
HOWARD TRACER, MD, Medical Officer, U.S. Preventive Services Task Force, Agency for Healthcare Research and Quality
LAURA CATON GILSTRAP, MD, Preventive Medicine and Biostatistics Residency, Uniformed Services University of the Health Sciences
Am Fam Physician. 2020;101(12):753-754
Related U.S. Preventive Services Task Force Recommendation Statement: Screening for Cognitive Impairment in Older Adults: Recommendation Statement
Author disclosure: No relevant financial affiliations.
L.C. is a 70-year-old Asian American woman who retired from teaching middle school five years ago. She lives independently and has a daughter and grandchildren who live within 30 miles of her. She presents for a follow-up visit to renew her hypertension medications. Her blood pressure is well controlled by an angiotensin receptor blocker, and she takes no other medications. She does not smoke, and she drinks two or three glasses of wine per week. She states that she is trying to stay active but is finding it difficult to make new friends and learn new skills. She is worried about her memory and wants to know whether she should be screened for cognitive impairment.

Case Study Questions
1 . Based on the U.S. Preventive Services Task Force (USPSTF) recommendation on screening for cognitive impairment, what would you recommend for L.C.?
A. L.C. should be screened because the USPSTF found convincing evidence that the net benefit of screening in adults 65 years and older is substantial.
B. L.C. should be screened because she has hypertension.
C. L.C. should not be screened because the USPSTF recommends screening only in older adults who are current smokers.
D. It is uncertain whether L.C. should be screened. The USPSTF found insufficient evidence to assess the balance of benefits and harms of screening for cognitive impairment in older adults.
E. L.C. should not be screened because the USPSTF found adequate evidence that screening for cognitive impairment is of no net benefit.
2 . According to the USPSTF recommendation statement, which of the following statements about screening tests for cognitive impairment are correct?
A. Some screening tools have relatively high sensitivity and specificity for the detection of dementia.
B. Screening tools have a positive predictive value approaching 80% for people who are in their 60s.
C. Screening tools generally have lower sensitivity and specificity for the detection of mild cognitive impairment than for the detection of dementia.
D. A positive result on a screening tool confirms the diagnosis of dementia.
3 . Based on the USPSTF recommendation, which one of the following statements about cognitive impairment is correct?
A. Mild cognitive impairment almost always progresses to dementia.
B. Increasing age is the highest risk factor for cognitive impairment.
C. Cardiovascular risk factors (e.g., hypertension, diabetes mellitus) are not associated with risk of dementia.
D. Mild cognitive impairment and dementia interfere with independent daily functioning.
E. Dementia currently affects an estimated 8 to 9 million people in the United States.
1. The correct answer is D . The USPSTF found insufficient evidence to assess the balance of benefits and harms of screening for cognitive impairment in older adults (I statement). 1 The USPSTF’s recommendation applies to community-dwelling adults 65 years or older without recognized signs or symptoms of cognitive impairment. The USPSTF’s I statement is not contingent on the presence or absence of factors that may be associated with dementia (e.g., cardiovascular risk factors, smoking). The I statement is neither a recommendation for nor against screening for cognitive impairment but is rather a call for more research.
2. The correct answers are A and C . The USPSTF found adequate evidence that some screening tools have relatively high sensitivity and specificity for the detection of dementia. 2 The USPSTF found that the sensitivity and specificity of screening tools are generally lower for the detection of mild cognitive impairment than they are for the detection of dementia. Based on the sensitivity and specificity of screening tools, the USPSTF estimates that when the prevalence of dementia is high (e.g., in adults 85 years or older), positive predictive values can be greater than 50%. However, because of lower prevalence, the positive predictive value can be closer to 20% in unselected populations of adults 65 to 74 years of age. Screening tools are not intended to diagnose dementia. A positive screening test result should lead to additional testing that can include blood tests, radiology examinations, and a medical and neuropsychological evaluation to confirm the diagnosis of dementia and to determine its subtype.
3. The correct answer is B . Increasing age is the highest known risk factor for cognitive impairment. Cardiovascular risk factors (e.g., diabetes, hypertension, hypercholesterolemia), depression, physical frailty, low education level, and low social support level have also been associated with risk of cognitive impairment. Mild cognitive impairment differs from dementia in that the impairment is not severe enough to interfere with independent daily functioning. Some people with mild cognitive impairment progress to dementia, but some do not. One systematic review found that 32% of people with mild cognitive impairment develop dementia over five years. 2 However, studies have also shown that 10% to 40% of people with mild cognitive impairment may return to normal cognition over approximately four to five years. Dementia currently affects an estimated 2.4 to 5.5 million people in the United States. 1 , 2
The views expressed in this work are those of the authors and do not reflect the official policy or position of the Department of Defense, the Uniformed Services University of the Health Sciences, the U.S. Department of Health and Human Services, or the U.S. government.
This PPIP quiz is based on the recommendations of the USPSTF. More information is available in the USPSTF Recommendation Statement and supporting documents on the USPSTF website ( https://www.uspreventiveservicestaskforce.org ). The practice recommendations in this activity are available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening#fullrecommendationstart .
U.S. Preventive Services Task Force. Screening for cognitive impairment in older adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323(8):757-763.
Patnode CD, Perdue LA, Rossom RC, et al. Screening for cognitive impairment in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(8):764-785.
This series is coordinated by Joanna Drowos, DO, contributing editor.
A collection of Putting Prevention Into Practice published in AFP is available at https://www.aafp.org/afp/ppip.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2020 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

- Previous Article
- Next Article
Case Presentation
Personal history, medical history, psychiatric history, clinical pearls, case study: cognitive impairment, depression, and severe hypoglycemia.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
John Zrebiec; Case Study: Cognitive Impairment, Depression, and Severe Hypoglycemia. Diabetes Spectr 1 October 2006; 19 (4): 212–215. https://doi.org/10.2337/diaspect.19.4.212
Download citation file:
- Ris (Zotero)
- Reference Manager
The following case study illustrates the clinical role of mental health professionals who specialize in the treatment of people with diabetes. This case describes the diagnostic dilemma presented by a patient with diabetes and a history of severe hypoglycemia complicated by other medical, psychiatric, social, and functional problems.
Cognitive impairment (e.g., memory loss, increased distractibility, and confusion) can present a difficult diagnostic problem for clinicians because it can be symptomatic of many underlying and undetected clinical conditions. Careful diagnosis is crucial because some causes may be progressive and irreversible, whereas other causes may be reversible with medical or psychiatric treatment. Some of the more noteworthy causes are related to medication, alcohol, aging, depression, and, as in this case study, the possible consequences of recurrent severe hypoglycemia related to diabetes. 1 Diabetes, of course, may cause an increase in cognitive decline over the course of time because of vascular risk factors. 2 Some evidence suggests that acute hyperglycemia may have a negative impact on cognitive performance. 3
Depression is the most common of the reversible causes of memory impairment, and people with diabetes are twice as likely to suffer from depression as those without diabetes. 4 Recent evidence suggests that diabetes may create alterations in regions of the brain that are associated with affect regulation and increase the risk for developing a depressive disorder. 5 Fortunately, problematic medications can be modified, and alcohol misuse or depression can be treated. Unfortunately, despite its relevance to the course of diabetes, depression is recognized and treated in fewer than one-third of people with diabetes. 4
The relationship between recurrent severe hypoglycemia and cognitive impairment remains unclear. Both prospective and longitudinal studies of cognitive function have been so plagued by methodological problems that it is difficult to unequivocally determine whether patients who experience repeated episodes of severe hypoglycemia are at risk for permanent brain injury or intellectual impairment. However, those with diabetes of long duration, especially with comorbid neuropathy, may be at higher risk for cognitive deficits resulting from hypoglycemia. 6 , 7 A meta-analysis of studies about cognitive performance in patients with type 1 diabetes found that lowered cognitive performance was associated with the presence of microvascular complications but not with the occurrence of severe hypoglycemia. 8 Recently, the Epidemiology of Diabetes Interventions and Complications study, a follow-up to the Diabetes Control and Complications Trial (DCCT) reported that multiple severe hypoglycemic episodes did not lead to increased risk for cognitive impairment in the subjects who had participated in the DCCT. 9
Steve is a 67-year-old white widower and retired accountant. He was referred for psychosocial evaluation at the diabetes clinic after an emergency room (ER) visit to a local hospital. He arrived at the ER with confusion and a severe hypoglycemic episode after taking an overdose of insulin. He denied suicidal intent or alcohol abuse and claimed to have mistakenly taken insulin lispro rather than his insulin glargine dose. The ER staff was suspicious about his claim because there had been eight similar ER visits for severe hypoglycemia within the last 2 years. He explained these previous events as a result of mixing up the types of insulin he injected.
After psychiatric assessment he was not judged to be a suicidal risk. He was discharged after his blood glucose levels stabilized, and he promised to pursue outpatient mental health treatment. His hemoglobin A 1c (A1C) at the time was 7.9%—his lowest on record for several years. Generally, his blood glucose levels displayed wide swings. He explained that high blood glucose levels made him feel more apathetic about eating and depressed about his diabetes self-management.
As a child, Steve attained developmental milestones at expected times. His father was in the Army, and as a result, Steve had moved 32 times before he graduated from high school. He was an excellent student throughout high school but only managed mediocre grades in college because of family conflict. He dropped out of college in his junior year and moved to a South Pacific island for 1 year.
After returning to the United States, he earned an undergraduate degree in English and then a second degree in accounting. After graduation, he married and worked for 20 years as an accountant in a group practice. Later, Steve started his own accounting firm, but he had difficulty keeping organized and recalls being constantly late for business meetings and failing to complete projects on time. In hindsight, Steve believes that he has struggled with depression on and off for > 30 years. He first recalls feeling depressed after his diagnosis with diabetes 36 years ago. He felt more depressed after he lost his 47-year-old sister to colon cancer in 1988, and then his 74-year-old father died from heart disease in 1991. But, he says his life “really fell apart” when his 54-year-old wife died from lung cancer in 1995. He contemplated suicide for 3 months but never acted. During this desperate period, he marginally functioned, lost many business clients, and was forced to close his company.
Overwhelmed by depression, he moved to the West Coast to live with his mother and worked at unskilled jobs. Diabetes complicated his emotional struggles, with blood glucose control fluctuating wildly and ranging from episodes of ketoacidosis that required hospitalization to severe hypoglycemic events that resulted in car crashes. Depression complicated his diabetes management, and after a hypoglycemia-related auto accident in which he ran over several pedestrians, he decided to stop working and was approved for social security because of psychiatric disability.
He came to the East Coast in 1998 to briefly visit his younger brother and decided to stay. Although he still lives near his brother, he says they have had only sporadic contact since a falling out after Steve “passed out” during a severe hypoglycemic episode. In 2000, Steve got engaged, but his fiancée left him to marry the father of her child. He says he felt devastated by the loss of yet another woman who had “become everything” to him. Since then, he has withdrawn socially and does not leave his apartment unless it is necessary. He has trouble managing his money, keeping his apartment neat and orderly, taking medications on time, and maintaining any structure in his day.
Steve punctually arrives at the correct hour but often on the wrong day for his medical appointments. He grapples with neuropathy, retinopathy, and unpredictable blood glucose levels. He monitors his blood glucose levels 8–12 times/day and tries to be careful about what he eats. He also has sleep apnea, and his sleep patterns are highly erratic. He frequently does not fall asleep until 4:00 a . m . and then may only be able to sleep for 2 hours. Often, he will then nap for several hours in the afternoon. He began continuous positive airway pressure treatment for his sleep problems in 2003 but did not tolerate treatment. He has switched to bilevel positive airway pressure (biPAP) within the last 18 months but only tolerates it for up to 3 hours each night. Additional diagnoses include hyperlipidemia, hypertension, atrial fibrillation, Meniere's disease, tinnitus, and arthritis. His medication list includes atorvastatin, lisinopril, hydrochlorothizide, warfarin, meclizine, and folic acid. He does not smoke and only rarely drinks alcohol. Only his paternal grandmother had diabetes.
Depression has plagued Steve since his diagnosis with diabetes. As noted earlier, his depression intensified after the deaths of his sister and father, but he did not descend into a suicidal mood until his wife died 10 years ago. Four years ago, he underwent electroconvulsive therapy (ECT), and although he continues to have occasional suicidal ideation, he has not made an attempt and has had no further psychiatric admissions. Both of his parents, his brother, and his sister suffered from depression. A maternal aunt suffered from dementia. His mother also struggled with alcohol abuse until her death from emphysema in 2004 at the age of 89. At the time of referral, he was taking fluoxetine, 40 mg, and venlafaxine, 37.5 mg, prescribed by a psychopharmacologist.
Was Steve's insulin overdose accidental or a suicide attempt?
What are the causes for his cognitive impairment?
How do his depression and cognitive problems affect his diabetes self-management?
What are the treatment recommendations?
When Steve started treatment, he was interested in learning how to alleviate his depression and improve his diabetes care. He was pleasant, cooperative, thoughtful, and tactful, and his language was eloquent but often emotionless. He tended to give very detailed and pensive answers to questions.
Careful clinical evaluation found that his insulin overdose was best explained by lack of attention rather than suicidal intent, desire for secondary gain, or fear of hyperglycemia. His eight previous severe hypoglycemic episodes raised the question of why this intelligent man kept repeating the same mistakes. His history hinted at troubles with complex cognitive functions (e.g., ability to plan, sequence, prioritize, organize, and initiate) that extended back to his college days. He reported that in the past year he had experienced more memory problems, sometimes forgetting names and having word-finding difficulties despite a sophisticated vocabulary base. He had also noticed increased short-term memory problems and a decline in attention span during the same period of time.
Earlier in the year, an episode of extremity weakness and fatigue had led to neuroimaging studies that revealed no evidence of neurological injury or stroke. Certainly, depression, perhaps further complicated by ECT, aging, 3 decades of diabetes, and recurrent episodes of severe hypoglycemia, may have contributed to his cognitive decline. In fact, he reported feeling more depressed within the past 6 months. He was referred for neuropsychological testing to further understand his changes in cognitive function and target treatable symptoms.
Neuropsychological tests indicated that his baseline functioning was in the superior range. He exhibited strengths across most cognitive domains, including memory, language, reasoning, and complex cognitive functioning. In contrast, he demonstrated relative weakness in mental speed, mental flexibility, word retrieval, and fine motor control. There was evidence for a moderate to severe level of clinical depression. Compared with prior testing (3 years previously), he exhibited a decline in processing speed, mental flexibility, word retrieval, and fine motor control. It was theorized that these changes were related to the cumulative effects of poor sleep, worsening depression, and multiple hypo- and hyperglycemic events. His care provider felt that unless his medical conditions were properly treated, his cognitive abilities would continue to fluctuate, and he would have even more difficulty circumventing these problems.
The care provider recommended that he try to regulate his sleep patterns, specifically avoiding long afternoon naps, and use his biPAP machine to help improve sleep. Better sleeping patterns should improve his attention span and overall cognitive functioning. The need for continuation of individual psychotherapy and psychiatric medications for depression was evident and eagerly accepted by Steve. Pursuing his therapy at a diabetes clinic easily opened the door for a referral to other members of the diabetes team (including an endocrinologist, dietitian, and nurse educator) to help regulate his erratic blood glucose levels. It also allowed for diabetes care to be truly collaborative.
It is widely accepted that depression can create more difficulties in maintaining treatment adherence and that the hardships of managing diabetes can lead to depression. 4 Steve lost his appetite when depressed and increased his risk of hypoglycemia. He was maintained on fast-acting insulin to provide more flexibility with meals and was prescribed an insulin pen to avoid mixing up different types of insulin.
Steve attended Blood Glucose Awareness Training sessions. This is a well-documented psychoeducational program that offers several empirically validated benefits for people with type 1 diabetes. Benefits include improved accuracy of blood glucose estimations, improved detection of hypoglycemia and hyperglycemia, improved judgments related to decisions about treatment when blood glucose is low, and a reduction in episodes of severe hypoglycemia. 10
Despite being quite likeable, Steve reported feeling isolated and lonely. He said he often felt “disengaged” from others, emotionally detached, and affectively flattened. To provide structure to his day, increase his level of cognitive and social stimulation, and learn from others about how to cope with diabetes and depression, he was referred to a hospital-based group for people suffering from depression and to one of the clinic's diabetes support groups. For additional social connection, he was encouraged to pursue his interest in photography.
Steve followed through on all recommendations. He has not had a severe hypoglycemic episode during the past 7 months. His A1C has changed from 7.9% at the time of referral to 8.3%, most likely reflecting the avoidance of severe lows. In individual therapy, he continues to work on his tendency to be too passive and mercilessly self-critical, and in the groups he is gaining more awareness of his tendency to feel either emotionally detached or overwhelmed by others. He has also enrolled in photography classes.
