Essay on Coronavirus Vaccine
500+ words essay on coronavirus vaccine.
The Coronavirus has infected millions of people so far all over the world. In addition to that, millions of people have lost their lives to it. Ever since the outbreak, researchers all over the world have been working constantly to develop vaccines that will work effectively against the virus. We will take a look at the Coronavirus vaccine that is present today. Vaccines have the ability to save people’s lives. Developing the vaccine for Coronavirus was a huge step to end the pandemic.


Working of Coronavirus Vaccine
As Coronavirus caused a lot of confusion and fear amongst people, it is natural people were not aware of how the vaccine works. To begin with, a vaccine will work by mimicking an infectious agent.
The agent can be viruses, bacteria or any other microorganisms. They carry the potential of causing disease. When it mimics that, our immune system learns how to respond against it rapidly and efficiently.
As per the traditional methods, vaccines have managed to do this as they introduce a weakened form of an infectious agent. It enables our immune system to basically build its memory.
As a result, our immune system can then identify it quickly and fight against it before it gets the chance to harm us or make us ill. Similarly, some of the coronavirus vaccines have been made like that.
On the other hand, there are other coronavirus vaccines that researchers have developed by making use of new approaches. We refer to them as messenger RNA or mRNA vaccines.
Over here, they do not introduce antigens in our bodies. Instead, mRNA vaccines give the genetic code our body needs to enable our immune system for producing the antigen itself.
For several years, researchers have been studying mRNA vaccine technology. Thus, they do not contain any live virus and also do not interfere with the human DNA .
Get the huge list of more than 500 Essay Topics and Ideas
Safety of Coronavirus Vaccine
While the vaccines are being developed at a fast pace, they also require rigorous testing. The tests are done in clinical trials to ensure that they meet the benchmarks for the safety and efficiency of international standards.
When they meet the standards, then only can they get the go-ahead from WHO and national regulatory agencies. UNICEF has said that it will attain and supply only those vaccines that meet the WHO guidelines and have met the regulatory approval.
As of now, the vaccine doses are limited in number. Thus, the healthcare workers, first responders, people over the age of 75 and residents of long-term care facilities will receive the first doses.
After that, everyone will be able to get it once more of them are available. To get the vaccine, a person may require to pay a fee. However, some government institutions are providing it free of cost.
In order to get the vaccine, one must check with their local and state health departments on a regular basis. When they get the chance, they must get the dose right away.
The Coronavirus outbreak has challenged the whole world. Constantly, the experts and authorities are working to develop the vaccines. Therefore, we can also do our bit and adopt preventive measures to limit the spread of this disease. The major goal is to get the vaccine to everyone so that we can go on and about with our normal lives.
FAQ on Essay on Coronavirus Vaccine
Question 1: What are some common side effects of the Coronavirus vaccine?
Answer 1: The most common side effect includes a sore arm, fever , headache, and fatigue. However, not to worry, side effects are good in this case. They indicate that your vaccine is starting to work as it triggers your immune system.
Question 2: When do Coronavirus vaccine side effects kick in?
Answer 2: Usually, most of the side effects start to kick in within the first 3 days after you get your vaccine. Moreover, they will last up to 1 to 2 days only.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

How to Write About Coronavirus in a College Essay
Students can share how they navigated life during the coronavirus pandemic in a full-length essay or an optional supplement.
Writing About COVID-19 in College Essays

Getty Images
Experts say students should be honest and not limit themselves to merely their experiences with the pandemic.
The global impact of COVID-19, the disease caused by the novel coronavirus, means colleges and prospective students alike are in for an admissions cycle like no other. Both face unprecedented challenges and questions as they grapple with their respective futures amid the ongoing fallout of the pandemic.
Colleges must examine applicants without the aid of standardized test scores for many – a factor that prompted many schools to go test-optional for now . Even grades, a significant component of a college application, may be hard to interpret with some high schools adopting pass-fail classes last spring due to the pandemic. Major college admissions factors are suddenly skewed.
"I can't help but think other (admissions) factors are going to matter more," says Ethan Sawyer, founder of the College Essay Guy, a website that offers free and paid essay-writing resources.
College essays and letters of recommendation , Sawyer says, are likely to carry more weight than ever in this admissions cycle. And many essays will likely focus on how the pandemic shaped students' lives throughout an often tumultuous 2020.
But before writing a college essay focused on the coronavirus, students should explore whether it's the best topic for them.
Writing About COVID-19 for a College Application
Much of daily life has been colored by the coronavirus. Virtual learning is the norm at many colleges and high schools, many extracurriculars have vanished and social lives have stalled for students complying with measures to stop the spread of COVID-19.
"For some young people, the pandemic took away what they envisioned as their senior year," says Robert Alexander, dean of admissions, financial aid and enrollment management at the University of Rochester in New York. "Maybe that's a spot on a varsity athletic team or the lead role in the fall play. And it's OK for them to mourn what should have been and what they feel like they lost, but more important is how are they making the most of the opportunities they do have?"
That question, Alexander says, is what colleges want answered if students choose to address COVID-19 in their college essay.
But the question of whether a student should write about the coronavirus is tricky. The answer depends largely on the student.
"In general, I don't think students should write about COVID-19 in their main personal statement for their application," Robin Miller, master college admissions counselor at IvyWise, a college counseling company, wrote in an email.
"Certainly, there may be exceptions to this based on a student's individual experience, but since the personal essay is the main place in the application where the student can really allow their voice to be heard and share insight into who they are as an individual, there are likely many other topics they can choose to write about that are more distinctive and unique than COVID-19," Miller says.
Opinions among admissions experts vary on whether to write about the likely popular topic of the pandemic.
"If your essay communicates something positive, unique, and compelling about you in an interesting and eloquent way, go for it," Carolyn Pippen, principal college admissions counselor at IvyWise, wrote in an email. She adds that students shouldn't be dissuaded from writing about a topic merely because it's common, noting that "topics are bound to repeat, no matter how hard we try to avoid it."
Above all, she urges honesty.
"If your experience within the context of the pandemic has been truly unique, then write about that experience, and the standing out will take care of itself," Pippen says. "If your experience has been generally the same as most other students in your context, then trying to find a unique angle can easily cross the line into exploiting a tragedy, or at least appearing as though you have."
But focusing entirely on the pandemic can limit a student to a single story and narrow who they are in an application, Sawyer says. "There are so many wonderful possibilities for what you can say about yourself outside of your experience within the pandemic."
He notes that passions, strengths, career interests and personal identity are among the multitude of essay topic options available to applicants and encourages them to probe their values to help determine the topic that matters most to them – and write about it.
That doesn't mean the pandemic experience has to be ignored if applicants feel the need to write about it.
Writing About Coronavirus in Main and Supplemental Essays
Students can choose to write a full-length college essay on the coronavirus or summarize their experience in a shorter form.
To help students explain how the pandemic affected them, The Common App has added an optional section to address this topic. Applicants have 250 words to describe their pandemic experience and the personal and academic impact of COVID-19.
"That's not a trick question, and there's no right or wrong answer," Alexander says. Colleges want to know, he adds, how students navigated the pandemic, how they prioritized their time, what responsibilities they took on and what they learned along the way.
If students can distill all of the above information into 250 words, there's likely no need to write about it in a full-length college essay, experts say. And applicants whose lives were not heavily altered by the pandemic may even choose to skip the optional COVID-19 question.
"This space is best used to discuss hardship and/or significant challenges that the student and/or the student's family experienced as a result of COVID-19 and how they have responded to those difficulties," Miller notes. Using the section to acknowledge a lack of impact, she adds, "could be perceived as trite and lacking insight, despite the good intentions of the applicant."
To guard against this lack of awareness, Sawyer encourages students to tap someone they trust to review their writing , whether it's the 250-word Common App response or the full-length essay.
Experts tend to agree that the short-form approach to this as an essay topic works better, but there are exceptions. And if a student does have a coronavirus story that he or she feels must be told, Alexander encourages the writer to be authentic in the essay.
"My advice for an essay about COVID-19 is the same as my advice about an essay for any topic – and that is, don't write what you think we want to read or hear," Alexander says. "Write what really changed you and that story that now is yours and yours alone to tell."
Sawyer urges students to ask themselves, "What's the sentence that only I can write?" He also encourages students to remember that the pandemic is only a chapter of their lives and not the whole book.
Miller, who cautions against writing a full-length essay on the coronavirus, says that if students choose to do so they should have a conversation with their high school counselor about whether that's the right move. And if students choose to proceed with COVID-19 as a topic, she says they need to be clear, detailed and insightful about what they learned and how they adapted along the way.
"Approaching the essay in this manner will provide important balance while demonstrating personal growth and vulnerability," Miller says.
Pippen encourages students to remember that they are in an unprecedented time for college admissions.
"It is important to keep in mind with all of these (admission) factors that no colleges have ever had to consider them this way in the selection process, if at all," Pippen says. "They have had very little time to calibrate their evaluations of different application components within their offices, let alone across institutions. This means that colleges will all be handling the admissions process a little bit differently, and their approaches may even evolve over the course of the admissions cycle."
Searching for a college? Get our complete rankings of Best Colleges.
10 Ways to Discover College Essay Ideas

Tags: students , colleges , college admissions , college applications , college search , Coronavirus
2024 Best Colleges

Search for your perfect fit with the U.S. News rankings of colleges and universities.
College Admissions: Get a Step Ahead!
Sign up to receive the latest updates from U.S. News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
Ask an Alum: Making the Most Out of College
You May Also Like
Law schools with the highest lsats.
Ilana Kowarski and Cole Claybourn April 11, 2024

Today NAIA, Tomorrow Title IX?
Lauren Camera April 9, 2024

Grad School Housing Options
Anayat Durrani April 9, 2024

How to Decide if an MBA Is Worth it
Sarah Wood March 27, 2024

What to Wear to a Graduation
LaMont Jones, Jr. March 27, 2024

FAFSA Delays Alarm Families, Colleges
Sarah Wood March 25, 2024

Help Your Teen With the College Decision
Anayat Durrani March 25, 2024

Toward Semiconductor Gender Equity
Alexis McKittrick March 22, 2024

March Madness in the Classroom
Cole Claybourn March 21, 2024

20 Lower-Cost Online Private Colleges
Sarah Wood March 21, 2024


- High contrast
- Press Centre
Search UNICEF
What you need to know about covid-19 vaccines, answers to the most common questions about coronavirus vaccines..

