Your session is about to expire
What is a contract research organization plus, top 5 cros to check out in 2022.


What is a CRO?
A contract research organization – CRO – is a company that provides outsourced services related to drug development and clinical research. Pharma and biotech firms and other sponsors may rely on a CRO for support in one or more aspects of developing and bringing new drugs and medical devices to market.
Keep reading as we explore the role of CROs in drug development, what services they offer, and why many sponsors work with one. We’ve also included a list of the top 5 CROs, and questions to ask before signing a contract with a CRO.
What Do CROs Do?

The main role of CROs is to carry out activities that the sponsoring pharmaceutical company cannot or chooses not to do in-house.
Pharmaceutical companies often count on both internal and external resources, and sometimes they need assistance with tasks that fall outside of their core capabilities. Most modern CROs provide services spanning the drug development pipeline, from running entire clinical trials to individual services such as data management, patient recruitment, protocol development, drug manufacturing, and the list goes on. CROs themselves might partner with other companies to provide robust services across the full spectrum of clinical trial activities. For example, CROs might partner with Power as a part of a multi-layered recruitment strategy for a trial.
The goal of a CRO is to provide streamlined services to pharmaceutical companies in order to help them get their new drugs developed, tested, and approved. It’s often more resource efficient and quicker to outsource certain aspects of clinical development, allowing sponsors to focus on their core operations.
For example, let’s say a biotech company has developed a new drug that looks promising in preclinical laboratory tests, but they’ve never conducted a clinical trial. It would generally be much more straightforward and efficient to partner with a CRO to conduct clinical trials than to dive into the complex regulatory landscape and risk inefficiencies or failures in the clinical testing of their promising new drug. Although the biotech firm would still be the sponsor of the trial, retaining responsibility for regulatory compliance, the CRO would be responsible for recruiting patients, administering the study drug, monitoring patients and overseeing the trial, and reporting the results back to the company. Each of these aspects is, in and of itself, a whole world that requires specialized expertise. It makes much more sense to outsource these aspects to dedicated professionals.
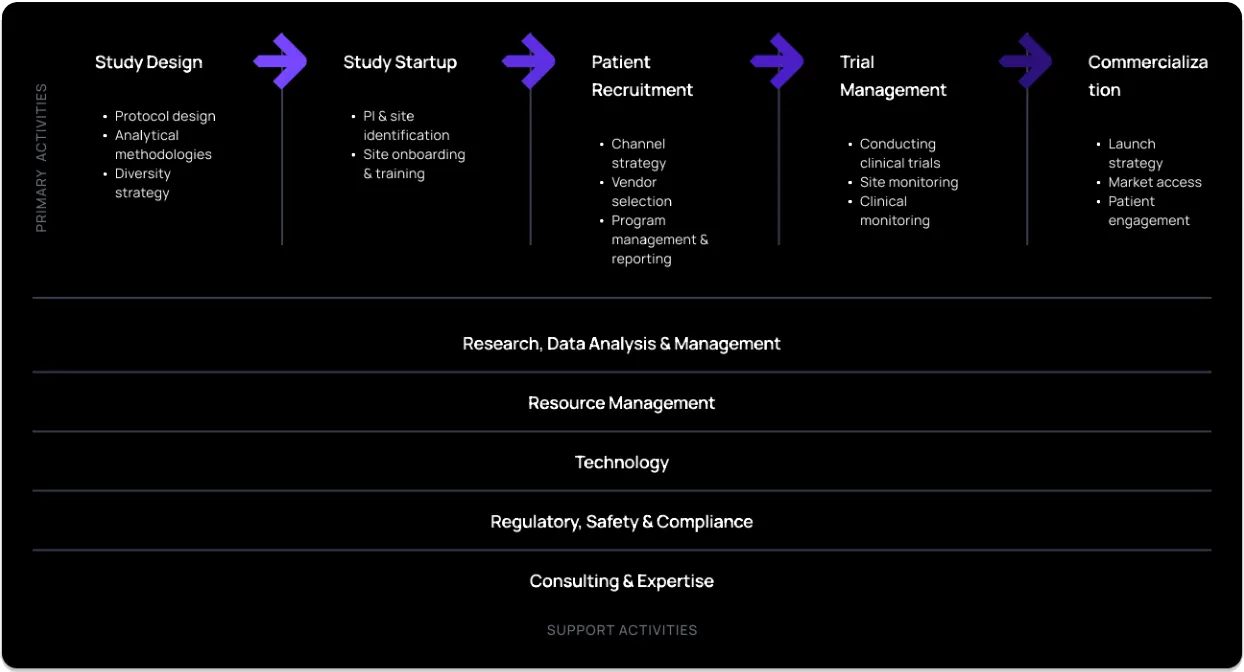
See the image below for some example services offered by CROs:
Are contract research organizations the same as clinical research organizations?
Contract research organizations (CROs) may get involved in all aspects of the clinical development process, from initial drug discovery through to pre-clinical and clinical trials and post-marketing surveillance studies. A CROs might help design a study, recruit patients for the study, perform laboratory tests, monitor patients during the study, analyze study data, prepare study reports for the sponsor, and present reports or summaries to regulatory bodies.
Clinical research organization is a less-common term which might be used to refer to a specific type of CRO that specializes in conducting clinical trials for pharmaceutical companies and other sponsor organizations. Thus, where a CRO might offer comprehensive service across the clinical development spectrum, a clinical research organization would be focused primarily on conducting phase I through phase IV studies for new drugs and medical devices.
Why do sponsors work with CROs?
CROs play an important role in pharmaceutical and biotechnology product development. Here are just some of the reasons why sponsors may decide to work with CROs:
1. Specialized operations and cost control
Building and managing clinical trial operations completely in-house is complicated, expensive, and requires specialization in a multitude of areas. The rise of CROs meant that pharmaceutical companies no longer needed to own all of their own scientific and clinical research facilities. Full-service CROs offer sponsors a complete set of solutions, allowing them to delegate as much or as little of the overall clinical development operation as they desire so as to optimize resource use and development timelines.
2. Expanded access to technology
CROs offer various technological solutions to support sponsors in designing, conducting, and managing clinical trials. Many of these tools are specific to clinical trials, and would not be commonplace in a lab-focused pharmaceutical company, for example. Sponsors can choose to leverage these technologies or product suites depending on the needs of each unique study. Some examples of eClinical technologies that sponsors may use include: planning tools (protocol design, patient enrollment, etc.); site management tools (activation, payments, etc.); modern patient recruitment tools (e.g., Power ); clinical trial management systems ( CTMS ); clinical data management systems (CDMS); analytics platforms.
3. Ability to handle large amounts of data
There are a lot of moving pieces involved in successfully running a clinical trial. CROs with experience in clinical trials will have efficient data handling procedures in place, making them a reliable partner for this complex aspect of modern trials which often draw from various data sources such as wearable devices, EHRs, eCOA / ePRO interfaces, etc.
4. Streamlined regulatory affairs (FDA regulatory compliance)
CROs typically have experience working with regulatory agencies such as the Food and Drug Administration (FDA). The FDA regulates the entirety of clinical trials, ensuring that ethical research guidelines and patient privacy safety standards are upheld. Sponsors must adhere to a multitude of FDA and HHS guidelines when conducting any clinical trial. Regulatory affairs is a complex landscape requiring familiarity with various parallel and overlapping regulations, as we discuss in our explainer on regulatory compliance in clinical trials . An experienced CRO will be able to help pharmaceutical companies get their products to market faster by facilitating regulatory submissions and navigating the intricacies of guidelines laid out in Title 21 and 45 CFR, GCP, HIPAA, etc. Partnering with a CRO offers an attractive alternative to hiring and training internal experts specializing in regulatory affairs, particularly for sponsors who aren’t conducting trials frequently.
5. Multi-disciplinary expertise leads to quicker and more effective trials
CROs often consist of a team of professionals with diverse backgrounds and specializations, which means that they can offer the appropriate expertise needed to effectively manage various aspects of clinical trial operations.
What to ask when choosing a CRO partner for clinical research

With the massive offer of CROs currently available, it’s important to realize the weight of the decision when selecting partners for clinical research. As the sponsor, regulatory compliance falls entirely under your responsibility, so choosing a trustworthy partner with a proven record of compliance can help you be sure your trial is in the right hands. Partnering with an incompetent or inexperienced CRO may have the opposite effect of streamlining trial timelines and resource usage, causing delays and risking valuable resources or even patient health.
The following 3 points act as a guideline for choosing a potential CRO before signing a provider agreement with them for a clinical trial, regardless of the extent of their involvement.
1. Obtain trustworthy information. It's important to gather as much information as possible about a potential research partner, as there is significant weight placed on such agreements. The website of the CRO itself will not tell the whole story; it is a good idea to look for real reviews and feedback from other companies who have worked with the CRO.
Make sure that the CRO you are considering has an established track record of success with past and current clients. Check that the CRO has an active online presence and transparent contact information, along with active client support.
The CRO should also have a good reputation among its employees; complaints by employees might be a red flag, particularly if they’re related to protocol deviations, non-compliance, mismanaged client disputes, internal uncleanliness or disorganization, or other issues that could impact patient safety and regulatory compliance.
2. Check that the CRO is up-to-date with regulatory standards and accreditations. The regulatory landscape is updated frequently, so it’s important to verify that the CRO in question has been active recently and has up-to-date authorization and accreditation for the specific operations you are considering contracting them for. If you can’t find this information on the website, contact them and have an open discussion to get clear answers.
3. Verify that the CRO has real expertise in the specific task you wish to outsource. This may seem obvious, but just because a given CRO is world-renowned in conducting clinical trials does not mean they will be experts in analyzing your specific data, particularly if you’re working in rare diseases or other niche areas. Direct experience in a similar therapeutic field and trial type is important, as it translates into efficiency and tells you that they will be comfortable navigating the particular waters you’re entering together. After all, the point is for the partnership to make your operations smoother, and for that, you need a partner with relevant experience.
What Are The Top CROs in 2022?
The contract research organization (CRO) industry is rapidly growing, and it's easy to see why due to the unique roles they fulfill in pharmaceutical and biomedical research.
We’ve curated a list of the top 5 contract research organizations (CROs) in 2022:
IQVIA is a leading healthcare technology company providing integrated services spanning the entire healthcare and clinical development spectrum. IQVIA offers solutions for clinical trials and research/development, real-world evidence studies, safety and regulatory compliance, and commercialization, and features a vast suite of technological tools powering “smarter healthcare.”
2) Labcorp Drug Development
Labcorp Drug Development, formerly Covance, is a contract research organization that offers drug development, clinical trial, and laboratory services to the pharmaceutical industry. The company's mission is to help advance patient care by providing clients with high-quality services and solutions.
Labcorp was founded in 1996 and has since grown into one of the world's largest CROs, with over 40 offices worldwide.
PPD is part of Thermo Fisher Scientific, serving as the clinical research services arm of the research giant. PPD is known internationally, having conducted clinical trials in over 100 countries, and currently consisting of over 35,000 employees worldwide.
PPD offers a comprehensive range of clinical development and laboratory services, providing sponsors with customizable solutions backed with a proven track record of professionalism and success.
ICON plc offers a full range of consulting, clinical development, and commercialization services to pharma, biotech, and other healthcare sector actors. Founded in Dublin, Ireland in 1990 and now counting on offices in 46 countries worldwide, ICON has a global team of experts and extensive experience in a wide range of therapeutic areas.
Medpace provides full-service clinical development services aimed particularly at biotech firms, with services ranging from study start-up through to monitoring, pharmacovigilance, regulatory affairs, and even medical writing. Founded in 1992, Medpace now operates in 6 continents and their teams of medical, operational, and regulatory specialists support their “therapeutically-aligned” scientific expertise and the delivery of their full-service, solution-oriented trial execution model.
CROs have become ubiquitous in clinical research amidst the trend toward partnering with third-party providers and the rise of specialized end-to-end clinical development services. A well-equipped CRO will be a valuable ally in managing drug development and clinical trial operations, bringing expertise and experience as well as access to state-of-the-art technology and facilities. Often, partnering with a CRO means optimizing resource allocation and thus keeping costs down. If you’re considering partnering with a CRO to enhance your research, development, or clinical trial efforts, it’s important to make an informed decision to ensure your operations are in the right hands. If you’re looking for a partner specializing in modern patient recruitment, reach out to us – we will be happy to show you the incredible power that lies within our patient recruitment marketplace and advanced recruitment solutions.
Need a predictable recruitment solution?
Tap into the largest source of independent patients looking for trials., other trials to consider.
Telephone-based early childhood developmental care coordination
Remotely supervised transcranial direct current stimulation, center for independent living seeks team, family navigator (fn), reflective process group, referral to harm reduction services, smoking cessation intervention, tcu opioid-treatment linkage model (o-tlm), enhanced services, popular categories.
Aricept Clinical Trials
Clinical Trials in Tampa, FL
Clinical Trials in Knoxville, TN
Clinical Trials in Florida
Clinical Trials in Delaware
Clinical Trials in Iowa
Paid Clinical Trials in Austin, TX
Paid Clinical Trials in Glendale, AZ
Diabetic Nephropathy Clinical Trials 2023
Anal Fistula Clinical Trials 2023
Popular guides.