How can one tell if a patient is depressed? Suspicion may be raised by history or reports of relatives, but most often it is the clinical discussion that discovers the cognitive and affective symptoms of depression, such as fatigue, insomnia, weight loss, poor concentration, loss of interest or pleasure in daily activities, sadness, helplessness, and hopelessness. Asking simple questions, such as “During the past month, have you been bothered by feeling down, depressed, or hopeless?” and “During the past month, have you been bothered by little interest or pleasure in doing things?” can be as successful as formal surveys when screening for depression. 11
How can one tell if a patient is cognitively impaired? Suspicion may be raised by difficulties with orientation, attention, reasoning, and memory problems, such as difficulty learning new information or remembering old information. Other clues include difficulties with calculations, perceptual disturbance, or language disturbance, such as word finding or perseveration. Complex cognitive functions, such as planning, organizing, sequencing, and abstracting, may be impaired. In a clinical interview, it is often difficult to follow the logical sense of a cognitively impaired patient's presentation. 12
How can one tell the difference between cognitive deficits caused by depression and those caused by brain injury? Depression can cause slowing of information processing, decreased attention and concentration, and learning problems. It can be difficult to know whether mild deficits in concentration or learning are caused by depression, mild traumatic brain injury, or both. Often, the most practical approach to such cases is to treat the depression first and then re-evaluate the patient for any residual neurocognitive deficits. Some areas of cognition are also not generally affected by depression, for example, language, problem solving, visual spatial analysis, complex cognitive functions, and visual or auditory perception.
When should one refer someone for neuropsychological testing? The most common reasons are when:
A medical condition is suspected to have affected brain health (e.g., recurrent severe hypoglycemia or hyperglycemia)
Situational explanations for changes in emotions or cognitive functioning cannot be readily identified
Relatively sudden, unexpected, and unaccounted for changes appear in mental or cognitive performance that affect work or daily functioning. 13
Who is appropriate to refer to a support group? Patients who are willing to listen to others and talk about themselves are good candidates for a support group. Exclusionary criteria include refusal to abide by group guidelines and serious problems with interpersonal relatedness. Contrary to popular opinion, patients who do not do well in groups are not good group candidates. Caution also needs to be exercised when including patients who are highly impulsive, acutely suicidal, or psychotic. 14
John Zrebiec, MSW, CDE, is associate director of the Behavioral and Mental Health Unit at the Joslin Diabetes Center and a lecturer in psychiatry at the Harvard Medical School in Boston, Mass.
Email alerts
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Case Report
- Open access
- Published: 01 July 2009
Male patient with mild cognitive impairment and extremely high P300 and Slow-wave latencies: a case report
- Vasileios T Papaliagkas 1 ,
- Magda N Tsolaki 2 ,
- Vasileios K Kimiskidis 2 &
- Georgios Anogianakis 1
Cases Journal volume 2 , Article number: 6157 ( 2009 ) Cite this article
3612 Accesses
Metrics details
We present a case of a 74-year-old Greek male who suffered from paraphasias, memory and orientation problems. The patient was assessed with neuropsychometric tests, auditory event-related potentials and cerebrospinal fluid proteins and was diagnosed with mild cognitive impairment. The emphasis on the case is on the unexplained high levels of P300 and Slow wave of the auditory event-related potentials.
P300 is believed to be delayed in Alzheimer's Disease (AD), however in our case it was extremely prolonged in baseline and follow-up examinations without AD being diagnosed. This might suggest that AD is a complex and multifactorial disease.
Case presentation
A 74-year-old Greek male patient was referred to the Memory outpatients clinic of the "G. Papanikolaou" Hospital in 7 February 2005 due to paraphasias, memory and orientation problems. The orientation problems were reported to be worse at night. The patient was 1.78 meters tall and 95 kg and had 6 years of education. His problems began 7 years before with initial symptoms mood and behavioural changes. One initiating factor that was mentioned was the loss of his brother.
From his past medical history the patient underwent prostate removal in 2001 and suffered from back pain. Moreover, the patient had high blood cholesterol levels for which he was receiving drug therapy from 2004 (Lipitor 40 mg 1 × 1). He was also taking Salospir 100 mg S = 1 × 1. No family history for AD or other form of dementia was reported.
Neuropsychological examination was performed. The score in the Mini-Mental State Examination (MMSE) [ 1 , 2 ] scale was 27/30 and the Clinical Dementia Rating (CDR) [ 3 ] scale was 0.5 suggesting that global cognitive function is satisfactory. The score in the Geriatric Depression Scale (GDS) [ 4 ] was 0/15, excluding depression as a diagnosis.
Neuroimaging studies (MRI) revealed slight microdegenerative changes of arterial type and slight atrophy of the right hippocampus. The patient was diagnosed as having Mild cognitive impairment (MCI).
Auditory event-related potentials were performed to the patient, who was included in the study of Papaliagkas et al. [ 5 ]. The P300 and SW latencies were found to be 591 ms and 779 ms respectively. Compared to the mean values of the latencies observed in a group of 91 MCI patients (mean ±SD value for P300: 406.4 ± 51.8 ms and for SW: 536.35 ± 62.11 ms) the patient's figures were higher by more than 3 standard deviations for P300 and by more than 4 for SW. On the contrary, the value of N200 latency (260 ms) was approximately equal to the respective mean value of the MCI patients (252 ms). A CSF sample was also obtained with lumbar puncture. The β-amyloid (1-42) and tau levels were determined, using the sandwich ELISA INNOTEST β-amyloid (1-42) and hTau-Antigen sandwich ELISA kits of Innogenetics, Ghent, Belgium. Both protein levels were within normal levels according to the kit manufacturer (β-amyloid (1-42) = 911 pg/ml, tau = 194 pg/ml). A follow-up examination was performed after 12 months. MMSE score was stable (27/30). P300 and SW latencies continued to be extremely high (P300 = 625 ms and SW = 751 ms), whereas N200 latency (268 ms) was still approximately equal to the mean value of the MCI patients (255 ms). Furthermore, CSF proteins continued to be within normal levels (β-amyloid (1-42) = 791 pg/ml, tau = 109 pg/ml).
Cognitive event-related potentials (ERPs) have been widely used in the study of dementias, including Alzheimer's disease. P300 component corresponds to mental processes such as recognition, categorization of stimuli, expectancy or short-term memory, while there are many regions in the brain, especially in the temporal lobe, the parietal lobe and the hippocampus which are thought to be responsible for its generation [ 6 ].
The numerous clinical P300 studies [ 6 ]-[ 11 ], strongly suggest that it may be clinically useful as an index of cognitive function and that it is prolonged in AD patients. N200 and P300 latencies were found to be significantly prolonged in AD patients when compared to either MCI patients or controls and this difference might help categorize patients into one of the three groups [ 7 ].
In our case study it is interesting to note that although the patient's P300 and SW latencies were extremely high, he did not suffer from AD. This increase might be due to the microdegenerative changes of arterial type and the atrophy of right hippocampus, however it cannot be verified if these slight changes can explain such extremely high latencies. P300 latency may increase slightly in minor ischemic stroke and this increase is associated with post-stroke depression [ 12 ], which was not diagnosed in our patient. No increased P300 latency has been observed in patients with vascular cognitive impairment [ 13 ]. It is also interesting to note that the high values of P300 and SW latencies were not accompanied by an increase in N200 latency, which implies that the mechanism of production of the N200 wave is independent of that of the two sequential waves.
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
Alzheimer's disease
Clinical Dementia Rating
Cerebrospinal fluid
Event-related potentials
Mild cognitive impairment
Mini-Mental State Examination
Magnetic Resonance Imaging
Folstein M, Folstein S, McHugh P: "Mini-Mental State". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975, 12: 189-198. 10.1016/0022-3956(75)90026-6.
Article CAS PubMed Google Scholar
Fountoulakis K, Tsolaki M, Chantzi H, Kazis A: Mini-Mental State Examination (MMSE): A validation study in the Greek elderly population. Encephalos (Greece). 1994, 31: 93-102.
Google Scholar
Morris JC: The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993, 43: 2412-2414.
Fountoulakis KN, Tsolaki M, Iacovides A, Yesavage J, O'Hara R, Kazis A, Ierodiakonou C: The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging (Milano). 1999, 11: 367-372.
CAS Google Scholar
Papaliagkas VT, Kimiskidis VK, Tsolaki MN, Anogianakis G: Usefulness of event-related potentials in the assessment of mild cognitive impairment. BMC Neurosci. 2008, 9: 107-10.1186/1471-2202-9-107.
Article PubMed Central PubMed Google Scholar
Yamaguchi S, Knight RT: P300 generation by novel somatosensory stimuli. Electroencephalogr Clin Neurophysiol. 1991, 78: 50-55. 10.1016/0013-4694(91)90018-Y.
Bennys K, Portet F, Touchon J, Rondouin G: Diagnostic value of event-related evoked potentials N200 and P300 subcomponents in early diagnosis of Alzheimer's disease and mild cognitive impairment. J Clin Neurophysiol. 2007, 24: 405-412. 10.1097/WNP.0b013e31815068d5.
Article PubMed Google Scholar
Golob EJ, Irimajiri R, Starr A: Auditory cortical activity in amnestic mild cognitive impairment: relationship to subtype and conversion to dementia. Brain. 2007, 130: 740-752. 10.1093/brain/awl375.
Frodl T, Hampel H, Juckel G, Bürger K, Padberg F, Engel RR, Möller HJ, Hegerl U: Value of event-related P300 subcomponents in the clinical diagnosis of mild cognitive impairment and Alzheimer's Disease. Psychophysiology. 2002, 39: 175-181. 10.1017/S0048577202010260.
Golob EJ, Johnson JK, Starr A: Auditory event-related potentials during target detection are abnormal in mild cognitive impairment. Clin Neurophysiol. 2002, 113: 151-161. 10.1016/S1388-2457(01)00713-1.
Irimajiri R, Golob EJ, Starr A: Auditory brain-stem, middle- and long-latency evoked potentials in mild cognitive impairment. Clin Neurophysiol. 2005, 116: 1918-1929. 10.1016/j.clinph.2005.04.010.
Korpelainen JT, Kauhanen ML, Tolonen U, Brusin E, Mononen H, Hiltunen P, Sotaniemi KA, Suominen K, Myllylä VV: Auditory P300 event related potential in minor ischemic stroke. Acta Neurol Scand. 2000, 101: 202-208. 10.1034/j.1600-0404.2000.101003202.x.
van Harten B, Laman DM, van Duijn H, Knol DL, Stam CJ, Scheltens P, Weinstein HC: The auditory oddball paradigm in patients with vascular cognitive impairment: a prolonged latency of the N2 complex. Dement Geriatr Cogn Disord. 2006, 21: 322-327. 10.1159/000091474.
Download references
Author information
Authors and affiliations.
Department of Experimental Physiology, Aristotle University of Thessaloniki, Thessaloniki, Greece
Vasileios T Papaliagkas & Georgios Anogianakis
Third Department of Neurology, Aristotle University of Thessaloniki, "G. Papanikolaou" Hospital, Thessaloniki, Greece
Magda N Tsolaki & Vasileios K Kimiskidis
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Vasileios T Papaliagkas .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
VTP contributed to the design of the study, carried out the event-related potentials, performed the statistical analysis and drafted the manuscript. VKK contributed to the design of the study, the event-related potentials and the manuscript preparation. MNT recruited the patients and the control subjects and provided the neurological diagnosis and interpretation. GAA contributed to the design and coordination of the study and the manuscript preparation. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Papaliagkas, V.T., Tsolaki, M.N., Kimiskidis, V.K. et al. Male patient with mild cognitive impairment and extremely high P300 and Slow-wave latencies: a case report. Cases Journal 2 , 6157 (2009). https://doi.org/10.4076/1757-1626-2-6157
Download citation
Received : 23 February 2009
Accepted : 25 May 2009
Published : 01 July 2009
DOI : https://doi.org/10.4076/1757-1626-2-6157
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- P300 Latency
- Mild Cognitive Impairment Patient
- Orientation Problem
- Vascular Cognitive Impairment
- N200 Latency
Cases Journal
ISSN: 1757-1626
The importance of attentive primary care in the early identification of mild cognitive impairment: case series
Affiliations.
- 1 Research and Development Unit, Hammersmith and Fulham Partnership, London, UK.
- 2 North End Medical Centre, London, UK.
- PMID: 38711887
- PMCID: PMC11070989
- DOI: 10.21037/acr-23-162
Background: Mild cognitive impairment (MCI) is a condition often preceding Alzheimer's disease and other dementias, characterized by subtle changes in cognitive function. While the importance of early detection is recognised, MCI is frequently underdiagnosed, especially when patients consult primary care physicians for non-cognitive health concerns. The case series aims to investigate the incidental identification of MCI in older patients who visit primary care settings for reasons unrelated to memory issues.
Case description: This is a retrospective case series comprising eight patients, ranging in age from 67 to 77 years, who initially presented in primary care settings for diverse non-memory-related concerns such as headaches, urinary tract infection (UTI) symptoms, and knee pain. Despite the lack of memory-related complaints, incidental findings suggestive of MCI were observed during clinical evaluations. The study explores the distinctions in clinical presentations and diagnostic pathways through thorough history taking and cognitive assessments, including the Montreal Cognitive Assessment (MoCA) and brain magnetic resonance imaging (MRI).
Conclusions: The study highlights the critical role that primary care settings can play in the early detection of MCI, even when patients present with non-cognitive complaints. It emphasizes the importance of comprehensive history taking as a tool for incidental identification of cognitive impairment. Although limited by sample size, the study calls for increased vigilance in primary care settings and suggests the need for future research aimed at optimizing early detection and management strategies for MCI in a primary care context.
Keywords: Frailty; case series; cognitive impairment; primary care.
2024 AME Case Reports. All rights reserved.
Publication types
- Case Reports
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 12, Issue 1
- Vision impairment and cognitive decline among older adults: a systematic review
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-2443-7058 Niranjani Nagarajan 1 ,
- http://orcid.org/0000-0001-5855-3896 Lama Assi 1 ,
- V Varadaraj 1 ,
- Mina Motaghi 2 ,
- Elizabeth Couser 3 ,
- http://orcid.org/0000-0002-0607-3564 Joshua R Ehrlich 4 , 5 ,
- Heather Whitson 6 ,
- Bonnielin K Swenor 1 , 7
- 1 Wilmer Eye Institute , Johns Hopkins University School of Medicine , Baltimore , Maryland , USA
- 2 Johns Hopkins School of Public Health , Baltimore , Maryland , USA
- 3 Johns Hopkins University School of Medicine , Baltimore , Maryland , USA
- 4 Ophthalmology and Visual Sciences , University of Michigan , Ann Arbor , Michigan , USA
- 5 Institute for healthcare policy and innovation , University of Michigan , Ann Arbor , Michigan , USA
- 6 Department of Medicine, Geriatrics , Duke University , Durham , North Carolina , USA
- 7 Epidemiology , Johns Hopkins University Bloomberg School of Public Health , Baltimore , Maryland , USA
- Correspondence to Professor Bonnielin K Swenor; bswenor{at}jhu.edu
Objectives There has been increasing epidemiological research examining the association between vision impairment (VI) and cognitive impairment and how poor vision may be a modifiable risk factor for cognitive decline. The objective of this systematic review is to synthesise the published literature on the association of VI with cognitive decline, cognitive impairment or dementia, to aid the development of interventions and guide public policies pertaining to the relationship between vision and cognition.
Methods A literature search was performed with Embase, Medline and Cochrane library databases from inception to March 2020, and included abstracts and articles published in peer-reviewed journals in English. Our inclusion criteria included publications that contained subjective/objective measures of vision and cognition, or a diagnosis of VI, cognitive impairment or dementia. Longitudinal or cross-sectional studies with ≥100 participants aged >50 years were included. The search identified 11 805 articles whose abstracts underwent screening by three teams of study authors. Data abstraction and quality assessment using the Effective Public Health Practice Project Quality Assessment Tool were performed by one author (NN). 10% of the articles underwent abstraction and appraisal by a second author (LA/VV), results were compared between both and were in agreement.
Results 110 full-text articles were selected for data extraction, of which 53 were cross-sectional, 43 longitudinal and 14 were case–control studies. The mean age of participants was 73.0 years (range 50–93.1). Ninety-one (83%) of these studies reported that VI was associated with cognitive impairment.
Conclusion Our systematic review indicates that a majority of studies examining the vision–cognition relationship report that VI is associated with more cognitive decline, cognitive impairment or dementia among older adults. This synthesis supports the need for additional research to understand the mechanisms underlying the association between VI and cognitive impairment and to test interventions that mitigate the cognitive consequences of VI.
- epidemiology
- delirium & cognitive disorders
- ophthalmology
- geriatric medicine
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2020-047929
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
There was heterogeneity in the measurement of cognitive and visual function among all included studies.
The quality assessment tool used for assessing quality of included studies penalised longitudinal studies that lost over 40% of participants due to drop-outs/withdrawals, which is common in studies that span over many years.
Majority of the included studies were cross-sectional, and these are prone to selection bias.
Introduction
Dementia is among the most pressing public health challenges of the 21st century. 1 In 2015, 46.8 million people were living with dementia, and the number is expected to double every 20 years. 2 Vision impairment (VI), another major global health problem, affects at least 2.2 billion people worldwide, 3 most of whom are aged 50 years and older. 4 Both cognitive and VI are projected to affect an increasing number of people over time, primarily due to population ageing. 4 5
Prior work has suggested that cognition and vision are associated, 6 7 and while there are shared risk factors (neuropathological/vascular), 8 there is also longitudinal evidence that VI is associated with cognitive changes. 9 The mechanisms underlying the vision-cognition relationship have not yet been fully characterised, but it is hypothesised that sensory loss, such as hearing impairment and VI, may lead to increased cognitive load, structural and functional changes in the brain, and decreased emotional and social well-being, all of which could potentially increase the risk of cognitive impairment. 9 10 While the role of treating hearing loss in preventing cognitive impairment has been acknowledged, VI has not yet been recognised as a potentially modifiable risk factor for cognitive impairment. 1 11
Since the majority of VI is due to correctable conditions, namely refractive error and cataract, 12 establishing the existence of an association between vision and cognitive impairment could present an additional opportunity to prevent cognitive impairment and dementia through interventions that optimise vision. In this systematic review of the literature, we examined the association between cognitive and vision impairment among older adults in existing observational studies. This qualitative review summarises the existing research examining the vision–cognition relationship, providing insight on data gaps and areas for continued investigation, as well as highlighting differences in methodological approaches that may impact the interpretation of results across studies.
