Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED TOPICS
The patient has been treated for hypertension for 10 years, currently with amlodipine 10 mg by mouth daily. She was once told that her cholesterol value was "borderline high" but does not know the value.
She denies symptoms of diabetes, chest pain, shortness of breath, heart disease, stroke, or circulatory problems of the lower extremities.
She estimates her current weight at 165 lbs (75 kg). She thinks she weighed 120 lbs (54 kg) at age 21 years but gained weight with each of her three pregnancies and did not return to her nonpregnant weight after each delivery. She weighed 155 lbs one year ago but gained weight following retirement from her job as an elementary school teacher. No family medical history is available because she was adopted. She does not eat breakfast, has a modest lunch, and consumes most of her calories at supper and in the evening.
On examination, blood pressure is 140/85 mmHg supine and 140/90 mmHg upright with a regular heart rate of 76 beats/minute. She weighs 169 lbs, with a body mass index (BMI) of 30.9 kg/m 2 . Fundoscopic examination reveals no evidence of retinopathy. Vibratory sensation is absent at the great toes, reduced at the medial malleoli, and normal at the tibial tubercles. Light touch sensation is reduced in the feet but intact more proximally. Knee jerks are 2+ bilaterally, but the ankle jerks are absent. The examination is otherwise within normal limits.

- Previous Article
- Next Article
Presentation
Clinical pearls, case study: treating hypertension in patients with diabetes.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Evan M. Benjamin; Case Study: Treating Hypertension in Patients With Diabetes. Clin Diabetes 1 July 2004; 22 (3): 137–138. https://doi.org/10.2337/diaclin.22.3.137
Download citation file:
- Ris (Zotero)
- Reference Manager
L.N. is a 49-year-old white woman with a history of type 2 diabetes,obesity, hypertension, and migraine headaches. The patient was diagnosed with type 2 diabetes 9 years ago when she presented with mild polyuria and polydipsia. L.N. is 5′4″ and has always been on the large side,with her weight fluctuating between 165 and 185 lb.
Initial treatment for her diabetes consisted of an oral sulfonylurea with the rapid addition of metformin. Her diabetes has been under fair control with a most recent hemoglobin A 1c of 7.4%.
Hypertension was diagnosed 5 years ago when blood pressure (BP) measured in the office was noted to be consistently elevated in the range of 160/90 mmHg on three occasions. L.N. was initially treated with lisinopril, starting at 10 mg daily and increasing to 20 mg daily, yet her BP control has fluctuated.
One year ago, microalbuminuria was detected on an annual urine screen, with 1,943 mg/dl of microalbumin identified on a spot urine sample. L.N. comes into the office today for her usual follow-up visit for diabetes. Physical examination reveals an obese woman with a BP of 154/86 mmHg and a pulse of 78 bpm.
What are the effects of controlling BP in people with diabetes?
What is the target BP for patients with diabetes and hypertension?
Which antihypertensive agents are recommended for patients with diabetes?
Diabetes mellitus is a major risk factor for cardiovascular disease (CVD). Approximately two-thirds of people with diabetes die from complications of CVD. Nearly half of middle-aged people with diabetes have evidence of coronary artery disease (CAD), compared with only one-fourth of people without diabetes in similar populations.
Patients with diabetes are prone to a number of cardiovascular risk factors beyond hyperglycemia. These risk factors, including hypertension,dyslipidemia, and a sedentary lifestyle, are particularly prevalent among patients with diabetes. To reduce the mortality and morbidity from CVD among patients with diabetes, aggressive treatment of glycemic control as well as other cardiovascular risk factors must be initiated.
Studies that have compared antihypertensive treatment in patients with diabetes versus placebo have shown reduced cardiovascular events. The United Kingdom Prospective Diabetes Study (UKPDS), which followed patients with diabetes for an average of 8.5 years, found that patients with tight BP control (< 150/< 85 mmHg) versus less tight control (< 180/< 105 mmHg) had lower rates of myocardial infarction (MI), stroke, and peripheral vascular events. In the UKPDS, each 10-mmHg decrease in mean systolic BP was associated with a 12% reduction in risk for any complication related to diabetes, a 15% reduction for death related to diabetes, and an 11% reduction for MI. Another trial followed patients for 2 years and compared calcium-channel blockers and angiotensin-converting enzyme (ACE) inhibitors,with or without hydrochlorothiazide against placebo and found a significant reduction in acute MI, congestive heart failure, and sudden cardiac death in the intervention group compared to placebo.
The Hypertension Optimal Treatment (HOT) trial has shown that patients assigned to lower BP targets have improved outcomes. In the HOT trial,patients who achieved a diastolic BP of < 80 mmHg benefited the most in terms of reduction of cardiovascular events. Other epidemiological studies have shown that BPs > 120/70 mmHg are associated with increased cardiovascular morbidity and mortality in people with diabetes. The American Diabetes Association has recommended a target BP goal of < 130/80 mmHg. Studies have shown that there is no lower threshold value for BP and that the risk of morbidity and mortality will continue to decrease well into the normal range.
Many classes of drugs have been used in numerous trials to treat patients with hypertension. All classes of drugs have been shown to be superior to placebo in terms of reducing morbidity and mortality. Often, numerous agents(three or more) are needed to achieve specific target levels of BP. Use of almost any drug therapy to reduce hypertension in patients with diabetes has been shown to be effective in decreasing cardiovascular risk. Keeping in mind that numerous agents are often required to achieve the target level of BP control, recommending specific agents becomes a not-so-simple task. The literature continues to evolve, and individual patient conditions and preferences also must come into play.
While lowering BP by any means will help to reduce cardiovascular morbidity, there is evidence that may help guide the selection of an antihypertensive regimen. The UKPDS showed no significant differences in outcomes for treatment for hypertension using an ACE inhibitor or aβ-blocker. In addition, both ACE inhibitors and angiotensin II receptor blockers (ARBs) have been shown to slow the development and progression of diabetic nephropathy. In the Heart Outcomes Prevention Evaluation (HOPE)trial, ACE inhibitors were found to have a favorable effect in reducing cardiovascular morbidity and mortality, whereas recent trials have shown a renal protective benefit from both ACE inhibitors and ARBs. ACE inhibitors andβ-blockers seem to be better than dihydropyridine calcium-channel blockers to reduce MI and heart failure. However, trials using dihydropyridine calcium-channel blockers in combination with ACE inhibitors andβ-blockers do not appear to show any increased morbidity or mortality in CVD, as has been implicated in the past for dihydropyridine calcium-channel blockers alone. Recently, the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) in high-risk hypertensive patients,including those with diabetes, demonstrated that chlorthalidone, a thiazide-type diuretic, was superior to an ACE inhibitor, lisinopril, in preventing one or more forms of CVD.
L.N. is a typical patient with obesity, diabetes, and hypertension. Her BP control can be improved. To achieve the target BP goal of < 130/80 mmHg, it may be necessary to maximize the dose of the ACE inhibitor and to add a second and perhaps even a third agent.
Diuretics have been shown to have synergistic effects with ACE inhibitors,and one could be added. Because L.N. has migraine headaches as well as diabetic nephropathy, it may be necessary to individualize her treatment. Adding a β-blocker to the ACE inhibitor will certainly help lower her BP and is associated with good evidence to reduce cardiovascular morbidity. Theβ-blocker may also help to reduce the burden caused by her migraine headaches. Because of the presence of microalbuminuria, the combination of ARBs and ACE inhibitors could also be considered to help reduce BP as well as retard the progression of diabetic nephropathy. Overall, more aggressive treatment to control L.N.'s hypertension will be necessary. Information obtained from recent trials and emerging new pharmacological agents now make it easier to achieve BP control targets.
Hypertension is a risk factor for cardiovascular complications of diabetes.
Clinical trials demonstrate that drug therapy versus placebo will reduce cardiovascular events when treating patients with hypertension and diabetes.
A target BP goal of < 130/80 mmHg is recommended.
Pharmacological therapy needs to be individualized to fit patients'needs.
ACE inhibitors, ARBs, diuretics, and β-blockers have all been documented to be effective pharmacological treatment.
Combinations of drugs are often necessary to achieve target levels of BP control.
ACE inhibitors and ARBs are agents best suited to retard progression of nephropathy.
Evan M. Benjamin, MD, FACP, is an assistant professor of medicine and Vice President of Healthcare Quality at Baystate Medical Center in Springfield, Mass.
Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Systematic review update
- Open access
- Published: 30 April 2024
Prevalence of thyroid dysfunction and associated factors among adult type 2 diabetes mellitus patients, 2000–2022: a systematic review and meta-analysis
- Rishan Hadgu ORCID: orcid.org/0009-0005-2040-6838 1 ,
- Abebaw Worede 2 &
- Sintayehu Ambachew 2 , 3
Systematic Reviews volume 13 , Article number: 119 ( 2024 ) Cite this article
Metrics details
Thyroid dysfunction (TD) and type 2 diabetes mellitus (T2DM) frequently co-occur and have overlapping pathologies, and their risk increases with age. Thyroid dysfunction along with T2DM will worsen macro- and microvascular complications, morbidity, and mortality.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guideline was followed. The databases used were Embase, ScienceDirect, PubMed, and Google Scholar. The Joana Briggs Institute (JBI) scale was used to assess the quality of the included studies. The data was extracted by Microsoft Excel and analyzed through STATA version 14 software. The overall pooled prevalence of TD and its main components were estimated using the random-effects model. The consistency of studies was assessed by I 2 test statistics. Pooled meta-logistic regression was used to present the pooled prevalence with a 95% confidence interval (CI). Besides, subgroup and sensitivity analyses were employed.
Thirty-eight studies were included. The pooled prevalence of TD was 20.24% (95% CI : 17.85, 22.64). The pooled prevalence of subclinical hypothyroidism, hypothyroidism, subclinical hyperthyroidism, and hyperthyroidism was found to be 11.87% (95% CI : 6.90, 16.84), 7.75% (95% CI : 5.71, 9.79), 2.49% (95% CI : 0.73, 4.25), and 2.51% (95% CI : 1.89, 3.13), respectively. Subgroup analysis based on continent revealed a higher prevalence of TD in Asia and Africa. Factors like being female, HbA1c ≥ 7%, DM duration > 5 years, family history of TD, central obesity, smoking, the presence of retinopathy, and neuropathy were found associated with TD.
The current systematic review and meta-analysis showed that the TD’s pooled prevalence was relatively higher than the general population. Therefore, regular screening of TD should be done for T2DM patients.
Peer Review reports
Introduction
Thyroid dysfunction (TD), the most common endocrinal pathology next to diabetes mellitus (DM) [ 1 ], is a condition characterized by an increased or decreased production of thyroid hormones (TH) [ 2 ]. TDs occur as hypothyroidism (clinical or subclinical) or hyperthyroidism (clinical or subclinical) and are reflected in circulating levels of free triiodothyronine (FT3), free thyroxine (FT4), and TSH [ 3 ]. Type 2 diabetes mellitus (T2DM) is characterized by hyperglycemia as a result of insulin resistance and impaired pancreatic beta-cell activity [ 4 , 5 ]. Obesity, a sedentary lifestyle, energy-dense foods, smoking, alcohol intake, and population aging are the key risk factors for T2DM [ 6 ].
Type 2 diabetes mellitus and TD often co-occur and have overlapping pathologies, and their risk increases with age. TDs are significantly more prevalent among T2DM patients [ 1 ]. TDs affect approximately 10 to 15% of the patients with diabetes, whereas in non-diabetes, the prevalence is approximately 6% [ 3 ]. The prevalence of TD in T2DM varies between studies, ranging from very low (5.5%) to very high (75%) [ 7 ]. Furthermore, studies have also recorded a higher prevalence of TD (31.4%) among females with T2DM [ 8 ]. Subclinical hypothyroidism is the most common type of TD among the diabetic population [ 9 , 10 , 11 ].
There is a complex relationship between TD and DM that has yet to be discovered. The pathophysiological link between T2DM and TD is thought to be the outcome of a complex interaction of biochemical, genetic, and hormonal abnormalities [ 12 ]. T2DM influences the TH in two sites, first at the level of hypothalamus by controlling TRH release and second at the peripheral tissues by impairing the conversion of T4 to T3 [ 13 , 14 ]. The hypothalamus–pituitary–thyroid axis may be disrupted by experimentally induced diabetes, which lowers plasma TRH and TSH levels, lowering TH synthesis. [ 15 ]. In addition to this, anti-diabetics such as sulfonylureas and thiazolidinedione group drugs (e.g., pioglitazone) can negatively impact thyroid function [ 12 ].
Thyroid dysfunction can also cause T2DM. Both hypothyroidism and hyperthyroidism have been investigated to be associated with DM [ 1 ]. Hypothyroidism is associated with reduced glucose absorption from GIT, and it is accompanied by prolonged peripheral glucose accumulation, diminished hepatic glucose output, and reduced utilization of glucose, which were considered hallmarks of diabetes [ 16 ]. On the other hand, hyperthyroidism promotes hyperglycemia, and several theories have been proposed to explain this impact. In a thyrotoxic environment, the half-life of insulin is shortened, which is assumed to be related to the accelerated degradation of the active hormone and the release of inactive precursors [ 17 ]. In addition, hyperthyroidism is also hypothesized to boost glucose production through a variety of processes, including upregulation of gluconeogenesis as a result of increased lipolysis and lactate overproduction, as well as increased hepatic output due to increased expression of the GLUT2 glucose transporter [ 18 ].
The coexistence of TD in T2DM patients will worsen the macro-vascular and microvascular complications, morbidity, mortality, and quality of life [ 11 ]. Evidence indicates that subclinical hypothyroidism compromises both micro- and macrovascular function, increasing the risk of peripheral neuropathy, peripheral artery disease, and diabetic nephropathy [ 13 , 19 ]. In addition to this, both subclinical hyperthyroidism and T2DM have been associated with an increase in cardiovascular disease risk and mortality [ 20 ]. Both TD and DM, especially uncontrolled diabetes, cause many health problems. Increased frequency of hypoglycemia in hypothyroidism and development of potentially life-threatening ketoacidosis in thyrotoxicosis are the most serious effects [ 21 ].
Detecting TD in T2DM patients would help clinicians provide the best treatment for metabolic problems, as TDs like hypothyroidism can make achieving a glycemic target and managing other comorbidities difficult [ 11 ]. Screening of TD, especially the subclinical dysfunction, in patients with DM is justified because most patients can be asymptomatic [ 22 ]. The strong link between diabetes and TD encouraged the American Diabetes Association to propose that people with diabetes must be checked periodically for TD [ 23 ].
There are different studies conducted to assess the prevalence and associated factors of TD among T2DM all over the world. Despite their results having a great disparity and inconsistent findings, moreover, there is no previous systematic review and meta-analysis that estimated the prevalence and associated factors of TD among T2DM. Therefore, the current systematic review and meta-analysis is designed to assess the pooled prevalence and associated factors of TD among T2DM patients.
Methods and materials
Eligibility criteria, inclusion criteria.
Studies on the prevalence and associated factors of TD among adult T2DM patients published in different peer-reviewed journals between 2000 and 2022 were included. All studies were original research published in English and contained the minimum information concerning sample size and status of TD, which helped to analyze a pooled estimate of the prevalence of TD and associated factors among adult T2DM patients. Besides, studies in which TD has been classified as hypothyroidism, hyperthyroidism, subclinical hypothyroidism, and subclinical hyperthyroidism using laboratory measurements of TSH, T4, and T3 were included.
Hypothyroidism is characterized by elevated serum TSH levels, a low serum FT4 level, and low FT3 [ 24 , 25 ], whereas hyperthyroidism is characterized by elevated serum FT4 and FT3 and low levels of TSH levels [ 26 ]. Subclinical hyperthyroidism is characterized by decreased serum TSH concentration in association with a normal serum FT4 and FT3 concentrations [ 26 ]. Subclinical hypothyroidism is defined as an elevated serum TSH level associated with normal total or FT4 and FT3 levels [ 20 ]. Studies that used International Diabetes Federation (IDF) criteria for diagnosing T2DM were included. The IDF criteria state diagnostic criteria for diabetes which is maintained fasting plasma glucose ≥ 7.0 mmol/l (126 mg/dl) or 2–h plasma glucose ≥ 11.1 mmol/l (200 mg/dl) [ 27 ].
Exclusion criteria
Articles written in another language other than English were excluded. Studies conducted among type 1 DM patients, and diabetic neuropathy patients, were excluded. Studies from non-original papers (literature reviews, books) were also excluded. Irrelevant and duplicated papers were excluded. Articles which lacked necessary information such as age and year of study were also excluded. Studies that did not show the diagnostic criteria for both T2DM and TD were omitted. Furthermore, articles that did not provide information on the overall prevalence of TD were omitted.
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guideline was used to report this systematic review and meta-analysis [ 28 ]. An electronic search was conducted to retrieve studies. Published articles of cross-sectional, case–control, cohort, prospective, case series, and retrospective studies were included. Embase, PubMed, Google Scholar, and ScienceDirect literature were the electronic databases used to identify studies conducted on the prevalence of TD and associated factors among T2DM patients published from 2000 to 2022. The search terms were used in agreement with the title/abstract using the arrangement of keywords that were used to select relevant studies. Figure 1 shows the flow chart used to describe the selection of studies.
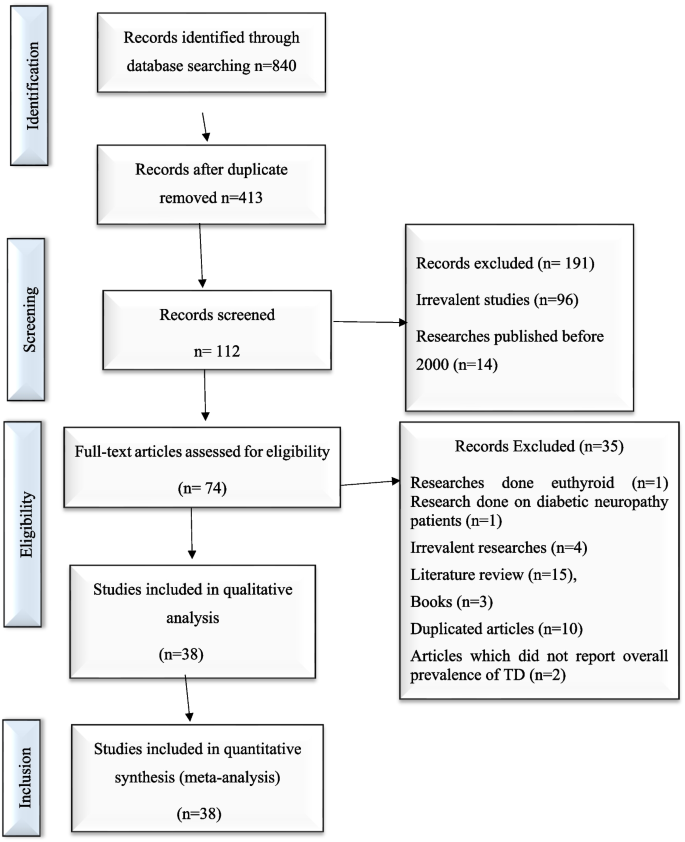
Flow chart to describe the selection of studies for the systematic review and meta-analysis of the prevalence of TD and associated factors among adult type 2 DM patients
Using Boolean operators like “OR” and “AND,” the search terms were utilized separately and in combination. An example of the search strategy used to retrieve relevant articles was as follows: (((((((prevalence[Title/Abstract]) AND (hypothyroidism[Title/Abstract])) AND (hyperthyroidism [Title/Abstract])) AND (thyroid disorders[Title/Abstract])) OR (thyroid dysfunction [Title/Abstract])) AND (adult[Title/Abstract])) AND (type 2 diabetes mellitus[Title/Abstract])) OR (insulin resistant diabetes[Title/Abstract]). Duplicated data were excluded. The software EndNote version X8 (Thomson Reuters, New York, NY, USA) was used to manage references and remove duplicated references.
Search method and quality assessment
An electronic search was conducted in Embase, PubMed, Google Scholar, and ScienceDirect literature using the keywords to include articles that were published from 2000 to 2022. Then, searched articles were screened by the title and abstract to consider the articles in the full-text review. Following the exclusion of duplicates, the abstracts and titles of 413 papers were screened for eligibility criteria, and 38 were chosen for full-text evaluation.
This systematic review and meta-analysis is based on original research articles. For maintaining the quality of the review, all duplications were checked thoroughly. The abstracts of these articles were checked deeply for the analysis and purification. A careful evaluation of each research paper was carried out at later stage.
The quality of the studies was assessed using the Joana Briggs Institute (JBI) standardized critical appraisal instrument for prevalence studies scale. The following items were used to appraise the included studies: (Q1) Was the sample frame appropriate to address the target population?, (Q2): Were study participants sampled in an appropriate way?, (Q3): Was the sample size adequate?, (Q4): Were the study subjects and the setting described in detail?, (Q5): Was the data analysis conducted with sufficient coverage of the identified sample?, (Q6): Were valid methods used for the identification of the condition?, (Q7): Was the condition measured in a standard, reliable way for all participants?, (Q8): Was there appropriate statistical analysis?, and (Q9): Was the response rate adequate, and if not, was the low response rate managed appropriately? Table 1 shows the methodological quality assessment of included studies using the Joana Briggs Institute (JBI) standardized critical appraisal instrument for prevalence studies scale.
Data extraction
An established data extraction tool, Microsoft Excel 2013 spreadsheet, was used for the data extraction. Three authors (R. H., A. W., and S. A.) independently conducted a search in Embase, PubMed, Google Scholar, and ScienceDirect databases. This tool extracted information such as the author’s name, publication year, study design, sample size, prevalence of TD, prevalence of subgroups of TD, and the laboratory diagnostic method used to diagnose TD, and T2DM were all extracted using this tool. PRISMA guideline was strictly followed when conducting this review.
Data processing and analysis
Data was entered and analyzed using STATA version 14 after extracting the data from all eligible studies. Overall, pooled prevalence of TD and its main components were estimated using the random-effects model. In the meta-analysis, to assess the consistency of studies, I 2 test statistics was used. This test examines the hypothesis of all the included studies is evaluated for the same effect. Consequently, since there was heterogeneity between the original studies ( I 2 = 93.5%, p < 0.001), a random-effect model was needed. The presence of publication bias was evaluated by using funnel plot test. Besides, study bias was evaluated using Egger’s test. Moreover, in this study, forest plots were used to estimate pooled effect size and effect of each study with their confidence interval (CI) to provide a visual image of the data. Pooled meta-logistic regression was used to present the pooled prevalence with a 95% confidence interval. Besides, subgroup and sensitivity analyses were employed.
Characteristics of the included studies
A total of 840 potential articles were identified through the systematic literature search. After removal of duplicates, 413 articles were screened by title and abstract, and 74 were found to be eligible for full-text assessment. Of these full-text-screened articles, 38 (including 19,803 study participants) were found to be eligible for meta-analysis. Table 2 shows the general characteristics and outcomes of included studies.
The studies that are included were done all over the globe and published between 2000 and 2022. The meta-analysis included 38 studies that revealed the prevalence and contributing factors of TD among T2DM patients. Among all the papers, 6 of them were from Africa [ 3 , 31 , 35 , 43 , 45 , 51 ], 28 were from Asia [ 3 , 7 , 8 , 9 , 11 , 14 , 16 , 23 , 24 , 29 , 32 , 33 , 34 , 37 , 38 , 39 , 40 , 41 , 42 , 44 , 46 , 47 , 49 , 50 , 52 , 53 , 54 , 55 , 56 , 57 , 58 ], 1 was from Australia [ 48 ], 2 were from Europe [ 10 , 30 ], and 1 was from South America [ 22 ]. Regarding the study design, 9 were case–control, 1 case series, 3 were cohort, 21 were cross-sectional, 1 prospective, and 3 retrospective studies. The minimum sample size was 40 participants in a case–control study conducted in India [ 55 ], while the highest sample size was 2219 participants, in a cohort study conducted in India [ 49 ].
The Joana JBI standardized critical appraisal instrument for prevalence studies indicated that none of the included studies was of poor quality. After quality assessment, the 38 studies were subjected to meta-analysis. Table 2 presents the characteristics and outcomes of the reviewed studies. The prevalence of TD was estimated based on measurement of blood levels of TSH, FT3, and FT4 among the T2DM patients from all over the world.
Prevalence of TD among adult T2DM patients
Thirty-eight published studies were included in this systematic review and meta-analysis, and all of these studies were used to estimate the pooled prevalence of TD among T2DM patients.
The minimum prevalence of TD was 8% from a retrospective study done in India [ 52 ], and the maximum prevalence of TD was found to be 35.4% in Nepal [ 32 ]. The I 2 test result showed high heterogeneity ( I 2 = 93.5%, = 0.000). The pooled prevalence of TD among T2DM was found to be 20.24% (95% CI : 17.85, 22.64) using random-effect model (Fig. 2 ).
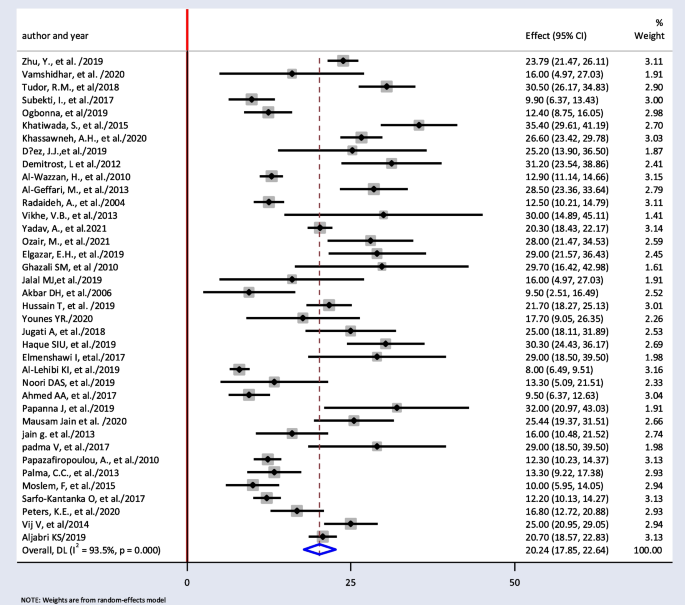
Pooled prevalence of thyroid dysfunction among T2DM patients from random effect model
Prevalence of types of TDs and subgroup analysis
Thirty-four papers were used to estimate the pooled prevalence of subgroups of TD. The pooled prevalence of subclinical hypothyroidism, hypothyroidism, subclinical hyperthyroidism, and hyperthyroidism were found to be 11.87% (95% CI : 6.90, 16.84), 7.75% (95% CI : 5.71, 9.79), 2.49% (95% CI : 0.73, 4.25), and 2.51% (95% CI : 1.89, 3.13), respectively.
Subgroup analysis based on the study design showed that the weighted pooled prevalence of TD was 18.97% (95% CI : 15.93, 22.01), 22.17% (95% CI : 16.41, 27.92), 21.32% (95% CI : 14.37, 28.27), 22.33% (95% CI : 4.34, 40.32), 21.70% (95% CI : 18.27, 25.13), and 25% (95% CI : 18.11, 31.89) among the cross-sectional, cohort, case control, retrospective, prospective, and case series respectively. Table 3 shows the summary of the subgroup analysis of studies.
Thirty-eight papers were used to estimate subgroup analysis based on continent. The result showed weighted pooled prevalence of TD was 21% (95% CI : 18.06, 23.94), 21.30% (95% CI : 3.46, 39.13), and 17.85% (95% CI : 12.68, 23.03) in Asia, Europe, and Africa, respectively (Fig. 3 ).
