Alcohol Use Disorders Identification Test (AUDIT) was developed by the World Health Organization (WHO) to identify risky or harmful alcohol consumption, and alcohol use disorder (AUD; Saunders et al., 1993 ). The self-report questionnaire consists of 10 items. Each item is scored between 0 and 4. The sum score ranges between 0 and 40 points, with scores above the cut-off of 15 reflecting hazardous alcohol consumption ( Conigrave et al., 1995 ). We used the German version of the AUDIT showing good psychometric properties in a German general practice population sample ( Dybek et al., 2006 ). The internal consistency was adequate, α=0.68, in the present sample.
Internet Use Disorder (AICA-S)
The Assessment of Internet and Computer Game Addiction Scale (AICA-S, Wölfling et al., 2016 ) was used to measure problematic and pathological internet use. The AICA-S is a self-report questionnaire, consisting of 17 items, of which 14 are relevant for the clinical classification of pathological internet use based on the DSM-5-criteria for SUD. Scores of 13 or greater indicate internet use disorder (IUD). Reliability (internal consistency of α=0.89) and validity of the AICA-S are determined in the general population ( Müller et al., 2014 ).
Psychological Distress (BSI-18)
The German short version of the Brief Symptom Inventory (BSI-18, Derogatis and Fitzpatrick, 2004 ) was used to assess psychological distress. The self-report questionnaire consists of 18 items which are rated on a five-point Likert scale ranging from not at all (0) to always (4). The total score indicates general distress (global severity index, GSI) and ranges between 0 and 72 points. The German translation of the scale showed good psychometric qualities in a sample of undergraduate students, non-clinical subjects and psychosomatic outpatients ( Spitzer et al., 2011 ). The BSI-18 showed an excellent internal consistency, α=0.85, in the present sample.
Impulsivity (BIS-15)
To assess impulsivity as a personality trait, the short version of the German Barratt Impulsiveness Scale (BIS-15, Meule et al., 2011 ) was used. The self-report questionnaire consists of 15 items, which are scored on a four-point scale from seldom/never (1) to almost always/always (4). The overall sum score of impulsivity ranges between 15 and 60, with higher scores indicating higher degrees of impulsivity. In our study, the overall impulsivity trait was analysed. The internal consistency was good, α=0.80, in the present sample.
Impulsive Eating Behaviour (FEV)
The subscale interference of the Eating Behaviour Questionnaire (FEV, Pudel and Westenhöfer, 1989 ) was used to assess impulsive eating behaviour. The FEV_In consists of 16 items, each answered with either true (1) or not true (2). Three items are inverted (8, 10, and 12). The internal consistency was adequate, α=0.71, in the present sample.
Food Related Inhibitory Control (FRIS)
The Food Related Inhibitory Control Scale (FRIS, C. Seiferth and colleagues, not yet published) is a newly developed questionnaire, currently revised and validated, which consists of 40 items, each of it answered on a scale from strongly disagree (0) to strongly agree (5). The total score ranges between 0 and 200, with higher scores indicating increased food related inhibitory control. Preliminary validation proposes a four-factor solution with the subscales Action Withdrawal (AW, e.g., item: “I eat unconsciously between the meals,” Cronbach’s α=0.82), Action Cancellation (AC, e.g., item: “Once I started eating, I cannot stop anymore.,” Cronbach’s α=0.86), Reward Sensitivity (RS, e.g., item: “I reward myself with eating,” Cronbach’s α=0.69) and Delay Discounting (e.g., item: “If I get hungry, I chose rapidly available food,” Cronbach’s α=0.72).
Emotional Eating Behaviour (DEBQ)
The subscale emotional eating of the German translation of the Dutch Eating Behaviour Questionnaire (DEBQ, Van Strien et al., 1986 ) was used to measure emotional eating behaviour. The original self-report questionnaire consists of 30 items. The emotional eating subscale (DEBQ_EE) consists of 10 items, all answered on a five-point Likert scale ranging from never (1) to very often (5). The subscale score ranges between 10 and 50 points. Good psychometric properties of the German version of the scale have been demonstrated by Nagl and colleagues ( Nagl et al., 2016 ). The internal consistency was excellent, α=0.92, in the present sample.
Weight Bias Internalization (WBI)
The German version of the Weight Bias Internalization Scale (WBIS, Hilbert et al., 2014 ) was used to measure internalized negative stereotypes and prejudice regarding overweight. The WBIS consists of 11 items which are rated on a seven-point scale ranging from strongly disagree (1) to strongly agree (7). The total score ranges between 11 and 77. The German version showed good psychometric properties ( Hilbert et al., 2014 ). The internal consistency was good, α=0.86, in the present sample.
Self-Efficacy (SWE)
To measure self-efficacy, the General Self-Efficacy Scale (SWE, Jerusalem and Schwarzer, 2003 ) was used. The self-report questionnaire consists of 10 items, each of it answered on a four-point scale ranging from not at all true (1) to exactly true (4), yielding a total score between 10 and 40 points. The SWE showed good psychometric properties in a representative German sample ( Hinz et al., 2006 ). The internal consistency was good, α=0.88, in the present sample.
Statistical Analysis
All analyses were conducted with IBM SPSS statistics for windows (Version 26.0, Armonk, NY: IBM Corp.) and Microsoft Excel (Version 16.0, Microsoft Corporation). Descriptive analyses were conducted using percentages and frequencies for categorical variables, as well as means and standard deviations for continuous variables. Chi-square distributions that compared categorical variables between groups (FA vs. NFA) were implemented as well as Bonferroni-adjusted independent t -tests to compare metrically scaled variables. Associations between metrically scaled variables were analysed using Pearson correlations .
The study was carried out in accordance with the Declaration of Helsinki. The Institutional Review Board of the Ruhr-University Bochum approved the study (Nr. 18-6415). All subjects were informed about the study and all provided written informed consent.
Prevalence of Food Addiction
The prevalence of Food Addiction was 15% ( N =32), with higher, although not statistically significant, prevalence rates in female (18.2%, N =26) compared to male (8.6%, N =6) participants.
Table 2 gives an overview about sociodemographic factors both of the total sample and the subsamples of individuals experiencing food addiction (FA) or not experiencing FA (NFA). No differences between the subsamples (FA vs. NFA) regarding gender, age, BMI, marital status and education could be found.
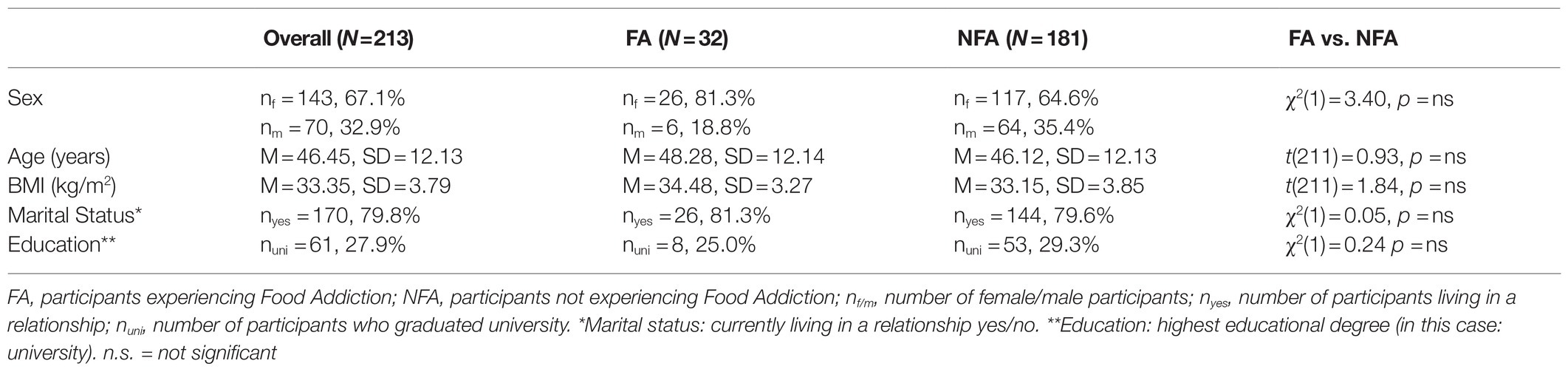
Table 2 . Sociodemographic factors (FA vs. NFA).
Food Addiction, Psychopathology and Weight- and Addiction-Related Constructs
Correlation analyses revealed a significant positive association ( r =0.17, p =0.014) between symptom severity of FA and BMI at baseline assessment ( Table 3 ). With regard to psychopathology, FA symptom severity was positively correlated to severity of depressive symptoms ( r =0.33, p <0.001), severity of IUD ( r =0.18, p =0.011) and psychological distress ( r =0.35, p <0.001). An impulsive ( r =0.48, p <0.001) and emotional eating behaviour ( r =0.36, p <0.001) as well as WBI ( r =0.44, p <0.001) was positively associated with FA. There were negative correlations between FA symptom severity and the subscales action withdrawal ( r =−49, p <0.001), action cancellation ( r =−0.41, p <0.001), reward sensitivity ( r =−0.46, p <0.001) and delay discounting ( r =−0.27, p <0.001) of the FRIS. A decrease in self-efficacy was also associated with FA symptom severity ( r =−0.21, p =0.002).
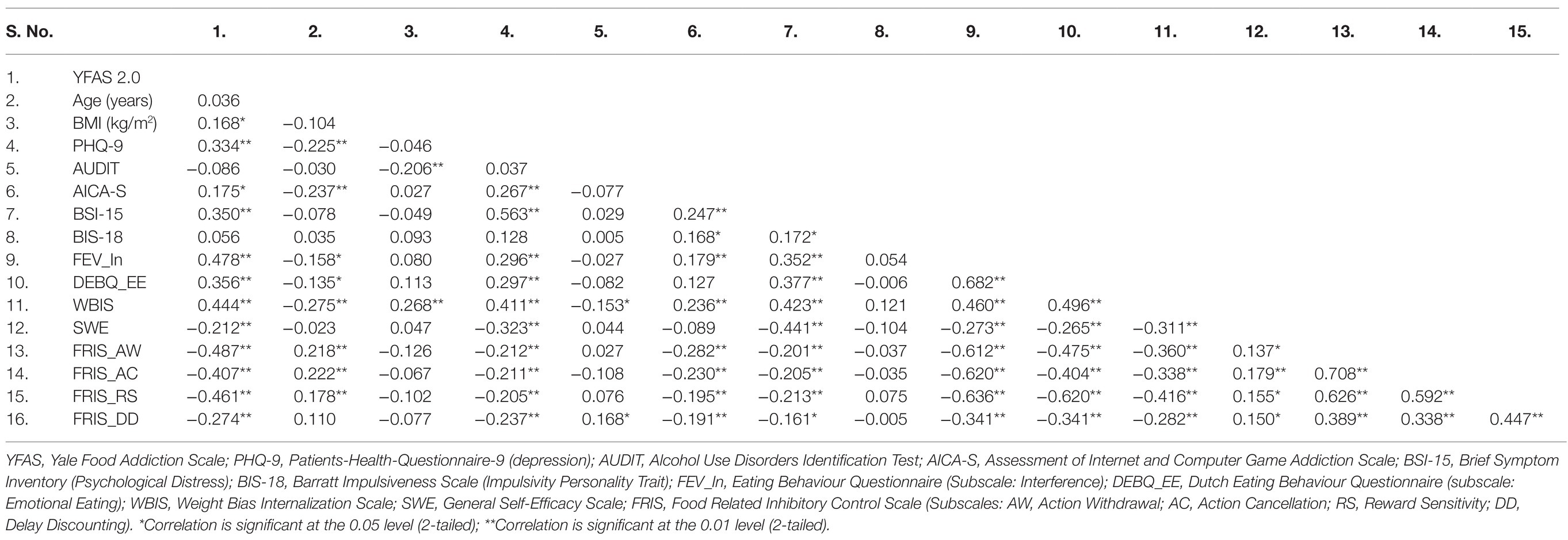
Table 3 . Pearson correlations among psychological test scores ( N =213).
Table 4 illustrates results from independent t-Tests between the subsamples (FA vs. NFA) on psychosocial measurements and psychopathology. FA participants showed significantly higher FA symptom severity (YFAS 2.0) than NFA participants did. Additionally, the GSI (BSI-15) was higher for FA compared to NFA participants, indicating higher psychological distress. Comparisons of psychosocial measurements associated with eating behaviour indicated that FA participants suffer from a more impulsive (FEV_In) and emotional eating behaviour (DEBQ_EE), as well as weight bias internalization (WBIS). Moreover, FA participants scored less on three of four FRIS subscales (AW, AC and RS), indicating impairments in behavioural inhibitory control and increased reward sensitivity. The groups did not differ on the subscale DD, reflecting impulsive choices.
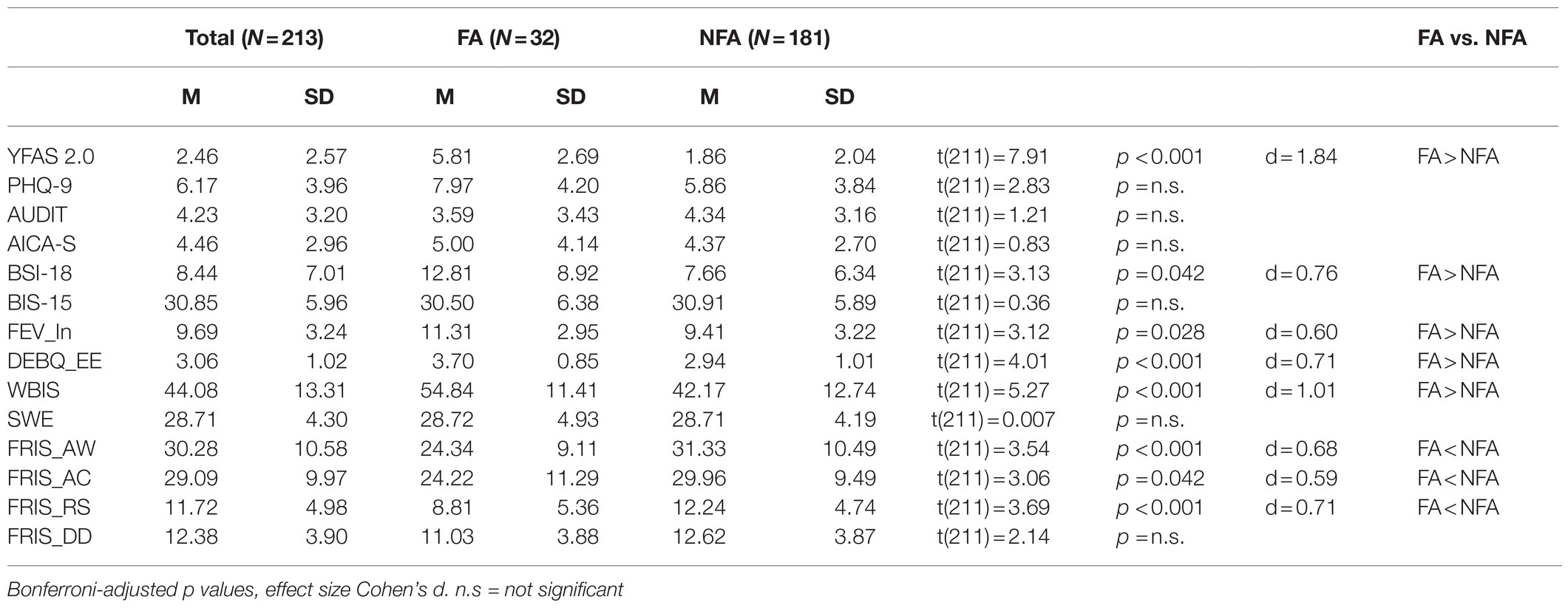
Table 4 . Psychopathology and weight- and addiction-related constructs (FA vs. NFA).
The main aim of the present study was to assess the prevalence of FA in a treatment-seeking sample of individuals with overweight or obesity and without comorbid BN or BED as previous studies did not control for these comorbid disorders. The prevalence of FA in our sample was 15%, with higher, although not statistically significant, rates in female (18.2%) compared to male participants (8.6%) as expected ( Pursey et al., 2014 ). Notably, the higher prevalence rates in female participants might also be explained by a general tendency of women to over-report health problems compared to men ( Boerma et al., 2016 ). Our findings fit the assumption that the prevalence of FA is higher in individuals with overweight and obesity (17.2%) compared to the general population (7.9%; Pursey et al., 2014 ; Hauck et al., 2017 ). Previous studies analysing FA in WLP samples, in which participants suffering from BED and BN were not excluded from participation, reported prevalence rates ranging between 15 and 38% ( Eichen et al., 2013 ; Meule et al., 2015 ). The lower prevalence rate in our study might therefore be due to the fact, that individuals suffering from BN or BED were excluded from participation. Still, since 15% of our sample experienced FA, we thus conclude that, even when adjusting for comorbid eating disorders, a relevant subgroup of WLP participants experiences an addiction-like eating behaviour.
FA severity was associated with increased BMI, which is in line with findings of previous studies ( Pedram et al., 2013 ; Gearhardt et al., 2014 ). A variety of studies reports associations between FA and SUDs ( Davis and Carter, 2009 ; García-García et al., 2014 ; Tinghino et al., 2020 ). In our study, AUD severity was not associated with FA. This might be because hazardous alcohol consumption was an exclusion criterion for participation, but the missing correlation could also be masked by the inverse relationship between AUD and BMI. However, in line with previous studies, FA was associated with severity of depressive symptoms and psychological distress ( Gearhardt et al., 2012 ; Burmeister et al., 2013 ). Although participants meeting the diagnostic criteria for major depression and AUD were excluded from participation, our results underline the strong relationship between FA symptomatology and mood disorders, and enlighten the high level of distress in individuals experiencing FA, which together might contribute to weight gain ( Bourdier et al., 2018 ). FA was linked to IUD severity. IUD is a behavioural addiction characterized by excessive internet usage and an impaired control over usage behaviour ( Young, 1996 ). Excessive internet usage is associated with unhealthy eating and sedentary behaviour and might subsequently aggravate FA symptomatology and contribute to weight gain ( Vandelanotte et al., 2009 ; Yildirim et al., 2018 ).