Methods/literature search
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines ( online esupplemental file 1 ).
Supplemental material
Cross-sectional and longitudinal studies reporting a measure of association between visual function and cognitive impairment were included if they had ≥100 participants aged of ≥50 years (mean) at baseline. Reasons for exclusion of studies were: (1) Outcome measure was not vision or cognition, (2) Association between vision and cognition was not explored, (3) Sample size <100, (4) Publication not in English, (5) Mean age <50 years, (6) No cognitive measure, (7) No vision measure and (8) Outcome was not part of inclusion criteria.
An academic librarian searched: Ovid Medline, Embase, Cochrane and PubMed from their inception to March 2020, and developed a search strategy that combined controlled vocabulary and keywords related to geriatrics, cognition and vision ( online esupplemental file 2 -complete Ovid Medline search strategy). Searches were limited to human studies published in English. Conference and poster abstracts, and short oral presentations were also included.
Search results were exported to Covidence (Veritas Health Innovation, Melbourne, Australia). Three teams of two reviewers each worked independently and in duplicate to screen titles, abstracts and full-text articles to determine inclusion (NN and MM; EC and YS; VV and LA). Disagreements were adjudicated by a member of the other study team.
Data were extracted from the included publications by one author (NN), and another (LA) extracted data from a random sample of 10% to compare results. Any discrepancies were adjudicated by a third author (VV). Data collected for each publication included: study design, participant characteristics, vision and cognition assessment methods and the summary measure that described the vision–cognition association.
The methodological quality of included studies was assessed by one author (NN) using the Effective Public Health Practice Project Quality Assessment Tool (EPHPP) 13 and the global quality ratings and findings were summarised qualitatively. A random 10% sample was reviewed by another author (VV) to ascertain consistency in quality assessment (QA).
Study Selection
Online esupplemental file 3 is a PRISMA flow chart that describes the results of the search strategy of articles that examined the association between VI and cognitive impairment or decline. Of the 11 805 studies that were imported for screening, 110 articles were included in our final systematic review. 7 14–122
Description of included studies
Table 1 describes the characteristics of the studies included. The total number of participants in this review was 9 799 329 (range: 112–7 210 535 per study), with a mean age of 73.0 years, (range: 50.0–93.1). Of the total 110 studies included, 53 were cross-sectional, 43 were longitudinal and 14 had a case–control study design. The range of follow-up time for the longitudinal studies was 2 months to 10 years.
- View inline
Patient demographics and study characteristics
Of the 110 studies included, 51 reported findings from participants enrolled in population-based studies. There were five studies each from the following large population based longitudinal studies: English Longitudinal Study of Aging (ELSA) and The Three-City Study. Three studies each from Fujiwara-Kyo Study, Salisbury Eye Evaluation Study, Irish Longitudinal Study on Aging and Singapore Epidemiology of Eye Diseases. Two studies each came from The Newcastle 85+ study, Study of Osteoporotic Fractures, Blue Mountain Eye Study, Australian Longitudinal Study on Aging, Health and Retirement Study, National Health and Aging Trends Study (NHATS), Leiden 85+ study, Health ABC study and the Singapore Malay Eye Study. Additionally, 10 studies used insurance claims data from different countries.
The studies in this review included participants from over 17 different countries ( table 1 ), 30 studies (27%) from the USA, followed by 25 studies from Europe (23%) including the UK, Germany, Ireland, Finland, Switzerland, France and Netherlands. Ninety of the studies were published between 2009 and 2020. All papers provided a description of sampling methods. 16 studies were included which were either conference abstracts or short oral presentations. 24 32 45 49–51 53 56 92 93 96 112–116
Assessment of cognitive function
To assess cognitive function, 89 studies used objective assessments, 13 used other assessment methods such as self-report and diagnosis codes, and 7 studies used a combination of both ( table 2 ); one study did not provide information about cognitive function assessment. 92 Mini-Mental State Examination (MMSE) was the most commonly used objective method to assess cognitive function (42 studies). Other methods used to objectively measure cognition included: Montreal-Cognitive Assessment test, 28 32 34 53 93 Addenbrooke’s Cognitive Examination-Revised, 45 61 79 Cognitive Performance Scale, 42 49 65 Blessed-Orientation-Memory-Concentration test, 25 62 Abbreviated Mental Test, 72 74 89 100 110 119 Blessed Dementia Scale, 86 Digit Symbol Substitution Test 22 122 and Cambridge Cognitive Examination test. 88 For the studies that used other assessment methods, four used self-reported cognitive measures, 18 22 39 105 and nine used diagnostic codes to define cognitive decline and/or dementia. 19–21 51 69 99 103 115 117 Among the studies that used a combination of objective and subjective methods, four used self-reported cognitive function along with an objective measure. 22 30 48 55
Measures of vision and cognition assessed in studies
Assessment of visual function
In order to assess visual function, 66 studies used objective assessments, 34 used other assessment methods such as self-report and diagnosis codes, and 8 studies used a combination of both ( table 2 ); no information was available from two studies. 24 116 Visual acuity (VA) was the most commonly measured visual function (42 studies), of which the Snellen acuity chart was the most commonly used method (18 studies). VA was also measured in combination with other visual functions, including: visual fields (VF) (six studies), 20 51 70 90 102 118 contrast sensitivity (CS) (eight studies), 29 52 86 93 94 102 118 122 macular pigment optical density (two studies) 14 34 and fundus photography (two studies). 63 118 Other methods used to objectively measure visual functions included: colour vision, 17 VF only, 28 CS only, 35 76 fundus photo with grading 38 100 and autorefraction. 74 Other assessment methods included: self-reported vision (24 studies) and diagnostic codes or patient records to define VI (10 studies). 19 21 46 69 83 99 104 115 117 121 The studies that used a combination of methods, eight studies used self-report along with an objective measure of visual function. 22 40 53 59 68 71 107 113
Quality of studies
The methodological quality of included studies was assessed using the EPHPP. 13 The tool assessed each study on five domains: (1) Selection bias, (2) Study design, (3) Confounders, (4) Data collection methods and (5) Analysis. For each included study the five relevant domains were ranked on a three-point Likert scale with three representing a low risk of bias (‘strong’), two a possible risk of bias (‘moderate’) and one a high risk of bias (‘weak’). An overall rating was derived following the EPHPP methodology. A study consisting of at least one ‘weak’ rating in a domain received an overall rating of ‘moderate,’ while those with two or more domains with ‘weak’ ratings were automatically classified as ‘weak’ overall. We present our studies in three different tables which is categorised based on the overall ratings, with ‘strong’, ‘moderate’ and ‘weak’ studies in tables 3–5 , respectively. In our sample, 17 studies received a rating of strong, 70 moderate and 23 weak.
Studies with a ‘strong’ rating
Studies with a ‘moderate’ rating
Studies with a ‘weak’ rating
Study findings
Of the 110 studies included ( tables 3–5 ), 91 found a significant positive association between VI and cognitive decline, cognitive impairment or dementia, and 13 studies found no significant association. 26 30 43 44 60 61 68 81 82 90 94 115 117 There were six studies that were inconclusive. 23 37 67 70 79 83 Of the 91 studies that found a significant association, 77 used objective methods to assess their vision or cognitive outcome. Of the 43 longitudinal studies, 35 found a significant association between VI and cognitive decline, cognitive impairment or dementia. The most commonly presented statistical measures were ORs and HRs. The random 10% of the study sample that was separately extracted by an independent author (LA) was found to be similar to elements from the primary extraction.
In this systematic review, we evaluated and synthesised the literature examining the association between VI and cognitive function among older adults, and found strong agreement that VI is associated with cognitive impairment, cognitive decline or dementia. Results from the longitudinal studies that found a positive association between vision and cognition supports our hypothesis that VI may be a risk factor for cognitive impairment, cognitive decline or dementia.
Ninety-one studies reported associations between decline in visual and cognitive functions. Garin et al , 40 who received a ‘moderate’ rating in the QA, performed a cross-sectional analysis in a representative sample of Spanish population and measured cognition objectively. They also measured distance and near vision and found that objective and subjectively measured poor distance and near VA were associated with worse cognitive functioning. Lin et al 47 used data from a large longitudinal cohort study of older women and found that VI was associated with greater odds of cognitive and functional decline over 2 years. This study used objective measures of assessment for both vision and cognition and received a ‘strong’ rating in the bias assessment. Luo et al , 48 who received a ‘moderate’ rating in QA, performed a cross-sectional analysis on a large population sample from China. They reported that those with VI and Dual Sensory Impairment (DSI) were more likely to have severe to extremely severe dementia compared with those without any sensory impairment. Another longitudinal study that received a ‘moderate’ rating in QA from Germany by Hajek et al 58 with a large sample size (n=2394) showed that the onset of severe VI was associated with a decline in cognitive function scores. Uhlmann et al 64 in their paired case–control study between VI and dementia patients concluded that VI was associated with both an increased risk and an increased clinical severity of AD. Although Frost et al 38 found a strong association between early age-related macular degeneration and AD, their study was cross-sectional, and the sample size was too low to derive an inference. Both these studies received ‘moderate’ rating in QA. Davies-Kershaw et al 95 in their longitudinal analysis using the ELSA wave 2 and wave 7 data found that individuals in the younger group (50–69 years) and with moderate and poor self-rated vision were at greater risk of developing dementia than those with normal self-rated vision. Hamedani et al 99 used Medicare claims data from 2014 consisting of 472 871 participants and concluded that blindness/low vision was associated with a greater odd of Alzheimer’s disease and all-cause dementia. Both these studies also received ‘moderate’ rating in QA. Soto-Perez-de-Celis et al62 in their cross-sectional study case–control study found that DSI was significantly associated with possible CI. However, the study received an overall ‘weak’ rating in QA.
Of the 91 studies that found an association between VI and cognitive function, 35 were longitudinal, 46 were cross-sectional and 10 were case–control studies. Of the 13 studies that found no association between VI and cognitive function, 6 were longitudinal, 5 were cross-sectional and 2 were case–control studies. Ihle et al 60 performed a cross-sectional analysis using a sample of 2812 participants from Switzerland. They objectively measured cognition and vision and concluded that their data did not support an increased relation of cognitive and sensory abilities in old age. This study received a ‘weak’ rating in QA. Hong et al 82 used data from Blue Mountain Eye Study, a longitudinal study from Australia that studied associations between VI and a decline in MMSE scores over a duration of 10 years. The study concluded that VI was not associated with cognitive decline over 5 years or 10 years. Although the study included a large number of participants overall (n=2334), only 152 individuals with VI were included in this analysis, suggesting that there may have been survival bias. Brenowitz et al 94 in their longitudinal study using the Health ABC data concluded that VA and CS independently were not significantly associated with incident dementia. However, Swenor et al 122 used data from the same study and found that impaired VA, CS and stereo acuity had a greater risk of incident cognitive impairment. This could be due to the different outcome measures assessed, that is, dementia 94 vs cognitive impairment. 122 These three studies received a ‘moderate’ rating in QA. Michalowsky et al , 117 who received a ‘strong’ rating in their case–control study concluded that VI was not significantly associated with dementia, a combination of both visual and hearing impairments was significantly associated with the risk of dementia.
There was considerable heterogeneity in the measurement and reporting of cognitive function. Studies measured cognitive function using a variety of instruments with the most common being MMSE. The MMSE is a paper‐based test with a maximum score of 30, with lower scores indicating more severe cognitive impairment. A score of 24 is often used as a threshold to define ‘normal’ cognitive function. 123 The MMSE has been found to be a valid and reliable tool as assesses by many studies. 123 124 Several studies used self-report, diagnosis codes and data from existing records to define cognitive status. Similarly, visual function was also assessed by various methods including self-report. While VA was assessed most commonly, there was significant variation in the charts and tools used to assess it. The parameters used to define cognitive decline and VI may have impacted results across and within studies.
Our systematic review has found that there is a strong consensus in the literature that VI is associated with cognitive decline, cognitive impairment or dementia. Two hypotheses may help explain this association. The first one is that a common pathological process (eg, vascular disease) might be responsible for both the sensory and cognitive impairment in older adults. The second one is that by increasing cognitive load, sensory impairments such as VI might cause cognitive impairment. 125 Literature also suggests that vision rehabilitation in the form of cataract surgery slows the rate of cognitive decline, and therefore, early vision interventions could potentially reduce risk of dementia. 126
Our review evaluated bias for all of the 110 included studies. The majority of studies included in our review were cross-sectional, and according to EPHPP guidelines, cross-sectional studies can only receive a low or moderate rating in the bias assessment. Cross-sectional studies are also prone to selection bias, thus yielding estimates that may not reflect true associations in the target population. Studies receiving a strong rating were all longitudinal. However, the tool penalises longitudinal studies that lose >40% of participants due to dropouts/withdrawals. This may, perhaps unfairly, affect longer longitudinal studies to a greater extent since they collect data over many years and can have more drop-outs due to deaths since they are conducted among older adults.
This review has several important implications. First, it highlights the need for standardised methods to assess and define both visual and cognitive function that will aid future research on these emerging public health issues. Second, it brings into focus the consistent association of VI with cognitive impairment in older adults and the need to better understand the mechanisms underlying this relationship. Third, as the longitudinal results support the sensory consequence theory, and suggest that VI may be a risk factor for cognitive decline, this points to a need for formulating preventive measures and vision rehabilitation models, such as prescription glasses, cataract surgery, low vision rehabilitation, etc, that could have the potential to improve overall health and well-being of older adults.

Limitations
Given the large number of studies included in this review and the heterogeneity of measures used to assess the outcome, it was not possible to compare and meta-analyse results across studies. Although 35 longitudinal studies found a positive association between VI and cognitive decline, we cannot establish temporality between this relationship due to the heterogeneous nature of the studies. The studies included diverse populations, with different disease processes, and variation in definitions of both cognitive and VI. There is also potential bias associated with studies that used different protocols for cognitive and sensory measurements. The MMSE, which was the most commonly used assessment method for testing cognition is sensory dependent and therefore one can argue that the results may be confounded with VI. 127 Further studies should examine the impact of using vision independent cognitive tests on the vision–cognition relationship. Our review examined all cause VI and dementia, and further study is needed to examine the vision–cognition relationship by dementia subtype and by different vision pathology. However, despite the heterogeneity in studies and assessment methods, we synthesised the evidence qualitatively and by taking into account study quality assessed using a validated tool. While our search strategy was robust, it may have been limited by the exclusion of studies that were not published in English.
The number of older adults with VI and dementia is increasing globally, and therefore, the elucidation of the relationship between vision and cognition is of particular public health importance. This systematic review found that the positive association of VI with cognitive decline, cognitive impairment or dementia is largely consistent across studies using different measures of vision and cognition, as well as between countries and cohorts. This overall agreement in the literature suggests that poor visual and cognitive function are associated, and that additional research is now needed to move beyond documenting these associations. The focus of this area of research should now turn to identifying the factors and strategies that mediate the vision–cognition relationship and identifying potential interventions, such as vision rehabilitation models and strategies tailored to people with VI, that may mitigate the cognitive implications of VI.
Ethics statements
Patient consent for publication.
Not applicable.