News & Articles
What is a contract research organization.

In the rapidly evolving landscape of medical research and development, the role of a Contract Research Organization (CRO) is becoming increasingly crucial. These organizations offer a comprehensive spectrum of services that enable pharmaceutical, biotechnology, and medical device companies to accelerate their product development process.
Contract Research Organization: Essential Partner in Medical Research
A Contract Research Organization (CRO) is a specialized service provider that offers end-to-end support for clinical trials, ranging from early development to post-approval studies. They leverage their expertise, resources, and global reach to help sponsors navigate through the complex processes of drug and device development.
Defining the Role of a CRO
The fundamental responsibility of a CRO is to assist sponsors in conducting clinical trials in a manner that is efficient, safe, and compliant with regulatory standards. This involves a wide range of activities, including but not limited to, clinical trial planning and design, site selection, data management, regulatory affairs, and project management.
Understanding the Significance of a CRO in Clinical Trials
The involvement of a CRO in clinical trials is essential for ensuring trial quality and compliance with national and international standards. They bring to the table innovative tools and strategies that can enhance efficiencies, thereby reducing timelines and costs. The choice of a suitable CRO is instrumental in determining the success of a clinical trial.
Vision Lifesciences as Your Trusted CRO Partner
At Vision Lifesciences, we pride ourselves on being a full-service Contract Research Organization with experience in designing and implementing clinical trials.
Our Expertise in Clinical Trial Services
Our suite of clinical trial services is designed to support your clinical study from inception to completion. We can either manage the entire study or assist with specific components, depending on your requirements.
Clinical Trial Planning
Our clinical trial planning services encompass creating the clinical plan and protocol, and facilitating the necessary approvals from regulatory authorities and ethics committees. We collaborate with clinical experts and other stakeholders to tailor a clinical study that suits your product’s specific needs, ensuring data is collected efficiently while complying with all regulatory requirements.
Clinical Data Management
Robust clinical data management is integral to the clinical trial process. We take meticulous measures to deliver high-quality clinical data management services to support your product development needs. This includes the creation of case report forms (CRFs), the establishment of efficient electronic data capture (EDC) systems, and the management of data entry and validation processes.
Clinical Project Management
We understand the importance of smooth project management in conducting clinical trials. We ensure efficiency while maintaining superior quality and compliance. Our project management services include site assessments, documentation for IRB/Ethics Committees, site initiation and training, and trial closeout steps.
Clinical Trial Monitoring
Clinical trial monitoring is crucial in ensuring that the trial is conducted, recorded, and reported in accordance with the protocol, ethics committees, and Good Clinical Practice (GCP). Our comprehensive monitoring plans help manage clinical trials and foster confidence in clinical trial integrity.
Choosing the Right Contract Research Organization
Selecting the right CRO for your clinical trial is a critical decision. It involves an evaluation of the qualification, experience, and quality system processes of the CRO. Here are a few questions to consider when choosing a CRO:
- Do they have a well-established quality system?
- Are they responsive and communicative throughout the project?
- Do they have a stable project team with low turnover?
- Can they assist with data management?
- Will they help in recruiting and managing safety boards or committees?
- Can they conduct audits to help you prepare for BIMO FDA inspections?
- Can they provide general site support and project management help in addition to clinical monitoring?
Why Choose Vision Lifesciences as Your CRO Partner?
At Vision Lifesciences , we bring our extensive experience and expertise to the table to ensure the success of your clinical trial. We understand that every trial is unique, and we tailor our services to meet your specific needs.
Commitment to Quality
We are committed to consistently delivering services of the highest quality that yield exceptional results and add value to your business. Our track record speaks volumes about our commitment to quality, with consistently high ratings from our clients in the past two quarters.
Comprehensive Services
Our comprehensive suite of services ensures that you have the support you need at every step of your clinical trial. From medical device engineering and testing to regulatory services and clinical trial services, we have you covered.
Global Reach
With a global presence, we have the capability to conduct clinical trials across multiple geographical locations, allowing for diverse patient populations and improved recruitment rates.
In conclusion, a Contract Research Organization is an invaluable partner in the realm of clinical trials. Their specialized skills and services allow sponsors to conduct trials efficiently, safely, and in compliance with regulatory standards.
At Vision Lifesciences, we are committed to being your trusted partner in your clinical trial journey. Contact us today to discuss your clinical trial needs. Our team of experts is ready and eager to support you in bringing your product to market.
Further Reading

The Top 11 Contract Research Organizations (CROs) Shaping the Future of Pharma, Biotech, and Medtech in 2023
Quick links.
- [email protected]
- Ulica Republike Austrije 23, Zagreb, Croatia
- World Trust Tower, 50 Stanley Street, Central, Hong Kong
Subscribe To Our Newsletter!
By submitting, you are agreeing to our terms and privacy policy
- Privacy Notice
- Terms and Conditions
Copyright © 2024 Vision Life Sciences. All Rights Reserved.
What is a CRO? Contract Research Organization

Alexander Doroshenko | Posted on September 13, 2023
Introduction
What do cros do, why are cros important, full-service cros, specialized cros, phases of clinical trials, regulatory compliance, benefits and challenges, how to choose a cro.
Ever wondered who’s behind the scenes, ensuring that the medicines you take are safe and effective? Meet the Contract Research Organizations (CROs), the unsung heroes of the pharmaceutical world. In this article, we’ll dive deep into what CROs are, why they’re indispensable, and how they operate.
So, are you ready to go behind the scenes?
The Basics of CROs
Contract Research Organizations (CROs) are the backbone of the pharmaceutical and biotechnological industries. They offer a plethora of services that span the entire drug development lifecycle. From the initial stages of research and development (R&D) to pre-clinical trials, clinical trials, and even post-market surveillance, CROs do it all. Imagine them as the Swiss Army knife in a researcher’s multi-functional, reliable, and absolutely indispensable toolkit.
Services Offered
Curious about how new APIs are discovered? Read This is how new APIs are discovered .
- Pre-Clinical Trials : Before a drug can be tested on humans, it undergoes rigorous testing in the lab. CROs manage these pre-clinical studies, ensuring they meet all regulatory guidelines.
- Clinical Trials : This is the phase most people are familiar with. CROs manage drug testing on human subjects, collecting and analyzing data to determine efficacy and safety.
- Regulatory Submissions : Once a drug has proven effective and safe, it needs governmental approval. CROs help in the preparation and submission of regulatory documents.
- Post-Market Surveillance : Even after a drug hits the market, it must be monitored for long-term effects, another area where CROs contribute.
The pharmaceutical industry is a behemoth, with global spending expected to reach $1.9 trillion by 2027. But did you know that the average cost to develop a new prescription drug is a staggering $2.6 billion? And that’s not even considering the time factor—it can take up to 12 years for a drug to go from the lab to the pharmacy shelf.
This is where CROs come in. They accelerate the drug development process, making it more efficient and cost-effective. By outsourcing specific tasks to experts, pharmaceutical companies can focus on what they do best, whether it’s R&D, marketing, or distribution.
Cost Savings
One of the most significant advantages of working with a CRO is cost savings. Running in-house clinical trials can be prohibitively expensive, requiring specialized staff, equipment, and facilities. CROs, with their expertise and resources, can often perform the same tasks at a fraction of the cost.
Global Reach
In today’s globalized world, clinical trials often span multiple countries, if not continents. CROs have the global reach and local knowledge to manage such complex, multi-site trials, ensuring they meet the regulatory requirements of each jurisdiction.
Expertise and Specialization
Another reason CROs are invaluable is their specialized expertise. Pharmaceutical companies often have broad focuses, from R&D to marketing. On the other hand, CROs specialize in specific aspects of drug development, bringing a level of expertise that’s hard to match.
Risk Mitigation
Drug development is fraught with risks, both financial and clinical. CROs help mitigate these risks by ensuring the trials are conducted ethically and scientifically, adhering to all regulatory guidelines. This not only safeguards the patients but also protects the pharmaceutical companies from potential legal issues.
Types of CROs
When you think of a one-stop shop for all your drug development needs, you think of a full-service CRO. These organizations offer end-to-end solutions, covering everything from pre-clinical trials to post-market surveillance.
- Simplicity : Working with a full-service CRO simplifies the drug development process, as you have a single point of contact for all stages.
- Consistency : With the same team overseeing the entire process, there’s greater consistency in data collection and analysis.
- Cost-Efficiency : Bundling services often leads to cost savings, making full-service CROs an economical choice for many companies.
Not all drug development projects require a full range of services. Sometimes, you might need specialized expertise in a particular area, such as bioanalytics or data management. This is where specialized CROs come in.
- Expertise : These CROs offer deep expertise in specific areas, making them the go-to choice for specialized projects.
- Quality : Specialized CROs often produce higher-quality data in their area of expertise, leading to more robust findings.
- Speed : With a focus on a specific aspect of drug development, specialized CROs can often complete tasks more quickly than their full-service counterparts.

The Role of CROs in Clinical Trials
Clinical trials are the make-or-break stage of drug development. It’s where a potential drug proves its efficacy and safety in human subjects. Given the high stakes, it’s no surprise that this is one of the most regulated aspects of the pharmaceutical industry.
Clinical trials are generally divided into four phases, each with its own set of objectives and methodologies. Here’s how CROs contribute to each:
- Phase I : This is the first time the drug is tested in humans. CROs help design the trial, recruit volunteers, and ensure all ethical guidelines are followed.
- Phase II : This phase tests the drug on a larger group of people to assess its efficacy and side effects. CROs manage the data collection and statistical analysis.
- Phase III : This is the final stage before the drug is submitted for regulatory approval. CROs often manage these large-scale, multi-site trials, ensuring they meet all regulatory requirements.
- Phase IV : Also known as post-market surveillance, this phase involves monitoring the drug’s long-term effects. CROs help design and manage these studies, collecting data that can lead to the drug being refined or even withdrawn from the market.
To learn more about API clinical trials, read EVERYTHING YOU NEED TO KNOW ABOUT API CLINICAL TRIALS .
Navigating the maze of regulations that govern clinical trials is no small feat. Different countries have different rules, and even within a country, regulations can vary by state or province. CROs are experts in regulatory compliance, ensuring that the trials they manage adhere to all local, national, and international guidelines.
Ethical Considerations
Beyond the legal requirements, clinical trials also have ethical considerations. CROs ensure that all trials are conducted with the participants. This involves obtaining informed consent, ensuring data privacy, and adhering to ethical standards like the Declaration of Helsinki, which sets ethical principles for medical researchers.
Quality Assurance
Quality assurance is another critical aspect of regulatory compliance. CROs implement rigorous quality control procedures to ensure that the data collected is accurate, reliable, and conducted in an ethical and scientific manner. This is crucial for the approval process and maintaining the research’s integrity.