Moreover, FA was linked to emotional eating and WBI and both were significantly higher in FA compared to NFA participants. There seems to be a shift from positive reinforcement to negative reinforcement in individuals experiencing FA regarding the consumption of palatable food ( Parylak et al., 2011 ). Given that palatable food can be both rewarding and stress reducing, food deprivation may lead to withdrawal (i.e., stress enhancement) and a subsequent shift from gratification to compulsive eating. In line with previous studies, self-efficacy was negatively correlated to FA symptom severity in our sample ( Burmeister et al., 2013 ; Cassin et al., 2019 ). Low self-efficacy and high WBI are linked to lower physical activity and WBI predicts reduced odds of achieving weight-loss in individuals with overweight and obesity ( Hübner et al., 2015 ; Pearl et al., 2019 ). Therefore, the reported associations between FA, WBI and self-efficacy fits prior observations that individuals experiencing FA suffer from weight cycling ( Gearhardt et al., 2014 ). It is assumed that the relationship between WBI and FA is moderated by emotional dysregulation, which should be further investigated ( Baldofski et al., 2016 ). Future studies might also consider assessing weight control or eating self-efficacy instead of global self-efficacy ( Linde et al., 2004 ; Cassin et al., 2019 ).
FA was associated with an impulsive eating behaviour and deficits in food-related inhibitory control. FA participants significantly differed from NFA participants in food-related impulsive actions (action withdrawal and cancelation), as well as reward sensitivity. This indicates that individuals experiencing FA have more problems in resisting impulses towards disposable and rewarding food and might therefore need specific training elements to enhance weight loss ( Meule, 2019a ). Delay discounting was associated with FA symptom severity, but the subgroup of FA participants did not significantly differ from NFA participants on this subscale. This might be due to the fact that delay discounting is generally increased in individuals with obesity ( Mole et al., 2015 ). Interestingly, FA was not associated with the impulsivity personality trait. This is in contrast to prior studies indicating that FA, as well as other SUDs and EDs are associated with higher impulsivity traits ( Mobbs et al., 2010 ; Churchill and Jessop, 2011 ; Murphy et al., 2014 ; VanderBroek-Stice et al., 2017 ). Since BN, BES and AUD were exclusion criteria in our study, it might be possible, that overlapping symptoms with this disorders confounded prior results. Based on our results, we hypothesize that not a global disposition towards impulsive behaviour, but rather a learning process that food is rewarding and disposable, may contribute to the impulsive eating behaviour in FA participants. In our study, impulsivity personality trait, impulsive eating behaviour and food-related inhibitory control were measured using self-reporting questionnaires. Since self-reported and behavioural results regarding disinhibition of eating behaviour in individuals with obesity can differ, the results of our study should be further analysed using behavioural tasks ( Loeber et al., 2012 ). Furthermore, potential moderating factors like restrained eating or current mood should be considered ( Loeber et al., 2018 ).
A particular strength of our study is that we analysed FA in a sample of individuals with overweight and obesity who did not suffer from eating disorders, which was verified by questionnaire and structured interview ( two stage design ). However, when interpreting the findings of the present study a few limitations should be acknowledged. Individuals with obesity class III (BMI>39.9kg/m 2 ) were excluded from participation due to the study design of the WLP. It can be assumed that the prevalence of FA would have been higher when including these participants, since FA is reported to be increased in individuals experiencing extreme obesity (ca. 30%; Hauck et al., 2017 ). In addition, since AUD and major depression were exclusion criteria, the reported associations between FA, AUD severity and severity of depressive symptoms might be confounded. Since the risk for FA increases in polyabusers, the exclusion of individuals suffering from hazardous alcohol consumption might subsequently cause a lower prevalence of FA in our study ( Tinghino et al., 2020 ). Finally, a broad test battery, i.e., with regard to different components of impulsivity and impulsive eating behaviour, was used. Still, our conclusions are based on results from self-reporting questionnaires and different results may be observed when using behavioural measurements as for example reported by Loeber et al. (2018) . In addition, typical pre-test self-report biases are known, which might have further influenced our results ( Aiken and West, 1990 ). Yet, despite its limitations, the YFAS is currently the “state-of-the-art” assessment of FA ( Schulte et al., 2020 ). With a view to a potential inclusion of FA in the diagnostic catalogues, it would make sense to develop and use objective diagnostic assessment tools, like structured interviews ( Gearhardt and Hebebrand, 2021 ; Hebebrand and Gearhardt, 2021 ). The interpretation of our results is aggravated due to our cross-sectional study design, i.e., with regard to causal analyses. It would subsequently be meaningful to verify the results in longitudinal studies, i.e., with regard to long-term weight management in individuals experiencing FA.
Summing up, our results support the view that, even when adjusting for BN and BED, a relevant subgroup of individuals with overweight and obesity experiences an addiction-like eating behaviour. This subgroup differs from non-addictive eaters on several weight- and addiction-related factors, like emotional eating, WBI, and impulsivity. Moreover, individuals experiencing FA suffer from depressive symptoms, addictive disorders and psychological distress. In sum, these impairments may contribute to weight gain. If so, our results underline that the lack of an officially approved FA diagnosis might currently cause an insufficient clinical care for individuals experiencing an addiction-like eating behaviour. It might therefore be reasonable to investigate the effect of FA on weight loss when adjusting for eating disorders and to further implicate addiction-specific therapeutic elements in WLPs to enhance weight loss and prevent weight regain in this subgroup.
Data Availability Statement
The datasets presented in this article are not readily available because data will be made available only on reasonable request. Requests to access the datasets should be directed to [email protected].
Ethics Statement
The studies involving human participants were reviewed and approved by The Institutional Review Board of the Ruhr-University Bochum (Nr. 18-6415). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MP: conceptualisation, acquisition of data, formal analysis, interpretation of data, and writing – original draft. SH: conceptualisation, writing- original draft, and study supervision. SS and CS: study design, acquisition of data, and review and editing. TF: acquisition of data and review and editing. JW: study design, study supervision, and review and editing. SS-L: study design and conceptualisation, study supervision, and review and editing. All authors contributed to the article and approved the submitted version.
The Federal Ministry of Education and Research, Germany (BMBF) funded the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.

Acknowledgments
The authors acknowledge support by the Open Access Publication Fund of the University of Bamberg. We acknowledge Tanja Roth, Sophia Everding, and Hannah Birk for research assistance, as well as Hans Maximilian Henrich for language proofreading.
Aiken, L. S., and West, S. G. (1990). Invalidity of true experiments: self-report pretest biases. Eval. Rev. 14, 374–390. doi: 10.1177/0193841X9001400403
CrossRef Full Text | Google Scholar
Avena, N. M., Bocarsly, M. E., Rada, P., Kim, A., and Hoebel, B. G. (2008). After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol. Behav. 94, 309–315. doi: 10.1016/j.physbeh.2008.01.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Baldofski, S., Rudolph, A., Tigges, W., Herbig, B., Jurowich, C., Kaiser, S., et al. (2016). Weight bias internalization, emotion dysregulation, and non-normative eating behaviors in prebariatric patients. Int. J. Eat. Disord. 49, 180–185. doi: 10.1002/eat.22484
Bari, A., and Robbins, T. W. (2013). Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 108, 44–79. doi: 10.1016/j.pneurobio.2013.06.005
Beitscher-Campbell, H., Blum, K., Febo, M., Madigan, M. A., Giordano, J., Badgaiyan, R. D., et al. (2016). Pilot clinical observations between food and drug seeking derived from fifty cases attending an eating disorder clinic. J. Behav. Addict. 5, 533–541. doi: 10.1556/2006.5.2016.055
Blaine, B., and Rodman, J. (2007). Responses to weight loss treatment among obese individuals with and without BED: A matched-study meta-analysis. Eat. Weight Disord. 12, 54–60. doi: 10.1007/BF03327579
Boerma, T., Hosseinpoor, A. R., Verdes, E., and Chatterji, S. (2016). A global assessment of the gender gap in self-reported health with survey data from 59 countries. BMC Public Health 16:675. doi: 10.1186/s12889-016-3352-y
Bourdier, L., Orri, M., Carre, A., Gearhardt, A. N., Romo, L., Dantzer, C., et al. (2018). Are emotionally driven and addictive-like eating behaviors the missing links between psychological distress and greater body weight? Appetite 120, 536–546. doi: 10.1016/j.appet.2017.10.013
Burger, K. S., and Stice, E. (2012). Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream–based milkshake. Am. J. Clin. Nutr. 95, 810–817. doi: 10.3945/ajcn.111.027003
Burmeister, J. M., Hinman, N., Koball, A., Hoffmann, D. A., and Carels, R. A. (2013). Food addiction in adults seeking weight loss treatment. Implications for psychosocial health and weight loss. Appetite 60, 103–110. doi: 10.1016/j.appet.2012.09.013
Burrows, T., Kay-Lambkin, F., Pursey, K., Skinner, J., and Dayas, C. (2018). Food addiction and associations with mental health symptoms: a systematic review with meta-analysis. J. Hum. Nutr. Diet. 31, 544–572. doi: 10.1111/jhn.12532
Burrows, T., Skinner, J., McKenna, R., and Rollo, M. (2017). Food addiction, binge eating disorder, and obesity: is there a relationship? Behav. Sci. 7:54. doi: 10.3390/bs7030054
Cassin, S. E., Buchman, D. Z., Leung, S. E., Kantarovich, K., Hawa, A., Carter, A., et al. (2019). Ethical, stigma, and policy implications of food addiction: a scoping review. Nutrients 11:710. doi: 10.3390/nu11040710
Chalk, M. B. (2004). Obesity: addressing a multifactorial disease. Case Manager 15, 47–49. doi: 10.1016/j.casemgr.2004.09.001
Chao, A. M., Wadden, T. A., Tronieri, J. S., Pearl, R. L., Alamuddin, N., Bakizada, Z. M., et al. (2019). Effects of addictive-like eating behaviors on weight loss with behavioral obesity treatment. J. Behav. Med. 42, 246–255. doi: 10.1007/s10865-018-9958-z
Chu, D.-T., Nguyet, N. T. M., Nga, V. T., Lien, N. V. T., Vo, D. D., Lien, N., et al. (2019). An update on obesity: mental consequences and psychological interventions. Diabetes Metab. Syndr. Clin. Res. Rev. 13, 155–160. doi: 10.1016/j.dsx.2018.07.015
Churchill, S., and Jessop, D. C. (2011). Reflective and non-reflective antecedents of health-related behaviour: exploring the relative contributions of impulsivity and implicit self-control to the prediction of dietary behaviour. Br. J. Health Psychol. 16, 257–272. doi: 10.1348/135910710X498688
Clark, S. M., and Saules, K. K. (2013). Validation of the Yale Food Addiction Scale among a weight-loss surgery population. Eat. Behav. 14, 216–219. doi: 10.1016/j.eatbeh.2013.01.002
Conigrave, K. M., Hall, W. D., and Saunders, J. B. (1995). The AUDIT questionnaire: choosing a cut-off score. Alcohol use disorder identification test. Addiction 90, 1349–1356. doi: 10.1111/j.1360-0443.1995.tb03552.x
Davis, C. (2015). The epidemiology and genetics of binge eating disorder (BED). CNS Spectr. 20, 522–529. doi: 10.1017/S1092852915000462
Davis, C., and Carter, J. C. (2009). Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite 53, 1–8. doi: 10.1016/j.appet.2009.05.018
Davis, C., Loxton, N. J., Levitan, R. D., Kaplan, A. S., Carter, J. C., and Kennedy, J. L. (2013). ‘Food addiction’and its association with a dopaminergic multilocus genetic profile. Physiol. Behav. 118, 63–69. doi: 10.1016/j.physbeh.2013.05.014
De Vries, S.-K., and Meule, A. (2016). Food addiction and bulimia nervosa: new data based on the Yale Food Addiction Scale 2.0. Eur. Eat. Disord. Rev. 24, 518–522. doi: 10.1002/erv.2470
Derogatis, L. R., and Fitzpatrick, M. (2004). “The SCL-90-R, the brief symptom inventory (BSI), and the BSI-18,” in The Use of Psychological Testing for Treatment Planning and Outcomes Assessment: Instruments for Adults. ed. M. E. Maruish (Lawrence Erlbaum Associates Publishers), 1–41.
Google Scholar
Dybek, I., Bischof, G., Grothues, J., Reinhardt, S., Meyer, C., Hapke, U., et al. (2006). The reliability and validity of the alcohol use disorders identification test (AUDIT) in a German general practice population sample. J. Stud. Alcohol 67, 473–481. doi: 10.15288/jsa.2006.67.473
Eichen, D. M., Lent, M. R., Goldbacher, E., and Foster, G. D. (2013). Exploration of “food addiction” in overweight and obese treatment-seeking adults. Appetite 67, 22–24. doi: 10.1016/j.appet.2013.03.008
Fichter, M. M., Quadflieg, N., Gierk, B., Voderholzer, U., and Heuser, J. (2015). The Munich eating and feeding disorder questionnaire (Munich ED-quest) DSM-5/ICD-10: validity, reliability, sensitivity to change and norms. Eur. Eat. Disord. Rev. 23, 229–240. doi: 10.1002/erv.2348
Fielding-Singh, P., Patel, M. L., King, A. C., and Gardner, C. D. (2019). Baseline psychosocial and demographic factors associated with study attrition and 12-month weight gain in the DIETFITS trial. Obesity 27, 1997–2004. doi: 10.1002/oby.22650
García-García, I., Horstmann, A., Jurado, M. A., Garolera, M., Chaudhry, S. J., Margulies, D. S., et al. (2014). Reward processing in obesity, substance addiction and non-substance addiction. Obes. Rev. 15, 853–869. doi: 10.1111/obr.12221
Gearhardt, A. N., Boswell, R. G., and White, M. A. (2014). The association of “food addiction” with disordered eating and body mass index. Eat. Behav. 15, 427–433. doi: 10.1016/j.eatbeh.2014.05.001
Gearhardt, A. N., Corbin, W. R., and Brownell, K. D. (2009). Preliminary validation of the Yale food addiction scale. Appetite 52, 430–436. doi: 10.1016/j.appet.2008.12.003
Gearhardt, A. N., Corbin, W. R., and Brownell, K. D. (2016). Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. 30:113. doi: 10.1037/adb0000136
Gearhardt, A. N., and Hebebrand, J. (2021). The concept of “food addiction” helps inform the understanding of overeating and obesity: YES. Am. J. Clin. Nutr. 113, 263–267. doi: 10.1093/ajcn/nqaa343
Gearhardt, A. N., White, M. A., Masheb, R. M., and Grilo, C. M. (2013). An examination of food addiction in a racially diverse sample of obese patients with binge eating disorder in primary care settings. Compr. Psychiatry 54, 500–505. doi: 10.1016/j.comppsych.2012.12.009
Gearhardt, A. N., White, M. A., Masheb, R. M., Morgan, P. T., Crosby, R. D., and Grilo, C. M. (2012). An examination of the food addiction construct in obese patients with binge eating disorder. Int. J. Eat. Disord. 45, 657–663. doi: 10.1002/eat.20957
Gearhardt, A. N., Yokum, S., Orr, P. T., Stice, E., Corbin, W. R., and Brownell, K. D. (2011). Neural correlates of food addiction. Arch. Gen. Psychiatry 68, 808–816. doi: 10.1001/archgenpsychiatry.2011.32
Grosshans, M., Vollmert, C., Vollstädt-Klein, S., Tost, H., Leber, S., Bach, P., et al. (2012). Association of leptin with food cue–induced activation in human reward pathways. Arch. Gen. Psychiatry 69, 529–537. doi: 10.1001/archgenpsychiatry.2011.1586
Hauck, C., Weiß, A., Schulte, E. M., Meule, A., and Ellrott, T. (2017). Prevalence of ‘food addiction’ as measured with the Yale Food Addiction Scale 2.0 in a representative German sample and its association with sex, age and weight categories. Obes. Facts 10, 12–24. doi: 10.1159/000456013
Hebebrand, J., and Gearhardt, A. N. (2021). The concept of “food addiction” helps inform the understanding of overeating and obesity: NO. Am. J. Clin. Nutr. 113, 268–273. doi: 10.1093/ajcn/nqaa344
Herpertz, S., Burgmer, R., Stang, A., de Zwaan, M., Wolf, A. M., Chen-Stute, A., et al. (2006). Prevalence of mental disorders in normal-weight and obese individuals with and without weight loss treatment in a German urban population. J. Psychosom. Res. 61, 95–103. doi: 10.1016/j.jpsychores.2005.10.003
Hilbert, A., Baldofski, S., Zenger, M., Löwe, B., Kersting, A., and Braehler, E. (2014). Weight bias internalization scale: psychometric properties and population norms. PLoS One 9:e86303. doi: 10.1371/journal.pone.0086303
Hilbert, A., and Tuschen-Caffier, B. (2016). Eating Disorder Examination-Questionnaire: Deutschsprachige Übersetzung [Eating Disorder Examination-Questionnaire: German version]. Dgvt-Verlag: Tübingen, Germany.