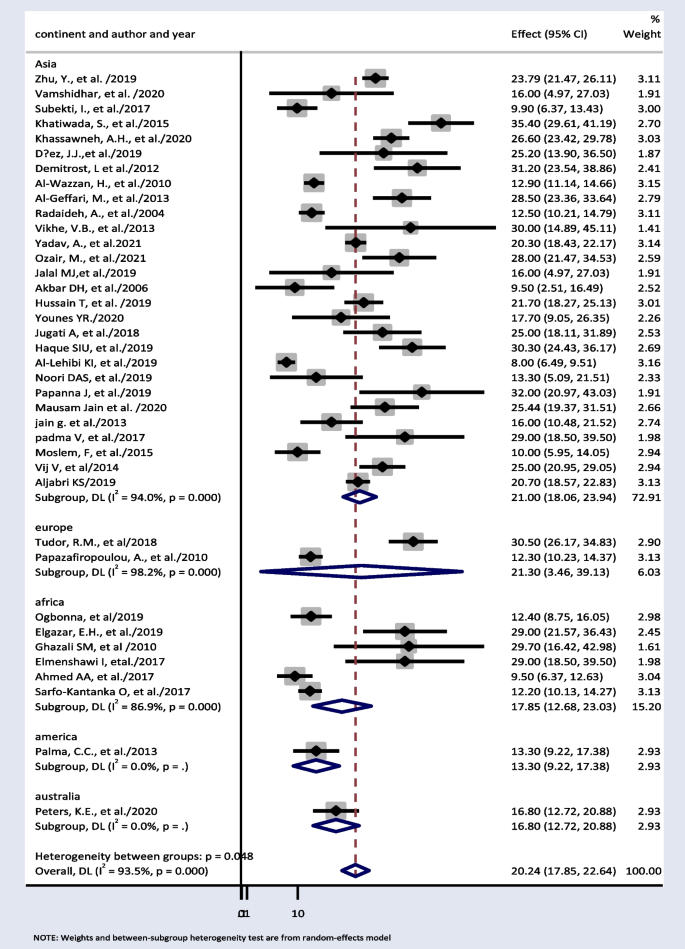
Pooled prevalence of thyroid dysfunction based on different continents
Factors associated with TD among T2DM patients
In this meta-analysis, seven studies were included to examine the factors associated with TD among T2DM [ 30 , 31 , 32 , 39 , 44 , 52 , 59 ]. Being female [ 23 , 30 , 31 , 32 , 39 , 44 , 59 ], central obesity [ 31 ], HbA1c ≥ 7% [ 31 , 44 ], > 5-year duration of DM [ 31 , 44 , 59 ], educational level [ 59 ], diabetic neuropathy and retinopathy [ 31 , 59 ], family history of TD [ 23 , 32 ], and smoking [ 32 , 39 , 44 ] were found to be associated with T2DM. Tables 4 and 5 shows summary statistics of the risk factors.
Sensitivity test
We did the sensitivity analysis of the prevalence of TD among T2DM by applying a random-effects model (Table 6 ). The analysis was done to evaluate the effect of each study on the pooled estimated prevalence of TD by excluding each study step by step. The result showed that excluded studies did not show a significant difference in the prevalence of TD among T2DM (Fig. 4 and Table 6 ).
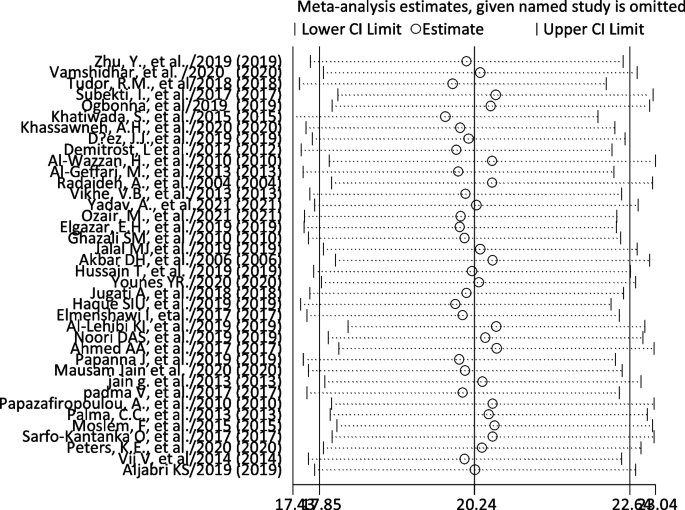
Sensitivity analysis of pooled prevalence of thyroid dysfunction for each study being removed one at a time
Publication bias
The included studies were assessed for potential publication bias visually by funnel plot. The funnel plot was asymmetrical which indicate the presence of publication bias (Figs. 4 and 5 ). Besides, the result of Egger’s test indicated there was publication bias, P -value < 0.05. The P -value was found to be 0.019 (Table 7 ).
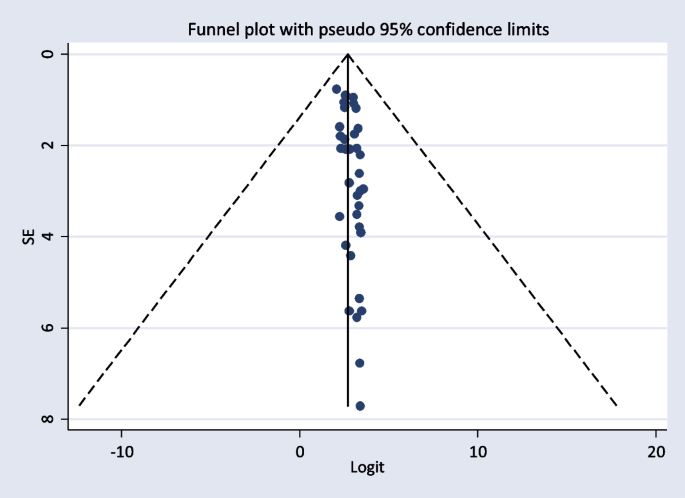
Funnel plot showing publication bias
The Egger’s test indicated that the unpublished findings might have shown a lower magnitude of TD. Adjusting the findings using the trim-and-fill method would provide a bias-adjusted effect estimate. Therefore, to do so, a trim-and-fill method analysis was conducted. A bias-adjusted effect estimate of TD showed 17.89 (15.611, 20.179), assuming there are missing studies (Fig. 6 ).
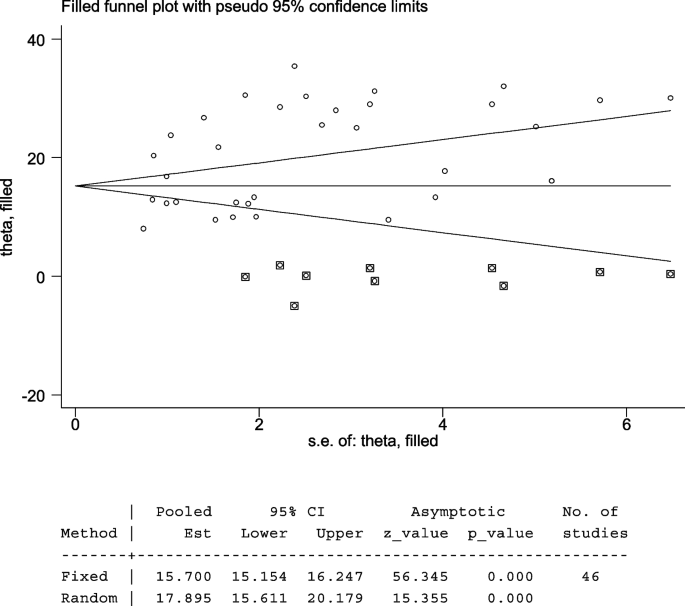
Trim-and-fill analysis of TD among T2DM patients
Meta-regression
Meta-regression was performed to determine the source of heterogeneity by considering sample size and year of publication as a covariate. There was no significant relationship between the year of publication and the prevalence of TD. In addition to this, meta-regression was also conducted that explains the linear prediction of the prevalence of TD and function of sample size. Similarly, there was no significant relationship between the sample size and the prevalence of TD (Table 8 ).
The pooled prevalence of TD in this systematic review and meta-analysis was found to be 20.24% (95% CI : 17.85, 22.64). Funnel plots and Egger’s tests showed there was publication bias among included studies. Trim-and-fill method was used to correct the results. A bias-adjusted effect estimate of TD showed 17.89 (15.611, 20.179), assuming there are missing studies. This result was higher than the 11.7% seen in a Colorado TD survey of 25,862 people who attended a state health fair [ 12 ]. It was also higher than the National Health and Nutrition Examination Survey (NHANES III Study), a survey of 17,353 subjects (5.9%) [ 60 ]. Type 2 diabetic patients have a higher prevalence of TD than non-diabetics; T2DM lowers TSH levels and impairs the conversion of T4 to T3 in the peripheral tissues. Poorly managed T2DM can lead to insulin resistance and hyperinsulinemia. This, in turn, promotes the growth of thyroid tissue and increases the formation of nodules and the size of goiters. In addition, while metformin can be beneficial in both T2DM and TD patients, there are some other antidiabetic drugs like sulfonylureas, and thiazolidinedione group drugs like pioglitazone can negatively impact thyroid function [ 12 ].
The most common type of TD seen in this systematic review and meta-analysis was subclinical hypothyroidism (11.87%, 95% CI : 6.90, 16.84). This result was in line with a systematic review and meta-analysis done globally (12% (95% CI : 10%, 14%) [ 61 ] and higher than results reported in general population (4–9%) [ 1 ]. More than half of TDs reported are undiagnosed or subclinical because symptoms of TD are easily mistaken for depression, menopause, or obesity [ 62 ]. The presence of subclinical hypothyroidism may increase cardiovascular risk by aggravating dyslipidemia, insulin resistance, obesity, and vascular endothelial dysfunction [ 1 , 63 ].
Hypothyroidism was the second most common form of TD found in this systematic review and meta-analysis (7.75% (95% CI : 5.71, 9.79). This finding was also higher than that of the NHANES III Study (4.6%) [ 1 ]. Overall hypothyroidism is the most common type of TD among T2DM patients. Worldwide, environmental iodine deficiency is the most common cause of hypothyroidism [ 64 ]. Globally, more than 1.9 billion individuals have inadequate iodine nutrition Despite the implementation of iodine supplementation programs (e.g., salt iodization), iodine intake remains suboptimal in large parts of the world [ 64 , 65 ].
In this review, the pooled prevalence of hyperthyroidism was 2.51% (95% CI : 1.89, 3.13). This was similar to the community-based study done in Wickham among 2779 participants (2%) [ 66 ].
Subgroup analysis based on continent showed 21% (95% CI : 18.06, 23.94) and 17.85% (95% CI : 12.68, 23.03) pooled prevalence of TD in Asia and Africa respectively. TDs have been documented in more than 110 countries, the most of which are in Africa, Asia, and Latin America [ 67 ]. In comparison with other continents, this result is high. This is because, in the developed world, the frequency of undiagnosed TD is anticipated to be declining as a result of extensive thyroid function testing and low treatment initiation thresholds. However, in continents such as Africa and Asia, this is challenge [ 68 ]. Iodine deficiency is a major public health problem throughout Africa and is the commonest cause of TDs in this continent [ 69 ]. At least 350 million Africans are at risk of iodine deficiency. A total of 25% of the global burden of iodine deficiency occurs in Africa [ 70 ].
Seven studies were included to examine the factors associated with TD among T2DM [ 30 , 31 , 32 , 39 , 44 , 52 , 59 ], and different factors were found associated with TD. Among them, sex was found to be the prominent determinant of TD. All of the studies indicated a statistically significant association between sex and TD that shows a higher risk of TD with being female. In a cross-sectional research of 411 T2DM patients in Saudi Arabia, it was discovered that being female has 1.95 higher odds of having TD as compared with males ( OR = 1.95, 95% CI : 1.36–2.78, p = 0.0001) [ 23 ]. Female gender was also a risk factor for TD, according to a study conducted in Greece among 1092 T2DM patients ( OR = 0.222, 95% CI = 0.141–0.352, p = 0.001) [ 30 ]. These results were also similar to the study conducted in Nepal and Kuwait ( RR = 1.44, 95% CI = 1.09–1.91, p = 0.01) and ( OR = 1.7, 95% CI : 1.2–2.9, p = < 0.001) respectively [ 32 , 39 ].
In a research done in Nigeria among 354 T2DM patients, it was found females who had T2DM were 3.8 times more likely to develop TD than their male counterparts ( OR = 3.8, 95% CI = 1.7–8.4, p = 0.002) [ 31 ]. Similar result was found in a case–control study conducted in Ethiopia. Being female had 2.5 times the odds of having TD than their male counterparts 2.5 ( OR = 2.5, 95% CI = 1.15–5.67, p = 0.022) [ 59 ].
The prevalence of TD in diabetic patients is influenced by female gender in which T2DM patients who are female are more likely to develop TD. This in because sex hormones and the skewed inactivation of the X chromosome are suspected to be triggers for hypothyroidism and hyperthyroidism [ 71 ]. Another factor contributing to the high prevalence of TD in women is the interaction between TH and hormones that change during the menstrual cycle [ 72 ].
Smoking was also found associated with TD among T2DM patients [ 32 , 39 ]. In the study conducted in Kuwait among 204 T2DM patients, ex-smokers and current smoker patients were more liable for TD ( OR = 18.1, 95% CI : 10.1–32.5) and ( OR = 7.8, 95% CI : 3.5–17.7) respectively [ 39 ]. Similar finding was also found in the study done in Nepal. Smokers had 2.32 higher odds of having TD ( OR = 2.32, 95% CI : 1.85–2.91) [ 32 ].
The reason behind this is that cigarette smoke contains cyanide which is converted to thiocyanate, which disrupts iodine uptake and blocks the production of THs [ 73 ]. Many other components of cigarettes also have antithyroid effects, such as decreasing T3 receptor binding or post-receptor activities in the liver, muscle, or both. According to reports, smoking/nicotine causes an unnaturally high metabolism, masking the fatigue/lethargy associated with hypothyroidism. When the smoker quits, this masking is removed, and the full effects of hypothyroidism on the metabolism and thyroid are felt. And, for smokers with undiagnosed TD, without proper TH treatment, smoking cessation seems to double weight gain whammy, as they lose the appetite suppressant, metabolism-upping effects of nicotine, and experience the full effects of the hypothyroidism [ 39 ].
Besides, among the seven papers used to assess associated factors, two of them reported that TD is associated with HbA1c ≥ 7% [ 31 , 44 ]. A study conducted in Nigeria found that T2DM patients with ≥ 7% HbA1c were 4.3 times more likely to develop TD than their counterparts with good glycemic control (H bA1c < 7%) ( OR = 4.3, 95% CI = 2.1–8.9, p = 0.025) [ 31 ]. A case–control study conducted in Jordan was also in line with this study. It was found that patients who had HBA1c ≥ 7% were found to have 2.55 higher odds of having TD when compared with patients who have HBA1c ≤ 7% ( OR = 2.55, 95% CI = 1.45–4.43, p = 0.001) [ 44 ]. The association of hyperglycemia with TD may be due to the adverse effects of chronic hyperglycemia on the hypothalamic-pituitary axis where it blunts or abolishes the nocturnal TSH peak [ 59 ].
It was also found central obesity (abnormal waist circumference) was significantly associated with TD in a case–control study done in Nigeria ( OR = 2.5, 95% CI = 1.5–5.2, p = 0.001) [ 31 ]. Leptin is known to be an important neuroendocrine regulator of the hypothalamo-pituitary-thyroid axis by regulating TRH gene expression in the paraventricular nucleus. Iodine deficiency, autoimmune thyroiditis, and mutations in the TSH receptor genes are some of the other hypotheses put forward to explain the association between increasing TSH, obesity, and subclinical hypothyroidism in some populations [ 31 ].
Duration of diabetes was found to be associated with TD in two of the studies [ 23 , 31 ]. In a research done in Nigeria among 354 T2DM patients, DM duration > 5 years ( OR = 3.3, p = 0.012) was a risk factor for TD [ 31 ]. A cross-sectional study conducted in Saudi Arabia diabetes also reported that duration of more than 10 years has been shown to be an important risk factor ( OR = 1.66, 95% CI : 1.06–2.61) [ 23 ]. This could indicate that the duration of diabetes mellitus (DM) is a risk factor for the development of TD, as persistent hyperglycemia inhibits the peripheral deiodination of T4 to T3, resulting in TD [ 31 ].
Educational level was also found associated with TD. It was found T2DM patients who attend primary school had 1.5 higher odds of having TD ( OR = 1.5, 95% CI : 1.03–1.67). On the other hand, T2DM patients who have secondary education and post-secondary education had less likely to have TD ( OR = 0.11, 95% CI = 0.06–0.48, p = 0.02) and ( OR = 0.21, 95% CI = 0.062–0.85, p = 0.028); this implies better educational levels being protective. This is logical because a higher educational level is linked to improved blood glucose control, which is linked to good thyroid function [ 59 ].
Among the seven papers used to assess associated factors, two studies showed that previous family history of TD was associated with TD. A cross-sectional study conducted in Saudi Arabia among 411 T2DM patients found that diabetic patients with a positive family history of TD had a higher chance of developing TD ( OR = 3.39, 95% CI : 2.47–4.63, p = < 0.0001) [ 23 ]. A study from Nepal also having previous family history of TD increased the risk by 2.57 ( RR = 2.57, 95% CI = 2–3.31, p < 0.001) [ 32 ].
Age > 50 was significant factor with OR of 3.9 (95% CI 2.151–7.052, p < 0.001). This can be explained by that elderly patients might have had undetected diabetes for a longer time [ 44 ]. About the factor presence of retinopathy [ 59 ] with an odds ratio of 9.3 (95% CI : 2.05–42.51, p =0.04), and for factor presence of neuropathy [ 59 ] with an odds ratio of ( OR =3.3, 95% CI =1.19–8.92, p =0.021) [ 32 ] showed the presence of retinopathy and neuropathy were risk factors of TD among T2DM patients respectively [ 39 ].
Conclusion and recommendation
The current systematic review and meta-analysis showed that the pooled prevalence of TD among T2DM patients was found to be higher compared with the general population. The pooled prevalence of TD among T2DM was found to be 20.24% (95% CI : 17.85, 22.64) using random-effect model. The pooled prevalence of subclinical hypothyroidism, hypothyroidism, subclinical hyperthyroidism, and hyperthyroidism was found to be 11.87% (95% CI : 6.90, 16.84), 7.75% (95% CI : 5.71, 9.79), 2.49% (95% CI : 0.73, 4.25), and 2.51% (95% CI : 1.89, 3.13), respectively. Being female, obesity, family history of TD, smoking, advanced age, and family history of DM were factors associated with TD among adult T2DM patients.
We recommend it is important to screen for TD in T2DM patients as each of these endocrinopathies and their complex interdependent interactions increase cardiovascular risks.
Strength and limitations
This systematic review and meta-analysis revealed the pooled figure on prevalence of TD, its subtypes, and associated factors of TD among T2DM patients. This will give researchers, policymakers, and public health stakeholders the empirical knowledge they need to develop health-promoting policies, allocate resources, and set priorities for monitoring future trends.
The limitations of this systematic review and meta-analysis is that the search strategy was limited only to published articles, but unpublished papers may be missed. Only free online databases were used. In addition to this, only papers written in English were included. Time barrier was also one of the limitations. Moreover, it is essential to highlight that the paucity of research conducted in the area of thyroid dysfunction among type 2 diabetes patients in Europe, America, and Australia resulted in a restricted number of articles being incorporated into our analysis.
Availability of data and materials
The main part of the data generated or analyzed during this study is included in this published article. Other data will be available from the corresponding author upon request.
Abbreviations
Body Mass Index
Diabetes Mellitus
Free Triiodothyronine
Free Thyroxine
Hemoglobin A1C
International Diabetes Federation
Milli-international Unit per Liter
National Health and Nutrition Examination Survey
Picomoles per Liter
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- Thyroid Dysfunction
Type 2 Diabetes Mellitus
Thyroid Hormone
Thyrotropin-releasing Hormone
Thyroid-stimulating Hormone
Wang C. The Relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res. 2013;2013:390534.
Article PubMed PubMed Central Google Scholar
Laulund AS, Nybo M, Brix TH, Abrahamsen B, Jørgensen HL, Hegedüs L. Duration of thyroid dysfunction correlates with all-cause mortality The OPENTHYRO Register Cohort. PloS one. 2014;9(10):e110437.
Ahmed AA, Mohamed SB, Elmadi SA, Abdorabo AA, Ismail IM, Ismail AM. Assessment of thyroid dysfunctions in type 2 diabetes mellitus patients in Surman, Western-Libya. Int J Clin Exp Med Sci. 2017;3:1–4.
Google Scholar
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1(1):1–22.
Article Google Scholar
Inzucchi SE, Sherwin RS. Type 2 diabetes mellitus. Philadelphia, Pa: Saunders Elsevier; 2011.
Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269.
Article CAS PubMed PubMed Central Google Scholar
Diez JJ, Sánchez P, Iglesias P. Prevalence of thyroid dysfunction in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119(04):201–7.
Papanna J, Bettegowda S. Thyroid dysfunction among type 2 diabetes mellitus patients: a study from rural hospital. J Med Sci Clin Res. 2019;7:433–6.
Vikhe VB, Kanitkar SA, Tamakuwala KK, Gaikwad AN, Kalyan M, Agarwal RR. Thyroid dysfunction in patients with type 2 diabetes mellitus at tertiary care centre. Natl J Med Res. 2013;3(4):377–80.
Tudor RM, Garrahy A, Woods CP, Crowley RK, Tormey WT, Smith D, et al. The prevalence and incidence of thyroid dysfunction in patients with diabetes - a longitudinal follow-up study. Ir J Med Sci. 2020;189(1):171–5.
Article PubMed Google Scholar
Subekti I, Pramono LA, Dewiasty E, Harbuwono DS. Thyroid dysfunction in type 2 diabetes mellitus patients. Acta Med Indones. 2017;49(4):314–23.
PubMed Google Scholar
Kalra S, Aggarwal S, Khandelwal D. Thyroid dysfunction and type 2 diabetes mellitus: screening strategies and implications for management. Diabetes Ther. 2019;10(6):2035–44.
Datchinamoorthi S, Rathanavel N, Rajagopalan B, Vanaja R. Study of thyroid dysfunction in type II diabetes mellitus. Int J Pharm Sci Res. 2016;7(9):3877.
Moslem F, Bithi TS, Biswas A. Prevalence of thyroid dysfunction among type-2 diabetes patients in an urban diabetes hospital. Bangladesh Open Sci J Clin Med. 2015;3(3):98–113.
Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev. 2019;40(3):789–824.
Vamshidhar IS, Rani SSS. A study of association of thyroid dysfunctions in patients with type 2 diabetes mellitus. Maedica. 2020;15(2):169–73.
CAS PubMed PubMed Central Google Scholar
Hage M, Zantout MS, Azar ST. Thyroid Disorders and Diabetes Mellitus. J Thyroid Res. 2011;2011:439463, 7:1–7.
Ward RJ, Heald AH, Ogunmekan S, Fryer AA, Duff CJ. Should we be screening for thyroid dysfunction in patients with type 2 diabetes mellitus? Br J Gen Pract. 2018;68(667):94–5.
Mohammed Hussein SM, AbdElmageed RM. The relationship between type 2 diabetes mellitus and related thyroid diseases. Cureus. 2021;13(12):e20697.
PubMed PubMed Central Google Scholar
Díez JJ, Iglesias P. Subclinical hyperthyroidism in patients with type 2 diabetes. Endocrine. 2012;42(1):157–63.
Vondra K, Vrbikova J, Dvorakova K. Thyroid gland diseases in adult patients with diabetes mellitus. Minerva Endocrinol. 2005;30(4):217–36.
CAS PubMed Google Scholar
Palma CC, Pavesi M, Nogueira VG, Clemente EL, Vasconcellos Mde F, Pereira LCJ, et al. Prevalence of thyroid dysfunction in patients with diabetes mellitus. Diabetol Metab Syndr. 2013;5(1):58.
Al-Geffari M, Ahmad NA, Al-Sharqawi AH, Youssef AM, AlNaqeb D, Al-Rubeaan K. Risk factors for thyroid dysfunction among type 2 diabetic patients in a highly diabetes mellitus prevalent society. Int J Endocrinol. 2013;2013:417920.
Akbar D, Ahmed M, Al-Mughales J. Thyroid dysfunction and thyroid autoimmunity in Saudi type 2 diabetics. Acta Diabetol. 2006;43(1):14–8.
Article CAS PubMed Google Scholar
Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. S Afr Fam Pract. 2012;54(5):384–90.
Reid JR, Wheeler SF. Hyperthyroidism: diagnosis and treatment. Am Fam Physician. 2005;72(4):623–30.
World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647.
Zhu Y, Xu F, Shen J, Liu Y, Bi C, Liu J, et al. Prevalence of thyroid dysfunction in older Chinese patients with type 2 diabetes-a multicenter cross-sectional observational study across China. PLoS ONE. 2019;14(5):e0216151.
Papazafiropoulou A, Sotiropoulos A, Kokolaki A, Kardara M, Stamataki P, Pappas S. Prevalence of thyroid dysfunction among Greek type 2 diabetic patients attending an outpatient clinic. J Clin Med Res. 2010;2(2):75.
Ogbonna SU, Ezeani IU. Risk factors of thyroid dysfunction in patients with type 2 diabetes mellitus. Front Endocrinol. 2019;10:440.
Khatiwada S, Kc R, Sah SK, Khan SA, Chaudhari RK, Baral N, et al. Thyroid dysfunction and associated risk factors among Nepalese diabetes mellitus patients. Int J Endocrinol. 2015;2015:570198.
Radaideh A, Mo M, Amari FL, Bateiha AE, El-Khateeb M, Naser P, et al. Diabetes mellitus in Jordan. Saudi Med J. 2004;25(8):1046–50.
Ozair M, Noor S, Raghav A, Siddiqi SS, Chugtai AM, Ahmad J. Prevalence of thyroid disorders in North Indian type 2 diabetic subjects: a cross sectional study. Diabetes Metab Syndr. 2018;12(3):301–4.
Elgazar EH, Esheba NE, Shalaby SA, Mohamed WF. Thyroid dysfunction prevalence and relation to glycemic control in patients with type 2 diabetes mellitus. Diabetes Metab Syndr. 2019;13(4):2513–7.
Younes YR. The prevalence of thyroid dysfunction in patients with type 2 diabetes mellitus. Chronic Dis J. 2020;8(1):45–8.
Haque SIU, Syed TM, Tahir A. Burden of thyroid dysfunction in patients of type 2 diabetes mellitus. PAFMJ. 2019;69(4):843–7.
Aljabri KS, Alnasser IM, Facharatz BS, Bokhari SA, Alshareef MA, Khan PM. The frequency of hypothyroidism in Saudi community-based hospital: A retrospective single centre study. Trends Diabetes Metab. 2019;2(1):1–4.
Al-Wazzan H, Daban A, Askar R, El-Shazly M. Prevalence and associated factors of thyroid dysfunction among type 2 diabetic patients. Kuwait Alexandria Journal of Medicine. 2010;46(2):141–8.
Jain G, Marwaha TS, Khurana A, Dhoat PS. Prevalence of thyroid disorders in patients of type 2 diabetes mellitus. Int J Med Dent Sci. 2013;2(2):153–61.
Nair A, Jayakumari C, Jabbar PK, Jayakumar RV, Raizada N, Gopi A, et al. Prevalence and associations of hypothyroidism in Indian patients with type 2 diabetes mellitus. J Thyroid Res. 2018;2018:5386129.
Jalal MJA, Riyas B, Kumar AP. Thyroid dysfunction in patients with type-2 diabetes mellitus in Kerala: a case–control study. Thyroid Res Pract. 2019;16(1):3.
Ghazali S, Abbiyesuku F. Thyroid dysfunction in type 2 diabetics seen at the University College Hospital, Ibadan. Nigeria Niger J Physiol Sci. 2010;25(2):173–9--9.
Khassawneh AH, Al-Mistarehi AH, Zein Alaabdin AM, Khasawneh L, AlQuran TM, Kheirallah KA, et al. Prevalence and predictors of thyroid dysfunction among type 2 diabetic patients: a case-control study. Int J Gen Med. 2020;13:803–16.
Sarfo-Kantanka O, Sarfo FS, Ansah EO, Yorke E, Akpalu J, Nkum BC, et al. Frequency and determinants of thyroid autoimmunity in Ghanaian type 2 diabetes patients: a case-control study. BMC Endocr Disord. 2017;17(1):2.
Padma V, Anand NN. Prevalence of Thyroid Dysfunction in Type 2 Diabetic Patients. Int J Pharm Biochem Sci. 2015;6(3):289–94.
Hussain T, Barik BS, Nayak AR, Das S, Khadanga UK, Yadav V, et al. Prevalence and predictors of thyroid dysfunction among patients with type 2 diabetes mellitus attending a tertiary care hospital in an urban area of Bhubaneswar, Odisha. Thyroid Res Pract. 2019;16(1):26.
Peters KE, Chubb SAP, Bruce DG, Davis WA, Davis TME. Prevalence and incidence of thyroid dysfunction in type 1 diabetes, type 2 diabetes and latent autoimmune diabetes of adults: the Fremantle Diabetes Study Phase II. Clin Endocrinol. 2020;92(4):373–82.
Article CAS Google Scholar
Yadav A, Yadav GAM, Narsingrao KK, Nanda Kumar LG, Yadav GSN. Prevalence of thyroid disorders among patients with diabetes in rural South India. Diabetes Metab Syndr. 2021;15(3):885–9.
Demitrost L, Ranabir S. Thyroid dysfunction in type 2 diabetes mellitus: a retrospective study. Indian J Endocrinol Metab. 2012;16(Suppl 2):S334.
Elmenshawi I, Alotaibi S, Alazmi A, Alazmi A, Alruwaili F, Alazmi N, et al. Prevalence of thyroid dysfunction in diabetic patients. J Diabetes Metab Disord. 2017;4:55–6.
Al-Lehibi KI, Abdulrahman MI, Albassam ENA-A. Thyroid dysfunction in type 2 diabetic patients and the effect of diabetes duration and anti-glycemic medications on mean tsh and a1c levels: a retrospective study. Int J Med Res Health Sci. 2019;8(9):117–22.
