Close panel
Press Enter
Predictive Search
- Analysis and Opinion
- Sustainability
- Life and Culture
- Coronavirus
- Seeds for the future
- BBVA Earnings
- Corporate information
- BBVA financial information
- Financial calendar
- Latest news
- BBVA Podcast
- Customer service via social networks
- Special reports
- Careers at BBVA
- Social media
- Vulnerability Disclosure Program
- Chair’s message
- CEO’s message
- History of BBVA
- BBVA in the world
- Organizational chart
- Sustainability and responsible banking model
- Corporate Presentation
- Code of Conduct
- BBVA’s tax strategy
- BBVA Due Diligence
- BBVA in 2023
- Annual Report
- Financial reports
- Relevant events
- Issuances and programs
- Biographies
- Photos Directors / Executive Leadership Team
- Download center
- Seeds the future
- The road to economic recovery: the evolution of COVID-19´s impact on consumption
- BBVA in Asia
- Sustainable Development Goals
- Annual General Meeting
- Trading floor
- Ciudad BBVA
- BBVA's Historical Archive
- BBVA Research
- Open Innovation
- BBVA Foundation
- Full website list
- English Español
- BBVA in brief
- Organization chart
- Business areas
- Strategy: BBVA’s transformation
- Presentations
- Financial data
- Risk management
- Share information
- Capital information
- Shareholders remuneration
- Equity analysts
- Significant events

Shareholders
- Financial products
- Shareholders club
- Shareholders and Investors Communication and Contact Policy
Debt investors
- Issuing companies
- Maturity profile
- Fixed Income Analysts
- Contact debt investors
Sustainability and Responsible Banking
- Sustainability strategy
- Sustainable Financing
- Principles and policies
- Presentation and Reports
- Sustainability ratings
- Responsible taxation
- Average payment period suppliers
- Contacts Responsible Banking
Corporate Governance and Remuneration Policy
- Corporate Bylaws
- General Meeting Regulations
- Information related AGM held
- Board Regulations
- Board of Directors
- Board Committees
- Director's Remuneration
- Information related to Identified Staff
- Annual Corporate Governance Report
- Information Circular 2/2016 of Bank of Spain
- BBVA Policy on Conduct in the Securities Markets
- Information related to integration transactions
- Shareholders and investors
- BBVA in the World
The importance and benefits of getting vaccinated against COVID-19
The pandemic has been one of the greatest health crises in recorded history. Thanks to rapid advances in science and technology, the light at the end of the tunnel is getting closer. Major vaccination efforts are currently underway to immunize the world's population. According to data from health authorities compiled by Our World in Data , 39.7% of the world's population has received at least one dose, while, as of today, almost 2.1 billion people across the globe are fully vaccinated.
There are many theories surrounding Covid-19 vaccination. However, the World Health Organization (WHO) and expert health authorities around the world are urging people to get vaccinated as the best solution to end the pandemic. The sooner people are immunized, the faster it will be possible not only to slow the spread of the disease, but also to limit its impact on the economy.
The benefits of vaccination
According to the WHO, vaccination is a simple, safe and effective way to protect against harmful diseases before coming into contact with them, as it activates the body's natural defenses to learn to resist specific infections and strengthen the immune system.
In this sense, vaccination against COVID-19 will reduce the risk of becoming seriously ill and dying, since the person will be better protected. Immunity will not be 100%, since a vaccinated person can still catch the disease; however, the consequences for the body are expected to be much less.
The main benefits are:
- COVID-19 vaccines can also prevent you from becoming seriously ill even if you contract the virus.
- TAll Covid-19 vaccines are safe and effective.
- By getting vaccinated yourself, you also protect the people around you.
- It is a safer way to develop immunity.
Accordingly, leading organizations such as the Centers for Disease Control and Prevention (CDC) of the United States state on their website that the vaccines are safe . They highlight three main points:
- Vaccines were developed based on scientific knowledge used for decades.
- They are not experimental. They went through all the required stages of clinical trials. Extensive testing and monitoring have shown these vaccines to be safe and effective.
- COVID-19 vaccines have undergone, and will continue to undergo, the most intensive safety monitoring in history..
Vaccination around the world
Worldwide, more than 3.19 billion doses of coronavirus vaccine have been administered so far, according to figures compiled by Our World in Data . According to these figures, almost 27% of the world's population has had both doses (approximately 2.1 billion people).
By country, China leads the global tally with more than two billion vaccines, followed by India, with more than 632 million doses administered. These countries are followed by Western nations such as the United States, which has administered 368 million injections; Brazil, with more than 187 million; and Turkey, with 93 million.
Keep reading about
- Current news
Share on Mastodon
Disclaimer: This translation was last updated on August 2, 2022. For up-to-date content, please visit the English version of this page.
Disclaimer: The Spanish COVID-19 site is currently undergoing significant updates which may lead to a delay in translated content. We apologize for any inconvenience.
Benefits of Getting A COVID-19 Vaccine
There are many benefits of getting vaccinated against COVID-19.
- Prevents serious illness: COVID-19 vaccines available in the United States are safe and effective at protecting people from getting seriously ill, being hospitalized, and dying.
- A safer way to build protection: Getting a COVID-19 vaccine is a safer, more reliable way to build protection than getting sick with COVID-19.
- Offers added protection: COVID-19 vaccines can offer added protection to people who had COVID-19, including protection against being hospitalized from a new infection.
How to be best protected: As with vaccines for other diseases, people are best protected when they stay up to date .
COVID-19 Vaccines Protect Your Health
COVID 19-vaccines are effective at protecting people from getting seriously ill, being hospitalized, and dying. Vaccination remains the safest strategy for avoiding hospitalizations, long-term health outcomes, and death.
What You Can Do Now to Prevent Severe Illness, Hospitalization, and Death
Use Vaccines.gov – to find a COVID-19 vaccine near you.
CDC recommends everyone aged 5 years and older get 1 updated COVID-19 vaccine . Children aged 6 months – 4 years may need more than 1 dose of updated COVID-19 to stay up to date . People aged 65 years and older who received 1 dose of any updated 2023-2024 COVID-19 vaccine (Pfizer-BioNTech, Moderna or Novavax) should receive 1 additional dose of an updated COVID-19 vaccine at least 4 months after the previous updated dose. For more Novavax information, click or tap here .
Severe Illness
COVID-19 vaccines are highly effective in preventing the most severe outcomes from a COVID-19 infection.
Myocarditis is a condition where the heart becomes inflamed in response to an infection or some other trigger. Myocarditis after COVID-19 vaccination is rare. This study shows that patients with COVID-19 had nearly 16 times the risk for myocarditis compared with patients who did not have COVID-19 .
Hospitalization
COVID-19 vaccines can help prevent you from becoming hospitalized if you do get infected with COVID-19.
COVID-19 vaccines can help prevent you from dying if you do get infected with COVID-19.
COVID-19 Vaccination is a Safer, More Reliable Way to Build Protection
Getting a COVID-19 vaccine is a safer, more reliable way to build protection than getting sick with COVID-19. COVID-19 vaccination helps protect people by creating an immune response without the potentially severe illness or post-COVID conditions that can be associated with COVID-19 infection.
- Getting sick with COVID-19 can cause severe illness or death, even in children, but it is not possible to determine who will experience mild or severe illness from COVID-19 infection.
- People may have long-term health issues after having COVID-19. Even people who do not have symptoms when they are first infected with COVID-19 can experience long-term health problems, also known as long COVID or post-COVID conditions .
- Complications can appear after mild or severe COVID-19, or after multisystem inflammatory syndrome in children (MIS-C) .
While people can get some protection from having COVID-19, the level and length of that protection varies, especially as COVID-19 variants continue to emerge .
- Immunity (protection) from infection can vary depending on how mild or severe someone’s illness was and their age.
- Immunity from infection decreases over time.
Importantly, there is no antibody test available that can reliably determine if a person is protected from further infection.
After vaccination, continue to follow all current prevention measures recommended by CDC based on latest COVID-19 hospital admission levels. Learn more about protecting your family from COVID-19.
- Facts about COVID-19 Vaccines
- Frequently Asked Questions about COVID-19 Vaccination
- COVID-19 Vaccines for People Who Would Like to Have a Baby
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Advertisement
Supported by
Personal Health
The Importance of Getting Fully Vaccinated
Covid remains a mortal threat not just for people like me in the upper decades of life but for almost anyone, no matter how young and healthy.
- Share full article

By Jane E. Brody
Too many Americans don’t seem to realize just how easily the novel coronavirus spreads and how awful Covid-19 can be. It is prompting far too many either to a) avoid getting any vaccine, b) skip the second dose if their first was Pfizer or Moderna or c) assume that the vaccine they got means they are now free to gather in any way they choose without taking any public health precautions.
Covid remains a mortal threat not just for people like me in the upper decades of life but also for almost anyone, no matter how young and healthy. Like the 37-year-old pregnant woman in Illinois who was put on life support after her baby was delivered by emergency C-section. Or the 26-year-old man in Maryland who was hospitalized on oxygen for five days and now tells everyone “ how bad it was and how scary it is. ” Although infections, hospitalizations and deaths are down from their dreadful peaks in 2020, we are still a long way from herd immunity — if we ever get there .
Sixty-one percent of people live in counties where the risk of infection right now is very high or extremely high, and whenever someone gets infected with the coronavirus, a mutation to an even more dangerous variant could arise.
After months of uncertainty about whether any vaccine that emerged from Operation Warp Speed would be safe and effective, the final highly reassuring results from the vaccine trials late last year were almost beyond belief. The members of the vaccine advisory committee who endorsed the Food and Drug Administration’s emergency use authorization of the vaccines are nongovernment experts with integrity and independent judgment. Had the government delayed the vaccine release until fully licensed, both the population and the economy likely would have been irreparably devastated.
I waited with bated breath for my turn to get immunized last winter and then for my two sons and daughters-in-law and four grandsons to become eligible this spring. All will be fully vaccinated by the end of the month when we gather for the first time in nearly two years to celebrate my 80th birthday. And all of us will continue to wear masks and maintain appropriate distance from others when we’re outdoors in close settings or indoors in public venues with people we don’t know.
In its advisory issued April 27 , the Centers for Disease Control and Prevention said that fully vaccinated people can visit indoors with others who are fully vaccinated without wearing a mask or physically distancing and can travel domestically without getting tested or self-quarantining. They can also now “gather or conduct activities outdoors without wearing a mask, except in certain crowded settings or venues” like a live performance, parade or sporting event.
But the agency warned unvaccinated people that they are least safe — and should remain masked — when going to an indoor movie, eating in an indoor restaurant or bar, participating in a high-intensity indoor exercise class or singing in an indoor chorus. Dr. Rochelle Walensky, the C.D.C.’s director, said that there’s an almost 20-fold increased risk of transmitting the virus indoors.
Even for vaccinated people, she said, “until more people are vaccinated and while we still have more than 50,000 cases a day, mask use indoors will provide extra protection.”
There are good reasons for continued precautions. More than half the population, including young children, are not yet immunized. It is not known whether immunized people can acquire the virus and remain symptom-free, then unwittingly spread it to others who are vulnerable. Not everyone who wants the vaccine is able to get it for logistical or health reasons, and the vaccines may not fully protect people with immune deficiencies.
Furthermore, even though the authorized vaccines result in a stronger immune response than natural infection, we don’t yet know how long their protection will last. The Excelsior Pass I got in New York State attests to my vaccination status, but it expires mid-August, six months after my second dose, at which time a booster shot may be needed to maintain my immunity.
Speaking of which, that second shot of the Pfizer or Moderna vaccine should not be skipped . Although a delay of a few weeks in getting it is likely not critical, the immune response after one dose is relatively weak and may leave people vulnerable, especially to the more virulent variants now circulating.
Two doses are 90 percent effective in preventing infection, and that protection is expected to last much longer. You should be given an appointment for the second dose when you sign up for the first dose or when you receive it.
Some people hesitate to get the second shot because they’ve heard the side effects can be nasty. But no matter how nasty, the vaccine side effects are short-lived and not nearly as severe or persistent as the disease the vaccine protects against. After recovery from even a mild case of Covid-19, a distressing legacy like a foggy brain or chronic fatigue can persist.
And, of course, the virus can also kill, even people who are relatively young and free of underlying health risks. The fatality rate from Covid-19 based on more than 32 million confirmed cases in the United States is 1.8 percent. Over 245 million doses of Covid vaccines were administered by May 3, and a federal review of adverse events found that no deaths resulted from the vaccine.
Nearly everyone gets a temporary sore arm from the shot, but at worst people may have flulike symptoms that last a day or two. If you have the option, consider planning a day off after the second shot in case you need to take it easy. Half my family had no reaction other than the expected arm pain. One daughter-in-law developed a fever of 102 degrees and one son was unusually tired, but I was like the Energizer Bunny the next day and accomplished twice as much as usual. Go figure!
If you have a smartphone, I urge you to sign up for the side effects monitoring system established by the C.D.C. I did and was asked repeatedly how I was faring after each vaccine dose. The system, called v-safe, can alert government health authorities to the frequency of side effects and to any previously unknown complications. It will also remind you to get your second dose of the Pfizer or Moderna vaccine.
And a final word: If you know people still struggling to get a vaccine appointment, please try to help them if you can.
Jane Brody is the Personal Health columnist, a position she has held since 1976. She has written more than a dozen books including the best sellers “Jane Brody’s Nutrition Book” and “Jane Brody’s Good Food Book.” More about Jane E. Brody

- High contrast
- Press Centre
Search UNICEF
What you need to know about covid-19 vaccines, answers to the most common questions about coronavirus vaccines..

- Available in:
Vaccines save millions of lives each year. The development of safe and effective COVID-19 vaccines are a crucial step in helping us get back to doing more of the things we enjoy with the people we love.
We’ve gathered the latest expert information to answer some of the most common questions about COVID-19 vaccines. Keep checking back as we will update this article as more information becomes available.
What are the benefits of getting vaccinated?
Vaccines save millions of lives each year and a COVID-19 vaccine could save yours. The COVID-19 vaccines are safe and effective, providing strong protection against serious illness and death. WHO reports that unvaccinated people have at least 10 times higher risk of death from COVID-19 than someone who has been vaccinated.
It is important to be vaccinated as soon as it’s your turn, even if you already had COVID-19. Getting vaccinated is a safer way for you to develop immunity from COVID-19 than getting infected.
The COVID-19 vaccines are highly effective, but no vaccine provides 100 per cent protection. Some people will still get ill from COVID-19 after vaccination or pass the virus onto someone else.
Therefore, it is important to continue practicing safety precautions to protect yourself and others, including avoiding crowded spaces, physical distancing, hand washing and wearing a mask.
Who should be vaccinated first?
Each country must identify priority populations, which WHO recommends are frontline health workers (to protect health systems) and those at highest risk of death due to COVID-19, such as older adults and people with certain medical conditions. Other essential workers, such as teachers and social workers, should then be prioritized, followed by additional groups as more vaccine doses become available.
The risk of severe illness from COVID-19 is very low amongst healthy children and adolescents, so unless they are part of a group at higher risk of severe COVID-19, it is less urgent to vaccinate them than these priority groups.
Children and adolescents who are at higher risk of developing severe illness from COVID-19, such as those with underlying illnesses, should be prioritized for COVID-19 vaccines.
When shouldn’t you be vaccinated against COVID-19?
If you have any questions about whether you should receive a COVID-19 vaccine, speak to your healthcare provider. At present, people with the following health conditions should not receive a COVID-19 vaccine to avoid any possible adverse effects:
- If you have a history of severe allergic reactions to any ingredients of a COVID-19 vaccine.
- If you are currently sick or experiencing symptoms of COVID-19 (although you can get vaccinated once you have recovered and your doctor has approved).
Should I get vaccinated if I already had COVID-19?
Yes, you should get vaccinated even if you’ve previously had COVID-19. While people who recover from COVID-19 may develop natural immunity to the virus, it is still not certain how long that immunity lasts or how well it protects you against COVID-19 reinfection. Vaccines offer more reliable protection, especially against severe illness and death. Vaccination policies after COVID-19 infection vary by country. Check with your health care provider on the recommendation where you live.
Which COVID-19 vaccine is best for me?
All WHO-approved vaccines have been shown to be highly effective at protecting you against severe illness and death from COVID-19. The best vaccine to get is the one most readily available to you.
You can find a list of those approved vaccines on WHO’s site .
Remember, if your vaccination involves two doses, it’s important to receive both to have the maximum protection.
How do COVID-19 vaccines work?
Vaccines work by mimicking an infectious agent – viruses, bacteria or other microorganisms that can cause a disease. This ‘teaches’ our immune system to rapidly and effectively respond against it.
Traditionally, vaccines have done this by introducing a weakened form of an infectious agent that allows our immune system to build a memory of it. This way, our immune system can quickly recognize and fight it before it makes us ill. That’s how some of the COVID-19 vaccines have been designed.
Other COVID-19 vaccines have been developed using new approaches, which are called messenger RNA, or mRNA, vaccines. Instead of introducing antigens (a substance that causes your immune system to produce antibodies), mRNA vaccines give our body the genetic code it needs to allow our immune system to produce the antigen itself. mRNA vaccine technology has been studied for several decades. They contain no live virus and do not interfere with human DNA.
For more information on how vaccines work, please visit WHO .
Are COVID-19 vaccines safe?
Yes, COVID-19 vaccines have been safely used to vaccinate billions of people. The COVID-19 vaccines were developed as rapidly as possible, but they had to go through rigorous testing in clinical trials to prove that they meet internationally agreed benchmarks for safety and effectiveness. Only if they meet these standards can a vaccine receive validation from WHO and national regulatory agencies.
UNICEF only procures and supplies COVID-19 vaccines that meet WHO’s established safety and efficacy criteria and that have received the required regulatory approval.
How were COVID-19 vaccines developed so quickly?
Scientists were able to develop safe effective vaccines in a relatively short amount of time due to a combination of factors that allowed them to scale up research and production without compromising safety:
- Because of the global pandemic, there was a larger sample size to study and tens of thousands of volunteers stepped forward
- Advancements in technology (like mRNA vaccines) that were years in the making
- Governments and other bodies came together to remove the obstacle of funding research and development
- Manufacturing of the vaccines occurred in parallel to the clinical trials to speed up production
Though they were developed quickly, all COVID-19 vaccines approved for use by the WHO are safe and effective.
What are the side effects of COVID-19 vaccines?
Vaccines are designed to give you immunity without the dangers of getting the disease. Not everyone does, but it’s common to experience some mild-to-moderate side effects that go away within a few days on their own.
Some of the mild-to-moderate side effects you may experience after vaccination include:
- Arm soreness at the injection site
- Muscle or joint aches
You can manage any side effects with rest, staying hydrated and taking medication to manage pain and fever, if needed.
If any symptoms continue for more than a few days then contact your healthcare provider for advice. More serious side effects are extremely rare, but if you experience a more severe reaction, then contact your healthcare provider immediately.
>> Read: What you need to know before, during and after receiving a COVID-19 vaccine
How do I find out more about a particular COVID-19 vaccine?
You can find out more about COVID-19 vaccines on WHO’s website .
Can I stop taking precautions after being vaccinated?
Keep taking precautions to protect yourself, family and friends if there is still COVID-19 in your area, even after getting vaccinated. The COVID-19 vaccines are highly effective against serious illness and death, but no vaccine is 100% effective.
The vaccines offer less protection against infection from the Omicron variant, which is now the dominant variant globally, but remain highly effective in preventing hospitalization, severe disease, and death. In addition to vaccination, it remains important to continue practicing safety precautions to protect yourself and others. These precautions include avoiding crowded spaces, physical distancing, hand washing, and wearing a mask (as per local policies).
Can I still get COVID-19 after I have been vaccinated? What are ‘breakthrough cases’?
A number of vaccinated people may get infected with COVID-19, which is called a breakthrough infection. In such cases, people are much more likely to only have milder symptoms. Vaccine protection against serious illness and death remains strong.
With more infectious virus variants such as Omicron, there have been more breakthrough infections. That’s why it's recommended to continue taking precautions such as avoiding crowded spaces, wearing a mask and washing your hands regularly, even if you are vaccinated.
And remember, it’s important to receive all of the recommended doses of vaccines to have the maximum protection.
How long does protection from COVID-19 vaccines last?
According to WHO, the effectiveness of COVID-19 vaccines wanes around 4-6 months after the primary series of vaccination has been completed. Taking a booster to strengthen your protection against serious disease is recommended if it is available to you.
Do the COVID-19 vaccines protect against variants?
The WHO-approved COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
However, the vaccines offer less protection against infection from Omicron, which is the dominant variant globally. That's why it's important to get vaccinated and continue measures to reduce the spread of the virus – which helps to reduce the chances for the virus to mutate – including physical distancing, mask wearing, good ventilation, regular handwashing and seeking care early if you have symptoms.
Do I need to get a booster shot?
Booster doses play an important role in protecting against severe disease, hospitalization and death.
WHO recommends that you take all COVID-19 vaccine doses recommended to you by your health authority as soon as it is your turn, including a booster dose if recommended.
Booster shots should be given first to high priority groups. Data shows that a booster shot plays a significant role in boosting waning immunity and protecting against severe disease from highly transmissible variants like Omicron.
If available, an additional second booster shot is also recommended for some groups of people, 4-6 months after the first booster. That includes older people, those who have weakened immune systems, pregnant women and healthcare workers.
Check with your local health authorities for guidance and the availability of booster shots where you live.
What do we know about the bivalent COVID-19 booster doses that have been developed to target Omicron?
Bivalent COVID-19 booster shots have now been developed with both the original strain of the coronavirus and a strain of Omicron. These have been designed to better match the Omicron subvariants that have proven to be particularly transmissible. Lab studies have shown that these doses help you to mount a higher antibody response against Omicron. Both Moderna and Pfizer have developed these bivalent vaccines, and some countries have now approved their use.
Check with your local health authorities for information about the availability of these doses and who can get them where you live. And it’s important to note that the original COVID-19 vaccines continue to work very well and provide strong protection against severe illness from Omicron.
Can I receive different types of COVID-19 vaccines?
Yes, however, policies on mixing vaccines vary by country. Some countries have used different vaccines for the primary vaccine series and the booster. Check with your local health authorities for guidance where you live and speak with your healthcare provider if you have any questions on what is best for you.
I’m pregnant. Can I get vaccinated against COVID-19?
Yes, you can get vaccinated if you are pregnant. COVID-19 during pregnancy puts you at higher risk of becoming severely ill and of giving birth prematurely.
Many people around the world have been vaccinated against COVID-19 while pregnant or breastfeeding. No safety concerns have been identified for them or their babies. Getting vaccinated while pregnant helps to protect your baby. For more information about receiving a COVID-19 vaccination while pregnant, speak to your healthcare provider.
>> Read: Navigating pregnancy during the COVID-19 pandemic
I’m breastfeeding. Should I get vaccinated against COVID-19?
Yes, if you are breastfeeding you should take the vaccine as soon as it is available to you. It is very safe and there is no risk to the mother or baby. None of the current COVID-19 vaccines have live virus in them, so there is no risk of you transmitting COVID-19 to your baby through your breastmilk from the vaccine. In fact, the antibodies that you have after vaccination may go through the breast milk and help protect your baby. >> Read: Breastfeeding safely during the COVID-19 pandemic
Can COVID-19 vaccines affect fertility?
No, you may have seen false claims on social media, but there is no evidence that any vaccine, including COVID-19 vaccines, can affect fertility in women or men. You should get vaccinated if you are currently trying to become pregnant.
Could a COVID-19 vaccine disrupt my menstrual cycle?
Some people have reported experiencing a disruption to their menstrual cycle after getting vaccinated against COVID-19. Although data is still limited, research is ongoing into the impact of vaccines on menstrual cycles.
Speak to your healthcare provider if you have concerns or questions about your periods.
Should my child or teen get a COVID-19 vaccine?
An increasing number of vaccines have been approved for use in children. They’ve been made available after examining the data on the safety and efficacy of these vaccines, and millions of children have been safely vaccinated around the world. Some COVID-19 vaccines have been approved for children from the age of 6 months old. Check with your local health authorities on what vaccines are authorized and available for children and adolescents where you live.
Children and adolescents tend to have milder disease compared to adults, so unless they are part of a group at higher risk of severe COVID-19, it is less urgent to vaccinate them than older people, those with chronic health conditions and health workers.
Remind your children of the importance of us all taking precautions to protect each other, such as avoiding crowded spaces, physical distancing, hand washing and wearing a mask.
It is critical that children continue to receive the recommended childhood vaccines.
How do I talk to my kids about COVID-19 vaccines?
News about COVID-19 vaccines is flooding our daily lives and it is only natural that curious young minds will have questions – lots of them. Read our explainer article for help explaining what can be a complicated topic in simple and reassuring terms.
It’s important to note that from the millions of children that have so far been vaccinated against COVID-19 globally, we know that side effects are very rare. Just like adults, children and adolescents might experience mild symptoms after receiving a dose, such as a slight fever and body aches. But these symptoms typically last for just a day or two. The authorized vaccines for adolescents and children are very safe.
My friend or family member is against COVID-19 vaccines. How do I talk to them?
The development of safe and effective COVID-19 vaccines is a huge step forward in our global effort to end the pandemic. This is exciting news, but there are still some people who are skeptical or hesitant about COVID-19 vaccines. Chances are you know a person who falls into this category.
We spoke to Dr. Saad Omer, Director at the Yale Institute for Global Health, to get his tips on how to navigate these challenging conversations. >> Read the interview
How can I protect my family until we are all vaccinated?
Safe and effective vaccines are a game changer, but even once vaccinated we need to continue taking precautions for the time being to protect ourselves and others. Variants like Omicron have proven that although COVID-19 vaccines are very effective at preventing severe disease, they’re not enough to stop the spread of the virus alone. The most important thing you can do is reduce your risk of exposure to the virus. To protect yourself and your loved ones, make sure to:
- Wear a mask where physical distancing from others is not possible.
- Keep a physical distance from others in public places.
- Avoid poorly ventilated or crowded spaces.
- Open windows to improve ventilation indoors.
- Try and focus on outdoor activities if possible.
- Wash your hands regularly with soap and water or an alcohol-based hand rub.
If you or a family member has a fever, cough or difficulty breathing, seek medical care early and avoid mixing with other children and adults.
Can COVID-19 vaccines affect your DNA?
No, none of the COVID-19 vaccines affect or interact with your DNA in any way. Messenger RNA, or mRNA, vaccines teach the cells how to make a protein that triggers an immune response inside the body. This response produces antibodies which keep you protected against the virus. mRNA is different from DNA and only stays inside the cell for about 72 hours before degrading. However, it never enters the nucleus of the cell, where DNA is kept.
Do the COVID-19 vaccines contain any animal products in them?
No, none of the WHO-approved COVID-19 vaccines contain animal products.
I’ve seen inaccurate information online about COVID-19 vaccines. What should I do?
Sadly, there is a lot of inaccurate information online about the COVID-19 virus and vaccines. A lot of what we’re experiencing is new to all of us, so there may be some occasions where information is shared, in a non-malicious way, that turns out to be inaccurate.
Misinformation in a health crisis can spread paranoia, fear and stigmatization. It can also result in people being left unprotected or more vulnerable to the virus. Get verified facts and advice from trusted sources like your local health authority, the UN, UNICEF, WHO.
If you see content online that you believe to be false or misleading, you can help stop it spreading by reporting it to the social media platform.
What is COVAX?
COVAX is a global effort committed to the development, production and equitable distribution of vaccines around the world. No country will be safe from COVID-19 until all countries are protected.
There are 190 countries and territories engaged in the COVAX Facility, which account for over 90 per cent of the world’s population. Working with CEPI, GAVI, WHO and other partners, UNICEF is leading efforts to procure and supply COVID-19 vaccines on behalf of COVAX.
Learn more about COVAX .
This article was last updated on 25 October 2022. It will continue to be updated to reflect the latest information.
Related topics
More to explore, covid-19 response.
Resources and information about UNICEF’s response to the COVID-19 pandemic
How to talk to your children about COVID-19 vaccines
Tips for navigating the conversation
How to talk to friends and family about vaccines
Tips for handling tough conversations with your loved ones
COVAX information centre
UNICEF and partners led the largest vaccine procurement and supply operation in history
Essay on Coronavirus Vaccine
500+ words essay on coronavirus vaccine.
The Coronavirus has infected millions of people so far all over the world. In addition to that, millions of people have lost their lives to it. Ever since the outbreak, researchers all over the world have been working constantly to develop vaccines that will work effectively against the virus. We will take a look at the Coronavirus vaccine that is present today. Vaccines have the ability to save people’s lives. Developing the vaccine for Coronavirus was a huge step to end the pandemic.

