- Open access
- Published: 13 July 2021

QbD approach to HPLC method development and validation of ceftriaxone sodium
- Krunal Y. Patel 1 ,
- Zarna R. Dedania 1 ,
- Ronak R. Dedania 1 &
- Unnati Patel 2
Future Journal of Pharmaceutical Sciences volume 7 , Article number: 141 ( 2021 ) Cite this article
19k Accesses
31 Citations
Metrics details
Quality by design (QbD) refers to the achievement of certain predictable quality with desired and predetermined specifications. A quality-by-design approach to method development can potentially lead to a more robust/rugged method due to emphasis on risk assessment and management than traditional or conventional approach. An important component of the QbD is the understanding of dependent variables, various factors, and their interaction effects by a desired set of experiments on the responses to be analyzed. The present study describes the risk based HPLC method development and validation of ceftriaxone sodium in pharmaceutical dosage form.
An efficient experimental design based on central composite design of two key components of the RP-HPLC method (mobile phase and pH) is presented. The chromatographic conditions were optimized with the Design Expert software 11.0 version, i.e., Phenomenex ODS column C18 (250 mm × 4.6 mm, 5.0 μ), mobile phase used acetonitrile to water (0.01% triethylamine with pH 6.5) (70:30, v/v), and the flow rate was 1 ml/min with retention time 4.15 min. The developed method was found to be linear with r 2 = 0.991 for range of 10–200 μg/ml at 270 nm detection wavelength. The system suitability test parameters, tailing factor and theoretical plates, were found to be 1.49 and 5236. The % RSD for intraday and inter day precision was found to be 0.70–0.94 and 0.55–0.95 respectively. The robustness values were less than 2%. The assay was found to be 99.73 ± 0.61%. The results of chromatographic peak purity indicate the absence of any coeluting peaks with the ceftriaxone sodium peak. The method validation parameters were in the prescribed limit as per ICH guidelines.
The central composite design experimental design describes the interrelationships of mobile phase and pH at three different level and responses to be observed were retention time, theoretical plates, and peak asymmetry with the help of the Design Expert 11.0 version. Here, a better understanding of the factors that influence chromatographic separation with greater confidence in the ability of the developed HPLC method to meet their intended purposes is done. The QbD approach to analytical method development was used for better understanding of method variables with different levels.
A QbD is defined as “A systemic approach to the method development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management [ 1 ].” The QbD approach emphasizes product and process understanding with quality risk management and controls, resulting in higher assurance of product quality, regulatory flexibility, and continual improvement. The QbD method was based on the understanding and implementation of guidelines ICH Q8 Pharmaceutical Development, ICH Q9 Quality Risk Management, and ICH Q10 Pharmaceutical Quality System [ 2 , 3 , 4 ]. Analytical science is considered to be an integral part of pharmaceutical product development and hence go simultaneously during the entire product life cycle. Analytical QbD defined as a science and risk-based paradigm for analytical method development, endeavoring for understanding the predefined objectives to control the critical method variables affecting the critical method attributes to achieve enhanced method performance, high robustness, ruggedness, and flexibility for continual improvement [ 5 , 6 ]. The result of analytical QbD is well known, fit for purpose, and robust method that reliably delivers the intended output over its lifecycle, similar to the process QbD [ 7 , 8 ]. For QbD, HPLC methods, robustness, and ruggedness should be tested earlier in the development stage of the method to ensure the efficiency of the method over the lifetime of the product [ 9 ]. Otherwise, it can take considerable time and energy to redevelop, revalidate, and retransfer analytical methods if a non-robust or non-rugged system is adapted. The major objective of AQbD has been to identify failure modes and establish robust method operable design region or design space within meaningful system suitability criteria and continuous life cycle management. Literature survey reveals QbD approaches for HPLC method were reported [ 10 , 11 , 12 , 13 ].
The current work intends to develop and optimize the HPLC method for ceftriaxone sodium in pharmaceutical dosage form by quality-by-design approach.
Ceftriaxone sodium was procured as gift sample Salvavidas Pharmaceutical Pvt. Ltd., Surat, Gujarat. All other reagents and chemicals used were of analytical grade, and solvents were used were of HPLC grade. The marketed formulations MONOCEF 250 mg by Aristo were used for assay.
Instruments and reference standards
The HPLC WATERS-2695 with Detector-UV VIS Dual Absorbance Detector WATERS-2487. C-18 column (150 mm × 4.6 mm × 5 μm particle size) was used at ambient temperature.
Chromatographic conditions
The Phenomenex C-18 column (250 mm × 4.6 mm having 5.0 μm particle size equilibrated with a mobile phase consisting of acetonitrile to water (70:30, v/v)) was used. The mobile phase pH 6.5 was adjusted with 0.01% triethylamine. The flow rate was kept at 1 ml/min, and column was set at ambient temperature. Eluents were supervised using a PDA detector at 270.0 nm. A satisfactory separation and peak symmetry for the drug were obtained with the above chromatographic condition. The HPLC method for ceftriaxone sodium was optimized for various parameters: mobile phase and pH as two variables at three different levels using central composite design.
Preparation of reference standard solution
The 1000 μg/ml standard stock solution was prepared by dissolving an accurately 25 mg of ceftriaxone sodium in 25 ml methanol. The stock solution was further diluted to a sub-stock 100 μg/ml. The 10 μg/ml solution was prepared by diluting 1 ml of sub-stock solution to 10 ml with methanol.
Selection of detection wavelength
Ten μg/ml ceftriaxone sodium was scanned in the range of 200–400 nm, and wavelength maxima 270 nm was selected as detection wavelength.
HPLC method development by QbD approach
HPLC method development by Analytical QbD was as follows.
Selection of quality target product profile
The QTPP plays an important role for identifying the variables that affect the QTPP parameters. The retention time, theoretical plates, and peak asymmetry were identified as QTPP for proposed HPLC method [ 14 , 15 ].
Determine critical quality attributes
The CQAs are the method parameters that are directly affect the QTPP. The mobile phase composition and pH of buffer were two critical method parameters required to be controlled to maintain the acceptable response range of QTPP [ 16 ].
Factorial design
After defining the QTPP and CQAs, the central composite experimental design was applied to optimization and selection of two key components: mobile phase and pH of HPLC method. The various interaction effects and quadratic effects of the mobile phase composition and pH of buffer solution on the retention time, theoretical plates, and peak asymmetry was studied using central composite statistical screening design.
A 2-factor, mobile phase composition and pH of buffer solution at 3 different levels, design was used with Design Expert® (Version 11.0, Stat-Ease Inc., and M M), the best suited response for second-order polynomial exploring quadratic response surfaces [ 15 ].
where A and B are independent variables coded for levels, Y is the measured response associated with each combination of factor level, β0 is an intercept, and β1 to β22 are regression coefficients derived from experimental runs of the observed experimental values of Y. Interaction and quadratic terms respectively represent the terms AB, A2, and B2.
Since multivariable interaction of variables and process parameter have been studied, the factors were selected based on preliminary analysis [ 17 ]. As independent variables, mobile phase composition and pH of buffer were chosen and shown in Table 1 . The dependent variables were retention time, peak area, and peak asymmetry as dependent variables for proposed independent variables [ 18 ].
Evaluation of experimental results and selection of final method conditions
Using the CCD approach, these method conditions were assessed. At the first step, the conditions for retention time, theoretical plates, and peak asymmetry were evaluated. For ceftriaxone sodium, this resulted in distinct chromatographic conditions. The proven acceptable ranges from robust regions where the deliberate variations in the method parameters do not affect the quality. This ensures that the method does not fail downstream during validation testing. If the modeling experiments do not have the desired response, the variable needs to be optimized at different levels until the responses were within the acceptable ranges [ 19 ]. The best suited chromatographic conditions shall be optimized using the Design Expert tools.
Risk assessment
The optimized final method is selected against the attributes of the method like that the developed method is efficient and will remain operational throughout the product’s lifetime. A risk-based approach based on the QbD principles set out in ICH Q8 and ICH Q9 guidelines was applied to the evaluation of method to study the robustness and ruggedness [ 20 ]. The parameters of the method or its performance under several circumstances, such as various laboratories, chemicals, analysts, instruments, reagents, and days, were evaluated for robustness and ruggedness studies [ 21 ].
Implement a control strategy
A control strategy should be implemented after the development of method. The analytical target profile was set for the development of the analytical control strategy. The analytical control strategy is the planned set of controls that was derived from the understanding of the various parameters, i.e., fitness for purpose, analytical procedure, and risk management. All these parameters ensure that both performance of the method and quality outputs are within the planned analytical target profile. Analytical control strategy was planned for sample preparation, measurement, and replicate control operations [ 22 ].
Continual improvement for managing analytical life cycle
The best way in the management of analysis lifecycle is doing a continual improvement that can be implemented by monitoring the quality consistency and periodic maintenance of HPLC instrument, computers, and updating of software and other related instrument and apparatus can be done within laboratory [ 23 ].
Analytical method validation
Method validation is a documented evidence which provides a high degree of assurance for a specific method that the process used to confirm the analytical process is suitable for its intended use. The developed HPLC method for estimation ceftriaxone sodium was validated as per ICH Q2 (R1) guidelines [ 24 ].
The linearity of ceftriaxone sodium was evaluated by analyzing 5 independent levels concentration range of 10–200 μg/ml. The calibration curve was constructed by plotting peak area on y axis versus concentration on x-axis. The regression line equation and correlation coefficient values were determined.
Repeatability calculated by the measurement of six samples 100 μg/ml ceftriaxone sodium. The intraday and interday precision were determined by analyzing three different concentrations of ceftriaxone sodium 100, 150, and 200 μg/ml concentrations at three times, on the same day at an interval of 2 h and for three different days. The acceptance limit for % RSD was less than 2.
The accuracy of the method was determined by calculating by recovery study from marketed formulation by at three levels 80%, 100%, and 120% of standard addition. The % recovery of ceftriaxone sodium was calculated. The acceptance limit for % recovery as per ICH guidelines was 98–102% of standard addition.
LOD and LOQ
The lowest drug concentration that can be accurately identified and separated from the background is referred to as a detection limit (LOD) and that can be quantified at the lowest concentration is referred to as LOQ, i.e., the quantification limit. The following equation was used to measure LOD and LOQ according to ICH guidelines.
where σ is the standard deviation of the y-intercept of the regression line, and SD is the slope of the calibration curve.