Acknowledgments
BKS and NN had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
- Livingston G ,
- Sommerlad A ,
- Orgeta V , et al
- Guerchet M ,
- World Health Organization
- Bourne RRA ,
- Flaxman SR ,
- Braithwaite T , et al
- Alzheimer’s Disease International
- Rogers S , et al
- Reyes-Ortiz CA ,
- DiNuzzo AR , et al
- Albers MW ,
- Gilmore GC ,
- Kaye J , et al
- Swenor BK ,
- Christ SL , et al
- Whitson HE ,
- Cronin-Golomb A ,
- Cruickshanks KJ , et al
- Organization, WH
- Resnikoff S , et al
- Effective Public Health Practice Project (EPHPP)
- Helmer C , et al
- Anstey KJ ,
- Luszcz MA ,
- Arnaoutoglou NA ,
- Arnaoutoglou M ,
- Nemtsas P , et al
- Arrighi HM ,
- McLaughlin T ,
- Schwarzkopf L ,
- Graessel E , et al
- Ferrari F ,
- Bhattacharya J ,
- Chriqui E ,
- Kergoat M-J , et al
- Cigolle C ,
- De Celis ESP ,
- de Kok DS ,
- Pillai A , et al
- Dearborn PJ ,
- Sullivan KJ , et al
- Diniz-Filho A ,
- Delano-Wood L ,
- Daga FB , et al
- Elliott AF ,
- Kline LB , et al
- Elyashiv SM ,
- Shabtai EL ,
- Finucane C ,
- Savva GM , et al
- Fischer ME ,
- Cruickshanks KJ ,
- Schubert CR , et al
- Formiga F ,
- Pérez-Castejón JM , et al
- Friedman DS ,
- Massof RW , et al
- Aung KZ , et al
- Fuller SD ,
- Siordia C , et al
- Lara E , et al
- Gaynes BI ,
- Leurgans S , et al
- Guthrie DM ,
- Davidson JGS ,
- Williams N , et al
- Jefferis JM ,
- Clarke MP ,
- Collerton D
- Taylor J-P ,
- Collerton J , et al
- Gutierrez PR ,
- Stone KL , et al
- Guo C , et al
- Maharani A ,
- Pendleton N ,
- Tampubolon G , et al
- Delcourt C ,
- Dartigues JF
- Rovner BW , et al
- Loughman J ,
- Spierer O ,
- Fischer N ,
- Barak A , et al
- Kergoat M-J ,
- Belleville S , et al
- Christ SL ,
- Gussekloo J ,
- de Craen AJM ,
- Oduber C , et al
- Brettschneider C ,
- Lühmann D , et al
- Hidalgo JL-T ,
- Martínez IP ,
- Bravo BN , et al
- Fagot D , et al
- Soto-Perez-de-Celis E ,
- Tew WP , et al
- Mitchell P , et al
- Uhlmann RF ,
- Larson EB ,
- Koepsell TD , et al
- Declercq A ,
- Finne-Soveri H , et al
- Harrabi H ,
- Rousseau J , et al
- Espeland MA ,
- Klein BE , et al
- Andersson L ,
- Ericsson K , et al
- Kao C-H , et al
- Morris P , et al
- Yoshikawa T ,
- Morikawa M , et al
- Haaland BA , et al
- Sourdet S ,
- Balardy L , et al
- Xu Y , et al
- Gelfand JM ,
- Lui L-Y , et al
- Nazroo J , et al
- Ogata Y , et al
- Rougier M-B , et al
- Lindenberger U ,
- Ghisletta P
- Mitchell P ,
- Burlutsky G , et al
- Collerton J ,
- Taylor J-P , et al
- MacDonald SWS ,
- Keller CJC ,
- Brewster PWH , et al
- Catte O , et al
- Mendola JD ,
- Corkin S , et al
- Obayashi K ,
- Saeki K , et al
- Magalhães MOdeC ,
- Peixoto JMdeS ,
- Frank MH , et al
- Cheung CY ,
- Li X , et al
- Mangione CM ,
- Seddon JM ,
- Cook EF , et al
- Riedel-Heller SG ,
- König H-H , et al
- Wittich W ,
- Al-Yawer F ,
- Brenowitz WD ,
- Lin FR , et al
- Davies-Kershaw HR ,
- Hackett RA ,
- Cadar D , et al
- Zhang Q , et al
- de la Fuente J ,
- Hjelmborg J ,
- Wod M , et al
- Hamalainen A ,
- Phillips N ,
- Wittich W , et al
- Hamedani AG ,
- VanderBeek BL ,
- Man REK , et al
- Wang W , et al
- Zhu LP , et al
- Richards M ,
- Chan WC , et al
- Gibbons LE , et al
- Varadaraj V ,
- Ramulu PY , et al
- Sagari A , et al
- Da Soh Z , et al
- Liljas AEM ,
- Walters K ,
- de Oliveira C , et al
- Longstreth WT ,
- Francis CE , et al
- Dartigues J-F , et al
- Hamedani A ,
- Michalowsky B ,
- Hoffmann W ,
- Sabanayagam C , et al
- Kuang W , et al
- Lin T-Y , et al
- Varadaraj V , et al
- Creavin ST ,
- Wisniewski S ,
- Noel-Storr AH
- Folstein MF ,
- Folstein SE ,
- Charalambous AP ,
- Leroi I , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
- Data supplement 3
Twitter @LamaAssi3
Contributors Study concept and design: BKS and HW. Collection, management, analysis and interpretation of the data: BKS, NN, EC, MM and YS. Drafting of manuscript: NN and LA. Preparation, review or approval of the manuscript: NN, VV, LA, BKS, JRE and HW. Decision to submit the manuscript for publication: BKS, JRE and HW.
Funding BKS is supported by funding from the National Institute on Ageing (K01AG052640). JRE is supported by the National Eye Institute (K23EY027848).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 29 April 2024
Cognitive profile in multiple sclerosis and post-COVID condition: a comparative study using a unified taxonomy
- Cristina Delgado-Alonso 1 ,
- Alfonso Delgado-Alvarez 1 ,
- María Díez-Cirarda 1 ,
- Silvia Oliver-Mas 1 ,
- Constanza Cuevas 1 ,
- Paloma Montero-Escribano 1 ,
- Ana Maria Ramos-Leví 2 ,
- María José Gil-Moreno 1 ,
- Juan Ignacio López-Carbonero 1 ,
- Bruce P. Hermann 3 ,
- Jorge Matias-Guiu 1 &
- Jordi A. Matias-Guiu ORCID: orcid.org/0000-0001-5520-2708 1
Scientific Reports volume 14 , Article number: 9806 ( 2024 ) Cite this article
1112 Accesses
63 Altmetric
Metrics details
- Central nervous system infections
- Multiple sclerosis
Post-COVID condition (PCC) and multiple sclerosis (MS) share some clinical and demographic features, including cognitive symptoms and fatigue. Some pathophysiological mechanisms well-known in MS, such as autoimmunity, neuroinflammation and myelin damage, have also been implicated in PCC. In this study, we aimed to compare the cognitive phenotypes of two large cohorts of patients with PCC and MS, and to evaluate the relationship between fatigue and cognitive performance. Cross-sectional study including 218 patients with PCC and 218 with MS matched by age, sex, and years of education. Patients were evaluated with a comprehensive neuropsychological protocol and were categorized according to the International Classification of Cognitive Disorders system. Fatigue and depression were also assessed. Cognitive profiles of PCC and MS largely overlapped, with a greater impairment in episodic memory in MS, but with small effect sizes. The most salient deficits in both disorders were in attention and processing speed. The severity of fatigue was greater in patients with PCC. Still, the correlations between fatigue severity and neuropsychological tests were more prominent in the case of MS. There were no differences in the severity of depression among groups. Our study found similar cognitive profiles in PCC and MS. Fatigue was more severe in PCC, but was more associated with cognitive performance in MS. Further comparative studies addressing the mechanisms related to cognitive dysfunction and fatigue may be of interest to advance the knowledge of these disorders and develop new therapies.
Similar content being viewed by others

APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease

An autoantibody signature predictive for multiple sclerosis

Introduction
Cognitive dysfunction and fatigue are commonly reported after the acute phase of SARS-CoV-2 infection and have been emphasized as the most frequent symptoms by the World Health Organization in the post-COVID condition or Long-COVID (PCC) 1 . Several studies have confirmed the presence of objective cognitive deficits in neuropsychological assessments 2 , 3 . PCC occurs mainly in middle and working age, and women are predominant 4 , 5 , 6 , 7 . Cognitive deficits are more prominent in attention and processing speed, episodic memory and executive function and have been linked to structural and functional brain changes in neuroimaging studies 8 , 9 , 10 , 11 , 12 , 13 . A longitudinal study showed greater reductions in cortical thickness and brain volumes in patients after COVID-19 than in healthy controls compared with neuroimaging acquired before the pandemic 14 . A recent study has also associated fatigue in PCC with structural imaging changes in the thalamus and basal ganglia 15 .
Similarly, most patients with Multiple Sclerosis (MS) also report fatigue and cognitive deficits. Cognitive deficits are especially focused on attention and processing speed impairment, followed by executive function and episodic memory. MS is a recognized autoimmune disorder, and cognitive deficits have been linked to cortical and subcortical structural and functional brain damage 16 , 17 .
Although the pathophysiology of PCC and the neurological symptoms of PCC is still unknown, several studies suggest mechanisms of neuroinflammation, autoimmune disorders, myelin dysregulation, and reactivation of another virus (such as Epstein-Barr infection) 18 , 19 . Although in a different clinical course and extent, these mechanisms are at least partially shared with MS. Besides, the role of Epstein-Barr virus or other viral infections in the development of MS and/or disease activity is supported by some studies 20 . Overall, this suggests an interest in evaluating the similarities and differences in the cognitive profiles of patients with PCC and MS. Comparative studies may also be useful to contextualize the cognitive deficits found in PCC, which have important socioeconomic consequences 21 . However, to our knowledge, there are no studies comparing cognitive dysfunction associated with PCC and MS. In addition, the relationship between fatigue and cognitive performance is still unclear. Previous studies in MS have found that cognitive tests assessing vigilance and alertness are more related to fatigue, which could be caused by shared mechanisms associated with brain atrophy and neurochemical dysfunction 22 . In PCC, fatigue has also correlated with some attentional tests 23 , 24 . Thus, this study aimed to compare the cognitive phenotypes of two large cohorts of patients with PCC and MS that were examined with the same neuropsychological protocol. We also aimed to evaluate the relationships between fatigue, and cognitive performance in the two cohorts. We also compared the frequency of depression, which is a relevant factor in both MS and PCC 25 , 26 , 27 .
Study design and participants
We conducted a cross-sectional investigation including patients with PCC and MS involved in previous research studies evaluating the cognitive characteristics of these disorders 28 , 29 . Patients were recruited from specific clinical programs dedicated to diagnosing and treating individuals with PCS and MS, where comprehensive neuropsychological assessment were integrated into the clinical protocol. The research protocol was approved by the Ethics Committee of our center (Comité de Ética de la Investigación con Medicamentos del Hospital Clínico San Carlos). Written informed consent was obtained from all participants.
Patients with PCC met the following criteria: (a) Diagnosis of COVID-19 confirmed by RT-PCR; (b) cognitive complaints or fatigue in close relationship with SARS-CoV-2 infection; (c) WHO criteria for Post-COVID-19 condition 1 . Exclusion criteria were as follows: (a) any cognitive complaint before COVID-19; (b) any medical, systemic, neurological or developmental comorbidity potentially linked to cognitive dysfunction; (c) history of alcohol or drug abuse; (d) neuropsychiatric disorders not attributable to PCC; (e) any sensory or motor disorder potentially biasing assessments.
Patients with MS met the following criteria: (a) diagnosis of MS according to the 2010 McDonald criteria 30 ; (2) age between 18 and 80 years. Exclusion criteria were as follows: (a) a relapse within the previous two months or active treatment with corticosteroids; (b) any other medical, systemic, neurological or developmental comorbidity potentially causing cognitive impairment; (c) history of alcohol or drug abuse; (d) neuropsychiatric disorders not attributable to MS; (e) sensory or motor disorder biasing assessments.
From an initial sample of 240 patients with PCC (mean age 48.42 ± 10.84 years, 77.9% of women, mean time since the acute infection of 17.48 ± 8.43 months), and 298 patients with MS (mean age 48.09 ± 9.84 years, 69.8% of women, mean duration of disease of 15.87 ± 7.85 years), a matched sample of 436 participants (218 per group) was obtained. The main clinical and demographic characteristics of each group and the vaccination status are presented in Table 1 . Time of SARS-CoV-2 infection (month and year) leading to PCC and time of assessments is shown in Supplementary Fig. 1 .
Neuropsychological and behavioral assessments
Patients were evaluated with a comprehensive neuropsychological protocol that is mainly based on the cognitive tests included in the Neuronorma battery. This was a set of neuropsychological tests co-normed in our country in older and young people 31 , 32 and has been validated in several diseases 33 . Previous works by our group implemented this battery to describe the cognitive profile in patients with MS and recently in PCC 28 , 29 . This battery was administered by trained neuropsychologists. The following tests were shared in the assessment of patients with PCC and MS and were included in the present study: forward and backward digit span, Corsi block-tapping test, Symbol Digit Modalities Test, Boston Naming Test (BNT), Judgment Line Orientation (JLO), Rey-Osterrieth Complex Figure (ROCF) (copy and recall at 3, 30 min, and recognition), Free and Cued Selective Reminding Test (FCSRT) (total free recall, total recall, delayed free recall, and delayed total recall), verbal fluencies (animals and words beginning with “ p ” and “m” in 1 min each one), Stroop Color-Word Interference Test.
Furthermore, patients were evaluated with the Modified Fatigue Impact Scale (MFIS) 34 . MFIS contains 21 items related to cognitive, physical, and psychosocial dimensions of fatigue, which are scored using a Likert-type scale. The assessment evaluates the impact of fatigue in the past 4 weeks. Additionally, depression was assessed with the Beck Depression Inventory-II 32 . Following previous literature, we used a cut-off of ≥ 38 to delineate fatigue and ≥ 19 to define moderate to severe depression 34 , 35 .
Statistical analysis
Statistical analysis was conducted using IBM(R) SPSS v26.0, JASP v0.16.1 and R software 36 . Figures were prepared using the ggplot2 package (v3.4.1). Using MedCalc 20.218, patients with MS and PCC were matched 1:1 according to sex, age (± 3 years), and years of education (± 3 years). The two independent samples t-test was used to compare the two groups. Effect sizes were estimated with Cohen’s d, and were classified as small (d = 0.2–0.49), moderate (d = 0.5–0.79), and large (d ≥ 0.8).
We calculated the percentage of impairment of each test according to the normative data correcting by age, years of education, and sex when needed. Normative data are based on a multicenter study conducted in Spain before the pandemic 31 , 32 . In addition, we used the criteria proposed by IC-CoDiMS and IC-CoDi-COVID groups to describe the cognitive phenotypes in patients with MS and PCC, respectively 37 , 38 . In this taxonomy, initially developed for epilepsy as IC-CoDE 39 , 40 , a domain is considered impaired when two tests within the same domain fall below the cutoff. For this study, we used -1 S.D as the cutoff to define impairment, according to the findings of the previous studies in both MS and PCC using these criteria 37 , 38 . Five cognitive domains are considered: attention/processing speed, executive function, language, visuospatial, and episodic memory. Then, according to the number of domains impaired, the patients are classified as: cognitively intact, single-domain impairment, bi-domain impairment, or multi-domain impairment (≥ 3 impaired domains). The tests specified in Table 2 were used to describe each cognitive domain. The chi-squared test was used to compare cognitive phenotypes between MS and PCC.
Pearson’s coefficient was used to estimate the correlations between fatigue and neuropsychological tests in PCC and MS. Coefficients < 0.40 were interpreted as a weak correlation, 0.40–0.69 as moderate, and > 0.69 as strong. Fisher r-to-z transformation was calculated to compare between correlation coefficients.
A p -value < 0.05 was considered statistically significant. Due to the number of cognitive tests in the neuropsychological protocol, we also specified those contrasts statistically significant after False-Discovery Rate (FDR) correction in the comparison of cognitive performance between PCC and MS.
Ethical approval
This study was approved by the Ethics and Research Committee from our centre and was performed according to the Declaration of Helsinki and its later amendments.
Comparison between PCC and MS
Patients with MS showed lower raw scores compared to PCC in Corsi forward and backwards, FCSRT (total free recall), ROCF memory at 3 and 30 min, and semantic verbal fluency. Conversely, PCC showed greater fatigue severity measured with MFIS. There were no statistically significant differences in the other neuropsychological tests and depressive symptoms. Effect size was moderate for fatigue, and low for the other significant neuropsychological tests. All results are shown in Table 3 .
Cognitive phenotypes
There were no statistically significant differences in the cognitive phenotypes (χ 2 3.014, p = 0.389). Specifically, 127 (58.25%) of PCC patients were regarded as cognitively intact, and 91 (41.74%) as cognitively impaired, 43 (19.72%) showed single-domain, 27 (12.38%) bi-domain, and 21 (9.63%) generalized impairment. Patients with MS were classified as cognitively intact in 112 cases (51.37%), and cognitively impaired in 106 (48.62%). Of those with impairment, 44 (20.18%) showed single-domain, 38 (17.43%) bi-domain, and 24 (11.00%) generalized impairment (Fig. 1 ).
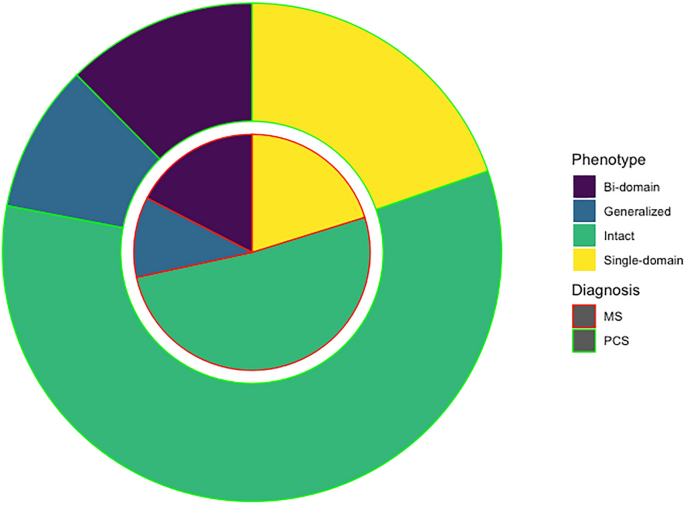
Circle chart representing the cognitive phenotypes in PCC and MS.