Working of Coronavirus Vaccine
As Coronavirus caused a lot of confusion and fear amongst people, it is natural people were not aware of how the vaccine works. To begin with, a vaccine will work by mimicking an infectious agent.
The agent can be viruses, bacteria or any other microorganisms. They carry the potential of causing disease. When it mimics that, our immune system learns how to respond against it rapidly and efficiently.
As per the traditional methods, vaccines have managed to do this as they introduce a weakened form of an infectious agent. It enables our immune system to basically build its memory.
As a result, our immune system can then identify it quickly and fight against it before it gets the chance to harm us or make us ill. Similarly, some of the coronavirus vaccines have been made like that.
On the other hand, there are other coronavirus vaccines that researchers have developed by making use of new approaches. We refer to them as messenger RNA or mRNA vaccines.
Over here, they do not introduce antigens in our bodies. Instead, mRNA vaccines give the genetic code our body needs to enable our immune system for producing the antigen itself.
For several years, researchers have been studying mRNA vaccine technology. Thus, they do not contain any live virus and also do not interfere with the human DNA .
Get the huge list of more than 500 Essay Topics and Ideas
Safety of Coronavirus Vaccine
While the vaccines are being developed at a fast pace, they also require rigorous testing. The tests are done in clinical trials to ensure that they meet the benchmarks for the safety and efficiency of international standards.
When they meet the standards, then only can they get the go-ahead from WHO and national regulatory agencies. UNICEF has said that it will attain and supply only those vaccines that meet the WHO guidelines and have met the regulatory approval.
As of now, the vaccine doses are limited in number. Thus, the healthcare workers, first responders, people over the age of 75 and residents of long-term care facilities will receive the first doses.
After that, everyone will be able to get it once more of them are available. To get the vaccine, a person may require to pay a fee. However, some government institutions are providing it free of cost.
In order to get the vaccine, one must check with their local and state health departments on a regular basis. When they get the chance, they must get the dose right away.
The Coronavirus outbreak has challenged the whole world. Constantly, the experts and authorities are working to develop the vaccines. Therefore, we can also do our bit and adopt preventive measures to limit the spread of this disease. The major goal is to get the vaccine to everyone so that we can go on and about with our normal lives.
FAQ on Essay on Coronavirus Vaccine
Question 1: What are some common side effects of the Coronavirus vaccine?
Answer 1: The most common side effect includes a sore arm, fever , headache, and fatigue. However, not to worry, side effects are good in this case. They indicate that your vaccine is starting to work as it triggers your immune system.
Question 2: When do Coronavirus vaccine side effects kick in?
Answer 2: Usually, most of the side effects start to kick in within the first 3 days after you get your vaccine. Moreover, they will last up to 1 to 2 days only.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

COVID-19 Vaccine: A comprehensive status report
The current COVID-19 pandemic has urged the scientific community internationally to find answers in terms of therapeutics and vaccines to control SARS-CoV-2. Published investigations mostly on SARS-CoV and to some extent on MERS has taught lessons on vaccination strategies to this novel coronavirus. This is attributed to the fact that SARS-CoV-2 uses the same receptor as SARS-CoV on the host cell i.e. human Angiotensin Converting Enzyme 2 (hACE2) and is approximately 79% similar genetically to SARS-CoV. Though the efforts on COVID-19 vaccines started very early, initially in China, as soon as the outbreak of novel coronavirus erupted and then world-over as the disease was declared a pandemic by WHO. But we will not be having an effective COVID-19 vaccine before September, 2020 as per very optimistic estimates. This is because a successful COVID-19 vaccine will require a cautious validation of efficacy and adverse reactivity as the target vaccinee population include high-risk individuals over the age of 60, particularly those with chronic co-morbid conditions, frontline healthcare workers and those involved in essentials industries. Various platforms for vaccine development are available namely: virus vectored vaccines, protein subunit vaccines, genetic vaccines, and monoclonal antibodies for passive immunization which are under evaluations for SARS-CoV-2, with each having discrete benefits and hindrances. The COVID-19 pandemic which probably is the most devastating one in the last 100 years after Spanish flu mandates the speedy evaluation of the multiple approaches for competence to elicit protective immunity and safety to curtail unwanted immune-potentiation which plays an important role in the pathogenesis of this virus. This review is aimed at providing an overview of the efforts dedicated to an effective vaccine for this novel coronavirus which has crippled the world in terms of economy, human health and life.
1. Introduction
The novel beta-coronavirus SARS-CoV-2 is believed to have emerged last year in 2019 in Wuhan from Bats. Crossing the species barrier it entered human beings with furtherance of infection through human to human transmission. The beta-coronaviruses have jumped between the species and have caused three zoonotic outbreaks namely, SARS CoV (2002-03), MERS-CoV (2012), and SARS-CoV-2 (2019- till date) in the last 2 decades. The existence of a myriad of coronaviruses in bats, including many SARS-related CoV (Severe Acute Respiratory Syndrome related Coronaviruses) and the sporadic crossing over of the species barriers of the coronaviruses to humans, suggest that the future occurrences of zoonotic transmission events may sustain ( Ou et al., 2020 ).
Since its emergence in Nov 2019, it has spread to 188 countries and 25 territories around the globe, despite elaborate efforts by WHO and Governments to contain the infection, primarily owing to the highly infectious nature of this virus ( Anon, 2020a ; Anon, 2020b ). As of 2 July 2020, 10,533,779 cases have been reported globally with 512,842 deaths ( (WHO) World Health Organisation, 2020 ). There has been a monumental increase in the number of infected patients, with a 7-day moving average of 210,209 cases per day, as of 2 July 2020 ( Anon, 2020a ). SARS-CoV-2, a highly contagious virus, tends to spread by the inhalation of the respiratory aerosols, direct human contact, and via fomites. Social distancing, personal hygiene, frequent hand washing or sanitizing using the alcohol (61-70%) based hand-sanitizers, and disinfection of the surfaces are some steps which can protect the individuals from getting infected ( (CDC), Centers for Disease Control and Prevention, 2020 ). R 0 is an epidemiological scale; used to measure the contagiousness of an infectious agent. Its magnitude depends upon various biological, environmental, and socio-behavioral factors. It can be defined as “the average number of secondary cases one would produce in a completely susceptible population in the absence of any deliberate intervention in disease transmission ( Delamater et al., 2019 ).” SARS-CoV-2 has an R 0 value range of 2-3 ( Park, 2020 ) which is significantly higher in comparison to Spanish flu for which the R 0 was recorded at 0.9-2.1 ( Pyrek, 2018 ). According to WHO, people living with non-communicable diseases (co-morbid conditions) are prone to severe illness due to COVID-19 infection. The incubation period of the virus ranges from 2-14 days with a median of 5.1 days ( Lauer et al., 2020 ). The symptoms include fever, dry cough, fatigue, shortness of breath, chills, muscles pain, headache, gastric disturbances and weight loss ( CDC, 2020 ). Some patients may have lymphopenia and bilateral ground-glass opacity changes in the chest CT scans. The histological examinations of the lungs’ biopsy samples have shown a bilaterally diffused alveolar damage with cellular fibromyxoid exudates. A few interstitial mononuclear inflammatory infiltrates were observed both in the liver and the heart specimens ( Xu et al., 2020 ). However, a large population of the infected patients have no or mild symptoms and remain asymptomatic ( Shang et al., 2020 ).
Structurally coronaviruses are pleomorphic, enveloped viruses with a characteristic fringe of projections composed of S protein on their surface. These viruses are equipped with a positive sense ssRNA genome, which is complexed with the nucleocapsid (N) protein forming helical nucleocapsids. The genome is both capped and polyadenylated ( Carter and Saunders, 2007 ). The genetic analysis of SARS-CoV-2 and SARS-CoV has revealed 79% similarity with a total of 380 amino acid substitutions condensed mainly within the NSP genes. Out of these substitutions, there are 27 amino acid replacements in the immune-dominant S protein while 102 and 61 amino acid substitutions are found in the NSP3 and NSP2. Whereas, NSP7, NSP13, E protein, and some accessory proteins are devoid of any amino acid substitutions ( Wu et al., 2020 ). SARS-CoV and SARS-CoV-2 bind a common host receptor, hACE2, to gain entry into the cell but SARS-CoV-2 binds the receptor with a higher affinity than the SARS-CoV. MERS-CoV uses an entirely different receptor that is, Dipeptidyl Peptidase 4 (DPP4) ( Wan et al., 2020 ) and the virus is distantly related to SARS-CoV-2 with around 50% similarity as per the sequence analysis of the two viruses ( Prof Roujian et al., 2020 ).
The genome of SARS-CoV-2 is transcribed in at least 10 Open Reading Frames (ORFs). ORF1ab translates into a polyprotein which is processed into 16 non-structural proteins (NSPs) ( Yoshimoto, 2020 ). The NSPs perform various functions like genome replication, inducing the cleavage of host mRNA, membrane rearrangement, generation of the autophagosome, cleavage of the NSP polyprotein, capping, tailing, methylation, unwinding of the RNA duplex, etc. which are essential for the viral life cycle ( da Silva et al., 2020 ). Besides, the SARS-CoV-2 virus contains four structural proteins namely, spike (S), nucleocapsid (N), envelope (E), and membrane (M) proteins which are encoded by the 3’-end of the viral genome ( Wrapp et al., 2020 ). Amongst the 4 structural proteins the S glycoprotein, being a large multi-functional trans-membrane protein, plays the vital role of viral attachment, fusion, and entry into the host cell ( Wrapp et al., 2020 ). The S protein consists of S1 and S2 subunits, which are further split into different functional domains. The S1 subunit has two functional domains viz. N-terminal Domain (NTD) and Receptor Binding Domain (RBD) and the latter contains conserved receptor binding motif (RBM) ( Jiang et al., 2020 ). The alignment studies have revealed that the region of RBD sequence lies between the residues 331 and 524 of the S protein ( Tai et al., 2020 ). Whereas, the S2 subunit has three operational domains namely, fusion peptide (FP), heptad repeat (HR) 1, and 2. The S1 protein trimer aligns itself at the top of the trimeric S2 stalk to form the immune-dominant S protein ( Jiang et al., 2020 ). Interestingly, a furin cleavage site is observed within the spike protein of SARS-CoV-2 while it is absent in the SARS-CoV which may be a possible explanation of the variation in the pathogenicity of the virus ( Walls et al., 2020 ). A host trans-membrane protease serine 2, (TMPRSS2) is responsible for the initial priming of the spike protein. The virus can utilize both TMPRSS2 and endosomal cysteine proteases cathepsin B and L (CatB/L) to initiate entry into the cell. The TMPRSS2 is responsible for the cleavage of the S protein to expose the FP region of the S2 subunit which is responsible for the initiation of the endosome mediated entry into the host cell. This indicates that TMPRSS2 is a host factor that is essential for viral entry; therefore, the drugs approved for the inhibition of this protease (like camostatmesylate) could be used for therapeutic purposes ( Hoffmann and Kleine-Weber, 2020 ). SARS-CoV-2 uses the human angiotensin-converting enzyme 2 (hACE2) receptor to seize the target cell through the spike glycoprotein (S-Protein), . It has been suggested that the coronaviruses exercise the use of conformational masking and glycan shielding of the spike protein to circumvent the host immune cells. The Cryo-EM structures have revealed the presence of two distinct: closed and open conformations of the S-Protein ectodomain trimer, as a consequence of the opening of the structure at the trimer apex. This conformational diversification is necessary for the receptor binding as the trimer opening exposes the RBM which is present at the interface between the protomers in the closed trimers ( Walls, 2020 ).
The E protein that forms E channels (called the viroporins), and is involved in a myriad of functions in the viral replication cycle involving assembly, release, pathogenesis, etc. ( Gralinski and Menachery, 2020 ). These reprobate ion channels exist in the form of homo-pentamers with each subunit containing 50-120 amino acids. E channels contain at least one trans-membrane domain (TMD) which facilitates the linkage in host cell membranes. SARS CoVs generally contain three categories of ion channels namely: E, 8a, and 3a. The E and 8a ion channels contain the PDZ (Post Synaptic Density Protein; Disc Large Tumor Suppressor; Zonula Occludens-1 Protein) Domain Binding Motif (PBM) which is responsible for the over-expression of the inflammatory cytokines which may result in the cytokine storm ( Pharmaceutical Targeting the Envelope Protein of SARS-CoV-2: the Screening for Inhibitors in Approved Drugs, 2020 ). From the sequence alignment study of the E protein, it was observed that a negatively charged glutamate residue (E69) in SARS-CoV corresponds to a positively charged arginine residue (R69) in SARS-CoV-2 ( Yoshimoto, 2020 ). However, this mutation is remote from the inhibitor binding site; therefore, E protein can be used as a pharmaceutical target ( Pharmaceutical Targeting the Envelope Protein of SARS-CoV-2: the Screening for Inhibitors in Approved Drugs, 2020 ).
M protein, the central organizer of CoV assembly, is most abundantly expressed in the virus particle. It functions crucially in the morphogenesis and assembly of the SARS-CoV-2 by interacting with the essential structural proteins ( Conserved Protein Domain Family: SARS-like-CoV_M, 2020 ). The binding of the M and N protein stabilizes the N protein and RNA complex, and the internal core of the virus. In case of SARS-CoV, the M protein has also been shown to induce the process of apoptosis in the host cell ( Yoshimoto, 2020 ).
In addition to stabilizing the ssRNA genome of the virus particle, the N protein is an antagonist of the antiviral RNAi. It is responsible for the inhibition of the cell cycle of the host cell as it can inhibit the entry of the cell into the S-phase ( Yoshimoto, 2020 ).
Immunotherapy is considered as an effective method for the prophylaxis and treatment of various infectious diseases and cancers, which involves the artificial triggering of the immune system to elicit the immune response ( Masihi, 2001 ). A vaccine that elicits the production of S protein neutralizing antibodies in the vaccinated subjects is the primary aim of all the programs for COVID-19 vaccines. Studies have revealed that there is a limited to no cross-neutralization between the sera of SARS-CoV and SARS-CoV-2, indicating that recovery from one infection may not shield against the other ( Ou et al., 2020 ). Furthermore, a database of approximately 5500 full-length genomes of SARS-CoV-2 isolated from various countries is now available at NCBI which facilitates delineating the polymorphisms in S protein and other important proteins of the virus concerning vaccine development. The rationale for writing this review is to gather all the information about the COVID-19 vaccine development programs and give the readers and researchers insight into types of vaccines being worked upon and the current status of the clinical trials of these vaccines for ready reference.
2. Vaccination strategies
Many efforts have been directed towards the development of the vaccines against COVID-19, to avert the pandemic and most of the developing vaccine candidates have been using the S-protein of SARS-CoV-2 ( Dhama et al., 2020 ). As of July 2, 2020, the worldwide SARS-CoV-2 vaccine landscape includes 158 vaccine candidates, out of which 135 are in the preclinical or the exploratory stage of their development. Currently, mRNA-1273 (Moderna), Ad5-nCoV (CanSino Biologicals), INO-4800 (Inovio, Inc.), LV-SMENP-DC, Pathogen-specific aAPC (ShinzenGeno-Immune Medical Institute), and ChAdOx1 (University of Oxford) have entered the phase I/II clinical trials ( WHO, 2020 ). The vaccines which are in the conduit are based upon inactivated or live attenuated viruses, protein sub-unit, virus-like particles (VLP), viral vector (replicating and non- replicating), DNA, RNA, nanoparticles, etc. with each exhibiting unique advantages and hindarances ( Table 1 ) ( Ning et al., 2020 ). COVID-19 vaccine landscape with percentage share of different types of vaccine is represented in Fig. 1 . To enhance the immunogenicity, various adjuvant technologies like AS03 (GSK), MF-59 (Novartis), CpG 1018 (Dynavax), etc. are now accessible to the researchers for the vaccine development ( Le et al., 2020 ). The immuno-informatics approach is also used for the epitope identification for the SARS-CoV-2 vaccine candidates. It can be used to identify the significant cytotoxic T cell and B-cell epitopes in the viral proteins ( Gupta et al., 2006 ; Baruah and Bose, 2020 ).
Outline of the vaccine production platforms for SARS-CoV-2 and their advantages and limitations