Robustness and ruggedness studies
The method’s robustness was calculated by subjecting the method to a minor change in the state of the method, such as pump flow rate and pH of mobile phase composition. The ruggedness studies were determined by changing the analyst as extraneous influencing factor. The acceptance limit for calculated %RSD of peak area was less than 2.
System suitability studies
The system suitability was evaluated by six replicate analyses of ceftriaxone sodium. The retention time, column efficiency, peak asymmetry, and theoretical plates were calculated for standard solutions.
Twenty tablets were weighed and powdered. Weigh an accurately about powder equivalent to 100 mg of ceftriaxone sodium, and transfer to 100 ml of volumetric flask. Add 25 ml of methanol, and perform sonication for 15 min until the powder dissolves. Then, make up the volume up to the mark with mobile phase. Filter the resulting solution with 0.42 μ Whatman filter paper. From the filtrate, dilute 0.5 ml to 10 ml to have a concentration of 100 μg/ml. The solution was analyzed by HPLC with same chromatographic condition as linearity. The mean of 3 different assay were used for calculation.
Initially, a mobile phase acetonitrile to water, 50:50 v/v, was tried; the peak was observed at far retention time. No single peak was observed with mobile phase acetonitrile to water, 80:20 v/v. The further mobile phase tried was acetonitrile to water, 40:60 v/v. The improvement of peak shape and symmetry was done by adjusting the buffer pH. The system suitability test parameters were satisfied with optimized chromatographic condition. The optimized mobile phase consisting of acetonitrile to water, 70:30 v/v, and pH 6.5 adjusted with 0.01% triethylamine. The central composite design was used further for the optimization of various parameters within the design space.
HPLC method development by QbD approach [ 25 ]
Quality target product profile
The QTPP selected were retention time, theoretical plates, and peak asymmetry for optimization of HPLC chromatographic condition.
Critical quality attributes
The mobile phase composition acetonitrile to water, 70:30, and pH of buffer solution adjusted with 0.01% triethylamine were identified.
Factorial design [ 21 ]
The CCD central composite design was selected for proposed HPLC method development. The optimization of various parameters is shown in Table 2 .
Design space
The response surface study type, central composite design, and quadric design model with 11 runs were used. The proposed CCD experimental design was applied, and the evaluation of mobile phase composition and pH of buffer was done against the three responses, retention time, theoretical plates, and peak asymmetry, and the result was summarized.
From Fig. 1 and equation retention time (for actual values) = 56.75 + 0.028 × A − 19.01 × B − 0.010 × AB + 0.000343 × A 2 + 1.70458 × B 2 , it was concluded that as β 1 positive coefficient (0.028) suggests that as the amount of acetonitrile in the mobile phase (A) increases and β 2 negative coefficient (− 19.01) suggests that as pH of buffer (B) decreases, the value of retention time was increased.
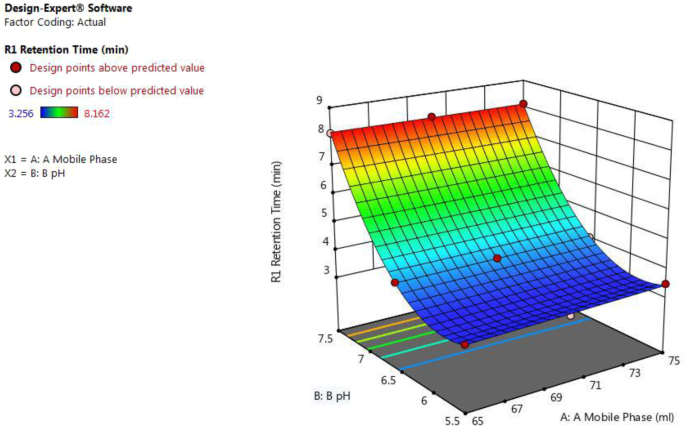
3D surface plot for effect of combination of factors on R1 retention time of ceftriaxone sodium by using central composite design
From Fig. 2 and equation theoretical plates (for actual values) = − 16774.36 − 4220.40 × A + 53225.20 × B + 56.05 × A × B + 26.83 × A 2 − 4380.60 × B 2 , it was concluded that as β 1 negative coefficient (− 4220.40) suggests that as the amount of acetonitrile in the mobile phase (A) decreases and β 2 positive coefficient (53225.20) suggests that as pH of buffer (B) increases, the value of theoretical plates was increased
3D surface plot for effect of combination of factors on R2 theoretical plate ceftriaxone sodium by using central composite design
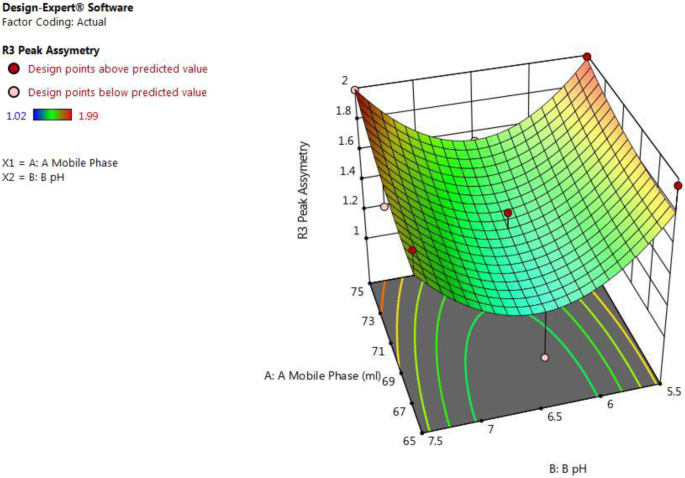
From Fig. 3 and equation peak asymmetry (for actual values) = 31.13 − 0.31 × A − 5.98 × B + 0.0055 × A × B + 0.0021 × A 2 + 0.429 × B 2 , it was concluded that as β 1 negative coefficient (− 0.31) suggests that as the amount of acetonitrile in the mobile phase (A) decreases and β 2 negative coefficient (− 5.98) suggests that as pH of buffer (B) decreases, the value of peak asymmetry was increased.
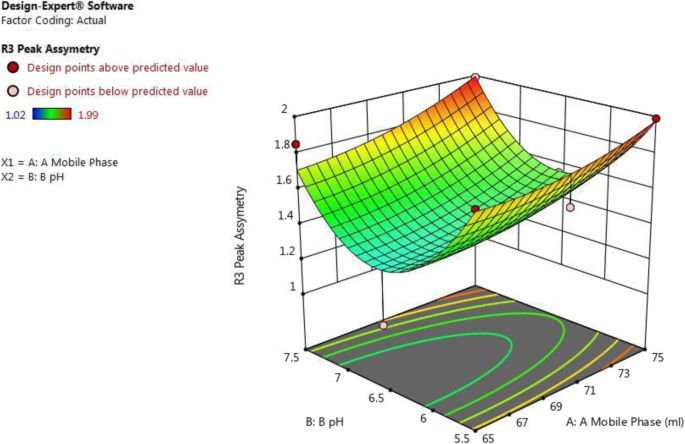
3D surface plot for effect of combination of factors on R3 peak asymmetry of ceftriaxone sodium by using central composite design
Optimized condition obtained
It was obtained by studying all responses in different experimental conditions using the Design expert 11.0 software, and optimized HPLC conditions and predicted responses are shown in Table 3 .
The observed value for responses was calculated by running the HPLC chromatogram for given set of mobile phase and pH of buffer and then compared with the predicted values to evaluate for % prediction error.
Method validation
System suitability.
The system suitability test was applied to a representative chromatogram to check the various parameters such as the retention time which was found to be 4.15 min, theoretical plates were 5263, peak asymmetry was 1.49, and % RSD of six replicate injections was 0.82. The 3D surface plot of desirability for obtaining optimized formulation is shown Fig. 4 .
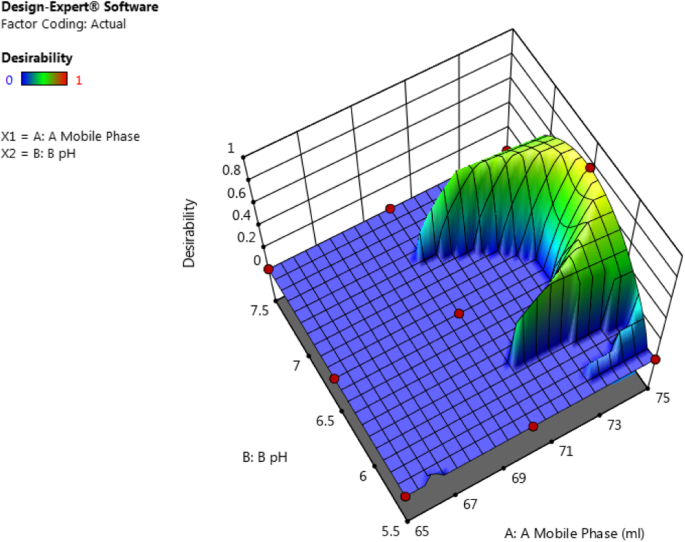
3D surface plot of desirability for obtaining optimized formulation
The constructed calibration curve for ceftriaxone sodium was linear over the concentration range of 10–200 μg/ml shown in Fig. 2 and Table 4 . Typically, the regression equation for the calibration curve was found to be y = 35441x + 60368 with a 0.991 correlation coefficient when graph was plotted with peak area verses concentration (Fig. 5 ).
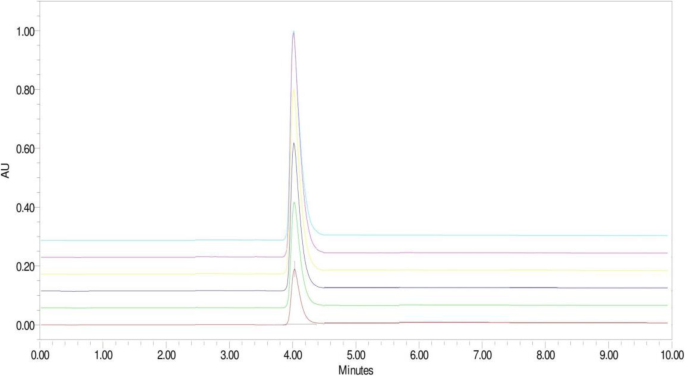
Linearity of 10–200 μg/ml ceftriaxone sodium
The % RSD for repeatability for ceftriaxone sodium based on six times the measurement of the same concentration (100 μg/ml) was found to be less than 0.082. Interday and intraday precisions were shown in Table 5 . The % RSD value less than 2 indicated that the developed method was found to be precise.