Hinz, A., Schumacher, J., Albani, C., Schmid, G., and Brähler, E. (2006). Bevölkerungsrepräsentative Normierung der Skala zur Allgemeinen Selbstwirksamkeitserwartung. Diagnostica 52, 26–32. doi: 10.1026/0012-1924.52.1.26
Hofmann, W., Friese, M., and Strack, F. (2009). Impulse and self-control from a dual-systems perspective. Perspect. Psychol. Sci. 4, 162–176. doi: 10.1111/j.1745-6924.2009.01116.x
Hübner, C., Baldofski, S., Zenger, M., Tigges, W., Herbig, B., Jurowich, C., et al. (2015). Influences of general self-efficacy and weight bias internalization on physical activity in bariatric surgery candidates. Surg. Obes. Relat. Dis. 11, 1371–1376. doi: 10.1016/j.soard.2014.11.013
Jeffery, R. W., Epstein, L. H., Wilson, G. T., Drewnowski, A., Stunkard, A. J., and Wing, R. R. (2000). Long-term maintenance of weight loss: current status. Health Psychol. 19:5. doi: 10.1037/0278-6133.19.Suppl1.5
Jensen, M. D., and Ryan, D. H. (2014). New obesity guidelines: promise and potential. JAMA 311, 23–24. doi: 10.1001/jama.2013.282546
Jerusalem, M., and Schwarzer, R. (2003). SWE-Skala zur Allgemeinen Selbstwirksamkeitserwartung. Psychology. doi: 10.23668/psycharchives.307
Kroenke, K., Spitzer, R. L., and Williams, J. B. (2001). The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613. doi: 10.1046/j.1525-1497.2001.016009606.x
Lent, M. R., Eichen, D. M., Goldbacher, E., Wadden, T. A., and Foster, G. D. (2014). Relationship of food addiction to weight loss and attrition during obesity treatment. Obesity 22, 52–55. doi: 10.1002/oby.20512
Linde, J. A., Jeffery, R. W., Levy, R. L., Sherwood, N. E., Utter, J., Pronk, N. P., et al. (2004). Binge eating disorder, weight control self-efficacy, and depression in overweight men and women. Int. J. Obes. 28, 418–425. doi: 10.1038/sj.ijo.0802570
Loeber, S., Grosshans, M., Korucuoglu, O., Vollmert, C., Vollstädt-Klein, S., Schneider, S., et al. (2012). Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int. J. Obes. 36, 1334–1339. doi: 10.1038/ijo.2011.184
Loeber, S., Rustemeier, M., Paslakis, G., Pietrowsky, R., Müller, A., and Herpertz, S. (2018). Mood and restrained eating moderate food-associated response inhibition in obese individuals with binge eating disorder. Psychiatry Res. 264, 346–353. doi: 10.1016/j.psychres.2018.03.081
Löwe, B., Spitzer, R. L., Zipfel, S., and Herzog, W. (2002). Gesundheitsfragebogen für Patienten (PHQ-D). Komplettversion und Kurzform. Karlsruhe: Pfizer GmbH.
Marmet, S., Studer, J., Wicki, M., Bertholet, N., Khazaal, Y., and Gmel, G. (2019). Unique versus shared associations between self-reported behavioral addictions and substance use disorders and mental health problems: a commonality analysis in a large sample of young Swiss men. J. Behav. Addict. 8, 664–677. doi: 10.1556/2006.8.2019.70
Meule, A. (2015). Focus: addiction: back by popular demand: a narrative review on the history of food addiction research. Yale J. Biol. Med. 88:295
PubMed Abstract | Google Scholar
Meule, A. (2017). Verlangen nach Süßem: Eine Evaluation der Suchtperspektive auf Zucker- und Süßstoffkonsum. Pädiatr. Pädol. 52, 156–161. doi: 10.1007/s00608-017-0489-6
Meule, A. (2019a). A critical examination of the practical implications derived from the food addiction concept. Curr. Obes. Rep. 8, 11–17. doi: 10.1007/s13679-019-0326-2
Meule, A. (2019b). “An addiction perspective on eating disorders and obesity,” in Eating Disorders and Obesity in Children and Adolescents. eds. J. Hebebrand and B. Herpertz-Dahlmann (Amsterdam: Elsevier), 99–104.
Meule, A., Hermann, T., and Kübler, A. (2015). Food addiction in overweight and obese adolescents seeking weight-loss treatment. Eur. Eat. Disord. Rev. 23, 193–198. doi: 10.1002/erv.2355
Meule, A., Müller, A., Gearhardt, A. N., and Blechert, J. (2017). German version of the Yale food addiction scale 2.0: prevalence and correlates of ‘food addiction’ in students and obese individuals. Appetite 115, 54–61. doi: 10.1016/j.appet.2016.10.003
Meule, A., Vögele, C., and Kübler, A. (2011). Psychometrische Evaluation der deutschen Barratt Impulsiveness Scale–Kurzversion (BIS-15). Diagnostica 57, 126–133. doi: 10.1026/0012-1924/a000042
Meule, A., von Rezori, V., and Blechert, J. (2014). Food addiction and bulimia nervosa. Eur. Eat. Disord. Rev. 22, 331–337. doi: 10.1002/erv.2306
Minhas, M., Murphy, C. M., Balodis, I. M., Samokhvalov, A. V., and MacKillop, J. (2021). Food addiction in a large community sample of Canadian adults: prevalence and relationship with obesity, body composition, quality of life and impulsivity. Addiction doi: 10.1111/add.15446 [Epub ahead of print].
Mobbs, O., Crépin, C., Thiéry, C., Golay, A., and Van der Linden, M. (2010). Obesity and the four facets of impulsivity. Patient Educ. Couns. 79, 372–377. doi: 10.1016/j.pec.2010.03.003
Mole, T. B., Irvine, M. A., Worbe, Y., Collins, P., Mitchell, S. P., Bolton, S., et al. (2015). Impulsivity in disorders of food and drug misuse. Psychol. Med. 45, 771–782. doi: 10.1017/S0033291714001834
Müller, K. W., Glaesmer, H., Brähler, E., Woelfling, K., and Beutel, M. E. (2014). Prevalence of internet addiction in the general population: results from a German population-based survey. Behav. Inform. Technol. 33, 757–766. doi: 10.1080/0144929X.2013.810778
Murphy, C. M., Stojek, M. K., and MacKillop, J. (2014). Interrelationships among impulsive personality traits, food addiction, and body mass index. Appetite 73, 45–50. doi: 10.1016/j.appet.2013.10.008
Nagl, M., Hilbert, A., de Zwaan, M., Braehler, E., and Kersting, A. (2016). The German version of the Dutch eating behavior questionnaire: psychometric properties, measurement invariance, and population-based norms. PLoS One 11:e0162510. doi: 10.1371/journal.pone.0162510
Naish, K. R., MacKillop, J., and Balodis, I. M. (2018). The concept of food addiction: A review of the current evidence. Curr. Behav. Neurosci. Rep. 5, 281–294. doi: 10.1007/s40473-018-0169-2
Novelle, M. G., and Diéguez, C. (2018). Food addiction and binge eating: lessons learned from animal models. Nutrients 10, 71. doi: 10.3390/nu10010071
Parylak, S. L., Koob, G. F., and Zorrilla, E. P. (2011). The dark side of food addiction. Physiol. Behav. 104, 149–156. doi: 10.1016/j.physbeh.2011.04.063
Pearl, R. L., Wadden, T. A., Chao, A. M., Walsh, O., Alamuddin, N., Berkowitz, R. I., et al. (2019). Weight bias internalization and long-term weight loss in patients with obesity. Ann. Behav. Med. 53, 782–787. doi: 10.1093/abm/kay084
Pedram, P., Wadden, D., Amini, P., Gulliver, W., Randell, E., Cahill, F., et al. (2013). Food addiction: its prevalence and significant association with obesity in the general population. PLoS One 8:e74832. doi: 10.1371/journal.pone.0074832
Preuss, H., Leister, L., Pinnow, M., and Legenbauer, T. (2019). Inhibitory control pathway to disinhibited eating: a matter of perspective? Appetite 141:104297. doi: 10.1016/j.appet.2019.05.028
Pudel, V., and Westenhöfer, J. (1989). Fragebogen zum Eßverhalten (FEV) Hogrefe. Göttingen, Germany.
Puhl, R. M., and Heuer, C. A. (2009). The stigma of obesity: a review and update. Obesity 17, 941–964. doi: 10.1038/oby.2008.636
Pursey, K. M., Stanwell, P., Gearhardt, A. N., Collins, C. E., and Burrows, T. L. (2014). The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients 6, 4552–4590. doi: 10.3390/nu6104552
Ross Middleton, K. M., Patidar, S. M., and Perri, M. G. (2012). The impact of extended care on the long-term maintenance of weight loss: a systematic review and meta-analysis. Obes. Rev. 13, 509–517. doi: 10.1111/j.1467-789X.2011.00972.x
Saunders, J. B., Aasland, O. G., Babor, T. F., De la Fuente, J. R., and Grant, M. (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88, 791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x
Schulte, E. M., Wadden, T. A., and Allison, K. C. (2020). An evaluation of food addiction as a distinct psychiatric disorder. Int. J. Eat. Disord. 53, 1610–1622. doi: 10.1002/eat.23350
Spitzer, C., Hammer, S., Löwe, B., Grabe, H. J., Barnow, S., Rose, M., et al. (2011). Die Kurzform des Brief Symptom Inventory (BSI-18): Erste Befunde zu den psychometrischen Kennwerten der deutschen Version. Fortschr. Neurol. Psychiatr. Grenzgeb. 79, 517–523. doi: 10.1055/s-0031-1281602
Swinburn, B., Egger, G., and Raza, F. (1999). Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev. Med. 29, 563–570. doi: 10.1006/pmed.1999.0585
Tinghino, B., Lugoboni, F., Amatulli, A., Biasin, C., Araldi, M. B., Cantiero, D., et al. (2020). The FODRAT study (FOod addiction, DRugs, alcohol and tobacco): first data on food addiction prevalence among patients with addiction to drugs, tobacco and alcohol. Eat. Weight Disord. 26, 449–455. doi: 10.1007/s40519-020-00865-z
Traversy, G., and Chaput, J.-P. (2015). Alcohol consumption and obesity: an update. Curr. Obes. Rep. 4, 122–130. doi: 10.1007/s13679-014-0129-4
Van Strien, T., Frijters, J. E., Bergers, G. P., and Defares, P. B. (1986). The Dutch eating behavior questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 5, 295–315. doi: 10.1002/1098-108X(198602)5:2<295::AID-EAT2260050209>3.0.CO;2-T
Vandelanotte, C., Sugiyama, T., Gardiner, P., and Owen, N. (2009). Associations of leisure-time internet and computer use with overweight and obesity, physical activity and sedentary behaviors: cross-sectional study. J. Med. Internet Res. 11:e28. doi: 10.2196/jmir.1084
VanderBroek-Stice, L., Stojek, M. K., Beach, S. R., and MacKillop, J. (2017). Multidimensional assessment of impulsivity in relation to obesity and food addiction. Appetite 112, 59–68. doi: 10.1016/j.appet.2017.01.009
Vidmar, A. P., Wee, C. P., and Salvy, S. J. (2021). Food addiction, executive function and mood in adolescents with obesity seeking treatment. Appetite 159:105049. doi: 10.1016/j.appet.2020.105049
Wölfling, K., Beutel, M. E., and Müller, K. W. (2016). “OSV-S—Skala zum Onlinesuchtverhalten,” in Diagnostische Verfahren in der Psychotherapie (Diagnostik für Klinik und Praxis) (Göttingen, Germany: Hogrefe), 362–366.
Yildirim, M. S., Sevincer, G. M., Kandeger, A., and Afacan, C. (2018). Investigation of the relationship between risk of internet addiction, food addiction, and self-esteem in high school students. Düşünen Adam 31:187. doi: 10.5350/DAJPN2018310206
Young, K. S. (1996). Internet addiction the emergence of a new clinical disorder. CyberPsychol. Behav. 1, 237–244. doi: 10.1089/cpb.1998.1.237
Keywords: food addiction, obesity, overweight, eating disorders, eating behaviour, weight loss treatment
Citation: Pape M, Herpertz S, Schroeder S, Seiferth C, Färber T, Wolstein J and Steins-Loeber S (2021) Food Addiction and Its Relationship to Weight- and Addiction-Related Psychological Parameters in Individuals With Overweight and Obesity. Front. Psychol . 12:736454. doi: 10.3389/fpsyg.2021.736454
Received: 05 July 2021; Accepted: 26 August 2021; Published: 21 September 2021.
Reviewed by:
Copyright © 2021 Pape, Herpertz, Schroeder, Seiferth, Färber, Wolstein and Steins-Loeber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Magdalena Pape, [email protected]
† These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Food Addiction: Its Prevalence and Significant Association with Obesity in the General Population
Affiliation Discipline of Medicine, Faculty of Medicine, Memorial University of Newfoundland, St. John's, Canada
Affiliation Discipline of Laboratory Medicine, Memorial University of Newfoundland, St. John's, Canada
Affiliation Department of Psychology, Faculty of Science, Memorial University of Newfoundland, St. John's, Canada
Affiliation Discipline of Genetics, Faculty of Medicine, Memorial University of Newfoundland, St. John's, Canada
* E-mail: [email protected]
- Pardis Pedram,
- Danny Wadden,
- Peyvand Amini,
- Wayne Gulliver,
- Edward Randell,
- Farrell Cahill,
- Sudesh Vasdev,
- Alan Goodridge,
- Jacqueline C. Carter,

- Published: September 4, 2013
- https://doi.org/10.1371/journal.pone.0074832
- Reader Comments
‘Food addiction’ shares a similar neurobiological and behavioral framework with substance addiction. However whether, and to what degree, ‘food addiction’ contributes to obesity in the general population is unknown.
to assess 1) the prevalence of ‘food addiction’ in the Newfoundland population; 2) if clinical symptom counts of ‘food addiction’ were significantly correlated with the body composition measurements; 3) if food addicts were significantly more obese than controls, and 4) if macronutrient intakes are associated with ‘food addiction’.
A total of 652 adults (415 women, 237 men) recruited from the general population participated in this study. Obesity was evaluated by Body Mass Index (BMI) and Body Fat percentage measured by dual-energy X-ray absorptiometry. ‘Food addiction’ was assessed using the Yale Food Addiction Scale and macronutrient intake was determined from the Willet Food Frequency Questionnaire.
The prevalence of ‘food addiction’ was 5.4% (6.7% in females and 3.0% in males) and increased with obesity status. The clinical symptom counts of ‘food addiction’ were positively correlated with all body composition measurements across the entire sample (p<0.001). Obesity measurements were significantly higher in food addicts than controls; Food addicts were 11.7 (kg) heavier, 4.6 BMI units higher, and had 8.2% more body fat and 8.5% more trunk fat. Furthermore, food addicts consumed more calories from fat and protein compared with controls.
Our results demonstrated that ‘food addiction’ contributes to severity of obesity and body composition measurements from normal weight to obese individuals in the general population with higher rate in women as compared to men.
Citation: Pedram P, Wadden D, Amini P, Gulliver W, Randell E, Cahill F, et al. (2013) Food Addiction: Its Prevalence and Significant Association with Obesity in the General Population. PLoS ONE 8(9): e74832. https://doi.org/10.1371/journal.pone.0074832
Editor: Jianping Ye, Pennington Biomedical Research Center, United States of America
Received: May 10, 2013; Accepted: August 5, 2013; Published: September 4, 2013
Copyright: © 2013 Pedram et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The study has been funded by a CIHR operating grant and CFI equipment grant to Dr. Guang Sun (CIHR: MOP192552). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Overweight and obesity are the abnormal or excessive accumulation of adipose tissue generally resulting from a chronic positive energy imbalance [1] , [2] . Recently it has been shown that globally approximately 1.0 billion adults are overweight, and a further 475 million are obese [3] . In the United States, the prevalence of obesity among adults increased by 1.1% between 2007 and 2009. If this trend continues, by 2050 close to 100% of Americans will be overweight or obese [4] . Obesity and overweight are the fifth leading cause of global death [1] and the second most preventable cause of death in the United States [5] . Obesity is a complex multifactorial disease but the causes are not yet completely known [6] . Weight gain is usually the result of a complex interaction between an individual's biology and environmental factors which lead to energy surplus [7] . In westernized society, one of the main causes of a chronic energy surplus is a reduced physical activity level owing to a sedentary lifestyle. Another equally important cause of energy surplus is overeating [8] , [9] . Overeating in some degree may occur in many individuals; however, a proportion may develop an obsessive/compulsive relationship to certain foods [10] . These individuals chronically consume more food than they need to maintain health and show compulsive intake behaviours associated with loss of control of eating [9] , [11] .
Accumulating research evidence has documented neurobiological and behavioural similarities between compulsive overeating and psychoactive drug dependence, leading researchers to use the term of ‘food addiction’ to describe this pattern of overeating [12] – [16] . In animal models, foods high in sugar and fat are particularly associated with addiction-like eating behaviour [17] – [19] . In human studies, it has also been suggested that the pattern of food intake in ‘food addiction’ may parallel substance dependence and this phenomenon might be understood with the same neurobiological, behavioral and clinical framework as conventional drug dependence [20] – [22] . Some researchers have argued that ‘food addiction’ should be included as a substance use disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM) [23] , [24] , although others have been critical of the clinical validity or utility of the ‘food addiction’ concept [9] , [25] . Recently, the Yale Food Addiction Scale (YFAS) has been developed, and validated, as a tool for the diagnosis of ‘food addiction’ [26] – [28] . The YFAS criteria have been used to explore the prevalence of ‘food addiction’ in eating disorder patients [29] , obese subjects [30] and junior college students [21] . There is a growing interest in the role of ‘food addiction’ in the increasing prevalence of human obesity which has reached an epidemic degree globally [14] . However, the exploration of ‘food addiction’ in humans is at an early stage and many fundamental questions are yet to be answered [25] , [26] .
First, the prevalence of ‘food addiction’ in the general population has not yet been assessed and this is an essential first step towards evaluating the potential contribution of ‘food addiction’ to human obesity. Only a few human studies are currently available and they were performed on specific cohorts like eating disorder patients [29] , small stratified groups such as obese adults seeking weight loss [31] or junior college students [21] . However no data are currently available regarding the role of ‘food addiction’ in the general population and there seems to be a high proportion of ‘food addiction’ in obese with binge eating and obese seeking weight loss. However the association of ‘food addiction’ with BMI in junior college students was negligibly weak. Therefore, a second equally important question to be answered is whether ‘food addiction’ is significantly correlated with the severity of obesity in the general population. A third question concerns the intake of macronutrient in ‘food addiction’, because data suggest that each macronutrient may play a different role [32] .
Hence the current study was designed to assess: 1) the prevalence of ‘food addiction’ in the Newfoundland population; 2) if the clinical symptom counts of ‘food addiction’ are significantly correlated with the severity of obesity in the general population; 3) if individuals classified as food addicted are significantly more obese than their non-food addicted counterparts; and 4) if food addicted subjects consumed more or less of any of the three macronutrients (i.e., fat, protein and carbohydrates).
Materials and Methods
Ethics statement.
This study was approved by the Health Research Ethics Authority (HREA), Memorial University of Newfoundland, Canada. All participants provided written informed consent.