Working with a CRO comes with many benefits that can make the drug development process smoother and more efficient. Let’s delve into some of these advantages:
- Scalability : One of the significant benefits of working with a CRO is the ability to scale operations up or down based on project requirements without the hassle of hiring or firing in-house staff.
- Access to Latest Technology : CROs are at the forefront of technological advancements in drug development. By outsourcing, companies can take advantage of cutting-edge technologies without the capital expenditure.
- Strategic Partnership : Many CROs go beyond being mere service providers to become strategic partners, offering insights and expertise that can shape the direction of research and development efforts.
While CROs offer numerous advantages, it’s essential to be aware of potential challenges:
- Lack of Control : When you outsource critical functions, you give up a certain level of control, which can concern some companies.
- Cultural and Language Barriers : If your CRO is based in a different country, cultural and language differences can pose challenges in communication and project management.
- Data Security : Handling sensitive data comes with the risk of data breaches or unauthorized access, so it’s crucial to vet the security protocols of a potential CRO carefully.
The first thing to consider when choosing a CRO is their expertise in your specific therapeutic area. Look for case studies, publications, or references that demonstrate their competence in your field.
Certifications
Certifications like Good Manufacturing Practices (GMP), Certificate of Suitability (CEP), and Food and Drug Administration (FDA) approval are not just jargon; they’re indicators of a CRO’s commitment to quality and regulatory compliance.
Word of mouth and client testimonials can provide valuable insights into a CRO’s reliability and quality of work. Don’t hesitate to ask for references or check online reviews.
Contract Research Organizations (CROs) are the unsung heroes of the pharmaceutical industry. They offer specialized services that streamline the drug development process, making it faster, more efficient, and more cost-effective. Whether you’re a pharmaceutical giant or a small biotech startup, understanding the role and benefits of CROs can offer valuable insights into how to bring your drug from the lab to the market successfully.
What services do CROs offer?
CROs offer a range of services from drug discovery to clinical trials and regulatory submissions.
Why are certifications important when choosing a CRO?
Certifications like GMP, CEP, and FDA indicate that the CRO meets industry standards for quality and reliability.
What are the benefits of working with a CRO?
Cost-effectiveness, specialized expertise, and global reach are some of the key benefits.
Are CROs only involved in clinical trials?
No, CROs offer a comprehensive range of services that extend beyond clinical trials.
- APIs & Manufacturing (14)
- Education & Career (5)
- Events & Exhibitions (3)
- Industry Insights & Trends (11)
- Interviews & Case Studies (7)
- Market Analysis & Data (13)
- Medicines & Access (16)
- Regulations & Standards (18)
- Sales & Marketing (15)
Latest posts
What is nicotinamide mononucleotide, top 10 india’s leading pharmaceutical giants, what is research and development (r&d), how long does it take to bring new medicines to the market, what is iso in pharma, recommended blogs.

Medicines & Access
Read time: 3 minutes
Explore how Nicotinamide Mononucleotide (NMN) could revolutionize anti-aging. Learn about its roles in energy, metabolism, and potentially reversing aging.

Market Analysis & Data
Explore the Top 10 Pharmaceutical Companies in India for 2024. Uncover key insights into their financials, innovations, and global impact.

APIs & Manufacturing , Medicines & Access , Regulations & Standards
Read time: 3.5 minutes
R&D in the pharmaceutical sector is a complex, yet thrilling journey from concept to reality. It’s where science meets innovation to create breakthroughs in medicine.

APIs & Manufacturing , Medicines & Access , Sales & Marketing
Read time: 2.5 minutes
Explore the intricate journey of drug development in the pharmaceutical industry. Understand the timeline, challenges, and stages from research to market.

Regulations & Standards
Explore the significance of ISO standards in the pharmaceutical industry. Learn how ISO compliance enhances quality, safety, and global market access in our detailed guide.

Drug Master File: What is the DMF?
Explore the essentials of Drug Master Files (DMFs) in the pharmaceutical industry. Learn about their types, significance, and role in regulatory compliance with our comprehensive guide.
Pharmaoffer is a B2B platform where you can find all qualified API suppliers in one place
Guide to Choosing & Collaborating with a CRO
Last Updated on
January 19, 2023
In the life sciences industry, the name of the game is often getting through preclinical studies and clinical trials. However, this feat is easier said than done. Many biotech, biopharma, pharma, and medical device companies need support to successfully conduct studies and trials.
Not every organization has the in-house capabilities, resources, and time to perform every trials-related task effectively and efficiently. When this is the case, businesses can rely on contract research organizations (CROs) for support with different stages of research and development.
This guide to CROs will help biotechnology, biopharmaceutical, and pharmaceutical companies select and collaborate with the right contract research organization, ensuring teams understand how CROs work, how to pick a good partner, and how to get full value out of these partnerships.
We’ll also review when it can make sense to lease lab equipment for in-house research and development alongside or in place of working with a CRO.
Need new or refurbished lab equipment? Excedr leases.
See our equipment list and browse a sample selection of what we can source. Or, if you’re ready, request an estimate.
What Is a Contract Research Organization?
A CRO is an individual or team that provides research services on a contract basis to other organizations in the life sciences, including pharmaceutical companies, biotechs, biopharmas, medical device companies, and even research institutions, government agencies, and foundations.
The services CROs provide to help develop drugs, medical devices, biologics, and other healthcare-based products include clinical trial support, laboratory testing, regulatory support, and more. Which services an organization or team offers will depend on their areas of focus and expertise, as well as the organization’s size and capabilities.
Outsourcing certain tasks and duties can allow companies to skip maintaining staff for these functions while still moving a drug or device from conception and development to FDA approval.
Contract research organizations typically work on a contract basis, providing services for specific projects or for a set period of time. This business model is designed to reduce drug development costs for companies and simplify entry into drug markets, and usually present a cost-effective alternative to in-house research and development for a wide variety of research-based labs and organizations that do not have the resources or expertise needed to perform certain research tasks on their own.
Working with a CRO can be an effective and efficient option if you’re working towards getting a product to market, but unsure if you want to take on the financial costs of building out an entire research team in addition to acquiring the necessary equipment and facilities to perform the tasks related to your clinical trials in-house.
In addition to a variety of services, CROs range in company size. This means, depending on your needs, you can find a small, specialized group or a larger, full-service organization that can handle all aspects of conducting research and clinical trials.
CROS will also carry out all of its work according to Good Clinical Practices (GCPs) that ensure high-quality studies and full compliance throughout your preclinical studies and clinical trials.
What Types of Services Do Contract Research Organizations Provide?
CROs offer a wide range of services to support the pharmaceutical, biotechnology, and medical device industries, from project, data, and site management, to clinical study management, research compliance and education, medicine and disease coding, validation, product development and commercialization, quality control, biostatistics, and much more.
Depending on the organization, the list can be quite extensive. Review the exact services each can provide. Understanding your requirements first and then assessing the scope of their services and resources needed can help you find a contract research organization that’s right for you.
Providing Laboratory Testing Services
CROs can provide specialized laboratory testing services, such as toxicology testing, microbiology testing, and pharmacokinetic studies. These services help companies assess the safety and efficacy of their products.
Supporting the Regulatory Process
Contract researchers can assist with the regulatory process and quality assurance, including preparing and submitting regulatory documents, such as registration and marketing applications, and providing guidance on compliance with regulatory requirements.
Conducting Preclinical Research
Contract researchers can also support drug discovery, product development, and conduct preclinical research on the safety and efficacy of new drugs, medical devices, and other products. This research typically involves animal testing and helps companies assess the potential risks and benefits of their products before they are tested in humans.
Conducting Clinical Trials
CROs can help design and manage clinical trials on behalf of other companies, from the initial planning stages to the analysis of the results. This may involve recruiting participants, collecting data, and monitoring the progress of the trial. When an organization offers clinical trial services and support, they might refer to themselves or be referred to as a clinical research organization.
Additional Research & Development Services
In addition to the services above, CROs can even help with other areas of research, including formulation development, process development, and analytical testing. These additional services can help laboratories improve the quality and effectiveness of their products.
What Types of Contract Research Organizations are There?
There are numerous types of CROs actively supporting the life sciences today. Their differences can range from the level of specialization—which types of studies they specialize in—the types of services they offer, company location or size, and much more.
Some organizations offer end-to-end services, providing support with preclinical studies, phase I, II, and III clinical research, and even commercialization They also tend to have expertise in a wider variety of therapeutic areas. Others tend to specialize in a certain stage of research and development, or may have expertise in a specific therapeutic area or industry.
Below are a few examples of different ways you can categorize contract research organizations.
Clinical Research Organizations
Clinical research organizations are CROs that specialize in conducting clinical trials on behalf of a client. They are usually responsible for designing the trial, recruiting participants, collecting data, and analyzing the results. In other words, they will handle clinical trial planning, data management, project management, site management, and trial monitoring.
These types of organizations can differ from other CROs who might provide services outside of clinical trials. Simply put, a contract research organization provides support to companies through a broad range of research support services, including study design, data management, and statistical analysis. These services can sometimes fall outside of clinical research and trials.
Laboratory Testing Organizations
Laboratory testing CROs provide specialized laboratory testing services, and may not provide clinical trial support. Instead, they’ll likely focus on a specific type of testing, such as toxicology or microbiology, or offer a range of services related to testing your product for a variety of reasons.
Regulatory Affairs Organizations
Some CROs specialize in regulatory affairs. This means they can provide support with a variety of the regulatory processes pharmaceutical, biotechnology, and medical device companies have to undertake and comply with. They can typically help companies prepare and submit regulatory documents, such as registration and marketing applications, and provide guidance on compliance with regulatory requirements.
Preclinical Research Organizations
Preclinical research CROs can help conduct research on the safety and efficacy of new drugs, medical devices, and other products before they are tested in humans during clinical trials. This type of research can involve animal testing and relies on a range of techniques, such as toxicity testing and pharmacokinetic studies. Some CROs that specialize in preclinical research can also help you when it comes time to conduct clinical trials, and vice versa.
How Are CROs Staffed?
Contract research organizations are staffed by a range of professionals with expertise in the pharmaceutical, biotechnology, and medical device industries. This can include clinical research professionals, laboratory technicians, regulatory affairs managers, preclinical research associates and scientists, and much more.
Clinical Research Professionals
Clinical research professionals, such as clinical research associates, clinical trial coordinators, and clinical trial managers, are responsible for planning, conducting, and managing a client’s clinical trials.

Regulatory Affairs Professionals
CROs that provide support with the regulatory process employ regulatory affairs professionals to help prepare and submit various regulatory documents. Regulatory affairs associates and managers can also provide guidance on compliance with different regulatory requirements the client will face.
Lab Professionals
Some CROS may employ laboratory professionals that are responsible for conducting tests and analyzing results. This can include laboratory technologists and technicians.
Preclinical Research Professionals
CROs that conduct preclinical research typically employ preclinical research professionals, such as preclinical research associates and preclinical research scientists. Like a clinical research coordinator or associate, these staff members are responsible for designing and conducting a client’s preclinical studies.
Additional Research & Development Professionals
In addition to various research associates, technicians, managers, and coordinators, CROs may also employ other types of R&D professionals. This can include formulation scientists, process engineers, and analytical chemists, who provide specialized research and development services for clients.
When Should You Use a Contract Research Organization?
There are several situations in which it can make sense to use a CRO to help with your business: lack of expertise or resources and cost or timeline inefficiencies are just two important reasons.
If you do not have the expertise or resources to conduct research and development in-house, CROs can provide the specialized expertise and resources needed to accelerate your research and fill in the gaps. This can be especially useful if you are a small company or if you are entering a new market or product area.
If you need to outsource research and development activities to save time and money, a contract researcher can—depending on the fees the organization charges—provide cost-effective R&D support, freeing up your team to focus on other tasks. Outsourcing can help you save time and money, and potentially help you get to market faster.
If you need support with the regulatory process, a contract research organization can provide guidance and support throughout the regulatory process, including preparing and submitting regulatory documents. With proper regulatory support, you’ll ensure you are in compliance with regulatory requirements and avoid costly delays or mistakes.
If you need to conduct clinical trials but lack the resources to do so, a CRO can design and manage the clinical trials on your behalf, from the initial planning stages to analysis of the results, making it easier to effectively and efficiently gather the data you need to support the safety and efficacy of your products.
Or, if you need to conduct preclinical research, a contract service provider can help conduct preclinical research on your products to assess their safety and efficacy before you go to clinical trials. Working with a CRO during your preclinical studies can help you more effectively identify any potential risks or benefits before moving on to test in humans, potentially saving you time and money in the long run.
How Do You Choose a CRO to Work With?
When it comes time to choose a CRO to partner with, there will be several factors you’ll want to consider, including their level of organizational expertise and experience, the quality and compliance of their work, the cost and value for money, the organization’s ability to communicate and collaborate, and its timeliness and flexibility.
You will also need to find a contract research organization that has a proven track record supporting R&D in your specific therapeutic area or industry. Working with a contractor that doesn’t have experience doing so may inevitably lead to failure in preclinical or clinical research.
Expertise & Experience
The CRO should have extensive expertise and experience in the specific area you are looking for support in, whether it’s conducting clinical trials or running laboratory tests. It can be helpful to look at each organization’s track record or the types of clients they have worked with in the past to assess their level of expertise.
Quality & Compliance
The organization should demonstrate a strong commitment to quality and compliance, and should be able to provide evidence of their compliance with relevant regulations and standards. Like assessing their track record or client list, you can review a CRO’s quality management system and any certifications they have, such as ISO 9001 certification, to determine their levels of quality and compliance.
Cost & Value for Money
A contract researcher’s fees should be competitive and provide real value for money. In other words, the fees you pay should align with what you receive in return. To assess whether or not the organization’s worth their prices, you can sometimes ask for cost estimates for the services you are interested in and compare them to other CROs.
Communication & Collaboration
The CRO should be able to communicate effectively and collaborate with your team throughout the project. Poor communication and collaborative efforts can result in poor results and failed trials, so it’s important to establish whether or not the CRO is a strong communicator. If possible, you can ask for references from other clients and to speak with the CRO’s team directly to assess their communication and collaboration skills.
Timeliness & Flexibility
An organization should also be able to deliver the services you need in a timely manner and should be flexible enough to adapt to any changing needs or requirements. Ask about the CRO’s availability and their approach to managing project timelines to get a better idea of whether or not they can meet deadlines and remain flexible in the face of changes.
Facility Locations
A CROs location can also be an important consideration when selecting a partner. If the drug or device you are developing together is expected to be approved in a variety of nations or locations, it will be beneficial—perhaps even necessary—to hire a CRO that has experience with the approval process in each location.
They may have facilities and staff positioned in the target location and will have knowledge about local regulations for clinical studies and approval.
Furthermore, because you’re outsourcing the work, you can easily lose oversight of the project. When a CRO’s team is conducting day-to-day tasks in another location, supervision can be more difficult, or even impossible. Comparing global CROs and local CROs and assessing how much supervision will be needed can help you understand whether or not location will be important in your selection.
Advantages & Disadvantages of Working with a CRO
Similar to working with a contract manufacturing organization (CMO), there are several advantages and disadvantages to working with a contract research organization.
The decision to use a CRO should be carefully considered, taking into account the potential drawbacks as well as the potential benefits.
- CROs have expertise and resources that may not be available in-house, such as specialized laboratory equipment or regulatory affairs experts. This can help you access the knowledge and capabilities you need to conduct research and development effectively.
- They can provide cost-effective support for research and development tasks, saving you time and money compared to conducting these activities in-house.
- They provide services on a contract basis, which can be more flexible than hiring permanent staff. This is especially useful if you have a specific project or a short-term need for research and development support.
- They typically have a strong commitment to quality and compliance, and are typically subject to strict regulatory requirements. This can help you ensure that your research and development activities are conducted according to the highest standards.
- They can often help you get to market faster by providing timely support for research and development activities, helping you stay ahead of the competition and capitalize on new opportunities.
- Using a CRO can sometimes be more expensive than conducting research in-house, as CROs typically charge a fee for their services.
- You may have less control over the research process, as the CRO will be responsible for conducting the study and collecting the data.
- If communication is poor, it can lead to misunderstandings and delays. Effective communication and collaboration between your company and the CRO is essential for the success of the research project.
- The quality of the research conducted by a CRO may vary depending on the experience and expertise of the individual CRO. It is important for the sponsoring company to carefully evaluate the CRO’s qualifications and track record before engaging them.
Lease with Excedr to Support In-House Research
Biotechnology, biopharmaceutical, and pharmaceutical companies often need support when it comes to preclinical, clinical trials, and other areas of research and development. There are a wide range of tasks, and not every company has systems and staff in place for every task or step.
However, rather than conduct the research in-house—spending the money to build out a team and acquire the necessary facilities and equipment and securing all the necessary compliance certifications—laboratories can outsource the research to contract research organizations.
CROs come in quite handy when startups, small- and medium-sized businesses, and even large enterprises need to increase R&D efficiencies and decrease the time and cost to approval and market. Using a CRO, on the other hand, can be a good option if you don’t have the expertise or resources to conduct the research yourself, or if you need access to specialized equipment or facilities.
CROs typically have extensive experience and expertise in conducting research studies, and can provide a range of services, including study design, data management, and statistical analysis.
However, using a CRO can be more expensive than conducting the research in-house, and you may have less control over the research process. If you do conduct research in-house, you will undoubtedly need to keep a variety of high-quality research equipment on-hand to gather and manage your data.
Not having the proper lab equipment, as you well know, can greatly affect the results of your work, making it necessary to keep up with technology and equip your lab with the right instrumentation.
Leasing lab equipment with Excedr can be a cost-effective option for life sciences and biotech companies when you want to reduce the upfront costs on the equipment, protect it with comprehensive service coverage, and remain flexible when a new and better technology inevitably becomes available.
Rather than sink your money into a fixed asset and take a huge chunk out of your R&D budget, leverage our leasing program instead. Doing so is an excellent choice when or if you need a specific piece of equipment that you don’t have in-house, or if you need to expand your existing laboratory facilities.
- Storyteller -->
- Storytalking
- Jaipur Bytes
- Vision-Nari
- LSP Home Join LSP

Contract Research Organization 101: The Essential Guide
What is a Contract Research Organization?
A contract research organization (CRO) is a company that provides services to the pharmaceutical, biotechnology, and medical device industries. Services provided by CROs can include clinical trial management, data management, biostatistics, and regulatory affairs.
CROs have become an increasingly important part of the drug development process as pharmaceutical companies look to outsource more of their research and development (R&D) activities. The global CRO market is expected to grow from $27.8 billion in 2016 to $41.5 billion by 2022, at a compound annual growth rate (CAGR) of 7.5%, according to a report by MarketsandMarkets.
The outsourcing of clinical trials by pharmaceutical companies to CROs is driven by a number of factors, including the increasing cost of conducting clinical trials, the need for speedier drug development timelines, and the desire to reduce the risk of failure during clinical trials.
CROs provide a number of benefits to pharmaceutical companies, including access to a global network of investigators, patients, and sites; expertise in trial design and execution; and flexibility in staffing and resourcing.
There are some drawbacks to working with CROs, including the loss of control over the clinical trial process and potential conflicts of interest. It is important for pharmaceutical companies to carefully consider whether outsourcing their clinical trials to a CRO is the right decision for their particular drug development program.
When selecting a CRO, pharmaceutical companies should consider a number of factors, including the CRO's experience in conducting similar trials, the quality of its data, its financial stability, and its geographic footprint.
2. What do Contract Research Organizations do?
A contract research organization (CRO) is a company that provides support to the pharmaceutical and biotechnology industries in the form of research services outsourced on a contract basis. CROs offer a variety of services, from preclinical research to clinical trials and post-marketing surveillance.
CROs first emerged in the 1970s as a way to help pharmaceutical companies reduce the cost of drug development. The industry has since grown to become a multi-billion-dollar business, with CROs now playing a vital role in the drug development process.
The global CRO market is expected to continue to grow in the coming years, driven by the increasing demand for outsourced research services and the continued trend of drug development costs.
CROs provide a variety of services to their clients, the most important of which are clinical research services. Clinical research is the process of conducting trials to test the safety and efficacy of new drugs.
CROs typically conduct Phase I-IV clinical trials on behalf of their clients. Phase I trials are the earliest and most important stage of drug development, as they are designed to assess the safety of a new drug.
CROs also play a vital role in post-marketing surveillance, which is the process of monitoring the safety and efficacy of a drug after it has been approved for sale.
In addition to clinical research services, CROs also offer a variety of other services, such as preclinical research, data management, and statistical analysis.
CROs are an important part of the pharmaceutical and biotechnology industries, and their services are essential to the drug development process Contract Research Organization .
3. The Benefits of Working with a Contract Research Organization
Working with a contract research organization (CRO) can offer a number of benefits to pharmaceutical and biotechnology companies. Here are three of the main benefits:
1. Access to expert knowledge and resources
Working with a CRO gives you access to a team of experts with extensive knowledge and experience in clinical research. This can be extremely helpful if you don't have an in-house clinical research team, or if your team is small and stretched thin.
The CRO can also provide access to valuable resources, such as a network of experienced investigators and specialized research facilities. This can save you time and money, and help ensure that your clinical trials are successful.
2. Reduced costs
Working with a CRO can save you money on the costs of clinical trials. The CRO can help you design and implement cost-effective clinical trials, and they can also negotiate favorable rates with investigators and research sites.
In addition, the CRO can manage all aspects of the clinical trial, from start to finish. This can free up your time and resources, so you can focus on other aspects of your business.
3. Increased efficiency
Working with a CRO can help you streamline the clinical trial process and make it more efficient. The CRO can help you develop an efficient trial protocol, and they can also manage the day-to-day operations of the trial.
This can save you time and money, and help ensure that your clinical trial is completed in a timely manner. In addition, the CRO can help you monitor the progress of the trial and identify any potential problems.
If you're considering working with a CRO, these are just a few of the benefits that you can expect. To learn more about the benefits of working with a CRO, contact a reputable CRO today.
4. The Different Types of Contract Research Organizations
A Contract Research Organization (CRO) is a company that provides services to the pharmaceutical, biotechnology, and medical device industries. The services provided by a CRO can range from drug development to clinical trials.
There are four main types of CROs:
1. Full-service CROs
2. Phase I CROs
3. Phase II-III CROs
4. Site Management Organizations (SMOs)
Full-service CROs offer a comprehensive range of services, from drug development to clinical trials. Full-service CROs are usually large companies with a global presence.
Phase I CROs specialize in early-stage clinical trials, also known as Phase I studies. Phase I studies are conducted on healthy volunteers to assess the safety and tolerability of a new drug or treatment.
Phase II-III CROs specialize in later-stage clinical trials, also known as Phase II and Phase III studies. Phase II studies are conducted on patients to assess the efficacy of a new drug or treatment. Phase III studies are conducted on large groups of patients to confirm the efficacy of a new drug or treatment and to compare it to existing treatments.
Site Management Organizations (SMOs) are companies that manage clinical trial sites. SMOs provide a variety of services to clinical trial sponsors, including site selection, site feasibility assessment, and site management.
5. How to Choose the Right Contract Research Organization
If you're looking to outsource your clinical research needs, you'll want to work with a contract research organization (CRO). But with so many CROs out there, how do you know which one is right for you?
Here are five things to consider when choosing a CRO:
1. Services Offered
The first thing you'll want to look at is the services offered by the CRO. Do they offer the specific services you're looking for? If not, they're probably not the right CRO for you.
2. Experience
When it comes to clinical research, experience matters. You'll want to work with a CRO that has extensive experience in the specific type of research you're conducting.
3. Geographic Location
Depending on the scope of your research project, you may need to work with a CRO that's located in a specific geographic region. For example, if you're conducting a multi-country study, you'll need to work with a CRO that has a presence in all of the countries involved.
Of course, you'll also need to consider cost when choosing a CRO. Make sure to get quotes from multiple CROs before making your final decision.
5. References
Finally, be sure to ask for references from each CRO you're considering. Talk to other companies that have worked with the CRO to get an idea of their experience.
By considering these five factors, you can be sure to choose the right CRO for your needs.
6. The Contract Research Organization Process
A contract research organization (CRO) is a company that provides research and development services to pharmaceutical, biotechnology, and medical device companies on a contract basis. CROs offer a wide range of services, from preclinical research to clinical trials and postmarketing surveillance.
The contract research organization process begins with the development of a research proposal. This proposal is then submitted to the CRO, which reviews it and provides a proposal for services. The client then reviews the proposal and, if satisfied, signs a contract with the CRO.
The CRO then begins to provide the services outlined in the contract. These services can include preclinical research, clinical trials, data management, and statistical analysis. The CRO works closely with the client to ensure that the project is completed on time and within budget.
Once the project is completed, the CRO provides the client with a report detailing the results of the research. The client then reviews the report and, if satisfied, pays the CRO for its services.
7. The Different Services Offered by Contract Research Organizations
A contract research organization (CRO) is a company that provides support to pharmaceutical and biotechnology companies in the form of research services outsourced on a contract basis. CROs offer a range of services, from preclinical research to clinical trials and post-marketing surveillance.
The global CRO market was valued at $26.2 billion in 2016 and is expected to grow to $45.8 billion by 2022, at a CAGR of 9.6%. The growth of the CRO market is driven by the increasing outsourcing of R&D activities by pharmaceutical and biotechnology companies, the increasing number of clinical trials, and the need to reduce the cost and time of drug development.
CROs offer a wide range of services, which can be broadly classified into the following categories:
1. Preclinical research services: These services include animal studies, in vitro studies, and pharmacokinetic/pharmacodynamic studies.
2. Clinical research services: These services include clinical trial management, data management, biostatistics, and medical writing.
3. Post-marketing surveillance services: These services include pharmacovigilance, epidemiological studies, and clinical outcome studies.
4. Regulatory affairs services: These services include regulatory submissions, clinical trial applications, and product registrations.
5. Quality assurance services: These services include auditing, inspections, and quality control.
6. Validation services: These services include computer systems validation, process validation, and cleaning validation.
7. Other services: These services include training, project management, and marketing.
CROs provide a wide range of services that can be customized to meet the specific needs of their clients. The services offered by CROs vary depending on their size, geographic location, and areas of expertise.
8. The Advantages of Working with a Contract Research Organization
A contract research organization (CRO) is a company that provides services to the pharmaceutical, biotechnology, and medical device industries. These services include clinical research, preclinical research, regulatory affairs, and more.
CROs play an important role in the development of new drugs and medical devices. They help to reduce the time and cost of clinical trials, and they also bring new therapies to market faster. In addition, CROs provide expertise and resources that may be unavailable in-house.
There are many advantages to working with a CRO. Here are eight of the most important:
1. CROs Have the Expertise and Resources You Need
CROs have a wealth of experience and expertise in clinical research. They also have access to the latest technology and resources. This means that they can provide a higher level of service than most in-house research teams.
2. CROs Can Help You Save Time and Money
CROs can help you save time and money by streamlining the clinical trial process. They have the experience and know-how to design efficient trials that get results. In addition, CROs have access to a network of patients and investigators. This can help you enroll patients faster and reduce your overall costs.
3. CROs Can Help You Reduce Risk
CROs can help you reduce the risks associated with clinical trials. They can design trials that minimize the risks of patient injury, for example. In addition, CROs can help you select the right investigational site and monitor the trial to ensure that it is being conducted ethically and in compliance with regulations.
4. CROs Can Help You Bring New Therapies to Market Faster
CROs can help you bring new therapies to market faster by streamlining the clinical trial process. In addition, CROs can help you navigate the regulatory process and get your product approved quickly.
5. CROs Can Help You Save on Regulatory Costs
CROs can help you save on regulatory costs by preparing and submitting regulatory documents. In addition, CROs can help you meet with FDA officials and answer any questions they may have.

clinical research organization (CRO). This was one of the best decisions I have ever made.
One of the hardest hit sectors has been the travel and tourism industry. As countries around
CRO services are important because they can help to improve the bottom line of a business by increas
Stay connected to your stories
- FBSHARE TwitterSHARE TumblrSHARE Copy Link
42 Launches
Part of the Life collection
Published on April 25, 2023
Recommended By
What's this story about.
Characters left :
- Life Love Poetry Happenings Mystery MyPlotTwist Culture Art Politics Letters To Juliet Society Universe Self-Help Modern Romance Fantasy Humor Something Else Adventure Commentary Confessions Crime Dark Fantasy Dear Diary Dear Mom Dreams Episodic/Serial Fan Fiction Flash Fiction Ideas Musings Parenting Play Screenplay Self-biography Songwriting Spirituality Travelogue Young Adult Science Fiction Children's Story Sci-Fantasy Poetry Wars Sponsored Horror
You can edit published STORIES
Delete Opinion
Are you sure you want to delete this opinion?
Delete Reply
Are you sure you want to delete this reply?
Report Content
Are you sure you want to report this content?
This content has been reported as inappropriate. Our team will look into it ASAP. Thank You!

By signing up you agree to Launchora's Terms & Policies.
Top 12 Clinical Research Organisations (CROs) in 2023

- Author Company: PharmiWeb.Jobs
- Author Name: Lucy Walters
- Author Email: [email protected]
- Author Website: https://www.pharmiweb.jobs/
The global clinical trials market was estimated to be worth $38.7 billion in 2021 and is expected to reach £52 billion by 2026. In this article, we look at the top 12 CROs in the world , highlighting the companies driving this considerable growth and accelerating research and development across the globe.
Recognised as the world’s largest and most comprehensive CRO powered by Healthcare Intelligence, ICON provides outsourced development and commercialisation services to pharmaceutical, biotechnology, medical device and government and public health organisations. ICON has a global network of offices in 46 countries, working on specialities including Medical Devices, Therapeutics, Government and Public Health Solutions, Clinical Research Services, Commercialisation and Outcomes, and more.
With approximately 70,000 employees, IQVIA is a leading provider of advanced analytics, technology solutions and clinical research services to the life sciences industry. Operating in more than 100 countries, IQVIA’s Clinical Research solutions include Protocol Design, Phase I Trials, Phase IIb / III Trials, Site Identification, Patient Recruitment and Global Laboratories.
With more than 20,000 employees globally, Parexel is one of the world’s largest CROs and provides contract research, consulting, and medical communications services to the worldwide pharmaceutical, biotechnology, and medical device industries. Parexel provides the full range of Phase I to IV clinical development services, with global study locations in Baltimore, US, London, UK, Los Angeles, US, and Berlin, Germany.
Syneos Health
Syneos Health is the only fully integrated biopharmaceutical solutions organisation built to accelerate customer success, with 29,000+ employees working across more than 110 countries. The company’s Clinical Development services include Decentralized Solutions, Bioanalytical Solutions, Early Phase, Phase II–III, Phase IIIb – IV, Real World and Late Phase, and Medical Device and Diagnostics. In 2022, Syneos Health was the winner of the Life Sciences Award for Most Innovative CRO.
Labcorp Drug Development
With more than 70,000 employees, Labcorp is a leading global life sciences company with unparalleled diagnostics and drug development capabilities. The company is driven to help medical, biotech, and pharmaceutical companies transform ideas into innovations, and supports clinical trial activities in approximately 100 countries. Labcorp plans to spin-off its clinical development business, to be named Fortrea, in mid-2023.
Part of Thermo Fisher Scientific, PPD is a leading global CRO with 130,000+ employees, working to make the world healthier, cleaner, and safer. The company offers a broad range of clinical development and analytical services, helping to accelerate medicines from early development through regulatory approval and market access. PPD works with a broad range of therapeutic areas, including Biosimilar Development, Cell & Gene Therapy, Immunology and Rheumatology, Infectious Diseases, Women’s Health, and more.
In 2022, Medpace celebrated 30 years of accelerating the development of medical therapeutics, with approximately 5,000 employees working across 41 countries. The company provides medical and regulatory leadership and guidance throughout the clinical development life cycle, along with efficient execution of studies around the world, from Early Phase to Late Phase. Medpace also earned the 2022 CRO Leadership Awards for Capability, Compatibility, Expertise, Quality, and Reliability.
Charles River Laboratories
With 20,000+ employees worldwide, Charles River Laboratories provides essential products and services that help pharmaceutical and biotechnology companies, government agencies and leading academic institutions across the globe to accelerate their research and drug development efforts. The company currently operates more than 110 facilities across 20+ countries, working on therapeutic areas including COVID-19, Cardiovascular Disease, Immunology, Infectious Diseases, and Rare Diseases.
WuXi AppTec
WuXi AppTec is a global company providing a broad portfolio of R&D and manufacturing services to help pharmaceutical and healthcare companies across the globe advance discoveries and deliver ground-breaking treatments. The company currently has more than 45,000 employees globally, including 42,000 scientists. WuXi Clinical, a wholly owned subsidiary of WuXi AppTec, is a global CRO providing a comprehensive range of clinical development services for the development of pharmaceuticals, biologics, and medical devices.
CTI is one of the 20 largest CROs in the world, with associates in 60+ countries across six continents, including 12 locations in Europe. The company is the highest-ranked CRO in the world after being named a winner in all 5 core categories of the 2022 CRO Leadership Award. CTI provides its partners with a full range of clinical services, across therapeutic areas including Rare / Orphan Diseases, Regenerative Medicine / Gene Therapy, Immunology, Transplantation, and Hematology / Oncology.
Worldwide Clinical Trials
With approximately 3,000 employees and operating in more than 60 countries, Worldwide Clinical Trials is a global, midsize CRO providing top-performing bioanalytical and Phase I-IV clinical development services to biotechnology and pharmaceutical companies. The company’s major therapeutic focus areas include Cardiovascular, Metabolic, Neuroscience, Oncology, and Rare Diseases. In 2022, they were recognised for excellence and honoured in 5 categories in the CRO Leadership Awards.
PSI CRO is a privately-owned, full-service CRO operating globally, with 27,000+ employees in more than 60 countries. Committed to being the best CRO in the world, the company specialises in the planning and execution of global pivotal registration clinical trials, supporting trials across multiple countries and continents. PSI was named a 2022 CRO Leadership Award Champion in the categories of Compatibility, Quality, and Reliability.
You are leaving PharmiWeb.com

Disclaimer: You are now leaving PharmiWeb.com website and are going to a website that is not operated by us. We are not responsible for the content or availability of linked sites.
ABOUT THIRD PARTY LINKS ON OUR SITE
PharmiWeb.com offers links to other third party websites that may be of interest to our website visitors. The links provided in our website are provided solely for your convenience and may assist you in locating other useful information on the Internet. When you click on these links you will leave the PharmiWeb.com website and will be redirected to another site. These sites are not under the control of PharmiWeb.com.
PharmiWeb.com is not responsible for the content of linked third party websites. We are not an agent for these third parties nor do we endorse or guarantee their products. We make no representation or warranty regarding the accuracy of the information contained in the linked sites. We suggest that you always verify the information obtained from linked websites before acting upon this information.
Also, please be aware that the security and privacy policies on these sites may be different than PharmiWeb.com policies, so please read third party privacy and security policies closely.
If you have any questions or concerns about the products and services offered on linked third party websites, please contact the third party directly.
Top 10 Clinical Research Organizations in the Pharma and Biotech Industry

Pharma IQ showcases the top 10 Contract Research Organizations across the Pharma IQ network.
Much of the world’s population is in desperate need of better medical care. This need is a large driving force behind drug discovery’s mission to uncover new medicines.
The drug discovery process is complex and stubborn. The clinical stage, in particular, is resource intensive, demanding and high risk . A fine balance is required to execute this stage correctly.
Clinical Research Organisations (CROs) supporting drug manufacturers on their road to discover and approve drugs of the future by absorbing some of the clinical stage’s the burdens. Some of the activities covered are:
- Data research
- Project management
- Trials that are run post approval
- Pre-clinical
This relationship, which is built on trust and skill, can award manufacturers with heightened expertise as well as cost and time efficiencies. These benefits come at the cost of duties such as intensive communication management , confidentiality concerns and regulatory considerations.
The trend towards outsourcing to CROs isn’t expected to fizzle out anytime soon, especially as the spread of clinical trials across the world is growing. The global market is forecasted for strong growth, after reaching US$36.2bn in 2017 it is projected to climb by another US$20bn over the next five years.
Read more: How to improve the success rates of clinical trials
What to look for in a cro.
Pharmaceutical and biologic firms need to select reliable partners who will add value to their medical research. This is a complex decision process involving the evaluation of many variables.
We invited the Pharma IQ community to vote for the leading CROs in the pharmaceutical and the biologic industry according to their own experiences.
Our research base is made up of participants from mainly big pharma or biotech, SME pharma and consultants. Other entities included in this research include government bodies, medical device manufacturers and public hospitals.