The accuracy was done by recovery study. Sample solutions were prepared by spiking at 3 levels, i.e., 80%, 100%, and 120%. The % recovery data obtained by the proposed HPLC method are shown in Table 6 . The % of recovery within 98–102% justify the developed method was accurate as per the ICH Q2 (R1) guidelines.
For robustness and ruggedness studies 100 μg/ml solution of ceftriaxone sodium was used. The robustness was studied by the slight but deliberate change in intrinsic method parameters like pH of mobile phase and flow rate. The ruggedness was studied by change in analyst as extraneous influencing factor. The % RSD for peak area were found to be less than 2 by change in pH of mobile phase, flow rate, and analyst.
The LOD and LOQ for ceftriaxone sodium based on standard deviation of slope and intercept were found to be 0.22 μg/ml and 0.67 μg/ml respectively.
The optimized chromatogram ceftriaxone sodium showed a resolved peak at retention time 4.15 min when performed assay from tablets. The % assay of drug content was found to be 99.73 ± 0.61 (n = 3) for label claim of ceftriaxone sodium. The assay result indicated the method’s ability to measure accurately and specifically in presence of excipients presents in tablet powder.
The analytical quality-by-design HPLC method for the estimation of ceftriaxone sodium in pharmaceutical formulation has been developed. The analytical target product profile were retention time, theoretical plates, and peak asymmetry for the analysis of ceftriaxone sodium by HPLC. The two variables namely the mobile phase composition and pH of buffer solution were identified as the critical quality attributes that affect the analytical target product profile. The central composite design was applied for two factors at three different levels with the use of the Design Expert Software Version 11.0. The risk assessment study identified the critical variables that have impact on analytical target profile [ 26 , 27 , 28 ]. In chromatographic separation, the variability in column selection, instrument configuration, and injection volume was kept controlled while variables such as pH of mobile phase, flow rate, and column temperature were assigned to robustness study.
The quality-by-design approach successfully developed the HPLC method for ceftriaxone sodium. The optimized RP-HPLC method for determination of ceftriaxone sodium used Phenomenex C18 column (250 × 4.6 mm, 5 μm particle size) and mobile phase consist of acetonitrile to water, 70:30 v/v, pH adjusted to 6.5 with 0.01% triethylamine buffer. The retention time for ceftriaxone sodium was found to be 4.15 min. The method was linear in the range of 10–200 μg/ml with 0.991 correlation coefficient. The % RSD for repeatability, intraday, and inter day precision was found to be less that 2% indicating the optimized method was precise. The LOD and LOQ were 0.22 μg/ml and 0.67 μg/ml, respectively. The % recovery of spiked samples was found to be 99.57 ± 1.47 to 100.79 ± 1.73 as per the acceptance criteria of the ICH guidelines. The method was developed as per the ICH guidelines.
A quality-by-design approach to HPLC method development has been described. The method goals are clarified based on the analytical target product profile. The experimental design describes the scouting of the key HPLC method components including mobile phase and pH. The analytical QbD concepts were extended to the HPLC method development for ceftriaxone sodium, and to determine the best performing system and the final design space, a multivariant study of several important process parameters such as the combination of 2 factors namely the mobile phase composition and pH of buffer at 3 different levels was performed. Their interrelationships were studied and optimized at different levels using central composite design. Here, a better understanding of the factors influencing chromatographic separation in the ability of the methods to meet their intended purposes is done. This approach offers a practical knowledge understanding that help for the development of a chromatographic optimization that can be used in the future. All the validated parameters were found within the acceptance criteria. The validated method was found to be linear, precise, accurate, specific, robust, and rugged for determination of ceftriaxone sodium. The QbD approach to method development has helped to better understand the method variables hence leading to less chance of failure during method validation and transfer. The automated QbD method development approach using the Design Expert software has provided a better performing more robust method in less time compared to manual method development. The statistical analysis of data indicates that the method is reproducible, selective, accurate, and robust. This method will be used further for routine analysis for quality control in pharmaceutical industry.
Availability of data and materials
All data and material are available upon request.
Abbreviations
- Quality by design
Active pharmaceutical ingredient
Central composite design
Critical quality attribute
High-performance liquid chromatography
Reverse phase high-performance liquid chromatography
Limit of quantitation
Limit of detection
Relative standard deviation
Sandipan R (2012) Quality by design: A holistic concept of building quality in pharmaceuticals. Int J Pharm Biomed Res 3:100–108
Google Scholar
The International Conference on Harmonisation ICH Technical Requirements for Registration of Pharmaceuticals for Human Use on Pharmaceutical Development Q8(R2) (2009) https://database.ich.org/sites/default/files/Q8%28R2%29%20Guideline.pdf
The International Conference on Harmonisation ICH Technical Requirements for Registration of Pharmaceuticals for Human Use on Quality Risk Management Q9 (2005) https://database.ich.org/sites/default/files/Q9%20Guideline.pdf
The International Conference on Harmonisation ICH Technical Requirements for Registration of Pharmaceuticals for Human Use on Pharmaceutical Quality System Q10 (2008) https://database.ich.org/sites/default/files/Q10%20Guideline.pdf
Borman P, Nethercote P, Chatfield M, Thompson D, Truman K (2007) The application of quality by design to analytical methods. Pharm Tech 31:142–152
Schweitzer M, Pohl M, Hanna BM, Nethercote P, Borman P, Hansen G, Smith K, Larew J (2010) Implications and opportunities of applying QbD principles to analytical measurements. Pharm Tech 34:52–59
CAS Google Scholar
Galen WE (2004) Analytical Instrumentation Handbook 2nd edn. Marcel Dekker Inc, New York
Snyder LR, Kirkland JJ, Glajch LJ (1997) Practical HPLC method development; 2nd edn. John Wiley & Sons Inc, New York. https://doi.org/10.1002/9781118592014
Book Google Scholar
Bhatt D, Rane S (2011) QbD approach to analytical RP-HPLC method development and its validation. Int J Pharm Pharm Sci 3:79–187
Rajkotwala A, Shaikh S, Dedania Z, Dedania R, Vijyendraswamy S (2016) QbD approach to analytical method development and validation of piracetam by HPLC. World J Pharmacy Pharmaceutical Sci 5:1771–1784
Singh P, Maurya J, Dedania Z, Dedania R (2017) QbD Approach for stability indicating HPLC method for determination of artemether and lumefantrine in combined dosage form. Int J Drug Reg Affairs 5:44–59
Article CAS Google Scholar
Prajapati R, Dedania Z, Jain V, Sutariya V, Dedania R, Chisti Z (2019) QbD approach to HPLC method development and validation for estimation of fluoxetine hydrochloride and olanzapine in pharmaceutical dosage form. J Emerging Tech Innovative Res 6:179–195
Dhand V, Dedania Z, Dedania R, Nakarani K (2020) QbD approach to method development and validation of orciprenaline sulphate by HPLC. J Global Trends Pharm Sci 11:8634–8640
Krull I, Swartz M, Turpin J, Lukulay P, Verseput R (2008) A quality-by-design methodology for rapid LC method development, part I. Liq Chroma Gas Chroma N Am 26:1190–1197
Myers R, Montgomery D, Anderson-Cook C (2016) Response surface methodology: process and product optimization using designed experiments. 4th edn. New York: Wiley
Yubing T (2011) Quality by design approaches to analytical methods- FDA perspective. https://www.fda.gov/files/about%20fda/published/Quality-by-Design-Approaches-to-Analytical-Methods%2D%2D%2D%2DFDA-Perspective%2D%2DYubing-Tang%2D%2DPh.D.%2D%2DOctober%2D%2D2011%2D%2DAAPS-Annual-Meeting.pdf . Accessed 15 Dec 2018.
Krull I, Swartz M, Turpin J, Lukulay P, Verseput R (2009) A quality-by-design methodology for rapid LC method development part II. Liq Chroma Gas Chroma N Am 27:48–69
Reid G, Morgado J, Barnett K, Harrington B, Wang J, Harwood J, Fortin D (2013) Analytical QbD in pharmaceutical development. https://www.waters.com/nextgen/in/en/library/application-notes/2019/analytical-quality-by-design-based-method-development-for-the-analysis-of-formoterol-budesonide-and-related-compounds-using-uhplc-ms.html . Accessed 10 June 2018.
Molnar RH, Monks K (2010) Aspects of the “Design Space” in high pressure liquid chromatography method development. J Chromatogra A 1217(19):3193–3200. https://doi.org/10.1016/j.chroma.2010.02.001
Monks K, Molnar I, Rieger H, Bogati B, Szabo E (2012) Quality by design: multidimensional exploration of the design space in high performance liquid chromatography method development for better robustness before validation. J Chromatogra A 1232:218–230. https://doi.org/10.1016/j.chroma.2011.12.041
Ramalingam P, Kalva B, Reddy Y (2015) Analytical quality by design: a tool for regulatory flexibility and robust analytics. Int J Ana Chem. https://doi.org/10.1155/2015/868727
The International Conference on Harmonisation ICH Technical Requirements for Registration of Pharmaceuticals for Human Use on Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities) Q11 (2012) https://database.ich.org/sites/default/files/Q11%20Guideline.pdf
Orlandini S, Pinzauti S, Furlanetto S (2013) Application of quality by design to the development of analytical separation methods. Ana Bioana Chem 405(2-3):443–450. https://doi.org/10.1007/s00216-012-6302-2
The International Conference on Harmonisation ICH Technical Requirements for Registration of Pharmaceuticals for Human Use on Validation of Analytical Procedures: Text and Methodology Q2(R1) (2005) https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
Reid G, Cheng G, Fortin D (2013) Reversed-phase liquid chromatographic method development in an analytical quality by design framework. J Liq Chrom Related Tech 36(18):2612–2638. https://doi.org/10.1080/10826076.2013.765457
Elder P, Borman P (2013) Improving analytical method reliability across the entire product lifecycle using QbD approaches. Pharmaceu Outsourcing, 14:14–19. http://www.pharmoutsourcing.com/Featured-Articles/142484-Improving-Analytical-Method-Reliability-Across-the-Entire-Product-Lifecycle-Using-QbD-Approaches/ . Accessed 2019.