Regarding the specific cognitive domains, 63 (28.89%) of PCC and 81 (37.15%) of MS showed impairment in attention/processing speed (χ 2 = 3.36, p = 0.067); 24 (11.00%) and 37 (16.97%) in episodic memory (χ 2 = 3.22, p = 0.073); 41 (18.8%) and 46 (21.10%) in executive function (χ 2 = 0.359, p = 0.549); 14 (6.42%) and 16 (7.33%) in visuospatial function (χ 2 = 0.143, p = 0.705); and 22 (10.09%) and 26 (11.92%) in language (χ 2 = 0.375, p = 0.541). The frequency of impairment of each individual test is shown in Supplementary Fig. 2 . Patients with MS showed higher frequency of impairment in Stroop trial 1 (χ 2 = 6.29, p = 0.012), semantic fluency (χ 2 = 9.86, p = 0.002), letter fluency (χ 2 = 9.42, p = 0.002), ROCF at 3 and 30 min (χ 2 = 6.12, p = 0.013 and χ 2 = 13.28, p < 0.001, respectively), and FCSRT total free recall (χ 2 = 7.20, p = 0.007). The other tests, including SDMT, showed no statistically significant differences ( p > 0.05).
Comparison of cognitive profiles within the groups with cognitive impairment
We also compared those patients meeting the criteria for cognitive impairment with PCC and MS. Patients with MS showed lower scores in ROCF memory at 3 min and 30 min and semantic fluency in age- and education-adjusted scaled scores (Supplementary Table 1 ). Patients with PCC showed greater severity of fatigue (59.95 ± 14.98 vs 54.47 ± 20.89, t = 2.13, p = 0.034). As depicted in Fig. 2 , the represented cognitive profile showed a more prominent impairment in those tests associated with attention and information processing speed.
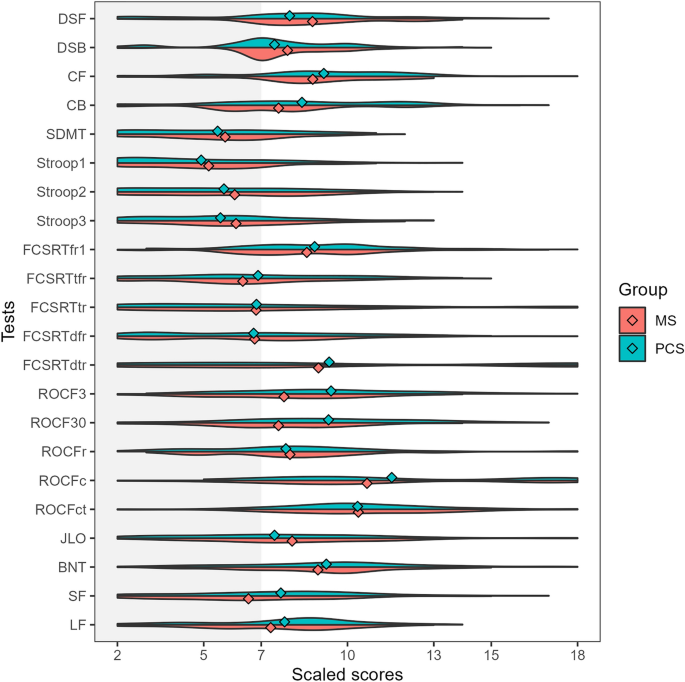
Violin plots representing the adjusted scaled scores (mean 10, standard deviation 3) in each cognitive test in patients with PCC (green) and MS (red) classified as cognitively impaired. The dots represent the mean of each group. DSF digit span forward, DSB digit span backward, CF Corsi forward, CB Corsi backward, SDMT symbol digit modalities test, FCSRT (free and cued selective reminding test, fr1 free recall 1, tfr total free recall, tr total recall, dfr delayed free recall, dtr delayed total recall, ROCF3 Rey-Osterrieth complex figure (memory at 3 min), ROCF30 Rey-Osterrieth complex figure (memory at 30 min), ROCFr Rey-Osterrieth complex figure (memory recognition), ROCFc Rey-Osterrieth complex figure (copy accuracy), ROCFct Rey-Osterrieth complex figure (copy time), JLO judgment line orientation, BNT Boston naming test, SF semantic fluency (animals), LF letter fluency (words beginning with “ p ”).
Correlations between fatigue and neuropsychological tests
All correlations are shown in Fig. 3 . In PCC, MFIS (total score) showed weak correlations with SDMT, FCSRT (total free recall and total recall), Stroop (parts 1, 2, and 3) and semantic and letter fluency. In MS, MFIS (total score) showed moderate correlations with SDMT, FCSRT (free delayed recall and total delayed recall), Stroop test (parts 1 and 2); and weak correlations were found with almost all the other tests.
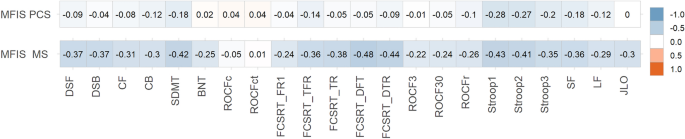
Heatmap showing correlations between MFIS (total score) and neuropsychological tests scores in PCC and MS. The size and direction of the correlation are shown in the right vertical label. DSF digit span forward, DSB digit span backward, CF Corsi forward, CB Corsi backward, SDMT symbol digit modalities test, FCSRT (free and cued selective reminding test), fr1 free recall 1, tfr total free recall, tr total recall, dfr delayed free recall, dtr delayed total recall, ROCF3 Rey-Osterrieth complex figure (memory at 3 min), ROCF30 Rey-Osterrieth complex figure (memory at 30 min), ROCFr Rey-Osterrieth complex figure (memory recognition), ROCFc Rey-Osterrieth complex figure (copy accuracy), ROCFct Rey-Osterrieth complex figure (copy time), JLO judgment line orientation, BNT Boston naming test, SF semantic fluency (animals), LF letter fluency (words beginning with “ p ”).
Patients with MS showed higher correlations than PCC in the following tests: digit span forward (z = 3.02, p = 0.002), digit span backward (r = 3.53, p < 0.001), Corsi forward (Z = 2.45, p = 0.014), SDMT (z = 2.82, p = 0.004), Boston Naming Test (z = 2.81, p = 0.005), FCSRT recall trial 1 (Z = 2.15, p = 0.015), FCSRT total free recall (Z = 2.38, p = 0.017), FCSRT total recall (z = 3.63, p < 0.001), FCSRT delayed free recall (Z = 4.94, p < 0.001), FCSRT delayed total recall (Z = 3.93, p < 0.001), ROCF (memory at 3 min) (Z = 2.26, p = 0.011), ROCF (memory at 30 min) (z = 2.1, p = 0.035), semantic fluency (Z = 2.03, p = 0.04), letter fluency (M-words) (z = 2.64, p = 0.008), letter fluency (R-words) (z = 3.02, p = 0.002), Judgment Line Orientation (Z = 3.26, p = 0.001). There were no statistically significant differences ( p > 0.05) in the comparison of correlation coefficients in Stroop (trials 1, 2, and 3), Corsi backward, ROCF (copy accuracy and time), ROCF (memory recognition), and letter fluency ( P -words).
In this study, we examined the existence of differences in cognitive characteristics between PCC and MS, and the relationship between cognitive function and fatigue. We used two large cohorts of patients that were evaluated with a common neuropsychological protocol. Our study found a significant overlap in cognitive profile between both diseases. Importantly, attention and processing speed were the most pronounced deficits in both disorders, which is consistent with previous studies 2 , 37 , 41 , 42 . Few differences were found in episodic memory tests, which were more impaired in the group of patients with MS than PCC. Similarly, semantic fluency was also more impaired, which could also be linked to the greater impairment of episodic memory 43 . However, effect sizes for these tests were small, confirming that MS and PCC present a very similar cognitive profile.
We applied a novel approach using an international classification of cognitive disorders that is being implemented across several disease groups 37 , 38 , 40 , 44 . This classification system is based on a five-domain cognitive model (attention/processing speed, executive function, episodic memory, visuospatial function, and language) and provides a working definition of impairment to identify cognitive phenotypes and improve cognitive diagnostics. This taxonomy has found reproducible findings across several independent cohorts examined with different neuropsychological batteries in epilepsy 40 , multiple sclerosis 37 and PCC 38 . It has also shown favorable cross-cultural properties in diverse settings 36 . Our study also supports the use of this taxonomy as a valid method for comparative research across disorders.
By comparing both disorders, the similarities in the cognitive characteristics and the severity of deficits contribute to contextualizing the cognitive dysfunction in PCC. In this regard, our findings suggest that cognitive deficits in PCC are almost as pronounced and prevalent as in MS, and fatigue is even more severe, supporting the mounting evidence that fatigue and cognitive dysfunction are associated with occupational issues and socioeconomic consequences 45 , 46 .
The severity and frequency of fatigue was greater in patients with PCC. Interestingly, correlations between MFIS total score (evaluating fatigue impact in the last 4 weeks) and neuropsychological tests were larger in the case of MS. However, the cognitive tests that showed stronger correlations with MFIS were similar in both disorders (e.g., Stroop). This may suggest common mechanisms and neural underpinnings in fatigue and cognitive dysfunction in both disorders, as has been recently described 9 . This opens the way to test new therapies for fatigue based on their association with functional brain changes, such as non-invasive brain stimulation, which have shown positive effects in two clinical trials 47 , 48 . However, at the same time, the lower correlation with neuropsychological tests and the greater severity of fatigue in PCC suggest the existence of other mechanisms (probably not dependent on the central nervous system and including systemic processes such as immune mechanisms, mitochondrial dysfunction or muscle abnormalities) involved in the pathophysiology of fatigue in PCC 24 , 49 , 50 . In contrast, fatigue in MS would be more dependent on central mechanisms.
Another interesting result is the lack of significant differences in the severity and frequency of depressive symptoms. Although neuropsychiatric symptoms have been especially emphasized in PCC, most studies did not include a control group 51 . The prevalence of depression is higher in MS than in the general population, and has been associated with several factors, including genetic and immunological factors, brain changes, and psychosocial factors 52 . Similarly, in PCC, proinflammatory factors and psychosocial factors have been hypothesized, but clear evidence about the pathophysiology of depression is still lacking.
Our study has some limitations. First, although the protocol includes several tests of the main cognitive domains, we acknowledge the possibility of differences between groups if other specific tests are used. In this regard, a more thorough analysis of attention and executive function subdomains may be of interest to further characterize the cognitive mechanisms impaired in each disorder. In this study, we selected only those tests shared by both cohorts to avoid potential differences in the frequency of impairment to the length of the battery or the number of neuropsychological tests and scores. Second, fatigue was only assessed with MFIS, which mainly evaluates the impact of fatigue in daily living. More comprehensive questionnaires may be of interest to evaluate potential differences in the clinical characteristics of fatigue across disorders. Additionally, it could also be of interest to evaluate the feeling of fatigue on the same day of the examination because MFIS considers the fatigue severity in the 4 weeks before the assessment. Third, our study is performed in a single center. However, demographic characteristics and degree of impairment in both PCC and MS are consistent with previous studies of the literature, suggesting that both cohorts are representative of these disorders. In this regard, the most important proportion were infected during the first waves (especially the first in March 2020) and before vaccines were available. Furthermore, we must acknowledge the possibility of selection bias, particularly concerning MS, where individuals with more pronounced motor and cognitive impairments may be less inclined to undergo extensive neuropsychological evaluations. Nevertheless, our study was conducted within a framework where comprehensive neuropsychological assessments are standard practice for both MS and PCS. Additionally, the demographic characteristics of our participants closely resemble those of other large-scale studies published in the field 53 , 54 .
In conclusion, our study finds similar cognitive profiles in PCC and MS, which are mainly characterized by attention and processing speed deficits. Fatigue was more severe in PCC, but the relationship between fatigue and cognitive function was greater in the case of MS. Further comparative studies addressing the mechanisms associated with cognitive dysfunction and fatigue may be of interest to advance the knowledge of these disorders and develop new therapies.
Data availability
The datasets generated and analyzed are available from the corresponding author on reasonable request.
Soriano, J. B., Murthy, S., Marshall, J. C., Relan, P. & Diaz, J. V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 22 , e102–e107 (2022).
Article CAS PubMed Google Scholar
Bertuccelli, M. et al. Cognitive impairment in people with previous COVID-19 infection: A scoping review. Cortex 154 , 212–230 (2022).
Article PubMed PubMed Central Google Scholar
Ceban, F. et al. Brain behavior and immunity fatigue and cognitive impairment in Post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 101 , 93–135 (2022).
Article ADS CAS PubMed Google Scholar
Crook, H., Raza, S., Nowell, J., Young, M. & Edison, P. Long covid—mechanisms, risk factors, and management. BMJ 374 , 1–18 (2021).
Google Scholar
Peter, R. S. et al. Post-acute sequelae of covid-19 six to 12 months after infection: Population based study. BMJ 379 , e071050 (2022).
Article PubMed Google Scholar
Bai, F. et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 28 , 611 (2022).
Article Google Scholar
Bahmer, T. et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. EClinicalMedicine 51 , 101549 (2022).
Caroli, A. et al. Brain diffusion alterations in patients with COVID-19 pathology and neurological manifestations. NeuroImage Clin. 37 , 103338 (2023).
Díez-Cirarda, M. et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain 146 , 2142–2152 (2023).
Lu, Y. et al. Cerebral micro-structural changes in COVID-19 patients–an MRI-based 3-month follow-up study. EClinicalMedicine 25 , 100484 (2020).
Ajcevic, M. et al. Cerebral hypoperfusion in post-COVID-19 cognitively impaired subjects revealed by arterial spin labeling MRI. Sci. Rep. 13 , 5808 (2023).
Article ADS CAS PubMed PubMed Central Google Scholar
Paolini, M. et al. Brain correlates of subjective cognitive complaints in COVID-19 survivors: A multimodal magnetic resonance imaging study. Eur. Neuropsychopharmacol. 68 , 1–10 (2023).
Zhao, S., Toniolo, S., Hampshire, A. & Husain, M. Effects of COVID-19 on cognition and brain health. Trends Cognit. Sci. 27 , 1053–1067 (2023).
Douaud, G. et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature https://doi.org/10.1038/s41586-022-04569-5 (2022).
Heine, J. et al. Articles structural brain changes in patients with post-COVID fatigue: A prospective observational study. eClinicalMedicine 58 , 101874 (2023).
Matías-Guiu, J. A. et al. Identification of cortical and subcortical correlates of cognitive performance in multiple sclerosis using voxel-based morphometry. Front. Neurol. 9 , 1–12 (2018).
Petracca, M. et al. Neuroimaging correlates of cognitive dysfunction in adults with multiple sclerosis. Brain Sci. 11 , 346 (2021).
Article CAS PubMed PubMed Central Google Scholar
Fernández-Castañeda, A. et al. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. BioRxiv (2022).
Gold, J. E., Okyay, R. A., Licht, W. E. & Hurley, D. J. Investigation of long covid prevalence and its relationship to epstein-barr virus reactivation. Pathogens 10 , 1–15 (2021).
Aloisi, F., Giovannoni, G. & Salvetti, M. Epstein–Barr virus as a cause of multiple sclerosis: Opportunities for prevention and therapy. Lancet. Neurol. 22 , 338–349 (2023).
Perlis, R. H. et al. Association of Post-COVID-19 condition symptoms and employment status. JAMA Netw. Open 6 , e2256152 (2023).
Hanken, K., Eling, P. & Hildebrandt, H. Is there a cognitive signature for MS-related fatigue?. Multiple Scler. 21 , 376–381 (2015).
Calabria, M. et al. Post-COVID-19 fatigue: The contribution of cognitive and neuropsychiatric symptoms. J. Neurol. 269 , 3990–3999 (2022).
Matias-Guiu, J. A. et al. Neuropsychological predictors of fatigue in Post-COVID syndrome. J. Clin. Med. 11 , 1–13 (2022).
Leavitt, V. M. et al. Dissociable cognitive patterns related to depression and anxiety in multiple sclerosis. Multiple Scler 26 , 1247–1255 (2020).
Article CAS Google Scholar
Premraj, L. et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID syndrome: A meta-analysis. J. Neurol. Sci. 434 , 120162 (2022).
Delgado-Alonso, C. et al. Unraveling brain fog in post-COVID syndrome: Relationship between subjective cognitive complaints and cognitive function, fatigue, and neuropsychiatric symptoms. Eur. J. Neurol. https://doi.org/10.1111/ene.16084 (2023).
Delgado-Alonso, C. et al. Cognitive dysfunction associated with COVID-19: A comprehensive neuropsychological study. J. Psychiatr. Res. 150 , 40–46 (2022).
Matías-Guiu, J. A. et al. Validation of the Neuronorma battery for neuropsychological assessment in multiple sclerosis. Multiple Scler. Relat. Disord. 42 , 102070 (2020).
Polman, C. H. et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 69 , 292–302 (2011).
Peña-Casanova, J. et al. Spanish multicenter normative studies (NEURONORMA project): Methods and sample characteristics. Arch. Clin. Neuropsychol. 24 , 307–319 (2009).
Peña-Casanova, J. et al. Estudios normativos españoles en población adulta joven (Proyecto NEURONORMA jóvenes): Métodos y características de la muestra. Neurologia 27 , 253–260 (2012).
