Clinical Trials
Adrenal cancer.
Displaying 10 studies
The purpose of this study is to investigate the safety and efficacy of Relacorilant in combination with Pembrolizumab for patients with Adrenocortical Carcinoma with Excess Glucocorticoid Production.
Adrenocortical carcinoma (ACC) is an aggressive, rare childhood cancer. Limited evidence exists on a definite histopathological criterion to differentiate ACC from adrenocortical adenomas. Early diagnosis and management is the key. Surgery is the mainstay of treatment. Even after complete resection, a high risk of recurrence of ACC remains. Despite multi-modality treatment strategies, ACC is associated with poor survival. Due to the rarity of pediatric ACC, limited evidence exists on morbidity and mortality of these patients as well as prognostic factors for survival. The aim of this study is to document morbidity and mortality of children with adrenocortical carcinoma (ACC) and ...
Despite recent improvements in outcome for children with newly diagnosed high-risk neuroblastoma, cure rates remain unsatisfactory.Further, these gains have been the result of interventions during the Consolidation (tandem autologous stem cell transplant) and Post-Consolidation (dinutuximab immunotherapy) phases of treatment, while rates of disease control during Induction have not improved in recent COG trials. The current phase 3 trial seeks to improve the event-free survival (EFS) for children with high-risk neuroblastoma through early integration of promising novel targeted therapies: targeted radiopharmaceutical therapy with 131I-MIBG or the ALK inhibitor, crizotinib. After enrollment, patients will receive one cycle of Induction chemotherapy. ...
The purpose of this study is to assess the effectiveness of Relacorilant for the treatment of hypercortisolism in patients with cortisol-secreting adrenal adenomas or hyperplasia, based on glycemic and blood pressure (BP) control at Week 22 compared with placebo. and to assess the safety of relacorilant for the treatment of hypercortisolism.
The purpose of this research is to follow people with adrenal disorders in order to make conclusions about the natural history of a particular adrenal disease as well as effect of various therapies and interventions decided on by you and your medical team. In addition, we will collect biomaterial from you at times you are being evaluated which will be used to discover novel biomarkers which can potentially improve the accuracy of current diagnostic tests and affect the management of patients with adrenal disorders. We will also include a control group (without known adrenal disease) to compare to the volunteers ...
The purpose of this study is to determine prevalence and incidence of osteopenia, osteoporosis, and fractures in patients with adenomas based on hormonal sub-type, and to determine the effect of abnormal steroid metabolome on bone density, bone metabolism, and fractures.
The purpose of this study is to create a research registry to prospectively collect research biospecimens and corresponding clinical data from subjects with an undiagnosed tumor or undifferentiated mass.
This study will be a Phase I/II, open-label, non-randomized, dose-finding trial conducted at multiple clinical centers. The study is designed to determine the safety, tolerability and PK of TKM-080301 in adult patients with solid tumors or lymphomas that are refractory to standard therapy or for whom there is no standard therapy. After the determination of the maximum tolerated dose this dose will be utilized in an expansion cohort or subjects with refractory neuroendocrine tumors (NET) or adrenocortical carcinoma (ACC) tumors.
This phase II trial is studying the side effects and how well cixutumumab works in treating patients with relapsed or refractory solid tumors. Monoclonal antibodies, such as cixutumumab, can block tumor growth in different ways. Some block the ability of tumor cells to grow and spread. Others find tumor cells and help kill them or carry tumor-killing substances to them.
The purpose of this study is to evaluate the challenges, behavioral patterns, and preferences of minority patient participation in clinical trials. Also, to develop and validate a personalized clinical trial educational platform to boost participation among underserved cancer patients.

Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Update on Biology and Genomics of Adrenocortical Carcinomas: Rationale for Emerging Therapies
Affiliations.
- 1 Department of Internal Medicine, Division of Metabolism, Endocrinology, and Diabetes, University of Michigan, Ann Arbor, Michigan 48109-2200, USA.
- 2 Medical Scientist Training Program, University of Michigan, Ann Arbor, Michigan 48109-2200, USA.
- 3 Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, Michigan 48109-2200, USA.
- 4 Rogel Cancer Center, University of Michigan, Ann Arbor, Michigan 48109-2200, USA.
- 5 Department of Cell & Developmental Biology, University of Michigan, Ann Arbor, Michigan 48109-2200, USA.
- PMID: 35551369
- PMCID: PMC9695111
- DOI: 10.1210/endrev/bnac012
The adrenal glands are paired endocrine organs that produce steroid hormones and catecholamines required for life. Adrenocortical carcinoma (ACC) is a rare and often fatal cancer of the peripheral domain of the gland, the adrenal cortex. Recent research in adrenal development, homeostasis, and disease have refined our understanding of the cellular and molecular programs controlling cortical growth and renewal, uncovering crucial clues into how physiologic programs are hijacked in early and late stages of malignant neoplasia. Alongside these studies, genome-wide approaches to examine adrenocortical tumors have transformed our understanding of ACC biology, and revealed that ACC is composed of distinct molecular subtypes associated with favorable, intermediate, and dismal clinical outcomes. The homogeneous transcriptional and epigenetic programs prevailing in each ACC subtype suggest likely susceptibility to any of a plethora of existing and novel targeted agents, with the caveat that therapeutic response may ultimately be limited by cancer cell plasticity. Despite enormous biomedical research advances in the last decade, the only potentially curative therapy for ACC to date is primary surgical resection, and up to 75% of patients will develop metastatic disease refractory to standard-of-care adjuvant mitotane and cytotoxic chemotherapy. A comprehensive, integrated, and current bench-to-bedside understanding of our field's investigations into adrenocortical physiology and neoplasia is crucial to developing novel clinical tools and approaches to equip the one-in-a-million patient fighting this devastating disease.
Keywords: adrenocortical carcinoma; adrenocortical development and homeostasis; genomics; molecular biomarkers; targeted therapies.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: [email protected].
Publication types
- Research Support, U.S. Gov't, Non-P.H.S.
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Adrenal Cortex Neoplasms* / drug therapy
- Adrenal Cortex Neoplasms* / genetics
- Adrenal Glands
- Adrenocortical Carcinoma* / drug therapy
- Adrenocortical Carcinoma* / genetics
- Mitotane / therapeutic use
Grants and funding
- R01 DK062027/DK/NIDDK NIH HHS/United States
- T32 GM007863/GM/NIGMS NIH HHS/United States
- R01 DK043140/NH/NIH HHS/United States

Personalize Your Experience
Log in or create an account for a personalized experience based on your selected interests.
Already have an account? Log In
Free standard shipping is valid on orders of $45 or more (after promotions and discounts are applied, regular shipping rates do not qualify as part of the $45 or more) shipped to US addresses only. Not valid on previous purchases or when combined with any other promotional offers.
Register for an enhanced, personalized experience.
Receive free access to exclusive content, a personalized homepage based on your interests, and a weekly newsletter with topics of your choice.
Home / Innovation & Research / A breakthrough in distinguishing benign adrenal tumors from cancerous ones
A breakthrough in distinguishing benign adrenal tumors from cancerous ones
Adrenal masses are found in 5% of the population, and while most are benign, it can be tricky for doctors to determine if the mass is cancerous. A new urine test can pinpoint the cases that are cancer so they can be treated.
Please login to bookmark

Approximately 80 million CT scans are performed in the United States every year. Adrenal tumors are found incidentally in about 5% of these scans. Most of these tumors will turn out to be benign, but a small fraction will be adrenal cortical carcinoma, a type of cancer with high mortality and frequent recurrence. Even for localized disease, the 5-year survival rates do not exceed 65%, and distant spread is associated with a more than 90% death rate within that time period. Early diagnosis of a malignant adrenal mass is therefore imperative to assure timely and appropriate therapy.
Unfortunately, CT imaging alone is very limited in its ability to distinguish benign from malignant adrenal tumors. Only very small and hypodense lesions can be easily dismissed as benign. The sizeable group of patients with larger or denser tumors end up with an arduous workup that frequently includes additional imaging studies, hormonal testing, and biopsy. However, even the latter has both a high diagnostic false positive and false negative rate, and ultimately the tumor is often removed, often unnecessarily. While, on the other hand, the delays due to the diagnostic work might also compromise optimal care for those tumors that prove malignant.
In addition, patients believed not to have adrenal cancer after their workup and those who opt out of biopsy or surgery often require long-term follow up with regular re-imaging and repeated hormone testing, with resultant radiation exposure and high health care costs.
Mayo Clinic’s Clinical Mass Spectrometry Laboratory has added a new, noninvasive, and more accurate test to diagnose malignant adrenal tumors through urinary steroid profiling. The test is the result of several years of analytical and clinical studies, some of which have been published in the journal Clinical Chemistry , and is based on cutting edge liquid chromatography, high-resolution, accurate-mass mass spectrometry measurement for 26 steroid metabolites in urine.
“Our new test for adrenal cortical carcinoma will differentiate this rare and lethal tumor from benign adrenocortical adenomas, including those that overproduce corticosteroids, or mineral steroids, or sex steroids, or those that are hormonally inactive. We also anticipate that the test will be able to aid in the diagnosis of inborn errors of steroid metabolism, such as congenital adrenal hyperplasia,” says Ravinder Singh, Ph.D . , co-director of the clinical mass spectrometry lab and senior author of a paper describing the test .
“The test will utilize both clinical and laboratory data. The clinical parameters are age at diagnosis and sex of the patient, the size of the tumor by CT scanning and its density in Hounsfield units, whether it was detected incidentally or not, and whether there is evidence of hormone overproduction,” says Dr. Singh. “All these data are readily available for almost all patients with an adrenal mass, and are used by our algorithm to calculate the pretest probability of having adrenal cortical carcinoma. The steroid profile testing is then performed and its results are added into the risk calculation algorithm to generate a posttest probability. The final result will provide the referring physicians a highly accurate probability for adrenal cortical carcinoma and will thereby facilitate the optimal choice of further investigation, if any, based on an informed discussion between doctor and patient.”
“In clinical diagnostics, we want to minimize the risks of false-positive or false-negative results,” says Dr. Singh. “Thanks to HRAM LC-MS technology, resolution is now high enough to allow us to measure many steroids simultaneously, instead of testing only a single steroid, and progress in complex data analysis has allowed us to combine all this data with clinical parameters to arrive at accurate ACC risk estimates.”
Understanding the adrenal glands
The human body has two adrenal glands, one above each kidney. Adrenal glands influence many processes and functions of our body, mainly through production of three types of steroid hormones:
- Mineralocorticoids (e.g., aldosterone, which helps control blood pressure)
- Glucocorticoids (e.g., cortisol, which is important for metabolism, immune response and stress)
- Sex steroids (e.g., DHEAS, a precursor of testosterone and estradiol)
These steroids are all synthesized from cholesterol, via enzymes in the adrenal glands. In benign adrenocortical adenomas, near-normal levels of precursor and bioactive steroids are produced. By contrast, adrenal cortical carcinoma frequently shows abnormal patterns of steroid production. By measuring 26 different steroid metabolites, even subtle abnormalities can be detected. This is the basis for the diagnostic capability of 26 steroid profiling.
Epidemiology of adrenal tumors
The timing for this new biomarker comes none too soon, since adrenal masses are found in 5% of the population. The prevalence increases with age, ranging between less than 0.5% in children and around 10% in 70-year-old patients.
“Radiologists are finding adrenal nodules incidentally all the time, during patient examinations for reasons other than evaluation for adrenal disease,” says Irina Bancos, M.D., an endocrinologist in Mayo’s Department of Internal Medicine, Division of Endocrinology, who co-authored the paper. “Although the majority of these tumors are benign, around 30% of adrenal tumors greater than 4 cm are malignant —most represented by adrenal cortical carcinoma, and the survival rate for these patients is very poor unless detected early.”
High vs. low resolution mass spectrometry
To conduct urinary steroid profiling, the investigators have used high resolution mass spectrometry. This allows the simultaneous detection of large numbers of different steroids, despite the fact that many of these compounds have the same, or very similar, molecular structures and molecular weights. At the same time, nonspecific interferences are also dramatically reduced and signal to noise ratios are improved, resulting in high analytical sensitivity and specificity.
“It allows us to distinguish between steroids with at least twice the accuracy of low-resolution mass spec,” says Dr. Singh. “In clinical diagnostics, especially adrenal cortical carcinoma, high accuracy, sensitivity and specificity are very important. We don’t want to put any unnecessary risks onto our patients.”
Although the use of high resolution is more costly than low resolution, the greater margin for error on a low-resolution instrument “can turn out to be quite expensive for a cancer patient,” adds Dr. Singh.
The current state: Diagnosis of small and medium tumors is an inexact science
As it stands, clinicians must rely on their acumen to determine the likelihood of an adrenal tumor being malignant or benign. This is a complex decision, which depends on tumor size, imaging characteristics, and production of a few steroid hormones that can be routinely tested. (Typically, existing lab tests do not add additional diagnostic values for confirming adrenal cortical carcinoma).
Larger malignant adrenal tumors are more readily recognized as malignant based on size, heterogeneity, areas of necrosis, and other imaging characteristics. It’s the smaller adrenal tumors that can present a diagnostic challenge, because when examined with current imaging technologies (e.g., CT scans and MRIs), benign adrenal tumors often “mimic” malignant ones.
“Clinical assessment of probability for malignancy works relatively well when expert physicians are involved and the tumors are relatively large,” says Dr. Bancos. “Having evidence-based clinical algorithms can in part improve this situation by standardizing risk assessment and enabling less specialized physicians to make better diagnostic decisions. However, for small and medium-sized tumors, even the experts are still frequently struggling to distinguish adrenal cortical carcinoma from adrenocortical adenomas or from other cancerous lesions in adrenal glands such as sarcomas, metastases, lymphomas, etc. This is why repeat imaging and extensive follow-up, and in some cases, adrenal biopsy or surgical exploration are often needed to arrive at a definitive diagnosis.”
Because of this uncertainty, a significant number of adrenal biopsies and adrenalectomies are performed on suspicious looking adrenal tumors unnecessarily, ultimately showing a benign tumor. On the other hand, adrenal biopsy of adrenal cortical carcinoma is not advised because of several reasons.
“Adrenal carcinoma has a capsule holding it together and, theoretically, this prevents the cancer from spreading locally,” says Dr. Bancos. “By biopsying it, we would open a door to dissemination, and that may significantly deteriorate the prognosis. Moreover, adrenal biopsy has a suboptimal accuracy for adrenal cortical carcinoma and is usually reserved for other cancers. Combining algorithmic clinical risk assessment with steroid profiling allows us to diagnosis adrenal cortical carcinoma in a noninvasive manner.”
Distinguishing adrenal cancer from adrenal metastases
Any time a cancerous lesion is found in an adrenal gland, clinicians have to differentiate between two scenarios:
- Adrenal cancer – many times this is a localized cancerous process, in which case adrenal surgery may cure the patient.
- Adrenal metastasis – cancer that has travelled to the adrenal gland from elsewhere in the body; since this type of cancer does not originate in the adrenal gland itself, it is not a localized process, but rather a systemic process.
“In the first situation, steroid profiling would allow us to diagnose adrenal cancer in a noninvasive manner, in which case a biopsy would be avoided and surgery would be scheduled ASAP,” says Dr. Bancos. “In the second situation, where cancer has travelled to the adrenal gland from somewhere else, steroid profiling will not tell us what kind of cancer it is. For example, is it lung cancer metastasis to the adrenal gland in a smoker, or colon cancer metastasis to the adrenal gland in someone without a colonoscopy? Or is it melanoma metastasis to the adrenal gland? In this situation, biopsy may be valuable, because it would tell us what type of primary cancer we are dealing with.”
Clinical impact of urinary steroid panel testing
With a simple, noninvasive urine test combined with clinical data that are regularly available, the new biomarker panel allows clinicians to make the diagnosis right away on these smaller, indeterminate tumors, and thus avoid unnecessary follow-up imaging visits, unneeded biopsies, or even adrenalectomy, where the entire mass is removed to reach a diagnosis.
“It goes without saying that a negative biomarker result should also avoid substantial health care costs,” says Dr. Bancos. “Conversely, in patients with a small ACC, a positive biomarker result will lead to an earlier intervention and will radically improve patient prognosis, because we would be removing a smaller cancer before it has a chance to grow and metastasize.”
The assay also promises to alleviate patient anxiety—patients and their families won’t have to wait so long, over repeated health care provider visits and tests, for an accurate diagnosis. It would help diagnose other adrenal diseases as well.
“We have promising preliminary data that this steroid profile could also be useful for the diagnosis and differential diagnosis of a number of functional adrenal diseases, from congenital adrenal hyperplasia to various syndromes of overproduction of certain bioactive steroids, such as cortisol or aldosterone,” says Stefan Grebe, M.D., Ph.D., a clinical biochemist at Mayo Clinic, and also a co-author.
Availability of urinary steroid profile testing
It would be overly expensive and complicated for most other institutions to implement this kind of metabolomics testing. Fortunately, the new assay will soon be available through Mayo Clinic Laboratories, the commercial laboratory testing wing of Mayo Clinic. Relevant clinical data will be collected at order entry, and the steroid profile data will be combined with the clinical risk data to arrive at an accurate risk adrenal cortical carcinoma risk assessment.
“Mayo Clinic Laboratories has historically been a leader in performing a wide range of endocrine testing,” says Dr. Singh. “We’re experts at using liquid chromatography and mass spectrometry, and now, we’re moving into this novel testing profile, which will be one of the first metabolomics multiple-analyte biomarker panels for cancer and will also pioneer algorithmic fusion of clinical and laboratory data in the cancer field.”

Relevant reading
Living Medicine
A sweeping biography of the visionary behind bone marrow transplantation and the story of the diseases cured by Don Thomas's discovery.

Discover more Innovation & Research content from articles, podcasts, to videos.
You May Also Enjoy

Privacy Policy
We've made some updates to our Privacy Policy. Please take a moment to review.
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
Adrenal Gland Tumor: About Clinical Trials
ON THIS PAGE: You will learn more about clinical trials, which are the main way that new medical approaches are studied to see how well they work. Use the menu to see other pages.
What are clinical trials?
Doctors and scientists are always looking for better ways to care for people with an adrenal gland tumor. To make scientific advances, doctors create research studies involving volunteers, called clinical trials. Every drug that is now approved by the U.S. Food and Drug Administration (FDA) was tested in clinical trials.
Clinical trials are used for all types of adrenal gland tumors and stages of adrenal gland cancer. Many focus on new treatments to learn if a new treatment is safe, effective, and possibly better than the existing treatments. These types of studies evaluate new drugs, different combinations of treatments, new approaches to radiation therapy or surgery, and new methods of treatment.
People who participate in clinical trials can be some of the first to get a treatment before it is available to the public. However, there are some risks with a clinical trial, including possible side effects and the chance that the new treatment may not work. People are encouraged to talk with their health care team about the pros and cons of joining a specific study.
Some clinical trials study new ways to relieve symptoms and side effects during treatment. Others study ways to manage the late effects that may happen a long time after treatment. Talk with your doctor about clinical trials for symptoms and side effects.
Deciding to join a clinical trial
People decide to participate in clinical trials for many reasons. For some, a clinical trial is the best treatment option available. Because standard treatments are not perfect, patients are often willing to face the added uncertainty of a clinical trial in the hope of a better result. Others volunteer for clinical trials because they know that these studies are the only way to make progress in treating adrenal gland tumors. Even if they do not benefit directly from the clinical trial, their participation may benefit future people with an adrenal gland tumor.
Insurance coverage and the costs of clinical trials differ by location and by study. In some programs, some of the expenses from participating in the clinical trial are reimbursed. In others, they are not. It is important to talk with the research team and your insurance company first to learn if and how your treatment in a clinical trial will be covered. Learn more about health insurance coverage of clinical trials.
Sometimes people have concerns that, in a clinical trial, they may receive no treatment by being given a placebo or a “sugar pill.” When used, placebos are usually combined with standard treatment in most cancer clinical trials. Study participants will always be told when a placebo is used in a study. Find out more about placebos in cancer clinical trials.
Patient safety and informed consent
To join a clinical trial, patients must participate in a process known as informed consent. During informed consent, the doctor should:
Describe all of the treatment options so that the person understands how the new treatment differs from the standard treatment.
List all of the risks of the new treatment, which may or may not be different from the risks of standard treatment.
Explain what will be required of each patient in order to participate in the clinical trial, including the number of doctor visits, tests, and the schedule of treatment.
Describe the purposes of the clinical trial and what researchers are trying to learn.
Clinical trials also have certain rules called “eligibility criteria” that help structure the research and keep patients safe. You and the research team will carefully review these criteria together. You will need to meet all of the eligibility criteria in order to participate in a clinical trial. Learn more about eligibility criteria in clinical trials. People who participate in a clinical trial may stop participating at any time for personal or medical reasons. This may include that the new treatment is not working or there are serious side effects. Clinical trials are also closely monitored by experts who watch for any problems with each study. It is important that people participating in a clinical trial talk with their doctor and researchers about who will be providing their treatment and care during the clinical trial, after the clinical trial ends, and/or if the patient chooses to leave the clinical trial before it ends.
Finding a clinical trial
Research through clinical trials is ongoing for all types of cancer. For specific topics being studied for adrenal gland tumors, learn more in the Latest Research section.
Cancer.Net offers more information about clinical trials in other areas of the website, including a complete section on clinical trials .
There are many resources and services to help you search for clinical trials for an adrenal gland tumor, including the following services. Please note that these links will take you to separate, independent websites:
ClinicalTrials.gov. This U.S. government database lists publicly and privately supported clinical trials.
World Health Organization (WHO) International Clinical Trials Registry Platform. The WHO coordinates health matters within the United Nations. This search portal gathers clinical trial information from many countries’ registries.
Read more about the basics of clinical trials matching services .
In addition, you can find a free video-based educational program about cancer clinical trials located in another section of this website.
The next section in this guide is Latest Research . It explains areas of scientific research for adrenal gland tumors. Use the menu to choose a different section to read in this guide.
Adrenal Gland Tumor Guide
Cancer.Net Guide Adrenal Gland Tumor
- Introduction
- Risk Factors
- Symptoms and Signs
- Types of Treatment
- About Clinical Trials
- Latest Research
- Coping with Treatment
- Follow-Up Care
- Questions to Ask the Health Care Team
- Additional Resources
View All Pages
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
- Patient Care & Health Information
- Diseases & Conditions
- Adrenal cancer
Adrenal glands
Located on top of each kidney, the adrenal glands make hormones that help regulate metabolism, the immune system, blood pressure and other important functions. Although small, these glands control much of what happens in the body.
Adrenal cancer is a rare cancer that begins in one or both of the small, triangular glands (adrenal glands) located on top of your kidneys. Adrenal glands produce hormones that give instructions to virtually every organ and tissue in your body.
Adrenal cancer, also called adrenocortical cancer, can occur at any age. But it's most likely to affect children younger than 5 and adults in their 40s and 50s.
When adrenal cancer is found early, there is a chance for cure. But if the cancer has spread to areas beyond the adrenal glands, cure becomes less likely. Treatment can be used to delay progression or recurrence.
Most growths that form in the adrenal glands are noncancerous (benign). Benign adrenal tumors, such as adenoma or pheochromocytoma, also can develop in the adrenal glands.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Signs and symptoms of adrenal cancer include:
- Weight gain
- Muscle weakness
- Pink or purple stretch marks on the skin
- Hormone changes in women that might cause excess facial hair, hair loss on the head and irregular periods
- Hormone changes in men that might cause enlarged breast tissue and shrinking testicles
- Abdominal bloating
- Loss of appetite
- Loss of weight without trying
It's not clear what causes adrenal cancer.
Adrenal cancer forms when something creates changes (mutations) in the DNA of an adrenal gland cell. A cell's DNA contains the instructions that tell a cell what to do. The mutations can tell the cell to multiply uncontrollably and to continue living when healthy cells would die. When this happens, the abnormal cells accumulate and form a tumor. The tumor cells can break away and spread (metastasize) to other parts of the body.
Risk factors
Adrenal cancer happens more often in people with inherited syndromes that increase the risk of certain cancers. These inherited syndromes include:
- Beckwith-Wiedemann syndrome
- Carney complex
- Li-Fraumeni syndrome
- Lynch syndrome
- Multiple endocrine neoplasia, type 1 (MEN 1)
Adrenal cancer care at Mayo Clinic
Living with adrenal cancer?
Connect with others like you for support and answers to your questions in the Neuroendocrine Tumors (NETs) support group on Mayo Clinic Connect, a patient community.
Neuroendocrine Tumors (NETs) Discussions

35 Replies Thu, Mar 28, 2024

144 Replies Tue, Mar 26, 2024

105 Replies Thu, Mar 28, 2024
- Neuroendocrine and adrenal tumors. Plymouth Meeting, Pa.: National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed Dec. 6, 2018.
- Jameson JL, et al., eds. Adrenocortical carcinoma. In: Endocrinology: Adult and Pediatric. 7th ed. Philadelphia, Pa.: Saunders Elsevier; 2016. https://www.clinicalkey.com. Accessed Dec. 6, 2018.
- Adrenocortical carcinoma treatment (PDQ). National Cancer Institute. https://www.cancer.gov/types/adrenocortical/patient/adrenocortical-treatment-pdq. Accessed Dec. 6, 2018.
- Warner KJ. Allscripts EPSi. Mayo Clinic, Rochester, Minn. July 17, 2018.
Associated Procedures
- Ablation therapy
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Let’s celebrate our doctors!
Join us in celebrating and honoring Mayo Clinic physicians on March 30th for National Doctor’s Day.
REVIEW article
Current status and future targeted therapy in adrenocortical cancer.

- Surgical Oncology Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, United States
Adrenocortical carcinoma (ACC) is a rare malignancy with a poor prognosis. The current treatment standards include complete surgical resection for localized resectable disease and systemic therapy with mitotane alone or in combination with etoposide, doxorubicin, and cisplatin in patients with advanced ACC. However, the efficacy of systemic therapy in ACC is very limited, with high rates of toxicities. The understanding of altered molecular pathways is critically important to identify effective treatment options that currently do not exist. In this review, we discuss the results of recent advanced in molecular profiling of ACC with the focus on dysregulated pathways from various genomic and epigenetic dysregulation. We discuss the potential translational therapeutic implication of molecular alterations. In addition, we review and summarize the results of recent clinical trials and ongoing trials.
Introduction
Adrenocortical carcinoma (ACC) is a rare malignancy arising from the adrenal cortex, with an annual worldwide incidence of 0.5–2 individuals per million population ( 1 , 2 ). The median age at diagnosis is 55 years, though the incidence follows a bimodal pattern of age distribution, with peaks before the age of five and between the fourth and fifth decades of life ( 3 , 4 ). Although the disease aggressiveness varies, the prognosis of patients with ACC is generally poor with a median overall survival of approximately 4 years ( 2 ), partly due to the late stage at presentation. Only one-third of patients with ACC in the US presented at TNM stage I or II ( 5 ). In a French study, five-year overall survival was 66, 58, 24, and 0% for stages 1, 2, 3, and 4 ACC, respectively ( 6 ). Alternatively, the prognosis of patients with ACC can be categorized by the extent of the disease. Five-year overall survival of patients with localized disease (limited to the adrenal gland), regionalized disease (locally advanced), and those with distant metastasis was 60–80%, 35–50%, and 0–28%, respectively ( 7 ). Cushing’s syndrome is observed in 50–60% of patients with ACC. Hyperandrogenism is seen in 20–30% of female patients, with a small number of those patients having estrogen and/or mineralocorticoid excess. Primary aldosteronism can also be seen in only 2.5% of patients with ACC ( 3 ). Patients may also experience weight loss, fatigue, night sweats, or fever ( 8 , 9 ). Prognosis also differs with regard to age, extent of surgical resection (R0, R1, R2), mitotic rate, and hormone secretion. A 10-year follow-up study of 180 patients that underwent resection of adrenocortical carcinoma clearly stratify this data ( 10 ). Data was stratified into cohorts by amount of time patients were alive after surgery (e.g., patients alive <2 years, alive 2–5 years, alive 5–10 years, alive >10 years). Of the 37 patients alive 5–10 years, 78.1% had an R0 resection, 21.9% had an R1 resection, and 0 patients were alive that had an R2 resection. Of these same patients, 48.5% had a non-secreting tumor, 24.2% had a cortisol-secreting tumor, and 18.2% had a non-cortisol secreting tumor. Neither age nor high mitotic rate did not seem to affect overall survival for any patient cohort in their review.
The current treatment scheme is patients with ACC is summarized in Figure 1 . Patients with localized and regionalized ACC have a potential for cure with complete surgical resection ( 7 ). Yet, even with an R0 resection, 50–80% of patients develop recurrent or metastatic disease ( 11 , 12 ). The role of surgery in patients with recurrent or metastatic disease remains a topic of debate. Some recent studies demonstrated a modest survival benefit in selected patients that underwent surgery for a recurrent disease if the disease-free interval was greater than 12 months ( 13 , 14 ). Because of the lack of effective systemic treatments, patients with resectable, recurrent and/or metastatic ACC should be evaluated for surgery when the disease progression is not rapid, such as those who do not develop new metastatic lesions within 6 months of diagnosis. Patient selection should be based on a thorough discussion of surgical risks, the benefits of achieving “no evidence of disease” status, and the risk and time of recurrence in the absence of level 1 data ( Figure 2 ). The selected patients with advanced ACC may benefit from metastasectomy ( 14 – 16 ). Since the efficacy and the options of systemic treatment are limited, an aggressive surgical approach may be recommended in patients with advanced ACC that follows a relatively more indolent course.
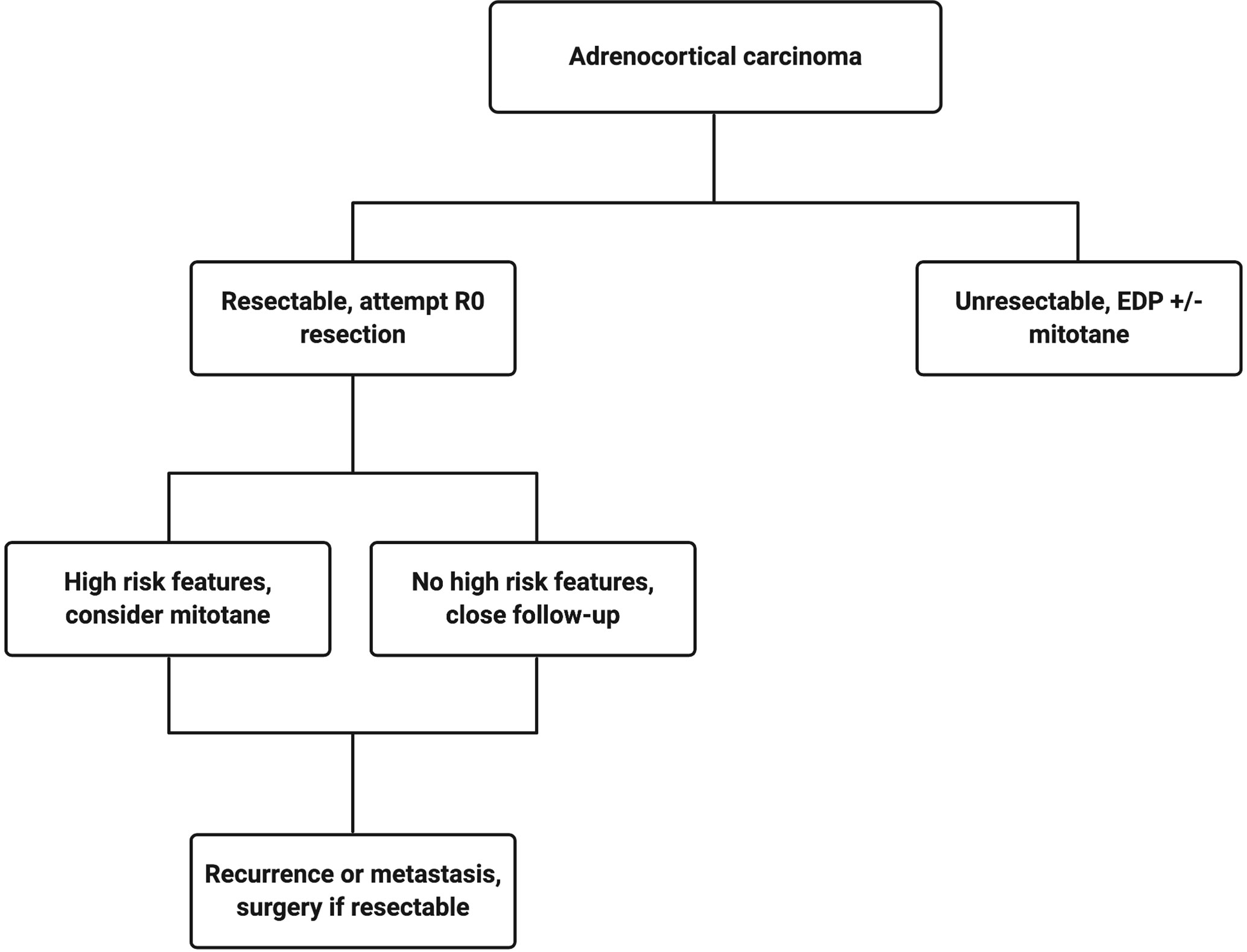
Figure 1 Treatment algorithm for a patient with confirmed adrenocortical carcinoma. Created with BioRender.com .
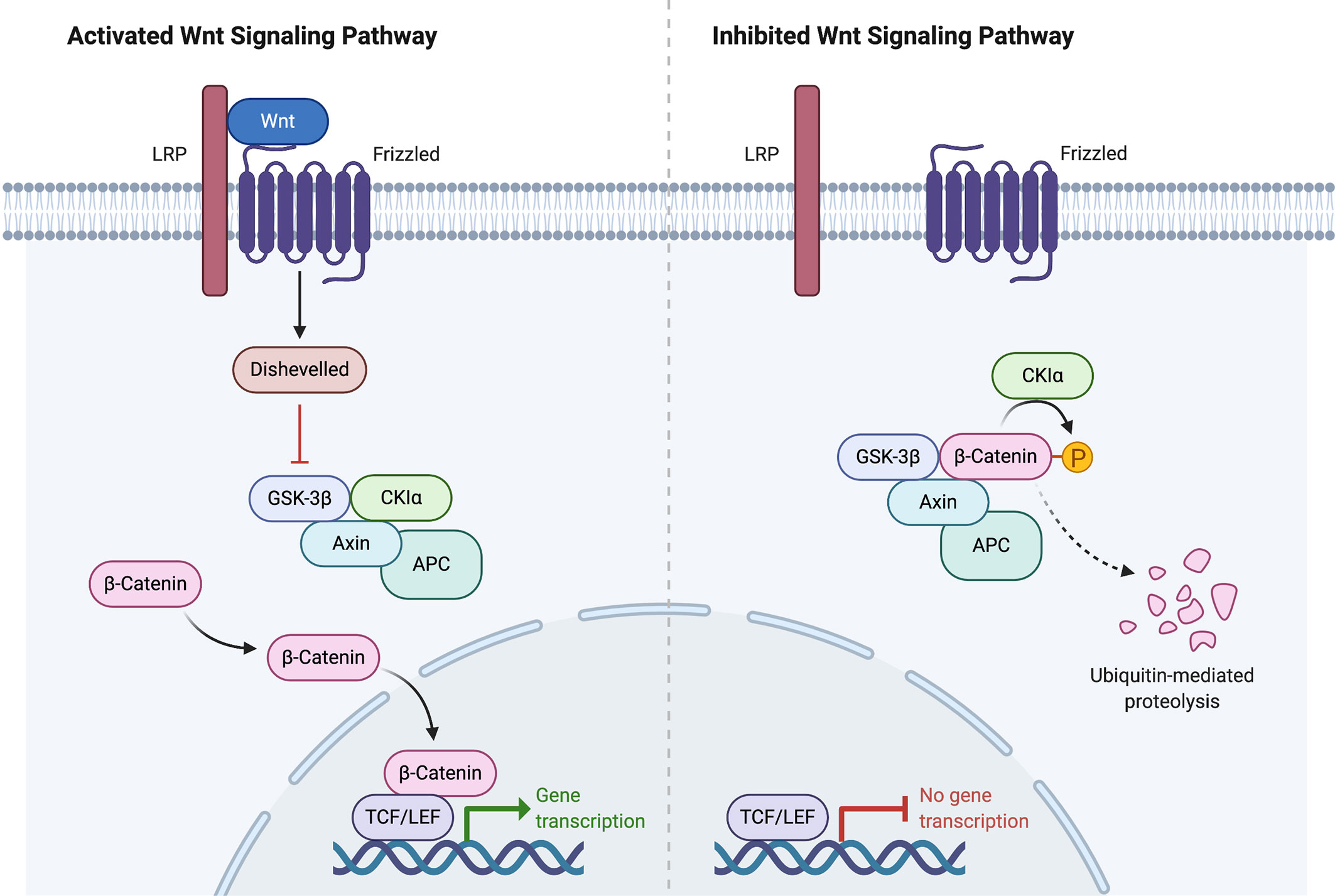
Figure 2 Wnt signaling pathway activation and inhibition. Created with BioRender.com .
Mitotane is the only FDA-approved systemic therapy in ACC. It is an adrenolytic agent derived from the insecticide dichlorodiphenyltrichloroethane (DDT). Mitotane has been the standard treatment for patients with advanced-stage ACC for decades to control tumor growth and hypercortisolemia. However, the response rates are only about 30% and the systemic toxicities make it difficult for patients to tolerate due to a narrow therapeutic window ( 17 – 19 ). In patients with advanced ACC who are not surgical candidates, mitotane combined with platinum-based chemotherapy is recommended as first-line treatment. The recommendation is based on data from the FIRM-ACT randomized clinical trial that compared mitotane plus streptozocin with mitotane plus etoposide, doxorubicin, and cisplatin (EDP-M). Patients who received EDP-M as the first-line therapy had a significantly higher rate of tumor response (23.2% vs. 9.2%, p < 0.001), but only translated to 3 months longer median progression-free survival, compared to the streptozocin group (5.0 vs. 2.1 months, p < 0.001). At five years, 15–20% of patients in the EDP-M group remained alive, compared to 5–10% of patients in the mitotane plus streptozocin cohort ( 20 ).
Patients with ACC mostly present sporadically; however, several hereditary syndromes are associated with the development of ACC. The insight into ACC carcinogenesis from these syndromes can be useful in identifying new treatments. These inherited syndromes include Li-Fraumeni, Beckwith-Wiedemann, multiple endocrine neoplasia type 1 (MEN1), Lynch, familial adenomatous polyposis syndromes, and Carney complex. Li-Fraumeni syndrome is inherited in the autosomal dominant pattern and is associated with inactivating pathogenic variant in TP53 on chromosome 17p13.1. In children, germline TP53 variants could be detected in 50–88% of patients with ACC ( 21 , 22 ). In adults with ACC, germline pathogenic variants in TP53 are seen in 4–6% of patients ( 23 , 24 ). Li-Fraumeni syndrome also confers susceptibility to breast carcinoma, soft tissue sarcoma, brain tumors, osteosarcoma, and leukemia ( 25 ). In Beckwith-Wiedemann syndrome, ACC tumors exhibit pathogenic variations or deletions of imprinted genes on chromosome 11p15. Patients with Beckwith-Wiedemann syndrome are also susceptible to developing congenital abnormalities (omphalocele, macroglossia, macrosomia, hemihypertrophy) or Wilm’s tumor. Patients with Lynch syndrome have a lifetime risk of colorectal cancer of 10–47%, depending on the mismatch repair gene that is mutated. Lynch syndrome has also been linked to increased rates of other cancers, including adrenocortical carcinoma, pancreatic, prostate, breast, and cervical cancer. Patients with MEN1 syndrome have inactivating pathogenic variants of the MEN1 gene that increase susceptibility to parathyroid tumors, pituitary tumors, pancreatic neuroendocrine tumors, and unilateral or bilateral adrenal tumors, including adrenocortical carcinoma. Familial adenomatous polyposis occurs secondary to a pathogenic germline variation in the APC gene and is associated with the development of multiple adenomatous polyps and cancer of the colon and rectum, along with extracolonic manifestations that include adrenocortical carcinoma ( 26 – 30 ). Lastly, Carney complex is a multiple neoplasia syndrome characterized by a pathogenic variant of the PRKAR1A gene resulting in spotty skin pigmentation, myxomas, pasmmomatous melanotic schwannomas, and endocrine tumors. A case report ( 31 ) and genetic analysis of a large family with Carney complex ( 32 ) point to an association between Carney complex and ACC.
Because of the lack of effective systemic treatment options, there is an urgent need for effective therapies in the management of adrenocortical carcinoma. A better understanding of the molecular drivers that contribute to ACC development is critical. In this review, we focus on the molecular alterations of ACC and the potential therapeutic implications.
Genetic Alterations
Two important studies that comprehensively analyzed ACC samples were from the European Network for the Study of Adrenal Tumors (ENSAT) ( 33 ) and The Cancer Genome Atlas ( 34 ) cohorts. These two studies integratively analyzed multiple molecular and genomic platforms. They discovered and confirmed several important molecular alterations in ACC tumorigenesis and progression. The publicly-available databases from both studies containing somatic mutations, DNA methylation, mRNA expression, miRNA expression, and proteomics in ACC have been an invaluable resource for researchers. The following sections will briefly summarize the molecular alterations associated with ACC.
Whole Exome Sequencing data
Assie et al. (ENSAT) performed the whole-exome sequencing (WES) on 45 ACC tumors and found 3,153 somatic mutations, 1,881 of which occurred in two tumors with a hypermutation phenotype ( 33 ). Zheng et al. (TCGA) performed WES on 91 tumors and found 8,841 mutations, 3,427 of which were found in two tumors with an ultramutator phenotype ( 34 ). The ultramutator phenotype was excluded from subsequent whole-exome analyses. Compared to other cancers with the median tumor mutation of 3.6 mutations per megabase ( 35 ), the tumor mutation burden in ACC is considered relatively low. ACC from ENSAT cohort displayed a mean somatic mutation rate of 0.6 mutations per megabase, whereas those from TCGA cohort showed a median somatic density of 0.9 mutations per megabase. However, both studies showed a linear relationship between the number of mutations in an ACC tumor and worse 5-year overall survival, higher Weiss score, and higher ENSAT stage ( 33 , 34 ).
Somatic Mutations
Copy number alterations were profiled via single-nucleotide polymorphism (SNP) array. In the ENSAT study, 16/22 autosomes showed the loss of heterozygosity in greater than 30% of cases. As previously reported ( 36 ), the IGF2 locus showed frequent loss of heterozygosity, and was seen in 82% of tumors in the ENSAT study. The TGCA study reported that IGF2 expression was unanimously high in 67/78 tumors, and the expression was independent of ACC classification ( 33 , 34 ).
In the ENSAT study, high-level amplifications were seen in TERT and CDK4 . Homozygous deletions were noted in CDKN2A , RB1 , ZNRF3 , 3q13.1, 4q34.3, and around a long noncoding RNA LINC00290 . Chromosomal analysis revealed hypodiploidy in 33% of tumors and polyploidy in 43% of tumors. The most frequently altered gene was ZNRF3 , with changes seen in 21% of ACC tumors in the ENSAT cohort. ZNRF3 encodes a cell surface membrane E3 ubiquitin ligase that is a negative feedback regulator of the Wnt/β-catenin signaling pathway by promoting the degradation of the LRP6 and Frizzled receptors ( Figure 2 ). Homozygous deletions of ZNRF3 were seen in 19 tumors, and somatic mutations were noted in 7 more tumors ( 33 , 34 ).
In the TCGA study, recurrent focal amplifications were similarly noted in TERT and CDK4 , but their analysis added TERF2 (Telomeric Repeat-Binding Factor2) and CCNE1 (Cyclin E1) to the list developed previously by the ENSAT study. Similar to ENSAT cohorts, TCGA study found deletions in CDKN2A , RB1 , ZNRF3 , and around LINC00290 in ACC. ABSOLUTE algorithm ( 37 ) was used to determine tumor purity, ploidy, and give insight into whole-genome doubling. Hypodiploidy was noted in 31% of tumors (which was higher than 11 other tumor types). The whole-genome doubling analysis led to an evaluation of telomere regulation. TERT expression was significantly higher in tumors that underwent the whole-genome doubling, leading the authors to postulate that the relationship between the whole-genome doubling and TERT expression suggests the important role TERT plays in maintaining telomere length in ACC ( 33 , 34 ).
MutSigCV ( 38 ) is a robust analytical methodology to identify gene mutations associated with cancers. This method overcomes the mutational heterogeneity. Using this method, CTNNB1 , TP53 , DAXX , MEN1 , PRKAR1A , RPL22 were all identified collectively between the ENSAT and TCGA cohorts. Of 122 ACC tumors evaluated in the ENSAT study, nine genes displayed damaging mutations, homozygous deletions, or high-level amplifications in ≥ 5% of ACCs: ZNRF3 , CTNNB1 , TP53 , CDKN2A , RB1 , MEN1 , DAXX , MED12 , and TERT . The TCGA group analyzed their data via the Cancer Gene Consensus and also noted that NF1 and MLL4 were mutated in more than 5% of the cohort ( 33 , 34 ).
Alterations in CTNNB1 , a gene that encodes the β-catenin protein, and ZNRF3 were noted to be mutually exclusive. β-catenin targets were activated via transcription in tumors with altered ZNRF3 were seen, but this activation was weaker than in CTNNB1- mutated tumors. Alterations of ZNRF3 , CTNNB1 , APC , and MEN1 resulted in modification of the Wnt/β-catenin pathway in 41% of TCGA tumors, and 39% of ENSAT tumors. Alterations in the p53-Rb pathway were noted in 33% of tumors in the ESNAT study. Histone modification ( MLL , MLL2, MLL4 ) and chromatin remodeling ( ATRX , DAXX ) were altered in 22% of tumors in the TCGA study ( 33 , 34 ).
These data suggest that the Wnt/β-catenin, cell-cycle regulators (CDKs), TERT, histone modification, and chromatin remodeling are the commonly dysregulated pathways in ACC. Our group demonstrated that high CDK1 expression in ACC was associated with adverse clinical features and shorter overall survival. We showed in vitro and in vivo efficacy of the synergistic combination of multi-CDK inhibitor and a proteasome inhibitor in ACC ( 39 ).
Methylome, Transcriptome, MicroRNA, and ACC Clustering
The ENSAT study incorporated the recursively partitioned mixture model ( 40 ) to show four different DNA methylation-based tumor clusters. Compared to benign adrenal cortical tumors, ACCs are globally more hypermethylated at the CpG islands in the promoter regions. Two of the clusters corresponded to previously described by the CpG Island Methylator Phenotype (CIMP) status based on their differential methylation profile. Consistent with several cancers such as gastric, ovarian, liver, and lung cancers, patients with hypermethylated ACC had a significantly shorter survival. The group that contained ACCs with differential hypermethylation was further divided into “CIMP-high” and “CIMP-low” subgroups. Patients with ACC in both subgroups had significantly shorter survival than that of the non-CIMP group, but those in the CIMP-high group had the shortest overall survival ( 41 ).
The clustering of mRNA expression profiles in the ENSAT study confirmed the existence of two main transcriptional clusters that are strongly correlated with survival. The C1A cluster displayed numerous pathogenic variants and DNA methylation alterations and is associated with poor outcomes. C1A group comprised largely of CIMP-high and CIMP-low, while almost all ACCs in the CIB group were non-CIMP. Interestingly, the C1B cluster displayed specific deregulation of two microRNA clusters and is associated with a good prognosis ( 33 ).
microRNA Illumina sequencing was performed on 45 ACCs and 3 normal adrenal gland samples in the ENSAT study. Confirming the previously reported data ( 42 – 44 ), MIR483 , located on intron 2 of the IGF2 locus, is overexpressed in ACC. Interestingly, a recent single-institution study of 48 patients suggested that mIR-483-5p measured after initial surgery for ACC can be a potential early post-operative biomarker for ACC prognosis to predict recurrence-free survival ( 45 ). In the ENSAT cohort, miRNA analysis revealed the upregulation of 11 miRNAs belonging to the miRNA-506-514 cluster (Xq27.3) and downregulation of 38 miRNAs to the imprinted DLK1-MEG3 cluster (14q32.2). miRNA clustering in the ENSAT cohort showed three distinct clusters: Mi1, Mi2, and Mi3. Cluster Mi1 showed the largest miRNA expression differences relative to normal adrenal samples. Interestingly, the ACCs in Mi1 cluster had the downregulation of 38 miRNAs belonging to the imprinted DLK1-MEG3 cluster (14q32.2). The SNP array identified the LOH of the 14q in all Mi1 tumors, with the associated transition from hemi to full-hypermethylated MEG3 long noncoding RNA. Thus, the loss of the maternal unmethylated allele resulted in the silencing of the miRNA in the DLK1-MEG3 cluster. Because the DLK1 is a non-canonical Notch ligand and was implicated in ACC tumorigenesis, targeting DLK1/Notch signaling may be further explored. Transcriptome clusters from the ENSAT group were strongly correlated with subgroups based on DNA methylation and miRNA expression. The C1A subgroup included almost all CIMP and Mi3 tumors, and the C1B subgroup was generally non-CIMP and belonged to Mi1 or Mi2 miRNA clusters. The mutation rate was noted to be higher in the C1A subgroup as compared to C1B (0.75 mutations/megabase vs 0.32 mutations/megabase; p = 7.5 × 10 -4 , Wilcoxon rank-sum test) ( 33 ).
Information from the clustering experiments described above contributed to further clustering experiments that were instrumental in formulating several molecular stratification systems. The clustering of 89 ACCs in the TCGA cohort based on their arm-level alterations produced three subgroups, named chromosomal (61%), noisy (30%), and quiet (9%). The chromosomal group had the highest frequency of whole-chromosome arm gains and losses, whereas the noisy group had a significantly higher number of chromosomal breaks, leading to worse overall survival. The quiet group had very few large copy-number alterations. Whole-genome doubling was seen in 68% of the noisy subtype, 51% of the chromosomal subtype, and 0% in the quiet subtype ( 34 ).
The TCGA study also performed clustering of ACC by genomic and transcriptomic characteristics that yielded a multitude of groups. A Cluster of Cluster (CoC) analysis was performed based on DNA copy-number, DNA methylation, mRNA-expression, and mi-RNA expression platforms. Three subtypes were delineated, named CoC I, II, and III. Disease progression rates were reported as 7%, 56%, and 96%, respectively. CoC I ACCs are characterized by low methylation of CIMP, steroid phenotype low pattern, and implicate the following genes: ZNRF3 , MEN1 , and MMR-related genes. CoC II ACCs have an intermediate level of methylation of CIMP, typically have steroid phenotype high pattern with/without proliferation pattern, and implicate the following genes: CDKN2A , CTNNB1 , NF1 , PRKAR1A , TP53 , and ZNRF3 . Lastly, CoC III ACCs show a high level of CIMP methylation, are steroid phenotype high with/without proliferation pattern, have the worst clinical outcomes, and have alterations in the following driver genes: CDK4 , CDKN2A , CTNNB1 , MLL4 , RB1 , TERT , TP53 , and ZNRF3 ( 34 ).
Another important study in understanding molecular markers of malignancy in ACC was performed by de Reyniès et al. ( 46 ). In their study, 153 unilateral adrenocortical tumors were analyzed by microarray or reverse transcription quantitative polymerase chain reaction. They discovered that among malignant tumors, the combined expression of BUB1B and PINK1 was the best predictor of overall survival. BUB1B encodes a protein called BUBR1 that is important for proper chromosome separation during cell division by ensuring that each sister chromatid is attached to a spindle microtubule. Impaired checkpoint function has been implicated in cancer predisposition ( 47 ). PINK1 encodes PTEN induced putative kinase 1, a protein located in the mitochondria and is thought to protect against malfunction during periods of cellular stress. Pathogenic variants in PINK1 cause one form of Parkinson’s disease ( 48 ). High levels of BUB1B-PINK1 is associated with a good overall prognosis and is classified as CoC I. On the contrary, low levels of BUB1B-PINK1 are associated with a worse overall prognosis and are typically classified as either CoC II or III.
More recently, Drelon et al. performed a retrospective analysis of publicly available microarray data from Cochin and Michigan ACC cohorts ( 49 ). Tumors in these cohorts had overexpression of EZH2 , and was further supported by mRNA sequence data from the TCGA program. E2F transcription factors are positive regulators of EZH2 expression. The knockdown of specific E2F transcription factors resulted in a decrease of ACC cells in vitro , and can be considered as a target for future study.

Recent Trials
ACC remains the neglected and lethal cancer because the progress in identifying clinically-effective therapy has been slow. Since the FIRM-ACT trial, no clinical trial has been successful to improve outcomes of patients with advanced ACC. Based on preclinical data above showing that 90% of ACC overexpressed IGF2 and the inhibition of IGF2/IGF1R was effective in vivo , several clinical trials were conducted using monoclonal antibodies (cixutumumab and figitumumab) and a small molecule inhibitor (linsitinib). None has shown promising efficacy in ACC. The GALACCTIC trial ( 50 ) was a phase III, double-blinded, randomized controlled trial that evaluated patients with advanced ACC treated with at least one but fewer than three previous drug regimens. Patients were administered either linsitinib (an inhibitor of IGF1R and insulin receptors) or placebo. The trial failed to show a survival benefit compared to placebo (median OS, 323 days vs 356 days, p = 0.77). The resistance likely occurs because of the downstream events. Thus, additional therapy to address Wnt/β-catenin and overexpressed CDKs may be needed.
Similar to other solid cancers, immunotherapy such as checkpoint inhibitors have been studied in ACC. One of the main challenges in using immunotherapy in ACC is the concomitant Cushing’s syndrome that occurs in 50% of patients with ACC. Glucocorticoid excess causes T cell depletion and is associated with an unfavorable prognosis ( 51 ). The JAVELIN trial ( 52 ) was a phase Ib single-arm study that included 1,758 patients with different types of advanced solid tumors. Avelumab, an anti-PD-L1 antibody, was the treatment of interest. A subgroup analysis of 50 patients with advanced ACC previously treated with platinum-based chemotherapy showed a partial response in 6% of patients, two of whom were also treated with mitotane concomitantly. Other trials have also failed to show the benefit of streptozocin monotherapy ( 20 ), the combination of IGF1R and mTOR pathway inhibitors (3.9% response, n = 181) ( 53 ), tyrosine-kinase inhibitors (1.4% response rate, n = 72) ( 53 ), and gemcitabine-based chemotherapy (4.9% response rate, n = 145) ( 54 ).
Bench to Bedside
Despite recent misses in ACC therapy development, there are many molecular targets currently being evaluated for the management of ACC. Each specific molecular dysregulation, whether it be from gene amplification, loss-of-function, or microsatellite instability, can potentially be treated with a specific targeted therapy. Because ACC is resistant to drug treatments for several reasons such as the overexpression of p-glycoprotein that effectively pumps the drugs out of the cells and multiple dysregulated pathways involved in tumorigenesis and cell survival, single-drug treatment is not likely to result in a durable long-term efficacy. The ideal treatments should target multiple critical dysregulated molecular features in ACC to induce a synergistic response tailored to patient’s tumor profile.
Our group demonstrated that the overexpression of CDK1 and CDK2 in multiple independent cohorts and was associated with poor prognosis. We found that patients with ACC overexpressing CDK1 and CDK2 had significantly higher rates of larger tumors, metastasis, recurrent disease, and shorter overall survival ( 39 ). Moreover, CDK4 amplification has a prevalence of approximately 19% in ACC ( 55 ). Treating the NCI-H295R ACC cell line and ACC primary cultures with a CDK4/6 inhibitor, palbociclib, induced a concentration-dependent decrease of cell viability in cell culture. Cell cycle analysis revealed an increase in the proportion of cells at G0/G1 phase, and palbociclib significantly decreased expression levels of cell cycle-related protein cyclin D1 ( 56 ). However, a different preclinical study evaluated the effects of palbociclib and ribociclib in NCI-H295R and SW-13 cell lines with different results. Treatment with palbociclib induced cell cycle arrest and senescence, similar to the previous experiment, in these two cell lines. Palbociclib induced apoptosis in the NCI-H295R cell line, but treatment with a different CDK4/6 inhibitor, ribociclib, did not. Neither drug-induced apoptosis in the SW-13 cell line ( 57 ). Thus, these two drugs may not be ideal candidates to test in a clinical trial. Palbociclib and ribociclib are currently used to treat HR-positive, HER2-negative breast cancer ( 58 ). Because there is an urgent need for effective systemic treatments in rare cancers such as ACC, traditional drug development is not feasible due to the prohibitive cost and time needed for developing a new therapy. We previously demonstrated that drug repurposing using quantitative high-throughput screening (qHTS) of a clinically approved drug library is an effective and efficient way to identify active drugs in rare cancers ( 59 – 61 ). However, the monotherapy using CDK inhibitors in cancer can induce the NF-kB signaling pathway to protect cells from lethal consequences ( 62 ). We showed that the combination of flavopiridol (multi-CDK inhibitor) and carfilzomib (proteasome inhibitor) was synergistically effective in vitro using NCI-H295R, SW13, and BD140A ACC cell lines, and in vivo using NCI-H295R human ACC xenografts via the reduction of XIAP (anti-apoptotic protein) ( 39 ). We are currently developing a clinical trial in patients with advanced ACC using the combination of multi-CDK and proteasome inhibitors.
Loss-of-function in ATRX has a prevalence of about 15% in ACC ( 55 ). Loss of ATRX in human cancer cells has been shown to prime cells for alternative lengthening of telomeres to achieve replicative mortality ( 63 ). Cells that undergo these changes are sensitive to serine/threonine-protein kinase ATR inhibitors (i.e. VE-821) or agents that produce double-strand breaks, such as radiation therapy ( 64 ). ATR inhibitors have also been shown to sensitize cells to topoisomerase inhibitors ( 65 ). A phase I study of ATR inhibitor M6620 in combination with topotecan (a topoisomerase 1 inhibitor) was performed for various advanced solid tumors. This combination is the first to use the ATR inhibitor-chemotherapy combination. The therapy was well-tolerated. Three of 5 patients with platinum-refractory small-cell lung cancer had a partial response or prolonged stable disease ( 66 ). However, no patients with adrenocortical carcinoma were included in this study. The combination of ATR inhibitor and topotecan should be studied in ACC with the loss of ATRX .
Loss-of-function mutations in NF1 or MAP2K1 have a reported prevalence of 12% in ACC ( 55 ). MEK inhibitors have been shown to be effective in a phase I study for NF1 -driven inoperative plexiform neurofibromas ( 67 ), melanoma, and glioma ( 58 ). There is no clinical trial in ACC using inhibitors of the MAPK signaling pathway.
Loss-of-function mutations in ATM , BRCA1 , or BRCA2 are uncommon in ACC with a prevalence of approximately 4% ( 55 ). PARP inhibitors are currently approved for BRCA1/BRCA2 mutation ovarian carcinoma ( 68 ). The efficacy of PARP inhibitor olaparib has also been observed in other cancers with a deficiency of the homologous DNA repair system, namely metastatic breast cancer and metastatic prostate cancer ( 69 , 70 ).
Microsatellite instability or hypermutator phenotype is seen in about 4% of ACC tumors. Solid tumors classified as hypermutated or microsatellite instability-high (MSI-H) have shown encouraging response rates (40% of 10 patients with mismatch repair-deficient colorectal cancers, 71% of 7 patients with mismatch repair-deficient non-colorectal cancers) to anti-PD-1 antibody ( 71 ). A recent phase II clinical trial studying pembrolizumab in advanced ACC showed an objective response rate of 14% (14 patients at 27 weeks evaluated; 2 patients had a partial response, 7 patients had stable disease, and 5 patients had progressive disease), though 13/14 tumor specimens were microsatellite-stable ( 72 ).
Loss-of-function mutations in PTCH-1 have a prevalence of 2% in ACC. Vismodegib is a Smoothened inhibitor that is currently approved for basal cell carcinoma, and other PTCH-1 mutated tumors have responded to this therapy in a recent phase II trial ( 73 ). The MyPathway trial is currently ongoing, and enrolling patients with molecular testing demonstrating an activating mutation of SMO or loss-of-function mutation of PTCH-1 .
JAG1 amplification or NOTCH1 loss-of-function has also been the topic of a recent trial. The prevalence of these two genetic alterations in ACC is currently unknown. JAG1 overexpressed in ACC has been linked to increased cell proliferation ( 74 ). NOTCH inhibition resulted in inhibition of cell proliferation in a Y1 mouse ACC cell line ( 75 ). In a recent phase I trial, four patients with ACC (3 with JAG1 amplification, 1 with NOTCH1 truncation) received a NOTCH inhibitor; one patient had a partial response ( 76 ).
The most common disease-driving mechanisms of ACC, as described in a previous section, are the p53-Rb and Wnt/β-catenin signaling pathways. Unfortunately, these pathways still lack specific targeted therapy on clinical trials at this time, though preclinical work shows some promise ( 77 ).
Other Treatments in ACC
Various additional therapies and strategies have been used in the treatment of ACC, including radiation therapy, radiofrequency ablation (RFA), and peptide receptor radionuclide therapy (PRRT). In preclinical studies ( 78 , 79 ), it has been suggested that mitotane has possible radiosensitizing properties, and when given in combination with ionizing radiation, can promote neoplastic growth inhibition. However, a retrospective SEER database study identified 74 patients that received radiotherapy out of 530 patients diagnosed with ACC. In their propensity score analysis, they concluded that radiotherapy did not improve overall or cancer-specific survival in patients with ACC ( 80 ). Radiation therapy seems best utilized in palliation of symtpoms rather than as a treatment modality ( 81 ). RFA has been utilitized in the management strategy of unresectable ACC tumors, and is most useful in tumors <5 cm ( 82 ). However, the long-term impact is not known. RFA is also used in the treatment of small liver metastases ( 82 ). PRRT has also been studied in patients with ACC ( 83 ). In a prospective study of 19 patients with metastatic ACC, 8 patients displayed radiometabolic uptake of any-grade intensity, and 2 patients displayed strong uptake in multiple lesions. The two patients with strong uptake were treated with PRRT, and both obtained overall disease control lasting 4 and 12 months, respectively. These are at best case reports with limited data, and should be properly studied in a clinical trial.
Current Clinical Trials
There are currently 12 ACC studies actively enrolling patients, as seen on clinicaltrials.gov ( Table 1 ). Three trials pertain to immunotherapy with anti-PD-1 antibodies: two phase II trials evaluating nivolumab plus ipilimumab; one phase II trial evaluating pembrolizumab for rare and inoperable tumors. ADIUVO-2 is a phase III trial comparing cisplatin/etoposide/mitotane and mitotane monotherapy after initial resection for ACC. ACACIA is evaluating the efficacy of cisplatin/etoposide as compared to observation/mitotane after primary resection of localized ACC. Three trials are currently evaluating the efficacy of cabozantinib, an inhibitor of receptor tyrosine kinase. One trial is evaluating cabazitaxel for ACC progression after previous chemotherapy lines. A cancer peptide therapeutic vaccine, administered with nivolumab, is being evaluated. Patients with ACC that has metastasized to the peritoneum can be enrolled in a phase II trial evaluating surgical resection and HIPEC (cisplatin intraperitoneally, sodium thiosulfate intravenously during HIPEC).
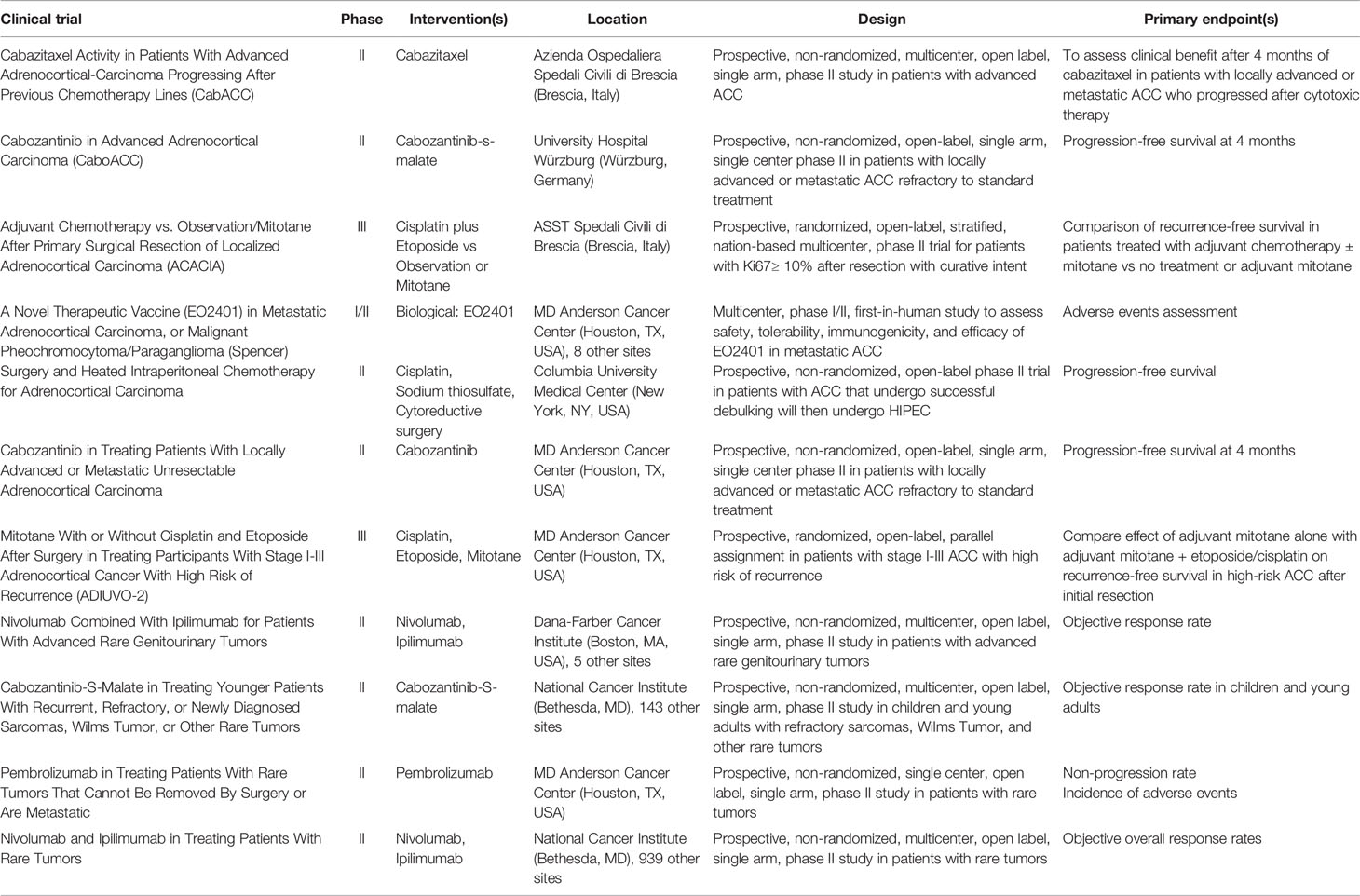
Table 1 Clinical trials that are actively recruiting for adrenocortical carcinoma.
Author Contributions
GA was tasked with outlining and writing the review article. NN was the principal investigator tasked with reviewing the article, making edits, and contributing ideas. All authors contributed to the article and approved the submitted version.
The research activity in this manuscript was supported by the Intramural Research Program, National Cancer Institute, NIH (ZIA BC 011286).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer (2008) 113(11):3130–6. doi: 10.1002/cncr.23886
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Kerkhofs TMA, Verhoeven RHA, Van der Zwan JM, Dieleman J, Kerstens MN, Links TP, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer (2013) 49(11):2579–86. doi: 10.1016/j.ejca.2013.02.034
3. Ng L, Libertino JM. Adrenocortical carcinoma: diagnosis, evaluation and treatment. J Urol (2003) 169(1):5–11. doi: 10.1016/S0022-5347(05)64023-2
4. Sharma E, Dahal S, Sharma P, Bhandari A, Gupta V, Amgai B, et al. The Characteristics and Trends in Adrenocortical Carcinoma: A United States Population Based Study. J Clin Med Res (2018) 10(8):636–40. doi: 10.14740/jocmr3503w
5. Wang S, Chen SS, Gao WC, Bai L, Luo L, Zheng XG, et al. Prognostic Factors of Adrenocortical Carcinoma: An Analysis of theSurveillance Epidemiology and End Results (SEER) Database. Asian Pac J Cancer Prev (2017) 18(10):2817–23. doi: 10.22034/APJCP.2017.18.10.2817
6. Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg (2001) 25(7):891–7. doi: 10.1007/s00268-001-0047-y
7. Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer (2009) 115(2):243–50. doi: 10.1002/cncr.24030
8. Fassnacht M, Dekkers OM, Else T, Gaudin E, Berruti A, de Krijger RR, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol (2018) 179(4):G1–G46. doi: 10.1530/EJE-18-0608
9. Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, et al. Clinical and biological features in the prognosis of adrenocortical cancer: Poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocr Metab (2006) 91(7):2650–5. doi: 10.1210/jc.2005-2730
10. Tran TB, Postlewait LM, Maithel SK, Prescott JD, Wang TS, Glenn J, et al. Actual 10-year survivors following resection of adrenocortical carcinoma. J Surg Oncol (2016) 114(8):971–6. doi: 10.1002/jso.24439
11. Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol (1999) 6(8):719–26. doi: 10.1007/s10434-999-0719-7
12. Fassnacht M, Johanssen S, Fenske W, Weismann D, Agha A, Beuschlein F, et al. Improved survival in patients with stage II adrenocortical carcinomafollowed up prospectively by specialized centers. J Clin EndocrinolMetab (2010) 95(11):4925–32. doi: 10.1210/jc.2010-0803
CrossRef Full Text | Google Scholar
13. Erdogan I, Deutschbein T, Jurowich C, Kroiss M, Ronchi C, Quinkler M, et al. The role of surgery in the management of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab (2013) 98(1):181–91. doi: 10.1210/jc.2012-2559
14. Datrice NM, Langan RC, Ripley RT, Kemp CD, Steinberg SM, Wood BJ, et al. Operative management for recurrent and metastatic adrenocortical carcinoma. J Surg Oncol (2012) 105(7):709–13. doi: 10.1002/jso.23015
15. Gaujoux S, Al-Ahmadie H, Allen PJ, Gonen M, Shia J, D'Angelica M, et al. Resection of adrenocortical carcinoma liver metastasis: is it justified? Ann Surg Oncol (2012) 19(8):2643–51. doi: 10.1245/s10434-012-2358-7
16. Kemp CD, Ripley RT, Mathur A, Steinberg SM, Nguyen DM, Fojo T, et al. Pulmonary resection for metastatic adrenocortical carcinoma: the National Cancer Institute experience. Ann Thorac Surg (2011) 92(4):1195–200. doi: 10.1016/j.athoracsur.2011.05.013
17. Haak HR, Hermans J, van de Velde CJ, Lentjes EG, Goslings BM, Fleuren GJ, et al. Optimal treatment of adrenocortical carcinoma with mitotane: results in a consecutive series of 96 patients. Br J Cancer (1994) 69(5):947–51. doi: 10.1038/bjc.1994.183
18. Baudin E, Pellegriti G, Bonnay M, Penfornis A, Laplanche A, Vassal G, et al. Impact of monitoring plasma 1,1-dichlorodiphenildichloroethane (o,p’DDD) levels on the treatment of patients with adrenocortical carcinoma. Cancer (2001) 92(6):1385–92. doi: 10.1002/1097-0142(20010915)92:6<1385::AID-CNCR1461>3.0.CO;2-2
19. Gonzalez RJ, Tamm EP, Ng C, Phan AT, Vassilopoulou-Sellin R, Perrier ND, et al. Response to mitotane predicts outcome in patients with recurrent adrenal cortical carcinoma. Surgery Discussion (2007) 142867-875(6):867–75. doi: 10.1016/j.surg.2007.09.006
20. Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, et al. Combination chemotherapy in advanced adrenocorticalcarcinoma. N Engl J Med (2012) 366(23):2189–97. doi: 10.1056/NEJMoa1200966
21. Wasserman JD, Novokmet A, Eichler-Jonsson C, Ribeiro RC, Rodriguez-Galindo C, Zambetti GP, et al. Prevalence and functional consequence of TP53 mutations in pediatric adrenocortical carcinoma: a children’s oncology group study. J Clin Oncol (2015) 33(6):602–9. doi: 10.1200/JCO.2013.52.6863
22. Malkin D, Li FP, Strong LC, Fraumeni JF Jr, Nelson CE, Kim DH, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science (1990) 250(4985):1233–8. doi: 10.1126/science.1978757
23. Raymond VM, Else T, Everett JN, Long JM, Gruber SB, Hammer GD. Prevalence of germline TP53 mutations in a prospective series of unselected patients with adrenocortical carcinoma. J Clin Endocrinol Metab (2013) 98(1):E119–125. doi: 10.1210/jc.2012-2198
24. Herrmann LJ, Heinze B, Fassnacht M, Willenberg HS, Quinkler M, Reisch N, et al. TP53 germline mutations in adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab (2012) 97(3):E476–85. doi: 10.1210/jc.2011-1982
25. Hisada M, Garber JE, Fung CY, Fraumeni JF, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. Jnci-J Natl Cancer I (1998) 90(8):606–11. doi: 10.1093/jnci/90.8.606
26. Raymond VM, Everett JN, Furtado LV, Gustafson SL, Jungbluth CR, Gruber SB, et al. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J Clin Oncol (2013) 31(24):3012–8. doi: 10.1200/JCO.2012.48.0988
27. Weksberg R, Shuman R, Beckwith JB. Beckwith-Wiedemann syndrome. Eur J Hum Genet (2010) 18(1):8–14. doi: 10.1038/ejhg.2009.106
28. Skogseid B, Rastad J, Gobl A, Larsson C, Backlin K, Juhlin C, et al. Adrenal lesion in multiple endocrine neoplasia type 1. Surgery (1995) 118(6):1077–82. doi: 10.1016/S0039-6060(05)80117-5
29. Gatta-Cherifi B, Chabre O, Murat A, Niccoli P, Cardot-Bauters C, Rohmer V, et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’etude des Tumeurs Endocrines database. Eur J Endocrinol (2012) 166(2):269–79. doi: 10.1530/EJE-11-0679
30. Bertherat J, Bertagna X. Pathogenesis of adrenocortical cancer. Best Pract Res Cl En (2009) 23(2):261–71. doi: 10.1016/j.beem.2008.10.006
31. Morin E, Mete O, Wasserman JD, Joshua AM, Asa SL, Ezzat S. Carney complex with adrenal cortical carcinoma. J Clin Endocrinol Metab (2012) 97(2):E202–6. doi: 10.1210/jc.2011-2321
32. Anselmo J, Medeiros S, Carneiro V, Greene E, Levy I, Nesterova M, et al. A Large Family with Carney Complex Caused by the S147G PRKAR1A Mutation Shows a Unique Spectrum of Disease Including Adrenocortical Cancer. J Clin Endocrinol Metab (2012) 97(2):351–9. doi: 10.1210/jc.2011-2244
33. Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, Barreau O, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet (2014) 46(6):607–12. doi: 10.1038/ng.2953
34. Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell (2016) 29(5):723–36. doi: 10.1016/j.ccell.2016.04.002
35. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med (2017) 9(1):34. doi: 10.1186/s13073-017-0424-2
36. Gicquel C, Bertagna X, Schneid H, Francillardleblond M, Luton JP, Girard F, et al. Rearrangements at the 11p15 Locus and Overexpression of Insulin-Like Growth Factor-Ii Gene in Sporadic Adrenocortical Tumors. J Clin Endocrinol Metab (1994) 78(6):1444–53. doi: 10.1210/jc.78.6.1444
37. Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol (2012) 30(5):413–21. doi: 10.1038/nbt.2203
38. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for newcancer-associated genes. Nature (2013) 499(7457):214–8. doi: 10.1038/nature12213
39. Nilubol N, Boufraqech M, Zhang L, Gaskins K, Shen M, Zhang YQ, et al. Synergistic combination of flavopiridol and carfilzomib targets commonly dysregulated pathways in adrenocortical carcinoma and has biomarkers of response. Oncotarget (2018) 9(68):33030–42. doi: 10.18632/oncotarget.26050
40. Houseman EA, Christensen BC, Yeh RF, Marsit CJ, Karagas MR, Wrensch M, et al. Model-based clustering of DNA methylation array data: a recursive-partitioning algorithm for high-dimensional data arising as a mixture of beta distributions. BMC Bioinf (2008) 9:365. doi: 10.1186/1471-2105-9-365
41. Barreau O, Assie G, Wilmot-Roussel H, Ragazzon B, Baudry C, Perlemoine K, et al. Identification of a CpG island methylator phenotype in adrenocortical carcinomas. J Clin Endocrinol Metab (2013) 98(1):E174–84. doi: 10.1210/jc.2012-2993
42. Soon PSH, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR, et al. miR-195 and miR-483-5p Identified as Predictors of Poor Prognosis in Adrenocortical Cancer. Clin Cancer Res (2009) 15(24):7684–92. doi: 10.1158/1078-0432.CCR-09-1587
43. Patterson EE, Holloway AK, Weng J, Fojo T, Kebebew E. MicroRNA Profiling of Adrenocortical Tumors Reveals miR-483 as a Marker of Malignancy. Cancer (2011) 117(8):1630–9. doi: 10.1002/cncr.25724
44. Chabre O, Libe R, Assie G, Barreau O, Bertherat J, Bertagna X, et al. Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocrine-Related Cancer (2013) 20(4):579–94. doi: 10.1530/ERC-13-0051
45. Oreglia M, Sbiera S, Fassnacht M, Guyon L, Denis J, Cristante J, et al. Early Postoperative Circulating miR-483-5p Is a Prognosis Marker forAdrenocortical Cancer. Cancers (2020)12(3):1–11. doi: 10.3390/cancers12030724
46. de Reynies A, Assie G, Rickman DS, Tissier F, Groussin L, Rene-Corail F, et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol (2009) 27(7):1108–15. doi: 10.1200/JCO.2008.18.5678
47. Hanks S, Coleman K, Reid S, Plaja A, Firth H, Fitzpatrick D, et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat Genet (2004) 36(11):1159–61. doi: 10.1038/ng1449
48. Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science (2004) 304(5674):1158–60. doi: 10.1126/science.1096284
49. Drelon C, Berthon A, Mathieu M, Ragazzon B, Kuick R, Tabbal H, et al. EZH2 is overexpressed in adrenocortical carcinoma and is associated with disease progression. Hum Mol Genet (2016) 25(13):2789–800. doi: 10.1093/hmg/ddw136
50. Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol (2015) 16(4):426–35. doi: 10.1016/S1470-2045(15)70081-1
51. Landwehr LS, Altieri B, Schreiner J, Sbiera I, Weigand I, Kroiss M, et al. Interplay between glucocorticoids and tumor-infiltrating lymphocyteson the prognosis of adrenocortical carcinoma. J Immunother Cancer (2020) 8(1):1–12. doi: 10.1136/jitc-2019-000469
52. Le Tourneau C, Hoimes C, Zarwan C, Wong DJ, Bauer S, Claus R, et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer (2018) 6(1):111. doi: 10.1186/s40425-018-0424-9
53. Creemers SG, Hofland LJ, Korpershoek E, Franssen GJ, van Kemenade FJ, de Herder WW, et al. Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocr Relat Cancer (2016) 23(1):R43–69. doi: 10.1530/ERC-15-0452
54. Henning JEK, Deutschbein T, Altieri B, Steinhauer S, Kircher S, Sbiera S, et al. Gemcitabine-Based Chemotherapy in Adrenocortical Carcinoma: A Multicenter Study of Efficacy and Predictive Factors. J Clin Endocrinol Metab (2017) 102(11):4323–32. doi: 10.1210/jc.2017-01624
55. Crona J, Beuschlein F. Adrenocortical carcinoma - towards genomics guided clinical care. Nat Rev Endocrinol (2019) 15(9):548–60. doi: 10.1038/s41574-019-0221-7
56. Fiorentini C, Fragni M, Tiberio GAM, Galli D, Roca E, Salvi V, et al. Palbociclib inhibits proliferation of human adrenocortical tumor cells. Endocrine (2018) 59(1):213–7. doi: 10.1007/s12020-017-1270-0
57. Hadjadj D, Kim SJ, Denecker T, Ben Driss L, Cadoret JC, Maric C, et al. A hypothesis-driven approach identifies CDK4 and CDK6 inhibitors as candidate drugs for treatments of adrenocortical carcinomas. Aging (Albany NY) (2017) 9(12):2695–716. doi: 10.18632/aging.101356
58. Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol (2017). PO.17.00011. doi: 10.1200/PO.17.00011
59. Satoh K, Zhang LS, Zhang YQ, Chelluri R, Boufraqech M, Nilubol N, et al. Identification of Niclosamide as a Novel Anticancer Agent for Adrenocortical Carcinoma. Clin Cancer Res (2016) 22(14):3458–66. doi: 10.1158/1078-0432.CCR-15-2256
60. Nilubol N, Zhang L, Shen M, Zhang YQ, He M, Austin CP, et al. Four clinically utilized drugs were identified and validated fortreatment of adrenocortical cancer using quantitative high-throughput screening. J Trans Med (2012) 10:1–15. doi: 10.1186/1479-5876-10-198
61. Zhang LS, He M, Zhang YQ, Nilubol N, Shen M, Kebebew E. Quantitative High-Throughput Drug Screening Identifies Novel Classes of Drugs with Anticancer Activity in Thyroid Cancer Cells: Opportunities for Repurposing. J Clin Endocrinol Metab (2012) 97(3):E319–28. doi: 10.1210/jc.2011-2671
62. Dai Y, Rahmani M, Grant S. Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-kappaB-dependent process. Oncogene (2003) 22(46):7108–22. doi: 10.1038/sj.onc.1206863
63. Flynn RL, Cox KE, Jeitany M, Wakimoto H, Bryll AR, Ganem NJ, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science (2015) 347(6219):273–7. doi: 10.1126/science.1257216
64. Koschmann C, Calinescu AA, Nunez FJ, Mackay A, Fazal-Salom J, Thomas D, et al. ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci Trans Med (2016) 8(328):328ra328. doi: 10.1126/scitranslmed.aac8228
65. Josse R, Martin SE, Guha R, Ormanoglu P, Pfister TD, Reaper PM, et al. ATR inhibitors VE-821 and VX-970 sensitize cancer cells to topoisomerase i inhibitors by disabling DNA replication initiation and fork elongation responses. Cancer Res (2014) 74(23):6968–79. doi: 10.1158/0008-5472.CAN-13-3369
66. Thomas A, Redon CE, Sciuto L, Padiernos E, Ji J, Lee MJ, et al. Phase I Study of ATR Inhibitor M6620 in Combination With Topotecanin Patients With Advanced Solid Tumors. J Clin Oncol (2018) 36(16):1594–602. doi: 10.1200/JCO.2017.76.6915
67. Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, et al. Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas. N Engl J Med (2016) 375(26):2550–60. doi: 10.1056/NEJMoa1605943
68. Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med (2012) 366(15):1382–92. doi: 10.1056/NEJMoa1105535
69. Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. New Engl J Med (2015) 373(18):1697–708. doi: 10.1056/NEJMoa1506859
70. Robson M, Im SA, Senkus E, Xu BH, Domchek SM, Masuda N, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. New Engl J Med (2017) 377(6):523–33. doi: 10.1056/NEJMoa1706450
71. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-RepairDeficiency. New Engl J Med (2015)372(26):2509–20. doi: 10.1056/NEJMoa1500596
72. Habra MA, Stephen B, Campbell M, Hess K, Tapia C, Xu M, et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J Immunother Cancer (2019) 7(1):253. doi: 10.1186/s40425-019-0722-x
73. Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J Clin Oncol (2018) 36(6):536–42. doi: 10.1200/JCO.2017.75.3780
74. Ronchi CL, Sbiera S, Altieri B, Steinhauer S, Wild V, Bekteshi M, et al. Notch1 pathway in adrenocortical carcinomas: correlations with clinical outcome. Endocr Relat Cancer (2015) 22(4):531–43. doi: 10.1530/ERC-15-0163
75. Simon DP, Giordano TJ, Hammer GD. Upregulated JAG1 Enhances Cell Proliferation in Adrenocortical Carcinoma. Clin Cancer Res (2012) 18(9):2452–64. doi: 10.1158/1078-0432.CCR-11-2371
76. Tuxen IV, Rohrberg KS, Oestrup O, Ahlborn LB, Schmidt AY, Spanggaard I, et al. Copenhagen Prospective Personalized Oncology (CoPPO)-Clinical Utility of Using Molecular Profiling to Select Patients to Phase I Trials. Clin Cancer Res (2019) 25(4):1239–47. doi: 10.1158/1078-0432.CCR-18-1780
77. Batisse-Lignier M, Sahut-Barnola I, Tissier F, Dumontet T, Mathieu M, Drelon C, et al. P53/Rb inhibition induces metastatic adrenocortical carcinomas in a preclinical transgenic model. Oncogene (2017) 36(31):4445–56. doi: 10.1038/onc.2017.54
78. Cerquetti L, Bucci B, Marchese R, Misitid S, De Paula U, Miceli R, et al. Mitotane increases the radiotherapy inhibitory effect and induces G2-arrest in combined treatment on both H295R and SW13 adrenocortical cell lines. Endocr Relat Cancer (2008) 15(2):623–34. doi: 10.1677/erc.1.1315
79. Cerquetti L, Carpinelli G, Lardo P, Proietti A, Saporito R. Antineoplastic Effect of a Combined Mitotane Treatment/IonizingRadiation in Adrenocortical Carcinoma: A Preclinical Study. Cancers (Basel) (2019) 11(11):1–13. doi: 10.3390/cancers11111768
80. Luo Y, Chen SS, Zheng XG, Luo L, Wang S. The efficacy of radiation therapy in adrenocortical carcinoma: A propensity score analysis of a population-based study. Med (Baltimore) (2017) 96(17):e6741. doi: 10.1097/MD.0000000000006741
81. Polat B, Fassnacht M, Pfreundner L, Guckenberger M, Bratengeier K, Johanssen S, et al. Radiotherapy in adrenocortical carcinoma. Cancer (2009) 115(13):2816–23. doi: 10.1002/cncr.24331
82. Wood BJ, Abraham J, Hvizda JL, Alexander HR, Fojo T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer (2003) 97(3):554–60. doi: 10.1002/cncr.11084
83. Grisanti S, Filice A, Basile V, Cosentini D, Rapa I, Albano D, et al. Treatment With 90Y/177Lu-DOTATOC in Patients With Metastatic Adrenocortical Carcinoma Expressing Somatostatin Receptors. J Clin Endocrinol Metab (2020) 105(3):E1‐5. doi: 10.1210/clinem/dgz091
Keywords: adrenocortical, cancer, drug discovery, clinical trial, genomics, molecular profiling, targeted therapy
Citation: Alyateem G and Nilubol N (2021) Current Status and Future Targeted Therapy in Adrenocortical Cancer. Front. Endocrinol. 12:613248. doi: 10.3389/fendo.2021.613248
Received: 01 October 2020; Accepted: 25 January 2021; Published: 01 March 2021.
Reviewed by:
Copyright © 2021 Alyateem and Nilubol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naris Nilubol, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Switch language:

Data Insights
Which drugs are most likely to be approved in adrenal gland cancer.

- Share on Linkedin
- Share on Facebook
Adrenal Gland Cancer is an indication for drug development with over 40 pipeline drugs currently active. According to GlobalData, preregistered drugs for Adrenal Gland Cancer have a 50% likelihood of approval (LoA) indication benchmark. GlobalData’s report assesses how phase transition success rate (PTSR) and likelihood of approval (LoA) scores for pipeline drugs in Adrenal Gland Cancer compared to historical benchmarks. Buy the report here.
Smarter leaders trust GlobalData
Premium insights likelihood of approval analysis for adrenal gland cancer.
Buy the Report
Premium Insights
The gold standard of business intelligence.
Find out more
GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval.
Adrenal Gland Cancer overview
Adrenal gland cancer is a rare type of neuroendocrine tumor that originates from the cells of the adrenal glands, which are located on top of the kidneys. The adrenal glands produce hormones that regulate various functions in the body, such as blood pressure, metabolism, stress response, and sex drive. Adrenal gland cancer can affect the production of these hormones, causing symptoms such as weight gain, muscle weakness, high blood pressure, hair growth, and mood changes. Adrenal gland cancer can be benign or malignant. The most common malignant adrenal gland cancer is adrenocortical carcinoma, which starts in the outer layer of the adrenal glands. Adrenal gland cancer can be diagnosed with blood tests, urine tests, imaging tests, and biopsy. Treatment options include surgery, radiation therapy, chemotherapy, and targeted therapy.
For a complete picture of PTSR and LoA scores for drugs in Adrenal Gland Cancer, buy the report here.
Blending expert knowledge with cutting-edge technology, GlobalData’s unrivalled proprietary data will enable you to decode what’s happening in your market. You can make better informed decisions and gain a future-proof advantage over your competitors.
Be better informed
G lobalDat a , the leading provider of industry intelligence, provided the underlying data, research, and analysis used to produce this article.
GlobalData’s Likelihood of Approval analytics tool dynamically assesses and predicts how likely a drug will move to the next stage in clinical development (PTSR), as well as how likely the drug will be approved (LoA). This is based on a proprietary algorithm built from the drugs’ sales forecast, regulatory milestones, cost forecasts, WACC rate and other proprietary data sources found on GlobalData’s Pharmaceutical Intelligence Center .

Data Insights Likelihood of Approval Analysis for Adrenal Gland Cancer
Related company profiles, more relevant.
ReAlta signs deal with NIAID to develop drug for radiation poisoning
Crispr drug licensing deals secure $21bn in top three therapy areas over five years, cheap drugs, inspections, and a future war in europe - what’s driving drug shortages, ose immunotherapeutics gets grant for anti-human sirpa antibody for treating cancer, sign up for our daily news round-up.
Give your business an edge with our leading industry insights.
Sign up to the newsletter: In Brief
Your corporate email address, i would also like to subscribe to:.
Pharma Technology Focus : Focus (monthly)
Thematic Take (monthly)
I consent to Verdict Media Limited collecting my details provided via this form in accordance with Privacy Policy
Thank you for subscribing
View all newsletters from across the GlobalData Media network.
- Share full article
Advertisement
Supported by
More Studies by Columbia Cancer Researchers Are Retracted
The studies, pulled because of copied data, illustrate the sluggishness of scientific publishers to address serious errors, experts said.

By Benjamin Mueller
Scientists in a prominent cancer lab at Columbia University have now had four studies retracted and a stern note added to a fifth accusing it of “severe abuse of the scientific publishing system,” the latest fallout from research misconduct allegations recently leveled against several leading cancer scientists.
A scientific sleuth in Britain last year uncovered discrepancies in data published by the Columbia lab, including the reuse of photos and other images across different papers. The New York Times reported last month that a medical journal in 2022 had quietly taken down a stomach cancer study by the researchers after an internal inquiry by the journal found ethics violations.
Despite that study’s removal, the researchers — Dr. Sam Yoon, chief of a cancer surgery division at Columbia University’s medical center, and Changhwan Yoon, a more junior biologist there — continued publishing studies with suspicious data. Since 2008, the two scientists have collaborated with other researchers on 26 articles that the sleuth, Sholto David, publicly flagged for misrepresenting experiments’ results.
One of those articles was retracted last month after The Times asked publishers about the allegations. In recent weeks, medical journals have retracted three additional studies, which described new strategies for treating cancers of the stomach, head and neck. Other labs had cited the articles in roughly 90 papers.
A major scientific publisher also appended a blunt note to the article that it had originally taken down without explanation in 2022. “This reuse (and in part, misrepresentation) of data without appropriate attribution represents a severe abuse of the scientific publishing system,” it said .
Still, those measures addressed only a small fraction of the lab’s suspect papers. Experts said the episode illustrated not only the extent of unreliable research by top labs, but also the tendency of scientific publishers to respond slowly, if at all, to significant problems once they are detected. As a result, other labs keep relying on questionable work as they pour federal research money into studies, allowing errors to accumulate in the scientific record.
“For every one paper that is retracted, there are probably 10 that should be,” said Dr. Ivan Oransky, co-founder of Retraction Watch, which keeps a database of 47,000-plus retracted studies. “Journals are not particularly interested in correcting the record.”
Columbia’s medical center declined to comment on allegations facing Dr. Yoon’s lab. It said the two scientists remained at Columbia and the hospital “is fully committed to upholding the highest standards of ethics and to rigorously maintaining the integrity of our research.”
The lab’s web page was recently taken offline. Columbia declined to say why. Neither Dr. Yoon nor Changhwan Yoon could be reached for comment. (They are not related.)
Memorial Sloan Kettering Cancer Center, where the scientists worked when much of the research was done, is investigating their work.
The Columbia scientists’ retractions come amid growing attention to the suspicious data that undergirds some medical research. Since late February, medical journals have retracted seven papers by scientists at Harvard’s Dana-Farber Cancer Institute . That followed investigations into data problems publicized by Dr. David , an independent molecular biologist who looks for irregularities in published images of cells, tumors and mice, sometimes with help from A.I. software.
The spate of misconduct allegations has drawn attention to the pressures on academic scientists — even those, like Dr. Yoon, who also work as doctors — to produce heaps of research.
Strong images of experiments’ results are often needed for those studies. Publishing them helps scientists win prestigious academic appointments and attract federal research grants that can pay dividends for themselves and their universities.
Dr. Yoon, a robotic surgery specialist noted for his treatment of stomach cancers, has helped bring in nearly $5 million in federal research money over his career.
The latest retractions from his lab included articles from 2020 and 2021 that Dr. David said contained glaring irregularities . Their results appeared to include identical images of tumor-stricken mice, despite those mice supposedly having been subjected to different experiments involving separate treatments and types of cancer cells.
The medical journal Cell Death & Disease retracted two of the latest studies, and Oncogene retracted the third. The journals found that the studies had also reused other images, like identical pictures of constellations of cancer cells.
The studies Dr. David flagged as containing image problems were largely overseen by the more senior Dr. Yoon. Changhwan Yoon, an associate research scientist who has worked alongside Dr. Yoon for a decade, was often a first author, which generally designates the scientist who ran the bulk of the experiments.
Kun Huang, a scientist in China who oversaw one of the recently retracted studies, a 2020 paper that did not include the more senior Dr. Yoon, attributed that study’s problematic sections to Changhwan Yoon. Dr. Huang, who made those comments this month on PubPeer, a website where scientists post about studies, did not respond to an email seeking comment.
But the more senior Dr. Yoon has long been made aware of problems in research he published alongside Changhwan Yoon: The two scientists were notified of the removal in January 2022 of their stomach cancer study that was found to have violated ethics guidelines.
Research misconduct is often pinned on the more junior researchers who conduct experiments. Other scientists, though, assign greater responsibility to the senior researchers who run labs and oversee studies, even as they juggle jobs as doctors or administrators.
“The research world’s coming to realize that with great power comes great responsibility and, in fact, you are responsible not just for what one of your direct reports in the lab has done, but for the environment you create,” Dr. Oransky said.
In their latest public retraction notices, medical journals said that they had lost faith in the results and conclusions. Imaging experts said some irregularities identified by Dr. David bore signs of deliberate manipulation, like flipped or rotated images, while others could have been sloppy copy-and-paste errors.
The little-noticed removal by a journal of the stomach cancer study in January 2022 highlighted some scientific publishers’ policy of not disclosing the reasons for withdrawing papers as long as they have not yet formally appeared in print. That study had appeared only online.
Roland Herzog, the editor of the journal Molecular Therapy, said that editors had drafted an explanation that they intended to publish at the time of the article’s removal. But Elsevier, the journal’s parent publisher, advised them that such a note was unnecessary, he said.
Only after the Times article last month did Elsevier agree to explain the article’s removal publicly with the stern note. In an editorial this week , the Molecular Therapy editors said that in the future, they would explain the removal of any articles that had been published only online.
But Elsevier said in a statement that it did not consider online articles “to be the final published articles of record.” As a result, company policy continues to advise that such articles be removed without an explanation when they are found to contain problems. The company said it allowed editors to provide additional information where needed.
Elsevier, which publishes nearly 3,000 journals and generates billions of dollars in annual revenue , has long been criticized for its opaque removals of online articles.
Articles by the Columbia scientists with data discrepancies that remain unaddressed were largely distributed by three major publishers: Elsevier, Springer Nature and the American Association for Cancer Research. Dr. David alerted many journals to the data discrepancies in October.
Each publisher said it was investigating the concerns. Springer Nature said investigations take time because they can involve consulting experts, waiting for author responses and analyzing raw data.
Dr. David has also raised concerns about studies published independently by scientists who collaborated with the Columbia researchers on some of their recently retracted papers. For example, Sandra Ryeom, an associate professor of surgical sciences at Columbia, published an article in 2003 while at Harvard that Dr. David said contained a duplicated image . As of 2021, she was married to the more senior Dr. Yoon, according to a mortgage document from that year.
A medical journal appended a formal notice to the article last week saying “appropriate editorial action will be taken” once data concerns had been resolved. Dr. Ryeom said in a statement that she was working with the paper’s senior author on “correcting the error.”
Columbia has sought to reinforce the importance of sound research practices. Hours after the Times article appeared last month, Dr. Michael Shelanski, the medical school’s senior vice dean for research, sent an email to faculty members titled “Research Fraud Accusations — How to Protect Yourself.” It warned that such allegations, whatever their merits, could take a toll on the university.
“In the months that it can take to investigate an allegation,” Dr. Shelanski wrote, “funding can be suspended, and donors can feel that their trust has been betrayed.”
Benjamin Mueller reports on health and medicine. He was previously a U.K. correspondent in London and a police reporter in New York. More about Benjamin Mueller
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Endocrine cancer articles from across Nature Portfolio
Endocrine cancers are a mixed group of diseases characterized by uncontrolled cellular proliferation of the hormone-producing glands of the endocrine system. These include the thyroid, adrenal, pancreas, parathyroid and pituitary glands.
Related Subjects
- Adrenal tumours
- Neuroendocrine cancer
- Pituitary tumours
- Thyroid cancer
Latest Research and Reviews

Elevated PDE4C level serves as a candidate diagnostic biomarker and correlates with poor survival in thyroid carcinoma
- Yongsheng Zhang
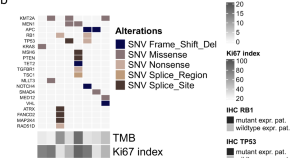
Patient derived tumoroids of high grade neuroendocrine neoplasms for more personalized therapies
- Simon L. April-Monn
- Philipp Kirchner
- Aurel Perren
Usefulness of video laryngoscopy in tracheal intubation at thyroid surgical position for intraoperative neuromonitoring
- Dongwook Won
- Jung-Man Lee
- Seong-Won Min
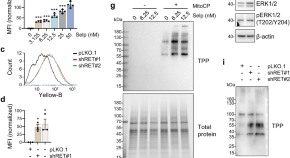
Selpercatinib combination with the mitochondria-targeted antioxidant MitoQ effectively suppresses RET –mutant thyroid cancer
- Wenjing Chen
- Sophie Dream
- Jong-In Park

Unraveling the role of the mitochondrial one-carbon pathway in undifferentiated thyroid cancer by multi-omics analyses
Different types of metabolic rewiring are reported to drive cancer development and as a potential therapeutic target. Here, the authors perform multi-omics analyses in a cohort of human normal and malignant thyroid samples and show association of mitochondrial one-carbon metabolism with undifferentiated thyroid cancer.
- Seong Eun Lee
- Seongyeol Park
- Yea Eun Kang
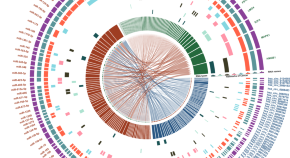
Emerging roles of circular RNAs in regulating the hallmarks of thyroid cancer
- Tianjiao Zhou
- Hongliang Yi
News and Comment
Firstmappp prospectively charts the efficacy of sunitinib for phaeochromocytoma and paraganglioma.
- David Killock
Differentiating high-grade neuroendocrine neoplasms
In this Journal Club, Taboada and Riechelmann discuss the importance of a study outlining a novel neuroendocrine neoplasm classification system.
- Rodrigo Gomes Taboada
- Rachel P. Riechelmann
The shift of therapeutic strategy for prolactinomas: surgery as the first-line option
Activity of selpercatinib confirmed in phase iii trials.
- Diana Romero
Shining a light on age-related adrenal cancer progression
- Shimona Starling
Capivasertib delays disease progression
- Peter Sidaway
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- International edition
- Australia edition
- Europe edition

Cancer charities praise ‘brave’ Princess of Wales for speaking about her diagnosis
Cancer Research UK’s chief executive says high-profile cancer cases such as Catherine’s can help others to seek help early
- Princess of Wales receiving chemotherapy treatment for cancer
- What we know about the Princess of Wales’s cancer diagnosis
- Catherine’s statement in full
Cancer charities have praised the Princess of Wales for her “brave” decision to speak out about her cancer diagnosis as a way to encourage others to get their symptoms checked.
In a video message released on Friday, Catherine, 42, spoke of how her condition was discovered after she underwent abdominal surgery in January. In the weeks that followed her procedure, wild rumours flew around her absence and silence – but she said she and her husband, Prince William, had needed time to explain the situation to their three children, George, 10, Charlotte, eight, and Louis, five.

“This of course came as a huge shock, and William and I have been doing everything we can to process and manage this privately for the sake of our young family,” she said.
“As you can imagine, this has taken time. It has taken me time to recover from major surgery in order to start my treatment. But, most importantly, it has taken us time to explain everything to George, Charlotte and Louis in a way that is appropriate for them, and to reassure them that I am going to be OK.”
Catherine’s announcement prompted the cancer charity Maggie’s to post tips on how to talk to young children about cancer.
“We are incredibly sorry to hear the Princess of Wales’s news and our thoughts are with her,” said Dame Laura Lee, Maggie’s chief executive. “We also know how challenging and worrying a cancer diagnosis can be for the whole family, especially with young children, and our thoughts are with them all.”
Cancer Research UK’s chief executive, Michelle Mitchell, said high-profile cancer cases such as the princess’s can help others to seek help early, when “treatment is more likely to be successful”.
“Nearly one in two of us will develop cancer during our lifetimes, but many more are affected when someone they love is diagnosed with cancer,” she said. “High-profile cancer cases often act as a prompt to encourage people to find out more or think about their own health. If people spot something that’s not normal for them or isn’t going away, they should check with their GP.”
after newsletter promotion
About 393,000 people in the UK are diagnosed with cancer each year, according to Macmillan Cancer Support.
“We hear from people all over the country, every day going through the experience the princess has described, and our thoughts are also with His Royal Highness the Prince of Wales and their children,” the charity said. “Many families will be sending solidarity to them.”
Amanda Pritchard, the chief executive of the NHS, said: “We know how difficult a diagnosis and treatment journey can be for patients and their families. Speaking out is really brave and can help others to get worrying symptoms checked.”
- Catherine, Princess of Wales
- Cancer research
- Medical research

Conspiracy theories tagged #kategate grow despite Kensington Palace video

Speculation about Princess of Wales was worst I’ve seen, says former adviser

Princess of Wales’ diagnosis: cancers in young are rising, but so are survival rates

I had cancer when my children were young. This is what Kate should know
Apologies for Kategate – but will the spirit of restraint on social media last?

On a garden bench, amid a sea of daffodils: how Kate dropped her bombshell news

Britain’s slimmed-down monarchy has been left vulnerable in wake of cancer diagnoses

Burden falls on Prince William to steer monarchy through next few months

‘Hardest conversation’: how to tell children about a cancer diagnosis
Most viewed.

IMAGES
VIDEO
COMMENTS
Important research on adrenal cancer currently is being done in hospitals and institutions around the world. Scientists are learning more about what causes the disease and how best to treat it. ... Przytulska J, Rogala N, Bednarek-Tupikowska G. Current and emerging therapies for adrenocortical carcinoma: Review. Adv Clin Exp Med. 2015;24(2):185 ...
Introduction. Adrenocortical carcinoma (ACC) is a rare endocrine malignancy that arises from the cortex of the adrenal gland and has an estimated worldwide incidence of ~1 case per million population per year. 1 -3 Despite its rarity, ACC generally portends a poor prognosis as the majority of patients develop locally recurrent or metastatic disease, despite undergoing seemingly curative ...
Adrenocortical Carcinoma in Children Rochester, MN. Adrenocortical carcinoma (ACC) is an aggressive, rare childhood cancer. Limited evidence exists on a definite histopathological criterion to differentiate ACC from adrenocortical adenomas. Early diagnosis and management is the key. Surgery is the mainstay of treatment.
The current high detection rate of adrenal tumors (4-10% of general population) is attributable to a widespread use of variety of imaging studies, especially a computed tomography. ... and usually originate from lung, kidney, breast, and colon cancer, or melanoma [1,33,34]. Cysts are rare lesions of the adrenal glands. ... This research ...
Latest Research and Reviews Advances in translational research of the rare cancer type adrenocortical carcinoma Adrenocortical carcinoma is a rare endocrine cancer with a dismal survival rate and ...
Introduction. Adrenocortical carcinoma (ACC) is a rare malignancy arising from the adrenal cortex, with an annual worldwide incidence of 0.5-2 individuals per million population (1, 2).The median age at diagnosis is 55 years, though the incidence follows a bimodal pattern of age distribution, with peaks before the age of five and between the fourth and fifth decades of life (3, 4).
Research Highlight; Published: ... "To test whether ZNRF3 was a tumour suppressor in adrenal cancer, ... Current issue Collections Follow us on Facebook ...
Adrenocortical carcinoma is a rare cancer (estimated incidence, 0.7 to 2.0 cases per 1 million population per year) 1,2 with a poor prognosis; the 5-year survival rate is less than 15% among ...
Adrenocortical carcinoma (ACC) is an aggressive and rare neoplasm that originates in the cortex of the adrenal gland. The disease is associated with heterogeneous but mostly poor outcomes and ...
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy that arises from the cortex of the adrenal gland and has an estimated worldwide incidence of ~1 case per million population per year. 1-3 Despite its rarity, ACC generally portends a poor prognosis as the majority of patients develop locally recurrent or metastatic disease, despite undergoing seemingly curative surgical resection ...
The adrenal glands are paired endocrine organs that produce steroid hormones and catecholamines required for life. Adrenocortical carcinoma (ACC) is a rare and often fatal cancer of the peripheral domain of the gland, the adrenal cortex. Recent research in adrenal development, homeostasis, and disea …
A breakthrough in distinguishing benign adrenal tumors from cancerous ones. Adrenal masses are found in 5% of the population, and while most are benign, it can be tricky for doctors to determine if the mass is cancerous. A new urine test can pinpoint the cases that are cancer so they can be treated. July 27, 2022 •. By Chris Bahnsen,
SDH is a specialized protein that plays an important role in the body's metabolism. Researchers think that when this gene is inactivated, it results in cancer, including adrenal gland tumors. Palliative and supportive care. Clinical trials are underway to find better ways of reducing symptoms and side effects of current adrenal gland tumor ...
This Research Topic welcomes original research, including clinical, translational, as well as molecular studies, review articles, and clinical trials on a range of topics related to current and new diagnostic approaches and treatments for adrenal tumors (pheochromocytoma, primary aldosteronism, adrenocortical carcinoma, adrenal tumors with ...
About Adrenal Cancer. Causes, Risk Factors, and Prevention. Early Detection, Diagnosis, and Staging. Treatment. After Treatment. Get an overview of adrenal cancer and the latest key statistics in the US.
Adrenal cancer affects everyone differently. Some people develop pain if the tumor grows and presses on nearby organs. Others may notice symptoms based on the extra hormones the tumor releases. General adrenal cancer symptoms may include: Abdominal pain. Back pain. Feeling of fullness in your belly. Muscle cramps.
This is Cancer.Net's Guide to Adrenal Gland Tumor. Use the menu below to choose the Introduction section to get started. Or, you can choose another section to learn more about a specific question you have. ... Latest Research. Coping with Treatment. Follow-Up Care. Survivorship. Questions to Ask the Health Care Team. Additional Resources ...
Research through clinical trials is ongoing for all types of cancer. For specific topics being studied for adrenal gland tumors, learn more in the Latest Research section. Cancer.Net offers more information about clinical trials in other areas of the website, including a complete section on clinical trials.
Symptoms. Signs and symptoms of adrenal cancer include: Weight gain. Muscle weakness. Pink or purple stretch marks on the skin. Hormone changes in women that might cause excess facial hair, hair loss on the head and irregular periods. Hormone changes in men that might cause enlarged breast tissue and shrinking testicles.
Researchers are enhancing immunotherapy effects against malignant tumors by developing and validating patent-ending poly (lactic-co-glycolic acid), or PLGA, nanoparticles modified with adenosine ...
Introduction. Adrenocortical carcinoma (ACC) is a rare malignancy arising from the adrenal cortex, with an annual worldwide incidence of 0.5-2 individuals per million population (1, 2).The median age at diagnosis is 55 years, though the incidence follows a bimodal pattern of age distribution, with peaks before the age of five and between the fourth and fifth decades of life (3, 4).
Adrenal Gland Cancer overview. Adrenal gland cancer is a rare type of neuroendocrine tumor that originates from the cells of the adrenal glands, which are located on top of the kidneys. The adrenal glands produce hormones that regulate various functions in the body, such as blood pressure, metabolism, stress response, and sex drive.
Clinical presentation. ACC is diagnosed more frequently in women than in men (ratio 1.5:1) and while the peak incidence is reported around the fourth to fifth decade, ACC may occur at any age. 6 The initial diagnosis is often incidental, and every adrenal tumor detected upon imaging should be subject to structured hormonal and radiological assessment [computed tomography (CT) or magnetic ...
Read the latest Research articles in Adrenal tumours from Nature Reviews Endocrinology. Skip to main content. ... 2013 was a good year for adrenocortical cancer, as the new knowledge gained holds ...
For nearly 25 years, Karen Peterson, PhD, the director of the Office of Scientific Career Development (OSCD) at Fred Hutch Cancer Center, has supported the career and professional development of postdoctoral researchers — at Fred Hutch, locally and nationally. At the National Postdoctoral Association (NPA) annual conference Friday, Peterson received the NPA Distinguished Service Award in ...
In a study, published today in Cancer Research, a journal of the American Association for Cancer Research, researchers say they have discovered that the protein puromycin-sensitive aminopeptidase ...
Dr. Yoon, a robotic surgery specialist noted for his treatment of stomach cancers, has helped bring in nearly $5 million in federal research money over his career.. The latest retractions from his ...
The preclinical research, led by Van Andel Institute scientists, determined how low doses of a DNMT inhibitor sensitize cancer cells to an EZH2 inhibitor, resulting in a one-two punch that combats ...
Endocrine cancer articles from across Nature Portfolio. Atom. RSS Feed. Endocrine cancers are a mixed group of diseases characterized by uncontrolled cellular proliferation of the hormone ...
Cancer Research UK's chief executive says high-profile cancer cases such as Catherine's can help others to seek help early Cancer charities have praised the Princess of Wales for her "brave ...