Smith J, Jones M Jr, Houghton L (1999) Future of health insurance. N Engl J Med 965:325–329
Schweitzer M, Pohl M, Hanna-Brown M (2010) Implications and opportunities of applying QbD principles to analytical measurements. Pharmaceu Tech 34:52–59
Download references
Acknowledgements
All authors are very thankful to the Bhagwan Mahavir College of Pharmacy, Surat, for providing necessary facilities to carry out the research work.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Quality Assurance, Bhagwan Mahavir College of Pharmacy, Vesu, Surat, Gujarat, India
Krunal Y. Patel, Zarna R. Dedania & Ronak R. Dedania
Department of Chemistry, The University of Alabama in Huntsville, 301 Sparkman Dr, Huntsville, AL-35899, USA
Unnati Patel
You can also search for this author in PubMed Google Scholar
Contributions
All authors associated with this research work declared that there is no conflict of interest for publication of work. All authors have read and approved the manuscript. The contribution of each author is mentioned below. KP: He is a M Pharm (Quality Assurance) Research Student and the above work has been carried out by him as dissertation work. ZD: She is Research Guide and HOD, Department of Quality Assurance and under her noble guidance the QbD approach for HPLC method has been developed and validated as per ICH guidelines. She is also giving training for ease of operation sophisticated instrument and involved in interpretation of data. RD: He is a co-guide and under his noble guidance student can understand the Design Expert Software and interpretation of statistical data. UP: She is a graduate teaching assistant at University of Alabama at Huntsville, USA and she has contributed for preparing the manuscript.
Corresponding author
Correspondence to Zarna R. Dedania .
Ethics declarations
Ethics approval and consent to participate.
Not applicable
Consent for publication
Competing interests.
No competing interests to declare.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Patel, K.Y., Dedania, Z.R., Dedania, R.R. et al. QbD approach to HPLC method development and validation of ceftriaxone sodium. Futur J Pharm Sci 7 , 141 (2021). https://doi.org/10.1186/s43094-021-00286-4
Download citation
Received : 04 December 2020
Accepted : 18 June 2021
Published : 13 July 2021
DOI : https://doi.org/10.1186/s43094-021-00286-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ceftriaxone sodium
- Design approach
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Development and Validation of an HPLC-UV Method for the Quantification of 4′-Hydroxydiclofenac Using Salicylic Acid: Future Applications for Measurement of In Vitro Drug–Drug Interaction in Rat Liver Microsomes
Associated data.
The data presented in this study are available in this article.
Salicylic acid is a key compound in nonsteroidal anti-inflammatory drugs that has been recently used for preventing the risk of hospitalization and death among COVID-19 patients and in preventing colorectal cancer (CRC) by suppressing two key proteins. Understanding drug–drug interaction pathways prevent the occurrence of adverse drug reactions in clinical trials. Drug–drug interactions can result in the variation of the pharmacodynamics and pharmacokinetic of the drug. Inhibition of the Cytochrome P450 enzyme activity leads to the withdrawal of the drug from the market. The aim of this paper was to develop and validate an HPLC-UV method for the quantification of 4′-hydroxydiclofenac as a CYP2C9 metabolite using salicylic acid as an inhibitor in rat liver microsomes. A CYP2C9 assay was developed and validated on the reversed phase C 18 column (SUPELCO 25 cm × 4.6 mm × 5 µm) using a low-pressure gradient elution programming at T = 30 °C, a wavelength of 282 nm, and a flow rate of 1 mL/min. 4′-hydroxydiclofenac demonstrated a good linearity (R 2 > 0.99), good reproducibility, low detection, and quantitation limit, and the inter and intra-day precision met the ICH guidelines (<15%). 4′-hydroxydiclofenac was stable for three days and showed an acceptable accuracy and recovery (80–120%) within the ICH guidelines in a rat liver microsome sample. This method will be beneficial for future applications of the in vitro inhibitory effect of salicylic acid on the CYP2C9 enzyme activity in rat microsomes and the in vivo administration of salicylic acid in clinical trials.
1. Introduction
A wide variety of clinical, physiological, and toxicological drugs are metabolized by human cytochrome P450 (CYP) enzymes [ 1 ]. Cytochrome P450 enzymes are a membrane-bound hemi-protein family that is biologically responsible for metabolizing a vast majority of hydrophobic xenobiotics into hydrophilic molecules [ 1 ]. Cytochrome P450 (CYP) enzymes are the main skeleton in clinical pharmacology and toxicology research [ 1 ].
The pharmacokinetic properties of a drug are a relatively important aspect in drug metabolism pathway identification [ 2 ]. The drug discovery and development pathways involve enzyme determination, which is responsible for the metabolism of a novel drug and the inhibition or induction of phase 1 and 2 enzymes by the new drug [ 2 ]. Inhibition occurs when the enzyme is inactivated or when the substrate does not bind to the catalytic site; thus, it can lead to a drug–drug interactions [ 3 ]. Currently, most drugs are oxidized or reduced by cytochrome P450s enzymes. Most P450s are expressed in the endoplasmic reticulum (ER) of organs such as the lungs, kidney, intestines, liver, and brain [ 2 ].
Interestingly, most marketed drugs are metabolized by six different P450 isozymes [ 4 ]. These isozymes are CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP2E1, and CYP3A4 [ 4 ]. The four CYP2C subfamilies in humans are known as CYP2C8, CYP2C18, CYP2C9, and CYP2C19 [ 5 ]. Diclofenac ( Figure 1 ) is one of many CYP2C9 drug substrates, such as naproxen, ibuprofen, phenytoin, tolbutamide, warfarin, suprofen, and piroxicam [ 5 ]. It is known as a nonsteroidal anti-inflammatory drug (NSID) used for the treatment of rheumatoid arthritis, osteoarthritis for long term treatment, and acute musculoskeletal injury for a short period of time [ 6 ].

Chemical structure of ( 1 ) salicylic acid, ( 2 ) diclofenac, ( 3 ) 4′-hydroxydiclofenac, and ( 4 ) 4-hydroxyoctanophenone.
Cytochrome P450 (CYP2C9) is a phase 1 polymorphic enzyme that works as a major pathway in metabolizing different clinically approved drugs such as anti-bacterial, anti-inflammatory, anticancer, and antihypertensive drugs [ 7 ]. Statistically, 70% of FDA approved drugs are metabolized by the CYP2C9 enzyme [ 8 ]. An investigational study showed that four major metabolites were detected in the human urine through HPLC analysis [ 9 ]. These metabolites are 3′-hydroxydiclofenac, 4′-hydroxydiclofenac, 5′-hydroxydiclofenac, and 3′-hydroxy-4′-methoxydiclofenac [ 9 ]. A previous paper showed that diclofenac is metabolized to 4′-hydroxydiclofenac and 5′-hydroxydiclofenac in rat liver microsomes, whereas in human liver microsomes, it metabolized to only 4′-hydroxydiclofenac [ 10 ]. The metabolite 5′-hydroxydiclofenac is formed predominantly by the CYP3A4 enzyme and by CYP2C19, CYP2C8, and CYP2C18 to a lesser extent [ 6 ].
A previous investigational study showed that diosmetin (flavone) competitively inhibited the CYP2C9 enzyme activity in pooled human liver microsomes [ 11 ]. Morusin is known as a herbal plant that possesses many pharmacological effects such as anti-tumor, anti-oxidant, anti-bacterial, and anti-inflammatory properties [ 12 ]. This study showed that morusin can inhibit the CYP2C9 enzyme activity non-competitively in human liver microsomes [ 12 ]. Another in vitro inhibition study done by Li et al. (2020) [ 13 ] showed that lysionotin (natural flavonoid) does not inhibit the CYP2C9, CYP1A2, CYP2A6, CYP2E1, and CYP2D6 enzyme activities. Therefore, lysionotin was safe to be taken with other drugs that are substrates for CYP2C9, CYP1A2, CYP2A6, CYP2E1, and CYP2D6 enzymes.
Salicylic acid ( Figure 1 ) is an anti-inflammatory drug excreted from wintergreen leaves and white willow trees that acts as an antioxidant in the pharmacological domain [ 14 ]. Aspirin is known as an antiplatelet agent that is recommended and encouraged to be taken by patients with COVID-19 [ 15 ]. A previous study demonstrated that salicylic acid and its metabolite (2,3-dihydroxybenzoic acid (2,3-DHBA), (2,4-DHBA), (2,5-DHBA), and (2,6-DHBA)) inhibited cyclin-dependent kinase (CDK 1,2,4, and 6) activity and downregulated the function of cyclin A2, B1, and D3 by targeting colon cancer tumor cells [ 14 ].
Our recent investigational studies revealed that salicylic acid has a low potential effect on CYP2C11 in rat liver microsomes [ 16 ]. In other words, salicylic acid non-competitively inhibited the CYP2C11 enzyme activity. Additionally, our previous research confirmed that salicylic acid acts as a mixed inhibitor (competitive and non-competitive) for the CYP2E1 enzyme in rat liver microsomes [ 17 ]. This means that salicylic acid has a low and high potential to cause interactions with other drugs that are a substrate of the CYP2E1 enzyme in the rat liver microsomes. According to our recent publication, salicylic acid uncompetitively inhibited the UGT2B17 enzyme activity in human supersomes [ 18 ]. This implies that salicylic acid has a negligible effect to cause a drug interaction with other UGT2B17 substrate drugs.
Recently, many studies have been reported regarding the method development and validation of diclofenac and 4′-hydroxydiclofenac in the plasma and rat serum [ 19 ]. The study by Kaphalia, et al. (2006) demonstrated a low detection limit of diclofenac and 4′-hydroxydiclofenac; however, this method was extracted by liquid–liquid extraction, which is not a simple and direct method compared with other methods for protein precipitation.
However, there are no studies regarding the method development and validation of 4′-hydroxydiclofenac with salicylic acid in the rat liver microsomes. Thus, the purpose of this paper was to develop and validate an efficient HPLC-UV method for the quantification of 4′-hydroxydiclofenac as a typical CYP2C9 metabolite using salicylic acid as an inhibitor in the rat liver microsome sample for the future evaluation of the inhibitory effect of salicylic acid on CYP2C9 enzyme activity. In this research paper, an HPLC-UV method was optimized and validated for a CYP2C9 assay using a salicylic acid inhibitor for the future investigation of the in vitro potential effect of salicylic acid on 4′-hydroxydiclofenac formation in rat microsomes.
2. Results and Discussion
2.1. method development, 2.1.1. uv–vis (ultraviolet-visible) spectroscopy.
Salicylic acid (100 µM), diclofenac (200 µM), 4′-hydroxydiclofenac (100 µM), and 4-hydroxyoctanophenone (50 µM) were analyzed by UV–VIS spectrophotometry for the measurement of the maximum wavelength of each component. All standard solutions were dissolved in pure methanol (wavelength cut-off of HPLC methanol was 210 nm).
The UV–VIS spectrum below illustrates the measurements of the maximum absorbance of each CYP2C9 components.