Sánchez-Benavides, G. et al. Cognitive and neuroimaging profiles in mild cognitive impairment and Alzheimer’s disease: Data from the Spanish Multicenter Normative Studies (NEURONORMA Project). J. Alzheimer’s Dis. 41 , 887–901 (2014).
Kos, D. et al. Evaluation of the modified fatigue impact scale in four different European countries. Multiple Scler. 11 , 76–80 (2005).
Beck, A. T. Manual for the beck depression inventory-II. (No Title) (1996).
R Core Team. R: A Language and Environment for Statistical Computing. (2022).
Hancock, L. M. et al. A proposed new taxonomy of cognitive phenotypes in multiple sclerosis: The International Classification of Cognitive Disorders in MS (IC-CoDiMS). Multiple Scler. J. 29 , 615–627 (2022).
Matias-Guiu, J. A. et al. Development of criteria for cognitive dysfunction in post-COVID syndrome: The IC-CoDi-COVID approach. Psychiatry Res. 319 , 115006 (2023).
Norman, M. et al. Addressing neuropsychological diagnostics in adults with epilepsy: Introducing the International Classification of Cognitive Disorders in Epilepsy: The IC CODE Initiative. Epilepsia Open 6 , 266–275 (2021).
McDonald, C. R. et al. Development and application of the International Classification of Cognitive Disorders in Epilepsy (IC-CoDE): Initial results from a multi-center study of adults with temporal lobe epilepsy. Neuropsychology 37 , 301–314 (2022).
Gonzalez-Fernandez, E. & Huang, J. Cognitive aspects of COVID-19. Curr. Neurol. Neurosci. Rep. 23 , 531–538 (2023).
Benedict, R. H. B., Amato, M. P., DeLuca, J. & Geurts, J. J. G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol 19 , 860–871 (2020).
Delgado-Álvarez, A. et al. Cognitive processes underlying verbal fluency in multiple sclerosis. Front. Neurol. 11 , 1–11 (2021).
Reyes, A. et al. Establishing the cross-cultural applicability of a harmonized approach to cognitive diagnostics in epilepsy: Initial results of the International Classification of Cognitive Disorders in Epilepsy in a Spanish-speaking sample. Epilepsia 64 , 728–741 (2023).
Delgado-Alonso, C. et al. Fatigue and cognitive dysfunction are associated with occupational status in post-COVID syndrome. Int. J. Environ. Res. Public Health 19 , 13368 (2022).
Moccia, M. et al. Determinants of early working impairments in multiple sclerosis. Front. Neurol. 13 , 1062847 (2022).
Oliver-Mas, S. et al. Transcranial direct current stimulation for post-COVID fatigue: A randomized, double-blind, controlled pilot study. Brain Commun. https://doi.org/10.1093/braincomms/fcad117 (2023).
Santana, K. et al. Non-invasive brain stimulation for fatigue in post-acute sequelae of SARS-CoV-2 (PASC). Brain Stimul. 16 , 100–107 (2023).
Paul, B. D., Lemle, M. D., Komaroff, A. L. & Snyder, S. H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. 118 , e2024358118 (2021).
Ramakrishnan, R. K., Kashour, T., Hamid, Q., Halwani, R. & Tleyjeh, I. M. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front. Immunol. 12 , 686029 (2021).
Renaud-Charest, O. et al. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J. Psychiatr. Res. 144 , 129–137 (2021).
Solaro, C., Gamberini, G. & Masuccio, F. G. Depression in multiple sclerosis: Epidemiology, aetiology, diagnosis and treatment. CNS Drugs 32 , 117–133 (2018).
Strober, L. et al. Symbol digit modalities test: A valid clinical trial endpoint for measuring cognition in multiple sclerosis. Multiple Scler 25 , 1781–1790 (2019).
Crivelli, L. et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement. 18 , 1047–1066 (2022).
Download references
Acknowledgements
The authors acknowledge all the participants in this study, and specifically the association of patients with long-COVID “Asociación Madrileña de Covid Persistente (AMACOP)” for their support.
This research has received funding from the Nominative Grant FIBHCSC 2020 COVID-19 (Department of Health, Community of Madrid) and Fundación para el Conocimiento Madri + d (Healthstart plus program, Community of Madrid, REACT-EU funds) through project G63-HEALTHSTARPLUS-HSP4. Jordi A Matias-Guiu is supported by Instituto de Salud Carlos III through the project INT20/00079 and INT23/00017 (co-funded by European Regional Development Fund “A way to make Europe”). María Valles-Salgado is supported by the Instituto de Salud Carlos III through a predoctoral contract (FI20/000145) (co-funded by European Regional Development Fund “A way to make Europe”). Maria Diez-Cirarda is funded by a Sara Borrell postdoctoral fellowship from the Instituto de Salud Carlos III (CD22/00043) (co-funded by European Regional Development Fund “A way to make Europe”). Silvia Mas-Oliver is supported by Fundación para el Conocimiento madri + d through project G63-HEALTHSTARPLUS-HSP4.
Author information
Authors and affiliations.
Department of Neurology, Hospital Clínico San Carlos, Instituto de Investigacion Sanitaria “San Carlos” (IdISSC), C/Profesor Martín Lagos, 28040, Madrid, Spain
Cristina Delgado-Alonso, Alfonso Delgado-Alvarez, María Díez-Cirarda, Silvia Oliver-Mas, Constanza Cuevas, Paloma Montero-Escribano, María José Gil-Moreno, Juan Ignacio López-Carbonero, Jorge Matias-Guiu & Jordi A. Matias-Guiu
Department of Endocrinology, Hospital Clínico San Carlos, Instituto de Investigacion Sanitaria “San Carlos” (IdISSC), Madrid, Spain
Ana Maria Ramos-Leví
Department of Neurology, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA
Bruce P. Hermann
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: C.D.A., J.M.G., J.A.M.G. Methodology: C.D.A., M.D.C., B.P.H., J.A.M.G. Data curation: all. Investigation: all. Formal analysis: C.D.A., A.M.R.L., J.A.M.G. Project administration: J.M.G., J.A.M.G. Writing original draft: C.D.A., J.A.M.G. Writing – review & editing: A.D.A., M.D.C., J.M.G., B.P.H.
Corresponding author
Correspondence to Jordi A. Matias-Guiu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary legends., supplementary table 1., supplementary figure 1., supplementary figure 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Delgado-Alonso, C., Delgado-Alvarez, A., Díez-Cirarda, M. et al. Cognitive profile in multiple sclerosis and post-COVID condition: a comparative study using a unified taxonomy. Sci Rep 14 , 9806 (2024). https://doi.org/10.1038/s41598-024-60368-0
Download citation
Received : 29 June 2023
Accepted : 22 April 2024
Published : 29 April 2024
DOI : https://doi.org/10.1038/s41598-024-60368-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Can Anxiety Relief Come at the Cost of Cognitive Health?
Recent research suggests that long term benzodiazepine use may increase dementia risk..
Posted May 10, 2024 | Reviewed by Devon Frye
- What Is Anxiety?
- Find counselling to overcome anxiety
- Long-term use of benzos can lead to addiction & cognitive impairments.
- Older people are particularly vulnerable to the impairing effects of long-term benzodiazepine use.
- A recent study revealed the mechanism by which benzos interfere with plasticity and cognitive abilities.
- Other treatments such as CBT & life-style changes can alleviate anxiety without the use of benzodiazepines.
Benzodiazepines, often referred to as “benzos,” are commonly prescribed medications used to treat conditions such as anxiety and insomnia . These include drugs like Ativan, Xanax, and Clonazepam. In the United States alone, millions of people use these anti- anxiety medications. Over the past decade, research has indicated that prolonged use of these drugs may be associated with addiction and cognitive impairments, including dementia , particularly in individuals aged 60 and above.
For instance, a study published in the British Medical Journal discovered a correlation between dementia and the use of anti-anxiety medications like benzos. The study followed nearly 9,000 older adults for six years after they began using the medication for insomnia or anxiety. The findings suggested that those who used benzos were 51 percent more likely to develop dementia, and the risk increased the longer the drugs were used (1).
Another study compared over 70,000 non-institutionalized Finnish men and women diagnosed with dementia from 2005-2010 with a larger demographic-matched group without dementia (2). The researchers concluded that individuals taking benzodiazepines and related drugs had a slightly increased risk of developing dementia.
The type of benzo taken did not affect the dementia risk. However, many of the individuals taking benzos were also on antidepressants or antipsychotic drugs, which could have also contributed to the development of dementia. The authors advised against the use of benzos, especially in older individuals.
In September 2020, the FDA announced that they would update the "black box warning" for benzodiazepines to include information about the risks of physical dependence, withdrawal reactions, misuse, abuse, and addiction (3). This was in addition to a previous warning about the risks of taking benzodiazepines concurrently with opiates, which could lead to death (4).
While these studies do not establish a cause-and-effect relationship, they do suggest a strong correlation between long-term benzo use and dementia. The mechanism for how long-term use of benzo could lead to cognitive impairments has been unknown.
However, a study published in 2022 shed some light on a possible mechanism by which benzos lead to dementia . Researchers at the Australian Nuclear Science and Technology Organization (ANSTO) conducted a study showing how diazepam, a commonly prescribed benzo, can impair the structural plasticity of dendritic spines, leading to cognitive impairments in mice.
The researchers used a unique lab model called “Guwiyang Wurra-TSPO knockout,” a healthy mouse lacking a protein present in the mitochondrion, the cell’s energy-providing organelle (5). Anti-anxiety drugs like diazepam bind to TSPO on the surface of microglial cell organelles. Microglial cells are the brain’s first immune responders and are implicated in dementia, long COVID , chronic fatigue and other cognitive impairments.
In animal models without TSPO, the physiological impairments associated with cognitive impairment from diazepam use were not observed. In addition, the study found that TSPO-mediated loss of dendritic spines accelerated cognitive decline . This is in contrast to the traditional view that benzos work by enhancing the inhibitory GABA synapses in the brain, thus calming the mind.
Anti-anxiety drugs may promote cognitive decline directly and indirectly in several ways:
- Impact on microglial cells: For example, promoting the movement of microglial cells interferes with dendritic spines' plasticity (areas in synaptic connections critical for learning, memory , and plasticity).
- Alteration to brain wiring: Long-term use of benzos alters the complex wiring of the brain. For example, the supporting microglial cells indirectly help maintain the functions of these circuits. Benzos interfere with glial cells.
- Side-effects of the medications: Benzos have side effects such as confusion, clouded thinking, and memory lapses. These side-effects can make learning, which is essential for protecting the brain against cognitive decline, challenging.
- Other mechanisms: Although one mechanism has been revealed, other mechanisms may also be involved.

Unsurprisingly, both the Harvard Health Publishing News and the American Geriatric Association have listed benzos as inappropriate for older adults. Fortunately, other options for treating anxiety exist. Alternative treatments include:
- Cognitive behavioral therapy (CBT): CBT is a well-established therapeutic approach for anxiety. It focuses on identifying and changing negative thought patterns and behaviors. A trained therapist can guide you through this process.
- Mindfulness and meditation : Mindfulness practices, such as meditation, deep breathing exercises, prayers, and progressive muscle relaxation, can help reduce anxiety. They promote relaxation and self-awareness.
- Physical activity : Regular exercise has been shown to reduce anxiety. It releases endorphins, which are natural mood lifters. Physical exercise also keeps the brain fit . Choose an activity you enjoy, whether it’s walking, yoga, dancing, or swimming.
- Limit caffeine and alcohol and avoid recreational drugs : Alcohol and recreational drugs can exacerbate anxiety. Reducing or avoiding them can make a difference. Caffeine can elicit many of the physiological symptoms that can trigger anxiety, such as rapid heartbeat.
- Sleep hygiene : Lack of sleep can worsen anxiety. Prioritize good sleep habits, such as maintaining a consistent sleep schedule and creating a relaxing bedtime routine. The authors of an article published in Neurotherapeutics in 2019 recommend that doctors be "very cautious" before prescribing benzos to older people and recommend encouraging good sleep hygiene to alleviate the symptoms of anxiety and insomnia (6).
In conclusion, while further research is needed to categorically conclude that long-term use of benzos causes dementia, the evidence strongly points to the perils of long-term use of benzos. This warning is even more credible after recent studies have shed light on the specific mechanisms by which benzos may lead to cognitive impairment. Fortunately, successful alternative treatments exist.
Note: Do not stop taking your prescribed anti-anxiety medications without consulting with a doctor.
(1) Billioti de Gage, S., Moride, Y., Ducruet, T., et al. (2014). Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 349, g5205.
(2) Tapiainen, V., Taipale, H., Tanskanen, A., et al. (2018). The risk of Alzheimer’s disease associated with benzodiazepines and related drugs: a nested case-control study. Acta Psychiatrica Scandinavia 138: 91-100 doi: 10.1111/acps.12909
(3) https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-bo…
(4) https://www.fda.gov/media/99689/download
(5) Shi, Y., Cui, M., Ochs, K. et al. Long-term diazepam treatment enhances microglial spine engulfment and impairs cognitive performance via the mitochondrial 18 kDa translocator protein (TSPO). Nat Neurosci 25, 317–329 (2022). https://doi.org/10.1038/s41593-022-01013-9
(6) DeKosky ST, Williamson JB. The long and the short of benzodiazepines and sleep medications: Short-term benefits, long-term harms? Neurotherapeutics . 2020;17(1):153-155. doi:10.1007/s13311-019-00827-z

Marwa Azab, Ph.D. , is an adjunct professor of psychology and human development at California State University, Long Beach.
- Find Counselling
- Find a Support Group
- Find Online Therapy
- Richmond - Tweed
- Newcastle - Maitland
- Canberra - ACT
- Sunshine Coast
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
- Introduction
- Conclusions
- Article Information
For illustrative purposes, 2 extreme high serum amyloid A (SAA) values (111.49 and 151.18 μg/mL) and 1 interleukin 1β (IL-1β) value (7.59 pg/mL) at the baseline measurement are not shown in the graphs. Repeated-measures analyses of variance were conducted to examine changes of time. The difference over time for IL-1β is based on the Wilcoxon signed rank test. The horizontal bar inside the boxes indicates the median, and the lower and upper ends of the boxes are the first and third quartiles. The whiskers indicate values within 1.5× the IQR from the upper or lower quartile (or the minium and maximum if within 1.5× the IQR of the quartiles), and data more extreme than the whiskers are plotted individually as outliers (circles). CRP indicates C-reactive protein; IL-6, interleukin 6; PAI-1, plasminogen activator inhibitor 1; and TNF, tumor necrosis factor. SI conversion factor: To convert CRP to mg/L, multiply by 10.0.
BARICO indicates Bariatric Surgery Rijnstate and Radboudumc Neuroimaging and Cognition in Obesity; BMI, body mass index; CRP, C-reactive protein; PAI-1, plasminogen activator inhibitor 1; RYGB, Roux-en-Y gastric bypass; and WC, waist circumference.
eTable 1. Baseline Characteristics and Cognitive Test Scores of Improvers and Non-improvers
eFigure 1. Flow Chart of the Study
eTable 2. Missing Data per Outcome Measure at Baseline and 6 Months After Bariatric Surgery
eTable 3. Plasma Concentrations of Adipokines and Inflammatory Markers Before and 6 Months After Bariatric Surgery
eFigure 2. Boxplots With Cognitive Outcomes Before and 6 Months After Bariatric Surgery (A-E)
eFigure 3. Boxplots With Plasma Concentrations of Adipokines and Inflammatory Markers per Group (Improvers and Non-improvers) Before and 6 Months After Bariatric Surgery (A-C)
eTable 4. Pearson and Spearman Correlation Coefficients Between Changes in Cognition and Changes in Anthropometric Measures, Plasma Levels, Mood and Physical Activity
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Vreeken D , Seidel F , Custers EM, et al. Factors Associated With Cognitive Improvement After Bariatric Surgery Among Patients With Severe Obesity in the Netherlands. JAMA Netw Open. 2023;6(5):e2315936. doi:10.1001/jamanetworkopen.2023.15936
Manage citations:
© 2024
- Permissions
Factors Associated With Cognitive Improvement After Bariatric Surgery Among Patients With Severe Obesity in the Netherlands
- 1 Department of Medical Imaging, Anatomy, and Radboud Alzheimer Center, Radboud University Medical Center, Donders Institute for Brain, Cognition, and Behavior, Center for Medical Neuroscience, Nijmegen, the Netherlands
- 2 Department of Bariatric Surgery, Vitalys, Rijnstate Hospital, Arnhem, the Netherlands
- 3 Department of Metabolic Health Research, Netherlands Organisation for Applied Scientific Research, Leiden, the Netherlands
- 4 Department of Surgery, WeightWorks Clinics, Amersfoort, the Netherlands
- 5 Department of Medical Psychology and Radboud Alzheimer Center, Radboud University Medical Center, Donders Institute for Brain, Cognition, and Behavior, Nijmegen, the Netherlands
- 6 Department of Neuropsychology and Rehabilitation Psychology, Centre for Cognition, Radboud University, Donders Institute for Brain, Cognition, and Behavior, Nijmegen, the Netherlands
- 7 Vincent van Gogh Institute for Psychiatry, Venray, the Netherlands
- 8 Division of Human Nutrition and Health, Wageningen University, Wageningen, the Netherlands
Question Are changes in adipokines, inflammatory factors, mood, and physical activity associated with cognitive improvement 6 months after bariatric surgery?