Jugati A, Biradar M. Thyroid dysfunction in patients with type 2 diabetes mellitus in a tertiary care center of North Karnataka. Medica. 2018;7(2):28.
Aljabri KS. The prevalence of thyroid disorders in patients with type 2 diabetes mellitus in Saudi community based hospital. Curr Res Diabetes Obes J. 2019;11(3):60–4.
Vij V, Chitnis P, Gupta VK. Evaluation of thyroid dysfunction among type II diabetic patients. Ijpbs. 2012;2(4):150–5.
CAS Google Scholar
Jali M, Kambar S, Jali SM, Pawar N, Nalawade P. Prevalence of thyroid dysfunction among type 2 diabetes mellitus patients. Diabetes Metab Syndr. 2017;11:S105–8.
Zeru MA, Tesfa E, Mitiku AA, Seyoum A, Bokoro TA. Prevalence and risk factors of type-2 diabetes mellitus in Ethiopia: systematic review and meta-analysis. Sci Rep. 2021;11(1):1–15.
Zafar M, Shahid SM, Alshammari RF, Kausar MA, Ginawi TA, Hatim AW, Wadi AM, Ali H, Hamed AA, Al-zahrani MS, Hussain A. Association of thyroid disorders with diabetes: A cross-sectional study. Nus Biosci. 2022;14(2).
Tekalign AM, Habte FB, Yimer RM. Determinants of Thyroid Dysfunction among Type 2 Diabetes Patients Attending Private Hospitals in Dire Dawa, Eastern Ethiopia. medRxiv. 2022:2022–02.
Grassetto G, Rubello D. Thyroid disorders and diabetes mellitus. Minerva Med. 2008;99(3):263–7.
Han C, He X, Xia X, Li Y, Shi X, Shan Z, et al. Subclinical hypothyroidism and type 2 diabetes: a systematic review and meta-analysis. PLoS ONE. 2015;10(8):e0135233.
Moini J, Pereira K, Samsam M. Epidemiology of thyroid disorders. Elsevier; 2020 Jan 8. 75-85
Joffe BI, Distiller LA. Diabetes mellitus and hypothyroidism: strange bedfellows or mutual companions? World J Diabetes. 2014;5(6):901.
Chiovato L, Magri F, Carlé A. Hypothyroidism in context: where we’ve been and where we’re going. Adv Ther. 2019;36(2):47–58.
de Benoist B, Andersson M, Takkouche B, Egli I. Prevalence of iodine deficiency worldwide. The Lancet. 2003;362(9398):1859–60.
Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol. 1977;7(6):481–93.
Alam Khan V, Khan MA, Akhtar S. Thyroid disorders, etiology and prevalence. J Med Sci. 2002;2(2):89–94.
Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301–16.
Ogbera AO, Kuku SF. Epidemiology of thyroid diseases in Africa. Indian journal of endocrinology and metabolism. 2011;15(Suppl2):S82.
Okosieme OE. Impact of iodination on thyroid pathology in Africa. J R Soc Med. 2006;99(8):396–401.
Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10(8):594–604.
Jacobson MH, Howards PP, Darrow LA, Meadows JW, Kesner JS, Spencer JB, et al. Thyroid hormones and menstrual cycle function in a longitudinal cohort of premenopausal women. Paediatr Perinat Epidemiol. 2018;32(3):225–34.
Babić Leko M, Gunjača I, Pleić N, Zemunik T. Environmental factors affecting thyroid-stimulating hormone and thyroid hormone levels. Int J Mol Sci. 2021;22(12):6521.
Download references
Author information
Authors and affiliations.
Department of Medical Laboratory Science, College of Medicine and Health Sciences, Arba Minch University, P.O. Box 21, Arba Minch, Ethiopia
Rishan Hadgu
Department of Clinical Chemistry, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, P.O. Box 196, Gondar, Ethiopia
Abebaw Worede & Sintayehu Ambachew
Adelaide Medical School, University of Adelaide, Adelaide, SA, Australia
Sintayehu Ambachew
You can also search for this author in PubMed Google Scholar
Contributions
Rishan Hadgu conducted study design, conception of research protocol, literature review, and data extraction. Sintayehu Ambachew, Abebaw Worede, and Rishan Hadgu were involved in data analysis and interpretation and manuscript drafting. Abebaw Worede and Sintayehu Ambachew made data interpretation and reviewed the manuscript. Rishan Hadgu and Sintayehu Ambachew were responsible for data extraction and quality assessment. All the authors critically revised the paper and agreed to be accountable for all aspects of the work.
Corresponding author
Correspondence to Rishan Hadgu .
Ethics declarations
Ethics approval and consent to participate.
All participants provided written informed consent to publish this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hadgu, R., Worede, A. & Ambachew, S. Prevalence of thyroid dysfunction and associated factors among adult type 2 diabetes mellitus patients, 2000–2022: a systematic review and meta-analysis. Syst Rev 13 , 119 (2024). https://doi.org/10.1186/s13643-024-02527-y
Download citation
Received : 08 May 2023
Accepted : 12 April 2024
Published : 30 April 2024
DOI : https://doi.org/10.1186/s13643-024-02527-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type two Diabetes mellitus
- Meta analysis
- Systematic review
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 17 April 2024
Navigating outpatient care of patients with type 2 diabetes after hospital discharge - a qualitative longitudinal study
- Léa Solh Dost 1 , 2 ,
- Giacomo Gastaldi 3 ,
- Marcelo Dos Santos Mamed 4 , 5 &
- Marie P. Schneider 1 , 2
BMC Health Services Research volume 24 , Article number: 476 ( 2024 ) Cite this article
268 Accesses
Metrics details
The transition from hospital to outpatient care is a particularly vulnerable period for patients as they move from regular health monitoring to self-management. This study aimed to map and investigate the journey of patients with polymorbidities, including type 2 diabetes (T2D), in the 2 months following hospital discharge and examine patients’ encounters with healthcare professionals (HCPs).
Patients discharged with T2D and at least two other comorbidities were recruited during hospitalization. This qualitative longitudinal study consisted of four semi-structured interviews per participant conducted from discharge up to 2 months after discharge. The interviews were based on a guide, transcribed verbatim, and thematically analyzed. Patient journeys through the healthcare system were represented using the patient journey mapping methodology.
Seventy-five interviews with 21 participants were conducted from October 2020 to July 2021. The participants had a median of 11 encounters (min–max: 6–28) with HCPs. The patient journey was categorized into six key steps: hospitalization, discharge, dispensing prescribed medications by the community pharmacist, follow-up calls, the first medical appointment, and outpatient care.
Conclusions
The outpatient journey in the 2 months following discharge is a complex and adaptive process. Despite the active role of numerous HCPs, navigation in outpatient care after discharge relies heavily on the involvement and responsibilities of patients. Preparation for discharge, post-hospitalization follow-up, and the first visit to the pharmacy and general practitioner are key moments for carefully considering patient care. Our findings underline the need for clarified roles and a standardized approach to discharge planning and post-discharge care in partnership with patients, family caregivers, and all stakeholders involved.
Peer Review reports
Care transition is defined as “the movement patients make between healthcare practitioners and settings as their condition and care needs change in the course of a chronic or acute illness” [ 1 ]. The transition from hospital to outpatient care is a particularly vulnerable period for patients as they move from a medical environment with regular health monitoring to self-management, where they must implement a large amount of information received during their hospital stay [ 2 , 3 , 4 , 5 , 6 ]. This transition period can be defined as “the post-hospital syndrome,” which corresponds to a transient period of vulnerability (e.g., 30 days) for various health problems, such as stress, immobility, confusion, and even cognitive decline in older adults, leading to complications [ 7 ]. Furthermore, discharged patients may experience a lack of care coordination, receive incomplete information, and inadequate follow-ups, leading to potential adverse events and hospital readmissions [ 8 , 9 , 10 ].
People with type 2 diabetes mellitus (T2D) represent a high proportion of hospitalized patients, and their condition and medications are associated with a higher rate of hospital readmission [ 11 , 12 , 13 ]. Moreover, T2D is generally associated with multiple comorbidities. This complex disease requires time-consuming self-management tasks such as polypharmacy, adaptations of medication dosages, diet, exercise, and medical follow-up, especially during care transition [ 14 , 15 , 16 ].
Various interventions and practices, such as enhanced patient education, discharge counseling, and timely follow-up, have been studied to improve care transition for patients with chronic diseases; however, they have shown mixed results in reducing costs and rehospitalization [ 17 , 18 , 19 , 20 ]. In addition, patient perspectives and patient-reported outcomes are rarely considered; however, their involvement and monitoring are essential for seamless and integrated care [ 21 , 22 ]. Care integration, an approach to strengthening healthcare systems in partnership with people, focuses on patient health needs, the quality of professional services, and interprofessional collaboration. This approach prevents care fragmentation for patients with complex needs [ 23 , 24 ]. Therefore, knowledge of healthcare system practices is essential to ensure integrated, coordinated, and high-quality care. Patient perspectives are critical, considering the lack of literature on how patients perceive their transition from hospital to autonomous care management [ 25 , 26 ].
Patients’ journeys during hospitalization have been described in the literature using various methods such as shadowing, personal diaries, and interviews; however, patients’ experiences after hospital discharge are rarely described [ 26 , 27 ]. Jackson et al. described the complexity of patient journeys in outpatient care after discharge using a multiple case study method to follow three patients with chronic obstructive pulmonary disease from hospitalization to 3 months post-discharge [ 26 ]. The literature does not provide an in-depth understanding of the experiences of patients with comorbidities during care transition upon hospital discharge. The assumption about the patient journey after discharge is that multiple and multi-professional encounters will ensure the transition of care from hospitalization to self-management, but often without care coordination.
This study aimed to investigate the healthcare trajectories of patients with comorbidities, including T2D, during the 2 months following hospital discharge and to examine patients’ encounters with healthcare professionals (HCPs).
While this article focuses on patients’ journeys to outpatient care, another article describes and analyzes patients’ medication management, knowledge, and adherence [ 28 ]. This study followed the Consolidated Criteria for Reporting Qualitative Research (COREQ).
Study design and population
A qualitative longitudinal research approach was adopted, with four individual semi-structured interviews over 2 months after discharge (approximately 3, 10, 30, and 60 days after discharge) that took place at home, by telephone, secured video call, or at the university at the participant’s convenience. Participants were recruited during hospitalization. The inclusion criteria were patients with T2D, with at least two other comorbidities, at least one medication change during hospitalization, hospitalization duration of at least 3 days, and those who returned home after discharge and self-managed their medications. A family caregiver could also participate in the interviews alongside to participants.
Researcher characteristics
All the researchers were trained in qualitative studies. The ward diabetologist and researcher (GG) who enrolled the patients in the study participated in most participants’ care during hospitalization. LS (Ph.D. student and community pharmacist) was unknown to participants and presented herself during hospitalization as a “researcher” rather than a pharmacist to avoid any risk of influencing participants’ answers. MS is a professor in pharmacy, whose research focuses on medication adherence in chronic diseases and aims at better understanding this behavior and its consequences for patients and the healthcare system. MDS is a researcher, linguist, and clinical psychologist, with a particular interest in patients living with chronic conditions such as diabetes and a strong experience in qualitative methodology and verbal data analysis.
Data collection
The interviews were based on four semi-structured interview guides based on existing frameworks and theories: the World Health Organization’s five dimensions for adherence, the Information-Motivation-Behavioral Skills model, and the Social Cognitive Theory [ 29 , 30 , 31 ]. For in-depth documentation of participants’ itinerary in the healthcare system, the interview guides included questions on the type, reason, and moment of the HCP’s encounters and patient relationships with HCPs. Interview guides are available in Supplementary File 1 . During the development phase of the study, the interview guides were reviewed for clarity and validity and adapted by two patient partners from the Geneva University Hospitals’ Patient Partner Platform for Research and Patient and Public Involvement. Thematic saturation was considered reached when no new code or theme emerged and new data repeated previously coded information [ 32 ]. Sociodemographic and clinical data were collected from hospital databases and patient questionnaires. The interviews were audio-recorded, anonymized, and transcribed verbatim.
Data analysis
The sociodemographic and clinical characteristics were descriptively analyzed. Transcriptions were double-coded until similar codes were obtained, and thematic analysis, as described by Braun and Clarke [ 33 , 34 ], was used in a systematic, iterative, and comparative manner. A patient journey mapping methodology was used to illustrate the trajectories of each participant and provide a comprehensive understanding of their experiences. Patient journey mapping is a visual method adapted from the marketing industry that is increasingly used in various health settings and contexts to illustrate and evaluate healthcare services and patient experiences [ 35 ]. In this analysis, we used the term “healthcare professionals” when more than one profession could be involved in participants’ healthcare. Otherwise, when a specific HCP was involved, we used the designated profession (e.g. physicians, pharmacists).
A. Participants description
Twenty-one participants were interviewed between October 2020 and September 2021, generating 75 interviews. All participants took part in Interview 1, 19 participants in Interview 2, 16 participants in Interview 3 and 19 participants in Interview 4, with a median duration of 41 minutes (IQR: 34-49) per interview. Interviews 1,2,3 and 4 took place respectively 5 days (IQR: 4-7), 14 days (13-20), 35 days (33-38), and 63 days (61-68) after discharge. Nine patients were newly diagnosed with T2D, and 12 had a previous diagnosis of T2D, two of whom were untreated. Further information on participants is described in Table 1 . The median number of comorbidities was six (range: 3–11), and participants newly diagnosed with diabetes tended to have fewer comorbidities (median: 4; range: 3–8). More detailed information regarding sociodemographic characteristics and medications has been published previously [ 28 ].
B. Journey mappings
Generic patient journey mapping, presented in Fig. 1 , summarizes the main and usual encounters participants had with their HCPs during the study period. Generic mapping results from all individual patient journey mappings from discharge to 2 months after discharge are available in Supplementary File 2 .
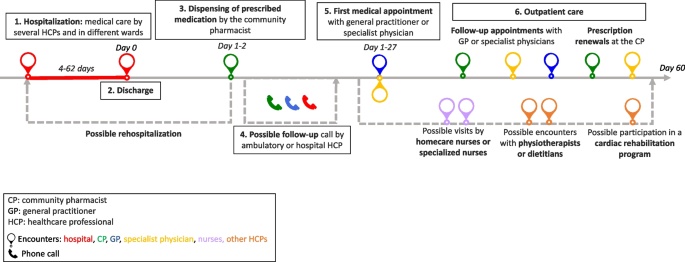
Generic patient journey mapping from hospitalization to two months after discharge
During the 2 months following discharge, the participants had a median number of 10 (range: 6–28) encounters with HCPs. The HCPs met by participants are represented in Fig. 2 . All participants visited their pharmacists at least once, and 16 of the 21 participants met their general practitioners (GPs) at least once. Five participants received home care assistance, four went to an outpatient cardiac rehabilitation program, and five were readmitted during the study period.
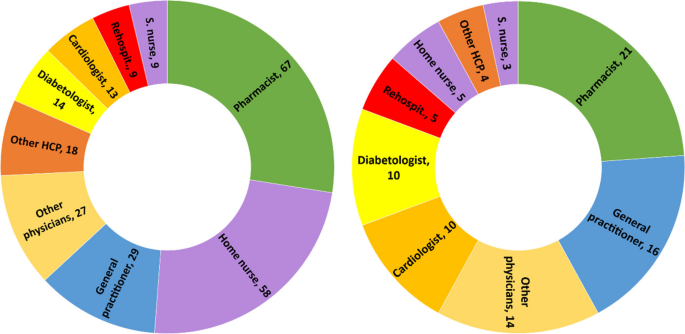
Healthcare professionals seen by participants during the study period. left: n=cumulative encounters; right: n=encountered at least once. Abbreviation: S.nurse: specialized nurse; Other physicians: ophthalmologists, neurologists, hematologists, immunologists, addictologists; other HCP: physiotherapists, dietitians, massage therapist
The first HCP encountered was at the community pharmacy on the same day or day after discharge, except for one participant who did not pick up her medication. The first medical appointment with a physician occurred between days 1 and 27 after discharge (median: 8; IQR: 6-14).
Participants newly diagnosed with diabetes had a closer follow-up after discharge than participants with a former diagnosis of T2D (median: 7; IQR: 6–10 vs median: 9; IQR: 5–19), fewer encounters with HCPs (median: 8; IQR: 7–10 vs. 11; IQR: 8–17), and fewer comorbidities (median: 4; IQR: 4–7 vs. 7; IQR: 5–9). Most participants newly diagnosed with T2D or receiving insulin treatment benefited from either a follow-up call, home visit by a nurse, or diabetes care appointment.
C. Qualitative analysis
Transcripts were analyzed longitudinally and categorized into six key steps based on the verbal data. These key steps, shown in Fig. 1 , represent the identified thematic categories and refer to the following elements: 1. Hospitalization, 2. Discharge, 3. Dispensing of prescribed medications at the pharmacy, 4. Possible follow-up call, 5. First medical appointment, and 6. Outpatient care.
Hospitalization: hospital constraints and care organization
Most participants thought they had benefited from adequate medical care by committed and attentive HCPs but highlighted different constraints and gaps. Some participants noted constraints related to the hospital environment, such as loss of autonomy during their stay, lack of privacy, and the large number of hospital staff encountered. This resulted in participants repeating the same information several times, causing frustration, misunderstanding and a lack of coordination for some participants:
“Twenty or thirty staff members come in during the day! So, it's hard to keep track of [what] is bein g said or done. The best thing for me [...] would be to have clear information from just one person.” Participant 8; interview 1 (P18.1)
Participants had different opinions on the hospital’s care organization. Some participants found that care coordination between the wards was well-organized. In contrast, others highlighted poor coordination and communication between the hospital wards, resulting in long waiting times, care fragmentation, and contradictory or unclear information. Some participants felt that they did not benefit from comprehensive and integrated care and that the hospital staff focused on the cause of their hospitalization, neglecting other comorbidities:
“They were not interested [in my diabetes and my sight]. I was there for the heart and that was where [my care] stopped.” P17.1
Patients’ involvement in decision-making regarding medical care varied. Some participants were involved in their care and took part in medical decisions. Written information, adequate communication, and health professionals’ interest in patients were highlighted by some participants:
“They took the information sheet and they explained everything to me. They didn't just come once; they came several times to explain everything to me.” P5.1
Other participants found the information difficult to understand, particularly because of their fatigue and because the information was provided orally.
Discharge: an unclear process
The discharge process was unclear for patients who could not identify a specific related outpatient medical visit or a key step that summarized their hospital stay and prepared them for discharge:
“Well, there's no real preparation [for discharge]. I was waiting for them to give me the go-ahead so I could go home, that’s all...” P7.4
For some participants, outpatient care follow-up was organized before discharge by the hospital team (generally by making an appointment with the patient’s GP before discharge), whereas others had no post-discharge follow-up scheduled during their hospitalization. Approximately half of the participants refused follow-ups during their hospitalization, such as home care services provided by a nurse, or a rehabilitation hospital stay. The main reason for this refusal was that patients did not perceive the need for follow-up:
“It's true that I was offered a lot of services, which I turned down because I didn't realize how I would manage back at home.” P22.2
Dispensing prescribed medications by the community pharmacist: the first HCP seen after discharge
On behalf of half the participants, a family caregiver went to the usual community or hospital outpatient pharmacy to pick up the medications. The main reasons for delegation were tiredness or difficulty moving. In some cases, this missed encounter would have allowed participants to discuss newly prescribed medications with the pharmacist:
“[My husband] went to get the medication. And I thought afterward, […] that I could have asked [the pharmacist]: “But listen, what is this medication for?” I would have asked questions” P2.3
Participants who met their pharmacist after hospital discharge reported a range of pharmaceutical practices, such as checking the prescribed medication against medication history, providing information and explanations, and offering services such as the preparation of pillboxes. For some, the pharmacists’ work at discharge did not differ from regular prescriptions, whereas others found that they received further support and explanations:
“She took the prescription […] checked thoroughly everything and then she wrote how, when, and how much to take on each medication box. She managed it very well and I had good explanations.” P20.3
Some participants experienced problems with generic substitution, the unavailability of medications, or dispensing errors, complicating their journey through the healthcare system.
Possible follow-up call by HCP: an unsystematic practice
Some participants received a call from their GP or hospital physician a few days after discharge to check their health or answer questions. These calls reassured participants and their caregivers, who knew they had a point of contact in case of difficulty. Occasionally, participants received calls from their community pharmacists to ensure proper understanding and validate medication changes issued during hospitalization. Some participants did not receive any calls and were disappointed by the lack of follow-up:
“There is no follow-up! Nobody called me from the hospital to see how I was doing […]” P8.2
First medical appointment: a key step in the transition of care
The first medical appointment was made in advance by the hospital staff or the patient after discharge. For some participants, this first appointment did not differ from usual care. For most, it was a crucial appointment that allowed them to discuss their hospitalization and new medications and organize their follow-up care. Being cared for by a trusted HCP enabled some patients to feel safe, relieved, and well-cared for, as illustrated by the exchange between a patient and her daughter:
Daughter: When [my mom] came back from the GP, she felt much better [...] It was as if a cork had popped. Was it psychological? Patient: Maybe… I just felt better. D: Do you think it was the fact that she paid attention to you as a doctor? P: She took care of me. She did it in a delicate way. [silence] - P23.2
Some participants complained that their physicians did not receive the hospital discharge letter, making it difficult to discuss hospitalization and sometimes resulting in delayed care.
Outpatient care: a multifaceted experience
During the 2 months after hospital discharge, participants visited several physicians (Fig. 2 ), such as their GP and specialist physicians, for follow-ups, routine check-ups, medical examinations, and new prescriptions. Most participants went to their regular pharmacies to renew their prescriptions, for additional medication information, or for health advice.
Some participants had home care nurses providing various services, such as toileting, care, checks on vital functions, or preparing weekly pill boxes. While some participants were satisfied with this service, others complained that home nurses were unreliable about appointment times or that this service was unnecessary. Some participants were reluctant to use these services:
“The [homecare nurse] makes you feel like you're sick... It's a bit humiliating.” P22.2
Specialized nurses, mostly in diabetology, were appreciated by patients who had dedicated time to talk about different issues concerning diabetes and medication and adapted explanations to the patient’s knowledge. Participants who participated in cardiac rehabilitation said that being in a group and talking to people with the same health problems motivated them to undertake lifestyle and dietary changes:
“In the rehabilitation program, I’m part of a team [of healthcare professionals and patients], I have companions who have gone through the same thing as me, so I’m not by myself. That's better for motivation.” P16.2
Navigating the outpatient healthcare system: the central role of patients
Managing medical appointments is time-consuming and complex for many participants. Some had difficulty knowing with whom to discuss and monitor their health problems. Others had difficulty scheduling medical appointments, especially with specialist physicians or during holidays. A few participants did not attend some of their appointments because of physical or mental vulnerabilities. Restrictions linked to the type of health insurance coverage made navigating the healthcare system difficult for some participants:
“Some medications weren't prescribed by my GP [...] but by the cardiologist. So, I must ask my GP for a delegation to see the cardiologist. And I have to do this for three or four specialists... Well, it’s a bit of a hassle […] it's not always easy or straightforward”. P11.2
Some participants had financial difficulties or constraints, such as expenses from their hospitalization, ambulance transportation, and medications not covered by their health insurance plans. This led to misunderstandings, stress, and anxiety, especially because some participants could not return to work or, to a lesser extent, because of their medical condition.
To ensure continuity of care, some participants were proactive in their case management, for example, by calling to confirm or obtain further information on an appointment or to ensure information transfer. Written convocations for upcoming medical appointments and tailored explanations helped the participants organize their care. Family caregivers were also key in taking participants to various consultations, reminding them, and managing their medical appointments.
Information transfer: incomplete and missing information
Information transfer between and within settings was occasionally lacking. Even weeks after hospitalization, some documents were not transmitted to outpatient physicians, sometimes delaying medical care. Some participants reported receiving incomplete, unclear, or contradictory information from different HCPs, sometimes leading to doubts, seeking a second medical opinion, or personal searches for information. A few proactive participants ensured good information transmission by making a copy of the prescription or sending copies of their documents to physicians:
“My GP hasn't received anything from the hospital yet. I’ve sent him the PDF with the medication I take before our appointment […] Yes, It’s the patient that does all the job.” P10.3
Interprofessional work: a practice highlighted by some participants
Several participants highlighted the interprofessional work they observed in the outpatient setting, especially because they had several comorbidities; therefore, several physicians followed their care:
“My case is very complex! For example, between the cardiologist and the diabetologist, they need to communicate closely because there could be consequences or interactions with the medications I take [for my heart and my diabetes].” P4.2
Health professionals referred their patients to the most appropriate provider for better follow-up (e.g., a nurse specializing in addictology referred a patient to a nurse specializing in diabetology for questions and follow-up on blood sugar levels). Interprofessional collaboration between physicians and pharmacists was noted by some participants, especially for prescription refills or ordering medications.
Patient-HCPs relationships: the importance of trust
Trust in the care relationship was discussed by the participants regarding different HCPs, especially GPs and community pharmacists. Most participants highlighted the communication skills and active listening of healthcare providers. Knowing an HCP for several years helped build trust and ensure an updated medical history:
“I've trusted this pharmacist for 20 years. I can phone her or go to the pharmacy to ask any question[...] I feel supported.” P3.2
Some participants experienced poor encounters owing to a lack of attentive listening or adapted communication, especially when delivering bad news (new diagnoses or deterioration of health status). Professional competencies were an important aspect of the patient-HCP relationship, and some participants lost confidence in their physician or pharmacist because of inadequate medical or pharmaceutical care management or errors, such as the physician prescribing the wrong medication dosage, the pharmacist delivering the wrong pillbox or the general practitioner refusing to see a patient:
“I think I'll find another doctor… In fact, the day I was hospitalized, I called before to make an appointment with her and she refused to see me […] because I had a fever, and I hadn’t done a [COVID] test.” P6.2
Most participants underlined the importance of their GP because they were available, attentive to their health issues, and had a comprehensive view of their medications and health, especially after hospitalization:
“Fortunately, there are general practitioners, who know everything. With some specialists, the body is fragmented, but my GP knows the whole body.” P14.1
After hospitalization, the GP’s role changed for some participants who saw their GP infrequently but now played a central role.
Community pharmacist: an indistinct role
Pharmacists and their teams were appreciated by most participants for their interpersonal competencies, such as kindness, availability, professional flexibility, and adaptability to patients’ needs to ensure medication continuity (e.g., extension of the prescription, home delivery, or extending time to pay for medications). The role of community pharmacists varied according to the participants. Some viewed pharmacists as simple salespeople:
“It's like a grocery store. [...] I go there, it's ordered, I take my medication, I pay and I leave.” P23.3
For others, the pharmacist provided medication and advice and was a timely source of information but did not play a central role in their care. For others, the pharmacist’s role is essential for medication monitoring and safety:
“I always go to the same pharmacy […] because I know I have protection: when [the pharmacist] enters the medications in his computer, if two medications are incompatible, he can verify. [...] There is this follow-up that I will not have if I go each time somewhere else.” P10.4
The patient journey mapping methodology, coupled with qualitative thematic analysis, enabled us to understand and shed light on the intricacies of the journey of polypharmacy patients with T2Din the healthcare system after discharge. This provided valuable insights into their experiences, challenges, and opportunities for improvement.
This study highlights the complex pathways of patients with comorbidities by considering the population of patients with T2D as an example. Our population included a wide variety of patients, both newly diagnosed and with known diabetes, hospitalized for T2D or other reasons. Navigating the healthcare system was influenced by the reason for hospitalization and diagnosis. For example, newly diagnosed participants with T2D had a closer follow-up after discharge, participants were more likely to undergo cardiac rehabilitation after infarction, and participants with a former T2D diagnosis were more complex, with more comorbidities and more HCP encounters. Our aim was not to compare these populations but to highlight particularities and differences in their health care and these qualitative data reveal the need for further studies to improve diabetes management during inpatient to outpatient care transition.
The variability in discharge practices and coordination with outpatient care highlights the lack of standardization during and after hospital discharge. Some participants had a planned appointment with their GP before discharge, others had a telephone call with a hospital or ambulatory physician, and some had no planned follow-up, causing confusion and stress. Although various local or national guidelines exist for managing patients discharged from the hospital [ 36 , 37 , 38 , 39 ], there are no standard practices regarding care coordination implemented in the setting of this study. The lack of local coordination has also been mentioned in other studies [ 5 , 40 , 41 ].
Our results also raise questions about the responsibility gap in the transition of care. Once discharged from the hospital, who is responsible for the patient until their first medical appointment? This responsibility is not clearly defined among hospital and outpatient care providers, with more than 25% of internal medicine residents indicating their responsibility for patients ending at discharge [ 42 , 43 ]. Importance should be given to clarifying when and who will take over the responsibility of guaranteeing patient safety and continuity of care and avoiding rehospitalization [ 44 ].