Pie Chart showing the different categories of SARS-CoV-2 vaccines under research ( Anon, 2020c ).
2.1. Protein Sub-unit vaccine
A subunit vaccine is the one which is based on the synthetic peptides or recombinant antigenic proteins, which are necessary for invigorating long-lasting protective and/or therapeutic immune response ( Ning et al., 2020 ). The subunit vaccine, however, exhibits low immunogenicity and requires auxiliary support of an adjuvant to potentiate the vaccine-induced immune responses. An adjuvant may enhance the biological half-life of the antigenic material, or it may ameliorate the immunomodulatory cytokine response. The addition of an adjuvant, therefore, helps in overcoming the shortcomings of the protein subunit vaccines ( Cao et al., 2018 ). The S protein of the SARS-CoV-2 is the most suitable antigen to induce the neutralizing antibodies against the pathogen. The S Protein consists of two subunits. The S1 subunit has the NTD, RBD, and RBM domains while the S2 subunit comprises of FP, HR 1, &2 ( Ou et al., 2020 ). The virus enters into the cell via endocytosis by utilizing the S-Protein mediated binding to the hACE2 receptor. Therefore, the S-Protein and its antigenic fragments are the prime targets for the institution of the subunit vaccine ( Ning et al., 2020 ). The S glycoprotein is a dynamic protein, possessing two conformational states i.e. pre-fusion and post-fusion state. Therefore, the antigen must maintain its surface chemistry and profile of the original pre-fusion spike protein to preserve the epitopes for igniting good quality antibody responses ( Graham, 2020 ). Moreover, means to target the masked RBM as an antigen will enhance the neutralizing antibody response and improve the overall efficacy of the vaccine.
2.1.1. NVX-CoV2373 (Novavax, Inc.| Emergent BioSolutions)
NVX-CoV2373 is a nano-particle based immunogenic vaccine which is based upon the recombinant expression of the stable pre-fusion, coronavirus S-Protein ( Coleman et al., 2020 ). The protein was stably expressed in the Baculovirus system ( Tu et al., 2020 ). The company plans to use the Matrix-M adjuvant to enhance the immune response against SARS-CoV-2 spike protein by the induction of high levels of neutralizing antibodies. In the animal models, a single immunization resulted in the high level of anti-spike protein antibodies which blocked the hACE2 receptor binding domain and could elicit SARS-CoV-2 wild type virus-neutralizing antibodies ( Novavax covid 19 vaccine trial, 2020 ).
2.1.2. Molecular Clamp Stabilized spike protein vaccine candidate
It is being developed by the University of Queensland in collaboration with GSK and Dynavax. The University will have access to vaccine adjuvant platform technology (AS03 Adjuvant system), which is believed to strengthen the vaccine response and minimize the amount of vaccine required per dose ( Lee, 2020 ). The University is developing a stabilized pre-fusion, recombinant viral protein sub-unit vaccine which is based upon the Molecular Clamp technology. This technology has been proved to induce the production of the neutralizing antibodies ( Tu et al., 2020 )
2.1.3. PittCoVacc (University of Pittsburgh)
It is a Micro-Needle Array (MNA) based recombinant SARS-CoV-2 vaccine which involves the administration of rSARS-CoV-2 S1 and rSARS-CoV-2-S1fRS09 (recombinant immunogens). A substantial increase in the antigen specific antibodies with a statistical significance was observed in the pre-clinical trials at the end of two weeks in the mice models. Furthermore, the immunogenicity of the vaccine was maintained even after the sterilization using gamma radiation. The statistically significant titers of antibodies at the early stages and also before boosting, support the feasibility of the MNA-SARS-CoV-2 vaccine ( Kim et al., 2020 ).
2.1.4. Triple Antigen Vaccine (Premas Biotech, India)
It is a multi-antigenic VLP vaccine prototype wherein the recombinant spike, membrane, and envelope protein of SARS-CoV-2 have been co-expressed in an engineered Saccharomyces cerevisiae expression platform (D-Crypt™). The proteins then undergo self-assembly as the VLP. The TEM and allied analytical data simultaneously furnished the biophysical characterization of the VLP. This prototype has the potential to enter the pre-clinical trials as a vaccine candidate after further research and development. Furthermore, it is thought to be safe and easy to manufacture on a mass scale, in a cost-effective manner ( Arora and Rastogi, 2020 ).
2.2. Viral Vectored vaccines
A vaccine based on viral vectors is a promising prophylactic solution against a pathogen. These vaccines are highly specific in delivering the genes to the target cells, highly efficient in the gene transduction, and efficiently induce the immune response, ( Ura et al., 2014 ). They offer a long term and high level of antigenic protein expression and therefore, have a great potential for prophylactic use as these vaccines trigger and prime the cytotoxic T cells (CTL) which ultimately leads to the elimination of the virus infected cells ( Le et al., 2020 ).
2.2.1. Ad5-nCoV (CanSino Biologics Inc | Beijing Institute of Biotechnology)
It is a recombinant, replication defective adenovirus type-5 vector (Ad5) expressing the recombinant spike protein of SARS-CoV-2. It was prepared by cloning an optimized full-length gene of the S Protein along with the plasminogen activator signal peptide gene in the Ad5 vector devoid of E1 and E3 genes. The vaccine was constructed using the Admax system from the Microbix Biosystem ( Zhu et al., 2020 ). The phase I clinical trials have established a positive antibody response or seroconversion. A four-fold increase in the RBD and S protein-specific neutralizing antibodies was noted within 14 days of immunization and peaked at day 28, post-vaccination. Furthermore, the CD4 + T cells and CD8 + T cells response peaked at day 14 post-vaccination. However, the pre-existing anti-Ad5 immunity partly limited both the antibody and the T cell responses ( Zhu et al., 2020 ). The study will further evaluate antibody response in the recipients who are between the age of 18 and 60, and received one of three study doses, with follow-up taking place at 3- and 6-months post-vaccination ( Anon, 2020d ).
2.2.2. Coroflu (University of Wisconsin-Madison | FluGen | Bharat Biotech)
M2SR, a self-limiting version of the influenza virus, which is modified by insertion of the SARS-CoV-2 gene sequence of the spike protein. Furthermore, the vaccine expresses the hemagglutinin protein of the influenza virus, thereby inducing immune response against both the viruses. The M2SR is self-limiting and does not undergo replication as it lacks the M2 gene. It is able to enter into the cell, thereby inducing the immunity against the virus. It shall be administered intra-nasally, mimicking the natural route of viral infection. This route activates several modes of the immune system and has higher immunogenicity as compared to the intramuscular injections ( Anon, 2020e ).
2.2.3. LV-SMENP-DC (Shenzhen Geno-Immune Medical Institute)
The LV-SMENP-DC vaccine is prepared by engineering the dendritic cells (DC) with the lentiviral vector expressing the conserved domains of the SARS-CoV-2 structural proteins and the protease using the SMENP minigenes. The subcutaneous inoculation of the vaccine presents the antigens on antigen presenting cells (APCs), that ultimately activate the Cytotoxic T cells and generate the immune response ( Le et al., 2020 ).
2.2.4. ChAdOx1 (University of Oxford)
ChAdOx1 recombinant adenovirus vaccine was developed using codon optimized S glycoprotein and synthesized with the tissue plasminogen activator (tPA) leader sequence at 5’ end. The sequence of SARS-CoV-2 coding for amino acids (2 to 1273) and the tPA leader and was propagated in the shuttle plasmid. This shuttle plasmid is responsible for encoding the major immediate early genes of the human cytomegalovirus (IE CMV) along with tetracycline operator (TetO) sites and polyadenylation signal from bovine growth hormone (BGH) between the Gateway® recombination cloning site. The Adenovirus vector genome is constructed in the Bacterial Artificial Chromosome by inserting the SARS-CoV-2 S gene into the E1 locus of ChAdOx1 adenovirus genome. The virus was then allowed to reproduce in the T-Rex 293 HEK (Human Embryonic Kidney 293) cell lines and purified by the CsCl gradient ultracentrifugation. The absence of any sub-genomic RNA (sgRNA) in the intra-muscularly vaccinated animals from the pre-clinical trials is indicative of the escalated immunity against the virus ( Doremalen et al., 2020 ). The previous studies have suggested that a single shot should marshal the immune response ( Ou et al., 2020 ). The vaccine has entered phase II clinical trials, where it shall be evaluated in a large sample of the population ( Anon, 2020f ).
2.3. mRNA Vaccine
mRNA is an emerging, non-infectious, and a non-integrating platform with almost no potential risk of insertional mutagenesis. Currently, the non-replicating RNA and the virus derived self-replicating RNAs are being studied. The immunogenicity of the mRNA can be minimized, and alterations can be made to increase the stability of these vaccines. Furthermore, the anti-vector immunity is also avoided as the mRNA is the minimally immunogenic genetic vector, allowing repeated administration of the vaccine ( Cuiling et al., 2020 ). This platform has empowered the rapid vaccine development program due to its flexibility and ability to mimic the antigen structure and expression as seen in the course of a natural infection ( Mulligan and Lyke, 2020 ).
2.3.1. mRNA-1273 (Moderna TX, Inc)
It is a vaccine composed of synthetic mRNA encapsulated in Lipid nanoparticle (LNP) which codes for the full-length, pre-fusion stabilized spike protein (S) of SARS-CoV-2. It has the potential to elicit a highly S-protein specific antiviral response. Furthermore, it is considered to be relatively safe as it is neither made up of the inactivated pathogen nor the sub-units of the live pathogen ( Tu et al., 2020 ). The vaccine has got a fast-track approval from FDA, to conduct the Phase II trials ( Anon, 2020g ).The company has released the interim phase I antibody data of eight participants who received various dose levels. The participants of the 25 μg dose group gave results comparable to the convalescent sera. Whereas, in participants who received the 100 μg dose, the levels of nAb essentially surpassed the levels found in convalescent sera. The vaccine was found to be predominantly safe and well tolerated in the 25 μg and 100 μg dose cohorts, while three participants experienced grade 3 systemic symptoms after the administration of the second dose of 250 μg dose levels ( Anon, 2020h ).
2.3.2. BNT162b1 (BioNTech| FosunPharma| Pfizer)
BNT162b1 is a codon-optimized mRNA vaccine that encodes for the trimerized SARS-CoV-2 RBD, a critical target of the virus nAb. The vaccine portrays an increased immunogenicity due to the addition of T4 fibritin-derived foldon trimerization domain to the RBD antigen. The mRNA is encapsulated in 80 nm ionizable cationic lipid nanoparticles, which ensures its efficient delivery. The Phase 1/2 clinical trials have revealed elevated RBD-specific IgG antibodies levels with a geometric mean concentration to be as high as 8 to 46.3 times titer of convalescent serum. Whereas, the geometric mean titers of the SARS-CoV-2 neutralizing antibodies were found to be 1.8 to 2.8 times the convalescent serum panel. Moderate and transient local reactions and systemic events were observed with no adverse effect. However, the data analysis did not evaluate the safety and immune responses beyond 2 weeks following the administration of the second dose ( Mulligan and Lyke, 2020 ).
2.4. DNA Vaccines
The most revolutionary approach to vaccination is the introduction of the DNA vaccine which encodes for the antigen and an adjuvant which induces the adaptive immune response. The transfected cells express the transgene which provides a steady supply of the transgene specific proteins which is quite similar to the live virus. Furthermore, the antigenic material is endocytosed by the immature Dendritic Cells which ultimately present the antigen to the CD4+ and CD8+ T cells in association with MHC 2 and MHC 1 antigens on the cell surface hence stimulating effective humoral as well as cell-mediated immune responses ( Hobernik and Bros, 2018 ).
2.4.1. INO-4800 (Inovio Pharmaceuticals)
It is a prophylactic DNA vaccine against SARS-CoV-2 ( Anon, 2020i ). It uses codon optimized S protein sequence of SARS-CoV-2 to which an IgE leader sequence is affixed. The SARS-CoV-2 IgE-spike sequence was synthesized and digested using BamHI and XhoI . The digested DNA was incorporated into the expression plasmid pGX0001 under the governance of IE CMV, and BGH polyadenylation signal. The presence of functional antibodies and T cell response in the preclinical trials suggest that the vaccine can produce an effective immune response within 7 days post-vaccination ( Smith et al., 2020 ). The vaccine has entered the Phase I clinical trials (Phase I: {"type":"clinical-trial","attrs":{"text":"NCT04336410","term_id":"NCT04336410"}} NCT04336410 ) and it is estimated to complete this phase of clinical trials by July, wherein the participants received 1.0 mg of INO-4800 by electroporation using CELLECTRA® 2000 device per dosing visit. The trial will evaluate the immunological profile, safety, and tolerability of the vaccine candidate upon intradermal injection and the electroporation in healthy human adults ( Anon, 2020i ).
2.5. Live Attenuated Vaccines
2.5.1. delns1-sars-cov2-rbd (university of hong kong).
This LAV is influenza-based vaccine strain with a deletion in the NS1 gene. It is re-organized to express the RBD domain of SARS-CoV-2 spike protein on its surface and, is cultivated in the chick embryo and/or Madin Darby Canine Kidney Cells (MDCK) cells. It is potentially more immunogenic than the wild type influenza virus and can be administered as a nasal spray ( Anon, 2020j ).
2.6. Others
The revelation of the structure and genome of the SARS-CoV-2 has led to the rapid development of various vaccine candidates with potential immunogenicity but also adverse reactogenicities. The task of vaccine development is long and cumbersome which requires evaluation in some long-lasting clinical trials. Various Biotech ventures are using different technologies for the development of their vaccine candidates; British and American Tobacco Company (BAT) recently unfolded the COVID-19 vaccine using their new, and fast-growing tobacco plant technology ( Anon, 2020k ), while Tianjin University has developed an oral vaccine which has successfully employed Saccharomyces cerevisiae to carry the S protein. The GRAS (Generally Regarded As Safe) status of the yeast provides high scalability, robustness, and cost-effective production of cosmic dosages required to fight off this pandemic ( Zhai et al., 2020 ). Furthermore, in silico studies, using various databases like VaxiJen, have revealed that the epitope sequences WTAGAAAYY and YDPLQPEL can be employed for the formulation of epitope-based peptide vaccines ( Garg et al., 2020 ).
2.6.1. Self Assembling Vaccine (HaloVax)
The vaccine uses a heat shock protein (hsp) to activate the immune system. It is composed of a fusion protein sandwiched between an hsp and Avidin. Biotinylated immunogenic peptides are also incorporated to customize the vaccine ( Voltron Therapeutics, Inc., 2020 ) Table 2 , Table 3 .
Rapidly progressing Anti COVID-19 vaccines. This table contains the information of rapidly developing vaccine candidates only, the list of all vaccine candidates in the pipeline can be accessed from: https://airtable.com/shrSAi6t5WFwqo3GM/tblEzPQS5fnc0FHYR/viweyymxOAtNvo7yH?blocks=bip
Legend: CCHF: Crimean-Congo Hemorrhagic Fever; CHIKV: Chikungunya Virus; DengV: Dengue Virus; FMD: Foot and Mouth Disease; EBOV: Ebola Virus; HAV: Hepatitis A Virus; HBV: Hepatitis B Virus; HIV: Human Immunodeficiency Virus; HPV: Human Papilloma Virus; Inf: Influenza; LASV: Lassa Fever Virus; MenB: Meningitis B; NIPV: Nipah Virus; NORV: Norovirus; RABV: Rabies Virus; RVF: Rift Valley Fever; SARS: Severe Acute Respiratory Syndrome; SIV: Simian Immunodeficiency Virus; TB: Tuberculosis; VEE: Venezuelan Equine; Encephalitis Virus; VZV: Varicella Vaccine (Chickenpox); YFV: Yellow Fever Virus; ZIKV: Zika Virus.
Latest developments in the status of the promising SARS-CoV-2 vaccines
3. Passive Immunization/adoptive immunity
It is the use of preformed antibodies in therapeutics of various diseases. It can be achieved by use of sera from convalescent patients, polyclonal serum raised in other animals such as horse, neutralizing monoclonal antibodies produced by hybridoma technology or humanized antibodies.
3.1. Convalescent Plasma therapy
To date, no distinct treatment has been proven to be efficacious against the COVID-19. Convalescent plasma (CP) therapy has been approved as an empirical treatment during the outbreaks ( (WHO), World Health Organisation, 2014 ). It is considered as the archetypal immunotherapy which has been used for the treatment and prevention of various viral diseases in the past such as SARS, MERS, H1N1 pandemic, measles, mumps, etc. ( Kai et al., 2020 ). A possible explanation for the efficacy of this classic adoptive immunotherapy is that the neutralizing immune-globulins from CP may conquer viremia, block new infection, and accelerate clearance of the infected cells.
Various studies conducted to evaluate therapeutic potential of CP have convincingly shown that administration of the neutralizing antibodies in the critically ill patients led to the amelioration of the clinical status in all patients without any deaths ( Kai et al., 2020 ; Shen et al., 2020a ; Ahn et al., 2020a ; Anon, 2020C ). The dosage prescribed for the CP therapy has not been standardized yet and needs Randomised Clinical Trials not only to eliminate the effect of other medicines but also to evaluate the efficacy and safety of CP therapy. ( Zhang et al., 2020 ). The patients who were considered critically ill with some of them having co-morbid conditions like hypertension, cardiovascular diseases, cerebrovascular diseases, chronic renal failure, etc. were included in the study. They were all admitted to the ICUs and were receiving either mechanical ventilation, high-flow nasal cannula oxygenation, or the low-flow nasal cannula oxygenation. All the patients in these studies were receiving antiviral or antibacterial or antifungal drugs for the treatment of co-infections ( Kai et al., 2020 ). Compared to the control group, the CP treatment group showed no notable differences in the baseline characteristics but exhibited a sizable difference in the clinical outcomes (i.e. normalization of the body temperature, absorption of pulmonary lesions, resolution of ARDS, weaning off the mechanical ventilators, etc.), and the death rates. The patients were tested negative for the viral loads after 7-37 days of CP infusion ( Shen et al., 2020b ). A reduction in the net quantity of inflammatory biomarkers CRP, procalcitonin, and Interleukin 6 (IL-6) in the trial group was observed along with a significant increase in the antibody titers (RBD specific IgM and IgG) post-convalescent plasma therapy ( Ahn et al., 2020b ). However, these uncontrolled and non-randomized trials for the CP therapy impede the researchers to come to a conclusive statement about the prospective potency of this treatment, and these observations require further evaluation which is ongoing in the clinical trials ( Yan, 2020 ).
3.2. Monoclonal Antibody
The monoclonal antibodies (mAb) or therapeutic antibodies, created in the laboratory are the clones of a unique parent which can bind to a single epitope, that is, they have a monovalent affinity ( Gelboin et al., 1999 ). The use of mAb in the prevention and treatment of infectious diseases can overcome various drawbacks which are cognate with the convalescent plasma therapy in terms of specificity, safety, low risk of blood-borne infection, purity, and other factors. A wide array of monoclonal antibodies have already been developed which are implemented in the anti-tumor, anti-platelet, or antiviral therapy ( Breedveld, 2000 ).
A SARS-CoV specific human mAb CR3022 has been found to bind with the RBD of the S protein of SARS-CoV-2, stipulating it as a prospective therapeutic agent, which can either be used alone or in combination therapy for the management of COVID-19 ( Tian et al., 2020 ). To achieve higher efficiency of disease prevention and treatment, a combinatorial effect of monoclonal antibodies recognizing different epitopes of the viral surface can be considered for the neutralization of the virus as it may prove to be more effective and prevent the viral escape ( Tian et al., 2020 ).
There are over 61 patents which claim to have prepared the SARS-specific, MERS-specific, and the diagnostic antibodies. Another group of 38 patents claims to have developed the antibodies that target the host proteins like IL-6/IL-6R, TLR3, CD16, ITAM (immune-receptor tyrosine-based activation motif), DC-SIGN (dendritic cell-specific intercellular adhesion molecule-grabbing non-integrin), ICAM-3 (intercellular adhesion molecule 3), or IP-10/CXCL10 (interferon γ-inducible protein 10). These antibodies can be used to counteract against the cytokine storm that has been reported to harmonize with the SARS-CoV-2 infection ( Liu et al., 2020 ). Tocilizumab, an anti-IL 6 receptor antibody is likely to control the hyper-inflammatory pulmonary symptoms which are coupled with the cytokine storm involving the chemokine dysregulation and various interleukins. Tocilizumab has been reported to block the cytokine axis IL6 hence inhibiting the inflammatory cascade. However, further clinical trials are essential to establish the effectiveness of the mAb ( Michot et al., 2020 ). Israel Institute for Biological Research (IIBR) claims to have successfully developed the mAb against SARS-CoV-2. The institute is in the process of patenting it which may soon be commercialized ( Upadhyay, 2020 ). A group led by Professor Vijay Chaudhary at the University of Delhi, Centre for Innovation in Infectious Disease Research, Education and Training (UDSC-CIIDRET), is isolating the genes encoding the antibodies responsible for the neutralization of the SARS-CoV-2. These genes will be employed to foster the recombinant Ab by exploiting the pre-existing in-house antibody library and a library fabricated from the cells of convalescent COVID-19 patients ( PIB, Delhi, 2020 ).
4. Limitations
The duration of clinical trials poses a sizable amount of hindrance to swift vaccine development. According to the norms laid down by the US Food and Drug Administration (FDA), and WHO, a vaccine candidate has to pass through at least three phases of placebo-controlled clinical trials for the validation of its safety and efficacy, which can take years to complete. Considering the severity of the pandemic, which has forced a complete shut-down of the global economy, speedy vaccine development is necessary. Some authors suggest that the controlled human challenge studies may be conducted to suitably divert the Phase 3 testing, and allow the rapid licensure of the immunogenic vaccines. However, in the expanded field study participants will be monitored constantly to look for any long-term implications posed by the vaccine. Furthermore, the safety trials for the special groups including, children and pregnant women, and immuno-compromised patients can be conducted before the extension of the vaccination to these groups ( Eyal et al., 2020 ).
The testing and development of safe and effective vaccines rely upon laboratory animal models. These animal models must show a similar course of the disease as in human beings. However, the standard inbred strains of mice are not susceptible to the COVID-19 infection, due to the difference between the humans and mice ACE2 receptors ( Anon, 2020D ). This calls for the development of transgenic mice, expressing the hACE2 receptor. Two animal models (hACE2 transgenic mice model and another, primate Macaques model) were previously developed for the SARS-CoV but the current situation requires steady breeding and distribution of these animal models to meet demands of the researchers around the globe ( Mice and Bao, 2020 ). The SARS-CoV-2 virus isolates can efficiently replicate in the lungs of the Syrian hamsters. The lungs of infected hamsters exhibit the pathological lesions analogous to the COVID-19 patients with pneumonia. Moreover, the nAb response exhibited by the infected hamster demonstrated immunity against the succeeding re-challenge studies. Furthermore, the transfusion of convalescent sera into the naïve hamsters mounted the antibody response and hence hindered the viral replication in the lungs. The assemblage of these experiments have illustrated the Syrian hamster may be a perfect model for comprehending SARS-CoV-2 pathogenesis, and evaluating antiviral drugs, and the immunotherapies ( Imai and Iwatsuki-Horimoto, 2020 ). Nevertheless, the assessment of the vaccine dependent immune enhancement cannot be extrapolated from the animal models and requires a legitimate survey from stage III human trials or the human challenge studies.
The Antibody dependent enhancement (ADE) is exploited by various viruses like Dengue, HIV, animal coronaviruses, etc. as an alternative method of infecting a variety of host cells. The virus-antibody complex can bind to the Fc receptors, activate the complement system, or induce a conformational change in the glycoprotein of the viral envelope ( Yip et al., 2016 ). This mechanism is observed when the vaccine-induced antibodies are either non-neutralizing or they are present in inadequate concentrations. This process triggers the viral entry into the cell due to the intensified binding efficiency of the virus-antibody complexes to FcR bearing cells. The clinical and preclinical trials of SARS-CoV vaccine candidates have demonstrated the aggravation of the disease due to ADE. Vaccine Associated Enhanced Respiratory Disease (VAERD) can also be induced by virus-antibody immune complex and T H 2-biased responses ( Graham, 2020 ).
The viral genome is vulnerable to mutations and can undergo the antigenic shift and the antigenic drift, as it continues to spread from one population to the next. The mutations may vary according to the environmental conditions of a geographical area, and the population density. By screening the 7500 samples of the infected patients, the scientists were able to figure out 198 mutations that may have materialized independently which may indicate the evolution of the virus inside the human host. These mutations may lead to different subtypes which may allow the virus to escape the immune system even after the administration of the vaccine ( Dorp et al., 2020 ).
5. Conclusion
SARS-CoV-2 has been the matter of the moment from the date it was declared as a pandemic, it has led to the termination of economic activities universally. Scientists across the continents are joining hands for the innovative tie-ups with both the pharmaceutical giants and the medical start-ups to repurpose drugs, develop vaccines, and devices to impede the progress of this overwhelming pandemic. A large number of COVID-19 vaccine candidates based upon various platforms have already been identified. Despite the undergoing efforts, a definitive answer does not exist. The process of vaccine development is quite laborious with various stages, including the pre-clinical stage, and clinical development which is a three-phase process. However, if sufficient data is already available, it has been recommended to skip a few stages, to accelerate the attainment of a vaccine faster with a quick regulatory review, approval, manufacturing, and quality control. This novel Coronavirus has therefore forced the scientific community to use unconventional approaches to accelerate the process of vaccine development. According to WHO: “vaccine must provide a highly favorable benefit-risk contour; with high efficacy, only mild or transient adverse effects and no serious ailments.” The vaccine must be suitable for all ages, pregnant, and lactating women and should provide a rapid onset of protection with a single dose and confer safety for at least up to one year of administration.
The use of novel technologies for vaccine development requires extensive testing for the safety and efficacy of a vaccine. The scientific community needs to construct various processes and capacities for the largescale manufacturing and administration of the coronavirus vaccines. The Coalition for Epidemic Preparedness Innovation (CEPI), an international non-governmental organization, which is funded by the Wellcome Trust, the European Commission, the Bill and Melinda Gates Foundation, and eight countries, is subsidizing the development of a large number of pandemic vaccine candidates around the globe. Moderna and the Vaccine Research Centre are co-developing an mRNA based vaccine candidate, wherein the mRNA is encapsulated in the lipid nanoparticles while Codagenix in collaboration with the Serum Institute of India is currently focused on developing the live attenuated viral vaccine. The pharmaceutical giants like Novavax, Sichuan Clover Biopharmaceuticals, iBio, and the University of Queensland are in the preclinical stage of the recombinant S glycoprotein vaccines. Additional strategies like the viral vector-based vaccines, targeting the S glycoprotein are being developed by the University of Oxford and CanSino Biologics, and other companies, Inovio and the Applied DNA Sciences are currently developing the DNA based vaccine candidates against the SARS-CoV-2 S Protein. Some of these vaccine candidates are at least months, away from being ready for human use, while others may take longer if at all approved for final use.
In India alone, six biotech ventures i.e. Serum Institute of India, ZydusCadila, Biological E, Indian Immunologicals, Bharat Biotech, and Mynvax are working in collaboration with various international vaccine developers. They are working on DNA vaccines, live attenuated recombinant measles vaccines, inactivated viral vaccines, subunit vaccines, and the vaccines developed by codon-optimization ( Coronavirus, 2020 ). Furthermore, the academic institutes like National Institute of Immunology (NII), Indian Institute of Science (IISc), International Center for Genetic Engineering and Biotechnology (ICGEB) New Delhi, Translational Health Science and Technology Institute (THSTI), etc. are attempting to develop the vaccines, and therapies, and the SARS-CoV-2 animal models to restrain the pandemic shortly ( Nandi, 2020 ).
The need of the hour is to develop a safe and effective COVID-19 vaccine which can induce an appropriate immune response to terminate this pandemic. It is the universal priority to spot the international funding mechanisms to support the development, manufacturing, and stockpiling of the coronavirus vaccines. This pandemic should serve as the guidepost to the international research community to not only acknowledge the outbreak but also indurate the following coronavirus crossing into mammals. A pan-coronavirus vaccine is urgently needed as the delay of vaccine rollout even by one week will accompany millions of deaths. Furthermore, it appears to be a scientifically feasible task if sufficient resources are made available in due time.
Funding Information
This work received no specific grant from any funding agency.
Declaration of Competing Interest
The author(s) declare that there are no conflicts of interest.
- Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., Jeong S.J., Kim J.H., Ku N.S., Yeom J.S., Roh J., Ahn M.Y., Chin B.S., Kim Y.S., Lee H., Yong D., Kim H.O., Kim S., Choi J.Y. Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea. J Korean Med Sci. 2020; 35 (14, April) doi: 10.3346/jkms.2020.35.e149. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahn J.Y., Sohn Y., Lee S.H., et al. Use of convalescent plasma therapy in two covid‐19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020 doi: 10.3346/jkms.2020.35.e149. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anon . 2020. Countries where COVID-19 has spread. www.worldometers.info. [Online] July 30, 2020. [Cited: July 31, 2020.] https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/ . [ Google Scholar ]
- Anon . 2020. Coronavirus Resource Center. https://coronavirus.jhu.edu/. [Online] Johns Hopkins University. [Cited: August 05, 2020.] https://coronavirus.jhu.edu/map.html . [ Google Scholar ]
- Anon . 2020. COVID-19 Treatment and Vaccine Tracker. https://airtable.com/shrSAi6t5WFwqo3GM/tblEzPQS5fnc0FHYR/viweyymxOAtNvo7yH?blocks=bipZFzhJ7wHPv7x9z https://airtable.com/. [Online] Milken Institute. [ Google Scholar ]
- Anon . 2020. A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Coronavirus Pneumonia vaccine (Vero cells) Registration no.: ChiCTR2000031809. http://www.chictr.org.cn/showprojen.aspx?proj=52227 http://www.chictr.org.cn. [Online] [ Google Scholar ]
- Anon . 2020. UW–Madison, FluGen, Bharat Biotech to develop CoroFlu, a coronavirus vaccine. https://www.businesswire.com/news/home/20200402005666/en/UW%E2%80%93Madison-FluGen-Bharat-Biotech-develop-CoroFlu-coronavirus https://www.businesswire.com. [Online] April 02. [ Google Scholar ]
- Anon . 2020. A Study of a Candidate COVID-19 Vaccine (COV001)https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04324606","term_id":"NCT04324606"}} NCT04324606 ?term=vaccine&cond=covid-19&draw=2 https://clinicaltrials.gov. [Online]. [Cited: June 8, 2020.] [ Google Scholar ]
- Anon . 2020. Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19)https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04283461","term_id":"NCT04283461"}} NCT04283461 ?term=vaccine&cond=covid-19&draw=2 https://clinicaltrials.gov/. [Online] [ Google Scholar ]
- Anon . 2020. Moderna Announces Positive Interim Phase 1 Data for its mRNA Vaccine (mRNA-1273) Against Novel Coronavirus. https://investors.modernatx.com/. [Online] Moderna, Inc., May 18, 2020. [Cited: June 15, 2020.] https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-positive-interim-phase-1-data-its-mrna-vaccine. [ Google Scholar ]
- Anon . 2020. Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04336410","term_id":"NCT04336410"}} NCT04336410 ?term=inovio&cond=covid-19&draw=2&rank=1 https://clinicaltrials.gov/. [Online] 2020. 1. [ Google Scholar ]
- Anon . The University of Hong Kong; 2020. HKU joins global partnership to develop COVID-19 vaccine. https://fightcovid19.hku.hk/hku-state-key-laboratory-for-emerging-infectious-diseases-joins-global-effort-to-develop-covid-19-vaccine/ https://fightcovid19.hku.hk/. [Online], March 18, 2020. [ Google Scholar ]
- Anon . 2020. (BAT), British and American Tobacco Company. Potential COVID-19 vaccine – BAT in the news. https://www.bat.com/group/sites/UK__9D9KCY.nsf/vwPagesWebLive/DOBNHBWR https://www.bat.com/. [Online] 2020. [Cited: June 1, 2020.] [ Google Scholar ]
- Anon . 2020. Immunity and Safety of Covid-19 Synthetic Minigene Vaccine.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04276896","term_id":"NCT04276896"}} NCT04276896 https://clinicaltrials.gov. [Online] [ Google Scholar ]
- Anon . 2020. Safety and Immunity of Covid-19 aAPC Vaccine.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04299724","term_id":"NCT04299724"}} NCT04299724 https://clinicaltrials.gov/. [Online] [ Google Scholar ]
- Anon . 2020. Sinovac gets regulatory approval to assess Covid-19 vaccine. https://www.clinicaltrialsarena.com/news/sinovac-covid-19-vaccine-trial-approval/ https://www.clinicaltrialsarena.com. [Online] April 15. [ Google Scholar ]
- Anon . 2020. Sinovac reports positive data from Phase I/II trials of CoronaVac. https://www.clinicaltrialsarena.com/news/sinovac-coronavac-data/ https://www.clinicaltrialsarena.com/. [Online] June 15, 2020. [Cited: June 20, 2020.] [ Google Scholar ]
- Anon . 2020. An Open Study of the Safety, Tolerability and Immunogenicity of the Drug "Gam-COVID-Vac" Vaccine Against COVID-19.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04436471","term_id":"NCT04436471"}} NCT04436471 ?term=vaccine&cond=covid-19&draw=4 https://clinicaltrials.gov/. [Online] June 22, 2020. [Cited: June 22, 2020.]. NCT04436471. [ Google Scholar ]
- Anon . 2020. An Open Study of the Safety, Tolerability and Immunogenicity of "Gam-COVID-Vac Lyo" Vaccine Against COVID-19.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04437875","term_id":"NCT04437875"}} NCT04437875 https://clinicaltrials.gov/. [Online] June 22, 2020. [Cited: June 22, 2020.]. NCT04437875. [ Google Scholar ]
- Anon . 2020. Vaxart Announces Positive Pre-Clinical Data for its Oral COVID-19 Vaccine Program. https://investors.vaxart.com/. [Online] Vaxart Inc., April 21, https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-positive-pre-clinical-data-its-oral-covid-19. [ Google Scholar ]
- Anon . 2020. Zydus Cadila looks to expedite Covid-19 vaccine development. https://www.pharmaceutical-technology.com/news/zydus-cadila-covid-19-vaccine/ https://www.pharmaceutical-technology.com. [Online] 17 February, [ Google Scholar ]
- Anon . 2020. Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines https://www.who.int/. [Online] June 22, 2020. [Cited: June 23, 2020.] [ Google Scholar ]
- Anon . 2020. Clinical trial to assess the safety of a coronavirus vaccine in healthy men and women. http://www.isrctn.com/ISRCTN17072692 http://www.isrctn.com/ [Online] June 17, 2020. [Cited: June 22, 2020.]. ISRCTN17072692. [ Google Scholar ]
- Anon . 2020. A Trial Investigating the Safety and Effects of Four BNT162 Vaccines Against COVID-2019 in Healthy Adults.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04380701","term_id":"NCT04380701"}} NCT04380701 https://clinicaltrials.gov/. [Online] May 8. [ Google Scholar ]
- Anon . BIOCAD Biotechnology Company; 2020. BIOCAD started working on mRNA vaccine against coronavirus. https://biocadglobal.com/index.php?posts&post=45 https://biocadglobal.com/. [Online], March 19. [ Google Scholar ]
- Anon . 2020. Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant.https://clinicaltrials.gov/ct2/show/record/ {"type":"clinical-trial","attrs":{"text":"NCT04368988","term_id":"NCT04368988"}} NCT04368988 https://clinicaltrials.gov/. [Online] May 27, 2020. [Cited: June 15, 2020.] [ Google Scholar ]
- Anon . 2020. Sanofi joins forces with U.S. Department of Health and Human Services to advance a novel coronavirus vaccine. http://www.news.sanofi.us/2020-02-18-Sanofi-joins-forces-with-U-S-Department-of-Health-and-Human-Services-to-advance-a-novel-coronavirus-vaccine http://www.news.sanofi.us/. [Online] Sanofi U.S., February 18, 2020. [ Google Scholar ]
- Anon . Medigaco Inc.; 2020. COVID-19 Vaccine Development Program. https://www.medicago.com/en/covid-19-programs/ https://www.medicago.com/. [Online] [ Google Scholar ]
- 2020. A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Coronavirus Pneumonia vaccine (Vero cells) http://www.chictr.org.cn/showprojen.aspx?proj=52227 http://www.chictr.org.cn. [Online] [ Google Scholar ]
- Anon . Sinovac Biotech Limited; 2020. Sinovac COVID-19 Vaccine Collaboration with Butantan Receives Approval from Brazilian Regulator for Phase III Trial. http://www.sinovac.com/?optionid=754&auto_id=907 http://www.sinovac.com/. [Online]July 06, 2020. [Cited: August 01, 2020.] [ Google Scholar ]
- Anon Treatment With Convalescent Plasma for Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Chest. 2020;(March) doi: 10.1016/j.chest.2020.03.039. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anon . 2020. COVID-19 / SARS-CoV-2. http://www.animalresearch.info/en/medical-advances/diseases-research/sars-cov-2/ http://www.animalresearch.info/. [Online] April 30, 2020. [Cited: June 11, 2020.] [ Google Scholar ]
- Arora Kajal, Rastogi Ruchir, et al. Multi-Antigenic Virus-like Particle of SARS CoV-2 produced in Saccharomyces cerevisiae as a vaccine candidate. Gurugram : s.n. bioRxiv. 2020;(May 19) doi: 10.1101/2020.05.18.099234. [ CrossRef ] [ Google Scholar ]
- Baruah V., Bose S. Immunoinformatics‐aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019‐nCoV. J Med Virol. 2020:495–500. doi: 10.1002/jmv.25698. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Breedveld F.C. Therapeutic monoclonal antibodies. The Lancet. 2000:735–740. doi: 10.1016/S0140-6736(00)01034-5. PMID 10703815. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- BROOK, STONY . 2020. Applied DNA Sciences Subsidiary, LineaRx, and Takis Biotech Collaborate for Development of a Linear DNA Vaccine Candidate Against Wuhan Coronavirus 2019-nCoV. https://adnas.com/coronoavirus-applied-dna-linearx-takis-biotech-vaccine/ https://adnas.com/. [Online] Applied DNA Sciences, February 07. [ Google Scholar ]
- Campbell Molly. 2020. Current Efforts in COVID-19 Vaccine Development. https://www.technologynetworks.com/biopharma/articles/current-efforts-in-covid-19-vaccine-development-332429 https://www.technologynetworks.com/. [Online] Technology Networks, March 23. [ Google Scholar ]
- Cao Y., Zhu X., Hossen M.N., et al. Augmentation of vaccine-induced humoral and cellular immunity by a physical radiofrequency adjuvant. Nat Commun. 2018 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carter John, Saunders Venitia. Wiley; Hoboken, New Jersey: 2007. Virology: Principles and Applications; p. 382. ISBN: (Paperback) 9780470023877 [ Google Scholar ]
- (CDC), Centers for Disease Control and Prevention . CDC; 2020. How COVID-19 Spreads. [Online] 2020. [Cited: June 1, 2020.] https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html? CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fprepare%2Ftransmission.html. [ Google Scholar ]
- CDC Coronavirus Disease 2019 (COVID-19)- Symptoms of Covid-19. Centres for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html [Online]. [ Google Scholar ]
- Coleman Christopher M., Liu Ye V., Mu Haiyan, Taylor Justin K., Massare Michael, Flyer David C., Glenn Gregory M., Smith Gale E., Frieman Matthew B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2020:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Conserved Protein Domain Family: SARS-like-CoV_M . 2020. NCBI. https://www.ncbi.nlm.nih.gov/Structure/cdd/cd21569 [Online]. [Cited: June 1, 2020.] [ Google Scholar ]
- Coronavirus . 2020. Around 30 Indian attempts at COVID-19 vaccine, says Principal Scientific Adviser. https://www.thehindu.com/ [Online] The Hindu, May 11, 2020. [Cited: June 11, 2020.] thehindu.com/sci-tech/health/coronavirus-around-30-indian-attempts-at-covid-19-vaccine-says-principal-scientific-adviser/article31560932.ece. [ Google Scholar ]
- CTRI/2020/07/026352. 2020 http://ctri.nic.in/. [Online] July 31, 2020. [Cited: August 01, 2020.] http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45306&EncHid=&userName=Zydus.
- Cuiling Zhang, Giulietta Maruggi, Hu Shan, Junwei Li. Advances in mRNA Vaccines for Infectious Diseases. Frontiers in Immunology. 2020:594. DOI=10.3389/fimmu.2019.00594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- da Silva S., da Silva C., Mendes R., Pena L. Role of Nonstructural Proteins in the Pathogenesis of SARS-CoV-2. Journal of medical virology. 2020 doi: 10.1002/jmv.25858. 10.1002/jmv.25858 p. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Delamater P.L., Street E.J., Leslie T.F., Yang Y., Jacobsen K.H. Complexity of the Basic Reproduction Number (R0) s.l. : Emerging Infectious Diseases. 2019; 25 doi: 10.3201/eid2501.171901. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020 doi: 10.1080/21645515.2020.1735227. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doremalen Neeltje van, Lambe Teresa, Spencer Alexandra, Belij-Rammerstorfer Sandra, Purushotham Jyothi N., Port Julia R., Avanzato Victoria, Bushmaker Trenton, Flaxman Amy, Ulaszewska Marta, Feldmann Friederike, Allen Elizabeth R., Sharpe Hannah. Jonathan ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. s.l. : bioRxiv. 2020 doi: 10.1101/2020.05.13.093195. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dorp Lucy van, Acman Mislav, Richard Damien, Shaw Liam P., Ford Charlotte E., Ormond Louise, Owen Christopher J., Pang Juanita, Tan Cedric C.S., Boshier Florencia A.T., Ortiz Arturo Torres, Balloux François. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. s.l. : Infection, Genetics and Evolution. 2020; 104351 doi: 10.1016/j.meegid.2020.104351. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eyal N., Lipsitch M., Smith P.G. Human Challenge Studies to Accelerate Coronavirus Vaccine Licensure. s.l. : The Journal of infectious diseases. 2020:1752–1756. doi: 10.1093/infdis/jiaa152. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Folegatti Pedro M, Ewer Katie J, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet. 2020;(July) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Garg P., Srivastava N., Srivastava P. An Integrated In-Silico Approach to Develop Epitope-Based Peptide Vaccine against SARS-CoV-2. s.l. : Preprints. 2020 doi: 10.20944/preprints202005.0401.v1). [ CrossRef ] [ Google Scholar ]
- Gelboin Harry V, et al. Inhibitory monoclonal antibodies to human cytochrome P450 enzymes: a new avenue for drug discovery. Trends in Pharmacological Sciences. 1999:432–438. doi: 10.1016/S01. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gralinski L.E., Menachery V.D. Return of the Coronavirus: 2019-nCoV. Viruses. 2020:135. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;(May 29):945–946. [ PubMed ] [ Google Scholar ]
- Gupta V., Tabiin T.M., Sun K., Chandrashekaran A., Anwar A., Yang K., Chikhlikar P., Salmon J., Brusic V., Marques E.T.A., Kellathur S.N., August T.J. SARS coronavirus nucleocapsid immunodominant T-cell epitope cluster is common to both exogenous recombinant and endogenous DNA-encoded immunogens. Virology. 2006; 347 (1):127–139. (impact factor: 2.657) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heat Biologics’ COVID-19 Vaccine Program . 2020. Heat Biologics. https://www.heatbio.com/product-pipeline/covid-19-vaccine https://www.heatbio.com/. [Online] [ Google Scholar ]
- Hobernik D., Bros M. DNA Vaccines-How Far From Clinical Use? Int J Mol Sci. 2018:3605. doi: 10.3390/ijms19113605. PMID: 30445702; PMCID: PMC6274812. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoffmann Markus, Kleine-Weber Hannah, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020:271–280. doi: 10.1016/j.cell.2020.02.052. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Imai Masaki, Iwatsuki-Horimoto Kiyoko, et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proceedings of the National Academy of Sciences. 2020;(May):16587–16595. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jackson Lisa A., Anderson, Evan J., et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N Engl J Med. 2020;(July) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jiang S., Du L., Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerging microbes & infections. 2020:275–277. doi: 10.1080/22221751.2020.1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Johnson & Johnson Announces a Lead Vaccine Candidate for COVID-19 . 2020. Landmark New Partnership with U.S. Department of Health & Human Services; and Commitment to Supply One Billion Vaccines Worldwide for Emergency Pandemic Use. https://www.prnewswire.com/news-releases/johnson--johnson-announces-a-lead-vaccine-candidate-for-covid-19-landmark-new-partnership-with-us-department-of-health--human-services-and-commitment-to-supply-one-billion-vaccines-worldwide-for-emergency-pandemic- https://www.prnewswire.com/. [Online] [ Google Scholar ]
- Kai Duan, Liu Bende, Li Cesheng, Zhang Huajun, Yu Ting, Qu Jieming, Zhou Min, Chen Li, Meng Shengli, Hu Yong, Peng Cheng, Yuan Mingchao, Huang Jinyan, Wang Zejun, Yu Jianhong, Gao Xiaoxiao, Wang Dan, Yu Xiaoqi, Li Li., Zhang Jiayou, Wu Xiao, Li Bei, Yanpin Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proceedings of the National Academy of Sciences. 2020;(April) doi: 10.1073/pnas.2004168117. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim E., et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102743. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lauer S.A., Grantz K.H., Bi Q., et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020:577–582. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Le Tung Thanh, Andreadakis Zacharias, Kumar Arun, Román Raúl Gómez, Tollefsen Stig, Saville Melanie, Mayhew Stephen. The COVID-19 vaccine development landscape. Nature Reviews Drug Discovery. 2020; 19 (5):305–306. [ PubMed ] [ Google Scholar ]
- Lee Jaimy. These 23 companies are working on coronavirus treatments or vaccines — here’s where things stand. Market watch. 2020 https://www.marketwatch.com/story/these-nine-companies-are-working-on-coronavirus-treatments-or-vaccines-heres-where-things-stand-2020-03-06 [Online] May 6, 2020. [Cited: June 1, 2020.] [ Google Scholar ]
- Liu Cynthia, Zhou Qiongqiong, Li Yingzhu, Garner Linda V., Watkins Steve P., Carter Linda J., Smoot Jeffrey, Gregg Anne C., Daniels Angela D., Jervey Susan, Albaiu Dana. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Central Science. 2020:315–331. doi: 10.1021/acscentsci.0c00272. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Masihi K Noel. Fighting infection using immunomodulatory agents. Expert Opinion on Biological Therapy. 2001:641–653. doi: 10.1517/14712598.1.4.641. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mice L., Bao, et al. The Pathogenicity of 2019 Novel Coronavirus in hACE2 Transgenic. s.l. : bioRxiv. 2020 doi: 10.1101/2020.02.07.939389. preprint. [ CrossRef ] [ Google Scholar ]
- Michot Jean-Marie, Albiges Laurence, Chaput Nathalie, Saada Veronique, Fanny Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Annals of Oncology. 2020 doi: 10.1016/j.annonc.2020.03.300. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mulligan Mark J., Lyke Kirsten E., et al. Phase 1/2 Study to Describe the Safety and Immunogenicity of a COVID-19 RNA Vaccine Candidate (BNT162b1) in Adults 18 to 55 Years of Age: Interim Report; preprint. s.l. : medRxiv. 2020 06.30.20142570, 2020, medRxiv. [ Google Scholar ]
- Myupchar . 2020. Race for COVID-19 vaccine: Covaxin and ZyCoV-D begin human trials in India, Moderna publishes preliminary data from phase 1. https://www.firstpost.com/health/race-for-covid-19-vaccine-covaxin-and-zycov-d-begin-human-trials-in-india-moderna-publishes-preliminary-data-from-phase-1-8600211.html/amp https://www.firstpost.com/. [Online] July 15, 2020. [Cited: August 01, 2020.] [ Google Scholar ]
- Nandi Jayashree. 2020. Top Indian scientists join global fight against coronavirus. https://www.hindustantimes.com/india-news/top-indian-scientists-join-global-fight-against-virus/story-CKRGfycsjBJD2ypLcbJPHM.html https://www.hindustantimes.com. [Online] Hindustan Times, New Delhi, March 30, 2020. [Cited: June 9, 2020.] [ Google Scholar ]
- Ning Wang, Jian Shang, Shibo Jiang, Lanying Du. Subunit Vaccines Against Emerging Pathogenic Human Coronaviruses. Frontiers in Microbiology. 2020 doi: 10.3389/fmicb.2020.00298. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Novavax covid 19 vaccine trial . 2020. Clinical Trials Arena. https://www.clinicaltrialsarena.com/news/novavax-covid-19-vaccine-trial/ [Online] [ Google Scholar ]
- Ou X., Liu Y., Lei X., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020; 1620 :11. doi: 10.1038/s41467-020-15562-9. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Park Su Eun. Epidemiology, virology, and clinical features of severe acute respiratory syndrome -coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19) Clin Exp Pediatr. 2020:119–124. doi: 10.3345/cep.2020.00493. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pharmaceutical Targeting the Envelope Protein of SARS-CoV-2: the Screening for Inhibitors in Approved Drugs . XR Pharmaceuticals Ltd.; Cambridge, New Zealand: 2020. Chernyshev, Anatoly. [ Google Scholar ]
- PIB, Delhi . 2020. DBT/ Anti-COVID consortium- Efforts underway to produce therapeutic antibodies against COVID-19: Isolating genes encoding antibodies for neutralising the SARS-CoV-2, COVID-19. https://pib.gov.in/. [Online] April 12, 2020. [Cited: June 07, 2020.] https://pib.gov.in/PressReleaseIframePage.aspx? PRID=1613531. [ Google Scholar ]
- Prof Roujian Lu, Zhao Xiang, Li Juan, Niu Peihua, Bo Yang, Honglong Wu, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020:565–574. doi: 10.1016/S0140-6736(20)30251-8. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pyrek Kelly M. 2018. 100 Years after the Spanish Flu: Lessons Learned and Challenges for the Future. https://www.infectioncontroltoday.com/ [Online] October 11, 2018. https://www.infectioncontroltoday.com/public-health/100-years-after-spanish-flu-lessons-learned-and-challenges-future. [ Google Scholar ]
- Shang W., Yang Y., Rao Y., et al. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 2020 doi: 10.1038/s41541-020-0170-0. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shen Chenguang, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020; 323 (16):1582–1589. doi: 10.1001/jama.2020.4783. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shen C., Wang Z., Zhao F., et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020; 323 (16):1582–1589. doi: 10.1001/jama.2020.4783. 2020. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smith T.R.F., Patel A., Ramos S., et al. Immunogenicity of a DNA vaccine candidate for COVID-19. 2601. s.l. : Nat Commun. 2020; 11 doi: 10.1038/s41467-020-16505-0. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tai W., He L., Zhang X., et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020 doi: 10.1038/s41. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- TNX-1800 (Coronavirus Vaccine) 2020. Online] Tonix Pharmaceuticals. https://www.tonixpharma.com/pipeline/tnx-1800-coronavirus-vaccine https://www.tonixpharma.com. [ Google Scholar ]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific. Emerg Microbes Infect. 2020:382–385. doi: 10.1080/22221751.2020.1729069. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tu Y.-F., Chien C.-S., Yarmishyn A.A., Lin Y.-Y., Luo Y.-H., Lin Y.-Y., Lai W.-Y., Yang D.-M., Chou S.-J., Yang Y.-P., Wang M.-L., Chiou S.-H. A Review of SARS-CoV-2 and the Ongoing Clinical Trials. International Journal of Molecular Sciences. 2020:2657. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Upadhyay Lipi. 2020. Italy claims to have developed the first COVID-19 vaccine: Here is what we know about all the potential coronavirus vaccines. https://timesofindia.indiatimes.com/life-style/health-fitness/health-news/italy-claims-to-develop-first-covid-19-vaccine-here-is-the-current-status-of-all-the-potential-coronavirus-vaccines/photostory/75575319.cms https://timesofindia.indiatimes.com/. [Online] May 8, 2020. [Cited: May 31, 2020.] [ Google Scholar ]
- Ura T., Okuda K., Shimada M. Developments in Viral Vector-Based Vaccines. Vaccines (Basel) 2014:624–641. doi: 10.3390/vaccines2030624. PMID: 26344749; PMCID: PMC4494222. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Voltron Therapeutics, Inc . 2020. Enters into Sponsored Research Agreement with The Vaccine & Immunotherapy Center at the Massachusetts General Hospital to Develop Potential COVID-19 Vaccine. https://www.prnewswire.com/news-releases/voltron-therapeutics-inc-enters-into-sponsored-research-agreement-with-the-vaccine--immunotherapy-center-at-the-massachusetts-general-hospital-to-develop-potential-covid-19-vaccine-301034225.html https://www.prnewswire.com/. [Online] April 02, 2020. [ Google Scholar ]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Vessler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020:281–292. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Walls Structure, Function, and Antigenicity of the SARSCoV-2 Spike Glycoprotein. Cell. 2020:281–292. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020 doi: 10.1128/JVI.00127-20. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- (WHO), World Health Organisation . s.l. : World Health Organisation; 2014. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease. September [ Google Scholar ]
- (WHO) World Health Organisation . s.l.: World Health Organisation; 2020. Coronavirus disease (Covid-19) Situation Report- 164. [ Google Scholar ]
- WHO . s.l. : World Health Organisation; 2020. Draft landscape of COVID-19 candidate vaccines. [ Google Scholar ]
- Wrapp Daniel, Wang Nianshuang, Corbett Kizzmekia S., Goldsmith Jory A., Hsieh Ching-Lin, Abiona Olubukola, Graham Barney S., Jason S. Mclellan Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;(13 March):1260–1263. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., et al. Commentary genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020:325–328. doi: 10.1016/j.chom.2020.02.001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yan, Xuebing. 2020 http://www.chictr.org.cn/showprojen.aspx?proj=49544. http://www.chictr.org.cn. [Online] February 2020.
- Yip M.S., Leung H.L., Li P.H., Cheung C.Y., Dutry I., Li D., Daëron M., Bruzzone R., Peiris J.S.M., Jaume M. Hong Kong Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS: s.n. Hong Kong medical journal = Xianggang yi xue za zhi / Hong Kong Academy of Medicine. 2016:25–31. [ PubMed ] [ Google Scholar ]
- Yoshimoto F.K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. The protein journal. 2020:198–216. doi: 10.1007/s10930-020-09901-4. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. International Journal of Antimicrobial Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105955. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang B., Liu S., Tan T., et al. Treatment with convalescent plasma for critically ill patients with SARS‐CoV‐2 infection. Chest. 2020; 2020 :30571–30577. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhu Feng-Cai, Li Yu-Hua, Guan Xu-Hua, Hou Li-Hua, Wang Wen-Juan, Li Jing-Xin, Wu Shi-Po, Wang Bu-Sen, Wang Zhao, Wang Lei, Jia Si-Yue, Jiang Hu-Dachuan, Wang Ling, Jiang Tao, Hu Yi, Gou Jin-Bo, Xu Sha-Bei, Xu Jun-Jie, Wang Xue-Wen, Wang Wei, Chen Wei. Safety, tolerability, and immunogenicity of a recombinan tadenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet. 2020 doi: 10.1016/S0140-6736(20)31208-3. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhu Feng-Cai, Guan Xu-Hua, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet. 2020;(July) [ PMC free article ] [ PubMed ] [ Google Scholar ]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2021
Public attitudes toward COVID-19 vaccination: The role of vaccine attributes, incentives, and misinformation
- Sarah Kreps 1 ,
- Nabarun Dasgupta 2 ,
- John S. Brownstein 3 , 4 ,
- Yulin Hswen 5 &
- Douglas L. Kriner ORCID: orcid.org/0000-0002-9353-2334 1
npj Vaccines volume 6 , Article number: 73 ( 2021 ) Cite this article
19k Accesses
72 Citations
43 Altmetric
Metrics details
- Health care
- Translational research
While efficacious vaccines have been developed to inoculate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as COVID-19), public vaccine hesitancy could still undermine efforts to combat the pandemic. Employing a survey of 1096 adult Americans recruited via the Lucid platform, we examined the relationships between vaccine attributes, proposed policy interventions such as financial incentives, and misinformation on public vaccination preferences. Higher degrees of vaccine efficacy significantly increased individuals’ willingness to receive a COVID-19 vaccine, while a high incidence of minor side effects, a co-pay, and Emergency Use Authorization to fast-track the vaccine decreased willingness. The vaccine manufacturer had no influence on public willingness to vaccinate. We also found no evidence that belief in misinformation about COVID-19 treatments was positively associated with vaccine hesitancy. The findings have implications for public health strategies intending to increase levels of community vaccination.
Similar content being viewed by others

Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA
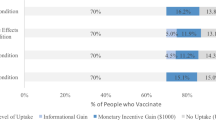
Vaccine hesitancy and monetary incentives
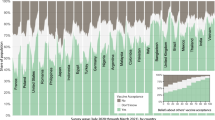
Providing normative information increases intentions to accept a COVID-19 vaccine
Introduction.
In less than a year, an array of vaccines was developed to bring an end to the SARS-CoV-2 pandemic. As impressive as the speed of development was the efficacy of vaccines such as Moderna and Pfizer, which are over 90%. Despite the growing availability and efficacy, however, vaccine hesitancy remains a potential impediment to widespread community uptake. While previous surveys indicate that overall levels of vaccine acceptance may be around 70% in the United States 1 , the case of Israel may offer a cautionary tale about self-reported preferences and vaccination in practice. Prospective studies 2 of vaccine acceptance in Israel showed that about 75% of the Israeli population would vaccinate, but Israel’s initial vaccination surge stalled around 42%. The government, which then augmented its vaccination efforts with incentive programs, attributed unexpected resistance to online misinformation 3 .
Research on vaccine hesitancy in the context of viruses such as influenza and measles, mumps, and rubella, suggests that misinformation surrounding vaccines is prevalent 4 , 5 . Emerging research on COVID-19 vaccine preferences, however, points to vaccine attributes as dominant determinants of attitudes toward vaccination. Higher efficacy is associated with greater likelihood of vaccinating 6 , 7 , whereas an FDA Emergency Use Authorization 6 or politicized approval timing 8 is associated with more hesitancy. Whether COVID-19 misinformation contributes to vaccine preferences or whether these attributes or policy interventions such as incentives play a larger role has not been studied. Further, while previous research has focused on a set of attributes that was relevant at one particular point in time, the evidence and context about the available vaccines has continued to shift in ways that could shape public willingness to accept the vaccine. For example, governments, employers, and economists have begun to think about or even devise ways to incentivize monetarily COVID-19 vaccine uptake, but researchers have not yet studied whether paying people to receive the COVID-19 vaccine would actually affect likely behavior. As supply problems wane and hesitancy becomes a limiting factor, understanding whether financial incentives can overcome hesitancy becomes a crucial question for public health. Further, as new vaccines such as Johnson and Johnson are authorized, knowing whether the vaccine manufacturer name elicits or deters interest in individuals is also important, as are the corresponding efficacy rates of different vaccines and the extent to which those affect vaccine preferences. The purpose of this study is to examine how information about vaccine attributes such as efficacy rates, the incidence of side effects, the nature of the governmental approval process, identity of the manufacturers, and policy interventions, including economic incentives, affect intention to vaccinate, and to examine the association between belief in an important category of misinformation—false claims concerning COVID-19 treatments—and willingness to vaccinate.
General characteristics of study population
Table 1 presents sample demographics, which largely reflect those of the US population as a whole. Of the 1335 US adults recruited for the study, a convenience sample of 1100 participants consented to begin the survey, and 1096 completed the full questionnaire. The sample was 51% female; 75% white; and had a median age of 43 with an interquartile range of 31–58. Comparisons of the sample demographics to those of other prominent social science surveys and U.S. Census figures are shown in Supplementary Table 1 .
Vaccination preferences
Each subject was asked to evaluate a series of seven hypothetical vaccines. For each hypothetical vaccine, our conjoint experiment randomly assigned values of five different vaccine attributes—efficacy, the incidence of minor side effects, government approval process, manufacturer, and cost/financial inducement. Descriptions of each attribute and the specific levels used in the experiment are summarized in Table 2 . After seeing the profile of each vaccine, the subject was asked whether she would choose to receive the vaccine described, or whether she would choose not to be vaccinated. Finally, subjects were asked to indicate how likely they would be to take the vaccine on a seven-point likert scale.
Across all choice sets, in 4419 cases (58%) subjects said they would choose the vaccine described in the profile rather than not being vaccinated. As shown in Fig. 1 , several characteristics of the vaccine significantly influenced willingness to vaccinate.
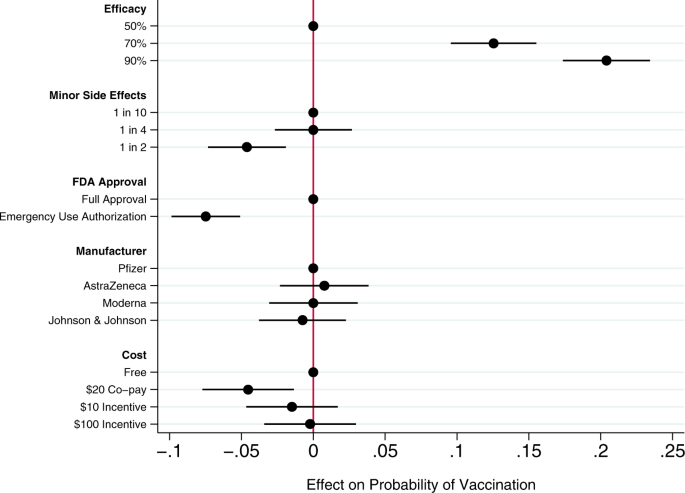
Circles present the estimated effect of each attribute level on the probability of a subject accepting vaccination from the attribute’s baseline level. Horizontal lines through points indicate 95% confidence intervals. Points without error bars denote the baseline value for each attribute. The average marginal component effects (AMCEs) are the regression coefficients reported in model 1 of Table 3 .
Efficacy had the largest effect on individual vaccine preferences. An efficacy rate of 90% increased uptake by about 20% relative to the baseline at 50% efficacy. Even a high incidence of minor side effects (1 in 2) had only a modest negative effect (about 5%) on willingness to vaccinate. Whether the vaccine went through full FDA approval or received an Emergency Use Authorization (EUA), an authority that allows the Food and Drug Administration mechanisms to accelerate the availability and use of treatments or medicines during medical emergencies 9 , significantly influenced willingness to vaccinate. An EUA decreased the likelihood of vaccination by 7% compared to a full FDA authorization; such a decline would translate into about 23 million Americans. While a $20 co-pay reduced the likelihood of vaccination relative to a no-cost baseline, financial incentives did not increase willingness to vaccinate. Lastly, the manufacturer had no effect on vaccination attitudes, despite the public pause of the AstraZeneca trial and prominence of Johnson & Johnson as a household name (our experiment was fielded before the pause in the administration of the Johnson & Johnson shot in the United States).
Model 2 of Table 3 presents an expanded model specification to investigate the association between misinformation and willingness to vaccinate. The primary additional independent variable of interest is a misinformation index that captures the extent to which each subject believes or rejects eight claims (five false; three true) about COVID-19 treatments. Additional analyses using alternate operationalizations of the misinformation index yield substantively similar results (Supplementary Table 4 ). This model also includes a number of demographic control variables, including indicators for political partisanship, gender, educational attainment, age, and race/ethnicity, all of which are also associated with belief in misinformation about the vaccine (Supplementary Table 2 ). Finally, the model also controls for subjects’ health insurance status, past experience vaccinating against seasonal influenza, attitudes toward the pharmaceutical industry, and beliefs about vaccine safety generally.
Greater levels of belief in misinformation about COVID-19 treatments were not associated with greater vaccine hesitancy. Instead, the relevant coefficient is positive and statistically significant, indicating that, all else being equal, individuals who scored higher on our index of misinformation about COVID-19 treatments were more willing to vaccinate than those who were less susceptible to believing false claims.
Strong beliefs that vaccines are safe generally was positively associated with willingness to accept a COVID-19 vaccine, as were past histories of frequent influenza vaccination and favorable attitudes toward the pharmaceutical industry. Women and older subjects were significantly less likely to report willingness to vaccinate than men and younger subjects, all else equal. Education was positively associated with willingness to vaccinate.
This research offers a comprehensive examination of attitudes toward COVID-19 vaccination, particularly the role of vaccine attributes, potential policy interventions, and misinformation. Several previous studies have analyzed the effects of vaccine characteristics on willingness to vaccinate, but the modal approach is to gauge willingness to accept a generic COVID-19 vaccine 10 , 11 . Large volumes of research show, however, that vaccine preferences hinge on specific vaccine attributes. Recent research considering the influence of attributes such as efficacy, side effects, and country of origin take a step toward understanding how properties affect individuals’ intentions to vaccinate 6 , 7 , 8 , 12 , 13 , but evidence about the attributes of actual vaccines, debates about how to promote vaccination within the population, and questions about the influence of misinformation have moved quickly 14 .
Our conjoint experiment therefore examined the influence of five vaccine attributes on vaccination willingness. The first category of attributes involved aspects of the vaccine itself. Since efficacy is one of the most common determinants of vaccine acceptance, we considered different levels of efficacy, 50%, 70%, and 90%, levels that are common in the literature 7 , 15 . Evidence from Phase III trials suggests that even the 90% efficacy level in our design, which is well above the 50% threshold from the FDA Guidance for minimal effectiveness for Emergency Use Authorization 16 , has been exceeded by both Pfizer’s and Moderna’s vaccines 17 , 18 . The 70% efficacy threshold is closer to the initial reports of the efficacy of the Johnson & Johnson vaccine, whose efficacy varied across regions 19 . Our analysis suggests that efficacy levels associated with recent mRNA vaccine trials increases public vaccine uptake by 20% over a baseline of a vaccine with 50% efficacy. A 70% efficacy rate increases public willingness to vaccinate by 13% over a baseline vaccine with 50% efficacy.
An additional set of epidemiological attributes consisted of the frequency of minor side effects. While severe side effects were plausible going into early clinical trials, evidence clearly suggests that minor side effects are more common, ranging from 10% to 100% of people vaccinated depending on the number of doses and the dose group (25–250 mcg) 20 . Since the 100 mcg dose was supported in Phase III trials 21 , we include the highest adverse event probability—approximating 60% as 1 in 2—and 1 in 10 as the lowest likelihood, approximating the number of people who experienced mild arthralgia 20 . Our findings suggest that a the prevalence of minor side effects associated with recent trials (i.e. a 1 in 2 chance), intention to vaccinate decreased by about 5% versus a 1 in 10 chance of minor side effects baseline. However, at a 25% rate of minor side effects, respondents did not indicate any lower likelihood of vaccination compared to the 10% baseline. Public communications about how to reduce well-known side effects, such as pain at the injection site, could contribute to improved acceptance of the vaccine, as it is unlikely that development of vaccine-related minor side effects will change.
We then considered the effect of EUA versus full FDA approval. The influenza H1N1 virus brought the process of EUA into public discourse 22 , and the COVID-19 virus has again raised the debate about whether and how to use EUA. Compared to recent studies also employing conjoint experimental designs that showed just a 3% decline in support conditional on EUA 6 , we found decreases in support of more than twice that with an EUA compared to full FDA approval. Statements made by the Trump administration promising an intensely rapid roll-out or isolated adverse events from vaccination in the UK may have exacerbated concerns about EUA versus full approval 8 , 23 , 24 , 25 . This negative effect is even greater among some subsets of the population. As shown in additional analyses reported in the Supplementary Information (Supplementary Fig. 5 ), the negative effects are greatest among those who believe vaccines are generally safe. Among those who believe vaccines generally are extremely safe, the EUA decreased willingness to vaccinate by 11%, all else equal. This suggests that outreach campaigns seeking to assure those troubled by the authorization process used for currently available vaccines should target their efforts on those who are generally predisposed to believe vaccines are safe.
Next, we compared receptiveness as a function of the manufacturer: Moderna, Pfizer, Johnson and Johnson, and AstraZeneca, all firms at advanced stages of vaccine development. Vaccine manufacturers in the US have not yet attempted to use trade names to differentiate their vaccines, instead relying on the association with manufacturer reputation. In other countries, vaccine brand names have been more intentionally publicized, such as Bharat Biotech’s Covaxin in India and Gamaleya Research Institute of Epidemiology and Microbiology Sputnik V in Russia. We found that manufacturer names had no impact on willingness to vaccinate. As with hepatitis and H. influenzae vaccines 26 , 27 , interchangeability has been an active topic of debate with coronavirus mRNA vaccines which require a second shot for full immunity. Our research suggests that at least as far as public receptiveness goes, interchangeability would not introduce concerns. We found no significant differences in vaccination uptake across any of the manufacturer treatments. Future research should investigate if a manufacturer preference develops as new evidence about efficacy and side effects becomes available, particularly depending on whether future booster shots, if needed, are deemed interchangeable with the initial vaccination.
Taking up the question of how cost and financial incentives shape behavior, we looked at paying and being paid to vaccinate. While existing research suggests that individuals are often willing to pay for vaccines 28 , 29 , some economists have proposed that the government pay individuals up to $1,000 to take the COVID-19 vaccine 30 . However, because a cost of $300 billion to vaccinate the population may be prohibitive, we posed a more modest $100 incentive. We also compared this with a $10 incentive, which previous studies suggest is sufficient for actions that do not require individuals to change behavior on a sustained basis 31 . While having to pay a $20 co-pay for the vaccine did deter individuals, the additional economic incentives had no positive effect although they did not discourage vaccination 32 . Consistent with past research 31 , 33 , further analysis shows that the negative effect of the $20 co-pay was concentrated among low-income earners (Supplementary Fig. 7 ). Financial incentives failed to increase vaccination willingness across income levels.
Our study also yields important insights into the relationship between one prominent category of COVID-19 misinformation and vaccination preferences. We find that susceptibility to misinformation about COVID-19 treatments—based on whether individuals can distinguish between factual and false information about efforts to combat COVID-19—is considerable. A quarter of subjects scored no higher on our misinformation index than random guessing or uniform abstention/unsure responses (for the full distribution, see Supplementary Fig. 2 ). However, subjects who scored higher on our misinformation index did not exhibit greater vaccination hesitancy. These subjects actually were more likely to believe in vaccine safety more generally and to accept a COVID-19 vaccine, all else being equal. These results run counter to recent findings of public opinion in France where greater conspiracy beliefs were negatively correlated with willingness to vaccinate against COVID-19 34 and in Korea where greater misinformation exposure and belief were negatively correlated with taking preventative actions 35 . Nevertheless, the results are robust to alternate operationalizations of belief in misinformation (i.e., constructing the index only using false claims, or measuring misinformation beliefs as the number of false claims believed: see Supplementary Table 4 ).
We recommend further study to understand the observed positive relationship between beliefs in COVID-19 misinformation about fake treatments and willingness to receive the COVID-19 vaccine. To be clear, we do not posit a causal relationship between the two. Rather, we suspect that belief in misinformation may be correlated with an omitted factor related to concerns about contracting COVID-19. For example, those who believe COVID-19 misinformation may have a higher perception of risk of COVID-19, and therefore be more willing to take a vaccine, all else equal 36 . Additional analyses reported in the Supplementary Information (Supplementary Fig. 6 ) show that the negative effect of an EUA on willingness to vaccinate was concentrated among those who scored low on the misinformation index. An EUA had little effect on the vaccination preferences of subjects most susceptible to misinformation. This pattern is consistent with the possibility that these subjects were more concerned with the disease and therefore more likely to vaccinate, regardless of the process through which the vaccine was brought to market.
We also observe that skepticism toward vaccines in general does not correlate perfectly with skepticism toward the COVID-19 vaccine. Therefore, it is important not to conflate people who are wary of the COVID-19 vaccine and those who are anti-vaccination, as even medically informed individuals may be hesitant because of the speed at which the COVID-19 vaccine was developed. For example, older people are more likely to believe vaccines are safe but less willing to receive the COVID-19 vaccine in our survey, perhaps following the high rates of vaccine skepticism among medical staff expressing concerns regarding the safety of a rapidly-developed vaccine 2 . This inverse relationship between age and willingness to vaccinate is also surprising. Most opinion surveys find older adults are more likely to vaccinate than younger adults 37 . However, most of these survey questions ask about willingness to take a generic vaccine. Two prior studies, both recruiting subjects from the Lucid platform and employing conjoint experiments to examine the effects of vaccine attributes on public willingness to vaccinate, also find greater vaccine hesitancy among older Americans 6 , 7 . Future research could explore whether these divergent results are a product of the characteristics of the sample or of the methodological design in which subjects have much more information about the vaccines when indicating their vaccination preferences.
An important limitation of our study is that it necessarily offers a snapshot in time, specifically prior to both the election and vaccine roll-out. We recommend further study to understand more how vaccine perceptions evolve both in terms of the perceived political ownership of the vaccine—now that President Biden is in office—and as evidence has emerged from the millions of people who have been vaccinated. Similarly, researchers should consider analyzing vaccine preferences in the context of online vaccine controversies that have been framed in terms of patient autonomy and right to refuse 38 , 39 . Vaccination mandates may evoke feelings of powerlessness, which may be exacerbated by misinformation about the vaccines themselves. Further, researchers should more fully consider how individual attributes such as political ideology and race intersect with vaccine preferences. Our study registered increased vaccine hesitancy among Blacks, but did not find that skepticism was directly related to misinformation. Perceptions and realities of race-based maltreatment could also be moderating factors worth exploring in future analyses 40 , 41 .
Overall, we found that the most important factor influencing vaccine preferences is vaccine efficacy, consistent with a number of previous studies about attitudes toward a range of vaccines 6 , 42 , 43 . Other attributes offer potential cautionary flags and opportunities for public outreach. The prospect of a 50% likelihood of mild side effects, consistent with the evidence about current COVID-19 vaccines being employed, dampens likelihood of uptake. Public health officials should reinforce the relatively mild nature of the side effects—pain at the injection site and fatigue being the most common 44 —and especially the temporary nature of these effects to assuage public concerns. Additionally, in considering policy interventions, public health authorities should recognize that a $20 co-pay will likely discourage uptake while financial incentives are unlikely to have a significant positive effect. Lastly, belief in misinformation about COVID-19 does not appear to be a strong predictor of vaccine hesitancy; belief in misinformation and willingness to vaccinate were positively correlated in our data. Future research should explore the possibility that exposure to and belief in misinformation is correlated with other factors associated with vaccine preferences.
Survey sample and procedures
This study was approved by the Cornell Institutional Review Board for Human Participant Research (protocol ID 2004009569). We conducted the study on October 29–30, 2020, prior to vaccine approval, which means we captured sentiments prospectively rather than based on information emerging from an ongoing vaccination campaign. We recruited a sample of 1096 adult Americans via the Lucid platform, which uses quota sampling to produce samples matched to the demographics of the U.S. population on age, gender, ethnicity, and geographic region. Research has shown that experimental effects observed in Lucid samples largely mirror those found using probability-based samples 45 . Supplementary Table 1 presents the demographics of our sample and comparisons to both the U.S. Census American Community Survey and the demographics of prominent social science surveys.
After providing informed consent on the first screen of the online survey, participants turned to a choice-based conjoint experiment that varied five attributes of the COVID-19 vaccine. Conjoint analyses are often used in marketing to research how different aspects of a product or service affect consumer choice. We build on public health studies that have analyzed the influence of vaccine characteristics on uptake within the population 42 , 46 .
Conjoint experiment
We first designed a choice-based conjoint experiment that allowed us to evaluate the relative influence of a range of vaccine attributes on respondents’ vaccine preferences. We examined five attributes summarized in Table 2 . Past research has shown that the first two attributes, efficacy and the incidence of side effects, are significant drivers of public preferences on a range of vaccines 47 , 48 , 49 , including COVID-19 6 , 7 , 13 , 50 . In this study, we increased the expected incidence of minor side effects from previous research 6 to reflect emerging evidence from Phase III trials. The third attribute, whether the vaccine received full FDA approval or an EUA, examines whether the speed of the approval process affects public vaccination preferences 6 . The fourth attribute, the manufacturer of the vaccine, allows us to examine whether the highly public pause in the AstraZeneca trial following an adverse event, and the significant differences in brand familiarity between smaller and less broadly known companies like Moderna and household name Johnson & Johnson affects public willingness to vaccinate. The fifth attribute examines the influence of a policy tool—offsetting the costs of vaccination or even incentivizing it financially—on public willingness to vaccinate.
Attribute levels and attribute order were randomly assigned across participants. A sample choice set is presented in Supplementary Fig. 1 . After viewing each profile individually, subjects were asked: “If you had to choose, would you choose to get this vaccine, or would you choose not to be vaccinated?” Subjects then made a binary choice, responding either that they “would choose to get this vaccine” or that they “would choose not to be vaccinated.” This is the dependent variable for the regression analyses in Table 3 . After making a binary choice to take the vaccine or not be vaccinated, we also asked subjects “how likely or unlikely would you be to get the vaccine described above?” Subjects indicated their vaccination preference on a seven-point scale ranging from “extremely likely” to “extremely unlikely.” Additional analyses using this ordinal dependent variable reported in Supplementary Table 3 yield substantively similar results to those presented in Table 3 .
To determine the effect of each attribute-level on willingness to vaccinate, we followed Hainmueller, Hopkins, and Yamamoto and employed an ordinary least squares (OLS) regression with standard errors clustered on respondent to estimate the average marginal component effects (AMCEs) for each attribute 51 . The AMCE represents the average difference in a subject choosing a vaccine when comparing two different attribute values—for example, 50% efficacy vs. 90% efficacy—averaged across all possible combinations of the other vaccine attribute values. The AMCEs are nonparametrically identified under a modest set of assumptions, many of which (such as randomization of attribute levels) are guaranteed by design. Model 1 in Table 3 estimates the AMCEs for each attribute. These AMCEs are illustrated in Fig. 1 .
Analyzing additional correlates of vaccine acceptance
To explore the association between respondents’ embrace of misinformation about COVID-19 treatments and vaccination willingness, the survey included an additional question battery. To measure the extent of belief in COVID-19 misinformation, we constructed a list of both accurate and inaccurate headlines about the coronavirus. We focused on treatments, relying on the World Health Organization’s list of myths, such as “Hand dryers are effective in killing the new coronavirus” and true headlines such as “Avoiding shaking hands can help limit the spread of the new coronavirus 52 .” Complete wording for each claim is provided in Supplementary Appendix 1 . Individuals read three true headlines and five myths, and then responded whether they believed each headline was true or false, or whether they were unsure. We coded responses to each headline so that an incorrect accuracy assessment yielded a 1; a correct accuracy assessment a -1; and a response of unsure was coded as 0. From this, we created an additive index of belief in misinformation that ranged from -8 to 8. The distribution of the misinformation index is presented in Supplementary Fig. 2 . A possible limitation of this measure is that because the survey was conducted online, some individuals could have searched for the answers to the questions before responding. However, the median misinformation index score for subjects in the top quartile in terms of time spent taking the survey was identical to the median for all other respondents. This may suggest that systematic searching for correct answers is unlikely.
To ensure that any association observed between belief in misinformation and willingness to vaccinate is not an artifact of how we operationalized susceptibility to misinformation, we also constructed two alternate measures of belief in misinformation. These measures are described in detail in the Supplementary Information (see Supplementary Figs. 3 and 4 ). Additional regression analyses using these alternate measures of misinformation beliefs yield substantively similar results (see Supplementary Table 4 ). Additional analyses examining whether belief in misinformation moderates the effect of efficacy and an FDA EUA on vaccine acceptance are presented in Supplementary Fig. 6 .
Finally, model 2 of Table 3 includes a range of additional control variables. Following past research, it includes a number of demographic variables, including indicator variables identifying subjects who identify as Democrats or Republicans; an indicator variable identifying females; a continuous variable measuring age (alternate analyses employing a categorical variable yield substantively similar results); an eight-point measure of educational attainment; and indicator variables identifying subjects who self-identify as Black or Latinx. Following previous research 6 , the model also controlled for three additional factors often associated with willingness to vaccinate: an indicator variable identifying whether each subject had health insurance; a variable measuring past frequency of influenza vaccination on a four-point scale ranging from “never” to “every year”; beliefs about the general safety of vaccines measured on a four-point scale ranging from “not at all safe” to “extremely safe”; and a measure of attitudes toward the pharmaceutical industry ranging from “very positive” to “very negative.”
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data and statistical code to reproduce the tables and figures in the manuscript and Supplementary Information are published at the Harvard Dataverse via this link: 10.7910/DVN/ZYU6CO.
Hamel, L., Kirzinger, A., Munana, C. & Brodie, M. KFF COVID-19 Vaccine Monitor: December 2020 | KFF (2020).
Dror, A. A. et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 35 , 775–779 (2020).
Article CAS PubMed Google Scholar
Scharf, I. & Ben Zion, I. As Vaccinations Lag, Israel Combats Online Misinformation. AP2 (21AD).
Nyhan, B. & Reifler, J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 33 , 459–464 (2015).
Article PubMed Google Scholar
Hussain, A., Ali, S., Ahmed, M. & Hussain, S. The anti-vaccination movement: a regression in modern medicine. Cureus 10 , e2919 (2018).
PubMed PubMed Central Google Scholar
Kreps, S. et al. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. open 3 , (2020).
Motta, M. Can a COVID-19 vaccine live up to Americans’ expectations? A conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc. Sci. Med . 113642, https://doi.org/10.1016/j.socscimed.2020.113642 (2021).
Bokemper, S. E., Huber, G. A., Gerber, A. S., James, E. K. & Omer, S. B. Timing of COVID-19 vaccine approval and endorsement by public figures. Vaccine , https://doi.org/10.1016/j.vaccine.2020.12.048 (2021).
Quinn, S. C., Jamison, A. M. & Freimuth, V. Communicating effectively about emergency use authorization and vaccines in the COVID-19 pandemic. Am. J. Public Health e1–e4 (2020).
Malik, A. A., McFadden, S. A. M., Elharake, J. & Omer, S. B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 26 , 100495 (2020).
Article PubMed PubMed Central Google Scholar
Fisher, K. A. et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann. Intern. Med. 173 , 964–973 (2020).
Lazarus, J. V. et al . A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med . 1–4 (2020).
Schwarzinger, M., Watson, V., Arwidson, P., Alla, F. & Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Heal ., https://doi.org/10.1016/s2468-2667(21)00012-8 (2021).
Wood, S. & Schulman, K. Beyond politics—promoting Covid-19 vaccination in the United States. N. Engl. J. Med . NEJMms2033790, https://doi.org/10.1056/NEJMms2033790 (2021).
Marshall, H. S., Chen, G., Clarke, M. & Ratcliffe, J. Adolescent, parent and societal preferences and willingness to pay for meningococcal B vaccine: a discrete choice experiment. Vaccine 34 , 671–677 (2016).
Administration, F. and D. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry . (2020).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383 , 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med ., https://doi.org/10.1056/nejmoa2035389 (2020).
Johnson & Johnson. Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial. (2020). Available at: https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial . (Accessed 28 Feb 2021).
Jackson, L. A. et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 383 , 1920–1931 (2020).
Anderson, E. J. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383 , 2427–2438 (2020).
Quinn, S. C., Kumar, S., Freimuth, V. S., Kidwell, K. & Musa, D. Public willingness to take a vaccine or drug under emergency use authorization during the 2009 H1N1 pandemic. Biosecurity Bioterrorism 7 , 275–290 (2009).
Holden Thorp, H. A dangerous rush for vaccines. Science 369 , 885 (2020).
Limaye, R. J., Sauer, M. & Truelove, S. A. Politicizing public health: the powder keg of rushing COVID-19 vaccines. Hum. Vaccines Immunother ., https://doi.org/10.1080/21645515.2020.1846400 (2020).
Feuer, W. Trump Says ‘No President’s Ever Pushed’ the FDA Like Jim, Vaccine Coming ‘Very Shortly’. CNBC (2020).
Soysal, A., Gokçe, I., Pehlivan, T. & Bakir, M. Interchangeability of a hepatitis A vaccine second dose: Avaxim 80 following a first dose of Vaqta 25 or Havrix 720 in children in Turkey. Eur. J. Pediatr. 166 , 533–539 (2007).
Greenberg, D. P. & Feldman, S. Vaccine interchangeability. Clin. Pediatrics 42 , 93–99 (2003).
Article Google Scholar
Bishai, D., Brice, R., Girod, I., Saleh, A. & Ehreth, J. Conjoint analysis of French and German parents’ willingness to pay for meningococcal vaccine. PharmacoEconomics 25 , 143–154 (2007).
Cameron, M. P., Newman, P. A., Roungprakhon, S. & Scarpa, R. The marginal willingness-to-pay for attributes of a hypothetical HIV vaccine. Vaccine 31 , 3712–3717 (2013).
Litan, R. Want herd immunity? Pay people to take the vaccine. Brookings (2020).
Kane, R. L., Johnson, P. E., Town, R. J. & Butler, M. A structured review of the effect of economic incentives on consumers’ preventive behavior. Am. J. Preventive Med. 27 , 327–352 (2004).
Volpp, K. G., Loewenstein, G. & Buttenheim, A. M. Behaviorally informed strategies for a National COVID-19 vaccine promotion program. JAMA - J. Am. Med. Assoc. 325 , 125–126 (2020).
Google Scholar
Nowalk, M. P. et al. Improving influenza vaccination rates in the workplace. A randomized trial. Am. J. Prev. Med. 38 , 237–246 (2010).
Bertin, P., Nera, K. & Delouvée, S. Conspiracy beliefs, rejection of vaccination, and support for hydroxychloroquine: a conceptual replication-extension in the COVID-19 pandemic context. Front. Psychol. 11 , 2471 (2020).
Lee, J. J. et al. Associations between COVID-19 misinformation exposure and belief with COVID-19 knowledge and preventive behaviors: cross-sectional online study. J. Med. Internet Res. 22 , e22205 (2020).
Reyna, V. F. Risk perception and communication in vaccination decisions: a fuzzy-trace theory approach. Vaccine 30 , 3790–3797 (2012).
Lin, C., Tu, P. & Beitsch, L. M. Confidence and receptivity for covid‐19 vaccines: a rapid systematic review. Vaccines 9 , 1–32 (2021).
Broniatowski, D. A. et al. Facebook pages, the ‘Disneyland’ measles outbreak, and promotion of vaccine refusal as a civil right, 2009–2019. Am. J. Public Health 110 , S312–S318 (2020).
Moon, K., Riege, A., Gourdon-Kanhukamwe, A. & Vallée-Tourangeau, G. The Moderating Effect of Autonomy on Promotional Health Messages Encouraging Flu Vaccination Uptake Among Healthcare Professionals . (PsyArXiv, 2020), https://doi.org/10.31234/OSF.IO/AJV4Q .
Ferdinand, K. C., Nedunchezhian, S. & Reddy, T. K. The COVID-19 and influenza “Twindemic”: barriers to influenza vaccination and potential acceptance of SARS-CoV2 vaccination in African Americans. J. Natl. Med. Assoc. 112 , 681–687 (2020).
PubMed Google Scholar
Jaiswal, J., LoSchiavo, C. & Perlman, D. C. Disinformation, misinformation and inequality-driven mistrust in the time of COVID-19: lessons unlearned from AIDS denialism. AIDS Behav. 24 , 2776–2780 (2020).
Determann, D. et al. Acceptance of vaccinations in pandemic outbreaks: a discrete choice experiment. PLoS ONE 9 , e102505 (2014).
Simpson, C. R., Ritchie, L. D., Robertson, C., Sheikh, A. & McMenamin, J. Effectiveness of H1N1 vaccine for the prevention of pandemic influenza in Scotland, UK: A retrospective observational cohort study. Lancet Infect. Dis. 12 , 696–702 (2012).
Remmel, A. COVID vaccines and safety: what the research says. Nature 590 , 538–540 (2021).
Coppock, A. & McClellan, O. A. Validating the demographic, political, psychological, and experimental results obtained from a new source of online survey respondents. Res. Polit. 6 , 1–14 (2019).
de Bekker-Grob, E. W. et al. The impact of vaccination and patient characteristics on influenza vaccination uptake of elderly people: A discrete choice experiment. Vaccine 36 , 1467–1476 (2018).
Determann, D. et al. Public preferences for vaccination programmes during pandemics caused by pathogens transmitted through respiratory droplets–A discrete choice experiment in four European countries, 2013. Eurosurveillance 21 , 1–13 (2016).
de Bekker-Grob, E. W. et al. Girls’ preferences for HPV vaccination: A discrete choice experiment. Vaccine 28 , 6692–6697 (2010).
Guo, N., Zhang, G., Zhu, D., Wang, J. & Shi, L. The effects of convenience and quality on the demand for vaccination: results from a discrete choice experiment. Vaccine 35 , 2848–2854 (2017).
Kaplan, R. M. & Milstein, A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc. Natl. Acad. Sci. USA 118 , 2021726118 (2021).
Hainmueller, J., Hopkins, D. J. & Yamamoto, T. Causal inference in conjoint analysis: Understanding multidimensional choices via stated preference experiments. Polit. Anal. 22 , 1–30 (2014).
Organization, W. H. Coronavirus disease (COVID-19) advice for the public: Mythbusters. (2020). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters . (Accessed 14 Jan 2021).
Download references
Acknowledgements
S.K. and D.K. would like to thank the Cornell Atkinson Center for Sustainability for financial support.
Author information
Authors and affiliations.
Department of Government, Cornell University, Ithaca, NY, USA
Sarah Kreps & Douglas L. Kriner
Injury Prevention Research Center, University of North Carolina, Chapel Hill, NC, USA
Nabarun Dasgupta
Department of Pediatrics, Harvard Medical School, Boston, MA, USA
John S. Brownstein
Computational Epidemiology Lab, Boston Children’s Hospital, Boston, MA, USA
Epidemiology and Biostatistics, University of California, San Francisco, CA, USA
Yulin Hswen
You can also search for this author in PubMed Google Scholar
Contributions
S.K. and D.K. designed the experiment/survey instrument and conducted the statistical analysis. S.K., N.D., J.B., Y.H., and D.K. all contributed to the conceptual design of the research and to the writing of the paper.
Corresponding author
Correspondence to Douglas L. Kriner .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions

About this article
Cite this article.
Kreps, S., Dasgupta, N., Brownstein, J.S. et al. Public attitudes toward COVID-19 vaccination: The role of vaccine attributes, incentives, and misinformation. npj Vaccines 6 , 73 (2021). https://doi.org/10.1038/s41541-021-00335-2
Download citation
Received : 25 January 2021
Accepted : 06 April 2021
Published : 14 May 2021
DOI : https://doi.org/10.1038/s41541-021-00335-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Progress with covid vaccine development and implementation.
- Richard W. Titball
- David I. Bernstein
- Alan D. T. Barrett
npj Vaccines (2024)
A synthesis of evidence for policy from behavioural science during COVID-19
- Kai Ruggeri
- Friederike Stock
- Robb Willer
Nature (2024)
The rapid progress in COVID vaccine development and implementation
- Nicolas V. J. Fanget
npj Vaccines (2022)
COVID-19 Vaccine Acceptability and Financial Incentives among Unhoused People in Los Angeles County: a Three-Stage Field Survey
- Allison D. Rosen
- Jacqueline Beltran
- Chelsea L. Shover
Journal of Urban Health (2022)
The shot, the message, and the messenger: COVID-19 vaccine acceptance in Latin America
- Pablo Argote
- Elena Barham
- Oscar Pocasangre
npj Vaccines (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Quantifying the Importance of COVID-19 Vaccination to Our Future Outlook
Affiliations.
- 1 Department of Health Sciences Research, Mayo Clinic, Rochester, MN. Electronic address: [email protected].
- 2 Department of Health Sciences Research, Mayo Clinic, Rochester, MN.
- 3 Department of Health Sciences Research, Mayo Clinic, Rochester, MN; Robert D. Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, MN.
- 4 Division of Health Care Policy and Research, Mayo Clinic, Rochester, MN; Robert D. Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, MN.
- 5 Department of Anesthesiology, Mayo Clinic, Rochester, MN; Division of Critical Care Medicine, Mayo Clinic, Rochester, MN.
- 6 Biostatistics, Mayo Clinic, Phoenix, AZ.
- 7 Department of Pulmonary and Critical Care Medicine.
- 8 Department of Neurology.
- 9 Department of Neurologic Surgery, Mayo Clinic, Rochester, MN.
- 10 Department of Transplant Critical Care Medicine, Mayo Clinic, Rochester, MN.
- 11 Division of Infectious Diseases, Mayo Clinic, Rochester, MN.
- 12 Department of Health Sciences Research, Mayo Clinic, Rochester, MN; Department of Medicine, Mayo Clinic, Rochester, MN.
- 13 Department of Gynecologic Surgery, Mayo Clinic College of Medicine, Rochester, MN.
- 14 Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN.
- PMID: 34218862
- PMCID: PMC8075811
- DOI: 10.1016/j.mayocp.2021.04.012
Predictive models have played a critical role in local, national, and international response to the COVID-19 pandemic. In the United States, health care systems and governmental agencies have relied on several models, such as the Institute for Health Metrics and Evaluation, Youyang Gu (YYG), Massachusetts Institute of Technology, and Centers for Disease Control and Prevention ensemble, to predict short- and long-term trends in disease activity. The Mayo Clinic Bayesian SIR model, recently made publicly available, has informed Mayo Clinic practice leadership at all sites across the United States and has been shared with Minnesota governmental leadership to help inform critical decisions during the past year. One key to the accuracy of the Mayo Clinic model is its ability to adapt to the constantly changing dynamics of the pandemic and uncertainties of human behavior, such as changes in the rate of contact among the population over time and by geographic location and now new virus variants. The Mayo Clinic model can also be used to forecast COVID-19 trends in different hypothetical worlds in which no vaccine is available, vaccinations are no longer being accepted from this point forward, and 75% of the population is already vaccinated. Surveys indicate that half of American adults are hesitant to receive a COVID-19 vaccine, and lack of understanding of the benefits of vaccination is an important barrier to use. The focus of this paper is to illustrate the stark contrast between these 3 scenarios and to demonstrate, mathematically, the benefit of high vaccine uptake on the future course of the pandemic.
Copyright © 2021. Published by Elsevier Inc.
- COVID-19 / epidemiology
- COVID-19 / prevention & control*
- COVID-19 Vaccines*
- Forecasting
- Hospitalization / statistics & numerical data
- Hospitalization / trends
- United States / epidemiology
- COVID-19 Vaccines