According to Figure 2 , the best-chosen wavelength on HPLC is 282 nm, as all of the compounds have a maximum absorption band at this wavelength. According to our previous study, salicylic acid absorbs at 282 nm wavelength (Salhab, H. et al. 2020). A study performed by Li and their colleague (2020) [ 13 ] showed that the CYP2C9 assay (diclofenac and 4′-hydroxydiclofenac) is absorbed at a 280 nm wavelength. Therefore, a 282 nm wavelength was chosen as an analytical parameter for our HPLC instrument.

UV–VIS spectrum of salicylic acid, diclofenac, 4′-hydroxydiclofenac, and 4-hydroxyoctanophenone in the CYP2C9 assay.
2.1.2. Robustness Test
A UV-HPLC method was employed to quantify the concentration of 4′-hydroxydiclofenac in rat liver microsomes. The application of this method led to higher sensitivity and lower detection limit compared with other papers in the literature. The chromatographic separation of salicylic acid, 4-hydroxyoctanophenone, diclofenac, and 4′-hydroxydiclofenac components were performed on a SUPELCO C18 column (25 cm × 4.6 mm, 5 µm particle size) using a gradient elution consisting of 0.1% formic acid in water as the mobile phase (A), acetonitrile as the mobile phase (B), and methanol as the mobile phase (C). A low-pressure isocratic elution made up of 80% methanol and 20% of water (0.1% formic acid) was used; however, the compounds were coeluted with each other. 4-hydroxyoctanophenone was chosen as an internal standard because various internal standards have been tested (caffeine and phenacetin) and the results showed that these compounds co-elute with the compound of interest when using this mobile phase. The total run time was 13 min, which was more rapid than the other method in the literature [ 19 ]. The spiked liver microsome samples were extracted by protein precipitation, which is more simple, direct, convenient, and time saving compared with the liquid–liquid extraction method [ 19 ].
Variation in Column Temperature
The retention time and area peak for each component were analyzed with HPLC using different sets of column temperature. Table 1 and Table 2 show the retention time and the average area peak of the components at T = 30 °C, T = 35 °C, and T = 25 °C. Based on Table 1 and Table 2 , components were eluted earlier at a temperature of 30 °C. A temperature of T = 30 °C was the best-chosen analytical parameter for these compounds, as it gives a better resolution between 4′-hydroxydiclofenac and diclofenac (2.701 min) compared with T = 25 °C (2.446 min) and between 4′-hydroxydiclofenac and salicylic acid (2.691 min) compared with T = 35 °C (2.447 min). The average area peak for each component at T = 30 °C was approximately the same at T = 25 °C and T = 35 °C.
The effect of decreasing temperature on both the area peak and retention time of each component in the rat liver microsomes.
The effect of decreasing the temperature on both the area peak and retention time of each compound in rat liver microsomes.
An ANOVA test with a single factor was performed to evaluate the effect of the average area peak and retention time of each component at T = 25 °C, T = 30 °C, and T = 35 °C. Based on the ANOVA test results, there was no statistically significant difference in the average area peak of each component at T = 25 °C, T = 30 °C, and T = 35 °C. The p -value = 0.9937 with a variance of 0.1091 at a significant level was set at α = 0.05 for T = 30 °C and the p -value = 0.9959 with a variance of 0.1091 was set at T = 35 °C. Therefore, changing the column temperature from T = 25 °C to T = 30 °C and T = 35 °C leaves the average area peak and retention time for each component unaffected. Thus, this method is robust for column temperature variation.
Variation in Flow Rate
The retention time and area peak for each component were analyzed with HPLC using different set of flow rate. According to Table 3 and Table 4 , the components were eluted much earlier at 1.2 mL/min compared with 0.8 mL/min and 1 mL/min. A flow rate of 1 mL/min was the best-chosen analytical parameter for the method, as these components were eluted earlier compared with a flow rate of 0.8 mL/min. In addition, a better resolution was obtained between 4-hydroxyoctanophenone and diclofenac (0.720 min) at a flow rate of 1 mL/min compared with 0.8 mL/min (0.709 min). The resolution between 4′-hydroxy diclofenac and salicylic acid at 1 mL/min (2.691 min) was greater than the resolution between 4′-hydroxydiclofenac and salicylic acid at 1.2 mL/min (2.232 min). The average area peak for each component at a flow rate of 1 mL/min was approximately the same at 0.8 mL/min and 1.2 mL/min.
The effect of changing flow rate on both area peak, and retention time of each component in rat liver microsomes.
The effect of changing the flow rate on both retention time and area peak of the components in rat liver microsomes.
An ANOVA test with a single factor was performed to evaluate the effect of the average area peak and retention time of each component at 0.8 mL/min, 1 mL/min, and 1.2 mL/min. Based on the ANOVA test results, there was a slight statistically significant difference in the average area peak of each component at 0.8 mL/min and 1mL/min. The p -value = 0.7522 and the variance (0.248 > 0.05) suggest that is not statistically significant at a significant level set at α = 0.05 at 1ml/min. The p -value = 0.7764 has a variance of (0.224 > 0.05) at 1.2 mL/min. Therefore, changing the flow rate from 1 mL/min to 0.8 mL/min and to 1.2 mL/min keeps the average area peak and retention time for each component mostly unaffected. Thus, this method is robust for flow rate variation.

2.2. Method Validation
2.2.1. linearity and range.
A set of 4′-hydroxydiclofenac concentrations (0, 5, 20, 40, 50, 80, and 100 μM) dissolved in HPLC methanol were added in each HPLC vial containing 50 μM of 4-hydroxyoctanophenone (Internal Standard) and were injected into the HPLC instrument, and each vial was analyzed three times (n = 3). A standard calibration curve of 4′-hydroxydiclofenac was constructed using the average peak area as a function of the concentration of 4′-hydroxydiclofenac using the Microsoft Excel 2010 software system. A linear calibration curve was obtained (y = 0.0121x + 0.004, R 2 = 0.9996) (standard error = 0.0055/intercept = 0.0064), as shown in Figure 3 below. The linear regression coefficient R 2 > 0.99 met the analytical ICH guidelines. The linear range of 4′-hydrodiclofenac was between 5 and 100 μM.

Calibration curve of 4′-hydroxydiclofenac.
2.2.2. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
The outcome summarized in Table 5 demonstrates that the CYP2C9 metabolite (4′-hydroxydiclofenac) has low detection and quantitation limit values. Moreover, the LOQ value (4.67 μM) for 4′-hydroxydiclofenac is less than the lowest concentration level of 4′-hydroxydiclofenac (5 μM). Therefore, these numerical numbers met the analytical ICH guidelines.
LOD and LOQ for 4′-hydroxydiclofenac in rat liver microsomes.
2.2.3. Precision
Intra-assay variation of 4′-hydroxydiclofenac in rat liver microsomes.
The intra-assay precision for 4′-hydroxydiclofenac was determined by quantifying three concentration levels (60, 30, and 10 μM) three times using an HPLC instrument. A calibration curve of 4′-hydroxydiclofenac at concentrations of 100, 80, 50, 40, 20, 5, and 0 μM was injected three times onto a HPLC instrument and a linear calibration curve was obtained (y = 0.0137x − 0.0003, R 2 = 0.999) (standard error = 0.0084/intercept = 0.0084). The linear regression R 2 > 0.99 met the ICH guidelines. The three concentration levels were determined from the calibration curve, as shown in Table 6 below. The percentage relative standard deviation was less than 5% in each concentration level according to ICH guidelines. In addition, this method showed that the protein matrix did not affect the analyte solution as it was <5%.
Intra-assay variation for 4′-hydroxydiclofenac (n = 3) in rat liver microsomes.
Inter-Assay Variation of 4′-Hydroxydiclofenac in Rat Liver Microsomes
Inter-assay precision for 4′-hydroxydiclofenac was determined by quantifying three concentration levels (60, 30, and 10 μM) three times on an HPLC instrument for three consecutive days (days 1, 2, and 3). A calibration curve of 4′-hydroxydiclofenac at concentrations of 100, 80, 50, 40, 20, 5, and 0 μM was injected three times using an HPLC instrument on day 1 and a linear calibration curve was obtained (y = 0.0137x − 0.0003, R 2 = 0.999) (standard error = 0.0084/intercept = 0.0084) (see Figure S1 ). A calibration curve of 4′-hydroxydiclofenac at concentrations of 100, 80, 50, 40, 20, 5, and 0 μM was injected three times using an HPLC instrument on day 2 and a linear calibration curve was obtained (y = 0.0121x + 0.004, R 2 =0.9996) (standard error = 0.0055/intercept = 0.0064) (see Figure S2 ). A calibration curve of 4′-hydroxydiclofenac at concentrations of 100, 80, 50, 40, 20, 5, and 0 μM was injected three times using an HPLC instrument on day 3 and a linear calibration curve was obtained (y = 0.0117x + 0.0041, R 2 = 0.9999) (standard error = 0.0036/intercept = 0.0044) (see Figure S3 ).
The linear regression R 2 > 0.99 for days 1, 2, and 3 met the ICH guidelines. The three calculated concentrations at each level were determined from the calibration curves on days 1, 2, and 3, as shown in Table 7 below. The percentage relative standard deviation was less than 10% in each concentration level according to ICH guidelines. In addition, this method showed that the protein matrix obtained ranging between 1–3% did not affect the analyte solution analysis.
Inter-assay precision of 4′-hydroxydiclofenc (n = 3) in the rat liver microsomes.
2.2.4. Specificity and Selectivity
A negative control blank of 50 µM of 4-hydroxyoctanophenone dissolved in HPLC acetonitrile with the spiked rat liver microsomes was injected three times to demonstrate that the method results were not affected by impurities, which determined that our method was selective.
Achievement of specificity was done by demonstrating the best mobile phase composition (50% H 2 O in 0.1% formic acid + 15% methanol + 35% acetonitrile) using a low-pressure gradient elution system ( Table 8 ). This resulted in a good analytical separation between the components at T = 30 °C using C18 (SUPELCO 25 cm × 4.6 mm, 5 µm) at a wavelength of 282 nm and a flow rate of 1 mL/min. Each compound containing spiked rat liver microsomes was run separately to determine the retention time of each compound at the above specified conditions (see Figure 4 , Figure 5 and Figures S4–S6 ).

HPLC chromatogram of the negative control blank containing rat liver microsomes.