The first visit with the community pharmacist after discharge and the referring physician were the key encounters. While the role of the GP at hospital discharge is well-defined, the community pharmacist’s role lacks clarity, even though they are the first HCP encountered upon hospital discharge. A meta-analysis showed the added value of community pharmacists and how their active participation during care transition can reduce readmission [ 18 ]. A better definition of the pharmacist’s role and integration into care coordination could benefit patient safety during the transition and should be assessed in future studies.
Our findings showed that the time elapsed between discharge and the first medical appointment varied widely (from 1 to 27 days), correlating with findings in the literature showing that more than 80% of patients see their GP within 30 days [ 45 ]. Despite the first medical appointment being within the first month after discharge, some patients in our study reported a lack of support and follow-up during the first few days after discharge. Care coordination at discharge is critical, as close outpatient follow-up within the first 7–10 days can reduce hospital readmission rates [ 46 , 47 ]. Furthermore, trust and communication skills are fundamental components of the patient-HCP relationship, underlined in our results, particularly during the first medical appointment. Relational continuity, especially with a particular HCP who has comprehensive patient knowledge, is crucial when patients interact with multiple clinicians and navigate various settings [ 48 , 49 ].
Navigating the outpatient healthcare system after discharge was complex for most participants and relied heavily on patient involvement and responsibility. While some participants who received clear information felt more empowered and engaged in their care, others highlighted the difficulty in organizing their care during this vulnerable period. Such difficulties in case management have been described previously [ 50 , 51 ]. Moreover, services proposed by HCPs (e.g., home assistance) do not always correspond to patient needs and are sometimes refused. This highlights the tension between HCPs’ medical recommendations, priorities, and patient expectations. This tension between medical priorities and patient needs was felt during hospitalization and shaped the 2 months following discharge. HCPs need to assess patient needs and preferences during hospitalization and transition for follow-up services. They must also ensure that the offered services meet at least the most relevant of patients’ perceived needs to improve seamless care and patient safety [ 52 , 53 ].
Examples of a lack of communication and information transfer were described in our results at different levels among HCPs, between participants or family caregivers, and HCPs, and these findings correlate with the literature [ 3 , 54 , 55 , 56 ]. Although family caregivers play an important role in supporting patients in the healthcare system, they are also additional interlocutors, leading to missed opportunities for patient-pharmacist interactions when dispensing discharged medication. Therefore, it is paramount to integrate and involve family caregivers in shared decision-making and communicate with patients remotely when they are not present [ 57 ].
Opportunities to improve the discharge of patients returning home after discharge without home care are highlighted in this article. Our insights can serve as a valuable foundation for healthcare providers and policymakers seeking to optimize patient experience and quality of care in the post-discharge phase. Different professionals should be integrated into standard practice through guidelines to ensure improved collaboration from hospital discharge to outpatient care. During hospitalization:
an appointment should be scheduled with the referring physician shortly after discharge to guarantee continuity of care
a hospital discharge interview should be conducted in a systematic way to summarize and securely close the hospitalization
the community pharmacist should be informed before the patient’s discharge to prepare and reconcile medications before and after hospitalization
In outpatient care:
an in-person or phone encounter with the pharmacy team should be scheduled for the patient and/or caregivers at discharge
a contact point (phone number, email, or virtual chat assistant) or scheduled follow-up should be implemented to answer questions and redirect patients before they can meet with the referring physician
a long-term and active communication channel between HCPs should be established.
In other countries, several outpatient services are already available for patients discharged home to enhance continuity of care and patient safety after discharge. The telehealth-based Transitional Care Management Programme, a local initiative in a New York hospital, involves contacting discharged patients 24 to 48 hours after discharge to support understanding of discharge instructions, medication access, follow-up appointments, and social needs [ 58 ]. The Australian Government has introduced the Transition Care Program that provides short-term care for older people, including social work, nursing support, personal care, and allied health care [ 59 ]. In England, the NHS has introduced the Discharge Medicines Service (DMS) in community pharmacies, which aims to improve communication between hospitals and community pharmacies and to ensure that patients understand changes to their medications [ 60 ].
Limitations
This study has several limitations. First, the accuracy of the encounter dates with HCPs, as described by the participants, could not be verified using a second data source (e.g., medical or pharmacy records). Additionally, recall biases cannot be excluded, especially during interviews 3 and 4, which took place at longer intervals (20 days between interviews 2 and 3 and 30 days between interviews 3 and 4). Nevertheless, our findings express a patient's representation of their healthcare system navigation experience. Secondly, these results may not be generalizable to populations with other long-term diseases, even though we recruited patients with different reasons for hospitalization, including age, sex, and comorbidities. In addition, the study region is predominantly an urban area with a high density of HCPs, which may influence patient journeys in the healthcare system. Finally, we excluded patients whose medications were managed by HCPs because these patients might have had different experiences, difficulties, and needs. This exclusion criterion was chosen because our objective was to investigate patients’ medication self-management, as described in another article [ 28 ].
A patient’s journey in the 2 months following discharge is unique for each individual and constitutes a complex and adaptive process. Despite the active role of numerous HCPs, navigation in outpatient care after discharge relies heavily on the involvement and responsibilities of polypharmacy. The findings of this study highlight the need to standardize the approach for discharge planning and post-discharge care in partnership with patients and caregivers. Preparation for discharge, the first visit to the pharmacy, and the first appointment with the GP are key moments for all patients, along with the involvement of other medical and nurse specialists, as needed. Standardizing practices, clarifying responsibilities, integrating community pharmacists during the transition, empowering patients, and enhancing interprofessional communication and collaboration should be explored and implemented to achieve better patient outcomes and a more seamless healthcare journey for individuals transitioning from the hospital to the community.
Availability of data and materials
The qualitative codes in French and anonymized patient datasets are available from the corresponding author on reasonable request. Individual patient journeys are provided in the Supplementary Files.
Abbreviations
General practitioner
Healthcare professional
type 2 diabetes mellitus
Coleman EA, Boult C. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–7.
Article PubMed Google Scholar
Allen J, Hutchinson AM, Brown R, Livingston PM. User experience and care for older people transitioning from hospital to home: Patients’ and carers’ perspectives. Health Expect. 2018;21(2):518–27.
Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. Jama. 2007;297(8):831–41.
Article CAS PubMed Google Scholar
Allen J, Hutchinson AM, Brown R, Livingston PM. Quality care outcomes following transitional care interventions for older people from hospital to home: a systematic review. BMC Health Serv Res. 2014;14:346.
Article PubMed PubMed Central Google Scholar
Hesselink G, Flink M, Olsson M, Barach P, Dudzik-Urbaniak E, Orrego C, et al. Are patients discharged with care? A qualitative study of perceptions and experiences of patients, family members and care providers. BMJ Qual Saf. 2012;21(Suppl 1):i39-49.
World Health Organization (WHO). Transitions of Care. 2016.
Google Scholar
Krumholz HM. Post-hospital syndrome–an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–2.
Article CAS PubMed PubMed Central Google Scholar
Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–28.
Banholzer S, Dunkelmann L, Haschke M, Derungs A, Exadaktylos A, Krähenbühl S, et al. Retrospective analysis of adverse drug reactions leading to short-term emergency hospital readmission. Swiss Med Wkly. 2021;151:w20400.
World Health Organization (WHO). Medication Safety in Transitions of Care. 2019.
Müller-Wieland D, Merkel M, Hamann A, Siegel E, Ottillinger B, Woker R, et al. Survey to estimate the prevalence of type 2 diabetes mellitus in hospital patients in Germany by systematic HbA1c measurement upon admission. Int J Clin Pract. 2018;72(12):e13273.
Blanc AL, Fumeaux T, Stirnemann J, Dupuis Lozeron E, Ourhamoune A, Desmeules J, et al. Development of a predictive score for potentially avoidable hospital readmissions for general internal medicine patients. PLoS One. 2019;14(7):e0219348.
Hansen LO, Greenwald JL, Budnitz T, Howell E, Halasyamani L, Maynard G, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421–7.
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10(1):107–11.
Iglay K, Hannachi H, Joseph Howie P, Xu J, Li X, Engel SS, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243–52.
Russell LB, Suh DC, Safford MA. Time requirements for diabetes self-management: too much for many? J Fam Pract. 2005;54(1):52–6.
PubMed Google Scholar
Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–8.
Lussier ME, Evans HJ, Wright EA, Gionfriddo MR. The impact of community pharmacist involvement on transitions of care: a systematic review and meta-analysis. J Am Pharm Assoc. 2020;60(1):153.
Article Google Scholar
Donzé J, John G, Genné D, Mancinetti M, Gouveia A, Méan M, et al. Effects of a Multimodal Transitional Care Intervention in Patients at High Risk of Readmission: The TARGET-READ Randomized Clinical Trial. JAMA Intern Med. 2023.
Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–107.
Noonan VK, Lyddiatt A, Ware P, Jaglal SB, Riopelle RJ, Bingham CO 3rd, et al. Montreal Accord on Patient-Reported Outcomes (PROs) use series - Paper 3: patient-reported outcomes can facilitate shared decision-making and guide self-management. J Clin Epidemiol. 2017;89:125–35.
Hesselink G, Schoonhoven L, Barach P, Spijker A, Gademan P, Kalkman C, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417–28.
(WHO) WHO. Systems in the who European region: framework for action on integrated health services delivery. Copenhagen: WHO; 2016.
Damery S, Flanagan S, Combes G. The effectiveness of interventions to achieve co-ordinated multidisciplinary care and reduce hospital use for people with chronic diseases: study protocol for a systematic review of reviews. Syst Revi. 2015;4(1):64.
Noor F, Gulis G, Karlsson LE. Exploration of understanding of integrated care from a public health perspective: a scoping review. J Public Health Res. 2023;12(3):22799036231181210.
Jackson K, Oelke ND, Besner J, Harrison A. Patient journey: implications for improving and integrating care for older adults with chronic obstructive pulmonary disease. Can J Aging. 2012;31(2):223–33.
Gualandi R, Masella C, Viglione D, Tartaglini D. Exploring the hospital patient journey: what does the patient experience? PLoS One. 2019;14(12):e0224899.
Solh Dost L, Gastaldi G, Schneider M. Patient medication management, understanding and adherence during the transition from hospital to ambulatory care – a qualitative longitudinal study in polymorbid type 2 diabetes patients. BMC Health Services Research, in press
World Health Organization (WHO). Adherence to long-term therapies: Evidence for action. 2003.
Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–73.
Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health. 1998;13(4):623–49.
Hennink MM, Kaiser BN, Marconi VC. Code saturation versus meaning saturation: how many interviews are enough? Qual Health Res. 2016;27(4):591–608.
Braun V, Clarke V. Reflecting on reflexive thematic analysis. Qual Res Sport Exercise Health. 2019;11(4):589–97.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Davies EL, Bulto LN, Walsh A, Pollock D, Langton VM, Laing RE, et al. Reporting and conducting patient journey mapping research in healthcare: a scoping review. J Adv Nurs. 2023;79(1):83–100.
California pharmacists association. Transitions of Care Resource Guide https://cdn.ymaws.com/www.cshp.org/resource/resmgr/Files/Practice-Policy/For_Pharmacists/transitions_of_care_final_10.pdf . Accessed 20 Nov 2023.
National Health Service (NHS). Guidance: Hospital discharge and community support guidance. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1087354/Hospital-Discharge-and-Community-Support-Guidance-2022-v2.pdf . Accessed 01 Apr 2024.
Winnipeg Regional Health Authority. Safe Patient Discahrge Guideline. 2017. https://wrha.mb.ca/files/guideline-safe-discharge.pdf . Accessed 01 Apr 2024.
Haute Autorité de Santé, France. Check-List de Sortie d'hospitalisation supérieure à 24 heures https://www.has-sante.fr/jcms/c_2035081/fr/check-list-de-sortie-d-hospitalisation-superieure-a-24h . Accessed 04 Apr 2024.
Wong ELY, Yam CHK, Cheung AWL, Leung MCM, Chan FWK, Wong FYY, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242.
Urban R, Paloumpi E, Rana N, Morgan J. Communicating medication changes to community pharmacy post-discharge: the good, the bad, and the improvements. Int J Clin Pharm. 2013;35(5):813–20.
Young E, Stickrath C, McNulty MC, Calderon AJ, Chapman E, Gonzalo JD, et al. Internal medicine residents’ perceived responsibility for patients at hospital discharge: a national survey. J Gen Intern Med. 2016;31(12):1490–5.
Jones CD, Vu MB, O’Donnell CM, Anderson ME, Patel S, Wald HL, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417–24.
Watts R, Pierson J, Gardner H. Co-ordination of the discharge planning process in critical care. J Clin Nurs. 2007;16(1):194–202.
Roughead EE, Kalisch LM, Ramsay EN, Ryan P, Gilbert AL. Continuity of care: when do patients visit community healthcare providers after leaving hospital? Intern Med J. 2011;41(9):662–7.
Riverin BD, Strumpf EC, Naimi AI, Li P. Optimal timing of physician visits after hospital discharge to reduce readmission. Health Serv Res. 2018;53(6):4682–703.
Coppa K, Kim EJ, Oppenheim MI, Bock KR, Conigliaro J, Hirsch JS. Examination of post-discharge follow-up appointment status and 30-day readmission. J Gen Intern Med. 2021;36(5):1214–21.
Haggerty JL, Roberge D, Freeman GK, Beaulieu C. Experienced continuity of care when patients see multiple clinicians: a qualitative metasummary. Ann Fam Med. 2013;11(3):262–71.
Baker R, Freeman G, Boulton M, Windridge K, Tarrant C, Low J, et al. Continuity of care: patients’ and carers’ views and choices in their use of primary care services. Report for the national co-ordinating center for NHS Service Delivery and Organisation R & D (NCCSDO). 2006.
Arora VM, Prochaska ML, Farnan JM, D’Arcy MJt, Schwanz KJ, Vinci LM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–91.
Allen J, Hutchinson AM, Brown R, Livingston PM. User experience and care integration in transitional care for older people from hospital to home: a meta-synthesis. Qual Health Res. 2016;27(1):24–36.
Krook M, Iwarzon M, Siouta E. The discharge process-from a patient’s perspective. SAGE Open Nurs. 2020;6:2377960819900707.
PubMed PubMed Central Google Scholar
Huber DL, McClelland E. Patient preferences and discharge planning transitions. J Prof Nurs. 2003;19(4):204–10.
Ravenscroft E. Navigating the health care system: insights from consumers with multimorbidity. J Nurs Healthcare Chronic Illness. 2010;2:215–24.
Ancker JS, Witteman HO, Hafeez B, Provencher T, Van de Graaf M, Wei E. The invisible work of personal health information management among people with multiple chronic conditions: qualitative interview study among patients and providers. J Med Internet Res. 2015;17(6):e137.
Manias E, Gerdtz M, Williams A, McGuiness J, Dooley M. Communicating about the management of medications as patients move across transition points of care: an observation and interview study. J Eval Clin Pract. 2016;22(5):635–43.
Mackie BR, Mitchell M, Marshall AP. Patient and family members’ perceptions of family participation in care on acute care wards. Scandinavian J Caring Sci. 2019;33(2):359–70.
Michelle E, Rachel CSF, Jonathan S, Priscilla H, Farrukh NJ. Telehealth-based transitional care management programme to improve access to care. BMJ Open Qual. 2023;12(4):e002495.
Department of Health and Aged Care, Australia. Transition Care Programme. https://www.health.gov.au/our-work/transition-care-programme . Accessed 03 Apr 2024
National Health Service (NHS) Discharge Medicines Service. 2021. https://www.england.nhs.uk/primary-care/pharmacy/pharmacy-services/nhs-discharge-medicines-service/ . Accessed 03 Apr 2024
Download references
Acknowledgments
The authors would like to thank all the patients who took part in this study. We would also like to thank the Geneva University Hospitals Patients Partners +3P platform as well as Mrs Tourane Corbière and Mr Joël Mermoud, patient partners, who reviewed interview guides for clarity and significance.
Open access funding provided by University of Geneva This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
School of Pharmaceutical Sciences, University of Geneva, Geneva, Switzerland
Léa Solh Dost & Marie P. Schneider
Institute of Pharmaceutical Sciences of Western Switzerland, University of Geneva, Geneva, Switzerland
Division of Endocrinology, Diabetes, Hypertension and Nutrition, Department of Medicine, Geneva University Hospitals, Geneva, Switzerland
Giacomo Gastaldi
Institute of Psychology and Education, University of Neuchatel, Neuchâtel, Switzerland
Marcelo Dos Santos Mamed
Institute of Psychology, University of Lausanne, Lausanne, Switzerland
You can also search for this author in PubMed Google Scholar
Contributions
LS, GG, and MS conceptualized and designed the study. LS and GG screened and recruited participants. LS conducted the interviews. LS, GG, and MS performed data analysis and interpretation. LS drafted the manuscript and LS and MS worked on the different versions. MDS contributed its expertise and external opinion as a clinical psychologist and linguist. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Léa Solh Dost or Marie P. Schneider .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval was sought and granted by the Cantonal Research Ethics Commission, Geneva (CCER) (2020-01779). Informed consent to participate was obtained for all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Interview guides.
Additional file 2.
Individual patient journey mappings from discharge to 2 months after discharge.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Solh Dost, L., Gastaldi, G., Dos Santos Mamed, M. et al. Navigating outpatient care of patients with type 2 diabetes after hospital discharge - a qualitative longitudinal study. BMC Health Serv Res 24 , 476 (2024). https://doi.org/10.1186/s12913-024-10959-4
Download citation
Received : 17 January 2024
Accepted : 07 April 2024
Published : 17 April 2024
DOI : https://doi.org/10.1186/s12913-024-10959-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type 2 diabetes mellitus
- Outpatient care
- Hospital discharge
- Qualitative research
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
- Open access
- Published: 22 April 2024
Association between diabetes mellitus and primary restenosis following endovascular treatment: a comprehensive meta-analysis of randomized controlled trials
- Xiaolei Sun 1 , 2 , 4 , 5 , 6 , 9 na1 ,
- Cheng Zhang 10 na1 ,
- Yarong Ma 7 na1 ,
- Yanzheng He 1 ,
- Xiaodong Zhang 8 &
- Jianbo Wu 3 , 5 , 6
Cardiovascular Diabetology volume 23 , Article number: 132 ( 2024 ) Cite this article
306 Accesses
2 Altmetric
Metrics details
Diabetes mellitus (DM) is thought to be closely related to arterial stenotic or occlusive disease caused by atherosclerosis. However, there is still no definitive clinical evidence to confirm that patients with diabetes have a higher risk of restenosis.
This meta-analysis was conducted to determine the effect of DM on restenosis among patients undergoing endovascular treatment, such as percutaneous transluminal angioplasty (PTA) or stenting.
Data sources and study selection
The PubMed/Medline, EMBASE and Cochrane Library electronic databases were searched from 01/1990 to 12/2022, without language restrictions. Trials were included if they satisfied the following eligibility criteria: (1) RCTs of patients with or without DM; (2) lesions confined to the coronary arteries or femoral popliteal artery; (3) endovascular treatment via PTA or stenting; and (4) an outcome of restenosis at the target lesion site. The exclusion criteria included the following: (1) greater than 20% of patients lost to follow-up and (2) a secondary restenosis operation.
Data extraction and synthesis
Two researchers independently screened the titles and abstracts for relevance, obtained full texts of potentially eligible studies, and assessed suitability based on inclusion and exclusion criteria.. Disagreements were resolved through consultation with a third researcher. Treatment effects were measured by relative ratios (RRs) with 95% confidence intervals (CIs) using random effects models. The quality of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria.
Main outcomes and measures
The main observation endpoint was restenosis, including > 50% stenosis at angiography, or TLR of the primary operation lesion during the follow-up period.
A total of 31,066 patients from 20 RCTs were included. Patients with DM had a higher risk of primary restenosis after endovascular treatment (RR = 1.43, 95% CI: 1.25–1.62; p = 0.001).
Conclusions and relevance
This meta-analysis of all currently available RCTs showed that patients with DM are more prone to primary restenosis after endovascular treatment.
Cardiovascular disease (CVD) has the highest mortality rate worldwide [ 1 ], and vascular stenotic and occlusive lesions are the main pathological cause of death and disability for CVD patients [ 2 ]. At present, the development of intravascular therapy technology provides a reliable method for the treatment of vascular occlusive diseases, and quite a few of the difficulties in treatment have been resolved. However, restenosis after endovascular repair is a major problem that confuses clinicians and affects their choice of treatment [ 3 ]. Studies have shown that 30% to 50% of patients with coronary ischemic disease experience restenosis after endovascular therapy within five years; although the application of drug-coated balloons or drug-eluting stents has clearly reduced the occurrence of restenosis, restenosis still occurs in 10% to 20% of patients within one year [ 4 , 5 ]. Restenosis after endovascular treatment causes a large number of patients to stop working or even die, which causes considerable damage to people's health and social and economic development. Thus, this is an urgent unsolved clinical problem. Identifying potential exposure and protective factors could make health care more effective in controlling restenosis after endovascular treatment.
Diabetes mellitus (DM) and its complications constitute one of the greatest human health problems worldwide. The prevalence of DM will increase globally from 371 million individuals in 2013 to 552 million individuals in 2030 [ 6 ]. DM is an important risk factor for the development of atherosclerotic diseases such as coronary heart disease (CHD), cerebrovascular disease, and peripheral artery disease (PAD). Cardiovascular complications are the leading cause of mortality among individuals with DM, and > 50% of patients die from a cardiovascular event, especially coronary artery disease but also stroke and peripheral vascular disease [ 7 ]. Insulin resistance and hyperglycemia in diabetes patients increase the risk of adverse cardiovascular events [ 8 ]. Studies have shown that hyperglycemia, insulin resistance, and an increase in advanced glycation end products are important conditions for a 2–fourfold increased risk of coronary artery disease (CAD) and PAD among people with diabetes [ 9 , 10 , 11 , 12 , 13 , 14 ]. However, at present, there is insufficient clinical evidence to confirm that diabetes increases the risk of restenosis after endovascular treatment. Therefore, to determine the effect of DM on restenosis among patients following endovascular interventional therapy, we designed this meta-analysis based on current clinical randomized controlled trial (RCT) data.
Study design and search strategy
We have designed the research and register for the study on the INPLASY website, and the registration number is INPLASY202370034 (DOI: 10.37766 / inplasy2023.7.0034). Ethical approval was acquired from the Ethic Committee of Southwest Medical University (No. 20220217–013). Two researchers independently scanned the titles and abstracts of the retrieved studies for the topic, and then obtained the full texts of potentially eligible studies and examined these independently for their suitability according to the inclusion criteria. In the case of disagreement between the two researchers, a third researcher was consulted to reach a consensus on whether to include the report or not. They documented the selection process with a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA [ 15 ]) flow chart. Trials were included if they satisfied the following eligibility criteria: (1) The studies included had to be randomized controlled studies (RCTs) including patients with or without DM; (2) had to involve lesions confined to the coronary arteries or femoral popliteal artery; (3) had to involve endovascular treatment via percutaneous transluminal angioplasty (PTA) or stenting; and (4) had to include an outcome of restenosis at the target lesion site. The exclusion criteria were as follows: (1) the proportion of patients lost to follow-up was higher than 20%; (2) a secondary restenosis operation; and (3) a sub-analysis or post-hoc analysis of RCTs. We developed and adhered to a standard protocol for study identification, inclusion, and data abstraction for all steps of our systematic review. Our major endpoint was restenosis, defined as a stenosis diameter > 50% in the in-segment area assessed by angiography, including the stent area as well as 5-mm margins proximal and distal to the stent. Meanwhile, clinically driven target lesion revascularization (CD-TLR) was also included, defined as any procedure performed to restore luminal patency after there has been late luminal loss of the target lesion (confirmed by angiography).
In this meta-analysis, we identified related published studies through a computerized literature search of the PubMed/Medline, Cochrane Library, and EMBASE (from 01/1990 to 12/2022) electronic databases. Two independent researchers checked citations for inclusion in the systematic review by using a hierarchical approach. The researchers assessed the title, abstract, and full text of these manuscripts. In addition, another reviewer manually searched the bibliographies of journal articles and relevant reviews to locate additional studies.
Reviewers extracted data using a data extraction form designed and piloted by the authors. If studies were reported in multiple publications, data were extracted from the different publications and then combined into a single data extraction form so that no data were omitted. The following characteristics of the included studies were registered in the data extraction form: methods and study design, participants, interventions and outcomes, including the outcome of restenosis. For all unclear restenosis analysis results, emails were sent to the corresponding authors to request the raw data; unfortunately, up to the time that the manuscript was written, no reply had been received.
Data synthesis and statistical analysis
For dichotomous data, we calculated Mantel‒Haenszel relative ratios (RRs) and 95% confidence intervals (CIs). Heterogeneity across studies was assessed by Cochran’s Q statistic with a P value set at 0.1. The I 2 statistic was also taken into account regardless of the P value. An I 2 of ≥ 50% was prespecified as the threshold considered too high to provide consistent analysis. A random effects model was used for the analysis. Tests were two-tailed, and a P value of < 0.05 was considered statistically significant. Funnel plots were used to assess publication bias. STATA 12.0 (StataCorp, USA) was used to analyze the data.

Assessment of the risk of bias in the included studies
Two reviewers assessed the risk of bias independently for the included studies using the Cochrane risk of bias assessment tool [ 16 ]. We evaluated all included studies for the following: adequacy of sequence generation and allocation concealment; adequacy of blinding of couples, providers and outcome assessors; completeness of outcome data; risk of selective outcome reporting; and risk of other potential sources of bias. The results of the risk of bias assessment are presented in Fig. 2 .
The results of the literature search
In this analysis, a total of 2,996 RCTs were retrieved from the database. After eliminating duplicate studies, 1,430 studies remained. After browsing titles and abstracts, 605 full-text articles were assessed for eligibility, of which 585 were excluded because of the absence of relevant endpoint data (no surveillance undertaken, restenosis rates not reported separately). Finally, 20 studies were included in the meta-analysis for qualitative and quantitative analyses (Fig. 1 ). As a result, 31,066 patients were enrolled in this study. Table 1 details the case numbers and baseline characteristics, inclusion/exclusion criteria, number and type of stent/PTA procedure, strategies for follow-up, criteria for diagnosing restenosis, and the number of cases of restenosis with DM for each RCT. All authors of the studies included in this meta-analysis were requested to supply missing data and details of their studies. Unfortunately, no author supplied the requested information.
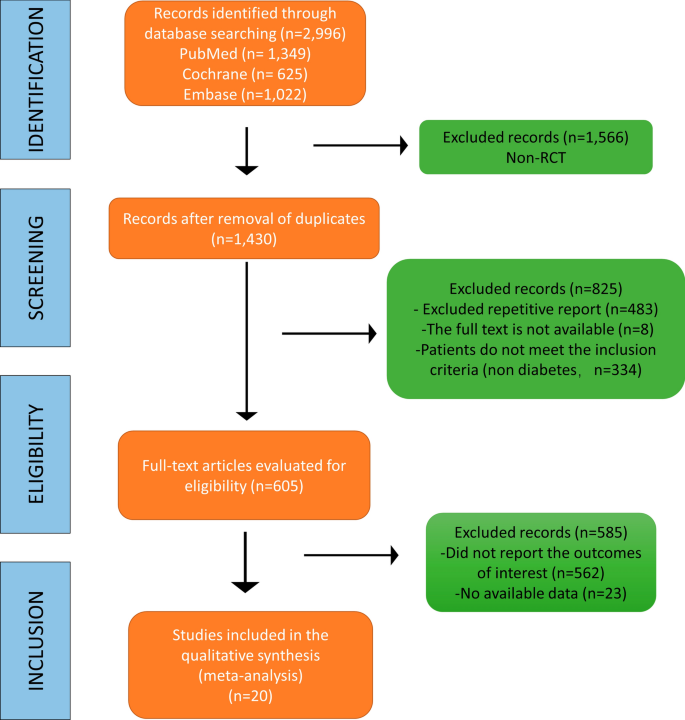
Flow diagram of the study selection process
Description of the prospective randomized pooled trials and quality assessment of the included studies
As the aim of this study was to analyze the PTA- or stenting-related outcomes among patients who suffered from cardiovascular stenotic or occlusive disease with or without DM, the efficacy endpoint was the rate of restenosis (> 50% stenosis at angiography or TLR during the follow-up period). As a result, 31,066 patients were enrolled in this study. Table 1 summarizes the characteristics of the 20 included RCTs. All trials met the inclusion criteria and had a low risk of bias according to the Cochrane tool [ 16 ] for assessing risk of bias in RCTs (Fig. 2 ). Four studies (TOSCA, SORT OUT III, ICE, HORIZONS AMI) were designed to be open-labeled, and the BASKET-SMALL 2 study did not describe its randomization method (Table 2 ).