- Find a Doctor
- Conditions & Treatments
- Pay Your Bill
- Patient Stories
- For Referring Providers
- Patient Login
- Make an Appointment
- Refer a Patient

573-882-4141
Conditions & Treatments

What are the Benefits of Getting the COVID-19 Vaccine?

Although experts are still learning a lot about the COVID-19 vaccines, there are some clear benefits to getting vaccinated.
If you’ve already received the vaccine, great job! Share these facts with others who might be hesitant. If you’re unsure whether the vaccine is right for you, consider these four benefits the vaccine could provide you and your loved ones.
The vaccine reduces your risk of infection.
Once you receive your first shot, your body begins producing antibodies to the coronavirus. These antibodies help your immune system fight the virus if you happen to be exposed, so it reduces your chance of getting the disease. There are four vaccines available for use in the United States, and they are all effective in preventing infection. Learn more about effectiveness .
It’s true that you can still become infected after being vaccinated, but once more of the population is vaccinated, those chances are further reduced thanks to something called herd immunity. So getting vaccinated not only reduces your chance of being infected, it also contributes to community protection, reducing the likelihood of virus transmission.
The vaccine can help your unborn baby or newborn.
Studies have found that expectant mothers who receive the COVID-19 vaccine create antibodies to the virus and pass those to their unborn baby through the placenta. Mothers were also shown to pass antibodies to their newborns through breast milk. This suggests those newborns have some immunity to the virus, which is especially important as young children cannot get the vaccine. Learn more about vaccine considerations for pregnant and nursing women .
The vaccine protects against severe illness.
During studies, the four vaccines — Johnson & Johnson, Moderna, Novavax and Pfizer — have shown to be effective at preventing severe illness from COVID-19. So if you are vaccinated and become infected, you are very unlikely to become severely ill.
The CDC tracks confirmed COVID-19 hospitalizations by vaccination status. For adults 18 and older, unvaccinated people were 3.5 times more likely to be hospitalized than fully vaccinated people. Among adolescents between ages 12-17, unvaccinated people are 2.1 times more likely to be hospitalized than fully vaccinated people.
Read more stories like this
Copyright © 2023 — Curators of the University of Missouri . All rights reserved. DMCA and other copyright information . Equal Opportunity/Access/Affirmative Action/Pro Disabled & Veteran Employer . For website information, contact the MU Health Care Communications . Disclaimer
Persuasive Essay Guide
Persuasive Essay About Covid19
How to Write a Persuasive Essay About Covid19 | Examples & Tips
11 min read

People also read
A Comprehensive Guide to Writing an Effective Persuasive Essay
200+ Persuasive Essay Topics to Help You Out
Learn How to Create a Persuasive Essay Outline
30+ Free Persuasive Essay Examples To Get You Started
Read Excellent Examples of Persuasive Essay About Gun Control
Crafting a Convincing Persuasive Essay About Abortion
Learn to Write Persuasive Essay About Business With Examples and Tips
Check Out 12 Persuasive Essay About Online Education Examples
Persuasive Essay About Smoking - Making a Powerful Argument with Examples
Are you looking to write a persuasive essay about the Covid-19 pandemic?
Writing a compelling and informative essay about this global crisis can be challenging. It requires researching the latest information, understanding the facts, and presenting your argument persuasively.
But don’t worry! with some guidance from experts, you’ll be able to write an effective and persuasive essay about Covid-19.
In this blog post, we’ll outline the basics of writing a persuasive essay . We’ll provide clear examples, helpful tips, and essential information for crafting your own persuasive piece on Covid-19.
Read on to get started on your essay.
- 1. Steps to Write a Persuasive Essay About Covid-19
- 2. Examples of Persuasive Essay About Covid19
- 3. Examples of Persuasive Essay About Covid-19 Vaccine
- 4. Examples of Persuasive Essay About Covid-19 Integration
- 5. Examples of Argumentative Essay About Covid 19
- 6. Examples of Persuasive Speeches About Covid-19
- 7. Tips to Write a Persuasive Essay About Covid-19
- 8. Common Topics for a Persuasive Essay on COVID-19
Steps to Write a Persuasive Essay About Covid-19
Here are the steps to help you write a persuasive essay on this topic, along with an example essay:
Step 1: Choose a Specific Thesis Statement
Your thesis statement should clearly state your position on a specific aspect of COVID-19. It should be debatable and clear. For example:
Step 2: Research and Gather Information
Collect reliable and up-to-date information from reputable sources to support your thesis statement. This may include statistics, expert opinions, and scientific studies. For instance:
- COVID-19 vaccination effectiveness data
- Information on vaccine mandates in different countries
- Expert statements from health organizations like the WHO or CDC
Step 3: Outline Your Essay
Create a clear and organized outline to structure your essay. A persuasive essay typically follows this structure:
- Introduction
- Background Information
- Body Paragraphs (with supporting evidence)
- Counterarguments (addressing opposing views)
Step 4: Write the Introduction
In the introduction, grab your reader's attention and present your thesis statement. For example:
Step 5: Provide Background Information
Offer context and background information to help your readers understand the issue better. For instance:
Step 6: Develop Body Paragraphs
Each body paragraph should present a single point or piece of evidence that supports your thesis statement. Use clear topic sentences, evidence, and analysis. Here's an example:
Step 7: Address Counterarguments
Acknowledge opposing viewpoints and refute them with strong counterarguments. This demonstrates that you've considered different perspectives. For example:
Step 8: Write the Conclusion
Summarize your main points and restate your thesis statement in the conclusion. End with a strong call to action or thought-provoking statement. For instance:
Step 9: Revise and Proofread
Edit your essay for clarity, coherence, grammar, and spelling errors. Ensure that your argument flows logically.
Step 10: Cite Your Sources
Include proper citations and a bibliography page to give credit to your sources.
Remember to adjust your approach and arguments based on your target audience and the specific angle you want to take in your persuasive essay about COVID-19.

Paper Due? Why Suffer? That's our Job!
Examples of Persuasive Essay About Covid19
When writing a persuasive essay about the Covid-19 pandemic, it’s important to consider how you want to present your argument. To help you get started, here are some example essays for you to read:
Check out some more PDF examples below:
Persuasive Essay About Covid-19 Pandemic
Sample Of Persuasive Essay About Covid-19
Persuasive Essay About Covid-19 In The Philippines - Example
If you're in search of a compelling persuasive essay on business, don't miss out on our “ persuasive essay about business ” blog!
Examples of Persuasive Essay About Covid-19 Vaccine
Covid19 vaccines are one of the ways to prevent the spread of Covid-19, but they have been a source of controversy. Different sides argue about the benefits or dangers of the new vaccines. Whatever your point of view is, writing a persuasive essay about it is a good way of organizing your thoughts and persuading others.
A persuasive essay about the Covid-19 vaccine could consider the benefits of getting vaccinated as well as the potential side effects.
Below are some examples of persuasive essays on getting vaccinated for Covid-19.
Covid19 Vaccine Persuasive Essay
Persuasive Essay on Covid Vaccines
Interested in thought-provoking discussions on abortion? Read our persuasive essay about abortion blog to eplore arguments!
Examples of Persuasive Essay About Covid-19 Integration
Covid19 has drastically changed the way people interact in schools, markets, and workplaces. In short, it has affected all aspects of life. However, people have started to learn to live with Covid19.
Writing a persuasive essay about it shouldn't be stressful. Read the sample essay below to get idea for your own essay about Covid19 integration.
Persuasive Essay About Working From Home During Covid19
Searching for the topic of Online Education? Our persuasive essay about online education is a must-read.
Examples of Argumentative Essay About Covid 19
Covid-19 has been an ever-evolving issue, with new developments and discoveries being made on a daily basis.
Writing an argumentative essay about such an issue is both interesting and challenging. It allows you to evaluate different aspects of the pandemic, as well as consider potential solutions.
Here are some examples of argumentative essays on Covid19.
Argumentative Essay About Covid19 Sample
Argumentative Essay About Covid19 With Introduction Body and Conclusion
Looking for a persuasive take on the topic of smoking? You'll find it all related arguments in out Persuasive Essay About Smoking blog!
Examples of Persuasive Speeches About Covid-19
Do you need to prepare a speech about Covid19 and need examples? We have them for you!
Persuasive speeches about Covid-19 can provide the audience with valuable insights on how to best handle the pandemic. They can be used to advocate for specific changes in policies or simply raise awareness about the virus.
Check out some examples of persuasive speeches on Covid-19:
Persuasive Speech About Covid-19 Example
Persuasive Speech About Vaccine For Covid-19
You can also read persuasive essay examples on other topics to master your persuasive techniques!
Tips to Write a Persuasive Essay About Covid-19
Writing a persuasive essay about COVID-19 requires a thoughtful approach to present your arguments effectively.
Here are some tips to help you craft a compelling persuasive essay on this topic:
Choose a Specific Angle
Start by narrowing down your focus. COVID-19 is a broad topic, so selecting a specific aspect or issue related to it will make your essay more persuasive and manageable. For example, you could focus on vaccination, public health measures, the economic impact, or misinformation.
Provide Credible Sources
Support your arguments with credible sources such as scientific studies, government reports, and reputable news outlets. Reliable sources enhance the credibility of your essay.
Use Persuasive Language
Employ persuasive techniques, such as ethos (establishing credibility), pathos (appealing to emotions), and logos (using logic and evidence). Use vivid examples and anecdotes to make your points relatable.
Organize Your Essay
Structure your essay involves creating a persuasive essay outline and establishing a logical flow from one point to the next. Each paragraph should focus on a single point, and transitions between paragraphs should be smooth and logical.
Emphasize Benefits
Highlight the benefits of your proposed actions or viewpoints. Explain how your suggestions can improve public health, safety, or well-being. Make it clear why your audience should support your position.
Use Visuals -H3
Incorporate graphs, charts, and statistics when applicable. Visual aids can reinforce your arguments and make complex data more accessible to your readers.
Call to Action
End your essay with a strong call to action. Encourage your readers to take a specific step or consider your viewpoint. Make it clear what you want them to do or think after reading your essay.
Revise and Edit
Proofread your essay for grammar, spelling, and clarity. Make sure your arguments are well-structured and that your writing flows smoothly.
Seek Feedback
Have someone else read your essay to get feedback. They may offer valuable insights and help you identify areas where your persuasive techniques can be improved.
Tough Essay Due? Hire Tough Writers!
Common Topics for a Persuasive Essay on COVID-19
Here are some persuasive essay topics on COVID-19:
- The Importance of Vaccination Mandates for COVID-19 Control
- Balancing Public Health and Personal Freedom During a Pandemic
- The Economic Impact of Lockdowns vs. Public Health Benefits
- The Role of Misinformation in Fueling Vaccine Hesitancy
- Remote Learning vs. In-Person Education: What's Best for Students?
- The Ethics of Vaccine Distribution: Prioritizing Vulnerable Populations
- The Mental Health Crisis Amidst the COVID-19 Pandemic
- The Long-Term Effects of COVID-19 on Healthcare Systems
- Global Cooperation vs. Vaccine Nationalism in Fighting the Pandemic
- The Future of Telemedicine: Expanding Healthcare Access Post-COVID-19
In search of more inspiring topics for your next persuasive essay? Our persuasive essay topics blog has plenty of ideas!
To sum it up,
You have read good sample essays and got some helpful tips. You now have the tools you needed to write a persuasive essay about Covid-19. So don't let the doubts stop you, start writing!
If you need professional writing help, don't worry! We've got that for you as well.
MyPerfectWords.com is a professional persuasive essay writing service that can help you craft an excellent persuasive essay on Covid-19. Our experienced essay writer will create a well-structured, insightful paper in no time!
So don't hesitate and place your ' write my essay online ' request today!
Frequently Asked Questions
Are there any ethical considerations when writing a persuasive essay about covid-19.
Yes, there are ethical considerations when writing a persuasive essay about COVID-19. It's essential to ensure the information is accurate, not contribute to misinformation, and be sensitive to the pandemic's impact on individuals and communities. Additionally, respecting diverse viewpoints and emphasizing public health benefits can promote ethical communication.
What impact does COVID-19 have on society?
The impact of COVID-19 on society is far-reaching. It has led to job and economic losses, an increase in stress and mental health disorders, and changes in education systems. It has also had a negative effect on social interactions, as people have been asked to limit their contact with others.
Write Essay Within 60 Seconds!

Caleb S. has been providing writing services for over five years and has a Masters degree from Oxford University. He is an expert in his craft and takes great pride in helping students achieve their academic goals. Caleb is a dedicated professional who always puts his clients first.

Paper Due? Why Suffer? That’s our Job!
Keep reading

- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 24, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
COVID-19 vaccine effectiveness and fewer common side effects most important factors in whether adults choose vaccination
by European Society of Clinical Microbiology and Infectious Diseases
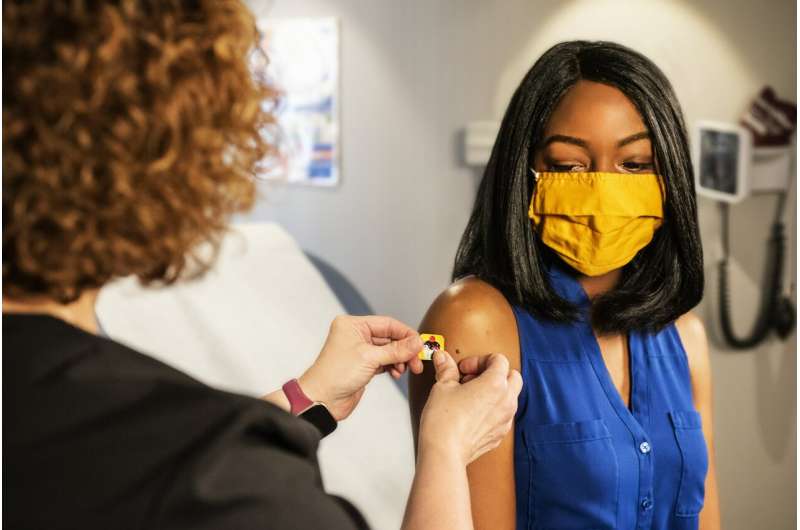
Concerns about the common side effects of COVID-19 vaccines and their effectiveness are key to determining whether adults in Germany and the UK choose to get vaccinated against the virus, according to new research being presented at this year's ESCMID Global Congress (formerly ECCMID) in Barcelona, Spain (27-30 April).
In contrast, timing of COVID-19 and influenza vaccines and their type have little influence on people's willingness to get vaccinated in both countries.
The survey and discrete choice experiment involved 1,000 adults (500 from Germany and 500 from the UK, with 250 who were fully vaccinated and 250 who were willing to receive a COVID-19 vaccine but were not up to date on their vaccine in each country. These "vaccine-hesitant" people included 230/250 participants from the UK who were partially vaccinated, and 20 who were unvaccinated, respectively; and 226/250 from Germany who were partially vaccinated, and 24 who were unvaccinated, respectively. Fully vaccinated was defined as participants who believed they were fully vaccinated, having received the initial primary series and additional COVID-19 booster doses. Un/partially vaccinated consisted of those who did not receive all primary series or booster doses available to them.
The study by Professor Jeffrey Lazarus from the Barcelona Institute for Global Health in Spain and international colleagues offers important insights into the drivers of behavior that might boost COVID-19 vaccine uptake, especially in those who are vaccine-hesitant.
"With vaccination fatigue growing alongside vaccine disinformation and hesitancy, our research suggests that educating the public about the benefits of vaccines, with messaging focusing largely on vaccine safety and efficacy, will get more people to roll up their sleeves," says Professor Lazarus. "What's more, a better understanding of the importance of the perceptions of possible vaccine side effects will be essential to developing more appropriate messaging to reduce vaccine hesitancy."
Despite medical evidence of the importance and safety of COVID-19 vaccines, some of the public is hesitant and/or opposed to COVID-19 vaccination. Understanding the public's preferences for different COVID-19 vaccines and drivers of vaccine hesitancy is critical for implementing effective strategies to increase vaccine uptake.
To identify the most important factors when choosing to be vaccinated against COVID-19, the researchers first conducted an online survey of 1,000 adults in Germany (average age 47 years; 50% women) and the UK (average age 50 years; 49% women) between July and August 2023, to find out their preferences and experiences with SARS-CoV-2 infections and COVID-19 vaccines.
Participants were recruited using a specialist patient recruitment agency called Global Perspectives (GP). GP identified eligible participants through their panel databases, as well as through support groups , word of mouth, internet advertising, email blasts, and social media. Recruitment messages were used to support this process. The sample was stratified by country, vaccination status, and disease risk status.
Then the study went a step further to examine which of six attributes of a COVID-19 vaccine were the most important in making a decision to be vaccinated or not—vaccine type (mRNA or protein), level of protection against COVID infection, level of protection against severe COVID-19 disease, chance of experiencing common side effects (i.e., reactogenicity events), risk of serious side effects (i.e., myocarditis/pericarditis), or joint and separate administration of influenza and COVID-19 vaccines.
This was done by giving each participant an illustrative choice task in which they viewed 11 unique vaccine profiles with a different combination of the six vaccine attributes. Participants were asked to choose between two different vaccine profiles at a time, and to pick which vaccine they would choose if there were only those two vaccine options, or they could select that they would prefer neither of the two options. Using this approach, researchers were able to understand the relative importance of each attribute to each participant.
In the baseline survey that asked participants how they felt about different attributes individually, 59% of German and 46% of UK respondents reported being moderately to extremely worried about COVID-19. More than three-quarters of those surveyed in both countries considered that being able to choose a COVID-19 vaccine to be moderately to extremely important. Additionally, around two-thirds of German and around half (45%) of UK participants reported that they were moderately to extremely worried about serious vaccine side effects.
The survey results differed substantially between the vaccinated and unvaccinated/partially vaccinated groups (based on ranking moderately to severely combined)—while concerns about COVID-19 were higher in the vaccinated group, having a choice of vaccine, vaccine type and concerns of side effects were all rated higher in the unvaccinated/partially vaccinated groups, with the trend followed in both countries.
However, when these attributes were put together in a combined profile in the discrete choice experiment (i.e., when considered together with efficacy, side effects, timing, etc.), the results showed that the most important considerations when deciding whether to be vaccinated in respondents from both countries were vaccine effectiveness against COVID-19 infection and severe disease, followed by common side effects.
Interestingly, the relative importance of common side effects was nearly 10% higher among Germany participants than their UK counterparts, while the importance of serious side effects was less than half as important as common side effects in both countries.
The researchers' next steps involve examining the rate at which participants experience common side effects and the impact on individuals' activities.
The authors note several limitations, including that the study used self-reported/stated preferences that might not always match preferences/decision-making in real-world situations.
Explore further
Feedback to editors

Study finds vitamin D alters mouse gut bacteria to give better cancer immunity
50 minutes ago

Food in sight? The liver is ready in minutes: Study shows how adapting sugar metabolism starts in the brain

Cancer drug trial provides lessons for future

Treatment for deadly superbug C. diff may be weakening
2 hours ago

Blocking gene may halt growth of breast cancer cells

Study finds RNA modification is responsible for disruption of mitochondrial protein synthesis in Alzheimer's disease

Nanomaterial that mimics proteins could be basis for new neurodegenerative disease treatments

How immune cells communicate to fight viruses: New mouse model enables identification of chemokine producers and sensors
3 hours ago

Differentiating cerebral cortical neurons to decipher molecular mechanisms of neurodegeneration

National trial safely scales back prescribing of a powerful antipsychotic for the elderly
Related stories.

Study identifies factors that shape vaccine preferences
May 4, 2022
Coronavirus vaccine Q&A: Are they safe? Are they effective? Will you need a booster?
Apr 14, 2021
One-third of U.S. adults continue to be hesitant about COVID-19 vaccines
Mar 3, 2021

Work to be done before people feel ready for COVID-19 vaccine, study finds
Oct 13, 2020

People were hesitant about rather than opposed to the COVID-19 vaccine, study finds
May 10, 2022

Lack of knowledge led to COVID-19 vaccination hesitancy during pregnancy
Apr 25, 2022
Recommended for you

Researchers publish final results of key clinical trial for gene therapy for sickle cell disease
5 hours ago

Alteration of brain network condition could predict painful vaso-occlusive crisis in patients with sickle cell disease
23 hours ago

New algorithm could provide early warning for asthma attacks
Apr 24, 2024

Solving the riddle of the sphingolipids in coronary artery disease
21 hours ago

COVID-19 pandemic alters view that doctors are obligated to provide care: Study
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

April 22, 2024
Professor honored for paper on willingness to get the COVID vaccine
Jeanine guidry, an affiliate faculty member, says improving our understanding of why people may or may not vaccinate remains ‘of great importance.’, share this story.
- Share on Twitter
- Share on Facebook
- Share on LinkedIn
By VCU News staff
Jeanine Guidry, Ph.D., an affiliate faculty member at the Department of Social and Behavioral Sciences in the School of Population Health and at the Richard T. Robertson School of Media and Culture in the College of Humanities and Sciences at Virginia Commonwealth University, is the recipient of the APIC/AJIC Award for Publication Excellence for her study “ Willingness to Get the COVID Vaccine With and Without Emergency Use Approval .”
The award recognizes an author who has published an article in the American Journal of Infection Control which was widely read and cited during the previous year.
Guidry's study, conducted at VCU with colleagues Linnea Laestadius , Ph.D.; Emily Vraga , Ph.D.; Carrie Miller , Ph.D.; Paul Perrin , Ph.D.; Candace Burton , Ph.D.; Mark Ryan , M.D.; Bernard Fuemmeler , Ph.D.; and Kellie Carlyle , Ph.D., involved a July 2020 survey of 788 adults that found that 59.9% of respondents were definitely or probably planning to receive a future coronavirus vaccine, but fewer than half of respondents planned to get it under emergency use authorization. It also found that white people were more likely than Black people to be vaccinated.
“With the ongoing presence of COVID-19 in our society, the risk of long COVID and the long-term threat of vaccine hesitancy, continuing to improve our understanding of why people may or may not vaccinate is of great importance,” said Guidry, who is now a faculty member at Tilburg University in the Netherlands. “My co-authors and I are grateful for APIC/AJIC’s support and commitment to deeper understanding in this field.”
Subscribe to VCU News
Subscribe to VCU News at newsletter.vcu.edu and receive a selection of stories, videos, photos, news clips and event listings in your inbox.
Related stories

Evangelical Christians were less likely to get COVID-19 vaccine after conversations with faith leaders

Patients undergoing treatment for cancer more susceptible to COVID-19 misinformation, study finds

Variants, misinformation, vaccine hesitancy: Jeanine Guidry’s work is more crucial than ever
Most popular

April 11, 2024
VCU celebrates ‘one of the best’ years for its growing and renowned research enterprise

April 16, 2024
A new home for creativity and collaboration at VCU

April 15, 2024
Richmonders, cast a ballot in a special election this month – and you can wear the winner this fall

April 17, 2024
Class of 2024: Deja Cooke returned to college to pursue a dream of a career in criminal justice
Latest headlines

April 25, 2024
Class of 2024: Lasting friendships put Angela-Marie Rone on a path to social work

VCU students and alums deliver the entrepreneurial pitch at Demo Day

Capital News Service and VCU InSight, two powerful proving grounds for journalism students, reach notable milestones

April 24, 2024
Class of 2024: Mohammed AlAwadh delves into the intricacies of drug development
- CATEGORIES OF CAUSATION
- EXPLORE CONCLUSIONS
- SHOULDER INJURIES
- GET THE REPORT
Consensus Study Digital Resource | APRIL 2024
Evidence Review of the Adverse Effects of COVID-19 Vaccination
A committee of experts reviewed hundreds of scientific research papers to make conclusions about the causal relationships between covid-19 vaccines and specific serious health conditions..
The report was requested by the federal Health Resources and Services Administration Division of Injury Compensation Programs (DICP), which houses the Vaccine Injury Compensation Program and the Countermeasures Injury Compensation Program. DICP uses reports from the National Academies as an important scientific contribution to its compensation decisions. This report includes research published before mid-October 2023; future research will bring more clarity to the conclusions as researchers continue to study these important vaccines.
The National Academies committee also investigated the causal relationships between intramuscular vaccine administration and shoulder injuries.
- Learn more about Shoulder Injuries from Intramuscular Administration of Vaccines
Categories of Causation
These definitions of causation describe the degrees of certainty that a condition has a causal relationship to a vaccine. The committee adopted the categories and definitions of causation used by previous National Academies committees. The conclusions are asymmetric. A conclusion that there is no causal relationship can never be as certain as a conclusion that there is a causal relationship.
Read more about how the committee assessed causality in Chapter 1
The totality of the evidence suggests that vaccination can cause this harm. Further research is unlikely to lead to a different conclusion.
The totality of the evidence suggests that vaccination might cause this harm, but meaningful uncertainty remains. Studies that better minimize bias and confounding, and studies that estimate effects more precisely, could lead to a different conclusion.
The available evidence is too limited (e.g., few studies in humans, biased, imprecise) or inconsistent to draw meaningful conclusions in support of or against causality. Future research could lead to a different conclusion. This conclusion also applies to situations in which no studies were identified.
The totality of the evidence suggests that vaccination does not cause this harm, but meaningful uncertainty remains. The committee acknowledges that individual causal effects are difficult to ascertain and the limitations of applying population average effects to draw conclusions about the causes of specific events in individual people. For example, it is possible that both vaccination and disease cause certain harms. Thus, (1) an event could be more common in an unvaccinated than a vaccinated population and (2) some of the events in the vaccinated population could be caused by vaccination. Research demonstrating a clear mechanism of action, or research demonstrating increased risk among vaccinated people compared with unvaccinated people, could lead to a different conclusion.
Conclusions by Adverse Effect, Vaccine, or Causal Category
Select a vaccine, and/or an adverse effect, and/or a causal category to view conclusions on evidence linking each of the four COVID vaccines to each of the adverse effects and their level of causation.
Learn more about the vaccines
RESET FILTERS
Viewing All Effects
Cardiac and Vascular Events
- Deep Vein Thrombosis
- Hemorrhagic Stroke
- Ischemic Stroke
- Myocardial Infarction
- Myocarditis
- Pericarditis
- Pulmonary Embolism
- Venous Thromboembolism
- Female Infertility
Hearing Conditions
- Sensorineural Hearing Loss
Immune-Mediated Events
- Capillary Leak Syndrome
- Immune Thrombocytopenic Purpura
- Thrombosis with Thrombocytopenia Syndrome
Neurologic Events
- Bell’s Palsy
- Chronic Headaches
- Chronic Inflammatory Demyelinating Polyneuropathy
- Guillain-Barré Syndrome
- Postural Orthostatic Tachycardia Syndrome
- Transverse Myelitis
- Sudden Death
About COVID-19 Vaccines Click on a vaccine name to read more

The Pfizer-BioNTech COVID-19 vaccine contains mRNA packaged inside a lipid nanoparticle. After injection, the mRNA enters cells and instructs them to produce a portion of the spike protein normally found on the surface of the SARS-CoV-2 virus. This leads the body to generate an immune response against the spike protein that protects the individual if they are later exposed to the virus.
It was granted Emergency Use Authorization (EUA) from the FDA on December 11, 2020. Full approval for individuals 16 years and older was granted on August 23, 2021. For pediatric use (ages 12-15), full approval was granted on May 10, 2021. For pediatric use (ages 5-11), full approval was granted on October 29, 2021.
The Moderna COVID-19 vaccine is an mRNA vaccine that contains mRNA packaged inside a lipid nanoparticle. After injection, the mRNA enters cells and instructs them to produce a portion of the spike protein normally found on the surface of the SARS-CoV-2 virus. This leads the body to generate an immune response against the spike protein that protects the individual if they are later exposed to the virus.
It was granted Emergency Use Authorization (EUA) by the FDA on December 18, 2020. Full approval for use in individuals 18 years and older was granted on August 23, 2021. For pediatric use (ages 12-17), full approval was granted on October 29, 2021.
BNT162b2 and mRNA-1273 COVID-19 vaccines are very similar in how they work with minor differences in how they are constructed. Neither vaccine integrates into the human genome.
mRNA vaccines, like other vaccines, are designed to trigger an immune response in the body without causing COVID infection.

The Janssen Pharmaceuticals’ (J&J) viral vector vaccine delivers genetic material (DNA) inside a modified (harmless) adenovirus. The DNA contains instructions for cells to produce a portion of the spike protein normally found on the surface of the SARS-CoV-2 virus. This leads the body to generate an immune response against the spike protein that protects the individual if they are later exposed to the virus.
It was granted Emergency Use Authorization (EUA) by the FDA on February 27, 2021. However, its EUA was revoked on July 12, 2021, due to concerns about a rare side effect involving blood clots.
Adenovirus vector, like other vaccines, are designed to trigger an immune response in the body without causing COVID infection.

The Novavax COVID-19 vaccine is a protein subunit vaccine that contains portions of the spike protein, a protein that is normally found on the surface of the SARS-CoV-2 virus. The vaccine also contains Matrix-M® (an “adjuvant”) that boosts the body’s immune response. This immune response against the spike protein then protects the individual if they are later exposed to the virus.
It was granted Emergency Use Authorization (EUA) by the FDA on July, 13 2022.
Protein subunit vaccines, like other vaccines, are designed to trigger an immune response in the body without causing COVID infection.
- Read Report
- Download Report PDF
- Download Report Highlights
- Learn More About the Project
Evidence Review of the Adverse Effects of COVID-19 Vaccination and Intramuscular Vaccine Administration
Vaccines are a public health success story, as they have prevented or lessened the effects of many infectious diseases. To address concerns around potential vaccine injuries, the Health Resources and Services Administration (HRSA) administers the Vaccine Injury Compensation Program (VICP) and the Countermeasures Injury Compensation Program (CICP), which provide compensation to those who assert that they were injured by routine vaccines or medical countermeasures, respectively. The National Academies of Sciences, Engineering, and Medicine have contributed to the scientific basis for VICP compensation decisions for decades.
HRSA asked the National Academies to convene an expert committee to review the epidemiological, clinical, and biological evidence about the relationship between COVID-19 vaccines and specific adverse events, as well as intramuscular administration of vaccines and shoulder injuries. This report outlines the committee findings and conclusions.
Read Full Description
- Digital Resource: Evidence Review of the Adverse Effects of COVID-19 Vaccination
- Digital Resource: Evidence Review of Shoulder Injuries from Intramuscular Administration of Vaccines
- Press Release
Recent News
Space Environmentalism: Toward a Circular Economy Approach for Orbital Space
The Challenge of Predicting Climate Migration
Celebrating Earth Day with the National Academies
Rohr Named U.S. Winner of Frontiers Planet Prize
- Load More...
COVID-19 vaccination performance of the U.S. states: a hybrid model of DEA and ensemble machine learning methods
- Original Research
- Published: 23 April 2024
Cite this article

- Ozlem Cosgun ORCID: orcid.org/0000-0003-1352-1764 1 ,
- Gamze Ogcu Kaya 2 &
- Cumhur Cosgun 3
Vaccination is seen as the most promising one among the efforts to stop COVID-19 and the U.S. government has given great importance to vaccination. However, which states have performed well in administering COVID-19 vaccines and which have not is an open significant question. Another important question is what makes a state more successful than others when evaluating vaccination performance. To answer both of these questions, we proposed a hybrid method that consists of Data Envelopment Analysis and Ensemble ML Methods. DEA was employed to find the vaccine efficiency of the states using the data aggregated from counties. ML techniques are then applied for the vaccine efficiency prediction and understanding the significance of the variables in the prediction. Our findings revealed that there are considerable differences between U.S. States’ performance and only 16 of the states were efficient in terms of their vaccination performance. Furthermore, Light GBM, Random Forest and XGBoost models provided the best results among the five ensemble machine learning methods that were applied. Therefore, an information fusion-based sensitivity analysis method was used to combine the results of each ML technique and ascertain the relative significance of the factors in the prediction of the efficiency. As the findings for factors affecting vaccination performance, percentage of vaccine doses delivered and COVID-19 deaths were found to be the major influential factors on the prediction of the efficiency and percentage of fully vaccinated people, the number of healthcare employees and human development index followed these variables.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Ardabili, S. F., Mosavi, A., Ghamisi, P., Ferdinand, F., Varkonyi-Koczy, A. R., Reuter, U., Rabczuk, T., & Atkinson, P. M. (2020). COVID-19 outbreak prediction with machine learning. Algorithms , 13 (10), 249.
Article Google Scholar
Basri, A., & Arif, M. (2021). Classification of seizure types using Random Forest Classifier. Advances in Science and Technology Research Journal , 15 (3), 167–178. https://doi.org/10.12913/22998624/140542 .
Batista, A. F. M., Miraglia, J. L., Donato, T. H. R., & Filho, A. D. P. (2021). COVID-19 diagnosis prediction in emergency care patients: A machine learning approach. medRxiv . https://doi.org/10.1101/2020.04.04.20052092 .
Brigato, L., & Iocchi, L. (2021). On the Effectiveness of Deep Ensembles for Small Data Tasks, International Conference on Learning Representations .
Brownlee, J. (2020). How to Develop a Light Gradient Boosted Machine (LightGBM) Ensemble. Accessed at September 28, 2021. https://machinelearningmastery.com/light-gradient-boosted-machine-lightgbm-ensemble/ .
U.S. Census Bureau (2023). County Population Totals and Components of Change: 2020–2022. https://www.census.gov/data/tables/time-series/demo/popest/2020s-counties-total.html .
Centers for Disease Control and Prevention (2021a). Cases and Deaths in the U.S., https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/us-cases-deaths . Accessed September, 2021.
Centers for Disease Control and Prevention (2021b). Cases and Deaths among Healthcare Personnel, https://covid.cdc.gov/covid-data-tracker/#health-care-personnel , Accessed September, 2021.
Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report. (2021c). Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥ 65 Years — COVID-NET, 13 States, February–April 2021, 70(32);1088–1093, https://www.cdc.gov/mmwr/volumes/70/wr/mm7032e3.htm .
Centers for Disease Control and Prevention. (2021d). COVID-19 risks and vaccine information for older adults .:text=The%20risk%20increases%20for%20people,having%20certain%20underlying%20medical%20conditions. https://www.cdc.gov/aging/covid19/covid19-older-adults.html#:~ .
Centers for Disease Control and Prevention (2023). COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-delivered-total .
Charnes, A., Cooper, W. W., & Rhodes, E. (1978). Measuring the efficiency of decision-making units. European Journal of Operational Research , 429–443.
Chen, Y., Wang, J., Zhu, J., Sherman, H. D., & Chou, S. (2019). How the great recession affects performance: A case of Pennsylvania hospitals using DEA. Annals of Operations Research , 77–99.
Chengsheng, T. U., Huacheng, L. I. U., & Bing, X. U. (2017). AdaBoost typical Algorithm and its application research. MATEC Web of Conferences 139, 00222, https://doi.org/10.1051/matecconf/201713900222 .
Colubri, A., Silver, T., Fradet, T., Retzepi, K., Fry, B., & Sabeti, P. (2016). Transforming clinical data into actionable prognosis models: Machine-learning framework and field-deployable app to predict outcome of Ebola patients. Plos Neglected Tropical Diseases .
Darabi, N., Ebrahimvandi, A., Hosseinichimeh, N., & Triantis, K. (2021). A DEA evaluation of US States’ Healthcare Systems in terms of their birth outcomes. Expert Systems with Applications , 182.
Data Central COVID-19 Vaccine tracker. (nd). https://data.news-leader.com/covid-19-vaccine-tracker/ .
Davis, G. (1989). Sensitivity analysis in neural net solutions. IEEE Transactions on Systems Man Cybern , 19 , 1078–1082.
de Oliveira, B. R. B., da Penha Sobral, A. I. G., Marinho, M. L. M., et al. (2021). Determinants of access to the SARS-CoV-2 vaccine: A preliminary approach. Int J Equity Health , 20 , 183. https://doi.org/10.1186/s12939-021-01520-4 .
Delen, D. (2010). A comparative analysis of machine learning techniques for student retention management. Decision Support Systems , 49 , 498–506.
Delen, D., Kuzey, C., & Uyar, A. (2013). Measuring firm performance using financial ratios: A decision tree approach. Expert Systems with Applications , 40 (10), 3970–3983.
U.S. Department of Commerce U.S. Census Bureau (2020). Resident Population for the 50 States, the District of Columbia, and Puerto Rico: 2020 . https://www2.census.gov / programs surveys / decennial/ 2020/ data/apportionment/apportionment-2020-Table 02.pdf.
Devi, S. S., Solanki, V. K., & Laskar, R. H. (2020). Recent advances on big data analysis for malaria prediction and various diagnosis methodologies. Handbook of Data Science Approaches for Biomedical Engineering, 153–184.
Food and Drug Administration (2020b). F DA Briefing Document Pfizer-BioNTech COVID-19 vaccine . https://www.fda.gov/media/144246/download .
Food and Drug Administration (2021). FDA issues Emergency Use Authorization for third COVID-19 vaccine . https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-COVID-19 -vaccine.
Food and Drug Administration (2020a). FDA Briefing Document Moderna COVID-19 vaccine . https://www.fda.gov/media/144434/download .
Fuller, C., Biros, D. P., & Delen, D. (2011). An investigation of data and text mining methods for real world deception detection. Expert Systems with Applications , 38 (7), 8392–8398.
Gallant, A. J., Nicholls, L. A. B., Rasmussen, S., Cogan, N., Young, D., & Williams, L. (2021). Changes in attitudes to vaccination as a result of the COVID-19 pandemic: A longitudinal study of older adults in the UK. PloS One , 16 (12), e0261844. https://doi.org/10.1371/journal.pone.0261844 .
Global Data Lab (2020). Human Development Indices (5.0) https://globaldatalab.org/shdi/shdi/?levels=1%2B4&interpolation=1&extrapolation=0&nearest_real=0
Götz, G., Herold, D., Klotz, P., & Schäfer, J. T. (2021). Efficiency in COVID-19 Vaccination Campaigns—A Comparison across Germany’s Federal States, Vaccines , 9, 788.
Han, S., & Jeong, J. (2020). An weighted CNN Ensemble Model with small amount of data for Bearing Fault diagnosis. Procedia Computer Science , 175 , 88–95.
Hancock, J. T., & Khoshgoftaar, T. M. (2020). CatBoost for big data: An interdisciplinary review. Journal of Big Data , 7 , 94. https://doi.org/10.1186/s40537-020-00369-8 .
Hu, X., Zhang, H., Mei, H., Xiao, D., Li, Y., & Li, M. (2020). Landslide susceptibility mapping using the Stacking Ensemble Machine Learning Method in Lushui, Southwest China. Applied Sciences , 10 (11), 4016.
Human Development Report (2020). https://hdr.undp.org/system/files/documents/technical-notes-calculating-human-development-indices.pdf .
Jeon, S., Lee, Y. F., & Koumi, K. (2022). COVID-19 vaccination: Sociopolitical and Economic Impact in the United States. Epidemiologia (Basel) , 3 (4), 502–517. https://doi.org/10.3390/epidemiologia3040038 PMID: 36416793; PMCID: PMC9680412.
Kaiser Health News (2020). Total Health Care Employment . https://www.kff.org/other/state-indicator/total-health-careemployment/current Timeframe = 0&selectedRows=%7B%22states%22:%7B%22all%22:%7B%7D%7D,22wrapups%22:%7B%22unitedstates%22:%7B%7D%7D%7D&sorodel=%7B%22colId%22:%22Location%22, %22sort%22:%22desc%22%7D.
Kamel, M. A., & Mousa, M. E. S. (2021). Measuring operational efficiency of isolation hospitals during COVID-19 pandemic using data envelopment analysis: A case of Egypt, Benchmarking. An International Journal , 28 (7), 2178–2201.
Google Scholar
Khanday, A. M., Rabani, S., Khan, Q., Rouf, N., & Din, M. M. (2020). Machine learning based approaches for detecting COVID-19 using clinical text data. Int J Inf Technol , 12 (3), 731–739.
Klebanova, T., Poluektova, N., & Rudachenk, O. (2021). Applying of Data Envelopment Analysis to study public administration effectiveness during a pandemic , online: http://ceur-ws.org/Vol-2927/paper8.pdf .
Krauss, C., Do, X. A., & Huck, N. (2017). Deep neural networks, gradient-boosted trees, random forests: Statistical arbitrage on the S&P 500. European Journal of Operational Research , 259 , 689–702.
Kumar, R. (2020). AdaBoost and Gradient Boost– Comparitive Study Between 2 Popular Ensemble Model Techniques. https://www.analyticsvidhya.com/blog/2020/10/adaboost-and-gradient-boost-comparitive-study-between-2-popular-ensemble-model-techniques/ .
Li, J. P., Haq, A. U., Din, S. U., Khan, J., Khan, A., & Saboor, A. (2020). Heart Disease Identification Method using machine learning classification in E-Healthcare. Ieee Access: Practical Innovations, Open Solutions , 8 , 107562–107582.
Machine Learning Mastery (2021). A Gentle Introduction to XGBoost for Applied Machine Learning . https://machinelearningmastery.com/gentle-introduction-xgboost-applied-machine-learning/ .
Malik, Z. A., & Senjiati, I. H. (2020). Efficiency Service Handling COVID 19 the Institute of Zakat by Method of Data Envelopment Analysis (DEA). Journal of Islamic Business and Economic Review , 3(2).
Mariano, E., Torres, B., Almeida, M., Ferraz, D., Rebelatto, D., & Mello, J. C. S. (2021). Brazilian States in the Context of COVID-19 Pandemic: An Index Proposition using Network Data Envelopment Analysis, I.E.E.E. Latin America Transactions, Vol. 19, No. 6.
Maya Clinic (2022). U.S. COVID-19 vaccine tracker: See your state’s progress, https://www.mayoclinic.org/coronavirus-COVID-19 /vaccine-tracker.
Mendola, M., Tonelli, F., Garletti, F. S., Greco, D., Fiscella, M., Cucchi, I., Costa, M. C., & Carrer, P. (2021). COVID-19 impact and vaccine effectiveness among healthcare workers of a large University Hospital in Lombardy, Italy. Med Lav. 23;112(6):453–464. https://doi.org/10.23749/mdl.v112i6.11983 . PMID: 34939623; PMCID: PMC8759047.
Misra, S., & Li, H. (2020). Noninvasive fracture characterization based on the classification of sonic wave travel times. Machine Learning for Subsurface Characterization , 243–287.
Mo, H., Sun, H., Liu, J., & Wei, S. (2019). Developing window behavior models for residential buildings using XGBoost algorithm. Energy and Buildings , 205 , 109564.
Mohanta, K. K., Sharanappa, D. S., & Aggarwal, A. (2021). Efficiency analysis in the management of COVID-19 pandemic in India based on data envelopment analysis, Current Research in Behavioral Sciences, 2.
Mourad, N., Habib, A. M., & Tharwat, A. (2021). Appraising healthcare systems’ efficiency in facing COVID-19 through data envelopment analysis. Decision Science Letters , 10 (3), 301–310.
Nahra, T. A., Mendez, D., & Alexander, J. A. (2009). Employing super-efficiency analysis as an alternative to DEA: An application in outpatient substance abuse treatment. European Journal of Operational Research , 196 , 3, 1097–1106.
Nepomuceno, T. C. C., Silva, W. M. N., Nepomuceno, K. T. C., & Barros, I. K. F. (2020). A DEA-Based complexity of needs Approach for Hospital beds Evacuation during the COVID-19 outbreak. Hindawi Journal of Healthcare Engineering .
Nistor, C. S., Ștefănescu, C. A., & Crișan, A. (2017). Performance through efficiency in the Public Healthcare System– A DEA Approach in an Emergent Country. Studia Universitatis Babes-Bolyai Oeconomica , 62 (1), 31–49.
Nti, I. K., Adekoya, A. F., & Weyori, B. A. (2020). A comprehensive evaluation of ensemble learning for stock-market prediction. Journal of Big Data 7(20).
Onen, Z., & Sayin, S. (2018). Evaluating Healthcare System Efficiency of OECD Countries: A DEA-Based Study, Operations Research Applications in Health Care Management , pp. 141–158.
Ontario Agency for Health Protection and Promotion (Public Health Ontario). (2021). COVID-19 real-world vaccine effectiveness– what we know so far . Queen’s Printer for Ontario.
Parbat, D. (2020). A Python based Support Vector Regression Model for prediction of Covid19 cases in India. Chaos, Solitons, and Fractals , 138.
Prompetchara, E., Ketloy, C., & Palaga, T. (2020). Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific Journal of Allergy and Immunology , 38 (1), 1–9.
Rasolomanana, O. M. (2021). Ensemble Neural Network Using A Small Dataset For The Prediction Of Bankruptcy: Combining Numerical And Textual Data, Hokkaido University Collection of Scholarly and Academic Papers. http://hdl.handle.net/2115/82952 .
Rays, Y. E., & Lemqeddem, H. A. (2021). Data Envelopment Analysis and Malmquist Index Application: Efficiency of Primary Health Care in Morocco and COVID-19. Turkish Journal of Computer and Mathematics Education , 12 (5), 971–983.
Regulatory Affairs Professionals Society (2020). https://www.raps.org/news-and-articles/news-articles/2020/3/COVID-19-vaccine-tracker .
Rustagi, V., Bajaj, M., Tanvi, Sgnh, P., Aggarwal, R., Alajmi, M. F., Hussain, A., Hassan, I., Gingh, A., & Singh, I. K. (2022). Analyzing the Effect of Vaccination over COVID cases and deaths in Asian Countries using machine learning models. Fron Cell Microbiol . https://doi.org/10.3389/fcimb.2021.806265 .
Rustam, F., et al. (2020). COVID-19 future forecasting using supervised machine learning models. Ieee Access: Practical Innovations, Open Solutions , 8 , 101489–101499.
Saltelli, A. (2002). Making best use of model evaluations to compute sensitivity indices. Computer Physics Communications , 145 (2), 280–297.
Samantha, A., Matthew, R., Olivia, P., Liz, H., & Cailey, M. (2020). COVID-19 Risks and Impacts Among Health Care Workers by Race/Ethnicity, Accessed September 27, 2021. https://www.kff.org/report-section/COVID-19-risks-and-impacts-among-health-care-workers-by-race-ethnicity-issue-brief/ .
Samut, P. K., & Cafrı, R. (2015). Analysis of the efficiency determinants of health systems in OECD countries by DEA and panel tobit. Social indicators research (pp. 1–20). Springer.
Sarwar, M. A., Kamal, N., Hamid, W., & Shah, M. A. (2018). Prediction of Diabetes Using Machine Learning Algorithms in Healthcare, 24th International Conference on Automation and Computing , 1–6.
Soares, P., Rocha, J. V., Moniz, M., Gama, A., Laires, P. A., Pedro, A. R., Dias, S., Leite, A., & Nunes, C. (2021). Factors Associated with COVID-19 vaccine hesitancy. Vaccines , 9 , 300. https://doi.org/10.3390/vaccines9030300 .
Stats America (2020). USA States in profile . http://www.statsamerica.org/sip/rank_list.aspx?rank_label=pop46&ct=S09
Su, E. C. Y., Hsiao, C. H., Chen, Y. T., & Yu, S. H. (2021). An examination of COVID-19 mitigation efficiency among 23 countries. Healthcare , 9.
Thammasiri, D., Delen, D., Meesad, P., & Kasap, N. (2014). A critical assessment of imbalanced class distribution problem: The case of predicting freshmen student attrition. Expert Systems with Applications , 41 (2), 321–330.
The New York Times (2021a). Covid in the U.S.: Latest Map and Case Count . https://www.nytimes.com/interactive/2021/us/covid-cases.html .
The New York Times (2021b). See How Vaccinations Are Going in Your County and State . https://www.nytimes.com/interactive/2020/us/COVID-19-vaccine-doses.html .
The Guardian (2021). West Virginia battles Covid surge after failing to build on early vaccine success. https://www.theguardian.com/us-news/2021/sep/15/west-virginia-covid-surge-vaccination .
United Nations, Department of Economic and Social Affairs Population Dynamics (2019). Data Query: Population by age and sex (thousands) . https://population.un.org/wpp/DataQuery/ . Published 2019. Accessed September 27, 2021.
US Cities Demographics (nd). https://public.opendatasoft.com/explore/dataset/us-cities-demographics/table/?refine.state=New+York .
Wu, Z., Zhou, C., Xu, F., & Lou, W. (2020). A CS-AdaBoost-BP model for product quality inspection. Annals of Operations Research . https://doi.org/10.1007/s10479-020-03798-z .
Yadav, M., Perumal, M., & Srinivas, M. (2020). Analysis on Novel Coronavirus (COVID-19) Using Machine Learning Methods, Chaos, Solitons and Fractals .
Zhu, Y., Xie, C., Wang, G., & Yan, X. (2017). Comparison of individual, ensemble and integrated ensemble machine learning methods to predict China’s SME credit risk in supply chain finance. Neural Comput & Applic , 28 , 41–50.
County Health Ranking and Roadmaps. (2023). Health Data . https://www.countyhealthrankings.org/health-data
Download references
Author information
Authors and affiliations.
Department of Information Management and Business Analytics, Montclair State University, 1 Normal Ave, Montclair, NJ, 07043, USA
Ozlem Cosgun
Department of Industrial Engineering, Sampoerna University, Jakarta, 12780, Indonesia
Gamze Ogcu Kaya
Pennsylvania Department of Transportation, Bureau of Construction and Materials, 81 Lab Lane, Harrisburg, PA, 17110-2543, USA
Cumhur Cosgun
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ozlem Cosgun .
Ethics declarations
Ethical approval.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cosgun, O., Ogcu Kaya, G. & Cosgun, C. COVID-19 vaccination performance of the U.S. states: a hybrid model of DEA and ensemble machine learning methods. Ann Oper Res (2024). https://doi.org/10.1007/s10479-024-06008-2
Download citation
Received : 11 November 2021
Accepted : 16 April 2024
Published : 23 April 2024
DOI : https://doi.org/10.1007/s10479-024-06008-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Machine learning
- Ensemble methods
- Find a journal
- Publish with us
- Track your research

IMAGES
COMMENTS
COVID-19 vaccines have undergone, and will continue to undergo, the most intensive safety monitoring in history.. Vaccination around the world. Worldwide, more than 3.19 billion doses of coronavirus vaccine have been administered so far, according to figures compiled by Our World in Data. According to these figures, almost 27% of the world's ...
There are many benefits of getting vaccinated against COVID-19. Prevents serious illness: COVID-19 vaccines available in the United States are safe and effective at protecting people from getting seriously ill, being hospitalized, and dying. A safer way to build protection: Getting a COVID-19 vaccine is a safer, more reliable way to build protection than getting sick with COVID-19.
Please use one of the following formats to cite this article in your essay, paper or report: APA. Moore, Sarah. (2022, January 17). The Importance of Global COVID-19 Vaccination.
The COVID-19 pandemic has led the world to undertake the largest vaccination campaign in human history. In record time, unprecedented scientific and governmental efforts have resulted in the acquisition of immunizers utilizing different technologies (nucleotide acids, viral vectors, inactivated and protein-based vaccines).
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement ...
For some COVID-19 vaccines, two doses are required . It's important to get the second dose if the vaccine requires two doses. For vaccines that require two doses, the first dose presents antigens - proteins that stimulate the production of antibodies - to the immune system for the first time. Scientists call this priming the immune response.
Instead, the most important goal of Covid-19 vaccination should be to provide long-term protection against severe disease, hospitalization, and death from current and future variants.
The fatality rate from Covid-19 based on more than 32 million confirmed cases in the United States is 1.8 percent. Over 245 million doses of Covid vaccines were administered by May 3, and a ...
The focus of this paper is to illustrate the stark contrast between these 3 scenarios and to demonstrate, mathematically, the benefit of high vaccine uptake on the future course of the pandemic. The rapid development and availability of vaccines have had a significant impact on the potential to control the COVID-19 pandemic.
This experience shed light on the need for real-time vaccine safety surveillance and the importance of context in decision making during a pandemic. In a study now reported in the Journal by Barda ...
The COVID-19 vaccines are highly effective, but no vaccine provides 100 per cent protection. Some people will still get ill from COVID-19 after vaccination or pass the virus onto someone else. Therefore, it is important to continue practicing safety precautions to protect yourself and others, including avoiding crowded spaces, physical ...
The COVID-19 vaccination will help keep you from getting the virus. COVID-19 vaccines were evaluated in clinical trials and have been approved because those studies show that the vaccine significantly reduces the probability of contracting the virus. Based on what has been proved about vaccines for other diseases, the COVID-19 vaccine may help ...
Today, 86% of the world's children receive essential, lifesaving vaccines, increasing from around 20% back in 1980. This protects them and their communities against a range of infectious diseases, including measles, diphtheria, tetanus, pertussis (whooping cough), hepatitis B and polio. The number of children paralysed by polio has been ...
The effectiveness of the mRNA vaccines in preventing COVID-19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. Efficacy observed in the trials was more than 90%.1,2 The efficacy of other vaccines evaluated in large randomised trials, such as the Oxford-AstraZeneca (70%) and Sputnik V (91%) vaccines, have been criticised for elements ...
500+ Words Essay on Coronavirus Vaccine. The Coronavirus has infected millions of people so far all over the world. In addition to that, millions of people have lost their lives to it. Ever since the outbreak, researchers all over the world have been working constantly to develop vaccines that will work effectively against the virus.
2. Vaccination strategies. Many efforts have been directed towards the development of the vaccines against COVID-19, to avert the pandemic and most of the developing vaccine candidates have been using the S-protein of SARS-CoV-2 (Dhama et al., 2020).As of July 2, 2020, the worldwide SARS-CoV-2 vaccine landscape includes 158 vaccine candidates, out of which 135 are in the preclinical or the ...
While efficacious vaccines have been developed to inoculate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as COVID-19), public vaccine hesitancy could still ...
The Mayo Clinic model can also be used to forecast COVID-19 trends in different hypothetical worlds in which no vaccine is available, vaccinations are no longer being accepted from this point forward, and 75% of the population is already vaccinated. Surveys indicate that half of American adults are hesitant to receive a COVID-19 vaccine, and ...
The vaccine can help your unborn baby or newborn. Studies have found that expectant mothers who receive the COVID-19 vaccine create antibodies to the virus and pass those to their unborn baby through the placenta. Mothers were also shown to pass antibodies to their newborns through breast milk. This suggests those newborns have some immunity to ...
Vaccines are safe, effective and an important part of the COVID-19 response - but stopping the spread of disease remains key. Remind people that they should keep taking other precautions, such as physical distancing, wearing a mask, keeping rooms well ventilated, avoiding crowds, cleaning hands, and coughing into a bent elbow or tissue.
Examples of Persuasive Essay About Covid-19 Vaccine. Covid19 vaccines are one of the ways to prevent the spread of Covid-19, but they have been a source of controversy. Different sides argue about the benefits or dangers of the new vaccines. ... The Importance of Vaccination Mandates for COVID-19 Control; Balancing Public Health and Personal ...
In contrast, timing of COVID-19 and influenza vaccines and their type have little influence on people's willingness to get vaccinated in both countries.. The survey and discrete choice experiment ...
Conclusion: Information on COVID-19 vaccine development and deployment is an important part of health communications that includes internet search. The paper contributes to understanding health communications during a pandemic, including mix of local and non-local sources and contingency on local social and health context.
The subsequent paragraphs are going to provide detailed explanations of the reasons why it is necessary for the COVID-19 vaccine to be taken. Firstly, the COVID-19 vaccine is necessary because it helps build protection against the virus. COVID-19 is a terrifying and dangerous communicable disease. The virus affects the respiratory organ of the ...
"With the ongoing presence of COVID-19 in our society, the risk of long COVID and the long-term threat of vaccine hesitancy, continuing to improve our understanding of why people may or may not vaccinate is of great importance," said Guidry, who is now a faculty member at Tilburg University in the Netherlands. "My co-authors and I are ...
The Pfizer and Moderna COVID-19 vaccines can cause myocarditis, but do not appear to cause infertility, Guillain-Barré syndrome, Bell's palsy, thrombosis with thrombocytopenia syndrome (TTS) or heart attack, according to a new National Academies of Sciences, Engineering, and Medicine report examining whether COVID-19 vaccines can cause certain harms.
What is the Importance of Vaccination Coverage? Vaccination programs save around 4-5 million people annually from unnecessary deaths. In 1796, Edward Jenner invented the world's first vaccine ...
The Novavax COVID-19 vaccine is a protein subunit vaccine that contains portions of the spike protein, a protein that is normally found on the surface of the SARS-CoV-2 virus. The vaccine also contains Matrix-M® (an "adjuvant") that boosts the body's immune response.
WASHINGTON — A new report from the National Academies of Sciences, Engineering, and Medicine reviews evidence for 19 potential harms of the COVID-19 vaccines, and for nine potential shoulder injuries from intramuscular administration of vaccines more broadly. The committee that conducted the review identified sufficient evidence to draw 20 conclusions about whether these vaccines could cause ...
Vaccination is seen as the most promising one among the efforts to stop COVID-19 and the U.S. government has given great importance to vaccination. However, which states have performed well in administering COVID-19 vaccines and which have not is an open significant question. Another important question is what makes a state more successful than others when evaluating vaccination performance ...