Findings In this cohort study of 156 participants with severe obesity who underwent bariatric surgery, 43.8% of the participants experienced overall cognitive improvement 6 months after surgery. This group had lower C-reactive protein and leptin levels and fewer depressive symptoms 6 months after surgery compared with the group of participants who did not show cognitive improvement.
Meaning This study suggests that reduced C-reactive protein and leptin levels, as well as fewer depressive symptoms, might partly explain the mechanisms behind cognitive improvement after bariatric surgery.
Importance Bariatric surgery–induced weight loss is often associated with improved cognitive function. However, improvement in cognitive function is not always exhibited by all patients, and the mechanisms behind cognitive improvement remain unknown.
Objective To investigate the association of changes in adipokines, inflammatory factors, mood, and physical activity with alterations in cognitive function after bariatric surgery among patients with severe obesity.
Design, Setting, and Participants This cohort study included 156 patients with severe obesity (body mass index [calculated as weight in kilograms divided by height in meters squared], >35) eligible for Roux-en-Y gastric bypass, aged between 35 and 55 years, who were enrolled in the BARICO (Bariatric Surgery Rijnstate and Radboudumc Neuroimaging and Cognition in Obesity) study between September 1, 2018, and December 31, 2020. Follow-up was completed July 31, 2021; 146 participants completed the 6-month follow-up and were included in the analysis.
Intervention Roux-en-Y gastric bypass.
Main Outcomes and Measures Overall cognitive performance (based on a 20% change index of the compound z score), inflammatory factors (eg, C-reactive protein and interleukin 6 levels), adipokines (eg, leptin and adiponectin levels), mood (assessed via the Beck Depression Inventory), and physical activity (assessed with the Baecke questionnaire).
Results A total of 146 patients (mean [SD] age, 46.1 [5.7] years; 124 women [84.9%]) completed the 6-month follow-up and were included. After bariatric surgery, all plasma levels of inflammatory markers, including C-reactive protein (median change, −0.32 mg/dL [IQR, –0.57 to –0.16 mg/dL]; P < .001) and leptin (median change, −51.5 pg/mL [IQR, –68.0 to –38.4 pg/mL]; P < .001), were lower, whereas adiponectin levels were higher (median change, 0.15 μg/mL [IQR, –0.20 to 0.62 µg/mL]; P < .001), depressive symptoms were (partly) resolved (median change in Beck Depression Inventory score, −3 [IQR, –6 to 0]; P < .001), and physical activity level was higher (mean [SD] change in Baecke score, 0.7 [1.1]; P < .001). Cognitive improvement was observed in 43.8% (57 of 130) of the participants overall. This group had lower C-reactive protein (0.11 vs 0.24 mg/dL; P = .04) and leptin levels (11.8 vs 14.5 pg/mL; P = .04) and fewer depressive symptoms at 6 months (4 vs 5; P = .045) compared with the group of participants who did not show cognitive improvement.
Conclusions and Relevance This study suggests that lower C-reactive protein and leptin levels, as well as fewer depressive symptoms, might partly explain the mechanisms behind cognitive improvement after bariatric surgery.
Obesity is a worldwide major health challenge. 1 It is associated with metabolic disorders, and obesity in midlife is considered a risk factor for cognitive decline and dementia at later ages. 2 , 3
Obesity seems to affect multiple cognitive domains, such as memory, verbal fluency, and executive functions. 4 Weight loss after bariatric surgery is associated with improved cognition. 5 , 6 Improvements in memory and executive functions have been found 3 months to 3 years after bariatric surgery. 7 , 8 However, not all studies of bariatric surgery reported cognitive improvement, 9 , 10 and the mechanisms behind such improvement are not well established yet, to our knowledge. Some proposed mechanisms are changes in white adipose tissue and related adipokines, reduced systemic inflammation, and improved mood and physical activity. 5 , 11 - 13 Classification of patients into those with improved cognition and those without cognitive improvement might reveal more information on the potential underlying mechanisms.
White adipose tissue is an endocrine organ regulating secretion of adipokines and cytokines, such as leptin, adiponectin, and proinflammatory cytokines. 14 In obesity, the secretion of proinflammatory adipokines is increased, resulting in systemic low-grade inflammation. 15 , 16 These adipokines and proinflammatory cytokines may influence structural and functional changes in the brain. 15 However, associations between other inflammatory factors, such as C-reactive protein (CRP) level, and cognitive function among people with obesity are inconsistent. An association between higher levels of CRP and impairment in cognitive flexibility was observed, 17 or it was found for women only, 18 while other studies failed to replicate this finding. 19 Leptin has been shown to be an important factor associated with attentional performance among individuals with obesity. 20 After bariatric surgery, reduced inflammation and changes in adipokines are observed, indicating that reduced inflammation may improve cognition after bariatric surgery. Furthermore, mood and physical activity play a major role in cognitive performance. 12 , 13 After surgery, changes in mood and increased physical activity are often observed; therefore, these lifestyle factors may be a mechanistic link between bariatric surgery and cognitive improvement. 5
In this study, we investigated the association between changes in adipokines, inflammatory factors, mood, and physical activity with cognitive function. Data were collected before and 6 months after bariatric surgery for adults with severe obesity (body mass index [BMI; calculated as weight in kilograms divided by height in meters squared], >35) enrolled in the BARICO (Bariatric Surgery Rijnstate and Radboudumc Neuroimaging and Cognition in Obesity) study. We also compared individuals with improved cognition with those without cognitive improvement. The results provide a better understanding of the potential mechanisms underlying cognitive improvement after bariatric surgery and may help to develop personalized strategies complementary to bariatric surgery.
Data from the BARICO study were analyzed. 21 Between September 1, 2018, and December 31, 2020, patients were recruited at the Rijnstate Hospital (Arnhem, the Netherlands). Participants were aged 35 to 55 years at recruitment and eligible for Roux-en-Y gastric bypass surgery. Neurologic or severe psychiatric illness, pregnancy, and treatment with any antibiotics, probiotics, or prebiotics 3 months before or at any point during the study (excluding preoperative prophylaxis) were exclusion criteria. Follow-up was completed July 31, 2021. Cognitive performance was assessed before and 6 months after surgery using neuropsychological tests. At both time points, blood samples were collected, anthropometric data were recorded, and participants filled out questionnaires. The study was approved by the Medical Ethics Committee CMO region Arnhem–Nijmegen and the local institutional ethics committee. The study was conducted according to the Declaration of Helsinki 22 and according to the ICH Harmonised Tripartite Guideline for Good Clinical Practice. 23 All participants provided written informed consent. The study was prospectively registered in the Dutch Trial Registry. 24 The Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline was followed.
Anthropometric measurements included blood pressure, body weight, BMI, waist circumference (WC), and percentage total body weight loss, defined as weight loss divided by total weight before Roux-en-Y gastric bypass surgery. Fasting (≥3 hours) blood samples were collected at both time points. Plasma levels of CRP, leptin, adiponectin, serum amyloid A (SAA), and plasminogen activator inhibitor 1 (PAI-1) were determined with human enzyme-linked immunosorbent assays. Tumor necrosis factor (TNF), interleukin 1β (IL-1β), and interleukin 6 (IL-6) levels were determined with single molecule array technology using the SP-X Imaging and Analysis system (Quanterix).
Cognitive performance was assessed by trained researchers using neuropsychological tests, described elsewhere. 21 All tests are standardized, demonstrate good validity, and have good retest reliability. In short, general cognition was assessed by the Montreal Cognitive Assessment. 25 Summed scores of the 3 trials (forward, backward, and sorting) of the Digit Span test (Wechsler Adult Intelligence Scale–Fourth Edition) were used to determine working memory. 26 Episodic memory was assessed via the immediate and delayed Story Recall Test from the Rivermead Behavioral Memory test. 27 The Controlled Oral Word Association Test (COWAT) was used to determine verbal fluency. 28 Last, the Flexibility subtest from the Tests of Attentional Performance, version 2.3, was used to study ability to shift attention. 29 Two different versions of the Montreal Cognitive Assessment, Story Recall Test, and COWAT for each time point were used to overcome material-specific practice effects. A compound score for global cognitive function was calculated by taking the mean of the z scores of the total score of the Digit Span Test, the Story Recall Test, and COWAT and the Flexibility subtest score from the Tests of Attentional Performance. The z scores at 6 months were based on the mean (SD) of each test score in the sample at baseline.
To examine individual associations of the surgery with cognitive test outcomes, we calculated the 20% change index. 30 This index assumes significant cognitive improvement if the postoperative test score is 20% higher than the preoperative test score. The following formula was used to calculate this index: 5( x 2 − x 1 )/ x 1 , where x 2 is the postoperative test score and x 1 the preoperative test score. This score was calculated for each domain separately and for the compound z score. An index of 1.00 or more indicates significant improvement.
Educational level was determined via the Verhage score, using 7 categories (1 = less than primary school; 7 = academic degree) based on the Dutch educational system that are comparable with the International Standard Classification of Education. 31 , 32 A score of 4 or less is defined as a low educational level, a score of 5 is defined as a middle educational level, and a score of 6 or 7 is defined as a high educational level.
Participants filled in standardized, online questionnaires at both time points. To assess depressive symptoms, the Beck Depression Inventory (BDI) (edition 1a) was used. 33 The BDI is a 21-item self-reported questionnaire determining the presence of depressive symptoms over the past 2 weeks. Each response is scored on a scale ranging from 0 (absence of symptom) to 3 (severe symptom). A total score from 0 to 9 indicates minimal depression, 10 to 20 indicates mild depression, 21 to 30 indicates moderate depression, and 31 or more indicates severe depression. To assess physical activity, the Baecke questionnaire was used, 34 which contains 16 questions with a 5-point Likert scale on the time spent on various activities. Three index scores for work, sport, and leisure were combined into 1 total score. The total score ranges from 3 to 15; higher scores indicate a higher physical activity level.
Statistical analyses were performed using IBM SPSS statistics, version 25 (SPSS Inc). Normality was checked for continuous variables. When assumptions on normality were not met, a natural log transformation or nonparametric test was used. To test changes over time, repeated-measures analyses of variance (ANOVA), the Wilcoxon signed rank test, or the χ 2 test were used for continuous parametric, continuous nonparametric, and categorical data, respectively. To compare changes in cognitive test scores over time, the following covariates were selected: age, sex, educational level, and preoperative BMI. None of the covariates had a significant association with the change in cognitive test scores or showed interaction with cognitive test scores over time. Therefore, no covariates were added. Statistical tests were 2-sided, and the α level was set at .05.
Next, we examined differences between participants showing overall cognitive improvement compared with those not showing cognitive improvement, based on the 20% change index for the compound z score. A participant was classified as improved when his or her index score was 1.00 or more. First, we tested whether the groups differed based on age, sex, and educational level (eTable 1 in Supplement 1 ). Since this was not the case, we did not add covariates in the model. Repeated-measures ANOVA was performed to test differences between groups regarding weight loss, circulating plasma markers, mood, and physical activity levels with time as within-participant variables and assigned groups as a between-participant variable. Furthermore, we explored the group × time interaction within the model. For nonnormally distributed data, the Mann-Whitney test at 6 months was used. Last, we explored whether changes in cognition were associated with changes in weight loss, circulating plasma markers, mood, and physical activity levels using bivariate correlations.
A total of 146 patients (mean [SD] age, 46.1 [5.7] years; 124 women [84.9%]) were included in the analyses (eFigure 1 and eTable 2 in Supplement 1 ). Participants’ characteristics are listed in Table 1 . 31 Mean weight, BMI, WC, and blood pressure were significantly lower after surgery. Participants also used significantly less medication for comorbidities, such as diabetes and hypertension, and fewer participants reported use of antidepressants.
Changes in plasma adipokine levels and inflammatory markers are depicted in Figure 1 . Less inflammation was observed after surgery (eTable 3 in Supplement 1 ). Furthermore, levels of leptin (median change, −51.5 pg/mL [IQR, –68.0 to –38.4 pg/mL]; P < .001), CRP (median change, −0.32 mg/dL [IQR, –0.57 to –0.16 mg/dL] [to convert to mg/L, multiply by 10.0]; P < .001), and PAI-1 (median change, –11.2 ng/mL [IQR, –28.0 to 7.6 ng/mL]) were lower, whereas adiponectin levels were higher (median change, 0.15 μg/mL [IQR, –0.20 to 0.62 µg/mL]; P < .001).
Cognitive test scores were higher 6 months after surgery for all cognitive domains ( Table 2 ; eFigure 2 in Supplement 1 ). Based on the 20% change index, 39.0% of the participants (57 of 146) showed improvement in episodic memory (Story Recall), and 42.3% of the participants (55 of 130) showed an ability to shift attention (Tests of Attentional Performance Flexibility index score). Only 11.0% of the participants (11 of 146) showed improvement in working memory (Digit Span), and 28.1% of the participants (41 of 146) showed improvement in verbal fluency (COWAT). Lower BDI scores were observed (median change, −3 [IQR, –6 to 0]; P < .001), indicating improved mood. At baseline, 27.5% of the participants (39 of 142) showed mild depressive symptoms, and 14.1% participants (20 of 142) showed moderate depressive symptoms. Only 8.0% (11 of 138) and 5.8% (8 of 138) of the participants continued to show symptoms of, respectively, mild and moderate depression after surgery. Furthermore, a higher Baecke score was observed after surgery (mean [SD] change, 0.7 [1.1]; P < .001), indicating higher physical activity levels.
Next, we divided the group into improvers and nonimprovers based on their overall cognitive improvement, defined as a 20% change index of 1.00 or more for their compound z score. In total, 43.8% of the participants (57 of 130) were classified as cognitive improvers who had lower cognitive test scores at baseline compared with nonimprovers (eTable 1 in Supplement 1 ). After surgery, both groups showed lower BMI, WC, CRP level, leptin level, SAA level, IL-1β level, PAI-1 level, and BDI score, as well as a higher Baecke score ( Table 3 ). Typical of improvers was a more pronounced reduction of median CRP and leptin levels compared with nonimprovers (CRP: improvers, from 0.42 mg/dL [IQR, 0.24-0.70 mg/dL] to 0.11 mg/dL [IQR, 0.05-0.23 mg/dL]; P < .001; nonimprovers, from 0.70 mg/dL [IQR, 0.41-1.22 mg/dL] to 0.24 mg/dL [IQR, 0.07-0.41 mg/dL]; P < .001; and leptin: improvers, from 63.7 pg/mL [IQR, 47.9-79.5 pg/mL] to 11.8 pg/mL [IQR, 8.0-19.8 pg/mL]; P < .001; nonimprovers, from 72.6 pg/mL [IQR, 58.9-90.6 pg/mL] to 14.5 pg/mL [IQR, 9.8-23.2 pg/mL]; P < .001). Improvers also showed a lower median BDI score after surgery compared with nonimprovers (improvers, from 8 [IQR, 4-11] to 4 [IQR, 3-6]; P < .001; nonimprovers, from 8 [IQR, 5-14] to 5 [IQR, 3-8]; P = .045).
Furthermore, a group × time interaction effect for adiponectin ( F 1,98 = 9.49; P = .003), TNF ( F 1,108 = 5.97; P = .02), and IL-6 ( F 1,108 = 7.27; P = .01) was shown. Only cognitive improvers showed higher median adiponectin levels after bariatric surgery (improvers, from 2.3 μg/mL [IQR, 1.7-2.9 μg/mL] to 2.6 μg/mL [IQR, 1.9-3.7 μg/mL]; P < .001; nonimprovers, from 2.4 μg/mL [IQR, 1.9-2.7 μg/mL] to 2.4 μg/mL [IQR, 2.1-2.9 μg/mL]; P = .38) ( Table 3 ; eFigure 3 in Supplement 1 ). However, no significant differences were observed between improvers and nonimprovers at both time points in adiponectin level (baseline, P = .11; at 6 months, P = .14). Only nonimprovers showed a significant change in median TNF and IL-6 levels over time (TNF: improvers, from 3.9 pg/mL [IQR, 3.0-5.2 pg/mL] to 3.8 pg/mL [IQR, 3.3-4.5 pg/mL]; P = .39; nonimprovers, from 4.4 pg/mL [IQR, 3.4-5.7 pg/mL] to 3.2 pg/mL [IQR, 2.7-4.2 pg/mL]; P < .001; and IL-6: improvers, from 1.7 pg/mL [IQR, 1.3-2.8 pg/mL] to 1.6 pg/mL [IQR, 1.2-2.4 pg/mL]; P = .16; nonimprovers, from 2.5 pg/mL [IQR, 1.4-3.6 pg/mL] to 1.6 pg/mL [IQR, 0.9-2.1 pg/mL]; P < .001) ( Table 3 ; eFigure 3 in Supplement 1 ). Anthropometric measurements and physical activity did not differ between both groups. eTable 4 in Supplement 1 shows the correlation coefficients between changes in cognitive test scores and changes in obesity indices, plasma markers, BDI, and physical activity. Change in BMI and WC were both positively correlated with change in verbal fluency (BMI: r = 0.21; P = .02; WC: r = 0.23; P = .02). Change in TNF level was positively correlated with improvement in working memory ( r = 0.18; P = .04), and change in IL-6 level was positively correlated with episodic memory ( r = 0.18; P = .049).