Study Sample
A total of 652 participants (415 female, 237 male) were recruited from the Canadian province of Newfoundland and Labrador (NL) via advertisements, posted flyers, and word of mouth. The inclusion criteria were: 1) age >19 years, 2) born in NL with family who lived in NL for at least three generations, 3) healthy without serious metabolic, cardiovascular or endocrine diseases, 4) not pregnant at the time of the study.
Anthropometric Measurements
Body weight, height, waist and hip circumference were measured after a 12 hours fasting period. Subjects were weighed to the nearest 0.1 (kg) in a standard hospital gown on a platform manual scale balance (Health O Meter, Bridgeview, IL). A fixed stadiometer was used to measure height to the nearest 0.1 (cm). Hip circumference was measured with the flexible measuring tape to the nearest 0.1 (cm) at the level of largest circumference between the waist and thighs while the participant was in a standing position. The same procedure was used to measure waist circumference at the level of the umbilicus, midway between the lowest rib and iliac crest. BMI was calculated by dividing participants' weight in kilograms by the square of his/her height in meter (kg/m 2 ). The subjects were classified as underweight/normal (BMI≤24.99) and overweight/obese (BMI≥25.00) based on BMI according to World Health Organization criteria [33] .
Body Composition Assessment
Whole body composition measurements including fat mass and lean body mass were measured using Dual-energy X-ray absorptiometry (DXA; Lunar Prodigy; GE Medical Systems, Madison, WI, USA). The measurements were performed in a supine position after 12 hours fasting. Total percent body fat (BF%) and percent trunk fat (TF%) were determined [34] . The subjects were also classified as under/normal weight and overweight/obese based on BF% according to the criteria recommended by Bray [35] .
‘Food Addiction’ Assessment
The diagnosis of ‘food addiction’ was based on the Yale Food Addiction Scale (YFAS) [26] . This questionnaire consists of 27 items that assess eating patterns over the past 12 months. The YFAS translates the Diagnostic and Statistical Manual IV TR (DSM-IV TR) substance dependence criteria in relation to eating behavior (including symptoms such as tolerance and withdrawal symptoms, vulnerability in social activities, difficulties cutting down or controlling substance use, etc.) by applying the DSM-IV TR. The scale uses a combination of Likert scale and dichotomous scoring options. The criteria for ‘food addiction’ are met when three or more symptoms are present within the past 12 months and clinically significant impairment or distress is present. The Likert scoring option is used for food addiction symptom counts (e.g. tolerance and withdrawal) ranging from 0 to 7 symptoms [26] , [29] .
Macronutrient intake and Physical Activity Assessment
Macronutrient intake (protein, fat and carbohydrate) during the past 12 months was assessed using the Willett Food Frequency Questionnaire (FFQ) [36] . Participants indicated their average use of a list of common food items, over the last 12 months. The amount of each selected food was converted to a mean daily intake value. The average daily intake for each food item consumed was entered into NutriBase Clinical Nutrition Manager (software version 9.0; CyberSoft Inc, Arizona). The total intake for each macronutrient per day was computed by the software for each subject [37] . The Baecke physical activity questionnaire was used to assess physical activity. This questionnaire assesses physical activity using three indices including work, sport and leisure [38] .
Statistical Analysis
Statistical analyses were performed using the R project for statistical computing version 2.15.2 (R Development Core Team). Data are presented as mean ± standard deviations (SD), maximum and minimum. Student t-test analyses were used to investigate the differences in measured variables between females and males. The prevalence of ‘food addiction’ was assessed in both the total cohort and different adiposity subgroups according to BMI and BF% by sexes. Relative risk ratios defined as the prevalence ratio were calculated to assess differences in the risk of ‘food addiction’ between sexes and between participants of different obesity status.
Student t-tests and Mann-Whitney-U tests (a non-parametric test) were employed to compare the anthropometric data related to obesity measures and macronutrients intake between ‘food addiction’ and non-food addiction groups. Furthermore, to take possible confounding factors into consideration, an ANCOVA was conducted to compare differences between food addicted and non-food addicted groups on obesity measurements with age, sex, smoking status, medication use and physical activity entered as covariates. Spearman partial correlation coefficients controlling for age, sex, smoking, medication use and physical activity were calculated to investigate the association between ‘food addiction’ and the severity of obesity. For all analyses, the alpha level was set at 0.05.
Physical Parameters and Prevalence of ‘Food Addiction’
Demographic and physical characteristics of the participants are presented in Table 1 . The prevalence of ‘food addiction’ according to the YFAS criteria was 5.4% in the entire population (in women and men it was 6.7% and 3.0%, respectively) ( Table 2 ). When participants were classified as under/normal weight or overweight/obese based on BMI, the prevalence of ‘food addiction’ was 1.6% and 7.7% in these two groups respectively. When subjects were classified as under/normal weight or overweight/obese based on BF% the prevalence of ‘food addiction’ was 2.9% and 6.8%, respectively. The percentage of ‘food addiction’ significantly increased with increasing obesity status regardless of how adiposity was defined (RR = 0.21, p<0.001 and RR = 0.42, p = 0.03, respectively). When the samples were split based on gender, this trend remained significant only in females whose adiposity was classified using BMI (RR = 0.13, p<0.001). The prevalence of ‘food addiction’ was higher in women than in men (RR = 2.28, p = 0.046). Additionally, when using BMI adiposity classifications, but not the BF% adiposity classifications, overweight/obese women had higher prevalence of ‘food addiction’ as compared to overweight/obese men (RR = 3.50, p = 0.002).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0074832.t001
https://doi.org/10.1371/journal.pone.0074832.t002
When food addicted subjects were classified by weight status based on BMI, 11.4% were under/normal weight, 88.6% were overweight/obese. When food addicted subjects were classified into adiposity group based on BF%, 20% were under/normal weight, 80% were overweight/obese ( Table 3 ).
https://doi.org/10.1371/journal.pone.0074832.t003
Correlations between clinical symptom counts of ‘food addiction’ and obesity
Spearman partial correlation coefficients controlling for sex and age were used to assess the relationship between the symptom counts of ‘food addiction’ and obesity measurements in the entire sample and in the non-food addicted subjects. All obesity related measurements (specifically markers related to central obesity) had strong positive correlations with YFAS symptom counts in both groups ( Table 4 ). Furthermore, when we controlled for potential confounding factors including smoking, medication use and physical activity, the correlations remained significant.
https://doi.org/10.1371/journal.pone.0074832.t004
Comparison of obesity measurements and macronutrient intake between ‘food addiction’ and non- food addiction groups
Both student t-test and Mann- Whiney U test showed significant differences in all obesity measurements between ‘food addiction’ and non-food addiction groups (p<0.001) ( Table 5 ). To take the other confounding factors into consideration, we conducted an ANCOVA controlling for sex, age, medication use, physical activity and smoking. All the differences remained significant. Food addicted subjects on average weighed 11.7 kg more and carried 4.6 more BMI than non- food addicted subjects. Additionally food addicted subjects had 8.2% greater body fat and 8.5% more trunk fat.
https://doi.org/10.1371/journal.pone.0074832.t005
Macronutrient intake was compared for the ‘food addiction’ and non-food addiction group ( Table 5 ). Overall, the amount of macronutrients consumed, expressed as gram per kilogram of body weight, was not significantly different between the food addicted and non-food addicted participants. However, the percent calorie intake from protein (p = 0.04 from Mann-Whitney-U test and p = 0.03 from ANCOVA) and the percent calorie intake from fat (p = 0.04 from Mann-Whitney-U test, p = 0.11 from ANCOVA) was significantly higher in food addicted as compared to non-food addiction participants
In general, regardless of the various genetic predispositions and environmental influences, overeating is the primary factor responsible for the increasing prevalence of human obesity [14] , [24] . To the best of our knowledge this is the first study reporting the contribution of ‘food addiction’ to the prevalence of human obesity in the general population [21] , [29] , [30] . One important finding is an estimation of the prevalence of ‘food addiction’ in the general Newfoundland population was at 5.4% (6.7% in women and 3.0% in men). In a previous study assessing obese patients with binge eating disorder (BED), the prevalence of ‘food addiction’ was reported to be as high as 56.8% [29] , suggesting an overlap between binge eating and ‘food addiction’. The prevalence of ‘food addiction’ in obese individuals seeking weight loss treatment was 25%, while in another study obese subjects not seeking weight loss, the prevalence of ‘food addiction’ was 15.2% [30] , [31] . In a cohort of junior college students with a normal BMI range, 8.8% met the YFAS criteria of ‘food addiction’; however the correlation between ‘food addiction’ clinical symptom counts and BMI was negligible [21] , [39] . Our results indicated that 80–88.6% of food addicted individuals were overweight/obese based on Bray or BMI criteria providing strong evidence that ‘food addiction’ has contributed to the rising prevalence of obesity in the general population. Of note, food addicted individuals were also observed in the underweight and normal weight cohort, however in a lower number. The current findings suggest that obesity featured with ‘food addiction’ may represent an important subgroup of the obese with a distinctive aetiology. The identification of this subgroup will open a novel avenue to assess the aetiology of obesity and thus aid in finding new effective methods to treat and prevent obesity.
The subjects in the present study were recruited from the general Newfoundland population. The prevalence of overweight/obesity in the current study is similar to data reported from Health Canada on the province of Newfoundland (62.1%) [40] . The prevalence of ‘food addiction’ revealed in our study on the Newfoundland population may, to some degree, represent the prevalence in other Canadian provinces. Moreover our findings also suggests a potential difference between men and women in regards to ‘food addiction’, as overweight/obese women classified using BMI had a significantly higher rate of ‘food addiction’ as compared to men. This is similar to the case with eating disorders in which women also are significantly more likely to suffer from an eating disorder than men [41] , [42] . Nevertheless larger studies in other populations are warranted to confirm the findings from our investigation.
The third major finding from the current study is the significant correlation between ‘food addiction’ and the severity of obesity in the general Newfoundland population. This finding appears to be robust as we were able to demonstrate this significant correlation throughout a number of analyses controlling for many confounding factors. Firstly, the clinical symptom counts of ‘food addiction’ was significantly correlated not only with BMI, but also with virtually all obesity related measurements including body weight, waist and hip circumferences, body fat and trunk fat percentage determined by DXA, an accurate measurement of body composition. This close correlation was seen in the non-food addicted group as well. We suggest that these robust and multiple correlations demonstrated a true association of ‘food addiction’ with human obesity. Additionally it was shown that obesity related variables were significantly different between food addicted and non-food addicted subjects. Participants who met criteria for ‘food addiction’ on average weighed 11.7 (kg) (25.79 lbs) more, had 4.6 higher BMI and possessed a 8.2% and 8.5% greater total body fat and trunk fat, respectively, as compared to non-food addicted subjects. These data provide the first direct evidence that ‘food addiction’ is strongly associated with obesity in the general population. Importantly, the individuals who met the criteria for ‘food addiction’ only represent between one fifth to one sixth of the total proportion of obese individuals in Newfoundland (25–30%) [40] . This suggests that ‘food addiction’ is likely an important factor in the development of human obesity but not the sole contributor.
Another important goal of our study was to examine differences in dietary patterns particularly macronutrients consumption between food addicted and non-food addicted subjects. Interestingly, the food addicted subjects' diet consisted of a higher percentage of calories from fat and protein, possibly suggesting that these types of foods are more likely to be associated with compulsive overeating. Given the significance of these findings will be important to verify these findings in other populations.
In the present study the YFAS was used as a diagnostic tool to classify participants with ‘food addiction’, as this set of measure and the criteria on which it is based have been validated [26] – [28] . Rather than directly asking if the subjects were addicted to food, the questionnaire assessed ‘food addiction’ based on DSM-IV-TR criteria [39] . Furthermore, using this set of criteria helped to distinguish subjects who regularly indulged in hyper palatable foods from those who have lost control over their eating behaviour [26] .
One limitation of the present study was that the number of female participants was larger than the number of males. Given the sex difference in the prevalence of ‘food addiction’ found in the present study, it is possible that the actual prevalence in the general population may be lower than 5.4% if the study had consisted of equal numbers of women and men. Future studies using cohorts with an equal number of females and males in the population are warranted.
In summary, our study has revealed for the first time that: 1) the prevalence of ‘food addiction’ in the general Newfoundland population was 5.4%; 2) women are at high risk of ‘food addiction’ than men; 3) ‘food addiction’ contributes to human obesity and is significantly associated with the severity of obesity/amount of body fat from normal to obese individuals in the general population. Our findings provide strong evidence that ‘food addiction’ may represent a distinct aetiology of human obesity in the general population.
Acknowledgments
We highly appreciated the contribution by all participated volunteers. We also wish to thank Jennifer Shea, Alicia Rideout, Hongwei Zhang, and our research collaborators.
Author Contributions
Conceived and designed the experiments: PP GS. Performed the experiments: PP GS DW PA FC. Analyzed the data: PP GS YJ. Contributed reagents/materials/analysis tools: PP GS DW PA FC. Wrote the paper: PP. Collaborators who helped in Data gathering: WG ER SV AG GZ. Psychologist consultant: JC.
- 1. World_Health_Organization (2013) Obesity and Overweight. World Health Organization. http://www.who.int/mediacentre/factsheets/fs311/en/index.html . Accessed 2013 Agu 12.
- View Article
- Google Scholar
- 3. International_Obesity_Taskforce (2010) The Global Epidemic. London: International Association for the Study of Obesity. http://www.iaso.org/iotf/obesity/obesitytheglobalepidemic/ . Accessed 2013 Agu 12.
- 15. von Deneen KM, Liu Y (2012) Food Addiction, Obesity and Neuroimaging. In: Belin D, editors. Addictions - From Pathophysiology To Treatment: InTech. 259–290.
- 33. World_Helath_Organization (2013) BMI classification. World Helath Organization. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html . Accessed 2013 Agu 12.
- 35. Bray GA (2003) Contemporary diagnosis and management of obesity and the Metabolic Syndrome. Newtown: Handbooks in Health Care.
- 40. Public_Health_Agency_of_Canada (2011) Obesity in Canada. Ottawa: Canadian Institute of Health Information. http://www.phac-aspc.gc.ca/hp-ps/hl-mvs/oic-oac/assets/pdf/oic-oac-eng.pdf . Accessed 2013 Agu 12.
Advertisement
Psychosocial Interventions for Food Addiction: a Systematic Review
- Food Addiction (A Meule, Section Editor)
- Published: 17 January 2020
- Volume 7 , pages 9–19, ( 2020 )
Cite this article
- Stephanie E. Cassin 1 , 2 , 3 ,
- Iris Sijercic 1 &
- Vanessa Montemarano 1
1443 Accesses
26 Citations
1 Altmetric
Explore all metrics
Purpose of Review
The current systematic review examined the empirical literature on psychosocial interventions for food addiction with the goal of providing recommendations for clinical practice and future research. A PsycINFO and PubMed search of publications was conducted in September 2019. Two authors assessed retrieved titles and abstracts to determine topic relevance and rated the quality of the included studies using an established checklist.
Recent Findings
Eight studies met the study inclusion criteria, and study quality ranged from “poor” to “fair”. Most studies were pilot and feasibility studies with limitations that impact the conclusions that can be drawn.
There are currently no empirically supported psychosocial interventions for food addiction. Additional research is warranted to develop and test the efficacy of interventions for food addiction. In the meantime, it is recommended that clinicians treating food addiction assess for comorbid eating disorders, and if present, first provide evidence-based treatments for those conditions.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
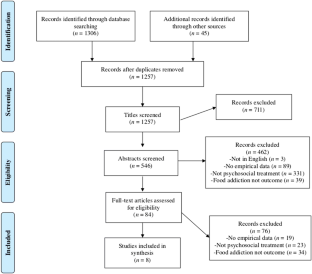
Similar content being viewed by others

Clinical Applications of the Food Addiction Concept

The Concept of Food Addiction: a Review of the Current Evidence
Katherine R. Naish, James MacKillop & Iris M. Balodis
A narrative review of potential treatment strategies for food addiction
Shae-Leigh C. Vella & Nagesh B. Pai
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gordon EL, Ariel-Donges AH, Bauman V, Merlo LJ. What is the evidence for “food addiction”? A systematic review. Nutrients. 2018;12:477. https://doi.org/10.3390/nu10040477 .
Article CAS Google Scholar
Fletcher PC, Kenny PJ. Food addiction: a valid concept? Neuropsychopharmacol. 2018;43:2506–13. https://doi.org/10.1038/s41386-018-0203-9 .
Article Google Scholar
Naish KR, MacKillop J, Balodis IM. The concept of food addiction: a review of the current evidence. Curr Behav Neurosci Rep. 2018;5:281–94. https://doi.org/10.1007/s40473-018-0169-2 .
Ziauddeen H, Fletcher PC. Is food addiction a valid and useful concept? Obes Rev. 2013;14:19–28. https://doi.org/10.1111/j.1467-789X.2012.01046.x .
Article CAS PubMed Google Scholar
Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale food addiction scale. Appetite. 2009;52:430–6. https://doi.org/10.1016/j.appet.2008.12.003 .
Article PubMed Google Scholar
Meule A. Back by popular demand: a narrative review on the history of food addiction research. Yale J Biol Med. 2015;88:295.
PubMed PubMed Central Google Scholar
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington: American Psychiatric Publishing; 2013.
Book Google Scholar
Meule A, Gearhardt AN. Five years of the Yale food addiction scale: taking stock and moving forward. Curr Addict Rep. 2014;1:193–205. https://doi.org/10.1007/s40429-014-0021-z .
Meule A, Geardhardt AN. Ten years of the Yale food addiction scale: a review of version 2.0. Curr Addict Rep. 2019;6:218–28. https://doi.org/10.1007/s40429-019-00261-3 .
Penzenstadler L, Soares C, Karila L, Khazaal Y. Systematic review of food addiction as measured with the Yale food addiction scale: implications for the food addiction construct. Curr Neuropharmacol. 2019;17:526–38. https://doi.org/10.2174/1570159X16666181108093520 .
Article CAS PubMed PubMed Central Google Scholar
Pursey KM, Stanwell P, Gearhardt AN, Collins CE, Burrows TL. The prevalence of food addiction as assessed by the Yale food addiction scale: a systematic review. Nutr. 2014;6:4552–90. https://doi.org/10.3390/nu6104552 .