Typical HPLC chromatogram of the components at 282 nm using gradient elution programming in rat liver microsome. The peaks marked are (1) salicylic acid (100 µM) (RT = 4.006 min), (2) 4′-hydroxydiclofenac (100 µM) (RT = 6.681 min), (3) diclofenac (200 µM) (RT = 9.353 min), and (4) 4-hydroxyoctanophenone (50 µM) (RT = 10.079 min), respectively.
Calculated analytical parameters for the CYP2C9 components.
N = 5.54 (R t /W 0.5 ); H = column Length/N. AsF = B/A, where A is the distance from the leading edge of the peak to the midpoint of the peak measured at 10% of peak height, and B is the distance from the midpoint of the peak to the trailing edge of the peak measured at 10% of the peak height. Rs = 2Δt R /0.5(W 1 +W 2 ), where W is the width at the peak base.
2.2.5. Chromatography Fundamentals Calculations for CYP2C9 Components
It is important to calculate the chromatography fundamentals for each component for the achievement of best separation. Table 8 illustrates the calculated column efficiency (N), height plate (H), asymmetry factor (AF), and resolution (R S ) for each CYP2C9 compound. All of the chromatography fundamentals were calculated at 100 µM for salicylic acid and 4-hydroxydiclofenac, 50 µM for 4′-hydroxyoctanophenone, and 200 µM for diclofenac.
According to Table 8 , the tailing factor for each analytical compound that was obtained was less than 1.5; ICH recommendations for system suitability are set at a tailing factor of <2. Thus, these chromatography fundamentals illustrate that our methodology is suitable with a run time of 11 min. In this method, the resolution between diclofenac and 4′-hydroxyoctanophenone was 3.30 higher than the resolution between diclofenac and ibuprofen (2.06) compared with the critical value of <1.5 [ 20 ].
2.2.6. Stability Test
Stability test of 4′-hydroxydiclofenac in rat liver microsomes.
Three concentration levels (10, 30, and 60 µM) of 4′-hydroxydiclofenac were assigned as low, medium, and high levels, respectively, in the rat liver microsomes stored for 48 h under natural light conditions and were injected three times with HPLC. A range of 4′-hydroxydiclofenac concentrations (100, 80, 60, 50, 40, 30, 20, 10, and 5 µM) for each day was determined in the presence of 4-hydroxyoctanophenone (C = 50 µM). The stability outcomes are summarized in Table 9 .
Stability test outcomes for 4′-hydroxydiclofenac in the rat liver microsomes.
a % recovery = (concentration of 4′-hydroxydiclofenac at 24 h/standard concentration of 4′-hydroxydiclofenac) × 100. b % Accuracy = 100 − ((calculated concentration − actual concentration)/actual concentration) × 100.
It is clearly shown that the calculated concentration of 4′-hydroxydiclofenac at each actual concentration level did not change significantly. Calibration curves were constructed at 0, 24, and 48 h, and they were as follows: y = 0.0115x + 0.0136 (r 2 = 0.9994) (day 1), y = 0.0126x + 0.0099 (r 2 = 0.9993) (day 2), and y = 0.0124x − 0.0012 (r 2 = 0.9991) (day 3) (see Figures S7 and S8 ) where r 2 met the ICH guidelines (r 2 > 0.99). As shown in Table 9 , the percentage recovery of three concentration levels at 24 and 48 h were within the acceptable recovery range (80–120%) according to ICH guidelines. In addition, the percentage accuracy of the three concentration levels of 4′-hydroxydiclofenac met the ICH guidelines (80–120%). Thus, our finding demonstrated that the 4′-hydroxydiclofenac solution was relatively stable for three consecutive days in the rat liver microsomes at room temperature, which is in agreement with the literature [ 21 ].
A previous study demonstrated that the HPLC method for the metabolism profile of diclofenac in liver slices was developed with a run time of 30 min [ 22 ]; however, the method was not optimized because of the long run time. Our method showed that diclofenac and 4′-hydroxydiclofenac using 4-hydroxyoctanophenone as an internal standard was developed and optimized with a run time of 13 min.
Various analytical techniques have been reported for the quantification of diclofenac in human plasma using the GC-MS technique [ 23 ]. The method optimized by Shah et al. (2016) showed a sensitive, robust, specific, and reproducible method for the quantification of diclofenac in human plasma. However, our method provided considerable improvement over this method, with increased accuracy and precision. Furthermore, precipitation of the protein through centrifuging without using derivatizing agents such as hexane, heptane, or benzene resulted in a high recovery >95%. In addition, the derivatizing agents used in GC-MS are quiet toxic extraction solvents [ 23 ].
A study performed on the separation of acyl glucuronide isomers of diclofenac by cyclic ion mobility spectrometry (cIM) mass spectrometry and LC-MS showed a high-throughput effective and rapid method compared with typical HPLC techniques [ 24 ]. However, the results demonstrated that 2/3 O-acyl and 4 O-acyl glucuronide isomers of diclofenac co-elute with each other, and the shape of the compound peaks is broad compared with the peak shape of the compounds.
Our HPLC method in the rat liver microsomes sample was found to be sensitive, direct, effective, low cost, and accurate for satisfying the bioanalytical method validation of ICH guidelines for the future application of pharmacokinetic in vitro and in vivo drug–drug interactions compared with other reported methods [ 25 ].
3. Materials and Methods
3.1. chemicals.
Chromatographic HPLC methanol, acetonitrile, and water were purchased from Sigma Aldrich, Co. (Old Brickyard, Gillingham, UK). HPLC analytical grade reagents 4-hydroxyoctanophenone (>98%), diclofenac (CYP2C9 substrate), and salicylic acid were procured from Sigma Aldrich, Co. (Old Brickyard, Gillingham, UK). 4′-Hydroxydiclofenac (CYP2C9 metabolite) was obtained from Carbosynth, Ltd. (Old Station Business Park, Compton, UK). Rat liver microsomes were purchased from Sigma Aldrich, Co. (Old Brickyard, Gillingham, UK) and stored at −80 °C.
3.2. Instruments
A UV–VIS spectrophotometry instrument with 1 cm length of quartz cuvette was used and was obtained from VWR International Ltd. (Magna Park, Lutterworth, Leicestershire, LE17 4XN, UK). The UV spectra were obtained using Chem Station Software from Agilent Technologies LDA (Cheadle Royal Business Park, Stockport, Cheshire, SK8 3GR, UK). A 570 pH meter was used and was obtained from Thermo-Fisher Scientific (Boundary Park, Hemel Hempstead, UK). An LC-2010A Shimadzu high performance liquid chromatography system (Shimadzu, Kyoto, Japan) combined with a degasser, a series 200 Peltier LC column oven, a series of 200 UV detector, a series of 200 autosampler, and an equipped quaternary gradient low-pressure pump was used for the chromatographing analysis. Target analytes were separated on a SUPELCO C18 column (25 cm × 4.6 mm, 5 µm particle size) that was purchased from Merck (Old Brickyard, Gillingham, UK).
3.3. Analytical Wavelength Selection
The UV–VIS spectrum was performed by preparing standard solutions of diclofenac (200 µM), 4′-hydroxydiclofenac (100 µM), and 4-hydroxyoctanophenone as an internal standard (50 µM) and salicylic acid (100 µM) in pure methanol.
3.4. High Performance Liquid Chromatography (HPLC) Analysis
3.4.1. rat liver microsomes sample preparation.
The frozen rat liver microsomes samples were thawed at room temperature and were vortex mixed. For the specificity and robustness of the method, an aliquot of 20 µL of rat liver microsomes was vortex mixed for 2 min with 1480 µL of standard solutions containing 50 µM of 4-hydroxyoctanophenone (internal standard), 100 µM of salicylic acid, 200 µM of diclofenac, and 100 µM of 4′-hydroxydiclofenac. After centrifuging at 14,000 rpm for 10 min, 1 mL of the supernatant was transferred for the HPLC analysis.
For the analysis of 4′-hydroxydiclofenac, an aliquot of 20 µL of rat liver microsomes was vortex-mixed for 2 min with 1480 µL of standard solution 4′-hydroxydiclofenac (0, 5, 10, 15, 20, 40, 50, 60, 80, and 100 µM) containing 50 µM of 4-hydroxyoctanophenone. After centrifuging at 14,000 rpm for 10 min, 1 mL of supernatant of each 4′-hydroxydiclofenac concentration was transferred for the HPLC analysis.
3.4.2. Preparation of Salicylic Acid, Diclofenac, 4-Hydroxyoctanophenone Stock and Standard Solutions in Rat Liver Microsomes
A 200 µM concentration of salicylic acid stock solution was prepared by dissolving 1.38 mg in 50 mL of HPLC-grade methanol. A 200 µM concentration of Diclofenac stock solution was prepared by dissolving it in 50 mL of methanol.
A 50 µM of 4-hydroxyoctanophenone solution was prepared by dissolving 1.1 mg powder in 100 mL of HPLC-grade acetonitrile.
3.4.3. Preparation of 4′-Hydroxydiclofenac Stock and Standard Solutions in Rat Liver Microsomes
A stock of 100 µM was prepared by dissolving 1.6 mg in 50 mL of HPLC-grade methanol. A calibration curve of different concentrations (10, 15, 20, 40, 60, and 80 µM) of 4′-hydroxydiclofenac standard solutions containing rat liver microsomes were obtained. Following centrifuging at 14,000 rpm for 10 min, 1 mL of supernatant of each of the concentration levels were stored at −20 °C for the method validation.
3.4.4. Liquid Chromatography (HPLC) Conditions
The chromatographic separation of salicylic acid, phenacetin, diclofenac, and 4′-hydroxydiclofenac components were performed on a SUPELCO C18 column (25 cm × 4.6 mm, 5 µm particle size) using a Shimadzu LC-2010A HT (200 UV-detector) system (Tokyo, Japan). The peak area for each component was obtained through a manual integration peak icon using the HPLC Lab Solution 2 software system. The HPLC mobile phase for separating 4-hydroxyoctanophenone (internal standard), diclofenac, 4′-hydroxydiclofenac, and salicylic acid was made up of low-pressure gradient elution system as shown in Table 10 below:
HPLC low gradient elution programming.
The flow rate was set at 1 mL/min using 282 nm as the wavelength for UV detection. The column temperature was set at 30 °C with an injection volume of 20 µL.
3.5. Data Analysis
The analytical parameters (linearity and range) for 4′-hydroxydiclofenac were determined from the calibration curves of 4′-hydroxydiclofenac using Microsoft Excel 2010 software. Calibration curve graph of 4′-hydroxydiclofenac was plotted using the average peak area ratio (average peak area of 4′-hydroxydiclofenac/average peak area of 4-hydroxyoctanophenone) as a function of the theoretical concentration of 4′-hydroxydiclofenac.