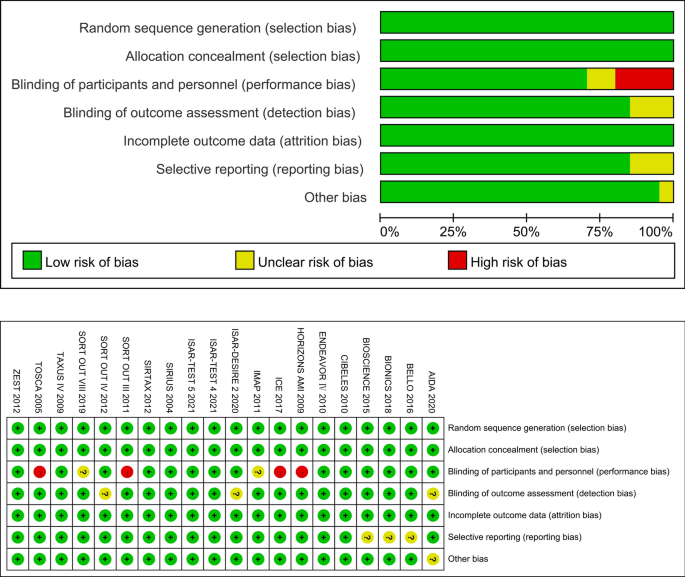
Risk of bias evaluated by the RoB 2 tool. All trials met the criteria and had a low risk of bias according to the Cochrane tool for assessing the risk of bias in RCTs
Meta-analysis results
There were 20 RCTs that included a total of 31,066 patients that reported data on DM and restenosis. Since there was high heterogeneity ( I 2 = 57.3%, P = 0.001) , a meta-analysis was conducted through a random effects model. Overall, the pooled results showed that DM was significantly associated with a higher risk of a major endpoint (RR = 1.43, 95% CI: 1.25–1.62; Fig. 3 ). The heterogeneity test showed significant differences among individual studies (P < 0.01, I 2 = 57.3%). The sensitivity analysis showed that heterogeneity mainly came from BIONICS (2018), but the outcome did not change with removal of this study (RR 1.40,95% CI: 1.23–1.59) (Fig. 9 ).
Forest plot of the association between DM and restenosis. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 3 (95% CI, 1.25–1.62; I 2 = 57.53%, P = 0.001). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
Subgroup analysis
To determine the relationship between glycemic control levels and the incidence of postoperative restenosis, we performed a subgroup analysis of the included studies. Since detailed blood glucose levels of patients could not be obtained, we divided the included studies into the "under control" group and the "unknown" group according to whether the proportion of overall medicine glycemic control (oral hypoglycemic agents or insulin) of diabetic patients in each study was more than 80% (Fig. 4 ). As a result, there was no significant difference in restenosis rates between the under-control group (RR = 1.53, 95% CI 1.26–1.84, P < 0.05) and the unknown group (RR = 1.37, 95% CI 1.16–1.63, P = 0.001).
Subgroup analysis of the association between restenosis and glycemic control levels. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 4 (‘Under Control’ subgroup, RR = 1.53, 95% CI, 1.25–1.62; I 2 = 36.9%, P < 0.05, ‘Unknown’ subgroup, RR = 1.37, 95% CI, 1.16–1.63; I 2 = 63%, P = 0.001.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
Antiplatelet therapy and anticoagulant therapy after vascular intervention are closely related to long-term efficacy [ 17 ]. Studies have demonstrated that an adequate treatment course of antiplatelet therapy after coronary stenting or angioplasty of lower extremity peripheral arteries is beneficial to improve long-term patency rates [ 18 , 19 ]. We also analyzed the relationship between the postoperative dual antiplatelet therapy (DAPT) duration and restenosis in the studies. The results showed that regardless of the duration of DAPT, either less than (RR = 1.53, 95% CI 1.23–1.90, P < 0.05) or more than 6 months (RR = 1.48, 95% CI 1.27–1.73, P = 0.006) had no effect on restenosis outcome (Fig. 5 ). In addition, subgroup analysis was performed based on a 1-year follow-up. The results showed that there was no significant difference in the incidence of restenosis based on follow-up duration in the more than 1 year group (RR = 1.41, 95% CI 1.16–1.70, P = 0.018) and the less than or equal to 1 year group (RR = 1.44, 95% CI 1.19–1.74, P = 0.003) (Fig. 6 ). Furthermore, in these 20 studies, the major target vessel was the coronary artery, except for the ICE study, which evaluated peripheral arteries. Then, we performed a subgroup analysis by different target lesions. As shown in the forest plot, the restenosis rate after endovascular therapy was related to the primary site of the vascular lesion (coronary artery (RR = 1.44, 95% CI 1.27–1.64) or lower limb artery (RR = 0.75, 95% CI 0.33–1.74)) (Fig. 7 ). Moreover, subgroup analysis was also performed to account for continental differences in the included populations. Since there was only one study conducted among Asians, our subgroup included European and American participants (without distinguishing between South and North America), and it showed no significant differences in the endpoints between Americans (RR = 1.53, 95% CI 1.22–1.92, P = 0.004) and Europeans (RR = 1.43, 95% CI 1.21–1.69, P < 0.05) (Fig. 8 ). According to the diagnosis of restenosis, including angiography and TLR, we conducted a subgroup analysis of the two modalities, and the results showed that patients with diabetes had a higher risk of TLR (RR = 1.53, 95% CI 1.34–1.76, P = 0.010) (Fig. 9 ).
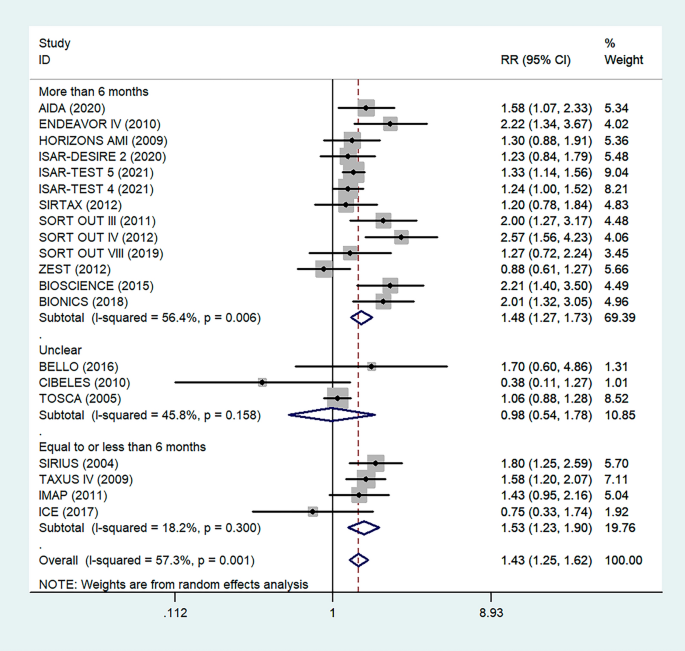
Subgroup analysis of the association between restenosis and the duration of DAPT. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 5 (‘More than 6 months’ subgroup, RR = 1.48, 95% CI, 1.27–1.73; I 2 = 56.4%, P = 0.006, ‘Equal to or less than 6 months’ subgroup, RR = 1.53, 95% CI, 1.23–1.90; I 2 = 18.2%, P < 0.05.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
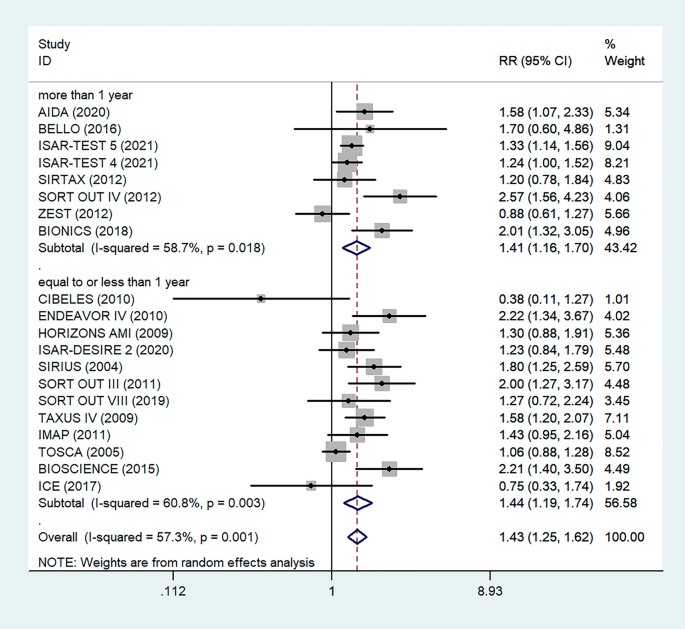
Subgroup analysis of the association between restenosis and different follow-up times. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 6 (‘More than 1 year’ subgroup, RR = 1.41, 95% CI, 1.16–1.70; I 2 = 58.7%, P = 0.018, ‘Equal to or less than 1 year’ subgroup, RR = 1.44, 95% CI, 1.19–1.74; I 2 = 60.8%, P = 0.003.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
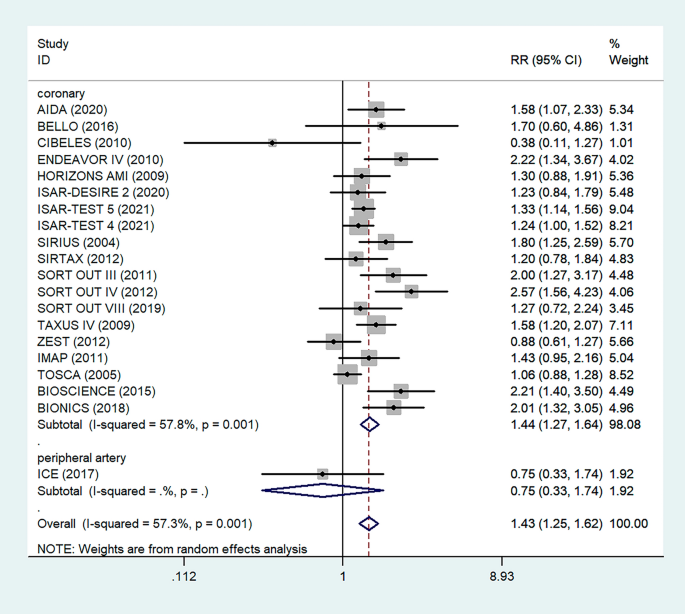
Subgroup analysis of the association between restenosis and types of lesion vessels. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 7 (‘Coronary’ subgroup, RR = 1.44, 95% CI, 1.27–1.64; I 2 = 57.8%, P = 0.001, ‘Peripheral’ subgroup, RR = 0.75, 95% CI, 0.33–1.74.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
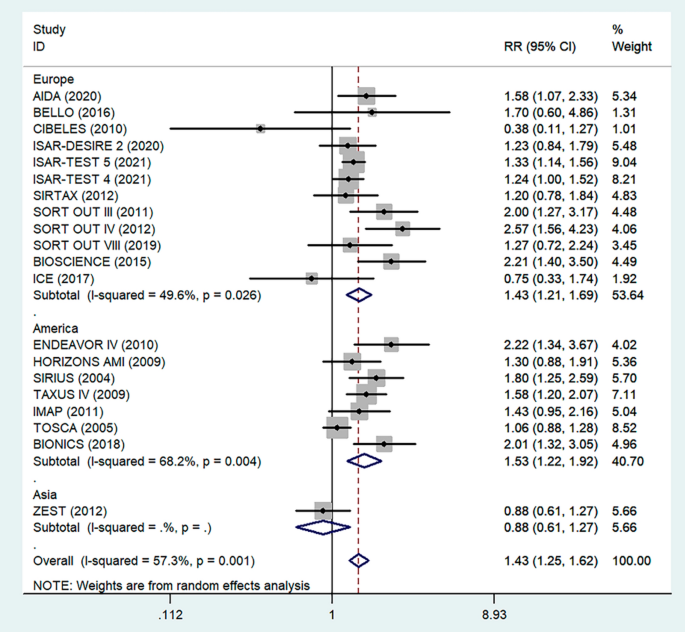
Subgroup analysis of the association between restenosis and different continents. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 8 (‘Europe’ subgroup, RR = 1.43, 95% CI, 1.21–1.69; I 2 = 49.6%, P < 0.05. ‘America’ subgroup, RR = 1.53, 95% CI, 1.22–1.92; I 2 = 68.2%, P = 0.004.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
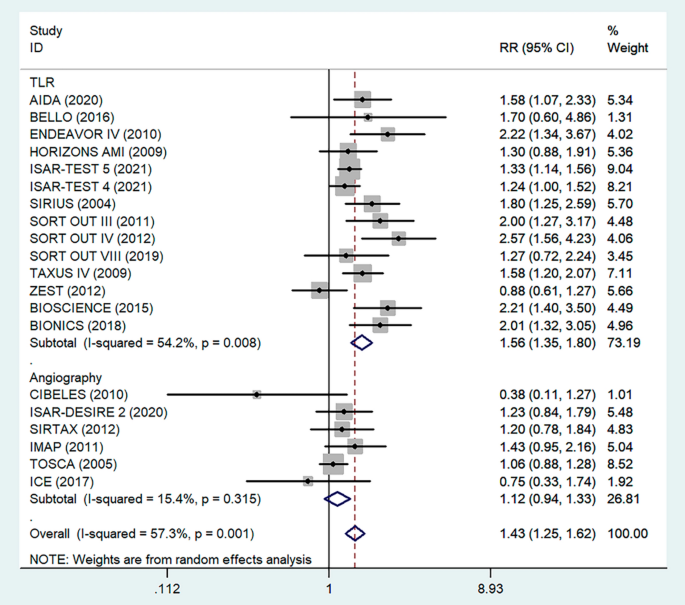
Subgroup analysis of the association between restenosis and diagnostic methods. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 9 (‘TLR’ subgroup, RR = 1.56, 95% CI, 1.35–1.80; I 2 = 49.6%, P = 0.008, ‘Angiography’ subgroup, RR = 1.12, 95% CI, 0.94–1.33; I 2 = 15.4%, P = 0.315.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
Quantified covariable analysis-meta-regression analyses
With the aim of performing a comprehensive literature review on restenosis after interventional endovascular treatments of PTA or stenting in patients with diabetes (1) across different “interventions” (we performed a regression analysis according to different interventional modalities, i.e., aspirin, PTA or stent placement) (2) among patients with different health conditions (the proportion of patients with smoking exposure, hypertension, or hyperlipemia, was distinguished and regression analysis was performed) (3) and in different periods of these interventional endovascular techniques (presented as the publication times), meta-regression was employed. RRs, using variable rates as the dependent variable, and the different interventions, the different health conditions, and the publication times as the independent variables, were determined. There was no evidence that the different interventions, the different health conditions and the publication times were confounding factors in this subgroup analysis. Data from the analyses of moderator variables are presented in Table 3 .
Sensitivity analysis
In the sensitivity analysis, each included study was removed one by one, and a summary analysis of the remaining studies was performed to assess whether a single included study had an excessive impact on the results of the entire meta-analysis (Fig. 10 ). None of the studies had an excessive impact on the results of the meta-analysis, indicating that the results of the meta-analysis were stable and reliable.
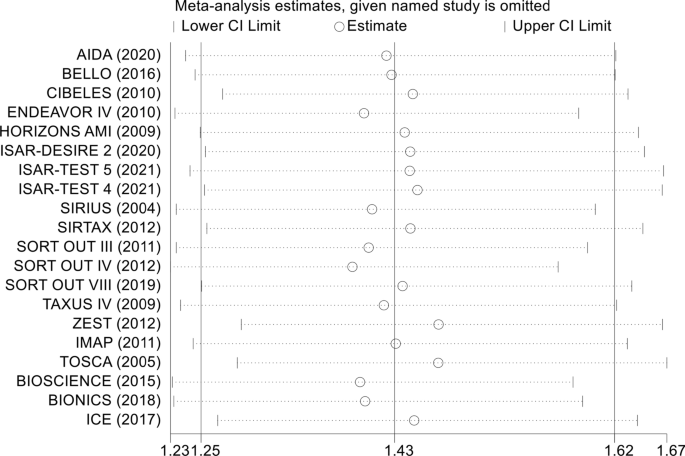
Sensitivity analysis of the association between diabetes mellitus and the endpoints
Publication bias
The probability of publication bias in the spread of the meta-analysis by funnel diagram and Begg’s test at a significance level of 0.05 indicated no bias of spread in the present study (p = 0.344) (Fig. 11 ). According to the results of the diagram, the publication offset of the included studies was small, and the results of the meta-analysis had high uniformity.
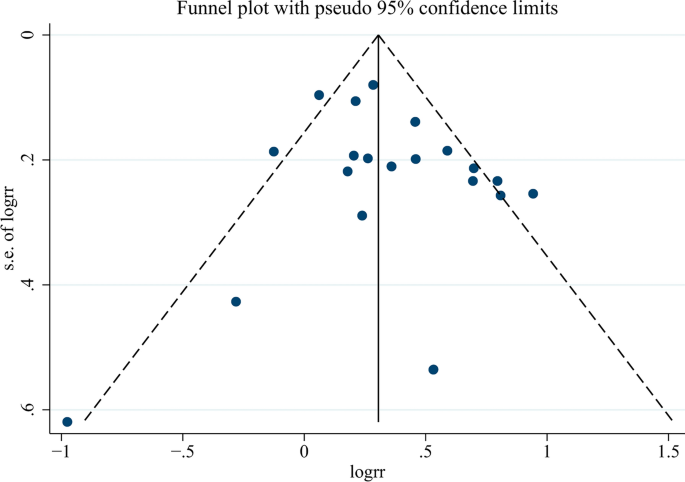
Funnel plot for assessing publication bias
Meta-analysis GRADE assessment
The evidence was assessed according to the GRADE process for the purposes of making clinical practice recommendations. We used GRADE to evaluate the quality of evidence, as shown in Fig. 12 . Judgments about evidence quality (high, moderate, low or very low) were made by two review authors who worked independently and resolved disagreements by discussion. Conclusions were justified, documented, and incorporated into the reporting of results for each outcome. A ‘high’ level of evidence score was obtained according to the GRADE scoring rule after assessing the risks of inconsistency, indirectness, imprecision and publication bias.
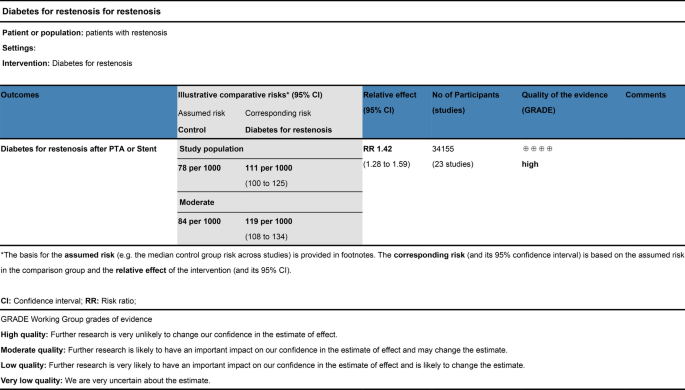
Meta-analysis GRADE assessment. Search strategy of PubMed/Medline. PubMed platform. Searched from 1990 to December 12, 2022. #1 (((diabetes mellitus) AND percutaneous transluminal angioplasty)) OR ((diabetes mellitus) AND stent) (798). #2 ((Percutaneous Transluminal Angioplasty) OR (Transluminal Angioplasty) OR (Endoluminal Repair) OR (Angioplasty) OR (Stent) OR (Endovascular Stent Grafting) OR (Stent Grafting) OR (Stents)) AND ((Diabetes mellitus) OR (Diabetes) OR (Diabetic)) AND ((Restenosis) OR (Graft Restenosis) OR (Restenoses)) AND ((random) OR (randomized) OR (randomised)) (235). #3 ((((diabetes mellitus) OR (diabetes)) OR (melituria)) OR (diabetic)) AND (restenosis) (316)
At present, PTA or stent implantation is the main treatment for cardiovascular stenosis or occlusion; however, restenosis after endovascular treatment is still a challenge [ 20 ]. Indeed, as the number of stent placements has risen to an estimate of over 3 million annually worldwide, revascularization procedures have become much more common [ 21 ]. However, restenosis after endovascular therapy is a major problem, and studies have shown that 30% to 50% of patients with coronary ischemic disease experience restenosis after endovascular therapy. To date, DM has been recognized as a high-risk factor for cardiovascular events [ 7 , 22 ]. Epidemiological investigations have shown that patients with concomitant DM and PAD are at high risk for major complications, such as amputation [ 23 ]. Technical progress, such as the application of drug-coated balloons or drug-eluting stents [ 20 , 24 ], has been found to potentially increase patency after endovascular treatment and thus reduce restenosis. The studies we enrolled included a large number of RCTs that involved drug-coated balloons and drug-eluting stents. Although the incidence of restenosis was significantly lower than that of traditional balloons or bare-metal stents, restenosis remained at ahigh rate and was difficult to resolve.
A study published in 1999 found that the long-term need for TLR increased with higher classes of in-stent restenosis (ISR) (hazard ratio (HR) = 1.7; P = 0.0380) and with the presence of diabetes (HR = 2.8; P = 0.0003) [ 25 ]. Taken together with other evidence, DM is suggested to be a strong determinant of restenosis (neointimal hyperplasia) [ 26 , 27 ]. Michael Jonas et al. [ 28 ] used the insulin resistance model of the Zucker fatty rat and found that insulin-resistant Zucker fatty rats developed a thicker neointima and a narrower lumen area 2 weeks after implantation of an abdominal aortic stent compared with normal Zucker lean rats. Additionally, Manikandan Panchatcharam et al. [ 29 ] established a femoral artery guide wire injury model in hyperglycemic mice and confirmed that hyperglycemia had an obvious accelerating effect on intimal regeneration after vascular injury by promoting smooth muscle cell proliferation and migration. These animal studies demonstrated the role of hyperglycemia and ISR in regulating the function of vascular smooth muscle cells (VSMCs) and promoting neointimal hyperplasia. Moreover, advanced glycation end products (AGEs) also play an important role in promoting the progression of diabetic vascular disease. Zhongmin Zhou et al. [ 30 ] demonstrated a prominently increased accumulation of AGEs and immunoreactivities of receptor for advanced glycation end products (RAGEs) in response to balloon injury in diabetic compared with nondiabetic rats. Additionally, blockade of RAGE/ligand interaction significantly decreased VSMC proliferation in vitro and bromodeoxyuridine (BrdU)-labeled proliferating VSMCs in vivo, suppressed neointimal formation and increased luminal area in both diabetic and nondiabetic rats. These animal studies demonstrated that the pathophysiological features of diabetes, including ISR, metabolic syndrome, hyperglycemia, and increased AGEs, are involved in neointima after balloon dilation or stent implantation.
The current mainstream view is that DM increases the risk of restenosis [ 31 ]. Studies have shown that patients with insulin-dependent DM are at particularly high risk for adverse events after percutaneous coronary intervention (PCI) [ 27 ]. The universally accepted hypothesis for this phenomenon is that hyperglycemia induces endothelial dysfunction and a proinflammatory state that promote the production of growth factors and cytokines, leading to extensive neointimal formation and thus contributing to the progression of restenosis [ 32 , 33 ]. Besides the endothelial cell inflammation hypothesis, researches indicated that endothelial progenitor cells played a significant role in restenosis [ 34 , 35 ]. Balestrieri ML et al [ 36 ] revealed that high glucose concentration decreased the quantity of endothelial progenitor cells via SIRT1 signaling pathway. In addition, the prethrombotic environment of patients with diabetes ultimately increases the risk of restenosis [ 37 ]. However, until now, there has been no conclusive, large-scale clinical evidence to support this view. Our meta-analysis involved 20 RCTs from multiple countries and time spans, with up to 31,066 patients. The results of the meta-analysis confirmed for the first time that DM is a high-risk factor for restenosis after endovascular treatment in a human cohort.
Studies [ 38 ] have shown that the type of glucose-controlling drug can affect postoperative restenosis in diabetic patients after coronary stent or balloon dilation. Their data, especially regarding metformin and thiazolidinediones, indicate beneficial results compared to insulin and sulfonylurea for restenosis. However, no large trials have been undertaken in which the effect of glucose-lowering agents on restenosis is associated with improved outcomes. Indeed, experts believe that maintaining proper glycemic control is crucial for diabetic patients who have undergone revascularization procedures [ 21 ]. In several recent prospective studies, high glycemic levels [ 39 , 40 ] and insulin resistance [ 41 ] have increased restenosis rates after coronary stenting or balloon angioplasty, but these results were based on statistical analysis of clinical phenomena, lacking evidence from high-quality controlled studies. Marfella R et al [ 42 ] conducted a RCT involving 165 patients with high blood glycemic and ST-segment elevation myocardial infarction (STEMI) undergoing PCI treatment, randomly assigning these patients to an interventional-glycemic-control group and an intensive-glycemic-control group. The results showed that the restenosis rate of patients in the intensive-glycemic-control group after PCI reduced by half (48% and 24%) at 6 months. The study confirmed the positive correlation between blood glucose levels and restenosis. However, it solely concentrated on blood glucose levels without addressing whether the patients had diabetes or specific subtypes. Our research indicates that restenosis of the diabetic patients after interventional treatment is not directly related to blood glucose level. This implies that diabetes may pose other risks aside from high blood glucose, such as AGEs and insulin resistance, which could potentially contribute to restenosis. Although Mone P et al [ 43 ] demonstrated the effect of high glucose on the risk of restenosis in STEMI patients without DM, the restenosis of high glucose-DM group (18.5%) is still higher than high glucose non-DM group (14.0%) at one-year follow-up, which indicates that diabetic patients may be influenced by additional pathogenic factors beyond elevated blood glucose levels. In animal studies, it has been demonstrated that hyperglycemia and insulin resistance lead to intimal hyperplasia after vascular injury in rats [ 28 , 29 , 30 , 44 , 45 ], which seems to be of crucial importance in determining exaggerated neointimal hyperplasia after balloon angioplasty in diabetic animals. However, our meta-analysis showed that blood glucose levels in diabetic patients did not affect the incidence of restenosis after endovascular therapy (Fig. 5 ). In animal experiments, hyperglycemia can contribute to neointimal hyperplasia, which was inconsistent with the results of our meta-analysis, suggesting that there may be other important risk factors involved in restenosis in diabetic patients. A prospective observational study involving 377 participants discovered that postoperative restenosis rates differed among patients with type 2 diabetes and acute myocardial infarction (AMI) based on whether they were prescribed oral sodium/glucose cotransporter 2 (SGLT2) inhibitors [ 46 ]. The study confirmed that the administration of SGLT2 inhibitors to type 2 diabetes patients was associated with a reduced frequency of ISR-related events, independent of glycemic control. This research highlights the importance of non-glycemic factors in the reduction of postoperative restenosis among diabetes patients. AGEs and their receptor isoforms seem to have an important contribution to both the pathogenesis and clinical outcome of restenosis. AGEs have been shown to promote carotid intimal regeneration in rats after balloon injury [ 30 ], suggesting that AGEs may act as a stimulus for restenosis. Cristiano Spadaccio et al. found that soluble RAGE (sRAGE) levels and total circulating AGEs were positively correlated with an increased risk of stent restenosis [ 47 , 48 ]. This suggests that AGEs may play a more important role in restenosis than hyperglycemia. Currently, there is a lack of clinical studies on the relationship between restenosis and AGE levels, and further RCT studies may be of great significance.
DAPT has become essential in daily clinical practice. In fact, current practice guidelines recommend aspirin and clopidogrel DAPT for patients suffering from CAD or monotherapy for patients with symptomatic PAD, regardless of clinical background [ 49 ]. However, there is controversy over the duration of antiplatelet therapy in view of the different lesion sites and stent types [ 50 ]. Cristian A Dámazo-Escobedo et al [ 51 ]. confirmed that long-term antiplatelet therapy after coronary stenting would be justified by the high incidence of thrombosis-restenosis through a prospective observational study. However, based on the current European guidelines for management after coronary stent placement, there is no consensus on the ideal duration of DAPT to prevent stent thrombosis-restenosis without a significant increase in bleeding risk. In our meta-analysis, based on the circumstances of antiplatelet therapy included in the study after revascularization, we took 6 months of DAPT as the line of demarcation and found that different durations of antiplatelet therapy had no significant effect on the incidence of restenosis after endovascular therapy. Moreover, anticoagulation therapy after revascularization in PAD is particularly important compared to that after coronary revascularization. The COMPASS and VOYAGER PAD RCTs showed that a low-dose oral anticoagulant combined with aspirin (Rivaroxaban 2.5 mg twice a day; Aspirin 100 mg once a day) improved the long-term patency rate of lower extremity artery disease after endovascular therapy, reduced the incidence of major limb adverse events and cardiovascular events, and did not increase the risk of fatal major bleeding [ 52 , 53 , 54 ]. Regrettably, due to the lack of available anticoagulant therapy regimens in the included studies, we did not perform a subgroup meta-analysis of anticoagulant therapy and restenosis in this study. Anticoagulation combined with antiplatelet therapy may be beneficial in future RCTs.