- Available in:
Vaccines save millions of lives each year. The development of safe and effective COVID-19 vaccines are a crucial step in helping us get back to doing more of the things we enjoy with the people we love.
We’ve gathered the latest expert information to answer some of the most common questions about COVID-19 vaccines. Keep checking back as we will update this article as more information becomes available.
What are the benefits of getting vaccinated?
Vaccines save millions of lives each year and a COVID-19 vaccine could save yours. The COVID-19 vaccines are safe and effective, providing strong protection against serious illness and death. WHO reports that unvaccinated people have at least 10 times higher risk of death from COVID-19 than someone who has been vaccinated.
It is important to be vaccinated as soon as it’s your turn, even if you already had COVID-19. Getting vaccinated is a safer way for you to develop immunity from COVID-19 than getting infected.
The COVID-19 vaccines are highly effective, but no vaccine provides 100 per cent protection. Some people will still get ill from COVID-19 after vaccination or pass the virus onto someone else.
Therefore, it is important to continue practicing safety precautions to protect yourself and others, including avoiding crowded spaces, physical distancing, hand washing and wearing a mask.
Who should be vaccinated first?
Each country must identify priority populations, which WHO recommends are frontline health workers (to protect health systems) and those at highest risk of death due to COVID-19, such as older adults and people with certain medical conditions. Other essential workers, such as teachers and social workers, should then be prioritized, followed by additional groups as more vaccine doses become available.
The risk of severe illness from COVID-19 is very low amongst healthy children and adolescents, so unless they are part of a group at higher risk of severe COVID-19, it is less urgent to vaccinate them than these priority groups.
Children and adolescents who are at higher risk of developing severe illness from COVID-19, such as those with underlying illnesses, should be prioritized for COVID-19 vaccines.
When shouldn’t you be vaccinated against COVID-19?
If you have any questions about whether you should receive a COVID-19 vaccine, speak to your healthcare provider. At present, people with the following health conditions should not receive a COVID-19 vaccine to avoid any possible adverse effects:
- If you have a history of severe allergic reactions to any ingredients of a COVID-19 vaccine.
- If you are currently sick or experiencing symptoms of COVID-19 (although you can get vaccinated once you have recovered and your doctor has approved).
Should I get vaccinated if I already had COVID-19?
Yes, you should get vaccinated even if you’ve previously had COVID-19. While people who recover from COVID-19 may develop natural immunity to the virus, it is still not certain how long that immunity lasts or how well it protects you against COVID-19 reinfection. Vaccines offer more reliable protection, especially against severe illness and death. Vaccination policies after COVID-19 infection vary by country. Check with your health care provider on the recommendation where you live.
Which COVID-19 vaccine is best for me?
All WHO-approved vaccines have been shown to be highly effective at protecting you against severe illness and death from COVID-19. The best vaccine to get is the one most readily available to you.
You can find a list of those approved vaccines on WHO’s site .
Remember, if your vaccination involves two doses, it’s important to receive both to have the maximum protection.
How do COVID-19 vaccines work?
Vaccines work by mimicking an infectious agent – viruses, bacteria or other microorganisms that can cause a disease. This ‘teaches’ our immune system to rapidly and effectively respond against it.
Traditionally, vaccines have done this by introducing a weakened form of an infectious agent that allows our immune system to build a memory of it. This way, our immune system can quickly recognize and fight it before it makes us ill. That’s how some of the COVID-19 vaccines have been designed.
Other COVID-19 vaccines have been developed using new approaches, which are called messenger RNA, or mRNA, vaccines. Instead of introducing antigens (a substance that causes your immune system to produce antibodies), mRNA vaccines give our body the genetic code it needs to allow our immune system to produce the antigen itself. mRNA vaccine technology has been studied for several decades. They contain no live virus and do not interfere with human DNA.
For more information on how vaccines work, please visit WHO .
Are COVID-19 vaccines safe?
Yes, COVID-19 vaccines have been safely used to vaccinate billions of people. The COVID-19 vaccines were developed as rapidly as possible, but they had to go through rigorous testing in clinical trials to prove that they meet internationally agreed benchmarks for safety and effectiveness. Only if they meet these standards can a vaccine receive validation from WHO and national regulatory agencies.
UNICEF only procures and supplies COVID-19 vaccines that meet WHO’s established safety and efficacy criteria and that have received the required regulatory approval.
How were COVID-19 vaccines developed so quickly?
Scientists were able to develop safe effective vaccines in a relatively short amount of time due to a combination of factors that allowed them to scale up research and production without compromising safety:
- Because of the global pandemic, there was a larger sample size to study and tens of thousands of volunteers stepped forward
- Advancements in technology (like mRNA vaccines) that were years in the making
- Governments and other bodies came together to remove the obstacle of funding research and development
- Manufacturing of the vaccines occurred in parallel to the clinical trials to speed up production
Though they were developed quickly, all COVID-19 vaccines approved for use by the WHO are safe and effective.
What are the side effects of COVID-19 vaccines?
Vaccines are designed to give you immunity without the dangers of getting the disease. Not everyone does, but it’s common to experience some mild-to-moderate side effects that go away within a few days on their own.
Some of the mild-to-moderate side effects you may experience after vaccination include:
- Arm soreness at the injection site
- Muscle or joint aches
You can manage any side effects with rest, staying hydrated and taking medication to manage pain and fever, if needed.
If any symptoms continue for more than a few days then contact your healthcare provider for advice. More serious side effects are extremely rare, but if you experience a more severe reaction, then contact your healthcare provider immediately.
>> Read: What you need to know before, during and after receiving a COVID-19 vaccine
How do I find out more about a particular COVID-19 vaccine?
You can find out more about COVID-19 vaccines on WHO’s website .
Can I stop taking precautions after being vaccinated?
Keep taking precautions to protect yourself, family and friends if there is still COVID-19 in your area, even after getting vaccinated. The COVID-19 vaccines are highly effective against serious illness and death, but no vaccine is 100% effective.
The vaccines offer less protection against infection from the Omicron variant, which is now the dominant variant globally, but remain highly effective in preventing hospitalization, severe disease, and death. In addition to vaccination, it remains important to continue practicing safety precautions to protect yourself and others. These precautions include avoiding crowded spaces, physical distancing, hand washing, and wearing a mask (as per local policies).
Can I still get COVID-19 after I have been vaccinated? What are ‘breakthrough cases’?
A number of vaccinated people may get infected with COVID-19, which is called a breakthrough infection. In such cases, people are much more likely to only have milder symptoms. Vaccine protection against serious illness and death remains strong.
With more infectious virus variants such as Omicron, there have been more breakthrough infections. That’s why it's recommended to continue taking precautions such as avoiding crowded spaces, wearing a mask and washing your hands regularly, even if you are vaccinated.
And remember, it’s important to receive all of the recommended doses of vaccines to have the maximum protection.
How long does protection from COVID-19 vaccines last?
According to WHO, the effectiveness of COVID-19 vaccines wanes around 4-6 months after the primary series of vaccination has been completed. Taking a booster to strengthen your protection against serious disease is recommended if it is available to you.
Do the COVID-19 vaccines protect against variants?
The WHO-approved COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
However, the vaccines offer less protection against infection from Omicron, which is the dominant variant globally. That's why it's important to get vaccinated and continue measures to reduce the spread of the virus – which helps to reduce the chances for the virus to mutate – including physical distancing, mask wearing, good ventilation, regular handwashing and seeking care early if you have symptoms.
Do I need to get a booster shot?
Booster doses play an important role in protecting against severe disease, hospitalization and death.
WHO recommends that you take all COVID-19 vaccine doses recommended to you by your health authority as soon as it is your turn, including a booster dose if recommended.
Booster shots should be given first to high priority groups. Data shows that a booster shot plays a significant role in boosting waning immunity and protecting against severe disease from highly transmissible variants like Omicron.
If available, an additional second booster shot is also recommended for some groups of people, 4-6 months after the first booster. That includes older people, those who have weakened immune systems, pregnant women and healthcare workers.
Check with your local health authorities for guidance and the availability of booster shots where you live.
What do we know about the bivalent COVID-19 booster doses that have been developed to target Omicron?
Bivalent COVID-19 booster shots have now been developed with both the original strain of the coronavirus and a strain of Omicron. These have been designed to better match the Omicron subvariants that have proven to be particularly transmissible. Lab studies have shown that these doses help you to mount a higher antibody response against Omicron. Both Moderna and Pfizer have developed these bivalent vaccines, and some countries have now approved their use.
Check with your local health authorities for information about the availability of these doses and who can get them where you live. And it’s important to note that the original COVID-19 vaccines continue to work very well and provide strong protection against severe illness from Omicron.
Can I receive different types of COVID-19 vaccines?
Yes, however, policies on mixing vaccines vary by country. Some countries have used different vaccines for the primary vaccine series and the booster. Check with your local health authorities for guidance where you live and speak with your healthcare provider if you have any questions on what is best for you.
I’m pregnant. Can I get vaccinated against COVID-19?
Yes, you can get vaccinated if you are pregnant. COVID-19 during pregnancy puts you at higher risk of becoming severely ill and of giving birth prematurely.
Many people around the world have been vaccinated against COVID-19 while pregnant or breastfeeding. No safety concerns have been identified for them or their babies. Getting vaccinated while pregnant helps to protect your baby. For more information about receiving a COVID-19 vaccination while pregnant, speak to your healthcare provider.
>> Read: Navigating pregnancy during the COVID-19 pandemic
I’m breastfeeding. Should I get vaccinated against COVID-19?
Yes, if you are breastfeeding you should take the vaccine as soon as it is available to you. It is very safe and there is no risk to the mother or baby. None of the current COVID-19 vaccines have live virus in them, so there is no risk of you transmitting COVID-19 to your baby through your breastmilk from the vaccine. In fact, the antibodies that you have after vaccination may go through the breast milk and help protect your baby. >> Read: Breastfeeding safely during the COVID-19 pandemic
Can COVID-19 vaccines affect fertility?
No, you may have seen false claims on social media, but there is no evidence that any vaccine, including COVID-19 vaccines, can affect fertility in women or men. You should get vaccinated if you are currently trying to become pregnant.
Could a COVID-19 vaccine disrupt my menstrual cycle?
Some people have reported experiencing a disruption to their menstrual cycle after getting vaccinated against COVID-19. Although data is still limited, research is ongoing into the impact of vaccines on menstrual cycles.
Speak to your healthcare provider if you have concerns or questions about your periods.
Should my child or teen get a COVID-19 vaccine?
An increasing number of vaccines have been approved for use in children. They’ve been made available after examining the data on the safety and efficacy of these vaccines, and millions of children have been safely vaccinated around the world. Some COVID-19 vaccines have been approved for children from the age of 6 months old. Check with your local health authorities on what vaccines are authorized and available for children and adolescents where you live.
Children and adolescents tend to have milder disease compared to adults, so unless they are part of a group at higher risk of severe COVID-19, it is less urgent to vaccinate them than older people, those with chronic health conditions and health workers.
Remind your children of the importance of us all taking precautions to protect each other, such as avoiding crowded spaces, physical distancing, hand washing and wearing a mask.
It is critical that children continue to receive the recommended childhood vaccines.
How do I talk to my kids about COVID-19 vaccines?
News about COVID-19 vaccines is flooding our daily lives and it is only natural that curious young minds will have questions – lots of them. Read our explainer article for help explaining what can be a complicated topic in simple and reassuring terms.
It’s important to note that from the millions of children that have so far been vaccinated against COVID-19 globally, we know that side effects are very rare. Just like adults, children and adolescents might experience mild symptoms after receiving a dose, such as a slight fever and body aches. But these symptoms typically last for just a day or two. The authorized vaccines for adolescents and children are very safe.
My friend or family member is against COVID-19 vaccines. How do I talk to them?
The development of safe and effective COVID-19 vaccines is a huge step forward in our global effort to end the pandemic. This is exciting news, but there are still some people who are skeptical or hesitant about COVID-19 vaccines. Chances are you know a person who falls into this category.
We spoke to Dr. Saad Omer, Director at the Yale Institute for Global Health, to get his tips on how to navigate these challenging conversations. >> Read the interview
How can I protect my family until we are all vaccinated?
Safe and effective vaccines are a game changer, but even once vaccinated we need to continue taking precautions for the time being to protect ourselves and others. Variants like Omicron have proven that although COVID-19 vaccines are very effective at preventing severe disease, they’re not enough to stop the spread of the virus alone. The most important thing you can do is reduce your risk of exposure to the virus. To protect yourself and your loved ones, make sure to:
- Wear a mask where physical distancing from others is not possible.
- Keep a physical distance from others in public places.
- Avoid poorly ventilated or crowded spaces.
- Open windows to improve ventilation indoors.
- Try and focus on outdoor activities if possible.
- Wash your hands regularly with soap and water or an alcohol-based hand rub.
If you or a family member has a fever, cough or difficulty breathing, seek medical care early and avoid mixing with other children and adults.
Can COVID-19 vaccines affect your DNA?
No, none of the COVID-19 vaccines affect or interact with your DNA in any way. Messenger RNA, or mRNA, vaccines teach the cells how to make a protein that triggers an immune response inside the body. This response produces antibodies which keep you protected against the virus. mRNA is different from DNA and only stays inside the cell for about 72 hours before degrading. However, it never enters the nucleus of the cell, where DNA is kept.
Do the COVID-19 vaccines contain any animal products in them?
No, none of the WHO-approved COVID-19 vaccines contain animal products.
I’ve seen inaccurate information online about COVID-19 vaccines. What should I do?
Sadly, there is a lot of inaccurate information online about the COVID-19 virus and vaccines. A lot of what we’re experiencing is new to all of us, so there may be some occasions where information is shared, in a non-malicious way, that turns out to be inaccurate.
Misinformation in a health crisis can spread paranoia, fear and stigmatization. It can also result in people being left unprotected or more vulnerable to the virus. Get verified facts and advice from trusted sources like your local health authority, the UN, UNICEF, WHO.
If you see content online that you believe to be false or misleading, you can help stop it spreading by reporting it to the social media platform.
What is COVAX?
COVAX is a global effort committed to the development, production and equitable distribution of vaccines around the world. No country will be safe from COVID-19 until all countries are protected.
There are 190 countries and territories engaged in the COVAX Facility, which account for over 90 per cent of the world’s population. Working with CEPI, GAVI, WHO and other partners, UNICEF is leading efforts to procure and supply COVID-19 vaccines on behalf of COVAX.
Learn more about COVAX .
This article was last updated on 25 October 2022. It will continue to be updated to reflect the latest information.
Related topics
More to explore, covid-19 response.
Resources and information about UNICEF’s response to the COVID-19 pandemic
How to talk to your children about COVID-19 vaccines
Tips for navigating the conversation
How to talk to friends and family about vaccines
Tips for handling tough conversations with your loved ones
COVAX information centre
UNICEF and partners led the largest vaccine procurement and supply operation in history
- CBSE Class 10th
- CBSE Class 12th
- UP Board 10th
- UP Board 12th
- Bihar Board 10th
- Bihar Board 12th
- Top Schools in India
- Top Schools in Delhi
- Top Schools in Mumbai
- Top Schools in Chennai
- Top Schools in Hyderabad
- Top Schools in Kolkata
- Top Schools in Pune
- Top Schools in Bangalore
Products & Resources
- JEE Main Knockout April
- Free Sample Papers
- Free Ebooks
- NCERT Notes
- NCERT Syllabus
- NCERT Books
- RD Sharma Solutions
- Navodaya Vidyalaya Admission 2024-25
- NCERT Solutions
- NCERT Solutions for Class 12
- NCERT Solutions for Class 11
- NCERT solutions for Class 10
- NCERT solutions for Class 9
- NCERT solutions for Class 8
- NCERT Solutions for Class 7
- JEE Main 2024
- JEE Advanced 2024
- BITSAT 2024
- View All Engineering Exams
- Colleges Accepting B.Tech Applications
- Top Engineering Colleges in India
- Engineering Colleges in India
- Engineering Colleges in Tamil Nadu
- Engineering Colleges Accepting JEE Main
- Top IITs in India
- Top NITs in India
- Top IIITs in India
- JEE Main College Predictor
- JEE Main Rank Predictor
- MHT CET College Predictor
- AP EAMCET College Predictor
- GATE College Predictor
- KCET College Predictor
- JEE Advanced College Predictor
- View All College Predictors
- JEE Main Question Paper
- JEE Main Mock Test
- JEE Main Registration
- JEE Main Syllabus
- Download E-Books and Sample Papers
- Compare Colleges
- B.Tech College Applications
- GATE 2024 Result
- MAH MBA CET Exam
- View All Management Exams
Colleges & Courses
- MBA College Admissions
- MBA Colleges in India
- Top IIMs Colleges in India
- Top Online MBA Colleges in India
- MBA Colleges Accepting XAT Score
- BBA Colleges in India
- XAT College Predictor 2024
- SNAP College Predictor
- NMAT College Predictor
- MAT College Predictor 2024
- CMAT College Predictor 2024
- CAT Percentile Predictor 2023
- CAT 2023 College Predictor
- CMAT 2024 Registration
- TS ICET 2024 Registration
- CMAT Exam Date 2024
- MAH MBA CET Cutoff 2024
- Download Helpful Ebooks
- List of Popular Branches
- QnA - Get answers to your doubts
- IIM Fees Structure
- AIIMS Nursing
- Top Medical Colleges in India
- Top Medical Colleges in India accepting NEET Score
- Medical Colleges accepting NEET
- List of Medical Colleges in India
- List of AIIMS Colleges In India
- Medical Colleges in Maharashtra
- Medical Colleges in India Accepting NEET PG
- NEET College Predictor
- NEET PG College Predictor
- NEET MDS College Predictor
- DNB CET College Predictor
- DNB PDCET College Predictor
- NEET Application Form 2024
- NEET PG Application Form 2024
- NEET Cut off
- NEET Online Preparation
- Download Helpful E-books
- LSAT India 2024
- Colleges Accepting Admissions
- Top Law Colleges in India
- Law College Accepting CLAT Score
- List of Law Colleges in India
- Top Law Colleges in Delhi
- Top Law Collages in Indore
- Top Law Colleges in Chandigarh
- Top Law Collages in Lucknow
Predictors & E-Books
- CLAT College Predictor
- MHCET Law ( 5 Year L.L.B) College Predictor
- AILET College Predictor
- Sample Papers
- Compare Law Collages
- Careers360 Youtube Channel
- CLAT Syllabus 2025
- CLAT Previous Year Question Paper
- AIBE 18 Result 2023
- NID DAT Exam
- Pearl Academy Exam
Animation Courses
- Animation Courses in India
- Animation Courses in Bangalore
- Animation Courses in Mumbai
- Animation Courses in Pune
- Animation Courses in Chennai
- Animation Courses in Hyderabad
- Design Colleges in India
- Fashion Design Colleges in Bangalore
- Fashion Design Colleges in Mumbai
- Fashion Design Colleges in Pune
- Fashion Design Colleges in Delhi
- Fashion Design Colleges in Hyderabad
- Fashion Design Colleges in India
- Top Design Colleges in India
- Free Design E-books
- List of Branches
- Careers360 Youtube channel
- NIFT College Predictor
- UCEED College Predictor
- NID DAT College Predictor
- IPU CET BJMC
- JMI Mass Communication Entrance Exam
- IIMC Entrance Exam
- Media & Journalism colleges in Delhi
- Media & Journalism colleges in Bangalore
- Media & Journalism colleges in Mumbai
- List of Media & Journalism Colleges in India
- CA Intermediate
- CA Foundation
- CS Executive
- CS Professional
- Difference between CA and CS
- Difference between CA and CMA
- CA Full form
- CMA Full form
- CS Full form
- CA Salary In India
Top Courses & Careers
- Bachelor of Commerce (B.Com)
- Master of Commerce (M.Com)
- Company Secretary
- Cost Accountant
- Charted Accountant
- Credit Manager
- Financial Advisor
- Top Commerce Colleges in India
- Top Government Commerce Colleges in India
- Top Private Commerce Colleges in India
- Top M.Com Colleges in Mumbai
- Top B.Com Colleges in India
- IT Colleges in Tamil Nadu
- IT Colleges in Uttar Pradesh
- MCA Colleges in India
- BCA Colleges in India
Quick Links
- Information Technology Courses
- Programming Courses
- Web Development Courses
- Data Analytics Courses
- Big Data Analytics Courses
- RUHS Pharmacy Admission Test
- Top Pharmacy Colleges in India
- Pharmacy Colleges in Pune
- Pharmacy Colleges in Mumbai
- Colleges Accepting GPAT Score
- Pharmacy Colleges in Lucknow
- List of Pharmacy Colleges in Nagpur
- GPAT Result
- GPAT 2024 Admit Card
- GPAT Question Papers
- NCHMCT JEE 2024
- Mah BHMCT CET
- Top Hotel Management Colleges in Delhi
- Top Hotel Management Colleges in Hyderabad
- Top Hotel Management Colleges in Mumbai
- Top Hotel Management Colleges in Tamil Nadu
- Top Hotel Management Colleges in Maharashtra
- B.Sc Hotel Management
- Hotel Management
- Diploma in Hotel Management and Catering Technology
Diploma Colleges
- Top Diploma Colleges in Maharashtra
- UPSC IAS 2024
- SSC CGL 2024
- IBPS RRB 2024
- Previous Year Sample Papers
- Free Competition E-books
- Sarkari Result
- QnA- Get your doubts answered
- UPSC Previous Year Sample Papers
- CTET Previous Year Sample Papers
- SBI Clerk Previous Year Sample Papers
- NDA Previous Year Sample Papers
Upcoming Events
- NDA Application Form 2024
- UPSC IAS Application Form 2024
- CDS Application Form 2024
- CTET Admit card 2024
- HP TET Result 2023
- SSC GD Constable Admit Card 2024
- UPTET Notification 2024
- SBI Clerk Result 2024
Other Exams
- SSC CHSL 2024
- UP PCS 2024
- UGC NET 2024
- RRB NTPC 2024
- IBPS PO 2024
- IBPS Clerk 2024
- IBPS SO 2024
- Top University in USA
- Top University in Canada
- Top University in Ireland
- Top Universities in UK
- Top Universities in Australia
- Best MBA Colleges in Abroad
- Business Management Studies Colleges
Top Countries
- Study in USA
- Study in UK
- Study in Canada
- Study in Australia
- Study in Ireland
- Study in Germany
- Study in China
- Study in Europe
Student Visas
- Student Visa Canada
- Student Visa UK
- Student Visa USA
- Student Visa Australia
- Student Visa Germany
- Student Visa New Zealand
- Student Visa Ireland
- CUET PG 2024
- IGNOU B.Ed Admission 2024
- DU Admission
- UP B.Ed JEE 2024
- DDU Entrance Exam
- IIT JAM 2024
- IGNOU Online Admission 2024
- Universities in India
- Top Universities in India 2024
- Top Colleges in India
- Top Universities in Uttar Pradesh 2024
- Top Universities in Bihar
- Top Universities in Madhya Pradesh 2024
- Top Universities in Tamil Nadu 2024
- Central Universities in India
- CUET PG Admit Card 2024
- IGNOU Date Sheet
- CUET Mock Test 2024
- CUET Application Form 2024
- CUET PG Syllabus 2024
- CUET Participating Universities 2024
- CUET Previous Year Question Paper
- CUET Syllabus 2024 for Science Students
- E-Books and Sample Papers
- CUET Exam Pattern 2024
- CUET Exam Date 2024
- CUET Syllabus 2024
- IGNOU Exam Form 2024
- IGNOU Result
- CUET PG Courses 2024
Engineering Preparation
- Knockout JEE Main 2024
- Test Series JEE Main 2024
- JEE Main 2024 Rank Booster
Medical Preparation
- Knockout NEET 2024
- Test Series NEET 2024
- Rank Booster NEET 2024
Online Courses
- JEE Main One Month Course
- NEET One Month Course
- IBSAT Free Mock Tests
- IIT JEE Foundation Course
- Knockout BITSAT 2024
- Career Guidance Tool
Top Streams
- IT & Software Certification Courses
- Engineering and Architecture Certification Courses
- Programming And Development Certification Courses
- Business and Management Certification Courses
- Marketing Certification Courses
- Health and Fitness Certification Courses
- Design Certification Courses
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
- UG Degree Courses
- PG Degree Courses
- Short Term Courses
- Free Courses
- Online Degrees and Diplomas
- Compare Courses
Top Providers
- Coursera Courses
- Udemy Courses
- Edx Courses
- Swayam Courses
- upGrad Courses
- Simplilearn Courses
- Great Learning Courses
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with
Plan, Prepare & Make the Best Career Choices
Covid 19 Essay in English
Essay on Covid -19: In a very short amount of time, coronavirus has spread globally. It has had an enormous impact on people's lives, economy, and societies all around the world, affecting every country. Governments have had to take severe measures to try and contain the pandemic. The virus has altered our way of life in many ways, including its effects on our health and our economy. Here are a few sample essays on ‘CoronaVirus’.
100 Words Essay on Covid 19
200 words essay on covid 19, 500 words essay on covid 19.
COVID-19 or Corona Virus is a novel coronavirus that was first identified in 2019. It is similar to other coronaviruses, such as SARS-CoV and MERS-CoV, but it is more contagious and has caused more severe respiratory illness in people who have been infected. The novel coronavirus became a global pandemic in a very short period of time. It has affected lives, economies and societies across the world, leaving no country untouched. The virus has caused governments to take drastic measures to try and contain it. From health implications to economic and social ramifications, COVID-19 impacted every part of our lives. It has been more than 2 years since the pandemic hit and the world is still recovering from its effects.
Since the outbreak of COVID-19, the world has been impacted in a number of ways. For one, the global economy has taken a hit as businesses have been forced to close their doors. This has led to widespread job losses and an increase in poverty levels around the world. Additionally, countries have had to impose strict travel restrictions in an attempt to contain the virus, which has resulted in a decrease in tourism and international trade. Furthermore, the pandemic has put immense pressure on healthcare systems globally, as hospitals have been overwhelmed with patients suffering from the virus. Lastly, the outbreak has led to a general feeling of anxiety and uncertainty, as people are fearful of contracting the disease.
My Experience of COVID-19
I still remember how abruptly colleges and schools shut down in March 2020. I was a college student at that time and I was under the impression that everything would go back to normal in a few weeks. I could not have been more wrong. The situation only got worse every week and the government had to impose a lockdown. There were so many restrictions in place. For example, we had to wear face masks whenever we left the house, and we could only go out for essential errands. Restaurants and shops were only allowed to operate at take-out capacity, and many businesses were shut down.
In the current scenario, coronavirus is dominating all aspects of our lives. The coronavirus pandemic has wreaked havoc upon people’s lives, altering the way we live and work in a very short amount of time. It has revolutionised how we think about health care, education, and even social interaction. This virus has had long-term implications on our society, including its impact on mental health, economic stability, and global politics. But we as individuals can help to mitigate these effects by taking personal responsibility to protect themselves and those around them from infection.
Effects of CoronaVirus on Education
The outbreak of coronavirus has had a significant impact on education systems around the world. In China, where the virus originated, all schools and universities were closed for several weeks in an effort to contain the spread of the disease. Many other countries have followed suit, either closing schools altogether or suspending classes for a period of time.
This has resulted in a major disruption to the education of millions of students. Some have been able to continue their studies online, but many have not had access to the internet or have not been able to afford the costs associated with it. This has led to a widening of the digital divide between those who can afford to continue their education online and those who cannot.
The closure of schools has also had a negative impact on the mental health of many students. With no face-to-face contact with friends and teachers, some students have felt isolated and anxious. This has been compounded by the worry and uncertainty surrounding the virus itself.
The situation with coronavirus has improved and schools have been reopened but students are still catching up with the gap of 2 years that the pandemic created. In the meantime, governments and educational institutions are working together to find ways to support students and ensure that they are able to continue their education despite these difficult circumstances.
Effects of CoronaVirus on Economy
The outbreak of the coronavirus has had a significant impact on the global economy. The virus, which originated in China, has spread to over two hundred countries, resulting in widespread panic and a decrease in global trade. As a result of the outbreak, many businesses have been forced to close their doors, leading to a rise in unemployment. In addition, the stock market has taken a severe hit.
Effects of CoronaVirus on Health
The effects that coronavirus has on one's health are still being studied and researched as the virus continues to spread throughout the world. However, some of the potential effects on health that have been observed thus far include respiratory problems, fever, and coughing. In severe cases, pneumonia, kidney failure, and death can occur. It is important for people who think they may have been exposed to the virus to seek medical attention immediately so that they can be treated properly and avoid any serious complications. There is no specific cure or treatment for coronavirus at this time, but there are ways to help ease symptoms and prevent the virus from spreading.
Explore Career Options (By Industry)
- Construction
- Entertainment
- Manufacturing
- Information Technology
Data Administrator
Database professionals use software to store and organise data such as financial information, and customer shipping records. Individuals who opt for a career as data administrators ensure that data is available for users and secured from unauthorised sales. DB administrators may work in various types of industries. It may involve computer systems design, service firms, insurance companies, banks and hospitals.
Bio Medical Engineer
The field of biomedical engineering opens up a universe of expert chances. An Individual in the biomedical engineering career path work in the field of engineering as well as medicine, in order to find out solutions to common problems of the two fields. The biomedical engineering job opportunities are to collaborate with doctors and researchers to develop medical systems, equipment, or devices that can solve clinical problems. Here we will be discussing jobs after biomedical engineering, how to get a job in biomedical engineering, biomedical engineering scope, and salary.
Ethical Hacker
A career as ethical hacker involves various challenges and provides lucrative opportunities in the digital era where every giant business and startup owns its cyberspace on the world wide web. Individuals in the ethical hacker career path try to find the vulnerabilities in the cyber system to get its authority. If he or she succeeds in it then he or she gets its illegal authority. Individuals in the ethical hacker career path then steal information or delete the file that could affect the business, functioning, or services of the organization.
GIS officer work on various GIS software to conduct a study and gather spatial and non-spatial information. GIS experts update the GIS data and maintain it. The databases include aerial or satellite imagery, latitudinal and longitudinal coordinates, and manually digitized images of maps. In a career as GIS expert, one is responsible for creating online and mobile maps.
Data Analyst
The invention of the database has given fresh breath to the people involved in the data analytics career path. Analysis refers to splitting up a whole into its individual components for individual analysis. Data analysis is a method through which raw data are processed and transformed into information that would be beneficial for user strategic thinking.
Data are collected and examined to respond to questions, evaluate hypotheses or contradict theories. It is a tool for analyzing, transforming, modeling, and arranging data with useful knowledge, to assist in decision-making and methods, encompassing various strategies, and is used in different fields of business, research, and social science.
Geothermal Engineer
Individuals who opt for a career as geothermal engineers are the professionals involved in the processing of geothermal energy. The responsibilities of geothermal engineers may vary depending on the workplace location. Those who work in fields design facilities to process and distribute geothermal energy. They oversee the functioning of machinery used in the field.
Database Architect
If you are intrigued by the programming world and are interested in developing communications networks then a career as database architect may be a good option for you. Data architect roles and responsibilities include building design models for data communication networks. Wide Area Networks (WANs), local area networks (LANs), and intranets are included in the database networks. It is expected that database architects will have in-depth knowledge of a company's business to develop a network to fulfil the requirements of the organisation. Stay tuned as we look at the larger picture and give you more information on what is db architecture, why you should pursue database architecture, what to expect from such a degree and what your job opportunities will be after graduation. Here, we will be discussing how to become a data architect. Students can visit NIT Trichy , IIT Kharagpur , JMI New Delhi .
Remote Sensing Technician
Individuals who opt for a career as a remote sensing technician possess unique personalities. Remote sensing analysts seem to be rational human beings, they are strong, independent, persistent, sincere, realistic and resourceful. Some of them are analytical as well, which means they are intelligent, introspective and inquisitive.
Remote sensing scientists use remote sensing technology to support scientists in fields such as community planning, flight planning or the management of natural resources. Analysing data collected from aircraft, satellites or ground-based platforms using statistical analysis software, image analysis software or Geographic Information Systems (GIS) is a significant part of their work. Do you want to learn how to become remote sensing technician? There's no need to be concerned; we've devised a simple remote sensing technician career path for you. Scroll through the pages and read.
Budget Analyst
Budget analysis, in a nutshell, entails thoroughly analyzing the details of a financial budget. The budget analysis aims to better understand and manage revenue. Budget analysts assist in the achievement of financial targets, the preservation of profitability, and the pursuit of long-term growth for a business. Budget analysts generally have a bachelor's degree in accounting, finance, economics, or a closely related field. Knowledge of Financial Management is of prime importance in this career.
Underwriter
An underwriter is a person who assesses and evaluates the risk of insurance in his or her field like mortgage, loan, health policy, investment, and so on and so forth. The underwriter career path does involve risks as analysing the risks means finding out if there is a way for the insurance underwriter jobs to recover the money from its clients. If the risk turns out to be too much for the company then in the future it is an underwriter who will be held accountable for it. Therefore, one must carry out his or her job with a lot of attention and diligence.
Finance Executive
Product manager.
A Product Manager is a professional responsible for product planning and marketing. He or she manages the product throughout the Product Life Cycle, gathering and prioritising the product. A product manager job description includes defining the product vision and working closely with team members of other departments to deliver winning products.
Operations Manager
Individuals in the operations manager jobs are responsible for ensuring the efficiency of each department to acquire its optimal goal. They plan the use of resources and distribution of materials. The operations manager's job description includes managing budgets, negotiating contracts, and performing administrative tasks.
Stock Analyst
Individuals who opt for a career as a stock analyst examine the company's investments makes decisions and keep track of financial securities. The nature of such investments will differ from one business to the next. Individuals in the stock analyst career use data mining to forecast a company's profits and revenues, advise clients on whether to buy or sell, participate in seminars, and discussing financial matters with executives and evaluate annual reports.
A Researcher is a professional who is responsible for collecting data and information by reviewing the literature and conducting experiments and surveys. He or she uses various methodological processes to provide accurate data and information that is utilised by academicians and other industry professionals. Here, we will discuss what is a researcher, the researcher's salary, types of researchers.
Welding Engineer
Welding Engineer Job Description: A Welding Engineer work involves managing welding projects and supervising welding teams. He or she is responsible for reviewing welding procedures, processes and documentation. A career as Welding Engineer involves conducting failure analyses and causes on welding issues.
Transportation Planner
A career as Transportation Planner requires technical application of science and technology in engineering, particularly the concepts, equipment and technologies involved in the production of products and services. In fields like land use, infrastructure review, ecological standards and street design, he or she considers issues of health, environment and performance. A Transportation Planner assigns resources for implementing and designing programmes. He or she is responsible for assessing needs, preparing plans and forecasts and compliance with regulations.
Environmental Engineer
Individuals who opt for a career as an environmental engineer are construction professionals who utilise the skills and knowledge of biology, soil science, chemistry and the concept of engineering to design and develop projects that serve as solutions to various environmental problems.
Safety Manager
A Safety Manager is a professional responsible for employee’s safety at work. He or she plans, implements and oversees the company’s employee safety. A Safety Manager ensures compliance and adherence to Occupational Health and Safety (OHS) guidelines.
Conservation Architect
A Conservation Architect is a professional responsible for conserving and restoring buildings or monuments having a historic value. He or she applies techniques to document and stabilise the object’s state without any further damage. A Conservation Architect restores the monuments and heritage buildings to bring them back to their original state.
Structural Engineer
A Structural Engineer designs buildings, bridges, and other related structures. He or she analyzes the structures and makes sure the structures are strong enough to be used by the people. A career as a Structural Engineer requires working in the construction process. It comes under the civil engineering discipline. A Structure Engineer creates structural models with the help of computer-aided design software.
Highway Engineer
Highway Engineer Job Description: A Highway Engineer is a civil engineer who specialises in planning and building thousands of miles of roads that support connectivity and allow transportation across the country. He or she ensures that traffic management schemes are effectively planned concerning economic sustainability and successful implementation.
Field Surveyor
Are you searching for a Field Surveyor Job Description? A Field Surveyor is a professional responsible for conducting field surveys for various places or geographical conditions. He or she collects the required data and information as per the instructions given by senior officials.
Orthotist and Prosthetist
Orthotists and Prosthetists are professionals who provide aid to patients with disabilities. They fix them to artificial limbs (prosthetics) and help them to regain stability. There are times when people lose their limbs in an accident. In some other occasions, they are born without a limb or orthopaedic impairment. Orthotists and prosthetists play a crucial role in their lives with fixing them to assistive devices and provide mobility.
Pathologist
A career in pathology in India is filled with several responsibilities as it is a medical branch and affects human lives. The demand for pathologists has been increasing over the past few years as people are getting more aware of different diseases. Not only that, but an increase in population and lifestyle changes have also contributed to the increase in a pathologist’s demand. The pathology careers provide an extremely huge number of opportunities and if you want to be a part of the medical field you can consider being a pathologist. If you want to know more about a career in pathology in India then continue reading this article.
Veterinary Doctor
Speech therapist, gynaecologist.
Gynaecology can be defined as the study of the female body. The job outlook for gynaecology is excellent since there is evergreen demand for one because of their responsibility of dealing with not only women’s health but also fertility and pregnancy issues. Although most women prefer to have a women obstetrician gynaecologist as their doctor, men also explore a career as a gynaecologist and there are ample amounts of male doctors in the field who are gynaecologists and aid women during delivery and childbirth.
Audiologist
The audiologist career involves audiology professionals who are responsible to treat hearing loss and proactively preventing the relevant damage. Individuals who opt for a career as an audiologist use various testing strategies with the aim to determine if someone has a normal sensitivity to sounds or not. After the identification of hearing loss, a hearing doctor is required to determine which sections of the hearing are affected, to what extent they are affected, and where the wound causing the hearing loss is found. As soon as the hearing loss is identified, the patients are provided with recommendations for interventions and rehabilitation such as hearing aids, cochlear implants, and appropriate medical referrals. While audiology is a branch of science that studies and researches hearing, balance, and related disorders.
An oncologist is a specialised doctor responsible for providing medical care to patients diagnosed with cancer. He or she uses several therapies to control the cancer and its effect on the human body such as chemotherapy, immunotherapy, radiation therapy and biopsy. An oncologist designs a treatment plan based on a pathology report after diagnosing the type of cancer and where it is spreading inside the body.
Are you searching for an ‘Anatomist job description’? An Anatomist is a research professional who applies the laws of biological science to determine the ability of bodies of various living organisms including animals and humans to regenerate the damaged or destroyed organs. If you want to know what does an anatomist do, then read the entire article, where we will answer all your questions.
For an individual who opts for a career as an actor, the primary responsibility is to completely speak to the character he or she is playing and to persuade the crowd that the character is genuine by connecting with them and bringing them into the story. This applies to significant roles and littler parts, as all roles join to make an effective creation. Here in this article, we will discuss how to become an actor in India, actor exams, actor salary in India, and actor jobs.
Individuals who opt for a career as acrobats create and direct original routines for themselves, in addition to developing interpretations of existing routines. The work of circus acrobats can be seen in a variety of performance settings, including circus, reality shows, sports events like the Olympics, movies and commercials. Individuals who opt for a career as acrobats must be prepared to face rejections and intermittent periods of work. The creativity of acrobats may extend to other aspects of the performance. For example, acrobats in the circus may work with gym trainers, celebrities or collaborate with other professionals to enhance such performance elements as costume and or maybe at the teaching end of the career.
Video Game Designer
Career as a video game designer is filled with excitement as well as responsibilities. A video game designer is someone who is involved in the process of creating a game from day one. He or she is responsible for fulfilling duties like designing the character of the game, the several levels involved, plot, art and similar other elements. Individuals who opt for a career as a video game designer may also write the codes for the game using different programming languages.
Depending on the video game designer job description and experience they may also have to lead a team and do the early testing of the game in order to suggest changes and find loopholes.
Radio Jockey
Radio Jockey is an exciting, promising career and a great challenge for music lovers. If you are really interested in a career as radio jockey, then it is very important for an RJ to have an automatic, fun, and friendly personality. If you want to get a job done in this field, a strong command of the language and a good voice are always good things. Apart from this, in order to be a good radio jockey, you will also listen to good radio jockeys so that you can understand their style and later make your own by practicing.
A career as radio jockey has a lot to offer to deserving candidates. If you want to know more about a career as radio jockey, and how to become a radio jockey then continue reading the article.
Choreographer
The word “choreography" actually comes from Greek words that mean “dance writing." Individuals who opt for a career as a choreographer create and direct original dances, in addition to developing interpretations of existing dances. A Choreographer dances and utilises his or her creativity in other aspects of dance performance. For example, he or she may work with the music director to select music or collaborate with other famous choreographers to enhance such performance elements as lighting, costume and set design.
Social Media Manager
A career as social media manager involves implementing the company’s or brand’s marketing plan across all social media channels. Social media managers help in building or improving a brand’s or a company’s website traffic, build brand awareness, create and implement marketing and brand strategy. Social media managers are key to important social communication as well.
Photographer
Photography is considered both a science and an art, an artistic means of expression in which the camera replaces the pen. In a career as a photographer, an individual is hired to capture the moments of public and private events, such as press conferences or weddings, or may also work inside a studio, where people go to get their picture clicked. Photography is divided into many streams each generating numerous career opportunities in photography. With the boom in advertising, media, and the fashion industry, photography has emerged as a lucrative and thrilling career option for many Indian youths.
An individual who is pursuing a career as a producer is responsible for managing the business aspects of production. They are involved in each aspect of production from its inception to deception. Famous movie producers review the script, recommend changes and visualise the story.
They are responsible for overseeing the finance involved in the project and distributing the film for broadcasting on various platforms. A career as a producer is quite fulfilling as well as exhaustive in terms of playing different roles in order for a production to be successful. Famous movie producers are responsible for hiring creative and technical personnel on contract basis.
Copy Writer
In a career as a copywriter, one has to consult with the client and understand the brief well. A career as a copywriter has a lot to offer to deserving candidates. Several new mediums of advertising are opening therefore making it a lucrative career choice. Students can pursue various copywriter courses such as Journalism , Advertising , Marketing Management . Here, we have discussed how to become a freelance copywriter, copywriter career path, how to become a copywriter in India, and copywriting career outlook.
In a career as a vlogger, one generally works for himself or herself. However, once an individual has gained viewership there are several brands and companies that approach them for paid collaboration. It is one of those fields where an individual can earn well while following his or her passion.
Ever since internet costs got reduced the viewership for these types of content has increased on a large scale. Therefore, a career as a vlogger has a lot to offer. If you want to know more about the Vlogger eligibility, roles and responsibilities then continue reading the article.
For publishing books, newspapers, magazines and digital material, editorial and commercial strategies are set by publishers. Individuals in publishing career paths make choices about the markets their businesses will reach and the type of content that their audience will be served. Individuals in book publisher careers collaborate with editorial staff, designers, authors, and freelance contributors who develop and manage the creation of content.
Careers in journalism are filled with excitement as well as responsibilities. One cannot afford to miss out on the details. As it is the small details that provide insights into a story. Depending on those insights a journalist goes about writing a news article. A journalism career can be stressful at times but if you are someone who is passionate about it then it is the right choice for you. If you want to know more about the media field and journalist career then continue reading this article.
Individuals in the editor career path is an unsung hero of the news industry who polishes the language of the news stories provided by stringers, reporters, copywriters and content writers and also news agencies. Individuals who opt for a career as an editor make it more persuasive, concise and clear for readers. In this article, we will discuss the details of the editor's career path such as how to become an editor in India, editor salary in India and editor skills and qualities.
Individuals who opt for a career as a reporter may often be at work on national holidays and festivities. He or she pitches various story ideas and covers news stories in risky situations. Students can pursue a BMC (Bachelor of Mass Communication) , B.M.M. (Bachelor of Mass Media) , or MAJMC (MA in Journalism and Mass Communication) to become a reporter. While we sit at home reporters travel to locations to collect information that carries a news value.
Corporate Executive
Are you searching for a Corporate Executive job description? A Corporate Executive role comes with administrative duties. He or she provides support to the leadership of the organisation. A Corporate Executive fulfils the business purpose and ensures its financial stability. In this article, we are going to discuss how to become corporate executive.
Multimedia Specialist
A multimedia specialist is a media professional who creates, audio, videos, graphic image files, computer animations for multimedia applications. He or she is responsible for planning, producing, and maintaining websites and applications.
Quality Controller
A quality controller plays a crucial role in an organisation. He or she is responsible for performing quality checks on manufactured products. He or she identifies the defects in a product and rejects the product.
A quality controller records detailed information about products with defects and sends it to the supervisor or plant manager to take necessary actions to improve the production process.
Production Manager
A QA Lead is in charge of the QA Team. The role of QA Lead comes with the responsibility of assessing services and products in order to determine that he or she meets the quality standards. He or she develops, implements and manages test plans.
Process Development Engineer
The Process Development Engineers design, implement, manufacture, mine, and other production systems using technical knowledge and expertise in the industry. They use computer modeling software to test technologies and machinery. An individual who is opting career as Process Development Engineer is responsible for developing cost-effective and efficient processes. They also monitor the production process and ensure it functions smoothly and efficiently.
AWS Solution Architect
An AWS Solution Architect is someone who specializes in developing and implementing cloud computing systems. He or she has a good understanding of the various aspects of cloud computing and can confidently deploy and manage their systems. He or she troubleshoots the issues and evaluates the risk from the third party.
Azure Administrator
An Azure Administrator is a professional responsible for implementing, monitoring, and maintaining Azure Solutions. He or she manages cloud infrastructure service instances and various cloud servers as well as sets up public and private cloud systems.
Computer Programmer
Careers in computer programming primarily refer to the systematic act of writing code and moreover include wider computer science areas. The word 'programmer' or 'coder' has entered into practice with the growing number of newly self-taught tech enthusiasts. Computer programming careers involve the use of designs created by software developers and engineers and transforming them into commands that can be implemented by computers. These commands result in regular usage of social media sites, word-processing applications and browsers.
Information Security Manager
Individuals in the information security manager career path involves in overseeing and controlling all aspects of computer security. The IT security manager job description includes planning and carrying out security measures to protect the business data and information from corruption, theft, unauthorised access, and deliberate attack
ITSM Manager
Automation test engineer.
An Automation Test Engineer job involves executing automated test scripts. He or she identifies the project’s problems and troubleshoots them. The role involves documenting the defect using management tools. He or she works with the application team in order to resolve any issues arising during the testing process.
Applications for Admissions are open.

Aakash iACST Scholarship Test 2024
Get up to 90% scholarship on NEET, JEE & Foundation courses

SAT® | CollegeBoard
Registeration closing on 19th Apr for SAT® | One Test-Many Universities | 90% discount on registrations fee | Free Practice | Multiple Attempts | no penalty for guessing

JEE Main Important Chemistry formulas
As per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

TOEFL ® Registrations 2024
Thinking of Studying Abroad? Think the TOEFL® test. Register now & Save 10% on English Proficiency Tests with Gift Cards

Resonance Coaching
Enroll in Resonance Coaching for success in JEE/NEET exams


ALLEN JEE Exam Prep
Start your JEE preparation with ALLEN
Everything about Education
Latest updates, Exclusive Content, Webinars and more.
Download Careers360 App's
Regular exam updates, QnA, Predictors, College Applications & E-books now on your Mobile
Cetifications
We Appeared in
Persuasive Essay Guide
Persuasive Essay About Covid19
How to Write a Persuasive Essay About Covid19 | Examples & Tips
11 min read

People also read
A Comprehensive Guide to Writing an Effective Persuasive Essay
200+ Persuasive Essay Topics to Help You Out
Learn How to Create a Persuasive Essay Outline
30+ Free Persuasive Essay Examples To Get You Started
Read Excellent Examples of Persuasive Essay About Gun Control
Crafting a Convincing Persuasive Essay About Abortion
Learn to Write Persuasive Essay About Business With Examples and Tips
Check Out 12 Persuasive Essay About Online Education Examples
Persuasive Essay About Smoking - Making a Powerful Argument with Examples
Are you looking to write a persuasive essay about the Covid-19 pandemic?
Writing a compelling and informative essay about this global crisis can be challenging. It requires researching the latest information, understanding the facts, and presenting your argument persuasively.
But don’t worry! with some guidance from experts, you’ll be able to write an effective and persuasive essay about Covid-19.
In this blog post, we’ll outline the basics of writing a persuasive essay . We’ll provide clear examples, helpful tips, and essential information for crafting your own persuasive piece on Covid-19.
Read on to get started on your essay.
- 1. Steps to Write a Persuasive Essay About Covid-19
- 2. Examples of Persuasive Essay About Covid19
- 3. Examples of Persuasive Essay About Covid-19 Vaccine
- 4. Examples of Persuasive Essay About Covid-19 Integration
- 5. Examples of Argumentative Essay About Covid 19
- 6. Examples of Persuasive Speeches About Covid-19
- 7. Tips to Write a Persuasive Essay About Covid-19
- 8. Common Topics for a Persuasive Essay on COVID-19
Steps to Write a Persuasive Essay About Covid-19
Here are the steps to help you write a persuasive essay on this topic, along with an example essay:
Step 1: Choose a Specific Thesis Statement
Your thesis statement should clearly state your position on a specific aspect of COVID-19. It should be debatable and clear. For example:
Step 2: Research and Gather Information
Collect reliable and up-to-date information from reputable sources to support your thesis statement. This may include statistics, expert opinions, and scientific studies. For instance:
- COVID-19 vaccination effectiveness data
- Information on vaccine mandates in different countries
- Expert statements from health organizations like the WHO or CDC
Step 3: Outline Your Essay
Create a clear and organized outline to structure your essay. A persuasive essay typically follows this structure:
- Introduction
- Background Information
- Body Paragraphs (with supporting evidence)
- Counterarguments (addressing opposing views)
Step 4: Write the Introduction
In the introduction, grab your reader's attention and present your thesis statement. For example:
Step 5: Provide Background Information
Offer context and background information to help your readers understand the issue better. For instance:
Step 6: Develop Body Paragraphs
Each body paragraph should present a single point or piece of evidence that supports your thesis statement. Use clear topic sentences, evidence, and analysis. Here's an example:
Step 7: Address Counterarguments
Acknowledge opposing viewpoints and refute them with strong counterarguments. This demonstrates that you've considered different perspectives. For example:
Step 8: Write the Conclusion
Summarize your main points and restate your thesis statement in the conclusion. End with a strong call to action or thought-provoking statement. For instance:
Step 9: Revise and Proofread
Edit your essay for clarity, coherence, grammar, and spelling errors. Ensure that your argument flows logically.
Step 10: Cite Your Sources
Include proper citations and a bibliography page to give credit to your sources.
Remember to adjust your approach and arguments based on your target audience and the specific angle you want to take in your persuasive essay about COVID-19.

Paper Due? Why Suffer? That's our Job!
Examples of Persuasive Essay About Covid19
When writing a persuasive essay about the Covid-19 pandemic, it’s important to consider how you want to present your argument. To help you get started, here are some example essays for you to read:
Check out some more PDF examples below:
Persuasive Essay About Covid-19 Pandemic
Sample Of Persuasive Essay About Covid-19
Persuasive Essay About Covid-19 In The Philippines - Example
If you're in search of a compelling persuasive essay on business, don't miss out on our “ persuasive essay about business ” blog!
Examples of Persuasive Essay About Covid-19 Vaccine
Covid19 vaccines are one of the ways to prevent the spread of Covid-19, but they have been a source of controversy. Different sides argue about the benefits or dangers of the new vaccines. Whatever your point of view is, writing a persuasive essay about it is a good way of organizing your thoughts and persuading others.
A persuasive essay about the Covid-19 vaccine could consider the benefits of getting vaccinated as well as the potential side effects.
Below are some examples of persuasive essays on getting vaccinated for Covid-19.
Covid19 Vaccine Persuasive Essay
Persuasive Essay on Covid Vaccines
Interested in thought-provoking discussions on abortion? Read our persuasive essay about abortion blog to eplore arguments!
Examples of Persuasive Essay About Covid-19 Integration
Covid19 has drastically changed the way people interact in schools, markets, and workplaces. In short, it has affected all aspects of life. However, people have started to learn to live with Covid19.
Writing a persuasive essay about it shouldn't be stressful. Read the sample essay below to get idea for your own essay about Covid19 integration.
Persuasive Essay About Working From Home During Covid19
Searching for the topic of Online Education? Our persuasive essay about online education is a must-read.
Examples of Argumentative Essay About Covid 19
Covid-19 has been an ever-evolving issue, with new developments and discoveries being made on a daily basis.
Writing an argumentative essay about such an issue is both interesting and challenging. It allows you to evaluate different aspects of the pandemic, as well as consider potential solutions.
Here are some examples of argumentative essays on Covid19.
Argumentative Essay About Covid19 Sample
Argumentative Essay About Covid19 With Introduction Body and Conclusion
Looking for a persuasive take on the topic of smoking? You'll find it all related arguments in out Persuasive Essay About Smoking blog!
Examples of Persuasive Speeches About Covid-19
Do you need to prepare a speech about Covid19 and need examples? We have them for you!
Persuasive speeches about Covid-19 can provide the audience with valuable insights on how to best handle the pandemic. They can be used to advocate for specific changes in policies or simply raise awareness about the virus.
Check out some examples of persuasive speeches on Covid-19:
Persuasive Speech About Covid-19 Example
Persuasive Speech About Vaccine For Covid-19
You can also read persuasive essay examples on other topics to master your persuasive techniques!
Tips to Write a Persuasive Essay About Covid-19
Writing a persuasive essay about COVID-19 requires a thoughtful approach to present your arguments effectively.
Here are some tips to help you craft a compelling persuasive essay on this topic:
Choose a Specific Angle
Start by narrowing down your focus. COVID-19 is a broad topic, so selecting a specific aspect or issue related to it will make your essay more persuasive and manageable. For example, you could focus on vaccination, public health measures, the economic impact, or misinformation.
Provide Credible Sources
Support your arguments with credible sources such as scientific studies, government reports, and reputable news outlets. Reliable sources enhance the credibility of your essay.
Use Persuasive Language
Employ persuasive techniques, such as ethos (establishing credibility), pathos (appealing to emotions), and logos (using logic and evidence). Use vivid examples and anecdotes to make your points relatable.
Organize Your Essay
Structure your essay involves creating a persuasive essay outline and establishing a logical flow from one point to the next. Each paragraph should focus on a single point, and transitions between paragraphs should be smooth and logical.
Emphasize Benefits
Highlight the benefits of your proposed actions or viewpoints. Explain how your suggestions can improve public health, safety, or well-being. Make it clear why your audience should support your position.
Use Visuals -H3
Incorporate graphs, charts, and statistics when applicable. Visual aids can reinforce your arguments and make complex data more accessible to your readers.
Call to Action
End your essay with a strong call to action. Encourage your readers to take a specific step or consider your viewpoint. Make it clear what you want them to do or think after reading your essay.
Revise and Edit
Proofread your essay for grammar, spelling, and clarity. Make sure your arguments are well-structured and that your writing flows smoothly.
Seek Feedback
Have someone else read your essay to get feedback. They may offer valuable insights and help you identify areas where your persuasive techniques can be improved.
Tough Essay Due? Hire Tough Writers!
Common Topics for a Persuasive Essay on COVID-19
Here are some persuasive essay topics on COVID-19:
- The Importance of Vaccination Mandates for COVID-19 Control
- Balancing Public Health and Personal Freedom During a Pandemic
- The Economic Impact of Lockdowns vs. Public Health Benefits
- The Role of Misinformation in Fueling Vaccine Hesitancy
- Remote Learning vs. In-Person Education: What's Best for Students?
- The Ethics of Vaccine Distribution: Prioritizing Vulnerable Populations
- The Mental Health Crisis Amidst the COVID-19 Pandemic
- The Long-Term Effects of COVID-19 on Healthcare Systems
- Global Cooperation vs. Vaccine Nationalism in Fighting the Pandemic
- The Future of Telemedicine: Expanding Healthcare Access Post-COVID-19
In search of more inspiring topics for your next persuasive essay? Our persuasive essay topics blog has plenty of ideas!
To sum it up,
You have read good sample essays and got some helpful tips. You now have the tools you needed to write a persuasive essay about Covid-19. So don't let the doubts stop you, start writing!
If you need professional writing help, don't worry! We've got that for you as well.
MyPerfectWords.com is a professional essay writing service that can help you craft an excellent persuasive essay on Covid-19. Our experienced essay writer will create a well-structured, insightful paper in no time!
So don't hesitate and get in touch with our persuasive essay writing service today!
Frequently Asked Questions
Are there any ethical considerations when writing a persuasive essay about covid-19.
Yes, there are ethical considerations when writing a persuasive essay about COVID-19. It's essential to ensure the information is accurate, not contribute to misinformation, and be sensitive to the pandemic's impact on individuals and communities. Additionally, respecting diverse viewpoints and emphasizing public health benefits can promote ethical communication.
What impact does COVID-19 have on society?
The impact of COVID-19 on society is far-reaching. It has led to job and economic losses, an increase in stress and mental health disorders, and changes in education systems. It has also had a negative effect on social interactions, as people have been asked to limit their contact with others.
Write Essay Within 60 Seconds!

Caleb S. has been providing writing services for over five years and has a Masters degree from Oxford University. He is an expert in his craft and takes great pride in helping students achieve their academic goals. Caleb is a dedicated professional who always puts his clients first.

Paper Due? Why Suffer? That’s our Job!
Keep reading


25,000+ students realised their study abroad dream with us. Take the first step today
Meet top uk universities from the comfort of your home, here’s your new year gift, one app for all your, study abroad needs, start your journey, track your progress, grow with the community and so much more.

Verification Code
An OTP has been sent to your registered mobile no. Please verify

Thanks for your comment !
Our team will review it before it's shown to our readers.

- School Education /
Essay On Covid-19: 100, 200 and 300 Words
- Updated on
- Sep 20, 2023

COVID-19, also known as the Coronavirus, is a global pandemic that has affected people all around the world. It first emerged in a lab in Wuhan, China, in late 2019 and quickly spread to countries around the world. This virus was reportedly caused by SARS-CoV-2. Since then, it has spread rapidly to many countries, causing widespread illness and impacting our lives in numerous ways. This blog talks about the details of this virus and also drafts an essay on COVID-19 in 100, 200 and 300 words for students and professionals.
Table of Contents
- 1 Essay On COVID-19 in English 100 Words
- 2 Essay On COVID-19 in 200 Words
- 3 Essay On COVID-19 in 300 Words
Also Read – Essay on Music
Essay On COVID-19 in English 100 Words
COVID-19, also known as the coronavirus, is a global pandemic. It started in late 2019 and has affected people all around the world. The virus spreads very quickly through someone’s sneeze and respiratory issues.
COVID-19 has had a significant impact on our lives, with lockdowns, travel restrictions, and changes in daily routines. To prevent the spread of COVID-19, we should wear masks, practice social distancing, and wash our hands frequently.
People should follow social distancing and other safety guidelines and also learn the tricks to be safe stay healthy and work the whole challenging time.
Essay On COVID-19 in 200 Words
COVID-19 also known as coronavirus, became a global health crisis in early 2020 and impacted mankind around the world. This virus is said to have originated in Wuhan, China in late 2019. It belongs to the coronavirus family and causes flu-like symptoms. It impacted the healthcare systems, economies and the daily lives of people all over the world.
The most crucial aspect of COVID-19 is its highly spreadable nature. It is a communicable disease that spreads through various means such as coughs from infected persons, sneezes and communication. Due to its easy transmission leading to its outbreaks, there were many measures taken by the government from all over the world such as Lockdowns, Social Distancing, and wearing masks.
There are many changes throughout the economic systems, and also in daily routines. Other measures such as schools opting for Online schooling, Remote work options available and restrictions on travel throughout the country and internationally. Subsequently, to cure and top its outbreak, the government started its vaccine campaigns, and other preventive measures.
In conclusion, COVID-19 tested the patience and resilience of the mankind. This pandemic has taught people the importance of patience, effort and humbleness.
Also Read – Essay on My Best Friend
Essay On COVID-19 in 300 Words
COVID-19, also known as the coronavirus, is a serious and contagious disease that has affected people worldwide. It was first discovered in late 2019 in Cina and then got spread in the whole world. It had a major impact on people’s life, their school, work and daily lives.
COVID-19 is primarily transmitted from person to person through respiratory droplets produced and through sneezes, and coughs of an infected person. It can spread to thousands of people because of its highly contagious nature. To cure the widespread of this virus, there are thousands of steps taken by the people and the government.
Wearing masks is one of the essential precautions to prevent the virus from spreading. Social distancing is another vital practice, which involves maintaining a safe distance from others to minimize close contact.
Very frequent handwashing is also very important to stop the spread of this virus. Proper hand hygiene can help remove any potential virus particles from our hands, reducing the risk of infection.
In conclusion, the Coronavirus has changed people’s perspective on living. It has also changed people’s way of interacting and how to live. To deal with this virus, it is very important to follow the important guidelines such as masks, social distancing and techniques to wash your hands. Getting vaccinated is also very important to go back to normal life and cure this virus completely. As we continue to battle this pandemic, it is crucial for everyone to do their part to protect themselves and their communities.
to write an essay on COVID-19, understand your word limit and make sure to cover all the stages and symptoms of this disease. You need to highlight all the challenges and impacts of COVID-19. Do not forget to conclude your essay with positive precautionary measures.
Writing an essay on COVID-19 in 200 words requires you to cover all the challenges, impacts and precautions of this disease. You don’t need to describe all of these factors in brief, but make sure to add as many options as your word limit allows.
The full form for COVID-19 is Corona Virus Disease of 2019.
Hence, we hope that this blog has assisted you in comprehending what an essay on COVID-19 in English 200 words must include. For more such essays, check our category essay writing .
Simran Popli
An avid writer and a creative person. With an experience of 1.5 years content writing, Simran has worked with different areas. From medical to working in a marketing agency with different clients to Ed-tech company, the journey has been diverse. Creative, vivacious and patient are the words that describe her personality.
Leave a Reply Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Contact no. *

Connect With Us

25,000+ students realised their study abroad dream with us. Take the first step today.

Resend OTP in

Need help with?
Study abroad.
UK, Canada, US & More
IELTS, GRE, GMAT & More
Scholarship, Loans & Forex
Country Preference
New Zealand
Which English test are you planning to take?
Which academic test are you planning to take.
Not Sure yet
When are you planning to take the exam?
Already booked my exam slot
Within 2 Months
Want to learn about the test
Which Degree do you wish to pursue?
When do you want to start studying abroad.
January 2024
September 2024
What is your budget to study abroad?

How would you describe this article ?
Please rate this article
We would like to hear more.
Have something on your mind?

Make your study abroad dream a reality in January 2022 with
India's Biggest Virtual University Fair

Essex Direct Admission Day
Why attend .

Don't Miss Out
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

COVID-19 Vaccine: A comprehensive status report
The current COVID-19 pandemic has urged the scientific community internationally to find answers in terms of therapeutics and vaccines to control SARS-CoV-2. Published investigations mostly on SARS-CoV and to some extent on MERS has taught lessons on vaccination strategies to this novel coronavirus. This is attributed to the fact that SARS-CoV-2 uses the same receptor as SARS-CoV on the host cell i.e. human Angiotensin Converting Enzyme 2 (hACE2) and is approximately 79% similar genetically to SARS-CoV. Though the efforts on COVID-19 vaccines started very early, initially in China, as soon as the outbreak of novel coronavirus erupted and then world-over as the disease was declared a pandemic by WHO. But we will not be having an effective COVID-19 vaccine before September, 2020 as per very optimistic estimates. This is because a successful COVID-19 vaccine will require a cautious validation of efficacy and adverse reactivity as the target vaccinee population include high-risk individuals over the age of 60, particularly those with chronic co-morbid conditions, frontline healthcare workers and those involved in essentials industries. Various platforms for vaccine development are available namely: virus vectored vaccines, protein subunit vaccines, genetic vaccines, and monoclonal antibodies for passive immunization which are under evaluations for SARS-CoV-2, with each having discrete benefits and hindrances. The COVID-19 pandemic which probably is the most devastating one in the last 100 years after Spanish flu mandates the speedy evaluation of the multiple approaches for competence to elicit protective immunity and safety to curtail unwanted immune-potentiation which plays an important role in the pathogenesis of this virus. This review is aimed at providing an overview of the efforts dedicated to an effective vaccine for this novel coronavirus which has crippled the world in terms of economy, human health and life.
1. Introduction
The novel beta-coronavirus SARS-CoV-2 is believed to have emerged last year in 2019 in Wuhan from Bats. Crossing the species barrier it entered human beings with furtherance of infection through human to human transmission. The beta-coronaviruses have jumped between the species and have caused three zoonotic outbreaks namely, SARS CoV (2002-03), MERS-CoV (2012), and SARS-CoV-2 (2019- till date) in the last 2 decades. The existence of a myriad of coronaviruses in bats, including many SARS-related CoV (Severe Acute Respiratory Syndrome related Coronaviruses) and the sporadic crossing over of the species barriers of the coronaviruses to humans, suggest that the future occurrences of zoonotic transmission events may sustain ( Ou et al., 2020 ).
Since its emergence in Nov 2019, it has spread to 188 countries and 25 territories around the globe, despite elaborate efforts by WHO and Governments to contain the infection, primarily owing to the highly infectious nature of this virus ( Anon, 2020a ; Anon, 2020b ). As of 2 July 2020, 10,533,779 cases have been reported globally with 512,842 deaths ( (WHO) World Health Organisation, 2020 ). There has been a monumental increase in the number of infected patients, with a 7-day moving average of 210,209 cases per day, as of 2 July 2020 ( Anon, 2020a ). SARS-CoV-2, a highly contagious virus, tends to spread by the inhalation of the respiratory aerosols, direct human contact, and via fomites. Social distancing, personal hygiene, frequent hand washing or sanitizing using the alcohol (61-70%) based hand-sanitizers, and disinfection of the surfaces are some steps which can protect the individuals from getting infected ( (CDC), Centers for Disease Control and Prevention, 2020 ). R 0 is an epidemiological scale; used to measure the contagiousness of an infectious agent. Its magnitude depends upon various biological, environmental, and socio-behavioral factors. It can be defined as “the average number of secondary cases one would produce in a completely susceptible population in the absence of any deliberate intervention in disease transmission ( Delamater et al., 2019 ).” SARS-CoV-2 has an R 0 value range of 2-3 ( Park, 2020 ) which is significantly higher in comparison to Spanish flu for which the R 0 was recorded at 0.9-2.1 ( Pyrek, 2018 ). According to WHO, people living with non-communicable diseases (co-morbid conditions) are prone to severe illness due to COVID-19 infection. The incubation period of the virus ranges from 2-14 days with a median of 5.1 days ( Lauer et al., 2020 ). The symptoms include fever, dry cough, fatigue, shortness of breath, chills, muscles pain, headache, gastric disturbances and weight loss ( CDC, 2020 ). Some patients may have lymphopenia and bilateral ground-glass opacity changes in the chest CT scans. The histological examinations of the lungs’ biopsy samples have shown a bilaterally diffused alveolar damage with cellular fibromyxoid exudates. A few interstitial mononuclear inflammatory infiltrates were observed both in the liver and the heart specimens ( Xu et al., 2020 ). However, a large population of the infected patients have no or mild symptoms and remain asymptomatic ( Shang et al., 2020 ).
Structurally coronaviruses are pleomorphic, enveloped viruses with a characteristic fringe of projections composed of S protein on their surface. These viruses are equipped with a positive sense ssRNA genome, which is complexed with the nucleocapsid (N) protein forming helical nucleocapsids. The genome is both capped and polyadenylated ( Carter and Saunders, 2007 ). The genetic analysis of SARS-CoV-2 and SARS-CoV has revealed 79% similarity with a total of 380 amino acid substitutions condensed mainly within the NSP genes. Out of these substitutions, there are 27 amino acid replacements in the immune-dominant S protein while 102 and 61 amino acid substitutions are found in the NSP3 and NSP2. Whereas, NSP7, NSP13, E protein, and some accessory proteins are devoid of any amino acid substitutions ( Wu et al., 2020 ). SARS-CoV and SARS-CoV-2 bind a common host receptor, hACE2, to gain entry into the cell but SARS-CoV-2 binds the receptor with a higher affinity than the SARS-CoV. MERS-CoV uses an entirely different receptor that is, Dipeptidyl Peptidase 4 (DPP4) ( Wan et al., 2020 ) and the virus is distantly related to SARS-CoV-2 with around 50% similarity as per the sequence analysis of the two viruses ( Prof Roujian et al., 2020 ).
The genome of SARS-CoV-2 is transcribed in at least 10 Open Reading Frames (ORFs). ORF1ab translates into a polyprotein which is processed into 16 non-structural proteins (NSPs) ( Yoshimoto, 2020 ). The NSPs perform various functions like genome replication, inducing the cleavage of host mRNA, membrane rearrangement, generation of the autophagosome, cleavage of the NSP polyprotein, capping, tailing, methylation, unwinding of the RNA duplex, etc. which are essential for the viral life cycle ( da Silva et al., 2020 ). Besides, the SARS-CoV-2 virus contains four structural proteins namely, spike (S), nucleocapsid (N), envelope (E), and membrane (M) proteins which are encoded by the 3’-end of the viral genome ( Wrapp et al., 2020 ). Amongst the 4 structural proteins the S glycoprotein, being a large multi-functional trans-membrane protein, plays the vital role of viral attachment, fusion, and entry into the host cell ( Wrapp et al., 2020 ). The S protein consists of S1 and S2 subunits, which are further split into different functional domains. The S1 subunit has two functional domains viz. N-terminal Domain (NTD) and Receptor Binding Domain (RBD) and the latter contains conserved receptor binding motif (RBM) ( Jiang et al., 2020 ). The alignment studies have revealed that the region of RBD sequence lies between the residues 331 and 524 of the S protein ( Tai et al., 2020 ). Whereas, the S2 subunit has three operational domains namely, fusion peptide (FP), heptad repeat (HR) 1, and 2. The S1 protein trimer aligns itself at the top of the trimeric S2 stalk to form the immune-dominant S protein ( Jiang et al., 2020 ). Interestingly, a furin cleavage site is observed within the spike protein of SARS-CoV-2 while it is absent in the SARS-CoV which may be a possible explanation of the variation in the pathogenicity of the virus ( Walls et al., 2020 ). A host trans-membrane protease serine 2, (TMPRSS2) is responsible for the initial priming of the spike protein. The virus can utilize both TMPRSS2 and endosomal cysteine proteases cathepsin B and L (CatB/L) to initiate entry into the cell. The TMPRSS2 is responsible for the cleavage of the S protein to expose the FP region of the S2 subunit which is responsible for the initiation of the endosome mediated entry into the host cell. This indicates that TMPRSS2 is a host factor that is essential for viral entry; therefore, the drugs approved for the inhibition of this protease (like camostatmesylate) could be used for therapeutic purposes ( Hoffmann and Kleine-Weber, 2020 ). SARS-CoV-2 uses the human angiotensin-converting enzyme 2 (hACE2) receptor to seize the target cell through the spike glycoprotein (S-Protein), . It has been suggested that the coronaviruses exercise the use of conformational masking and glycan shielding of the spike protein to circumvent the host immune cells. The Cryo-EM structures have revealed the presence of two distinct: closed and open conformations of the S-Protein ectodomain trimer, as a consequence of the opening of the structure at the trimer apex. This conformational diversification is necessary for the receptor binding as the trimer opening exposes the RBM which is present at the interface between the protomers in the closed trimers ( Walls, 2020 ).
The E protein that forms E channels (called the viroporins), and is involved in a myriad of functions in the viral replication cycle involving assembly, release, pathogenesis, etc. ( Gralinski and Menachery, 2020 ). These reprobate ion channels exist in the form of homo-pentamers with each subunit containing 50-120 amino acids. E channels contain at least one trans-membrane domain (TMD) which facilitates the linkage in host cell membranes. SARS CoVs generally contain three categories of ion channels namely: E, 8a, and 3a. The E and 8a ion channels contain the PDZ (Post Synaptic Density Protein; Disc Large Tumor Suppressor; Zonula Occludens-1 Protein) Domain Binding Motif (PBM) which is responsible for the over-expression of the inflammatory cytokines which may result in the cytokine storm ( Pharmaceutical Targeting the Envelope Protein of SARS-CoV-2: the Screening for Inhibitors in Approved Drugs, 2020 ). From the sequence alignment study of the E protein, it was observed that a negatively charged glutamate residue (E69) in SARS-CoV corresponds to a positively charged arginine residue (R69) in SARS-CoV-2 ( Yoshimoto, 2020 ). However, this mutation is remote from the inhibitor binding site; therefore, E protein can be used as a pharmaceutical target ( Pharmaceutical Targeting the Envelope Protein of SARS-CoV-2: the Screening for Inhibitors in Approved Drugs, 2020 ).
M protein, the central organizer of CoV assembly, is most abundantly expressed in the virus particle. It functions crucially in the morphogenesis and assembly of the SARS-CoV-2 by interacting with the essential structural proteins ( Conserved Protein Domain Family: SARS-like-CoV_M, 2020 ). The binding of the M and N protein stabilizes the N protein and RNA complex, and the internal core of the virus. In case of SARS-CoV, the M protein has also been shown to induce the process of apoptosis in the host cell ( Yoshimoto, 2020 ).
In addition to stabilizing the ssRNA genome of the virus particle, the N protein is an antagonist of the antiviral RNAi. It is responsible for the inhibition of the cell cycle of the host cell as it can inhibit the entry of the cell into the S-phase ( Yoshimoto, 2020 ).
Immunotherapy is considered as an effective method for the prophylaxis and treatment of various infectious diseases and cancers, which involves the artificial triggering of the immune system to elicit the immune response ( Masihi, 2001 ). A vaccine that elicits the production of S protein neutralizing antibodies in the vaccinated subjects is the primary aim of all the programs for COVID-19 vaccines. Studies have revealed that there is a limited to no cross-neutralization between the sera of SARS-CoV and SARS-CoV-2, indicating that recovery from one infection may not shield against the other ( Ou et al., 2020 ). Furthermore, a database of approximately 5500 full-length genomes of SARS-CoV-2 isolated from various countries is now available at NCBI which facilitates delineating the polymorphisms in S protein and other important proteins of the virus concerning vaccine development. The rationale for writing this review is to gather all the information about the COVID-19 vaccine development programs and give the readers and researchers insight into types of vaccines being worked upon and the current status of the clinical trials of these vaccines for ready reference.
2. Vaccination strategies
Many efforts have been directed towards the development of the vaccines against COVID-19, to avert the pandemic and most of the developing vaccine candidates have been using the S-protein of SARS-CoV-2 ( Dhama et al., 2020 ). As of July 2, 2020, the worldwide SARS-CoV-2 vaccine landscape includes 158 vaccine candidates, out of which 135 are in the preclinical or the exploratory stage of their development. Currently, mRNA-1273 (Moderna), Ad5-nCoV (CanSino Biologicals), INO-4800 (Inovio, Inc.), LV-SMENP-DC, Pathogen-specific aAPC (ShinzenGeno-Immune Medical Institute), and ChAdOx1 (University of Oxford) have entered the phase I/II clinical trials ( WHO, 2020 ). The vaccines which are in the conduit are based upon inactivated or live attenuated viruses, protein sub-unit, virus-like particles (VLP), viral vector (replicating and non- replicating), DNA, RNA, nanoparticles, etc. with each exhibiting unique advantages and hindarances ( Table 1 ) ( Ning et al., 2020 ). COVID-19 vaccine landscape with percentage share of different types of vaccine is represented in Fig. 1 . To enhance the immunogenicity, various adjuvant technologies like AS03 (GSK), MF-59 (Novartis), CpG 1018 (Dynavax), etc. are now accessible to the researchers for the vaccine development ( Le et al., 2020 ). The immuno-informatics approach is also used for the epitope identification for the SARS-CoV-2 vaccine candidates. It can be used to identify the significant cytotoxic T cell and B-cell epitopes in the viral proteins ( Gupta et al., 2006 ; Baruah and Bose, 2020 ).
Outline of the vaccine production platforms for SARS-CoV-2 and their advantages and limitations

Pie Chart showing the different categories of SARS-CoV-2 vaccines under research ( Anon, 2020c ).
2.1. Protein Sub-unit vaccine
A subunit vaccine is the one which is based on the synthetic peptides or recombinant antigenic proteins, which are necessary for invigorating long-lasting protective and/or therapeutic immune response ( Ning et al., 2020 ). The subunit vaccine, however, exhibits low immunogenicity and requires auxiliary support of an adjuvant to potentiate the vaccine-induced immune responses. An adjuvant may enhance the biological half-life of the antigenic material, or it may ameliorate the immunomodulatory cytokine response. The addition of an adjuvant, therefore, helps in overcoming the shortcomings of the protein subunit vaccines ( Cao et al., 2018 ). The S protein of the SARS-CoV-2 is the most suitable antigen to induce the neutralizing antibodies against the pathogen. The S Protein consists of two subunits. The S1 subunit has the NTD, RBD, and RBM domains while the S2 subunit comprises of FP, HR 1, &2 ( Ou et al., 2020 ). The virus enters into the cell via endocytosis by utilizing the S-Protein mediated binding to the hACE2 receptor. Therefore, the S-Protein and its antigenic fragments are the prime targets for the institution of the subunit vaccine ( Ning et al., 2020 ). The S glycoprotein is a dynamic protein, possessing two conformational states i.e. pre-fusion and post-fusion state. Therefore, the antigen must maintain its surface chemistry and profile of the original pre-fusion spike protein to preserve the epitopes for igniting good quality antibody responses ( Graham, 2020 ). Moreover, means to target the masked RBM as an antigen will enhance the neutralizing antibody response and improve the overall efficacy of the vaccine.
2.1.1. NVX-CoV2373 (Novavax, Inc.| Emergent BioSolutions)
NVX-CoV2373 is a nano-particle based immunogenic vaccine which is based upon the recombinant expression of the stable pre-fusion, coronavirus S-Protein ( Coleman et al., 2020 ). The protein was stably expressed in the Baculovirus system ( Tu et al., 2020 ). The company plans to use the Matrix-M adjuvant to enhance the immune response against SARS-CoV-2 spike protein by the induction of high levels of neutralizing antibodies. In the animal models, a single immunization resulted in the high level of anti-spike protein antibodies which blocked the hACE2 receptor binding domain and could elicit SARS-CoV-2 wild type virus-neutralizing antibodies ( Novavax covid 19 vaccine trial, 2020 ).
2.1.2. Molecular Clamp Stabilized spike protein vaccine candidate
It is being developed by the University of Queensland in collaboration with GSK and Dynavax. The University will have access to vaccine adjuvant platform technology (AS03 Adjuvant system), which is believed to strengthen the vaccine response and minimize the amount of vaccine required per dose ( Lee, 2020 ). The University is developing a stabilized pre-fusion, recombinant viral protein sub-unit vaccine which is based upon the Molecular Clamp technology. This technology has been proved to induce the production of the neutralizing antibodies ( Tu et al., 2020 )
2.1.3. PittCoVacc (University of Pittsburgh)
It is a Micro-Needle Array (MNA) based recombinant SARS-CoV-2 vaccine which involves the administration of rSARS-CoV-2 S1 and rSARS-CoV-2-S1fRS09 (recombinant immunogens). A substantial increase in the antigen specific antibodies with a statistical significance was observed in the pre-clinical trials at the end of two weeks in the mice models. Furthermore, the immunogenicity of the vaccine was maintained even after the sterilization using gamma radiation. The statistically significant titers of antibodies at the early stages and also before boosting, support the feasibility of the MNA-SARS-CoV-2 vaccine ( Kim et al., 2020 ).
2.1.4. Triple Antigen Vaccine (Premas Biotech, India)
It is a multi-antigenic VLP vaccine prototype wherein the recombinant spike, membrane, and envelope protein of SARS-CoV-2 have been co-expressed in an engineered Saccharomyces cerevisiae expression platform (D-Crypt™). The proteins then undergo self-assembly as the VLP. The TEM and allied analytical data simultaneously furnished the biophysical characterization of the VLP. This prototype has the potential to enter the pre-clinical trials as a vaccine candidate after further research and development. Furthermore, it is thought to be safe and easy to manufacture on a mass scale, in a cost-effective manner ( Arora and Rastogi, 2020 ).
2.2. Viral Vectored vaccines
A vaccine based on viral vectors is a promising prophylactic solution against a pathogen. These vaccines are highly specific in delivering the genes to the target cells, highly efficient in the gene transduction, and efficiently induce the immune response, ( Ura et al., 2014 ). They offer a long term and high level of antigenic protein expression and therefore, have a great potential for prophylactic use as these vaccines trigger and prime the cytotoxic T cells (CTL) which ultimately leads to the elimination of the virus infected cells ( Le et al., 2020 ).
2.2.1. Ad5-nCoV (CanSino Biologics Inc | Beijing Institute of Biotechnology)
It is a recombinant, replication defective adenovirus type-5 vector (Ad5) expressing the recombinant spike protein of SARS-CoV-2. It was prepared by cloning an optimized full-length gene of the S Protein along with the plasminogen activator signal peptide gene in the Ad5 vector devoid of E1 and E3 genes. The vaccine was constructed using the Admax system from the Microbix Biosystem ( Zhu et al., 2020 ). The phase I clinical trials have established a positive antibody response or seroconversion. A four-fold increase in the RBD and S protein-specific neutralizing antibodies was noted within 14 days of immunization and peaked at day 28, post-vaccination. Furthermore, the CD4 + T cells and CD8 + T cells response peaked at day 14 post-vaccination. However, the pre-existing anti-Ad5 immunity partly limited both the antibody and the T cell responses ( Zhu et al., 2020 ). The study will further evaluate antibody response in the recipients who are between the age of 18 and 60, and received one of three study doses, with follow-up taking place at 3- and 6-months post-vaccination ( Anon, 2020d ).
2.2.2. Coroflu (University of Wisconsin-Madison | FluGen | Bharat Biotech)
M2SR, a self-limiting version of the influenza virus, which is modified by insertion of the SARS-CoV-2 gene sequence of the spike protein. Furthermore, the vaccine expresses the hemagglutinin protein of the influenza virus, thereby inducing immune response against both the viruses. The M2SR is self-limiting and does not undergo replication as it lacks the M2 gene. It is able to enter into the cell, thereby inducing the immunity against the virus. It shall be administered intra-nasally, mimicking the natural route of viral infection. This route activates several modes of the immune system and has higher immunogenicity as compared to the intramuscular injections ( Anon, 2020e ).
2.2.3. LV-SMENP-DC (Shenzhen Geno-Immune Medical Institute)
The LV-SMENP-DC vaccine is prepared by engineering the dendritic cells (DC) with the lentiviral vector expressing the conserved domains of the SARS-CoV-2 structural proteins and the protease using the SMENP minigenes. The subcutaneous inoculation of the vaccine presents the antigens on antigen presenting cells (APCs), that ultimately activate the Cytotoxic T cells and generate the immune response ( Le et al., 2020 ).
2.2.4. ChAdOx1 (University of Oxford)
ChAdOx1 recombinant adenovirus vaccine was developed using codon optimized S glycoprotein and synthesized with the tissue plasminogen activator (tPA) leader sequence at 5’ end. The sequence of SARS-CoV-2 coding for amino acids (2 to 1273) and the tPA leader and was propagated in the shuttle plasmid. This shuttle plasmid is responsible for encoding the major immediate early genes of the human cytomegalovirus (IE CMV) along with tetracycline operator (TetO) sites and polyadenylation signal from bovine growth hormone (BGH) between the Gateway® recombination cloning site. The Adenovirus vector genome is constructed in the Bacterial Artificial Chromosome by inserting the SARS-CoV-2 S gene into the E1 locus of ChAdOx1 adenovirus genome. The virus was then allowed to reproduce in the T-Rex 293 HEK (Human Embryonic Kidney 293) cell lines and purified by the CsCl gradient ultracentrifugation. The absence of any sub-genomic RNA (sgRNA) in the intra-muscularly vaccinated animals from the pre-clinical trials is indicative of the escalated immunity against the virus ( Doremalen et al., 2020 ). The previous studies have suggested that a single shot should marshal the immune response ( Ou et al., 2020 ). The vaccine has entered phase II clinical trials, where it shall be evaluated in a large sample of the population ( Anon, 2020f ).
2.3. mRNA Vaccine
mRNA is an emerging, non-infectious, and a non-integrating platform with almost no potential risk of insertional mutagenesis. Currently, the non-replicating RNA and the virus derived self-replicating RNAs are being studied. The immunogenicity of the mRNA can be minimized, and alterations can be made to increase the stability of these vaccines. Furthermore, the anti-vector immunity is also avoided as the mRNA is the minimally immunogenic genetic vector, allowing repeated administration of the vaccine ( Cuiling et al., 2020 ). This platform has empowered the rapid vaccine development program due to its flexibility and ability to mimic the antigen structure and expression as seen in the course of a natural infection ( Mulligan and Lyke, 2020 ).
2.3.1. mRNA-1273 (Moderna TX, Inc)
It is a vaccine composed of synthetic mRNA encapsulated in Lipid nanoparticle (LNP) which codes for the full-length, pre-fusion stabilized spike protein (S) of SARS-CoV-2. It has the potential to elicit a highly S-protein specific antiviral response. Furthermore, it is considered to be relatively safe as it is neither made up of the inactivated pathogen nor the sub-units of the live pathogen ( Tu et al., 2020 ). The vaccine has got a fast-track approval from FDA, to conduct the Phase II trials ( Anon, 2020g ).The company has released the interim phase I antibody data of eight participants who received various dose levels. The participants of the 25 μg dose group gave results comparable to the convalescent sera. Whereas, in participants who received the 100 μg dose, the levels of nAb essentially surpassed the levels found in convalescent sera. The vaccine was found to be predominantly safe and well tolerated in the 25 μg and 100 μg dose cohorts, while three participants experienced grade 3 systemic symptoms after the administration of the second dose of 250 μg dose levels ( Anon, 2020h ).
2.3.2. BNT162b1 (BioNTech| FosunPharma| Pfizer)
BNT162b1 is a codon-optimized mRNA vaccine that encodes for the trimerized SARS-CoV-2 RBD, a critical target of the virus nAb. The vaccine portrays an increased immunogenicity due to the addition of T4 fibritin-derived foldon trimerization domain to the RBD antigen. The mRNA is encapsulated in 80 nm ionizable cationic lipid nanoparticles, which ensures its efficient delivery. The Phase 1/2 clinical trials have revealed elevated RBD-specific IgG antibodies levels with a geometric mean concentration to be as high as 8 to 46.3 times titer of convalescent serum. Whereas, the geometric mean titers of the SARS-CoV-2 neutralizing antibodies were found to be 1.8 to 2.8 times the convalescent serum panel. Moderate and transient local reactions and systemic events were observed with no adverse effect. However, the data analysis did not evaluate the safety and immune responses beyond 2 weeks following the administration of the second dose ( Mulligan and Lyke, 2020 ).
2.4. DNA Vaccines
The most revolutionary approach to vaccination is the introduction of the DNA vaccine which encodes for the antigen and an adjuvant which induces the adaptive immune response. The transfected cells express the transgene which provides a steady supply of the transgene specific proteins which is quite similar to the live virus. Furthermore, the antigenic material is endocytosed by the immature Dendritic Cells which ultimately present the antigen to the CD4+ and CD8+ T cells in association with MHC 2 and MHC 1 antigens on the cell surface hence stimulating effective humoral as well as cell-mediated immune responses ( Hobernik and Bros, 2018 ).
2.4.1. INO-4800 (Inovio Pharmaceuticals)
It is a prophylactic DNA vaccine against SARS-CoV-2 ( Anon, 2020i ). It uses codon optimized S protein sequence of SARS-CoV-2 to which an IgE leader sequence is affixed. The SARS-CoV-2 IgE-spike sequence was synthesized and digested using BamHI and XhoI . The digested DNA was incorporated into the expression plasmid pGX0001 under the governance of IE CMV, and BGH polyadenylation signal. The presence of functional antibodies and T cell response in the preclinical trials suggest that the vaccine can produce an effective immune response within 7 days post-vaccination ( Smith et al., 2020 ). The vaccine has entered the Phase I clinical trials (Phase I: {"type":"clinical-trial","attrs":{"text":"NCT04336410","term_id":"NCT04336410"}} NCT04336410 ) and it is estimated to complete this phase of clinical trials by July, wherein the participants received 1.0 mg of INO-4800 by electroporation using CELLECTRA® 2000 device per dosing visit. The trial will evaluate the immunological profile, safety, and tolerability of the vaccine candidate upon intradermal injection and the electroporation in healthy human adults ( Anon, 2020i ).
2.5. Live Attenuated Vaccines
2.5.1. delns1-sars-cov2-rbd (university of hong kong).
This LAV is influenza-based vaccine strain with a deletion in the NS1 gene. It is re-organized to express the RBD domain of SARS-CoV-2 spike protein on its surface and, is cultivated in the chick embryo and/or Madin Darby Canine Kidney Cells (MDCK) cells. It is potentially more immunogenic than the wild type influenza virus and can be administered as a nasal spray ( Anon, 2020j ).
2.6. Others
The revelation of the structure and genome of the SARS-CoV-2 has led to the rapid development of various vaccine candidates with potential immunogenicity but also adverse reactogenicities. The task of vaccine development is long and cumbersome which requires evaluation in some long-lasting clinical trials. Various Biotech ventures are using different technologies for the development of their vaccine candidates; British and American Tobacco Company (BAT) recently unfolded the COVID-19 vaccine using their new, and fast-growing tobacco plant technology ( Anon, 2020k ), while Tianjin University has developed an oral vaccine which has successfully employed Saccharomyces cerevisiae to carry the S protein. The GRAS (Generally Regarded As Safe) status of the yeast provides high scalability, robustness, and cost-effective production of cosmic dosages required to fight off this pandemic ( Zhai et al., 2020 ). Furthermore, in silico studies, using various databases like VaxiJen, have revealed that the epitope sequences WTAGAAAYY and YDPLQPEL can be employed for the formulation of epitope-based peptide vaccines ( Garg et al., 2020 ).
2.6.1. Self Assembling Vaccine (HaloVax)
The vaccine uses a heat shock protein (hsp) to activate the immune system. It is composed of a fusion protein sandwiched between an hsp and Avidin. Biotinylated immunogenic peptides are also incorporated to customize the vaccine ( Voltron Therapeutics, Inc., 2020 ) Table 2 , Table 3 .
Rapidly progressing Anti COVID-19 vaccines. This table contains the information of rapidly developing vaccine candidates only, the list of all vaccine candidates in the pipeline can be accessed from: https://airtable.com/shrSAi6t5WFwqo3GM/tblEzPQS5fnc0FHYR/viweyymxOAtNvo7yH?blocks=bip
Legend: CCHF: Crimean-Congo Hemorrhagic Fever; CHIKV: Chikungunya Virus; DengV: Dengue Virus; FMD: Foot and Mouth Disease; EBOV: Ebola Virus; HAV: Hepatitis A Virus; HBV: Hepatitis B Virus; HIV: Human Immunodeficiency Virus; HPV: Human Papilloma Virus; Inf: Influenza; LASV: Lassa Fever Virus; MenB: Meningitis B; NIPV: Nipah Virus; NORV: Norovirus; RABV: Rabies Virus; RVF: Rift Valley Fever; SARS: Severe Acute Respiratory Syndrome; SIV: Simian Immunodeficiency Virus; TB: Tuberculosis; VEE: Venezuelan Equine; Encephalitis Virus; VZV: Varicella Vaccine (Chickenpox); YFV: Yellow Fever Virus; ZIKV: Zika Virus.
Latest developments in the status of the promising SARS-CoV-2 vaccines
3. Passive Immunization/adoptive immunity
It is the use of preformed antibodies in therapeutics of various diseases. It can be achieved by use of sera from convalescent patients, polyclonal serum raised in other animals such as horse, neutralizing monoclonal antibodies produced by hybridoma technology or humanized antibodies.
3.1. Convalescent Plasma therapy
To date, no distinct treatment has been proven to be efficacious against the COVID-19. Convalescent plasma (CP) therapy has been approved as an empirical treatment during the outbreaks ( (WHO), World Health Organisation, 2014 ). It is considered as the archetypal immunotherapy which has been used for the treatment and prevention of various viral diseases in the past such as SARS, MERS, H1N1 pandemic, measles, mumps, etc. ( Kai et al., 2020 ). A possible explanation for the efficacy of this classic adoptive immunotherapy is that the neutralizing immune-globulins from CP may conquer viremia, block new infection, and accelerate clearance of the infected cells.
Various studies conducted to evaluate therapeutic potential of CP have convincingly shown that administration of the neutralizing antibodies in the critically ill patients led to the amelioration of the clinical status in all patients without any deaths ( Kai et al., 2020 ; Shen et al., 2020a ; Ahn et al., 2020a ; Anon, 2020C ). The dosage prescribed for the CP therapy has not been standardized yet and needs Randomised Clinical Trials not only to eliminate the effect of other medicines but also to evaluate the efficacy and safety of CP therapy. ( Zhang et al., 2020 ). The patients who were considered critically ill with some of them having co-morbid conditions like hypertension, cardiovascular diseases, cerebrovascular diseases, chronic renal failure, etc. were included in the study. They were all admitted to the ICUs and were receiving either mechanical ventilation, high-flow nasal cannula oxygenation, or the low-flow nasal cannula oxygenation. All the patients in these studies were receiving antiviral or antibacterial or antifungal drugs for the treatment of co-infections ( Kai et al., 2020 ). Compared to the control group, the CP treatment group showed no notable differences in the baseline characteristics but exhibited a sizable difference in the clinical outcomes (i.e. normalization of the body temperature, absorption of pulmonary lesions, resolution of ARDS, weaning off the mechanical ventilators, etc.), and the death rates. The patients were tested negative for the viral loads after 7-37 days of CP infusion ( Shen et al., 2020b ). A reduction in the net quantity of inflammatory biomarkers CRP, procalcitonin, and Interleukin 6 (IL-6) in the trial group was observed along with a significant increase in the antibody titers (RBD specific IgM and IgG) post-convalescent plasma therapy ( Ahn et al., 2020b ). However, these uncontrolled and non-randomized trials for the CP therapy impede the researchers to come to a conclusive statement about the prospective potency of this treatment, and these observations require further evaluation which is ongoing in the clinical trials ( Yan, 2020 ).
3.2. Monoclonal Antibody
The monoclonal antibodies (mAb) or therapeutic antibodies, created in the laboratory are the clones of a unique parent which can bind to a single epitope, that is, they have a monovalent affinity ( Gelboin et al., 1999 ). The use of mAb in the prevention and treatment of infectious diseases can overcome various drawbacks which are cognate with the convalescent plasma therapy in terms of specificity, safety, low risk of blood-borne infection, purity, and other factors. A wide array of monoclonal antibodies have already been developed which are implemented in the anti-tumor, anti-platelet, or antiviral therapy ( Breedveld, 2000 ).
A SARS-CoV specific human mAb CR3022 has been found to bind with the RBD of the S protein of SARS-CoV-2, stipulating it as a prospective therapeutic agent, which can either be used alone or in combination therapy for the management of COVID-19 ( Tian et al., 2020 ). To achieve higher efficiency of disease prevention and treatment, a combinatorial effect of monoclonal antibodies recognizing different epitopes of the viral surface can be considered for the neutralization of the virus as it may prove to be more effective and prevent the viral escape ( Tian et al., 2020 ).
There are over 61 patents which claim to have prepared the SARS-specific, MERS-specific, and the diagnostic antibodies. Another group of 38 patents claims to have developed the antibodies that target the host proteins like IL-6/IL-6R, TLR3, CD16, ITAM (immune-receptor tyrosine-based activation motif), DC-SIGN (dendritic cell-specific intercellular adhesion molecule-grabbing non-integrin), ICAM-3 (intercellular adhesion molecule 3), or IP-10/CXCL10 (interferon γ-inducible protein 10). These antibodies can be used to counteract against the cytokine storm that has been reported to harmonize with the SARS-CoV-2 infection ( Liu et al., 2020 ). Tocilizumab, an anti-IL 6 receptor antibody is likely to control the hyper-inflammatory pulmonary symptoms which are coupled with the cytokine storm involving the chemokine dysregulation and various interleukins. Tocilizumab has been reported to block the cytokine axis IL6 hence inhibiting the inflammatory cascade. However, further clinical trials are essential to establish the effectiveness of the mAb ( Michot et al., 2020 ). Israel Institute for Biological Research (IIBR) claims to have successfully developed the mAb against SARS-CoV-2. The institute is in the process of patenting it which may soon be commercialized ( Upadhyay, 2020 ). A group led by Professor Vijay Chaudhary at the University of Delhi, Centre for Innovation in Infectious Disease Research, Education and Training (UDSC-CIIDRET), is isolating the genes encoding the antibodies responsible for the neutralization of the SARS-CoV-2. These genes will be employed to foster the recombinant Ab by exploiting the pre-existing in-house antibody library and a library fabricated from the cells of convalescent COVID-19 patients ( PIB, Delhi, 2020 ).
4. Limitations
The duration of clinical trials poses a sizable amount of hindrance to swift vaccine development. According to the norms laid down by the US Food and Drug Administration (FDA), and WHO, a vaccine candidate has to pass through at least three phases of placebo-controlled clinical trials for the validation of its safety and efficacy, which can take years to complete. Considering the severity of the pandemic, which has forced a complete shut-down of the global economy, speedy vaccine development is necessary. Some authors suggest that the controlled human challenge studies may be conducted to suitably divert the Phase 3 testing, and allow the rapid licensure of the immunogenic vaccines. However, in the expanded field study participants will be monitored constantly to look for any long-term implications posed by the vaccine. Furthermore, the safety trials for the special groups including, children and pregnant women, and immuno-compromised patients can be conducted before the extension of the vaccination to these groups ( Eyal et al., 2020 ).
The testing and development of safe and effective vaccines rely upon laboratory animal models. These animal models must show a similar course of the disease as in human beings. However, the standard inbred strains of mice are not susceptible to the COVID-19 infection, due to the difference between the humans and mice ACE2 receptors ( Anon, 2020D ). This calls for the development of transgenic mice, expressing the hACE2 receptor. Two animal models (hACE2 transgenic mice model and another, primate Macaques model) were previously developed for the SARS-CoV but the current situation requires steady breeding and distribution of these animal models to meet demands of the researchers around the globe ( Mice and Bao, 2020 ). The SARS-CoV-2 virus isolates can efficiently replicate in the lungs of the Syrian hamsters. The lungs of infected hamsters exhibit the pathological lesions analogous to the COVID-19 patients with pneumonia. Moreover, the nAb response exhibited by the infected hamster demonstrated immunity against the succeeding re-challenge studies. Furthermore, the transfusion of convalescent sera into the naïve hamsters mounted the antibody response and hence hindered the viral replication in the lungs. The assemblage of these experiments have illustrated the Syrian hamster may be a perfect model for comprehending SARS-CoV-2 pathogenesis, and evaluating antiviral drugs, and the immunotherapies ( Imai and Iwatsuki-Horimoto, 2020 ). Nevertheless, the assessment of the vaccine dependent immune enhancement cannot be extrapolated from the animal models and requires a legitimate survey from stage III human trials or the human challenge studies.
The Antibody dependent enhancement (ADE) is exploited by various viruses like Dengue, HIV, animal coronaviruses, etc. as an alternative method of infecting a variety of host cells. The virus-antibody complex can bind to the Fc receptors, activate the complement system, or induce a conformational change in the glycoprotein of the viral envelope ( Yip et al., 2016 ). This mechanism is observed when the vaccine-induced antibodies are either non-neutralizing or they are present in inadequate concentrations. This process triggers the viral entry into the cell due to the intensified binding efficiency of the virus-antibody complexes to FcR bearing cells. The clinical and preclinical trials of SARS-CoV vaccine candidates have demonstrated the aggravation of the disease due to ADE. Vaccine Associated Enhanced Respiratory Disease (VAERD) can also be induced by virus-antibody immune complex and T H 2-biased responses ( Graham, 2020 ).
The viral genome is vulnerable to mutations and can undergo the antigenic shift and the antigenic drift, as it continues to spread from one population to the next. The mutations may vary according to the environmental conditions of a geographical area, and the population density. By screening the 7500 samples of the infected patients, the scientists were able to figure out 198 mutations that may have materialized independently which may indicate the evolution of the virus inside the human host. These mutations may lead to different subtypes which may allow the virus to escape the immune system even after the administration of the vaccine ( Dorp et al., 2020 ).
5. Conclusion
SARS-CoV-2 has been the matter of the moment from the date it was declared as a pandemic, it has led to the termination of economic activities universally. Scientists across the continents are joining hands for the innovative tie-ups with both the pharmaceutical giants and the medical start-ups to repurpose drugs, develop vaccines, and devices to impede the progress of this overwhelming pandemic. A large number of COVID-19 vaccine candidates based upon various platforms have already been identified. Despite the undergoing efforts, a definitive answer does not exist. The process of vaccine development is quite laborious with various stages, including the pre-clinical stage, and clinical development which is a three-phase process. However, if sufficient data is already available, it has been recommended to skip a few stages, to accelerate the attainment of a vaccine faster with a quick regulatory review, approval, manufacturing, and quality control. This novel Coronavirus has therefore forced the scientific community to use unconventional approaches to accelerate the process of vaccine development. According to WHO: “vaccine must provide a highly favorable benefit-risk contour; with high efficacy, only mild or transient adverse effects and no serious ailments.” The vaccine must be suitable for all ages, pregnant, and lactating women and should provide a rapid onset of protection with a single dose and confer safety for at least up to one year of administration.
The use of novel technologies for vaccine development requires extensive testing for the safety and efficacy of a vaccine. The scientific community needs to construct various processes and capacities for the largescale manufacturing and administration of the coronavirus vaccines. The Coalition for Epidemic Preparedness Innovation (CEPI), an international non-governmental organization, which is funded by the Wellcome Trust, the European Commission, the Bill and Melinda Gates Foundation, and eight countries, is subsidizing the development of a large number of pandemic vaccine candidates around the globe. Moderna and the Vaccine Research Centre are co-developing an mRNA based vaccine candidate, wherein the mRNA is encapsulated in the lipid nanoparticles while Codagenix in collaboration with the Serum Institute of India is currently focused on developing the live attenuated viral vaccine. The pharmaceutical giants like Novavax, Sichuan Clover Biopharmaceuticals, iBio, and the University of Queensland are in the preclinical stage of the recombinant S glycoprotein vaccines. Additional strategies like the viral vector-based vaccines, targeting the S glycoprotein are being developed by the University of Oxford and CanSino Biologics, and other companies, Inovio and the Applied DNA Sciences are currently developing the DNA based vaccine candidates against the SARS-CoV-2 S Protein. Some of these vaccine candidates are at least months, away from being ready for human use, while others may take longer if at all approved for final use.
In India alone, six biotech ventures i.e. Serum Institute of India, ZydusCadila, Biological E, Indian Immunologicals, Bharat Biotech, and Mynvax are working in collaboration with various international vaccine developers. They are working on DNA vaccines, live attenuated recombinant measles vaccines, inactivated viral vaccines, subunit vaccines, and the vaccines developed by codon-optimization ( Coronavirus, 2020 ). Furthermore, the academic institutes like National Institute of Immunology (NII), Indian Institute of Science (IISc), International Center for Genetic Engineering and Biotechnology (ICGEB) New Delhi, Translational Health Science and Technology Institute (THSTI), etc. are attempting to develop the vaccines, and therapies, and the SARS-CoV-2 animal models to restrain the pandemic shortly ( Nandi, 2020 ).
The need of the hour is to develop a safe and effective COVID-19 vaccine which can induce an appropriate immune response to terminate this pandemic. It is the universal priority to spot the international funding mechanisms to support the development, manufacturing, and stockpiling of the coronavirus vaccines. This pandemic should serve as the guidepost to the international research community to not only acknowledge the outbreak but also indurate the following coronavirus crossing into mammals. A pan-coronavirus vaccine is urgently needed as the delay of vaccine rollout even by one week will accompany millions of deaths. Furthermore, it appears to be a scientifically feasible task if sufficient resources are made available in due time.
Funding Information
This work received no specific grant from any funding agency.
Declaration of Competing Interest
The author(s) declare that there are no conflicts of interest.
- Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., Jeong S.J., Kim J.H., Ku N.S., Yeom J.S., Roh J., Ahn M.Y., Chin B.S., Kim Y.S., Lee H., Yong D., Kim H.O., Kim S., Choi J.Y. Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea. J Korean Med Sci. 2020; 35 (14, April) doi: 10.3346/jkms.2020.35.e149. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahn J.Y., Sohn Y., Lee S.H., et al. Use of convalescent plasma therapy in two covid‐19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020 doi: 10.3346/jkms.2020.35.e149. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anon . 2020. Countries where COVID-19 has spread. www.worldometers.info. [Online] July 30, 2020. [Cited: July 31, 2020.] https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/ . [ Google Scholar ]
- Anon . 2020. Coronavirus Resource Center. https://coronavirus.jhu.edu/. [Online] Johns Hopkins University. [Cited: August 05, 2020.] https://coronavirus.jhu.edu/map.html . [ Google Scholar ]
- Anon . 2020. COVID-19 Treatment and Vaccine Tracker. https://airtable.com/shrSAi6t5WFwqo3GM/tblEzPQS5fnc0FHYR/viweyymxOAtNvo7yH?blocks=bipZFzhJ7wHPv7x9z https://airtable.com/. [Online] Milken Institute. [ Google Scholar ]
- Anon . 2020. A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Coronavirus Pneumonia vaccine (Vero cells) Registration no.: ChiCTR2000031809. http://www.chictr.org.cn/showprojen.aspx?proj=52227 http://www.chictr.org.cn. [Online] [ Google Scholar ]
- Anon . 2020. UW–Madison, FluGen, Bharat Biotech to develop CoroFlu, a coronavirus vaccine. https://www.businesswire.com/news/home/20200402005666/en/UW%E2%80%93Madison-FluGen-Bharat-Biotech-develop-CoroFlu-coronavirus https://www.businesswire.com. [Online] April 02. [ Google Scholar ]
- Anon . 2020. A Study of a Candidate COVID-19 Vaccine (COV001)https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04324606","term_id":"NCT04324606"}} NCT04324606 ?term=vaccine&cond=covid-19&draw=2 https://clinicaltrials.gov. [Online]. [Cited: June 8, 2020.] [ Google Scholar ]
- Anon . 2020. Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19)https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04283461","term_id":"NCT04283461"}} NCT04283461 ?term=vaccine&cond=covid-19&draw=2 https://clinicaltrials.gov/. [Online] [ Google Scholar ]
- Anon . 2020. Moderna Announces Positive Interim Phase 1 Data for its mRNA Vaccine (mRNA-1273) Against Novel Coronavirus. https://investors.modernatx.com/. [Online] Moderna, Inc., May 18, 2020. [Cited: June 15, 2020.] https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-positive-interim-phase-1-data-its-mrna-vaccine. [ Google Scholar ]
- Anon . 2020. Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04336410","term_id":"NCT04336410"}} NCT04336410 ?term=inovio&cond=covid-19&draw=2&rank=1 https://clinicaltrials.gov/. [Online] 2020. 1. [ Google Scholar ]
- Anon . The University of Hong Kong; 2020. HKU joins global partnership to develop COVID-19 vaccine. https://fightcovid19.hku.hk/hku-state-key-laboratory-for-emerging-infectious-diseases-joins-global-effort-to-develop-covid-19-vaccine/ https://fightcovid19.hku.hk/. [Online], March 18, 2020. [ Google Scholar ]
- Anon . 2020. (BAT), British and American Tobacco Company. Potential COVID-19 vaccine – BAT in the news. https://www.bat.com/group/sites/UK__9D9KCY.nsf/vwPagesWebLive/DOBNHBWR https://www.bat.com/. [Online] 2020. [Cited: June 1, 2020.] [ Google Scholar ]
- Anon . 2020. Immunity and Safety of Covid-19 Synthetic Minigene Vaccine.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04276896","term_id":"NCT04276896"}} NCT04276896 https://clinicaltrials.gov. [Online] [ Google Scholar ]
- Anon . 2020. Safety and Immunity of Covid-19 aAPC Vaccine.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04299724","term_id":"NCT04299724"}} NCT04299724 https://clinicaltrials.gov/. [Online] [ Google Scholar ]
- Anon . 2020. Sinovac gets regulatory approval to assess Covid-19 vaccine. https://www.clinicaltrialsarena.com/news/sinovac-covid-19-vaccine-trial-approval/ https://www.clinicaltrialsarena.com. [Online] April 15. [ Google Scholar ]
- Anon . 2020. Sinovac reports positive data from Phase I/II trials of CoronaVac. https://www.clinicaltrialsarena.com/news/sinovac-coronavac-data/ https://www.clinicaltrialsarena.com/. [Online] June 15, 2020. [Cited: June 20, 2020.] [ Google Scholar ]
- Anon . 2020. An Open Study of the Safety, Tolerability and Immunogenicity of the Drug "Gam-COVID-Vac" Vaccine Against COVID-19.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04436471","term_id":"NCT04436471"}} NCT04436471 ?term=vaccine&cond=covid-19&draw=4 https://clinicaltrials.gov/. [Online] June 22, 2020. [Cited: June 22, 2020.]. NCT04436471. [ Google Scholar ]
- Anon . 2020. An Open Study of the Safety, Tolerability and Immunogenicity of "Gam-COVID-Vac Lyo" Vaccine Against COVID-19.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04437875","term_id":"NCT04437875"}} NCT04437875 https://clinicaltrials.gov/. [Online] June 22, 2020. [Cited: June 22, 2020.]. NCT04437875. [ Google Scholar ]
- Anon . 2020. Vaxart Announces Positive Pre-Clinical Data for its Oral COVID-19 Vaccine Program. https://investors.vaxart.com/. [Online] Vaxart Inc., April 21, https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-positive-pre-clinical-data-its-oral-covid-19. [ Google Scholar ]
- Anon . 2020. Zydus Cadila looks to expedite Covid-19 vaccine development. https://www.pharmaceutical-technology.com/news/zydus-cadila-covid-19-vaccine/ https://www.pharmaceutical-technology.com. [Online] 17 February, [ Google Scholar ]
- Anon . 2020. Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines https://www.who.int/. [Online] June 22, 2020. [Cited: June 23, 2020.] [ Google Scholar ]
- Anon . 2020. Clinical trial to assess the safety of a coronavirus vaccine in healthy men and women. http://www.isrctn.com/ISRCTN17072692 http://www.isrctn.com/ [Online] June 17, 2020. [Cited: June 22, 2020.]. ISRCTN17072692. [ Google Scholar ]
- Anon . 2020. A Trial Investigating the Safety and Effects of Four BNT162 Vaccines Against COVID-2019 in Healthy Adults.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04380701","term_id":"NCT04380701"}} NCT04380701 https://clinicaltrials.gov/. [Online] May 8. [ Google Scholar ]
- Anon . BIOCAD Biotechnology Company; 2020. BIOCAD started working on mRNA vaccine against coronavirus. https://biocadglobal.com/index.php?posts&post=45 https://biocadglobal.com/. [Online], March 19. [ Google Scholar ]
- Anon . 2020. Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant.https://clinicaltrials.gov/ct2/show/record/ {"type":"clinical-trial","attrs":{"text":"NCT04368988","term_id":"NCT04368988"}} NCT04368988 https://clinicaltrials.gov/. [Online] May 27, 2020. [Cited: June 15, 2020.] [ Google Scholar ]
- Anon . 2020. Sanofi joins forces with U.S. Department of Health and Human Services to advance a novel coronavirus vaccine. http://www.news.sanofi.us/2020-02-18-Sanofi-joins-forces-with-U-S-Department-of-Health-and-Human-Services-to-advance-a-novel-coronavirus-vaccine http://www.news.sanofi.us/. [Online] Sanofi U.S., February 18, 2020. [ Google Scholar ]
- Anon . Medigaco Inc.; 2020. COVID-19 Vaccine Development Program. https://www.medicago.com/en/covid-19-programs/ https://www.medicago.com/. [Online] [ Google Scholar ]
- 2020. A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Coronavirus Pneumonia vaccine (Vero cells) http://www.chictr.org.cn/showprojen.aspx?proj=52227 http://www.chictr.org.cn. [Online] [ Google Scholar ]
- Anon . Sinovac Biotech Limited; 2020. Sinovac COVID-19 Vaccine Collaboration with Butantan Receives Approval from Brazilian Regulator for Phase III Trial. http://www.sinovac.com/?optionid=754&auto_id=907 http://www.sinovac.com/. [Online]July 06, 2020. [Cited: August 01, 2020.] [ Google Scholar ]
- Anon Treatment With Convalescent Plasma for Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Chest. 2020;(March) doi: 10.1016/j.chest.2020.03.039. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anon . 2020. COVID-19 / SARS-CoV-2. http://www.animalresearch.info/en/medical-advances/diseases-research/sars-cov-2/ http://www.animalresearch.info/. [Online] April 30, 2020. [Cited: June 11, 2020.] [ Google Scholar ]
- Arora Kajal, Rastogi Ruchir, et al. Multi-Antigenic Virus-like Particle of SARS CoV-2 produced in Saccharomyces cerevisiae as a vaccine candidate. Gurugram : s.n. bioRxiv. 2020;(May 19) doi: 10.1101/2020.05.18.099234. [ CrossRef ] [ Google Scholar ]
- Baruah V., Bose S. Immunoinformatics‐aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019‐nCoV. J Med Virol. 2020:495–500. doi: 10.1002/jmv.25698. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Breedveld F.C. Therapeutic monoclonal antibodies. The Lancet. 2000:735–740. doi: 10.1016/S0140-6736(00)01034-5. PMID 10703815. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- BROOK, STONY . 2020. Applied DNA Sciences Subsidiary, LineaRx, and Takis Biotech Collaborate for Development of a Linear DNA Vaccine Candidate Against Wuhan Coronavirus 2019-nCoV. https://adnas.com/coronoavirus-applied-dna-linearx-takis-biotech-vaccine/ https://adnas.com/. [Online] Applied DNA Sciences, February 07. [ Google Scholar ]
- Campbell Molly. 2020. Current Efforts in COVID-19 Vaccine Development. https://www.technologynetworks.com/biopharma/articles/current-efforts-in-covid-19-vaccine-development-332429 https://www.technologynetworks.com/. [Online] Technology Networks, March 23. [ Google Scholar ]
- Cao Y., Zhu X., Hossen M.N., et al. Augmentation of vaccine-induced humoral and cellular immunity by a physical radiofrequency adjuvant. Nat Commun. 2018 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carter John, Saunders Venitia. Wiley; Hoboken, New Jersey: 2007. Virology: Principles and Applications; p. 382. ISBN: (Paperback) 9780470023877 [ Google Scholar ]
- (CDC), Centers for Disease Control and Prevention . CDC; 2020. How COVID-19 Spreads. [Online] 2020. [Cited: June 1, 2020.] https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html? CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fprepare%2Ftransmission.html. [ Google Scholar ]
- CDC Coronavirus Disease 2019 (COVID-19)- Symptoms of Covid-19. Centres for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html [Online]. [ Google Scholar ]
- Coleman Christopher M., Liu Ye V., Mu Haiyan, Taylor Justin K., Massare Michael, Flyer David C., Glenn Gregory M., Smith Gale E., Frieman Matthew B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2020:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Conserved Protein Domain Family: SARS-like-CoV_M . 2020. NCBI. https://www.ncbi.nlm.nih.gov/Structure/cdd/cd21569 [Online]. [Cited: June 1, 2020.] [ Google Scholar ]
- Coronavirus . 2020. Around 30 Indian attempts at COVID-19 vaccine, says Principal Scientific Adviser. https://www.thehindu.com/ [Online] The Hindu, May 11, 2020. [Cited: June 11, 2020.] thehindu.com/sci-tech/health/coronavirus-around-30-indian-attempts-at-covid-19-vaccine-says-principal-scientific-adviser/article31560932.ece. [ Google Scholar ]
- CTRI/2020/07/026352. 2020 http://ctri.nic.in/. [Online] July 31, 2020. [Cited: August 01, 2020.] http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45306&EncHid=&userName=Zydus.
- Cuiling Zhang, Giulietta Maruggi, Hu Shan, Junwei Li. Advances in mRNA Vaccines for Infectious Diseases. Frontiers in Immunology. 2020:594. DOI=10.3389/fimmu.2019.00594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- da Silva S., da Silva C., Mendes R., Pena L. Role of Nonstructural Proteins in the Pathogenesis of SARS-CoV-2. Journal of medical virology. 2020 doi: 10.1002/jmv.25858. 10.1002/jmv.25858 p. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Delamater P.L., Street E.J., Leslie T.F., Yang Y., Jacobsen K.H. Complexity of the Basic Reproduction Number (R0) s.l. : Emerging Infectious Diseases. 2019; 25 doi: 10.3201/eid2501.171901. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020 doi: 10.1080/21645515.2020.1735227. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doremalen Neeltje van, Lambe Teresa, Spencer Alexandra, Belij-Rammerstorfer Sandra, Purushotham Jyothi N., Port Julia R., Avanzato Victoria, Bushmaker Trenton, Flaxman Amy, Ulaszewska Marta, Feldmann Friederike, Allen Elizabeth R., Sharpe Hannah. Jonathan ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. s.l. : bioRxiv. 2020 doi: 10.1101/2020.05.13.093195. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dorp Lucy van, Acman Mislav, Richard Damien, Shaw Liam P., Ford Charlotte E., Ormond Louise, Owen Christopher J., Pang Juanita, Tan Cedric C.S., Boshier Florencia A.T., Ortiz Arturo Torres, Balloux François. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. s.l. : Infection, Genetics and Evolution. 2020; 104351 doi: 10.1016/j.meegid.2020.104351. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eyal N., Lipsitch M., Smith P.G. Human Challenge Studies to Accelerate Coronavirus Vaccine Licensure. s.l. : The Journal of infectious diseases. 2020:1752–1756. doi: 10.1093/infdis/jiaa152. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Folegatti Pedro M, Ewer Katie J, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet. 2020;(July) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Garg P., Srivastava N., Srivastava P. An Integrated In-Silico Approach to Develop Epitope-Based Peptide Vaccine against SARS-CoV-2. s.l. : Preprints. 2020 doi: 10.20944/preprints202005.0401.v1). [ CrossRef ] [ Google Scholar ]
- Gelboin Harry V, et al. Inhibitory monoclonal antibodies to human cytochrome P450 enzymes: a new avenue for drug discovery. Trends in Pharmacological Sciences. 1999:432–438. doi: 10.1016/S01. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gralinski L.E., Menachery V.D. Return of the Coronavirus: 2019-nCoV. Viruses. 2020:135. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;(May 29):945–946. [ PubMed ] [ Google Scholar ]
- Gupta V., Tabiin T.M., Sun K., Chandrashekaran A., Anwar A., Yang K., Chikhlikar P., Salmon J., Brusic V., Marques E.T.A., Kellathur S.N., August T.J. SARS coronavirus nucleocapsid immunodominant T-cell epitope cluster is common to both exogenous recombinant and endogenous DNA-encoded immunogens. Virology. 2006; 347 (1):127–139. (impact factor: 2.657) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heat Biologics’ COVID-19 Vaccine Program . 2020. Heat Biologics. https://www.heatbio.com/product-pipeline/covid-19-vaccine https://www.heatbio.com/. [Online] [ Google Scholar ]
- Hobernik D., Bros M. DNA Vaccines-How Far From Clinical Use? Int J Mol Sci. 2018:3605. doi: 10.3390/ijms19113605. PMID: 30445702; PMCID: PMC6274812. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoffmann Markus, Kleine-Weber Hannah, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020:271–280. doi: 10.1016/j.cell.2020.02.052. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Imai Masaki, Iwatsuki-Horimoto Kiyoko, et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proceedings of the National Academy of Sciences. 2020;(May):16587–16595. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jackson Lisa A., Anderson, Evan J., et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N Engl J Med. 2020;(July) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jiang S., Du L., Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerging microbes & infections. 2020:275–277. doi: 10.1080/22221751.2020.1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Johnson & Johnson Announces a Lead Vaccine Candidate for COVID-19 . 2020. Landmark New Partnership with U.S. Department of Health & Human Services; and Commitment to Supply One Billion Vaccines Worldwide for Emergency Pandemic Use. https://www.prnewswire.com/news-releases/johnson--johnson-announces-a-lead-vaccine-candidate-for-covid-19-landmark-new-partnership-with-us-department-of-health--human-services-and-commitment-to-supply-one-billion-vaccines-worldwide-for-emergency-pandemic- https://www.prnewswire.com/. [Online] [ Google Scholar ]
- Kai Duan, Liu Bende, Li Cesheng, Zhang Huajun, Yu Ting, Qu Jieming, Zhou Min, Chen Li, Meng Shengli, Hu Yong, Peng Cheng, Yuan Mingchao, Huang Jinyan, Wang Zejun, Yu Jianhong, Gao Xiaoxiao, Wang Dan, Yu Xiaoqi, Li Li., Zhang Jiayou, Wu Xiao, Li Bei, Yanpin Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proceedings of the National Academy of Sciences. 2020;(April) doi: 10.1073/pnas.2004168117. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim E., et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102743. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lauer S.A., Grantz K.H., Bi Q., et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020:577–582. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Le Tung Thanh, Andreadakis Zacharias, Kumar Arun, Román Raúl Gómez, Tollefsen Stig, Saville Melanie, Mayhew Stephen. The COVID-19 vaccine development landscape. Nature Reviews Drug Discovery. 2020; 19 (5):305–306. [ PubMed ] [ Google Scholar ]
- Lee Jaimy. These 23 companies are working on coronavirus treatments or vaccines — here’s where things stand. Market watch. 2020 https://www.marketwatch.com/story/these-nine-companies-are-working-on-coronavirus-treatments-or-vaccines-heres-where-things-stand-2020-03-06 [Online] May 6, 2020. [Cited: June 1, 2020.] [ Google Scholar ]
- Liu Cynthia, Zhou Qiongqiong, Li Yingzhu, Garner Linda V., Watkins Steve P., Carter Linda J., Smoot Jeffrey, Gregg Anne C., Daniels Angela D., Jervey Susan, Albaiu Dana. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Central Science. 2020:315–331. doi: 10.1021/acscentsci.0c00272. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Masihi K Noel. Fighting infection using immunomodulatory agents. Expert Opinion on Biological Therapy. 2001:641–653. doi: 10.1517/14712598.1.4.641. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mice L., Bao, et al. The Pathogenicity of 2019 Novel Coronavirus in hACE2 Transgenic. s.l. : bioRxiv. 2020 doi: 10.1101/2020.02.07.939389. preprint. [ CrossRef ] [ Google Scholar ]
- Michot Jean-Marie, Albiges Laurence, Chaput Nathalie, Saada Veronique, Fanny Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Annals of Oncology. 2020 doi: 10.1016/j.annonc.2020.03.300. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mulligan Mark J., Lyke Kirsten E., et al. Phase 1/2 Study to Describe the Safety and Immunogenicity of a COVID-19 RNA Vaccine Candidate (BNT162b1) in Adults 18 to 55 Years of Age: Interim Report; preprint. s.l. : medRxiv. 2020 06.30.20142570, 2020, medRxiv. [ Google Scholar ]
- Myupchar . 2020. Race for COVID-19 vaccine: Covaxin and ZyCoV-D begin human trials in India, Moderna publishes preliminary data from phase 1. https://www.firstpost.com/health/race-for-covid-19-vaccine-covaxin-and-zycov-d-begin-human-trials-in-india-moderna-publishes-preliminary-data-from-phase-1-8600211.html/amp https://www.firstpost.com/. [Online] July 15, 2020. [Cited: August 01, 2020.] [ Google Scholar ]
- Nandi Jayashree. 2020. Top Indian scientists join global fight against coronavirus. https://www.hindustantimes.com/india-news/top-indian-scientists-join-global-fight-against-virus/story-CKRGfycsjBJD2ypLcbJPHM.html https://www.hindustantimes.com. [Online] Hindustan Times, New Delhi, March 30, 2020. [Cited: June 9, 2020.] [ Google Scholar ]
- Ning Wang, Jian Shang, Shibo Jiang, Lanying Du. Subunit Vaccines Against Emerging Pathogenic Human Coronaviruses. Frontiers in Microbiology. 2020 doi: 10.3389/fmicb.2020.00298. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Novavax covid 19 vaccine trial . 2020. Clinical Trials Arena. https://www.clinicaltrialsarena.com/news/novavax-covid-19-vaccine-trial/ [Online] [ Google Scholar ]
- Ou X., Liu Y., Lei X., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020; 1620 :11. doi: 10.1038/s41467-020-15562-9. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Park Su Eun. Epidemiology, virology, and clinical features of severe acute respiratory syndrome -coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19) Clin Exp Pediatr. 2020:119–124. doi: 10.3345/cep.2020.00493. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pharmaceutical Targeting the Envelope Protein of SARS-CoV-2: the Screening for Inhibitors in Approved Drugs . XR Pharmaceuticals Ltd.; Cambridge, New Zealand: 2020. Chernyshev, Anatoly. [ Google Scholar ]
- PIB, Delhi . 2020. DBT/ Anti-COVID consortium- Efforts underway to produce therapeutic antibodies against COVID-19: Isolating genes encoding antibodies for neutralising the SARS-CoV-2, COVID-19. https://pib.gov.in/. [Online] April 12, 2020. [Cited: June 07, 2020.] https://pib.gov.in/PressReleaseIframePage.aspx? PRID=1613531. [ Google Scholar ]
- Prof Roujian Lu, Zhao Xiang, Li Juan, Niu Peihua, Bo Yang, Honglong Wu, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020:565–574. doi: 10.1016/S0140-6736(20)30251-8. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pyrek Kelly M. 2018. 100 Years after the Spanish Flu: Lessons Learned and Challenges for the Future. https://www.infectioncontroltoday.com/ [Online] October 11, 2018. https://www.infectioncontroltoday.com/public-health/100-years-after-spanish-flu-lessons-learned-and-challenges-future. [ Google Scholar ]
- Shang W., Yang Y., Rao Y., et al. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 2020 doi: 10.1038/s41541-020-0170-0. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shen Chenguang, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020; 323 (16):1582–1589. doi: 10.1001/jama.2020.4783. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shen C., Wang Z., Zhao F., et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020; 323 (16):1582–1589. doi: 10.1001/jama.2020.4783. 2020. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smith T.R.F., Patel A., Ramos S., et al. Immunogenicity of a DNA vaccine candidate for COVID-19. 2601. s.l. : Nat Commun. 2020; 11 doi: 10.1038/s41467-020-16505-0. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tai W., He L., Zhang X., et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020 doi: 10.1038/s41. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- TNX-1800 (Coronavirus Vaccine) 2020. Online] Tonix Pharmaceuticals. https://www.tonixpharma.com/pipeline/tnx-1800-coronavirus-vaccine https://www.tonixpharma.com. [ Google Scholar ]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific. Emerg Microbes Infect. 2020:382–385. doi: 10.1080/22221751.2020.1729069. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tu Y.-F., Chien C.-S., Yarmishyn A.A., Lin Y.-Y., Luo Y.-H., Lin Y.-Y., Lai W.-Y., Yang D.-M., Chou S.-J., Yang Y.-P., Wang M.-L., Chiou S.-H. A Review of SARS-CoV-2 and the Ongoing Clinical Trials. International Journal of Molecular Sciences. 2020:2657. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Upadhyay Lipi. 2020. Italy claims to have developed the first COVID-19 vaccine: Here is what we know about all the potential coronavirus vaccines. https://timesofindia.indiatimes.com/life-style/health-fitness/health-news/italy-claims-to-develop-first-covid-19-vaccine-here-is-the-current-status-of-all-the-potential-coronavirus-vaccines/photostory/75575319.cms https://timesofindia.indiatimes.com/. [Online] May 8, 2020. [Cited: May 31, 2020.] [ Google Scholar ]
- Ura T., Okuda K., Shimada M. Developments in Viral Vector-Based Vaccines. Vaccines (Basel) 2014:624–641. doi: 10.3390/vaccines2030624. PMID: 26344749; PMCID: PMC4494222. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Voltron Therapeutics, Inc . 2020. Enters into Sponsored Research Agreement with The Vaccine & Immunotherapy Center at the Massachusetts General Hospital to Develop Potential COVID-19 Vaccine. https://www.prnewswire.com/news-releases/voltron-therapeutics-inc-enters-into-sponsored-research-agreement-with-the-vaccine--immunotherapy-center-at-the-massachusetts-general-hospital-to-develop-potential-covid-19-vaccine-301034225.html https://www.prnewswire.com/. [Online] April 02, 2020. [ Google Scholar ]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Vessler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020:281–292. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Walls Structure, Function, and Antigenicity of the SARSCoV-2 Spike Glycoprotein. Cell. 2020:281–292. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020 doi: 10.1128/JVI.00127-20. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- (WHO), World Health Organisation . s.l. : World Health Organisation; 2014. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease. September [ Google Scholar ]
- (WHO) World Health Organisation . s.l.: World Health Organisation; 2020. Coronavirus disease (Covid-19) Situation Report- 164. [ Google Scholar ]
- WHO . s.l. : World Health Organisation; 2020. Draft landscape of COVID-19 candidate vaccines. [ Google Scholar ]
- Wrapp Daniel, Wang Nianshuang, Corbett Kizzmekia S., Goldsmith Jory A., Hsieh Ching-Lin, Abiona Olubukola, Graham Barney S., Jason S. Mclellan Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;(13 March):1260–1263. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., et al. Commentary genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020:325–328. doi: 10.1016/j.chom.2020.02.001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yan, Xuebing. 2020 http://www.chictr.org.cn/showprojen.aspx?proj=49544. http://www.chictr.org.cn. [Online] February 2020.
- Yip M.S., Leung H.L., Li P.H., Cheung C.Y., Dutry I., Li D., Daëron M., Bruzzone R., Peiris J.S.M., Jaume M. Hong Kong Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS: s.n. Hong Kong medical journal = Xianggang yi xue za zhi / Hong Kong Academy of Medicine. 2016:25–31. [ PubMed ] [ Google Scholar ]
- Yoshimoto F.K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. The protein journal. 2020:198–216. doi: 10.1007/s10930-020-09901-4. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. International Journal of Antimicrobial Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105955. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang B., Liu S., Tan T., et al. Treatment with convalescent plasma for critically ill patients with SARS‐CoV‐2 infection. Chest. 2020; 2020 :30571–30577. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhu Feng-Cai, Li Yu-Hua, Guan Xu-Hua, Hou Li-Hua, Wang Wen-Juan, Li Jing-Xin, Wu Shi-Po, Wang Bu-Sen, Wang Zhao, Wang Lei, Jia Si-Yue, Jiang Hu-Dachuan, Wang Ling, Jiang Tao, Hu Yi, Gou Jin-Bo, Xu Sha-Bei, Xu Jun-Jie, Wang Xue-Wen, Wang Wei, Chen Wei. Safety, tolerability, and immunogenicity of a recombinan tadenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet. 2020 doi: 10.1016/S0140-6736(20)31208-3. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhu Feng-Cai, Guan Xu-Hua, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet. 2020;(July) [ PMC free article ] [ PubMed ] [ Google Scholar ]
Loading metrics
Open Access
Peer-reviewed
Research Article
COVID-19 vaccine brand hesitancy and other challenges to vaccination in the Philippines
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation School of Medicine and Public Health, Ateneo de Manila University, Manila, Philippines
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing
Affiliations School of Medicine and Public Health, Ateneo de Manila University, Manila, Philippines, The Medical City, Manila, Philippines
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing – review & editing
- Arianna Maever L. Amit,
- Veincent Christian F. Pepito,
- Lourdes Sumpaico-Tanchanco,
- Manuel M. Dayrit