All of the experimental outcomes are represented as average ± error. All HPLC validation parameters (LOD, LOQ, % error, % accuracy, precision, % recovery, and matrix effect) were calculated using Microsoft Excel 2010 software. LOD and LOQ were calculated mathematically using the following equations: LOD = 3.3б/S and LOQ = 10б/S, where б is the standard deviation of the response and S is the slope of the calibration curve.
4. Conclusions
In conclusion, a new HPLC-UV method for the quantification of a metabolic product (4′-hydroxydiclofenac) was developed with salicylic acid. All of the analytical parameters for 4′-hydroxydiclofenac were validated according to ICH guidelines. In addition, this method was found to be robust. All of the analytical parameters (LOD, LOQ, accuracy, precision, % error, and recovery) for 4′-hydroxydiclofenac met the ICH guidelines. Therefore, this method (extraction by protein precipitation) is more easy, direct, time saving, and convenient than liquid–liquid extraction. This method needs to urgently be developed and validated for the future application of the in vitro potential assessment of salicylic acid on the CYP2C9 enzyme and the for the safety administration of salicylic acid with other drugs in clinical trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27113587/s1 . Figure S1: Calibration curve of 4′-hydroxydiclofenac for intra-assay and inter-assay Day 1. Figure S2: Calibration curve of 4′-hydroxydiclofenac for inter-assay Day 2. Figure S3: Calibration curve of 4′-hydroxydiclofenac for inter-assay Day 3. Figure S4: HPLC chromatogram of salicylic acid (100 µM). Figure S5: HPLC chromatogram of 4′-hydroxydiclofenac (100 µM), Figure S6: HPLC chromatogram of Diclofenac (200 µM). Figure S7: Calibration curve of 4′-hydroxydiclofenac stability test day 1. Figure S8: Calibration curve of 4′-hydroxydiclofenac stability test day 2. Figure S9: Calibration curve of 4′-hydroxydiclofenac stability test day 3.
Funding Statement
No funding has been received from any commercial or profit sectors.
Author Contributions
H.S. and J.B. designed and conceived the experiments, and H.S. conducted the laboratory analysis. Formal data analysis, interpretations, and writing of the original draft were performed by H.S. The final manuscript was edited, reviewed, and approved by all of the authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all of the subjects involved in the study.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest, financial or otherwise, for this work.
Sample Availability
Samples of the compounds are not available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- [email protected]
- Submit Manuscript
- ISSN0975-4725

- Review Paper | Open Access
- Volume 02 | Issue 01 | Article Id IJPS/240201110
A Comprehensive Overview Of HPLC Method Development And Validation

Department of Pharmaceutical Quality Assurance, P. Wadhwani College of Pharmacy, Yavatmal, Maharashtra, India
- View Article
HPLC has become the workhorse of analytical separations due to its versatility, sensitivity, and precision. However, optimizing and validating an HPLC method for specific analytes requires an intricate adjustment of parameters. This review provides a comprehensive overview of the key steps involved in developing and validating a robust HPLC method. Initially, we delve into the critical factors influencing method development, including analyte properties, sample preparation strategies, column selection, mobile phase optimization, and detector choice. We detail the importance of resolution, peak shape, and retention time control in achieving optimal separation. Next, we dissect the validation process, highlighting essential parameters like linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision, specificity, robustness, and system suitability. We discuss established protocols and regulatory guidelines for each parameter, emphasizing the principles behind their evaluation. Furthermore, we explore advanced method development approaches, such as hyphenation with mass spectrometry (MS) for enhanced analyte identification and quantitative analysis. We also briefly touch upon emerging trends in HPLC, including microfluidic chips and green chromatography practices. This review serves as a valuable resource for both novice and experienced analysts, offering a roadmap for navigating the intricacies of HPLC method development and validation, ultimately paving the way for reliable and reproducible analytical results.
- Sharma BK. Instrumental Methods of Chemical Analysis, 24th Edition, Pg: 68-110.
- Gurdeep R Chatwal, Sham Anand. Instrumental Methods of Chemical Analysis, pg:185-190.
- Khan MC, Reddy NK, Ravindra G, Reddy KVSRK, Dubey PK. Development and validation of a stability indicating HPLC method for simultaneous determination of four novel fluoroquinolone dimers as potential antibacterial agents. J Pharmaceut Biomed Anal, 59, 2012, 162–166.
- Blanchet B, Sabourea C, Benichou AS, Billemont B, Taieb SR, Alain D. Development and validation of an HPLC-UV- visible method for sunitinibquantifcation in human plasma. ClinChimActa, 404, 2009, 134–139.
- FDA Guidance for Industry. Analytical Procedures and Method Validation, Chemistry, Manufacturing, and Controls Documentation, Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER), 2000.
- Korany MA, Mahgoub H, Ossama TF, Hadir MM. Application of artificial neural networks for response surface modeling in HPLC method development. J Adv Res, 3, 2012, 53–63.
- Ferrarini A, Huidobro AL, Pellati F, Barbas C. Development and validation of a HPLC method for the determination of sertraline and three non-chiral related impurities. J Pharmaceut Biomed Anal, 53, 2010, 122–129.
- Collier JW, Shah RB, Bryant AR, Habib MJ, Khan MA, Faustino PJ. Development and application of a validated HPLC method for the analysis of dissolution samples of levothyroxine sodium drug products. J Pharmaceut Biomed Anal, 54, 2011, 433–438.
- Singh S, Bakshi M. Guidance on conduct of stress tests to determine inherent stability of drugs. Phrama Tech, 24, 2000, 1- 14.
- Swartz ME, Jone MD, Fowler P, Andrew MA. Automated HPLC method development and transfer. LcGc N. Am, 75, 2002, 49-50.
- Synder LR, Kirkland JJ, Glajach JLX. In Practical HPLC Methods Development. John Wiley, New York, 295, 1997, 643- 712.
- Swartz M, Murphy MB. New Fronties in Chromatography. Am Lab, 37, 2005, 22-27.
- Debebe Z, Nekhai S, Ashenaf M, David BL, Kalinowski DS, RG Victor, Byrnes WM, Richardson DR, Karla PK. Development of a sensitive HPLC method to measure invitro permeability of E- and Z-isomeric forms of thiosemicarbazones in Caco-2 monolayers. J Chromatogram B, 906, 2012, 25–32.
- www.agilent.com/chem/store (Accessed on 11/01/2024)
- Dolan JW. Peak tailing and resolution. LcGc N. Am, 20, 2002, 430-436.
- Qiang Fu, Shou M, Chien D, Markovich R, Rustum AM. Development and validation of a stability-indicating RP-HPLC method for assay of betamethasone and estimation of its related compounds. J Pharmaceut Biomed Anal, 51, 2010, 617– 625.
- Nguyen AT, Aerts T, Dam DW, Deyn PPD. Biogenic amines and their metabolites in mouse brain tissue: Development, optimization and validation of an analytical HPLC method. J Chromatogra B, 878, 2010, 3003–3014.
- International Conference on Harmonization of technical Requirements for Registration of Pharmaceuticals for Human use, ICH
- Harmonized Tripartite guideline-Validation of Analytical procedures: Text and methodology Q2 (R1), Current step 4 version., London 2005.
- Singh R. HPLC method development and validation-an overview. Journal of Pharmaceutical Education & Research. 2013 Jun 1;4(1).
- Sonia K, Lakshmi KS. HPTLC method development and validation: An overview. Journal of Pharmaceutical Sciences and Research. 2017 May 1;9(5):652.
- Ahuja S, Rasmussen H, editors. HPLC method development for pharmaceuticals. Elsevier; 2011 Sep 21

Sayali V. Ganjiwale

A. P. Dewani
A. v. chandewar.
Sayali V. Ganjiwale*, A. P. Dewani, A. V. Chandewar, A Comprehensive Overview of HPLC Method Development and Validation, Int. J. of Pharm. Sci., 2024, Vol 2, Issue 1, 802-811. https://doi.org/10.5281/zenodo.10581387
More related articles
A review on herbal mosquito repellent ..., robotic surgical revolution exploring the future o..., narrative review: multifunction pharmaceutical exc..., clinical trial design in drug enhance safety and efficacy..., revolutionizing herbal therapeutics: exploring advanced nanotechnology drug deli..., design development and evaluation of floating drug delivery system for lornoxica....

- Received 22 Jan, 2024
- Accepted 26 Jan, 2024
- Published 29 Jan, 2024
Related Articles
Development and characterization of phytosome as a novel carrier by qbd approach..., regulating artificial intelligence: developments and challenges..., nsaid induced acute kidney injury (aki): a case study..., formulation of dosage forms with rabeprazole: challenges and future perspectives..., robotic surgical revolution exploring the future of precision medicine in the op..., narrative review: multifunction pharmaceutical excipients in tablet formulation....