Ifland JR, Preuss HG, Marcus MT, Rourke KM, Taylor WC, Burau K, et al. Refined food addiction: a classic substance use disorder. Med Hypotheses. 2009;72:518–26. https://doi.org/10.1016/j.mehy.2008.11.035 .
National Institute for Health and Care Excellence. Alcohol-use disorders: diagnosis, assessment, and management of harmful drinking (high-risk drinking) and alcohol dependence [Internet]. NICE; 2011 [updated 2019 July 19; cited 2019 Oct 28]. Available from https://www.nice.org.uk/guidance/cg115
National Institute for Health and Care Excellence. Eating disorders: recognition and treatment [Internet]. NICE; 2017 [updated 2017 May; cited 2019 Oct 28]. Available from https://www.nice.org.uk/guidance/ng69
Schulte EM, Joyner MA, Potenza MN, Grilo CM, Gearhardt AN. Current considerations regarding food addiction. Curr Psychiatry Rep. 2015;17:563. https://doi.org/10.1007/s11920-015-0563-3 .
• Schulte EM, Grilo CM, Gearhardt AN. Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clin Psychol Rev. 2016;44:125–39. https://doi.org/10.1016/j.cpr.2016.02.001 . This review paper examines the shared mechanisms and unique aspects of addiction and eating disorders and provides a guiding framework for future research. One recommendation is to evaluate whether treatment approaches for addictive disorders, such as abstinence-based or harm reduction approaches, are beneficial for food addiction.
Article PubMed PubMed Central Google Scholar
Wilson GT. Eating disorders, obesity and addiction. Eur Eat Disord Rev. 2010;18:341–51. https://doi.org/10.1002/erv.1048 .
•• Treasure J, Leslie M, Chami R, Fernández-Aranda F. Are trans diagnostic models of eating disorders fit for purpose? A consideration of the evidence for food addiction. Eur Eat Disord Rev. 2018;26:83–91. https://doi.org/10.1002/erv.2578 . This paper proposes a food addiction model of binge eating behaviour and recommends specific targets for intervention, including reducing access to processed foods, reducing habit-based eating, reducing restriction of healthy foods, and improving emotion regulation.
•• Wiss DA, Brewerton TD. Incorporating food addiction into disordered eating: the disordered eating food addiction nutrition guide (DEFANG). Eat Weight Disord. 2017;22:49–59. https://doi.org/10.1007/s40519-016-0344-y . This paper examines the relationship between eating disorders and addictions and proposes a new model of conceptualizing and treating eating disorders that incorporates the concept of food addiction.
Adams RC, Sedgmond J, Maizey L, Chambers CD, Lawrence NS. Food addiction: implications for the diagnosis and treatment of overeating. Nutr. 2019;11:2086. https://doi.org/10.3390/nu11092086 .
Davis C, Carter JC. If certain foods are addictive, how might this change the treatment of compulsive overeating and obesity? Curr Addict Rep. 2014;1:89–95. https://doi.org/10.1007/s40429-014-0013-z .
• Meule A. A critical examination of the practical implications derived from the food addiction concept. Curr Obes Rep. 2019;8:11–7. https://doi.org/10.1007/s13679-019-0326-2 . This paper provides a thoughtful analysis of the potential clinical implications that arise from the concept of food addiction. The author suggests that food addiction may be a useful metaphor in the treatment of binge eating disorder, but calls into question the current clinical utility of food addiction.
• Schulte EM, Joyner MA, Schiestl ET, Gearhardt AN. Future directions in “food addiction”: next steps and treatment implications. Curr Addict Rep. 2017;4:165–71. https://doi.org/10.1007/s40429-017-0140-4 . This paper discusses potential treatment implications of food addiction, including harm reduction approaches, exposure to food cues, coping skills for managing cravings, and strategies for improving emotion regulation.
• Vella SC, Pai NB. A narrative review of potential treatment strategies for food addiction. Eat Weight Disord. 2017;22:387–93. https://doi.org/10.1007/s40519-017-0400-2 . This review article proposes treatment strategies for food addiction that target four key behaviours of the addiction phenotype: cravings, impulsivity, compulsivity, and motivation .
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. https://doi.org/10.1136/jech.52.6.377 .
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:1–6. https://doi.org/10.1371/journal.pmed.1000097 .
Trac MH, McArthur E, Jandoc R, Dixon SN, Nash DM, Hackam DG, et al. Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. Can Med Assoc J. 2016;188:E120–9. https://doi.org/10.1503/cmaj.150901 .
Hooper P, Jutai J, Strong G, Russell-Minda E. Age-related macular degeneration and low-vision rehabilitation: a systematic review. Can J Ophthalmol. 2008;43:180–7. https://doi.org/10.3129/i08-001 .
Krippendorff K. Content analysis: an introduction to its methodology. Beverly Hills, CA: Sage; 1980.
Google Scholar
Mount R, Neziroglu F, Taylor CJ. An obsessive-compulsive view of obesity and its treatment. J Clin Psychol. 1990;46:68–78. https://doi.org/10.1002/1097-4679(199001)46:1<68::AID-JCLP2270460112>3.0.CO;2-V .
Mason AE, Lustig RH, Brown RR, Acree M, Bacchetti P, Moran PJ, et al. Acute responses to opioidergic blockade as a biomarker of hedonic eating among obese women enrolled in a mindfulness-based weight loss intervention trial. Appetite. 2015;91:311–20. https://doi.org/10.1016/j.appet.2015.04.062 .
Weinstein A, Zlatkes M, Gingis A, Lejoyeux M. The effects of a 12-step self-help group for compulsive eating on measures of food addiction, anxiety, depression, and self-efficacy. J Groups Addict Recovery. 2015;10:190–200. https://doi.org/10.1080/1556035X.2015.1034825 .
Hilker I, Sánchez I, Steward T, Jiménez-Murcia S, Granero R, Gearhardt AN, et al. Food addiction in bulimia nervosa: clinical correlates and association with response to a brief psychoeducational intervention. Eur Eat Disord Rev. 2016;24:482–8. https://doi.org/10.1002/erv.2473 .
Sawamoto R, Nozaki T, Nishihara T, Furukawa T, Hata T, Komaki G, et al. Predictors of successful long-term weight loss maintenance: a two-year follow-up. Biopsychosoc Med. 2017;11:14. https://doi.org/10.1186/s13030-017-0099-3 .
Vidmar AP, Pretlow R, Borzutzky C, Wee CP, Fox DS, Fink C, et al. An addiction model-based mobile health weight loss intervention in adolescents with obesity. Pediatr Obes. 2019;14:e12464. https://doi.org/10.1111/ijpo.12464 .
Webber KH, Mellin L, Mayes L, Mitrovic I, Saulnier M. Pilot investigation of 2 nondiet approaches to improve weight and health. Altern Ther Health Med. 2017;24:16–20.
Miller-Matero LR, Brescacin C, Clark SM, Troncone CL, Tobin ET. Why WAIT? Preliminary evaluation of the weight assistance and intervention techniques (WAIT) group. Psychol Health Med. 2019;24:1029–37. https://doi.org/10.1080/13548506.2019.1587478 .
Chambless DL, Hollon SD. Defining empirically supported therapies. J Consul Clin Psychol. 1998;66:7–18.
Tolin DF, McKay D, Forman EM, Klonsky ED, Thombs BD. Empirically supported treatment: recommendations for a new model. Clin Psychol Sci Pract. 2015;22:317–38. https://doi.org/10.1111/cpsp.12122 .
Davis CA. A commentary on the associations among ‘food addiction’, binge eating disorder, and obesity: overlapping conditions with idiosyncratic clinical features. Appetite. 2017;115:3–8. https://doi.org/10.1016/j.appet.2016.11.001 .
Carter JC, van Wijk M, Rowsell M. Symptoms of ‘food addiction’ in binge eating disorder using the Yale food addiction scale version 2.0. Appetite. 2019;133:362–9. https://doi.org/10.1016/j.appet.2018.11.032 .
Cassin SE, von Ranson KM. Is binge eating experienced as an addiction? Appetite. 2007;49:687–90. https://doi.org/10.1016/j.appet.2007.06.012 .
Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7:47–55. https://doi.org/10.1016/0306-4603(82)90024-7 .
Latner JD, Mond JM, Kelly MC, Haynes SN, Hay PJ. The loss of control over eating scale: development and psychometric evaluation. Int J Eat Disord. 2014;47:647–59. https://doi.org/10.1002/eat.22296 .
Owen P, Marlatt GA. Should abstinence be the goal for alcohol treatment? Am J Addict. 2001;10:289–95. https://doi.org/10.1080/aja.10.4.289.295 .
Saladin ME, Santa Ana EJ. Controlled drinking: more than just a controversy. Curr Opin Psychiatry. 2004;17:175–87. https://doi.org/10.1097/01.yco.0000126547.69286.12 .
Sackett DL, Rosenberg WM, Gray JM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–2. https://doi.org/10.1136/bmj.312.7023.71 .
Peterson CB, Becker CB, Treasure J, Shafran R, Bryant-Waugh R. The three-legged stool of evidence-based practice in eating disorder treatment: research, clinical, and patient perspectives. BMC Med. 2016;14:69. https://doi.org/10.1186/s12916-016-0615-5 .
Cassin SE, von Ranson KM, Heng K, Brar J, Wojtowicz AE. Adapted motivational interviewing for women with binge eating disorder: a randomized controlled trial. Psychol Addict Behav. 2008;22:417–25. https://doi.org/10.1037/0893-164X.22.3.417 .
Knowles L, Anokhina A, Serpell L. Motivational interventions in the eating disorders: what is the evidence? Int J Eat Disord. 2013;46:97–107. https://doi.org/10.1002/eat.22053 .
Linardon J, Messer M, Fuller-Tyszkiewicz M. Meta-analysis of the effects of cognitive-behavioral therapy for binge-eating-type disorders on abstinence rates in nonrandomized effectiveness studies: comparable outcomes to randomized, controlled trials? Int J Eat Disord. 2018;51:1303–11. https://doi.org/10.1002/eat.22986 .
Linardon J. Rates of abstinence following psychological or behavioral treatments for binge-eating disorder: meta-analysis. Int J Eat Disord. 2018;51:785–97. https://doi.org/10.1002/eat.22897 .
Download references
Author information
Authors and affiliations.
Department of Psychology, Ryerson University, Toronto, ON, M5B 2K3, Canada
Stephanie E. Cassin, Iris Sijercic & Vanessa Montemarano
Department of Psychiatry, University of Toronto, Toronto, Canada
Stephanie E. Cassin
Centre for Mental Health, University Health Network, Toronto, Canada
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stephanie E. Cassin .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Food Addiction
Rights and permissions
Reprints and permissions
About this article
Cassin, S.E., Sijercic, I. & Montemarano, V. Psychosocial Interventions for Food Addiction: a Systematic Review. Curr Addict Rep 7 , 9–19 (2020). https://doi.org/10.1007/s40429-020-00295-y
Download citation
Published : 17 January 2020
Issue Date : March 2020
DOI : https://doi.org/10.1007/s40429-020-00295-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Food addiction
- Compulsive eating
- Intervention
- Psychotherapy
- Find a journal
- Publish with us
- Track your research
- Share full article
Advertisement
Supported by
Unhealthy Foods Aren’t Just Bad For You, They May Also Be Addictive
Food researchers debate whether highly processed foods like potato chips and ice cream are addictive, triggering our brains to overeat.

By Anahad O’Connor
Five years ago, a group of nutrition scientists studied what Americans eat and reached a striking conclusion: More than half of all the calories that the average American consumes comes from ultra-processed foods, which they defined as “industrial formulations” that combine large amounts of sugar, salt, oils, fats and other additives.
Highly processed foods continue to dominate the American diet, despite being linked to obesity, heart disease, Type 2 diabetes and other health problems. They are cheap and convenient, and engineered to taste good. They are aggressively marketed by the food industry. But a growing number of scientists say another reason these foods are so heavily consumed is that for many people they are not just tempting but addictive, a notion that has sparked controversy among researchers.
Recently, the American Journal of Clinical Nutrition explored the science behind food addiction and whether ultra-processed foods might be contributing to overeating and obesity. It featured a debate between two of the leading experts on the subject, Ashley Gearhardt, associate professor in the psychology department at the University of Michigan, and Dr. Johannes Hebebrand, head of the department of child and adolescent psychiatry, psychosomatics and psychotherapy at the University of Duisburg-Essen in Germany.
Dr. Gearhardt, a clinical psychologist, helped develop the Yale Food Addiction Scale , a survey that is used to determine whether a person shows signs of addictive behavior toward food. In one study involving more than 500 people , she and her colleagues found that certain foods were especially likely to elicit “addictive-like” eating behaviors, such as intense cravings, a loss of control, and an inability to cut back despite experiencing harmful consequences and a strong desire to stop eating them.
At the top of the list were pizza, chocolate, potato chips, cookies, ice cream, French fries and cheeseburgers. Dr. Gearhardt has found in her research that these highly processed foods share much in common with addictive substances. Like cigarettes and cocaine, their ingredients are derived from naturally occurring plants and foods that are stripped of components that slow their absorption, such as fiber, water and protein. Then their most pleasurable ingredients are refined and processed into products that are rapidly absorbed into the bloodstream, enhancing their ability to light up regions of the brain that regulate reward, emotion and motivation.
Salt, thickeners, artificial flavors and other additives in highly processed foods strengthen their pull by enhancing properties like texture and mouth-feel, similar to the way that cigarettes contain an array of additives designed to increase their addictive potential, said Dr. Gearhardt. Menthol helps to mask the bitter flavor of nicotine , for example, while another ingredient used in some cigarettes, cocoa, dilates the airways and increases nicotine’s absorption.
A common denominator among the most irresistible ultra-processed foods is that they contain large amounts of fat and refined carbohydrates, a potent combination that is rarely seen in naturally occurring foods that humans evolved to eat, such as fruits, vegetables, meat, nuts, honey, beans and seeds, said Dr. Gearhardt. Many foods found in nature are rich in either fat or carbs, but typically they are not high in both.
“People don’t experience an addictive behavioral response to naturally occurring foods that are good for our health, like strawberries,” said Dr. Gearhardt, director of the Food and Addiction Science and Treatment lab at the University of Michigan. “It’s this subset of highly processed foods that are engineered in a way that’s so similar to how we create other addictive substances. These are the foods that can trigger a loss of control and compulsive, problematic behaviors that parallel what we see with alcohol and cigarettes.”
In one study , Dr. Gearhardt found that when people cut back on highly processed foods, they experienced symptoms that were comparable to the withdrawal seen in drug abusers, such as irritability, fatigue, feelings of sadness and cravings. Other researchers have found in brain imaging studies that people who frequently consume junk foods can develop a tolerance to them over time, leading them to require larger and larger amounts to get the same enjoyment.
In her clinical practice, Dr. Gearhardt has encountered patients — some obese and some not — who struggle in vain to control their intake of highly processed foods. Some attempt to eat them in moderation, only to find that they lose control and eat to the point of feeling ill and distraught. Many of her patients find that they cannot quit these foods despite struggling with uncontrolled diabetes, excessive weight gain and other health problems.
“The striking thing is that my clients are almost always acutely aware of the negative consequences of their highly processed food consumption, and they have typically tried dozens of strategies like crash diets and cleanses to try and get their relationship with these foods under control,” she said. “While these attempts might work for a short time, they almost always end up relapsing.”
But Dr. Hebebrand disputes the notion that any food is addictive. While potato chips and pizza can seem irresistible to some, he argues that they do not cause an altered state of mind, a hallmark of addictive substances. Smoking a cigarette, drinking a glass of wine or taking a hit of heroin, for instance, causes an immediate sensation in the brain that foods do not, he says.
“You can take any addictive drug, and it’s always the same story that almost everyone will have an altered state of mind after ingesting it,” said Dr. Hebebrand. “That indicates that the substance is having an effect on your central nervous system. But we are all ingesting highly processed foods, and none of us is experiencing this altered state of mind because there’s no direct hit of a substance in the brain.”
In substance use disorders, people become dependent on a specific chemical that acts on the brain, like the nicotine in cigarettes or the ethanol in wine and liquor. They initially seek out this chemical to get a high, and then become dependent on it to alleviate depressed and negative emotions. But in highly processed foods, there is no one compound that can be singled out as addictive, Dr. Hebebrand said. In fact, evidence suggests that obese people who overeat tend to consume a wide range of foods with different textures, flavors and compositions. Dr. Hebebrand argued that overeating is driven in part by the food industry marketing more than 20,000 new products every year, giving people access to a seemingly endless variety of foods and beverages.
“It’s the diversity of foods that is so appealing and causing the problem, not a single substance in these foods,” he added.
Those who argue against food addiction also point out that most people consume highly processed foods on a daily basis without showing any signs of addiction. But Dr. Gearhardt notes that addictive substances do not hook everyone who consumes them. According to research , about two-thirds of people who smoke cigarettes go on to become addicted, while a third do not. Only about 21 percent of people who use cocaine in their lifetimes become addicted, while just 23 percent of people who drink alcohol develop a dependence on it. Studies suggests that a wide range of factors determine whether people become addicted, including their genetics, family histories, exposure to trauma, and environmental and socioeconomic backgrounds.
“Most people try addictive substances and they don’t become addicted,” Dr. Gearhardt said. “So if these foods are addictive, we wouldn’t expect that 100 percent of society is going to be addicted to them.”
For people who struggle with limiting their intake of highly processed foods, Dr. Gearhardt recommends keeping a journal of what you eat so you can identify the foods that have the most pull — the ones that cause intense cravings and that you can’t stop eating once you start. Keep those foods out of your home, while stocking your fridge and pantry with healthier alternatives that you enjoy, she said.
Keep track of the triggers that lead to cravings and binges. They could be emotions like stress, boredom and loneliness. Or it could be the Dunkin’ Donuts that you drive by three times a week. Make a plan to manage those triggers by a taking a different route home, for example, or by using nonfood activities to alleviate stress and boredom. And avoid skipping meals, because hunger can set off cravings that lead to regrettable decisions, she said.
“Making sure you are regularly fueling your body with nutritious, minimally processed foods that you enjoy can be important for helping you navigate a very challenging food environment,” said Dr. Gearhardt.
Anahad O’Connor is a staff reporter covering health, science, nutrition and other topics. He is also a bestselling author of consumer health books such as “Never Shower in a Thunderstorm” and “The 10 Things You Need to Eat.” More about Anahad O’Connor
A Guide to Better Nutrition
A viral TikTok trend touts “Oatzempic,” a half cup of rolled oats with a cup of water and the juice of half a lime, as a weight-loss hack. We asked the experts if there’s anything to it .
How much salt is too much? Should I cut back? We asked experts these and other questions about sodium .
Patients were told for years that cutting calories would ease the symptoms of polycystic ovary syndrome. But research suggests dieting may not help at all .
We asked a nutrition expert how she keeps up healthy habits without stressing about food. Here are seven tips she shared for maintaining that balance.
There are many people who want to lose a few pounds for whom weight loss drugs are not the right choice. Is old-fashioned dieting a good option ?
Read these books to shift into a healthier way of thinking about food .
In today’s fast-paced and increasingly disconnected world, feelings of loneliness and social isolation have become all too common. While we often think of loneliness as an emotional state, new research from UCLA Health suggests that it can have profound effects on our physical health as well, particularly when it comes to our eating habits and risk of obesity. “Researching how the brain processes loneliness and how this is related to obesity and health outcomes hasn’t been done,” says senior study author Arpana Gupta, a researcher and co-director of the UCLA Goodman-Luskin Microbiome Center, in a media release.
Using functional magnetic resonance imaging (fMRI), UCLA Health researchers observed that when shown images of food, especially sweet treats, the brains of lonely individuals showed heightened activity in regions associated with processing internal states, such as hunger and appetite, as well as increased attention and motivation toward external food cues. At the same time, brain areas responsible for exerting self-control and making healthy decisions showed decreased activity. This imbalance in brain function may help explain why people who feel socially isolated are more likely to engage in unhealthy eating behaviors, such as having intense food cravings, using food as a reward, and even displaying signs of food addiction.
The study found that these altered brain responses were associated with a higher body fat percentage in lonely individuals. However, the impact of loneliness goes beyond just physical health. Researchers also discovered that the brain changes linked to social isolation were associated with poorer mental health outcomes, including increased anxiety and reduced positive emotions and psychological resilience. This suggests that loneliness may create a vicious cycle where unhealthy eating behaviors are used as a coping mechanism for dealing with the negative feelings that arise from feeling disconnected from others.
Dr. Gupta explains, “These findings are interesting because they provide evidence for what we intuitively know. When people are alone or lonely, it impacts more than how they are feeling; they underreport what they eat, their desire to eat, and their cravings, especially for unhealthy foods.” So why might feelings of loneliness lead to such dramatic changes in brain function and behavior? One theory is that when we feel socially isolated, our brains may enter a sort of “self-preservation mode.” This heightened state of vigilance and reactivity could drive us to seek out quick sources of comfort and energy, such as sugary or high-calorie foods. Over time, this pattern of eating can lead to weight gain and increase the risk of obesity-related health problems.
“If you have more cravings, you eat more and may have more anxiety or depression, which may lead you to eat more,” says study lead author Xiaobei Zhang, a postdoctoral researcher at UCLA Health Sciences. The findings of this study underscore the importance of addressing the growing problem of loneliness and social isolation in our society. While it may be tempting to turn to food for comfort during times of stress or loneliness, it’s crucial to find healthier ways to cope with these feelings and maintain social connections.
How drugs affect addicts’ brains so that they can even forget to eat and drink
A study on mice suggests that a large part of neurons that regulate thirst and hunger are impacted by the impulses generated by cocaine and morphine.

The consumption of certain substances produces such an intense effect on the brain’s reward circuits that it can make a person forget hunger and thirst. That’s a conclusion easily drawn after witnessing a person suffering from addiction, but this week, a team of scientists from several U.S. institutions published an article in the journal Science that describes part of the mechanism responsible for such behavior, and suggests the possibility of new solutions for addicts.
Drugs like cocaine and heroin can hook people because they generate changes in the brain, taking control of systems that make us crave water and food, survival basics. These substances intensify desire to consume more of them, and reduce the pleasure felt from other rewards that help us to lead a healthy life. Several previous studies have analyzed these mechanisms, but the authors of the report published on Thursday sought to combine the latest technology using animal subjects in order to understand the biological basis for narcotics’ power to affect the priorities of those who consume such substances.
To identify the place in the brain that is activated by drugs, researchers used mice, who were given cocaine and morphine. Later, the rodents were observed with techniques that measured their brains and saw how both drugs increased the activity in their nucleus accumbens, a group of neurons related to basic survival activities like sexual desire and hunger. Cocaine prevents bodies from reabsorbing dopamine, which intensifies the activation of reward circuits. Morphine becomes attached to opioid receptors, which can also free up dopamine in the nucleus accumbens. In both cases, the more times the drugs were administered, the greater the neuronal activity in the region.
When they used techniques like optogenetics, which employ light to activate the neurons of the nucleus accumbens so that they would react as though the mouse had received a drug, the scientists observed that the rodents lost their appetite, as if they had ingested addictive substances. Employing other tools that followed the activity of individual neurons, researchers found that, in most cases, there was an overlap between the pleasure response to eating and drinking and that of consuming narcotic drugs.
Scientists observed that some circuits were activated with the consumption of large quantities of food, and that this activation increased consumption, a vicious cycle. Still, researchers saw that the hunger mechanism self-limited when it came to natural rewards, and did not reach the same levels as the amplification of desire that accompanied the consumption of drugs.
Eric Nestler, the study’s co-author, explains that identifying the biochemical methods that are used when drugs take control of reward circuits teaches us that, “based on these studies on mice, the manipulation of these new paths block the damaging effects of the drugs and simultaneously repair responses to natural rewards.” “This offers tangible paths towards developing new treatments for addiction,” continues Nestler, who is the director of The Friedman Brain Institute at New York’s Mount Sinai Hospital.
Nonetheless, Nestler acknowledges that the same overlap shows the difficulty of finding new ways to treat addiction, because the goal of these treatments is to counteract the effect of the drugs, “without affecting the person’s response to natural rewards.”
Elena Martín, researcher at Barcelona’s Pompeu Fabra University and addictions specialist, thinks that the study touches on factors that were previously understood, but that it does use many new techniques for a much greater precision in that knowledge. In her opinion, “These results are important for understanding other addictions, such as food addiction.” She continues, “There are researchers who doubt that food can cause addiction, because it is a natural reinforcer, but this overlap in the activation of neurons that we see between cocaine, morphine and food leads us to think that food addiction is possible.”
Addiction is possible, in part, because of the brain’s plasticity, its ability to adapt to new circumstances and even reorganize our priorities when necessary. These changes begin by intensely increasing dopamine levels in the nucleus accumbens, but end up producing longer-lasting changes in the prefrontal cortex, the part of the brain that determines personality and the ability to control oneself. Until recently, the most serious effects of drug-induced brain changes were thought to be irreversible, but work like that of researcher Nora Volkow has changed that perspective. Now, treatments such as cognitive behavioral therapy, which offer tools to regain control, are used to combat food and drug addiction. The study by Nestler and his colleagues illustrates the biological basis that give such common treatments validity.
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
More information

What happens to your liver when you quit alcohol
/cloudfront-eu-central-1.images.arcpublishing.com/prisa/NCX3JHNI4BBJPPWZOTZXIGRY7Q.jpg)
Can you really be allergic to alcohol?
- Francés online
- Inglés online
- Italiano online
- Alemán online
- Crucigramas & Juegos

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Yale J Biol Med
- v.88(3); 2015 Sep

Focus: Addiction
Back by popular demand: a narrative review on the history of food addiction research.
In recent years, the concept of food addiction has gained more and more popularity. This approach acknowledges the apparent parallels between substance use disorders and overeating of highly palatable, high-caloric foods. Part of this discussion includes that “hyperpalatable” foods may have an addictive potential because of increased potency due to certain nutrients or additives. Although this idea seems to be relatively new, research on food addiction actually encompasses several decades, a fact that often remains unrecognized. Scientific use of the term addiction in reference to chocolate even dates back to the 19th century. In the 20th century, food addiction research underwent several paradigm shifts, which include changing foci on anorexia nervosa, bulimia nervosa, obesity, or binge eating disorder. Thus, the purpose of this review is to describe the history and state of the art of food addiction research and to demonstrate its development and refinement of definitions and methodologies.
Introduction
In recent years, the concept of food addiction has become increasingly popular. This concept includes the idea that certain foods (usually highly processed, highly palatable, and highly caloric foods) may have an addictive potential and that certain forms of overeating may represent an addicted behavior. This increased popularity is reflected not only in a high number of media reports and lay literature [ 1 , 2 ], but also in a substantial increase in the number of scientific publications ( Figure 1 ) [ 3 , 4 ]. In 2012, for example, a comprehensive handbook on food and addiction was published because “science has reached a critical mass to the point where an edited book is warranted” [ 5 ]. This increased interest appears to have created the impression that the idea of food addiction only became relevant in the 21st century because of the increasing availability of highly processed foods and that the concept of food addiction was developed in an effort to explain increasing prevalence rates of obesity [ 6 ]. Some researchers even refer to alleged pioneering work in food addiction research by citing articles that were published in this century [ 7 , 8 ].

Number of scientific publications on food addiction in the years 1990-2014. Values represent the number of hits based on a Web of Science search conducted for each year separately, using the search term “food addiction” and selecting “topic” (which searches the title, abstract, and keywords within a record).
As will be demonstrated throughout this paper, this notion about food addiction being a new idea, which originated in recent years and may explain the obesity pandemic, is wrong. Therefore, this article briefly presents the development of food addiction research. One aim is to demonstrate that its history, although it is a relatively new field of research, actually encompasses several decades and the association between food and addiction even dates back to the 19th century. In the 20th century, focus areas of and opinions about food addiction changed dynamically, such as the types of foods and eating disorders that were proposed to be related to addiction and the methods that were used to investigate eating behavior from an addiction perspective ( Figure 2 ). The current article, however, does not intend to outline the various phenomenological and neurobiological parallels between overeating and substance use or speculate about possible consequences and implications of the food addiction concept for treatment, prevention, and public policy. All of these issues have been extensively discussed elsewhere [ 9 - 21 ]. Finally, this article does not intend to evaluate the validity of the food addiction concept.

Some focus areas with selected references in the history of food addiction research.
Late 19th and Early 20th Century: First Beginnings
The Journal of Inebriety was one of the first addiction journals and was published from 1876 to 1914 [ 22 ]. During this time, different terms were used to describe excessive alcohol and drug use (e.g., habitual drunkenness, inebriety, ebriosity, dipsomania, narcomania, oinomania, alcoholism, and addiction ). Interestingly, the term addiction as used in the Journal of Inebriety primarily referred to dependence upon drugs other than alcohol and first appeared in 1890 in reference to chocolate [ 22 ]. Subsequently, the addictive properties of “stimulating” foods were also mentioned in other issues of the journal [ 17 ]. For instance, Clouston [ 23 ] stated that when “a brain has depended on stimulating diet and drink for its restoration when exhausted, there is an intense and irresistible craving set up for such food and drink stimulants whenever there is fatigue.”
In 1932, Mosche Wulff, one of the pioneers of psychoanalysis, published an article in German, the title of which may be translated as “On an Interesting Oral Symptom Complex and Its Relationship to Addiction” [ 24 ]. Later, Thorner [ 25 ] referred to this work, stating that “Wulff links overeating, which he calls food addiction, with a constitutional oral factor and differentiates it from melancholia insofar as the food addict simply introjects erotically in place of a genital relationship while the melancholic incorporates in a sadistic and destructive manner.” While this psychoanalytical perspective on overeating is certainly outdated and appears disconcerting nowadays, it is nonetheless remarkable to see that the idea of describing overeating as an addiction was already existent in the 1930s.
1950s: Coining of the Term ‘Food Addiction’
The term food addiction was first introduced in the scientific literature by Theron Randolph in 1956 [ 26 ]. He described it as “a specific adaptation to one or more regularly consumed foods to which a person is highly sensitive [which] produces a common pattern of symptoms descriptively similar to those of other addictive processes.” He also noted, however, that “most often involved are corn, wheat, coffee, milk, eggs, potatoes and other frequently eaten foods.” This view has changed, as nowadays highly processed foods with high sugar and/or fat content are discussed as being potentially addictive [ 27 ].
Randolph was not the only one using the term food addiction around this time. In an article published in 1959, a panel discussion that revolved around the role of environment and personality in the management of diabetes was reported [ 28 ]. During this discussion, Albert J. Stunkard (1922-2014) [ 29 ], a psychiatrist whose article in which he first described binge eating disorder (BED) was published in the same year [ 30 ], was interviewed. For instance, he was asked, “One of the most common and difficult problems we face is that of food addiction, both in the genesis of diabetes and its treatment. Are there physiological factors involved in this mechanism or is it all psychological? What is its relation to alcohol addiction and addiction to narcotics?” [ 28 ]. Stunkard replied that he does not think that the term food addiction “is justified in terms of what we know about addiction to alcohol and drugs.” However, what is more important for the historical examination in the present article is that he also stated that the term food addiction is widely used, which further supports that the idea of food addiction was well-known among scientists and the general public as early as the 1950s.
1960s and 1970s: Overeaters Anonymous and Occasional Mentions
Overeaters Anonymous (OA), a self-help organization based on the 12-step program of Alcoholics Anonymous, was founded in 1960. Accordingly, OA advocates an addiction framework of overeating, and the group’s primary purpose is to abstain from using the identified addictive substance (i.e., certain foods). Little research has been conducted on OA in its more than 50 years of existence, and although participants agree that OA was helpful to them, there is no consensus regarding how OA “works” [ 31 , 32 ]. Nevertheless, OA would not remain the only self-help organization with an addiction perspective on overeating, as similar self-help groups were established in the decades that followed [ 17 ].
Scientific research on the concept of food addiction, however, was virtually non-existent in the 1960s and 1970s, but some researchers sporadically used the term in their articles. For example, food addiction was mentioned along with other substance use problems in two papers by Bell in the 1960s [ 33 , 34 ] and was mentioned in the context of food allergies and otitis media in 1966 [ 35 ]. In 1970, Swanson and Dinello referred to food addiction in the context of high rates of weight regain after weight loss in obese individuals [ 36 ]. To conclude, although there were no efforts to systematically investigate the concept of food addiction in the 1960s and 1970s, it was already used by self-help groups with the aim of reducing overeating and used in scientific articles in the context of or even as a synonym for obesity.
1980s: Focus on Anorexia and Bulimia Nervosa
In the 1980s, some researchers attempted to describe the food restriction displayed by individuals with anorexia nervosa (AN) as an addictive behavior (or “starvation dependence”) [ 37 ]. For example, Szmukler and Tantam [ 38 ] argued that “patients with AN are dependent on the psychological and possibly physiological effects of starvation. Increased weight loss results from tolerance to starvation necessitating greater restriction of food to obtain the desired effect, and the later development of unpleasant ‘withdrawal’ symptoms on eating.” This idea was later facilitated by the discovery of the role of endogenous opioid systems in AN [ 39 , 40 ]. Of note, however, the role of endorphins also was discussed in the opposite condition, that is, obesity [ 41 , 42 ]. Similarly, obesity was investigated under the food addiction framework in a study published in 1989, in which obese persons were compared with normal-weight controls on their level of “object representation” [ 43 ].
There were also some studies on bulimia nervosa (BN) from an addiction perspective, which originated from the field of personality psychology. These studies were preluded by two articles from 1979, which reported elevated scores on a measure of addictive personality in obese individuals [ 44 ] but lower scores in both anorexic and obese individuals as compared to smokers [ 45 ]. Comparative studies between groups of substance dependent and bulimic patients also produced inconsistent findings, with some studies finding similar scores on personality measures across groups and some studies finding differences [ 46 - 49 ]. These studies on addictive personality in BN were accompanied by a case study, in which substance abuse was found to be a useful metaphor in the treatment of BN [ 50 ] and the development of the “Foodaholics Group Treatment Program” [ 51 ].
1990s: Chocoholics and Critical Remarks
Following these first attempts to describe eating disorders as an addiction, there were some comprehensive reviews published in the 1990s and in 2000, in which the addiction model of eating disorders was critically discussed based on conceptual, physiological, and other considerations [ 52 - 55 ]. However, with the exception of a few articles, two in which addictive personality in individuals with eating disorders or obesity were investigated [ 56 , 57 ] and two in which unusual cases of addiction-like carrot consumption were reported [ 58 , 59 ], a new research focus seemed to have emerged: chocolate.
Chocolate is the most often craved food in Western societies, particularly among women [ 60 , 61 ], and the food that people most often have problems with controlling consumption [ 27 , 62 ]. It was already noted in 1989 that chocolate has a combination of high fat and high sugar content, which makes it a “hedonically ideal substance” [ 63 ] — an idea which is similar to speculations about “hyperpalatable” addictive foods some 25 years later [ 3 , 27 ]. In addition to chocolate’s macronutrient composition, other factors like its sensory properties or psychoactive ingredients such as caffeine and theobromine also were discussed as contributors to the addictive-like nature of chocolate [ 64 , 65 ]. However, the xanthine-based effects of chocolate have been found to be unlikely to explain liking for chocolate or its addiction-like consumption [ 61 ].
Few studies were conducted in which so-called “chocoholics” or “chocolate addicts” were investigated. One was a descriptive study reporting craving and consumption patterns among other variables [ 66 ]; another one compared similar measures between “chocolate addicts” and controls [ 67 ]; and one study compared such groups on subjective and physiological responses to chocolate exposure [ 68 ]. A major shortcoming of these studies was, however, that “chocolate addiction” status was based on self-identification, which is vulnerable to bias and validity and is limited by the fact that most nonprofessional participants do not have a precise definition of addiction. Finally, two studies examined associations between “chocolate addiction” and addiction to other substances and behaviors and found positive, but very small, relationships [ 69 , 70 ].
2000s: Animal Models and Neuroimaging
In the early 2000s — approximately 40 years after OA was founded — a pilot study was published in which the treatment of bulimic and obese patients with a 12-step program was reported [ 71 ]. Besides this therapeutic approach, however, the focus of this decade was the examination of neural mechanisms underlying overeating and obesity that may parallel findings from substance dependence. In humans, these neural mechanisms were primarily investigated by positron emission tomography and functional magnetic resonance imaging. For example, a groundbreaking article by Wang and colleagues [ 72 ] reported lower striatal dopamine D 2 receptor availability in obese individuals as compared to controls, which the authors interpreted as a correlate of a “reward deficiency syndrome” similar to what has been found in individuals with substance dependence [ 73 , 74 ]. Other studies, for example, found that similar brain areas are activated during the experience of food and drug craving, and studies in which neural responses to high-calorie food stimuli were investigated found that individuals with BN and BED exhibit higher activation in reward-related brain areas as compared to controls, just like individuals with substance dependence show higher reward-related activity in response to substance-related cues [ 75 , 76 ].
Another important line of food addiction research in this decade was rodent models. In one of these paradigms, rats are food deprived daily for 12 hours and then given 12-hour access to both a sugar solution and chow [ 77 ]. Rats who underwent this schedule of intermittent access to sugar and chow for several weeks were found to exhibit behavioral symptoms of addiction such as withdrawal when access to sugar was removed, and they also showed neurochemical changes [ 77 , 78 ]. Other studies found that rats provided with a high-calorie “cafeteria” diet gained weight, which was accompanied by a downregulation of striatal dopamine D 2 receptors and continued consumption of palatable foods despite aversive consequences [ 79 ]. To conclude, these studies suggest that consumption of high amounts of sugar may indeed lead to addiction-like behavior and, in combination with high fat intake, to weight gain in rodents [ 80 ] and that overlapping neural circuits are involved in the processing of food- and drug-related cues and in the control of eating behavior and substance use, respectively.
2010s: Assessment of Food Addiction in Humans and Progress in Animal Research
In recent years, researchers have tried to more precisely define and assess food addiction. For example, Cassin and von Ranson [ 81 ] substituted references to “substance” with “binge eating” in a structured interview of the substance dependence criteria in the fourth revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) and found that 92 percent of participants with BED met the full criteria for substance dependence. Another approach was the development of the Yale Food Addiction Scale (YFAS), which is a self-report measure for the assessment of symptoms of food addiction based on the diagnostic criteria for substance dependence in the DSM-IV [ 82 ]. Specifically, the YFAS measures the seven symptoms for substance dependence as stated in the DSM-IV with all items referring to food and eating: 1) taking the substance in larger amounts or for a longer period than intended (e.g., “I find myself continuing to consume certain foods even though I am no longer hungry.”); 2) persistent desire or repeated unsuccessful attempts to quit (e.g., “Not eating certain types of food or cutting down on certain types of food is something I worry about.”); 3) spending much time to obtain or use the substance or recover from its effects (e.g., “I find that when certain foods are not available, I will go out of my way to obtain them. For example, I will drive to the store to purchase certain foods even though I have other options available to me at home.”); 4) giving up important social, occupational, or recreational activities due to substance use (e.g., “There have been times when I consumed certain foods so often or in such large quantities that I started to eat food instead of working, spending time with my family or friends, or engaging in other important activities or recreational activities I enjoy.”); 5) continued substance use despite psychological or physical problems (e.g., “I kept consuming the same types of food or the same amount of food even though I was having emotional and/or physical problems.”); 6) tolerance (e.g., “Over time, I have found that I need to eat more and more to get the feeling I want, such as reduced negative emotions or increased pleasure.”); and 7) withdrawal symptoms (e.g., “I have had withdrawal symptoms such as agitation, anxiety, or other physical symptoms when I cut down or stopped eating certain foods.”). Two additional items assess the presence of a clinically significant impairment or distress resulting from overeating. Similar to the DSM-IV, food addiction can be “diagnosed” if at least three symptoms are met and a clinically significant impairment or distress is present [ 82 , 83 ].
The YFAS has been employed in a considerable number of studies in the past 6 years, which show that individuals with a food addiction “diagnosis” can be differentiated from those without a “diagnosis” on numerous variables ranging from self-report measures of eating pathology, psychopathology, emotion regulation, or impulsivity to physiological and behavioral measures such as a multilocus genetic profile associated with dopaminergic signaling or motor responses to high-calorie food-cues [ 62 ]. Although the YFAS has proved to be a useful tool for the investigation of addictive-like eating, it is, of course, not perfect and its validity has been questioned [ 84 ]. For example, it has been found that approximately 50 percent of obese adults with BED receive a YFAS diagnosis and that these individuals show higher eating-related and general psychopathology than obese adults with BED who do not receive a YFAS diagnosis [ 85 , 86 ]. In the light of these findings, it has been argued that food addiction as measured with the YFAS may merely represent a more severe form of BED [ 87 , 88 ]. Furthermore, the food addiction model continues to be a heavily debated topic with some researchers strongly supporting its validity [ 3 , 7 , 21 , 89 - 91 ], while others argue against it based on different physiological effects of drugs of abuse and specific nutrients such as sugar, conceptual considerations, and other issues [ 84 , 92 - 97 ]. Most recently, it has been proposed that even if there is a kind of eating behavior that may be called an addiction, the term food addiction is misguided as there is no clear addictive agent, and, thus, it should be rather considered as a behavioral addiction (i.e., “eating addiction”) [ 98 ].
Animal research on food addiction has progressed in recent years as well. This includes, for example, a plethora of studies showing differential effects of specific nutrient components (e.g., high-fat diet, high-sugar diet, combined high-fat and high-sugar diet, or high-protein diet) on eating behavior and neurochemistry [ 99 , 100 ]. Other research demonstrates that certain eating regimes also can affect offspring in rodents. For instance, it has been found that in utero exposure to a highly palatable diet influences food preferences, metabolic dysregulations, brain-reward functioning, and the risk for obesity [ 99 , 101 ]. New paradigms for the assessment of food addiction-like behavior have been employed, which measure, for example, compulsive food intake under aversive circumstances [ 102 ]. Finally, application of certain drugs, which reduces substance use in rats, has been found to reduce addiction-like intake of palatable foods [ 103 ].
Conclusions and Future Directions
The term addiction was already used in reference to food by the end of the 19th century. In the middle of the 20th century, the term food addiction was widely used, not only among laypersons but also among scientists. However, it was also poorly (if at all) defined, and the term often was used without scrutiny. Empirical articles aiming at validating the concept of food addiction in humans were lacking in most decades of the 20th century, and an addiction model of eating disorders and obesity was more critically discussed by the end of the century. Food addiction research underwent several paradigm shifts, which involved, for example, a focus on obesity in the middle of the 20th century, a focus on AN and BN in the 1980s, a focus on chocolate in the 1990s, and a focus on BED and — again — obesity in the 2000s in light of results from animal and neuroimaging studies.
Thus, although research on food addiction has increased substantially in recent years, neither is it a new idea nor was it conceptualized to explain the rising prevalence rates of obesity. The aim of this article is to increase awareness of the long history of the food addiction concept and its dynamically changing scientific paradigms and methods. If researchers reflect on this history, it may be easier to find a consensus about what is actually meant by food addiction and it may inspire important next steps that have to be taken, and, thus, progress in this field of research will be facilitated [ 104 ].
For example, many themes that revived in the last couple of years were already discussed a few decades ago. These include, for example, studies on an addictive personality underlying both overeating and substance use [ 105 , 106 ] or the idea of considering AN as an addiction [ 107 , 108 ], with both topics being present as early as the 1980s. The idea of considering BN as an addiction [ 109 ] also dates back several decades. Thus, it appears that the focus on obesity in the context of food addiction in recent years (e.g., [ 13 , 110 ]) seems somewhat misguided, considering that researchers stated decades ago that addiction-like eating is neither restricted to individuals with obesity nor can obesity be equated with food addiction [ 28 , 50 ].
Another recurring theme seems to concern the measurement of food addiction. As stated above, there were some studies in the 1990s in which food addiction was based on self-identification. This issue was taken up again in recent studies, which show that there is a large mismatch between food addiction classification based on the YFAS and self-perceived food addiction [ 111 , 112 ], thus implying that individuals’ own definition or experience of food addiction is not consistent with the substance use model proposed by the YFAS. Although researchers do not agree about the precise definitions of food addiction symptoms yet [ 84 , 113 ], it appears that standardized measures such as the YFAS are necessary to prevent over-classification of food addiction. Although the rationale behind the YFAS, namely translating substance dependence criteria of the DSM to food and eating, is straightforward, it also has been criticized as it differs from definitions that other researchers have about addiction [ 93 , 98 ]. Thus, an important future direction may be if and how food addiction can be measured in humans other than using the YFAS.
If food addiction research will be guided by the translation of DSM substance dependence criteria to food and eating in the future, an important question will be which implications arise from the changes in the diagnostic criteria for substance dependence in the fifth revision of the DSM for food addiction [ 114 ]. For example, are all addiction criteria (as described in the DSM-5) equally applicable to human eating behavior? If not, does this obliterate the concept of food addiction?
Besides these basic questions about the definition and measurement of food addiction, other important avenues for future research may include, but are not limited to: How relevant is the concept of food addiction for the treatment of obesity or binge eating and in public policy making? If it is relevant, how can it be implemented best [ 17 , 91 ]? What are the disadvantages (if any) of the concept of food addiction [ 115 - 119 ]? How can animal models of addiction-like eating be improved to more specifically reflect relevant processes in humans [ 120 ]? Can addiction-like eating actually be reduced to the addictive effects of one or more substances or should “food addiction” be replaced by “eating addiction” [ 98 ]?
Although food addiction has been discussed in the scientific community for decades, it remains a highly controversial and heavily debated topic, which, of course, makes it an exciting field of research. Notwithstanding that scientific output on this topic rapidly increased in the last couple of years, its systematic investigation is still in its infancy, and, thus, research efforts will most likely increase in the years to come.
Acknowledgments
The author is supported by a grant of the European Research Council (ERC-StG-2014 639445 NewEat).
Abbreviations
- Tarman V, Werdell P. Food Junkies: The truth about food addiction. Toronto, Canada: Dundurn; 2014. [ Google Scholar ]
- Avena NM, Talbott JR. Why diets fail (because you're addicted to sugar) New York: Ten Speed Press; 2014. [ Google Scholar ]
- Gearhardt AN, Davis C, Kuschner R, Brownell KD. The addiction potential of hyperpalatable foods. Curr Drug Abuse Rev. 2011; 4 :140–145. [ PubMed ] [ Google Scholar ]
- Krashes MJ, Kravitz AV. Optogenetic and chemogenetic insights into the food addiction hypothesis. Front Behav Neurosci. 2014; 8 (57):1–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brownell KD, Gold MS. Food and addiction - a comprehensive handbook. New York: Oxford University Press; 2012. p. xxii. [ Google Scholar ]
- Cocores JA, Gold MS. The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Med Hypotheses. 2009; 73 :892–899. [ PubMed ] [ Google Scholar ]
- Shriner R, Gold M. Food addiction: an evolving nonlinear science. Nutrients. 2014; 6 :5370–5391. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shriner RL. Food addiction: detox and abstinence reinterpreted? Exp Gerontol. 2013; 48 :1068–1074. [ PubMed ] [ Google Scholar ]
- Ifland JR, Preuss HG, Marcus MT, Rourk KM, Taylor WC, Burau K. et al. Refined food addiction: a classic substance use disorder. Med Hypotheses. 2009; 72 :518–526. [ PubMed ] [ Google Scholar ]
- Thornley S, McRobbie H, Eyles H, Walker N, Simmons G. The obesity epidemic: is glycemic index the key to unlocking a hidden addiction? Med Hypotheses. 2008; 71 :709–714. [ PubMed ] [ Google Scholar ]
- Pelchat ML. Food addiction in humans. J Nutr. 2009; 139 :620–622. [ PubMed ] [ Google Scholar ]
- Corsica JA, Pelchat ML. Food addiction: true or false? Curr Opin Gastroenterol. 2010; 26 (2):165–169. [ PubMed ] [ Google Scholar ]
- Barry D, Clarke M, Petry NM. Obesity and its relationship to addictions: is overeating a form of addictive behavior? Am J Addict. 2009; 18 :439–451. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. The addictive dimensionality of obesity. Biol Psychiatry. 2013; 73 :811–818. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013; 14 :2–18. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009; 53 :1–8. [ PubMed ] [ Google Scholar ]
- Davis C, Carter JC. If certain foods are addictive, how might this change the treatment of compulsive overeating and obesity? Curr Addict Rep. 2014; 1 :89–95. [ Google Scholar ]
- Lee NM, Carter A, Owen N, Hall WD. The neurobiology of overeating. Embo Rep. 2012; 13 :785–790. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gearhardt AN, Bragg MA, Pearl RL, Schvey NA, Roberto CA, Brownell KD. Obesity and public policy. Annu Rev Clin Psychol. 2012; 8 :405–430. [ PubMed ] [ Google Scholar ]
- Gearhardt AN, Corbin WR, Brownell KD. Food addiction - an examination of the diagnostic criteria for dependence. J Addict Med. 2009; 3 :1–7. [ PubMed ] [ Google Scholar ]
- Gearhardt AN, Grilo CM, Corbin WR, DiLeone RJ, Brownell KD, Potenza MN. Can food be addictive? Public health and policy implications. Addiction. 2011; 106 :1208–1212. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Weiner B, White W. The Journal of Inebriety (1876-1914): history, topical analysis, and photographic images. Addiction. 2007; 102 :15–23. [ PubMed ] [ Google Scholar ]
- Clouston TS. Diseased cravings and paralyzed control: dipsomania; morphinomania; chloralism; cocainism. J Inebr. 1890; 12 :203–245. [ Google Scholar ]
- Wulff M. Über einen interessanten oralen Symptomenkomplex und seine Beziehungen zur Sucht. Int Z Psychoanal. 1932; 18 :281–302. [ Google Scholar ]
- Thorner HA. On compulsive eating. J Psychsom Res. 1970; 14 :321–325. [ PubMed ] [ Google Scholar ]
- Randolph TG. The descriptive features of food addiction: Addictive eating and drinking. Q J Stud Alcohol. 1956; 17 :198–224. [ PubMed ] [ Google Scholar ]
- Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE. 2015; 10 (2):e0117959. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hinkle LE, Knowles HC, Fischer A, Stunkard AJ. Role of environment and personality in management of the difficult patient with diabetes mellitus - panel discussion. Diabetes. 1959; 8 :371–378. [ PubMed ] [ Google Scholar ]
- Allison KC, Berkowitz RI, Brownell KD, Foster GD, Wadden TA. Albert J. (“Mickey”) Stunkard, M.D. Obesity. 2014; 22 :1937–1938. [ PubMed ] [ Google Scholar ]
- Stunkard AJ. Eating patterns and obesity. Psychiatr Q. 1959; 33 :284–295. [ PubMed ] [ Google Scholar ]
- Russel-Mayhew S, von Ranson KM, Masson PC. How does Overeaters Anonymous help its members? A qualitative analysis. Eur Eat Disord Rev. 2010; 18 :33–42. [ PubMed ] [ Google Scholar ]
- Weiner S. The addiction of overeating: self-help groups as treatment models. J Clin Psychol. 1998; 54 :163–167. [ PubMed ] [ Google Scholar ]
- Bell RG. A method of clinical orientation to alcohol addiction. Can Med Assoc J. 1960; 83 :1346–1352. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bell RG. Defensive thinking in alcohol addicts. Can Med Assoc J. 1965; 92 :228–231. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Clemis JD, Shambaugh GE Jr., Derlacki EL. Withdrawal reactions in chronic food addiction as related to chronic secretory otitis media. Ann Otol Rhinol Laryngol. 1966; 75 :793–797. [ PubMed ] [ Google Scholar ]
- Swanson DW, Dinello FA. Follow-up of patients starved for obesity. Psychosom Med. 1970; 32 :209–214. [ PubMed ] [ Google Scholar ]
- Scott DW. Alcohol and food abuse: some comparisons. Br J Addict. 1983; 78 :339–349. [ PubMed ] [ Google Scholar ]
- Szmukler GI, Tantam D. Anorexia nervosa: Starvation dependence. Br J Med Psychol. 1984; 57 :303–310. [ PubMed ] [ Google Scholar ]
- Marrazzi MA, Luby ED. An auto-addiction opioid model of chronic anorexia nervosa. lnt J Eat Disord. 1986; 5 :191–208. [ Google Scholar ]
- Marrazzi MA, Mullingsbritton J, Stack L, Powers RJ, Lawhorn J, Graham V. et al. Atypical endogenous opioid systems in mice in relation to an auto-addiction opioid model of anorexia nervosa. Life Sci. 1990; 47 :1427–1435. [ PubMed ] [ Google Scholar ]
- Gold MS, Sternbach HA. Endorphins in obesity and in the regulation of appetite and weight. Integr Psychiatry. 1984; 2 :203–207. [ Google Scholar ]
- Wise J. Endorphins and metabolic control in the obese: a mechanism for food addiction. J Obes Weight Reg. 1981; 1 :165–181. [ Google Scholar ]
- Raynes E, Auerbach C, Botyanski NC. Level of object representation and psychic structure deficit in obese persons. Psychol Rep. 1989; 64 :291–294. [ PubMed ] [ Google Scholar ]
- Leon GR, Eckert ED, Teed D, Buchwald H. Changes in body image and other psychological factors after intestinal bypass surgery for massive obesity. J Behav Med. 1979; 2 :39–55. [ PubMed ] [ Google Scholar ]
- Leon GR, Kolotkin R, Korgeski G. MacAndrew Addiction Scale and other MMPI characteristics associated with obesity, anorexia and smoking behavior. Addict Behav. 1979; 4 :401–407. [ PubMed ] [ Google Scholar ]
- Feldman J, Eysenck S. Addictive personality traits in bulimic patients. Pers Indiv Diff. 1986; 7 :923–926. [ Google Scholar ]