It is worth mentioning that according to the subgroup analysis, patients with diabetes mellitus had a higher TLR rate, while patients with restenosis detected by angiographic follow-up showed no significant difference. This implies that patients with diabetes may be more prone to symptomatic restenosis and require surgical reintervention, suggesting to clinicians that patients with diabetes may have increased reoperation rates. Therefore, diabetic patients should take this characteristic into full consideration when choosing the type of balloon or stent, such as the choice of a drug-eluting balloon to reduce restenosis. Since this study did not involve comparisons of balloon types or stent types, research in this direction may be of great significance for diabetic patients.
The findings of the meta-analysis involving 31,066 individuals affirm that patients with diabetes mellitus (DM) have a higher risk of restenosis following intravascular treatment. Nevertheless, there are still limitations to this meta-analysis. More detailed baseline characteristics of patients were not obtained from some of the included studies, even after contact these corresponding authors, such as the medical history time of DM, detailed blood glucose levels and hypoglycemic methods (such as diet, exercise or drug therapy), which may be important factors and could affect the analysis of restenosis. Due to the high rank of the GRADE results, we also suggest a cautious interpretation for this meta-analysis, and further high-quality RCTs are needed to improve the current conclusion.
Conclusions
In summary, the findings of this systematic review and meta-analysis provided convincing evidence that patients with DM had an increased risk of primary restenosis after PTA or stenting, suggesting that DM is a high-risk factor for restenosis after endovascular treatment, irrespective of blood glucose level, antiplatelet therapy duration, targeted lesion vessel and continent. In conclusion, our meta-analysis provides a reliable suggestion for the health management of diabetic patients with vascular occlusive disease after endovascular therapy.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
Angiographic binary restenosis
Advanced glycation end products
Bromodeoxyuridine
Coronary artery disease
Confidence interval
Dual antiplatelet therapy
Diabetes mellitus
Follow-up time
Grading of Recommendations Assessment, Development and Evaluation
Hazard ratio
In-stent restenosis
Major adverse cardiac event(s)
Peripheral artery disease
Percutaneous coronary intervention
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Percutaneous transluminal angioplasty
Quantitative angiography-confirmed restenosis
Receptor for advanced glycation end products
Randomized controlled trials
Relative ratio
Sodium/glucose cotransporter 2
Soluble RAGE
ST-segment elevation myocardial infarction
Targeted lesion revascularization
Vascular smooth muscle cells
Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–40. https://doi.org/10.1038/nrcardio.2017.154 .
Article PubMed Google Scholar
Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010 .
Article PubMed PubMed Central Google Scholar
Gori T. Restenosis after coronary stent implantation: cellular mechanisms and potential of endothelial progenitor cells (A Short Guide for the Interventional Cardiologist). Cells. 2022. https://doi.org/10.3390/cells11132094 .
Kereiakes DJ, Kandzari DE, Zidar JP. Drug-coated balloons for in-stent restenosis. J Am Coll Cardiol. 2020;76:1391–2. https://doi.org/10.1016/j.jacc.2020.06.082 .
Article CAS PubMed Google Scholar
Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–725. https://doi.org/10.1161/CIR.0000000000000470 .
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302. https://doi.org/10.1038/nrendo.2014.29 .
Newman JD, Schwartzbard AZ, Weintraub HS, Goldberg IJ, Berger JS. Primary prevention of cardiovascular disease in diabetes mellitus. J Am Coll Cardiol. 2017;70:883–93. https://doi.org/10.1016/j.jacc.2017.07.001 .
Eckel RH, Bornfeldt KE, Goldberg IJ. Cardiovascular disease in diabetes, beyond glucose. Cell Metab. 2021;33:1519–45. https://doi.org/10.1016/j.cmet.2021.07.001 .
Article CAS PubMed PubMed Central Google Scholar
Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018;24:59. https://doi.org/10.1186/s10020-018-0060-3 .
Henry H. Ruiz1, Ravichandran Ramasamy1, Ann Marie Schmidt1. Advanced Glycation End Products: Building on the Concept of the “Common Soil” in Metabolic Disease. Endocrine Society 2019. doi: https://doi.org/10.1210/endocr/bqz006/5602541
Jankauskas SS, Kansakar U, Varzideh F, et al. Heart failure in diabetes. Metabolism. 2021;125: 154910. https://doi.org/10.1016/j.metabol.2021.154910 .
Shu J, Matarese A, Santulli G. Diabetes, body fat, skeletal muscle, and hypertension: the ominous chiasmus? J Clin Hypertens (Greenwich). 2019;21:239–42. https://doi.org/10.1111/jch.13453 .
Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14:453. https://doi.org/10.1007/s11892-013-0453-1 .
Shu J, Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis. 2018;275:379–81. https://doi.org/10.1016/j.atherosclerosis.2018.05.033 .
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700. https://doi.org/10.1136/bmj.b2700 .
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928. https://doi.org/10.1136/bmj.d5928 .
Miyazaki Y, Suwannasom P, Sotomi Y, et al. Single or dual antiplatelet therapy after PCI. Nat Rev Cardiol. 2017;14:294–303. https://doi.org/10.1038/nrcardio.2017.12 .
Mohan J, Yelamanchili VS, Zacharias SK. Acute Coronary Syndrome Catheter Interventions. In. StatPearls. Treasure Island (FL); 2022.
De Luca L, Bonaca MP, Magnani G. Antithrombotic strategies for patients with coronary and lower extremity peripheral artery diseases: a narrative review. Expert Rev Cardiovasc Ther. 2020;18:881–9. https://doi.org/10.1080/14779072.2020.1833719 .
Wang J, Jin X, Huang Y, et al. Endovascular stent-induced alterations in host artery mechanical environments and their roles in stent restenosis and late thrombosis. Regen Biomater. 2018;5:177–87. https://doi.org/10.1093/rb/rby006 .
Wilson S, Mone P, Kansakar U, et al. Diabetes and restenosis. Cardiovasc Diabetol. 2022;21:23. https://doi.org/10.1186/s12933-022-01460-5 .
Balakumar P, Maung UK, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113:600–9. https://doi.org/10.1016/j.phrs.2016.09.040 .
Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40:1808–17. https://doi.org/10.1161/ATVBAHA.120.314595 .
Nestelberger T, Kaiser C, Jeger R. Drug-coated balloons in cardiovascular disease: benefits, challenges, and clinical applications. Expert Opin Drug Deliv. 2020;17:201–11. https://doi.org/10.1080/17425247.2020.1714590 .
Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–8. https://doi.org/10.1161/01.cir.100.18.1872 .
Liu MW, Roubin GS, King SB. Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–87. https://doi.org/10.1161/01.cir.79.6.1374 .
Abizaid A, Kornowski R, Mintz GS, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol. 1998;32:584–9. https://doi.org/10.1016/s0735-1097(98)00286-1 .
Jonas M, Edelman ER, Groothuis A, et al. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res. 2005;97:725–33. https://doi.org/10.1161/01.RES.0000183730.52908.C6 .
Panchatcharam M, Miriyala S, Yang F, et al. Enhanced proliferation and migration of vascular smooth muscle cells in response to vascular injury under hyperglycemic conditions is controlled by beta3 integrin signaling. Int J Biochem Cell Biol. 2010;42:965–74. https://doi.org/10.1016/j.biocel.2010.02.009 .
Zhou Z, Wang K, Penn MS, et al. Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury. Circulation. 2003;107:2238–43. https://doi.org/10.1161/01.CIR.0000063577.32819.23 .
Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2011;9:53–62. https://doi.org/10.1038/nrcardio.2011.132 .
Lee MS, David EM, Makkar RR, Wilentz JR. Molecular and cellular basis of restenosis after percutaneous coronary intervention: the intertwining roles of platelets, leukocytes, and the coagulation-fibrinolysis system. J Pathol. 2004;203:861–70. https://doi.org/10.1002/path.1598 .
Aronson D, Edelman ER. Revascularization for coronary artery disease in diabetes mellitus: angioplasty, stents and coronary artery bypass grafting. Rev Endocr Metab Disord. 2010;11:75–86. https://doi.org/10.1007/s11154-010-9135-3 .
Hibbert B, O’Brien ER. Circulating endothelial progenitor cells and in-stent restenosis: friend, foe, or none of the above? Can J Cardiol. 2014;30:6–7. https://doi.org/10.1016/j.cjca.2013.11.010 .
Park KS, Kang SN, Kim DH, et al. Late endothelial progenitor cell-capture stents with CD146 antibody and nanostructure reduce in-stent restenosis and thrombosis. Acta Biomater. 2020;111:91–101. https://doi.org/10.1016/j.actbio.2020.05.011 .
Balestrieri ML, Servillo L, Esposito A, et al. Poor glycaemic control in type 2 diabetes patients reduces endothelial progenitor cell number by influencing SIRT1 signalling via platelet-activating factor receptor activation. Diabetologia. 2013;56:162–72. https://doi.org/10.1007/s00125-012-2749-0 .
Elezi S, Kastrati A, Pache J, et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol. 1998;32:1866–73. https://doi.org/10.1016/s0735-1097(98)00467-7 .
Lexis CP, Rahel BM, Meeder JG, Zijlstra F, van der Horst IC. The role of glucose lowering agents on restenosis after percutaneous coronary intervention in patients with diabetes mellitus. Cardiovasc Diabetol. 2009;8:41. https://doi.org/10.1186/1475-2840-8-41 .
Liu C, Zhao Q, Zhao Z, et al. Correlation between estimated glucose disposal rate and in-stent restenosis following percutaneous coronary intervention in individuals with non-ST-segment elevation acute coronary syndrome. Front Endocrinol (Lausanne). 2022;13:1033354. https://doi.org/10.3389/fendo.2022.1033354 .
Zhu Y, Liu K, Chen M, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20:137. https://doi.org/10.1186/s12933-021-01332-4 .
Sasso FC, Pafundi PC, Marfella R, et al. Adiponectin and insulin resistance are related to restenosis and overall new PCI in subjects with normal glucose tolerance: the prospective AIRE Study. Cardiovasc Diabetol. 2019;18:24. https://doi.org/10.1186/s12933-019-0826-0 .
Marfella R, Sasso FC, Siniscalchi M, et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention is associated with a lower rate of in-stent restenosis in patients with acute ST-elevation myocardial infarction. J Clin Endocrinol Metab. 2012;97:2862–71. https://doi.org/10.1210/jc.2012-1364 .
Mone P, Gambardella J, Minicucci F, et al. Hyperglycemia Drives Stent Restenosis in STEMI Patients. Diabetes Care. 2021;44:e192–3. https://doi.org/10.2337/dc21-0939 .
Desouza CV, Gerety M, Hamel FG. Neointimal hyperplasia and vascular endothelial growth factor expression are increased in normoglycemic, insulin resistant, obese fatty rats. Atherosclerosis. 2006;184:283–9. https://doi.org/10.1016/j.atherosclerosis.2005.04.015 .
Indolfi C, Torella D, Cavuto L, et al. Effects of balloon injury on neointimal hyperplasia in streptozotocin-induced diabetes and in hyperinsulinemic nondiabetic pancreatic islet-transplanted rats. Circulation. 2001;103:2980–6. https://doi.org/10.1161/01.cir.103.24.2980 .
Marfella R, Sardu C, D’Onofrio N, et al. SGLT-2 inhibitors and in-stent restenosis-related events after acute myocardial infarction: an observational study in patients with type 2 diabetes. BMC Med. 2023;21:71. https://doi.org/10.1186/s12916-023-02781-2 .
Spadaccio C, Patti G, De Marco F, et al. Usefulness of preprocedural levels of advanced glycation end products to predict restenosis in patients with controlled diabetes mellitus undergoing drug-eluting stent implantation for stable angina pectoris (from the Prospective ARMYDA-AGEs Study). Am J Cardiol. 2013;112:21–6. https://doi.org/10.1016/j.amjcard.2013.02.046 .
Basta G, Berti S, Cocci F, et al. Plasma N-epsilon-(carboxymethyl)lysine levels are associated with the extent of vessel injury after coronary arterial stenting. Coron Artery Dis. 2008;19:299–305. https://doi.org/10.1097/MCA.0b013e3282fec058 .
Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–79. https://doi.org/10.1161/CIR.0000000000000471 .
Zhang Y, Zhang X, Dong Q, et al. Duration of dual antiplatelet therapy after implantation of drug-coated balloon. Front Cardiovasc Med. 2021;8: 762391. https://doi.org/10.3389/fcvm.2021.762391 .
Damazo-Escobedo CA, Vieyra-Herrera G, Martinez-Rios MA, Garcia-Navarrete MG, Silva-Ruz C. Coronary stent and prolonged dual antiplatelet therapy. A longitudinal study. Gac Med Mex. 2022;158:216–21. https://doi.org/10.24875/GMM.M22000677 .
Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–29. https://doi.org/10.1016/S0140-6736(17)32409-1 .
Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N Engl J Med. 2020;382:1994–2004. https://doi.org/10.1056/NEJMoa2000052 .
Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71:2306–15. https://doi.org/10.1016/j.jacc.2018.03.008 .
Kerkmeijer LSM, Tijssen RYG, Hofma SH, et al. Three-year clinical outcomes of the absorb bioresorbable vascular scaffold compared to Xience everolimus-eluting stent in routine PCI in patients with diabetes mellitus-AIDA sub-study. Catheter Cardiovasc Interv. 2021;98:713–20. https://doi.org/10.1002/ccd.29329 .
Giannini F, Latib A, Jabbour RJ, et al. Comparison of paclitaxel drug-eluting balloon and paclitaxel-eluting stent in small coronary vessels in diabetic and nondiabetic patients—results from the BELLO (balloon elution and late loss optimization) trial. Cardiovasc Revasc Med. 2017;18:4–9. https://doi.org/10.1016/j.carrev.2016.12.008 .
Konigstein M, Ben-Yehuda O, Smits PC, et al. Outcomes among diabetic patients undergoing percutaneous coronary intervention with contemporary drug-eluting stents: analysis from the BIONICS randomized trial. JACC Cardiovasc Interv. 2018;11:2467–76. https://doi.org/10.1016/j.jcin.2018.09.033 .
Franzone A, Pilgrim T, Heg D, et al. Clinical outcomes according to diabetic status in patients treated with biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents: prespecified subgroup analysis of the BIOSCIENCE trial. Circ Cardiovasc Interv. 2015. https://doi.org/10.1161/CIRCINTERVENTIONS.114.002319 .
Ruiz-Garcia J, Teles R, Rumoroso JR, et al. Comparison between diabetic and non-diabetic patients after successful percutaneous coronary intervention for chronic total occlusions in the drug-eluting stent era. Rev Port Cardiol. 2015;34:263–70. https://doi.org/10.1016/j.repc.2014.10.009 .
Kirtane AJ, Patel R, O’Shaughnessy C, et al. Clinical and angiographic outcomes in diabetics from the ENDEAVOR IV trial: randomized comparison of zotarolimus- and paclitaxel-eluting stents in patients with coronary artery disease. JACC Cardiovasc Interv. 2009;2:967–76. https://doi.org/10.1016/j.jcin.2009.08.008 .
Brener SJ, Mehran R, Dressler O, Cristea E, Stone GW. Diabetes mellitus, myocardial reperfusion, and outcome in patients with acute ST-elevation myocardial infarction treated with primary angioplasty (from HORIZONS AMI). Am J Cardiol. 2012;109:1111–6. https://doi.org/10.1016/j.amjcard.2011.11.046 .
Krankenberg H, Zeller T, Ingwersen M, et al. Self-expanding versus balloon-expandable stents for iliac artery occlusive disease: the randomized ICE trial. JACC Cardiovasc Interv. 2017;10:1694–704. https://doi.org/10.1016/j.jcin.2017.05.015 .
Abreu Filho LM, Forte AA, Sumita MK, Favarato D, Meireles GC. Influence of metal alloy and the profile of coronary stents in patients with multivessel coronary disease. Clinics (Sao Paulo). 2011;66:985–9. https://doi.org/10.1590/s1807-59322011000600011 .
Kufner S, Byrne RA, de Waha A, et al. Sirolimus-eluting versus paclitaxel-eluting stents in diabetic and non-diabetic patients within sirolimus-eluting stent restenosis: results from the ISAR-DESIRE 2 trial. Cardiovasc Revasc Med. 2014;15:69–75. https://doi.org/10.1016/j.carrev.2014.02.001 .
Lenz T, Koch T, Joner M, et al. Ten-year clinical outcomes of biodegradable versus durable polymer new-generation drug-eluting stent in patients with coronary artery disease with and without diabetes mellitus. J Am Heart Assoc. 2021;10: e020165. https://doi.org/10.1161/JAHA.120.020165 .
Koch T, Lenz T, Joner M, et al. Ten-year clinical outcomes of polymer-free versus durable polymer new-generation drug-eluting stent in patients with coronary artery disease with and without diabetes mellitus : results of the intracoronary stenting and angiographic results: test Efficacy of Sirolimus- and Probucol- and Zotarolimus-Eluting Stents (ISAR-TEST 5) trial. Clin Res Cardiol. 2021;110:1586–98. https://doi.org/10.1007/s00392-021-01854-7 .
Moussa I, Leon MB, Baim DS, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004;109:2273–8. https://doi.org/10.1161/01.CIR.0000129767.45513.71 .
Billinger M, Beutler J, Taghetchian KR, et al. Two-year clinical outcome after implantation of sirolimus-eluting and paclitaxel-eluting stents in diabetic patients. Eur Heart J. 2008;29:718–25. https://doi.org/10.1093/eurheartj/ehn021 .
Maeng M, Jensen LO, Tilsted HH, et al. Outcome of sirolimus-eluting versus zotarolimus-eluting coronary stent implantation in patients with and without diabetes mellitus (a SORT OUT III Substudy). Am J Cardiol. 2011;108:1232–7. https://doi.org/10.1016/j.amjcard.2011.06.037 .
Jensen LO, Thayssen P, Junker A, et al. Comparison of outcomes in patients with versus without diabetes mellitus after revascularization with everolimus- and sirolimus-eluting stents (from the SORT OUT IV trial). Am J Cardiol. 2012;110:1585–91. https://doi.org/10.1016/j.amjcard.2012.07.022 .
Gyldenkerne C, Olesen KKW, Jensen LO, et al. Everolimus-eluting versus biolimus-eluting coronary stent implantation in patients with and without diabetes mellitus. Am J Cardiol. 2019;124:671–7. https://doi.org/10.1016/j.amjcard.2019.05.060 .
Hermiller JB, Raizner A, Cannon L, et al. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: the TAXUS-IV trial. J Am Coll Cardiol. 2005;45:1172–9. https://doi.org/10.1016/j.jacc.2004.10.075 .
Yee KM, Buller CE, Catellier D, et al. Effect of bare metal stenting on angiographic and clinical outcomes in diabetic and nondiabetic patients undergoing percutaneous coronary intervention of nonacute occluded coronary arteries: a report from the Total Occlusion Study of Canada (TOSCA). Catheter Cardiovasc Interv. 2005;66:178–84. https://doi.org/10.1002/ccd.20410 .
Jang S-J, Park D-W, Kim W-J, et al. Differential long-term outcomes of zotarolimus-eluting stents compared with sirolimus-eluting and paclitaxel-eluting stents in diabetic and nondiabetic patients: two-year subgroup analysis of the ZEST randomized trial. Catheter Cardiovasc Interv. 2013;81:1106–14. https://doi.org/10.1002/ccd.24603 .
Download references
Acknowledgements
This study was supported by the International Science and Technology Innovation Cooperation Project of Sichuan Province (22GJHZ0278), the Sichuan Science and Technology Program (2022YFS0614; 2022YFS0617), the Medical Research Project of Sichuan Province (S21020), the Science and Technology Strategic Cooperation Project of Luzhou Municipal People's Government and Southwest Medical University (2021LZXNYD-D10; 23YKDCXY0003), and the Doctoral Research Initiation Program of the Affiliated Hospital of Southwest Medical University (19041).
Author information
Xiaolei Sun, Cheng Zhang and Yarong Ma contributed equally to this study.
Authors and Affiliations
Department of General Surgery (Vascular Surgery), Affiliated Hospital of Southwest Medical University, Luzhou, 646000, China
Xiaolei Sun & Yanzheng He
Department of Interventional Medicine, Affiliated Hospital of Southwest Medical University, Luzhou, 646000, China
Xiaolei Sun
Department of Pharmacology, Basic Medicine Research Innovation Center for Cardiometabolic Diseases, Ministry of Education, and Laboratory for Cardiovascular Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, 646000, China
Laboratory of Nucleic Acids in Medicine for National High-Level Talents, Nucleic Acid Medicine of Luzhou Key Laboratory, Southwest Medical University, Luzhou, 646000, China
Key Laboratory of Medical Electrophysiology, Ministry of Education and Medical Electrophysiological Key Laboratory of Sichuan Province, Collaborative Innovation Center for Prevention and Treatment of Cardiovascular Disease of Sichuan Province, Institute of Cardiovascular Research, Southwest Medical University, Luzhou, 646000, China
Xiaolei Sun & Jianbo Wu
Cardiovascular and Metabolic Diseases Key Laboratory of Luzhou, Luzhou, 646000, China
Department of Ophthalmology, Affiliated Hospital of Southwest Medical University, Luzhou, 646000, China
Chongqing Clinical Research Center for Reproductive Medicine, Center for Reproductive Medicine, Women and Children’s Hospital of Chongqing Medical University, Chongqing, China
Xiaodong Zhang
School of Cardiovascular Medicine and Sciences, Faculty of Life Science and Medicine, King’s College London British Heart Foundation Centre of Research Excellence, King’s College London, London, SE5 9NU, UK
Department of General Surgery, Center of Vascular and Interventional Surgery, The Third People’s Hospital of Chengdu, The Affiliated Hospital of Southwest Jiaotong University &The Second Affiliated Hospital of Chengdu, Chongqing Medical University, Chengdu, 610031, China
Cheng Zhang
You can also search for this author in PubMed Google Scholar
Contributions
X.S. conceptualized and designed this study. J.W., X.Z., and X.S. revised and edited the manuscript. Y.M. and C.Z. collected and analyzed the data. C.Z. and X.S. prepared the draft of the manuscript. J.W. and Y.H. reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Correspondence to Xiaolei Sun , Xiaodong Zhang or Jianbo Wu .
Ethics declarations
Competing interests.
The authors have no conflicts of interest to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sun, X., Zhang, C., Ma, Y. et al. Association between diabetes mellitus and primary restenosis following endovascular treatment: a comprehensive meta-analysis of randomized controlled trials. Cardiovasc Diabetol 23 , 132 (2024). https://doi.org/10.1186/s12933-024-02201-6
Download citation
Received : 28 January 2024
Accepted : 14 March 2024
Published : 22 April 2024
DOI : https://doi.org/10.1186/s12933-024-02201-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Cardiovascular Diabetology
ISSN: 1475-2840
- Submission enquiries: [email protected]
- Diabetes & Primary Care
- Vol:23 | No:04
Interactive case study: Hypoglycaemia and type 2 diabetes
- 12 Aug 2021
Share this article + Add to reading list – Remove from reading list ↓ Download pdf

Diabetes & Primary Care ’s series of interactive case studies is aimed at GPs, practice nurses and other professionals in primary and community care who would like to broaden their understanding of type 2 diabetes.
The four mini-case studies created for this issue of the journal cover various aspects relating to hypoglycaemia and type 2 diabetes.
The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Working through the case studies will improve your knowledge and problem-solving skills in type 2 diabetes by encouraging you to make evidence-based decisions in the context of individual cases.
You are invited to respond to the questions by typing in your answers. In this way, you are actively involved in the learning process, which is hopefully a much more effective way to learn. By actively engaging with these case histories, I hope you will feel more confident and empowered to manage such presentations effectively in the future.
Active, 76-year-old Jean, who has type 2 diabetes, has experienced dizziness, confusion and speech slurring after gardening for several hours. A capillary blood glucose reading of 2.3 mmol/L was found.
How would you respond to her episode?
John, a 49-year-old HGV driver, uses metformin, gliclazide and alogliptin for his type 2 diabetes. Occasionally, he experiences mild symptoms of hypoglycaemia.
Would you make any changes to his medication?
Chinua has had type 2 diabetes for 13 years. He has recently switched from a basal insulin to a twice-daily premixed insulin. He has heard that his risk of experiencing hypoglycaemia may be higher.
What symptoms should Chinua be looking out for?
65-year-old Candice has collapsed at her type 2 diabetes review. Her capillary glucose reading is 1.7 mmol/L.
How can you manage this episode of severe hypoglycaemia?
By working through these interactive cases, you will consider the following issues and more:
- What constitutes hypoglycaemia.
- Its causes and risk factors in type 2 diabetes.
- Practical advice on its detection and management.
- Strategies for minimising the risk.
Click here to access new interactive case studies
How to diagnose and treat hypertension in adults with type 2 diabetes
Diabetes distilled: statin heart benefits outweigh diabetes risks, interactive case study: non-diabetic hyperglycaemia – prediabetes, diabetes distilled: smoking cessation cuts excess mortality rates after as little as 3 years, impact of freestyle libre 2 on diabetes distress and glycaemic control in people on twice-daily pre-mixed insulin, updated guidance from the pcds and abcd: managing the national glp-1 ra shortage, diabetes distilled: fib-4 – a diagnostic and prognostic marker for liver and cardiovascular events and mortality.

Diagnosing and treating hypertension in accordance with updated NICE guidelines.
24 Apr 2024
Quantifying the risk of worsening glycaemia, and how should healthcare professionals respond?
22 Apr 2024

Diagnosing and managing non-diabetic hyperglycaemia.
17 Apr 2024

The mortality benefits of smoking cessation may be greater and accrue more rapidly than previously understood.
Sign up to all DiabetesontheNet journals
- CPD Learning
- Journal of Diabetes Nursing
- Diabetes Care for Children & Young People
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 29 April 2024
SGLT2i impact on HCC incidence in patients with fatty liver disease and diabetes: a nation-wide cohort study in South Korea
- Hyo Jung Cho 1 ,
- Eunyoung Lee 2 ,
- Soon Sun Kim 1 &
- Jae Youn Cheong 1
Scientific Reports volume 14 , Article number: 9761 ( 2024 ) Cite this article
1 Altmetric
Metrics details
- Drug discovery
- Endocrinology
- Gastroenterology
This study evaluated the effect of sodium-glucose cotransporter-2 inhibitors (SGLT2i) on cancer development, particularly in hepatocellular carcinoma (HCC), in individuals with concomitant fatty liver disease (FLD) and type 2 diabetes mellitus (T2DM). Using data from Korea's Health Insurance Review and Assessment Service, we performed Kaplan–Meier and Cox regression analyses in patients with non-alcoholic fatty liver disease (NAFLD) and T2DM (NAFLD-T2DM cohort) and those with chronic viral hepatitis (CVH) alongside FLD and T2DM (FLD-T2DM-CVH cohort). In the propensity score (PS) matched NAFLD-T2DM cohort (N = 107,972), SGLT2i use was not associated with the occurrence of overall cancer, including HCC. However, old age, male sex, liver cirrhosis, and hypothyroidism were identified as independent risk factors for HCC occurrence, whereas statin and fibrate usage were associated with reduced HCC risk in this cohort in multivariate Cox analysis. In the PS-matched FLD-T2DM-CVH cohort (N = 2798), a significant decrease in HCC occurrence was observed among SGLT2i users ( P = 0.03). This finding remained consistent in the multivariate Cox regression analysis (Hazard ratio = 2.21, 95% confidence interval = 1.01–4.85, P = 0.048). In conclusion, SGLT2i may be a beneficial option for diabetes management in patients with concomitant T2DM, FLD, and CVH while affirming the overall safety of SGLT2i in other types of cancer.
Similar content being viewed by others
Efficacy of sodium glucose cotransporter 2 inhibitors on hepatic fibrosis and steatosis in non-alcoholic fatty liver disease: an updated systematic review and meta-analysis
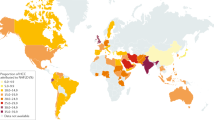
Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention

Association of statin treatment with hepatocellular carcinoma risk in end-stage kidney disease patients with chronic viral hepatitis
Introduction.
Fatty liver disease (FLD), which is characterized by the accumulation of fat in the liver, is a prevalent liver disorder with significant global impact 1 , 2 . This condition is a hepatic manifestation of metabolic syndrome and is closely linked to insulin resistance and type 2 diabetes mellitus (T2DM) 2 , 3 , 4 . Individuals with diabetes are at a higher risk of developing FLD, and diabetes increases the risk of progression to more severe liver diseases, such as liver cirrhosis or hepatocellular carcinoma (HCC) 5 , 6 . Various studies have indicated that patients with concurrent FLD and T2DM are significantly more likely to develop HCC 6 , 7 , 8 . With the incidence of FLD and T2DM increasing worldwide, managing the risk of progression to HCC in these patient populations is becoming a critical concern.