In this study, we wanted to reveal the underlying processes involved in cognitive improvement after bariatric surgery. Cognitive improvement was observed for all cognitive domains after surgery. Taking repeated testing into account, 43.8% of the participants showed improvement in overall cognitive function. Furthermore, 6 months after bariatric surgery, significant improvements in general health were observed, such as less inflammation; lowered leptin, SAA, and PAI-1 levels; higher adiponectin level; lower blood pressure; less use of medication for comorbidities (such as diabetes and hypertension); less reported use of antidepressants; improved mood; and higher physical activity levels ( Figure 2 ). Participants classified as cognitive improvers had lower CRP and leptin levels and fewer depressive symptoms after surgery than nonimprovers. This association was independent of physical activity and anthropometric measures.
Consistent with past studies, our results indicate an association between cognitive improvement and bariatric surgery. 5 , 7 , 8 Most earlier studies did not control for repeated testing. In this study, we used the 20% change index, which demonstrated that only part of the participants showed reliable cognitive improvement according to this relatively strict definition, although the reliable change index or the inclusion of a control group remains the preferred method to study cognitive changes. Studies that were performed earlier often described improvement in memory, executive function, and cognitive control after bariatric surgery. 5 , 6 The largest effect sizes in our study were found for the cognitive domains of verbal fluency and ability to shift attention (ie, executive functions). Larger changes in BMI and WC were associated with a higher change in the ability to shift attention as well. This finding is supported by a meta-analysis showing deficits in executive functions associated with obesity. 35 Our results suggest that obesity-associated cognitive decline is at least partly reversible by bariatric surgery. Based on the 20% change index, we found reliable cognitive improvement in approximately 39% of the participants. These participants had lower baseline cognitive test scores, indicating either a higher range of improvement in these lower-scoring individuals or a higher test sensitivity toward change in this group. They also showed improved liver (CRP) and white adipose tissue–related (leptin) biomarkers, confirming large heterogeneity in obesity. 36
Lower CRP and leptin levels and fewer depressive symptoms were observed in participants with cognitive improvement compared with nonimprovers, although nonimprovers did show lower CRP and leptin levels and fewer depressive symptoms as well. CRP is a liver-specific and highly sensitive inflammation marker. Results regarding CRP with higher leptin levels suggest that concerted beneficial associations with biomarkers of the liver and white adipose tissue are seen among cognitive improvers. This finding indicates the importance of improvement in metabolic function for cognitive improvement. Furthermore, an interaction was observed for adiponectin, TNF, and IL-6. Cognitive improvers did not show less TNF and IL-6 over time, while nonimprovers did. However, TNF and IL-6 values were within the normal range for healthy adults for both groups, making these differences less relevant. 37 , 38 Adiponectin levels were higher among improvers after bariatric surgery, but not among nonimprovers. Adiponectin is an important adipokine with multiple functions, such as anti-inflammatory effects, inhibiting formation of atherosclerosis, and glucose regulation, but it also shows neuroprotective effects. 15 , 39 , 40 Adiponectin seems to be associated with memory performance. 41 Altogether, our results indicate the importance of improvement in liver and white adipose tissue functioning for cognitive improvement.
One of the most pronounced outcomes of bariatric surgery that was also observed in our study is reduced obesity-related comorbidities, such as hypertension and diabetes. Remission of hypertension has a positive influence on vascular wall health and might be associated with improved blood circulation and cerebral blood flow. 42 , 43 Cognitive functioning is generally correlated with cerebral blood flow, 44 implying that cerebrovascular changes after bariatric surgery may be associated with cognitive improvement. Structural brain changes in obesity, such as reduced hippocampal and gray matter volume, have been linked to impaired glucose and insulin regulation, 45 suggesting that improvement in diabetes and glucose regulation might also be associated with cognitive improvement after bariatric surgery. Whether cognitive improvement after bariatric surgery is associated with cerebrovascular function and glycemic control needs to be further investigated.
Earlier studies focused on separate associations of CRP and leptin levels with cognitive functioning after bariatric surgery. A study in a comparable patient population did not find an association between CRP level and cognitive improvement after surgery; however, there were some differences compared with our study 19 (ie, differences in age range of patients and statistical analysis), and they tested multiple domains separately instead of using 1 global cognitive score. 19 These differences and the fact that we divided the participants into 2 groups based on overall cognitive performance might explain the discrepancy in results. Other studies support the hypothesis that lower cognitive function among individuals with obesity is associated with systemic inflammation. 17 , 18 , 46 Our results regarding leptin are consistent with an earlier study in which lower leptin levels 12 months after bariatric surgery were associated with better executive function. 47
Fewer depressive symptoms were observed among cognitive improvers, indicating that cognitive functioning is associated with mood. 12 The exact mechanism linking obesity, depression, and cognitive function is most likely complex because these factors are all interrelated, making causality difficult to establish. 4 , 12 , 48 Physical activity did not differ between participants with and participants without cognitive improvement, ruling out physical activity as a potential factor underlying cognitive improvement. Overall, we did not find many differences between participants regarding change in physical activity level. The physical activity results might have been influenced by social desirability because self-reported data were used 49 ; therefore, a more objective measurement for physical activity is advised in future studies.
Our study has some limitations. First, due to a relatively short follow-up, it is not possible to suggest longer-term associations of weight loss with reductions in the risk of neurogenerative diseases. Second, beause this study lacks a control group, we had to control for possible practice or learning effects of repeated testing using standardized multiple versions of cognitive tests and the 20% change index. We believe that combining these 2 methods gives a good representation of actual cognitive improvement. Third, because this study is an observational study, it is not possible to draw conclusions on causality, and follow-up intervention studies are therefore planned. The study also has some strengths, including a large sample size, the dividing of the groups into cognitive improvers and nonimprovers, and the inclusion of multiple variables for better understanding of the factors associated with cognitive improvement after bariatric surgery.
The results of this cohort study suggest an association between bariatric surgery and cognitive improvement for approximately 39% of the participants. Furthermore, lower inflammation, changes in adipokines, improved mood, and higher physical activity levels were seen. The observed changes in liver-specific inflammation (CRP levels), adipokines (leptin levels), and depressive symptoms might partly explain the mechanism behind cognitive improvement after bariatric surgery. The further reductions in CRP and leptin levels specifically among cognitive improvers indicate the importance of improving the metabolic-inflammatory condition of both organs to improve cognition. The exact interaction between individual biomarkers and how they are mechanistically associated with cognitive function in multiple domains remains unsolved. Future studies should include a control group and multiple other potential mechanisms to clarify cognitive improvement after bariatric surgery, such as cerebrovascular function, glycemic control, microbiota, eating disorders, or nutrition (eg, caloric restriction). Furthermore, longitudinal studies are needed to study cognition and weight loss over time and to investigate whether weight loss might reduce the risk of neurodegenerative diseases.
Accepted for Publication: April 17, 2023.
Published: May 30, 2023. doi:10.1001/jamanetworkopen.2023.15936
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Vreeken D et al. JAMA Network Open .
Corresponding Author: Amanda J. Kiliaan, PhD, Department of Medical Imaging, Anatomy, Preclinical Imaging Centre, Radboud University Medical Center, Donders Institute for Brain, Cognition and Behaviour, Geert Grooteplein 21N, 6525 EZ Nijmegen, the Netherlands ( [email protected] ).
Author Contributions: Ms Vreeken and Dr Kiliaan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Hazebroek and Kiliaan contributed equally to this work.
Concept and design: Vreeken, Custers, Aarts, Kessels, Hazebroek, Kiliaan.
Acquisition, analysis, or interpretation of data: Vreeken, Seidel, Olsthoorn, Cools, Kleemann, Wiesmann, Hazebroek, Kiliaan.
Drafting of the manuscript: Vreeken, Custers, Cools, Aarts, Kiliaan.
Critical revision of the manuscript for important intellectual content: Seidel, Olsthoorn, Aarts, Kleemann, Kessels, Wiesmann, Hazebroek, Kiliaan.
Statistical analysis: Vreeken, Cools, Kessels, Wiesmann, Kiliaan.
Obtained funding: Aarts, Kleemann, Kiliaan.
Administrative, technical, or material support: Seidel, Custers, Cools, Kleemann.
Supervision: Aarts, Kessels, Wiesmann, Hazebroek, Kiliaan.
Conflict of Interest Disclosures: Dr Aarts reported receiving grants from Rijnstate/Radboud Vriendenfonds during the conduct of the study. Dr Kleemann reported serving as director for the Early Research Program of the Netherlands Organisation for Applied Scientific Research (TNO) “Body Brain Interactions,” where this work was performed. Dr Kessels reported receiving scientific consultant fees from the University of Michigan and Pearson Assessment Netherlands; and royalties from Hogrefe Publishing, BoomLemma Publishing, and Bohn Stafleu van Loghum outside the submitted work. No other disclosures were reported.
Funding/Support: This work was supported by a grant of the Rijnstate-Radboudumc Promotion Fund. The study was performed in collaboration with the Netherlands Organisation for Applied Scientific Research (TNO) Metabolic Health Research (Leiden, the Netherlands) with support from TNOs Research programs Biomedical Health (PMC13), ERP Body Brain Interactions and the Shared Research Program GLoBAL, an initiative of Radboudumc, Rijnstate, and TNO.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: The authors thank W. van Duyvenvoorde, BSc, Department of Metabolic Health Research, Netherlands Organisation for Applied Scientific Research, for processing human tissues; J. M. Snabel, BSc, Department of Metabolic Health Research, Netherlands Organisation for Applied Scientific Research, for technical support with the biomarker analyses; and A. Hofboer, BSc, Department of Bariatric Surgery, Vitalys, Rijnstate Hospital, for her contribution on data collection. They were not compensated for their contributions during the study period (September 1, 2018, to December 31, 2021).
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
VIDEO
COMMENTS
In addition to a standard laboratory work-up for cognitive impairment, it is important to determine whether imaging of the brain provides evidence of neurodegeneration in a topographical distribution consistent with the clinical presentation. ... 16 Dickerson BC, McGinnis SM, Xia C, et al.: Approach to atypical Alzheimer's disease and case ...
Other differential diagnoses and potential contributors to her cognitive impairments included cerebrovascular disease—which has an increased incidence among patients with hypertension, hypercholesterolemia, obesity, and smoking history—and hypothyroidism-related cognitive impairment. Both vascular cognitive impairment and hypothyroidism can ...
Discussion of her case reviews mild cognitive impairment with emphasis on an evidence-based ap-proach to evaluation and treatment, including manage- ... cross-sectional population study to affect 16.8% of an el-derly population.10 A more restrictively defined syndrome with lower expected prevalence, mild cognitive impair-ment (MCI), has been ...
Abstract. We report a case of a man aged 67 years presenting with recent depressive symptoms and paranoid ideations in addition to 1-year cognitive impairment. He has vascular risk factors and family history of memory loss. An episode of depression 2 decades ago resolved spontaneously but was followed by occupational decline.
Introduction. Cognitive impairment is highly prevalent in the elderly and increases with advancing age.1, 2, 3 Worldwide, dementia is estimated to affect 1.8% of people in their 60s, 5.1% of people in their 70s, 15.1% of people in their 80s, and 35.7% of people in their 90s. 3 A study from the Centers for Disease Control and Prevention using the 2011 Behavioral Risk Factor Surveillance survey ...
Case Study. L.C. is a 70-year-old Asian American woman who retired from teaching middle school five years ago. ... One systematic review found that 32% of people with mild cognitive impairment ...
Summary. An 85-year-old woman with hypertension and hyperlipidemia presented with gradual and progressive cognitive impairment for more than 2 years, involving cognitive domains of memory, executive function, visuospatial and mood. She has short-term memory loss such as forgetting whether she has eaten or showered.
Case Studies in Cognitive Dysfuncation - University of Michigan
This single-subject case study is an investigation into the potential cognitive benefit of TMT and can be used to inform a future more rigorous study. The participant was an adult male diagnosed with cognitive impairment as a result of a severe brain injury following an automobile accident.
The estimated prevalence of mild cognitive impairment in population-based studies ranges from 10 to 20% in persons older than 65 years of age. 6-10 In the Mayo Clinic Study of Aging, a ...
Mild cognitive impairment (MCI) is the prodromal phase of dementia, and it is highly underdiagnosed in the community. We aimed to develop an automated, rapid (< 5 min), electronic screening tool ...
The post-COVID-19 cases exhibit statistically significant cognitive deficits, chronic fatigue, and impairment of the senses of appetite, taste, smell, and hearing. Among the 19 post-COVID cases, the frequency of post-COVID-19 cognitive impairment is 57 (32.3%) with a statistically significant higher mean age (44.6±16.9) (P<0.001) and ...
Background: Cognitive impairment (CI) is a common symptom contributing to functional loss in major depressive disorder (MDD). However, the features of CI and its related risk factors in young and middle-aged MDD patients remain unclear. Methods: In this case-control study, 18- to 55-year-old acute-onset MDD patients and healthy controls (HCs) were recruited from nine centers in China.
Objectives: This review summarized the applicability of various decision-making tools for helping people with dementia or mild cognitive impairment (MCI) and their families make decisions. Design: This study was a narrative literature review. The protocol of this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42020182259).
The relationship between recurrent severe hypoglycemia and cognitive impairment remains unclear. Both prospective and longitudinal studies of cognitive function have been so plagued by methodological problems that it is difficult to unequivocally determine whether patients who experience repeated episodes of severe hypoglycemia are at risk for permanent brain injury or intellectual impairment.
Scenario 1: Excluding Individuals with Cognitive Impairment. Study Summary: This study aims to document upper limb muscle function among stroke patients in order to inform the development of a future motion assistive device. Individuals ages 65 and over who have been diagnosed with a stroke occurrence and have no cognitive impairment will be ...
In a small study (N=109) of stroke and transient ischemic attack survivors at 7 years after the index event, 37% had mild cognitive impairment and 22% had dementia. 35 Another study reported an overall prevalence of cognitive impairment 3 months after stroke and at annual follow-up up to 14 years after stroke that remained relatively unchanged ...
However, the literature shows that patients with cognitive impairment tend to have inconsistent pain management, leading to worse outcomes. We conducted a case-control study looking at 296 patients who presented with hip fractures to a major trauma centre between 1 December 2019 and 30 May 2020. Cognition was assessed using pre-recorded ...
We present a case of a 74-year-old Greek male who suffered from paraphasias, memory and orientation problems. The patient was assessed with neuropsychometric tests, auditory event-related potentials and cerebrospinal fluid proteins and was diagnosed with mild cognitive impairment. The emphasis on the case is on the unexplained high levels of P300 and Slow wave of the auditory event-related ...
A case controlled design was used to compare the patterns of cognitive function in a sample of patients with chronic HF and matched community-dwelling control subjects. A battery of neuropsychologic tests, including 3 that were computer based that allowed millisecond-measurement of reaction times, were used to assess cognitive function.
ing cognitive function is absent. Recent studies have reported that 3- n-butylphthalide (NBP) has a positive effect on improving cognitive impairment; however, its clinical efficacy and safety is unclear. Therefore, we conducted a meta-analysis to assess its efficacy and safety for cognitive impairment.
The study highlights the critical role that primary care settings can play in the early detection of MCI, even when patients present with non-cognitive complaints. ... The importance of attentive primary care in the early identification of mild cognitive impairment: case series AME Case Rep. 2024 Mar 19:8:56. doi: 10.21037/acr-23-162 ...
Results 110 full-text articles were selected for data extraction, of which 53 were cross-sectional, 43 longitudinal and 14 were case-control studies. The mean age of participants was 73.0 years (range 50-93.1). Ninety-one (83%) of these studies reported that VI was associated with cognitive impairment.
The included studies demonstrate that non-immersive electronic brain gaming is a safe, feasible, user-friendly, and potentially effective CCT intervention to maintain or improve cognitive functions among older adults with cognitive impairments. 10 Although some differences were found in intervention dose, type of brain gaming, and cognitive ...
For this study, we used -1 S.D as the cutoff to define impairment, according to the findings of the previous studies in both MS and PCC using these criteria 37,38. Five cognitive domains are ...
A recent study revealed the mechanism by which benzos interfere with plasticity and cognitive abilities. Other treatments such as CBT & life-style changes can alleviate anxiety without the use of ...
We post RCTs, prospective cohorts, epidemiology, and case studies and discuss the pro's and con's of each. We discuss type 2 diabetes, gout, Alzheimer's, mild cognitive impairment, obesity, epilepsy, mental illness, autoimmune diseases, metabolic syndrome, sugar, omega 6 polyunsaturated seed oils, & more!
Introduction. An estimated 2.4 to 5.5 million older Americans live with cognitive impairment (Plassman et al., 2008) in the United States (U.S.) and that number will increase in the coming decades (Hebert, Weuve, Scherr, & Evans, 2013).A number of high-quality, rigorous research studies aimed at providing effective health services specifically to these individuals exist (Hoffmann et al., 2015 ...
Post-COVID condition (PCC) and multiple sclerosis (MS) share some clinical and demographic features, including cognitive symptoms and fatigue. Some pathophysiological mechanisms well-known in MS, such as autoimmunity, neuroinflammation and myelin damage, have also been implicated in PCC. In this study, we aimed to compare the cognitive phenotypes of two large cohorts of patients with PCC and ...
Since this was not the case, we did not add covariates in the model. ... although the reliable change index or the inclusion of a control group remains the preferred method to study cognitive changes. ... The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi ...