During planning and operationalization stages, before enrollment starts, research teams should discuss what forms will be needed for their study. These could be data collection forms, source documentation, even forms or logs for study management like visit checklists and progress notes.
In general, templates are an efficient way to build study documents but care needs to be taken that edits are made that are relevant and appropriate for each study. Study teams can use these templated tools and edit for each new study or can build their own templates based on their usual needs to use for all future studies.
The CRRO templated tools are not meant to be static, unchanging documents, but must be edited for each study to align with IRB-approved procedures that are in the INSPIR application, protocol, or other study document. However, not all templated tools that are available will be required or should be used on every study. Study teams should review all available tools and what will be needed for compliant and complete documentation.
CRRO templated tools are built upon a mix of institutional policies, federal regulations, ICH Good Clinical Practice, and current best practices. The CRRO cannot make any guarantee that these templated tools follow current policy or regulations as those may change without warning or announcement. These templated tools are not meant to be used as a policy or guidance document.
It is strongly recommended that study teams review the Overview and Instructions prior to using any of the templated tools. General questions on how to use these forms or implement them for a specific study can be answered by contacting the CRRO . Specific guidance on building these tools within Word, REDCap, or other systems are available from the CRRO .
Suggestions for additional tools or changes to current tools are welcome and can be submitted on our Tool Recommendation Form .
Adverse Event Form Adverse Event Log Biospecimen Storage and Tracking Log Delegation of Authority and Responsibilities Eligibility Criteria Checklist Essential Documents Location Cover Page Informed Consent Documentation IRB Submissions Log Note to File Template Participant Completion Form Participant Identification Log Phone Call Summary Report Pregnancy Testing Documentation Protocol Deviation Form Protocol Deviation Log Regulatory Binder Cover Page Schedule of Events Screening and Enrollment Log Site Visit Log SOP or MOP Template Staff License Log Training Log – Group Training Log – Individual Visit Checklist
FAQs on Regulatory Documentation Regulatory Binder Tabs REDCap e-Reg Lite AdobeSign Guidance


Regulatory Binder
Essential documents.
The ICH GCP Guidelines define Essential Documents as those documents which individually and collectively permit evaluation of the conduct of a trial and the quality of data produced. These documents serve to demonstrate compliance with standards of Good Clinical Practice and with all applicable regulatory requirements. Filing essential documents in a timely manner can greatly assist in the successful management of a clinical trial.
The Regulatory Binder is often the first document reviewed during audits and inspections. Not all the essential documents are available at the start of the study. Documents can be grouped into those that are generated before study initiation, those that are generated during trial conduct and those that are generated after study completion.
Not all documents have to be filed in one single binder. The Regulatory Binder may sometimes consist of several binders that are stored in the same or different locations. It is important to know where all these documents are located to be able to pull them out when needed in a timely manner. The Regulatory Binder is referred to synonymously as the Study Files, Investigator Files or Investigator Binder.
Organizing Your Regulatory Binder
Instructions: Create tabs for each section listed below and place the appropriate documents in each corresponding section in a binder. Be sure to label the outside of the binder (cover and spine) with the protocol number, PI name, and study site. Use multiple binders or master binders to maintain documentation if needed.
Anytime information is kept in a master binder, place a note to file (in the section of the Binder) referencing the location of the separate binder.
1. Site Visit (Monitoring) Log
This provides documentation at the site that the study was monitored and the frequency of monitoring. The monitor and designated site staff both sign the log to verify the date the monitor was present. For consecutive days, each day is entered separately.
2. Delegation of Authority (Responsibilities) Log
This log documents responsibilites assigned to research team members and their dates of involvement in the project. It helps ensure the appropriate delegation of study related tasks.
3. Site Personnel Signature Log
This documents the names and provides handwriting samples of all personnel involved in the conduct of the study
4. Study Personnel Education
All personnel involved in research with human subjects are required to complete the following:
Human Subject Protection Training HIPAA Training
When adding personnel to the study, they must complete all of the above and their addition must be IRB approved prior to participating in the study.
4.1 Training Log
This is a record of training provided, e.g. protocol training or other study-specific training of staff. This should include a site initiation visit (SIV) attendance log.
5. CVs/Financial Disclosures/Investigator Statements
This section should include:
Curricula Vitae, licenses, and certifications for all study staff Disclosure information, including each Study Specific Disclosure form submitted to the IRB. FDA Form 1572 (if applicable): Date and sign all versions FDA Form 1571 (if applicable): for Investigator initiated INDs Signed investigator agreement (if applicable): for device studies
6. Public Registration of Research Studies (PRS) (If applicable)
All research studies that are applicable clinical trial must be registered at www.clinicaltrials.gov as per the International Committee of Medical Journal Editors (ICMJE), the FDA Amendment Act of 2007, and institutional policy.
Contact the Georgetown PRS Administrator, Patricia Mazar at [email protected] to set up a PRS user account.
Note: For commercially funded, multi-center studies, public registration is typically handled by the study sponsor or CRO.
Place the registration receipt in this section for initial registration and for any updates.
7. Screening/Enrollment Log
This section should include a log of subjects who were screened (and reason for screen failure) and enrolled. Some studies allow for re-screening of subjects.
8. Subject Visit Tracking Log
This log tracks all enrolled subjects’ visits, reason for early termination and keeps visits scheduled as per protocol.
9. Subject Identification Code List
This is a confidential list of the names of all the subjects that provides a link between their identity and their study code to allow the Investigator to reveal the identity of any subject, if necessary.
10. Consent Forms
This section should include consent form document(s) (all IRB approved and stamped versions) stored in reverse chronological order with the current approved version first.
Place most currently approved consent form in a plastic sleeve
Note: Any changes to the consent form must be submitted to and approved by the IRB prior to use. Submit consent amendments through eRIC .
Guidance for consent of Non-English speaking subjects can be found here .
10.1 HIPAA Forms
(Authorization, Waiver, and/or Research Preparation Purposes)
This section includes all IRB approved and stamped versions of any of the HIPAA forms (as applicable).
HIPAA regulatory information
11. Protocol
This section should include the protocol (and protocol signature page) and all amendments (and amendment signature page or pages), stored in reverse chronological order with the current approved version first.
Note: Any changes to the protocol must be submitted to and approved by the IRB prior to implementation. Submit amendments through eRIC .
12. IRB Federal Wide Assurance Letter
This section should contain the most current IRB assurance letter
13. IRB Approval(s) /Communication
This section should include copies of the original IRB application/submission, IRB approval letters (contingent and final approval), and all correspondence with the IRB (including emails).
It includes IRB Membership Rosters , Continuing Review Submissions, protocol modifications and DSMB reports and close-out (final study) reports.
Contact the IRB for a copy of any missing documents.
14. Investigational Product Information (as applicable)
Investigator’s Brochure (IB)
This section must include all versions of the IB (may be maintained separately with note in section explaining location of IB) and receipt forms.
Evidence of IRB submission and review of all versions must be maintained.
Package Insert
For FDA approved agents, file a copy of the package insert.
Device Manual
Fo device studies this section should have a device information sheet/manual.
15. Study Termination If your research study is being terminated or if the PI is leaving and the study will no longer be continued, inform the IRB through eRIC .
16. Protocol Deviations / Protocol Exceptions
This section should include correspondence relevant to the issue and copies of the documents stored in reverse chronological order with the most current documents first.
Please note that some Sponsor approved waivers may need to be approved by the IRB prior to implementation.
GU IRB Manual-Policy on Reporting Protocol Deviations
CAPA Template (Corrective Action and Prevention Plan)
17. Adverse Events and Unanticipated Problems
This section should include correspondence, copies and acknowledgements of reports for internal AEs and unanticipated problems reported to the IRB and Sponsor and regulatory authorities as applicable.
AE/SAE Log : Adverse events encompass both physical and psychological harms. They can occur in the context of medical, behavioral and social research.
Unanticipated Problem Log : Click here for examples of unanticipated problems that do not involve adverse events.
GU IRB Manual-Policy on Reporting Adverse Events and Unanticipated Problems
Submit reportable events through eRIC .
18. IND Safety Reports
This section should include correspondence (including IRB acknowledgement) and copies of Safety Reports for external AEs reported to the IRB.
19. Advertising/Educational Materials (if applicable)
This section should include: Any IRB approved advertisements, recruitment flyers, written educational, or other materials provided to study participants, stored in reverse chronological order with the most current documents first.
Note: For marketing materials used to recruit through mass media (e.g. newspaper, TV, radio, some internet postings, & etc.) you must contact the Georgetown University Medical Center Communications Office to ensure logo/branding is appropriate.
20. Sample Tracking and Shipping (if applicable)
This section should include a master log that allows tracking of research specimen sample collection, shipment (or transport), and storage, and packing and shipping training certification (from Saf-T-Pak or other approved equivalent program).
Shippers or receipts can be placed in this section or in individual subject files.
Note: All biological materials must be handled, stored, and shipped in compliance with FAA and IATA regulations as well as GUMC policies on hazardous materials.
21. Temperature Logs for Refrigerator/Freezer
Temperature logs document compliance with Protocol /Study Procedures requirements and GCP.
22. Investigational/Test Article
This section includes:
Shipment records (usually requires site signature of receipt and Sponsor notification of receipt)
Site Accountability Records (inventory of overall supply of drug/device, promps reordering of supply)
Subject Drug Accountability Records / Device Log (documents the date and quantity of drug/device dispensed to subject and return of drug/device from subject
Blind Break Instructions (instructions for revealing the identity of the treatment, if blinded)
Interactive Voice Response System Instructions (IVRS), if applicable
**Maintain drug accountability in the Research Pharmacy over the course of the study; at the trial completion file all records here.
23. Local Lab Certificates/Reference Ranges
For every lab listed on FDA Form 1572, place a copy of (maintain current certifications for duration of study):
Lab certificate(s) and reference ranges (for the duration of study) Lab director’s CV
Note: The above is not required for research labs that perform testing where results will not be shared with subjects or their treatment providers.
For studies that use MedStar/Georgetown University Hospital Laboratories, click HERE
24. Correspondence
Please document and maintain all relevant, significant communication from the sponsor, the CRO or monitor in this section. Study related Newsletters may be placed in this section.
25. Blank Set of Case Report Forms
26. Notes To File (NTF)
These may include site generated and/or sponsor generated notes to file. Sponsor generated NTF may be global or site specific.
27. Other Documents
Other necessary approvals (e.g. Radiation Safety Committee)
Place other important study documents in this section. This can include: Certificates of Confidentiality, literature or publications, correspondence from the FDA or NIH, and other general correspondence.
28. Additional Tools
We’d like to hear from you. If you have suggestions, comments, or questions about this regulatory binder, please contact Bronwyn Murray at [email protected] or at 202-687-1350.
Research Rules & Policies
Clinical research record retention.
Federal regulation and International Conference on Harmonization (ICH) Good Clinical Practice (E-6) requires investigators and sponsors to retain specific study records associated with the conduct of clinical research. These documents are often referred to as Essential Documents. The Division of Microbiology and Infectious Diseases (DMID) guidances below are specifically directed to DMID clinical trials conducted under an Investigational New Drug Application (IND), Investigational Device Exemption (IDE) or international equivalent. The general principles apply to all clinical research.
45 Code of Regulations (CFR) part74.53 also requires awardees to retain records pertinent to an award for a period of three years from the date of submission of the final expenditure report or, for awards that are renewed quarterly or annually, from the date of the submission of the quarterly or annual financial report. Investigators should reference the regulation for details.
Investigators should also be aware that they may also have local government or institutional policies for record retention. The most stringent requirement would apply.
Additional Information
- DMID Regulatory File Guidelines
- DMID Source Documentation Standards
- FDA heading for Investigation New Drug application, sections 312.57 and 312.62 and IDE regulations, section 812.140
- Human Subjects Protections heading- 45CFR46.115
- Institutional Review Boards and Ethical Committees heading
- International Conference on Harmonisation heading-ICH GCP Guidelines E-6 section 8
- Record Retention heading
Good Clinical Practice Study Documentation
The Department of Medicine Clinical Research Unit has prepared this document is to provide guidance to all faculty and staff involved in the conduct of research on the best practices related to documentation .
Good study documentation will allow for an individual with basic knowledge of the particular project to recreate the events of the study.
General Information
- Maintain records of all data and observations pertinent to the research subject. These records should be identifiable to a particular participant.
- Remember that source documents are where the information is first recorded.
- All data must be verifiable.
- Study documentation should be able to recreate the study for any reviewer.
- Attributable – Can you tell who wrote and/or did this
- Legible – Can it be read?
- Contemporaneous- – Is the data current, and in the correct time frame? The notation, signature and date should occur at the same time.
- Original – Has the data been altered?
- Accurate – Are there conflicting data elsewhere? Content should precisely reflect the event.
- Use a signed Note to File to explain any discrepancies, missing or incomplete data.
- The same standards maintained for medical documentation should be followed for research documentation
- All documents require 2 identifiers on each page.
- All entries are to be signed and dated in real time.
- Error corrections are made by drawing a single line through the incorrect entry, initial and date.
- Never obliterate entries that require correction.
- Subject records need to be secure but accessible.
- Do not alter past-dated notes by writing alongside or adding to prior entries. Updates may be made through addenda.
- Use dark ink, do not use pencil.
- Never use whiteout.
- If the source data is incomplete or deficient, it may be completed or corrected using an addendum. This late entry must be signed and dated at the time it is created.
Note to File
- May be used to correct errors, or as an explanation to a departure from the protocol. Reasons for any departure should be documented and attempts to correct or prevent in the future should be included.
- This should not be used as a panacea to correct any error.
Informed Consent
- The process requires documentation and should reflect the process approved by the IRB in a narrative form or through the use of a checklist.
- Signature and date and time must be of the person obtaining the consent, at the time of the process. (Not added later)
Case Report Forms as Source
Case report forms may be used as source only when this practice is clearly outlined in the protocol, and they represent the data collected for the research are where the data were initially recorded.
Medical Records From Outside Source
- Copies of records from an outside source may be used if they support endpoints, inclusion/exclusion criteria or adverse events.
- Attempts to obtain medical records should be recorded in the research chart.
Questionnaires
- Documentation must reflect who completed the questionnaire, in compliance with the protocol.
- For questionnaires completed by staff, a note should reflect how the information was obtained ie: direct interview with participant, phone call, chart abstraction.
An official website of the United States government
Here’s how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Dept. of Health & Human Services
Clinical Research Study Investigator's Toolbox
The purpose of the NIA Clinical Research Toolbox is to provide a Web-based informational repository for investigators and staff involved in clinical research. The Toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research studies.
Issued by: National Institutes of Health (NIH)
NIA Clinical Research Investigator's Toolbox
Supporting clinical research, study startup.
- NIA Guidance on Clinical Trials
Forms and Templates
- Glossary of Terms
Data Safety and Monitoring
As depicted in the NIA Guidance on Clinical Trials , NIA is responsible for overseeing the data and safety monitoring of the clinical research it supports. Data and safety monitoring of a clinical trial is commensurate with the risks posed to the study participants and with the size and complexity of the study.
Applicants requesting support for any intervention study must complete "PHS Human Subjects and Clinical Trials Information" form of the SF424 (R&R), describe a data and safety monitoring plan (DSMP), which discusses the need for an independent data and safety monitoring body or justifies why such a body is not needed to monitor the study and proposes an alternative safety monitoring mechanism. For example, for a single-site, low risk study, the PI may propose a local safety monitor, while a multi-site, higher risk study might propose a Data and Safety Monitoring Board (DSMB).
- Data and Safety Monitoring Plan (DSMP) Template and Guidelines (MS Word, 37K) and DSMP Checklist (MS Word, 43K) were developed to assist investigators in preparation of a sound data and safety monitoring plan. All clinical trials require study-specific monitoring procedures to ensure participant safety and data integrity. The DSMP outlines procedures that investigators and study staff will follow when implementing a clinical trial. Investigators submitting grant applications for clinical trials are required to include a general description of the DSMP as part of the research grant application.
- Guideline for Budgeting for Data and Safety Monitoring Activities (MS Word, 25K) aids investigators in budgeting for an independent DSMB or a Safety Officer when preparing the budget section of a grant application.
Data Sharing
The National Institutes of Health (NIH) advocates making available to the public the results and accomplishments of the activities that it funds. NIH assures that research resources developed with public funds become readily available to the broader research community in a timely manner for further research, development, application, and secondary data analysis. The expectation is that this will lead to products and knowledge of benefit to public health. To ensure that future research can build on previous efforts and discoveries, the National Institutes of Health (NIH) has developed a data sharing policy effective October 1, 2003, for applicants seeking NIH funding of $500,000 or more in direct costs in any one year. The policy expects final research data, especially unique data, from NIH-supported research efforts be made available to the investigators. The NIH policy on data sharing applies to:
- Basic research, clinical studies, surveys, and other types of research supported by the NIH.
- Human subjects and laboratory research.
- Data not produced with NIH funding but used in an NIH-supported activity in some instances.
Investigators are expected to include in their grant application a brief description of how final research data will be shared, or explain why data-sharing is not possible (for example: human subject protection concerns). Please see NIH’s Example Plan (MS Word, 55K) for a template you may modify to fit the data you plan to share.
Initial Proposal Concept Form (MS Word, 39K) - This form should be used to advocate for an initiative by the Division of Geriatrics and Clinical Gerontology (DGCG) for a clinical trial or trials that exceed $2 million in direct costs in any year of funding. DGCG Clinical Trials Advisory Panel, a task force of the National Advisory Council on Aging (NACA), will evaluate the concept proposals in October – November of each Fiscal Year and will provide its recommendations to DGCG, NACA, and to the NIA Director on initiatives for large clinical trials.
Back to top
The clinical protocol is a document that describes how a clinical study will be conducted by detailing the objective(s), design, methodology, statistical considerations and organization of a clinical study, and describes methods used to ensure the safety of the study participants and integrity of the data collected.
Protocol (MS Word, 93K) - The Clinical Intervention Study Protocol Template outlines a clinical study protocol and provides guidance on important content to include in each section. The template can be downloaded as an MS Word file for adaptation by the study investigator.
Manual of Procedures
A Manual of Procedures (MOP) is a handbook that details a study’s conduct and operations as well as facilitates consistency in protocol implementation and data collection across study participants and sites. It operationalizes the study protocol and describes each step of the study and how it is to be executed. A copy of the MOP should be provided to each member of the Study Team. Ideally, the MOP would contain an adequate amount of detail that any individual(s) at any site(s) could run the study consistently with only the information contained in the MOP and its appendices.
Get resources to support your study recruitment
Visit NIA’s ADORE (Alzheimer’s and Dementia Outreach, Recruitment, and Engagement) Resources for a searchable collection of materials for clinical trials recruitment and retention.
The NIA recognizes the importance of a MOP and has developed documents to assist principal investigators in writing their study MOP. Investigators with a multi-site study are required to submit a MOP, while single-site study investigators are strongly encouraged to review the MOP and determine which sections are necessary in order to ensure the study procedures are performed as intended. The Guidelines below provide details on each section of the MOP, while the MOP Outlines are an overview listing the sections that are most relevant in those types of studies.
- Manual of Procedures (MOP) Outline – Multi-Site (MS Word, 30K)
- Manual of Procedures (MOP) Guidelines – Multi-Site (MS Word, 179K)
- Manual of Procedures (MOP) Outline – Single-Site (MS Word, 27K)
- Manual of Procedures (MOP) Guidelines - Single-Site (MS Word, 170K)
The following documents can also be found within the MOP template:
- Screening Log provides documentation of all individuals who were evaluated for participation in a research study. The log typically contains a unique identification number for each person screened along with individuals’ initials, age, gender, race and ethnicity, screening date, and eligibility status.
- Schedule of Events presents the activities that take place at each contact with the participant.
- Protocol Deviation Log provides participant-specific documentation of missed visits and other actions that deviate from the protocol.
Informed Consent
The consent process provides individuals with sufficient information for making informed decisions about participation in a clinical research study. The following documents are provided as a tool to assist NIA investigators for developing a comprehensive informed consent:
- Informed Consent Template (MS Word, 115K) provides a general outline of a study specific informed consent form (ICF). It is critical that investigators consult with their local IRB for any institution-specific templates and/or requirements regarding the format and content of the consent form.
- Informed Consent Checklist (MS Word, 55K) presents required and additional elements of the consent forms as set forth in Code of Federal Regulations.
- Informed Consent Version Tracker (MS Excel, 20K) provides a template with two examples of tools that sites may use to track informed consent versions; this helps minimize the use of expired versions and the occurrence of consent deviations.
Data Safety and Monitoring Boards
The Data and Safety Monitoring Board (DSMB) is an independent group of experts that advises the NIA Director and the study investigators. The members of the DSMB serve in an individual capacity and provide their expertise and recommendations. The need for DSMB oversight is based on assessment of the study’s overall risk. Investigators may propose a DSMB in their grant application, or NIA may require that a DSMB be established following consideration of review panel’s comments, NIA’s National Advisory Council on Aging (NACA) advice, and/or input from NIA staff.
- Sample Data and Safety Monitoring Board Charter (MS Word, 24K) The DSMB Charter describes the responsibilities of the DSMB to ensure ongoing, independent study review and assure the study is conducted according to the highest scientific and ethical standards.
- DSMB Conflict of Interest and Confidentiality Statement (MS Word, 20K) - All members of the DSMB are required to be independent of the studies being reviewed and need to certify this by signing a DSMB Conflict of Interest and Confidentiality statement.
- DSMB Report - Single Site Open (MS Word, 323K)
- DSMB Report - Single Site Closed (MS Word, 342K)
- DSMB Report - Multi Site Open (MS Word, 449K)
- DSMB Report - Multi Site Closed (MS Word, 348K)
Additional Startup Tools
- Recruitment and Retention Tips (MS Word, 33K) describe approaches to recruitment and retention of older individuals from diverse ethnic and racial groups in clinical research studies.
- Data Management Tips (MS Word, 30K) help to ensure adequate data management processes and procedures in a clinical study. Investigators are encouraged to use Data Management Tips to describe how data will be handled in the study.
- Best Practices for Data Coordinating Centers – This Compendium, developed by the National Heart Lung and Blood Institute (NHLBI) provides helpful tips for clinical researchers and other stakeholders for developing large, multisite clinical trial programs.
Investigators must include in their application proposed adverse event (AE) and serious adverse event (SAE) definitions and discuss their monitoring and reporting. All clinical trials of drugs and biological products conducted under an Investigational New Drug Application (IND) must use definitions of adverse events and adverse reactions and follow the reporting requirements established by 21 Code of Federal Regulations (CFR) Part 312.32. Trials of medical devices conducted under an Investigational Device Exemption (IDE) must use the definitions and reporting requirements established by 21 CFR 812. All other interventional studies must propose their definitions of adverse events and their reporting procedures. See the NIA Guidance on Clinical Trials for additional information .
- Adverse Event Form ( MS Word , 38K or screen-readable PDF , 69K) provides a template for a study form for collecting information about adverse events that is reviewed by safety monitoring bodies.
- Serious Adverse Event Form ( MS Word , 31K or screen-readable PDF , 769K) provides a template for a study form for collecting information about serious adverse events. The form includes major components of the Food and Drug Administration (FDA) Form 3500.
- AE/SAE Process Flow ( MS Word , 79K or screen-readable plain text file , 4K) illustrates how adverse events and serious adverse events are handled within a study.
The NIA Safety Training Course (available below), an online training venue, provides an overview of human subject safety surveillance and reporting requirements in clinical research studies. The intent of the course is to help clinical study investigators and staff understand and implement NIA and regulatory requirements for safe, high quality clinical research. The topics covered include Good Clinical Practice (GCP), Human Subject Protections, Adverse Events and Unanticipated Problems, Safety Monitoring and Reporting Requirements, Safety Monitoring and Oversight: Data and Safety Monitoring Boards (DSMBs) and Safety Officers, Regulatory Requirements and Responsibilities of Principal Investigators, and Data and Safety Monitoring Plans. The course requires about 40 minutes to complete.
Administrative Forms
Site Signature Log - Delegation of Authority Log ( MS Excel, 47K or screen-readable PDF, 294K ) A record of all study personnel and their specific responsibilities, signatures, and dates of involvement during the conduct of a clinical research study.
Note to File Template (MS Word, 20K) - Used by clinical site staff to document protocol deviations or other discrepancies identified during the conduct of the clinical research study and plans for resolution/prevention.
Sample Visit Flow and Schedule (MS Word, 25K) – The visit schedule tracks an individual participant’s progress through the study and helps to ensure that visits take place during the protocol-specified timeframe. The visit flow provides an overview of the activities that take place at each study visit, and may be customized for each study site.
Study Drug/Investigational Product Tracker (MS Excel, 12K) - Used to track study drug/investigational product disposition and accountability by the clinical research site. For multi-site studies under an investigational new drug (IND) application, this tracker could be used by coordinating centers to track the overall distribution of investigational product.
Study Drug/Investigational Product Compliance Log (MS Word, 30K) - Used to track study drug/investigational product disposition and accountability for each individual participant. This form may be used to track protocol adherence via amount dispensed and returned and is designed to be used in conjunction with the Study Drug/Investigational Product Tracker. May also be used to track study drug/investigational return or destruction.
Study-wide Forms
Adverse Events Form ( MS Word, 38K or screen-readable PDF, 68K )
Prior and Concomitant Medications ( MS Word, 34K or screen-readable PDF, 58K )
Protocol Deviations Form ( MS Word, 46K or screen-readable PDF, 80K )
Serious Adverse Events Form ( MS Word, 31K or screen-readable PDF, 769K )
Study Disposition Form ( MS Word, 32K or screen-readable PDF, 56K )
Baseline Visit Forms
Visit Checklist ( MS Word, 34K or screen-readable PDF, 53K )
Eligibility Form ( MS Word, 29K or screen-readable PDF, 184K )
Demographics Form ( MS Word, 32K or screen-readable PDF, 661K )
Medical History Form ( MS Word, 50K or screen-readable PDF, 87K )
Medical History Conventional ( MS Word, 54K or screen-readable PDF,184 K )
Vital Signs Form ( MS Word, 33K or screen-readable PDF, 101K )
Physical Exam Form ( MS Word, 73K or screen-readable PDF, 193K )
Randomization and Enrollment Form ( MS Word, 32K or screen-readable PDF, 806K )
HHS is committed to making its websites and documents accessible to the widest possible audience, including individuals with disabilities. We are in the process of retroactively making some documents accessible. If you need assistance accessing an accessible version of this document, please reach out to the [email protected] .
DISCLAIMER: The contents of this database lack the force and effect of law, except as authorized by law (including Medicare Advantage Rate Announcements and Advance Notices) or as specifically incorporated into a contract. The Department may not cite, use, or rely on any guidance that is not posted on the guidance repository, except to establish historical facts.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Perspect Clin Res
- v.2(2); Apr-Jun 2011
Good documentation practice in clinical research
Chitra bargaje.
Department of Clinical Trials and Safety, Global Quality and Regulatory Compliance, Bristol Myers Squibb, Mumbai, India
One of the most common inspection findings in investigator site inspections is lack of reliable, accurate and adequate source documentation. This also happens to be the most common pitfall identified during sponsor audits. The importance of good documentation practice needs to be emphasized to investigator sites to ensure that the study results are built on the foundation of credible and valid data. This article focuses on the key principles of good documentation practice and offers suggestions for improvement.
INTRODUCTION
Inadequate/inaccurate case histories form the second most commonly cited deficiency in US-FDA inspections of clinical investigator sites.
Similarly, source documentation issues ranked 5th among the top 10 findings from European Medicines Agency (EMA) inspections of investigator sites in 2009[ 1 ] and in some instances the findings were classified ‘critical’. Not surprisingly, clinical trial monitors and auditors also report documentation issues as a frequent area of GCP concern.
I would like to share an experience at a recent investigator site audit.
During the audit opening meeting we were informed that all the source data is on paper and no electronic documentation is used. The site was actually using MS word to document the data collected during the study. In normal practice the site did not use MS word to generate medical records. This method was adopted only for clinical trial subjects. For the trial subjects there were no other hand-written progress notes which the site would normally use for routine patients.
There were two underlying potential issues here:
- First, the site was following a different practice for documenting progress for clinical research subjects. Were the subjects’ records missing any elements of standard care because of the deviation from routine practice?
- Second, the site thought they had no electronic documentation, although MS word was used to record all subject data.
This example, illustrates a common occurrence in clinical trial research where a lack of understanding of basic GCP principles may have a negative impact on the quality of the study.
WHAT IS THE PURPOSE OF SOURCE DOCUMENTATION?
To understand the importance of good source documentation we should first review the purpose of source documentation. The most important purpose of source documentation in a clinical trial is to reconstruct the trial as it happened. It should enable an independent observer to reconfirm the data. Documentation should be such that it is able to provide audit trail to permit investigation if and when required.
Source documentation is the medical record of the subject before, during and after the trial.
It is the tool which confirms the eligibility criteria of the subject in the given trial.
It documents the progress of the subject from consenting till the subject completes the study. It records the accountability of the investigational product dispensed, consumed and returned by the subject. It serves as the complete medical record of the subject as the reference to the treating physician at any point of time.
Finally it forms a strong foundation for the data that gets transcribed into a CRF which ultimately gets translated into a clinical study report.
Irrespective of clinical trial, accurate documentation supports the fundamental principle of protecting subject’s rights, safety and well-being.
There can not be two thoughts to emphasize the need for reliable and quality documentation.
PRINCIPLES OF GOOD DOCUMENTATION PRACTICE
So, what does it mean when we say ‘Good Documentation’ and how do we practice it?
Any basic training in clinical research will definitely include these phrases:
‘What is not documented is not done!’
‘Document what is done as well as what is not done!’
Roots of good documentation principles are in the ICH-GCP where source data and source document is first defined.
ICH E6 1.51 source data
All information in original records and certified copies of original records of clinical findings, observations, or other activities in a clinical trial necessary for the reconstruction and evaluation of the trial. Source data are contained in source documents (original records or certified copies).
The words in italics describe some inherent qualities of source data.
ICH E6 1.52 source documents
Original documents, data and records (e.g., hospital records, clinical and office charts, laboratory notes, memoranda, subjects’ diaries or evaluation checklists, pharmacy dispensing records, recorded data from automated instruments, copies or transcriptions certified after verification as being accurate copies, microfiches, photographic negatives, microfilm or magnetic media, X-rays, subject files, and records kept at the pharmacy, at the laboratories and at medico-technical departments involved in the clinical trial).
This definition describes the various types of documents which collectively form the source document.
Key attributes for good documentation were first described by US-FDA in the form of ALCOA -attributable, legible, contemporaneous, original and accurate. These are also adapted by World Health Organization (WHO). These criteria evolved with time. EMA has added some more ‘letters’ to describe qualities of good source documentation particularly for electronic documentation.[ 2 – 4 ]
Let‘s look at these attributes described by different authorities collectively.
Attributable
It should be clear who has documented the data.
Readable and signatures identifiable.
Contemporaneous
The information should be documented in the correct time frame along with the flow of events. If a clinical observation cannot be entered when made, chronology should be recorded. Acceptable amount of delay should be defined and justified.[ 4 ]
Original, if not original should be exact copy; the first record made by the appropriate person. The investigator should have the original source document.
Accurate, consistent and real representation of facts.
Long-lasting and durable.
Available and accessible
Easily available for review of treating physicians and during audits/inspections. The documents should be retrievable in reasonable time.
Complete till that point in time.
Demonstrate the required attributes consistently.
Based on real and reliable facts.
Corroborated
The data should be backed up by evidence.
Interestingly, it should be noted that the Drug Controller General India (DCGI) would emphasize on the condition in addition to the completeness, legibility and accessibility of investigator source data file as noted in DCGI’s guidance document for inspections.[ 5 ] My understanding of ‘condition’ is the state of the source documents, in terms of filing, storing and readability.
The degree to which the data fulfills the data quality criteria establishes acceptability of the data. It also determines the degree of excellence of the data quality. Qualities like consistency, credibility and corroboration help establish data integrity along with the data quality.
These are the expectations from clinical trial documentation however in reality many issues are observed in terms of quality of source documentation.
COMMON FINDINGS WITH RESPECT TO SOURCE DOCUMENTATION
‘Failure to maintain adequate and accurate case histories that record all observations and other data pertinent to the investigation on each individual administered the investigational drug or employed as a control in the investigation’ is cited in 6 out of the 10 warning letters issued by US-FDA to clinical investigators in 2010.[ 6 ]
At one investigator site source documents were not available because the computer ‘crashed’ . So in the absence of availability, adequacy of the records could not be evaluated. The investigator was warned for ‘failure to retain records required to be maintained for the required timeframe per regulations’ .
I would like to highlight some of the findings from the warning letters in detail here. These findings give an idea of regulatory expectations and lacunae in documentation noted during inspections. I am sure readers would be able to relate to some of these findings with their personal experience.
- Eligibility criteria could not be confirmed. For e.g., (a)IVRS user manual states “Complete call worksheets prior to contacting the IVRS; then file completed worksheets with each subject’s source documentation.” The IVRS worksheets were not kept in the subjects’ files or maintained at the site and as such it could not be confirmed that patients were stratified in the right arm and received the medication they were assigned to. (b) All the items in the exclusion criteria checklist are checked except for the exclusion criterion related to the history of thrombocytopenia, including heparin-induced thrombocytopenia, or a platelet count <100,000 cells/microliter. In the absence of lab report this exclusion criteria could not be confirmed on the basis of the incomplete checklists.
- Multiple records for same data points making it unable to determine which served as the accurate source record, for e.g., multiple versions of visual analog scales completed for same visit with different values.
- Discrepancies in records to confirm primary efficacy endpoint of the study, for e.g., the total administered dose of morphine, as reflected in hospital records was different from the Case Report Form. The primary efficacy endpoint of the protocol was to measure the reduction in the requirement for morphine use in the 24 hours following surgery measured by total morphine usage compared to placebo.
- Clinical significance for out of range lab values not documented on the lab reports or conflicting information found in the source documentation-e.g., significant high glucose value marked as clinically nonsignificant on the lab report although the subject was referred to for primary physician for further follow-up.
- Missing pages from subject interview scales, numerous unexplained corrections months after the initial entries and conflicting information; incorrect subject identifiers, incorrect date e.g., same date on screening visit, visit week 1 and week 4.
- Numerous AEs not reported in CRFs, delays in transcribing data in CRFs, discrepancies between source and the CRF. Lack of timely reporting of AEs in eCRFs jeopardizes subject safety and reliability and integrity of data captured at the site.
- Incorrect/incomplete documentation regarding the disposition of drugs-dates, quantity and use by subjects.
Although some of these issues may appear minor prima facie such as some checkboxes not checked, a lab report not marked for significance for out of range value, some discrepancies in source and CRF, unexplained corrections, these issues point toward lack of understanding of good documentation requirements. For an independent observer such data would fail to provide confidence and assurance of data quality and safety of the subjects enrolled. The data may be deemed unfit for use. All exposure of patients to new drugs and the efforts and time spent by the investigator team would be wasted.
Systematic deficiencies in documentation can lead to questions about the integrity of the data, potentially resulting in health authority decisions to exclude the data from analysis.
In essence, we can definitely say that the quality of documentation can make or break the study at a given site.

WHAT ARE THE POSSIBLE ROOT CAUSES FOR REPEATED DEFICIENCIES IN SOURCE DOCUMENTATION?
Clinical research documentation involves a variety of documents from various sources and is often completed by several people. Thus rendering this process to be complicated and posing challenges to meet requirements. Moreover clinical research happens over a long period of time which adds to the challenge of maintaining continuity in the documentation practice.
Inadequacies in documentation could be the result of lack of training and experience in good understanding of clinical research and documentation requirements. As a result the principal investigator (PI) and staff may continue documentation per the routine medical practice. In India, the documentation in routine medical practice may not be as extensive as what would be expected for clinical research.
Additional unmonitored medical records are discovered at the time of audits/inspections. Such as: Diaries of coordinator, inpatient records of the hospital, electronic records, etc., for the simple reason that the staff does not realize that these form a part of source record. These unmonitored records may have important data which do not find its way to the CRF. This would have an impact on the availability of important information in CRFs. Reliability and integrity of data might me affected as a result.
In many FDA warning letters one can observe that inadequate case histories, consenting or drug disposal records are often attributed to the lack of investigator’s supervision in ensuring compliance. The PI delegates responsibilities to the study team and may not provide adequate time to review the source data due to lack of time or commitment. The study documentation is completely left on the shoulders of study coordinator’s.
Various tools are used for data collection. At times sponsor provides source document worksheets to ensure complete documentation. If these worksheets are not designed accurately to align with the protocol and CRF source data quality is directly impacted. These worksheets are often completed as checkboxes without any additional notes, comments or supporting documents. Source document worksheets sometimes also result in multiple records. The sites continue to maintain the clinical practice routine documentation and worksheets are completed in addition for the study. As such, these worksheets are no longer a primary source and thus no source document.
Workload of the existing staff can be another important reason leading to poor documentation. This may cause errors like source data for one subject entered in another subject record, pages misfiled, use of incorrect consent forms and similar issues.
Study coordinator/PI work with various sponsors/CROs at a time. These different sponsors/CROs communicate different level of expectations regarding source documentation. If the site is not experienced enough and they do not have a standard procedure to follow they may get confused with variations in guidance they receive. This may negatively impact the quality of data.
Certain technical inadequacies may also lead to poor source documentation. For e.g., the ECG machine is old and does not print the date, time and subject identifiers, printer or fax machine does not work. If the fax is not working it may result in not receiving important data i.e., lab reports, data queries, investigational product allocation confirmations, SAE transmission confirmations, etc. Important email correspondence with sponsor/CROs if not printed and archived may get lost.
HOW CAN THE DOCUMENTATION BE IMPROVED?
Based on the various causes noted above, I would like to offer some suggestions to improve the quality of source documentation at sites.
- PI should delegate responsibilities to staff adequately trained in protocol and GCP. Particular training should be provided on ALCOA and other good documentation practice requirements. Medical decisions should be delegated to medically qualified staff. Training of site staff should be repeated at defined frequency. New hires should be adequately trained before trial participation.
- PI should commit for involvement, and supervision throughout the entire duration of the study. There should be an agreed and documented procedure for PI to ensure supervision of the study by meetings with site staff, monitors; review of documentation, timely resolution of medical, ethical or GCP issues. The PI or designated subinvestigators should validate the medical data. The PI should also supervise the work of SMO staff and external facilities if used. In case there are performance issues with SMO staff or external facility PI should immediately inform the supervisor as well as sponsor.
- Site should develop a SOP for good documentation. This SOP should be shared with the sponsor/CRO and agreed upon before the start of the trial. This SOP should address aspects including but not limited to consenting process, verifying eligibility, use of right tools such as diaries, source document worksheets, OPD papers, copies of prescriptions, etc; ways to avoid multiple records and in case of multiple records should define the source for the study, method of corrections, review of safety labs and other reports. Documented procedure at site level should encompass management, maintenance, archival and retrieval of source documentation. Sites should have measures for continuous improvement and maintaining high-quality data. Sites should develop process for quality control.
- Before the trial commences all technical aspects such as for e-CRFs, fax, printers, etc. should be clarified and issues resolved. In case of any difficulties during the trial, sponsor should be informed and back-up plans agreed upon till the issue is resolved. In case when original lab records or investigational records are sent to central location for assessment, process should be in place to ensure a duplicate copy or certified copy is available in the site source records.
- Sponsor/CRO also plays an important role in ensuring quality of source documentation. Sponsor/CRO should ensure PI’s commitment and involvement throughout the study. Sponsor/CRO should assess the site’s documentation practice during pre-study visit and during the study; provide training to the site staff to reinforce expectations. Time spent effectively during pre-study evaluation on source documentation would help a great deal to minimize documentation issues later. The source data and their respective capture methods should be clearly defined prior to trial recruitment i.e. in the protocol or study specific source data agreement.
CONCLUSIONS
Source documentation should demonstrate the ALCOA and other attributes as described by regulatory authorities and GCP. Source documentation related findings are the most commonly cited during inspections and audits. PI’s commitment and involvement in the trial makes a huge difference. Efforts to train the sites, understand the sites practices right from the pre-study visit and continuous monitoring and training would definitely help in improving and maintaining the quality of site source documentation practices.
Ultimately the source document should speak for itself. It should narrate the medical journey of the patient as it happened to an independent observer-an auditor or inspector and thus form a strong foundation for a good clinical research.
Acknowledgments
I would like to thank Jessica Parchman (Group Director) and Kristel Van De Voorde (Director) from Global Quality and Regulatory Compliance, Bristol Myers Squibb for reviewing the article and providing valuable suggestions in shaping this article.

- Clinical research
13. DESIGNING OF CLINICAL STUDY DOCUMENTS (Protocol, CRF, ICF)
PATH: PHARMD/ PHARMD NOTES/ PHARMD FIFTH YEAR NOTES/ CLINICAL RESEARCH/ DESIGNING OF CLINICAL STUDY DOCUMENTS (Protocol, CRF, ICF)
Leave a Reply Cancel Reply
Your email address will not be published. Required fields are marked *
Name *
Email *
Add Comment *
Save my name, email, and website in this browser for the next time I comment.
Post Comment
Trending now


Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Science News
- Meetings and Events
- Social Media
- Press Resources
- Email Updates
- Innovation Speaker Series
Revolutionizing the Study of Mental Disorders
March 27, 2024 • Feature Story • 75th Anniversary
At a Glance:
- The Research Domain Criteria framework (RDoC) was created in 2010 by the National Institute of Mental Health.
- The framework encourages researchers to examine functional processes that are implemented by the brain on a continuum from normal to abnormal.
- This way of researching mental disorders can help overcome inherent limitations in using all-or-nothing diagnostic systems for research.
- Researchers worldwide have taken up the principles of RDoC.
- The framework continues to evolve and update as new information becomes available.
President George H. W. Bush proclaimed the 1990s “ The Decade of the Brain ,” urging the National Institutes of Health, the National Institute of Mental Health (NIMH), and others to raise awareness about the benefits of brain research.
“Over the years, our understanding of the brain—how it works, what goes wrong when it is injured or diseased—has increased dramatically. However, we still have much more to learn,” read the president’s proclamation. “The need for continued study of the brain is compelling: millions of Americans are affected each year by disorders of the brain…Today, these individuals and their families are justifiably hopeful, for a new era of discovery is dawning in brain research.”
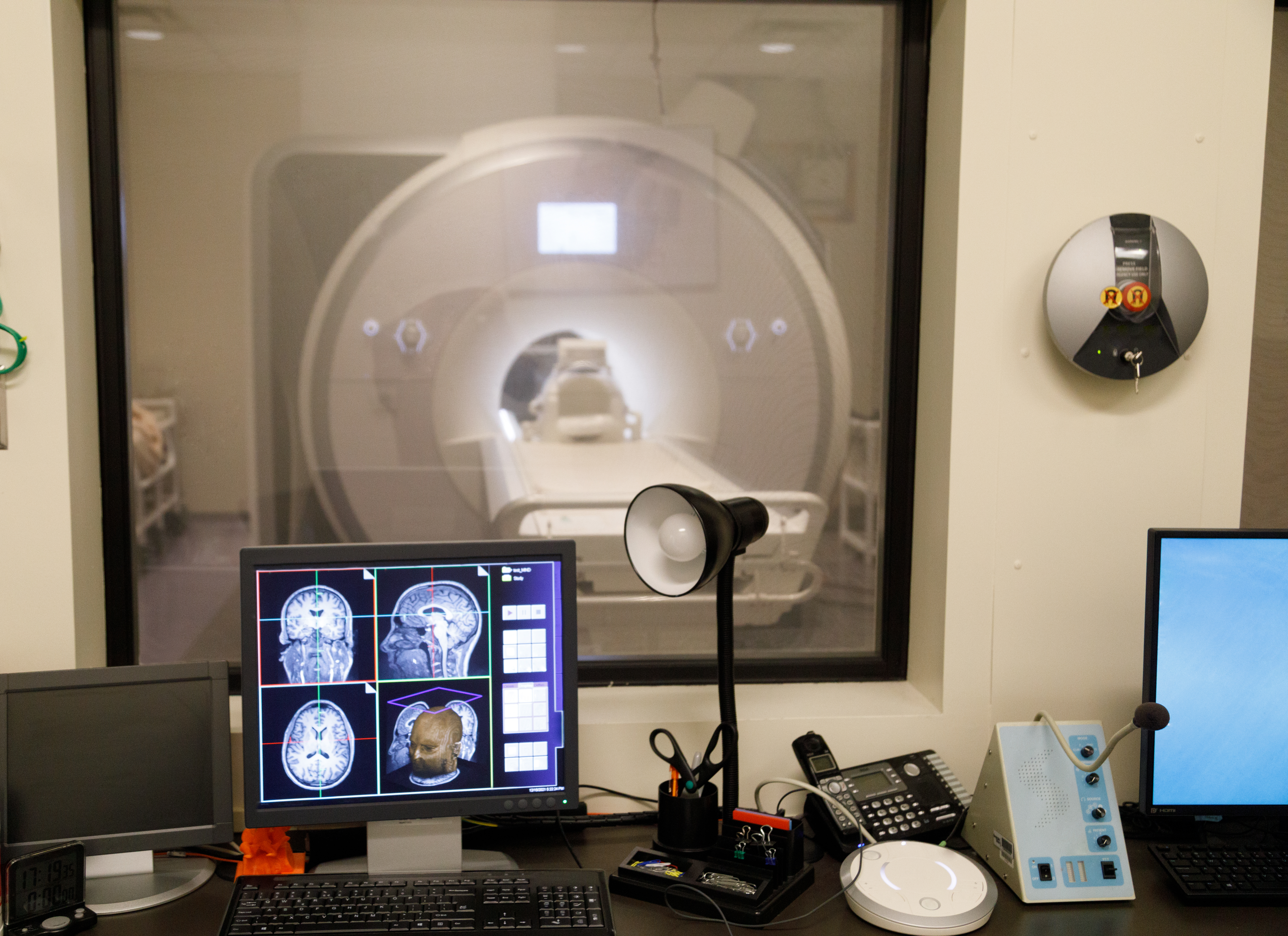
Still, despite the explosion of new techniques and tools for studying the brain, such as functional magnetic resonance imaging (fMRI), many mental health researchers were growing frustrated that their field was not progressing as quickly as they had hoped.
For decades, researchers have studied mental disorders using diagnoses based on the Diagnostic and Statistical Manual of Mental Disorders (DSM)—a handbook that lists the symptoms of mental disorders and the criteria for diagnosing a person with a disorder. But, among many researchers, suspicion was growing that the system used to diagnose mental disorders may not be the best way to study them.
“There are many benefits to using the DSM in medical settings—it provides reliability and ease of diagnosis. It also provides a clear-cut diagnosis for patients, which can be necessary to request insurance-based coverage of healthcare or job- or school-based accommodations,” said Bruce Cuthbert, Ph.D., who headed the workgroup that developed NIMH’s Research Domain Criteria Initiative. “However, when used in research, this approach is not always ideal.”
Researchers would often test people with a specific diagnosed DSM disorder against those with a different disorder or with no disorder and see how the groups differed. However, different mental disorders can have similar symptoms, and people can be diagnosed with several different disorders simultaneously. In addition, a diagnosis using the DSM is all or none—patients either qualify for the disorder based on their number of symptoms, or they don’t. This black-and-white approach means there may be people who experience symptoms of a mental disorder but just miss the cutoff for diagnosis.
Dr. Cuthbert, who is now the senior member of the RDoC Unit which orchestrates RDoC work, stated that “Diagnostic systems are based on clinical signs and symptoms, but signs and symptoms can’t really tell us much about what is going on in the brain or the underlying causes of a disorder. With modern neuroscience, we were seeing that information on genetic, pathophysiological, and psychological causes of mental disorders did not line up well with the current diagnostic disorder categories, suggesting that there were central processes that relate to mental disorders that were not being reflected in DMS-based research.”
Road to evolution
Concerned about the limits of using the DSM for research, Dr. Cuthbert, a professor of clinical psychology at the University of Minnesota at the time, approached Dr. Thomas Insel (then NIMH director) during a conference in the autumn of 2008. Dr. Cuthbert recalled saying, “I think it’s really important that we start looking at dimensions of functions related to mental disorders such as fear, working memory, and reward systems because we know that these dimensions cut across various disorders. I think NIMH really needs to think about mental disorders in this new way.”
Dr. Cuthbert didn’t know it then, but he was suggesting something similar to ideas that NIMH was considering. Just months earlier, Dr. Insel had spearheaded the inclusion of a goal in NIMH’s 2008 Strategic Plan for Research to “develop, for research purposes, new ways of classifying mental disorders based on dimensions of observable behavior and neurobiological measures.”
Unaware of the new strategic goal, Dr. Cuthbert was surprised when Dr. Insel's senior advisor, Marlene Guzman, called a few weeks later to ask if he’d be interested in taking a sabbatical to help lead this new effort. Dr. Cuthbert soon transitioned into a full-time NIMH employee, joining the Institute at an exciting time to lead the development of what became known as the Research Domain Criteria (RDoC) Framework. The effort began in 2009 with the creation of an internal working group of interdisciplinary NIMH staff who identified core functional areas that could be used as examples of what research using this new conceptual framework looked like.
The workgroup members conceived a bold change in how investigators studied mental disorders.
“We wanted researchers to transition from looking at mental disorders as all or none diagnoses based on groups of symptoms. Instead, we wanted to encourage researchers to understand how basic core functions of the brain—like fear processing and reward processing—work at a biological and behavioral level and how these core functions contribute to mental disorders,” said Dr. Cuthbert.
This approach would incorporate biological and behavioral measures of mental disorders and examine processes that cut across and apply to all mental disorders. From Dr. Cuthbert’s standpoint, this could help remedy some of the frustrations mental health researchers were experiencing.
Around the same time the workgroup was sharing its plans and organizing the first steps, Sarah Morris, Ph.D., was a researcher focusing on schizophrenia at the University of Maryland School of Medicine in Baltimore. When she first read these papers, she wondered what this new approach would mean for her research, her grants, and her lab.
She also remembered feeling that this new approach reflected what she was seeing in her data.
“When I grouped my participants by those with and without schizophrenia, there was a lot of overlap, and there was a lot of variability across the board, and so it felt like RDoC provided the pathway forward to dissect that and sort it out,” said Dr. Morris.
Later that year, Dr. Morris joined NIMH and the RDoC workgroup, saying, “I was bumping up against a wall every day in my own work and in the data in front of me. And the idea that someone would give the field permission to try something new—that was super exciting.”
The five original RDoC domains of functioning were introduced to the broader scientific community in a series of articles published in 2010 .
To establish the new framework, the RDoC workgroup (including Drs. Cuthbert and Morris) began a series of workshops in 2011 to collect feedback from experts in various areas from the larger scientific community. Five workshops were held over the next two years, each with a different broad domain of functioning based upon prior basic behavioral neuroscience. The five domains were called:
- Negative valence (which included processes related to things like fear, threat, and loss)
- Positive valence (which included processes related to working for rewards and appreciating rewards)
- Cognitive processes
- Social processes
- Arousal and regulation processes (including arousal systems for the body and sleep).
At each workshop, experts defined several specific functions, termed constructs, that fell within the domain of interest. For instance, constructs in the cognitive processes domain included attention, memory, cognitive control, and others.
The result of these feedback sessions was a framework that described mental disorders as the interaction between different functional processes—processes that could occur on a continuum from normal to abnormal. Researchers could measure these functional processes in a variety of complementary ways—for example, by looking at genes associated with these processes, the brain circuits that implement these processes, tests or observations of behaviors that represent these functional processes, and what patients report about their concerns. Also included in the framework was an understanding that functional processes associated with mental disorders are impacted and altered by the environment and a person’s developmental stage.
Preserving momentum
Over time, the Framework continued evolving and adapting to the changing science. In 2018, a sixth functional area called sensorimotor processes was added to the Framework, and in 2019, a workshop was held to better incorporate developmental and environmental processes into the framework.;
Since its creation, the use of RDoC principles in mental health research has spread across the U.S. and the rest of the world. For example, the Psychiatric Ratings using Intermediate Stratified Markers project (PRISM) , which receives funding from the European Union’s Innovative Medicines Initiative, is seeking to link biological markers of social withdrawal with clinical diagnoses using RDoC-style principles. Similarly, the Roadmap for Mental Health Research in Europe (ROAMER) project by the European Commission sought to integrate mental health research across Europe using principles similar to those in the RDoC Framework.;
Dr. Morris, who has acceded to the Head of the RDoC Unit, commented: “The fact that investigators and science funders outside the United States are also pursuing similar approaches gives me confidence that we’ve been on the right pathway. I just think that this has got to be how nature works and that we are in better alignment with the basic fundamental processes that are of interest to understanding mental disorders.”
The RDoC framework will continue to adapt and change with emerging science to remain relevant as a resource for researchers now and in the future. For instance, NIMH continues to work toward the development and optimization of tools to assess RDoC constructs and supports data-driven efforts to measure function within and across domains.
“For the millions of people impacted by mental disorders, research means hope. The RDoC framework helps us study mental disorders in a different way and has already driven considerable change in the field over the past decade,” said Joshua A. Gordon, M.D., Ph.D., director of NIMH. “We hope this and other innovative approaches will continue to accelerate research progress, paving the way for prevention, recovery, and cure.”
Publications
Cuthbert, B. N., & Insel, T. R. (2013). Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine , 11 , 126. https://doi.org/10.1186/1741-7015-11-126
Cuthbert B. N. (2014). Translating intermediate phenotypes to psychopathology: The NIMH Research Domain Criteria. Psychophysiology , 51 (12), 1205–1206. https://doi.org/10.1111/psyp.12342
Cuthbert, B., & Insel, T. (2010). The data of diagnosis: New approaches to psychiatric classification. Psychiatry , 73 (4), 311–314. https://doi.org/10.1521/psyc.2010.73.4.311
Cuthbert, B. N., & Kozak, M. J. (2013). Constructing constructs for psychopathology: The NIMH research domain criteria. Journal of Abnormal Psychology , 122 (3), 928–937. https://doi.org/10.1037/a0034028
Garvey, M. A., & Cuthbert, B. N. (2017). Developing a motor systems domain for the NIMH RDoC program. Schizophrenia Bulletin , 43 (5), 935–936. https://doi.org/10.1093/schbul/sbx095
Insel, T. (2013). Transforming diagnosis . http://www.nimh.nih.gov/about/director/2013/transforming-diagnosis.shtml
Kozak, M. J., & Cuthbert, B. N. (2016). The NIMH Research Domain Criteria initiative: Background, issues, and pragmatics. Psychophysiology , 53 (3), 286–297. https://doi.org/10.1111/psyp.12518
Morris, S. E., & Cuthbert, B. N. (2012). Research Domain Criteria: Cognitive systems, neural circuits, and dimensions of behavior. Dialogues in Clinical Neuroscience , 14 (1), 29–37. https://doi.org/10.31887/DCNS.2012.14.1/smorris
Sanislow, C. A., Pine, D. S., Quinn, K. J., Kozak, M. J., Garvey, M. A., Heinssen, R. K., Wang, P. S., & Cuthbert, B. N. (2010). Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology , 119 (4), 631–639. https://doi.org/10.1037/a0020909
- Presidential Proclamation 6158 (The Decade of the Brain)
- Research Domain Criteria Initiative website
- Psychiatric Ratings using Intermediate Stratified Markers (PRISM)
- Roadmap for Mental Health Research in Europe (ROAMER)
- Accelerating Clinical Trials in the EU (ACT EU)
ACT EU aims to transform how clinical trials are initiated, designed and run to further promote the development of high quality, safe and effective medicines, and to better integrate clinical research in the European health system.
The initiative seeks to deliver on the clinical trial innovation recommendations of the European medicines agencies network strategy and the European Commission’s Pharmaceutical strategy for Europe .
It builds on the Clinical Trials Regulation (CTR) and Clinical Trials Information System's (CTIS) launch on 31 January 2022.
The European Commission, EMA and Heads of Medicines Agencies (HMA) launched ACT EU in January 2022.
For more information, see the ACT EU website:
The ACT EU workplan sets out deliverables and timelines for the programme for 2022-26.
The deliverables for 2023 include the following:
- Establishing a process to support academic sponsors in enabling large multinational clinical trials
- Supporting clinical trial sponsors to make best use of available CTIS and CTR training activities
- Setting up a multi-stakeholder platform to facilitate dialogue between clinical trial stakeholders, including patients, healthcare professionals and academia
- Modernising good clinical practice by supporting the adoption and implementation of revised EU guidelines in clinical trial design
- Facilitating innovation in clinical trial methods by publishing a methodology roadmap and further developing guidance on decentralised clinical trials
EMA, the European Commision and the Heads of Medicines Agencies (HMA) published the workplan in August 2022.
ACT EU multi-annual workplan 2022-2026
Priority actions
The programme's strategy paper features ten priority action (PA) areas that are the basis for the ACT EU workplan.
The PAs are listed and detailed below.
Accelerating clinical trials in the EU (ACT EU) - Delivering an EU clinical trials transformation initiative
Priority action 1: Mapping and governance
The priority action refers to mapping existing clinical trial activities and developing a governance rationalisation strategy.
This aims to clarify the roles and responsibilities of the various expert groups working within the European medicines regulatory network.
For more information, see the ACT EU workplan .
Priority action 2: Successful implementation of CTR
The priority action aims to oversee the successful and timely implementation of the Clinical Trials Regulation (CTR) and its implementing acts.
This includes aspects such as:
- tracking the performance of the European clinical trials environment;
- raising awareness of training;
- addressing implementation issues in a swift manner.
For more information, see the ACT EU workplan .
A public consultation on the CTIS transparency rules was open from 3 May to 28 June 2023. EMA is currently reviewing the comments received, with the aim of publishing a concept paper with the proposed simplified transparency rules later this year.
Related guidance is available, taking into account feedback received during a public consultation concluded in 2022:
- CTIS: Protection of personal data and commercially confidential information
To find out more, see:
- Clinical Trials Regulation: progress on implementation
- Clinical Trials Information System: training and support - Protection of personal data and commercially confidential information
- Review of transparency rules for the EU Clinical Trials Information System (CTIS) (03/05/2023)
Under ACT EU, the European medicines regulatory network publishes statistics on the authorisation of clinical trials in the EU / EEA every month. This information provides an insight into how the CTR is transforming clinical trials.
A report is available summarising the findings of a survey for sponsors who submitted trials under the CTR:
- Summary report on targeted consultation on the implementation of the Clinical Trials Regulation (EU) No 536/2014
The survey was conducted in 2022.
Priority action 3: Multi-stakeholder platform
This priority action aims to establish a platform for stakeholders involved in designing, regulating, performing and participating in clinical trials.
It should help identify relevant scientific, methodological and technological advances to develop the clinical trials environment in the EU.
A concept paper is available on the importance of engaging stakeholders in all phases of a clinical trial, including trial design, and the objectives, composition, governance and transparency rules for the multi-stakeholder platform:
EMA invited stakeholders to provide input on these areas, priority topics for the multi-stakeholder platform and to express their interest in participating.
The report on the outcome of this public consultation is now available:
For more information:
- ACT EU workplan
- ACT EU multi-stakeholder platform kick-off workshop (22-23/06/2023)
Priority action 4: Good clinical practice modernisation
The priority action is meant to support the modernisation of good clinical practice in order to align with the increasingly diverse range of clinical trial types and data sources .
This will support the implementation of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E6 guideline.
For more information, see:
- ACT EU PA04 - Multi-stakeholder Workshop on ICH E6 R3 - Public Consultation (13-14/07/2023)
Priority action 5: Clinical trials analytics
The priority action intends to maximise the value of clinical trials data to support evidence-based decision-making.
It aims to enable the development of a publicly accessible EU clinical trials dashboard .
Priority action 6: Targeted communication campaign
The priority action aims to plan and launch a targeted communication campaign to engage all stakeholders who enable clinical trials.
They include data protection experts, academia, small and medium-sized enterprises (SMEs), funders, health technology assessment (HTA) bodies and healthcare professionals.
- Clinical trials highlights
Priority action 7: Scientific advice
The priority action aims to reinforce scientific advice coordination between clinical trial approval and clinical trial design , so as to facilitate the development of safe and effective medicines.
This will further foster collaboration between EMA's Agency's Scientific Advice Working Party (SAWP) or Emergency Task Force (ETF), on one side, and the HMA's Clinical Trial Coordination Group (CTCG) on the other side.
The project will include a number of pilot phases planned until the end of 2025.
For more information, see the ACT EU workplan .
In order to clarify the scope of scientific advice activities, ACT EU mapped information on voluntary procedures available within the European medicines regulatory network:
- Science advice on medicines for Human use in the EU medicines regulatory network
The programme also collated updates on other scientific advice procedures, as shown below.
EMA centralised scientific advice
For scientific advice provided by SAWP and the Committee for Medicinal Products for Human Use (CHMP), EMA clarifies which questions are outside the scope through the following resources:
- Scientific advice and protocol assistance: Questions outside the scope of scientific advice
- European Medicines Agency guidance for applicants seeking scientific advice and protocol assistance (clean version)
- European Medicines Agency guidance for applicants seeking scientific advice and protocol assistance with track-changes (version with track changes)
EMA centralised scientific advice for public health emergencies
EMA's ETF and CHMP also provide centralised scienfic advice for medicines targeting a declared or potential public health emergency, and for preparedness, based on articles 15 and 16 of Regulation 123/2022 .
For related guidance for developers, see:
- COVID-19 guidance: research and development
- Scientific advice and protocol assistance: Medicines intended for a disease causing public health emergency
Simultaneous national scientific advice (SNSA)
The EU Innovation Network (EU IN) consulted ACT EU to launch the second phase of its pilot on simultaneous national scientific advice (SNSA). The launch took place in November 2022.
For more information on SNSA's purpose, and to access relevant documents such as guidance and application forms, see:
- Heads of Medicines Agency: Simultaneous national scientific advice (SNSA) pilot phase 2 launch
- Innovation in medicines: EU Innovation Network - Simultaneous national scientific advice
The SNSA pilot focuses specifically on scientific advice to facilitate clinical trials within the EU.
The optimised SNSA process will continue to complement and provide a bridge between purely national scientific advice and centralised European scientific advice procedures from EMA.
The pilot is expected to inform the development of a final consolidated process for the provision of clinical trial-related advice .
Priority action 8: Methodologies
This priority action aims to develop and publish guidance on key methodologies.
This will ensure that all relevant EU expert groups pool their expertise and align their priorities in order to accelerate the development of future guidance, in collaboration with relevant stakeholders.
Guidance is already available for complex clinical trials and decentralised clinical trials .
A reflection paper on single-arm clinical trials submitted as pivotal evidence in a marketing authorisation application is available for public consultation until 30 September 2023.
ACT EU PA08 multi-stakeholder methodology workshop will take place 23 November.
For more information, see the ACT EU workplan .
Priority action 9: Clinical trials safety
The priority action aims to successfully establish clinical trials safety monitoring in the EU.
This would see Member States working together to improve trial safety through coordinated work-sharing assessment, in line with the EU4Health Joint Action .
To support these activities this priority action will focus on training for safety assessors, with the development of a curriculum to harmonise expertise.
It will also enable the implementation of ICH E19 guideline on selective safety data collection .
For more information:
- Risk proportionate approaches in clinical trials: Recommendations of the expert group on clinical trials for the implementation of Regulation (EU) No 536/2014 on clinical trials on medicinal products for human use
Priority action 10: Training curriculum
The priority action aims to provide a training curriculum informed by regulatory experience , with modules on drug development and regulatory science.
This curriculum is expected to engage universities and SMEs, and serve as an educational ecosystem.
A training strategy is available, setting out high-level objectives for the development of a training curriculum.
This document highlights the importance of accessing appropriate expertise in medicines development:
- Priority Action 10: Training strategy - Accelerating Clinical Trials in the European Union (ACT EU)
EMA published this strategy in February 2022.
- Public consultation on a multi-stakeholder platform to improve clinical trials in the EU (03/02/2023)
- Single-arm trials as pivotal evidence for the authorisation of medicines in the EU (21/04/2023)
- Review of transparency rules for the EU Clinical Trials Information System (CTIS) (03/05/2023)
Related content
- Innovation in medicines
- Clinical trials in human medicines
External links
- Heads of Medicines Agencies
Share this page
How useful do you find this page.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Learn About Drug and Device Approvals
- The Drug Development Process
Step 3: Clinical Research
While preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will interact with the human body. “Clinical research” refers to studies, or trials, that are done in people. As the developers design the clinical study, they will consider what they want to accomplish for each of the different Clinical Research Phases and begin the Investigational New Drug Process (IND), a process they must go through before clinical research begins.
On this page you will find information on:
Designing Clinical Trials
Clinical Research Phase Studies
The Investigational New Drug Process
Asking for FDA Assistance
FDA IND Review Team
Researchers design clinical trials to answer specific research questions related to a medical product. These trials follow a specific study plan, called a protocol , that is developed by the researcher or manufacturer. Before a clinical trial begins, researchers review prior information about the drug to develop research questions and objectives. Then, they decide:
Who qualifies to participate (selection criteria)
How many people will be part of the study
How long the study will last
Whether there will be a control group and other ways to limit research bias
How the drug will be given to patients and at what dosage
What assessments will be conducted, when, and what data will be collected
How the data will be reviewed and analyzed
Clinical trials follow a typical series from early, small-scale, Phase 1 studies to late-stage, large scale, Phase 3 studies.
What are the Clinical Trial Phases?
Watch this video to learn about the three phases of clinical trials.
Study Participants: 20 to 100 healthy volunteers or people with the disease/condition.
Length of Study: Several months
Purpose: Safety and dosage
During Phase 1 studies, researchers test a new drug in normal volunteers (healthy people). In most cases, 20 to 80 healthy volunteers or people with the disease/condition participate in Phase 1. However, if a new drug is intended for use in cancer patients, researchers conduct Phase 1 studies in patients with that type of cancer.
Phase 1 studies are closely monitored and gather information about how a drug interacts with the human body. Researchers adjust dosing schemes based on animal data to find out how much of a drug the body can tolerate and what its acute side effects are.
As a Phase 1 trial continues, researchers answer research questions related to how it works in the body, the side effects associated with increased dosage, and early information about how effective it is to determine how best to administer the drug to limit risks and maximize possible benefits. This is important to the design of Phase 2 studies.
Approximately 70% of drugs move to the next phase
Study Participants: Up to several hundred people with the disease/condition.
Length of Study: Several months to 2 years
Purpose: Efficacy and side effects
In Phase 2 studies, researchers administer the drug to a group of patients with the disease or condition for which the drug is being developed. Typically involving a few hundred patients, these studies aren't large enough to show whether the drug will be beneficial.
Instead, Phase 2 studies provide researchers with additional safety data. Researchers use these data to refine research questions, develop research methods, and design new Phase 3 research protocols.
Approximately 33% of drugs move to the next phase
Study Participants: 300 to 3,000 volunteers who have the disease or condition
Length of Study: 1 to 4 years
Purpose: Efficacy and monitoring of adverse reactions
Researchers design Phase 3 studies to demonstrate whether or not a product offers a treatment benefit to a specific population. Sometimes known as pivotal studies, these studies involve 300 to 3,000 participants.
Phase 3 studies provide most of the safety data. In previous studies, it is possible that less common side effects might have gone undetected. Because these studies are larger and longer in duration, the results are more likely to show long-term or rare side effects
Approximately 25-30% of drugs move to the next phase
Study Participants: Several thousand volunteers who have the disease/condition
Purpose: Safety and efficacy
Phase 4 trials are carried out once the drug or device has been approved by FDA during the Post-Market Safety Monitoring
Learn more about Clinical Trials .
Drug developers, or sponsors , must submit an Investigational New Drug (IND) application to FDA before beginning clinical research.
In the IND application, developers must include:
Animal study data and toxicity (side effects that cause great harm) data
Manufacturing information
Clinical protocols (study plans) for studies to be conducted
Data from any prior human research
Information about the investigator
Drug developers are free to ask for help from FDA at any point in the drug development process, including:
Pre-IND application, to review FDA guidance documents and get answers to questions that may help enhance their research
After Phase 2, to obtain guidance on the design of large Phase 3 studies
Any time during the process, to obtain an assessment of the IND application
Even though FDA offers extensive technical assistance, drug developers are not required to take FDA’s suggestions. As long as clinical trials are thoughtfully designed, reflect what developers know about a product, safeguard participants, and otherwise meet Federal standards, FDA allows wide latitude in clinical trial design.
The review team consists of a group of specialists in different scientific fields. Each member has different responsibilities.
Project Manager: Coordinates the team’s activities throughout the review process, and is the primary contact for the sponsor.
Medical Officer: Reviews all clinical study information and data before, during, and after the trial is complete.
Statistician: Interprets clinical trial designs and data, and works closely with the medical officer to evaluate protocols and safety and efficacy data.
Pharmacologist: Reviews preclinical studies.
Pharmakineticist: Focuses on the drug’s absorption, distribution, metabolism, and excretion processes.Interprets blood-level data at different time intervals from clinical trials, as a way to assess drug dosages and administration schedules.
Chemist: Evaluates a drug’s chemical compounds. Analyzes how a drug was made and its stability, quality control, continuity, the presence of impurities, etc.
Microbiologist: Reviews the data submitted, if the product is an antimicrobial product, to assess response across different classes of microbes.
The FDA review team has 30 days to review the original IND submission. The process protects volunteers who participate in clinical trials from unreasonable and significant risk in clinical trials. FDA responds to IND applications in one of two ways:
Approval to begin clinical trials.
Clinical hold to delay or stop the investigation. FDA can place a clinical hold for specific reasons, including:
Participants are exposed to unreasonable or significant risk.
Investigators are not qualified.
Materials for the volunteer participants are misleading.
The IND application does not include enough information about the trial’s risks.
A clinical hold is rare; instead, FDA often provides comments intended to improve the quality of a clinical trial. In most cases, if FDA is satisfied that the trial meets Federal standards, the applicant is allowed to proceed with the proposed study.
The developer is responsible for informing the review team about new protocols, as well as serious side effects seen during the trial. This information ensures that the team can monitor the trials carefully for signs of any problems. After the trial ends, researchers must submit study reports.
This process continues until the developer decides to end clinical trials or files a marketing application. Before filing a marketing application, a developer must have adequate data from two large, controlled clinical trials.
News & Media
- News Releases
- In The News
- Organization News
- Social Media
- Duke Health Blog
Fortified Eggs Did Not Raise Cholesterol in Modest-Sized Cardiology Study
Further study needed to investigate secondary findings. .
DURHAM, NC – There are often conflicting headlines about whether certain foods are good or bad for you, and the news about eggs has been especially confusing. Search the topic online and you’ll find a wealth of articles spanning back decades.
A study presented at the American College of Cardiology’s Annual Scientific Session and led by researchers at Duke, offers new evidence on fortified eggs, which are eggs enriched with various vitamins or nutrients. In a modest-sized randomized trial, researchers found that fortified eggs did not have a negative impact on bad cholesterol (LDL cholesterol) or good cholesterol (HDL cholesterol) over the course of the four-month study.
The study was sponsored by Eggland’s Best, a company that makes and sells fortified eggs. It also provided the eggs used in the research.
The study had 140 participants, all people aged 50 or older, who had experienced at least one cardiac event in the past or had risk factors for cardiovascular disease such as diabetes. Researchers randomized participants into two groups, asking half to eat two or fewer eggs per week for four months. The other half were provided with fortified eggs and asked to eat 12 per week for the same period of time.
While no significant changes in bad or good cholesterol were found, a secondary finding hinted there could be some benefit associated with fortified egg consumption for older patients and patients with diabetes.
That secondary finding was not statistically significant due to the number of study participants, but senior researcher, Robert Mentz, M.D. , associate professor in the Department of Medicine at the Duke University School of Medicine , said it’s an interesting signal that the researchers would like to investigate in future work.
“If we can explore this area further, in a larger study, specifically focusing on the type of patients who appear to have potentially experienced some benefit, and over a longer period of time, we could see if it is possible for fortified eggs to improve cholesterol,” Mentz said.
The study’s first author, Nina Nouhravesh, M.D., a cardiology fellow at the Duke Clinical Research Institute , said the study can be viewed as a pilot study.
“While it was modest in size, it did include a broadly generalized population,” Nouravesh said. “The average age of participants was 66 years, half were women, and more than 25% identified as Black.”
Mentz said the enrollment was representative of the community, especially for a study aimed at cardiology patients.
He said he would like to move forward with a larger study assessing clinical outcomes, particularly when considering the topic of equity and food access.
“There are disparities around access to food,” Mentz said. “Individuals who are the most socially disadvantaged (and likely have more instances of high blood pressure and diabetes), often have less access to healthy foods. Often what we hear described in the community is access to fresh fruits and vegetables. Those are really time-limited foods that may go bad quickly. Fortified eggs can be safely stored in the refrigerator for longer periods of time. Investigating potential health benefits of an easily accessible and less time-limited food is something we should be doing.”
“I think we are in this exciting time where people think of food as medicine,” Mentz said. “Some foods are fortified and nutritionally optimized before they’re disseminated, similar to medications, so it’s exciting to use the same rigor that’s applied in medication trials to food science.”
In addition to Mentz and Nouhravesh, study authors include Josephine Harrington, Laura H. Aberle, Cynthia L. Green, Kathleen Voss, Dave Holdsworth, Kurt Misialek, Bartel T. Slaugh, Mandee Wieand, William S. Yancy and Neha Pagidipati.
Press Release: Novel Study Compares Fracture Patterning in Fatal, Survived Intimate Partner Violence Cases
BU researchers first to document intimate partner homicide-related fractures from a forensic anthropological perspective
Boston—Intimate partner violence (IPV) is an underreported global human rights issue that affects approximately 25% of women and 10% of men and is the leading cause of homicides of women worldwide. Multiple interventional studies have been conducted to screen for IPV, however, fractures associated with intimate partner homicide (IPH) have not been studied from a forensic anthropological perspective.
A new study from researchers at Boston University Chobanian & Avedisian School of Medicine has found that cases of IPH presented similarly to IPV cases in that injuries were concentrated in the middle and lower face, but fractures were notably more frequent in the upper face and cranial vault regions (space that encases and protects the brain together with the base of the skull) in cases of IPH.
Furthermore, the study which also sought to identify the fracture classifications most frequently associated with IPH and the number of craniofacial fractures, found the overwhelming majority of fractures were either comminuted (broken into more than two pieces) or linear (a less serious skull fracture that doesn’t splinter, depress or distort the bone) fractures in the bone. Contrary to expectations, blunt force trauma IPH was not necessarily associated with extensive fracturing, as most individuals (75.8%) presented with five or fewer fractures, with a range from zero to 26 fractures per individual.
“Understanding fracture patterns associated with certain mechanisms of homicide are beneficial in forensic cases by aiding medical examiners and law enforcement in directing their investigations and in providing skeletal evidence for underreported IPH,” explained corresponding author Sean Tallman, PhD, assistant professor of anatomy & neurobiology at the school.
The researchers reviewed 10 years of blunt force trauma homicide case reports from the New Mexico Office of the Medical Examiner to identify cases of IPH. They then obtained the postmortem CT scans from these cases. Using DICOM software, they viewed the CT slides individually and as a 3-D rendered model to note the location and number of fractures as well as the fracture classification. These observations were then compared to published numbers of fracture count and location from studies focusing on survived cases of intimate partner violence from medical and dental professionals.
According to the researchers, this study may help forensic pathologists and anthropologists to better identify victims of intimate partner violence through understanding the variability of fractures to the head, which is a common target for intimate violence. “However, our work demonstrates that there is considerable variability in the manifestations of skeletal trauma associated with intimate partner violence. No one type of fracture or constellation of fractures is indicative of IPV as contextual/investigative evidence is also crucial for identifying when intimate partner violence occurred,” added Tallman.
These findings appear online in the journal Forensic Science International.
Share this:
View all posts

IMAGES
VIDEO
COMMENTS
CLINICAL STUDY REPORT . To document results and interpretation of trial. X (if applicable) X . Author: ©European Medicines Agency, 2018. ... Upcoming Clinical Research Training and Conferences. SCDM 2024 Annual Conference. 29 September 2023 - 02 October 2024. SCDM (Society of Clinical Data Management)
ICH Guidance Documents. This International Conference on Harmonization (ICH) document makes recommendations on information that should be included in a core clinical study report of an individual ...
Study teams can use these templated tools and edit for each new study or can build their own templates based on their usual needs to use for all future studies. The CRRO templated tools are not meant to be static, unchanging documents, but must be edited for each study to align with IRB-approved procedures that are in the INSPIR application ...
Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice and human subject protection. Guidance documents are not binding ...
The clinical protocol is a document that describes how a clinical study will be conducted by detailing the objective(s), design, methodology, statistical considerations and organization of a clinical study, and describes methods used to ensure the safety of the study participants and integrity of the data collected.
Regulatory Document Checklists by Study Type ... The CREST Program aims to ensure that the reported clinical research study data are accurate, complete, and verifiable, the conduct of the study is in compliance with the study protocol, Good Clinical Practice (GCP) and the regulations of applicable agencies, and the rights and well-being of ...
1.Maintain the CV and/or other relevant documents indicating the qualifications and eligibility of investigators and other key personnel to conduct a trial and/or to provide medical supervision of subjects. 2. Valid licenses & certifications for all professional study staff (e.g., medical or nursing license) 3.
The Regulatory Binder is often the first document reviewed during audits and inspections. Not all the essential documents are available at the start of the study. Documents can be grouped into those that are generated before study initiation, those that are generated during trial conduct and those that are generated after study completion.
4.3.1 What. The Manual of Operational Procedures (MOP) is a handbook of instructions designed to guide the research team to successfully carry out all aspects of a particular research project according to that study's research protocol. It clearly spells out the "who, what, where, when and how" of the RCT's conduct.
binder (synonyms: Regulatory Binder, Investigator Binder, Investigational Site File (ISF), and Study Binder) Details: This document clarifies the standard content of the binder. It is the responsibility of the investigator to ensure compliance with good clinical. practice (GCP), institutional review board (IRB), and applicable regulatory ...
Federal regulation and International Conference on Harmonization (ICH) Good Clinical Practice (E-6) requires investigators and sponsors to retain specific study records associated with the conduct of clinical research. These documents are often referred to as Essential Documents.
The purpose of this guidance is to aid study teams in determining what essential documents are required to be in the investigator site file (ISF) versus the trial master file (TMF). Every time a study is conducted, it is expected that the study team maintains an ISF that is audit ready. An ISF is required regardless of if it is an investigator ...
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study.
Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current ...
Study documents are submitted to fulfill one of two requirements: 1. The . clinical protocol (IRB-approved) and the . statistical analysis plan . must be included with ClinicalTrials.gov. results, 1. otherwise they do not have to be submitted. These can be a single combined file, or two different files. 2. An . informed consent form (ICF) must ...
The Department of Medicine Clinical Research Unit has prepared this document is to provide guidance to all faculty and staff involved in the conduct of research on the best practices related to documentation. Good study documentation will allow for an individual with basic knowledge of the particular project to recreate the events of the study.
Good Clinical Research Practice (GCP) is a process that incorporates established ethical and scientifi c quality standards for the design, conduct, recording and reporting of clinical research involving the participation of human subjects. Compliance with GCP provides public assurance that the rights, safety, and well-being of research
A. Prior to Clinical Research Implementation The PI or delegated research team members will create and maintain regulatory study binders for each clinical research study that will contain required, original , and revised essential documents ( See Attachment A: Essential Document Checklist and Attachment B: Regulatory File Checklist).
The clinical protocol is a document that describes how a clinical study will be conducted by detailing the objective(s), design, methodology, statistical considerations and organization of a clinical study, and describes methods used to ensure the safety of the study participants and integrity of the data collected.
FDA-regulated research is required to conform to CGP standards, which define the essential documents that trial investigators are responsible for creating and maintaining. Collectively, these key documents are referred to as the regulatory binder. Other names for the regulatory binder include: Clinical study files. Investigator files.
The site was actually using MS word to document the data collected during the study. In normal practice the site did not use MS word to generate medical records. ... Clinical research documentation involves a variety of documents from various sources and is often completed by several people. Thus rendering this process to be complicated and ...
Clinical trials are studies intended to discover or verify the effects of one or more investigational medicines.. The regulation of clinical trials aims to ensure that the rights, safety and well-being of trial participants are protected and the results of clinical trials are credible.. Regardless of where they are conducted, all clinical trials included in applications for marketing ...
PATH: PHARMD/ PHARMD NOTES/ PHARMD FIFTH YEAR NOTES/ CLINICAL RESEARCH/ DESIGNING OF CLINICAL STUDY DOCUMENTS (Protocol, CRF, ICF) 9. INDICATIONS FOR TDM. PROTOCOL FOR TDM October 31, 2021 In "TDM". 2. NOMOGRAMS AND TABULATIONS IN DESIGNING DOSAGE REGIMEN October 31, 2021 In "TDM". 2. INTRODUCTION TO CLINICAL TRIALS October 31, 2021 In ...
The Research Domain Criteria framework (RDoC) was created in 2010 by the National Institute of Mental Health. The framework encourages researchers to examine functional processes that are implemented by the brain on a continuum from normal to abnormal. This way of researching mental disorders can help overcome inherent limitations in using all ...
The ACT EU workplan sets out deliverables and timelines for the programme for 2022-26.. The deliverables for 2023 include the following: Establishing a process to support academic sponsors in enabling large multinational clinical trials; Supporting clinical trial sponsors to make best use of available CTIS and CTR training activities Setting up a multi-stakeholder platform to facilitate ...
By comparison, the FDA inspected 976 clinical study sites in 2017. And the FDA was unable to complete about 30% of one type of common inspection within the requested time frames from fiscal year ...
After the trial ends, researchers must submit study reports. This process continues until the developer decides to end clinical trials or files a marketing application. Before filing a marketing ...
The study's first author, Nina Nouhravesh, M.D., a cardiology fellow at the Duke Clinical Research Institute, said the study can be viewed as a pilot study. "While it was modest in size, it did include a broadly generalized population," Nouravesh said. "The average age of participants was 66 years, half were women, and more than 25% ...
A new study from researchers at Boston University Chobanian & Avedisian School of Medicine has found that cases of IPH presented similarly to IPV cases in that injuries were concentrated in the middle and lower face, but fractures were notably more frequent in the upper face and cranial vault regions (space that encases and protects the brain ...
Last week, the Health Research Authority posted complete registration information for 1,545 trials, including the name of the study, the sponsor, and registration number for the trial. Until now ...