What criteria makes an excellent CRO?
The rising public pressure on medicine prices and spend by pharmaceutical firms, drives the appeal of outsourcing as a way to cool costs.
However, as pharma continues to wrestle with stagnant innovation levels, despite the funding boost to R&D pipelines, cheaper running costs are no longer the primary motive for outsourcing. In fact, this was considered as the last priority for our response base.
Read more: How to automate the data lifecycle to accelerate drug discovery
(respondent’s could vote for more than one answer) .
One respondent noted that on top of having a combination of all the listed attributes, they desired the ‘right’ culture and expertise within the actual group of people proposed for the study.
Availability in certain regions, emerging markets, for example, and being update to date with recent regulatory changes were highlighted as key factors by some participants.
Just over 55% of our base agreed that quality is the most important differentiator when selecting a CRO .
Regarding the importance of quality, in association with the Trial Master File ( TMF) and Inspection Readiness event , Ivan Walrath, Head of Audit and Inspection Quality at Pfizer noted how important it is CROs recognized the significance of this file. The TMF is what regulators will consult to evaluate the quality of the science behind the product at hand.
He added: “What I’ve seen when these partnerships have worked well is a good level of communication and integration between the people from the CRO and the sponsor working together to execute a trial.
“When you have a well-functioning team, even across those organizational boundaries, everybody gets behind what needs to happen from a quality standpoint, from a compliance standpoint and other important aspects like hitting timelines.”
Our 2018 study found that Clinical trial supply has seen a reduction in wastage rates thanks to smarter integration and alignment with enrollment rates.
This improvement was achieved through Interactive Voice Response Systems (IVRS) and Interactive Response Technology (IRT), these tools were one of the top spend priorities.
Read more: Top five countries running the most clinical trials
The majority of the base noted that contract reviews would happen only when an issue came up or on an annual basis.
A few participants clarified that reviews are ongoing as part of their oversight to ensure compliance with contract services, but “not for the purpose of switching CROs, that would only be done in extreme circumstances of failure to perform.”

Ending a partnership with a CRO
Similar to 2017’s results, lack of quality was the aspect voted most likely to end a partnership with a CRO, attracting 65% of the votes. A high level of mistakes was the next option with 12% of the vote. One participant proceeded to clarify a legitimate reason being:
“Significant failure to meet obligations of contract, missing multiple milestones, without reasonable cause or appropriate risk mitigation.”
Pharma IQ top 10 CROs
1. iqvia, 2. parexel, 3. syneos health, 4. covance, 5. icon, 6. pra health sciences, 7. ppd, 8. chiltern, 9. fisher clinical services, 10. medpace, read our updated guide to the 10 biggest cros in the pharma sector for 2022.
Firms that attracted recognition from the network as rising stars include:
· Charles river laboratories
· pivotal, · psi, · pharmolam.
Further comments included: “After 25 years in the business, always with small Biotechs, I have learned that niche CROs provide the best service for our needs (Argint and CONET). I tend to avoid large CROs when at all possible.”
Quick links
- Why R&D must digitalize and become data-centric
- The benefits of drug reformulation
Get exclusive access to member-only articles, reports, videos, interviews, webinars and other premium content from industry experts and thought leaders by signing up to Pharma IQ here .
FIND CONTENT BY TYPE
- Infographics
Pharma IQ COMMUNITY
- Advertise With Us
- User Agreement
- Cookie Policy
- Become a Member Today
- Pharma IQ App
ADVERTISE WITH US
Reach Pharmaceuticals & Biotechnology professionals through cost-effective marketing opportunities to deliver your message, position yourself as a thought leader, and introduce new products, techniques and strategies to the market.
JOIN THE Pharma IQ COMMUNITY
Join Pharma & Biotech today and interact with a vibrant network of professionals, keeping up to date with the industry by accessing our wealth of articles, videos, live conferences and more.

Pharma IQ, a division of IQPC
Careers With IQPC | Contact Us | About Us | Cookie Policy
Become a Member today!
PLEASE ENTER YOUR EMAIL TO JOIN FOR FREE
Already an IQPC Community Member? Sign in Here or Forgot Password Sign up now and get FREE access to our extensive library of reports, infographics, whitepapers, webinars and online events from the world’s foremost thought leaders.
We respect your privacy, by clicking 'Subscribe' you will receive our e-newsletter, including information on Podcasts, Webinars, event discounts, online learning opportunities and agree to our User Agreement. You have the right to object. For further information on how we process and monitor your personal data click here . You can unsubscribe at any time.
Clinical Researcher
Leave No Site Behind: How Sites, Sponsors, and CROs Can Speed Clinical Research Together
Clinical Researcher January 14, 2020

Clinical Researcher—January 2020 (Volume 34, Issue 1)
SITE STRATEGIES
As the age of blockbuster drugs has given way to precise, impactful treatments that are customized for rare diseases and individual patients, the volume of clinical trials has compounded. In 2007, there were 48,290 clinical trials recorded worldwide while more than 322,100 trials are expected to be tallied for 2019.{1}
With clinical studies flourishing, sponsors have shifted to an outsourcing model to drive greater efficiency. More than 70% of all clinical trials are expected to involve a contract research organization (CRO) in 2020,{2} and analysts predict the CRO market will reach nearly $55 billion by 2025.{3}
Clinical research sites are also becoming increasingly vital in helping sponsors and CROs keep pace with bringing new treatments to market. Sites, however, face significant hurdles in meeting the demands of this new era of drug innovation—hurdles that may discourage the all-important participation of principal investigators in studies. In fact, more than half of first-time investigators do not conduct a subsequent trial.{4}
Going forward, there is a significant opportunity for the industry to improve collaboration and execution of clinical trials. Sites have the most to gain, especially with improving productivity and reducing delays that impact trial execution. Recently, leading investigators from a range of different sites identified three key opportunities for improvement: communication and collaboration, information sharing, and streamlining common trial processes.
Improve Communication and Collaboration to Drive Productivity
Good communication often starts with mutual understanding. By getting to know one another and establishing common ground, clinical trial partners can be more productive and develop stronger working relationships. While the ability of sponsors and CROs to meet the needs of sites has progressed in recent years, most sites say there is room to improve communication,{5} especially around the day-to-day responsibilities and workloads that impact timelines at sites.
“Sponsors don’t have visibility into the many things on my plate to understand that their timelines are sometimes not achievable,” said Katie Seehusen, a regulatory specialist with the Iowa Diabetes & Endocrinology Research Center . “I am the only regulatory specialist at my site, and we currently have 30 ongoing drug and device studies with seven new studies coming soon. So, it’s important that I structure my time to get everything done.”
Seehusen frequently balances the critical deadlines of various sponsors and CROs, and ultimately has to make difficult judgement calls about which jobs get done first based on priorities and available resources. “I am pretty good at multitasking,” she said, “but sometimes there just isn’t enough time in the day. Just because we work at high volume, doesn’t mean we have a high volume of staff.”
The demand on sites to get more done, faster can be overwhelming. Sites acknowledge that better communication on timelines and even collaborating with sponsors and CROs on how to work more efficiently can alleviate some of the pressure they’re feeling.
Jeff Kingsley, CEO of IACT Health , a clinical research network based in Columbus, Ga., said part of the communication and collaboration breakdown often stems from the outsourcing model itself. “There’s sometimes a big gap between the sponsor and clinical trial sites with separate companies handling labs, oversight, and so forth,” he said. “If sites and sponsors become more strongly connected, we could better support the tremendous growth happening throughout the industry. Bridging the communication gap is critical to this.”
Streamline Information Exchange for Faster Trials
One of the most common needs among researchers is improving information exchange with sponsors and CROs.{6} Key drivers included reducing manual processes, improving collaboration among study partners, and better visibility and oversight (see Figure 1).
Figure 1: Top Drivers to Streamline Information Exchange
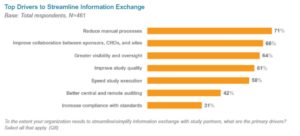
Sites often depend on sponsors and CROs to provide technology for managing trial activities and collaborating during a study. Sponsors and CROs on average use three different systems—including e-mail, portals, file sharing, and eTMF applications—to exchange trial data and documents with study partners.
Meanwhile, sites support multiple studies at one time. With each sponsor providing a unique system to sites running dozens or even hundreds of trials, the use of technology can become overwhelming. In fact, the average site uses a minimum of 12 different electronic systems to collect and capture clinical study data.{7}
“One of the biggest challenges we have as a site is that most technology comes from sponsors, and the sponsors select the technology that serves their needs,” said Rachel Sheppard, clinical research director for the Clinical Trials Units at the University of Louisville . “We’re running trials for hundreds of sponsors, so we have to learn all those various systems and be agile in them. So, it’s been difficult for us to transition to electronic regulatory systems because of that.”
Several sites noted that better information sharing could be addressed by newer cloud-based tools that are capable of streamlining trial activities and accelerating research.{8} Sheppard said that administrative minutiae are holding sites back and preventing trials from getting started quickly. “These setbacks could be resolved with better technology,” she added.
Nelson Rutrick, CEO of Adams Clinical , a site outside Boston specializing in psychiatric research, said sponsors collect large amounts of information on site data quality during patient enrollment. Making these data available helps sites understand where they’re doing well and where they need to improve.
“Sites know how well they are doing relative to other sites on recruitment metrics, but they often lack visibility into their performance on quality metrics,” Rutrick said. “Most electronic data capture and eSource products have reporting features that display some statistics to sites, but these features are usually turned off. Sponsors and CROs should turn these features on so sites can see where improvement is needed.”
Some sites also said making information sharing more efficient would enable them to focus more time and energy on other important matters—most notably training.
“I never want to be so busy that I stop learning,” said Seehusen. “Research is always changing. There are always new things to learn. We have a job where we constantly need to learn about what’s changing. I would love to have more time to train and be better at my job.”
Better training is an opportunity to improve clinical trials. Researchers want a roadmap for the right qualifications to do their job—the skills they should have and the related competencies. There’s a big movement among sites around competency-based roles. With so many specialized roles, the industry could benefit from sites having more ways to get credentialed for their unique roles.
Katrina Quidley, a regulatory manager with IACT, said she would like more sponsors and CROs to consider waiving redundant requirements for good clinical practice (GCP) training for staff with Certified Clinical Research Coordinator (CCRC) or Certified Principal Investigator (CPI) credentials through the Association of Clinical Research Professionals (ACRP). In 2014, the TransCelerate BioPharma Inc. initiative began acknowledging these ACRP certifications as an equivalent to any GCP course. Accepting ACRP certifications for GCP fulfillment streamlines the training process and allows sites to move forward with completing all personnel requirements and meeting expected timelines.
Simplify Operational Processes to Reduce Delays
With more study partners in the mix, trials have become increasingly complex. More people are involved in trial processes, each wanting to review and approve a site’s work. This often leads to trials being underbudgeted or delayed.
“There are many parties involved these days,” said Kingsley. “Innovation can stagnate because of so many in-betweens. CROs typically prefer that all communication be filtered through them, so we often lose the emotional connection with sponsors. Sponsors and sites don’t have much contact anymore.”
Seehusen said she often finds herself dealing with more people than she can count—all needing the same information. “Sponsors and CROs have a lot of people working on the trial, so there can often be a lot of redundant steps that cause delays,” she noted. “Centralizing coordination and improving organization could save me hours; and if you multiply that across all the studies happening at any given time, it could add up to days of improved productivity.”
Working Better Together to Improve Patients’ Lives
The pressure to innovate specialized products and get them to market quickly will only become harder as the ecosystem of clinical trial stakeholders continues to expand. Improving efficiency among study partners will enable the entire industry to better support the growing number of trials and, ultimately, bring innovative new therapies and drugs to market much faster for the patients who need them.
In our conversations with expert sources at sites, they underscored their desire to work with sponsors and CROs to improve trial processes. In the end, they said everyone just wants to achieve more positive results.
“Our biggest focus is on improving the lives of patients,” Seehusen said. “So, if we’re working at a capacity of maybe 15 studies a year and we can get to 20 studies a year because we’re working more efficiently and effectively, then we can see more patients and help more people. That’s really our end goal—just being able to help more people.”
- ClinicalTrials.gov. 2019. Number of Registered Studies Over Time. https://clinicaltrials.gov/ct2/resources/trends
- Business Wire. 2015. Research and Markets: The New 2015 Trends of Global Clinical Development Outsourcing Market. https://www.businesswire.com/news/home/20150130005621/en/Research-Markets-New-2015-Trends-Global-Clinical
- Grand View Research. 2019. Healthcare CRO Market Size Worth $54.7 Billion by 2025. https://www.grandviewresearch.com/press-release/global-healthcare-cro-market
- ScienceDirect. 2017. One and done: Reasons principal investigators conduct only one FDA-regulated drug trial. https://www.sciencedirect.com/science/article/pii/S245186541630093X
- CenterWatch. 2019. Sponsors, CROs Doing Better, Sites Say, But More Work Is Needed. https://www.centerwatch.com/articles/24465-sponsors-cros-doing-better-sites-say-but-more-work-is-needed
- Veeva Systems, Inc. 2019. Veeva 2019 Unified Clinical Operations Survey Report. https://www.veeva.com/resources/veeva-2019-unified-clinical-operations-survey-report/
- BioSpace. 2016. New CenterWatch Study Finds that E-Clinical Technologies are Increasing Investigative Site Work Burden and Performance Inefficiencies. https://www.biospace.com/article/releases/new-centerwatch-inc-study-finds-that-e-clinical-technologies-are-increasing-investigative-site-work-burden-and-performance-inefficiencies-/?keywords=centerwatch+study+finds+that+e-clinical
- Veeva Systems. 2019. Veeva announces free eRegulatory solution for Clinical Trial Sites. https://www.veeva.com/resources/veeva-announces-free-eregulatory-solution-for-clinical-research-sites/