- Published: January 13, 2022
- https://doi.org/10.1371/journal.pgph.0000165
- See the preprint
- Peer Review
- Reader Comments
Effective and safe COVID-19 vaccines have been developed at a rapid and unprecedented pace to control the spread of the virus, and prevent hospitalisations and deaths. However, COVID-19 vaccine uptake is challenged by vaccine hesitancy and anti-vaccination sentiments, a global shortage of vaccine supply, and inequitable vaccine distribution especially among low- and middle-income countries including the Philippines. In this paper, we explored vaccination narratives and challenges experienced and observed by Filipinos during the early vaccination period. We interviewed 35 individuals from a subsample of 1,599 survey respondents 18 years and older in the Philippines. The interviews were conducted in Filipino, Cebuano, and/or English via online platforms such as Zoom or via phone call. All interviews were recorded, transcribed verbatim, translated, and analysed using inductive content analysis. To highlight the complex reasons for delaying and/or refusing COVID-19 vaccines, we embedded our findings within the social ecological model. Our analysis showed that individual perceptions play a major role in the decision to vaccinate. Such perceptions are shaped by exposure to (mis)information amplified by the media, the community, and the health system. Social networks may either positively or negatively impact vaccination uptake, depending on their views on vaccines. Political issues contribute to vaccine brand hesitancy, resulting in vaccination delays and refusals. Perceptions about the inefficiency and inflexibility of the system also create additional barriers to the vaccine rollout in the country, especially among vulnerable and marginalised groups. Recognising and addressing concerns at all levels are needed to improve COVID-19 vaccination uptake and reach. Strengthening health literacy is a critical tool to combat misinformation that undermines vaccine confidence. Vaccination systems must also consider the needs of marginalised and vulnerable groups to ensure their access to vaccines. In all these efforts to improve vaccine uptake, governments will need to engage with communities to ‘co-create’ solutions.
Citation: Amit AML, Pepito VCF, Sumpaico-Tanchanco L, Dayrit MM (2022) COVID-19 vaccine brand hesitancy and other challenges to vaccination in the Philippines. PLOS Glob Public Health 2(1): e0000165. https://doi.org/10.1371/journal.pgph.0000165
Editor: Dione Benjumea-Bedoya, Corporacion Universitaria Remington, COLOMBIA
Received: October 27, 2021; Accepted: December 22, 2021; Published: January 13, 2022
Copyright: © 2022 Amit et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data relevant to the study are included in the article.
Funding: AMLA/VCFP/LST/MMD are funded by the Ateneo de Manila University Research Council COVID-19 Research Grant (Grant No. COVID-URC 01 2021). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: We have read the journal’s policy and the authors of this manuscript have the following competing interests: VCFP owns shares of GMA Network, Inc., a Philippine Stock Exchange-listed company with interests in mass media. AMLA, VCFP, and MMD receive funding from Sanofi to conduct research on self-care.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic continues to burden health systems and communities globally, with millions of cases and deaths [ 1 ]. Because of the significant and continued impact of COVID-19, vaccines have been developed at a rapid and unprecedented pace to control the spread of the virus, and prevent hospitalisations and deaths [ 2 ]. Many vaccines have been shown to be safe and effective with high-income countries having vaccinated more than half of their population [ 3 ]. Despite the availability of these vaccines, countries are faced with various challenges including vaccine hesitancy and anti-vaccination sentiments, limited global supply, and inefficient vaccine deployment [ 4 , 5 ]. These issues in vaccine uptake, together with declining community acceptance of other public health interventions, will mean a delayed recovery and prolonged pandemic [ 6 ].
The World Health Organization (WHO) in 2019 identified vaccine hesitancy or the reluctance to vaccinate as one of the top ten threats to global health despite evidence of the important role of vaccines in improving population health outcomes [ 7 ]. Together with weak primary health care and other health challenges, countries especially low- and middle-income countries (LMICs) will struggle to meet the demands of the communities within their health system capacity. With the pandemic, countries are further burdened with many health systems overwhelmed throughout its course. The Philippines presently faces these challenges: vaccine hesitancy and increasing anti-vaccination sentiments, a weak primary health care system with efforts to strengthen it through the recently implemented Universal Health Care Law, and an overwhelmed health system because of the demands of COVID-19 and other public health problems [ 8 – 13 ]. These challenges are further compounded by a global shortage of vaccine supply with inequitable vaccine distributions [ 14 ].
Historically, the Philippines was one of the countries with generally high vaccine confidence rates [ 15 ]. Following the dengue vaccine controversy in 2017 however, confidence levels have dramatically dropped and have impacted succeeding vaccination efforts including the COVID-19 vaccination campaign [ 9 , 12 , 15 – 17 ]. Dengvaxia, the world’s first commercially available dengue vaccine developed by Sanofi Pasteur, was introduced as part of a national school-based immunization programme despite the lack of empirical data on the risks associated with administration of the vaccine among those not previously infected with dengue or seronegative children [ 9 , 12 , 15 – 17 ]. By the time reports were released that the vaccine may cause more severe disease among seronegatives, the Philippines had already inoculated more than 800,000 Filipino school-age children [ 9 ]. This was highly politicised, and damaged trust in vaccines and the health sector [ 9 , 12 , 15 – 17 ]. As a result, immunisation rates dropped and the country saw outbreaks of previously controlled vaccine-preventable diseases such as measles and polio [ 18 , 19 ]. In addition to vaccine hesitancy, the Philippine health system is not prepared for additional health care demands. As early as the first phase of the pandemic, critical care capacity was overwhelmed with the influx of patients in hospitals [ 10 , 11 ]. As of 16 September 2021, the Philippines ranks third among countries with the highest number of newly confirmed cases per one million population [ 1 , 20 ]. Globally, 42.9% of the world population have received one dose of a COVID-19 vaccine, with much lower rates in LMICs like the Philippines [ 20 , 21 ]. Only 55% of Filipinos have expressed willingness to be vaccinated against COVID-19, and as of 16 September 2021, only 30% of the population have been fully vaccinated[ 21 , 22 ].
To end this pandemic, it is critical to implement all possible public health interventions and strategies from face masks, physical distancing, to getting vaccinated [ 4 , 23 ]. However, there is a need to recognise that the adoption of all these interventions is influenced by individual risk perceptions, and these perceptions are shaped by various sources of information and experiences [ 24 ]. Additionally, there are interpersonal and structural factors that influence health decisions of individuals. Recognising the multiple dimensions in which behaviours and decisions occur, theories and models have been proposed to explain how individuals make decisions on their health based on factors that change over time and context [ 25 , 26 ]. The social ecological model provides a useful framework for investigating health behaviours and decisions by recognising that a multiplicity of factors interacts to influence health of individuals [ 26 ]. These include individual factors representing biological or behavioural characteristics, interpersonal factors representing networks and social capital operating within a defined boundary, and structural factors that include health systems and are mediated through laws and policies [ 26 ]. Published studies on vaccination that utilised this model reported that vaccine intentions and attitudes operate along multiple dimensions, with a series of events influencing decisions related to vaccination [ 17 , 27 , 28 ]. Improving adherence to interventions and vaccination rates therefore requires a better understanding of the different reasons behind vaccine mistrust and not just determining their individual beliefs, knowledge, and levels of trust [ 17 , 27 , 28 ]. A recently published scoping review supports the use of the social ecological model in understanding attitudes towards COVID-19 vaccination [ 29 ]. The review showed that influencing factors are embedded within the social ecological model and that multilevel interventions are needed to improve uptake of vaccines [ 29 ]. This scoping review of 50 articles had representation from various countries, but did not include data from the Philippines. We address this gap by exploring the vaccination narratives and challenges experienced and observed by Filipinos during the early COVID-19 vaccination period. We used qualitative data from a mixed-methods study conducted from June to August 2021 that aimed to understand how people in the Philippines view COVID-19 and what influences their behaviours. With these findings, we hope to provide insights to possible avenues of future research and directions for improving COVID-19 vaccine uptake and reach.
Material and methods
Design and setting.
We conducted an online survey among adults ages 18 and older in the Philippines (n = 1,599) from June to August 2021. A subsample participated in the semi-structured interviews (n = 35) with representation from the general population and health workforce from July to August 2021. Data from the interviews informed the findings of this paper.
Participants and recruitment
We aimed to interview participants from different regions in the Philippines, various age groups, socio-economic classes, and vaccination status and attitudes. This allowed us to ensure maximum variation sampling, which aims to capture as many population contexts as possible. We contacted a total of 115 individuals through the information they provided (i.e., mobile number, phone number, e-mail). Out of the 115, 35 participants completed the interviews. The remaining 80 either refused or could not be contacted after a maximum of three attempts. We classified participants according to their vaccination priority group based on the COVID-19 Vaccination Program’s prioritisation framework [ 30 ]. Those in the first priority group (A1) were frontline workers in health facilities; other priority groups (A2 to C) comprised and represented the general population ( Table 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pgph.0000165.t001
Data collection
We conducted the interviews in Filipino, Cebuano, and/or English via online platforms such as Zoom or via phone call. The interview guide included questions about their views on COVID-19, vaccines, and their risk perceptions and behaviours. We recruited interview participants until saturation was reached (i.e., no new information was being obtained from the interviews) [ 31 ]. The interviews lasted between 60 to 90 minutes with a token amounting to USD 6 provided to each participant. All participants consented to the interview being recorded.
Data analysis
The interviews were digitally recorded, transcribed verbatim, and translated from Filipino or Cebuano to English. The research team are native and/or fluent speakers of the three languages, and checked for linguistic and conceptual equivalence in the translated documents. We de-identified all participants and assigned pseudonyms. We analysed the data using inductive content analysis focusing on the experiences and views towards vaccination [ 32 ]. Our analysis was guided by principles of grounded theory. Transcripts of the interviews were read to identify themes and two investigators (AMLA, VCFP) independently coded the interviews according to emergent themes in Microsoft Excel [ 33 ]. We used coding language that was close to the participants’ terms and phrases to ensure that we were co-constructing accurate categories reflective of their responses [ 34 ]. The codes were reviewed, and areas of disagreement were resolved between the two investigators. Themes from the interviews were further explored through discussions with the other members of the team. We considered reflexivity throughout data collection and analysis, acknowledging that our preconceptions and experiences about vaccination as public health practitioners and health professionals may influence the way we analyse and interpret data. Our use of the grounded theory allowed us to explore the experiences of our participants and our own shared experiences, and avoided being limited by how we view COVID-19 vaccination [ 35 ]. To highlight the complex reasons for delaying and/or refusing COVID-19 vaccination, we embedded our findings within the social ecological model with three broad themes: individual factors (attitudes, beliefs, knowledge, behaviours), interpersonal factors (relationships and social networks), and structural factors (health systems and service delivery; media; and policies, regulations, and laws at the local, national, and global level) [ 26 ] ( Fig 1 ). The quotes presented in this paper are either in the original English or translated from Filipino or Cebuano.
This figure shows the three main tiers of factors influencing vaccination intention and uptake: individual (beliefs, attitudes, knowledge, health literacy), interpersonal (relationships, networks), and structural (health systems and service delivery, media, policies). These three dimensions are jointly or individually impacted by misinformation (white circles).
https://doi.org/10.1371/journal.pgph.0000165.g001
Patients and public involvement
The public were not directly involved in the design, recruitment, conduct, reporting, or dissemination plans of this research. Their only involvement was as research participants.
Ethics statement
This study was approved by the University Research Ethics Office of Ateneo de Manila University (Study No. SMPH CORISK 2021). All participants were informed about the aims and objectives of the study by including the written consent form in the email correspondence. Prior the interview, the research team thoroughly explained the study to them and provided them the opportunity to ask questions they may have. Written digital consent was taken from study participants before the interview.
We interviewed 35 participants with representation from different vaccination priority groups working in various parts of the country. Our participants also had different educational backgrounds, employment status, and vaccination attitude ( Table 2 ). There was an almost equal proportion of females and males (females: 19; males: 16) with a median age of 38 years old (range: 21 to 74 years old) in the overall study population.
https://doi.org/10.1371/journal.pgph.0000165.t002
Participant views on the barriers to COVID-19 vaccination are presented below, organised using the three tiers of the social ecological model. Individual barriers include perceptions; attitudes; and beliefs about the science, about vaccines, about the health system and government. Interpersonal barriers are the networks and social capital that influence health beliefs and decisions. Vaccine procurement, supply, and logistics, together with media- and policy-related issues, comprise the structural barriers. Where there are differences between the general population and health workers, these are highlighted in the text.
Individual barriers
Vaccine brand hesitancy and brand preferences..
Vaccine brand hesitancy or delay in getting the vaccine due to brand preferences was a common theme among the participants. The country’s first administered vaccine was Sinovac-CoronaVac, which is manufactured by a Chinese biopharmaceutical company. This was given to health workers despite lack of published data on effectiveness at the time and initial announcements that these were not recommended for high-risk individuals ( Quote I1, Table 3 ). In addition to concerns about the effectiveness of the vaccine, participants also read and heard information on how this vaccine was made. They believed this specific vaccine was using the same virus to ‘immunise’ an individual’s system, which may have unintended effects ( Quote I2, Table 3 ). Other participants cited that this specific brand was not recognised by other countries, and therefore wanted and waited for other vaccines. Meanwhile, others refused to receive mRNA vaccines due to beliefs about its safety and effectiveness.
https://doi.org/10.1371/journal.pgph.0000165.t003
Negative experiences with the health system as source of vaccine hesitancy and anti-vaccination sentiments.
The participants cited negative experiences in the past, whether these happened recently or decades ago, as causes of their negative attitude towards vaccines. Three participants who identified themselves as COVID-19 ‘anti-vaxxers’ or those opposed to vaccines, had different sources of anti-vaccination sentiments. These three participants belong to different priority groups. One belongs to the A1 or frontliner group and is working as a Barangay Health/Emergency Response Team (BHERT) member who responds to COVID-19 related health care needs in the community. The second is a retired professional (A2 or senior citizen group) while the third is an environmental protection officer who oversees implementation of public health standards in the community (B2 or other government workers). These participants experienced an undesired event related to vaccines and/or medical care from four years to more than three decades prior the pandemic ( Quotes I3-I5, Table 3 ). Except for one anti-vaxxer, no other health worker reported negative experiences that caused mistrust in the COVID-19 vaccines and vaccination campaign.
Vaccines are viewed as unsafe and deadly.
Perceptions on risk of getting infection with and dying from the virus varied among the participants. However, for those who were opposed to the vaccines, their fear of the COVID-19 vaccine and its effects was greater than their fear of the virus and outcomes ( Quote I6, Table 3 ). This fear and their view of vaccines being unsafe and deadly resulted to vaccine refusals or delays. According to them, the deaths observed after administration of the vaccine are caused by the vaccine; however, medical doctors and hospitals report the death as being caused by underlying conditions such as comorbidities ( Quotes I7-I8, Table 3 ). Some participants also believed the circulating theory that the life span of those who are vaccinated is shortened and they only have two to three years to live: “ you are healthy but because of the vaccine , you suddenly die ”. In addition to the belief that vaccines cause death or shorten an individual’s life span, participants also had doubts about the COVID-19 vaccines particularly the mRNA vaccines that use a relatively new technology ( Quote I9, Table 3 ). These concerns about the safety profile of vaccines either caused delays in vaccine acceptance and uptake or refusals. The reverse was reported among most of the health workers and other participants who viewed vaccines positively. They believed that the vaccine protects them from severe illness, hospitalisation, and death, and that vaccines only have minimal risk.
Vaccines are viewed as unnecessary and insufficient to prevent disease.
Vaccines were viewed as unnecessary by some participants, especially those in older age groups who are not allowed to go out ( Quote I10, Table 3 ). Those in lower priority groups felt that others needed the vaccine more than them. Younger participants shared that they were COVID-19 survivors even without the vaccine; but those at high risk especially the elderly and persons with comorbidities will need the vaccine to protect them ( Quote I11, Table 3 ) . The participants also viewed vaccines as insufficient–they expected that getting vaccinated means no longer needing other public health interventions but were disappointed to learn that vaccines are only one part of the solution. Participants therefore questioned the need for the vaccines given the information they have read and/or watched about still being at risk of getting infected despite being vaccinated ( Quote I12, Table 3 ). The lack of clarity in the role of the vaccines has negatively influenced people’s decisions on getting the vaccine.
Skepticism towards vaccine incentives.
Vaccine incentives in the country, such as promotions and offers for those vaccinated, created skepticism among some of the participants. These incentives ‘bothered’ participants and raised questions about the role of vaccines and the intentions of the government. As a result, these incentives ‘disincentivised’ participants from getting the vaccine as participants felt being forced to take it ( Quote I13, Table 3 ).
Use of vaccines not fully approved by the Food and Drug Administration (FDA).
Participants viewed decisions to vaccinate individuals as ‘rash’ and expressed concerns about vaccines not yet being fully approved by the Food and Drug Administration (FDA). Some also shared concerns about the rapid development of vaccines compared to other vaccines that took decades to develop ( Quote I14, Table 3 ). Participants felt that they were being experimented on using an unproven vaccine, relating this with the dengue vaccine controversy ( Quote I15, Table 3 ). This caused delay or refusal in getting the vaccines when it was offered to them.
Low health literacy and lack of critical skills to evaluate health information.
Health literacy or how people acquire, evaluate, and apply health information to inform their decisions, including getting the vaccine, is an important but underestimated tool to combat misinformation. Participants shared that Filipinos seemed to know a lot about vaccines, but only superficially. They shared that those among low-resource communities and older population groups were especially vulnerable to misinformation ( Quote I16, Table 3 ). This lack of awareness and critical skills to evaluate information, together with the rapid spread of misinformation, influences people’s decisions to get their first dose, to return to their second and get fully vaccinated ( Quote I17, Table 3 ). There were also several participants who shared that they were confused with the contradictory information they were reading and hearing ( Quotes I18, Table 3 ).
Religious beliefs do not support vaccines.
‘Antichrist’–this was how one participant described the vaccines against COVID-19. Another participant shared concerns about the vaccines and how they would replace antibodies created by God ( Quote I19, Table 3 ). She mentioned that these vaccines have active chemicals that are causing unintended side effects and deaths.
Interpersonal barriers
Family influence and opposition to vaccines..
Participants recognised the influence of their family on their health decisions including getting vaccinated. One participant who was opposed to COVID-19 vaccines shared that everyone in their family was unvaccinated because they believed her (A1, 51–60 years old, female, Misamis Oriental). Similarly, a mother who had a negative experience related to the dengue vaccine that was administered to her child, refused to have herself and her family vaccinated against COVID-19 (B2, 41–50 years old, female, National Capital Region).
Misinformation spread by networks.
Rumours and misinformation about COVID-19 vaccines are easily spread by networks, whether by word of mouth or through social media. A participant said her “ eyes have been opened only now because of YouTube ” (A2, 61–70 years old, female, Camarines Norte). Participants believed that this affected vaccine uptake, especially among individuals who do not have the opportunity to receive accurate information from official sources including the Department of Health ( Quote IC1, Table 4 ).
https://doi.org/10.1371/journal.pgph.0000165.t004
Perceived conflicts of interest of health professionals.
Participants viewed key figures in the response to the pandemic as having conflicts of interests. This perception of having ‘hidden agenda’ created mistrust in the information provided health professionals, health organisations, and other figures and institutions. These conflicts of interest, whether financial or non-financial, subject evidence and data to bias especially if there are undesired adverse effects to the treatment or vaccine ( Quote IC2, Table 4 ).
Structural barriers: Health systems and service delivery
Inadequate supply of vaccines..
Observations of participants regarding supply of vaccines varied according to location and membership to the vaccine priority groups. Participants, especially those from cities and provinces outside of metropolitan areas, reported that the supply of vaccines was insufficient to meet the demands and needs of the communities ( Quote S-HS1, Table 5 ). However, even within highly urbanised areas, participants shared that there were those who did not get their second doses on time because no vaccines arrived ( Quote S-HS2, Table 5 ). Health workers found that vaccines for them were easily accessible, however those in other groups had to wait longer before getting the vaccine ( Quote S-HS3, Table 5 ).
https://doi.org/10.1371/journal.pgph.0000165.t005
Perceived inefficiencies of the vaccination system.
Participants highlighted issues with the system including the slow rollout of vaccines, long waiting time, inefficient registration systems, and lack of a centralised system. Participants mentioned getting frustrated with the speed at which vaccines are being distributed and administered in the country ( Quote S-HS4, Table 5 ). Participants also mentioned issues with the waiting process to get a slot after registration and the waiting time at the day of the vaccination, with some being asked to stay at vaccination sites for two hours to watch a seminar on COVID-19 and vaccines ( Quotes S-HS5-6, Table 5 ). There was perceived risk of exposure, which could be lessened if the process was faster and more efficient. There were also glitches in the online registration systems used by local governments that caused additional delays in getting people vaccinated ( Quote S-HS7, Table 5 ). Local governments are responsible for the distribution and administration of vaccines among their constituents, and individuals may register with various local governments depending on their place of residence or work. This lack of a centralised system makes it difficult to track who have already been vaccinated and where they have been vaccinated such that those who are still waiting for a slot are unable to secure one ( Quote S-HS8, Table 5 ).
View that the vaccination system is inflexible and excludes vulnerable and marginalised populations.
The current vaccination system of some local governments is viewed as inflexible that excludes vulnerable and marginalised populations. There are individuals who lack access to technology and digital platforms. Especially in rural areas and among the elderly, their exclusion due to access issues is further compounded by their low digital health literacy. These individuals are then unable to register online and get the vaccine ( Quote S-HS9, Table 5 ). While registration is online, even those in older age groups who are part of highly prioritised groups because of their susceptibility to the virus are required to go to the vaccination centre ( Quote S-HS10, Table 5 ). Similarly, those belonging to marginalised groups and communities also encounter considerable challenges to getting the vaccine ( Quote S-HS11, Table 5 ).
Logistical challenges.
A participant recognised that there are also logistical constraints in the distribution of vaccines, in addition to problems with supply. The COVID-19 vaccines have different temperature requirements with some requiring special distribution systems ( S-HS12, Table 5 ). These logistical challenges influence the distribution of vaccine brands to areas that have the capability to store them and affect decisions to delay getting the vaccine especially among those who prefer other brands ( S-HS13, Table 5 ).
Health professionals seen as amplifiers of misinformation.
Misinformation on vaccines and treatment were not only observed within families and social networks, but also within the medical community reported by participants who are health professionals themselves. There have been debates about Ivermectin as treatment for COVID-19, as well as vaccines, which have created factions within the group ( S-HS14, Table 5 ). Some of these health professionals who are anti-vaxxers or opposed to vaccines publicly share their views in media and in their practice ( S-HS15, Table 5 ). Because of the stature and credibility of health professionals, their views, whether backed by science or not, get amplified in the media and communities.
Pandemic response deemed as ineffective affects trust in health institutions.
The response and messaging of health organisations, together with other key figures and institutions in the country, were viewed by participants as ineffective ( S-HS16, Table 5 ). As a result, there is declining trust in these organisations with participants doubting information provided, such that Filipinos no longer take the pandemic seriously ( S-HS17, S-HS18, Table 5 ). In turn, participants turn to other sources of information that they think are more credible and trustworthy.
Structural barriers: Media and policies
Traditional and digital media accelerating the infodemic..
Information on the virus and vaccines are easily and effectively amplified by the media. With the infodemic (portmanteau of information and epidemic) or the exponential production of information whether scientifically accurate or not, traditional media and digital media become drivers of (mis)information or fear towards vaccines ( Quotes S-MP1-S-MP2, Table 6 ). Information that participants were receiving from these sources influenced their health beliefs and vaccine decisions ( Quote S-MP3, Table 6 ).
https://doi.org/10.1371/journal.pgph.0000165.t006
Perceived poor policy implementation and lack of evidence-based policies contributing to loss of confidence in vaccines and health institutions.
The government developed the Philippine “National Deployment and Vaccination Plan for COVID-19 Vaccines” that identifies population groups to be prioritised ensure vaccine equity accounting for different risks and needs [ 36 ]. This plan also stated that only vaccines granted with emergency use authorisation (EUA) or certificate of product registration (CPR) by the Philippine FDA will be purchased by the government. However, this was reported by participants to be poorly implemented with others using connections also known as ‘ palakasan ’ system to get the vaccine ahead of those in the priority list ( Quote S-MP4, Table 6 ). Even within the government, the Presidential Security Group were given vaccines even without EUA and/or CPR registration from the FDA ( Quote S-MP5, Table 6 ). In addition, the government purchased vaccines that did not publish their results, and reportedly had lower efficacy rates but more expensive ( Quote S-MP6, Table 6 ). As a result, participants felt that the government was ‘settling for less’ and that Filipinos deserved better (A4, 21–30 years old, female, National Capital Region). These issues contributed to declining confidence in vaccines and health institutions, with Filipinos questioning the safety of such vaccines and the implementation of these prioritisation frameworks.
National and local political issues.
Past and current political issues contributed to refusals to specific vaccine brands. Together with reports of how the virus emerged from Wuhan, China, these triggered skepticism towards vaccines manufactured in their country. Participants mentioned the dispute of the Philippines and China regarding contested territory at the West Philippine Sea (South China Sea) as a reason for not preferring and/or refusing vaccines from their country, even when donations of Sinovac from China were the first vaccines to be available ( Quote S-MP7, Table 6 ). This dispute also influenced how participants thought about the origins of the virus and why other countries developed their own vaccines ( Quote S-MP8, Table 6 ). Locally, participants viewed politics to have influence on which cities or provinces receive preferred vaccine brands. They mentioned that these ‘favored hospitals and provinces’ were prioritised, which was perceived as unfair and causing further delays in the vaccination rollout ( Quote S-MP9, Table 6 ).
One of the most effective public health strategies, vaccination, has been the focus of false and inaccurate information with rapidly declining rates of acceptance. [ 37 ]. In the Philippines, vaccine confidence plummeted after the Dengue vaccine controversy [ 9 , 12 , 15 – 17 ]. While anti-vaccination views and vaccine hesitancy are not yet the main barrier to vaccination in the Philippines which still struggles with vaccine access and distribution, lessons from other countries indicate that these equally and urgently need to be addressed in addition to other challenges [ 38 ]. Our study supports the findings of other published research that report a host of individual, interpersonal, and structural barriers that work individually or collectively against vaccination uptake and reach [ 29 ]. Therefore, there is a need for a holistic approach to promote COVID-19 vaccination that not only addresses barriers at the individual level, but also at the interpersonal and structural levels [ 38 , 39 ].
Individual perceptions, beliefs, and experiences play a major role on the decision to vaccinate. These are shaped by exposure to (mis)information spread by networks, by key health figures and institutions, and through the media [ 40 – 43 ]. Misinformation regarding vaccines have been present since vaccines were first developed [ 44 – 46 ], but the advent of social media made its propagation much easier [ 43 , 45 , 47 ]. Unique to the Philippine context is vaccine brand hesitancy, specifically towards Chinese manufactured vaccines and mRNA vaccines. This is caused in part by lack of transparency and scientific information, and spread through networks and the media. Further aggravating the issue is how some people attempt to correct misconceptions in a way that alienates people instead of addressing misinformation. People involved in vaccine promotion activities, especially primary care providers, may need to be trained on how to engage with vocal vaccine deniers and promote vaccination. The World Health Organization document outlining how to respond to vaccine misinformation would be an important resource in such an endeavour [ 48 ]. Celebrities and social media influencers may also play a role in promoting vaccination [ 41 ], but it is essential that they disclose conflicts of interest to develop trust with their audience. The media also needs to be trained on how to present news regarding adverse effects following immunsation, and regarding COVID-19 in general, so as not to create unnecessary panic and dissuade people from getting vaccinated. A study reported that there may be a need to use first-person, people-centred narratives to prevent ‘psychic numbing’ and give faces to numbers [ 49 ]. In all these, it is vital to engage with the public, especially those who are vaccine hesitant, in order to promote vaccination using language that is inclusive and applicable to their context [ 48 ].
The health system and one’s interactions with it also contribute to one’s decision to get vaccinated. As in this study, trust in the health system has been found to be a major factor in getting COVID-19 vaccine [ 41 , 50 ]. The Philippine government has instituted several health system confidence-building policies. The recent COVID-19 Vaccination Program Act stipulates the provision of free COVID-19 vaccines to all Filipinos and the establishment of an indemnification fund for people who could possibly develop adverse effects following immunisation [ 51 ]. Perceptions of ‘ palakasan ’ (i.e., use of political connections), stemming from instances during the course of the pandemic where powerful individuals seem to be above the law [ 52 ], contribute to vaccine hesitancy and poor uptake of vaccines. These negative impacts are further compounded by the highly politicised Dengvaxia controversy where individuals, especially parents of school-age children, felt that health institutions and governments were experimenting on them [ 9 , 12 ] with our participants relating the COVID-19 vaccine ‘experiment’ with the dengue vaccine. In addition, inadequate supply, logistical challenges, and perceptions about the inefficiency and inflexibility of the system negatively impact vaccination rates in the country. As of 16 September 2021, only 3 in 10 Filipinos received one dose with significant differences between population groups: almost all frontline and health workers have been vaccinated while only 2 in 5 elderly Filipinos received their first dose [ 21 ]. Those in the third priority group have higher rates than the elderly population group, which were offered the vaccines earlier. Apart from individual reasons, marginalised and vulnerable groups such as the elderly have reported not being able to get their vaccine due to lack of home vaccination services and guidance in using online registration systems. The system will need to consider needs of all population groups to improve vaccination uptake. In all these, trust in the health system needs to be maintained, while disregarding regulations and policies in place can erode trust in the vaccination process.
In the Philippines, the national government has the responsibility to procure, allocate, and distribute the vaccines to the different provinces and municipalities, but it is the local government that is responsible for last-mile transport and actual inoculation. This results in wide variations in client registration and procedures between different localities. This underlines the need to identify best practices in vaccine rollout systems to implement a system that is efficient and inclusive to ensure that access to technology and mobility will not be barriers to vaccination.
There are a number of limitations that need to be considered when interpreting our findings. First, we were not able to have representation from the A5 priority group (indigent population). While we initially were able to get a participant from this group based on the survey response, we later found during the interview that this individual belonged to a different vaccination priority classification. This may point to issues with online data collection where researchers are unable to reach individuals from low-resource households. Second, there may be social desirability bias because we were unable to ensure if the respondent had other people with them that may have caused a change in their responses. Additionally, we did not disclose any political affiliations and interests, but participants may have been cautious in mentioning negative experiences related to vaccination. Participants may also have chosen more positive responses considering our background as health researchers. However, we emphasised that they will remain anonymous and their data treated with utmost confidentiality. Lastly, factors influencing COVID-19 vaccination uptake is context-specific, and this paper does not aim to represent all situations and circumstances.
Challenges to COVID-19 vaccination may be individual, interpersonal, and/or structural, which interact to influence decisions. Individual perceptions play a major role in the decision to vaccinate, and such perceptions are shaped by exposure to (mis)information amplified by the media, the community, and the health system. In the Philippines, vaccine brand hesitancy and misinformation are prevalent due to their rapid spread through social media and sensationalism in traditional media. Information on the effectiveness of safety of vaccines regardless of brand needs to be communicated to the public to increase COVID-19 vaccine confidence. At the interpersonal level, exposure to networks and health workers who are opposed to vaccines heightens public skepticism of vaccination. Structural barriers including political issues and poor implementation further contribute to vaccine refusals. The ongoing infodemic and anti-vaccination sentiments operating at all three levels (individual, interpersonal, structural) require empowering individuals to evaluate health information, and therefore health literacy becomes a critical tool to combat misinformation. Families and peers also need to be involved in these discussions as they influence vaccine uptake. Individuals engaged in vaccine promotion activities may need to be retrained on how to engage with vocal vaccine deniers in public. Given the involvement of traditional media, trainings on public health and science communication may be helpful in reporting vaccination-related news. Public figures need to disclose conflicts of interests and be transparent to the public, laying out the risks and benefits of vaccines. Laws should be well-implemented and equally implemented regardless of socioeconomic class or social position to encourage trust in the health care system and in vaccination initiatives. There is also a need to study best practices in vaccine rollout to implement systems that are efficient and inclusive so that we can vaccinate as many people against COVID-19 as quickly and as inclusively as possible: provide technological support particularly among older populations and allow flexible options for receiving the vaccine such as home vaccination. Given resource limitations, the vaccination rollout could also be improved by increasing the role of the private sector in the rollout and administration of the vaccine. The government and health organisations will need to connect with individuals, communities, and other institutions, including those who are against vaccines or hesitant towards vaccines, to co-create effective and sustainable solutions.
Acknowledgments
We would like to thank Michelle Edillon, Kriselle Abcede, Ryan Molen, and Josef Bondoc for their invaluable support to this project. We provide credit to BioRender.com for the figures illustrated in this paper. Finally, we are grateful to our participants who generously shared their stories with us.
- View Article
- Google Scholar
- PubMed/NCBI
- 34. Charmaz K. Constructing grounded theory: a practical guide through qualitative analysis. London, Great Britain: SAGE Publications Ltd; 2006.
- 35. Strauss A, Corbin J. Grounded theory methodology. Handbook of qualitative research. New York: Sage; 1994. pp. 273–85.
- 51. Department of Health (Philippines). Implementing rules and regulations of Republic Act No. 11525 known as "An Act Establishing the COVID-19 Vaccination Program Expediting the Vaccine Procurement and Administration Process, Providing Funds Therefor, and For Other Purposes | Department of Health website. 2021 [Cited 16 Sep 2021]. Available from: https://doh.gov.ph/IRR-of-RA11525-An-Act-Establishing-the-COVID-19-Vaccination-Program-Expediting-the-Vaccine-Procurement-and-Administration-Process-Providing-Funds-Therefor-and-For-Other-Purposes
REVIEW article
The effect of covid-19 vaccine to the omicron variant in children and adolescents: a systematic review and meta-analysis.