- de Silva P, Eysenck S. Personality and addictiveness in anorexic and bulimic patients. Pers Indiv Diff. 1987; 8 :749–751. [ Google Scholar ]
- Hatsukami D, Owen P, Pyle R, Mitchell J. Similarities and differences on the MMPI between women with bulimia and women with alcohol or drug abuse problems. Addict Behav. 1982; 7 :435–439. [ PubMed ] [ Google Scholar ]
- Kagan DM, Albertson LM. Scores on MacAndrew Factors - Bulimics and other addictive populations. Int J Eat Disord. 1986; 5 :1095–1101. [ Google Scholar ]
- Slive A, Young F. Bulimia as substance abuse: a metaphor for strategic treatment. J Strategic Syst Ther. 1986; 5 :71–84. [ Google Scholar ]
- Stoltz SG. Recovering from foodaholism. J Special Group Work. 1984; 9 :51–61. [ Google Scholar ]
- Vandereycken W. The addiction model in eating disorders: some critical remarks and a selected bibliography. Int J Eat Disord. 1990; 9 :95–101. [ Google Scholar ]
- Wilson GT. The addiction model of eating disorders: a critical analysis. Adv Behav Res Ther. 1991; 13 :27–72. [ Google Scholar ]
- Wilson GT. Eating disorders and addiction. Drugs Soc. 1999; 15 :87–101. [ Google Scholar ]
- Rogers PJ, Smit HJ. Food craving and food “addiction”: a critical review of the evidence from a biopsychosocial perspective. Pharmacol Biochem Behav. 2000; 66 :3–14. [ PubMed ] [ Google Scholar ]
- Kayloe JC. Food addiction. Psychotherapy. 1993; 30 :269–275. [ Google Scholar ]
- Davis C, Claridge G. The eating disorders as addiction: A psychobiological perspective. Addict Behav. 1998; 23 :463–475. [ PubMed ] [ Google Scholar ]
- Černý L, Černý K. Can carrots be addictive? An extraordinary form of drug dependence. Br J Addict. 1992; 87 :1195–1197. [ PubMed ] [ Google Scholar ]
- Kaplan R. Carrot addiction. Aust N Z J Psychiatry. 1996; 30 :698–700. [ PubMed ] [ Google Scholar ]
- Weingarten HP, Elston D. Food cravings in a college population. Appetite. 1991; 17 :167–175. [ PubMed ] [ Google Scholar ]
- Rozin P, Levine E, Stoess C. Chocolate craving and liking. Appetite. 1991; 17 :199–212. [ PubMed ] [ Google Scholar ]
- Meule A, Gearhardt AN. Five years of the Yale Food Addiction Scale: taking stock and moving forward. Curr Addict Rep. 2014; 1 :193–205. [ Google Scholar ]
- Max B. This and that: chocolate addiction, the dual pharmacogenetics of asparagus eaters, and the arithmetic of freedom. Trends Pharmacol Sci. 1989; 10 :390–393. [ PubMed ] [ Google Scholar ]
- Bruinsma K, Taren DL. Chocolate: food or drug? J Am Diet Assoc. 1999; 99 :1249–1256. [ PubMed ] [ Google Scholar ]
- Patterson R. Recovery from this addiction was sweet indeed. Can Med Assoc J. 1993; 148 :1028–1032. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hetherington MM, Macdiarmid JI. “Chocolate addiction”: a preliminary study of its description and its relationship to problem eating. Appetite. 1993; 21 :233–246. [ PubMed ] [ Google Scholar ]
- Macdiarmid JI, Hetherington MM. Mood modulation by food: an exploration of affect and cravings in ‘chocolate addicts’ Br J Clin Psychol. 1995; 34 :129–138. [ PubMed ] [ Google Scholar ]
- Tuomisto T, Hetherington MM, Morris MF, Tuomisto MT, Turjanmaa V, Lappalainen R. Psychological and physiological characteristics of sweet food “addiction” Int J Eat Disord. 1999; 25 :169–175. [ PubMed ] [ Google Scholar ]
- Rozin P, Stoess C. Is there a general tendency to become addicted? Addict Behav. 1993; 18 :81–87. [ PubMed ] [ Google Scholar ]
- Greenberg JL, Lewis SE, Dodd DK. Overlapping addictions and self-esteem among college men and women. Addict Behav. 1999; 24 :565–571. [ PubMed ] [ Google Scholar ]
- Trotzky AS. The treatment of eating disorders as addiction among adolescent females. Int J Adolesc Med Health. 2002; 14 :269–274. [ PubMed ] [ Google Scholar ]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W. et al. Brain dopamine and obesity. Lancet. 2001; 357 :354–357. [ PubMed ] [ Google Scholar ]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc B. 2008; 363 :3191–3200. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005; 8 :555–560. [ PubMed ] [ Google Scholar ]
- Schienle A, Schäfer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009; 65 :654–661. [ PubMed ] [ Google Scholar ]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004; 23 :1486–1493. [ PubMed ] [ Google Scholar ]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008; 32 :20–39. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Avena NM. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp Clin Psychopharmacol. 2007; 15 :481–491. [ PubMed ] [ Google Scholar ]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010; 13 :635–641. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009; 139 :623–628. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cassin SE, von Ranson KM. Is binge eating experienced as an addiction? Appetite. 2007; 49 :687–690. [ PubMed ] [ Google Scholar ]
- Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009; 52 :430–436. [ PubMed ] [ Google Scholar ]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [ Google Scholar ]
- Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. 2012; 13 :279–286. [ PubMed ] [ Google Scholar ]
- Gearhardt AN, White MA, Masheb RM, Grilo CM. An examination of food addiction in a racially diverse sample of obese patients with binge eating disorder in primary care settings. Compr Psychiatry. 2013; 54 :500–505. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gearhardt AN, White MA, Masheb RM, Morgan PT, Crosby RD, Grilo CM. An examination of the food addiction construct in obese patients with binge eating disorder. Int J Eat Disord. 2012; 45 :657–663. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Davis C. Compulsive overeating as an addictive behavior: overlap between food addiction and Binge Eating Disorder. Curr Obes Rep. 2013; 2 :171–178. [ Google Scholar ]
- Davis C. From passive overeating to “food addiction”: A spectrum of compulsion and severity. ISRN Obesity. 2013; 2013 (435027):1–20. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Avena NM, Gearhardt AN, Gold MS, Wang GJ, Potenza MN. Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data. Nat Rev Neurosci. 2012; 13 :514. [ PubMed ] [ Google Scholar ]
- Avena NM, Gold MS. Food and addiction - sugars, fats and hedonic overeating. Addiction. 2011; 106 :1214–1215. [ PubMed ] [ Google Scholar ]
- Gearhardt AN, Brownell KD. Can food and addiction change the game? Biol Psychiatry. 2013; 73 :802–803. [ PubMed ] [ Google Scholar ]
- Ziauddeen H, Farooqi IS, Fletcher PC. Food addiction: is there a baby in the bathwater? Nat Rev Neurosci. 2012; 13 :514. [ Google Scholar ]
- Ziauddeen H, Fletcher PC. Is food addiction a valid and useful concept? Obes Rev. 2013; 14 :19–28. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Benton D. The plausibility of sugar addiction and its role in obesity and eating disorders. Clin Nutr. 2010; 29 :288–303. [ PubMed ] [ Google Scholar ]
- Wilson GT. Eating disorders, obesity and addiction. Eur Eat Disord Rev. 2010; 18 :341–351. [ PubMed ] [ Google Scholar ]
- Rogers PJ. Obesity - is food addiction to blame? Addiction. 2011; 106 :1213–1214. [ PubMed ] [ Google Scholar ]
- Blundell JE, Finlayson G. Food addiction not helpful: the hedonic component - implicit wanting - is important. Addiction. 2011; 106 :1216–1218. [ PubMed ] [ Google Scholar ]
- Hebebrand J, Albayrak O, Adan R, Antel J, Dieguez C, de Jong J. et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci Biobehav Rev. 2014; 47 :295–306. [ PubMed ] [ Google Scholar ]
- Avena NM, Gold JA, Kroll C, Gold MS. Further developments in the neurobiology of food and addiction: update on the state of the science. Nutrition. 2012; 28 :341–343. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tulloch AJ, Murray S, Vaicekonyte R, Avena NM. Neural responses to macronutrients: hedonic and homeostatic mechanisms. Gastroenterology. 2015; 148 :1205–1218. [ PubMed ] [ Google Scholar ]
- Borengasser SJ, Kang P, Faske J, Gomez-Acevedo H, Blackburn ML, Badger TM. et al. High fat diet and in utero exposure to maternal obesity disrupts circadian rhythm and leads to metabolic programming of liver in rat offspring. PLoS ONE. 2014; 9 (1):e84209. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Velázquez-Sánchez C, Ferragud A, Moore CF, Everitt BJ, Sabino V, Cottone P. High trait impulsivity predicts food addiction-like behavior in the rat. Neuropsychopharmacology. 2014; 39 :2463–2472. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bocarsly ME, Hoebel BG, Paredes D, von Loga I, Murray SM, Wang M. et al. GS 455534 selectively suppresses binge eating of palatable food and attenuates dopamine release in the accumbens of sugar-bingeing rats. Behav Pharmacol. 2014; 25 :147–157. [ PubMed ] [ Google Scholar ]
- Schulte EM, Joyner MA, Potenza MN, Grilo CM, Gearhardt A. Current considerations regarding food addiction. Curr Psychiat Rep. 2015; 17 (19):1–8. [ PubMed ] [ Google Scholar ]
- Lent MR, Swencionis C. Addictive personality and maladaptive eating behaviors in adults seeking bariatric surgery. Eat Behav. 2012; 13 :67–70. [ PubMed ] [ Google Scholar ]
- Davis C. A narrative review of binge eating and addictive behaviors: shared associations with seasonality and personality factors. Front Psychiatry. 2013; 4 (183):1–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barbarich-Marsteller NC, Foltin RW, Walsh BT. Does anorexia nervosa resemble an addiction? Curr Drug Abuse Rev. 2011; 4 :197–200. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Speranza M, Revah-Levy A, Giquel L, Loas G, Venisse JL, Jeammet P. et al. An investigation of Goodman's addictive disorder criteria in eating disorders. Eur Eat Disord Rev. 2012; 20 :182–189. [ PubMed ] [ Google Scholar ]
- Umberg EN, Shader RI, Hsu LK, Greenblatt DJ. From disordered eating to addiction: the “food drug” in bulimia nervosa. J Clin Psychopharmacol. 2012; 32 :376–389. [ PubMed ] [ Google Scholar ]
- Grosshans M, Loeber S, Kiefer F. Implications from addiction research towards the understanding and treatment of obesity. Addict Biol. 2011; 16 :189–198. [ PubMed ] [ Google Scholar ]
- Hardman CA, Rogers PJ, Dallas R, Scott J, Ruddock HK, Robinson E. “Food addiction is real”. The effects of exposure to this message on self-diagnosed food addiction and eating behaviour. Appetite. 2015; 91 :179–184. [ PubMed ] [ Google Scholar ]
- Meadows A, Higgs S. I think, therefore I am? Characteristics of a non-clinical population of self-perceived food addicts. Appetite. 2013; 71 :482. [ Google Scholar ]
- Meule A, Kübler A. The translation of substance dependence criteria to food-related behaviors: different views and interpretations. Front Psychiatry. 2012; 3 (64):1–2. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Meule A, Gearhardt AN. Food addiction in the light of DSM-5. Nutrients. 2014; 6 :3653–3671. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- DePierre JA, Puhl RM, Luedicke J. A new stigmatized identity? Comparisons of a “food addict” label with other stigmatized health conditions. Basic Appl Soc Psych. 2013; 35 :10–21. [ Google Scholar ]
- DePierre JA, Puhl RM, Luedicke J. Public perceptions of food addiction: a comparison with alcohol and tobacco. J Subst Use. 2014; 19 :1–6. [ Google Scholar ]
- Latner JD, Puhl RM, Murakami JM, O’Brien KS. Food addiction as a causal model of obesity. Effects on stigma, blame, and perceived psychopathology. Appetite. 2014; 77 :77–82. [ PubMed ] [ Google Scholar ]
- Lee NM, Hall WD, Lucke J, Forlini C, Carter A. Food addiction and its impact on weight-based stigma and the treatment of obese individuals in the U.S. and Australia. Nutrients. 2014; 6 :5312–5326. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lee NM, Lucke J, Hall WD, Meurk C, Boyle FM, Carter A. Public views on food addiction and obesity: implications for policy and treatment. PLoS ONE. 2013; 8 (9):e74836. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Avena NM. The study of food addiction using animal models of binge eating. Appetite. 2010; 55 :734–737. [ PMC free article ] [ PubMed ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Abstract. Food addiction is considered an important link for a better understanding of psychiatric and medical problems triggered by dysfunctions of eating behaviors, e. g., obesity, metabolic syndrome, binge eating disorder, or bulimia nervosa. At behavioral level, food addiction has high degrees of similarity with other eating disorders, a ...
Due to the proliferative nature of research on food addiction, two searches were done: the first was completed on 29 June 2016, and the second was completed on 8 January 2018. Protocols were followed for both searches exactly as described above, with the exception that the second search included only articles published since 30 June 2016. ...
However, with several DSM-5 criteria having limited application to overeating, the term 'food addiction' is likely to apply only in a minority of cases. Nevertheless, research investigating the underlying psychological causes of overeating within the context of food addiction has led to some novel and potentially effective interventions.
Purpose of Review The majority of existing research discusses food addiction (FA) classification, which provides information for different groups and which groups may or may not be affected to differing degrees. Fewer studies report FA symptom scores, and fewer still report on individual symptoms. This paper discusses the symptoms of craving and loss of control as they are common FA symptoms ...
An increasing number of studies have established the prevalence and correlates of food addiction. Moreover, food addiction may be associated with obesity and disordered eating. Thus, intervening on food addiction may be helpful in the prevention and therapy of obesity and eating disorders. However, controversy exists about if this phenomenon is ...
Abstract. As ultraprocessed foods (i.e., foods composed of mostly cheap industrial sources of dietary energy and nutrients plus additives) have become more abundant in our food supply, rates of obesity and diet-related disease have increased simultaneously. Food addiction has emerged as a phenotype of significant empirical interest within the ...
The past decade has seen a surge in research on the topic of "food addiction" [].Although the rewarding nature of food has been recognized for some time [], it is only recently that an empirical measure of food addiction was developed [].Sophisticated neuroimaging techniques and behavioral paradigms have revealed overlap in responses to food and addictive drugs, and studies in both humans ...
Interestingly, a scale of food addiction—the Yale Food Addiction Scale (YFAS)—has been developed based on similar measures. This is a 25-point self-report questionnaire meant to discover the impact of addictive behaviors related to food, based on substance dependence criteria from the DSM. Table 2 shows this scale. The survey examines ...
Purpose of Review To summarize recent neurobiological evidence for (1) the addictive potential of ultra-processed foods and (2) the utility of food addiction, defined by behavioral criteria, as a clinically meaningful type of disordered eating. Recent Findings Ultra-processed foods appear to be capable of triggering biobehavioral mechanisms associated with addiction (e.g., dopaminergic ...
The application of the term "food addiction" in humans is based on a set of features, held to resemble substance addictions. It carries the claim that this resemblance occurs because certain ...
Current state of research on food addiction. The concept of food addiction refers to specific food-related behaviours characterised by excessive and dysregulated consumption of high-energy food (Reference Imperatori, Fabbricatore and Vumbaca 9).Research on food addiction began in 1956 (Reference Randolph 10).However, much attention has been given to this concept in recent years as new methods ...
Editorial on the Research Topic. Neurobiology of food addiction. Food addiction (FA) is an intriguing issue that has received significant attention in recent years. The concept of "food addiction," which refers to food as an addictive-potential factor, was first described in Randolph (1956). However, in the last two decades, this topic has ...
Back by popular demand: A narrative review on the history of food addiction research. The Yale Journal of Biology and Medicine. 2015; 88 (3):295-302. [PMC free article] [Google Scholar] Meule A, Kubler A. Food cravings in food addiction: The distinct role of positive reinforcement.
In 2009 the Yale Food Addiction Scale emerged. It is used to assess whether a person displays behavioral patterns that would merit fries, shakes and other palatable foods being classified as ...
Food Addiction, Psychopathology and Weight- and Addiction-Related Constructs. Correlation analyses revealed a significant positive association (r=0.17, p=0.014) between symptom severity of FA and BMI at baseline assessment ().With regard to psychopathology, FA symptom severity was positively correlated to severity of depressive symptoms (r=0.33, p<0.001), severity of IUD (r=0.18, p=0.011) and ...
Background 'Food addiction' shares a similar neurobiological and behavioral framework with substance addiction. However whether, and to what degree, 'food addiction' contributes to obesity in the general population is unknown. Objectives to assess 1) the prevalence of 'food addiction' in the Newfoundland population; 2) if clinical symptom counts of 'food addiction' were ...
Purpose of Review The current systematic review examined the empirical literature on psychosocial interventions for food addiction with the goal of providing recommendations for clinical practice and future research. A PsycINFO and PubMed search of publications was conducted in September 2019. Two authors assessed retrieved titles and abstracts to determine topic relevance and rated the ...
But Dr. Hebebrand disputes the notion that any food is addictive. While potato chips and pizza can seem irresistible to some, he argues that they do not cause an altered state of mind, a hallmark ...
Food addiction (FA) is characterised by diminished control over the consumption of certain foods (e.g., hyper-palatable energy-dense foods), which persists despite growing negative health consequences [ 1, 2 ]. Research on the concept of FA has steadily been growing [ 3] and self-reported FA is estimated to affect between 16-20% of the ...
Loneliness and social isolation can be both contributing factors to — or consequences of — food addiction. In the Michigan Medicine research, addiction to highly processed foods was seen in 51 ...
According to 2019 research, three positions summarize the current debate around food addiction:. The addictive potential of certain foods, such as those with high levels of carbohydrates or fat ...
Over time, this pattern of eating can lead to weight gain and increase the risk of obesity-related health problems. "If you have more cravings, you eat more and may have more anxiety or ...
Food addiction, as an explanation for the often-distressing cravings, loss of control, ... And yet, there is a continued willingness to diagnose, measure, and apply food addiction as a validated concept in research. The strong narrative around food addiction is, of course, understandable. A person prone to binges or overeating in general feels ...
That's a conclusion easily drawn after witnessing a person suffering from addiction, but this week, a team of scientists from several U.S. institutions published an article in the journal Science that describes part of the mechanism responsible for such behavior, and suggests the possibility of new solutions for addicts.
Scientific research on the concept of food addiction, however, was virtually non-existent in the 1960s and 1970s, but some researchers sporadically used the term in their articles. For example, food addiction was mentioned along with other substance use problems in two papers by Bell in the 1960s [33,34] and was mentioned in the context of food ...