Bree Burks ( [email protected] ) is Vice President, Site Strategy for Veeva Systems, with more than 12 years of experience in research in sponsor and site settings.
Sorry, we couldn't find any jobs that match your criteria.

Barriers to Clinical Trial Enrollment: Focus on Underrepresented Populations

Using Simulation to Teach Research

An Approach to a Benefit-Risk Framework

Why Catalyst Clinical Research?
You need someone to listen to your needs. You need a catalyst for your drug development needs.
Catalyst provides highly customizable clinical trial and drug development solutions to the global biotechnology and biopharmaceutical industries through our Catalyst Oncology and Catalyst Flex. Through these two solutions, Catalyst offers a full-service oncology CRO and multi-therapeutic functional service provider services.
With Catalyst, you can always count on us. We are a catalyst.
Get the latest news and press releases from Catalyst Clinical Research

- Privacy Overview
- Strictly Necessary Cookies
- 3rd Party Cookies
- Additional Cookies
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!
This website uses the following additional cookies:
(List the cookies that you are using on the website here.)

Guest Column | June 14, 2018
Cro selection 101 — how to get started.
By Joelle Herman , president, NeoTrials, LLC

This three-part article series will look at how best to procure, manage, and implement best practices in this complicated market.
As companies continue to look for smarter ways to develop drugs, biologics, and devices, outsourcing clinical trials to contract research organizations (CROs) and niche providers continues to grow. A recent report from Statistica projects that global CRO revenues will reach $52.3 billion by 2021 just in locations outside of the Asia-Pacific (APAC) region. While that is impressive, APAC, which is one of the fastest growing markets in the world, is projected to grow at a 20 percent compound annual growth rate (CAGR), compared to 11.4 percent “rest of world” revenue. 1 This growth is substantial and plays into the critical nature of how product developers procure and utilize CROs. This is a complex process whether you are a large biopharma or a small one poised to initiate clinical trials. The complexity of a successful procurement can be managed with good early planning and a strategic approach.
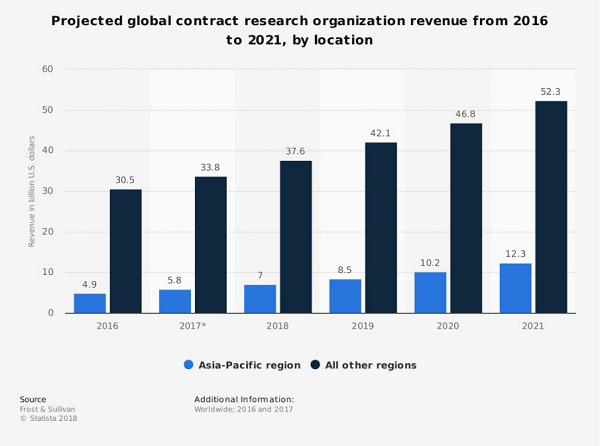
Champion/Selection Committee
Before you can go out and get bids, the needs of the project/program must be defined by an internal champion or selection committee. A person or team closest to the science or the person responsible for leading the program team or, in a small company, the person managing the clinical trial(s) often are best positioned to lead this effort. A champion will coordinate the entire process across multiple functions (project management, medical, finance, clinical operations, etc.) for the sole purpose of planning the best outsourcing strategy. The champion will ensure a process workflow, define roles/responsibilities, identify challenges (i.e., to eliminate silos or divisions because of competing priorities or lack of vested interest), and ensure goals are defined and agreed to by the selection committee.
A committee that is composed of multidisciplinary leaders often can determine the CRO that is the best fit for the organization. Not all CROs are aligned with your organizational culture, practices, or expertise, so it is a best practice to define these parameters. The selection committee must also have a vested and committed interest in this process. Depending on the size of the organization, the committee may want to define and agree on a communication strategy. This ensures the workflow is moving ahead as per plan and that all members are accountable for their roles and tasks. Additionally, good planning establishes how to maximize core assets and utilize internal resources, supplemented with external resources. A champion and selection committee will define exactly what tasks the CRO will perform and which procedures/processes will govern various tasks. For example, the sponsoring company will establish how the investigator site contracts will be negotiated and who will legally be bound by the agreement(s). CROs bidding on the work need to know exactly what tasks they will perform in order to provide their best approach. With that, the champion/committee will create a specifications document to communicate standard processes, tasks, and tools it is looking to procure. By further defining the scope of the services being procured, whether full service or partial service, companies create a strategic sourcing approach for the clinical trial.
Procurement/Strategic Sourcing
In order to establish and assess the best fit, the committee may want to utilize its vendor network, current CROs, and/or conduct a competition through public announcement of the procurement. A competition is not a new concept, but how you conduct the competition is key, especially if the project is partnered with another commercial entity or federal agency (i.e., federally funded). Also, as the committee considers long-term planning or portfolio management, it seems logical to maximize efficiencies with relationship management. How can teams reduce cost and time in this repetitive process? Commercial procurement models generally take one of three forms. The preferred provider model, which creates a more collaborative relationship with a CRO, ensures repeat business and allows for streamlined buying power. The performance-based model or managed services model drives accountability through incentives/penalties using milestones/target achievements. Another model is used when sponsors want to harness innovation to select the best value-added solution. Some CROs have new technology or innovative approaches aligned with the company’s goals to augment internal resources. Making these requirements clear to CROs that range from generalists to specialists with partial- to full-service clinical research functions allows the CRO procurement filter process to be maximized for best fit. What does this mean for the selection committee? What level of detail and criteria are needed?
The request for proposal (RFP) documentation is a vital piece in communicating the sponsor/client’s needs. The RFP will be addressed in Part 2 of this series, but before you can create an RFP, the committee must define criteria for selection. Matching chemistry like perception or perceived value is important on the surface, but the real measures of potential performance are the following criteria.

Risk management practices are now expected to be incorporated into the selection and oversight of CROs and other vendors. Find out more in SAM Sather's webinar:
CRO Oversight Post ICH GCP E6 (R2) Addendum
Selection Criteria For CRO Selection
- Specific therapeutic area and indication experience
- Team member experience in the indication
- Approach to current challenges (demonstrated understanding of enrollment, feasibility, access to KOLs, and investigative sites with the right population and experience)
- Responsiveness and ability to be limber with internal and external processes/systems (don’t forget to conduct a qualification audit as a contingency to an agreement)
- Quality/consistency of performance/financial stability/inspection history 2
- Value (determine the best service for best price)
- Transparency (confirm the scope of service delineates the client will have access to systems)
- Collaboration and communication planning (ask if they have escalation strategies)
- How do they plan (ask for examples of schedules, what plans will be part of the service, i.e., project plans, risk management plan, subcontractor management plan, monitoring plan, statistical analysis plan, data and pharmacovigilance plans)
According to Comprehend, 3 a clinical intelligence software provider, the three most common overlooked selection criteria are transparency, collaborative partnership, and real-time responsiveness. These intangible factors should be part of any strategic selection process. Sponsors struggle to quantify these factors, but to win the business, CROs can proactively demonstrate these intangibles with solid evidence. This isn’t one-sided. CROs can only provide their best services when all parties model transparent and collaborative relationships.
Future articles in this series will cover: developing a CRO selection workflow and checklist, addressing documentation and regulation concerns, and applying best practices to overcome the major challenges in outsourcing.
References:
- https://www.statista.com/statistics/817599/revenue-forecast-for-pharma-cros-by-location/
- https://www.pharmoutsourcing.com/Featured-Articles/172751-What-to-Look-for-in-Selecting-a-CRO-CMO-and-How-to-Ensure-the-Right-Choice-A-Quality-Assurance-Perspective/
- https://www.comprehend.com/overlooked-criteria-in-cro-selection/
About the Author:

Like what you are reading?
Sign up for our free newsletter, newsletter signup.


Reimagining CRO Partnerships: The Emerging Preference for Midsize Organizations

As the clinical research landscape continually evolves, the choice of a Contract Research Organization (CRO) partner can significantly impact the success of drug development programs. In fact, recent industry dynamics indicate a considerable shift in preference towards midsize CROs, driven by the need for more personalized, agile, and cost-effective partnerships.
Worldwide Clinical Trials recently conducted a comprehensive research study, where we included 140 key industry-decision makers from biotech and pharmaceutical companies of all sizes. Through this study, we unveiled critical insights into this trend, shedding light on the key client challenges associated with large CROs and the distinct advantages midsize CROs can offer.
Key Client Challenges with Large CROs
Our research reveals a growing hesitation among industry leaders to partner with large CROs, with nearly half of the respondents citing instability due to mergers, acquisitions, and downsizing initiatives. Other concerns respondents are apprehensive about include:
- 77% of respondents: Large CROs promised broad services all under one roof, but services are typically not integrated seamlessly into service delivery.
- 74% of respondents: Lack of personalized attention
- 71% of respondents: Less continuity on large CRO project teams for the duration of the trial
- 71% of respondents: High clinical development costs
- 70% of respondents: Project team disruptions leading to extended timelines
These findings underscore a critical need for change in how CRO partnerships are viewed and valued within the industry.
The Advantages of Midsize CROs
In contrast to large CROs, midsize CROs like Worldwide Clinical Trials offer a unique value proposition to the market. Respondents in our research highlighted several key benefits, including:
- 87% of respondents: Being treated like a high-priority client
- 83% of respondents: Agility
- 81% of respondents: Rapid escalation of study-related issues, minimizing delays
- 80% of respondents: Responsiveness and collaboration
- 80% of respondents: Access to senior-level expertise
- 77% of respondents: Demonstration of a shared sense of ownership for clinical development programs
- 74% of respondents: Personalized attention
The Shift Towards Midsize CROs
Reflecting on the capabilities of Worldwide, an impressive 80% of respondents expressed their intention to explore our services further. From our strengths in personalized attention and responsiveness to our collaborative partnerships and therapeutic-focused expertise, our services have made us the preferred partner for future endeavors. This industry shift represents not just a change in sponsor preference but rather a broader reevaluation of what attributes are most valued in a CRO partnership.
To gain deeper insights into this pivotal shift and how it can benefit your clinical development strategy, we invite you to download our full industry survey report . Discover how Worldwide is positioned to meet your needs with agility, expertise, and a truly personalized approach.
You may also be interested in

Schizophrenia Clinical Development at Worldwide Clinical Trials

Revolutionizing Drug Development Through Bioanalytical Excellence: Enabling Automation in Bioanalysis for Optimal Results

In-House Clinical Assessment Technologies at Worldwide
Want to learn more about worldwide clinical trials, cookies on the worldwide clinical trials website.
We use cookies to improve your browsing experience and help us improve our websites. We have carefully selected third parties that use cookies to achieve purposes illustrated in the cookie policy. For more information. Read more about online privacy statement . By closing this banner or continuing to browse our website, you agree to our use of such cookies.