- 1 Institute of Respiratory Health and Multimorbidity, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2 Integrated Care Management Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3 Department of Obstetrics and Gynecology, National Clinical Research Center for Obstetrics and Gynecology (Peking University Third Hospital), National Center for Healthcare Quality Management in Obstetrics, Peking University Third Hospital, Peking University, Beijing, China
- 4 General Practice Ward/International Medical Center Ward, General Practice Medical Center, West China Hospital, Sichuan University, Chengdu, China
- 5 Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 6 Key Laboratory of Obstetrics & Gynecologic and Pediatric Diseases and Birth Defects of the Ministry of Education, Sichuan University, Chengdu, China
Background: Omicron (B.1.1.529), a variant of SARS-CoV-2, has emerged as a dominant strain in COVID-19 pandemic. This development has raised concerns about the effectiveness of vaccination to Omicron, particularly in the context of children and adolescents. Our study evaluated the efficacy of different COVID-19 vaccination regimens in children and adolescents during the Omicron epidemic phase.
Methods: We searched PubMed, Cochrane, Web of Science, and Embase electronic databases for studies published through March 2023 on the association between COVID-19 vaccination and vaccine effectiveness (VE) against SARS-CoV-2 infection in children and adolescents at the Omicron variant period. The effectiveness outcomes included mild COVID-19 and severe COVID-19. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and was prospectively registered in PROSPERO (CRD42023390481).
Results: A total of 33 studies involving 16,532,536 children were included in the analysis. First, in children and adolescents aged 0–19 years, the overall VE of the COVID-19 vaccine is 45% (95% confidence interval [CI]: 40 to 50%). Subgroup analysis of VE during Omicron epidemic phase for different dosage regimens demonstrated that the VE was 50% (95% CI: 44 to 55%) for the 2-dose vaccination and 61% (95% CI: 45 to 73%) for the booster vaccination. Upon further analysis of different effectiveness outcomes during the 2-dose vaccination showed that the VE was 41% (95% CI: 35 to 47%) against mild COVID-19 and 71% (95% CI: 60 to 79%) against severe COVID-19. In addition, VE exhibited a gradual decrease over time, with the significant decline in the efficacy of Omicron for infection before and after 90 days following the 2-dose vaccination, registering 54% (95% CI: 48 to 59%) and 34% (95% CI: 21 to 56%), respectively.
Conclusion: During the Omicron variant epidemic, the vaccine provided protection against SARS-CoV-2 infection in children and adolescents aged 0–19 years. Two doses of vaccination can provide effective protection severe COVID-19, with booster vaccination additionally enhancing VE.
1 Introduction
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019, its global impact has been profound ( 1 ), causing millions of infections and significantly affecting both human lives and socio-economic stability ( 2 ). As the epidemic evolves, the Omicron variant became the predominant strain of novel coronavirus pneumonia worldwide since November 2021 ( 3 , 4 ). With the emergence of Omicron variant, the incidence of SARS-CoV-2 infections is growing among children ( 5 ), including mild COVID-19 (fever, fatigue, persistent dry cough, decreased or loss the sense of taste or smell and other symptoms) and severe COVID-19 (pneumonia, or life-threatening complications affecting the gastrointestinal, neurological, cardiovascular systems, or hospitalizations) ( 6 ).
Vaccination is the most economically efficient means to guard against COVID-19 ( 7 , 8 ). And the efficacy of vaccination is linked to the vaccination dosages and the vaccination interval ( 9 – 13 ). The vaccination regimen currently comprises complete vaccination (two doses), and the booster vaccination (three doses) in children ( 14 , 15 ). Comprehending the efficacy of vaccines in children is crucial for informed decision-making regarding vaccine policies, including the necessity, timing, and dosages of vaccination for children. Piechottal et al. found that in children aged 5–11 years, mRNA vaccines are moderately effective against infections with the omicron variant and protect well against COVID-19 hospitalizations ( 16 ). However, there remains limited understanding regarding the protective efficacy of vaccines against the omicron variant infection in children of a wider age range. In addition, it is unclear that the reasonable time interval after the administration of two doses vaccination and the efficacy of vaccination in preventing both mild and severe infections among individuals aged 0–19 years. Therefore, we conducted the meta-analysis to explore the efficacy of COVID-19 vaccine in children and adolescents aged 0–19 years during Omicron epidemic phase.
The study explored the association between vaccine effectiveness (VE) of COVID-19 vaccine and children SARS-CoV-2 infections during the Omicron variant outbreak. Additional, subgroup analyses were conducted to identify potential factors including various vaccination dosages, diverse SARS-CoV-2 outcomes, and different time intervals after the two doses vaccination. The findings provided a reference for the vaccination strategy of children against COVID-19 during the Omicron variant period and offered robust support for safeguarding the health and safety of the pediatric population.
2.1 Registration
The present investigation adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and was prospectively registered in PROSPERO under the registration number CRD42023390481. Initially, the PROSPERO protocol was designed to evaluate the effectiveness of various vaccine types. However, the most articles meeting our inclusion criteria focused on the BNT162b2 vaccine, with limited data available for other vaccine types. Consequently, we modified the protocol to explore the vaccine efficacy concerning dosage, infection severity, and vaccination intervals.
2.2 Information sources and search strategies
We conducted comprehensive systematic literature searches utilizing the PubMed, Cochrane, Web of Science, and Embase electronic databases/platforms, spanning until Feb. 2023. A structured search strategy was meticulously devised, encompassing pertinent Medical Subject Headings (MeSH) search terms such as “COVID-19 Vaccines,” and text words such as “COVID19 Virus Vaccines,” “Coronavirus Disease 2019 Vaccine.” As well as Supplementary Concept “SARS-CoV-2 variants,” or text words like “Omicron,” “SARS-CoV-2 BA.5 variant,” “COVID-19 Virus variant B.1.1.529,” and “SARS-CoV-2 omicron variant.” Moreover, we included MeSH term “Child,” or text words like “Child,” “Children,” and MeSH term “Pediatrics,” with corresponding text words “pediatric.” To ensure comprehensive coverage, we adapted the search strategy accordingly for the other electronic databases employed. The specific search strategy for each database/platform is shown in the Supplementary materials . Additionally, we meticulously examined the reference lists of the included studies to identify further relevant literature for inclusion.
2.3 Eligibility criteria
We conducted a systematic review of studies that investigated the effectiveness of COVID-19 vaccines in preventing Omicron variant infections among children and adolescents. Our study population comprised individuals aged 0–19 years, with no restrictions on vaccine types or dosages administered. For precise analysis, included studies must explicitly specify COVID-19 infection attributed to the Omicron variant (PCR-confirmed or antigen-test confirmed) as the outcome measure and provide accessible data on VE. Our study excluded reviews, case series, case reports, and studies involving non-human subjects.
2.4 Study selection process
A single investigator conducted the initial database search and diligently screened for any duplicate entries. Following the elimination of duplicates, two reviewers (TR and WL) meticulously evaluated the titles and abstracts of all records, subsequently scrutinizing the full texts of the eligible articles.
2.5 Data collection
Data pertaining to study design and methodology, author names, publication year, study location, sample size, age range, dosages of vaccination, different outcomes of SARS-CoV-2 infection during Omicron-dominant period, and potential confounding variables were meticulously extracted from the incorporated studies. The extraction process was carried out by two independent reviewers (TR and WL).
2.6 Study risk of bias assessment
The risk of bias for all chosen studies was independently evaluated by two reviewers (TR and WL) utilizing the Newcastle-Ottawa Scale (NOS) score. Subsequently, the quality of each study was categorized into three grades: low (0–3), moderate ( 4 – 6 ), and high ( 7 – 9 ).
2.7 Statistical analysis
Data from the including studies were meticulously extracted into Microsoft Excel and then imported into Stata 12 software (Stata Corp) and Review Manager 5.3 for conducting the meta-analyses. VE is defined as the reduction in disease incidence among vaccinated individuals compared to unvaccinated individuals. The VE and its accompanying 95% confidence intervals (CIs) were computed utilizing either adjusted or unadjusted risk ratios (RR): VE = (1 - RR) × 100%. The VE expressed in percentage values exceeding 0% indicate a potential protective impact of the vaccine. We employed pooled RR and VE to evaluate the correlation between COVID-19 vaccination in children and adolescents and SARS-CoV-2 infections during the Omicron-dominant period. To quantify inconsistency across studies and ascertain the percentage of variability in effect estimates potentially arising from heterogeneity rather than sampling error, the I 2 statistic and Q test were used to evaluate each study heterogeneity. If the heterogeneity was significant and I 2 > 50%, a random effects model was used; otherwise, a fixed effects model was used. p < 0.05 was considered statistically significant. Additionally, sensitivity analysis was performed to assess the robustness of associations by excluding one study at a time. To gauge publication bias, a funnel plot was constructed, and Egger’s and Begg’s tests were conducted.
Furthermore, we performed subgroup analyses by stratifying the different vaccination dosages, varying time intervals after the 2-dose vaccination, and distinct outcome of SARS-CoV-2 infections. Based on the information provided in the original studies, the dosages of vaccination were categorized into three subgroups: one dose indicating incomplete vaccination, two doses representing complete vaccination, and three doses administered as booster vaccination. The classification of outcomes was divided into two subgroups: mild COVID-19 (fever, fatigue, persistent dry cough, decreased or loss the sense of taste or smell and other symptoms) and severe COVID-19 (pneumonia, or life-threatening complications affecting the gastrointestinal, neurological, cardiovascular systems, or hospitalization) based on the outcome indicators reported in the original study data. The time intervals were divided into two subgroups, as reported in the original studies: ≤90 days and > 90 days following the two doses vaccination.
The study selection process is shown in Figure 1 . In our study, a total of 1731 records were searched in the databases to explore the efficacy of the COVID-19 vaccine in children aged 0–19 years during the Omicron-dominant period. In the course of our initial literature search and screening process, a total of 214 records underwent full-text evaluation. Among these, 13 records were recognized as reviews or editorials, 89 records lacked pertinent or valuable data, 55 records did not involve children or adolescents, and 25 records were unavailable in full text. Consequently, 32 records were eligible for inclusion in our study ( Supplementary Table 1 ). Among these, we identified 15 cohort studies ( 10 , 17 – 30 ) and 18 case–control studies ( 11 , 12 , 31 – 45 ), all utilizing non-vaccination as a control group, comprising an expansive cohort of 17,177,822 individuals. Of the 33 studies (one record contains two studies) included, 29 (87.88%) evaluated the effectiveness of the BNT162b2 vaccine, six (18.18%) involved the efficacy of the CoronaVac vaccine, two each on the effectiveness of the mRNA-1273 and the BBIBP-CorV vaccine, and one on the ChAdOx1nCoV-19 vaccine.
Figure 1 . PRISMA flowchart.
The NOS scores indicated that all the studies included in the analysis demonstrated moderate to high methodological quality. Among them, 17 studies ( 10 , 17 – 19 , 23 – 26 , 28 , 29 , 32 , 34 , 35 , 38 , 43 , 45 ) were rated as high quality, while 16 studies ( 11 , 12 , 20 – 22 , 27 , 30 , 31 , 33 , 36 , 37 , 39 – 42 , 44 ) were considered to be of medium quality ( Supplementary Table 2 ).
We next conducted a meta-analysis on 33 studies with eligible data to explore the VE for COVID-19 vaccine among children in Omicron-dominant period. The overall RR was 0.55 (95% CI: 0.50 to 0.60, I 2 = 89%, p < 0.01; Figure 2 ; VE: 45, 95% CI: 40 to 50%; Table 1 ). Moreover, we evaluated the possibility of publication bias. The funnel plot resembles an asymmetrical distribution ( Supplementary Figure 1 ). Egger’s test ( p = 0.01; Supplementary Figure 2 ) and Begg’s test ( p = 0.04; Supplementary Figure 3 ) showed publication bias. Therefore, we employed the trim-and-fill method to address publication bias, and we found that the results remained statistically significant after applying the trim-and-fill method ( Supplementary Figure 4 ). This indicates the stability and reliability of our results, further supporting the validity of our conclusions. Then we performed sensitivity analysis indicated that the results were robust through removing a single study each time ( Supplementary Figure 5 ).
Figure 2 . Forest plot for risk ratios on preventing Omicron infections. The red square symbolizes the point estimate for each study, with its size proportional to the study’s weight relative to the summary estimate. The black diamond symbol represents the overall effect estimate derived from the meta-analysis. Meta-analysis based on Random Effects model, inverse variance method (IV). Effect size estimates expressed in Log Risk Ratio [95%CI].
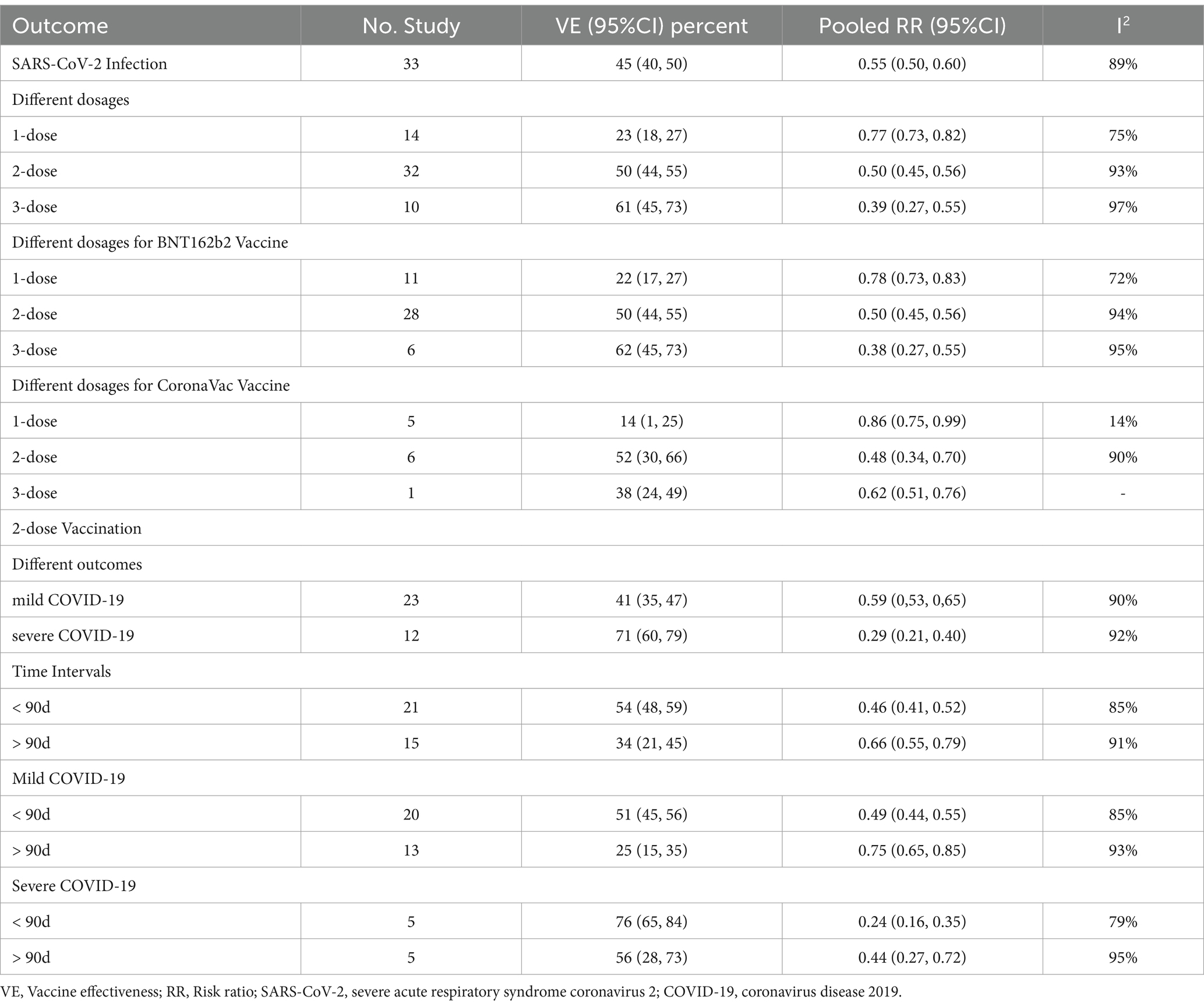
Table 1 . Overall effectiveness and vaccine effectiveness results of different vaccination regimens.
Additionally, subgroup analysis was conducted to identify potential factors that may influence the relationship between children’s vaccination and vaccine efficacy in preventing infections during the Omicron-dominant period. These factors included the dosages of vaccination, the classification of outcomes, and the interval between vaccine dosages.
Regardless of vaccination type, 14 studies ( 20 , 26 , 27 , 29 , 34 , 37 – 41 , 43 – 45 ) investigated VE of incomplete vaccination (1-dose) compared to non-vaccination individuals, revealing an overall RR of 0.77 (95% CI: 0.73 to 0.82, I 2 = 75%, p < 0.01; Figure 3 ; VE: 23, 95% CI: 18 to 27%; Table 1 ). And a total of 32 studies ( 10 – 12 , 17 – 41 , 43 – 45 ) explored complete vaccination (2-dose), yielding an overall RR of 0.50 (95% CI: 0.45 to 0.56, I 2 = 93%, p < 0.01; Figure 3 ; VE: 50, 95% CI: 44 to 55%; Table 1 ). Additionally, 10 studies ( 12 , 17 , 19 , 25 , 28 – 30 , 36 , 39 , 42 ) focused on booster vaccination (3-dose), presenting an overall RR of 0.39 (95% CI: 0.27 to 0.55, I 2 = 97%, p < 0.01; Figure 3 ; VE: 61, 95% CI: 45 to 73%; Table 1 ).
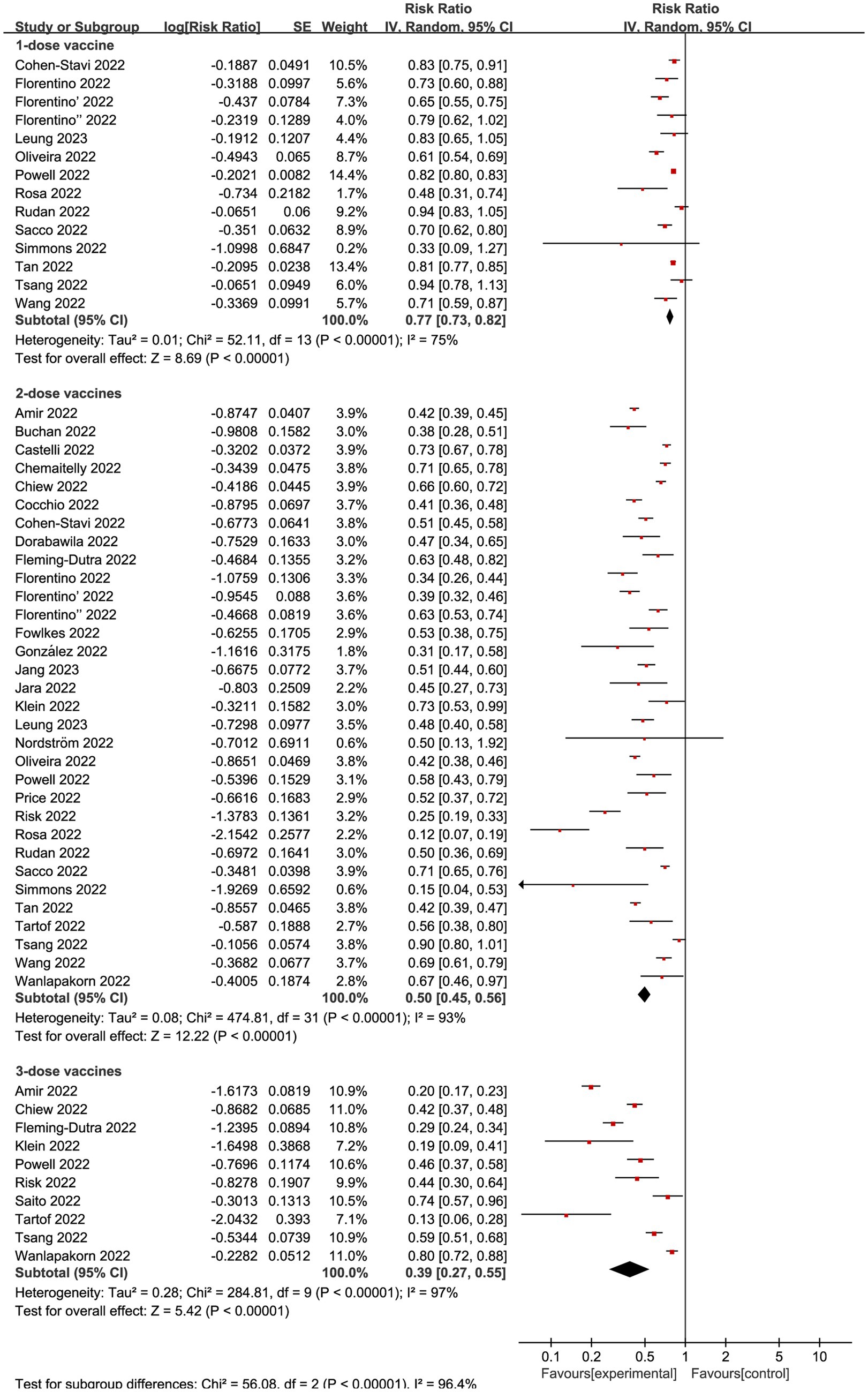
Figure 3 . Forest plot for risk ratios of different vaccines dosages on preventing Omicron infections. The red square symbolizes the point estimate for each study, with its size proportional to the study’s weight relative to the summary estimate. The black diamond symbol represents the overall effect estimate derived from the meta-analysis. Meta-analysis based on Random Effects model, inverse variance method (IV). Effect size estimates expressed in Log Risk Ratio [95%CI].
For the VE of vaccine dosages among different vaccine types (BNT162b2 and CoronaVac), we performed a subgroup analysis. 11 studies analyzed VE of 1-dose BNT162b2 vaccine, revealing an overall RR of 0.78 (95% CI: 0.73 to 0.83, I 2 = 72%, p < 0.01; Supplementary Figure 6 ; VE: 22, 95% CI: 17 to 27%; Table 1 ) compared to non-vaccination individuals. And a total of 28 studies explored 2-dose BNT162b2 vaccine compared to non-vaccination individuals, yielding an overall RR of 0.50 (95% CI: 0.45 to 0.56, I 2 = 94%, p < 0.01; Supplementary Figure 6 ; VE: 50, 95% CI: 44 to 55%; Table 1 ). Additionally, seven studies focused on 3-dose BNT162b2 vaccine, presenting an overall RR of 0.38 (95% CI: 0.27 to 0.55, I 2 = 95%, p < 0.01; Supplementary Figure 6 ; VE: 62, 95% CI: 45 to 73%; Table 1 ).
The effectiveness of the CoronaVac vaccine with 1-dose was investigated in five studies, demonstrating an overall RR of 0.86 (95% CI: 0.75 to 0.99, I 2 = 14%, p = 0.32; Supplementary Figure 7 ; VE: 14, 95% CI: 1 to 25%; Table 1 ). For the 2-dose regimen, a total of six studies were evaluated, revealing an overall RR of 0.48 (95% CI: 0.34 to 0.70, I 2 = 90%, p < 0.01; Supplementary Figure 7 ; VE: 52, 95% CI: 30 to 66%; Table 1 ). In addition, only one study looked at the efficacy of 3-dose CoronaVac vaccine, yielding a RR of 0.62 (95% CI: 0.51 to 0.76; Supplementary Figure 7 ; VE: 38, 95% CI: 24 to 49%; Table 1 ).
We subsequently conducted subgroup analyses within the complete vaccination group, focusing on distinct outcome measures. Nine studies ( 12 , 20 , 21 , 26 , 29 , 31 , 34 , 39 , 45 ) made mild COVID-19 as the outcome, presenting an overall RR of 0.59 (95% CI: 0.53 to 0.65, I 2 = 90%, p < 0.01; Figure 4 ; VE: 41, 95% CI:35 to 47%; Table 1 ). Meanwhile, complete vaccination could decrease the risks of Omicron associated severe COVID-19 with RR of 0.29 (95% CI: 0.21 to 0.40, I 2 = 92%, p < 0.01; Figure 4 ; VE: 71, 95% CI: 60 to 79%; Table 1 ).
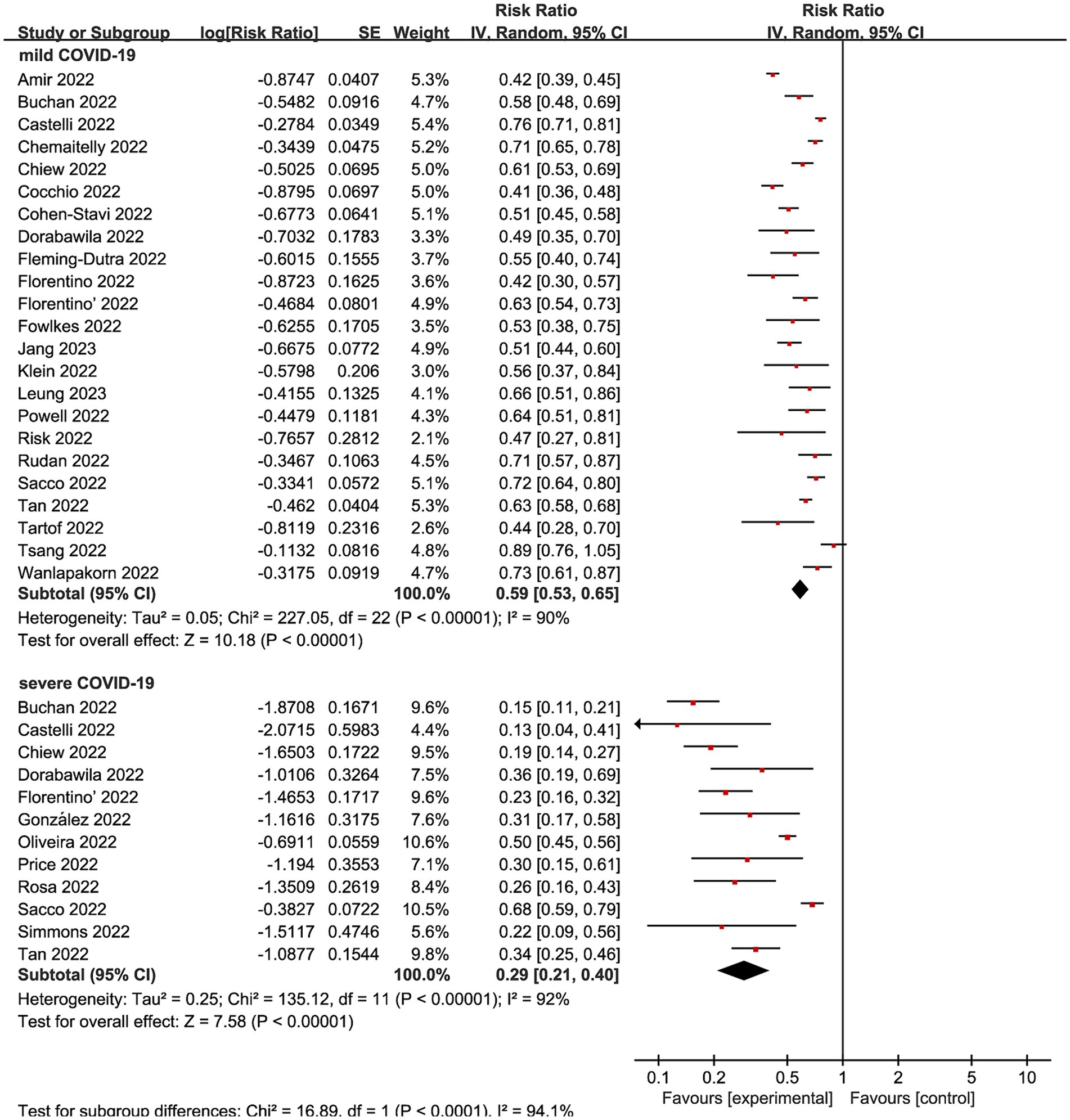
Figure 4 . Forest plot for risk ratios on various outcomes following Omicron infections. The red square symbolizes the point estimate for each study, with its size proportional to the study’s weight relative to the summary estimate. The black diamond symbol represents the overall effect estimate derived from the meta-analysis. Meta-analysis based on Random Effects model, inverse variance method (IV). Effect size estimates expressed in Log Risk Ratio [95%CI].
Many studies showed that the vaccine demonstrates its optimal protective effect within about 90 days following the second dose ( 13 , 18 , 46 , 47 ). Therefore, we conducted subgroup analyses in the complete vaccinated group using 90 days as a reference time point. Among the 33 studies included, 23 studies incorporated time-based monitoring of outcome indicators, while the remaining 10 studies did not specify temporal conditions. When combining all VE evaluations of complete vaccination within 90 days, the vaccination decreased infection by an overall RR of 0.46 (95% CI: 0.41 to 0.52, I 2 = 85%, p < 0.01; Figure 5 ; VE: 54, 95% CI: 48 to 59%; Table 1 ). The cumulative effectiveness of vaccination over 90 days after the complete vaccination was 0.66 (95% CI 0.55 to 0.79, I 2 = 91%, p < 0.01; Figure 5 ; VE: 34, 95% CI: 21 to 45%; Table 1 ) in the vaccinated cohort.
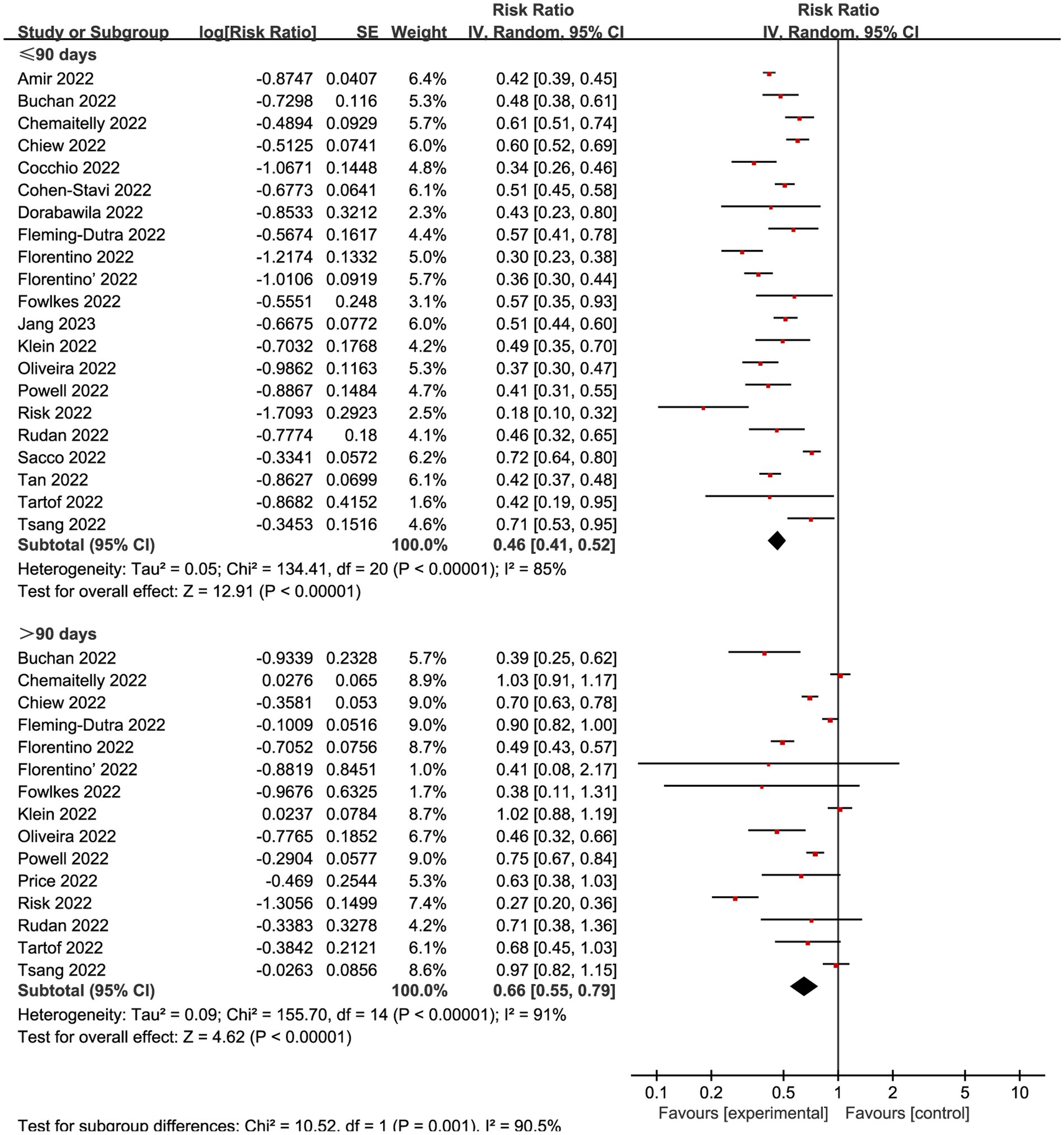
Figure 5 . Forest plot for risk ratios of different time intervals after 2-dose vaccination on preventing Omicron infections. The red square symbolizes the point estimate for each study, with its size proportional to the study’s weight relative to the summary estimate. The black diamond symbol represents the overall effect estimate derived from the meta-analysis. Meta-analysis based on Random Effects model, inverse variance method (IV). Effect size estimates expressed in Log Risk Ratio [95%CI].
We also explored the effects of vaccination at different time intervals across two outcomes. The VE against omicron mild COVID-19 in 90 days before and after were 0.49 (95% CI: 0.44 to 0.55, I 2 = 85%, p < 0.01; Figure 6 ; VE: 51, 95% CI: 45 to 56%; Table 1 ) and 0.75 (95% CI: 0.65 to 0.85, I 2 = 93%, p < 0.01; Figure 6 ; VE: 25, 95% CI: 15 to 35%; Table 1 ), respectively. Studies evaluated the VE, which decreased with time after receipt of the second dose, over time for the recent vaccination. As for the severe COVID-19, intervals less than 90 days or more than 90 days was associated with a decreased risk for Omicron with RR 0.24 (95% CI 0.16 to 0.35, I 2 = 79%, p < 0.01; Figure 7 ; VE: 76, 95% CI: 65 to 84%; Table 1 ) and RR 0.44 (95% CI 0.27 to 0.72, I 2 = 95%, p < 0.01; Figure 7 ; VE: 56, 95% CI: 28 to 73%; Table 1 ), respectively.
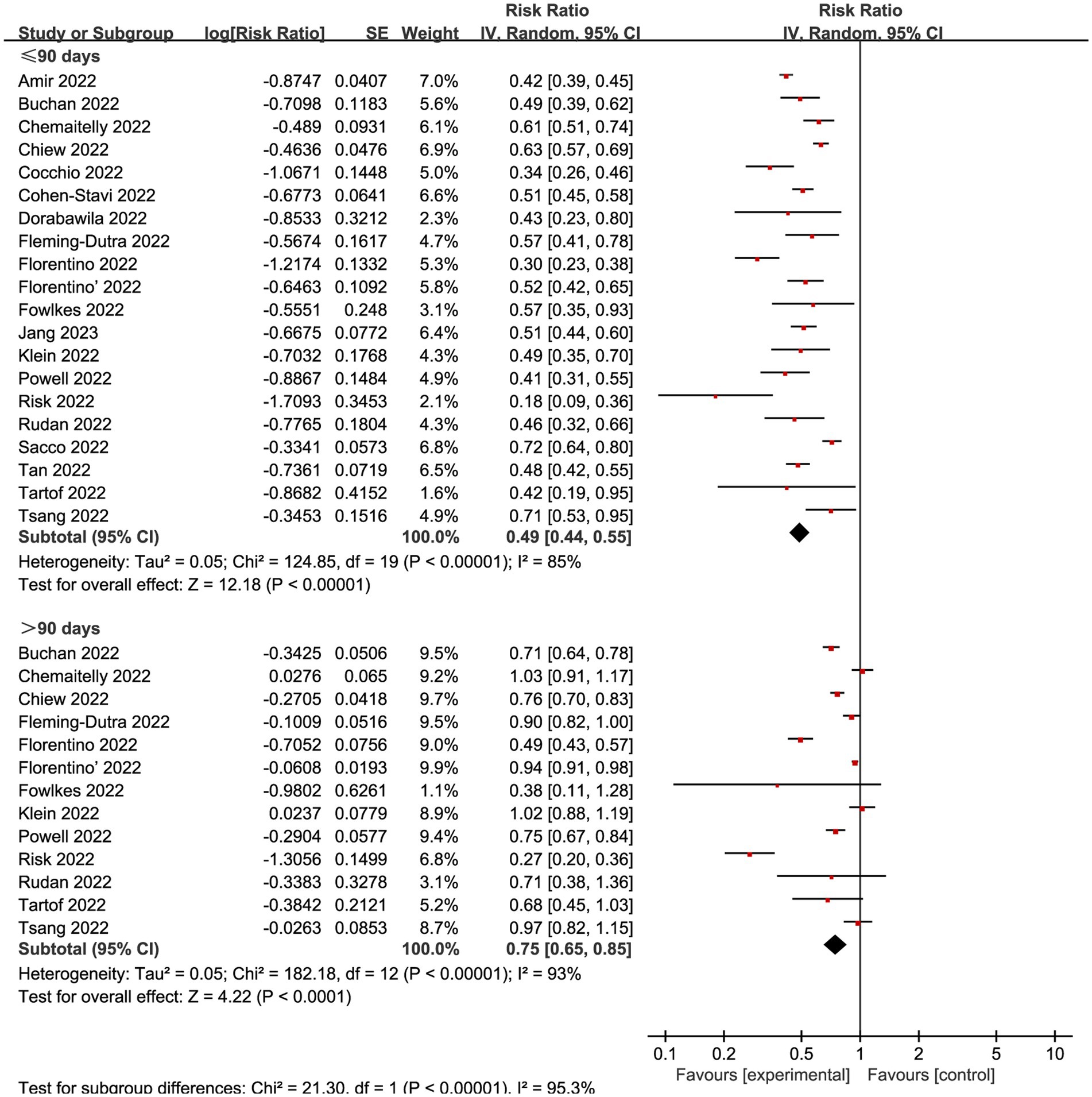
Figure 6 . Forest plot for risk ratios of different time intervals after 2-dose vaccination on preventing mild COVID-19. The red square symbolizes the point estimate for each study, with its size proportional to the study’s weight relative to the summary estimate. The black diamond symbol represents the overall effect estimate derived from the meta-analysis. Meta-analysis based on Random Effects model, inverse variance method (IV). Effect size estimates expressed in Log Risk Ratio [95%CI].
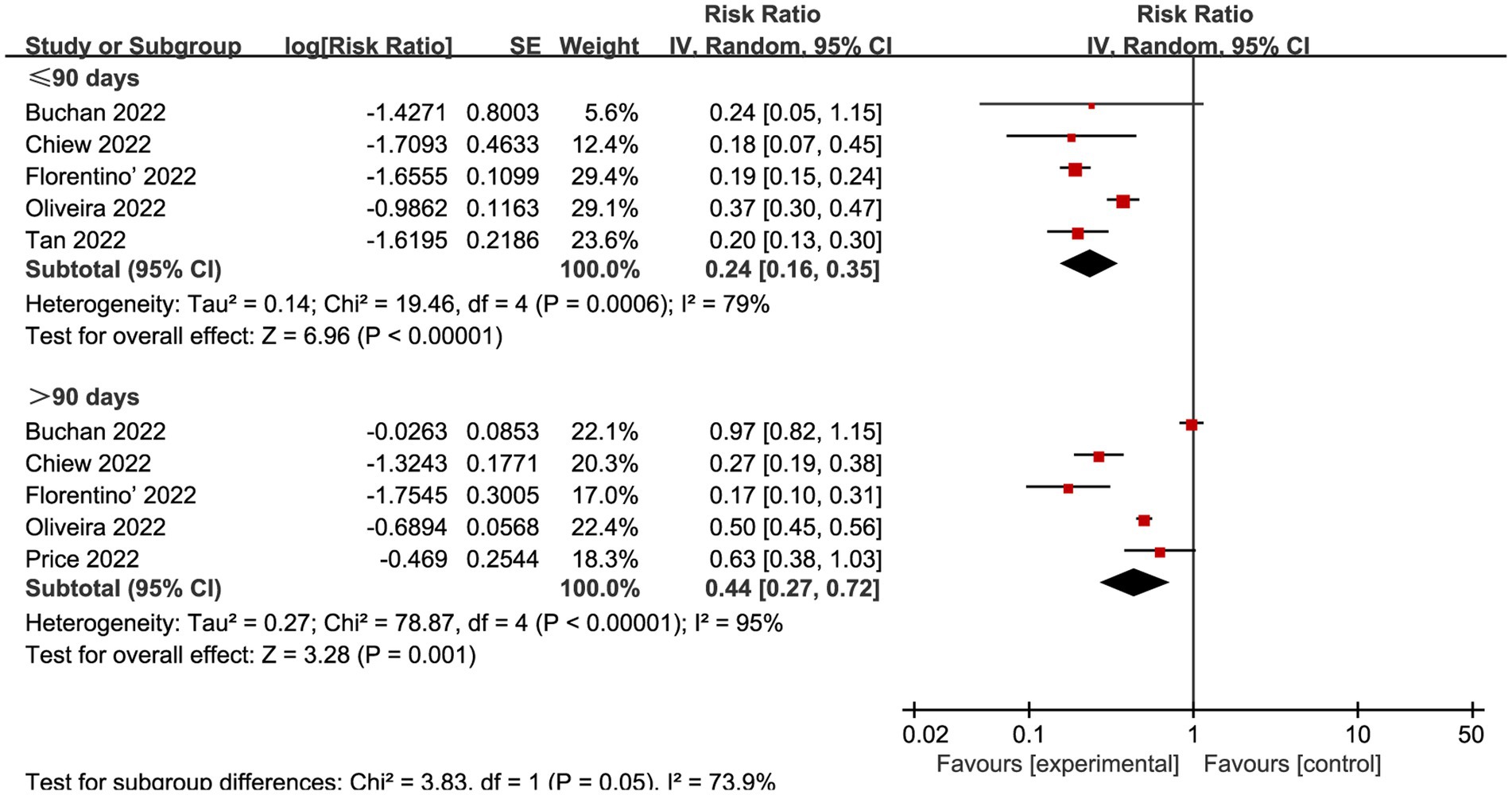
Figure 7 . Forest plot for risk ratios of different time intervals after 2-dose vaccination on preventing severe COVID-19. The red square symbolizes the point estimate for each study, with its size proportional to the study’s weight relative to the summary estimate. The black diamond symbol represents the overall effect estimate derived from the meta-analysis. Meta-analysis based on Random Effects model, inverse variance method (IV). Effect size estimates expressed in Log Risk Ratio [95%CI].
4 Discussion
The study focuses on the efficacy of COVID-19 vaccination among children and adolescents aged 0–19 during the Omicron-dominant period. The study shows that the vaccine provided protection against SARS-CoV-2 infections during the Omicron-dominant period. The VE trend is increasing with the additional booster vaccination regimen, and its efficacy varies for distinct SARS-CoV-2 infections within the 2-dose vaccination. The vaccine offered greater protection against severe COVID-19 during Omicron epidemic phase compared to mild COVID-19. A gradual decline in the efficacy of the COVID-19 vaccination against both mild and severe COVID-19 was observed over time, with a notable decline occurring after 90 days.
Our study estimates the VE against SARS-CoV-2 infections during Omicron-dominant phase was significantly lower than the VE during Delta-dominant phase ( 14 , 48 ). The decline in VE during the Omicron-dominant period may be due to the increased incidence of breakthrough infections associated with the Omicron variant, along with the rapid and infectious transmission of this variant ( 49 , 50 ). Furthermore, the enhanced potential of Omicron variant for immune evasion compared to the Delta variant may be involved in this phenomenon ( 51 – 53 ).
According to our study findings, there was a positive correlation between VE and the number of vaccinations administered. As the number of vaccinations increases, the VE gradually increases as well. It is well established that vaccine-induced immunity decreases over time ( 32 ). However, the increased dosages of the vaccine could maintain and generate favorable antibody, B-cell, and T-cell responses, thus providing robust protection to the body ( 54 ).