Copyright © 2023 IJPS. All rights reserved
Stability-indicating HPTLC method development for determination of arterolane maleate and piperaquine phosphate in combined dosage form
- Original Research Paper
- Published: 04 May 2020
- Volume 33 , pages 131–139, ( 2020 )
Cite this article
- Shubhangee Gaikwad 1 ,
- Amol Bansode 1 ,
- Nisha Patade 2 &
- Shraddha Tathe 1
58 Accesses
Explore all metrics
A stability-indicating high-performance thin-layer chromatography (HPTLC) method was developed and validated for determination of arterolane maleate and piperaquine phosphate in combined dosage form. This study was carried out by using the mobile phase containing isopropyl alcohol: n -butanol:methanol:triethylamine (3:6:1:0.2 v/v ). The method was validated as per the International Conference on Harmonization (ICH) guidelines. The correlation coefficient was found to be 0.9961 and 0.9914 in the concentration range of 150–900 and 750–4500 ng/band for arterolane maleate and piperaquine phosphate, respectively. The method had an accuracy of 98.25% for arterolane maleate and 97.15% for piperaquine phosphate. For stability study, arterolane maleate and piperaquine phosphate were subjected to acid, base, oxidation, heat, and photo-degradation studies. As the HPTLC method could effectively separate the drugs from their degradation products, it can be used for stability-indicating analysis.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Stability-indicating RP-UPLC method for determination of antihypertensive drugs and their degradation products in tablets: application to content uniformity and dissolution studies
Mahmoud A. Mohamed & Hossam F. Nassar
Stability Indicating RP-HPLC Simultaneous Estimation of Hyoscine Butylbromide and Paracetamol in Tablets
Nalini Calambur Nagarajan, Amuthalakshmi Sivaperumanan & Priya

Development and Validation of a Stability-Indicating High-Performance Thin-Layer Chromatographic Method for Determination of Pyridostigmine Bromide in the Presence of Its Alkaline-Induced Degradation Product
Nouruddin W. Ali, Eglal A. Abdelaleem, … Fatma F. Abdallah
Bhavsar AS, Patel SD, Patel JR (2015) Development and validation of stability indicating RP-HPLC method for simultaneous estimation of Arterolane Maleate and Piperaquine Phosphate in pharmaceutical dosage form. Der Pharmacia Sinica 6(4):30–37
CAS Google Scholar
Parulkar AS, Shabaraya R (2018) A novel approach for the use of Arterolane Maleate in treatment of malaria. World Journal of Pharmacy and Pharmaceutical Sciences 7(5):702–718
Patel SD, Tiwari N, Marvaniya V (2017) Development and validation of Q-absorbance ratio method for simultaneous estimation of Arterolane Maleate and Piperaquine Phosphate in pharmaceutical dosage form. International Journal of Allied Medical Science and Clinical Research 5(2):648–656
Google Scholar
Valecha N, Savargaonkar D, Srivastava B, Rao BH, Tripathi S, Gogtay N, Kochar SK, Kumar BV, Rajadhyaksha G, Lakhani J, Solanki B, Jalali K, Arora S, Roy K, Saha N, Iyer S, Sharma P, Anvikar R (2016) Comparison of the safety and efficacy of fixed-dose combination of Arterolane Maleate and Piperaquine Phosphate with Chloroquine in acute, uncomplicated Plasmodium vivax malaria: a phase III, multicentric, openlabel study. Malar J 15(42):1–13
Indian Pharmacopoeia, 2014, Ministry of health and family welfare, 7th edition, Indian pharmacopeia commission, Ghaziabad India, 1084
United States Pharmacopoeia 2014. NF 34, Pharmacopoeia Convention Rockville: The United States, 1258
Central Drug Standard Control Organization, List of Drug Approved For Marketing in India, 2011. http://www.cdsco.nic.in/writereaddata/LISTOFAPPROVED-DRUG-FROM-2011.pdf .
Zhang Q, Yuan L, Haung C (2008) Quality control of piperaquine in pharmaceutical formulations by capillary zone electrophoresis. Talanta 76(1):44–48
Article CAS Google Scholar
Sethi P. D., (1996), “High performance thin layer chromatography”, first ed. CBS publication, New Delhi, 5-21
Sonia K, Beddi B. S. and Dr. Lakshmi K., (2017). HPTLC method development and validation: an overview. Journal of Pharmaceutical Science and Research, 9(5): 652–657
Chauhan A, Mittu B, Chauhan P (2015) Analytical method development and validation: a concise review. Journal of Analytical and Bioanalytical Techniques 6(1):1–5
ICH, Validation of analytical procedures: Text and Methodology Q2 (R1) (2005) International conference on harmonization. IFPMA, Geneva
Daksh S, Goyal A, Pandiya CK (2015) Validation of analytical methods: strategies and significance. International Journal of Research and Development in Pharmacy and Life Sciences 4(3):1489–1497
ICH, Stability Testing of New Drug Substances and Products Q1A (R2) (2003) International conference on harmonization. IFPMA, Geneva
Download references
Acknowledgments
The authors would like to thank to Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune, and also Sinhgad Institute of Pharmacy, Narhe, for providing the necessary infrastructural facilities to perform this study.
Author information
Authors and affiliations.
Department of Pharmaceutical Chemistry, Sinhgad Institute of Pharmacy, Narhe, Pune, Maharashtra, 411041, India
Shubhangee Gaikwad, Amol Bansode & Shraddha Tathe
Department of Quality Assurance Techniques, Sinhgad Institute of Pharmacy, Narhe, Pune, India
Nisha Patade
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Shubhangee Gaikwad .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Gaikwad, S., Bansode, A., Patade, N. et al. Stability-indicating HPTLC method development for determination of arterolane maleate and piperaquine phosphate in combined dosage form. JPC-J Planar Chromat 33 , 131–139 (2020). https://doi.org/10.1007/s00764-020-00021-4
Download citation
Received : 18 December 2019
Accepted : 01 April 2020
Published : 04 May 2020
Issue Date : April 2020
DOI : https://doi.org/10.1007/s00764-020-00021-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Arterolane maleate
- Piperaquine phosphate
- Stability-indicating
- ICH guidelines
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
In this paper, we developed and validated a simple, accurate, and reliable analytical HPLC-UV-ESI-IT-MS method, carried out on a C18 column, which is potentially suitable to quantify carnosol in ...
According to the ICH Q2 (R1), in a robust method, small variations in certain method parameters do not affect the reliability and results of the method. 47 These small variations are important for the pharmaceutical industry in terms of the transfer of the analytical method from research and development to the quality control laboratory or from ...
Quality by design (QbD) refers to the achievement of certain predictable quality with desired and predetermined specifications. A quality-by-design approach to method development can potentially lead to a more robust/rugged method due to emphasis on risk assessment and management than traditional or conventional approach. An important component of the QbD is the understanding of dependent ...
A fruitful implementation of experimental design in HPLC can be executed through four common stages; i.e.: (i) choosing the convenient design, (ii) suitable software, (iii) experimental trials, data analysis, and (iv) interpretation. The use of Optimization Designs in HPLC method development is summarized in Table 3.
development effort goes into validating a stability indicating HPLC-method. The goal of the HPLC-method is to separate quantify the main active drug, any reaction impurities, all available synthetic inter-mediates and any degradants. Steps involve in method development are: 1. Qualified and calibrated instrument 2. Documented methods 3.
The issues pertinent to designing HPLC method development and validation and identification of the influences which may change these characteristics and to what extent are discussed. High performance liquid chromatography (HPLC) is an essential analytical tool in assessing drug product. HPLC methods should be able to separate, detect, and quantify the various drugs and drug related degradants ...
4.4.2.6 SPE Method Development, 127 4.4.2.7 Column Chromatography for Sample Pretreatment, 139 4.4.3 Membrane Separations, 139 4.5 Sample Pretreatment for Solid Samples, 144 4.5.1 Traditional Extraction Methods, 145 4.5.2 Newer Extraction Methods, 147 4.5.2.1 Supercritical Fluid Extraction, 147 4.5.2.2 Microwave-Assisted Solvent Extraction, 151
This chapter provides an overview of high-pressure liquid chromatography (HPLC) method development focusing on reversed-phase stability-indicating methods for small-molecule drugs. It describes the traditional approach to method development, starting with the initial selection of a column, detector, and mobile phase, followed by parameter ...
High-performance liquid chromatography (HPLC) has become an indispensable tool for modern analytical chemistry, and reversed-phase (RP) HPLC is one of the most widely used techniques for the separation and analysis of complex mixtures. In recent years, there have been numerous advances in RP HPLC method development, including the development of new stationary phases, improved column technology ...
HPLC method development: The following is a step in HPLC method development: Recognizing the Physicochemical Properties of Drug Molecules . www.ijppr.humanjournals.com Citation: R. D. Chakole et al. Ijppr.Human, 2021; Vol. 21 (4): 66-82. 71 Choosing chromatographic conditions
A Novel, efficient and convenient reversed-phase high-performance liquid chromatography method was developed for eluxadoline (EXDL) drug in the presence of its impurities 1 and 4. Successful separation of EXDL drug from the its impurities was achieved on Prontosil ODS C18 column (5 µm 250 × 4 mm) with isocratic elution of Acetonitrile:Methanol:0.1 M Sodium acetate 40:40:20 (v/v) as a mobile ...
In this research paper, an HPLC-UV method was optimized and validated for a CYP2C9 assay using a salicylic acid inhibitor for the future investigation of the in vitro potential effect of salicylic acid on 4′-hydroxydiclofenac formation in rat microsomes.
Development of HPLC method. In Fig. 1, chromatogram A represents the blank mobile phase, and chromatogram B represents DS with an average retention time of 1.345 ± 0.127 min and with no interfering peaks. This is an indication of the specificity of the developed HPLC method. ... These HPLC methods reported in have several disadvantages ...
HPLC has become the workhorse of analytical separations due to its versatility, sensitivity, and precision. However, optimizing and validating an HPLC method for specific analytes requires an intricate adjustment of parameters. This review provides a comprehensive overview of the key steps involved in developing and validating a robust HPLC method. Initially, we delve into the critical factors ...
METHOD DEVELOPMENT ON HPLC A step involved in method development of HPLC is as follows: 1 Understanding the Physicochemical properties of drug molecule. 2 Selection of chromatographic conditions. 3 Developing the approach of analysis. 4 Sample preparations 5 Method optimization 6 Method validation 1.
What are Some Standard Method Development Practices? 1. Follow preferred method development scheme and do "hands-on" method development - based on selectivity-changing parameters— e g pH column or mobile phase typese.g., pH, column, or mobile phase types 2. Use method development software - run a few predictive runs and get prediction ...
The development and approval of a demonstration strategy are key elements of any drug development program [13]. The HPLC investigation method is developed to detect, measure or purify premium compounds. This special topic will focus on the development and approval of exercise as it is associated with medical equipment.
Section A-Research paper RP-HPLC Method Development and Validation of Metformin, Vildagliptin and Remogliflozin in Bulk and Pharmaceutical Dosage form Eur. Chem. Bull. 2023, 12 (s3), 6526 -6540 6530 Trial - 5: Figure 4: Method Development Trials 2.3.2. Final Chromatographic Conditions: Table No. 4: Final Chromatographic Condition ...
A stability-indicating high-performance thin-layer chromatography (HPTLC) method was developed and validated for determination of arterolane maleate and piperaquine phosphate in combined dosage form. This study was carried out by using the mobile phase containing isopropyl alcohol:n-butanol:methanol:triethylamine (3:6:1:0.2 v/v). The method was validated as per the International Conference on ...
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1430 ISSN 2229-5518 ... EXPERIMENTAL METHODS . 2.1. HPLC method development:- 2.1.1. Chemicals and reagents . Clopidogrel and Ritonavir were kindly providedas gift sample from IPCA (Mumbai) and ...
The swift growth of cities worldwide poses significant challenges in ensuring a sufficient water, energy, and food supply. The Nexus has innovated valuable systems to address these challenges. However, a crucial issue is the potential for pollution resulting from these systems, which directly and indirectly impacts public health and the overall quality of urban living.