Melior Discovery Earns Prestigious 'CRO of the Year' Title from Life Sciences Review
EXTON, PA — Melior Discovery, Inc., a standout in the pre-clinical pharmacology sector, has been lauded as the Contract Research Organization (CRO) of the Year by Life Sciences Review magazine . The accolade, announced in the publication’s year-end edition, places Melior at the forefront of the industry, highlighting its innovative contributions to drug discovery and development.
Life Sciences Review’s annual coverage showcases 10 leading CROs, with Melior clinching the top spot for its exceptional service offerings. Central to Melior’s acclaim is its proprietary theraTRACE® platform, a phenotypic screening tool that has significantly advanced the identification of new therapeutic uses for existing drugs. This approach, known as drug repositioning, can dramatically reduce the time and cost associated with bringing treatments to market.
Additionally, Melior’s specialized platforms, immuno-theraTRACE® for oncology and opioidTRACE® for analgesics, further underscore the company’s commitment to addressing critical needs in healthcare. These platforms, alongside a comprehensive suite of animal models of human disease, provide invaluable resources for pharmaceutical and biopharmaceutical companies seeking to validate the efficacy and safety of their compounds in pre-clinical stages.
Andrew Reaume, President and CEO of Melior, expressed gratitude for the recognition, viewing it as a testament to the company’s growing influence and innovation within the life sciences sector. “This honor, together with the distinction that we received last year from Pharma Tech Outlook as a Top 20 CRO, are a testimony to the dynamic strides that Melior is making towards becoming one of the most important players in providing pre-clinical in vivo proof-of-concept for pharma and biopharmaceutical companies of all sorts,” said Reaume.
The selection of Melior as CRO of the Year is significant for several reasons. First, it highlights the increasing importance of CROs in the drug development process. As pharmaceutical companies face escalating costs and complexities in bringing new therapies to market, partnerships with CROs like Melior offer a more efficient path through the critical stages of drug discovery and development.
Second, Melior’s recognition shines a spotlight on the value of phenotypic screening and drug repositioning. By leveraging existing compounds with known safety profiles, companies can potentially bring life-saving treatments to patients more quickly and at lower costs than traditional drug discovery methods allow.
As the pharmaceutical industry continues to evolve, the role of innovative CROs will undoubtedly expand. Melior Discovery’s recent accolade from Life Sciences Review not only celebrates the company’s achievements but also signals a broader shift towards more collaborative and efficient approaches to drug development.


- Messages from the board
- Vision and ambition
- From vision to action
- Research strategy
- Organization
- The Building
- Key Process and target
- Center of e-Learning
- Digital Library Knowledge Center
- Indonesia Museum of Health and Medicine
- Medical Education Center
- Simulation Based Medical Education & Research Center
- Center for Sport and Exercise Studies
- Drug Development
- Evidence-based Health Policy Center
- Human Cancer
- Human Genetics
- Human Nutrition
- Human Reproduction, Fertility and Family Planning
- Infectious Disease and Immunology
- Medical Technology
- Metabolic Disorder, Cardiovascular and Aging
- Neuroscience and Brain Development
- Occupational and Environmental Health
- Primary Health Care Research and Innovation Center
- Stem Cells and Tissue Engineering
- Animal Research Facilities
- Big Data Center
- Bio-Informatica
- Clinical Research Supporting Unit
- Molecular Biology and Proteomics Core Facilities
- Writing Center
- Class & Seminar Room
- Executive Lounge
- Integrated Teaching Theatre
- Post Graduate Room
- Our Services
- High-end Equipments
Medical Research Core Facilities
Clinical research supporting unit (crsu).
Is a contract research organization (CRO) with the main mission to organize and conduct clinical studies in Indonesia and overseas and ensure that they are in accordance with International Conference Harmonization and Good Clinical Practices (ICH-GCP). CRSU has a good networking with industry and related institutions both in Indonesia and overseas. CRSU mainly focuses on improving the quality of clinical trials in Indonesia through conducting clinical research according to GCP guidelines. In line with this, CRSU has established good collaboration with various stakeholders.
Ongoing Research
- Biosimilar clinical trial of erythropoietin.
- Patch Test Study: Sensitization and Hypoallergenic for Baby Products.
- Clinical trial of infertility supplement.
Publication
Collaboration.
- Pharmacies PT. Novartis. PT. Bayer. PT. Sanofi Aventis. PT. Otsuka. PT. Abbot. PT. Takeda. PT. Daewoong. PT. Roche. PT. Morinaga. Kohjin Life Sciences Co., Ltd. Alert Asia. PT. BSN. OrchidLife Sdn Bhd. PT.Astra Zeneca. PT. Dexa Medica. PT. Rohto. PT. Etana. PT. Sari Husada Generasi Mahardhika. PT. Ika Pharmindo. PT. Kino Care. PT. Deltomed Lab. PT. Kalbe Pharma.PT. Tempo Research.PT. Mecosin Indonesia.PT. Novell Pharmaceutical Lab. PT. Sanghiang Perkasa. PT. Combiphar. PT. Soho Industri Pharmacies. PT. Fukuhara Health Care Indonesia. PT. Triyasa Nagamas Farma.
- Hospital RSUPN DR. Cipto Mangunkusumo. RSUP Dr. Kariadi. Pusat Jantung Nasional Harapan Kita. RS Kanker Dharmais. RSIA Budi Kemuliaan. Klinik Makara Universitas Indonesia.
- University Sekolah Farmasi Institut Teknologi Bandung. UIN Syarif Hidayatullah Jakarta.
- Laboratory Laboratorium Prodia. Pharma metric. Equilab. Laboratorium Terpadu FKUI.
Prof. dr. Frans D. Suyatna, PhD, SpFK

Lin Gustina

Prof. Arini Setiawati, PhD

Desi Sakawulan, STP, M.Sc

Prof. Dr. dr. Rianto Setiabudy, SpFK

Taruna Dibya

Dra. Yanti Mariana, MS

Mirantika Oktarina

Asiva Noor Rachmayani, SKM
Privacy preference center, privacy preferences.

IMAGES
VIDEO
COMMENTS
Research Assist, is a boutique contract research organization (CRO) providing exceptional clinical research services to industry (pharmaceutical, biotech, and device) clients since 2000. Our seasoned staff of professionals are experienced in the mana...
Clinical research organization is a less-common term which might be used to refer to a specific type of CRO that specializes in conducting clinical trials for pharmaceutical companies and other sponsor organizations. Thus, where a CRO might offer comprehensive service across the clinical development spectrum, a clinical research organization ...
A Contract Research Organization (CRO) is a specialized service provider that offers end-to-end support for clinical trials, ranging from early development to post-approval studies. They leverage their expertise, resources, and global reach to help sponsors navigate through the complex processes of drug and device development.
Learn the key considerations for selecting a clinical research organization (CRO) outsourcing partner for your clinical trial as well as how to mitigate the four common challenges while working with CROs. ... When deciding on a CRO specializing in clinical research, sponsors will find a wide range of choices, from global players who cover every ...
At Worldwide, we believe a personalized approach is the only way to unlock the power of everyone working on a study - from operational and therapeutic experts to site partners and scientists. That means you'll always have direct access to our experts. You'll be able to tap into more than 30 years of therapeutic experience on a global scale.
Contract Research Organizations (CROs) are the backbone of the pharmaceutical and biotechnological industries. They offer a plethora of services that span the entire drug development lifecycle. From the initial stages of research and development (R&D) to pre-clinical trials, clinical trials, and even post-market surveillance, CROs do it all.
Stakeholders, Resources, and Documents in Clinical Research. Deepak C. Chilkoti, in Clinical Pharmacy Education, Practice and Research, 2019 Contract Research Organization. A CRO is a person or an organization (commercial, academic, or other) contracted by the sponsor to perform one or more of a sponsor's trial-related duties and functions.
What Is a Contract Research Organization? A CRO is an individual or team that provides research services on a contract basis to other organizations in the life sciences, including pharmaceutical companies, biotechs, biopharmas, medical device companies, and even research institutions, government agencies, and foundations. ... Clinical Research ...
To advance new therapies, pharmaceutical, biotech, and medical device companies engage contract research organizations (CROs) for their know-how in navigating the complex landscape of drug development and regulatory pathways and to run clinical trials.CROs assist in increasing the speed with which new interventions, including drugs, devices, and technology, are brought to market.
A contract research organization (CRO) is a company that provides support to the pharmaceutical and biotechnology industries in the form of research services outsourced on a contract basis. CROs offer a variety of services, from preclinical research to clinical trials and post-marketing surveillance. CROs first emerged in the 1970s as a way to ...
The global clinical trials market was estimated to be worth $38.7 billion in 2021 and is expected to reach £52 billion by 2026. In this article, we look at the top 12 CROs in the world, highlighting the companies driving this considerable growth and accelerating research and development across the globe.. ICON. Recognised as the world's largest and most comprehensive CRO powered by ...
The core activities a CRO can provide include (but are not limited to) clinical trial management, data research and project management. Despite an initial downturn at the start of the pandemic, Covid-19 created a spike in the number of clinical trials taking place due to the need for vaccines and drugs to tackle the virus.
Chanice Henry. 03/27/2018. Pharma IQ showcases the top 10 Contract Research Organizations across the Pharma IQ network. Much of the world's population is in desperate need of better medical care. This need is a large driving force behind drug discovery's mission to uncover new medicines. The drug discovery process is complex and stubborn.
More than 70% of all clinical trials are expected to involve a contract research organization (CRO) in 2020,{2} and analysts predict the CRO market will reach nearly $55 billion by 2025.{3} Clinical research sites are also becoming increasingly vital in helping sponsors and CROs keep pace with bringing new treatments to market.
Catalyst is a premier clinical contract research organization offering innovative solutions. We provide customizable solutions through Catalyst Oncology, a full-service oncology CRO for biotech, and Catalyst Flex, a multi-therapeutic FSP provider for biopharma.
Selection Criteria For CRO Selection. According to Comprehend, 3 a clinical intelligence software provider, the three most common overlooked selection criteria are transparency, collaborative partnership, and real-time responsiveness. These intangible factors should be part of any strategic selection process.
A clinical research associate works on behalf of the sponsor (pharmaceutical company, university, or health organization) or for a contract research organization (CRO) that funds the research. Clinical trials are the long, scientific process of ensuring that certain drugs, therapies, and devices are safe and effective for public consumption and ...
The U.S. biopharmaceutical CMO & CRO market size was estimated at USD 10.82 billion in 2023 and is projected to hit around USD 19.75 billion by 2033, growing at a CAGR of 6.2% during the forecast period from 2024 to 2033.. The contract manufacturing organization (CMO) and contract research organization (CRO) industries have seen significant growth in recent years, particularly in the U.S.
Alcanza Clinical Research's buying spree continues with the acquisition of FDI Clinical Research, a contract research organization based in Puerto Rico, an April 22 press release announced. The ...
As the clinical research landscape continually evolves, the choice of a Contract Research Organization (CRO) partner can significantly impact the success of drug development programs. In fact, recent industry dynamics indicate a considerable shift in preference towards midsize CROs, driven by the need for more personalized, agile, and cost-effective partnerships.
EXTON, PA — Melior Discovery, Inc., a standout in the pre-clinical pharmacology sector, has been lauded as the Contract Research Organization (CRO) of the Year by Life Sciences Review magazine. …
Key Functions in Clinical Operations • Project Management. • Managing and coordination of study conduct • Monitoring and tracking of project milestones to ensure that the project runs within timelines. • Participation as appropriate to CORE TEAMS to expedite the feasibility and conduct of global trials
Clinical Research Supporting Unit (CRSU) Is a contract research organization (CRO) with the main mission to organize and conduct clinical studies in Indonesia and overseas and ensure that they are in accordance with International Conference Harmonization and Good Clinical Practices (ICH-GCP). CRSU has a good networking with industry and related ...