For vaccination intervals, some studies have suggested that a 3-month interval may be preferable to a vaccination program with the shorter intervals, which protects the largest number of individuals in the population as early as possible in case of supply shortages. The vaccination also improving protection after receiving the second dose ( 13 ). Furthermore, regulatory authorities in countries such as the United Kingdom have approved 2-dose intervals of up to 3 months for viral vector and mRNA vaccines ( 55 , 56 ). The relative VE of the booster vaccination given more than 3 months after the second vaccination was 84.4% and the absolute VE for symptomatic SARS-CoV-2 infection was 94.0% in adults over 50 years of age compared with unvaccinated participants ( 57 ). However, some studies have found that the highest antibody response could occur in the first month after vaccination, but immunity declined rapidly in the next 3–4 months, with the peak antibody titers decreased by almost 4–5.5 times ( 58 , 59 ). They supported that vaccine protection against Omicron variant infection waned within 3 months after the second dose, suggesting that a shorter interval between the second vaccination and booster may be beneficial ( 60 ). However, our study found no significant decline in VE for severe COVID-19 observed over the 3-month interval after the second vaccination. The most significant decline of VE was observed against mild COVID-19, where efficacy decreased by approximately 50% over the 3-month interval after second vaccination.
While COVID-19 vaccines provide steady protection against severe SARS-CoV-2 infections, their efficacy in preventing mild SARS-CoV-2 infections has been reduced, particularly during the Omicron-dominant period ( 61 – 63 ). It is well-established that antibodies or localized memory immune responses are the primary determinants of infection. COVID-19 vaccines primarily generate a systemic immune response, where immunoglobulin G (IgG) circulates in serum and body fluids as the primary functional component ( 64 ). With prophylactic vaccination, IgG antibodies remain in the serum for a certain duration. To prevent viral infection effectively, vaccine-induced serum IgG antibodies must enter the respiratory tract, coming into direct contact with lung endothelial cell surfaces to neutralize a viral infection ( 65 , 66 ). However, due to a limited number of specific antibodies reaching the upper respiratory tract and gradual decreases in antibody concentrations over time ( 67 ), the COVID-19 vaccine’s immune response is ineffective to prevent virus replication in the upper respiratory tract. The reduction of local antibodies in the upper respiratory tract weakens the protective effect against antiviral infection, leading to decreased defense against mild COVID-19. However, the antibody library present in circulating blood in the lung efficiently blocks the virus from attacking the alveolar epithelium and capillary endothelium ( 68 , 69 ), limiting severe pulmonary infections. In addition, Omicron cross-reactive T-cells and immune memory B-cells located throughout the body can be swiftly engaged upon encounter with a viral infection ( 70 ), producing an effective B and T cell-specific immune response ( 71 ). The immune memory B-cells produce large amounts of targeted antibodies to protect against the spread and replication of viruses, helping to prevent the onset of severe COVID-19 ( 72 , 73 ). Accordingly, children who received complete vaccination during the Omicron-dominant period experienced a reduced risk of severe SARS-CoV-2 infection.
This meta-analysis has several merits. First, eligible studies were retrieved from current major literature databases to minimize the risk of omitting relevant studies. Second, all included studies were published after the emergence and spread of Omicron variants, and these data were representative of the Omicron epidemic period. Third, the research data of included studies was obtained from national electronic medical databases, providing a representative population sample and a large sample size. Finally, all included studies were of high or moderate methodological quality, providing high reliability for the meta-analysis.
Our study also had several limitations. Firstly, meta-analyses of VE show a high degree of heterogeneity. Although sensitivity and subgroup analyses were performed to identify possible sources of heterogeneity, it appears that heterogeneity was not because of the degrees of infection, dosages of vaccination, or vaccination intervals. The type of vaccination, the body’s immune response, and variations in population characteristics might be responsible for the heterogeneity. However, due to insufficient data available from the included studies to stratify these variations, identifying the source of heterogeneity was challenging. Secondly, the findings regarding VE against SARS-CoV-2 infections in children and adolescents during the Omicron-dominant period may exhibit minor bias. However, further studies with larger sample sizes are warranted. In addition, some of the included studies did not provide exact time after second vaccination to evaluation of VE. Therefore, the evaluation of efficacy at longer time nodes in this study is relatively limited. The VE at different time nodes is still uncertain. Although boosters may improve efficacy, the timing of boosters remain to be further investigated.
5 Conclusion
During the period dominated by the Omicron variant, vaccination has demonstrated its ability to reduce the risk of SARS-CoV-2 infection in children and adolescents aged 0 to 19 years. The effectiveness of the vaccine becomes more pronounced as the number of dosages increases. Two doses vaccination significantly reduces the risk of severe COVID-19. The protection was still present but decreased over 90 days after the second vaccine regimen.
Author contributions
WL: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SZ: Data curation, Investigation, Writing – original draft. YY: Data curation, Investigation, Writing – original draft. YL: Data curation, Formal analysis, Writing – original draft. TR: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was funded by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD22009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1338208/full#supplementary-material
1. McKee, M, and Stuckler, D. If the world fails to protect the economy, COVID-19 will damage health not just now but also in the future. Nat Med . (2020) 26:640–2. doi: 10.1038/s41591-020-0863-y
PubMed Abstract | Crossref Full Text | Google Scholar
2. Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) (2023). Available from: https://coronavirus.jhu.edu/map.html .
Google Scholar
3. Singh, J, Pandit, P, McArthur, AG, Banerjee, A, and Mossman, K. Evolutionary trajectory of SARS-CoV-2 and emerging variants. Virol J . (2021) 18:166. doi: 10.1186/s12985-021-01633-w
4. Karim, SSA, and Karim, QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet . (2021) 398:2126–8. doi: 10.1016/S0140-6736(21)02758-6
5. Kozlov, M . Does omicron hit kids harder? Scientists are trying to find out. Nature . (2022). doi: 10.1038/d41586-022-00309-x
Crossref Full Text | Google Scholar
6. Shi, DS, Whitaker, M, Marks, KJ, Anglin, O, Milucky, J, Patel, K, et al. Hospitalizations of children aged 5-11 years with laboratory-confirmed COVID-19 - COVID-NET, 14 states, march 2020-February 2022. MMWR Morb Mortal Wkly Rep . (2022) 71:574–81. doi: 10.15585/mmwr.mm7116e1
7. Watson, OJ, Barnsley, G, Toor, J, Hogan, AB, Winskill, P, and Ghani, AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis . (2022) 22:1293–302. doi: 10.1016/S1473-3099(22)00320-6
8. Voysey, M, Clemens, SAC, Madhi, SA, Weckx, LY, Folegatti, PM, Aley, PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet . (2021) 397:99–111. doi: 10.1016/S0140-6736(20)32661-1
9. Yang, ZR, Jiang, YW, Li, FX, Liu, D, Lin, TF, Zhao, ZY, et al. Efficacy of SARS-CoV-2 vaccines and the dose-response relationship with three major antibodies: a systematic review and meta-analysis of randomised controlled trials. Lancet Microbe . (2023) 4:e236–46. doi: 10.1016/S2666-5247(22)00390-1
10. Jara, A, Undurraga, EA, Zubizarreta, JR, González, C, Acevedo, J, Pizarro, A, et al. Effectiveness of CoronaVac in children 3-5 years of age during the SARS-CoV-2 omicron outbreak in Chile. Nat Med . (2022) 28:1377–80. doi: 10.1038/s41591-022-01874-4
11. Price, AM, Olson, SM, Newhams, MM, Halasa, NB, Boom, JA, Sahni, LC, et al. BNT162b2 protection against the omicron variant in children and adolescents. N Engl J Med . (2022) 386:1899–909. doi: 10.1056/NEJMoa2202826
12. Fleming-Dutra, KE, Britton, A, Shang, N, Derado, G, Link-Gelles, R, Accorsi, EK, et al. Association of Prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during omicron predominance. JAMA . (2022) 327:2210–9. doi: 10.1001/jama.2022.7493
13. Voysey, M, Costa Clemens, SA, Madhi, SA, Weckx, LY, Folegatti, PM, Aley, PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet . (2021) 397:881–91. doi: 10.1016/S0140-6736(21)00432-3
14. Polack, FP, Thomas, SJ, Kitchin, N, Absalon, J, Gurtman, A, Lockhart, S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med . (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
15. Olson, SM, Newhams, MM, Halasa, NB, Price, AM, Boom, JA, Sahni, LC, et al. Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N Engl J Med . (2022) 386:713–23. doi: 10.1056/NEJMoa2117995
16. Piechotta, V, Siemens, W, Thielemann, I, Toews, M, Koch, J, Vygen-Bonnet, S, et al. Safety and effectiveness of vaccines against COVID-19 in children aged 5-11 years: a systematic review and meta-analysis. Lancet Child & adolescent heal . (2023) 7:379–91. doi: 10.1016/S2352-4642(23)00078-0
17. Amir, O, Goldberg, Y, Mandel, M, Bar-On, YM, Bodenheimer, O, Freedman, L, et al. Initial protection against SARS-CoV-2 omicron lineage infection in children and adolescents by BNT162b2 in Israel: an observational study. Lancet Infect Dis . (2023) 23:67–73. doi: 10.1016/S1473-3099(22)00527-8
18. Chemaitelly, H, AlMukdad, S, Ayoub, HH, Altarawneh, HN, Coyle, P, Tang, P, et al. Covid-19 vaccine protection among children and adolescents in Qatar. N Engl J Med . (2022) 387:1865–76. doi: 10.1056/NEJMoa2210058
19. Chiew, CJ, Premikha, M, Chong, CY, Wei, WE, Ong, B, Lye, DC, et al. Effectiveness of primary series and booster vaccination against SARS-CoV-2 infection and hospitalisation among adolescents aged 12-17 years in Singapore: a national cohort study. Lancet Infect Dis . (2022) 23:177–82. doi: 10.1016/S1473-3099(22)00573-4
20. Cohen-Stavi, CJ, Magen, O, Barda, N, Yaron, S, Peretz, A, Netzer, D, et al. BNT162b2 vaccine effectiveness against omicron in children 5 to 11 years of age. N Engl J Med . (2022) 387:227–36. doi: 10.1056/NEJMoa2205011
21. Dorabawila, V, Hoefer, D, Bauer, UE, Bassett, MT, Lutterloh, E, and Rosenberg, ES. Risk of infection and hospitalization among vaccinated and unvaccinated children and adolescents in New York after the emergence of the omicron variant. JAMA . (2022) 327:2242–4. doi: 10.1001/jama.2022.7319
22. Fowlkes, AL, Yoon, SK, Lutrick, K, Gwynn, L, Burns, J, Grant, L, et al. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5-11 years and adolescents aged 12-15 years - PROTECT cohort, July 2021-February 2022. MMWR Morb Mortal Wkly Rep . (2022) 71:422–8. doi: 10.15585/mmwr.mm7111e1
23. González, S, Olszevicki, S, Gaiano, A, Baino, ANV, Regairaz, L, Salazar, M, et al. Effectiveness of BBIBP-CorV, BNT162b2 and mRNA-1273 vaccines against hospitalisations among children and adolescents during the omicron outbreak in Argentina: a retrospective cohort study. Lancet Reg Health Am . (2022) 13:100316. doi: 10.2139/ssrn.4087375
24. Nordström, P, Ballin, M, and Nordström, A. Safety and effectiveness monovalent of COVID-19 mRNA vaccination and risk factors for hospitalisation caused by the omicron variant in 0.8 million adolescents: A nationwide cohort study in Sweden. PLoS Med . (2023) 20:e1004127. doi: 10.1371/journal.pmed.1004127
25. Risk, M, Miao, H, Freed, G, and Shen, C. Vaccine effectiveness, school reopening, and Risk of omicron infection among adolescents aged 12–17 years. J Adolesc Health . (2022) 72:147–52. doi: 10.1016/j.jadohealth.2022.09.006
26. Rudan, I, Millington, T, Antal, K, Grange, Z, Fenton, L, Sullivan, C, et al. BNT162b2 COVID-19 vaccination uptake, safety, effectiveness and waning in children and young people aged 12-17 years in Scotland. Lancet Regional Health-Europe . (2022) 23:100513. doi: 10.1016/j.lanepe.2022.100513
27. Tan, SHX, Cook, AR, Heng, D, Ong, B, Lye, DC, and Tan, KB. Effectiveness of BNT162b2 vaccine against omicron in children 5 to 11 years of age. N Engl J Med . (2022) 387:525–32. doi: 10.1056/NEJMoa2203209
28. Tartof, SY, Frankland, TB, Slezak, JM, Puzniak, L, Hong, V, Xie, F, et al. Effectiveness associated with BNT162b2 vaccine against emergency department and urgent care encounters for Delta and omicron SARS-CoV-2 infection among adolescents aged 12 to 17 years. JAMA Netw Open . (2022) 5:E2225162. doi: 10.1001/jamanetworkopen.2022.25162
29. Tsang, NNY, So, HC, Cowling, BJ, Leung, GM, and Ip, DKM. Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study. Lancet Infect Dis . (2023) 23:421–434. doi: 10.2139/ssrn.4200539
30. Wanlapakorn, N, Kanokudom, S, Phowatthanasathian, H, Chansaenroj, J, Suntronwong, N, Assawakosri, S, et al. Comparison of the reactogenicity and immunogenicity between two-dose mRNA COVID-19 vaccine and inactivated COVID-19 vaccine followed by an mRNA vaccine in children aged 5-11 years. J Med Virol . (2023). 95:e28758. doi: 10.1002/jmv.28758
31. Buchan, SA, Nguyen, L, Wilson, SE, Kitchen, SA, and Kwong, JC. Vaccine effectiveness of BNT162b2 against Delta and Omicron Variants in Adolescents. Pediatrics . (2022) 150:e2022057634. doi: 10.1542/peds.2022-057634
32. Castelli, JM, Rearte, A, Olszevicki, S, Voto, C, Del Valle, JM, Pesce, M, et al. Effectiveness of mRNA-1273, BNT162b2, and BBIBP-CorV vaccines against infection and mortality in children in Argentina, during predominance of delta and omicron covid-19 variants: test negative, case-control study. BMJ . (2022) 379:e073070. doi: 10.1136/bmj-2022-073070
33. Cocchio, S, Zabeo, F, Tremolada, G, Facchin, G, Venturato, G, Marcon, T, et al. COVID-19 vaccine effectiveness against omicron variant among underage subjects: the Veneto Region’s experience. Vaccine . (2022) 10:1362. doi: 10.3390/vaccines10081362
34. Florentino, PTV, Millington, T, Cerqueira-Silva, T, Robertson, C, de Araújo, OV, Júnior, JBS, et al. Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: a test-negative case-control study. Lancet Infect Dis . (2022) 22:1577–86. doi: 10.1016/S1473-3099(22)00451-0
35. Jang, EJ, Choe, YJ, Kim, RK, and Park, YJ. BNT162b2 vaccine effectiveness against the SARS-CoV-2 omicron variant in children aged 5 to 11 years. JAMA Pediatr . (2023) 177:319–20. doi: 10.1001/jamapediatrics.2022.5221
36. Klein, NP, Stockwell, MS, Demarco, M, Gaglani, M, Kharbanda, AB, Irving, SA, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5-17 years - VISION network, 10 states, April 2021-January 2022. MMWR Morb Mortal Wkly Rep . (2022) 71:352–8. doi: 10.15585/mmwr.mm7109e3
37. Leung, D, Rosa Duque, JS, Yip, KM, So, HK, Wong, WHS, and Lau, YL. Effectiveness of BNT162b2 and CoronaVac in children and adolescents against SARS-CoV-2 infection during omicron BA.2 wave in Hong Kong. Commun Med . (2023) 3:3. doi: 10.1038/s43856-022-00233-1
38. Oliveira, EA, Oliveira, MCL, Colosimo, EA, Simões, ESAC, Mak, RH, Vasconcelos, MA, et al. Vaccine effectiveness against SARS-CoV-2 variants in adolescents from 15 to 90 days after second dose: a population-based test-negative case-control study. J Pediatr . (2022) 253:189–196.e2. doi: 10.1016/j.jpeds.2022.09.039
39. Powell, AA, Kirsebom, F, Stowe, J, Ramsay, ME, Lopez-Bernal, J, Andrews, N, et al. Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, august, 2021-march, 2022: a national, observational, test-negative, case-control study. Lancet Infect Dis . (2022) 23:435–44. doi: 10.1016/S1473-3099(22)00729-0
40. Rosa Duque, JS, Leung, D, Yip, KM, Lee, DHL, So, HK, Wong, WHS, et al. COVID-19 vaccines versus pediatric hospitalization. Cell Rep Med . (2023). 4:100936. doi: 10.1016/j.xcrm.2023.100936
41. Sacco, C, Del Manso, M, Mateo-Urdiales, A, Rota, MC, Petrone, D, Riccardo, F, et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5-11 years in Italy: a retrospective analysis of January-April, 2022. Lancet . (2022) 400:97–103. doi: 10.1016/S0140-6736(22)01185-0
42. Saito, Y, Yamamoto, K, Takita, M, Kami, M, Tsubokura, M, and Shibuya, K. Effectiveness of the booster of SARS-CoV-2 vaccine among Japanese adolescents: a cohort study. Vaccine . (2022) 10:1914. doi: 10.3390/vaccines10111914
43. Simmons, AE, Amoako, A, Grima, AA, Murison, KR, Buchan, SA, Fisman, DN, et al. Vaccine effectiveness against hospitalization among adolescent and pediatric SARS-CoV-2 cases between May 2021 and January 2022 in Ontario, Canada: A retrospective cohort study. PLoS One . (2023) 18:e0283715. doi: 10.1371/journal.pone.0283715
44. Wang, X, Chang, H, Tian, H, Zhu, Y, Li, J, Wei, Z, et al. Epidemiological and clinical features of SARS-CoV-2 infection in children during the outbreak of omicron variant in Shanghai, march 7-31, 2022. Influenza Other Respir Viruses . (2022) 16:1059–65. doi: 10.1111/irv.13044
45. Florentino, PTV, Alves, FJO, Cerqueira-Silva, T, Oliveira, VA, Junior, JBS, Jantsch, AG, et al. Vaccine effectiveness of CoronaVac against COVID-19 among children in Brazil during the omicron period. Nat Commun . (2022) 13:4756. doi: 10.1038/s41467-022-32524-5
46. Prunas, O, Warren, JL, Crawford, FW, Gazit, S, Patalon, T, Weinberger, DM, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. Science . (2022) 375:1151–4. doi: 10.1126/science.abl4292
47. Creech, CB, Anderson, E, Berthaud, V, Yildirim, I, Atz, AM, Melendez Baez, I, et al. Evaluation of mRNA-1273 Covid-19 vaccine in children 6 to 11 years of age. N Engl J Med . (2022) 386:2011–23. doi: 10.1056/NEJMoa2203315
48. Frenck, RW Jr, Klein, NP, Kitchin, N, Gurtman, A, Absalon, J, Lockhart, S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med . (2021) 385:239–50. doi: 10.1056/NEJMoa2107456
49. Viana, R, Moyo, S, Amoako, DG, Tegally, H, Scheepers, C, Althaus, CL, et al. Rapid epidemic expansion of the SARS-CoV-2 omicron variant in southern Africa. Nature . (2022) 603:679–86. doi: 10.1038/s41586-022-04411-y
50. Lyngse, FP, Mortensen, LH, Denwood, MJ, Christiansen, LE, Møller, CH, Skov, RL, et al. Household transmission of the SARS-CoV-2 omicron variant in Denmark. Nat Commun . (2022) 13:5573. doi: 10.1038/s41467-022-33328-3
51. Hoffmann, M, Krüger, N, Schulz, S, Cossmann, A, Rocha, C, Kempf, A, et al. The omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell . (2022) 185:447–56.e11. doi: 10.1016/j.cell.2021.12.032
52. Planas, D, Saunders, N, Maes, P, Guivel-Benhassine, F, Planchais, C, Buchrieser, J, et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature . (2022) 602:671–5. doi: 10.1038/s41586-021-04389-z
53. Ai, J, Zhang, H, Zhang, Y, Lin, K, Zhang, Y, Wu, J, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect . (2022) 11:337–43. doi: 10.1080/22221751.2021.2022440
54. Payne, RP, Longet, S, Austin, JA, Skelly, DT, Dejnirattisai, W, Adele, S, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell . (2021) 184:5699–714.e11. doi: 10.1016/j.cell.2021.10.011
55. Department of Health & Social Care GU. Statement from the UK chief medical officers on the prioritisation of first doses of COVID-19 vaccines (2020) Available from: https://www.gov.uk/government/news/statement-from-the-uk-chief-medical-officers-on-the-prioritisation-of-first-doses-of-covid-19-vaccines .
56. Department of Health & Social Care GU. Oxford university/AstraZeneca vaccine authorised by UK medicines regulator (2020). Available from: https://www.gov.uk/government/news/oxford-universityastrazeneca-vaccine-authorised-by-uk-medicines-regulator .
57. Andrews, N, Stowe, J, Kirsebom, F, Toffa, S, Sachdeva, R, Gower, C, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med . (2022) 28:831–7. doi: 10.1038/s41591-022-01699-1
58. Regev-Yochay, G, Gonen, T, Gilboa, M, Mandelboim, M, Indenbaum, V, Amit, S, et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against omicron. N Engl J Med . (2022) 386:1377–80. doi: 10.1056/NEJMc2202542
59. Agrawal, U, Bedston, S, McCowan, C, Oke, J, Patterson, L, Robertson, C, et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet . (2022) 400:1305–20. doi: 10.1016/S0140-6736(22)01656-7
60. Tseng, HF, Ackerson, BK, Luo, Y, Sy, LS, Talarico, CA, Tian, Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and Delta variants. Nat Med . (2022) 28:1063–71. doi: 10.1038/s41591-022-01753-y
61. Rosenberg, ES, Holtgrave, DR, Dorabawila, V, Conroy, M, Greene, D, Lutterloh, E, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status - New York, may 3-July 25, 2021. MMWR Morb Mortal Wkly Rep . (2021) 70:1150–5. doi: 10.15585/mmwr.mm7034e1
62. Feikin, DR, Higdon, MM, Abu-Raddad, LJ, Andrews, N, Araos, R, Goldberg, Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet . (2022) 399:924–44. doi: 10.1016/S0140-6736(22)00152-0
63. Hodgson, SH, Mansatta, K, Mallett, G, Harris, V, Emary, KRW, and Pollard, AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis . (2021) 21:e26–35. doi: 10.1016/S1473-3099(20)30773-8
64. Lund, FE, and Randall, TD. Scent of a vaccine. Science . (2021) 373:397–9. doi: 10.1126/science.abg9857
65. Reynolds, HY . Immunoglobulin G and its function in the human respiratory tract. Mayo Clin Proc . (1988) 63:161–74. doi: 10.1016/S0025-6196(12)64949-0
66. Mades, A, Chellamathu, P, Kojima, N, Lopez, L, MacMullan, MA, Denny, N, et al. Detection of persistent SARS-CoV-2 IgG antibodies in oral mucosal fluid and upper respiratory tract specimens following COVID-19 mRNA vaccination. Sci Rep . (2021) 11:24448. doi: 10.1038/s41598-021-03931-3
67. Yang, H, Xie, Y, and Li, C. Understanding the mechanisms for COVID-19 vaccine's protection against infection and severe disease. Expert Rev Vaccines . (2023) 22:186–92. doi: 10.1080/14760584.2023.2174529
68. Varona, JF, Muñiz, J, Balboa-Barreiro, V, Peñalver, F, Abarca, E, Almirall, C, et al. Persistence and waning of natural SARS-CoV-2 antibodies over 18 months: long-term durability of IgG humoral response in healthcare workers. J Gen Intern Med . (2022) 37:2614–6. doi: 10.1007/s11606-022-07652-9
69. Hewitt, RJ, and Lloyd, CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol . (2021) 21:347–62. doi: 10.1038/s41577-020-00477-9
70. Hawman, DW, Meade-White, K, Archer, J, Leventhal, SS, Wilson, D, Shaia, C, et al. SARS-CoV2 variant-specific replicating RNA vaccines protect from disease following challenge with heterologous variants of concern. eLife . (2022) 11:11. doi: 10.7554/eLife.75537
71. Cinicola, BL, Piano Mortari, E, Zicari, AM, Agrati, C, Bordoni, V, Albano, C, et al. The BNT162b2 vaccine induces humoral and cellular immune memory to SARS-CoV-2 Wuhan strain and the omicron variant in children 5 to 11 years of age. Front Immunol . (2022) 13:1094727. doi: 10.3389/fimmu.2022.1094727
72. Khoury, DS, Docken, SS, Subbarao, K, Kent, SJ, Davenport, MP, and Cromer, D. Predicting the efficacy of variant-modified COVID-19 vaccine boosters. Nat Med . (2023) 29:574–8. doi: 10.1038/s41591-023-02228-4
73. Yegiazaryan, A, Abnousian, A, Alexander, LJ, Badaoui, A, Flaig, B, Sheren, N, et al. Recent developments in the understanding of immunity, pathogenesis and management of COVID-19. Int J Mol Sci . (2022) 23:9297. doi: 10.3390/ijms23169297
Keywords: SARS-CoV-2 variants, Omicron, COVID-19 vaccines, child, adolescent
Citation: Lu W, Zeng S, Yao Y, Luo Y and Ruan T (2024) The effect of COVID-19 vaccine to the Omicron variant in children and adolescents: a systematic review and meta-analysis. Front. Public Health . 12:1338208. doi: 10.3389/fpubh.2024.1338208
Received: 14 November 2023; Accepted: 27 March 2024; Published: 10 April 2024.
Reviewed by:
Copyright © 2024 Lu, Zeng, Yao, Luo and Ruan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiechao Ruan, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