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) are a class of drugs primarily used to treat T2DM 9 . Beyond their role in lowering blood glucose levels, emerging research suggests SGLT2i may offer additional benefits in liver diseases, including non-alcoholic fatty liver disease (NAFLD) and HCC 10 , 11 , 12 . Pre-clinical studies have shown that SGLT2i can decrease hepatic steatosis, enhance insulin sensitivity, and reduce liver inflammation and fibrosis 13 , 14 . Several clinical studies have shown that the use of SGLT2i in patients with NAFLD improves liver function and serum markers of liver injury 11 , 15 . Additionally, the use of SGLT2i in T2DM patients has been observed to reduce the risk of HCC development 16 . However, the relationship between SGLT2i and other cancer types has yielded mixed outcomes; while some studies report a reduced risk of cancer, such as lung and gastrointestinal cancers, others have raised concerns over increased risks of bladder cancer 17 . Given the relatively recent introduction of SGLT2i in the market, there is a critical need for further research involving long-term follow-up and the use of clinical big data to more thoroughly investigate the cancer incidence associated with SGLT2i use.
In healthcare research, the use of big data has become increasingly vital, particularly for identifying trends, patterns, and correlations within vast datasets 18 . Our study used data from the Health Insurance Review and Assessment Service (HIRA) of Korea, which is an extensive database encompassing a wide array of patient information 19 . The use of such big datasets offers a unique opportunity to conduct comprehensive and detailed analyses of large populations. This approach enabled us to observe real-world outcomes, overcome the limitations of small sample sizes, and enhance the generalizability of our findings.
Our study aimed to evaluate the impact of SGLT2i on cancer development, with a specific focus on HCC, in patients with co-existing FLD and T2DM, using a nationwide Korean cohort from the HIRA. We conducted our analysis on two distinct subpopulations of patients coexisting with FLD and T2DM. The first cohort included patients with T2DM and NAFLD, after excluding those with other chronic liver diseases, such as chronic viral hepatitis (CVH), alcoholic liver disease, and autoimmune liver disease from patients with FLD and T2DM. The second cohort comprised high-risk individuals with HCC who were diagnosed with CVH among patients with FLD and T2DM. In addition to assessing the impact of SGLT2i on HCC incidence, we also examined various demographic and clinical factors to identify independent risk factors for HCC in these patient groups, leveraging an extensive dataset to provide insights into effective HCC risk management strategies.
Baseline characteristics and incidence rate of cancers in the NAFLD-T2DM cohort
We identified 201,542 patients with co-existing NAFLD and T2DM. Of these patients, 55,770 (27.7%) were in the SGLT2i group and 145,772 (72.3%) were in the non-SGLT2i group. The median [interquartile range (IQR)] of the follow-up time was 3.56 (2.17–5.10) years for all, 3.01 (1.94–4.52) years for SLGT2i group, and 3.77 (2.30–5.34) years for Non-SGLT2i group. This selection was conducted after excluding patients diagnosed with chronic liver diseases including CVH, alcoholic liver disease, and autoimmune liver disease. After 1:1 PS matching, a balanced cohort of 107,972 patients was established for analysis and evenly divided into 53,986 patients (50.0%) in the SGLT2i group and 53,986 patients (50.0%) in the non-SGLT2i group (Fig. 1 ). In PS-matched cohort, the median (IQR) of the follow-up time was 3.04 (1.94–4.55) years for all, 3.05 (1.95–4.56) years for SLGT2i group, and 3.03 (1.93–4.54) years for Non-SGLT2i group. There was no significant difference in follow-up period between the two groups ( P = 0.445).
Schematic representation of cohort derivation for this study. T2DM type 2 diabetes mellitus, NAFLD non-alcoholic fatty liver disease, FLD fatty liver disease, CVH chronic viral hepatitis, SGLT2i sodium-glucose cotransporter-2 inhibitor, PSM propensity score matching.
Supplementary Table 1 and Figure S1 A (Love plot) confirm the successful adjustment of covariate differences between groups following PS matching. In this cohort, PS matching effectively standardized the mean differences, with all variables achieving an aSMD of less than 0.1, demonstrating excellent balance across covariates. These results underscore the robustness of the matching process and comparability of the groups for subsequent analyses. Table 1 illustrates comprehensive patient characteristics before and after PS matching.
Table 2 displays the number of cancer cases, person-years, and IR per 10,000 person-years [95% CI] for each type of cancer according to SGLT2i exposure status in the NAFLD-T2DM cohort both before and after PS matching. Figure 3 A shows a forest plot of the HRs for each cancer type. In the pre-matching analysis, non-SGLT2i users exhibited significant HRs for the occurrence of “total cancer”, HCC, CCC, stomach cancer, colorectal cancer, pancreatic cancer, lung cancer, prostate cancer, and “other cancers”. However, after PS matching, the statistically significant differences in cancer risk between the two groups disappeared.
Survival analysis of HCC and other cancers in the PS-matched NAFLD-T2DM cohort according to SGLT2i usage
Figure 2 A shows the Kaplan–Meier curves comparing the probability of HCC incidence between SGLT2i users and non-SGLT2i users within the PS-matched NAFLD-T2DM cohort. No significant differences were observed not only in HCC development but also in other types of cancer, indicating that SGLT2i usage does not statistically influence cancer incidence in this cohort (Fig. S1 ).
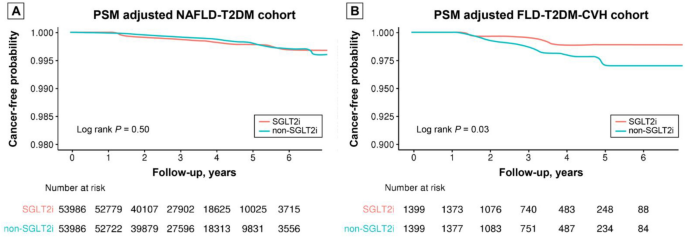
Comparison of Kaplan–Meier curves of HCC occurrence according to SGLT2i exposure in PS-matched NAFLD-T2DM cohort and PS-matched FLD-T2DM-CVH cohort. HCC hepatocellular carcinoma, SGLT2i sodium-glucose cotransporter-2 inhibitor, PS propensity score, NAFLD non-alcoholic fatty liver disease, T2DM type 2 diabetes mellitus, FLD fatty liver disease, CVH chronic viral hepatitis.
Subsequently, Cox proportional hazards analysis was conducted to identify the independent variables affecting HCC occurrence in this cohort (Table 3 ). In the univariate Cox regression analysis, older age; male sex; comorbidities such as hypertension, hypothyroidism, and liver cirrhosis; and the use of aspirin, beta-blockers, calcium channel blockers, and fibrates were identified as significant risk factors for HCC occurrence. In the multivariate Cox regression analysis, which included variables with a P value < 0.1 and SGLT2i used from the univariate analysis, older age (HR = 1.08, 95% CI = 1.06–1.10, P < 0.001), male sex (HR = 2.79, 95% CI = 1.87–4.14, P < 0.001), hypothyroidism (HR = 2.43, 95% CI = 1.21–4.87, P = 0.013), liver cirrhosis (HR = 17.88, 95% CI = 8.19–39.03, P < 0.001), statin use (HR = 0.59, 95% CI = 0.36–0.96, P = 0.035), and fibrate use (HR = 0.14, 95% CI = 0.02–0.99, P = 0.049) were identified as independent risk factors for HCC occurrence. The Concordance index of this model was 0.805, with a standard error (SE) of 0.029.
Baseline characteristics and incidence rate of cancers in the FLD-T2DM-CVH cohort
In this subset, 4936 patients with CVH along with co-existing NAFLD and T2DM were identified. Among them, 1440 (29.2%) were categorized into the SGLT2i group and 3,496 (70.8%) into the non-SGLT2i group. The median (IQR) of the follow-up period was 3.50 (2.18–4.95) years for all, 3.06 (2.04–4.46) years for SLGT2i group, and 3.67 (2.25–5.17) years for Non-SGLT2i group. Following 1:1 PS matching, an eligible cohort for analysis was formed, consisting of patients with an equal distribution of 1,399 patients (50.0%) in both the SGLT2i and non-SGLT2i groups (Fig. 1 ). In the PS-matched FLD-T2DM-CVH cohort, the median [IQR] of the follow-up time was 3.13 (2.07–4.48) years for all, 3.07 (2.03–4.48) years for SLGT2i group, and 3.19 (2.10–4.48) years for non-SGLT2i group. There was no significant difference in follow-up period between the two groups ( P = 0.529).
Supplementary Table 2 and Fig. S1 B (Love plot) confirm the successful adjustment of covariate differences between groups following PS matching. Significant discrepancies between groups were noted before PS matching; however, PS matching effectively standardized the mean differences in the PS-matched cohort, with all variables achieving an aSMD of less than 0.1, demonstrating excellent balance across covariates. These results underscore the robustness of the matching process and comparability of the groups for subsequent analyses. Table 4 illustrates comprehensive patient characteristics before and after PS matching.
Table 5 shows the number of cancer cases, person-years, and IR per 10,000 person-years for each cancer type according to SGLT2i exposure in the FLD-T2DM-CVH cohort before and after PS matching. Notably, the crude IR per 10,000 person-years of HCC was significantly higher in the FLD-T2DM-CVH cohort (IR per 10,000 person-years: 57.3, 95% CI 18.4–71.6) compared to the NAFLD-T2DM cohort (IR per 10,000 person-years: 5.2, 95% CI 3.6–5.7). Interestingly, in both pre-and post-PS matching, the IR per 10,000 person-years of HCC was markedly higher in the non-SGLT2i group (Pre-PS matching: 18.4 vs 71.6, and post-PS matching: 18.8 vs 41.7, for SGLT2i users and non-SGLT2i users, respectively).
While the IR per 10,000 person-years for HCC increased by more than tenfold in the FLD-T2DM-CVH cohort, the number of cases of other cancer types decreased as the cohort size diminished. As shown in Table 5 , for several cancer types, the number of cases was less than 10. Due to concerns such as lack of statistical power, risk of overestimation, adherence to the Events Per Variable rule, and model fit issues, HRs could not be calculated for these types of cancer. We could analyze HR of HCC, “total cancer”, and “other cancer” in both pre- and post-PM matched cohorts. The risk of HCC occurrence in non-SGLT2i users was significantly higher in both cohorts; before matching [crude HR = 3.58 (1.80–7.09)] and in the PS-matched cohort [adjusted HR = 2.32 (1.06–5.06)]. The risk of “total cancer” showed significant HR in the pre-matched cohort; however, this significance disappeared in the post-PS-matched cohort (Fig. 3 B).
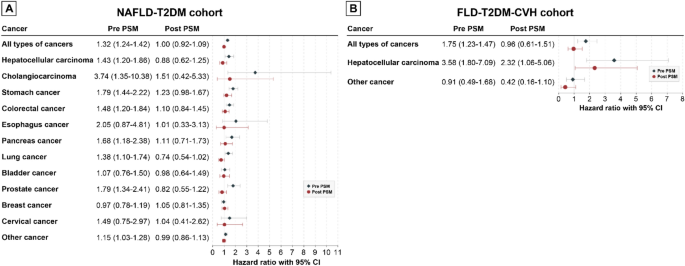
Forrest plots of the hazard ratio of each cancer according to SGLT2i usage in the NAFLD-T2DM cohort and FLD-T2DM-CVH cohort. ( A ) NAFLD-T2DM cohort. ( B ) FLD-T2DM-CVH cohort. SGLT2i sodium-glucose cotransporter-2 inhibitor, PSM propensity score matching, NAFLD non-alcoholic fatty liver disease, T2DM type 2 diabetes mellitus, FLD fatty liver disease, CVH chronic viral hepatitis.
Survival analysis of HCC and other cancers in the PS matched FLD-T2DM-CVH cohort according to SGLT2i usage
Figure 2 b displays the Kaplan–Meier curves comparing HCC occurrence between SGLT2i and non-SGLT2i users within the PS-matched FLD-T2DM-CVH cohort. SGLT2i users had a significantly lower risk of developing HCC ( P = 0.03). There were no significant differences in the occurrence of “total cancers” and “other cancers” between the two groups (Fig. S3 ).
Subsequently, Cox proportional hazards analysis was conducted in the PS-matched FLD-T2DM-CVH cohort (Table 6 ). In the univariate Cox regression analysis, older age and comorbidities such as dyslipidemia, heart failure, and liver cirrhosis, as well as the use of SGLT2i, statins, and antiviral treatment, were significantly associated with the occurrence of HCC. To adjust for covariates, we performed a multivariate Cox regression analysis by entering variables with a P value < 0.1 from the univariate analysis. Sex was also included in the multivariate analysis, although it was not a significant factor in the univariate analysis. This is because it is considered a basic variable for adjustment. In multivariate analysis, SGLT2i usage [HR = 2.22 (1.01–4.87), P = 0.047] was identified as an independent risk factor of HCC occurrence along with older age [HR = 1.07 (1.03–1.10), P < 0.001], male sex [HR = 2.23 (1.00–5.26), P = 0.049], and liver cirrhosis [HR = 7.33 (3.31–16.21), P < 0.001]. The C-index of this model was 0.882 with an SE of 0.056.
This study undertook a comprehensive analysis using large-scale healthcare data to investigate the influence of SGLT2i on cancer development, with emphasis on HCC, in a cohort with co-existing FLD and T2DM. By leveraging high-quality data from the HIRA Service of Korea. This study enriches the field with valuable insights into practical implications and outcomes in the clinical setting. Our findings in the NAFLD-T2DM cohort indicated no significant differences in the incidence of HCC and other types of cancers based on SGLT2i use. However, in the HCC high-risk group of patients, the FLD-T2DM-CVH cohort, the use of SGLT2i was significantly associated with a lower incidence of HCC, even after PS matching and multivariate Cox analysis, highlighting its potential protective effect in this particular subgroup.
A previous systematic review and meta-analysis investigating the association between SGLT2i and cancer risk in T2DM patients found no significant increase in the overall cancer risk, consistent with our findings in the NAFLD-T2DM cohort 17 . This prior research, encompassing 46 randomized controlled trials, indicated an increased risk of bladder cancer with SGLT2 inhibitor use but suggested a potential protective effect against gastrointestinal cancers. However, the authors state that further long-term studies are recommended owing to the short-term nature of the trials included in the study. In our study on patients with FLD and T2DM, the use of SGLT2i was not associated with an increased risk of bladder cancer, and the potential protective effect against gastrointestinal cancer was not statistically significant. Chou et al. 20 reported a protective effect of SGLT2i against HCC compared to dipeptidyl peptidase-4 inhibitors in T2DM patients using data from Hong Kong's National Health Care System. In our study, using data from the Korean HIRA Service, we initially observed a trend towards lower crude IRs of HCC and other cancer types among SGLT2i users within the NAFLD-T2DM cohort. In addition, a significant increase in the HRs of various types of cancers, including HCC, was observed in non-SGLT2i users before matching. However, this trend did not reach statistical significance after PS matching, which was adjusted for discrepancies in person-years attributable to the relatively recent introduction of SGLT2i compared to other oral hypoglycemic agents (OHA). This may be due to differences in the observed person-years between SGLT2i users and non-users. Specifically, SGLT2i users demonstrated relatively shorter person-years than non-users, resulting in an apparent increase in the IR of various cancers in the SGLT2i user group before matching.
While SGLT2i did not demonstrate a statistically significant association with HCC incidence in patients with NAFLD and T2DM, multivariate Cox analysis identified several factors associated with increased HCC risk in this population. These included older age, male sex, presence of hypothyroidism, and liver cirrhosis. Furthermore, the use of statins and fibrates has been associated with a lower incidence of HCC. This observation aligns with the existing research, underscoring the potential protective effects of statins and fibrates against HCC. Previous research has demonstrated that statins may confer a protective benefit in the chemoprevention and treatment of several cancers, including HCC 21 , 22 , 23 , 24 . Recently, Zou et al. 25 suggested an association between statin and reduced risk of HCC development in NAFLD patients by using the Optum de-identified Clinformatics database. Additionally, a large-scale case–control study in Taiwan revealed a significant inverse association between fibrate use and the incidence of liver cancer 26 . The study demonstrated that fibrate use was associated with significantly lower odds of liver cancer in a dose-dependent manner, indicating a protective effect of fibrates against liver cancer. While our study contributes to the understanding of SGLT2i's role in various types of cancer risk, particularly in a specific cohort of patients with NAFLD and T2DM, it also highlights the importance of considering the protective effects of other medications, such as statins and fibrates, in managing HCC risk in this cohort.
In our FLD-T2DM-CVH cohort, we noted a notably higher crude incidence rate of HCC compared to the NAFLD-T2DM cohort. This difference is attributed not only to viral infection but also to variations in HCC screening strategies for both cohorts. CVH is a well-known risk factor for HCC, and it is recommended by various expert groups that patients with CVH should undergo biannual HCC screening 27 , 28 , 29 . On the other hand, in patients without CVH or liver cirrhosis, regular HCC screening is not recommended. Considering the significant differences in HCC risk and HCC screening strategies based on CVH status, we conducted separate analyses for patients with CVH and those NAFLD patients without CVH to minimize potential biases. Interestingly, within the CVH cohort with higher HCC risk, we noted a pronounced protective effect of SGLT2i against development of HCC. This finding is in line with the concepts of risk difference effect and relative risk reduction, suggesting that therapeutic interventions might offer greater absolute benefits in populations at a higher baseline risk 30 , 31 . The underlying theory suggests that individuals at an elevated risk of a condition may gain more from interventions due to their higher initial risk, potentially preventing a greater number of adverse outcomes 31 . Despite the limitations of our study design and dataset which prevent a detailed statistical analysis to fully quantify this effect, the observed trend highlights the importance of considering baseline risk when evaluating treatment outcomes. This insight is particularly pertinent for clinicians seeking to optimize therapeutic strategies for patients with diverse risk profiles, emphasizing the need for tailored approaches based on individual patient risk factors. Further research is needed to explore this differential effect more comprehensively, possibly by incorporating more detailed data on baseline risk and utilizing statistical methods to assess the interaction effects between treatment efficacy and specific risk factors for HCC in patients. This finding aligns with a territory-wide cohort study conducted in Hong Kong, which reported that SGLT2i use was associated with a lower risk of HCC development in patients with co-existing T2DM and chronic hepatitis B infection 32 . These results suggest the potential protective effects of SGLT2 inhibitors against HCC development in high-risk patients, reinforcing the importance of targeted therapeutic strategies for managing HCC risk in patients with diabetes and chronic viral hepatitis.
The strength of our study lies in its large sample size and utilization of a national database, enabling a robust statistical approach and enhancing the generalizability of our findings. Nevertheless, we acknowledge the presence of inherent limitations, notably the study's retrospective and observational nature, which could introduce biases and the potential for residual confounding factors that might not be fully eliminated through statistical adjustments. In addition to, critical individual patient variables, such as height, weight, and blood glucose levels, which can significantly influence the outcomes, were not directly measured in our study. To mitigate these constraints, we incorporated several variables capable of indirectly representing the baseline health status of patients, including diagnoses related to obesity and the intensity of glycemic control treatments. Notably, in the Korean healthcare system, the prescription of OHAs and insulin is determined by initial HbA1c levels, offering a surrogate marker for assessing patients' baseline glycemic control. This methodology, while not directly measuring each variable, provides a practical and indirect assessment of patients' health conditions that could address, at least partially, some of the limitations mentioned. Furthermore, the potential underdiagnosis of early-stage HCC among non-cirrhotic patients without CVH presents an additional limitation. Our reliance on ICD-10 codes for identifying FLD, T2DM, and any cancers might not capture all instances of early-stage HCC, especially given the lack of established recommendations for HCC screening in non-cirrhotic patients. To address this concern, we employed a wash-out period strategy, however, we recognize that this measure cannot fully overcome the challenges associated with underdiagnosis of early-stage HCC. It indicates the need for future studies to develop more precise diagnostic criteria and screening protocols for this patient population.
In conclusion, within the NAFLD-T2DM cohort, SGLT2i did not demonstrate a statistically significant effect in reducing the risk of developing HCC. In contrast, our analysis within the FLD-T2DM-CVH cohort indicates a significant association between SGLT2i use and a decreased risk of HCC, highlighting their potential as a preventive strategy in patients with a higher risk profile of HCC. Nevertheless, it is important to recognize that our study is based on retrospective cohort data, underscoring the need for future research through prospective cohort studies to further validate these findings.
Data source
We used a dataset from the HIRA database of the Republic of Korea between January 1, 2014, and December 31, 2021. The dataset contained comprehensive information from both inpatient and outpatient medical claims, including details such as prescription drug utilization, diagnostic and treatment codes, and primary and secondary diagnosis codes.
Study design
This study was designed as a comparative cohort study to evaluate the implications of SGLT2 inhibitor prescription on HCC incidence in patients diagnosed with FLD and T2DM. Figure 1 shows the flowchart of this study. Data were extracted from eligible patients. The eligibility criteria for the study were as follows: (1) patients diagnosed with co-existing FLD and T2DM, and (2) patients receiving treatment with one to three types of OHA. Patients diagnosed with FLD or T2DM were identified based on medical diagnoses according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Individuals who met the following criteria were excluded: those diagnosed with any malignancy or those who underwent liver transplantation before cohort entry or within the year after cohort entry, considering the lag period to eliminate the possibility of detection of already existing cancers. Patients with cohort entry day in 2014 were excluded because they did not meet the criteria for assessing baseline characteristics in the year prior to cohort entry. Patients with a cohort entry day of 2021 were also excluded due to the lack of a minimum one-year follow-up period to evaluate cancer development. Patients who had a history of any OHA or insulin prescription within 1 year before cohort entry were also excluded.
Patients treated with SGLT2i for more than 90 days since cohort entry were categorized into SGLT2i users, while those who never used SGLT2i during 2014–2021 were categorized into the comparative group non-SGLT2i users. The index date was defined as the cohort entry day, which was set as the first date of SGLT2i or other OHA prescriptions. In South Korea, OHA is prescribed according to the insurance coverage criteria of the National Health Insurance Service. The insurance coverage criteria were based on the patient's glycemic control status, as represented by hemoglobin A1c(HbA1c) 33 . Thus, the number of prescribed OHA or insulin use was closely related to the glycemic control status in each patient. Therefore, the use of multiple OHA or insulin suggests that patients with diabetes require more intensive treatment to achieve adequate glycemic control. Furthermore, we classified the patients according to the number of prescribed OHA and insulin usage during the 90 days after cohort entry to reflect the glycemic control level at the time of cohort entry; level 1—one or two OHA had been taken, Level 2—three classes of OHA had been taken without insulin, and level 3—administration of insulin in combination with other OHA. The index year, age at cohort entry, sex, level of antidiabetic treatment 90 days after cohort entry, comorbidities, Charlson Comorbidity Index (CCI), and prescribed drugs during the year prior to cohort entry were analyzed as baseline characteristics.
Cohort definition
We analyzed two distinct patient cohorts with concurrent FLD and T2DM. The patients presenting with both FLD and T2DM, who also have CVH, are categorized into a higher risk group for HCC, necessitating bi-annual HCC screenings for this population. Conversely, T2DM-NAFLD patients without CVH or liver cirrhosis are not classified as being at high risk for HCC, and thus, regular HCC screenings using ultrasound are not routinely recommended for them. To address the disparities in risk and screening frequencies between patients with CVH and those with only NAFLD, we conducted separate analyses for these groups to mitigate any biases arising from these differences. The first, termed the NAFLD-T2DM cohort, was identified by excluding patients with other causes of chronic liver diseases at baseline, such as CVH, alcoholic liver disease, and autoimmune liver disease including primary biliary cholangitis and autoimmune hepatitis, aligning with the definition of NAFLD. The second cohort, the FLD-T2DM-CVH cohort, included patients diagnosed with CVH in addition to concurrent FLD and T2DM. CVH, alcoholic liver disease, primary biliary cholangitis, and autoimmune hepatitis were diagnosed based on the presence of these diagnoses in medical records during the year prior to cohort entry. Additionally, patients were considered to have received antiviral treatment if they had been prescribed antiviral agents for hepatitis B or C within the year prior to cohort entry.
The primary outcome of the present study was a diagnosis of any malignancy, which was indicated by the C code in the ICD-10, and registration of catastrophic illness coverage in the national health insurance system for the corresponding malignancies. All eligible patients were followed up from the index date until the occurrence of the primary outcome or the study end date (31st December 2021), whichever occurred first. In this study, we evaluated the occurrence of a spectrum of cancer types: HCC, Cholangiocarcinoma (CCC), and various gastrointestinal cancers (stomach, colorectal, esophageal, and pancreatic), along with lung, bladder, prostate, breast, and cervical cancers. We also included a category termed “other cancers” to encompass less common or unspecified cancer sites. Furthermore, we assessed the combined incidence rate of these malignancies, referred to as “total cancer” incidence, to provide an aggregate measure of cancer diagnoses in our study.
Statistical analyses
To thoroughly evaluate the baseline characteristics across differing groups in our study, we meticulously applied descriptive statistical techniques. These techniques were used to analyze a wide array of baseline covariates, including age, sex, the intensity of antidiabetic treatment, an array of comorbid conditions, the Charlson Comorbidity Index (CCI), and any co-medication regimes. By employing the absolute standardized mean difference (aSMD) with a threshold set at 0.1 or higher, we successfully pinpointed notable discrepancies between the study groups, ensuring a rigorous comparison basis.
To rigorously adjust for potential confounding factors and balance the comparison groups, we meticulously calculated propensity scores. This was achieved using logistic regression, factoring in critical variables such as age, sex, the index year of study entry, the CCI score, medical histories of hypertension and liver cirrhosis, and the specific level of antidiabetic treatment within the NAFLD-T2DM cohort. Similarly, for the FLD-T2DM-CVH cohort, additional variables including medical histories of hypertension, dyslipidemia, heart failure, coronary artery disease, alcoholic liver disease, chronic hepatitis B, chronic hepatitis C, and the administration history of ACE inhibitors, ARBs, statins, and ezetimibe were considered, alongside the level of antidiabetic treatment. Following this, a precise 1:1 propensity score (PS) matching was executed without replacement using the nearest-neighbor matching algorithm, applying a caliper width of 0.02 to ensure close matches.
Subsequently, we determined the incidence rate (IR) of each cancer type within the study groups, presenting these rates as cases per 10,000 person-years to provide a clear understanding of cancer development risk.
For a comparative analysis of the effect of SGLT2 inhibitors on HCC and other cancer types’ development, Kaplan–Meier curves were plotted, and log-rank tests were utilized, offering a visual and statistical representation of the time-to-event data. To further refine our understanding, both univariate and multivariate Cox proportional hazard regression analyses were conducted. These analyses aimed to estimate hazard ratios [HR] and their 95% confidence intervals [CI] based on baseline variables such as sex, age at cohort entry, detailed comorbidities, and the use of SGLT2i, along with antiplatelet, antihypertensive, and antidyslipidemic agents. The multivariate Cox regression analysis included variables that exhibited a P value of < 0.1 in the univariate analysis, a strategic choice to ensure that all potential predictors of interest showing a trend towards association were considered, even if they did not meet the conventional significance threshold.
These comprehensive statistical analyses were performed using advanced software tools, namely SAS version v9.4 (SAS Institute, Inc., Cary, NC, USA) and R version 4.3.2 (Boston, MA, USA), to ensure the utmost accuracy and reliability of our findings.
Ethics approval statement
This study was performed according to the Declaration of Helsinki. This retrospective study utilized data from the Health Insurance Review and Assessment Service (HIRA) in South Korea. The institutional review board (IRB) of Ajou university hospital granted an informed consent waiver due to the study's nature and use of de-identified data. Ethical approval was given by the Ajou University IRB, recognizing that patient confidentiality and privacy were upheld, in line with ethical guidelines for retrospective research (AJOUIRB-EX-2023-179).
Patient consent statement
Patient consent was waived for this study as it exclusively utilized anonymized data, ensuring the privacy and confidentiality of individual participants.
Data availability
The data that support the findings of this study are available from the Health Insurance Review and Assessment Service (HIRA) of South Korea but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Health Insurance Review and Assessment Service (HIRA) of South Korea.
Cotter, T. G. & Rinella, M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 158 , 1851–1864 (2020).
Article CAS PubMed Google Scholar
Han, S. K., Baik, S. K. & Kim, M. Y. Non-alcoholic fatty liver disease: Definition and subtypes. Clin. Mol. Hepatol. 29 , S5-s16 (2023).
Article PubMed Google Scholar
Dharmalingam, M. & Yamasandhi, P. G. Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 22 , 421–428 (2018).
Article CAS PubMed PubMed Central Google Scholar
Cho, H. J. et al. Improvement of nonalcoholic fatty liver disease reduces the risk of type 2 diabetes mellitus. Gut Liver 13 , 440–449 (2019).
Kanwal, F. et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 155 , 1828-1837.e2 (2018).
Nakatsuka, T. & Tateishi, R. Development and prognosis of hepatocellular carcinoma in patients with diabetes. Clin. Mol. Hepatol. 29 , 51–64 (2023).
Wang, C. et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: A systematic review and meta-analysis of cohort studies. Int. J. Cancer 130 , 1639–1648 (2012).
Torres, M. C. P. et al. Diabetes medications and risk of HCC. Hepatology 76 , 1880–1897 (2022).
Article Google Scholar
van Baar, M. J. B. et al. SGLT2 inhibitors in combination therapy: From mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care 41 , 1543–1556 (2018).
Shao, S. C., Kuo, L. T., Chien, R. N., Hung, M. J. & Lai, E. C. SGLT2 inhibitors in patients with type 2 diabetes with non-alcoholic fatty liver diseases: An umbrella review of systematic reviews. BMJ Open Diabetes Res. Care 8 , e001956 (2020).
Article PubMed PubMed Central Google Scholar
Dwinata, M., Putera, D. D., Hasan, I. & Raharjo, M. SGLT2 inhibitors for improving hepatic fibrosis and steatosis in non-alcoholic fatty liver disease complicated with type 2 diabetes mellitus: A systematic review. Clin. Exp. Hepatol. 6 , 339–346 (2020).
Arvanitakis, K., Koufakis, T., Kotsa, K. & Germanidis, G. The effects of sodium-glucose cotransporter 2 inhibitors on hepatocellular carcinoma: From molecular mechanisms to potential clinical implications. Pharmacol. Res. 181 , 106261 (2022).
Komiya, C. et al. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS One 11 , e0151511 (2016).
Finck, B. N. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes 67 , 2485–2493 (2018).
Wong, C. et al. Sodium-glucose co-transporter 2 inhibitors for non-alcoholic fatty liver disease in Asian patients with type 2 diabetes: a meta-analysis. Front. Endocrinol. (Lausanne) 11 , 609135 (2021).
Bea, S. et al. Sodium-glucose cotransporter-2 inhibitors and risk of hepatocellular carcinoma among patients with type 2 diabetes. Clin. Gastroenterol. Hepatol. 21 , 3451-3454.e4 (2023).
Tang, H. et al. SGLT2 inhibitors and risk of cancer in type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Diabetologia 60 , 1862–1872 (2017).
Belle, A. et al. Big data analytics in healthcare. Biomed. Res. Int. 2015 , 370194 (2015).
Kim, J. A., Yoon, S., Kim, L. Y. & Kim, D. S. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J. Korean Med. Sci. 32 , 718–728 (2017).
Chou, O. H. I. et al. Lower risks of sodium glucose cotransporter 2 (SGLT2) inhibitors compared to dipeptidyl peptidase-4 (DPP4) inhibitors for new-onset non-alcoholic fatty liver disease and hepatocellular carcinoma in type 2 diabetes mellitus: A population-based study. Preprint https://doi.org/10.1101/2022.08.16.22278847 (2022).
Lonardo, A. & Loria, P. Potential for statins in the chemoprevention and management of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 27 , 1654–1664 (2012).
Demierre, M. F., Higgins, P. D., Gruber, S. B., Hawk, E. & Lippman, S. M. Statins and cancer prevention. Nat. Rev. Cancer 5 , 930–942 (2005).
Mansourian, P. G. et al. Effects of statins on the risk of hepatocellular carcinoma. Gastroenterol. Hepatol. (N. Y.) 10 , 417–426 (2014).
PubMed Google Scholar
Goh, M. J. & Sinn, D. H. Statin and aspirin for chemoprevention of hepatocellular carcinoma: Time to use or wait further?. Clin. Mol. Hepatol. 28 , 380–395 (2022).
Zou, B., Odden, M. C. & Nguyen, M. H. Statin use and reduced hepatocellular carcinoma risk in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 21 , 435-444.e6 (2023).
Li, I. H. et al. Inverse association of fibrates and liver cancer: A population-based case-control study in Taiwan. J. Clin. Pharmacol. 59 , 1170–1176 (2019).
Cho, Y., Kim, B. H. & Park, J. W. Overview of Asian clinical practice guidelines for the management of hepatocellular carcinoma: An Asian perspective comparison. Clin. Mol. Hepatol. 29 , 252–262 (2023).
Singal, A. G. et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 78 , 1922–1965 (2023).
Kinsey, E. & Lee, H. M. Management of hepatocellular carcinoma in 2024: The multidisciplinary paradigm in an evolving treatment landscape. Cancers (Basel) 16 , 666 (2024).
Schechtman, E. Odds ratio, relative risk, absolute risk reduction, and the number needed to treat—Which of these should we use?. Value Health 5 , 431–436 (2002).
Austin, P. C. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. J. Clin. Epidemiol. 63 , 2–6 (2010).
Lee, C. H. et al. SGLT2i reduces risk of developing HCC in patients with co-existing type 2 diabetes and hepatitis B infection: A territory-wide cohort study in Hong Kong. Hepatology 78 , 1569–1580 (2023).
Choi, J. H. et al. 2023 clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab. J. 47 , 575–594 (2023).
Download references
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HR22C1734, HR21C1003, HI23C0901), and National IT Industry Promotion Agency (NIPA) grant funded by the Korea government (MSIT) (No. S0252-21-1001).
Author information
Authors and affiliations.
Department of Gastroenterology, Ajou University School of Medicine, Worldcup-ro 164, Yeongtong-gu, Suwon, 16499, Republic of Korea
Hyo Jung Cho, Soon Sun Kim & Jae Youn Cheong
Department of Neurology, McGovern Medical School at UTHealth, Houston, TX, USA
Eunyoung Lee
You can also search for this author in PubMed Google Scholar
Contributions
Concept and design: Jae Youn Cheong, Hyo Jung Cho. Acquisition, analysis, or interpretation of data: Hyo Jung Cho, Eunyoung Lee. Drafting of the manuscript: Hyo Jung Cho. Critical review of the manuscript for important intellectual content: Soon Sun Kim. Statistical analysis: Hyo Jung Cho, Eunyoung Lee. Supervision: Soon Sun Kim. Obtained funding: Jae Youn Cheong, Hyo Jung Cho.
Corresponding author
Correspondence to Jae Youn Cheong .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cho, H.J., Lee, E., Kim, S.S. et al. SGLT2i impact on HCC incidence in patients with fatty liver disease and diabetes: a nation-wide cohort study in South Korea. Sci Rep 14 , 9761 (2024). https://doi.org/10.1038/s41598-024-60133-3
Download citation
Received : 16 January 2024
Accepted : 19 April 2024
Published : 29 April 2024
DOI : https://doi.org/10.1038/s41598-024-60133-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hepatocellular carcinoma
- Sodium-glucose cotransporter-2 inhibitors (SGLT2i)
- Fatty liver
- Type 2 diabetes mellitus
- Chronic viral hepatitis
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Pathophysiology

https://slideplayer.nl/slide/2005829/
According to McCance and Huether (2019), 9.3 % of the adult population in the United States is affected by Type 2 diabetes mellitus. Risk factors for developing Type 2 diabetes are family history, hypertension, obesity, and increased age. Lifestyle choices, genetic factors, and environmental factors combined can all contribute to the development of Type 2 diabetes mellitus. One main issue leading to Type 2 diabetes is insulin resistance in peripheral tissues specifically the muscle, liver, and adipose tissue (McCance & Huether, 2019).
Alpha cells and beta cells are islet cells that are found in the pancreas. The beta cells are responsible for creating insulin and the alpha cells are responsible for creating glucagon. The increasingly high glucagon levels cause blood glucose levels to increase leading to the stimulation of gluconeogenesis and glycogenolysis (McCance & Huether, 2019). Due to the decreased reactiveness of the alpha cells to glucose, the glucagon secretion begins increasing as well. Amylin which is a beta-cell hormone is responsible for repressing the alpha cells release of glucagon (McCance & Huether, 2019). In Type 2 diabetes the cells begin to become insulin resistant. This means the needed glucose is unable to get inside of the cells which causes it to accumulate in the blood. In this case, the insulin receptors are abnormal or missing causing glucose to be locked out of the cells.
The beta cells attempt to keep up with the increased demand for insulin but eventually lose the ability to produce enough. The beta cells begin to decrease in number and size and eventually fail due to exhaustion (McCance & Huether, 2019). This leads to hyperglycemia which is the buildup of glucose in the bloodstream. In an attempt to compensate for hyperglycemia, the pancreas will produce more insulin. The pancreas will eventually reach exhaustion and no longer be able to compete with the body’s increased demand for insulin.
Our GI hormones (gut hormones) contribute to diabetes & insulin resistance as well. Ghrelin is a hormone made in the stomach and pancreatic islets that control food intake. Insulin resistance has been associated with reduced levels of ghrelin. Incretins are released from the GI tract to increase insulin release, regenerate the beta-cell and provide a barrier to beta-cell damage (McCance & Huether, 2019). Studies show the incretin glucagon-like peptide 1, (GLP-1) depicts a decrease in beta-cell responsiveness in type 2 diabetes (McCance & Huether, 2019).
Due to hyperglycemia and the current lack of insulin polyphagia, polydipsia and polyuria are classic signs that appear while recurrent infections and visual changes occur later on. If hyperglycemia continues to progress without treatment microvascular complications such as nephropathy, neuropathy, and retinopathy can occur along with macrovascular complications: cerebrovascular disease, coronary artery disease, and peripheral artery disease (McCance & Huether, 2019).
According to the American Diabetes Association (2015), there are four ways to diagnose Type 2 diabetes
- Glycated hemoglobin (A1C) test: Diabetics diagnosed using this test will have an A1C of 6.5% or higher
- Random blood sugar test: Diabetics diagnosed using this test will have a blood sugar of > 200 mg/dL
- Fasting plasma glucose (FPG): Diabetics diagnosed using this test will have a FPG of 126 mg/dL or higher
- Oral glucose tolerance test (OGTT): Diabetics diagnosed using this test will have an OGTT of 200 mg/dL or higher.
American Diabetes Association. (2015, January 1). 2. Classification and Diagnosis of Diabetes. Retrieved from https://care.diabetesjournals.org/content/38/Supplement_1/S8.
McCance, K. L., Huether, S. E., Brashers, V. L., & Rote, N. S. (2019). Pathophysiology: the biologic basis for disease in adults and children (8th ed.). St. Louis, MO: Elsevier.
Non-diabetic nephropathy in diabetic patients: incidence, HbA1c variability and other predictive factors, and implications
- Nephrology – Original Paper
- Published: 25 April 2024
Cite this article

- Bülent Demirelli ORCID: orcid.org/0000-0001-9110-868X 1 ,
- Burcu Boztepe ORCID: orcid.org/0000-0002-3479-4801 2 ,
- Elif Gülcan Şenol 2 ,
- Başak Boynueğri ORCID: orcid.org/0000-0003-4054-2069 2 ,
- Yelda Deligöz Bildacı ORCID: orcid.org/0000-0001-9888-995X 3 ,
- Gülistan Gümrükçü ORCID: orcid.org/0000-0002-5704-6160 4 ,
- Mustafa Canbakan 2 &
- Melike Betül Öğütmen ORCID: orcid.org/0000-0002-6623-9669 2
40 Accesses
Explore all metrics
Diabetes mellitus (DM) is the leading cause of chronic kidney disease (CKD) in the population. In patients with diabetes mellitus, the incidence of non-diabetic nephropathy (NDNP) has been estimated to range from 3% to 69.5%. Personal judgment is frequently employed while deciding whether or not to do a kidney biopsy (KB) on diabetic patients. NDNP alters the prognosis and course of treatment for people with DM. In our study, we examined the incidence of NDNP concurrent with the progression of diabetes mellitus, as well as the laboratory and clinical indicators that could be utilized to forecast it.
A retrospective analysis of 76 diabetic patients who underwent KB was conducted. Based on the pathological diagnoses of these patients, they were categorized as DNP (diabetic nephropathy) or NDNP. The definition of HbA1c variability was determined by calculating the mean HbA1c and the average value of the HbA1c measurements, as well as the standard deviation (SD) for each participant.
NDNP was detected in 50% of 76 patients. Among patients with NDNP, 36.8% had focal segmental glomerulosclerosis (FSGS), 23.6% had membranous glomerulonephritis, and 7.8% had IgA nephritis. The NDNP group exhibited significantly higher rates of female gender, absence of diabetic retinopathy, shorter time to diagnosis of diabetes mellitus, chronic kidney disease, and proteinuria, less intensive medication for diabetes mellitus, presence of hematuria and leukociduria, immunological serological marker positivity, and non-HbA1C variability. Risk factors for predicting non-diabetic nephropathy, as determined by multivariate analysis, included female gender, the absence of diabetic retinopathy, non-HbA1c variability and a positive immunological serological test.
In this study, a significant number of diabetic patients with chronic kidney disease were diagnosed with NDNP. Identifying these patients allows for treatment of the specific underlying disease. Factors such as the absence of DR, non-HbA1c variability, female gender, and immunological serological test positivity can predict NDNP and guide the clinician’s decision on kidney biopsy. Further prospective studies are warranted to validate the efficacy of potential predictive factors like HbA1c variability.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Characterizing predictors of non-diabetic kidney disease (NDKD) in diabetic patients
Identification of clinical predictors of diabetic nephropathy and non-diabetic renal disease in Chinese patients with type 2 diabetes, with reference to disease course and outcome

Nondiabetic Renal Disease (NDRD) and Diabetic Kidney Disease (DKD)
Data availability.
The data generated in this study has been included in the article, and further inquiries can be directed to the corresponding author.
Atlas, D., IDF diabetes atlas. International Diabetes Federation (9th editio). Retrieved from. 2019 http://www.idf.org/about-diabetes/facts-figures
Sartore G et al (2023) Long-term HbA1c variability and macro-/micro-vascular complications in type 2 diabetes mellitus: a meta-analysis update. Acta Diabetol 60:1–18
Article Google Scholar
Zimmet PZ et al (2014) Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol 2(1):56–64
Article PubMed Google Scholar
Koçyiğit İ, Seyahi N, Ateş K (2023) Current status of kidney replacement therapy in türkiye: a summary of 2021 Turkish society of nephrology registry report. Turk J Nephrol (Online) 32:174
Hermann JM et al (2014) HbA1c variability as an independent risk factor for diabetic retinopathy in type 1 diabetes: a German/Austrian multicenter analysis on 35,891 patients. PLoS ONE 9(3):e91137
Article PubMed PubMed Central Google Scholar
Lin C-C et al (2013) Risks of diabetic nephropathy with variation in hemoglobin A1c and fasting plasma glucose. Am J Med 126:1017.e1-1017.e10
Article CAS PubMed Google Scholar
Fiorentino M et al (2017) Renal biopsy in patients with diabetes: a pooled meta-analysis of 48 studies. Nephrol Dial Transplant 32(1):97–110
CAS PubMed Google Scholar
Zhuo L et al (2013) Evaluation of renal biopsies in type 2 diabetic patients with kidney disease: a clinicopathological study of 216 cases. Int Urol Nephrol 45:173–179
Zukowska-Szczechowska E, Tomaszewski M (1995) Renal affection in patients with diabetes mellitus is not always caused by diabetic nephropathy. Rocz Akad Med Bialymst 2004(49):185–189
Google Scholar
Suarez MLG et al (2013) Diabetic nephropathy: is it time yet for routine kidney biopsy? World J Diabetes 4(6):245
Sharma SG et al (2013) The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol 8(10):1718–1724
Wu T-E, Su Y-W, Chen H-S (2022) Mean HbA1c and HbA1c variability are associated with differing diabetes-related complications in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 192:110069
Akselrod D, Friger M, Biderman A (2021) HbA1C variability among type 2 diabetic patients: a retrospective cohort study. Diabetol Metab Syndr 13:1–7
Inker LA et al (2021) New creatinine-and cystatin C–based equations to estimate GFR without race. N Engl J Med 385(19):1737–1749
Article CAS PubMed PubMed Central Google Scholar
Santoro D et al (2021) Kidney biopsy in type 2 diabetic patients: critical reflections on present indications and diagnostic alternatives. Int J Mol Sci 22(11):5425
Gambara V et al (1993) Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol 3(8):1458–1466
Christensen PK et al (2000) Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int 58(4):1719–1731
Castellano I et al (2002) Renal histological lesions in patients with type II diabetes mellitus. Nefrol:Publ Of de la Soc Espanola Nefrol 22:162–169
CAS Google Scholar
Bi H et al (2011) Nondiabetic renal disease in type 2 diabetic patients: a review of our experience in 220 cases. Ren Fail 33(1):26–30
Ghani AA et al (2009) Renal biopsy in patients with type 2 diabetes mellitus: indications and nature of the lesions. Ann Saudi Med 29(6):450–453
Zhou J et al (2008) A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant 23(6):1940–1945
Dong Z et al (2016) Clinical predictors differentiating non-diabetic renal diseases from diabetic nephropathy in a large population of type 2 diabetes patients. Diabetes Res Clin Pract 121:112–118
Soni SS et al (2006) Non diabetic renal disease in type 2 diabetes mellitus. Nephrology 11(6):533–537
Jalalah SM (2008) Non-diabetic renal disease in diabetic patients. Saud J Kidney Dis Transplant 19(5):813
Erdogmus S et al (2017) Non-diabetic kidney disease in type 2 diabetic patients: prevalence, clinical predictors and outcomes. Kidney Blood Press Res 42(5):886–893
Heybeli C et al (2019) Predictors and histopathological characteristics of non-diabetic renal disorders in diabetes: a look from the tubulointerstitial point of view. Intern Med J 49(12):1524–1533
Segelmark M, Weiner M, Peters B (2023) # 5414 clinical utility of serological test at time of biopsy. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfad063c_5414
Kritmetapak K et al (2018) Clinical and pathological characteristics of non-diabetic renal disease in type 2 diabetes patients. Clin Kidney J 11(3):342–347
Shadab S et al (2022) Characterizing predictors of non-diabetic kidney disease (NDKD) in diabetic patients. Int Urol Nephrol 54(6):1303–1309
Al-Rubeaan K et al (2014) Diabetic nephropathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. PLoS ONE 9(2):e88956
Arici M et al (2023) # 5047 diabetes and chronic kidney disease in Turkey (diakit): the study on chronic kidney disease in diabetes mellitus patients in the cappadocia cohort. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfad063d_5047
Kronzer VL, Bridges SL Jr, Davis JM III (2021) Why women have more autoimmune diseases than men: an evolutionary perspective. Evol Appl 14(3):629–633
Bagriacik N et al (2009) Obesity profile in Turkey. Int J Diabetes Metab 17(1):5–8
Initiative NKFKDOQ (2007) Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kindney Dis. 49:S62–S73
Powers AC, Niswender KD, Evans-Molina C (2018) Diabetes mellitus: diagnosis, classification, and pathophysiology. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J (eds) Harrison's principles of internalmedicine, 20th edn. McGraw-Hill Education, New York, pp 2850–2859
Tan J et al (2017) Presentation, pathology and prognosis of renal disease in type 2 diabetes. BMJ Open Diabetes Res Care 5(1):e000412
Pallayova M et al (2015) Predicting non-diabetic renal disease in type 2 diabetic adults: the value of glycated hemoglobin. J Diabetes Complicat 29(5):718–723
Group, A.t.C.C.R.i.D.S. (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559
Sheng C-S et al (2020) Prognostic significance of long-term HbA1c variability for all-cause mortality in the ACCORD trial. Diabetes Care 43(6):1185–1190
Prentice J, Pizer S, Conlin P (2016) Identifying the independent effect of HbA1c variability on adverse health outcomes in patients with type 2 diabetes. Diabet Med 33(12):1640–1648
Low S et al (2017) Effect of long-term glycemic variability on estimated glomerular filtration rate decline among patients with type 2 diabetes mellitus: I nsights from the D iabetic N ephropathy C ohort in S ingapore: 2 型糖尿病患者长期血糖波动对估算的肾小球滤过率下降的影响: 来自新加坡糖尿病肾病队列研究的启示. J Diabetes 9(10):908–919
Hironaka K et al (1991) Nondiabetic renal disease complicating diabetic nephropathy. J Diabetic Complicat 5(2–3):148–149
Article CAS Google Scholar
Download references
The authors received no specific funding for this work
Author information
Authors and affiliations.
Department of Nephrology, Marmara Unıversity Pendik Training and Research Hospital, Nephrology Clinic, Fevzi Çakmak Quarter Muhsin Yazıcıoğlu Street No: 10 Üst Kaynarca, Pendik, İstanbul, Turkey
Bülent Demirelli
Department of Nephrology, Haydarpaşa Numune Training and Research Hospital, Nephrology Clinic, İstanbul, Turkey
Burcu Boztepe, Elif Gülcan Şenol, Başak Boynueğri, Mustafa Canbakan & Melike Betül Öğütmen
Department of Nephrology, Dokuz Eylül University Research and Application Hospital, Nephrology Clinic, İstanbul, Turkey
Yelda Deligöz Bildacı
Department of Pathology, Haydarpaşa Numune Training and Research Hospital, Izmir, Turkey
Gülistan Gümrükçü
You can also search for this author in PubMed Google Scholar
Contributions
B.D., Bu.B., Ba. B. wrote the main manuscript text B.D. prepared figure E.G.S prepared tables. Y.D.B., G.G., collected data B.D. analyzed the data Conception or design, authorship: M.B.Ö., M.C. All authors reviewed the manuscript
Corresponding author
Correspondence to Bülent Demirelli .
Ethics declarations
Conflict of interest.
The authors have declared that no conflict of interest exists.
Ethical approval
The methods utilized in this investigation were authorized by the Haydarpaşa Numune Research and Training Hospital Clinical Research Ethics Committee (Approval No. HNEAH-KAEK 2022/205), and all procedures conducted in the study adhered to the ethical standards outlined in the 1964 Declaration of Helsinki
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Demirelli, B., Boztepe, B., Şenol, E.G. et al. Non-diabetic nephropathy in diabetic patients: incidence, HbA1c variability and other predictive factors, and implications. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-04066-w
Download citation
Received : 22 March 2024
Accepted : 18 April 2024
Published : 25 April 2024
DOI : https://doi.org/10.1007/s11255-024-04066-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Non-diabetic nephropathy
- Kidney biopsy
- Kidney disease
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
The patient received a diagnosis of type 2 diabetes mellitus; lifestyle modification was recommended. ... as to whether LADA truly represents an entity that is distinct from type 1 diabetes. 6 Both patients with type 1 diabetes and patients with LADA commonly have elevated levels of ... observational case control studies. PLoS One 2013; 8
The following case study illustrates the clinical role of advanced practice nurses in the management of a patient with type 2 diabetes. Case Presentation A.B. is a retired 69-year-old man with a 5-year history of type 2 diabetes.
CASE. A 47-year-old woman was found to have hyperglycemia at a health fair when a random blood glucose level was 227 mg/dL (12.6 mmol/L). Several days later, a fasting blood glucose value was 147 mg/dL (8.2 mmol/L). She has no previous history of diabetes, is alarmed by the possibility of having this disorder, and seeks your advice.
Presentation of Case. Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia. Eleven years before this presentation, the blood ...
Commonly, controlling hyperglycemia leads to a decrease in triglycerides.1 However, in this patient, the clearing of serum triglycerides, the restricted saturated fat, and the weight loss had a substantial impact on improving glucose tolerance without adding further diabetes oral agents. Studies have shown that dietary fat, primarily saturated ...
Patient Presentation and History. Chief Complaint: the patient's wife is bringing the patient in after a fall at their home. Presentation: J.S. a 50-year-old African American male who presents with his wife after he fell at home. After the fall, he told his wife "I will be fine, I think my vision just needs checked.".
A 59-year-old woman with type 1 diabetes and a 2-year history of cognitive decline presented with obtundation. There was diffuse, symmetric hypointensity in the brain on T2-weighted images and abno...
The following case study illustrates the pharmacotherapeutic challenges of diabetes with other comorbidities, which can lead to potential drug-drug and drug-disease interactions. Although it does not offer detailed solutions to such problems, this case does describe the process of patient care and problem resolution as approached by advanced ...
Abstract. Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. Screening for GDM is usually done at 24-28 weeks of gestation. In this case, we report a 31-year-old woman who developed gestational diabetes at 6 weeks in two successive pregnancies.
Results. Results of the lunch study for a type 2 diabetic subject indicate that the recovery time of the post-prandial blood glucose level can be adjusted to 4 hours, which is comparable to the typical time interval for non-diabetics: 3 to 4 hours. A moderate lifestyle adjustment of light supper coupled with morning swimming of 20 laps in a 25 ...
Diabetes mellitus currently affects 6.4% or 285 million adults worldwide, and that number is expected to increase to 7.7% or 439 million by 2030. 1 In the United States, the prevalence of diabetes in adults increased from 11.3% in 2010 to 12.3% in 2012. 2 The current type 2 diabetes mellitus (T2DM) epidemic is closely associated with a parallel obesity epidemic, with more than 85% of patients ...
Case Studies /. Hyperglycemia in a 56-Year-Old Woman. Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada) — dedicated to using leading-edge science to save and improve lives around the world. Learn more about the MSD Manuals and our commitment to Global Medical Knowledge.
The three mini-case studies presented with this issue of the journal take you through what to consider in making an accurate diagnosis of type 2 diabetes. The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Treatment. Treatment of type 2 diabetes mellitus (T2DM) focuses on decreasing blood glucose, increasing insulin secretion, or countering insulin resistance. Treatment of symptoms, such as diabetic retinopathy, nephropathy, or neuropathy requires additional and involved patient education, medications, and therapies.
Correct Diagnosis: The correct diagnosis for J.S. is type 2 diabetes mellitus. J.S. has a Hb A1c of 10% and a fasting blood glucose of 240 mg/dL. Both of these values fit in the diagnostic criteria for DM. The patient also has obesity, a lack of a balanced diet, and he is a smoker. These are all risk factors for DM.
Diabetes Mellitus: A Case Study . Ain Q and Sadeeqa S* Institute of Pharmacy, ... Accordingly, metformin is widely regarded as the drug of choice for most patients with type 2 diabetes. Concern ...
B. Administer a dose of IV antibiotics to a diabetic with an infected foot ulcer. C. Administer IV dextrose to a diabetic client with a blood glucose level of 25 mg/dl (1.39 mmol/L). D. Hang a new bag of normal saline on a diabetic with a blood glucose level of 275 mg/dl (14.26 mmol/L).
Studies that have compared antihypertensive treatment in patients with diabetes versus placebo have shown reduced cardiovascular events. The United Kingdom Prospective Diabetes Study (UKPDS), which followed patients with diabetes for an average of 8.5 years, found that patients with tight BP control (< 150/< 85 mmHg) versus less tight control (< 180/< 105 mmHg) had lower rates of myocardial ...
Papanna J, Bettegowda S. Thyroid dysfunction among type 2 diabetes mellitus patients: a study from rural hospital. J Med Sci Clin Res. 2019;7:433-6. ... Thyroid dysfunction in patients with type-2 diabetes mellitus in Kerala: a case-control study. Thyroid Res Pract. 2019;16(1):3. Article Google Scholar
The transition from hospital to outpatient care is a particularly vulnerable period for patients as they move from regular health monitoring to self-management. This study aimed to map and investigate the journey of patients with polymorbidities, including type 2 diabetes (T2D), in the 2 months following hospital discharge and examine patients' encounters with healthcare professionals (HCPs).
The study used 50 hypothetical cases of diabetes mellitus. Patient data from real world was not used in this study due to ethical concerns. Each case summary contained patient details, status of diabetes, it's complications and comorbidities, laboratory reports, current medications and proposed future management plan.
Diabetes mellitus (DM) is thought to be closely related to arterial stenotic or occlusive disease caused by atherosclerosis. However, there is still no definitive clinical evidence to confirm that patients with diabetes have a higher risk of restenosis. This meta-analysis was conducted to determine the effect of DM on restenosis among patients undergoing endovascular treatment, such as ...
Diabetes & Primary Care 's series of interactive case studies is aimed at GPs, practice nurses and other professionals in primary and community care who would like to broaden their understanding of type 2 diabetes. The four mini-case studies created for this issue of the journal cover various aspects relating to hypoglycaemia and type 2 ...
Schematic representation of cohort derivation for this study. T2DM type 2 diabetes mellitus, ... a large-scale case-control study in Taiwan revealed a ... in patients with diabetes mellitus: ...
Studies show the incretin glucagon-like peptide 1, (GLP-1) depicts a decrease in beta-cell responsiveness in type 2 diabetes (McCance & Huether, 2019). Due to hyperglycemia and the current lack of insulin polyphagia, polydipsia and polyuria are classic signs that appear while recurrent infections and visual changes occur later on.
Purpose Diabetes mellitus (DM) is the leading cause of chronic kidney disease (CKD) in the population. In patients with diabetes mellitus, the incidence of non-diabetic nephropathy (NDNP) has been estimated to range from 3% to 69.5%. Personal judgment is frequently employed while deciding whether or not to do a kidney biopsy (KB) on diabetic patients. NDNP alters the prognosis and course of ...