Call us @ 08069405205

Search Here

- An Introduction to the CSE Exam
- Personality Test
- Annual Calendar by UPSC-2024
- Common Myths about the Exam
- About Insights IAS
- Our Mission, Vision & Values
- Director's Desk
- Meet Our Team
- Our Branches
- Careers at Insights IAS
- Daily Current Affairs+PIB Summary
- Insights into Editorials
- Insta Revision Modules for Prelims
- Current Affairs Quiz
- Static Quiz
- Current Affairs RTM
- Insta-DART(CSAT)
- Insta 75 Days Revision Tests for Prelims 2024
- Secure (Mains Answer writing)
- Secure Synopsis
- Ethics Case Studies
- Insta Ethics
- Weekly Essay Challenge
- Insta Revision Modules-Mains
- Insta 75 Days Revision Tests for Mains
- Secure (Archive)
- Anthropology
- Law Optional
- Kannada Literature
- Public Administration
- English Literature
- Medical Science
- Mathematics
- Commerce & Accountancy
- Monthly Magazine: CURRENT AFFAIRS 30
- Content for Mains Enrichment (CME)
- InstaMaps: Important Places in News
- Weekly CA Magazine
- The PRIME Magazine
- Insta Revision Modules-Prelims
- Insta-DART(CSAT) Quiz
- Insta 75 days Revision Tests for Prelims 2022
- Insights SECURE(Mains Answer Writing)
- Interview Transcripts
- Previous Years' Question Papers-Prelims
- Answer Keys for Prelims PYQs
- Solve Prelims PYQs
- Previous Years' Question Papers-Mains
- UPSC CSE Syllabus
- Toppers from Insights IAS
- Testimonials
- Felicitation
- UPSC Results
- Indian Heritage & Culture
- Ancient Indian History
- Medieval Indian History
- Modern Indian History
- World History
- World Geography
- Indian Geography
- Indian Society
- Social Justice
- International Relations
- Agriculture
- Environment & Ecology
- Disaster Management
- Science & Technology
- Security Issues
- Ethics, Integrity and Aptitude

- Indian Heritage & Culture
- Enivornment & Ecology

Explain the concept of mRNA vaccines, outlining their potential benefits in preventing infectious diseases like COVID-19, and discuss their limitations and challenges.
Topic: Awareness in the fields of IT, Space, Computers, robotics, Nano-technology, biotechnology and issues relating to intellectual property rights.
4. Explain the concept of mRNA vaccines, outlining their potential benefits in preventing infectious diseases like COVID-19, and discuss their limitations and challenges. (250 words)
Difficulty level: Moderate
Reference: Insights on India
Why the question: The question is part of the static syllabus of General studies paper – 3 and mentioned as part of Mission-2024 Secure timetable. Key Demand of the question: To write about mRNA vaccines, their potential benefits and challenges and limitations associated with their use. Directive word: Discuss – This is an all-encompassing directive – you must debate on paper by going through the details of the issues concerned by examining each one of them. You must give reasons for both for and against arguments. Structure of the answer: Introduction: Begin by introducing the concept of mRNA vaccines and their relevance, especially in the context of recent vaccine developments. Body: Firstly, explain the fundamental concept of mRNA vaccines, including how they work to stimulate an immune response. Next, write about the advantages of mRNA vaccines, such as their rapid development potential, versatility against various pathogens, and effectiveness in preventing infectious diseases. Highlight their role in addressing public health emergencies, like the COVID-19 pandemic, through quick vaccine development. Next, write about the limitations associated with mRNA vaccines, such as the need for ultra-cold storage, potential short-term side effects, and the relatively recent nature of this technology. Conclusion: Conclude by writing a way forward.

- Our Mission, Vision & Values
- Director’s Desk
- Commerce & Accountancy
- Previous Years’ Question Papers-Prelims
- Previous Years’ Question Papers-Mains
- Environment & Ecology
- Science & Technology
- Share full article
Advertisement
Supported by
Robert MacNeil, Earnest News Anchor for PBS, Dies at 93
With his longtime co-host Jim Lehrer, he delivered thoughtful reports that stood in stark contrast to the commercial networks’ ever more sensational newscasts.

By Elizabeth Jensen
Robert MacNeil, the Canadian-born journalist who delivered sober evening newscasts for more than two decades on PBS as the co-anchor of “The MacNeil/Lehrer Report,” later expanded as “The MacNeil/Lehrer NewsHour,” died early Friday in Manhattan. He was 93.
His death, at NewYork–Presbyterian Hospital, was confirmed by his daughter Alison MacNeil.
Mr. MacNeil spent time at NBC News early in his career and was a reporter for the network in Dallas on the day President John F. Kennedy was assassinated. But he came to reject the flashier style of the commercial American networks, and in 1971 he joined the fledgling Public Broadcasting Service.
He brought with him a news sensibility honed at the BBC, where he had worked in the interim, and became a key figure in shaping U.S. public television’s in-depth and evenhanded approach to news coverage.
A pairing with Jim Lehrer in 1973 to cover the Senate Watergate hearings for PBS was unpopular with the operators of many local public stations, who thought the prime-time broadcasts weren’t appropriate evening fare. But the two men’s serious demeanor was a hit with viewers, and the broadcasts won an Emmy Award and eventually launched an enduring collaboration.
In October 1975, some major public stations began carrying the “The Robert MacNeil Report,” a half-hour of Mr. MacNeil’s design that examined a single issue each night and shunned showy production values. Within a year the program was renamed “The MacNeil/Lehrer Report.” It was expanded again in 1983 to become “The MacNeil/Lehrer NewsHour,” a multitopic program that was the nation’s first full hour of evening news.
The program offered a stark counterpoint to the ever-frothier newscasts on the commercial networks’ local affiliates and was honored with every major broadcast journalism award.
Intensely private in public, Mr. MacNeil was known to friends as engaging and wickedly funny. He was proud of his no-nonsense style on air, which critics called boring but which he called civilized discourse in the public interest. One memorable example was his hourlong interview in 1985 with Fidel Castro, in which Mr. Castro reluctantly defended the Soviet invasion of Afghanistan in 1979, in part because he would never “be on the side of the United States.’’
Mr. MacNeil defended his interviewing style and his program’s unsensational approach to weighty topics. “I cannot stand the theatrical, prosecutorial interview, the interview designed to draw attention to the interviewer, full of either mawkish, false sentiment or theatrically belligerent questioning,” he told The New York Times in 1995, when he retired from the daily newscast.
“Every journalist in this country has a stake in the democratic system working, and I think institutions of democracy are worth taking seriously,” he added. “It’s a very old-fashioned, corny view, but Jim and I both feel that strongly, which is one of the reasons our show is the way it is.”
Robert Breckenridge Ware MacNeil, known as Robin, was born on Jan. 19, 1931, in Montreal and raised in the port city of Halifax, Nova Scotia. His father, Robert A.S. MacNeil, served in the Royal Canadian Navy during World War II, commanding convoy escort ships, and later joined the Royal Canadian Mounted Police. His mother Margaret (Oxner) MacNeil, was left to raise her children alone for several years while her husband was at war.
While Mr. MacNeil was attending Dalhousie University in Halifax, a producer for the Canadian Broadcasting Corp. saw him in a school production of “Othello,” and he was hired to act in CBC radio productions and eventually a daily radio soap opera.
He soon dropped out of college to try his hand full time at stage acting, but decided that he was better suited to be a playwright and returned to school, this time at Carleton University in Ottawa. While still a student he worked as a national radio announcer for the CBC and then for the CBC’s new television service, where he also hosted a children’s program.
After graduating, he moved to England to write plays, but quickly turned to journalism to make money. He told The Times in 1995, “I had one of those golden careers; it just floated.”
In 1960, after five years at the Reuters news agency in London, Mr. MacNeil joined NBC News, eventually replacing John Chancellor as a wide-ranging foreign correspondent, covering wars in Africa and the Cuban missile crisis. (For about a week after that October 1962 episode, he and five other journalists were held under house arrest in a Havana hotel by the Castro government.) He was present at the construction of the Berlin Wall and later covered its dismantling in 1989.
Mr. MacNeil was assigned to cover Washington in 1963 and was on his first presidential trip on Nov. 22 when President Kennedy was assassinated in Dallas. While his work covering the killing was overshadowed by that of his NBC News colleagues, he may have had his own brush with the drama of that day.
After the shots were fired in Dealey Plaza, Mr. MacNeil made his way to the nearest building, the Texas School Book Depository — the building from which the fatal shots had been fired. There, he asked a man who was leaving and another in the lobby where the nearest telephone was. Kennedy’s accused assassin, Lee Harvey Oswald, later told the Dallas police that he had encountered a Secret Service agent at the building. The historian William Manchester concluded in his 1967 book, “The Death of a President,” that the man in the suit, crew cut and press badge was, in fact, Mr. MacNeil.
In his autobiography, “The Right Place at the Right Time” (1982), Mr. MacNeil wrote that “it was possible, but I had no way of confirming that either of the young men I had spoken to was Oswald.”
In 1965, Mr. MacNeil became the co-anchor, with Ray Scherer, of NBC’s half-hour weekend news broadcast, “The Scherer-MacNeil Report.” But two years later he returned to London, reporting for the BBC’s “Panorama” program, before joining PBS in 1971.
Mr. MacNeil, who had homes in Manhattan and Nova Scotia, became an American citizen in 1997 and was made an officer in the Order of Canada the same year. He reflected on his life as a dual citizen in a 2003 memoir, “Looking for My Country: Finding Myself in America.”
His wife, Donna MacNeil, died in 2015 . His first marriage, to Rosemarie Coopland, ended in divorce, as did his second marriage, to Jane Doherty.
He is survived by two children from his first marriage, Ian MacNeil, a theatrical set designer who won a Tony in 2009 for his work on the musical “Billy Elliott,” and Cathy MacNeil; two children from his second marriage, Alison and Will MacNeil; and five grandchildren.
After retiring from the daily newscast, Mr. MacNeil continued to work with PBS, including hosting the “America at a Crossroads” series of documentaries in 2007, which examined the nation’s challenges in the post-9/11 world. With Mr. Lehrer, his close friend, he remained a partner in MacNeil/Lehrer Productions, which produced their newscast until 2014, when WETA, the Washington, D.C., public media station where the “NewsHour” is based, assumed ownership. Mr. Lehrer died in 2020 at 85.
Mr. MacNeil found himself at the center of controversy in 2011 when, returning to “NewsHour” for a six-part series on autism, he featured the story of his grandson Nick. He was criticized for allowing his daughter Alison to question whether her son’s autism was linked to vaccines. (He did qualify her comments by noting that “public health authorities say there is no scientifically valid evidence that vaccines cause autism.”)
Mr. MacNeil chaired the board of the MacDowell Colony (now known as MacDowell), the retreat for artists, writers and musicians in Peterborough, N.H., from 1993 to 2010. After leaving the “NewsHour,” he returned to his first love, writing. He was the author of “The People Machine” (1968), about the relationship between television and politics; three memoirs; and four novels — “Burden of Desire” (1992), “The Voyage” (1995), “Breaking News” (1998) and “Portrait of Julia” (2013).
He was a co-author of “The Story of English,” a companion volume to the 1986 BBC-PBS television series that he hosted, and he wrote its 2005 sequel, “Do You Speak American?”
Mr. MacNeil remained proud of his early evening newscast. In interviews for the Archive of American Television in 2000 and 2001, he was asked how he wanted to be remembered.
“Television has changed journalism, utterly, not just for television, but for print and everybody else,” he said. “It’s changed the whole culture and ethos of journalism. And to have been able hold the line — perhaps Canute-like — against a tide that’s going to engulf us all in the end, for a few years, has been a source of gratification to me.”
Sofia Poznansky contributed reporting

IMAGES
VIDEO
COMMENTS
500+ Words Essay on Coronavirus Vaccine. The Coronavirus has infected millions of people so far all over the world. In addition to that, millions of people have lost their lives to it. Ever since the outbreak, researchers all over the world have been working constantly to develop vaccines that will work effectively against the virus.
Please use one of the following formats to cite this article in your essay, paper or report: APA. Moore, Sarah. (2022, January 17). The Importance of Global COVID-19 Vaccination.
A novel coronavirus (CoV) named '2019-nCoV' or '2019 novel coronavirus' or 'COVID-19' by the World Health Organization (WHO) is in charge of the current outbreak of pneumonia that began at the beginning of December 2019 near in Wuhan City, Hubei Province, China [1-4]. COVID-19 is a pathogenic virus. From the phylogenetic analysis ...
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
Writing About COVID-19 in College Essays. Experts say students should be honest and not limit themselves to merely their experiences with the pandemic. The global impact of COVID-19, the disease ...
العربية. 25 October 2022. Vaccines save millions of lives each year. The development of safe and effective COVID-19 vaccines are a crucial step in helping us get back to doing more of the things we enjoy with the people we love. We've gathered the latest expert information to answer some of the most common questions about COVID-19 ...
Amid the staggering amount of suffering and death during this historic pandemic of COVID-19, a remarkable success story stands out. The development of several highly efficacious vaccines against a previously unknown viral pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in less than 1 year from the identification of the virus is unprecedented in the history of vaccinology.
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement — in 18 months, researchers, manufacturers and ...
This experience shed light on the need for real-time vaccine safety surveillance and the importance of context in decision making during a pandemic. In a study now reported in the Journal by Barda ...
COVID-19 vaccines. The development and roll-out of COVID-19 vaccines have been fundamental in saving lives and protecting people from severe disease and death —especially those most at-risk and vulnerable to COVID-19, protecting health systems, and reducing widescale social disruption. WHO in the Western Pacific continues to work with ...
100 Words Essay on Covid 19. COVID-19 or Corona Virus is a novel coronavirus that was first identified in 2019. It is similar to other coronaviruses, such as SARS-CoV and MERS-CoV, but it is more contagious and has caused more severe respiratory illness in people who have been infected. The novel coronavirus became a global pandemic in a very ...
For some COVID-19 vaccines, two doses are required . It's important to get the second dose if the vaccine requires two doses. For vaccines that require two doses, the first dose presents antigens - proteins that stimulate the production of antibodies - to the immune system for the first time. Scientists call this priming the immune response.
1. Listen with empathy. Start by listening with empathy to those who have questions around vaccination. Don't dismiss them, and acknowledge how they're feeling (without necessarily agreeing, for example "it's okay to have questions, or want more information before getting a vaccine"). 2. Ask open-ended questions.
Step 1: Choose a Specific Thesis Statement. Your thesis statement should clearly state your position on a specific aspect of COVID-19. It should be debatable and clear. For example: Thesis Statement: "COVID-19 vaccination mandates are necessary for public health and safety."
Background Despite reliable evidence-based research supporting the COVID-19 vaccines, population-wide confidence and trust remain limited. We sought to expand prior knowledge about COVID-19 vaccine perceptions, while determining which population groups are at greatest risk for not getting a vaccine. Methods Study participants in the U.S. (79% female, median age group 46-60 years) were ...
The first essay series under this theme delves into different dimensions of COVID-19 Vaccines in Southeast Asia, including issues related to vaccine supply, confidence, delivery, distribution and beyond. Visit the SDGHI website to read the other essays in the series. For any enquiries, please contact: [email protected].
A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 ( SARS-CoV-2 ), the virus that causes coronavirus disease 2019 ( COVID‑19 ). Prior to the COVID‑19 pandemic, an established body of knowledge existed about the structure and function of coronaviruses causing ...
Essay On Covid-19: 100, 200 and 300 Words. COVID-19, also known as the Coronavirus, is a global pandemic that has affected people all around the world. It first emerged in a lab in Wuhan, China, in late 2019 and quickly spread to countries around the world. This virus was reportedly caused by SARS-CoV-2. Since then, it has spread rapidly to ...
The availability of a safe and effective vaccine for COVID-19 is well-recognized as an additional tool to contribute to the control of the pandemic. At the same time, the challenges and efforts needed to rapidly develop, evaluate and produce this at scale are enormous. It is vital that we evaluate as many vaccines as possible as we cannot ...
2. Vaccination strategies. Many efforts have been directed towards the development of the vaccines against COVID-19, to avert the pandemic and most of the developing vaccine candidates have been using the S-protein of SARS-CoV-2 (Dhama et al., 2020).As of July 2, 2020, the worldwide SARS-CoV-2 vaccine landscape includes 158 vaccine candidates, out of which 135 are in the preclinical or the ...
Effective and safe COVID-19 vaccines have been developed at a rapid and unprecedented pace to control the spread of the virus, and prevent hospitalisations and deaths. However, COVID-19 vaccine uptake is challenged by vaccine hesitancy and anti-vaccination sentiments, a global shortage of vaccine supply, and inequitable vaccine distribution especially among low- and middle-income countries ...
Topic: To what extent do you support or oppose the governments' mandatory vaccination policies against the Covid-19 pandemic? For more than a year, people from many countries have been living through the peak of the global COVID-19 pandemic around the world, which has caused serious and terrible consequences, because of this, there is now a chase for the development of the necessary vaccines.
Keywords: SARS-CoV-2 variants, Omicron, COVID-19 vaccines, child, adolescent. Citation: Lu W, Zeng S, Yao Y, Luo Y and Ruan T (2024) The effect of COVID-19 vaccine to the Omicron variant in children and adolescents: a systematic review and meta-analysis. Front. Public Health. 12:1338208. doi: 10.3389/fpubh.2024.1338208
Topic: Awareness in the fields of IT, Space, Computers, robotics, Nano-technology, biotechnology and issues relating to intellectual property rights. 4. Explain the concept of mRNA vaccines, outlining their potential benefits in preventing infectious diseases like COVID-19, and discuss their limitations and challenges. (250 words) Difficulty level: Moderate Reference: Insights on India Why the ...
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. Most people infected with the virus will experience mild to moderate respiratory illness and recover without requiring special treatment. However, some will become seriously ill and require medical attention. Older people and those with underlying medical ...
Associated Press. Robert MacNeil, the Canadian-born journalist who delivered sober evening newscasts for more than two decades on PBS as the co-anchor of "The MacNeil/Lehrer Report," later ...